User login
The Pros and Cons of Using Larger Femoral Heads in Total Hip Arthroplasty
A feasibility study of caregiver-provided massage as supportive care for Veterans with cancer
Purpose To assess the feasibility of using a multimedia program to teach caregivers of Veterans with cancer how to offer basic massage for supportive care at home.
Methods Feasibility was assessed according to partner availability, compliance with watching training materials and practicing massage regularly, compliance with data collection; perceived study materials burden; clarity of instructional and other study materials. Pre- and post-massage changes in patients’ symptom scores were measured using a numerical rate scale. A semistructured exit interview was answered by patient and caregiver at the end of the study.
Results A total of 27 dyads were recruited. Veterans were 78% male. Forty-eight percent were diagnosed with hematologic malignancies (85%, advanced stage); 52% were diagnosed with solid tumors (64% advanced stage). Caregivers were 78% female; 81% were spouses. Out of the 27 pairs, 11 completed 8 weeks of data and practiced massage weekly. The majority of attrition (69%) was due to caregivers’ burden. Caregivers reported instructional materials were clear, high quality, and easy to use. Patients were highly satisfied with receiving touch from their partners regularly. Post-massage symptom scores showed statistically significant decreases in pain, stress/anxiety, and fatigue. Perceived burden of data collection instruments was high, particularly for patients.
Conclusion It is feasible to use the TCC program to train caregivers of Veterans with cancer to offer massage for supportive
care at home. Future studies should evaluate ways of providing support to caregivers, including offering massage to them, and
easing the burden of data collection for patients.
*For a PDF of the full article, click on the link to the left of this introduction.
Purpose To assess the feasibility of using a multimedia program to teach caregivers of Veterans with cancer how to offer basic massage for supportive care at home.
Methods Feasibility was assessed according to partner availability, compliance with watching training materials and practicing massage regularly, compliance with data collection; perceived study materials burden; clarity of instructional and other study materials. Pre- and post-massage changes in patients’ symptom scores were measured using a numerical rate scale. A semistructured exit interview was answered by patient and caregiver at the end of the study.
Results A total of 27 dyads were recruited. Veterans were 78% male. Forty-eight percent were diagnosed with hematologic malignancies (85%, advanced stage); 52% were diagnosed with solid tumors (64% advanced stage). Caregivers were 78% female; 81% were spouses. Out of the 27 pairs, 11 completed 8 weeks of data and practiced massage weekly. The majority of attrition (69%) was due to caregivers’ burden. Caregivers reported instructional materials were clear, high quality, and easy to use. Patients were highly satisfied with receiving touch from their partners regularly. Post-massage symptom scores showed statistically significant decreases in pain, stress/anxiety, and fatigue. Perceived burden of data collection instruments was high, particularly for patients.
Conclusion It is feasible to use the TCC program to train caregivers of Veterans with cancer to offer massage for supportive
care at home. Future studies should evaluate ways of providing support to caregivers, including offering massage to them, and
easing the burden of data collection for patients.
*For a PDF of the full article, click on the link to the left of this introduction.
Purpose To assess the feasibility of using a multimedia program to teach caregivers of Veterans with cancer how to offer basic massage for supportive care at home.
Methods Feasibility was assessed according to partner availability, compliance with watching training materials and practicing massage regularly, compliance with data collection; perceived study materials burden; clarity of instructional and other study materials. Pre- and post-massage changes in patients’ symptom scores were measured using a numerical rate scale. A semistructured exit interview was answered by patient and caregiver at the end of the study.
Results A total of 27 dyads were recruited. Veterans were 78% male. Forty-eight percent were diagnosed with hematologic malignancies (85%, advanced stage); 52% were diagnosed with solid tumors (64% advanced stage). Caregivers were 78% female; 81% were spouses. Out of the 27 pairs, 11 completed 8 weeks of data and practiced massage weekly. The majority of attrition (69%) was due to caregivers’ burden. Caregivers reported instructional materials were clear, high quality, and easy to use. Patients were highly satisfied with receiving touch from their partners regularly. Post-massage symptom scores showed statistically significant decreases in pain, stress/anxiety, and fatigue. Perceived burden of data collection instruments was high, particularly for patients.
Conclusion It is feasible to use the TCC program to train caregivers of Veterans with cancer to offer massage for supportive
care at home. Future studies should evaluate ways of providing support to caregivers, including offering massage to them, and
easing the burden of data collection for patients.
*For a PDF of the full article, click on the link to the left of this introduction.
Gender differences in the evolution of illness understanding among patients with advanced cancer
Background Patient understanding of advanced metastatic disease is central to decisions about care near death. Prior studies have focused on gender differences in communication style rather than on illness understanding.
Objectives To evaluate gender differences in terminal illness acknowledgement (TIA), understanding that the disease is incurable and the advanced stage of the disease. To evaluate gender differences in patients’ reports of discussions of life expectancy with oncology providers and its effect on differences in illness understanding.
Methods Coping with Cancer 2 patients (N 68) were interviewed before and after a visit with their oncology providers to discuss scan results.
Results At the prescan interview, there were no statistically significant gender differences in patient measures of illness understanding. At the postscan interview, women were more likely than men to recognize that their illness was incurable (Adjusted Odds Ratio, [AOR] 5.29; P .038), know that their cancer was at an advanced stage (AOR, 6.38; P, .013), and report having had discussions of life expectancy with their oncologist (AOR, 4.77; P, .021). Controlling discussions of life expectancy, women were more likely than men to report that their cancer was at an advanced stage (AOR, 9.53; P .050). Controlling for gender, discussions of life expectancy were associated with higher rates of TIA (AOR, 4.65; P, .036) and higher rates of understanding that the cancer was incurable (AOR, 4.09; P .085).
Conclusions Due largely to gender differences in communication, women over time have a better understanding of their illness than men. More frequent discussions of life expectancy should enhance illness understanding and reduce gender differences.
*For a PDF of the full article, click on the link to the left of this introduction.
Background Patient understanding of advanced metastatic disease is central to decisions about care near death. Prior studies have focused on gender differences in communication style rather than on illness understanding.
Objectives To evaluate gender differences in terminal illness acknowledgement (TIA), understanding that the disease is incurable and the advanced stage of the disease. To evaluate gender differences in patients’ reports of discussions of life expectancy with oncology providers and its effect on differences in illness understanding.
Methods Coping with Cancer 2 patients (N 68) were interviewed before and after a visit with their oncology providers to discuss scan results.
Results At the prescan interview, there were no statistically significant gender differences in patient measures of illness understanding. At the postscan interview, women were more likely than men to recognize that their illness was incurable (Adjusted Odds Ratio, [AOR] 5.29; P .038), know that their cancer was at an advanced stage (AOR, 6.38; P, .013), and report having had discussions of life expectancy with their oncologist (AOR, 4.77; P, .021). Controlling discussions of life expectancy, women were more likely than men to report that their cancer was at an advanced stage (AOR, 9.53; P .050). Controlling for gender, discussions of life expectancy were associated with higher rates of TIA (AOR, 4.65; P, .036) and higher rates of understanding that the cancer was incurable (AOR, 4.09; P .085).
Conclusions Due largely to gender differences in communication, women over time have a better understanding of their illness than men. More frequent discussions of life expectancy should enhance illness understanding and reduce gender differences.
*For a PDF of the full article, click on the link to the left of this introduction.
Background Patient understanding of advanced metastatic disease is central to decisions about care near death. Prior studies have focused on gender differences in communication style rather than on illness understanding.
Objectives To evaluate gender differences in terminal illness acknowledgement (TIA), understanding that the disease is incurable and the advanced stage of the disease. To evaluate gender differences in patients’ reports of discussions of life expectancy with oncology providers and its effect on differences in illness understanding.
Methods Coping with Cancer 2 patients (N 68) were interviewed before and after a visit with their oncology providers to discuss scan results.
Results At the prescan interview, there were no statistically significant gender differences in patient measures of illness understanding. At the postscan interview, women were more likely than men to recognize that their illness was incurable (Adjusted Odds Ratio, [AOR] 5.29; P .038), know that their cancer was at an advanced stage (AOR, 6.38; P, .013), and report having had discussions of life expectancy with their oncologist (AOR, 4.77; P, .021). Controlling discussions of life expectancy, women were more likely than men to report that their cancer was at an advanced stage (AOR, 9.53; P .050). Controlling for gender, discussions of life expectancy were associated with higher rates of TIA (AOR, 4.65; P, .036) and higher rates of understanding that the cancer was incurable (AOR, 4.09; P .085).
Conclusions Due largely to gender differences in communication, women over time have a better understanding of their illness than men. More frequent discussions of life expectancy should enhance illness understanding and reduce gender differences.
*For a PDF of the full article, click on the link to the left of this introduction.
Chlorpromazine bioavailability from a topical gel formulation in volunteers
Background Symptom management medications are often compounded into topical gel formulations providing an alternative route of administration for hospice and palliative care patients. Though commonly used, transdermal absorption and bioavailability studies of these gel products are lacking. Chlorpromazine was studied because it is FDA approved for treatment of nausea and vomiting and is used off-label for treatment of agitation and delirium.
Objective The objective of this study is to determine the transdermal absorption of chlorpromazine PLO gel in healthy adults.
Methods Twenty-five milligrams of chlorpromazine in PLO gel was applied to 10 subjects’ wrists and 100 mg was applied to 1 subject’s wrist. Blood draws were completed preapplication and 1, 2, and 4 hours postapplication. This single-center unblinded study recruited healthy adults between 18 and 70 years of age. Participants were not pregnant, did not have an allergy to any component of the study medication, and were not taking a phenothiazine medication.
Results Chlorpromazine was undetected in any of the 11 subjects’ blood samples.
Limitations There is an assumption of equivalent medication absorption in healthy patients and palliative care or hospice patients.
Conclusion Rapid relief of symptoms at end of life is essential. Chlorpromazine in PLO gel may not be an effective treatment option since blood levels were undetectable at 1, 2, and 4 hours after topical application.
*For a PDF of the full article, click on the link to the left of this introduction.
Background Symptom management medications are often compounded into topical gel formulations providing an alternative route of administration for hospice and palliative care patients. Though commonly used, transdermal absorption and bioavailability studies of these gel products are lacking. Chlorpromazine was studied because it is FDA approved for treatment of nausea and vomiting and is used off-label for treatment of agitation and delirium.
Objective The objective of this study is to determine the transdermal absorption of chlorpromazine PLO gel in healthy adults.
Methods Twenty-five milligrams of chlorpromazine in PLO gel was applied to 10 subjects’ wrists and 100 mg was applied to 1 subject’s wrist. Blood draws were completed preapplication and 1, 2, and 4 hours postapplication. This single-center unblinded study recruited healthy adults between 18 and 70 years of age. Participants were not pregnant, did not have an allergy to any component of the study medication, and were not taking a phenothiazine medication.
Results Chlorpromazine was undetected in any of the 11 subjects’ blood samples.
Limitations There is an assumption of equivalent medication absorption in healthy patients and palliative care or hospice patients.
Conclusion Rapid relief of symptoms at end of life is essential. Chlorpromazine in PLO gel may not be an effective treatment option since blood levels were undetectable at 1, 2, and 4 hours after topical application.
*For a PDF of the full article, click on the link to the left of this introduction.
Background Symptom management medications are often compounded into topical gel formulations providing an alternative route of administration for hospice and palliative care patients. Though commonly used, transdermal absorption and bioavailability studies of these gel products are lacking. Chlorpromazine was studied because it is FDA approved for treatment of nausea and vomiting and is used off-label for treatment of agitation and delirium.
Objective The objective of this study is to determine the transdermal absorption of chlorpromazine PLO gel in healthy adults.
Methods Twenty-five milligrams of chlorpromazine in PLO gel was applied to 10 subjects’ wrists and 100 mg was applied to 1 subject’s wrist. Blood draws were completed preapplication and 1, 2, and 4 hours postapplication. This single-center unblinded study recruited healthy adults between 18 and 70 years of age. Participants were not pregnant, did not have an allergy to any component of the study medication, and were not taking a phenothiazine medication.
Results Chlorpromazine was undetected in any of the 11 subjects’ blood samples.
Limitations There is an assumption of equivalent medication absorption in healthy patients and palliative care or hospice patients.
Conclusion Rapid relief of symptoms at end of life is essential. Chlorpromazine in PLO gel may not be an effective treatment option since blood levels were undetectable at 1, 2, and 4 hours after topical application.
*For a PDF of the full article, click on the link to the left of this introduction.
Complementary and alternative medicine (CAM) use in advanced cancer: a systematic review
This systematic review synthesizes knowledge about the use of complementary and alternative medicine (CAM) among advanced cancer patients. EBSCO and Ovid databases were searched using core concepts, including advanced cancer, CAM, integrative medicine, and decision-making. Articles included in the final review were analyzed using narrative synthesis methods, including thematic analysis, concept mapping, and critical reflection on the synthesis process. Results demonstrate that advanced cancer patients who are younger, female, more educated, have longer duration of disease, and have previously used CAM are more likely to use CAM during this stage of illness. Key themes identified include patterns of and reasons for use; and barriers and facilitators to informed CAM decision-making. Knowledge regarding the use of CAM in advanced cancer remains in its nascent stages. Findings suggest a need for more research on understanding the dynamic process of CAM decision-making in the advanced cancer population from the patients’ perspective.
*For a PDF of the full article, click on the link to the left of this introduction.
This systematic review synthesizes knowledge about the use of complementary and alternative medicine (CAM) among advanced cancer patients. EBSCO and Ovid databases were searched using core concepts, including advanced cancer, CAM, integrative medicine, and decision-making. Articles included in the final review were analyzed using narrative synthesis methods, including thematic analysis, concept mapping, and critical reflection on the synthesis process. Results demonstrate that advanced cancer patients who are younger, female, more educated, have longer duration of disease, and have previously used CAM are more likely to use CAM during this stage of illness. Key themes identified include patterns of and reasons for use; and barriers and facilitators to informed CAM decision-making. Knowledge regarding the use of CAM in advanced cancer remains in its nascent stages. Findings suggest a need for more research on understanding the dynamic process of CAM decision-making in the advanced cancer population from the patients’ perspective.
*For a PDF of the full article, click on the link to the left of this introduction.
This systematic review synthesizes knowledge about the use of complementary and alternative medicine (CAM) among advanced cancer patients. EBSCO and Ovid databases were searched using core concepts, including advanced cancer, CAM, integrative medicine, and decision-making. Articles included in the final review were analyzed using narrative synthesis methods, including thematic analysis, concept mapping, and critical reflection on the synthesis process. Results demonstrate that advanced cancer patients who are younger, female, more educated, have longer duration of disease, and have previously used CAM are more likely to use CAM during this stage of illness. Key themes identified include patterns of and reasons for use; and barriers and facilitators to informed CAM decision-making. Knowledge regarding the use of CAM in advanced cancer remains in its nascent stages. Findings suggest a need for more research on understanding the dynamic process of CAM decision-making in the advanced cancer population from the patients’ perspective.
*For a PDF of the full article, click on the link to the left of this introduction.
Best practices for pediatric palliative cancer care: a primer for clinical providers
Cancer is the leading cause of disease-related death in children and adolescents. Pediatric patients with cancer suffer greatly at the end of life. However, palliative care interventions can reduce suffering and significantly improve the care of these patients and their families. A large percentage of pediatric deaths occur outside of the hospital setting where pediatric palliative resources may not be readily available. This review focuses on the principles of best practice in the provision of palliative care for children and adolescents with cancer.
Click on the PDF icon at the top of this introduction to read the full article.
Cancer is the leading cause of disease-related death in children and adolescents. Pediatric patients with cancer suffer greatly at the end of life. However, palliative care interventions can reduce suffering and significantly improve the care of these patients and their families. A large percentage of pediatric deaths occur outside of the hospital setting where pediatric palliative resources may not be readily available. This review focuses on the principles of best practice in the provision of palliative care for children and adolescents with cancer.
Click on the PDF icon at the top of this introduction to read the full article.
Cancer is the leading cause of disease-related death in children and adolescents. Pediatric patients with cancer suffer greatly at the end of life. However, palliative care interventions can reduce suffering and significantly improve the care of these patients and their families. A large percentage of pediatric deaths occur outside of the hospital setting where pediatric palliative resources may not be readily available. This review focuses on the principles of best practice in the provision of palliative care for children and adolescents with cancer.
Click on the PDF icon at the top of this introduction to read the full article.
Calcium Pyrophosphate Dihydrate Crystal Deposition Disease (Pseudogout) of Lumbar Spine Mimicking Osteomyelitis-Discitis With Epidural Phlegmon
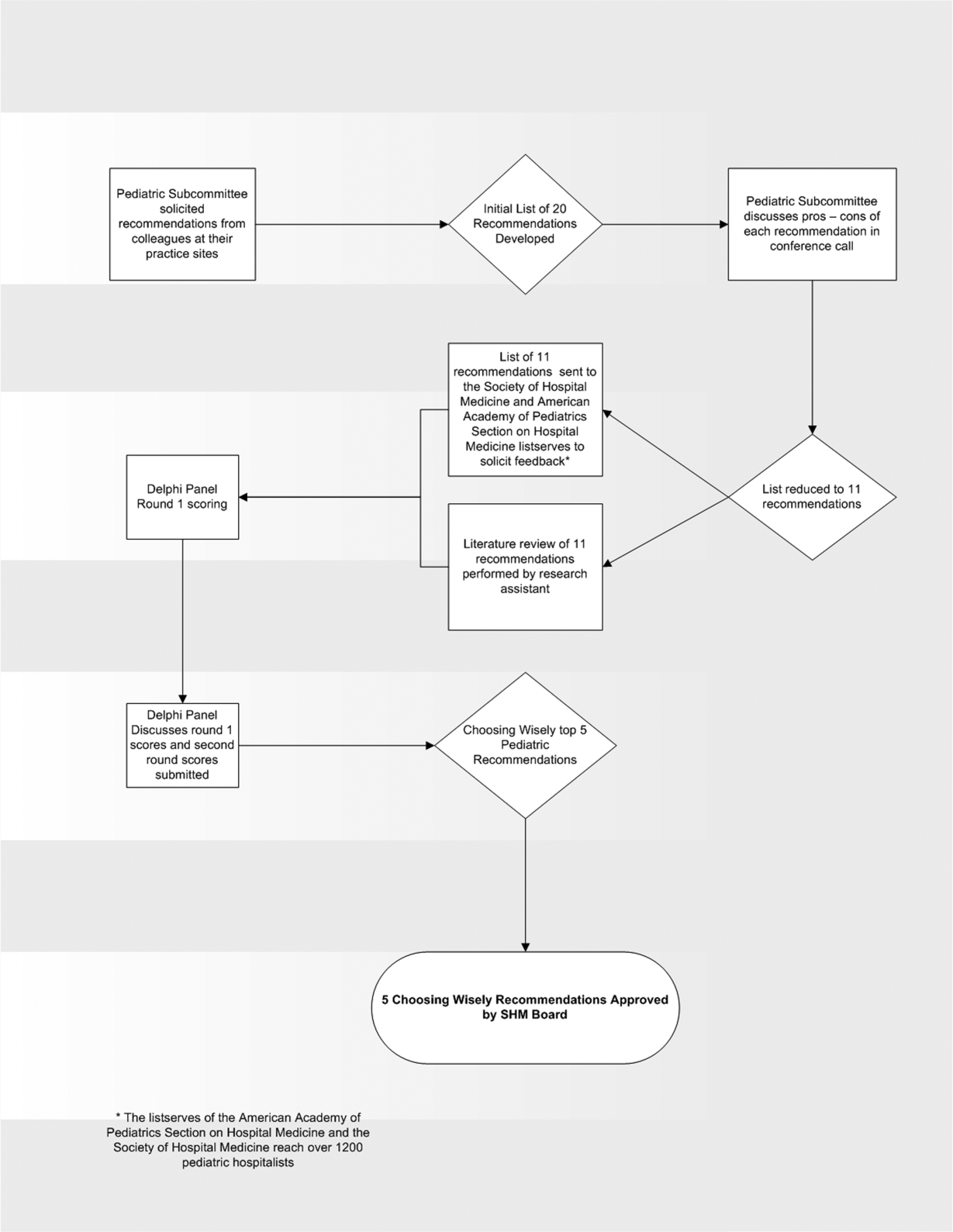
Record Attendance, Key Issues Highlight Pediatric Hospital Medicine's 10th Anniversary
With a record number of attendees, Pediatric Hospital Medicine 2013 (PHM) swept into New Orleans last month, carrying with it unbridled enthusiasm about the past, present, and future.
Virginia Moyer, MD, MPH, vice president for maintenance of certification and quality for the American Board of Pediatrics (ABP) and professor of pediatrics and chief of academic general pediatrics at Texas Children’s Hospital, delivered a keynote address to 700 attendees that focused on the challenges and opportunities of providing evidence-based, high-quality care in the hospital, as well as ABP’s role in meeting these challenges.
“If evidence-based medicine is an individual sport,” Dr. Moyer said, “then quality improvement is a team sport.”
Barriers to quality improvement (QI)— such as lack of will, lack of data, and lack of training—can be surmounted in a team environment, she said. ABP is continuing in its efforts to support QI education through its Maintenance of Certification (MOC) Part 4 modules, as well as other educational activities.
Other highlights of the 10th annual Pediatric Hospital Medicine meeting:
- The addition of a new “Community Hospitalists” track was given high marks by those in attendance. It covered such topics as perioperative management of medically complex pediatric patients, community-acquired pneumonia, and osteomyelitis.
- A 10-year retrospective of pediatric hospital medicine was given by a panel of notable pediatric hospitalists, including Erin Stucky Fisher, MD, FAAP, MHM, chief of hospital medicine at Rady Children’s Hospital in San Diego; Mary Ottolini, MD, MPH, chief of hospital medicine at Children’s National Medical Center in Washington; Jack Percelay, MD, MPH, FAAP, associate clinical professor at Pace University; and Daniel Rauch, MD, FAAP, pediatric hospitalist program director at the NYU School of Medicine in New York City. A host of new programs has been established by the PHM community, including the Quality Improvement Innovation Networks (QuIIN); the Value in Pediatrics (VIP) network; the International Network for Simulation-Based Pediatric Innovation, Research, and Education (INSPIRE); patient- and family-centered rounds; and the I-PASS Handoff Program. The panel also discussed future challenges, including reduction of unnecessary treatments, interfacing, and perhaps incorporating “hyphen hospitalists,” and learning from advances made by the adult HM community.
- The ever-popular “Top Articles in Pediatric Hospital Medicine” session was presented by H. Barrett Fromme, MD, associate professor of pediatrics at the University of Chicago, and Ben Bauer, MD, director of pediatric hospital medicine at Riley Hospital for Children at Indiana University Health in Indianapolis, which was met with raucous approval by the audience. The presentation not only educated those in attendance about the most cutting-edge pediatric literature, but it also included dance moves most likely to attract the opposite sex and clothing appropriate for the Australian pediatric hospitalist.
- The three presidents of the sponsoring societies—Thomas McInerney, MD, FAAP, of the American Academy of Pediatrics, David Keller, MD, of the Academic Pediatric Association, and Eric Howell, MD, SFHM, of SHM—presented each society’s contributions to the growth of PHM, as well as future areas for cooperative sponsorship. These include the development of the AAP Section of Hospital Medicine Library website, the APA Quality Scholars program, and SHM’s efforts to increase interest in hospital medicine in medical students and trainees. “Ask not what hospital medicine can do for you,” Dr. Howell implored, “ask what you can do for hospital medicine!”
- Members of the Joint Council of Pediatric Hospital Medicine (JCPHM) presented the recent recommendations of the council arising from an April 2013 meeting with the ABP in Chapel Hill, N.C. Despite acknowledgements that no decision will be met with uniform satisfaction by all the stakeholders, the JCPHM concluded that the path that would best advance the field of PHM, provide for high-quality care of hospitalized children, and ensure the public trust would be a two-year fellowship sponsored by ABP. This would ultimately lead to approved certification eligibility for fellowship graduates by the American Board of Medical Specialties (ABMS); it would also make provisions for “grandfathering” in current pediatric hospitalists. Concerns from med-peds, community hospitalists, and recent residency graduate communities were addressed by the panel.
- A recurrent theme of reducing unnecessary treatments, interventions, and, perhaps, hospitalizations was summarized eloquently by Alan Schroeder, MD, director of the pediatric ICU and chief of pediatric inpatient care at Santa Clara (Calif.) Valley Health. Barriers to reducing unnecessary care can be substantial, including pressure from families, pressure from colleagues, profit motive, and the “n’s of 1,” according to Dr. Schroeder. Ultimately, however, avoiding testing and treatments that have no benefit to children will improve care. “Ask, ‘How will this test benefit my patient?’ not ‘How will this test change management?’” Dr. Schroeder advised. TH
10: years in existence
720: attendees
220: scientific abstracts
9: tracks
Dr. Chang is The Hospitalist’s pediatric editor and a med-peds-trained hospitalist working at the University of California San Diego and Rady Children’s Hospital.
With a record number of attendees, Pediatric Hospital Medicine 2013 (PHM) swept into New Orleans last month, carrying with it unbridled enthusiasm about the past, present, and future.
Virginia Moyer, MD, MPH, vice president for maintenance of certification and quality for the American Board of Pediatrics (ABP) and professor of pediatrics and chief of academic general pediatrics at Texas Children’s Hospital, delivered a keynote address to 700 attendees that focused on the challenges and opportunities of providing evidence-based, high-quality care in the hospital, as well as ABP’s role in meeting these challenges.
“If evidence-based medicine is an individual sport,” Dr. Moyer said, “then quality improvement is a team sport.”
Barriers to quality improvement (QI)— such as lack of will, lack of data, and lack of training—can be surmounted in a team environment, she said. ABP is continuing in its efforts to support QI education through its Maintenance of Certification (MOC) Part 4 modules, as well as other educational activities.
Other highlights of the 10th annual Pediatric Hospital Medicine meeting:
- The addition of a new “Community Hospitalists” track was given high marks by those in attendance. It covered such topics as perioperative management of medically complex pediatric patients, community-acquired pneumonia, and osteomyelitis.
- A 10-year retrospective of pediatric hospital medicine was given by a panel of notable pediatric hospitalists, including Erin Stucky Fisher, MD, FAAP, MHM, chief of hospital medicine at Rady Children’s Hospital in San Diego; Mary Ottolini, MD, MPH, chief of hospital medicine at Children’s National Medical Center in Washington; Jack Percelay, MD, MPH, FAAP, associate clinical professor at Pace University; and Daniel Rauch, MD, FAAP, pediatric hospitalist program director at the NYU School of Medicine in New York City. A host of new programs has been established by the PHM community, including the Quality Improvement Innovation Networks (QuIIN); the Value in Pediatrics (VIP) network; the International Network for Simulation-Based Pediatric Innovation, Research, and Education (INSPIRE); patient- and family-centered rounds; and the I-PASS Handoff Program. The panel also discussed future challenges, including reduction of unnecessary treatments, interfacing, and perhaps incorporating “hyphen hospitalists,” and learning from advances made by the adult HM community.
- The ever-popular “Top Articles in Pediatric Hospital Medicine” session was presented by H. Barrett Fromme, MD, associate professor of pediatrics at the University of Chicago, and Ben Bauer, MD, director of pediatric hospital medicine at Riley Hospital for Children at Indiana University Health in Indianapolis, which was met with raucous approval by the audience. The presentation not only educated those in attendance about the most cutting-edge pediatric literature, but it also included dance moves most likely to attract the opposite sex and clothing appropriate for the Australian pediatric hospitalist.
- The three presidents of the sponsoring societies—Thomas McInerney, MD, FAAP, of the American Academy of Pediatrics, David Keller, MD, of the Academic Pediatric Association, and Eric Howell, MD, SFHM, of SHM—presented each society’s contributions to the growth of PHM, as well as future areas for cooperative sponsorship. These include the development of the AAP Section of Hospital Medicine Library website, the APA Quality Scholars program, and SHM’s efforts to increase interest in hospital medicine in medical students and trainees. “Ask not what hospital medicine can do for you,” Dr. Howell implored, “ask what you can do for hospital medicine!”
- Members of the Joint Council of Pediatric Hospital Medicine (JCPHM) presented the recent recommendations of the council arising from an April 2013 meeting with the ABP in Chapel Hill, N.C. Despite acknowledgements that no decision will be met with uniform satisfaction by all the stakeholders, the JCPHM concluded that the path that would best advance the field of PHM, provide for high-quality care of hospitalized children, and ensure the public trust would be a two-year fellowship sponsored by ABP. This would ultimately lead to approved certification eligibility for fellowship graduates by the American Board of Medical Specialties (ABMS); it would also make provisions for “grandfathering” in current pediatric hospitalists. Concerns from med-peds, community hospitalists, and recent residency graduate communities were addressed by the panel.
- A recurrent theme of reducing unnecessary treatments, interventions, and, perhaps, hospitalizations was summarized eloquently by Alan Schroeder, MD, director of the pediatric ICU and chief of pediatric inpatient care at Santa Clara (Calif.) Valley Health. Barriers to reducing unnecessary care can be substantial, including pressure from families, pressure from colleagues, profit motive, and the “n’s of 1,” according to Dr. Schroeder. Ultimately, however, avoiding testing and treatments that have no benefit to children will improve care. “Ask, ‘How will this test benefit my patient?’ not ‘How will this test change management?’” Dr. Schroeder advised. TH
10: years in existence
720: attendees
220: scientific abstracts
9: tracks
Dr. Chang is The Hospitalist’s pediatric editor and a med-peds-trained hospitalist working at the University of California San Diego and Rady Children’s Hospital.
With a record number of attendees, Pediatric Hospital Medicine 2013 (PHM) swept into New Orleans last month, carrying with it unbridled enthusiasm about the past, present, and future.
Virginia Moyer, MD, MPH, vice president for maintenance of certification and quality for the American Board of Pediatrics (ABP) and professor of pediatrics and chief of academic general pediatrics at Texas Children’s Hospital, delivered a keynote address to 700 attendees that focused on the challenges and opportunities of providing evidence-based, high-quality care in the hospital, as well as ABP’s role in meeting these challenges.
“If evidence-based medicine is an individual sport,” Dr. Moyer said, “then quality improvement is a team sport.”
Barriers to quality improvement (QI)— such as lack of will, lack of data, and lack of training—can be surmounted in a team environment, she said. ABP is continuing in its efforts to support QI education through its Maintenance of Certification (MOC) Part 4 modules, as well as other educational activities.
Other highlights of the 10th annual Pediatric Hospital Medicine meeting:
- The addition of a new “Community Hospitalists” track was given high marks by those in attendance. It covered such topics as perioperative management of medically complex pediatric patients, community-acquired pneumonia, and osteomyelitis.
- A 10-year retrospective of pediatric hospital medicine was given by a panel of notable pediatric hospitalists, including Erin Stucky Fisher, MD, FAAP, MHM, chief of hospital medicine at Rady Children’s Hospital in San Diego; Mary Ottolini, MD, MPH, chief of hospital medicine at Children’s National Medical Center in Washington; Jack Percelay, MD, MPH, FAAP, associate clinical professor at Pace University; and Daniel Rauch, MD, FAAP, pediatric hospitalist program director at the NYU School of Medicine in New York City. A host of new programs has been established by the PHM community, including the Quality Improvement Innovation Networks (QuIIN); the Value in Pediatrics (VIP) network; the International Network for Simulation-Based Pediatric Innovation, Research, and Education (INSPIRE); patient- and family-centered rounds; and the I-PASS Handoff Program. The panel also discussed future challenges, including reduction of unnecessary treatments, interfacing, and perhaps incorporating “hyphen hospitalists,” and learning from advances made by the adult HM community.
- The ever-popular “Top Articles in Pediatric Hospital Medicine” session was presented by H. Barrett Fromme, MD, associate professor of pediatrics at the University of Chicago, and Ben Bauer, MD, director of pediatric hospital medicine at Riley Hospital for Children at Indiana University Health in Indianapolis, which was met with raucous approval by the audience. The presentation not only educated those in attendance about the most cutting-edge pediatric literature, but it also included dance moves most likely to attract the opposite sex and clothing appropriate for the Australian pediatric hospitalist.
- The three presidents of the sponsoring societies—Thomas McInerney, MD, FAAP, of the American Academy of Pediatrics, David Keller, MD, of the Academic Pediatric Association, and Eric Howell, MD, SFHM, of SHM—presented each society’s contributions to the growth of PHM, as well as future areas for cooperative sponsorship. These include the development of the AAP Section of Hospital Medicine Library website, the APA Quality Scholars program, and SHM’s efforts to increase interest in hospital medicine in medical students and trainees. “Ask not what hospital medicine can do for you,” Dr. Howell implored, “ask what you can do for hospital medicine!”
- Members of the Joint Council of Pediatric Hospital Medicine (JCPHM) presented the recent recommendations of the council arising from an April 2013 meeting with the ABP in Chapel Hill, N.C. Despite acknowledgements that no decision will be met with uniform satisfaction by all the stakeholders, the JCPHM concluded that the path that would best advance the field of PHM, provide for high-quality care of hospitalized children, and ensure the public trust would be a two-year fellowship sponsored by ABP. This would ultimately lead to approved certification eligibility for fellowship graduates by the American Board of Medical Specialties (ABMS); it would also make provisions for “grandfathering” in current pediatric hospitalists. Concerns from med-peds, community hospitalists, and recent residency graduate communities were addressed by the panel.
- A recurrent theme of reducing unnecessary treatments, interventions, and, perhaps, hospitalizations was summarized eloquently by Alan Schroeder, MD, director of the pediatric ICU and chief of pediatric inpatient care at Santa Clara (Calif.) Valley Health. Barriers to reducing unnecessary care can be substantial, including pressure from families, pressure from colleagues, profit motive, and the “n’s of 1,” according to Dr. Schroeder. Ultimately, however, avoiding testing and treatments that have no benefit to children will improve care. “Ask, ‘How will this test benefit my patient?’ not ‘How will this test change management?’” Dr. Schroeder advised. TH
10: years in existence
720: attendees
220: scientific abstracts
9: tracks
Dr. Chang is The Hospitalist’s pediatric editor and a med-peds-trained hospitalist working at the University of California San Diego and Rady Children’s Hospital.
Delirium Superimposed on Dementia
Much attention has been given recently to hospitalized older adults, the critical 30‐day period, and posthospital syndrome.[1] What is missing from this dialogue is the contribution and significance of underlying cognitive impairment. By 2050, 14 million older persons in the United States are expected to have dementia.[2] Increasing numbers of older adults diagnosed with dementia are hospitalized and are at increased risk of developing delirium; in fact, delirium occurs in over half of hospitalized persons with dementia.[3] Further, current evidence suggests that delirium may accelerate the clinical course and trajectory of cognitive decline, and may be associated with considerably worse long‐term outcomes, including prolonged hospitalization, rehospitalization within 30 days, nursing home placement, and death.[3, 4, 5, 6] However, the problem of delirium superimposed on dementia (DSD) remains a neglected area of investigation in hospitalized patients. Delirium is superimposed on dementia when an acute change in mental status (characterized by a fluctuating course, inattention, and either disorganized thinking or altered level of consciousness) is layered on top of preexisting dementia.[4]
Despite the poor outcomes and high prevalence of DSD, little is known about the natural history in hospitalized older adults with dementia. Delirium studies often exclude persons with dementia, even though the prevalence of DSD is extremely high in both community (13%19%) and hospital (40%89%) populations and associated with higher costs and utilization compared to dementia and delirium alone.[4, 5, 7] In 1 study, annual costs for DSD were $9566 compared to $7557 for dementia alone.[7] The few risk‐factor studies of DSD were conducted in intensive care unit (ICU) or long‐term care settings.[8, 9]
The purpose of this study was to describe the incidence, risk factors, and outcomes associated with incident delirium in a prospective cohort of hospitalized older adults with dementia. The study aims were to: (1) estimate the incidence of new delirium in hospitalized persons with dementia, (2) identify the risk factors associated with incident delirium superimposed on dementia in this sample, (3) describe the outcomes associated with development of delirium, and (4) evaluate the contributions of delirium severity and duration to outcomes.
METHODS
This 24‐month prospective cohort study recruited and enrolled consecutive hospital admissions with dementia in a 300‐bed community hospital in central Pennsylvania from July 2006 through November 2008. Data were collected daily from patients during hospitalization, followed by a 1‐month posthospitalization interview with patients and their caregivers in the community setting. Patients were included if they spoke English, had been hospitalized fewer than 24 hours, and met the screening criteria for dementia. Patients were excluded if they had any significant neurological condition associated with cognitive impairment other than dementia (eg, brain tumor), a major acute psychiatric disorder, were unable to communicate, or had no caregiver to interview. The interviewers included experienced research assistants (RAs) who were either registered nurses or trained in a health‐related field. All staff training of instruments were done with scripted training manuals and video training using manuals for the Confusion Assessment Method (CAM). After training was completed, final inter‐rater reliability assessments were conducted until staff reached 100% agreement. The RAs were blinded to the aims and completed over 10 hours of training. Inter‐rater reliability checks were conducted on 10% of the sample in the field with >90% agreement attained on all instruments. This study was reviewed by and approved by The Pennsylvania State University institutional review board, and consent was received from all subjects.
Study Measures
Dementia was defined by meeting all 3 criteria of a Modified Blessed Dementia Rating Score of >3, an Informant Questionnaire on Cognitive Decline in the Elderly of 3.3, and documented dementia symptoms of at least 6 months' duration prior to current illness.[10, 11, 12] The Mini‐Mental State Examination (MMSE), purchased from Psychological Assessment Resources, Inc. (Lutz, FL), was used to measure change from day to day and aid in the measurement of delirium, but was not used to establish the diagnosis of dementia. Both the Clinical Dementia Rating Scale[13] and the Global Deterioration Scale (GDS)[14] were used to measure dementia stage and severity.
Delirium and delirium severity were defined according to the validated CAM algorithm;[15] the Delirium Rating Scale‐Revised‐98 was used for delirium severity.[16] In a recent review, the CAM showed an overall sensitivity of 94% and specificity of 89%.[17] In the present study, delirium was measured in a comprehensive and structured interview that involved the MMSE and CAM criteria, and was based on a 24‐hour period of observations, interviews with nurses and family members, and chart review. The CAM was completed daily during patient hospitalization and the follow‐up interviews. The CAM assesses 4 criteria including acute and fluctuating nature, inattention, disorganized thought, and altered level of consciousness. Delirium was recorded by the research staff as present or absent each day based on full CAM criteria. Because the goal of the present study focused on full CAM delirium, subsyndromal delirium was not presented in this article.
Delirium duration was defined as the number of days with a positive rating. Data were collected daily from patients during hospitalization, followed by a single interview at 1‐month posthospitalization with patients and their caregivers. Most interviews were in person.
Delirium Risk Factors
Central nervous system‐active drug use was defined by 2005 American Hospital Formulary Services classification.[18] The Beers criteria were used to define potentially inappropriate medication use.[19] The Cornell scale for depression in persons with dementia was used, with a cut point of 12 indicating depression.[20] Functional status change was measured via the Katz Index of Activities of Daily Living (ADLs) and Lawton Instrumental Activities Of Daily Living (IADLs) change scores.[21] Comorbid conditions were classified with a weighted index that took into account both the number and seriousness of different comorbid diseases.[22] Pain was measured using the Pain Assessment in Advanced Dementia (PAINAD) scale.[23] Dehydration was defined using the blood urea nitrogen (BUN)/creatinine ratio and/or any chart diagnosis of dehydration. Admission lab values (BUN/creatinine) were abstracted from the medical records.
Primary Outcomes
The primary outcomes measured were full CAM delirium, index hospitalization length of stay, cognitive decline (change in MMSE and GDS scores), death, and functional status change (change from baseline to discharge score). One‐month mortality was measured by chart review and follow‐up family interviews performed at 1 month via telephone or in‐person interviews. Mortality was not verified by additional methods.
Statistical Analysis
All statistical analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC), and statistical significance was assessed using an level of 0.05 unless otherwise noted. Descriptive statistics were calculated on all characteristics by incident delirium status.
Potential risk factors for incident delirium were examined using [2] and t tests, where appropriate. Simple proportional hazards models were used to estimate the relative risk (RR) and 95% confidence interval (CI) for incident delirium. A stepwise model‐building procedure under a proportional hazards model was used to build a final model for incident delirium that contained all variables that were statistically significant at the 0.05 level or that had an RR of 1.5 or greater. Adjusted RR and corresponding 95% CI were determined. The outcome in each model was the number of days from admission to an incident delirium diagnosis. Subjects without incident delirium were censored using their length of stay as the total number of days they were at risk for developing delirium.
Finally, to examine the relationships between incident delirium, maximum incident delirium severity and the number of inpatient days positive for delirium with the outcomes of death, impaired in 2 or more IADLs at follow‐up, impaired in 2 or more ADLs at follow‐up, length of stay, change in IADLs from admission to follow‐up, and change in ADLs from admission to follow‐up, logistic regression (for the dichotomous outcome of mortality), analysis of covariance or linear regression (depending on the whether the independent variable was categorical or continuous) was performed controlling for age, gender, and GDS score.
RESULTS
Of 256 eligible patients, dual consent was obtained from 154 patient and 154 family research subjects (308 consents). The refusal rate was 39% (n=102). Fourteen subjects were consented and enrolled but later dropped out due to family/proxy concerns regarding the patient's ability to participate in interviews. Thus, the final sample included 139 patients.
Descriptive statistics for baseline measures are given in Table 1. Briefly, the average age of subjects was 83 years (standard deviation [SD]=7); 41% were male; 57% were single, divorced, or widowed; and the average number of years of education was 12 years (SD=3). Thirty‐three percent were dehydrated on admission, and 33% had fallen within 2 weeks prior to admission. Thirty‐four percent had an infection at baseline, and 36% had some sensory impairment.
| Factor | Delirium, N=44, 31.7% | No Delirium, N=95, 68.3% | Relative Risk | 95% CI | P Value |
|---|---|---|---|---|---|
| |||||
| Demographic covariates | |||||
| Age, y, mean (SD) | 85.9 (5.9) | 82.4 (7.0) | 1.07 | 1.021.12 | 0.0051 |
| Male gender, n (%) | 23 (52.3) | 33 (34.7) | 1.83 | 1.013.31 | 0.0456 |
| Single/divorced/widowed, n (%) | 23 (52.3) | 56 (60.2) | 0.81 | 0.451.47 | 0.4882 |
| Education, y, mean (SD) | 12.6 (3.2) | 12.1 (3.0) | 1.06 | 0.951.17 | 0.3146 |
| Clinical covariates | |||||
| Dehydration, n (%) | 12 (30.8) | 30 (33.7) | 0.88 | 0.451.74 | 0.7152 |
| Fall in last 2 weeks, n (%) | 14 (41.2) | 21 (29.6) | 1.73* | 0.873.43 | 0.1186 |
| Infection, n (%) | 13 (40.6) | 21 (30.9) | 1.42 | 0.702.88 | 0.3328 |
| Sensory impairment, n (%) | 16 (36.4) | 33 (34.7) | 1.04 | 0.561.91 | 0.9132 |
| Lawton score, mean (SD) | 1.6 (1.3) | 2.3 (2.0) | 0.84 | 0.701.01 | 0.0592 |
| Katz impaired score, mean (SD) | 2.3 (2.0) | 3.4 (2.1) | 0.82 | 0.710.95 | 0.0072 |
| Charlson score, mean (SD) | 2.5 (1.8) | 2.3 (1.4) | 1.06 | 0.861.30 | 0.6013 |
| BUN, mean (SD) | 28.2 (17.6) | 25.6 (15.3) | 1.01 | 0.991.03 | 0.4175 |
| Creatinine, mean (SD) | 1.6 (1.3) | 2.4 (6.8) | 0.99 | 0.901.08 | 0.7356 |
| Cornell Depression score, mean (SD) | 1.6 (0.8) | 1.2 (0.9) | 1.35 | 0.991.83 | 0.0553 |
| Global Deterioration score, mean (SD) | 4.7 (1.2) | 3.9 (1.3) | 1.45* | 1.141.86 | 0.0027 |
| PAINAD score, mean (SD) | 2.1 (3.0) | 2.0 (2.9) | 1.01 | 0.911.12 | 0.8540 |
| Total number of regular medications, mean (SD) | 11.5 (4.6) | 11.0 (5.0) | 1.00 | 0.941.67 | 0.9771 |
| Total number of Beers medications, mean (SD) | 0.3 (0.7) | 0.4 (0.7) | 0.76 | 0.461.27 | 0.2933 |
| Cognitive impairment covariates | |||||
| MMSE score, mean (SD) | 12.7 (6.8) | 17.1 (6.6) | 0.94 | 0.900.98 | 0.0019 |
| Blessed score, mean (SD) | 9.5 (3.5) | 7.7 (2.9) | 1.14 | 1.041.24 | 0.0038 |
| Measures of deliriumcovariates for follow‐up outcomes | |||||
| Maximum incident delirium severity, mean (SD) | 15.4 (5.6) | 8.7 (6.1) | <0.0001 | ||
| Inpatient days with positive CAM, mean (SD) | 2.0 (1.1) | 0.2 (1.4) | <0.0001 | ||
| Follow‐up outcomes | |||||
| Mortality, n (%) | 11 (25.0) | 9 (9.5) | 0.0153 | ||
| Length of stay, mean (SD) | 9.1 (4.4) | 5.7 (4.1) | <0.0001 | ||
| Change in Lawton IADLs from admission to follow‐up, mean (SD) | 0.4 (1.5) | 0.2 (1.8) | 0.5094 | ||
| Change in Katz impaired ADLs from admission to follow‐up, mean (SD) | 0.3 (1.7) | 0.4 (1.6) | 0.6919 | ||
The overall incidence of delirium was 32% (44/139) and the range of days to incident delirium was 1 to 8 days. During the baseline period (Table 1), subjects with delirium were older, more likely to be male, had lower Katz impairment scores, higher GDS score, lower MMSE scores on admission, and higher Blessed scores than subjects without delirium. Slightly more persons with delirium had a prior fall, although the RR was not statistically significant. Length of stay measured at discharge was significantly higher for those with delirium (mean=9.1) than those without delirium (mean=5.7) (P<0.0001). Subjects with delirium were more likely to have died at 1 month than those without delirium (P=0.0153).
In addition, we analyzed the adjusted relative risk estimates for the final model of incident delirium. Significant risk factors or risk factors with RR estimates at least 1.5 (or <0.66 if protective [Table 1]) that were examined in a more comprehensive multiple proportional hazards model included age, gender, having had a fall in the last 2 weeks, number of impaired ADLs (based on Katz), GDS scores, MMSE scores at baseline, and Blessed scores at baseline. The final proportional hazards included gender and GDS score. Males were nearly 1.8 times as likely to develop delirium than females, and for every 1 unit increase in the GDS, subjects were 1.5 times more likely to develop delirium.
Finally, Table 2 gives the results of examining outcomes related to incident delirium measures. For mortality, there were no statistically significant predictors of death after controlling for age, gender, or GDS. For length of stay, subjects with incident delirium had significantly longer lengths of stay, as incident delirium severity increased by 1 unit the length of stay increased by 0.4 days, and as the number of inpatient days with delirium increased by 1 day the length of stay increased by 1.8 days. For change in the impaired Katz ADLs from admission to follow‐up, as incident delirium severity increased by 1 unit the change in impaired Katz ADLs increased by 0.05 units.
| Variable | |||
|---|---|---|---|
| Outcome mortality | Level | Adjusted Estimate of Associationa | P Value |
| |||
| Incident delirium, OR (95% CI)b | Yes | 2.33 (0.82‐6.61) | 0.1130 |
| No | 1.00 | ||
| Maximum incident delirium severity, OR (95% CI)b | 1.05 (0.961.14) | 0.2719 | |
| Number of inpatient days with positive delirium, OR (95% CI)b | 1.15 (0.891.49) | 0.2871 | |
| Outcome LOS | |||
| Incident delirium, mean (SE)c | Yes | 9.2 (0.7) | <0.0001 |
| No | 5.6 (0.5) | ||
| Maximum incident delirium severity, slope (SE)d | 0.43 (0.06) | <0.0001 | |
| Number of inpatient days with positive delirium, slope (SE)d | 1.80 (0.21) | <0.0001 | |
| Outcomechange in Lawton IADLs from admission to follow‐up | |||
| Incident delirium, mean (SE)c | Yes | 0.51 (0.33) | 0.3787 |
| No | 0.15 (0.20) | ||
| Maximum incident delirium severity, slope (SE)d | 0.003 (0.03) | 0.9260 | |
| Number of inpatient days with positive delirium, slope (SE)d | 0.16 (0.11) | 0.1497 | |
| Outcomechange in Katz impaired ADLs from admission to follow‐up | |||
| Incident delirium, mean (SE)c | Yes | 0.19 (0.26) | 0.5086 |
| No | 0.40 (0.17) | ||
| Maximum incident delirium severity, slope (SE)d | 0.05 (0.03) | 0.0437 | |
| Number of inpatient days with positive delirium, slope (SE)d | 0.13 (0.09) | 0.1717 | |
DISCUSSION
The most compelling finding from this study is the high incidence of delirium in hospitalized older adults with dementia and the association with poor clinical outcomes in those who develop delirium superimposed on dementia. DSD is difficult to detect and prevent; persons with DSD are at risk for poor quality of life. Those with delirium had a 25% short‐term mortality rate (P=0.0153), substantially increased length of stay (9.1 vs 5.1 days with an odds ratio of 1.8) and poorer physical function at discharge and follow‐up. At 1 month follow‐up, subjects with delirium had greater functional decline and lower GDS scores than those without delirium.
The incidence of delirium in this study was high (32%). Being delirious any time was associated with death and poor function. Delirium was also associated with the stage of the persons' baseline dementia, advanced age, lower MMSE scores, and falling before admission.
Previous studies have found delirium associated with increased mortality. Three studies found that within 1 year of a delirium episode, a significant number of persons died or were institutionalized.[24, 25, 26] Other research has reported death within 1 year of documented delirium episodes, and a 3‐fold increased rate of death in the ICU.[24, 27, 28, 29, 30, 31, 32] This study is 1 of only a few to focus on increased mortality with DSD and to focus uniquely on hospitalized patients with delirium and dementia.
The main risk factors for delirium in this study were male sex and severity of dementia. Our results, combined with those from other recent studies by Voyer and colleagues,[8, 33, 34] point to the critical importance of screening for dementia in hospitalized older adults as dementia severity is a significant indicator of delirium severity. For instance, Voyer and colleagues[34] reported that persons with mild dementia were likely to experience a mild delirium, whereas those with a more severe level of dementia were more likely to experience moderate to severe delirium. Our findings show that those who experienced episodes of delirium represented a highly vulnerable population with advanced dementia, sensory impairment, more falls and dehydration at admission, and higher Blessed scores. A recent study by Saczynski and colleagues[35] found 40% of patients who had experienced postoperative delirium did not return to their baseline at 6 months. Clearly, preventing delirium should be a critical priority to prevent such deterioration in the highly vulnerable population of hospitalized patients with dementia.
Patients in this study were on a mean of over 11 medications. One‐third of dementia patients in our study had also experienced a fall and dehydration at baseline. Other studies have found a relationship between cognitive decline, falling, and medications.[36] Many of these patients came into the hospital with potentially modifiable and preventable community or ambulatory care conditions of polypharmacy, falling, sensory impairment, and dehydration.
Importantly, in our study, length of stay was significantly higher (9.1 vs 5.7) for those with delirium compared to those without delirium. This finding is alarming when examining the economic impact of preventing delirium. Previous studies have found the cost of delirious episodes rivals those for diabetes and heart disease, and that decreasing length of stay by just 1 day would save over $20 million dollars per year.[4, 37]
In summary, this study is 1 of the first to report a high incidence of DSD and poorer outcomes for persons who experience delirium compared to those with dementia alone. This is 1 of only a few studies examining unique risk factors and delirium severity for DSD in the acute care setting. Findings from the current study report potential risk factors for development of incident delirium and highlight the challenge of preventing DSD before and during hospitalization. The generalizability of this study may be limited by the use of a nondiverse study population drawn from a single hospital in the northeast United States, though the use of a community hospital increases the relevance to real‐world practice settings. Determination of baseline cognitive status and the differentiation of delirium and dementia are difficult, but validated, state‐of‐the‐art methods were used that have been applied in previous studies.
This study provides fundamental methodological improvements over previous work, and advances the science by providing valuable data on the natural history, correlates, and outcomes of DSD. The strengths of this study include the prospective cohort design, the daily assessment for delirium based on a 24‐hour period, methods for determining cognitive status at baseline in this difficult population, and utilizing strict blinding of the well‐trained outcome assessors.
This study lays the groundwork for future studies to improve care for persons with dementia who present to acute care and to plan prevention programs for delirium before they are admitted to the hospital. We must be able to translate best practice for DSD into the acute care and community settings to prevent or minimize effects of delirium in persons with dementia. Interventions to increase early detection of delirium by hospital staff have the potential to decrease the severity and duration of delirium and prevent unnecessary suffering and costs from the complications of delirium and preventable readmissions to the hospital.
Thus, this study holds substantial clinical and economic implications for this population in the acute care setting, and will direct future studies leading to changes in real‐world practice settings for persons with dementia.
Disclosures
Drs. Fick, Inouye, and Steis acknowledge support for this project described by grants number R03 AG023216 (DMF) and number P01AG031720 (SKI) from the National Institute of Aging (NIA). This study and its contents are solely the responsibilities of the authors and do not necessarily represent the official views of the National Institutes of Health/NIA. The principal investigator, Dr. Fick, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest.
funding section: Dr. Inouye holds the Milton and Shirley F. Levy Family Chair.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8(2):131–168.
- , . Delirium superimposed on dementia: accuracy of nurse documentation. J Gerontol Nurs. 2012;38(1):32–42.
- , , . Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723–1732.
- , , , et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575.
- , , , et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156(12):848–856, W296.
- , , , . Delirium superimposed on dementia in a community‐dwelling managed care population: a 3‐year retrospective study of occurrence, costs, and utilization. J Gerontol. 2005;60A(6):748–753.
- , , , . Factors associated with delirium severity among older persons with dementia. J Neurosci Nurs. 2011;43(2):62–69.
- , , . Delirium in the intensive care unit. Crit Care 2008;12(suppl 3):S3.
- , , . Correlations of Mini‐Mental State and Modified Rating Scale to measures of transitional health status in dementia. J Gerontol. 1987;42(1):33–36.
- , , , . Population‐based norms for the Mini‐Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391.
- , . The mini‐mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935.
- , , , , . A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572.
- , . The GDS/FAST staging system. Int Psychogeriatr. 1997;9(suppl 1):167–171.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948.
- , , , , , . Validation of the Delirium Rating Scale‐Revised‐98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–242.
- , , , . The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823–830.
- American Society of Health‐System Pharmacists. AHFS Drug Information. Bethesda, MD: American Society of Health‐System Pharmacists; 2005.
- , , , , , . Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts [published correction appears in Arch Intern Med. 2004;164:298]. Arch Intern Med 2003;163(22):2716–2724.
- , , , . Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23(3):271–284.
- . Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–726.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , . Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc. 2003;4(1):9–15.
- , , . Prevalence and outcomes of delirium in community and non‐acute care settings in people without dementia: a report from the Canadian Study of Health and Aging. BMC Med. 2006;4:15.
- , , , . Prognostic significance of delirium in frail older people. Dementia. 2005;19(2‐3):158–163.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
- , , , . Delirium subtypes and 1‐year mortality among elderly patients discharged from a post‐acute rehabilitation facility. J Gerontol. 2007;62A(10):1182–1183.
- , , , , , . Delirium in older patients admitted to general internal medicine. J Geriatr Psychiatry Neurol. 2006;19(2):83–90.
- , , , . Association between psychomotor activity delirium subtypes and mortality among newly admitted postacute facility patients. J Gerontol. 2007;62A(2):174–179.
- , , , , , . Premature death associated with delirium at 1‐year follow‐up. Arch Intern Med. 2005;165:1657–1662.
- , , , et al. Older adults discharged from the hospital with delirium: 1‐year outcomes. J Am Geriatr Soc. 2006;54(8):1245–1250.
- , , , . Influence of prior cognitive impairment on the severity of delirium symptoms among older patients. J Neurosci Nurs. 2006;38(2):90–101.
- , , , , . Factors associated with delirium severity among older patients. J Clin Nurs. 2007;16:819–831.
- , , , et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39.
- , , , et al. Effect of central nervous system medication use on decline in cognition in community‐dwelling older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2009;57(2):243–250.
- , , , , . One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27.
Much attention has been given recently to hospitalized older adults, the critical 30‐day period, and posthospital syndrome.[1] What is missing from this dialogue is the contribution and significance of underlying cognitive impairment. By 2050, 14 million older persons in the United States are expected to have dementia.[2] Increasing numbers of older adults diagnosed with dementia are hospitalized and are at increased risk of developing delirium; in fact, delirium occurs in over half of hospitalized persons with dementia.[3] Further, current evidence suggests that delirium may accelerate the clinical course and trajectory of cognitive decline, and may be associated with considerably worse long‐term outcomes, including prolonged hospitalization, rehospitalization within 30 days, nursing home placement, and death.[3, 4, 5, 6] However, the problem of delirium superimposed on dementia (DSD) remains a neglected area of investigation in hospitalized patients. Delirium is superimposed on dementia when an acute change in mental status (characterized by a fluctuating course, inattention, and either disorganized thinking or altered level of consciousness) is layered on top of preexisting dementia.[4]
Despite the poor outcomes and high prevalence of DSD, little is known about the natural history in hospitalized older adults with dementia. Delirium studies often exclude persons with dementia, even though the prevalence of DSD is extremely high in both community (13%19%) and hospital (40%89%) populations and associated with higher costs and utilization compared to dementia and delirium alone.[4, 5, 7] In 1 study, annual costs for DSD were $9566 compared to $7557 for dementia alone.[7] The few risk‐factor studies of DSD were conducted in intensive care unit (ICU) or long‐term care settings.[8, 9]
The purpose of this study was to describe the incidence, risk factors, and outcomes associated with incident delirium in a prospective cohort of hospitalized older adults with dementia. The study aims were to: (1) estimate the incidence of new delirium in hospitalized persons with dementia, (2) identify the risk factors associated with incident delirium superimposed on dementia in this sample, (3) describe the outcomes associated with development of delirium, and (4) evaluate the contributions of delirium severity and duration to outcomes.
METHODS
This 24‐month prospective cohort study recruited and enrolled consecutive hospital admissions with dementia in a 300‐bed community hospital in central Pennsylvania from July 2006 through November 2008. Data were collected daily from patients during hospitalization, followed by a 1‐month posthospitalization interview with patients and their caregivers in the community setting. Patients were included if they spoke English, had been hospitalized fewer than 24 hours, and met the screening criteria for dementia. Patients were excluded if they had any significant neurological condition associated with cognitive impairment other than dementia (eg, brain tumor), a major acute psychiatric disorder, were unable to communicate, or had no caregiver to interview. The interviewers included experienced research assistants (RAs) who were either registered nurses or trained in a health‐related field. All staff training of instruments were done with scripted training manuals and video training using manuals for the Confusion Assessment Method (CAM). After training was completed, final inter‐rater reliability assessments were conducted until staff reached 100% agreement. The RAs were blinded to the aims and completed over 10 hours of training. Inter‐rater reliability checks were conducted on 10% of the sample in the field with >90% agreement attained on all instruments. This study was reviewed by and approved by The Pennsylvania State University institutional review board, and consent was received from all subjects.
Study Measures
Dementia was defined by meeting all 3 criteria of a Modified Blessed Dementia Rating Score of >3, an Informant Questionnaire on Cognitive Decline in the Elderly of 3.3, and documented dementia symptoms of at least 6 months' duration prior to current illness.[10, 11, 12] The Mini‐Mental State Examination (MMSE), purchased from Psychological Assessment Resources, Inc. (Lutz, FL), was used to measure change from day to day and aid in the measurement of delirium, but was not used to establish the diagnosis of dementia. Both the Clinical Dementia Rating Scale[13] and the Global Deterioration Scale (GDS)[14] were used to measure dementia stage and severity.
Delirium and delirium severity were defined according to the validated CAM algorithm;[15] the Delirium Rating Scale‐Revised‐98 was used for delirium severity.[16] In a recent review, the CAM showed an overall sensitivity of 94% and specificity of 89%.[17] In the present study, delirium was measured in a comprehensive and structured interview that involved the MMSE and CAM criteria, and was based on a 24‐hour period of observations, interviews with nurses and family members, and chart review. The CAM was completed daily during patient hospitalization and the follow‐up interviews. The CAM assesses 4 criteria including acute and fluctuating nature, inattention, disorganized thought, and altered level of consciousness. Delirium was recorded by the research staff as present or absent each day based on full CAM criteria. Because the goal of the present study focused on full CAM delirium, subsyndromal delirium was not presented in this article.
Delirium duration was defined as the number of days with a positive rating. Data were collected daily from patients during hospitalization, followed by a single interview at 1‐month posthospitalization with patients and their caregivers. Most interviews were in person.
Delirium Risk Factors
Central nervous system‐active drug use was defined by 2005 American Hospital Formulary Services classification.[18] The Beers criteria were used to define potentially inappropriate medication use.[19] The Cornell scale for depression in persons with dementia was used, with a cut point of 12 indicating depression.[20] Functional status change was measured via the Katz Index of Activities of Daily Living (ADLs) and Lawton Instrumental Activities Of Daily Living (IADLs) change scores.[21] Comorbid conditions were classified with a weighted index that took into account both the number and seriousness of different comorbid diseases.[22] Pain was measured using the Pain Assessment in Advanced Dementia (PAINAD) scale.[23] Dehydration was defined using the blood urea nitrogen (BUN)/creatinine ratio and/or any chart diagnosis of dehydration. Admission lab values (BUN/creatinine) were abstracted from the medical records.
Primary Outcomes
The primary outcomes measured were full CAM delirium, index hospitalization length of stay, cognitive decline (change in MMSE and GDS scores), death, and functional status change (change from baseline to discharge score). One‐month mortality was measured by chart review and follow‐up family interviews performed at 1 month via telephone or in‐person interviews. Mortality was not verified by additional methods.
Statistical Analysis
All statistical analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC), and statistical significance was assessed using an level of 0.05 unless otherwise noted. Descriptive statistics were calculated on all characteristics by incident delirium status.
Potential risk factors for incident delirium were examined using [2] and t tests, where appropriate. Simple proportional hazards models were used to estimate the relative risk (RR) and 95% confidence interval (CI) for incident delirium. A stepwise model‐building procedure under a proportional hazards model was used to build a final model for incident delirium that contained all variables that were statistically significant at the 0.05 level or that had an RR of 1.5 or greater. Adjusted RR and corresponding 95% CI were determined. The outcome in each model was the number of days from admission to an incident delirium diagnosis. Subjects without incident delirium were censored using their length of stay as the total number of days they were at risk for developing delirium.
Finally, to examine the relationships between incident delirium, maximum incident delirium severity and the number of inpatient days positive for delirium with the outcomes of death, impaired in 2 or more IADLs at follow‐up, impaired in 2 or more ADLs at follow‐up, length of stay, change in IADLs from admission to follow‐up, and change in ADLs from admission to follow‐up, logistic regression (for the dichotomous outcome of mortality), analysis of covariance or linear regression (depending on the whether the independent variable was categorical or continuous) was performed controlling for age, gender, and GDS score.
RESULTS
Of 256 eligible patients, dual consent was obtained from 154 patient and 154 family research subjects (308 consents). The refusal rate was 39% (n=102). Fourteen subjects were consented and enrolled but later dropped out due to family/proxy concerns regarding the patient's ability to participate in interviews. Thus, the final sample included 139 patients.
Descriptive statistics for baseline measures are given in Table 1. Briefly, the average age of subjects was 83 years (standard deviation [SD]=7); 41% were male; 57% were single, divorced, or widowed; and the average number of years of education was 12 years (SD=3). Thirty‐three percent were dehydrated on admission, and 33% had fallen within 2 weeks prior to admission. Thirty‐four percent had an infection at baseline, and 36% had some sensory impairment.
| Factor | Delirium, N=44, 31.7% | No Delirium, N=95, 68.3% | Relative Risk | 95% CI | P Value |
|---|---|---|---|---|---|
| |||||
| Demographic covariates | |||||
| Age, y, mean (SD) | 85.9 (5.9) | 82.4 (7.0) | 1.07 | 1.021.12 | 0.0051 |
| Male gender, n (%) | 23 (52.3) | 33 (34.7) | 1.83 | 1.013.31 | 0.0456 |
| Single/divorced/widowed, n (%) | 23 (52.3) | 56 (60.2) | 0.81 | 0.451.47 | 0.4882 |
| Education, y, mean (SD) | 12.6 (3.2) | 12.1 (3.0) | 1.06 | 0.951.17 | 0.3146 |
| Clinical covariates | |||||
| Dehydration, n (%) | 12 (30.8) | 30 (33.7) | 0.88 | 0.451.74 | 0.7152 |
| Fall in last 2 weeks, n (%) | 14 (41.2) | 21 (29.6) | 1.73* | 0.873.43 | 0.1186 |
| Infection, n (%) | 13 (40.6) | 21 (30.9) | 1.42 | 0.702.88 | 0.3328 |
| Sensory impairment, n (%) | 16 (36.4) | 33 (34.7) | 1.04 | 0.561.91 | 0.9132 |
| Lawton score, mean (SD) | 1.6 (1.3) | 2.3 (2.0) | 0.84 | 0.701.01 | 0.0592 |
| Katz impaired score, mean (SD) | 2.3 (2.0) | 3.4 (2.1) | 0.82 | 0.710.95 | 0.0072 |
| Charlson score, mean (SD) | 2.5 (1.8) | 2.3 (1.4) | 1.06 | 0.861.30 | 0.6013 |
| BUN, mean (SD) | 28.2 (17.6) | 25.6 (15.3) | 1.01 | 0.991.03 | 0.4175 |
| Creatinine, mean (SD) | 1.6 (1.3) | 2.4 (6.8) | 0.99 | 0.901.08 | 0.7356 |
| Cornell Depression score, mean (SD) | 1.6 (0.8) | 1.2 (0.9) | 1.35 | 0.991.83 | 0.0553 |
| Global Deterioration score, mean (SD) | 4.7 (1.2) | 3.9 (1.3) | 1.45* | 1.141.86 | 0.0027 |
| PAINAD score, mean (SD) | 2.1 (3.0) | 2.0 (2.9) | 1.01 | 0.911.12 | 0.8540 |
| Total number of regular medications, mean (SD) | 11.5 (4.6) | 11.0 (5.0) | 1.00 | 0.941.67 | 0.9771 |
| Total number of Beers medications, mean (SD) | 0.3 (0.7) | 0.4 (0.7) | 0.76 | 0.461.27 | 0.2933 |
| Cognitive impairment covariates | |||||
| MMSE score, mean (SD) | 12.7 (6.8) | 17.1 (6.6) | 0.94 | 0.900.98 | 0.0019 |
| Blessed score, mean (SD) | 9.5 (3.5) | 7.7 (2.9) | 1.14 | 1.041.24 | 0.0038 |
| Measures of deliriumcovariates for follow‐up outcomes | |||||
| Maximum incident delirium severity, mean (SD) | 15.4 (5.6) | 8.7 (6.1) | <0.0001 | ||
| Inpatient days with positive CAM, mean (SD) | 2.0 (1.1) | 0.2 (1.4) | <0.0001 | ||
| Follow‐up outcomes | |||||
| Mortality, n (%) | 11 (25.0) | 9 (9.5) | 0.0153 | ||
| Length of stay, mean (SD) | 9.1 (4.4) | 5.7 (4.1) | <0.0001 | ||
| Change in Lawton IADLs from admission to follow‐up, mean (SD) | 0.4 (1.5) | 0.2 (1.8) | 0.5094 | ||
| Change in Katz impaired ADLs from admission to follow‐up, mean (SD) | 0.3 (1.7) | 0.4 (1.6) | 0.6919 | ||
The overall incidence of delirium was 32% (44/139) and the range of days to incident delirium was 1 to 8 days. During the baseline period (Table 1), subjects with delirium were older, more likely to be male, had lower Katz impairment scores, higher GDS score, lower MMSE scores on admission, and higher Blessed scores than subjects without delirium. Slightly more persons with delirium had a prior fall, although the RR was not statistically significant. Length of stay measured at discharge was significantly higher for those with delirium (mean=9.1) than those without delirium (mean=5.7) (P<0.0001). Subjects with delirium were more likely to have died at 1 month than those without delirium (P=0.0153).
In addition, we analyzed the adjusted relative risk estimates for the final model of incident delirium. Significant risk factors or risk factors with RR estimates at least 1.5 (or <0.66 if protective [Table 1]) that were examined in a more comprehensive multiple proportional hazards model included age, gender, having had a fall in the last 2 weeks, number of impaired ADLs (based on Katz), GDS scores, MMSE scores at baseline, and Blessed scores at baseline. The final proportional hazards included gender and GDS score. Males were nearly 1.8 times as likely to develop delirium than females, and for every 1 unit increase in the GDS, subjects were 1.5 times more likely to develop delirium.
Finally, Table 2 gives the results of examining outcomes related to incident delirium measures. For mortality, there were no statistically significant predictors of death after controlling for age, gender, or GDS. For length of stay, subjects with incident delirium had significantly longer lengths of stay, as incident delirium severity increased by 1 unit the length of stay increased by 0.4 days, and as the number of inpatient days with delirium increased by 1 day the length of stay increased by 1.8 days. For change in the impaired Katz ADLs from admission to follow‐up, as incident delirium severity increased by 1 unit the change in impaired Katz ADLs increased by 0.05 units.
| Variable | |||
|---|---|---|---|
| Outcome mortality | Level | Adjusted Estimate of Associationa | P Value |
| |||
| Incident delirium, OR (95% CI)b | Yes | 2.33 (0.82‐6.61) | 0.1130 |
| No | 1.00 | ||
| Maximum incident delirium severity, OR (95% CI)b | 1.05 (0.961.14) | 0.2719 | |
| Number of inpatient days with positive delirium, OR (95% CI)b | 1.15 (0.891.49) | 0.2871 | |
| Outcome LOS | |||
| Incident delirium, mean (SE)c | Yes | 9.2 (0.7) | <0.0001 |
| No | 5.6 (0.5) | ||
| Maximum incident delirium severity, slope (SE)d | 0.43 (0.06) | <0.0001 | |
| Number of inpatient days with positive delirium, slope (SE)d | 1.80 (0.21) | <0.0001 | |
| Outcomechange in Lawton IADLs from admission to follow‐up | |||
| Incident delirium, mean (SE)c | Yes | 0.51 (0.33) | 0.3787 |
| No | 0.15 (0.20) | ||
| Maximum incident delirium severity, slope (SE)d | 0.003 (0.03) | 0.9260 | |
| Number of inpatient days with positive delirium, slope (SE)d | 0.16 (0.11) | 0.1497 | |
| Outcomechange in Katz impaired ADLs from admission to follow‐up | |||
| Incident delirium, mean (SE)c | Yes | 0.19 (0.26) | 0.5086 |
| No | 0.40 (0.17) | ||
| Maximum incident delirium severity, slope (SE)d | 0.05 (0.03) | 0.0437 | |
| Number of inpatient days with positive delirium, slope (SE)d | 0.13 (0.09) | 0.1717 | |
DISCUSSION
The most compelling finding from this study is the high incidence of delirium in hospitalized older adults with dementia and the association with poor clinical outcomes in those who develop delirium superimposed on dementia. DSD is difficult to detect and prevent; persons with DSD are at risk for poor quality of life. Those with delirium had a 25% short‐term mortality rate (P=0.0153), substantially increased length of stay (9.1 vs 5.1 days with an odds ratio of 1.8) and poorer physical function at discharge and follow‐up. At 1 month follow‐up, subjects with delirium had greater functional decline and lower GDS scores than those without delirium.
The incidence of delirium in this study was high (32%). Being delirious any time was associated with death and poor function. Delirium was also associated with the stage of the persons' baseline dementia, advanced age, lower MMSE scores, and falling before admission.
Previous studies have found delirium associated with increased mortality. Three studies found that within 1 year of a delirium episode, a significant number of persons died or were institutionalized.[24, 25, 26] Other research has reported death within 1 year of documented delirium episodes, and a 3‐fold increased rate of death in the ICU.[24, 27, 28, 29, 30, 31, 32] This study is 1 of only a few to focus on increased mortality with DSD and to focus uniquely on hospitalized patients with delirium and dementia.
The main risk factors for delirium in this study were male sex and severity of dementia. Our results, combined with those from other recent studies by Voyer and colleagues,[8, 33, 34] point to the critical importance of screening for dementia in hospitalized older adults as dementia severity is a significant indicator of delirium severity. For instance, Voyer and colleagues[34] reported that persons with mild dementia were likely to experience a mild delirium, whereas those with a more severe level of dementia were more likely to experience moderate to severe delirium. Our findings show that those who experienced episodes of delirium represented a highly vulnerable population with advanced dementia, sensory impairment, more falls and dehydration at admission, and higher Blessed scores. A recent study by Saczynski and colleagues[35] found 40% of patients who had experienced postoperative delirium did not return to their baseline at 6 months. Clearly, preventing delirium should be a critical priority to prevent such deterioration in the highly vulnerable population of hospitalized patients with dementia.
Patients in this study were on a mean of over 11 medications. One‐third of dementia patients in our study had also experienced a fall and dehydration at baseline. Other studies have found a relationship between cognitive decline, falling, and medications.[36] Many of these patients came into the hospital with potentially modifiable and preventable community or ambulatory care conditions of polypharmacy, falling, sensory impairment, and dehydration.
Importantly, in our study, length of stay was significantly higher (9.1 vs 5.7) for those with delirium compared to those without delirium. This finding is alarming when examining the economic impact of preventing delirium. Previous studies have found the cost of delirious episodes rivals those for diabetes and heart disease, and that decreasing length of stay by just 1 day would save over $20 million dollars per year.[4, 37]
In summary, this study is 1 of the first to report a high incidence of DSD and poorer outcomes for persons who experience delirium compared to those with dementia alone. This is 1 of only a few studies examining unique risk factors and delirium severity for DSD in the acute care setting. Findings from the current study report potential risk factors for development of incident delirium and highlight the challenge of preventing DSD before and during hospitalization. The generalizability of this study may be limited by the use of a nondiverse study population drawn from a single hospital in the northeast United States, though the use of a community hospital increases the relevance to real‐world practice settings. Determination of baseline cognitive status and the differentiation of delirium and dementia are difficult, but validated, state‐of‐the‐art methods were used that have been applied in previous studies.
This study provides fundamental methodological improvements over previous work, and advances the science by providing valuable data on the natural history, correlates, and outcomes of DSD. The strengths of this study include the prospective cohort design, the daily assessment for delirium based on a 24‐hour period, methods for determining cognitive status at baseline in this difficult population, and utilizing strict blinding of the well‐trained outcome assessors.
This study lays the groundwork for future studies to improve care for persons with dementia who present to acute care and to plan prevention programs for delirium before they are admitted to the hospital. We must be able to translate best practice for DSD into the acute care and community settings to prevent or minimize effects of delirium in persons with dementia. Interventions to increase early detection of delirium by hospital staff have the potential to decrease the severity and duration of delirium and prevent unnecessary suffering and costs from the complications of delirium and preventable readmissions to the hospital.
Thus, this study holds substantial clinical and economic implications for this population in the acute care setting, and will direct future studies leading to changes in real‐world practice settings for persons with dementia.
Disclosures
Drs. Fick, Inouye, and Steis acknowledge support for this project described by grants number R03 AG023216 (DMF) and number P01AG031720 (SKI) from the National Institute of Aging (NIA). This study and its contents are solely the responsibilities of the authors and do not necessarily represent the official views of the National Institutes of Health/NIA. The principal investigator, Dr. Fick, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest.
funding section: Dr. Inouye holds the Milton and Shirley F. Levy Family Chair.
Much attention has been given recently to hospitalized older adults, the critical 30‐day period, and posthospital syndrome.[1] What is missing from this dialogue is the contribution and significance of underlying cognitive impairment. By 2050, 14 million older persons in the United States are expected to have dementia.[2] Increasing numbers of older adults diagnosed with dementia are hospitalized and are at increased risk of developing delirium; in fact, delirium occurs in over half of hospitalized persons with dementia.[3] Further, current evidence suggests that delirium may accelerate the clinical course and trajectory of cognitive decline, and may be associated with considerably worse long‐term outcomes, including prolonged hospitalization, rehospitalization within 30 days, nursing home placement, and death.[3, 4, 5, 6] However, the problem of delirium superimposed on dementia (DSD) remains a neglected area of investigation in hospitalized patients. Delirium is superimposed on dementia when an acute change in mental status (characterized by a fluctuating course, inattention, and either disorganized thinking or altered level of consciousness) is layered on top of preexisting dementia.[4]
Despite the poor outcomes and high prevalence of DSD, little is known about the natural history in hospitalized older adults with dementia. Delirium studies often exclude persons with dementia, even though the prevalence of DSD is extremely high in both community (13%19%) and hospital (40%89%) populations and associated with higher costs and utilization compared to dementia and delirium alone.[4, 5, 7] In 1 study, annual costs for DSD were $9566 compared to $7557 for dementia alone.[7] The few risk‐factor studies of DSD were conducted in intensive care unit (ICU) or long‐term care settings.[8, 9]
The purpose of this study was to describe the incidence, risk factors, and outcomes associated with incident delirium in a prospective cohort of hospitalized older adults with dementia. The study aims were to: (1) estimate the incidence of new delirium in hospitalized persons with dementia, (2) identify the risk factors associated with incident delirium superimposed on dementia in this sample, (3) describe the outcomes associated with development of delirium, and (4) evaluate the contributions of delirium severity and duration to outcomes.
METHODS
This 24‐month prospective cohort study recruited and enrolled consecutive hospital admissions with dementia in a 300‐bed community hospital in central Pennsylvania from July 2006 through November 2008. Data were collected daily from patients during hospitalization, followed by a 1‐month posthospitalization interview with patients and their caregivers in the community setting. Patients were included if they spoke English, had been hospitalized fewer than 24 hours, and met the screening criteria for dementia. Patients were excluded if they had any significant neurological condition associated with cognitive impairment other than dementia (eg, brain tumor), a major acute psychiatric disorder, were unable to communicate, or had no caregiver to interview. The interviewers included experienced research assistants (RAs) who were either registered nurses or trained in a health‐related field. All staff training of instruments were done with scripted training manuals and video training using manuals for the Confusion Assessment Method (CAM). After training was completed, final inter‐rater reliability assessments were conducted until staff reached 100% agreement. The RAs were blinded to the aims and completed over 10 hours of training. Inter‐rater reliability checks were conducted on 10% of the sample in the field with >90% agreement attained on all instruments. This study was reviewed by and approved by The Pennsylvania State University institutional review board, and consent was received from all subjects.
Study Measures
Dementia was defined by meeting all 3 criteria of a Modified Blessed Dementia Rating Score of >3, an Informant Questionnaire on Cognitive Decline in the Elderly of 3.3, and documented dementia symptoms of at least 6 months' duration prior to current illness.[10, 11, 12] The Mini‐Mental State Examination (MMSE), purchased from Psychological Assessment Resources, Inc. (Lutz, FL), was used to measure change from day to day and aid in the measurement of delirium, but was not used to establish the diagnosis of dementia. Both the Clinical Dementia Rating Scale[13] and the Global Deterioration Scale (GDS)[14] were used to measure dementia stage and severity.
Delirium and delirium severity were defined according to the validated CAM algorithm;[15] the Delirium Rating Scale‐Revised‐98 was used for delirium severity.[16] In a recent review, the CAM showed an overall sensitivity of 94% and specificity of 89%.[17] In the present study, delirium was measured in a comprehensive and structured interview that involved the MMSE and CAM criteria, and was based on a 24‐hour period of observations, interviews with nurses and family members, and chart review. The CAM was completed daily during patient hospitalization and the follow‐up interviews. The CAM assesses 4 criteria including acute and fluctuating nature, inattention, disorganized thought, and altered level of consciousness. Delirium was recorded by the research staff as present or absent each day based on full CAM criteria. Because the goal of the present study focused on full CAM delirium, subsyndromal delirium was not presented in this article.
Delirium duration was defined as the number of days with a positive rating. Data were collected daily from patients during hospitalization, followed by a single interview at 1‐month posthospitalization with patients and their caregivers. Most interviews were in person.
Delirium Risk Factors
Central nervous system‐active drug use was defined by 2005 American Hospital Formulary Services classification.[18] The Beers criteria were used to define potentially inappropriate medication use.[19] The Cornell scale for depression in persons with dementia was used, with a cut point of 12 indicating depression.[20] Functional status change was measured via the Katz Index of Activities of Daily Living (ADLs) and Lawton Instrumental Activities Of Daily Living (IADLs) change scores.[21] Comorbid conditions were classified with a weighted index that took into account both the number and seriousness of different comorbid diseases.[22] Pain was measured using the Pain Assessment in Advanced Dementia (PAINAD) scale.[23] Dehydration was defined using the blood urea nitrogen (BUN)/creatinine ratio and/or any chart diagnosis of dehydration. Admission lab values (BUN/creatinine) were abstracted from the medical records.
Primary Outcomes
The primary outcomes measured were full CAM delirium, index hospitalization length of stay, cognitive decline (change in MMSE and GDS scores), death, and functional status change (change from baseline to discharge score). One‐month mortality was measured by chart review and follow‐up family interviews performed at 1 month via telephone or in‐person interviews. Mortality was not verified by additional methods.
Statistical Analysis
All statistical analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC), and statistical significance was assessed using an level of 0.05 unless otherwise noted. Descriptive statistics were calculated on all characteristics by incident delirium status.
Potential risk factors for incident delirium were examined using [2] and t tests, where appropriate. Simple proportional hazards models were used to estimate the relative risk (RR) and 95% confidence interval (CI) for incident delirium. A stepwise model‐building procedure under a proportional hazards model was used to build a final model for incident delirium that contained all variables that were statistically significant at the 0.05 level or that had an RR of 1.5 or greater. Adjusted RR and corresponding 95% CI were determined. The outcome in each model was the number of days from admission to an incident delirium diagnosis. Subjects without incident delirium were censored using their length of stay as the total number of days they were at risk for developing delirium.
Finally, to examine the relationships between incident delirium, maximum incident delirium severity and the number of inpatient days positive for delirium with the outcomes of death, impaired in 2 or more IADLs at follow‐up, impaired in 2 or more ADLs at follow‐up, length of stay, change in IADLs from admission to follow‐up, and change in ADLs from admission to follow‐up, logistic regression (for the dichotomous outcome of mortality), analysis of covariance or linear regression (depending on the whether the independent variable was categorical or continuous) was performed controlling for age, gender, and GDS score.
RESULTS
Of 256 eligible patients, dual consent was obtained from 154 patient and 154 family research subjects (308 consents). The refusal rate was 39% (n=102). Fourteen subjects were consented and enrolled but later dropped out due to family/proxy concerns regarding the patient's ability to participate in interviews. Thus, the final sample included 139 patients.
Descriptive statistics for baseline measures are given in Table 1. Briefly, the average age of subjects was 83 years (standard deviation [SD]=7); 41% were male; 57% were single, divorced, or widowed; and the average number of years of education was 12 years (SD=3). Thirty‐three percent were dehydrated on admission, and 33% had fallen within 2 weeks prior to admission. Thirty‐four percent had an infection at baseline, and 36% had some sensory impairment.
| Factor | Delirium, N=44, 31.7% | No Delirium, N=95, 68.3% | Relative Risk | 95% CI | P Value |
|---|---|---|---|---|---|
| |||||
| Demographic covariates | |||||
| Age, y, mean (SD) | 85.9 (5.9) | 82.4 (7.0) | 1.07 | 1.021.12 | 0.0051 |
| Male gender, n (%) | 23 (52.3) | 33 (34.7) | 1.83 | 1.013.31 | 0.0456 |
| Single/divorced/widowed, n (%) | 23 (52.3) | 56 (60.2) | 0.81 | 0.451.47 | 0.4882 |
| Education, y, mean (SD) | 12.6 (3.2) | 12.1 (3.0) | 1.06 | 0.951.17 | 0.3146 |
| Clinical covariates | |||||
| Dehydration, n (%) | 12 (30.8) | 30 (33.7) | 0.88 | 0.451.74 | 0.7152 |
| Fall in last 2 weeks, n (%) | 14 (41.2) | 21 (29.6) | 1.73* | 0.873.43 | 0.1186 |
| Infection, n (%) | 13 (40.6) | 21 (30.9) | 1.42 | 0.702.88 | 0.3328 |
| Sensory impairment, n (%) | 16 (36.4) | 33 (34.7) | 1.04 | 0.561.91 | 0.9132 |
| Lawton score, mean (SD) | 1.6 (1.3) | 2.3 (2.0) | 0.84 | 0.701.01 | 0.0592 |
| Katz impaired score, mean (SD) | 2.3 (2.0) | 3.4 (2.1) | 0.82 | 0.710.95 | 0.0072 |
| Charlson score, mean (SD) | 2.5 (1.8) | 2.3 (1.4) | 1.06 | 0.861.30 | 0.6013 |
| BUN, mean (SD) | 28.2 (17.6) | 25.6 (15.3) | 1.01 | 0.991.03 | 0.4175 |
| Creatinine, mean (SD) | 1.6 (1.3) | 2.4 (6.8) | 0.99 | 0.901.08 | 0.7356 |
| Cornell Depression score, mean (SD) | 1.6 (0.8) | 1.2 (0.9) | 1.35 | 0.991.83 | 0.0553 |
| Global Deterioration score, mean (SD) | 4.7 (1.2) | 3.9 (1.3) | 1.45* | 1.141.86 | 0.0027 |
| PAINAD score, mean (SD) | 2.1 (3.0) | 2.0 (2.9) | 1.01 | 0.911.12 | 0.8540 |
| Total number of regular medications, mean (SD) | 11.5 (4.6) | 11.0 (5.0) | 1.00 | 0.941.67 | 0.9771 |
| Total number of Beers medications, mean (SD) | 0.3 (0.7) | 0.4 (0.7) | 0.76 | 0.461.27 | 0.2933 |
| Cognitive impairment covariates | |||||
| MMSE score, mean (SD) | 12.7 (6.8) | 17.1 (6.6) | 0.94 | 0.900.98 | 0.0019 |
| Blessed score, mean (SD) | 9.5 (3.5) | 7.7 (2.9) | 1.14 | 1.041.24 | 0.0038 |
| Measures of deliriumcovariates for follow‐up outcomes | |||||
| Maximum incident delirium severity, mean (SD) | 15.4 (5.6) | 8.7 (6.1) | <0.0001 | ||
| Inpatient days with positive CAM, mean (SD) | 2.0 (1.1) | 0.2 (1.4) | <0.0001 | ||
| Follow‐up outcomes | |||||
| Mortality, n (%) | 11 (25.0) | 9 (9.5) | 0.0153 | ||
| Length of stay, mean (SD) | 9.1 (4.4) | 5.7 (4.1) | <0.0001 | ||
| Change in Lawton IADLs from admission to follow‐up, mean (SD) | 0.4 (1.5) | 0.2 (1.8) | 0.5094 | ||
| Change in Katz impaired ADLs from admission to follow‐up, mean (SD) | 0.3 (1.7) | 0.4 (1.6) | 0.6919 | ||
The overall incidence of delirium was 32% (44/139) and the range of days to incident delirium was 1 to 8 days. During the baseline period (Table 1), subjects with delirium were older, more likely to be male, had lower Katz impairment scores, higher GDS score, lower MMSE scores on admission, and higher Blessed scores than subjects without delirium. Slightly more persons with delirium had a prior fall, although the RR was not statistically significant. Length of stay measured at discharge was significantly higher for those with delirium (mean=9.1) than those without delirium (mean=5.7) (P<0.0001). Subjects with delirium were more likely to have died at 1 month than those without delirium (P=0.0153).
In addition, we analyzed the adjusted relative risk estimates for the final model of incident delirium. Significant risk factors or risk factors with RR estimates at least 1.5 (or <0.66 if protective [Table 1]) that were examined in a more comprehensive multiple proportional hazards model included age, gender, having had a fall in the last 2 weeks, number of impaired ADLs (based on Katz), GDS scores, MMSE scores at baseline, and Blessed scores at baseline. The final proportional hazards included gender and GDS score. Males were nearly 1.8 times as likely to develop delirium than females, and for every 1 unit increase in the GDS, subjects were 1.5 times more likely to develop delirium.
Finally, Table 2 gives the results of examining outcomes related to incident delirium measures. For mortality, there were no statistically significant predictors of death after controlling for age, gender, or GDS. For length of stay, subjects with incident delirium had significantly longer lengths of stay, as incident delirium severity increased by 1 unit the length of stay increased by 0.4 days, and as the number of inpatient days with delirium increased by 1 day the length of stay increased by 1.8 days. For change in the impaired Katz ADLs from admission to follow‐up, as incident delirium severity increased by 1 unit the change in impaired Katz ADLs increased by 0.05 units.
| Variable | |||
|---|---|---|---|
| Outcome mortality | Level | Adjusted Estimate of Associationa | P Value |
| |||
| Incident delirium, OR (95% CI)b | Yes | 2.33 (0.82‐6.61) | 0.1130 |
| No | 1.00 | ||
| Maximum incident delirium severity, OR (95% CI)b | 1.05 (0.961.14) | 0.2719 | |
| Number of inpatient days with positive delirium, OR (95% CI)b | 1.15 (0.891.49) | 0.2871 | |
| Outcome LOS | |||
| Incident delirium, mean (SE)c | Yes | 9.2 (0.7) | <0.0001 |
| No | 5.6 (0.5) | ||
| Maximum incident delirium severity, slope (SE)d | 0.43 (0.06) | <0.0001 | |
| Number of inpatient days with positive delirium, slope (SE)d | 1.80 (0.21) | <0.0001 | |
| Outcomechange in Lawton IADLs from admission to follow‐up | |||
| Incident delirium, mean (SE)c | Yes | 0.51 (0.33) | 0.3787 |
| No | 0.15 (0.20) | ||
| Maximum incident delirium severity, slope (SE)d | 0.003 (0.03) | 0.9260 | |
| Number of inpatient days with positive delirium, slope (SE)d | 0.16 (0.11) | 0.1497 | |
| Outcomechange in Katz impaired ADLs from admission to follow‐up | |||
| Incident delirium, mean (SE)c | Yes | 0.19 (0.26) | 0.5086 |
| No | 0.40 (0.17) | ||
| Maximum incident delirium severity, slope (SE)d | 0.05 (0.03) | 0.0437 | |
| Number of inpatient days with positive delirium, slope (SE)d | 0.13 (0.09) | 0.1717 | |
DISCUSSION
The most compelling finding from this study is the high incidence of delirium in hospitalized older adults with dementia and the association with poor clinical outcomes in those who develop delirium superimposed on dementia. DSD is difficult to detect and prevent; persons with DSD are at risk for poor quality of life. Those with delirium had a 25% short‐term mortality rate (P=0.0153), substantially increased length of stay (9.1 vs 5.1 days with an odds ratio of 1.8) and poorer physical function at discharge and follow‐up. At 1 month follow‐up, subjects with delirium had greater functional decline and lower GDS scores than those without delirium.
The incidence of delirium in this study was high (32%). Being delirious any time was associated with death and poor function. Delirium was also associated with the stage of the persons' baseline dementia, advanced age, lower MMSE scores, and falling before admission.
Previous studies have found delirium associated with increased mortality. Three studies found that within 1 year of a delirium episode, a significant number of persons died or were institutionalized.[24, 25, 26] Other research has reported death within 1 year of documented delirium episodes, and a 3‐fold increased rate of death in the ICU.[24, 27, 28, 29, 30, 31, 32] This study is 1 of only a few to focus on increased mortality with DSD and to focus uniquely on hospitalized patients with delirium and dementia.
The main risk factors for delirium in this study were male sex and severity of dementia. Our results, combined with those from other recent studies by Voyer and colleagues,[8, 33, 34] point to the critical importance of screening for dementia in hospitalized older adults as dementia severity is a significant indicator of delirium severity. For instance, Voyer and colleagues[34] reported that persons with mild dementia were likely to experience a mild delirium, whereas those with a more severe level of dementia were more likely to experience moderate to severe delirium. Our findings show that those who experienced episodes of delirium represented a highly vulnerable population with advanced dementia, sensory impairment, more falls and dehydration at admission, and higher Blessed scores. A recent study by Saczynski and colleagues[35] found 40% of patients who had experienced postoperative delirium did not return to their baseline at 6 months. Clearly, preventing delirium should be a critical priority to prevent such deterioration in the highly vulnerable population of hospitalized patients with dementia.
Patients in this study were on a mean of over 11 medications. One‐third of dementia patients in our study had also experienced a fall and dehydration at baseline. Other studies have found a relationship between cognitive decline, falling, and medications.[36] Many of these patients came into the hospital with potentially modifiable and preventable community or ambulatory care conditions of polypharmacy, falling, sensory impairment, and dehydration.
Importantly, in our study, length of stay was significantly higher (9.1 vs 5.7) for those with delirium compared to those without delirium. This finding is alarming when examining the economic impact of preventing delirium. Previous studies have found the cost of delirious episodes rivals those for diabetes and heart disease, and that decreasing length of stay by just 1 day would save over $20 million dollars per year.[4, 37]
In summary, this study is 1 of the first to report a high incidence of DSD and poorer outcomes for persons who experience delirium compared to those with dementia alone. This is 1 of only a few studies examining unique risk factors and delirium severity for DSD in the acute care setting. Findings from the current study report potential risk factors for development of incident delirium and highlight the challenge of preventing DSD before and during hospitalization. The generalizability of this study may be limited by the use of a nondiverse study population drawn from a single hospital in the northeast United States, though the use of a community hospital increases the relevance to real‐world practice settings. Determination of baseline cognitive status and the differentiation of delirium and dementia are difficult, but validated, state‐of‐the‐art methods were used that have been applied in previous studies.
This study provides fundamental methodological improvements over previous work, and advances the science by providing valuable data on the natural history, correlates, and outcomes of DSD. The strengths of this study include the prospective cohort design, the daily assessment for delirium based on a 24‐hour period, methods for determining cognitive status at baseline in this difficult population, and utilizing strict blinding of the well‐trained outcome assessors.
This study lays the groundwork for future studies to improve care for persons with dementia who present to acute care and to plan prevention programs for delirium before they are admitted to the hospital. We must be able to translate best practice for DSD into the acute care and community settings to prevent or minimize effects of delirium in persons with dementia. Interventions to increase early detection of delirium by hospital staff have the potential to decrease the severity and duration of delirium and prevent unnecessary suffering and costs from the complications of delirium and preventable readmissions to the hospital.
Thus, this study holds substantial clinical and economic implications for this population in the acute care setting, and will direct future studies leading to changes in real‐world practice settings for persons with dementia.
Disclosures
Drs. Fick, Inouye, and Steis acknowledge support for this project described by grants number R03 AG023216 (DMF) and number P01AG031720 (SKI) from the National Institute of Aging (NIA). This study and its contents are solely the responsibilities of the authors and do not necessarily represent the official views of the National Institutes of Health/NIA. The principal investigator, Dr. Fick, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest.
funding section: Dr. Inouye holds the Milton and Shirley F. Levy Family Chair.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8(2):131–168.
- , . Delirium superimposed on dementia: accuracy of nurse documentation. J Gerontol Nurs. 2012;38(1):32–42.
- , , . Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723–1732.
- , , , et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575.
- , , , et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156(12):848–856, W296.
- , , , . Delirium superimposed on dementia in a community‐dwelling managed care population: a 3‐year retrospective study of occurrence, costs, and utilization. J Gerontol. 2005;60A(6):748–753.
- , , , . Factors associated with delirium severity among older persons with dementia. J Neurosci Nurs. 2011;43(2):62–69.
- , , . Delirium in the intensive care unit. Crit Care 2008;12(suppl 3):S3.
- , , . Correlations of Mini‐Mental State and Modified Rating Scale to measures of transitional health status in dementia. J Gerontol. 1987;42(1):33–36.
- , , , . Population‐based norms for the Mini‐Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391.
- , . The mini‐mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935.
- , , , , . A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572.
- , . The GDS/FAST staging system. Int Psychogeriatr. 1997;9(suppl 1):167–171.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948.
- , , , , , . Validation of the Delirium Rating Scale‐Revised‐98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–242.
- , , , . The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823–830.
- American Society of Health‐System Pharmacists. AHFS Drug Information. Bethesda, MD: American Society of Health‐System Pharmacists; 2005.
- , , , , , . Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts [published correction appears in Arch Intern Med. 2004;164:298]. Arch Intern Med 2003;163(22):2716–2724.
- , , , . Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23(3):271–284.
- . Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–726.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , . Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc. 2003;4(1):9–15.
- , , . Prevalence and outcomes of delirium in community and non‐acute care settings in people without dementia: a report from the Canadian Study of Health and Aging. BMC Med. 2006;4:15.
- , , , . Prognostic significance of delirium in frail older people. Dementia. 2005;19(2‐3):158–163.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
- , , , . Delirium subtypes and 1‐year mortality among elderly patients discharged from a post‐acute rehabilitation facility. J Gerontol. 2007;62A(10):1182–1183.
- , , , , , . Delirium in older patients admitted to general internal medicine. J Geriatr Psychiatry Neurol. 2006;19(2):83–90.
- , , , . Association between psychomotor activity delirium subtypes and mortality among newly admitted postacute facility patients. J Gerontol. 2007;62A(2):174–179.
- , , , , , . Premature death associated with delirium at 1‐year follow‐up. Arch Intern Med. 2005;165:1657–1662.
- , , , et al. Older adults discharged from the hospital with delirium: 1‐year outcomes. J Am Geriatr Soc. 2006;54(8):1245–1250.
- , , , . Influence of prior cognitive impairment on the severity of delirium symptoms among older patients. J Neurosci Nurs. 2006;38(2):90–101.
- , , , , . Factors associated with delirium severity among older patients. J Clin Nurs. 2007;16:819–831.
- , , , et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39.
- , , , et al. Effect of central nervous system medication use on decline in cognition in community‐dwelling older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2009;57(2):243–250.
- , , , , . One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8(2):131–168.
- , . Delirium superimposed on dementia: accuracy of nurse documentation. J Gerontol Nurs. 2012;38(1):32–42.
- , , . Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723–1732.
- , , , et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575.
- , , , et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156(12):848–856, W296.
- , , , . Delirium superimposed on dementia in a community‐dwelling managed care population: a 3‐year retrospective study of occurrence, costs, and utilization. J Gerontol. 2005;60A(6):748–753.
- , , , . Factors associated with delirium severity among older persons with dementia. J Neurosci Nurs. 2011;43(2):62–69.
- , , . Delirium in the intensive care unit. Crit Care 2008;12(suppl 3):S3.
- , , . Correlations of Mini‐Mental State and Modified Rating Scale to measures of transitional health status in dementia. J Gerontol. 1987;42(1):33–36.
- , , , . Population‐based norms for the Mini‐Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391.
- , . The mini‐mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935.
- , , , , . A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572.
- , . The GDS/FAST staging system. Int Psychogeriatr. 1997;9(suppl 1):167–171.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948.
- , , , , , . Validation of the Delirium Rating Scale‐Revised‐98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–242.
- , , , . The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823–830.
- American Society of Health‐System Pharmacists. AHFS Drug Information. Bethesda, MD: American Society of Health‐System Pharmacists; 2005.
- , , , , , . Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts [published correction appears in Arch Intern Med. 2004;164:298]. Arch Intern Med 2003;163(22):2716–2724.
- , , , . Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23(3):271–284.
- . Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–726.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , . Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc. 2003;4(1):9–15.
- , , . Prevalence and outcomes of delirium in community and non‐acute care settings in people without dementia: a report from the Canadian Study of Health and Aging. BMC Med. 2006;4:15.
- , , , . Prognostic significance of delirium in frail older people. Dementia. 2005;19(2‐3):158–163.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
- , , , . Delirium subtypes and 1‐year mortality among elderly patients discharged from a post‐acute rehabilitation facility. J Gerontol. 2007;62A(10):1182–1183.
- , , , , , . Delirium in older patients admitted to general internal medicine. J Geriatr Psychiatry Neurol. 2006;19(2):83–90.
- , , , . Association between psychomotor activity delirium subtypes and mortality among newly admitted postacute facility patients. J Gerontol. 2007;62A(2):174–179.
- , , , , , . Premature death associated with delirium at 1‐year follow‐up. Arch Intern Med. 2005;165:1657–1662.
- , , , et al. Older adults discharged from the hospital with delirium: 1‐year outcomes. J Am Geriatr Soc. 2006;54(8):1245–1250.
- , , , . Influence of prior cognitive impairment on the severity of delirium symptoms among older patients. J Neurosci Nurs. 2006;38(2):90–101.
- , , , , . Factors associated with delirium severity among older patients. J Clin Nurs. 2007;16:819–831.
- , , , et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39.
- , , , et al. Effect of central nervous system medication use on decline in cognition in community‐dwelling older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2009;57(2):243–250.
- , , , , . One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27.
Copyright © 2013 Society of Hospital Medicine
Choosing Wisely in Pediatric Medicine
Overuse in medicine is a significant and under‐recognized problem. Don Berwick estimated that waste accounts for at least 20% of healthcare expenditures in the United States, with overtreatment as one of the largest categories.[1] A commentary by Schroeder et al. challenged pediatricians to incorporate this knowledge into our own patient safety and quality movement.[2] Recently published data suggest that we are far from achieving the patient safety goals set forth in the Institute of Medicine's landmark To Err is Human[3] report, despite more than a decade of national, local, and regional efforts.[4] One way to reduce waste and improve patient safety is to eliminate practices of unproven benefit. Therapies or tests that may initially seem promising are often proven to be not only unhelpful but actually harmful. The recommendation of the US Preventive Services Task Force against routine screening for prostate specific antigen is an example of how a common test initially thought of as lifesaving actually increases harm.[5]
The American Board of Internal Medicine Foundation (ABIM‐F) recently announced the Choosing Wisely campaign. Through this campaign the Foundation encourages physicians, patients and other healthcare stakeholders to think and talk about medical tests and procedures that may be unnecessary.[6] The primary output of this challenge is the development of a list of 5 tests and or therapies that physicians and patients should question. The ABIM‐F approached different medical societies to develop these lists within their own specialties. The Society of Hospital Medicine (SHM) joined the Choosing Wisely campaign in April 2012, and agreed to develop a list of 5 therapies and tests for adult hospital medicine and pediatric hospital medicine. Here we present the contribution of the pediatric workgroup detailing the methodology and process for developing the list, as well as summarizing the evidence supporting each recommendation.
METHODS
In the spring of 2012, the pediatric committee of the SHM convened a workgroup of pediatric hospitalists to develop a top 5 list for the field. This workgroup was composed of experienced pediatric hospitalists representing diverse geographic locations of the United States and a mix of academic and nonacademic practice settings. The group, consisting of 4 women and 9 men, began by proposing candidate recommendations after discussion with colleagues at their different practice sites. The group was charged to maintain a focus on overuse practices that had a strong basis in evidence, were frequently encountered at their practice sites, and achieved significant consensus among their colleagues. Figure 1 shows the process map describing the method for the development of the pediatric recommendations. All workgroup participants were queried as to conflict of interest relevant to this work and none were identified.
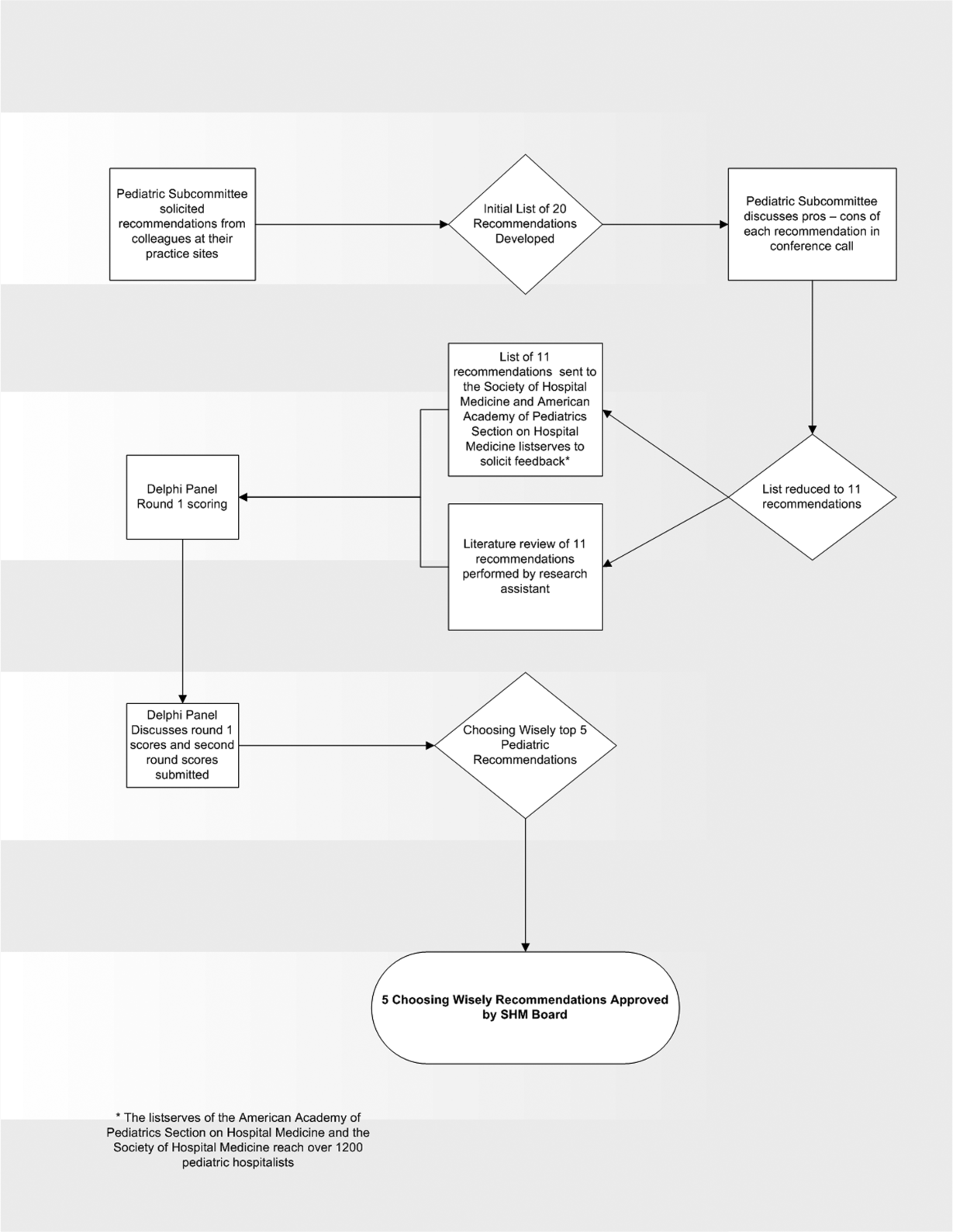
Literature Review
After the generation of the initial top 20 list, 2 reviewers conducted independent literature searches in PubMed, MEDLINE, and the Cochrane Library on the proposed topics. The reviewers also conducted generic Internet searches. Key search terms included pediatric asthma, bronchiolitis, chest radiograph, systemic corticosteroids, gastroesophageal reflux disease (GERD), infant, child, acid suppression therapy, continuous pulse oximetry, pneumonia, gastroenteritis, viral testing, blood culture, and soft tissue infections. To ensure that the reviewers included all studies relevant to the searches, they utilized broad terms. The search included all literature published through 2012, and nonEnglish language publications were included in the search. Studies selected and included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, included a pediatric population in the guidelines or study, reviewed the harm associated with the administration of a particular test or treatment, and explored the cost associated with the test or treatment.
The Delphi Panel
Members of the workgroup formed a Delphi panel except for 1 member (R.Q.) who served as the nonvoting moderator. The members of the Delphi panel considered the results of the literature search for each recommendation along with the collated feedback from hospitalist listserves as described in Figure 1. Each panel member received a voting instrument with the candidate tests and treatments for the first round of Delphi voting. The panel utilized a modified Delphi method or the RAND Corporation (RAND)/University of California at Los Angeles (UCLA) appropriateness method as described in previous publications of quality indicator development in pediatrics.[7] Each panelist scored the candidate tests and treatments and forwarded the scores to the moderator. Subsequently, all the members of the Delphi panel met through a conference call to carry out the second round of voting. The deidentified collated results of the first round of Delphi voting were made available and discussed during the call. The moderator collated the final results, and the final 5 recommendations were those that had the highest score after the second round of Delphi voting.
Volume and Costs
During deliberations, the committee took into account the prevalence and cost rankings of our most common pediatric inpatient diagnoses. This was done using the Agency for Healthcare Research and Quality's (AHRQ) Healthcare Utilization Project (HCUP), specifically, the Kids' Inpatient Database (KID). HCUP includes the largest collection of longitudinal hospital care data in the United States, encompassing all‐payer discharge‐level information. We excluded normal newborn hospitalizations, and looked at the top 10 acute inpatient diagnoses in terms of both volume and aggregate costs.
RESULTS
The initial list of 20 candidate tests and treatments as well as the refined list of 11 recommendations can be found as electronic supplements to this publication (see Supporting Table 1 and Supporting Table 2 in the online version of this article). The format and language of the list of 11 recommendations were chosen to mesh with that typically used in the ABIM‐F Choosing Wisely campaign. During the Delphi panel, there was strong group consensus about combining items 1 and 2 (chest radiographs in asthma and bronchiolitis) into a single recommendation.
| Do not order chest radiographs in children with asthma or bronchiolitis. |
| Do not use bronchodilators in children with bronchiolitis. |
| Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection. |
| Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
The top 5 recommendations based on the result of the second round of Delphi scoring are shown in Table 1 and described below along with a detailed evidence summary.
Do not order chest radiographs in children with asthma or bronchiolitis.
The National Heart and Lung Institute's guidelines for the management of asthma, published in 1987, recommend against routinely obtaining chest radiographs in patients with asthma or asthma exacerbations.[8] Supporting this recommendation are several studies that show a low overall yield when obtaining chest radiographs for wheezing patients.[9, 10, 11] Most relevant, studies that evaluated the clinical utility of radiographs in patients with asthma have demonstrated that they influence clinical management in less than 2% of cases.[12] A quality improvement project aimed at decreasing the rate of chest radiographs obtained in patients with asthma demonstrated that close to 60% of patients admitted to the hospital had chest radiographs performed, and that significant overall reductions can be achieved (45.3%28.9%, P=0.0005) without impacting clinical outcomes negatively.[13]
Similarly, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely obtaining radiographs during the evaluation for bronchiolitis.[14] Studies assessing the utility of chest x‐rays in these children demonstrate an even lower incidence of abnormalities (0.75%) and indicate that, despite this low incidence, physicians are more likely to treat with antibiotics when radiographs are obtained.[15] There is also evidence that chest radiographs in patients with bronchiolitis are not useful in predicting severity of illness.[16] Furthermore, cost‐effective analyses have demonstrated that omitting chest radiographs in bronchiolitis is actually cost‐effective, without compromising diagnostic accuracy.[17] In a recently published national benchmarking inpatient collaborative, Ralston et al. demonstrated that the majority of patients admitted to the hospital with bronchiolitis have chest radiographs performed at a rate of 64% (interquartile range [IQR], 54%81%).[18]
In both bronchiolitis and asthma, the elimination of unnecessary radiographs has the potential to decrease costs, reduce radiation exposure, and minimize the overuse of antibiotics that often occurs secondary to false positive results.
Do not use bronchodilators in children with bronchiolitis.
Ralston showed that 70% (IQR, 59%83%) of admitted bronchiolitis patients received bronchodilators with an average of 7.9 doses per patient (IQR, 4.69.8). National guidelines for bronchiolitis suggest a very limited role of bronchodilators in patients with bronchiolitis.[14] The first meta‐analyses of studies related to the question of ‐agonist efficacy in bronchiolitis were published in the late 1990s, revealing minimal or no treatment effects.[19, 20] Since then, further research has solidified these findings, and fairly definitive statements can be made based on a recent comprehensive meta‐analysis.[21] The pooled data do not show any effect on hospitalization rates, hospital length of stay, or other inpatient outcomes in bronchiolitis. They do show a small change in clinical scores documented in the outpatient setting, though these scores have not correlated with any detectable difference in outcomes. Routine use of ‐agonists in the inpatient setting has no proven benefit, and given the large amount of consistent data, there is no compelling reason for further study of this therapy in the inpatient setting.
Epinephrine, a combined ‐ and ‐agonist, has been extensively evaluated in bronchiolitis as well. Like albuterol, epinephrine has been reported to have no effect on hospital length of stay in bronchiolitis.[22] The issue of admission rates after epinephrine is complicated by 1 very large study that combined epinephrine with dexamethasone and reported a decreased admission rate, though only at 7 days after therapy; however, this effect was nullified after adjustment for multiple comparisons.[23] When the end point is improvement of respiratory scores, epinephrine may perform better than albuterol in studies where they are directly compared; however, there is no evidence that repeated usage of epinephrine has any impact on any clinical outcome for inpatients.[24, 25]
Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection
In their summary of evidence, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely using systemic corticosteroids for infants with bronchiolitis.[14] The previously reference bronchiolitis benchmarking study demonstrated that admitted patients received steroids at a rate of 21% (IQR, 14%26%). The poor efficacy of corticosteroids in children with bronchiolitis under 2 years of age is well demonstrated in the literature. A large, blinded, randomized, controlled study compared systemic oral corticosteroids to placebo in hospitalized children 10 months to 6 years of age with viral wheezing.[26] This study showed no benefit of corticosteroids over placebo in length of stay or parental report of symptoms 1 week later. In the study, a subanalysis of children with eczema and family history of asthma also demonstrated no benefit of systemic corticosteroids. Large systematic reviews further argue that there is no effect of corticosteroids on the likelihood of admission or length of stay in infants with bronchiolitis.[27, 28] One 4‐armed prospective study of children 6 weeks to 12 months of age found no efficacy of dexamethasone over placebo.[23] There was modest benefit of dexamethasone in conjunction with racemic epinephrine; however, this benefit disappeared after adjustment for multiple comparisons. Three smaller studies showing benefit of systemic corticosteroids, however, were highly problematic. They have included older children, were retrospective, or demonstrated inconsistent results.[29, 30] A smaller study showed benefit for children over 2 years of age, but none for children under 2 years of age.[31] Premature infants are at increased risk of asthma, which typically responds well to corticosteroids as these children get older. However, a retrospective study of premature infants under 2 years of age with bronchiolitis demonstrated no association between corticosteroid use and length of stay, even in the subset of premature infants responding to albuterol.[32]
Systemic corticosteroid use in children is not harmless. Children under 2 years of age are especially vulnerable to the decreased growth velocity seen as a side effect of systemic corticosteroids.[33] Corticosteroids may also negatively impact the course of infectious illness. For instance, in children hospitalized with pneumonia but not receiving ‐agonists (ie, patients who are unlikely to have asthma), length of stay is prolonged and readmission is higher in those who receive corticosteroids.[34]
Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy.
From 2000 to 2005, the incidence of infants diagnosed with gastroeshopaheal reflux (GER) tripled (3.4%12.3%), and the use of proton pump inhibitors (PPIs) doubled (31.5%62.6%).[35] Patients diagnosed with GER and treated with antireflux medication incurred 1.8 times higher healthcare costs in 1 study compared to healthy controls.[36] Though common, the use of acid suppressive medications in infants lacks evidence for efficacy in the majority of the clinical scenarios in which they are prescribed.[37, 38] PPIs have failed to outperform placebo for typical infant reflux, which is generally developmental and not pathologic.[39, 40] Furthermore, prompted by findings in adults, multiple pediatric investigators have now catalogued the potential risks associated with acid blockade in children in multiple clinical settings. Specifically, increased risk of pneumonia has been documented in inpatients and outpatients, and increased risk of necrotizing enterocolitis and other serious infections have been documented in intensive care unit settings.[41] In the absence of data supporting efficacy and given the emerging data on risk, empiric acid suppression in infants with reflux is wasteful and potentially harmful.
Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
Pulse oximetry use has become widespread in the management of infants with bronchiolitis and likely accounts for the dramatic increase in bronchiolitis hospitalization rates in recent years.[14, 42, 43, 44, 45, 46, 47] Despite this increase in hospitalization rate, there was no change in mortality from bronchiolitis between 1979 and 1997.[48] The continuous monitoring of oxygen saturations in hospitalized infants with bronchiolitis may lead to overdiagnosis of hypoxemia and subsequent oxygen use that is of no apparent benefit to the child. Schroeder et al. demonstrated that 26% of a sample of infants hospitalized with bronchiolitis had a prolonged length of stay because of a perceived need for oxygen based on pulse oximetry readings.[43] Unger and Cunningham showed that the need for oxygen was the final determinant of length of stay in 58% of cases, and Cunningham and Murray suggested that using an oxygen saturation cutoff of 94% instead of 90% might increase the length of stay by 22 hours.[44, 49]
It has been previously shown that hypoxia is normative in infants. Healthy infants experience multiple episodes of SpO2 90% while sleeping.[50] This finding strengthens the notion that detection of low saturations in infants convalescing from bronchiolitis may simply reflect overdiagnosis. Among children with chronic severe asthma, who presumably have experienced episodes of hypoxia throughout childhood, there is no difference in school performance compared to healthy controls.[51]
The practice parameter on bronchiolitis from the American Academy of Pediatrics states: as the child's clinical course improves, continuous measurement of SpO2 is not routinely needed, which is a recommendation based on expert consensus.[14] There is at least one ongoing randomized trial comparing the use of continuous versus intermittent pulse oximetry in hospitalized infants with bronchiolitis who are weaned off oxygen (
DISCUSSION
Berwick and Hackbarth define overtreatment as: waste that comes from subjecting patients to care that, according to sound science and the patients' own preferences, cannot possibly help themcare rooted in outmoded habits, supply‐driven behaviors, and ignoring science.[1] With this project, we tried to capture common clinical sources of waste in the inpatient pediatric setting. This is an inherently difficult project because of the absence of solid evidence to inform every decision point in medicine. Although there is always room for improvement in our evidence base, our group intentionally gravitated to areas where the evidence was robust.
The primary strength of this work is the use of the RAND/UCLA appropriateness method or modified Delphi method. Several publications have validated this methodology as a sound strategy to assess quality indicators and issues related to overuse.[7, 53] To our knowledge, we are the first group to report the use of this methodology to develop a list such as the list reported here.
There were some challenges inherent to this project that can be considered limitations of the work. One perceived limitation of our list is the heavy concentration on respiratory diagnoses, especially bronchiolitis and asthma. We do not feel this is a genuine limitation, as the recommendations were partly driven by volume and costs as assessed by the KID database. Among the top 10 acute inpatient diagnoses in pediatrics, respiratory diagnoses are the most common, including bronchiolitis, pneumonia, and asthma. Pneumonia or bronchiolitis has been the most common medical diagnosis in inpatient pediatrics for the past decade, and both are always in the top 10 for costs as well.[54] Thus, the impact of decreasing overuse for these conditions will be highly significant from a simple volume standpoint.
The primary limitation of this work is the lack of implementation strategies. Although the Choosing Wisely campaign has plans for dissemination of the lists, compliance with the recommendations may be suboptimal. Although the development process followed an accepted methodology, shortcomings include the lack of wide, local, multidisciplinary (including parents or caretakers) consultation. Other barriers to compliance with these recommendations exist. Despite evidence that bronchiolitis is a benign self‐limited disease that does not respond to bronchodilators and steroids, the drive to identify and correct all abnormalities, such as wheezing or low oxygen saturation in a nontoxic infant with bronchiolitis, seems to trump the obligation to do no harm in daily practice.[55] This behavior may result from pressure by patients, families, nurses, or peers and is deeply embedded in our medical culture, where action is preferred to inaction without full knowledge or consideration of risks. Doctors and nurses have become attached to the pulse oximeter, believing somehow that the number displayed is less subjective and holds more predictive value than careful evaluation of the patient's respiratory status. Other pressures, such as direct to consumer marketing have made acid reflux a household term that is easily treated with over‐the‐counter medications. Considerations of the care continuum will also serve as barriers. Chest x‐rays, for example, are frequently obtained prior to admission to the hospital before the hospitalist is involved.
To overcome these limitations, the study of individual and organizational adoption of innovation might be relevant. Though it is complex and often more descriptive than proscriptive, a few salient features have emerged. Champions and opinion leaders make a difference, local culture is dominant, social networking is important, simple innovations that can be trialed on a small scale are adaptable by the user and have observable benefits, are more likely to be adopted.[56] Fortunately, the top 5 list meets many of these criteria, but also faces the daunting challenges of inertia, lack of financial incentive, inability to break with old habits, and fear of lawsuits and perceived patient/parent dissatisfaction. Ongoing evaluation, feedback, and audit will be necessary to detect and sustain change.
CONCLUSION
We have identified 5 tests or therapies overused in inpatient general pediatrics. One goal of the Choosing Wisely campaign is to begin to change social norms related to physician behavior. We hope by asking clinicians to consider doing less for common conditions in inpatient pediatrics, that they will increasingly consider the known and unanticipated risks of any medical interventions they choose to use. Finally, we would like to encourage all pediatricians to embrace the idea of good stewardship and join us in prioritizing and addressing waste and overuse as important patient safety issues as well as threats to the sustainability of our healthcare system.
Acknowledgments
The authors thank Drs. Doug Carlson, James O'Callaghan, and Karen Smith from the Society of Hospital Medicine's Pediatric and Quality and Safety Committees for their support of this effort.
Disclosure: Nothing to report.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- , , . Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128:e1596–e1597.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- , , , , , . Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363:2124–2134.
- . Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- , , , et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357:1515–1523.
- National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138.
- , . The chest x‐ray and childhood acute asthma. Aust Clin Rev. 1993;13:153–156.
- , , , . Clinical factors associated with focal infiltrates in wheezing infants and toddlers. Clin Pediatr (Phila). 2000;39:387–393.
- , , , . Chest radiographs in the pediatric emergency department for children < or = 18 months of age with wheezing. Clin Pediatr (Phila). 1999;38:395–399.
- , , , , , . Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009;124:e29–e36.
- , . Reduce the rads: a quality assurance project on reducing unnecessary chest X‐rays in children with asthma. J Paediatr Child Health. 2005;41:107–111.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- , , , et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150:429–433.
- , , , et al. Incidence and predisposing factors for severe disease in previously healthy term infants experiencing their first episode of bronchiolitis. Acta Paediatr. 2011;100:e17–e23.
- , , , et al. A cost effectiveness analysis of omitting radiography in diagnosis of acute bronchiolitis. Pediatr Pulmonol. 2009;44:122–127.
- , , , et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25–30.
- , , , . Efficacy of bronchodilator therapy in bronchiolitis. A meta‐analysis. Arch Pediatr Adolesc Med. 1996;150:1166–1172.
- , . Efficacy of beta2‐agonists in bronchiolitis: a reappraisal and meta‐analysis. Pediatrics. 1997;100:233–239.
- , . Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2010;(12):CD001266.
- , , , et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 2011;(6):CD003123.
- , , , et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079–2089.
- , , , et al. A multicenter, randomized, double‐blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349:27–35.
- , , , . A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr. 2002;141:818–824.
- , , , et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. N Engl J Med. 2009;360:329–338.
- , , , et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2010;(10):CD004878.
- , , , , . Systemic corticosteroids in infant bronchiolitis: a meta‐analysis. Pediatrics. 2000;105:E44.
- , , , . Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518.
- , , . Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356.
- , , , , . Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet. 1987;1:879–882.
- , , , , . The clinical management of preterm infants with bronchiolitis. Hosp Pediatr. 2013;3:244–250.
- , . Glucocorticoids and growth in asthmatic children. Pediatr Allergy Immunol. 1995;6:145–154.
- , , , , , . Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia. Pediatrics. 2011;127:e255–e263.
- , , , , , . Pediatric gastroesophageal reflux disease and acid‐related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12:348–355.
- , , , , , . Healthcare costs of GERD and acid‐related conditions in pediatric patients, with comparison between histamine‐2 receptor antagonists and proton pump inhibitors. Curr Med Res Opin. 2009;25:2703–2709.
- , , , . Are we overprescribing antireflux medications for infants with regurgitation? Pediatrics. 2007;120:946–949.
- , , , , . Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45:421–427.
- , , , , , . Efficacy of proton‐pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127:925–935.
- . Effectiveness and safety of proton pump inhibitors in infantile gastroesophageal reflux disease. Ann Pharmacother. 2010;44:572–576.
- . Are there risks associated with empric acid suppression treatment of infants and children suspected of having gastroesophageal reflux disease? Hosp Pediatr. 2013;3:16–23.
- , , , . Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics. 2003;111:e45–e51.
- , , , . Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158:527–530.
- , . Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121:470–475.
- . Oxygen therapy for bronchiolitis. Pediatrics. 2007;120:686–687; author reply 687–688.
- , , , , , . Bronchiolitis‐associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446.
- , . Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342–349.
- , , , , . Bronchiolitis‐associated mortality and estimates of respiratory syncytial virus‐associated deaths among US children, 1979–1997. J Infect Dis. 2001;183:16–22.
- , . Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97:361–363.
- , , , et al. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J Pediatr. 2011;159:377–383.e1.
- , . The impact of severe asthma on schoolchildren. J Asthma. 1999;36:409–417.
- , . Multi‐center, randomized trial of pulse oximetry monitoring strategies for children hospitalized for bronchiolitis. Abstract presented at: ID Week 2012; October 2012; San Diego, CA.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- Agency for Healthcare Research and Quality. HCUPnet. Kids inpatient database 2009. Available at: http://hcupnet.ahrq.gov. Accessed November 6, 2012.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- . How to implement change in clinical practice. Paediatr Respir Rev. 2003;4:340–346.
Overuse in medicine is a significant and under‐recognized problem. Don Berwick estimated that waste accounts for at least 20% of healthcare expenditures in the United States, with overtreatment as one of the largest categories.[1] A commentary by Schroeder et al. challenged pediatricians to incorporate this knowledge into our own patient safety and quality movement.[2] Recently published data suggest that we are far from achieving the patient safety goals set forth in the Institute of Medicine's landmark To Err is Human[3] report, despite more than a decade of national, local, and regional efforts.[4] One way to reduce waste and improve patient safety is to eliminate practices of unproven benefit. Therapies or tests that may initially seem promising are often proven to be not only unhelpful but actually harmful. The recommendation of the US Preventive Services Task Force against routine screening for prostate specific antigen is an example of how a common test initially thought of as lifesaving actually increases harm.[5]
The American Board of Internal Medicine Foundation (ABIM‐F) recently announced the Choosing Wisely campaign. Through this campaign the Foundation encourages physicians, patients and other healthcare stakeholders to think and talk about medical tests and procedures that may be unnecessary.[6] The primary output of this challenge is the development of a list of 5 tests and or therapies that physicians and patients should question. The ABIM‐F approached different medical societies to develop these lists within their own specialties. The Society of Hospital Medicine (SHM) joined the Choosing Wisely campaign in April 2012, and agreed to develop a list of 5 therapies and tests for adult hospital medicine and pediatric hospital medicine. Here we present the contribution of the pediatric workgroup detailing the methodology and process for developing the list, as well as summarizing the evidence supporting each recommendation.
METHODS
In the spring of 2012, the pediatric committee of the SHM convened a workgroup of pediatric hospitalists to develop a top 5 list for the field. This workgroup was composed of experienced pediatric hospitalists representing diverse geographic locations of the United States and a mix of academic and nonacademic practice settings. The group, consisting of 4 women and 9 men, began by proposing candidate recommendations after discussion with colleagues at their different practice sites. The group was charged to maintain a focus on overuse practices that had a strong basis in evidence, were frequently encountered at their practice sites, and achieved significant consensus among their colleagues. Figure 1 shows the process map describing the method for the development of the pediatric recommendations. All workgroup participants were queried as to conflict of interest relevant to this work and none were identified.

Literature Review
After the generation of the initial top 20 list, 2 reviewers conducted independent literature searches in PubMed, MEDLINE, and the Cochrane Library on the proposed topics. The reviewers also conducted generic Internet searches. Key search terms included pediatric asthma, bronchiolitis, chest radiograph, systemic corticosteroids, gastroesophageal reflux disease (GERD), infant, child, acid suppression therapy, continuous pulse oximetry, pneumonia, gastroenteritis, viral testing, blood culture, and soft tissue infections. To ensure that the reviewers included all studies relevant to the searches, they utilized broad terms. The search included all literature published through 2012, and nonEnglish language publications were included in the search. Studies selected and included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, included a pediatric population in the guidelines or study, reviewed the harm associated with the administration of a particular test or treatment, and explored the cost associated with the test or treatment.
The Delphi Panel
Members of the workgroup formed a Delphi panel except for 1 member (R.Q.) who served as the nonvoting moderator. The members of the Delphi panel considered the results of the literature search for each recommendation along with the collated feedback from hospitalist listserves as described in Figure 1. Each panel member received a voting instrument with the candidate tests and treatments for the first round of Delphi voting. The panel utilized a modified Delphi method or the RAND Corporation (RAND)/University of California at Los Angeles (UCLA) appropriateness method as described in previous publications of quality indicator development in pediatrics.[7] Each panelist scored the candidate tests and treatments and forwarded the scores to the moderator. Subsequently, all the members of the Delphi panel met through a conference call to carry out the second round of voting. The deidentified collated results of the first round of Delphi voting were made available and discussed during the call. The moderator collated the final results, and the final 5 recommendations were those that had the highest score after the second round of Delphi voting.
Volume and Costs
During deliberations, the committee took into account the prevalence and cost rankings of our most common pediatric inpatient diagnoses. This was done using the Agency for Healthcare Research and Quality's (AHRQ) Healthcare Utilization Project (HCUP), specifically, the Kids' Inpatient Database (KID). HCUP includes the largest collection of longitudinal hospital care data in the United States, encompassing all‐payer discharge‐level information. We excluded normal newborn hospitalizations, and looked at the top 10 acute inpatient diagnoses in terms of both volume and aggregate costs.
RESULTS
The initial list of 20 candidate tests and treatments as well as the refined list of 11 recommendations can be found as electronic supplements to this publication (see Supporting Table 1 and Supporting Table 2 in the online version of this article). The format and language of the list of 11 recommendations were chosen to mesh with that typically used in the ABIM‐F Choosing Wisely campaign. During the Delphi panel, there was strong group consensus about combining items 1 and 2 (chest radiographs in asthma and bronchiolitis) into a single recommendation.
| Do not order chest radiographs in children with asthma or bronchiolitis. |
| Do not use bronchodilators in children with bronchiolitis. |
| Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection. |
| Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
The top 5 recommendations based on the result of the second round of Delphi scoring are shown in Table 1 and described below along with a detailed evidence summary.
Do not order chest radiographs in children with asthma or bronchiolitis.
The National Heart and Lung Institute's guidelines for the management of asthma, published in 1987, recommend against routinely obtaining chest radiographs in patients with asthma or asthma exacerbations.[8] Supporting this recommendation are several studies that show a low overall yield when obtaining chest radiographs for wheezing patients.[9, 10, 11] Most relevant, studies that evaluated the clinical utility of radiographs in patients with asthma have demonstrated that they influence clinical management in less than 2% of cases.[12] A quality improvement project aimed at decreasing the rate of chest radiographs obtained in patients with asthma demonstrated that close to 60% of patients admitted to the hospital had chest radiographs performed, and that significant overall reductions can be achieved (45.3%28.9%, P=0.0005) without impacting clinical outcomes negatively.[13]
Similarly, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely obtaining radiographs during the evaluation for bronchiolitis.[14] Studies assessing the utility of chest x‐rays in these children demonstrate an even lower incidence of abnormalities (0.75%) and indicate that, despite this low incidence, physicians are more likely to treat with antibiotics when radiographs are obtained.[15] There is also evidence that chest radiographs in patients with bronchiolitis are not useful in predicting severity of illness.[16] Furthermore, cost‐effective analyses have demonstrated that omitting chest radiographs in bronchiolitis is actually cost‐effective, without compromising diagnostic accuracy.[17] In a recently published national benchmarking inpatient collaborative, Ralston et al. demonstrated that the majority of patients admitted to the hospital with bronchiolitis have chest radiographs performed at a rate of 64% (interquartile range [IQR], 54%81%).[18]
In both bronchiolitis and asthma, the elimination of unnecessary radiographs has the potential to decrease costs, reduce radiation exposure, and minimize the overuse of antibiotics that often occurs secondary to false positive results.
Do not use bronchodilators in children with bronchiolitis.
Ralston showed that 70% (IQR, 59%83%) of admitted bronchiolitis patients received bronchodilators with an average of 7.9 doses per patient (IQR, 4.69.8). National guidelines for bronchiolitis suggest a very limited role of bronchodilators in patients with bronchiolitis.[14] The first meta‐analyses of studies related to the question of ‐agonist efficacy in bronchiolitis were published in the late 1990s, revealing minimal or no treatment effects.[19, 20] Since then, further research has solidified these findings, and fairly definitive statements can be made based on a recent comprehensive meta‐analysis.[21] The pooled data do not show any effect on hospitalization rates, hospital length of stay, or other inpatient outcomes in bronchiolitis. They do show a small change in clinical scores documented in the outpatient setting, though these scores have not correlated with any detectable difference in outcomes. Routine use of ‐agonists in the inpatient setting has no proven benefit, and given the large amount of consistent data, there is no compelling reason for further study of this therapy in the inpatient setting.
Epinephrine, a combined ‐ and ‐agonist, has been extensively evaluated in bronchiolitis as well. Like albuterol, epinephrine has been reported to have no effect on hospital length of stay in bronchiolitis.[22] The issue of admission rates after epinephrine is complicated by 1 very large study that combined epinephrine with dexamethasone and reported a decreased admission rate, though only at 7 days after therapy; however, this effect was nullified after adjustment for multiple comparisons.[23] When the end point is improvement of respiratory scores, epinephrine may perform better than albuterol in studies where they are directly compared; however, there is no evidence that repeated usage of epinephrine has any impact on any clinical outcome for inpatients.[24, 25]
Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection
In their summary of evidence, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely using systemic corticosteroids for infants with bronchiolitis.[14] The previously reference bronchiolitis benchmarking study demonstrated that admitted patients received steroids at a rate of 21% (IQR, 14%26%). The poor efficacy of corticosteroids in children with bronchiolitis under 2 years of age is well demonstrated in the literature. A large, blinded, randomized, controlled study compared systemic oral corticosteroids to placebo in hospitalized children 10 months to 6 years of age with viral wheezing.[26] This study showed no benefit of corticosteroids over placebo in length of stay or parental report of symptoms 1 week later. In the study, a subanalysis of children with eczema and family history of asthma also demonstrated no benefit of systemic corticosteroids. Large systematic reviews further argue that there is no effect of corticosteroids on the likelihood of admission or length of stay in infants with bronchiolitis.[27, 28] One 4‐armed prospective study of children 6 weeks to 12 months of age found no efficacy of dexamethasone over placebo.[23] There was modest benefit of dexamethasone in conjunction with racemic epinephrine; however, this benefit disappeared after adjustment for multiple comparisons. Three smaller studies showing benefit of systemic corticosteroids, however, were highly problematic. They have included older children, were retrospective, or demonstrated inconsistent results.[29, 30] A smaller study showed benefit for children over 2 years of age, but none for children under 2 years of age.[31] Premature infants are at increased risk of asthma, which typically responds well to corticosteroids as these children get older. However, a retrospective study of premature infants under 2 years of age with bronchiolitis demonstrated no association between corticosteroid use and length of stay, even in the subset of premature infants responding to albuterol.[32]
Systemic corticosteroid use in children is not harmless. Children under 2 years of age are especially vulnerable to the decreased growth velocity seen as a side effect of systemic corticosteroids.[33] Corticosteroids may also negatively impact the course of infectious illness. For instance, in children hospitalized with pneumonia but not receiving ‐agonists (ie, patients who are unlikely to have asthma), length of stay is prolonged and readmission is higher in those who receive corticosteroids.[34]
Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy.
From 2000 to 2005, the incidence of infants diagnosed with gastroeshopaheal reflux (GER) tripled (3.4%12.3%), and the use of proton pump inhibitors (PPIs) doubled (31.5%62.6%).[35] Patients diagnosed with GER and treated with antireflux medication incurred 1.8 times higher healthcare costs in 1 study compared to healthy controls.[36] Though common, the use of acid suppressive medications in infants lacks evidence for efficacy in the majority of the clinical scenarios in which they are prescribed.[37, 38] PPIs have failed to outperform placebo for typical infant reflux, which is generally developmental and not pathologic.[39, 40] Furthermore, prompted by findings in adults, multiple pediatric investigators have now catalogued the potential risks associated with acid blockade in children in multiple clinical settings. Specifically, increased risk of pneumonia has been documented in inpatients and outpatients, and increased risk of necrotizing enterocolitis and other serious infections have been documented in intensive care unit settings.[41] In the absence of data supporting efficacy and given the emerging data on risk, empiric acid suppression in infants with reflux is wasteful and potentially harmful.
Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
Pulse oximetry use has become widespread in the management of infants with bronchiolitis and likely accounts for the dramatic increase in bronchiolitis hospitalization rates in recent years.[14, 42, 43, 44, 45, 46, 47] Despite this increase in hospitalization rate, there was no change in mortality from bronchiolitis between 1979 and 1997.[48] The continuous monitoring of oxygen saturations in hospitalized infants with bronchiolitis may lead to overdiagnosis of hypoxemia and subsequent oxygen use that is of no apparent benefit to the child. Schroeder et al. demonstrated that 26% of a sample of infants hospitalized with bronchiolitis had a prolonged length of stay because of a perceived need for oxygen based on pulse oximetry readings.[43] Unger and Cunningham showed that the need for oxygen was the final determinant of length of stay in 58% of cases, and Cunningham and Murray suggested that using an oxygen saturation cutoff of 94% instead of 90% might increase the length of stay by 22 hours.[44, 49]
It has been previously shown that hypoxia is normative in infants. Healthy infants experience multiple episodes of SpO2 90% while sleeping.[50] This finding strengthens the notion that detection of low saturations in infants convalescing from bronchiolitis may simply reflect overdiagnosis. Among children with chronic severe asthma, who presumably have experienced episodes of hypoxia throughout childhood, there is no difference in school performance compared to healthy controls.[51]
The practice parameter on bronchiolitis from the American Academy of Pediatrics states: as the child's clinical course improves, continuous measurement of SpO2 is not routinely needed, which is a recommendation based on expert consensus.[14] There is at least one ongoing randomized trial comparing the use of continuous versus intermittent pulse oximetry in hospitalized infants with bronchiolitis who are weaned off oxygen (
DISCUSSION
Berwick and Hackbarth define overtreatment as: waste that comes from subjecting patients to care that, according to sound science and the patients' own preferences, cannot possibly help themcare rooted in outmoded habits, supply‐driven behaviors, and ignoring science.[1] With this project, we tried to capture common clinical sources of waste in the inpatient pediatric setting. This is an inherently difficult project because of the absence of solid evidence to inform every decision point in medicine. Although there is always room for improvement in our evidence base, our group intentionally gravitated to areas where the evidence was robust.
The primary strength of this work is the use of the RAND/UCLA appropriateness method or modified Delphi method. Several publications have validated this methodology as a sound strategy to assess quality indicators and issues related to overuse.[7, 53] To our knowledge, we are the first group to report the use of this methodology to develop a list such as the list reported here.
There were some challenges inherent to this project that can be considered limitations of the work. One perceived limitation of our list is the heavy concentration on respiratory diagnoses, especially bronchiolitis and asthma. We do not feel this is a genuine limitation, as the recommendations were partly driven by volume and costs as assessed by the KID database. Among the top 10 acute inpatient diagnoses in pediatrics, respiratory diagnoses are the most common, including bronchiolitis, pneumonia, and asthma. Pneumonia or bronchiolitis has been the most common medical diagnosis in inpatient pediatrics for the past decade, and both are always in the top 10 for costs as well.[54] Thus, the impact of decreasing overuse for these conditions will be highly significant from a simple volume standpoint.
The primary limitation of this work is the lack of implementation strategies. Although the Choosing Wisely campaign has plans for dissemination of the lists, compliance with the recommendations may be suboptimal. Although the development process followed an accepted methodology, shortcomings include the lack of wide, local, multidisciplinary (including parents or caretakers) consultation. Other barriers to compliance with these recommendations exist. Despite evidence that bronchiolitis is a benign self‐limited disease that does not respond to bronchodilators and steroids, the drive to identify and correct all abnormalities, such as wheezing or low oxygen saturation in a nontoxic infant with bronchiolitis, seems to trump the obligation to do no harm in daily practice.[55] This behavior may result from pressure by patients, families, nurses, or peers and is deeply embedded in our medical culture, where action is preferred to inaction without full knowledge or consideration of risks. Doctors and nurses have become attached to the pulse oximeter, believing somehow that the number displayed is less subjective and holds more predictive value than careful evaluation of the patient's respiratory status. Other pressures, such as direct to consumer marketing have made acid reflux a household term that is easily treated with over‐the‐counter medications. Considerations of the care continuum will also serve as barriers. Chest x‐rays, for example, are frequently obtained prior to admission to the hospital before the hospitalist is involved.
To overcome these limitations, the study of individual and organizational adoption of innovation might be relevant. Though it is complex and often more descriptive than proscriptive, a few salient features have emerged. Champions and opinion leaders make a difference, local culture is dominant, social networking is important, simple innovations that can be trialed on a small scale are adaptable by the user and have observable benefits, are more likely to be adopted.[56] Fortunately, the top 5 list meets many of these criteria, but also faces the daunting challenges of inertia, lack of financial incentive, inability to break with old habits, and fear of lawsuits and perceived patient/parent dissatisfaction. Ongoing evaluation, feedback, and audit will be necessary to detect and sustain change.
CONCLUSION
We have identified 5 tests or therapies overused in inpatient general pediatrics. One goal of the Choosing Wisely campaign is to begin to change social norms related to physician behavior. We hope by asking clinicians to consider doing less for common conditions in inpatient pediatrics, that they will increasingly consider the known and unanticipated risks of any medical interventions they choose to use. Finally, we would like to encourage all pediatricians to embrace the idea of good stewardship and join us in prioritizing and addressing waste and overuse as important patient safety issues as well as threats to the sustainability of our healthcare system.
Acknowledgments
The authors thank Drs. Doug Carlson, James O'Callaghan, and Karen Smith from the Society of Hospital Medicine's Pediatric and Quality and Safety Committees for their support of this effort.
Disclosure: Nothing to report.
Overuse in medicine is a significant and under‐recognized problem. Don Berwick estimated that waste accounts for at least 20% of healthcare expenditures in the United States, with overtreatment as one of the largest categories.[1] A commentary by Schroeder et al. challenged pediatricians to incorporate this knowledge into our own patient safety and quality movement.[2] Recently published data suggest that we are far from achieving the patient safety goals set forth in the Institute of Medicine's landmark To Err is Human[3] report, despite more than a decade of national, local, and regional efforts.[4] One way to reduce waste and improve patient safety is to eliminate practices of unproven benefit. Therapies or tests that may initially seem promising are often proven to be not only unhelpful but actually harmful. The recommendation of the US Preventive Services Task Force against routine screening for prostate specific antigen is an example of how a common test initially thought of as lifesaving actually increases harm.[5]
The American Board of Internal Medicine Foundation (ABIM‐F) recently announced the Choosing Wisely campaign. Through this campaign the Foundation encourages physicians, patients and other healthcare stakeholders to think and talk about medical tests and procedures that may be unnecessary.[6] The primary output of this challenge is the development of a list of 5 tests and or therapies that physicians and patients should question. The ABIM‐F approached different medical societies to develop these lists within their own specialties. The Society of Hospital Medicine (SHM) joined the Choosing Wisely campaign in April 2012, and agreed to develop a list of 5 therapies and tests for adult hospital medicine and pediatric hospital medicine. Here we present the contribution of the pediatric workgroup detailing the methodology and process for developing the list, as well as summarizing the evidence supporting each recommendation.
METHODS
In the spring of 2012, the pediatric committee of the SHM convened a workgroup of pediatric hospitalists to develop a top 5 list for the field. This workgroup was composed of experienced pediatric hospitalists representing diverse geographic locations of the United States and a mix of academic and nonacademic practice settings. The group, consisting of 4 women and 9 men, began by proposing candidate recommendations after discussion with colleagues at their different practice sites. The group was charged to maintain a focus on overuse practices that had a strong basis in evidence, were frequently encountered at their practice sites, and achieved significant consensus among their colleagues. Figure 1 shows the process map describing the method for the development of the pediatric recommendations. All workgroup participants were queried as to conflict of interest relevant to this work and none were identified.

Literature Review
After the generation of the initial top 20 list, 2 reviewers conducted independent literature searches in PubMed, MEDLINE, and the Cochrane Library on the proposed topics. The reviewers also conducted generic Internet searches. Key search terms included pediatric asthma, bronchiolitis, chest radiograph, systemic corticosteroids, gastroesophageal reflux disease (GERD), infant, child, acid suppression therapy, continuous pulse oximetry, pneumonia, gastroenteritis, viral testing, blood culture, and soft tissue infections. To ensure that the reviewers included all studies relevant to the searches, they utilized broad terms. The search included all literature published through 2012, and nonEnglish language publications were included in the search. Studies selected and included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, included a pediatric population in the guidelines or study, reviewed the harm associated with the administration of a particular test or treatment, and explored the cost associated with the test or treatment.
The Delphi Panel
Members of the workgroup formed a Delphi panel except for 1 member (R.Q.) who served as the nonvoting moderator. The members of the Delphi panel considered the results of the literature search for each recommendation along with the collated feedback from hospitalist listserves as described in Figure 1. Each panel member received a voting instrument with the candidate tests and treatments for the first round of Delphi voting. The panel utilized a modified Delphi method or the RAND Corporation (RAND)/University of California at Los Angeles (UCLA) appropriateness method as described in previous publications of quality indicator development in pediatrics.[7] Each panelist scored the candidate tests and treatments and forwarded the scores to the moderator. Subsequently, all the members of the Delphi panel met through a conference call to carry out the second round of voting. The deidentified collated results of the first round of Delphi voting were made available and discussed during the call. The moderator collated the final results, and the final 5 recommendations were those that had the highest score after the second round of Delphi voting.
Volume and Costs
During deliberations, the committee took into account the prevalence and cost rankings of our most common pediatric inpatient diagnoses. This was done using the Agency for Healthcare Research and Quality's (AHRQ) Healthcare Utilization Project (HCUP), specifically, the Kids' Inpatient Database (KID). HCUP includes the largest collection of longitudinal hospital care data in the United States, encompassing all‐payer discharge‐level information. We excluded normal newborn hospitalizations, and looked at the top 10 acute inpatient diagnoses in terms of both volume and aggregate costs.
RESULTS
The initial list of 20 candidate tests and treatments as well as the refined list of 11 recommendations can be found as electronic supplements to this publication (see Supporting Table 1 and Supporting Table 2 in the online version of this article). The format and language of the list of 11 recommendations were chosen to mesh with that typically used in the ABIM‐F Choosing Wisely campaign. During the Delphi panel, there was strong group consensus about combining items 1 and 2 (chest radiographs in asthma and bronchiolitis) into a single recommendation.
| Do not order chest radiographs in children with asthma or bronchiolitis. |
| Do not use bronchodilators in children with bronchiolitis. |
| Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection. |
| Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
The top 5 recommendations based on the result of the second round of Delphi scoring are shown in Table 1 and described below along with a detailed evidence summary.
Do not order chest radiographs in children with asthma or bronchiolitis.
The National Heart and Lung Institute's guidelines for the management of asthma, published in 1987, recommend against routinely obtaining chest radiographs in patients with asthma or asthma exacerbations.[8] Supporting this recommendation are several studies that show a low overall yield when obtaining chest radiographs for wheezing patients.[9, 10, 11] Most relevant, studies that evaluated the clinical utility of radiographs in patients with asthma have demonstrated that they influence clinical management in less than 2% of cases.[12] A quality improvement project aimed at decreasing the rate of chest radiographs obtained in patients with asthma demonstrated that close to 60% of patients admitted to the hospital had chest radiographs performed, and that significant overall reductions can be achieved (45.3%28.9%, P=0.0005) without impacting clinical outcomes negatively.[13]
Similarly, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely obtaining radiographs during the evaluation for bronchiolitis.[14] Studies assessing the utility of chest x‐rays in these children demonstrate an even lower incidence of abnormalities (0.75%) and indicate that, despite this low incidence, physicians are more likely to treat with antibiotics when radiographs are obtained.[15] There is also evidence that chest radiographs in patients with bronchiolitis are not useful in predicting severity of illness.[16] Furthermore, cost‐effective analyses have demonstrated that omitting chest radiographs in bronchiolitis is actually cost‐effective, without compromising diagnostic accuracy.[17] In a recently published national benchmarking inpatient collaborative, Ralston et al. demonstrated that the majority of patients admitted to the hospital with bronchiolitis have chest radiographs performed at a rate of 64% (interquartile range [IQR], 54%81%).[18]
In both bronchiolitis and asthma, the elimination of unnecessary radiographs has the potential to decrease costs, reduce radiation exposure, and minimize the overuse of antibiotics that often occurs secondary to false positive results.
Do not use bronchodilators in children with bronchiolitis.
Ralston showed that 70% (IQR, 59%83%) of admitted bronchiolitis patients received bronchodilators with an average of 7.9 doses per patient (IQR, 4.69.8). National guidelines for bronchiolitis suggest a very limited role of bronchodilators in patients with bronchiolitis.[14] The first meta‐analyses of studies related to the question of ‐agonist efficacy in bronchiolitis were published in the late 1990s, revealing minimal or no treatment effects.[19, 20] Since then, further research has solidified these findings, and fairly definitive statements can be made based on a recent comprehensive meta‐analysis.[21] The pooled data do not show any effect on hospitalization rates, hospital length of stay, or other inpatient outcomes in bronchiolitis. They do show a small change in clinical scores documented in the outpatient setting, though these scores have not correlated with any detectable difference in outcomes. Routine use of ‐agonists in the inpatient setting has no proven benefit, and given the large amount of consistent data, there is no compelling reason for further study of this therapy in the inpatient setting.
Epinephrine, a combined ‐ and ‐agonist, has been extensively evaluated in bronchiolitis as well. Like albuterol, epinephrine has been reported to have no effect on hospital length of stay in bronchiolitis.[22] The issue of admission rates after epinephrine is complicated by 1 very large study that combined epinephrine with dexamethasone and reported a decreased admission rate, though only at 7 days after therapy; however, this effect was nullified after adjustment for multiple comparisons.[23] When the end point is improvement of respiratory scores, epinephrine may perform better than albuterol in studies where they are directly compared; however, there is no evidence that repeated usage of epinephrine has any impact on any clinical outcome for inpatients.[24, 25]
Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection
In their summary of evidence, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely using systemic corticosteroids for infants with bronchiolitis.[14] The previously reference bronchiolitis benchmarking study demonstrated that admitted patients received steroids at a rate of 21% (IQR, 14%26%). The poor efficacy of corticosteroids in children with bronchiolitis under 2 years of age is well demonstrated in the literature. A large, blinded, randomized, controlled study compared systemic oral corticosteroids to placebo in hospitalized children 10 months to 6 years of age with viral wheezing.[26] This study showed no benefit of corticosteroids over placebo in length of stay or parental report of symptoms 1 week later. In the study, a subanalysis of children with eczema and family history of asthma also demonstrated no benefit of systemic corticosteroids. Large systematic reviews further argue that there is no effect of corticosteroids on the likelihood of admission or length of stay in infants with bronchiolitis.[27, 28] One 4‐armed prospective study of children 6 weeks to 12 months of age found no efficacy of dexamethasone over placebo.[23] There was modest benefit of dexamethasone in conjunction with racemic epinephrine; however, this benefit disappeared after adjustment for multiple comparisons. Three smaller studies showing benefit of systemic corticosteroids, however, were highly problematic. They have included older children, were retrospective, or demonstrated inconsistent results.[29, 30] A smaller study showed benefit for children over 2 years of age, but none for children under 2 years of age.[31] Premature infants are at increased risk of asthma, which typically responds well to corticosteroids as these children get older. However, a retrospective study of premature infants under 2 years of age with bronchiolitis demonstrated no association between corticosteroid use and length of stay, even in the subset of premature infants responding to albuterol.[32]
Systemic corticosteroid use in children is not harmless. Children under 2 years of age are especially vulnerable to the decreased growth velocity seen as a side effect of systemic corticosteroids.[33] Corticosteroids may also negatively impact the course of infectious illness. For instance, in children hospitalized with pneumonia but not receiving ‐agonists (ie, patients who are unlikely to have asthma), length of stay is prolonged and readmission is higher in those who receive corticosteroids.[34]
Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy.
From 2000 to 2005, the incidence of infants diagnosed with gastroeshopaheal reflux (GER) tripled (3.4%12.3%), and the use of proton pump inhibitors (PPIs) doubled (31.5%62.6%).[35] Patients diagnosed with GER and treated with antireflux medication incurred 1.8 times higher healthcare costs in 1 study compared to healthy controls.[36] Though common, the use of acid suppressive medications in infants lacks evidence for efficacy in the majority of the clinical scenarios in which they are prescribed.[37, 38] PPIs have failed to outperform placebo for typical infant reflux, which is generally developmental and not pathologic.[39, 40] Furthermore, prompted by findings in adults, multiple pediatric investigators have now catalogued the potential risks associated with acid blockade in children in multiple clinical settings. Specifically, increased risk of pneumonia has been documented in inpatients and outpatients, and increased risk of necrotizing enterocolitis and other serious infections have been documented in intensive care unit settings.[41] In the absence of data supporting efficacy and given the emerging data on risk, empiric acid suppression in infants with reflux is wasteful and potentially harmful.
Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
Pulse oximetry use has become widespread in the management of infants with bronchiolitis and likely accounts for the dramatic increase in bronchiolitis hospitalization rates in recent years.[14, 42, 43, 44, 45, 46, 47] Despite this increase in hospitalization rate, there was no change in mortality from bronchiolitis between 1979 and 1997.[48] The continuous monitoring of oxygen saturations in hospitalized infants with bronchiolitis may lead to overdiagnosis of hypoxemia and subsequent oxygen use that is of no apparent benefit to the child. Schroeder et al. demonstrated that 26% of a sample of infants hospitalized with bronchiolitis had a prolonged length of stay because of a perceived need for oxygen based on pulse oximetry readings.[43] Unger and Cunningham showed that the need for oxygen was the final determinant of length of stay in 58% of cases, and Cunningham and Murray suggested that using an oxygen saturation cutoff of 94% instead of 90% might increase the length of stay by 22 hours.[44, 49]
It has been previously shown that hypoxia is normative in infants. Healthy infants experience multiple episodes of SpO2 90% while sleeping.[50] This finding strengthens the notion that detection of low saturations in infants convalescing from bronchiolitis may simply reflect overdiagnosis. Among children with chronic severe asthma, who presumably have experienced episodes of hypoxia throughout childhood, there is no difference in school performance compared to healthy controls.[51]
The practice parameter on bronchiolitis from the American Academy of Pediatrics states: as the child's clinical course improves, continuous measurement of SpO2 is not routinely needed, which is a recommendation based on expert consensus.[14] There is at least one ongoing randomized trial comparing the use of continuous versus intermittent pulse oximetry in hospitalized infants with bronchiolitis who are weaned off oxygen (
DISCUSSION
Berwick and Hackbarth define overtreatment as: waste that comes from subjecting patients to care that, according to sound science and the patients' own preferences, cannot possibly help themcare rooted in outmoded habits, supply‐driven behaviors, and ignoring science.[1] With this project, we tried to capture common clinical sources of waste in the inpatient pediatric setting. This is an inherently difficult project because of the absence of solid evidence to inform every decision point in medicine. Although there is always room for improvement in our evidence base, our group intentionally gravitated to areas where the evidence was robust.
The primary strength of this work is the use of the RAND/UCLA appropriateness method or modified Delphi method. Several publications have validated this methodology as a sound strategy to assess quality indicators and issues related to overuse.[7, 53] To our knowledge, we are the first group to report the use of this methodology to develop a list such as the list reported here.
There were some challenges inherent to this project that can be considered limitations of the work. One perceived limitation of our list is the heavy concentration on respiratory diagnoses, especially bronchiolitis and asthma. We do not feel this is a genuine limitation, as the recommendations were partly driven by volume and costs as assessed by the KID database. Among the top 10 acute inpatient diagnoses in pediatrics, respiratory diagnoses are the most common, including bronchiolitis, pneumonia, and asthma. Pneumonia or bronchiolitis has been the most common medical diagnosis in inpatient pediatrics for the past decade, and both are always in the top 10 for costs as well.[54] Thus, the impact of decreasing overuse for these conditions will be highly significant from a simple volume standpoint.
The primary limitation of this work is the lack of implementation strategies. Although the Choosing Wisely campaign has plans for dissemination of the lists, compliance with the recommendations may be suboptimal. Although the development process followed an accepted methodology, shortcomings include the lack of wide, local, multidisciplinary (including parents or caretakers) consultation. Other barriers to compliance with these recommendations exist. Despite evidence that bronchiolitis is a benign self‐limited disease that does not respond to bronchodilators and steroids, the drive to identify and correct all abnormalities, such as wheezing or low oxygen saturation in a nontoxic infant with bronchiolitis, seems to trump the obligation to do no harm in daily practice.[55] This behavior may result from pressure by patients, families, nurses, or peers and is deeply embedded in our medical culture, where action is preferred to inaction without full knowledge or consideration of risks. Doctors and nurses have become attached to the pulse oximeter, believing somehow that the number displayed is less subjective and holds more predictive value than careful evaluation of the patient's respiratory status. Other pressures, such as direct to consumer marketing have made acid reflux a household term that is easily treated with over‐the‐counter medications. Considerations of the care continuum will also serve as barriers. Chest x‐rays, for example, are frequently obtained prior to admission to the hospital before the hospitalist is involved.
To overcome these limitations, the study of individual and organizational adoption of innovation might be relevant. Though it is complex and often more descriptive than proscriptive, a few salient features have emerged. Champions and opinion leaders make a difference, local culture is dominant, social networking is important, simple innovations that can be trialed on a small scale are adaptable by the user and have observable benefits, are more likely to be adopted.[56] Fortunately, the top 5 list meets many of these criteria, but also faces the daunting challenges of inertia, lack of financial incentive, inability to break with old habits, and fear of lawsuits and perceived patient/parent dissatisfaction. Ongoing evaluation, feedback, and audit will be necessary to detect and sustain change.
CONCLUSION
We have identified 5 tests or therapies overused in inpatient general pediatrics. One goal of the Choosing Wisely campaign is to begin to change social norms related to physician behavior. We hope by asking clinicians to consider doing less for common conditions in inpatient pediatrics, that they will increasingly consider the known and unanticipated risks of any medical interventions they choose to use. Finally, we would like to encourage all pediatricians to embrace the idea of good stewardship and join us in prioritizing and addressing waste and overuse as important patient safety issues as well as threats to the sustainability of our healthcare system.
Acknowledgments
The authors thank Drs. Doug Carlson, James O'Callaghan, and Karen Smith from the Society of Hospital Medicine's Pediatric and Quality and Safety Committees for their support of this effort.
Disclosure: Nothing to report.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- , , . Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128:e1596–e1597.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- , , , , , . Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363:2124–2134.
- . Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- , , , et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357:1515–1523.
- National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138.
- , . The chest x‐ray and childhood acute asthma. Aust Clin Rev. 1993;13:153–156.
- , , , . Clinical factors associated with focal infiltrates in wheezing infants and toddlers. Clin Pediatr (Phila). 2000;39:387–393.
- , , , . Chest radiographs in the pediatric emergency department for children < or = 18 months of age with wheezing. Clin Pediatr (Phila). 1999;38:395–399.
- , , , , , . Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009;124:e29–e36.
- , . Reduce the rads: a quality assurance project on reducing unnecessary chest X‐rays in children with asthma. J Paediatr Child Health. 2005;41:107–111.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- , , , et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150:429–433.
- , , , et al. Incidence and predisposing factors for severe disease in previously healthy term infants experiencing their first episode of bronchiolitis. Acta Paediatr. 2011;100:e17–e23.
- , , , et al. A cost effectiveness analysis of omitting radiography in diagnosis of acute bronchiolitis. Pediatr Pulmonol. 2009;44:122–127.
- , , , et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25–30.
- , , , . Efficacy of bronchodilator therapy in bronchiolitis. A meta‐analysis. Arch Pediatr Adolesc Med. 1996;150:1166–1172.
- , . Efficacy of beta2‐agonists in bronchiolitis: a reappraisal and meta‐analysis. Pediatrics. 1997;100:233–239.
- , . Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2010;(12):CD001266.
- , , , et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 2011;(6):CD003123.
- , , , et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079–2089.
- , , , et al. A multicenter, randomized, double‐blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349:27–35.
- , , , . A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr. 2002;141:818–824.
- , , , et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. N Engl J Med. 2009;360:329–338.
- , , , et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2010;(10):CD004878.
- , , , , . Systemic corticosteroids in infant bronchiolitis: a meta‐analysis. Pediatrics. 2000;105:E44.
- , , , . Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518.
- , , . Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356.
- , , , , . Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet. 1987;1:879–882.
- , , , , . The clinical management of preterm infants with bronchiolitis. Hosp Pediatr. 2013;3:244–250.
- , . Glucocorticoids and growth in asthmatic children. Pediatr Allergy Immunol. 1995;6:145–154.
- , , , , , . Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia. Pediatrics. 2011;127:e255–e263.
- , , , , , . Pediatric gastroesophageal reflux disease and acid‐related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12:348–355.
- , , , , , . Healthcare costs of GERD and acid‐related conditions in pediatric patients, with comparison between histamine‐2 receptor antagonists and proton pump inhibitors. Curr Med Res Opin. 2009;25:2703–2709.
- , , , . Are we overprescribing antireflux medications for infants with regurgitation? Pediatrics. 2007;120:946–949.
- , , , , . Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45:421–427.
- , , , , , . Efficacy of proton‐pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127:925–935.
- . Effectiveness and safety of proton pump inhibitors in infantile gastroesophageal reflux disease. Ann Pharmacother. 2010;44:572–576.
- . Are there risks associated with empric acid suppression treatment of infants and children suspected of having gastroesophageal reflux disease? Hosp Pediatr. 2013;3:16–23.
- , , , . Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics. 2003;111:e45–e51.
- , , , . Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158:527–530.
- , . Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121:470–475.
- . Oxygen therapy for bronchiolitis. Pediatrics. 2007;120:686–687; author reply 687–688.
- , , , , , . Bronchiolitis‐associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446.
- , . Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342–349.
- , , , , . Bronchiolitis‐associated mortality and estimates of respiratory syncytial virus‐associated deaths among US children, 1979–1997. J Infect Dis. 2001;183:16–22.
- , . Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97:361–363.
- , , , et al. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J Pediatr. 2011;159:377–383.e1.
- , . The impact of severe asthma on schoolchildren. J Asthma. 1999;36:409–417.
- , . Multi‐center, randomized trial of pulse oximetry monitoring strategies for children hospitalized for bronchiolitis. Abstract presented at: ID Week 2012; October 2012; San Diego, CA.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- Agency for Healthcare Research and Quality. HCUPnet. Kids inpatient database 2009. Available at: http://hcupnet.ahrq.gov. Accessed November 6, 2012.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- . How to implement change in clinical practice. Paediatr Respir Rev. 2003;4:340–346.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- , , . Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128:e1596–e1597.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- , , , , , . Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363:2124–2134.
- . Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- , , , et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357:1515–1523.
- National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138.
- , . The chest x‐ray and childhood acute asthma. Aust Clin Rev. 1993;13:153–156.
- , , , . Clinical factors associated with focal infiltrates in wheezing infants and toddlers. Clin Pediatr (Phila). 2000;39:387–393.
- , , , . Chest radiographs in the pediatric emergency department for children < or = 18 months of age with wheezing. Clin Pediatr (Phila). 1999;38:395–399.
- , , , , , . Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009;124:e29–e36.
- , . Reduce the rads: a quality assurance project on reducing unnecessary chest X‐rays in children with asthma. J Paediatr Child Health. 2005;41:107–111.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- , , , et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150:429–433.
- , , , et al. Incidence and predisposing factors for severe disease in previously healthy term infants experiencing their first episode of bronchiolitis. Acta Paediatr. 2011;100:e17–e23.
- , , , et al. A cost effectiveness analysis of omitting radiography in diagnosis of acute bronchiolitis. Pediatr Pulmonol. 2009;44:122–127.
- , , , et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25–30.
- , , , . Efficacy of bronchodilator therapy in bronchiolitis. A meta‐analysis. Arch Pediatr Adolesc Med. 1996;150:1166–1172.
- , . Efficacy of beta2‐agonists in bronchiolitis: a reappraisal and meta‐analysis. Pediatrics. 1997;100:233–239.
- , . Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2010;(12):CD001266.
- , , , et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 2011;(6):CD003123.
- , , , et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079–2089.
- , , , et al. A multicenter, randomized, double‐blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349:27–35.
- , , , . A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr. 2002;141:818–824.
- , , , et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. N Engl J Med. 2009;360:329–338.
- , , , et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2010;(10):CD004878.
- , , , , . Systemic corticosteroids in infant bronchiolitis: a meta‐analysis. Pediatrics. 2000;105:E44.
- , , , . Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518.
- , , . Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356.
- , , , , . Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet. 1987;1:879–882.
- , , , , . The clinical management of preterm infants with bronchiolitis. Hosp Pediatr. 2013;3:244–250.
- , . Glucocorticoids and growth in asthmatic children. Pediatr Allergy Immunol. 1995;6:145–154.
- , , , , , . Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia. Pediatrics. 2011;127:e255–e263.
- , , , , , . Pediatric gastroesophageal reflux disease and acid‐related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12:348–355.
- , , , , , . Healthcare costs of GERD and acid‐related conditions in pediatric patients, with comparison between histamine‐2 receptor antagonists and proton pump inhibitors. Curr Med Res Opin. 2009;25:2703–2709.
- , , , . Are we overprescribing antireflux medications for infants with regurgitation? Pediatrics. 2007;120:946–949.
- , , , , . Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45:421–427.
- , , , , , . Efficacy of proton‐pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127:925–935.
- . Effectiveness and safety of proton pump inhibitors in infantile gastroesophageal reflux disease. Ann Pharmacother. 2010;44:572–576.
- . Are there risks associated with empric acid suppression treatment of infants and children suspected of having gastroesophageal reflux disease? Hosp Pediatr. 2013;3:16–23.
- , , , . Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics. 2003;111:e45–e51.
- , , , . Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158:527–530.
- , . Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121:470–475.
- . Oxygen therapy for bronchiolitis. Pediatrics. 2007;120:686–687; author reply 687–688.
- , , , , , . Bronchiolitis‐associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446.
- , . Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342–349.
- , , , , . Bronchiolitis‐associated mortality and estimates of respiratory syncytial virus‐associated deaths among US children, 1979–1997. J Infect Dis. 2001;183:16–22.
- , . Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97:361–363.
- , , , et al. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J Pediatr. 2011;159:377–383.e1.
- , . The impact of severe asthma on schoolchildren. J Asthma. 1999;36:409–417.
- , . Multi‐center, randomized trial of pulse oximetry monitoring strategies for children hospitalized for bronchiolitis. Abstract presented at: ID Week 2012; October 2012; San Diego, CA.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- Agency for Healthcare Research and Quality. HCUPnet. Kids inpatient database 2009. Available at: http://hcupnet.ahrq.gov. Accessed November 6, 2012.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- . How to implement change in clinical practice. Paediatr Respir Rev. 2003;4:340–346.
Copyright © 2013 Society of Hospital Medicine