User login
Obesity Intervention With Follow‐up
Obesity‐related medical care remains a substantial driver in escalating healthcare costs. Not surprisingly, healthcare costs for obese patients are 40% higher annually than those for normal‐weight individuals.[1] In 2002, the morbidity attributable to obesity was calculated to equal, if not exceed, that associated with smoking.[2] Though inpatient outcomes appear similar for obese individuals, nearly all obesity‐related comorbidities can lead to hospitalization, and obesity has been linked to early mortality.[3, 4, 5] As obesity‐related costs continue to grow, so does the need to intervene in this at‐risk patient population.[3, 4, 5] Though significant efforts have focused on obesity interventions in the outpatient setting, a paucity of data exists on how best to address obesity during inpatient hospitalization.
Hospitalization itself has often been described as a teachable moment, a time during which a life event leads to increased receptivity to behavior change.[6, 7, 8] The positive effects of inpatient smoking cessation efforts are well recognized. Such initiatives typically include an inpatient counseling session, followed by supportive contact postdischarge.[9, 10] Features common to successful outpatient weight loss interventions include ongoing patient contact of variable duration, frequent self‐weighing, diet modifications, and increased activity.[11, 12, 13, 14, 15] To date, little is known about the effectiveness of such programs in the inpatient setting, though research has shown that obese inpatients are receptive to weight loss initiatives.[16] Accomplishing even modest weight reductions in such patients has the potential to lead to significant health and cost benefits.[1, 17, 18, 19]
In this study we sought to determine whether inpatient weight loss counseling with post discharge phone follow‐up would result in significant weight loss at 6 months when compared to controls. Secondary end points included weight change from baseline and changes in waist‐to‐hip ratios (WHRs). To our knowledge, this is the first randomized trial designed to evaluate the effect of an inpatient obesity intervention with postdischarge follow‐up in a general medicine population.
METHODS
Setting/Participants
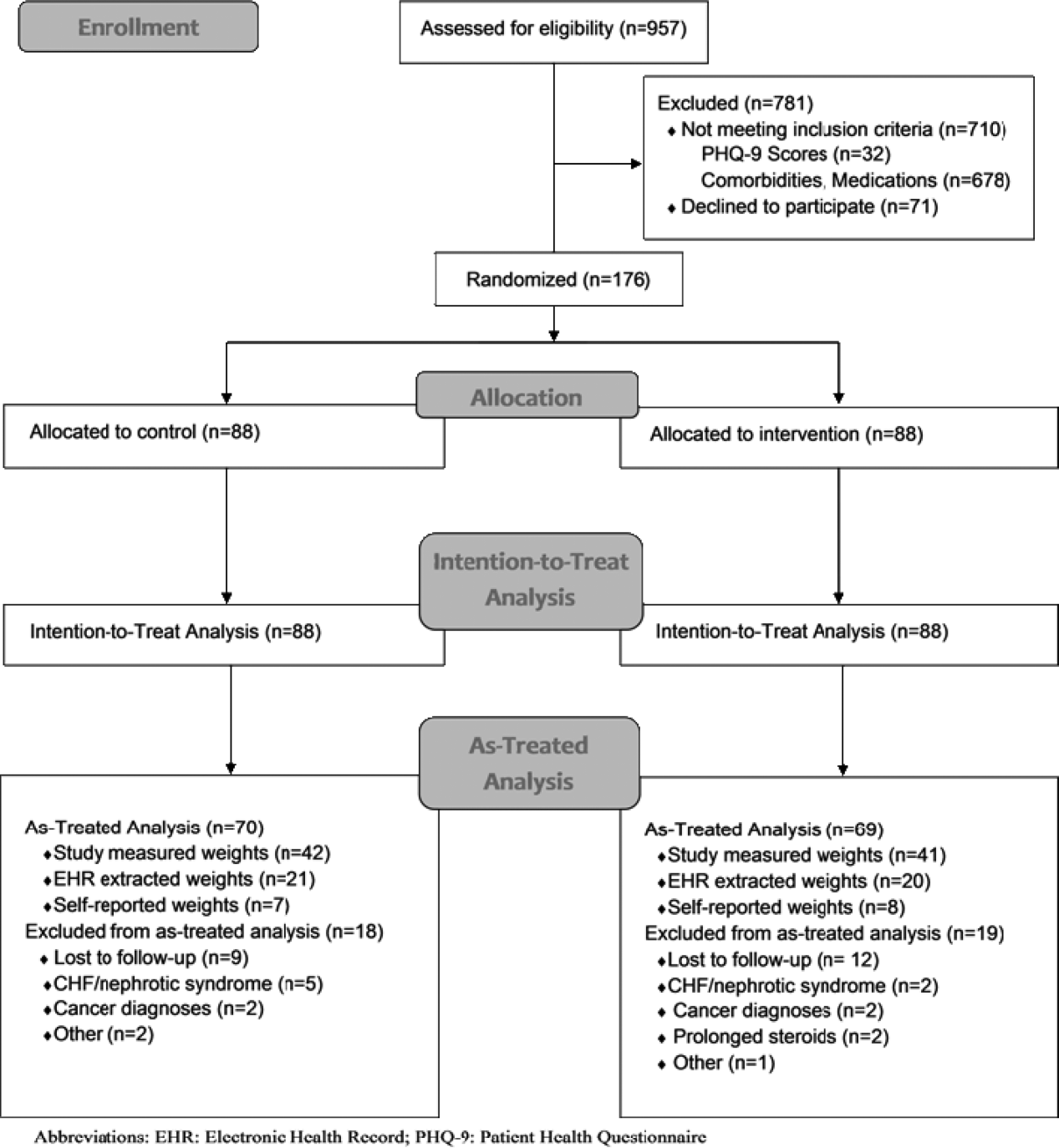
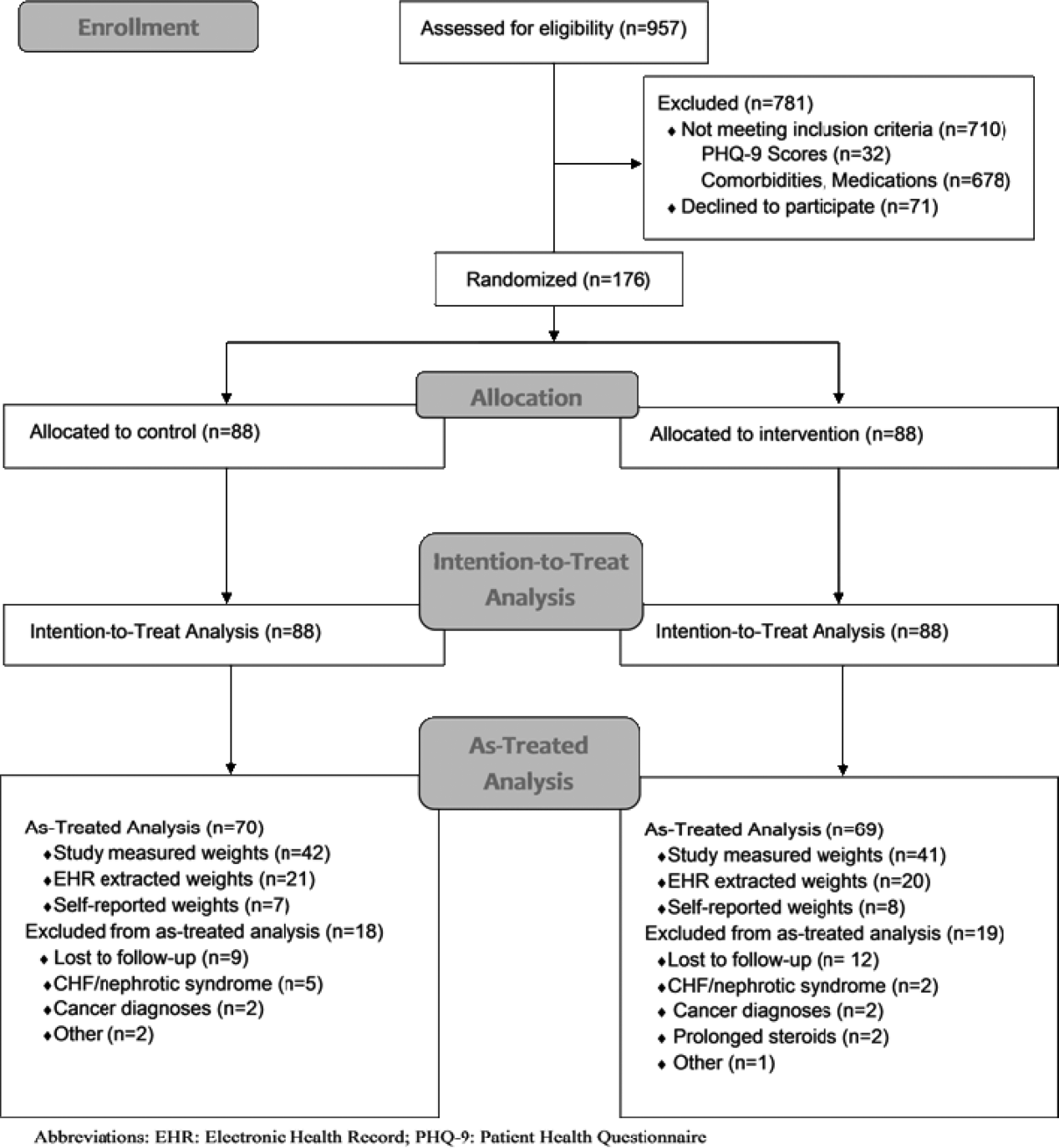
We conducted a prospective, randomized controlled trial from January 2011 to May 2012 at a single, large (854‐bed), academic medical center in Chicago, Illinois. Eligible subjects were those with a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) between 30 and 50 kg/m2, ages 18 to 65 years old, admitted to an internal medicine service. Exclusion criteria included the presence of acute medical conditions known to affect weight, Charlson comorbidity index >3, moderate to severe major depression, prolonged steroid use (>2 weeks), initiation of medications known to affect weight (eg, diuretics), non‐English speaking, and precontemplation stage of change. Upon enrollment, subjects were randomly assigned to either the control or intervention group. A computer‐generated block randomization scheme was used to generate group assignments. Study research assistants sequentially assigned enrolled patients according to the computer‐generated randomization scheme. Group assignment was only revealed to each study participant after enrollment was complete. Figure 1 summarizes subject recruitment, randomization, and follow‐up. Informed written consent was obtained from all participants. Study participants, physicians, and investigators were unblinded. Study subjects were informed that they were participating in an obesity study as outlined on the study consent form. Study protocols and procedures were approved by the institutional review board at Northwestern University.
Interventions
After enrollment, all subjects had body weight measured on a calibrated study scale in light clothing or hospital gown without shoes. Waist circumference (narrowest circumference between the ribs and iliac crest) and hip circumference (maximum circumference of the hips) were measured to the nearest 0.1 cm. Measurements were taken in triplicate and averaged. WHR was calculated as waist circumference divided by hip circumference. All participants completed a demographic questionnaire and rated their level of agreement with 6 statements relating to weight perceptions and weight loss using a Likert scale from 1 (strongly disagree) to 10 (strongly agree).
Participants in the control group were not provided with any specific instructions regarding weight loss, diet, or exercise prior to discharge. Intervention group subjects were asked to view a 13‐minute weight loss education video (addressed specific caloric intake goals for weight loss, portion sizes), undergo a 25‐minute personalized counseling session with a certified health educator or study physician, and to set 3 specific lifestyle goals prior to discharge (weight loss, dietary, and fitness). A personal weight loss goal of 10% baseline body weight was set for intervention subjects based on obesity treatment guidelines suggesting subjects could safely lose 1 to 2 lb per week over the course of the study.[20] Clinically significant weight loss was defined as weight loss of 5% or more from baseline body weight based on literature illustrating health benefits with this amount of weight loss.[17, 18, 19]
All study subjects received a phone call schedule and weight‐tracking sheet prior to discharge, with calls scheduled at weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24. Phone calls for both groups were used to obtain weight and identify changes in medications or health condition and were conducted by a certified health educator or study physician. No problem solving, motivational support, or other specific instruction was provided to the control group, whereas phone calls for intervention subjects utilized motivational interviewing and problem‐solving techniques.
Study subjects were asked to return for an in‐person follow‐up visit at 6 months. Weight was reassessed with subjects in light clothing and without shoes on the same calibrated study scale by a certified health educator. Follow‐up WHRs were also collected.
Outcomes
The primary outcome of the study was the difference in mean weight change (change in kilograms from baseline) between control and intervention groups at 6 months. Secondary outcome measures included intragroup weight change from baseline and changes in WHR.
Measured weights were obtained for subjects who returned for 6‐month follow‐up. For those unable or unwilling to return at 6 months, measured weights were obtained from the electronic health record (EHR) and self‐reported weights requested for use in imputed weight calculations. Imputation weights for missing weight values were prioritized as follows: (1) in‐person 6‐month follow‐up weight used if available, (2) inpatient or outpatient EHR obtained weight used if in‐person weight unavailable, and (3) if neither an in‐person or EHR weight was available, a self‐reported weight was used.[21] For intention‐to‐treat analysis, baseline weight was carried forward for subjects lacking follow‐up data after enrollment, historically considered a conservative strategy in weight loss trials.[22, 23]
Statistical Analysis
Baseline patient characteristics were compared using 2 tests for categorical variables and 2‐sample t tests for continuous variables. The primary study outcome of weight change over time for each group was assessed for all study participants using an intention‐to‐treat analysis. Separate as‐treated analyses were also performed utilizing imputed weights for those who failed to follow‐up at 6 months and for study completers who had a measured study weight documented at 6 months.
Three analyzable datasets were computed: intention‐to‐treat (using all participants randomized to the study), as‐treated analysis with imputed weights, and as treated analysis with measured 6‐month study weights only. Intent‐to‐treat analysis provides the unbiased comparisons among the treatment groups. To avoid dilution of treatment effect, as‐treated analyses with imputed weights (including measured weights at 6‐month follow‐up obtained from other sources [eg, clinic visit]) and with measured study weights (completers only) were performed.
Weight change over time was analyzed with a longitudinal covariance pattern model, using an unstructured variance‐covariance matrix. Specifically, weight was modeled at all time points (baseline and weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24) using a priori contrasts and treating baseline as the reference cell to assess weight change, relative to baseline, at the 4 postbaseline time points.[24] Group effects on these a priori time contrasts were included to test for weight change differences between groups, and we specifically tested whether the group effect on weight change was equal or varied across the postbaseline time points.
We aimed to obtain a sample size of 176 subjects (88 in each group) in order to achieve 80% power to detect a 5‐kg weight loss in the intervention group after 6 months (at most standard deviation [SD]=15) and a 5‐kg difference in weight loss between groups (SD=10), assuming an of 0.05 using 2‐tailed testing and an attrition rate of 20%.
RESULTS
Over a period of 18 months we were able to recruit 176 subjects. We found no significant differences in baseline characteristics between groups (Table 1). Sixteen subjects developed exclusionary conditions after enrollment and were subsequently excluded from as‐treated data analyses. Follow‐up weight data for as‐treated analysis were available for 139 study subjects through the use of in‐person (n=83), EHR (n=41), and self‐reported (n=15) weights.
| Intervention, N=88 | Control, N=88 | |
|---|---|---|
| ||
| Age, y, mean (SD) | 48.9 (10.5) | 48.7 (10.3) |
| Female, % | 67.1 | 62.5 |
| Race/ethnicity, % | ||
| African American | 50.0 | 41.4 |
| Caucasian | 36.4 | 46.5 |
| Other | 13.6 | 11.6 |
| Education level, % | ||
| High school | 11.4 | 11.5 |
| College | 68.2 | 64.4 |
| Graduate level | 20.5 | 24.1 |
| Annual income, % | ||
| <$50,000 | 43.0 | 45.2 |
| $50,000$100,000 | 45.4 | 33.3 |
| >$100,000 | 11.6 | 21.4 |
| BMI, mean (SD), kg/m2 | 38.0 (5.1) | 37.5 (4.9) |
| BMI category, % | ||
| 3034.9 | 34.1 | 34.1 |
| 3539.9 | 28.4 | 37.5 |
| 40 | 37.5 | 28.4 |
| Waist‐hip ratio, mean (SD)a | 0.95 (0.08) | 0.96 (0.08) |
| Length of stay, d, median (interquartile range) | 2.0 (1.13.0) | 2.2 (1.33.3) |
| Diabetes, % | 27.3 | 25.0 |
| Admit diagnosis, % | ||
| Cardiovascular | 34.1 | 25.0 |
| Gastrointestinal | 15.9 | 18.2 |
| Pulmonary | 10.2 | 5.7 |
| Infectious | 11.4 | 13.6 |
| Endocrine | 3.4 | 2.3 |
| Other | 25.0 | 35.2 |
Change in Weight Loss and WHR
For the 176 participants included in the intent‐to‐treat analysis, mean weight loss for the intervention group and control groups was 1.08 kg (SD=4.33) and 1.35 kg (SD=3.64) at 6 months, respectively. We found no significant difference in weight loss between groups at 6 months (P=0.26), though there was statistically significant weight loss from baseline noted in both groups (P=0.02 and P=0.0008, respectively) (Table 2).
| Characteristic | Intervention Group | Control Group | P Valuea |
|---|---|---|---|
| |||
| Intent‐to‐treat analysis (all participants), kg (SD) | |||
| No. | 88 | 88 | |
| Baseline | 107.7 (16.7) | 105.1 (17.4) | 0.23 |
| 6‐month follow‐up | 106.6 (16.1) | 103.8 (17.1) | 0.16 |
| Weight change | 1.08 (4.33) | 1.35 (3.64) | 0.26 |
| As treated analysis with imputed weights, kg (SD) | |||
| No. | 69 | 70 | |
| Baseline | 108.9 (16.7) | 104.0 (16.2) | 0.08 |
| 6‐month follow‐up | 106.1 (17.2) | 102.4 (15.9) | 0.18 |
| Weight change | 2.88 (5.77) | 1.69 (5.09) | 0.12 |
| As treated analysis with measured 6‐month weights (completers), kg (SD) | |||
| No. | 41 | 42 | |
| Baseline | 109.8 (16.2) | 107.0 (18.0) | 0.47 |
| 6‐month follow‐up | 107.4 (15.0) | 104.2 (17.7) | 0.37 |
| Weight change | 2.32 (6.16) | 2.83 (4.88) | 0.68 |
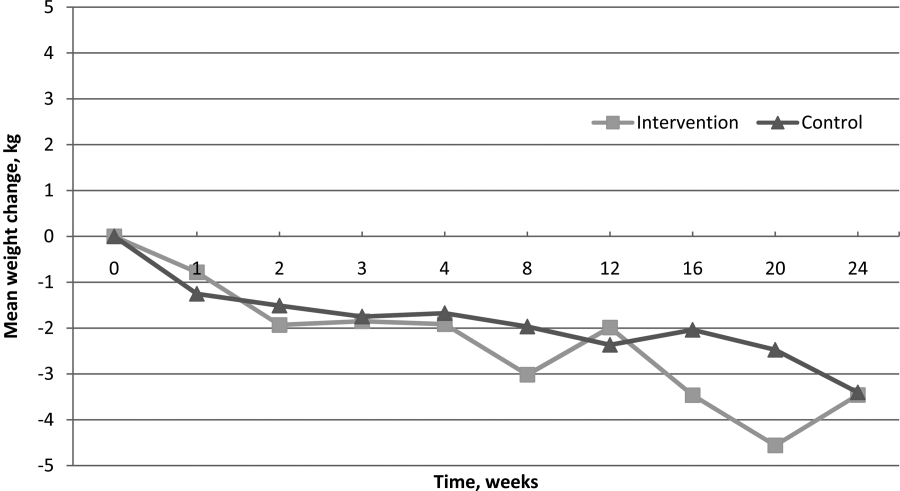
Of 139 participants in the as‐treated analysis utilizing imputed weights, weight loss for the intervention group and control groups was 2.88 kg (SD=5.77) and 1.69 kg (SD=5.09). There was statistically significant weight loss at the 6‐month follow‐up from baseline in both groups (P=0.006, P=0.004, respectively). However, there were neither statistically nor clinically significant differences between the 2 groups (1.19 kg, P=0.12). Finally, for the 83 completers in the as‐treated analysis with measured study weights only, weight loss for the intervention group and control group was 2.32 kg (SD=6.16) and 2.83 kg (SD=4.88), respectively. Though we again noted statistically significant weight loss at the 6‐month follow‐up from baseline in both groups (P=0.02, P=0.0005, respectively), we found neither statistically nor clinically significant differences in weight loss between the 2 groups (0.51 kg, P=0.68). Figure 2 illustrates weight change over time for the intervention and control subjects who returned for in‐person follow‐up at 6 months.
For WHRs, we found no difference in WHR change between groups at 6 months (0.04 vs 0.04, P=0.59). However, among those who completed the study, there was a statistically significant decrease in WHR from baseline within both groups, decreasing 0.040.06 (P=0.006) in the intervention group and 0.040.04 (P<0.001) among controls.
Weight Perceptions
Only 34% of participants accurately perceived their weight and correctly identified themselves as either obese or morbidly obese. Nearly half of the study participants (47%) classified themselves as overweight rather than obese, though all met criteria for obesity. We found weight perception was most accurate among Caucasians (48%) and least accurate among African Americans (24%) and morbidly obese individuals (26%). Nearly all subjects felt weight loss was important (99%), and most assumed weight had contributed to their hospitalization (64%).
DISCUSSION
We hypothesized that intervention group subjects would lose more weight than those assigned to control given that they received weight loss interventions previously shown to be effective.[13, 25, 26, 27] However, intention‐to‐treat analysis showed no difference in weight loss between intervention and control subjects at 6 months. Interestingly, as‐treated analyses did suggest that subjects in both study arms lost a modest amount of weight over the duration of the study. Though modest weight reductions have been shown to give rise to health benefits, neither group met our prespecified goal for clinically significant weight loss (5% of baseline body weight).[18, 19] There were also no differences in WHRs noted between the intervention and control groups. The modest reductions in WHRs from baseline in both groups are of uncertain clinical significance but of interest given the well‐established graded relationship between WHR and risk of cardiovascular disease.[28, 29, 30, 31]
Though the control group subjects received no specific instruction regarding weight loss, we suspect that the influences of study enrollment, discussion of obesity while an inpatient, regular phone contacts, and weight tracking may have been sufficient to affect weight behaviors. Certainly, this exceeds usual care for hospitalized patients suffering from obesity. Though it is possible that all of obese patients lose weight over the 6‐month period following hospitalization, we feel this is unlikely. The exclusion of subjects with an elevated Charlson comorbidity index lessened the likelihood of weight loss due to chronic disease, and without intervention, obese individuals tend to gain rather than lose weight over time.[32] Nonetheless, the lack of significant weight loss between groups suggests that the specific weight loss instruction provided to the intervention group did not promote more weight loss than the general education and regular phone calls provided to controls.
Our findings related to weight perception were similar to those established in prior studies. Individuals frequently misperceive their weight and weight perceptions are least accurate among severely obese individuals and nonwhites.[16, 33, 34] Contrary to prior studies, we found that the majority of participants felt their weight negatively impacted their health, and most thought their hospitalization was weight‐related.[35] Interestingly, research suggests that weight‐related perception of health risk correlates with the likelihood of making a weight loss attempt, another factor that may have influenced the behavior of study participants.[35]
This study has several limitations. It was conducted and based on practices at a single institution, thus limiting generalizability. Additionally, the percentage of subjects who returned for 6‐month follow‐up was lower than desired at 50%. However, high attrition rates commonly plague obesity trials, and we are unaware of any existing studies documenting expected attrition rates among obese inpatients.[23, 36, 37, 38] To help address this, we used imputed weights in our as‐treated analysis to obtain follow‐up weight values on 79% of subjects. Further, the intentional exclusion of subjects in the precontemplation stage of change likely resulted in selection of a more motivated patient population. However, this was done assuming that most inpatient obesity interventions would primarily target patients interested in losing weight. Finally, the lack of a usual care group that more accurately reflects the experience of most hospitalized obese patientsno regular postdischarge interactionsdoes limit interpretation of the modest weight loss noted in both study groups.
In conclusion, an inpatient obesity intervention with post‐discharge follow‐up did not result in intervention subjects losing more weight than controls over a 6‐month period. However, the finding of modest weight loss among both groups is of interest and may warrant further investigation. It remains unclear whether this is a naturally occurring phenomenon or whether other factors influence behavior change in this patient population. Additional studies will be needed to clarify the impact of hospitalization, obesity recognition, perception of health risk, weight tracking, and follow‐up on weight behaviors. Given the proven benefits of even modest weight reductions, encouraging any amount of weight loss in these at‐risk individuals would appear to be a step in the right direction. We have yet to determine whether inpatient obesity interventions represent a lost opportunity.
- , , , . The impact of weight loss among seniors on Medicare spending. Health Econ Rev. 2013;3(1):7.
- . The effects of obesity, smoking, and drinking on medical problems and costs. Health Aff (Millwood). 2002;21(2):245–253.
- , , , , , . The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9:88.
- , , , , , . The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103(10):1895–1901.
- , , , . Assessment of excess mortality in obesity. Am J Epidemiol. 1998;147(1):42–48.
- , , . Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure‐a review. Am J Cardiol. 2004;94(9):1155–1160.
- . In‐hospital initiation of statins: taking advantage of the “teachable moment”. Cleve Clin J Med. 2003;70(6):502, 504–506.
- , , . Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170.
- , , . Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med. 2008;168(18):1950–1960.
- , . Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial. CMAJ. 2009;180(13):1297–1303.
- , , , , , . Phone and e‐mail counselling are effective for weight management in an overweight working population: a randomized controlled trial. BMC Public Health. 2009;9:6.
- , . Weighing the evidence: benefits of regular weight monitoring for weight control. J Nutr Educ Behav. 2005;37(6):319–322.
- , , , et al. Comparative effectiveness of weight‐loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–1968.
- , , , et al. Weight‐loss outcomes: a systematic review and meta‐analysis of weight‐loss clinical trials with a minimum 1‐year follow‐up. J Am Diet Assoc. 2007;107(10):1755–1767.
- , , . Self‐monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102.
- , , , . Willingness for weight loss intervention among overweight and obese inpatients. South Med J. 2011;104(6):397–400.
- , , , et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486.
- , , . The beneficial effects of modest weight loss on cardiovascular risk factors. Int J Obes Relat Metab Disord. 1997;21(suppl 1):S5–S9.
- . Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16(6):397–415.
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(suppl 2):51S–209S.
- , , , et al. Validity of clinical body weight measures as substitutes for missing data in a randomized trial. Obes Res Clin Pract. 2008;2(4):277–281.
- , , . Advances in analysis of longitudinal data. Ann Rev Clin Psychol. 2010;6:79–107.
- , , , et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PloS One. 2009;4(8):e6624.
- , . Longitudinal Data Analysis. Hoboken, NJ: Wiley‐Interscience; 2006.
- , , , . Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150(4):255–262.
- . Clinical practice. Nonsurgical management of obesity in adults. N Engl J Med. 2008;358(18):1941–1950.
- , , . A meta‐analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord. 1997;21(10):941–947.
- , , , et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population‐based prospective study. Circulation. 2007;116(25):2933–2943.
- , , , et al. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50(8):752–759.
- , , , et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case‐control study. Lancet. 2005;366(9497):1640–1649.
- , , , . Waist circumference and waist‐to‐hip ratio as predictors of cardiovascular events: meta‐regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–856.
- , , , . Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855.
- , . Self‐perception of weight appropriateness in the United States. Am J Prev Med. 2003;24(4):332–339.
- , , , et al. Differences in weight perception among blacks and whites. J Womens Health (Larchmt). 2011;20(12):1805–1811.
- , , , , . Perceived health risk of excess body weight among overweight and obese men and women: differences by sex. Prev Med. 2008;47(1):46–52.
- , , . Obesity research—limitations of methods, measurements, and medications. JAMA. 2006;295(7):826–828.
- . Interpreting incomplete data in studies of diet and weight loss. N Engl J Med. 2003;348(21):2136–2137.
- , , , . Predictors of attrition in a large clinic‐based weight‐loss program. Obes Res. 2003;11(7):888–894.
Obesity‐related medical care remains a substantial driver in escalating healthcare costs. Not surprisingly, healthcare costs for obese patients are 40% higher annually than those for normal‐weight individuals.[1] In 2002, the morbidity attributable to obesity was calculated to equal, if not exceed, that associated with smoking.[2] Though inpatient outcomes appear similar for obese individuals, nearly all obesity‐related comorbidities can lead to hospitalization, and obesity has been linked to early mortality.[3, 4, 5] As obesity‐related costs continue to grow, so does the need to intervene in this at‐risk patient population.[3, 4, 5] Though significant efforts have focused on obesity interventions in the outpatient setting, a paucity of data exists on how best to address obesity during inpatient hospitalization.
Hospitalization itself has often been described as a teachable moment, a time during which a life event leads to increased receptivity to behavior change.[6, 7, 8] The positive effects of inpatient smoking cessation efforts are well recognized. Such initiatives typically include an inpatient counseling session, followed by supportive contact postdischarge.[9, 10] Features common to successful outpatient weight loss interventions include ongoing patient contact of variable duration, frequent self‐weighing, diet modifications, and increased activity.[11, 12, 13, 14, 15] To date, little is known about the effectiveness of such programs in the inpatient setting, though research has shown that obese inpatients are receptive to weight loss initiatives.[16] Accomplishing even modest weight reductions in such patients has the potential to lead to significant health and cost benefits.[1, 17, 18, 19]
In this study we sought to determine whether inpatient weight loss counseling with post discharge phone follow‐up would result in significant weight loss at 6 months when compared to controls. Secondary end points included weight change from baseline and changes in waist‐to‐hip ratios (WHRs). To our knowledge, this is the first randomized trial designed to evaluate the effect of an inpatient obesity intervention with postdischarge follow‐up in a general medicine population.
METHODS
Setting/Participants
We conducted a prospective, randomized controlled trial from January 2011 to May 2012 at a single, large (854‐bed), academic medical center in Chicago, Illinois. Eligible subjects were those with a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) between 30 and 50 kg/m2, ages 18 to 65 years old, admitted to an internal medicine service. Exclusion criteria included the presence of acute medical conditions known to affect weight, Charlson comorbidity index >3, moderate to severe major depression, prolonged steroid use (>2 weeks), initiation of medications known to affect weight (eg, diuretics), non‐English speaking, and precontemplation stage of change. Upon enrollment, subjects were randomly assigned to either the control or intervention group. A computer‐generated block randomization scheme was used to generate group assignments. Study research assistants sequentially assigned enrolled patients according to the computer‐generated randomization scheme. Group assignment was only revealed to each study participant after enrollment was complete. Figure 1 summarizes subject recruitment, randomization, and follow‐up. Informed written consent was obtained from all participants. Study participants, physicians, and investigators were unblinded. Study subjects were informed that they were participating in an obesity study as outlined on the study consent form. Study protocols and procedures were approved by the institutional review board at Northwestern University.

Interventions
After enrollment, all subjects had body weight measured on a calibrated study scale in light clothing or hospital gown without shoes. Waist circumference (narrowest circumference between the ribs and iliac crest) and hip circumference (maximum circumference of the hips) were measured to the nearest 0.1 cm. Measurements were taken in triplicate and averaged. WHR was calculated as waist circumference divided by hip circumference. All participants completed a demographic questionnaire and rated their level of agreement with 6 statements relating to weight perceptions and weight loss using a Likert scale from 1 (strongly disagree) to 10 (strongly agree).
Participants in the control group were not provided with any specific instructions regarding weight loss, diet, or exercise prior to discharge. Intervention group subjects were asked to view a 13‐minute weight loss education video (addressed specific caloric intake goals for weight loss, portion sizes), undergo a 25‐minute personalized counseling session with a certified health educator or study physician, and to set 3 specific lifestyle goals prior to discharge (weight loss, dietary, and fitness). A personal weight loss goal of 10% baseline body weight was set for intervention subjects based on obesity treatment guidelines suggesting subjects could safely lose 1 to 2 lb per week over the course of the study.[20] Clinically significant weight loss was defined as weight loss of 5% or more from baseline body weight based on literature illustrating health benefits with this amount of weight loss.[17, 18, 19]
All study subjects received a phone call schedule and weight‐tracking sheet prior to discharge, with calls scheduled at weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24. Phone calls for both groups were used to obtain weight and identify changes in medications or health condition and were conducted by a certified health educator or study physician. No problem solving, motivational support, or other specific instruction was provided to the control group, whereas phone calls for intervention subjects utilized motivational interviewing and problem‐solving techniques.
Study subjects were asked to return for an in‐person follow‐up visit at 6 months. Weight was reassessed with subjects in light clothing and without shoes on the same calibrated study scale by a certified health educator. Follow‐up WHRs were also collected.
Outcomes
The primary outcome of the study was the difference in mean weight change (change in kilograms from baseline) between control and intervention groups at 6 months. Secondary outcome measures included intragroup weight change from baseline and changes in WHR.
Measured weights were obtained for subjects who returned for 6‐month follow‐up. For those unable or unwilling to return at 6 months, measured weights were obtained from the electronic health record (EHR) and self‐reported weights requested for use in imputed weight calculations. Imputation weights for missing weight values were prioritized as follows: (1) in‐person 6‐month follow‐up weight used if available, (2) inpatient or outpatient EHR obtained weight used if in‐person weight unavailable, and (3) if neither an in‐person or EHR weight was available, a self‐reported weight was used.[21] For intention‐to‐treat analysis, baseline weight was carried forward for subjects lacking follow‐up data after enrollment, historically considered a conservative strategy in weight loss trials.[22, 23]
Statistical Analysis
Baseline patient characteristics were compared using 2 tests for categorical variables and 2‐sample t tests for continuous variables. The primary study outcome of weight change over time for each group was assessed for all study participants using an intention‐to‐treat analysis. Separate as‐treated analyses were also performed utilizing imputed weights for those who failed to follow‐up at 6 months and for study completers who had a measured study weight documented at 6 months.
Three analyzable datasets were computed: intention‐to‐treat (using all participants randomized to the study), as‐treated analysis with imputed weights, and as treated analysis with measured 6‐month study weights only. Intent‐to‐treat analysis provides the unbiased comparisons among the treatment groups. To avoid dilution of treatment effect, as‐treated analyses with imputed weights (including measured weights at 6‐month follow‐up obtained from other sources [eg, clinic visit]) and with measured study weights (completers only) were performed.
Weight change over time was analyzed with a longitudinal covariance pattern model, using an unstructured variance‐covariance matrix. Specifically, weight was modeled at all time points (baseline and weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24) using a priori contrasts and treating baseline as the reference cell to assess weight change, relative to baseline, at the 4 postbaseline time points.[24] Group effects on these a priori time contrasts were included to test for weight change differences between groups, and we specifically tested whether the group effect on weight change was equal or varied across the postbaseline time points.
We aimed to obtain a sample size of 176 subjects (88 in each group) in order to achieve 80% power to detect a 5‐kg weight loss in the intervention group after 6 months (at most standard deviation [SD]=15) and a 5‐kg difference in weight loss between groups (SD=10), assuming an of 0.05 using 2‐tailed testing and an attrition rate of 20%.
RESULTS
Over a period of 18 months we were able to recruit 176 subjects. We found no significant differences in baseline characteristics between groups (Table 1). Sixteen subjects developed exclusionary conditions after enrollment and were subsequently excluded from as‐treated data analyses. Follow‐up weight data for as‐treated analysis were available for 139 study subjects through the use of in‐person (n=83), EHR (n=41), and self‐reported (n=15) weights.
| Intervention, N=88 | Control, N=88 | |
|---|---|---|
| ||
| Age, y, mean (SD) | 48.9 (10.5) | 48.7 (10.3) |
| Female, % | 67.1 | 62.5 |
| Race/ethnicity, % | ||
| African American | 50.0 | 41.4 |
| Caucasian | 36.4 | 46.5 |
| Other | 13.6 | 11.6 |
| Education level, % | ||
| High school | 11.4 | 11.5 |
| College | 68.2 | 64.4 |
| Graduate level | 20.5 | 24.1 |
| Annual income, % | ||
| <$50,000 | 43.0 | 45.2 |
| $50,000$100,000 | 45.4 | 33.3 |
| >$100,000 | 11.6 | 21.4 |
| BMI, mean (SD), kg/m2 | 38.0 (5.1) | 37.5 (4.9) |
| BMI category, % | ||
| 3034.9 | 34.1 | 34.1 |
| 3539.9 | 28.4 | 37.5 |
| 40 | 37.5 | 28.4 |
| Waist‐hip ratio, mean (SD)a | 0.95 (0.08) | 0.96 (0.08) |
| Length of stay, d, median (interquartile range) | 2.0 (1.13.0) | 2.2 (1.33.3) |
| Diabetes, % | 27.3 | 25.0 |
| Admit diagnosis, % | ||
| Cardiovascular | 34.1 | 25.0 |
| Gastrointestinal | 15.9 | 18.2 |
| Pulmonary | 10.2 | 5.7 |
| Infectious | 11.4 | 13.6 |
| Endocrine | 3.4 | 2.3 |
| Other | 25.0 | 35.2 |
Change in Weight Loss and WHR
For the 176 participants included in the intent‐to‐treat analysis, mean weight loss for the intervention group and control groups was 1.08 kg (SD=4.33) and 1.35 kg (SD=3.64) at 6 months, respectively. We found no significant difference in weight loss between groups at 6 months (P=0.26), though there was statistically significant weight loss from baseline noted in both groups (P=0.02 and P=0.0008, respectively) (Table 2).
| Characteristic | Intervention Group | Control Group | P Valuea |
|---|---|---|---|
| |||
| Intent‐to‐treat analysis (all participants), kg (SD) | |||
| No. | 88 | 88 | |
| Baseline | 107.7 (16.7) | 105.1 (17.4) | 0.23 |
| 6‐month follow‐up | 106.6 (16.1) | 103.8 (17.1) | 0.16 |
| Weight change | 1.08 (4.33) | 1.35 (3.64) | 0.26 |
| As treated analysis with imputed weights, kg (SD) | |||
| No. | 69 | 70 | |
| Baseline | 108.9 (16.7) | 104.0 (16.2) | 0.08 |
| 6‐month follow‐up | 106.1 (17.2) | 102.4 (15.9) | 0.18 |
| Weight change | 2.88 (5.77) | 1.69 (5.09) | 0.12 |
| As treated analysis with measured 6‐month weights (completers), kg (SD) | |||
| No. | 41 | 42 | |
| Baseline | 109.8 (16.2) | 107.0 (18.0) | 0.47 |
| 6‐month follow‐up | 107.4 (15.0) | 104.2 (17.7) | 0.37 |
| Weight change | 2.32 (6.16) | 2.83 (4.88) | 0.68 |
Of 139 participants in the as‐treated analysis utilizing imputed weights, weight loss for the intervention group and control groups was 2.88 kg (SD=5.77) and 1.69 kg (SD=5.09). There was statistically significant weight loss at the 6‐month follow‐up from baseline in both groups (P=0.006, P=0.004, respectively). However, there were neither statistically nor clinically significant differences between the 2 groups (1.19 kg, P=0.12). Finally, for the 83 completers in the as‐treated analysis with measured study weights only, weight loss for the intervention group and control group was 2.32 kg (SD=6.16) and 2.83 kg (SD=4.88), respectively. Though we again noted statistically significant weight loss at the 6‐month follow‐up from baseline in both groups (P=0.02, P=0.0005, respectively), we found neither statistically nor clinically significant differences in weight loss between the 2 groups (0.51 kg, P=0.68). Figure 2 illustrates weight change over time for the intervention and control subjects who returned for in‐person follow‐up at 6 months.

For WHRs, we found no difference in WHR change between groups at 6 months (0.04 vs 0.04, P=0.59). However, among those who completed the study, there was a statistically significant decrease in WHR from baseline within both groups, decreasing 0.040.06 (P=0.006) in the intervention group and 0.040.04 (P<0.001) among controls.
Weight Perceptions
Only 34% of participants accurately perceived their weight and correctly identified themselves as either obese or morbidly obese. Nearly half of the study participants (47%) classified themselves as overweight rather than obese, though all met criteria for obesity. We found weight perception was most accurate among Caucasians (48%) and least accurate among African Americans (24%) and morbidly obese individuals (26%). Nearly all subjects felt weight loss was important (99%), and most assumed weight had contributed to their hospitalization (64%).
DISCUSSION
We hypothesized that intervention group subjects would lose more weight than those assigned to control given that they received weight loss interventions previously shown to be effective.[13, 25, 26, 27] However, intention‐to‐treat analysis showed no difference in weight loss between intervention and control subjects at 6 months. Interestingly, as‐treated analyses did suggest that subjects in both study arms lost a modest amount of weight over the duration of the study. Though modest weight reductions have been shown to give rise to health benefits, neither group met our prespecified goal for clinically significant weight loss (5% of baseline body weight).[18, 19] There were also no differences in WHRs noted between the intervention and control groups. The modest reductions in WHRs from baseline in both groups are of uncertain clinical significance but of interest given the well‐established graded relationship between WHR and risk of cardiovascular disease.[28, 29, 30, 31]
Though the control group subjects received no specific instruction regarding weight loss, we suspect that the influences of study enrollment, discussion of obesity while an inpatient, regular phone contacts, and weight tracking may have been sufficient to affect weight behaviors. Certainly, this exceeds usual care for hospitalized patients suffering from obesity. Though it is possible that all of obese patients lose weight over the 6‐month period following hospitalization, we feel this is unlikely. The exclusion of subjects with an elevated Charlson comorbidity index lessened the likelihood of weight loss due to chronic disease, and without intervention, obese individuals tend to gain rather than lose weight over time.[32] Nonetheless, the lack of significant weight loss between groups suggests that the specific weight loss instruction provided to the intervention group did not promote more weight loss than the general education and regular phone calls provided to controls.
Our findings related to weight perception were similar to those established in prior studies. Individuals frequently misperceive their weight and weight perceptions are least accurate among severely obese individuals and nonwhites.[16, 33, 34] Contrary to prior studies, we found that the majority of participants felt their weight negatively impacted their health, and most thought their hospitalization was weight‐related.[35] Interestingly, research suggests that weight‐related perception of health risk correlates with the likelihood of making a weight loss attempt, another factor that may have influenced the behavior of study participants.[35]
This study has several limitations. It was conducted and based on practices at a single institution, thus limiting generalizability. Additionally, the percentage of subjects who returned for 6‐month follow‐up was lower than desired at 50%. However, high attrition rates commonly plague obesity trials, and we are unaware of any existing studies documenting expected attrition rates among obese inpatients.[23, 36, 37, 38] To help address this, we used imputed weights in our as‐treated analysis to obtain follow‐up weight values on 79% of subjects. Further, the intentional exclusion of subjects in the precontemplation stage of change likely resulted in selection of a more motivated patient population. However, this was done assuming that most inpatient obesity interventions would primarily target patients interested in losing weight. Finally, the lack of a usual care group that more accurately reflects the experience of most hospitalized obese patientsno regular postdischarge interactionsdoes limit interpretation of the modest weight loss noted in both study groups.
In conclusion, an inpatient obesity intervention with post‐discharge follow‐up did not result in intervention subjects losing more weight than controls over a 6‐month period. However, the finding of modest weight loss among both groups is of interest and may warrant further investigation. It remains unclear whether this is a naturally occurring phenomenon or whether other factors influence behavior change in this patient population. Additional studies will be needed to clarify the impact of hospitalization, obesity recognition, perception of health risk, weight tracking, and follow‐up on weight behaviors. Given the proven benefits of even modest weight reductions, encouraging any amount of weight loss in these at‐risk individuals would appear to be a step in the right direction. We have yet to determine whether inpatient obesity interventions represent a lost opportunity.
Obesity‐related medical care remains a substantial driver in escalating healthcare costs. Not surprisingly, healthcare costs for obese patients are 40% higher annually than those for normal‐weight individuals.[1] In 2002, the morbidity attributable to obesity was calculated to equal, if not exceed, that associated with smoking.[2] Though inpatient outcomes appear similar for obese individuals, nearly all obesity‐related comorbidities can lead to hospitalization, and obesity has been linked to early mortality.[3, 4, 5] As obesity‐related costs continue to grow, so does the need to intervene in this at‐risk patient population.[3, 4, 5] Though significant efforts have focused on obesity interventions in the outpatient setting, a paucity of data exists on how best to address obesity during inpatient hospitalization.
Hospitalization itself has often been described as a teachable moment, a time during which a life event leads to increased receptivity to behavior change.[6, 7, 8] The positive effects of inpatient smoking cessation efforts are well recognized. Such initiatives typically include an inpatient counseling session, followed by supportive contact postdischarge.[9, 10] Features common to successful outpatient weight loss interventions include ongoing patient contact of variable duration, frequent self‐weighing, diet modifications, and increased activity.[11, 12, 13, 14, 15] To date, little is known about the effectiveness of such programs in the inpatient setting, though research has shown that obese inpatients are receptive to weight loss initiatives.[16] Accomplishing even modest weight reductions in such patients has the potential to lead to significant health and cost benefits.[1, 17, 18, 19]
In this study we sought to determine whether inpatient weight loss counseling with post discharge phone follow‐up would result in significant weight loss at 6 months when compared to controls. Secondary end points included weight change from baseline and changes in waist‐to‐hip ratios (WHRs). To our knowledge, this is the first randomized trial designed to evaluate the effect of an inpatient obesity intervention with postdischarge follow‐up in a general medicine population.
METHODS
Setting/Participants
We conducted a prospective, randomized controlled trial from January 2011 to May 2012 at a single, large (854‐bed), academic medical center in Chicago, Illinois. Eligible subjects were those with a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) between 30 and 50 kg/m2, ages 18 to 65 years old, admitted to an internal medicine service. Exclusion criteria included the presence of acute medical conditions known to affect weight, Charlson comorbidity index >3, moderate to severe major depression, prolonged steroid use (>2 weeks), initiation of medications known to affect weight (eg, diuretics), non‐English speaking, and precontemplation stage of change. Upon enrollment, subjects were randomly assigned to either the control or intervention group. A computer‐generated block randomization scheme was used to generate group assignments. Study research assistants sequentially assigned enrolled patients according to the computer‐generated randomization scheme. Group assignment was only revealed to each study participant after enrollment was complete. Figure 1 summarizes subject recruitment, randomization, and follow‐up. Informed written consent was obtained from all participants. Study participants, physicians, and investigators were unblinded. Study subjects were informed that they were participating in an obesity study as outlined on the study consent form. Study protocols and procedures were approved by the institutional review board at Northwestern University.

Interventions
After enrollment, all subjects had body weight measured on a calibrated study scale in light clothing or hospital gown without shoes. Waist circumference (narrowest circumference between the ribs and iliac crest) and hip circumference (maximum circumference of the hips) were measured to the nearest 0.1 cm. Measurements were taken in triplicate and averaged. WHR was calculated as waist circumference divided by hip circumference. All participants completed a demographic questionnaire and rated their level of agreement with 6 statements relating to weight perceptions and weight loss using a Likert scale from 1 (strongly disagree) to 10 (strongly agree).
Participants in the control group were not provided with any specific instructions regarding weight loss, diet, or exercise prior to discharge. Intervention group subjects were asked to view a 13‐minute weight loss education video (addressed specific caloric intake goals for weight loss, portion sizes), undergo a 25‐minute personalized counseling session with a certified health educator or study physician, and to set 3 specific lifestyle goals prior to discharge (weight loss, dietary, and fitness). A personal weight loss goal of 10% baseline body weight was set for intervention subjects based on obesity treatment guidelines suggesting subjects could safely lose 1 to 2 lb per week over the course of the study.[20] Clinically significant weight loss was defined as weight loss of 5% or more from baseline body weight based on literature illustrating health benefits with this amount of weight loss.[17, 18, 19]
All study subjects received a phone call schedule and weight‐tracking sheet prior to discharge, with calls scheduled at weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24. Phone calls for both groups were used to obtain weight and identify changes in medications or health condition and were conducted by a certified health educator or study physician. No problem solving, motivational support, or other specific instruction was provided to the control group, whereas phone calls for intervention subjects utilized motivational interviewing and problem‐solving techniques.
Study subjects were asked to return for an in‐person follow‐up visit at 6 months. Weight was reassessed with subjects in light clothing and without shoes on the same calibrated study scale by a certified health educator. Follow‐up WHRs were also collected.
Outcomes
The primary outcome of the study was the difference in mean weight change (change in kilograms from baseline) between control and intervention groups at 6 months. Secondary outcome measures included intragroup weight change from baseline and changes in WHR.
Measured weights were obtained for subjects who returned for 6‐month follow‐up. For those unable or unwilling to return at 6 months, measured weights were obtained from the electronic health record (EHR) and self‐reported weights requested for use in imputed weight calculations. Imputation weights for missing weight values were prioritized as follows: (1) in‐person 6‐month follow‐up weight used if available, (2) inpatient or outpatient EHR obtained weight used if in‐person weight unavailable, and (3) if neither an in‐person or EHR weight was available, a self‐reported weight was used.[21] For intention‐to‐treat analysis, baseline weight was carried forward for subjects lacking follow‐up data after enrollment, historically considered a conservative strategy in weight loss trials.[22, 23]
Statistical Analysis
Baseline patient characteristics were compared using 2 tests for categorical variables and 2‐sample t tests for continuous variables. The primary study outcome of weight change over time for each group was assessed for all study participants using an intention‐to‐treat analysis. Separate as‐treated analyses were also performed utilizing imputed weights for those who failed to follow‐up at 6 months and for study completers who had a measured study weight documented at 6 months.
Three analyzable datasets were computed: intention‐to‐treat (using all participants randomized to the study), as‐treated analysis with imputed weights, and as treated analysis with measured 6‐month study weights only. Intent‐to‐treat analysis provides the unbiased comparisons among the treatment groups. To avoid dilution of treatment effect, as‐treated analyses with imputed weights (including measured weights at 6‐month follow‐up obtained from other sources [eg, clinic visit]) and with measured study weights (completers only) were performed.
Weight change over time was analyzed with a longitudinal covariance pattern model, using an unstructured variance‐covariance matrix. Specifically, weight was modeled at all time points (baseline and weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24) using a priori contrasts and treating baseline as the reference cell to assess weight change, relative to baseline, at the 4 postbaseline time points.[24] Group effects on these a priori time contrasts were included to test for weight change differences between groups, and we specifically tested whether the group effect on weight change was equal or varied across the postbaseline time points.
We aimed to obtain a sample size of 176 subjects (88 in each group) in order to achieve 80% power to detect a 5‐kg weight loss in the intervention group after 6 months (at most standard deviation [SD]=15) and a 5‐kg difference in weight loss between groups (SD=10), assuming an of 0.05 using 2‐tailed testing and an attrition rate of 20%.
RESULTS
Over a period of 18 months we were able to recruit 176 subjects. We found no significant differences in baseline characteristics between groups (Table 1). Sixteen subjects developed exclusionary conditions after enrollment and were subsequently excluded from as‐treated data analyses. Follow‐up weight data for as‐treated analysis were available for 139 study subjects through the use of in‐person (n=83), EHR (n=41), and self‐reported (n=15) weights.
| Intervention, N=88 | Control, N=88 | |
|---|---|---|
| ||
| Age, y, mean (SD) | 48.9 (10.5) | 48.7 (10.3) |
| Female, % | 67.1 | 62.5 |
| Race/ethnicity, % | ||
| African American | 50.0 | 41.4 |
| Caucasian | 36.4 | 46.5 |
| Other | 13.6 | 11.6 |
| Education level, % | ||
| High school | 11.4 | 11.5 |
| College | 68.2 | 64.4 |
| Graduate level | 20.5 | 24.1 |
| Annual income, % | ||
| <$50,000 | 43.0 | 45.2 |
| $50,000$100,000 | 45.4 | 33.3 |
| >$100,000 | 11.6 | 21.4 |
| BMI, mean (SD), kg/m2 | 38.0 (5.1) | 37.5 (4.9) |
| BMI category, % | ||
| 3034.9 | 34.1 | 34.1 |
| 3539.9 | 28.4 | 37.5 |
| 40 | 37.5 | 28.4 |
| Waist‐hip ratio, mean (SD)a | 0.95 (0.08) | 0.96 (0.08) |
| Length of stay, d, median (interquartile range) | 2.0 (1.13.0) | 2.2 (1.33.3) |
| Diabetes, % | 27.3 | 25.0 |
| Admit diagnosis, % | ||
| Cardiovascular | 34.1 | 25.0 |
| Gastrointestinal | 15.9 | 18.2 |
| Pulmonary | 10.2 | 5.7 |
| Infectious | 11.4 | 13.6 |
| Endocrine | 3.4 | 2.3 |
| Other | 25.0 | 35.2 |
Change in Weight Loss and WHR
For the 176 participants included in the intent‐to‐treat analysis, mean weight loss for the intervention group and control groups was 1.08 kg (SD=4.33) and 1.35 kg (SD=3.64) at 6 months, respectively. We found no significant difference in weight loss between groups at 6 months (P=0.26), though there was statistically significant weight loss from baseline noted in both groups (P=0.02 and P=0.0008, respectively) (Table 2).
| Characteristic | Intervention Group | Control Group | P Valuea |
|---|---|---|---|
| |||
| Intent‐to‐treat analysis (all participants), kg (SD) | |||
| No. | 88 | 88 | |
| Baseline | 107.7 (16.7) | 105.1 (17.4) | 0.23 |
| 6‐month follow‐up | 106.6 (16.1) | 103.8 (17.1) | 0.16 |
| Weight change | 1.08 (4.33) | 1.35 (3.64) | 0.26 |
| As treated analysis with imputed weights, kg (SD) | |||
| No. | 69 | 70 | |
| Baseline | 108.9 (16.7) | 104.0 (16.2) | 0.08 |
| 6‐month follow‐up | 106.1 (17.2) | 102.4 (15.9) | 0.18 |
| Weight change | 2.88 (5.77) | 1.69 (5.09) | 0.12 |
| As treated analysis with measured 6‐month weights (completers), kg (SD) | |||
| No. | 41 | 42 | |
| Baseline | 109.8 (16.2) | 107.0 (18.0) | 0.47 |
| 6‐month follow‐up | 107.4 (15.0) | 104.2 (17.7) | 0.37 |
| Weight change | 2.32 (6.16) | 2.83 (4.88) | 0.68 |
Of 139 participants in the as‐treated analysis utilizing imputed weights, weight loss for the intervention group and control groups was 2.88 kg (SD=5.77) and 1.69 kg (SD=5.09). There was statistically significant weight loss at the 6‐month follow‐up from baseline in both groups (P=0.006, P=0.004, respectively). However, there were neither statistically nor clinically significant differences between the 2 groups (1.19 kg, P=0.12). Finally, for the 83 completers in the as‐treated analysis with measured study weights only, weight loss for the intervention group and control group was 2.32 kg (SD=6.16) and 2.83 kg (SD=4.88), respectively. Though we again noted statistically significant weight loss at the 6‐month follow‐up from baseline in both groups (P=0.02, P=0.0005, respectively), we found neither statistically nor clinically significant differences in weight loss between the 2 groups (0.51 kg, P=0.68). Figure 2 illustrates weight change over time for the intervention and control subjects who returned for in‐person follow‐up at 6 months.

For WHRs, we found no difference in WHR change between groups at 6 months (0.04 vs 0.04, P=0.59). However, among those who completed the study, there was a statistically significant decrease in WHR from baseline within both groups, decreasing 0.040.06 (P=0.006) in the intervention group and 0.040.04 (P<0.001) among controls.
Weight Perceptions
Only 34% of participants accurately perceived their weight and correctly identified themselves as either obese or morbidly obese. Nearly half of the study participants (47%) classified themselves as overweight rather than obese, though all met criteria for obesity. We found weight perception was most accurate among Caucasians (48%) and least accurate among African Americans (24%) and morbidly obese individuals (26%). Nearly all subjects felt weight loss was important (99%), and most assumed weight had contributed to their hospitalization (64%).
DISCUSSION
We hypothesized that intervention group subjects would lose more weight than those assigned to control given that they received weight loss interventions previously shown to be effective.[13, 25, 26, 27] However, intention‐to‐treat analysis showed no difference in weight loss between intervention and control subjects at 6 months. Interestingly, as‐treated analyses did suggest that subjects in both study arms lost a modest amount of weight over the duration of the study. Though modest weight reductions have been shown to give rise to health benefits, neither group met our prespecified goal for clinically significant weight loss (5% of baseline body weight).[18, 19] There were also no differences in WHRs noted between the intervention and control groups. The modest reductions in WHRs from baseline in both groups are of uncertain clinical significance but of interest given the well‐established graded relationship between WHR and risk of cardiovascular disease.[28, 29, 30, 31]
Though the control group subjects received no specific instruction regarding weight loss, we suspect that the influences of study enrollment, discussion of obesity while an inpatient, regular phone contacts, and weight tracking may have been sufficient to affect weight behaviors. Certainly, this exceeds usual care for hospitalized patients suffering from obesity. Though it is possible that all of obese patients lose weight over the 6‐month period following hospitalization, we feel this is unlikely. The exclusion of subjects with an elevated Charlson comorbidity index lessened the likelihood of weight loss due to chronic disease, and without intervention, obese individuals tend to gain rather than lose weight over time.[32] Nonetheless, the lack of significant weight loss between groups suggests that the specific weight loss instruction provided to the intervention group did not promote more weight loss than the general education and regular phone calls provided to controls.
Our findings related to weight perception were similar to those established in prior studies. Individuals frequently misperceive their weight and weight perceptions are least accurate among severely obese individuals and nonwhites.[16, 33, 34] Contrary to prior studies, we found that the majority of participants felt their weight negatively impacted their health, and most thought their hospitalization was weight‐related.[35] Interestingly, research suggests that weight‐related perception of health risk correlates with the likelihood of making a weight loss attempt, another factor that may have influenced the behavior of study participants.[35]
This study has several limitations. It was conducted and based on practices at a single institution, thus limiting generalizability. Additionally, the percentage of subjects who returned for 6‐month follow‐up was lower than desired at 50%. However, high attrition rates commonly plague obesity trials, and we are unaware of any existing studies documenting expected attrition rates among obese inpatients.[23, 36, 37, 38] To help address this, we used imputed weights in our as‐treated analysis to obtain follow‐up weight values on 79% of subjects. Further, the intentional exclusion of subjects in the precontemplation stage of change likely resulted in selection of a more motivated patient population. However, this was done assuming that most inpatient obesity interventions would primarily target patients interested in losing weight. Finally, the lack of a usual care group that more accurately reflects the experience of most hospitalized obese patientsno regular postdischarge interactionsdoes limit interpretation of the modest weight loss noted in both study groups.
In conclusion, an inpatient obesity intervention with post‐discharge follow‐up did not result in intervention subjects losing more weight than controls over a 6‐month period. However, the finding of modest weight loss among both groups is of interest and may warrant further investigation. It remains unclear whether this is a naturally occurring phenomenon or whether other factors influence behavior change in this patient population. Additional studies will be needed to clarify the impact of hospitalization, obesity recognition, perception of health risk, weight tracking, and follow‐up on weight behaviors. Given the proven benefits of even modest weight reductions, encouraging any amount of weight loss in these at‐risk individuals would appear to be a step in the right direction. We have yet to determine whether inpatient obesity interventions represent a lost opportunity.
- , , , . The impact of weight loss among seniors on Medicare spending. Health Econ Rev. 2013;3(1):7.
- . The effects of obesity, smoking, and drinking on medical problems and costs. Health Aff (Millwood). 2002;21(2):245–253.
- , , , , , . The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9:88.
- , , , , , . The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103(10):1895–1901.
- , , , . Assessment of excess mortality in obesity. Am J Epidemiol. 1998;147(1):42–48.
- , , . Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure‐a review. Am J Cardiol. 2004;94(9):1155–1160.
- . In‐hospital initiation of statins: taking advantage of the “teachable moment”. Cleve Clin J Med. 2003;70(6):502, 504–506.
- , , . Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170.
- , , . Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med. 2008;168(18):1950–1960.
- , . Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial. CMAJ. 2009;180(13):1297–1303.
- , , , , , . Phone and e‐mail counselling are effective for weight management in an overweight working population: a randomized controlled trial. BMC Public Health. 2009;9:6.
- , . Weighing the evidence: benefits of regular weight monitoring for weight control. J Nutr Educ Behav. 2005;37(6):319–322.
- , , , et al. Comparative effectiveness of weight‐loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–1968.
- , , , et al. Weight‐loss outcomes: a systematic review and meta‐analysis of weight‐loss clinical trials with a minimum 1‐year follow‐up. J Am Diet Assoc. 2007;107(10):1755–1767.
- , , . Self‐monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102.
- , , , . Willingness for weight loss intervention among overweight and obese inpatients. South Med J. 2011;104(6):397–400.
- , , , et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486.
- , , . The beneficial effects of modest weight loss on cardiovascular risk factors. Int J Obes Relat Metab Disord. 1997;21(suppl 1):S5–S9.
- . Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16(6):397–415.
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(suppl 2):51S–209S.
- , , , et al. Validity of clinical body weight measures as substitutes for missing data in a randomized trial. Obes Res Clin Pract. 2008;2(4):277–281.
- , , . Advances in analysis of longitudinal data. Ann Rev Clin Psychol. 2010;6:79–107.
- , , , et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PloS One. 2009;4(8):e6624.
- , . Longitudinal Data Analysis. Hoboken, NJ: Wiley‐Interscience; 2006.
- , , , . Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150(4):255–262.
- . Clinical practice. Nonsurgical management of obesity in adults. N Engl J Med. 2008;358(18):1941–1950.
- , , . A meta‐analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord. 1997;21(10):941–947.
- , , , et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population‐based prospective study. Circulation. 2007;116(25):2933–2943.
- , , , et al. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50(8):752–759.
- , , , et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case‐control study. Lancet. 2005;366(9497):1640–1649.
- , , , . Waist circumference and waist‐to‐hip ratio as predictors of cardiovascular events: meta‐regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–856.
- , , , . Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855.
- , . Self‐perception of weight appropriateness in the United States. Am J Prev Med. 2003;24(4):332–339.
- , , , et al. Differences in weight perception among blacks and whites. J Womens Health (Larchmt). 2011;20(12):1805–1811.
- , , , , . Perceived health risk of excess body weight among overweight and obese men and women: differences by sex. Prev Med. 2008;47(1):46–52.
- , , . Obesity research—limitations of methods, measurements, and medications. JAMA. 2006;295(7):826–828.
- . Interpreting incomplete data in studies of diet and weight loss. N Engl J Med. 2003;348(21):2136–2137.
- , , , . Predictors of attrition in a large clinic‐based weight‐loss program. Obes Res. 2003;11(7):888–894.
- , , , . The impact of weight loss among seniors on Medicare spending. Health Econ Rev. 2013;3(1):7.
- . The effects of obesity, smoking, and drinking on medical problems and costs. Health Aff (Millwood). 2002;21(2):245–253.
- , , , , , . The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9:88.
- , , , , , . The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103(10):1895–1901.
- , , , . Assessment of excess mortality in obesity. Am J Epidemiol. 1998;147(1):42–48.
- , , . Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure‐a review. Am J Cardiol. 2004;94(9):1155–1160.
- . In‐hospital initiation of statins: taking advantage of the “teachable moment”. Cleve Clin J Med. 2003;70(6):502, 504–506.
- , , . Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170.
- , , . Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med. 2008;168(18):1950–1960.
- , . Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial. CMAJ. 2009;180(13):1297–1303.
- , , , , , . Phone and e‐mail counselling are effective for weight management in an overweight working population: a randomized controlled trial. BMC Public Health. 2009;9:6.
- , . Weighing the evidence: benefits of regular weight monitoring for weight control. J Nutr Educ Behav. 2005;37(6):319–322.
- , , , et al. Comparative effectiveness of weight‐loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–1968.
- , , , et al. Weight‐loss outcomes: a systematic review and meta‐analysis of weight‐loss clinical trials with a minimum 1‐year follow‐up. J Am Diet Assoc. 2007;107(10):1755–1767.
- , , . Self‐monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102.
- , , , . Willingness for weight loss intervention among overweight and obese inpatients. South Med J. 2011;104(6):397–400.
- , , , et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486.
- , , . The beneficial effects of modest weight loss on cardiovascular risk factors. Int J Obes Relat Metab Disord. 1997;21(suppl 1):S5–S9.
- . Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16(6):397–415.
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(suppl 2):51S–209S.
- , , , et al. Validity of clinical body weight measures as substitutes for missing data in a randomized trial. Obes Res Clin Pract. 2008;2(4):277–281.
- , , . Advances in analysis of longitudinal data. Ann Rev Clin Psychol. 2010;6:79–107.
- , , , et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PloS One. 2009;4(8):e6624.
- , . Longitudinal Data Analysis. Hoboken, NJ: Wiley‐Interscience; 2006.
- , , , . Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150(4):255–262.
- . Clinical practice. Nonsurgical management of obesity in adults. N Engl J Med. 2008;358(18):1941–1950.
- , , . A meta‐analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord. 1997;21(10):941–947.
- , , , et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population‐based prospective study. Circulation. 2007;116(25):2933–2943.
- , , , et al. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50(8):752–759.
- , , , et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case‐control study. Lancet. 2005;366(9497):1640–1649.
- , , , . Waist circumference and waist‐to‐hip ratio as predictors of cardiovascular events: meta‐regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–856.
- , , , . Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855.
- , . Self‐perception of weight appropriateness in the United States. Am J Prev Med. 2003;24(4):332–339.
- , , , et al. Differences in weight perception among blacks and whites. J Womens Health (Larchmt). 2011;20(12):1805–1811.
- , , , , . Perceived health risk of excess body weight among overweight and obese men and women: differences by sex. Prev Med. 2008;47(1):46–52.
- , , . Obesity research—limitations of methods, measurements, and medications. JAMA. 2006;295(7):826–828.
- . Interpreting incomplete data in studies of diet and weight loss. N Engl J Med. 2003;348(21):2136–2137.
- , , , . Predictors of attrition in a large clinic‐based weight‐loss program. Obes Res. 2003;11(7):888–894.
© 2014 Society of Hospital Medicine
Much to like on the stroke guidelines menu
The devastation of an acute stroke is something relatively few of us have experienced personally, but professionally we see it very regularly. An estimated 690,000-plus adults in the United States suffer an ischemic stroke annually, and an additional 240,000 experience a transient ischemic attack.
The good news is that the current estimated annual rate of future stroke in this patient population (3%-4%) is historically low, thanks to preventive measures, according to the new "Guidelines for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals," which was published online in Stroke in May (Stroke 2014 May 1 [doi: 10.1161/STR.0000000000000024]). This updated guideline gives evidence-based recommendations on secondary stroke prevention as well as primary prevention in those who have suffered a transient ischemic attack (TIA).
This very extensive guide from the American Heart Association and the American Stroke Association addresses a wide variety of scenarios, ranging from general risk factor modification to specific circumstances, such as myocardial infarction and thrombus, cardiomyopathy, pregnancy, arterial dissection, and aortic arch atherosclerosis.
I welcome the recommendation to consider adding clopidogrel 75 mg/day to aspirin for 90 days in patients with a recent (within 30 days) stroke or TIA attributable to high-grade stenosis (70%-99%) of a major intracranial artery. I used to feel rather helpless to improve the long-term outcome in these patients, but now there seems to be something more we can do, other than just using statins and single antiplatelet therapy.
Other new recommendations stress nutrition. One item suggests performing a nutritional assessment for patients with a history of ischemic stroke or TIA. While many patients may never get around to seeing a nutritionist as an outpatient, no matter how often their primary care physician stresses the importance, when they are in the hospital we have a captive audience. So why not order a nutrition consult, along with the consult for physical, occupational, and speech therapy?
After having experienced an acute neurologic event, many patients and their families are highly motivated to make whatever changes are necessary to prevent a future, potentially catastrophic stroke. Reduction of sodium from 3.3 g/day to 2.5 g/day or less is reasonable, according to the guidelines, though lowering intake to less than 1.5 g/day will lower blood pressure even further. A nutritionist’s input into how to attain these levels without eating a diet that tastes like cardboard can be invaluable. The new guidelines also suggest counseling patients to follow a Mediterranean-type diet – emphasizing whole grains, fruits, vegetables, nuts, olive oil, legumes, fish, poultry, and even low-fat dairy products – instead of the traditional low fat diet.
These new recommendations are only the tip of the iceberg, and this document is highly worthwhile for all practicing clinicians.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
The devastation of an acute stroke is something relatively few of us have experienced personally, but professionally we see it very regularly. An estimated 690,000-plus adults in the United States suffer an ischemic stroke annually, and an additional 240,000 experience a transient ischemic attack.
The good news is that the current estimated annual rate of future stroke in this patient population (3%-4%) is historically low, thanks to preventive measures, according to the new "Guidelines for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals," which was published online in Stroke in May (Stroke 2014 May 1 [doi: 10.1161/STR.0000000000000024]). This updated guideline gives evidence-based recommendations on secondary stroke prevention as well as primary prevention in those who have suffered a transient ischemic attack (TIA).
This very extensive guide from the American Heart Association and the American Stroke Association addresses a wide variety of scenarios, ranging from general risk factor modification to specific circumstances, such as myocardial infarction and thrombus, cardiomyopathy, pregnancy, arterial dissection, and aortic arch atherosclerosis.
I welcome the recommendation to consider adding clopidogrel 75 mg/day to aspirin for 90 days in patients with a recent (within 30 days) stroke or TIA attributable to high-grade stenosis (70%-99%) of a major intracranial artery. I used to feel rather helpless to improve the long-term outcome in these patients, but now there seems to be something more we can do, other than just using statins and single antiplatelet therapy.
Other new recommendations stress nutrition. One item suggests performing a nutritional assessment for patients with a history of ischemic stroke or TIA. While many patients may never get around to seeing a nutritionist as an outpatient, no matter how often their primary care physician stresses the importance, when they are in the hospital we have a captive audience. So why not order a nutrition consult, along with the consult for physical, occupational, and speech therapy?
After having experienced an acute neurologic event, many patients and their families are highly motivated to make whatever changes are necessary to prevent a future, potentially catastrophic stroke. Reduction of sodium from 3.3 g/day to 2.5 g/day or less is reasonable, according to the guidelines, though lowering intake to less than 1.5 g/day will lower blood pressure even further. A nutritionist’s input into how to attain these levels without eating a diet that tastes like cardboard can be invaluable. The new guidelines also suggest counseling patients to follow a Mediterranean-type diet – emphasizing whole grains, fruits, vegetables, nuts, olive oil, legumes, fish, poultry, and even low-fat dairy products – instead of the traditional low fat diet.
These new recommendations are only the tip of the iceberg, and this document is highly worthwhile for all practicing clinicians.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
The devastation of an acute stroke is something relatively few of us have experienced personally, but professionally we see it very regularly. An estimated 690,000-plus adults in the United States suffer an ischemic stroke annually, and an additional 240,000 experience a transient ischemic attack.
The good news is that the current estimated annual rate of future stroke in this patient population (3%-4%) is historically low, thanks to preventive measures, according to the new "Guidelines for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals," which was published online in Stroke in May (Stroke 2014 May 1 [doi: 10.1161/STR.0000000000000024]). This updated guideline gives evidence-based recommendations on secondary stroke prevention as well as primary prevention in those who have suffered a transient ischemic attack (TIA).
This very extensive guide from the American Heart Association and the American Stroke Association addresses a wide variety of scenarios, ranging from general risk factor modification to specific circumstances, such as myocardial infarction and thrombus, cardiomyopathy, pregnancy, arterial dissection, and aortic arch atherosclerosis.
I welcome the recommendation to consider adding clopidogrel 75 mg/day to aspirin for 90 days in patients with a recent (within 30 days) stroke or TIA attributable to high-grade stenosis (70%-99%) of a major intracranial artery. I used to feel rather helpless to improve the long-term outcome in these patients, but now there seems to be something more we can do, other than just using statins and single antiplatelet therapy.
Other new recommendations stress nutrition. One item suggests performing a nutritional assessment for patients with a history of ischemic stroke or TIA. While many patients may never get around to seeing a nutritionist as an outpatient, no matter how often their primary care physician stresses the importance, when they are in the hospital we have a captive audience. So why not order a nutrition consult, along with the consult for physical, occupational, and speech therapy?
After having experienced an acute neurologic event, many patients and their families are highly motivated to make whatever changes are necessary to prevent a future, potentially catastrophic stroke. Reduction of sodium from 3.3 g/day to 2.5 g/day or less is reasonable, according to the guidelines, though lowering intake to less than 1.5 g/day will lower blood pressure even further. A nutritionist’s input into how to attain these levels without eating a diet that tastes like cardboard can be invaluable. The new guidelines also suggest counseling patients to follow a Mediterranean-type diet – emphasizing whole grains, fruits, vegetables, nuts, olive oil, legumes, fish, poultry, and even low-fat dairy products – instead of the traditional low fat diet.
These new recommendations are only the tip of the iceberg, and this document is highly worthwhile for all practicing clinicians.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
NIDA releases updated tools to help parents talk to teens about marijuana
The National Institute on Drug Abuse has released an updated set of resources to help parents "sort out marijuana myths from science based facts," according to a statement released May 20 by the National Institutes of Health.
Two updated booklets are being released in conjunction with the Substance Abuse and Mental Health Services Administration’s National Prevention Week 2014, which takes place May 18-24.
"Marijuana Facts for Teens" covers the topics of marijuana’s health consequences in teens, its effect on the developing brain, addiction risk, and updated information about its potential medical benefits. "Marijuana: Facts Parents Need to Know" contains updated guidelines for parents on how to tell if their child is using marijuana and how to discuss the topic with their children.
Both publications have been updated to include new sections on the dangers of synthetic marijuana, the effect of marijuana use on teens’ IQ, and potential therapeutic uses of the drug.
According to the 2013 Monitoring the Future survey, 45.5% of teens will have tried marijuana at least once by the time they graduate high school, and more than 6% of high school seniors report smoking daily.
For more information, visit http://teens.drugabuse.gov/.
The National Institute on Drug Abuse has released an updated set of resources to help parents "sort out marijuana myths from science based facts," according to a statement released May 20 by the National Institutes of Health.
Two updated booklets are being released in conjunction with the Substance Abuse and Mental Health Services Administration’s National Prevention Week 2014, which takes place May 18-24.
"Marijuana Facts for Teens" covers the topics of marijuana’s health consequences in teens, its effect on the developing brain, addiction risk, and updated information about its potential medical benefits. "Marijuana: Facts Parents Need to Know" contains updated guidelines for parents on how to tell if their child is using marijuana and how to discuss the topic with their children.
Both publications have been updated to include new sections on the dangers of synthetic marijuana, the effect of marijuana use on teens’ IQ, and potential therapeutic uses of the drug.
According to the 2013 Monitoring the Future survey, 45.5% of teens will have tried marijuana at least once by the time they graduate high school, and more than 6% of high school seniors report smoking daily.
For more information, visit http://teens.drugabuse.gov/.
The National Institute on Drug Abuse has released an updated set of resources to help parents "sort out marijuana myths from science based facts," according to a statement released May 20 by the National Institutes of Health.
Two updated booklets are being released in conjunction with the Substance Abuse and Mental Health Services Administration’s National Prevention Week 2014, which takes place May 18-24.
"Marijuana Facts for Teens" covers the topics of marijuana’s health consequences in teens, its effect on the developing brain, addiction risk, and updated information about its potential medical benefits. "Marijuana: Facts Parents Need to Know" contains updated guidelines for parents on how to tell if their child is using marijuana and how to discuss the topic with their children.
Both publications have been updated to include new sections on the dangers of synthetic marijuana, the effect of marijuana use on teens’ IQ, and potential therapeutic uses of the drug.
According to the 2013 Monitoring the Future survey, 45.5% of teens will have tried marijuana at least once by the time they graduate high school, and more than 6% of high school seniors report smoking daily.
For more information, visit http://teens.drugabuse.gov/.
What Matters: Anxiolytics, hypnotics and eternal sleep
Data from the Centers for Disease Control and Prevention’s National Ambulatory Medical Center Survey reveal benzodiazepine prescriptions grew by 12.5% per year between 2002 and 2009. Data from the National Health and Nutrition Examination Survey suggest that prescriptions for sleep aids (sedatives and hypnotics) tripled between 1998 and 2006. Four percent of U.S. adults age 20 years or older and 7% of adults age 80 years or older report using a prescription sleep aid in the past month.
Aside from the addictive potential and their limited long-term effectiveness, they may be associated with an increased risk of death.
Dr. Scott Weich and his colleagues at University of Warwick, Coventry, England, analyzed data from a retrospective matched cohort study involving 34,727 patients aged at least 16 years who received prescriptions for anxiolytics or hypnotics and 69,418 patients who did not (BMJ 2014;348:g1996). To reduce the likelihood that patients received a prescription that they did not fill, only patients receiving at least two prescriptions were included. The average follow-up period was 7.6 years. The most commonly prescribed drugs were diazepam (48%), temazepam (35%), zopiclone (34%), and zolpidem (8%).
Significantly higher ratios for mortality were observed with the use of these drugs. Adjusting for potential confounders, the hazard ratio for mortality during the whole follow-up period was significantly elevated for the group receiving any sedative or hypnotic in the first year of recruitment (hazard ratio, 3.32; 95% confidence interval: 3.19-3.45).
Dose responses were observed for study drugs. For example, the HR for patients receiving more than 90 doses during the first year was 4.51 (95% CI: 4.22-4.82). Patients who did not receive study drugs beyond 1 year were less likely to die than those who continued to take them. The authors point out that these data translate into four excess deaths linked to use of these drugs per 100 people over 7.6 years after the initial prescription.
The biggest challenge will be to figure out how best to incorporate this information into our counseling of patients without sounding like we are "fear-mongering." Fear-mongering doesn’t work – it just makes our patients more anxious, when what we really need to do is calm them down.
Cognitive-behavioral therapy works for insomnia, but patients report not having the time. I always start the discussion by telling patients to read the book "No More Sleepless Nights" and to start a sleep log. Amazing what we can learn from this. This as least gets the ball rolling.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. Dr. Ebbert does not receive royalties from the sale of "No More Sleepless Nights."
Data from the Centers for Disease Control and Prevention’s National Ambulatory Medical Center Survey reveal benzodiazepine prescriptions grew by 12.5% per year between 2002 and 2009. Data from the National Health and Nutrition Examination Survey suggest that prescriptions for sleep aids (sedatives and hypnotics) tripled between 1998 and 2006. Four percent of U.S. adults age 20 years or older and 7% of adults age 80 years or older report using a prescription sleep aid in the past month.
Aside from the addictive potential and their limited long-term effectiveness, they may be associated with an increased risk of death.
Dr. Scott Weich and his colleagues at University of Warwick, Coventry, England, analyzed data from a retrospective matched cohort study involving 34,727 patients aged at least 16 years who received prescriptions for anxiolytics or hypnotics and 69,418 patients who did not (BMJ 2014;348:g1996). To reduce the likelihood that patients received a prescription that they did not fill, only patients receiving at least two prescriptions were included. The average follow-up period was 7.6 years. The most commonly prescribed drugs were diazepam (48%), temazepam (35%), zopiclone (34%), and zolpidem (8%).
Significantly higher ratios for mortality were observed with the use of these drugs. Adjusting for potential confounders, the hazard ratio for mortality during the whole follow-up period was significantly elevated for the group receiving any sedative or hypnotic in the first year of recruitment (hazard ratio, 3.32; 95% confidence interval: 3.19-3.45).
Dose responses were observed for study drugs. For example, the HR for patients receiving more than 90 doses during the first year was 4.51 (95% CI: 4.22-4.82). Patients who did not receive study drugs beyond 1 year were less likely to die than those who continued to take them. The authors point out that these data translate into four excess deaths linked to use of these drugs per 100 people over 7.6 years after the initial prescription.
The biggest challenge will be to figure out how best to incorporate this information into our counseling of patients without sounding like we are "fear-mongering." Fear-mongering doesn’t work – it just makes our patients more anxious, when what we really need to do is calm them down.
Cognitive-behavioral therapy works for insomnia, but patients report not having the time. I always start the discussion by telling patients to read the book "No More Sleepless Nights" and to start a sleep log. Amazing what we can learn from this. This as least gets the ball rolling.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. Dr. Ebbert does not receive royalties from the sale of "No More Sleepless Nights."
Data from the Centers for Disease Control and Prevention’s National Ambulatory Medical Center Survey reveal benzodiazepine prescriptions grew by 12.5% per year between 2002 and 2009. Data from the National Health and Nutrition Examination Survey suggest that prescriptions for sleep aids (sedatives and hypnotics) tripled between 1998 and 2006. Four percent of U.S. adults age 20 years or older and 7% of adults age 80 years or older report using a prescription sleep aid in the past month.
Aside from the addictive potential and their limited long-term effectiveness, they may be associated with an increased risk of death.
Dr. Scott Weich and his colleagues at University of Warwick, Coventry, England, analyzed data from a retrospective matched cohort study involving 34,727 patients aged at least 16 years who received prescriptions for anxiolytics or hypnotics and 69,418 patients who did not (BMJ 2014;348:g1996). To reduce the likelihood that patients received a prescription that they did not fill, only patients receiving at least two prescriptions were included. The average follow-up period was 7.6 years. The most commonly prescribed drugs were diazepam (48%), temazepam (35%), zopiclone (34%), and zolpidem (8%).
Significantly higher ratios for mortality were observed with the use of these drugs. Adjusting for potential confounders, the hazard ratio for mortality during the whole follow-up period was significantly elevated for the group receiving any sedative or hypnotic in the first year of recruitment (hazard ratio, 3.32; 95% confidence interval: 3.19-3.45).
Dose responses were observed for study drugs. For example, the HR for patients receiving more than 90 doses during the first year was 4.51 (95% CI: 4.22-4.82). Patients who did not receive study drugs beyond 1 year were less likely to die than those who continued to take them. The authors point out that these data translate into four excess deaths linked to use of these drugs per 100 people over 7.6 years after the initial prescription.
The biggest challenge will be to figure out how best to incorporate this information into our counseling of patients without sounding like we are "fear-mongering." Fear-mongering doesn’t work – it just makes our patients more anxious, when what we really need to do is calm them down.
Cognitive-behavioral therapy works for insomnia, but patients report not having the time. I always start the discussion by telling patients to read the book "No More Sleepless Nights" and to start a sleep log. Amazing what we can learn from this. This as least gets the ball rolling.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. Dr. Ebbert does not receive royalties from the sale of "No More Sleepless Nights."
Chemists discover true structure of anticancer agent

Credit: NIH
Chemists say they have determined the correct structure of a compound that has shown activity against lymphoma and a range of other cancers.
Their research, published in Angewandte Chemie, focused on a compound called TIC10.
The team showed that TIC10’s structure differs subtly from a version described by another group last year, and the previous structure associated with TIC10 actually describes a molecule that lacks TIC10’s anticancer activity.
The newly identified structure describes a molecule with potent anticancer effects in animals, representing a new family of biologically active structures that can now be explored for possible therapeutic uses.
“This new structure should generate much interest in the cancer research community,” said study author Kim D. Janda, PhD, of The Scripps Research Institute in La Jolla, California.
Antitumor potential
TIC10 was first described in Science Translational Medicine in early 2013. The authors identified the compound, within a library of thousands of molecules maintained by the National Cancer Institute (NCI), for its ability to boost cells’ production of the natural antitumor protein TRAIL. (TIC10 stands for TRAIL-inducing compound #10.)
As a small molecule, TIC10 would be easier to deliver in a therapy than the TRAIL protein itself. The paper’s authors reported that TIC10 was orally active and dramatically shrank a variety of tumors in mice.
Tumors can develop resistance to TRAIL, but Dr Janda had been studying compounds that defeat this resistance. The news about TIC10 therefore got his attention.
“I thought, ‘They have this molecule for upregulating TRAIL, and we have these molecules that can overcome tumor-cell TRAIL resistance—the combination could be important,’” he said.
The original publication on TIC10 included a figure showing its predicted structure. So Dr Janda asked one of his postdoctoral researchers, Jonathan Lockner, to make TIC10 using that information.
Although the original TIC10 research team had seemingly confirmed the predicted structure with mass spectrometry, no one had published a thorough characterization of the TIC10 molecule.
“There were no nuclear magnetic resonance data or X-ray crystallography data, and there was definitely no procedure for the synthesis,” Dr Lockner said. “My background was chemistry, though, so I was able to find a way to synthesize it starting from simple compounds.”
Surprising inactivity
There was just one problem with Dr Lockner’s newly synthesized “TIC10.” When tested, it failed to induce TRAIL expression in cells, even at high doses.
“Of course, I was nervous,” Dr Lockner said. “As a chemist, you never want to make a mistake and give biologists the wrong material.”
To try and verify they had the right material, Dr Janda’s team obtained a sample of TIC10 directly from the NCI.
“When we got that sample and tested it, we saw that it had the expected TRAIL-upregulating effect,” said Nicholas Jacob, a graduate student in the Janda Lab and coauthor of the new paper.
“That prompted us to look more closely at the structures of these 2 compounds.”
The researchers spent months characterizing their own synthesized material and the NCI material, using an array of sophisticated structural analysis tools. They also tested the 2 compounds’ biological effects.
The team eventually concluded that the TIC10 compound from the NCI library does boost TRAIL production in cells and remains promising as the basis for anticancer therapies, but it does not have the structure that was originally published.
The right structure
The originally published structure has a core made of 3 carbon-nitrogen rings in a straight line and does not induce TRAIL activity. The correct, TRAIL-inducing structure differs subtly, with an end ring that sticks out at an angle.
In chemists’ parlance, the 2 compounds are constitutional isomers: a linear imidazolinopyrimidinone and an angular imidazolinopyrimidinone.
And Dr Lockner found that the angular, TRAIL-inducing structure was easier to synthesize than the one originally described.
Now, with the correct molecule in hand and a solid understanding of its structure and synthesis, Dr Janda and his team are moving forward with their original plan to study TIC10 in combination with TRAIL-resistance-thwarting molecules as an anticancer therapy.
The therapeutic implications of TIC10 may even go beyond cancer, according to the researchers. The angular core of the TRAIL-inducing molecule Dr Janda’s team discovered is a novel type of a biologically active structure, or pharmacophore, from which chemists may now be able to build a new class of candidate drugs, possibly for a variety of ailments. ![]()

Credit: NIH
Chemists say they have determined the correct structure of a compound that has shown activity against lymphoma and a range of other cancers.
Their research, published in Angewandte Chemie, focused on a compound called TIC10.
The team showed that TIC10’s structure differs subtly from a version described by another group last year, and the previous structure associated with TIC10 actually describes a molecule that lacks TIC10’s anticancer activity.
The newly identified structure describes a molecule with potent anticancer effects in animals, representing a new family of biologically active structures that can now be explored for possible therapeutic uses.
“This new structure should generate much interest in the cancer research community,” said study author Kim D. Janda, PhD, of The Scripps Research Institute in La Jolla, California.
Antitumor potential
TIC10 was first described in Science Translational Medicine in early 2013. The authors identified the compound, within a library of thousands of molecules maintained by the National Cancer Institute (NCI), for its ability to boost cells’ production of the natural antitumor protein TRAIL. (TIC10 stands for TRAIL-inducing compound #10.)
As a small molecule, TIC10 would be easier to deliver in a therapy than the TRAIL protein itself. The paper’s authors reported that TIC10 was orally active and dramatically shrank a variety of tumors in mice.
Tumors can develop resistance to TRAIL, but Dr Janda had been studying compounds that defeat this resistance. The news about TIC10 therefore got his attention.
“I thought, ‘They have this molecule for upregulating TRAIL, and we have these molecules that can overcome tumor-cell TRAIL resistance—the combination could be important,’” he said.
The original publication on TIC10 included a figure showing its predicted structure. So Dr Janda asked one of his postdoctoral researchers, Jonathan Lockner, to make TIC10 using that information.
Although the original TIC10 research team had seemingly confirmed the predicted structure with mass spectrometry, no one had published a thorough characterization of the TIC10 molecule.
“There were no nuclear magnetic resonance data or X-ray crystallography data, and there was definitely no procedure for the synthesis,” Dr Lockner said. “My background was chemistry, though, so I was able to find a way to synthesize it starting from simple compounds.”
Surprising inactivity
There was just one problem with Dr Lockner’s newly synthesized “TIC10.” When tested, it failed to induce TRAIL expression in cells, even at high doses.
“Of course, I was nervous,” Dr Lockner said. “As a chemist, you never want to make a mistake and give biologists the wrong material.”
To try and verify they had the right material, Dr Janda’s team obtained a sample of TIC10 directly from the NCI.
“When we got that sample and tested it, we saw that it had the expected TRAIL-upregulating effect,” said Nicholas Jacob, a graduate student in the Janda Lab and coauthor of the new paper.
“That prompted us to look more closely at the structures of these 2 compounds.”
The researchers spent months characterizing their own synthesized material and the NCI material, using an array of sophisticated structural analysis tools. They also tested the 2 compounds’ biological effects.
The team eventually concluded that the TIC10 compound from the NCI library does boost TRAIL production in cells and remains promising as the basis for anticancer therapies, but it does not have the structure that was originally published.
The right structure
The originally published structure has a core made of 3 carbon-nitrogen rings in a straight line and does not induce TRAIL activity. The correct, TRAIL-inducing structure differs subtly, with an end ring that sticks out at an angle.
In chemists’ parlance, the 2 compounds are constitutional isomers: a linear imidazolinopyrimidinone and an angular imidazolinopyrimidinone.
And Dr Lockner found that the angular, TRAIL-inducing structure was easier to synthesize than the one originally described.
Now, with the correct molecule in hand and a solid understanding of its structure and synthesis, Dr Janda and his team are moving forward with their original plan to study TIC10 in combination with TRAIL-resistance-thwarting molecules as an anticancer therapy.
The therapeutic implications of TIC10 may even go beyond cancer, according to the researchers. The angular core of the TRAIL-inducing molecule Dr Janda’s team discovered is a novel type of a biologically active structure, or pharmacophore, from which chemists may now be able to build a new class of candidate drugs, possibly for a variety of ailments. ![]()

Credit: NIH
Chemists say they have determined the correct structure of a compound that has shown activity against lymphoma and a range of other cancers.
Their research, published in Angewandte Chemie, focused on a compound called TIC10.
The team showed that TIC10’s structure differs subtly from a version described by another group last year, and the previous structure associated with TIC10 actually describes a molecule that lacks TIC10’s anticancer activity.
The newly identified structure describes a molecule with potent anticancer effects in animals, representing a new family of biologically active structures that can now be explored for possible therapeutic uses.
“This new structure should generate much interest in the cancer research community,” said study author Kim D. Janda, PhD, of The Scripps Research Institute in La Jolla, California.
Antitumor potential
TIC10 was first described in Science Translational Medicine in early 2013. The authors identified the compound, within a library of thousands of molecules maintained by the National Cancer Institute (NCI), for its ability to boost cells’ production of the natural antitumor protein TRAIL. (TIC10 stands for TRAIL-inducing compound #10.)
As a small molecule, TIC10 would be easier to deliver in a therapy than the TRAIL protein itself. The paper’s authors reported that TIC10 was orally active and dramatically shrank a variety of tumors in mice.
Tumors can develop resistance to TRAIL, but Dr Janda had been studying compounds that defeat this resistance. The news about TIC10 therefore got his attention.
“I thought, ‘They have this molecule for upregulating TRAIL, and we have these molecules that can overcome tumor-cell TRAIL resistance—the combination could be important,’” he said.
The original publication on TIC10 included a figure showing its predicted structure. So Dr Janda asked one of his postdoctoral researchers, Jonathan Lockner, to make TIC10 using that information.
Although the original TIC10 research team had seemingly confirmed the predicted structure with mass spectrometry, no one had published a thorough characterization of the TIC10 molecule.
“There were no nuclear magnetic resonance data or X-ray crystallography data, and there was definitely no procedure for the synthesis,” Dr Lockner said. “My background was chemistry, though, so I was able to find a way to synthesize it starting from simple compounds.”
Surprising inactivity
There was just one problem with Dr Lockner’s newly synthesized “TIC10.” When tested, it failed to induce TRAIL expression in cells, even at high doses.
“Of course, I was nervous,” Dr Lockner said. “As a chemist, you never want to make a mistake and give biologists the wrong material.”
To try and verify they had the right material, Dr Janda’s team obtained a sample of TIC10 directly from the NCI.
“When we got that sample and tested it, we saw that it had the expected TRAIL-upregulating effect,” said Nicholas Jacob, a graduate student in the Janda Lab and coauthor of the new paper.
“That prompted us to look more closely at the structures of these 2 compounds.”
The researchers spent months characterizing their own synthesized material and the NCI material, using an array of sophisticated structural analysis tools. They also tested the 2 compounds’ biological effects.
The team eventually concluded that the TIC10 compound from the NCI library does boost TRAIL production in cells and remains promising as the basis for anticancer therapies, but it does not have the structure that was originally published.
The right structure
The originally published structure has a core made of 3 carbon-nitrogen rings in a straight line and does not induce TRAIL activity. The correct, TRAIL-inducing structure differs subtly, with an end ring that sticks out at an angle.
In chemists’ parlance, the 2 compounds are constitutional isomers: a linear imidazolinopyrimidinone and an angular imidazolinopyrimidinone.
And Dr Lockner found that the angular, TRAIL-inducing structure was easier to synthesize than the one originally described.
Now, with the correct molecule in hand and a solid understanding of its structure and synthesis, Dr Janda and his team are moving forward with their original plan to study TIC10 in combination with TRAIL-resistance-thwarting molecules as an anticancer therapy.
The therapeutic implications of TIC10 may even go beyond cancer, according to the researchers. The angular core of the TRAIL-inducing molecule Dr Janda’s team discovered is a novel type of a biologically active structure, or pharmacophore, from which chemists may now be able to build a new class of candidate drugs, possibly for a variety of ailments. ![]()
Drug gains orphan designation for DLBCL

The US Food and Drug Administration (FDA) has granted orphan designation to selinexor (KPT-330) for the treatment of diffuse large B-cell lymphoma (DLBCL).
The drug elicited responses in patients with non-Hodgkin lymphoma (NHL), including DLBCL, in an ongoing phase 1 study.
Selinexor is a selective inhibitor of nuclear transport that functions by binding to the nuclear export protein XPO1 (also called CRM1). This leads to the accumulation of tumor suppressor proteins in the cell nucleus, which is thought to cause apoptosis in cancer cells while largely sparing normal cells.
The FDA grants orphan designation to promote the development of drugs that target conditions affecting 200,000 or fewer US patients annually and are expected to provide significant therapeutic advantage over existing treatments.
Selinexor’s orphan designation for DLBCL qualifies the drug’s developer, Karyopharm Therapeutics, Inc., for benefits that apply across all stages of development, including an accelerated approval process, 7 years of market exclusivity following marketing approval, tax credits on US clinical trials, eligibility for orphan drug grants, and a waiver of certain administrative fees.
The FDA has also granted selinexor orphan status for the treatment of acute myeloid leukemia.
Phase 1 study
Researchers evaluated selinexor in an ongoing phase 1 study of patients with NHL or chronic lymphocytic leukemia (CLL) and presented results at the 2013 ASH Annual Meeting (abstract 90).
At that time, the study included 18 patients with NHL or CLL. They had a median age of 66.5 years and had received a median of 4.5 prior treatment regimens.
Patients received selinexor at 6 different dose levels. There were no clinically significant cumulative toxicities or cases of major organ dysfunction, and the maximum-tolerated dose was not reached. Researchers continued dosing at 35 mg/m2 twice weekly.
Ten patients experienced drug-related grade 3/4 adverse events, including thrombocytopenia without bleeding (n=6), neutropenia (n=5), dehydration (n=1), syncope (n=1), hypotension (n=1), and fatigue (n=1).
The most common grade 1/2 events were anorexia (n=10), fatigue (n=9), diarrhea (n=6), vomiting (n=6), neutropenia (n=5), malaise (n=3), anemia (n=3), and weight loss (n=3).
Response was evaluable in 15 patients. Eighty percent of patients, all of whom had progressive disease on study entry, experienced tumor shrinkage or disease stabilization on selinexor. The other 20% of patients progressed.
Of 3 patients with DLBCL, 1 progressed, 1 had stable disease, and 1 achieved 93% tumor shrinkage.
“We are encouraged by the response data in patients with DLBCL who have received selinexor in our ongoing phase 1 clinical trial in advanced hematological malignancies,” said Michael G. Kauffman, MD, PhD, Karyopharm’s Chief Executive Officer.
“We plan to present updated clinical data for selinexor across multiple indications, including DLBCL, at ASCO 2014.” ![]()

The US Food and Drug Administration (FDA) has granted orphan designation to selinexor (KPT-330) for the treatment of diffuse large B-cell lymphoma (DLBCL).
The drug elicited responses in patients with non-Hodgkin lymphoma (NHL), including DLBCL, in an ongoing phase 1 study.
Selinexor is a selective inhibitor of nuclear transport that functions by binding to the nuclear export protein XPO1 (also called CRM1). This leads to the accumulation of tumor suppressor proteins in the cell nucleus, which is thought to cause apoptosis in cancer cells while largely sparing normal cells.
The FDA grants orphan designation to promote the development of drugs that target conditions affecting 200,000 or fewer US patients annually and are expected to provide significant therapeutic advantage over existing treatments.
Selinexor’s orphan designation for DLBCL qualifies the drug’s developer, Karyopharm Therapeutics, Inc., for benefits that apply across all stages of development, including an accelerated approval process, 7 years of market exclusivity following marketing approval, tax credits on US clinical trials, eligibility for orphan drug grants, and a waiver of certain administrative fees.
The FDA has also granted selinexor orphan status for the treatment of acute myeloid leukemia.
Phase 1 study
Researchers evaluated selinexor in an ongoing phase 1 study of patients with NHL or chronic lymphocytic leukemia (CLL) and presented results at the 2013 ASH Annual Meeting (abstract 90).
At that time, the study included 18 patients with NHL or CLL. They had a median age of 66.5 years and had received a median of 4.5 prior treatment regimens.
Patients received selinexor at 6 different dose levels. There were no clinically significant cumulative toxicities or cases of major organ dysfunction, and the maximum-tolerated dose was not reached. Researchers continued dosing at 35 mg/m2 twice weekly.
Ten patients experienced drug-related grade 3/4 adverse events, including thrombocytopenia without bleeding (n=6), neutropenia (n=5), dehydration (n=1), syncope (n=1), hypotension (n=1), and fatigue (n=1).
The most common grade 1/2 events were anorexia (n=10), fatigue (n=9), diarrhea (n=6), vomiting (n=6), neutropenia (n=5), malaise (n=3), anemia (n=3), and weight loss (n=3).
Response was evaluable in 15 patients. Eighty percent of patients, all of whom had progressive disease on study entry, experienced tumor shrinkage or disease stabilization on selinexor. The other 20% of patients progressed.
Of 3 patients with DLBCL, 1 progressed, 1 had stable disease, and 1 achieved 93% tumor shrinkage.
“We are encouraged by the response data in patients with DLBCL who have received selinexor in our ongoing phase 1 clinical trial in advanced hematological malignancies,” said Michael G. Kauffman, MD, PhD, Karyopharm’s Chief Executive Officer.
“We plan to present updated clinical data for selinexor across multiple indications, including DLBCL, at ASCO 2014.” ![]()

The US Food and Drug Administration (FDA) has granted orphan designation to selinexor (KPT-330) for the treatment of diffuse large B-cell lymphoma (DLBCL).
The drug elicited responses in patients with non-Hodgkin lymphoma (NHL), including DLBCL, in an ongoing phase 1 study.
Selinexor is a selective inhibitor of nuclear transport that functions by binding to the nuclear export protein XPO1 (also called CRM1). This leads to the accumulation of tumor suppressor proteins in the cell nucleus, which is thought to cause apoptosis in cancer cells while largely sparing normal cells.
The FDA grants orphan designation to promote the development of drugs that target conditions affecting 200,000 or fewer US patients annually and are expected to provide significant therapeutic advantage over existing treatments.
Selinexor’s orphan designation for DLBCL qualifies the drug’s developer, Karyopharm Therapeutics, Inc., for benefits that apply across all stages of development, including an accelerated approval process, 7 years of market exclusivity following marketing approval, tax credits on US clinical trials, eligibility for orphan drug grants, and a waiver of certain administrative fees.
The FDA has also granted selinexor orphan status for the treatment of acute myeloid leukemia.
Phase 1 study
Researchers evaluated selinexor in an ongoing phase 1 study of patients with NHL or chronic lymphocytic leukemia (CLL) and presented results at the 2013 ASH Annual Meeting (abstract 90).
At that time, the study included 18 patients with NHL or CLL. They had a median age of 66.5 years and had received a median of 4.5 prior treatment regimens.
Patients received selinexor at 6 different dose levels. There were no clinically significant cumulative toxicities or cases of major organ dysfunction, and the maximum-tolerated dose was not reached. Researchers continued dosing at 35 mg/m2 twice weekly.
Ten patients experienced drug-related grade 3/4 adverse events, including thrombocytopenia without bleeding (n=6), neutropenia (n=5), dehydration (n=1), syncope (n=1), hypotension (n=1), and fatigue (n=1).
The most common grade 1/2 events were anorexia (n=10), fatigue (n=9), diarrhea (n=6), vomiting (n=6), neutropenia (n=5), malaise (n=3), anemia (n=3), and weight loss (n=3).
Response was evaluable in 15 patients. Eighty percent of patients, all of whom had progressive disease on study entry, experienced tumor shrinkage or disease stabilization on selinexor. The other 20% of patients progressed.
Of 3 patients with DLBCL, 1 progressed, 1 had stable disease, and 1 achieved 93% tumor shrinkage.
“We are encouraged by the response data in patients with DLBCL who have received selinexor in our ongoing phase 1 clinical trial in advanced hematological malignancies,” said Michael G. Kauffman, MD, PhD, Karyopharm’s Chief Executive Officer.
“We plan to present updated clinical data for selinexor across multiple indications, including DLBCL, at ASCO 2014.” ![]()
Cancer trial publications often omit minority accrual rates

Credit: Rhoda Baer
A review of clinical trial data from 2012 suggests Hispanic patients are underrepresented in US cancer studies, and many trial publications fail to provide information on patients’ racial/ethnic backgrounds.
Researchers analyzed 159 reports of phase 2 and 3 trials and found that roughly 21% included information on the number of minority patients enrolled.
About 8% of the publications included data on the number of Hispanic patients enrolled.
And from this data, the investigators found the accrual rate for Hispanic patients was about 4%.
According to the researchers, this lack of information and low representation inhibits physicians’ ability to provide optimal treatment to Hispanic cancer patients and patients belonging to other minority groups.
“We have a major responsibility to ensure adequate representation,” said study author Ian M. Thompson Jr, MD, of The University of Texas Health Science Center at San Antonio.
“How else will we know how best to treat our patients, and how else are we going to reduce the health disparities in [the Hispanic] population?”
Dr Thompson and his colleagues have a particular interest in the Hispanic population because 58% of San Antonio residents are Hispanic, as are 68% of residents in South Texas as a whole.
So the investigators wanted to determine Hispanic accrual rates in randomized trials of cancer patients. The team evaluated data from phase 2 and 3 cancer trials published in 2012. They focused on studies that were considered likely to change the standard of care and were published in “high-impact” journals.
The researchers identified 159 trials—68 phase 2 studies and 91 phase 3 studies. They discovered that 33 of the trial publications—about 21%—disclosed data on minority accrual. And 13 publications—about 8%—included data on the accrual of Hispanic cancer patients.
Of the 4154 patients enrolled on those 13 trials, 162 were Hispanic, which translates to an overall accrual rate of 3.9%. The enrollment of Hispanic patients ranged from 1 patient (0.5%) in a phase 2 trial of lung cancer to 17 patients (26%) in a phase 2 study of acute lymphoblastic leukemia.
“Fundamentally, in the most recent published cancer clinical trials, either the number and proportion of Hispanics are not reported or are far below their actual representation in the national population,” Dr Thompson summarized.
“For institutions like ours that serve a ‘minority-majority’ population, it’s a major responsibility for us to ensure adequate representation so that we can tell our patients how they can best be treated and how we can reduce the disparities of this rapidly growing population.”
Dr Thompson and his colleagues described this research in the Journal of Clinical Oncology. ![]()

Credit: Rhoda Baer
A review of clinical trial data from 2012 suggests Hispanic patients are underrepresented in US cancer studies, and many trial publications fail to provide information on patients’ racial/ethnic backgrounds.
Researchers analyzed 159 reports of phase 2 and 3 trials and found that roughly 21% included information on the number of minority patients enrolled.
About 8% of the publications included data on the number of Hispanic patients enrolled.
And from this data, the investigators found the accrual rate for Hispanic patients was about 4%.
According to the researchers, this lack of information and low representation inhibits physicians’ ability to provide optimal treatment to Hispanic cancer patients and patients belonging to other minority groups.
“We have a major responsibility to ensure adequate representation,” said study author Ian M. Thompson Jr, MD, of The University of Texas Health Science Center at San Antonio.
“How else will we know how best to treat our patients, and how else are we going to reduce the health disparities in [the Hispanic] population?”
Dr Thompson and his colleagues have a particular interest in the Hispanic population because 58% of San Antonio residents are Hispanic, as are 68% of residents in South Texas as a whole.
So the investigators wanted to determine Hispanic accrual rates in randomized trials of cancer patients. The team evaluated data from phase 2 and 3 cancer trials published in 2012. They focused on studies that were considered likely to change the standard of care and were published in “high-impact” journals.
The researchers identified 159 trials—68 phase 2 studies and 91 phase 3 studies. They discovered that 33 of the trial publications—about 21%—disclosed data on minority accrual. And 13 publications—about 8%—included data on the accrual of Hispanic cancer patients.
Of the 4154 patients enrolled on those 13 trials, 162 were Hispanic, which translates to an overall accrual rate of 3.9%. The enrollment of Hispanic patients ranged from 1 patient (0.5%) in a phase 2 trial of lung cancer to 17 patients (26%) in a phase 2 study of acute lymphoblastic leukemia.
“Fundamentally, in the most recent published cancer clinical trials, either the number and proportion of Hispanics are not reported or are far below their actual representation in the national population,” Dr Thompson summarized.
“For institutions like ours that serve a ‘minority-majority’ population, it’s a major responsibility for us to ensure adequate representation so that we can tell our patients how they can best be treated and how we can reduce the disparities of this rapidly growing population.”
Dr Thompson and his colleagues described this research in the Journal of Clinical Oncology. ![]()

Credit: Rhoda Baer
A review of clinical trial data from 2012 suggests Hispanic patients are underrepresented in US cancer studies, and many trial publications fail to provide information on patients’ racial/ethnic backgrounds.
Researchers analyzed 159 reports of phase 2 and 3 trials and found that roughly 21% included information on the number of minority patients enrolled.
About 8% of the publications included data on the number of Hispanic patients enrolled.
And from this data, the investigators found the accrual rate for Hispanic patients was about 4%.
According to the researchers, this lack of information and low representation inhibits physicians’ ability to provide optimal treatment to Hispanic cancer patients and patients belonging to other minority groups.
“We have a major responsibility to ensure adequate representation,” said study author Ian M. Thompson Jr, MD, of The University of Texas Health Science Center at San Antonio.
“How else will we know how best to treat our patients, and how else are we going to reduce the health disparities in [the Hispanic] population?”
Dr Thompson and his colleagues have a particular interest in the Hispanic population because 58% of San Antonio residents are Hispanic, as are 68% of residents in South Texas as a whole.
So the investigators wanted to determine Hispanic accrual rates in randomized trials of cancer patients. The team evaluated data from phase 2 and 3 cancer trials published in 2012. They focused on studies that were considered likely to change the standard of care and were published in “high-impact” journals.
The researchers identified 159 trials—68 phase 2 studies and 91 phase 3 studies. They discovered that 33 of the trial publications—about 21%—disclosed data on minority accrual. And 13 publications—about 8%—included data on the accrual of Hispanic cancer patients.
Of the 4154 patients enrolled on those 13 trials, 162 were Hispanic, which translates to an overall accrual rate of 3.9%. The enrollment of Hispanic patients ranged from 1 patient (0.5%) in a phase 2 trial of lung cancer to 17 patients (26%) in a phase 2 study of acute lymphoblastic leukemia.
“Fundamentally, in the most recent published cancer clinical trials, either the number and proportion of Hispanics are not reported or are far below their actual representation in the national population,” Dr Thompson summarized.
“For institutions like ours that serve a ‘minority-majority’ population, it’s a major responsibility for us to ensure adequate representation so that we can tell our patients how they can best be treated and how we can reduce the disparities of this rapidly growing population.”
Dr Thompson and his colleagues described this research in the Journal of Clinical Oncology. ![]()
mAb gets breakthrough designation for MM

Credit: Linda Bartlett
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for elotuzumab, a humanized monoclonal antibody
(mAb).
The designation is for elotuzumab used in combination with lenalidomide and dexamethasone to treat patients with multiple myeloma (MM) who have received 1 or more prior therapies.
The FDA’s decision is based on findings from a phase 2 trial in which MM patients received that treatment combination.
According to the FDA, breakthrough designation is intended to expedite the development and review of drugs for serious or life-threatening conditions. For a treatment to receive this designation, there must be preliminary clinical evidence that demonstrates the drug may offer substantial improvement over currently available therapy on at least 1 clinically significant endpoint.
About elotuzumab
Elotuzumab is a humanized IgG1 mAb targeting signaling lymphocyte activation molecule (SLAMF7, also known as CS1), a glycoprotein expressed on myeloma and natural killer cells but not detectable in normal tissue.
Researchers are investigating whether, through both direct activation and engagement of natural killer cells, elotuzumab may selectively target and kill SLAMF7-expressing myeloma cells.
Elotuzumab is under investigation as a monotherapy in smoldering myeloma and in combination with other therapies in first-line and relapsed or refractory MM.
A clinical development program for the mAb is underway, including phase 3 trials in first-line MM (ELOQUENT-1) and relapsed or refractory MM (ELOQUENT-2). The agent is also under investigation in a randomized, phase 2 study of bortezomib and dexamethasone in patients with relapsed or refractory MM.
Elotuzumab is under development by AbbVie and Bristol-Myers Squibb.
Phase 2 trial results
The breakthrough therapy designation for elotuzumab is based on results of a randomized, phase 2 trial presented at the EHA 2013 Annual Congress (abstract 14). Study investigators tested 2 doses of the mAb in combination with lenalidomide and low-dose dexamethasone in patients with previously treated MM.
Patients were randomized 1:1 to receive elotuzumab at 10 mg/kg or 20 mg/kg (intravenous infusion on days 1, 8, 15, and 22 of a 28-day cycle in the first 2 cycles and then days 1 and 15 of subsequent cycles) in combination with oral lenalidomide at 25 mg/day on days 1 to 21 and oral dexamethasone at 40 mg/week. Patients were treated until their disease progressed or they developed unacceptable toxicity.
In the 10 mg/kg arm (n=36), which is the dose used in the ongoing phase 3 trials, the median progression-free survival was 33 months, after a median follow-up of 20.8 months. And the objective response rate was 92%.
In the 20 mg/kg arm (n=37), the median progression-free survival was 18 months, after a median follow-up of 17.1 months. And the objective response rate was 76%.
The safety data were consistent with previously reported results for elotuzumab from this trial. In patients receiving elotuzumab at 10 mg/kg or 20 mg/kg, most treatment-emergent adverse events occurred within 18 months of starting therapy.
The most common grade 3/4 adverse events for the 10 mg/kg and 20 mg/kg arms, respectively, were lymphopenia (26% and 9%), neutropenia (21% and 22%), thrombocytopenia (21% and 17%), anemia (13% and 12%), leukopenia (8% and 7%), hyperglycemia (5% and 12%), pneumonia (8% and 5%), diarrhea (10% and 5%), fatigue (8% and 9%), and hypokalemia (8% and 5%).
Two deaths occurred on study. One patient died of pneumonia, multiple organ failure, and sepsis. The other died of disease progression. ![]()

Credit: Linda Bartlett
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for elotuzumab, a humanized monoclonal antibody
(mAb).
The designation is for elotuzumab used in combination with lenalidomide and dexamethasone to treat patients with multiple myeloma (MM) who have received 1 or more prior therapies.
The FDA’s decision is based on findings from a phase 2 trial in which MM patients received that treatment combination.
According to the FDA, breakthrough designation is intended to expedite the development and review of drugs for serious or life-threatening conditions. For a treatment to receive this designation, there must be preliminary clinical evidence that demonstrates the drug may offer substantial improvement over currently available therapy on at least 1 clinically significant endpoint.
About elotuzumab
Elotuzumab is a humanized IgG1 mAb targeting signaling lymphocyte activation molecule (SLAMF7, also known as CS1), a glycoprotein expressed on myeloma and natural killer cells but not detectable in normal tissue.
Researchers are investigating whether, through both direct activation and engagement of natural killer cells, elotuzumab may selectively target and kill SLAMF7-expressing myeloma cells.
Elotuzumab is under investigation as a monotherapy in smoldering myeloma and in combination with other therapies in first-line and relapsed or refractory MM.
A clinical development program for the mAb is underway, including phase 3 trials in first-line MM (ELOQUENT-1) and relapsed or refractory MM (ELOQUENT-2). The agent is also under investigation in a randomized, phase 2 study of bortezomib and dexamethasone in patients with relapsed or refractory MM.
Elotuzumab is under development by AbbVie and Bristol-Myers Squibb.
Phase 2 trial results
The breakthrough therapy designation for elotuzumab is based on results of a randomized, phase 2 trial presented at the EHA 2013 Annual Congress (abstract 14). Study investigators tested 2 doses of the mAb in combination with lenalidomide and low-dose dexamethasone in patients with previously treated MM.
Patients were randomized 1:1 to receive elotuzumab at 10 mg/kg or 20 mg/kg (intravenous infusion on days 1, 8, 15, and 22 of a 28-day cycle in the first 2 cycles and then days 1 and 15 of subsequent cycles) in combination with oral lenalidomide at 25 mg/day on days 1 to 21 and oral dexamethasone at 40 mg/week. Patients were treated until their disease progressed or they developed unacceptable toxicity.
In the 10 mg/kg arm (n=36), which is the dose used in the ongoing phase 3 trials, the median progression-free survival was 33 months, after a median follow-up of 20.8 months. And the objective response rate was 92%.
In the 20 mg/kg arm (n=37), the median progression-free survival was 18 months, after a median follow-up of 17.1 months. And the objective response rate was 76%.
The safety data were consistent with previously reported results for elotuzumab from this trial. In patients receiving elotuzumab at 10 mg/kg or 20 mg/kg, most treatment-emergent adverse events occurred within 18 months of starting therapy.
The most common grade 3/4 adverse events for the 10 mg/kg and 20 mg/kg arms, respectively, were lymphopenia (26% and 9%), neutropenia (21% and 22%), thrombocytopenia (21% and 17%), anemia (13% and 12%), leukopenia (8% and 7%), hyperglycemia (5% and 12%), pneumonia (8% and 5%), diarrhea (10% and 5%), fatigue (8% and 9%), and hypokalemia (8% and 5%).
Two deaths occurred on study. One patient died of pneumonia, multiple organ failure, and sepsis. The other died of disease progression. ![]()

Credit: Linda Bartlett
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for elotuzumab, a humanized monoclonal antibody
(mAb).
The designation is for elotuzumab used in combination with lenalidomide and dexamethasone to treat patients with multiple myeloma (MM) who have received 1 or more prior therapies.
The FDA’s decision is based on findings from a phase 2 trial in which MM patients received that treatment combination.
According to the FDA, breakthrough designation is intended to expedite the development and review of drugs for serious or life-threatening conditions. For a treatment to receive this designation, there must be preliminary clinical evidence that demonstrates the drug may offer substantial improvement over currently available therapy on at least 1 clinically significant endpoint.
About elotuzumab
Elotuzumab is a humanized IgG1 mAb targeting signaling lymphocyte activation molecule (SLAMF7, also known as CS1), a glycoprotein expressed on myeloma and natural killer cells but not detectable in normal tissue.
Researchers are investigating whether, through both direct activation and engagement of natural killer cells, elotuzumab may selectively target and kill SLAMF7-expressing myeloma cells.
Elotuzumab is under investigation as a monotherapy in smoldering myeloma and in combination with other therapies in first-line and relapsed or refractory MM.
A clinical development program for the mAb is underway, including phase 3 trials in first-line MM (ELOQUENT-1) and relapsed or refractory MM (ELOQUENT-2). The agent is also under investigation in a randomized, phase 2 study of bortezomib and dexamethasone in patients with relapsed or refractory MM.
Elotuzumab is under development by AbbVie and Bristol-Myers Squibb.
Phase 2 trial results
The breakthrough therapy designation for elotuzumab is based on results of a randomized, phase 2 trial presented at the EHA 2013 Annual Congress (abstract 14). Study investigators tested 2 doses of the mAb in combination with lenalidomide and low-dose dexamethasone in patients with previously treated MM.
Patients were randomized 1:1 to receive elotuzumab at 10 mg/kg or 20 mg/kg (intravenous infusion on days 1, 8, 15, and 22 of a 28-day cycle in the first 2 cycles and then days 1 and 15 of subsequent cycles) in combination with oral lenalidomide at 25 mg/day on days 1 to 21 and oral dexamethasone at 40 mg/week. Patients were treated until their disease progressed or they developed unacceptable toxicity.
In the 10 mg/kg arm (n=36), which is the dose used in the ongoing phase 3 trials, the median progression-free survival was 33 months, after a median follow-up of 20.8 months. And the objective response rate was 92%.
In the 20 mg/kg arm (n=37), the median progression-free survival was 18 months, after a median follow-up of 17.1 months. And the objective response rate was 76%.
The safety data were consistent with previously reported results for elotuzumab from this trial. In patients receiving elotuzumab at 10 mg/kg or 20 mg/kg, most treatment-emergent adverse events occurred within 18 months of starting therapy.
The most common grade 3/4 adverse events for the 10 mg/kg and 20 mg/kg arms, respectively, were lymphopenia (26% and 9%), neutropenia (21% and 22%), thrombocytopenia (21% and 17%), anemia (13% and 12%), leukopenia (8% and 7%), hyperglycemia (5% and 12%), pneumonia (8% and 5%), diarrhea (10% and 5%), fatigue (8% and 9%), and hypokalemia (8% and 5%).
Two deaths occurred on study. One patient died of pneumonia, multiple organ failure, and sepsis. The other died of disease progression. ![]()
Pasireotide decreases incidence of postoperative fistula
The somatostatin analogue pasireotide reduced postoperative pancreatic fistula leak or abscess by 56%, compared with placebo, a randomized study has determined.
Pasireotide (Signifor) was effective after both pancreaticoduodenectomy and distal pancreatectomy, whether or not the pancreatic duct was dilated, Dr. Peter J. Allen and his colleagues wrote in the May 21 issue of the New England Journal of Medicine (N. Engl. J. Med. 2014;370:2014-22).
In those patients who did develop fistulas or leaks, pasireotide was associated with fewer grade 3 occurrences.
"These results suggest that ... not only were many leaks and fistulas prevented, but when they did occur they were less clinically relevant," wrote Dr. Allen of the Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The study randomized 300 patients to subcutaneous injections of either placebo or pasireotide twice daily for 7 days after pancreatic surgery. The primary endpoint was the development of a pancreatic leak, fistula, or abscess of at least grade 3. Secondary endpoints included the overall rate of pancreatic complications (all grades) and the rate of grade B or grade C pancreatic fistula.
Patients were a mean of 64 years old. Most (73%) underwent a pancreaticoduodenectomy. The average length of stay for these patients was about 10 days. The active group received 900 mcg of pasireotide subcutaneously twice daily for 7 days, beginning on the morning of surgery.
Mean postoperative serum glucose levels were significantly higher in patients taking pasireotide (258 mg/dL vs. 215 mg/dL). Readmission occurred in significantly fewer pasireotide patients (17% vs. 29%).
Significantly fewer of those taking the active drug were able to finish the entire course of 14 doses (76% vs. 86% given placebo). The lower completion rate was mostly due to nausea and vomiting, which caused 26 patients in the active group and 3 in the placebo group to withdraw from the study.
A leak or fistula of grade 3 or higher developed in 45 patients. The outcome was significantly less common among those taking pasireotide than among those on placebo (9% vs. 21%; relative risk, 0.44). "This corresponded to an absolute risk reduction of 11.7 percentage points," with a number needed to treat of 8, the investigators said.
Pasireotide was significantly more effective than placebo in surgical subgroups, including pancreaticoduodenectomy (RR, 0.49) and distal pancreatectomy (RR, 0.32). The effect was also positive whether the pancreatic duct was dilated (RR, 0.11) or nondilated (RR, 0.55).
The secondary outcome (grade B or C postoperative fistula) occurred in 37 patients (12%). In the pasireotide group, there were 12 grade B fistulas and no grade C fistulas. In the placebo group, there were 20 grade B and 5 grade C fistulas.
Overall 60-day mortality was 0.7% (one death in each treatment group). Grade 3 and 4 complications were common, occurring in 92% of the pasireotide group and 90% of the placebo group. Most of these were expected postoperative serum abnormalities.
The investigators said that the other approved somatostatin analogue, octreotide, has not been clearly associated with pancreatic leak reduction. They suggested that pasireotide may be more effective because it has a longer half-life and binds to four of the five somatostatin-receptor subtypes, rather than just two, as octreotide does.
They added that the octreotide studies were conducted before 2005, when there was no consistent definition of postoperative pancreatic fistula. Therefore, they concluded, the extant data cannot be used to identify octreotide efficacy in this application.
Pasireotide, which is made by Novartis Pharmaceuticals, is currently approved as an injection for the treatment of Cushing’s disease patients who cannot be helped through surgery.
Novartis Pharmaceuticals sponsored the trial. Dr. Allen received Novartis grant funding but had no other financial ties with the company.
The somatostatin analogue pasireotide reduced postoperative pancreatic fistula leak or abscess by 56%, compared with placebo, a randomized study has determined.
Pasireotide (Signifor) was effective after both pancreaticoduodenectomy and distal pancreatectomy, whether or not the pancreatic duct was dilated, Dr. Peter J. Allen and his colleagues wrote in the May 21 issue of the New England Journal of Medicine (N. Engl. J. Med. 2014;370:2014-22).
In those patients who did develop fistulas or leaks, pasireotide was associated with fewer grade 3 occurrences.
"These results suggest that ... not only were many leaks and fistulas prevented, but when they did occur they were less clinically relevant," wrote Dr. Allen of the Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The study randomized 300 patients to subcutaneous injections of either placebo or pasireotide twice daily for 7 days after pancreatic surgery. The primary endpoint was the development of a pancreatic leak, fistula, or abscess of at least grade 3. Secondary endpoints included the overall rate of pancreatic complications (all grades) and the rate of grade B or grade C pancreatic fistula.
Patients were a mean of 64 years old. Most (73%) underwent a pancreaticoduodenectomy. The average length of stay for these patients was about 10 days. The active group received 900 mcg of pasireotide subcutaneously twice daily for 7 days, beginning on the morning of surgery.
Mean postoperative serum glucose levels were significantly higher in patients taking pasireotide (258 mg/dL vs. 215 mg/dL). Readmission occurred in significantly fewer pasireotide patients (17% vs. 29%).
Significantly fewer of those taking the active drug were able to finish the entire course of 14 doses (76% vs. 86% given placebo). The lower completion rate was mostly due to nausea and vomiting, which caused 26 patients in the active group and 3 in the placebo group to withdraw from the study.
A leak or fistula of grade 3 or higher developed in 45 patients. The outcome was significantly less common among those taking pasireotide than among those on placebo (9% vs. 21%; relative risk, 0.44). "This corresponded to an absolute risk reduction of 11.7 percentage points," with a number needed to treat of 8, the investigators said.
Pasireotide was significantly more effective than placebo in surgical subgroups, including pancreaticoduodenectomy (RR, 0.49) and distal pancreatectomy (RR, 0.32). The effect was also positive whether the pancreatic duct was dilated (RR, 0.11) or nondilated (RR, 0.55).
The secondary outcome (grade B or C postoperative fistula) occurred in 37 patients (12%). In the pasireotide group, there were 12 grade B fistulas and no grade C fistulas. In the placebo group, there were 20 grade B and 5 grade C fistulas.
Overall 60-day mortality was 0.7% (one death in each treatment group). Grade 3 and 4 complications were common, occurring in 92% of the pasireotide group and 90% of the placebo group. Most of these were expected postoperative serum abnormalities.
The investigators said that the other approved somatostatin analogue, octreotide, has not been clearly associated with pancreatic leak reduction. They suggested that pasireotide may be more effective because it has a longer half-life and binds to four of the five somatostatin-receptor subtypes, rather than just two, as octreotide does.
They added that the octreotide studies were conducted before 2005, when there was no consistent definition of postoperative pancreatic fistula. Therefore, they concluded, the extant data cannot be used to identify octreotide efficacy in this application.
Pasireotide, which is made by Novartis Pharmaceuticals, is currently approved as an injection for the treatment of Cushing’s disease patients who cannot be helped through surgery.
Novartis Pharmaceuticals sponsored the trial. Dr. Allen received Novartis grant funding but had no other financial ties with the company.
The somatostatin analogue pasireotide reduced postoperative pancreatic fistula leak or abscess by 56%, compared with placebo, a randomized study has determined.
Pasireotide (Signifor) was effective after both pancreaticoduodenectomy and distal pancreatectomy, whether or not the pancreatic duct was dilated, Dr. Peter J. Allen and his colleagues wrote in the May 21 issue of the New England Journal of Medicine (N. Engl. J. Med. 2014;370:2014-22).
In those patients who did develop fistulas or leaks, pasireotide was associated with fewer grade 3 occurrences.
"These results suggest that ... not only were many leaks and fistulas prevented, but when they did occur they were less clinically relevant," wrote Dr. Allen of the Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The study randomized 300 patients to subcutaneous injections of either placebo or pasireotide twice daily for 7 days after pancreatic surgery. The primary endpoint was the development of a pancreatic leak, fistula, or abscess of at least grade 3. Secondary endpoints included the overall rate of pancreatic complications (all grades) and the rate of grade B or grade C pancreatic fistula.
Patients were a mean of 64 years old. Most (73%) underwent a pancreaticoduodenectomy. The average length of stay for these patients was about 10 days. The active group received 900 mcg of pasireotide subcutaneously twice daily for 7 days, beginning on the morning of surgery.
Mean postoperative serum glucose levels were significantly higher in patients taking pasireotide (258 mg/dL vs. 215 mg/dL). Readmission occurred in significantly fewer pasireotide patients (17% vs. 29%).
Significantly fewer of those taking the active drug were able to finish the entire course of 14 doses (76% vs. 86% given placebo). The lower completion rate was mostly due to nausea and vomiting, which caused 26 patients in the active group and 3 in the placebo group to withdraw from the study.
A leak or fistula of grade 3 or higher developed in 45 patients. The outcome was significantly less common among those taking pasireotide than among those on placebo (9% vs. 21%; relative risk, 0.44). "This corresponded to an absolute risk reduction of 11.7 percentage points," with a number needed to treat of 8, the investigators said.
Pasireotide was significantly more effective than placebo in surgical subgroups, including pancreaticoduodenectomy (RR, 0.49) and distal pancreatectomy (RR, 0.32). The effect was also positive whether the pancreatic duct was dilated (RR, 0.11) or nondilated (RR, 0.55).
The secondary outcome (grade B or C postoperative fistula) occurred in 37 patients (12%). In the pasireotide group, there were 12 grade B fistulas and no grade C fistulas. In the placebo group, there were 20 grade B and 5 grade C fistulas.
Overall 60-day mortality was 0.7% (one death in each treatment group). Grade 3 and 4 complications were common, occurring in 92% of the pasireotide group and 90% of the placebo group. Most of these were expected postoperative serum abnormalities.
The investigators said that the other approved somatostatin analogue, octreotide, has not been clearly associated with pancreatic leak reduction. They suggested that pasireotide may be more effective because it has a longer half-life and binds to four of the five somatostatin-receptor subtypes, rather than just two, as octreotide does.
They added that the octreotide studies were conducted before 2005, when there was no consistent definition of postoperative pancreatic fistula. Therefore, they concluded, the extant data cannot be used to identify octreotide efficacy in this application.
Pasireotide, which is made by Novartis Pharmaceuticals, is currently approved as an injection for the treatment of Cushing’s disease patients who cannot be helped through surgery.
Novartis Pharmaceuticals sponsored the trial. Dr. Allen received Novartis grant funding but had no other financial ties with the company.
FROM NEJM
Key clinical point: Pasireotide reduced the incidence of postoperative pancreatic fistula, leak, or abscess.
Major finding: Compared with placebo, pasireotide reduced the rate of fistula, leak, or abscess by 56%.
Data source: The randomized, placebo-controlled study included 300 patients.
Disclosures: Novartis Pharmaceuticals sponsored the trial. Dr. Allen received Novartis grant funding but had no other financial ties with the company.
MERS Cases Put Hospitalists on Alert for Infectious Disease
Patients diagnosed with Middle East Respiratory Syndrome (MERS) in Indiana and Florida have healthcare workers and hospitalists on the lookout for additional cases of the potentially fatal respiratory infection.
Hospitalists should pay attention to patients exhibiting fever and respiratory symptoms who traveled to the Arabian Peninsula in the 14 days prior to disease onset, and contact the hospital epidemiologist if MERS is suspected, says James Pile, MD, vice chair of the department of hospital medicine at the Cleveland Clinic.
The CDC has reported three cases of MERS this month. The first, reported on May 2, involved a healthcare worker from Saudi Arabia who traveled to Indiana to visit family. The second was reported on May 11, when another visiting healthcare worker from Saudi Arabia checked into the emergency department in Orlando, Fla., after he fell ill with fever, chills, and a slight cough. Both patients are considered to be fully recovered.
The third MERS case is in an Illinois man who had a business meeting with the patient from Indiana and represents the first case of the virus being contracted in the U.S. A blood test confirmed that the Illinois man had been infected with the virus, but he’s reported that he no longer feels sick.
Caused by a coronavirus called MERS-CoV, MERS was first reported in Saudi Arabia in 2012. So far, there have been more than 600 confirmed cases around the world and 181 people have died, according to the World Health Organization.
"The CDC suggests that the index U.S. case represents a very low threat to the general population in this country, and my sense is that that this will not turn out to be a major issue for the U.S. healthcare system, but there’s still a lot we don’t know about MERS," Dr. Pile says. “The scope of the issue should become much clearer over the next couple of months.”
For more information on MERS, check out this CDC fact sheet.
Read physician editor Danielle Scheurer's recent blog post on the MERS situation.
Visit our website for more information about hospitalists and infectious disease care.
Patients diagnosed with Middle East Respiratory Syndrome (MERS) in Indiana and Florida have healthcare workers and hospitalists on the lookout for additional cases of the potentially fatal respiratory infection.
Hospitalists should pay attention to patients exhibiting fever and respiratory symptoms who traveled to the Arabian Peninsula in the 14 days prior to disease onset, and contact the hospital epidemiologist if MERS is suspected, says James Pile, MD, vice chair of the department of hospital medicine at the Cleveland Clinic.
The CDC has reported three cases of MERS this month. The first, reported on May 2, involved a healthcare worker from Saudi Arabia who traveled to Indiana to visit family. The second was reported on May 11, when another visiting healthcare worker from Saudi Arabia checked into the emergency department in Orlando, Fla., after he fell ill with fever, chills, and a slight cough. Both patients are considered to be fully recovered.
The third MERS case is in an Illinois man who had a business meeting with the patient from Indiana and represents the first case of the virus being contracted in the U.S. A blood test confirmed that the Illinois man had been infected with the virus, but he’s reported that he no longer feels sick.
Caused by a coronavirus called MERS-CoV, MERS was first reported in Saudi Arabia in 2012. So far, there have been more than 600 confirmed cases around the world and 181 people have died, according to the World Health Organization.
"The CDC suggests that the index U.S. case represents a very low threat to the general population in this country, and my sense is that that this will not turn out to be a major issue for the U.S. healthcare system, but there’s still a lot we don’t know about MERS," Dr. Pile says. “The scope of the issue should become much clearer over the next couple of months.”
For more information on MERS, check out this CDC fact sheet.
Read physician editor Danielle Scheurer's recent blog post on the MERS situation.
Visit our website for more information about hospitalists and infectious disease care.
Patients diagnosed with Middle East Respiratory Syndrome (MERS) in Indiana and Florida have healthcare workers and hospitalists on the lookout for additional cases of the potentially fatal respiratory infection.
Hospitalists should pay attention to patients exhibiting fever and respiratory symptoms who traveled to the Arabian Peninsula in the 14 days prior to disease onset, and contact the hospital epidemiologist if MERS is suspected, says James Pile, MD, vice chair of the department of hospital medicine at the Cleveland Clinic.
The CDC has reported three cases of MERS this month. The first, reported on May 2, involved a healthcare worker from Saudi Arabia who traveled to Indiana to visit family. The second was reported on May 11, when another visiting healthcare worker from Saudi Arabia checked into the emergency department in Orlando, Fla., after he fell ill with fever, chills, and a slight cough. Both patients are considered to be fully recovered.
The third MERS case is in an Illinois man who had a business meeting with the patient from Indiana and represents the first case of the virus being contracted in the U.S. A blood test confirmed that the Illinois man had been infected with the virus, but he’s reported that he no longer feels sick.
Caused by a coronavirus called MERS-CoV, MERS was first reported in Saudi Arabia in 2012. So far, there have been more than 600 confirmed cases around the world and 181 people have died, according to the World Health Organization.
"The CDC suggests that the index U.S. case represents a very low threat to the general population in this country, and my sense is that that this will not turn out to be a major issue for the U.S. healthcare system, but there’s still a lot we don’t know about MERS," Dr. Pile says. “The scope of the issue should become much clearer over the next couple of months.”
For more information on MERS, check out this CDC fact sheet.
Read physician editor Danielle Scheurer's recent blog post on the MERS situation.
Visit our website for more information about hospitalists and infectious disease care.