User login
Cancer doesn’t increase a child’s risk of PTSD

Credit: Bill Branson
Childhood cancer patients are no more likely than their healthy peers to develop post-traumatic stress disorder (PTSD), according to research published in the Journal of Clinical Oncology.
“A cancer diagnosis is a highly significant and challenging event, but this study highlights the impressive capacity of children to adjust to changes in their lives and, in most cases, do just fine or even thrive emotionally as a result,” said study author Sean Phipps, PhD, of St Jude Children’s Research Hospital in Memphis.
PTSD is a treatable anxiety disorder that can develop following terrifying events that result in real or potential physical harm. The diagnosis is based on patient reports of certain symptoms, including persistent frightening thoughts, flashbacks, numbness, detachment, and sleep disturbances.
For this study, researchers used 3 established PTSD screening methods on 255 pediatric cancer patients (aged 8 to 17 at diagnosis) and 101 of their healthy, demographically matched peers.
This included a symptom check list and a structured diagnostic interview about the event in a child’s life he or she identified as the most traumatic. The researchers also interviewed parents about PTSD symptoms in themselves and their children.
Based on self-reported symptoms, 2.8% of cancer patients (n=7) met the criteria for a diagnosis of PTSD, either when the study was conducted or in the past.
The PTSD was cancer-related in 2 of these patients. In the other 5 patients, the disorder was linked to a drive-by shooting, Hurricane Katrina, or other stressful events.
By diagnostic interview, 0.4% of the cancer patients met PTSD criteria. According to parents’ reports, 1.6% of the cancer patients met criteria for current PTSD, and 5.9% met lifetime criteria.
These rates of PTSD were not significantly different from the rates reported in the healthy control subjects (all P values were greater than 0.1).
Unlike many previous studies of PTSD in cancer patients, researchers initially refrained from asking patients specifically about their diagnosis. Investigators wanted to avoid suggesting to patients that their cancer diagnoses were traumatic.
“We know such suggestions, called ‘focusing illusions,’ prime individuals to think about their cancer experience as traumatic and leaves them prone to exaggerating its impact in subjective reports,” Dr Phipps said. ![]()

Credit: Bill Branson
Childhood cancer patients are no more likely than their healthy peers to develop post-traumatic stress disorder (PTSD), according to research published in the Journal of Clinical Oncology.
“A cancer diagnosis is a highly significant and challenging event, but this study highlights the impressive capacity of children to adjust to changes in their lives and, in most cases, do just fine or even thrive emotionally as a result,” said study author Sean Phipps, PhD, of St Jude Children’s Research Hospital in Memphis.
PTSD is a treatable anxiety disorder that can develop following terrifying events that result in real or potential physical harm. The diagnosis is based on patient reports of certain symptoms, including persistent frightening thoughts, flashbacks, numbness, detachment, and sleep disturbances.
For this study, researchers used 3 established PTSD screening methods on 255 pediatric cancer patients (aged 8 to 17 at diagnosis) and 101 of their healthy, demographically matched peers.
This included a symptom check list and a structured diagnostic interview about the event in a child’s life he or she identified as the most traumatic. The researchers also interviewed parents about PTSD symptoms in themselves and their children.
Based on self-reported symptoms, 2.8% of cancer patients (n=7) met the criteria for a diagnosis of PTSD, either when the study was conducted or in the past.
The PTSD was cancer-related in 2 of these patients. In the other 5 patients, the disorder was linked to a drive-by shooting, Hurricane Katrina, or other stressful events.
By diagnostic interview, 0.4% of the cancer patients met PTSD criteria. According to parents’ reports, 1.6% of the cancer patients met criteria for current PTSD, and 5.9% met lifetime criteria.
These rates of PTSD were not significantly different from the rates reported in the healthy control subjects (all P values were greater than 0.1).
Unlike many previous studies of PTSD in cancer patients, researchers initially refrained from asking patients specifically about their diagnosis. Investigators wanted to avoid suggesting to patients that their cancer diagnoses were traumatic.
“We know such suggestions, called ‘focusing illusions,’ prime individuals to think about their cancer experience as traumatic and leaves them prone to exaggerating its impact in subjective reports,” Dr Phipps said. ![]()

Credit: Bill Branson
Childhood cancer patients are no more likely than their healthy peers to develop post-traumatic stress disorder (PTSD), according to research published in the Journal of Clinical Oncology.
“A cancer diagnosis is a highly significant and challenging event, but this study highlights the impressive capacity of children to adjust to changes in their lives and, in most cases, do just fine or even thrive emotionally as a result,” said study author Sean Phipps, PhD, of St Jude Children’s Research Hospital in Memphis.
PTSD is a treatable anxiety disorder that can develop following terrifying events that result in real or potential physical harm. The diagnosis is based on patient reports of certain symptoms, including persistent frightening thoughts, flashbacks, numbness, detachment, and sleep disturbances.
For this study, researchers used 3 established PTSD screening methods on 255 pediatric cancer patients (aged 8 to 17 at diagnosis) and 101 of their healthy, demographically matched peers.
This included a symptom check list and a structured diagnostic interview about the event in a child’s life he or she identified as the most traumatic. The researchers also interviewed parents about PTSD symptoms in themselves and their children.
Based on self-reported symptoms, 2.8% of cancer patients (n=7) met the criteria for a diagnosis of PTSD, either when the study was conducted or in the past.
The PTSD was cancer-related in 2 of these patients. In the other 5 patients, the disorder was linked to a drive-by shooting, Hurricane Katrina, or other stressful events.
By diagnostic interview, 0.4% of the cancer patients met PTSD criteria. According to parents’ reports, 1.6% of the cancer patients met criteria for current PTSD, and 5.9% met lifetime criteria.
These rates of PTSD were not significantly different from the rates reported in the healthy control subjects (all P values were greater than 0.1).
Unlike many previous studies of PTSD in cancer patients, researchers initially refrained from asking patients specifically about their diagnosis. Investigators wanted to avoid suggesting to patients that their cancer diagnoses were traumatic.
“We know such suggestions, called ‘focusing illusions,’ prime individuals to think about their cancer experience as traumatic and leaves them prone to exaggerating its impact in subjective reports,” Dr Phipps said. ![]()
Mandatory disclosures may make docs avoid COIs

Credit: Rhoda Baer
Previous research has indicated that requiring conflict of interest (COI) disclosures may lead advisers to give more biased advice.
However, virtually all of the prior studies questioned the effectiveness of COI disclosures that advisers were unable to avoid.
With a new study, researchers examined situations in which advisers have the ability to avoid COIs—such as doctors who can decide whether to accept gifts or payments from pharmaceutical companies.
And the results showed that when COIs can be avoided, disclosure successfully deters advisers from accepting COIs, so they have nothing to disclose except the absence of conflicts.
The research was published in Psychological Science.
“Prior research has cast doubt as to the effectiveness of disclosure for managing conflicts of interest, particularly when consumers have the burden of interpreting and reacting to the information,” said study author Sunita Sah, PhD, MB ChB, of Georgetown University in Washington, DC.
“Our findings suggest that disclosure can become a successful intervention to managing some conflicts of interest if it motivates professionals or providers to avoid such conflicts.”
For this study, the researchers conducted 3 experiments to determine how COIs influence advisers. In the first experiment, 97 adviser–advisee pairs participated in an online game with Amazon.com gift cards at stake.
Advisers informed the advisees regarding the number of filled dots on a grid. The estimators were paid based on their accuracy, but advisers had a conflict. They were paid more if advisees gave an estimate that was higher than the true value.
The set up—with advisees only seeing a small subset of the complete grid—was designed to simulate a situation in which a consumer receives advice from a better-informed but conflicted professional. The results replicated previous research and showed that disclosure led advisers to give higher (and more biased) recommendations than nondisclosure.
In the second experiment, the researchers again randomly assigned pairs of advisees and advisers to conditions in which the conflict was either disclosed or not disclosed.
There was, however, an important change from the first study. Advisers were given a choice of whether to accept or reject the COI.
Without disclosure, a majority of advisers (63%) chose the incentives that created a COI. But with disclosure, a minority (33%) accepted the conflict.
Advice was higher (and more biased) for those who chose incentives with conflicts than for those who did not, and advisers in the disclosure condition gave significantly less biased advice than those in the nondisclosure condition.
Finally, in a study with 248 participants, the researchers added a third condition to the second experiment: voluntary disclosure. In this third condition, advisers decided both whether to choose incentives that entailed a conflict and whether to disclose if they had a conflict.
Similar to mandatory disclosure, voluntary disclosure led advisers to avoid COIs and then disclose their freedom from conflicts to advisees.
“Disclosure doesn’t seem to be much good when conflicts are unavoidable, but it does seem to help when advisers have a choice about whether to subject themselves to conflicts,” said study author George Loewenstein, PhD, of Carnegie Mellon University in Pittsburgh.
“A nice feature of disclosure is that it is, in effect, ‘self-calibrating.’ Doctors, for example, are unlikely to find it worth it to accept small gifts such as pens or calendars if the gifts are going to be disclosed. Although larger gifts would be more tempting, doctors are likely to be deterred from accepting them because disclosure of large gifts would be more damaging to their reputations.” ![]()

Credit: Rhoda Baer
Previous research has indicated that requiring conflict of interest (COI) disclosures may lead advisers to give more biased advice.
However, virtually all of the prior studies questioned the effectiveness of COI disclosures that advisers were unable to avoid.
With a new study, researchers examined situations in which advisers have the ability to avoid COIs—such as doctors who can decide whether to accept gifts or payments from pharmaceutical companies.
And the results showed that when COIs can be avoided, disclosure successfully deters advisers from accepting COIs, so they have nothing to disclose except the absence of conflicts.
The research was published in Psychological Science.
“Prior research has cast doubt as to the effectiveness of disclosure for managing conflicts of interest, particularly when consumers have the burden of interpreting and reacting to the information,” said study author Sunita Sah, PhD, MB ChB, of Georgetown University in Washington, DC.
“Our findings suggest that disclosure can become a successful intervention to managing some conflicts of interest if it motivates professionals or providers to avoid such conflicts.”
For this study, the researchers conducted 3 experiments to determine how COIs influence advisers. In the first experiment, 97 adviser–advisee pairs participated in an online game with Amazon.com gift cards at stake.
Advisers informed the advisees regarding the number of filled dots on a grid. The estimators were paid based on their accuracy, but advisers had a conflict. They were paid more if advisees gave an estimate that was higher than the true value.
The set up—with advisees only seeing a small subset of the complete grid—was designed to simulate a situation in which a consumer receives advice from a better-informed but conflicted professional. The results replicated previous research and showed that disclosure led advisers to give higher (and more biased) recommendations than nondisclosure.
In the second experiment, the researchers again randomly assigned pairs of advisees and advisers to conditions in which the conflict was either disclosed or not disclosed.
There was, however, an important change from the first study. Advisers were given a choice of whether to accept or reject the COI.
Without disclosure, a majority of advisers (63%) chose the incentives that created a COI. But with disclosure, a minority (33%) accepted the conflict.
Advice was higher (and more biased) for those who chose incentives with conflicts than for those who did not, and advisers in the disclosure condition gave significantly less biased advice than those in the nondisclosure condition.
Finally, in a study with 248 participants, the researchers added a third condition to the second experiment: voluntary disclosure. In this third condition, advisers decided both whether to choose incentives that entailed a conflict and whether to disclose if they had a conflict.
Similar to mandatory disclosure, voluntary disclosure led advisers to avoid COIs and then disclose their freedom from conflicts to advisees.
“Disclosure doesn’t seem to be much good when conflicts are unavoidable, but it does seem to help when advisers have a choice about whether to subject themselves to conflicts,” said study author George Loewenstein, PhD, of Carnegie Mellon University in Pittsburgh.
“A nice feature of disclosure is that it is, in effect, ‘self-calibrating.’ Doctors, for example, are unlikely to find it worth it to accept small gifts such as pens or calendars if the gifts are going to be disclosed. Although larger gifts would be more tempting, doctors are likely to be deterred from accepting them because disclosure of large gifts would be more damaging to their reputations.” ![]()

Credit: Rhoda Baer
Previous research has indicated that requiring conflict of interest (COI) disclosures may lead advisers to give more biased advice.
However, virtually all of the prior studies questioned the effectiveness of COI disclosures that advisers were unable to avoid.
With a new study, researchers examined situations in which advisers have the ability to avoid COIs—such as doctors who can decide whether to accept gifts or payments from pharmaceutical companies.
And the results showed that when COIs can be avoided, disclosure successfully deters advisers from accepting COIs, so they have nothing to disclose except the absence of conflicts.
The research was published in Psychological Science.
“Prior research has cast doubt as to the effectiveness of disclosure for managing conflicts of interest, particularly when consumers have the burden of interpreting and reacting to the information,” said study author Sunita Sah, PhD, MB ChB, of Georgetown University in Washington, DC.
“Our findings suggest that disclosure can become a successful intervention to managing some conflicts of interest if it motivates professionals or providers to avoid such conflicts.”
For this study, the researchers conducted 3 experiments to determine how COIs influence advisers. In the first experiment, 97 adviser–advisee pairs participated in an online game with Amazon.com gift cards at stake.
Advisers informed the advisees regarding the number of filled dots on a grid. The estimators were paid based on their accuracy, but advisers had a conflict. They were paid more if advisees gave an estimate that was higher than the true value.
The set up—with advisees only seeing a small subset of the complete grid—was designed to simulate a situation in which a consumer receives advice from a better-informed but conflicted professional. The results replicated previous research and showed that disclosure led advisers to give higher (and more biased) recommendations than nondisclosure.
In the second experiment, the researchers again randomly assigned pairs of advisees and advisers to conditions in which the conflict was either disclosed or not disclosed.
There was, however, an important change from the first study. Advisers were given a choice of whether to accept or reject the COI.
Without disclosure, a majority of advisers (63%) chose the incentives that created a COI. But with disclosure, a minority (33%) accepted the conflict.
Advice was higher (and more biased) for those who chose incentives with conflicts than for those who did not, and advisers in the disclosure condition gave significantly less biased advice than those in the nondisclosure condition.
Finally, in a study with 248 participants, the researchers added a third condition to the second experiment: voluntary disclosure. In this third condition, advisers decided both whether to choose incentives that entailed a conflict and whether to disclose if they had a conflict.
Similar to mandatory disclosure, voluntary disclosure led advisers to avoid COIs and then disclose their freedom from conflicts to advisees.
“Disclosure doesn’t seem to be much good when conflicts are unavoidable, but it does seem to help when advisers have a choice about whether to subject themselves to conflicts,” said study author George Loewenstein, PhD, of Carnegie Mellon University in Pittsburgh.
“A nice feature of disclosure is that it is, in effect, ‘self-calibrating.’ Doctors, for example, are unlikely to find it worth it to accept small gifts such as pens or calendars if the gifts are going to be disclosed. Although larger gifts would be more tempting, doctors are likely to be deterred from accepting them because disclosure of large gifts would be more damaging to their reputations.” ![]()
Challenges in Training: Open repair in 2020
Since FDA approval of endovascular aneurysm repair in 1999, the management of abdominal aortic aneurysms has transformed. Only 5.2% of AAAs were repaired by EVAR in 2000 compared to 74% in 2010. While the volume of AAA cases has remained constant at about 45,000 cases annually, the increase in EVAR has led to a 34% drop in open AAA cases. This national decline in open AAA cases is paralleled by a 33% decline in open AAA cases completed by vascular trainees since 2001, as reported in national Accreditation Council for Graduate Medical Education case logs.
In conjunction with this decrease in open AAA repair volume, trainees are reporting low confidence in independently performing open aneurysm cases, with nearly 40% of 2014 graduating vascular trainees having expressed low confidence in their ability on a 3-point Likert scale (1 – not confident, 2 – somewhat confident, 3 – very confident).
Over the past decade, we have also seen a threefold increase in adjudicated cases against vascular surgeons due to complications arising from open AAA cases, along with an increase in litigation against vascular surgeons who have been in practice for fewer than 3 years.
Open AAA surgery volume will continue to decrease as branched and fenestrated technology improves, and as expanded utilization in patients with complex anatomy occurs with the next generation of endografts.
By 2020, based on prediction models, vascular trainees will complete only five open aneurysm cases during their training, This raises serious questions about how we will educate the next generation of vascular surgeons to safely and effectively manage patients who can be treated only by open repair.
Since FDA approval of endovascular aneurysm repair in 1999, the management of abdominal aortic aneurysms has transformed. Only 5.2% of AAAs were repaired by EVAR in 2000 compared to 74% in 2010. While the volume of AAA cases has remained constant at about 45,000 cases annually, the increase in EVAR has led to a 34% drop in open AAA cases. This national decline in open AAA cases is paralleled by a 33% decline in open AAA cases completed by vascular trainees since 2001, as reported in national Accreditation Council for Graduate Medical Education case logs.
In conjunction with this decrease in open AAA repair volume, trainees are reporting low confidence in independently performing open aneurysm cases, with nearly 40% of 2014 graduating vascular trainees having expressed low confidence in their ability on a 3-point Likert scale (1 – not confident, 2 – somewhat confident, 3 – very confident).
Over the past decade, we have also seen a threefold increase in adjudicated cases against vascular surgeons due to complications arising from open AAA cases, along with an increase in litigation against vascular surgeons who have been in practice for fewer than 3 years.
Open AAA surgery volume will continue to decrease as branched and fenestrated technology improves, and as expanded utilization in patients with complex anatomy occurs with the next generation of endografts.
By 2020, based on prediction models, vascular trainees will complete only five open aneurysm cases during their training, This raises serious questions about how we will educate the next generation of vascular surgeons to safely and effectively manage patients who can be treated only by open repair.
Since FDA approval of endovascular aneurysm repair in 1999, the management of abdominal aortic aneurysms has transformed. Only 5.2% of AAAs were repaired by EVAR in 2000 compared to 74% in 2010. While the volume of AAA cases has remained constant at about 45,000 cases annually, the increase in EVAR has led to a 34% drop in open AAA cases. This national decline in open AAA cases is paralleled by a 33% decline in open AAA cases completed by vascular trainees since 2001, as reported in national Accreditation Council for Graduate Medical Education case logs.
In conjunction with this decrease in open AAA repair volume, trainees are reporting low confidence in independently performing open aneurysm cases, with nearly 40% of 2014 graduating vascular trainees having expressed low confidence in their ability on a 3-point Likert scale (1 – not confident, 2 – somewhat confident, 3 – very confident).
Over the past decade, we have also seen a threefold increase in adjudicated cases against vascular surgeons due to complications arising from open AAA cases, along with an increase in litigation against vascular surgeons who have been in practice for fewer than 3 years.
Open AAA surgery volume will continue to decrease as branched and fenestrated technology improves, and as expanded utilization in patients with complex anatomy occurs with the next generation of endografts.
By 2020, based on prediction models, vascular trainees will complete only five open aneurysm cases during their training, This raises serious questions about how we will educate the next generation of vascular surgeons to safely and effectively manage patients who can be treated only by open repair.
Calcium Hydroxylapatite Filler Foreign Body Granulomas: A Case Report of a Rare Occurrence and Review of the Literature
CEA vs. stenting in the elderly: The debate continues
For elderly patients with carotid disease, carotid endarterectomy carries a lower risk of perioperative stroke or transient ischemic attack, the same risk of perioperative MI, and a slightly higher risk of perioperative death compared with carotid artery stenting, according to a meta-analysis.
However, the individual elderly patient’s vascular anatomy plays a crucial role in determining perioperative risk, as does his or her overall health and clinical profile.
"The results of [our] analysis suggest that careful consideration of a constellation of clinical and anatomic factors is required before an appropriate treatment of carotid disease in elderly patients is selected. The cardiovascular disease burden and general health of the individual patient should be meticulously evaluated before interventional instead of optimal medical treatment is applied," said Dr. George A. Antoniou of the department of vascular surgery, Hellenic Red Cross Hospital, Athens, and his associates.
Which treatment is the most appropriate for elderly patients with carotid disease is still much debated. Dr. Antoniou and his colleagues performed a comprehensive review of the medical literature since 1986 and a meta-analysis of 44 articles that directly compared outcomes in elderly patients with those of younger patients after carotid endarterectomy (39 studies) or carotid stenting (18 articles).
"Elderly" was defined as older than 80 years in most of these studies, and as older than 75 years in many, but there was great variability among the studies, and some even considered "older than 65 years" to be elderly.
Overall, the meta-analysis included 269,596 endarterectomies in elderly patients against 243,089 in younger patients, and 38,751 carotid stenting procedures in elderly patients against 36,450 in younger patients.
For endarterectomy, the rate of perioperative stroke was not significantly different between elderly (0.9%) and younger (1.2%) patients, nor was the rate of TIA (1.9% vs 1.8%, respectively). However, perioperative mortality was significantly higher in elderly (0.5%) than in younger (0.4%) patients.
In contrast, for carotid stenting, the rate of perioperative stroke was significantly higher for elderly patients (2.4%) than for younger patients (1.7%), as was the rate of TIA (3.6% vs 2.1%). And mortality was not significantly different between elderly patients (0.6%) and younger patients (0.7%), the researchers wrote (JAMA Surg. 2013 Oct. 23 [doi:10.1001/jamasurg.2013.4135]).
Both procedures were associated with an increased rate of perioperative MI in elderly patients, compared with younger patients. These rates were 2.2% in elderly patients, compared with 1.4% in younger patients undergoing endarterectomy; and 2.3% in elderly patients, compared with 1.5% in younger patients undergoing carotid stenting.
These findings remained robust in sensitivity analyses.
"It seems that endarterectomy is associated with improved neurologic outcomes compared with carotid stenting in elderly patients, at the expense of increased perioperative mortality." However, the small increase in mortality seen with endarterectomy – one-tenth of 1% – may not be clinically significant, Dr. Antoniou and his associates said.
Moreover, neurologic risk is closely tied to vascular anatomy. Elderly patients tend to have more unfavorable anatomy than do younger patients, but should be assessed on an individual basis. Unfavorable traits include heavily calcified and tortuous supra-aortic branches, as well as adverse morphology of the aortic arch such as elongation, distortion, and stenosis.
Manipulating the stenting instruments through such features may in itself raise the risk of neurologic sequelae. It also makes the procedure more technically difficult, which increases the risk of endothelial trauma, thrombus dislodgment, and thromboembolic events.
"In addition, elderly patients with significant extracranial atherosclerotic disease are likely to have a compromised cerebrovascular reserve, which makes them more susceptible to ischemic events from cerebral microembolization," the researchers said.
They reported having no conflicts.
This study’s conclusions are not surprising, given that most clinicians have already seen them both in randomized prospective studies and in their own practices, said Dr. R. Clement Darling III.
However, the variation in the definition of "elderly" among the trials in this meta-analysis is a real concern: 64% used 80 years as the cutoff, 31% used 75 years, and some used 70 or even 65 years as the cutoff.
"The bottom line is, carotid endarterectomy and carotid stenting seem to work equally well in younger patients, in expert hands. However, in the ‘elderly’ (at any age), endarterectomy has better outcomes with low morbidity, mortality, and stroke rate, and it remains the standard of care."
Dr. Darling of the Vascular Group, Albany, N.Y., made these remarks in an invited commentary (JAMA Surgery 2013 Oct. 23 [doi:10.1001/jamasurg.2013.4160]). He reported no financial conflicts of interest.
This study’s conclusions are not surprising, given that most clinicians have already seen them both in randomized prospective studies and in their own practices, said Dr. R. Clement Darling III.
However, the variation in the definition of "elderly" among the trials in this meta-analysis is a real concern: 64% used 80 years as the cutoff, 31% used 75 years, and some used 70 or even 65 years as the cutoff.
"The bottom line is, carotid endarterectomy and carotid stenting seem to work equally well in younger patients, in expert hands. However, in the ‘elderly’ (at any age), endarterectomy has better outcomes with low morbidity, mortality, and stroke rate, and it remains the standard of care."
Dr. Darling of the Vascular Group, Albany, N.Y., made these remarks in an invited commentary (JAMA Surgery 2013 Oct. 23 [doi:10.1001/jamasurg.2013.4160]). He reported no financial conflicts of interest.
This study’s conclusions are not surprising, given that most clinicians have already seen them both in randomized prospective studies and in their own practices, said Dr. R. Clement Darling III.
However, the variation in the definition of "elderly" among the trials in this meta-analysis is a real concern: 64% used 80 years as the cutoff, 31% used 75 years, and some used 70 or even 65 years as the cutoff.
"The bottom line is, carotid endarterectomy and carotid stenting seem to work equally well in younger patients, in expert hands. However, in the ‘elderly’ (at any age), endarterectomy has better outcomes with low morbidity, mortality, and stroke rate, and it remains the standard of care."
Dr. Darling of the Vascular Group, Albany, N.Y., made these remarks in an invited commentary (JAMA Surgery 2013 Oct. 23 [doi:10.1001/jamasurg.2013.4160]). He reported no financial conflicts of interest.
For elderly patients with carotid disease, carotid endarterectomy carries a lower risk of perioperative stroke or transient ischemic attack, the same risk of perioperative MI, and a slightly higher risk of perioperative death compared with carotid artery stenting, according to a meta-analysis.
However, the individual elderly patient’s vascular anatomy plays a crucial role in determining perioperative risk, as does his or her overall health and clinical profile.
"The results of [our] analysis suggest that careful consideration of a constellation of clinical and anatomic factors is required before an appropriate treatment of carotid disease in elderly patients is selected. The cardiovascular disease burden and general health of the individual patient should be meticulously evaluated before interventional instead of optimal medical treatment is applied," said Dr. George A. Antoniou of the department of vascular surgery, Hellenic Red Cross Hospital, Athens, and his associates.
Which treatment is the most appropriate for elderly patients with carotid disease is still much debated. Dr. Antoniou and his colleagues performed a comprehensive review of the medical literature since 1986 and a meta-analysis of 44 articles that directly compared outcomes in elderly patients with those of younger patients after carotid endarterectomy (39 studies) or carotid stenting (18 articles).
"Elderly" was defined as older than 80 years in most of these studies, and as older than 75 years in many, but there was great variability among the studies, and some even considered "older than 65 years" to be elderly.
Overall, the meta-analysis included 269,596 endarterectomies in elderly patients against 243,089 in younger patients, and 38,751 carotid stenting procedures in elderly patients against 36,450 in younger patients.
For endarterectomy, the rate of perioperative stroke was not significantly different between elderly (0.9%) and younger (1.2%) patients, nor was the rate of TIA (1.9% vs 1.8%, respectively). However, perioperative mortality was significantly higher in elderly (0.5%) than in younger (0.4%) patients.
In contrast, for carotid stenting, the rate of perioperative stroke was significantly higher for elderly patients (2.4%) than for younger patients (1.7%), as was the rate of TIA (3.6% vs 2.1%). And mortality was not significantly different between elderly patients (0.6%) and younger patients (0.7%), the researchers wrote (JAMA Surg. 2013 Oct. 23 [doi:10.1001/jamasurg.2013.4135]).
Both procedures were associated with an increased rate of perioperative MI in elderly patients, compared with younger patients. These rates were 2.2% in elderly patients, compared with 1.4% in younger patients undergoing endarterectomy; and 2.3% in elderly patients, compared with 1.5% in younger patients undergoing carotid stenting.
These findings remained robust in sensitivity analyses.
"It seems that endarterectomy is associated with improved neurologic outcomes compared with carotid stenting in elderly patients, at the expense of increased perioperative mortality." However, the small increase in mortality seen with endarterectomy – one-tenth of 1% – may not be clinically significant, Dr. Antoniou and his associates said.
Moreover, neurologic risk is closely tied to vascular anatomy. Elderly patients tend to have more unfavorable anatomy than do younger patients, but should be assessed on an individual basis. Unfavorable traits include heavily calcified and tortuous supra-aortic branches, as well as adverse morphology of the aortic arch such as elongation, distortion, and stenosis.
Manipulating the stenting instruments through such features may in itself raise the risk of neurologic sequelae. It also makes the procedure more technically difficult, which increases the risk of endothelial trauma, thrombus dislodgment, and thromboembolic events.
"In addition, elderly patients with significant extracranial atherosclerotic disease are likely to have a compromised cerebrovascular reserve, which makes them more susceptible to ischemic events from cerebral microembolization," the researchers said.
They reported having no conflicts.
For elderly patients with carotid disease, carotid endarterectomy carries a lower risk of perioperative stroke or transient ischemic attack, the same risk of perioperative MI, and a slightly higher risk of perioperative death compared with carotid artery stenting, according to a meta-analysis.
However, the individual elderly patient’s vascular anatomy plays a crucial role in determining perioperative risk, as does his or her overall health and clinical profile.
"The results of [our] analysis suggest that careful consideration of a constellation of clinical and anatomic factors is required before an appropriate treatment of carotid disease in elderly patients is selected. The cardiovascular disease burden and general health of the individual patient should be meticulously evaluated before interventional instead of optimal medical treatment is applied," said Dr. George A. Antoniou of the department of vascular surgery, Hellenic Red Cross Hospital, Athens, and his associates.
Which treatment is the most appropriate for elderly patients with carotid disease is still much debated. Dr. Antoniou and his colleagues performed a comprehensive review of the medical literature since 1986 and a meta-analysis of 44 articles that directly compared outcomes in elderly patients with those of younger patients after carotid endarterectomy (39 studies) or carotid stenting (18 articles).
"Elderly" was defined as older than 80 years in most of these studies, and as older than 75 years in many, but there was great variability among the studies, and some even considered "older than 65 years" to be elderly.
Overall, the meta-analysis included 269,596 endarterectomies in elderly patients against 243,089 in younger patients, and 38,751 carotid stenting procedures in elderly patients against 36,450 in younger patients.
For endarterectomy, the rate of perioperative stroke was not significantly different between elderly (0.9%) and younger (1.2%) patients, nor was the rate of TIA (1.9% vs 1.8%, respectively). However, perioperative mortality was significantly higher in elderly (0.5%) than in younger (0.4%) patients.
In contrast, for carotid stenting, the rate of perioperative stroke was significantly higher for elderly patients (2.4%) than for younger patients (1.7%), as was the rate of TIA (3.6% vs 2.1%). And mortality was not significantly different between elderly patients (0.6%) and younger patients (0.7%), the researchers wrote (JAMA Surg. 2013 Oct. 23 [doi:10.1001/jamasurg.2013.4135]).
Both procedures were associated with an increased rate of perioperative MI in elderly patients, compared with younger patients. These rates were 2.2% in elderly patients, compared with 1.4% in younger patients undergoing endarterectomy; and 2.3% in elderly patients, compared with 1.5% in younger patients undergoing carotid stenting.
These findings remained robust in sensitivity analyses.
"It seems that endarterectomy is associated with improved neurologic outcomes compared with carotid stenting in elderly patients, at the expense of increased perioperative mortality." However, the small increase in mortality seen with endarterectomy – one-tenth of 1% – may not be clinically significant, Dr. Antoniou and his associates said.
Moreover, neurologic risk is closely tied to vascular anatomy. Elderly patients tend to have more unfavorable anatomy than do younger patients, but should be assessed on an individual basis. Unfavorable traits include heavily calcified and tortuous supra-aortic branches, as well as adverse morphology of the aortic arch such as elongation, distortion, and stenosis.
Manipulating the stenting instruments through such features may in itself raise the risk of neurologic sequelae. It also makes the procedure more technically difficult, which increases the risk of endothelial trauma, thrombus dislodgment, and thromboembolic events.
"In addition, elderly patients with significant extracranial atherosclerotic disease are likely to have a compromised cerebrovascular reserve, which makes them more susceptible to ischemic events from cerebral microembolization," the researchers said.
They reported having no conflicts.
Ibrutinib trial stopped early

Credit: Steven Harbour
The phase 3 RESONATE study has been stopped early due to positive results in patients receiving ibrutinib.
In this trial, researchers compared the BTK inhibitor ibrutinib to the CD20-directed antibody ofatumumab in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
The study has ended early because 2 key endpoints were met—namely, ibrutinib significantly improved progression-free and overall survival rates.
The RESONATE study enrolled 391 patients with relapsed or refractory CLL or SLL with measurable nodal disease who were not eligible for treatment with purine-analog-based therapy. Patients had received at least 1 prior therapy.
The researchers randomized patients to receive 420 mg of ibrutinib orally once daily or intravenous doses of ofatumumab over the course of 24 weeks until disease progression or unacceptable toxicity.
At the planned interim analysis, ibrutinib had significantly improved progression-free survival (the primary endpoint) and overall survival (a secondary endpoint).
And the safety profile of ibrutinib was acceptable, according to the companies developing the drug (Pharmacyclics, Inc. and Janssen Research and Development, LLC).
Based on these results, an independent data monitoring committee recommended that patients in the ofatumumab arm be given access to ibrutinib.
Pharmacyclics has informed the US Food and Drug Administration of this recommendation, and Janssen has informed the European Medicines Agency. Both companies are in talks with the health authorities to define the next regulatory steps.
Pharmacyclics has said detailed data from the RESONATE study will be presented at an upcoming oncology conference.
Ibrutinib was recently approved by the US Food and Drug Administration to treat mantle cell lymphoma. ![]()

Credit: Steven Harbour
The phase 3 RESONATE study has been stopped early due to positive results in patients receiving ibrutinib.
In this trial, researchers compared the BTK inhibitor ibrutinib to the CD20-directed antibody ofatumumab in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
The study has ended early because 2 key endpoints were met—namely, ibrutinib significantly improved progression-free and overall survival rates.
The RESONATE study enrolled 391 patients with relapsed or refractory CLL or SLL with measurable nodal disease who were not eligible for treatment with purine-analog-based therapy. Patients had received at least 1 prior therapy.
The researchers randomized patients to receive 420 mg of ibrutinib orally once daily or intravenous doses of ofatumumab over the course of 24 weeks until disease progression or unacceptable toxicity.
At the planned interim analysis, ibrutinib had significantly improved progression-free survival (the primary endpoint) and overall survival (a secondary endpoint).
And the safety profile of ibrutinib was acceptable, according to the companies developing the drug (Pharmacyclics, Inc. and Janssen Research and Development, LLC).
Based on these results, an independent data monitoring committee recommended that patients in the ofatumumab arm be given access to ibrutinib.
Pharmacyclics has informed the US Food and Drug Administration of this recommendation, and Janssen has informed the European Medicines Agency. Both companies are in talks with the health authorities to define the next regulatory steps.
Pharmacyclics has said detailed data from the RESONATE study will be presented at an upcoming oncology conference.
Ibrutinib was recently approved by the US Food and Drug Administration to treat mantle cell lymphoma. ![]()

Credit: Steven Harbour
The phase 3 RESONATE study has been stopped early due to positive results in patients receiving ibrutinib.
In this trial, researchers compared the BTK inhibitor ibrutinib to the CD20-directed antibody ofatumumab in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
The study has ended early because 2 key endpoints were met—namely, ibrutinib significantly improved progression-free and overall survival rates.
The RESONATE study enrolled 391 patients with relapsed or refractory CLL or SLL with measurable nodal disease who were not eligible for treatment with purine-analog-based therapy. Patients had received at least 1 prior therapy.
The researchers randomized patients to receive 420 mg of ibrutinib orally once daily or intravenous doses of ofatumumab over the course of 24 weeks until disease progression or unacceptable toxicity.
At the planned interim analysis, ibrutinib had significantly improved progression-free survival (the primary endpoint) and overall survival (a secondary endpoint).
And the safety profile of ibrutinib was acceptable, according to the companies developing the drug (Pharmacyclics, Inc. and Janssen Research and Development, LLC).
Based on these results, an independent data monitoring committee recommended that patients in the ofatumumab arm be given access to ibrutinib.
Pharmacyclics has informed the US Food and Drug Administration of this recommendation, and Janssen has informed the European Medicines Agency. Both companies are in talks with the health authorities to define the next regulatory steps.
Pharmacyclics has said detailed data from the RESONATE study will be presented at an upcoming oncology conference.
Ibrutinib was recently approved by the US Food and Drug Administration to treat mantle cell lymphoma. ![]()
Music therapy helps AYAs undergoing HSCT

Credit: Chad McNeeley
A music therapy intervention can help adolescents and young adults (AYAs) cope with cancer and its treatment, according to research published in the journal Cancer.
The intervention consisted of writing song lyrics and producing music videos.
It helped AYA cancer patients communicate their feelings about their disease and its treatment, hematopoietic stem cell transplant (HSCT).
The program also had positive effects on patients’ social integration and family environment.
About the intervention
The therapeutic music video (TMV) intervention was designed to improve resilience in AYA cancer patients undergoing HSCT. Resilience is the process of positively adjusting to stressors.
“Adolescents and young adults who are resilient have the ability to rise above their illness, gain a sense of mastery and confidence in how they have dealt with their cancer, and demonstrate a desire to reach out and help others,” said study author Joan Haase, PhD, RN, of the Indiana University School of Nursing.
Dr Haase and her colleagues wanted to use the TMV intervention to help AYAs explore and express thoughts and emotions about their disease and treatment that might otherwise go unspoken.
The patients did this by writing song lyrics and producing videos with the help of a board-certified music therapist. As they moved through phases of the intervention—making sound recordings, collecting video images, and storyboarding—patients had opportunities to involve family, friends, and healthcare providers in their project.
Results of the study
To test the intervention, Dr Haase and her colleagues enrolled 113 cancer patients (aged 11 to 24 years) who were undergoing HSCT.
The patients were randomized to the TMV intervention group or a control group that received audiobooks. All patients completed 6 sessions over 3 weeks.
After the intervention, the TMV group reported significantly better courageous coping. And at 100 days after HSCT, the TMV group reported significantly better social integration and family environments.
Parents reported that the videos gave them insight into their children’s cancer experiences. However, parents needed help to initiate and sustain conversations about messages shared through their children’s videos.
The investigators said these findings provide evidence supporting the use of a music-based intervention delivered by a music therapist to help AYAs cope with high-risk, high-intensity cancer treatments.
“The availability of music therapy services from a board-certified music therapist in the United States has become more widespread, and, through studies like this one, we hope to see increased availability and access to this important allied health service,” said study author Sheri L. Robb, PhD, also of the Indiana University School of Nursing.
“One of our team’s next steps is to disseminate findings, train professional music therapists on this intervention, and then conduct an implementation study to examine how the intervention may change as it moves into the standard care setting and whether, in the presence of these changes, patient benefits are maintained.” ![]()

Credit: Chad McNeeley
A music therapy intervention can help adolescents and young adults (AYAs) cope with cancer and its treatment, according to research published in the journal Cancer.
The intervention consisted of writing song lyrics and producing music videos.
It helped AYA cancer patients communicate their feelings about their disease and its treatment, hematopoietic stem cell transplant (HSCT).
The program also had positive effects on patients’ social integration and family environment.
About the intervention
The therapeutic music video (TMV) intervention was designed to improve resilience in AYA cancer patients undergoing HSCT. Resilience is the process of positively adjusting to stressors.
“Adolescents and young adults who are resilient have the ability to rise above their illness, gain a sense of mastery and confidence in how they have dealt with their cancer, and demonstrate a desire to reach out and help others,” said study author Joan Haase, PhD, RN, of the Indiana University School of Nursing.
Dr Haase and her colleagues wanted to use the TMV intervention to help AYAs explore and express thoughts and emotions about their disease and treatment that might otherwise go unspoken.
The patients did this by writing song lyrics and producing videos with the help of a board-certified music therapist. As they moved through phases of the intervention—making sound recordings, collecting video images, and storyboarding—patients had opportunities to involve family, friends, and healthcare providers in their project.
Results of the study
To test the intervention, Dr Haase and her colleagues enrolled 113 cancer patients (aged 11 to 24 years) who were undergoing HSCT.
The patients were randomized to the TMV intervention group or a control group that received audiobooks. All patients completed 6 sessions over 3 weeks.
After the intervention, the TMV group reported significantly better courageous coping. And at 100 days after HSCT, the TMV group reported significantly better social integration and family environments.
Parents reported that the videos gave them insight into their children’s cancer experiences. However, parents needed help to initiate and sustain conversations about messages shared through their children’s videos.
The investigators said these findings provide evidence supporting the use of a music-based intervention delivered by a music therapist to help AYAs cope with high-risk, high-intensity cancer treatments.
“The availability of music therapy services from a board-certified music therapist in the United States has become more widespread, and, through studies like this one, we hope to see increased availability and access to this important allied health service,” said study author Sheri L. Robb, PhD, also of the Indiana University School of Nursing.
“One of our team’s next steps is to disseminate findings, train professional music therapists on this intervention, and then conduct an implementation study to examine how the intervention may change as it moves into the standard care setting and whether, in the presence of these changes, patient benefits are maintained.” ![]()

Credit: Chad McNeeley
A music therapy intervention can help adolescents and young adults (AYAs) cope with cancer and its treatment, according to research published in the journal Cancer.
The intervention consisted of writing song lyrics and producing music videos.
It helped AYA cancer patients communicate their feelings about their disease and its treatment, hematopoietic stem cell transplant (HSCT).
The program also had positive effects on patients’ social integration and family environment.
About the intervention
The therapeutic music video (TMV) intervention was designed to improve resilience in AYA cancer patients undergoing HSCT. Resilience is the process of positively adjusting to stressors.
“Adolescents and young adults who are resilient have the ability to rise above their illness, gain a sense of mastery and confidence in how they have dealt with their cancer, and demonstrate a desire to reach out and help others,” said study author Joan Haase, PhD, RN, of the Indiana University School of Nursing.
Dr Haase and her colleagues wanted to use the TMV intervention to help AYAs explore and express thoughts and emotions about their disease and treatment that might otherwise go unspoken.
The patients did this by writing song lyrics and producing videos with the help of a board-certified music therapist. As they moved through phases of the intervention—making sound recordings, collecting video images, and storyboarding—patients had opportunities to involve family, friends, and healthcare providers in their project.
Results of the study
To test the intervention, Dr Haase and her colleagues enrolled 113 cancer patients (aged 11 to 24 years) who were undergoing HSCT.
The patients were randomized to the TMV intervention group or a control group that received audiobooks. All patients completed 6 sessions over 3 weeks.
After the intervention, the TMV group reported significantly better courageous coping. And at 100 days after HSCT, the TMV group reported significantly better social integration and family environments.
Parents reported that the videos gave them insight into their children’s cancer experiences. However, parents needed help to initiate and sustain conversations about messages shared through their children’s videos.
The investigators said these findings provide evidence supporting the use of a music-based intervention delivered by a music therapist to help AYAs cope with high-risk, high-intensity cancer treatments.
“The availability of music therapy services from a board-certified music therapist in the United States has become more widespread, and, through studies like this one, we hope to see increased availability and access to this important allied health service,” said study author Sheri L. Robb, PhD, also of the Indiana University School of Nursing.
“One of our team’s next steps is to disseminate findings, train professional music therapists on this intervention, and then conduct an implementation study to examine how the intervention may change as it moves into the standard care setting and whether, in the presence of these changes, patient benefits are maintained.” ![]()
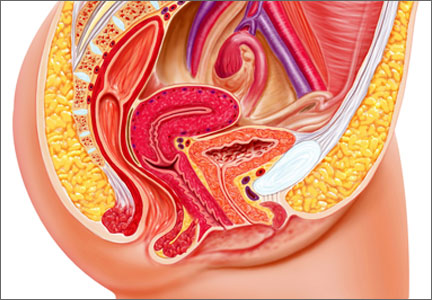
Does vaginal prolapse repair using synthetic mesh confer long-term benefit over native-tissue colpopexy?
This is the third report from Gutman and colleagues on the outcomes of a double-blind, multicenter, randomized, controlled trial of vaginal prolapse repair using synthetic mesh versus native-tissue colpopexy in women with significant vaginal prolapse.
The trial involved 33 women who underwent mesh repair and 32 who underwent repair without mesh. (The mesh-free repair consisted primarily of uterosacral suspension and concurrent colporrhaphy.) It was halted when it reached a predetermined threshold for discontinuation, which was a mesh erosion rate of 15% or more.
Investigators found no difference in long-term cure rates between the mesh and no-mesh groups, regardless of the definition of cure (ie, anatomic, symptomatic, or combined). Nor was there a difference in the overall recurrence rate.
Summary of earlier reports
Three-month outcomes. The first report from this trial described 3-month objective treatment outcomes, with success described as prolapse no greater than stage 1.1 It found a high erosion rate (15.6%) for vaginal mesh, with no differences between groups in overall subjective or objective cure rates, with an overall recurrence rate of 59.4% (19 cases) in the mesh group versus 70.4% (24 cases) in the no-mesh group (P = .28), with recurrence defined as prolapse beyond stage 1 in any compartment. Investigators also observed potential benefit in the mesh group in the anterior vaginal wall at point Ba at a median of 9.7 months after surgery.
Related Article: Stop using synthetic mesh for routine repair of pelvic organ prolapse Cheryl B. Iglesia, MD (Stop/Start, April 2013)
One-year outcomes. The second report described 1-year objective and functional outcomes in all participants of the trial.2 It found comparable objective and subjective cure rates between groups but a higher reoperation rate for mesh repairs. Prolapse recurred in the anterior department in 46.9% of women in the mesh group versus 60.6% in the no-mesh group (P = .40).
Subjective quality-of-life assessments continued to reflect significant improvement in symptoms from baseline. Vaginal bulging was relieved in 96.2% of women in the mesh group, compared with 90.9% in the no-mesh group (P = .62).
More women in the mesh group required reoperation for recurrent prolapse or mesh exposure (5 in the mesh group vs 0 in the no-mesh group; P = .017).
Strengths and limitations of the trial
Gutman and colleagues are to be congratulated for continuing to monitor longer-term outcomes of vaginal prolapse repairs augmented with synthetic mesh, as data are sorely needed on both early complications and those more remote from surgery. However, it is regrettable that continued attrition in this trial led to minimal power to compare outcomes between groups.
Cure rates were assessed three ways: anatomically, by virtue of symptoms, and by a combination of the two measures. Participants had documentation of at least 2-year anatomic outcomes and 3-year subjective outcomes using validated measures.
Forty-one (63%) of the original 65 women in the trial had anatomic outcomes (20 in the mesh group vs 21 in the no-mesh group), and 51 (78%) of the original 65 women had evaluable subjective outcomes (25 in the mesh group vs 26 in the no-mesh group).
Women who underwent reoperation for recurrent prolapse were removed from any outcomes analysis and considered to have failed composite outcomes measures (anatomic and subjective assessment and whether reoperation or a pessary was required for recurrent prolapse).
The length of follow-up was similar between groups (median, 3 years; interquartile range, 2.97–3.15), and both groups demonstrated significant anatomic and subjective improvement from baseline.
No difference was observed between groups in the original primary anatomic outcome, which was a POP-Q stage no greater than 1 (45% in the mesh group vs 43% in the no-mesh group; P >.99). Nor was there a difference between groups in any other anatomic outcome, including POP-Q point Ba (median, –1.5 for mesh [range, –2.5, 1.0] vs –0.5 [range, –3.0, 4.0] for the no-mesh group; P = .21) and bulge symptoms (92% for the mesh group vs 81% for the no-mesh group; relative risk, 1.4; 95% confidence interval, 0.91–1.42).
Despite small numbers and markedly reduced comparative validity (readily acknowledged by the investigators), these longer-term outcomes were assessed by examiners blinded to treatment and using validated objective and subjective outcome measures.
The only other randomized trial of mesh versus native-tissue repair with 3-year outcomes had a much larger sample size and follow-up but addressed only anterior-compartment prolapse.3
What this evidence means for practice
The 3-year data presented by Gutman and colleagues should be viewed with caution, owing to the trial’s reduced sample size and power. However, they may be useful in designing future trials.
In the meantime, given the limited longer-term outcomes data available at present, I would recommend continued individualized use of mesh versus native-tissue repair in women presenting with prolapse, including educating patients about the risks and benefits of both approaches. It also is important that outcomes be followed in all of our patients in a robust, unbiased fashion. The new American Urogynecologic Society Pelvic Floor Disorders Registry provides the opportunity for this.
Holly E. Richter, PhD, MD
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
- Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: A randomized controlled trial. Obstet Gynecol. 2010;116(2 Pt 1):293–303.
- Sokol AI, Iglesia CB, Kudish BI, et al. One-year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. Am J Obstet Gynecol. 2012;206(1):86.e1–e9.
- Nieminen K, Hiltunen R, Takala T, et al. Outcomes after anterior vaginal wall repair with mesh: A randomized, controlled trial with a 3-year follow-up. Am J Obstet Gynecol. 2010;203(3):235.e1–e8.
This is the third report from Gutman and colleagues on the outcomes of a double-blind, multicenter, randomized, controlled trial of vaginal prolapse repair using synthetic mesh versus native-tissue colpopexy in women with significant vaginal prolapse.
The trial involved 33 women who underwent mesh repair and 32 who underwent repair without mesh. (The mesh-free repair consisted primarily of uterosacral suspension and concurrent colporrhaphy.) It was halted when it reached a predetermined threshold for discontinuation, which was a mesh erosion rate of 15% or more.
Investigators found no difference in long-term cure rates between the mesh and no-mesh groups, regardless of the definition of cure (ie, anatomic, symptomatic, or combined). Nor was there a difference in the overall recurrence rate.
Summary of earlier reports
Three-month outcomes. The first report from this trial described 3-month objective treatment outcomes, with success described as prolapse no greater than stage 1.1 It found a high erosion rate (15.6%) for vaginal mesh, with no differences between groups in overall subjective or objective cure rates, with an overall recurrence rate of 59.4% (19 cases) in the mesh group versus 70.4% (24 cases) in the no-mesh group (P = .28), with recurrence defined as prolapse beyond stage 1 in any compartment. Investigators also observed potential benefit in the mesh group in the anterior vaginal wall at point Ba at a median of 9.7 months after surgery.
Related Article: Stop using synthetic mesh for routine repair of pelvic organ prolapse Cheryl B. Iglesia, MD (Stop/Start, April 2013)
One-year outcomes. The second report described 1-year objective and functional outcomes in all participants of the trial.2 It found comparable objective and subjective cure rates between groups but a higher reoperation rate for mesh repairs. Prolapse recurred in the anterior department in 46.9% of women in the mesh group versus 60.6% in the no-mesh group (P = .40).
Subjective quality-of-life assessments continued to reflect significant improvement in symptoms from baseline. Vaginal bulging was relieved in 96.2% of women in the mesh group, compared with 90.9% in the no-mesh group (P = .62).
More women in the mesh group required reoperation for recurrent prolapse or mesh exposure (5 in the mesh group vs 0 in the no-mesh group; P = .017).
Strengths and limitations of the trial
Gutman and colleagues are to be congratulated for continuing to monitor longer-term outcomes of vaginal prolapse repairs augmented with synthetic mesh, as data are sorely needed on both early complications and those more remote from surgery. However, it is regrettable that continued attrition in this trial led to minimal power to compare outcomes between groups.
Cure rates were assessed three ways: anatomically, by virtue of symptoms, and by a combination of the two measures. Participants had documentation of at least 2-year anatomic outcomes and 3-year subjective outcomes using validated measures.
Forty-one (63%) of the original 65 women in the trial had anatomic outcomes (20 in the mesh group vs 21 in the no-mesh group), and 51 (78%) of the original 65 women had evaluable subjective outcomes (25 in the mesh group vs 26 in the no-mesh group).
Women who underwent reoperation for recurrent prolapse were removed from any outcomes analysis and considered to have failed composite outcomes measures (anatomic and subjective assessment and whether reoperation or a pessary was required for recurrent prolapse).
The length of follow-up was similar between groups (median, 3 years; interquartile range, 2.97–3.15), and both groups demonstrated significant anatomic and subjective improvement from baseline.
No difference was observed between groups in the original primary anatomic outcome, which was a POP-Q stage no greater than 1 (45% in the mesh group vs 43% in the no-mesh group; P >.99). Nor was there a difference between groups in any other anatomic outcome, including POP-Q point Ba (median, –1.5 for mesh [range, –2.5, 1.0] vs –0.5 [range, –3.0, 4.0] for the no-mesh group; P = .21) and bulge symptoms (92% for the mesh group vs 81% for the no-mesh group; relative risk, 1.4; 95% confidence interval, 0.91–1.42).
Despite small numbers and markedly reduced comparative validity (readily acknowledged by the investigators), these longer-term outcomes were assessed by examiners blinded to treatment and using validated objective and subjective outcome measures.
The only other randomized trial of mesh versus native-tissue repair with 3-year outcomes had a much larger sample size and follow-up but addressed only anterior-compartment prolapse.3
What this evidence means for practice
The 3-year data presented by Gutman and colleagues should be viewed with caution, owing to the trial’s reduced sample size and power. However, they may be useful in designing future trials.
In the meantime, given the limited longer-term outcomes data available at present, I would recommend continued individualized use of mesh versus native-tissue repair in women presenting with prolapse, including educating patients about the risks and benefits of both approaches. It also is important that outcomes be followed in all of our patients in a robust, unbiased fashion. The new American Urogynecologic Society Pelvic Floor Disorders Registry provides the opportunity for this.
Holly E. Richter, PhD, MD
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
This is the third report from Gutman and colleagues on the outcomes of a double-blind, multicenter, randomized, controlled trial of vaginal prolapse repair using synthetic mesh versus native-tissue colpopexy in women with significant vaginal prolapse.
The trial involved 33 women who underwent mesh repair and 32 who underwent repair without mesh. (The mesh-free repair consisted primarily of uterosacral suspension and concurrent colporrhaphy.) It was halted when it reached a predetermined threshold for discontinuation, which was a mesh erosion rate of 15% or more.
Investigators found no difference in long-term cure rates between the mesh and no-mesh groups, regardless of the definition of cure (ie, anatomic, symptomatic, or combined). Nor was there a difference in the overall recurrence rate.
Summary of earlier reports
Three-month outcomes. The first report from this trial described 3-month objective treatment outcomes, with success described as prolapse no greater than stage 1.1 It found a high erosion rate (15.6%) for vaginal mesh, with no differences between groups in overall subjective or objective cure rates, with an overall recurrence rate of 59.4% (19 cases) in the mesh group versus 70.4% (24 cases) in the no-mesh group (P = .28), with recurrence defined as prolapse beyond stage 1 in any compartment. Investigators also observed potential benefit in the mesh group in the anterior vaginal wall at point Ba at a median of 9.7 months after surgery.
Related Article: Stop using synthetic mesh for routine repair of pelvic organ prolapse Cheryl B. Iglesia, MD (Stop/Start, April 2013)
One-year outcomes. The second report described 1-year objective and functional outcomes in all participants of the trial.2 It found comparable objective and subjective cure rates between groups but a higher reoperation rate for mesh repairs. Prolapse recurred in the anterior department in 46.9% of women in the mesh group versus 60.6% in the no-mesh group (P = .40).
Subjective quality-of-life assessments continued to reflect significant improvement in symptoms from baseline. Vaginal bulging was relieved in 96.2% of women in the mesh group, compared with 90.9% in the no-mesh group (P = .62).
More women in the mesh group required reoperation for recurrent prolapse or mesh exposure (5 in the mesh group vs 0 in the no-mesh group; P = .017).
Strengths and limitations of the trial
Gutman and colleagues are to be congratulated for continuing to monitor longer-term outcomes of vaginal prolapse repairs augmented with synthetic mesh, as data are sorely needed on both early complications and those more remote from surgery. However, it is regrettable that continued attrition in this trial led to minimal power to compare outcomes between groups.
Cure rates were assessed three ways: anatomically, by virtue of symptoms, and by a combination of the two measures. Participants had documentation of at least 2-year anatomic outcomes and 3-year subjective outcomes using validated measures.
Forty-one (63%) of the original 65 women in the trial had anatomic outcomes (20 in the mesh group vs 21 in the no-mesh group), and 51 (78%) of the original 65 women had evaluable subjective outcomes (25 in the mesh group vs 26 in the no-mesh group).
Women who underwent reoperation for recurrent prolapse were removed from any outcomes analysis and considered to have failed composite outcomes measures (anatomic and subjective assessment and whether reoperation or a pessary was required for recurrent prolapse).
The length of follow-up was similar between groups (median, 3 years; interquartile range, 2.97–3.15), and both groups demonstrated significant anatomic and subjective improvement from baseline.
No difference was observed between groups in the original primary anatomic outcome, which was a POP-Q stage no greater than 1 (45% in the mesh group vs 43% in the no-mesh group; P >.99). Nor was there a difference between groups in any other anatomic outcome, including POP-Q point Ba (median, –1.5 for mesh [range, –2.5, 1.0] vs –0.5 [range, –3.0, 4.0] for the no-mesh group; P = .21) and bulge symptoms (92% for the mesh group vs 81% for the no-mesh group; relative risk, 1.4; 95% confidence interval, 0.91–1.42).
Despite small numbers and markedly reduced comparative validity (readily acknowledged by the investigators), these longer-term outcomes were assessed by examiners blinded to treatment and using validated objective and subjective outcome measures.
The only other randomized trial of mesh versus native-tissue repair with 3-year outcomes had a much larger sample size and follow-up but addressed only anterior-compartment prolapse.3
What this evidence means for practice
The 3-year data presented by Gutman and colleagues should be viewed with caution, owing to the trial’s reduced sample size and power. However, they may be useful in designing future trials.
In the meantime, given the limited longer-term outcomes data available at present, I would recommend continued individualized use of mesh versus native-tissue repair in women presenting with prolapse, including educating patients about the risks and benefits of both approaches. It also is important that outcomes be followed in all of our patients in a robust, unbiased fashion. The new American Urogynecologic Society Pelvic Floor Disorders Registry provides the opportunity for this.
Holly E. Richter, PhD, MD
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
- Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: A randomized controlled trial. Obstet Gynecol. 2010;116(2 Pt 1):293–303.
- Sokol AI, Iglesia CB, Kudish BI, et al. One-year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. Am J Obstet Gynecol. 2012;206(1):86.e1–e9.
- Nieminen K, Hiltunen R, Takala T, et al. Outcomes after anterior vaginal wall repair with mesh: A randomized, controlled trial with a 3-year follow-up. Am J Obstet Gynecol. 2010;203(3):235.e1–e8.
- Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: A randomized controlled trial. Obstet Gynecol. 2010;116(2 Pt 1):293–303.
- Sokol AI, Iglesia CB, Kudish BI, et al. One-year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. Am J Obstet Gynecol. 2012;206(1):86.e1–e9.
- Nieminen K, Hiltunen R, Takala T, et al. Outcomes after anterior vaginal wall repair with mesh: A randomized, controlled trial with a 3-year follow-up. Am J Obstet Gynecol. 2010;203(3):235.e1–e8.
Circumcision accident: $1.3M verdict
CIRCUMCISION ACCIDENT: $1.3M VERDICT
A newborn underwent circumcision when 12 hours old. The ObGyn removed adhesions present between the foreskin and glans. After locking the Mogen clamp, the ObGyn amputated a 9-mm by 8-mm portion of the top of the penis along with the foreskin. The newborn was rushed to a children’s hospital where a pediatric urologist surgically reattached the amputated glans. The child’s penis is not cosmetically normal, with permanent scars and disfigurement. He has altered nerve sensation at and above the area of the amputation.
PARENTS’ CLAIM The ObGyn improperly performed the circumcision. He failed to remove a sufficient amount of adhesions, pulled too much into the clamp, and amputated 30% of the distal portion of the glans.
PHYSICIAN’S DEFENSE The ObGyn circumcised this child the same way he had performed more than 1,000 circumcisions. Multiple dense adhesions between the glans and foreskin caused the top of the penis to be inadvertently pulled through the clamp. Amputation is a known risk of the procedure.
VERDICT A $1,357,901 Illinois verdict was returned.
WHAT CAUSED CHILD’S KIDNEY DISEASE?
At 36 weeks’ gestation, a mother came to the emergency department (ED) with abdominal pain. She had proteinuria, elevated liver enzymes, and a low-normal platelet count. An ObGyn determined that the fetus was normal, and discharged her.
The patient returned 2 days later with internal bleeding and placental abruption. She was diagnosed with hemolysis, elevated liver enzymes, and low platelet count (HELLP syndrome). The child, born by cesarean delivery, had kidney failure that caused growth retardation. The child has received a kidney transplant.
PARENTS’ CLAIM The mother should not have been discharged from the hospital with abnormal findings.
DEFENDANTS’ DEFENSE The case was settled during trial.
VERDICT A $1 million New Jersey settlement was reached, of which $100,000 was provided to the mother.
Related Article: A stepwise approach to managing eclampsia and other hypertensive emergencies Baha M. Sibai, MD (October 2013)
EXCESSIVE FORCE BLAMED FOR ERB’S PALSY
Shoulder dystocia was encountered during delivery. The child suffered a brachial plexus injury with Erb’s palsy. She received botulinum toxin injections and underwent nerve-graft surgery to restore some function. She has limited use of her right arm and a protruding right elbow.
PARENTS’ CLAIM The ObGyn used excessive force in response to shoulder dystocia.
PHYSICIAN’S DEFENSE The case was settled at trial.
VERDICT A $1 million New Jersey settlement was placed in a structured payment fund to provide a net $1.78 million over the child’s lifetime.
Related Article: You are the second responder to a shoulder dystocia emergency. What do you do first? Robert L. Barbieri, MD (Editorial, May 2013)
WAS WOMAN UNLAWFULLY SEEKING DRUGS?
A 30-year-old woman went to an ED with pelvic pain and vaginal discharge. An ED physician conducted a physical exam. Blood tests indicated the patient had taken barbiturates, but the patient could not explain the findings. Determining no cause for her symptoms, the ED physician discharged the patient.
Just after she left, the ED physician found that the patient had several narcotics prescriptions and called the police. The patient was arrested and charged with unlawfully seeking drugs at the hospital. Criminal charges were later dismissed.
PATIENT’S CLAIM The ED physician did not properly examine her; she was found to have endometriosis and underwent surgery a few weeks later. The ED physician was negligent for divulging her personal information to police.
The ED physician had had his physician’s license suspended due to substance abuse and had also been arrested for driving under the influence after his license was restored.
PHYSICIAN’S DEFENSE The ED physician’s examination and treatment were proper. The phone call to police was not part of treatment. The patient had a malicious prosecution basis for any claims.
VERDICT A $125,000 Kentucky verdict was returned.
CASCADING PROBLEMS: MOTHER AND BABY DIE
A pregnant woman was admitted to an ED, where the on-call physician determined that she had pneumonia. The patient’s ObGyn, 45 miles away, refused to come to the hospital or arrange for another ObGyn to take the case.
Several hours later, after the mother was found to have fulminant preeclampsia, the ObGyn demanded the patient be moved to the hospital’s internal medicine (IM) service. However, the IM service refused to admit the patient because she needed obstetric care. The ObGyn tried to transfer the patient to a maternal-fetal medicine (MFM) specialist at a tertiary care center; transfer was refused because the patient was too unstable and needed an emergency cesarean delivery. The ObGyn continued to refuse to relinquish care to another ObGyn.
The ED physician decided to transfer the patient to another hospital 50 miles away even though she was now in active labor. An MFM specialist accepted transfer. After 5 hours in the ED, the mother left by ambulance, but, during transport, she suffered placental abruption and internal hemorrhaging. She was in critical condition upon arrival. An emergency cesarean delivery was performed, but the mother died. The baby, born with severe brain damage, also died.
ESTATES’ CLAIM The ED physician failed to properly and timely determine that the mother had preeclampsia; no treatment for hypertension was provided. The ED physician withheld critical information, including the patient’s severe hypertension, proteinurea, and edema, when speaking to the MFM specialist who accepted transfer. The ED physician did not evaluate the mother before departure and certified the transfer although the patient was highly unstable.
The ObGyn was negligent in not transferring care to another ObGyn and not coming to the hospital. The ObGyn did not inform the ED physician of the rejected attempt to transfer the patient or of the first MFM specialist’s recommendation for emergency cesarean. Both mother and baby could have survived with proper treatment.
DEFENDANTS’ DEFENSE The case was settled at trial.
VERDICT A $900,000 Michigan settlement was reached.
FECAL INCONTINENCE AFTER EPISIOTOMY
A 26-year-old woman gave birth after her ObGyn created an episiotomy to facilitate delivery. The incision was repaired and the ObGyn prescribed docusate (Colace) to soften her stools.
A month later, the patient report-ed fecal incontinence. The ObGyn determined that the incontinence was related to the episiotomy, but did not feel that immediate attention was needed. When the condition did not improve, the patient saw a colorectal surgeon, who diagnosed a significant sphincter defect. The patient underwent a sphincteroplasty, with minor improvement.
PATIENT’S CLAIM The ObGyn failed to properly manage episiotomy healing. The patient remembers being told to stop docusate after she had passed one stool after delivery. A 10-day regimen of docusate and a diet to reduce defecation frequency should have been prescribed. Incontinence should have prompted an immediate referral to a colorectal surgeon.
PHYSICIAN’S DEFENSE Prompt surgical intervention was not necessary. Sphincteroplasty can be delayed until conservative methods have been tried. Episiotomy healing was properly addressed. Permanent incontinence is a known risk of the procedure.
VERDICT A $6 million New York verdict was returned.
Related Article: Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery? Errol R. Norwitz, MD, PhD (Examining the Evidence, August 2012)
MECONIUM ASPIRATION SYNDROME
A baby stayed in hospital for 3 weeks postdelivery due to meconium aspiration syndrome.
PARENTS’ CLAIM The resident who followed the mother during her pregnancy was negligent in allowing the pregnancy to progress to 46 weeks’ gestation before delivery.
DEFENDANTS’ DEFENSE The estimated date of conception was disputed. The resident claimed that the baby was born at 42 weeks’ gestation. An attending physician reviewed all prenatal visits with the resident. The mother’s cervix was never ripe before induction of labor. Aspiration occurred despite aggressive suctioning. The child has had no further respiratory issues since her neonatal discharge.
VERDICT An Illinois defense verdict was returned.
BOWEL INJURY AFTER HYSTERECTOMY
A woman underwent laparoscopic-assisted vaginal hysterectomy and was discharged the following day. Two days later, she went to an ED in acute distress. A bowel perforation was found during emergency surgery, and her colon was repaired. She made a full recovery.
PATIENT’S CLAIM The ObGyn was negligent in failing to properly evaluate the patient after surgery. The ObGyn also failed to explain the signs of a possible perforation to the patient before she left the hospital.
PHYSICIAN’S DEFENSE The patient’s postoperative course was normal while she was hospitalized. Bowel perforation is a known complication of the procedure. The patient had been informed of all the signs and symptoms of a bowel perforation and had been instructed to call the ObGyn or return to the hospital if she began to have any symptoms.
VERDICT A South Carolina defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
CIRCUMCISION ACCIDENT: $1.3M VERDICT
A newborn underwent circumcision when 12 hours old. The ObGyn removed adhesions present between the foreskin and glans. After locking the Mogen clamp, the ObGyn amputated a 9-mm by 8-mm portion of the top of the penis along with the foreskin. The newborn was rushed to a children’s hospital where a pediatric urologist surgically reattached the amputated glans. The child’s penis is not cosmetically normal, with permanent scars and disfigurement. He has altered nerve sensation at and above the area of the amputation.
PARENTS’ CLAIM The ObGyn improperly performed the circumcision. He failed to remove a sufficient amount of adhesions, pulled too much into the clamp, and amputated 30% of the distal portion of the glans.
PHYSICIAN’S DEFENSE The ObGyn circumcised this child the same way he had performed more than 1,000 circumcisions. Multiple dense adhesions between the glans and foreskin caused the top of the penis to be inadvertently pulled through the clamp. Amputation is a known risk of the procedure.
VERDICT A $1,357,901 Illinois verdict was returned.
WHAT CAUSED CHILD’S KIDNEY DISEASE?
At 36 weeks’ gestation, a mother came to the emergency department (ED) with abdominal pain. She had proteinuria, elevated liver enzymes, and a low-normal platelet count. An ObGyn determined that the fetus was normal, and discharged her.
The patient returned 2 days later with internal bleeding and placental abruption. She was diagnosed with hemolysis, elevated liver enzymes, and low platelet count (HELLP syndrome). The child, born by cesarean delivery, had kidney failure that caused growth retardation. The child has received a kidney transplant.
PARENTS’ CLAIM The mother should not have been discharged from the hospital with abnormal findings.
DEFENDANTS’ DEFENSE The case was settled during trial.
VERDICT A $1 million New Jersey settlement was reached, of which $100,000 was provided to the mother.
Related Article: A stepwise approach to managing eclampsia and other hypertensive emergencies Baha M. Sibai, MD (October 2013)
EXCESSIVE FORCE BLAMED FOR ERB’S PALSY
Shoulder dystocia was encountered during delivery. The child suffered a brachial plexus injury with Erb’s palsy. She received botulinum toxin injections and underwent nerve-graft surgery to restore some function. She has limited use of her right arm and a protruding right elbow.
PARENTS’ CLAIM The ObGyn used excessive force in response to shoulder dystocia.
PHYSICIAN’S DEFENSE The case was settled at trial.
VERDICT A $1 million New Jersey settlement was placed in a structured payment fund to provide a net $1.78 million over the child’s lifetime.
Related Article: You are the second responder to a shoulder dystocia emergency. What do you do first? Robert L. Barbieri, MD (Editorial, May 2013)
WAS WOMAN UNLAWFULLY SEEKING DRUGS?
A 30-year-old woman went to an ED with pelvic pain and vaginal discharge. An ED physician conducted a physical exam. Blood tests indicated the patient had taken barbiturates, but the patient could not explain the findings. Determining no cause for her symptoms, the ED physician discharged the patient.
Just after she left, the ED physician found that the patient had several narcotics prescriptions and called the police. The patient was arrested and charged with unlawfully seeking drugs at the hospital. Criminal charges were later dismissed.
PATIENT’S CLAIM The ED physician did not properly examine her; she was found to have endometriosis and underwent surgery a few weeks later. The ED physician was negligent for divulging her personal information to police.
The ED physician had had his physician’s license suspended due to substance abuse and had also been arrested for driving under the influence after his license was restored.
PHYSICIAN’S DEFENSE The ED physician’s examination and treatment were proper. The phone call to police was not part of treatment. The patient had a malicious prosecution basis for any claims.
VERDICT A $125,000 Kentucky verdict was returned.
CASCADING PROBLEMS: MOTHER AND BABY DIE
A pregnant woman was admitted to an ED, where the on-call physician determined that she had pneumonia. The patient’s ObGyn, 45 miles away, refused to come to the hospital or arrange for another ObGyn to take the case.
Several hours later, after the mother was found to have fulminant preeclampsia, the ObGyn demanded the patient be moved to the hospital’s internal medicine (IM) service. However, the IM service refused to admit the patient because she needed obstetric care. The ObGyn tried to transfer the patient to a maternal-fetal medicine (MFM) specialist at a tertiary care center; transfer was refused because the patient was too unstable and needed an emergency cesarean delivery. The ObGyn continued to refuse to relinquish care to another ObGyn.
The ED physician decided to transfer the patient to another hospital 50 miles away even though she was now in active labor. An MFM specialist accepted transfer. After 5 hours in the ED, the mother left by ambulance, but, during transport, she suffered placental abruption and internal hemorrhaging. She was in critical condition upon arrival. An emergency cesarean delivery was performed, but the mother died. The baby, born with severe brain damage, also died.
ESTATES’ CLAIM The ED physician failed to properly and timely determine that the mother had preeclampsia; no treatment for hypertension was provided. The ED physician withheld critical information, including the patient’s severe hypertension, proteinurea, and edema, when speaking to the MFM specialist who accepted transfer. The ED physician did not evaluate the mother before departure and certified the transfer although the patient was highly unstable.
The ObGyn was negligent in not transferring care to another ObGyn and not coming to the hospital. The ObGyn did not inform the ED physician of the rejected attempt to transfer the patient or of the first MFM specialist’s recommendation for emergency cesarean. Both mother and baby could have survived with proper treatment.
DEFENDANTS’ DEFENSE The case was settled at trial.
VERDICT A $900,000 Michigan settlement was reached.
FECAL INCONTINENCE AFTER EPISIOTOMY
A 26-year-old woman gave birth after her ObGyn created an episiotomy to facilitate delivery. The incision was repaired and the ObGyn prescribed docusate (Colace) to soften her stools.
A month later, the patient report-ed fecal incontinence. The ObGyn determined that the incontinence was related to the episiotomy, but did not feel that immediate attention was needed. When the condition did not improve, the patient saw a colorectal surgeon, who diagnosed a significant sphincter defect. The patient underwent a sphincteroplasty, with minor improvement.
PATIENT’S CLAIM The ObGyn failed to properly manage episiotomy healing. The patient remembers being told to stop docusate after she had passed one stool after delivery. A 10-day regimen of docusate and a diet to reduce defecation frequency should have been prescribed. Incontinence should have prompted an immediate referral to a colorectal surgeon.
PHYSICIAN’S DEFENSE Prompt surgical intervention was not necessary. Sphincteroplasty can be delayed until conservative methods have been tried. Episiotomy healing was properly addressed. Permanent incontinence is a known risk of the procedure.
VERDICT A $6 million New York verdict was returned.
Related Article: Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery? Errol R. Norwitz, MD, PhD (Examining the Evidence, August 2012)
MECONIUM ASPIRATION SYNDROME
A baby stayed in hospital for 3 weeks postdelivery due to meconium aspiration syndrome.
PARENTS’ CLAIM The resident who followed the mother during her pregnancy was negligent in allowing the pregnancy to progress to 46 weeks’ gestation before delivery.
DEFENDANTS’ DEFENSE The estimated date of conception was disputed. The resident claimed that the baby was born at 42 weeks’ gestation. An attending physician reviewed all prenatal visits with the resident. The mother’s cervix was never ripe before induction of labor. Aspiration occurred despite aggressive suctioning. The child has had no further respiratory issues since her neonatal discharge.
VERDICT An Illinois defense verdict was returned.
BOWEL INJURY AFTER HYSTERECTOMY
A woman underwent laparoscopic-assisted vaginal hysterectomy and was discharged the following day. Two days later, she went to an ED in acute distress. A bowel perforation was found during emergency surgery, and her colon was repaired. She made a full recovery.
PATIENT’S CLAIM The ObGyn was negligent in failing to properly evaluate the patient after surgery. The ObGyn also failed to explain the signs of a possible perforation to the patient before she left the hospital.
PHYSICIAN’S DEFENSE The patient’s postoperative course was normal while she was hospitalized. Bowel perforation is a known complication of the procedure. The patient had been informed of all the signs and symptoms of a bowel perforation and had been instructed to call the ObGyn or return to the hospital if she began to have any symptoms.
VERDICT A South Carolina defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
CIRCUMCISION ACCIDENT: $1.3M VERDICT
A newborn underwent circumcision when 12 hours old. The ObGyn removed adhesions present between the foreskin and glans. After locking the Mogen clamp, the ObGyn amputated a 9-mm by 8-mm portion of the top of the penis along with the foreskin. The newborn was rushed to a children’s hospital where a pediatric urologist surgically reattached the amputated glans. The child’s penis is not cosmetically normal, with permanent scars and disfigurement. He has altered nerve sensation at and above the area of the amputation.
PARENTS’ CLAIM The ObGyn improperly performed the circumcision. He failed to remove a sufficient amount of adhesions, pulled too much into the clamp, and amputated 30% of the distal portion of the glans.
PHYSICIAN’S DEFENSE The ObGyn circumcised this child the same way he had performed more than 1,000 circumcisions. Multiple dense adhesions between the glans and foreskin caused the top of the penis to be inadvertently pulled through the clamp. Amputation is a known risk of the procedure.
VERDICT A $1,357,901 Illinois verdict was returned.
WHAT CAUSED CHILD’S KIDNEY DISEASE?
At 36 weeks’ gestation, a mother came to the emergency department (ED) with abdominal pain. She had proteinuria, elevated liver enzymes, and a low-normal platelet count. An ObGyn determined that the fetus was normal, and discharged her.
The patient returned 2 days later with internal bleeding and placental abruption. She was diagnosed with hemolysis, elevated liver enzymes, and low platelet count (HELLP syndrome). The child, born by cesarean delivery, had kidney failure that caused growth retardation. The child has received a kidney transplant.
PARENTS’ CLAIM The mother should not have been discharged from the hospital with abnormal findings.
DEFENDANTS’ DEFENSE The case was settled during trial.
VERDICT A $1 million New Jersey settlement was reached, of which $100,000 was provided to the mother.
Related Article: A stepwise approach to managing eclampsia and other hypertensive emergencies Baha M. Sibai, MD (October 2013)
EXCESSIVE FORCE BLAMED FOR ERB’S PALSY
Shoulder dystocia was encountered during delivery. The child suffered a brachial plexus injury with Erb’s palsy. She received botulinum toxin injections and underwent nerve-graft surgery to restore some function. She has limited use of her right arm and a protruding right elbow.
PARENTS’ CLAIM The ObGyn used excessive force in response to shoulder dystocia.
PHYSICIAN’S DEFENSE The case was settled at trial.
VERDICT A $1 million New Jersey settlement was placed in a structured payment fund to provide a net $1.78 million over the child’s lifetime.
Related Article: You are the second responder to a shoulder dystocia emergency. What do you do first? Robert L. Barbieri, MD (Editorial, May 2013)
WAS WOMAN UNLAWFULLY SEEKING DRUGS?
A 30-year-old woman went to an ED with pelvic pain and vaginal discharge. An ED physician conducted a physical exam. Blood tests indicated the patient had taken barbiturates, but the patient could not explain the findings. Determining no cause for her symptoms, the ED physician discharged the patient.
Just after she left, the ED physician found that the patient had several narcotics prescriptions and called the police. The patient was arrested and charged with unlawfully seeking drugs at the hospital. Criminal charges were later dismissed.
PATIENT’S CLAIM The ED physician did not properly examine her; she was found to have endometriosis and underwent surgery a few weeks later. The ED physician was negligent for divulging her personal information to police.
The ED physician had had his physician’s license suspended due to substance abuse and had also been arrested for driving under the influence after his license was restored.
PHYSICIAN’S DEFENSE The ED physician’s examination and treatment were proper. The phone call to police was not part of treatment. The patient had a malicious prosecution basis for any claims.
VERDICT A $125,000 Kentucky verdict was returned.
CASCADING PROBLEMS: MOTHER AND BABY DIE
A pregnant woman was admitted to an ED, where the on-call physician determined that she had pneumonia. The patient’s ObGyn, 45 miles away, refused to come to the hospital or arrange for another ObGyn to take the case.
Several hours later, after the mother was found to have fulminant preeclampsia, the ObGyn demanded the patient be moved to the hospital’s internal medicine (IM) service. However, the IM service refused to admit the patient because she needed obstetric care. The ObGyn tried to transfer the patient to a maternal-fetal medicine (MFM) specialist at a tertiary care center; transfer was refused because the patient was too unstable and needed an emergency cesarean delivery. The ObGyn continued to refuse to relinquish care to another ObGyn.
The ED physician decided to transfer the patient to another hospital 50 miles away even though she was now in active labor. An MFM specialist accepted transfer. After 5 hours in the ED, the mother left by ambulance, but, during transport, she suffered placental abruption and internal hemorrhaging. She was in critical condition upon arrival. An emergency cesarean delivery was performed, but the mother died. The baby, born with severe brain damage, also died.
ESTATES’ CLAIM The ED physician failed to properly and timely determine that the mother had preeclampsia; no treatment for hypertension was provided. The ED physician withheld critical information, including the patient’s severe hypertension, proteinurea, and edema, when speaking to the MFM specialist who accepted transfer. The ED physician did not evaluate the mother before departure and certified the transfer although the patient was highly unstable.
The ObGyn was negligent in not transferring care to another ObGyn and not coming to the hospital. The ObGyn did not inform the ED physician of the rejected attempt to transfer the patient or of the first MFM specialist’s recommendation for emergency cesarean. Both mother and baby could have survived with proper treatment.
DEFENDANTS’ DEFENSE The case was settled at trial.
VERDICT A $900,000 Michigan settlement was reached.
FECAL INCONTINENCE AFTER EPISIOTOMY
A 26-year-old woman gave birth after her ObGyn created an episiotomy to facilitate delivery. The incision was repaired and the ObGyn prescribed docusate (Colace) to soften her stools.
A month later, the patient report-ed fecal incontinence. The ObGyn determined that the incontinence was related to the episiotomy, but did not feel that immediate attention was needed. When the condition did not improve, the patient saw a colorectal surgeon, who diagnosed a significant sphincter defect. The patient underwent a sphincteroplasty, with minor improvement.
PATIENT’S CLAIM The ObGyn failed to properly manage episiotomy healing. The patient remembers being told to stop docusate after she had passed one stool after delivery. A 10-day regimen of docusate and a diet to reduce defecation frequency should have been prescribed. Incontinence should have prompted an immediate referral to a colorectal surgeon.
PHYSICIAN’S DEFENSE Prompt surgical intervention was not necessary. Sphincteroplasty can be delayed until conservative methods have been tried. Episiotomy healing was properly addressed. Permanent incontinence is a known risk of the procedure.
VERDICT A $6 million New York verdict was returned.
Related Article: Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery? Errol R. Norwitz, MD, PhD (Examining the Evidence, August 2012)
MECONIUM ASPIRATION SYNDROME
A baby stayed in hospital for 3 weeks postdelivery due to meconium aspiration syndrome.
PARENTS’ CLAIM The resident who followed the mother during her pregnancy was negligent in allowing the pregnancy to progress to 46 weeks’ gestation before delivery.
DEFENDANTS’ DEFENSE The estimated date of conception was disputed. The resident claimed that the baby was born at 42 weeks’ gestation. An attending physician reviewed all prenatal visits with the resident. The mother’s cervix was never ripe before induction of labor. Aspiration occurred despite aggressive suctioning. The child has had no further respiratory issues since her neonatal discharge.
VERDICT An Illinois defense verdict was returned.
BOWEL INJURY AFTER HYSTERECTOMY
A woman underwent laparoscopic-assisted vaginal hysterectomy and was discharged the following day. Two days later, she went to an ED in acute distress. A bowel perforation was found during emergency surgery, and her colon was repaired. She made a full recovery.
PATIENT’S CLAIM The ObGyn was negligent in failing to properly evaluate the patient after surgery. The ObGyn also failed to explain the signs of a possible perforation to the patient before she left the hospital.
PHYSICIAN’S DEFENSE The patient’s postoperative course was normal while she was hospitalized. Bowel perforation is a known complication of the procedure. The patient had been informed of all the signs and symptoms of a bowel perforation and had been instructed to call the ObGyn or return to the hospital if she began to have any symptoms.
VERDICT A South Carolina defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
Mutation could be target for MDS/AML treatment
Scientists have found evidence to suggest that a genetic alteration in osteoblasts can induce acute myeloid leukemia (AML).
And this provides a potential therapeutic target for AML and myelodysplastic syndromes (MDS).
Stavroula Kousteni, PhD, of Columbia University Medical Center in New York, and her colleagues described these findings in Nature.
The researchers discovered that an activating mutation of beta-catenin in mouse osteoblasts induces AML.
This mutation leads to cancer in adjacent hematopoietic stem cells (HSCs) through a series of events. First, the mutated beta-catenin protein moves from its normal location on the exterior of the osteoblast to the cell’s nucleus, where it activates production of the protein jagged1.
Jagged1 proteins are then shipped to the osteoblast’s exterior membrane, where they can bind to Notch proteins—which activate signaling pathways—on neighboring HSCs. When this happens, Notch transmits signals inside the HSCs that enable leukemic transformation.
To confirm the role of jagged1 in AML development, the investigators removed 1 allele of jagged1 in osteoblasts. This decreased Notch signaling in Lin-Sca+c-Kit+ cells, rescued anemia and deregulation of HSC lineage differentiation, and prevented AML development.
The researchers then evaluated the effects of blocking Notch signaling using a gamma-secretase inhibitor. The treatment reversed hematopoietic deregulation and myeloid expansion in the blood, marrow, and spleens of the mice and reversed their AML.
“If the [process] works the same way in humans, our study suggests practical ways that we may be able to intervene with a drug or an antibody,” Dr Kousteni said.
With this in mind, she and her colleagues analyzed cells from 107 patients with AML or MDS. About 38% of the patients had changes in beta-catenin, jagged1, and Notch signaling that mirrored the changes in the mice. But none of the 56 healthy control subjects studied had these changes.
The investigators therefore concluded that these findings provide new insight into AML/MDS pathogenesis and may have implications for treatment. ![]()

Scientists have found evidence to suggest that a genetic alteration in osteoblasts can induce acute myeloid leukemia (AML).
And this provides a potential therapeutic target for AML and myelodysplastic syndromes (MDS).
Stavroula Kousteni, PhD, of Columbia University Medical Center in New York, and her colleagues described these findings in Nature.
The researchers discovered that an activating mutation of beta-catenin in mouse osteoblasts induces AML.
This mutation leads to cancer in adjacent hematopoietic stem cells (HSCs) through a series of events. First, the mutated beta-catenin protein moves from its normal location on the exterior of the osteoblast to the cell’s nucleus, where it activates production of the protein jagged1.
Jagged1 proteins are then shipped to the osteoblast’s exterior membrane, where they can bind to Notch proteins—which activate signaling pathways—on neighboring HSCs. When this happens, Notch transmits signals inside the HSCs that enable leukemic transformation.
To confirm the role of jagged1 in AML development, the investigators removed 1 allele of jagged1 in osteoblasts. This decreased Notch signaling in Lin-Sca+c-Kit+ cells, rescued anemia and deregulation of HSC lineage differentiation, and prevented AML development.
The researchers then evaluated the effects of blocking Notch signaling using a gamma-secretase inhibitor. The treatment reversed hematopoietic deregulation and myeloid expansion in the blood, marrow, and spleens of the mice and reversed their AML.
“If the [process] works the same way in humans, our study suggests practical ways that we may be able to intervene with a drug or an antibody,” Dr Kousteni said.
With this in mind, she and her colleagues analyzed cells from 107 patients with AML or MDS. About 38% of the patients had changes in beta-catenin, jagged1, and Notch signaling that mirrored the changes in the mice. But none of the 56 healthy control subjects studied had these changes.
The investigators therefore concluded that these findings provide new insight into AML/MDS pathogenesis and may have implications for treatment. ![]()

Scientists have found evidence to suggest that a genetic alteration in osteoblasts can induce acute myeloid leukemia (AML).
And this provides a potential therapeutic target for AML and myelodysplastic syndromes (MDS).
Stavroula Kousteni, PhD, of Columbia University Medical Center in New York, and her colleagues described these findings in Nature.
The researchers discovered that an activating mutation of beta-catenin in mouse osteoblasts induces AML.
This mutation leads to cancer in adjacent hematopoietic stem cells (HSCs) through a series of events. First, the mutated beta-catenin protein moves from its normal location on the exterior of the osteoblast to the cell’s nucleus, where it activates production of the protein jagged1.
Jagged1 proteins are then shipped to the osteoblast’s exterior membrane, where they can bind to Notch proteins—which activate signaling pathways—on neighboring HSCs. When this happens, Notch transmits signals inside the HSCs that enable leukemic transformation.
To confirm the role of jagged1 in AML development, the investigators removed 1 allele of jagged1 in osteoblasts. This decreased Notch signaling in Lin-Sca+c-Kit+ cells, rescued anemia and deregulation of HSC lineage differentiation, and prevented AML development.
The researchers then evaluated the effects of blocking Notch signaling using a gamma-secretase inhibitor. The treatment reversed hematopoietic deregulation and myeloid expansion in the blood, marrow, and spleens of the mice and reversed their AML.
“If the [process] works the same way in humans, our study suggests practical ways that we may be able to intervene with a drug or an antibody,” Dr Kousteni said.
With this in mind, she and her colleagues analyzed cells from 107 patients with AML or MDS. About 38% of the patients had changes in beta-catenin, jagged1, and Notch signaling that mirrored the changes in the mice. But none of the 56 healthy control subjects studied had these changes.
The investigators therefore concluded that these findings provide new insight into AML/MDS pathogenesis and may have implications for treatment. ![]()