User login
Management of Complications Following Radiofrequency Ablation of a Pedicle Osteoid Osteoma
Aggressive and delusional about his alien origins, but refusing treatment
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
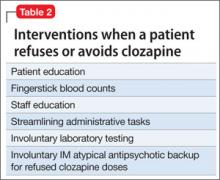
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
Effect of a Preoperative Protocol of Aerobic Physical Therapy on the Quality of Life of Patients With Adolescent Idiopathic Scoliosis: A Randomized Clinical Study
Using CBT effectively for treating depression and anxiety
Fewer than 20% of people seeking help for depression and anxiety disorders receive cognitive-behavioral therapy (CBT), the most established evidence-based psychotherapeutic treatment.1 Efforts are being made to increase access to CBT,2 but a substantial barrier remains: therapist training is a strong predictor of treatment outcome, and many therapists offering CBT services are not sufficiently trained to deliver multiple manual-based interventions with adequate fidelity to the model. Proposed solutions to this barrier include:
• abbreviated versions of CBT training for practitioners in primary care and community settings
• culturally adapted CBT training for community health workers3
• Internet-based CBT and telemedicine (telephone and video conferencing)2
• mobile phone applications that use text messaging, social support, and physiological monitoring as adjuncts to clinical practice or stand-alone interventions.4
New models of CBT also are emerging, including transdiagnostic CBT and metacognitive approaches (mindfulness-based cognitive therapy and acceptance and commitment therapy), and several new foci for exposure therapy.
In light of these ongoing modulations, this article is intended to help clinicians make informed decisions about CBT when selecting treatment for patients with depressive and anxiety disorders (Box5 ). We review the evidence of CBT’s efficacy for acute-phase treatment and relapse prevention; explain the common elements considered essential to CBT practice; describe CBT adaptations for specific anxiety disorders; and provide an overview of recent advances in conceptualizing and adapting CBT.
Efficacy for mood and anxiety disorders
Depression. Dozens of randomized controlled trials (RCT) and other studies support CBT’s efficacy in treating major depressive disorder (MDD). For acute treatment:
• CBT is more effective in producing remission when compared with no treatment, treatment as usual, or nonspecific psychotherapy.
• For mild to moderate depression, CBT is equivalent to antidepressant medication in terms of response and remission rates.
• Combining antidepressant therapy with CBT increases treatment adherence.6
Less well known may be that a successful response to CBT in the acute phase may have a protective effect against depression recurrences. A 2013 meta-analysis that totaled 506 individuals with depressive disorders found a trend toward significantly lower relapse rates when CBT was discontinued after acute therapy, compared with antidepressant therapy that continued beyond the acute phase.7
Anxiety. Among psychotherapies, CBT’s superior efficacy for anxiety disorders is well-established. CBT and its specific-disorder adaptations are considered first-line treatment.8
CBT’s essential elements
CBT focuses on distorted cognitions about the self, the world, and the future, and on behaviors that lead to or maintain symptoms.
Cognitive interventions seek to identify thoughts and beliefs that trigger emotional and behavioral reactions. A person with social anxiety disorder, for example, might believe that people will notice if he makes even a minor social mistake and then reject him, which will make him feel worthless. CBT can help him subject these beliefs to rational analysis and develop more adaptive beliefs, such as: “It is not certain that I will behave so badly that people would notice, but if that happened, the likelihood of being outright rejected is probably low. If—in the worst-case scenario—I was rejected, I am not worthless; I’m just a fallible human being.”
CBT’s behavioral component can be conceptualized as behavioral activation (BA), a structured approach to help the patient:
• increase behaviors and experiences that are rewarding
• overcome barriers to engaging in these new behaviors
• and decrease behaviors that maintain symptoms.
BA can be a useful intervention for individuals with depression characterized by lack of engagement or capacity for pleasurable experiences. During pregnancy and the postpartum period, for example, a woman undergoes physical, social, and environmental changes that might gradually deprive her of sources of pleasure and other reinforcing activities. BA would focus on developing creative solutions to regain access to or create new opportunities for rewarding experiences and to avoid behaviors (such as social withdrawal or physical activity restriction) that perpetuate depressed mood.
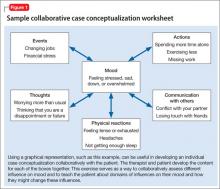
Common elements. Cognitive and behavioral interventions focus on problem solving, individualized case conceptualization (Figure 1), and collaborative empiricism.9
Individualized case conceptualization lays the foundation for the course of CBT, and may be thought of as a map for therapy. Case conceptualization brings in several domains of assessment including symptoms and diagnosis, the patient’s strengths, formative experiences (including biopsychosocial aspects), contextual factors, and cognitive factors that influence diagnosis and treatment, such as automatic thoughts or schemas. The case formulation leads to a working hypothesis about the optimal course and focus of CBT.
Collaborative empiricism is the way in which the patient and therapist work together to continually refine this working hypothesis. The pair works together to investigate the hypotheses and all aspects of the therapeutic relationship.
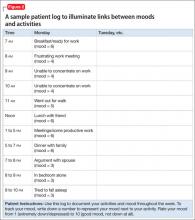
Although no specific technique defines CBT, a common practice is to educate a person about interrelationships between behaviors/activities, thoughts, and mood. A mood activity log (Figure 2) can illuminate links between moods and activities and be useful with targeting interventions. For a person with social anxiety, for example, a mood activity log could assist in developing a hierarchy of feared social situations and avoidance intensity. Systematic exposure therapy would follow, beginning with the least frightening/intense situation, accompanied by teaching new coping skills (such as relaxation strategies).
CBT adaptations for anxiety disorders
Elements of CBT have been adapted for a variety of anxiety disorders, based on specific symptoms and features (Table).10-15
Panic disorder. Panic control treatment is considered the first-line intervention for panic disorder’s defining features: spontaneous panic attacks, worry about future occurrence of attacks, and perceived catastrophic consequences (such as heart attack, fainting).10 This CBT adaptation includes:
• patient education about the nature of panic
• breathing retraining to foster exposure to feared bodily sensations and avoided activities and places
• cognitive restructuring of danger-related thoughts (such as “I’m going to faint,” or “It would be catastrophic if I did”).
Obsessive-compulsive disorder. Exposure and response prevention (ERP) is the first-line treatment for obsessive-compulsive disorder (OCD).11 In traditional therapist-guided ERP, patients expose themselves to perceived contaminants while refraining from inappropriate compulsive behaviors (such as hand washing).
Cognitive interventions also can be an effective treatment of obsessions, without patients having to engage in exposure to their horrific thoughts and images.16 Consider, for example, a new mother who upon seeing the kitchen knife has the intrusive thought, “What if I stabbed my baby?” Instead of the traditional exposure approach for OCD (ie, having her vividly imagine stabbing her baby until her anxiety level subsided), the cognitive intervention would be to educate her about the normalcy of intrusive thoughts, particularly in the postpartum period.
Generalized anxiety disorder. CBT for generalized anxiety disorder (GAD) targets patients’ overestimation of the likelihood of negative events and the belief that these events, should they occur, would be catastrophic and render them unable to cope.12
Motivational interviewing (MI) appears to be a useful adjunct to precede traditional CBT, particularly for severe worriers.17 MI attempts to help individuals with GAD recognize their ambivalence about giving up worry. This technique acknowledges and validates perceived benefits of worry (eg, “It helps me prepare for the worst, so I won’t be emotionally devastated if it happens”), but also explores how worry is destructive.
Emerging CBT models for anxiety disorders
Metacognitive treatment. Evidence, such as presented by Dobson,18 suggests that the field of CBT is shifting towards a metacognitive model of change and treatment. A metacognitive approach goes beyond changing thinking and emphasizes thoughts about thoughts and experiences. Examples include mindfulness-based cognitive therapy (MBCT) and acceptance and commitment therapy (ACT).
MBCT typically consists of an 8-week program of 2-hour sessions each week and 1 full-day retreat. MBCT is modeled after Kabat-Zinn’s widely disseminated and empirically supported mindfulness based stress reduction course.19 MBCT was developed as a relapse prevention program for patients who had recovered from depression. Unlike traditional cognitive therapy for depression that targets changing the content of automatic thoughts and core beliefs, in MBCT patients are aware of negative automatic thoughts and find ways to change their relationship with these thoughts, learning that thoughts are not facts. This process mainly is carried out by practicing mindfulness meditation exercises. Importantly, MBCT goes beyond mindful acceptance of negative thoughts and teaches patients mindful acceptance of all internal experiences.
A fundamental difference between ACT and traditional CBT is the approach to cognitions.20 Although CBT focuses on changing the content of maladaptive thoughts, such as “I am a worthless person,” ACT focuses on changing the function of thoughts. ACT strives to help patients to accept their internal experiences—whether unwanted thoughts, feelings, bodily sensations, or memories—while committing themselves to pursuing their life goals and values. Strategies aim to help patients step back from their thoughts and observe them as just thoughts. The patient who thinks, “I am worthless” would be instructed to practice saying “I am having the thought I am worthless.” Therefore the thought no longer controls the person’s behavior.
These approaches train the patient to keenly observe distressing thoughts and experiences—not necessarily with the goal of changing them but to accept them and act in a way that is consistent with his (her) goals and values. A meta-analysis of 39 studies found mindfulness-based therapy effective in improving symptoms in participants with anxiety and mood disorders.21 Similarly, ACT has demonstrated efficacy with mixed anxiety disorders.22
Transdiagnostic CBT. Recent research18 suggests that mood and anxiety disorders may have more commonalities than differences in underlying biological and psychological traits. Because the symptoms of anxiety and depressive disorders tend to overlap, and their rate of comorbidity may be as high as 55%,23 so-called transdiagnostic treatments have been developed. Transdiagnostic treatments target impairing symptoms that cut across different diagnoses. For example, patients with depression, anxiety, or substance abuse might share a common difficulty with regulating and coping with negative emotions.
In a preliminary comparison trial,24 46 patients with social anxiety disorder, panic disorder, or GAD were randomly assigned to transdiagnostic CBT (n = 23) or diagnosis-specific CBT (n = 23). Treatments were based on widely used manuals and offered in 2-hour group sessions across 12 weeks. Transdiagnostic CBT was found to be as effective as specific CBT protocols in terms of symptom improvement. Participants attended an average of 8.46 sessions, with similar attendance in each protocol. Fourteen participants (30%) discontinued treatment, similar to attrition rates reported in other trials of transdiagnostic and diagnosis-specific CBT.
Transdiagnostic treatments may facilitate the dissemination of empirically supported treatments because therapists would not be required to have training and supervision to competency in delivering multiple manuals for specific anxiety disorders. This could be attractive to busy practitioners with limited time to learn new treatments.
Bottom Line
Efficacy of cognitive-behavioral therapy (CBT) for depression and anxiety is well established. Although no specific technique defines CBT, a common practice is to educate an individual about interrelationships between behaviors/activities, thoughts, and mood. CBT techniques can be customized to treat specific anxiety disorders, such as panic disorder, obsessive-compulsive disorder, and generalized anxiety disorder.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Collins K, Westra H, Dozois D, et al. Gaps in accessing treatment for anxiety and depressions: challenges for the delivery of care. Clin Psychol Rev. 2004;24(5):583-616.
2. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychological Science in the Public Interest. 2013;14(2):65-111.
3. Rahman A, Malik A, Sikander S, et al. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008;372(9642):902-909.
4. Aguilera A, Muench F. There’s an app for that: information technology applications for cognitive behavioral practitioners. Behavior Therapist. 2012;35(4):65-73.
5. Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006; 74(4):658-670.
6. Hollon SD, Jarrett RB, Nierenberg AA, et al. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J Clin Psychiatry. 2005;66(4):455-468.
7. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4):1-8.
8. Stewart R, Chambless D. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77(4): 595-606.
9. Wright JH, Basco MR, Thase M. Learning cognitive behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing; 2006.
10. Barlow DH, Craske MG. Mastery of your anxiety and panic. 4th ed. New York, NY: Oxford University Press, Inc.; 2007.
11. Foa EB, Yadin E, Lichner TK. Exposure and response prevention for obsessive-compulsive disorder: therapist guide. New York, NY: Oxford University Press, Inc.; 2012.
12. Dugas MJ, Robichaud M. Cognitive-behavioral treatment for generalized anxiety disorder. New York, NY: Routledge; 2007.
13. Zlomke K, Davis TE. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008;39(3):207-223.
14. Foa EB, Hembree E, Rothbaum B. Prolonged exposure therapy for PTSD: emotional processing of traumatic experiences. Therapist guide. New York, NY: Oxford University Press, Inc.; 2007.
15. Resick PA, Schnicke MK. Cognitive processing therapy for rape victims. London, United Kingdom: Sage Publications; 1996.
16. Whittal ML, Robichaud M, Woody SR. Cognitive treatment of obsessions: enhancing dissemination with video components. Cognitive and Behavioral Practice. 2010;17(1):1-8.
17. Westra H, Arkowitz H, Dozois D. Adding a motivational interviewing pretreatment to cognitive behavioral therapy for generalized anxiety disorder: a preliminary randomized controlled trial. J Anxiety Disord. 2009;23(2): 1106-1117.
18. Dobson KS. The science of CBT: toward a metacognitive model of change? Behav Ther. 2013;44(2):224-227.
19. Kabat-Zinn J. Full catastrophe living. Using the wisdom of your body and mind to face stress, pain, and illness. Revised edition. New York, NY: Bantam Books; 2013.
20. Hayes SC, Strosahl KD. Acceptance and commitment therapy. The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2012.
21. Hofmann S, Sawyer A, Witt A, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78(2): 169-183.
22. Arch J, Eifert G, Davies C, et al. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. J Consult Clin Psychol. 2012;80(5):750-765.
23. Brown TA, Campbell LA, Lehman CL, et al. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585-599.
24. Norton P, Barrera T. Transdiagnostic versus diagnosis-specific CBT for anxiety disorders: a preliminary randomized controlled noninferiority trial. Depress Anxiety. 2012;29(10):874-882.
Fewer than 20% of people seeking help for depression and anxiety disorders receive cognitive-behavioral therapy (CBT), the most established evidence-based psychotherapeutic treatment.1 Efforts are being made to increase access to CBT,2 but a substantial barrier remains: therapist training is a strong predictor of treatment outcome, and many therapists offering CBT services are not sufficiently trained to deliver multiple manual-based interventions with adequate fidelity to the model. Proposed solutions to this barrier include:
• abbreviated versions of CBT training for practitioners in primary care and community settings
• culturally adapted CBT training for community health workers3
• Internet-based CBT and telemedicine (telephone and video conferencing)2
• mobile phone applications that use text messaging, social support, and physiological monitoring as adjuncts to clinical practice or stand-alone interventions.4
New models of CBT also are emerging, including transdiagnostic CBT and metacognitive approaches (mindfulness-based cognitive therapy and acceptance and commitment therapy), and several new foci for exposure therapy.
In light of these ongoing modulations, this article is intended to help clinicians make informed decisions about CBT when selecting treatment for patients with depressive and anxiety disorders (Box5 ). We review the evidence of CBT’s efficacy for acute-phase treatment and relapse prevention; explain the common elements considered essential to CBT practice; describe CBT adaptations for specific anxiety disorders; and provide an overview of recent advances in conceptualizing and adapting CBT.
Efficacy for mood and anxiety disorders
Depression. Dozens of randomized controlled trials (RCT) and other studies support CBT’s efficacy in treating major depressive disorder (MDD). For acute treatment:
• CBT is more effective in producing remission when compared with no treatment, treatment as usual, or nonspecific psychotherapy.
• For mild to moderate depression, CBT is equivalent to antidepressant medication in terms of response and remission rates.
• Combining antidepressant therapy with CBT increases treatment adherence.6
Less well known may be that a successful response to CBT in the acute phase may have a protective effect against depression recurrences. A 2013 meta-analysis that totaled 506 individuals with depressive disorders found a trend toward significantly lower relapse rates when CBT was discontinued after acute therapy, compared with antidepressant therapy that continued beyond the acute phase.7
Anxiety. Among psychotherapies, CBT’s superior efficacy for anxiety disorders is well-established. CBT and its specific-disorder adaptations are considered first-line treatment.8
CBT’s essential elements
CBT focuses on distorted cognitions about the self, the world, and the future, and on behaviors that lead to or maintain symptoms.
Cognitive interventions seek to identify thoughts and beliefs that trigger emotional and behavioral reactions. A person with social anxiety disorder, for example, might believe that people will notice if he makes even a minor social mistake and then reject him, which will make him feel worthless. CBT can help him subject these beliefs to rational analysis and develop more adaptive beliefs, such as: “It is not certain that I will behave so badly that people would notice, but if that happened, the likelihood of being outright rejected is probably low. If—in the worst-case scenario—I was rejected, I am not worthless; I’m just a fallible human being.”
CBT’s behavioral component can be conceptualized as behavioral activation (BA), a structured approach to help the patient:
• increase behaviors and experiences that are rewarding
• overcome barriers to engaging in these new behaviors
• and decrease behaviors that maintain symptoms.
BA can be a useful intervention for individuals with depression characterized by lack of engagement or capacity for pleasurable experiences. During pregnancy and the postpartum period, for example, a woman undergoes physical, social, and environmental changes that might gradually deprive her of sources of pleasure and other reinforcing activities. BA would focus on developing creative solutions to regain access to or create new opportunities for rewarding experiences and to avoid behaviors (such as social withdrawal or physical activity restriction) that perpetuate depressed mood.
Common elements. Cognitive and behavioral interventions focus on problem solving, individualized case conceptualization (Figure 1), and collaborative empiricism.9
Individualized case conceptualization lays the foundation for the course of CBT, and may be thought of as a map for therapy. Case conceptualization brings in several domains of assessment including symptoms and diagnosis, the patient’s strengths, formative experiences (including biopsychosocial aspects), contextual factors, and cognitive factors that influence diagnosis and treatment, such as automatic thoughts or schemas. The case formulation leads to a working hypothesis about the optimal course and focus of CBT.
Collaborative empiricism is the way in which the patient and therapist work together to continually refine this working hypothesis. The pair works together to investigate the hypotheses and all aspects of the therapeutic relationship.
Although no specific technique defines CBT, a common practice is to educate a person about interrelationships between behaviors/activities, thoughts, and mood. A mood activity log (Figure 2) can illuminate links between moods and activities and be useful with targeting interventions. For a person with social anxiety, for example, a mood activity log could assist in developing a hierarchy of feared social situations and avoidance intensity. Systematic exposure therapy would follow, beginning with the least frightening/intense situation, accompanied by teaching new coping skills (such as relaxation strategies).
CBT adaptations for anxiety disorders
Elements of CBT have been adapted for a variety of anxiety disorders, based on specific symptoms and features (Table).10-15
Panic disorder. Panic control treatment is considered the first-line intervention for panic disorder’s defining features: spontaneous panic attacks, worry about future occurrence of attacks, and perceived catastrophic consequences (such as heart attack, fainting).10 This CBT adaptation includes:
• patient education about the nature of panic
• breathing retraining to foster exposure to feared bodily sensations and avoided activities and places
• cognitive restructuring of danger-related thoughts (such as “I’m going to faint,” or “It would be catastrophic if I did”).
Obsessive-compulsive disorder. Exposure and response prevention (ERP) is the first-line treatment for obsessive-compulsive disorder (OCD).11 In traditional therapist-guided ERP, patients expose themselves to perceived contaminants while refraining from inappropriate compulsive behaviors (such as hand washing).
Cognitive interventions also can be an effective treatment of obsessions, without patients having to engage in exposure to their horrific thoughts and images.16 Consider, for example, a new mother who upon seeing the kitchen knife has the intrusive thought, “What if I stabbed my baby?” Instead of the traditional exposure approach for OCD (ie, having her vividly imagine stabbing her baby until her anxiety level subsided), the cognitive intervention would be to educate her about the normalcy of intrusive thoughts, particularly in the postpartum period.
Generalized anxiety disorder. CBT for generalized anxiety disorder (GAD) targets patients’ overestimation of the likelihood of negative events and the belief that these events, should they occur, would be catastrophic and render them unable to cope.12
Motivational interviewing (MI) appears to be a useful adjunct to precede traditional CBT, particularly for severe worriers.17 MI attempts to help individuals with GAD recognize their ambivalence about giving up worry. This technique acknowledges and validates perceived benefits of worry (eg, “It helps me prepare for the worst, so I won’t be emotionally devastated if it happens”), but also explores how worry is destructive.
Emerging CBT models for anxiety disorders
Metacognitive treatment. Evidence, such as presented by Dobson,18 suggests that the field of CBT is shifting towards a metacognitive model of change and treatment. A metacognitive approach goes beyond changing thinking and emphasizes thoughts about thoughts and experiences. Examples include mindfulness-based cognitive therapy (MBCT) and acceptance and commitment therapy (ACT).
MBCT typically consists of an 8-week program of 2-hour sessions each week and 1 full-day retreat. MBCT is modeled after Kabat-Zinn’s widely disseminated and empirically supported mindfulness based stress reduction course.19 MBCT was developed as a relapse prevention program for patients who had recovered from depression. Unlike traditional cognitive therapy for depression that targets changing the content of automatic thoughts and core beliefs, in MBCT patients are aware of negative automatic thoughts and find ways to change their relationship with these thoughts, learning that thoughts are not facts. This process mainly is carried out by practicing mindfulness meditation exercises. Importantly, MBCT goes beyond mindful acceptance of negative thoughts and teaches patients mindful acceptance of all internal experiences.
A fundamental difference between ACT and traditional CBT is the approach to cognitions.20 Although CBT focuses on changing the content of maladaptive thoughts, such as “I am a worthless person,” ACT focuses on changing the function of thoughts. ACT strives to help patients to accept their internal experiences—whether unwanted thoughts, feelings, bodily sensations, or memories—while committing themselves to pursuing their life goals and values. Strategies aim to help patients step back from their thoughts and observe them as just thoughts. The patient who thinks, “I am worthless” would be instructed to practice saying “I am having the thought I am worthless.” Therefore the thought no longer controls the person’s behavior.
These approaches train the patient to keenly observe distressing thoughts and experiences—not necessarily with the goal of changing them but to accept them and act in a way that is consistent with his (her) goals and values. A meta-analysis of 39 studies found mindfulness-based therapy effective in improving symptoms in participants with anxiety and mood disorders.21 Similarly, ACT has demonstrated efficacy with mixed anxiety disorders.22
Transdiagnostic CBT. Recent research18 suggests that mood and anxiety disorders may have more commonalities than differences in underlying biological and psychological traits. Because the symptoms of anxiety and depressive disorders tend to overlap, and their rate of comorbidity may be as high as 55%,23 so-called transdiagnostic treatments have been developed. Transdiagnostic treatments target impairing symptoms that cut across different diagnoses. For example, patients with depression, anxiety, or substance abuse might share a common difficulty with regulating and coping with negative emotions.
In a preliminary comparison trial,24 46 patients with social anxiety disorder, panic disorder, or GAD were randomly assigned to transdiagnostic CBT (n = 23) or diagnosis-specific CBT (n = 23). Treatments were based on widely used manuals and offered in 2-hour group sessions across 12 weeks. Transdiagnostic CBT was found to be as effective as specific CBT protocols in terms of symptom improvement. Participants attended an average of 8.46 sessions, with similar attendance in each protocol. Fourteen participants (30%) discontinued treatment, similar to attrition rates reported in other trials of transdiagnostic and diagnosis-specific CBT.
Transdiagnostic treatments may facilitate the dissemination of empirically supported treatments because therapists would not be required to have training and supervision to competency in delivering multiple manuals for specific anxiety disorders. This could be attractive to busy practitioners with limited time to learn new treatments.
Bottom Line
Efficacy of cognitive-behavioral therapy (CBT) for depression and anxiety is well established. Although no specific technique defines CBT, a common practice is to educate an individual about interrelationships between behaviors/activities, thoughts, and mood. CBT techniques can be customized to treat specific anxiety disorders, such as panic disorder, obsessive-compulsive disorder, and generalized anxiety disorder.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Fewer than 20% of people seeking help for depression and anxiety disorders receive cognitive-behavioral therapy (CBT), the most established evidence-based psychotherapeutic treatment.1 Efforts are being made to increase access to CBT,2 but a substantial barrier remains: therapist training is a strong predictor of treatment outcome, and many therapists offering CBT services are not sufficiently trained to deliver multiple manual-based interventions with adequate fidelity to the model. Proposed solutions to this barrier include:
• abbreviated versions of CBT training for practitioners in primary care and community settings
• culturally adapted CBT training for community health workers3
• Internet-based CBT and telemedicine (telephone and video conferencing)2
• mobile phone applications that use text messaging, social support, and physiological monitoring as adjuncts to clinical practice or stand-alone interventions.4
New models of CBT also are emerging, including transdiagnostic CBT and metacognitive approaches (mindfulness-based cognitive therapy and acceptance and commitment therapy), and several new foci for exposure therapy.
In light of these ongoing modulations, this article is intended to help clinicians make informed decisions about CBT when selecting treatment for patients with depressive and anxiety disorders (Box5 ). We review the evidence of CBT’s efficacy for acute-phase treatment and relapse prevention; explain the common elements considered essential to CBT practice; describe CBT adaptations for specific anxiety disorders; and provide an overview of recent advances in conceptualizing and adapting CBT.
Efficacy for mood and anxiety disorders
Depression. Dozens of randomized controlled trials (RCT) and other studies support CBT’s efficacy in treating major depressive disorder (MDD). For acute treatment:
• CBT is more effective in producing remission when compared with no treatment, treatment as usual, or nonspecific psychotherapy.
• For mild to moderate depression, CBT is equivalent to antidepressant medication in terms of response and remission rates.
• Combining antidepressant therapy with CBT increases treatment adherence.6
Less well known may be that a successful response to CBT in the acute phase may have a protective effect against depression recurrences. A 2013 meta-analysis that totaled 506 individuals with depressive disorders found a trend toward significantly lower relapse rates when CBT was discontinued after acute therapy, compared with antidepressant therapy that continued beyond the acute phase.7
Anxiety. Among psychotherapies, CBT’s superior efficacy for anxiety disorders is well-established. CBT and its specific-disorder adaptations are considered first-line treatment.8
CBT’s essential elements
CBT focuses on distorted cognitions about the self, the world, and the future, and on behaviors that lead to or maintain symptoms.
Cognitive interventions seek to identify thoughts and beliefs that trigger emotional and behavioral reactions. A person with social anxiety disorder, for example, might believe that people will notice if he makes even a minor social mistake and then reject him, which will make him feel worthless. CBT can help him subject these beliefs to rational analysis and develop more adaptive beliefs, such as: “It is not certain that I will behave so badly that people would notice, but if that happened, the likelihood of being outright rejected is probably low. If—in the worst-case scenario—I was rejected, I am not worthless; I’m just a fallible human being.”
CBT’s behavioral component can be conceptualized as behavioral activation (BA), a structured approach to help the patient:
• increase behaviors and experiences that are rewarding
• overcome barriers to engaging in these new behaviors
• and decrease behaviors that maintain symptoms.
BA can be a useful intervention for individuals with depression characterized by lack of engagement or capacity for pleasurable experiences. During pregnancy and the postpartum period, for example, a woman undergoes physical, social, and environmental changes that might gradually deprive her of sources of pleasure and other reinforcing activities. BA would focus on developing creative solutions to regain access to or create new opportunities for rewarding experiences and to avoid behaviors (such as social withdrawal or physical activity restriction) that perpetuate depressed mood.
Common elements. Cognitive and behavioral interventions focus on problem solving, individualized case conceptualization (Figure 1), and collaborative empiricism.9
Individualized case conceptualization lays the foundation for the course of CBT, and may be thought of as a map for therapy. Case conceptualization brings in several domains of assessment including symptoms and diagnosis, the patient’s strengths, formative experiences (including biopsychosocial aspects), contextual factors, and cognitive factors that influence diagnosis and treatment, such as automatic thoughts or schemas. The case formulation leads to a working hypothesis about the optimal course and focus of CBT.
Collaborative empiricism is the way in which the patient and therapist work together to continually refine this working hypothesis. The pair works together to investigate the hypotheses and all aspects of the therapeutic relationship.
Although no specific technique defines CBT, a common practice is to educate a person about interrelationships between behaviors/activities, thoughts, and mood. A mood activity log (Figure 2) can illuminate links between moods and activities and be useful with targeting interventions. For a person with social anxiety, for example, a mood activity log could assist in developing a hierarchy of feared social situations and avoidance intensity. Systematic exposure therapy would follow, beginning with the least frightening/intense situation, accompanied by teaching new coping skills (such as relaxation strategies).
CBT adaptations for anxiety disorders
Elements of CBT have been adapted for a variety of anxiety disorders, based on specific symptoms and features (Table).10-15
Panic disorder. Panic control treatment is considered the first-line intervention for panic disorder’s defining features: spontaneous panic attacks, worry about future occurrence of attacks, and perceived catastrophic consequences (such as heart attack, fainting).10 This CBT adaptation includes:
• patient education about the nature of panic
• breathing retraining to foster exposure to feared bodily sensations and avoided activities and places
• cognitive restructuring of danger-related thoughts (such as “I’m going to faint,” or “It would be catastrophic if I did”).
Obsessive-compulsive disorder. Exposure and response prevention (ERP) is the first-line treatment for obsessive-compulsive disorder (OCD).11 In traditional therapist-guided ERP, patients expose themselves to perceived contaminants while refraining from inappropriate compulsive behaviors (such as hand washing).
Cognitive interventions also can be an effective treatment of obsessions, without patients having to engage in exposure to their horrific thoughts and images.16 Consider, for example, a new mother who upon seeing the kitchen knife has the intrusive thought, “What if I stabbed my baby?” Instead of the traditional exposure approach for OCD (ie, having her vividly imagine stabbing her baby until her anxiety level subsided), the cognitive intervention would be to educate her about the normalcy of intrusive thoughts, particularly in the postpartum period.
Generalized anxiety disorder. CBT for generalized anxiety disorder (GAD) targets patients’ overestimation of the likelihood of negative events and the belief that these events, should they occur, would be catastrophic and render them unable to cope.12
Motivational interviewing (MI) appears to be a useful adjunct to precede traditional CBT, particularly for severe worriers.17 MI attempts to help individuals with GAD recognize their ambivalence about giving up worry. This technique acknowledges and validates perceived benefits of worry (eg, “It helps me prepare for the worst, so I won’t be emotionally devastated if it happens”), but also explores how worry is destructive.
Emerging CBT models for anxiety disorders
Metacognitive treatment. Evidence, such as presented by Dobson,18 suggests that the field of CBT is shifting towards a metacognitive model of change and treatment. A metacognitive approach goes beyond changing thinking and emphasizes thoughts about thoughts and experiences. Examples include mindfulness-based cognitive therapy (MBCT) and acceptance and commitment therapy (ACT).
MBCT typically consists of an 8-week program of 2-hour sessions each week and 1 full-day retreat. MBCT is modeled after Kabat-Zinn’s widely disseminated and empirically supported mindfulness based stress reduction course.19 MBCT was developed as a relapse prevention program for patients who had recovered from depression. Unlike traditional cognitive therapy for depression that targets changing the content of automatic thoughts and core beliefs, in MBCT patients are aware of negative automatic thoughts and find ways to change their relationship with these thoughts, learning that thoughts are not facts. This process mainly is carried out by practicing mindfulness meditation exercises. Importantly, MBCT goes beyond mindful acceptance of negative thoughts and teaches patients mindful acceptance of all internal experiences.
A fundamental difference between ACT and traditional CBT is the approach to cognitions.20 Although CBT focuses on changing the content of maladaptive thoughts, such as “I am a worthless person,” ACT focuses on changing the function of thoughts. ACT strives to help patients to accept their internal experiences—whether unwanted thoughts, feelings, bodily sensations, or memories—while committing themselves to pursuing their life goals and values. Strategies aim to help patients step back from their thoughts and observe them as just thoughts. The patient who thinks, “I am worthless” would be instructed to practice saying “I am having the thought I am worthless.” Therefore the thought no longer controls the person’s behavior.
These approaches train the patient to keenly observe distressing thoughts and experiences—not necessarily with the goal of changing them but to accept them and act in a way that is consistent with his (her) goals and values. A meta-analysis of 39 studies found mindfulness-based therapy effective in improving symptoms in participants with anxiety and mood disorders.21 Similarly, ACT has demonstrated efficacy with mixed anxiety disorders.22
Transdiagnostic CBT. Recent research18 suggests that mood and anxiety disorders may have more commonalities than differences in underlying biological and psychological traits. Because the symptoms of anxiety and depressive disorders tend to overlap, and their rate of comorbidity may be as high as 55%,23 so-called transdiagnostic treatments have been developed. Transdiagnostic treatments target impairing symptoms that cut across different diagnoses. For example, patients with depression, anxiety, or substance abuse might share a common difficulty with regulating and coping with negative emotions.
In a preliminary comparison trial,24 46 patients with social anxiety disorder, panic disorder, or GAD were randomly assigned to transdiagnostic CBT (n = 23) or diagnosis-specific CBT (n = 23). Treatments were based on widely used manuals and offered in 2-hour group sessions across 12 weeks. Transdiagnostic CBT was found to be as effective as specific CBT protocols in terms of symptom improvement. Participants attended an average of 8.46 sessions, with similar attendance in each protocol. Fourteen participants (30%) discontinued treatment, similar to attrition rates reported in other trials of transdiagnostic and diagnosis-specific CBT.
Transdiagnostic treatments may facilitate the dissemination of empirically supported treatments because therapists would not be required to have training and supervision to competency in delivering multiple manuals for specific anxiety disorders. This could be attractive to busy practitioners with limited time to learn new treatments.
Bottom Line
Efficacy of cognitive-behavioral therapy (CBT) for depression and anxiety is well established. Although no specific technique defines CBT, a common practice is to educate an individual about interrelationships between behaviors/activities, thoughts, and mood. CBT techniques can be customized to treat specific anxiety disorders, such as panic disorder, obsessive-compulsive disorder, and generalized anxiety disorder.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Collins K, Westra H, Dozois D, et al. Gaps in accessing treatment for anxiety and depressions: challenges for the delivery of care. Clin Psychol Rev. 2004;24(5):583-616.
2. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychological Science in the Public Interest. 2013;14(2):65-111.
3. Rahman A, Malik A, Sikander S, et al. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008;372(9642):902-909.
4. Aguilera A, Muench F. There’s an app for that: information technology applications for cognitive behavioral practitioners. Behavior Therapist. 2012;35(4):65-73.
5. Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006; 74(4):658-670.
6. Hollon SD, Jarrett RB, Nierenberg AA, et al. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J Clin Psychiatry. 2005;66(4):455-468.
7. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4):1-8.
8. Stewart R, Chambless D. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77(4): 595-606.
9. Wright JH, Basco MR, Thase M. Learning cognitive behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing; 2006.
10. Barlow DH, Craske MG. Mastery of your anxiety and panic. 4th ed. New York, NY: Oxford University Press, Inc.; 2007.
11. Foa EB, Yadin E, Lichner TK. Exposure and response prevention for obsessive-compulsive disorder: therapist guide. New York, NY: Oxford University Press, Inc.; 2012.
12. Dugas MJ, Robichaud M. Cognitive-behavioral treatment for generalized anxiety disorder. New York, NY: Routledge; 2007.
13. Zlomke K, Davis TE. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008;39(3):207-223.
14. Foa EB, Hembree E, Rothbaum B. Prolonged exposure therapy for PTSD: emotional processing of traumatic experiences. Therapist guide. New York, NY: Oxford University Press, Inc.; 2007.
15. Resick PA, Schnicke MK. Cognitive processing therapy for rape victims. London, United Kingdom: Sage Publications; 1996.
16. Whittal ML, Robichaud M, Woody SR. Cognitive treatment of obsessions: enhancing dissemination with video components. Cognitive and Behavioral Practice. 2010;17(1):1-8.
17. Westra H, Arkowitz H, Dozois D. Adding a motivational interviewing pretreatment to cognitive behavioral therapy for generalized anxiety disorder: a preliminary randomized controlled trial. J Anxiety Disord. 2009;23(2): 1106-1117.
18. Dobson KS. The science of CBT: toward a metacognitive model of change? Behav Ther. 2013;44(2):224-227.
19. Kabat-Zinn J. Full catastrophe living. Using the wisdom of your body and mind to face stress, pain, and illness. Revised edition. New York, NY: Bantam Books; 2013.
20. Hayes SC, Strosahl KD. Acceptance and commitment therapy. The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2012.
21. Hofmann S, Sawyer A, Witt A, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78(2): 169-183.
22. Arch J, Eifert G, Davies C, et al. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. J Consult Clin Psychol. 2012;80(5):750-765.
23. Brown TA, Campbell LA, Lehman CL, et al. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585-599.
24. Norton P, Barrera T. Transdiagnostic versus diagnosis-specific CBT for anxiety disorders: a preliminary randomized controlled noninferiority trial. Depress Anxiety. 2012;29(10):874-882.
1. Collins K, Westra H, Dozois D, et al. Gaps in accessing treatment for anxiety and depressions: challenges for the delivery of care. Clin Psychol Rev. 2004;24(5):583-616.
2. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychological Science in the Public Interest. 2013;14(2):65-111.
3. Rahman A, Malik A, Sikander S, et al. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008;372(9642):902-909.
4. Aguilera A, Muench F. There’s an app for that: information technology applications for cognitive behavioral practitioners. Behavior Therapist. 2012;35(4):65-73.
5. Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006; 74(4):658-670.
6. Hollon SD, Jarrett RB, Nierenberg AA, et al. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J Clin Psychiatry. 2005;66(4):455-468.
7. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4):1-8.
8. Stewart R, Chambless D. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77(4): 595-606.
9. Wright JH, Basco MR, Thase M. Learning cognitive behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing; 2006.
10. Barlow DH, Craske MG. Mastery of your anxiety and panic. 4th ed. New York, NY: Oxford University Press, Inc.; 2007.
11. Foa EB, Yadin E, Lichner TK. Exposure and response prevention for obsessive-compulsive disorder: therapist guide. New York, NY: Oxford University Press, Inc.; 2012.
12. Dugas MJ, Robichaud M. Cognitive-behavioral treatment for generalized anxiety disorder. New York, NY: Routledge; 2007.
13. Zlomke K, Davis TE. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008;39(3):207-223.
14. Foa EB, Hembree E, Rothbaum B. Prolonged exposure therapy for PTSD: emotional processing of traumatic experiences. Therapist guide. New York, NY: Oxford University Press, Inc.; 2007.
15. Resick PA, Schnicke MK. Cognitive processing therapy for rape victims. London, United Kingdom: Sage Publications; 1996.
16. Whittal ML, Robichaud M, Woody SR. Cognitive treatment of obsessions: enhancing dissemination with video components. Cognitive and Behavioral Practice. 2010;17(1):1-8.
17. Westra H, Arkowitz H, Dozois D. Adding a motivational interviewing pretreatment to cognitive behavioral therapy for generalized anxiety disorder: a preliminary randomized controlled trial. J Anxiety Disord. 2009;23(2): 1106-1117.
18. Dobson KS. The science of CBT: toward a metacognitive model of change? Behav Ther. 2013;44(2):224-227.
19. Kabat-Zinn J. Full catastrophe living. Using the wisdom of your body and mind to face stress, pain, and illness. Revised edition. New York, NY: Bantam Books; 2013.
20. Hayes SC, Strosahl KD. Acceptance and commitment therapy. The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2012.
21. Hofmann S, Sawyer A, Witt A, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78(2): 169-183.
22. Arch J, Eifert G, Davies C, et al. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. J Consult Clin Psychol. 2012;80(5):750-765.
23. Brown TA, Campbell LA, Lehman CL, et al. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585-599.
24. Norton P, Barrera T. Transdiagnostic versus diagnosis-specific CBT for anxiety disorders: a preliminary randomized controlled noninferiority trial. Depress Anxiety. 2012;29(10):874-882.
Do biomarkers for Alzheimer’s disease have utility in everyday practice?
Guidelines for diagnosing Alzheimer’s disease (AD) are undergoing the first major changes since they were developed 30 years ago. The National Institute on Aging (NIA) and the Alzheimer’s Association (AA) have established workgroups to revise guidelines that were written in 1984.1
One of the major changes to these new guidelines is mention of research on biomarkers for diagnosing and monitoring progression of dementia in AD. This is an exciting and provocative development, but the questions practitioners who diagnose and treat AD should be asking are whether such biomarkers have utility in clinical practice today, or whether their application is a distant promise of continuing research.
Principles put forward in the guidelines
The new AD guidelines set forth in 3 major papers by the workgroups created by the NIA and AA include a change in nomenclature of AD.2 The workgroups have sought to define AD with specific stages that include:
• a preclinical/prodromal phase, in which the pathophysiology responsible for future cognitive changes is ongoing but lacks clinical manifestations3
• mild cognitive impairment, now considered a distinct entity from dementia and diagnosed when a person has early signs of AD; manifestations of impaired cognition in early disease are not significant enough to affect daily functioning.4
These newly formulated stages of AD rely on clinical judgment, and AD remains a clinical diagnosis. However, the new diagnostic guidelines include the use of biomarkers to measure disease progression.
Biomarkers of normal biologic function and pathology
The Biomarkers Definitions Working Group defines a biomarker as:
… a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.5
These characteristics include imaging studies and body fluids, such as serum and cerebrospinal fluid (CSF).
In AD, biomarkers are meant to measure the pathogenic processes of:
• accumulation and deposition of amyloid β _protein (Aβ42) plaques
• neuronal degeneration characterized by an increase in phosphorylated tau protein and neurofibrillary tangles.6
The purpose of these biomarkers is to identify ongoing disease and help the clinician stage patients who display a spectrum of symptoms.
Four classes of biomarkers (Table7)have been identified for use in the diagnosis of, and research on, AD:
• neuroimaging
• CSF
• serum
• genetic markers.
Neuroimaging
The basic purpose of CT and MRI of the head in the workup of cognitive impairment is to rule out a lesion in the brain, such as a tumor or hemorrhage, as the cause of, or contributor to, the impairment. Several neuroimaging studies are available to aid in diagnosing AD and distinguishing it from other causes of dementia, including:
• Fludeoxyglucose (FDG) positron-emission tomography (PET) scanning
• MRI
• Florbetapir F 18 Injection for PET.
FDG PET identifies areas of the brain in which glucose metabolism is decreased. This finding is thought to represent synaptic dysfunction.8 The true clinical utility of FDG PET appears to be as an aid in distinguishing cases of AD from frontotemporal dementia, by identifying regions of metabolic dysfunction.9 (Note: Medicare will reimburse for FDG PET only if 1) the patient has met diagnostic criteria for both AD and frontotemporal dementia for at least 6 months and 2) the cause of symptoms is uncertain.10)
FDG PET also can be useful in patients with mild cognitive impairment by identifying hypometabolism in the temporal and parietal regions of the brain years before clinical AD develops.In addition to FDG, 2 other imaging probes—Pittsburgh compound and 2-(1-{6-[(2-[fluorine-18]fluoroethyl)(methyl) amino]-2-naphthyl}-ethylidene) malononitrile (more commonly, FDDNP)—have been used with PET as research tools to demonstrate evidence of AD.11
MRI has been used to measure hippocampal atrophy and cortical thinning that occurs as a patient progresses from normal cognitive function or mild cognitive impairment to full dementia.5 The degree of atrophy has not been well correlated with the degree of functional impairment.
Florbetapir F 18 Injection was approved by the FDA in October 2013, under the brand name AMYViD, for measuring the quantity of Aβ42 deposition in the brain. When injected, this radiopharmaceutical binds to Aβ42 and can be detected on PET.12 Use criteria for AMYViD PET recently were developed13; the technique is indicated as an additional diagnostic tool for ruling out AD.
A negative AMYViD scan indicates sparse or no Aβ42 plaques, and is inconsistent with AD. However, a positive AMYViD scan does not establish a diagnosis of AD or other cognitive disorder.14 This lack of specificity decreases the potential utility of the scan in clinical practice.
Use of AMYViD PET in general practice also is constrained by cost, which varies by location, based on the fee for the PET scan ($1,000 to $3,000)15; to that, add the cost of a dose of AMYViD ($1,600, wholesale).16 The technique is not reimbursable, and the total out-of-pocket expense can be as much as $5,000—making an AMYViD PET prohibitive.
Cerebrospinal fluid markers
CSF biomarkers used in the evaluation of AD are Aβ42, t-tau protein, and p-tau protein.6,17 It is generally thought that the level of Aβ42 in CSF decreases in AD—indicative of Aβ42 being deposited in the brain.8 Tau proteins are elevated in CSF as neurons are destroyed. P-tau is associated with the neurofibrillary tangles of AD; its presence in CSF is thought to represent an increase in those tangles. The combination of a low level of Aβ42 and an elevated level of p-tau in CSF is considered the signature CSF biomarker of AD.6
Serum markers
The search for reliable serum biomarkers of AD is the area of greatest research interest because a blood test is a less invasive form of screening. Regrettably, the utility of serum biomarkers for clinical practice has not been established.
Aβ42 can be measured in serum, but levels do not correlate well with CSF levels.18 Other serum markers that have been evaluated for clinical utility include measures of lipid metabolism, oxidation, and inflammation. With none of these is there clear correlation between the level of protein and AD.18
Fourth front: Genetics
Several alleles are associated with AD. Mutations in amyloid precursor protein, presenilin 1, and presenilin 2 have been shown to cause a change in the processing of Aβ42 and thus lead to AD.19 These mutations are inherited in an autosomal-dominant fashion and are detected in early-onset (age <65) AD.
Mutations in apolipoprotein 4-β4 also has been the subject of much research; this allele usually is associated with increased risk of the more common, later-onset AD.20 Some evidence suggests that apolipoprotein 4-β4 carriers who develop AD might be at risk of earlier onset of symptoms, compared to noncarriers,21 but the clinical significance of that increased risk has not been established.
What utility do biomarkers have?
As we said at the beginning of this article, the question that clinicians should be asking is: “What is the current clinical utility of these sophisticated biomarkers and genetic testing?”
The answer is “little utility.” Diagnosing AD is a clinical enterprise, with, as we’ve outlined, specific and narrow exceptions.
Recently, researchers demonstrated biomarker evidence of AD before symptom onset in patients who have known autosomal-dominant gene mutations for AD.19 There is no evidence, however, that these biomarkers are useful for screening the general population to identify people who 1) are at risk of, or who have, AD and 2) do not have AD.
That being said, CSF and imaging biomarkers of AD are being used in clinical settings in some European countries to aid investigation of cognitive decline.
In conclusion
Here are key points to take away from this discussion of biomarkers of AD:
• The utility of these biomarkers today is in research—although some of them might, on occasion, be useful to distinguish dementia caused by AD from other dementias.
• The ultimate goal of research is to uncover a serum biomarker that can identify patients in the preclinical/prodromal stage of AD, so that disease-modifying therapies and preventive measures can be initiated before symptoms manifest.
• Science is a long way from making this goal a reality, but recent changes in the diagnostic criteria for AD will encourage research in this area of study.
Bottom Line
Researchers are working to uncover biomarkers that will identify patients in the preclinical or prodromal stage of Alzheimer’s disease, but diagnosis remains clinical. Recent changes to diagnostic criteria will encourage research in this area.
Related Resources
• Blennow K, Dubois B, Fagan AM, et al. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease [published online May 5, 2014]. Alzheimers Dement. doi: 10.1016/j.jalz.2014.02.004.
• Chase A. Alzheimer disease: Advances in imaging of AD biomarkers could aid early diagnosis. Nat Rev Neurol. 2014;10(5):239.
• De Riva V, Galloni E, Marcon M, et al. Analysis of combined CSF biomarkers in AD diagnosis. Clin Lab. 2014;60(4):629-634.
• Kristofikova Z, Ricny J, Kolarova M, et al. Interactions between amyloid-β and tau in cerebrospinal fluid of people with mild cognitive impairment and Alzheimer’s disease [published online March 26, 2014]. J Alzheimers Dis. doi: 10.3233/ JAD-132393.
Drug Brand Name
Florbetapir F 18 Injection • AMYViD
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):257-262.
2. McKhann GM, Knopman DS. Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
3. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
4. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):270-279.
5. Cummings JL. Biomarkers in Alzheimer’s disease– perspectives for the future. US Neurology. 2010;6(1):23-27.
6. Sperling R, Keith J. Biomarkers of Alzheimer disease: current and future applications to diagnostic criteria. Continuum (Minneap Minn). 2013;19(2 Dementia):325-338.
7. Craig-Shapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiol Dis. 2009;35(2):128-140.
8. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128.
9. Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130(pt 10):2616-2635.
10. National Coverage Determination (NCD) for FDG PET for Dementia and Neurodegenerative Diseases (220.6.13). Centers for Medicare and Medicaid Services. http://www. cms.gov/medicare-coverage-database/details/ncd-details. aspx?NCDId=288&ncdver=3&bc=BAABAAAAAAAA&. Accessed May 9, 2014.
11. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161-172. 12. Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir- PET for imaging beta-amyloid pathology. JAMA. 2011;305(3): 275-283.
13. Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9(4):e106-e109.
14. AMYViD [package insert]. Indianapolis, IN: Eli Lilly & Co; 2012.
15. First guidelines published for brain amyloid imaging in Alzheimer’s. Alzheimer’s Association. http://www.alz.org/ news_and_events_60578.asp. Published January 28, 2013. Accessed May 9, 2014.
16. Zakaib GD. FDA approves Amyvid for clinical use. Alzforum. http://www.alzforum.org/news/research-news/ fda-approves-amyvid-clinical-use. Published April 9, 2012. Accessed May 16, 2014.
17. Skillbäck T, Zetterberg H, Blennow K, et al. Cerebrospinal fluid biomarkers for Alzheimer disease and subcortical axonal damage in 5,542 clinical samples. Alzheimers Res Ther. 2013;5(5):47.
18. Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1(2):226-234.
19. Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804.
20. Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature Genetics. 2007;39(1):17-23.
21. Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53-77.
Guidelines for diagnosing Alzheimer’s disease (AD) are undergoing the first major changes since they were developed 30 years ago. The National Institute on Aging (NIA) and the Alzheimer’s Association (AA) have established workgroups to revise guidelines that were written in 1984.1
One of the major changes to these new guidelines is mention of research on biomarkers for diagnosing and monitoring progression of dementia in AD. This is an exciting and provocative development, but the questions practitioners who diagnose and treat AD should be asking are whether such biomarkers have utility in clinical practice today, or whether their application is a distant promise of continuing research.
Principles put forward in the guidelines
The new AD guidelines set forth in 3 major papers by the workgroups created by the NIA and AA include a change in nomenclature of AD.2 The workgroups have sought to define AD with specific stages that include:
• a preclinical/prodromal phase, in which the pathophysiology responsible for future cognitive changes is ongoing but lacks clinical manifestations3
• mild cognitive impairment, now considered a distinct entity from dementia and diagnosed when a person has early signs of AD; manifestations of impaired cognition in early disease are not significant enough to affect daily functioning.4
These newly formulated stages of AD rely on clinical judgment, and AD remains a clinical diagnosis. However, the new diagnostic guidelines include the use of biomarkers to measure disease progression.
Biomarkers of normal biologic function and pathology
The Biomarkers Definitions Working Group defines a biomarker as:
… a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.5
These characteristics include imaging studies and body fluids, such as serum and cerebrospinal fluid (CSF).
In AD, biomarkers are meant to measure the pathogenic processes of:
• accumulation and deposition of amyloid β _protein (Aβ42) plaques
• neuronal degeneration characterized by an increase in phosphorylated tau protein and neurofibrillary tangles.6
The purpose of these biomarkers is to identify ongoing disease and help the clinician stage patients who display a spectrum of symptoms.
Four classes of biomarkers (Table7)have been identified for use in the diagnosis of, and research on, AD:
• neuroimaging
• CSF
• serum
• genetic markers.
Neuroimaging
The basic purpose of CT and MRI of the head in the workup of cognitive impairment is to rule out a lesion in the brain, such as a tumor or hemorrhage, as the cause of, or contributor to, the impairment. Several neuroimaging studies are available to aid in diagnosing AD and distinguishing it from other causes of dementia, including:
• Fludeoxyglucose (FDG) positron-emission tomography (PET) scanning
• MRI
• Florbetapir F 18 Injection for PET.
FDG PET identifies areas of the brain in which glucose metabolism is decreased. This finding is thought to represent synaptic dysfunction.8 The true clinical utility of FDG PET appears to be as an aid in distinguishing cases of AD from frontotemporal dementia, by identifying regions of metabolic dysfunction.9 (Note: Medicare will reimburse for FDG PET only if 1) the patient has met diagnostic criteria for both AD and frontotemporal dementia for at least 6 months and 2) the cause of symptoms is uncertain.10)
FDG PET also can be useful in patients with mild cognitive impairment by identifying hypometabolism in the temporal and parietal regions of the brain years before clinical AD develops.In addition to FDG, 2 other imaging probes—Pittsburgh compound and 2-(1-{6-[(2-[fluorine-18]fluoroethyl)(methyl) amino]-2-naphthyl}-ethylidene) malononitrile (more commonly, FDDNP)—have been used with PET as research tools to demonstrate evidence of AD.11
MRI has been used to measure hippocampal atrophy and cortical thinning that occurs as a patient progresses from normal cognitive function or mild cognitive impairment to full dementia.5 The degree of atrophy has not been well correlated with the degree of functional impairment.
Florbetapir F 18 Injection was approved by the FDA in October 2013, under the brand name AMYViD, for measuring the quantity of Aβ42 deposition in the brain. When injected, this radiopharmaceutical binds to Aβ42 and can be detected on PET.12 Use criteria for AMYViD PET recently were developed13; the technique is indicated as an additional diagnostic tool for ruling out AD.
A negative AMYViD scan indicates sparse or no Aβ42 plaques, and is inconsistent with AD. However, a positive AMYViD scan does not establish a diagnosis of AD or other cognitive disorder.14 This lack of specificity decreases the potential utility of the scan in clinical practice.
Use of AMYViD PET in general practice also is constrained by cost, which varies by location, based on the fee for the PET scan ($1,000 to $3,000)15; to that, add the cost of a dose of AMYViD ($1,600, wholesale).16 The technique is not reimbursable, and the total out-of-pocket expense can be as much as $5,000—making an AMYViD PET prohibitive.
Cerebrospinal fluid markers
CSF biomarkers used in the evaluation of AD are Aβ42, t-tau protein, and p-tau protein.6,17 It is generally thought that the level of Aβ42 in CSF decreases in AD—indicative of Aβ42 being deposited in the brain.8 Tau proteins are elevated in CSF as neurons are destroyed. P-tau is associated with the neurofibrillary tangles of AD; its presence in CSF is thought to represent an increase in those tangles. The combination of a low level of Aβ42 and an elevated level of p-tau in CSF is considered the signature CSF biomarker of AD.6
Serum markers
The search for reliable serum biomarkers of AD is the area of greatest research interest because a blood test is a less invasive form of screening. Regrettably, the utility of serum biomarkers for clinical practice has not been established.
Aβ42 can be measured in serum, but levels do not correlate well with CSF levels.18 Other serum markers that have been evaluated for clinical utility include measures of lipid metabolism, oxidation, and inflammation. With none of these is there clear correlation between the level of protein and AD.18
Fourth front: Genetics
Several alleles are associated with AD. Mutations in amyloid precursor protein, presenilin 1, and presenilin 2 have been shown to cause a change in the processing of Aβ42 and thus lead to AD.19 These mutations are inherited in an autosomal-dominant fashion and are detected in early-onset (age <65) AD.
Mutations in apolipoprotein 4-β4 also has been the subject of much research; this allele usually is associated with increased risk of the more common, later-onset AD.20 Some evidence suggests that apolipoprotein 4-β4 carriers who develop AD might be at risk of earlier onset of symptoms, compared to noncarriers,21 but the clinical significance of that increased risk has not been established.
What utility do biomarkers have?
As we said at the beginning of this article, the question that clinicians should be asking is: “What is the current clinical utility of these sophisticated biomarkers and genetic testing?”
The answer is “little utility.” Diagnosing AD is a clinical enterprise, with, as we’ve outlined, specific and narrow exceptions.
Recently, researchers demonstrated biomarker evidence of AD before symptom onset in patients who have known autosomal-dominant gene mutations for AD.19 There is no evidence, however, that these biomarkers are useful for screening the general population to identify people who 1) are at risk of, or who have, AD and 2) do not have AD.
That being said, CSF and imaging biomarkers of AD are being used in clinical settings in some European countries to aid investigation of cognitive decline.
In conclusion
Here are key points to take away from this discussion of biomarkers of AD:
• The utility of these biomarkers today is in research—although some of them might, on occasion, be useful to distinguish dementia caused by AD from other dementias.
• The ultimate goal of research is to uncover a serum biomarker that can identify patients in the preclinical/prodromal stage of AD, so that disease-modifying therapies and preventive measures can be initiated before symptoms manifest.
• Science is a long way from making this goal a reality, but recent changes in the diagnostic criteria for AD will encourage research in this area of study.
Bottom Line
Researchers are working to uncover biomarkers that will identify patients in the preclinical or prodromal stage of Alzheimer’s disease, but diagnosis remains clinical. Recent changes to diagnostic criteria will encourage research in this area.
Related Resources
• Blennow K, Dubois B, Fagan AM, et al. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease [published online May 5, 2014]. Alzheimers Dement. doi: 10.1016/j.jalz.2014.02.004.
• Chase A. Alzheimer disease: Advances in imaging of AD biomarkers could aid early diagnosis. Nat Rev Neurol. 2014;10(5):239.
• De Riva V, Galloni E, Marcon M, et al. Analysis of combined CSF biomarkers in AD diagnosis. Clin Lab. 2014;60(4):629-634.
• Kristofikova Z, Ricny J, Kolarova M, et al. Interactions between amyloid-β and tau in cerebrospinal fluid of people with mild cognitive impairment and Alzheimer’s disease [published online March 26, 2014]. J Alzheimers Dis. doi: 10.3233/ JAD-132393.
Drug Brand Name
Florbetapir F 18 Injection • AMYViD
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Guidelines for diagnosing Alzheimer’s disease (AD) are undergoing the first major changes since they were developed 30 years ago. The National Institute on Aging (NIA) and the Alzheimer’s Association (AA) have established workgroups to revise guidelines that were written in 1984.1
One of the major changes to these new guidelines is mention of research on biomarkers for diagnosing and monitoring progression of dementia in AD. This is an exciting and provocative development, but the questions practitioners who diagnose and treat AD should be asking are whether such biomarkers have utility in clinical practice today, or whether their application is a distant promise of continuing research.
Principles put forward in the guidelines
The new AD guidelines set forth in 3 major papers by the workgroups created by the NIA and AA include a change in nomenclature of AD.2 The workgroups have sought to define AD with specific stages that include:
• a preclinical/prodromal phase, in which the pathophysiology responsible for future cognitive changes is ongoing but lacks clinical manifestations3
• mild cognitive impairment, now considered a distinct entity from dementia and diagnosed when a person has early signs of AD; manifestations of impaired cognition in early disease are not significant enough to affect daily functioning.4
These newly formulated stages of AD rely on clinical judgment, and AD remains a clinical diagnosis. However, the new diagnostic guidelines include the use of biomarkers to measure disease progression.
Biomarkers of normal biologic function and pathology
The Biomarkers Definitions Working Group defines a biomarker as:
… a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.5
These characteristics include imaging studies and body fluids, such as serum and cerebrospinal fluid (CSF).
In AD, biomarkers are meant to measure the pathogenic processes of:
• accumulation and deposition of amyloid β _protein (Aβ42) plaques
• neuronal degeneration characterized by an increase in phosphorylated tau protein and neurofibrillary tangles.6
The purpose of these biomarkers is to identify ongoing disease and help the clinician stage patients who display a spectrum of symptoms.
Four classes of biomarkers (Table7)have been identified for use in the diagnosis of, and research on, AD:
• neuroimaging
• CSF
• serum
• genetic markers.
Neuroimaging
The basic purpose of CT and MRI of the head in the workup of cognitive impairment is to rule out a lesion in the brain, such as a tumor or hemorrhage, as the cause of, or contributor to, the impairment. Several neuroimaging studies are available to aid in diagnosing AD and distinguishing it from other causes of dementia, including:
• Fludeoxyglucose (FDG) positron-emission tomography (PET) scanning
• MRI
• Florbetapir F 18 Injection for PET.
FDG PET identifies areas of the brain in which glucose metabolism is decreased. This finding is thought to represent synaptic dysfunction.8 The true clinical utility of FDG PET appears to be as an aid in distinguishing cases of AD from frontotemporal dementia, by identifying regions of metabolic dysfunction.9 (Note: Medicare will reimburse for FDG PET only if 1) the patient has met diagnostic criteria for both AD and frontotemporal dementia for at least 6 months and 2) the cause of symptoms is uncertain.10)
FDG PET also can be useful in patients with mild cognitive impairment by identifying hypometabolism in the temporal and parietal regions of the brain years before clinical AD develops.In addition to FDG, 2 other imaging probes—Pittsburgh compound and 2-(1-{6-[(2-[fluorine-18]fluoroethyl)(methyl) amino]-2-naphthyl}-ethylidene) malononitrile (more commonly, FDDNP)—have been used with PET as research tools to demonstrate evidence of AD.11
MRI has been used to measure hippocampal atrophy and cortical thinning that occurs as a patient progresses from normal cognitive function or mild cognitive impairment to full dementia.5 The degree of atrophy has not been well correlated with the degree of functional impairment.
Florbetapir F 18 Injection was approved by the FDA in October 2013, under the brand name AMYViD, for measuring the quantity of Aβ42 deposition in the brain. When injected, this radiopharmaceutical binds to Aβ42 and can be detected on PET.12 Use criteria for AMYViD PET recently were developed13; the technique is indicated as an additional diagnostic tool for ruling out AD.
A negative AMYViD scan indicates sparse or no Aβ42 plaques, and is inconsistent with AD. However, a positive AMYViD scan does not establish a diagnosis of AD or other cognitive disorder.14 This lack of specificity decreases the potential utility of the scan in clinical practice.
Use of AMYViD PET in general practice also is constrained by cost, which varies by location, based on the fee for the PET scan ($1,000 to $3,000)15; to that, add the cost of a dose of AMYViD ($1,600, wholesale).16 The technique is not reimbursable, and the total out-of-pocket expense can be as much as $5,000—making an AMYViD PET prohibitive.
Cerebrospinal fluid markers
CSF biomarkers used in the evaluation of AD are Aβ42, t-tau protein, and p-tau protein.6,17 It is generally thought that the level of Aβ42 in CSF decreases in AD—indicative of Aβ42 being deposited in the brain.8 Tau proteins are elevated in CSF as neurons are destroyed. P-tau is associated with the neurofibrillary tangles of AD; its presence in CSF is thought to represent an increase in those tangles. The combination of a low level of Aβ42 and an elevated level of p-tau in CSF is considered the signature CSF biomarker of AD.6
Serum markers
The search for reliable serum biomarkers of AD is the area of greatest research interest because a blood test is a less invasive form of screening. Regrettably, the utility of serum biomarkers for clinical practice has not been established.
Aβ42 can be measured in serum, but levels do not correlate well with CSF levels.18 Other serum markers that have been evaluated for clinical utility include measures of lipid metabolism, oxidation, and inflammation. With none of these is there clear correlation between the level of protein and AD.18
Fourth front: Genetics
Several alleles are associated with AD. Mutations in amyloid precursor protein, presenilin 1, and presenilin 2 have been shown to cause a change in the processing of Aβ42 and thus lead to AD.19 These mutations are inherited in an autosomal-dominant fashion and are detected in early-onset (age <65) AD.
Mutations in apolipoprotein 4-β4 also has been the subject of much research; this allele usually is associated with increased risk of the more common, later-onset AD.20 Some evidence suggests that apolipoprotein 4-β4 carriers who develop AD might be at risk of earlier onset of symptoms, compared to noncarriers,21 but the clinical significance of that increased risk has not been established.
What utility do biomarkers have?
As we said at the beginning of this article, the question that clinicians should be asking is: “What is the current clinical utility of these sophisticated biomarkers and genetic testing?”
The answer is “little utility.” Diagnosing AD is a clinical enterprise, with, as we’ve outlined, specific and narrow exceptions.
Recently, researchers demonstrated biomarker evidence of AD before symptom onset in patients who have known autosomal-dominant gene mutations for AD.19 There is no evidence, however, that these biomarkers are useful for screening the general population to identify people who 1) are at risk of, or who have, AD and 2) do not have AD.
That being said, CSF and imaging biomarkers of AD are being used in clinical settings in some European countries to aid investigation of cognitive decline.
In conclusion
Here are key points to take away from this discussion of biomarkers of AD:
• The utility of these biomarkers today is in research—although some of them might, on occasion, be useful to distinguish dementia caused by AD from other dementias.
• The ultimate goal of research is to uncover a serum biomarker that can identify patients in the preclinical/prodromal stage of AD, so that disease-modifying therapies and preventive measures can be initiated before symptoms manifest.
• Science is a long way from making this goal a reality, but recent changes in the diagnostic criteria for AD will encourage research in this area of study.
Bottom Line
Researchers are working to uncover biomarkers that will identify patients in the preclinical or prodromal stage of Alzheimer’s disease, but diagnosis remains clinical. Recent changes to diagnostic criteria will encourage research in this area.
Related Resources
• Blennow K, Dubois B, Fagan AM, et al. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease [published online May 5, 2014]. Alzheimers Dement. doi: 10.1016/j.jalz.2014.02.004.
• Chase A. Alzheimer disease: Advances in imaging of AD biomarkers could aid early diagnosis. Nat Rev Neurol. 2014;10(5):239.
• De Riva V, Galloni E, Marcon M, et al. Analysis of combined CSF biomarkers in AD diagnosis. Clin Lab. 2014;60(4):629-634.
• Kristofikova Z, Ricny J, Kolarova M, et al. Interactions between amyloid-β and tau in cerebrospinal fluid of people with mild cognitive impairment and Alzheimer’s disease [published online March 26, 2014]. J Alzheimers Dis. doi: 10.3233/ JAD-132393.
Drug Brand Name
Florbetapir F 18 Injection • AMYViD
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):257-262.
2. McKhann GM, Knopman DS. Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
3. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
4. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):270-279.
5. Cummings JL. Biomarkers in Alzheimer’s disease– perspectives for the future. US Neurology. 2010;6(1):23-27.
6. Sperling R, Keith J. Biomarkers of Alzheimer disease: current and future applications to diagnostic criteria. Continuum (Minneap Minn). 2013;19(2 Dementia):325-338.
7. Craig-Shapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiol Dis. 2009;35(2):128-140.
8. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128.
9. Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130(pt 10):2616-2635.
10. National Coverage Determination (NCD) for FDG PET for Dementia and Neurodegenerative Diseases (220.6.13). Centers for Medicare and Medicaid Services. http://www. cms.gov/medicare-coverage-database/details/ncd-details. aspx?NCDId=288&ncdver=3&bc=BAABAAAAAAAA&. Accessed May 9, 2014.
11. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161-172. 12. Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir- PET for imaging beta-amyloid pathology. JAMA. 2011;305(3): 275-283.
13. Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9(4):e106-e109.
14. AMYViD [package insert]. Indianapolis, IN: Eli Lilly & Co; 2012.
15. First guidelines published for brain amyloid imaging in Alzheimer’s. Alzheimer’s Association. http://www.alz.org/ news_and_events_60578.asp. Published January 28, 2013. Accessed May 9, 2014.
16. Zakaib GD. FDA approves Amyvid for clinical use. Alzforum. http://www.alzforum.org/news/research-news/ fda-approves-amyvid-clinical-use. Published April 9, 2012. Accessed May 16, 2014.
17. Skillbäck T, Zetterberg H, Blennow K, et al. Cerebrospinal fluid biomarkers for Alzheimer disease and subcortical axonal damage in 5,542 clinical samples. Alzheimers Res Ther. 2013;5(5):47.
18. Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1(2):226-234.
19. Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804.
20. Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature Genetics. 2007;39(1):17-23.
21. Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53-77.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):257-262.
2. McKhann GM, Knopman DS. Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
3. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
4. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):270-279.
5. Cummings JL. Biomarkers in Alzheimer’s disease– perspectives for the future. US Neurology. 2010;6(1):23-27.
6. Sperling R, Keith J. Biomarkers of Alzheimer disease: current and future applications to diagnostic criteria. Continuum (Minneap Minn). 2013;19(2 Dementia):325-338.
7. Craig-Shapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiol Dis. 2009;35(2):128-140.
8. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128.
9. Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130(pt 10):2616-2635.
10. National Coverage Determination (NCD) for FDG PET for Dementia and Neurodegenerative Diseases (220.6.13). Centers for Medicare and Medicaid Services. http://www. cms.gov/medicare-coverage-database/details/ncd-details. aspx?NCDId=288&ncdver=3&bc=BAABAAAAAAAA&. Accessed May 9, 2014.
11. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161-172. 12. Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir- PET for imaging beta-amyloid pathology. JAMA. 2011;305(3): 275-283.
13. Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9(4):e106-e109.
14. AMYViD [package insert]. Indianapolis, IN: Eli Lilly & Co; 2012.
15. First guidelines published for brain amyloid imaging in Alzheimer’s. Alzheimer’s Association. http://www.alz.org/ news_and_events_60578.asp. Published January 28, 2013. Accessed May 9, 2014.
16. Zakaib GD. FDA approves Amyvid for clinical use. Alzforum. http://www.alzforum.org/news/research-news/ fda-approves-amyvid-clinical-use. Published April 9, 2012. Accessed May 16, 2014.
17. Skillbäck T, Zetterberg H, Blennow K, et al. Cerebrospinal fluid biomarkers for Alzheimer disease and subcortical axonal damage in 5,542 clinical samples. Alzheimers Res Ther. 2013;5(5):47.
18. Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1(2):226-234.
19. Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804.
20. Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature Genetics. 2007;39(1):17-23.
21. Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53-77.
Billing and Coding Knowledge: A Comparative Survey of Professional Coders, Practicing Orthopedic Surgeons, and Orthopedic Residents
Progressive Valgus Angulation of the Ankle Secondary to Loss of Fibular Congruity Treated With Medial Tibial Hemiepiphysiodesis and Fibular Reconstruction
Delayed Presentation of a Cervical Spine Fracture Dislocation With Posterior Ligamentous Disruption in a Gymnast
BPD and the broader landscape of neuropsychiatric illness
Dr. Henry A. Nasrallah’s recent Editorial on borderline personality disorder (BPD) (Current Psychiatry, From the Editor, April 2014, p. 19-20, 32 [http://bit.ly/1e8yAwE]) describes BPD as a heritable brain disease. I have been arguing this point for many years, often finding support from my colleague, Hagop Akiskal, MD, and opposition from my psychoanalytic colleagues.
In recent papers1,2 on brain changes in BPD and the connection between BPD and bipolar disorders, I wrote that there often is a heritable aspect to the condition. There are exceptions to such heritability, as in the setting of a horrific environment (eg, father-daughter incest, parental brutality), where the same symptoms seen in BPD develop primarily from post-natal influences. Dr. Akiskal and I were discussing this a long time back, before MRI. Now I feel vindicated, with generous help from someone of Dr. Nasrallah’s prestige and influence.
There also is electrophysiological (including evoked potential) evidence for neural pathology in BPD, as well as data derived from single photon emission CT scanning. The burgeoning literature on MRI and functional MRI studies of BPD is in good agreement about the brain changes most relevant to BPD and that are found with regularity in this condition.
Particularly when BPD is diagnosed in people (usually women) who do not have a history of neglect, sexual molestation, parental humiliation or cruelty, or head injury, what else is there, if not genetically predisposed alterations in the frontolimbic structures (and maybe the periaqueductal gray) that underlie the so-called “personality disorder,” and, not surprisingly, bipolar disorders, especially bipolar II disorder, which often is the other side of the coin as BPD, and amenable to the same combination of medication and psychotherapy?
Michael H. Stone, MD
Professor of Clinical Psychiatry Columbia College of Physicians and Surgeons
New York, New York
----------------------------------------------------------------------------------------------------------
As a psychiatrist/psychoanalyst who works with BPD patients, I read Dr. Nasrallah’s April 2014 Editorial with great interest and enthusiasm. Over the past 10 years, I have been impressed with the number of patients with BPD whose nonverbal learning disorders and auditory and visual processing disorders have gone undiagnosed. Recently, I lectured on this topic to the staff of a school for children with a range of neuropsychiatric disorders; the staff found my observations about such comorbidity consistent with their observations. These dysfunctions, or neurological variations—unknown to the parent and the child—interfere with early object-relation formation, attachment capacity, and learning. Neuropsychiatry and psychological development are, in fact, part of the same system.
An example: For 12 years, I have been treating a patient who has auditory processing and working memory problems, meaning that she could not process the connections among different ideas. This difficulty frustrated her parents, who, in their frustration, criticized her for not paying attention. She was labeled “bad” and assumed the role of the “black sheep” in her family. Although she was intelligent, she was often wrong in her judgments and choices, and easily frustrated. In therapy, as I realized what part of her problem was, I changed my technique.
When my patient asked me to tell her the sequence of understandings that we had just put together, I invited her to take my pad and write down her sense of it. As she described each part of that sequence to me, we would discuss it and I would remind her of lost fragments. Gradually, she learned to put ideas together; however, I also watched her struggle to hold these ideas in working memory and to use them.
Over time, she has improved and is more functional. After several years of disability, she returned to work, although she still struggles interpersonally.
With many of such patients, I have had to modify traditional techniques of psychotherapy. I am fascinated by, and enjoy, such intensive psychotherapy. I am also amazed to see the impact of previously unknown neuropathologic variations on development. The more I learn about the impact of neuropsychiatry on psychological development, the more I can help my patients.
Howard Wishnie, MD
Cambridge, Massachusetts
Dr. Nasrallah responds
I appreciate Dr. Stone’s kind words and concurrence with my thinking about BPD. It would have been appropriate to include discussion of neurophysiological findings in my Editorial, but I opted to use my limited space to focus on structural and functional neuroimaging and genetics.
Henry A. Nasrallah, MD
Professor and Chairman Department of Neurology & Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
1. Stone MH. The spectrum of borderline personality disorder: a neurophysiological view. Neuropsychiatric Electrophysiology. In press.
2. Stone MH. A new look at borderline personality disorder and related disorders: hyper-activity in the limbic system and lower centers. Psychodyn Psychiatry. 2013;41(3):437-466.
Dr. Henry A. Nasrallah’s recent Editorial on borderline personality disorder (BPD) (Current Psychiatry, From the Editor, April 2014, p. 19-20, 32 [http://bit.ly/1e8yAwE]) describes BPD as a heritable brain disease. I have been arguing this point for many years, often finding support from my colleague, Hagop Akiskal, MD, and opposition from my psychoanalytic colleagues.
In recent papers1,2 on brain changes in BPD and the connection between BPD and bipolar disorders, I wrote that there often is a heritable aspect to the condition. There are exceptions to such heritability, as in the setting of a horrific environment (eg, father-daughter incest, parental brutality), where the same symptoms seen in BPD develop primarily from post-natal influences. Dr. Akiskal and I were discussing this a long time back, before MRI. Now I feel vindicated, with generous help from someone of Dr. Nasrallah’s prestige and influence.
There also is electrophysiological (including evoked potential) evidence for neural pathology in BPD, as well as data derived from single photon emission CT scanning. The burgeoning literature on MRI and functional MRI studies of BPD is in good agreement about the brain changes most relevant to BPD and that are found with regularity in this condition.
Particularly when BPD is diagnosed in people (usually women) who do not have a history of neglect, sexual molestation, parental humiliation or cruelty, or head injury, what else is there, if not genetically predisposed alterations in the frontolimbic structures (and maybe the periaqueductal gray) that underlie the so-called “personality disorder,” and, not surprisingly, bipolar disorders, especially bipolar II disorder, which often is the other side of the coin as BPD, and amenable to the same combination of medication and psychotherapy?
Michael H. Stone, MD
Professor of Clinical Psychiatry Columbia College of Physicians and Surgeons
New York, New York
----------------------------------------------------------------------------------------------------------
As a psychiatrist/psychoanalyst who works with BPD patients, I read Dr. Nasrallah’s April 2014 Editorial with great interest and enthusiasm. Over the past 10 years, I have been impressed with the number of patients with BPD whose nonverbal learning disorders and auditory and visual processing disorders have gone undiagnosed. Recently, I lectured on this topic to the staff of a school for children with a range of neuropsychiatric disorders; the staff found my observations about such comorbidity consistent with their observations. These dysfunctions, or neurological variations—unknown to the parent and the child—interfere with early object-relation formation, attachment capacity, and learning. Neuropsychiatry and psychological development are, in fact, part of the same system.
An example: For 12 years, I have been treating a patient who has auditory processing and working memory problems, meaning that she could not process the connections among different ideas. This difficulty frustrated her parents, who, in their frustration, criticized her for not paying attention. She was labeled “bad” and assumed the role of the “black sheep” in her family. Although she was intelligent, she was often wrong in her judgments and choices, and easily frustrated. In therapy, as I realized what part of her problem was, I changed my technique.
When my patient asked me to tell her the sequence of understandings that we had just put together, I invited her to take my pad and write down her sense of it. As she described each part of that sequence to me, we would discuss it and I would remind her of lost fragments. Gradually, she learned to put ideas together; however, I also watched her struggle to hold these ideas in working memory and to use them.
Over time, she has improved and is more functional. After several years of disability, she returned to work, although she still struggles interpersonally.
With many of such patients, I have had to modify traditional techniques of psychotherapy. I am fascinated by, and enjoy, such intensive psychotherapy. I am also amazed to see the impact of previously unknown neuropathologic variations on development. The more I learn about the impact of neuropsychiatry on psychological development, the more I can help my patients.
Howard Wishnie, MD
Cambridge, Massachusetts
Dr. Nasrallah responds
I appreciate Dr. Stone’s kind words and concurrence with my thinking about BPD. It would have been appropriate to include discussion of neurophysiological findings in my Editorial, but I opted to use my limited space to focus on structural and functional neuroimaging and genetics.
Henry A. Nasrallah, MD
Professor and Chairman Department of Neurology & Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
Dr. Henry A. Nasrallah’s recent Editorial on borderline personality disorder (BPD) (Current Psychiatry, From the Editor, April 2014, p. 19-20, 32 [http://bit.ly/1e8yAwE]) describes BPD as a heritable brain disease. I have been arguing this point for many years, often finding support from my colleague, Hagop Akiskal, MD, and opposition from my psychoanalytic colleagues.
In recent papers1,2 on brain changes in BPD and the connection between BPD and bipolar disorders, I wrote that there often is a heritable aspect to the condition. There are exceptions to such heritability, as in the setting of a horrific environment (eg, father-daughter incest, parental brutality), where the same symptoms seen in BPD develop primarily from post-natal influences. Dr. Akiskal and I were discussing this a long time back, before MRI. Now I feel vindicated, with generous help from someone of Dr. Nasrallah’s prestige and influence.
There also is electrophysiological (including evoked potential) evidence for neural pathology in BPD, as well as data derived from single photon emission CT scanning. The burgeoning literature on MRI and functional MRI studies of BPD is in good agreement about the brain changes most relevant to BPD and that are found with regularity in this condition.
Particularly when BPD is diagnosed in people (usually women) who do not have a history of neglect, sexual molestation, parental humiliation or cruelty, or head injury, what else is there, if not genetically predisposed alterations in the frontolimbic structures (and maybe the periaqueductal gray) that underlie the so-called “personality disorder,” and, not surprisingly, bipolar disorders, especially bipolar II disorder, which often is the other side of the coin as BPD, and amenable to the same combination of medication and psychotherapy?
Michael H. Stone, MD
Professor of Clinical Psychiatry Columbia College of Physicians and Surgeons
New York, New York
----------------------------------------------------------------------------------------------------------
As a psychiatrist/psychoanalyst who works with BPD patients, I read Dr. Nasrallah’s April 2014 Editorial with great interest and enthusiasm. Over the past 10 years, I have been impressed with the number of patients with BPD whose nonverbal learning disorders and auditory and visual processing disorders have gone undiagnosed. Recently, I lectured on this topic to the staff of a school for children with a range of neuropsychiatric disorders; the staff found my observations about such comorbidity consistent with their observations. These dysfunctions, or neurological variations—unknown to the parent and the child—interfere with early object-relation formation, attachment capacity, and learning. Neuropsychiatry and psychological development are, in fact, part of the same system.
An example: For 12 years, I have been treating a patient who has auditory processing and working memory problems, meaning that she could not process the connections among different ideas. This difficulty frustrated her parents, who, in their frustration, criticized her for not paying attention. She was labeled “bad” and assumed the role of the “black sheep” in her family. Although she was intelligent, she was often wrong in her judgments and choices, and easily frustrated. In therapy, as I realized what part of her problem was, I changed my technique.
When my patient asked me to tell her the sequence of understandings that we had just put together, I invited her to take my pad and write down her sense of it. As she described each part of that sequence to me, we would discuss it and I would remind her of lost fragments. Gradually, she learned to put ideas together; however, I also watched her struggle to hold these ideas in working memory and to use them.
Over time, she has improved and is more functional. After several years of disability, she returned to work, although she still struggles interpersonally.
With many of such patients, I have had to modify traditional techniques of psychotherapy. I am fascinated by, and enjoy, such intensive psychotherapy. I am also amazed to see the impact of previously unknown neuropathologic variations on development. The more I learn about the impact of neuropsychiatry on psychological development, the more I can help my patients.
Howard Wishnie, MD
Cambridge, Massachusetts
Dr. Nasrallah responds
I appreciate Dr. Stone’s kind words and concurrence with my thinking about BPD. It would have been appropriate to include discussion of neurophysiological findings in my Editorial, but I opted to use my limited space to focus on structural and functional neuroimaging and genetics.
Henry A. Nasrallah, MD
Professor and Chairman Department of Neurology & Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
1. Stone MH. The spectrum of borderline personality disorder: a neurophysiological view. Neuropsychiatric Electrophysiology. In press.
2. Stone MH. A new look at borderline personality disorder and related disorders: hyper-activity in the limbic system and lower centers. Psychodyn Psychiatry. 2013;41(3):437-466.
1. Stone MH. The spectrum of borderline personality disorder: a neurophysiological view. Neuropsychiatric Electrophysiology. In press.
2. Stone MH. A new look at borderline personality disorder and related disorders: hyper-activity in the limbic system and lower centers. Psychodyn Psychiatry. 2013;41(3):437-466.