User login
A Different Source of Elbow Pain
ANSWER
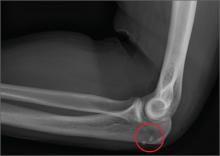
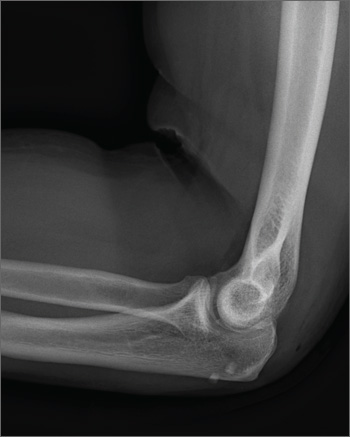
The radiograph shows no obvious fracture or dislocation. However, there are two small, radiopaque densities noted within the soft tissue. These are most likely consistent with broken glass pieces.
When the laceration was irrigated and the wound probed, the glass bits were found and removed prior to wound closure.
ANSWER
The radiograph shows no obvious fracture or dislocation. However, there are two small, radiopaque densities noted within the soft tissue. These are most likely consistent with broken glass pieces.
When the laceration was irrigated and the wound probed, the glass bits were found and removed prior to wound closure.
ANSWER
The radiograph shows no obvious fracture or dislocation. However, there are two small, radiopaque densities noted within the soft tissue. These are most likely consistent with broken glass pieces.
When the laceration was irrigated and the wound probed, the glass bits were found and removed prior to wound closure.
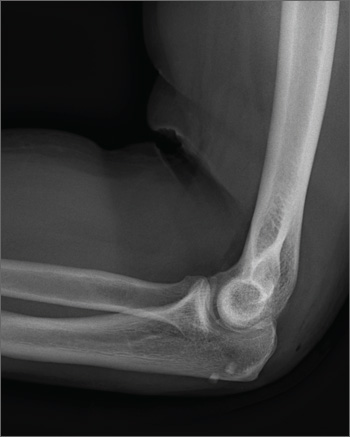
A 30-year-old man is brought to your facility after being in a motor vehicle collision. He was an unrestrained driver who lost control of his vehicle, went off the road, and hit a tree. Emergency personnel on the scene indicated there was moderate damage to his vehicle, including a broken windshield, and no air bag deployment. The patient is complaining of right shoulder, chest, hip, and left elbow pain. His medical history is unremarkable. His vital signs are normal. On physical examination, he has superficial lacerations on his forehead, face, and both forearms. Bleeding from all wounds is controlled. Palpation reveals some bruising of the right shoulder, chest, right hip, and left elbow; no obvious deformity or neurovascular compromise is noted. Multiple radiographs are ordered; the left elbow is shown. What is your impression?
New and Noteworthy Information—August 2013
Patients with Alzheimer’s disease are less likely to have cancer, and patients with cancer are less likely to have Alzheimer’s disease, according to data published online ahead of print July 10 in Neurology. Researchers conducted a cohort study of more than one million people in northern Italy. They derived cancer incidence using the local health authority’s tumor registry and calculated the incidence of Alzheimer’s dementia from registries of drug prescriptions, hospitalizations, and payment exemptions. The risk of cancer in patients with Alzheimer’s dementia was reduced by 50%, and the risk of Alzheimer’s dementia in patients with cancer was reduced by 35%. The investigators observed this relationship in almost all subgroup analyses, suggesting that anticipated potential confounding factors did not significantly influence the results.
Children exposed to antiepileptic drugs in utero may have an increased risk of adverse development within their first three years of life, according to research published online ahead of print July 19 in Epilepsia. From mid-1999 through December 2008, researchers followed children born to mothers who had been recruited at 13 to 17 weeks of pregnancy. Mothers reported their children’s motor development, language, social skills, and autistic traits at 18 months and 36 months. A total of 333 children were exposed to antiepileptic drugs in utero. At 18 months, exposed children had an increased risk of abnormal scores for gross motor skills and autistic traits, compared with nonexposed children. At 36 months, exposed children had an increased risk of abnormal scores for gross motor skills, sentence skills, and autistic traits.
The spatial pattern of amyloid deposition may be related to cognitive performance, according to a study published online ahead of print July 15 in Neurobiology of Aging. Researchers examined the spatial patterns of amyloid deposition throughout the brain using Pittsburgh Compound Blue PET data from the Baltimore Longitudinal Study of Aging. The group approximated spatial patterns of the temporal progression of amyloid plaque deposition from cross-sectional data. Results were consistent with patterns of progression known from autopsy studies. When the investigators categorized participants into subgroups based on longitudinal change in memory performance, they found significantly different spatial patterns of the estimated progression of amyloid deposition between these subgroups. This finding may affect the use of amyloid imaging as a biomarker in research and clinical applications, said the researchers.
A low frequency of physical activity may be associated with an increased risk of stroke, according to data published online ahead of print July 18 in Stroke. Researchers analyzed data for 27,348 participants in the Reasons for Geographic And Racial Differences in Stroke study who had no prior diagnosis of stroke. Participants reported their frequency of moderate-to-vigorous physical activity at baseline according to three categories. Physical inactivity was reported by 33% of participants and was associated with a 20% higher risk of stroke. Adjustment for demographics and socioeconomic factors did not affect the risk, but further adjustment for traditional stroke risk factors completely attenuated the risk. Effects of physical activity are likely to be mediated through the reduction of traditional risk factors, said the researchers.
FTY-720, an immunomodulator for treating multiple sclerosis, may alleviate existing cardiac hypertrophy, according to research published in the July 1 issue of Circulation: Heart Failure. Investigators subjected male C57/Bl6 mice to transverse aortic constriction (TAC) for one week. The researchers treated the mice with FTY-720 for two subsequent weeks while continuing to subject them to TAC. Mice treated with FTY-720 had significantly reduced ventricular mass, ameliorated fibrosis, and improved cardiac performance, compared with mice that received vehicle. Mechanistic studies suggested that FTY-720 appreciably inhibited nuclear factor of activated T-cells activity. In addition, pertussis toxin (Gi-coupled receptor inhibitor) substantially blocked the antihypertrophic effect of FTY-720 in primary rat and human cardiomyocytes. FTY-720 or its analogs could be a promising approach for treating hypertrophic heart disease, said the investigators.
For patients with cardiac arrest who require vasopressors, a combination of vasopressin, epinephrine, and methylprednisolone during cardiopulmonary resuscitation (CPR) and stress-dose hydrocortisone in postresuscitation shock may improve survival to hospital discharge and result in favorable neurologic status, according to data published in the July 17 JAMA. Researchers studied 268 consecutive patients with cardiac arrest requiring epinephrine. Patients received vasopressin plus epinephrine or saline placebo plus epinephrine for the first five CPR cycles after randomization, followed by additional epinephrine if needed. During the first CPR cycle after randomization, patients in the intervention group received methylprednisolone, and patients in the control group received saline placebo. Patients in the treatment arm had higher probability for return of spontaneous circulation of 20 minutes or longer and survival to hospital discharge with cerebral performance category score of 1 or 2.
Chinese people may have a higher risk of stroke than Caucasians, according to research published in the July 16 Neurology. Investigators analyzed studies conducted since 1990 in Chinese populations of first-ever stroke incidence and pathologic types and subtypes of stroke. The team examined hospital- and community-based studies. They also examined community-based stroke studies in Caucasian populations. Age-standardized, annual, first-ever stroke incidence in community-based studies was higher among Chinese than among Caucasian populations. Intracerebral hemorrhage accounted for a larger, more variable proportion of strokes in China than in Taiwan, in Chinese community-based than in hospital-based studies, and in community-based Chinese than in Caucasian studies. The overall proportion of lacunar ischemic stroke was higher in Chinese than in Caucasian populations, but variable study methodologies precluded reliable comparisons.
Narcolepsy in humans may be triggered partly by a proliferation of cells containing histamine, according to data published online ahead of print July 2 in Annals of Neurology. Investigators used immunohistochemistry for histidine decarboxylase (HDC) and quantitative microscopy to detect histamine cells in patients with narcolepsy, Hcrt receptor-2 mutant dogs, and three mouse narcolepsy models. The researchers found an average 64% increase in the number of histamine neurons in human narcolepsy with cataplexy, with no overlap between patients with narcolepsy and controls. The investigators did not observe altered numbers of HDC cells in any of the animal models of narcolepsy, however. The increased histamine cell numbers observed in human narcolepsy may be related to the process causing the human disorder, said the study authors.
Epilepsy may be associated with an early onset of cognitive decline, according to data published online ahead of print on July 8 in JAMA Neurology. Investigators conducted a retrospective observational study of patients at a memory and aging center from 2007 to 2012. Twelve participants had amnestic mild cognitive impairment (aMCI) plus epilepsy, 35 had Alzheimer’s disease plus epilepsy, and seven had Alzheimer’s disease plus subclinical epileptiform activity. Patients with aMCI and epilepsy presented with symptoms of cognitive decline 6.8 years earlier than patients with aMCI who did not have epilepsy. Patients with Alzheimer’s disease who had epilepsy presented with cognitive decline 5.5 years earlier than patients with Alzheimer’s disease who did not have epilepsy. Patients with Alzheimer’s disease and subclinical epileptiform activity also had an early onset of cognitive decline.
Globus pallidus interna (GPi) deep brain stimulation (DBS) may be an effective therapy for DYT1-associated torsion dystonia, according to a study published in the July issue of Neurosurgery. Researchers conducted a retrospective chart review of 47 consecutive patients with DYT1 who were treated by a single surgical team during a 10-year period and followed for up to 96 months. Symptom severity was quantified with the Burke–Fahn–Marsden Dystonia Rating Scale. Motor symptom severity was reduced to less than 20% of baseline after two years of DBS therapy. Disability scores were reduced to less than 30% of baseline. Symptomatic improvement was sustained throughout follow-up. Sixty-one percent of patients had discontinued all dystonia-related medications at their last follow-up. Ninety-one percent had discontinued at least one class of medication.
Concurrent cerebrovascular disease is a common neuropathologic finding in older individuals with dementia, according to an article published online ahead of print July 10 in Brain. Cerebrovascular disease, vascular pathology, and vascular risk factors were studied in 5,715 cases from the National Alzheimer’s Coordinating Centre database who had a single neurodegenerative disease diagnosis based on a neuropathologic examination with or without cerebrovascular disease. After controlling for age and gender, the researchers found a lower prevalence of coincident cerebrovascular disease among patients with α-synucleinopathies, frontotemporal lobar degeneration due to tau and TAR DNA-binding protein 43, and prion disease than among patients with Alzheimer’s disease. This result was more significant in younger patients. Data suggest that these disorders should be targeted by treatments for cerebrovascular disease, according to the researchers.
Athletes who do not get enough sleep the night before undergoing baseline concussion testing may not perform as well as they expect, according to research presented at the 2013 Annual Meeting of the American Orthopaedic Society for Sports Medicine in Chicago. Researchers reviewed 3,686 nonconcussed athletes (1,315 female) with baseline symptom and ImPACT neurocognitive scores. Individuals reported their previous night’s sleep duration as fewer than seven hours, seven to nine hours, or more than nine hours. The study authors observed significant differences in reaction time, verbal memory, and visual memory in the group that had slept less than seven hours. Visual-motor speed scores did not seem to be affected. Significant differences in the total number of reported symptoms were associated with sleeping fewer than seven hours.
Continuation of lipophilic statin therapy may be associated with a decreased risk of Parkinson’s disease, compared with discontinuation, according to research published online ahead of print July 24 in Neurology. Between 2001 and 2008, investigators recruited participants without Parkinson’s disease who had initiated statin therapy. Among 43,810 subjects, the incidence rate for Parkinson’s disease was 1.68 and 3.52 per 1,000,000 person-days for lipophilic and hydrophilic statins, respectively. Continuation of lipophilic statins was associated with a decreased risk of Parkinson’s disease, compared with statin discontinuation. No association between hydrophilic statins and occurrence of Parkinson’s disease was observed. Among lipophilic statins, a significant association was observed for simvastatin and atorvastatin, especially in females. Long-term use of lipophilic or hydrophilic statins was not significantly associated with Parkinson’s disease.
—Erik Greb
Senior Associate Editor
Patients with Alzheimer’s disease are less likely to have cancer, and patients with cancer are less likely to have Alzheimer’s disease, according to data published online ahead of print July 10 in Neurology. Researchers conducted a cohort study of more than one million people in northern Italy. They derived cancer incidence using the local health authority’s tumor registry and calculated the incidence of Alzheimer’s dementia from registries of drug prescriptions, hospitalizations, and payment exemptions. The risk of cancer in patients with Alzheimer’s dementia was reduced by 50%, and the risk of Alzheimer’s dementia in patients with cancer was reduced by 35%. The investigators observed this relationship in almost all subgroup analyses, suggesting that anticipated potential confounding factors did not significantly influence the results.
Children exposed to antiepileptic drugs in utero may have an increased risk of adverse development within their first three years of life, according to research published online ahead of print July 19 in Epilepsia. From mid-1999 through December 2008, researchers followed children born to mothers who had been recruited at 13 to 17 weeks of pregnancy. Mothers reported their children’s motor development, language, social skills, and autistic traits at 18 months and 36 months. A total of 333 children were exposed to antiepileptic drugs in utero. At 18 months, exposed children had an increased risk of abnormal scores for gross motor skills and autistic traits, compared with nonexposed children. At 36 months, exposed children had an increased risk of abnormal scores for gross motor skills, sentence skills, and autistic traits.
The spatial pattern of amyloid deposition may be related to cognitive performance, according to a study published online ahead of print July 15 in Neurobiology of Aging. Researchers examined the spatial patterns of amyloid deposition throughout the brain using Pittsburgh Compound Blue PET data from the Baltimore Longitudinal Study of Aging. The group approximated spatial patterns of the temporal progression of amyloid plaque deposition from cross-sectional data. Results were consistent with patterns of progression known from autopsy studies. When the investigators categorized participants into subgroups based on longitudinal change in memory performance, they found significantly different spatial patterns of the estimated progression of amyloid deposition between these subgroups. This finding may affect the use of amyloid imaging as a biomarker in research and clinical applications, said the researchers.
A low frequency of physical activity may be associated with an increased risk of stroke, according to data published online ahead of print July 18 in Stroke. Researchers analyzed data for 27,348 participants in the Reasons for Geographic And Racial Differences in Stroke study who had no prior diagnosis of stroke. Participants reported their frequency of moderate-to-vigorous physical activity at baseline according to three categories. Physical inactivity was reported by 33% of participants and was associated with a 20% higher risk of stroke. Adjustment for demographics and socioeconomic factors did not affect the risk, but further adjustment for traditional stroke risk factors completely attenuated the risk. Effects of physical activity are likely to be mediated through the reduction of traditional risk factors, said the researchers.
FTY-720, an immunomodulator for treating multiple sclerosis, may alleviate existing cardiac hypertrophy, according to research published in the July 1 issue of Circulation: Heart Failure. Investigators subjected male C57/Bl6 mice to transverse aortic constriction (TAC) for one week. The researchers treated the mice with FTY-720 for two subsequent weeks while continuing to subject them to TAC. Mice treated with FTY-720 had significantly reduced ventricular mass, ameliorated fibrosis, and improved cardiac performance, compared with mice that received vehicle. Mechanistic studies suggested that FTY-720 appreciably inhibited nuclear factor of activated T-cells activity. In addition, pertussis toxin (Gi-coupled receptor inhibitor) substantially blocked the antihypertrophic effect of FTY-720 in primary rat and human cardiomyocytes. FTY-720 or its analogs could be a promising approach for treating hypertrophic heart disease, said the investigators.
For patients with cardiac arrest who require vasopressors, a combination of vasopressin, epinephrine, and methylprednisolone during cardiopulmonary resuscitation (CPR) and stress-dose hydrocortisone in postresuscitation shock may improve survival to hospital discharge and result in favorable neurologic status, according to data published in the July 17 JAMA. Researchers studied 268 consecutive patients with cardiac arrest requiring epinephrine. Patients received vasopressin plus epinephrine or saline placebo plus epinephrine for the first five CPR cycles after randomization, followed by additional epinephrine if needed. During the first CPR cycle after randomization, patients in the intervention group received methylprednisolone, and patients in the control group received saline placebo. Patients in the treatment arm had higher probability for return of spontaneous circulation of 20 minutes or longer and survival to hospital discharge with cerebral performance category score of 1 or 2.
Chinese people may have a higher risk of stroke than Caucasians, according to research published in the July 16 Neurology. Investigators analyzed studies conducted since 1990 in Chinese populations of first-ever stroke incidence and pathologic types and subtypes of stroke. The team examined hospital- and community-based studies. They also examined community-based stroke studies in Caucasian populations. Age-standardized, annual, first-ever stroke incidence in community-based studies was higher among Chinese than among Caucasian populations. Intracerebral hemorrhage accounted for a larger, more variable proportion of strokes in China than in Taiwan, in Chinese community-based than in hospital-based studies, and in community-based Chinese than in Caucasian studies. The overall proportion of lacunar ischemic stroke was higher in Chinese than in Caucasian populations, but variable study methodologies precluded reliable comparisons.
Narcolepsy in humans may be triggered partly by a proliferation of cells containing histamine, according to data published online ahead of print July 2 in Annals of Neurology. Investigators used immunohistochemistry for histidine decarboxylase (HDC) and quantitative microscopy to detect histamine cells in patients with narcolepsy, Hcrt receptor-2 mutant dogs, and three mouse narcolepsy models. The researchers found an average 64% increase in the number of histamine neurons in human narcolepsy with cataplexy, with no overlap between patients with narcolepsy and controls. The investigators did not observe altered numbers of HDC cells in any of the animal models of narcolepsy, however. The increased histamine cell numbers observed in human narcolepsy may be related to the process causing the human disorder, said the study authors.
Epilepsy may be associated with an early onset of cognitive decline, according to data published online ahead of print on July 8 in JAMA Neurology. Investigators conducted a retrospective observational study of patients at a memory and aging center from 2007 to 2012. Twelve participants had amnestic mild cognitive impairment (aMCI) plus epilepsy, 35 had Alzheimer’s disease plus epilepsy, and seven had Alzheimer’s disease plus subclinical epileptiform activity. Patients with aMCI and epilepsy presented with symptoms of cognitive decline 6.8 years earlier than patients with aMCI who did not have epilepsy. Patients with Alzheimer’s disease who had epilepsy presented with cognitive decline 5.5 years earlier than patients with Alzheimer’s disease who did not have epilepsy. Patients with Alzheimer’s disease and subclinical epileptiform activity also had an early onset of cognitive decline.
Globus pallidus interna (GPi) deep brain stimulation (DBS) may be an effective therapy for DYT1-associated torsion dystonia, according to a study published in the July issue of Neurosurgery. Researchers conducted a retrospective chart review of 47 consecutive patients with DYT1 who were treated by a single surgical team during a 10-year period and followed for up to 96 months. Symptom severity was quantified with the Burke–Fahn–Marsden Dystonia Rating Scale. Motor symptom severity was reduced to less than 20% of baseline after two years of DBS therapy. Disability scores were reduced to less than 30% of baseline. Symptomatic improvement was sustained throughout follow-up. Sixty-one percent of patients had discontinued all dystonia-related medications at their last follow-up. Ninety-one percent had discontinued at least one class of medication.
Concurrent cerebrovascular disease is a common neuropathologic finding in older individuals with dementia, according to an article published online ahead of print July 10 in Brain. Cerebrovascular disease, vascular pathology, and vascular risk factors were studied in 5,715 cases from the National Alzheimer’s Coordinating Centre database who had a single neurodegenerative disease diagnosis based on a neuropathologic examination with or without cerebrovascular disease. After controlling for age and gender, the researchers found a lower prevalence of coincident cerebrovascular disease among patients with α-synucleinopathies, frontotemporal lobar degeneration due to tau and TAR DNA-binding protein 43, and prion disease than among patients with Alzheimer’s disease. This result was more significant in younger patients. Data suggest that these disorders should be targeted by treatments for cerebrovascular disease, according to the researchers.
Athletes who do not get enough sleep the night before undergoing baseline concussion testing may not perform as well as they expect, according to research presented at the 2013 Annual Meeting of the American Orthopaedic Society for Sports Medicine in Chicago. Researchers reviewed 3,686 nonconcussed athletes (1,315 female) with baseline symptom and ImPACT neurocognitive scores. Individuals reported their previous night’s sleep duration as fewer than seven hours, seven to nine hours, or more than nine hours. The study authors observed significant differences in reaction time, verbal memory, and visual memory in the group that had slept less than seven hours. Visual-motor speed scores did not seem to be affected. Significant differences in the total number of reported symptoms were associated with sleeping fewer than seven hours.
Continuation of lipophilic statin therapy may be associated with a decreased risk of Parkinson’s disease, compared with discontinuation, according to research published online ahead of print July 24 in Neurology. Between 2001 and 2008, investigators recruited participants without Parkinson’s disease who had initiated statin therapy. Among 43,810 subjects, the incidence rate for Parkinson’s disease was 1.68 and 3.52 per 1,000,000 person-days for lipophilic and hydrophilic statins, respectively. Continuation of lipophilic statins was associated with a decreased risk of Parkinson’s disease, compared with statin discontinuation. No association between hydrophilic statins and occurrence of Parkinson’s disease was observed. Among lipophilic statins, a significant association was observed for simvastatin and atorvastatin, especially in females. Long-term use of lipophilic or hydrophilic statins was not significantly associated with Parkinson’s disease.
—Erik Greb
Senior Associate Editor
Patients with Alzheimer’s disease are less likely to have cancer, and patients with cancer are less likely to have Alzheimer’s disease, according to data published online ahead of print July 10 in Neurology. Researchers conducted a cohort study of more than one million people in northern Italy. They derived cancer incidence using the local health authority’s tumor registry and calculated the incidence of Alzheimer’s dementia from registries of drug prescriptions, hospitalizations, and payment exemptions. The risk of cancer in patients with Alzheimer’s dementia was reduced by 50%, and the risk of Alzheimer’s dementia in patients with cancer was reduced by 35%. The investigators observed this relationship in almost all subgroup analyses, suggesting that anticipated potential confounding factors did not significantly influence the results.
Children exposed to antiepileptic drugs in utero may have an increased risk of adverse development within their first three years of life, according to research published online ahead of print July 19 in Epilepsia. From mid-1999 through December 2008, researchers followed children born to mothers who had been recruited at 13 to 17 weeks of pregnancy. Mothers reported their children’s motor development, language, social skills, and autistic traits at 18 months and 36 months. A total of 333 children were exposed to antiepileptic drugs in utero. At 18 months, exposed children had an increased risk of abnormal scores for gross motor skills and autistic traits, compared with nonexposed children. At 36 months, exposed children had an increased risk of abnormal scores for gross motor skills, sentence skills, and autistic traits.
The spatial pattern of amyloid deposition may be related to cognitive performance, according to a study published online ahead of print July 15 in Neurobiology of Aging. Researchers examined the spatial patterns of amyloid deposition throughout the brain using Pittsburgh Compound Blue PET data from the Baltimore Longitudinal Study of Aging. The group approximated spatial patterns of the temporal progression of amyloid plaque deposition from cross-sectional data. Results were consistent with patterns of progression known from autopsy studies. When the investigators categorized participants into subgroups based on longitudinal change in memory performance, they found significantly different spatial patterns of the estimated progression of amyloid deposition between these subgroups. This finding may affect the use of amyloid imaging as a biomarker in research and clinical applications, said the researchers.
A low frequency of physical activity may be associated with an increased risk of stroke, according to data published online ahead of print July 18 in Stroke. Researchers analyzed data for 27,348 participants in the Reasons for Geographic And Racial Differences in Stroke study who had no prior diagnosis of stroke. Participants reported their frequency of moderate-to-vigorous physical activity at baseline according to three categories. Physical inactivity was reported by 33% of participants and was associated with a 20% higher risk of stroke. Adjustment for demographics and socioeconomic factors did not affect the risk, but further adjustment for traditional stroke risk factors completely attenuated the risk. Effects of physical activity are likely to be mediated through the reduction of traditional risk factors, said the researchers.
FTY-720, an immunomodulator for treating multiple sclerosis, may alleviate existing cardiac hypertrophy, according to research published in the July 1 issue of Circulation: Heart Failure. Investigators subjected male C57/Bl6 mice to transverse aortic constriction (TAC) for one week. The researchers treated the mice with FTY-720 for two subsequent weeks while continuing to subject them to TAC. Mice treated with FTY-720 had significantly reduced ventricular mass, ameliorated fibrosis, and improved cardiac performance, compared with mice that received vehicle. Mechanistic studies suggested that FTY-720 appreciably inhibited nuclear factor of activated T-cells activity. In addition, pertussis toxin (Gi-coupled receptor inhibitor) substantially blocked the antihypertrophic effect of FTY-720 in primary rat and human cardiomyocytes. FTY-720 or its analogs could be a promising approach for treating hypertrophic heart disease, said the investigators.
For patients with cardiac arrest who require vasopressors, a combination of vasopressin, epinephrine, and methylprednisolone during cardiopulmonary resuscitation (CPR) and stress-dose hydrocortisone in postresuscitation shock may improve survival to hospital discharge and result in favorable neurologic status, according to data published in the July 17 JAMA. Researchers studied 268 consecutive patients with cardiac arrest requiring epinephrine. Patients received vasopressin plus epinephrine or saline placebo plus epinephrine for the first five CPR cycles after randomization, followed by additional epinephrine if needed. During the first CPR cycle after randomization, patients in the intervention group received methylprednisolone, and patients in the control group received saline placebo. Patients in the treatment arm had higher probability for return of spontaneous circulation of 20 minutes or longer and survival to hospital discharge with cerebral performance category score of 1 or 2.
Chinese people may have a higher risk of stroke than Caucasians, according to research published in the July 16 Neurology. Investigators analyzed studies conducted since 1990 in Chinese populations of first-ever stroke incidence and pathologic types and subtypes of stroke. The team examined hospital- and community-based studies. They also examined community-based stroke studies in Caucasian populations. Age-standardized, annual, first-ever stroke incidence in community-based studies was higher among Chinese than among Caucasian populations. Intracerebral hemorrhage accounted for a larger, more variable proportion of strokes in China than in Taiwan, in Chinese community-based than in hospital-based studies, and in community-based Chinese than in Caucasian studies. The overall proportion of lacunar ischemic stroke was higher in Chinese than in Caucasian populations, but variable study methodologies precluded reliable comparisons.
Narcolepsy in humans may be triggered partly by a proliferation of cells containing histamine, according to data published online ahead of print July 2 in Annals of Neurology. Investigators used immunohistochemistry for histidine decarboxylase (HDC) and quantitative microscopy to detect histamine cells in patients with narcolepsy, Hcrt receptor-2 mutant dogs, and three mouse narcolepsy models. The researchers found an average 64% increase in the number of histamine neurons in human narcolepsy with cataplexy, with no overlap between patients with narcolepsy and controls. The investigators did not observe altered numbers of HDC cells in any of the animal models of narcolepsy, however. The increased histamine cell numbers observed in human narcolepsy may be related to the process causing the human disorder, said the study authors.
Epilepsy may be associated with an early onset of cognitive decline, according to data published online ahead of print on July 8 in JAMA Neurology. Investigators conducted a retrospective observational study of patients at a memory and aging center from 2007 to 2012. Twelve participants had amnestic mild cognitive impairment (aMCI) plus epilepsy, 35 had Alzheimer’s disease plus epilepsy, and seven had Alzheimer’s disease plus subclinical epileptiform activity. Patients with aMCI and epilepsy presented with symptoms of cognitive decline 6.8 years earlier than patients with aMCI who did not have epilepsy. Patients with Alzheimer’s disease who had epilepsy presented with cognitive decline 5.5 years earlier than patients with Alzheimer’s disease who did not have epilepsy. Patients with Alzheimer’s disease and subclinical epileptiform activity also had an early onset of cognitive decline.
Globus pallidus interna (GPi) deep brain stimulation (DBS) may be an effective therapy for DYT1-associated torsion dystonia, according to a study published in the July issue of Neurosurgery. Researchers conducted a retrospective chart review of 47 consecutive patients with DYT1 who were treated by a single surgical team during a 10-year period and followed for up to 96 months. Symptom severity was quantified with the Burke–Fahn–Marsden Dystonia Rating Scale. Motor symptom severity was reduced to less than 20% of baseline after two years of DBS therapy. Disability scores were reduced to less than 30% of baseline. Symptomatic improvement was sustained throughout follow-up. Sixty-one percent of patients had discontinued all dystonia-related medications at their last follow-up. Ninety-one percent had discontinued at least one class of medication.
Concurrent cerebrovascular disease is a common neuropathologic finding in older individuals with dementia, according to an article published online ahead of print July 10 in Brain. Cerebrovascular disease, vascular pathology, and vascular risk factors were studied in 5,715 cases from the National Alzheimer’s Coordinating Centre database who had a single neurodegenerative disease diagnosis based on a neuropathologic examination with or without cerebrovascular disease. After controlling for age and gender, the researchers found a lower prevalence of coincident cerebrovascular disease among patients with α-synucleinopathies, frontotemporal lobar degeneration due to tau and TAR DNA-binding protein 43, and prion disease than among patients with Alzheimer’s disease. This result was more significant in younger patients. Data suggest that these disorders should be targeted by treatments for cerebrovascular disease, according to the researchers.
Athletes who do not get enough sleep the night before undergoing baseline concussion testing may not perform as well as they expect, according to research presented at the 2013 Annual Meeting of the American Orthopaedic Society for Sports Medicine in Chicago. Researchers reviewed 3,686 nonconcussed athletes (1,315 female) with baseline symptom and ImPACT neurocognitive scores. Individuals reported their previous night’s sleep duration as fewer than seven hours, seven to nine hours, or more than nine hours. The study authors observed significant differences in reaction time, verbal memory, and visual memory in the group that had slept less than seven hours. Visual-motor speed scores did not seem to be affected. Significant differences in the total number of reported symptoms were associated with sleeping fewer than seven hours.
Continuation of lipophilic statin therapy may be associated with a decreased risk of Parkinson’s disease, compared with discontinuation, according to research published online ahead of print July 24 in Neurology. Between 2001 and 2008, investigators recruited participants without Parkinson’s disease who had initiated statin therapy. Among 43,810 subjects, the incidence rate for Parkinson’s disease was 1.68 and 3.52 per 1,000,000 person-days for lipophilic and hydrophilic statins, respectively. Continuation of lipophilic statins was associated with a decreased risk of Parkinson’s disease, compared with statin discontinuation. No association between hydrophilic statins and occurrence of Parkinson’s disease was observed. Among lipophilic statins, a significant association was observed for simvastatin and atorvastatin, especially in females. Long-term use of lipophilic or hydrophilic statins was not significantly associated with Parkinson’s disease.
—Erik Greb
Senior Associate Editor
Survey suggests docs could improve trial enrollment
Results of a nationwide poll indicate that many Americans believe participation in clinical trials is important.
And most of the individuals surveyed said they would participate in a trial if their doctor recommended it.
However, many respondents said they learned of clinical trials via the Internet, not from discussions with their physician. And most said a physician’s recommendation was an important factor in the decision to enroll in a clinical trial.
This poll was conducted online by Zogby Analytics. The results are available on the Research!America website.
The goal of the survey was to evaluate respondents’ perceptions about clinical trials and determine if perceptions differed according to racial groups. The survey included 684 non-Hispanic whites, 406 Hispanics, 403 African-Americans, and 300 Asians.
A willingness to participate
Most of the African-Americans (62%) and Hispanics (57%) polled said it’s very important to participate in a clinical trial to improve the health of others. Fifty percent of Asians agreed, as did 49% of non-Hispanic whites.
Furthermore, 75% of Hispanics, 72% of African-Americans, 71% of non-Hispanic whites, and 65% of Asians said they would likely participate in a clinical trial if it was recommended by a doctor.
And about three-quarters of all respondents said, assuming the correct privacy protections were in place, they would be willing to share personal health information to advance medical research.
“The poll reveals a willingness among minorities to participate in clinical trials to improve the quality of healthcare, but enrollment remains stubbornly low,” said Mary Woolley, president and CEO of Research!America.
Among those individuals surveyed, only 17% of Hispanics, 15% of African-Americans, 15% of non-Hispanic whites, and 11% of Asians said they or a family member had participated in a clinical trial.
When asked to give a reason for low enrollment in clinical trials, most respondents cited a lack of trust—61% of African-Americans, 54% of non-Hispanic whites, 52% of Hispanics, and 51% of Asians.
The importance of physicians, institutions
Many of the individuals surveyed said they had learned of clinical trials via the Internet—56% of Asians, 52% of non-Hispanic whites, 50% of African-Americans, and 45% of Hispanics.
Far fewer said they had heard of trials during discussions with their doctors—22% of Hispanics, 22% of non-Hispanic whites, 20% of Asians, and 17% of African-Americans.
And most respondents said a doctor’s recommendation is a “very” or “somewhat” important factor in the decision to participate in a clinical trial—81% of Hispanics, 81% of non-Hispanic whites, 80% of Asians, and 78% of African-Americans.
Institutional reputation was also deemed an important factor by 74% of African-Americans, 72% of non-Hispanic whites, 66% of Hispanics, and 66% of Asians.
And many respondents said healthcare providers should play a major role in raising awareness of clinical trials. In fact, 42% of non-Hispanic whites, 38% of Hispanics, 36% of Asians, and 33% of African-Americans said providers have the greatest responsibility in educating the public about clinical trials.
“It’s imperative that healthcare providers and others help patients gain a deeper knowledge of clinical trials so all Americans can benefit from life-saving treatments,” Woolley said.
This survey was conducted by Zogby Analytics for Research!America, the Association of Clinical Research Organizations, the Clinical Research Forum, the Friends of the National Library of Medicine, and the Clinical Trials Transformation Initiative. ![]()
Results of a nationwide poll indicate that many Americans believe participation in clinical trials is important.
And most of the individuals surveyed said they would participate in a trial if their doctor recommended it.
However, many respondents said they learned of clinical trials via the Internet, not from discussions with their physician. And most said a physician’s recommendation was an important factor in the decision to enroll in a clinical trial.
This poll was conducted online by Zogby Analytics. The results are available on the Research!America website.
The goal of the survey was to evaluate respondents’ perceptions about clinical trials and determine if perceptions differed according to racial groups. The survey included 684 non-Hispanic whites, 406 Hispanics, 403 African-Americans, and 300 Asians.
A willingness to participate
Most of the African-Americans (62%) and Hispanics (57%) polled said it’s very important to participate in a clinical trial to improve the health of others. Fifty percent of Asians agreed, as did 49% of non-Hispanic whites.
Furthermore, 75% of Hispanics, 72% of African-Americans, 71% of non-Hispanic whites, and 65% of Asians said they would likely participate in a clinical trial if it was recommended by a doctor.
And about three-quarters of all respondents said, assuming the correct privacy protections were in place, they would be willing to share personal health information to advance medical research.
“The poll reveals a willingness among minorities to participate in clinical trials to improve the quality of healthcare, but enrollment remains stubbornly low,” said Mary Woolley, president and CEO of Research!America.
Among those individuals surveyed, only 17% of Hispanics, 15% of African-Americans, 15% of non-Hispanic whites, and 11% of Asians said they or a family member had participated in a clinical trial.
When asked to give a reason for low enrollment in clinical trials, most respondents cited a lack of trust—61% of African-Americans, 54% of non-Hispanic whites, 52% of Hispanics, and 51% of Asians.
The importance of physicians, institutions
Many of the individuals surveyed said they had learned of clinical trials via the Internet—56% of Asians, 52% of non-Hispanic whites, 50% of African-Americans, and 45% of Hispanics.
Far fewer said they had heard of trials during discussions with their doctors—22% of Hispanics, 22% of non-Hispanic whites, 20% of Asians, and 17% of African-Americans.
And most respondents said a doctor’s recommendation is a “very” or “somewhat” important factor in the decision to participate in a clinical trial—81% of Hispanics, 81% of non-Hispanic whites, 80% of Asians, and 78% of African-Americans.
Institutional reputation was also deemed an important factor by 74% of African-Americans, 72% of non-Hispanic whites, 66% of Hispanics, and 66% of Asians.
And many respondents said healthcare providers should play a major role in raising awareness of clinical trials. In fact, 42% of non-Hispanic whites, 38% of Hispanics, 36% of Asians, and 33% of African-Americans said providers have the greatest responsibility in educating the public about clinical trials.
“It’s imperative that healthcare providers and others help patients gain a deeper knowledge of clinical trials so all Americans can benefit from life-saving treatments,” Woolley said.
This survey was conducted by Zogby Analytics for Research!America, the Association of Clinical Research Organizations, the Clinical Research Forum, the Friends of the National Library of Medicine, and the Clinical Trials Transformation Initiative. ![]()
Results of a nationwide poll indicate that many Americans believe participation in clinical trials is important.
And most of the individuals surveyed said they would participate in a trial if their doctor recommended it.
However, many respondents said they learned of clinical trials via the Internet, not from discussions with their physician. And most said a physician’s recommendation was an important factor in the decision to enroll in a clinical trial.
This poll was conducted online by Zogby Analytics. The results are available on the Research!America website.
The goal of the survey was to evaluate respondents’ perceptions about clinical trials and determine if perceptions differed according to racial groups. The survey included 684 non-Hispanic whites, 406 Hispanics, 403 African-Americans, and 300 Asians.
A willingness to participate
Most of the African-Americans (62%) and Hispanics (57%) polled said it’s very important to participate in a clinical trial to improve the health of others. Fifty percent of Asians agreed, as did 49% of non-Hispanic whites.
Furthermore, 75% of Hispanics, 72% of African-Americans, 71% of non-Hispanic whites, and 65% of Asians said they would likely participate in a clinical trial if it was recommended by a doctor.
And about three-quarters of all respondents said, assuming the correct privacy protections were in place, they would be willing to share personal health information to advance medical research.
“The poll reveals a willingness among minorities to participate in clinical trials to improve the quality of healthcare, but enrollment remains stubbornly low,” said Mary Woolley, president and CEO of Research!America.
Among those individuals surveyed, only 17% of Hispanics, 15% of African-Americans, 15% of non-Hispanic whites, and 11% of Asians said they or a family member had participated in a clinical trial.
When asked to give a reason for low enrollment in clinical trials, most respondents cited a lack of trust—61% of African-Americans, 54% of non-Hispanic whites, 52% of Hispanics, and 51% of Asians.
The importance of physicians, institutions
Many of the individuals surveyed said they had learned of clinical trials via the Internet—56% of Asians, 52% of non-Hispanic whites, 50% of African-Americans, and 45% of Hispanics.
Far fewer said they had heard of trials during discussions with their doctors—22% of Hispanics, 22% of non-Hispanic whites, 20% of Asians, and 17% of African-Americans.
And most respondents said a doctor’s recommendation is a “very” or “somewhat” important factor in the decision to participate in a clinical trial—81% of Hispanics, 81% of non-Hispanic whites, 80% of Asians, and 78% of African-Americans.
Institutional reputation was also deemed an important factor by 74% of African-Americans, 72% of non-Hispanic whites, 66% of Hispanics, and 66% of Asians.
And many respondents said healthcare providers should play a major role in raising awareness of clinical trials. In fact, 42% of non-Hispanic whites, 38% of Hispanics, 36% of Asians, and 33% of African-Americans said providers have the greatest responsibility in educating the public about clinical trials.
“It’s imperative that healthcare providers and others help patients gain a deeper knowledge of clinical trials so all Americans can benefit from life-saving treatments,” Woolley said.
This survey was conducted by Zogby Analytics for Research!America, the Association of Clinical Research Organizations, the Clinical Research Forum, the Friends of the National Library of Medicine, and the Clinical Trials Transformation Initiative. ![]()
Listen to John Vazquez, MD, discuss neurophobia and tips for adjusting to discomfort in treating neuro patients
Click here to listen to more of our interview with Dr. Vazquez
Click here to listen to more of our interview with Dr. Vazquez
Click here to listen to more of our interview with Dr. Vazquez
“Telestroke” care and “teleneurology” give hospitalists additional resources
To deal with the demand for neurologists, hospitals are increasingly turning to a new idea: patching a neurologist into the exam room from afar, Skype-style. This approach to caring for patients with strokes and other neurologic conditions—“telestroke” care or “teleneurology”—is an emerging valuable resource, says Edgar Kenton, MD, who is in the process of expanding such a system at Geisinger Health System in Danville, Pa.
It’s a hub-and-spoke system, with a central hospital as the “hub” and other hospitals as the “spokes” that connect with the hub, using computers and real-time video. Geisinger has six spokes, with plans for about another half-dozen.
“We’re able to talk to the physician, we can see the patient, we can see the families if they’re there, and get a lot of information,” says Dr. Kenton, director of the stroke program at Geisinger.
It’s crucial to see patients when they’re suspected of having stroke symptoms, he says, so that the neurologist can get crucial insight they can’t get over the phone. With a laptop, tablet, or smartphone, the neurologist can do a consult regardless of location.
Kevin Barrett, MD, assistant professor of neurology and a neurohospitalist at Mayo Clinic in Jacksonville, Fla., says he expects that one paradoxical effect of telestroke care is that more patients with neurological conditions ultimately will end up under the care of hospitalists. That’s because centers will be more likely to take on a patient, consult with a neurologist using telestroke care or teleneurology, then admit the patient—after the neurologist has logged off.
“I do think that we are going to see an increase in the number of neurologic-type patients that are cared for primarily by hospitalists,” he says. “I’m there for the acute consultation. But often those patients are then subsequently admitted to a hospitalist service for the very reason that I was called. They don’t have local resources for neurology or a neurohospitalist.
“I actually think there’s going to be more involvement on the hospitalist front for neurologic patients, even patients with neurologic emergencies, because of telemedicine,” Dr. Barrett says. TH
Tom Collins is a freelance writer in South Florida.
To deal with the demand for neurologists, hospitals are increasingly turning to a new idea: patching a neurologist into the exam room from afar, Skype-style. This approach to caring for patients with strokes and other neurologic conditions—“telestroke” care or “teleneurology”—is an emerging valuable resource, says Edgar Kenton, MD, who is in the process of expanding such a system at Geisinger Health System in Danville, Pa.
It’s a hub-and-spoke system, with a central hospital as the “hub” and other hospitals as the “spokes” that connect with the hub, using computers and real-time video. Geisinger has six spokes, with plans for about another half-dozen.
“We’re able to talk to the physician, we can see the patient, we can see the families if they’re there, and get a lot of information,” says Dr. Kenton, director of the stroke program at Geisinger.
It’s crucial to see patients when they’re suspected of having stroke symptoms, he says, so that the neurologist can get crucial insight they can’t get over the phone. With a laptop, tablet, or smartphone, the neurologist can do a consult regardless of location.
Kevin Barrett, MD, assistant professor of neurology and a neurohospitalist at Mayo Clinic in Jacksonville, Fla., says he expects that one paradoxical effect of telestroke care is that more patients with neurological conditions ultimately will end up under the care of hospitalists. That’s because centers will be more likely to take on a patient, consult with a neurologist using telestroke care or teleneurology, then admit the patient—after the neurologist has logged off.
“I do think that we are going to see an increase in the number of neurologic-type patients that are cared for primarily by hospitalists,” he says. “I’m there for the acute consultation. But often those patients are then subsequently admitted to a hospitalist service for the very reason that I was called. They don’t have local resources for neurology or a neurohospitalist.
“I actually think there’s going to be more involvement on the hospitalist front for neurologic patients, even patients with neurologic emergencies, because of telemedicine,” Dr. Barrett says. TH
Tom Collins is a freelance writer in South Florida.
To deal with the demand for neurologists, hospitals are increasingly turning to a new idea: patching a neurologist into the exam room from afar, Skype-style. This approach to caring for patients with strokes and other neurologic conditions—“telestroke” care or “teleneurology”—is an emerging valuable resource, says Edgar Kenton, MD, who is in the process of expanding such a system at Geisinger Health System in Danville, Pa.
It’s a hub-and-spoke system, with a central hospital as the “hub” and other hospitals as the “spokes” that connect with the hub, using computers and real-time video. Geisinger has six spokes, with plans for about another half-dozen.
“We’re able to talk to the physician, we can see the patient, we can see the families if they’re there, and get a lot of information,” says Dr. Kenton, director of the stroke program at Geisinger.
It’s crucial to see patients when they’re suspected of having stroke symptoms, he says, so that the neurologist can get crucial insight they can’t get over the phone. With a laptop, tablet, or smartphone, the neurologist can do a consult regardless of location.
Kevin Barrett, MD, assistant professor of neurology and a neurohospitalist at Mayo Clinic in Jacksonville, Fla., says he expects that one paradoxical effect of telestroke care is that more patients with neurological conditions ultimately will end up under the care of hospitalists. That’s because centers will be more likely to take on a patient, consult with a neurologist using telestroke care or teleneurology, then admit the patient—after the neurologist has logged off.
“I do think that we are going to see an increase in the number of neurologic-type patients that are cared for primarily by hospitalists,” he says. “I’m there for the acute consultation. But often those patients are then subsequently admitted to a hospitalist service for the very reason that I was called. They don’t have local resources for neurology or a neurohospitalist.
“I actually think there’s going to be more involvement on the hospitalist front for neurologic patients, even patients with neurologic emergencies, because of telemedicine,” Dr. Barrett says. TH
Tom Collins is a freelance writer in South Florida.
Oklahoma Hospitalist Discusses Medical Center Devastation, Community Response
Click here to listen to more of our interview with Dr. Womble
Click here to listen to more of our interview with Dr. Womble
Click here to listen to more of our interview with Dr. Womble
Listen to Project BOOST lead analyst Luke Hansen, MD, MPH, discuss the outcomes study published in JHM
Click here to listen to our interview with Dr. Hansen
Click here to listen to our interview with Dr. Hansen
Click here to listen to our interview with Dr. Hansen
Devastating Superstorm Gone, But Not Forgotten in Moore, Okla.

–Joe R. Womble, MD
The first bit of feedback was fantastic: Everyone who had been inside the hospital—roughly 200 to 300 people, including 30 patients—had survived.
“Everyone was fine,” he said. “All the patients and staff, no one got injured. I was thinking that either the hospital was missed by the storm or that it must not have really damaged it very significantly.”
Unfortunately, the hospital was not OK. He watched as local TV painted a very different picture.
“They started showing aerial shots and I was just shocked. My jaw was just dropped,” Dr. Womble said. “The main entrance that I go in every day was literally stacked with three or four cars deep. A huge stack of about 30 cars was piled up on the main entrance, essentially.”
It was as though they were “toy cars.”
The May 20 tornado, a two-mile-wide superstorm boasting 200-mph winds that struck just south of Oklahoma City, claimed 24 lives and left the regional health system with a void in its network. It also left hospitalists mourning the loss of the place they called a second home several times a week. About a week after the storm, officials announced that Moore Medical Center would have to be demolished.
Miraculous Moments
Despite the terrible events, hospitalists and hospital officials were astounded by the good fortune of the hospital’s inhabitants. Dr. Womble said about 100 people from nearby neighborhoods and businesses used the hospital as shelter.
Senthil Raju, MD, a hospitalist who had done rounds at Moore Medical earlier that day, said the protocol was to take shelter in the hallways. But at some point, probably only minutes before the storm hit, the chief nurse and the house supervisor made the decision to move all the patients to the ground floor because they were in “reasonably stable condition,” according to Dr. Womble, who relayed accounts by staffers who were there. Most of the people in the hospital rode out the storm in the first-floor cafeteria.
After the storm, patient rooms on the second floor were either no longer there or had been reduced to their steel innards.
The decision to move everyone undoubtedly saved lives. “If any of our patients stayed there, they’re probably all dead,” Dr. Raju said.
David Whitaker, CEO of Norman Regional Health System, which includes Moore Medical, marveled at the outcome.
“We had some bumps and bruises, some scratches, but no major lacerations, no broken bones, no injuries that people couldn’t ambulate. It’s totally amazing,” he said. “The leadership that was in place, the employees that were working at that time, they sprang into action, they took command and control of the situation. They got people into the proper areas.”
Dr. Womble said hospital staff at Moore Medical had still more amazing stories of death-defiance. They told him 30 people refused to leave the chapel. Somehow, the chapel remained intact, even though the hospital all around it was destroyed. Whitaker confirmed this.
One woman in active labor was kept in a second-floor operating room—which the medical staff thought was the best place for her, all things considered. Nurses covered the woman with pillows, blankets, and their own bodies as the tornado barreled through the town. She survived and gave birth to a boy several hours later. The parents gave him the middle name Emmanuel, which means “God is with us.”
As the tornado approached, an elderly volunteer had gone outside to get something from a van he used to transport elderly patients to and from a physical therapy program. “Nobody inside knew he had gone outside,” Dr. Womble said. By the time he tried to get back in, the power had gone out, and the doors wouldn’t open. He huddled behind a concrete pillar and ended up with just one minor laceration.
Patients eventually were taken to another hospital, Norman’s HealthPlex, about five miles south. Both Dr. Womble and Dr. Raju have begun working full time at the HealthPlex.
Dr. Raju said that he avoided being at Moore Medical during the tornado only by a turn of luck. He normally rounds at Moore in the afternoon and at the HealthPlex in the morning. But on that day, there were three new admissions at Moore, and only one at HealthPlex. So he went to Moore first, and was gone by the time the tornado hit.
“So lucky,” he said.

–David Whitaker, CEO of Norman Regional Health System
The Aftermath
It remains to be seen what kind of medical facility will be built to replace Moore Medical Center.
“Nobody knows what will happen next, but a lot of us speculate that they will not rebuild an inpatient facility,” said Dr. Womble, who had worked at Moore Medical Center for four years.
Whitaker said the first priority was to re-establish the clinics located at Moore Medical, and that has been done. The next step is, possibly, a temporary building in Moore for urgent care. The long-term plan remains in the discussion phase.
“We’ve already started having some meetings,” Whitaker said. “We’re going to determine what type of facility, what service levels it will be offering as we go back.”
It’s hard knowing that his hospital is no longer there, Dr. Raju said.
“We are going to miss it,” he said. “It’s unimaginable.”
Dr. Womble said those first few hours, when he wasn’t sure of where he’d be working, were difficult. He struggles to describe the feeling of not being able to provide care at his hospital at the time it’s most needed.
“It’s really hard to put it into words,” he said. “It’s the only hospital in that city, and it’s just me and my partner to take care of virtually everyone that comes in with any kind of medical problem. I definitely feel a tie to the community.
“It’s devastating. What is the rest of the city going to do for their hospital care? They essentially will not have a hospital in their city. They’ll have to drive to another city for care.”
Tom Collins is a freelance writer in South Florida.

–Joe R. Womble, MD
The first bit of feedback was fantastic: Everyone who had been inside the hospital—roughly 200 to 300 people, including 30 patients—had survived.
“Everyone was fine,” he said. “All the patients and staff, no one got injured. I was thinking that either the hospital was missed by the storm or that it must not have really damaged it very significantly.”
Unfortunately, the hospital was not OK. He watched as local TV painted a very different picture.
“They started showing aerial shots and I was just shocked. My jaw was just dropped,” Dr. Womble said. “The main entrance that I go in every day was literally stacked with three or four cars deep. A huge stack of about 30 cars was piled up on the main entrance, essentially.”
It was as though they were “toy cars.”
The May 20 tornado, a two-mile-wide superstorm boasting 200-mph winds that struck just south of Oklahoma City, claimed 24 lives and left the regional health system with a void in its network. It also left hospitalists mourning the loss of the place they called a second home several times a week. About a week after the storm, officials announced that Moore Medical Center would have to be demolished.
Miraculous Moments
Despite the terrible events, hospitalists and hospital officials were astounded by the good fortune of the hospital’s inhabitants. Dr. Womble said about 100 people from nearby neighborhoods and businesses used the hospital as shelter.
Senthil Raju, MD, a hospitalist who had done rounds at Moore Medical earlier that day, said the protocol was to take shelter in the hallways. But at some point, probably only minutes before the storm hit, the chief nurse and the house supervisor made the decision to move all the patients to the ground floor because they were in “reasonably stable condition,” according to Dr. Womble, who relayed accounts by staffers who were there. Most of the people in the hospital rode out the storm in the first-floor cafeteria.
After the storm, patient rooms on the second floor were either no longer there or had been reduced to their steel innards.
The decision to move everyone undoubtedly saved lives. “If any of our patients stayed there, they’re probably all dead,” Dr. Raju said.
David Whitaker, CEO of Norman Regional Health System, which includes Moore Medical, marveled at the outcome.
“We had some bumps and bruises, some scratches, but no major lacerations, no broken bones, no injuries that people couldn’t ambulate. It’s totally amazing,” he said. “The leadership that was in place, the employees that were working at that time, they sprang into action, they took command and control of the situation. They got people into the proper areas.”
Dr. Womble said hospital staff at Moore Medical had still more amazing stories of death-defiance. They told him 30 people refused to leave the chapel. Somehow, the chapel remained intact, even though the hospital all around it was destroyed. Whitaker confirmed this.
One woman in active labor was kept in a second-floor operating room—which the medical staff thought was the best place for her, all things considered. Nurses covered the woman with pillows, blankets, and their own bodies as the tornado barreled through the town. She survived and gave birth to a boy several hours later. The parents gave him the middle name Emmanuel, which means “God is with us.”
As the tornado approached, an elderly volunteer had gone outside to get something from a van he used to transport elderly patients to and from a physical therapy program. “Nobody inside knew he had gone outside,” Dr. Womble said. By the time he tried to get back in, the power had gone out, and the doors wouldn’t open. He huddled behind a concrete pillar and ended up with just one minor laceration.
Patients eventually were taken to another hospital, Norman’s HealthPlex, about five miles south. Both Dr. Womble and Dr. Raju have begun working full time at the HealthPlex.
Dr. Raju said that he avoided being at Moore Medical during the tornado only by a turn of luck. He normally rounds at Moore in the afternoon and at the HealthPlex in the morning. But on that day, there were three new admissions at Moore, and only one at HealthPlex. So he went to Moore first, and was gone by the time the tornado hit.
“So lucky,” he said.

–David Whitaker, CEO of Norman Regional Health System
The Aftermath
It remains to be seen what kind of medical facility will be built to replace Moore Medical Center.
“Nobody knows what will happen next, but a lot of us speculate that they will not rebuild an inpatient facility,” said Dr. Womble, who had worked at Moore Medical Center for four years.
Whitaker said the first priority was to re-establish the clinics located at Moore Medical, and that has been done. The next step is, possibly, a temporary building in Moore for urgent care. The long-term plan remains in the discussion phase.
“We’ve already started having some meetings,” Whitaker said. “We’re going to determine what type of facility, what service levels it will be offering as we go back.”
It’s hard knowing that his hospital is no longer there, Dr. Raju said.
“We are going to miss it,” he said. “It’s unimaginable.”
Dr. Womble said those first few hours, when he wasn’t sure of where he’d be working, were difficult. He struggles to describe the feeling of not being able to provide care at his hospital at the time it’s most needed.
“It’s really hard to put it into words,” he said. “It’s the only hospital in that city, and it’s just me and my partner to take care of virtually everyone that comes in with any kind of medical problem. I definitely feel a tie to the community.
“It’s devastating. What is the rest of the city going to do for their hospital care? They essentially will not have a hospital in their city. They’ll have to drive to another city for care.”
Tom Collins is a freelance writer in South Florida.

–Joe R. Womble, MD
The first bit of feedback was fantastic: Everyone who had been inside the hospital—roughly 200 to 300 people, including 30 patients—had survived.
“Everyone was fine,” he said. “All the patients and staff, no one got injured. I was thinking that either the hospital was missed by the storm or that it must not have really damaged it very significantly.”
Unfortunately, the hospital was not OK. He watched as local TV painted a very different picture.
“They started showing aerial shots and I was just shocked. My jaw was just dropped,” Dr. Womble said. “The main entrance that I go in every day was literally stacked with three or four cars deep. A huge stack of about 30 cars was piled up on the main entrance, essentially.”
It was as though they were “toy cars.”
The May 20 tornado, a two-mile-wide superstorm boasting 200-mph winds that struck just south of Oklahoma City, claimed 24 lives and left the regional health system with a void in its network. It also left hospitalists mourning the loss of the place they called a second home several times a week. About a week after the storm, officials announced that Moore Medical Center would have to be demolished.
Miraculous Moments
Despite the terrible events, hospitalists and hospital officials were astounded by the good fortune of the hospital’s inhabitants. Dr. Womble said about 100 people from nearby neighborhoods and businesses used the hospital as shelter.
Senthil Raju, MD, a hospitalist who had done rounds at Moore Medical earlier that day, said the protocol was to take shelter in the hallways. But at some point, probably only minutes before the storm hit, the chief nurse and the house supervisor made the decision to move all the patients to the ground floor because they were in “reasonably stable condition,” according to Dr. Womble, who relayed accounts by staffers who were there. Most of the people in the hospital rode out the storm in the first-floor cafeteria.
After the storm, patient rooms on the second floor were either no longer there or had been reduced to their steel innards.
The decision to move everyone undoubtedly saved lives. “If any of our patients stayed there, they’re probably all dead,” Dr. Raju said.
David Whitaker, CEO of Norman Regional Health System, which includes Moore Medical, marveled at the outcome.
“We had some bumps and bruises, some scratches, but no major lacerations, no broken bones, no injuries that people couldn’t ambulate. It’s totally amazing,” he said. “The leadership that was in place, the employees that were working at that time, they sprang into action, they took command and control of the situation. They got people into the proper areas.”
Dr. Womble said hospital staff at Moore Medical had still more amazing stories of death-defiance. They told him 30 people refused to leave the chapel. Somehow, the chapel remained intact, even though the hospital all around it was destroyed. Whitaker confirmed this.
One woman in active labor was kept in a second-floor operating room—which the medical staff thought was the best place for her, all things considered. Nurses covered the woman with pillows, blankets, and their own bodies as the tornado barreled through the town. She survived and gave birth to a boy several hours later. The parents gave him the middle name Emmanuel, which means “God is with us.”
As the tornado approached, an elderly volunteer had gone outside to get something from a van he used to transport elderly patients to and from a physical therapy program. “Nobody inside knew he had gone outside,” Dr. Womble said. By the time he tried to get back in, the power had gone out, and the doors wouldn’t open. He huddled behind a concrete pillar and ended up with just one minor laceration.
Patients eventually were taken to another hospital, Norman’s HealthPlex, about five miles south. Both Dr. Womble and Dr. Raju have begun working full time at the HealthPlex.
Dr. Raju said that he avoided being at Moore Medical during the tornado only by a turn of luck. He normally rounds at Moore in the afternoon and at the HealthPlex in the morning. But on that day, there were three new admissions at Moore, and only one at HealthPlex. So he went to Moore first, and was gone by the time the tornado hit.
“So lucky,” he said.

–David Whitaker, CEO of Norman Regional Health System
The Aftermath
It remains to be seen what kind of medical facility will be built to replace Moore Medical Center.
“Nobody knows what will happen next, but a lot of us speculate that they will not rebuild an inpatient facility,” said Dr. Womble, who had worked at Moore Medical Center for four years.
Whitaker said the first priority was to re-establish the clinics located at Moore Medical, and that has been done. The next step is, possibly, a temporary building in Moore for urgent care. The long-term plan remains in the discussion phase.
“We’ve already started having some meetings,” Whitaker said. “We’re going to determine what type of facility, what service levels it will be offering as we go back.”
It’s hard knowing that his hospital is no longer there, Dr. Raju said.
“We are going to miss it,” he said. “It’s unimaginable.”
Dr. Womble said those first few hours, when he wasn’t sure of where he’d be working, were difficult. He struggles to describe the feeling of not being able to provide care at his hospital at the time it’s most needed.
“It’s really hard to put it into words,” he said. “It’s the only hospital in that city, and it’s just me and my partner to take care of virtually everyone that comes in with any kind of medical problem. I definitely feel a tie to the community.
“It’s devastating. What is the rest of the city going to do for their hospital care? They essentially will not have a hospital in their city. They’ll have to drive to another city for care.”
Tom Collins is a freelance writer in South Florida.
11 Things Neurologists Think Hospitalists Need To Know
11 Things: At a Glance
- You might be overdiagnosing transient ischemic attacks (TIA).
- Early mobilization after a stroke might be better for some patients.
- MRI is the best tool to evaluate TIA patients.
- Consider focal seizure or complex partial seizure as one of the possible causes of confusion or speech disturbance, or both.
- Tracking the time a hospitalized patient was last seen to be normal is crucial.
- Consider neuromuscular disorders when a patient presents with weakness.
- Urinary tract infections (UTIs) are not the only cause of altered mental status.
- Take care in distinguishing aphasia from general confusion.
- A simple checklist might eliminate the need to consult the neurologist.
- Calling a neurologist earlier is way better than calling later.
- Hire a neurohospitalist if your institution doesn’t have one already.
When a patient is admitted to the hospital with neurological symptoms, such as altered mental status, he or she might not be the only one who is confused. Hospitalists might be a little confused, too.
Of all the subspecialties to which hospitalists are exposed, none might make them more uncomfortable than neurology. Because of what often is a dearth of training in this area, and because of the vexing and sometimes fleeting nature of symptoms, hospitalists might be inclined to lean on neurologists more than other specialists.
The Hospitalist spoke with a half-dozen experts, gathering their words of guidance and clinical tips. Here’s hoping they give you a little extra confidence the next time you see a patient with altered mental status.
You might be overdiagnosing transient ischemic attacks (TIA).
Ira Chang, MD, a neurohospitalist with Blue Sky Neurology in Englewood, Colo., and assistant clinical professor at the University of Colorado Health Sciences Center in Denver, says TIA is all too commonly a go-to diagnosis, frequently when there’s another cause.
“I think that hospitalists, and maybe medical internists in general, are very quick to diagnose anything that has a neurologic symptom that comes and goes as a TIA,” she says. “Patients have to have specific neurologic symptoms that we think are due to arterial blood flow or ischemia problems.”
Near-fainting spells and dizzy spells involving confusion commonly are diagnosed as TIA when these symptoms could be due to “a number of other causes,” Dr. Chang adds.
Kevin Barrett, MD, assistant professor of neurology and a neurohospitalist at Mayo Clinic in Jacksonville, Fla., says the suspicion of a TIA should be greater if the patient is older or has traditional cardiovascular risk factors, such as hyptertension, diabetes, hyperlipidemia, or tobacco use.
A TIA typically causes symptoms referable to common arterial distributions. Carotid-distribution TIA often causes ipsilateral loss of vision and contralateral weakness or numbness. Posterior-circulation TIAs bring on symptoms such as ataxia, unilateral or bilateral limb weakness, diplopia, and slurred or slow speech.
TIA diagnoses can be tricky even for those trained in neurology, Dr. Barrett says.
“Even among fellowship-trained vascular neurologists, TIA can be a challenging diagnosis, often with poor inter-observer agreement,” he notes.
Early mobilization after a stroke might be better for some patients.
After receiving tissue plasminogen activator (tPA) therapy for stroke, patients historically were kept on bed rest for 24 hours to reduce the risk of hemorrhage. Evidence now is coming to light that some patients might benefit from getting out of bed sooner, Dr. Barrett says.1
“We’re learning that in selected patients, they can actually be mobilized at 12 hours,” he says. “In some cases, that would not only reduce the risk of complications related to immobilization like DVT but shorten length of stay. These are all important metrics for anybody who practices primarily within an inpatient setting.”
Early mobilization generally is more suitable for patients with less severe deficits and who are hemodynamically stable.
MRI is the best tool to evaluate TIA patients.
TIA patients who have transient symptoms and normal diffusion-weighted imaging (DWI) abnormalities on an MRI are at a very low risk. “Less than 1% of those patients have a stroke within the subsequent seven days,” Dr. Barrett says.2 “But those patients who do have a DWI abnormality, they’re at very high risk: 7.1% at seven days.
“The utility of MRI following TIA is becoming very much apparent. It is something that hospitalists should be aware of.”
Consider focal seizure or complex partial seizure as one of the possible causes of confusion or speech disturbance, or both.
Patients experiencing confusion or speech disturbance or altered mentation—particularly if they’re elderly or have dementia—could be having a partial seizure, Dr. Chang says. Dementia patients have a 10% to 15% incidence of complex partial seizures, she says.
“I see that underdiagnosed a lot,” she says. “They keep coming back, and everybody diagnoses them with TIAs. So they keep getting put on aspirin, and they get switched to Aggrenox [to prevent clotting]. They keep coming back with the same symptoms.”
Tracking the time a hospitalized patient was last seen to be normal is crucial.
About 10% to 15% of strokes occur in patients who are in the hospital.
“While a lot of those strokes are perioperative, there also are patients who are going to be on hospitalist services,” says Eric Adelman, MD, assistant professor of neurology at the University of Michigan in Ann Arbor.
Hospitalists should note that patients suffering strokes are found not just in the ED but also on the floor, where all the tools for treatment might not be as readily available. That makes those cases a challenge and makes forethought that much more important, Dr. Adelman says.
“It’s a matter of trying to track down last normal times,” he says. “If they’re eligible for tPA and they’re within the therapeutic window, we should be able to do that within a hospital.”
Establishing a neurological baseline is particularly important for patients who are at higher stroke risk, like those with atrial fibrillation and other cardiovascular risk factors.
“In case something does happen,” Dr. Adelman says, “at least you have a baseline so you can [know that] at time X, we knew they had full strength in their right arm, and now they don’t.”
Consider neuromuscular disorders when a patient presents with weakness.
It’s safe to say some hospitalists might miss a neuromuscular disorder, Dr. Chang says.
“A lot of disorders that are harder for hospitalists to diagnose and that tend to take longer to call a neurologist [on] are things that are due to myasthenia gravis [a breakdown between nerves and muscles leading to muscle fatigue], myopathy, or ALS,” she says. “Many patients present with weakness. I think a lot of times there will be a lot of tests on and a lot of treatment for general medical conditions that can cause weakness.”
And that might be a case of misdirected attention. Patients with weakness accompanied by persistent swallowing problems, slurred speech with no other obvious cause, or the inability to lift their head off the bed without an obvious cause may end up with a neuromuscular diagnosis, she says.
It would be helpful to have a neurologist’s input in these cases, she says, where “nothing’s getting better, and three, four, five days later, the patient’s still weak.
“I think a neurologist would be more in tune with something like that,” she adds.
Urinary tract infections (UTIs) are not the only cause of altered mental status.
That might seem obvious, but too often, a UTI can be pegged as the source of altered mental status when it should not be, Dr. Chang says.
“We get a lot of people who come in with confusion and they have a slightly abnormal urinalysis and they diagnose them with UTI,” Dr. Chang says. “And it turns out that they actually had a stroke or they had a seizure.”
Significantly altered mentation should show a significantly abnormal urine with a positive culture, she says. “They ought to have significant laboratory support for a urinary tract infection.”
Dr. Barrett says a neurologic review of systems, or at least a neurologic exam, should be the physician’s guide.
“Those are key parts of a hospitalist’s practice,” he says, “because that’s what’s truly going to guide them to consider primary neurological causes of altered mental status.”
Take care in distinguishing aphasia from general confusion.
If a patient is still talking and is fairly fluent, that doesn’t mean they aren’t suffering from certain types of aphasia, a disorder caused by damage to parts of the brain that control language, Dr. Adelman says.
“Oftentimes, when you’re dealing with a patient with confusion, you want to make sure that it’s confusion, or encephalopathy, rather than a focal neurologic problem like aphasia,” he says. “Frequently patients with aphasia will have other symptoms such as a facial drop or weakness in the arm, but stroke can present as isolated aphasia.”
A good habit to get into is to determine whether the patient can repeat a phrase, follow a command, or name objects, he says. If they can, they probably do not have aphasia.
“The thing that you worry about with aphasia, particularly acute onset aphasia, is an ischemic stroke,” Dr. Adelman says.
A simple checklist might eliminate the need to consult the neurologist.
When Edgar Kenton, MD, now director of the stroke program at Geisinger Health System in Danville, Pa., was at Emory University Hospital in Atlanta, he found he was getting snowed under with consults from hospitalists. There were about 15 hospitalists for just one or two neurologists.
“There was no way I was able to see these patients, particularly in follow-up, because you might get five consults every day,” he says. “By the middle of the week, that’s 15 consults. You don’t get a chance to go back and see the patients because you’re just going from one consult to the other.”
The situation improved with a checklist of things to consider when a patient presents with altered mental status. Before seeking a consult, neurologists suggested the hospitalists check the electrolytes, blood pressure, and urine, and use CT scans as a screening test. That might uncover the root of the patient’s problems. If those are clear, by all means get the neurologist involved, he says.
“We were able to educate the hospitalists so they knew when to call; they knew when it was beyond their expertise to take care of the patient, so we weren’t getting called for every patient with altered mental status when all they needed to do was to check the electrolytes,” Dr. Kenton says.
Calling a neurologist earlier is way better than calling later.
Once the decision is made to consult with a neurologist, the consult should be done right away, Dr. Kenton says, not after a few days when symptoms don’t appear to be improving.
“We’ll get the call on a Friday afternoon because they thought, finally, ‘Well, you know, we need to get neurology involved because we a) haven’t solved the problem and b) there may be some other tests we should be getting,’” he says of common situations. “That has been a problem. If you don’t have a neurohospitalist involved day by day, working with the patient and the general hospitalist, neurology becomes an afterthought.”
He says accurate and early diagnosis is paramount to the patient.
“If the diagnosis is delayed, obviously there’s more insult to the patients, more persistent insult,” he says, noting the timing is particularly important in neurological conditions “because things can get bad in a hurry.”
He strongly urges hospitalists to consult with a neurologist before ordering an entire battery of tests.
At Geisinger, neurologists are encouraging hospitalists to chat informally with neurosurgeons about cases for guidance at the outset rather than after several days.
Hire a neurohospitalist if your institution doesn’t have one already.
At the top of the list of Dr. Kenton’s suggestions on caring for hospitalized neurology patients is this declaration: “Get a neurohospitalist.”
“It’s important to have the neurologist involved from the time the patient’s admitted,” he says. “That’s the value of connecting the general hospitalist with neurologists.”
S. Andrew Josephson, MD, director of the neurohospitalist program at the University of California at San Francisco, says his colleagues are team players and improve patient care.
“Neurology consultations can be called very quickly, and a nice partnership can develop between internal medicine hospitalists and neurohospitalists to care for those patients who have those medical and neurologic problems,” he says.
He also says having a neurohospitalist on board can ease some of the tension.
“No longer if there’s a neurologic condition does a hospitalist have to think about, ‘Well, does this rise to the level of something that I need to get the neurologist to drive across the city to come see?’” he explains. “‘Or is this something we should try to manage ourselves?’”
Tom Collins is a freelance writer in South Florida.
References
- Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G. A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke. 2008;39;390-396.
- Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs. time-defined TIA: a multicenter study. Neurology. 2011;77(13):1222-1228.
- Zinchuk AV, Flanagan EP, Tubridy NJ, Miller WA, McCullough LD. Attitudes of US medical trainees towards neurology education: “Neurophobia”—a global issue. BMC Med Educ. 2010;10:49.
11 Things: At a Glance
- You might be overdiagnosing transient ischemic attacks (TIA).
- Early mobilization after a stroke might be better for some patients.
- MRI is the best tool to evaluate TIA patients.
- Consider focal seizure or complex partial seizure as one of the possible causes of confusion or speech disturbance, or both.
- Tracking the time a hospitalized patient was last seen to be normal is crucial.
- Consider neuromuscular disorders when a patient presents with weakness.
- Urinary tract infections (UTIs) are not the only cause of altered mental status.
- Take care in distinguishing aphasia from general confusion.
- A simple checklist might eliminate the need to consult the neurologist.
- Calling a neurologist earlier is way better than calling later.
- Hire a neurohospitalist if your institution doesn’t have one already.
When a patient is admitted to the hospital with neurological symptoms, such as altered mental status, he or she might not be the only one who is confused. Hospitalists might be a little confused, too.
Of all the subspecialties to which hospitalists are exposed, none might make them more uncomfortable than neurology. Because of what often is a dearth of training in this area, and because of the vexing and sometimes fleeting nature of symptoms, hospitalists might be inclined to lean on neurologists more than other specialists.
The Hospitalist spoke with a half-dozen experts, gathering their words of guidance and clinical tips. Here’s hoping they give you a little extra confidence the next time you see a patient with altered mental status.
You might be overdiagnosing transient ischemic attacks (TIA).
Ira Chang, MD, a neurohospitalist with Blue Sky Neurology in Englewood, Colo., and assistant clinical professor at the University of Colorado Health Sciences Center in Denver, says TIA is all too commonly a go-to diagnosis, frequently when there’s another cause.
“I think that hospitalists, and maybe medical internists in general, are very quick to diagnose anything that has a neurologic symptom that comes and goes as a TIA,” she says. “Patients have to have specific neurologic symptoms that we think are due to arterial blood flow or ischemia problems.”
Near-fainting spells and dizzy spells involving confusion commonly are diagnosed as TIA when these symptoms could be due to “a number of other causes,” Dr. Chang adds.
Kevin Barrett, MD, assistant professor of neurology and a neurohospitalist at Mayo Clinic in Jacksonville, Fla., says the suspicion of a TIA should be greater if the patient is older or has traditional cardiovascular risk factors, such as hyptertension, diabetes, hyperlipidemia, or tobacco use.
A TIA typically causes symptoms referable to common arterial distributions. Carotid-distribution TIA often causes ipsilateral loss of vision and contralateral weakness or numbness. Posterior-circulation TIAs bring on symptoms such as ataxia, unilateral or bilateral limb weakness, diplopia, and slurred or slow speech.
TIA diagnoses can be tricky even for those trained in neurology, Dr. Barrett says.
“Even among fellowship-trained vascular neurologists, TIA can be a challenging diagnosis, often with poor inter-observer agreement,” he notes.
Early mobilization after a stroke might be better for some patients.
After receiving tissue plasminogen activator (tPA) therapy for stroke, patients historically were kept on bed rest for 24 hours to reduce the risk of hemorrhage. Evidence now is coming to light that some patients might benefit from getting out of bed sooner, Dr. Barrett says.1
“We’re learning that in selected patients, they can actually be mobilized at 12 hours,” he says. “In some cases, that would not only reduce the risk of complications related to immobilization like DVT but shorten length of stay. These are all important metrics for anybody who practices primarily within an inpatient setting.”
Early mobilization generally is more suitable for patients with less severe deficits and who are hemodynamically stable.
MRI is the best tool to evaluate TIA patients.
TIA patients who have transient symptoms and normal diffusion-weighted imaging (DWI) abnormalities on an MRI are at a very low risk. “Less than 1% of those patients have a stroke within the subsequent seven days,” Dr. Barrett says.2 “But those patients who do have a DWI abnormality, they’re at very high risk: 7.1% at seven days.
“The utility of MRI following TIA is becoming very much apparent. It is something that hospitalists should be aware of.”
Consider focal seizure or complex partial seizure as one of the possible causes of confusion or speech disturbance, or both.
Patients experiencing confusion or speech disturbance or altered mentation—particularly if they’re elderly or have dementia—could be having a partial seizure, Dr. Chang says. Dementia patients have a 10% to 15% incidence of complex partial seizures, she says.
“I see that underdiagnosed a lot,” she says. “They keep coming back, and everybody diagnoses them with TIAs. So they keep getting put on aspirin, and they get switched to Aggrenox [to prevent clotting]. They keep coming back with the same symptoms.”
Tracking the time a hospitalized patient was last seen to be normal is crucial.
About 10% to 15% of strokes occur in patients who are in the hospital.
“While a lot of those strokes are perioperative, there also are patients who are going to be on hospitalist services,” says Eric Adelman, MD, assistant professor of neurology at the University of Michigan in Ann Arbor.
Hospitalists should note that patients suffering strokes are found not just in the ED but also on the floor, where all the tools for treatment might not be as readily available. That makes those cases a challenge and makes forethought that much more important, Dr. Adelman says.
“It’s a matter of trying to track down last normal times,” he says. “If they’re eligible for tPA and they’re within the therapeutic window, we should be able to do that within a hospital.”
Establishing a neurological baseline is particularly important for patients who are at higher stroke risk, like those with atrial fibrillation and other cardiovascular risk factors.
“In case something does happen,” Dr. Adelman says, “at least you have a baseline so you can [know that] at time X, we knew they had full strength in their right arm, and now they don’t.”
Consider neuromuscular disorders when a patient presents with weakness.
It’s safe to say some hospitalists might miss a neuromuscular disorder, Dr. Chang says.
“A lot of disorders that are harder for hospitalists to diagnose and that tend to take longer to call a neurologist [on] are things that are due to myasthenia gravis [a breakdown between nerves and muscles leading to muscle fatigue], myopathy, or ALS,” she says. “Many patients present with weakness. I think a lot of times there will be a lot of tests on and a lot of treatment for general medical conditions that can cause weakness.”
And that might be a case of misdirected attention. Patients with weakness accompanied by persistent swallowing problems, slurred speech with no other obvious cause, or the inability to lift their head off the bed without an obvious cause may end up with a neuromuscular diagnosis, she says.
It would be helpful to have a neurologist’s input in these cases, she says, where “nothing’s getting better, and three, four, five days later, the patient’s still weak.
“I think a neurologist would be more in tune with something like that,” she adds.
Urinary tract infections (UTIs) are not the only cause of altered mental status.
That might seem obvious, but too often, a UTI can be pegged as the source of altered mental status when it should not be, Dr. Chang says.
“We get a lot of people who come in with confusion and they have a slightly abnormal urinalysis and they diagnose them with UTI,” Dr. Chang says. “And it turns out that they actually had a stroke or they had a seizure.”
Significantly altered mentation should show a significantly abnormal urine with a positive culture, she says. “They ought to have significant laboratory support for a urinary tract infection.”
Dr. Barrett says a neurologic review of systems, or at least a neurologic exam, should be the physician’s guide.
“Those are key parts of a hospitalist’s practice,” he says, “because that’s what’s truly going to guide them to consider primary neurological causes of altered mental status.”
Take care in distinguishing aphasia from general confusion.
If a patient is still talking and is fairly fluent, that doesn’t mean they aren’t suffering from certain types of aphasia, a disorder caused by damage to parts of the brain that control language, Dr. Adelman says.
“Oftentimes, when you’re dealing with a patient with confusion, you want to make sure that it’s confusion, or encephalopathy, rather than a focal neurologic problem like aphasia,” he says. “Frequently patients with aphasia will have other symptoms such as a facial drop or weakness in the arm, but stroke can present as isolated aphasia.”
A good habit to get into is to determine whether the patient can repeat a phrase, follow a command, or name objects, he says. If they can, they probably do not have aphasia.
“The thing that you worry about with aphasia, particularly acute onset aphasia, is an ischemic stroke,” Dr. Adelman says.
A simple checklist might eliminate the need to consult the neurologist.
When Edgar Kenton, MD, now director of the stroke program at Geisinger Health System in Danville, Pa., was at Emory University Hospital in Atlanta, he found he was getting snowed under with consults from hospitalists. There were about 15 hospitalists for just one or two neurologists.
“There was no way I was able to see these patients, particularly in follow-up, because you might get five consults every day,” he says. “By the middle of the week, that’s 15 consults. You don’t get a chance to go back and see the patients because you’re just going from one consult to the other.”
The situation improved with a checklist of things to consider when a patient presents with altered mental status. Before seeking a consult, neurologists suggested the hospitalists check the electrolytes, blood pressure, and urine, and use CT scans as a screening test. That might uncover the root of the patient’s problems. If those are clear, by all means get the neurologist involved, he says.
“We were able to educate the hospitalists so they knew when to call; they knew when it was beyond their expertise to take care of the patient, so we weren’t getting called for every patient with altered mental status when all they needed to do was to check the electrolytes,” Dr. Kenton says.
Calling a neurologist earlier is way better than calling later.
Once the decision is made to consult with a neurologist, the consult should be done right away, Dr. Kenton says, not after a few days when symptoms don’t appear to be improving.
“We’ll get the call on a Friday afternoon because they thought, finally, ‘Well, you know, we need to get neurology involved because we a) haven’t solved the problem and b) there may be some other tests we should be getting,’” he says of common situations. “That has been a problem. If you don’t have a neurohospitalist involved day by day, working with the patient and the general hospitalist, neurology becomes an afterthought.”
He says accurate and early diagnosis is paramount to the patient.
“If the diagnosis is delayed, obviously there’s more insult to the patients, more persistent insult,” he says, noting the timing is particularly important in neurological conditions “because things can get bad in a hurry.”
He strongly urges hospitalists to consult with a neurologist before ordering an entire battery of tests.
At Geisinger, neurologists are encouraging hospitalists to chat informally with neurosurgeons about cases for guidance at the outset rather than after several days.
Hire a neurohospitalist if your institution doesn’t have one already.
At the top of the list of Dr. Kenton’s suggestions on caring for hospitalized neurology patients is this declaration: “Get a neurohospitalist.”
“It’s important to have the neurologist involved from the time the patient’s admitted,” he says. “That’s the value of connecting the general hospitalist with neurologists.”
S. Andrew Josephson, MD, director of the neurohospitalist program at the University of California at San Francisco, says his colleagues are team players and improve patient care.
“Neurology consultations can be called very quickly, and a nice partnership can develop between internal medicine hospitalists and neurohospitalists to care for those patients who have those medical and neurologic problems,” he says.
He also says having a neurohospitalist on board can ease some of the tension.
“No longer if there’s a neurologic condition does a hospitalist have to think about, ‘Well, does this rise to the level of something that I need to get the neurologist to drive across the city to come see?’” he explains. “‘Or is this something we should try to manage ourselves?’”
Tom Collins is a freelance writer in South Florida.
References
- Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G. A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke. 2008;39;390-396.
- Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs. time-defined TIA: a multicenter study. Neurology. 2011;77(13):1222-1228.
- Zinchuk AV, Flanagan EP, Tubridy NJ, Miller WA, McCullough LD. Attitudes of US medical trainees towards neurology education: “Neurophobia”—a global issue. BMC Med Educ. 2010;10:49.
11 Things: At a Glance
- You might be overdiagnosing transient ischemic attacks (TIA).
- Early mobilization after a stroke might be better for some patients.
- MRI is the best tool to evaluate TIA patients.
- Consider focal seizure or complex partial seizure as one of the possible causes of confusion or speech disturbance, or both.
- Tracking the time a hospitalized patient was last seen to be normal is crucial.
- Consider neuromuscular disorders when a patient presents with weakness.
- Urinary tract infections (UTIs) are not the only cause of altered mental status.
- Take care in distinguishing aphasia from general confusion.
- A simple checklist might eliminate the need to consult the neurologist.
- Calling a neurologist earlier is way better than calling later.
- Hire a neurohospitalist if your institution doesn’t have one already.
When a patient is admitted to the hospital with neurological symptoms, such as altered mental status, he or she might not be the only one who is confused. Hospitalists might be a little confused, too.
Of all the subspecialties to which hospitalists are exposed, none might make them more uncomfortable than neurology. Because of what often is a dearth of training in this area, and because of the vexing and sometimes fleeting nature of symptoms, hospitalists might be inclined to lean on neurologists more than other specialists.
The Hospitalist spoke with a half-dozen experts, gathering their words of guidance and clinical tips. Here’s hoping they give you a little extra confidence the next time you see a patient with altered mental status.
You might be overdiagnosing transient ischemic attacks (TIA).
Ira Chang, MD, a neurohospitalist with Blue Sky Neurology in Englewood, Colo., and assistant clinical professor at the University of Colorado Health Sciences Center in Denver, says TIA is all too commonly a go-to diagnosis, frequently when there’s another cause.
“I think that hospitalists, and maybe medical internists in general, are very quick to diagnose anything that has a neurologic symptom that comes and goes as a TIA,” she says. “Patients have to have specific neurologic symptoms that we think are due to arterial blood flow or ischemia problems.”
Near-fainting spells and dizzy spells involving confusion commonly are diagnosed as TIA when these symptoms could be due to “a number of other causes,” Dr. Chang adds.
Kevin Barrett, MD, assistant professor of neurology and a neurohospitalist at Mayo Clinic in Jacksonville, Fla., says the suspicion of a TIA should be greater if the patient is older or has traditional cardiovascular risk factors, such as hyptertension, diabetes, hyperlipidemia, or tobacco use.
A TIA typically causes symptoms referable to common arterial distributions. Carotid-distribution TIA often causes ipsilateral loss of vision and contralateral weakness or numbness. Posterior-circulation TIAs bring on symptoms such as ataxia, unilateral or bilateral limb weakness, diplopia, and slurred or slow speech.
TIA diagnoses can be tricky even for those trained in neurology, Dr. Barrett says.
“Even among fellowship-trained vascular neurologists, TIA can be a challenging diagnosis, often with poor inter-observer agreement,” he notes.
Early mobilization after a stroke might be better for some patients.
After receiving tissue plasminogen activator (tPA) therapy for stroke, patients historically were kept on bed rest for 24 hours to reduce the risk of hemorrhage. Evidence now is coming to light that some patients might benefit from getting out of bed sooner, Dr. Barrett says.1
“We’re learning that in selected patients, they can actually be mobilized at 12 hours,” he says. “In some cases, that would not only reduce the risk of complications related to immobilization like DVT but shorten length of stay. These are all important metrics for anybody who practices primarily within an inpatient setting.”
Early mobilization generally is more suitable for patients with less severe deficits and who are hemodynamically stable.
MRI is the best tool to evaluate TIA patients.
TIA patients who have transient symptoms and normal diffusion-weighted imaging (DWI) abnormalities on an MRI are at a very low risk. “Less than 1% of those patients have a stroke within the subsequent seven days,” Dr. Barrett says.2 “But those patients who do have a DWI abnormality, they’re at very high risk: 7.1% at seven days.
“The utility of MRI following TIA is becoming very much apparent. It is something that hospitalists should be aware of.”
Consider focal seizure or complex partial seizure as one of the possible causes of confusion or speech disturbance, or both.
Patients experiencing confusion or speech disturbance or altered mentation—particularly if they’re elderly or have dementia—could be having a partial seizure, Dr. Chang says. Dementia patients have a 10% to 15% incidence of complex partial seizures, she says.
“I see that underdiagnosed a lot,” she says. “They keep coming back, and everybody diagnoses them with TIAs. So they keep getting put on aspirin, and they get switched to Aggrenox [to prevent clotting]. They keep coming back with the same symptoms.”
Tracking the time a hospitalized patient was last seen to be normal is crucial.
About 10% to 15% of strokes occur in patients who are in the hospital.
“While a lot of those strokes are perioperative, there also are patients who are going to be on hospitalist services,” says Eric Adelman, MD, assistant professor of neurology at the University of Michigan in Ann Arbor.
Hospitalists should note that patients suffering strokes are found not just in the ED but also on the floor, where all the tools for treatment might not be as readily available. That makes those cases a challenge and makes forethought that much more important, Dr. Adelman says.
“It’s a matter of trying to track down last normal times,” he says. “If they’re eligible for tPA and they’re within the therapeutic window, we should be able to do that within a hospital.”
Establishing a neurological baseline is particularly important for patients who are at higher stroke risk, like those with atrial fibrillation and other cardiovascular risk factors.
“In case something does happen,” Dr. Adelman says, “at least you have a baseline so you can [know that] at time X, we knew they had full strength in their right arm, and now they don’t.”
Consider neuromuscular disorders when a patient presents with weakness.
It’s safe to say some hospitalists might miss a neuromuscular disorder, Dr. Chang says.
“A lot of disorders that are harder for hospitalists to diagnose and that tend to take longer to call a neurologist [on] are things that are due to myasthenia gravis [a breakdown between nerves and muscles leading to muscle fatigue], myopathy, or ALS,” she says. “Many patients present with weakness. I think a lot of times there will be a lot of tests on and a lot of treatment for general medical conditions that can cause weakness.”
And that might be a case of misdirected attention. Patients with weakness accompanied by persistent swallowing problems, slurred speech with no other obvious cause, or the inability to lift their head off the bed without an obvious cause may end up with a neuromuscular diagnosis, she says.
It would be helpful to have a neurologist’s input in these cases, she says, where “nothing’s getting better, and three, four, five days later, the patient’s still weak.
“I think a neurologist would be more in tune with something like that,” she adds.
Urinary tract infections (UTIs) are not the only cause of altered mental status.
That might seem obvious, but too often, a UTI can be pegged as the source of altered mental status when it should not be, Dr. Chang says.
“We get a lot of people who come in with confusion and they have a slightly abnormal urinalysis and they diagnose them with UTI,” Dr. Chang says. “And it turns out that they actually had a stroke or they had a seizure.”
Significantly altered mentation should show a significantly abnormal urine with a positive culture, she says. “They ought to have significant laboratory support for a urinary tract infection.”
Dr. Barrett says a neurologic review of systems, or at least a neurologic exam, should be the physician’s guide.
“Those are key parts of a hospitalist’s practice,” he says, “because that’s what’s truly going to guide them to consider primary neurological causes of altered mental status.”
Take care in distinguishing aphasia from general confusion.
If a patient is still talking and is fairly fluent, that doesn’t mean they aren’t suffering from certain types of aphasia, a disorder caused by damage to parts of the brain that control language, Dr. Adelman says.
“Oftentimes, when you’re dealing with a patient with confusion, you want to make sure that it’s confusion, or encephalopathy, rather than a focal neurologic problem like aphasia,” he says. “Frequently patients with aphasia will have other symptoms such as a facial drop or weakness in the arm, but stroke can present as isolated aphasia.”
A good habit to get into is to determine whether the patient can repeat a phrase, follow a command, or name objects, he says. If they can, they probably do not have aphasia.
“The thing that you worry about with aphasia, particularly acute onset aphasia, is an ischemic stroke,” Dr. Adelman says.
A simple checklist might eliminate the need to consult the neurologist.
When Edgar Kenton, MD, now director of the stroke program at Geisinger Health System in Danville, Pa., was at Emory University Hospital in Atlanta, he found he was getting snowed under with consults from hospitalists. There were about 15 hospitalists for just one or two neurologists.
“There was no way I was able to see these patients, particularly in follow-up, because you might get five consults every day,” he says. “By the middle of the week, that’s 15 consults. You don’t get a chance to go back and see the patients because you’re just going from one consult to the other.”
The situation improved with a checklist of things to consider when a patient presents with altered mental status. Before seeking a consult, neurologists suggested the hospitalists check the electrolytes, blood pressure, and urine, and use CT scans as a screening test. That might uncover the root of the patient’s problems. If those are clear, by all means get the neurologist involved, he says.
“We were able to educate the hospitalists so they knew when to call; they knew when it was beyond their expertise to take care of the patient, so we weren’t getting called for every patient with altered mental status when all they needed to do was to check the electrolytes,” Dr. Kenton says.
Calling a neurologist earlier is way better than calling later.
Once the decision is made to consult with a neurologist, the consult should be done right away, Dr. Kenton says, not after a few days when symptoms don’t appear to be improving.
“We’ll get the call on a Friday afternoon because they thought, finally, ‘Well, you know, we need to get neurology involved because we a) haven’t solved the problem and b) there may be some other tests we should be getting,’” he says of common situations. “That has been a problem. If you don’t have a neurohospitalist involved day by day, working with the patient and the general hospitalist, neurology becomes an afterthought.”
He says accurate and early diagnosis is paramount to the patient.
“If the diagnosis is delayed, obviously there’s more insult to the patients, more persistent insult,” he says, noting the timing is particularly important in neurological conditions “because things can get bad in a hurry.”
He strongly urges hospitalists to consult with a neurologist before ordering an entire battery of tests.
At Geisinger, neurologists are encouraging hospitalists to chat informally with neurosurgeons about cases for guidance at the outset rather than after several days.
Hire a neurohospitalist if your institution doesn’t have one already.
At the top of the list of Dr. Kenton’s suggestions on caring for hospitalized neurology patients is this declaration: “Get a neurohospitalist.”
“It’s important to have the neurologist involved from the time the patient’s admitted,” he says. “That’s the value of connecting the general hospitalist with neurologists.”
S. Andrew Josephson, MD, director of the neurohospitalist program at the University of California at San Francisco, says his colleagues are team players and improve patient care.
“Neurology consultations can be called very quickly, and a nice partnership can develop between internal medicine hospitalists and neurohospitalists to care for those patients who have those medical and neurologic problems,” he says.
He also says having a neurohospitalist on board can ease some of the tension.
“No longer if there’s a neurologic condition does a hospitalist have to think about, ‘Well, does this rise to the level of something that I need to get the neurologist to drive across the city to come see?’” he explains. “‘Or is this something we should try to manage ourselves?’”
Tom Collins is a freelance writer in South Florida.
References
- Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G. A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke. 2008;39;390-396.
- Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs. time-defined TIA: a multicenter study. Neurology. 2011;77(13):1222-1228.
- Zinchuk AV, Flanagan EP, Tubridy NJ, Miller WA, McCullough LD. Attitudes of US medical trainees towards neurology education: “Neurophobia”—a global issue. BMC Med Educ. 2010;10:49.
Obituary: Laura Mirkinson, MD, MSc, FAAP
Laura Mirkinson, MD, MSc, FAAP, pediatric hospitalist and a founder of the American Academy of Pediatrics’ (AAP) Section on Hospital Medicine Executive Committee, died April 29 of ovarian cancer. She was 60.
Dr. Mirkinson received her medical degree from Uniformed Services University of the Health Sciences (USUHS) in Bethesda, Md., before serving her pediatrics residency at Bethesda Naval Hospital and Walter Reed Army Medical Center. She served as an active-duty medical officer in the U.S. Naval Medical Corps Reserves before retiring as a captain in 2000. She then worked at Children’s Hospital of Washington and the pediatric hospitalist group at Holy Cross Hospital in Silver Spring, Md.
Dr. Mirkinson played a role in the development of pediatric hospital medicine during and after her military service. In 2007, she was elected chief of pediatrics at Blythedale Children’s Hospital in Valhalla, N.Y. She also served as director of education for the AAP’s Section on Hospital Medicine and was its second chairperson, following the section’s co-founder Jack Percelay, MD, FAAP. Through this work, she helped promote the AAP Section on Hospital Medicine newsletter, as well as the Hospital Pediatrics journal.
Dr. Mirkinson’s colleagues and students remember her as a caring, insightful teacher and mentor who offered wise advice on all matters professional and personal. Her devotion to patient care was evident throughout her career. Even in her administrative roles, she would often practice clinical medicine to help fill scheduling gaps or cover other physicians’ vacations.
Laura Mirkinson, MD, MSc, FAAP, pediatric hospitalist and a founder of the American Academy of Pediatrics’ (AAP) Section on Hospital Medicine Executive Committee, died April 29 of ovarian cancer. She was 60.
Dr. Mirkinson received her medical degree from Uniformed Services University of the Health Sciences (USUHS) in Bethesda, Md., before serving her pediatrics residency at Bethesda Naval Hospital and Walter Reed Army Medical Center. She served as an active-duty medical officer in the U.S. Naval Medical Corps Reserves before retiring as a captain in 2000. She then worked at Children’s Hospital of Washington and the pediatric hospitalist group at Holy Cross Hospital in Silver Spring, Md.
Dr. Mirkinson played a role in the development of pediatric hospital medicine during and after her military service. In 2007, she was elected chief of pediatrics at Blythedale Children’s Hospital in Valhalla, N.Y. She also served as director of education for the AAP’s Section on Hospital Medicine and was its second chairperson, following the section’s co-founder Jack Percelay, MD, FAAP. Through this work, she helped promote the AAP Section on Hospital Medicine newsletter, as well as the Hospital Pediatrics journal.
Dr. Mirkinson’s colleagues and students remember her as a caring, insightful teacher and mentor who offered wise advice on all matters professional and personal. Her devotion to patient care was evident throughout her career. Even in her administrative roles, she would often practice clinical medicine to help fill scheduling gaps or cover other physicians’ vacations.
Laura Mirkinson, MD, MSc, FAAP, pediatric hospitalist and a founder of the American Academy of Pediatrics’ (AAP) Section on Hospital Medicine Executive Committee, died April 29 of ovarian cancer. She was 60.
Dr. Mirkinson received her medical degree from Uniformed Services University of the Health Sciences (USUHS) in Bethesda, Md., before serving her pediatrics residency at Bethesda Naval Hospital and Walter Reed Army Medical Center. She served as an active-duty medical officer in the U.S. Naval Medical Corps Reserves before retiring as a captain in 2000. She then worked at Children’s Hospital of Washington and the pediatric hospitalist group at Holy Cross Hospital in Silver Spring, Md.
Dr. Mirkinson played a role in the development of pediatric hospital medicine during and after her military service. In 2007, she was elected chief of pediatrics at Blythedale Children’s Hospital in Valhalla, N.Y. She also served as director of education for the AAP’s Section on Hospital Medicine and was its second chairperson, following the section’s co-founder Jack Percelay, MD, FAAP. Through this work, she helped promote the AAP Section on Hospital Medicine newsletter, as well as the Hospital Pediatrics journal.
Dr. Mirkinson’s colleagues and students remember her as a caring, insightful teacher and mentor who offered wise advice on all matters professional and personal. Her devotion to patient care was evident throughout her career. Even in her administrative roles, she would often practice clinical medicine to help fill scheduling gaps or cover other physicians’ vacations.