User login
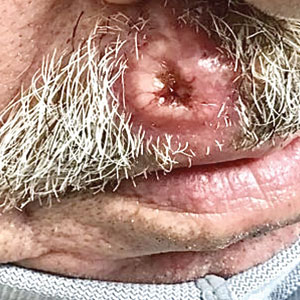
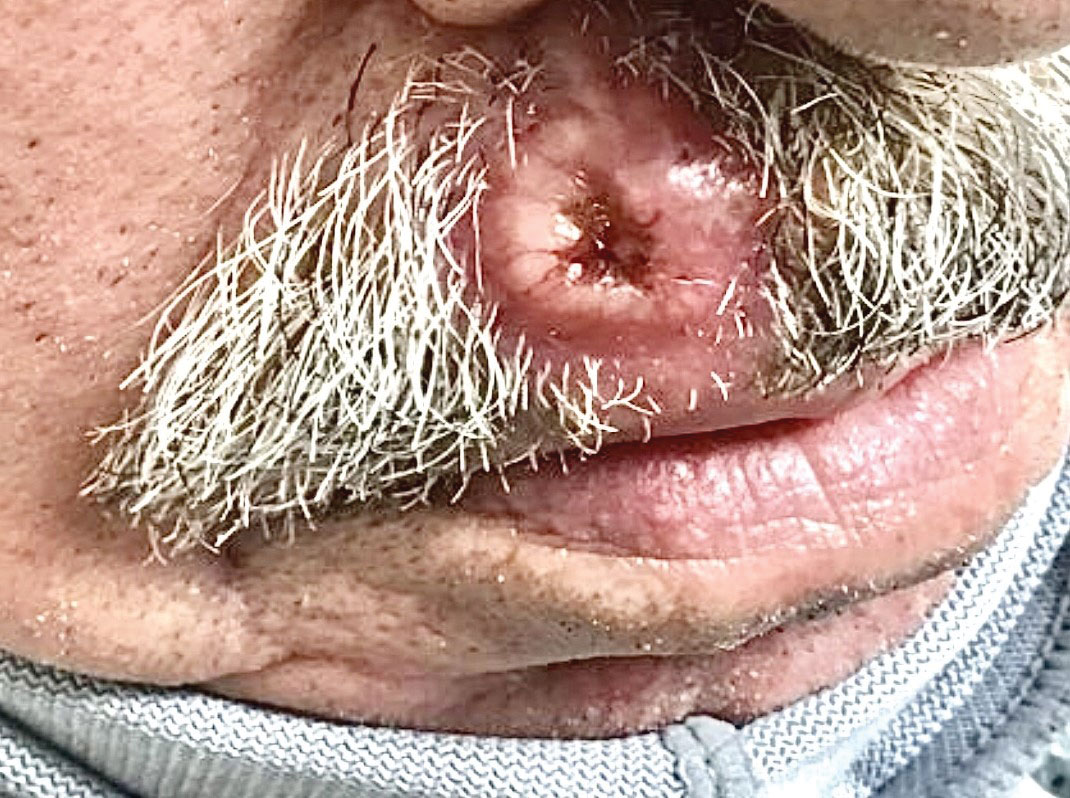
Ulcerated Nodule on the Lip
The Diagnosis: Cutaneous Metastasis
A shave biopsy of the lip revealed a diffuse cellular infiltrate filling the superficial and deep dermis (Figure 1A). Morphologically, the cells had abundant clear cytoplasm with eccentrically located, pleomorphic, hyperchromatic nuclei with occasional prominent nucleoli (Figure 1B). The cells stained positive for AE1/ AE3 on immunohistochemistry (Figure 2). A punch biopsy of the nodule in the right axillary vault revealed a morphologically similar proliferation of cells. A colonoscopy revealed a completely obstructing circumferential mass in the distal ascending colon. A biopsy of the mass confirmed invasive adenocarcinoma, supporting a diagnosis of cutaneous metastases from adenocarcinoma of the colon. The patient underwent resection of the lip tumor and started multiagent chemotherapy for his newly diagnosed stage IV adenocarcinoma of the colon. The patient died, despite therapy.

Cutaneous metastasis from solid malignancies is uncommon, as only 1.3% of them exhibit cutaneous manifestations at presentation.1 Cutaneous metastasis from signet ring cell adenocarcinoma (SRCA) of the colon is uncommon, and cutaneous metastasis of colorectal SRCA rarely precedes the diagnosis of the primary lesion.2 Among the colorectal cancers that metastasize to the skin, metastasis to the face occurs in only 0.5% of patients.3

Signet ring cell adenocarcinomas are poorly differentiated adenocarcinomas histologically characterized by the neoplastic cells’ circular to ovoid appearance with a flattened top.4,5 This distinctive shape is from the displacement of the nucleus to the periphery of the cell due to the accumulation of intracytoplasmic mucin.4 Classically, malignancies are characterized as an SRCA if more than 50% of the cells have a signet ring cell morphology; if the signet ring cells comprise less than 50% of the neoplasm, the tumor is designated as an adenocarcinoma with signet ring morphology.4 The most common cause of cutaneous metastasis with signet ring morphology is gastric cancer, while colorectal carcinoma is less common.1 Colorectal SRCAs usually are found in the right colon or the rectum in comparison to other colonic sites.6
Clinically, cutaneous metastasis can present in a variety of ways. The most common presentation is nodular lesions that may coalesce to become zosteriform in configuration or lesions that mimic inflammatory dermatoses.7 Cutaneous metastasis is more common in breast and lung cancer, and when it occurs secondary to colorectal cancer, cutaneous metastasis rarely predates the detection of the primary neoplasm.2
The clinical appearance of metastasis is not specific and can mimic many entities8; therefore, a high index of suspicion must be employed when managing patients, even those without a history of internal malignancy. In our patient, the smooth nodular lesion appeared similar to a basal cell carcinoma; however, basal cell carcinomas appear more pearly, and arborizing telangiectasia often is seen.9 Merkel cell carcinoma is common on sundamaged skin of the head and neck but clinically appears more violaceous than the lesion seen in our patient.10 Paracoccidioidomycosis may form ulcerated papulonodules or plaques, especially around the nose and mouth. In many of these cases, lesions develop from contiguous lesions of the oral mucosa; therefore, the presence of oral lesions will help distinguish this infectious entity from cutaneous metastasis. Multiple lesions usually are identified when there is hematogenous dissemination.11 Mycosis fungoides is a subtype of cutaneous T-cell lymphoma and is characterized by multiple patches, plaques, and nodules on sun-protected areas. Involvement of the head and neck is not common, except in the folliculotropic subtype, which has a separate and distinct clinical morphology.12
The development of signet ring morphology from an adenocarcinoma can be attributed to the activation of phosphatidylinositol 3-kinase (PI3K), which leads to downstream activation of mitogen-activated protein kinase (MAPK) and the subsequent loss of intercellular tight junctions. The mucin 4 gene, MUC4, also is upregulated by PI3K activation and possesses antiapoptotic and mitogenic effects in addition to its mucin secretory function.13
The neoplastic cells in SRCAs stain positive for mucicarmine, Alcian blue, and periodic acid–Schiff, which highlights the mucinous component of the cells.7 Immunohistochemical stains with CK7, CK20, AE1/AE3, and epithelial membrane antigen can be implemented to confirm an epithelial origin of the primary cancer.7,13 CK20 is a low-molecular-weight cytokeratin normally expressed by Merkel cells and by the epithelium of the gastrointestinal tract and urothelium, whereas CK7 expression typically is expressed in the lungs, ovaries, endometrium, and breasts, but not in the lower gastrointestinal tract.14 Differentiating primary cutaneous adenocarcinoma from cutaneous metastasis can be accomplished with a thorough clinical history; however, p63 positivity supports a primary cutaneous lesion and may be useful in certain situations.7 CDX2 stains can be utilized to aid in localizing the primary neoplasm when it is unknown, and when positive, it suggests a lower gastrointestinal tract origin. However, special AT-rich sequence-binding protein 2 (SATB2) recently has been proposed as a replacement immunohistochemical marker for CDX2, as it has greater specificity for SRCA of the lower gastrointestinal tract.15 Benign entities with signet ring cell morphology are difficult to distinguish from SRCA; however, malignant lesions are more likely to demonstrate an infiltrative growth pattern, frequent mitotic figures, and apoptosis. Immunohistochemistry also can be utilized to support the diagnosis of benign proliferation with signet ring morphology, as benign lesions often will demonstrate E-cadherin positivity and negativity for p53 and Ki-67.13
Cutaneous metastasis usually correlates to advanced disease and generally indicates a worse prognosis.13 Signet ring cell morphology in both gastric and colorectal cancer portends a poor prognosis, and there is a lower overall survival in patients with these malignancies compared to cancers of the same organ with non–signet ring cell morphology.4,8
- Mandzhieva B, Jalil A, Nadeem M, et al. Most common pathway of metastasis of rectal signet ring cell carcinoma to the skin: hematogenous. Cureus. 2020;12:E6890.
- Parente P, Ciardiello D, Reggiani Bonetti L, et al. Cutaneous metastasis from colorectal cancer: making light on an unusual and misdiagnosed event. Life. 2021;11:954.
- Picciariello A, Tomasicchio G, Lantone G, et al. Synchronous “skip” facial metastases from colorectal adenocarcinoma: a case report and review of literature. BMC Gastroenterol. 2022;22:68.
- Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers. 2020;12:1544.
- Xu Q, Karouji Y, Kobayashi M, et al. The PI 3-kinase-Rac-p38 MAP kinase pathway is involved in the formation of signet-ring cell carcinoma. Oncogene. 2003;22:5537-5544.
- Morales-Cruz M, Salgado-Nesme N, Trolle-Silva AM, et al. Signet ring cell carcinoma of the rectum: atypical metastatic presentation. BMJ Case Rep CP. 2019;12:E229135.
- Demirciog˘lu D, Öztürk Durmaz E, Demirkesen C, et al. Livedoid cutaneous metastasis of signet‐ring cell gastric carcinoma. J Cutan Pathol. 2021;48:785-788.
- Dong X, Sun G, Qu H, et al. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg. 2021;8:642468.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract. 2017;4:2.
- Marques, SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610-615.
- Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome. Hematol Oncol Clin. 2019;33:103-120.
- Gündüz Ö, Emeksiz MC, Atasoy P, et al. Signet-ring cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8:6819.
- Al-Taee A, Almukhtar R, Lai J, et al. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016;4:283.
- Ma C, Lowenthal BM, Pai RK. SATB2 is superior to CDX2 in distinguishing signet ring cell carcinoma of the upper gastrointestinal tract and lower gastrointestinal tract. Am J Surg Pathol. 2018; 42:1715-1722.
The Diagnosis: Cutaneous Metastasis
A shave biopsy of the lip revealed a diffuse cellular infiltrate filling the superficial and deep dermis (Figure 1A). Morphologically, the cells had abundant clear cytoplasm with eccentrically located, pleomorphic, hyperchromatic nuclei with occasional prominent nucleoli (Figure 1B). The cells stained positive for AE1/ AE3 on immunohistochemistry (Figure 2). A punch biopsy of the nodule in the right axillary vault revealed a morphologically similar proliferation of cells. A colonoscopy revealed a completely obstructing circumferential mass in the distal ascending colon. A biopsy of the mass confirmed invasive adenocarcinoma, supporting a diagnosis of cutaneous metastases from adenocarcinoma of the colon. The patient underwent resection of the lip tumor and started multiagent chemotherapy for his newly diagnosed stage IV adenocarcinoma of the colon. The patient died, despite therapy.

Cutaneous metastasis from solid malignancies is uncommon, as only 1.3% of them exhibit cutaneous manifestations at presentation.1 Cutaneous metastasis from signet ring cell adenocarcinoma (SRCA) of the colon is uncommon, and cutaneous metastasis of colorectal SRCA rarely precedes the diagnosis of the primary lesion.2 Among the colorectal cancers that metastasize to the skin, metastasis to the face occurs in only 0.5% of patients.3

Signet ring cell adenocarcinomas are poorly differentiated adenocarcinomas histologically characterized by the neoplastic cells’ circular to ovoid appearance with a flattened top.4,5 This distinctive shape is from the displacement of the nucleus to the periphery of the cell due to the accumulation of intracytoplasmic mucin.4 Classically, malignancies are characterized as an SRCA if more than 50% of the cells have a signet ring cell morphology; if the signet ring cells comprise less than 50% of the neoplasm, the tumor is designated as an adenocarcinoma with signet ring morphology.4 The most common cause of cutaneous metastasis with signet ring morphology is gastric cancer, while colorectal carcinoma is less common.1 Colorectal SRCAs usually are found in the right colon or the rectum in comparison to other colonic sites.6
Clinically, cutaneous metastasis can present in a variety of ways. The most common presentation is nodular lesions that may coalesce to become zosteriform in configuration or lesions that mimic inflammatory dermatoses.7 Cutaneous metastasis is more common in breast and lung cancer, and when it occurs secondary to colorectal cancer, cutaneous metastasis rarely predates the detection of the primary neoplasm.2
The clinical appearance of metastasis is not specific and can mimic many entities8; therefore, a high index of suspicion must be employed when managing patients, even those without a history of internal malignancy. In our patient, the smooth nodular lesion appeared similar to a basal cell carcinoma; however, basal cell carcinomas appear more pearly, and arborizing telangiectasia often is seen.9 Merkel cell carcinoma is common on sundamaged skin of the head and neck but clinically appears more violaceous than the lesion seen in our patient.10 Paracoccidioidomycosis may form ulcerated papulonodules or plaques, especially around the nose and mouth. In many of these cases, lesions develop from contiguous lesions of the oral mucosa; therefore, the presence of oral lesions will help distinguish this infectious entity from cutaneous metastasis. Multiple lesions usually are identified when there is hematogenous dissemination.11 Mycosis fungoides is a subtype of cutaneous T-cell lymphoma and is characterized by multiple patches, plaques, and nodules on sun-protected areas. Involvement of the head and neck is not common, except in the folliculotropic subtype, which has a separate and distinct clinical morphology.12
The development of signet ring morphology from an adenocarcinoma can be attributed to the activation of phosphatidylinositol 3-kinase (PI3K), which leads to downstream activation of mitogen-activated protein kinase (MAPK) and the subsequent loss of intercellular tight junctions. The mucin 4 gene, MUC4, also is upregulated by PI3K activation and possesses antiapoptotic and mitogenic effects in addition to its mucin secretory function.13
The neoplastic cells in SRCAs stain positive for mucicarmine, Alcian blue, and periodic acid–Schiff, which highlights the mucinous component of the cells.7 Immunohistochemical stains with CK7, CK20, AE1/AE3, and epithelial membrane antigen can be implemented to confirm an epithelial origin of the primary cancer.7,13 CK20 is a low-molecular-weight cytokeratin normally expressed by Merkel cells and by the epithelium of the gastrointestinal tract and urothelium, whereas CK7 expression typically is expressed in the lungs, ovaries, endometrium, and breasts, but not in the lower gastrointestinal tract.14 Differentiating primary cutaneous adenocarcinoma from cutaneous metastasis can be accomplished with a thorough clinical history; however, p63 positivity supports a primary cutaneous lesion and may be useful in certain situations.7 CDX2 stains can be utilized to aid in localizing the primary neoplasm when it is unknown, and when positive, it suggests a lower gastrointestinal tract origin. However, special AT-rich sequence-binding protein 2 (SATB2) recently has been proposed as a replacement immunohistochemical marker for CDX2, as it has greater specificity for SRCA of the lower gastrointestinal tract.15 Benign entities with signet ring cell morphology are difficult to distinguish from SRCA; however, malignant lesions are more likely to demonstrate an infiltrative growth pattern, frequent mitotic figures, and apoptosis. Immunohistochemistry also can be utilized to support the diagnosis of benign proliferation with signet ring morphology, as benign lesions often will demonstrate E-cadherin positivity and negativity for p53 and Ki-67.13
Cutaneous metastasis usually correlates to advanced disease and generally indicates a worse prognosis.13 Signet ring cell morphology in both gastric and colorectal cancer portends a poor prognosis, and there is a lower overall survival in patients with these malignancies compared to cancers of the same organ with non–signet ring cell morphology.4,8
The Diagnosis: Cutaneous Metastasis
A shave biopsy of the lip revealed a diffuse cellular infiltrate filling the superficial and deep dermis (Figure 1A). Morphologically, the cells had abundant clear cytoplasm with eccentrically located, pleomorphic, hyperchromatic nuclei with occasional prominent nucleoli (Figure 1B). The cells stained positive for AE1/ AE3 on immunohistochemistry (Figure 2). A punch biopsy of the nodule in the right axillary vault revealed a morphologically similar proliferation of cells. A colonoscopy revealed a completely obstructing circumferential mass in the distal ascending colon. A biopsy of the mass confirmed invasive adenocarcinoma, supporting a diagnosis of cutaneous metastases from adenocarcinoma of the colon. The patient underwent resection of the lip tumor and started multiagent chemotherapy for his newly diagnosed stage IV adenocarcinoma of the colon. The patient died, despite therapy.

Cutaneous metastasis from solid malignancies is uncommon, as only 1.3% of them exhibit cutaneous manifestations at presentation.1 Cutaneous metastasis from signet ring cell adenocarcinoma (SRCA) of the colon is uncommon, and cutaneous metastasis of colorectal SRCA rarely precedes the diagnosis of the primary lesion.2 Among the colorectal cancers that metastasize to the skin, metastasis to the face occurs in only 0.5% of patients.3

Signet ring cell adenocarcinomas are poorly differentiated adenocarcinomas histologically characterized by the neoplastic cells’ circular to ovoid appearance with a flattened top.4,5 This distinctive shape is from the displacement of the nucleus to the periphery of the cell due to the accumulation of intracytoplasmic mucin.4 Classically, malignancies are characterized as an SRCA if more than 50% of the cells have a signet ring cell morphology; if the signet ring cells comprise less than 50% of the neoplasm, the tumor is designated as an adenocarcinoma with signet ring morphology.4 The most common cause of cutaneous metastasis with signet ring morphology is gastric cancer, while colorectal carcinoma is less common.1 Colorectal SRCAs usually are found in the right colon or the rectum in comparison to other colonic sites.6
Clinically, cutaneous metastasis can present in a variety of ways. The most common presentation is nodular lesions that may coalesce to become zosteriform in configuration or lesions that mimic inflammatory dermatoses.7 Cutaneous metastasis is more common in breast and lung cancer, and when it occurs secondary to colorectal cancer, cutaneous metastasis rarely predates the detection of the primary neoplasm.2
The clinical appearance of metastasis is not specific and can mimic many entities8; therefore, a high index of suspicion must be employed when managing patients, even those without a history of internal malignancy. In our patient, the smooth nodular lesion appeared similar to a basal cell carcinoma; however, basal cell carcinomas appear more pearly, and arborizing telangiectasia often is seen.9 Merkel cell carcinoma is common on sundamaged skin of the head and neck but clinically appears more violaceous than the lesion seen in our patient.10 Paracoccidioidomycosis may form ulcerated papulonodules or plaques, especially around the nose and mouth. In many of these cases, lesions develop from contiguous lesions of the oral mucosa; therefore, the presence of oral lesions will help distinguish this infectious entity from cutaneous metastasis. Multiple lesions usually are identified when there is hematogenous dissemination.11 Mycosis fungoides is a subtype of cutaneous T-cell lymphoma and is characterized by multiple patches, plaques, and nodules on sun-protected areas. Involvement of the head and neck is not common, except in the folliculotropic subtype, which has a separate and distinct clinical morphology.12
The development of signet ring morphology from an adenocarcinoma can be attributed to the activation of phosphatidylinositol 3-kinase (PI3K), which leads to downstream activation of mitogen-activated protein kinase (MAPK) and the subsequent loss of intercellular tight junctions. The mucin 4 gene, MUC4, also is upregulated by PI3K activation and possesses antiapoptotic and mitogenic effects in addition to its mucin secretory function.13
The neoplastic cells in SRCAs stain positive for mucicarmine, Alcian blue, and periodic acid–Schiff, which highlights the mucinous component of the cells.7 Immunohistochemical stains with CK7, CK20, AE1/AE3, and epithelial membrane antigen can be implemented to confirm an epithelial origin of the primary cancer.7,13 CK20 is a low-molecular-weight cytokeratin normally expressed by Merkel cells and by the epithelium of the gastrointestinal tract and urothelium, whereas CK7 expression typically is expressed in the lungs, ovaries, endometrium, and breasts, but not in the lower gastrointestinal tract.14 Differentiating primary cutaneous adenocarcinoma from cutaneous metastasis can be accomplished with a thorough clinical history; however, p63 positivity supports a primary cutaneous lesion and may be useful in certain situations.7 CDX2 stains can be utilized to aid in localizing the primary neoplasm when it is unknown, and when positive, it suggests a lower gastrointestinal tract origin. However, special AT-rich sequence-binding protein 2 (SATB2) recently has been proposed as a replacement immunohistochemical marker for CDX2, as it has greater specificity for SRCA of the lower gastrointestinal tract.15 Benign entities with signet ring cell morphology are difficult to distinguish from SRCA; however, malignant lesions are more likely to demonstrate an infiltrative growth pattern, frequent mitotic figures, and apoptosis. Immunohistochemistry also can be utilized to support the diagnosis of benign proliferation with signet ring morphology, as benign lesions often will demonstrate E-cadherin positivity and negativity for p53 and Ki-67.13
Cutaneous metastasis usually correlates to advanced disease and generally indicates a worse prognosis.13 Signet ring cell morphology in both gastric and colorectal cancer portends a poor prognosis, and there is a lower overall survival in patients with these malignancies compared to cancers of the same organ with non–signet ring cell morphology.4,8
- Mandzhieva B, Jalil A, Nadeem M, et al. Most common pathway of metastasis of rectal signet ring cell carcinoma to the skin: hematogenous. Cureus. 2020;12:E6890.
- Parente P, Ciardiello D, Reggiani Bonetti L, et al. Cutaneous metastasis from colorectal cancer: making light on an unusual and misdiagnosed event. Life. 2021;11:954.
- Picciariello A, Tomasicchio G, Lantone G, et al. Synchronous “skip” facial metastases from colorectal adenocarcinoma: a case report and review of literature. BMC Gastroenterol. 2022;22:68.
- Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers. 2020;12:1544.
- Xu Q, Karouji Y, Kobayashi M, et al. The PI 3-kinase-Rac-p38 MAP kinase pathway is involved in the formation of signet-ring cell carcinoma. Oncogene. 2003;22:5537-5544.
- Morales-Cruz M, Salgado-Nesme N, Trolle-Silva AM, et al. Signet ring cell carcinoma of the rectum: atypical metastatic presentation. BMJ Case Rep CP. 2019;12:E229135.
- Demirciog˘lu D, Öztürk Durmaz E, Demirkesen C, et al. Livedoid cutaneous metastasis of signet‐ring cell gastric carcinoma. J Cutan Pathol. 2021;48:785-788.
- Dong X, Sun G, Qu H, et al. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg. 2021;8:642468.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract. 2017;4:2.
- Marques, SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610-615.
- Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome. Hematol Oncol Clin. 2019;33:103-120.
- Gündüz Ö, Emeksiz MC, Atasoy P, et al. Signet-ring cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8:6819.
- Al-Taee A, Almukhtar R, Lai J, et al. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016;4:283.
- Ma C, Lowenthal BM, Pai RK. SATB2 is superior to CDX2 in distinguishing signet ring cell carcinoma of the upper gastrointestinal tract and lower gastrointestinal tract. Am J Surg Pathol. 2018; 42:1715-1722.
- Mandzhieva B, Jalil A, Nadeem M, et al. Most common pathway of metastasis of rectal signet ring cell carcinoma to the skin: hematogenous. Cureus. 2020;12:E6890.
- Parente P, Ciardiello D, Reggiani Bonetti L, et al. Cutaneous metastasis from colorectal cancer: making light on an unusual and misdiagnosed event. Life. 2021;11:954.
- Picciariello A, Tomasicchio G, Lantone G, et al. Synchronous “skip” facial metastases from colorectal adenocarcinoma: a case report and review of literature. BMC Gastroenterol. 2022;22:68.
- Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers. 2020;12:1544.
- Xu Q, Karouji Y, Kobayashi M, et al. The PI 3-kinase-Rac-p38 MAP kinase pathway is involved in the formation of signet-ring cell carcinoma. Oncogene. 2003;22:5537-5544.
- Morales-Cruz M, Salgado-Nesme N, Trolle-Silva AM, et al. Signet ring cell carcinoma of the rectum: atypical metastatic presentation. BMJ Case Rep CP. 2019;12:E229135.
- Demirciog˘lu D, Öztürk Durmaz E, Demirkesen C, et al. Livedoid cutaneous metastasis of signet‐ring cell gastric carcinoma. J Cutan Pathol. 2021;48:785-788.
- Dong X, Sun G, Qu H, et al. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg. 2021;8:642468.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract. 2017;4:2.
- Marques, SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610-615.
- Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome. Hematol Oncol Clin. 2019;33:103-120.
- Gündüz Ö, Emeksiz MC, Atasoy P, et al. Signet-ring cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8:6819.
- Al-Taee A, Almukhtar R, Lai J, et al. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016;4:283.
- Ma C, Lowenthal BM, Pai RK. SATB2 is superior to CDX2 in distinguishing signet ring cell carcinoma of the upper gastrointestinal tract and lower gastrointestinal tract. Am J Surg Pathol. 2018; 42:1715-1722.
A 79-year-old man with a medical history of type 2 diabetes mellitus, hypothyroidism, and atrial fibrillation presented with an enlarging lesion on the right side of the upper cutaneous lip of 5 weeks’ duration. He had no personal history of skin cancer or other malignancy and was up to date on all routine cancer screenings. He reported associated lip and oral cavity tenderness, weakness, and a 13.6-kg (30-lb) unintentional weight loss over the last 6 months. He had used over-the-counter bacitracin ointment on the lesion without relief. A full-body skin examination revealed a firm, mobile, flesh-colored, nondraining nodule in the right axillary vault.
‘Landmark’ trial shows opioids for back, neck pain no better than placebo
Opioids do not relieve acute low back or neck pain in the short term and lead to worse outcomes in the long term, results of the first randomized controlled trial testing the efficacy and safety of a short course of opioids for acute nonspecific low back/neck pain suggest.
After 6 weeks, there was no significant difference in pain scores of patients who took opioids, compared with those who took placebo. After 1 year, patients given the placebo had slightly lower pain scores. Also, patients using opioids were at greater risk of opioid misuse after 1 year.
This is a “landmark” trial with “practice-changing” results, senior author Christine Lin, PhD, with the University of Sydney, told this news organization.
“Before this trial, we did not have good evidence on whether opioids were effective for acute low back pain or neck pain, yet opioids were one of the most commonly used medicines for these conditions,” Dr. Lin explained.
On the basis of these results, “opioids should not be recommended at all for acute low back pain and neck pain,” Dr. Lin said.
Results of the OPAL study were published online in The Lancet.
Rigorous trial
The trial was conducted in 157 primary care or emergency department sites in Australia and involved 347 adults who had been experiencing low back pain, neck pain, or both for 12 weeks or less.
They were randomly allocated (1:1) to receive guideline-recommended care (reassurance and advice to stay active) plus an opioid (oxycodone up to 20 mg daily) or identical placebo for up to 6 weeks. Naloxone was provided to help prevent opioid-induced constipation and improve blinding.
The primary outcome was pain severity at 6 weeks, measured with the pain severity subscale of the Brief Pain Inventory (10-point scale).
After 6 weeks, opioid therapy offered no more relief for acute back/neck pain or functional improvement than placebo.
The mean pain score at 6 weeks was 2.78 in the opioid group, versus 2.25 in the placebo group (adjusted mean difference, 0.53; 95% confidence interval, –0.00 to 1.07; P = .051). At 1 year, mean pain scores in the placebo group were slightly lower than in the opioid group (1.8 vs. 2.4).
In addition, there was a doubling of the risk of opioid misuse at 1 year among patients randomly allocated to receive opioid therapy for 6 weeks, compared with those allocated to receive placebo for 6 weeks.
At 1 year, 24 (20%) of 123 of the patients who received opioids were at risk of misuse, as indicated by the Current Opioid Misuse Measure scale, compared with 13 (10%) of 128 patients in the placebo group (P = .049). The COMM is a widely used measure of current aberrant drug-related behavior among patients with chronic pain who are being prescribed opioid therapy.
Results raise ‘serious questions’
“I believe the findings of the study will need to be disseminated to the doctors and patients, so they receive this latest evidence on opioids,” Dr. Lin said in an interview.
“We need to reassure doctors and patients that most people with acute low back pain and neck pain recover well with time (usually by 6 weeks), so management is simple – staying active, avoiding bed rest, and, if necessary, using a heat pack for short term pain relief. If drugs are required, consider anti-inflammatory drugs,” Dr. Lin added.
The authors of a linked comment say the OPAL trial “raises serious questions about the use of opioid therapy for acute low back and neck pain.”
Mark Sullivan, MD, PhD, and Jane Ballantyne, MD, with the University of Washington, Seattle, note that current clinical guidelines recommend opioids for patients with acute back and neck pain when other drug treatments fail or are contraindicated.
“As many as two-thirds of patients might receive an opioid when presenting for care of back or neck pain. It is time to re-examine these guidelines and these practices,” Dr. Sullivan and Dr. Ballantyne conclude.
Funding for the OPAL study was provided by the National Health and Medical Research Council, the University of Sydney Faculty of Medicine and Health, and SafeWork SA. The study authors have disclosed no relevant financial relationships. Dr. Sullivan and Dr. Ballantyne are board members (unpaid) of Physicians for Responsible Opioid Prescribing and have been paid consultants in opioid litigation.
A version of this article originally appeared on Medscape.com.
Opioids do not relieve acute low back or neck pain in the short term and lead to worse outcomes in the long term, results of the first randomized controlled trial testing the efficacy and safety of a short course of opioids for acute nonspecific low back/neck pain suggest.
After 6 weeks, there was no significant difference in pain scores of patients who took opioids, compared with those who took placebo. After 1 year, patients given the placebo had slightly lower pain scores. Also, patients using opioids were at greater risk of opioid misuse after 1 year.
This is a “landmark” trial with “practice-changing” results, senior author Christine Lin, PhD, with the University of Sydney, told this news organization.
“Before this trial, we did not have good evidence on whether opioids were effective for acute low back pain or neck pain, yet opioids were one of the most commonly used medicines for these conditions,” Dr. Lin explained.
On the basis of these results, “opioids should not be recommended at all for acute low back pain and neck pain,” Dr. Lin said.
Results of the OPAL study were published online in The Lancet.
Rigorous trial
The trial was conducted in 157 primary care or emergency department sites in Australia and involved 347 adults who had been experiencing low back pain, neck pain, or both for 12 weeks or less.
They were randomly allocated (1:1) to receive guideline-recommended care (reassurance and advice to stay active) plus an opioid (oxycodone up to 20 mg daily) or identical placebo for up to 6 weeks. Naloxone was provided to help prevent opioid-induced constipation and improve blinding.
The primary outcome was pain severity at 6 weeks, measured with the pain severity subscale of the Brief Pain Inventory (10-point scale).
After 6 weeks, opioid therapy offered no more relief for acute back/neck pain or functional improvement than placebo.
The mean pain score at 6 weeks was 2.78 in the opioid group, versus 2.25 in the placebo group (adjusted mean difference, 0.53; 95% confidence interval, –0.00 to 1.07; P = .051). At 1 year, mean pain scores in the placebo group were slightly lower than in the opioid group (1.8 vs. 2.4).
In addition, there was a doubling of the risk of opioid misuse at 1 year among patients randomly allocated to receive opioid therapy for 6 weeks, compared with those allocated to receive placebo for 6 weeks.
At 1 year, 24 (20%) of 123 of the patients who received opioids were at risk of misuse, as indicated by the Current Opioid Misuse Measure scale, compared with 13 (10%) of 128 patients in the placebo group (P = .049). The COMM is a widely used measure of current aberrant drug-related behavior among patients with chronic pain who are being prescribed opioid therapy.
Results raise ‘serious questions’
“I believe the findings of the study will need to be disseminated to the doctors and patients, so they receive this latest evidence on opioids,” Dr. Lin said in an interview.
“We need to reassure doctors and patients that most people with acute low back pain and neck pain recover well with time (usually by 6 weeks), so management is simple – staying active, avoiding bed rest, and, if necessary, using a heat pack for short term pain relief. If drugs are required, consider anti-inflammatory drugs,” Dr. Lin added.
The authors of a linked comment say the OPAL trial “raises serious questions about the use of opioid therapy for acute low back and neck pain.”
Mark Sullivan, MD, PhD, and Jane Ballantyne, MD, with the University of Washington, Seattle, note that current clinical guidelines recommend opioids for patients with acute back and neck pain when other drug treatments fail or are contraindicated.
“As many as two-thirds of patients might receive an opioid when presenting for care of back or neck pain. It is time to re-examine these guidelines and these practices,” Dr. Sullivan and Dr. Ballantyne conclude.
Funding for the OPAL study was provided by the National Health and Medical Research Council, the University of Sydney Faculty of Medicine and Health, and SafeWork SA. The study authors have disclosed no relevant financial relationships. Dr. Sullivan and Dr. Ballantyne are board members (unpaid) of Physicians for Responsible Opioid Prescribing and have been paid consultants in opioid litigation.
A version of this article originally appeared on Medscape.com.
Opioids do not relieve acute low back or neck pain in the short term and lead to worse outcomes in the long term, results of the first randomized controlled trial testing the efficacy and safety of a short course of opioids for acute nonspecific low back/neck pain suggest.
After 6 weeks, there was no significant difference in pain scores of patients who took opioids, compared with those who took placebo. After 1 year, patients given the placebo had slightly lower pain scores. Also, patients using opioids were at greater risk of opioid misuse after 1 year.
This is a “landmark” trial with “practice-changing” results, senior author Christine Lin, PhD, with the University of Sydney, told this news organization.
“Before this trial, we did not have good evidence on whether opioids were effective for acute low back pain or neck pain, yet opioids were one of the most commonly used medicines for these conditions,” Dr. Lin explained.
On the basis of these results, “opioids should not be recommended at all for acute low back pain and neck pain,” Dr. Lin said.
Results of the OPAL study were published online in The Lancet.
Rigorous trial
The trial was conducted in 157 primary care or emergency department sites in Australia and involved 347 adults who had been experiencing low back pain, neck pain, or both for 12 weeks or less.
They were randomly allocated (1:1) to receive guideline-recommended care (reassurance and advice to stay active) plus an opioid (oxycodone up to 20 mg daily) or identical placebo for up to 6 weeks. Naloxone was provided to help prevent opioid-induced constipation and improve blinding.
The primary outcome was pain severity at 6 weeks, measured with the pain severity subscale of the Brief Pain Inventory (10-point scale).
After 6 weeks, opioid therapy offered no more relief for acute back/neck pain or functional improvement than placebo.
The mean pain score at 6 weeks was 2.78 in the opioid group, versus 2.25 in the placebo group (adjusted mean difference, 0.53; 95% confidence interval, –0.00 to 1.07; P = .051). At 1 year, mean pain scores in the placebo group were slightly lower than in the opioid group (1.8 vs. 2.4).
In addition, there was a doubling of the risk of opioid misuse at 1 year among patients randomly allocated to receive opioid therapy for 6 weeks, compared with those allocated to receive placebo for 6 weeks.
At 1 year, 24 (20%) of 123 of the patients who received opioids were at risk of misuse, as indicated by the Current Opioid Misuse Measure scale, compared with 13 (10%) of 128 patients in the placebo group (P = .049). The COMM is a widely used measure of current aberrant drug-related behavior among patients with chronic pain who are being prescribed opioid therapy.
Results raise ‘serious questions’
“I believe the findings of the study will need to be disseminated to the doctors and patients, so they receive this latest evidence on opioids,” Dr. Lin said in an interview.
“We need to reassure doctors and patients that most people with acute low back pain and neck pain recover well with time (usually by 6 weeks), so management is simple – staying active, avoiding bed rest, and, if necessary, using a heat pack for short term pain relief. If drugs are required, consider anti-inflammatory drugs,” Dr. Lin added.
The authors of a linked comment say the OPAL trial “raises serious questions about the use of opioid therapy for acute low back and neck pain.”
Mark Sullivan, MD, PhD, and Jane Ballantyne, MD, with the University of Washington, Seattle, note that current clinical guidelines recommend opioids for patients with acute back and neck pain when other drug treatments fail or are contraindicated.
“As many as two-thirds of patients might receive an opioid when presenting for care of back or neck pain. It is time to re-examine these guidelines and these practices,” Dr. Sullivan and Dr. Ballantyne conclude.
Funding for the OPAL study was provided by the National Health and Medical Research Council, the University of Sydney Faculty of Medicine and Health, and SafeWork SA. The study authors have disclosed no relevant financial relationships. Dr. Sullivan and Dr. Ballantyne are board members (unpaid) of Physicians for Responsible Opioid Prescribing and have been paid consultants in opioid litigation.
A version of this article originally appeared on Medscape.com.
FDA approves first gene therapy for hemophilia A
Valoctocogene roxaparvovec, a one-time, single-dose IV infusion, is the first gene therapy approved in the United States for severe hemophilia A and will cost around $2.9 million. BioMarin has said the price tag reflects “the possibility of freedom from years” of infusions, which come to about $800,000 each year.
However, last December, the Institute for Clinical and Economic Review (ICER) set the upper bounds for the gene therapy at about $1.96 million. The extent to which the gene therapy will provide freedom from infusions, for how long, and in which patients are not completely understood.
Hemophilia A is caused by a mutation in the gene that produces a protein called coagulation factor VIII, which is essential for blood clotting. Valoctocogene roxaparvovec delivers a functional gene to liver cells via an adeno-associated virus serotype 5; the gene instructs the cells to make the missing clotting factor.
“Adults with severe hemophilia A face a lifelong burden, with frequent infusions and a high risk of health complications, including uncontrolled bleeding and irreversible joint damage,” Steven Pipe, MD, professor of pediatrics and pathology at the University of Michigan, Ann Arbor, and an investigator for the phase 3 study that led to the approval, said in a statement. The approval of valoctocogene roxaparvovec “has the potential to transform the way we treat adults based on years of bleed control following a single, one-time infusion.”
About 6,500 U.S. adults live with severe hemophilia A, and BioMarin said it anticipates approximately 2,500 will be eligible to receive the gene therapy following the approval. The U.S. indication is limited to patients without a history of factor VIII inhibitors and without detectable antibodies to the adeno-associated virus serotype 5.
Last August, the European Medicines Agency authorized the gene therapy for use in Europe, but according to Forbes and PharmaPhorum, uptake in Europe has been delayed because of reimbursement issues, given the cost of treatment and clinical uncertainties.
Data to date, however, are promising for most patients. Approval was based on BioMarin’s open-label, single-arm GENEr8-1 study in 134 men with severe congenital hemophilia A. Patients received a single infusion of 6 x 1013 vector genomes per kilogram.
Among the 132 patients available for 2-year evaluation, median factor VIII activity was in the range for mild hemophilia (6%-39% of normal) with an 84.5% reduction in bleeding events from baseline.
More than 80% of participants had no bleeding events requiring treatment, and there was a 98% reduction from baseline in mean use of exogenous factor VIII.
Overall, at 2 years, only 4.5% of patients had factor VIII activity consistent with severe hemophilia A; 9.1% had activity consistent with moderate disease; 59.8% had activity consistent with mild disease, and 26.5% had activity in the normal range above 40 IU/dL.
Trial investigators estimated that the typical half-life of the transgene-derived factor VIII production system is 123 weeks.
Among the six men who resumed prophylaxis, most had fewer bleeding events than when they were on prophylaxis before the infusion. All patients developed antibodies to the virus delivery vector, precluding retreatment.
Elevated alanine aminotransferase levels were the most common adverse event, occurring in 88.8% of patients, who were treated with immunosuppressants for a median of 33 weeks. Elevations persisted at 2 years in 29% of patients.
The other most common adverse events were headache (38.1%), nausea (37.3%), and increases in aspartate aminotransferase (35.1%).
A version of this article first appeared on Medscape.com.
Valoctocogene roxaparvovec, a one-time, single-dose IV infusion, is the first gene therapy approved in the United States for severe hemophilia A and will cost around $2.9 million. BioMarin has said the price tag reflects “the possibility of freedom from years” of infusions, which come to about $800,000 each year.
However, last December, the Institute for Clinical and Economic Review (ICER) set the upper bounds for the gene therapy at about $1.96 million. The extent to which the gene therapy will provide freedom from infusions, for how long, and in which patients are not completely understood.
Hemophilia A is caused by a mutation in the gene that produces a protein called coagulation factor VIII, which is essential for blood clotting. Valoctocogene roxaparvovec delivers a functional gene to liver cells via an adeno-associated virus serotype 5; the gene instructs the cells to make the missing clotting factor.
“Adults with severe hemophilia A face a lifelong burden, with frequent infusions and a high risk of health complications, including uncontrolled bleeding and irreversible joint damage,” Steven Pipe, MD, professor of pediatrics and pathology at the University of Michigan, Ann Arbor, and an investigator for the phase 3 study that led to the approval, said in a statement. The approval of valoctocogene roxaparvovec “has the potential to transform the way we treat adults based on years of bleed control following a single, one-time infusion.”
About 6,500 U.S. adults live with severe hemophilia A, and BioMarin said it anticipates approximately 2,500 will be eligible to receive the gene therapy following the approval. The U.S. indication is limited to patients without a history of factor VIII inhibitors and without detectable antibodies to the adeno-associated virus serotype 5.
Last August, the European Medicines Agency authorized the gene therapy for use in Europe, but according to Forbes and PharmaPhorum, uptake in Europe has been delayed because of reimbursement issues, given the cost of treatment and clinical uncertainties.
Data to date, however, are promising for most patients. Approval was based on BioMarin’s open-label, single-arm GENEr8-1 study in 134 men with severe congenital hemophilia A. Patients received a single infusion of 6 x 1013 vector genomes per kilogram.
Among the 132 patients available for 2-year evaluation, median factor VIII activity was in the range for mild hemophilia (6%-39% of normal) with an 84.5% reduction in bleeding events from baseline.
More than 80% of participants had no bleeding events requiring treatment, and there was a 98% reduction from baseline in mean use of exogenous factor VIII.
Overall, at 2 years, only 4.5% of patients had factor VIII activity consistent with severe hemophilia A; 9.1% had activity consistent with moderate disease; 59.8% had activity consistent with mild disease, and 26.5% had activity in the normal range above 40 IU/dL.
Trial investigators estimated that the typical half-life of the transgene-derived factor VIII production system is 123 weeks.
Among the six men who resumed prophylaxis, most had fewer bleeding events than when they were on prophylaxis before the infusion. All patients developed antibodies to the virus delivery vector, precluding retreatment.
Elevated alanine aminotransferase levels were the most common adverse event, occurring in 88.8% of patients, who were treated with immunosuppressants for a median of 33 weeks. Elevations persisted at 2 years in 29% of patients.
The other most common adverse events were headache (38.1%), nausea (37.3%), and increases in aspartate aminotransferase (35.1%).
A version of this article first appeared on Medscape.com.
Valoctocogene roxaparvovec, a one-time, single-dose IV infusion, is the first gene therapy approved in the United States for severe hemophilia A and will cost around $2.9 million. BioMarin has said the price tag reflects “the possibility of freedom from years” of infusions, which come to about $800,000 each year.
However, last December, the Institute for Clinical and Economic Review (ICER) set the upper bounds for the gene therapy at about $1.96 million. The extent to which the gene therapy will provide freedom from infusions, for how long, and in which patients are not completely understood.
Hemophilia A is caused by a mutation in the gene that produces a protein called coagulation factor VIII, which is essential for blood clotting. Valoctocogene roxaparvovec delivers a functional gene to liver cells via an adeno-associated virus serotype 5; the gene instructs the cells to make the missing clotting factor.
“Adults with severe hemophilia A face a lifelong burden, with frequent infusions and a high risk of health complications, including uncontrolled bleeding and irreversible joint damage,” Steven Pipe, MD, professor of pediatrics and pathology at the University of Michigan, Ann Arbor, and an investigator for the phase 3 study that led to the approval, said in a statement. The approval of valoctocogene roxaparvovec “has the potential to transform the way we treat adults based on years of bleed control following a single, one-time infusion.”
About 6,500 U.S. adults live with severe hemophilia A, and BioMarin said it anticipates approximately 2,500 will be eligible to receive the gene therapy following the approval. The U.S. indication is limited to patients without a history of factor VIII inhibitors and without detectable antibodies to the adeno-associated virus serotype 5.
Last August, the European Medicines Agency authorized the gene therapy for use in Europe, but according to Forbes and PharmaPhorum, uptake in Europe has been delayed because of reimbursement issues, given the cost of treatment and clinical uncertainties.
Data to date, however, are promising for most patients. Approval was based on BioMarin’s open-label, single-arm GENEr8-1 study in 134 men with severe congenital hemophilia A. Patients received a single infusion of 6 x 1013 vector genomes per kilogram.
Among the 132 patients available for 2-year evaluation, median factor VIII activity was in the range for mild hemophilia (6%-39% of normal) with an 84.5% reduction in bleeding events from baseline.
More than 80% of participants had no bleeding events requiring treatment, and there was a 98% reduction from baseline in mean use of exogenous factor VIII.
Overall, at 2 years, only 4.5% of patients had factor VIII activity consistent with severe hemophilia A; 9.1% had activity consistent with moderate disease; 59.8% had activity consistent with mild disease, and 26.5% had activity in the normal range above 40 IU/dL.
Trial investigators estimated that the typical half-life of the transgene-derived factor VIII production system is 123 weeks.
Among the six men who resumed prophylaxis, most had fewer bleeding events than when they were on prophylaxis before the infusion. All patients developed antibodies to the virus delivery vector, precluding retreatment.
Elevated alanine aminotransferase levels were the most common adverse event, occurring in 88.8% of patients, who were treated with immunosuppressants for a median of 33 weeks. Elevations persisted at 2 years in 29% of patients.
The other most common adverse events were headache (38.1%), nausea (37.3%), and increases in aspartate aminotransferase (35.1%).
A version of this article first appeared on Medscape.com.
For psoriasis, review finds several biosimilars as safe and effective as biologics
The effectiveness and safety of biosimilars for psoriasis appear to be similar to the originator biologics, reported the authors of a review of studies comparing the two.
“This systematic review found that there was no clinically or statistically significant difference in the efficacy and safety between biosimilars and originators of adalimumab, etanercept, infliximab, and ustekinumab for the treatment of psoriasis,” senior study author and clinical lecturer Zenas Z. N. Yiu, MBChB, PhD, and his colleagues at the University of Manchester, England, wrote in JAMA Dermatology.“The biosimilars evaluated in this study could be considered alongside originators for biologic-naive patients to improve the accessibility of biological treatments,” they added. “Switching patients currently on originators to biosimilars could be considered where clinically appropriate to reduce treatment costs.”
Biologics versus biosimilars
In contrast to most chemically synthesized drugs, biologics are created from living organisms, and they have complex structures that can vary slightly from batch to batch, Luigi Naldi, MD, director of the department of dermatology of Ospedale San Bortolo, Vicenza, Italy, and Antonio Addis, PharmD, researcher in the department of epidemiology, Regione Lazio, in Rome, wrote in an accompanying editorial.
Once the patent on the “originator” biologic expires, U.S. and European regulators allow other manufacturers to develop similar molecules – biosimilars – through an abbreviated approval process. If the results of a limited number of equivalence or noninferiority clinical trials are acceptable, registration for all the indications of the originator is allowed for its biosimilars. Referring to the expense of biologics, Dr. Naldi and Dr. Addis noted that in the United States, “biologics comprise less than 3% of the volume of drugs on the market, but account for more than one-third of all drug spending.”
Systematic review
Dr. Yiu and his colleagues queried standard medical research databases in August 2022, and included 14 randomized clinical trials (10 adalimumab, 2 etanercept, 1 infliximab, and 1 ustekinumab) and 3 cohort studies (1 adalimumab, 1 etanercept, 1 infliximab and etanercept) in their review.
Twelve trials compared biosimilars vs. originators in originator-naive patients, and 11 trials compared switching from originators to biosimilars vs. continuous treatment with the originator.
The researchers found the following:
At week 16, mean PASI75 (Psoriasis Area and Severity Index) response rates ranges from 60.7% to 90.6% for adalimumab biosimilars, vs. 61.5% to 91.7% for the originator. Mean PASI75 responses for the two etanercept biosimilars were 56.1% and 76.7% vs. 55.5% and 73.4% for the originator. In the ustekinumab study, mean PASI75 responses were 86.1% for the biosimilar vs. 84.0% for the originator.
At week 52, mean PASI75 responses were between 86.3% and 92.8% for adalimumab biosimilars vs. 84.9% and 93.9% for the originator. In the one comparison of an etanercept biosimilar, mean PAS175 responses were 80.9% for the biosimilar vs. 82.9% for the originator.
In studies involving patients switching from the originator to a biosimilar vs. continuing treatment with the originator, 32-week response rates ranged from 87.0% to 91.3% for adalimumab biosimilars and from 88.2% to 93.2% for the originator. In the one ustekinumab study, the 32-week mean PASI75 response was 92.6% after switching from the originator to a biosimilar vs. 92.9% with continuous treatment with the originator.
At week 52, mean PASI75 responses to adalimumab were between 84.2% and 94.8% for patients who switched to biosimilars and between 88.1% and 93.9% for those who stayed on the originator.
At week 52, in all the randomized trials, the incidence of adverse events and serious adverse events among those who switched to the biosimilar and those who continued with the originator were similar. Two cohort studies showed similar safety outcomes between originators and biosimilars, but one reported more adverse events in patients who switched to adalimumab biosimilars (P = .04).
Three clinical trials showed low risk for bias, 11 had moderate risk, and all cohort studies had moderate to high risk for bias.
Experts weigh in
Asked to comment on the study, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., told this news organization that he expects that the results will affect patient care.
However, he added, “I believe the decision of whether to use a biosimilar instead of the originator biologic may be more in the hands of the insurers than in the hands of physicians and patients.
“Biologics for psoriasis are so complicated that even the originator products vary from batch to batch. A biosimilar is basically like another batch of the innovative product,” explained Dr. Feldman, who was not involved in the study. “If we’re comfortable with patients being on different batches of the innovator product, we probably should be comfortable with them being on a biosimilar, as we have more evidence for the similarity of the biosimilar than we do for the current batch of the originator product.”
Aída Lugo-Somolinos, MD, professor of dermatology and director of the Contact Dermatitis Clinic at the University of North Carolina, Chapel Hill, said that “biologics have become the treatment of choice for moderate to severe psoriasis, and the use of biosimilars may be an alternative to reduce psoriasis treatment costs.
“Unfortunately, this study included a comparison of the existing biosimilars, which are drugs that are not the first line of treatment for psoriasis any longer,” added Dr. Lugo-Somolinos, who was not involved in the study.
Neil J. Korman, MD, PhD, professor of dermatology and codirector of the Skin Study Center at Case Western Reserve University, Cleveland, said the study was an important systematic review.
“This is a very timely publication because in the United States, several biosimilars are reaching the market in 2023,” he said. “The costs of the originator biologics are extraordinarily high, and the promise of biosimilars is that their costs will be significantly lower.”
Because all the studies were short term, Dr. Korman, who was not involved in the study, joins the study authors in recommending further related research into the long-term safety and efficacy of these agents.
Dr. Feldman, as well as one study author and one editorial author, reported relevant relationships with various pharmaceutical companies, including those that develop biosimilars. The remaining study authors, as well as Dr. Lugo-Somolinos and Dr. Korman, reported no relevant relationships. The study was funded by the Psoriasis Association and supported by the NIHR (National Institute for Health and Care Research) Manchester Biomedical Research Centre. All outside experts commented by email.
The effectiveness and safety of biosimilars for psoriasis appear to be similar to the originator biologics, reported the authors of a review of studies comparing the two.
“This systematic review found that there was no clinically or statistically significant difference in the efficacy and safety between biosimilars and originators of adalimumab, etanercept, infliximab, and ustekinumab for the treatment of psoriasis,” senior study author and clinical lecturer Zenas Z. N. Yiu, MBChB, PhD, and his colleagues at the University of Manchester, England, wrote in JAMA Dermatology.“The biosimilars evaluated in this study could be considered alongside originators for biologic-naive patients to improve the accessibility of biological treatments,” they added. “Switching patients currently on originators to biosimilars could be considered where clinically appropriate to reduce treatment costs.”
Biologics versus biosimilars
In contrast to most chemically synthesized drugs, biologics are created from living organisms, and they have complex structures that can vary slightly from batch to batch, Luigi Naldi, MD, director of the department of dermatology of Ospedale San Bortolo, Vicenza, Italy, and Antonio Addis, PharmD, researcher in the department of epidemiology, Regione Lazio, in Rome, wrote in an accompanying editorial.
Once the patent on the “originator” biologic expires, U.S. and European regulators allow other manufacturers to develop similar molecules – biosimilars – through an abbreviated approval process. If the results of a limited number of equivalence or noninferiority clinical trials are acceptable, registration for all the indications of the originator is allowed for its biosimilars. Referring to the expense of biologics, Dr. Naldi and Dr. Addis noted that in the United States, “biologics comprise less than 3% of the volume of drugs on the market, but account for more than one-third of all drug spending.”
Systematic review
Dr. Yiu and his colleagues queried standard medical research databases in August 2022, and included 14 randomized clinical trials (10 adalimumab, 2 etanercept, 1 infliximab, and 1 ustekinumab) and 3 cohort studies (1 adalimumab, 1 etanercept, 1 infliximab and etanercept) in their review.
Twelve trials compared biosimilars vs. originators in originator-naive patients, and 11 trials compared switching from originators to biosimilars vs. continuous treatment with the originator.
The researchers found the following:
At week 16, mean PASI75 (Psoriasis Area and Severity Index) response rates ranges from 60.7% to 90.6% for adalimumab biosimilars, vs. 61.5% to 91.7% for the originator. Mean PASI75 responses for the two etanercept biosimilars were 56.1% and 76.7% vs. 55.5% and 73.4% for the originator. In the ustekinumab study, mean PASI75 responses were 86.1% for the biosimilar vs. 84.0% for the originator.
At week 52, mean PASI75 responses were between 86.3% and 92.8% for adalimumab biosimilars vs. 84.9% and 93.9% for the originator. In the one comparison of an etanercept biosimilar, mean PAS175 responses were 80.9% for the biosimilar vs. 82.9% for the originator.
In studies involving patients switching from the originator to a biosimilar vs. continuing treatment with the originator, 32-week response rates ranged from 87.0% to 91.3% for adalimumab biosimilars and from 88.2% to 93.2% for the originator. In the one ustekinumab study, the 32-week mean PASI75 response was 92.6% after switching from the originator to a biosimilar vs. 92.9% with continuous treatment with the originator.
At week 52, mean PASI75 responses to adalimumab were between 84.2% and 94.8% for patients who switched to biosimilars and between 88.1% and 93.9% for those who stayed on the originator.
At week 52, in all the randomized trials, the incidence of adverse events and serious adverse events among those who switched to the biosimilar and those who continued with the originator were similar. Two cohort studies showed similar safety outcomes between originators and biosimilars, but one reported more adverse events in patients who switched to adalimumab biosimilars (P = .04).
Three clinical trials showed low risk for bias, 11 had moderate risk, and all cohort studies had moderate to high risk for bias.
Experts weigh in
Asked to comment on the study, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., told this news organization that he expects that the results will affect patient care.
However, he added, “I believe the decision of whether to use a biosimilar instead of the originator biologic may be more in the hands of the insurers than in the hands of physicians and patients.
“Biologics for psoriasis are so complicated that even the originator products vary from batch to batch. A biosimilar is basically like another batch of the innovative product,” explained Dr. Feldman, who was not involved in the study. “If we’re comfortable with patients being on different batches of the innovator product, we probably should be comfortable with them being on a biosimilar, as we have more evidence for the similarity of the biosimilar than we do for the current batch of the originator product.”
Aída Lugo-Somolinos, MD, professor of dermatology and director of the Contact Dermatitis Clinic at the University of North Carolina, Chapel Hill, said that “biologics have become the treatment of choice for moderate to severe psoriasis, and the use of biosimilars may be an alternative to reduce psoriasis treatment costs.
“Unfortunately, this study included a comparison of the existing biosimilars, which are drugs that are not the first line of treatment for psoriasis any longer,” added Dr. Lugo-Somolinos, who was not involved in the study.
Neil J. Korman, MD, PhD, professor of dermatology and codirector of the Skin Study Center at Case Western Reserve University, Cleveland, said the study was an important systematic review.
“This is a very timely publication because in the United States, several biosimilars are reaching the market in 2023,” he said. “The costs of the originator biologics are extraordinarily high, and the promise of biosimilars is that their costs will be significantly lower.”
Because all the studies were short term, Dr. Korman, who was not involved in the study, joins the study authors in recommending further related research into the long-term safety and efficacy of these agents.
Dr. Feldman, as well as one study author and one editorial author, reported relevant relationships with various pharmaceutical companies, including those that develop biosimilars. The remaining study authors, as well as Dr. Lugo-Somolinos and Dr. Korman, reported no relevant relationships. The study was funded by the Psoriasis Association and supported by the NIHR (National Institute for Health and Care Research) Manchester Biomedical Research Centre. All outside experts commented by email.
The effectiveness and safety of biosimilars for psoriasis appear to be similar to the originator biologics, reported the authors of a review of studies comparing the two.
“This systematic review found that there was no clinically or statistically significant difference in the efficacy and safety between biosimilars and originators of adalimumab, etanercept, infliximab, and ustekinumab for the treatment of psoriasis,” senior study author and clinical lecturer Zenas Z. N. Yiu, MBChB, PhD, and his colleagues at the University of Manchester, England, wrote in JAMA Dermatology.“The biosimilars evaluated in this study could be considered alongside originators for biologic-naive patients to improve the accessibility of biological treatments,” they added. “Switching patients currently on originators to biosimilars could be considered where clinically appropriate to reduce treatment costs.”
Biologics versus biosimilars
In contrast to most chemically synthesized drugs, biologics are created from living organisms, and they have complex structures that can vary slightly from batch to batch, Luigi Naldi, MD, director of the department of dermatology of Ospedale San Bortolo, Vicenza, Italy, and Antonio Addis, PharmD, researcher in the department of epidemiology, Regione Lazio, in Rome, wrote in an accompanying editorial.
Once the patent on the “originator” biologic expires, U.S. and European regulators allow other manufacturers to develop similar molecules – biosimilars – through an abbreviated approval process. If the results of a limited number of equivalence or noninferiority clinical trials are acceptable, registration for all the indications of the originator is allowed for its biosimilars. Referring to the expense of biologics, Dr. Naldi and Dr. Addis noted that in the United States, “biologics comprise less than 3% of the volume of drugs on the market, but account for more than one-third of all drug spending.”
Systematic review
Dr. Yiu and his colleagues queried standard medical research databases in August 2022, and included 14 randomized clinical trials (10 adalimumab, 2 etanercept, 1 infliximab, and 1 ustekinumab) and 3 cohort studies (1 adalimumab, 1 etanercept, 1 infliximab and etanercept) in their review.
Twelve trials compared biosimilars vs. originators in originator-naive patients, and 11 trials compared switching from originators to biosimilars vs. continuous treatment with the originator.
The researchers found the following:
At week 16, mean PASI75 (Psoriasis Area and Severity Index) response rates ranges from 60.7% to 90.6% for adalimumab biosimilars, vs. 61.5% to 91.7% for the originator. Mean PASI75 responses for the two etanercept biosimilars were 56.1% and 76.7% vs. 55.5% and 73.4% for the originator. In the ustekinumab study, mean PASI75 responses were 86.1% for the biosimilar vs. 84.0% for the originator.
At week 52, mean PASI75 responses were between 86.3% and 92.8% for adalimumab biosimilars vs. 84.9% and 93.9% for the originator. In the one comparison of an etanercept biosimilar, mean PAS175 responses were 80.9% for the biosimilar vs. 82.9% for the originator.
In studies involving patients switching from the originator to a biosimilar vs. continuing treatment with the originator, 32-week response rates ranged from 87.0% to 91.3% for adalimumab biosimilars and from 88.2% to 93.2% for the originator. In the one ustekinumab study, the 32-week mean PASI75 response was 92.6% after switching from the originator to a biosimilar vs. 92.9% with continuous treatment with the originator.
At week 52, mean PASI75 responses to adalimumab were between 84.2% and 94.8% for patients who switched to biosimilars and between 88.1% and 93.9% for those who stayed on the originator.
At week 52, in all the randomized trials, the incidence of adverse events and serious adverse events among those who switched to the biosimilar and those who continued with the originator were similar. Two cohort studies showed similar safety outcomes between originators and biosimilars, but one reported more adverse events in patients who switched to adalimumab biosimilars (P = .04).
Three clinical trials showed low risk for bias, 11 had moderate risk, and all cohort studies had moderate to high risk for bias.
Experts weigh in
Asked to comment on the study, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., told this news organization that he expects that the results will affect patient care.
However, he added, “I believe the decision of whether to use a biosimilar instead of the originator biologic may be more in the hands of the insurers than in the hands of physicians and patients.
“Biologics for psoriasis are so complicated that even the originator products vary from batch to batch. A biosimilar is basically like another batch of the innovative product,” explained Dr. Feldman, who was not involved in the study. “If we’re comfortable with patients being on different batches of the innovator product, we probably should be comfortable with them being on a biosimilar, as we have more evidence for the similarity of the biosimilar than we do for the current batch of the originator product.”
Aída Lugo-Somolinos, MD, professor of dermatology and director of the Contact Dermatitis Clinic at the University of North Carolina, Chapel Hill, said that “biologics have become the treatment of choice for moderate to severe psoriasis, and the use of biosimilars may be an alternative to reduce psoriasis treatment costs.
“Unfortunately, this study included a comparison of the existing biosimilars, which are drugs that are not the first line of treatment for psoriasis any longer,” added Dr. Lugo-Somolinos, who was not involved in the study.
Neil J. Korman, MD, PhD, professor of dermatology and codirector of the Skin Study Center at Case Western Reserve University, Cleveland, said the study was an important systematic review.
“This is a very timely publication because in the United States, several biosimilars are reaching the market in 2023,” he said. “The costs of the originator biologics are extraordinarily high, and the promise of biosimilars is that their costs will be significantly lower.”
Because all the studies were short term, Dr. Korman, who was not involved in the study, joins the study authors in recommending further related research into the long-term safety and efficacy of these agents.
Dr. Feldman, as well as one study author and one editorial author, reported relevant relationships with various pharmaceutical companies, including those that develop biosimilars. The remaining study authors, as well as Dr. Lugo-Somolinos and Dr. Korman, reported no relevant relationships. The study was funded by the Psoriasis Association and supported by the NIHR (National Institute for Health and Care Research) Manchester Biomedical Research Centre. All outside experts commented by email.
FROM JAMA DERMATOLOGY
FDA pilot program aims to reduce risk of diagnostic tests for cancer
The Food and Drug Administration recently released final guidance on a voluntary pilot program to help reduce the risks associated with certain diagnostic biomarker tests used to guide cancer treatment decisions for patients.
These laboratory-developed tests were designed to detect cancer biomarkers to help clinicians find the most appropriate cancer treatments for their patients. But the agency explained it has “become increasingly concerned that some tests made by laboratories and not authorized by the FDA may not provide accurate and reliable test results or perform as well as FDA-authorized tests.”
Currently, in most circumstances, an in vitro companion diagnostic would be granted marketing authorization alongside the approval of a corresponding cancer therapy. Under limited circumstances, however, the FDA may decide to approve a cancer therapy that requires a diagnostic test, which has not yet received marketing authorization. In these instances, “the benefits from the use of the therapeutic product are so pronounced as to outweigh the risks from the lack of an [in vitro companion diagnostic] with marketing authorization,” the FDA explained in 2014 in a guidance titled, In Vitro Companion Diagnostic Devices .
The new pilot program now aims to “address concerns and questions around the use of unauthorized diagnostics” and help improve cancer care for patients, Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, said in a press announcement.
The voluntary program will seek to provide greater transparency surrounding performance recommendations for these diagnostic tests. More specifically, the FDA will ask drug manufacturers to provide performance information for tests used to enroll patients in clinical trials that support drug approval. The agency will assess the performance information and establish the minimum performance criteria recommended for similar tests used to select cancer treatments for patients. The results, posted to the FDA’s website, may be used by laboratories to guide their development of diagnostic tests.
The FDA plans to evaluate no more than nine drug sponsors for the pilot program. This initial phase of the program is anticipated to last up to a year.
“We believe this guidance and the launch of the pilot program are important steps toward addressing safety risks posed by the use of poorly performing laboratory developed tests,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration recently released final guidance on a voluntary pilot program to help reduce the risks associated with certain diagnostic biomarker tests used to guide cancer treatment decisions for patients.
These laboratory-developed tests were designed to detect cancer biomarkers to help clinicians find the most appropriate cancer treatments for their patients. But the agency explained it has “become increasingly concerned that some tests made by laboratories and not authorized by the FDA may not provide accurate and reliable test results or perform as well as FDA-authorized tests.”
Currently, in most circumstances, an in vitro companion diagnostic would be granted marketing authorization alongside the approval of a corresponding cancer therapy. Under limited circumstances, however, the FDA may decide to approve a cancer therapy that requires a diagnostic test, which has not yet received marketing authorization. In these instances, “the benefits from the use of the therapeutic product are so pronounced as to outweigh the risks from the lack of an [in vitro companion diagnostic] with marketing authorization,” the FDA explained in 2014 in a guidance titled, In Vitro Companion Diagnostic Devices .
The new pilot program now aims to “address concerns and questions around the use of unauthorized diagnostics” and help improve cancer care for patients, Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, said in a press announcement.
The voluntary program will seek to provide greater transparency surrounding performance recommendations for these diagnostic tests. More specifically, the FDA will ask drug manufacturers to provide performance information for tests used to enroll patients in clinical trials that support drug approval. The agency will assess the performance information and establish the minimum performance criteria recommended for similar tests used to select cancer treatments for patients. The results, posted to the FDA’s website, may be used by laboratories to guide their development of diagnostic tests.
The FDA plans to evaluate no more than nine drug sponsors for the pilot program. This initial phase of the program is anticipated to last up to a year.
“We believe this guidance and the launch of the pilot program are important steps toward addressing safety risks posed by the use of poorly performing laboratory developed tests,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration recently released final guidance on a voluntary pilot program to help reduce the risks associated with certain diagnostic biomarker tests used to guide cancer treatment decisions for patients.
These laboratory-developed tests were designed to detect cancer biomarkers to help clinicians find the most appropriate cancer treatments for their patients. But the agency explained it has “become increasingly concerned that some tests made by laboratories and not authorized by the FDA may not provide accurate and reliable test results or perform as well as FDA-authorized tests.”
Currently, in most circumstances, an in vitro companion diagnostic would be granted marketing authorization alongside the approval of a corresponding cancer therapy. Under limited circumstances, however, the FDA may decide to approve a cancer therapy that requires a diagnostic test, which has not yet received marketing authorization. In these instances, “the benefits from the use of the therapeutic product are so pronounced as to outweigh the risks from the lack of an [in vitro companion diagnostic] with marketing authorization,” the FDA explained in 2014 in a guidance titled, In Vitro Companion Diagnostic Devices .
The new pilot program now aims to “address concerns and questions around the use of unauthorized diagnostics” and help improve cancer care for patients, Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, said in a press announcement.
The voluntary program will seek to provide greater transparency surrounding performance recommendations for these diagnostic tests. More specifically, the FDA will ask drug manufacturers to provide performance information for tests used to enroll patients in clinical trials that support drug approval. The agency will assess the performance information and establish the minimum performance criteria recommended for similar tests used to select cancer treatments for patients. The results, posted to the FDA’s website, may be used by laboratories to guide their development of diagnostic tests.
The FDA plans to evaluate no more than nine drug sponsors for the pilot program. This initial phase of the program is anticipated to last up to a year.
“We believe this guidance and the launch of the pilot program are important steps toward addressing safety risks posed by the use of poorly performing laboratory developed tests,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
A version of this article first appeared on Medscape.com.
FDA OKs Suflave, a lower-volume colonoscopy prep drink
, the manufacturer, Sebela Pharmaceuticals, has announced.
Suflave comes in a carton containing two bottles and two flavor packets. Each bottle contains 178.7 g polyethylene glycol 3350, 7.3 g sodium sulfate, 1.12 g potassium chloride, 0.9 g magnesium sulfate, and 0.5 g sodium chloride. One bottle and one flavor packet are equivalent to one dose.
Administration of both doses is required for a complete preparation for colonoscopy. After each dose, an additional 16 ounces of water must be consumed.
In a clinical trial, 94% of patients achieved successful bowel cleansing with Suflave, the company said in a news release.
Most patients reported that Suflave tastes like a sports drink and described the taste as “neutral to very pleasant.” Most patients also reported that Suflave was “tolerable to very easy” to consume and indicated they would request it for a subsequent colonoscopy.
“Patients frequently struggle with the taste and volume of traditional bowel preparations – and fear related to the preparation can also negatively affect patient willingness to undergo follow-up colonoscopy if it is indicated,” Douglas K. Rex, MD, gastroenterologist and distinguished professor emeritus at Indiana University, Indianapolis, said in the release.
“I believe the palatable lemon-lime flavor of Suflave will be a welcomed option for patients – reducing preparation hesitancy and giving more people the chance to feel comfortable during preparation and getting a successful and effective procedure,” Dr. Rex added.
Suflave will be available by prescription to patients in the United States in early August.
A version of this article originally appeared on Medscape.com.
, the manufacturer, Sebela Pharmaceuticals, has announced.
Suflave comes in a carton containing two bottles and two flavor packets. Each bottle contains 178.7 g polyethylene glycol 3350, 7.3 g sodium sulfate, 1.12 g potassium chloride, 0.9 g magnesium sulfate, and 0.5 g sodium chloride. One bottle and one flavor packet are equivalent to one dose.
Administration of both doses is required for a complete preparation for colonoscopy. After each dose, an additional 16 ounces of water must be consumed.
In a clinical trial, 94% of patients achieved successful bowel cleansing with Suflave, the company said in a news release.
Most patients reported that Suflave tastes like a sports drink and described the taste as “neutral to very pleasant.” Most patients also reported that Suflave was “tolerable to very easy” to consume and indicated they would request it for a subsequent colonoscopy.
“Patients frequently struggle with the taste and volume of traditional bowel preparations – and fear related to the preparation can also negatively affect patient willingness to undergo follow-up colonoscopy if it is indicated,” Douglas K. Rex, MD, gastroenterologist and distinguished professor emeritus at Indiana University, Indianapolis, said in the release.
“I believe the palatable lemon-lime flavor of Suflave will be a welcomed option for patients – reducing preparation hesitancy and giving more people the chance to feel comfortable during preparation and getting a successful and effective procedure,” Dr. Rex added.
Suflave will be available by prescription to patients in the United States in early August.
A version of this article originally appeared on Medscape.com.
, the manufacturer, Sebela Pharmaceuticals, has announced.
Suflave comes in a carton containing two bottles and two flavor packets. Each bottle contains 178.7 g polyethylene glycol 3350, 7.3 g sodium sulfate, 1.12 g potassium chloride, 0.9 g magnesium sulfate, and 0.5 g sodium chloride. One bottle and one flavor packet are equivalent to one dose.
Administration of both doses is required for a complete preparation for colonoscopy. After each dose, an additional 16 ounces of water must be consumed.
In a clinical trial, 94% of patients achieved successful bowel cleansing with Suflave, the company said in a news release.
Most patients reported that Suflave tastes like a sports drink and described the taste as “neutral to very pleasant.” Most patients also reported that Suflave was “tolerable to very easy” to consume and indicated they would request it for a subsequent colonoscopy.
“Patients frequently struggle with the taste and volume of traditional bowel preparations – and fear related to the preparation can also negatively affect patient willingness to undergo follow-up colonoscopy if it is indicated,” Douglas K. Rex, MD, gastroenterologist and distinguished professor emeritus at Indiana University, Indianapolis, said in the release.
“I believe the palatable lemon-lime flavor of Suflave will be a welcomed option for patients – reducing preparation hesitancy and giving more people the chance to feel comfortable during preparation and getting a successful and effective procedure,” Dr. Rex added.
Suflave will be available by prescription to patients in the United States in early August.
A version of this article originally appeared on Medscape.com.
WHO plans to declare common sweetener as possible carcinogen
The World Health Organization is set to list the artificial sweetener aspartame as a possible carcinogen.
The move, reported by multiple media sources, is expected during a July 14 meeting of WHO research experts – the International Agency for Research on Cancer. Reuters cited two unnamed sources “with knowledge of the process,” noting that aspartame is one of the world’s most commonly used sweeteners.
Aspartame is 200 times sweeter than sugar and was first approved by the Food and Drug Administration in 1974 for use as a tabletop sweetener and in chewing gum and cold breakfast cereals, as well as instant coffee, gelatins, puddings and fillings, and dairy products. Up to 95% of carbonated soft drinks that have a sweetener use aspartame, and the substance is often added by consumers to beverages (it’s the blue packet of sweetener in the array of packets that appear on diner and restaurant tables), The Washington Post reported.
The WHO currently lists 126 agents as known to be carcinogenic to humans, ranging from alcohol and tobacco to outdoor air pollution. The WHO also lists 94 agents as “probably” carcinogenic to humans and 322 agents as “possibly” carcinogenic to humans. Aspartame would join the “possibly” group, which includes gasoline engine exhaust and working as a dry cleaner.
Earlier this year, the WHO warned that people should not use nonsugar sweeteners to control their weight because of potential health risks.
A version of this article originally appeared on WebMD.com.
The World Health Organization is set to list the artificial sweetener aspartame as a possible carcinogen.
The move, reported by multiple media sources, is expected during a July 14 meeting of WHO research experts – the International Agency for Research on Cancer. Reuters cited two unnamed sources “with knowledge of the process,” noting that aspartame is one of the world’s most commonly used sweeteners.
Aspartame is 200 times sweeter than sugar and was first approved by the Food and Drug Administration in 1974 for use as a tabletop sweetener and in chewing gum and cold breakfast cereals, as well as instant coffee, gelatins, puddings and fillings, and dairy products. Up to 95% of carbonated soft drinks that have a sweetener use aspartame, and the substance is often added by consumers to beverages (it’s the blue packet of sweetener in the array of packets that appear on diner and restaurant tables), The Washington Post reported.
The WHO currently lists 126 agents as known to be carcinogenic to humans, ranging from alcohol and tobacco to outdoor air pollution. The WHO also lists 94 agents as “probably” carcinogenic to humans and 322 agents as “possibly” carcinogenic to humans. Aspartame would join the “possibly” group, which includes gasoline engine exhaust and working as a dry cleaner.
Earlier this year, the WHO warned that people should not use nonsugar sweeteners to control their weight because of potential health risks.
A version of this article originally appeared on WebMD.com.
The World Health Organization is set to list the artificial sweetener aspartame as a possible carcinogen.
The move, reported by multiple media sources, is expected during a July 14 meeting of WHO research experts – the International Agency for Research on Cancer. Reuters cited two unnamed sources “with knowledge of the process,” noting that aspartame is one of the world’s most commonly used sweeteners.
Aspartame is 200 times sweeter than sugar and was first approved by the Food and Drug Administration in 1974 for use as a tabletop sweetener and in chewing gum and cold breakfast cereals, as well as instant coffee, gelatins, puddings and fillings, and dairy products. Up to 95% of carbonated soft drinks that have a sweetener use aspartame, and the substance is often added by consumers to beverages (it’s the blue packet of sweetener in the array of packets that appear on diner and restaurant tables), The Washington Post reported.
The WHO currently lists 126 agents as known to be carcinogenic to humans, ranging from alcohol and tobacco to outdoor air pollution. The WHO also lists 94 agents as “probably” carcinogenic to humans and 322 agents as “possibly” carcinogenic to humans. Aspartame would join the “possibly” group, which includes gasoline engine exhaust and working as a dry cleaner.
Earlier this year, the WHO warned that people should not use nonsugar sweeteners to control their weight because of potential health risks.
A version of this article originally appeared on WebMD.com.
Residency match process under scrutiny again, this time by AMA
The American Medical Association is considering whether to study alternatives to the current residency matching program in an effort to improve residents’ compensation and other job-related issues. A recent call-to-action resolution by the AMA’s House of Delegates is the latest in a long string of debates about whether to change the annual process that matches future doctors with compatible residency programs.
AMA’s Resident and Fellow Section introduced the resolution in March, and the delegates approved it earlier in June at AMA’s annual meeting. The resolution states that the match process of the National Resident Matching Program (NRMP) “poses significant anticompetition concerns.” Those include preventing residents from negotiating for higher wages, better benefits, and improved working conditions, according to the approved resolution.
The full AMA board still has to consider the resolution and hasn’t set a date for that review, though it’s expected to be in the next few months, according to Jennifer Sellers, AMA’s public information officer. She said in an interview that the organization declined to comment, wanting to hold off until the board decides how to proceed.
The NRMP, which oversees the matching process, told this news organization that the AMA doesn’t play a role in the Match.
The organization doesn’t believe studying alternative placement methods benefits applicants and residents, and returning to a pre-Match environment, would harm applicants and programs, according to Donna Lamb, DHSc, MBA, BSN, president and CEO.
“The NRMP has no role in determining, publishing, or setting resident salaries nor does the NRMP have a role in the contracting or employment of residents, and it never has.”
Dr. Lamb said changing the Match would “subject applicants to undue pressure and coercion to accept an offer of training. This will exacerbate disparities in candidate selection already evident in medical education and potentially result in salary reductions in more competitive specialties and in more desirable geographic locations.”
The latest push to reform the match process dates back two decades to a 2002 class action antitrust lawsuit by residents and doctors against the NRMP and other organizations involved in the Match.
The residents argued at that time that by restraining competition among teaching hospitals, the matching system allowed hospitals to keep residents’ wages artificially low. The defendants, which included large teaching hospitals, successfully lobbied Congress for an exemption to the antitrust laws, and the case was subsequently dismissed.
The AMA was one of the defendants, so if it moves forward to review the match process, it likely would pit the organization against the NRMP.
Sherman Marek, the attorney who represented the residents, said in an interview that he was not surprised by the latest AMA resolution. “Maybe the AMA leadership has come around to the idea that it’s better for young physicians to not have the match in place,” he says. “I would applaud that sort of evolution.”
Tyler Ramsey, DO, an internal medicine resident and AMA member, said he believes the group’s current president, Jesse Ehrenfeld, MD, MPH, empathizes with doctors in training. “I think he understands [our] views and is more progressive.”
The NRMP also has considered ways to improve the match process to make it easier and more equitable for applicants. In its latest effort, the organization is studying whether programs should certify their rank order list in advance of applicants. This change would give applicants more flexibility to visit residency locations before the programs consider changing their rankings, Dr. Lamb explained. The NRMP also is mulling the possibilities of a two-phase match after deciding in 2022 not to move forward with a previous version of the proposal.
The recent House of Delegates resolution states that “residents are using other means to obtain fair wages, safe working conditions, and other benefits that are unable to be negotiated within the current system.”
Dr. Ramsey, who trains in North Carolina, said the “other means” may include negotiating through a union. “The AMA realizes that there is a problem and that people are unionizing,” he said. “Obviously, as an organization, we’re not doing something correctly, to the point where people are feeling the need to get their rights a different way.”
The Committee of Interns and Residents, which represents 30,000 members, reported a rise in medical trainee unions across the country in 2022.
Not everyone believes that ditching the Match would benefit applicants and residents. Sam Payabvash, MD, assistant professor of radiology at Yale, New Haven, Conn., School of Medicine, tweeted about the resolution as part of a larger Twitter discussion that alternatives are likely to be “more onerous and expensive for applicants.”
An advantage of the match program, Dr. Lamb argued, is that it “improves the reach of applicants into medically underserved communities through widespread program participation.”
Dr. Ramsey agreed that the match program has benefits and drawbacks, but he believes it favors programs over residents. “It comes as no surprise that numerous residents suffer from depression and our suicide rates are the highest amongst all professions due to the lack of control or negotiation of fair salary and working conditions. Overall, the way things are now, residents just do not have a lot of rights.”
A version of this article originally appeared on Medscape.com.
The American Medical Association is considering whether to study alternatives to the current residency matching program in an effort to improve residents’ compensation and other job-related issues. A recent call-to-action resolution by the AMA’s House of Delegates is the latest in a long string of debates about whether to change the annual process that matches future doctors with compatible residency programs.
AMA’s Resident and Fellow Section introduced the resolution in March, and the delegates approved it earlier in June at AMA’s annual meeting. The resolution states that the match process of the National Resident Matching Program (NRMP) “poses significant anticompetition concerns.” Those include preventing residents from negotiating for higher wages, better benefits, and improved working conditions, according to the approved resolution.
The full AMA board still has to consider the resolution and hasn’t set a date for that review, though it’s expected to be in the next few months, according to Jennifer Sellers, AMA’s public information officer. She said in an interview that the organization declined to comment, wanting to hold off until the board decides how to proceed.
The NRMP, which oversees the matching process, told this news organization that the AMA doesn’t play a role in the Match.
The organization doesn’t believe studying alternative placement methods benefits applicants and residents, and returning to a pre-Match environment, would harm applicants and programs, according to Donna Lamb, DHSc, MBA, BSN, president and CEO.
“The NRMP has no role in determining, publishing, or setting resident salaries nor does the NRMP have a role in the contracting or employment of residents, and it never has.”
Dr. Lamb said changing the Match would “subject applicants to undue pressure and coercion to accept an offer of training. This will exacerbate disparities in candidate selection already evident in medical education and potentially result in salary reductions in more competitive specialties and in more desirable geographic locations.”
The latest push to reform the match process dates back two decades to a 2002 class action antitrust lawsuit by residents and doctors against the NRMP and other organizations involved in the Match.
The residents argued at that time that by restraining competition among teaching hospitals, the matching system allowed hospitals to keep residents’ wages artificially low. The defendants, which included large teaching hospitals, successfully lobbied Congress for an exemption to the antitrust laws, and the case was subsequently dismissed.
The AMA was one of the defendants, so if it moves forward to review the match process, it likely would pit the organization against the NRMP.
Sherman Marek, the attorney who represented the residents, said in an interview that he was not surprised by the latest AMA resolution. “Maybe the AMA leadership has come around to the idea that it’s better for young physicians to not have the match in place,” he says. “I would applaud that sort of evolution.”
Tyler Ramsey, DO, an internal medicine resident and AMA member, said he believes the group’s current president, Jesse Ehrenfeld, MD, MPH, empathizes with doctors in training. “I think he understands [our] views and is more progressive.”
The NRMP also has considered ways to improve the match process to make it easier and more equitable for applicants. In its latest effort, the organization is studying whether programs should certify their rank order list in advance of applicants. This change would give applicants more flexibility to visit residency locations before the programs consider changing their rankings, Dr. Lamb explained. The NRMP also is mulling the possibilities of a two-phase match after deciding in 2022 not to move forward with a previous version of the proposal.
The recent House of Delegates resolution states that “residents are using other means to obtain fair wages, safe working conditions, and other benefits that are unable to be negotiated within the current system.”
Dr. Ramsey, who trains in North Carolina, said the “other means” may include negotiating through a union. “The AMA realizes that there is a problem and that people are unionizing,” he said. “Obviously, as an organization, we’re not doing something correctly, to the point where people are feeling the need to get their rights a different way.”
The Committee of Interns and Residents, which represents 30,000 members, reported a rise in medical trainee unions across the country in 2022.
Not everyone believes that ditching the Match would benefit applicants and residents. Sam Payabvash, MD, assistant professor of radiology at Yale, New Haven, Conn., School of Medicine, tweeted about the resolution as part of a larger Twitter discussion that alternatives are likely to be “more onerous and expensive for applicants.”
An advantage of the match program, Dr. Lamb argued, is that it “improves the reach of applicants into medically underserved communities through widespread program participation.”
Dr. Ramsey agreed that the match program has benefits and drawbacks, but he believes it favors programs over residents. “It comes as no surprise that numerous residents suffer from depression and our suicide rates are the highest amongst all professions due to the lack of control or negotiation of fair salary and working conditions. Overall, the way things are now, residents just do not have a lot of rights.”
A version of this article originally appeared on Medscape.com.
The American Medical Association is considering whether to study alternatives to the current residency matching program in an effort to improve residents’ compensation and other job-related issues. A recent call-to-action resolution by the AMA’s House of Delegates is the latest in a long string of debates about whether to change the annual process that matches future doctors with compatible residency programs.
AMA’s Resident and Fellow Section introduced the resolution in March, and the delegates approved it earlier in June at AMA’s annual meeting. The resolution states that the match process of the National Resident Matching Program (NRMP) “poses significant anticompetition concerns.” Those include preventing residents from negotiating for higher wages, better benefits, and improved working conditions, according to the approved resolution.
The full AMA board still has to consider the resolution and hasn’t set a date for that review, though it’s expected to be in the next few months, according to Jennifer Sellers, AMA’s public information officer. She said in an interview that the organization declined to comment, wanting to hold off until the board decides how to proceed.
The NRMP, which oversees the matching process, told this news organization that the AMA doesn’t play a role in the Match.
The organization doesn’t believe studying alternative placement methods benefits applicants and residents, and returning to a pre-Match environment, would harm applicants and programs, according to Donna Lamb, DHSc, MBA, BSN, president and CEO.
“The NRMP has no role in determining, publishing, or setting resident salaries nor does the NRMP have a role in the contracting or employment of residents, and it never has.”
Dr. Lamb said changing the Match would “subject applicants to undue pressure and coercion to accept an offer of training. This will exacerbate disparities in candidate selection already evident in medical education and potentially result in salary reductions in more competitive specialties and in more desirable geographic locations.”
The latest push to reform the match process dates back two decades to a 2002 class action antitrust lawsuit by residents and doctors against the NRMP and other organizations involved in the Match.
The residents argued at that time that by restraining competition among teaching hospitals, the matching system allowed hospitals to keep residents’ wages artificially low. The defendants, which included large teaching hospitals, successfully lobbied Congress for an exemption to the antitrust laws, and the case was subsequently dismissed.
The AMA was one of the defendants, so if it moves forward to review the match process, it likely would pit the organization against the NRMP.
Sherman Marek, the attorney who represented the residents, said in an interview that he was not surprised by the latest AMA resolution. “Maybe the AMA leadership has come around to the idea that it’s better for young physicians to not have the match in place,” he says. “I would applaud that sort of evolution.”
Tyler Ramsey, DO, an internal medicine resident and AMA member, said he believes the group’s current president, Jesse Ehrenfeld, MD, MPH, empathizes with doctors in training. “I think he understands [our] views and is more progressive.”
The NRMP also has considered ways to improve the match process to make it easier and more equitable for applicants. In its latest effort, the organization is studying whether programs should certify their rank order list in advance of applicants. This change would give applicants more flexibility to visit residency locations before the programs consider changing their rankings, Dr. Lamb explained. The NRMP also is mulling the possibilities of a two-phase match after deciding in 2022 not to move forward with a previous version of the proposal.
The recent House of Delegates resolution states that “residents are using other means to obtain fair wages, safe working conditions, and other benefits that are unable to be negotiated within the current system.”
Dr. Ramsey, who trains in North Carolina, said the “other means” may include negotiating through a union. “The AMA realizes that there is a problem and that people are unionizing,” he said. “Obviously, as an organization, we’re not doing something correctly, to the point where people are feeling the need to get their rights a different way.”
The Committee of Interns and Residents, which represents 30,000 members, reported a rise in medical trainee unions across the country in 2022.
Not everyone believes that ditching the Match would benefit applicants and residents. Sam Payabvash, MD, assistant professor of radiology at Yale, New Haven, Conn., School of Medicine, tweeted about the resolution as part of a larger Twitter discussion that alternatives are likely to be “more onerous and expensive for applicants.”
An advantage of the match program, Dr. Lamb argued, is that it “improves the reach of applicants into medically underserved communities through widespread program participation.”
Dr. Ramsey agreed that the match program has benefits and drawbacks, but he believes it favors programs over residents. “It comes as no surprise that numerous residents suffer from depression and our suicide rates are the highest amongst all professions due to the lack of control or negotiation of fair salary and working conditions. Overall, the way things are now, residents just do not have a lot of rights.”
A version of this article originally appeared on Medscape.com.
Does colchicine have a role in treating excess ASCVD risk in patients with chronic inflammatory conditions?
The recent Food and Drug Administration approval of colchicine 0.5 mg (Lodoco) for use in atherosclerotic cardiovascular disease (ASCVD) prevention will possibly create opportunities to use the drug to treat residual risk for ASCVD in some patients with immune-mediated inflammatory diseases, particularly in rheumatology.
Potential in rheumatology
The 0.5-mg dose is just a shade under the 0.6-mg, twice daily dosing rheumatologists typically prescribe for gout, Christie Bartels, MD, MS, chief of rheumatology at the University of Wisconsin–Madison, said in an interview. Clinicians also use the 0.6-mg dose off-label for pseudogout or calcium pyrophosphate deposition disease (CPPD), Dr. Bartels noted.
The new formulation opens the consideration for using colchicine more in patients with psoriatic arthritis, lupus, and rheumatoid arthritis, she said. “I think we could certainly discuss it, particularly, in secondary prevention patients who already had an event or who are at the highest risk and already on optimal traditional agents,” she said.
She cited previous comments by Paul Ridker, MD, director of the center for cardiovascular disease prevention at Brigham and Women’s Hospital in Boston, and developer of the high-sensitivity C-reactive protein (hsCRP) test for measuring inflammatory markers. “We might not know the answer because Dr. Ridker pointed out he used colchicine 0.5 mg in patients that had a high-sensitivity CRP that was high; we need patients who have had inflammation of unknown origin, so those patients presumably weren’t already on another anti-inflammatory,” she said, noting that hydroxychloroquine, methotrexate, and some biologics provide some protection from cardiovascular risks.
However, a potential role for long-term colchicine 0.5 mg in ASCVD prevention may cause consideration for changing the drug’s role in gout treatment, Dr. Bartels said. “In gout, where we do have an FDA-approved indication for colchicine, we used to use it only for the first 6 months while we were getting patients to goal on allopurinol, which was usually then monotherapy after the first 6 months,” she said. “I think this will likely change how I treat gout patients in that I may also offer to continue both medications [colchicine and allopurinol] if they are tolerating them well.
“And then in patients where I’m using it off-label in CPPD, I might again share with them that in addition to possibly helping their CPPD, there may be this added benefit to reduce inflammation just in discussing the risks and benefits of the medicine.”
However, rheumatologists must be careful in using colchicine beyond the typical 6-month cycle, Dr. Bartels said. “One of the tricky things with colchicine, and part of the reason we did not traditionally continue it specifically past the first 6 months, was that it can cause myopathies or cytopenias, so we still have to counsel patients regarding these risks and monitor that,” she said.
Additionally, colchicine can have drug interactions with statins or calcium channel blockers that can change colchicine levels. “I think the dose here is so low, the 0.5 mg, that it’s probably still safe, but again, it’s something that we have to take a look at in the patient’s whole picture and the rest of their burden of their meds in order to make a decision with them,” Dr. Bartels said.
Possibilities in dermatology
The LoDoCo2 trial one of two major randomized trials that supported approval of colchicine 0.5 mg, reported that treated patients had a 60% lower rate of gout than the placebo group (1.4% vs. 3.4%). Joel Gelfand, MD, MSCE, the James J. Leyden professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, pointed to this in evaluating the dermatologic implications of the drug’s approval. “This may be of particular interest as people with psoriasis have an increased risk of gout,” he said in emailed comments.
Colchicine’s mechanism of action to reduce inflammation parallels that of tumor necrosis factor (TNF) inhibitors used for dermatologic indications, namely by inhibiting leukocyte adhesion to disrupt the downregulation of TNF receptors, Dr. Gelfand said.
“Interestingly, observational data suggests biologics that target TNF such as adalimumab, etanercept, etc., are associated with a reduction in CV events, and in placebo-controlled trials we conducted in psoriasis patients, it reduced key inflammatory mediators of cardiovascular disease, including IL [interleukin]-6,” he said. “Randomized clinical trials to evaluate the ability of TNF inhibitors, which are now available as biosimilars, to prevent cardiovascular events in high-risk patients, should be conducted, and more work is needed to identify which additional immune-targeted treatments may lower CV risk with an acceptable safety profile.”
Colchicine currently has few indications for rare conditions in dermatology, Dr. Gelfand said, including Sweets syndrome, subcorneal pustular dermatosis, and cutaneous vasculitis. “There are some reports to suggest it may help psoriatic disease, but current data are limited and insufficient to recommend its use for psoriasis and/or psoriatic arthritis,” he said.
The approval of colchicine 0.5 mg for ASCVD could be meaningful for people with psoriasis who are also being treated for CV risk factors, Dr. Gelfand said. “Additional considerations such as signs of residual inflammation (elevated hsCRP) and CV imaging findings may be used to further guide shared decision-making for optimal use,” he said.
Another consideration he noted: “This is also a novel 0.5-mg formulation, and thus cost may be an issue.”
Would side effects bar use in gastroenterology?
Colchicine 0.5 mg may not move the needle much for expanding treatment of ASCVD in patients with inflammatory bowel disease (IBD) and potentially other gastrointestinal conditions, Edward Loftus Jr., MD, the Maxine and Jack Zarrow Family professor of gastroenterology specifically for IBD at the Mayo Clinic in Rochester, Minn., told MDEdge in emailed comments. “Given the GI side effect profile [of colchicine], I am not sure I would go there,” he said.
“Hopefully, the prescribers of this low-dose formulation are aware of the gastrointestinal side effects, such as diarrhea and nausea, and educate patients about these side effects so that a proper risk-benefit discussion can ensue,” he said.
Dr. Bartels reporting a previous financial relationship with Pfizer. Dr. Gelfand said he has financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, GlaxoSmithKline, Twill, Lilly, Leo, Moonlake, Janssen Biologics, Novartis, Pfizer, UCB, Neuroderm, and Veolia North America. Dr. Loftus disclosed relationships with AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Receptos, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Pfizer, Sun, Surrozen, Takeda, Theravance, and UCB.
The recent Food and Drug Administration approval of colchicine 0.5 mg (Lodoco) for use in atherosclerotic cardiovascular disease (ASCVD) prevention will possibly create opportunities to use the drug to treat residual risk for ASCVD in some patients with immune-mediated inflammatory diseases, particularly in rheumatology.
Potential in rheumatology
The 0.5-mg dose is just a shade under the 0.6-mg, twice daily dosing rheumatologists typically prescribe for gout, Christie Bartels, MD, MS, chief of rheumatology at the University of Wisconsin–Madison, said in an interview. Clinicians also use the 0.6-mg dose off-label for pseudogout or calcium pyrophosphate deposition disease (CPPD), Dr. Bartels noted.
The new formulation opens the consideration for using colchicine more in patients with psoriatic arthritis, lupus, and rheumatoid arthritis, she said. “I think we could certainly discuss it, particularly, in secondary prevention patients who already had an event or who are at the highest risk and already on optimal traditional agents,” she said.
She cited previous comments by Paul Ridker, MD, director of the center for cardiovascular disease prevention at Brigham and Women’s Hospital in Boston, and developer of the high-sensitivity C-reactive protein (hsCRP) test for measuring inflammatory markers. “We might not know the answer because Dr. Ridker pointed out he used colchicine 0.5 mg in patients that had a high-sensitivity CRP that was high; we need patients who have had inflammation of unknown origin, so those patients presumably weren’t already on another anti-inflammatory,” she said, noting that hydroxychloroquine, methotrexate, and some biologics provide some protection from cardiovascular risks.
However, a potential role for long-term colchicine 0.5 mg in ASCVD prevention may cause consideration for changing the drug’s role in gout treatment, Dr. Bartels said. “In gout, where we do have an FDA-approved indication for colchicine, we used to use it only for the first 6 months while we were getting patients to goal on allopurinol, which was usually then monotherapy after the first 6 months,” she said. “I think this will likely change how I treat gout patients in that I may also offer to continue both medications [colchicine and allopurinol] if they are tolerating them well.
“And then in patients where I’m using it off-label in CPPD, I might again share with them that in addition to possibly helping their CPPD, there may be this added benefit to reduce inflammation just in discussing the risks and benefits of the medicine.”
However, rheumatologists must be careful in using colchicine beyond the typical 6-month cycle, Dr. Bartels said. “One of the tricky things with colchicine, and part of the reason we did not traditionally continue it specifically past the first 6 months, was that it can cause myopathies or cytopenias, so we still have to counsel patients regarding these risks and monitor that,” she said.
Additionally, colchicine can have drug interactions with statins or calcium channel blockers that can change colchicine levels. “I think the dose here is so low, the 0.5 mg, that it’s probably still safe, but again, it’s something that we have to take a look at in the patient’s whole picture and the rest of their burden of their meds in order to make a decision with them,” Dr. Bartels said.
Possibilities in dermatology
The LoDoCo2 trial one of two major randomized trials that supported approval of colchicine 0.5 mg, reported that treated patients had a 60% lower rate of gout than the placebo group (1.4% vs. 3.4%). Joel Gelfand, MD, MSCE, the James J. Leyden professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, pointed to this in evaluating the dermatologic implications of the drug’s approval. “This may be of particular interest as people with psoriasis have an increased risk of gout,” he said in emailed comments.
Colchicine’s mechanism of action to reduce inflammation parallels that of tumor necrosis factor (TNF) inhibitors used for dermatologic indications, namely by inhibiting leukocyte adhesion to disrupt the downregulation of TNF receptors, Dr. Gelfand said.
“Interestingly, observational data suggests biologics that target TNF such as adalimumab, etanercept, etc., are associated with a reduction in CV events, and in placebo-controlled trials we conducted in psoriasis patients, it reduced key inflammatory mediators of cardiovascular disease, including IL [interleukin]-6,” he said. “Randomized clinical trials to evaluate the ability of TNF inhibitors, which are now available as biosimilars, to prevent cardiovascular events in high-risk patients, should be conducted, and more work is needed to identify which additional immune-targeted treatments may lower CV risk with an acceptable safety profile.”
Colchicine currently has few indications for rare conditions in dermatology, Dr. Gelfand said, including Sweets syndrome, subcorneal pustular dermatosis, and cutaneous vasculitis. “There are some reports to suggest it may help psoriatic disease, but current data are limited and insufficient to recommend its use for psoriasis and/or psoriatic arthritis,” he said.
The approval of colchicine 0.5 mg for ASCVD could be meaningful for people with psoriasis who are also being treated for CV risk factors, Dr. Gelfand said. “Additional considerations such as signs of residual inflammation (elevated hsCRP) and CV imaging findings may be used to further guide shared decision-making for optimal use,” he said.
Another consideration he noted: “This is also a novel 0.5-mg formulation, and thus cost may be an issue.”
Would side effects bar use in gastroenterology?
Colchicine 0.5 mg may not move the needle much for expanding treatment of ASCVD in patients with inflammatory bowel disease (IBD) and potentially other gastrointestinal conditions, Edward Loftus Jr., MD, the Maxine and Jack Zarrow Family professor of gastroenterology specifically for IBD at the Mayo Clinic in Rochester, Minn., told MDEdge in emailed comments. “Given the GI side effect profile [of colchicine], I am not sure I would go there,” he said.
“Hopefully, the prescribers of this low-dose formulation are aware of the gastrointestinal side effects, such as diarrhea and nausea, and educate patients about these side effects so that a proper risk-benefit discussion can ensue,” he said.
Dr. Bartels reporting a previous financial relationship with Pfizer. Dr. Gelfand said he has financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, GlaxoSmithKline, Twill, Lilly, Leo, Moonlake, Janssen Biologics, Novartis, Pfizer, UCB, Neuroderm, and Veolia North America. Dr. Loftus disclosed relationships with AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Receptos, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Pfizer, Sun, Surrozen, Takeda, Theravance, and UCB.
The recent Food and Drug Administration approval of colchicine 0.5 mg (Lodoco) for use in atherosclerotic cardiovascular disease (ASCVD) prevention will possibly create opportunities to use the drug to treat residual risk for ASCVD in some patients with immune-mediated inflammatory diseases, particularly in rheumatology.
Potential in rheumatology
The 0.5-mg dose is just a shade under the 0.6-mg, twice daily dosing rheumatologists typically prescribe for gout, Christie Bartels, MD, MS, chief of rheumatology at the University of Wisconsin–Madison, said in an interview. Clinicians also use the 0.6-mg dose off-label for pseudogout or calcium pyrophosphate deposition disease (CPPD), Dr. Bartels noted.
The new formulation opens the consideration for using colchicine more in patients with psoriatic arthritis, lupus, and rheumatoid arthritis, she said. “I think we could certainly discuss it, particularly, in secondary prevention patients who already had an event or who are at the highest risk and already on optimal traditional agents,” she said.
She cited previous comments by Paul Ridker, MD, director of the center for cardiovascular disease prevention at Brigham and Women’s Hospital in Boston, and developer of the high-sensitivity C-reactive protein (hsCRP) test for measuring inflammatory markers. “We might not know the answer because Dr. Ridker pointed out he used colchicine 0.5 mg in patients that had a high-sensitivity CRP that was high; we need patients who have had inflammation of unknown origin, so those patients presumably weren’t already on another anti-inflammatory,” she said, noting that hydroxychloroquine, methotrexate, and some biologics provide some protection from cardiovascular risks.
However, a potential role for long-term colchicine 0.5 mg in ASCVD prevention may cause consideration for changing the drug’s role in gout treatment, Dr. Bartels said. “In gout, where we do have an FDA-approved indication for colchicine, we used to use it only for the first 6 months while we were getting patients to goal on allopurinol, which was usually then monotherapy after the first 6 months,” she said. “I think this will likely change how I treat gout patients in that I may also offer to continue both medications [colchicine and allopurinol] if they are tolerating them well.
“And then in patients where I’m using it off-label in CPPD, I might again share with them that in addition to possibly helping their CPPD, there may be this added benefit to reduce inflammation just in discussing the risks and benefits of the medicine.”
However, rheumatologists must be careful in using colchicine beyond the typical 6-month cycle, Dr. Bartels said. “One of the tricky things with colchicine, and part of the reason we did not traditionally continue it specifically past the first 6 months, was that it can cause myopathies or cytopenias, so we still have to counsel patients regarding these risks and monitor that,” she said.
Additionally, colchicine can have drug interactions with statins or calcium channel blockers that can change colchicine levels. “I think the dose here is so low, the 0.5 mg, that it’s probably still safe, but again, it’s something that we have to take a look at in the patient’s whole picture and the rest of their burden of their meds in order to make a decision with them,” Dr. Bartels said.
Possibilities in dermatology
The LoDoCo2 trial one of two major randomized trials that supported approval of colchicine 0.5 mg, reported that treated patients had a 60% lower rate of gout than the placebo group (1.4% vs. 3.4%). Joel Gelfand, MD, MSCE, the James J. Leyden professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, pointed to this in evaluating the dermatologic implications of the drug’s approval. “This may be of particular interest as people with psoriasis have an increased risk of gout,” he said in emailed comments.
Colchicine’s mechanism of action to reduce inflammation parallels that of tumor necrosis factor (TNF) inhibitors used for dermatologic indications, namely by inhibiting leukocyte adhesion to disrupt the downregulation of TNF receptors, Dr. Gelfand said.
“Interestingly, observational data suggests biologics that target TNF such as adalimumab, etanercept, etc., are associated with a reduction in CV events, and in placebo-controlled trials we conducted in psoriasis patients, it reduced key inflammatory mediators of cardiovascular disease, including IL [interleukin]-6,” he said. “Randomized clinical trials to evaluate the ability of TNF inhibitors, which are now available as biosimilars, to prevent cardiovascular events in high-risk patients, should be conducted, and more work is needed to identify which additional immune-targeted treatments may lower CV risk with an acceptable safety profile.”
Colchicine currently has few indications for rare conditions in dermatology, Dr. Gelfand said, including Sweets syndrome, subcorneal pustular dermatosis, and cutaneous vasculitis. “There are some reports to suggest it may help psoriatic disease, but current data are limited and insufficient to recommend its use for psoriasis and/or psoriatic arthritis,” he said.
The approval of colchicine 0.5 mg for ASCVD could be meaningful for people with psoriasis who are also being treated for CV risk factors, Dr. Gelfand said. “Additional considerations such as signs of residual inflammation (elevated hsCRP) and CV imaging findings may be used to further guide shared decision-making for optimal use,” he said.
Another consideration he noted: “This is also a novel 0.5-mg formulation, and thus cost may be an issue.”
Would side effects bar use in gastroenterology?
Colchicine 0.5 mg may not move the needle much for expanding treatment of ASCVD in patients with inflammatory bowel disease (IBD) and potentially other gastrointestinal conditions, Edward Loftus Jr., MD, the Maxine and Jack Zarrow Family professor of gastroenterology specifically for IBD at the Mayo Clinic in Rochester, Minn., told MDEdge in emailed comments. “Given the GI side effect profile [of colchicine], I am not sure I would go there,” he said.
“Hopefully, the prescribers of this low-dose formulation are aware of the gastrointestinal side effects, such as diarrhea and nausea, and educate patients about these side effects so that a proper risk-benefit discussion can ensue,” he said.
Dr. Bartels reporting a previous financial relationship with Pfizer. Dr. Gelfand said he has financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, GlaxoSmithKline, Twill, Lilly, Leo, Moonlake, Janssen Biologics, Novartis, Pfizer, UCB, Neuroderm, and Veolia North America. Dr. Loftus disclosed relationships with AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Receptos, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Pfizer, Sun, Surrozen, Takeda, Theravance, and UCB.
MRI identifies rectal cancer patients who can skip CRT
Guidelines recommend chemoradiation (CRT) before surgery for patients with clinical stage II-III rectal cancer in order to lower the risk of locoregional recurrence, but there is a growing concern among oncologists that the approach leads to overtreatment.
However, the issue has been how best to identify patients who would do well with less intensive treatment.
A German team reported a promising approach to this issue, describing the use of preoperative MRI to assess the mesorectal fascia (MRF) for the presence of a tumor. The paper was published in the Journal of Clinical Oncology.
The thinking is that with uninvolved MRF, the tumor is removed by total mesorectal excision (TME) alone, while patients with involved MRF need neoadjuvant chemoradiation therapy to shrink the tumor before resection.
The team put the idea to the test in 884 patients with cT2-4 rectal cancer.
There were 530 patients (60%) with clear MRFs, and they proceeded directly to total mesorectal excision. The 5-year locoregional recurrence rate was just 2.9% in this group.
In comparison, almost 6% of the 354 patients who received neoadjuvant CRT in this study had a locoregional recurrence within 5 years of TME.
Neoadjuvant chemoradiation offers “no advantage over optimized surgery” for such patients “if a 5-year [locoregional recurrence] rate of approximately 5% is acceptable,” said investigators, led by Reinhard Ruppert, MD, of the department of general and visceral surgery, endocrine surgery, and coloproctology at the Municipal Hospital of Munich-Neuperlach.
If so, neoadjuvant chemoradiation “and its adverse effects can be avoided in 60% of the total population and in 45% of patients with clinical stage II and III cancer” as found in the study, they said.
“The risk of undertreatment because of the omission of” neoadjuvant chemoradiation is low, they commented. Of the 10 patients who had a negative MRF but turned out to have positive resection margins at surgery, only one had a recurrence, the team noted.
Overall, the study suggests that neoadjuvant chemoradiation therapy can be restricted to patients at high risk of locoregional recurrence. “These findings may be used for guiding clinical surgical practice and the administration of neoadjuvant radiotherapy or neoadjuvant chemoradiotherapy,” the investigators said.
Concern about reproducibility
Approached for comment, Alan Venook, MD, a gastrointestinal oncologist at the University of California, San Francisco, said, “This is another paper that pretty much confirms the assumption that we overtreat many patients with rectal cancer.”
These “data support the principle that many, if not most, patients with localized rectal cancer do not require trimodality therapy (chemotherapy, radiation, surgery) to achieve cure. That said, it remains a real challenge to figure out which of the modalities can or should be omitted in the average patient,” Dr. Venook told this news organization.
Overall, the German results “are excellent” but it’s unknown if the results can be replicated in community settings, given the expertise needed to discern MRF involvement on MRI and the fact that not every patient gets TME, the gold-standard surgery used in the trial, he said.
Venook said that at his university, given the rapidly evolving literature on de-escalating treatment, every rectal cancer case is discussed at a multidisciplinary tumor board to decide the best course of action.
Study details
Patients in the trial were treated at 14 centers in Germany from 2007 to 2016; nodal involvement was allowed, but subjects had no distant metastases. The call on whether or not they had MRF involvement was based on the distance between the MRF on preoperative MRI and their tumor, suspicious lymph nodes, and tumor deposits.
Patients with a distance greater than 1 mm were considered low risk for recurrence and underwent upfront total mesorectal excision. Those with a distance of 1 mm or fewer as well as patients with cT4 tumors and cT3 tumors in the lower rectal third – a location that makes it difficult to assess the MRF involvement – received up to 50.4 Gy radiation plus fluorouracil before surgery.
The 5-year rate of distant metastases was 15.9% in the upfront surgery group versus 30.5% in the nCRT arm; 11% of the upfront surgery group died of rectal cancer during follow-up versus 21.8% of the nCRT arm.
The work was funded by Johannes Gutenberg University Mainz. Dr. Ruppert and Dr. Venook report no relevant financial relationships. Three investigators reported honoraria and/or travel expenses from Intuitive Surgical, AbbVie, Johnson & Johnson, and other companies.
A version of this article originally appeared on Medscape.com.
Guidelines recommend chemoradiation (CRT) before surgery for patients with clinical stage II-III rectal cancer in order to lower the risk of locoregional recurrence, but there is a growing concern among oncologists that the approach leads to overtreatment.
However, the issue has been how best to identify patients who would do well with less intensive treatment.
A German team reported a promising approach to this issue, describing the use of preoperative MRI to assess the mesorectal fascia (MRF) for the presence of a tumor. The paper was published in the Journal of Clinical Oncology.
The thinking is that with uninvolved MRF, the tumor is removed by total mesorectal excision (TME) alone, while patients with involved MRF need neoadjuvant chemoradiation therapy to shrink the tumor before resection.
The team put the idea to the test in 884 patients with cT2-4 rectal cancer.
There were 530 patients (60%) with clear MRFs, and they proceeded directly to total mesorectal excision. The 5-year locoregional recurrence rate was just 2.9% in this group.
In comparison, almost 6% of the 354 patients who received neoadjuvant CRT in this study had a locoregional recurrence within 5 years of TME.
Neoadjuvant chemoradiation offers “no advantage over optimized surgery” for such patients “if a 5-year [locoregional recurrence] rate of approximately 5% is acceptable,” said investigators, led by Reinhard Ruppert, MD, of the department of general and visceral surgery, endocrine surgery, and coloproctology at the Municipal Hospital of Munich-Neuperlach.
If so, neoadjuvant chemoradiation “and its adverse effects can be avoided in 60% of the total population and in 45% of patients with clinical stage II and III cancer” as found in the study, they said.
“The risk of undertreatment because of the omission of” neoadjuvant chemoradiation is low, they commented. Of the 10 patients who had a negative MRF but turned out to have positive resection margins at surgery, only one had a recurrence, the team noted.
Overall, the study suggests that neoadjuvant chemoradiation therapy can be restricted to patients at high risk of locoregional recurrence. “These findings may be used for guiding clinical surgical practice and the administration of neoadjuvant radiotherapy or neoadjuvant chemoradiotherapy,” the investigators said.
Concern about reproducibility
Approached for comment, Alan Venook, MD, a gastrointestinal oncologist at the University of California, San Francisco, said, “This is another paper that pretty much confirms the assumption that we overtreat many patients with rectal cancer.”
These “data support the principle that many, if not most, patients with localized rectal cancer do not require trimodality therapy (chemotherapy, radiation, surgery) to achieve cure. That said, it remains a real challenge to figure out which of the modalities can or should be omitted in the average patient,” Dr. Venook told this news organization.
Overall, the German results “are excellent” but it’s unknown if the results can be replicated in community settings, given the expertise needed to discern MRF involvement on MRI and the fact that not every patient gets TME, the gold-standard surgery used in the trial, he said.
Venook said that at his university, given the rapidly evolving literature on de-escalating treatment, every rectal cancer case is discussed at a multidisciplinary tumor board to decide the best course of action.
Study details
Patients in the trial were treated at 14 centers in Germany from 2007 to 2016; nodal involvement was allowed, but subjects had no distant metastases. The call on whether or not they had MRF involvement was based on the distance between the MRF on preoperative MRI and their tumor, suspicious lymph nodes, and tumor deposits.
Patients with a distance greater than 1 mm were considered low risk for recurrence and underwent upfront total mesorectal excision. Those with a distance of 1 mm or fewer as well as patients with cT4 tumors and cT3 tumors in the lower rectal third – a location that makes it difficult to assess the MRF involvement – received up to 50.4 Gy radiation plus fluorouracil before surgery.
The 5-year rate of distant metastases was 15.9% in the upfront surgery group versus 30.5% in the nCRT arm; 11% of the upfront surgery group died of rectal cancer during follow-up versus 21.8% of the nCRT arm.
The work was funded by Johannes Gutenberg University Mainz. Dr. Ruppert and Dr. Venook report no relevant financial relationships. Three investigators reported honoraria and/or travel expenses from Intuitive Surgical, AbbVie, Johnson & Johnson, and other companies.
A version of this article originally appeared on Medscape.com.
Guidelines recommend chemoradiation (CRT) before surgery for patients with clinical stage II-III rectal cancer in order to lower the risk of locoregional recurrence, but there is a growing concern among oncologists that the approach leads to overtreatment.
However, the issue has been how best to identify patients who would do well with less intensive treatment.
A German team reported a promising approach to this issue, describing the use of preoperative MRI to assess the mesorectal fascia (MRF) for the presence of a tumor. The paper was published in the Journal of Clinical Oncology.
The thinking is that with uninvolved MRF, the tumor is removed by total mesorectal excision (TME) alone, while patients with involved MRF need neoadjuvant chemoradiation therapy to shrink the tumor before resection.
The team put the idea to the test in 884 patients with cT2-4 rectal cancer.
There were 530 patients (60%) with clear MRFs, and they proceeded directly to total mesorectal excision. The 5-year locoregional recurrence rate was just 2.9% in this group.
In comparison, almost 6% of the 354 patients who received neoadjuvant CRT in this study had a locoregional recurrence within 5 years of TME.
Neoadjuvant chemoradiation offers “no advantage over optimized surgery” for such patients “if a 5-year [locoregional recurrence] rate of approximately 5% is acceptable,” said investigators, led by Reinhard Ruppert, MD, of the department of general and visceral surgery, endocrine surgery, and coloproctology at the Municipal Hospital of Munich-Neuperlach.
If so, neoadjuvant chemoradiation “and its adverse effects can be avoided in 60% of the total population and in 45% of patients with clinical stage II and III cancer” as found in the study, they said.
“The risk of undertreatment because of the omission of” neoadjuvant chemoradiation is low, they commented. Of the 10 patients who had a negative MRF but turned out to have positive resection margins at surgery, only one had a recurrence, the team noted.
Overall, the study suggests that neoadjuvant chemoradiation therapy can be restricted to patients at high risk of locoregional recurrence. “These findings may be used for guiding clinical surgical practice and the administration of neoadjuvant radiotherapy or neoadjuvant chemoradiotherapy,” the investigators said.
Concern about reproducibility
Approached for comment, Alan Venook, MD, a gastrointestinal oncologist at the University of California, San Francisco, said, “This is another paper that pretty much confirms the assumption that we overtreat many patients with rectal cancer.”
These “data support the principle that many, if not most, patients with localized rectal cancer do not require trimodality therapy (chemotherapy, radiation, surgery) to achieve cure. That said, it remains a real challenge to figure out which of the modalities can or should be omitted in the average patient,” Dr. Venook told this news organization.
Overall, the German results “are excellent” but it’s unknown if the results can be replicated in community settings, given the expertise needed to discern MRF involvement on MRI and the fact that not every patient gets TME, the gold-standard surgery used in the trial, he said.
Venook said that at his university, given the rapidly evolving literature on de-escalating treatment, every rectal cancer case is discussed at a multidisciplinary tumor board to decide the best course of action.
Study details
Patients in the trial were treated at 14 centers in Germany from 2007 to 2016; nodal involvement was allowed, but subjects had no distant metastases. The call on whether or not they had MRF involvement was based on the distance between the MRF on preoperative MRI and their tumor, suspicious lymph nodes, and tumor deposits.
Patients with a distance greater than 1 mm were considered low risk for recurrence and underwent upfront total mesorectal excision. Those with a distance of 1 mm or fewer as well as patients with cT4 tumors and cT3 tumors in the lower rectal third – a location that makes it difficult to assess the MRF involvement – received up to 50.4 Gy radiation plus fluorouracil before surgery.
The 5-year rate of distant metastases was 15.9% in the upfront surgery group versus 30.5% in the nCRT arm; 11% of the upfront surgery group died of rectal cancer during follow-up versus 21.8% of the nCRT arm.
The work was funded by Johannes Gutenberg University Mainz. Dr. Ruppert and Dr. Venook report no relevant financial relationships. Three investigators reported honoraria and/or travel expenses from Intuitive Surgical, AbbVie, Johnson & Johnson, and other companies.
A version of this article originally appeared on Medscape.com.