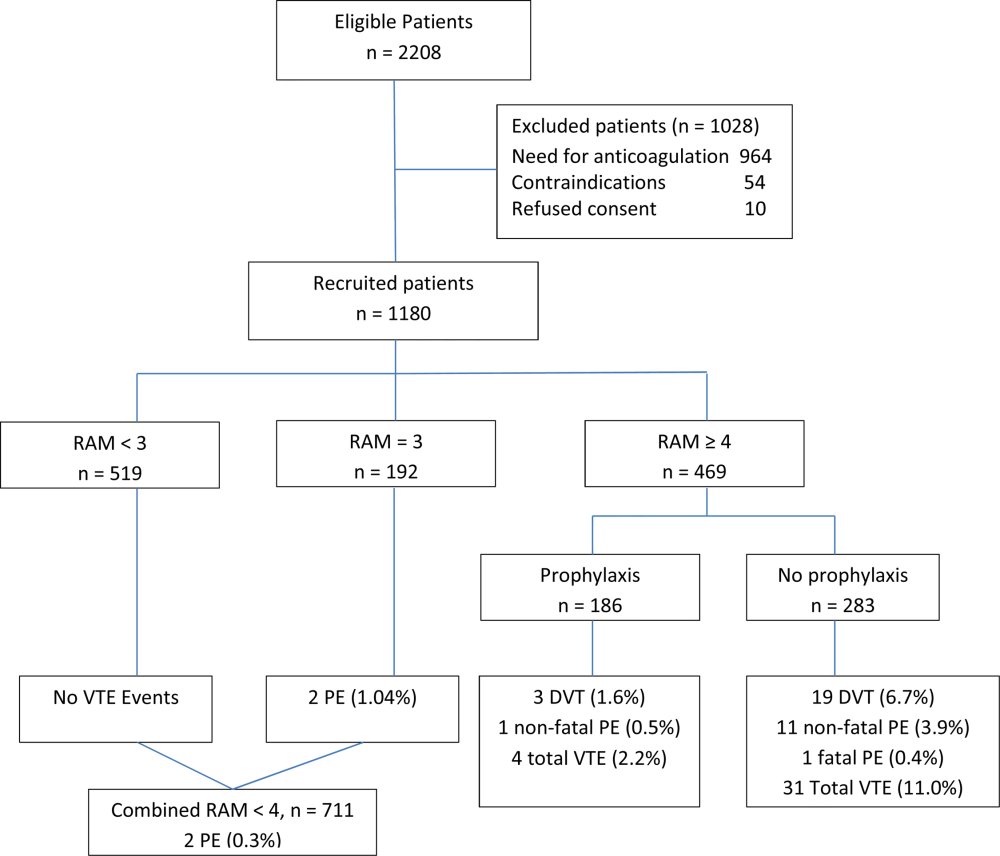
User login
When is an answer not an answer?
When your beloved authors were studying research and statistics, around the time that Methuselah was celebrating his first birthday, we thought we knew the difference between hypothesis testing and hypothesis generating. With the former, you begin with a question, design a study to answer it, carry it out, and then do some statistical mumbo-jumbo on the data to determine if you have reasonable evidence to answer the question. With the latter, usually done after you’ve answered the main questions, you don’t have any preconceived idea of what’s going on, so you analyze anything that moves. We know that’s not really kosher, because the probability of finding something just by chance (a Type I error) increases astronomically as you do more tests.1 So, in the hypothesis generating phase, you don’t come to any conclusions; you just say, “That’s an interesting finding. Now we’ll have to do a real study to see if our observation holds up.”
Click on the PDF icon at the top of this introduction to read the full article.
When your beloved authors were studying research and statistics, around the time that Methuselah was celebrating his first birthday, we thought we knew the difference between hypothesis testing and hypothesis generating. With the former, you begin with a question, design a study to answer it, carry it out, and then do some statistical mumbo-jumbo on the data to determine if you have reasonable evidence to answer the question. With the latter, usually done after you’ve answered the main questions, you don’t have any preconceived idea of what’s going on, so you analyze anything that moves. We know that’s not really kosher, because the probability of finding something just by chance (a Type I error) increases astronomically as you do more tests.1 So, in the hypothesis generating phase, you don’t come to any conclusions; you just say, “That’s an interesting finding. Now we’ll have to do a real study to see if our observation holds up.”
Click on the PDF icon at the top of this introduction to read the full article.
When your beloved authors were studying research and statistics, around the time that Methuselah was celebrating his first birthday, we thought we knew the difference between hypothesis testing and hypothesis generating. With the former, you begin with a question, design a study to answer it, carry it out, and then do some statistical mumbo-jumbo on the data to determine if you have reasonable evidence to answer the question. With the latter, usually done after you’ve answered the main questions, you don’t have any preconceived idea of what’s going on, so you analyze anything that moves. We know that’s not really kosher, because the probability of finding something just by chance (a Type I error) increases astronomically as you do more tests.1 So, in the hypothesis generating phase, you don’t come to any conclusions; you just say, “That’s an interesting finding. Now we’ll have to do a real study to see if our observation holds up.”
Click on the PDF icon at the top of this introduction to read the full article.
Adaptability and Resiliency of Military Families During Reunification: Results of a Longitudinal Study
Medicare Beneficiaries Likely Readmitted
For at least 25 years, approximately 20% of Medicare fee‐for‐service discharges have been followed by a hospital readmission within 30 days.[1, 2] Section 3025 of the Patient Protection and Affordable Care Act (ACA)[3] created escalating penalties for hospitals with higher than expected 30‐day readmission rates, and the Congressional Budget Office estimated this will reduce Medicare spending by over $7 billion between 2010 and 2019.[4]
Hospitals and physicians have begun developing strategies to identify which Medicare beneficiaries are most likely to be readmitted and use this information to design programs to reduce their readmission rate. Initially, penalties will be based on readmission rates after an index discharge with heart failure, myocardial infarction, and pneumonia.[5] Recently, the Centers for Medicare and Medicaid Services (CMS) released the Inpatient Prospective Payment System FY2014 proposed rule, which proposes to add 2 new readmission penalties beginning in FY2015: readmissions for hip/knee arthroplasty and chronic obstructive pulmonary disease.[6] Other countries are already penalizing hospitals with high readmission rates; for example, Germany is penalizing all readmissions that occur within a 30‐day period following admission.[7] In this brief report, we examine the characteristics of Medicare beneficiaries most likely to be readmitted within 30 days. We focus on readmission rates for all discharge conditions and all patient readmission rates, because we believe the language in the ACA ultimately points to an all‐inclusive approach.
METHODS
We used a nationally random 5% sample of all Medicare beneficiaries for the period between January 1, 2008 and September 30, 2008. To be included, beneficiaries must have both Part A and B coverage and live within the United States. Medicare Advantage patients were excluded because Medicare Advantage plans do not report the data in the same way as fee for service. We calculated the readmission rate as the number of admissions that were preceded by an at‐risk discharge within 30 days divided by the total number of at‐risk discharges. This definition included admissions to and discharges from sole community providers, Medicare‐dependent small rural hospitals, and critical access hospitals. We counted as at risk all live discharges from short‐term acute care hospitals that were not discharged against medical advice, discharged to a rehabilitation unit within an acute care hospital, or readmitted on day 0 (due to inconsistency with use of transfer coding). We only included discharges and readmissions to acute care hospitals and excluded hospitalizations in long‐term care facilities, rehabilitation facilities, skilled nursing homes, and other non‐acute care hospital facilities from being an index hospitalization. However, if the beneficiary was discharged to 1 of these facilities and then readmitted to an acute care hospital, the readmission was counted.
Each discharge was recorded as an independent event and we reset the readmission clock for a fresh 30‐day count each time the beneficiary was discharged. We examined the admission and readmission rate to determine if the rates varied by age, gender, reason for entitlement, racial characteristics, region of the country, number of chronic conditions, and whether the beneficiary is also enrolled in Medicaid (dual eligibles). We calculated the mean readmission rate for each diagnosis‐related group (DRG) and then used the probability of having a readmission for each DRG to calculate a case mix adjustment for each hospital. To calculate the chronic illness burden, we used a previously developed methodology for counting the number of chronic disease categories reported for the patient in the preceding year (2007).[8, 9] The classification system is maintained by the Agency for Health Care Research and Quality. We then used logistic regression to calculate the odds ratio of a discharge being readmitted based on these factors. We preformed statistical analysis using SAS version 9.1.3 (SAS Institute Inc., Cary, NC).
RESULTS
There were 434,999 hospital discharges that occurred in the first 9 months of 2008 in the 5% sample. There were 20.6% of Medicare beneficiaries hospitalized, and the overall readmission rate was 19.5%. Table 1 shows the odds ratios and 95% confidence intervals for the probability that a Medicare beneficiary will be readmitted within 30 days for variables including: age, sex, race, dual‐eligibility status, number of comorbid conditions, geographic region, and reason for entitlement. Of note, beneficiaries with 10 or more chronic conditions were more than 6 times more likely, and beneficiaries with 5 to 9 chronic conditions were more than 2.5 times more likely, to be readmitted than beneficiaries with 1 to 4 chronic conditions.
| Variable | Estimate | 95% Confidence Limits |
|---|---|---|
| ||
| Age 144 years | 1.634 | 1.5071.771 |
| Age 4564 years | 1.231 | 1.1421.327 |
| Age 7584 years | 1.048 | 1.0271.069 |
| Age 85+ years | 1.141 | 1.1151.168 |
| Age 6574 years | REF | |
| Male | 1.201 | 1.1831.220 |
| Black | 1.250 | 1.2211.280 |
| Other race | 1.071 | 1.0331.111 |
| White | REF | |
| Dual eligibles | 1.173 | 1.1511.195 |
| Northeast region | 1.146 | 1.1151.178 |
| Midwest region | 1.092 | 1.0631.122 |
| South region | 1.037 | 1.0111.063 |
| West region | REF | |
| 0 comorbidities | 0.255 | 0.1480.441 |
| 59 comorbidities | 2.533 | 2.4492.621 |
| 10+ comorbidities | 6.119 | 5.9136.332 |
| 14 comorbidities | REF | |
| Disabled | 0.817 | 0.7570.880 |
| ESRD | 1.327 | 1.2231.440 |
| Age >64 years | REF | |
DISCUSSION
The most interesting finding is that beneficiaries with 10 or more chronic conditions were more than 6 times more likely to be readmitted than beneficiaries with 1 to 4 chronic conditions. Beneficiaries with 10 or more chronic conditions represent only 8.9% of all Medicare beneficiaries (31.0% of all hospitalizations), but they were responsible for 50.2% of all readmissions. The 31.8% of beneficiaries with 5 to 9 chronic conditions (55.5% of all hospitalizations) had the second highest odds ratio (2.5) and were responsible for 45% of all readmissions. The 59.3% of beneficiaries with 5 comorbidities (13.6% of all hospitalizations) were associated with only 4.7% of all readmissions. This strongly suggests that hospitals focus their attention on beneficiaries with 10 or more comorbidities. These results were despite correction for DRG diagnosis in the model.
We recognize that the number of chronic conditions is a crude measure of health status because it weighs hundreds of different clinical conditions equally; however, it seems a good proxy for 3 closely allied concepts: (1) the overall burden of chronic illness carried by the patient, (2) the patient's level of engagement with the healthcare system (including number of unique providers), and (3) the number of conditions being treated. By providing a 1‐year window of a patient's health status, it is a more complete picture than any single hospital claim submission or indices based solely on hospital discharge data.
The other variables are less predictive of 30‐day readmissions. Beneficiaries over 85 years old are only 14% more likely, whereas disabled Medicare beneficiaries 44 years old are 63% more likely to be readmitted than beneficiaries between 65 and 74 years old. Men are 20% more likely to be readmitted than women. Black race and dual‐eligibility slightly increase rates of readmission. Beneficiaries located in the West have the lowest readmission rates. In comparison to those who are aged, those with end‐stage renal disease (ESRD) have a higher rate of readmission, and those with a disability have a lower rate of readmission. In considering the age and reason for entitlement findings, one would assume that ESRD was the driver of higher readmission rates in the younger Medicare population.
CMS will need to analyze which hospitals have higher than expected readmission rates, and this will require risk adjustment at each hospital. In addition to the number of chronic conditions and other variables shown in Table 1, other factors CMS might want to include when it starts doing readmissions for all discharges is the discharge diagnosis (because our results suggest there are significant differences in the probability of a readmission across DRGs). In addition, CMS will need to consider how to capture additional data not currently in the claims data, such as social factors like homelessness.
We recognize significant limitations to these findings. First, this analysis uses only information that is available from Medicare claims and administrative data. Claims give almost no information on how well the hospital planned the discharge, instructed the patient and family, or engaged follow‐up providers. Also, claims data tell us virtually nothing about a patient's health literacy or social situation. Second, the analysis relies on claims data, but this has little clinical detail. Third, these data are limited to persons enrolled in fee‐for‐service Medicare. Fourth, we included all readmissions, including some readmissions (such as chemotherapy and staged percutaneous coronary interventions) that were part of a planned treatment protocol.[10] Fifth, we were unable to distinguish same‐day readmissions versus transfers, and therefore excluded all same‐day readmissions from measurement.
As hospitals and physicians begin to plan for the regulations that will penalize hospitals with high readmission rates, they will need to strongly consider targeting beneficiaries with more than 10 chronic conditions.
Acknowledgments
The Commonwealth Fund provided a grant to Dr. Anderson to help support this work. The authors report no conflicts of interest.
- , . Hospital readmissions in the Medicare population. N Engl J Med. 1984;311:1349–1353.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428.
- Patient Protection and Affordable Care Act. Section 3025. Available at: http://www.gpo.gov/fdsys/pkg/PLAW‐111publ148/pdf/PLAW‐111publ148.pdf. Accessed April 8, 2013.
- Congressional Budget Office.Patient Protection and Affordable Care Act. Available at: http://www.cbo.gov/doc.cfm?index=10868. Accessed April 8, 2013.
- , , , et al.2012 measures maintenance technical report: acute myocardial infarction, heart failure, and pneumonia 30‐day risk‐standardized readmission measures. Available at: http://www.qualitynet.org/dcs/ContentServer?c=Page78:27597–27599.
- , , , , . Hospital payment based on diagnosis‐related groups differs in Europe and holds lessons for the United States. Health Aff (Millwood). 2013;32:713–723.
- , , , . Out‐of‐pocket medical spending for care of chronic conditions. Health Aff (Millwood). 2001;20:267–278.
- MEPS data documentation HC‐006: 1996 medical conditions. Pub. no. 99‐DP06. Rockville, MD: AHRQ; 1999.
- , . Planned readmissions: a potential solution. Arch Intern Med. 2012;172:269–270.
For at least 25 years, approximately 20% of Medicare fee‐for‐service discharges have been followed by a hospital readmission within 30 days.[1, 2] Section 3025 of the Patient Protection and Affordable Care Act (ACA)[3] created escalating penalties for hospitals with higher than expected 30‐day readmission rates, and the Congressional Budget Office estimated this will reduce Medicare spending by over $7 billion between 2010 and 2019.[4]
Hospitals and physicians have begun developing strategies to identify which Medicare beneficiaries are most likely to be readmitted and use this information to design programs to reduce their readmission rate. Initially, penalties will be based on readmission rates after an index discharge with heart failure, myocardial infarction, and pneumonia.[5] Recently, the Centers for Medicare and Medicaid Services (CMS) released the Inpatient Prospective Payment System FY2014 proposed rule, which proposes to add 2 new readmission penalties beginning in FY2015: readmissions for hip/knee arthroplasty and chronic obstructive pulmonary disease.[6] Other countries are already penalizing hospitals with high readmission rates; for example, Germany is penalizing all readmissions that occur within a 30‐day period following admission.[7] In this brief report, we examine the characteristics of Medicare beneficiaries most likely to be readmitted within 30 days. We focus on readmission rates for all discharge conditions and all patient readmission rates, because we believe the language in the ACA ultimately points to an all‐inclusive approach.
METHODS
We used a nationally random 5% sample of all Medicare beneficiaries for the period between January 1, 2008 and September 30, 2008. To be included, beneficiaries must have both Part A and B coverage and live within the United States. Medicare Advantage patients were excluded because Medicare Advantage plans do not report the data in the same way as fee for service. We calculated the readmission rate as the number of admissions that were preceded by an at‐risk discharge within 30 days divided by the total number of at‐risk discharges. This definition included admissions to and discharges from sole community providers, Medicare‐dependent small rural hospitals, and critical access hospitals. We counted as at risk all live discharges from short‐term acute care hospitals that were not discharged against medical advice, discharged to a rehabilitation unit within an acute care hospital, or readmitted on day 0 (due to inconsistency with use of transfer coding). We only included discharges and readmissions to acute care hospitals and excluded hospitalizations in long‐term care facilities, rehabilitation facilities, skilled nursing homes, and other non‐acute care hospital facilities from being an index hospitalization. However, if the beneficiary was discharged to 1 of these facilities and then readmitted to an acute care hospital, the readmission was counted.
Each discharge was recorded as an independent event and we reset the readmission clock for a fresh 30‐day count each time the beneficiary was discharged. We examined the admission and readmission rate to determine if the rates varied by age, gender, reason for entitlement, racial characteristics, region of the country, number of chronic conditions, and whether the beneficiary is also enrolled in Medicaid (dual eligibles). We calculated the mean readmission rate for each diagnosis‐related group (DRG) and then used the probability of having a readmission for each DRG to calculate a case mix adjustment for each hospital. To calculate the chronic illness burden, we used a previously developed methodology for counting the number of chronic disease categories reported for the patient in the preceding year (2007).[8, 9] The classification system is maintained by the Agency for Health Care Research and Quality. We then used logistic regression to calculate the odds ratio of a discharge being readmitted based on these factors. We preformed statistical analysis using SAS version 9.1.3 (SAS Institute Inc., Cary, NC).
RESULTS
There were 434,999 hospital discharges that occurred in the first 9 months of 2008 in the 5% sample. There were 20.6% of Medicare beneficiaries hospitalized, and the overall readmission rate was 19.5%. Table 1 shows the odds ratios and 95% confidence intervals for the probability that a Medicare beneficiary will be readmitted within 30 days for variables including: age, sex, race, dual‐eligibility status, number of comorbid conditions, geographic region, and reason for entitlement. Of note, beneficiaries with 10 or more chronic conditions were more than 6 times more likely, and beneficiaries with 5 to 9 chronic conditions were more than 2.5 times more likely, to be readmitted than beneficiaries with 1 to 4 chronic conditions.
| Variable | Estimate | 95% Confidence Limits |
|---|---|---|
| ||
| Age 144 years | 1.634 | 1.5071.771 |
| Age 4564 years | 1.231 | 1.1421.327 |
| Age 7584 years | 1.048 | 1.0271.069 |
| Age 85+ years | 1.141 | 1.1151.168 |
| Age 6574 years | REF | |
| Male | 1.201 | 1.1831.220 |
| Black | 1.250 | 1.2211.280 |
| Other race | 1.071 | 1.0331.111 |
| White | REF | |
| Dual eligibles | 1.173 | 1.1511.195 |
| Northeast region | 1.146 | 1.1151.178 |
| Midwest region | 1.092 | 1.0631.122 |
| South region | 1.037 | 1.0111.063 |
| West region | REF | |
| 0 comorbidities | 0.255 | 0.1480.441 |
| 59 comorbidities | 2.533 | 2.4492.621 |
| 10+ comorbidities | 6.119 | 5.9136.332 |
| 14 comorbidities | REF | |
| Disabled | 0.817 | 0.7570.880 |
| ESRD | 1.327 | 1.2231.440 |
| Age >64 years | REF | |
DISCUSSION
The most interesting finding is that beneficiaries with 10 or more chronic conditions were more than 6 times more likely to be readmitted than beneficiaries with 1 to 4 chronic conditions. Beneficiaries with 10 or more chronic conditions represent only 8.9% of all Medicare beneficiaries (31.0% of all hospitalizations), but they were responsible for 50.2% of all readmissions. The 31.8% of beneficiaries with 5 to 9 chronic conditions (55.5% of all hospitalizations) had the second highest odds ratio (2.5) and were responsible for 45% of all readmissions. The 59.3% of beneficiaries with 5 comorbidities (13.6% of all hospitalizations) were associated with only 4.7% of all readmissions. This strongly suggests that hospitals focus their attention on beneficiaries with 10 or more comorbidities. These results were despite correction for DRG diagnosis in the model.
We recognize that the number of chronic conditions is a crude measure of health status because it weighs hundreds of different clinical conditions equally; however, it seems a good proxy for 3 closely allied concepts: (1) the overall burden of chronic illness carried by the patient, (2) the patient's level of engagement with the healthcare system (including number of unique providers), and (3) the number of conditions being treated. By providing a 1‐year window of a patient's health status, it is a more complete picture than any single hospital claim submission or indices based solely on hospital discharge data.
The other variables are less predictive of 30‐day readmissions. Beneficiaries over 85 years old are only 14% more likely, whereas disabled Medicare beneficiaries 44 years old are 63% more likely to be readmitted than beneficiaries between 65 and 74 years old. Men are 20% more likely to be readmitted than women. Black race and dual‐eligibility slightly increase rates of readmission. Beneficiaries located in the West have the lowest readmission rates. In comparison to those who are aged, those with end‐stage renal disease (ESRD) have a higher rate of readmission, and those with a disability have a lower rate of readmission. In considering the age and reason for entitlement findings, one would assume that ESRD was the driver of higher readmission rates in the younger Medicare population.
CMS will need to analyze which hospitals have higher than expected readmission rates, and this will require risk adjustment at each hospital. In addition to the number of chronic conditions and other variables shown in Table 1, other factors CMS might want to include when it starts doing readmissions for all discharges is the discharge diagnosis (because our results suggest there are significant differences in the probability of a readmission across DRGs). In addition, CMS will need to consider how to capture additional data not currently in the claims data, such as social factors like homelessness.
We recognize significant limitations to these findings. First, this analysis uses only information that is available from Medicare claims and administrative data. Claims give almost no information on how well the hospital planned the discharge, instructed the patient and family, or engaged follow‐up providers. Also, claims data tell us virtually nothing about a patient's health literacy or social situation. Second, the analysis relies on claims data, but this has little clinical detail. Third, these data are limited to persons enrolled in fee‐for‐service Medicare. Fourth, we included all readmissions, including some readmissions (such as chemotherapy and staged percutaneous coronary interventions) that were part of a planned treatment protocol.[10] Fifth, we were unable to distinguish same‐day readmissions versus transfers, and therefore excluded all same‐day readmissions from measurement.
As hospitals and physicians begin to plan for the regulations that will penalize hospitals with high readmission rates, they will need to strongly consider targeting beneficiaries with more than 10 chronic conditions.
Acknowledgments
The Commonwealth Fund provided a grant to Dr. Anderson to help support this work. The authors report no conflicts of interest.
For at least 25 years, approximately 20% of Medicare fee‐for‐service discharges have been followed by a hospital readmission within 30 days.[1, 2] Section 3025 of the Patient Protection and Affordable Care Act (ACA)[3] created escalating penalties for hospitals with higher than expected 30‐day readmission rates, and the Congressional Budget Office estimated this will reduce Medicare spending by over $7 billion between 2010 and 2019.[4]
Hospitals and physicians have begun developing strategies to identify which Medicare beneficiaries are most likely to be readmitted and use this information to design programs to reduce their readmission rate. Initially, penalties will be based on readmission rates after an index discharge with heart failure, myocardial infarction, and pneumonia.[5] Recently, the Centers for Medicare and Medicaid Services (CMS) released the Inpatient Prospective Payment System FY2014 proposed rule, which proposes to add 2 new readmission penalties beginning in FY2015: readmissions for hip/knee arthroplasty and chronic obstructive pulmonary disease.[6] Other countries are already penalizing hospitals with high readmission rates; for example, Germany is penalizing all readmissions that occur within a 30‐day period following admission.[7] In this brief report, we examine the characteristics of Medicare beneficiaries most likely to be readmitted within 30 days. We focus on readmission rates for all discharge conditions and all patient readmission rates, because we believe the language in the ACA ultimately points to an all‐inclusive approach.
METHODS
We used a nationally random 5% sample of all Medicare beneficiaries for the period between January 1, 2008 and September 30, 2008. To be included, beneficiaries must have both Part A and B coverage and live within the United States. Medicare Advantage patients were excluded because Medicare Advantage plans do not report the data in the same way as fee for service. We calculated the readmission rate as the number of admissions that were preceded by an at‐risk discharge within 30 days divided by the total number of at‐risk discharges. This definition included admissions to and discharges from sole community providers, Medicare‐dependent small rural hospitals, and critical access hospitals. We counted as at risk all live discharges from short‐term acute care hospitals that were not discharged against medical advice, discharged to a rehabilitation unit within an acute care hospital, or readmitted on day 0 (due to inconsistency with use of transfer coding). We only included discharges and readmissions to acute care hospitals and excluded hospitalizations in long‐term care facilities, rehabilitation facilities, skilled nursing homes, and other non‐acute care hospital facilities from being an index hospitalization. However, if the beneficiary was discharged to 1 of these facilities and then readmitted to an acute care hospital, the readmission was counted.
Each discharge was recorded as an independent event and we reset the readmission clock for a fresh 30‐day count each time the beneficiary was discharged. We examined the admission and readmission rate to determine if the rates varied by age, gender, reason for entitlement, racial characteristics, region of the country, number of chronic conditions, and whether the beneficiary is also enrolled in Medicaid (dual eligibles). We calculated the mean readmission rate for each diagnosis‐related group (DRG) and then used the probability of having a readmission for each DRG to calculate a case mix adjustment for each hospital. To calculate the chronic illness burden, we used a previously developed methodology for counting the number of chronic disease categories reported for the patient in the preceding year (2007).[8, 9] The classification system is maintained by the Agency for Health Care Research and Quality. We then used logistic regression to calculate the odds ratio of a discharge being readmitted based on these factors. We preformed statistical analysis using SAS version 9.1.3 (SAS Institute Inc., Cary, NC).
RESULTS
There were 434,999 hospital discharges that occurred in the first 9 months of 2008 in the 5% sample. There were 20.6% of Medicare beneficiaries hospitalized, and the overall readmission rate was 19.5%. Table 1 shows the odds ratios and 95% confidence intervals for the probability that a Medicare beneficiary will be readmitted within 30 days for variables including: age, sex, race, dual‐eligibility status, number of comorbid conditions, geographic region, and reason for entitlement. Of note, beneficiaries with 10 or more chronic conditions were more than 6 times more likely, and beneficiaries with 5 to 9 chronic conditions were more than 2.5 times more likely, to be readmitted than beneficiaries with 1 to 4 chronic conditions.
| Variable | Estimate | 95% Confidence Limits |
|---|---|---|
| ||
| Age 144 years | 1.634 | 1.5071.771 |
| Age 4564 years | 1.231 | 1.1421.327 |
| Age 7584 years | 1.048 | 1.0271.069 |
| Age 85+ years | 1.141 | 1.1151.168 |
| Age 6574 years | REF | |
| Male | 1.201 | 1.1831.220 |
| Black | 1.250 | 1.2211.280 |
| Other race | 1.071 | 1.0331.111 |
| White | REF | |
| Dual eligibles | 1.173 | 1.1511.195 |
| Northeast region | 1.146 | 1.1151.178 |
| Midwest region | 1.092 | 1.0631.122 |
| South region | 1.037 | 1.0111.063 |
| West region | REF | |
| 0 comorbidities | 0.255 | 0.1480.441 |
| 59 comorbidities | 2.533 | 2.4492.621 |
| 10+ comorbidities | 6.119 | 5.9136.332 |
| 14 comorbidities | REF | |
| Disabled | 0.817 | 0.7570.880 |
| ESRD | 1.327 | 1.2231.440 |
| Age >64 years | REF | |
DISCUSSION
The most interesting finding is that beneficiaries with 10 or more chronic conditions were more than 6 times more likely to be readmitted than beneficiaries with 1 to 4 chronic conditions. Beneficiaries with 10 or more chronic conditions represent only 8.9% of all Medicare beneficiaries (31.0% of all hospitalizations), but they were responsible for 50.2% of all readmissions. The 31.8% of beneficiaries with 5 to 9 chronic conditions (55.5% of all hospitalizations) had the second highest odds ratio (2.5) and were responsible for 45% of all readmissions. The 59.3% of beneficiaries with 5 comorbidities (13.6% of all hospitalizations) were associated with only 4.7% of all readmissions. This strongly suggests that hospitals focus their attention on beneficiaries with 10 or more comorbidities. These results were despite correction for DRG diagnosis in the model.
We recognize that the number of chronic conditions is a crude measure of health status because it weighs hundreds of different clinical conditions equally; however, it seems a good proxy for 3 closely allied concepts: (1) the overall burden of chronic illness carried by the patient, (2) the patient's level of engagement with the healthcare system (including number of unique providers), and (3) the number of conditions being treated. By providing a 1‐year window of a patient's health status, it is a more complete picture than any single hospital claim submission or indices based solely on hospital discharge data.
The other variables are less predictive of 30‐day readmissions. Beneficiaries over 85 years old are only 14% more likely, whereas disabled Medicare beneficiaries 44 years old are 63% more likely to be readmitted than beneficiaries between 65 and 74 years old. Men are 20% more likely to be readmitted than women. Black race and dual‐eligibility slightly increase rates of readmission. Beneficiaries located in the West have the lowest readmission rates. In comparison to those who are aged, those with end‐stage renal disease (ESRD) have a higher rate of readmission, and those with a disability have a lower rate of readmission. In considering the age and reason for entitlement findings, one would assume that ESRD was the driver of higher readmission rates in the younger Medicare population.
CMS will need to analyze which hospitals have higher than expected readmission rates, and this will require risk adjustment at each hospital. In addition to the number of chronic conditions and other variables shown in Table 1, other factors CMS might want to include when it starts doing readmissions for all discharges is the discharge diagnosis (because our results suggest there are significant differences in the probability of a readmission across DRGs). In addition, CMS will need to consider how to capture additional data not currently in the claims data, such as social factors like homelessness.
We recognize significant limitations to these findings. First, this analysis uses only information that is available from Medicare claims and administrative data. Claims give almost no information on how well the hospital planned the discharge, instructed the patient and family, or engaged follow‐up providers. Also, claims data tell us virtually nothing about a patient's health literacy or social situation. Second, the analysis relies on claims data, but this has little clinical detail. Third, these data are limited to persons enrolled in fee‐for‐service Medicare. Fourth, we included all readmissions, including some readmissions (such as chemotherapy and staged percutaneous coronary interventions) that were part of a planned treatment protocol.[10] Fifth, we were unable to distinguish same‐day readmissions versus transfers, and therefore excluded all same‐day readmissions from measurement.
As hospitals and physicians begin to plan for the regulations that will penalize hospitals with high readmission rates, they will need to strongly consider targeting beneficiaries with more than 10 chronic conditions.
Acknowledgments
The Commonwealth Fund provided a grant to Dr. Anderson to help support this work. The authors report no conflicts of interest.
- , . Hospital readmissions in the Medicare population. N Engl J Med. 1984;311:1349–1353.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428.
- Patient Protection and Affordable Care Act. Section 3025. Available at: http://www.gpo.gov/fdsys/pkg/PLAW‐111publ148/pdf/PLAW‐111publ148.pdf. Accessed April 8, 2013.
- Congressional Budget Office.Patient Protection and Affordable Care Act. Available at: http://www.cbo.gov/doc.cfm?index=10868. Accessed April 8, 2013.
- , , , et al.2012 measures maintenance technical report: acute myocardial infarction, heart failure, and pneumonia 30‐day risk‐standardized readmission measures. Available at: http://www.qualitynet.org/dcs/ContentServer?c=Page78:27597–27599.
- , , , , . Hospital payment based on diagnosis‐related groups differs in Europe and holds lessons for the United States. Health Aff (Millwood). 2013;32:713–723.
- , , , . Out‐of‐pocket medical spending for care of chronic conditions. Health Aff (Millwood). 2001;20:267–278.
- MEPS data documentation HC‐006: 1996 medical conditions. Pub. no. 99‐DP06. Rockville, MD: AHRQ; 1999.
- , . Planned readmissions: a potential solution. Arch Intern Med. 2012;172:269–270.
- , . Hospital readmissions in the Medicare population. N Engl J Med. 1984;311:1349–1353.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428.
- Patient Protection and Affordable Care Act. Section 3025. Available at: http://www.gpo.gov/fdsys/pkg/PLAW‐111publ148/pdf/PLAW‐111publ148.pdf. Accessed April 8, 2013.
- Congressional Budget Office.Patient Protection and Affordable Care Act. Available at: http://www.cbo.gov/doc.cfm?index=10868. Accessed April 8, 2013.
- , , , et al.2012 measures maintenance technical report: acute myocardial infarction, heart failure, and pneumonia 30‐day risk‐standardized readmission measures. Available at: http://www.qualitynet.org/dcs/ContentServer?c=Page78:27597–27599.
- , , , , . Hospital payment based on diagnosis‐related groups differs in Europe and holds lessons for the United States. Health Aff (Millwood). 2013;32:713–723.
- , , , . Out‐of‐pocket medical spending for care of chronic conditions. Health Aff (Millwood). 2001;20:267–278.
- MEPS data documentation HC‐006: 1996 medical conditions. Pub. no. 99‐DP06. Rockville, MD: AHRQ; 1999.
- , . Planned readmissions: a potential solution. Arch Intern Med. 2012;172:269–270.
Dear Doctor: A Patient‐Centered Tool
In their seminal report Crossing the Quality Chasm, the Institute of Medicine outlined patient‐centered care as 1 of its 6 aims to improve the healthcare delivery system.[1] Patients who are more involved in their diagnosis and treatment plan are more likely to feel respected, be satisfied with their healthcare experience, and ultimately have better outcomes.[2, 3] In a study of hospitalized patients, only 42% were able to state their diagnosis at the time of discharge, suggesting that hospital providers could communicate better with patients about their hospital care.[4] Additionally, only 28% of hospitalized patients were able to list their medications, and only 37% were able to state the purpose of their medications. Although hospitals have taken great strides to improve the quality of patient care, publicly reported patient care surveys, such as the Hospital Consumer Assessment of Hospitals and Health Systems (HCAHPS), suggest that physician communication with patients could be further improved.[1, 5] Furthermore, a recent report by the Institute of Medicine stresses the need to get patients and families involved in their care.[6] Thus, hospital‐based providers should seek to enhance the quality of their communication with patients.
With greater emphasis placed on patient‐ and family‐centered care at many health systems, simple and easy‐to‐implement strategies to improve communication with patients need to be developed and tested.[7, 8] Patients who actively participate in their healthcare by asking questions of their doctor are able to control the focus of their interaction and adjust the amount of information provided.[9] Simply asking questions can have a critical impact, as 1 study found that the frequency with which patients asked questions was significantly related to the amount of information received about general and specific medical matters.[10] The notepad is a common tool for reminders and personal interactions that is used in everyday life, but has not been formalized in the hospital. We introduced Dear Doctor (DD) notes, a bedside notepad designed to prompt patient questions, with the goal of facilitating patient communication with their hospitalist physicians (Figure 1). As hospitalists provide direct and indirect care to a growing number of hospitalized patients, they are likely to be asked questions and opinions about the patients' diagnoses and plans. Furthermore, hospitalists are poised to lead institutional quality, safety, efficiency, and service improvement efforts in the inpatient setting. Becoming familiar with communication‐enhancing tools, such as the DD notes, may help hospitalists in their improvement team roles.

METHODS
Setting
We conducted a study between July 2009 and September 2009 on inpatient medical wards at a large academic medical center with 610 beds and over 44,000 annual discharges.[11] The internal medicine services served by attending physicians and residents comprise a large proportion of hospitalized patients, accounting for over 17,000 discharges per year. Each medical unit includes 32 beds.
Population
Patients over the age of 18 years admitted to a general medicine or cardiology unit and who were able to verbally communicate in English were eligible to be surveyed in the study. Patients with a length of stay <24 hours were excluded. A total of 664 patients were surveyed for inclusion in the study, 440 patients in the intervention group and 224 patients in the control group.
Intervention
The DD notepad included sample questions and informational prompts derived with input from a community focus group. The community focus group consisted of current and formerly hospitalized patients and family members who were asked by members of the study group what they thought would be important to include on a notepad provided to patients. From their answers the study team developed the DD notepad prototype. The DD notepad included 3 general categories of questions: (1) diagnosis and treatment, (2) tests and procedures, and (3) medications. To address other miscellaneous topics such as discharge and posthospital care needs, a section was designated for the patient to check off as I have a few more questions (Use the back of the sheet).
All patients admitted to the study units were intended to receive the DD notepad and pen, which were placed on the bedside table during the room change by our custodial staff. Patients who did not receive DD notepads in the intervention group during their first hospitalization day were provided with 1 by the clinical assistants working with the hospitalists. These patients did not initially receive the notepad due to logistical reasons from temporary rotating staff who were not instructed to provide the notepads. Patients were not formally prompted to use the notepad. Hospitalists, residents, and nurses on the study units were informed about the distribution of DD notes to patients on these units; however, they were not provided with any specific instructions on how they should incorporate the DD notes into their interactions with their patients. The use of the DD notepad was left to each healthcare professional's own discretion.
Members of the study team surveyed patients who had been in the hospital for a minimum of 24 hours in the intervention and control groups twice weekly. All responses were deidentified of any personal or health information. Patients were asked to rate on a scale from 1 to 5 their use of the DD notepads, their perceived value, the circumstances in which the notepads were used, and their level of satisfaction with how their physicians communicated and answered their questions (1 = no improvement, 5 = significant improvement). For control patients, questions pertaining to DD notepads were not applicable and were therefore excluded.
Statistical Analysis
The data were analyzed in an intention‐to‐treat analysis of all 440 patients in the intervention group. Intervention and control groups were compared using 2, rank sum, and Fisher exact statistical tests, with significance assigned as P < 0.05, using SPSS software version 17.0 (SPSS, Inc., Chicago, IL). Our project was approved by the University of Michigan's institutional review board.
RESULTS
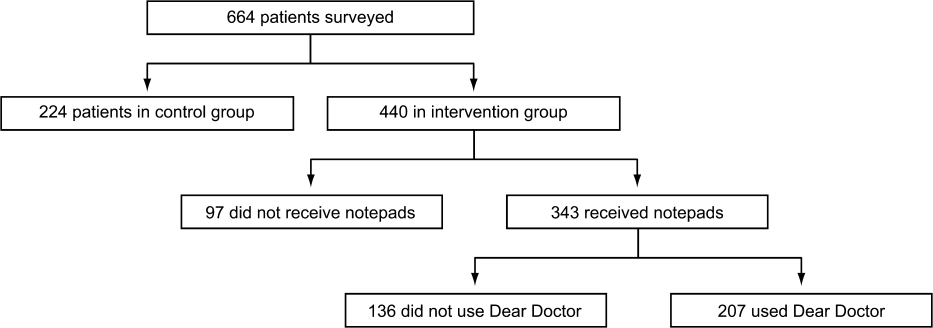
Of the 440 patients surveyed in the intervention group (1 general medicine and 1 cardiology unit), 343 (78%) received the notepads in their rooms and 207 (47%) used them (Figure 2). Not every patient in the intervention group received DD notepads due to inconsistent placement of DD notepads upon every room turnover. Of the patients admitted to the control group (1 general medicine and 1 cardiology unit), 224 were surveyed. Fifty‐four percent of the 440 patients in the intervention group reported that they took notes related to their hospital care, compared to only 22% of the 224 patients in the control group (P < 0.001). Of the patients who took notes within the intervention group (n = 207), 91% of them utilized the DD notepads.
Patients in the intervention group who received and used the DD notes (n = 207) compared to patients in the control group (n = 224) were more likely to report that their questions were answered by their physicians (4.63 vs 4.45, P < 0.001). In an intention‐to‐treat analysis of all 440 patients in the intervention group, the overall satisfaction with physician communication was not significantly different between the intervention and control groups as measured on a 5‐point Likert scale (4.55 vs 4.55, P = 0.89). However, 89% of the patients in the intervention group who used the notepads felt that DD notepads either moderately or significantly improved their communication with their providers (Figure 3).

When the 207 patients who received DD notepads were asked how they used this tool, 99% of these patients used DD to write down questions, 82% to keep track of tests and procedures, and 54% indicated that their family and friends also used the notepads during the hospital stay (Table 1). Among these patients who utilized the DD notepads, 93% reported that they would use them again in the future.
| Wrote Notes? (P < 0.001) (%) | Used DD? (%) | Use in Future? (%) | Frequency of Questions Answered (P < 0.001) | DD Improved Communication | Satisfied With Communication? (P = 0.89) | |
|---|---|---|---|---|---|---|
| ||||||
| Intervention (n = 440) | 54 | 91 | 93.2 | 4.63 | 3.76 | 4.55 |
| Control (n = 224) | 22 | 4.45 | 4.55 | |||
Of the 97 patients in the intervention group who did not receive a DD notepad, we asked if they would use the DD notepad if they were made aware of such a tool. Of these patients, 77% agreed that they would use DD notes if they were made available in the future, 100% of them said that they would use DD to write down questions, 97% indicated that they would write notes about tests and procedures, and 88% of them believed that their families and friends would use DD notes.
DISCUSSION
As hospitals place greater emphasis and value on patient‐centered care as part of their clinical mission, it is important to develop tools to help facilitate the doctor‐patient relationship. We found that patients who were provided the Dear Doctor notepad were more satisfied that their doctors answered their questions and felt this tool enhanced their ability to communicate with their physicians. Employing the use of a familiar tool such as the notepad to remind patients about specific issues in their interactions with their providers can be a powerful intervention. Our study demonstrated that the DD notepad was widely accepted by patients, and that almost all of them would use this tool if it were made available to them in the future.
Other tools and methods to enhance the quality of communication between patients and their healthcare providers have included using whiteboards in the patients' rooms to relay the care plan to the patients, implementing bedside rounds by the healthcare team, and multidisciplinary huddling to coordinate information to the patient.[12, 13] Studies of these communication tools have shown potential to improve teamwork, interaction, and patient care. All of these have their own merit and value, and our DD notepad should be considered an adjunct to existing methods to enhance the patient care experience. A bedside tool that is familiar in form to most patients also needs to have the feature of easy access and use. Once this barrier has been removed for the patient and their family members, tools such as the DD notepad can impact the patient‐centeredness by fostering increased and better quality dialogue between the patients, their family members, and healthcare providers.
The DD notepad represents a means of communication that may have the potential to empower patients. It is possible that through question prompts, the DD notepad stimulates the patient to be an active partner with his or her healthcare team. This may enable patients to have some sense of control and accountability of their care in a setting where they would otherwise feel overwhelmed or powerless. The 3 general categories to help patients write down their questions included diagnosis, treatment plan, and medications. In the inpatient setting, where patient‐care activities can be fast paced, and patients are unable to recall some details when speaking to the healthcare team, these notes may remind the patient to write their thoughts down so that they may be remembered for a future time. In situations where patients may not know which questions to ask, the question prompts may be particularly helpful. We did not assess whether our particular question prompts were the key elements that resulted in their perceived value, or whether simply placing a blank notepad at the bedside would also have been successful. However, the specific questions were suggested by the focus groups. Enhanced communication, focusing on the patients' understanding of their condition, and the need to pursue certain diagnostic or therapeutic interventions, may help patients to be better prepared for the next course of plan. These topics of reasons for hospitalization, treatment plan, and medication changes are also important for patients to be active participants in their care, in particular as they transition from 1 site of care to the next, and their healthcare will be delivered by different providers.
There are several limitations to our study. First, this intervention was performed at a single hospital site with only 2 clinical services (general medicine, cardiology) represented in the study groups. Although we do not have any causal reasons to believe this tool would be looked upon differently by patients on other clinical services, it is possible that patients on a different clinical unit or service may view this tool as less or more useful. Second, as the patients were not randomized to intervention, but rather based on the units to which they were admitted, it is possible that other variables, such as the experience of the unit staff, the patient's condition, and housestaff‐based service versus hospitalist‐based service may have played a role in how the patients perceived the use of DD notes. Third, patients were only surveyed if they were able to verbally communicate in English. These notes may not be as useful in hospital settings to populations with language or literacy barriers. Fourth, the logistical implementation of DD notes limited our ability to deliver the DD notepads in every patient's rooms, where only 78% of the intervention group received the DD notepads. This may be the reason that we did not find that overall satisfaction with physician communication differed between intervention and control groups. Nonetheless, we performed an intention‐to‐treat analysis to minimize any biases in our analysis. Last, although our survey of patients asked about their satisfaction in using the DD note pads, we did not compare these results with those of Press‐Ganey or HCAHPS scores of patients on the intervention group versus the control group. Additionally, lack of data about type, quality, and quantity of questions asked by a control group to see if the notepads actually improved quality of questions asked is a limitation; however, we believe our outcomes of interest were most specifically evaluated through our survey questions.
DD notes show that the majority of patients who use this tool feel a modest to significant improvement in communication with their providers. Although the quality of medical care is undoubtedly the first priority, the patients' view of their care, which includes communication, is arguably just as important. An often‐forgotten goal of hospitals and clinics is to provide service excellence along with high‐quality care. Thus, it is imperative for hospitals and their care providers to not only focus on the quality and safety of the clinical care, but also be mindful of the patient's entire experience throughout their hospital stay. Many of the categories of questions asked in the HCAHPS address the patient's experience and perspectives of hospital care. Furthermore, the role of the HCAHPS survey in the Value‐Based Purchasing rules may enhance the importance of these notepads. As the results of HCAHPS are becoming more transparent and available to the public, the impact of such results will have a greater significance to the future of the hospital's clinical mission.
CONCLUSION
DD notepads are a simple, low‐cost, patient‐centered tool that can be an effective reminder for patients to ask their healthcare providers questions related to their hospital care. Utilizing a common tool such as the notepad, redesigned for the healthcare setting, can serve to help healthcare providers interact with their patients. Patient satisfaction may be higher in patients who use the DD notepad.
Disclosures: Aaron S. Farberg, MD, and Andrew M. Lin, MD, contributed equally in every way and should be considered co‐first authors. This work was supported by a University of Michigan Fostering Innovations Grant. The authors have no conflicting financial interests.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , , . An evidence base for patient‐centered cancer care: a meta‐analysis of studies of observed communication between cancer specialists and their patients. Patient Educ Couns. 2009;77(3):379–383.
- . Effective physician‐patient communication and health outcomes: a review. Can Med Assoc J. 1995;152:1423–1433.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359:1921–1931.
- Institute of Medicine of the National Academies.Best care at lower cost: the path to continuously learning health care in America. Available at: http://www.iom.edu/Reports/2012/Best‐Care‐at‐Lower‐Cost‐The‐Path‐to‐Continuously‐Learning‐Health‐Care‐in‐America.aspx. Accessed October 5, 2012.
- , . An introduction to technology for patient‐centered collaborative care. J Ambul Care Manage. 2006;29:195–198.
- , , , , , . Effect on health‐related outcome of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2(6):595–608
- , , , , . Characteristics of physicians with participatory decision‐making styles. Ann Intern Med. 1996;124(5):497–504.
- . Information‐giving consultations: the influence of patients' communicative styles and personal characteristics. Soc Sci Med. 1991:32(5):541–548.
- University of Michigan Health System.Patient care and University of Michigan Health System. Available at: http://www.uofmhealth.org/about%2Bumhs/about‐clinical‐care. Accessed August 31, 2012.
- , , , et al. It's the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual. 2011;26(2):127–131
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5(4):234–239.
In their seminal report Crossing the Quality Chasm, the Institute of Medicine outlined patient‐centered care as 1 of its 6 aims to improve the healthcare delivery system.[1] Patients who are more involved in their diagnosis and treatment plan are more likely to feel respected, be satisfied with their healthcare experience, and ultimately have better outcomes.[2, 3] In a study of hospitalized patients, only 42% were able to state their diagnosis at the time of discharge, suggesting that hospital providers could communicate better with patients about their hospital care.[4] Additionally, only 28% of hospitalized patients were able to list their medications, and only 37% were able to state the purpose of their medications. Although hospitals have taken great strides to improve the quality of patient care, publicly reported patient care surveys, such as the Hospital Consumer Assessment of Hospitals and Health Systems (HCAHPS), suggest that physician communication with patients could be further improved.[1, 5] Furthermore, a recent report by the Institute of Medicine stresses the need to get patients and families involved in their care.[6] Thus, hospital‐based providers should seek to enhance the quality of their communication with patients.
With greater emphasis placed on patient‐ and family‐centered care at many health systems, simple and easy‐to‐implement strategies to improve communication with patients need to be developed and tested.[7, 8] Patients who actively participate in their healthcare by asking questions of their doctor are able to control the focus of their interaction and adjust the amount of information provided.[9] Simply asking questions can have a critical impact, as 1 study found that the frequency with which patients asked questions was significantly related to the amount of information received about general and specific medical matters.[10] The notepad is a common tool for reminders and personal interactions that is used in everyday life, but has not been formalized in the hospital. We introduced Dear Doctor (DD) notes, a bedside notepad designed to prompt patient questions, with the goal of facilitating patient communication with their hospitalist physicians (Figure 1). As hospitalists provide direct and indirect care to a growing number of hospitalized patients, they are likely to be asked questions and opinions about the patients' diagnoses and plans. Furthermore, hospitalists are poised to lead institutional quality, safety, efficiency, and service improvement efforts in the inpatient setting. Becoming familiar with communication‐enhancing tools, such as the DD notes, may help hospitalists in their improvement team roles.

METHODS
Setting
We conducted a study between July 2009 and September 2009 on inpatient medical wards at a large academic medical center with 610 beds and over 44,000 annual discharges.[11] The internal medicine services served by attending physicians and residents comprise a large proportion of hospitalized patients, accounting for over 17,000 discharges per year. Each medical unit includes 32 beds.
Population
Patients over the age of 18 years admitted to a general medicine or cardiology unit and who were able to verbally communicate in English were eligible to be surveyed in the study. Patients with a length of stay <24 hours were excluded. A total of 664 patients were surveyed for inclusion in the study, 440 patients in the intervention group and 224 patients in the control group.
Intervention
The DD notepad included sample questions and informational prompts derived with input from a community focus group. The community focus group consisted of current and formerly hospitalized patients and family members who were asked by members of the study group what they thought would be important to include on a notepad provided to patients. From their answers the study team developed the DD notepad prototype. The DD notepad included 3 general categories of questions: (1) diagnosis and treatment, (2) tests and procedures, and (3) medications. To address other miscellaneous topics such as discharge and posthospital care needs, a section was designated for the patient to check off as I have a few more questions (Use the back of the sheet).
All patients admitted to the study units were intended to receive the DD notepad and pen, which were placed on the bedside table during the room change by our custodial staff. Patients who did not receive DD notepads in the intervention group during their first hospitalization day were provided with 1 by the clinical assistants working with the hospitalists. These patients did not initially receive the notepad due to logistical reasons from temporary rotating staff who were not instructed to provide the notepads. Patients were not formally prompted to use the notepad. Hospitalists, residents, and nurses on the study units were informed about the distribution of DD notes to patients on these units; however, they were not provided with any specific instructions on how they should incorporate the DD notes into their interactions with their patients. The use of the DD notepad was left to each healthcare professional's own discretion.
Members of the study team surveyed patients who had been in the hospital for a minimum of 24 hours in the intervention and control groups twice weekly. All responses were deidentified of any personal or health information. Patients were asked to rate on a scale from 1 to 5 their use of the DD notepads, their perceived value, the circumstances in which the notepads were used, and their level of satisfaction with how their physicians communicated and answered their questions (1 = no improvement, 5 = significant improvement). For control patients, questions pertaining to DD notepads were not applicable and were therefore excluded.
Statistical Analysis
The data were analyzed in an intention‐to‐treat analysis of all 440 patients in the intervention group. Intervention and control groups were compared using 2, rank sum, and Fisher exact statistical tests, with significance assigned as P < 0.05, using SPSS software version 17.0 (SPSS, Inc., Chicago, IL). Our project was approved by the University of Michigan's institutional review board.
RESULTS
Of the 440 patients surveyed in the intervention group (1 general medicine and 1 cardiology unit), 343 (78%) received the notepads in their rooms and 207 (47%) used them (Figure 2). Not every patient in the intervention group received DD notepads due to inconsistent placement of DD notepads upon every room turnover. Of the patients admitted to the control group (1 general medicine and 1 cardiology unit), 224 were surveyed. Fifty‐four percent of the 440 patients in the intervention group reported that they took notes related to their hospital care, compared to only 22% of the 224 patients in the control group (P < 0.001). Of the patients who took notes within the intervention group (n = 207), 91% of them utilized the DD notepads.

Patients in the intervention group who received and used the DD notes (n = 207) compared to patients in the control group (n = 224) were more likely to report that their questions were answered by their physicians (4.63 vs 4.45, P < 0.001). In an intention‐to‐treat analysis of all 440 patients in the intervention group, the overall satisfaction with physician communication was not significantly different between the intervention and control groups as measured on a 5‐point Likert scale (4.55 vs 4.55, P = 0.89). However, 89% of the patients in the intervention group who used the notepads felt that DD notepads either moderately or significantly improved their communication with their providers (Figure 3).

When the 207 patients who received DD notepads were asked how they used this tool, 99% of these patients used DD to write down questions, 82% to keep track of tests and procedures, and 54% indicated that their family and friends also used the notepads during the hospital stay (Table 1). Among these patients who utilized the DD notepads, 93% reported that they would use them again in the future.
| Wrote Notes? (P < 0.001) (%) | Used DD? (%) | Use in Future? (%) | Frequency of Questions Answered (P < 0.001) | DD Improved Communication | Satisfied With Communication? (P = 0.89) | |
|---|---|---|---|---|---|---|
| ||||||
| Intervention (n = 440) | 54 | 91 | 93.2 | 4.63 | 3.76 | 4.55 |
| Control (n = 224) | 22 | 4.45 | 4.55 | |||
Of the 97 patients in the intervention group who did not receive a DD notepad, we asked if they would use the DD notepad if they were made aware of such a tool. Of these patients, 77% agreed that they would use DD notes if they were made available in the future, 100% of them said that they would use DD to write down questions, 97% indicated that they would write notes about tests and procedures, and 88% of them believed that their families and friends would use DD notes.
DISCUSSION
As hospitals place greater emphasis and value on patient‐centered care as part of their clinical mission, it is important to develop tools to help facilitate the doctor‐patient relationship. We found that patients who were provided the Dear Doctor notepad were more satisfied that their doctors answered their questions and felt this tool enhanced their ability to communicate with their physicians. Employing the use of a familiar tool such as the notepad to remind patients about specific issues in their interactions with their providers can be a powerful intervention. Our study demonstrated that the DD notepad was widely accepted by patients, and that almost all of them would use this tool if it were made available to them in the future.
Other tools and methods to enhance the quality of communication between patients and their healthcare providers have included using whiteboards in the patients' rooms to relay the care plan to the patients, implementing bedside rounds by the healthcare team, and multidisciplinary huddling to coordinate information to the patient.[12, 13] Studies of these communication tools have shown potential to improve teamwork, interaction, and patient care. All of these have their own merit and value, and our DD notepad should be considered an adjunct to existing methods to enhance the patient care experience. A bedside tool that is familiar in form to most patients also needs to have the feature of easy access and use. Once this barrier has been removed for the patient and their family members, tools such as the DD notepad can impact the patient‐centeredness by fostering increased and better quality dialogue between the patients, their family members, and healthcare providers.
The DD notepad represents a means of communication that may have the potential to empower patients. It is possible that through question prompts, the DD notepad stimulates the patient to be an active partner with his or her healthcare team. This may enable patients to have some sense of control and accountability of their care in a setting where they would otherwise feel overwhelmed or powerless. The 3 general categories to help patients write down their questions included diagnosis, treatment plan, and medications. In the inpatient setting, where patient‐care activities can be fast paced, and patients are unable to recall some details when speaking to the healthcare team, these notes may remind the patient to write their thoughts down so that they may be remembered for a future time. In situations where patients may not know which questions to ask, the question prompts may be particularly helpful. We did not assess whether our particular question prompts were the key elements that resulted in their perceived value, or whether simply placing a blank notepad at the bedside would also have been successful. However, the specific questions were suggested by the focus groups. Enhanced communication, focusing on the patients' understanding of their condition, and the need to pursue certain diagnostic or therapeutic interventions, may help patients to be better prepared for the next course of plan. These topics of reasons for hospitalization, treatment plan, and medication changes are also important for patients to be active participants in their care, in particular as they transition from 1 site of care to the next, and their healthcare will be delivered by different providers.
There are several limitations to our study. First, this intervention was performed at a single hospital site with only 2 clinical services (general medicine, cardiology) represented in the study groups. Although we do not have any causal reasons to believe this tool would be looked upon differently by patients on other clinical services, it is possible that patients on a different clinical unit or service may view this tool as less or more useful. Second, as the patients were not randomized to intervention, but rather based on the units to which they were admitted, it is possible that other variables, such as the experience of the unit staff, the patient's condition, and housestaff‐based service versus hospitalist‐based service may have played a role in how the patients perceived the use of DD notes. Third, patients were only surveyed if they were able to verbally communicate in English. These notes may not be as useful in hospital settings to populations with language or literacy barriers. Fourth, the logistical implementation of DD notes limited our ability to deliver the DD notepads in every patient's rooms, where only 78% of the intervention group received the DD notepads. This may be the reason that we did not find that overall satisfaction with physician communication differed between intervention and control groups. Nonetheless, we performed an intention‐to‐treat analysis to minimize any biases in our analysis. Last, although our survey of patients asked about their satisfaction in using the DD note pads, we did not compare these results with those of Press‐Ganey or HCAHPS scores of patients on the intervention group versus the control group. Additionally, lack of data about type, quality, and quantity of questions asked by a control group to see if the notepads actually improved quality of questions asked is a limitation; however, we believe our outcomes of interest were most specifically evaluated through our survey questions.
DD notes show that the majority of patients who use this tool feel a modest to significant improvement in communication with their providers. Although the quality of medical care is undoubtedly the first priority, the patients' view of their care, which includes communication, is arguably just as important. An often‐forgotten goal of hospitals and clinics is to provide service excellence along with high‐quality care. Thus, it is imperative for hospitals and their care providers to not only focus on the quality and safety of the clinical care, but also be mindful of the patient's entire experience throughout their hospital stay. Many of the categories of questions asked in the HCAHPS address the patient's experience and perspectives of hospital care. Furthermore, the role of the HCAHPS survey in the Value‐Based Purchasing rules may enhance the importance of these notepads. As the results of HCAHPS are becoming more transparent and available to the public, the impact of such results will have a greater significance to the future of the hospital's clinical mission.
CONCLUSION
DD notepads are a simple, low‐cost, patient‐centered tool that can be an effective reminder for patients to ask their healthcare providers questions related to their hospital care. Utilizing a common tool such as the notepad, redesigned for the healthcare setting, can serve to help healthcare providers interact with their patients. Patient satisfaction may be higher in patients who use the DD notepad.
Disclosures: Aaron S. Farberg, MD, and Andrew M. Lin, MD, contributed equally in every way and should be considered co‐first authors. This work was supported by a University of Michigan Fostering Innovations Grant. The authors have no conflicting financial interests.
In their seminal report Crossing the Quality Chasm, the Institute of Medicine outlined patient‐centered care as 1 of its 6 aims to improve the healthcare delivery system.[1] Patients who are more involved in their diagnosis and treatment plan are more likely to feel respected, be satisfied with their healthcare experience, and ultimately have better outcomes.[2, 3] In a study of hospitalized patients, only 42% were able to state their diagnosis at the time of discharge, suggesting that hospital providers could communicate better with patients about their hospital care.[4] Additionally, only 28% of hospitalized patients were able to list their medications, and only 37% were able to state the purpose of their medications. Although hospitals have taken great strides to improve the quality of patient care, publicly reported patient care surveys, such as the Hospital Consumer Assessment of Hospitals and Health Systems (HCAHPS), suggest that physician communication with patients could be further improved.[1, 5] Furthermore, a recent report by the Institute of Medicine stresses the need to get patients and families involved in their care.[6] Thus, hospital‐based providers should seek to enhance the quality of their communication with patients.
With greater emphasis placed on patient‐ and family‐centered care at many health systems, simple and easy‐to‐implement strategies to improve communication with patients need to be developed and tested.[7, 8] Patients who actively participate in their healthcare by asking questions of their doctor are able to control the focus of their interaction and adjust the amount of information provided.[9] Simply asking questions can have a critical impact, as 1 study found that the frequency with which patients asked questions was significantly related to the amount of information received about general and specific medical matters.[10] The notepad is a common tool for reminders and personal interactions that is used in everyday life, but has not been formalized in the hospital. We introduced Dear Doctor (DD) notes, a bedside notepad designed to prompt patient questions, with the goal of facilitating patient communication with their hospitalist physicians (Figure 1). As hospitalists provide direct and indirect care to a growing number of hospitalized patients, they are likely to be asked questions and opinions about the patients' diagnoses and plans. Furthermore, hospitalists are poised to lead institutional quality, safety, efficiency, and service improvement efforts in the inpatient setting. Becoming familiar with communication‐enhancing tools, such as the DD notes, may help hospitalists in their improvement team roles.

METHODS
Setting
We conducted a study between July 2009 and September 2009 on inpatient medical wards at a large academic medical center with 610 beds and over 44,000 annual discharges.[11] The internal medicine services served by attending physicians and residents comprise a large proportion of hospitalized patients, accounting for over 17,000 discharges per year. Each medical unit includes 32 beds.
Population
Patients over the age of 18 years admitted to a general medicine or cardiology unit and who were able to verbally communicate in English were eligible to be surveyed in the study. Patients with a length of stay <24 hours were excluded. A total of 664 patients were surveyed for inclusion in the study, 440 patients in the intervention group and 224 patients in the control group.
Intervention
The DD notepad included sample questions and informational prompts derived with input from a community focus group. The community focus group consisted of current and formerly hospitalized patients and family members who were asked by members of the study group what they thought would be important to include on a notepad provided to patients. From their answers the study team developed the DD notepad prototype. The DD notepad included 3 general categories of questions: (1) diagnosis and treatment, (2) tests and procedures, and (3) medications. To address other miscellaneous topics such as discharge and posthospital care needs, a section was designated for the patient to check off as I have a few more questions (Use the back of the sheet).
All patients admitted to the study units were intended to receive the DD notepad and pen, which were placed on the bedside table during the room change by our custodial staff. Patients who did not receive DD notepads in the intervention group during their first hospitalization day were provided with 1 by the clinical assistants working with the hospitalists. These patients did not initially receive the notepad due to logistical reasons from temporary rotating staff who were not instructed to provide the notepads. Patients were not formally prompted to use the notepad. Hospitalists, residents, and nurses on the study units were informed about the distribution of DD notes to patients on these units; however, they were not provided with any specific instructions on how they should incorporate the DD notes into their interactions with their patients. The use of the DD notepad was left to each healthcare professional's own discretion.
Members of the study team surveyed patients who had been in the hospital for a minimum of 24 hours in the intervention and control groups twice weekly. All responses were deidentified of any personal or health information. Patients were asked to rate on a scale from 1 to 5 their use of the DD notepads, their perceived value, the circumstances in which the notepads were used, and their level of satisfaction with how their physicians communicated and answered their questions (1 = no improvement, 5 = significant improvement). For control patients, questions pertaining to DD notepads were not applicable and were therefore excluded.
Statistical Analysis
The data were analyzed in an intention‐to‐treat analysis of all 440 patients in the intervention group. Intervention and control groups were compared using 2, rank sum, and Fisher exact statistical tests, with significance assigned as P < 0.05, using SPSS software version 17.0 (SPSS, Inc., Chicago, IL). Our project was approved by the University of Michigan's institutional review board.
RESULTS
Of the 440 patients surveyed in the intervention group (1 general medicine and 1 cardiology unit), 343 (78%) received the notepads in their rooms and 207 (47%) used them (Figure 2). Not every patient in the intervention group received DD notepads due to inconsistent placement of DD notepads upon every room turnover. Of the patients admitted to the control group (1 general medicine and 1 cardiology unit), 224 were surveyed. Fifty‐four percent of the 440 patients in the intervention group reported that they took notes related to their hospital care, compared to only 22% of the 224 patients in the control group (P < 0.001). Of the patients who took notes within the intervention group (n = 207), 91% of them utilized the DD notepads.

Patients in the intervention group who received and used the DD notes (n = 207) compared to patients in the control group (n = 224) were more likely to report that their questions were answered by their physicians (4.63 vs 4.45, P < 0.001). In an intention‐to‐treat analysis of all 440 patients in the intervention group, the overall satisfaction with physician communication was not significantly different between the intervention and control groups as measured on a 5‐point Likert scale (4.55 vs 4.55, P = 0.89). However, 89% of the patients in the intervention group who used the notepads felt that DD notepads either moderately or significantly improved their communication with their providers (Figure 3).

When the 207 patients who received DD notepads were asked how they used this tool, 99% of these patients used DD to write down questions, 82% to keep track of tests and procedures, and 54% indicated that their family and friends also used the notepads during the hospital stay (Table 1). Among these patients who utilized the DD notepads, 93% reported that they would use them again in the future.
| Wrote Notes? (P < 0.001) (%) | Used DD? (%) | Use in Future? (%) | Frequency of Questions Answered (P < 0.001) | DD Improved Communication | Satisfied With Communication? (P = 0.89) | |
|---|---|---|---|---|---|---|
| ||||||
| Intervention (n = 440) | 54 | 91 | 93.2 | 4.63 | 3.76 | 4.55 |
| Control (n = 224) | 22 | 4.45 | 4.55 | |||
Of the 97 patients in the intervention group who did not receive a DD notepad, we asked if they would use the DD notepad if they were made aware of such a tool. Of these patients, 77% agreed that they would use DD notes if they were made available in the future, 100% of them said that they would use DD to write down questions, 97% indicated that they would write notes about tests and procedures, and 88% of them believed that their families and friends would use DD notes.
DISCUSSION
As hospitals place greater emphasis and value on patient‐centered care as part of their clinical mission, it is important to develop tools to help facilitate the doctor‐patient relationship. We found that patients who were provided the Dear Doctor notepad were more satisfied that their doctors answered their questions and felt this tool enhanced their ability to communicate with their physicians. Employing the use of a familiar tool such as the notepad to remind patients about specific issues in their interactions with their providers can be a powerful intervention. Our study demonstrated that the DD notepad was widely accepted by patients, and that almost all of them would use this tool if it were made available to them in the future.
Other tools and methods to enhance the quality of communication between patients and their healthcare providers have included using whiteboards in the patients' rooms to relay the care plan to the patients, implementing bedside rounds by the healthcare team, and multidisciplinary huddling to coordinate information to the patient.[12, 13] Studies of these communication tools have shown potential to improve teamwork, interaction, and patient care. All of these have their own merit and value, and our DD notepad should be considered an adjunct to existing methods to enhance the patient care experience. A bedside tool that is familiar in form to most patients also needs to have the feature of easy access and use. Once this barrier has been removed for the patient and their family members, tools such as the DD notepad can impact the patient‐centeredness by fostering increased and better quality dialogue between the patients, their family members, and healthcare providers.
The DD notepad represents a means of communication that may have the potential to empower patients. It is possible that through question prompts, the DD notepad stimulates the patient to be an active partner with his or her healthcare team. This may enable patients to have some sense of control and accountability of their care in a setting where they would otherwise feel overwhelmed or powerless. The 3 general categories to help patients write down their questions included diagnosis, treatment plan, and medications. In the inpatient setting, where patient‐care activities can be fast paced, and patients are unable to recall some details when speaking to the healthcare team, these notes may remind the patient to write their thoughts down so that they may be remembered for a future time. In situations where patients may not know which questions to ask, the question prompts may be particularly helpful. We did not assess whether our particular question prompts were the key elements that resulted in their perceived value, or whether simply placing a blank notepad at the bedside would also have been successful. However, the specific questions were suggested by the focus groups. Enhanced communication, focusing on the patients' understanding of their condition, and the need to pursue certain diagnostic or therapeutic interventions, may help patients to be better prepared for the next course of plan. These topics of reasons for hospitalization, treatment plan, and medication changes are also important for patients to be active participants in their care, in particular as they transition from 1 site of care to the next, and their healthcare will be delivered by different providers.
There are several limitations to our study. First, this intervention was performed at a single hospital site with only 2 clinical services (general medicine, cardiology) represented in the study groups. Although we do not have any causal reasons to believe this tool would be looked upon differently by patients on other clinical services, it is possible that patients on a different clinical unit or service may view this tool as less or more useful. Second, as the patients were not randomized to intervention, but rather based on the units to which they were admitted, it is possible that other variables, such as the experience of the unit staff, the patient's condition, and housestaff‐based service versus hospitalist‐based service may have played a role in how the patients perceived the use of DD notes. Third, patients were only surveyed if they were able to verbally communicate in English. These notes may not be as useful in hospital settings to populations with language or literacy barriers. Fourth, the logistical implementation of DD notes limited our ability to deliver the DD notepads in every patient's rooms, where only 78% of the intervention group received the DD notepads. This may be the reason that we did not find that overall satisfaction with physician communication differed between intervention and control groups. Nonetheless, we performed an intention‐to‐treat analysis to minimize any biases in our analysis. Last, although our survey of patients asked about their satisfaction in using the DD note pads, we did not compare these results with those of Press‐Ganey or HCAHPS scores of patients on the intervention group versus the control group. Additionally, lack of data about type, quality, and quantity of questions asked by a control group to see if the notepads actually improved quality of questions asked is a limitation; however, we believe our outcomes of interest were most specifically evaluated through our survey questions.
DD notes show that the majority of patients who use this tool feel a modest to significant improvement in communication with their providers. Although the quality of medical care is undoubtedly the first priority, the patients' view of their care, which includes communication, is arguably just as important. An often‐forgotten goal of hospitals and clinics is to provide service excellence along with high‐quality care. Thus, it is imperative for hospitals and their care providers to not only focus on the quality and safety of the clinical care, but also be mindful of the patient's entire experience throughout their hospital stay. Many of the categories of questions asked in the HCAHPS address the patient's experience and perspectives of hospital care. Furthermore, the role of the HCAHPS survey in the Value‐Based Purchasing rules may enhance the importance of these notepads. As the results of HCAHPS are becoming more transparent and available to the public, the impact of such results will have a greater significance to the future of the hospital's clinical mission.
CONCLUSION
DD notepads are a simple, low‐cost, patient‐centered tool that can be an effective reminder for patients to ask their healthcare providers questions related to their hospital care. Utilizing a common tool such as the notepad, redesigned for the healthcare setting, can serve to help healthcare providers interact with their patients. Patient satisfaction may be higher in patients who use the DD notepad.
Disclosures: Aaron S. Farberg, MD, and Andrew M. Lin, MD, contributed equally in every way and should be considered co‐first authors. This work was supported by a University of Michigan Fostering Innovations Grant. The authors have no conflicting financial interests.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , , . An evidence base for patient‐centered cancer care: a meta‐analysis of studies of observed communication between cancer specialists and their patients. Patient Educ Couns. 2009;77(3):379–383.
- . Effective physician‐patient communication and health outcomes: a review. Can Med Assoc J. 1995;152:1423–1433.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359:1921–1931.
- Institute of Medicine of the National Academies.Best care at lower cost: the path to continuously learning health care in America. Available at: http://www.iom.edu/Reports/2012/Best‐Care‐at‐Lower‐Cost‐The‐Path‐to‐Continuously‐Learning‐Health‐Care‐in‐America.aspx. Accessed October 5, 2012.
- , . An introduction to technology for patient‐centered collaborative care. J Ambul Care Manage. 2006;29:195–198.
- , , , , , . Effect on health‐related outcome of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2(6):595–608
- , , , , . Characteristics of physicians with participatory decision‐making styles. Ann Intern Med. 1996;124(5):497–504.
- . Information‐giving consultations: the influence of patients' communicative styles and personal characteristics. Soc Sci Med. 1991:32(5):541–548.
- University of Michigan Health System.Patient care and University of Michigan Health System. Available at: http://www.uofmhealth.org/about%2Bumhs/about‐clinical‐care. Accessed August 31, 2012.
- , , , et al. It's the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual. 2011;26(2):127–131
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5(4):234–239.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , , . An evidence base for patient‐centered cancer care: a meta‐analysis of studies of observed communication between cancer specialists and their patients. Patient Educ Couns. 2009;77(3):379–383.
- . Effective physician‐patient communication and health outcomes: a review. Can Med Assoc J. 1995;152:1423–1433.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359:1921–1931.
- Institute of Medicine of the National Academies.Best care at lower cost: the path to continuously learning health care in America. Available at: http://www.iom.edu/Reports/2012/Best‐Care‐at‐Lower‐Cost‐The‐Path‐to‐Continuously‐Learning‐Health‐Care‐in‐America.aspx. Accessed October 5, 2012.
- , . An introduction to technology for patient‐centered collaborative care. J Ambul Care Manage. 2006;29:195–198.
- , , , , , . Effect on health‐related outcome of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2(6):595–608
- , , , , . Characteristics of physicians with participatory decision‐making styles. Ann Intern Med. 1996;124(5):497–504.
- . Information‐giving consultations: the influence of patients' communicative styles and personal characteristics. Soc Sci Med. 1991:32(5):541–548.
- University of Michigan Health System.Patient care and University of Michigan Health System. Available at: http://www.uofmhealth.org/about%2Bumhs/about‐clinical‐care. Accessed August 31, 2012.
- , , , et al. It's the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual. 2011;26(2):127–131
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5(4):234–239.
© 2013 Society of Hospital Medicine
VTE Prevention Guidelines for Inpatients
Patients hospitalized for acute medical illness have more than a 10‐fold increased risk for venous thromboembolism (VTE),[1] with an undeniably dramatic, negative impact on the lives of those afflicted, including fatal pulmonary embolism (PE), which most commonly affects patients on the medical service.[2, 3, 4] Yet estimates for the overall rate of VTE in this population are relatively low, raising questions about which subsets of medical patients warrant the risk and cost of prophylaxis.
Recently, the American College of Physicians published guidelines (ACP‐1)[5] and a supporting review[6] addressing VTE prophylaxis in nonsurgical inpatients, followed by publication of the American College of Chest Physicians (ACCP) 9th Edition of the Chest Guidelines on Antithrombotic Therapy and Prevention of Thrombosis (AT9),[7] which divides VTE prevention into 3 articles,[8, 9, 10] including 1 on nonsurgical patients.[8] Both ACP‐1 and AT9 differ significantly from the 2008 ACCP guidelines (AT8),[11] but took different approaches to methodology, risk assessment, and several other aspects of thromboprophylaxis (Table 1). This narrative review summarizes and compares these recommendations and the methods used to arrive at them, with a final section focusing on implications for improvement teams designing order sets and system changes to address VTE prophylaxis.
| 2008 ACCP VTE Guideline AT8 | 2012 ACCP VTE Guideline AT9 | 2011 ACP Guideline | |
|---|---|---|---|
| |||
| Stance on asymptomatic VTE end points | Because of the strong concordance between asymptomatic DVT and clinically important VTE, we believe that DVT detected by a sensitive screening tesis an appropriate outcome in the early assessment of new thromboprophylaxis interventions. | Use of this surrogate (asymptomatic, screening‐detected thrombosis) creates major problems in making the trade‐off between patient‐important outcomes (thrombosis and serious bleeding). | Surrogate outcomes of asymptomatic screening detected‐thrombosis should not be used. |
| Who should be prophylaxed? | 6.0.0: For acutely ill medical patients admitted to hospital with congestive heart failure or severe respiratory disease, or who are confined to bed and have one or more additional risk factors, including active cancer, previous VTE, sepsis, neurologic disease, or inflammatory bowel disease, we recommend thromboprophylaxis with LMWH (1A), UFH (1A), or fondaparinux (1A). | 2.3: For acutely ill hospitalized medical patients at increased risk for thrombosis, we recommend anticoagulant thromboprophylaxis, with LMWH, UFH bid, UFH tid, or fondaparinux (1B). | ACP recommends pharmacologic prophylaxis with heparin or a related drug for venous thromboembolism in medical (including stroke) patients unless the assessed risk for bleeding outweighs the likely benefits (grade: strong recommendation, moderate‐quality evidence). |
| 2.4: For acutely ill hospitalized medical patients at low risk of thrombosis, we recommend against the use of pharmacologic or mechanical prophylaxis. (1B) | |||
| Choice of anticoagulant prophylaxis | There is no compelling evidence that UFH should be administered three times daily in preference to twice daily in medical patients, although these two regimens have never been directly compared. | In choosing the specific anticoagulant drug to be used for pharmacoprophylaxis, choices should be based on patient preference, compliance, and ease of administration (eg, daily vs bid vs tid dosing), as well as on local factors affecting acquisition costs. | [T]he choice of agent for prophylaxis of VTE should be based on ease of use, adverse effect profile, and cost of medication. |
| No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | |
| Mechanical prophylaxis | 1.4.3.1: We recommend that mechanical methods of prophylaxis be used primarily in patients at high risk of bleeding (grade 1A), or possibly as an adjunct to anticoagulant‐based thromboprophylaxis (grade 2A). | 2.7.2: For acutely ill hospitalized medical patients at increased risk of thrombosis who are bleeding or at high risk for major bleeding, we suggest the optimal use of mechanical thromboprophylaxis with GCS (grade 2C) or IPC (grade 2C). | ACP recommends against the use of mechanical prophylaxis with graduated compression stockings for prevention of venous thromboembolism (grade: strong recommendation, moderate‐quality evidence). |
| No strong evidence for IPC vs GCS | |||
| Duration | [T]he optimal duration of thromboprophylaxis remains unclear. To the end of hospitalization for most patients. | 2.8: [W]e suggest against extending the duration of thromboprophylaxis beyond the period of patient immobilization or acute hospital stay (2B). | The optimal duration of heparin prophylaxis is uncertain. |
| Risk Stratification | The approach of individual prophylaxis prescribing based on formal RAMs is not used routinely by most clinicians because it has not been adequately validated and is cumbersome. Individual RAMs may not be worth the effort, because there are only a limited number of thromboprophylaxis options, and one of the principles of effective thromboprophylaxis is to reduce complexity in decision making. | (Noncritical care) No formal risk assessment recommendation. Padua point‐based model is inherent in definitions of baseline VTE risk. | ACP does not support the application of performance measures in medical (including stroke) patients that promotes universal venous thromboembolism prophylaxis regardless of risk. |
| Another approach involves implementation of group‐specific thromboprophylaxis routinely for all patients who belong to each of the major target groups. We support this approach. | There are no validated risk assessment models to stratify VTE risk in critically ill patients. | Many risk assessment tools are available for estimating thromboembolism risk, but the current evidence is insufficient to recommend a validated tool. | |
| [T]he decision is best left to physician judgment, and performance measures targeting all patients are inappropriate. | |||
WHY ARE THE NEW GUIDELINES DIFFERENT?
Major randomized controlled trials (RCTs)[12, 13, 14] of thromboprophylaxis used routine deep vein thrombosis (DVT) surveillance and included both symptomatic (S‐VTE) and asymptomatic VTE (A‐VTE) end points. These studies consistently demonstrated 44% to 63% reductions in VTE without increases in major bleeding.[11] Because of the strong relationship between A‐VTE and S‐VTE outcomes, and a paucity of studies using only S‐VTE outcomes, AT8 judged that A‐VTE outcomes were valid to include, whereas the new guidelines reject the use of asymptomatic VTE end points.[5, 8, 15] To minimize financial and intellectual conflicts of interest, AT9 also used methodologists rather than VTE experts as topic editors, excluded conflicted experts from voting on recommendations, and attempted to estimate patient values and preferences.[15] As a result, AT9 makes fewer strong recommendations (182 1A recommendations in 2008, but only 29 in 2012), replacing them with weak suggestions.
WHAT DO THE NEW GUIDELINES RECOMMEND?
AT8 recommended anticoagulant prophylaxis for acutely ill medical inpatients with known risk factors, but did not recommend routine thromboprophylaxis. However, because of well‐known problems with underprophylaxis,[16, 17, 18, 19] particularly in medical patients, the low risk of bleeding, and difficulties with explicitly defining low‐risk patients, many discounted the need for VTE risk stratification.
Both new guidelines recommend prophylaxis for many nonsurgical patients, but discourage routine thromboprophylaxis for nonsurgical inpatients. AT9 specifically recommends against any thromboprophylaxis for low‐risk medical inpatients, implying that many nonsurgical, non‐critical care patients belong in this category, citing lower estimates of benefit, lower estimates of VTE risk, and potential bleeding risks.
The guidelines[5, 8] agree that, when indicated and absent contraindications, anticoagulant prophylaxis is preferred over mechanical prophylaxis, and agree there is insufficient evidence to recommend 1 anticoagulant over another.
For patients at risk of both VTE and bleeding, ACP‐1 states that intermittent pneumatic compression (IPC) devices are a reasonable option, given the evidence showing benefit in surgical patients. However, ACP‐1 recommends against graduated compression stockings (GCS) in nonsurgical patients based on a meta‐analysis dominated by the CLOTS‐1 (Clots in Legs Or sTockings after Stroke) trial, which found that thigh‐high GCS increased the risk of skin breakdown without reducing VTE[20] in immobilized stroke patients. AT9 does not recommend against GCS for patients facing bleeding and VTE risk. AT9 notes the hazards of generalizing results from stroke patients, and also considers the somewhat contradictory results from the CLOTS‐2 trial in stroke patients, which found a lower rate of VTE with thigh‐high GCS than with knee‐high GCS.[21] AT9 designates a recommendation of 2C for either IPC devices or thigh‐high GCS for those at VTE risk when anticoagulants are contraindicated.
Combination mechanical‐pharmacologic prophylaxis has proven superior in some surgical populations, and many hospitals use combined prophylaxis in high‐risk medical patients. However, combination prophylaxis has not been studied in this population. ACP‐1 does not comment on the practice; AT9 does not recommend for or against it. Institutions that use combination prophylaxis should be aware that although it may seem logical to extrapolate estimates of benefit seen in selected surgical patients, this is not a recommended practice.
RCTs for thromboprophylaxis in nonsurgical inpatients provided prophylaxis for 6 to 21 days. Neither ACP‐1 or AT9 recommend routinely extending prophylaxis beyond the hospital stay, citing an RCT[22] in which the benefit of extended duration low molecular weight heparin was limited to selected subsets of patients and offset by bleeding complications. AT9 suggests prophylaxis for 6 to 21 days, until full mobility is restored, or until dischargewhichever comes first.[8] However, we know of no study that establishes a mobility level at which prophylaxis can be safely discontinued, especially in inpatients with multiple risk factors.
ESTIMATING RISK AND BENEFIT OF PROPHYLAXIS AND LIMITATIONS OF METHODS
Calculating risk/benefit ratios for thromboprophylaxis requires estimates of baseline VTE and bleeding risks, and estimates of the impact of prophylaxis on those baseline risks. Methods to estimate the impact of prophylaxis on S‐VTE from studies relying on A‐VTE all have limitations, as acknowledged by the AT9 introduction.[15]
The ACP‐1 review found the only significant effect of prophylaxis on medical inpatients was a modest reduction in PE and a modest increase in total bleeding events, without effects on major bleeding, DVT, or mortality.[6] The authors summarized the findings as indicative of little or no net benefit for the medical population as a whole. The ACP‐1 review derives estimates of S‐VTE risk, bleeding, and mortality from control (baseline) and interventional arms of RCTs that used routine VTE screening, and included A‐VTE end points. The baseline risk of VTE could potentially be overestimated, because the populations in the trials are not representative of the entire medical population.
On the other hand, pooling trials with screening‐detected VTE to estimate S‐VTE outcomes is a questionable practice that may falsely lower estimates of VTE prophylaxis benefit. Screening‐detected VTE may be treated or declared a study end point before it becomes symptomatic. MEDENOX (Medical Patients With Enoxaparin) is an illustrative example.[12] The 263 placebo recipients suffered 37 A‐VTEs and 4 S‐VTEs. The 272 enoxaparin recipients suffered 17 A‐VTEs and 3 S‐VTEs. Patients at the highest risk of S‐VTE were counted as reaching an end point before they could develop symptoms; this happened more than twice as often in the placebo arm. This decreases both estimates of baseline VTE risk and the measured benefit of prophylaxis for S‐VTE. Screening could conceivably reduce measured effects on mortality as well, because patients begin VTE therapy earlier. Per ACP‐1, the estimated risk for DVT is lower than for PE, running counter to literature experience[8, 16] and raising issues of face validity. The ACP‐1 review accepts all original definitions of major bleeding, including a 2 g/dL drop in hemoglobin,[12] which commonly occurs without any bleeding or clinical consequence, and bleeding events were ascribed to heparins up to 120 days after randomization, long after they could have been responsible.
Previous meta‐analyses of thromboprophylaxis studies[23, 24] shared many of these same limitations, but did not ascribe bleeding complications to heparins for this extended duration, and had point estimates that suggested a larger impact from prophylaxis than ACP‐1. Dentali et al., for example, showed statistically significant impact on PE (relative risk [RR] 0.43), fatal PE (RR 0.38), and a nearly statistically significant large impact on DVT (RR 0.47, 95% confidence interval [CI]: 0.22‐1.00),[24] whereas ACP‐1 estimated a smaller significant impact on PE (RR 0.69), no significant difference in fatal PE, and a much smaller estimate of the impact on DVT (RR 0.78, 95% CI: 0.45‐1.35) (Table 2).
| Baseline Risk | Relative Effect (95% CI) | Absolute Effect per 1000 Patients Treated (95% CI) | |
|---|---|---|---|
| |||
| ACP guideline review (Lederle), UFH or LMWH vs placebo/no treatment, medical patients | |||
| Mortality | 6.6 | OR 0.94 (0.84‐1.04) | 4 fewer (11 fewer to 3 more) |
| Major bleeding | 0.25 | OR 1.49 (0.91‐2.43) | 1 more (no effect to 3 more) |
| Symptomatic DVT | 0.96 | OR 0.78 (0.45‐1.35) | 2 fewer (6 fewer to 4 more) |
| PE | 1.2 | OR 0.69 (0.52‐0.90) | 4 fewer (6 fewer to 1 fewer) |
| Fatal PE | 0.30 | OR 0.77 (0.43‐1.37) | 1 fewer (2 fewer to 1 more) |
| ACCP AT9 (Kahn), non‐critical care medical inpatients, anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Mortality | 4.5 | OR 0.97 (0.79‐1.19) | 1 fewer (9 fewer to 8 more) |
| Major bleeding | 0.40 | OR 1.32 (0.73‐2.37) | 1 more (1 fewer to 6 more) |
| Thrombocytopenia | 0.13 | OR 0.92 (0.54‐1.53) | 1 fewer (6 fewer to 7 more) |
| Symptomatic DVT | |||
| Padua score 4 | 0.2 | RR 0.47 (0.22‐1) | 1 fewer (1 fewer to no effect) |
| Padua score 4 | 6.7 | 34 fewer (51 fewer to no effect) | |
| ACCP AT9 (Kahn) non‐critical care medical inpatients, Anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Nonfatal PE | |||
| Padua score 4 | 0.2 | RR 0.61 (0.23‐1.67) | 1 fewer (1 fewer to 1 more) |
| Padua score 4 | 3.9 | 15 fewer (30 fewer to 36 more) | |
| Fatal PE | 0.4 | RR 0.41 (0.22‐0.76) | 2 fewer (1 fewer to 3 fewer) |
| ACCP AT9 (Kahn), critical care medical inpatients, any heparin (LMWH, UFH) vs placebo/no treatment) | |||
| Mortality | 9.4 | RR 1.01 (0.04‐2.57) | 1 more (56 fewer to 148 more) |
| Major bleeding | 2.7 | RR 2.09 (0.54‐8.16) | 29 more (12 fewer to 190 more) |
| Symptomatic DVT | 5.8 | RR 0.86 (0.59‐1.25) | 4 fewer (12 fewer to 8 more) |
| Pulmonary embolus | 4.2 | RR, 0.73 (0.26‐2.11) | 11 fewer (31 fewer to 47 more) |
AT9 used a variety of methods to estimate each component of the risk/benefit equation. Critical care and non‐critical care estimates were generated independently, but because of limited data, the critical care estimates were highly imprecise. In non‐critical care patients, as in ACP‐1, treatment effects were estimated from RCTs that routinely screened for A‐VTE, and they adapted the Dentali et al. estimate of DVT risk reduction. The baseline risk for bleeding and mortality were derived from the control population of the same meta‐analysis.[24]
Using a novel approach, AT9 estimated baseline nonsurgical VTE risk from a prospective observational cohort study of 1180 medical inpatients divided into high‐ and low‐risk groups by a point‐scoring system.[25] Deriving risk estimates from an observational cohort has theoretical advantages. Many patients did not receive prophylaxis, allowing for unadjusted risk estimates; they represented a cross‐section of medical inpatients rather than a selected trial population, and risk estimates were not reduced by the culling of screen‐detected A‐VTE.
The Padua risk‐assessment model (RAM) (Table 3) defines high VTE risk as a cumulative score 4. There were 60.3% of patients at low risk and 39.7% at high risk using this threshold. Among unprophylaxed patients, VTE occurred in 11% of high‐risk patients versus 0.3% of low‐risk patients (hazard ratio 32.0, 95% CI: 4.1251.0).
| Baseline Features | Score |
|---|---|
| |
| Active cancer* | 3 |
| Previous VTE (excluding superficial thrombosis) | 3 |
| Reduced mobility | 3 |
| Already known thrombophilic condition | 3 |
| Recent (1 month) trauma and/or surgery | 2 |
| Elderly age (70 years) | 1 |
| Heart and/or respiratory failure | 1 |
| Acute myocardial infarction or stroke | 1 |
| Acute infection and/or rheumatologic disorder | 1 |
| Obesity (BMI 30) | 1 |
| Ongoing hormonal treatment | 1 |
PADUA: A CLOSER LOOK
In the Padua study, 60% of the population appeared to be at such low risk for VTE that prophylaxis would seem unnecessary, but closer scrutiny should raise concern about generalizing these results. Of the 711 low‐risk patients, 1% were immobile, only 6% had cancer, 6% were obese, and only 12% had any acute infection or inflammatory condition, yet their mean length of stay was 7.9 days. These characteristics do not apply to 60% of American inpatients. Furthermore, 964 of 2208 eligible patients (44%) were excluded because they required therapeutic anticoagulation.[25]
Correspondence with the authors revealed that the 2 PEs in patients with Padua scores 4 occurred among 192 patients with a risk score of 3 (Figure 1), a 1% (2/192) risk of PE. This is a very small sample, and the true risk of VTE for medical inpatients with a risk score of 3 may be lower or significantly higher. In the Padua population, a risk score of 3 equated to a VTE risk of 6.9%, whereas those with a score of 0 to 2 had no VTE. For those adapting the Padua model, careful consideration of using a cutoff of 3, versus 4, is warranted.
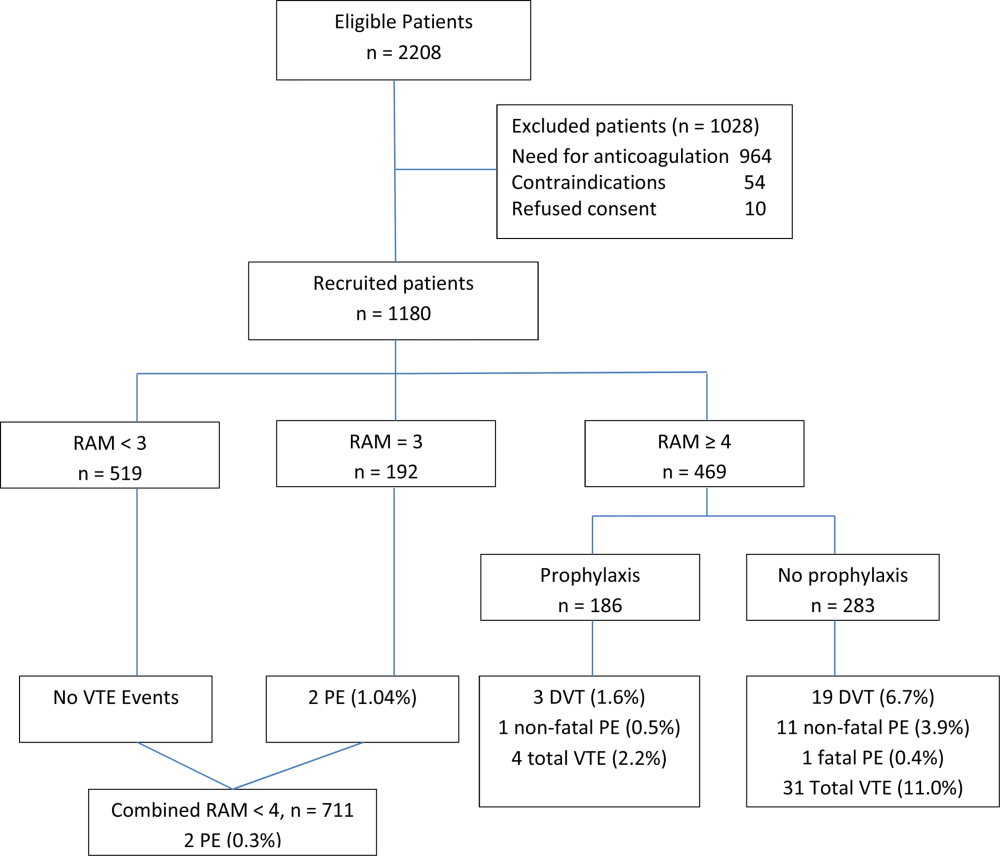
IMPLICATIONS FOR VTE PROTOCOL IMPLEMENTATION AND IMPROVEMENT TEAMS
AT9 and ACP‐1 sought to focus on S‐VTE, remove bias from recommendations, and highlight potential risks of unnecessary prophylaxis in low‐risk patients. They have largely succeeded in these important goals. However, the complexity of the new guidelines and lack of consensus about VTE risk assessment pose significant challenges to improvement teams tasked with implementing the guidelines in real‐world settings.
CHOOSING A VTE RAM
The fundamental question is: How can hospitals assess VTE risk, assure adequate prophylaxis for patients who need it, while minimizing excess prophylaxis, in a practical, efficient way?
Approach 1: Opt Out Approach
Both guidelines discourage universal prophylaxis for inpatients without contraindications unless the physician opts out. Although the simplicity of this approach is appealing, the low rate of VTE in a substantial segment of the medical inpatient population and known risks of thromboprophylaxis make this strategy suboptimal.
Approach 2: No VTE RAM
ACP‐1 notes that evidence is not sufficient to recommend 1 RAM over another, and essentially advises leaving prophylaxis decisions up to an individual physician's judgment. Although the evidence may not prove which system is best, prophylaxis reliability is dismal when there is no system or when hospitals offer prophylaxis options without guidance.[26, 27] Widespread, well‐documented underprophylaxis[16, 17, 18, 19] is largely the result of relying on unguided physician judgment and relatively passive interventions like educational sessions and pocket cards.[8] This approach also deprives improvement teams of standard definitions of VTE risk, bleeding risk, and adequate prophylaxis necessary to measure and improve VTE prophylaxis. Because of significant gray areas in the literature and varied infrastructure, institutions will not implement identical VTE prevention programs, but institutional standardization remains a cornerstone of improvement.
Approach 3: Buckets of Risk
The AT8 approach to risk assessment was to place patients into VTE risk groups described in the text, rather than have an individualized point‐scoring system.[11] These assessments can be made in seconds with high levels of interobserver agreement, implemented without undue effort, and spur high levels of compliance.[28, 29] Most importantly, implementation was associated with a 40% reduction in hospital‐associated VTE (RR 0.69, 95% CI: 0.470.79) without detectable increases in bleeding or heparin‐induced thrombocytopenia. Although this strategy has not been tested in randomized trials, it has been replicated in multiple real‐world settings that avoid concerns about generalizability due to imperfect trial populations.[28, 30]
The most popular bucket model in common use, derived from a table in the AT8 guidelines, is similar to models presented in UK National Institute for Health and Care Excellence guidelines for medical inpatients.[31] These models are potentially less precise than point‐based systems, but offer simplicity, ease of use, and improved physician acceptance, and thus may be more effective than point‐based models in settings without advanced clinical decision support. The models are flexible to reflect greater or lesser degrees of aggressiveness in defining risk categories, and can be used to approximate some point‐based systems.
Approach 4: Individualized Point‐Based RAM
AT9 authors used the Padua VTE RAM to define low‐ and high‐risk patients for VTE in their recommendation for medical inpatients. The Padua model appears relatively simple, but it does require calculations, and there is a paucity of data for implementation experience with it. As mentioned above, if teams use the Padua model, the optimal cutoff (3 vs 4) for recommending prophylaxis is uncertain, and both should be considered.
The Caprini point‐based system is not mentioned in the guideline for thromboprophylaxis in medical inpatients, but in our collaborative improvement experience, it is perhaps the most commonly used point‐based model for medical inpatients.[28, 30] It is also embedded in AT9 recommendations for prophylaxis in the nonorthopedic surgical population,[9] and thus is tempting to use for both medical and surgical patients. There are several caveats to those considering the use of these more complex point‐based models. Complex point‐based RAM suffer from poor interobserver agreement.[32] They have also had limited ability to exclude low‐risk patients from prophylaxis in validation studies,[33] and have not been tested extensively in medical populations. Although AT9 considers the Caprini RAM relatively easy to use,[9] our experience in collaboratives suggests that for many hospitals, the model is too complex to be used reliably.[28, 30] Clinicians often simply bypass the clinical decision support offered in the tool, rather than checking off all risk factors, adding up the point total, and identifying the appropriate prophylaxis choices based on the point total.[28] Other point‐based RAM (reviewed elsewhere[34, 35, 36]) pose similar implementation challenges.
On the other hand, centers with more sophisticated clinical decision support and a strong improvement framework can overcome some of these challenges to get good results with complex point‐based models. A forcing function can ensure that practitioners complete all risk‐assessment tasks. Providers can check off the VTE risk factors and bleeding risk factors on 1 screen, and several factors like age, body mass index, and renal function can be autopopulated. Instead of asking the provider to add up points, the combination of answers checked off on the first screen can drive behind‐the‐scenes calculations and seamlessly lead providers to prophylaxis choices appropriate for that combination of VTE and bleeding risks. Customized models can be designed for a wide variety of services. Similar strategies can ease adaption with more complex qualitative models as well.[37]
BOTTOM LINE IN CHOOSING A VTE RAM
Many medical inpatients are at high risk for VTE, but others are not at sufficient risk to warrant prophylaxis. VTE risk assessment should be embedded in admission, transfer, and perioperative order sets and may need a hard stop to insure completion. There is a trend to favor individualized point‐based models over models that place patients in groups of risk, but evidence is insufficient to recommend 1 type of RAM over another, and more complex point‐based models often require extensive local customization and algorithmic clinical decision support to effectively implement them. Centers without advanced capability may find the bucket models more effective. We urge improvement teams to trial their RAM with common patient case scenarios, and to make a choice based on an effort‐benefit analysis, feedback from their clinicians, and the level of customization in clinical decision support available to them.
OTHER IMPLEMENTATION STRATEGIES
VTE and bleeding risk change during hospitalization. We have used ongoing daily surveillance and measurement of patients on no prophylaxis to prompt concurrent intervention (ie, measure‐vention) to increase prophylaxis for patients at risk.[28] Improvement teams should focus not only on increasing prophylaxis for those at risk, but should also use measure‐vention, checklists, or other techniques to identify low‐risk (eg, ambulating) patients for cessation of overly aggressive prophylaxis. Efforts to improve early progressive ambulation, limit central venous catheters to those who truly need them, and improve adherence to mechanical prophylaxis can also reduce VTE, as well as benefitting patient populations in other ways.
We recognize there are several approaches to close the implementation gap in delivering thromboprophylaxis judiciously but reliably, and encourage research and publication of varied strategies. Last, we hope efforts to limit unnecessary prophylaxis and challenges inherent in implementing new and complex guidelines do not increase the morbidity and mortality of hospital‐acquired VTE, by derailing the delivery of prophylaxis to those in whom the benefits outweigh the risks.
Disclosures: Dr. Merli has conducted research for Johnson & Johnson, Bristol Myers Squibb, and Portala Scientific and has been a consultant for Johnson & Johnson and Bristol Myers Squibb.
- US Department of Health and Human Services. Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. 2008. Available at: http://www.surgeongeneral.gov/topics/deepvein/index.html. Accessed January 29, 2013.
- , , , et al. Incidence of venous thromboembolism in hospitalized patients vs. community residents. Mayo Clin Proc. 2001;76:1102–1110.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815.
- , , . New onset of venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118(6):1680–1684.
- , , , , . venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625–632.
- , , , . Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , et al. Prevention of VTE in nonsurgical patients. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Prevention of VTE in nonorthopedic surgical patients. Chest. 2012;141(2 suppl):e227S–e277S.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S–453S.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- , , , et al. The outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registry. J Thromb Haemost. 2004;2:1892–1898.
- , , , et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936–945.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387–394.
- , , , et al.; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost. 2010;103(4):736–748.
- , , , et al; CLOTS Trials Collaboration. Effectiveness of thigh‐length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomized controlled trial. Lancet. 2009;373(9679):1958–1965.
- CLOTS (Clots in Legs Or sTockings after Stroke) Trial Collaboration. Thigh‐length versus below‐knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med. 2010;153(9):553–562.
- , , , et al. Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
- , , , , . Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2007;167(1)476–486.
- , , , , . Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007; 46(4):278–288.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , . Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes. J Hosp Med. 2009;4(2):81–89.
- . Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? J Hosp Med. 2009;4(2):77–80.
- , . Designing and implementing effective VTE prevention protocols: lessons from collaboratives. J Thromb Thrombolysis. 2010;29(2):159–166.
- , , , et al. Optimizing prevention of hospital acquired venous thromboembolism: prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10–18.
- , , , et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38(7):301–310.
- NHS National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. NICE Clinical Guideline 92. 2010. Available at: http://www.nice.org.uk/guidance/CG92. Accessed April 18, 2013.
- , , , , . Reliability of a point‐based VTE risk assessment tool in the hands of medical residents. J Hosp Med. 2011;6:195–201.
- , , , , , . A validation of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251(2):344–350.
- , , , . Risk assessment models for thromboprophylaxis of medical patients. Thromb Res. 2012;129:127–132.
- , , , , . Risk‐assessment models for predicting venous thromboembolism among hospitalized non‐surgical patients: a systematic review. J Thromb Thrombolysis. 2013;35:67–80.
- , , . The use of weighted and scored risk assessment models for venous thromboembolism. Thromb Haemost. 2012;108(6):1072–1076.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
Patients hospitalized for acute medical illness have more than a 10‐fold increased risk for venous thromboembolism (VTE),[1] with an undeniably dramatic, negative impact on the lives of those afflicted, including fatal pulmonary embolism (PE), which most commonly affects patients on the medical service.[2, 3, 4] Yet estimates for the overall rate of VTE in this population are relatively low, raising questions about which subsets of medical patients warrant the risk and cost of prophylaxis.
Recently, the American College of Physicians published guidelines (ACP‐1)[5] and a supporting review[6] addressing VTE prophylaxis in nonsurgical inpatients, followed by publication of the American College of Chest Physicians (ACCP) 9th Edition of the Chest Guidelines on Antithrombotic Therapy and Prevention of Thrombosis (AT9),[7] which divides VTE prevention into 3 articles,[8, 9, 10] including 1 on nonsurgical patients.[8] Both ACP‐1 and AT9 differ significantly from the 2008 ACCP guidelines (AT8),[11] but took different approaches to methodology, risk assessment, and several other aspects of thromboprophylaxis (Table 1). This narrative review summarizes and compares these recommendations and the methods used to arrive at them, with a final section focusing on implications for improvement teams designing order sets and system changes to address VTE prophylaxis.
| 2008 ACCP VTE Guideline AT8 | 2012 ACCP VTE Guideline AT9 | 2011 ACP Guideline | |
|---|---|---|---|
| |||
| Stance on asymptomatic VTE end points | Because of the strong concordance between asymptomatic DVT and clinically important VTE, we believe that DVT detected by a sensitive screening tesis an appropriate outcome in the early assessment of new thromboprophylaxis interventions. | Use of this surrogate (asymptomatic, screening‐detected thrombosis) creates major problems in making the trade‐off between patient‐important outcomes (thrombosis and serious bleeding). | Surrogate outcomes of asymptomatic screening detected‐thrombosis should not be used. |
| Who should be prophylaxed? | 6.0.0: For acutely ill medical patients admitted to hospital with congestive heart failure or severe respiratory disease, or who are confined to bed and have one or more additional risk factors, including active cancer, previous VTE, sepsis, neurologic disease, or inflammatory bowel disease, we recommend thromboprophylaxis with LMWH (1A), UFH (1A), or fondaparinux (1A). | 2.3: For acutely ill hospitalized medical patients at increased risk for thrombosis, we recommend anticoagulant thromboprophylaxis, with LMWH, UFH bid, UFH tid, or fondaparinux (1B). | ACP recommends pharmacologic prophylaxis with heparin or a related drug for venous thromboembolism in medical (including stroke) patients unless the assessed risk for bleeding outweighs the likely benefits (grade: strong recommendation, moderate‐quality evidence). |
| 2.4: For acutely ill hospitalized medical patients at low risk of thrombosis, we recommend against the use of pharmacologic or mechanical prophylaxis. (1B) | |||
| Choice of anticoagulant prophylaxis | There is no compelling evidence that UFH should be administered three times daily in preference to twice daily in medical patients, although these two regimens have never been directly compared. | In choosing the specific anticoagulant drug to be used for pharmacoprophylaxis, choices should be based on patient preference, compliance, and ease of administration (eg, daily vs bid vs tid dosing), as well as on local factors affecting acquisition costs. | [T]he choice of agent for prophylaxis of VTE should be based on ease of use, adverse effect profile, and cost of medication. |
| No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | |
| Mechanical prophylaxis | 1.4.3.1: We recommend that mechanical methods of prophylaxis be used primarily in patients at high risk of bleeding (grade 1A), or possibly as an adjunct to anticoagulant‐based thromboprophylaxis (grade 2A). | 2.7.2: For acutely ill hospitalized medical patients at increased risk of thrombosis who are bleeding or at high risk for major bleeding, we suggest the optimal use of mechanical thromboprophylaxis with GCS (grade 2C) or IPC (grade 2C). | ACP recommends against the use of mechanical prophylaxis with graduated compression stockings for prevention of venous thromboembolism (grade: strong recommendation, moderate‐quality evidence). |
| No strong evidence for IPC vs GCS | |||
| Duration | [T]he optimal duration of thromboprophylaxis remains unclear. To the end of hospitalization for most patients. | 2.8: [W]e suggest against extending the duration of thromboprophylaxis beyond the period of patient immobilization or acute hospital stay (2B). | The optimal duration of heparin prophylaxis is uncertain. |
| Risk Stratification | The approach of individual prophylaxis prescribing based on formal RAMs is not used routinely by most clinicians because it has not been adequately validated and is cumbersome. Individual RAMs may not be worth the effort, because there are only a limited number of thromboprophylaxis options, and one of the principles of effective thromboprophylaxis is to reduce complexity in decision making. | (Noncritical care) No formal risk assessment recommendation. Padua point‐based model is inherent in definitions of baseline VTE risk. | ACP does not support the application of performance measures in medical (including stroke) patients that promotes universal venous thromboembolism prophylaxis regardless of risk. |
| Another approach involves implementation of group‐specific thromboprophylaxis routinely for all patients who belong to each of the major target groups. We support this approach. | There are no validated risk assessment models to stratify VTE risk in critically ill patients. | Many risk assessment tools are available for estimating thromboembolism risk, but the current evidence is insufficient to recommend a validated tool. | |
| [T]he decision is best left to physician judgment, and performance measures targeting all patients are inappropriate. | |||
WHY ARE THE NEW GUIDELINES DIFFERENT?
Major randomized controlled trials (RCTs)[12, 13, 14] of thromboprophylaxis used routine deep vein thrombosis (DVT) surveillance and included both symptomatic (S‐VTE) and asymptomatic VTE (A‐VTE) end points. These studies consistently demonstrated 44% to 63% reductions in VTE without increases in major bleeding.[11] Because of the strong relationship between A‐VTE and S‐VTE outcomes, and a paucity of studies using only S‐VTE outcomes, AT8 judged that A‐VTE outcomes were valid to include, whereas the new guidelines reject the use of asymptomatic VTE end points.[5, 8, 15] To minimize financial and intellectual conflicts of interest, AT9 also used methodologists rather than VTE experts as topic editors, excluded conflicted experts from voting on recommendations, and attempted to estimate patient values and preferences.[15] As a result, AT9 makes fewer strong recommendations (182 1A recommendations in 2008, but only 29 in 2012), replacing them with weak suggestions.
WHAT DO THE NEW GUIDELINES RECOMMEND?
AT8 recommended anticoagulant prophylaxis for acutely ill medical inpatients with known risk factors, but did not recommend routine thromboprophylaxis. However, because of well‐known problems with underprophylaxis,[16, 17, 18, 19] particularly in medical patients, the low risk of bleeding, and difficulties with explicitly defining low‐risk patients, many discounted the need for VTE risk stratification.
Both new guidelines recommend prophylaxis for many nonsurgical patients, but discourage routine thromboprophylaxis for nonsurgical inpatients. AT9 specifically recommends against any thromboprophylaxis for low‐risk medical inpatients, implying that many nonsurgical, non‐critical care patients belong in this category, citing lower estimates of benefit, lower estimates of VTE risk, and potential bleeding risks.
The guidelines[5, 8] agree that, when indicated and absent contraindications, anticoagulant prophylaxis is preferred over mechanical prophylaxis, and agree there is insufficient evidence to recommend 1 anticoagulant over another.
For patients at risk of both VTE and bleeding, ACP‐1 states that intermittent pneumatic compression (IPC) devices are a reasonable option, given the evidence showing benefit in surgical patients. However, ACP‐1 recommends against graduated compression stockings (GCS) in nonsurgical patients based on a meta‐analysis dominated by the CLOTS‐1 (Clots in Legs Or sTockings after Stroke) trial, which found that thigh‐high GCS increased the risk of skin breakdown without reducing VTE[20] in immobilized stroke patients. AT9 does not recommend against GCS for patients facing bleeding and VTE risk. AT9 notes the hazards of generalizing results from stroke patients, and also considers the somewhat contradictory results from the CLOTS‐2 trial in stroke patients, which found a lower rate of VTE with thigh‐high GCS than with knee‐high GCS.[21] AT9 designates a recommendation of 2C for either IPC devices or thigh‐high GCS for those at VTE risk when anticoagulants are contraindicated.
Combination mechanical‐pharmacologic prophylaxis has proven superior in some surgical populations, and many hospitals use combined prophylaxis in high‐risk medical patients. However, combination prophylaxis has not been studied in this population. ACP‐1 does not comment on the practice; AT9 does not recommend for or against it. Institutions that use combination prophylaxis should be aware that although it may seem logical to extrapolate estimates of benefit seen in selected surgical patients, this is not a recommended practice.
RCTs for thromboprophylaxis in nonsurgical inpatients provided prophylaxis for 6 to 21 days. Neither ACP‐1 or AT9 recommend routinely extending prophylaxis beyond the hospital stay, citing an RCT[22] in which the benefit of extended duration low molecular weight heparin was limited to selected subsets of patients and offset by bleeding complications. AT9 suggests prophylaxis for 6 to 21 days, until full mobility is restored, or until dischargewhichever comes first.[8] However, we know of no study that establishes a mobility level at which prophylaxis can be safely discontinued, especially in inpatients with multiple risk factors.
ESTIMATING RISK AND BENEFIT OF PROPHYLAXIS AND LIMITATIONS OF METHODS
Calculating risk/benefit ratios for thromboprophylaxis requires estimates of baseline VTE and bleeding risks, and estimates of the impact of prophylaxis on those baseline risks. Methods to estimate the impact of prophylaxis on S‐VTE from studies relying on A‐VTE all have limitations, as acknowledged by the AT9 introduction.[15]
The ACP‐1 review found the only significant effect of prophylaxis on medical inpatients was a modest reduction in PE and a modest increase in total bleeding events, without effects on major bleeding, DVT, or mortality.[6] The authors summarized the findings as indicative of little or no net benefit for the medical population as a whole. The ACP‐1 review derives estimates of S‐VTE risk, bleeding, and mortality from control (baseline) and interventional arms of RCTs that used routine VTE screening, and included A‐VTE end points. The baseline risk of VTE could potentially be overestimated, because the populations in the trials are not representative of the entire medical population.
On the other hand, pooling trials with screening‐detected VTE to estimate S‐VTE outcomes is a questionable practice that may falsely lower estimates of VTE prophylaxis benefit. Screening‐detected VTE may be treated or declared a study end point before it becomes symptomatic. MEDENOX (Medical Patients With Enoxaparin) is an illustrative example.[12] The 263 placebo recipients suffered 37 A‐VTEs and 4 S‐VTEs. The 272 enoxaparin recipients suffered 17 A‐VTEs and 3 S‐VTEs. Patients at the highest risk of S‐VTE were counted as reaching an end point before they could develop symptoms; this happened more than twice as often in the placebo arm. This decreases both estimates of baseline VTE risk and the measured benefit of prophylaxis for S‐VTE. Screening could conceivably reduce measured effects on mortality as well, because patients begin VTE therapy earlier. Per ACP‐1, the estimated risk for DVT is lower than for PE, running counter to literature experience[8, 16] and raising issues of face validity. The ACP‐1 review accepts all original definitions of major bleeding, including a 2 g/dL drop in hemoglobin,[12] which commonly occurs without any bleeding or clinical consequence, and bleeding events were ascribed to heparins up to 120 days after randomization, long after they could have been responsible.
Previous meta‐analyses of thromboprophylaxis studies[23, 24] shared many of these same limitations, but did not ascribe bleeding complications to heparins for this extended duration, and had point estimates that suggested a larger impact from prophylaxis than ACP‐1. Dentali et al., for example, showed statistically significant impact on PE (relative risk [RR] 0.43), fatal PE (RR 0.38), and a nearly statistically significant large impact on DVT (RR 0.47, 95% confidence interval [CI]: 0.22‐1.00),[24] whereas ACP‐1 estimated a smaller significant impact on PE (RR 0.69), no significant difference in fatal PE, and a much smaller estimate of the impact on DVT (RR 0.78, 95% CI: 0.45‐1.35) (Table 2).
| Baseline Risk | Relative Effect (95% CI) | Absolute Effect per 1000 Patients Treated (95% CI) | |
|---|---|---|---|
| |||
| ACP guideline review (Lederle), UFH or LMWH vs placebo/no treatment, medical patients | |||
| Mortality | 6.6 | OR 0.94 (0.84‐1.04) | 4 fewer (11 fewer to 3 more) |
| Major bleeding | 0.25 | OR 1.49 (0.91‐2.43) | 1 more (no effect to 3 more) |
| Symptomatic DVT | 0.96 | OR 0.78 (0.45‐1.35) | 2 fewer (6 fewer to 4 more) |
| PE | 1.2 | OR 0.69 (0.52‐0.90) | 4 fewer (6 fewer to 1 fewer) |
| Fatal PE | 0.30 | OR 0.77 (0.43‐1.37) | 1 fewer (2 fewer to 1 more) |
| ACCP AT9 (Kahn), non‐critical care medical inpatients, anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Mortality | 4.5 | OR 0.97 (0.79‐1.19) | 1 fewer (9 fewer to 8 more) |
| Major bleeding | 0.40 | OR 1.32 (0.73‐2.37) | 1 more (1 fewer to 6 more) |
| Thrombocytopenia | 0.13 | OR 0.92 (0.54‐1.53) | 1 fewer (6 fewer to 7 more) |
| Symptomatic DVT | |||
| Padua score 4 | 0.2 | RR 0.47 (0.22‐1) | 1 fewer (1 fewer to no effect) |
| Padua score 4 | 6.7 | 34 fewer (51 fewer to no effect) | |
| ACCP AT9 (Kahn) non‐critical care medical inpatients, Anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Nonfatal PE | |||
| Padua score 4 | 0.2 | RR 0.61 (0.23‐1.67) | 1 fewer (1 fewer to 1 more) |
| Padua score 4 | 3.9 | 15 fewer (30 fewer to 36 more) | |
| Fatal PE | 0.4 | RR 0.41 (0.22‐0.76) | 2 fewer (1 fewer to 3 fewer) |
| ACCP AT9 (Kahn), critical care medical inpatients, any heparin (LMWH, UFH) vs placebo/no treatment) | |||
| Mortality | 9.4 | RR 1.01 (0.04‐2.57) | 1 more (56 fewer to 148 more) |
| Major bleeding | 2.7 | RR 2.09 (0.54‐8.16) | 29 more (12 fewer to 190 more) |
| Symptomatic DVT | 5.8 | RR 0.86 (0.59‐1.25) | 4 fewer (12 fewer to 8 more) |
| Pulmonary embolus | 4.2 | RR, 0.73 (0.26‐2.11) | 11 fewer (31 fewer to 47 more) |
AT9 used a variety of methods to estimate each component of the risk/benefit equation. Critical care and non‐critical care estimates were generated independently, but because of limited data, the critical care estimates were highly imprecise. In non‐critical care patients, as in ACP‐1, treatment effects were estimated from RCTs that routinely screened for A‐VTE, and they adapted the Dentali et al. estimate of DVT risk reduction. The baseline risk for bleeding and mortality were derived from the control population of the same meta‐analysis.[24]
Using a novel approach, AT9 estimated baseline nonsurgical VTE risk from a prospective observational cohort study of 1180 medical inpatients divided into high‐ and low‐risk groups by a point‐scoring system.[25] Deriving risk estimates from an observational cohort has theoretical advantages. Many patients did not receive prophylaxis, allowing for unadjusted risk estimates; they represented a cross‐section of medical inpatients rather than a selected trial population, and risk estimates were not reduced by the culling of screen‐detected A‐VTE.
The Padua risk‐assessment model (RAM) (Table 3) defines high VTE risk as a cumulative score 4. There were 60.3% of patients at low risk and 39.7% at high risk using this threshold. Among unprophylaxed patients, VTE occurred in 11% of high‐risk patients versus 0.3% of low‐risk patients (hazard ratio 32.0, 95% CI: 4.1251.0).
| Baseline Features | Score |
|---|---|
| |
| Active cancer* | 3 |
| Previous VTE (excluding superficial thrombosis) | 3 |
| Reduced mobility | 3 |
| Already known thrombophilic condition | 3 |
| Recent (1 month) trauma and/or surgery | 2 |
| Elderly age (70 years) | 1 |
| Heart and/or respiratory failure | 1 |
| Acute myocardial infarction or stroke | 1 |
| Acute infection and/or rheumatologic disorder | 1 |
| Obesity (BMI 30) | 1 |
| Ongoing hormonal treatment | 1 |
PADUA: A CLOSER LOOK
In the Padua study, 60% of the population appeared to be at such low risk for VTE that prophylaxis would seem unnecessary, but closer scrutiny should raise concern about generalizing these results. Of the 711 low‐risk patients, 1% were immobile, only 6% had cancer, 6% were obese, and only 12% had any acute infection or inflammatory condition, yet their mean length of stay was 7.9 days. These characteristics do not apply to 60% of American inpatients. Furthermore, 964 of 2208 eligible patients (44%) were excluded because they required therapeutic anticoagulation.[25]
Correspondence with the authors revealed that the 2 PEs in patients with Padua scores 4 occurred among 192 patients with a risk score of 3 (Figure 1), a 1% (2/192) risk of PE. This is a very small sample, and the true risk of VTE for medical inpatients with a risk score of 3 may be lower or significantly higher. In the Padua population, a risk score of 3 equated to a VTE risk of 6.9%, whereas those with a score of 0 to 2 had no VTE. For those adapting the Padua model, careful consideration of using a cutoff of 3, versus 4, is warranted.

IMPLICATIONS FOR VTE PROTOCOL IMPLEMENTATION AND IMPROVEMENT TEAMS
AT9 and ACP‐1 sought to focus on S‐VTE, remove bias from recommendations, and highlight potential risks of unnecessary prophylaxis in low‐risk patients. They have largely succeeded in these important goals. However, the complexity of the new guidelines and lack of consensus about VTE risk assessment pose significant challenges to improvement teams tasked with implementing the guidelines in real‐world settings.
CHOOSING A VTE RAM
The fundamental question is: How can hospitals assess VTE risk, assure adequate prophylaxis for patients who need it, while minimizing excess prophylaxis, in a practical, efficient way?
Approach 1: Opt Out Approach
Both guidelines discourage universal prophylaxis for inpatients without contraindications unless the physician opts out. Although the simplicity of this approach is appealing, the low rate of VTE in a substantial segment of the medical inpatient population and known risks of thromboprophylaxis make this strategy suboptimal.
Approach 2: No VTE RAM
ACP‐1 notes that evidence is not sufficient to recommend 1 RAM over another, and essentially advises leaving prophylaxis decisions up to an individual physician's judgment. Although the evidence may not prove which system is best, prophylaxis reliability is dismal when there is no system or when hospitals offer prophylaxis options without guidance.[26, 27] Widespread, well‐documented underprophylaxis[16, 17, 18, 19] is largely the result of relying on unguided physician judgment and relatively passive interventions like educational sessions and pocket cards.[8] This approach also deprives improvement teams of standard definitions of VTE risk, bleeding risk, and adequate prophylaxis necessary to measure and improve VTE prophylaxis. Because of significant gray areas in the literature and varied infrastructure, institutions will not implement identical VTE prevention programs, but institutional standardization remains a cornerstone of improvement.
Approach 3: Buckets of Risk
The AT8 approach to risk assessment was to place patients into VTE risk groups described in the text, rather than have an individualized point‐scoring system.[11] These assessments can be made in seconds with high levels of interobserver agreement, implemented without undue effort, and spur high levels of compliance.[28, 29] Most importantly, implementation was associated with a 40% reduction in hospital‐associated VTE (RR 0.69, 95% CI: 0.470.79) without detectable increases in bleeding or heparin‐induced thrombocytopenia. Although this strategy has not been tested in randomized trials, it has been replicated in multiple real‐world settings that avoid concerns about generalizability due to imperfect trial populations.[28, 30]
The most popular bucket model in common use, derived from a table in the AT8 guidelines, is similar to models presented in UK National Institute for Health and Care Excellence guidelines for medical inpatients.[31] These models are potentially less precise than point‐based systems, but offer simplicity, ease of use, and improved physician acceptance, and thus may be more effective than point‐based models in settings without advanced clinical decision support. The models are flexible to reflect greater or lesser degrees of aggressiveness in defining risk categories, and can be used to approximate some point‐based systems.
Approach 4: Individualized Point‐Based RAM
AT9 authors used the Padua VTE RAM to define low‐ and high‐risk patients for VTE in their recommendation for medical inpatients. The Padua model appears relatively simple, but it does require calculations, and there is a paucity of data for implementation experience with it. As mentioned above, if teams use the Padua model, the optimal cutoff (3 vs 4) for recommending prophylaxis is uncertain, and both should be considered.
The Caprini point‐based system is not mentioned in the guideline for thromboprophylaxis in medical inpatients, but in our collaborative improvement experience, it is perhaps the most commonly used point‐based model for medical inpatients.[28, 30] It is also embedded in AT9 recommendations for prophylaxis in the nonorthopedic surgical population,[9] and thus is tempting to use for both medical and surgical patients. There are several caveats to those considering the use of these more complex point‐based models. Complex point‐based RAM suffer from poor interobserver agreement.[32] They have also had limited ability to exclude low‐risk patients from prophylaxis in validation studies,[33] and have not been tested extensively in medical populations. Although AT9 considers the Caprini RAM relatively easy to use,[9] our experience in collaboratives suggests that for many hospitals, the model is too complex to be used reliably.[28, 30] Clinicians often simply bypass the clinical decision support offered in the tool, rather than checking off all risk factors, adding up the point total, and identifying the appropriate prophylaxis choices based on the point total.[28] Other point‐based RAM (reviewed elsewhere[34, 35, 36]) pose similar implementation challenges.
On the other hand, centers with more sophisticated clinical decision support and a strong improvement framework can overcome some of these challenges to get good results with complex point‐based models. A forcing function can ensure that practitioners complete all risk‐assessment tasks. Providers can check off the VTE risk factors and bleeding risk factors on 1 screen, and several factors like age, body mass index, and renal function can be autopopulated. Instead of asking the provider to add up points, the combination of answers checked off on the first screen can drive behind‐the‐scenes calculations and seamlessly lead providers to prophylaxis choices appropriate for that combination of VTE and bleeding risks. Customized models can be designed for a wide variety of services. Similar strategies can ease adaption with more complex qualitative models as well.[37]
BOTTOM LINE IN CHOOSING A VTE RAM
Many medical inpatients are at high risk for VTE, but others are not at sufficient risk to warrant prophylaxis. VTE risk assessment should be embedded in admission, transfer, and perioperative order sets and may need a hard stop to insure completion. There is a trend to favor individualized point‐based models over models that place patients in groups of risk, but evidence is insufficient to recommend 1 type of RAM over another, and more complex point‐based models often require extensive local customization and algorithmic clinical decision support to effectively implement them. Centers without advanced capability may find the bucket models more effective. We urge improvement teams to trial their RAM with common patient case scenarios, and to make a choice based on an effort‐benefit analysis, feedback from their clinicians, and the level of customization in clinical decision support available to them.
OTHER IMPLEMENTATION STRATEGIES
VTE and bleeding risk change during hospitalization. We have used ongoing daily surveillance and measurement of patients on no prophylaxis to prompt concurrent intervention (ie, measure‐vention) to increase prophylaxis for patients at risk.[28] Improvement teams should focus not only on increasing prophylaxis for those at risk, but should also use measure‐vention, checklists, or other techniques to identify low‐risk (eg, ambulating) patients for cessation of overly aggressive prophylaxis. Efforts to improve early progressive ambulation, limit central venous catheters to those who truly need them, and improve adherence to mechanical prophylaxis can also reduce VTE, as well as benefitting patient populations in other ways.
We recognize there are several approaches to close the implementation gap in delivering thromboprophylaxis judiciously but reliably, and encourage research and publication of varied strategies. Last, we hope efforts to limit unnecessary prophylaxis and challenges inherent in implementing new and complex guidelines do not increase the morbidity and mortality of hospital‐acquired VTE, by derailing the delivery of prophylaxis to those in whom the benefits outweigh the risks.
Disclosures: Dr. Merli has conducted research for Johnson & Johnson, Bristol Myers Squibb, and Portala Scientific and has been a consultant for Johnson & Johnson and Bristol Myers Squibb.
Patients hospitalized for acute medical illness have more than a 10‐fold increased risk for venous thromboembolism (VTE),[1] with an undeniably dramatic, negative impact on the lives of those afflicted, including fatal pulmonary embolism (PE), which most commonly affects patients on the medical service.[2, 3, 4] Yet estimates for the overall rate of VTE in this population are relatively low, raising questions about which subsets of medical patients warrant the risk and cost of prophylaxis.
Recently, the American College of Physicians published guidelines (ACP‐1)[5] and a supporting review[6] addressing VTE prophylaxis in nonsurgical inpatients, followed by publication of the American College of Chest Physicians (ACCP) 9th Edition of the Chest Guidelines on Antithrombotic Therapy and Prevention of Thrombosis (AT9),[7] which divides VTE prevention into 3 articles,[8, 9, 10] including 1 on nonsurgical patients.[8] Both ACP‐1 and AT9 differ significantly from the 2008 ACCP guidelines (AT8),[11] but took different approaches to methodology, risk assessment, and several other aspects of thromboprophylaxis (Table 1). This narrative review summarizes and compares these recommendations and the methods used to arrive at them, with a final section focusing on implications for improvement teams designing order sets and system changes to address VTE prophylaxis.
| 2008 ACCP VTE Guideline AT8 | 2012 ACCP VTE Guideline AT9 | 2011 ACP Guideline | |
|---|---|---|---|
| |||
| Stance on asymptomatic VTE end points | Because of the strong concordance between asymptomatic DVT and clinically important VTE, we believe that DVT detected by a sensitive screening tesis an appropriate outcome in the early assessment of new thromboprophylaxis interventions. | Use of this surrogate (asymptomatic, screening‐detected thrombosis) creates major problems in making the trade‐off between patient‐important outcomes (thrombosis and serious bleeding). | Surrogate outcomes of asymptomatic screening detected‐thrombosis should not be used. |
| Who should be prophylaxed? | 6.0.0: For acutely ill medical patients admitted to hospital with congestive heart failure or severe respiratory disease, or who are confined to bed and have one or more additional risk factors, including active cancer, previous VTE, sepsis, neurologic disease, or inflammatory bowel disease, we recommend thromboprophylaxis with LMWH (1A), UFH (1A), or fondaparinux (1A). | 2.3: For acutely ill hospitalized medical patients at increased risk for thrombosis, we recommend anticoagulant thromboprophylaxis, with LMWH, UFH bid, UFH tid, or fondaparinux (1B). | ACP recommends pharmacologic prophylaxis with heparin or a related drug for venous thromboembolism in medical (including stroke) patients unless the assessed risk for bleeding outweighs the likely benefits (grade: strong recommendation, moderate‐quality evidence). |
| 2.4: For acutely ill hospitalized medical patients at low risk of thrombosis, we recommend against the use of pharmacologic or mechanical prophylaxis. (1B) | |||
| Choice of anticoagulant prophylaxis | There is no compelling evidence that UFH should be administered three times daily in preference to twice daily in medical patients, although these two regimens have never been directly compared. | In choosing the specific anticoagulant drug to be used for pharmacoprophylaxis, choices should be based on patient preference, compliance, and ease of administration (eg, daily vs bid vs tid dosing), as well as on local factors affecting acquisition costs. | [T]he choice of agent for prophylaxis of VTE should be based on ease of use, adverse effect profile, and cost of medication. |
| No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | |
| Mechanical prophylaxis | 1.4.3.1: We recommend that mechanical methods of prophylaxis be used primarily in patients at high risk of bleeding (grade 1A), or possibly as an adjunct to anticoagulant‐based thromboprophylaxis (grade 2A). | 2.7.2: For acutely ill hospitalized medical patients at increased risk of thrombosis who are bleeding or at high risk for major bleeding, we suggest the optimal use of mechanical thromboprophylaxis with GCS (grade 2C) or IPC (grade 2C). | ACP recommends against the use of mechanical prophylaxis with graduated compression stockings for prevention of venous thromboembolism (grade: strong recommendation, moderate‐quality evidence). |
| No strong evidence for IPC vs GCS | |||
| Duration | [T]he optimal duration of thromboprophylaxis remains unclear. To the end of hospitalization for most patients. | 2.8: [W]e suggest against extending the duration of thromboprophylaxis beyond the period of patient immobilization or acute hospital stay (2B). | The optimal duration of heparin prophylaxis is uncertain. |
| Risk Stratification | The approach of individual prophylaxis prescribing based on formal RAMs is not used routinely by most clinicians because it has not been adequately validated and is cumbersome. Individual RAMs may not be worth the effort, because there are only a limited number of thromboprophylaxis options, and one of the principles of effective thromboprophylaxis is to reduce complexity in decision making. | (Noncritical care) No formal risk assessment recommendation. Padua point‐based model is inherent in definitions of baseline VTE risk. | ACP does not support the application of performance measures in medical (including stroke) patients that promotes universal venous thromboembolism prophylaxis regardless of risk. |
| Another approach involves implementation of group‐specific thromboprophylaxis routinely for all patients who belong to each of the major target groups. We support this approach. | There are no validated risk assessment models to stratify VTE risk in critically ill patients. | Many risk assessment tools are available for estimating thromboembolism risk, but the current evidence is insufficient to recommend a validated tool. | |
| [T]he decision is best left to physician judgment, and performance measures targeting all patients are inappropriate. | |||
WHY ARE THE NEW GUIDELINES DIFFERENT?
Major randomized controlled trials (RCTs)[12, 13, 14] of thromboprophylaxis used routine deep vein thrombosis (DVT) surveillance and included both symptomatic (S‐VTE) and asymptomatic VTE (A‐VTE) end points. These studies consistently demonstrated 44% to 63% reductions in VTE without increases in major bleeding.[11] Because of the strong relationship between A‐VTE and S‐VTE outcomes, and a paucity of studies using only S‐VTE outcomes, AT8 judged that A‐VTE outcomes were valid to include, whereas the new guidelines reject the use of asymptomatic VTE end points.[5, 8, 15] To minimize financial and intellectual conflicts of interest, AT9 also used methodologists rather than VTE experts as topic editors, excluded conflicted experts from voting on recommendations, and attempted to estimate patient values and preferences.[15] As a result, AT9 makes fewer strong recommendations (182 1A recommendations in 2008, but only 29 in 2012), replacing them with weak suggestions.
WHAT DO THE NEW GUIDELINES RECOMMEND?
AT8 recommended anticoagulant prophylaxis for acutely ill medical inpatients with known risk factors, but did not recommend routine thromboprophylaxis. However, because of well‐known problems with underprophylaxis,[16, 17, 18, 19] particularly in medical patients, the low risk of bleeding, and difficulties with explicitly defining low‐risk patients, many discounted the need for VTE risk stratification.
Both new guidelines recommend prophylaxis for many nonsurgical patients, but discourage routine thromboprophylaxis for nonsurgical inpatients. AT9 specifically recommends against any thromboprophylaxis for low‐risk medical inpatients, implying that many nonsurgical, non‐critical care patients belong in this category, citing lower estimates of benefit, lower estimates of VTE risk, and potential bleeding risks.
The guidelines[5, 8] agree that, when indicated and absent contraindications, anticoagulant prophylaxis is preferred over mechanical prophylaxis, and agree there is insufficient evidence to recommend 1 anticoagulant over another.
For patients at risk of both VTE and bleeding, ACP‐1 states that intermittent pneumatic compression (IPC) devices are a reasonable option, given the evidence showing benefit in surgical patients. However, ACP‐1 recommends against graduated compression stockings (GCS) in nonsurgical patients based on a meta‐analysis dominated by the CLOTS‐1 (Clots in Legs Or sTockings after Stroke) trial, which found that thigh‐high GCS increased the risk of skin breakdown without reducing VTE[20] in immobilized stroke patients. AT9 does not recommend against GCS for patients facing bleeding and VTE risk. AT9 notes the hazards of generalizing results from stroke patients, and also considers the somewhat contradictory results from the CLOTS‐2 trial in stroke patients, which found a lower rate of VTE with thigh‐high GCS than with knee‐high GCS.[21] AT9 designates a recommendation of 2C for either IPC devices or thigh‐high GCS for those at VTE risk when anticoagulants are contraindicated.
Combination mechanical‐pharmacologic prophylaxis has proven superior in some surgical populations, and many hospitals use combined prophylaxis in high‐risk medical patients. However, combination prophylaxis has not been studied in this population. ACP‐1 does not comment on the practice; AT9 does not recommend for or against it. Institutions that use combination prophylaxis should be aware that although it may seem logical to extrapolate estimates of benefit seen in selected surgical patients, this is not a recommended practice.
RCTs for thromboprophylaxis in nonsurgical inpatients provided prophylaxis for 6 to 21 days. Neither ACP‐1 or AT9 recommend routinely extending prophylaxis beyond the hospital stay, citing an RCT[22] in which the benefit of extended duration low molecular weight heparin was limited to selected subsets of patients and offset by bleeding complications. AT9 suggests prophylaxis for 6 to 21 days, until full mobility is restored, or until dischargewhichever comes first.[8] However, we know of no study that establishes a mobility level at which prophylaxis can be safely discontinued, especially in inpatients with multiple risk factors.
ESTIMATING RISK AND BENEFIT OF PROPHYLAXIS AND LIMITATIONS OF METHODS
Calculating risk/benefit ratios for thromboprophylaxis requires estimates of baseline VTE and bleeding risks, and estimates of the impact of prophylaxis on those baseline risks. Methods to estimate the impact of prophylaxis on S‐VTE from studies relying on A‐VTE all have limitations, as acknowledged by the AT9 introduction.[15]
The ACP‐1 review found the only significant effect of prophylaxis on medical inpatients was a modest reduction in PE and a modest increase in total bleeding events, without effects on major bleeding, DVT, or mortality.[6] The authors summarized the findings as indicative of little or no net benefit for the medical population as a whole. The ACP‐1 review derives estimates of S‐VTE risk, bleeding, and mortality from control (baseline) and interventional arms of RCTs that used routine VTE screening, and included A‐VTE end points. The baseline risk of VTE could potentially be overestimated, because the populations in the trials are not representative of the entire medical population.
On the other hand, pooling trials with screening‐detected VTE to estimate S‐VTE outcomes is a questionable practice that may falsely lower estimates of VTE prophylaxis benefit. Screening‐detected VTE may be treated or declared a study end point before it becomes symptomatic. MEDENOX (Medical Patients With Enoxaparin) is an illustrative example.[12] The 263 placebo recipients suffered 37 A‐VTEs and 4 S‐VTEs. The 272 enoxaparin recipients suffered 17 A‐VTEs and 3 S‐VTEs. Patients at the highest risk of S‐VTE were counted as reaching an end point before they could develop symptoms; this happened more than twice as often in the placebo arm. This decreases both estimates of baseline VTE risk and the measured benefit of prophylaxis for S‐VTE. Screening could conceivably reduce measured effects on mortality as well, because patients begin VTE therapy earlier. Per ACP‐1, the estimated risk for DVT is lower than for PE, running counter to literature experience[8, 16] and raising issues of face validity. The ACP‐1 review accepts all original definitions of major bleeding, including a 2 g/dL drop in hemoglobin,[12] which commonly occurs without any bleeding or clinical consequence, and bleeding events were ascribed to heparins up to 120 days after randomization, long after they could have been responsible.
Previous meta‐analyses of thromboprophylaxis studies[23, 24] shared many of these same limitations, but did not ascribe bleeding complications to heparins for this extended duration, and had point estimates that suggested a larger impact from prophylaxis than ACP‐1. Dentali et al., for example, showed statistically significant impact on PE (relative risk [RR] 0.43), fatal PE (RR 0.38), and a nearly statistically significant large impact on DVT (RR 0.47, 95% confidence interval [CI]: 0.22‐1.00),[24] whereas ACP‐1 estimated a smaller significant impact on PE (RR 0.69), no significant difference in fatal PE, and a much smaller estimate of the impact on DVT (RR 0.78, 95% CI: 0.45‐1.35) (Table 2).
| Baseline Risk | Relative Effect (95% CI) | Absolute Effect per 1000 Patients Treated (95% CI) | |
|---|---|---|---|
| |||
| ACP guideline review (Lederle), UFH or LMWH vs placebo/no treatment, medical patients | |||
| Mortality | 6.6 | OR 0.94 (0.84‐1.04) | 4 fewer (11 fewer to 3 more) |
| Major bleeding | 0.25 | OR 1.49 (0.91‐2.43) | 1 more (no effect to 3 more) |
| Symptomatic DVT | 0.96 | OR 0.78 (0.45‐1.35) | 2 fewer (6 fewer to 4 more) |
| PE | 1.2 | OR 0.69 (0.52‐0.90) | 4 fewer (6 fewer to 1 fewer) |
| Fatal PE | 0.30 | OR 0.77 (0.43‐1.37) | 1 fewer (2 fewer to 1 more) |
| ACCP AT9 (Kahn), non‐critical care medical inpatients, anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Mortality | 4.5 | OR 0.97 (0.79‐1.19) | 1 fewer (9 fewer to 8 more) |
| Major bleeding | 0.40 | OR 1.32 (0.73‐2.37) | 1 more (1 fewer to 6 more) |
| Thrombocytopenia | 0.13 | OR 0.92 (0.54‐1.53) | 1 fewer (6 fewer to 7 more) |
| Symptomatic DVT | |||
| Padua score 4 | 0.2 | RR 0.47 (0.22‐1) | 1 fewer (1 fewer to no effect) |
| Padua score 4 | 6.7 | 34 fewer (51 fewer to no effect) | |
| ACCP AT9 (Kahn) non‐critical care medical inpatients, Anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Nonfatal PE | |||
| Padua score 4 | 0.2 | RR 0.61 (0.23‐1.67) | 1 fewer (1 fewer to 1 more) |
| Padua score 4 | 3.9 | 15 fewer (30 fewer to 36 more) | |
| Fatal PE | 0.4 | RR 0.41 (0.22‐0.76) | 2 fewer (1 fewer to 3 fewer) |
| ACCP AT9 (Kahn), critical care medical inpatients, any heparin (LMWH, UFH) vs placebo/no treatment) | |||
| Mortality | 9.4 | RR 1.01 (0.04‐2.57) | 1 more (56 fewer to 148 more) |
| Major bleeding | 2.7 | RR 2.09 (0.54‐8.16) | 29 more (12 fewer to 190 more) |
| Symptomatic DVT | 5.8 | RR 0.86 (0.59‐1.25) | 4 fewer (12 fewer to 8 more) |
| Pulmonary embolus | 4.2 | RR, 0.73 (0.26‐2.11) | 11 fewer (31 fewer to 47 more) |
AT9 used a variety of methods to estimate each component of the risk/benefit equation. Critical care and non‐critical care estimates were generated independently, but because of limited data, the critical care estimates were highly imprecise. In non‐critical care patients, as in ACP‐1, treatment effects were estimated from RCTs that routinely screened for A‐VTE, and they adapted the Dentali et al. estimate of DVT risk reduction. The baseline risk for bleeding and mortality were derived from the control population of the same meta‐analysis.[24]
Using a novel approach, AT9 estimated baseline nonsurgical VTE risk from a prospective observational cohort study of 1180 medical inpatients divided into high‐ and low‐risk groups by a point‐scoring system.[25] Deriving risk estimates from an observational cohort has theoretical advantages. Many patients did not receive prophylaxis, allowing for unadjusted risk estimates; they represented a cross‐section of medical inpatients rather than a selected trial population, and risk estimates were not reduced by the culling of screen‐detected A‐VTE.
The Padua risk‐assessment model (RAM) (Table 3) defines high VTE risk as a cumulative score 4. There were 60.3% of patients at low risk and 39.7% at high risk using this threshold. Among unprophylaxed patients, VTE occurred in 11% of high‐risk patients versus 0.3% of low‐risk patients (hazard ratio 32.0, 95% CI: 4.1251.0).
| Baseline Features | Score |
|---|---|
| |
| Active cancer* | 3 |
| Previous VTE (excluding superficial thrombosis) | 3 |
| Reduced mobility | 3 |
| Already known thrombophilic condition | 3 |
| Recent (1 month) trauma and/or surgery | 2 |
| Elderly age (70 years) | 1 |
| Heart and/or respiratory failure | 1 |
| Acute myocardial infarction or stroke | 1 |
| Acute infection and/or rheumatologic disorder | 1 |
| Obesity (BMI 30) | 1 |
| Ongoing hormonal treatment | 1 |
PADUA: A CLOSER LOOK
In the Padua study, 60% of the population appeared to be at such low risk for VTE that prophylaxis would seem unnecessary, but closer scrutiny should raise concern about generalizing these results. Of the 711 low‐risk patients, 1% were immobile, only 6% had cancer, 6% were obese, and only 12% had any acute infection or inflammatory condition, yet their mean length of stay was 7.9 days. These characteristics do not apply to 60% of American inpatients. Furthermore, 964 of 2208 eligible patients (44%) were excluded because they required therapeutic anticoagulation.[25]
Correspondence with the authors revealed that the 2 PEs in patients with Padua scores 4 occurred among 192 patients with a risk score of 3 (Figure 1), a 1% (2/192) risk of PE. This is a very small sample, and the true risk of VTE for medical inpatients with a risk score of 3 may be lower or significantly higher. In the Padua population, a risk score of 3 equated to a VTE risk of 6.9%, whereas those with a score of 0 to 2 had no VTE. For those adapting the Padua model, careful consideration of using a cutoff of 3, versus 4, is warranted.

IMPLICATIONS FOR VTE PROTOCOL IMPLEMENTATION AND IMPROVEMENT TEAMS
AT9 and ACP‐1 sought to focus on S‐VTE, remove bias from recommendations, and highlight potential risks of unnecessary prophylaxis in low‐risk patients. They have largely succeeded in these important goals. However, the complexity of the new guidelines and lack of consensus about VTE risk assessment pose significant challenges to improvement teams tasked with implementing the guidelines in real‐world settings.
CHOOSING A VTE RAM
The fundamental question is: How can hospitals assess VTE risk, assure adequate prophylaxis for patients who need it, while minimizing excess prophylaxis, in a practical, efficient way?
Approach 1: Opt Out Approach
Both guidelines discourage universal prophylaxis for inpatients without contraindications unless the physician opts out. Although the simplicity of this approach is appealing, the low rate of VTE in a substantial segment of the medical inpatient population and known risks of thromboprophylaxis make this strategy suboptimal.
Approach 2: No VTE RAM
ACP‐1 notes that evidence is not sufficient to recommend 1 RAM over another, and essentially advises leaving prophylaxis decisions up to an individual physician's judgment. Although the evidence may not prove which system is best, prophylaxis reliability is dismal when there is no system or when hospitals offer prophylaxis options without guidance.[26, 27] Widespread, well‐documented underprophylaxis[16, 17, 18, 19] is largely the result of relying on unguided physician judgment and relatively passive interventions like educational sessions and pocket cards.[8] This approach also deprives improvement teams of standard definitions of VTE risk, bleeding risk, and adequate prophylaxis necessary to measure and improve VTE prophylaxis. Because of significant gray areas in the literature and varied infrastructure, institutions will not implement identical VTE prevention programs, but institutional standardization remains a cornerstone of improvement.
Approach 3: Buckets of Risk
The AT8 approach to risk assessment was to place patients into VTE risk groups described in the text, rather than have an individualized point‐scoring system.[11] These assessments can be made in seconds with high levels of interobserver agreement, implemented without undue effort, and spur high levels of compliance.[28, 29] Most importantly, implementation was associated with a 40% reduction in hospital‐associated VTE (RR 0.69, 95% CI: 0.470.79) without detectable increases in bleeding or heparin‐induced thrombocytopenia. Although this strategy has not been tested in randomized trials, it has been replicated in multiple real‐world settings that avoid concerns about generalizability due to imperfect trial populations.[28, 30]
The most popular bucket model in common use, derived from a table in the AT8 guidelines, is similar to models presented in UK National Institute for Health and Care Excellence guidelines for medical inpatients.[31] These models are potentially less precise than point‐based systems, but offer simplicity, ease of use, and improved physician acceptance, and thus may be more effective than point‐based models in settings without advanced clinical decision support. The models are flexible to reflect greater or lesser degrees of aggressiveness in defining risk categories, and can be used to approximate some point‐based systems.
Approach 4: Individualized Point‐Based RAM
AT9 authors used the Padua VTE RAM to define low‐ and high‐risk patients for VTE in their recommendation for medical inpatients. The Padua model appears relatively simple, but it does require calculations, and there is a paucity of data for implementation experience with it. As mentioned above, if teams use the Padua model, the optimal cutoff (3 vs 4) for recommending prophylaxis is uncertain, and both should be considered.
The Caprini point‐based system is not mentioned in the guideline for thromboprophylaxis in medical inpatients, but in our collaborative improvement experience, it is perhaps the most commonly used point‐based model for medical inpatients.[28, 30] It is also embedded in AT9 recommendations for prophylaxis in the nonorthopedic surgical population,[9] and thus is tempting to use for both medical and surgical patients. There are several caveats to those considering the use of these more complex point‐based models. Complex point‐based RAM suffer from poor interobserver agreement.[32] They have also had limited ability to exclude low‐risk patients from prophylaxis in validation studies,[33] and have not been tested extensively in medical populations. Although AT9 considers the Caprini RAM relatively easy to use,[9] our experience in collaboratives suggests that for many hospitals, the model is too complex to be used reliably.[28, 30] Clinicians often simply bypass the clinical decision support offered in the tool, rather than checking off all risk factors, adding up the point total, and identifying the appropriate prophylaxis choices based on the point total.[28] Other point‐based RAM (reviewed elsewhere[34, 35, 36]) pose similar implementation challenges.
On the other hand, centers with more sophisticated clinical decision support and a strong improvement framework can overcome some of these challenges to get good results with complex point‐based models. A forcing function can ensure that practitioners complete all risk‐assessment tasks. Providers can check off the VTE risk factors and bleeding risk factors on 1 screen, and several factors like age, body mass index, and renal function can be autopopulated. Instead of asking the provider to add up points, the combination of answers checked off on the first screen can drive behind‐the‐scenes calculations and seamlessly lead providers to prophylaxis choices appropriate for that combination of VTE and bleeding risks. Customized models can be designed for a wide variety of services. Similar strategies can ease adaption with more complex qualitative models as well.[37]
BOTTOM LINE IN CHOOSING A VTE RAM
Many medical inpatients are at high risk for VTE, but others are not at sufficient risk to warrant prophylaxis. VTE risk assessment should be embedded in admission, transfer, and perioperative order sets and may need a hard stop to insure completion. There is a trend to favor individualized point‐based models over models that place patients in groups of risk, but evidence is insufficient to recommend 1 type of RAM over another, and more complex point‐based models often require extensive local customization and algorithmic clinical decision support to effectively implement them. Centers without advanced capability may find the bucket models more effective. We urge improvement teams to trial their RAM with common patient case scenarios, and to make a choice based on an effort‐benefit analysis, feedback from their clinicians, and the level of customization in clinical decision support available to them.
OTHER IMPLEMENTATION STRATEGIES
VTE and bleeding risk change during hospitalization. We have used ongoing daily surveillance and measurement of patients on no prophylaxis to prompt concurrent intervention (ie, measure‐vention) to increase prophylaxis for patients at risk.[28] Improvement teams should focus not only on increasing prophylaxis for those at risk, but should also use measure‐vention, checklists, or other techniques to identify low‐risk (eg, ambulating) patients for cessation of overly aggressive prophylaxis. Efforts to improve early progressive ambulation, limit central venous catheters to those who truly need them, and improve adherence to mechanical prophylaxis can also reduce VTE, as well as benefitting patient populations in other ways.
We recognize there are several approaches to close the implementation gap in delivering thromboprophylaxis judiciously but reliably, and encourage research and publication of varied strategies. Last, we hope efforts to limit unnecessary prophylaxis and challenges inherent in implementing new and complex guidelines do not increase the morbidity and mortality of hospital‐acquired VTE, by derailing the delivery of prophylaxis to those in whom the benefits outweigh the risks.
Disclosures: Dr. Merli has conducted research for Johnson & Johnson, Bristol Myers Squibb, and Portala Scientific and has been a consultant for Johnson & Johnson and Bristol Myers Squibb.
- US Department of Health and Human Services. Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. 2008. Available at: http://www.surgeongeneral.gov/topics/deepvein/index.html. Accessed January 29, 2013.
- , , , et al. Incidence of venous thromboembolism in hospitalized patients vs. community residents. Mayo Clin Proc. 2001;76:1102–1110.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815.
- , , . New onset of venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118(6):1680–1684.
- , , , , . venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625–632.
- , , , . Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , et al. Prevention of VTE in nonsurgical patients. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Prevention of VTE in nonorthopedic surgical patients. Chest. 2012;141(2 suppl):e227S–e277S.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S–453S.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- , , , et al. The outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registry. J Thromb Haemost. 2004;2:1892–1898.
- , , , et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936–945.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387–394.
- , , , et al.; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost. 2010;103(4):736–748.
- , , , et al; CLOTS Trials Collaboration. Effectiveness of thigh‐length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomized controlled trial. Lancet. 2009;373(9679):1958–1965.
- CLOTS (Clots in Legs Or sTockings after Stroke) Trial Collaboration. Thigh‐length versus below‐knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med. 2010;153(9):553–562.
- , , , et al. Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
- , , , , . Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2007;167(1)476–486.
- , , , , . Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007; 46(4):278–288.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , . Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes. J Hosp Med. 2009;4(2):81–89.
- . Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? J Hosp Med. 2009;4(2):77–80.
- , . Designing and implementing effective VTE prevention protocols: lessons from collaboratives. J Thromb Thrombolysis. 2010;29(2):159–166.
- , , , et al. Optimizing prevention of hospital acquired venous thromboembolism: prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10–18.
- , , , et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38(7):301–310.
- NHS National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. NICE Clinical Guideline 92. 2010. Available at: http://www.nice.org.uk/guidance/CG92. Accessed April 18, 2013.
- , , , , . Reliability of a point‐based VTE risk assessment tool in the hands of medical residents. J Hosp Med. 2011;6:195–201.
- , , , , , . A validation of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251(2):344–350.
- , , , . Risk assessment models for thromboprophylaxis of medical patients. Thromb Res. 2012;129:127–132.
- , , , , . Risk‐assessment models for predicting venous thromboembolism among hospitalized non‐surgical patients: a systematic review. J Thromb Thrombolysis. 2013;35:67–80.
- , , . The use of weighted and scored risk assessment models for venous thromboembolism. Thromb Haemost. 2012;108(6):1072–1076.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- US Department of Health and Human Services. Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. 2008. Available at: http://www.surgeongeneral.gov/topics/deepvein/index.html. Accessed January 29, 2013.
- , , , et al. Incidence of venous thromboembolism in hospitalized patients vs. community residents. Mayo Clin Proc. 2001;76:1102–1110.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815.
- , , . New onset of venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118(6):1680–1684.
- , , , , . venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625–632.
- , , , . Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , et al. Prevention of VTE in nonsurgical patients. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Prevention of VTE in nonorthopedic surgical patients. Chest. 2012;141(2 suppl):e227S–e277S.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S–453S.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- , , , et al. The outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registry. J Thromb Haemost. 2004;2:1892–1898.
- , , , et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936–945.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387–394.
- , , , et al.; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost. 2010;103(4):736–748.
- , , , et al; CLOTS Trials Collaboration. Effectiveness of thigh‐length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomized controlled trial. Lancet. 2009;373(9679):1958–1965.
- CLOTS (Clots in Legs Or sTockings after Stroke) Trial Collaboration. Thigh‐length versus below‐knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med. 2010;153(9):553–562.
- , , , et al. Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
- , , , , . Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2007;167(1)476–486.
- , , , , . Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007; 46(4):278–288.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , . Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes. J Hosp Med. 2009;4(2):81–89.
- . Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? J Hosp Med. 2009;4(2):77–80.
- , . Designing and implementing effective VTE prevention protocols: lessons from collaboratives. J Thromb Thrombolysis. 2010;29(2):159–166.
- , , , et al. Optimizing prevention of hospital acquired venous thromboembolism: prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10–18.
- , , , et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38(7):301–310.
- NHS National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. NICE Clinical Guideline 92. 2010. Available at: http://www.nice.org.uk/guidance/CG92. Accessed April 18, 2013.
- , , , , . Reliability of a point‐based VTE risk assessment tool in the hands of medical residents. J Hosp Med. 2011;6:195–201.
- , , , , , . A validation of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251(2):344–350.
- , , , . Risk assessment models for thromboprophylaxis of medical patients. Thromb Res. 2012;129:127–132.
- , , , , . Risk‐assessment models for predicting venous thromboembolism among hospitalized non‐surgical patients: a systematic review. J Thromb Thrombolysis. 2013;35:67–80.
- , , . The use of weighted and scored risk assessment models for venous thromboembolism. Thromb Haemost. 2012;108(6):1072–1076.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
Recent onset of rash, dehydration, and nonbloody diarrhea in an elderly man
An 80-year-old Hawaiian man of Chinese ancestry arrives at the emergency department with diarrhea and dehydration. You are called to admit him for acute renal failure. On entering the patient’s room, you note that he has a diffuse maculopapular rash and is wheezing.
Twenty-one months earlier, the patient suffered his first episode of gout. Since that time, he has been asymptomatic. Two months ago, his primary care physician obtained a uric acid level and found it elevated at 10.6 mg/dL. She started the patient on allopurinol 300 mg PO daily.
Twenty days ago (approximately 6 weeks after initiation of allopurinol), the rash developed along with generalized pruritus. The patient’s primary care physician referred him to a dermatologist for skin biopsy, and he discontinued allopurinol 11 days ago.
Just in the past week, the patient began to experience a metallic taste in his mouth, as well as anorexia, malaise, chills, dysuria, and nonbloody diarrhea. He became nauseous and decreased his oral intake, which led to dehydration and progressive weakness.
Additional medical history
- The patient’s medical history is significant for renal insufficiency, type 2 diabetes mellitus, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, chronic nasal allergies, benign prostatic hypertrophy, osteoarthritis, and gout.
- He is taking the following medications: fluticasone inhalant 110 mcg daily; fluticasone propionate one spray in both nostrils daily; montelukast 10 mg PO daily; irbesartan 300 mg PO daily; amlodipine 5 mg PO daily; glyburide 2.5 mg PO BID; triamterene/hydrochlorothiazide 37.5/25 mg PO QOD; albuterol 90 mcg 2 puffs q6h prn; terazosin 2 mg PO qhs; simvastatin 40 mg PO daily; azelastine 137 mcg 1 spray in both nostrils prn; meclizine 25 mg PO daily prn; fexofenadine 150 mg PO qPM; cyclobenzaprine 10 mg PO qhs prn; and hydrocodone/acetamino- phen 5/500 mg 2 tabs PO daily prn.
Social history
- The patient recently arrived from Hawaii to visit his wife’s family.
- He does not drink alcohol, but he smokes 4 cigarettes a day.
Review of systems
- A review of systems is negative for the following: fever, sick contacts, history of renal calculi, hemoptysis, ocular or ENT symptoms, history of hepatic disease, peripheral neuropathy, and neurologic symptoms.
Physical examination
- The patient is alert and cooperative.
- Temperature is 97.9oF, blood pressure 110/52 mm Hg, pulse 112 beats per minute, respiratory rate 16 breaths per minute, oxygen saturation 96% on room air.
- Mucous membranes are dry.
- Auscultation of the heart is normal.
- Significant wheezing is present in bilateral lung fields, but requiring no use of accessory muscles during respiration.
- Abdominal exam is remarkable only for obesity.
- Trace pitting edema is present in both lower extremities.
- The maculopapular rash is diffuse and nonblanching. Scaling on the trunk, areas of erythema below the umbilicus, and coalescing macular lesions on bilateral lower extremities are present.
- Joints are not swollen or tender, and there are no tophi.
- There are no focal neurologic deficits, and deep tendon reflexes are normal.
Laboratory studies completed in the ED
- Blood urea nitrogen, 112 mg/dL ; creatinine, 3 mg/dL ; glomerular filtration rate (GFR), 22 mL/min (baseline ratio of blood urea nitrogen/creatinine, 36:1.57; baseline GFR, 43 mL/min)
- White blood cell count, 15 k/uL
- Hemoglobin, 14.3 g/dL ; hematocrit, 43.6%; platelet count, 307/uL
- Alanine aminotransferase and aspartate aminotransferase, 177 and 139 IU/L, respectively
- Direct, indirect, and total bilirubin, 0.60, 1.19, and 1.79 mg/dL, respectively
- Serum eosinophils, 22% (normal <6%)
- Erythrocyte sedimentation rate, 50 mm/h.
Radiology
- Chest radiographs (posterior-anterior, lateral) show a bilateral process consistent with atelectasis or lung scarring.
- Noncontrast computed tomography (CT) of the thorax confirms parenchymal scarring but no acute process.
- Hepatic sonography reveals increased echogenicity of the liver parenchyma consistent with an acute hepatocellular process.
- Magnetic resonance imaging (MRI) of the abdomen shows a diffuse process in the liver with trace parahepatic ascites.
- Renal ultrasound shows bilateral renal cysts with no hydronephrosis or urolithiasis.
- Cardiac echocardiography reveals left ventricular hypertrophy but normal ejection fraction.
- Noncontrast CT (head) and MRI (brain) show an acute right frontoparietal cerebrovascular accident and an old lacunar infarct.
Dermatologist’s report
- A skin biopsy reveals lymphocytic perivascular infiltrate with scattered eosinophils and mild spongiosis consistent with vasculitis.
Follow-up laboratory data
- Acute hepatitis panel is negative
- Uric acid, 13.8 mg/dL
- Urine eosinophils, 31% (normal <1%)
- Anti-neutrophil cytoplasmic antibody IgG, 1:40 mildly elevated (normal<1:20)
- Anti-nuclear antibody (ANA) IgG, none
- Glycosylated hemoglobin, 7.1%
- High-sensitivity C-reactive protein, 207.96 mg/L (>10 mg/L is very high)
In summary, this patient’s erythematous rash is a biopsy-confirmed vasculitis. Additional findings are hepatitis, acute on chronic renal failure, eosinophilia, and leukocytosis.
Q/ What is your presumptive diagnosis?
Allopurinol hypersensitivity syndrome
Allopurinol hypersensitivity syndrome (AHS) is a diffuse vasculitis induced by a type III hypersensitivity reaction, possibly to oxypurinol, allopurinol’s toxic metabolite. The exact pathophysiology is unknown, but oxypurinol levels correlate positively with the risk of AHS.1 Thiazides may increase oxypurinol levels.2
Signs and symptoms of AHS include fever, erythematous skin rash, eosinophilia, hepatitis, progressive renal insufficiency, and leukocytosis. Case reports have also attributed septic shock, myocardial infarction, and Guillain-Barré syndrome to AHS.3-6 The incidence of AHS is 0.1% to 0.4% of patients treated with allopurinol; mortality approaches 25%.1
Q/ What are the diagnostic criteria for AHS?
Singer and Wallace have outlined diagnostic criteria for AHS,7 the first being a clear history of exposure to allopurinol.
Second, the clinical profile usually takes one of the following forms:
- The patient exhibits at least 2 of the following major features: worsening renal function, acute hepatocellular injury, or a rash (toxic epidermal necrolysis, erythema multiforme, or diffuse maculopapular or exfoliative dermatitis) or
- The patient exhibits just one of the major features and at least one of the following minor features: fever, eosinophilia, or leukocytosis.
Third, there is no history of exposure to another drug that may cause a similar clinical picture.
Q/ What is the accepted treatment for ahS?
Although there is no well-established treatment plan, the standard of care is to discontinue allopurinol, administer parenteral corticosteroids followed by oral taper, and offer supportive management. Desensitization protocols are difficult and hypersensitivity reactions may recur. Early withdrawal of steroids has been reported to result in recurrence of symptoms. However, no strong data exist for determining an optimal length of corticosteroid therapy, or the number of days that constitutes “early” withdrawal of steroids. Mortality is high, even with seemingly adequate treatment.
Q/ Which patients are most at risk for ahS?
Definite risk factors for AHS include recent onset of allopurinol therapy, the presence of HLA-B5801 allele in patients of Han Chinese and European ancestry, and chronic kidney disease.1,8,9 Suggested risk factors include concomitant use of thiazide diuretics with allopurinol, treatment of asymptomatic hyperuricemia, and high allopurinol dose relative to renal function.
Q/ What are the indications for allopurinol therapy?
Indications for allopurinol therapy:1,10
- Failure of uricosuric drugs or contraindications to their use
- Frequent attacks of gouty arthritis (≥3 per year)
- Nephrolithiasis
- Marked overproduction of urate, such as seen in tumor lysis syndrome
- The presence of tophi.
For patients with appropriate indications for allopurinol therapy, treat with the minimum effective dose. Initiation of allopurinol is controversial for a patient with a single lifetime episode of gout. However, most patients with one episode of gout will develop recurrent gout.8
In patients with creatinine clearance >60 mL/min, allopurinol is usually started at 100 mg oral daily and titrated every 2 to 3 weeks until reaching the desired effectiveness.8 One retrospective study suggests that initiating allopurinol at a dose of 1.5 mg per unit of estimated GFR may reduce the risk of AHS.11
Our patient’s case: Treatment, discharge, readmission
With our patient, we started intravenous normal saline fluid boluses and parenteral methylprednisolone, 60 mg q6h. We discontinued triamterene/hydrochlorothiazide due to his hyperuricemia, and irbesartan and glyburide due to renal failure. Basal and rapid-acting insulins maintained good glycemic control. To control his wheezing and dyspnea, we began albuterol/ipratropium nebulizer treatments and oxygen delivery via nasal cannula. Nephrology, pulmonology, and rheumatology consultants agreed with these management decisions.
Over the next 9 days, the patient’s renal function improved and his rash started to resolve. His wheezing fluctuated but persisted throughout the hospital stay. We stopped nasal oxygen delivery, and his oxygen saturation remained normal (96%-98%) on room air. He was subsequently discharged in stable condition on a 2-week prednisone taper starting at 30 mg bid.
Thirteen days later, he was readmitted with a right frontoparietal cerebrovascular accident (CVA). He developed respiratory distress, which led to respiratory arrest, and was ventilated. He became hypotensive, lapsed into shock, and died a few days later (approximately one month after initial presentation).
The appropriate use of allopurinol—a second look
This case raised many questions for our inpatient team concerning not just AHS, but the appropriate use and dosing of allopurinol. Allopurinol is widely used for hyperuricemia and gout because it is effective for all causes of hyperuricemia and is inexpensive. Given that 3.9%12 of the general population has gout and that its prevalence has increased with rising rates of obesity, the importance of AHS is not abstract.
Revisiting initial treatment choices. Our patient was at risk for this syndrome due to his Chinese ancestry, recent onset of allopurinol therapy, concomitant use of triamterene/hydrochlorothiazide, and impaired renal function. Although his uric acid level was >10 mg/dL when treatment was started, his risk factors may have precluded initiation of allopurinol. Furthermore, the prescribed dose of allopurinol (300 mg) was too high for his baseline GFR; 100 mg daily would have been more appropriate. It could be argued that hydrochlorothiazide would not be an antihypertensive of choice due to its hyperuricemic effects. Switching the thiazide to a loop diuretic would offer no benefit, because loop diuretics also cause hyperuricemia. (More on which antihypertensive agent would have been appropriate in a bit.)
The role of AHS in the patient’s death is unclear. He was at high risk for stroke considering his age, comorbidities, and evidence of an old lacunar infarct. Myocardial infarctions have been reported as one cause of death in AHS, but we have found no reports of associated stroke. However, the temporal proximity of the CVA suggests a contributing effect of AHS. The adequacy of treatment is also brought into question. Early withdrawal of glucocorticoids can be associated with relapse of AHS; in retrospect, the prednisone dose may have been too low or the taper too short, or both.
Therapeutic alternatives to allopurinol were available. The most appropriate initial option for this patient likely was no pharmacologic intervention at all but a focus on weight loss and diet. The risk of gouty attacks in men increases as body mass index rises above normal (≥25 kg/m2) and decreases with weight loss. Lower calorie diets with decreased saturated fat, higher complex carbohydrates, and allowed proteins (low-fat dairy products) are more palatable and more effective than strict low-purine diets.
A urate-lowering antihypertensive may have been a better option—specifically, losartan rather than irbesartan and triamterene/hydrochlorothiazide.8,13 Losartan has uricosuric properties at the 50-mg dose.14 When compared in different studies with irbesartan, enalapril, and candesartan, losartan alone lowered serum urate levels. Increasing losartan to 100 mg could provide better hypertensive control but has not been found to lower urate levels more dramatically than the lower dose.13
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers have been found to blunt the urate-elevating effects of thiazides. Therefore, if the patient’s blood pressure was not adequately controlled on losartan alone, the combination of losartan/hydrochlorothiazide would be a reasonable choice because minimal or no change in serum uric acid levels would be expected.15
Febuxostat, a nonpurine selective inhibitor of xanthine oxidase, is a potential alternative to allopurinol in patients with gout. It is a more potent urate-lowering agent than allopurinol and can be used without dosage reduction in mild (creatinine clearance [CrCl], 60-89 mL/min) to moderate (CrCl, 30-59 mL/ min) renal insufficiency. Febuxostat is metabolized primarily in the liver—in contrast to allopurinol, which is excreted by the kidneys—and may increase liver transaminase levels.10 Although febuxostat was not available at the time our patient developed AHS, it would not have been indicated in asymptomatic hyperuricemia.
This compelling case reminds us to carefully consider the indications and risk factors in using allopurinol, and to be aware of the rare but sometimes devastating consequences of this commonly used drug.
CORRESPONDENCE
Tahirah Tyrell, MD, Rochester General Medical Group, 1425 Portland Avenue, Rochester, NY 14621;
[email protected].
1. Lee HY, Ariyasinghe J, Thirumoorthy T. Allopurinol hypersensitivity syndrome: a preventable severe cutaneous adverse reaction? Singapore Med J. 2008;49:384-387.
2. Markel A. Allopurinol hypersensitivity and DRESS syndrome. Am J Med. 2008;121:e25.
3. Koike K, et al. Adverse reaction case reports. React Wkly. 2008 Sept 6;1218:5.
4. Mete N, Yilmaz F, et al. Adverse reaction case reports. React Wkly. 2004 June 26;1007:7.
5. Benito-León J, Porta-Etessam J. Guillain-Barré syndrome and allopurinol- induced hypersensitivity. Eur Neurol. 2001;45:186-187.
6. Makar-Ausperger KMA. Allopurinol/furosemide. Hypersensitivity syndrome: case report. Reactions Wkly. 2007 Oct 13;1173:5.
7. Singer JZ, Wallace SL. The allopurinol hypersensitivity syndrome. Unnecessary morbidity and mortality. Arthritis Rheum. 1986; 29: 82-87.
8. Becker M. Prevention of recurrent gout. UpToDate. April 10, 2013. Available at: http://www.uptodate.com/contents/prevention-ofrecurrent- gout?detectedLanguage=en&source=search_result&s earch=Prevention+of+recurrent+gout&selectedTitle=1%7E8&pr ovider=noProvider. Accessed August 1, 2013.
9. Ramasamy SN, Korb-Wells CS, Kannangara DR, et al. Allopurinol hypersensitivity: a systematic review of all published cases, 1950- 2012. Drug Saf. 2013;July 20. [Epub ahead of print].
10. Moreland LW. Febuxostat–treatment for hyperuricemia and gout? N Engl J Med. 2005;353:2505-2507.
11. Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64:2529-2536.
12. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63: 3136-3141.
13. Würzner G, Gerster JC, Chiolero A, et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricemia and gout. J Hypertens. 2001;19:1855-1860.
14. Terkeltaub RA. Clinical practice. gout. N Engl J Med. 2003;349:1647-1655.
15. Manolis AJ, Grossman E, Jelakovic B, et al. Effects of losartan and candesartan monotherapy and losartan/hydrochlorothiazide combination therapy in patients with mild to moderate hypertension. Losartan Trial Investigators. Clin Ther. 2000;22:1186-1203.
An 80-year-old Hawaiian man of Chinese ancestry arrives at the emergency department with diarrhea and dehydration. You are called to admit him for acute renal failure. On entering the patient’s room, you note that he has a diffuse maculopapular rash and is wheezing.
Twenty-one months earlier, the patient suffered his first episode of gout. Since that time, he has been asymptomatic. Two months ago, his primary care physician obtained a uric acid level and found it elevated at 10.6 mg/dL. She started the patient on allopurinol 300 mg PO daily.
Twenty days ago (approximately 6 weeks after initiation of allopurinol), the rash developed along with generalized pruritus. The patient’s primary care physician referred him to a dermatologist for skin biopsy, and he discontinued allopurinol 11 days ago.
Just in the past week, the patient began to experience a metallic taste in his mouth, as well as anorexia, malaise, chills, dysuria, and nonbloody diarrhea. He became nauseous and decreased his oral intake, which led to dehydration and progressive weakness.
Additional medical history
- The patient’s medical history is significant for renal insufficiency, type 2 diabetes mellitus, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, chronic nasal allergies, benign prostatic hypertrophy, osteoarthritis, and gout.
- He is taking the following medications: fluticasone inhalant 110 mcg daily; fluticasone propionate one spray in both nostrils daily; montelukast 10 mg PO daily; irbesartan 300 mg PO daily; amlodipine 5 mg PO daily; glyburide 2.5 mg PO BID; triamterene/hydrochlorothiazide 37.5/25 mg PO QOD; albuterol 90 mcg 2 puffs q6h prn; terazosin 2 mg PO qhs; simvastatin 40 mg PO daily; azelastine 137 mcg 1 spray in both nostrils prn; meclizine 25 mg PO daily prn; fexofenadine 150 mg PO qPM; cyclobenzaprine 10 mg PO qhs prn; and hydrocodone/acetamino- phen 5/500 mg 2 tabs PO daily prn.
Social history
- The patient recently arrived from Hawaii to visit his wife’s family.
- He does not drink alcohol, but he smokes 4 cigarettes a day.
Review of systems
- A review of systems is negative for the following: fever, sick contacts, history of renal calculi, hemoptysis, ocular or ENT symptoms, history of hepatic disease, peripheral neuropathy, and neurologic symptoms.
Physical examination
- The patient is alert and cooperative.
- Temperature is 97.9oF, blood pressure 110/52 mm Hg, pulse 112 beats per minute, respiratory rate 16 breaths per minute, oxygen saturation 96% on room air.
- Mucous membranes are dry.
- Auscultation of the heart is normal.
- Significant wheezing is present in bilateral lung fields, but requiring no use of accessory muscles during respiration.
- Abdominal exam is remarkable only for obesity.
- Trace pitting edema is present in both lower extremities.
- The maculopapular rash is diffuse and nonblanching. Scaling on the trunk, areas of erythema below the umbilicus, and coalescing macular lesions on bilateral lower extremities are present.
- Joints are not swollen or tender, and there are no tophi.
- There are no focal neurologic deficits, and deep tendon reflexes are normal.
Laboratory studies completed in the ED
- Blood urea nitrogen, 112 mg/dL ; creatinine, 3 mg/dL ; glomerular filtration rate (GFR), 22 mL/min (baseline ratio of blood urea nitrogen/creatinine, 36:1.57; baseline GFR, 43 mL/min)
- White blood cell count, 15 k/uL
- Hemoglobin, 14.3 g/dL ; hematocrit, 43.6%; platelet count, 307/uL
- Alanine aminotransferase and aspartate aminotransferase, 177 and 139 IU/L, respectively
- Direct, indirect, and total bilirubin, 0.60, 1.19, and 1.79 mg/dL, respectively
- Serum eosinophils, 22% (normal <6%)
- Erythrocyte sedimentation rate, 50 mm/h.
Radiology
- Chest radiographs (posterior-anterior, lateral) show a bilateral process consistent with atelectasis or lung scarring.
- Noncontrast computed tomography (CT) of the thorax confirms parenchymal scarring but no acute process.
- Hepatic sonography reveals increased echogenicity of the liver parenchyma consistent with an acute hepatocellular process.
- Magnetic resonance imaging (MRI) of the abdomen shows a diffuse process in the liver with trace parahepatic ascites.
- Renal ultrasound shows bilateral renal cysts with no hydronephrosis or urolithiasis.
- Cardiac echocardiography reveals left ventricular hypertrophy but normal ejection fraction.
- Noncontrast CT (head) and MRI (brain) show an acute right frontoparietal cerebrovascular accident and an old lacunar infarct.
Dermatologist’s report
- A skin biopsy reveals lymphocytic perivascular infiltrate with scattered eosinophils and mild spongiosis consistent with vasculitis.
Follow-up laboratory data
- Acute hepatitis panel is negative
- Uric acid, 13.8 mg/dL
- Urine eosinophils, 31% (normal <1%)
- Anti-neutrophil cytoplasmic antibody IgG, 1:40 mildly elevated (normal<1:20)
- Anti-nuclear antibody (ANA) IgG, none
- Glycosylated hemoglobin, 7.1%
- High-sensitivity C-reactive protein, 207.96 mg/L (>10 mg/L is very high)
In summary, this patient’s erythematous rash is a biopsy-confirmed vasculitis. Additional findings are hepatitis, acute on chronic renal failure, eosinophilia, and leukocytosis.
Q/ What is your presumptive diagnosis?
Allopurinol hypersensitivity syndrome
Allopurinol hypersensitivity syndrome (AHS) is a diffuse vasculitis induced by a type III hypersensitivity reaction, possibly to oxypurinol, allopurinol’s toxic metabolite. The exact pathophysiology is unknown, but oxypurinol levels correlate positively with the risk of AHS.1 Thiazides may increase oxypurinol levels.2
Signs and symptoms of AHS include fever, erythematous skin rash, eosinophilia, hepatitis, progressive renal insufficiency, and leukocytosis. Case reports have also attributed septic shock, myocardial infarction, and Guillain-Barré syndrome to AHS.3-6 The incidence of AHS is 0.1% to 0.4% of patients treated with allopurinol; mortality approaches 25%.1
Q/ What are the diagnostic criteria for AHS?
Singer and Wallace have outlined diagnostic criteria for AHS,7 the first being a clear history of exposure to allopurinol.
Second, the clinical profile usually takes one of the following forms:
- The patient exhibits at least 2 of the following major features: worsening renal function, acute hepatocellular injury, or a rash (toxic epidermal necrolysis, erythema multiforme, or diffuse maculopapular or exfoliative dermatitis) or
- The patient exhibits just one of the major features and at least one of the following minor features: fever, eosinophilia, or leukocytosis.
Third, there is no history of exposure to another drug that may cause a similar clinical picture.
Q/ What is the accepted treatment for ahS?
Although there is no well-established treatment plan, the standard of care is to discontinue allopurinol, administer parenteral corticosteroids followed by oral taper, and offer supportive management. Desensitization protocols are difficult and hypersensitivity reactions may recur. Early withdrawal of steroids has been reported to result in recurrence of symptoms. However, no strong data exist for determining an optimal length of corticosteroid therapy, or the number of days that constitutes “early” withdrawal of steroids. Mortality is high, even with seemingly adequate treatment.
Q/ Which patients are most at risk for ahS?
Definite risk factors for AHS include recent onset of allopurinol therapy, the presence of HLA-B5801 allele in patients of Han Chinese and European ancestry, and chronic kidney disease.1,8,9 Suggested risk factors include concomitant use of thiazide diuretics with allopurinol, treatment of asymptomatic hyperuricemia, and high allopurinol dose relative to renal function.
Q/ What are the indications for allopurinol therapy?
Indications for allopurinol therapy:1,10
- Failure of uricosuric drugs or contraindications to their use
- Frequent attacks of gouty arthritis (≥3 per year)
- Nephrolithiasis
- Marked overproduction of urate, such as seen in tumor lysis syndrome
- The presence of tophi.
For patients with appropriate indications for allopurinol therapy, treat with the minimum effective dose. Initiation of allopurinol is controversial for a patient with a single lifetime episode of gout. However, most patients with one episode of gout will develop recurrent gout.8
In patients with creatinine clearance >60 mL/min, allopurinol is usually started at 100 mg oral daily and titrated every 2 to 3 weeks until reaching the desired effectiveness.8 One retrospective study suggests that initiating allopurinol at a dose of 1.5 mg per unit of estimated GFR may reduce the risk of AHS.11
Our patient’s case: Treatment, discharge, readmission
With our patient, we started intravenous normal saline fluid boluses and parenteral methylprednisolone, 60 mg q6h. We discontinued triamterene/hydrochlorothiazide due to his hyperuricemia, and irbesartan and glyburide due to renal failure. Basal and rapid-acting insulins maintained good glycemic control. To control his wheezing and dyspnea, we began albuterol/ipratropium nebulizer treatments and oxygen delivery via nasal cannula. Nephrology, pulmonology, and rheumatology consultants agreed with these management decisions.
Over the next 9 days, the patient’s renal function improved and his rash started to resolve. His wheezing fluctuated but persisted throughout the hospital stay. We stopped nasal oxygen delivery, and his oxygen saturation remained normal (96%-98%) on room air. He was subsequently discharged in stable condition on a 2-week prednisone taper starting at 30 mg bid.
Thirteen days later, he was readmitted with a right frontoparietal cerebrovascular accident (CVA). He developed respiratory distress, which led to respiratory arrest, and was ventilated. He became hypotensive, lapsed into shock, and died a few days later (approximately one month after initial presentation).
The appropriate use of allopurinol—a second look
This case raised many questions for our inpatient team concerning not just AHS, but the appropriate use and dosing of allopurinol. Allopurinol is widely used for hyperuricemia and gout because it is effective for all causes of hyperuricemia and is inexpensive. Given that 3.9%12 of the general population has gout and that its prevalence has increased with rising rates of obesity, the importance of AHS is not abstract.
Revisiting initial treatment choices. Our patient was at risk for this syndrome due to his Chinese ancestry, recent onset of allopurinol therapy, concomitant use of triamterene/hydrochlorothiazide, and impaired renal function. Although his uric acid level was >10 mg/dL when treatment was started, his risk factors may have precluded initiation of allopurinol. Furthermore, the prescribed dose of allopurinol (300 mg) was too high for his baseline GFR; 100 mg daily would have been more appropriate. It could be argued that hydrochlorothiazide would not be an antihypertensive of choice due to its hyperuricemic effects. Switching the thiazide to a loop diuretic would offer no benefit, because loop diuretics also cause hyperuricemia. (More on which antihypertensive agent would have been appropriate in a bit.)
The role of AHS in the patient’s death is unclear. He was at high risk for stroke considering his age, comorbidities, and evidence of an old lacunar infarct. Myocardial infarctions have been reported as one cause of death in AHS, but we have found no reports of associated stroke. However, the temporal proximity of the CVA suggests a contributing effect of AHS. The adequacy of treatment is also brought into question. Early withdrawal of glucocorticoids can be associated with relapse of AHS; in retrospect, the prednisone dose may have been too low or the taper too short, or both.
Therapeutic alternatives to allopurinol were available. The most appropriate initial option for this patient likely was no pharmacologic intervention at all but a focus on weight loss and diet. The risk of gouty attacks in men increases as body mass index rises above normal (≥25 kg/m2) and decreases with weight loss. Lower calorie diets with decreased saturated fat, higher complex carbohydrates, and allowed proteins (low-fat dairy products) are more palatable and more effective than strict low-purine diets.
A urate-lowering antihypertensive may have been a better option—specifically, losartan rather than irbesartan and triamterene/hydrochlorothiazide.8,13 Losartan has uricosuric properties at the 50-mg dose.14 When compared in different studies with irbesartan, enalapril, and candesartan, losartan alone lowered serum urate levels. Increasing losartan to 100 mg could provide better hypertensive control but has not been found to lower urate levels more dramatically than the lower dose.13
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers have been found to blunt the urate-elevating effects of thiazides. Therefore, if the patient’s blood pressure was not adequately controlled on losartan alone, the combination of losartan/hydrochlorothiazide would be a reasonable choice because minimal or no change in serum uric acid levels would be expected.15
Febuxostat, a nonpurine selective inhibitor of xanthine oxidase, is a potential alternative to allopurinol in patients with gout. It is a more potent urate-lowering agent than allopurinol and can be used without dosage reduction in mild (creatinine clearance [CrCl], 60-89 mL/min) to moderate (CrCl, 30-59 mL/ min) renal insufficiency. Febuxostat is metabolized primarily in the liver—in contrast to allopurinol, which is excreted by the kidneys—and may increase liver transaminase levels.10 Although febuxostat was not available at the time our patient developed AHS, it would not have been indicated in asymptomatic hyperuricemia.
This compelling case reminds us to carefully consider the indications and risk factors in using allopurinol, and to be aware of the rare but sometimes devastating consequences of this commonly used drug.
CORRESPONDENCE
Tahirah Tyrell, MD, Rochester General Medical Group, 1425 Portland Avenue, Rochester, NY 14621;
[email protected].
An 80-year-old Hawaiian man of Chinese ancestry arrives at the emergency department with diarrhea and dehydration. You are called to admit him for acute renal failure. On entering the patient’s room, you note that he has a diffuse maculopapular rash and is wheezing.
Twenty-one months earlier, the patient suffered his first episode of gout. Since that time, he has been asymptomatic. Two months ago, his primary care physician obtained a uric acid level and found it elevated at 10.6 mg/dL. She started the patient on allopurinol 300 mg PO daily.
Twenty days ago (approximately 6 weeks after initiation of allopurinol), the rash developed along with generalized pruritus. The patient’s primary care physician referred him to a dermatologist for skin biopsy, and he discontinued allopurinol 11 days ago.
Just in the past week, the patient began to experience a metallic taste in his mouth, as well as anorexia, malaise, chills, dysuria, and nonbloody diarrhea. He became nauseous and decreased his oral intake, which led to dehydration and progressive weakness.
Additional medical history
- The patient’s medical history is significant for renal insufficiency, type 2 diabetes mellitus, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, chronic nasal allergies, benign prostatic hypertrophy, osteoarthritis, and gout.
- He is taking the following medications: fluticasone inhalant 110 mcg daily; fluticasone propionate one spray in both nostrils daily; montelukast 10 mg PO daily; irbesartan 300 mg PO daily; amlodipine 5 mg PO daily; glyburide 2.5 mg PO BID; triamterene/hydrochlorothiazide 37.5/25 mg PO QOD; albuterol 90 mcg 2 puffs q6h prn; terazosin 2 mg PO qhs; simvastatin 40 mg PO daily; azelastine 137 mcg 1 spray in both nostrils prn; meclizine 25 mg PO daily prn; fexofenadine 150 mg PO qPM; cyclobenzaprine 10 mg PO qhs prn; and hydrocodone/acetamino- phen 5/500 mg 2 tabs PO daily prn.
Social history
- The patient recently arrived from Hawaii to visit his wife’s family.
- He does not drink alcohol, but he smokes 4 cigarettes a day.
Review of systems
- A review of systems is negative for the following: fever, sick contacts, history of renal calculi, hemoptysis, ocular or ENT symptoms, history of hepatic disease, peripheral neuropathy, and neurologic symptoms.
Physical examination
- The patient is alert and cooperative.
- Temperature is 97.9oF, blood pressure 110/52 mm Hg, pulse 112 beats per minute, respiratory rate 16 breaths per minute, oxygen saturation 96% on room air.
- Mucous membranes are dry.
- Auscultation of the heart is normal.
- Significant wheezing is present in bilateral lung fields, but requiring no use of accessory muscles during respiration.
- Abdominal exam is remarkable only for obesity.
- Trace pitting edema is present in both lower extremities.
- The maculopapular rash is diffuse and nonblanching. Scaling on the trunk, areas of erythema below the umbilicus, and coalescing macular lesions on bilateral lower extremities are present.
- Joints are not swollen or tender, and there are no tophi.
- There are no focal neurologic deficits, and deep tendon reflexes are normal.
Laboratory studies completed in the ED
- Blood urea nitrogen, 112 mg/dL ; creatinine, 3 mg/dL ; glomerular filtration rate (GFR), 22 mL/min (baseline ratio of blood urea nitrogen/creatinine, 36:1.57; baseline GFR, 43 mL/min)
- White blood cell count, 15 k/uL
- Hemoglobin, 14.3 g/dL ; hematocrit, 43.6%; platelet count, 307/uL
- Alanine aminotransferase and aspartate aminotransferase, 177 and 139 IU/L, respectively
- Direct, indirect, and total bilirubin, 0.60, 1.19, and 1.79 mg/dL, respectively
- Serum eosinophils, 22% (normal <6%)
- Erythrocyte sedimentation rate, 50 mm/h.
Radiology
- Chest radiographs (posterior-anterior, lateral) show a bilateral process consistent with atelectasis or lung scarring.
- Noncontrast computed tomography (CT) of the thorax confirms parenchymal scarring but no acute process.
- Hepatic sonography reveals increased echogenicity of the liver parenchyma consistent with an acute hepatocellular process.
- Magnetic resonance imaging (MRI) of the abdomen shows a diffuse process in the liver with trace parahepatic ascites.
- Renal ultrasound shows bilateral renal cysts with no hydronephrosis or urolithiasis.
- Cardiac echocardiography reveals left ventricular hypertrophy but normal ejection fraction.
- Noncontrast CT (head) and MRI (brain) show an acute right frontoparietal cerebrovascular accident and an old lacunar infarct.
Dermatologist’s report
- A skin biopsy reveals lymphocytic perivascular infiltrate with scattered eosinophils and mild spongiosis consistent with vasculitis.
Follow-up laboratory data
- Acute hepatitis panel is negative
- Uric acid, 13.8 mg/dL
- Urine eosinophils, 31% (normal <1%)
- Anti-neutrophil cytoplasmic antibody IgG, 1:40 mildly elevated (normal<1:20)
- Anti-nuclear antibody (ANA) IgG, none
- Glycosylated hemoglobin, 7.1%
- High-sensitivity C-reactive protein, 207.96 mg/L (>10 mg/L is very high)
In summary, this patient’s erythematous rash is a biopsy-confirmed vasculitis. Additional findings are hepatitis, acute on chronic renal failure, eosinophilia, and leukocytosis.
Q/ What is your presumptive diagnosis?
Allopurinol hypersensitivity syndrome
Allopurinol hypersensitivity syndrome (AHS) is a diffuse vasculitis induced by a type III hypersensitivity reaction, possibly to oxypurinol, allopurinol’s toxic metabolite. The exact pathophysiology is unknown, but oxypurinol levels correlate positively with the risk of AHS.1 Thiazides may increase oxypurinol levels.2
Signs and symptoms of AHS include fever, erythematous skin rash, eosinophilia, hepatitis, progressive renal insufficiency, and leukocytosis. Case reports have also attributed septic shock, myocardial infarction, and Guillain-Barré syndrome to AHS.3-6 The incidence of AHS is 0.1% to 0.4% of patients treated with allopurinol; mortality approaches 25%.1
Q/ What are the diagnostic criteria for AHS?
Singer and Wallace have outlined diagnostic criteria for AHS,7 the first being a clear history of exposure to allopurinol.
Second, the clinical profile usually takes one of the following forms:
- The patient exhibits at least 2 of the following major features: worsening renal function, acute hepatocellular injury, or a rash (toxic epidermal necrolysis, erythema multiforme, or diffuse maculopapular or exfoliative dermatitis) or
- The patient exhibits just one of the major features and at least one of the following minor features: fever, eosinophilia, or leukocytosis.
Third, there is no history of exposure to another drug that may cause a similar clinical picture.
Q/ What is the accepted treatment for ahS?
Although there is no well-established treatment plan, the standard of care is to discontinue allopurinol, administer parenteral corticosteroids followed by oral taper, and offer supportive management. Desensitization protocols are difficult and hypersensitivity reactions may recur. Early withdrawal of steroids has been reported to result in recurrence of symptoms. However, no strong data exist for determining an optimal length of corticosteroid therapy, or the number of days that constitutes “early” withdrawal of steroids. Mortality is high, even with seemingly adequate treatment.
Q/ Which patients are most at risk for ahS?
Definite risk factors for AHS include recent onset of allopurinol therapy, the presence of HLA-B5801 allele in patients of Han Chinese and European ancestry, and chronic kidney disease.1,8,9 Suggested risk factors include concomitant use of thiazide diuretics with allopurinol, treatment of asymptomatic hyperuricemia, and high allopurinol dose relative to renal function.
Q/ What are the indications for allopurinol therapy?
Indications for allopurinol therapy:1,10
- Failure of uricosuric drugs or contraindications to their use
- Frequent attacks of gouty arthritis (≥3 per year)
- Nephrolithiasis
- Marked overproduction of urate, such as seen in tumor lysis syndrome
- The presence of tophi.
For patients with appropriate indications for allopurinol therapy, treat with the minimum effective dose. Initiation of allopurinol is controversial for a patient with a single lifetime episode of gout. However, most patients with one episode of gout will develop recurrent gout.8
In patients with creatinine clearance >60 mL/min, allopurinol is usually started at 100 mg oral daily and titrated every 2 to 3 weeks until reaching the desired effectiveness.8 One retrospective study suggests that initiating allopurinol at a dose of 1.5 mg per unit of estimated GFR may reduce the risk of AHS.11
Our patient’s case: Treatment, discharge, readmission
With our patient, we started intravenous normal saline fluid boluses and parenteral methylprednisolone, 60 mg q6h. We discontinued triamterene/hydrochlorothiazide due to his hyperuricemia, and irbesartan and glyburide due to renal failure. Basal and rapid-acting insulins maintained good glycemic control. To control his wheezing and dyspnea, we began albuterol/ipratropium nebulizer treatments and oxygen delivery via nasal cannula. Nephrology, pulmonology, and rheumatology consultants agreed with these management decisions.
Over the next 9 days, the patient’s renal function improved and his rash started to resolve. His wheezing fluctuated but persisted throughout the hospital stay. We stopped nasal oxygen delivery, and his oxygen saturation remained normal (96%-98%) on room air. He was subsequently discharged in stable condition on a 2-week prednisone taper starting at 30 mg bid.
Thirteen days later, he was readmitted with a right frontoparietal cerebrovascular accident (CVA). He developed respiratory distress, which led to respiratory arrest, and was ventilated. He became hypotensive, lapsed into shock, and died a few days later (approximately one month after initial presentation).
The appropriate use of allopurinol—a second look
This case raised many questions for our inpatient team concerning not just AHS, but the appropriate use and dosing of allopurinol. Allopurinol is widely used for hyperuricemia and gout because it is effective for all causes of hyperuricemia and is inexpensive. Given that 3.9%12 of the general population has gout and that its prevalence has increased with rising rates of obesity, the importance of AHS is not abstract.
Revisiting initial treatment choices. Our patient was at risk for this syndrome due to his Chinese ancestry, recent onset of allopurinol therapy, concomitant use of triamterene/hydrochlorothiazide, and impaired renal function. Although his uric acid level was >10 mg/dL when treatment was started, his risk factors may have precluded initiation of allopurinol. Furthermore, the prescribed dose of allopurinol (300 mg) was too high for his baseline GFR; 100 mg daily would have been more appropriate. It could be argued that hydrochlorothiazide would not be an antihypertensive of choice due to its hyperuricemic effects. Switching the thiazide to a loop diuretic would offer no benefit, because loop diuretics also cause hyperuricemia. (More on which antihypertensive agent would have been appropriate in a bit.)
The role of AHS in the patient’s death is unclear. He was at high risk for stroke considering his age, comorbidities, and evidence of an old lacunar infarct. Myocardial infarctions have been reported as one cause of death in AHS, but we have found no reports of associated stroke. However, the temporal proximity of the CVA suggests a contributing effect of AHS. The adequacy of treatment is also brought into question. Early withdrawal of glucocorticoids can be associated with relapse of AHS; in retrospect, the prednisone dose may have been too low or the taper too short, or both.
Therapeutic alternatives to allopurinol were available. The most appropriate initial option for this patient likely was no pharmacologic intervention at all but a focus on weight loss and diet. The risk of gouty attacks in men increases as body mass index rises above normal (≥25 kg/m2) and decreases with weight loss. Lower calorie diets with decreased saturated fat, higher complex carbohydrates, and allowed proteins (low-fat dairy products) are more palatable and more effective than strict low-purine diets.
A urate-lowering antihypertensive may have been a better option—specifically, losartan rather than irbesartan and triamterene/hydrochlorothiazide.8,13 Losartan has uricosuric properties at the 50-mg dose.14 When compared in different studies with irbesartan, enalapril, and candesartan, losartan alone lowered serum urate levels. Increasing losartan to 100 mg could provide better hypertensive control but has not been found to lower urate levels more dramatically than the lower dose.13
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers have been found to blunt the urate-elevating effects of thiazides. Therefore, if the patient’s blood pressure was not adequately controlled on losartan alone, the combination of losartan/hydrochlorothiazide would be a reasonable choice because minimal or no change in serum uric acid levels would be expected.15
Febuxostat, a nonpurine selective inhibitor of xanthine oxidase, is a potential alternative to allopurinol in patients with gout. It is a more potent urate-lowering agent than allopurinol and can be used without dosage reduction in mild (creatinine clearance [CrCl], 60-89 mL/min) to moderate (CrCl, 30-59 mL/ min) renal insufficiency. Febuxostat is metabolized primarily in the liver—in contrast to allopurinol, which is excreted by the kidneys—and may increase liver transaminase levels.10 Although febuxostat was not available at the time our patient developed AHS, it would not have been indicated in asymptomatic hyperuricemia.
This compelling case reminds us to carefully consider the indications and risk factors in using allopurinol, and to be aware of the rare but sometimes devastating consequences of this commonly used drug.
CORRESPONDENCE
Tahirah Tyrell, MD, Rochester General Medical Group, 1425 Portland Avenue, Rochester, NY 14621;
[email protected].
1. Lee HY, Ariyasinghe J, Thirumoorthy T. Allopurinol hypersensitivity syndrome: a preventable severe cutaneous adverse reaction? Singapore Med J. 2008;49:384-387.
2. Markel A. Allopurinol hypersensitivity and DRESS syndrome. Am J Med. 2008;121:e25.
3. Koike K, et al. Adverse reaction case reports. React Wkly. 2008 Sept 6;1218:5.
4. Mete N, Yilmaz F, et al. Adverse reaction case reports. React Wkly. 2004 June 26;1007:7.
5. Benito-León J, Porta-Etessam J. Guillain-Barré syndrome and allopurinol- induced hypersensitivity. Eur Neurol. 2001;45:186-187.
6. Makar-Ausperger KMA. Allopurinol/furosemide. Hypersensitivity syndrome: case report. Reactions Wkly. 2007 Oct 13;1173:5.
7. Singer JZ, Wallace SL. The allopurinol hypersensitivity syndrome. Unnecessary morbidity and mortality. Arthritis Rheum. 1986; 29: 82-87.
8. Becker M. Prevention of recurrent gout. UpToDate. April 10, 2013. Available at: http://www.uptodate.com/contents/prevention-ofrecurrent- gout?detectedLanguage=en&source=search_result&s earch=Prevention+of+recurrent+gout&selectedTitle=1%7E8&pr ovider=noProvider. Accessed August 1, 2013.
9. Ramasamy SN, Korb-Wells CS, Kannangara DR, et al. Allopurinol hypersensitivity: a systematic review of all published cases, 1950- 2012. Drug Saf. 2013;July 20. [Epub ahead of print].
10. Moreland LW. Febuxostat–treatment for hyperuricemia and gout? N Engl J Med. 2005;353:2505-2507.
11. Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64:2529-2536.
12. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63: 3136-3141.
13. Würzner G, Gerster JC, Chiolero A, et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricemia and gout. J Hypertens. 2001;19:1855-1860.
14. Terkeltaub RA. Clinical practice. gout. N Engl J Med. 2003;349:1647-1655.
15. Manolis AJ, Grossman E, Jelakovic B, et al. Effects of losartan and candesartan monotherapy and losartan/hydrochlorothiazide combination therapy in patients with mild to moderate hypertension. Losartan Trial Investigators. Clin Ther. 2000;22:1186-1203.
1. Lee HY, Ariyasinghe J, Thirumoorthy T. Allopurinol hypersensitivity syndrome: a preventable severe cutaneous adverse reaction? Singapore Med J. 2008;49:384-387.
2. Markel A. Allopurinol hypersensitivity and DRESS syndrome. Am J Med. 2008;121:e25.
3. Koike K, et al. Adverse reaction case reports. React Wkly. 2008 Sept 6;1218:5.
4. Mete N, Yilmaz F, et al. Adverse reaction case reports. React Wkly. 2004 June 26;1007:7.
5. Benito-León J, Porta-Etessam J. Guillain-Barré syndrome and allopurinol- induced hypersensitivity. Eur Neurol. 2001;45:186-187.
6. Makar-Ausperger KMA. Allopurinol/furosemide. Hypersensitivity syndrome: case report. Reactions Wkly. 2007 Oct 13;1173:5.
7. Singer JZ, Wallace SL. The allopurinol hypersensitivity syndrome. Unnecessary morbidity and mortality. Arthritis Rheum. 1986; 29: 82-87.
8. Becker M. Prevention of recurrent gout. UpToDate. April 10, 2013. Available at: http://www.uptodate.com/contents/prevention-ofrecurrent- gout?detectedLanguage=en&source=search_result&s earch=Prevention+of+recurrent+gout&selectedTitle=1%7E8&pr ovider=noProvider. Accessed August 1, 2013.
9. Ramasamy SN, Korb-Wells CS, Kannangara DR, et al. Allopurinol hypersensitivity: a systematic review of all published cases, 1950- 2012. Drug Saf. 2013;July 20. [Epub ahead of print].
10. Moreland LW. Febuxostat–treatment for hyperuricemia and gout? N Engl J Med. 2005;353:2505-2507.
11. Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64:2529-2536.
12. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63: 3136-3141.
13. Würzner G, Gerster JC, Chiolero A, et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricemia and gout. J Hypertens. 2001;19:1855-1860.
14. Terkeltaub RA. Clinical practice. gout. N Engl J Med. 2003;349:1647-1655.
15. Manolis AJ, Grossman E, Jelakovic B, et al. Effects of losartan and candesartan monotherapy and losartan/hydrochlorothiazide combination therapy in patients with mild to moderate hypertension. Losartan Trial Investigators. Clin Ther. 2000;22:1186-1203.
Consider this strategy for upper GI bleeds
Do not order transfusions of red blood cells for patients with acute upper gastrointestinal bleeding unless their hemoglobin level <7 g/dL.
Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.1
A: Based on a single randomized controlled trial (RCT) consistent with other RCTs on recommendations for transfusion.
ILLUSTRATED CASE
An 82-year-old patient presents to the emergency department with several episodes of melena over the past week and one episode of hematemesis this morning. He denies any shortness of breath, dizziness, lightheadedness, or fatigue. He is tachycardic but normotensive. Lab results note a hemoglobin level of 8.3 g/dL. Should you order a transfusion of red blood cells?
Acute upper gastrointestinal bleeding (UGIB) commonly requires hospital admission, with approximately 61 cases per 100,000 population in the United States in 2009.2 Gastroduodenal peptic ulcer disease accounts for the majority of these cases.3 Although trends indicate an overall decrease in cases requiring hospitalization, UGIB remains a condition associated with a mortality rate of 2.5% and inpatient costs of $2 billion annually.2,3
Studies have been inconclusive—until now
An RCT published in 1999 showed a restrictive transfusion strategy (hemoglobin threshold of 7 g/dL) to be at least as effective as—and possibly superior to—a liberal strategy (threshold of 10 g/dL) in critically ill patients.4 In 2010, an RCT demonstrated that a liberal transfusion strategy (also defined as a transfusion threshold of 10 g/dL) did not reduce the rates of death or in-hospital morbidity in elderly patients after hip surgery.5 A recent Cochrane review of transfusion strategies for UGIB included only 3 small studies (N=93), so its authors could not draw any firm conclusions.6 The results of a new RCT, detailed below, are more conclusive.
STUDY SUMMARY: Restrictive transfusion policy lowers mortality risk
Villanueva et al conducted a nonblinded RCT comparing outcomes in patients admitted to the hospital with moderate-risk acute UGIB transfused on a liberal vs a restrictive strategy.1 The restrictive group used a transfusion hemoglobin threshold of 7 g/dL and a posttransfusion target of 7 to 9 g/dL; the liberal group used a threshold of 9 g/dL, with a posttransfusion target of 9 to 11 g/dL. Patients received one unit of red blood cells at a time until their hemoglobin was above the predetermined threshold.
Patients were excluded if they declined blood transfusion; had massive exsanguinating bleeding, acute coronary syndrome, symptomatic peripheral vasculopathy, stroke, lower GI bleeding, or a transient ischemic attack; had received a transfusion within the previous 90 days; or had a recent history of surgery or trauma. Patients at low risk of rebleeding (as defined by the Rockall risk scoring system) were also excluded. Randomization was stratified by the presence or absence of cirrhosis of the liver.
Participants (N=921) had confirmed hematemesis and/or melena on admission. All underwent emergency gastroscopy within 6 hours of admission, with subsequent interventions based on endoscopic findings. In addition to established hemoglobin levels, patients received a transfusion anytime they developed signs or symptoms related to anemia, massive bleeding, or the need for surgery. Staff monitored hemoglobin levels every 8 hours during the first 48 hours, then daily thereafter.
Both groups had similar baseline characteristics, including hemoglobin on admission and source of bleeding. The authors used intention-to-treat analysis to identify the primary outcome: death from any cause at 45 days. Secondary outcomes were further bleeding and in-hospital complications.
During hospitalization, 49% of patients in the restrictive group and 86% of those in the liberal group received a blood transfusion (P<.001). Thirty-two patients (17 from the restrictive group and 15 from the liberal group) withdrew from the study, leaving 889 patients for overall analysis.
At 45 days, overall mortality from any cause was 5% in the restrictive group and 9% in the liberal group (P=.02; number needed to treat [NNT]=25). Sub-group analysis revealed a lower risk of death in patients with cirrhosis and Child-Pugh class A or B disease assigned to the restrictive transfusion group vs the liberal group. The results showed a trend toward a lower risk of death in patients with bleeding from varices or peptic ulcers for the restrictive group, as well.
In addition, the restrictive transfusion group had a significantly lower rate of adverse events (40% vs 48% for the liberal transfusion group; P=.02, NNT=13), with a significant reduction in transfusion reactions (3% vs 9%; P=.001, NNT=17) and cardiac complications (11% vs 16%; P=.04, NNT=20). The restrictive group had a lower rate of further bleeding (10% vs 16% for the liberal transfusion group; P=.01, NNT=17), as well.
WHAT'S NEW: Many reasons to limit transfusions for acute upper GI bleed
This RCT provides evidence that patients with acute UGIB have improved survival rates and fewer adverse events when a restrictive transfusion strategy is used. In addition to improving patient outcomes, a restrictive strategy will likely reduce costs and overall use of blood products. Thus, the study, along with other recent evaluations, adds evidence to support more restrictive transfusion thresholds.
The AABB (formerly named the American Association of Blood Banks) recently
released guidelines calling for restrictive transfusion thresholds (7-8 g/dL) in stable hospitalized patients.7 In 2012, the American College of Gastroenterology published a practice guideline with a recommended target hemoglobin level of ≥7 g/dL in the management of patients who have ulcer bleeding but no signs of intravascular depletion or comorbidities such as coronary artery disease.8
CAVEATS: Results might differ when endoscopy is delayed
The patients in the study detailed here underwent emergency gastroscopy within 6 hours of admission, and both groups received the same therapies based on endoscopic findings. It remains unclear whether the benefits of a restrictive transfusion strategy would persist in patients who do not undergo endoscopy within that timeframe. And, because the reported baseline characteristics of the patients did not include the prevalence of cardiac disease, caution should be exercised before extrapolating these results to patients with underlying (active or historical) cardiac disease.
CHALLENGES TO IMPLEMENTATION: Changing long-held policies may be difficult
Although RCTs as well as clinical guidelines suggest that restrictive transfusion policies are safe and effective, changing long-held clinical practices is never easy.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med.2013;368:11-21.
2. Laine L, Yang H, Chang SC,et al. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol 2012; 107:1190-1195.
3. Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928-937.
4. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409-417.
5. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453-2462.
6. Jairath V, Hearnshaw S, Brunskill SJ, et al. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database of Systematic Reviews 2010;CD006613.
7. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49-58.
8. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012; 107:345-360.
Do not order transfusions of red blood cells for patients with acute upper gastrointestinal bleeding unless their hemoglobin level <7 g/dL.
Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.1
A: Based on a single randomized controlled trial (RCT) consistent with other RCTs on recommendations for transfusion.
ILLUSTRATED CASE
An 82-year-old patient presents to the emergency department with several episodes of melena over the past week and one episode of hematemesis this morning. He denies any shortness of breath, dizziness, lightheadedness, or fatigue. He is tachycardic but normotensive. Lab results note a hemoglobin level of 8.3 g/dL. Should you order a transfusion of red blood cells?
Acute upper gastrointestinal bleeding (UGIB) commonly requires hospital admission, with approximately 61 cases per 100,000 population in the United States in 2009.2 Gastroduodenal peptic ulcer disease accounts for the majority of these cases.3 Although trends indicate an overall decrease in cases requiring hospitalization, UGIB remains a condition associated with a mortality rate of 2.5% and inpatient costs of $2 billion annually.2,3
Studies have been inconclusive—until now
An RCT published in 1999 showed a restrictive transfusion strategy (hemoglobin threshold of 7 g/dL) to be at least as effective as—and possibly superior to—a liberal strategy (threshold of 10 g/dL) in critically ill patients.4 In 2010, an RCT demonstrated that a liberal transfusion strategy (also defined as a transfusion threshold of 10 g/dL) did not reduce the rates of death or in-hospital morbidity in elderly patients after hip surgery.5 A recent Cochrane review of transfusion strategies for UGIB included only 3 small studies (N=93), so its authors could not draw any firm conclusions.6 The results of a new RCT, detailed below, are more conclusive.
STUDY SUMMARY: Restrictive transfusion policy lowers mortality risk
Villanueva et al conducted a nonblinded RCT comparing outcomes in patients admitted to the hospital with moderate-risk acute UGIB transfused on a liberal vs a restrictive strategy.1 The restrictive group used a transfusion hemoglobin threshold of 7 g/dL and a posttransfusion target of 7 to 9 g/dL; the liberal group used a threshold of 9 g/dL, with a posttransfusion target of 9 to 11 g/dL. Patients received one unit of red blood cells at a time until their hemoglobin was above the predetermined threshold.
Patients were excluded if they declined blood transfusion; had massive exsanguinating bleeding, acute coronary syndrome, symptomatic peripheral vasculopathy, stroke, lower GI bleeding, or a transient ischemic attack; had received a transfusion within the previous 90 days; or had a recent history of surgery or trauma. Patients at low risk of rebleeding (as defined by the Rockall risk scoring system) were also excluded. Randomization was stratified by the presence or absence of cirrhosis of the liver.
Participants (N=921) had confirmed hematemesis and/or melena on admission. All underwent emergency gastroscopy within 6 hours of admission, with subsequent interventions based on endoscopic findings. In addition to established hemoglobin levels, patients received a transfusion anytime they developed signs or symptoms related to anemia, massive bleeding, or the need for surgery. Staff monitored hemoglobin levels every 8 hours during the first 48 hours, then daily thereafter.
Both groups had similar baseline characteristics, including hemoglobin on admission and source of bleeding. The authors used intention-to-treat analysis to identify the primary outcome: death from any cause at 45 days. Secondary outcomes were further bleeding and in-hospital complications.
During hospitalization, 49% of patients in the restrictive group and 86% of those in the liberal group received a blood transfusion (P<.001). Thirty-two patients (17 from the restrictive group and 15 from the liberal group) withdrew from the study, leaving 889 patients for overall analysis.
At 45 days, overall mortality from any cause was 5% in the restrictive group and 9% in the liberal group (P=.02; number needed to treat [NNT]=25). Sub-group analysis revealed a lower risk of death in patients with cirrhosis and Child-Pugh class A or B disease assigned to the restrictive transfusion group vs the liberal group. The results showed a trend toward a lower risk of death in patients with bleeding from varices or peptic ulcers for the restrictive group, as well.
In addition, the restrictive transfusion group had a significantly lower rate of adverse events (40% vs 48% for the liberal transfusion group; P=.02, NNT=13), with a significant reduction in transfusion reactions (3% vs 9%; P=.001, NNT=17) and cardiac complications (11% vs 16%; P=.04, NNT=20). The restrictive group had a lower rate of further bleeding (10% vs 16% for the liberal transfusion group; P=.01, NNT=17), as well.
WHAT'S NEW: Many reasons to limit transfusions for acute upper GI bleed
This RCT provides evidence that patients with acute UGIB have improved survival rates and fewer adverse events when a restrictive transfusion strategy is used. In addition to improving patient outcomes, a restrictive strategy will likely reduce costs and overall use of blood products. Thus, the study, along with other recent evaluations, adds evidence to support more restrictive transfusion thresholds.
The AABB (formerly named the American Association of Blood Banks) recently
released guidelines calling for restrictive transfusion thresholds (7-8 g/dL) in stable hospitalized patients.7 In 2012, the American College of Gastroenterology published a practice guideline with a recommended target hemoglobin level of ≥7 g/dL in the management of patients who have ulcer bleeding but no signs of intravascular depletion or comorbidities such as coronary artery disease.8
CAVEATS: Results might differ when endoscopy is delayed
The patients in the study detailed here underwent emergency gastroscopy within 6 hours of admission, and both groups received the same therapies based on endoscopic findings. It remains unclear whether the benefits of a restrictive transfusion strategy would persist in patients who do not undergo endoscopy within that timeframe. And, because the reported baseline characteristics of the patients did not include the prevalence of cardiac disease, caution should be exercised before extrapolating these results to patients with underlying (active or historical) cardiac disease.
CHALLENGES TO IMPLEMENTATION: Changing long-held policies may be difficult
Although RCTs as well as clinical guidelines suggest that restrictive transfusion policies are safe and effective, changing long-held clinical practices is never easy.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Do not order transfusions of red blood cells for patients with acute upper gastrointestinal bleeding unless their hemoglobin level <7 g/dL.
Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.1
A: Based on a single randomized controlled trial (RCT) consistent with other RCTs on recommendations for transfusion.
ILLUSTRATED CASE
An 82-year-old patient presents to the emergency department with several episodes of melena over the past week and one episode of hematemesis this morning. He denies any shortness of breath, dizziness, lightheadedness, or fatigue. He is tachycardic but normotensive. Lab results note a hemoglobin level of 8.3 g/dL. Should you order a transfusion of red blood cells?
Acute upper gastrointestinal bleeding (UGIB) commonly requires hospital admission, with approximately 61 cases per 100,000 population in the United States in 2009.2 Gastroduodenal peptic ulcer disease accounts for the majority of these cases.3 Although trends indicate an overall decrease in cases requiring hospitalization, UGIB remains a condition associated with a mortality rate of 2.5% and inpatient costs of $2 billion annually.2,3
Studies have been inconclusive—until now
An RCT published in 1999 showed a restrictive transfusion strategy (hemoglobin threshold of 7 g/dL) to be at least as effective as—and possibly superior to—a liberal strategy (threshold of 10 g/dL) in critically ill patients.4 In 2010, an RCT demonstrated that a liberal transfusion strategy (also defined as a transfusion threshold of 10 g/dL) did not reduce the rates of death or in-hospital morbidity in elderly patients after hip surgery.5 A recent Cochrane review of transfusion strategies for UGIB included only 3 small studies (N=93), so its authors could not draw any firm conclusions.6 The results of a new RCT, detailed below, are more conclusive.
STUDY SUMMARY: Restrictive transfusion policy lowers mortality risk
Villanueva et al conducted a nonblinded RCT comparing outcomes in patients admitted to the hospital with moderate-risk acute UGIB transfused on a liberal vs a restrictive strategy.1 The restrictive group used a transfusion hemoglobin threshold of 7 g/dL and a posttransfusion target of 7 to 9 g/dL; the liberal group used a threshold of 9 g/dL, with a posttransfusion target of 9 to 11 g/dL. Patients received one unit of red blood cells at a time until their hemoglobin was above the predetermined threshold.
Patients were excluded if they declined blood transfusion; had massive exsanguinating bleeding, acute coronary syndrome, symptomatic peripheral vasculopathy, stroke, lower GI bleeding, or a transient ischemic attack; had received a transfusion within the previous 90 days; or had a recent history of surgery or trauma. Patients at low risk of rebleeding (as defined by the Rockall risk scoring system) were also excluded. Randomization was stratified by the presence or absence of cirrhosis of the liver.
Participants (N=921) had confirmed hematemesis and/or melena on admission. All underwent emergency gastroscopy within 6 hours of admission, with subsequent interventions based on endoscopic findings. In addition to established hemoglobin levels, patients received a transfusion anytime they developed signs or symptoms related to anemia, massive bleeding, or the need for surgery. Staff monitored hemoglobin levels every 8 hours during the first 48 hours, then daily thereafter.
Both groups had similar baseline characteristics, including hemoglobin on admission and source of bleeding. The authors used intention-to-treat analysis to identify the primary outcome: death from any cause at 45 days. Secondary outcomes were further bleeding and in-hospital complications.
During hospitalization, 49% of patients in the restrictive group and 86% of those in the liberal group received a blood transfusion (P<.001). Thirty-two patients (17 from the restrictive group and 15 from the liberal group) withdrew from the study, leaving 889 patients for overall analysis.
At 45 days, overall mortality from any cause was 5% in the restrictive group and 9% in the liberal group (P=.02; number needed to treat [NNT]=25). Sub-group analysis revealed a lower risk of death in patients with cirrhosis and Child-Pugh class A or B disease assigned to the restrictive transfusion group vs the liberal group. The results showed a trend toward a lower risk of death in patients with bleeding from varices or peptic ulcers for the restrictive group, as well.
In addition, the restrictive transfusion group had a significantly lower rate of adverse events (40% vs 48% for the liberal transfusion group; P=.02, NNT=13), with a significant reduction in transfusion reactions (3% vs 9%; P=.001, NNT=17) and cardiac complications (11% vs 16%; P=.04, NNT=20). The restrictive group had a lower rate of further bleeding (10% vs 16% for the liberal transfusion group; P=.01, NNT=17), as well.
WHAT'S NEW: Many reasons to limit transfusions for acute upper GI bleed
This RCT provides evidence that patients with acute UGIB have improved survival rates and fewer adverse events when a restrictive transfusion strategy is used. In addition to improving patient outcomes, a restrictive strategy will likely reduce costs and overall use of blood products. Thus, the study, along with other recent evaluations, adds evidence to support more restrictive transfusion thresholds.
The AABB (formerly named the American Association of Blood Banks) recently
released guidelines calling for restrictive transfusion thresholds (7-8 g/dL) in stable hospitalized patients.7 In 2012, the American College of Gastroenterology published a practice guideline with a recommended target hemoglobin level of ≥7 g/dL in the management of patients who have ulcer bleeding but no signs of intravascular depletion or comorbidities such as coronary artery disease.8
CAVEATS: Results might differ when endoscopy is delayed
The patients in the study detailed here underwent emergency gastroscopy within 6 hours of admission, and both groups received the same therapies based on endoscopic findings. It remains unclear whether the benefits of a restrictive transfusion strategy would persist in patients who do not undergo endoscopy within that timeframe. And, because the reported baseline characteristics of the patients did not include the prevalence of cardiac disease, caution should be exercised before extrapolating these results to patients with underlying (active or historical) cardiac disease.
CHALLENGES TO IMPLEMENTATION: Changing long-held policies may be difficult
Although RCTs as well as clinical guidelines suggest that restrictive transfusion policies are safe and effective, changing long-held clinical practices is never easy.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med.2013;368:11-21.
2. Laine L, Yang H, Chang SC,et al. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol 2012; 107:1190-1195.
3. Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928-937.
4. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409-417.
5. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453-2462.
6. Jairath V, Hearnshaw S, Brunskill SJ, et al. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database of Systematic Reviews 2010;CD006613.
7. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49-58.
8. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012; 107:345-360.
1. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med.2013;368:11-21.
2. Laine L, Yang H, Chang SC,et al. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol 2012; 107:1190-1195.
3. Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928-937.
4. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409-417.
5. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453-2462.
6. Jairath V, Hearnshaw S, Brunskill SJ, et al. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database of Systematic Reviews 2010;CD006613.
7. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49-58.
8. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012; 107:345-360.
Copyright 2013. The Family Physicians Inquiries Network. All rights reserved.
When to worry about incidental renal and adrenal masses
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.
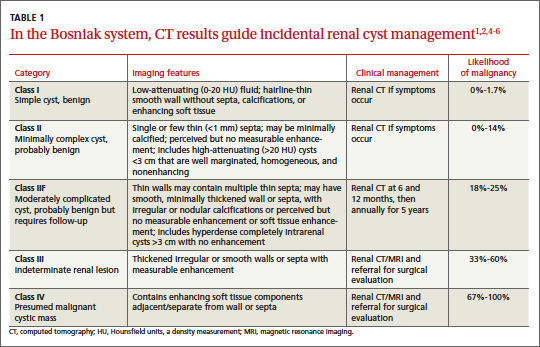
Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.

Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.

Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
Hip fracture in older patients: Tips and tools to speed recovery
› Ensure that surgical stabilization of hip fracture is performed as soon as possible—ideally within 48 hours of injury. A
› To reduce the risk of delirium, orient the patient frequently; get her out of bed as soon as possible, and avoid prolonged catheter use. A
› Order protein supplements for patients recovering from hip fracture and take steps to facilitate an early return to eating. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
The patient and family request a consultation with Ms. J’s primary care physician. If you were her physician, what would you advise?
Hip fracture in a frail elderly patient is an injury that, while common, can be difficult to manage. With good reason. Geriatric hip fracture is associated with increased morbidity, functional decline, and use of nursing home services, as well as a higher mortality rate: One in 5 hip fracture patients dies within a year of the injury.1
As the population ages, we are seeing more hip fractures in the “oldest old” those who, like Ms. J, are older than 85. While the incidence increases exponentially with age in both men and women, women are 3 times more likely than men to sustain a hip fracture.2 White women ages 85 to 95 face the highest risk, with an incidence of more than 3%.3
In addition to managing the acute phase of hip fracture and helping patients and families make decisions about optimal treatment, there is much you can do to boost the likelihood of a rapid rehabilitation and a successful outcome.
What type of fracture? How best to treat it?
Two types of hip fractures are responsible for the vast majority of cases: About 45% of hip fractures are intracapsular, involving the femoral head and neck; another 45% are intertrochanteric fractures. Both usually involve low-energy trauma, such as a fall from a chair or tripping over a rug. Intertrochanteric and subtrochanteric fractures (the latter accounting for the remaining 10%) are extracapsular.2,4,5
Typically associated with high-energy trauma such as a motor vehicle accident, or with metastatic lesions, subtrochanteric fractures have a bimodal distribution: They are most common in individuals between the ages of 20 and 40 and those older than 60.2
Fractures involving the femoral neck can disrupt the vascular supply to the femoral head and result in avascular necrosis (AVN) or nonunion.2,4,5 A meta-analysis of the outcome of displaced femoral neck fractures found the rates of osteonecrosis and nonunion to be as high as 20% to 30%.5 Intertrochanteric fractures rarely lead to AVN or nonunion, but patients may develop complications associated with degenerative changes.2,4,5 Nonunion is a potential complication of subtrochanteric fracture.2
For most patients, surgical management is preferred
The main goals of treatment are to stabilize the hip, decrease pain and restore the level of prefracture function. Surgery is the preferred treatment for hip fracture because it provides stable fixation, facilitating full weight bearing and decreasing the risk of complications. Surgery is also associated with a shorter stay in the hospital and improved rehabilitation and recovery.6
Surgical stabilization should be performed as soon as possible—ideally, within 48 hours.5 A recent study found conflicting evidence of the effect of delayed surgery on mortality, but demonstrated that surgery within 24 hours of injury minimizes the rate of chest infections, urinary tract infections, and pressure sores, as well as the duration of the hospital stay.7 (To learn more about surgical stabilization of hip fracture, see “What type of surgery? Age is just one consideration” 5,8-10 below.)
When surgery is contraindicated
Nonoperative management is reserved for patients who stand to gain only minimal function from surgical stabilization, because they either were not ambulatory to begin with or have severe dementia. In addition, medical management is used for patients with contraindications to anesthesia, those who delay seeking medical care until the fracture has begun to heal, and patients who refuse surgical fixation.5,11
The choice of surgical intervention depends on multiple factors, including the:
- type and severity of the fracture
- preference of the orthopedic surgeon
- age of the patient
- comorbid conditions
- prognosis.
For femoral neck fractures, patients younger than 65 years are candidates for internal fixation; for older individuals and those who already had limited mobility, arthroplasty should be considered.5 Studies of pain and functional outcomes show a modest tendency for total hip arthroplasty to have better results than internal fixation in patients older than 65.8
Intertrochanteric fractures can be treated with either sliding hip screws or
intramedullary nails. Intramedullary nail implants are done percutaneously, resulting in a shorter duration for surgery, less blood loss, and an earlier return to full weight bearing.5 A recent study suggests that intramedullary nails result in more reoperations than hip screws.9 No evidence is conclusive about the superiority of either type of hardware.
Subtrochanteric fractures are typically repaired by hemiarthroplasty.
A Cochrane review of randomized controlled trials found insufficient evidence to determine whether replacement arthroplasty has any advantage over internal fixation for extracapsular hip fractures.10
CASE After a careful review of Ms. J’s health status, radiographs of the fracture (FIGURE 1A), and consultation with an orthopedic surgeon and a geriatrician, you recommend surgery as soon as the patient is fully stabilized. Without it, she would be at high risk for urinary tract infection, pressure sores, and thromboembolism associated with long-term immobility.
The next day, Ms. J undergoes surgical fixation with a sliding hip screw (FIGURE 1B). Her Foley catheter is removed the same day, and physical therapy is begun the following day. On postoperative day 4 she is discharged to an in patient rehabilitation facility.
Begin rehabilitation without delay
Whether a patient has surgery or is treated nonoperatively for hip fracture, the goal of rehabilitation is the same—to restore mobility as quickly as possible. A clinical review found no significant difference in mortality rates between those who underwent surgical fixation and those who were treated medically with early mobilization, consisting of immediate bed-to-chair transfer (with assistance), followed by progression to ambulation as tolerated.12
For patients who undergo surgery for hip fracture, increased immobility is linked to poorer functioning in the areas of self-care and transfers at 2 months and to higher mortality rates at 6 months.13 Physical therapy should be initiated on the first postoperative day and should start with bed mobility range of motion, followed by independent transfers from bed to chair, and ultimately achieving full weight bearing.5
Many complications are predictable, and often preventable
The term “hip fracture syndrome”4 is often used in reference to a cluster of common (and often preventable) complications of hip fracture, with delirium, venous thromboembolism (VTE), and malnutrition foremost among them.
Take steps to prevent—or treat—delirium
Delirium is among the most common complication, occurring in up to 62% of older patients with hip fracture.4 The highest predictor of delirium is preexisting cognitive impairment.
Other risk factors for delirium include advanced age, vision or hearing impairment, concurrent alcohol abuse, malnutrition, comorbidity, and polypharmacy.4,14 Delirium is associated with increased morbidity and mortality, decreased rehabilitation potential, and poor functional recovery independent of prior frailty.4,15,16
Hypoactive delirium is easily missed. While agitated, or hyperactive, delirium is more easily recognized, it is crucial to be aware of hypoactive delirium, as well. Patients with hypoactive delirium tend to become more withdrawn and their delirium is easily missed, leading to worse outcomes.15 The Confusion Assessment Method (TABLE 1)17 is an easy-to-use validated tool developed to aid in the diagnosis of delirium at the bedside.
Many factors contribute to the development of delirium. Medical complications, such as infection, electrolyte and volume imbalances, hypoxia, and myocardial infarction, are obvious precipitants.15 Disturbances in sleep-wake cycles, an unfamiliar environment, physical restraints, and the use of Foley catheters—all of which can impair an older patient’s sensory awareness—are less well-known contributing factors.
Tips for preventing delirium. Early mobilization, in addition to boosting physical recovery, can help prevent delirium.
Other tips:
- discontinue catheterization as soon as possible; this may help prevent delirium, and lessen the risk of urinary tract infection.
- remind nurses and family members to continuously reorient patients to their surroundings.
- treat pain aggressively.
- consult a geriatrician early on.
While opioids are often thought to cause delirium, several studies have shown an inverse relationship—that is, hip fracture patients who were given opioids for pain were actually less likely to develop delirium than those who did not receive opioids. This raises 2 important points:
1. untreated pain may itself be a significant risk factor for delirium,15,18 and
2. delirium itself is not a contraindication to opioids.18
CASE In her first week at the inpatient rehabilitation center, Ms. J requires slightly more narcotic medication for pain control. The staff notices increased confusion and a decrease in the number of bowel movements. Ms. J is started on a regimen of sennosides and docusate twice daily. Her mental status improves quickly and she has no further complications while at the rehab center.
Nonopioid pain medications such as acetaminophen should be scheduled at appropriate doses (eg, 1 g tid). Ensure that patients recovering from hip fracture are not given benzodiazepines, anticholinergics, or antihistamines15— which are sometimes included in a facility’s PRN protocol. In clinical trials, prophylactic administration of antipsychotics or anticholinesterase therapy to high-risk patients has had conflicting results.19,20
Arrange for a geriatric consult before problems occur. Several studies have shown that a geriatric consultation and concurrent management by a geriatrician using structured protocols to evaluate for common risk factors known to precipitate delirium (eg, pain, bowel/bladder function, nutrition, mobilization) can reduce the risk of delirium.16
Provide supportive care. Although treatment of the underlying cause is the definitive treatment for delirium, there are times when supportive care is all that’s needed. Reassurance from family members or staff is the recommended first step. Physical restraints should be avoided unless patient safety is threatened despite attempts to provide supportive care.
If treatment for delirium is needed, lowdose antipsychotics are recommended. The most studied agent is haloperidol, which can be administered intravenously (IV), intramuscularly (IM), or orally. Monitoring the corrected QT (QTc) interval is recommended for patients taking haloperidol, and discontinuation of the drug—or a cardiology consult— is recommended if the QTc interval is prolonged (>450 ms or >25% of baseline).21
There is a slightly higher risk of cardiac arrhythmias with IV administration of haloperidol compared with IM or oral dosing. Despite this risk, haloperidol IV is the treatment of choice for delirium.21 Newer atypical antipsychotics have also been used to treat delirium, but data are limited.21
Guard against VTE
Studies have shown rates of VTE to be as high as 40% to 60% after orthopedic procedures, and prophylaxis has long been the standard of care.22 In its 2012 consensus guidelines for antithrombotic therapy, the American College of Chest Physicians (ACCP) recommends fondiparinux, apixaban, rivaroxaban, dabigatran, low-molecular-weight heparin (LMWH), low-dose unfractionated heparin, aspirin, warfarin, or an intermittent pneumatic compression device (IPCD) as prophylaxis.23 Portable battery-powered IPCDs are recommended for 18 hours postop.23
The guideline authors prefer LMWH to the other treatments, and recommend dual prophylaxis with an IPCD and an antithrombotic agent while the patient is in the hospital and for a minimum of 10 to 14 days (and up to 35 days) after discharge. If surgery for hip fracture is delayed, the ACCP recommends that LMWH be administered after admission, but withheld for at least 12 hours before surgery. In patients with a high risk of bleeding, the ACCP recommends either an IPCD alone or no prophylaxis and notes that inferior vena cava filters should not be placed in high-risk patients.23
Take steps to ensure ample protein intake
Malnourishment is another common complication, affecting up to 20% of hip fracture patients.24 In many cases, a catabolic state predisposes patients to protein depletion, leading to decreased wound healing and an increase in other postop complications.24,25 Protein supplementation is associated with decreased length of stay and a reduction in postop complications.26
This complication can often be avoided by encouraging an early return to eating. Specific steps: Ensure that patients have their dentures available and are able to use them; are positioned properly for eating; and receive high-caloric supplemental drinks. Nutritional assessments should also be done to ensure that their intake of calcium and vitamin D is sufficient to prevent future falls and reduce fracture risk. (For more information, see “Vitamin D: When it helps, when it harms” [J Fam Pract. 2013;62:368-370.])
Combat hip fracture by stressing avoidance
Prevention of hip fracture, of course, is the ideal way to reduce the burden of disease for older patients. Along these lines, there are many ways you can help.
Start with fall reduction
Hip fracture is associated with a fall 90% of the time,27 and care for older patients should be focused on reducing the risk for falls and improving bone health and muscular function. While a complete review of preventive measures is beyond the scope of this article, we offer some highlights here and in TABLE 2.
Encourage physical activity In addition to helping to reduce falls, physical activity—particularly repetitive weight-bearing exercise—can help maintain bone density and improve muscle mass, strength, and balance.28
Rather than focus on a single exercise, however, a combination of activities—Tai Chi and walking, for instance, or weight lifting and cycling —appears to have the best likelihood of fall reduction.29 Whenever possible, physical activity for older patients should include challenges in executive function, as well. In a recent study comparing regular walking with trail-walking between sequentially marked flags, participants in the more complex activity had a greater decrease in fall rates.30
Review vitamin D and calcium intake. Elderly patients with low levels of vitamin D are at increased risk of muscle mass decline, and therefore increased risk of fracture.31 A systematic review and meta-analysis of vitamin D supplementation in older adults found the relative risk of falling was 0.86 (95% confidence interval [CI], 0.79-0.93) for those assigned to vitamin D therapy compared with those on placebo. Risk reduction was greater in groups taking 800 IU or more of vitamin D daily and those taking adjunctive calcium supplementation.32
Maximizing vitamin D for falls reduction is supported by the American Geriatrics Society, 33 the Agency for Healthcare Research and Quality (AHRQ),34 and the US Preventive Services Task Force (USPSTF).35 The USPSTF recently released a recommendation for exercise or physical therapy and vitamin D supplementation (800 IU) to prevent falls in community-dwelling adults ages 65 and over who are at an increased risk for falls.36
However, the USPSTF advises against daily supplementation with vitamin D and calcium at doses ?400 IU and 1000 mg, respectively, for noninstitutionalized postmenopausal women for primary fracture prevention. Calcium supplementation has not been shown to reduce hip fractures, but has been found to improve hip bone density.37
Consider bisphosphonates. Order a dual energy x-ray absorptiometry (DEXA) scan for older patients to identify osteoporosis. Most hip fractures are osteoporotic, and patients should be started on bisphosphonates within 2 to 12 weeks of injury38 to reduce the risk of mortality associated with hip fracture.39 The most studied bisphosphonates in geriatric hip fracture are alendronate, risedronate, and zoledronate; all were found to have a number needed to treat of 91 to prevent one hip fracture.40
Focus on the home environment. In addition to addressing the bone and muscular health of older patients, focus should be placed on the home environment. A Cochrane review of fall prevention for those living in the community found that home safety interventions reduced the risk of falls, but only for those with severe vision impairment and a high risk of falls.29 A 2010 American Geriatric Society (AGS) and British Geriatric Society (BGS) review of fall prevention gave an A recommendation—the highest rating— to home assessment and intervention by a health care professional to identify home hazards and promote safe performance of daily activities.33
Conduct brown-bag reviews. Polypharmacy is a well-documented (and growing) problem among the elderly.41 Both the AGS and BGS encourage a review of medications (including over-the-counter products) and interactions at each office visit,33 with specific attention paid to drugs that may cause dizziness, drowsiness, and near syncopal or syncopal episodes.
To reduce the risk of medication interactions and adverse effects, look for opportunities to reduce the number of drugs your elderly patients are taking. Consider involving a clinical pharmacist in medication reviews—an intervention that has been shown to be cost effective and lead to better patient outcomes.42
CASE After 4 weeks, Ms. J is ready to return home. Rather than a return to independent living, however, her children convince her to move to an assisted living facility—a move you strongly support. You schedule a visit in 2 weeks.
CORRESPONDENCE
Jeremy D. Close, MD, Department of Family and Community Medicine, Thomas Jefferson University, 833 Chestnut Street #301, Philadelphia, PA 19107; [email protected]
1. Leibson CL, Toteson ANA, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644-50.
2. Brunner LC, Eshilian-Oates L, Kuo TY. Hip fractures in adults. Am Fam Physician. 2003;67:537-542.
3. Jacobsen SJ, Goldberg J, Miles TP, et al. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health. 1990;80:871-873.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24:701-719.
5. Jackman JM, Watson JT. Hip fractures in older men. Clin Geriatr Med. 2010;26:311-329.
6. Handoll HH, Parker MJ. Conservative versus operative treatment for hip fractures in adults. Cochrane Database Syst Rev. 2008;(3):CD000337.
7. Leung F, Lau W, Kwan K, et al. Does timing of surgery matter in fragility hip fractures? Osteoporos Int. 2010; 21(suppl 4):S529-S534.
8. Butler M, Forte ML, Joglekar SB, et al. Evidence summary: systematic review of surgical treatments for geriatric hip fractures. J Bone Joint Surg Am. 2011;93:1104-1115.
9. Matre K, Havelin LI, Gjertsen JE, et al. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop Relat Res. 2013;471: 1379-1386.
10. Parker MJ, Handoll HH. Replacement arthroplasty versus internal fixation for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2006;(2):CD000086.
11. Cummings-Vaughn LA, Gammack JK. Falls, osteoporosis, and hip fractures. Med Clin North Am. 2011;95:495-506.
12. Jain R, Basinski A, Kreder HJ. Nonoperative treatment of hip fractures. Int Orthop. 2003;27:11-17.
13. Siu A, Penrod J, Boockvar K, et al. Early ambulation after hip fracture: effects on function and mortality. Arch Intern Med. 2006;166:766-771.
14. Juliebø V, Bjøro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354-1361.
15. Flinn DR, Deihl KM, Seyfried LS, et al. Prevention, diagnosis, and management of postoperative delirium in older adults. J Am Coll Surg. 2009;209:261-268.
16. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
17. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
18. Sieber FE, Mears S, Lee H, et al. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc. 2011;59:2256-2262.
19. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for Rather than focus on a single exercise, a combination of activities—eg, Tai Chi and walking, or weight lifting and cycling—have the greatest likelihood of fall reduction. prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35:714-719.
20. Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343-349.
21. Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119-141.
22. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference of Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
23. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):7S-47S.
24. Garcia Lazaro M, Montero Perez-Barquero M, Carpintero Benitez P. The role of malnutrition and other medical factors in the evolution of patients with hip fracture [article in Spanish]. An Med Interna. 2004;21:557-563.
25. Lavernia CJ, Sierra RJ, Baerga L. Nutritional parameters and short term outcome in arthroplasty. J Am Coll Nutr. 1999;18:274-278.
26. Huddleston JM, Whitford KJ. Medical care of elderly patients with hip fractures. Mayo Clin Proc. 2001;76:295-298.
27. Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178-208.
28. Nelson ME, Fiatarone MA, Morganti CM, et al. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures a randomized controlled trial. JAMA. 1994;272:1909-1914.
29. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
30. Yamada M, Tanaka B, Nagai K, et al. Trail-walking exercise and fall risk factors in community-dwelling older adults: preliminary results of a randomized controlled trial. J Am Geriatr Soc. 2010;58:1946-1951.
31. Visser M, Deeg DJ, Lips P; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766-5772.
32. Kalyani RR, Stein B, Valiyil R, et al. Vitamin D treatment for the prevention of falls in older adults: systematic review and metaanalysis. J Am Geriatr Soc. 2010;58:1299-1310.
33. The American Geriatrics Society. Prevention of falls in older persons [clinical practice guideline]. 2010. Available at: http:// www.americangeriatrics.org/health_care_professionals/clinical_practice/clinical_guidelines_recommendations/ 2010/. Accessed August 16, 2013.
34. Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;(158):1-235.
35. Michael YL, Whitlock EP, Lin JS, et al. Primary care-relevant interventions to prevent falling in older adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:815-825.
36. USPSTF. Prevention of falls in community-dwelling older adults. US Preventive Services Task Force recommendation statement. May 2012. Available at: www.uspreventiveservices taskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed August 19, 2013.
37. Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
38. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; for the HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
39. Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011;22:983-991.
40. Ringe, JD, Doherty, JG. Absolute risk reduction in osteoporosis: assessing treatment efficacy by number needed to treat. Rheumatol Int. 2010;30:863-869.
41. Veehof L, Stewart R, Haaijer-Ruskamp F, et al. The development of polypharmacy. A longitudinal study. Fam Pract. 2000;17:261-267.
42. Choe HM, Farris KB, Stevenson JG, et al. Patient-centered medical home: developing, expanding, and sustaining a role for pharmacists. Am J Health Syst Pharm. 2012;69:1063-1071.
› Ensure that surgical stabilization of hip fracture is performed as soon as possible—ideally within 48 hours of injury. A
› To reduce the risk of delirium, orient the patient frequently; get her out of bed as soon as possible, and avoid prolonged catheter use. A
› Order protein supplements for patients recovering from hip fracture and take steps to facilitate an early return to eating. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
The patient and family request a consultation with Ms. J’s primary care physician. If you were her physician, what would you advise?
Hip fracture in a frail elderly patient is an injury that, while common, can be difficult to manage. With good reason. Geriatric hip fracture is associated with increased morbidity, functional decline, and use of nursing home services, as well as a higher mortality rate: One in 5 hip fracture patients dies within a year of the injury.1
As the population ages, we are seeing more hip fractures in the “oldest old” those who, like Ms. J, are older than 85. While the incidence increases exponentially with age in both men and women, women are 3 times more likely than men to sustain a hip fracture.2 White women ages 85 to 95 face the highest risk, with an incidence of more than 3%.3
In addition to managing the acute phase of hip fracture and helping patients and families make decisions about optimal treatment, there is much you can do to boost the likelihood of a rapid rehabilitation and a successful outcome.
What type of fracture? How best to treat it?
Two types of hip fractures are responsible for the vast majority of cases: About 45% of hip fractures are intracapsular, involving the femoral head and neck; another 45% are intertrochanteric fractures. Both usually involve low-energy trauma, such as a fall from a chair or tripping over a rug. Intertrochanteric and subtrochanteric fractures (the latter accounting for the remaining 10%) are extracapsular.2,4,5
Typically associated with high-energy trauma such as a motor vehicle accident, or with metastatic lesions, subtrochanteric fractures have a bimodal distribution: They are most common in individuals between the ages of 20 and 40 and those older than 60.2
Fractures involving the femoral neck can disrupt the vascular supply to the femoral head and result in avascular necrosis (AVN) or nonunion.2,4,5 A meta-analysis of the outcome of displaced femoral neck fractures found the rates of osteonecrosis and nonunion to be as high as 20% to 30%.5 Intertrochanteric fractures rarely lead to AVN or nonunion, but patients may develop complications associated with degenerative changes.2,4,5 Nonunion is a potential complication of subtrochanteric fracture.2
For most patients, surgical management is preferred
The main goals of treatment are to stabilize the hip, decrease pain and restore the level of prefracture function. Surgery is the preferred treatment for hip fracture because it provides stable fixation, facilitating full weight bearing and decreasing the risk of complications. Surgery is also associated with a shorter stay in the hospital and improved rehabilitation and recovery.6
Surgical stabilization should be performed as soon as possible—ideally, within 48 hours.5 A recent study found conflicting evidence of the effect of delayed surgery on mortality, but demonstrated that surgery within 24 hours of injury minimizes the rate of chest infections, urinary tract infections, and pressure sores, as well as the duration of the hospital stay.7 (To learn more about surgical stabilization of hip fracture, see “What type of surgery? Age is just one consideration” 5,8-10 below.)
When surgery is contraindicated
Nonoperative management is reserved for patients who stand to gain only minimal function from surgical stabilization, because they either were not ambulatory to begin with or have severe dementia. In addition, medical management is used for patients with contraindications to anesthesia, those who delay seeking medical care until the fracture has begun to heal, and patients who refuse surgical fixation.5,11
The choice of surgical intervention depends on multiple factors, including the:
- type and severity of the fracture
- preference of the orthopedic surgeon
- age of the patient
- comorbid conditions
- prognosis.
For femoral neck fractures, patients younger than 65 years are candidates for internal fixation; for older individuals and those who already had limited mobility, arthroplasty should be considered.5 Studies of pain and functional outcomes show a modest tendency for total hip arthroplasty to have better results than internal fixation in patients older than 65.8
Intertrochanteric fractures can be treated with either sliding hip screws or
intramedullary nails. Intramedullary nail implants are done percutaneously, resulting in a shorter duration for surgery, less blood loss, and an earlier return to full weight bearing.5 A recent study suggests that intramedullary nails result in more reoperations than hip screws.9 No evidence is conclusive about the superiority of either type of hardware.
Subtrochanteric fractures are typically repaired by hemiarthroplasty.
A Cochrane review of randomized controlled trials found insufficient evidence to determine whether replacement arthroplasty has any advantage over internal fixation for extracapsular hip fractures.10
CASE After a careful review of Ms. J’s health status, radiographs of the fracture (FIGURE 1A), and consultation with an orthopedic surgeon and a geriatrician, you recommend surgery as soon as the patient is fully stabilized. Without it, she would be at high risk for urinary tract infection, pressure sores, and thromboembolism associated with long-term immobility.
The next day, Ms. J undergoes surgical fixation with a sliding hip screw (FIGURE 1B). Her Foley catheter is removed the same day, and physical therapy is begun the following day. On postoperative day 4 she is discharged to an in patient rehabilitation facility.
Begin rehabilitation without delay
Whether a patient has surgery or is treated nonoperatively for hip fracture, the goal of rehabilitation is the same—to restore mobility as quickly as possible. A clinical review found no significant difference in mortality rates between those who underwent surgical fixation and those who were treated medically with early mobilization, consisting of immediate bed-to-chair transfer (with assistance), followed by progression to ambulation as tolerated.12
For patients who undergo surgery for hip fracture, increased immobility is linked to poorer functioning in the areas of self-care and transfers at 2 months and to higher mortality rates at 6 months.13 Physical therapy should be initiated on the first postoperative day and should start with bed mobility range of motion, followed by independent transfers from bed to chair, and ultimately achieving full weight bearing.5
Many complications are predictable, and often preventable
The term “hip fracture syndrome”4 is often used in reference to a cluster of common (and often preventable) complications of hip fracture, with delirium, venous thromboembolism (VTE), and malnutrition foremost among them.
Take steps to prevent—or treat—delirium
Delirium is among the most common complication, occurring in up to 62% of older patients with hip fracture.4 The highest predictor of delirium is preexisting cognitive impairment.
Other risk factors for delirium include advanced age, vision or hearing impairment, concurrent alcohol abuse, malnutrition, comorbidity, and polypharmacy.4,14 Delirium is associated with increased morbidity and mortality, decreased rehabilitation potential, and poor functional recovery independent of prior frailty.4,15,16
Hypoactive delirium is easily missed. While agitated, or hyperactive, delirium is more easily recognized, it is crucial to be aware of hypoactive delirium, as well. Patients with hypoactive delirium tend to become more withdrawn and their delirium is easily missed, leading to worse outcomes.15 The Confusion Assessment Method (TABLE 1)17 is an easy-to-use validated tool developed to aid in the diagnosis of delirium at the bedside.
Many factors contribute to the development of delirium. Medical complications, such as infection, electrolyte and volume imbalances, hypoxia, and myocardial infarction, are obvious precipitants.15 Disturbances in sleep-wake cycles, an unfamiliar environment, physical restraints, and the use of Foley catheters—all of which can impair an older patient’s sensory awareness—are less well-known contributing factors.
Tips for preventing delirium. Early mobilization, in addition to boosting physical recovery, can help prevent delirium.
Other tips:
- discontinue catheterization as soon as possible; this may help prevent delirium, and lessen the risk of urinary tract infection.
- remind nurses and family members to continuously reorient patients to their surroundings.
- treat pain aggressively.
- consult a geriatrician early on.
While opioids are often thought to cause delirium, several studies have shown an inverse relationship—that is, hip fracture patients who were given opioids for pain were actually less likely to develop delirium than those who did not receive opioids. This raises 2 important points:
1. untreated pain may itself be a significant risk factor for delirium,15,18 and
2. delirium itself is not a contraindication to opioids.18
CASE In her first week at the inpatient rehabilitation center, Ms. J requires slightly more narcotic medication for pain control. The staff notices increased confusion and a decrease in the number of bowel movements. Ms. J is started on a regimen of sennosides and docusate twice daily. Her mental status improves quickly and she has no further complications while at the rehab center.
Nonopioid pain medications such as acetaminophen should be scheduled at appropriate doses (eg, 1 g tid). Ensure that patients recovering from hip fracture are not given benzodiazepines, anticholinergics, or antihistamines15— which are sometimes included in a facility’s PRN protocol. In clinical trials, prophylactic administration of antipsychotics or anticholinesterase therapy to high-risk patients has had conflicting results.19,20
Arrange for a geriatric consult before problems occur. Several studies have shown that a geriatric consultation and concurrent management by a geriatrician using structured protocols to evaluate for common risk factors known to precipitate delirium (eg, pain, bowel/bladder function, nutrition, mobilization) can reduce the risk of delirium.16
Provide supportive care. Although treatment of the underlying cause is the definitive treatment for delirium, there are times when supportive care is all that’s needed. Reassurance from family members or staff is the recommended first step. Physical restraints should be avoided unless patient safety is threatened despite attempts to provide supportive care.
If treatment for delirium is needed, lowdose antipsychotics are recommended. The most studied agent is haloperidol, which can be administered intravenously (IV), intramuscularly (IM), or orally. Monitoring the corrected QT (QTc) interval is recommended for patients taking haloperidol, and discontinuation of the drug—or a cardiology consult— is recommended if the QTc interval is prolonged (>450 ms or >25% of baseline).21
There is a slightly higher risk of cardiac arrhythmias with IV administration of haloperidol compared with IM or oral dosing. Despite this risk, haloperidol IV is the treatment of choice for delirium.21 Newer atypical antipsychotics have also been used to treat delirium, but data are limited.21
Guard against VTE
Studies have shown rates of VTE to be as high as 40% to 60% after orthopedic procedures, and prophylaxis has long been the standard of care.22 In its 2012 consensus guidelines for antithrombotic therapy, the American College of Chest Physicians (ACCP) recommends fondiparinux, apixaban, rivaroxaban, dabigatran, low-molecular-weight heparin (LMWH), low-dose unfractionated heparin, aspirin, warfarin, or an intermittent pneumatic compression device (IPCD) as prophylaxis.23 Portable battery-powered IPCDs are recommended for 18 hours postop.23
The guideline authors prefer LMWH to the other treatments, and recommend dual prophylaxis with an IPCD and an antithrombotic agent while the patient is in the hospital and for a minimum of 10 to 14 days (and up to 35 days) after discharge. If surgery for hip fracture is delayed, the ACCP recommends that LMWH be administered after admission, but withheld for at least 12 hours before surgery. In patients with a high risk of bleeding, the ACCP recommends either an IPCD alone or no prophylaxis and notes that inferior vena cava filters should not be placed in high-risk patients.23
Take steps to ensure ample protein intake
Malnourishment is another common complication, affecting up to 20% of hip fracture patients.24 In many cases, a catabolic state predisposes patients to protein depletion, leading to decreased wound healing and an increase in other postop complications.24,25 Protein supplementation is associated with decreased length of stay and a reduction in postop complications.26
This complication can often be avoided by encouraging an early return to eating. Specific steps: Ensure that patients have their dentures available and are able to use them; are positioned properly for eating; and receive high-caloric supplemental drinks. Nutritional assessments should also be done to ensure that their intake of calcium and vitamin D is sufficient to prevent future falls and reduce fracture risk. (For more information, see “Vitamin D: When it helps, when it harms” [J Fam Pract. 2013;62:368-370.])
Combat hip fracture by stressing avoidance
Prevention of hip fracture, of course, is the ideal way to reduce the burden of disease for older patients. Along these lines, there are many ways you can help.
Start with fall reduction
Hip fracture is associated with a fall 90% of the time,27 and care for older patients should be focused on reducing the risk for falls and improving bone health and muscular function. While a complete review of preventive measures is beyond the scope of this article, we offer some highlights here and in TABLE 2.
Encourage physical activity In addition to helping to reduce falls, physical activity—particularly repetitive weight-bearing exercise—can help maintain bone density and improve muscle mass, strength, and balance.28
Rather than focus on a single exercise, however, a combination of activities—Tai Chi and walking, for instance, or weight lifting and cycling —appears to have the best likelihood of fall reduction.29 Whenever possible, physical activity for older patients should include challenges in executive function, as well. In a recent study comparing regular walking with trail-walking between sequentially marked flags, participants in the more complex activity had a greater decrease in fall rates.30
Review vitamin D and calcium intake. Elderly patients with low levels of vitamin D are at increased risk of muscle mass decline, and therefore increased risk of fracture.31 A systematic review and meta-analysis of vitamin D supplementation in older adults found the relative risk of falling was 0.86 (95% confidence interval [CI], 0.79-0.93) for those assigned to vitamin D therapy compared with those on placebo. Risk reduction was greater in groups taking 800 IU or more of vitamin D daily and those taking adjunctive calcium supplementation.32
Maximizing vitamin D for falls reduction is supported by the American Geriatrics Society, 33 the Agency for Healthcare Research and Quality (AHRQ),34 and the US Preventive Services Task Force (USPSTF).35 The USPSTF recently released a recommendation for exercise or physical therapy and vitamin D supplementation (800 IU) to prevent falls in community-dwelling adults ages 65 and over who are at an increased risk for falls.36
However, the USPSTF advises against daily supplementation with vitamin D and calcium at doses ?400 IU and 1000 mg, respectively, for noninstitutionalized postmenopausal women for primary fracture prevention. Calcium supplementation has not been shown to reduce hip fractures, but has been found to improve hip bone density.37
Consider bisphosphonates. Order a dual energy x-ray absorptiometry (DEXA) scan for older patients to identify osteoporosis. Most hip fractures are osteoporotic, and patients should be started on bisphosphonates within 2 to 12 weeks of injury38 to reduce the risk of mortality associated with hip fracture.39 The most studied bisphosphonates in geriatric hip fracture are alendronate, risedronate, and zoledronate; all were found to have a number needed to treat of 91 to prevent one hip fracture.40
Focus on the home environment. In addition to addressing the bone and muscular health of older patients, focus should be placed on the home environment. A Cochrane review of fall prevention for those living in the community found that home safety interventions reduced the risk of falls, but only for those with severe vision impairment and a high risk of falls.29 A 2010 American Geriatric Society (AGS) and British Geriatric Society (BGS) review of fall prevention gave an A recommendation—the highest rating— to home assessment and intervention by a health care professional to identify home hazards and promote safe performance of daily activities.33
Conduct brown-bag reviews. Polypharmacy is a well-documented (and growing) problem among the elderly.41 Both the AGS and BGS encourage a review of medications (including over-the-counter products) and interactions at each office visit,33 with specific attention paid to drugs that may cause dizziness, drowsiness, and near syncopal or syncopal episodes.
To reduce the risk of medication interactions and adverse effects, look for opportunities to reduce the number of drugs your elderly patients are taking. Consider involving a clinical pharmacist in medication reviews—an intervention that has been shown to be cost effective and lead to better patient outcomes.42
CASE After 4 weeks, Ms. J is ready to return home. Rather than a return to independent living, however, her children convince her to move to an assisted living facility—a move you strongly support. You schedule a visit in 2 weeks.
CORRESPONDENCE
Jeremy D. Close, MD, Department of Family and Community Medicine, Thomas Jefferson University, 833 Chestnut Street #301, Philadelphia, PA 19107; [email protected]
› Ensure that surgical stabilization of hip fracture is performed as soon as possible—ideally within 48 hours of injury. A
› To reduce the risk of delirium, orient the patient frequently; get her out of bed as soon as possible, and avoid prolonged catheter use. A
› Order protein supplements for patients recovering from hip fracture and take steps to facilitate an early return to eating. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
The patient and family request a consultation with Ms. J’s primary care physician. If you were her physician, what would you advise?
Hip fracture in a frail elderly patient is an injury that, while common, can be difficult to manage. With good reason. Geriatric hip fracture is associated with increased morbidity, functional decline, and use of nursing home services, as well as a higher mortality rate: One in 5 hip fracture patients dies within a year of the injury.1
As the population ages, we are seeing more hip fractures in the “oldest old” those who, like Ms. J, are older than 85. While the incidence increases exponentially with age in both men and women, women are 3 times more likely than men to sustain a hip fracture.2 White women ages 85 to 95 face the highest risk, with an incidence of more than 3%.3
In addition to managing the acute phase of hip fracture and helping patients and families make decisions about optimal treatment, there is much you can do to boost the likelihood of a rapid rehabilitation and a successful outcome.
What type of fracture? How best to treat it?
Two types of hip fractures are responsible for the vast majority of cases: About 45% of hip fractures are intracapsular, involving the femoral head and neck; another 45% are intertrochanteric fractures. Both usually involve low-energy trauma, such as a fall from a chair or tripping over a rug. Intertrochanteric and subtrochanteric fractures (the latter accounting for the remaining 10%) are extracapsular.2,4,5
Typically associated with high-energy trauma such as a motor vehicle accident, or with metastatic lesions, subtrochanteric fractures have a bimodal distribution: They are most common in individuals between the ages of 20 and 40 and those older than 60.2
Fractures involving the femoral neck can disrupt the vascular supply to the femoral head and result in avascular necrosis (AVN) or nonunion.2,4,5 A meta-analysis of the outcome of displaced femoral neck fractures found the rates of osteonecrosis and nonunion to be as high as 20% to 30%.5 Intertrochanteric fractures rarely lead to AVN or nonunion, but patients may develop complications associated with degenerative changes.2,4,5 Nonunion is a potential complication of subtrochanteric fracture.2
For most patients, surgical management is preferred
The main goals of treatment are to stabilize the hip, decrease pain and restore the level of prefracture function. Surgery is the preferred treatment for hip fracture because it provides stable fixation, facilitating full weight bearing and decreasing the risk of complications. Surgery is also associated with a shorter stay in the hospital and improved rehabilitation and recovery.6
Surgical stabilization should be performed as soon as possible—ideally, within 48 hours.5 A recent study found conflicting evidence of the effect of delayed surgery on mortality, but demonstrated that surgery within 24 hours of injury minimizes the rate of chest infections, urinary tract infections, and pressure sores, as well as the duration of the hospital stay.7 (To learn more about surgical stabilization of hip fracture, see “What type of surgery? Age is just one consideration” 5,8-10 below.)
When surgery is contraindicated
Nonoperative management is reserved for patients who stand to gain only minimal function from surgical stabilization, because they either were not ambulatory to begin with or have severe dementia. In addition, medical management is used for patients with contraindications to anesthesia, those who delay seeking medical care until the fracture has begun to heal, and patients who refuse surgical fixation.5,11
The choice of surgical intervention depends on multiple factors, including the:
- type and severity of the fracture
- preference of the orthopedic surgeon
- age of the patient
- comorbid conditions
- prognosis.
For femoral neck fractures, patients younger than 65 years are candidates for internal fixation; for older individuals and those who already had limited mobility, arthroplasty should be considered.5 Studies of pain and functional outcomes show a modest tendency for total hip arthroplasty to have better results than internal fixation in patients older than 65.8
Intertrochanteric fractures can be treated with either sliding hip screws or
intramedullary nails. Intramedullary nail implants are done percutaneously, resulting in a shorter duration for surgery, less blood loss, and an earlier return to full weight bearing.5 A recent study suggests that intramedullary nails result in more reoperations than hip screws.9 No evidence is conclusive about the superiority of either type of hardware.
Subtrochanteric fractures are typically repaired by hemiarthroplasty.
A Cochrane review of randomized controlled trials found insufficient evidence to determine whether replacement arthroplasty has any advantage over internal fixation for extracapsular hip fractures.10
CASE After a careful review of Ms. J’s health status, radiographs of the fracture (FIGURE 1A), and consultation with an orthopedic surgeon and a geriatrician, you recommend surgery as soon as the patient is fully stabilized. Without it, she would be at high risk for urinary tract infection, pressure sores, and thromboembolism associated with long-term immobility.
The next day, Ms. J undergoes surgical fixation with a sliding hip screw (FIGURE 1B). Her Foley catheter is removed the same day, and physical therapy is begun the following day. On postoperative day 4 she is discharged to an in patient rehabilitation facility.
Begin rehabilitation without delay
Whether a patient has surgery or is treated nonoperatively for hip fracture, the goal of rehabilitation is the same—to restore mobility as quickly as possible. A clinical review found no significant difference in mortality rates between those who underwent surgical fixation and those who were treated medically with early mobilization, consisting of immediate bed-to-chair transfer (with assistance), followed by progression to ambulation as tolerated.12
For patients who undergo surgery for hip fracture, increased immobility is linked to poorer functioning in the areas of self-care and transfers at 2 months and to higher mortality rates at 6 months.13 Physical therapy should be initiated on the first postoperative day and should start with bed mobility range of motion, followed by independent transfers from bed to chair, and ultimately achieving full weight bearing.5
Many complications are predictable, and often preventable
The term “hip fracture syndrome”4 is often used in reference to a cluster of common (and often preventable) complications of hip fracture, with delirium, venous thromboembolism (VTE), and malnutrition foremost among them.
Take steps to prevent—or treat—delirium
Delirium is among the most common complication, occurring in up to 62% of older patients with hip fracture.4 The highest predictor of delirium is preexisting cognitive impairment.
Other risk factors for delirium include advanced age, vision or hearing impairment, concurrent alcohol abuse, malnutrition, comorbidity, and polypharmacy.4,14 Delirium is associated with increased morbidity and mortality, decreased rehabilitation potential, and poor functional recovery independent of prior frailty.4,15,16
Hypoactive delirium is easily missed. While agitated, or hyperactive, delirium is more easily recognized, it is crucial to be aware of hypoactive delirium, as well. Patients with hypoactive delirium tend to become more withdrawn and their delirium is easily missed, leading to worse outcomes.15 The Confusion Assessment Method (TABLE 1)17 is an easy-to-use validated tool developed to aid in the diagnosis of delirium at the bedside.
Many factors contribute to the development of delirium. Medical complications, such as infection, electrolyte and volume imbalances, hypoxia, and myocardial infarction, are obvious precipitants.15 Disturbances in sleep-wake cycles, an unfamiliar environment, physical restraints, and the use of Foley catheters—all of which can impair an older patient’s sensory awareness—are less well-known contributing factors.
Tips for preventing delirium. Early mobilization, in addition to boosting physical recovery, can help prevent delirium.
Other tips:
- discontinue catheterization as soon as possible; this may help prevent delirium, and lessen the risk of urinary tract infection.
- remind nurses and family members to continuously reorient patients to their surroundings.
- treat pain aggressively.
- consult a geriatrician early on.
While opioids are often thought to cause delirium, several studies have shown an inverse relationship—that is, hip fracture patients who were given opioids for pain were actually less likely to develop delirium than those who did not receive opioids. This raises 2 important points:
1. untreated pain may itself be a significant risk factor for delirium,15,18 and
2. delirium itself is not a contraindication to opioids.18
CASE In her first week at the inpatient rehabilitation center, Ms. J requires slightly more narcotic medication for pain control. The staff notices increased confusion and a decrease in the number of bowel movements. Ms. J is started on a regimen of sennosides and docusate twice daily. Her mental status improves quickly and she has no further complications while at the rehab center.
Nonopioid pain medications such as acetaminophen should be scheduled at appropriate doses (eg, 1 g tid). Ensure that patients recovering from hip fracture are not given benzodiazepines, anticholinergics, or antihistamines15— which are sometimes included in a facility’s PRN protocol. In clinical trials, prophylactic administration of antipsychotics or anticholinesterase therapy to high-risk patients has had conflicting results.19,20
Arrange for a geriatric consult before problems occur. Several studies have shown that a geriatric consultation and concurrent management by a geriatrician using structured protocols to evaluate for common risk factors known to precipitate delirium (eg, pain, bowel/bladder function, nutrition, mobilization) can reduce the risk of delirium.16
Provide supportive care. Although treatment of the underlying cause is the definitive treatment for delirium, there are times when supportive care is all that’s needed. Reassurance from family members or staff is the recommended first step. Physical restraints should be avoided unless patient safety is threatened despite attempts to provide supportive care.
If treatment for delirium is needed, lowdose antipsychotics are recommended. The most studied agent is haloperidol, which can be administered intravenously (IV), intramuscularly (IM), or orally. Monitoring the corrected QT (QTc) interval is recommended for patients taking haloperidol, and discontinuation of the drug—or a cardiology consult— is recommended if the QTc interval is prolonged (>450 ms or >25% of baseline).21
There is a slightly higher risk of cardiac arrhythmias with IV administration of haloperidol compared with IM or oral dosing. Despite this risk, haloperidol IV is the treatment of choice for delirium.21 Newer atypical antipsychotics have also been used to treat delirium, but data are limited.21
Guard against VTE
Studies have shown rates of VTE to be as high as 40% to 60% after orthopedic procedures, and prophylaxis has long been the standard of care.22 In its 2012 consensus guidelines for antithrombotic therapy, the American College of Chest Physicians (ACCP) recommends fondiparinux, apixaban, rivaroxaban, dabigatran, low-molecular-weight heparin (LMWH), low-dose unfractionated heparin, aspirin, warfarin, or an intermittent pneumatic compression device (IPCD) as prophylaxis.23 Portable battery-powered IPCDs are recommended for 18 hours postop.23
The guideline authors prefer LMWH to the other treatments, and recommend dual prophylaxis with an IPCD and an antithrombotic agent while the patient is in the hospital and for a minimum of 10 to 14 days (and up to 35 days) after discharge. If surgery for hip fracture is delayed, the ACCP recommends that LMWH be administered after admission, but withheld for at least 12 hours before surgery. In patients with a high risk of bleeding, the ACCP recommends either an IPCD alone or no prophylaxis and notes that inferior vena cava filters should not be placed in high-risk patients.23
Take steps to ensure ample protein intake
Malnourishment is another common complication, affecting up to 20% of hip fracture patients.24 In many cases, a catabolic state predisposes patients to protein depletion, leading to decreased wound healing and an increase in other postop complications.24,25 Protein supplementation is associated with decreased length of stay and a reduction in postop complications.26
This complication can often be avoided by encouraging an early return to eating. Specific steps: Ensure that patients have their dentures available and are able to use them; are positioned properly for eating; and receive high-caloric supplemental drinks. Nutritional assessments should also be done to ensure that their intake of calcium and vitamin D is sufficient to prevent future falls and reduce fracture risk. (For more information, see “Vitamin D: When it helps, when it harms” [J Fam Pract. 2013;62:368-370.])
Combat hip fracture by stressing avoidance
Prevention of hip fracture, of course, is the ideal way to reduce the burden of disease for older patients. Along these lines, there are many ways you can help.
Start with fall reduction
Hip fracture is associated with a fall 90% of the time,27 and care for older patients should be focused on reducing the risk for falls and improving bone health and muscular function. While a complete review of preventive measures is beyond the scope of this article, we offer some highlights here and in TABLE 2.
Encourage physical activity In addition to helping to reduce falls, physical activity—particularly repetitive weight-bearing exercise—can help maintain bone density and improve muscle mass, strength, and balance.28
Rather than focus on a single exercise, however, a combination of activities—Tai Chi and walking, for instance, or weight lifting and cycling —appears to have the best likelihood of fall reduction.29 Whenever possible, physical activity for older patients should include challenges in executive function, as well. In a recent study comparing regular walking with trail-walking between sequentially marked flags, participants in the more complex activity had a greater decrease in fall rates.30
Review vitamin D and calcium intake. Elderly patients with low levels of vitamin D are at increased risk of muscle mass decline, and therefore increased risk of fracture.31 A systematic review and meta-analysis of vitamin D supplementation in older adults found the relative risk of falling was 0.86 (95% confidence interval [CI], 0.79-0.93) for those assigned to vitamin D therapy compared with those on placebo. Risk reduction was greater in groups taking 800 IU or more of vitamin D daily and those taking adjunctive calcium supplementation.32
Maximizing vitamin D for falls reduction is supported by the American Geriatrics Society, 33 the Agency for Healthcare Research and Quality (AHRQ),34 and the US Preventive Services Task Force (USPSTF).35 The USPSTF recently released a recommendation for exercise or physical therapy and vitamin D supplementation (800 IU) to prevent falls in community-dwelling adults ages 65 and over who are at an increased risk for falls.36
However, the USPSTF advises against daily supplementation with vitamin D and calcium at doses ?400 IU and 1000 mg, respectively, for noninstitutionalized postmenopausal women for primary fracture prevention. Calcium supplementation has not been shown to reduce hip fractures, but has been found to improve hip bone density.37
Consider bisphosphonates. Order a dual energy x-ray absorptiometry (DEXA) scan for older patients to identify osteoporosis. Most hip fractures are osteoporotic, and patients should be started on bisphosphonates within 2 to 12 weeks of injury38 to reduce the risk of mortality associated with hip fracture.39 The most studied bisphosphonates in geriatric hip fracture are alendronate, risedronate, and zoledronate; all were found to have a number needed to treat of 91 to prevent one hip fracture.40
Focus on the home environment. In addition to addressing the bone and muscular health of older patients, focus should be placed on the home environment. A Cochrane review of fall prevention for those living in the community found that home safety interventions reduced the risk of falls, but only for those with severe vision impairment and a high risk of falls.29 A 2010 American Geriatric Society (AGS) and British Geriatric Society (BGS) review of fall prevention gave an A recommendation—the highest rating— to home assessment and intervention by a health care professional to identify home hazards and promote safe performance of daily activities.33
Conduct brown-bag reviews. Polypharmacy is a well-documented (and growing) problem among the elderly.41 Both the AGS and BGS encourage a review of medications (including over-the-counter products) and interactions at each office visit,33 with specific attention paid to drugs that may cause dizziness, drowsiness, and near syncopal or syncopal episodes.
To reduce the risk of medication interactions and adverse effects, look for opportunities to reduce the number of drugs your elderly patients are taking. Consider involving a clinical pharmacist in medication reviews—an intervention that has been shown to be cost effective and lead to better patient outcomes.42
CASE After 4 weeks, Ms. J is ready to return home. Rather than a return to independent living, however, her children convince her to move to an assisted living facility—a move you strongly support. You schedule a visit in 2 weeks.
CORRESPONDENCE
Jeremy D. Close, MD, Department of Family and Community Medicine, Thomas Jefferson University, 833 Chestnut Street #301, Philadelphia, PA 19107; [email protected]
1. Leibson CL, Toteson ANA, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644-50.
2. Brunner LC, Eshilian-Oates L, Kuo TY. Hip fractures in adults. Am Fam Physician. 2003;67:537-542.
3. Jacobsen SJ, Goldberg J, Miles TP, et al. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health. 1990;80:871-873.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24:701-719.
5. Jackman JM, Watson JT. Hip fractures in older men. Clin Geriatr Med. 2010;26:311-329.
6. Handoll HH, Parker MJ. Conservative versus operative treatment for hip fractures in adults. Cochrane Database Syst Rev. 2008;(3):CD000337.
7. Leung F, Lau W, Kwan K, et al. Does timing of surgery matter in fragility hip fractures? Osteoporos Int. 2010; 21(suppl 4):S529-S534.
8. Butler M, Forte ML, Joglekar SB, et al. Evidence summary: systematic review of surgical treatments for geriatric hip fractures. J Bone Joint Surg Am. 2011;93:1104-1115.
9. Matre K, Havelin LI, Gjertsen JE, et al. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop Relat Res. 2013;471: 1379-1386.
10. Parker MJ, Handoll HH. Replacement arthroplasty versus internal fixation for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2006;(2):CD000086.
11. Cummings-Vaughn LA, Gammack JK. Falls, osteoporosis, and hip fractures. Med Clin North Am. 2011;95:495-506.
12. Jain R, Basinski A, Kreder HJ. Nonoperative treatment of hip fractures. Int Orthop. 2003;27:11-17.
13. Siu A, Penrod J, Boockvar K, et al. Early ambulation after hip fracture: effects on function and mortality. Arch Intern Med. 2006;166:766-771.
14. Juliebø V, Bjøro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354-1361.
15. Flinn DR, Deihl KM, Seyfried LS, et al. Prevention, diagnosis, and management of postoperative delirium in older adults. J Am Coll Surg. 2009;209:261-268.
16. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
17. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
18. Sieber FE, Mears S, Lee H, et al. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc. 2011;59:2256-2262.
19. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for Rather than focus on a single exercise, a combination of activities—eg, Tai Chi and walking, or weight lifting and cycling—have the greatest likelihood of fall reduction. prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35:714-719.
20. Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343-349.
21. Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119-141.
22. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference of Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
23. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):7S-47S.
24. Garcia Lazaro M, Montero Perez-Barquero M, Carpintero Benitez P. The role of malnutrition and other medical factors in the evolution of patients with hip fracture [article in Spanish]. An Med Interna. 2004;21:557-563.
25. Lavernia CJ, Sierra RJ, Baerga L. Nutritional parameters and short term outcome in arthroplasty. J Am Coll Nutr. 1999;18:274-278.
26. Huddleston JM, Whitford KJ. Medical care of elderly patients with hip fractures. Mayo Clin Proc. 2001;76:295-298.
27. Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178-208.
28. Nelson ME, Fiatarone MA, Morganti CM, et al. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures a randomized controlled trial. JAMA. 1994;272:1909-1914.
29. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
30. Yamada M, Tanaka B, Nagai K, et al. Trail-walking exercise and fall risk factors in community-dwelling older adults: preliminary results of a randomized controlled trial. J Am Geriatr Soc. 2010;58:1946-1951.
31. Visser M, Deeg DJ, Lips P; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766-5772.
32. Kalyani RR, Stein B, Valiyil R, et al. Vitamin D treatment for the prevention of falls in older adults: systematic review and metaanalysis. J Am Geriatr Soc. 2010;58:1299-1310.
33. The American Geriatrics Society. Prevention of falls in older persons [clinical practice guideline]. 2010. Available at: http:// www.americangeriatrics.org/health_care_professionals/clinical_practice/clinical_guidelines_recommendations/ 2010/. Accessed August 16, 2013.
34. Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;(158):1-235.
35. Michael YL, Whitlock EP, Lin JS, et al. Primary care-relevant interventions to prevent falling in older adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:815-825.
36. USPSTF. Prevention of falls in community-dwelling older adults. US Preventive Services Task Force recommendation statement. May 2012. Available at: www.uspreventiveservices taskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed August 19, 2013.
37. Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
38. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; for the HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
39. Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011;22:983-991.
40. Ringe, JD, Doherty, JG. Absolute risk reduction in osteoporosis: assessing treatment efficacy by number needed to treat. Rheumatol Int. 2010;30:863-869.
41. Veehof L, Stewart R, Haaijer-Ruskamp F, et al. The development of polypharmacy. A longitudinal study. Fam Pract. 2000;17:261-267.
42. Choe HM, Farris KB, Stevenson JG, et al. Patient-centered medical home: developing, expanding, and sustaining a role for pharmacists. Am J Health Syst Pharm. 2012;69:1063-1071.
1. Leibson CL, Toteson ANA, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644-50.
2. Brunner LC, Eshilian-Oates L, Kuo TY. Hip fractures in adults. Am Fam Physician. 2003;67:537-542.
3. Jacobsen SJ, Goldberg J, Miles TP, et al. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health. 1990;80:871-873.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24:701-719.
5. Jackman JM, Watson JT. Hip fractures in older men. Clin Geriatr Med. 2010;26:311-329.
6. Handoll HH, Parker MJ. Conservative versus operative treatment for hip fractures in adults. Cochrane Database Syst Rev. 2008;(3):CD000337.
7. Leung F, Lau W, Kwan K, et al. Does timing of surgery matter in fragility hip fractures? Osteoporos Int. 2010; 21(suppl 4):S529-S534.
8. Butler M, Forte ML, Joglekar SB, et al. Evidence summary: systematic review of surgical treatments for geriatric hip fractures. J Bone Joint Surg Am. 2011;93:1104-1115.
9. Matre K, Havelin LI, Gjertsen JE, et al. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop Relat Res. 2013;471: 1379-1386.
10. Parker MJ, Handoll HH. Replacement arthroplasty versus internal fixation for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2006;(2):CD000086.
11. Cummings-Vaughn LA, Gammack JK. Falls, osteoporosis, and hip fractures. Med Clin North Am. 2011;95:495-506.
12. Jain R, Basinski A, Kreder HJ. Nonoperative treatment of hip fractures. Int Orthop. 2003;27:11-17.
13. Siu A, Penrod J, Boockvar K, et al. Early ambulation after hip fracture: effects on function and mortality. Arch Intern Med. 2006;166:766-771.
14. Juliebø V, Bjøro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354-1361.
15. Flinn DR, Deihl KM, Seyfried LS, et al. Prevention, diagnosis, and management of postoperative delirium in older adults. J Am Coll Surg. 2009;209:261-268.
16. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
17. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
18. Sieber FE, Mears S, Lee H, et al. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc. 2011;59:2256-2262.
19. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for Rather than focus on a single exercise, a combination of activities—eg, Tai Chi and walking, or weight lifting and cycling—have the greatest likelihood of fall reduction. prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35:714-719.
20. Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343-349.
21. Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119-141.
22. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference of Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
23. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):7S-47S.
24. Garcia Lazaro M, Montero Perez-Barquero M, Carpintero Benitez P. The role of malnutrition and other medical factors in the evolution of patients with hip fracture [article in Spanish]. An Med Interna. 2004;21:557-563.
25. Lavernia CJ, Sierra RJ, Baerga L. Nutritional parameters and short term outcome in arthroplasty. J Am Coll Nutr. 1999;18:274-278.
26. Huddleston JM, Whitford KJ. Medical care of elderly patients with hip fractures. Mayo Clin Proc. 2001;76:295-298.
27. Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178-208.
28. Nelson ME, Fiatarone MA, Morganti CM, et al. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures a randomized controlled trial. JAMA. 1994;272:1909-1914.
29. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
30. Yamada M, Tanaka B, Nagai K, et al. Trail-walking exercise and fall risk factors in community-dwelling older adults: preliminary results of a randomized controlled trial. J Am Geriatr Soc. 2010;58:1946-1951.
31. Visser M, Deeg DJ, Lips P; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766-5772.
32. Kalyani RR, Stein B, Valiyil R, et al. Vitamin D treatment for the prevention of falls in older adults: systematic review and metaanalysis. J Am Geriatr Soc. 2010;58:1299-1310.
33. The American Geriatrics Society. Prevention of falls in older persons [clinical practice guideline]. 2010. Available at: http:// www.americangeriatrics.org/health_care_professionals/clinical_practice/clinical_guidelines_recommendations/ 2010/. Accessed August 16, 2013.
34. Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;(158):1-235.
35. Michael YL, Whitlock EP, Lin JS, et al. Primary care-relevant interventions to prevent falling in older adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:815-825.
36. USPSTF. Prevention of falls in community-dwelling older adults. US Preventive Services Task Force recommendation statement. May 2012. Available at: www.uspreventiveservices taskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed August 19, 2013.
37. Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
38. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; for the HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
39. Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011;22:983-991.
40. Ringe, JD, Doherty, JG. Absolute risk reduction in osteoporosis: assessing treatment efficacy by number needed to treat. Rheumatol Int. 2010;30:863-869.
41. Veehof L, Stewart R, Haaijer-Ruskamp F, et al. The development of polypharmacy. A longitudinal study. Fam Pract. 2000;17:261-267.
42. Choe HM, Farris KB, Stevenson JG, et al. Patient-centered medical home: developing, expanding, and sustaining a role for pharmacists. Am J Health Syst Pharm. 2012;69:1063-1071.
Painful ear nodules
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
A 49-year-old man with a history of hypertension, hypercholesterolemia, polysubstance use, recurrent methicillin-resistant Staphylococcus aureus skin infections, and chronic hepatitis C infection sought care at our emergency department (ED) because parts of his ears had started turning black 3 days earlier. They were also painful to the touch. He denied fever, any similar skin lesions, injury to his ears, or a history of easy bleeding or bruising. A recovering alcoholic, he admitted to regular marijuana use and twice-weekly cocaine use. He had last used cocaine 3 days ago.
The patient was thin and in no acute distress. His vital signs and cardiopulmonary exams were normal. Examination of his ears revealed bilateral violaceous firm, tender purpura on the pinnae (FIGURE).
A complete blood count (CBC) revealed mild leukopenia (white blood cell [WBC] count, 2.0 × 109/L), neutropenia (0.9 × 109/L), and a normal platelet count (264 × 109/L). A chemistry panel, liver function tests, and prothrombin time were normal. Erythrocyte sedimentation rate (ESR) was elevated to 69 mm/h. The patient’s cholesterol level was not elevated. Urine toxicology was positive for cocaine and opioids. A human immunodeficiency virus test was negative.
Figure
Tender purpura on the pinnae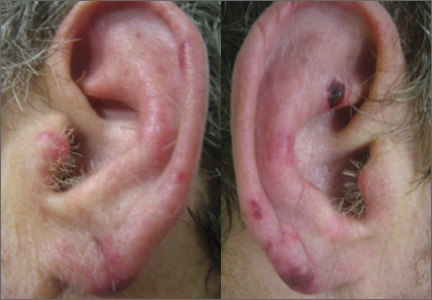
What is your diagnosis?
How would you treat this patient?
Diagnosis: Levamisole toxicity
The patient was diagnosed with levamisole toxicity based on his clinical presentation and the fact that he had used cocaine around the time his ear lesions appeared.
Levamisole—primarily a veterinary antihelmintic medication—is used on rare occasions to treat nephrotic syndrome in children.1 Levamisole is frequently added to cocaine or heroin to increase the street drug’s potency. The Drug Enforcement Administration reports that 69% of seized cocaine lots in the United States contain levamisole.2
The compound is thought to cause a vasculitis and bone marrow suppression resulting in neutropenia. The vasculitis targets small vessels, resulting in thrombosis, which can lead to tissue necrosis.1
Other possibilities in the differential Dx
The differential diagnosis includes a variety of vasculitides and other microvascular pathologies.
Cholesterol emboli arise when cholesterol crystals are released from atherosclerotic plaques, typically after invasive cardiac procedures. In addition, anticoagulants can cause the release of these crystals by inhibiting the formation of protective clots around unstable plaques.3 These emboli can seed the microvasculature anywhere, but the kidneys and skin are most frequently affected. These crystals not only clog the vasculature, causing tissue ischemia, but also activate the complement cascade, triggering a series of inflammatory responses that can lead to luminal fibrosis and narrowing.3
Affected patients have a history of atherosclerotic disease or predisposing factors such as hypertension or diabetes. Ulcerations or frank cyanosis may be found at the tips of the fingers or toes. In severe cases, gangrene will form in these regions. Patients may also have livido reticularis, a lace-like hyperpigmented rash over the lower extremities. Laboratory analysis may indicate acute renal failure or eosinophilia.3
Bacterial endocarditis results from the seeding of bacterial emboli primarily from the mitral or tricuspid valves.4 Streptococci are the primary infectious agent, with staphylococci being more common among intravenous drug users. High-risk populations include patients with artificial valves, the elderly, and the immunocompromised.4
Clinical manifestations include Janeway lesions (asymptomatic hemorrhagic papules on the palms) and Osler’s nodes (tender nodules on the fingertips). Splinter hemorrhages, or linear nonblanching lesions, may be present within the nail beds. Palpable purpura and petechiae may also be found.
Patients may have positive blood cultures, leukocytosis, an elevated ESR, or vegetations on a transesophageal echocardiogram.4 The physical exam may reveal a new cardiac murmur.
High circulating levels of cryoglobulins can arise in the setting of hepatitis C infection, but can also be seen in a number of autoimmune disorders and other infectious diseases.5 Cryoglobulins are immune complexes that are deposited into the lumen of microvasculature. In cold temperatures, these cryoglobulins precipitate, resulting in vasculitis. While most patients are asymptomatic, cutaneous findings in the distal extremities can include palpable purpura, ulcerations, and livido reticularis.5 Patients may complain of arthritis or symptoms consistent with Raynaud’s phenomenon.
Detection of specific serum cryoprecipitates isolated by immunofixation is pathognomonic for this condition, provided the sample is collected in a warm tube. Elevated rheumatoid factor and decreased complement levels may also be seen.5
Henoch-Schönlein purpura (HSP) is a small vessel vasculitis caused by IgA deposition that predominantly affects children. HSP has a host of systemic symptoms, often preceded by a benign upper respiratory infection, consisting of palpable purpura, arthritis, abdominal pain, and glomerulonephritis.6 Palpable purpura will generally be found in dependent portions of the body—especially the buttocks and lower legs.
While the diagnosis is primarily clinical, serum IgA levels and ESR can be elevated, urinalysis may demonstrate hematuria or proteinuria, and a CBC may reveal a leukocytosis with normal platelets.6
Suspect levamisole toxicity in patients using cocaine
Patients with levamisole toxicity present with sudden-onset tender plaques or bullae with necrotic centers within days of cocaine use. Case reports cite lesions primarily on the ears and cheeks. However, they can appear almost anywhere on the body.2,7-9 Physicians should have a high index of suspicion for levamisole toxicity in patients using cocaine who present with unexplained neutropenia or vasculitis.
Laboratory tests. If needed, tissue biopsy and urine detection of levamisole can be used to confirm the diagnosis.1
Management is straight-forward, but not simple
Skin lesions have been reported to improve several weeks after discontinuing use of contaminated cocaine1 (strength of recommendation [SOR]: C). Known users should be referred to drug treatment centers and counseled on the risks of use.
Our patient required hospitalization
When our patient came into the ED, he also complained of left thigh pain and swelling. A computed tomography scan revealed a deep sartorius abscess. The patient was admitted for ultrasound-guided aspiration of the abscess and IV antibiotics. His bilateral painful ear nodules persisted throughout his hospitalization, although his neutropenia resolved after 3 days.
Correspondence: Katherine Winter, MD, 101 Manning Drive, Chapel Hill, NC 27514; [email protected]
1. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
2. CDC. Agranulocytosis associated with cocaine use - four States, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631-641.
4. Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-1330.
5. Tedeschi A, Barate C, Minola E, et al. Cryoglobulinemia. Blood Rev. 2007;21:183-200.
6. Trapani S, Micheli A, Grisolia F, et al. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum. 2005;35:143-153.
7. Muirhead TT, Eide MJ. Images in clinical medicine. Toxic effects of levamisole in a cocaine user. N Engl J Med. 2011;364:e52.
8. Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med. 2010;152: 758-759.
9. Chung C, Tumeh P, Birnbaum R. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
A 49-year-old man with a history of hypertension, hypercholesterolemia, polysubstance use, recurrent methicillin-resistant Staphylococcus aureus skin infections, and chronic hepatitis C infection sought care at our emergency department (ED) because parts of his ears had started turning black 3 days earlier. They were also painful to the touch. He denied fever, any similar skin lesions, injury to his ears, or a history of easy bleeding or bruising. A recovering alcoholic, he admitted to regular marijuana use and twice-weekly cocaine use. He had last used cocaine 3 days ago.
The patient was thin and in no acute distress. His vital signs and cardiopulmonary exams were normal. Examination of his ears revealed bilateral violaceous firm, tender purpura on the pinnae (FIGURE).
A complete blood count (CBC) revealed mild leukopenia (white blood cell [WBC] count, 2.0 × 109/L), neutropenia (0.9 × 109/L), and a normal platelet count (264 × 109/L). A chemistry panel, liver function tests, and prothrombin time were normal. Erythrocyte sedimentation rate (ESR) was elevated to 69 mm/h. The patient’s cholesterol level was not elevated. Urine toxicology was positive for cocaine and opioids. A human immunodeficiency virus test was negative.
Figure
Tender purpura on the pinnae
What is your diagnosis?
How would you treat this patient?
Diagnosis: Levamisole toxicity
The patient was diagnosed with levamisole toxicity based on his clinical presentation and the fact that he had used cocaine around the time his ear lesions appeared.
Levamisole—primarily a veterinary antihelmintic medication—is used on rare occasions to treat nephrotic syndrome in children.1 Levamisole is frequently added to cocaine or heroin to increase the street drug’s potency. The Drug Enforcement Administration reports that 69% of seized cocaine lots in the United States contain levamisole.2
The compound is thought to cause a vasculitis and bone marrow suppression resulting in neutropenia. The vasculitis targets small vessels, resulting in thrombosis, which can lead to tissue necrosis.1
Other possibilities in the differential Dx
The differential diagnosis includes a variety of vasculitides and other microvascular pathologies.
Cholesterol emboli arise when cholesterol crystals are released from atherosclerotic plaques, typically after invasive cardiac procedures. In addition, anticoagulants can cause the release of these crystals by inhibiting the formation of protective clots around unstable plaques.3 These emboli can seed the microvasculature anywhere, but the kidneys and skin are most frequently affected. These crystals not only clog the vasculature, causing tissue ischemia, but also activate the complement cascade, triggering a series of inflammatory responses that can lead to luminal fibrosis and narrowing.3
Affected patients have a history of atherosclerotic disease or predisposing factors such as hypertension or diabetes. Ulcerations or frank cyanosis may be found at the tips of the fingers or toes. In severe cases, gangrene will form in these regions. Patients may also have livido reticularis, a lace-like hyperpigmented rash over the lower extremities. Laboratory analysis may indicate acute renal failure or eosinophilia.3
Bacterial endocarditis results from the seeding of bacterial emboli primarily from the mitral or tricuspid valves.4 Streptococci are the primary infectious agent, with staphylococci being more common among intravenous drug users. High-risk populations include patients with artificial valves, the elderly, and the immunocompromised.4
Clinical manifestations include Janeway lesions (asymptomatic hemorrhagic papules on the palms) and Osler’s nodes (tender nodules on the fingertips). Splinter hemorrhages, or linear nonblanching lesions, may be present within the nail beds. Palpable purpura and petechiae may also be found.
Patients may have positive blood cultures, leukocytosis, an elevated ESR, or vegetations on a transesophageal echocardiogram.4 The physical exam may reveal a new cardiac murmur.
High circulating levels of cryoglobulins can arise in the setting of hepatitis C infection, but can also be seen in a number of autoimmune disorders and other infectious diseases.5 Cryoglobulins are immune complexes that are deposited into the lumen of microvasculature. In cold temperatures, these cryoglobulins precipitate, resulting in vasculitis. While most patients are asymptomatic, cutaneous findings in the distal extremities can include palpable purpura, ulcerations, and livido reticularis.5 Patients may complain of arthritis or symptoms consistent with Raynaud’s phenomenon.
Detection of specific serum cryoprecipitates isolated by immunofixation is pathognomonic for this condition, provided the sample is collected in a warm tube. Elevated rheumatoid factor and decreased complement levels may also be seen.5
Henoch-Schönlein purpura (HSP) is a small vessel vasculitis caused by IgA deposition that predominantly affects children. HSP has a host of systemic symptoms, often preceded by a benign upper respiratory infection, consisting of palpable purpura, arthritis, abdominal pain, and glomerulonephritis.6 Palpable purpura will generally be found in dependent portions of the body—especially the buttocks and lower legs.
While the diagnosis is primarily clinical, serum IgA levels and ESR can be elevated, urinalysis may demonstrate hematuria or proteinuria, and a CBC may reveal a leukocytosis with normal platelets.6
Suspect levamisole toxicity in patients using cocaine
Patients with levamisole toxicity present with sudden-onset tender plaques or bullae with necrotic centers within days of cocaine use. Case reports cite lesions primarily on the ears and cheeks. However, they can appear almost anywhere on the body.2,7-9 Physicians should have a high index of suspicion for levamisole toxicity in patients using cocaine who present with unexplained neutropenia or vasculitis.
Laboratory tests. If needed, tissue biopsy and urine detection of levamisole can be used to confirm the diagnosis.1
Management is straight-forward, but not simple
Skin lesions have been reported to improve several weeks after discontinuing use of contaminated cocaine1 (strength of recommendation [SOR]: C). Known users should be referred to drug treatment centers and counseled on the risks of use.
Our patient required hospitalization
When our patient came into the ED, he also complained of left thigh pain and swelling. A computed tomography scan revealed a deep sartorius abscess. The patient was admitted for ultrasound-guided aspiration of the abscess and IV antibiotics. His bilateral painful ear nodules persisted throughout his hospitalization, although his neutropenia resolved after 3 days.
Correspondence: Katherine Winter, MD, 101 Manning Drive, Chapel Hill, NC 27514; [email protected]
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
A 49-year-old man with a history of hypertension, hypercholesterolemia, polysubstance use, recurrent methicillin-resistant Staphylococcus aureus skin infections, and chronic hepatitis C infection sought care at our emergency department (ED) because parts of his ears had started turning black 3 days earlier. They were also painful to the touch. He denied fever, any similar skin lesions, injury to his ears, or a history of easy bleeding or bruising. A recovering alcoholic, he admitted to regular marijuana use and twice-weekly cocaine use. He had last used cocaine 3 days ago.
The patient was thin and in no acute distress. His vital signs and cardiopulmonary exams were normal. Examination of his ears revealed bilateral violaceous firm, tender purpura on the pinnae (FIGURE).
A complete blood count (CBC) revealed mild leukopenia (white blood cell [WBC] count, 2.0 × 109/L), neutropenia (0.9 × 109/L), and a normal platelet count (264 × 109/L). A chemistry panel, liver function tests, and prothrombin time were normal. Erythrocyte sedimentation rate (ESR) was elevated to 69 mm/h. The patient’s cholesterol level was not elevated. Urine toxicology was positive for cocaine and opioids. A human immunodeficiency virus test was negative.
Figure
Tender purpura on the pinnae
What is your diagnosis?
How would you treat this patient?
Diagnosis: Levamisole toxicity
The patient was diagnosed with levamisole toxicity based on his clinical presentation and the fact that he had used cocaine around the time his ear lesions appeared.
Levamisole—primarily a veterinary antihelmintic medication—is used on rare occasions to treat nephrotic syndrome in children.1 Levamisole is frequently added to cocaine or heroin to increase the street drug’s potency. The Drug Enforcement Administration reports that 69% of seized cocaine lots in the United States contain levamisole.2
The compound is thought to cause a vasculitis and bone marrow suppression resulting in neutropenia. The vasculitis targets small vessels, resulting in thrombosis, which can lead to tissue necrosis.1
Other possibilities in the differential Dx
The differential diagnosis includes a variety of vasculitides and other microvascular pathologies.
Cholesterol emboli arise when cholesterol crystals are released from atherosclerotic plaques, typically after invasive cardiac procedures. In addition, anticoagulants can cause the release of these crystals by inhibiting the formation of protective clots around unstable plaques.3 These emboli can seed the microvasculature anywhere, but the kidneys and skin are most frequently affected. These crystals not only clog the vasculature, causing tissue ischemia, but also activate the complement cascade, triggering a series of inflammatory responses that can lead to luminal fibrosis and narrowing.3
Affected patients have a history of atherosclerotic disease or predisposing factors such as hypertension or diabetes. Ulcerations or frank cyanosis may be found at the tips of the fingers or toes. In severe cases, gangrene will form in these regions. Patients may also have livido reticularis, a lace-like hyperpigmented rash over the lower extremities. Laboratory analysis may indicate acute renal failure or eosinophilia.3
Bacterial endocarditis results from the seeding of bacterial emboli primarily from the mitral or tricuspid valves.4 Streptococci are the primary infectious agent, with staphylococci being more common among intravenous drug users. High-risk populations include patients with artificial valves, the elderly, and the immunocompromised.4
Clinical manifestations include Janeway lesions (asymptomatic hemorrhagic papules on the palms) and Osler’s nodes (tender nodules on the fingertips). Splinter hemorrhages, or linear nonblanching lesions, may be present within the nail beds. Palpable purpura and petechiae may also be found.
Patients may have positive blood cultures, leukocytosis, an elevated ESR, or vegetations on a transesophageal echocardiogram.4 The physical exam may reveal a new cardiac murmur.
High circulating levels of cryoglobulins can arise in the setting of hepatitis C infection, but can also be seen in a number of autoimmune disorders and other infectious diseases.5 Cryoglobulins are immune complexes that are deposited into the lumen of microvasculature. In cold temperatures, these cryoglobulins precipitate, resulting in vasculitis. While most patients are asymptomatic, cutaneous findings in the distal extremities can include palpable purpura, ulcerations, and livido reticularis.5 Patients may complain of arthritis or symptoms consistent with Raynaud’s phenomenon.
Detection of specific serum cryoprecipitates isolated by immunofixation is pathognomonic for this condition, provided the sample is collected in a warm tube. Elevated rheumatoid factor and decreased complement levels may also be seen.5
Henoch-Schönlein purpura (HSP) is a small vessel vasculitis caused by IgA deposition that predominantly affects children. HSP has a host of systemic symptoms, often preceded by a benign upper respiratory infection, consisting of palpable purpura, arthritis, abdominal pain, and glomerulonephritis.6 Palpable purpura will generally be found in dependent portions of the body—especially the buttocks and lower legs.
While the diagnosis is primarily clinical, serum IgA levels and ESR can be elevated, urinalysis may demonstrate hematuria or proteinuria, and a CBC may reveal a leukocytosis with normal platelets.6
Suspect levamisole toxicity in patients using cocaine
Patients with levamisole toxicity present with sudden-onset tender plaques or bullae with necrotic centers within days of cocaine use. Case reports cite lesions primarily on the ears and cheeks. However, they can appear almost anywhere on the body.2,7-9 Physicians should have a high index of suspicion for levamisole toxicity in patients using cocaine who present with unexplained neutropenia or vasculitis.
Laboratory tests. If needed, tissue biopsy and urine detection of levamisole can be used to confirm the diagnosis.1
Management is straight-forward, but not simple
Skin lesions have been reported to improve several weeks after discontinuing use of contaminated cocaine1 (strength of recommendation [SOR]: C). Known users should be referred to drug treatment centers and counseled on the risks of use.
Our patient required hospitalization
When our patient came into the ED, he also complained of left thigh pain and swelling. A computed tomography scan revealed a deep sartorius abscess. The patient was admitted for ultrasound-guided aspiration of the abscess and IV antibiotics. His bilateral painful ear nodules persisted throughout his hospitalization, although his neutropenia resolved after 3 days.
Correspondence: Katherine Winter, MD, 101 Manning Drive, Chapel Hill, NC 27514; [email protected]
1. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
2. CDC. Agranulocytosis associated with cocaine use - four States, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631-641.
4. Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-1330.
5. Tedeschi A, Barate C, Minola E, et al. Cryoglobulinemia. Blood Rev. 2007;21:183-200.
6. Trapani S, Micheli A, Grisolia F, et al. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum. 2005;35:143-153.
7. Muirhead TT, Eide MJ. Images in clinical medicine. Toxic effects of levamisole in a cocaine user. N Engl J Med. 2011;364:e52.
8. Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med. 2010;152: 758-759.
9. Chung C, Tumeh P, Birnbaum R. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
1. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
2. CDC. Agranulocytosis associated with cocaine use - four States, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631-641.
4. Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-1330.
5. Tedeschi A, Barate C, Minola E, et al. Cryoglobulinemia. Blood Rev. 2007;21:183-200.
6. Trapani S, Micheli A, Grisolia F, et al. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum. 2005;35:143-153.
7. Muirhead TT, Eide MJ. Images in clinical medicine. Toxic effects of levamisole in a cocaine user. N Engl J Med. 2011;364:e52.
8. Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med. 2010;152: 758-759.
9. Chung C, Tumeh P, Birnbaum R. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.