User login
Use of the Retroauricular Pull-Through Sandwich Flap for Repair of an Extensive Conchal Bowl Defect With Complete Cartilage Loss
Practice Gap
Repair of a conchal defect requires careful consideration to achieve an optimal outcome. Reconstruction should resurface exposed cartilage, restore the natural projection of the auricle, and direct sound into the external auditory meatus. Patients also should be able to wear glasses and a hearing aid.
The reconstructive ladder for most conchal bowl defects includes secondary intention healing, full-thickness skin grafting (FTSG), and either a revolving-door flap or a flip-flop flap. Secondary intention and FTSG are appropriate for superficial defects, in which the loss of cartilage is not substantial.1,2 Revolving-door and flip-flop flaps are single-stage retroauricular approaches used to repair relatively small defects of the conchal bowl.3 However, reconstructive options are limited for a large defect in which there is extensive loss of cartilage; 3-stage retroauricular approaches have been utilized. The anterior pedicled retroauricular flap is a 3-stage repair that can be utilized to reconstruct a through-and-through defect of the central ear:
- Stage 1: an anteriorly based retroauricular pedicle is incised, hinged over, and sutured to the medial aspect of the defect, resurfacing the posterior ear.
- Stage 2: the pedicle is severed and the flap is folded on itself to resurface the anterior ear.
- Stage 3: the folded edge is de-epithelialized and set into the lateral defect.4
The revolving-door flap also uses a 3-stage approach and is utilized for a full-thickness central auricular defect:
- Stage 1: a revolving-door flap is used to resurface the anterior ear.
- Stage 2: a cartilage graft provides structural support.
- Stage 3: division and inset with an FTSG is used to resurface the posterior ear.
The anterior pedicled retroauricular flap and revolving-door flap techniques are useful for defects when there is intact posterior auricular skin but not when there is extensive loss of cartilage. Other downsides to these 3-stage approaches are the time and multiple procedures required.5
We describe the technique of a retroauricular pull-through sandwich flap for repair of a large conchal bowl defect with extensive cartilage loss and intact posterior auricular skin.
Technique
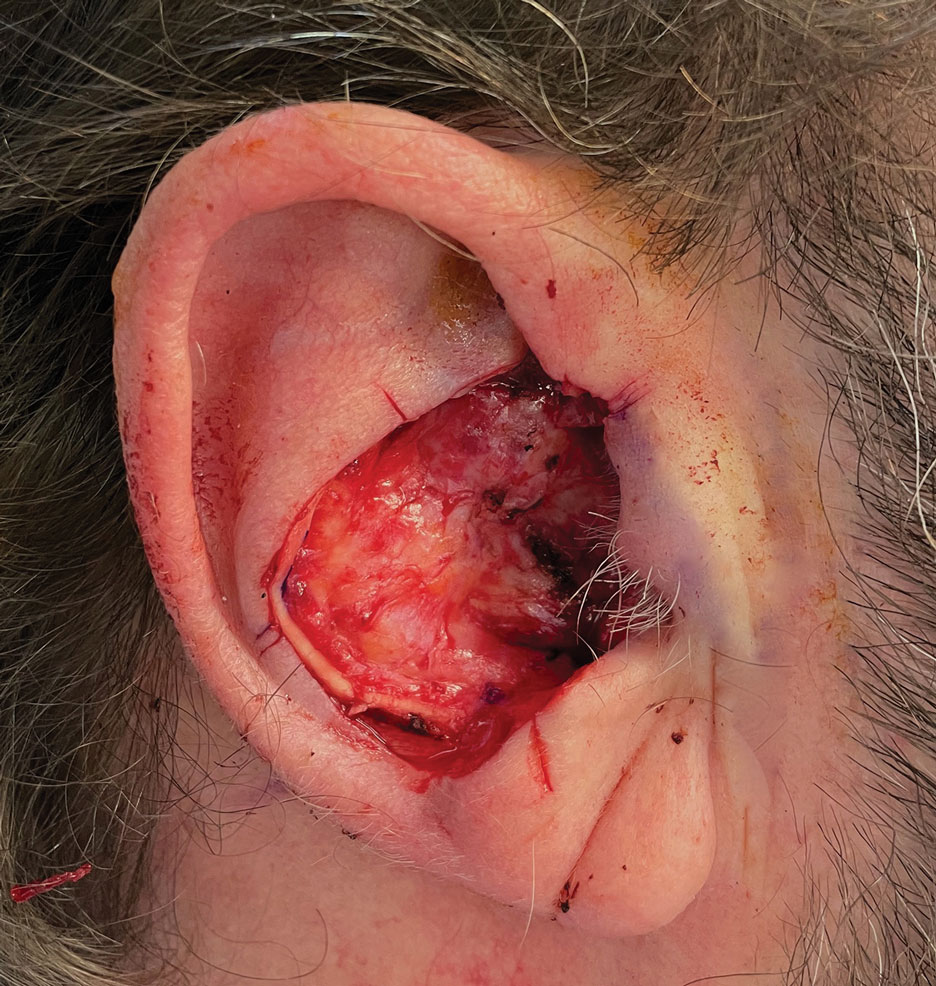
A 62-year-old man presented for treatment of a 2.6×2.4-cm nodular and infiltrative basal cell carcinoma of the right conchal bowl. The tumor was cleared with 3 stages of Mohs micrographic surgery, resulting in a 5.5×4.2-cm defect with complete loss of cartilage throughout the concha, helical crus, and inner rim of the antihelix (Figure 1). A 2-stage repair was performed utilizing a cartilage graft and a pull-through retroauricular interpolation flap.
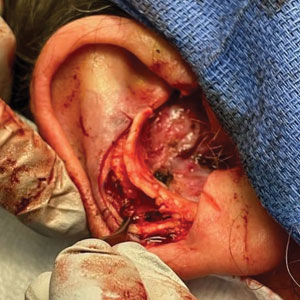
Stage 1—A cartilage graft was harvested from the left concha and sutured into the central defect for structural support (Figure 2). An incision was then made through the posterior auricular skin, just medial to the residual antihelical cartilage, and a retroauricular interpolation flap was pulled through this incision to resurface the lateral two-thirds of the conchal bowl defect. This created a “sandwich” of tissue, with the following layers (ordered from anterior to posterior): retroauricular interpolation flap, cartilage graft, and intact posterior auricular skin.
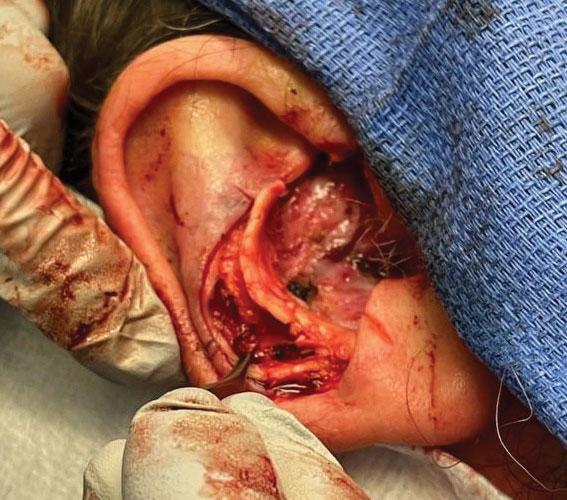
A preauricular banner transposition flap was used to repair the medial one-third of the conchal defect. A small area was left to heal by secondary intention (Figure 3).
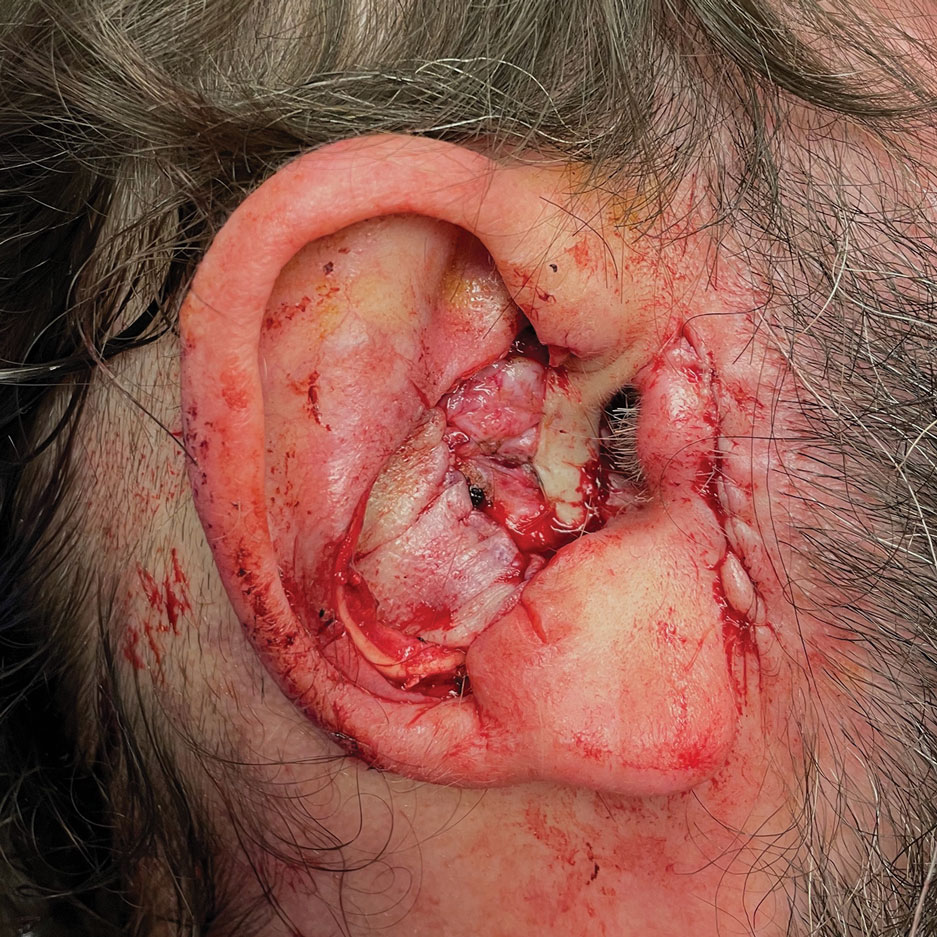
Stage 2—The patient returned 3 weeks later for division and inset of the retroauricular interpolation flap. The pedicle of the flap was severed and its free edge was sutured into the lateral aspect of the defect. The posterior auricular incision that the flap had been pulled through in stage 1 of the repair was closed in a layered fashion, and the secondary defect of the postauricular scalp was left to heal by secondary intention (Figure 4).
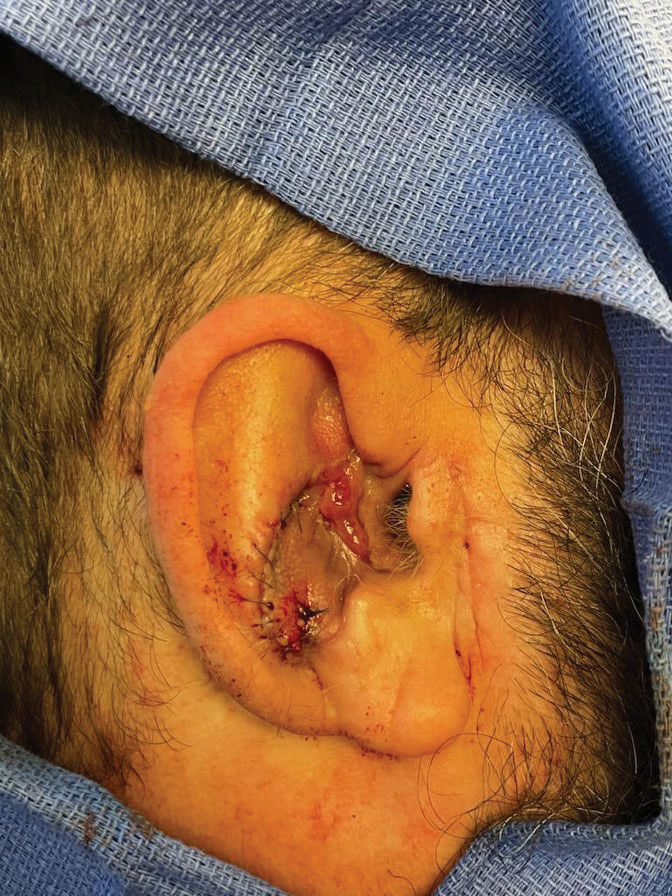
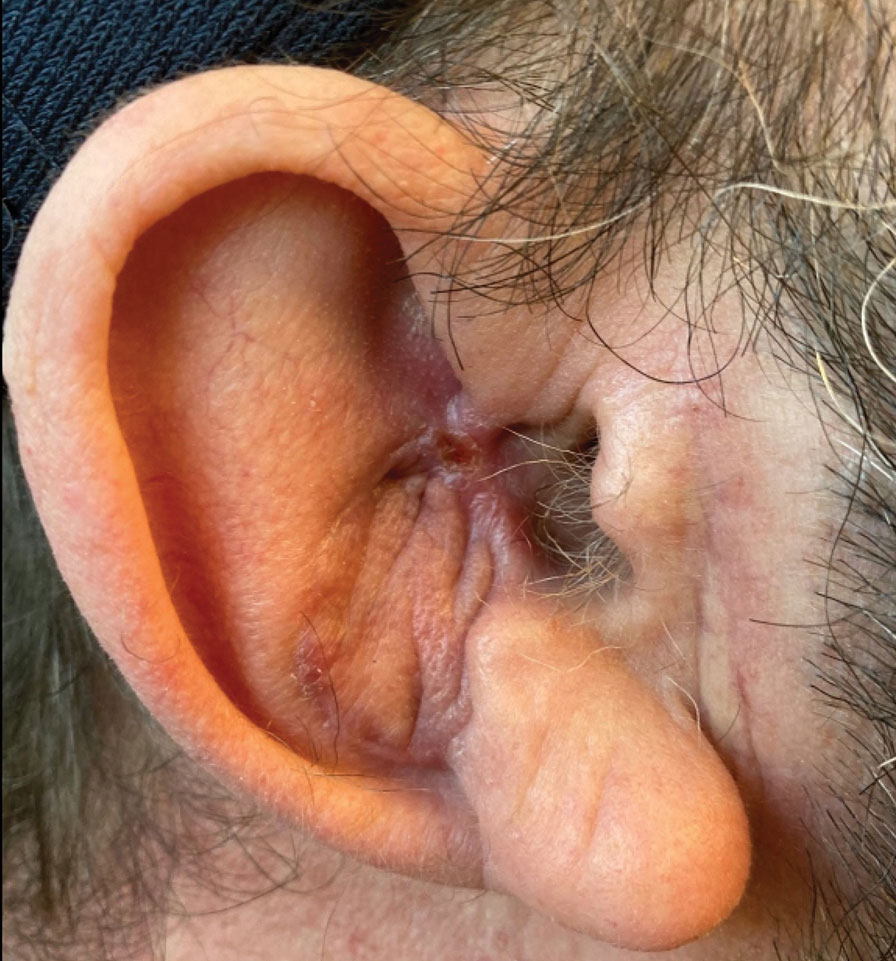
Final Results—At follow-up 1 month later, the patient was noted to have good aesthetic and functional outcomes (Figure 5).
Practice Implications
The retroauricular pull-through sandwich flap combines a cartilage graft and a retroauricular interpolation flap pulled through an incision in the posterior auricular skin to resurface the anterior ear. This repair is most useful for a large conchal bowl defect in which there is extensive missing cartilage but intact posterior auricular skin.
The retroauricular scalp is a substantial tissue reservoir with robust vasculature; an interpolation flap from this area frequently is used to repair an extensive ear defect. The most common use of an interpolation flap is for a large helical defect; however, the flap also can be pulled through an incision in the posterior auricular skin to the front of the ear in a manner similar to revolving-door and flip-flop flaps, thus allowing for increased flap reach.
A cartilage graft provides structural support, helping to maintain auricular projection. The helical arcades provide a robust vascular supply and maintain viability of the helical rim tissue, despite the large aperture created for the pull-through flap.
We recommend this 2-stage repair for large conchal bowl defects with extensive cartilage loss and intact posterior auricular skin.
- Clark DP, Hanke CW. Neoplasms of the conchal bowl: treatment with Mohs micrographic surgery. J Dermatol Surg Oncol. 1988;14:1223-1228. doi:10.1111/j.1524-4725.1988.tb03479.x
- Dessy LA, Figus A, Fioramonti P, et al. Reconstruction of anterior auricular conchal defect after malignancy excision: revolving-door flap versus full-thickness skin graft. J Plast Reconstr Aesthet Surg. 2010;63:746-752. doi:10.1016/j.bjps.2009.01.073
- Golash A, Bera S, Kanoi AV, et al. The revolving door flap: revisiting an elegant but forgotten flap for ear defect reconstruction. Indian J Plast Surg. 2020;53:64-70. doi:10.1055/s-0040-1709531
- Heinz MB, Hölzle F, Ghassemi A. Repairing a non-marginal full-thickness auricular defect using a reversed flap from the postauricular area. J Oral Maxillofac Surg. 2015;73:764-768. doi:10.1016/j.joms.2014.11.005
- Leitenberger JJ, Golden SK. Reconstruction after full-thickness loss of the antihelix, scapha, and triangular fossa. Dermatol Surg. 2016;42:893-896. doi:10.1097/DSS.0000000000000664
Practice Gap
Repair of a conchal defect requires careful consideration to achieve an optimal outcome. Reconstruction should resurface exposed cartilage, restore the natural projection of the auricle, and direct sound into the external auditory meatus. Patients also should be able to wear glasses and a hearing aid.
The reconstructive ladder for most conchal bowl defects includes secondary intention healing, full-thickness skin grafting (FTSG), and either a revolving-door flap or a flip-flop flap. Secondary intention and FTSG are appropriate for superficial defects, in which the loss of cartilage is not substantial.1,2 Revolving-door and flip-flop flaps are single-stage retroauricular approaches used to repair relatively small defects of the conchal bowl.3 However, reconstructive options are limited for a large defect in which there is extensive loss of cartilage; 3-stage retroauricular approaches have been utilized. The anterior pedicled retroauricular flap is a 3-stage repair that can be utilized to reconstruct a through-and-through defect of the central ear:
- Stage 1: an anteriorly based retroauricular pedicle is incised, hinged over, and sutured to the medial aspect of the defect, resurfacing the posterior ear.
- Stage 2: the pedicle is severed and the flap is folded on itself to resurface the anterior ear.
- Stage 3: the folded edge is de-epithelialized and set into the lateral defect.4
The revolving-door flap also uses a 3-stage approach and is utilized for a full-thickness central auricular defect:
- Stage 1: a revolving-door flap is used to resurface the anterior ear.
- Stage 2: a cartilage graft provides structural support.
- Stage 3: division and inset with an FTSG is used to resurface the posterior ear.
The anterior pedicled retroauricular flap and revolving-door flap techniques are useful for defects when there is intact posterior auricular skin but not when there is extensive loss of cartilage. Other downsides to these 3-stage approaches are the time and multiple procedures required.5
We describe the technique of a retroauricular pull-through sandwich flap for repair of a large conchal bowl defect with extensive cartilage loss and intact posterior auricular skin.
Technique
A 62-year-old man presented for treatment of a 2.6×2.4-cm nodular and infiltrative basal cell carcinoma of the right conchal bowl. The tumor was cleared with 3 stages of Mohs micrographic surgery, resulting in a 5.5×4.2-cm defect with complete loss of cartilage throughout the concha, helical crus, and inner rim of the antihelix (Figure 1). A 2-stage repair was performed utilizing a cartilage graft and a pull-through retroauricular interpolation flap.

Stage 1—A cartilage graft was harvested from the left concha and sutured into the central defect for structural support (Figure 2). An incision was then made through the posterior auricular skin, just medial to the residual antihelical cartilage, and a retroauricular interpolation flap was pulled through this incision to resurface the lateral two-thirds of the conchal bowl defect. This created a “sandwich” of tissue, with the following layers (ordered from anterior to posterior): retroauricular interpolation flap, cartilage graft, and intact posterior auricular skin.

A preauricular banner transposition flap was used to repair the medial one-third of the conchal defect. A small area was left to heal by secondary intention (Figure 3).

Stage 2—The patient returned 3 weeks later for division and inset of the retroauricular interpolation flap. The pedicle of the flap was severed and its free edge was sutured into the lateral aspect of the defect. The posterior auricular incision that the flap had been pulled through in stage 1 of the repair was closed in a layered fashion, and the secondary defect of the postauricular scalp was left to heal by secondary intention (Figure 4).

Final Results—At follow-up 1 month later, the patient was noted to have good aesthetic and functional outcomes (Figure 5).

Practice Implications
The retroauricular pull-through sandwich flap combines a cartilage graft and a retroauricular interpolation flap pulled through an incision in the posterior auricular skin to resurface the anterior ear. This repair is most useful for a large conchal bowl defect in which there is extensive missing cartilage but intact posterior auricular skin.
The retroauricular scalp is a substantial tissue reservoir with robust vasculature; an interpolation flap from this area frequently is used to repair an extensive ear defect. The most common use of an interpolation flap is for a large helical defect; however, the flap also can be pulled through an incision in the posterior auricular skin to the front of the ear in a manner similar to revolving-door and flip-flop flaps, thus allowing for increased flap reach.
A cartilage graft provides structural support, helping to maintain auricular projection. The helical arcades provide a robust vascular supply and maintain viability of the helical rim tissue, despite the large aperture created for the pull-through flap.
We recommend this 2-stage repair for large conchal bowl defects with extensive cartilage loss and intact posterior auricular skin.
Practice Gap
Repair of a conchal defect requires careful consideration to achieve an optimal outcome. Reconstruction should resurface exposed cartilage, restore the natural projection of the auricle, and direct sound into the external auditory meatus. Patients also should be able to wear glasses and a hearing aid.
The reconstructive ladder for most conchal bowl defects includes secondary intention healing, full-thickness skin grafting (FTSG), and either a revolving-door flap or a flip-flop flap. Secondary intention and FTSG are appropriate for superficial defects, in which the loss of cartilage is not substantial.1,2 Revolving-door and flip-flop flaps are single-stage retroauricular approaches used to repair relatively small defects of the conchal bowl.3 However, reconstructive options are limited for a large defect in which there is extensive loss of cartilage; 3-stage retroauricular approaches have been utilized. The anterior pedicled retroauricular flap is a 3-stage repair that can be utilized to reconstruct a through-and-through defect of the central ear:
- Stage 1: an anteriorly based retroauricular pedicle is incised, hinged over, and sutured to the medial aspect of the defect, resurfacing the posterior ear.
- Stage 2: the pedicle is severed and the flap is folded on itself to resurface the anterior ear.
- Stage 3: the folded edge is de-epithelialized and set into the lateral defect.4
The revolving-door flap also uses a 3-stage approach and is utilized for a full-thickness central auricular defect:
- Stage 1: a revolving-door flap is used to resurface the anterior ear.
- Stage 2: a cartilage graft provides structural support.
- Stage 3: division and inset with an FTSG is used to resurface the posterior ear.
The anterior pedicled retroauricular flap and revolving-door flap techniques are useful for defects when there is intact posterior auricular skin but not when there is extensive loss of cartilage. Other downsides to these 3-stage approaches are the time and multiple procedures required.5
We describe the technique of a retroauricular pull-through sandwich flap for repair of a large conchal bowl defect with extensive cartilage loss and intact posterior auricular skin.
Technique
A 62-year-old man presented for treatment of a 2.6×2.4-cm nodular and infiltrative basal cell carcinoma of the right conchal bowl. The tumor was cleared with 3 stages of Mohs micrographic surgery, resulting in a 5.5×4.2-cm defect with complete loss of cartilage throughout the concha, helical crus, and inner rim of the antihelix (Figure 1). A 2-stage repair was performed utilizing a cartilage graft and a pull-through retroauricular interpolation flap.

Stage 1—A cartilage graft was harvested from the left concha and sutured into the central defect for structural support (Figure 2). An incision was then made through the posterior auricular skin, just medial to the residual antihelical cartilage, and a retroauricular interpolation flap was pulled through this incision to resurface the lateral two-thirds of the conchal bowl defect. This created a “sandwich” of tissue, with the following layers (ordered from anterior to posterior): retroauricular interpolation flap, cartilage graft, and intact posterior auricular skin.

A preauricular banner transposition flap was used to repair the medial one-third of the conchal defect. A small area was left to heal by secondary intention (Figure 3).

Stage 2—The patient returned 3 weeks later for division and inset of the retroauricular interpolation flap. The pedicle of the flap was severed and its free edge was sutured into the lateral aspect of the defect. The posterior auricular incision that the flap had been pulled through in stage 1 of the repair was closed in a layered fashion, and the secondary defect of the postauricular scalp was left to heal by secondary intention (Figure 4).

Final Results—At follow-up 1 month later, the patient was noted to have good aesthetic and functional outcomes (Figure 5).

Practice Implications
The retroauricular pull-through sandwich flap combines a cartilage graft and a retroauricular interpolation flap pulled through an incision in the posterior auricular skin to resurface the anterior ear. This repair is most useful for a large conchal bowl defect in which there is extensive missing cartilage but intact posterior auricular skin.
The retroauricular scalp is a substantial tissue reservoir with robust vasculature; an interpolation flap from this area frequently is used to repair an extensive ear defect. The most common use of an interpolation flap is for a large helical defect; however, the flap also can be pulled through an incision in the posterior auricular skin to the front of the ear in a manner similar to revolving-door and flip-flop flaps, thus allowing for increased flap reach.
A cartilage graft provides structural support, helping to maintain auricular projection. The helical arcades provide a robust vascular supply and maintain viability of the helical rim tissue, despite the large aperture created for the pull-through flap.
We recommend this 2-stage repair for large conchal bowl defects with extensive cartilage loss and intact posterior auricular skin.
- Clark DP, Hanke CW. Neoplasms of the conchal bowl: treatment with Mohs micrographic surgery. J Dermatol Surg Oncol. 1988;14:1223-1228. doi:10.1111/j.1524-4725.1988.tb03479.x
- Dessy LA, Figus A, Fioramonti P, et al. Reconstruction of anterior auricular conchal defect after malignancy excision: revolving-door flap versus full-thickness skin graft. J Plast Reconstr Aesthet Surg. 2010;63:746-752. doi:10.1016/j.bjps.2009.01.073
- Golash A, Bera S, Kanoi AV, et al. The revolving door flap: revisiting an elegant but forgotten flap for ear defect reconstruction. Indian J Plast Surg. 2020;53:64-70. doi:10.1055/s-0040-1709531
- Heinz MB, Hölzle F, Ghassemi A. Repairing a non-marginal full-thickness auricular defect using a reversed flap from the postauricular area. J Oral Maxillofac Surg. 2015;73:764-768. doi:10.1016/j.joms.2014.11.005
- Leitenberger JJ, Golden SK. Reconstruction after full-thickness loss of the antihelix, scapha, and triangular fossa. Dermatol Surg. 2016;42:893-896. doi:10.1097/DSS.0000000000000664
- Clark DP, Hanke CW. Neoplasms of the conchal bowl: treatment with Mohs micrographic surgery. J Dermatol Surg Oncol. 1988;14:1223-1228. doi:10.1111/j.1524-4725.1988.tb03479.x
- Dessy LA, Figus A, Fioramonti P, et al. Reconstruction of anterior auricular conchal defect after malignancy excision: revolving-door flap versus full-thickness skin graft. J Plast Reconstr Aesthet Surg. 2010;63:746-752. doi:10.1016/j.bjps.2009.01.073
- Golash A, Bera S, Kanoi AV, et al. The revolving door flap: revisiting an elegant but forgotten flap for ear defect reconstruction. Indian J Plast Surg. 2020;53:64-70. doi:10.1055/s-0040-1709531
- Heinz MB, Hölzle F, Ghassemi A. Repairing a non-marginal full-thickness auricular defect using a reversed flap from the postauricular area. J Oral Maxillofac Surg. 2015;73:764-768. doi:10.1016/j.joms.2014.11.005
- Leitenberger JJ, Golden SK. Reconstruction after full-thickness loss of the antihelix, scapha, and triangular fossa. Dermatol Surg. 2016;42:893-896. doi:10.1097/DSS.0000000000000664
CLL combo treatment: Phase-3 study inconclusive
The difference in PFS between the IVO arm, 85%, versus 87% in the IO arm was statistically insignificant.
“Due to the early read-out and the futility boundaries being crossed, long-term follow-up will be critical to understand if there are any long-term benefits to IVO,” said study principal investigator Jennifer A. Woyach MD, professor in the division of hematology at The Ohio State University Comprehensive Care Center (OSUCCC – The James) in Columbus.
The 14-month follow-up data includes results from 465 CLL patients aged 65+ (median age 74 years, 67.5% male) who were treatment naive. The IO and IVO arms had 232 and 233 participants respectively, patients across both arms had Eastern Cooperative Oncology Group scores of 0-1 (97%), occurrence of Del (17p) was 13%, and a Rai stage status of III/IV was 55%, slightly more patients in the IO arm had unmutated IGHV 55% vs. 47% in the IVO arm. Researchers noted that, as expected, patients in the IVO group had a greater occurrence of hematologic adverse events graded at 3 or above, 61% VS 48% in the IO arm, P =.006.
The trial was spurred by the fact that many CLL patients on IO therapy must remain on treatment indefinitely, and an earlier phase II trial suggested that IVO therapy could lead to deep remission and therapy discontinuation.
Looking at the complete response (CR) rates and undetectable minimal residual disease (uMRD) rates across both arms suggested that there may be some hope that IVO could help CLL patients achieve deep remissions and discontinue therapy. Patients in the IVO arm had a CR of 68.5% and uMRD of 86.8% while only 31.3% of those in the IO arm had a CR and 33.3% achieved uMRD status.
“Despite the impressive CR and uMRD results, this study demonstrates that IVO is not superior to IO in terms of progression-free survival. However, because many patients in the IVO arm have discontinued treatment while those in the IO arm remain on ibrutinib, we think that it will be very important to continue to follow these patients long term, to see if there are advantages to this time limited therapy, especially in terms of toxicity, that we cannot appreciate with this follow-up,” said Dr. Woyach.
The Alliance for Clinical Trials in Oncology cooperative group, including OSUCCC James, is currently working to design the next frontline CLL study for older patients that builds on this work.
Dr. Woyach disclosed ties with Abbvie, AstraZeneca, Beigene, Genentech, Janssen, Loxo/Lilly, Merck, Newave, Pharmacyclics, and Schrodinger.
The difference in PFS between the IVO arm, 85%, versus 87% in the IO arm was statistically insignificant.
“Due to the early read-out and the futility boundaries being crossed, long-term follow-up will be critical to understand if there are any long-term benefits to IVO,” said study principal investigator Jennifer A. Woyach MD, professor in the division of hematology at The Ohio State University Comprehensive Care Center (OSUCCC – The James) in Columbus.
The 14-month follow-up data includes results from 465 CLL patients aged 65+ (median age 74 years, 67.5% male) who were treatment naive. The IO and IVO arms had 232 and 233 participants respectively, patients across both arms had Eastern Cooperative Oncology Group scores of 0-1 (97%), occurrence of Del (17p) was 13%, and a Rai stage status of III/IV was 55%, slightly more patients in the IO arm had unmutated IGHV 55% vs. 47% in the IVO arm. Researchers noted that, as expected, patients in the IVO group had a greater occurrence of hematologic adverse events graded at 3 or above, 61% VS 48% in the IO arm, P =.006.
The trial was spurred by the fact that many CLL patients on IO therapy must remain on treatment indefinitely, and an earlier phase II trial suggested that IVO therapy could lead to deep remission and therapy discontinuation.
Looking at the complete response (CR) rates and undetectable minimal residual disease (uMRD) rates across both arms suggested that there may be some hope that IVO could help CLL patients achieve deep remissions and discontinue therapy. Patients in the IVO arm had a CR of 68.5% and uMRD of 86.8% while only 31.3% of those in the IO arm had a CR and 33.3% achieved uMRD status.
“Despite the impressive CR and uMRD results, this study demonstrates that IVO is not superior to IO in terms of progression-free survival. However, because many patients in the IVO arm have discontinued treatment while those in the IO arm remain on ibrutinib, we think that it will be very important to continue to follow these patients long term, to see if there are advantages to this time limited therapy, especially in terms of toxicity, that we cannot appreciate with this follow-up,” said Dr. Woyach.
The Alliance for Clinical Trials in Oncology cooperative group, including OSUCCC James, is currently working to design the next frontline CLL study for older patients that builds on this work.
Dr. Woyach disclosed ties with Abbvie, AstraZeneca, Beigene, Genentech, Janssen, Loxo/Lilly, Merck, Newave, Pharmacyclics, and Schrodinger.
The difference in PFS between the IVO arm, 85%, versus 87% in the IO arm was statistically insignificant.
“Due to the early read-out and the futility boundaries being crossed, long-term follow-up will be critical to understand if there are any long-term benefits to IVO,” said study principal investigator Jennifer A. Woyach MD, professor in the division of hematology at The Ohio State University Comprehensive Care Center (OSUCCC – The James) in Columbus.
The 14-month follow-up data includes results from 465 CLL patients aged 65+ (median age 74 years, 67.5% male) who were treatment naive. The IO and IVO arms had 232 and 233 participants respectively, patients across both arms had Eastern Cooperative Oncology Group scores of 0-1 (97%), occurrence of Del (17p) was 13%, and a Rai stage status of III/IV was 55%, slightly more patients in the IO arm had unmutated IGHV 55% vs. 47% in the IVO arm. Researchers noted that, as expected, patients in the IVO group had a greater occurrence of hematologic adverse events graded at 3 or above, 61% VS 48% in the IO arm, P =.006.
The trial was spurred by the fact that many CLL patients on IO therapy must remain on treatment indefinitely, and an earlier phase II trial suggested that IVO therapy could lead to deep remission and therapy discontinuation.
Looking at the complete response (CR) rates and undetectable minimal residual disease (uMRD) rates across both arms suggested that there may be some hope that IVO could help CLL patients achieve deep remissions and discontinue therapy. Patients in the IVO arm had a CR of 68.5% and uMRD of 86.8% while only 31.3% of those in the IO arm had a CR and 33.3% achieved uMRD status.
“Despite the impressive CR and uMRD results, this study demonstrates that IVO is not superior to IO in terms of progression-free survival. However, because many patients in the IVO arm have discontinued treatment while those in the IO arm remain on ibrutinib, we think that it will be very important to continue to follow these patients long term, to see if there are advantages to this time limited therapy, especially in terms of toxicity, that we cannot appreciate with this follow-up,” said Dr. Woyach.
The Alliance for Clinical Trials in Oncology cooperative group, including OSUCCC James, is currently working to design the next frontline CLL study for older patients that builds on this work.
Dr. Woyach disclosed ties with Abbvie, AstraZeneca, Beigene, Genentech, Janssen, Loxo/Lilly, Merck, Newave, Pharmacyclics, and Schrodinger.
Evaluation of Laboratory Follow-up in Acne Patients Treated With Isotretinoin
Isotretinoin is used in the treatment of nodulocystic and severe papulopustular acne. During the treatment period, laboratory monitoring is recommended to identify the risk for complications such as hepatotoxicity, teratogenicity, rhabdomyolysis, hyperlipidemia, and pancreatitis.1 There is a lack of consensus of the frequency of follow-up of laboratory parameters during isotretinoin treatment. This study evaluated the changes in laboratory parameters used in daily practice for patients with acne who were treated with isotretinoin to determine the optimum test repetition frequency.
Materials and Methods
Statistical Analysis—The descriptive statistics of the measurements were presented as means, standard deviations, or medians (first and third quartiles). With respect to the normal distribution, the consistency of the measurements was evaluated with the Kolmogorov-Smirnov test, and small deviations from the normal distribution were observed. Changes in laboratory measurements were evaluated with simple repeated-measures analysis of variance, and changes that differed significantly were determined by a Holm-Sidak post hoc test. Relationships between total cumulative doses and laboratory measurements at second visits were evaluated by the Pearson correlation analysis. The statistical significance level was P<.05. SPSS Statistics 23 (IBM) was used in the calculations.
Results
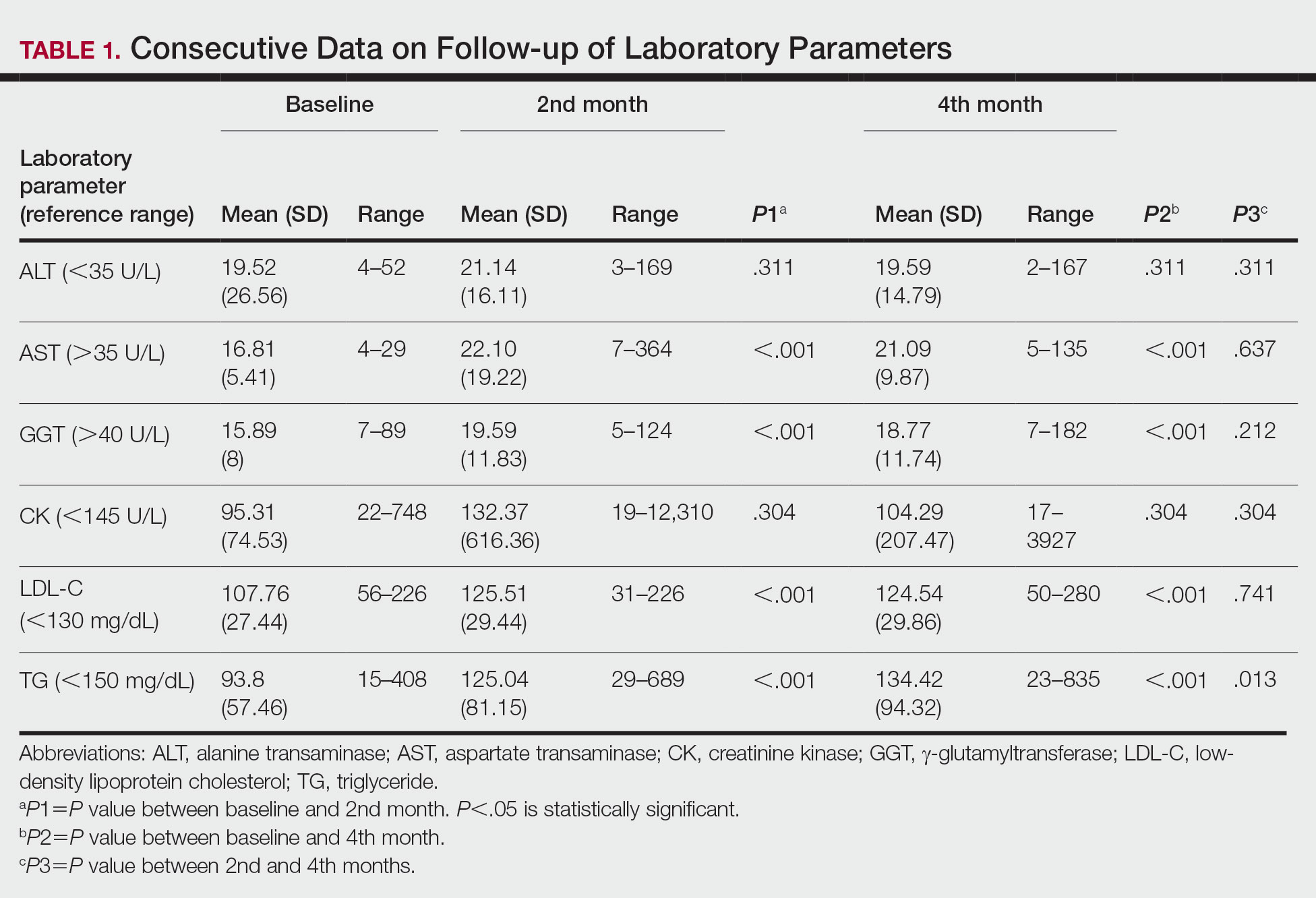
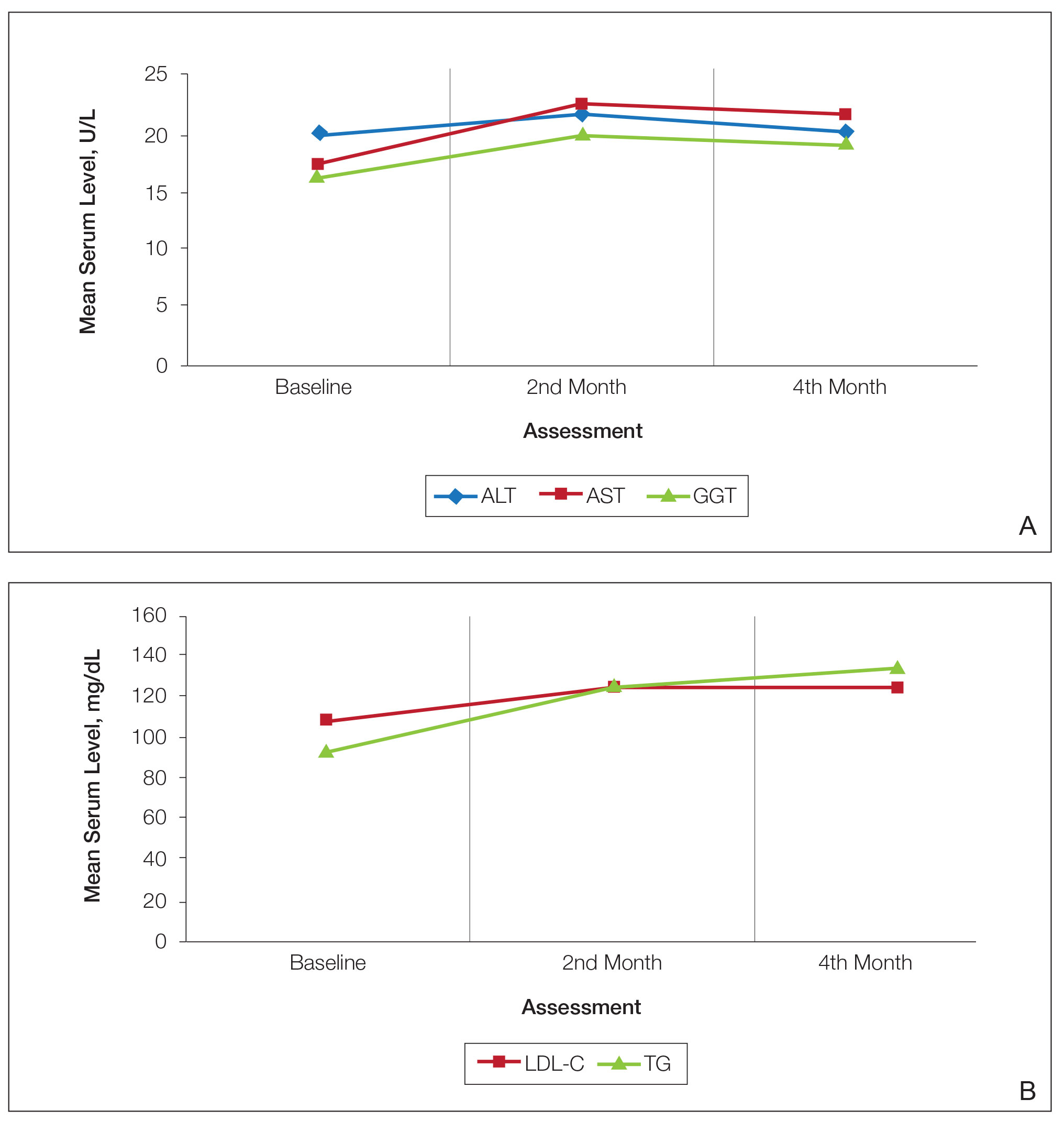
Consecutive Data at Baseline and Follow-up—A total of 415 patients with a mean age (SD) of 21.49 (7.25) years (range, 12–53 years) were included in our study. The mean total cumulative dose (SD) of the patients was 7267.27 (1878.4) mg. The consecutive data of the means of the laboratory parameters are shown in Table 1 and Figure 1. There was no significant change in the ALT levels between baseline and the fourth month as well as between the second- and fourth-month assessments (both P=.311).
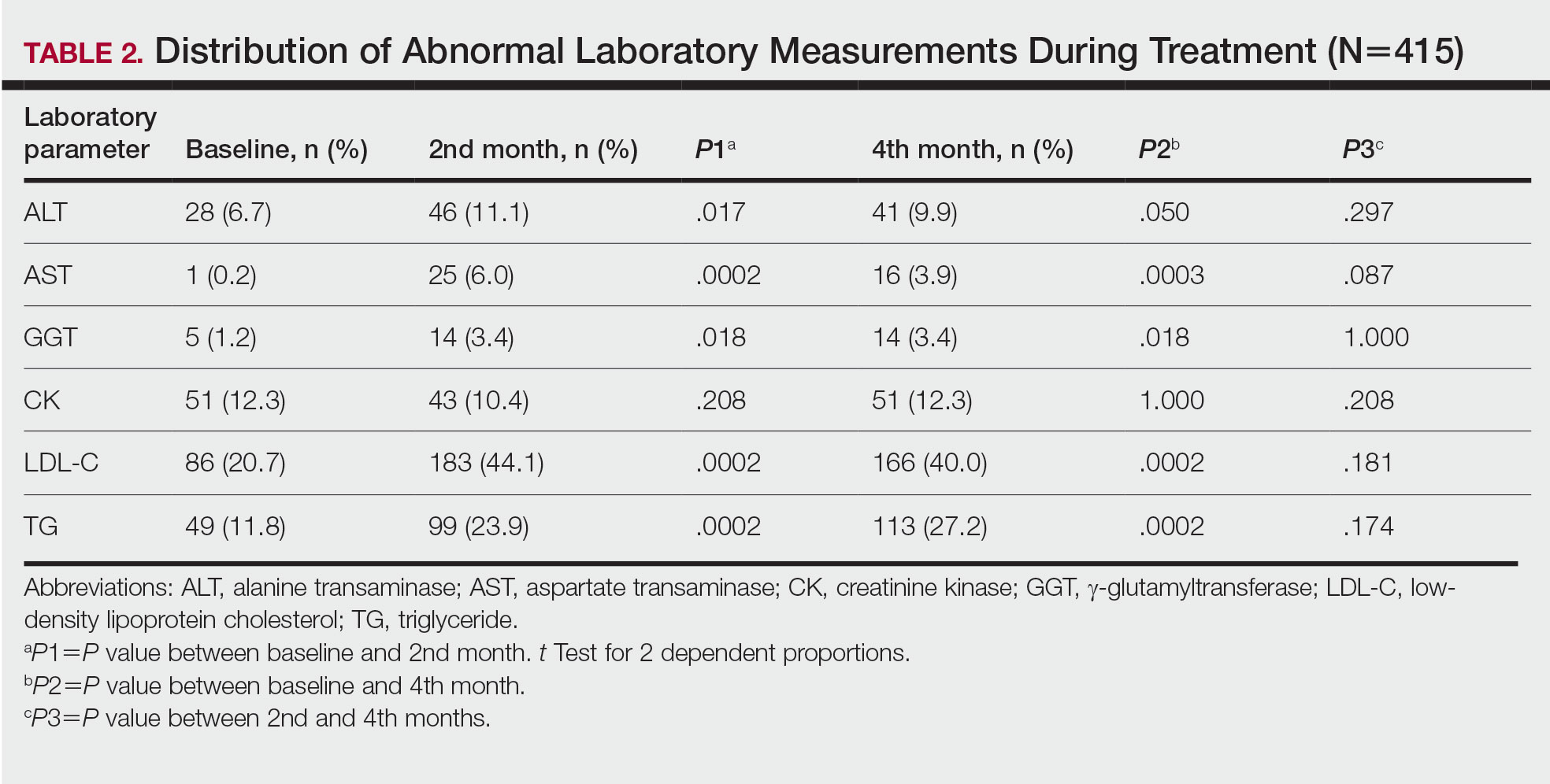
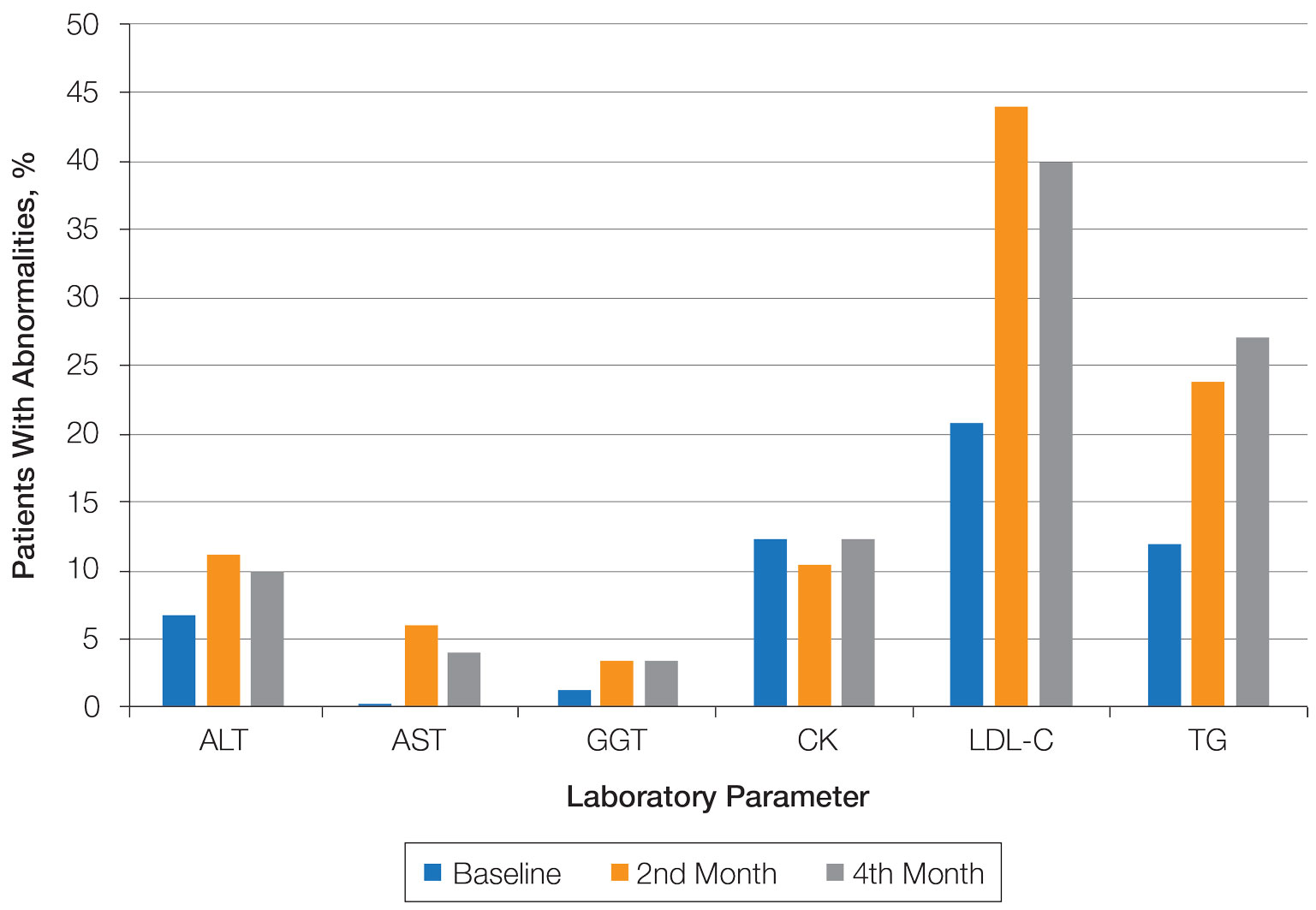
Abnormal Laboratory Measurements—The distribution of abnormal laboratory measurements during treatment is shown in Table 2 and Figure 2. Grade 3 or higher elevations of liver transaminases (ALT, AST) and GGT were observed in fewer than 2% of patients during treatment compared with baseline (grade 3 elevations of ALT and AST together in 2 patients; grade 4 AST elevation in 1 patient; grade 3 elevations of ALT, AST, and GGT combined in 1 patient; isolated grade 3 GGT elevation in 1 patient). All of the patients who developed grade 3 liver transaminases and isolated grade 3 GGT elevation had improved values when these were rechecked within 2 weeks.
In the patient who developed hepatotoxicity in the second month, the ALT level rose from a baseline of 19 U/L to 169 U/L, the AST level from a baseline of 19 U/L to 61 U/L, and the GGT level from a baseline of 24 U/L to 124 U/L. The patient was asymptomatic. Liver function test levels returned to reference range 4 weeks after discontinuation of therapy. Hepatotoxicity did not recur after treatment was re-administered.
The patient who developed grade 4 AST elevation (364 U/L) experienced fatigue and myalgia. He had done vigorous exercise up to 2 days before the test and also had a grade 4 CK elevation (12,310 U/L). He was thought to have isotretinoin-related rhabdomyolysis. His treatment was discontinued, and he was advised to hydrate and rest. Treatment was re-started after 2 weeks. With frequent laboratory monitoring and avoidance of vigorous physical activity, the patient completed the remaining course of isotretinoin without any laboratory abnormalities or symptoms.
Creatinine kinase abnormalities in the second and fourth months compared with baseline were not statistically significant. The patients with grade 3 or higher CK elevations, except for the case with rhabdomyolysis, had no clinical signs or other characteristic laboratory findings of rhabdomyolysis.
Hypercholesterolemia (LDL-C ≥130 mg/dL) occurred most frequently, with a maximum of 280 mg/dL in 1 patient (in the fourth month) and less than 250 mg/dL in all other patients. Hypercholesterolemia occurred in 183 (44.1%) patients in the second month and in 166 (40.0%) patients in the fourth month. However, baseline abnormalities also were frequent (86 [20.7%]), and hypercholesterolemia persisted in the second and fourth months in all of these patients.
It was observed that the patients with TG abnormalities increased continuously in the second (99 [23.9%]) and fourth (113 [27.2%]) months compared with baseline (49 [11.8%]). Grade 3 TG elevations were observed in 2.2% of patients (n=9; 5 patients in the second month, 4 patients in the fourth month) during treatment compared with baseline, and all patients had grade 1 or 2 hypertriglyceridemia at baseline. Of the patients with grade 3 TG elevation, 3 patients in the second month and 2 patients in the fourth month were obese at baseline. No grade 4 TG elevations were observed. Complications related to hyperlipidemia, such as pancreatitis, were observed in 1 patient. No patient terminated treatment because of lipid abnormalities. The treatment of our patients with major hypercholesterolemia and/or grade 3 hypertriglyceridemia was interrupted. The hyperlipidemia of these patients was controlled by a low-fat diet and a short-term dose reduction.
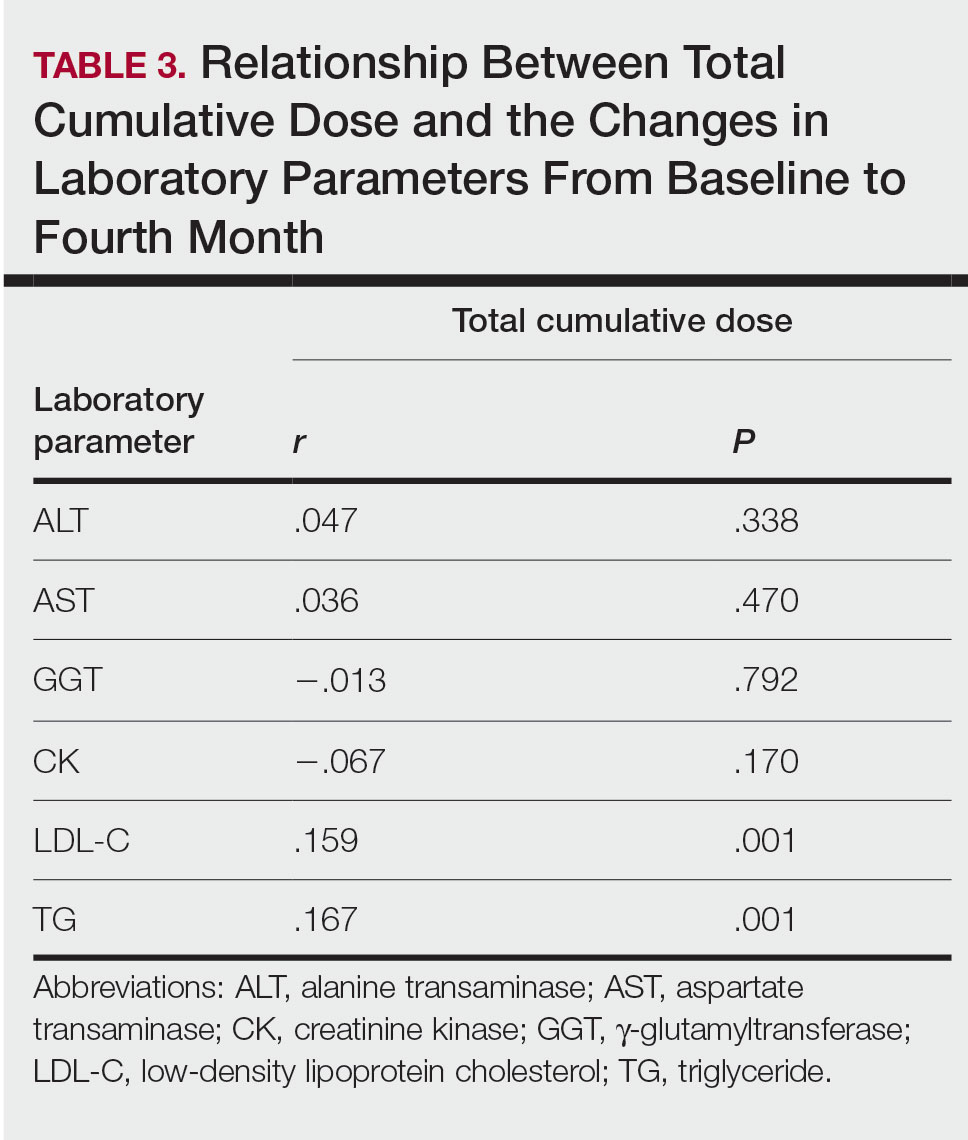
Relationship Between Total Cumulative Dose and Laboratory Parameters—The relationships between the total cumulative dose and changes up to the fourth month are presented in Table 3. As the total dose increased, the changes in TG and LDL-C levels significantly increased in the fourth month (both P=.001). However, the degree of these relationships was weak. No significant correlation was found between the periodic changes of other laboratory parameters and the total dose.
Comment
The parameters followed in our study show that TG levels tend to increase continuously from baseline during isotretinoin treatment, while ALT, AST, GGT, and LDL-C levels increase in the second month and decrease at 4 months. Although this same trend occurs with CK levels, the change was not statistically significant. The most common laboratory abnormality in our study was hyperlipidemia. Levels of LDL-C and TG were both found to be statistically elevated in the second and fourth months of treatment compared with baseline. Parthasarathy et al3 reported that obesity had an important role in the increase of lipid levels in patients using isotretinoin at baseline. In our study, 5 of 9 patients (55.6%) with grade 3 TG elevation were obese, which supports the theory that obesity plays an important role in the increase in lipid levels. Up-to-date laboratory follow-up of lipids suggests that there is no need to follow up serum lipids after the second month of treatment. Patients with risk factors for hyperlipidemia, such as abdominal obesity and familial hyperlipidemia, do not require further follow-up if there is no increase in serum lipids in the first month of treatment.1 The presence of grade 1 or 2 hypertriglyceridemia at baseline in all our patients with grade 3 TG elevation may suggest that periodic laboratory follow-up during isotretinoin treatment is necessary to detect patients with grade 3 and higher TG levels.
The lack of knowledge of other risk factors (eg, familial hyperlipidemia, insulin resistance) for hyperlipidemia in all patients at baseline may be a limitation of our study. Although hypercholesterolemia persisted in the follow-up of our patients with initial LDL-C abnormalities, hypercholesterolemia over 250 mg/dL was very rare (1 patient). Possible complications associated with serum lipid abnormalities are pancreatitis and metabolic syndrome.4 In our study, none of the patients with lipid abnormalities had any relevant clinical sequelae. The dose-dependent elevation of the changes in LDL-C and TG (Table 3) may be important to predict the significant elevation of lipids and the associated complications in patients with a high total cumulative dose target that may require a long treatment duration. However, considering the short follow-up periods in our patients, the absence of clinical sequelae may be misleading. There are differences in recommendations between the US and European guidelines for isotretinoin dosage. Although the US guidelines recommend a total cumulative dose target, the European guidelines recommend low-dose isotretinoin daily for at least 6 months instead of a cumulative dose.
Most liver transaminase abnormalities were detected in the second month. Abnormalities in GGT were seen in the second month and remained elevated at the next follow-up. However, clinically important grade 3 transaminase and GGT elevations were rare. It has been reported that GGT levels are more specific than transaminases in measuring hepatotoxicity.7 The fact that our patient with hepatotoxicity had a grade 3 GGT elevation in addition to grade 3 transaminase elevations supports that GGT elevation is more specific than transaminase levels in measuring hepatotoxicity. When these parameters were rechecked in our patients with grade 3 transaminase elevations, except in the case of hepatotoxicity, transaminase elevations did not recur, and GGT elevations did not accompany elevated transaminases, which suggested that transaminases may be elevated due to an extrahepatic origin (eg, hemolysis, exercise).
Rhabdomyolysis secondary to isotretinoin is rare in the literature of acne studies. In addition to clinical findings such as myalgia and fatigue, increased CK and abnormal liver enzymes, specifically AST, suggest the development of rhabdomyolysis.8 Our patient who developed rhabdomyolysis also had a recent history of vigorous exercise, grade 4 CK, and AST elevations. Other patients with isolated grade 3 CK elevations were informed about possible clinical signs of rhabdomyolysis, and they were able to complete their courses without any incident. According to a study by Landau et al,9 isotretinoin-associated hyperCKemia has been reported as benign. Similarly, our study found that isolated CK elevation during isotretinoin treatment may be misleading as a sign of rhabdomyolysis. Instead, CK monitoring may be more appropriate and cost-effective in patients with suspected clinical signs of rhabdomyolysis or in those with major elevations in transaminases, especially AST.
Conclusion
According to our study, hyperlipidemia was the most common complication in acne patients using isotretinoin. It may be appropriate to monitor the TG level at 2-month intervals in patients with grade 1 or 2 TG elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia. Periodic monitoring of LDL-C and TG levels may be appropriate, especially in patients who require a high total cumulative dose of isotretinoin. Clinically important liver enzyme abnormalities were rare in our study. Our findings support the idea that routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Additionally, periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 9, 2006. Accessed June 12, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Parthasarathy V, Shah N, Kirkorian AY. The utility of laboratory testing for pediatric patients undergoing isotretinoin treatment. Pediatr Dermatol. 2022;39:731-733.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7:171-174.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30:1261-1268.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017;23:13030/qt7rv7j80p.
- Raneses E, Schmidgal EC. Rhabdomyolysis caused by isotretinoin and exercise in an otherwise healthy female patient. Cureus. 2022;14:E25981.
- Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venereol. 2001;81:350-352.
Isotretinoin is used in the treatment of nodulocystic and severe papulopustular acne. During the treatment period, laboratory monitoring is recommended to identify the risk for complications such as hepatotoxicity, teratogenicity, rhabdomyolysis, hyperlipidemia, and pancreatitis.1 There is a lack of consensus of the frequency of follow-up of laboratory parameters during isotretinoin treatment. This study evaluated the changes in laboratory parameters used in daily practice for patients with acne who were treated with isotretinoin to determine the optimum test repetition frequency.
Materials and Methods

Statistical Analysis—The descriptive statistics of the measurements were presented as means, standard deviations, or medians (first and third quartiles). With respect to the normal distribution, the consistency of the measurements was evaluated with the Kolmogorov-Smirnov test, and small deviations from the normal distribution were observed. Changes in laboratory measurements were evaluated with simple repeated-measures analysis of variance, and changes that differed significantly were determined by a Holm-Sidak post hoc test. Relationships between total cumulative doses and laboratory measurements at second visits were evaluated by the Pearson correlation analysis. The statistical significance level was P<.05. SPSS Statistics 23 (IBM) was used in the calculations.
Results
Consecutive Data at Baseline and Follow-up—A total of 415 patients with a mean age (SD) of 21.49 (7.25) years (range, 12–53 years) were included in our study. The mean total cumulative dose (SD) of the patients was 7267.27 (1878.4) mg. The consecutive data of the means of the laboratory parameters are shown in Table 1 and Figure 1. There was no significant change in the ALT levels between baseline and the fourth month as well as between the second- and fourth-month assessments (both P=.311).

Abnormal Laboratory Measurements—The distribution of abnormal laboratory measurements during treatment is shown in Table 2 and Figure 2. Grade 3 or higher elevations of liver transaminases (ALT, AST) and GGT were observed in fewer than 2% of patients during treatment compared with baseline (grade 3 elevations of ALT and AST together in 2 patients; grade 4 AST elevation in 1 patient; grade 3 elevations of ALT, AST, and GGT combined in 1 patient; isolated grade 3 GGT elevation in 1 patient). All of the patients who developed grade 3 liver transaminases and isolated grade 3 GGT elevation had improved values when these were rechecked within 2 weeks.

In the patient who developed hepatotoxicity in the second month, the ALT level rose from a baseline of 19 U/L to 169 U/L, the AST level from a baseline of 19 U/L to 61 U/L, and the GGT level from a baseline of 24 U/L to 124 U/L. The patient was asymptomatic. Liver function test levels returned to reference range 4 weeks after discontinuation of therapy. Hepatotoxicity did not recur after treatment was re-administered.

The patient who developed grade 4 AST elevation (364 U/L) experienced fatigue and myalgia. He had done vigorous exercise up to 2 days before the test and also had a grade 4 CK elevation (12,310 U/L). He was thought to have isotretinoin-related rhabdomyolysis. His treatment was discontinued, and he was advised to hydrate and rest. Treatment was re-started after 2 weeks. With frequent laboratory monitoring and avoidance of vigorous physical activity, the patient completed the remaining course of isotretinoin without any laboratory abnormalities or symptoms.
Creatinine kinase abnormalities in the second and fourth months compared with baseline were not statistically significant. The patients with grade 3 or higher CK elevations, except for the case with rhabdomyolysis, had no clinical signs or other characteristic laboratory findings of rhabdomyolysis.
Hypercholesterolemia (LDL-C ≥130 mg/dL) occurred most frequently, with a maximum of 280 mg/dL in 1 patient (in the fourth month) and less than 250 mg/dL in all other patients. Hypercholesterolemia occurred in 183 (44.1%) patients in the second month and in 166 (40.0%) patients in the fourth month. However, baseline abnormalities also were frequent (86 [20.7%]), and hypercholesterolemia persisted in the second and fourth months in all of these patients.
It was observed that the patients with TG abnormalities increased continuously in the second (99 [23.9%]) and fourth (113 [27.2%]) months compared with baseline (49 [11.8%]). Grade 3 TG elevations were observed in 2.2% of patients (n=9; 5 patients in the second month, 4 patients in the fourth month) during treatment compared with baseline, and all patients had grade 1 or 2 hypertriglyceridemia at baseline. Of the patients with grade 3 TG elevation, 3 patients in the second month and 2 patients in the fourth month were obese at baseline. No grade 4 TG elevations were observed. Complications related to hyperlipidemia, such as pancreatitis, were observed in 1 patient. No patient terminated treatment because of lipid abnormalities. The treatment of our patients with major hypercholesterolemia and/or grade 3 hypertriglyceridemia was interrupted. The hyperlipidemia of these patients was controlled by a low-fat diet and a short-term dose reduction.
Relationship Between Total Cumulative Dose and Laboratory Parameters—The relationships between the total cumulative dose and changes up to the fourth month are presented in Table 3. As the total dose increased, the changes in TG and LDL-C levels significantly increased in the fourth month (both P=.001). However, the degree of these relationships was weak. No significant correlation was found between the periodic changes of other laboratory parameters and the total dose.

Comment
The parameters followed in our study show that TG levels tend to increase continuously from baseline during isotretinoin treatment, while ALT, AST, GGT, and LDL-C levels increase in the second month and decrease at 4 months. Although this same trend occurs with CK levels, the change was not statistically significant. The most common laboratory abnormality in our study was hyperlipidemia. Levels of LDL-C and TG were both found to be statistically elevated in the second and fourth months of treatment compared with baseline. Parthasarathy et al3 reported that obesity had an important role in the increase of lipid levels in patients using isotretinoin at baseline. In our study, 5 of 9 patients (55.6%) with grade 3 TG elevation were obese, which supports the theory that obesity plays an important role in the increase in lipid levels. Up-to-date laboratory follow-up of lipids suggests that there is no need to follow up serum lipids after the second month of treatment. Patients with risk factors for hyperlipidemia, such as abdominal obesity and familial hyperlipidemia, do not require further follow-up if there is no increase in serum lipids in the first month of treatment.1 The presence of grade 1 or 2 hypertriglyceridemia at baseline in all our patients with grade 3 TG elevation may suggest that periodic laboratory follow-up during isotretinoin treatment is necessary to detect patients with grade 3 and higher TG levels.
The lack of knowledge of other risk factors (eg, familial hyperlipidemia, insulin resistance) for hyperlipidemia in all patients at baseline may be a limitation of our study. Although hypercholesterolemia persisted in the follow-up of our patients with initial LDL-C abnormalities, hypercholesterolemia over 250 mg/dL was very rare (1 patient). Possible complications associated with serum lipid abnormalities are pancreatitis and metabolic syndrome.4 In our study, none of the patients with lipid abnormalities had any relevant clinical sequelae. The dose-dependent elevation of the changes in LDL-C and TG (Table 3) may be important to predict the significant elevation of lipids and the associated complications in patients with a high total cumulative dose target that may require a long treatment duration. However, considering the short follow-up periods in our patients, the absence of clinical sequelae may be misleading. There are differences in recommendations between the US and European guidelines for isotretinoin dosage. Although the US guidelines recommend a total cumulative dose target, the European guidelines recommend low-dose isotretinoin daily for at least 6 months instead of a cumulative dose.
Most liver transaminase abnormalities were detected in the second month. Abnormalities in GGT were seen in the second month and remained elevated at the next follow-up. However, clinically important grade 3 transaminase and GGT elevations were rare. It has been reported that GGT levels are more specific than transaminases in measuring hepatotoxicity.7 The fact that our patient with hepatotoxicity had a grade 3 GGT elevation in addition to grade 3 transaminase elevations supports that GGT elevation is more specific than transaminase levels in measuring hepatotoxicity. When these parameters were rechecked in our patients with grade 3 transaminase elevations, except in the case of hepatotoxicity, transaminase elevations did not recur, and GGT elevations did not accompany elevated transaminases, which suggested that transaminases may be elevated due to an extrahepatic origin (eg, hemolysis, exercise).
Rhabdomyolysis secondary to isotretinoin is rare in the literature of acne studies. In addition to clinical findings such as myalgia and fatigue, increased CK and abnormal liver enzymes, specifically AST, suggest the development of rhabdomyolysis.8 Our patient who developed rhabdomyolysis also had a recent history of vigorous exercise, grade 4 CK, and AST elevations. Other patients with isolated grade 3 CK elevations were informed about possible clinical signs of rhabdomyolysis, and they were able to complete their courses without any incident. According to a study by Landau et al,9 isotretinoin-associated hyperCKemia has been reported as benign. Similarly, our study found that isolated CK elevation during isotretinoin treatment may be misleading as a sign of rhabdomyolysis. Instead, CK monitoring may be more appropriate and cost-effective in patients with suspected clinical signs of rhabdomyolysis or in those with major elevations in transaminases, especially AST.
Conclusion
According to our study, hyperlipidemia was the most common complication in acne patients using isotretinoin. It may be appropriate to monitor the TG level at 2-month intervals in patients with grade 1 or 2 TG elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia. Periodic monitoring of LDL-C and TG levels may be appropriate, especially in patients who require a high total cumulative dose of isotretinoin. Clinically important liver enzyme abnormalities were rare in our study. Our findings support the idea that routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Additionally, periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
Isotretinoin is used in the treatment of nodulocystic and severe papulopustular acne. During the treatment period, laboratory monitoring is recommended to identify the risk for complications such as hepatotoxicity, teratogenicity, rhabdomyolysis, hyperlipidemia, and pancreatitis.1 There is a lack of consensus of the frequency of follow-up of laboratory parameters during isotretinoin treatment. This study evaluated the changes in laboratory parameters used in daily practice for patients with acne who were treated with isotretinoin to determine the optimum test repetition frequency.
Materials and Methods

Statistical Analysis—The descriptive statistics of the measurements were presented as means, standard deviations, or medians (first and third quartiles). With respect to the normal distribution, the consistency of the measurements was evaluated with the Kolmogorov-Smirnov test, and small deviations from the normal distribution were observed. Changes in laboratory measurements were evaluated with simple repeated-measures analysis of variance, and changes that differed significantly were determined by a Holm-Sidak post hoc test. Relationships between total cumulative doses and laboratory measurements at second visits were evaluated by the Pearson correlation analysis. The statistical significance level was P<.05. SPSS Statistics 23 (IBM) was used in the calculations.
Results
Consecutive Data at Baseline and Follow-up—A total of 415 patients with a mean age (SD) of 21.49 (7.25) years (range, 12–53 years) were included in our study. The mean total cumulative dose (SD) of the patients was 7267.27 (1878.4) mg. The consecutive data of the means of the laboratory parameters are shown in Table 1 and Figure 1. There was no significant change in the ALT levels between baseline and the fourth month as well as between the second- and fourth-month assessments (both P=.311).

Abnormal Laboratory Measurements—The distribution of abnormal laboratory measurements during treatment is shown in Table 2 and Figure 2. Grade 3 or higher elevations of liver transaminases (ALT, AST) and GGT were observed in fewer than 2% of patients during treatment compared with baseline (grade 3 elevations of ALT and AST together in 2 patients; grade 4 AST elevation in 1 patient; grade 3 elevations of ALT, AST, and GGT combined in 1 patient; isolated grade 3 GGT elevation in 1 patient). All of the patients who developed grade 3 liver transaminases and isolated grade 3 GGT elevation had improved values when these were rechecked within 2 weeks.

In the patient who developed hepatotoxicity in the second month, the ALT level rose from a baseline of 19 U/L to 169 U/L, the AST level from a baseline of 19 U/L to 61 U/L, and the GGT level from a baseline of 24 U/L to 124 U/L. The patient was asymptomatic. Liver function test levels returned to reference range 4 weeks after discontinuation of therapy. Hepatotoxicity did not recur after treatment was re-administered.

The patient who developed grade 4 AST elevation (364 U/L) experienced fatigue and myalgia. He had done vigorous exercise up to 2 days before the test and also had a grade 4 CK elevation (12,310 U/L). He was thought to have isotretinoin-related rhabdomyolysis. His treatment was discontinued, and he was advised to hydrate and rest. Treatment was re-started after 2 weeks. With frequent laboratory monitoring and avoidance of vigorous physical activity, the patient completed the remaining course of isotretinoin without any laboratory abnormalities or symptoms.
Creatinine kinase abnormalities in the second and fourth months compared with baseline were not statistically significant. The patients with grade 3 or higher CK elevations, except for the case with rhabdomyolysis, had no clinical signs or other characteristic laboratory findings of rhabdomyolysis.
Hypercholesterolemia (LDL-C ≥130 mg/dL) occurred most frequently, with a maximum of 280 mg/dL in 1 patient (in the fourth month) and less than 250 mg/dL in all other patients. Hypercholesterolemia occurred in 183 (44.1%) patients in the second month and in 166 (40.0%) patients in the fourth month. However, baseline abnormalities also were frequent (86 [20.7%]), and hypercholesterolemia persisted in the second and fourth months in all of these patients.
It was observed that the patients with TG abnormalities increased continuously in the second (99 [23.9%]) and fourth (113 [27.2%]) months compared with baseline (49 [11.8%]). Grade 3 TG elevations were observed in 2.2% of patients (n=9; 5 patients in the second month, 4 patients in the fourth month) during treatment compared with baseline, and all patients had grade 1 or 2 hypertriglyceridemia at baseline. Of the patients with grade 3 TG elevation, 3 patients in the second month and 2 patients in the fourth month were obese at baseline. No grade 4 TG elevations were observed. Complications related to hyperlipidemia, such as pancreatitis, were observed in 1 patient. No patient terminated treatment because of lipid abnormalities. The treatment of our patients with major hypercholesterolemia and/or grade 3 hypertriglyceridemia was interrupted. The hyperlipidemia of these patients was controlled by a low-fat diet and a short-term dose reduction.
Relationship Between Total Cumulative Dose and Laboratory Parameters—The relationships between the total cumulative dose and changes up to the fourth month are presented in Table 3. As the total dose increased, the changes in TG and LDL-C levels significantly increased in the fourth month (both P=.001). However, the degree of these relationships was weak. No significant correlation was found between the periodic changes of other laboratory parameters and the total dose.

Comment
The parameters followed in our study show that TG levels tend to increase continuously from baseline during isotretinoin treatment, while ALT, AST, GGT, and LDL-C levels increase in the second month and decrease at 4 months. Although this same trend occurs with CK levels, the change was not statistically significant. The most common laboratory abnormality in our study was hyperlipidemia. Levels of LDL-C and TG were both found to be statistically elevated in the second and fourth months of treatment compared with baseline. Parthasarathy et al3 reported that obesity had an important role in the increase of lipid levels in patients using isotretinoin at baseline. In our study, 5 of 9 patients (55.6%) with grade 3 TG elevation were obese, which supports the theory that obesity plays an important role in the increase in lipid levels. Up-to-date laboratory follow-up of lipids suggests that there is no need to follow up serum lipids after the second month of treatment. Patients with risk factors for hyperlipidemia, such as abdominal obesity and familial hyperlipidemia, do not require further follow-up if there is no increase in serum lipids in the first month of treatment.1 The presence of grade 1 or 2 hypertriglyceridemia at baseline in all our patients with grade 3 TG elevation may suggest that periodic laboratory follow-up during isotretinoin treatment is necessary to detect patients with grade 3 and higher TG levels.
The lack of knowledge of other risk factors (eg, familial hyperlipidemia, insulin resistance) for hyperlipidemia in all patients at baseline may be a limitation of our study. Although hypercholesterolemia persisted in the follow-up of our patients with initial LDL-C abnormalities, hypercholesterolemia over 250 mg/dL was very rare (1 patient). Possible complications associated with serum lipid abnormalities are pancreatitis and metabolic syndrome.4 In our study, none of the patients with lipid abnormalities had any relevant clinical sequelae. The dose-dependent elevation of the changes in LDL-C and TG (Table 3) may be important to predict the significant elevation of lipids and the associated complications in patients with a high total cumulative dose target that may require a long treatment duration. However, considering the short follow-up periods in our patients, the absence of clinical sequelae may be misleading. There are differences in recommendations between the US and European guidelines for isotretinoin dosage. Although the US guidelines recommend a total cumulative dose target, the European guidelines recommend low-dose isotretinoin daily for at least 6 months instead of a cumulative dose.
Most liver transaminase abnormalities were detected in the second month. Abnormalities in GGT were seen in the second month and remained elevated at the next follow-up. However, clinically important grade 3 transaminase and GGT elevations were rare. It has been reported that GGT levels are more specific than transaminases in measuring hepatotoxicity.7 The fact that our patient with hepatotoxicity had a grade 3 GGT elevation in addition to grade 3 transaminase elevations supports that GGT elevation is more specific than transaminase levels in measuring hepatotoxicity. When these parameters were rechecked in our patients with grade 3 transaminase elevations, except in the case of hepatotoxicity, transaminase elevations did not recur, and GGT elevations did not accompany elevated transaminases, which suggested that transaminases may be elevated due to an extrahepatic origin (eg, hemolysis, exercise).
Rhabdomyolysis secondary to isotretinoin is rare in the literature of acne studies. In addition to clinical findings such as myalgia and fatigue, increased CK and abnormal liver enzymes, specifically AST, suggest the development of rhabdomyolysis.8 Our patient who developed rhabdomyolysis also had a recent history of vigorous exercise, grade 4 CK, and AST elevations. Other patients with isolated grade 3 CK elevations were informed about possible clinical signs of rhabdomyolysis, and they were able to complete their courses without any incident. According to a study by Landau et al,9 isotretinoin-associated hyperCKemia has been reported as benign. Similarly, our study found that isolated CK elevation during isotretinoin treatment may be misleading as a sign of rhabdomyolysis. Instead, CK monitoring may be more appropriate and cost-effective in patients with suspected clinical signs of rhabdomyolysis or in those with major elevations in transaminases, especially AST.
Conclusion
According to our study, hyperlipidemia was the most common complication in acne patients using isotretinoin. It may be appropriate to monitor the TG level at 2-month intervals in patients with grade 1 or 2 TG elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia. Periodic monitoring of LDL-C and TG levels may be appropriate, especially in patients who require a high total cumulative dose of isotretinoin. Clinically important liver enzyme abnormalities were rare in our study. Our findings support the idea that routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Additionally, periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 9, 2006. Accessed June 12, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Parthasarathy V, Shah N, Kirkorian AY. The utility of laboratory testing for pediatric patients undergoing isotretinoin treatment. Pediatr Dermatol. 2022;39:731-733.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7:171-174.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30:1261-1268.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017;23:13030/qt7rv7j80p.
- Raneses E, Schmidgal EC. Rhabdomyolysis caused by isotretinoin and exercise in an otherwise healthy female patient. Cureus. 2022;14:E25981.
- Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venereol. 2001;81:350-352.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 9, 2006. Accessed June 12, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Parthasarathy V, Shah N, Kirkorian AY. The utility of laboratory testing for pediatric patients undergoing isotretinoin treatment. Pediatr Dermatol. 2022;39:731-733.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7:171-174.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30:1261-1268.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017;23:13030/qt7rv7j80p.
- Raneses E, Schmidgal EC. Rhabdomyolysis caused by isotretinoin and exercise in an otherwise healthy female patient. Cureus. 2022;14:E25981.
- Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venereol. 2001;81:350-352.
Practice Points
- Hyperlipidemia was the most common complication in patients with acne using isotretinoin.
- It may be appropriate to monitor triglyceride levels at 2-month intervals in patients with grade 1 or 2 triglyceride elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia.
- Routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
The Growing Pains of Changing Times for Acne and Rosacea Pathophysiology: Where Will It All End Up?
It is interesting to observe the changes in dermatology that have occurred over the last 1 to 2 decades, especially as major advances in basic science research techniques have rapidly expanded our current understanding of the pathophysiology of many disease states—psoriasis, psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, hidradenitis suppurativa, and lichen planus.1 Although acne vulgaris (AV) and rosacea do not make front-page news quite as often as some of these other aforementioned disease states in the pathophysiology arena, advances still have been made in understanding the pathophysiology, albeit slower and often less popularized in dermatology publications and other forms of media.2-4
If one looks at our fundamental understanding of AV, most of the discussion over multiple decades has been driven by new treatments and in some cases new formulations and packaging differences with topical agents. Although we understood that adrenarche, a subsequent increase in androgen synthesis, and the ensuing sebocyte development with formation of sebum were prerequisites for the development of AV, the absence of therapeutic options to address these vital components of AV—especially US Food and Drug Administration (FDA)–approved therapies—resulted in limited discussion about this specific area.5 Rather, the discussion was dominated by the notable role of Propionibacterium acnes (now called Cutibacterium acnes) in AV pathophysiology, as we had therapies such as benzoyl peroxide and antibiotics that improved AV in direct correlation with reductions in P acnes.6 This was soon coupled with an advanced understanding of how to reduce follicular hyperkeratinization with the development of topical tretinoin, followed by 3 other topical retinoids over time—adapalene, tazarotene, and trifarotene. Over subsequent years, slowly emerging basic science developments and collective data reviews added to our understanding of AV and how different therapies appear to work, including the role of toll-like receptors, anti-inflammatory properties of tetracyclines, and inflammasomes.7-9 Without a doubt, the availability of oral isotretinoin revolutionized AV therapy, especially in patients with severe refractory disease, with advanced formulations allowing for optimization of sustained remission without the need for high dietary fat intake.10-12
Progress in the pathophysiology of rosacea has been slower to develop, with the first true discussion of specific clinical presentations published after the new millennium.13 This was followed by more advanced basic science and clinical research, which led to an improved ability to understand modes of action of various therapies and to correlate treatment selection with specific visible manifestations of rosacea, including incorporation of physical devices.14-16 A newer perspective on evaluation and management of rosacea moved away from the “buckets” of rosacea subtypes to phenotypes observed at the time of clinical presentation.17,18
I could elaborate on research advancements with both diseases, but the bottom line is that information, developments, and current perspectives change over time. Keeping up is a challenge for all who study and practice dermatology. It is human nature to revert to what we already believe and do, which sometimes remains valid and other times is quite outdated and truly replaced by more optimal approaches. With AV and rosacea, progress is much slower in availability of newer agents. With AV, new agents have included topical dapsone, oral sarecycline, and topical clascoterone, with the latter being the first FDA-approved topical agent to mitigate the effects of androgens and sebum in both males and females. For rosacea, the 2 most recent FDA-approved therapies are minocycline foam and microencapsulated benzoyl peroxide. All of these therapies are proven to be effective for the modes of action and skin manifestations they specifically manage. Over the upcoming year, we are hoping to see the first triple-combination topical product come to market for AV, which will prompt our minds to consider if and how 3 established agents can work together to further augment treatment efficacy with favorable tolerability and safety.
Where will all of this end up? It is hard to say. We still have several other areas to tackle with both disease states, including establishing a well-substantiated understanding of the pathophysiologic role of the microbiome, sorting out the role of antibiotic use due to concerns about bacterial resistance, integration of FDA-approved physical devices in AV, and data on both diet and optimized skin care, to name a few.19-21
There is a lot on the plate to accomplish and digest. I have remained very involved in this subject matter for almost 3 decades and am still feeling the growing pains. Fortunately, the satisfaction of being part of a process so important to the lives of millions of patients makes this worth every moment. Stay tuned—more valuable information is to come.
- Wu J, Fang Z, Liu T, et al. Maximizing the utility of transcriptomics data in inflammatory skin diseases. Front Immunol. 2021;12:761890.
- Firlej E, Kowalska W, Szymaszek K, et al. The role of skin immune system in acne. J Clin Med. 2022;11:1579.
- Mias C, Mengeaud V, Bessou-Touya S, et al. Recent advances in understanding inflammatory acne: deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. 2023;(37 suppl 2):3-11.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. doi:10.12688/f1000research.16537.1
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. doi:10.12688/f1000research.15659.1
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-198.
- Del Rosso JQ, Webster G, Weiss JS, et al. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14:14-21.
- Zhu W, Wang HL, Bu XL, et al. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. 2022;10:645.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 suppl):S3-S21.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, treatment, crossover study. J Am Acad Dermatol. 2013;69:762-767.
- Del Rosso JQ, Stein Gold L, Seagal J, et al. An open-label, phase IV study evaluating Lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12:13-18.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 suppl):1-8.
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182:1269-1276.
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344.
- Daou H, Paradiso M, Hennessy K. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Kayiran MA, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
It is interesting to observe the changes in dermatology that have occurred over the last 1 to 2 decades, especially as major advances in basic science research techniques have rapidly expanded our current understanding of the pathophysiology of many disease states—psoriasis, psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, hidradenitis suppurativa, and lichen planus.1 Although acne vulgaris (AV) and rosacea do not make front-page news quite as often as some of these other aforementioned disease states in the pathophysiology arena, advances still have been made in understanding the pathophysiology, albeit slower and often less popularized in dermatology publications and other forms of media.2-4
If one looks at our fundamental understanding of AV, most of the discussion over multiple decades has been driven by new treatments and in some cases new formulations and packaging differences with topical agents. Although we understood that adrenarche, a subsequent increase in androgen synthesis, and the ensuing sebocyte development with formation of sebum were prerequisites for the development of AV, the absence of therapeutic options to address these vital components of AV—especially US Food and Drug Administration (FDA)–approved therapies—resulted in limited discussion about this specific area.5 Rather, the discussion was dominated by the notable role of Propionibacterium acnes (now called Cutibacterium acnes) in AV pathophysiology, as we had therapies such as benzoyl peroxide and antibiotics that improved AV in direct correlation with reductions in P acnes.6 This was soon coupled with an advanced understanding of how to reduce follicular hyperkeratinization with the development of topical tretinoin, followed by 3 other topical retinoids over time—adapalene, tazarotene, and trifarotene. Over subsequent years, slowly emerging basic science developments and collective data reviews added to our understanding of AV and how different therapies appear to work, including the role of toll-like receptors, anti-inflammatory properties of tetracyclines, and inflammasomes.7-9 Without a doubt, the availability of oral isotretinoin revolutionized AV therapy, especially in patients with severe refractory disease, with advanced formulations allowing for optimization of sustained remission without the need for high dietary fat intake.10-12
Progress in the pathophysiology of rosacea has been slower to develop, with the first true discussion of specific clinical presentations published after the new millennium.13 This was followed by more advanced basic science and clinical research, which led to an improved ability to understand modes of action of various therapies and to correlate treatment selection with specific visible manifestations of rosacea, including incorporation of physical devices.14-16 A newer perspective on evaluation and management of rosacea moved away from the “buckets” of rosacea subtypes to phenotypes observed at the time of clinical presentation.17,18
I could elaborate on research advancements with both diseases, but the bottom line is that information, developments, and current perspectives change over time. Keeping up is a challenge for all who study and practice dermatology. It is human nature to revert to what we already believe and do, which sometimes remains valid and other times is quite outdated and truly replaced by more optimal approaches. With AV and rosacea, progress is much slower in availability of newer agents. With AV, new agents have included topical dapsone, oral sarecycline, and topical clascoterone, with the latter being the first FDA-approved topical agent to mitigate the effects of androgens and sebum in both males and females. For rosacea, the 2 most recent FDA-approved therapies are minocycline foam and microencapsulated benzoyl peroxide. All of these therapies are proven to be effective for the modes of action and skin manifestations they specifically manage. Over the upcoming year, we are hoping to see the first triple-combination topical product come to market for AV, which will prompt our minds to consider if and how 3 established agents can work together to further augment treatment efficacy with favorable tolerability and safety.
Where will all of this end up? It is hard to say. We still have several other areas to tackle with both disease states, including establishing a well-substantiated understanding of the pathophysiologic role of the microbiome, sorting out the role of antibiotic use due to concerns about bacterial resistance, integration of FDA-approved physical devices in AV, and data on both diet and optimized skin care, to name a few.19-21
There is a lot on the plate to accomplish and digest. I have remained very involved in this subject matter for almost 3 decades and am still feeling the growing pains. Fortunately, the satisfaction of being part of a process so important to the lives of millions of patients makes this worth every moment. Stay tuned—more valuable information is to come.
It is interesting to observe the changes in dermatology that have occurred over the last 1 to 2 decades, especially as major advances in basic science research techniques have rapidly expanded our current understanding of the pathophysiology of many disease states—psoriasis, psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, hidradenitis suppurativa, and lichen planus.1 Although acne vulgaris (AV) and rosacea do not make front-page news quite as often as some of these other aforementioned disease states in the pathophysiology arena, advances still have been made in understanding the pathophysiology, albeit slower and often less popularized in dermatology publications and other forms of media.2-4
If one looks at our fundamental understanding of AV, most of the discussion over multiple decades has been driven by new treatments and in some cases new formulations and packaging differences with topical agents. Although we understood that adrenarche, a subsequent increase in androgen synthesis, and the ensuing sebocyte development with formation of sebum were prerequisites for the development of AV, the absence of therapeutic options to address these vital components of AV—especially US Food and Drug Administration (FDA)–approved therapies—resulted in limited discussion about this specific area.5 Rather, the discussion was dominated by the notable role of Propionibacterium acnes (now called Cutibacterium acnes) in AV pathophysiology, as we had therapies such as benzoyl peroxide and antibiotics that improved AV in direct correlation with reductions in P acnes.6 This was soon coupled with an advanced understanding of how to reduce follicular hyperkeratinization with the development of topical tretinoin, followed by 3 other topical retinoids over time—adapalene, tazarotene, and trifarotene. Over subsequent years, slowly emerging basic science developments and collective data reviews added to our understanding of AV and how different therapies appear to work, including the role of toll-like receptors, anti-inflammatory properties of tetracyclines, and inflammasomes.7-9 Without a doubt, the availability of oral isotretinoin revolutionized AV therapy, especially in patients with severe refractory disease, with advanced formulations allowing for optimization of sustained remission without the need for high dietary fat intake.10-12
Progress in the pathophysiology of rosacea has been slower to develop, with the first true discussion of specific clinical presentations published after the new millennium.13 This was followed by more advanced basic science and clinical research, which led to an improved ability to understand modes of action of various therapies and to correlate treatment selection with specific visible manifestations of rosacea, including incorporation of physical devices.14-16 A newer perspective on evaluation and management of rosacea moved away from the “buckets” of rosacea subtypes to phenotypes observed at the time of clinical presentation.17,18
I could elaborate on research advancements with both diseases, but the bottom line is that information, developments, and current perspectives change over time. Keeping up is a challenge for all who study and practice dermatology. It is human nature to revert to what we already believe and do, which sometimes remains valid and other times is quite outdated and truly replaced by more optimal approaches. With AV and rosacea, progress is much slower in availability of newer agents. With AV, new agents have included topical dapsone, oral sarecycline, and topical clascoterone, with the latter being the first FDA-approved topical agent to mitigate the effects of androgens and sebum in both males and females. For rosacea, the 2 most recent FDA-approved therapies are minocycline foam and microencapsulated benzoyl peroxide. All of these therapies are proven to be effective for the modes of action and skin manifestations they specifically manage. Over the upcoming year, we are hoping to see the first triple-combination topical product come to market for AV, which will prompt our minds to consider if and how 3 established agents can work together to further augment treatment efficacy with favorable tolerability and safety.
Where will all of this end up? It is hard to say. We still have several other areas to tackle with both disease states, including establishing a well-substantiated understanding of the pathophysiologic role of the microbiome, sorting out the role of antibiotic use due to concerns about bacterial resistance, integration of FDA-approved physical devices in AV, and data on both diet and optimized skin care, to name a few.19-21
There is a lot on the plate to accomplish and digest. I have remained very involved in this subject matter for almost 3 decades and am still feeling the growing pains. Fortunately, the satisfaction of being part of a process so important to the lives of millions of patients makes this worth every moment. Stay tuned—more valuable information is to come.
- Wu J, Fang Z, Liu T, et al. Maximizing the utility of transcriptomics data in inflammatory skin diseases. Front Immunol. 2021;12:761890.
- Firlej E, Kowalska W, Szymaszek K, et al. The role of skin immune system in acne. J Clin Med. 2022;11:1579.
- Mias C, Mengeaud V, Bessou-Touya S, et al. Recent advances in understanding inflammatory acne: deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. 2023;(37 suppl 2):3-11.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. doi:10.12688/f1000research.16537.1
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. doi:10.12688/f1000research.15659.1
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-198.
- Del Rosso JQ, Webster G, Weiss JS, et al. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14:14-21.
- Zhu W, Wang HL, Bu XL, et al. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. 2022;10:645.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 suppl):S3-S21.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, treatment, crossover study. J Am Acad Dermatol. 2013;69:762-767.
- Del Rosso JQ, Stein Gold L, Seagal J, et al. An open-label, phase IV study evaluating Lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12:13-18.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 suppl):1-8.
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182:1269-1276.
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344.
- Daou H, Paradiso M, Hennessy K. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Kayiran MA, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Wu J, Fang Z, Liu T, et al. Maximizing the utility of transcriptomics data in inflammatory skin diseases. Front Immunol. 2021;12:761890.
- Firlej E, Kowalska W, Szymaszek K, et al. The role of skin immune system in acne. J Clin Med. 2022;11:1579.
- Mias C, Mengeaud V, Bessou-Touya S, et al. Recent advances in understanding inflammatory acne: deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. 2023;(37 suppl 2):3-11.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. doi:10.12688/f1000research.16537.1
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. doi:10.12688/f1000research.15659.1
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-198.
- Del Rosso JQ, Webster G, Weiss JS, et al. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14:14-21.
- Zhu W, Wang HL, Bu XL, et al. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. 2022;10:645.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 suppl):S3-S21.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, treatment, crossover study. J Am Acad Dermatol. 2013;69:762-767.
- Del Rosso JQ, Stein Gold L, Seagal J, et al. An open-label, phase IV study evaluating Lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12:13-18.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 suppl):1-8.
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182:1269-1276.
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344.
- Daou H, Paradiso M, Hennessy K. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Kayiran MA, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
Florida GI gets candid about imposter syndrome, insurers, starting a GI fellowship
Her first faculty position as assistant program director for the gastroenterology fellowship program at the University of Iowa offered some inspiration. “I loved teaching and working with trainees and knew I always wanted to remain in this realm,” Dr. Naveed said.
When she moved to Orlando to join AdventHealth, she noticed there was no gastroenterology training program. “I was strictly in private practice. Though I love working with patients, I constantly felt like something was missing. When the opportunity to start a fellowship program came, I was highly motivated to bring it to fruition.”
The AdventHealth fellowship is almost done with its inaugural year.
“Starting a fellowship at a new institution is a very challenging yet incredibly rewarding experience,” she said. In this Q&A, she discusses her strategies for dealing with insurance companies and imposter syndrome, and why she looks to her father as her role model in medicine.
Q: Why did you choose GI?
Dr. Naveed: Gastroenterology is a rapidly evolving field which makes it incredibly fascinating. The initial draw was that I was always excited to learn about GI physiology and disease. I also was fortunate to train with amazing gastroenterologists during residency. I had great examples of strong and successful female GIs to look up to. Lastly, for the most part, gastroenterologists are all fairly laid back and have an interesting sense of humor.
Q: What gives you the most joy in your day-to-day practice?
Dr. Naveed: I love learning and teaching. As a program director, I am directly involved with fellows, residents, and students, but there are always additional enrichment opportunities beyond these interactions. I value teaching clinic medical assistants so they feel more confident and empowered in their work. I also try to educate my nurse practitioners. The best compliment at the end of a long day is that they learned something valuable.
Q: How do you stay current with advances in your field?
Dr. Naveed: Between my role as a physician and as an educator, I owe it to my patients and trainees to stay current with advances in the field. But of course, this is challenging, and at times it feels like there are not enough hours in the day. While reading journal articles and attending conferences are great ways to refresh one’s knowledge, the winner for me has been social media (specifically Twitter). It’s easy to find a “Tweetorial” on almost any topic. There are some excellent initiatives on Twitter such as Monday Night IBD, ACG Evidence-Based GI Doc, Scoping Sundays, and GI Journal Club where important articles, new treatment options, and challenging cases are discussed. Of course, I also learn a lot from my fellows and residents.
Q: What fears did you have to push past to get to where you are in your career?
Dr. Naveed: Pushing past imposter syndrome, which is a feeling of self-doubt despite education, experience, and accomplishments. It is something many of us deal with. I’ve had to retire the notion that I am not experienced enough to achieve a particular career goal.
Q: What habits have you established that have benefited your career most?
Dr. Naveed: It’s a challenge to not immediately say “yes” to every opportunity or project. It’s also difficult to learn to delegate. I am lucky to have a great team, and I have learned that delegating certain tasks or projects helps everyone grow. Also, if I say no to an opportunity, I still try to suggest another colleague or mentee who may be interested and/or a good fit.
Q: Describe your biggest practice-related challenge and what you are doing to address it.
Dr. Naveed: Pushback from insurance companies to approve medications or interventions is incredibly frustrating for myself and the patient. It is also incredibly time consuming and requires significant clinical bandwidth that could otherwise be used in other capacities. While not a solution, I at least try to make sure the patient is kept updated and understands causes of delay, and more importantly, what we are doing to address the issue. I have realized that it’s always preferable to empower the patient, rather than leave them uninformed, which can foster frustration and dissatisfaction.
Q: What teacher or mentor had the greatest impact on you?
Dr. Naveed: I have been blessed with many mentors at different points in my medical career that have greatly impacted and shaped my journey. During my fellowship at University of Texas Southwestern (UTSW), Nisa Kubiliun, MD, was not only a mentor, but also an incredible sponsor. She saw potential in me and encouraged involvement in activities critical for career advancement. Arjmand Mufti, MD, the former program director of the UTSW GI fellowship, is still always just a call away when I need advice regarding my GI fellowship program at AdventHealth. I also have mentors and sponsors within my own institution who invest time and energy into my success.
Q: Outside of teachers and mentors, who or what has had the strongest influence in your life?
Dr. Naveed: My father, who is also a physician, has had a profound influence on my personal and professional development. His own medical journey has been incredibly unique. He has practiced medicine internationally, trained and worked in a traditional academic setting, established a very successful private practice, and now has transitioned to running a hospital-based practice. He has seen it all (and he’s also a brilliant physician), and he is always able to talk me through any situation.
Q: What principles guide you?
Dr. Naveed: Treating my patients how I would want a physician to treat my family is central to my practice. Also, I try to approach any successes with gratitude, and likewise, be patient with inevitable failures. It can be challenging, but I try to find the lesson in every failed venture.
Q: What would you do differently if you had a chance?
Dr. Naveed: I have always had an interest in international medical missions but have yet to participate in one. I have previously passed on such opportunities, thinking it was not the right time, but in hindsight I wish I had taken the leap. I still hope to eventually accomplish this goal.
Q: Describe a scene of your vision for the future.
Dr. Naveed: I hope that our GI fellowship continues to flourish and attract exceptional faculty and candidates. I want to remain involved in graduate medical education, but I hope to continue to challenge myself and advance within this domain. Most importantly, I hope I can continue to balance my career aspirations with my personal goals. I want to continue to be present for my family and kids.
Q: Describe how you would spend a free Saturday afternoon.
Dr. Naveed: You can usually find me at the local farmer’s market with my husband and kids. Afterwards, we’re definitely going to get Chick-fil-A followed by ice cream.
Lightning round
If you weren’t a gastroenterologist, what would you be?
International event planner.
How many cups of coffee do you drink per day?
Usually three.
Favorite breakfast?
Eggs, corned beef hash, toast.
Texting or talking?
Texting always unless it’s Mom or Dad. They always get a call.
Place you most want to travel?
Japan.
Follow Dr. Naveed on Twitter at @MN_GIMD
Her first faculty position as assistant program director for the gastroenterology fellowship program at the University of Iowa offered some inspiration. “I loved teaching and working with trainees and knew I always wanted to remain in this realm,” Dr. Naveed said.
When she moved to Orlando to join AdventHealth, she noticed there was no gastroenterology training program. “I was strictly in private practice. Though I love working with patients, I constantly felt like something was missing. When the opportunity to start a fellowship program came, I was highly motivated to bring it to fruition.”
The AdventHealth fellowship is almost done with its inaugural year.
“Starting a fellowship at a new institution is a very challenging yet incredibly rewarding experience,” she said. In this Q&A, she discusses her strategies for dealing with insurance companies and imposter syndrome, and why she looks to her father as her role model in medicine.
Q: Why did you choose GI?
Dr. Naveed: Gastroenterology is a rapidly evolving field which makes it incredibly fascinating. The initial draw was that I was always excited to learn about GI physiology and disease. I also was fortunate to train with amazing gastroenterologists during residency. I had great examples of strong and successful female GIs to look up to. Lastly, for the most part, gastroenterologists are all fairly laid back and have an interesting sense of humor.
Q: What gives you the most joy in your day-to-day practice?
Dr. Naveed: I love learning and teaching. As a program director, I am directly involved with fellows, residents, and students, but there are always additional enrichment opportunities beyond these interactions. I value teaching clinic medical assistants so they feel more confident and empowered in their work. I also try to educate my nurse practitioners. The best compliment at the end of a long day is that they learned something valuable.
Q: How do you stay current with advances in your field?
Dr. Naveed: Between my role as a physician and as an educator, I owe it to my patients and trainees to stay current with advances in the field. But of course, this is challenging, and at times it feels like there are not enough hours in the day. While reading journal articles and attending conferences are great ways to refresh one’s knowledge, the winner for me has been social media (specifically Twitter). It’s easy to find a “Tweetorial” on almost any topic. There are some excellent initiatives on Twitter such as Monday Night IBD, ACG Evidence-Based GI Doc, Scoping Sundays, and GI Journal Club where important articles, new treatment options, and challenging cases are discussed. Of course, I also learn a lot from my fellows and residents.
Q: What fears did you have to push past to get to where you are in your career?
Dr. Naveed: Pushing past imposter syndrome, which is a feeling of self-doubt despite education, experience, and accomplishments. It is something many of us deal with. I’ve had to retire the notion that I am not experienced enough to achieve a particular career goal.
Q: What habits have you established that have benefited your career most?
Dr. Naveed: It’s a challenge to not immediately say “yes” to every opportunity or project. It’s also difficult to learn to delegate. I am lucky to have a great team, and I have learned that delegating certain tasks or projects helps everyone grow. Also, if I say no to an opportunity, I still try to suggest another colleague or mentee who may be interested and/or a good fit.
Q: Describe your biggest practice-related challenge and what you are doing to address it.
Dr. Naveed: Pushback from insurance companies to approve medications or interventions is incredibly frustrating for myself and the patient. It is also incredibly time consuming and requires significant clinical bandwidth that could otherwise be used in other capacities. While not a solution, I at least try to make sure the patient is kept updated and understands causes of delay, and more importantly, what we are doing to address the issue. I have realized that it’s always preferable to empower the patient, rather than leave them uninformed, which can foster frustration and dissatisfaction.
Q: What teacher or mentor had the greatest impact on you?
Dr. Naveed: I have been blessed with many mentors at different points in my medical career that have greatly impacted and shaped my journey. During my fellowship at University of Texas Southwestern (UTSW), Nisa Kubiliun, MD, was not only a mentor, but also an incredible sponsor. She saw potential in me and encouraged involvement in activities critical for career advancement. Arjmand Mufti, MD, the former program director of the UTSW GI fellowship, is still always just a call away when I need advice regarding my GI fellowship program at AdventHealth. I also have mentors and sponsors within my own institution who invest time and energy into my success.
Q: Outside of teachers and mentors, who or what has had the strongest influence in your life?
Dr. Naveed: My father, who is also a physician, has had a profound influence on my personal and professional development. His own medical journey has been incredibly unique. He has practiced medicine internationally, trained and worked in a traditional academic setting, established a very successful private practice, and now has transitioned to running a hospital-based practice. He has seen it all (and he’s also a brilliant physician), and he is always able to talk me through any situation.
Q: What principles guide you?
Dr. Naveed: Treating my patients how I would want a physician to treat my family is central to my practice. Also, I try to approach any successes with gratitude, and likewise, be patient with inevitable failures. It can be challenging, but I try to find the lesson in every failed venture.
Q: What would you do differently if you had a chance?
Dr. Naveed: I have always had an interest in international medical missions but have yet to participate in one. I have previously passed on such opportunities, thinking it was not the right time, but in hindsight I wish I had taken the leap. I still hope to eventually accomplish this goal.
Q: Describe a scene of your vision for the future.
Dr. Naveed: I hope that our GI fellowship continues to flourish and attract exceptional faculty and candidates. I want to remain involved in graduate medical education, but I hope to continue to challenge myself and advance within this domain. Most importantly, I hope I can continue to balance my career aspirations with my personal goals. I want to continue to be present for my family and kids.
Q: Describe how you would spend a free Saturday afternoon.
Dr. Naveed: You can usually find me at the local farmer’s market with my husband and kids. Afterwards, we’re definitely going to get Chick-fil-A followed by ice cream.
Lightning round
If you weren’t a gastroenterologist, what would you be?
International event planner.
How many cups of coffee do you drink per day?
Usually three.
Favorite breakfast?
Eggs, corned beef hash, toast.
Texting or talking?
Texting always unless it’s Mom or Dad. They always get a call.
Place you most want to travel?
Japan.
Follow Dr. Naveed on Twitter at @MN_GIMD
Her first faculty position as assistant program director for the gastroenterology fellowship program at the University of Iowa offered some inspiration. “I loved teaching and working with trainees and knew I always wanted to remain in this realm,” Dr. Naveed said.
When she moved to Orlando to join AdventHealth, she noticed there was no gastroenterology training program. “I was strictly in private practice. Though I love working with patients, I constantly felt like something was missing. When the opportunity to start a fellowship program came, I was highly motivated to bring it to fruition.”
The AdventHealth fellowship is almost done with its inaugural year.
“Starting a fellowship at a new institution is a very challenging yet incredibly rewarding experience,” she said. In this Q&A, she discusses her strategies for dealing with insurance companies and imposter syndrome, and why she looks to her father as her role model in medicine.
Q: Why did you choose GI?
Dr. Naveed: Gastroenterology is a rapidly evolving field which makes it incredibly fascinating. The initial draw was that I was always excited to learn about GI physiology and disease. I also was fortunate to train with amazing gastroenterologists during residency. I had great examples of strong and successful female GIs to look up to. Lastly, for the most part, gastroenterologists are all fairly laid back and have an interesting sense of humor.
Q: What gives you the most joy in your day-to-day practice?
Dr. Naveed: I love learning and teaching. As a program director, I am directly involved with fellows, residents, and students, but there are always additional enrichment opportunities beyond these interactions. I value teaching clinic medical assistants so they feel more confident and empowered in their work. I also try to educate my nurse practitioners. The best compliment at the end of a long day is that they learned something valuable.
Q: How do you stay current with advances in your field?
Dr. Naveed: Between my role as a physician and as an educator, I owe it to my patients and trainees to stay current with advances in the field. But of course, this is challenging, and at times it feels like there are not enough hours in the day. While reading journal articles and attending conferences are great ways to refresh one’s knowledge, the winner for me has been social media (specifically Twitter). It’s easy to find a “Tweetorial” on almost any topic. There are some excellent initiatives on Twitter such as Monday Night IBD, ACG Evidence-Based GI Doc, Scoping Sundays, and GI Journal Club where important articles, new treatment options, and challenging cases are discussed. Of course, I also learn a lot from my fellows and residents.
Q: What fears did you have to push past to get to where you are in your career?
Dr. Naveed: Pushing past imposter syndrome, which is a feeling of self-doubt despite education, experience, and accomplishments. It is something many of us deal with. I’ve had to retire the notion that I am not experienced enough to achieve a particular career goal.
Q: What habits have you established that have benefited your career most?
Dr. Naveed: It’s a challenge to not immediately say “yes” to every opportunity or project. It’s also difficult to learn to delegate. I am lucky to have a great team, and I have learned that delegating certain tasks or projects helps everyone grow. Also, if I say no to an opportunity, I still try to suggest another colleague or mentee who may be interested and/or a good fit.
Q: Describe your biggest practice-related challenge and what you are doing to address it.
Dr. Naveed: Pushback from insurance companies to approve medications or interventions is incredibly frustrating for myself and the patient. It is also incredibly time consuming and requires significant clinical bandwidth that could otherwise be used in other capacities. While not a solution, I at least try to make sure the patient is kept updated and understands causes of delay, and more importantly, what we are doing to address the issue. I have realized that it’s always preferable to empower the patient, rather than leave them uninformed, which can foster frustration and dissatisfaction.
Q: What teacher or mentor had the greatest impact on you?
Dr. Naveed: I have been blessed with many mentors at different points in my medical career that have greatly impacted and shaped my journey. During my fellowship at University of Texas Southwestern (UTSW), Nisa Kubiliun, MD, was not only a mentor, but also an incredible sponsor. She saw potential in me and encouraged involvement in activities critical for career advancement. Arjmand Mufti, MD, the former program director of the UTSW GI fellowship, is still always just a call away when I need advice regarding my GI fellowship program at AdventHealth. I also have mentors and sponsors within my own institution who invest time and energy into my success.
Q: Outside of teachers and mentors, who or what has had the strongest influence in your life?
Dr. Naveed: My father, who is also a physician, has had a profound influence on my personal and professional development. His own medical journey has been incredibly unique. He has practiced medicine internationally, trained and worked in a traditional academic setting, established a very successful private practice, and now has transitioned to running a hospital-based practice. He has seen it all (and he’s also a brilliant physician), and he is always able to talk me through any situation.
Q: What principles guide you?
Dr. Naveed: Treating my patients how I would want a physician to treat my family is central to my practice. Also, I try to approach any successes with gratitude, and likewise, be patient with inevitable failures. It can be challenging, but I try to find the lesson in every failed venture.
Q: What would you do differently if you had a chance?
Dr. Naveed: I have always had an interest in international medical missions but have yet to participate in one. I have previously passed on such opportunities, thinking it was not the right time, but in hindsight I wish I had taken the leap. I still hope to eventually accomplish this goal.
Q: Describe a scene of your vision for the future.
Dr. Naveed: I hope that our GI fellowship continues to flourish and attract exceptional faculty and candidates. I want to remain involved in graduate medical education, but I hope to continue to challenge myself and advance within this domain. Most importantly, I hope I can continue to balance my career aspirations with my personal goals. I want to continue to be present for my family and kids.
Q: Describe how you would spend a free Saturday afternoon.
Dr. Naveed: You can usually find me at the local farmer’s market with my husband and kids. Afterwards, we’re definitely going to get Chick-fil-A followed by ice cream.
Lightning round
If you weren’t a gastroenterologist, what would you be?
International event planner.
How many cups of coffee do you drink per day?
Usually three.
Favorite breakfast?
Eggs, corned beef hash, toast.
Texting or talking?
Texting always unless it’s Mom or Dad. They always get a call.
Place you most want to travel?
Japan.
Follow Dr. Naveed on Twitter at @MN_GIMD
Breakthroughs and challenges in hepatology
CHICAGO – The most notable is the progress from discovery of the hepatitis C virus (previously non-A, non-B hepatitis) in 1989 to a near 100% cure with 8-12 weeks of oral medications in just 30 years, culminating in the The Nobel Prize in Physiology or Medicine in 2020.
This remarkable feat is matched by the progress from discovery of the hepatitis B virus (initially coined Australia antigen) and a 1976 Nobel Prize to an effective vaccine in 1981, to a list of antiviral drugs approved beginning in 1992 (with currently available nucleos(t)ide analogues associated with near zero risk of antiviral drug resistance even when used as monotherapy), to demonstration that both hepatitis B vaccine and antivirals can prevent liver cancer. Other major breakthroughs include blood-based and image-based noninvasive assessment of liver fibrosis obviating the need for liver biopsy to stage liver disease, and multiple systemic therapies for advanced liver cancer.
However, there remain many challenges. While we have the tools to eliminate hepatitis B and hepatitis C, resources and coordinated efforts are needed to realize this feasible goal. Development of a vaccine for hepatitis C and a cure for hepatitis B will facilitate this goal.
The biggest present-day challenges in hepatology are the epidemics of nonalcoholic fatty liver disease (NAFLD) and alcohol-associated liver disease (ALD), particularly since both are increasingly impacting young people, socially disadvantaged populations, and those with mental health problems. Social isolation and mental and financial stressors associated with the COVID pandemic have aggravated both NAFLD and ALD, which have now become the leading indications for liver transplantation. Multi-pronged approaches, including public policies and education, destigmatization, easy access to mental health care, provider training, and provision of multi-disciplinary care, are needed to curb this tsunami. NAFLD affects more than 30% of the global population, and screening with simple biomarker(s) followed by liver stiffness measurement using elastography has been proposed to identify patients with or at high risk of advanced fibrosis or cirrhosis for specialist care. NAFLD is a heterogeneous disease and the requirement for paired liver biopsies to demonstrate benefit have made drug development challenging. Better phenotyping of disease and surrogates for outcomes are needed. Liver cancer is one of the top cancer killers globally, but it is also a preventable cancer making prevention and early treatment of liver disease a top public health priority.
Dr. Lok is director of clinical hepatology and assistant dean for clinical research, University of Michigan Medical School, Ann Arbor. She disclosed research grants with AstraZeneca, Kowa, and Target. She has served as a consultant/adviser to Abbott, Chroma, GlaxoSmithKline, Roche, Virion, and Novo Nordisk. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2023. DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – The most notable is the progress from discovery of the hepatitis C virus (previously non-A, non-B hepatitis) in 1989 to a near 100% cure with 8-12 weeks of oral medications in just 30 years, culminating in the The Nobel Prize in Physiology or Medicine in 2020.
This remarkable feat is matched by the progress from discovery of the hepatitis B virus (initially coined Australia antigen) and a 1976 Nobel Prize to an effective vaccine in 1981, to a list of antiviral drugs approved beginning in 1992 (with currently available nucleos(t)ide analogues associated with near zero risk of antiviral drug resistance even when used as monotherapy), to demonstration that both hepatitis B vaccine and antivirals can prevent liver cancer. Other major breakthroughs include blood-based and image-based noninvasive assessment of liver fibrosis obviating the need for liver biopsy to stage liver disease, and multiple systemic therapies for advanced liver cancer.
However, there remain many challenges. While we have the tools to eliminate hepatitis B and hepatitis C, resources and coordinated efforts are needed to realize this feasible goal. Development of a vaccine for hepatitis C and a cure for hepatitis B will facilitate this goal.
The biggest present-day challenges in hepatology are the epidemics of nonalcoholic fatty liver disease (NAFLD) and alcohol-associated liver disease (ALD), particularly since both are increasingly impacting young people, socially disadvantaged populations, and those with mental health problems. Social isolation and mental and financial stressors associated with the COVID pandemic have aggravated both NAFLD and ALD, which have now become the leading indications for liver transplantation. Multi-pronged approaches, including public policies and education, destigmatization, easy access to mental health care, provider training, and provision of multi-disciplinary care, are needed to curb this tsunami. NAFLD affects more than 30% of the global population, and screening with simple biomarker(s) followed by liver stiffness measurement using elastography has been proposed to identify patients with or at high risk of advanced fibrosis or cirrhosis for specialist care. NAFLD is a heterogeneous disease and the requirement for paired liver biopsies to demonstrate benefit have made drug development challenging. Better phenotyping of disease and surrogates for outcomes are needed. Liver cancer is one of the top cancer killers globally, but it is also a preventable cancer making prevention and early treatment of liver disease a top public health priority.
Dr. Lok is director of clinical hepatology and assistant dean for clinical research, University of Michigan Medical School, Ann Arbor. She disclosed research grants with AstraZeneca, Kowa, and Target. She has served as a consultant/adviser to Abbott, Chroma, GlaxoSmithKline, Roche, Virion, and Novo Nordisk. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2023. DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – The most notable is the progress from discovery of the hepatitis C virus (previously non-A, non-B hepatitis) in 1989 to a near 100% cure with 8-12 weeks of oral medications in just 30 years, culminating in the The Nobel Prize in Physiology or Medicine in 2020.
This remarkable feat is matched by the progress from discovery of the hepatitis B virus (initially coined Australia antigen) and a 1976 Nobel Prize to an effective vaccine in 1981, to a list of antiviral drugs approved beginning in 1992 (with currently available nucleos(t)ide analogues associated with near zero risk of antiviral drug resistance even when used as monotherapy), to demonstration that both hepatitis B vaccine and antivirals can prevent liver cancer. Other major breakthroughs include blood-based and image-based noninvasive assessment of liver fibrosis obviating the need for liver biopsy to stage liver disease, and multiple systemic therapies for advanced liver cancer.
However, there remain many challenges. While we have the tools to eliminate hepatitis B and hepatitis C, resources and coordinated efforts are needed to realize this feasible goal. Development of a vaccine for hepatitis C and a cure for hepatitis B will facilitate this goal.
The biggest present-day challenges in hepatology are the epidemics of nonalcoholic fatty liver disease (NAFLD) and alcohol-associated liver disease (ALD), particularly since both are increasingly impacting young people, socially disadvantaged populations, and those with mental health problems. Social isolation and mental and financial stressors associated with the COVID pandemic have aggravated both NAFLD and ALD, which have now become the leading indications for liver transplantation. Multi-pronged approaches, including public policies and education, destigmatization, easy access to mental health care, provider training, and provision of multi-disciplinary care, are needed to curb this tsunami. NAFLD affects more than 30% of the global population, and screening with simple biomarker(s) followed by liver stiffness measurement using elastography has been proposed to identify patients with or at high risk of advanced fibrosis or cirrhosis for specialist care. NAFLD is a heterogeneous disease and the requirement for paired liver biopsies to demonstrate benefit have made drug development challenging. Better phenotyping of disease and surrogates for outcomes are needed. Liver cancer is one of the top cancer killers globally, but it is also a preventable cancer making prevention and early treatment of liver disease a top public health priority.
Dr. Lok is director of clinical hepatology and assistant dean for clinical research, University of Michigan Medical School, Ann Arbor. She disclosed research grants with AstraZeneca, Kowa, and Target. She has served as a consultant/adviser to Abbott, Chroma, GlaxoSmithKline, Roche, Virion, and Novo Nordisk. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2023. DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
AT DDW 2023
Disconnecting to reconnect
I recently returned from a bucket list trip rafting the full length of the Grand Canyon via the Colorado River. It was a spectacular trip, filled with thrilling rapids, awe-inspiring hikes through slot canyons, and swimming in the turquoise waters of Havasu Falls.
For those of you who are fortunate to have experienced a similar adventure, I think you’ll agree one of the best things about the trip (aside from the breathtaking scenery) was the ability to completely unplug. Not only did I travel without my trusty laptop, but cell service was nonexistent. The effect of this forced digital detox was magical. By mentally disconnecting from work without the constant ping of my email and EHR inbox, our group had deeper conversations and formed genuine connections without the distractions of technology. In the frenetically paced world of modern health care where clinicians are reachable wherever they are in the world (even on vacation) as the boundaries between work and life blur, there are increasingly fewer times like this when we can fully disconnect. Yet, periodically disconnecting from work is critical, particularly for the clinician community, which is grappling with increasing levels of burnout and its consequences. As you embark on your well-deserved summer vacations, I hope you have an opportunity to set aside your devices to reconnect more fully with your family and friends, but also yourself.
In this month’s issue of GI&Hepatology News, we update you on AGA’s ongoing advocacy efforts to challenge UnitedHealthcare’s plans to impose increased administrative burdens on GI practices relating to routine GI procedures. We also highlight a landmark clinical trial in pediatric Crohn’s disease recently published in Gastroenterology. In our quarterly Perspectives column, Dr. Mariam Naveed and Dr. Petr Protiva outline important considerations regarding when to stop surveillance for colorectal neoplasia in elderly patients. Finally, our July Member Spotlight features gastroenterologist Dr. Russ Arjal, who shares his experiences launching Telebelly Health, an entirely virtual GI practice.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
I recently returned from a bucket list trip rafting the full length of the Grand Canyon via the Colorado River. It was a spectacular trip, filled with thrilling rapids, awe-inspiring hikes through slot canyons, and swimming in the turquoise waters of Havasu Falls.
For those of you who are fortunate to have experienced a similar adventure, I think you’ll agree one of the best things about the trip (aside from the breathtaking scenery) was the ability to completely unplug. Not only did I travel without my trusty laptop, but cell service was nonexistent. The effect of this forced digital detox was magical. By mentally disconnecting from work without the constant ping of my email and EHR inbox, our group had deeper conversations and formed genuine connections without the distractions of technology. In the frenetically paced world of modern health care where clinicians are reachable wherever they are in the world (even on vacation) as the boundaries between work and life blur, there are increasingly fewer times like this when we can fully disconnect. Yet, periodically disconnecting from work is critical, particularly for the clinician community, which is grappling with increasing levels of burnout and its consequences. As you embark on your well-deserved summer vacations, I hope you have an opportunity to set aside your devices to reconnect more fully with your family and friends, but also yourself.
In this month’s issue of GI&Hepatology News, we update you on AGA’s ongoing advocacy efforts to challenge UnitedHealthcare’s plans to impose increased administrative burdens on GI practices relating to routine GI procedures. We also highlight a landmark clinical trial in pediatric Crohn’s disease recently published in Gastroenterology. In our quarterly Perspectives column, Dr. Mariam Naveed and Dr. Petr Protiva outline important considerations regarding when to stop surveillance for colorectal neoplasia in elderly patients. Finally, our July Member Spotlight features gastroenterologist Dr. Russ Arjal, who shares his experiences launching Telebelly Health, an entirely virtual GI practice.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
I recently returned from a bucket list trip rafting the full length of the Grand Canyon via the Colorado River. It was a spectacular trip, filled with thrilling rapids, awe-inspiring hikes through slot canyons, and swimming in the turquoise waters of Havasu Falls.
For those of you who are fortunate to have experienced a similar adventure, I think you’ll agree one of the best things about the trip (aside from the breathtaking scenery) was the ability to completely unplug. Not only did I travel without my trusty laptop, but cell service was nonexistent. The effect of this forced digital detox was magical. By mentally disconnecting from work without the constant ping of my email and EHR inbox, our group had deeper conversations and formed genuine connections without the distractions of technology. In the frenetically paced world of modern health care where clinicians are reachable wherever they are in the world (even on vacation) as the boundaries between work and life blur, there are increasingly fewer times like this when we can fully disconnect. Yet, periodically disconnecting from work is critical, particularly for the clinician community, which is grappling with increasing levels of burnout and its consequences. As you embark on your well-deserved summer vacations, I hope you have an opportunity to set aside your devices to reconnect more fully with your family and friends, but also yourself.
In this month’s issue of GI&Hepatology News, we update you on AGA’s ongoing advocacy efforts to challenge UnitedHealthcare’s plans to impose increased administrative burdens on GI practices relating to routine GI procedures. We also highlight a landmark clinical trial in pediatric Crohn’s disease recently published in Gastroenterology. In our quarterly Perspectives column, Dr. Mariam Naveed and Dr. Petr Protiva outline important considerations regarding when to stop surveillance for colorectal neoplasia in elderly patients. Finally, our July Member Spotlight features gastroenterologist Dr. Russ Arjal, who shares his experiences launching Telebelly Health, an entirely virtual GI practice.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Surveillance colonoscopy: When and how to stop
Dear colleagues,
Colonoscopy is the bread and butter of endoscopy. Multidisciplinary updates continue to support screening colonoscopy in reducing the risk of developing colorectal cancer. But there has been debate about the best uses of resources, especially with increased recognition of colorectal cancer (CRC) in younger patients, and successive guidelines lengthening the intervals for most surveillance colonoscopy.
In particular, when do we feel comfortable recommending cessation of surveillance colonoscopy especially in those who are 75-85 years old? Routine colonoscopy remains a very low-risk procedure even in older patients with multiple comorbidities.
In this issue of Perspectives, Dr. Petr Protiva and Dr. Mariam Naveed address this issue and provide differing perspectives on approaching surveillance colonoscopy in the elderly.
We welcome your thoughts on this issue on Twitter at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Striking a balance: Deciding the optimal age to cease surveillance for colorectal neoplasia
BY PETR PROTIVA, MD, MPH
The appropriate age to stop surveillance for colorectal neoplasia remains uncertain. Screening for average-risk individuals is typically stopped at age 75, but personalized screening with shared decision-making may continue until age 85.1 Evidence suggests that any survival benefit of screening past age 86 would be outweighed by the harm of screening and/or natural mortality. Nevertheless, determining the optimal age for surveillance in those with a history of neoplasia still poses some challenges. The issue is confounded as many clinicians use the terms “screening” and “surveillance” interchangeably. It should be noted that screening implies the individual is at average risk, while surveillance refers to those at elevated risk because of a personal history of colonic adenomas or cancer
Comorbidities and life expectancy
Despite recent staggering setbacks and a drop in the average life expectancy in the United States, the proportion of individuals older than 65 years old has been steadily increasing to a currently estimated 58.9 million – 16.8% of the U.S. population – and is projected to increase in the future.2 However, the prevalence of comorbidities also increases with age, and these are crucial factors to weigh in the decision-making process. Severe comorbid conditions, such as chronic obstructive pulmonary disease, cirrhosis, chronic hepatitis, chronic renal failure, dementia, and congestive heart failure can limit a patient’s ability to undergo surveillance and diminish or negate its benefits. There are online tools that help clinicians estimate life expectancy and time to benefit (that is, time between the intervention and its benefit), such as the Lee or Schonberg index.3 Consensus on time to colorectal screening benefit is about 9-10 years, but may be much shorter for surveillance. Striking a balance between the potential benefits of continued surveillance and the risks and burdens imposed on older adults with limited life expectancy is essential for making well-informed decisions.
Age-related risk increase and risk of neoplasia in the surveillance population:
The absolute risk of developing colorectal cancer is dependent on age. In adults aged 45-49, it is 33.4/100,000, rising to 135.6 in those 70-74 years old; in persons aged 85 and older, it is 234.7/100,000.4 A significant challenge in determining the appropriate age to stop surveillance is the additional individual risk based on baseline polyp characteristics. It seems reasonable to treat low-risk adenomas similarly to screening and stop surveillance by 85 or 86 years old in most cases. The decision process is less clear in individuals with advanced lesions or a personal history of colon cancer. The prevalence of advanced adenomas on the baseline exam is about 15% and greater than 20% on the follow-up exam if the index adenoma(s) were at least 20 mm in diameter. For five or more adenomas at baseline, the prevalence of advanced lesions on follow-up exam is about 20%. On the other hand, a negative surveillance colonoscopy (that is, no polyps found) is associated with far fewer advanced lesions on follow-up.
Colonoscopy
The safety of colonoscopy in older adults should be considered. The colonoscopy procedure is generally very safe, but is associated with a higher risk of post-procedure complications after outpatient colonoscopy in patients 75 years and older. In addition, comorbidities such as anemia, cardiac arrhythmia, heart failure, hypertension, chronic kidney disease, liver disease, smoking history, and obesity are also risk factors. It should be noted that high-quality colonoscopy is key to the detection and full removal of neoplastic lesions and risk reduction. The inability to achieve adequate colon preparation for any reason or undertaking colonoscopy in patients at high risk for complications reduces its benefit.
Who should oversee surveillance, the primary care physician or gastroenterologist
Should a gastroenterologist oversee the decision on surveillance in older adults based on a combination of age, estimated procedure risk and benefits, patient preferences, and current guidelines? Certainly, it seems appropriate and that this is what most specialists think, according to a recent survey of gastroenterologists and primary care physicians (PCPs) on this topic.5 Perhaps, not surprisingly, most PCPs disagreed – PCPs are thoroughly familiar with their patients’ up-to-date comorbidities, functional status, and preferences, and they have the benefit of knowing them for a long time. They also integrate diagnostic results from multiple subspecialists, sometimes from different states. The role of PCPs is critical in centers that offer open-access colonoscopy. Gastroenterologists may be the most appropriate authority to evaluate older individuals for continued surveillance, but in most busy practices these patients are seen by mid-level practitioners. Specialists play an important role if the PCP is uncertain whether surveillance is still indicated or in older patients with a history of advanced adenoma. Therefore, the colonoscopy report or subsequent communication after pathology results are returned should include a recommendation for future surveillance and a clear provision for discontinuing the surveillance in case of future health decline.
Conclusion
Determining the optimal age to discontinue surveillance for colorectal neoplasia involves evaluating multiple factors. Although the age limit is clearer for average-risk screening and low-risk lesion surveillance, uncertainty remains for individuals with advanced neoplasia history. Significant factors in this decision-making process include subsequent neoplasia risk, comorbidities, life expectancy, and age-related risks associated with colonoscopy. Cooperation between PCPs and subspecialty physicians is essential in making surveillance decisions for older adults. PCPs are well positioned to consider detailed patient comorbidities, functional status, and patient preferences, especially with the help of online life-expectancy estimators for most elderly or comorbid patients. To assist in this process, gastroenterologists should state clearly in their procedure report and subsequent pathology letters whether surveillance is recommended and that it is conditional on future comorbidities and should be discontinued if the patient’s health significantly declines.
Dr. Protiva is associate professor of medicine, Yale University, and director of the colon cancer screening program, VA Connecticut Healthcare System, West Haven. Dr. Protiva has no relevant disclosures.
References
1. Patel SG et al. Gastroenterology. 2022;162:285-99
2. U.S. Census Bureau. Measuring America’s People, Places, and Economy
3. University of California, San Francisco. ePrognosis. 4. Siegel RL et al. CA Cancer J Clin. 2023 May-Jun;73(3):233-54.
5. Schoenborn NL et al. Am J Gastroenterol. 2023; 118(3):523-30.
The gastroenterologist should guide the decision to maintain, or halt, surveillance in older adults
BY MARIAM NAVEED, MD
Endoscopic screening and surveillance for CRC in older adults (≥ 75 years old) is a medical “gray area” that needs more high-quality data to inform clinical decision making. In the most recent 2022 clinical guideline update from the U.S. Multisociety Task Force on Colorectal Cancer, the recommendation to stop CRC screening in average risk patients older than 86 years is well supported because of colonoscopy-associated mortality risk outweighing the benefits of adenoma detection and removal. By comparison, screening recommendations for average risk individuals between 76-85 years old are ambiguous and ultimately the decision to proceed with colonoscopy in this clinical population should be individualized based on shared decision making between the provider and patient. Of note, the same guideline provides no specific guidance for ongoing surveillance in the same age group and similarly suggests a shared decision-making approach.1
As a practicing gastroenterologist in the retirement capital of Florida, older adults comprise a large portion of my clinical practice. I have noticed several aspects unique to this demographic that merit special consideration. For example, a significant percentage of these patients are seasonal (that is, “snowbird”) patients that have multiple sets of doctors (set of physicians in their home state and another set in Florida). Consequently, fragmentation of clinical data enables opportunities for colonoscopies to be wrongly ordered (either in an inappropriate time frame and/or for inaccurate indications). In my own practice, when such a patient is referred for consideration of CRC surveillance, any/all external records must first be obtained and validated as a prerequisite for appropriate clinical counseling and informed decision-making. Additionally, consideration of periprocedural risks is particularly relevant in older adults, secondary to both the increased rate of direct complications and the likelihood of pre-existing comorbidities affecting completion of a safe colonoscopy. Factors that can be easily overlooked include higher rates of poor bowel preparation and corresponding decreased completion rates. Moreover, if the patient has a history of high-risk adenomas or worrisome family history warranting ongoing evaluation, but they also have high-risk comorbidities, I will frequently involve the patient’s cardiologist or pulmonologist to provide medical clearance prior to offering CRC screening/surveillance.
In addition to the clinical ambiguity of appropriateness of continued CRC screening/surveillance in the setting of advanced age, there is also the question of which provider is best positioned to counsel patients regarding this decision-making. Does the onus fall on the gastroenterologist (the proceduralist ultimately performing the procedure) or the PCP (who is likely more familiar with the patient’s overall health profile)? In a recent survey, more than 50% of PCPs reported feeling uncertain in their understanding of risk versus benefit stratification of continued CRC screening in older adults.2 While there may be justification for both classes of providers to be involved, in my opinion, the decision to maintain or halt surveillance in older adults should be primarily guided by the gastroenterologist who is better equipped to provide individualized guidance regarding the nuanced risks of disease progression in these patients with prior history of adenomas, and who is clinically responsible for any procedure-related complications.
In an era of cost containment, insurance companies are increasingly placing barriers for approving surveillance and diagnostic colonoscopies. Thus, we need to be ever mindful of appropriately allocating resources to best benefit patients. The data on incidence of polyps and CRC in older adults is inconsistent and even difficult at times for a gastroenterologist to interpret. Therefore, in my opinion, the onus should not fall solely on the PCPs who are not routinely familiar with this information. We as gastroenterologists typically have greater domain-specific knowledge regarding current data and updated guidelines.
Gastroenterologists should wield this expertise to regulate overly liberalized recommendations for continued surveillance in fragile patients, or conversely to intervene in settings of prematurely halting surveillance in high-risk populations with appropriate life expectancy to experience disease progression. It is critical to carefully consider patient-individualized life expectancies, avoid surveillance in patients without clinically significant polyps, avoid over-weighting previous abnormal prior colonoscopies without reviewing more current procedure results, and take time to discuss patient preferences. As proceduralists, we must also be mindful of intrinsic biases towards performing surveillance in patients who are not likely to benefit from this intervention, and several studies have reported on the overuse of surveillance colonoscopies in the form of repeating surveillance earlier than recommended or in the context of limited life expectancy.3
Finally, it is necessary to emphasize that PCPs are critical allies for promoting overall patient health, especially in scenarios where recommendations to discontinue surveillance may not coincide with patient preference. It has been reported that patients usually do not consider poor overall health relevant to decisions regarding CRC surveillance (which I have also experienced to be true).4 In these scenarios, partnering with the PCP can be strategic, as patients may be more inclined to trust the guidance of their more familiar physician. At the end of the day, regardless of which provider takes ownership of initiating the discussion surrounding surveillance colonoscopy in older adults, communication is key between all providers and the patient to ensure optimal outcomes.
As the U.S. population continues to age, the demographic of patients aged 65 and older is projected to nearly double by 2060.5 Decisions regarding ongoing surveillance for CRC will continue to be frequent and increasingly relevant. The importance of studies generating high-quality data to inform appropriate guidelines specific to this population cannot be understated.
Dr. Naveed is a gastroenterologist and director of the gastroenterology and hepatology fellowship program at AdventHealth Medical Group, Altamonte Springs, Fla. Dr. Naveed had no relevant disclosures.
References
1. Patel SG et al. Gastroenterology. 2022 Jan;162(1):285-99.
2 Schoenborn NL et al. Am J Gastroenterol. 2023 Mar 1;118(3):523-30.
3 Calderwood AH et al. JAMA Intern Med. 2023 May 1;183(5):426-34.
4 Schoenborn NL et al. Am J Gastroenterol. 2023 Mar 1;118(3):523-30.
5 U.S. Census Bureau. Population Projections.
Dear colleagues,
Colonoscopy is the bread and butter of endoscopy. Multidisciplinary updates continue to support screening colonoscopy in reducing the risk of developing colorectal cancer. But there has been debate about the best uses of resources, especially with increased recognition of colorectal cancer (CRC) in younger patients, and successive guidelines lengthening the intervals for most surveillance colonoscopy.
In particular, when do we feel comfortable recommending cessation of surveillance colonoscopy especially in those who are 75-85 years old? Routine colonoscopy remains a very low-risk procedure even in older patients with multiple comorbidities.
In this issue of Perspectives, Dr. Petr Protiva and Dr. Mariam Naveed address this issue and provide differing perspectives on approaching surveillance colonoscopy in the elderly.
We welcome your thoughts on this issue on Twitter at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Striking a balance: Deciding the optimal age to cease surveillance for colorectal neoplasia
BY PETR PROTIVA, MD, MPH
The appropriate age to stop surveillance for colorectal neoplasia remains uncertain. Screening for average-risk individuals is typically stopped at age 75, but personalized screening with shared decision-making may continue until age 85.1 Evidence suggests that any survival benefit of screening past age 86 would be outweighed by the harm of screening and/or natural mortality. Nevertheless, determining the optimal age for surveillance in those with a history of neoplasia still poses some challenges. The issue is confounded as many clinicians use the terms “screening” and “surveillance” interchangeably. It should be noted that screening implies the individual is at average risk, while surveillance refers to those at elevated risk because of a personal history of colonic adenomas or cancer
Comorbidities and life expectancy
Despite recent staggering setbacks and a drop in the average life expectancy in the United States, the proportion of individuals older than 65 years old has been steadily increasing to a currently estimated 58.9 million – 16.8% of the U.S. population – and is projected to increase in the future.2 However, the prevalence of comorbidities also increases with age, and these are crucial factors to weigh in the decision-making process. Severe comorbid conditions, such as chronic obstructive pulmonary disease, cirrhosis, chronic hepatitis, chronic renal failure, dementia, and congestive heart failure can limit a patient’s ability to undergo surveillance and diminish or negate its benefits. There are online tools that help clinicians estimate life expectancy and time to benefit (that is, time between the intervention and its benefit), such as the Lee or Schonberg index.3 Consensus on time to colorectal screening benefit is about 9-10 years, but may be much shorter for surveillance. Striking a balance between the potential benefits of continued surveillance and the risks and burdens imposed on older adults with limited life expectancy is essential for making well-informed decisions.
Age-related risk increase and risk of neoplasia in the surveillance population:
The absolute risk of developing colorectal cancer is dependent on age. In adults aged 45-49, it is 33.4/100,000, rising to 135.6 in those 70-74 years old; in persons aged 85 and older, it is 234.7/100,000.4 A significant challenge in determining the appropriate age to stop surveillance is the additional individual risk based on baseline polyp characteristics. It seems reasonable to treat low-risk adenomas similarly to screening and stop surveillance by 85 or 86 years old in most cases. The decision process is less clear in individuals with advanced lesions or a personal history of colon cancer. The prevalence of advanced adenomas on the baseline exam is about 15% and greater than 20% on the follow-up exam if the index adenoma(s) were at least 20 mm in diameter. For five or more adenomas at baseline, the prevalence of advanced lesions on follow-up exam is about 20%. On the other hand, a negative surveillance colonoscopy (that is, no polyps found) is associated with far fewer advanced lesions on follow-up.
Colonoscopy
The safety of colonoscopy in older adults should be considered. The colonoscopy procedure is generally very safe, but is associated with a higher risk of post-procedure complications after outpatient colonoscopy in patients 75 years and older. In addition, comorbidities such as anemia, cardiac arrhythmia, heart failure, hypertension, chronic kidney disease, liver disease, smoking history, and obesity are also risk factors. It should be noted that high-quality colonoscopy is key to the detection and full removal of neoplastic lesions and risk reduction. The inability to achieve adequate colon preparation for any reason or undertaking colonoscopy in patients at high risk for complications reduces its benefit.
Who should oversee surveillance, the primary care physician or gastroenterologist
Should a gastroenterologist oversee the decision on surveillance in older adults based on a combination of age, estimated procedure risk and benefits, patient preferences, and current guidelines? Certainly, it seems appropriate and that this is what most specialists think, according to a recent survey of gastroenterologists and primary care physicians (PCPs) on this topic.5 Perhaps, not surprisingly, most PCPs disagreed – PCPs are thoroughly familiar with their patients’ up-to-date comorbidities, functional status, and preferences, and they have the benefit of knowing them for a long time. They also integrate diagnostic results from multiple subspecialists, sometimes from different states. The role of PCPs is critical in centers that offer open-access colonoscopy. Gastroenterologists may be the most appropriate authority to evaluate older individuals for continued surveillance, but in most busy practices these patients are seen by mid-level practitioners. Specialists play an important role if the PCP is uncertain whether surveillance is still indicated or in older patients with a history of advanced adenoma. Therefore, the colonoscopy report or subsequent communication after pathology results are returned should include a recommendation for future surveillance and a clear provision for discontinuing the surveillance in case of future health decline.
Conclusion
Determining the optimal age to discontinue surveillance for colorectal neoplasia involves evaluating multiple factors. Although the age limit is clearer for average-risk screening and low-risk lesion surveillance, uncertainty remains for individuals with advanced neoplasia history. Significant factors in this decision-making process include subsequent neoplasia risk, comorbidities, life expectancy, and age-related risks associated with colonoscopy. Cooperation between PCPs and subspecialty physicians is essential in making surveillance decisions for older adults. PCPs are well positioned to consider detailed patient comorbidities, functional status, and patient preferences, especially with the help of online life-expectancy estimators for most elderly or comorbid patients. To assist in this process, gastroenterologists should state clearly in their procedure report and subsequent pathology letters whether surveillance is recommended and that it is conditional on future comorbidities and should be discontinued if the patient’s health significantly declines.
Dr. Protiva is associate professor of medicine, Yale University, and director of the colon cancer screening program, VA Connecticut Healthcare System, West Haven. Dr. Protiva has no relevant disclosures.
References
1. Patel SG et al. Gastroenterology. 2022;162:285-99
2. U.S. Census Bureau. Measuring America’s People, Places, and Economy
3. University of California, San Francisco. ePrognosis. 4. Siegel RL et al. CA Cancer J Clin. 2023 May-Jun;73(3):233-54.
5. Schoenborn NL et al. Am J Gastroenterol. 2023; 118(3):523-30.
The gastroenterologist should guide the decision to maintain, or halt, surveillance in older adults
BY MARIAM NAVEED, MD
Endoscopic screening and surveillance for CRC in older adults (≥ 75 years old) is a medical “gray area” that needs more high-quality data to inform clinical decision making. In the most recent 2022 clinical guideline update from the U.S. Multisociety Task Force on Colorectal Cancer, the recommendation to stop CRC screening in average risk patients older than 86 years is well supported because of colonoscopy-associated mortality risk outweighing the benefits of adenoma detection and removal. By comparison, screening recommendations for average risk individuals between 76-85 years old are ambiguous and ultimately the decision to proceed with colonoscopy in this clinical population should be individualized based on shared decision making between the provider and patient. Of note, the same guideline provides no specific guidance for ongoing surveillance in the same age group and similarly suggests a shared decision-making approach.1
As a practicing gastroenterologist in the retirement capital of Florida, older adults comprise a large portion of my clinical practice. I have noticed several aspects unique to this demographic that merit special consideration. For example, a significant percentage of these patients are seasonal (that is, “snowbird”) patients that have multiple sets of doctors (set of physicians in their home state and another set in Florida). Consequently, fragmentation of clinical data enables opportunities for colonoscopies to be wrongly ordered (either in an inappropriate time frame and/or for inaccurate indications). In my own practice, when such a patient is referred for consideration of CRC surveillance, any/all external records must first be obtained and validated as a prerequisite for appropriate clinical counseling and informed decision-making. Additionally, consideration of periprocedural risks is particularly relevant in older adults, secondary to both the increased rate of direct complications and the likelihood of pre-existing comorbidities affecting completion of a safe colonoscopy. Factors that can be easily overlooked include higher rates of poor bowel preparation and corresponding decreased completion rates. Moreover, if the patient has a history of high-risk adenomas or worrisome family history warranting ongoing evaluation, but they also have high-risk comorbidities, I will frequently involve the patient’s cardiologist or pulmonologist to provide medical clearance prior to offering CRC screening/surveillance.
In addition to the clinical ambiguity of appropriateness of continued CRC screening/surveillance in the setting of advanced age, there is also the question of which provider is best positioned to counsel patients regarding this decision-making. Does the onus fall on the gastroenterologist (the proceduralist ultimately performing the procedure) or the PCP (who is likely more familiar with the patient’s overall health profile)? In a recent survey, more than 50% of PCPs reported feeling uncertain in their understanding of risk versus benefit stratification of continued CRC screening in older adults.2 While there may be justification for both classes of providers to be involved, in my opinion, the decision to maintain or halt surveillance in older adults should be primarily guided by the gastroenterologist who is better equipped to provide individualized guidance regarding the nuanced risks of disease progression in these patients with prior history of adenomas, and who is clinically responsible for any procedure-related complications.
In an era of cost containment, insurance companies are increasingly placing barriers for approving surveillance and diagnostic colonoscopies. Thus, we need to be ever mindful of appropriately allocating resources to best benefit patients. The data on incidence of polyps and CRC in older adults is inconsistent and even difficult at times for a gastroenterologist to interpret. Therefore, in my opinion, the onus should not fall solely on the PCPs who are not routinely familiar with this information. We as gastroenterologists typically have greater domain-specific knowledge regarding current data and updated guidelines.
Gastroenterologists should wield this expertise to regulate overly liberalized recommendations for continued surveillance in fragile patients, or conversely to intervene in settings of prematurely halting surveillance in high-risk populations with appropriate life expectancy to experience disease progression. It is critical to carefully consider patient-individualized life expectancies, avoid surveillance in patients without clinically significant polyps, avoid over-weighting previous abnormal prior colonoscopies without reviewing more current procedure results, and take time to discuss patient preferences. As proceduralists, we must also be mindful of intrinsic biases towards performing surveillance in patients who are not likely to benefit from this intervention, and several studies have reported on the overuse of surveillance colonoscopies in the form of repeating surveillance earlier than recommended or in the context of limited life expectancy.3
Finally, it is necessary to emphasize that PCPs are critical allies for promoting overall patient health, especially in scenarios where recommendations to discontinue surveillance may not coincide with patient preference. It has been reported that patients usually do not consider poor overall health relevant to decisions regarding CRC surveillance (which I have also experienced to be true).4 In these scenarios, partnering with the PCP can be strategic, as patients may be more inclined to trust the guidance of their more familiar physician. At the end of the day, regardless of which provider takes ownership of initiating the discussion surrounding surveillance colonoscopy in older adults, communication is key between all providers and the patient to ensure optimal outcomes.
As the U.S. population continues to age, the demographic of patients aged 65 and older is projected to nearly double by 2060.5 Decisions regarding ongoing surveillance for CRC will continue to be frequent and increasingly relevant. The importance of studies generating high-quality data to inform appropriate guidelines specific to this population cannot be understated.
Dr. Naveed is a gastroenterologist and director of the gastroenterology and hepatology fellowship program at AdventHealth Medical Group, Altamonte Springs, Fla. Dr. Naveed had no relevant disclosures.
References
1. Patel SG et al. Gastroenterology. 2022 Jan;162(1):285-99.
2 Schoenborn NL et al. Am J Gastroenterol. 2023 Mar 1;118(3):523-30.
3 Calderwood AH et al. JAMA Intern Med. 2023 May 1;183(5):426-34.
4 Schoenborn NL et al. Am J Gastroenterol. 2023 Mar 1;118(3):523-30.
5 U.S. Census Bureau. Population Projections.
Dear colleagues,
Colonoscopy is the bread and butter of endoscopy. Multidisciplinary updates continue to support screening colonoscopy in reducing the risk of developing colorectal cancer. But there has been debate about the best uses of resources, especially with increased recognition of colorectal cancer (CRC) in younger patients, and successive guidelines lengthening the intervals for most surveillance colonoscopy.
In particular, when do we feel comfortable recommending cessation of surveillance colonoscopy especially in those who are 75-85 years old? Routine colonoscopy remains a very low-risk procedure even in older patients with multiple comorbidities.
In this issue of Perspectives, Dr. Petr Protiva and Dr. Mariam Naveed address this issue and provide differing perspectives on approaching surveillance colonoscopy in the elderly.
We welcome your thoughts on this issue on Twitter at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Striking a balance: Deciding the optimal age to cease surveillance for colorectal neoplasia
BY PETR PROTIVA, MD, MPH
The appropriate age to stop surveillance for colorectal neoplasia remains uncertain. Screening for average-risk individuals is typically stopped at age 75, but personalized screening with shared decision-making may continue until age 85.1 Evidence suggests that any survival benefit of screening past age 86 would be outweighed by the harm of screening and/or natural mortality. Nevertheless, determining the optimal age for surveillance in those with a history of neoplasia still poses some challenges. The issue is confounded as many clinicians use the terms “screening” and “surveillance” interchangeably. It should be noted that screening implies the individual is at average risk, while surveillance refers to those at elevated risk because of a personal history of colonic adenomas or cancer
Comorbidities and life expectancy
Despite recent staggering setbacks and a drop in the average life expectancy in the United States, the proportion of individuals older than 65 years old has been steadily increasing to a currently estimated 58.9 million – 16.8% of the U.S. population – and is projected to increase in the future.2 However, the prevalence of comorbidities also increases with age, and these are crucial factors to weigh in the decision-making process. Severe comorbid conditions, such as chronic obstructive pulmonary disease, cirrhosis, chronic hepatitis, chronic renal failure, dementia, and congestive heart failure can limit a patient’s ability to undergo surveillance and diminish or negate its benefits. There are online tools that help clinicians estimate life expectancy and time to benefit (that is, time between the intervention and its benefit), such as the Lee or Schonberg index.3 Consensus on time to colorectal screening benefit is about 9-10 years, but may be much shorter for surveillance. Striking a balance between the potential benefits of continued surveillance and the risks and burdens imposed on older adults with limited life expectancy is essential for making well-informed decisions.
Age-related risk increase and risk of neoplasia in the surveillance population:
The absolute risk of developing colorectal cancer is dependent on age. In adults aged 45-49, it is 33.4/100,000, rising to 135.6 in those 70-74 years old; in persons aged 85 and older, it is 234.7/100,000.4 A significant challenge in determining the appropriate age to stop surveillance is the additional individual risk based on baseline polyp characteristics. It seems reasonable to treat low-risk adenomas similarly to screening and stop surveillance by 85 or 86 years old in most cases. The decision process is less clear in individuals with advanced lesions or a personal history of colon cancer. The prevalence of advanced adenomas on the baseline exam is about 15% and greater than 20% on the follow-up exam if the index adenoma(s) were at least 20 mm in diameter. For five or more adenomas at baseline, the prevalence of advanced lesions on follow-up exam is about 20%. On the other hand, a negative surveillance colonoscopy (that is, no polyps found) is associated with far fewer advanced lesions on follow-up.
Colonoscopy
The safety of colonoscopy in older adults should be considered. The colonoscopy procedure is generally very safe, but is associated with a higher risk of post-procedure complications after outpatient colonoscopy in patients 75 years and older. In addition, comorbidities such as anemia, cardiac arrhythmia, heart failure, hypertension, chronic kidney disease, liver disease, smoking history, and obesity are also risk factors. It should be noted that high-quality colonoscopy is key to the detection and full removal of neoplastic lesions and risk reduction. The inability to achieve adequate colon preparation for any reason or undertaking colonoscopy in patients at high risk for complications reduces its benefit.
Who should oversee surveillance, the primary care physician or gastroenterologist
Should a gastroenterologist oversee the decision on surveillance in older adults based on a combination of age, estimated procedure risk and benefits, patient preferences, and current guidelines? Certainly, it seems appropriate and that this is what most specialists think, according to a recent survey of gastroenterologists and primary care physicians (PCPs) on this topic.5 Perhaps, not surprisingly, most PCPs disagreed – PCPs are thoroughly familiar with their patients’ up-to-date comorbidities, functional status, and preferences, and they have the benefit of knowing them for a long time. They also integrate diagnostic results from multiple subspecialists, sometimes from different states. The role of PCPs is critical in centers that offer open-access colonoscopy. Gastroenterologists may be the most appropriate authority to evaluate older individuals for continued surveillance, but in most busy practices these patients are seen by mid-level practitioners. Specialists play an important role if the PCP is uncertain whether surveillance is still indicated or in older patients with a history of advanced adenoma. Therefore, the colonoscopy report or subsequent communication after pathology results are returned should include a recommendation for future surveillance and a clear provision for discontinuing the surveillance in case of future health decline.
Conclusion
Determining the optimal age to discontinue surveillance for colorectal neoplasia involves evaluating multiple factors. Although the age limit is clearer for average-risk screening and low-risk lesion surveillance, uncertainty remains for individuals with advanced neoplasia history. Significant factors in this decision-making process include subsequent neoplasia risk, comorbidities, life expectancy, and age-related risks associated with colonoscopy. Cooperation between PCPs and subspecialty physicians is essential in making surveillance decisions for older adults. PCPs are well positioned to consider detailed patient comorbidities, functional status, and patient preferences, especially with the help of online life-expectancy estimators for most elderly or comorbid patients. To assist in this process, gastroenterologists should state clearly in their procedure report and subsequent pathology letters whether surveillance is recommended and that it is conditional on future comorbidities and should be discontinued if the patient’s health significantly declines.
Dr. Protiva is associate professor of medicine, Yale University, and director of the colon cancer screening program, VA Connecticut Healthcare System, West Haven. Dr. Protiva has no relevant disclosures.
References
1. Patel SG et al. Gastroenterology. 2022;162:285-99
2. U.S. Census Bureau. Measuring America’s People, Places, and Economy
3. University of California, San Francisco. ePrognosis. 4. Siegel RL et al. CA Cancer J Clin. 2023 May-Jun;73(3):233-54.
5. Schoenborn NL et al. Am J Gastroenterol. 2023; 118(3):523-30.
The gastroenterologist should guide the decision to maintain, or halt, surveillance in older adults
BY MARIAM NAVEED, MD
Endoscopic screening and surveillance for CRC in older adults (≥ 75 years old) is a medical “gray area” that needs more high-quality data to inform clinical decision making. In the most recent 2022 clinical guideline update from the U.S. Multisociety Task Force on Colorectal Cancer, the recommendation to stop CRC screening in average risk patients older than 86 years is well supported because of colonoscopy-associated mortality risk outweighing the benefits of adenoma detection and removal. By comparison, screening recommendations for average risk individuals between 76-85 years old are ambiguous and ultimately the decision to proceed with colonoscopy in this clinical population should be individualized based on shared decision making between the provider and patient. Of note, the same guideline provides no specific guidance for ongoing surveillance in the same age group and similarly suggests a shared decision-making approach.1
As a practicing gastroenterologist in the retirement capital of Florida, older adults comprise a large portion of my clinical practice. I have noticed several aspects unique to this demographic that merit special consideration. For example, a significant percentage of these patients are seasonal (that is, “snowbird”) patients that have multiple sets of doctors (set of physicians in their home state and another set in Florida). Consequently, fragmentation of clinical data enables opportunities for colonoscopies to be wrongly ordered (either in an inappropriate time frame and/or for inaccurate indications). In my own practice, when such a patient is referred for consideration of CRC surveillance, any/all external records must first be obtained and validated as a prerequisite for appropriate clinical counseling and informed decision-making. Additionally, consideration of periprocedural risks is particularly relevant in older adults, secondary to both the increased rate of direct complications and the likelihood of pre-existing comorbidities affecting completion of a safe colonoscopy. Factors that can be easily overlooked include higher rates of poor bowel preparation and corresponding decreased completion rates. Moreover, if the patient has a history of high-risk adenomas or worrisome family history warranting ongoing evaluation, but they also have high-risk comorbidities, I will frequently involve the patient’s cardiologist or pulmonologist to provide medical clearance prior to offering CRC screening/surveillance.
In addition to the clinical ambiguity of appropriateness of continued CRC screening/surveillance in the setting of advanced age, there is also the question of which provider is best positioned to counsel patients regarding this decision-making. Does the onus fall on the gastroenterologist (the proceduralist ultimately performing the procedure) or the PCP (who is likely more familiar with the patient’s overall health profile)? In a recent survey, more than 50% of PCPs reported feeling uncertain in their understanding of risk versus benefit stratification of continued CRC screening in older adults.2 While there may be justification for both classes of providers to be involved, in my opinion, the decision to maintain or halt surveillance in older adults should be primarily guided by the gastroenterologist who is better equipped to provide individualized guidance regarding the nuanced risks of disease progression in these patients with prior history of adenomas, and who is clinically responsible for any procedure-related complications.
In an era of cost containment, insurance companies are increasingly placing barriers for approving surveillance and diagnostic colonoscopies. Thus, we need to be ever mindful of appropriately allocating resources to best benefit patients. The data on incidence of polyps and CRC in older adults is inconsistent and even difficult at times for a gastroenterologist to interpret. Therefore, in my opinion, the onus should not fall solely on the PCPs who are not routinely familiar with this information. We as gastroenterologists typically have greater domain-specific knowledge regarding current data and updated guidelines.
Gastroenterologists should wield this expertise to regulate overly liberalized recommendations for continued surveillance in fragile patients, or conversely to intervene in settings of prematurely halting surveillance in high-risk populations with appropriate life expectancy to experience disease progression. It is critical to carefully consider patient-individualized life expectancies, avoid surveillance in patients without clinically significant polyps, avoid over-weighting previous abnormal prior colonoscopies without reviewing more current procedure results, and take time to discuss patient preferences. As proceduralists, we must also be mindful of intrinsic biases towards performing surveillance in patients who are not likely to benefit from this intervention, and several studies have reported on the overuse of surveillance colonoscopies in the form of repeating surveillance earlier than recommended or in the context of limited life expectancy.3
Finally, it is necessary to emphasize that PCPs are critical allies for promoting overall patient health, especially in scenarios where recommendations to discontinue surveillance may not coincide with patient preference. It has been reported that patients usually do not consider poor overall health relevant to decisions regarding CRC surveillance (which I have also experienced to be true).4 In these scenarios, partnering with the PCP can be strategic, as patients may be more inclined to trust the guidance of their more familiar physician. At the end of the day, regardless of which provider takes ownership of initiating the discussion surrounding surveillance colonoscopy in older adults, communication is key between all providers and the patient to ensure optimal outcomes.
As the U.S. population continues to age, the demographic of patients aged 65 and older is projected to nearly double by 2060.5 Decisions regarding ongoing surveillance for CRC will continue to be frequent and increasingly relevant. The importance of studies generating high-quality data to inform appropriate guidelines specific to this population cannot be understated.
Dr. Naveed is a gastroenterologist and director of the gastroenterology and hepatology fellowship program at AdventHealth Medical Group, Altamonte Springs, Fla. Dr. Naveed had no relevant disclosures.
References
1. Patel SG et al. Gastroenterology. 2022 Jan;162(1):285-99.
2 Schoenborn NL et al. Am J Gastroenterol. 2023 Mar 1;118(3):523-30.
3 Calderwood AH et al. JAMA Intern Med. 2023 May 1;183(5):426-34.
4 Schoenborn NL et al. Am J Gastroenterol. 2023 Mar 1;118(3):523-30.
5 U.S. Census Bureau. Population Projections.
Launching an entirely virtual health care GI practice
that partners with health systems to offer GI care services throughout the country.
Dr. Arjal, who as a cofounder of Telebelly Health also serves as chief medical officer and president of the practice, previously served as vice president of Puget Sound Gastroenterology and practiced in the Seattle area for 13 years. He served as vice president of clinical affairs for Gastro Health, the nation’s second-largest gastroenterology group, which acquired the Puget Sound practice in 2019. But then in 2021, he founded Telebelly with Sheri Rudberg, MBA, JD, who serves as CEO of the business; Alex Brown, who leads product development; and Nakort Valles, who serves as the company’s chief technology officer.
Building a new business whose goal is to transform GI health care delivery has been his biggest challenge to date. “I am proud of Telebelly because its goals are goals we all share, which is to try to get people in the door and take good care of them,” Dr. Arjal said.
Through virtual care clinics like Telebelly Health, patients can see a provider who is affiliated with a practice, even if the provider is in another state provided he or she is licensed in the patient’s home state. Some states have passed legislation to permanently allow out-of-state physicians to practice telehealth in their state if they follow the state’s requirements. In some states, that may amount to accepting an out-of-state medical license or requiring out-of-state clinicians to pass an exam.
Telebelly Health has served thousands of patients since September when the practice was launched. “We are scaling pretty quickly and will be doubling the number of providers in the next couple of months,” Dr. Arjal said.
In this Q&A, he talks more about his new business venture and his vision for the future of medicine.
Question: Why did you choose GI?
Answer: I wanted to do something that was cognitive where I interacted with and really got to know patients. I also wanted to be a proceduralist. I never wanted to be a surgeon – I knew that wasn’t for me. I fell in love with GI the first year in med school. I thought the pathology was interesting, and what GIs did in the acute setting as well as the outpatient setting was compelling.
Q. What achievement are you most proud of?
A. Prior to Telebelly, I led a large regional GI group in a competitive marketplace. Now, with Telebelly, building a team with a vision to transform the space has been the biggest challenge I have taken on. It’s still a work in progress, but we’ve had a great start. Starting a company wasn’t easy. It was something that I didn’t know a lot about, so I had to take a fair bit of risk. I wasn’t sure if I had it in me at the beginning. It’s not something I’d ever done before, so I was testing myself. I am proud that we were able to launch the company and have successfully scaled it. It’s been more successful than I expected.
Q. Describe your biggest practice-related challenge and what you are doing to address it.
A. Access to care. I think it’s very hard to see somebody with GI expertise and it certainly got worse during the pandemic. In my previous role, we used advanced practice providers. We tried to implement technology, sometimes effectively, sometimes not. But in general, we wanted to try to increase the supply of providers and compress these patient journeys to get people in the door. But that’s still a very difficult challenge we’re all trying to solve.
Q. What teacher or mentor had the greatest impact on you?
A. I would say two: James Trotter, MD, a hepatologist at the University of Colorado where I trained. He had a terrific impact in the sense that he was 100% focused on patients and got to know them as people. This taught me what it meant to be a clinician that was sort of a humanist. He cared so much for his patients that I still think about what Jim would do in a room today, 15 years after I finished my fellowship.
When I started my first job at Puget Sound Gastroenterology in the Seattle area, Robin Sloane, MD, was one of the senior partners of the group. I had a lot to learn after finishing fellowship. He was wonderful and gracious and really taught me a ton about the practical aspects of medicine. I felt this was an extension of my training in that he was a real clinician who really cared deeply for his patients. If I hadn’t met those two, my career and maybe my view of just what I did day-to-day would be different. They were both very, very impactful for me.
Q. Outside of teachers and mentors, who has had the strongest influence on your life?
A. Two people: my mother and my wife. My mother was a single parent and we were immigrants to the country. She was an ambitious woman who didn’t let anything stop her. I certainly learned a ton about resilience, work ethic. She’s somebody who always treated people well. My wife also supported and believed in me, and without her, I would not have had the courage to start a company.
Q. Describe a scene of your vision for the future.
A. I think we need to change our mindset in terms of how we interact with patients. I think there’s going to be a lot of clinical testing that is performed away from the physician’s office. It’s going to become more democratized and more decentralized. And I think in the future, patients will have more agency in how they interact with the system. I think artificial intelligence will potentially augment all of this as well. We’ll have patients who are more engaged, have more choice and easier access to expert care. They’ll come in with more information on their hands and they won’t have to wait as long. I think the wait times to get to a GI clinic now are way too long.
What I’d also like to see are providers spending more time doing things that they’re trained to do rather than documentation, summarizing data, and dealing with administrative headaches. I think almost everybody has that goal, but I think that’s achievable.
I want providers to have an iron man or iron woman suit when they see a patient, to have more data at their fingertips, to spend more time with the patients and have smarter visits.
Q. What did you fear most early in your career?
A. Failure for the most part, and comfort. For a long time, I wanted to start a company and change the space. Fear of failure has been ingrained in me and I think that’s true for a lot of physicians. I had always been a perfectionist.
Q. What gives you the most joy in your day-to-day practice?
A. Seeing patients is by far the thing I enjoy most. I don’t love documenting or digging up information, but I like getting to know folks. In general, I’m a social person and my outpatient clinic gives me the most joy, probably more than anything else.
Q. How do you stay current with advances in your field?
A. I’m curious about all new things, so I stay current through traditional means: I go to conferences regularly, I take postgraduate courses, I listen to podcasts, talk to colleagues, and read journals on a regular basis. But there are a lot of adjacent sources I pay attention to as well, such as nonmedical journals and nonmedical podcasts. I talk to folks outside the space and try to learn from them as well.
Q. What habits have you established that have benefited your career?
A. I do the same thing every day before my clinic days or my endoscopy days. I make reading a part of each day so I can slow down and be more present. Every day I try not to perform just what I do workwise, but I try to find some balance either with my family, or through exercise. I think I’ve been pretty good at separating work life from personal life.
Lightning round questions
Texting or talking? Talking.
Favorite junk food? Peanut butter M&Ms.
How many cups of coffee do you drink per day? Three.
If you weren’t a gastroenterologist, what would you be? Venture capitalist.
Introvert or extrovert? Both.
that partners with health systems to offer GI care services throughout the country.
Dr. Arjal, who as a cofounder of Telebelly Health also serves as chief medical officer and president of the practice, previously served as vice president of Puget Sound Gastroenterology and practiced in the Seattle area for 13 years. He served as vice president of clinical affairs for Gastro Health, the nation’s second-largest gastroenterology group, which acquired the Puget Sound practice in 2019. But then in 2021, he founded Telebelly with Sheri Rudberg, MBA, JD, who serves as CEO of the business; Alex Brown, who leads product development; and Nakort Valles, who serves as the company’s chief technology officer.
Building a new business whose goal is to transform GI health care delivery has been his biggest challenge to date. “I am proud of Telebelly because its goals are goals we all share, which is to try to get people in the door and take good care of them,” Dr. Arjal said.
Through virtual care clinics like Telebelly Health, patients can see a provider who is affiliated with a practice, even if the provider is in another state provided he or she is licensed in the patient’s home state. Some states have passed legislation to permanently allow out-of-state physicians to practice telehealth in their state if they follow the state’s requirements. In some states, that may amount to accepting an out-of-state medical license or requiring out-of-state clinicians to pass an exam.
Telebelly Health has served thousands of patients since September when the practice was launched. “We are scaling pretty quickly and will be doubling the number of providers in the next couple of months,” Dr. Arjal said.
In this Q&A, he talks more about his new business venture and his vision for the future of medicine.
Question: Why did you choose GI?
Answer: I wanted to do something that was cognitive where I interacted with and really got to know patients. I also wanted to be a proceduralist. I never wanted to be a surgeon – I knew that wasn’t for me. I fell in love with GI the first year in med school. I thought the pathology was interesting, and what GIs did in the acute setting as well as the outpatient setting was compelling.
Q. What achievement are you most proud of?
A. Prior to Telebelly, I led a large regional GI group in a competitive marketplace. Now, with Telebelly, building a team with a vision to transform the space has been the biggest challenge I have taken on. It’s still a work in progress, but we’ve had a great start. Starting a company wasn’t easy. It was something that I didn’t know a lot about, so I had to take a fair bit of risk. I wasn’t sure if I had it in me at the beginning. It’s not something I’d ever done before, so I was testing myself. I am proud that we were able to launch the company and have successfully scaled it. It’s been more successful than I expected.
Q. Describe your biggest practice-related challenge and what you are doing to address it.
A. Access to care. I think it’s very hard to see somebody with GI expertise and it certainly got worse during the pandemic. In my previous role, we used advanced practice providers. We tried to implement technology, sometimes effectively, sometimes not. But in general, we wanted to try to increase the supply of providers and compress these patient journeys to get people in the door. But that’s still a very difficult challenge we’re all trying to solve.
Q. What teacher or mentor had the greatest impact on you?
A. I would say two: James Trotter, MD, a hepatologist at the University of Colorado where I trained. He had a terrific impact in the sense that he was 100% focused on patients and got to know them as people. This taught me what it meant to be a clinician that was sort of a humanist. He cared so much for his patients that I still think about what Jim would do in a room today, 15 years after I finished my fellowship.
When I started my first job at Puget Sound Gastroenterology in the Seattle area, Robin Sloane, MD, was one of the senior partners of the group. I had a lot to learn after finishing fellowship. He was wonderful and gracious and really taught me a ton about the practical aspects of medicine. I felt this was an extension of my training in that he was a real clinician who really cared deeply for his patients. If I hadn’t met those two, my career and maybe my view of just what I did day-to-day would be different. They were both very, very impactful for me.
Q. Outside of teachers and mentors, who has had the strongest influence on your life?
A. Two people: my mother and my wife. My mother was a single parent and we were immigrants to the country. She was an ambitious woman who didn’t let anything stop her. I certainly learned a ton about resilience, work ethic. She’s somebody who always treated people well. My wife also supported and believed in me, and without her, I would not have had the courage to start a company.
Q. Describe a scene of your vision for the future.
A. I think we need to change our mindset in terms of how we interact with patients. I think there’s going to be a lot of clinical testing that is performed away from the physician’s office. It’s going to become more democratized and more decentralized. And I think in the future, patients will have more agency in how they interact with the system. I think artificial intelligence will potentially augment all of this as well. We’ll have patients who are more engaged, have more choice and easier access to expert care. They’ll come in with more information on their hands and they won’t have to wait as long. I think the wait times to get to a GI clinic now are way too long.
What I’d also like to see are providers spending more time doing things that they’re trained to do rather than documentation, summarizing data, and dealing with administrative headaches. I think almost everybody has that goal, but I think that’s achievable.
I want providers to have an iron man or iron woman suit when they see a patient, to have more data at their fingertips, to spend more time with the patients and have smarter visits.
Q. What did you fear most early in your career?
A. Failure for the most part, and comfort. For a long time, I wanted to start a company and change the space. Fear of failure has been ingrained in me and I think that’s true for a lot of physicians. I had always been a perfectionist.
Q. What gives you the most joy in your day-to-day practice?
A. Seeing patients is by far the thing I enjoy most. I don’t love documenting or digging up information, but I like getting to know folks. In general, I’m a social person and my outpatient clinic gives me the most joy, probably more than anything else.
Q. How do you stay current with advances in your field?
A. I’m curious about all new things, so I stay current through traditional means: I go to conferences regularly, I take postgraduate courses, I listen to podcasts, talk to colleagues, and read journals on a regular basis. But there are a lot of adjacent sources I pay attention to as well, such as nonmedical journals and nonmedical podcasts. I talk to folks outside the space and try to learn from them as well.
Q. What habits have you established that have benefited your career?
A. I do the same thing every day before my clinic days or my endoscopy days. I make reading a part of each day so I can slow down and be more present. Every day I try not to perform just what I do workwise, but I try to find some balance either with my family, or through exercise. I think I’ve been pretty good at separating work life from personal life.
Lightning round questions
Texting or talking? Talking.
Favorite junk food? Peanut butter M&Ms.
How many cups of coffee do you drink per day? Three.
If you weren’t a gastroenterologist, what would you be? Venture capitalist.
Introvert or extrovert? Both.
that partners with health systems to offer GI care services throughout the country.
Dr. Arjal, who as a cofounder of Telebelly Health also serves as chief medical officer and president of the practice, previously served as vice president of Puget Sound Gastroenterology and practiced in the Seattle area for 13 years. He served as vice president of clinical affairs for Gastro Health, the nation’s second-largest gastroenterology group, which acquired the Puget Sound practice in 2019. But then in 2021, he founded Telebelly with Sheri Rudberg, MBA, JD, who serves as CEO of the business; Alex Brown, who leads product development; and Nakort Valles, who serves as the company’s chief technology officer.
Building a new business whose goal is to transform GI health care delivery has been his biggest challenge to date. “I am proud of Telebelly because its goals are goals we all share, which is to try to get people in the door and take good care of them,” Dr. Arjal said.
Through virtual care clinics like Telebelly Health, patients can see a provider who is affiliated with a practice, even if the provider is in another state provided he or she is licensed in the patient’s home state. Some states have passed legislation to permanently allow out-of-state physicians to practice telehealth in their state if they follow the state’s requirements. In some states, that may amount to accepting an out-of-state medical license or requiring out-of-state clinicians to pass an exam.
Telebelly Health has served thousands of patients since September when the practice was launched. “We are scaling pretty quickly and will be doubling the number of providers in the next couple of months,” Dr. Arjal said.
In this Q&A, he talks more about his new business venture and his vision for the future of medicine.
Question: Why did you choose GI?
Answer: I wanted to do something that was cognitive where I interacted with and really got to know patients. I also wanted to be a proceduralist. I never wanted to be a surgeon – I knew that wasn’t for me. I fell in love with GI the first year in med school. I thought the pathology was interesting, and what GIs did in the acute setting as well as the outpatient setting was compelling.
Q. What achievement are you most proud of?
A. Prior to Telebelly, I led a large regional GI group in a competitive marketplace. Now, with Telebelly, building a team with a vision to transform the space has been the biggest challenge I have taken on. It’s still a work in progress, but we’ve had a great start. Starting a company wasn’t easy. It was something that I didn’t know a lot about, so I had to take a fair bit of risk. I wasn’t sure if I had it in me at the beginning. It’s not something I’d ever done before, so I was testing myself. I am proud that we were able to launch the company and have successfully scaled it. It’s been more successful than I expected.
Q. Describe your biggest practice-related challenge and what you are doing to address it.
A. Access to care. I think it’s very hard to see somebody with GI expertise and it certainly got worse during the pandemic. In my previous role, we used advanced practice providers. We tried to implement technology, sometimes effectively, sometimes not. But in general, we wanted to try to increase the supply of providers and compress these patient journeys to get people in the door. But that’s still a very difficult challenge we’re all trying to solve.
Q. What teacher or mentor had the greatest impact on you?
A. I would say two: James Trotter, MD, a hepatologist at the University of Colorado where I trained. He had a terrific impact in the sense that he was 100% focused on patients and got to know them as people. This taught me what it meant to be a clinician that was sort of a humanist. He cared so much for his patients that I still think about what Jim would do in a room today, 15 years after I finished my fellowship.
When I started my first job at Puget Sound Gastroenterology in the Seattle area, Robin Sloane, MD, was one of the senior partners of the group. I had a lot to learn after finishing fellowship. He was wonderful and gracious and really taught me a ton about the practical aspects of medicine. I felt this was an extension of my training in that he was a real clinician who really cared deeply for his patients. If I hadn’t met those two, my career and maybe my view of just what I did day-to-day would be different. They were both very, very impactful for me.
Q. Outside of teachers and mentors, who has had the strongest influence on your life?
A. Two people: my mother and my wife. My mother was a single parent and we were immigrants to the country. She was an ambitious woman who didn’t let anything stop her. I certainly learned a ton about resilience, work ethic. She’s somebody who always treated people well. My wife also supported and believed in me, and without her, I would not have had the courage to start a company.
Q. Describe a scene of your vision for the future.
A. I think we need to change our mindset in terms of how we interact with patients. I think there’s going to be a lot of clinical testing that is performed away from the physician’s office. It’s going to become more democratized and more decentralized. And I think in the future, patients will have more agency in how they interact with the system. I think artificial intelligence will potentially augment all of this as well. We’ll have patients who are more engaged, have more choice and easier access to expert care. They’ll come in with more information on their hands and they won’t have to wait as long. I think the wait times to get to a GI clinic now are way too long.
What I’d also like to see are providers spending more time doing things that they’re trained to do rather than documentation, summarizing data, and dealing with administrative headaches. I think almost everybody has that goal, but I think that’s achievable.
I want providers to have an iron man or iron woman suit when they see a patient, to have more data at their fingertips, to spend more time with the patients and have smarter visits.
Q. What did you fear most early in your career?
A. Failure for the most part, and comfort. For a long time, I wanted to start a company and change the space. Fear of failure has been ingrained in me and I think that’s true for a lot of physicians. I had always been a perfectionist.
Q. What gives you the most joy in your day-to-day practice?
A. Seeing patients is by far the thing I enjoy most. I don’t love documenting or digging up information, but I like getting to know folks. In general, I’m a social person and my outpatient clinic gives me the most joy, probably more than anything else.
Q. How do you stay current with advances in your field?
A. I’m curious about all new things, so I stay current through traditional means: I go to conferences regularly, I take postgraduate courses, I listen to podcasts, talk to colleagues, and read journals on a regular basis. But there are a lot of adjacent sources I pay attention to as well, such as nonmedical journals and nonmedical podcasts. I talk to folks outside the space and try to learn from them as well.
Q. What habits have you established that have benefited your career?
A. I do the same thing every day before my clinic days or my endoscopy days. I make reading a part of each day so I can slow down and be more present. Every day I try not to perform just what I do workwise, but I try to find some balance either with my family, or through exercise. I think I’ve been pretty good at separating work life from personal life.
Lightning round questions
Texting or talking? Talking.
Favorite junk food? Peanut butter M&Ms.
How many cups of coffee do you drink per day? Three.
If you weren’t a gastroenterologist, what would you be? Venture capitalist.
Introvert or extrovert? Both.
TAVI turmoil: Did an ANP perform transcatheter aortic valve replacement in the U.K.?
In the United Kingdom, John Steele, an advanced nurse practitioner (ANP) at Glenfield Hospital, part of the University Hospitals of Leicester NHS Trust (UHL), was congratulated on Twitter as “the first nurse-ANP who has performed the whole TAVI procedure as the first operator – true transformation addressing NHS needs.”
The now-deleted tweet from @GHCardiology is still visible in the Twitter thread of Mamas A. Mamas, a professor of interventional cardiology at Keele University, England. “This is so inappropriate on so many levels,” Dr. Mamas tweeted. “This is not safe for patients particularly given that there are numerous TAVI trained medically qualified operators in UK. You have also taken away training opportunities for medical / surgical trainees.”
Other followers also responded, largely negatively.
“This is crazy. Is this @TheOnion???” tweeted Martha Gulati, MD, director of preventive cardiology in the Smidt Heart Institute at Cedars-Sinai, Los Angeles, Calif., in response to Dr. Mamas, referring to the popular satirical news outlet. “Seriously I can’t see this as an actuality given the potential for so many other issues they wouldn’t know how to deal with.”
Could it happen in the U.S.?
Could a U.S.-based nurse practitioner perform TAVR? Possibly. Should they? No, says Andrew M. Goldsweig, MD, chair of the U.S. Society for Cardiovascular Angiography and Interventions Structural Heart Council. “Experienced nurse practitioners who have participated as secondary operators in many TAVR procedures and have observed the primary physician operators likely know the technical steps involved in an uncomplicated transfemoral TAVR procedure,” he told this news organization.
“However, a physician’s depth and breadth of training are absolutely required both to recognize and to address any periprocedural issues,” said Dr. Goldsweig, who is also director of the cardiac catheterization laboratory and director of cardiovascular clinical research at Baystate Medical Center in Springfield, Mass.
What it takes to do TAVR
Transcatheter aortic valves were first approved by the FDA in 2011 for use in patients with severe, inoperable, aortic stenosis. The procedure is now increasingly used as an alternative to surgical AVR in intermediate- and low-risk patients and has a longer history in Europe.
Dr. Goldsweig notes that “TAVR is a complex procedure with many potential challenges. Physicians are trained to diagnose and manage vascular access complications, heart failure and respiratory complications, rhythm disturbances, stroke, paravalvular leak, valve malpositioning/embolization, cardiogenic shock, and any other issues that may arise in the peri-TAVR period.
“Physicians can perform vascular imaging and interventions, transition to alternative access, manage intubation and ventilation, facilitate embolectomy, place a pacemaker, close a paravalvular leak, capture a misplaced valve, deploy mechanical circulatory support, and perform other diagnostic and interventional procedures as necessary that are required for TAVR operators and vastly exceed the training and scope of a nurse practitioner.”
The 2023 ACC/AHA/SCAI advanced training statement on interventional cardiology defines select competencies for interventional cardiologists who choose to focus their career on peripheral, vascular, or structural heart interventions.
In a recent article in Structural Heart, Dr. Goldsweig and colleagues write, “Training in SHD [structural heart disease] has historically been fragmented and informal. Current modes of SHD training include unaccredited fellowship training, industry-sponsored forums and device-specific training, and training through on-site proctorship.”
Such programs have grown “exponentially,” they write, “despite the conspicuous absence of formalized training requirements.”
In response to the John Steele uproar, the British Cardiovascular Intervention Society posted a statement on its website, noting, “As medicine has changed so there has increasingly been a role for allied health practitioners with advanced skills to take on responsibilities that were previously considered to be the domain of doctors ...
“TAVI procedures however carry a mortality risk, and the responsibility for undertaking a successful TAVI procedure will always lie with a Cardiologist who has had the breadth of training to manage the various complications that may occur during or after a procedure. This requires years of training, and there is no short-cut, or substitute.”
The BCIS promises a statement “later in the year [on] the expected training route for undertaking TAVI and other structural heart procedures.”
Why it matters: Scope creep
Despite the current upheaval, it’s not the first time that a nurse in the United Kingdom has performed a procedure normally performed by a medical doctor. A 3-year-old Reddit post on r/JuniorDoctorsUK points to a 2017 Guardian article titled, “Meet the nurse who will soon perform surgery on patients alone.” Although the “surgical care practitioner” seems to be performing within the scope of her practice, people responding to the post say it’s an example of “mid-level [scope] creep.”
More recently, a Reddit post in the same group points to a congratulatory post for a “nurse-led radial access.” One person commented, “Today they do the access. Tomorrow they do the full diagnostic. Day after they do the pressure wire. Next week they do the PCI [percutaneous coronary intervention].”
Broadly, “scope creep” refers to scope-of-practice expansions, but not turf wars, according to Rebekah Bernard, MD, a family physician in Fort Myers, Fla., who cowrote, “Patients at Risk: The Rise of the Nurse Practitioner and Physician Assistant in Healthcare,” with Niran Al-Agba, MD, a pediatrician in Silverdale, Wash.
The reasons behind U.K. scope creep aren’t clear. Some believe it’s money. Some say the system is broken and that doctors are being exploited.
In relation to the NP-TAVI case, the British Junior Cardiologist Association commented that it reflects a lack of support and advocacy for medical/surgical trainees who need the training opportunities that are going instead to allied health professionals.
In the United States, scope creep is being taken seriously (some may say too seriously) by the American Medical Association. The AMA is lobbying to stop “inappropriate scope expansions,” bolstered by its AMA Scope of Practice Partnership.
Pointing to a scope creep video produced by the AMA, one JuniorDoctorsUK Reddit post asks, “why isn’t the BMA doing anything similar?”
Time for a rethink?
Back to Glenfield Hospital. Not only has Cardiology Glenfield deleted the controversial tweet; it is now is backtracking on its congratulations to ANP Steele, tweeting, “We want to make clear that the lead operator for the procedure was a consultant structural interventionist. However, we are looking into the circumstances, including a review of clinical governance.” From the responses, few clinicians are buying that explanation.
In response to a request for a comment from Glenfield, Andrew Furlong, UHL medical director, reiterated to this news organization through communications manager Gareth Duggan, “We are investigating the circumstances of the procedure with our cardiology team and reviewing our governance processes.”
Dr. Goldsweig participated in a past speaking engagement for Edwards Lifesciences.
A version of this article originally appeared on Medscape.com.
In the United Kingdom, John Steele, an advanced nurse practitioner (ANP) at Glenfield Hospital, part of the University Hospitals of Leicester NHS Trust (UHL), was congratulated on Twitter as “the first nurse-ANP who has performed the whole TAVI procedure as the first operator – true transformation addressing NHS needs.”
The now-deleted tweet from @GHCardiology is still visible in the Twitter thread of Mamas A. Mamas, a professor of interventional cardiology at Keele University, England. “This is so inappropriate on so many levels,” Dr. Mamas tweeted. “This is not safe for patients particularly given that there are numerous TAVI trained medically qualified operators in UK. You have also taken away training opportunities for medical / surgical trainees.”
Other followers also responded, largely negatively.
“This is crazy. Is this @TheOnion???” tweeted Martha Gulati, MD, director of preventive cardiology in the Smidt Heart Institute at Cedars-Sinai, Los Angeles, Calif., in response to Dr. Mamas, referring to the popular satirical news outlet. “Seriously I can’t see this as an actuality given the potential for so many other issues they wouldn’t know how to deal with.”
Could it happen in the U.S.?
Could a U.S.-based nurse practitioner perform TAVR? Possibly. Should they? No, says Andrew M. Goldsweig, MD, chair of the U.S. Society for Cardiovascular Angiography and Interventions Structural Heart Council. “Experienced nurse practitioners who have participated as secondary operators in many TAVR procedures and have observed the primary physician operators likely know the technical steps involved in an uncomplicated transfemoral TAVR procedure,” he told this news organization.
“However, a physician’s depth and breadth of training are absolutely required both to recognize and to address any periprocedural issues,” said Dr. Goldsweig, who is also director of the cardiac catheterization laboratory and director of cardiovascular clinical research at Baystate Medical Center in Springfield, Mass.
What it takes to do TAVR
Transcatheter aortic valves were first approved by the FDA in 2011 for use in patients with severe, inoperable, aortic stenosis. The procedure is now increasingly used as an alternative to surgical AVR in intermediate- and low-risk patients and has a longer history in Europe.
Dr. Goldsweig notes that “TAVR is a complex procedure with many potential challenges. Physicians are trained to diagnose and manage vascular access complications, heart failure and respiratory complications, rhythm disturbances, stroke, paravalvular leak, valve malpositioning/embolization, cardiogenic shock, and any other issues that may arise in the peri-TAVR period.
“Physicians can perform vascular imaging and interventions, transition to alternative access, manage intubation and ventilation, facilitate embolectomy, place a pacemaker, close a paravalvular leak, capture a misplaced valve, deploy mechanical circulatory support, and perform other diagnostic and interventional procedures as necessary that are required for TAVR operators and vastly exceed the training and scope of a nurse practitioner.”
The 2023 ACC/AHA/SCAI advanced training statement on interventional cardiology defines select competencies for interventional cardiologists who choose to focus their career on peripheral, vascular, or structural heart interventions.
In a recent article in Structural Heart, Dr. Goldsweig and colleagues write, “Training in SHD [structural heart disease] has historically been fragmented and informal. Current modes of SHD training include unaccredited fellowship training, industry-sponsored forums and device-specific training, and training through on-site proctorship.”
Such programs have grown “exponentially,” they write, “despite the conspicuous absence of formalized training requirements.”
In response to the John Steele uproar, the British Cardiovascular Intervention Society posted a statement on its website, noting, “As medicine has changed so there has increasingly been a role for allied health practitioners with advanced skills to take on responsibilities that were previously considered to be the domain of doctors ...
“TAVI procedures however carry a mortality risk, and the responsibility for undertaking a successful TAVI procedure will always lie with a Cardiologist who has had the breadth of training to manage the various complications that may occur during or after a procedure. This requires years of training, and there is no short-cut, or substitute.”
The BCIS promises a statement “later in the year [on] the expected training route for undertaking TAVI and other structural heart procedures.”
Why it matters: Scope creep
Despite the current upheaval, it’s not the first time that a nurse in the United Kingdom has performed a procedure normally performed by a medical doctor. A 3-year-old Reddit post on r/JuniorDoctorsUK points to a 2017 Guardian article titled, “Meet the nurse who will soon perform surgery on patients alone.” Although the “surgical care practitioner” seems to be performing within the scope of her practice, people responding to the post say it’s an example of “mid-level [scope] creep.”
More recently, a Reddit post in the same group points to a congratulatory post for a “nurse-led radial access.” One person commented, “Today they do the access. Tomorrow they do the full diagnostic. Day after they do the pressure wire. Next week they do the PCI [percutaneous coronary intervention].”
Broadly, “scope creep” refers to scope-of-practice expansions, but not turf wars, according to Rebekah Bernard, MD, a family physician in Fort Myers, Fla., who cowrote, “Patients at Risk: The Rise of the Nurse Practitioner and Physician Assistant in Healthcare,” with Niran Al-Agba, MD, a pediatrician in Silverdale, Wash.
The reasons behind U.K. scope creep aren’t clear. Some believe it’s money. Some say the system is broken and that doctors are being exploited.
In relation to the NP-TAVI case, the British Junior Cardiologist Association commented that it reflects a lack of support and advocacy for medical/surgical trainees who need the training opportunities that are going instead to allied health professionals.
In the United States, scope creep is being taken seriously (some may say too seriously) by the American Medical Association. The AMA is lobbying to stop “inappropriate scope expansions,” bolstered by its AMA Scope of Practice Partnership.
Pointing to a scope creep video produced by the AMA, one JuniorDoctorsUK Reddit post asks, “why isn’t the BMA doing anything similar?”
Time for a rethink?
Back to Glenfield Hospital. Not only has Cardiology Glenfield deleted the controversial tweet; it is now is backtracking on its congratulations to ANP Steele, tweeting, “We want to make clear that the lead operator for the procedure was a consultant structural interventionist. However, we are looking into the circumstances, including a review of clinical governance.” From the responses, few clinicians are buying that explanation.
In response to a request for a comment from Glenfield, Andrew Furlong, UHL medical director, reiterated to this news organization through communications manager Gareth Duggan, “We are investigating the circumstances of the procedure with our cardiology team and reviewing our governance processes.”
Dr. Goldsweig participated in a past speaking engagement for Edwards Lifesciences.
A version of this article originally appeared on Medscape.com.
In the United Kingdom, John Steele, an advanced nurse practitioner (ANP) at Glenfield Hospital, part of the University Hospitals of Leicester NHS Trust (UHL), was congratulated on Twitter as “the first nurse-ANP who has performed the whole TAVI procedure as the first operator – true transformation addressing NHS needs.”
The now-deleted tweet from @GHCardiology is still visible in the Twitter thread of Mamas A. Mamas, a professor of interventional cardiology at Keele University, England. “This is so inappropriate on so many levels,” Dr. Mamas tweeted. “This is not safe for patients particularly given that there are numerous TAVI trained medically qualified operators in UK. You have also taken away training opportunities for medical / surgical trainees.”
Other followers also responded, largely negatively.
“This is crazy. Is this @TheOnion???” tweeted Martha Gulati, MD, director of preventive cardiology in the Smidt Heart Institute at Cedars-Sinai, Los Angeles, Calif., in response to Dr. Mamas, referring to the popular satirical news outlet. “Seriously I can’t see this as an actuality given the potential for so many other issues they wouldn’t know how to deal with.”
Could it happen in the U.S.?
Could a U.S.-based nurse practitioner perform TAVR? Possibly. Should they? No, says Andrew M. Goldsweig, MD, chair of the U.S. Society for Cardiovascular Angiography and Interventions Structural Heart Council. “Experienced nurse practitioners who have participated as secondary operators in many TAVR procedures and have observed the primary physician operators likely know the technical steps involved in an uncomplicated transfemoral TAVR procedure,” he told this news organization.
“However, a physician’s depth and breadth of training are absolutely required both to recognize and to address any periprocedural issues,” said Dr. Goldsweig, who is also director of the cardiac catheterization laboratory and director of cardiovascular clinical research at Baystate Medical Center in Springfield, Mass.
What it takes to do TAVR
Transcatheter aortic valves were first approved by the FDA in 2011 for use in patients with severe, inoperable, aortic stenosis. The procedure is now increasingly used as an alternative to surgical AVR in intermediate- and low-risk patients and has a longer history in Europe.
Dr. Goldsweig notes that “TAVR is a complex procedure with many potential challenges. Physicians are trained to diagnose and manage vascular access complications, heart failure and respiratory complications, rhythm disturbances, stroke, paravalvular leak, valve malpositioning/embolization, cardiogenic shock, and any other issues that may arise in the peri-TAVR period.
“Physicians can perform vascular imaging and interventions, transition to alternative access, manage intubation and ventilation, facilitate embolectomy, place a pacemaker, close a paravalvular leak, capture a misplaced valve, deploy mechanical circulatory support, and perform other diagnostic and interventional procedures as necessary that are required for TAVR operators and vastly exceed the training and scope of a nurse practitioner.”
The 2023 ACC/AHA/SCAI advanced training statement on interventional cardiology defines select competencies for interventional cardiologists who choose to focus their career on peripheral, vascular, or structural heart interventions.
In a recent article in Structural Heart, Dr. Goldsweig and colleagues write, “Training in SHD [structural heart disease] has historically been fragmented and informal. Current modes of SHD training include unaccredited fellowship training, industry-sponsored forums and device-specific training, and training through on-site proctorship.”
Such programs have grown “exponentially,” they write, “despite the conspicuous absence of formalized training requirements.”
In response to the John Steele uproar, the British Cardiovascular Intervention Society posted a statement on its website, noting, “As medicine has changed so there has increasingly been a role for allied health practitioners with advanced skills to take on responsibilities that were previously considered to be the domain of doctors ...
“TAVI procedures however carry a mortality risk, and the responsibility for undertaking a successful TAVI procedure will always lie with a Cardiologist who has had the breadth of training to manage the various complications that may occur during or after a procedure. This requires years of training, and there is no short-cut, or substitute.”
The BCIS promises a statement “later in the year [on] the expected training route for undertaking TAVI and other structural heart procedures.”
Why it matters: Scope creep
Despite the current upheaval, it’s not the first time that a nurse in the United Kingdom has performed a procedure normally performed by a medical doctor. A 3-year-old Reddit post on r/JuniorDoctorsUK points to a 2017 Guardian article titled, “Meet the nurse who will soon perform surgery on patients alone.” Although the “surgical care practitioner” seems to be performing within the scope of her practice, people responding to the post say it’s an example of “mid-level [scope] creep.”
More recently, a Reddit post in the same group points to a congratulatory post for a “nurse-led radial access.” One person commented, “Today they do the access. Tomorrow they do the full diagnostic. Day after they do the pressure wire. Next week they do the PCI [percutaneous coronary intervention].”
Broadly, “scope creep” refers to scope-of-practice expansions, but not turf wars, according to Rebekah Bernard, MD, a family physician in Fort Myers, Fla., who cowrote, “Patients at Risk: The Rise of the Nurse Practitioner and Physician Assistant in Healthcare,” with Niran Al-Agba, MD, a pediatrician in Silverdale, Wash.
The reasons behind U.K. scope creep aren’t clear. Some believe it’s money. Some say the system is broken and that doctors are being exploited.
In relation to the NP-TAVI case, the British Junior Cardiologist Association commented that it reflects a lack of support and advocacy for medical/surgical trainees who need the training opportunities that are going instead to allied health professionals.
In the United States, scope creep is being taken seriously (some may say too seriously) by the American Medical Association. The AMA is lobbying to stop “inappropriate scope expansions,” bolstered by its AMA Scope of Practice Partnership.
Pointing to a scope creep video produced by the AMA, one JuniorDoctorsUK Reddit post asks, “why isn’t the BMA doing anything similar?”
Time for a rethink?
Back to Glenfield Hospital. Not only has Cardiology Glenfield deleted the controversial tweet; it is now is backtracking on its congratulations to ANP Steele, tweeting, “We want to make clear that the lead operator for the procedure was a consultant structural interventionist. However, we are looking into the circumstances, including a review of clinical governance.” From the responses, few clinicians are buying that explanation.
In response to a request for a comment from Glenfield, Andrew Furlong, UHL medical director, reiterated to this news organization through communications manager Gareth Duggan, “We are investigating the circumstances of the procedure with our cardiology team and reviewing our governance processes.”
Dr. Goldsweig participated in a past speaking engagement for Edwards Lifesciences.
A version of this article originally appeared on Medscape.com.