User login
What Is the Best Management of Hereditary Angioedema?
Case
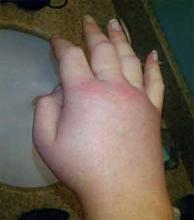
A 36-year-old man with a known history of hereditary angioedema (HAE) presents with severe orofacial swelling and laryngeal angioedema, requiring expectant management, including endotracheal intubation. His previous angioedema (AE) episodes involved his hands, feet, and genitalia; episodes generally occurred after physical trauma. Ten years prior to admission, he had an episode of secondary small bowel obstruction. The patient had been prescribed prophylactic danazol (Danacrine) 100 mg BID but he had gradually been reducing the dosage due to mood changes; at the time of presentation, he had already tapered to 100 mg danazol three times per week (Monday, Wednesday, and Friday).
Overview
HAE is an autosomal dominant condition characterized by localized, episodic swelling of the deeper dermal layers and/or mucosal tissue. Its acute presentation can vary in severity; presentations can be lethal.
HAE is generally unresponsive to conventional treatments used for other causes of AE (e.g. food or drug reactions) including glucocorticoids, antihistamines, and epinephrine. The pharmacologic treatment of acute attacks, as well as for short- and long-term prophylaxis of HAE, has evolved significantly in recent years and now includes several forms of C1 inhibitor (C1INH) protein replacement, as well as a bradykinin antagonist, and a kallikrein inhibitor.
Review of the Data
Epidemiology. HAE is an autosomal dominant disease with prevalence in the U.S. of 1 in 10,000 to 1 in 50,000 patients. All ethnic groups are equally affected, with no gender predilection. In most cases, a positive family history is present; however, in 25% of cases, spontaneous mutations occur such that an unremarkable family history does not rule out the diagnosis.1
Pathophysiology. In the past decade, there has been substantial advancement in our understanding of HAE pathophysiology. HAE occurs as a result of functional or quantitative C1 esterase inhibitor (C1INH) deficiency.
C1INH belongs to a group of proteins known as serpins (serine protease inhibitors). The C1INH gene is located on chromosome 11, and has several polymorphic sites, which predispose to spontaneous mutations.1
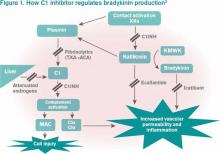
Bradykinin is the core bioactive mediator, which causes vasodilation, smooth muscle contraction, and subsequent edema.1 C1INH regulates bradykinin production by blocking kallikrein’s conversion of factor XII into XIIa, prekallikrein to kallikrein, and cleavage of high-molecular-weight kininogen by activated kallikrein to form bradykinin (see Figure 1).1,2
Clinical Manifestations
HAE is characterized by recurrent episodes of swelling, the frequency and severity of which are quite variable. Virtually all HAE patients have abdominal- and extremity-swelling episodes, and 50% will have episodes of laryngeal swelling; other involved areas might include the face, oropharynx, and genitalia.4 These episodes are usually unilateral; edema is nonpruritic, nonpitting, and often painless. Episodes involving the oropharynx, larynx, and abdomen can be associated with potentially serious morbidity and mortality.1, 3
HAE episodes usually commence during late childhood and early puberty (on average at age 11). Approximately half of HAE patients will have oropharyngeal involvement that might occur many years, even decades, after the initial onset of the disease. The annual rate of severe, life-threatening laryngeal edema was 0.9% in a recent retrospective study.4
Severity of the disease is variable. Attacks are episodic, and occur on average every 10 to 20 days in untreated patients. These attacks typically peak over 24 hours, then usually resolve after 48 to 72 hours. However, the complete resolution of signs and symptoms can last for up to one week after the attacks.5
There is no concomitant pruritus or urticaria that accompanies the AE. However, erythema marginatum, an evanescent nonpruritic rash with serpiginous borders involving the trunk and inner surface of extremities but sparing the face, might herald the onset of an episode. This rash usually has central pallor that blanches with pressure and worsens with heat.
HAE can be triggered by stressful events, including trauma, surgery, menstruation, and viral infections. However, in many instances, HAE attacks occur without an identifiable cause.5
Differential Diagnosis from Other Causes of Angioedema
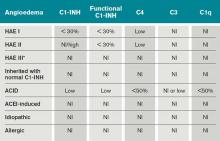
Type I HAE is characterized by a quantitative C1INH deficiency (which is functionally abnormal as well), and occurs in 85% of patients. Type II HAE occurs in 15% of patients, and results from a functionally abnormal C1INH.
In patients with Type I and II HAE, as well as acquired C1 inhibitor deficiency (ACID), C4 levels are low during and between attacks. C2 levels are also low during acute attacks. In ACID, levels of C1q are also reduced; these patients require further workup to rule out an undiagnosed malignancy or an autoimmune process. In contrast, patients with ACE-induced, idiopathic, and allergic AE have normal complement profiles.3,6
Type III is a more recently described type of HAE that is rare, not well understood, and generally affects women.3,6 Clinically, it resembles Type I and Type II HAE but complement levels, including C1 inhibitor, are normal (see Table 1).
Treatment
HAE types I, II, III, and ACID are generally unresponsive to glucocorticoids, antihistamines, and epinephrine. These forms of AE may be exacerbated by exogenous estrogen.1,8 For this reason, HAE patients should avoid oral hormonal contraception and estrogen replacement therapy. In addition, ACE inhibitors should also be avoided based on their effect on bradykinin degradation.
Until the introduction of newer therapeutic choices, as noted in our case, the treatment of acute attacks of AE was essentially supportive. Patients with impending laryngeal obstruction were managed with intubation prior to progression of the AE to limit airway patency. Prior to the modern era, a substantial proportion of HAE patients died of asphyxiation.
Fresh frozen plasma (FFP) has been used to treat acute HAE attacks, but given its content of contact system proteins (in addition to C1INH), FFP might also pose a risk for worsening of HAE; for this reason, it must be given cautiously to patients who are symptomatic.9
In the past decade, there has been significant progress in the available treatments for HAE. Currently in the U.S., there are several agents recently approved by, or have pending approvals from, the FDA, including several forms of C1INH replacement, a bradykinin antagonist, and a kallikrein inhibitor.
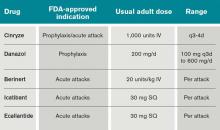
The C1 esterase inhibitor (human) drugs are administered intravenously; both have been shown to be efficacious and safe. Nanofiltered C1 inhibitor provided relief in a median time of two hours when used acutely; when used as prophylaxis, it decreased the number of attacks in a three-month period by 50% (six vs. 12 with placebo, P<0.001).11
The other C1INH is rhucin, still not approved in U.S. This drug is characterized by a short half-life (approximately two to four hours) compared with the plasma-derived C1INH agents (24 to 48 hours). It is contraindicated in patients with rabbit hypersensitivity, as it is purified from rabbit breast milk.10
Ecallantide is a kallikrein inhibitor for acute therapy that is administered via three subcutaneous injections. This agent has been linked to allergic/anaphylactic reactions in a minority of patients (approximately 4%); therefore, it should be administered cautiously, by a health-care provider, and in a setting where anaphylaxis can be successfully managed.12 Icatibant is a bradykinin antagonist recently approved in the U.S. and administered SC via a single injection.10
In light of the development of these new agents, there is a need for updated guidelines for the long- and short-term prophylaxis and acute management of HAE. A recent guideline focused on the management of HAE in gynecologic and obstetric patients recommended the use of plasma-derived C1INH C1 esterase inhibitor (human) (Cinryze) for short- and long-term prophylaxis and acute treatment of HAE.13 The effect of pregnancy on HAE is variable: Some women worsen and other women have less swelling during their pregnancy. Swelling at the time of parturition is rare; however, the risk rises during the post-partum period.
Type III HAE. An additional form of HAE has been recognized with a pattern of AE episodes that mimics Type I or Type II HAE but with unremarkable laboratory studies of the complement cascade, including C1 inhibitor level and function. At this time, there is no laboratory test with which a diagnosis of Type III HAE can be confirmed. The diagnosis should be suspected in patients with a strong family history of AE reflecting autosomal dominant inheritance. In some, but not all, cases, the condition is manifest in association with high estrogen levels (e.g. pregnancy or administration of oral contraceptives). Type III HAE patients have a salutary response to the same agents that are efficacious for Type I and II HAE.
Acquired C1 inhibitor deficiency (ACID). ACID generally occurs in adults and is clinically indistinguishable from HAE. ACID is not associated with a remarkable family history of AE. In contrast to HAE, this is a consumptive deficiency of C1 inhibitor and results from enhanced catabolism that exceeds the capacity for regenerating C1 inhibitor protein. It is often associated with neoplastic (usually lymphoproliferative) or autoimmune disorders; treatment of the underlying condition frequently leads to improvement in ACID. Although its management is similar to HAE, it tends to be more responsive to anti-fibrinolytics. A salutary response to C1INH replacement therapy might not occur in patients with autoantibodies to C1 inhibitor, but efficacy of ecallantide and icatibant for the treatment of acquired AE has been reported.14, 15
ACEI angioedema. Treatment with angiotensin-converting enzyme inhibitors (ACE-I) has been associated with recurrent AE without urticaria in 0.1 to 0.7% of patients exposed to these drugs.16 Angioedema from ACE-I more frequently occurs within the first few months of therapy, but it might occur even after years of continuous therapy. ACEI-induced AE is secondary to impaired degradation of bradykinin. The main treatment is to discontinue the offending agent and avoid all other ACE-I, as this is a class-specific reaction.17
Angiotensin receptor blockers (ARBs) have been associated less commonly with AE. The mechanism for ARB-associated AE has not been elucidated. A meta-analysis showed that in 2% to 17% of patients who were switched to ARBs, recurrence of AE was observed.18 From the pooling of these data with two randomized controlled trials, it is estimated that approximately 10% or less of patients with ACEI-associated AE who switched to ARBs will develop AE.19 In the majority of cases, patients can be switched to ARBs with no recurrence of AE; however, the decision to prescribe an ARB to a patient who has had AE while receiving ACEI should be made carefully on an individualized risk/benefit basis.19
Preventive Treatment
The 17 α-alkylated androgens that can be used for treatment of HAE are danazol (Danacrine), stanozolol (Winstrol), oxandralone (Oxandrine) and methyltestosterone (Android). In patients with HAE, attenuated androgens can significantly reduce the frequency and severity of attacks; however, their use is limited by risk for untoward effects (virilization, abnormal liver function tests, change in libido, anxiety, etc.).21 There is also a risk for hepatotoxicity, including development of hepatic adenomas and hepatic carcinoma.
Antifibrinolytics also may have efficacy for HAE, but these agents have been associated with a variety of adverse effects, including nausea and diarrhea, postural hypotension, fatigue, enhanced thrombosis, retinal changes, and teratogenicity.8, 22, 23
In 2009, long-term prophylaxis with C1-INH concentrate was recommended for patients with HAE with frequent or disabling attacks, a history of laryngeal attacks, and poor quality of life. The 2007 International Consensus Algorithm for the Diagnosis, Therapy, and Management of HAE recommended long-term prophylaxis in patients with more than one monthly severe HAE attack, more than five days of disability per month, or any history of airway compromise.24, 25
The decision to prescribe long-term prophylaxis, and the dose/frequency of medication required, should be individualized based on clinical parameters, such as frequency and severity of attacks, and not on C1 INH or C4 levels.
Perioperative Considerations
It is well established that any trauma, including dental procedures or surgery, can precipitate HAE attacks. For this reason, short-term prophylactic treatment in HAE patients undergoing procedures is recommended. Ideally, avoiding endotracheal intubation is the best approach; however, if intubation cannot be avoided, then adequate prophylaxis should be administered.2
Attenuated androgens can be given up to seven days before a procedure, or C1 INH can be administered 24 hours in advance. If C1 INH is unavailable, FFP can be given six to 12 hours in advance in patients who are not symptomatic; in case of endotracheal intubation, either FFP or C1 INH should be administered immediately before.2
Several case reports in multiple specialty surgical patients (abdominal surgery, cardiopulmonary bypass, orthopedic surgery, etc.) have confirmed the successful use of C1 INH in the prevention of acute attacks with favorable outcomes.2
There is no need to follow C1 INH levels, as it has no clinical relevance.
Back to the Case
The patient was admitted to the ICU and received a total of eight units of FFP. He was transferred to our institution and was able to be extubated three days after initial presentation. Laboratory studies revealed C4 10mg/dL and C1 esterase inhibitor 10mg/dL (both low).
Danazol was resumed. However, within several months after discharge, Cinryze became available in the U.S. market and was eventually prescribed. The patient has not had further significant attacks requiring inpatient management.
Dr. Auron is an assistant professor of medicine and pediatrics at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University. Dr. Lang is co-director of the Asthma Center and director of the Allergy/Immunology Fellowship Training Program at the Cleveland Clinic.
References
- Bernstein, JA. Update on angioedema: evaluation, diagnosis, and treatment. Allergy Asthma Proc. 2011;32(6):408-412.
- Levy JH, Freiberger DJ, Roback J. Hereditary angioedema: current and emerging treatment options. Anesth Analg. 2010;110(5):1271-1280.
- Busse PJ. Angioedema: Differential diagnosis and treatment. Allergy Asthma Proc. 2011;32:Suppl 1:S3-S11.
- Khan DA. Hereditary angioedema: historical aspects, classification, pathophysiology, clinical presentation, and laboratory diagnosis. Allergy Asthma Proc. 2011;32(1):1-10.
- Bork K, Meng G, Staubach P, Hardt, J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006;119(3):267-274.
- Zuraw BL, Christiansen SC. Pathogenesis and laboratory diagnosis of hereditary angioedema. Allergy Asthma Proc. 2009;30:487-492.
- Frazer-Abel A, Giclas PC. Update on laboratory tests for the diagnosis and differentiation of hereditary angioedema and acquired angioedema. Allergy Asthma Proc. 2011;32:Suppl 1:S17-S21.
- Banerjee A. Current treatment of hereditary angioedema: an update on clinical studies. Allergy Asthma Proc. 2010;31:398-406.
- Donaldson VH. Therapy of "the neurotic edema." N Engl J Med. 1972;286(15):835-836.
- Riedl MA. Update on the acute treatment of hereditary angioedema. Allergy Asthma Proc. 2011;32:11-16.
- Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. 2010;363:513-522.
- Cicardi M, Levy RJ, McNeil DL. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med. 2010;363:523-531.
- Caballero T, Farkas H, Bouillet L, et al. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol. 2012;129(2):308-320.
- Cicardi M, Zanichelli A. Acquired angioedema. J Allergy Clin Immunol. 2010;6(1):14.
- Zanichelli A, Badini M, Nataloni I, Montano N, Cicardi M. Treatment of acquired angioedema with icatibant: a case report. Intern Emerg Med. 2011;6(3):279-280.
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26(4):725-737.
- Haymore BR, Yoon J, Mikita CP, Klote MM, DeZee KJ. Risk of angioedema with angiotensin receptor blockers in patients with prior angioedema associated with angiotensin-converting enzyme inhibitors: a meta-analysis. Ann Allergy Asthma Immunol. 2008;101(5):495-499.
- Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2011;45(4):520-524.
- Nzeako UC. Diagnosis and management of angioedema with abdominal involvement: a gastroenterology perspective. World J Gastroenterol. 2010; 16(39):4913-4921.
- Banerji A, Sloane DE, Sheffer AL. Hereditary angioedema: a current state-of-the-art review, V: attenuated androgens for the treatment of hereditary angioedema. Ann Allergy Asthma Immunol. 2008;100(1) (Suppl 2):S19-22.
- Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med. 2008; 359(10):1027-1036.
- Zuraw BL. Hereditary angioedema: a current state-of-the-art review, IV: short- and long-term treatment of hereditary angioedema: out with the old and in with the new? Ann Allergy Asthma Immunol. 2008;100(1) (Suppl 2):S13-S18.
- Bowen T, Cicardi M, Bork K, et al. Hereditary angioedema: a current state-of-the-art review, VII: Canadian Hungarian 2007 International Consensus Algorithm for the Diagnosis, Therapy, and Management of Hereditary Angioedema. Ann Allergy Asthma Immunol. 2008;100(1)(Suppl 2):S30-40.
- Craig T, Riedl M, Dykewicz M, et al. When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol. 2009.102(5):366-372.
- Frank MM. Update on preventive therapy (prophylaxis) of hereditary angioedema. Allergy Asthma Proc. 2011;32(1):17-21.
Case
A 36-year-old man with a known history of hereditary angioedema (HAE) presents with severe orofacial swelling and laryngeal angioedema, requiring expectant management, including endotracheal intubation. His previous angioedema (AE) episodes involved his hands, feet, and genitalia; episodes generally occurred after physical trauma. Ten years prior to admission, he had an episode of secondary small bowel obstruction. The patient had been prescribed prophylactic danazol (Danacrine) 100 mg BID but he had gradually been reducing the dosage due to mood changes; at the time of presentation, he had already tapered to 100 mg danazol three times per week (Monday, Wednesday, and Friday).
Overview
HAE is an autosomal dominant condition characterized by localized, episodic swelling of the deeper dermal layers and/or mucosal tissue. Its acute presentation can vary in severity; presentations can be lethal.
HAE is generally unresponsive to conventional treatments used for other causes of AE (e.g. food or drug reactions) including glucocorticoids, antihistamines, and epinephrine. The pharmacologic treatment of acute attacks, as well as for short- and long-term prophylaxis of HAE, has evolved significantly in recent years and now includes several forms of C1 inhibitor (C1INH) protein replacement, as well as a bradykinin antagonist, and a kallikrein inhibitor.
Review of the Data
Epidemiology. HAE is an autosomal dominant disease with prevalence in the U.S. of 1 in 10,000 to 1 in 50,000 patients. All ethnic groups are equally affected, with no gender predilection. In most cases, a positive family history is present; however, in 25% of cases, spontaneous mutations occur such that an unremarkable family history does not rule out the diagnosis.1
Pathophysiology. In the past decade, there has been substantial advancement in our understanding of HAE pathophysiology. HAE occurs as a result of functional or quantitative C1 esterase inhibitor (C1INH) deficiency.
C1INH belongs to a group of proteins known as serpins (serine protease inhibitors). The C1INH gene is located on chromosome 11, and has several polymorphic sites, which predispose to spontaneous mutations.1
Bradykinin is the core bioactive mediator, which causes vasodilation, smooth muscle contraction, and subsequent edema.1 C1INH regulates bradykinin production by blocking kallikrein’s conversion of factor XII into XIIa, prekallikrein to kallikrein, and cleavage of high-molecular-weight kininogen by activated kallikrein to form bradykinin (see Figure 1).1,2
Clinical Manifestations
HAE is characterized by recurrent episodes of swelling, the frequency and severity of which are quite variable. Virtually all HAE patients have abdominal- and extremity-swelling episodes, and 50% will have episodes of laryngeal swelling; other involved areas might include the face, oropharynx, and genitalia.4 These episodes are usually unilateral; edema is nonpruritic, nonpitting, and often painless. Episodes involving the oropharynx, larynx, and abdomen can be associated with potentially serious morbidity and mortality.1, 3
HAE episodes usually commence during late childhood and early puberty (on average at age 11). Approximately half of HAE patients will have oropharyngeal involvement that might occur many years, even decades, after the initial onset of the disease. The annual rate of severe, life-threatening laryngeal edema was 0.9% in a recent retrospective study.4
Severity of the disease is variable. Attacks are episodic, and occur on average every 10 to 20 days in untreated patients. These attacks typically peak over 24 hours, then usually resolve after 48 to 72 hours. However, the complete resolution of signs and symptoms can last for up to one week after the attacks.5
There is no concomitant pruritus or urticaria that accompanies the AE. However, erythema marginatum, an evanescent nonpruritic rash with serpiginous borders involving the trunk and inner surface of extremities but sparing the face, might herald the onset of an episode. This rash usually has central pallor that blanches with pressure and worsens with heat.
HAE can be triggered by stressful events, including trauma, surgery, menstruation, and viral infections. However, in many instances, HAE attacks occur without an identifiable cause.5
Differential Diagnosis from Other Causes of Angioedema
Type I HAE is characterized by a quantitative C1INH deficiency (which is functionally abnormal as well), and occurs in 85% of patients. Type II HAE occurs in 15% of patients, and results from a functionally abnormal C1INH.
In patients with Type I and II HAE, as well as acquired C1 inhibitor deficiency (ACID), C4 levels are low during and between attacks. C2 levels are also low during acute attacks. In ACID, levels of C1q are also reduced; these patients require further workup to rule out an undiagnosed malignancy or an autoimmune process. In contrast, patients with ACE-induced, idiopathic, and allergic AE have normal complement profiles.3,6
Type III is a more recently described type of HAE that is rare, not well understood, and generally affects women.3,6 Clinically, it resembles Type I and Type II HAE but complement levels, including C1 inhibitor, are normal (see Table 1).
Treatment
HAE types I, II, III, and ACID are generally unresponsive to glucocorticoids, antihistamines, and epinephrine. These forms of AE may be exacerbated by exogenous estrogen.1,8 For this reason, HAE patients should avoid oral hormonal contraception and estrogen replacement therapy. In addition, ACE inhibitors should also be avoided based on their effect on bradykinin degradation.
Until the introduction of newer therapeutic choices, as noted in our case, the treatment of acute attacks of AE was essentially supportive. Patients with impending laryngeal obstruction were managed with intubation prior to progression of the AE to limit airway patency. Prior to the modern era, a substantial proportion of HAE patients died of asphyxiation.
Fresh frozen plasma (FFP) has been used to treat acute HAE attacks, but given its content of contact system proteins (in addition to C1INH), FFP might also pose a risk for worsening of HAE; for this reason, it must be given cautiously to patients who are symptomatic.9
In the past decade, there has been significant progress in the available treatments for HAE. Currently in the U.S., there are several agents recently approved by, or have pending approvals from, the FDA, including several forms of C1INH replacement, a bradykinin antagonist, and a kallikrein inhibitor.
The C1 esterase inhibitor (human) drugs are administered intravenously; both have been shown to be efficacious and safe. Nanofiltered C1 inhibitor provided relief in a median time of two hours when used acutely; when used as prophylaxis, it decreased the number of attacks in a three-month period by 50% (six vs. 12 with placebo, P<0.001).11
The other C1INH is rhucin, still not approved in U.S. This drug is characterized by a short half-life (approximately two to four hours) compared with the plasma-derived C1INH agents (24 to 48 hours). It is contraindicated in patients with rabbit hypersensitivity, as it is purified from rabbit breast milk.10
Ecallantide is a kallikrein inhibitor for acute therapy that is administered via three subcutaneous injections. This agent has been linked to allergic/anaphylactic reactions in a minority of patients (approximately 4%); therefore, it should be administered cautiously, by a health-care provider, and in a setting where anaphylaxis can be successfully managed.12 Icatibant is a bradykinin antagonist recently approved in the U.S. and administered SC via a single injection.10
In light of the development of these new agents, there is a need for updated guidelines for the long- and short-term prophylaxis and acute management of HAE. A recent guideline focused on the management of HAE in gynecologic and obstetric patients recommended the use of plasma-derived C1INH C1 esterase inhibitor (human) (Cinryze) for short- and long-term prophylaxis and acute treatment of HAE.13 The effect of pregnancy on HAE is variable: Some women worsen and other women have less swelling during their pregnancy. Swelling at the time of parturition is rare; however, the risk rises during the post-partum period.
Type III HAE. An additional form of HAE has been recognized with a pattern of AE episodes that mimics Type I or Type II HAE but with unremarkable laboratory studies of the complement cascade, including C1 inhibitor level and function. At this time, there is no laboratory test with which a diagnosis of Type III HAE can be confirmed. The diagnosis should be suspected in patients with a strong family history of AE reflecting autosomal dominant inheritance. In some, but not all, cases, the condition is manifest in association with high estrogen levels (e.g. pregnancy or administration of oral contraceptives). Type III HAE patients have a salutary response to the same agents that are efficacious for Type I and II HAE.
Acquired C1 inhibitor deficiency (ACID). ACID generally occurs in adults and is clinically indistinguishable from HAE. ACID is not associated with a remarkable family history of AE. In contrast to HAE, this is a consumptive deficiency of C1 inhibitor and results from enhanced catabolism that exceeds the capacity for regenerating C1 inhibitor protein. It is often associated with neoplastic (usually lymphoproliferative) or autoimmune disorders; treatment of the underlying condition frequently leads to improvement in ACID. Although its management is similar to HAE, it tends to be more responsive to anti-fibrinolytics. A salutary response to C1INH replacement therapy might not occur in patients with autoantibodies to C1 inhibitor, but efficacy of ecallantide and icatibant for the treatment of acquired AE has been reported.14, 15
ACEI angioedema. Treatment with angiotensin-converting enzyme inhibitors (ACE-I) has been associated with recurrent AE without urticaria in 0.1 to 0.7% of patients exposed to these drugs.16 Angioedema from ACE-I more frequently occurs within the first few months of therapy, but it might occur even after years of continuous therapy. ACEI-induced AE is secondary to impaired degradation of bradykinin. The main treatment is to discontinue the offending agent and avoid all other ACE-I, as this is a class-specific reaction.17
Angiotensin receptor blockers (ARBs) have been associated less commonly with AE. The mechanism for ARB-associated AE has not been elucidated. A meta-analysis showed that in 2% to 17% of patients who were switched to ARBs, recurrence of AE was observed.18 From the pooling of these data with two randomized controlled trials, it is estimated that approximately 10% or less of patients with ACEI-associated AE who switched to ARBs will develop AE.19 In the majority of cases, patients can be switched to ARBs with no recurrence of AE; however, the decision to prescribe an ARB to a patient who has had AE while receiving ACEI should be made carefully on an individualized risk/benefit basis.19
Preventive Treatment
The 17 α-alkylated androgens that can be used for treatment of HAE are danazol (Danacrine), stanozolol (Winstrol), oxandralone (Oxandrine) and methyltestosterone (Android). In patients with HAE, attenuated androgens can significantly reduce the frequency and severity of attacks; however, their use is limited by risk for untoward effects (virilization, abnormal liver function tests, change in libido, anxiety, etc.).21 There is also a risk for hepatotoxicity, including development of hepatic adenomas and hepatic carcinoma.
Antifibrinolytics also may have efficacy for HAE, but these agents have been associated with a variety of adverse effects, including nausea and diarrhea, postural hypotension, fatigue, enhanced thrombosis, retinal changes, and teratogenicity.8, 22, 23
In 2009, long-term prophylaxis with C1-INH concentrate was recommended for patients with HAE with frequent or disabling attacks, a history of laryngeal attacks, and poor quality of life. The 2007 International Consensus Algorithm for the Diagnosis, Therapy, and Management of HAE recommended long-term prophylaxis in patients with more than one monthly severe HAE attack, more than five days of disability per month, or any history of airway compromise.24, 25
The decision to prescribe long-term prophylaxis, and the dose/frequency of medication required, should be individualized based on clinical parameters, such as frequency and severity of attacks, and not on C1 INH or C4 levels.
Perioperative Considerations
It is well established that any trauma, including dental procedures or surgery, can precipitate HAE attacks. For this reason, short-term prophylactic treatment in HAE patients undergoing procedures is recommended. Ideally, avoiding endotracheal intubation is the best approach; however, if intubation cannot be avoided, then adequate prophylaxis should be administered.2
Attenuated androgens can be given up to seven days before a procedure, or C1 INH can be administered 24 hours in advance. If C1 INH is unavailable, FFP can be given six to 12 hours in advance in patients who are not symptomatic; in case of endotracheal intubation, either FFP or C1 INH should be administered immediately before.2
Several case reports in multiple specialty surgical patients (abdominal surgery, cardiopulmonary bypass, orthopedic surgery, etc.) have confirmed the successful use of C1 INH in the prevention of acute attacks with favorable outcomes.2
There is no need to follow C1 INH levels, as it has no clinical relevance.
Back to the Case
The patient was admitted to the ICU and received a total of eight units of FFP. He was transferred to our institution and was able to be extubated three days after initial presentation. Laboratory studies revealed C4 10mg/dL and C1 esterase inhibitor 10mg/dL (both low).
Danazol was resumed. However, within several months after discharge, Cinryze became available in the U.S. market and was eventually prescribed. The patient has not had further significant attacks requiring inpatient management.
Dr. Auron is an assistant professor of medicine and pediatrics at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University. Dr. Lang is co-director of the Asthma Center and director of the Allergy/Immunology Fellowship Training Program at the Cleveland Clinic.
References
- Bernstein, JA. Update on angioedema: evaluation, diagnosis, and treatment. Allergy Asthma Proc. 2011;32(6):408-412.
- Levy JH, Freiberger DJ, Roback J. Hereditary angioedema: current and emerging treatment options. Anesth Analg. 2010;110(5):1271-1280.
- Busse PJ. Angioedema: Differential diagnosis and treatment. Allergy Asthma Proc. 2011;32:Suppl 1:S3-S11.
- Khan DA. Hereditary angioedema: historical aspects, classification, pathophysiology, clinical presentation, and laboratory diagnosis. Allergy Asthma Proc. 2011;32(1):1-10.
- Bork K, Meng G, Staubach P, Hardt, J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006;119(3):267-274.
- Zuraw BL, Christiansen SC. Pathogenesis and laboratory diagnosis of hereditary angioedema. Allergy Asthma Proc. 2009;30:487-492.
- Frazer-Abel A, Giclas PC. Update on laboratory tests for the diagnosis and differentiation of hereditary angioedema and acquired angioedema. Allergy Asthma Proc. 2011;32:Suppl 1:S17-S21.
- Banerjee A. Current treatment of hereditary angioedema: an update on clinical studies. Allergy Asthma Proc. 2010;31:398-406.
- Donaldson VH. Therapy of "the neurotic edema." N Engl J Med. 1972;286(15):835-836.
- Riedl MA. Update on the acute treatment of hereditary angioedema. Allergy Asthma Proc. 2011;32:11-16.
- Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. 2010;363:513-522.
- Cicardi M, Levy RJ, McNeil DL. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med. 2010;363:523-531.
- Caballero T, Farkas H, Bouillet L, et al. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol. 2012;129(2):308-320.
- Cicardi M, Zanichelli A. Acquired angioedema. J Allergy Clin Immunol. 2010;6(1):14.
- Zanichelli A, Badini M, Nataloni I, Montano N, Cicardi M. Treatment of acquired angioedema with icatibant: a case report. Intern Emerg Med. 2011;6(3):279-280.
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26(4):725-737.
- Haymore BR, Yoon J, Mikita CP, Klote MM, DeZee KJ. Risk of angioedema with angiotensin receptor blockers in patients with prior angioedema associated with angiotensin-converting enzyme inhibitors: a meta-analysis. Ann Allergy Asthma Immunol. 2008;101(5):495-499.
- Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2011;45(4):520-524.
- Nzeako UC. Diagnosis and management of angioedema with abdominal involvement: a gastroenterology perspective. World J Gastroenterol. 2010; 16(39):4913-4921.
- Banerji A, Sloane DE, Sheffer AL. Hereditary angioedema: a current state-of-the-art review, V: attenuated androgens for the treatment of hereditary angioedema. Ann Allergy Asthma Immunol. 2008;100(1) (Suppl 2):S19-22.
- Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med. 2008; 359(10):1027-1036.
- Zuraw BL. Hereditary angioedema: a current state-of-the-art review, IV: short- and long-term treatment of hereditary angioedema: out with the old and in with the new? Ann Allergy Asthma Immunol. 2008;100(1) (Suppl 2):S13-S18.
- Bowen T, Cicardi M, Bork K, et al. Hereditary angioedema: a current state-of-the-art review, VII: Canadian Hungarian 2007 International Consensus Algorithm for the Diagnosis, Therapy, and Management of Hereditary Angioedema. Ann Allergy Asthma Immunol. 2008;100(1)(Suppl 2):S30-40.
- Craig T, Riedl M, Dykewicz M, et al. When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol. 2009.102(5):366-372.
- Frank MM. Update on preventive therapy (prophylaxis) of hereditary angioedema. Allergy Asthma Proc. 2011;32(1):17-21.
Case
A 36-year-old man with a known history of hereditary angioedema (HAE) presents with severe orofacial swelling and laryngeal angioedema, requiring expectant management, including endotracheal intubation. His previous angioedema (AE) episodes involved his hands, feet, and genitalia; episodes generally occurred after physical trauma. Ten years prior to admission, he had an episode of secondary small bowel obstruction. The patient had been prescribed prophylactic danazol (Danacrine) 100 mg BID but he had gradually been reducing the dosage due to mood changes; at the time of presentation, he had already tapered to 100 mg danazol three times per week (Monday, Wednesday, and Friday).
Overview
HAE is an autosomal dominant condition characterized by localized, episodic swelling of the deeper dermal layers and/or mucosal tissue. Its acute presentation can vary in severity; presentations can be lethal.
HAE is generally unresponsive to conventional treatments used for other causes of AE (e.g. food or drug reactions) including glucocorticoids, antihistamines, and epinephrine. The pharmacologic treatment of acute attacks, as well as for short- and long-term prophylaxis of HAE, has evolved significantly in recent years and now includes several forms of C1 inhibitor (C1INH) protein replacement, as well as a bradykinin antagonist, and a kallikrein inhibitor.
Review of the Data
Epidemiology. HAE is an autosomal dominant disease with prevalence in the U.S. of 1 in 10,000 to 1 in 50,000 patients. All ethnic groups are equally affected, with no gender predilection. In most cases, a positive family history is present; however, in 25% of cases, spontaneous mutations occur such that an unremarkable family history does not rule out the diagnosis.1
Pathophysiology. In the past decade, there has been substantial advancement in our understanding of HAE pathophysiology. HAE occurs as a result of functional or quantitative C1 esterase inhibitor (C1INH) deficiency.
C1INH belongs to a group of proteins known as serpins (serine protease inhibitors). The C1INH gene is located on chromosome 11, and has several polymorphic sites, which predispose to spontaneous mutations.1
Bradykinin is the core bioactive mediator, which causes vasodilation, smooth muscle contraction, and subsequent edema.1 C1INH regulates bradykinin production by blocking kallikrein’s conversion of factor XII into XIIa, prekallikrein to kallikrein, and cleavage of high-molecular-weight kininogen by activated kallikrein to form bradykinin (see Figure 1).1,2
Clinical Manifestations
HAE is characterized by recurrent episodes of swelling, the frequency and severity of which are quite variable. Virtually all HAE patients have abdominal- and extremity-swelling episodes, and 50% will have episodes of laryngeal swelling; other involved areas might include the face, oropharynx, and genitalia.4 These episodes are usually unilateral; edema is nonpruritic, nonpitting, and often painless. Episodes involving the oropharynx, larynx, and abdomen can be associated with potentially serious morbidity and mortality.1, 3
HAE episodes usually commence during late childhood and early puberty (on average at age 11). Approximately half of HAE patients will have oropharyngeal involvement that might occur many years, even decades, after the initial onset of the disease. The annual rate of severe, life-threatening laryngeal edema was 0.9% in a recent retrospective study.4
Severity of the disease is variable. Attacks are episodic, and occur on average every 10 to 20 days in untreated patients. These attacks typically peak over 24 hours, then usually resolve after 48 to 72 hours. However, the complete resolution of signs and symptoms can last for up to one week after the attacks.5
There is no concomitant pruritus or urticaria that accompanies the AE. However, erythema marginatum, an evanescent nonpruritic rash with serpiginous borders involving the trunk and inner surface of extremities but sparing the face, might herald the onset of an episode. This rash usually has central pallor that blanches with pressure and worsens with heat.
HAE can be triggered by stressful events, including trauma, surgery, menstruation, and viral infections. However, in many instances, HAE attacks occur without an identifiable cause.5
Differential Diagnosis from Other Causes of Angioedema
Type I HAE is characterized by a quantitative C1INH deficiency (which is functionally abnormal as well), and occurs in 85% of patients. Type II HAE occurs in 15% of patients, and results from a functionally abnormal C1INH.
In patients with Type I and II HAE, as well as acquired C1 inhibitor deficiency (ACID), C4 levels are low during and between attacks. C2 levels are also low during acute attacks. In ACID, levels of C1q are also reduced; these patients require further workup to rule out an undiagnosed malignancy or an autoimmune process. In contrast, patients with ACE-induced, idiopathic, and allergic AE have normal complement profiles.3,6
Type III is a more recently described type of HAE that is rare, not well understood, and generally affects women.3,6 Clinically, it resembles Type I and Type II HAE but complement levels, including C1 inhibitor, are normal (see Table 1).
Treatment
HAE types I, II, III, and ACID are generally unresponsive to glucocorticoids, antihistamines, and epinephrine. These forms of AE may be exacerbated by exogenous estrogen.1,8 For this reason, HAE patients should avoid oral hormonal contraception and estrogen replacement therapy. In addition, ACE inhibitors should also be avoided based on their effect on bradykinin degradation.
Until the introduction of newer therapeutic choices, as noted in our case, the treatment of acute attacks of AE was essentially supportive. Patients with impending laryngeal obstruction were managed with intubation prior to progression of the AE to limit airway patency. Prior to the modern era, a substantial proportion of HAE patients died of asphyxiation.
Fresh frozen plasma (FFP) has been used to treat acute HAE attacks, but given its content of contact system proteins (in addition to C1INH), FFP might also pose a risk for worsening of HAE; for this reason, it must be given cautiously to patients who are symptomatic.9
In the past decade, there has been significant progress in the available treatments for HAE. Currently in the U.S., there are several agents recently approved by, or have pending approvals from, the FDA, including several forms of C1INH replacement, a bradykinin antagonist, and a kallikrein inhibitor.
The C1 esterase inhibitor (human) drugs are administered intravenously; both have been shown to be efficacious and safe. Nanofiltered C1 inhibitor provided relief in a median time of two hours when used acutely; when used as prophylaxis, it decreased the number of attacks in a three-month period by 50% (six vs. 12 with placebo, P<0.001).11
The other C1INH is rhucin, still not approved in U.S. This drug is characterized by a short half-life (approximately two to four hours) compared with the plasma-derived C1INH agents (24 to 48 hours). It is contraindicated in patients with rabbit hypersensitivity, as it is purified from rabbit breast milk.10
Ecallantide is a kallikrein inhibitor for acute therapy that is administered via three subcutaneous injections. This agent has been linked to allergic/anaphylactic reactions in a minority of patients (approximately 4%); therefore, it should be administered cautiously, by a health-care provider, and in a setting where anaphylaxis can be successfully managed.12 Icatibant is a bradykinin antagonist recently approved in the U.S. and administered SC via a single injection.10
In light of the development of these new agents, there is a need for updated guidelines for the long- and short-term prophylaxis and acute management of HAE. A recent guideline focused on the management of HAE in gynecologic and obstetric patients recommended the use of plasma-derived C1INH C1 esterase inhibitor (human) (Cinryze) for short- and long-term prophylaxis and acute treatment of HAE.13 The effect of pregnancy on HAE is variable: Some women worsen and other women have less swelling during their pregnancy. Swelling at the time of parturition is rare; however, the risk rises during the post-partum period.
Type III HAE. An additional form of HAE has been recognized with a pattern of AE episodes that mimics Type I or Type II HAE but with unremarkable laboratory studies of the complement cascade, including C1 inhibitor level and function. At this time, there is no laboratory test with which a diagnosis of Type III HAE can be confirmed. The diagnosis should be suspected in patients with a strong family history of AE reflecting autosomal dominant inheritance. In some, but not all, cases, the condition is manifest in association with high estrogen levels (e.g. pregnancy or administration of oral contraceptives). Type III HAE patients have a salutary response to the same agents that are efficacious for Type I and II HAE.
Acquired C1 inhibitor deficiency (ACID). ACID generally occurs in adults and is clinically indistinguishable from HAE. ACID is not associated with a remarkable family history of AE. In contrast to HAE, this is a consumptive deficiency of C1 inhibitor and results from enhanced catabolism that exceeds the capacity for regenerating C1 inhibitor protein. It is often associated with neoplastic (usually lymphoproliferative) or autoimmune disorders; treatment of the underlying condition frequently leads to improvement in ACID. Although its management is similar to HAE, it tends to be more responsive to anti-fibrinolytics. A salutary response to C1INH replacement therapy might not occur in patients with autoantibodies to C1 inhibitor, but efficacy of ecallantide and icatibant for the treatment of acquired AE has been reported.14, 15
ACEI angioedema. Treatment with angiotensin-converting enzyme inhibitors (ACE-I) has been associated with recurrent AE without urticaria in 0.1 to 0.7% of patients exposed to these drugs.16 Angioedema from ACE-I more frequently occurs within the first few months of therapy, but it might occur even after years of continuous therapy. ACEI-induced AE is secondary to impaired degradation of bradykinin. The main treatment is to discontinue the offending agent and avoid all other ACE-I, as this is a class-specific reaction.17
Angiotensin receptor blockers (ARBs) have been associated less commonly with AE. The mechanism for ARB-associated AE has not been elucidated. A meta-analysis showed that in 2% to 17% of patients who were switched to ARBs, recurrence of AE was observed.18 From the pooling of these data with two randomized controlled trials, it is estimated that approximately 10% or less of patients with ACEI-associated AE who switched to ARBs will develop AE.19 In the majority of cases, patients can be switched to ARBs with no recurrence of AE; however, the decision to prescribe an ARB to a patient who has had AE while receiving ACEI should be made carefully on an individualized risk/benefit basis.19
Preventive Treatment
The 17 α-alkylated androgens that can be used for treatment of HAE are danazol (Danacrine), stanozolol (Winstrol), oxandralone (Oxandrine) and methyltestosterone (Android). In patients with HAE, attenuated androgens can significantly reduce the frequency and severity of attacks; however, their use is limited by risk for untoward effects (virilization, abnormal liver function tests, change in libido, anxiety, etc.).21 There is also a risk for hepatotoxicity, including development of hepatic adenomas and hepatic carcinoma.
Antifibrinolytics also may have efficacy for HAE, but these agents have been associated with a variety of adverse effects, including nausea and diarrhea, postural hypotension, fatigue, enhanced thrombosis, retinal changes, and teratogenicity.8, 22, 23
In 2009, long-term prophylaxis with C1-INH concentrate was recommended for patients with HAE with frequent or disabling attacks, a history of laryngeal attacks, and poor quality of life. The 2007 International Consensus Algorithm for the Diagnosis, Therapy, and Management of HAE recommended long-term prophylaxis in patients with more than one monthly severe HAE attack, more than five days of disability per month, or any history of airway compromise.24, 25
The decision to prescribe long-term prophylaxis, and the dose/frequency of medication required, should be individualized based on clinical parameters, such as frequency and severity of attacks, and not on C1 INH or C4 levels.
Perioperative Considerations
It is well established that any trauma, including dental procedures or surgery, can precipitate HAE attacks. For this reason, short-term prophylactic treatment in HAE patients undergoing procedures is recommended. Ideally, avoiding endotracheal intubation is the best approach; however, if intubation cannot be avoided, then adequate prophylaxis should be administered.2
Attenuated androgens can be given up to seven days before a procedure, or C1 INH can be administered 24 hours in advance. If C1 INH is unavailable, FFP can be given six to 12 hours in advance in patients who are not symptomatic; in case of endotracheal intubation, either FFP or C1 INH should be administered immediately before.2
Several case reports in multiple specialty surgical patients (abdominal surgery, cardiopulmonary bypass, orthopedic surgery, etc.) have confirmed the successful use of C1 INH in the prevention of acute attacks with favorable outcomes.2
There is no need to follow C1 INH levels, as it has no clinical relevance.
Back to the Case
The patient was admitted to the ICU and received a total of eight units of FFP. He was transferred to our institution and was able to be extubated three days after initial presentation. Laboratory studies revealed C4 10mg/dL and C1 esterase inhibitor 10mg/dL (both low).
Danazol was resumed. However, within several months after discharge, Cinryze became available in the U.S. market and was eventually prescribed. The patient has not had further significant attacks requiring inpatient management.
Dr. Auron is an assistant professor of medicine and pediatrics at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University. Dr. Lang is co-director of the Asthma Center and director of the Allergy/Immunology Fellowship Training Program at the Cleveland Clinic.
References
- Bernstein, JA. Update on angioedema: evaluation, diagnosis, and treatment. Allergy Asthma Proc. 2011;32(6):408-412.
- Levy JH, Freiberger DJ, Roback J. Hereditary angioedema: current and emerging treatment options. Anesth Analg. 2010;110(5):1271-1280.
- Busse PJ. Angioedema: Differential diagnosis and treatment. Allergy Asthma Proc. 2011;32:Suppl 1:S3-S11.
- Khan DA. Hereditary angioedema: historical aspects, classification, pathophysiology, clinical presentation, and laboratory diagnosis. Allergy Asthma Proc. 2011;32(1):1-10.
- Bork K, Meng G, Staubach P, Hardt, J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006;119(3):267-274.
- Zuraw BL, Christiansen SC. Pathogenesis and laboratory diagnosis of hereditary angioedema. Allergy Asthma Proc. 2009;30:487-492.
- Frazer-Abel A, Giclas PC. Update on laboratory tests for the diagnosis and differentiation of hereditary angioedema and acquired angioedema. Allergy Asthma Proc. 2011;32:Suppl 1:S17-S21.
- Banerjee A. Current treatment of hereditary angioedema: an update on clinical studies. Allergy Asthma Proc. 2010;31:398-406.
- Donaldson VH. Therapy of "the neurotic edema." N Engl J Med. 1972;286(15):835-836.
- Riedl MA. Update on the acute treatment of hereditary angioedema. Allergy Asthma Proc. 2011;32:11-16.
- Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. 2010;363:513-522.
- Cicardi M, Levy RJ, McNeil DL. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med. 2010;363:523-531.
- Caballero T, Farkas H, Bouillet L, et al. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol. 2012;129(2):308-320.
- Cicardi M, Zanichelli A. Acquired angioedema. J Allergy Clin Immunol. 2010;6(1):14.
- Zanichelli A, Badini M, Nataloni I, Montano N, Cicardi M. Treatment of acquired angioedema with icatibant: a case report. Intern Emerg Med. 2011;6(3):279-280.
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26(4):725-737.
- Haymore BR, Yoon J, Mikita CP, Klote MM, DeZee KJ. Risk of angioedema with angiotensin receptor blockers in patients with prior angioedema associated with angiotensin-converting enzyme inhibitors: a meta-analysis. Ann Allergy Asthma Immunol. 2008;101(5):495-499.
- Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2011;45(4):520-524.
- Nzeako UC. Diagnosis and management of angioedema with abdominal involvement: a gastroenterology perspective. World J Gastroenterol. 2010; 16(39):4913-4921.
- Banerji A, Sloane DE, Sheffer AL. Hereditary angioedema: a current state-of-the-art review, V: attenuated androgens for the treatment of hereditary angioedema. Ann Allergy Asthma Immunol. 2008;100(1) (Suppl 2):S19-22.
- Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med. 2008; 359(10):1027-1036.
- Zuraw BL. Hereditary angioedema: a current state-of-the-art review, IV: short- and long-term treatment of hereditary angioedema: out with the old and in with the new? Ann Allergy Asthma Immunol. 2008;100(1) (Suppl 2):S13-S18.
- Bowen T, Cicardi M, Bork K, et al. Hereditary angioedema: a current state-of-the-art review, VII: Canadian Hungarian 2007 International Consensus Algorithm for the Diagnosis, Therapy, and Management of Hereditary Angioedema. Ann Allergy Asthma Immunol. 2008;100(1)(Suppl 2):S30-40.
- Craig T, Riedl M, Dykewicz M, et al. When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol. 2009.102(5):366-372.
- Frank MM. Update on preventive therapy (prophylaxis) of hereditary angioedema. Allergy Asthma Proc. 2011;32(1):17-21.
Nonurgent Pediatric Admissions on Weekends Bump Up Hospital Costs
Clinical question: Do weekend admissions for failure to thrive (FTT) result in higher costs and length of stay (LOS)?
Background: FTT accounts for up to 5% of all admissions for children younger than 2 years of age. The optimal approach to inpatient or outpatient care is not well defined. Hospitalizations sometimes are used to facilitate costly and intense workups for organic disease. Given the nonurgent nature of this condition and expected barriers to efficient workup on weekends, it is likely that weekend admissions for FTT might not add much value.
Study design: Retrospective cohort study.
Setting: Forty-two tertiary-care pediatric hospitals.
Synopsis: A total of 23,332 children younger than 2 were studied over an eight-year period. Saturday and Sunday admissions resulted in an average increase in LOS by 1.93 days and an increase in cost by $2,785 when compared with weekday admissions. Patients admitted on weekends were more likely to have imaging studies and lab tests performed, but were less likely to have a discharge diagnosis of FTT. The authors estimate that if one-half of the weekend admissions from 2010 with a consistent FTT diagnosis at admission and discharge were converted to a Monday admission, $534,145 in savings to the health-care system would result.
One notable limitation of the authors’ conclusions is that patients admitted on weekends appeared to have more organic diagnoses documented and might in fact have been more acutely ill, requiring more workup and intervention. Researchers were not able to further explore this using the administrative data. Nonetheless, a subset of weekend admissions with a consistent FTT diagnosis appeared to represent no value added to the system, and potentially could have resulted in a $3.5 million cost savings had they simply been admitted instead on a weekday.
Bottom line: Nonurgent weekend admissions for FTT are inefficient.
Citation: Thompson RT, Bennett WE, Finnell SME, Downs SM. Increased length of stay and costs associated with weekend admissions for failure to thrive. Pediatrics. 2012;131:e805-e810.
Reviewed by Pediatric Editor Mark Shen, MD, SFHM, medical director of hospital medicine at Dell Children's Medical Center, Austin, Texas.
Clinical question: Do weekend admissions for failure to thrive (FTT) result in higher costs and length of stay (LOS)?
Background: FTT accounts for up to 5% of all admissions for children younger than 2 years of age. The optimal approach to inpatient or outpatient care is not well defined. Hospitalizations sometimes are used to facilitate costly and intense workups for organic disease. Given the nonurgent nature of this condition and expected barriers to efficient workup on weekends, it is likely that weekend admissions for FTT might not add much value.
Study design: Retrospective cohort study.
Setting: Forty-two tertiary-care pediatric hospitals.
Synopsis: A total of 23,332 children younger than 2 were studied over an eight-year period. Saturday and Sunday admissions resulted in an average increase in LOS by 1.93 days and an increase in cost by $2,785 when compared with weekday admissions. Patients admitted on weekends were more likely to have imaging studies and lab tests performed, but were less likely to have a discharge diagnosis of FTT. The authors estimate that if one-half of the weekend admissions from 2010 with a consistent FTT diagnosis at admission and discharge were converted to a Monday admission, $534,145 in savings to the health-care system would result.
One notable limitation of the authors’ conclusions is that patients admitted on weekends appeared to have more organic diagnoses documented and might in fact have been more acutely ill, requiring more workup and intervention. Researchers were not able to further explore this using the administrative data. Nonetheless, a subset of weekend admissions with a consistent FTT diagnosis appeared to represent no value added to the system, and potentially could have resulted in a $3.5 million cost savings had they simply been admitted instead on a weekday.
Bottom line: Nonurgent weekend admissions for FTT are inefficient.
Citation: Thompson RT, Bennett WE, Finnell SME, Downs SM. Increased length of stay and costs associated with weekend admissions for failure to thrive. Pediatrics. 2012;131:e805-e810.
Reviewed by Pediatric Editor Mark Shen, MD, SFHM, medical director of hospital medicine at Dell Children's Medical Center, Austin, Texas.
Clinical question: Do weekend admissions for failure to thrive (FTT) result in higher costs and length of stay (LOS)?
Background: FTT accounts for up to 5% of all admissions for children younger than 2 years of age. The optimal approach to inpatient or outpatient care is not well defined. Hospitalizations sometimes are used to facilitate costly and intense workups for organic disease. Given the nonurgent nature of this condition and expected barriers to efficient workup on weekends, it is likely that weekend admissions for FTT might not add much value.
Study design: Retrospective cohort study.
Setting: Forty-two tertiary-care pediatric hospitals.
Synopsis: A total of 23,332 children younger than 2 were studied over an eight-year period. Saturday and Sunday admissions resulted in an average increase in LOS by 1.93 days and an increase in cost by $2,785 when compared with weekday admissions. Patients admitted on weekends were more likely to have imaging studies and lab tests performed, but were less likely to have a discharge diagnosis of FTT. The authors estimate that if one-half of the weekend admissions from 2010 with a consistent FTT diagnosis at admission and discharge were converted to a Monday admission, $534,145 in savings to the health-care system would result.
One notable limitation of the authors’ conclusions is that patients admitted on weekends appeared to have more organic diagnoses documented and might in fact have been more acutely ill, requiring more workup and intervention. Researchers were not able to further explore this using the administrative data. Nonetheless, a subset of weekend admissions with a consistent FTT diagnosis appeared to represent no value added to the system, and potentially could have resulted in a $3.5 million cost savings had they simply been admitted instead on a weekday.
Bottom line: Nonurgent weekend admissions for FTT are inefficient.
Citation: Thompson RT, Bennett WE, Finnell SME, Downs SM. Increased length of stay and costs associated with weekend admissions for failure to thrive. Pediatrics. 2012;131:e805-e810.
Reviewed by Pediatric Editor Mark Shen, MD, SFHM, medical director of hospital medicine at Dell Children's Medical Center, Austin, Texas.
Physician Reviews of Hospital Medicine-Related Research
In This Edition
Literature At A Glance
A guide to this month’s studies
- Prices for common hospital procedures not readily available
- Antibiotics associated with decreased mortality in acute COPD exacerbation
- Endovascular therapy has no benefit to systemic t-PA in acute stroke
- Net harm observed with rivaroxaban for thromboprophylaxis
- Noninvasive ventilation more effective, safer for AECOPD patients
- Synthetic cannabinoid use and acute kidney injury
- Dabigatran vs. warfarin in the extended treatment of VTE
- Spironolactone improves diastolic function
- Real-time EMR-based prediction tool for clinical deterioration
- Hypothermia protocol and cardiac arrest
Prices for Common Hospital Procedures Not Readily Available
Clinical question: Are patients able to select health-care providers based on price of service?
Background: With health-care costs rising, patients are encouraged to take a more active role in cost containment. Many initiatives call for greater pricing transparency in the health-care system. This study evaluated price availability for a common surgical procedure.
Study design: Telephone inquiries with standardized interview script.
Setting: Twenty top-ranked orthopedic hospitals and 102 non-top-ranked U.S. hospitals.
Synopsis: Hospitals were contacted by phone with a standardized, scripted request for their price of an elective total hip arthroplasty. The script described the patient as a 62-year-old grandmother without insurance who is able to pay out of pocket and wishes to compare hospital prices. On the first or second attempt, 40% of top-ranked and 32% of non-top-ranked hospitals were able to provide their price; after five attempts, authors were unable to obtain full pricing information (both hospital and physician fee) from 40% of top-ranked and 37% of non-top-ranked hospitals. Neither fee was made available by 15% of top-ranked and 16% of non-top-ranked hospitals. Wide variation of pricing was found across hospitals. The authors commented on the difficulties they encountered, such as the transfer of calls between departments and the uncertainty of representatives on how to assist.
Bottom line: For individual patients, applying basic economic principles as a consumer might be tiresome and often impossible, with no major differences between top-ranked and non-top-ranked hospitals.
Citation: Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
Antibiotics Associated with Decreased Mortality in Acute COPD Exacerbation
Clinical question: Do antibiotics when added to systemic steroids provide clinical benefit for patients with acute COPD exacerbation?
Background: Despite widespread use of antibiotics in the treatment of acute COPD, their benefit is not clear.
Study design: Retrospective cohort study.Setting: Four hundred ten U.S. hospitals participating in Perspective, an inpatient administrative database.
Synopsis: More than 50,000 patients treated with systemic steroids for acute COPD exacerbation were included in this study; 85% of them received empiric antibiotics within the first two hospital days. They were compared with those treated with systemic steroids alone. In-hospital mortality was 1.02% for patients on steroids plus antibiotics versus 1.78% on steroids alone. Use of antibiotics was associated with a 40% reduction in the odds of in-hospital mortality (OR, 0.60; 95% CI, 0.50-0.74) and reduced readmissions. In an analysis of matched cohorts, hospital mortality was 1% for patients on antibiotics and 1.8% for patients without antibiotics (OR, 0.53; 95% CI, 0.40-0.71). The risk for readmission for Clostridium difficile colitis was not different between the groups, but other potential adverse effects of antibiotic use, such as development of resistant micro-organisms, were not studied. In a subset analysis, three groups of antibiotics were compared: macrolides, quinolones, and cephalosporins. None was better than another, but those treated with macrolides had a lower readmission rate for C. diff.
Bottom line: Treatment with antibiotics when added to systemic steroids is associated with improved outcomes in acute COPD exacerbations, but there is no clear advantage of one antibiotic class over another.
Citation: Stefan MS, Rothberg MB, Shieh M, Pekow PS, Lindenauer PK. Association between antibiotic treatment and outcomes in patients hospitalized with acute exacerbation of COPD treated with systemic steroids. Chest. 2013;143(1):82-90.
Endovascular Therapy Added to Systemic t-PA Has No Benefit in Acute Stroke
Clinical question: Does adding endovascular therapy to intravenous tissue plasminogen activator (t-PA) reduce disability in acute stroke?
Background: Early systemic t-PA is the only proven reperfusion therapy in acute stroke, but it is unknown if adding localized endovascular therapy is beneficial.
Study design: Randomized, open-label, blinded-outcome trial.
Setting: Fifty-eight centers in North America, Europe, and Australia.
Synopsis: Patients aged 18 to 82 with acute ischemic stroke were eligible if they received t-PA within three hours of enrollment and had moderate to severe neurologic deficit (National Institutes of Health Stroke Scale [NIHSS] >10, or NIHSS 8 or 9 with CT angiographic evidence of specific major artery occlusion). All patients received standard-dose t-PA; those randomized to endovascular treatment underwent angiography, and, if indicated, underwent one of the endovascular treatments chosen by the neurointerventionalist. The primary outcome measure was a modified Rankin score of 2 or lower (indicating functional independence) at 90 days (assessed by a neurologist).
After enrollment of 656 patients, the trial was terminated early for futility. There was no significant difference in the primary outcome, with 40.8% of patients in the endovascular intervention group and 38.7% in the t-PA-only group having a modified Rankin score of 2 or lower. There was also no difference in mortality or other secondary outcomes, even when the analysis was limited to patients presenting with more severe neurologic deficits.
Bottom line: The addition of endovascular therapy to systemic t-PA in acute ischemic stroke does not improve functional outcomes or mortality.
Citation: Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893-903.
Rivaroxaban Compared with Enoxaparin for Thromboprophylaxis
Clinical question: Is extended-duration rivaroxaban more effective than standard-duration enoxaparin in preventing deep venous thrombosis in acutely ill medical patients?
Background: Trials have shown benefits of thromboprophylaxis in acutely ill medical patients at increased risk of venous thrombosis, but the optimal duration and type of anticoagulation is unknown.
Study design: Prospective, randomized, double-blinded, active-comparator controlled trial.
Setting: Five hundred fifty-two centers in 52 countries.
Synopsis: The trial enrolled 8,428 patients hospitalized with an acute medical condition and reduced mobility. Patients were randomized to receive either enoxaparin for 10 (+/-4) days or rivaroxaban for 35 (+/-4) days. The co-primary composite outcomes were the incidence of asymptomatic proximal deep venous thrombosis, symptomatic deep venous thrombosis, pulmonary embolism, or death related to venous thromboembolism over 10 days and over 35 days.
Both groups had a 2.7% incidence of the primary endpoint over 10 days; over 35 days, the extended-duration rivaroxaban group had a reduced incidence of the primary endpoint of 4.4% compared with 5.7% for enoxaparin. However, there was an increase of clinically relevant bleeding in the rivaroxaban group, occurring in 2.8% and 4.1% of patients over 10 and 35 days, respectively, compared with 1.2% and 1.7% for enoxaparin.
Overall, there was net harm with rivaroxaban over both time periods: 6.6% and 9.4% of patients in the rivaroxaban group had a negative outcome at 10 and 35 days, compared with 4.4% and 7.8% with enoxaparin.
Bottom line: There was net harm with extended-duration rivaroxaban versus standard-duration enoxaparin for thromboprophylaxis in hospitalized medical patients.
Citation: Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513-523.
Noninvasive Ventilation More Effective, Safer for Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD)
Clinical question: What are the patterns in use of noninvasive ventilation (NIV) and invasive mechanical ventilation (IMV) in patients with AECOPD, and which method produces better outcomes?
Background: Multiple randomized controlled trials and meta-analyses have suggested a mortality benefit with NIV compared to standard medical care in AECOPD. However, little evidence exists on head-to-head comparisons of NIV and IMV.
Study design: Retrospective cohort study.
Setting: Non-federal, short-term, general, and other specialty hospitals nationwide.
Synopsis: A sample of 67,651 ED visits for AECOPD with acute respiratory failure was analyzed from the National Emergency Department Sample (NEDS) database between 2006 and 2008. The study found that NIV use increased to 16% in 2008 from 14% in 2006. Use varied widely between hospitals and was more utilized in higher-case-volume, nonmetropolitan, and Northeastern hospitals. Compared with IMV, NIV was associated with 46% lower inpatient mortality (risk ratio 0.54, 95% confidence interval [CI] 0.50-0.59), shortened hospital length of stay (-3.2 days, 95% CI -3.4 to -2.9), lower hospital charges (-$35,012, 95% CI -$36,848 to -$33,176), and lower risk of iatrogenic pneumothorax (0.05% vs. 0.5%, P<0.001). Causality could not be established given the observational study design.
Bottom line: NIV is associated with better outcomes than IMV in the management of AECOPD, and might be underutilized.
Citation: Tsai CL, Lee WY, Delclos GL, Hanania NA, Camargo CA Jr. Comparative effectiveness of noninvasive ventilation vs. invasive mechanical ventilation in chronic obstructive pulmonary disease patients with acute respiratory failure. J Hosp Med. 2013;8(4):165-172.
Synthetic Cannabinoid Use May Cause Acute Kidney Injury
Clinical question: Are synthetic cannabinoids associated with acute kidney injury (AKI)?
Background: Synthetic cannabinoids are designer drugs of abuse with a growing popularity in the U.S.
Study design: Retrospective case series.
Setting: Hospitals in Wyoming, Oklahoma, Rhode Island, New York, Kansas, and Oregon.
Synopsis: The Centers for Disease Control and Prevention (CDC) issued an alert when 16 cases of unexplained AKI after exposure to synthetic cannabinoids were reported between March and December 2012. Synthetic cannabinoid use is associated with neurologic, sympathomimetic, and cardiovascular toxicity; however, this is the first case series reporting renal toxicity. The 16 patients included 15 males and one female, aged 15-33 years, with no pre-existing renal disease or nephrotoxic medication consumption. All used synthetic cannabinoids within hours to days before developing nausea, vomiting, abdominal, and flank and/or back pain.
Creatinine peaked one to six days after symptom onset. Five patients required hemodialysis, and all 16 recovered. There was no finding specific for all cases on ultrasound and/or biopsy. Toxicologic analysis of specimens was possible in seven cases and revealed a previously unreported fluorinated synthetic cannabinoid compound XLR-11 in all clinical specimens of patients who used the drug within two days of being tested.
Overall, the analysis did not reveal any single compound or brand that could explain all cases.
Bottom line: Clinicians should be aware of the potential for renal or other toxicities in users of synthetic cannabinoid products and should ask about their use in cases of unexplained AKI.
Citation: Murphy TD, Weidenbach KN, Van Houten C, et al. Centers for Disease Control and Prevention. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(6):93-98.
Dabigatran versus Warfarin in Extended VTE Treatment
Clinical question: Is dabigatran suitable for extended treatment VTE?
Background: In contrast to warfarin (Coumadin), dabigatran (Pradaxa) is given in a fixed dose and does not require frequent laboratory monitoring. Dabigatran has been shown to be noninferior to warfarin in the initial six-month treatment of VTE.
Study design: Two double-blinded, randomized trials: an active-control study and a placebo-control study.
Setting: Two hundred sixty-five sites in 33 countries for the active-control study, and 147 sites in 21 countries for the placebo-control study.
Synopsis: This study enrolled 4,299 adult patients with objectively confirmed, symptomatic, proximal deep vein thrombosis or pulmonary embolism. In the active-control study comparing warfarin and dabigatran, recurrent objectively confirmed symptomatic or fatal VTE was observed in 1.8% of patients in the dabigatran group compared with 1.3% of patients in the warfarin group (P=0.01 for noninferiority). While major or clinically relevant bleeding was less frequent with dabigatran compared to warfarin (hazard ratio, 0.54), more acute coronary syndromes were observed with dabigatran (0.9% vs. 0.2%, P=0.02). In the placebo-control study, symptomatic or fatal VTE was observed in 0.4% of patients in the dabigatran group compared with 5.6% in the placebo group. Clinically relevant bleeding was more common with dabigatran (5.3% vs. 1.8%; hazard ratio 2.92).
Bottom line: Treatment with dabigatran met the pre-specified noninferiority margin in this study. However, it is worth noting that patients with VTE given extended treatment with dabigatran had significantly higher rates of recurrent symptomatic or fatal VTE than patients treated with warfarin.
Citation: Schulman S, Kearson C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709-718.
Spironolactone Improves Diastolic Function
Clinical question: What is the efficacy of aldosterone receptor blockers on diastolic function and exercise capacity?
Background: Mineralocorticoid receptor activation by aldosterone contributes to the pathophysiology of heart failure (HF) in patients with and without reduced ejection fraction (EF). Aldosterone receptor blockers (spironolactone) reduce overall and cardiovascular mortality in HF patients with reduced EF; however, its effect on HF patients with preserved EF is unknown.
Study design: Prospective, randomized, double-blinded, placebo-controlled trial.
Setting: Ten ambulatory sites in Germany and Austria.
Synopsis: This study enrolled 422 men and women, aged 50 or older, with New York Heart Association (NYHA) Class II or III HF and preserved EF, and randomized them to receive spironolactone 25 mg daily or placebo for one year.
The endpoints included echocardiographic measures of diastolic function and remodeling; N-terminal pro b-type natriuretic peptide (NT pro-BNP) levels; exercise capacity; quality of life; and HF symptoms.
In the spironolactone group, there was significant improvement in echocardiographic measures of diastolic function and remodeling as well as NT pro-BNP levels. However, there was no difference in exercise capacity, quality of life, or HF symptoms when compared to placebo.
The spironolactone group had significantly lower blood pressure than the control group, which may account for some of the remodeling effects. The study may have been underpowered, and the study population might not have had severe enough disease to detect a difference in clinical measures. It remains unknown if structural changes on echocardiography will translate into clinical benefits.
Bottom line: Compared to placebo, spironolactone did improve diastolic function by echo but did not improve exercise capacity.
Citation: Edelmann F, Wachter R, Schmidt A, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791.
Real-Time, EMR-Based Prediction Tool Accurately Predicts ICU Transfer Risks
Clinical question: Can clinical deterioration be accurately predicted using real-time data from an electronic medical record (EMR), and can it lead to better outcomes using a floor-based intervention?
Background: Research has shown that EMR-based prediction tools can help identify real-time clinical deterioration in ward patients, but an intervention based on these computer-based alerts has not been shown to be effective.
Study design: Randomized controlled crossover study.
Setting: Eight adult medicine wards in an academic medical center in the Midwest.
Synopsis: There were 20,031 patients assigned to intervention versus control based on their floor location. Computerized alerts were generated using a prediction algorithm. For patients admitted to intervention wards, the alerts were sent to the charge nurse via pager. Patients meeting the alert threshold based on the computerized prediction tool had five times higher risk of ICU transfer, and nine times higher risk of death than patients not meeting the alert threshold. Intervention of charge nurse notification via pager did not result in any significant change in length of stay (LOS), ICU transfer, or mortality. Charge nurses in the intervention group were supposed to alert a physician after receiving the computer alert, but the authors did not measure how often this occurred.
Bottom line: A real-time EMR-based prediction tool accurately predicts higher risk of ICU transfer and death, as well as LOS, but a floor-based intervention to alert the charge nurse in real time did not lead to better outcomes.
Citation: Bailey TC, Chen Y, Mao Y, et al. A trial of a real-time alert for clinical deterioration in patients hospitalized on general medicine wards. J Hosp Med. 2013 Feb 25. doi: 10.1002/jhm.2009 [Epub ahead of print].
Hypothermia Protocol and Cardiac Arrest
Clinical question: Is mild therapeutic hypothermia (MTH) following cardiac arrest beneficial and safe?
Background: Those with sudden cardiac arrest often have poor neurologic outcome. There are some studies that show benefit with hypothermia, but information on safety is limited.
Study design: Meta-analysis.
Setting: Europe, the United Kingdom, the U.S., Canada, Japan, and South Korea.
Synopsis: This study pooled data from 63 studies that looked at MTH protocols in the setting of comatose patients as a result of cardiac arrest. The end points included mortality and any complication potentially attributed to the MTH.
When restricting the analysis to include only randomized controlled trials, MTH was associated with decreased risk of in-hospital mortality (RR=0.75, 95% CI: 0.62-0.92), 30-day mortality (RR=0.61, 95% CI 0.45-0.81), and six-month mortality (RR=0.73, 95% CI 0.61-0.88). MTH was associated with increased risk of arrhythmia (RR=1.25, 95% CI: 1.00-1.55) and hypokalemia (RR=2.35, 95% CI: 1.35-4.11); all other complications were similar between groups.
There were inconsistent results regarding benefit in pediatric patients, as well as comatose patients with non-ventricular fibrillation (non-v-fib) arrest (i.e. asystole or pulseless electrical activity).
Bottom line: Mild therapeutic hypothermia can improve survival of comatose patients after v-fib cardiac arrest and is generally safe.
Citation: Xiao G, Guo Q, Xie X, et al. Safety profile and outcome of mild therapeutic hypothermia in patients following cardiac arrest: systematic review and meta-analysis. Emerg Med J. 2013;30:90-100.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Prices for common hospital procedures not readily available
- Antibiotics associated with decreased mortality in acute COPD exacerbation
- Endovascular therapy has no benefit to systemic t-PA in acute stroke
- Net harm observed with rivaroxaban for thromboprophylaxis
- Noninvasive ventilation more effective, safer for AECOPD patients
- Synthetic cannabinoid use and acute kidney injury
- Dabigatran vs. warfarin in the extended treatment of VTE
- Spironolactone improves diastolic function
- Real-time EMR-based prediction tool for clinical deterioration
- Hypothermia protocol and cardiac arrest
Prices for Common Hospital Procedures Not Readily Available
Clinical question: Are patients able to select health-care providers based on price of service?
Background: With health-care costs rising, patients are encouraged to take a more active role in cost containment. Many initiatives call for greater pricing transparency in the health-care system. This study evaluated price availability for a common surgical procedure.
Study design: Telephone inquiries with standardized interview script.
Setting: Twenty top-ranked orthopedic hospitals and 102 non-top-ranked U.S. hospitals.
Synopsis: Hospitals were contacted by phone with a standardized, scripted request for their price of an elective total hip arthroplasty. The script described the patient as a 62-year-old grandmother without insurance who is able to pay out of pocket and wishes to compare hospital prices. On the first or second attempt, 40% of top-ranked and 32% of non-top-ranked hospitals were able to provide their price; after five attempts, authors were unable to obtain full pricing information (both hospital and physician fee) from 40% of top-ranked and 37% of non-top-ranked hospitals. Neither fee was made available by 15% of top-ranked and 16% of non-top-ranked hospitals. Wide variation of pricing was found across hospitals. The authors commented on the difficulties they encountered, such as the transfer of calls between departments and the uncertainty of representatives on how to assist.
Bottom line: For individual patients, applying basic economic principles as a consumer might be tiresome and often impossible, with no major differences between top-ranked and non-top-ranked hospitals.
Citation: Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
Antibiotics Associated with Decreased Mortality in Acute COPD Exacerbation
Clinical question: Do antibiotics when added to systemic steroids provide clinical benefit for patients with acute COPD exacerbation?
Background: Despite widespread use of antibiotics in the treatment of acute COPD, their benefit is not clear.
Study design: Retrospective cohort study.Setting: Four hundred ten U.S. hospitals participating in Perspective, an inpatient administrative database.
Synopsis: More than 50,000 patients treated with systemic steroids for acute COPD exacerbation were included in this study; 85% of them received empiric antibiotics within the first two hospital days. They were compared with those treated with systemic steroids alone. In-hospital mortality was 1.02% for patients on steroids plus antibiotics versus 1.78% on steroids alone. Use of antibiotics was associated with a 40% reduction in the odds of in-hospital mortality (OR, 0.60; 95% CI, 0.50-0.74) and reduced readmissions. In an analysis of matched cohorts, hospital mortality was 1% for patients on antibiotics and 1.8% for patients without antibiotics (OR, 0.53; 95% CI, 0.40-0.71). The risk for readmission for Clostridium difficile colitis was not different between the groups, but other potential adverse effects of antibiotic use, such as development of resistant micro-organisms, were not studied. In a subset analysis, three groups of antibiotics were compared: macrolides, quinolones, and cephalosporins. None was better than another, but those treated with macrolides had a lower readmission rate for C. diff.
Bottom line: Treatment with antibiotics when added to systemic steroids is associated with improved outcomes in acute COPD exacerbations, but there is no clear advantage of one antibiotic class over another.
Citation: Stefan MS, Rothberg MB, Shieh M, Pekow PS, Lindenauer PK. Association between antibiotic treatment and outcomes in patients hospitalized with acute exacerbation of COPD treated with systemic steroids. Chest. 2013;143(1):82-90.
Endovascular Therapy Added to Systemic t-PA Has No Benefit in Acute Stroke
Clinical question: Does adding endovascular therapy to intravenous tissue plasminogen activator (t-PA) reduce disability in acute stroke?
Background: Early systemic t-PA is the only proven reperfusion therapy in acute stroke, but it is unknown if adding localized endovascular therapy is beneficial.
Study design: Randomized, open-label, blinded-outcome trial.
Setting: Fifty-eight centers in North America, Europe, and Australia.
Synopsis: Patients aged 18 to 82 with acute ischemic stroke were eligible if they received t-PA within three hours of enrollment and had moderate to severe neurologic deficit (National Institutes of Health Stroke Scale [NIHSS] >10, or NIHSS 8 or 9 with CT angiographic evidence of specific major artery occlusion). All patients received standard-dose t-PA; those randomized to endovascular treatment underwent angiography, and, if indicated, underwent one of the endovascular treatments chosen by the neurointerventionalist. The primary outcome measure was a modified Rankin score of 2 or lower (indicating functional independence) at 90 days (assessed by a neurologist).
After enrollment of 656 patients, the trial was terminated early for futility. There was no significant difference in the primary outcome, with 40.8% of patients in the endovascular intervention group and 38.7% in the t-PA-only group having a modified Rankin score of 2 or lower. There was also no difference in mortality or other secondary outcomes, even when the analysis was limited to patients presenting with more severe neurologic deficits.
Bottom line: The addition of endovascular therapy to systemic t-PA in acute ischemic stroke does not improve functional outcomes or mortality.
Citation: Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893-903.
Rivaroxaban Compared with Enoxaparin for Thromboprophylaxis
Clinical question: Is extended-duration rivaroxaban more effective than standard-duration enoxaparin in preventing deep venous thrombosis in acutely ill medical patients?
Background: Trials have shown benefits of thromboprophylaxis in acutely ill medical patients at increased risk of venous thrombosis, but the optimal duration and type of anticoagulation is unknown.
Study design: Prospective, randomized, double-blinded, active-comparator controlled trial.
Setting: Five hundred fifty-two centers in 52 countries.
Synopsis: The trial enrolled 8,428 patients hospitalized with an acute medical condition and reduced mobility. Patients were randomized to receive either enoxaparin for 10 (+/-4) days or rivaroxaban for 35 (+/-4) days. The co-primary composite outcomes were the incidence of asymptomatic proximal deep venous thrombosis, symptomatic deep venous thrombosis, pulmonary embolism, or death related to venous thromboembolism over 10 days and over 35 days.
Both groups had a 2.7% incidence of the primary endpoint over 10 days; over 35 days, the extended-duration rivaroxaban group had a reduced incidence of the primary endpoint of 4.4% compared with 5.7% for enoxaparin. However, there was an increase of clinically relevant bleeding in the rivaroxaban group, occurring in 2.8% and 4.1% of patients over 10 and 35 days, respectively, compared with 1.2% and 1.7% for enoxaparin.
Overall, there was net harm with rivaroxaban over both time periods: 6.6% and 9.4% of patients in the rivaroxaban group had a negative outcome at 10 and 35 days, compared with 4.4% and 7.8% with enoxaparin.
Bottom line: There was net harm with extended-duration rivaroxaban versus standard-duration enoxaparin for thromboprophylaxis in hospitalized medical patients.
Citation: Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513-523.
Noninvasive Ventilation More Effective, Safer for Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD)
Clinical question: What are the patterns in use of noninvasive ventilation (NIV) and invasive mechanical ventilation (IMV) in patients with AECOPD, and which method produces better outcomes?
Background: Multiple randomized controlled trials and meta-analyses have suggested a mortality benefit with NIV compared to standard medical care in AECOPD. However, little evidence exists on head-to-head comparisons of NIV and IMV.
Study design: Retrospective cohort study.
Setting: Non-federal, short-term, general, and other specialty hospitals nationwide.
Synopsis: A sample of 67,651 ED visits for AECOPD with acute respiratory failure was analyzed from the National Emergency Department Sample (NEDS) database between 2006 and 2008. The study found that NIV use increased to 16% in 2008 from 14% in 2006. Use varied widely between hospitals and was more utilized in higher-case-volume, nonmetropolitan, and Northeastern hospitals. Compared with IMV, NIV was associated with 46% lower inpatient mortality (risk ratio 0.54, 95% confidence interval [CI] 0.50-0.59), shortened hospital length of stay (-3.2 days, 95% CI -3.4 to -2.9), lower hospital charges (-$35,012, 95% CI -$36,848 to -$33,176), and lower risk of iatrogenic pneumothorax (0.05% vs. 0.5%, P<0.001). Causality could not be established given the observational study design.
Bottom line: NIV is associated with better outcomes than IMV in the management of AECOPD, and might be underutilized.
Citation: Tsai CL, Lee WY, Delclos GL, Hanania NA, Camargo CA Jr. Comparative effectiveness of noninvasive ventilation vs. invasive mechanical ventilation in chronic obstructive pulmonary disease patients with acute respiratory failure. J Hosp Med. 2013;8(4):165-172.
Synthetic Cannabinoid Use May Cause Acute Kidney Injury
Clinical question: Are synthetic cannabinoids associated with acute kidney injury (AKI)?
Background: Synthetic cannabinoids are designer drugs of abuse with a growing popularity in the U.S.
Study design: Retrospective case series.
Setting: Hospitals in Wyoming, Oklahoma, Rhode Island, New York, Kansas, and Oregon.
Synopsis: The Centers for Disease Control and Prevention (CDC) issued an alert when 16 cases of unexplained AKI after exposure to synthetic cannabinoids were reported between March and December 2012. Synthetic cannabinoid use is associated with neurologic, sympathomimetic, and cardiovascular toxicity; however, this is the first case series reporting renal toxicity. The 16 patients included 15 males and one female, aged 15-33 years, with no pre-existing renal disease or nephrotoxic medication consumption. All used synthetic cannabinoids within hours to days before developing nausea, vomiting, abdominal, and flank and/or back pain.
Creatinine peaked one to six days after symptom onset. Five patients required hemodialysis, and all 16 recovered. There was no finding specific for all cases on ultrasound and/or biopsy. Toxicologic analysis of specimens was possible in seven cases and revealed a previously unreported fluorinated synthetic cannabinoid compound XLR-11 in all clinical specimens of patients who used the drug within two days of being tested.
Overall, the analysis did not reveal any single compound or brand that could explain all cases.
Bottom line: Clinicians should be aware of the potential for renal or other toxicities in users of synthetic cannabinoid products and should ask about their use in cases of unexplained AKI.
Citation: Murphy TD, Weidenbach KN, Van Houten C, et al. Centers for Disease Control and Prevention. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(6):93-98.
Dabigatran versus Warfarin in Extended VTE Treatment
Clinical question: Is dabigatran suitable for extended treatment VTE?
Background: In contrast to warfarin (Coumadin), dabigatran (Pradaxa) is given in a fixed dose and does not require frequent laboratory monitoring. Dabigatran has been shown to be noninferior to warfarin in the initial six-month treatment of VTE.
Study design: Two double-blinded, randomized trials: an active-control study and a placebo-control study.
Setting: Two hundred sixty-five sites in 33 countries for the active-control study, and 147 sites in 21 countries for the placebo-control study.
Synopsis: This study enrolled 4,299 adult patients with objectively confirmed, symptomatic, proximal deep vein thrombosis or pulmonary embolism. In the active-control study comparing warfarin and dabigatran, recurrent objectively confirmed symptomatic or fatal VTE was observed in 1.8% of patients in the dabigatran group compared with 1.3% of patients in the warfarin group (P=0.01 for noninferiority). While major or clinically relevant bleeding was less frequent with dabigatran compared to warfarin (hazard ratio, 0.54), more acute coronary syndromes were observed with dabigatran (0.9% vs. 0.2%, P=0.02). In the placebo-control study, symptomatic or fatal VTE was observed in 0.4% of patients in the dabigatran group compared with 5.6% in the placebo group. Clinically relevant bleeding was more common with dabigatran (5.3% vs. 1.8%; hazard ratio 2.92).
Bottom line: Treatment with dabigatran met the pre-specified noninferiority margin in this study. However, it is worth noting that patients with VTE given extended treatment with dabigatran had significantly higher rates of recurrent symptomatic or fatal VTE than patients treated with warfarin.
Citation: Schulman S, Kearson C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709-718.
Spironolactone Improves Diastolic Function
Clinical question: What is the efficacy of aldosterone receptor blockers on diastolic function and exercise capacity?
Background: Mineralocorticoid receptor activation by aldosterone contributes to the pathophysiology of heart failure (HF) in patients with and without reduced ejection fraction (EF). Aldosterone receptor blockers (spironolactone) reduce overall and cardiovascular mortality in HF patients with reduced EF; however, its effect on HF patients with preserved EF is unknown.
Study design: Prospective, randomized, double-blinded, placebo-controlled trial.
Setting: Ten ambulatory sites in Germany and Austria.
Synopsis: This study enrolled 422 men and women, aged 50 or older, with New York Heart Association (NYHA) Class II or III HF and preserved EF, and randomized them to receive spironolactone 25 mg daily or placebo for one year.
The endpoints included echocardiographic measures of diastolic function and remodeling; N-terminal pro b-type natriuretic peptide (NT pro-BNP) levels; exercise capacity; quality of life; and HF symptoms.
In the spironolactone group, there was significant improvement in echocardiographic measures of diastolic function and remodeling as well as NT pro-BNP levels. However, there was no difference in exercise capacity, quality of life, or HF symptoms when compared to placebo.
The spironolactone group had significantly lower blood pressure than the control group, which may account for some of the remodeling effects. The study may have been underpowered, and the study population might not have had severe enough disease to detect a difference in clinical measures. It remains unknown if structural changes on echocardiography will translate into clinical benefits.
Bottom line: Compared to placebo, spironolactone did improve diastolic function by echo but did not improve exercise capacity.
Citation: Edelmann F, Wachter R, Schmidt A, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791.
Real-Time, EMR-Based Prediction Tool Accurately Predicts ICU Transfer Risks
Clinical question: Can clinical deterioration be accurately predicted using real-time data from an electronic medical record (EMR), and can it lead to better outcomes using a floor-based intervention?
Background: Research has shown that EMR-based prediction tools can help identify real-time clinical deterioration in ward patients, but an intervention based on these computer-based alerts has not been shown to be effective.
Study design: Randomized controlled crossover study.
Setting: Eight adult medicine wards in an academic medical center in the Midwest.
Synopsis: There were 20,031 patients assigned to intervention versus control based on their floor location. Computerized alerts were generated using a prediction algorithm. For patients admitted to intervention wards, the alerts were sent to the charge nurse via pager. Patients meeting the alert threshold based on the computerized prediction tool had five times higher risk of ICU transfer, and nine times higher risk of death than patients not meeting the alert threshold. Intervention of charge nurse notification via pager did not result in any significant change in length of stay (LOS), ICU transfer, or mortality. Charge nurses in the intervention group were supposed to alert a physician after receiving the computer alert, but the authors did not measure how often this occurred.
Bottom line: A real-time EMR-based prediction tool accurately predicts higher risk of ICU transfer and death, as well as LOS, but a floor-based intervention to alert the charge nurse in real time did not lead to better outcomes.
Citation: Bailey TC, Chen Y, Mao Y, et al. A trial of a real-time alert for clinical deterioration in patients hospitalized on general medicine wards. J Hosp Med. 2013 Feb 25. doi: 10.1002/jhm.2009 [Epub ahead of print].
Hypothermia Protocol and Cardiac Arrest
Clinical question: Is mild therapeutic hypothermia (MTH) following cardiac arrest beneficial and safe?
Background: Those with sudden cardiac arrest often have poor neurologic outcome. There are some studies that show benefit with hypothermia, but information on safety is limited.
Study design: Meta-analysis.
Setting: Europe, the United Kingdom, the U.S., Canada, Japan, and South Korea.
Synopsis: This study pooled data from 63 studies that looked at MTH protocols in the setting of comatose patients as a result of cardiac arrest. The end points included mortality and any complication potentially attributed to the MTH.
When restricting the analysis to include only randomized controlled trials, MTH was associated with decreased risk of in-hospital mortality (RR=0.75, 95% CI: 0.62-0.92), 30-day mortality (RR=0.61, 95% CI 0.45-0.81), and six-month mortality (RR=0.73, 95% CI 0.61-0.88). MTH was associated with increased risk of arrhythmia (RR=1.25, 95% CI: 1.00-1.55) and hypokalemia (RR=2.35, 95% CI: 1.35-4.11); all other complications were similar between groups.
There were inconsistent results regarding benefit in pediatric patients, as well as comatose patients with non-ventricular fibrillation (non-v-fib) arrest (i.e. asystole or pulseless electrical activity).
Bottom line: Mild therapeutic hypothermia can improve survival of comatose patients after v-fib cardiac arrest and is generally safe.
Citation: Xiao G, Guo Q, Xie X, et al. Safety profile and outcome of mild therapeutic hypothermia in patients following cardiac arrest: systematic review and meta-analysis. Emerg Med J. 2013;30:90-100.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Prices for common hospital procedures not readily available
- Antibiotics associated with decreased mortality in acute COPD exacerbation
- Endovascular therapy has no benefit to systemic t-PA in acute stroke
- Net harm observed with rivaroxaban for thromboprophylaxis
- Noninvasive ventilation more effective, safer for AECOPD patients
- Synthetic cannabinoid use and acute kidney injury
- Dabigatran vs. warfarin in the extended treatment of VTE
- Spironolactone improves diastolic function
- Real-time EMR-based prediction tool for clinical deterioration
- Hypothermia protocol and cardiac arrest
Prices for Common Hospital Procedures Not Readily Available
Clinical question: Are patients able to select health-care providers based on price of service?
Background: With health-care costs rising, patients are encouraged to take a more active role in cost containment. Many initiatives call for greater pricing transparency in the health-care system. This study evaluated price availability for a common surgical procedure.
Study design: Telephone inquiries with standardized interview script.
Setting: Twenty top-ranked orthopedic hospitals and 102 non-top-ranked U.S. hospitals.
Synopsis: Hospitals were contacted by phone with a standardized, scripted request for their price of an elective total hip arthroplasty. The script described the patient as a 62-year-old grandmother without insurance who is able to pay out of pocket and wishes to compare hospital prices. On the first or second attempt, 40% of top-ranked and 32% of non-top-ranked hospitals were able to provide their price; after five attempts, authors were unable to obtain full pricing information (both hospital and physician fee) from 40% of top-ranked and 37% of non-top-ranked hospitals. Neither fee was made available by 15% of top-ranked and 16% of non-top-ranked hospitals. Wide variation of pricing was found across hospitals. The authors commented on the difficulties they encountered, such as the transfer of calls between departments and the uncertainty of representatives on how to assist.
Bottom line: For individual patients, applying basic economic principles as a consumer might be tiresome and often impossible, with no major differences between top-ranked and non-top-ranked hospitals.
Citation: Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
Antibiotics Associated with Decreased Mortality in Acute COPD Exacerbation
Clinical question: Do antibiotics when added to systemic steroids provide clinical benefit for patients with acute COPD exacerbation?
Background: Despite widespread use of antibiotics in the treatment of acute COPD, their benefit is not clear.
Study design: Retrospective cohort study.Setting: Four hundred ten U.S. hospitals participating in Perspective, an inpatient administrative database.
Synopsis: More than 50,000 patients treated with systemic steroids for acute COPD exacerbation were included in this study; 85% of them received empiric antibiotics within the first two hospital days. They were compared with those treated with systemic steroids alone. In-hospital mortality was 1.02% for patients on steroids plus antibiotics versus 1.78% on steroids alone. Use of antibiotics was associated with a 40% reduction in the odds of in-hospital mortality (OR, 0.60; 95% CI, 0.50-0.74) and reduced readmissions. In an analysis of matched cohorts, hospital mortality was 1% for patients on antibiotics and 1.8% for patients without antibiotics (OR, 0.53; 95% CI, 0.40-0.71). The risk for readmission for Clostridium difficile colitis was not different between the groups, but other potential adverse effects of antibiotic use, such as development of resistant micro-organisms, were not studied. In a subset analysis, three groups of antibiotics were compared: macrolides, quinolones, and cephalosporins. None was better than another, but those treated with macrolides had a lower readmission rate for C. diff.
Bottom line: Treatment with antibiotics when added to systemic steroids is associated with improved outcomes in acute COPD exacerbations, but there is no clear advantage of one antibiotic class over another.
Citation: Stefan MS, Rothberg MB, Shieh M, Pekow PS, Lindenauer PK. Association between antibiotic treatment and outcomes in patients hospitalized with acute exacerbation of COPD treated with systemic steroids. Chest. 2013;143(1):82-90.
Endovascular Therapy Added to Systemic t-PA Has No Benefit in Acute Stroke
Clinical question: Does adding endovascular therapy to intravenous tissue plasminogen activator (t-PA) reduce disability in acute stroke?
Background: Early systemic t-PA is the only proven reperfusion therapy in acute stroke, but it is unknown if adding localized endovascular therapy is beneficial.
Study design: Randomized, open-label, blinded-outcome trial.
Setting: Fifty-eight centers in North America, Europe, and Australia.
Synopsis: Patients aged 18 to 82 with acute ischemic stroke were eligible if they received t-PA within three hours of enrollment and had moderate to severe neurologic deficit (National Institutes of Health Stroke Scale [NIHSS] >10, or NIHSS 8 or 9 with CT angiographic evidence of specific major artery occlusion). All patients received standard-dose t-PA; those randomized to endovascular treatment underwent angiography, and, if indicated, underwent one of the endovascular treatments chosen by the neurointerventionalist. The primary outcome measure was a modified Rankin score of 2 or lower (indicating functional independence) at 90 days (assessed by a neurologist).
After enrollment of 656 patients, the trial was terminated early for futility. There was no significant difference in the primary outcome, with 40.8% of patients in the endovascular intervention group and 38.7% in the t-PA-only group having a modified Rankin score of 2 or lower. There was also no difference in mortality or other secondary outcomes, even when the analysis was limited to patients presenting with more severe neurologic deficits.
Bottom line: The addition of endovascular therapy to systemic t-PA in acute ischemic stroke does not improve functional outcomes or mortality.
Citation: Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893-903.
Rivaroxaban Compared with Enoxaparin for Thromboprophylaxis
Clinical question: Is extended-duration rivaroxaban more effective than standard-duration enoxaparin in preventing deep venous thrombosis in acutely ill medical patients?
Background: Trials have shown benefits of thromboprophylaxis in acutely ill medical patients at increased risk of venous thrombosis, but the optimal duration and type of anticoagulation is unknown.
Study design: Prospective, randomized, double-blinded, active-comparator controlled trial.
Setting: Five hundred fifty-two centers in 52 countries.
Synopsis: The trial enrolled 8,428 patients hospitalized with an acute medical condition and reduced mobility. Patients were randomized to receive either enoxaparin for 10 (+/-4) days or rivaroxaban for 35 (+/-4) days. The co-primary composite outcomes were the incidence of asymptomatic proximal deep venous thrombosis, symptomatic deep venous thrombosis, pulmonary embolism, or death related to venous thromboembolism over 10 days and over 35 days.
Both groups had a 2.7% incidence of the primary endpoint over 10 days; over 35 days, the extended-duration rivaroxaban group had a reduced incidence of the primary endpoint of 4.4% compared with 5.7% for enoxaparin. However, there was an increase of clinically relevant bleeding in the rivaroxaban group, occurring in 2.8% and 4.1% of patients over 10 and 35 days, respectively, compared with 1.2% and 1.7% for enoxaparin.
Overall, there was net harm with rivaroxaban over both time periods: 6.6% and 9.4% of patients in the rivaroxaban group had a negative outcome at 10 and 35 days, compared with 4.4% and 7.8% with enoxaparin.
Bottom line: There was net harm with extended-duration rivaroxaban versus standard-duration enoxaparin for thromboprophylaxis in hospitalized medical patients.
Citation: Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513-523.
Noninvasive Ventilation More Effective, Safer for Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD)
Clinical question: What are the patterns in use of noninvasive ventilation (NIV) and invasive mechanical ventilation (IMV) in patients with AECOPD, and which method produces better outcomes?
Background: Multiple randomized controlled trials and meta-analyses have suggested a mortality benefit with NIV compared to standard medical care in AECOPD. However, little evidence exists on head-to-head comparisons of NIV and IMV.
Study design: Retrospective cohort study.
Setting: Non-federal, short-term, general, and other specialty hospitals nationwide.
Synopsis: A sample of 67,651 ED visits for AECOPD with acute respiratory failure was analyzed from the National Emergency Department Sample (NEDS) database between 2006 and 2008. The study found that NIV use increased to 16% in 2008 from 14% in 2006. Use varied widely between hospitals and was more utilized in higher-case-volume, nonmetropolitan, and Northeastern hospitals. Compared with IMV, NIV was associated with 46% lower inpatient mortality (risk ratio 0.54, 95% confidence interval [CI] 0.50-0.59), shortened hospital length of stay (-3.2 days, 95% CI -3.4 to -2.9), lower hospital charges (-$35,012, 95% CI -$36,848 to -$33,176), and lower risk of iatrogenic pneumothorax (0.05% vs. 0.5%, P<0.001). Causality could not be established given the observational study design.
Bottom line: NIV is associated with better outcomes than IMV in the management of AECOPD, and might be underutilized.
Citation: Tsai CL, Lee WY, Delclos GL, Hanania NA, Camargo CA Jr. Comparative effectiveness of noninvasive ventilation vs. invasive mechanical ventilation in chronic obstructive pulmonary disease patients with acute respiratory failure. J Hosp Med. 2013;8(4):165-172.
Synthetic Cannabinoid Use May Cause Acute Kidney Injury
Clinical question: Are synthetic cannabinoids associated with acute kidney injury (AKI)?
Background: Synthetic cannabinoids are designer drugs of abuse with a growing popularity in the U.S.
Study design: Retrospective case series.
Setting: Hospitals in Wyoming, Oklahoma, Rhode Island, New York, Kansas, and Oregon.
Synopsis: The Centers for Disease Control and Prevention (CDC) issued an alert when 16 cases of unexplained AKI after exposure to synthetic cannabinoids were reported between March and December 2012. Synthetic cannabinoid use is associated with neurologic, sympathomimetic, and cardiovascular toxicity; however, this is the first case series reporting renal toxicity. The 16 patients included 15 males and one female, aged 15-33 years, with no pre-existing renal disease or nephrotoxic medication consumption. All used synthetic cannabinoids within hours to days before developing nausea, vomiting, abdominal, and flank and/or back pain.
Creatinine peaked one to six days after symptom onset. Five patients required hemodialysis, and all 16 recovered. There was no finding specific for all cases on ultrasound and/or biopsy. Toxicologic analysis of specimens was possible in seven cases and revealed a previously unreported fluorinated synthetic cannabinoid compound XLR-11 in all clinical specimens of patients who used the drug within two days of being tested.
Overall, the analysis did not reveal any single compound or brand that could explain all cases.
Bottom line: Clinicians should be aware of the potential for renal or other toxicities in users of synthetic cannabinoid products and should ask about their use in cases of unexplained AKI.
Citation: Murphy TD, Weidenbach KN, Van Houten C, et al. Centers for Disease Control and Prevention. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(6):93-98.
Dabigatran versus Warfarin in Extended VTE Treatment
Clinical question: Is dabigatran suitable for extended treatment VTE?
Background: In contrast to warfarin (Coumadin), dabigatran (Pradaxa) is given in a fixed dose and does not require frequent laboratory monitoring. Dabigatran has been shown to be noninferior to warfarin in the initial six-month treatment of VTE.
Study design: Two double-blinded, randomized trials: an active-control study and a placebo-control study.
Setting: Two hundred sixty-five sites in 33 countries for the active-control study, and 147 sites in 21 countries for the placebo-control study.
Synopsis: This study enrolled 4,299 adult patients with objectively confirmed, symptomatic, proximal deep vein thrombosis or pulmonary embolism. In the active-control study comparing warfarin and dabigatran, recurrent objectively confirmed symptomatic or fatal VTE was observed in 1.8% of patients in the dabigatran group compared with 1.3% of patients in the warfarin group (P=0.01 for noninferiority). While major or clinically relevant bleeding was less frequent with dabigatran compared to warfarin (hazard ratio, 0.54), more acute coronary syndromes were observed with dabigatran (0.9% vs. 0.2%, P=0.02). In the placebo-control study, symptomatic or fatal VTE was observed in 0.4% of patients in the dabigatran group compared with 5.6% in the placebo group. Clinically relevant bleeding was more common with dabigatran (5.3% vs. 1.8%; hazard ratio 2.92).
Bottom line: Treatment with dabigatran met the pre-specified noninferiority margin in this study. However, it is worth noting that patients with VTE given extended treatment with dabigatran had significantly higher rates of recurrent symptomatic or fatal VTE than patients treated with warfarin.
Citation: Schulman S, Kearson C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709-718.
Spironolactone Improves Diastolic Function
Clinical question: What is the efficacy of aldosterone receptor blockers on diastolic function and exercise capacity?
Background: Mineralocorticoid receptor activation by aldosterone contributes to the pathophysiology of heart failure (HF) in patients with and without reduced ejection fraction (EF). Aldosterone receptor blockers (spironolactone) reduce overall and cardiovascular mortality in HF patients with reduced EF; however, its effect on HF patients with preserved EF is unknown.
Study design: Prospective, randomized, double-blinded, placebo-controlled trial.
Setting: Ten ambulatory sites in Germany and Austria.
Synopsis: This study enrolled 422 men and women, aged 50 or older, with New York Heart Association (NYHA) Class II or III HF and preserved EF, and randomized them to receive spironolactone 25 mg daily or placebo for one year.
The endpoints included echocardiographic measures of diastolic function and remodeling; N-terminal pro b-type natriuretic peptide (NT pro-BNP) levels; exercise capacity; quality of life; and HF symptoms.
In the spironolactone group, there was significant improvement in echocardiographic measures of diastolic function and remodeling as well as NT pro-BNP levels. However, there was no difference in exercise capacity, quality of life, or HF symptoms when compared to placebo.
The spironolactone group had significantly lower blood pressure than the control group, which may account for some of the remodeling effects. The study may have been underpowered, and the study population might not have had severe enough disease to detect a difference in clinical measures. It remains unknown if structural changes on echocardiography will translate into clinical benefits.
Bottom line: Compared to placebo, spironolactone did improve diastolic function by echo but did not improve exercise capacity.
Citation: Edelmann F, Wachter R, Schmidt A, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791.
Real-Time, EMR-Based Prediction Tool Accurately Predicts ICU Transfer Risks
Clinical question: Can clinical deterioration be accurately predicted using real-time data from an electronic medical record (EMR), and can it lead to better outcomes using a floor-based intervention?
Background: Research has shown that EMR-based prediction tools can help identify real-time clinical deterioration in ward patients, but an intervention based on these computer-based alerts has not been shown to be effective.
Study design: Randomized controlled crossover study.
Setting: Eight adult medicine wards in an academic medical center in the Midwest.
Synopsis: There were 20,031 patients assigned to intervention versus control based on their floor location. Computerized alerts were generated using a prediction algorithm. For patients admitted to intervention wards, the alerts were sent to the charge nurse via pager. Patients meeting the alert threshold based on the computerized prediction tool had five times higher risk of ICU transfer, and nine times higher risk of death than patients not meeting the alert threshold. Intervention of charge nurse notification via pager did not result in any significant change in length of stay (LOS), ICU transfer, or mortality. Charge nurses in the intervention group were supposed to alert a physician after receiving the computer alert, but the authors did not measure how often this occurred.
Bottom line: A real-time EMR-based prediction tool accurately predicts higher risk of ICU transfer and death, as well as LOS, but a floor-based intervention to alert the charge nurse in real time did not lead to better outcomes.
Citation: Bailey TC, Chen Y, Mao Y, et al. A trial of a real-time alert for clinical deterioration in patients hospitalized on general medicine wards. J Hosp Med. 2013 Feb 25. doi: 10.1002/jhm.2009 [Epub ahead of print].
Hypothermia Protocol and Cardiac Arrest
Clinical question: Is mild therapeutic hypothermia (MTH) following cardiac arrest beneficial and safe?
Background: Those with sudden cardiac arrest often have poor neurologic outcome. There are some studies that show benefit with hypothermia, but information on safety is limited.
Study design: Meta-analysis.
Setting: Europe, the United Kingdom, the U.S., Canada, Japan, and South Korea.
Synopsis: This study pooled data from 63 studies that looked at MTH protocols in the setting of comatose patients as a result of cardiac arrest. The end points included mortality and any complication potentially attributed to the MTH.
When restricting the analysis to include only randomized controlled trials, MTH was associated with decreased risk of in-hospital mortality (RR=0.75, 95% CI: 0.62-0.92), 30-day mortality (RR=0.61, 95% CI 0.45-0.81), and six-month mortality (RR=0.73, 95% CI 0.61-0.88). MTH was associated with increased risk of arrhythmia (RR=1.25, 95% CI: 1.00-1.55) and hypokalemia (RR=2.35, 95% CI: 1.35-4.11); all other complications were similar between groups.
There were inconsistent results regarding benefit in pediatric patients, as well as comatose patients with non-ventricular fibrillation (non-v-fib) arrest (i.e. asystole or pulseless electrical activity).
Bottom line: Mild therapeutic hypothermia can improve survival of comatose patients after v-fib cardiac arrest and is generally safe.
Citation: Xiao G, Guo Q, Xie X, et al. Safety profile and outcome of mild therapeutic hypothermia in patients following cardiac arrest: systematic review and meta-analysis. Emerg Med J. 2013;30:90-100.
High mortality seen in acute-on-chronic liver failure
An attempt to define and classify acute-on-chronic liver failure showed that the syndrome carries a 28-day mortality rate that is 15 times greater than in cirrhosis patients who do not have the syndrome.
Moreover, the syndrome is extremely common, and may be found in nearly one-third of acutely decompensated cirrhosis patients, wrote Dr. Richard Moreau and colleagues. The study was published in the June issue of Gastroenterology.
Source: American Gastroenterological Association
"A universally accepted and used definition of acute-on-chronic liver failure (ACLF) is still lacking," said Dr. Moreau of Université Paris Diderot, Paris.
"Defining ACLF is not only a matter of nosology, but also is of great importance because it would allow early identification of patients at high risk for end-organ failure–related death, requiring specific treatments and/or intensive management," he added.
To that end, Dr. Moreau looked at 1,343 adult patients hospitalized for at least 1 day who had an acute decompensation of cirrhosis as defined by the acute development of large ascites, hepatic encephalopathy, gastrointestinal hemorrhage, bacterial infection, or any combination of the above.
Patients who were admitted for a scheduled procedure or treatment were excluded from the analysis, as were patients with severe chronic extrahepatic disease and patients with HIV infection.
The study subjects were then divided into four groups. The first group, which was judged not to have ACLF, had no organ failure, a serum creatinine less than 1.5 mg/dL, and no hepatic encephalopathy. This group made up 1,040 of the 1,343 enrolled patients (77.4%), and had 28-day and 90-day mortality rates of 4.7% and 14%, respectively.
The next cohort, called ACLF grade 1, comprised patients with a single coagulation, circulatory or respiratory failure; a serum creatinine between 1.5 and 1.9 mg/dL; and/or mild to moderate hepatic encephalopathy. The 148 patients in this class (11.0%) had 28- and 90-day mortality rates of 22.1% and 40.7%, respectively.
ACLF grade 2 was more severe, with two organ failures; 108 patients (8%) had this at enrollment, and exhibited 28- and 90-day mortality rates of 32.0% and 40.7%, respectively.
The most severely ill patients were classed as having ACLF grade 3, with three organ failures or more. A total of 47 patients (3.5%) fell into this category, and they had 28- and 90-day mortality rates of 76.7% and 79.1%, respectively.
Overall, according to the investigators, patients with ACLF were younger (mean age 56 years versus 58 years in patients without ACLF; P = .02), had a lower mean arterial blood pressure on admittance to the hospital (79 mm Hg versus 85 mm Hg in non-ACLF patients; P less than .001) and more frequently were actively alcoholic.
They also found that patients with ACLF had a significantly higher white cell count (9.7 x 109 compared with 6.6 x 109/L; P less than .001) and plasma C-reactive protein level (40.3 versus 24.9 mg/L; P less than .001) than the group without ACLF.
And finally, in what they called an "outstanding observation," the authors determined that up to 43.6% of patients with ACLF had no precipitating event leading to their acute decompensation, including gastrointestinal hemorrhage, bacterial infection, or active alcoholism.
The authors concluded that their novel diagnostic criteria show that ACLF is "distinct from ‘mere’ AD."
They conceded that their study was not designed to assess ideal management for these patients. "Whether patients with ACLF should be admitted or not to the intensive care unit is controversial," they wrote. "Nevertheless, our results can serve as a resource for designing studies aimed to investigate the appropriate site of hospitalization for patients with ACLF."
The authors disclosed that pharmaceutical companies provided funding for a chronic liver failure consortium, which provided the initiative for this study; several other investigators also disclosed ties with pharmaceutical companies.
The study by Moreau and colleagues represents a culmination of efforts to bring together a continent’s worth of experience in research and patient care into defining an important issue that has been struggling to find a definition: acute-on-chronic liver failure or ACLF. This important study crucially separates ACLF as an entity distinct
from mere decompensation of cirrhosis, something that has long confused the
picture among clinicians. The definition of organ failure, the basis for ACLF,
was determined a priori by expert opinion. The team found a significantly poorer
prognosis in patients with more than two organ failures, especially renal
failure. An intriguing finding was the better prognosis of previously
decompensated cirrhotic patients compared with those without prior
decompensation, which should form the basis for future investigation into the
relationship of ACLF with potential immune tolerance. Other interesting points
raised, such as the absence of precipitating factors in almost half of the ACLF
cases, and correlation of outcomes with leukocyte count and C-reaction protein,
need further corroboration since a large proportion of patients had an
alcoholic etiology. In a large study, it is not always possible to have uniform
precipitating factor investigations. Importantly, these results have also been
corroborated by the initial reports on ACLF using simpler criteria for organ
failure in the North American Consortium for End-Stage Liver Disease (NACSELD).
As the cirrhotic population in Western countries ages along with a scarcity of
donor organs and the emergence of resistant pathogens, the knowledge of ACLF is
going to be increasingly relevant and further large, collaborative studies are
needed along the lines of this paper to tackle this growing issue.
Dr. Jasmohan Bajaj is in the division of gastroenterology, hepatology,
and nutrition at Virginia Commonwealth University and at McGuire VA Medical
Center, Richmond.
His institution has received grants from, and he has been a consultant to,
Grifols, Salix, and Otsuka. He has received an honorarium from Merz.
The study by Moreau and colleagues represents a culmination of efforts to bring together a continent’s worth of experience in research and patient care into defining an important issue that has been struggling to find a definition: acute-on-chronic liver failure or ACLF. This important study crucially separates ACLF as an entity distinct
from mere decompensation of cirrhosis, something that has long confused the
picture among clinicians. The definition of organ failure, the basis for ACLF,
was determined a priori by expert opinion. The team found a significantly poorer
prognosis in patients with more than two organ failures, especially renal
failure. An intriguing finding was the better prognosis of previously
decompensated cirrhotic patients compared with those without prior
decompensation, which should form the basis for future investigation into the
relationship of ACLF with potential immune tolerance. Other interesting points
raised, such as the absence of precipitating factors in almost half of the ACLF
cases, and correlation of outcomes with leukocyte count and C-reaction protein,
need further corroboration since a large proportion of patients had an
alcoholic etiology. In a large study, it is not always possible to have uniform
precipitating factor investigations. Importantly, these results have also been
corroborated by the initial reports on ACLF using simpler criteria for organ
failure in the North American Consortium for End-Stage Liver Disease (NACSELD).
As the cirrhotic population in Western countries ages along with a scarcity of
donor organs and the emergence of resistant pathogens, the knowledge of ACLF is
going to be increasingly relevant and further large, collaborative studies are
needed along the lines of this paper to tackle this growing issue.
Dr. Jasmohan Bajaj is in the division of gastroenterology, hepatology,
and nutrition at Virginia Commonwealth University and at McGuire VA Medical
Center, Richmond.
His institution has received grants from, and he has been a consultant to,
Grifols, Salix, and Otsuka. He has received an honorarium from Merz.
The study by Moreau and colleagues represents a culmination of efforts to bring together a continent’s worth of experience in research and patient care into defining an important issue that has been struggling to find a definition: acute-on-chronic liver failure or ACLF. This important study crucially separates ACLF as an entity distinct
from mere decompensation of cirrhosis, something that has long confused the
picture among clinicians. The definition of organ failure, the basis for ACLF,
was determined a priori by expert opinion. The team found a significantly poorer
prognosis in patients with more than two organ failures, especially renal
failure. An intriguing finding was the better prognosis of previously
decompensated cirrhotic patients compared with those without prior
decompensation, which should form the basis for future investigation into the
relationship of ACLF with potential immune tolerance. Other interesting points
raised, such as the absence of precipitating factors in almost half of the ACLF
cases, and correlation of outcomes with leukocyte count and C-reaction protein,
need further corroboration since a large proportion of patients had an
alcoholic etiology. In a large study, it is not always possible to have uniform
precipitating factor investigations. Importantly, these results have also been
corroborated by the initial reports on ACLF using simpler criteria for organ
failure in the North American Consortium for End-Stage Liver Disease (NACSELD).
As the cirrhotic population in Western countries ages along with a scarcity of
donor organs and the emergence of resistant pathogens, the knowledge of ACLF is
going to be increasingly relevant and further large, collaborative studies are
needed along the lines of this paper to tackle this growing issue.
Dr. Jasmohan Bajaj is in the division of gastroenterology, hepatology,
and nutrition at Virginia Commonwealth University and at McGuire VA Medical
Center, Richmond.
His institution has received grants from, and he has been a consultant to,
Grifols, Salix, and Otsuka. He has received an honorarium from Merz.
An attempt to define and classify acute-on-chronic liver failure showed that the syndrome carries a 28-day mortality rate that is 15 times greater than in cirrhosis patients who do not have the syndrome.
Moreover, the syndrome is extremely common, and may be found in nearly one-third of acutely decompensated cirrhosis patients, wrote Dr. Richard Moreau and colleagues. The study was published in the June issue of Gastroenterology.
Source: American Gastroenterological Association
"A universally accepted and used definition of acute-on-chronic liver failure (ACLF) is still lacking," said Dr. Moreau of Université Paris Diderot, Paris.
"Defining ACLF is not only a matter of nosology, but also is of great importance because it would allow early identification of patients at high risk for end-organ failure–related death, requiring specific treatments and/or intensive management," he added.
To that end, Dr. Moreau looked at 1,343 adult patients hospitalized for at least 1 day who had an acute decompensation of cirrhosis as defined by the acute development of large ascites, hepatic encephalopathy, gastrointestinal hemorrhage, bacterial infection, or any combination of the above.
Patients who were admitted for a scheduled procedure or treatment were excluded from the analysis, as were patients with severe chronic extrahepatic disease and patients with HIV infection.
The study subjects were then divided into four groups. The first group, which was judged not to have ACLF, had no organ failure, a serum creatinine less than 1.5 mg/dL, and no hepatic encephalopathy. This group made up 1,040 of the 1,343 enrolled patients (77.4%), and had 28-day and 90-day mortality rates of 4.7% and 14%, respectively.
The next cohort, called ACLF grade 1, comprised patients with a single coagulation, circulatory or respiratory failure; a serum creatinine between 1.5 and 1.9 mg/dL; and/or mild to moderate hepatic encephalopathy. The 148 patients in this class (11.0%) had 28- and 90-day mortality rates of 22.1% and 40.7%, respectively.
ACLF grade 2 was more severe, with two organ failures; 108 patients (8%) had this at enrollment, and exhibited 28- and 90-day mortality rates of 32.0% and 40.7%, respectively.
The most severely ill patients were classed as having ACLF grade 3, with three organ failures or more. A total of 47 patients (3.5%) fell into this category, and they had 28- and 90-day mortality rates of 76.7% and 79.1%, respectively.
Overall, according to the investigators, patients with ACLF were younger (mean age 56 years versus 58 years in patients without ACLF; P = .02), had a lower mean arterial blood pressure on admittance to the hospital (79 mm Hg versus 85 mm Hg in non-ACLF patients; P less than .001) and more frequently were actively alcoholic.
They also found that patients with ACLF had a significantly higher white cell count (9.7 x 109 compared with 6.6 x 109/L; P less than .001) and plasma C-reactive protein level (40.3 versus 24.9 mg/L; P less than .001) than the group without ACLF.
And finally, in what they called an "outstanding observation," the authors determined that up to 43.6% of patients with ACLF had no precipitating event leading to their acute decompensation, including gastrointestinal hemorrhage, bacterial infection, or active alcoholism.
The authors concluded that their novel diagnostic criteria show that ACLF is "distinct from ‘mere’ AD."
They conceded that their study was not designed to assess ideal management for these patients. "Whether patients with ACLF should be admitted or not to the intensive care unit is controversial," they wrote. "Nevertheless, our results can serve as a resource for designing studies aimed to investigate the appropriate site of hospitalization for patients with ACLF."
The authors disclosed that pharmaceutical companies provided funding for a chronic liver failure consortium, which provided the initiative for this study; several other investigators also disclosed ties with pharmaceutical companies.
An attempt to define and classify acute-on-chronic liver failure showed that the syndrome carries a 28-day mortality rate that is 15 times greater than in cirrhosis patients who do not have the syndrome.
Moreover, the syndrome is extremely common, and may be found in nearly one-third of acutely decompensated cirrhosis patients, wrote Dr. Richard Moreau and colleagues. The study was published in the June issue of Gastroenterology.
Source: American Gastroenterological Association
"A universally accepted and used definition of acute-on-chronic liver failure (ACLF) is still lacking," said Dr. Moreau of Université Paris Diderot, Paris.
"Defining ACLF is not only a matter of nosology, but also is of great importance because it would allow early identification of patients at high risk for end-organ failure–related death, requiring specific treatments and/or intensive management," he added.
To that end, Dr. Moreau looked at 1,343 adult patients hospitalized for at least 1 day who had an acute decompensation of cirrhosis as defined by the acute development of large ascites, hepatic encephalopathy, gastrointestinal hemorrhage, bacterial infection, or any combination of the above.
Patients who were admitted for a scheduled procedure or treatment were excluded from the analysis, as were patients with severe chronic extrahepatic disease and patients with HIV infection.
The study subjects were then divided into four groups. The first group, which was judged not to have ACLF, had no organ failure, a serum creatinine less than 1.5 mg/dL, and no hepatic encephalopathy. This group made up 1,040 of the 1,343 enrolled patients (77.4%), and had 28-day and 90-day mortality rates of 4.7% and 14%, respectively.
The next cohort, called ACLF grade 1, comprised patients with a single coagulation, circulatory or respiratory failure; a serum creatinine between 1.5 and 1.9 mg/dL; and/or mild to moderate hepatic encephalopathy. The 148 patients in this class (11.0%) had 28- and 90-day mortality rates of 22.1% and 40.7%, respectively.
ACLF grade 2 was more severe, with two organ failures; 108 patients (8%) had this at enrollment, and exhibited 28- and 90-day mortality rates of 32.0% and 40.7%, respectively.
The most severely ill patients were classed as having ACLF grade 3, with three organ failures or more. A total of 47 patients (3.5%) fell into this category, and they had 28- and 90-day mortality rates of 76.7% and 79.1%, respectively.
Overall, according to the investigators, patients with ACLF were younger (mean age 56 years versus 58 years in patients without ACLF; P = .02), had a lower mean arterial blood pressure on admittance to the hospital (79 mm Hg versus 85 mm Hg in non-ACLF patients; P less than .001) and more frequently were actively alcoholic.
They also found that patients with ACLF had a significantly higher white cell count (9.7 x 109 compared with 6.6 x 109/L; P less than .001) and plasma C-reactive protein level (40.3 versus 24.9 mg/L; P less than .001) than the group without ACLF.
And finally, in what they called an "outstanding observation," the authors determined that up to 43.6% of patients with ACLF had no precipitating event leading to their acute decompensation, including gastrointestinal hemorrhage, bacterial infection, or active alcoholism.
The authors concluded that their novel diagnostic criteria show that ACLF is "distinct from ‘mere’ AD."
They conceded that their study was not designed to assess ideal management for these patients. "Whether patients with ACLF should be admitted or not to the intensive care unit is controversial," they wrote. "Nevertheless, our results can serve as a resource for designing studies aimed to investigate the appropriate site of hospitalization for patients with ACLF."
The authors disclosed that pharmaceutical companies provided funding for a chronic liver failure consortium, which provided the initiative for this study; several other investigators also disclosed ties with pharmaceutical companies.
FROM GASTROENTEROLOGY
Major finding: Acute-on-chronic liver failure syndrome can be divided into three classes and is distinct from acute decompensation of chronic liver failure.
Data source: Data from 1,343 hospitalized patients with cirrhosis and acute decompensation from February to September 2011 at 29 liver units in eight European countries.
Disclosures: The authors disclosed that pharmaceutical companies provided funding for a chronic liver failure consortium, which provided the initiative for this study; several other investigators also disclosed ties with pharmaceutical companies.
Augmentin implicated in drug-induced liver injury
The crude incidence of drug-induced liver injury is roughly 19.1 cases per 100,000 inhabitants, with amoxicillin-clavulanate the most commonly implicated agent.
That’s according to the second published population-based cohort study of drug-induced liver injury (DILI), wrote Dr. Einar S. Björnsson. The study was published in the June issue of Gastroenterology.
Dr. Björnsson, of the University of Iceland, and colleagues looked at all patients aged older than 15 years hospitalized for liver disease with suspected DILI, plus outpatients at the National University Hospital of Iceland, and all those seen in private practice between March 1, 2010, and Feb. 29, 2012.
Source: American Gastroenterological Association
According to the authors, "In Iceland, every citizen is issued a specific personal identification number that is, among other things, connected to a nationwide pharmaceutical database on outpatient prescriptions."
Therefore, "The study examined the Icelandic Medicines Registry records of prescriptions for all drugs associated with DILI that had at least a possible causal relationship" according to the Roussel Uclaf Causality Assessment Method.
DILI was defined as aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels greater than three times the upper limit of normal, and/or alkaline phosphatase (ALP) levels greater than two times the upper limit of normal.
Acetaminophen toxicity cases were excluded, though patients with preexisting chronic liver injury were not, if they were considered to have developed superimposed DILI on top of their baseline liver enzyme values.
The authors found that over the study period there were 96 cases eligible for inclusion, including 49 cases in the first year and 47 in the second. That translated into a crude annual incidence during the study period of 19.1 cases per 100,000 inhabitants.
Roughly half were female (56%), and the median age was 55 years (range, 16-91 years).
Looking at the clinical characteristics of the cohort, the authors calculated that only 27% of patients developed jaundice, while 10% of patients complained of rash and 6% of fever. Four of the patients had preexisting liver disease.
Overall, liver injury was judged to be due to a single prescription medication in 75% of cases, most commonly amoxicillin-clavulanate (22%), followed by diclofenac (6%), azathioprine (4%) infliximab (4%), and nitrofurantoin (4%).
By tying the injury to Iceland’s prescription drug database, that meant an incidence of DILI among outpatients of 1 per 133 filled azathioprine prescriptions and 1 in 2,350 amoxicillin-clavulanate users; among inpatients, the incidence of injury attributed to amoxicillin-clavulanate was 1 per 729 patients.
By drug classes, after antibiotics, immunosuppressants were found to be commonly associated with DILI (10%), followed by psychotropic drugs, which accounted for 7% of cases, and then nonsteroidal anti-inflammatory drugs, at 6%, "with diclofenac as the only agent."
Single-drug antineoplastic agents were the causes of DILI in 5% of the cohort, and lipid-lowering agents were the cause in just 3.1% of patients (atorvastatin, n = 2; simvastatin, n = 1).
After injuries due to a single agent, dietary supplements were assumed to be the culprit in 16% of cases, and the use of multiple agents was implicated in 9% of cases.
Looking at outcomes, the researchers reported that DILI was mild in 35 patients (36%), moderate in 55 patients (58%), and severe in 5 patients (5%); there was 1 death, in an 82-year-old patient.
Finally, the median duration from diagnosis of DILI to the normalization of liver enzymes was 64 days, and 7% still had abnormal liver tests 6 months after DILI diagnosis.
According to the authors, the only previously published population-based study, done in France, found an annual crude incidence rate of 13.9 cases per 100,000 inhabitants per year (Hepatology 2002;36:451-5).
They conceded that their rate is somewhat higher; however, "the French study provided no information about the patients at risk for DILI because information about drug consumption was not available," they wrote.
The authors stated that the study was funded by a grant from the National University Hospital of Iceland Research Fund; they disclosed no individual financial conflicts of interest.
The crude incidence of drug-induced liver injury is roughly 19.1 cases per 100,000 inhabitants, with amoxicillin-clavulanate the most commonly implicated agent.
That’s according to the second published population-based cohort study of drug-induced liver injury (DILI), wrote Dr. Einar S. Björnsson. The study was published in the June issue of Gastroenterology.
Dr. Björnsson, of the University of Iceland, and colleagues looked at all patients aged older than 15 years hospitalized for liver disease with suspected DILI, plus outpatients at the National University Hospital of Iceland, and all those seen in private practice between March 1, 2010, and Feb. 29, 2012.
Source: American Gastroenterological Association
According to the authors, "In Iceland, every citizen is issued a specific personal identification number that is, among other things, connected to a nationwide pharmaceutical database on outpatient prescriptions."
Therefore, "The study examined the Icelandic Medicines Registry records of prescriptions for all drugs associated with DILI that had at least a possible causal relationship" according to the Roussel Uclaf Causality Assessment Method.
DILI was defined as aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels greater than three times the upper limit of normal, and/or alkaline phosphatase (ALP) levels greater than two times the upper limit of normal.
Acetaminophen toxicity cases were excluded, though patients with preexisting chronic liver injury were not, if they were considered to have developed superimposed DILI on top of their baseline liver enzyme values.
The authors found that over the study period there were 96 cases eligible for inclusion, including 49 cases in the first year and 47 in the second. That translated into a crude annual incidence during the study period of 19.1 cases per 100,000 inhabitants.
Roughly half were female (56%), and the median age was 55 years (range, 16-91 years).
Looking at the clinical characteristics of the cohort, the authors calculated that only 27% of patients developed jaundice, while 10% of patients complained of rash and 6% of fever. Four of the patients had preexisting liver disease.
Overall, liver injury was judged to be due to a single prescription medication in 75% of cases, most commonly amoxicillin-clavulanate (22%), followed by diclofenac (6%), azathioprine (4%) infliximab (4%), and nitrofurantoin (4%).
By tying the injury to Iceland’s prescription drug database, that meant an incidence of DILI among outpatients of 1 per 133 filled azathioprine prescriptions and 1 in 2,350 amoxicillin-clavulanate users; among inpatients, the incidence of injury attributed to amoxicillin-clavulanate was 1 per 729 patients.
By drug classes, after antibiotics, immunosuppressants were found to be commonly associated with DILI (10%), followed by psychotropic drugs, which accounted for 7% of cases, and then nonsteroidal anti-inflammatory drugs, at 6%, "with diclofenac as the only agent."
Single-drug antineoplastic agents were the causes of DILI in 5% of the cohort, and lipid-lowering agents were the cause in just 3.1% of patients (atorvastatin, n = 2; simvastatin, n = 1).
After injuries due to a single agent, dietary supplements were assumed to be the culprit in 16% of cases, and the use of multiple agents was implicated in 9% of cases.
Looking at outcomes, the researchers reported that DILI was mild in 35 patients (36%), moderate in 55 patients (58%), and severe in 5 patients (5%); there was 1 death, in an 82-year-old patient.
Finally, the median duration from diagnosis of DILI to the normalization of liver enzymes was 64 days, and 7% still had abnormal liver tests 6 months after DILI diagnosis.
According to the authors, the only previously published population-based study, done in France, found an annual crude incidence rate of 13.9 cases per 100,000 inhabitants per year (Hepatology 2002;36:451-5).
They conceded that their rate is somewhat higher; however, "the French study provided no information about the patients at risk for DILI because information about drug consumption was not available," they wrote.
The authors stated that the study was funded by a grant from the National University Hospital of Iceland Research Fund; they disclosed no individual financial conflicts of interest.
The crude incidence of drug-induced liver injury is roughly 19.1 cases per 100,000 inhabitants, with amoxicillin-clavulanate the most commonly implicated agent.
That’s according to the second published population-based cohort study of drug-induced liver injury (DILI), wrote Dr. Einar S. Björnsson. The study was published in the June issue of Gastroenterology.
Dr. Björnsson, of the University of Iceland, and colleagues looked at all patients aged older than 15 years hospitalized for liver disease with suspected DILI, plus outpatients at the National University Hospital of Iceland, and all those seen in private practice between March 1, 2010, and Feb. 29, 2012.
Source: American Gastroenterological Association
According to the authors, "In Iceland, every citizen is issued a specific personal identification number that is, among other things, connected to a nationwide pharmaceutical database on outpatient prescriptions."
Therefore, "The study examined the Icelandic Medicines Registry records of prescriptions for all drugs associated with DILI that had at least a possible causal relationship" according to the Roussel Uclaf Causality Assessment Method.
DILI was defined as aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels greater than three times the upper limit of normal, and/or alkaline phosphatase (ALP) levels greater than two times the upper limit of normal.
Acetaminophen toxicity cases were excluded, though patients with preexisting chronic liver injury were not, if they were considered to have developed superimposed DILI on top of their baseline liver enzyme values.
The authors found that over the study period there were 96 cases eligible for inclusion, including 49 cases in the first year and 47 in the second. That translated into a crude annual incidence during the study period of 19.1 cases per 100,000 inhabitants.
Roughly half were female (56%), and the median age was 55 years (range, 16-91 years).
Looking at the clinical characteristics of the cohort, the authors calculated that only 27% of patients developed jaundice, while 10% of patients complained of rash and 6% of fever. Four of the patients had preexisting liver disease.
Overall, liver injury was judged to be due to a single prescription medication in 75% of cases, most commonly amoxicillin-clavulanate (22%), followed by diclofenac (6%), azathioprine (4%) infliximab (4%), and nitrofurantoin (4%).
By tying the injury to Iceland’s prescription drug database, that meant an incidence of DILI among outpatients of 1 per 133 filled azathioprine prescriptions and 1 in 2,350 amoxicillin-clavulanate users; among inpatients, the incidence of injury attributed to amoxicillin-clavulanate was 1 per 729 patients.
By drug classes, after antibiotics, immunosuppressants were found to be commonly associated with DILI (10%), followed by psychotropic drugs, which accounted for 7% of cases, and then nonsteroidal anti-inflammatory drugs, at 6%, "with diclofenac as the only agent."
Single-drug antineoplastic agents were the causes of DILI in 5% of the cohort, and lipid-lowering agents were the cause in just 3.1% of patients (atorvastatin, n = 2; simvastatin, n = 1).
After injuries due to a single agent, dietary supplements were assumed to be the culprit in 16% of cases, and the use of multiple agents was implicated in 9% of cases.
Looking at outcomes, the researchers reported that DILI was mild in 35 patients (36%), moderate in 55 patients (58%), and severe in 5 patients (5%); there was 1 death, in an 82-year-old patient.
Finally, the median duration from diagnosis of DILI to the normalization of liver enzymes was 64 days, and 7% still had abnormal liver tests 6 months after DILI diagnosis.
According to the authors, the only previously published population-based study, done in France, found an annual crude incidence rate of 13.9 cases per 100,000 inhabitants per year (Hepatology 2002;36:451-5).
They conceded that their rate is somewhat higher; however, "the French study provided no information about the patients at risk for DILI because information about drug consumption was not available," they wrote.
The authors stated that the study was funded by a grant from the National University Hospital of Iceland Research Fund; they disclosed no individual financial conflicts of interest.
FROM GASTROENTEROLOGY
Major finding: Drug-induced liver injury has an incidence of 19.1 per 100,000 persons, with the incidence per outpatient users of amoxicillin-clavulanate at 1 per 2,350.
Data source: A population-based cohort study of 251,000 Icelanders.
Disclosures: The authors stated that the study was funded by a grant from the National University Hospital of Iceland Research Fund; they disclosed no individual conflicts.
Post-transplant Lymphoproliferative Disorders
There is an increased risk of malignancy after both solid organ transplantation (SOT) and hematopoietic cell transplantation (HCT). In patients who undergo SOT, the second most common malignancy after nonmelanoma skin cancers is post-transplant lymphoproliferative disorders (PTLD). The term PTLD includes disorders ranging from benign hyperplasia to malignant lymphomas occurring in the setting of immunosuppression during SOT and HCT. The first cases of PTLD were described in renal transplant recipients in the late 1960s. Since then, PTLD has remained a serious and sometimes fatal complication in the posttransplant setting.
To read the full article in PDF:
There is an increased risk of malignancy after both solid organ transplantation (SOT) and hematopoietic cell transplantation (HCT). In patients who undergo SOT, the second most common malignancy after nonmelanoma skin cancers is post-transplant lymphoproliferative disorders (PTLD). The term PTLD includes disorders ranging from benign hyperplasia to malignant lymphomas occurring in the setting of immunosuppression during SOT and HCT. The first cases of PTLD were described in renal transplant recipients in the late 1960s. Since then, PTLD has remained a serious and sometimes fatal complication in the posttransplant setting.
To read the full article in PDF:
There is an increased risk of malignancy after both solid organ transplantation (SOT) and hematopoietic cell transplantation (HCT). In patients who undergo SOT, the second most common malignancy after nonmelanoma skin cancers is post-transplant lymphoproliferative disorders (PTLD). The term PTLD includes disorders ranging from benign hyperplasia to malignant lymphomas occurring in the setting of immunosuppression during SOT and HCT. The first cases of PTLD were described in renal transplant recipients in the late 1960s. Since then, PTLD has remained a serious and sometimes fatal complication in the posttransplant setting.
To read the full article in PDF:
Evaluation and management of premature ventricular complexes
Premature ventricular complexes (PVCs) are a common cause of palpitations, and are also often detected incidentally on electrocardiography (ECG), ambulatory monitoring, or inpatient telemetry. At the cellular level, ventricular myocytes spontaneously depolarize to create an extra systole that is “out of sync” with the cardiac cycle.
Although nearly everyone has some PVCs from time to time, people vary widely in their frequency of PVCs and their sensitivity to them.1,2 Some patients are exquisitely sensitive to even a small number of PVCs, while others are completely unaware of PVCs in a bigeminal pattern (ie, every other heartbeat). This article will review the evaluation and management of PVCs with a focus on clinical aspects.
DIAGNOSTIC EVALUATION
Personal and family history
Symptoms. The initial history should establish the presence, extent, timing, and duration of symptoms. Patients may use the word “palpitations” to describe their symptoms, but they also describe them as “hard” heartbeats, “chest-thumping,” or as a “catch” or “skipped” heartbeat. Related symptoms may include difficulty breathing, chest pain, fatigue, and dizziness.
The interview should determine whether the symptoms represent a minor nuisance or a major quality-of-life issue to the patient, and whether there are any specific associations or triggers. For example, it is very common for patients to become aware of PVCs at night, particularly in certain positions, such as lying on the left side. Patients often associate PVC symptoms with emotional stress, exercise, or caffeine or stimulant use.
Medication use. An accurate and up-to-date list of prescription medications should be screened for alpha-, beta-, or dopamine-receptor agonist drugs. Similarly, any use of over-the-counter sympathomimetic medications and nonprescription supplements should be elicited, including compounded elixirs or beverages. Many commercially available products designed to treat fatigue or increase alertness contain large doses of caffeine or other stimulants. It is also important to consider the use of illicit substances such as cocaine, amphetamine, methamphetamine, and their derivatives.
The patient’s medical and surgical history should be queried for any known structural heart disease, including coronary artery disease, myocardial infarction, congestive heart failure, valvular heart disease, congenital heart disease, and heritable conditions such as hypertrophic cardiomyopathy, prolonged QT syndromes, or other channel disorders. Pulmonary disorders such as sarcoidosis, pulmonary hypertension, or obstructive sleep apnea are also relevant. Similarly, it is important to identify endocrine disorders, including thyroid problems, sex hormone abnormalities, or adrenal gland conditions.
A careful family history should include any instance of sudden death in first-degree relatives, any heritable cardiac conditions, or coronary artery disease at an early age.
Physical examination
The physical examination should focus on findings that suggest underlying structural heart disease. Findings suggestive of congestive heart failure include elevated jugular venous pressures, abnormal cardiac sounds, pulmonary rales, abnormal arterial pulses, or peripheral edema. A murmur or a pathologic heart sound should raise suspicion of valvular or congenital heart disease when present in a young patient.
Inspection and palpation of the thyroid can reveal a related disorder. Obvious skin changes or neurologic findings can similarly reveal a systemic and possibly related clinical disorder that can have cardiac manifestations (eg, muscular dystrophy).
Electrocardiography, Holter monitoring, and other monitoring
Assessment of the cardiac rhythm includes 12-lead ECG and ambulatory Holter monitoring, typically for 24 or 48 hours.
Holter monitoring provides a continuous recording, usually in at least two or three leads. Patients are given a symptom journal or are asked to keep a diary of symptoms experienced during the monitoring period. The monitor is worn underneath clothing and is returned for download upon completion. Technicians process the data with the aid of computer software, and the final output is reviewed and interpreted by a cardiologist or cardiac electrophysiologist.
Holter monitoring for at least 24 hours is a critical step in assessing any patient with known or suspected PVCs, as it can both quantify the total burden of ventricular ectopy and identify the presence of any related ventricular tachycardia. In addition, it can detect additional supraventricular arrhythmias or bradycardia during the monitoring period. The PVC burden is an important measurement; it is expressed as the percentage of heartbeats that were ventricular extrasystoles during the monitoring period.
Both ECG and Holter monitoring are limited in that they are only snapshots of the rhythm during the period when a patient is actually hooked up. Many patients experience PVCs in clusters every very few days or weeks. Such a pattern is unlikely to be detected by a single ECG or 24- or 48-hour Holter monitoring.
A 30-day ambulatory event monitor (also known as a wearable loop recorder) is an important diagnostic tool in these scenarios. The concept is very similar to that of Holter monitoring, except that the device provides a continuous loop recording of the cardiac rhythm that is digitally stored in clips when the patient activates the device. Some wearable loop recorders also have auto-save features for heart rates falling outside of a programmed range.
Mobile outpatient cardiac telemetry is the most comprehensive form of noninvasive rhythm monitoring available. This is essentially the equivalent of continuous inpatient cardiac telemetry, but in a patient who is not hospitalized. It is a wearable ambulatory device providing continuous recordings, real-time automatic detections, and patient-activated symptom recordings. It can be used for up to 6 weeks. Advantages include detection and quantification of asymptomatic events, and real-time transmissions that the physician can act upon. The major disadvantage is cost, including coverage denial by many third-party payers.
This test is rarely indicated as part of a PVC evaluation and is typically ordered only by a cardiologist or cardiac electrophysiologist.
Noninvasive cardiac evaluation
Surface echocardiography is indicated to look for overt structural heart disease and can reliably detect abnormalities in cardiac chamber size, wall thickness, and function. Valvular heart disease is concomitantly identified by two-dimensional imaging as well as by color Doppler. The finding of significant structural heart disease in conjunction with PVCs should prompt a cardiology referral, as this carries significant prognostic implications.3–5
Exercise treadmill stress testing is appropriate for patients who experience PVCs with exercise or for whom an evaluation for coronary artery disease is indicated. The expected finding would be an increase in PVCs or ventricular tachycardia with exercise or in the subsequent recovery period. Exercise testing can be combined with either echocardiographic or nuclear perfusion imaging to evaluate the possibility of myocardial ischemia. For patients unable to exercise, pharmacologic stress testing with dobutamine or a vasodilator agent can be performed.
Advanced noninvasive cardiac imaging— such as computed tomography, magnetic resonance imaging, or positron-emission tomography—should be reserved for specific clinical indications such as congenital heart disease, suspected cardiac sarcoidosis, and infiltrative heart disease, and for specific cardiomyopathies, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. For example, frequent PVCs with a left bundle branch block morphology and superior axis raise the concern for a right ventricular disorder and may prompt cardiac magnetic resonance imaging for either arrhythmogenic right ventricular cardiomyopathy or sarcoidosis.
PVCs WITHOUT STRUCTURAL HEART DISEASE
Outflow tract PVCs and ventricular tachycardia
The right or left ventricular outflow tracts, or the epicardial tissue immediately adjacent to the aortic sinuses of Valsalva are the most common sites of origin for ventricular ectopy in the absence of structural heart disease.6–9 Affected cells often demonstrate a triggered activity mechanism due to cyclic adenosine monophosphate-mediated and calcium-dependent delayed after-depolarizations.7,8
Most of these foci are in the right ventricular outflow tract, producing a left bundle branch block morphology with an inferior axis (positive R waves in limb leads II, III, and aVF) and typical precordial R-wave transition in V3 and V4 (Figure 1). A minority are in the left ventricular outflow tract, producing a right bundle branch block with an inferior axis pattern, or in the aortic sinuses with a left bundle branch block pattern but with early precordial R transition in V2 and V3.
A study in 122 patients showed that right and left outflow tract arrhythmias had similar electrophysiologic properties and pharmacologic sensitivities, providing evidence for shared mechanisms possibly due to the common embryologic origin of these structures.9
Such arrhythmias are typically catecholamine-sensitive and are sometimes inducible with burst pacing in the electrophysiology laboratory. The short ventricular coupling intervals can promote intracellular calcium overload in the affected cells, leading to triggered activity.
Therefore, outflow tract PVCs and ventricular tachycardia are commonly encountered clinically during exercise and, to an even greater extent, in the postexercise cool-down period. Similarly, they can be worse during periods of emotional stress or fatigue, when the body’s endogenous catecholamine production is elevated. However, it is worthwhile to note that there are exceptions to this principle in which faster sinus rates seem to overdrive the PVCs in some patients, causing them to become paradoxically more frequent at rest, or even during sleep.
Outflow tract PVCs can be managed medically with beta-blockers, nondihydropyridine calcium channel blockers (verapamil or diltiazem), or, less commonly, class IC drugs such as flecainide. They are also highly curable by catheter ablation (Figure 2), with procedure success rates greater than 90%.9.10
However, a subset of outflow tract PVCs nested deep in a triangle of epicardial tissue between the right and left endocardial surface and underneath the left main coronary artery can be challenging. This region has been labeled the left ventricular summit, and is shielded from ablation by an epicardial fat pad in the adjacent pericardial space.11 Ablation attempts made from the right and left endocardial surfaces as well as the epicardial surface (pericardial space) sometimes cannot adequately penetrate the tissue deep enough to reach the originating focus deep within this triangle. While ablation cannot always fully eliminate the PVC, ablation from more than one of the sites listed can generally reduce its burden, often in combination with suppressive medical therapy (Figure 3).
Fascicular PVCs
Fascicular PVCs originate from within the left ventricular His-Purkinje system12 and produce a right bundle branch block morphology with either an anterior or posterior hemiblock pattern (Figure 4). Exit from the posterior fascicle causes an anterior hemiblock pattern, and exit from the anterior fascicle a posterior hemiblock pattern. Utilization of the rapidly conducting His-Purkinje system gives these PVCs a very narrow QRS duration, sometimes approaching 120 milliseconds or shorter. This occasionally causes them to be mistaken for aberrantly conducted supraventricular beats. Such spontaneous PVCs are commonly associated with both sustained and nonsustained ventricular tachycardia and are usually sensitive to verapamil.13
Special issues relating to mapping and catheter ablation of fascicular arrhythmias involve the identification of Purkinje fiber potentials and associated procedural diagnostic maneuvers during tachycardia.14
Other sites for PVCs
Other sites of origin for PVCs in the absence of structural heart disease include ventricular tissue adjacent to the aortomitral continuity,15 the tricuspid annulus,16 the mitral valve annulus, 17 papillary muscles,18 and other Purkinje-adjacent structures such as left ventricular false tendons.19 An example of a papillary muscle PVC is shown in Figures 5 and 6.
Curable by catheter ablation
Any of these PVCs can potentially be cured by catheter ablation when present at a sufficient burden to allow for activation mapping in the electrophysiology laboratory. The threshold for offering ablation varies among operators, but is generally around 10% or greater. Pacemapping is a technique applied in the electrophysiology laboratory when medically refractory symptomatic PVCs occurring at a lower burden require ablation.
PVCs WITH AN UNDERLYING CARDIAC CONDITION
Coronary artery disease
Tissue injury and death caused by acute myocardial infarction has long been recognized as a common cause of spontaneous ventricular ectopy attributed to infarct border zones of ischemic or hibernating myocardium.20,21
Suppression has not been associated with improved outcomes, as shown for class IC drugs in the landmark Cardiac Arrhythmia Suppression Trial (CAST),22 or in the amiodarone treatment arm of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II).23 Therefore, treatment of ventricular ectopy in this patient population is usually symptom-driven unless there is hemodynamic intolerance, tachycardia-related cardiomyopathy, or a very high burden of PVCs in a patient who may be at risk of developing tachycardia-related cardiomyopathy. Antiarrhythmic drug treatment, when required, usually involves beta-blockers or class III medications such as sotalol or amiodarone.
Nonischemic dilated cardiomyopathy
This category includes patients with a wide variety of disease states including valvular heart disease, lymphocytic and other viral myocarditis, cardiac sarcoidosis, amyloidosis and other infiltrative diseases, familial conditions, and idiopathic dilated cardiomyopathy (ie, etiology unknown). Although it is a heterogeneous group, a common theme is that PVCs in this patient cohort may require epicardial mapping and ablation.24 Similarly, epicardial PVCs and ventricular tachycardia cluster at the basal posterolateral left ventricle near the mitral annulus, for unclear reasons.25
While specific criteria have been published, an epicardial focus is suggested by slowing of the initial QRS segment, pseudo-delta waves, a wider overall QRS, and Q waves in limb lead I.26
Treatment is symptom-driven unless the patient has a tachycardia-related cardiomyopathy or a high burden associated with the risk for its development. Antiarrhythmic drug therapy, when required, typically involves a beta-blocker or a class III drug such as sotalol or amiodarone. Sotalol is used in this population but has limited safety data and should be used cautiously in patients without an implantable cardioverter-defibrillator.
Arrhythmogenic right ventricular cardiomyopathy
Spontaneous ventricular ectopy and tachycardia are common, if not expected, in patients with this heritable autosomal dominant disorder. This condition is progressive and associated with the risk of sudden cardiac death. Criteria for diagnosis were established in 2010, and patients with suspected arrhythmogenic right ventricular cardiomyopathy often undergo cardiac magnetic resonance imaging.27 Diagnostic findings include fibro-fatty tissue replacement, which usually starts in the right ventricle but can progress to involve the left ventricle. PVCs and ventricular tachycardia can involve the right ventricular free wall and are often epicardial.
Catheter ablation is usually palliative, as future arrhythmias are expected. Many patients with this condition require an implantable cardioverter-defibrillator for prevention of sudden cardiac death, and some go on to cardiac transplantation as the disease progresses and ventricular arrhythmias become incessant.
Other conditions
Spontaneous ventricular ectopy is common in other heritable and acquired cardiomyopathies including hypertrophic cardiomyopathy and in infiltrative or inflammatory disorders such as cardiac amyloidosis and sarcoidosis. While technically falling under the rubric of nonischemic heart disease, the presence of spontaneous ventricular ectopy carries specific prognostic implications depending on the underlying diagnosis. Therefore, an appropriate referral for complete cardiac evaluation should be considered when a heritable disorder or other acquired structural heart disease is suspected.
TACHYCARDIA-RELATED CARDIOMYOPATHY
Tachycardia-related cardiomyopathy refers to left ventricular systolic dysfunction that is primarily caused by arrhythmias. This includes frequent PVCs or ventricular tachycardia but also atrial arrhythmias occurring at a high burden that directly weaken myocardial function over time. Although much research has been devoted to this condition, our understanding of its etiology and pathology is incomplete.
PVCs and ventricular ectopy burdens in excess of 15% to 20% have been associated with the development of this condition.28,29 However, it is important to note that cardiomyopathy can also develop at lower burdens.30 One study found that a burden greater than 24% was 79% sensitive and 78% specific for development of tachycardia-related cardiomyopathy.31 Additional studies have demonstrated specific PVC morphologic features such as slurring in the initial QRS segment and also PVCs occurring at shorter coupling intervals as being associated with cardiomyopathy.32–34
For these reasons, both quantification of the total burden and careful evaluation of available electrocardiograms and rhythm strips are important even in asymptomatic patients with frequent PVCs. Similarly, unexplained left ventricular dysfunction in patients with PVC burdens in these discussed ranges should raise suspicion for this diagnosis. Patients with tachycardia-related cardiomyopathy usually have at least partially reversible left ventricular dysfunction when identified or treated early.29,35
MEDICAL AND ABLATIVE TREATMENT
Available treatments include medical suppression and catheter ablation. One needs to exercise clinical judgment and incorporate all of the PVC-related data to make treatment decisions.
Little data for trigger avoidance and behavioral modification
Some patients report a strong association between palpitations related to PVCs and caffeine intake, other stimulants, or other dietary triggers. However, few data exist to support the role of trigger avoidance and behavioral modification in treatment. In fact, an older randomized trial in 81 men found no benefit in a program of total abstinence from caffeine and smoking, moderation of alcohol intake, and physical conditioning.36
Nonetheless, some argue in favor of advising patients to make these dietary and lifestyle changes, given the overall health benefits of aggressive risk-factor modification for cardiovascular disease.37 Certainly, a trial of trigger avoidance and behavioral modification seems reasonable for patients who have strongly associated historical triggers in the absence of structural heart disease and PVCs occurring at a low to modest burden.
Beta-blockers are the mainstay
Beta-blockers are the mainstay of medical suppression of PVCs, primarily through their effect on beta-1 adrenergic receptors to reduce intracellular cyclic adenosine monophosphate and thus decrease automaticity. Blocking beta-1 receptors also causes a negative chronotropic effect, reducing the resting sinus rate in addition to slowing atrioventricular nodal conduction.
Cardioselective beta-blockers include atenolol, betaxolol, metoprolol, and nadolol. These drugs are effective in suppressing PVCs, or at least in reducing the burden to more tolerable levels.
Beta-blockers are most strongly indicated in patients who require PVC suppression and who have concomitant coronary artery disease, prior myocardial infarction, or other cardiomyopathy, as this drug class favorably affects long-term prognosis in these conditions.
Common side effects of beta-blockers include fatigue, shortness of breath, depressed mood, and loss of libido. Side effects can present a significant challenge, particularly for younger patients. Noncardioselective beta-blockers are less commonly prescribed, with the exception of propranolol, which is an effective sympatholytic drug that blocks both beta-1 and beta-2 receptors.
Many patients with asthma or peripheral arterial disease can tolerate these drugs well despite concerns about provoked bronchospasm or claudication, respectively, and neither of these conditions is considered an absolute contraindication. Excessive bradycardia with beta-blocker therapy can lead to dizziness, lightheadedness, or overt syncope, and these drugs should be used with caution in patients with baseline sinus node dysfunction or atrioventricular nodal disease.
Nondihydropyridine calcium channel blockers
Nondihydropyridine calcium channel blockers are particularly effective for PVC suppression in patients without structural heart disease by the mechanisms previously described involving intracellular calcium channels. In particular, they are highly effective and are considered the drugs of choice in treating fascicular PVCs.
Verapamil is a potent drug in this class, but it also commonly causes constipation as a side effect. Diltiazem is less constipating but can cause fatigue, drowsiness, and headaches. Both drugs reduce the resting heart rate and slow atrioventricular nodal conduction. Patients predisposed to bradycardia or atrioventricular block can develop dizziness or overt syncope. Calcium channel blockers are also used cautiously in patients with congestive heart failure, given their potential negative inotropic effects.
Overall, calcium channel blockers are a very reasonable choice for young patients without structural heart disease who need PVC suppression.
Other antiarrhythmic drugs
Sotalol merits special consideration because it has both beta-blocker and class III antiarrhythmic properties, blocking potassium channels and prolonging cardiac repolarization. It can be very effective in PVC suppression but also creates some degree of QT prolongation. The QT-prolonging effect is accentuated in patients with baseline QT prolongation or abnormal renal function. Rarely, this can lead to torsades de pointes. As a safety precaution, some patients are admitted to the hospital when they start sotalol therapy so that they can be monitored with continuous telemetry and ECG to detect excessive QT prolongation.
Amiodarone is a versatile drug with mixed pharmacologic properties that include a predominantly potassium channel-blocking class III drug effect. However, this effect is balanced by its other pharmacologic properties that make QT prolongation less of a clinical concern. Excessive QT prolongation may still occur when used concomitantly with other QT-prolonging drugs.
Amiodarone is very effective in suppressing PVCs and ventricular arrhythmias but has considerable short-term and long-term side effects. Cumulative toxicity risks include damage to the thyroid gland, liver, skin, eyes, and lungs. Routine thyroid function testing, pulmonary function testing, and eye examinations are often considered for patients on long-term amiodarone therapy. Short-term use of this drug does not typically require such surveillance.
Catheter ablation
As mentioned in the previous sections, catheter ablation is a safe and effective treatment for PVCs. It is curative in most cases, and significantly reduces the PVC burden in others.
Procedure. Patients are brought to the electrophysiology laboratory in a fasted state and are partially sedated with an intravenous drug such as midazolam or fentanyl, or both. Steerable catheters are placed into appropriate cardiac chambers from femoral access sites, which are infiltrated with local anesthesia. Sometimes sedative or analgesic drugs must be limited if they are known to suppress PVCs.
Most operators prefer a technique called activation mapping, in which the catheter is maneuvered to home in on the precise PVC origin within the heart, which is subsequently ablated. This technique has very high success rates, but having enough spontaneous PVCs to map during the procedure is essential for the technique to succeed. Conversely, not having sufficient PVCs on the day of the procedure is a common reason that ablation fails or cannot be performed at all.
Pace-mapping is an alternate technique that does not require a continuous stream of PVCs. This involves pacing from different candidate locations inside the heart in an effort to precisely match the ECG appearance of the clinical PVC and to ablate at this site. Although activation mapping generally yields higher success rates and is preferred by most operators, pace-mapping can be successful when a perfect 12–12 match is elicited. In many cases, the two techniques are used together during the same procedure, particularly if the patient’s PVCs spontaneously wax and wane, as they often do.
Risks. Like any medical procedure, catheter ablation carries some inherent risks, including rare but potentially serious events. Unstable arrhythmias may require pace-termination from the catheter or, rarely, shock-termination externally. Even more rare is cardiac arrest requiring cardiopulmonary resuscitation. Uncommon but life-threatening complications also include pericardial effusion or cardiac tamponade requiring percutaneous drainage or, rarely, emergency surgical correction. Although such events are life-threatening, death is extremely rare.
Complications causing permanent disability are also very uncommon but include the risk of collateral injury to the conduction system requiring permanent pacemaker placement, injury to the coronary vessels requiring urgent treatment, or diaphragmatic injury affecting breathing. Left-sided cardiac ablation also carries a small risk of stroke, which is mitigated by giving intravenous heparin during the procedure.
More common but generally non-life-threatening complications include femoral vascular events such as hematomas, pseudoaneurysms, or fistulas that sometimes require subsequent treatment. These complications are generally treatable but can significantly prolong the recovery period.
Catheter ablation procedures are typically 2 to 6 hours in duration, depending on the chambers involved, PVC frequency, and other considerations. Postprocedure bed rest is required for a number of hours. A Foley catheter is sometimes used for patient comfort when a prolonged procedure is anticipated. This carries a small risk of urinary tract infection. Epicardial catheter ablation that requires access to the surface of the heart (ie, the pericardial space) is uncommon but carries some unique risks, including rare injury to coronary vessels or adjacent organs such as the liver or stomach.
Overall, both endocardial and epicardial catheter ablation can be performed safely and effectively in the overwhelming majority of patients, but understanding and explaining the potential risks remains a crucial part of the informed consent process.
TAKE-HOME POINTS
- PVCs are a common cause of palpitations but are also noted as incidental findings by ECG, Holter monitoring, and inpatient telemetry.
- The diagnostic evaluation includes an assessment for underlying structural heart disease and quantification of the total PVC burden.
- Patients without structural heart disease and with low-to-modest PVC burdens may not require specific treatment. PVCs at greater burdens, typically 15% to 20%, or with specific high-risk features carry a risk of tachycardia-related cardiomyopathy and may require treatment even if they are asymptomatic. These high-risk features include initial QRS slurring and PVCs occurring at shorter coupling intervals.
- Treatment involves medical therapy with a beta-blocker, a calcium channel blocker, or another antiarrhythmic drug, and catheter ablation in selected cases.
- Catheter ablation can be curative but is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
- Kostis JB, McCrone K, Moreyra AE, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation 1981; 63:1351–1356.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95:1230–1237.
- Simpson RJ, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002; 143:535–540.
- Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985; 109:497–504.
- Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30:5–15.
- Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation 1986; 74:270–280.
- Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995; 92:421–429.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007; 49:2035–2043.
- Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 2010; 3:616–623.
- Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macro-reentry within the Purkinje network. Circulation 2002; 105:462–469.
- Iwai S, Lerman BB. Management of ventricular tachycardia in patients with clinically normal hearts. Curr Cardiol Rep 2000; 2:515–521.
- Nogami A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011; 34:624–650.
- Letsas KP, Efremidis M, Kollias G, Xydonas S, Sideris A. Electrocardiographic and electrophysiologic characteristics of ventricular extrasystoles arising from the aortomitral continuity. Cardiol Res Pract 2011; 2011:864964.
- Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007; 4:7–16.
- Tada H, Ito S, Naito S, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005; 45:877–886.
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008; 1:23–29.
- Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009; 6:1050–1058.
- Bigger JT, Dresdale FJ, Heissenbuttel RH, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis 1977; 19:255–300.
- Eldar M, Sievner Z, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Primary ventricular tachycardia in acute myocardial infarction: clinical characteristics and mortality. The SPRINT Study Group. Ann Intern Med 1992; 117:31–36.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321:406–412.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009; 54:799–808.
- Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation 2007; 116:1998–2001.
- Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2010; 3:63–71.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010; 121:1533–1541.
- Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol 2012; 5:229–236.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008; 13:81–85.
- Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010; 7:865–869.
- Moulton KP, Medcalf T, Lazzara R. Premature ventricular complex morphology. A marker for left ventricular structure and function. Circulation 1990; 81:1245–1251.
- Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011; 8:1046–1049.
- Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003; 19:295–299.
- Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009; 6:1543–1549.
- DeBacker G, Jacobs D, Prineas R, et al. Ventricular premature contractions: a randomized non-drug intervention trial in normal men. Circulation 1979; 59:762–769.
- Glatter KA, Myers R, Chiamvimonvat N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: what do you tell your patients to do or not to do? Curr Treat Options Cardiovasc Med 2012; 14:529–535.
Premature ventricular complexes (PVCs) are a common cause of palpitations, and are also often detected incidentally on electrocardiography (ECG), ambulatory monitoring, or inpatient telemetry. At the cellular level, ventricular myocytes spontaneously depolarize to create an extra systole that is “out of sync” with the cardiac cycle.
Although nearly everyone has some PVCs from time to time, people vary widely in their frequency of PVCs and their sensitivity to them.1,2 Some patients are exquisitely sensitive to even a small number of PVCs, while others are completely unaware of PVCs in a bigeminal pattern (ie, every other heartbeat). This article will review the evaluation and management of PVCs with a focus on clinical aspects.
DIAGNOSTIC EVALUATION
Personal and family history
Symptoms. The initial history should establish the presence, extent, timing, and duration of symptoms. Patients may use the word “palpitations” to describe their symptoms, but they also describe them as “hard” heartbeats, “chest-thumping,” or as a “catch” or “skipped” heartbeat. Related symptoms may include difficulty breathing, chest pain, fatigue, and dizziness.
The interview should determine whether the symptoms represent a minor nuisance or a major quality-of-life issue to the patient, and whether there are any specific associations or triggers. For example, it is very common for patients to become aware of PVCs at night, particularly in certain positions, such as lying on the left side. Patients often associate PVC symptoms with emotional stress, exercise, or caffeine or stimulant use.
Medication use. An accurate and up-to-date list of prescription medications should be screened for alpha-, beta-, or dopamine-receptor agonist drugs. Similarly, any use of over-the-counter sympathomimetic medications and nonprescription supplements should be elicited, including compounded elixirs or beverages. Many commercially available products designed to treat fatigue or increase alertness contain large doses of caffeine or other stimulants. It is also important to consider the use of illicit substances such as cocaine, amphetamine, methamphetamine, and their derivatives.
The patient’s medical and surgical history should be queried for any known structural heart disease, including coronary artery disease, myocardial infarction, congestive heart failure, valvular heart disease, congenital heart disease, and heritable conditions such as hypertrophic cardiomyopathy, prolonged QT syndromes, or other channel disorders. Pulmonary disorders such as sarcoidosis, pulmonary hypertension, or obstructive sleep apnea are also relevant. Similarly, it is important to identify endocrine disorders, including thyroid problems, sex hormone abnormalities, or adrenal gland conditions.
A careful family history should include any instance of sudden death in first-degree relatives, any heritable cardiac conditions, or coronary artery disease at an early age.
Physical examination
The physical examination should focus on findings that suggest underlying structural heart disease. Findings suggestive of congestive heart failure include elevated jugular venous pressures, abnormal cardiac sounds, pulmonary rales, abnormal arterial pulses, or peripheral edema. A murmur or a pathologic heart sound should raise suspicion of valvular or congenital heart disease when present in a young patient.
Inspection and palpation of the thyroid can reveal a related disorder. Obvious skin changes or neurologic findings can similarly reveal a systemic and possibly related clinical disorder that can have cardiac manifestations (eg, muscular dystrophy).
Electrocardiography, Holter monitoring, and other monitoring
Assessment of the cardiac rhythm includes 12-lead ECG and ambulatory Holter monitoring, typically for 24 or 48 hours.
Holter monitoring provides a continuous recording, usually in at least two or three leads. Patients are given a symptom journal or are asked to keep a diary of symptoms experienced during the monitoring period. The monitor is worn underneath clothing and is returned for download upon completion. Technicians process the data with the aid of computer software, and the final output is reviewed and interpreted by a cardiologist or cardiac electrophysiologist.
Holter monitoring for at least 24 hours is a critical step in assessing any patient with known or suspected PVCs, as it can both quantify the total burden of ventricular ectopy and identify the presence of any related ventricular tachycardia. In addition, it can detect additional supraventricular arrhythmias or bradycardia during the monitoring period. The PVC burden is an important measurement; it is expressed as the percentage of heartbeats that were ventricular extrasystoles during the monitoring period.
Both ECG and Holter monitoring are limited in that they are only snapshots of the rhythm during the period when a patient is actually hooked up. Many patients experience PVCs in clusters every very few days or weeks. Such a pattern is unlikely to be detected by a single ECG or 24- or 48-hour Holter monitoring.
A 30-day ambulatory event monitor (also known as a wearable loop recorder) is an important diagnostic tool in these scenarios. The concept is very similar to that of Holter monitoring, except that the device provides a continuous loop recording of the cardiac rhythm that is digitally stored in clips when the patient activates the device. Some wearable loop recorders also have auto-save features for heart rates falling outside of a programmed range.
Mobile outpatient cardiac telemetry is the most comprehensive form of noninvasive rhythm monitoring available. This is essentially the equivalent of continuous inpatient cardiac telemetry, but in a patient who is not hospitalized. It is a wearable ambulatory device providing continuous recordings, real-time automatic detections, and patient-activated symptom recordings. It can be used for up to 6 weeks. Advantages include detection and quantification of asymptomatic events, and real-time transmissions that the physician can act upon. The major disadvantage is cost, including coverage denial by many third-party payers.
This test is rarely indicated as part of a PVC evaluation and is typically ordered only by a cardiologist or cardiac electrophysiologist.
Noninvasive cardiac evaluation
Surface echocardiography is indicated to look for overt structural heart disease and can reliably detect abnormalities in cardiac chamber size, wall thickness, and function. Valvular heart disease is concomitantly identified by two-dimensional imaging as well as by color Doppler. The finding of significant structural heart disease in conjunction with PVCs should prompt a cardiology referral, as this carries significant prognostic implications.3–5
Exercise treadmill stress testing is appropriate for patients who experience PVCs with exercise or for whom an evaluation for coronary artery disease is indicated. The expected finding would be an increase in PVCs or ventricular tachycardia with exercise or in the subsequent recovery period. Exercise testing can be combined with either echocardiographic or nuclear perfusion imaging to evaluate the possibility of myocardial ischemia. For patients unable to exercise, pharmacologic stress testing with dobutamine or a vasodilator agent can be performed.
Advanced noninvasive cardiac imaging— such as computed tomography, magnetic resonance imaging, or positron-emission tomography—should be reserved for specific clinical indications such as congenital heart disease, suspected cardiac sarcoidosis, and infiltrative heart disease, and for specific cardiomyopathies, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. For example, frequent PVCs with a left bundle branch block morphology and superior axis raise the concern for a right ventricular disorder and may prompt cardiac magnetic resonance imaging for either arrhythmogenic right ventricular cardiomyopathy or sarcoidosis.
PVCs WITHOUT STRUCTURAL HEART DISEASE
Outflow tract PVCs and ventricular tachycardia
The right or left ventricular outflow tracts, or the epicardial tissue immediately adjacent to the aortic sinuses of Valsalva are the most common sites of origin for ventricular ectopy in the absence of structural heart disease.6–9 Affected cells often demonstrate a triggered activity mechanism due to cyclic adenosine monophosphate-mediated and calcium-dependent delayed after-depolarizations.7,8
Most of these foci are in the right ventricular outflow tract, producing a left bundle branch block morphology with an inferior axis (positive R waves in limb leads II, III, and aVF) and typical precordial R-wave transition in V3 and V4 (Figure 1). A minority are in the left ventricular outflow tract, producing a right bundle branch block with an inferior axis pattern, or in the aortic sinuses with a left bundle branch block pattern but with early precordial R transition in V2 and V3.
A study in 122 patients showed that right and left outflow tract arrhythmias had similar electrophysiologic properties and pharmacologic sensitivities, providing evidence for shared mechanisms possibly due to the common embryologic origin of these structures.9
Such arrhythmias are typically catecholamine-sensitive and are sometimes inducible with burst pacing in the electrophysiology laboratory. The short ventricular coupling intervals can promote intracellular calcium overload in the affected cells, leading to triggered activity.
Therefore, outflow tract PVCs and ventricular tachycardia are commonly encountered clinically during exercise and, to an even greater extent, in the postexercise cool-down period. Similarly, they can be worse during periods of emotional stress or fatigue, when the body’s endogenous catecholamine production is elevated. However, it is worthwhile to note that there are exceptions to this principle in which faster sinus rates seem to overdrive the PVCs in some patients, causing them to become paradoxically more frequent at rest, or even during sleep.
Outflow tract PVCs can be managed medically with beta-blockers, nondihydropyridine calcium channel blockers (verapamil or diltiazem), or, less commonly, class IC drugs such as flecainide. They are also highly curable by catheter ablation (Figure 2), with procedure success rates greater than 90%.9.10
However, a subset of outflow tract PVCs nested deep in a triangle of epicardial tissue between the right and left endocardial surface and underneath the left main coronary artery can be challenging. This region has been labeled the left ventricular summit, and is shielded from ablation by an epicardial fat pad in the adjacent pericardial space.11 Ablation attempts made from the right and left endocardial surfaces as well as the epicardial surface (pericardial space) sometimes cannot adequately penetrate the tissue deep enough to reach the originating focus deep within this triangle. While ablation cannot always fully eliminate the PVC, ablation from more than one of the sites listed can generally reduce its burden, often in combination with suppressive medical therapy (Figure 3).
Fascicular PVCs
Fascicular PVCs originate from within the left ventricular His-Purkinje system12 and produce a right bundle branch block morphology with either an anterior or posterior hemiblock pattern (Figure 4). Exit from the posterior fascicle causes an anterior hemiblock pattern, and exit from the anterior fascicle a posterior hemiblock pattern. Utilization of the rapidly conducting His-Purkinje system gives these PVCs a very narrow QRS duration, sometimes approaching 120 milliseconds or shorter. This occasionally causes them to be mistaken for aberrantly conducted supraventricular beats. Such spontaneous PVCs are commonly associated with both sustained and nonsustained ventricular tachycardia and are usually sensitive to verapamil.13
Special issues relating to mapping and catheter ablation of fascicular arrhythmias involve the identification of Purkinje fiber potentials and associated procedural diagnostic maneuvers during tachycardia.14
Other sites for PVCs
Other sites of origin for PVCs in the absence of structural heart disease include ventricular tissue adjacent to the aortomitral continuity,15 the tricuspid annulus,16 the mitral valve annulus, 17 papillary muscles,18 and other Purkinje-adjacent structures such as left ventricular false tendons.19 An example of a papillary muscle PVC is shown in Figures 5 and 6.
Curable by catheter ablation
Any of these PVCs can potentially be cured by catheter ablation when present at a sufficient burden to allow for activation mapping in the electrophysiology laboratory. The threshold for offering ablation varies among operators, but is generally around 10% or greater. Pacemapping is a technique applied in the electrophysiology laboratory when medically refractory symptomatic PVCs occurring at a lower burden require ablation.
PVCs WITH AN UNDERLYING CARDIAC CONDITION
Coronary artery disease
Tissue injury and death caused by acute myocardial infarction has long been recognized as a common cause of spontaneous ventricular ectopy attributed to infarct border zones of ischemic or hibernating myocardium.20,21
Suppression has not been associated with improved outcomes, as shown for class IC drugs in the landmark Cardiac Arrhythmia Suppression Trial (CAST),22 or in the amiodarone treatment arm of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II).23 Therefore, treatment of ventricular ectopy in this patient population is usually symptom-driven unless there is hemodynamic intolerance, tachycardia-related cardiomyopathy, or a very high burden of PVCs in a patient who may be at risk of developing tachycardia-related cardiomyopathy. Antiarrhythmic drug treatment, when required, usually involves beta-blockers or class III medications such as sotalol or amiodarone.
Nonischemic dilated cardiomyopathy
This category includes patients with a wide variety of disease states including valvular heart disease, lymphocytic and other viral myocarditis, cardiac sarcoidosis, amyloidosis and other infiltrative diseases, familial conditions, and idiopathic dilated cardiomyopathy (ie, etiology unknown). Although it is a heterogeneous group, a common theme is that PVCs in this patient cohort may require epicardial mapping and ablation.24 Similarly, epicardial PVCs and ventricular tachycardia cluster at the basal posterolateral left ventricle near the mitral annulus, for unclear reasons.25
While specific criteria have been published, an epicardial focus is suggested by slowing of the initial QRS segment, pseudo-delta waves, a wider overall QRS, and Q waves in limb lead I.26
Treatment is symptom-driven unless the patient has a tachycardia-related cardiomyopathy or a high burden associated with the risk for its development. Antiarrhythmic drug therapy, when required, typically involves a beta-blocker or a class III drug such as sotalol or amiodarone. Sotalol is used in this population but has limited safety data and should be used cautiously in patients without an implantable cardioverter-defibrillator.
Arrhythmogenic right ventricular cardiomyopathy
Spontaneous ventricular ectopy and tachycardia are common, if not expected, in patients with this heritable autosomal dominant disorder. This condition is progressive and associated with the risk of sudden cardiac death. Criteria for diagnosis were established in 2010, and patients with suspected arrhythmogenic right ventricular cardiomyopathy often undergo cardiac magnetic resonance imaging.27 Diagnostic findings include fibro-fatty tissue replacement, which usually starts in the right ventricle but can progress to involve the left ventricle. PVCs and ventricular tachycardia can involve the right ventricular free wall and are often epicardial.
Catheter ablation is usually palliative, as future arrhythmias are expected. Many patients with this condition require an implantable cardioverter-defibrillator for prevention of sudden cardiac death, and some go on to cardiac transplantation as the disease progresses and ventricular arrhythmias become incessant.
Other conditions
Spontaneous ventricular ectopy is common in other heritable and acquired cardiomyopathies including hypertrophic cardiomyopathy and in infiltrative or inflammatory disorders such as cardiac amyloidosis and sarcoidosis. While technically falling under the rubric of nonischemic heart disease, the presence of spontaneous ventricular ectopy carries specific prognostic implications depending on the underlying diagnosis. Therefore, an appropriate referral for complete cardiac evaluation should be considered when a heritable disorder or other acquired structural heart disease is suspected.
TACHYCARDIA-RELATED CARDIOMYOPATHY
Tachycardia-related cardiomyopathy refers to left ventricular systolic dysfunction that is primarily caused by arrhythmias. This includes frequent PVCs or ventricular tachycardia but also atrial arrhythmias occurring at a high burden that directly weaken myocardial function over time. Although much research has been devoted to this condition, our understanding of its etiology and pathology is incomplete.
PVCs and ventricular ectopy burdens in excess of 15% to 20% have been associated with the development of this condition.28,29 However, it is important to note that cardiomyopathy can also develop at lower burdens.30 One study found that a burden greater than 24% was 79% sensitive and 78% specific for development of tachycardia-related cardiomyopathy.31 Additional studies have demonstrated specific PVC morphologic features such as slurring in the initial QRS segment and also PVCs occurring at shorter coupling intervals as being associated with cardiomyopathy.32–34
For these reasons, both quantification of the total burden and careful evaluation of available electrocardiograms and rhythm strips are important even in asymptomatic patients with frequent PVCs. Similarly, unexplained left ventricular dysfunction in patients with PVC burdens in these discussed ranges should raise suspicion for this diagnosis. Patients with tachycardia-related cardiomyopathy usually have at least partially reversible left ventricular dysfunction when identified or treated early.29,35
MEDICAL AND ABLATIVE TREATMENT
Available treatments include medical suppression and catheter ablation. One needs to exercise clinical judgment and incorporate all of the PVC-related data to make treatment decisions.
Little data for trigger avoidance and behavioral modification
Some patients report a strong association between palpitations related to PVCs and caffeine intake, other stimulants, or other dietary triggers. However, few data exist to support the role of trigger avoidance and behavioral modification in treatment. In fact, an older randomized trial in 81 men found no benefit in a program of total abstinence from caffeine and smoking, moderation of alcohol intake, and physical conditioning.36
Nonetheless, some argue in favor of advising patients to make these dietary and lifestyle changes, given the overall health benefits of aggressive risk-factor modification for cardiovascular disease.37 Certainly, a trial of trigger avoidance and behavioral modification seems reasonable for patients who have strongly associated historical triggers in the absence of structural heart disease and PVCs occurring at a low to modest burden.
Beta-blockers are the mainstay
Beta-blockers are the mainstay of medical suppression of PVCs, primarily through their effect on beta-1 adrenergic receptors to reduce intracellular cyclic adenosine monophosphate and thus decrease automaticity. Blocking beta-1 receptors also causes a negative chronotropic effect, reducing the resting sinus rate in addition to slowing atrioventricular nodal conduction.
Cardioselective beta-blockers include atenolol, betaxolol, metoprolol, and nadolol. These drugs are effective in suppressing PVCs, or at least in reducing the burden to more tolerable levels.
Beta-blockers are most strongly indicated in patients who require PVC suppression and who have concomitant coronary artery disease, prior myocardial infarction, or other cardiomyopathy, as this drug class favorably affects long-term prognosis in these conditions.
Common side effects of beta-blockers include fatigue, shortness of breath, depressed mood, and loss of libido. Side effects can present a significant challenge, particularly for younger patients. Noncardioselective beta-blockers are less commonly prescribed, with the exception of propranolol, which is an effective sympatholytic drug that blocks both beta-1 and beta-2 receptors.
Many patients with asthma or peripheral arterial disease can tolerate these drugs well despite concerns about provoked bronchospasm or claudication, respectively, and neither of these conditions is considered an absolute contraindication. Excessive bradycardia with beta-blocker therapy can lead to dizziness, lightheadedness, or overt syncope, and these drugs should be used with caution in patients with baseline sinus node dysfunction or atrioventricular nodal disease.
Nondihydropyridine calcium channel blockers
Nondihydropyridine calcium channel blockers are particularly effective for PVC suppression in patients without structural heart disease by the mechanisms previously described involving intracellular calcium channels. In particular, they are highly effective and are considered the drugs of choice in treating fascicular PVCs.
Verapamil is a potent drug in this class, but it also commonly causes constipation as a side effect. Diltiazem is less constipating but can cause fatigue, drowsiness, and headaches. Both drugs reduce the resting heart rate and slow atrioventricular nodal conduction. Patients predisposed to bradycardia or atrioventricular block can develop dizziness or overt syncope. Calcium channel blockers are also used cautiously in patients with congestive heart failure, given their potential negative inotropic effects.
Overall, calcium channel blockers are a very reasonable choice for young patients without structural heart disease who need PVC suppression.
Other antiarrhythmic drugs
Sotalol merits special consideration because it has both beta-blocker and class III antiarrhythmic properties, blocking potassium channels and prolonging cardiac repolarization. It can be very effective in PVC suppression but also creates some degree of QT prolongation. The QT-prolonging effect is accentuated in patients with baseline QT prolongation or abnormal renal function. Rarely, this can lead to torsades de pointes. As a safety precaution, some patients are admitted to the hospital when they start sotalol therapy so that they can be monitored with continuous telemetry and ECG to detect excessive QT prolongation.
Amiodarone is a versatile drug with mixed pharmacologic properties that include a predominantly potassium channel-blocking class III drug effect. However, this effect is balanced by its other pharmacologic properties that make QT prolongation less of a clinical concern. Excessive QT prolongation may still occur when used concomitantly with other QT-prolonging drugs.
Amiodarone is very effective in suppressing PVCs and ventricular arrhythmias but has considerable short-term and long-term side effects. Cumulative toxicity risks include damage to the thyroid gland, liver, skin, eyes, and lungs. Routine thyroid function testing, pulmonary function testing, and eye examinations are often considered for patients on long-term amiodarone therapy. Short-term use of this drug does not typically require such surveillance.
Catheter ablation
As mentioned in the previous sections, catheter ablation is a safe and effective treatment for PVCs. It is curative in most cases, and significantly reduces the PVC burden in others.
Procedure. Patients are brought to the electrophysiology laboratory in a fasted state and are partially sedated with an intravenous drug such as midazolam or fentanyl, or both. Steerable catheters are placed into appropriate cardiac chambers from femoral access sites, which are infiltrated with local anesthesia. Sometimes sedative or analgesic drugs must be limited if they are known to suppress PVCs.
Most operators prefer a technique called activation mapping, in which the catheter is maneuvered to home in on the precise PVC origin within the heart, which is subsequently ablated. This technique has very high success rates, but having enough spontaneous PVCs to map during the procedure is essential for the technique to succeed. Conversely, not having sufficient PVCs on the day of the procedure is a common reason that ablation fails or cannot be performed at all.
Pace-mapping is an alternate technique that does not require a continuous stream of PVCs. This involves pacing from different candidate locations inside the heart in an effort to precisely match the ECG appearance of the clinical PVC and to ablate at this site. Although activation mapping generally yields higher success rates and is preferred by most operators, pace-mapping can be successful when a perfect 12–12 match is elicited. In many cases, the two techniques are used together during the same procedure, particularly if the patient’s PVCs spontaneously wax and wane, as they often do.
Risks. Like any medical procedure, catheter ablation carries some inherent risks, including rare but potentially serious events. Unstable arrhythmias may require pace-termination from the catheter or, rarely, shock-termination externally. Even more rare is cardiac arrest requiring cardiopulmonary resuscitation. Uncommon but life-threatening complications also include pericardial effusion or cardiac tamponade requiring percutaneous drainage or, rarely, emergency surgical correction. Although such events are life-threatening, death is extremely rare.
Complications causing permanent disability are also very uncommon but include the risk of collateral injury to the conduction system requiring permanent pacemaker placement, injury to the coronary vessels requiring urgent treatment, or diaphragmatic injury affecting breathing. Left-sided cardiac ablation also carries a small risk of stroke, which is mitigated by giving intravenous heparin during the procedure.
More common but generally non-life-threatening complications include femoral vascular events such as hematomas, pseudoaneurysms, or fistulas that sometimes require subsequent treatment. These complications are generally treatable but can significantly prolong the recovery period.
Catheter ablation procedures are typically 2 to 6 hours in duration, depending on the chambers involved, PVC frequency, and other considerations. Postprocedure bed rest is required for a number of hours. A Foley catheter is sometimes used for patient comfort when a prolonged procedure is anticipated. This carries a small risk of urinary tract infection. Epicardial catheter ablation that requires access to the surface of the heart (ie, the pericardial space) is uncommon but carries some unique risks, including rare injury to coronary vessels or adjacent organs such as the liver or stomach.
Overall, both endocardial and epicardial catheter ablation can be performed safely and effectively in the overwhelming majority of patients, but understanding and explaining the potential risks remains a crucial part of the informed consent process.
TAKE-HOME POINTS
- PVCs are a common cause of palpitations but are also noted as incidental findings by ECG, Holter monitoring, and inpatient telemetry.
- The diagnostic evaluation includes an assessment for underlying structural heart disease and quantification of the total PVC burden.
- Patients without structural heart disease and with low-to-modest PVC burdens may not require specific treatment. PVCs at greater burdens, typically 15% to 20%, or with specific high-risk features carry a risk of tachycardia-related cardiomyopathy and may require treatment even if they are asymptomatic. These high-risk features include initial QRS slurring and PVCs occurring at shorter coupling intervals.
- Treatment involves medical therapy with a beta-blocker, a calcium channel blocker, or another antiarrhythmic drug, and catheter ablation in selected cases.
- Catheter ablation can be curative but is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
Premature ventricular complexes (PVCs) are a common cause of palpitations, and are also often detected incidentally on electrocardiography (ECG), ambulatory monitoring, or inpatient telemetry. At the cellular level, ventricular myocytes spontaneously depolarize to create an extra systole that is “out of sync” with the cardiac cycle.
Although nearly everyone has some PVCs from time to time, people vary widely in their frequency of PVCs and their sensitivity to them.1,2 Some patients are exquisitely sensitive to even a small number of PVCs, while others are completely unaware of PVCs in a bigeminal pattern (ie, every other heartbeat). This article will review the evaluation and management of PVCs with a focus on clinical aspects.
DIAGNOSTIC EVALUATION
Personal and family history
Symptoms. The initial history should establish the presence, extent, timing, and duration of symptoms. Patients may use the word “palpitations” to describe their symptoms, but they also describe them as “hard” heartbeats, “chest-thumping,” or as a “catch” or “skipped” heartbeat. Related symptoms may include difficulty breathing, chest pain, fatigue, and dizziness.
The interview should determine whether the symptoms represent a minor nuisance or a major quality-of-life issue to the patient, and whether there are any specific associations or triggers. For example, it is very common for patients to become aware of PVCs at night, particularly in certain positions, such as lying on the left side. Patients often associate PVC symptoms with emotional stress, exercise, or caffeine or stimulant use.
Medication use. An accurate and up-to-date list of prescription medications should be screened for alpha-, beta-, or dopamine-receptor agonist drugs. Similarly, any use of over-the-counter sympathomimetic medications and nonprescription supplements should be elicited, including compounded elixirs or beverages. Many commercially available products designed to treat fatigue or increase alertness contain large doses of caffeine or other stimulants. It is also important to consider the use of illicit substances such as cocaine, amphetamine, methamphetamine, and their derivatives.
The patient’s medical and surgical history should be queried for any known structural heart disease, including coronary artery disease, myocardial infarction, congestive heart failure, valvular heart disease, congenital heart disease, and heritable conditions such as hypertrophic cardiomyopathy, prolonged QT syndromes, or other channel disorders. Pulmonary disorders such as sarcoidosis, pulmonary hypertension, or obstructive sleep apnea are also relevant. Similarly, it is important to identify endocrine disorders, including thyroid problems, sex hormone abnormalities, or adrenal gland conditions.
A careful family history should include any instance of sudden death in first-degree relatives, any heritable cardiac conditions, or coronary artery disease at an early age.
Physical examination
The physical examination should focus on findings that suggest underlying structural heart disease. Findings suggestive of congestive heart failure include elevated jugular venous pressures, abnormal cardiac sounds, pulmonary rales, abnormal arterial pulses, or peripheral edema. A murmur or a pathologic heart sound should raise suspicion of valvular or congenital heart disease when present in a young patient.
Inspection and palpation of the thyroid can reveal a related disorder. Obvious skin changes or neurologic findings can similarly reveal a systemic and possibly related clinical disorder that can have cardiac manifestations (eg, muscular dystrophy).
Electrocardiography, Holter monitoring, and other monitoring
Assessment of the cardiac rhythm includes 12-lead ECG and ambulatory Holter monitoring, typically for 24 or 48 hours.
Holter monitoring provides a continuous recording, usually in at least two or three leads. Patients are given a symptom journal or are asked to keep a diary of symptoms experienced during the monitoring period. The monitor is worn underneath clothing and is returned for download upon completion. Technicians process the data with the aid of computer software, and the final output is reviewed and interpreted by a cardiologist or cardiac electrophysiologist.
Holter monitoring for at least 24 hours is a critical step in assessing any patient with known or suspected PVCs, as it can both quantify the total burden of ventricular ectopy and identify the presence of any related ventricular tachycardia. In addition, it can detect additional supraventricular arrhythmias or bradycardia during the monitoring period. The PVC burden is an important measurement; it is expressed as the percentage of heartbeats that were ventricular extrasystoles during the monitoring period.
Both ECG and Holter monitoring are limited in that they are only snapshots of the rhythm during the period when a patient is actually hooked up. Many patients experience PVCs in clusters every very few days or weeks. Such a pattern is unlikely to be detected by a single ECG or 24- or 48-hour Holter monitoring.
A 30-day ambulatory event monitor (also known as a wearable loop recorder) is an important diagnostic tool in these scenarios. The concept is very similar to that of Holter monitoring, except that the device provides a continuous loop recording of the cardiac rhythm that is digitally stored in clips when the patient activates the device. Some wearable loop recorders also have auto-save features for heart rates falling outside of a programmed range.
Mobile outpatient cardiac telemetry is the most comprehensive form of noninvasive rhythm monitoring available. This is essentially the equivalent of continuous inpatient cardiac telemetry, but in a patient who is not hospitalized. It is a wearable ambulatory device providing continuous recordings, real-time automatic detections, and patient-activated symptom recordings. It can be used for up to 6 weeks. Advantages include detection and quantification of asymptomatic events, and real-time transmissions that the physician can act upon. The major disadvantage is cost, including coverage denial by many third-party payers.
This test is rarely indicated as part of a PVC evaluation and is typically ordered only by a cardiologist or cardiac electrophysiologist.
Noninvasive cardiac evaluation
Surface echocardiography is indicated to look for overt structural heart disease and can reliably detect abnormalities in cardiac chamber size, wall thickness, and function. Valvular heart disease is concomitantly identified by two-dimensional imaging as well as by color Doppler. The finding of significant structural heart disease in conjunction with PVCs should prompt a cardiology referral, as this carries significant prognostic implications.3–5
Exercise treadmill stress testing is appropriate for patients who experience PVCs with exercise or for whom an evaluation for coronary artery disease is indicated. The expected finding would be an increase in PVCs or ventricular tachycardia with exercise or in the subsequent recovery period. Exercise testing can be combined with either echocardiographic or nuclear perfusion imaging to evaluate the possibility of myocardial ischemia. For patients unable to exercise, pharmacologic stress testing with dobutamine or a vasodilator agent can be performed.
Advanced noninvasive cardiac imaging— such as computed tomography, magnetic resonance imaging, or positron-emission tomography—should be reserved for specific clinical indications such as congenital heart disease, suspected cardiac sarcoidosis, and infiltrative heart disease, and for specific cardiomyopathies, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. For example, frequent PVCs with a left bundle branch block morphology and superior axis raise the concern for a right ventricular disorder and may prompt cardiac magnetic resonance imaging for either arrhythmogenic right ventricular cardiomyopathy or sarcoidosis.
PVCs WITHOUT STRUCTURAL HEART DISEASE
Outflow tract PVCs and ventricular tachycardia
The right or left ventricular outflow tracts, or the epicardial tissue immediately adjacent to the aortic sinuses of Valsalva are the most common sites of origin for ventricular ectopy in the absence of structural heart disease.6–9 Affected cells often demonstrate a triggered activity mechanism due to cyclic adenosine monophosphate-mediated and calcium-dependent delayed after-depolarizations.7,8
Most of these foci are in the right ventricular outflow tract, producing a left bundle branch block morphology with an inferior axis (positive R waves in limb leads II, III, and aVF) and typical precordial R-wave transition in V3 and V4 (Figure 1). A minority are in the left ventricular outflow tract, producing a right bundle branch block with an inferior axis pattern, or in the aortic sinuses with a left bundle branch block pattern but with early precordial R transition in V2 and V3.
A study in 122 patients showed that right and left outflow tract arrhythmias had similar electrophysiologic properties and pharmacologic sensitivities, providing evidence for shared mechanisms possibly due to the common embryologic origin of these structures.9
Such arrhythmias are typically catecholamine-sensitive and are sometimes inducible with burst pacing in the electrophysiology laboratory. The short ventricular coupling intervals can promote intracellular calcium overload in the affected cells, leading to triggered activity.
Therefore, outflow tract PVCs and ventricular tachycardia are commonly encountered clinically during exercise and, to an even greater extent, in the postexercise cool-down period. Similarly, they can be worse during periods of emotional stress or fatigue, when the body’s endogenous catecholamine production is elevated. However, it is worthwhile to note that there are exceptions to this principle in which faster sinus rates seem to overdrive the PVCs in some patients, causing them to become paradoxically more frequent at rest, or even during sleep.
Outflow tract PVCs can be managed medically with beta-blockers, nondihydropyridine calcium channel blockers (verapamil or diltiazem), or, less commonly, class IC drugs such as flecainide. They are also highly curable by catheter ablation (Figure 2), with procedure success rates greater than 90%.9.10
However, a subset of outflow tract PVCs nested deep in a triangle of epicardial tissue between the right and left endocardial surface and underneath the left main coronary artery can be challenging. This region has been labeled the left ventricular summit, and is shielded from ablation by an epicardial fat pad in the adjacent pericardial space.11 Ablation attempts made from the right and left endocardial surfaces as well as the epicardial surface (pericardial space) sometimes cannot adequately penetrate the tissue deep enough to reach the originating focus deep within this triangle. While ablation cannot always fully eliminate the PVC, ablation from more than one of the sites listed can generally reduce its burden, often in combination with suppressive medical therapy (Figure 3).
Fascicular PVCs
Fascicular PVCs originate from within the left ventricular His-Purkinje system12 and produce a right bundle branch block morphology with either an anterior or posterior hemiblock pattern (Figure 4). Exit from the posterior fascicle causes an anterior hemiblock pattern, and exit from the anterior fascicle a posterior hemiblock pattern. Utilization of the rapidly conducting His-Purkinje system gives these PVCs a very narrow QRS duration, sometimes approaching 120 milliseconds or shorter. This occasionally causes them to be mistaken for aberrantly conducted supraventricular beats. Such spontaneous PVCs are commonly associated with both sustained and nonsustained ventricular tachycardia and are usually sensitive to verapamil.13
Special issues relating to mapping and catheter ablation of fascicular arrhythmias involve the identification of Purkinje fiber potentials and associated procedural diagnostic maneuvers during tachycardia.14
Other sites for PVCs
Other sites of origin for PVCs in the absence of structural heart disease include ventricular tissue adjacent to the aortomitral continuity,15 the tricuspid annulus,16 the mitral valve annulus, 17 papillary muscles,18 and other Purkinje-adjacent structures such as left ventricular false tendons.19 An example of a papillary muscle PVC is shown in Figures 5 and 6.
Curable by catheter ablation
Any of these PVCs can potentially be cured by catheter ablation when present at a sufficient burden to allow for activation mapping in the electrophysiology laboratory. The threshold for offering ablation varies among operators, but is generally around 10% or greater. Pacemapping is a technique applied in the electrophysiology laboratory when medically refractory symptomatic PVCs occurring at a lower burden require ablation.
PVCs WITH AN UNDERLYING CARDIAC CONDITION
Coronary artery disease
Tissue injury and death caused by acute myocardial infarction has long been recognized as a common cause of spontaneous ventricular ectopy attributed to infarct border zones of ischemic or hibernating myocardium.20,21
Suppression has not been associated with improved outcomes, as shown for class IC drugs in the landmark Cardiac Arrhythmia Suppression Trial (CAST),22 or in the amiodarone treatment arm of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II).23 Therefore, treatment of ventricular ectopy in this patient population is usually symptom-driven unless there is hemodynamic intolerance, tachycardia-related cardiomyopathy, or a very high burden of PVCs in a patient who may be at risk of developing tachycardia-related cardiomyopathy. Antiarrhythmic drug treatment, when required, usually involves beta-blockers or class III medications such as sotalol or amiodarone.
Nonischemic dilated cardiomyopathy
This category includes patients with a wide variety of disease states including valvular heart disease, lymphocytic and other viral myocarditis, cardiac sarcoidosis, amyloidosis and other infiltrative diseases, familial conditions, and idiopathic dilated cardiomyopathy (ie, etiology unknown). Although it is a heterogeneous group, a common theme is that PVCs in this patient cohort may require epicardial mapping and ablation.24 Similarly, epicardial PVCs and ventricular tachycardia cluster at the basal posterolateral left ventricle near the mitral annulus, for unclear reasons.25
While specific criteria have been published, an epicardial focus is suggested by slowing of the initial QRS segment, pseudo-delta waves, a wider overall QRS, and Q waves in limb lead I.26
Treatment is symptom-driven unless the patient has a tachycardia-related cardiomyopathy or a high burden associated with the risk for its development. Antiarrhythmic drug therapy, when required, typically involves a beta-blocker or a class III drug such as sotalol or amiodarone. Sotalol is used in this population but has limited safety data and should be used cautiously in patients without an implantable cardioverter-defibrillator.
Arrhythmogenic right ventricular cardiomyopathy
Spontaneous ventricular ectopy and tachycardia are common, if not expected, in patients with this heritable autosomal dominant disorder. This condition is progressive and associated with the risk of sudden cardiac death. Criteria for diagnosis were established in 2010, and patients with suspected arrhythmogenic right ventricular cardiomyopathy often undergo cardiac magnetic resonance imaging.27 Diagnostic findings include fibro-fatty tissue replacement, which usually starts in the right ventricle but can progress to involve the left ventricle. PVCs and ventricular tachycardia can involve the right ventricular free wall and are often epicardial.
Catheter ablation is usually palliative, as future arrhythmias are expected. Many patients with this condition require an implantable cardioverter-defibrillator for prevention of sudden cardiac death, and some go on to cardiac transplantation as the disease progresses and ventricular arrhythmias become incessant.
Other conditions
Spontaneous ventricular ectopy is common in other heritable and acquired cardiomyopathies including hypertrophic cardiomyopathy and in infiltrative or inflammatory disorders such as cardiac amyloidosis and sarcoidosis. While technically falling under the rubric of nonischemic heart disease, the presence of spontaneous ventricular ectopy carries specific prognostic implications depending on the underlying diagnosis. Therefore, an appropriate referral for complete cardiac evaluation should be considered when a heritable disorder or other acquired structural heart disease is suspected.
TACHYCARDIA-RELATED CARDIOMYOPATHY
Tachycardia-related cardiomyopathy refers to left ventricular systolic dysfunction that is primarily caused by arrhythmias. This includes frequent PVCs or ventricular tachycardia but also atrial arrhythmias occurring at a high burden that directly weaken myocardial function over time. Although much research has been devoted to this condition, our understanding of its etiology and pathology is incomplete.
PVCs and ventricular ectopy burdens in excess of 15% to 20% have been associated with the development of this condition.28,29 However, it is important to note that cardiomyopathy can also develop at lower burdens.30 One study found that a burden greater than 24% was 79% sensitive and 78% specific for development of tachycardia-related cardiomyopathy.31 Additional studies have demonstrated specific PVC morphologic features such as slurring in the initial QRS segment and also PVCs occurring at shorter coupling intervals as being associated with cardiomyopathy.32–34
For these reasons, both quantification of the total burden and careful evaluation of available electrocardiograms and rhythm strips are important even in asymptomatic patients with frequent PVCs. Similarly, unexplained left ventricular dysfunction in patients with PVC burdens in these discussed ranges should raise suspicion for this diagnosis. Patients with tachycardia-related cardiomyopathy usually have at least partially reversible left ventricular dysfunction when identified or treated early.29,35
MEDICAL AND ABLATIVE TREATMENT
Available treatments include medical suppression and catheter ablation. One needs to exercise clinical judgment and incorporate all of the PVC-related data to make treatment decisions.
Little data for trigger avoidance and behavioral modification
Some patients report a strong association between palpitations related to PVCs and caffeine intake, other stimulants, or other dietary triggers. However, few data exist to support the role of trigger avoidance and behavioral modification in treatment. In fact, an older randomized trial in 81 men found no benefit in a program of total abstinence from caffeine and smoking, moderation of alcohol intake, and physical conditioning.36
Nonetheless, some argue in favor of advising patients to make these dietary and lifestyle changes, given the overall health benefits of aggressive risk-factor modification for cardiovascular disease.37 Certainly, a trial of trigger avoidance and behavioral modification seems reasonable for patients who have strongly associated historical triggers in the absence of structural heart disease and PVCs occurring at a low to modest burden.
Beta-blockers are the mainstay
Beta-blockers are the mainstay of medical suppression of PVCs, primarily through their effect on beta-1 adrenergic receptors to reduce intracellular cyclic adenosine monophosphate and thus decrease automaticity. Blocking beta-1 receptors also causes a negative chronotropic effect, reducing the resting sinus rate in addition to slowing atrioventricular nodal conduction.
Cardioselective beta-blockers include atenolol, betaxolol, metoprolol, and nadolol. These drugs are effective in suppressing PVCs, or at least in reducing the burden to more tolerable levels.
Beta-blockers are most strongly indicated in patients who require PVC suppression and who have concomitant coronary artery disease, prior myocardial infarction, or other cardiomyopathy, as this drug class favorably affects long-term prognosis in these conditions.
Common side effects of beta-blockers include fatigue, shortness of breath, depressed mood, and loss of libido. Side effects can present a significant challenge, particularly for younger patients. Noncardioselective beta-blockers are less commonly prescribed, with the exception of propranolol, which is an effective sympatholytic drug that blocks both beta-1 and beta-2 receptors.
Many patients with asthma or peripheral arterial disease can tolerate these drugs well despite concerns about provoked bronchospasm or claudication, respectively, and neither of these conditions is considered an absolute contraindication. Excessive bradycardia with beta-blocker therapy can lead to dizziness, lightheadedness, or overt syncope, and these drugs should be used with caution in patients with baseline sinus node dysfunction or atrioventricular nodal disease.
Nondihydropyridine calcium channel blockers
Nondihydropyridine calcium channel blockers are particularly effective for PVC suppression in patients without structural heart disease by the mechanisms previously described involving intracellular calcium channels. In particular, they are highly effective and are considered the drugs of choice in treating fascicular PVCs.
Verapamil is a potent drug in this class, but it also commonly causes constipation as a side effect. Diltiazem is less constipating but can cause fatigue, drowsiness, and headaches. Both drugs reduce the resting heart rate and slow atrioventricular nodal conduction. Patients predisposed to bradycardia or atrioventricular block can develop dizziness or overt syncope. Calcium channel blockers are also used cautiously in patients with congestive heart failure, given their potential negative inotropic effects.
Overall, calcium channel blockers are a very reasonable choice for young patients without structural heart disease who need PVC suppression.
Other antiarrhythmic drugs
Sotalol merits special consideration because it has both beta-blocker and class III antiarrhythmic properties, blocking potassium channels and prolonging cardiac repolarization. It can be very effective in PVC suppression but also creates some degree of QT prolongation. The QT-prolonging effect is accentuated in patients with baseline QT prolongation or abnormal renal function. Rarely, this can lead to torsades de pointes. As a safety precaution, some patients are admitted to the hospital when they start sotalol therapy so that they can be monitored with continuous telemetry and ECG to detect excessive QT prolongation.
Amiodarone is a versatile drug with mixed pharmacologic properties that include a predominantly potassium channel-blocking class III drug effect. However, this effect is balanced by its other pharmacologic properties that make QT prolongation less of a clinical concern. Excessive QT prolongation may still occur when used concomitantly with other QT-prolonging drugs.
Amiodarone is very effective in suppressing PVCs and ventricular arrhythmias but has considerable short-term and long-term side effects. Cumulative toxicity risks include damage to the thyroid gland, liver, skin, eyes, and lungs. Routine thyroid function testing, pulmonary function testing, and eye examinations are often considered for patients on long-term amiodarone therapy. Short-term use of this drug does not typically require such surveillance.
Catheter ablation
As mentioned in the previous sections, catheter ablation is a safe and effective treatment for PVCs. It is curative in most cases, and significantly reduces the PVC burden in others.
Procedure. Patients are brought to the electrophysiology laboratory in a fasted state and are partially sedated with an intravenous drug such as midazolam or fentanyl, or both. Steerable catheters are placed into appropriate cardiac chambers from femoral access sites, which are infiltrated with local anesthesia. Sometimes sedative or analgesic drugs must be limited if they are known to suppress PVCs.
Most operators prefer a technique called activation mapping, in which the catheter is maneuvered to home in on the precise PVC origin within the heart, which is subsequently ablated. This technique has very high success rates, but having enough spontaneous PVCs to map during the procedure is essential for the technique to succeed. Conversely, not having sufficient PVCs on the day of the procedure is a common reason that ablation fails or cannot be performed at all.
Pace-mapping is an alternate technique that does not require a continuous stream of PVCs. This involves pacing from different candidate locations inside the heart in an effort to precisely match the ECG appearance of the clinical PVC and to ablate at this site. Although activation mapping generally yields higher success rates and is preferred by most operators, pace-mapping can be successful when a perfect 12–12 match is elicited. In many cases, the two techniques are used together during the same procedure, particularly if the patient’s PVCs spontaneously wax and wane, as they often do.
Risks. Like any medical procedure, catheter ablation carries some inherent risks, including rare but potentially serious events. Unstable arrhythmias may require pace-termination from the catheter or, rarely, shock-termination externally. Even more rare is cardiac arrest requiring cardiopulmonary resuscitation. Uncommon but life-threatening complications also include pericardial effusion or cardiac tamponade requiring percutaneous drainage or, rarely, emergency surgical correction. Although such events are life-threatening, death is extremely rare.
Complications causing permanent disability are also very uncommon but include the risk of collateral injury to the conduction system requiring permanent pacemaker placement, injury to the coronary vessels requiring urgent treatment, or diaphragmatic injury affecting breathing. Left-sided cardiac ablation also carries a small risk of stroke, which is mitigated by giving intravenous heparin during the procedure.
More common but generally non-life-threatening complications include femoral vascular events such as hematomas, pseudoaneurysms, or fistulas that sometimes require subsequent treatment. These complications are generally treatable but can significantly prolong the recovery period.
Catheter ablation procedures are typically 2 to 6 hours in duration, depending on the chambers involved, PVC frequency, and other considerations. Postprocedure bed rest is required for a number of hours. A Foley catheter is sometimes used for patient comfort when a prolonged procedure is anticipated. This carries a small risk of urinary tract infection. Epicardial catheter ablation that requires access to the surface of the heart (ie, the pericardial space) is uncommon but carries some unique risks, including rare injury to coronary vessels or adjacent organs such as the liver or stomach.
Overall, both endocardial and epicardial catheter ablation can be performed safely and effectively in the overwhelming majority of patients, but understanding and explaining the potential risks remains a crucial part of the informed consent process.
TAKE-HOME POINTS
- PVCs are a common cause of palpitations but are also noted as incidental findings by ECG, Holter monitoring, and inpatient telemetry.
- The diagnostic evaluation includes an assessment for underlying structural heart disease and quantification of the total PVC burden.
- Patients without structural heart disease and with low-to-modest PVC burdens may not require specific treatment. PVCs at greater burdens, typically 15% to 20%, or with specific high-risk features carry a risk of tachycardia-related cardiomyopathy and may require treatment even if they are asymptomatic. These high-risk features include initial QRS slurring and PVCs occurring at shorter coupling intervals.
- Treatment involves medical therapy with a beta-blocker, a calcium channel blocker, or another antiarrhythmic drug, and catheter ablation in selected cases.
- Catheter ablation can be curative but is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
- Kostis JB, McCrone K, Moreyra AE, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation 1981; 63:1351–1356.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95:1230–1237.
- Simpson RJ, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002; 143:535–540.
- Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985; 109:497–504.
- Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30:5–15.
- Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation 1986; 74:270–280.
- Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995; 92:421–429.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007; 49:2035–2043.
- Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 2010; 3:616–623.
- Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macro-reentry within the Purkinje network. Circulation 2002; 105:462–469.
- Iwai S, Lerman BB. Management of ventricular tachycardia in patients with clinically normal hearts. Curr Cardiol Rep 2000; 2:515–521.
- Nogami A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011; 34:624–650.
- Letsas KP, Efremidis M, Kollias G, Xydonas S, Sideris A. Electrocardiographic and electrophysiologic characteristics of ventricular extrasystoles arising from the aortomitral continuity. Cardiol Res Pract 2011; 2011:864964.
- Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007; 4:7–16.
- Tada H, Ito S, Naito S, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005; 45:877–886.
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008; 1:23–29.
- Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009; 6:1050–1058.
- Bigger JT, Dresdale FJ, Heissenbuttel RH, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis 1977; 19:255–300.
- Eldar M, Sievner Z, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Primary ventricular tachycardia in acute myocardial infarction: clinical characteristics and mortality. The SPRINT Study Group. Ann Intern Med 1992; 117:31–36.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321:406–412.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009; 54:799–808.
- Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation 2007; 116:1998–2001.
- Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2010; 3:63–71.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010; 121:1533–1541.
- Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol 2012; 5:229–236.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008; 13:81–85.
- Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010; 7:865–869.
- Moulton KP, Medcalf T, Lazzara R. Premature ventricular complex morphology. A marker for left ventricular structure and function. Circulation 1990; 81:1245–1251.
- Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011; 8:1046–1049.
- Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003; 19:295–299.
- Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009; 6:1543–1549.
- DeBacker G, Jacobs D, Prineas R, et al. Ventricular premature contractions: a randomized non-drug intervention trial in normal men. Circulation 1979; 59:762–769.
- Glatter KA, Myers R, Chiamvimonvat N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: what do you tell your patients to do or not to do? Curr Treat Options Cardiovasc Med 2012; 14:529–535.
- Kostis JB, McCrone K, Moreyra AE, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation 1981; 63:1351–1356.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95:1230–1237.
- Simpson RJ, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002; 143:535–540.
- Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985; 109:497–504.
- Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30:5–15.
- Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation 1986; 74:270–280.
- Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995; 92:421–429.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007; 49:2035–2043.
- Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 2010; 3:616–623.
- Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macro-reentry within the Purkinje network. Circulation 2002; 105:462–469.
- Iwai S, Lerman BB. Management of ventricular tachycardia in patients with clinically normal hearts. Curr Cardiol Rep 2000; 2:515–521.
- Nogami A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011; 34:624–650.
- Letsas KP, Efremidis M, Kollias G, Xydonas S, Sideris A. Electrocardiographic and electrophysiologic characteristics of ventricular extrasystoles arising from the aortomitral continuity. Cardiol Res Pract 2011; 2011:864964.
- Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007; 4:7–16.
- Tada H, Ito S, Naito S, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005; 45:877–886.
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008; 1:23–29.
- Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009; 6:1050–1058.
- Bigger JT, Dresdale FJ, Heissenbuttel RH, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis 1977; 19:255–300.
- Eldar M, Sievner Z, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Primary ventricular tachycardia in acute myocardial infarction: clinical characteristics and mortality. The SPRINT Study Group. Ann Intern Med 1992; 117:31–36.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321:406–412.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009; 54:799–808.
- Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation 2007; 116:1998–2001.
- Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2010; 3:63–71.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010; 121:1533–1541.
- Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol 2012; 5:229–236.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008; 13:81–85.
- Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010; 7:865–869.
- Moulton KP, Medcalf T, Lazzara R. Premature ventricular complex morphology. A marker for left ventricular structure and function. Circulation 1990; 81:1245–1251.
- Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011; 8:1046–1049.
- Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003; 19:295–299.
- Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009; 6:1543–1549.
- DeBacker G, Jacobs D, Prineas R, et al. Ventricular premature contractions: a randomized non-drug intervention trial in normal men. Circulation 1979; 59:762–769.
- Glatter KA, Myers R, Chiamvimonvat N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: what do you tell your patients to do or not to do? Curr Treat Options Cardiovasc Med 2012; 14:529–535.
KEY POINTS
- Diagnostic evaluation should include an assessment for structural heart disease and quantification of the total PVC burden by ambulatory Holter monitoring.
- Patients without structural heart disease and low-to-modest PVC burdens do not always require treatment. PVCs at higher burdens (typically more than 15% to 20% of heartbeats) or strung together in runs of ventricular tachycardia pose a higher risk of tachycardia-related cardiomyopathy and heart failure, even if asymptomatic.
- When necessary, treatment for PVCs involves beta-blockers, calcium channel blockers, or other antiarrhythmic drugs and catheter ablation in selected cases.
- Catheter ablation can be curative, but it is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
Which patients may benefit from coronary artery calcification scoring?
Although we still have no evidence from randomized trials that patients have better outcomes if we measure the calcification in their coronary arteries, a growing body of evidence shows that we can estimate risk more accurately than with a risk model score alone if we also score coronary artery calcification in asymptomatic patients, especially those at intermediate risk.
Current guidelines1 recommend using the Framingham Risk Score or a similar tool to estimate coronary risk in asymptomatic patients, but these tools have only modest accuracy. Calcification scoring is accurate, inexpensive, quick, widely available, low-risk, and does not appear to increase medical costs afterward. In addition to improving risk stratification, it may also encourage patients to adhere better to drug therapy and lifestyle modification.
HOW IS CORONARY ARTERY CALCIFICATION MEASURED?
Calcification of the coronary arteries is synonymous with atherosclerosis. It can easily be detected with computed tomography without contrast (Figure 1), and the amount can be quantified with a scoring system such as the volumetric score or the Agatston score. The latter, which is more commonly used, is based on the product of the area of the calcium deposits and the x-ray attenuation in Hounsfield units.
Scores can be roughly categorized (with some overlap owing to data from different studies) as:
- Low risk: 0 Agatston units (AU)
- Average risk: 1–112 AU
- Moderate risk: 100–400 AU
- High risk: 400–999 AU
- Very high risk: 1,000 AU.2
The actual test takes only a few seconds, and the patient can usually be out the door in 15 minutes or less. It does not require iodinated contrast and the radiation dose is minimal, usually less than 1 mSv, equivalent to fewer than 10 chest radiographs.3
The cost is typically between $200 and $500. The test is usually not covered by health insurance, but this differs by insurer and by state; for example, coverage is mandated in Texas, and the test is covered by United Healthcare.
WHAT IS THE EVIDENCE IN FAVOR OF CALCIFICATION SCORING?
Cohort studies with long-term follow-up show that calcification scoring has robust prognostic ability. A pooled analysis of several of these studies2 showed that a higher score strongly correlated with a higher risk of cardiac events over 3 to 5 years. Compared with the risk in people with a score of 0, the risk was twice as high in those with a score of 1 to 112, four times as high with a score of 100 to 400, seven times as high with a score of 400 to 499, and 10 times as high with a score greater than 1,000.2
A cohort study of more than 25,000 patients had similar conclusions about the magnitude of risk associated with coronary calcification.4 It also found that the 10-year risk of death was 0.6% in patients with a score of 0, 3.4% with a score of 101 to 399, 5.3% with a score of 400 to 699, 6.1% with a score of 700 to 999, and 12.2% with a score greater than 1,000.
Although progression of coronary artery calcification may predict the risk of death from any cause,5 the clinical utility of serial measurements is not yet apparent, especially since statin therapy—our front-line treatment for coronary disease—has not been shown to slow the progression of calcification.
Improving the accuracy of risk prediction
If a patient’s 10-year coronary risk is intermediate (10% to 20%), calcification scoring can reclassify the risk as low or high in about 50% of cases and can improve the accuracy of risk prediction.6–8
For example, Elias-Smale et al6 evaluated the effect of calcification scoring in 2,028 asymptomatic patients, with median follow-up of 9.2 years and 135 coronary events observed. Adding the calcification score to the Framingham model significantly improved risk classification, with a net reclassification improvement (NRI) of 0.14 (P < .01). (NRI is a measure of discriminatory performance for a diagnostic test; higher is better.9) Reclassification was most robust in those at intermediate risk, 52% of whom were reclassified, with 30% reclassified to low risk and 22% reclassified to high risk.
Erbel et al7 reported data from the Heinz Nixdorf Recall study, which used calcification scoring to estimate the NRI in 4,129 patients followed for 5 years. During this time there were 93 coronary deaths and non-fatal myocardial infarctions. The addition of the calcification score to the Framingham risk model resulted in an NRI of 0.21 (P = .0002) for patients with a risk of 6% to 20% and 0.31 (P < .0001) for those with a risk of 10% to 20%. Erbel et al also estimated the C statistic (area under the receiver operating characteristic curve; the maximum value is 1.0 and the higher the value the better) for the addition of the calcification score to the Framingham risk model and to the Adult Treatment Panel (ATP) III algorithm. They reported a significant increase of 0.681 to 0.749 with the Framingham model and 0.653 to 0.755 with the ATP III algorithm.
Polonsky et al8 studied a cohort of 5,878 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) and estimated the event risk using a model based on Framingham risk characteristics. When the calcification score was added to the prediction model, 26% of the sample was reclassified to a new risk category. In intermediate-risk patients, 292 (16%) were reclassified as high risk, and 712 (39%) were reclassified as low risk, achieving an NRI of 0.55 (95% confidence interval 0.41 to 0.69; P < .001). In addition, the C statistic for the prediction of cardiovascular events was 0.76 for the model based on Framingham risk characteristics and increased to 0.81 (P < .001) with the addition of calcification scoring.
Improving adherence and care
Knowing that a patient has a higher calcification score, physicians are more likely to prescribe lipid-lowering and antihypertensive drugs (Table 1),10–12 and patients with a higher score are also more often adherent to recommendations regarding diet and exercise.13
Rozanski et al,14 in a randomized controlled trial, showed that measuring coronary artery calcification did not increase downstream medical spending. A modest improvement in systolic blood pressure (P = .02), serum low-density lipoprotein level (P = .04), and waist circumference (P = .01) was observed in patients who had their calcification measured. Patients with the highest scores had the greatest improvement in coronary risk factors, including blood pressure, cholesterol, weight, and regular exercise.
On the other hand, other analyses have suggested that imaging tests are not effective for motivating behavioral changes. This topic deserves more research.15
Less utility in symptomatic disease
Coronary artery calcification scoring has less clinical utility in patients who already have coronary symptoms. Villines et al16 described a cohort of 10,037 patients with coronary symptoms who underwent calcification scoring and computed tomographic coronary angiography and found that stenosis of greater than 50% was present in 3.5% of those who had a score of 0 and in 29% of those with a score higher than 0. Therefore, a score of 0 does not rule out obstructive coronary heart disease if the patient has symptoms. Conversely, these patients may still have coronary artery calcification even if perfusion stress imaging is normal,17,18 and calcification scoring may have a role in the evaluation of equivocal stress tests.19
CALCIFICATION SCORING GUIDELINES
In their most recent (2010) joint guidelines for assessing risk of coronary heart disease in asymptomatic patients,20 the American College of Cardiology and the American Heart Association say coronary artery calcification scoring:
- Is recommended for asymptomatic patients at intermediate 10-year risk (10% to 20%) of coronary heart disease (class IIa recommendation, level of evidence B)
- May be acceptable for asymptomatic patients at low to intermediate risk (6% to 10%) (class IIb recommendation)
- Is discouraged for those at low risk (< 6%) (class III recommendation).
The most recent (2010) criteria for the appropriate use of cardiac computed tomography21 provide similar recommendations. Specifically, coronary artery calcification scoring with noncontrast computed tomography was rated as appropriate for patients at intermediate risk (10% to 20%) of coronary heart disease and for the specific subset of patients who are at low risk (6% to 10%) but who have a family history of premature coronary heart disease.
These recommendations are based on multiple lines of evidence that calcification scoring is a robust risk-predictor, can enhance risk estimates beyond traditional scoring strategies, and may—in theory—improve outcomes.
CALCIFICATION SCORING’S LIMITATIONS
The images used for measuring coronary calcification do predict risk of cardiovascular events, but they are not adequate to assess the severity of coronary stenosis. Further, calcification scoring often leads to incidental findings, which can cause anxiety and possibly lead to more imaging, entailing more radiation exposure and expense. And as noted, there are no randomized trial data demonstrating a reduction in cardiovascular events with the use of calcification scoring.
- Redberg RF, Benjamin EJ, Bittner V, et al. ACCF/AHA 2009 performance measures for primary prevention of cardiovascular disease in adults. J Am Coll Cardiol 2009; 54:1364–1405.
- Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain. J Am Coll Cardiol 2007; 49:378–402.
- Winchester DE, Wymer DC, Shifrin RY, Kraft SM, Hill JA. Responsible use of computed tomography in the evaluation of coronary artery disease and chest pain. Mayo Clin Proc 2010; 85:358–364.
- Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007; 49:1860–1870.
- Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010; 3:1229–1236.
- Elias-Smale SE, Proença RV, Koller MT, et al. Coronary calcium score improves classification of coronary heart disease risk in the elderly: The Rotterdam study. J Am Coll Cardiol 2010; 56:1407–1414.
- Erbel R, Möhlenkamp S, Moebus S, et al; Heinz Nixdorf Recall Study Investigative Group. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010; 56:1397–1406.
- Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 2010; 303:1610–1616.
- Pencina MJ, Agostino RB, Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statist Med 2008; 27:157–172.
- Kalia NK, Miller LG, Nasir K, Blumenthal RS, Agrawal N, Budoff MJ. Visualizing coronary calcium is associated with improvements in adherence to statin therapy. Atherosclerosis 2006; 185:394–399.
- Nasir K, McClelland RL, Blumenthal RS, et al. Coronary artery calcium in relation to initiation and continuation of cardiovascular preventive medications: the Multi-Ethnic Study of Atherosclerosis (MESA). Circ Cardiovasc Qual Outcomes 2010; 3:228–235.
- Taylor AJ, Bindeman J, Feuerstein I, et al. Community-based provision of statin and aspirin after the detection of coronary artery calcium within a community-based screening cohort. J Am Coll Cardiol 2008; 51:1337–1341.
- Orakzai RH, Nasir K, Orakzai SH, et al. Effect of patient visualization of coronary calcium by electron beam computed tomography on changes in beneficial lifestyle behaviors. Am J Cardiol 2008; 101:999–1002.
- Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol 2011; 57:1622–1632.
- Hackam DG, Shojania KG, Spence JD, et al. Influence of noninvasive cardiovascular imaging in primary prevention: systematic review and meta-analysis of randomized trials. Arch Intern Med 2011; 171:977–982.
- Villines TC, Hulten EA, Shaw LJ, et al; CONFIRM Registry Investigators. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography. J Am Coll Cardiol 2011; 58:2533–2540.
- Schenker MP, Dorbala S, Hong EC, et al. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation 2008; 117:1693–1700.
- Bybee KA, Lee J, Markiewicz R, et al. Diagnostic and clinical benefit of combined coronary calcium and perfusion assessment in patients undergoing PET/CT myocardial perfusion stress imaging. J Nucl Cardiol 2010; 17:188–196.
- Schmermund A, Baumgart D, Sack S, et al. Assessment of coronary calcification by electron-beam computed tomography in symptomatic patients with normal, abnormal or equivocal exercise stress test. Eur Heart J 2000; 21:1674–1682.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults. J Am Coll Cardiol 2010; 56:e50–e103.
- Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. J Am Coll Cardiol 2010; 56:1864–1894.
Although we still have no evidence from randomized trials that patients have better outcomes if we measure the calcification in their coronary arteries, a growing body of evidence shows that we can estimate risk more accurately than with a risk model score alone if we also score coronary artery calcification in asymptomatic patients, especially those at intermediate risk.
Current guidelines1 recommend using the Framingham Risk Score or a similar tool to estimate coronary risk in asymptomatic patients, but these tools have only modest accuracy. Calcification scoring is accurate, inexpensive, quick, widely available, low-risk, and does not appear to increase medical costs afterward. In addition to improving risk stratification, it may also encourage patients to adhere better to drug therapy and lifestyle modification.
HOW IS CORONARY ARTERY CALCIFICATION MEASURED?
Calcification of the coronary arteries is synonymous with atherosclerosis. It can easily be detected with computed tomography without contrast (Figure 1), and the amount can be quantified with a scoring system such as the volumetric score or the Agatston score. The latter, which is more commonly used, is based on the product of the area of the calcium deposits and the x-ray attenuation in Hounsfield units.
Scores can be roughly categorized (with some overlap owing to data from different studies) as:
- Low risk: 0 Agatston units (AU)
- Average risk: 1–112 AU
- Moderate risk: 100–400 AU
- High risk: 400–999 AU
- Very high risk: 1,000 AU.2
The actual test takes only a few seconds, and the patient can usually be out the door in 15 minutes or less. It does not require iodinated contrast and the radiation dose is minimal, usually less than 1 mSv, equivalent to fewer than 10 chest radiographs.3
The cost is typically between $200 and $500. The test is usually not covered by health insurance, but this differs by insurer and by state; for example, coverage is mandated in Texas, and the test is covered by United Healthcare.
WHAT IS THE EVIDENCE IN FAVOR OF CALCIFICATION SCORING?
Cohort studies with long-term follow-up show that calcification scoring has robust prognostic ability. A pooled analysis of several of these studies2 showed that a higher score strongly correlated with a higher risk of cardiac events over 3 to 5 years. Compared with the risk in people with a score of 0, the risk was twice as high in those with a score of 1 to 112, four times as high with a score of 100 to 400, seven times as high with a score of 400 to 499, and 10 times as high with a score greater than 1,000.2
A cohort study of more than 25,000 patients had similar conclusions about the magnitude of risk associated with coronary calcification.4 It also found that the 10-year risk of death was 0.6% in patients with a score of 0, 3.4% with a score of 101 to 399, 5.3% with a score of 400 to 699, 6.1% with a score of 700 to 999, and 12.2% with a score greater than 1,000.
Although progression of coronary artery calcification may predict the risk of death from any cause,5 the clinical utility of serial measurements is not yet apparent, especially since statin therapy—our front-line treatment for coronary disease—has not been shown to slow the progression of calcification.
Improving the accuracy of risk prediction
If a patient’s 10-year coronary risk is intermediate (10% to 20%), calcification scoring can reclassify the risk as low or high in about 50% of cases and can improve the accuracy of risk prediction.6–8
For example, Elias-Smale et al6 evaluated the effect of calcification scoring in 2,028 asymptomatic patients, with median follow-up of 9.2 years and 135 coronary events observed. Adding the calcification score to the Framingham model significantly improved risk classification, with a net reclassification improvement (NRI) of 0.14 (P < .01). (NRI is a measure of discriminatory performance for a diagnostic test; higher is better.9) Reclassification was most robust in those at intermediate risk, 52% of whom were reclassified, with 30% reclassified to low risk and 22% reclassified to high risk.
Erbel et al7 reported data from the Heinz Nixdorf Recall study, which used calcification scoring to estimate the NRI in 4,129 patients followed for 5 years. During this time there were 93 coronary deaths and non-fatal myocardial infarctions. The addition of the calcification score to the Framingham risk model resulted in an NRI of 0.21 (P = .0002) for patients with a risk of 6% to 20% and 0.31 (P < .0001) for those with a risk of 10% to 20%. Erbel et al also estimated the C statistic (area under the receiver operating characteristic curve; the maximum value is 1.0 and the higher the value the better) for the addition of the calcification score to the Framingham risk model and to the Adult Treatment Panel (ATP) III algorithm. They reported a significant increase of 0.681 to 0.749 with the Framingham model and 0.653 to 0.755 with the ATP III algorithm.
Polonsky et al8 studied a cohort of 5,878 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) and estimated the event risk using a model based on Framingham risk characteristics. When the calcification score was added to the prediction model, 26% of the sample was reclassified to a new risk category. In intermediate-risk patients, 292 (16%) were reclassified as high risk, and 712 (39%) were reclassified as low risk, achieving an NRI of 0.55 (95% confidence interval 0.41 to 0.69; P < .001). In addition, the C statistic for the prediction of cardiovascular events was 0.76 for the model based on Framingham risk characteristics and increased to 0.81 (P < .001) with the addition of calcification scoring.
Improving adherence and care
Knowing that a patient has a higher calcification score, physicians are more likely to prescribe lipid-lowering and antihypertensive drugs (Table 1),10–12 and patients with a higher score are also more often adherent to recommendations regarding diet and exercise.13
Rozanski et al,14 in a randomized controlled trial, showed that measuring coronary artery calcification did not increase downstream medical spending. A modest improvement in systolic blood pressure (P = .02), serum low-density lipoprotein level (P = .04), and waist circumference (P = .01) was observed in patients who had their calcification measured. Patients with the highest scores had the greatest improvement in coronary risk factors, including blood pressure, cholesterol, weight, and regular exercise.
On the other hand, other analyses have suggested that imaging tests are not effective for motivating behavioral changes. This topic deserves more research.15
Less utility in symptomatic disease
Coronary artery calcification scoring has less clinical utility in patients who already have coronary symptoms. Villines et al16 described a cohort of 10,037 patients with coronary symptoms who underwent calcification scoring and computed tomographic coronary angiography and found that stenosis of greater than 50% was present in 3.5% of those who had a score of 0 and in 29% of those with a score higher than 0. Therefore, a score of 0 does not rule out obstructive coronary heart disease if the patient has symptoms. Conversely, these patients may still have coronary artery calcification even if perfusion stress imaging is normal,17,18 and calcification scoring may have a role in the evaluation of equivocal stress tests.19
CALCIFICATION SCORING GUIDELINES
In their most recent (2010) joint guidelines for assessing risk of coronary heart disease in asymptomatic patients,20 the American College of Cardiology and the American Heart Association say coronary artery calcification scoring:
- Is recommended for asymptomatic patients at intermediate 10-year risk (10% to 20%) of coronary heart disease (class IIa recommendation, level of evidence B)
- May be acceptable for asymptomatic patients at low to intermediate risk (6% to 10%) (class IIb recommendation)
- Is discouraged for those at low risk (< 6%) (class III recommendation).
The most recent (2010) criteria for the appropriate use of cardiac computed tomography21 provide similar recommendations. Specifically, coronary artery calcification scoring with noncontrast computed tomography was rated as appropriate for patients at intermediate risk (10% to 20%) of coronary heart disease and for the specific subset of patients who are at low risk (6% to 10%) but who have a family history of premature coronary heart disease.
These recommendations are based on multiple lines of evidence that calcification scoring is a robust risk-predictor, can enhance risk estimates beyond traditional scoring strategies, and may—in theory—improve outcomes.
CALCIFICATION SCORING’S LIMITATIONS
The images used for measuring coronary calcification do predict risk of cardiovascular events, but they are not adequate to assess the severity of coronary stenosis. Further, calcification scoring often leads to incidental findings, which can cause anxiety and possibly lead to more imaging, entailing more radiation exposure and expense. And as noted, there are no randomized trial data demonstrating a reduction in cardiovascular events with the use of calcification scoring.
Although we still have no evidence from randomized trials that patients have better outcomes if we measure the calcification in their coronary arteries, a growing body of evidence shows that we can estimate risk more accurately than with a risk model score alone if we also score coronary artery calcification in asymptomatic patients, especially those at intermediate risk.
Current guidelines1 recommend using the Framingham Risk Score or a similar tool to estimate coronary risk in asymptomatic patients, but these tools have only modest accuracy. Calcification scoring is accurate, inexpensive, quick, widely available, low-risk, and does not appear to increase medical costs afterward. In addition to improving risk stratification, it may also encourage patients to adhere better to drug therapy and lifestyle modification.
HOW IS CORONARY ARTERY CALCIFICATION MEASURED?
Calcification of the coronary arteries is synonymous with atherosclerosis. It can easily be detected with computed tomography without contrast (Figure 1), and the amount can be quantified with a scoring system such as the volumetric score or the Agatston score. The latter, which is more commonly used, is based on the product of the area of the calcium deposits and the x-ray attenuation in Hounsfield units.
Scores can be roughly categorized (with some overlap owing to data from different studies) as:
- Low risk: 0 Agatston units (AU)
- Average risk: 1–112 AU
- Moderate risk: 100–400 AU
- High risk: 400–999 AU
- Very high risk: 1,000 AU.2
The actual test takes only a few seconds, and the patient can usually be out the door in 15 minutes or less. It does not require iodinated contrast and the radiation dose is minimal, usually less than 1 mSv, equivalent to fewer than 10 chest radiographs.3
The cost is typically between $200 and $500. The test is usually not covered by health insurance, but this differs by insurer and by state; for example, coverage is mandated in Texas, and the test is covered by United Healthcare.
WHAT IS THE EVIDENCE IN FAVOR OF CALCIFICATION SCORING?
Cohort studies with long-term follow-up show that calcification scoring has robust prognostic ability. A pooled analysis of several of these studies2 showed that a higher score strongly correlated with a higher risk of cardiac events over 3 to 5 years. Compared with the risk in people with a score of 0, the risk was twice as high in those with a score of 1 to 112, four times as high with a score of 100 to 400, seven times as high with a score of 400 to 499, and 10 times as high with a score greater than 1,000.2
A cohort study of more than 25,000 patients had similar conclusions about the magnitude of risk associated with coronary calcification.4 It also found that the 10-year risk of death was 0.6% in patients with a score of 0, 3.4% with a score of 101 to 399, 5.3% with a score of 400 to 699, 6.1% with a score of 700 to 999, and 12.2% with a score greater than 1,000.
Although progression of coronary artery calcification may predict the risk of death from any cause,5 the clinical utility of serial measurements is not yet apparent, especially since statin therapy—our front-line treatment for coronary disease—has not been shown to slow the progression of calcification.
Improving the accuracy of risk prediction
If a patient’s 10-year coronary risk is intermediate (10% to 20%), calcification scoring can reclassify the risk as low or high in about 50% of cases and can improve the accuracy of risk prediction.6–8
For example, Elias-Smale et al6 evaluated the effect of calcification scoring in 2,028 asymptomatic patients, with median follow-up of 9.2 years and 135 coronary events observed. Adding the calcification score to the Framingham model significantly improved risk classification, with a net reclassification improvement (NRI) of 0.14 (P < .01). (NRI is a measure of discriminatory performance for a diagnostic test; higher is better.9) Reclassification was most robust in those at intermediate risk, 52% of whom were reclassified, with 30% reclassified to low risk and 22% reclassified to high risk.
Erbel et al7 reported data from the Heinz Nixdorf Recall study, which used calcification scoring to estimate the NRI in 4,129 patients followed for 5 years. During this time there were 93 coronary deaths and non-fatal myocardial infarctions. The addition of the calcification score to the Framingham risk model resulted in an NRI of 0.21 (P = .0002) for patients with a risk of 6% to 20% and 0.31 (P < .0001) for those with a risk of 10% to 20%. Erbel et al also estimated the C statistic (area under the receiver operating characteristic curve; the maximum value is 1.0 and the higher the value the better) for the addition of the calcification score to the Framingham risk model and to the Adult Treatment Panel (ATP) III algorithm. They reported a significant increase of 0.681 to 0.749 with the Framingham model and 0.653 to 0.755 with the ATP III algorithm.
Polonsky et al8 studied a cohort of 5,878 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) and estimated the event risk using a model based on Framingham risk characteristics. When the calcification score was added to the prediction model, 26% of the sample was reclassified to a new risk category. In intermediate-risk patients, 292 (16%) were reclassified as high risk, and 712 (39%) were reclassified as low risk, achieving an NRI of 0.55 (95% confidence interval 0.41 to 0.69; P < .001). In addition, the C statistic for the prediction of cardiovascular events was 0.76 for the model based on Framingham risk characteristics and increased to 0.81 (P < .001) with the addition of calcification scoring.
Improving adherence and care
Knowing that a patient has a higher calcification score, physicians are more likely to prescribe lipid-lowering and antihypertensive drugs (Table 1),10–12 and patients with a higher score are also more often adherent to recommendations regarding diet and exercise.13
Rozanski et al,14 in a randomized controlled trial, showed that measuring coronary artery calcification did not increase downstream medical spending. A modest improvement in systolic blood pressure (P = .02), serum low-density lipoprotein level (P = .04), and waist circumference (P = .01) was observed in patients who had their calcification measured. Patients with the highest scores had the greatest improvement in coronary risk factors, including blood pressure, cholesterol, weight, and regular exercise.
On the other hand, other analyses have suggested that imaging tests are not effective for motivating behavioral changes. This topic deserves more research.15
Less utility in symptomatic disease
Coronary artery calcification scoring has less clinical utility in patients who already have coronary symptoms. Villines et al16 described a cohort of 10,037 patients with coronary symptoms who underwent calcification scoring and computed tomographic coronary angiography and found that stenosis of greater than 50% was present in 3.5% of those who had a score of 0 and in 29% of those with a score higher than 0. Therefore, a score of 0 does not rule out obstructive coronary heart disease if the patient has symptoms. Conversely, these patients may still have coronary artery calcification even if perfusion stress imaging is normal,17,18 and calcification scoring may have a role in the evaluation of equivocal stress tests.19
CALCIFICATION SCORING GUIDELINES
In their most recent (2010) joint guidelines for assessing risk of coronary heart disease in asymptomatic patients,20 the American College of Cardiology and the American Heart Association say coronary artery calcification scoring:
- Is recommended for asymptomatic patients at intermediate 10-year risk (10% to 20%) of coronary heart disease (class IIa recommendation, level of evidence B)
- May be acceptable for asymptomatic patients at low to intermediate risk (6% to 10%) (class IIb recommendation)
- Is discouraged for those at low risk (< 6%) (class III recommendation).
The most recent (2010) criteria for the appropriate use of cardiac computed tomography21 provide similar recommendations. Specifically, coronary artery calcification scoring with noncontrast computed tomography was rated as appropriate for patients at intermediate risk (10% to 20%) of coronary heart disease and for the specific subset of patients who are at low risk (6% to 10%) but who have a family history of premature coronary heart disease.
These recommendations are based on multiple lines of evidence that calcification scoring is a robust risk-predictor, can enhance risk estimates beyond traditional scoring strategies, and may—in theory—improve outcomes.
CALCIFICATION SCORING’S LIMITATIONS
The images used for measuring coronary calcification do predict risk of cardiovascular events, but they are not adequate to assess the severity of coronary stenosis. Further, calcification scoring often leads to incidental findings, which can cause anxiety and possibly lead to more imaging, entailing more radiation exposure and expense. And as noted, there are no randomized trial data demonstrating a reduction in cardiovascular events with the use of calcification scoring.
- Redberg RF, Benjamin EJ, Bittner V, et al. ACCF/AHA 2009 performance measures for primary prevention of cardiovascular disease in adults. J Am Coll Cardiol 2009; 54:1364–1405.
- Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain. J Am Coll Cardiol 2007; 49:378–402.
- Winchester DE, Wymer DC, Shifrin RY, Kraft SM, Hill JA. Responsible use of computed tomography in the evaluation of coronary artery disease and chest pain. Mayo Clin Proc 2010; 85:358–364.
- Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007; 49:1860–1870.
- Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010; 3:1229–1236.
- Elias-Smale SE, Proença RV, Koller MT, et al. Coronary calcium score improves classification of coronary heart disease risk in the elderly: The Rotterdam study. J Am Coll Cardiol 2010; 56:1407–1414.
- Erbel R, Möhlenkamp S, Moebus S, et al; Heinz Nixdorf Recall Study Investigative Group. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010; 56:1397–1406.
- Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 2010; 303:1610–1616.
- Pencina MJ, Agostino RB, Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statist Med 2008; 27:157–172.
- Kalia NK, Miller LG, Nasir K, Blumenthal RS, Agrawal N, Budoff MJ. Visualizing coronary calcium is associated with improvements in adherence to statin therapy. Atherosclerosis 2006; 185:394–399.
- Nasir K, McClelland RL, Blumenthal RS, et al. Coronary artery calcium in relation to initiation and continuation of cardiovascular preventive medications: the Multi-Ethnic Study of Atherosclerosis (MESA). Circ Cardiovasc Qual Outcomes 2010; 3:228–235.
- Taylor AJ, Bindeman J, Feuerstein I, et al. Community-based provision of statin and aspirin after the detection of coronary artery calcium within a community-based screening cohort. J Am Coll Cardiol 2008; 51:1337–1341.
- Orakzai RH, Nasir K, Orakzai SH, et al. Effect of patient visualization of coronary calcium by electron beam computed tomography on changes in beneficial lifestyle behaviors. Am J Cardiol 2008; 101:999–1002.
- Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol 2011; 57:1622–1632.
- Hackam DG, Shojania KG, Spence JD, et al. Influence of noninvasive cardiovascular imaging in primary prevention: systematic review and meta-analysis of randomized trials. Arch Intern Med 2011; 171:977–982.
- Villines TC, Hulten EA, Shaw LJ, et al; CONFIRM Registry Investigators. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography. J Am Coll Cardiol 2011; 58:2533–2540.
- Schenker MP, Dorbala S, Hong EC, et al. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation 2008; 117:1693–1700.
- Bybee KA, Lee J, Markiewicz R, et al. Diagnostic and clinical benefit of combined coronary calcium and perfusion assessment in patients undergoing PET/CT myocardial perfusion stress imaging. J Nucl Cardiol 2010; 17:188–196.
- Schmermund A, Baumgart D, Sack S, et al. Assessment of coronary calcification by electron-beam computed tomography in symptomatic patients with normal, abnormal or equivocal exercise stress test. Eur Heart J 2000; 21:1674–1682.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults. J Am Coll Cardiol 2010; 56:e50–e103.
- Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. J Am Coll Cardiol 2010; 56:1864–1894.
- Redberg RF, Benjamin EJ, Bittner V, et al. ACCF/AHA 2009 performance measures for primary prevention of cardiovascular disease in adults. J Am Coll Cardiol 2009; 54:1364–1405.
- Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain. J Am Coll Cardiol 2007; 49:378–402.
- Winchester DE, Wymer DC, Shifrin RY, Kraft SM, Hill JA. Responsible use of computed tomography in the evaluation of coronary artery disease and chest pain. Mayo Clin Proc 2010; 85:358–364.
- Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007; 49:1860–1870.
- Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010; 3:1229–1236.
- Elias-Smale SE, Proença RV, Koller MT, et al. Coronary calcium score improves classification of coronary heart disease risk in the elderly: The Rotterdam study. J Am Coll Cardiol 2010; 56:1407–1414.
- Erbel R, Möhlenkamp S, Moebus S, et al; Heinz Nixdorf Recall Study Investigative Group. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010; 56:1397–1406.
- Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 2010; 303:1610–1616.
- Pencina MJ, Agostino RB, Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statist Med 2008; 27:157–172.
- Kalia NK, Miller LG, Nasir K, Blumenthal RS, Agrawal N, Budoff MJ. Visualizing coronary calcium is associated with improvements in adherence to statin therapy. Atherosclerosis 2006; 185:394–399.
- Nasir K, McClelland RL, Blumenthal RS, et al. Coronary artery calcium in relation to initiation and continuation of cardiovascular preventive medications: the Multi-Ethnic Study of Atherosclerosis (MESA). Circ Cardiovasc Qual Outcomes 2010; 3:228–235.
- Taylor AJ, Bindeman J, Feuerstein I, et al. Community-based provision of statin and aspirin after the detection of coronary artery calcium within a community-based screening cohort. J Am Coll Cardiol 2008; 51:1337–1341.
- Orakzai RH, Nasir K, Orakzai SH, et al. Effect of patient visualization of coronary calcium by electron beam computed tomography on changes in beneficial lifestyle behaviors. Am J Cardiol 2008; 101:999–1002.
- Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol 2011; 57:1622–1632.
- Hackam DG, Shojania KG, Spence JD, et al. Influence of noninvasive cardiovascular imaging in primary prevention: systematic review and meta-analysis of randomized trials. Arch Intern Med 2011; 171:977–982.
- Villines TC, Hulten EA, Shaw LJ, et al; CONFIRM Registry Investigators. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography. J Am Coll Cardiol 2011; 58:2533–2540.
- Schenker MP, Dorbala S, Hong EC, et al. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation 2008; 117:1693–1700.
- Bybee KA, Lee J, Markiewicz R, et al. Diagnostic and clinical benefit of combined coronary calcium and perfusion assessment in patients undergoing PET/CT myocardial perfusion stress imaging. J Nucl Cardiol 2010; 17:188–196.
- Schmermund A, Baumgart D, Sack S, et al. Assessment of coronary calcification by electron-beam computed tomography in symptomatic patients with normal, abnormal or equivocal exercise stress test. Eur Heart J 2000; 21:1674–1682.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults. J Am Coll Cardiol 2010; 56:e50–e103.
- Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. J Am Coll Cardiol 2010; 56:1864–1894.
Does coronary artery calcification scoring still have a role in practice?
To try to identify and treat people who are at highest risk of cardiovascular events, including death, we use comprehensive risk-prediction models. Unfortunately, these models have limited accuracy and precision and do not predict very well.
Attractive, then, is the idea of using a noninvasive imaging test to measure coronary atherosclerosis before it causes trouble and thereby individualize the risk assessment. Noncontrast computed tomography (CT) can measure the amount of calcification in the coronary arteries, and therefore it can estimate the coronary atherosclerotic burden. It seems like an ideal test, and calcification as a marker of subclinical atherosclerosis has been extensively investigated.
However, despite more than 2 decades of use and data from hundreds of thousands of patients, the test remains poorly understood. Many physicians seem to use it solely as a means of placating “worried well” patients and do not truly appreciate its implications. Others proceed to ordering CT angiography, a more expensive test that involves the added risks of using higher x-ray doses and iodinated contrast, even when a correctly interpreted calcification score would provide ample information.
In this issue of the Cleveland Clinic Journal of Medicine, Chauffe and Winchester review the utility of coronary artery calcification scoring in current practice. We wish to supplement their review by suggesting some considerations to take into account before ordering this test:
- Does the patient have symptoms of coronary artery disease, and what is his or her risk-factor profile? Baseline patient characteristics are important to consider if we are to use this test appropriately.
- How should the result be interpreted, and does the ordering physician have the confidence to accept the result?
BEST USED IN ASYMPTOMATIC PATIENTS AT INTERMEDIATE RISK
Many large retrospective and prospective registries have demonstrated the predictive value of coronary artery calcification in diverse cohorts of patients without symptoms.
In three prospective registries—the Multi-Ethnic Study of Atherosclerosis1 (MESA) with 6,722 patients, the Coronary CT Angiography Evaluation for Clinical Outcomes2 (CONFIRM) with 7,590 patients, and the Heinz Nixdorf Recall (NHR) study3 with 4,129 patients—most of the patients who had heart attacks had a calcification score greater than 100. And conversely, data from more than 100,000 people show that the absence of calcification (ie, a score of 0) denotes a very low risk (< 1% over 5 years).1–6
The pretest probability of coronary artery disease needs to be considered. The data clearly indicate that a Bayesian approach is warranted and that coronary artery calcification scoring should mainly be done in patients at intermediate or low-intermediate risk. Trials have shown that calcification scoring will reclassify more than 50% of intermediate-risk patients into the high-risk or low-risk category.3
The implications of these findings were eloquently assessed in the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER). In this trial, it was estimated that for patients with no calcification who would otherwise fulfill the criteria for treatment with a statin, 549 patients would need to be treated to prevent one coronary event, compared with 24 similar patients with a calcification score greater than 100.7
Although such analyses have potential shortcomings, in this era of greater concern about how to allocate finite resources, using a simple, inexpensive test to individualize long-term treatment is an attractive idea. Further, measuring calcification does not appear to increase testing “downstream” and indeed reduces it as compared with no calcification scoring. It also results in better adherence to drug therapy and lifestyle changes.
Because calcification scoring provides additional prognostic data and accurately discriminates and reclassifies risk, the American College of Cardiology and the American Heart Association have awarded it a class IIa recommendation for asymptomatic patients at intermediate risk, meaning that there is conflicting evidence or a divergence of opinion about its usefulness, but the weight of evidence or opinion favors it.8
ITS ROLE IS MORE CONTROVERSIAL IN SYMPTOMATIC PATIENTS
Perhaps a less established and more controversial use of coronary artery calcification scoring is in patients who are having coronary symptoms. In patients at high cardiovascular risk, this test by itself may miss an unacceptable number of those who truly have significant stenoses.9 However, when the appropriate population is selected, there is substantial evidence that it can be an important means of risk stratification.
In patients at low to intermediate risk, the absence of calcification indicates a very low likelihood of significant coronary artery stenosis, as demonstrated in the Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter (CONFIRM) registry.10 In the 10,037 symptomatic patients evaluated, a score of 0 had a 99% negative predictive value for excluding stenosis greater than 70% and was associated with a 2-year event rate less than 1%. These data were supported by a meta-analysis of nearly 1,000 symptomatic patients with a score of 0, in whom the 2-year event rate was less than 2%.4
Taken together, these data suggest that the absence of coronary calcification in people at low to intermediate risk indicates a very low likelihood of significant stenotic coronary artery disease and foretells an excellent prognosis.
These data have already been incorporated into the British National Institute for Health and Clinical Excellence (NICE) guidelines, in which calcification scoring is an integral part of the management algorithm in patients with chest pain who are at low risk.
WHY NOT JUST DO CT ANGIOGRAPHY?
But why bother with coronary artery calcification scoring when we can do CT angiography instead? The angiography scanners we have today can cover the entire heart in a single gantry rotation. Dual-source scanners provide temporal resolution as low as 75 ms, and sequential, prospective electrocardiographic gating and iterative reconstruction can routinely achieve scans with doses of radiation as low as 3 mSv that provide coronary artery images of exquisite quality.
On the other hand, calcification scoring is fast and easy to perform and poses less potential harm to the patient, since it uses lower doses of radiation and no contrast agents. In addition, the quantification is semi-automated, so the results can be interpreted quickly and are reproducible.
In the CONFIRM trial, prediction by CT angiography was no better than calcification scoring in asymptomatic patients, so it is not recommended in this population.2 In symptomatic patients, the CONFIRM trial data suggest that almost 1,000 additional CT angiography procedures would need to be done to identify one myocardial infarction and more than 1,500 procedures to identify one patient at risk of death missed by calcification scoring of 0 in patients at low to intermediate risk.11
Chauffe and Winchester nicely summarize the limitations of calcification scoring. However, we would emphasize the potential implications of the above findings. Appropriately utilized, calcification scoring is safe, reproducible, and inexpensive and helps individualize treatment in asymptomatic patients at low to intermediate risk, thereby avoiding under- and overtreatment and potentially reducing downstream costs while improving compliance.
In patients at low to intermediate risk who present with chest pain, documenting the absence of calcification can rationalize downstream testing and reliably, quickly, and safely permit patient discharge from emergency departments. In a time of increasing costs and patient demands and finite resources, clinicians should remain cognizant of the usefulness of evaluating coronary artery calcification.
- Budoff MJ, McClelland RL, Nasir K, et al. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 2009; 158:554–561.
- Cho I, Chang HJ, Sung JM, et al; CONFIRM Investigators. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry. Circulation 2012; 126:304–313.
- Erbel R, Möhlenkamp S, Moebus S, et al; Heinz Nixdorf Recall Study Investigative Group. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010; 56:1397–1406.
- Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2009; 2:675–688.
- Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging 2009; 2:692–700.
- Graham G, Blaha MJ, Budoff MJ, et al. Impact of coronary artery calcification on all-cause mortality in individuals with and without hypertension. Atherosclerosis 2012; 225:432–437.
- Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet 2011; 378:684–692.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults. J Am Coll Cardiol 2010; 56:e50–e103.
- Gottlieb I, Miller JM, Arbab-Zadeh A, et al. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol 2010; 55:627–634.
- Villines TC, Hulten EA, Shaw LJ, et al; CONFIRM Registry Investigators. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM registry. J Am Coll Cardiol 2011; 58:2533–2540.
- Joshi PH, Blaha MJ, Blumenthal RS, Blankstein R, Nasir K. What is the role of calcium scoring in the age of coronary computed tomographic angiography? J Nucl Cardiol 2012; 19:1226–1235.
To try to identify and treat people who are at highest risk of cardiovascular events, including death, we use comprehensive risk-prediction models. Unfortunately, these models have limited accuracy and precision and do not predict very well.
Attractive, then, is the idea of using a noninvasive imaging test to measure coronary atherosclerosis before it causes trouble and thereby individualize the risk assessment. Noncontrast computed tomography (CT) can measure the amount of calcification in the coronary arteries, and therefore it can estimate the coronary atherosclerotic burden. It seems like an ideal test, and calcification as a marker of subclinical atherosclerosis has been extensively investigated.
However, despite more than 2 decades of use and data from hundreds of thousands of patients, the test remains poorly understood. Many physicians seem to use it solely as a means of placating “worried well” patients and do not truly appreciate its implications. Others proceed to ordering CT angiography, a more expensive test that involves the added risks of using higher x-ray doses and iodinated contrast, even when a correctly interpreted calcification score would provide ample information.
In this issue of the Cleveland Clinic Journal of Medicine, Chauffe and Winchester review the utility of coronary artery calcification scoring in current practice. We wish to supplement their review by suggesting some considerations to take into account before ordering this test:
- Does the patient have symptoms of coronary artery disease, and what is his or her risk-factor profile? Baseline patient characteristics are important to consider if we are to use this test appropriately.
- How should the result be interpreted, and does the ordering physician have the confidence to accept the result?
BEST USED IN ASYMPTOMATIC PATIENTS AT INTERMEDIATE RISK
Many large retrospective and prospective registries have demonstrated the predictive value of coronary artery calcification in diverse cohorts of patients without symptoms.
In three prospective registries—the Multi-Ethnic Study of Atherosclerosis1 (MESA) with 6,722 patients, the Coronary CT Angiography Evaluation for Clinical Outcomes2 (CONFIRM) with 7,590 patients, and the Heinz Nixdorf Recall (NHR) study3 with 4,129 patients—most of the patients who had heart attacks had a calcification score greater than 100. And conversely, data from more than 100,000 people show that the absence of calcification (ie, a score of 0) denotes a very low risk (< 1% over 5 years).1–6
The pretest probability of coronary artery disease needs to be considered. The data clearly indicate that a Bayesian approach is warranted and that coronary artery calcification scoring should mainly be done in patients at intermediate or low-intermediate risk. Trials have shown that calcification scoring will reclassify more than 50% of intermediate-risk patients into the high-risk or low-risk category.3
The implications of these findings were eloquently assessed in the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER). In this trial, it was estimated that for patients with no calcification who would otherwise fulfill the criteria for treatment with a statin, 549 patients would need to be treated to prevent one coronary event, compared with 24 similar patients with a calcification score greater than 100.7
Although such analyses have potential shortcomings, in this era of greater concern about how to allocate finite resources, using a simple, inexpensive test to individualize long-term treatment is an attractive idea. Further, measuring calcification does not appear to increase testing “downstream” and indeed reduces it as compared with no calcification scoring. It also results in better adherence to drug therapy and lifestyle changes.
Because calcification scoring provides additional prognostic data and accurately discriminates and reclassifies risk, the American College of Cardiology and the American Heart Association have awarded it a class IIa recommendation for asymptomatic patients at intermediate risk, meaning that there is conflicting evidence or a divergence of opinion about its usefulness, but the weight of evidence or opinion favors it.8
ITS ROLE IS MORE CONTROVERSIAL IN SYMPTOMATIC PATIENTS
Perhaps a less established and more controversial use of coronary artery calcification scoring is in patients who are having coronary symptoms. In patients at high cardiovascular risk, this test by itself may miss an unacceptable number of those who truly have significant stenoses.9 However, when the appropriate population is selected, there is substantial evidence that it can be an important means of risk stratification.
In patients at low to intermediate risk, the absence of calcification indicates a very low likelihood of significant coronary artery stenosis, as demonstrated in the Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter (CONFIRM) registry.10 In the 10,037 symptomatic patients evaluated, a score of 0 had a 99% negative predictive value for excluding stenosis greater than 70% and was associated with a 2-year event rate less than 1%. These data were supported by a meta-analysis of nearly 1,000 symptomatic patients with a score of 0, in whom the 2-year event rate was less than 2%.4
Taken together, these data suggest that the absence of coronary calcification in people at low to intermediate risk indicates a very low likelihood of significant stenotic coronary artery disease and foretells an excellent prognosis.
These data have already been incorporated into the British National Institute for Health and Clinical Excellence (NICE) guidelines, in which calcification scoring is an integral part of the management algorithm in patients with chest pain who are at low risk.
WHY NOT JUST DO CT ANGIOGRAPHY?
But why bother with coronary artery calcification scoring when we can do CT angiography instead? The angiography scanners we have today can cover the entire heart in a single gantry rotation. Dual-source scanners provide temporal resolution as low as 75 ms, and sequential, prospective electrocardiographic gating and iterative reconstruction can routinely achieve scans with doses of radiation as low as 3 mSv that provide coronary artery images of exquisite quality.
On the other hand, calcification scoring is fast and easy to perform and poses less potential harm to the patient, since it uses lower doses of radiation and no contrast agents. In addition, the quantification is semi-automated, so the results can be interpreted quickly and are reproducible.
In the CONFIRM trial, prediction by CT angiography was no better than calcification scoring in asymptomatic patients, so it is not recommended in this population.2 In symptomatic patients, the CONFIRM trial data suggest that almost 1,000 additional CT angiography procedures would need to be done to identify one myocardial infarction and more than 1,500 procedures to identify one patient at risk of death missed by calcification scoring of 0 in patients at low to intermediate risk.11
Chauffe and Winchester nicely summarize the limitations of calcification scoring. However, we would emphasize the potential implications of the above findings. Appropriately utilized, calcification scoring is safe, reproducible, and inexpensive and helps individualize treatment in asymptomatic patients at low to intermediate risk, thereby avoiding under- and overtreatment and potentially reducing downstream costs while improving compliance.
In patients at low to intermediate risk who present with chest pain, documenting the absence of calcification can rationalize downstream testing and reliably, quickly, and safely permit patient discharge from emergency departments. In a time of increasing costs and patient demands and finite resources, clinicians should remain cognizant of the usefulness of evaluating coronary artery calcification.
To try to identify and treat people who are at highest risk of cardiovascular events, including death, we use comprehensive risk-prediction models. Unfortunately, these models have limited accuracy and precision and do not predict very well.
Attractive, then, is the idea of using a noninvasive imaging test to measure coronary atherosclerosis before it causes trouble and thereby individualize the risk assessment. Noncontrast computed tomography (CT) can measure the amount of calcification in the coronary arteries, and therefore it can estimate the coronary atherosclerotic burden. It seems like an ideal test, and calcification as a marker of subclinical atherosclerosis has been extensively investigated.
However, despite more than 2 decades of use and data from hundreds of thousands of patients, the test remains poorly understood. Many physicians seem to use it solely as a means of placating “worried well” patients and do not truly appreciate its implications. Others proceed to ordering CT angiography, a more expensive test that involves the added risks of using higher x-ray doses and iodinated contrast, even when a correctly interpreted calcification score would provide ample information.
In this issue of the Cleveland Clinic Journal of Medicine, Chauffe and Winchester review the utility of coronary artery calcification scoring in current practice. We wish to supplement their review by suggesting some considerations to take into account before ordering this test:
- Does the patient have symptoms of coronary artery disease, and what is his or her risk-factor profile? Baseline patient characteristics are important to consider if we are to use this test appropriately.
- How should the result be interpreted, and does the ordering physician have the confidence to accept the result?
BEST USED IN ASYMPTOMATIC PATIENTS AT INTERMEDIATE RISK
Many large retrospective and prospective registries have demonstrated the predictive value of coronary artery calcification in diverse cohorts of patients without symptoms.
In three prospective registries—the Multi-Ethnic Study of Atherosclerosis1 (MESA) with 6,722 patients, the Coronary CT Angiography Evaluation for Clinical Outcomes2 (CONFIRM) with 7,590 patients, and the Heinz Nixdorf Recall (NHR) study3 with 4,129 patients—most of the patients who had heart attacks had a calcification score greater than 100. And conversely, data from more than 100,000 people show that the absence of calcification (ie, a score of 0) denotes a very low risk (< 1% over 5 years).1–6
The pretest probability of coronary artery disease needs to be considered. The data clearly indicate that a Bayesian approach is warranted and that coronary artery calcification scoring should mainly be done in patients at intermediate or low-intermediate risk. Trials have shown that calcification scoring will reclassify more than 50% of intermediate-risk patients into the high-risk or low-risk category.3
The implications of these findings were eloquently assessed in the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER). In this trial, it was estimated that for patients with no calcification who would otherwise fulfill the criteria for treatment with a statin, 549 patients would need to be treated to prevent one coronary event, compared with 24 similar patients with a calcification score greater than 100.7
Although such analyses have potential shortcomings, in this era of greater concern about how to allocate finite resources, using a simple, inexpensive test to individualize long-term treatment is an attractive idea. Further, measuring calcification does not appear to increase testing “downstream” and indeed reduces it as compared with no calcification scoring. It also results in better adherence to drug therapy and lifestyle changes.
Because calcification scoring provides additional prognostic data and accurately discriminates and reclassifies risk, the American College of Cardiology and the American Heart Association have awarded it a class IIa recommendation for asymptomatic patients at intermediate risk, meaning that there is conflicting evidence or a divergence of opinion about its usefulness, but the weight of evidence or opinion favors it.8
ITS ROLE IS MORE CONTROVERSIAL IN SYMPTOMATIC PATIENTS
Perhaps a less established and more controversial use of coronary artery calcification scoring is in patients who are having coronary symptoms. In patients at high cardiovascular risk, this test by itself may miss an unacceptable number of those who truly have significant stenoses.9 However, when the appropriate population is selected, there is substantial evidence that it can be an important means of risk stratification.
In patients at low to intermediate risk, the absence of calcification indicates a very low likelihood of significant coronary artery stenosis, as demonstrated in the Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter (CONFIRM) registry.10 In the 10,037 symptomatic patients evaluated, a score of 0 had a 99% negative predictive value for excluding stenosis greater than 70% and was associated with a 2-year event rate less than 1%. These data were supported by a meta-analysis of nearly 1,000 symptomatic patients with a score of 0, in whom the 2-year event rate was less than 2%.4
Taken together, these data suggest that the absence of coronary calcification in people at low to intermediate risk indicates a very low likelihood of significant stenotic coronary artery disease and foretells an excellent prognosis.
These data have already been incorporated into the British National Institute for Health and Clinical Excellence (NICE) guidelines, in which calcification scoring is an integral part of the management algorithm in patients with chest pain who are at low risk.
WHY NOT JUST DO CT ANGIOGRAPHY?
But why bother with coronary artery calcification scoring when we can do CT angiography instead? The angiography scanners we have today can cover the entire heart in a single gantry rotation. Dual-source scanners provide temporal resolution as low as 75 ms, and sequential, prospective electrocardiographic gating and iterative reconstruction can routinely achieve scans with doses of radiation as low as 3 mSv that provide coronary artery images of exquisite quality.
On the other hand, calcification scoring is fast and easy to perform and poses less potential harm to the patient, since it uses lower doses of radiation and no contrast agents. In addition, the quantification is semi-automated, so the results can be interpreted quickly and are reproducible.
In the CONFIRM trial, prediction by CT angiography was no better than calcification scoring in asymptomatic patients, so it is not recommended in this population.2 In symptomatic patients, the CONFIRM trial data suggest that almost 1,000 additional CT angiography procedures would need to be done to identify one myocardial infarction and more than 1,500 procedures to identify one patient at risk of death missed by calcification scoring of 0 in patients at low to intermediate risk.11
Chauffe and Winchester nicely summarize the limitations of calcification scoring. However, we would emphasize the potential implications of the above findings. Appropriately utilized, calcification scoring is safe, reproducible, and inexpensive and helps individualize treatment in asymptomatic patients at low to intermediate risk, thereby avoiding under- and overtreatment and potentially reducing downstream costs while improving compliance.
In patients at low to intermediate risk who present with chest pain, documenting the absence of calcification can rationalize downstream testing and reliably, quickly, and safely permit patient discharge from emergency departments. In a time of increasing costs and patient demands and finite resources, clinicians should remain cognizant of the usefulness of evaluating coronary artery calcification.
- Budoff MJ, McClelland RL, Nasir K, et al. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 2009; 158:554–561.
- Cho I, Chang HJ, Sung JM, et al; CONFIRM Investigators. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry. Circulation 2012; 126:304–313.
- Erbel R, Möhlenkamp S, Moebus S, et al; Heinz Nixdorf Recall Study Investigative Group. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010; 56:1397–1406.
- Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2009; 2:675–688.
- Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging 2009; 2:692–700.
- Graham G, Blaha MJ, Budoff MJ, et al. Impact of coronary artery calcification on all-cause mortality in individuals with and without hypertension. Atherosclerosis 2012; 225:432–437.
- Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet 2011; 378:684–692.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults. J Am Coll Cardiol 2010; 56:e50–e103.
- Gottlieb I, Miller JM, Arbab-Zadeh A, et al. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol 2010; 55:627–634.
- Villines TC, Hulten EA, Shaw LJ, et al; CONFIRM Registry Investigators. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM registry. J Am Coll Cardiol 2011; 58:2533–2540.
- Joshi PH, Blaha MJ, Blumenthal RS, Blankstein R, Nasir K. What is the role of calcium scoring in the age of coronary computed tomographic angiography? J Nucl Cardiol 2012; 19:1226–1235.
- Budoff MJ, McClelland RL, Nasir K, et al. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 2009; 158:554–561.
- Cho I, Chang HJ, Sung JM, et al; CONFIRM Investigators. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry. Circulation 2012; 126:304–313.
- Erbel R, Möhlenkamp S, Moebus S, et al; Heinz Nixdorf Recall Study Investigative Group. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010; 56:1397–1406.
- Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2009; 2:675–688.
- Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging 2009; 2:692–700.
- Graham G, Blaha MJ, Budoff MJ, et al. Impact of coronary artery calcification on all-cause mortality in individuals with and without hypertension. Atherosclerosis 2012; 225:432–437.
- Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet 2011; 378:684–692.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults. J Am Coll Cardiol 2010; 56:e50–e103.
- Gottlieb I, Miller JM, Arbab-Zadeh A, et al. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol 2010; 55:627–634.
- Villines TC, Hulten EA, Shaw LJ, et al; CONFIRM Registry Investigators. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM registry. J Am Coll Cardiol 2011; 58:2533–2540.
- Joshi PH, Blaha MJ, Blumenthal RS, Blankstein R, Nasir K. What is the role of calcium scoring in the age of coronary computed tomographic angiography? J Nucl Cardiol 2012; 19:1226–1235.
A 74-year-old man with abdominal pain
A 74-year-old man presented to the emergency department in December 2011 with a 1-week history of worsening abdominal pain, nausea with emesis, and decreased appetite. The pain was dull, diffuse, and not related to oral intake or bowel movements. He denied any bloody stools, melena, or hematemesis, but he had not had a bowel movement in the past week.
He was already known to have stage IV colon cancer with metastases to the lungs and liver. He had undergone a partial colectomy in 2009 and was receiving chemotherapy at the time of admission.
He also had an infrarenal abdominal aortic aneurysm that had been repaired in 2003 with endovascular placement of a Gore Excluder stent graft. This was complicated by a type II endoleak, treated with coil embolization. The same endoleak later recurred and was treated with injection of Onyx liquid embolic agent.
His medical history also included hypertension, type 2 diabetes mellitus, and hyperlipidemia. He had undergone a laparoscopic cholecystectomy in 2007.
He denied any fevers, chills, headache, lightheadedness, or change in vision. He had no respiratory, cardiac, or urinary symptoms. He had been constipated for the past few weeks and had recently been started on a bowel regimen, with mild relief. There had been no other changes to his medications.
His temperature on presentation was 97.5°F (36.4°C), blood pressure 120/64 mm Hg, pulse 96, respiratory rate 22, and oxygen saturation 95% on room air. He was awake, alert, oriented, and in no acute distress. His mucous membranes were dry. His lungs were clear to auscultation, and his heart sounds were normal. His bowel sounds were hyperactive and his abdomen was slightly tender diffusely, but there was no abdominal distention, rebound tenderness, guarding, or palpable masses. His joints, muscle strength, and muscle tone were normal. Table 1 shows his initial laboratory values.
Given the patient’s history of colon cancer, the emergency department physician ordered computed tomography (CT) of the abdomen to assess the state of his disease and to evaluate for bowel obstruction. The scan revealed a large abdominal aortic aneurysm with foci of gas within the aneurysmal sac. Metastases in the liver, lung, and retroperitoneum appeared stable; abundant colonic stool suggested constipation (Figure 1).
CAUSES OF PERIAORTIC GAS AFTER ANEURYSM REPAIR
1. What is the most common cause of periaortic ectopic gas in a patient with a repaired abdominal aortic aneurysm?
- Endoleak
- Stent graft infection
- Retroperitoneal fibrosis
- Aortoenteric fistula
Endoleak
Endoleak, a complication of endovascular abdominal aortic aneurysm repair, is defined as blood flow within the aneurysm sac but outside the endoluminal graft.1 It occurs in up to 15% of patients after endograft placement in the first month alone, and in up to 47% of patients eventually.2 It can lead to aneurysm enlargement and rupture. Endoleaks are classified into five types, each with different causes and management options.3,4 Contrast-enhanced CT is the most commonly used diagnostic tool.5
Endoleak cannot be ruled out in our patient, since CT was done without contrast. However, gas within the aneurysm is not consistent with this diagnosis.
Stent graft infection
Infection has been reported in 1% to 6% of patients receiving a stent graft for aortic aneurysm.6 They occur most commonly in the first year after placement; one study showed that 42% of patients diagnosed with graft infection presented within 3 months of endovascular repair.7
The leading cause of graft infection is contamination during the original procedure, but secondary infection from hematologic seeding and contamination from adjacent bowel are also possible.8 In our patient, who underwent graft placement followed by endovascular repairs of endoleaks, bacterial seeding of his aortic aneurysm from the procedures should be considered.9
The most common organisms are staphylococcal species, with Staphylococcus aureus more common in early infection and coagulase-negative staphylococci more common in late infection.10 Methicillin-resistant S aureus has been reported in as many as 25% of cases of graft infection. Diphtheroids and gram-negative enteric organisms should also be considered.11
CT is the most effective imaging test for graft infection. Perigraft soft tissue, fluid, and gas are the major CT findings.12
Given that our patient presented with abdominal pain, leukocytosis, and the CT finding of perigraft gas, graft infection should be high on our list differential diagnoses.
Retroperitoneal fibrosis
Retroperitoneal fibrosis is most often idiopathic, although many believe it is due to an exaggerated local inflammatory reaction to aortic atherosclerosis or is a manifestation of a systemic autoimmune disease.13 Secondary retroperitoneal fibrosis may be due to drugs, infection, or malignancy.
Pathologic findings include sclerotic plaques, typically around the abdominal vessels and ureters. Clinical presentations are often nonspecific, with early symptoms that include back or abdominal pain, malaise, anorexia, edema, and hematuria.14,15 Progressive ureteral obstruction can occur in later stages. CT with contrast is the imaging test of choice to visualize the extent of disease, with the fibrosis exhibiting attenuation similar to that of muscle.16
Initial treatment of idiopathic retroperitoneal fibrosis is with a glucocorticoid or other immunosuppressive agent, whereas treatment of secondary retroperitoneal fibrosis is aimed at the underlying cause.17 Late stages complicated by ureteral obstruction typically require surgery.18
Our patient did have some nonspecific complaints that could be due to retroperitoneal fibrosis. He also had an intra-abdominal malignancy, which could lead to secondary retroperitoneal fibrosis. However, his CT findings of periaortic gas are not consistent with this diagnosis.19
Aortoenteric fistula
Aortoenteric fistulas can be either primary or secondary.
Primary aortoenteric fistulas occur de novo in patients who have never undergone any surgery or procedure in the aorta. This type of fistula usually results from pressure erosion of an atherosclerotic abdominal aortic aneurysm into the gastrointestinal tract. They are rare, with an annual incidence of 0.04% to 0.07% in the general population.20,21
Secondary aortoenteric fistulas are complications of aortic reconstructive therapy. After open repair, a perianastomotic or pseudoaneurysmal fistula can develop into the gastrointestinal tract.4 Endovascular repair leaves the aortic wall intact with no exposed suture lines, but an aortoenteric fistula can still develop22 and in fact occur in 0.4% to 3.1% of recipients of stent grafts for abdominal aortic aneurysm repair.23 In such cases, it is commonly thought that graft infection can lead to formation of an aortoenteric fistula, but a penetrating gastrointestinal ulcer, tumor invasion, radiation therapy, and trauma have also been implicated.19,24–26 An aortoenteric fistula can present several months to several years after either open or endovascular abdominal aortic aneurysm repair.4,23
One of the main CT signs of an aortoenteric fistula is periaortic ectopic gas at least 3 to 4 weeks after surgery or endovascular repair.19 Gas around the stent graft is most commonly caused by infection, but an aortoenteric fistula must also be considered in our patient, as roughly one-third of graft infections present as aortoenteric fistula.27 Our patient denied having any gastrointestinal bleeding, but his hemoglobin concentration at presentation was 8.9 g/dL.
Highlight point. Perigraft gas after abdominal aortic aneurysm repair can be seen in graft infection and aortoenteric fistula.
SIGNS AND SYMPTOMS OF AORTOENTERIC FISTULA
2. What is the most common clinical sign or symptom of an aortoenteric fistula?
- Gastrointestinal bleeding
- Sepsis
- Abdominal pain
- Back pain
Gastrointestinal bleeding occurs in 80% of patients who have an aortoenteric fistula, sepsis in 40%, abdominal pain in 30%, and back pain in 15%.19 The classic triad of symptoms is gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass. However, symptoms can vary widely, and the classic triad is present in fewer than 25% of cases.28 Sepsis may be the predominant clinical manifestation, particularly in the early stages of fistula formation. Unexplained fever is an underrecognized early manifestation.24
Highlight point. The classic triad of symptoms of an aortoenteric fistula (gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass) is seen in fewer than 25% of cases.
Case continued: The patient develops frank bleeding
The vascular surgery service was consulted because of concern for an aortic graft infection, since surgical removal of the infected material is recommended.10 The patient was deemed to be a poor surgical candidate, given his stage IV colon cancer, so he was treated conservatively with broad-spectrum antibiotics.
Over the next 2 days, he had two episodes of dark, bloody bowel movements, but he remained hemodynamically stable. He subsequently developed frank bleeding per rectum with symptoms of lightheadedness, and his hemoglobin concentration fell to 6.9 g/dL. He was given a total of 3 units of packed red blood cells, which raised his hemoglobin level, but only to 8.3 g/dL. The gastroenterology service was consulted to evaluate for the source of the bleeding.
Comment. In a situation like this, an aortoenteric fistula is high on our list of differential diagnoses as the cause of bleeding, but other causes of frank bleeding per rectum such as diverticulosis, arteriovenous malformation, hemorrhoids, or a rapid upper-gastrointestinal bleed cannot be ruled out.
Upper-gastrointestinal endoscopy is the most commonly used diagnostic test for aortoenteric fistulas. It can also find other possible sources of gastrointestinal bleeding. CT with contrast is another option. It can depict the fistula itself or reveal signs of infection, such as gas or liquid surrounding the graft. In an emergency, when there is not enough time for diagnostic testing and an aortoenteric fistula is strongly suspected on clinical grounds, emergency surgical exploration is warranted.4,24
In our patient, the gastrointestinal service elected to first perform endoscopy to look for an aortoenteric fistula.
WHERE DO AORTOENTERIC FISTULAS OCCUR?
3. In which part of the gastrointestinal tract is an aortoenteric fistula most commonly located?
- Esophagus
- Stomach
- Duodenum
- Jejunum
Aortoenteric fistulas can occur at any of these locations, but 80% of cases of secondary aortoenteric fistula are in the duodenum, most often in the third or fourth (horizontal or ascending) part.19 Endoscopic visualization of a pulsatile bleeding mass in this area is diagnostic. However, even if no fistula is seen, upper endoscopy cannot rule out an aortoenteric fistula because the lesion can be located more distal than the scope can reach, which is typically no farther than the first or second parts.4,24
Case continued: What endoscopy showed
The esophagus was normal. There was old clotted blood in the stomach, but no lesions or ulcers. The duodenal bulb and second portion of the duodenum were normal. Three ulcers were noted in the third and fourth portions of the duodenum. The largest and deepest ulcer had an adherent blood clot, and the bowel wall was pulsatile in this region (Figure 2). These findings revealed the source of the gastrointestinal bleeding and were consistent with an aortoenteric fistula.
The patient’s initial bloody bowel movements were herald bleeds, ie, transient and self-limited episodes resulting from necrosis and mucosal ulceration. Herald bleeds can precede a massive gastrointestinal hemorrhage resulting from a true aortoenteric communication.19
Highlight point. Herald bleeds are self-limited and precede hemorrhage that results from a true aortoenteric communication.
TREATMENT OF AORTOENTERIC FISTULA
4. How are aortoenteric fistulas treated?
- Surgery
- Antibiotics
- Endoscopic intervention
Surgery is the definitive treatment. The traditional procedure is open surgical resection of the affected portion of the aorta followed by extra-anatomic (axillobifemoral) bypass or in situ aortic reconstruction using an antibiotic-impregnated prosthetic graft, autogenous femoral vein graft, or cryopreserved allograft.9,29 There have been cases of successful endovascular repair of aortoenteric fistulas, but this approach is generally used as a palliative bridge to definitive surgery.30
Antibiotics should be used if graft infection is suspected, ie, in most cases. However, surgery is still needed to repair the fistula and remove the source of infection. Cultures taken during surgical repair can help guide the choice of antibiotic after surgery.
Endoscopy can aid in diagnosing an aortoenteric fistula, as in the case of our patient. However, vascular surgery is necessary to close the communication between the aorta and the gastrointestinal tract.
Case continued: The patient declines treatment
In view of the patient’s enteroscopic findings, the vascular surgery service was again consulted for surgical correction of the aortoenteric fistula. Treatment was discussed with the patient and his family, but they declined any intervention in view of the high risk of morbidity and death that surgery would entail. Nearing the end of life with advanced cancer and a newly diagnosed aortoenteric fistula, the patient preferred comfort measures with hospice care.
Take-home points
Abdominal pain is the reason for 5% to 10% of emergency department visits, and between 35% to 41% of patients admitted to the hospital because of abdominal pain do not have a definitive diagnosis.31 It is crucial to think about an aortoenteric fistula in such patients who have a history of abdominal aortic aneurysm repair and gastrointestinal bleeding. Timely diagnosis and intervention are necessary to manage this otherwise-fatal condition.
- Hong C, Heiken JP, Sicard GA, Pilgram TK, Bae KT. Clinical significance of endoleak detected on follow-up CT after endovascular repair of abdominal aortic aneurysm. AJR Am J Roentgenol 2008; 191:808–813.
- Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002; 35:1029–1035.
- Corriere MA, Feurer ID, Becker SY, et al. Endoleak following endovascular abdominal aortic aneurysm repair: implications for duration of screening. Ann Surg 2004; 239:800–805.
- Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther 2008; 15:441–448.
- Demko TM, Diamond JR, Groff J. Obstructive nephropathy as a result of retroperitoneal fibrosis: a review of its pathogenesis and associations. J Am Soc Nephrol 1997; 8:684–688.
- Zetrenne E, McIntosh BC, McRae MH, Gusberg R, Evans GR, Narayan D. Prosthetic vascular graft infection: a multi-center review of surgical management. Yale J Biol Med 2007; 80:113–121.
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg 2008; 47:264–269.
- Swain TW, Calligaro KD, Dougherty MD. Management of infected aortic prosthetic grafts. Vasc Endovascular Surg 2004; 38:75–82.
- Cernohorsky P, Reijnen MM, Tielliu IF, van Sterkenburg SM, van den Dungen JJ, Zeebregts CJ. The relevance of aortic endograft prosthetic infection. J Vasc Surg 2011; 54:327–333.
- FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother 2005; 56:996–999.
- Orton DF, LeVeen RF, Saigh JA, et al. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics 2000; 20:977–993.
- Pacanowski JP, Dieter RS, Stevens SL, Freeman MB, Goldman MH. Endoleak: the achilles heel of endovascular abdominal aortic aneurysm exclusion—a case report. WMJ 2002; 101:57–58,63.
- van Bommel EF. Retroperitoneal fibrosis. Neth J Med 2002; 60:231–242.
- Utz DC, Henry JD. Retroperitoneal fibrosis. Med Clin North Am 1966; 50:1091–1099.
- Dalla-Palma L, Rocca-Rossetti S, Pozzi-Mucelli RS, Rizzatto G. Computed tomography in the diagnosis of retroperitoneal fibrosis. Urol Radiol 1981; 3:77–83.
- Harreby M, Bilde T, Helin P, Meyhoff HH, Vinterberg H, Nielsen VA. Retroperitoneal fibrosis treated with methylprednisolon pulse and disease-modifying antirheumatic drugs. Scand J Urol Nephrol 1994; 28:237–242.
- Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology (Oxford) 2004; 43:1441–1446.
- Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg 2005; 92:143–152.
- Baril DT, Carroccio A, Ellozy SH, et al. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg 2006; 44:250–257.
- Vu QD, Menias CO, Bhalla S, Peterson C, Wang LL, Balfe DM. Aortoenteric fistulas: CT features and potential mimics. Radiographics 2009; 29:197–209.
- Jayarajan S, Napolitano LM, Rectenwald JE, Upchurch GR. Primary aortoenteric fistula and endovascular repair. Vasc Endovascular Surg 2009; 43:592–596.
- Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg 2007; 45:834–836.
- Senadhi V, Brown JC, Arora D, Shaffer R, Shetty D, Mackrell P. A mysterious cause of gastrointestinal bleeding disguising itself as diverticulosis and peptic ulcer disease: a review of diagnostic modalities for aortoenteric fistula. Case Rep Gastroenterol 2010; 4:510–517.
- Simon T, Feller E. Diverse presentation of secondary aortoenteric fistulae. Case Report Med 2011; 2011:406730.
- Schwab CW, McMahon DJ, Phillips G, Pentecost MJ. Aortic balloon control of a traumatic aortoenteric fistula after damage control laparotomy: a case report. J Trauma 1996; 40:1021–1023.
- Napoli PJ, Meade PC, Adams CW. Primary aortoenteric fistula from a posttraumatic pseudoaneurysm. J Trauma 1996; 41:149–152.
- Laser A, Baker N, Rectenwald J, Eliason JL, Criado-Pallares E, Upchurch GR. Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2011; 54:58–63.
- Luo CY, Lai CH, Wen JS, Lin BW. Secondary aortocolic fistula: case report and review of the literature. Ann Vasc Surg 2010; 24:256.e5–256.e12.
- Kim JY, Kim YW, Kim CJ, Lim HI, Kim DI, Huh S. Successful surgical treatment of aortoenteric fistula. J Korean Med Sci 2007; 22:846–850.
- Verhey P, Best A, Lakin P, Nachiondo J, Petersen B. Successful endovascular treatment of aortoenteric fistula secondary to eroding duodenal stent. J Vasc Interv Radiol 2006; 17:1345–1348.
- Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. In:Hockberger RS, editor: UpToDate. Waltham, MA: UpToDate, 2012.
A 74-year-old man presented to the emergency department in December 2011 with a 1-week history of worsening abdominal pain, nausea with emesis, and decreased appetite. The pain was dull, diffuse, and not related to oral intake or bowel movements. He denied any bloody stools, melena, or hematemesis, but he had not had a bowel movement in the past week.
He was already known to have stage IV colon cancer with metastases to the lungs and liver. He had undergone a partial colectomy in 2009 and was receiving chemotherapy at the time of admission.
He also had an infrarenal abdominal aortic aneurysm that had been repaired in 2003 with endovascular placement of a Gore Excluder stent graft. This was complicated by a type II endoleak, treated with coil embolization. The same endoleak later recurred and was treated with injection of Onyx liquid embolic agent.
His medical history also included hypertension, type 2 diabetes mellitus, and hyperlipidemia. He had undergone a laparoscopic cholecystectomy in 2007.
He denied any fevers, chills, headache, lightheadedness, or change in vision. He had no respiratory, cardiac, or urinary symptoms. He had been constipated for the past few weeks and had recently been started on a bowel regimen, with mild relief. There had been no other changes to his medications.
His temperature on presentation was 97.5°F (36.4°C), blood pressure 120/64 mm Hg, pulse 96, respiratory rate 22, and oxygen saturation 95% on room air. He was awake, alert, oriented, and in no acute distress. His mucous membranes were dry. His lungs were clear to auscultation, and his heart sounds were normal. His bowel sounds were hyperactive and his abdomen was slightly tender diffusely, but there was no abdominal distention, rebound tenderness, guarding, or palpable masses. His joints, muscle strength, and muscle tone were normal. Table 1 shows his initial laboratory values.
Given the patient’s history of colon cancer, the emergency department physician ordered computed tomography (CT) of the abdomen to assess the state of his disease and to evaluate for bowel obstruction. The scan revealed a large abdominal aortic aneurysm with foci of gas within the aneurysmal sac. Metastases in the liver, lung, and retroperitoneum appeared stable; abundant colonic stool suggested constipation (Figure 1).
CAUSES OF PERIAORTIC GAS AFTER ANEURYSM REPAIR
1. What is the most common cause of periaortic ectopic gas in a patient with a repaired abdominal aortic aneurysm?
- Endoleak
- Stent graft infection
- Retroperitoneal fibrosis
- Aortoenteric fistula
Endoleak
Endoleak, a complication of endovascular abdominal aortic aneurysm repair, is defined as blood flow within the aneurysm sac but outside the endoluminal graft.1 It occurs in up to 15% of patients after endograft placement in the first month alone, and in up to 47% of patients eventually.2 It can lead to aneurysm enlargement and rupture. Endoleaks are classified into five types, each with different causes and management options.3,4 Contrast-enhanced CT is the most commonly used diagnostic tool.5
Endoleak cannot be ruled out in our patient, since CT was done without contrast. However, gas within the aneurysm is not consistent with this diagnosis.
Stent graft infection
Infection has been reported in 1% to 6% of patients receiving a stent graft for aortic aneurysm.6 They occur most commonly in the first year after placement; one study showed that 42% of patients diagnosed with graft infection presented within 3 months of endovascular repair.7
The leading cause of graft infection is contamination during the original procedure, but secondary infection from hematologic seeding and contamination from adjacent bowel are also possible.8 In our patient, who underwent graft placement followed by endovascular repairs of endoleaks, bacterial seeding of his aortic aneurysm from the procedures should be considered.9
The most common organisms are staphylococcal species, with Staphylococcus aureus more common in early infection and coagulase-negative staphylococci more common in late infection.10 Methicillin-resistant S aureus has been reported in as many as 25% of cases of graft infection. Diphtheroids and gram-negative enteric organisms should also be considered.11
CT is the most effective imaging test for graft infection. Perigraft soft tissue, fluid, and gas are the major CT findings.12
Given that our patient presented with abdominal pain, leukocytosis, and the CT finding of perigraft gas, graft infection should be high on our list differential diagnoses.
Retroperitoneal fibrosis
Retroperitoneal fibrosis is most often idiopathic, although many believe it is due to an exaggerated local inflammatory reaction to aortic atherosclerosis or is a manifestation of a systemic autoimmune disease.13 Secondary retroperitoneal fibrosis may be due to drugs, infection, or malignancy.
Pathologic findings include sclerotic plaques, typically around the abdominal vessels and ureters. Clinical presentations are often nonspecific, with early symptoms that include back or abdominal pain, malaise, anorexia, edema, and hematuria.14,15 Progressive ureteral obstruction can occur in later stages. CT with contrast is the imaging test of choice to visualize the extent of disease, with the fibrosis exhibiting attenuation similar to that of muscle.16
Initial treatment of idiopathic retroperitoneal fibrosis is with a glucocorticoid or other immunosuppressive agent, whereas treatment of secondary retroperitoneal fibrosis is aimed at the underlying cause.17 Late stages complicated by ureteral obstruction typically require surgery.18
Our patient did have some nonspecific complaints that could be due to retroperitoneal fibrosis. He also had an intra-abdominal malignancy, which could lead to secondary retroperitoneal fibrosis. However, his CT findings of periaortic gas are not consistent with this diagnosis.19
Aortoenteric fistula
Aortoenteric fistulas can be either primary or secondary.
Primary aortoenteric fistulas occur de novo in patients who have never undergone any surgery or procedure in the aorta. This type of fistula usually results from pressure erosion of an atherosclerotic abdominal aortic aneurysm into the gastrointestinal tract. They are rare, with an annual incidence of 0.04% to 0.07% in the general population.20,21
Secondary aortoenteric fistulas are complications of aortic reconstructive therapy. After open repair, a perianastomotic or pseudoaneurysmal fistula can develop into the gastrointestinal tract.4 Endovascular repair leaves the aortic wall intact with no exposed suture lines, but an aortoenteric fistula can still develop22 and in fact occur in 0.4% to 3.1% of recipients of stent grafts for abdominal aortic aneurysm repair.23 In such cases, it is commonly thought that graft infection can lead to formation of an aortoenteric fistula, but a penetrating gastrointestinal ulcer, tumor invasion, radiation therapy, and trauma have also been implicated.19,24–26 An aortoenteric fistula can present several months to several years after either open or endovascular abdominal aortic aneurysm repair.4,23
One of the main CT signs of an aortoenteric fistula is periaortic ectopic gas at least 3 to 4 weeks after surgery or endovascular repair.19 Gas around the stent graft is most commonly caused by infection, but an aortoenteric fistula must also be considered in our patient, as roughly one-third of graft infections present as aortoenteric fistula.27 Our patient denied having any gastrointestinal bleeding, but his hemoglobin concentration at presentation was 8.9 g/dL.
Highlight point. Perigraft gas after abdominal aortic aneurysm repair can be seen in graft infection and aortoenteric fistula.
SIGNS AND SYMPTOMS OF AORTOENTERIC FISTULA
2. What is the most common clinical sign or symptom of an aortoenteric fistula?
- Gastrointestinal bleeding
- Sepsis
- Abdominal pain
- Back pain
Gastrointestinal bleeding occurs in 80% of patients who have an aortoenteric fistula, sepsis in 40%, abdominal pain in 30%, and back pain in 15%.19 The classic triad of symptoms is gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass. However, symptoms can vary widely, and the classic triad is present in fewer than 25% of cases.28 Sepsis may be the predominant clinical manifestation, particularly in the early stages of fistula formation. Unexplained fever is an underrecognized early manifestation.24
Highlight point. The classic triad of symptoms of an aortoenteric fistula (gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass) is seen in fewer than 25% of cases.
Case continued: The patient develops frank bleeding
The vascular surgery service was consulted because of concern for an aortic graft infection, since surgical removal of the infected material is recommended.10 The patient was deemed to be a poor surgical candidate, given his stage IV colon cancer, so he was treated conservatively with broad-spectrum antibiotics.
Over the next 2 days, he had two episodes of dark, bloody bowel movements, but he remained hemodynamically stable. He subsequently developed frank bleeding per rectum with symptoms of lightheadedness, and his hemoglobin concentration fell to 6.9 g/dL. He was given a total of 3 units of packed red blood cells, which raised his hemoglobin level, but only to 8.3 g/dL. The gastroenterology service was consulted to evaluate for the source of the bleeding.
Comment. In a situation like this, an aortoenteric fistula is high on our list of differential diagnoses as the cause of bleeding, but other causes of frank bleeding per rectum such as diverticulosis, arteriovenous malformation, hemorrhoids, or a rapid upper-gastrointestinal bleed cannot be ruled out.
Upper-gastrointestinal endoscopy is the most commonly used diagnostic test for aortoenteric fistulas. It can also find other possible sources of gastrointestinal bleeding. CT with contrast is another option. It can depict the fistula itself or reveal signs of infection, such as gas or liquid surrounding the graft. In an emergency, when there is not enough time for diagnostic testing and an aortoenteric fistula is strongly suspected on clinical grounds, emergency surgical exploration is warranted.4,24
In our patient, the gastrointestinal service elected to first perform endoscopy to look for an aortoenteric fistula.
WHERE DO AORTOENTERIC FISTULAS OCCUR?
3. In which part of the gastrointestinal tract is an aortoenteric fistula most commonly located?
- Esophagus
- Stomach
- Duodenum
- Jejunum
Aortoenteric fistulas can occur at any of these locations, but 80% of cases of secondary aortoenteric fistula are in the duodenum, most often in the third or fourth (horizontal or ascending) part.19 Endoscopic visualization of a pulsatile bleeding mass in this area is diagnostic. However, even if no fistula is seen, upper endoscopy cannot rule out an aortoenteric fistula because the lesion can be located more distal than the scope can reach, which is typically no farther than the first or second parts.4,24
Case continued: What endoscopy showed
The esophagus was normal. There was old clotted blood in the stomach, but no lesions or ulcers. The duodenal bulb and second portion of the duodenum were normal. Three ulcers were noted in the third and fourth portions of the duodenum. The largest and deepest ulcer had an adherent blood clot, and the bowel wall was pulsatile in this region (Figure 2). These findings revealed the source of the gastrointestinal bleeding and were consistent with an aortoenteric fistula.
The patient’s initial bloody bowel movements were herald bleeds, ie, transient and self-limited episodes resulting from necrosis and mucosal ulceration. Herald bleeds can precede a massive gastrointestinal hemorrhage resulting from a true aortoenteric communication.19
Highlight point. Herald bleeds are self-limited and precede hemorrhage that results from a true aortoenteric communication.
TREATMENT OF AORTOENTERIC FISTULA
4. How are aortoenteric fistulas treated?
- Surgery
- Antibiotics
- Endoscopic intervention
Surgery is the definitive treatment. The traditional procedure is open surgical resection of the affected portion of the aorta followed by extra-anatomic (axillobifemoral) bypass or in situ aortic reconstruction using an antibiotic-impregnated prosthetic graft, autogenous femoral vein graft, or cryopreserved allograft.9,29 There have been cases of successful endovascular repair of aortoenteric fistulas, but this approach is generally used as a palliative bridge to definitive surgery.30
Antibiotics should be used if graft infection is suspected, ie, in most cases. However, surgery is still needed to repair the fistula and remove the source of infection. Cultures taken during surgical repair can help guide the choice of antibiotic after surgery.
Endoscopy can aid in diagnosing an aortoenteric fistula, as in the case of our patient. However, vascular surgery is necessary to close the communication between the aorta and the gastrointestinal tract.
Case continued: The patient declines treatment
In view of the patient’s enteroscopic findings, the vascular surgery service was again consulted for surgical correction of the aortoenteric fistula. Treatment was discussed with the patient and his family, but they declined any intervention in view of the high risk of morbidity and death that surgery would entail. Nearing the end of life with advanced cancer and a newly diagnosed aortoenteric fistula, the patient preferred comfort measures with hospice care.
Take-home points
Abdominal pain is the reason for 5% to 10% of emergency department visits, and between 35% to 41% of patients admitted to the hospital because of abdominal pain do not have a definitive diagnosis.31 It is crucial to think about an aortoenteric fistula in such patients who have a history of abdominal aortic aneurysm repair and gastrointestinal bleeding. Timely diagnosis and intervention are necessary to manage this otherwise-fatal condition.
A 74-year-old man presented to the emergency department in December 2011 with a 1-week history of worsening abdominal pain, nausea with emesis, and decreased appetite. The pain was dull, diffuse, and not related to oral intake or bowel movements. He denied any bloody stools, melena, or hematemesis, but he had not had a bowel movement in the past week.
He was already known to have stage IV colon cancer with metastases to the lungs and liver. He had undergone a partial colectomy in 2009 and was receiving chemotherapy at the time of admission.
He also had an infrarenal abdominal aortic aneurysm that had been repaired in 2003 with endovascular placement of a Gore Excluder stent graft. This was complicated by a type II endoleak, treated with coil embolization. The same endoleak later recurred and was treated with injection of Onyx liquid embolic agent.
His medical history also included hypertension, type 2 diabetes mellitus, and hyperlipidemia. He had undergone a laparoscopic cholecystectomy in 2007.
He denied any fevers, chills, headache, lightheadedness, or change in vision. He had no respiratory, cardiac, or urinary symptoms. He had been constipated for the past few weeks and had recently been started on a bowel regimen, with mild relief. There had been no other changes to his medications.
His temperature on presentation was 97.5°F (36.4°C), blood pressure 120/64 mm Hg, pulse 96, respiratory rate 22, and oxygen saturation 95% on room air. He was awake, alert, oriented, and in no acute distress. His mucous membranes were dry. His lungs were clear to auscultation, and his heart sounds were normal. His bowel sounds were hyperactive and his abdomen was slightly tender diffusely, but there was no abdominal distention, rebound tenderness, guarding, or palpable masses. His joints, muscle strength, and muscle tone were normal. Table 1 shows his initial laboratory values.
Given the patient’s history of colon cancer, the emergency department physician ordered computed tomography (CT) of the abdomen to assess the state of his disease and to evaluate for bowel obstruction. The scan revealed a large abdominal aortic aneurysm with foci of gas within the aneurysmal sac. Metastases in the liver, lung, and retroperitoneum appeared stable; abundant colonic stool suggested constipation (Figure 1).
CAUSES OF PERIAORTIC GAS AFTER ANEURYSM REPAIR
1. What is the most common cause of periaortic ectopic gas in a patient with a repaired abdominal aortic aneurysm?
- Endoleak
- Stent graft infection
- Retroperitoneal fibrosis
- Aortoenteric fistula
Endoleak
Endoleak, a complication of endovascular abdominal aortic aneurysm repair, is defined as blood flow within the aneurysm sac but outside the endoluminal graft.1 It occurs in up to 15% of patients after endograft placement in the first month alone, and in up to 47% of patients eventually.2 It can lead to aneurysm enlargement and rupture. Endoleaks are classified into five types, each with different causes and management options.3,4 Contrast-enhanced CT is the most commonly used diagnostic tool.5
Endoleak cannot be ruled out in our patient, since CT was done without contrast. However, gas within the aneurysm is not consistent with this diagnosis.
Stent graft infection
Infection has been reported in 1% to 6% of patients receiving a stent graft for aortic aneurysm.6 They occur most commonly in the first year after placement; one study showed that 42% of patients diagnosed with graft infection presented within 3 months of endovascular repair.7
The leading cause of graft infection is contamination during the original procedure, but secondary infection from hematologic seeding and contamination from adjacent bowel are also possible.8 In our patient, who underwent graft placement followed by endovascular repairs of endoleaks, bacterial seeding of his aortic aneurysm from the procedures should be considered.9
The most common organisms are staphylococcal species, with Staphylococcus aureus more common in early infection and coagulase-negative staphylococci more common in late infection.10 Methicillin-resistant S aureus has been reported in as many as 25% of cases of graft infection. Diphtheroids and gram-negative enteric organisms should also be considered.11
CT is the most effective imaging test for graft infection. Perigraft soft tissue, fluid, and gas are the major CT findings.12
Given that our patient presented with abdominal pain, leukocytosis, and the CT finding of perigraft gas, graft infection should be high on our list differential diagnoses.
Retroperitoneal fibrosis
Retroperitoneal fibrosis is most often idiopathic, although many believe it is due to an exaggerated local inflammatory reaction to aortic atherosclerosis or is a manifestation of a systemic autoimmune disease.13 Secondary retroperitoneal fibrosis may be due to drugs, infection, or malignancy.
Pathologic findings include sclerotic plaques, typically around the abdominal vessels and ureters. Clinical presentations are often nonspecific, with early symptoms that include back or abdominal pain, malaise, anorexia, edema, and hematuria.14,15 Progressive ureteral obstruction can occur in later stages. CT with contrast is the imaging test of choice to visualize the extent of disease, with the fibrosis exhibiting attenuation similar to that of muscle.16
Initial treatment of idiopathic retroperitoneal fibrosis is with a glucocorticoid or other immunosuppressive agent, whereas treatment of secondary retroperitoneal fibrosis is aimed at the underlying cause.17 Late stages complicated by ureteral obstruction typically require surgery.18
Our patient did have some nonspecific complaints that could be due to retroperitoneal fibrosis. He also had an intra-abdominal malignancy, which could lead to secondary retroperitoneal fibrosis. However, his CT findings of periaortic gas are not consistent with this diagnosis.19
Aortoenteric fistula
Aortoenteric fistulas can be either primary or secondary.
Primary aortoenteric fistulas occur de novo in patients who have never undergone any surgery or procedure in the aorta. This type of fistula usually results from pressure erosion of an atherosclerotic abdominal aortic aneurysm into the gastrointestinal tract. They are rare, with an annual incidence of 0.04% to 0.07% in the general population.20,21
Secondary aortoenteric fistulas are complications of aortic reconstructive therapy. After open repair, a perianastomotic or pseudoaneurysmal fistula can develop into the gastrointestinal tract.4 Endovascular repair leaves the aortic wall intact with no exposed suture lines, but an aortoenteric fistula can still develop22 and in fact occur in 0.4% to 3.1% of recipients of stent grafts for abdominal aortic aneurysm repair.23 In such cases, it is commonly thought that graft infection can lead to formation of an aortoenteric fistula, but a penetrating gastrointestinal ulcer, tumor invasion, radiation therapy, and trauma have also been implicated.19,24–26 An aortoenteric fistula can present several months to several years after either open or endovascular abdominal aortic aneurysm repair.4,23
One of the main CT signs of an aortoenteric fistula is periaortic ectopic gas at least 3 to 4 weeks after surgery or endovascular repair.19 Gas around the stent graft is most commonly caused by infection, but an aortoenteric fistula must also be considered in our patient, as roughly one-third of graft infections present as aortoenteric fistula.27 Our patient denied having any gastrointestinal bleeding, but his hemoglobin concentration at presentation was 8.9 g/dL.
Highlight point. Perigraft gas after abdominal aortic aneurysm repair can be seen in graft infection and aortoenteric fistula.
SIGNS AND SYMPTOMS OF AORTOENTERIC FISTULA
2. What is the most common clinical sign or symptom of an aortoenteric fistula?
- Gastrointestinal bleeding
- Sepsis
- Abdominal pain
- Back pain
Gastrointestinal bleeding occurs in 80% of patients who have an aortoenteric fistula, sepsis in 40%, abdominal pain in 30%, and back pain in 15%.19 The classic triad of symptoms is gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass. However, symptoms can vary widely, and the classic triad is present in fewer than 25% of cases.28 Sepsis may be the predominant clinical manifestation, particularly in the early stages of fistula formation. Unexplained fever is an underrecognized early manifestation.24
Highlight point. The classic triad of symptoms of an aortoenteric fistula (gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass) is seen in fewer than 25% of cases.
Case continued: The patient develops frank bleeding
The vascular surgery service was consulted because of concern for an aortic graft infection, since surgical removal of the infected material is recommended.10 The patient was deemed to be a poor surgical candidate, given his stage IV colon cancer, so he was treated conservatively with broad-spectrum antibiotics.
Over the next 2 days, he had two episodes of dark, bloody bowel movements, but he remained hemodynamically stable. He subsequently developed frank bleeding per rectum with symptoms of lightheadedness, and his hemoglobin concentration fell to 6.9 g/dL. He was given a total of 3 units of packed red blood cells, which raised his hemoglobin level, but only to 8.3 g/dL. The gastroenterology service was consulted to evaluate for the source of the bleeding.
Comment. In a situation like this, an aortoenteric fistula is high on our list of differential diagnoses as the cause of bleeding, but other causes of frank bleeding per rectum such as diverticulosis, arteriovenous malformation, hemorrhoids, or a rapid upper-gastrointestinal bleed cannot be ruled out.
Upper-gastrointestinal endoscopy is the most commonly used diagnostic test for aortoenteric fistulas. It can also find other possible sources of gastrointestinal bleeding. CT with contrast is another option. It can depict the fistula itself or reveal signs of infection, such as gas or liquid surrounding the graft. In an emergency, when there is not enough time for diagnostic testing and an aortoenteric fistula is strongly suspected on clinical grounds, emergency surgical exploration is warranted.4,24
In our patient, the gastrointestinal service elected to first perform endoscopy to look for an aortoenteric fistula.
WHERE DO AORTOENTERIC FISTULAS OCCUR?
3. In which part of the gastrointestinal tract is an aortoenteric fistula most commonly located?
- Esophagus
- Stomach
- Duodenum
- Jejunum
Aortoenteric fistulas can occur at any of these locations, but 80% of cases of secondary aortoenteric fistula are in the duodenum, most often in the third or fourth (horizontal or ascending) part.19 Endoscopic visualization of a pulsatile bleeding mass in this area is diagnostic. However, even if no fistula is seen, upper endoscopy cannot rule out an aortoenteric fistula because the lesion can be located more distal than the scope can reach, which is typically no farther than the first or second parts.4,24
Case continued: What endoscopy showed
The esophagus was normal. There was old clotted blood in the stomach, but no lesions or ulcers. The duodenal bulb and second portion of the duodenum were normal. Three ulcers were noted in the third and fourth portions of the duodenum. The largest and deepest ulcer had an adherent blood clot, and the bowel wall was pulsatile in this region (Figure 2). These findings revealed the source of the gastrointestinal bleeding and were consistent with an aortoenteric fistula.
The patient’s initial bloody bowel movements were herald bleeds, ie, transient and self-limited episodes resulting from necrosis and mucosal ulceration. Herald bleeds can precede a massive gastrointestinal hemorrhage resulting from a true aortoenteric communication.19
Highlight point. Herald bleeds are self-limited and precede hemorrhage that results from a true aortoenteric communication.
TREATMENT OF AORTOENTERIC FISTULA
4. How are aortoenteric fistulas treated?
- Surgery
- Antibiotics
- Endoscopic intervention
Surgery is the definitive treatment. The traditional procedure is open surgical resection of the affected portion of the aorta followed by extra-anatomic (axillobifemoral) bypass or in situ aortic reconstruction using an antibiotic-impregnated prosthetic graft, autogenous femoral vein graft, or cryopreserved allograft.9,29 There have been cases of successful endovascular repair of aortoenteric fistulas, but this approach is generally used as a palliative bridge to definitive surgery.30
Antibiotics should be used if graft infection is suspected, ie, in most cases. However, surgery is still needed to repair the fistula and remove the source of infection. Cultures taken during surgical repair can help guide the choice of antibiotic after surgery.
Endoscopy can aid in diagnosing an aortoenteric fistula, as in the case of our patient. However, vascular surgery is necessary to close the communication between the aorta and the gastrointestinal tract.
Case continued: The patient declines treatment
In view of the patient’s enteroscopic findings, the vascular surgery service was again consulted for surgical correction of the aortoenteric fistula. Treatment was discussed with the patient and his family, but they declined any intervention in view of the high risk of morbidity and death that surgery would entail. Nearing the end of life with advanced cancer and a newly diagnosed aortoenteric fistula, the patient preferred comfort measures with hospice care.
Take-home points
Abdominal pain is the reason for 5% to 10% of emergency department visits, and between 35% to 41% of patients admitted to the hospital because of abdominal pain do not have a definitive diagnosis.31 It is crucial to think about an aortoenteric fistula in such patients who have a history of abdominal aortic aneurysm repair and gastrointestinal bleeding. Timely diagnosis and intervention are necessary to manage this otherwise-fatal condition.
- Hong C, Heiken JP, Sicard GA, Pilgram TK, Bae KT. Clinical significance of endoleak detected on follow-up CT after endovascular repair of abdominal aortic aneurysm. AJR Am J Roentgenol 2008; 191:808–813.
- Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002; 35:1029–1035.
- Corriere MA, Feurer ID, Becker SY, et al. Endoleak following endovascular abdominal aortic aneurysm repair: implications for duration of screening. Ann Surg 2004; 239:800–805.
- Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther 2008; 15:441–448.
- Demko TM, Diamond JR, Groff J. Obstructive nephropathy as a result of retroperitoneal fibrosis: a review of its pathogenesis and associations. J Am Soc Nephrol 1997; 8:684–688.
- Zetrenne E, McIntosh BC, McRae MH, Gusberg R, Evans GR, Narayan D. Prosthetic vascular graft infection: a multi-center review of surgical management. Yale J Biol Med 2007; 80:113–121.
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg 2008; 47:264–269.
- Swain TW, Calligaro KD, Dougherty MD. Management of infected aortic prosthetic grafts. Vasc Endovascular Surg 2004; 38:75–82.
- Cernohorsky P, Reijnen MM, Tielliu IF, van Sterkenburg SM, van den Dungen JJ, Zeebregts CJ. The relevance of aortic endograft prosthetic infection. J Vasc Surg 2011; 54:327–333.
- FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother 2005; 56:996–999.
- Orton DF, LeVeen RF, Saigh JA, et al. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics 2000; 20:977–993.
- Pacanowski JP, Dieter RS, Stevens SL, Freeman MB, Goldman MH. Endoleak: the achilles heel of endovascular abdominal aortic aneurysm exclusion—a case report. WMJ 2002; 101:57–58,63.
- van Bommel EF. Retroperitoneal fibrosis. Neth J Med 2002; 60:231–242.
- Utz DC, Henry JD. Retroperitoneal fibrosis. Med Clin North Am 1966; 50:1091–1099.
- Dalla-Palma L, Rocca-Rossetti S, Pozzi-Mucelli RS, Rizzatto G. Computed tomography in the diagnosis of retroperitoneal fibrosis. Urol Radiol 1981; 3:77–83.
- Harreby M, Bilde T, Helin P, Meyhoff HH, Vinterberg H, Nielsen VA. Retroperitoneal fibrosis treated with methylprednisolon pulse and disease-modifying antirheumatic drugs. Scand J Urol Nephrol 1994; 28:237–242.
- Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology (Oxford) 2004; 43:1441–1446.
- Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg 2005; 92:143–152.
- Baril DT, Carroccio A, Ellozy SH, et al. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg 2006; 44:250–257.
- Vu QD, Menias CO, Bhalla S, Peterson C, Wang LL, Balfe DM. Aortoenteric fistulas: CT features and potential mimics. Radiographics 2009; 29:197–209.
- Jayarajan S, Napolitano LM, Rectenwald JE, Upchurch GR. Primary aortoenteric fistula and endovascular repair. Vasc Endovascular Surg 2009; 43:592–596.
- Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg 2007; 45:834–836.
- Senadhi V, Brown JC, Arora D, Shaffer R, Shetty D, Mackrell P. A mysterious cause of gastrointestinal bleeding disguising itself as diverticulosis and peptic ulcer disease: a review of diagnostic modalities for aortoenteric fistula. Case Rep Gastroenterol 2010; 4:510–517.
- Simon T, Feller E. Diverse presentation of secondary aortoenteric fistulae. Case Report Med 2011; 2011:406730.
- Schwab CW, McMahon DJ, Phillips G, Pentecost MJ. Aortic balloon control of a traumatic aortoenteric fistula after damage control laparotomy: a case report. J Trauma 1996; 40:1021–1023.
- Napoli PJ, Meade PC, Adams CW. Primary aortoenteric fistula from a posttraumatic pseudoaneurysm. J Trauma 1996; 41:149–152.
- Laser A, Baker N, Rectenwald J, Eliason JL, Criado-Pallares E, Upchurch GR. Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2011; 54:58–63.
- Luo CY, Lai CH, Wen JS, Lin BW. Secondary aortocolic fistula: case report and review of the literature. Ann Vasc Surg 2010; 24:256.e5–256.e12.
- Kim JY, Kim YW, Kim CJ, Lim HI, Kim DI, Huh S. Successful surgical treatment of aortoenteric fistula. J Korean Med Sci 2007; 22:846–850.
- Verhey P, Best A, Lakin P, Nachiondo J, Petersen B. Successful endovascular treatment of aortoenteric fistula secondary to eroding duodenal stent. J Vasc Interv Radiol 2006; 17:1345–1348.
- Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. In:Hockberger RS, editor: UpToDate. Waltham, MA: UpToDate, 2012.
- Hong C, Heiken JP, Sicard GA, Pilgram TK, Bae KT. Clinical significance of endoleak detected on follow-up CT after endovascular repair of abdominal aortic aneurysm. AJR Am J Roentgenol 2008; 191:808–813.
- Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002; 35:1029–1035.
- Corriere MA, Feurer ID, Becker SY, et al. Endoleak following endovascular abdominal aortic aneurysm repair: implications for duration of screening. Ann Surg 2004; 239:800–805.
- Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther 2008; 15:441–448.
- Demko TM, Diamond JR, Groff J. Obstructive nephropathy as a result of retroperitoneal fibrosis: a review of its pathogenesis and associations. J Am Soc Nephrol 1997; 8:684–688.
- Zetrenne E, McIntosh BC, McRae MH, Gusberg R, Evans GR, Narayan D. Prosthetic vascular graft infection: a multi-center review of surgical management. Yale J Biol Med 2007; 80:113–121.
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg 2008; 47:264–269.
- Swain TW, Calligaro KD, Dougherty MD. Management of infected aortic prosthetic grafts. Vasc Endovascular Surg 2004; 38:75–82.
- Cernohorsky P, Reijnen MM, Tielliu IF, van Sterkenburg SM, van den Dungen JJ, Zeebregts CJ. The relevance of aortic endograft prosthetic infection. J Vasc Surg 2011; 54:327–333.
- FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother 2005; 56:996–999.
- Orton DF, LeVeen RF, Saigh JA, et al. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics 2000; 20:977–993.
- Pacanowski JP, Dieter RS, Stevens SL, Freeman MB, Goldman MH. Endoleak: the achilles heel of endovascular abdominal aortic aneurysm exclusion—a case report. WMJ 2002; 101:57–58,63.
- van Bommel EF. Retroperitoneal fibrosis. Neth J Med 2002; 60:231–242.
- Utz DC, Henry JD. Retroperitoneal fibrosis. Med Clin North Am 1966; 50:1091–1099.
- Dalla-Palma L, Rocca-Rossetti S, Pozzi-Mucelli RS, Rizzatto G. Computed tomography in the diagnosis of retroperitoneal fibrosis. Urol Radiol 1981; 3:77–83.
- Harreby M, Bilde T, Helin P, Meyhoff HH, Vinterberg H, Nielsen VA. Retroperitoneal fibrosis treated with methylprednisolon pulse and disease-modifying antirheumatic drugs. Scand J Urol Nephrol 1994; 28:237–242.
- Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology (Oxford) 2004; 43:1441–1446.
- Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg 2005; 92:143–152.
- Baril DT, Carroccio A, Ellozy SH, et al. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg 2006; 44:250–257.
- Vu QD, Menias CO, Bhalla S, Peterson C, Wang LL, Balfe DM. Aortoenteric fistulas: CT features and potential mimics. Radiographics 2009; 29:197–209.
- Jayarajan S, Napolitano LM, Rectenwald JE, Upchurch GR. Primary aortoenteric fistula and endovascular repair. Vasc Endovascular Surg 2009; 43:592–596.
- Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg 2007; 45:834–836.
- Senadhi V, Brown JC, Arora D, Shaffer R, Shetty D, Mackrell P. A mysterious cause of gastrointestinal bleeding disguising itself as diverticulosis and peptic ulcer disease: a review of diagnostic modalities for aortoenteric fistula. Case Rep Gastroenterol 2010; 4:510–517.
- Simon T, Feller E. Diverse presentation of secondary aortoenteric fistulae. Case Report Med 2011; 2011:406730.
- Schwab CW, McMahon DJ, Phillips G, Pentecost MJ. Aortic balloon control of a traumatic aortoenteric fistula after damage control laparotomy: a case report. J Trauma 1996; 40:1021–1023.
- Napoli PJ, Meade PC, Adams CW. Primary aortoenteric fistula from a posttraumatic pseudoaneurysm. J Trauma 1996; 41:149–152.
- Laser A, Baker N, Rectenwald J, Eliason JL, Criado-Pallares E, Upchurch GR. Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2011; 54:58–63.
- Luo CY, Lai CH, Wen JS, Lin BW. Secondary aortocolic fistula: case report and review of the literature. Ann Vasc Surg 2010; 24:256.e5–256.e12.
- Kim JY, Kim YW, Kim CJ, Lim HI, Kim DI, Huh S. Successful surgical treatment of aortoenteric fistula. J Korean Med Sci 2007; 22:846–850.
- Verhey P, Best A, Lakin P, Nachiondo J, Petersen B. Successful endovascular treatment of aortoenteric fistula secondary to eroding duodenal stent. J Vasc Interv Radiol 2006; 17:1345–1348.
- Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. In:Hockberger RS, editor: UpToDate. Waltham, MA: UpToDate, 2012.