User login
Patchy hair loss on the scalp
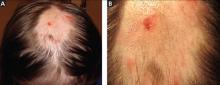
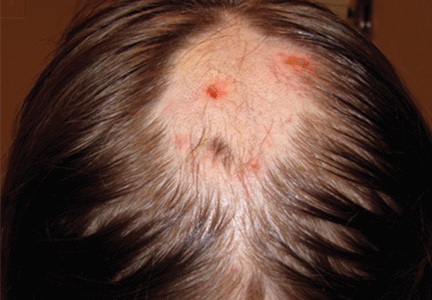
A 12-year-old girl has a large, irregular area of hair loss over the central frontoparietal scalp. Physical examination reveals scattered short hairs of varying lengths and a few small crusts throughout the area of alopecia (Figure 1). The remainder of the scalp appears normal.
Q: Which diagnosis is most likely?
- Alopecia areata
- Lichen planopilaris
- Discoid lupus erythematosus
- Trichotillomania
- Follicular degeneration syndrome
A: The correct answer is trichotillomania, the compulsive pulling out of one’s own hair. Irregularly shaped areas of alopecia containing short hairs of varied lengths and excoriation should raise clinical suspicion of trichotillomania. Biopsy can confirm the diagnosis when follicles devoid of hair shafts, hemorrhage, and misshapen fragments of scalp hair (pigment casts) are seen.
DIAGNOSTIC CLUES
Trichotillomania may present as striking hair loss (alopecia) with an irregular pattern, often with sharp angles or scalloped borders.1 Short and broken hairs within involved areas are typically seen because regenerating hairs are too short to be grasped and pulled out.2 Although hair loss on the scalp may be most evident, hair loss on any hair-bearing area of the body may be noted, including eyebrows and eyelashes.
Family members and the affected individual are often aware of compulsive manipulation of hair.
Depression, anxiety, and other grooming behaviors such as skin-picking and nail-biting may be associated with trichotillomania. Affected individuals often feel a sense of gratification from pulling out hairs. Although systemic complications are rare, some individuals ingest the removed hairs (trichophagy), and the hairs may be caught in the gastric folds and eventually form a trichobezoar.3
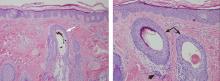
The diagnosis is usually based on clinical findings and by asking the patient about hair-pulling. Asking the patient if the habit is due to the feel of the hair, a need to calm himself or herself, or other factors may be revealing. The majority of cases can be diagnosed without biopsy. Biopsy from affected areas reveals changes related to trauma such as empty hair follicles, hemorrhage, and hair shaft fragments in the dermis2 (Figure 2). The number of catagen follicles is increased. Other causes of patchy alopecia are associated with different findings on biopsy.
Alopecia areata may be associated with an increased number of catagen hairs but is characterized by a peribulbar lymphocytic infiltrate.
Biopsy of lichen planopilaris typically reveals vacuolar changes along the dermal-follicular junction and necrotic keratinocytes.
Cutaneous lupus erythematosus is associated with thickening of the basement membrane zone, increased mucin in the dermis, follicular plugging by keratin, and vacuolar changes along the dermal-epidermal junction.
Biopsy of follicular degeneration syndrome exhibits premature desquamation of the internal root sheath as well as an increased number of fibrous tracts marking the sites of lost hairs.
The etiology of trichotillomania remains largely unknown, and the prognosis varies.4,5 There may be a family history, as there appears to be a genetic component to this disease. The disorder may also occur in the absence of external stressors.5
TREATMENT OPTIONS
Young children often develop trichotillomania that is transient in nature and most often does not require formal intervention. Older children may benefit from psychotherapy.5
Clomipramine (Anafranil) has been shown to be more effective than placebo.6 Selective serotonin reuptake inhibitors are no more effective than placebo.6,7 Pimozide (Orap), haloperidol (Haldol), and other agents have been reported to be of benefit in some instances. Although no large randomized clinical trials in children have been performed, N-acetylcysteine (Acetadote) seems to be a very promising form of therapy in adults.8 A multidisciplinary approach is usually helpful in finding the best treatment option for a particular patient.
- Shah KN, Fried RG. Factitial dermatoses in children. Curr Opin Pediatr 2006; 18:403–409.
- Hautmann G, Hercogova J, Lotti T. Trichotillomania. J Am Acad Dermatol 2002; 46:807–821.
- Lynch KA, Feola PG, Guenther E. Gastric trichobezoar: an important cause of abdominal pain presenting to the pediatric emergency department. Pediatr Emerg Care 2003; 19:343–347.
- Franklin ME, Tolin DF, editors. In: Treating Trichotillomania: Cognitive-Behavioral Therapy for Hairpulling and Related Problems. New York, NY: Springer; 2007.
- Duke DC, Keeley ML, Geffken GR, Storch EA. Trichotillomania: a current review. Clin Psychol Rev 2010; 30:181–193.
- Bloch MH, Landeros-Weisenberger A, Dombrowski P, et al. Systematic review: pharmacological and behavioral treatment for trichotillomania. Biol Psychiatry 2007; 62:839–846.
- Bloch MH. Trichotillomania across the life span. J Am Acad Child Adolesc Psychiatry 2009; 48:879–883.
- Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry 2009; 66:756–763.
A 12-year-old girl has a large, irregular area of hair loss over the central frontoparietal scalp. Physical examination reveals scattered short hairs of varying lengths and a few small crusts throughout the area of alopecia (Figure 1). The remainder of the scalp appears normal.
Q: Which diagnosis is most likely?
- Alopecia areata
- Lichen planopilaris
- Discoid lupus erythematosus
- Trichotillomania
- Follicular degeneration syndrome
A: The correct answer is trichotillomania, the compulsive pulling out of one’s own hair. Irregularly shaped areas of alopecia containing short hairs of varied lengths and excoriation should raise clinical suspicion of trichotillomania. Biopsy can confirm the diagnosis when follicles devoid of hair shafts, hemorrhage, and misshapen fragments of scalp hair (pigment casts) are seen.
DIAGNOSTIC CLUES
Trichotillomania may present as striking hair loss (alopecia) with an irregular pattern, often with sharp angles or scalloped borders.1 Short and broken hairs within involved areas are typically seen because regenerating hairs are too short to be grasped and pulled out.2 Although hair loss on the scalp may be most evident, hair loss on any hair-bearing area of the body may be noted, including eyebrows and eyelashes.
Family members and the affected individual are often aware of compulsive manipulation of hair.
Depression, anxiety, and other grooming behaviors such as skin-picking and nail-biting may be associated with trichotillomania. Affected individuals often feel a sense of gratification from pulling out hairs. Although systemic complications are rare, some individuals ingest the removed hairs (trichophagy), and the hairs may be caught in the gastric folds and eventually form a trichobezoar.3
The diagnosis is usually based on clinical findings and by asking the patient about hair-pulling. Asking the patient if the habit is due to the feel of the hair, a need to calm himself or herself, or other factors may be revealing. The majority of cases can be diagnosed without biopsy. Biopsy from affected areas reveals changes related to trauma such as empty hair follicles, hemorrhage, and hair shaft fragments in the dermis2 (Figure 2). The number of catagen follicles is increased. Other causes of patchy alopecia are associated with different findings on biopsy.
Alopecia areata may be associated with an increased number of catagen hairs but is characterized by a peribulbar lymphocytic infiltrate.
Biopsy of lichen planopilaris typically reveals vacuolar changes along the dermal-follicular junction and necrotic keratinocytes.
Cutaneous lupus erythematosus is associated with thickening of the basement membrane zone, increased mucin in the dermis, follicular plugging by keratin, and vacuolar changes along the dermal-epidermal junction.
Biopsy of follicular degeneration syndrome exhibits premature desquamation of the internal root sheath as well as an increased number of fibrous tracts marking the sites of lost hairs.
The etiology of trichotillomania remains largely unknown, and the prognosis varies.4,5 There may be a family history, as there appears to be a genetic component to this disease. The disorder may also occur in the absence of external stressors.5
TREATMENT OPTIONS
Young children often develop trichotillomania that is transient in nature and most often does not require formal intervention. Older children may benefit from psychotherapy.5
Clomipramine (Anafranil) has been shown to be more effective than placebo.6 Selective serotonin reuptake inhibitors are no more effective than placebo.6,7 Pimozide (Orap), haloperidol (Haldol), and other agents have been reported to be of benefit in some instances. Although no large randomized clinical trials in children have been performed, N-acetylcysteine (Acetadote) seems to be a very promising form of therapy in adults.8 A multidisciplinary approach is usually helpful in finding the best treatment option for a particular patient.
A 12-year-old girl has a large, irregular area of hair loss over the central frontoparietal scalp. Physical examination reveals scattered short hairs of varying lengths and a few small crusts throughout the area of alopecia (Figure 1). The remainder of the scalp appears normal.
Q: Which diagnosis is most likely?
- Alopecia areata
- Lichen planopilaris
- Discoid lupus erythematosus
- Trichotillomania
- Follicular degeneration syndrome
A: The correct answer is trichotillomania, the compulsive pulling out of one’s own hair. Irregularly shaped areas of alopecia containing short hairs of varied lengths and excoriation should raise clinical suspicion of trichotillomania. Biopsy can confirm the diagnosis when follicles devoid of hair shafts, hemorrhage, and misshapen fragments of scalp hair (pigment casts) are seen.
DIAGNOSTIC CLUES
Trichotillomania may present as striking hair loss (alopecia) with an irregular pattern, often with sharp angles or scalloped borders.1 Short and broken hairs within involved areas are typically seen because regenerating hairs are too short to be grasped and pulled out.2 Although hair loss on the scalp may be most evident, hair loss on any hair-bearing area of the body may be noted, including eyebrows and eyelashes.
Family members and the affected individual are often aware of compulsive manipulation of hair.
Depression, anxiety, and other grooming behaviors such as skin-picking and nail-biting may be associated with trichotillomania. Affected individuals often feel a sense of gratification from pulling out hairs. Although systemic complications are rare, some individuals ingest the removed hairs (trichophagy), and the hairs may be caught in the gastric folds and eventually form a trichobezoar.3
The diagnosis is usually based on clinical findings and by asking the patient about hair-pulling. Asking the patient if the habit is due to the feel of the hair, a need to calm himself or herself, or other factors may be revealing. The majority of cases can be diagnosed without biopsy. Biopsy from affected areas reveals changes related to trauma such as empty hair follicles, hemorrhage, and hair shaft fragments in the dermis2 (Figure 2). The number of catagen follicles is increased. Other causes of patchy alopecia are associated with different findings on biopsy.
Alopecia areata may be associated with an increased number of catagen hairs but is characterized by a peribulbar lymphocytic infiltrate.
Biopsy of lichen planopilaris typically reveals vacuolar changes along the dermal-follicular junction and necrotic keratinocytes.
Cutaneous lupus erythematosus is associated with thickening of the basement membrane zone, increased mucin in the dermis, follicular plugging by keratin, and vacuolar changes along the dermal-epidermal junction.
Biopsy of follicular degeneration syndrome exhibits premature desquamation of the internal root sheath as well as an increased number of fibrous tracts marking the sites of lost hairs.
The etiology of trichotillomania remains largely unknown, and the prognosis varies.4,5 There may be a family history, as there appears to be a genetic component to this disease. The disorder may also occur in the absence of external stressors.5
TREATMENT OPTIONS
Young children often develop trichotillomania that is transient in nature and most often does not require formal intervention. Older children may benefit from psychotherapy.5
Clomipramine (Anafranil) has been shown to be more effective than placebo.6 Selective serotonin reuptake inhibitors are no more effective than placebo.6,7 Pimozide (Orap), haloperidol (Haldol), and other agents have been reported to be of benefit in some instances. Although no large randomized clinical trials in children have been performed, N-acetylcysteine (Acetadote) seems to be a very promising form of therapy in adults.8 A multidisciplinary approach is usually helpful in finding the best treatment option for a particular patient.
- Shah KN, Fried RG. Factitial dermatoses in children. Curr Opin Pediatr 2006; 18:403–409.
- Hautmann G, Hercogova J, Lotti T. Trichotillomania. J Am Acad Dermatol 2002; 46:807–821.
- Lynch KA, Feola PG, Guenther E. Gastric trichobezoar: an important cause of abdominal pain presenting to the pediatric emergency department. Pediatr Emerg Care 2003; 19:343–347.
- Franklin ME, Tolin DF, editors. In: Treating Trichotillomania: Cognitive-Behavioral Therapy for Hairpulling and Related Problems. New York, NY: Springer; 2007.
- Duke DC, Keeley ML, Geffken GR, Storch EA. Trichotillomania: a current review. Clin Psychol Rev 2010; 30:181–193.
- Bloch MH, Landeros-Weisenberger A, Dombrowski P, et al. Systematic review: pharmacological and behavioral treatment for trichotillomania. Biol Psychiatry 2007; 62:839–846.
- Bloch MH. Trichotillomania across the life span. J Am Acad Child Adolesc Psychiatry 2009; 48:879–883.
- Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry 2009; 66:756–763.
- Shah KN, Fried RG. Factitial dermatoses in children. Curr Opin Pediatr 2006; 18:403–409.
- Hautmann G, Hercogova J, Lotti T. Trichotillomania. J Am Acad Dermatol 2002; 46:807–821.
- Lynch KA, Feola PG, Guenther E. Gastric trichobezoar: an important cause of abdominal pain presenting to the pediatric emergency department. Pediatr Emerg Care 2003; 19:343–347.
- Franklin ME, Tolin DF, editors. In: Treating Trichotillomania: Cognitive-Behavioral Therapy for Hairpulling and Related Problems. New York, NY: Springer; 2007.
- Duke DC, Keeley ML, Geffken GR, Storch EA. Trichotillomania: a current review. Clin Psychol Rev 2010; 30:181–193.
- Bloch MH, Landeros-Weisenberger A, Dombrowski P, et al. Systematic review: pharmacological and behavioral treatment for trichotillomania. Biol Psychiatry 2007; 62:839–846.
- Bloch MH. Trichotillomania across the life span. J Am Acad Child Adolesc Psychiatry 2009; 48:879–883.
- Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry 2009; 66:756–763.
A farmer with chest pain and lung nodules
A 50-year-old farmer reports having bilateral pleuritic chest pain for the past week. He was treated 25 years ago for brucellosis, with neither clinical nor radiologic lung involvement. He is a 30-pack-year smoker. He lives in a rural area. He reports no other symptoms.
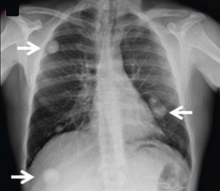
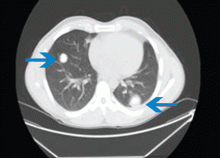
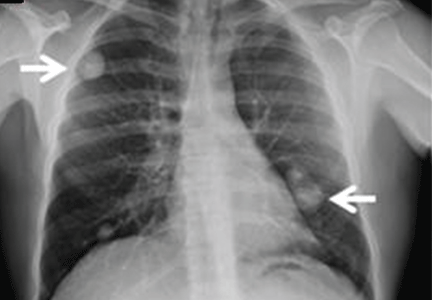
The physical examination is normal except for mild hepatomegaly. Laboratory tests (including transaminases) were normal, with the exception of the C-reactive protein level (7 mg/dL). Tumor markers, beta-2-microglobulin level, serologic tests for atypical bacteria and Brucella organisms, Mantoux test, protein electrophoresis, and tests for autoimmune antibodies were normal or negative. Echocardiography revealed no vegetations. However, chest radiography revealed multiple nodules in both lungs (Figure 1, arrows). Thoracic computed tomography showed well-defined nodules 2 to 3 cm in diameter suggestive of calcified granuloma (Figure 2, arrows).
Q: Which is the most likely diagnosis?
- Pulmonary tuberculosis
- Metastatic lung disease
- Pulmonary brucellosis
- Septic pulmonary emboli
- Lymphoma
A: The most likely diagnosis is pulmonary brucellosis. The patient lives in a rural area where brucellosis is endemic, and his occupation has meant that he also has had decades of daily exposure to farm animals, mainly sheep.
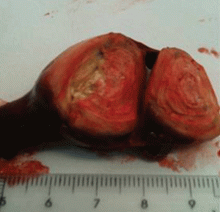
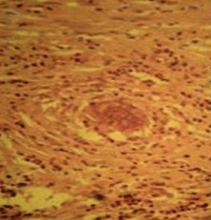
Lung biopsy specimens were obtained by minimally invasive thoracoscopy (Figure 3), and histologic study revealed noncaseating granulomas with central necrosis (Figure 4). Lastly, cultures of the resected nodule were positive for Brucella melitensis.
Once the diagnosis of pulmonary brucellosis was made, the following treatment regimen was started: rifampicin 600 mg daily for 2 months, doxycycline 100 mg twice daily for 2 months, and intramuscular gentamicin 240 mg daily for 2 weeks. The chest pain gradually improved and resolved completely by 1 month after treatment was started; the lung lesions disappeared 8 weeks later. The patient remains disease-free at 6 months.
TYPICAL FEATURES OF BRUCELLOSIS
Brucellosis is a zoonotic disease transmitted to humans not only by ingestion of infected dairy products, but also by direct contact with infected animals or by inhalation of contaminated aerosols. This latter physiopathologic mechanism of acquiring the disease seems to be the most probable when the lungs are involved, 1 and it is common in people such as our patient, whose occupation exposes them to Brucella species.
Although brucellosis can initially present with mild respiratory tract symptoms, true pulmonary involvement (characterized by a more aggressive and prolonged course) is very uncommon, with a reported incidence of 1% to 7%.1,2 Respiratory involvement in brucellosis may appear as part of a systemic illness, as the presenting symptom of the disease, or even as a solitary abnormality on chest radiography.1 Bronchopneumonia, interstitial pneumonia, empyema, pleural effusion, paratracheal lymphadenopathy, and lung nodules have all been reported.2
Reinfection or a late relapse?
In our patient, a question was whether the second episode of brucellosis was a reinfection or a late relapse of the disease. Reinfection seemed the most feasible explanation, supported by his continuous occupational exposure, the properly treated first episode (rifampicin 600 mg daily and doxycycline 100 mg twice daily, both for 45 days), the long symptom-free period, and the fact that most relapses have been reported to occur during the first 6 months after therapy.3 However, late reactivation of an asymptomatic chronic lung infection was also possible, given the ability of Brucella species to survive inside the phagocytic mononuclear cells; brucellosis reactivation has been reported even 28 years after the first episode.4
DIAGNOSTIC CHALLENGES
The diagnosis of brucellosis with laboratory testing is challenging. The organism is difficult to isolate in sputum culture (only one case has been described until now),5 and serologic tests can be falsely negative, although this is rare.6,7 In fact, serologic testing in patients with focal brucellosis may be falsely negative when the serum agglutination test is performed,4,7 as could have occurred in our patient. In several studies, pleural fluid culture has been shown as a good method to isolate Brucella organisms,8 but biopsy is often the only way to establish the diagnosis.6
Complications of lung involvement in brucellosis are seldom severe and, when they appear, usually respond to the same treatment as for uncomplicated brucellosis.2
The combination of respiratory symptoms, epidemiologic risk factors, an endemic setting, and a history of a previous episode all raise clinical suspicion of brucellosis. If clinical suspicion is high, negative results of sputum, serology, or pleural fluid cultures should never rule out the disease; biopsy of the respiratory region affected is warranted.
- Hatipoglu CA, Bilgin G, Tulek N, Kosar U. Pulmonary involvement in brucellosis. J Infect 2005; 51:116–119.
- Pappas G, Bosilkovski M, Akritidis N, Mastora M, Krteva L, Tsianos E. Brucellosis and the respiratory system. Clin Infect Dis 2003; 37:e95–e99.
- Ariza J, Corredoira J, Pallares R, et al. Characteristics of and risk factors for relapse of brucellosis in humans. Clin Infect Dis 1995; 20:1241–1249.
- Ögredici Ö, Erb S, Langer I, et al. Brucellosis reactivation after 28 years. Emerg Infect Dis 2010; 16:2021–2022.
- Gattas N, Loberant N, Rimon D. Miliary and reticulo-nodular pulmonary brucellosis. [in Hebrew]. Harefuah 1998; 135:357–359,407.
- Theegarten D, Albrecht S, Tötsch M, Teschler H, Neubauer H, Al Dahouk S. Brucellosis of the lung: case report and review of the literature. Virchows Arch 2008; 452:97–101.
- Celik AD, Yulugkural Z, Kilincer C, Hamamcioglu MK, Kuloglu F, Akata F. Negative serology: could exclude the diagnosis of brucellosis? Rheumatol Int 2010; Epub ahead of print.
- Kerem E, Diav O, Navon P, Branski D. Pleural fluid characteristics in pulmonary brucellosis. Thorax 1994; 49:89–90.
A 50-year-old farmer reports having bilateral pleuritic chest pain for the past week. He was treated 25 years ago for brucellosis, with neither clinical nor radiologic lung involvement. He is a 30-pack-year smoker. He lives in a rural area. He reports no other symptoms.
The physical examination is normal except for mild hepatomegaly. Laboratory tests (including transaminases) were normal, with the exception of the C-reactive protein level (7 mg/dL). Tumor markers, beta-2-microglobulin level, serologic tests for atypical bacteria and Brucella organisms, Mantoux test, protein electrophoresis, and tests for autoimmune antibodies were normal or negative. Echocardiography revealed no vegetations. However, chest radiography revealed multiple nodules in both lungs (Figure 1, arrows). Thoracic computed tomography showed well-defined nodules 2 to 3 cm in diameter suggestive of calcified granuloma (Figure 2, arrows).
Q: Which is the most likely diagnosis?
- Pulmonary tuberculosis
- Metastatic lung disease
- Pulmonary brucellosis
- Septic pulmonary emboli
- Lymphoma
A: The most likely diagnosis is pulmonary brucellosis. The patient lives in a rural area where brucellosis is endemic, and his occupation has meant that he also has had decades of daily exposure to farm animals, mainly sheep.
Lung biopsy specimens were obtained by minimally invasive thoracoscopy (Figure 3), and histologic study revealed noncaseating granulomas with central necrosis (Figure 4). Lastly, cultures of the resected nodule were positive for Brucella melitensis.
Once the diagnosis of pulmonary brucellosis was made, the following treatment regimen was started: rifampicin 600 mg daily for 2 months, doxycycline 100 mg twice daily for 2 months, and intramuscular gentamicin 240 mg daily for 2 weeks. The chest pain gradually improved and resolved completely by 1 month after treatment was started; the lung lesions disappeared 8 weeks later. The patient remains disease-free at 6 months.
TYPICAL FEATURES OF BRUCELLOSIS
Brucellosis is a zoonotic disease transmitted to humans not only by ingestion of infected dairy products, but also by direct contact with infected animals or by inhalation of contaminated aerosols. This latter physiopathologic mechanism of acquiring the disease seems to be the most probable when the lungs are involved, 1 and it is common in people such as our patient, whose occupation exposes them to Brucella species.
Although brucellosis can initially present with mild respiratory tract symptoms, true pulmonary involvement (characterized by a more aggressive and prolonged course) is very uncommon, with a reported incidence of 1% to 7%.1,2 Respiratory involvement in brucellosis may appear as part of a systemic illness, as the presenting symptom of the disease, or even as a solitary abnormality on chest radiography.1 Bronchopneumonia, interstitial pneumonia, empyema, pleural effusion, paratracheal lymphadenopathy, and lung nodules have all been reported.2
Reinfection or a late relapse?
In our patient, a question was whether the second episode of brucellosis was a reinfection or a late relapse of the disease. Reinfection seemed the most feasible explanation, supported by his continuous occupational exposure, the properly treated first episode (rifampicin 600 mg daily and doxycycline 100 mg twice daily, both for 45 days), the long symptom-free period, and the fact that most relapses have been reported to occur during the first 6 months after therapy.3 However, late reactivation of an asymptomatic chronic lung infection was also possible, given the ability of Brucella species to survive inside the phagocytic mononuclear cells; brucellosis reactivation has been reported even 28 years after the first episode.4
DIAGNOSTIC CHALLENGES
The diagnosis of brucellosis with laboratory testing is challenging. The organism is difficult to isolate in sputum culture (only one case has been described until now),5 and serologic tests can be falsely negative, although this is rare.6,7 In fact, serologic testing in patients with focal brucellosis may be falsely negative when the serum agglutination test is performed,4,7 as could have occurred in our patient. In several studies, pleural fluid culture has been shown as a good method to isolate Brucella organisms,8 but biopsy is often the only way to establish the diagnosis.6
Complications of lung involvement in brucellosis are seldom severe and, when they appear, usually respond to the same treatment as for uncomplicated brucellosis.2
The combination of respiratory symptoms, epidemiologic risk factors, an endemic setting, and a history of a previous episode all raise clinical suspicion of brucellosis. If clinical suspicion is high, negative results of sputum, serology, or pleural fluid cultures should never rule out the disease; biopsy of the respiratory region affected is warranted.
A 50-year-old farmer reports having bilateral pleuritic chest pain for the past week. He was treated 25 years ago for brucellosis, with neither clinical nor radiologic lung involvement. He is a 30-pack-year smoker. He lives in a rural area. He reports no other symptoms.
The physical examination is normal except for mild hepatomegaly. Laboratory tests (including transaminases) were normal, with the exception of the C-reactive protein level (7 mg/dL). Tumor markers, beta-2-microglobulin level, serologic tests for atypical bacteria and Brucella organisms, Mantoux test, protein electrophoresis, and tests for autoimmune antibodies were normal or negative. Echocardiography revealed no vegetations. However, chest radiography revealed multiple nodules in both lungs (Figure 1, arrows). Thoracic computed tomography showed well-defined nodules 2 to 3 cm in diameter suggestive of calcified granuloma (Figure 2, arrows).
Q: Which is the most likely diagnosis?
- Pulmonary tuberculosis
- Metastatic lung disease
- Pulmonary brucellosis
- Septic pulmonary emboli
- Lymphoma
A: The most likely diagnosis is pulmonary brucellosis. The patient lives in a rural area where brucellosis is endemic, and his occupation has meant that he also has had decades of daily exposure to farm animals, mainly sheep.
Lung biopsy specimens were obtained by minimally invasive thoracoscopy (Figure 3), and histologic study revealed noncaseating granulomas with central necrosis (Figure 4). Lastly, cultures of the resected nodule were positive for Brucella melitensis.
Once the diagnosis of pulmonary brucellosis was made, the following treatment regimen was started: rifampicin 600 mg daily for 2 months, doxycycline 100 mg twice daily for 2 months, and intramuscular gentamicin 240 mg daily for 2 weeks. The chest pain gradually improved and resolved completely by 1 month after treatment was started; the lung lesions disappeared 8 weeks later. The patient remains disease-free at 6 months.
TYPICAL FEATURES OF BRUCELLOSIS
Brucellosis is a zoonotic disease transmitted to humans not only by ingestion of infected dairy products, but also by direct contact with infected animals or by inhalation of contaminated aerosols. This latter physiopathologic mechanism of acquiring the disease seems to be the most probable when the lungs are involved, 1 and it is common in people such as our patient, whose occupation exposes them to Brucella species.
Although brucellosis can initially present with mild respiratory tract symptoms, true pulmonary involvement (characterized by a more aggressive and prolonged course) is very uncommon, with a reported incidence of 1% to 7%.1,2 Respiratory involvement in brucellosis may appear as part of a systemic illness, as the presenting symptom of the disease, or even as a solitary abnormality on chest radiography.1 Bronchopneumonia, interstitial pneumonia, empyema, pleural effusion, paratracheal lymphadenopathy, and lung nodules have all been reported.2
Reinfection or a late relapse?
In our patient, a question was whether the second episode of brucellosis was a reinfection or a late relapse of the disease. Reinfection seemed the most feasible explanation, supported by his continuous occupational exposure, the properly treated first episode (rifampicin 600 mg daily and doxycycline 100 mg twice daily, both for 45 days), the long symptom-free period, and the fact that most relapses have been reported to occur during the first 6 months after therapy.3 However, late reactivation of an asymptomatic chronic lung infection was also possible, given the ability of Brucella species to survive inside the phagocytic mononuclear cells; brucellosis reactivation has been reported even 28 years after the first episode.4
DIAGNOSTIC CHALLENGES
The diagnosis of brucellosis with laboratory testing is challenging. The organism is difficult to isolate in sputum culture (only one case has been described until now),5 and serologic tests can be falsely negative, although this is rare.6,7 In fact, serologic testing in patients with focal brucellosis may be falsely negative when the serum agglutination test is performed,4,7 as could have occurred in our patient. In several studies, pleural fluid culture has been shown as a good method to isolate Brucella organisms,8 but biopsy is often the only way to establish the diagnosis.6
Complications of lung involvement in brucellosis are seldom severe and, when they appear, usually respond to the same treatment as for uncomplicated brucellosis.2
The combination of respiratory symptoms, epidemiologic risk factors, an endemic setting, and a history of a previous episode all raise clinical suspicion of brucellosis. If clinical suspicion is high, negative results of sputum, serology, or pleural fluid cultures should never rule out the disease; biopsy of the respiratory region affected is warranted.
- Hatipoglu CA, Bilgin G, Tulek N, Kosar U. Pulmonary involvement in brucellosis. J Infect 2005; 51:116–119.
- Pappas G, Bosilkovski M, Akritidis N, Mastora M, Krteva L, Tsianos E. Brucellosis and the respiratory system. Clin Infect Dis 2003; 37:e95–e99.
- Ariza J, Corredoira J, Pallares R, et al. Characteristics of and risk factors for relapse of brucellosis in humans. Clin Infect Dis 1995; 20:1241–1249.
- Ögredici Ö, Erb S, Langer I, et al. Brucellosis reactivation after 28 years. Emerg Infect Dis 2010; 16:2021–2022.
- Gattas N, Loberant N, Rimon D. Miliary and reticulo-nodular pulmonary brucellosis. [in Hebrew]. Harefuah 1998; 135:357–359,407.
- Theegarten D, Albrecht S, Tötsch M, Teschler H, Neubauer H, Al Dahouk S. Brucellosis of the lung: case report and review of the literature. Virchows Arch 2008; 452:97–101.
- Celik AD, Yulugkural Z, Kilincer C, Hamamcioglu MK, Kuloglu F, Akata F. Negative serology: could exclude the diagnosis of brucellosis? Rheumatol Int 2010; Epub ahead of print.
- Kerem E, Diav O, Navon P, Branski D. Pleural fluid characteristics in pulmonary brucellosis. Thorax 1994; 49:89–90.
- Hatipoglu CA, Bilgin G, Tulek N, Kosar U. Pulmonary involvement in brucellosis. J Infect 2005; 51:116–119.
- Pappas G, Bosilkovski M, Akritidis N, Mastora M, Krteva L, Tsianos E. Brucellosis and the respiratory system. Clin Infect Dis 2003; 37:e95–e99.
- Ariza J, Corredoira J, Pallares R, et al. Characteristics of and risk factors for relapse of brucellosis in humans. Clin Infect Dis 1995; 20:1241–1249.
- Ögredici Ö, Erb S, Langer I, et al. Brucellosis reactivation after 28 years. Emerg Infect Dis 2010; 16:2021–2022.
- Gattas N, Loberant N, Rimon D. Miliary and reticulo-nodular pulmonary brucellosis. [in Hebrew]. Harefuah 1998; 135:357–359,407.
- Theegarten D, Albrecht S, Tötsch M, Teschler H, Neubauer H, Al Dahouk S. Brucellosis of the lung: case report and review of the literature. Virchows Arch 2008; 452:97–101.
- Celik AD, Yulugkural Z, Kilincer C, Hamamcioglu MK, Kuloglu F, Akata F. Negative serology: could exclude the diagnosis of brucellosis? Rheumatol Int 2010; Epub ahead of print.
- Kerem E, Diav O, Navon P, Branski D. Pleural fluid characteristics in pulmonary brucellosis. Thorax 1994; 49:89–90.
POLST: An improvement over traditional advance directives
An 89-year-old woman with advanced dementia is living in a nursing home and is fully dependent in all aspects of personal care, including feeding. She has a health care proxy and a living will.
Her husband is her health care agent and has established that the primary goal of her care should be to keep her comfortable. He has repeatedly discussed this goal with her attending physician and the nursing-home staff and has reiterated that when his wife had capacity, she wanted “no heroics,” “no feeding tube,” and no life-sustaining treatment that would prolong her dying. He has requested that she not be transferred to the hospital and that she receive all further care at the nursing home. These preferences are consistent with her living will.
One evening, she becomes somnolent and febrile, with rapid breathing. The physician covering for the attending physician does not know the patient, cannot reach her husband, and sends her to the hospital, where she is admitted with aspiration pneumonia.
Her level of alertness improves with hydration. However, the hospital nurses have a difficult time feeding her. She does not seem to want to eat, “pockets” food in her cheeks, is slow to swallow, and sometimes coughs during feeding. This is nothing new—at the nursing home, her feeding pattern had been the same for nearly 6 months. During this time she always had a cough; fevers came and went. She has slowly lost weight; she now weighs 100 lb (45 kg), down 30 lb (14 kg) in 3 years.
With treatment, her respiratory distress and fever resolve. The physician orders a swallowing evaluation by a speech therapist, who determines that she needs a feeding tube. After that, a meeting is scheduled with her husband and physician to discuss the speech therapist’s assessment. The patient’s husband emphatically refuses the feeding tube and is upset that she was transferred to the hospital against his expressed wishes.
Why did this happen?
TRADITIONAL ADVANCE DIRECTIVES ARE OFTEN NOT ENOUGH
Even when patients fill out advance directives in accordance with state law, their preferences for care at the end of life are not consistently followed.
Problems with living wills
Living wills state patients’ wishes about medical care in the event that they develop an irreversible condition that prevents them from making their own medical decisions. The living will becomes effective if they become terminally ill, permanently unconscious, or minimally conscious due to brain damage and will never regain the ability to make decisions. People who want to indicate under what set of circumstances they favor or object to receiving any specific treatments use a living will.
The Patient Self-Determination Act of 1990 states that on admission to a hospital or nursing home, patients have to be informed of their rights, including the right to accept or refuse treatment.1 However, the current system of communicating wishes about end-of-life care using solely traditional advance directives such as the living will has proven insufficient. This is because traditional advance directives, being general statements of patients’ preferences, need to be carried out through specifications in medical orders when the need arises.2
Further, traditional advance directives require patients to recognize the importance of advance care planning, understand medical interventions, evaluate their own personal values and beliefs, and communicate their wishes to their agents, loved ones, physicians, and health care providers. Moreover, these documents apply to future circumstances, require further interpretation by the agent and health care professionals, and do not result in actionable medical orders. Decisions about care depend on interpreting earlier conversations, the physician’s estimates of prognosis, and, possibly, the personal convictions of the physician, agent, and loved ones, even though ethically, all involved need to focus on the patient’s stated wishes or best interest. A living will does not help clarify the patient’s wishes in the absence of antecedent conversation with the family, close friends, and the patient’s personal physician. And living wills cannot be read and interpreted in an emergency.
The situation is further complicated by difficulty in defining “terminal” or “irreversible” conditions and accounting for the different perspective that physicians, agents, and loved ones bring to the situation. For example, imagine a patient with dementia nearing the end of life who eats less, has difficulty managing secretions, aspirates, and develops pneumonia. While end-stage dementia is terminal, pneumonia may be reversible.
Increasingly, therefore, people are being counseled to appoint a health care agent (see below).3
The importance of a health care proxy (durable power of attorney for health care)
In a health care proxy document (also known as durable power of attorney for health care), the patient names a health care agent. This person has authority to make decisions about the patient’s medical care, including life-sustaining treatment. In other words, you the patient appoint someone to speak for you in the event you are unable to make your own medical decisions (not only at the end of life).
Since anyone may face a sudden and unexpected acute illness or injury with the risk of becoming incapacitated and unable to make medical decisions, everyone age 18 and older should be encouraged to complete a health care proxy document and to engage in advance care planning discussions with family and loved ones. Physicians can initiate this process as a wellness initiative and can help patients and families understand advance care planning. In all health care settings, trained and qualified health care professionals can provide education on advance care planning to patients, families, and loved ones.
A key issue when naming a health care agent is choosing the right one, someone who will make decisions in accordance with the person’s current values and beliefs and who can separate his or her personal values from the patient’s values. Another key issue: people need to have proactive discussions about their personal values, beliefs, and goals of care, which many are reluctant to do, and the health care agent must be willing to talk about sensitive issues ahead of time. Even when a health care agent is available in an emergency, emergency medical services personnel cannot follow directions from a health care agent. Most importantly, a health care agent must be able to handle potential conflicts between family and providers.
POLST ENSURES PATIENT PREFERENCES ARE HONORED AT THE END OF LIFE
Approximately 20 years ago, a team of health care professionals at the University of Oregon recognized these problems and realized that physicians needed to be more involved in discussions with patients about end-of-life care and in translating the patient’s preferences and values into concrete medical orders. The result was the Physician Orders for Life-Sustaining Treatment (POLST) Paradigm Program.4
What is POLST?
POLST is an end-of-life-care transitions program that focuses on patient-centered goals for care and shared informed medical decision-making.5,6 It offers a mechanism to communicate the wishes of seriously ill patients to have or to limit medical treatment as they move from one care setting to another. Table 1 lists the differences between traditional advance directives and POLST.
The aim is to improve the quality of care that seriously ill patients receive at the end of life. POLST is based on effective communication of the patient’s wishes, with actionable medical orders documented on a brightly colored form (www.ohsu.edu/polst/programs/sample-forms.htm; Figure 1) and a promise by health care professionals to honor these wishes.7 Key features of the program include education, training, and a quality-improvement process.
Who is POLST for?
POLST is for patients with serious life-limiting illness who have a life expectancy of less than 1 year, or anyone of advanced age interested in defining their end-of-life care wishes. Qualified and trained health care professionals (physicians, physician’s assistants, nurse practitioners, and social workers) participate in discussions leading to the completion of a POLST form in all settings, particularly along the long-term care continuum and for home hospice.
The key element of the POLST process: Shared, informed medical decision-making
Health care professionals working as an interdisciplinary team play a key role in educating patients and their families about advance care planning and shared, informed medical decision-making, as well as in resolving conflict. To be effective, shared medical decision-making must be well-informed. The decision-maker (patient, health care agent, or surrogate) must weigh the following questions (Table 2):
- Will treatment make a difference?
- Do the burdens of treatment outweigh its benefits?
- Is there hope of recovery? If so, what will life be like afterward?
- What does the patient value? What is the patient’s goal for his or her care?
In-depth discussions with patients, family members, and surrogates are needed, and these people are often reluctant to ask these questions and afraid to discuss the dying process. Even if they are informed of their diagnosis and prognosis, they may not know what they mean in terms of their everyday experience and future.
Health care professionals engaging in these conversations can use the eight-step POLST protocol (Table 3) to elicit their preferences at the end of life. Table 4 lists tools and resources to enhance the understanding of advance care planning and POLST.
What does the POLST form cover?
The POLST form (Figure 1) provides instructions about resuscitation if the patient has no pulse and is not breathing. Additionally, the medical orders indicate decisions about the level of medical intervention that the patient wants or does not want, eg, intubation, mechanical ventilation, transport to the hospital, intensive care, artificial nutrition and hydration, and antibiotics.
Thus, POLST is outcome-neutral and can be used either to limit medical interventions or to clarify a request for any or all medically indicated treatments.
Both the practitioner and the patient or patient’s surrogate sign the form. The original goes into the patient’s chart, and a copy should accompany the patient if he or she is transferred or discharged. Additionally, if the state has a POLST registry, the POLST information should be entered into the registry.
POLST is expanding across the country
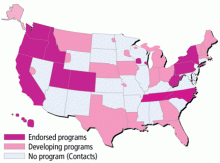
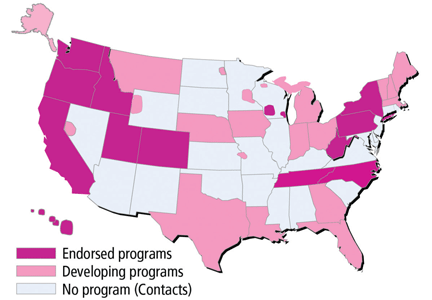
The use of POLST has been expanding across the United States, with POLST programs now implemented in all or part of at least 30 states. There are endorsed programs in 14 states, and programs are being developed in 26 more. Requirements for endorsement are found at www.polst.org. Figure 2 shows the status of POLST in the 50 states.
Oregon’s POLST form is the original model for other forms designed to meet specific legislative or regulatory requirements in other states. POLST-like programs are known by different names in different states: eg, New York’s Medical Orders for Life-Sustaining Treatment (MOLST) and West Virginia’s Physicians Orders for Scope of Treatment (POST), but all endorsed programs share common core elements.
POLST research
A number of studies in the past 10 years have shown that POLST improves the documentation and honoring of patient preferences, whatever they may be.4,8–16
Emergency medical technicians in Oregon reported that the POLST form provides clear instructions about patient preferences and is useful when deciding which treatments to provide. In contrast to the single-intervention focus of out-of-hospital do-not-resuscitate orders, the POLST form provides patients the opportunity to document treatment goals and preferences for interventions across a range of treatment options, thus permitting greater individualization.13
Comfort care is not sacrificed if a POLST document is in place. Most hospice patients choose at least one life-sustaining treatment on their POLST form.14
In a multistate study published in 2010, the medical records of residents in 90 randomly chosen Medicaid-eligible nursing homes were reviewed.15 POLST was compared with traditional advance care planning in terms of the effect on the presence of medical orders reflecting treatment preferences, symptom management, and use of life-sustaining treatments. The study found that residents with POLST forms had significantly more medical orders about life-sustaining treatments than residents with traditional advance directives. There were no differences between residents with or without POLST forms on symptom assessment or management measures. POLST was more effective than traditional advance planning at limiting unwanted life-sustaining treatments. The study suggests that POLST offers significant advantages over traditional advance directives in nursing facilities.15,16
In summary, more than a decade of research has shown that the POLST Paradigm Program serves as an emerging national model for implementing shared, informed medical decision-making. Furthermore, POLST more accurately conveys end-of-life care preferences for patients with advanced chronic illness and for dying patients than traditional advance directives and yields higher adherence by medical professionals.
CLINICAL CASE REVISITED
Let’s consider if the physician for our 89-year-old woman with dementia had completed a POLST form with orders indicating “do not attempt resuscitation (DNR/no CPR)” and “comfort measures only, do not transfer to hospital for life-sustaining treatment and transfer if comfort needs cannot be met in current location.”
The patient’s respiratory distress and fever would have been treated at her nursing home with medication and oxygen. She would have been transferred to the hospital only if her comfort needs would not have been met at the nursing home. Unwanted life-sustaining treatment would have been avoided. The wishes of the patient, based on her values and careful consideration of options, would have been respected.
- Dunn PM, Tolle SW, Moss AH, Black JS. The POLST paradigm: respecting the wishes of patients and families. Ann Long-Term Care 2007; 15:33–40.
- Patient Self-Determination Act of 1990. Pub. L. No. 101-508, ss 4206, 104 Stat. 1388.
- Bomba PA, Sabatino CP. POLST: an emerging model for end-of-life care planning. The ElderLaw Report 2009; 20:1–5.
- Karp Sabatino C. AARP Public Policy Institute, Improving advance illness care: the evolution of state POLST programs 2011. http://assets.aarp.org/rgcenter/ppi/cons-prot/POLST-Report-04-11.pdf. Accessed May 30, 2012.
- Bomba PA. Discussing patient p and end of life care, Journal of the Monroe County Medical Society, 7th District Branch, MSSNY 2011;12–15. www.compassionandsupport.org/index.php/research_/. Accessed May 30, 2012.
- Citko J, Moss AH, Carley M, Tolle SW. The National POLST Paradigm Initiative, 2ND ed. Fast Facts and Concepts 2010;178. www.eperc.mcw.edu/fastfact/ff_178.htm. Accessed May 30, 2012.
- Center for Ethics in Health Care, Oregon Health & Science University. www.ohsu.edu/polst/. Accessed May 30, 2012.
- Lee MA, Brummel-Smith K, Meyer J, Drew N, London MR. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. Program of All-Inclusive Care for the Elderly. J Am Geriatr Soc 2000; 48:1219–1225.
- Meyers JL, Moore C, McGrory A, Sparr J, Ahern M. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Gerontol Nurs 2004; 30:37–46.
- Dunn PM, Schmidt TA, Carley MM, Donius M, Weinstein MA, Dull VT. A method to communicate patient p about medically indicated life-sustaining treatment in the out-of-hospital setting. J Am Geriatr Soc 1996; 44:785–791.
- Cantor MD. Improving advance care planning: lessons from POLST. Physician Orders for Life-Sustaining Treatment (comment). J Am Geriatr Soc 2000; 48:1343–1344.
- Tolle SW, Tilden VP, Nelson CA, Dunn PM. A prospective study of the efficacy of the physician order form for life-sustaining treatment. J Am Geriatr Soc 1998; 46:1097–1102.
- Schmidt TA, Hickman SE, Tolle SW, Brooks HS. The Physician Orders for Life-Sustaining Treatment program: Oregon emergency medical technicians’ practical experiences and attitudes. J Am Geriatr Soc 2004; 52:1430–1434.
- Hickman SE, Nelson CA, Moss AH, et al. Use of the Physician Orders for Life-Sustaining Treatment (POLST) paradigm program in the hospice setting. J Palliat Med 2009; 12:133–141.
- Hickman SE, Nelson CA, Perrin NA, Moss AH, Hammes BJ, Tolle SW. A comparison of methods to communicate treatment p in nursing facilities: traditional practices versus the physician orders for life-sustaining treatment program. J Am Geriatr Soc 2010; 58:1241–1248.
- Hickman SE, Nelson CA, Moss AH, Tolle SW, Perrin NA, Hammes BJ. The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc 2011; 59:2091–2099.
An 89-year-old woman with advanced dementia is living in a nursing home and is fully dependent in all aspects of personal care, including feeding. She has a health care proxy and a living will.
Her husband is her health care agent and has established that the primary goal of her care should be to keep her comfortable. He has repeatedly discussed this goal with her attending physician and the nursing-home staff and has reiterated that when his wife had capacity, she wanted “no heroics,” “no feeding tube,” and no life-sustaining treatment that would prolong her dying. He has requested that she not be transferred to the hospital and that she receive all further care at the nursing home. These preferences are consistent with her living will.
One evening, she becomes somnolent and febrile, with rapid breathing. The physician covering for the attending physician does not know the patient, cannot reach her husband, and sends her to the hospital, where she is admitted with aspiration pneumonia.
Her level of alertness improves with hydration. However, the hospital nurses have a difficult time feeding her. She does not seem to want to eat, “pockets” food in her cheeks, is slow to swallow, and sometimes coughs during feeding. This is nothing new—at the nursing home, her feeding pattern had been the same for nearly 6 months. During this time she always had a cough; fevers came and went. She has slowly lost weight; she now weighs 100 lb (45 kg), down 30 lb (14 kg) in 3 years.
With treatment, her respiratory distress and fever resolve. The physician orders a swallowing evaluation by a speech therapist, who determines that she needs a feeding tube. After that, a meeting is scheduled with her husband and physician to discuss the speech therapist’s assessment. The patient’s husband emphatically refuses the feeding tube and is upset that she was transferred to the hospital against his expressed wishes.
Why did this happen?
TRADITIONAL ADVANCE DIRECTIVES ARE OFTEN NOT ENOUGH
Even when patients fill out advance directives in accordance with state law, their preferences for care at the end of life are not consistently followed.
Problems with living wills
Living wills state patients’ wishes about medical care in the event that they develop an irreversible condition that prevents them from making their own medical decisions. The living will becomes effective if they become terminally ill, permanently unconscious, or minimally conscious due to brain damage and will never regain the ability to make decisions. People who want to indicate under what set of circumstances they favor or object to receiving any specific treatments use a living will.
The Patient Self-Determination Act of 1990 states that on admission to a hospital or nursing home, patients have to be informed of their rights, including the right to accept or refuse treatment.1 However, the current system of communicating wishes about end-of-life care using solely traditional advance directives such as the living will has proven insufficient. This is because traditional advance directives, being general statements of patients’ preferences, need to be carried out through specifications in medical orders when the need arises.2
Further, traditional advance directives require patients to recognize the importance of advance care planning, understand medical interventions, evaluate their own personal values and beliefs, and communicate their wishes to their agents, loved ones, physicians, and health care providers. Moreover, these documents apply to future circumstances, require further interpretation by the agent and health care professionals, and do not result in actionable medical orders. Decisions about care depend on interpreting earlier conversations, the physician’s estimates of prognosis, and, possibly, the personal convictions of the physician, agent, and loved ones, even though ethically, all involved need to focus on the patient’s stated wishes or best interest. A living will does not help clarify the patient’s wishes in the absence of antecedent conversation with the family, close friends, and the patient’s personal physician. And living wills cannot be read and interpreted in an emergency.
The situation is further complicated by difficulty in defining “terminal” or “irreversible” conditions and accounting for the different perspective that physicians, agents, and loved ones bring to the situation. For example, imagine a patient with dementia nearing the end of life who eats less, has difficulty managing secretions, aspirates, and develops pneumonia. While end-stage dementia is terminal, pneumonia may be reversible.
Increasingly, therefore, people are being counseled to appoint a health care agent (see below).3
The importance of a health care proxy (durable power of attorney for health care)
In a health care proxy document (also known as durable power of attorney for health care), the patient names a health care agent. This person has authority to make decisions about the patient’s medical care, including life-sustaining treatment. In other words, you the patient appoint someone to speak for you in the event you are unable to make your own medical decisions (not only at the end of life).
Since anyone may face a sudden and unexpected acute illness or injury with the risk of becoming incapacitated and unable to make medical decisions, everyone age 18 and older should be encouraged to complete a health care proxy document and to engage in advance care planning discussions with family and loved ones. Physicians can initiate this process as a wellness initiative and can help patients and families understand advance care planning. In all health care settings, trained and qualified health care professionals can provide education on advance care planning to patients, families, and loved ones.
A key issue when naming a health care agent is choosing the right one, someone who will make decisions in accordance with the person’s current values and beliefs and who can separate his or her personal values from the patient’s values. Another key issue: people need to have proactive discussions about their personal values, beliefs, and goals of care, which many are reluctant to do, and the health care agent must be willing to talk about sensitive issues ahead of time. Even when a health care agent is available in an emergency, emergency medical services personnel cannot follow directions from a health care agent. Most importantly, a health care agent must be able to handle potential conflicts between family and providers.
POLST ENSURES PATIENT PREFERENCES ARE HONORED AT THE END OF LIFE
Approximately 20 years ago, a team of health care professionals at the University of Oregon recognized these problems and realized that physicians needed to be more involved in discussions with patients about end-of-life care and in translating the patient’s preferences and values into concrete medical orders. The result was the Physician Orders for Life-Sustaining Treatment (POLST) Paradigm Program.4
What is POLST?
POLST is an end-of-life-care transitions program that focuses on patient-centered goals for care and shared informed medical decision-making.5,6 It offers a mechanism to communicate the wishes of seriously ill patients to have or to limit medical treatment as they move from one care setting to another. Table 1 lists the differences between traditional advance directives and POLST.
The aim is to improve the quality of care that seriously ill patients receive at the end of life. POLST is based on effective communication of the patient’s wishes, with actionable medical orders documented on a brightly colored form (www.ohsu.edu/polst/programs/sample-forms.htm; Figure 1) and a promise by health care professionals to honor these wishes.7 Key features of the program include education, training, and a quality-improvement process.
Who is POLST for?
POLST is for patients with serious life-limiting illness who have a life expectancy of less than 1 year, or anyone of advanced age interested in defining their end-of-life care wishes. Qualified and trained health care professionals (physicians, physician’s assistants, nurse practitioners, and social workers) participate in discussions leading to the completion of a POLST form in all settings, particularly along the long-term care continuum and for home hospice.
The key element of the POLST process: Shared, informed medical decision-making
Health care professionals working as an interdisciplinary team play a key role in educating patients and their families about advance care planning and shared, informed medical decision-making, as well as in resolving conflict. To be effective, shared medical decision-making must be well-informed. The decision-maker (patient, health care agent, or surrogate) must weigh the following questions (Table 2):
- Will treatment make a difference?
- Do the burdens of treatment outweigh its benefits?
- Is there hope of recovery? If so, what will life be like afterward?
- What does the patient value? What is the patient’s goal for his or her care?
In-depth discussions with patients, family members, and surrogates are needed, and these people are often reluctant to ask these questions and afraid to discuss the dying process. Even if they are informed of their diagnosis and prognosis, they may not know what they mean in terms of their everyday experience and future.
Health care professionals engaging in these conversations can use the eight-step POLST protocol (Table 3) to elicit their preferences at the end of life. Table 4 lists tools and resources to enhance the understanding of advance care planning and POLST.
What does the POLST form cover?
The POLST form (Figure 1) provides instructions about resuscitation if the patient has no pulse and is not breathing. Additionally, the medical orders indicate decisions about the level of medical intervention that the patient wants or does not want, eg, intubation, mechanical ventilation, transport to the hospital, intensive care, artificial nutrition and hydration, and antibiotics.
Thus, POLST is outcome-neutral and can be used either to limit medical interventions or to clarify a request for any or all medically indicated treatments.
Both the practitioner and the patient or patient’s surrogate sign the form. The original goes into the patient’s chart, and a copy should accompany the patient if he or she is transferred or discharged. Additionally, if the state has a POLST registry, the POLST information should be entered into the registry.
POLST is expanding across the country
The use of POLST has been expanding across the United States, with POLST programs now implemented in all or part of at least 30 states. There are endorsed programs in 14 states, and programs are being developed in 26 more. Requirements for endorsement are found at www.polst.org. Figure 2 shows the status of POLST in the 50 states.
Oregon’s POLST form is the original model for other forms designed to meet specific legislative or regulatory requirements in other states. POLST-like programs are known by different names in different states: eg, New York’s Medical Orders for Life-Sustaining Treatment (MOLST) and West Virginia’s Physicians Orders for Scope of Treatment (POST), but all endorsed programs share common core elements.
POLST research
A number of studies in the past 10 years have shown that POLST improves the documentation and honoring of patient preferences, whatever they may be.4,8–16
Emergency medical technicians in Oregon reported that the POLST form provides clear instructions about patient preferences and is useful when deciding which treatments to provide. In contrast to the single-intervention focus of out-of-hospital do-not-resuscitate orders, the POLST form provides patients the opportunity to document treatment goals and preferences for interventions across a range of treatment options, thus permitting greater individualization.13
Comfort care is not sacrificed if a POLST document is in place. Most hospice patients choose at least one life-sustaining treatment on their POLST form.14
In a multistate study published in 2010, the medical records of residents in 90 randomly chosen Medicaid-eligible nursing homes were reviewed.15 POLST was compared with traditional advance care planning in terms of the effect on the presence of medical orders reflecting treatment preferences, symptom management, and use of life-sustaining treatments. The study found that residents with POLST forms had significantly more medical orders about life-sustaining treatments than residents with traditional advance directives. There were no differences between residents with or without POLST forms on symptom assessment or management measures. POLST was more effective than traditional advance planning at limiting unwanted life-sustaining treatments. The study suggests that POLST offers significant advantages over traditional advance directives in nursing facilities.15,16
In summary, more than a decade of research has shown that the POLST Paradigm Program serves as an emerging national model for implementing shared, informed medical decision-making. Furthermore, POLST more accurately conveys end-of-life care preferences for patients with advanced chronic illness and for dying patients than traditional advance directives and yields higher adherence by medical professionals.
CLINICAL CASE REVISITED
Let’s consider if the physician for our 89-year-old woman with dementia had completed a POLST form with orders indicating “do not attempt resuscitation (DNR/no CPR)” and “comfort measures only, do not transfer to hospital for life-sustaining treatment and transfer if comfort needs cannot be met in current location.”
The patient’s respiratory distress and fever would have been treated at her nursing home with medication and oxygen. She would have been transferred to the hospital only if her comfort needs would not have been met at the nursing home. Unwanted life-sustaining treatment would have been avoided. The wishes of the patient, based on her values and careful consideration of options, would have been respected.
An 89-year-old woman with advanced dementia is living in a nursing home and is fully dependent in all aspects of personal care, including feeding. She has a health care proxy and a living will.
Her husband is her health care agent and has established that the primary goal of her care should be to keep her comfortable. He has repeatedly discussed this goal with her attending physician and the nursing-home staff and has reiterated that when his wife had capacity, she wanted “no heroics,” “no feeding tube,” and no life-sustaining treatment that would prolong her dying. He has requested that she not be transferred to the hospital and that she receive all further care at the nursing home. These preferences are consistent with her living will.
One evening, she becomes somnolent and febrile, with rapid breathing. The physician covering for the attending physician does not know the patient, cannot reach her husband, and sends her to the hospital, where she is admitted with aspiration pneumonia.
Her level of alertness improves with hydration. However, the hospital nurses have a difficult time feeding her. She does not seem to want to eat, “pockets” food in her cheeks, is slow to swallow, and sometimes coughs during feeding. This is nothing new—at the nursing home, her feeding pattern had been the same for nearly 6 months. During this time she always had a cough; fevers came and went. She has slowly lost weight; she now weighs 100 lb (45 kg), down 30 lb (14 kg) in 3 years.
With treatment, her respiratory distress and fever resolve. The physician orders a swallowing evaluation by a speech therapist, who determines that she needs a feeding tube. After that, a meeting is scheduled with her husband and physician to discuss the speech therapist’s assessment. The patient’s husband emphatically refuses the feeding tube and is upset that she was transferred to the hospital against his expressed wishes.
Why did this happen?
TRADITIONAL ADVANCE DIRECTIVES ARE OFTEN NOT ENOUGH
Even when patients fill out advance directives in accordance with state law, their preferences for care at the end of life are not consistently followed.
Problems with living wills
Living wills state patients’ wishes about medical care in the event that they develop an irreversible condition that prevents them from making their own medical decisions. The living will becomes effective if they become terminally ill, permanently unconscious, or minimally conscious due to brain damage and will never regain the ability to make decisions. People who want to indicate under what set of circumstances they favor or object to receiving any specific treatments use a living will.
The Patient Self-Determination Act of 1990 states that on admission to a hospital or nursing home, patients have to be informed of their rights, including the right to accept or refuse treatment.1 However, the current system of communicating wishes about end-of-life care using solely traditional advance directives such as the living will has proven insufficient. This is because traditional advance directives, being general statements of patients’ preferences, need to be carried out through specifications in medical orders when the need arises.2
Further, traditional advance directives require patients to recognize the importance of advance care planning, understand medical interventions, evaluate their own personal values and beliefs, and communicate their wishes to their agents, loved ones, physicians, and health care providers. Moreover, these documents apply to future circumstances, require further interpretation by the agent and health care professionals, and do not result in actionable medical orders. Decisions about care depend on interpreting earlier conversations, the physician’s estimates of prognosis, and, possibly, the personal convictions of the physician, agent, and loved ones, even though ethically, all involved need to focus on the patient’s stated wishes or best interest. A living will does not help clarify the patient’s wishes in the absence of antecedent conversation with the family, close friends, and the patient’s personal physician. And living wills cannot be read and interpreted in an emergency.
The situation is further complicated by difficulty in defining “terminal” or “irreversible” conditions and accounting for the different perspective that physicians, agents, and loved ones bring to the situation. For example, imagine a patient with dementia nearing the end of life who eats less, has difficulty managing secretions, aspirates, and develops pneumonia. While end-stage dementia is terminal, pneumonia may be reversible.
Increasingly, therefore, people are being counseled to appoint a health care agent (see below).3
The importance of a health care proxy (durable power of attorney for health care)
In a health care proxy document (also known as durable power of attorney for health care), the patient names a health care agent. This person has authority to make decisions about the patient’s medical care, including life-sustaining treatment. In other words, you the patient appoint someone to speak for you in the event you are unable to make your own medical decisions (not only at the end of life).
Since anyone may face a sudden and unexpected acute illness or injury with the risk of becoming incapacitated and unable to make medical decisions, everyone age 18 and older should be encouraged to complete a health care proxy document and to engage in advance care planning discussions with family and loved ones. Physicians can initiate this process as a wellness initiative and can help patients and families understand advance care planning. In all health care settings, trained and qualified health care professionals can provide education on advance care planning to patients, families, and loved ones.
A key issue when naming a health care agent is choosing the right one, someone who will make decisions in accordance with the person’s current values and beliefs and who can separate his or her personal values from the patient’s values. Another key issue: people need to have proactive discussions about their personal values, beliefs, and goals of care, which many are reluctant to do, and the health care agent must be willing to talk about sensitive issues ahead of time. Even when a health care agent is available in an emergency, emergency medical services personnel cannot follow directions from a health care agent. Most importantly, a health care agent must be able to handle potential conflicts between family and providers.
POLST ENSURES PATIENT PREFERENCES ARE HONORED AT THE END OF LIFE
Approximately 20 years ago, a team of health care professionals at the University of Oregon recognized these problems and realized that physicians needed to be more involved in discussions with patients about end-of-life care and in translating the patient’s preferences and values into concrete medical orders. The result was the Physician Orders for Life-Sustaining Treatment (POLST) Paradigm Program.4
What is POLST?
POLST is an end-of-life-care transitions program that focuses on patient-centered goals for care and shared informed medical decision-making.5,6 It offers a mechanism to communicate the wishes of seriously ill patients to have or to limit medical treatment as they move from one care setting to another. Table 1 lists the differences between traditional advance directives and POLST.
The aim is to improve the quality of care that seriously ill patients receive at the end of life. POLST is based on effective communication of the patient’s wishes, with actionable medical orders documented on a brightly colored form (www.ohsu.edu/polst/programs/sample-forms.htm; Figure 1) and a promise by health care professionals to honor these wishes.7 Key features of the program include education, training, and a quality-improvement process.
Who is POLST for?
POLST is for patients with serious life-limiting illness who have a life expectancy of less than 1 year, or anyone of advanced age interested in defining their end-of-life care wishes. Qualified and trained health care professionals (physicians, physician’s assistants, nurse practitioners, and social workers) participate in discussions leading to the completion of a POLST form in all settings, particularly along the long-term care continuum and for home hospice.
The key element of the POLST process: Shared, informed medical decision-making
Health care professionals working as an interdisciplinary team play a key role in educating patients and their families about advance care planning and shared, informed medical decision-making, as well as in resolving conflict. To be effective, shared medical decision-making must be well-informed. The decision-maker (patient, health care agent, or surrogate) must weigh the following questions (Table 2):
- Will treatment make a difference?
- Do the burdens of treatment outweigh its benefits?
- Is there hope of recovery? If so, what will life be like afterward?
- What does the patient value? What is the patient’s goal for his or her care?
In-depth discussions with patients, family members, and surrogates are needed, and these people are often reluctant to ask these questions and afraid to discuss the dying process. Even if they are informed of their diagnosis and prognosis, they may not know what they mean in terms of their everyday experience and future.
Health care professionals engaging in these conversations can use the eight-step POLST protocol (Table 3) to elicit their preferences at the end of life. Table 4 lists tools and resources to enhance the understanding of advance care planning and POLST.
What does the POLST form cover?
The POLST form (Figure 1) provides instructions about resuscitation if the patient has no pulse and is not breathing. Additionally, the medical orders indicate decisions about the level of medical intervention that the patient wants or does not want, eg, intubation, mechanical ventilation, transport to the hospital, intensive care, artificial nutrition and hydration, and antibiotics.
Thus, POLST is outcome-neutral and can be used either to limit medical interventions or to clarify a request for any or all medically indicated treatments.
Both the practitioner and the patient or patient’s surrogate sign the form. The original goes into the patient’s chart, and a copy should accompany the patient if he or she is transferred or discharged. Additionally, if the state has a POLST registry, the POLST information should be entered into the registry.
POLST is expanding across the country
The use of POLST has been expanding across the United States, with POLST programs now implemented in all or part of at least 30 states. There are endorsed programs in 14 states, and programs are being developed in 26 more. Requirements for endorsement are found at www.polst.org. Figure 2 shows the status of POLST in the 50 states.
Oregon’s POLST form is the original model for other forms designed to meet specific legislative or regulatory requirements in other states. POLST-like programs are known by different names in different states: eg, New York’s Medical Orders for Life-Sustaining Treatment (MOLST) and West Virginia’s Physicians Orders for Scope of Treatment (POST), but all endorsed programs share common core elements.
POLST research
A number of studies in the past 10 years have shown that POLST improves the documentation and honoring of patient preferences, whatever they may be.4,8–16
Emergency medical technicians in Oregon reported that the POLST form provides clear instructions about patient preferences and is useful when deciding which treatments to provide. In contrast to the single-intervention focus of out-of-hospital do-not-resuscitate orders, the POLST form provides patients the opportunity to document treatment goals and preferences for interventions across a range of treatment options, thus permitting greater individualization.13
Comfort care is not sacrificed if a POLST document is in place. Most hospice patients choose at least one life-sustaining treatment on their POLST form.14
In a multistate study published in 2010, the medical records of residents in 90 randomly chosen Medicaid-eligible nursing homes were reviewed.15 POLST was compared with traditional advance care planning in terms of the effect on the presence of medical orders reflecting treatment preferences, symptom management, and use of life-sustaining treatments. The study found that residents with POLST forms had significantly more medical orders about life-sustaining treatments than residents with traditional advance directives. There were no differences between residents with or without POLST forms on symptom assessment or management measures. POLST was more effective than traditional advance planning at limiting unwanted life-sustaining treatments. The study suggests that POLST offers significant advantages over traditional advance directives in nursing facilities.15,16
In summary, more than a decade of research has shown that the POLST Paradigm Program serves as an emerging national model for implementing shared, informed medical decision-making. Furthermore, POLST more accurately conveys end-of-life care preferences for patients with advanced chronic illness and for dying patients than traditional advance directives and yields higher adherence by medical professionals.
CLINICAL CASE REVISITED
Let’s consider if the physician for our 89-year-old woman with dementia had completed a POLST form with orders indicating “do not attempt resuscitation (DNR/no CPR)” and “comfort measures only, do not transfer to hospital for life-sustaining treatment and transfer if comfort needs cannot be met in current location.”
The patient’s respiratory distress and fever would have been treated at her nursing home with medication and oxygen. She would have been transferred to the hospital only if her comfort needs would not have been met at the nursing home. Unwanted life-sustaining treatment would have been avoided. The wishes of the patient, based on her values and careful consideration of options, would have been respected.
- Dunn PM, Tolle SW, Moss AH, Black JS. The POLST paradigm: respecting the wishes of patients and families. Ann Long-Term Care 2007; 15:33–40.
- Patient Self-Determination Act of 1990. Pub. L. No. 101-508, ss 4206, 104 Stat. 1388.
- Bomba PA, Sabatino CP. POLST: an emerging model for end-of-life care planning. The ElderLaw Report 2009; 20:1–5.
- Karp Sabatino C. AARP Public Policy Institute, Improving advance illness care: the evolution of state POLST programs 2011. http://assets.aarp.org/rgcenter/ppi/cons-prot/POLST-Report-04-11.pdf. Accessed May 30, 2012.
- Bomba PA. Discussing patient p and end of life care, Journal of the Monroe County Medical Society, 7th District Branch, MSSNY 2011;12–15. www.compassionandsupport.org/index.php/research_/. Accessed May 30, 2012.
- Citko J, Moss AH, Carley M, Tolle SW. The National POLST Paradigm Initiative, 2ND ed. Fast Facts and Concepts 2010;178. www.eperc.mcw.edu/fastfact/ff_178.htm. Accessed May 30, 2012.
- Center for Ethics in Health Care, Oregon Health & Science University. www.ohsu.edu/polst/. Accessed May 30, 2012.
- Lee MA, Brummel-Smith K, Meyer J, Drew N, London MR. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. Program of All-Inclusive Care for the Elderly. J Am Geriatr Soc 2000; 48:1219–1225.
- Meyers JL, Moore C, McGrory A, Sparr J, Ahern M. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Gerontol Nurs 2004; 30:37–46.
- Dunn PM, Schmidt TA, Carley MM, Donius M, Weinstein MA, Dull VT. A method to communicate patient p about medically indicated life-sustaining treatment in the out-of-hospital setting. J Am Geriatr Soc 1996; 44:785–791.
- Cantor MD. Improving advance care planning: lessons from POLST. Physician Orders for Life-Sustaining Treatment (comment). J Am Geriatr Soc 2000; 48:1343–1344.
- Tolle SW, Tilden VP, Nelson CA, Dunn PM. A prospective study of the efficacy of the physician order form for life-sustaining treatment. J Am Geriatr Soc 1998; 46:1097–1102.
- Schmidt TA, Hickman SE, Tolle SW, Brooks HS. The Physician Orders for Life-Sustaining Treatment program: Oregon emergency medical technicians’ practical experiences and attitudes. J Am Geriatr Soc 2004; 52:1430–1434.
- Hickman SE, Nelson CA, Moss AH, et al. Use of the Physician Orders for Life-Sustaining Treatment (POLST) paradigm program in the hospice setting. J Palliat Med 2009; 12:133–141.
- Hickman SE, Nelson CA, Perrin NA, Moss AH, Hammes BJ, Tolle SW. A comparison of methods to communicate treatment p in nursing facilities: traditional practices versus the physician orders for life-sustaining treatment program. J Am Geriatr Soc 2010; 58:1241–1248.
- Hickman SE, Nelson CA, Moss AH, Tolle SW, Perrin NA, Hammes BJ. The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc 2011; 59:2091–2099.
- Dunn PM, Tolle SW, Moss AH, Black JS. The POLST paradigm: respecting the wishes of patients and families. Ann Long-Term Care 2007; 15:33–40.
- Patient Self-Determination Act of 1990. Pub. L. No. 101-508, ss 4206, 104 Stat. 1388.
- Bomba PA, Sabatino CP. POLST: an emerging model for end-of-life care planning. The ElderLaw Report 2009; 20:1–5.
- Karp Sabatino C. AARP Public Policy Institute, Improving advance illness care: the evolution of state POLST programs 2011. http://assets.aarp.org/rgcenter/ppi/cons-prot/POLST-Report-04-11.pdf. Accessed May 30, 2012.
- Bomba PA. Discussing patient p and end of life care, Journal of the Monroe County Medical Society, 7th District Branch, MSSNY 2011;12–15. www.compassionandsupport.org/index.php/research_/. Accessed May 30, 2012.
- Citko J, Moss AH, Carley M, Tolle SW. The National POLST Paradigm Initiative, 2ND ed. Fast Facts and Concepts 2010;178. www.eperc.mcw.edu/fastfact/ff_178.htm. Accessed May 30, 2012.
- Center for Ethics in Health Care, Oregon Health & Science University. www.ohsu.edu/polst/. Accessed May 30, 2012.
- Lee MA, Brummel-Smith K, Meyer J, Drew N, London MR. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. Program of All-Inclusive Care for the Elderly. J Am Geriatr Soc 2000; 48:1219–1225.
- Meyers JL, Moore C, McGrory A, Sparr J, Ahern M. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Gerontol Nurs 2004; 30:37–46.
- Dunn PM, Schmidt TA, Carley MM, Donius M, Weinstein MA, Dull VT. A method to communicate patient p about medically indicated life-sustaining treatment in the out-of-hospital setting. J Am Geriatr Soc 1996; 44:785–791.
- Cantor MD. Improving advance care planning: lessons from POLST. Physician Orders for Life-Sustaining Treatment (comment). J Am Geriatr Soc 2000; 48:1343–1344.
- Tolle SW, Tilden VP, Nelson CA, Dunn PM. A prospective study of the efficacy of the physician order form for life-sustaining treatment. J Am Geriatr Soc 1998; 46:1097–1102.
- Schmidt TA, Hickman SE, Tolle SW, Brooks HS. The Physician Orders for Life-Sustaining Treatment program: Oregon emergency medical technicians’ practical experiences and attitudes. J Am Geriatr Soc 2004; 52:1430–1434.
- Hickman SE, Nelson CA, Moss AH, et al. Use of the Physician Orders for Life-Sustaining Treatment (POLST) paradigm program in the hospice setting. J Palliat Med 2009; 12:133–141.
- Hickman SE, Nelson CA, Perrin NA, Moss AH, Hammes BJ, Tolle SW. A comparison of methods to communicate treatment p in nursing facilities: traditional practices versus the physician orders for life-sustaining treatment program. J Am Geriatr Soc 2010; 58:1241–1248.
- Hickman SE, Nelson CA, Moss AH, Tolle SW, Perrin NA, Hammes BJ. The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc 2011; 59:2091–2099.
KEY POINTS
- Failures and opportunities for improvement in current advance care planning processes highlight the need for change.
- Differences exist between traditional advance directives and actionable medical orders.
- Advance care planning discussions can be initiated by physicians as a wellness initiative for everyone 18 years of age and older and can help patients and families understand advance care planning.
- POLST is outcome-neutral and may be used either to limit medical interventions or to clarify a request for any or all medically indicated treatments.
- Shared, informed medical decision-making is an essential element of the POLST process.
Kidneys have a lot of nerve
Wearing my rheumatologist hat, I know that patients are not sent to me for management of their hypertension. Certainly, I play an active role in dictating aggressive blood pressure control in patients with renal vasculitis and lupus nephritis as an integral part of their therapy, and conversely, I contribute to the difficulty in controlling blood pressures of those relatively few patients to whom I recommend full-dose nonsteroidal anti-inflammatory drugs. But for the most part, I am an (occasionally silent) voyeur, observing the blood pressure management of patients who are managed by others.
It is striking how many patients show up in my office with blood pressures outside the range advocated by current guidelines. Some pressures “normalize” when I recheck them after quiet conversation, sometimes using a larger, more appropriately sized cuff. But most do not.
Many explanations are offered. The usual is that their pressure is “just up in the doctor’s office” (when else are they carefully checked?), but few of these patients have undergone 24-hour ambulatory monitoring to diagnose “white coat hypertension” or to assess whether a normal physiologic pattern of nocturnal “dipping” is present. Some are already taking one or more antihypertensive drugs, yet their blood pressure is above the recommended target. Infrequently are the drugs pushed to their maximally tolerated dose.
From my practice experience, it seems that most patients with imperfectly controlled blood pressure do not fit the definition of resistant hypertension (inadequate response to three appropriate drugs in maximally tolerated doses). But resistant hypertension is also a problem affecting many patients and is in need of a solution.
In this issue, Thomas et al describe a novel approach undergoing clinical testing—catheter-based renal denervation. Early results are encouraging. But hypertension is a heterogeneous condition, and in a physiologically based therapy, the underlying pathophysiology may dictate the response and side effects of denervation in specific patients.
A recent study showed that denervation was effective in a few patients with chronic kidney disease, normalizing nocturnal dipping without further reducing renal function.1 But careful attention will need to be focused on patients who are likely reliant on interorgan neural communication. What will be the systemic effect if a patient who has undergone renal denervation develops severe cirrhosis and is in need of hepatorenal reflexes, or if a treated patient develops new severe congestive heart failure or sleep apnea? As appropriately stated in this issue by Thomas et al and by Bhatt, some optimism for the promise of this technique is justifiable, but we really will need studies large enough to include appropriate subsets for the analysis of both safety and efficacy.
- Hering D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012[Epub ahead of print].
Wearing my rheumatologist hat, I know that patients are not sent to me for management of their hypertension. Certainly, I play an active role in dictating aggressive blood pressure control in patients with renal vasculitis and lupus nephritis as an integral part of their therapy, and conversely, I contribute to the difficulty in controlling blood pressures of those relatively few patients to whom I recommend full-dose nonsteroidal anti-inflammatory drugs. But for the most part, I am an (occasionally silent) voyeur, observing the blood pressure management of patients who are managed by others.
It is striking how many patients show up in my office with blood pressures outside the range advocated by current guidelines. Some pressures “normalize” when I recheck them after quiet conversation, sometimes using a larger, more appropriately sized cuff. But most do not.
Many explanations are offered. The usual is that their pressure is “just up in the doctor’s office” (when else are they carefully checked?), but few of these patients have undergone 24-hour ambulatory monitoring to diagnose “white coat hypertension” or to assess whether a normal physiologic pattern of nocturnal “dipping” is present. Some are already taking one or more antihypertensive drugs, yet their blood pressure is above the recommended target. Infrequently are the drugs pushed to their maximally tolerated dose.
From my practice experience, it seems that most patients with imperfectly controlled blood pressure do not fit the definition of resistant hypertension (inadequate response to three appropriate drugs in maximally tolerated doses). But resistant hypertension is also a problem affecting many patients and is in need of a solution.
In this issue, Thomas et al describe a novel approach undergoing clinical testing—catheter-based renal denervation. Early results are encouraging. But hypertension is a heterogeneous condition, and in a physiologically based therapy, the underlying pathophysiology may dictate the response and side effects of denervation in specific patients.
A recent study showed that denervation was effective in a few patients with chronic kidney disease, normalizing nocturnal dipping without further reducing renal function.1 But careful attention will need to be focused on patients who are likely reliant on interorgan neural communication. What will be the systemic effect if a patient who has undergone renal denervation develops severe cirrhosis and is in need of hepatorenal reflexes, or if a treated patient develops new severe congestive heart failure or sleep apnea? As appropriately stated in this issue by Thomas et al and by Bhatt, some optimism for the promise of this technique is justifiable, but we really will need studies large enough to include appropriate subsets for the analysis of both safety and efficacy.
Wearing my rheumatologist hat, I know that patients are not sent to me for management of their hypertension. Certainly, I play an active role in dictating aggressive blood pressure control in patients with renal vasculitis and lupus nephritis as an integral part of their therapy, and conversely, I contribute to the difficulty in controlling blood pressures of those relatively few patients to whom I recommend full-dose nonsteroidal anti-inflammatory drugs. But for the most part, I am an (occasionally silent) voyeur, observing the blood pressure management of patients who are managed by others.
It is striking how many patients show up in my office with blood pressures outside the range advocated by current guidelines. Some pressures “normalize” when I recheck them after quiet conversation, sometimes using a larger, more appropriately sized cuff. But most do not.
Many explanations are offered. The usual is that their pressure is “just up in the doctor’s office” (when else are they carefully checked?), but few of these patients have undergone 24-hour ambulatory monitoring to diagnose “white coat hypertension” or to assess whether a normal physiologic pattern of nocturnal “dipping” is present. Some are already taking one or more antihypertensive drugs, yet their blood pressure is above the recommended target. Infrequently are the drugs pushed to their maximally tolerated dose.
From my practice experience, it seems that most patients with imperfectly controlled blood pressure do not fit the definition of resistant hypertension (inadequate response to three appropriate drugs in maximally tolerated doses). But resistant hypertension is also a problem affecting many patients and is in need of a solution.
In this issue, Thomas et al describe a novel approach undergoing clinical testing—catheter-based renal denervation. Early results are encouraging. But hypertension is a heterogeneous condition, and in a physiologically based therapy, the underlying pathophysiology may dictate the response and side effects of denervation in specific patients.
A recent study showed that denervation was effective in a few patients with chronic kidney disease, normalizing nocturnal dipping without further reducing renal function.1 But careful attention will need to be focused on patients who are likely reliant on interorgan neural communication. What will be the systemic effect if a patient who has undergone renal denervation develops severe cirrhosis and is in need of hepatorenal reflexes, or if a treated patient develops new severe congestive heart failure or sleep apnea? As appropriately stated in this issue by Thomas et al and by Bhatt, some optimism for the promise of this technique is justifiable, but we really will need studies large enough to include appropriate subsets for the analysis of both safety and efficacy.
- Hering D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012[Epub ahead of print].
- Hering D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012[Epub ahead of print].
The promise of renal denervation
Resistant hypertension has become the focus of intense medical interest. The most commonly accepted definition of resistant hypertension is uncontrolled blood pressure despite the use of drugs from three or more antihypertensive classes, one of which is a diuretic, at maximally tolerated doses. About 1 in 50 patients with a new diagnosis of hypertension will develop resistant hypertension.1
In the 1950s, surgical renal denervation was shown to be a highly effective treatment for resistant hypertension, but the procedure was abandoned because of intolerable side effects such as bladder dysfunction and orthostasis. More recently, carotid baroreceptor surgery for resistant hypertension was investigated; results were encouraging, but this currently remains a surgical procedure.2 Now, catheter-based renal denervation has emerged as a potential minimally invasive strategy to treat resistant hypertension.
In this issue of Cleveland Clinic Journal of Medicine, Thomas et al provide an elegant review of catheter-based renal denervation to treat resistant hypertension.3 The authors nicely summarize the available data for renal denervation for resistant hypertension. A reduction in office systolic blood pressure of about 30 mm Hg has been observed.4,5 In the published studies to date, there have been no major complications beyond those associated with any angiographic procedure.
Of note, this procedure is investigational in the United States, though it is available outside of research studies in other parts of the world. Symplicity HTN-3, a pivotal trial for potential US Food and Drug Administration approval of catheter-based renal denervation, is ongoing.6
The review by Thomas et al is relevant to primary care physicians, cardiologists, nephrologists, and endocrinologists, all of whom manage patients with resistant and refractory hypertension. It explains the potential indications and referral patterns for the procedure, if approved. This review brings clinicians quickly up to speed about the exciting developments in renal denervation.
UNANSWERED QUESTIONS
As should be evident, there are many unanswered questions about renal denervation.
The long-term durability of catheter-based renal denervation remains to be determined. The available data support a sustained effect out to at least 2 years.7 Further study will be necessary to determine if there are some patients in whom the effects wear out over time. But even if that happens, assuming the beneficial effect lasts at least a few years, it may be reasonable to repeat the procedure.
Another important question is whether the reductions in blood pressure with denervation translate into reductions in stroke, heart failure, renal failure, myocardial infarction, and death. It is logical to think that this relationship holds for catheter-based denervation as it does for medical therapy, though more study is needed to see if this is true.
CAVEATS
As with coronary artery disease, it will be important to ensure that patients labeled as having resistant hypertension truly have the disease. The diagnosis requires a careful history, evaluation of potential causes of secondary hypertension, and increased use of ambulatory blood pressure monitoring to rule out white-coat and masked hypertension.
If a patient truly has resistant hypertension, appropriate lifestyle modifications (primarily salt restriction to levels well below 2.4 g/day) and aggressive pharmacotherapy should first be attempted.8 Aldosterone blockade clearly has an important role, especially in obese patients, as it has been shown to markedly lower blood pressure in this phenotype.9
Imitation is the greatest form of flattery, and this is certainly true in the world of drugs and medical devices. Accordingly, a number of systems for renal denervation are being developed. This will likely spur further innovation and refinement in the technology.
On the other hand, as with coronary artery stents, it is important to realize that there is a fair amount of engineering sophistication behind catheter-based renal denervation. As has already happened in some parts of the world, taking a radiofrequency catheter designed for electrophysiology procedures and indiscriminately using it for renal denervation could be dangerous for patients.
Furthermore, if practitioners rapidly adopt this procedure but do not adhere to the indications and protocols used in the clinical trials, the outcomes could be worse, and the net result might be a setback for this promising field of research.
OTHER INDICATIONS AND BENEFITS?
As Thomas et al point out, in addition to resistant hypertension, renal denervation has also been studied in heart failure, chronic renal failure, diabetes mellitus, and sleep apnea.10–12 Sympathetic nerve overactivity appears to have a pathologic role in all these diseases. In small studies, renal denervation has already been shown to improve systolic and diastolic dysfunction, to cause regression of left ventricular hypertrophy, and to improve glycemic control. Since these cardiovascular risk factors often cluster in the same patient, a treatment that addresses several risk factors simultaneously would be expected to have a profound benefit on cardiovascular outcomes, though this remains to be established.
Several studies are under way. Symplicity-HF will study renal denervation in 40 patients with chronic heart failure and renal impairment. The Symplicity registry will follow more than 5,000 patients undergoing catheter-based renal denervation for resistant hypertension and other conditions marked by sympathetic nerve overactivity. If an important role for renal denervation is validated in Symplicity HTN-3, it would be easy to imagine trials of renal denervation in patients with lesser degrees of hypertension.
Only with further careful randomized trials of renal denervation will its full promise be realized.
- Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012; e-pub ahead of print.
- Bisognano JD, Bakris G, Nadim MK, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal Trial. J Am Coll Cardiol 2011; 58:765–773.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension. Cleve Clin J Med 2012; 79:501–510.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol 2012; in press.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
Resistant hypertension has become the focus of intense medical interest. The most commonly accepted definition of resistant hypertension is uncontrolled blood pressure despite the use of drugs from three or more antihypertensive classes, one of which is a diuretic, at maximally tolerated doses. About 1 in 50 patients with a new diagnosis of hypertension will develop resistant hypertension.1
In the 1950s, surgical renal denervation was shown to be a highly effective treatment for resistant hypertension, but the procedure was abandoned because of intolerable side effects such as bladder dysfunction and orthostasis. More recently, carotid baroreceptor surgery for resistant hypertension was investigated; results were encouraging, but this currently remains a surgical procedure.2 Now, catheter-based renal denervation has emerged as a potential minimally invasive strategy to treat resistant hypertension.
In this issue of Cleveland Clinic Journal of Medicine, Thomas et al provide an elegant review of catheter-based renal denervation to treat resistant hypertension.3 The authors nicely summarize the available data for renal denervation for resistant hypertension. A reduction in office systolic blood pressure of about 30 mm Hg has been observed.4,5 In the published studies to date, there have been no major complications beyond those associated with any angiographic procedure.
Of note, this procedure is investigational in the United States, though it is available outside of research studies in other parts of the world. Symplicity HTN-3, a pivotal trial for potential US Food and Drug Administration approval of catheter-based renal denervation, is ongoing.6
The review by Thomas et al is relevant to primary care physicians, cardiologists, nephrologists, and endocrinologists, all of whom manage patients with resistant and refractory hypertension. It explains the potential indications and referral patterns for the procedure, if approved. This review brings clinicians quickly up to speed about the exciting developments in renal denervation.
UNANSWERED QUESTIONS
As should be evident, there are many unanswered questions about renal denervation.
The long-term durability of catheter-based renal denervation remains to be determined. The available data support a sustained effect out to at least 2 years.7 Further study will be necessary to determine if there are some patients in whom the effects wear out over time. But even if that happens, assuming the beneficial effect lasts at least a few years, it may be reasonable to repeat the procedure.
Another important question is whether the reductions in blood pressure with denervation translate into reductions in stroke, heart failure, renal failure, myocardial infarction, and death. It is logical to think that this relationship holds for catheter-based denervation as it does for medical therapy, though more study is needed to see if this is true.
CAVEATS
As with coronary artery disease, it will be important to ensure that patients labeled as having resistant hypertension truly have the disease. The diagnosis requires a careful history, evaluation of potential causes of secondary hypertension, and increased use of ambulatory blood pressure monitoring to rule out white-coat and masked hypertension.
If a patient truly has resistant hypertension, appropriate lifestyle modifications (primarily salt restriction to levels well below 2.4 g/day) and aggressive pharmacotherapy should first be attempted.8 Aldosterone blockade clearly has an important role, especially in obese patients, as it has been shown to markedly lower blood pressure in this phenotype.9
Imitation is the greatest form of flattery, and this is certainly true in the world of drugs and medical devices. Accordingly, a number of systems for renal denervation are being developed. This will likely spur further innovation and refinement in the technology.
On the other hand, as with coronary artery stents, it is important to realize that there is a fair amount of engineering sophistication behind catheter-based renal denervation. As has already happened in some parts of the world, taking a radiofrequency catheter designed for electrophysiology procedures and indiscriminately using it for renal denervation could be dangerous for patients.
Furthermore, if practitioners rapidly adopt this procedure but do not adhere to the indications and protocols used in the clinical trials, the outcomes could be worse, and the net result might be a setback for this promising field of research.
OTHER INDICATIONS AND BENEFITS?
As Thomas et al point out, in addition to resistant hypertension, renal denervation has also been studied in heart failure, chronic renal failure, diabetes mellitus, and sleep apnea.10–12 Sympathetic nerve overactivity appears to have a pathologic role in all these diseases. In small studies, renal denervation has already been shown to improve systolic and diastolic dysfunction, to cause regression of left ventricular hypertrophy, and to improve glycemic control. Since these cardiovascular risk factors often cluster in the same patient, a treatment that addresses several risk factors simultaneously would be expected to have a profound benefit on cardiovascular outcomes, though this remains to be established.
Several studies are under way. Symplicity-HF will study renal denervation in 40 patients with chronic heart failure and renal impairment. The Symplicity registry will follow more than 5,000 patients undergoing catheter-based renal denervation for resistant hypertension and other conditions marked by sympathetic nerve overactivity. If an important role for renal denervation is validated in Symplicity HTN-3, it would be easy to imagine trials of renal denervation in patients with lesser degrees of hypertension.
Only with further careful randomized trials of renal denervation will its full promise be realized.
Resistant hypertension has become the focus of intense medical interest. The most commonly accepted definition of resistant hypertension is uncontrolled blood pressure despite the use of drugs from three or more antihypertensive classes, one of which is a diuretic, at maximally tolerated doses. About 1 in 50 patients with a new diagnosis of hypertension will develop resistant hypertension.1
In the 1950s, surgical renal denervation was shown to be a highly effective treatment for resistant hypertension, but the procedure was abandoned because of intolerable side effects such as bladder dysfunction and orthostasis. More recently, carotid baroreceptor surgery for resistant hypertension was investigated; results were encouraging, but this currently remains a surgical procedure.2 Now, catheter-based renal denervation has emerged as a potential minimally invasive strategy to treat resistant hypertension.
In this issue of Cleveland Clinic Journal of Medicine, Thomas et al provide an elegant review of catheter-based renal denervation to treat resistant hypertension.3 The authors nicely summarize the available data for renal denervation for resistant hypertension. A reduction in office systolic blood pressure of about 30 mm Hg has been observed.4,5 In the published studies to date, there have been no major complications beyond those associated with any angiographic procedure.
Of note, this procedure is investigational in the United States, though it is available outside of research studies in other parts of the world. Symplicity HTN-3, a pivotal trial for potential US Food and Drug Administration approval of catheter-based renal denervation, is ongoing.6
The review by Thomas et al is relevant to primary care physicians, cardiologists, nephrologists, and endocrinologists, all of whom manage patients with resistant and refractory hypertension. It explains the potential indications and referral patterns for the procedure, if approved. This review brings clinicians quickly up to speed about the exciting developments in renal denervation.
UNANSWERED QUESTIONS
As should be evident, there are many unanswered questions about renal denervation.
The long-term durability of catheter-based renal denervation remains to be determined. The available data support a sustained effect out to at least 2 years.7 Further study will be necessary to determine if there are some patients in whom the effects wear out over time. But even if that happens, assuming the beneficial effect lasts at least a few years, it may be reasonable to repeat the procedure.
Another important question is whether the reductions in blood pressure with denervation translate into reductions in stroke, heart failure, renal failure, myocardial infarction, and death. It is logical to think that this relationship holds for catheter-based denervation as it does for medical therapy, though more study is needed to see if this is true.
CAVEATS
As with coronary artery disease, it will be important to ensure that patients labeled as having resistant hypertension truly have the disease. The diagnosis requires a careful history, evaluation of potential causes of secondary hypertension, and increased use of ambulatory blood pressure monitoring to rule out white-coat and masked hypertension.
If a patient truly has resistant hypertension, appropriate lifestyle modifications (primarily salt restriction to levels well below 2.4 g/day) and aggressive pharmacotherapy should first be attempted.8 Aldosterone blockade clearly has an important role, especially in obese patients, as it has been shown to markedly lower blood pressure in this phenotype.9
Imitation is the greatest form of flattery, and this is certainly true in the world of drugs and medical devices. Accordingly, a number of systems for renal denervation are being developed. This will likely spur further innovation and refinement in the technology.
On the other hand, as with coronary artery stents, it is important to realize that there is a fair amount of engineering sophistication behind catheter-based renal denervation. As has already happened in some parts of the world, taking a radiofrequency catheter designed for electrophysiology procedures and indiscriminately using it for renal denervation could be dangerous for patients.
Furthermore, if practitioners rapidly adopt this procedure but do not adhere to the indications and protocols used in the clinical trials, the outcomes could be worse, and the net result might be a setback for this promising field of research.
OTHER INDICATIONS AND BENEFITS?
As Thomas et al point out, in addition to resistant hypertension, renal denervation has also been studied in heart failure, chronic renal failure, diabetes mellitus, and sleep apnea.10–12 Sympathetic nerve overactivity appears to have a pathologic role in all these diseases. In small studies, renal denervation has already been shown to improve systolic and diastolic dysfunction, to cause regression of left ventricular hypertrophy, and to improve glycemic control. Since these cardiovascular risk factors often cluster in the same patient, a treatment that addresses several risk factors simultaneously would be expected to have a profound benefit on cardiovascular outcomes, though this remains to be established.
Several studies are under way. Symplicity-HF will study renal denervation in 40 patients with chronic heart failure and renal impairment. The Symplicity registry will follow more than 5,000 patients undergoing catheter-based renal denervation for resistant hypertension and other conditions marked by sympathetic nerve overactivity. If an important role for renal denervation is validated in Symplicity HTN-3, it would be easy to imagine trials of renal denervation in patients with lesser degrees of hypertension.
Only with further careful randomized trials of renal denervation will its full promise be realized.
- Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012; e-pub ahead of print.
- Bisognano JD, Bakris G, Nadim MK, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal Trial. J Am Coll Cardiol 2011; 58:765–773.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension. Cleve Clin J Med 2012; 79:501–510.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol 2012; in press.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
- Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012; e-pub ahead of print.
- Bisognano JD, Bakris G, Nadim MK, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal Trial. J Am Coll Cardiol 2011; 58:765–773.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension. Cleve Clin J Med 2012; 79:501–510.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol 2012; in press.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
Renal denervation to treat resistant hypertension: Guarded optimism
Can a percutaneous catheter-based procedure effectively treat resistant hypertension?
Radiofrequency ablation of the renal sympathetic nerves is undergoing randomized controlled trials in patients who have resistant hypertension and other disorders that involve the sympathetic nervous system. Remarkably, the limited results available so far look good.
This article discusses the physiologic rationale for renal denervation, the evidence from studies in humans of the benefits, risks, and complications of the procedure, upcoming trials, and areas for future research.
DESPITE MANY TREATMENT OPTIONS, RESISTANT HYPERTENSION IS COMMON
Hypertension is a leading reason for visits to physicians in the United States and is associated with increased rates of cardiovascular disease and death.1,2 A variety of antihypertensive agents are available, and the percentage of people with hypertension whose blood pressure is under control has increased over the past 2 decades. Nevertheless, population-based studies show that the control rate remains suboptimal.3 Effective pharmacologic treatment may be limited by inadequate doses or inappropriate combinations of antihypertensive drugs, concurrent use of agents that raise the blood pressure, noncompliance with dietary restrictions, and side effects that result in poor compliance with drug therapy.
Resistant hypertension is defined as failure to achieve goal blood pressure in patients who are adhering to full tolerated doses of an appropriate three-drug regimen that includes a diuretic.1,4,5 If we use these criteria, many patients labelled as having resistant hypertension probably do not truly have it; instead, they are nonadherent to therapy or are on an inadequate or inappropriate regimen. Although the true prevalence of resistant hypertension is not clear, estimates from large clinical trials suggest that about 20% to 30% of hypertensive patients may meet the criteria for it.4 For the subset of patients who have truly resistant hypertension, nonpharmacologic treatments such as renal sympathetic denervation are an intriguing avenue.
SURGICAL SYMPATHETIC DENERVATION: TRIED AND ABANDONED IN THE 1950s
More than a half century ago, a surgical procedure, thoracolumbar sympathectomy (in which sympathetic nerve trunks and splanchnic nerves were removed), was sometimes performed to control blood pressure in patients with malignant hypertension. This was effective but caused debilitating side effects such as postural hypotension, erectile dysfunction, and syncope.
Smithwick and Thompson6 reported that, in 1,266 hypertensive patients who underwent this procedure and 467 medically treated controls, the 5-year mortality rates were 19% and 54%, respectively. Forty-five percent of those who survived the surgery had significantly lower blood pressure afterward, and the antihypertensive effect lasted 10 years or more.
The procedure fell out of favor due to the morbidity associated with this nonselective approach and to the increased availability of drug therapy.
THE SYMPATHETIC NERVOUS SYSTEM IS A DRIVER OF HYPERTENSION
A variety of evidence suggests that hyperactivation of the sympathetic nervous system plays a major role in initiating and maintaining hypertension. For example, drugs that inhibit the sympathetic drive at various levels have a blood-pressure-lowering effect. Further, direct intraneural recordings show a high level of sympathetic nerve activity in the muscles of hypertensive patients, who also have high levels of cardiac and renal norepinephrine “spillover”—ie, the amount of this neurotransmitter that escapes neuronal uptake and local metabolism and spills over into the circulation.7
The kidneys are supplied with postganglionic sympathetic nerve fibers that end in the efferent and afferent renal arterioles, the juxtaglomerular apparatus, and the renal tubular system. Studies in animals and humans have shown that an increase in efferent signals (ie, from the brain to the kidney) leads to renal vasoconstriction and decreased renal blood flow, increased renin release, and sodium retention.8,9 Afferent signals (from the kidney to the central nervous system), which are increased in states of renal ischemia, renal parenchymal injury, and hypoxia, disinhibit the vasomotor center (the nuclei tractus solitarii) in the central nervous system, leading to increased efferent signals to the kidneys, heart, and peripheral blood vessels (Figure 1).10
Enhanced sympathetic activity in patients with hypertension may play a role in subsequent target-organ damage such as left ventricular hypertrophy, congestive heart failure, and progressive renal damage.11
Studies of renal denervation in animals, using surgical and chemical techniques, have further helped to establish the role of renal sympathetic nerves in hypertension.12,13
CATHETER-BASED RENAL DENERVATION
Renal sympathetic nerves run through the adventitia of the renal arteries in a mesh-like pattern.
In the renal denervation procedure, a specially designed catheter is inserted into a femoral artery and advanced into one of the renal arteries. There, radiofrequency energy is applied to the endoluminal surface according to a proprietary algorithm, thereby delivering thermal injury selectively to the renal sympathetic nerves without affecting the abdominal, pelvic, or lower-extremity nerves. The energy delivered is lower than that used for cardiac electrophysiologic procedures.
The nerves are not imaged or mapped before treatment. The procedure is performed on both sides, with four to six sites ablated in a longitudinal and rotational manner in 2-minute treatments at each site, to cover the full circumference (Figure 1).
In the United States, the device (Symplicity Renal Denervation System; Medtronic, Inc, Mountain View, CA) is available only for investigational use.
Below, we briefly review the studies of renal denervation to date. SYMPLICITY HTN-1 Symplicity HTN-1 was a proof-of-principle study in 45 patients with resistant hypertension (Table 1).14,15
Effect on blood pressure. Six months after renal denervation, blood pressure was significantly lower than at baseline (−22/−11 mm Hg, 95% confidence interval [CI] 10/5 mm Hg) in 26 patients available for follow-up. At 12 months, the difference from baseline was −27/−10 mm Hg (95% CI 16/11 mm Hg) in 9 patients available for follow-up (Table 2).14
Evidence of the durability of blood pressure reduction came from an expanded cohort of 153 patients followed for 2 years after denervation.16
Further follow-up data showed a sustained and significant blood pressure reduction through 3 years after denervation (unpublished results presented at the 2012 annual meeting of the American College of Cardiology). Notably, patients who were initially considered to be nonresponders (defined as failure of their blood pressure to go down by at least 10 mm Hg) were all reported to have a clinical response at 36 months.
Adverse events. In the initial and expanded cohorts combined, one patient suffered a renal artery dissection due to manipulation of the guiding catheter before the radiofrequency energy was delivered, and three patients developed a femoral pseudoaneurysm. No other long-term arterial complications were observed.
Comments. Limitations of this study included a small number of patients, no control group, and a primary outcome of a reduction in office blood pressure rather than in ambulatory blood pressure.
Additionally, although the authors concluded that there was no significant deterioration in renal function during the study period, we should note that in an additional follow-up period in this cohort, 10 patients with available 2-year data had a decrease in estimated glomerular filtration rate (eGFR) of −16.0 mL/min/1.73 m2. In 5 patients who did not have spironolactone (Aldactone) or another diuretic added after the first year of followup, a lesser but significant decrease (−7.8 mL/min/1.73 m2) was noted. The investigators surmised that denervation may enhance diuretic sensitivity, leading to prerenal azotemia in some patients.17
SYMPLICITY HTN-2
The Symplicity HTN-2 trial was a larger, randomized, efficacy study that built on the earlier results, providing additional evidence of therapeutic benefit.15
An international cohort of 106 patients with resistant hypertension, defined as systolic blood pressure of 160 mm Hg or higher (or ≥ 150 mm Hg in patients with type 2 diabetes) despite the use of three or more antihypertensive medications, were randomly assigned to undergo renal denervation with the Symplicity device (n = 52) or to continue their previous treatment with antihypertensive medications alone (n = 54). The primary effectiveness end point was the change in seated office blood pressure from baseline to 6 months (Table 1).
Effect on blood pressure. In the denervation group, at 6 months, office blood pressure had changed by a mean of −32/−12 mm Hg (standard deviation [SD] 23/11 mm Hg) compared with a mean change of 1/0 mm Hg (SD 21/10 mm Hg) in the control group. Fortyone (84%) of the 49 patients who underwent denervation had a decrease in systolic blood pressure of 10 mm Hg or more at 6 months compared with baseline values, while five (10%) had no decline in systolic blood pressure. Nineteen patients had a reduction in systolic pressure to less than 140 mm Hg in the denervation group.
A subset of patients (20 in the denervation group and 25 in the control group) underwent 24-hour ambulatory blood pressure monitoring at 6 months. This showed a similar though less pronounced fall in blood pressure in the denervation group and no change in the controls. A subanalysis that censored all data for patients whose medication was increased during the follow-up period showed a blood pressure reduction of −31/−12 mm Hg (SD 22/11 mm Hg) in the renal denervation group.
Adverse events. Procedure-related adverse events included a single femoral artery pseudoaneurysm, one case of postprocedural hypotension requiring a reduction in antihypertensive medications, and 7 (13%) of 52 patients who experienced intraprocedural bradycardia requiring atropine.
Effect on renal function. No significant difference was noted between groups in the mean change in renal function at 6 months, whether assessed by eGFR, serum creatinine level, or cystatin C level. At 6 months, no patient had a decrease of more than 50% in eGFR, although two patients who underwent renal denervation and three controls had more than a 25% decrease in eGFR.
At 6 months, the urine albumin-to-creatinine ratio had changed by a median of −3 mg/g (range −1,089 to 76) in 38 patients in the treatment group and by 1 mg/g (range −538 to 227) in 37 controls.
Most patients (88%) undergoing renal denervation underwent renal arterial imaging at 6 months, on which a single patient showed possible progression of an underlying atherosclerotic lesion that was unrelated to the procedure and that did not require intervention.
Denervation and the normal stress response. Whether renal denervation negatively affects the body’s physiologic response to stress that is normally mediated by sympathetic nerve activity was addressed in an extended investigation of Symplicity HTN-2 using cardiopulmonary exercise tests at baseline and 3 months after renal denervation.18 In the denervation group, blood pressure during exercise was significantly lower at 3 months than at baseline, but the heart rate increase at different levels of exercise was not affected. Additionally, the resting heart rate was lower and heart rate recovery after exercise improved after the procedure, particularly in patients without diabetes.
Comments. The Symplicity HTN-2 trial benefited from a randomized trial design and strict inclusion criteria of treatment resistance, but it still had notable limitations. A pretrial evaluation for causes of secondary hypertension or white-coat hypertension was not explicitly described. The control group did not undergo a sham procedure, and data analyzers were not masked to treatment assignment. Although not analyzed as a primary end point, the use of home-based and 24-hour ambulatory blood pressure assessment—measures important for determining white-coat hypertension—revealed substantial differences in blood pressure changes relative to office measurements. Because nearly all the patients (97%) were white, the generalizability of treatment results to black patients with resistant hypertension may be limited. Isolated diastolic hypertension (defined as diastolic pressure ≥ 90 mm Hg with systolic pressure < 140 mm Hg), which is more common in younger patients, was not studied.
DOES RENAL DENERVATION REDUCE SYMPATHETIC TONE?
A subgroup of 10 patients in the Symplicity HTN-1 trial whose mean 6-month office blood pressure was reduced by 22/12 mm Hg underwent assessment of renal norepinephrine spillover. A substantial (47%) reduction in renal norepinephrine spillover was noted 1 month after the procedure.14
The investigators additionally described a marked reduction in renal norepinephrine spillover from both kidneys in one patient, with a reduction of 48% from the left kidney and 75% from the right kidney 1 month after the procedure. Whole-body norepinephrine spillover in this patient was reduced by 42%. This effect was accompanied by a 50% decrease in plasma renin activity and by an increase in renal plasma flow. Aldosterone levels were not reported.19
Thus, the decrease in renal norepinephrine spillover suggests a reduction of renal efferent activity, and the decrease in total body norepinephrine spillover suggests a reduction in central sympathetic drive via the renal afferent pathway.
Microneurography in this same patient showed a gradual reduction in muscle sympathetic nerve activity to normal levels, from 56 bursts per minute at baseline to 41 at 30 days and 19 at 12 months).19 Decreased renin secretion, via circulating angiotensin II, may affect central sympathetic outflow as well.
Comments. While these findings address some of the underlying mechanisms, the small number of patients in whom these studies were done limits the generalizability of the results. The impact of the procedure on renal hemodynamics will need to be studied, including possible direct effects of the procedure, and whether there are differences in different study populations or differences based on blood pressure levels.
WHICH PATIENTS RESPOND BEST TO THIS PROCEDURE?
Although the Symplicity HTN-2 investigators report some predictors of increased reduction in blood pressure on multivariate analysis, including increased blood pressure at baseline and reduced heart rate at baseline, these are not specific enough to enable patient selection.
Interestingly, results from the expanded cohort of the Symplicity HTN-1 study found that patients on central sympatholytic agents such as clonidine had a greater reduction in blood pressure, although the reason for this is unclear.16 Identifying specific predictors of treatment success at baseline will be essential in future studies.
The earlier Symplicity trials and the ongoing Symplicity HTN-3 trial are in patients who have high blood pressure not responding to three or more antihypertensive drugs. The mean baseline systolic blood pressure in the Symplicity HTN-1 and HTN-2 trials was 178 mm Hg, and patients were taking an average of five antihypertensive drugs (Table 1). It is not known whether denervation will produce similar blood-pressure-lowering results across the spectrum of hypertension severity.
WHAT ARE THE LONG-TERM RESULTS OF DENERVATION?
Enthusiasm for the results from the Symplicity trials is tempered by concerns about the durability of the effects of the procedure, the need for better understanding of the impact of renal denervation on a wide array of pathophysiologic cascades leading to hypertension, and the effect on renal hemodynamics.
Antihypertensive efficacy has been reported to persist up to 2 years after the procedure,16 with recent unpublished data suggesting efficacy up to 3 years, but longer follow-up is needed to address whether these effects are finite.
Although reinnervation of afferent renal nerves has not been described, transplant models have shown anatomic regrowth of efferent nerves; the impact of this efferent reinnervation on blood pressure remains unclear. Experience from renal transplantation also shows that implanted kidneys that are “denervated” can still maintain fluid and electrolyte regulation.
Follow-up renal imaging in the Symplicity trials did not indicate renal artery stenosis at the sites of denervation in patients who underwent the procedure. Animal studies using the Symplicity catheter system showed renal nerve injury as evidenced by nerve fibrosis and thickened epineurium and perineurium, but no significant smooth muscle hyperplasia, arterial stenosis, or thrombosis by angiography or histology at 6 months.20
WHAT ARE THE RISKS?
Adverse effects that were noted in the short term are detailed under discussion of the trials and in Table 2.
Long-term adverse events in the Symplicity HTN-2 trial that required hospitalization were reported in five patients in the denervation group and three patients in the control group (Table 2). These included transient ischemic attacks, hypertensive crises, hypotensive episodes, angina, and nausea.
Renal function was maintained for the duration of both trials, and details regarding eGFR change have been described above under the discussion of the trials.
Diffuse visceral pain at the time of the procedure is reported as an expected occurrence, managed with intravenous analgesic medications.
DOES SYMPATHETIC DENERVATION HAVE A ROLE IN OTHER CONDITIONS?
Interestingly, other sympathetically driven diseases, such as diabetes mellitus and polycystic ovary syndrome, may prove to be targets for this therapy in the future.21
Mahfoud et al22 conducted a pilot study in 37 patients with resistant hypertension undergoing renal denervation and 13 control patients. Fasting glucose levels declined from 118 ± 3.4 mg/dL to 108 ± 3.8 mg/dL after 3 months in the intervention group (P = .039), compared with no change in the control group. Insulin and C-peptide levels were also lower in the intervention group. The reported improvement in glucose metabolism and insulin sensitivity suggests that the beneficial effects of this procedure may extend beyond blood pressure reduction.
Brandt et al23 reported regression of left ventricular hypertrophy and significantly improved cardiac functional parameters, including increase in ejection fraction and improved diastolic dysfunction, in a study of 46 patients who underwent renal denervation. This findings suggests a potential beneficial effect on cardiac remodeling.
Witkowski et al24 reported lowering of blood pressure in 10 patients with refractory hypertension and obstructive sleep apnea who underwent renal denervation, which was accompanied by improvement of sleep apnea severity.
Ukena et al25 reported reduction in ventricular tachyarrhythmias in two patients with congestive heart failure who had therapy-resistant electrical storm.
A recent pilot study in 15 patients with stage 3 and 4 chronic kidney disease (mean eGFR 31 mL/min/1.73 m2) showed significantly improved office blood pressure control up to 1 year, restoration of nocturnal dipping on 24-hour monitoring, as well as a nonsignificant trend towards increased hemoglobin levels and decreased proteinuria. No additional deterioration of renal function was reported in these patients (2 patients had renal function assessed up to 1 year).26
Thus, the benefits of this procedure may extend to other diseases that have a common underlying thread of elevated sympathetic activity, by targeting the “sympathorenal” axis.27
GUARDED OPTIMISM AND FUTURE DIRECTIONS
Given the well-known cardiovascular risks and health care costs associated with uncontrolled hypertension and the continued challenge that physicians face in managing it, novel therapies such as renal denervation may provide an adjunct to existing pharmacologic approaches.
While there is certainly cause for guarded optimism, especially with the striking blood pressure-lowering results seen in trials so far, it should be kept in mind that the mechanisms leading to the hypertensive response are complex and multifactorial, and further understanding of this therapy with long-term follow-up is needed. A comparison study with spironolactone, which is increasingly being used to treat resistant hypertension (in the absence of a diagnosis of primary aldosteronism)28,29 would help to further establish the role of this procedure.
Studies of carotid baroreceptor stimulation via an implantable device have shown sustained reduction in blood pressure in patients with resistant hypertension. A study comparing this technique with renal denervation for efficacy and safety end points could be considered in the future.30,31
The planned Symplicity HTN-3 study in the United States will be the largest trial to date, with a targeted randomization of more than 500 patients using strict enrollment criteria, including the use of maximally tolerated doses of diuretics and more focus on the use of ambulatory blood pressure monitoring and on the blinding of participants. This study will help further analysis of this technology in a more diverse population.32,33
Future studies should be designed to clarify pathophysiologic mechanisms, patient selection criteria, effects on target organ damage, and efficacy in patients with chronic kidney disease, obesity, congestive heart failure, and in less severe forms of hypertension.
A CALL FOR PARTICIPANTS IN A CLINICAL TRIAL
The Departments of Cardiology and Nephrology and Hypertension at Cleveland Clinic are currently enrolling patients in the Symplicity HTN-3 trial. For more information, please contact George Thomas, MD ([email protected]), or Mehdi Shishehbor, DO, MPH ([email protected]), or visit www.symplifybptrial.com.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Vital Health Stat 13( 169) 2011. http://www.cdc.gov/nchs/data/series/sr_13/sr13_169.pdf. Accessed April 24, 2012.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc 1953; 152:1501–1504.
- Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 2009; 54:1195–1201.
- Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation 1977; 56:691–698.
- Kon V. Neural control of renal circulation. Miner Electrolyte Metab 1989; 15:33–43.
- Campese VM. Neurogenic factors and hypertension in renal disease. Kidney Int Suppl 2000; 75:S2–S6.
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34:724–728.
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 2004; 287:H695–H703.
- Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol 1983; 245:F1–F14.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatmentresistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Petidis K, Anyfanti P, Doumas M. Renal sympathetic denervation: renal function concerns. Hypertension 2011; 58:e19; author replye20.
- Ukena C, Mahfoud F, Kindermann I, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol 2011; 58:1176–1182.
- Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension (letter). N Engl J Med 2009; 361:932–934.
- Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol 2011; 100:1095–1101.
- Schlaich MP, Straznicky N, Grima M, et al. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens 2011; 29:991–996.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
- Ukena C, Bauer A, Mahfoud F, et al. Renal sympathetic denervation for treatment of electrical storm: first-inman experience. Clin Res Cardiol 2012; 101:63–67.
- Herring D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012; May 17[Epub ahead of print]
- Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Böhm M, Krum H. Sympatho-renal axis in chronic disease. Clin Res Cardiol 2011; 100:1049–1057.
- Chapman N, Dobson J, Wilson S, et al; Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003; 16:925–930.
- Papademetriou V, Doumas M, Faselis C, et al. Carotid baroreceptor stimulation for the treatment of resistant hypertension. Int J Hypertens 2011; 2011:964394.
- Ng MM, Sica DA, Frishman WH. Rheos: an implantable carotid sinus stimulation device for the nonpharmacologic treatment of resistant hypertension. Cardiol Rev 2011; 19:52–57.
- US National Institutes of Health. Renal denervation in patients with uncontrolled hypertension (SYMPLICITY HTN-3). http://www.clinicaltrials.gov/ct2/show/NCT01418261. Accessed June 7, 2012.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the Symplicity HTN-3 trial. Clin Cardiol 2012 May 9. [Epub ahead of print]
Can a percutaneous catheter-based procedure effectively treat resistant hypertension?
Radiofrequency ablation of the renal sympathetic nerves is undergoing randomized controlled trials in patients who have resistant hypertension and other disorders that involve the sympathetic nervous system. Remarkably, the limited results available so far look good.
This article discusses the physiologic rationale for renal denervation, the evidence from studies in humans of the benefits, risks, and complications of the procedure, upcoming trials, and areas for future research.
DESPITE MANY TREATMENT OPTIONS, RESISTANT HYPERTENSION IS COMMON
Hypertension is a leading reason for visits to physicians in the United States and is associated with increased rates of cardiovascular disease and death.1,2 A variety of antihypertensive agents are available, and the percentage of people with hypertension whose blood pressure is under control has increased over the past 2 decades. Nevertheless, population-based studies show that the control rate remains suboptimal.3 Effective pharmacologic treatment may be limited by inadequate doses or inappropriate combinations of antihypertensive drugs, concurrent use of agents that raise the blood pressure, noncompliance with dietary restrictions, and side effects that result in poor compliance with drug therapy.
Resistant hypertension is defined as failure to achieve goal blood pressure in patients who are adhering to full tolerated doses of an appropriate three-drug regimen that includes a diuretic.1,4,5 If we use these criteria, many patients labelled as having resistant hypertension probably do not truly have it; instead, they are nonadherent to therapy or are on an inadequate or inappropriate regimen. Although the true prevalence of resistant hypertension is not clear, estimates from large clinical trials suggest that about 20% to 30% of hypertensive patients may meet the criteria for it.4 For the subset of patients who have truly resistant hypertension, nonpharmacologic treatments such as renal sympathetic denervation are an intriguing avenue.
SURGICAL SYMPATHETIC DENERVATION: TRIED AND ABANDONED IN THE 1950s
More than a half century ago, a surgical procedure, thoracolumbar sympathectomy (in which sympathetic nerve trunks and splanchnic nerves were removed), was sometimes performed to control blood pressure in patients with malignant hypertension. This was effective but caused debilitating side effects such as postural hypotension, erectile dysfunction, and syncope.
Smithwick and Thompson6 reported that, in 1,266 hypertensive patients who underwent this procedure and 467 medically treated controls, the 5-year mortality rates were 19% and 54%, respectively. Forty-five percent of those who survived the surgery had significantly lower blood pressure afterward, and the antihypertensive effect lasted 10 years or more.
The procedure fell out of favor due to the morbidity associated with this nonselective approach and to the increased availability of drug therapy.
THE SYMPATHETIC NERVOUS SYSTEM IS A DRIVER OF HYPERTENSION
A variety of evidence suggests that hyperactivation of the sympathetic nervous system plays a major role in initiating and maintaining hypertension. For example, drugs that inhibit the sympathetic drive at various levels have a blood-pressure-lowering effect. Further, direct intraneural recordings show a high level of sympathetic nerve activity in the muscles of hypertensive patients, who also have high levels of cardiac and renal norepinephrine “spillover”—ie, the amount of this neurotransmitter that escapes neuronal uptake and local metabolism and spills over into the circulation.7
The kidneys are supplied with postganglionic sympathetic nerve fibers that end in the efferent and afferent renal arterioles, the juxtaglomerular apparatus, and the renal tubular system. Studies in animals and humans have shown that an increase in efferent signals (ie, from the brain to the kidney) leads to renal vasoconstriction and decreased renal blood flow, increased renin release, and sodium retention.8,9 Afferent signals (from the kidney to the central nervous system), which are increased in states of renal ischemia, renal parenchymal injury, and hypoxia, disinhibit the vasomotor center (the nuclei tractus solitarii) in the central nervous system, leading to increased efferent signals to the kidneys, heart, and peripheral blood vessels (Figure 1).10
Enhanced sympathetic activity in patients with hypertension may play a role in subsequent target-organ damage such as left ventricular hypertrophy, congestive heart failure, and progressive renal damage.11
Studies of renal denervation in animals, using surgical and chemical techniques, have further helped to establish the role of renal sympathetic nerves in hypertension.12,13
CATHETER-BASED RENAL DENERVATION
Renal sympathetic nerves run through the adventitia of the renal arteries in a mesh-like pattern.
In the renal denervation procedure, a specially designed catheter is inserted into a femoral artery and advanced into one of the renal arteries. There, radiofrequency energy is applied to the endoluminal surface according to a proprietary algorithm, thereby delivering thermal injury selectively to the renal sympathetic nerves without affecting the abdominal, pelvic, or lower-extremity nerves. The energy delivered is lower than that used for cardiac electrophysiologic procedures.
The nerves are not imaged or mapped before treatment. The procedure is performed on both sides, with four to six sites ablated in a longitudinal and rotational manner in 2-minute treatments at each site, to cover the full circumference (Figure 1).
In the United States, the device (Symplicity Renal Denervation System; Medtronic, Inc, Mountain View, CA) is available only for investigational use.
Below, we briefly review the studies of renal denervation to date. SYMPLICITY HTN-1 Symplicity HTN-1 was a proof-of-principle study in 45 patients with resistant hypertension (Table 1).14,15
Effect on blood pressure. Six months after renal denervation, blood pressure was significantly lower than at baseline (−22/−11 mm Hg, 95% confidence interval [CI] 10/5 mm Hg) in 26 patients available for follow-up. At 12 months, the difference from baseline was −27/−10 mm Hg (95% CI 16/11 mm Hg) in 9 patients available for follow-up (Table 2).14
Evidence of the durability of blood pressure reduction came from an expanded cohort of 153 patients followed for 2 years after denervation.16
Further follow-up data showed a sustained and significant blood pressure reduction through 3 years after denervation (unpublished results presented at the 2012 annual meeting of the American College of Cardiology). Notably, patients who were initially considered to be nonresponders (defined as failure of their blood pressure to go down by at least 10 mm Hg) were all reported to have a clinical response at 36 months.
Adverse events. In the initial and expanded cohorts combined, one patient suffered a renal artery dissection due to manipulation of the guiding catheter before the radiofrequency energy was delivered, and three patients developed a femoral pseudoaneurysm. No other long-term arterial complications were observed.
Comments. Limitations of this study included a small number of patients, no control group, and a primary outcome of a reduction in office blood pressure rather than in ambulatory blood pressure.
Additionally, although the authors concluded that there was no significant deterioration in renal function during the study period, we should note that in an additional follow-up period in this cohort, 10 patients with available 2-year data had a decrease in estimated glomerular filtration rate (eGFR) of −16.0 mL/min/1.73 m2. In 5 patients who did not have spironolactone (Aldactone) or another diuretic added after the first year of followup, a lesser but significant decrease (−7.8 mL/min/1.73 m2) was noted. The investigators surmised that denervation may enhance diuretic sensitivity, leading to prerenal azotemia in some patients.17
SYMPLICITY HTN-2
The Symplicity HTN-2 trial was a larger, randomized, efficacy study that built on the earlier results, providing additional evidence of therapeutic benefit.15
An international cohort of 106 patients with resistant hypertension, defined as systolic blood pressure of 160 mm Hg or higher (or ≥ 150 mm Hg in patients with type 2 diabetes) despite the use of three or more antihypertensive medications, were randomly assigned to undergo renal denervation with the Symplicity device (n = 52) or to continue their previous treatment with antihypertensive medications alone (n = 54). The primary effectiveness end point was the change in seated office blood pressure from baseline to 6 months (Table 1).
Effect on blood pressure. In the denervation group, at 6 months, office blood pressure had changed by a mean of −32/−12 mm Hg (standard deviation [SD] 23/11 mm Hg) compared with a mean change of 1/0 mm Hg (SD 21/10 mm Hg) in the control group. Fortyone (84%) of the 49 patients who underwent denervation had a decrease in systolic blood pressure of 10 mm Hg or more at 6 months compared with baseline values, while five (10%) had no decline in systolic blood pressure. Nineteen patients had a reduction in systolic pressure to less than 140 mm Hg in the denervation group.
A subset of patients (20 in the denervation group and 25 in the control group) underwent 24-hour ambulatory blood pressure monitoring at 6 months. This showed a similar though less pronounced fall in blood pressure in the denervation group and no change in the controls. A subanalysis that censored all data for patients whose medication was increased during the follow-up period showed a blood pressure reduction of −31/−12 mm Hg (SD 22/11 mm Hg) in the renal denervation group.
Adverse events. Procedure-related adverse events included a single femoral artery pseudoaneurysm, one case of postprocedural hypotension requiring a reduction in antihypertensive medications, and 7 (13%) of 52 patients who experienced intraprocedural bradycardia requiring atropine.
Effect on renal function. No significant difference was noted between groups in the mean change in renal function at 6 months, whether assessed by eGFR, serum creatinine level, or cystatin C level. At 6 months, no patient had a decrease of more than 50% in eGFR, although two patients who underwent renal denervation and three controls had more than a 25% decrease in eGFR.
At 6 months, the urine albumin-to-creatinine ratio had changed by a median of −3 mg/g (range −1,089 to 76) in 38 patients in the treatment group and by 1 mg/g (range −538 to 227) in 37 controls.
Most patients (88%) undergoing renal denervation underwent renal arterial imaging at 6 months, on which a single patient showed possible progression of an underlying atherosclerotic lesion that was unrelated to the procedure and that did not require intervention.
Denervation and the normal stress response. Whether renal denervation negatively affects the body’s physiologic response to stress that is normally mediated by sympathetic nerve activity was addressed in an extended investigation of Symplicity HTN-2 using cardiopulmonary exercise tests at baseline and 3 months after renal denervation.18 In the denervation group, blood pressure during exercise was significantly lower at 3 months than at baseline, but the heart rate increase at different levels of exercise was not affected. Additionally, the resting heart rate was lower and heart rate recovery after exercise improved after the procedure, particularly in patients without diabetes.
Comments. The Symplicity HTN-2 trial benefited from a randomized trial design and strict inclusion criteria of treatment resistance, but it still had notable limitations. A pretrial evaluation for causes of secondary hypertension or white-coat hypertension was not explicitly described. The control group did not undergo a sham procedure, and data analyzers were not masked to treatment assignment. Although not analyzed as a primary end point, the use of home-based and 24-hour ambulatory blood pressure assessment—measures important for determining white-coat hypertension—revealed substantial differences in blood pressure changes relative to office measurements. Because nearly all the patients (97%) were white, the generalizability of treatment results to black patients with resistant hypertension may be limited. Isolated diastolic hypertension (defined as diastolic pressure ≥ 90 mm Hg with systolic pressure < 140 mm Hg), which is more common in younger patients, was not studied.
DOES RENAL DENERVATION REDUCE SYMPATHETIC TONE?
A subgroup of 10 patients in the Symplicity HTN-1 trial whose mean 6-month office blood pressure was reduced by 22/12 mm Hg underwent assessment of renal norepinephrine spillover. A substantial (47%) reduction in renal norepinephrine spillover was noted 1 month after the procedure.14
The investigators additionally described a marked reduction in renal norepinephrine spillover from both kidneys in one patient, with a reduction of 48% from the left kidney and 75% from the right kidney 1 month after the procedure. Whole-body norepinephrine spillover in this patient was reduced by 42%. This effect was accompanied by a 50% decrease in plasma renin activity and by an increase in renal plasma flow. Aldosterone levels were not reported.19
Thus, the decrease in renal norepinephrine spillover suggests a reduction of renal efferent activity, and the decrease in total body norepinephrine spillover suggests a reduction in central sympathetic drive via the renal afferent pathway.
Microneurography in this same patient showed a gradual reduction in muscle sympathetic nerve activity to normal levels, from 56 bursts per minute at baseline to 41 at 30 days and 19 at 12 months).19 Decreased renin secretion, via circulating angiotensin II, may affect central sympathetic outflow as well.
Comments. While these findings address some of the underlying mechanisms, the small number of patients in whom these studies were done limits the generalizability of the results. The impact of the procedure on renal hemodynamics will need to be studied, including possible direct effects of the procedure, and whether there are differences in different study populations or differences based on blood pressure levels.
WHICH PATIENTS RESPOND BEST TO THIS PROCEDURE?
Although the Symplicity HTN-2 investigators report some predictors of increased reduction in blood pressure on multivariate analysis, including increased blood pressure at baseline and reduced heart rate at baseline, these are not specific enough to enable patient selection.
Interestingly, results from the expanded cohort of the Symplicity HTN-1 study found that patients on central sympatholytic agents such as clonidine had a greater reduction in blood pressure, although the reason for this is unclear.16 Identifying specific predictors of treatment success at baseline will be essential in future studies.
The earlier Symplicity trials and the ongoing Symplicity HTN-3 trial are in patients who have high blood pressure not responding to three or more antihypertensive drugs. The mean baseline systolic blood pressure in the Symplicity HTN-1 and HTN-2 trials was 178 mm Hg, and patients were taking an average of five antihypertensive drugs (Table 1). It is not known whether denervation will produce similar blood-pressure-lowering results across the spectrum of hypertension severity.
WHAT ARE THE LONG-TERM RESULTS OF DENERVATION?
Enthusiasm for the results from the Symplicity trials is tempered by concerns about the durability of the effects of the procedure, the need for better understanding of the impact of renal denervation on a wide array of pathophysiologic cascades leading to hypertension, and the effect on renal hemodynamics.
Antihypertensive efficacy has been reported to persist up to 2 years after the procedure,16 with recent unpublished data suggesting efficacy up to 3 years, but longer follow-up is needed to address whether these effects are finite.
Although reinnervation of afferent renal nerves has not been described, transplant models have shown anatomic regrowth of efferent nerves; the impact of this efferent reinnervation on blood pressure remains unclear. Experience from renal transplantation also shows that implanted kidneys that are “denervated” can still maintain fluid and electrolyte regulation.
Follow-up renal imaging in the Symplicity trials did not indicate renal artery stenosis at the sites of denervation in patients who underwent the procedure. Animal studies using the Symplicity catheter system showed renal nerve injury as evidenced by nerve fibrosis and thickened epineurium and perineurium, but no significant smooth muscle hyperplasia, arterial stenosis, or thrombosis by angiography or histology at 6 months.20
WHAT ARE THE RISKS?
Adverse effects that were noted in the short term are detailed under discussion of the trials and in Table 2.
Long-term adverse events in the Symplicity HTN-2 trial that required hospitalization were reported in five patients in the denervation group and three patients in the control group (Table 2). These included transient ischemic attacks, hypertensive crises, hypotensive episodes, angina, and nausea.
Renal function was maintained for the duration of both trials, and details regarding eGFR change have been described above under the discussion of the trials.
Diffuse visceral pain at the time of the procedure is reported as an expected occurrence, managed with intravenous analgesic medications.
DOES SYMPATHETIC DENERVATION HAVE A ROLE IN OTHER CONDITIONS?
Interestingly, other sympathetically driven diseases, such as diabetes mellitus and polycystic ovary syndrome, may prove to be targets for this therapy in the future.21
Mahfoud et al22 conducted a pilot study in 37 patients with resistant hypertension undergoing renal denervation and 13 control patients. Fasting glucose levels declined from 118 ± 3.4 mg/dL to 108 ± 3.8 mg/dL after 3 months in the intervention group (P = .039), compared with no change in the control group. Insulin and C-peptide levels were also lower in the intervention group. The reported improvement in glucose metabolism and insulin sensitivity suggests that the beneficial effects of this procedure may extend beyond blood pressure reduction.
Brandt et al23 reported regression of left ventricular hypertrophy and significantly improved cardiac functional parameters, including increase in ejection fraction and improved diastolic dysfunction, in a study of 46 patients who underwent renal denervation. This findings suggests a potential beneficial effect on cardiac remodeling.
Witkowski et al24 reported lowering of blood pressure in 10 patients with refractory hypertension and obstructive sleep apnea who underwent renal denervation, which was accompanied by improvement of sleep apnea severity.
Ukena et al25 reported reduction in ventricular tachyarrhythmias in two patients with congestive heart failure who had therapy-resistant electrical storm.
A recent pilot study in 15 patients with stage 3 and 4 chronic kidney disease (mean eGFR 31 mL/min/1.73 m2) showed significantly improved office blood pressure control up to 1 year, restoration of nocturnal dipping on 24-hour monitoring, as well as a nonsignificant trend towards increased hemoglobin levels and decreased proteinuria. No additional deterioration of renal function was reported in these patients (2 patients had renal function assessed up to 1 year).26
Thus, the benefits of this procedure may extend to other diseases that have a common underlying thread of elevated sympathetic activity, by targeting the “sympathorenal” axis.27
GUARDED OPTIMISM AND FUTURE DIRECTIONS
Given the well-known cardiovascular risks and health care costs associated with uncontrolled hypertension and the continued challenge that physicians face in managing it, novel therapies such as renal denervation may provide an adjunct to existing pharmacologic approaches.
While there is certainly cause for guarded optimism, especially with the striking blood pressure-lowering results seen in trials so far, it should be kept in mind that the mechanisms leading to the hypertensive response are complex and multifactorial, and further understanding of this therapy with long-term follow-up is needed. A comparison study with spironolactone, which is increasingly being used to treat resistant hypertension (in the absence of a diagnosis of primary aldosteronism)28,29 would help to further establish the role of this procedure.
Studies of carotid baroreceptor stimulation via an implantable device have shown sustained reduction in blood pressure in patients with resistant hypertension. A study comparing this technique with renal denervation for efficacy and safety end points could be considered in the future.30,31
The planned Symplicity HTN-3 study in the United States will be the largest trial to date, with a targeted randomization of more than 500 patients using strict enrollment criteria, including the use of maximally tolerated doses of diuretics and more focus on the use of ambulatory blood pressure monitoring and on the blinding of participants. This study will help further analysis of this technology in a more diverse population.32,33
Future studies should be designed to clarify pathophysiologic mechanisms, patient selection criteria, effects on target organ damage, and efficacy in patients with chronic kidney disease, obesity, congestive heart failure, and in less severe forms of hypertension.
A CALL FOR PARTICIPANTS IN A CLINICAL TRIAL
The Departments of Cardiology and Nephrology and Hypertension at Cleveland Clinic are currently enrolling patients in the Symplicity HTN-3 trial. For more information, please contact George Thomas, MD ([email protected]), or Mehdi Shishehbor, DO, MPH ([email protected]), or visit www.symplifybptrial.com.
Can a percutaneous catheter-based procedure effectively treat resistant hypertension?
Radiofrequency ablation of the renal sympathetic nerves is undergoing randomized controlled trials in patients who have resistant hypertension and other disorders that involve the sympathetic nervous system. Remarkably, the limited results available so far look good.
This article discusses the physiologic rationale for renal denervation, the evidence from studies in humans of the benefits, risks, and complications of the procedure, upcoming trials, and areas for future research.
DESPITE MANY TREATMENT OPTIONS, RESISTANT HYPERTENSION IS COMMON
Hypertension is a leading reason for visits to physicians in the United States and is associated with increased rates of cardiovascular disease and death.1,2 A variety of antihypertensive agents are available, and the percentage of people with hypertension whose blood pressure is under control has increased over the past 2 decades. Nevertheless, population-based studies show that the control rate remains suboptimal.3 Effective pharmacologic treatment may be limited by inadequate doses or inappropriate combinations of antihypertensive drugs, concurrent use of agents that raise the blood pressure, noncompliance with dietary restrictions, and side effects that result in poor compliance with drug therapy.
Resistant hypertension is defined as failure to achieve goal blood pressure in patients who are adhering to full tolerated doses of an appropriate three-drug regimen that includes a diuretic.1,4,5 If we use these criteria, many patients labelled as having resistant hypertension probably do not truly have it; instead, they are nonadherent to therapy or are on an inadequate or inappropriate regimen. Although the true prevalence of resistant hypertension is not clear, estimates from large clinical trials suggest that about 20% to 30% of hypertensive patients may meet the criteria for it.4 For the subset of patients who have truly resistant hypertension, nonpharmacologic treatments such as renal sympathetic denervation are an intriguing avenue.
SURGICAL SYMPATHETIC DENERVATION: TRIED AND ABANDONED IN THE 1950s
More than a half century ago, a surgical procedure, thoracolumbar sympathectomy (in which sympathetic nerve trunks and splanchnic nerves were removed), was sometimes performed to control blood pressure in patients with malignant hypertension. This was effective but caused debilitating side effects such as postural hypotension, erectile dysfunction, and syncope.
Smithwick and Thompson6 reported that, in 1,266 hypertensive patients who underwent this procedure and 467 medically treated controls, the 5-year mortality rates were 19% and 54%, respectively. Forty-five percent of those who survived the surgery had significantly lower blood pressure afterward, and the antihypertensive effect lasted 10 years or more.
The procedure fell out of favor due to the morbidity associated with this nonselective approach and to the increased availability of drug therapy.
THE SYMPATHETIC NERVOUS SYSTEM IS A DRIVER OF HYPERTENSION
A variety of evidence suggests that hyperactivation of the sympathetic nervous system plays a major role in initiating and maintaining hypertension. For example, drugs that inhibit the sympathetic drive at various levels have a blood-pressure-lowering effect. Further, direct intraneural recordings show a high level of sympathetic nerve activity in the muscles of hypertensive patients, who also have high levels of cardiac and renal norepinephrine “spillover”—ie, the amount of this neurotransmitter that escapes neuronal uptake and local metabolism and spills over into the circulation.7
The kidneys are supplied with postganglionic sympathetic nerve fibers that end in the efferent and afferent renal arterioles, the juxtaglomerular apparatus, and the renal tubular system. Studies in animals and humans have shown that an increase in efferent signals (ie, from the brain to the kidney) leads to renal vasoconstriction and decreased renal blood flow, increased renin release, and sodium retention.8,9 Afferent signals (from the kidney to the central nervous system), which are increased in states of renal ischemia, renal parenchymal injury, and hypoxia, disinhibit the vasomotor center (the nuclei tractus solitarii) in the central nervous system, leading to increased efferent signals to the kidneys, heart, and peripheral blood vessels (Figure 1).10
Enhanced sympathetic activity in patients with hypertension may play a role in subsequent target-organ damage such as left ventricular hypertrophy, congestive heart failure, and progressive renal damage.11
Studies of renal denervation in animals, using surgical and chemical techniques, have further helped to establish the role of renal sympathetic nerves in hypertension.12,13
CATHETER-BASED RENAL DENERVATION
Renal sympathetic nerves run through the adventitia of the renal arteries in a mesh-like pattern.
In the renal denervation procedure, a specially designed catheter is inserted into a femoral artery and advanced into one of the renal arteries. There, radiofrequency energy is applied to the endoluminal surface according to a proprietary algorithm, thereby delivering thermal injury selectively to the renal sympathetic nerves without affecting the abdominal, pelvic, or lower-extremity nerves. The energy delivered is lower than that used for cardiac electrophysiologic procedures.
The nerves are not imaged or mapped before treatment. The procedure is performed on both sides, with four to six sites ablated in a longitudinal and rotational manner in 2-minute treatments at each site, to cover the full circumference (Figure 1).
In the United States, the device (Symplicity Renal Denervation System; Medtronic, Inc, Mountain View, CA) is available only for investigational use.
Below, we briefly review the studies of renal denervation to date. SYMPLICITY HTN-1 Symplicity HTN-1 was a proof-of-principle study in 45 patients with resistant hypertension (Table 1).14,15
Effect on blood pressure. Six months after renal denervation, blood pressure was significantly lower than at baseline (−22/−11 mm Hg, 95% confidence interval [CI] 10/5 mm Hg) in 26 patients available for follow-up. At 12 months, the difference from baseline was −27/−10 mm Hg (95% CI 16/11 mm Hg) in 9 patients available for follow-up (Table 2).14
Evidence of the durability of blood pressure reduction came from an expanded cohort of 153 patients followed for 2 years after denervation.16
Further follow-up data showed a sustained and significant blood pressure reduction through 3 years after denervation (unpublished results presented at the 2012 annual meeting of the American College of Cardiology). Notably, patients who were initially considered to be nonresponders (defined as failure of their blood pressure to go down by at least 10 mm Hg) were all reported to have a clinical response at 36 months.
Adverse events. In the initial and expanded cohorts combined, one patient suffered a renal artery dissection due to manipulation of the guiding catheter before the radiofrequency energy was delivered, and three patients developed a femoral pseudoaneurysm. No other long-term arterial complications were observed.
Comments. Limitations of this study included a small number of patients, no control group, and a primary outcome of a reduction in office blood pressure rather than in ambulatory blood pressure.
Additionally, although the authors concluded that there was no significant deterioration in renal function during the study period, we should note that in an additional follow-up period in this cohort, 10 patients with available 2-year data had a decrease in estimated glomerular filtration rate (eGFR) of −16.0 mL/min/1.73 m2. In 5 patients who did not have spironolactone (Aldactone) or another diuretic added after the first year of followup, a lesser but significant decrease (−7.8 mL/min/1.73 m2) was noted. The investigators surmised that denervation may enhance diuretic sensitivity, leading to prerenal azotemia in some patients.17
SYMPLICITY HTN-2
The Symplicity HTN-2 trial was a larger, randomized, efficacy study that built on the earlier results, providing additional evidence of therapeutic benefit.15
An international cohort of 106 patients with resistant hypertension, defined as systolic blood pressure of 160 mm Hg or higher (or ≥ 150 mm Hg in patients with type 2 diabetes) despite the use of three or more antihypertensive medications, were randomly assigned to undergo renal denervation with the Symplicity device (n = 52) or to continue their previous treatment with antihypertensive medications alone (n = 54). The primary effectiveness end point was the change in seated office blood pressure from baseline to 6 months (Table 1).
Effect on blood pressure. In the denervation group, at 6 months, office blood pressure had changed by a mean of −32/−12 mm Hg (standard deviation [SD] 23/11 mm Hg) compared with a mean change of 1/0 mm Hg (SD 21/10 mm Hg) in the control group. Fortyone (84%) of the 49 patients who underwent denervation had a decrease in systolic blood pressure of 10 mm Hg or more at 6 months compared with baseline values, while five (10%) had no decline in systolic blood pressure. Nineteen patients had a reduction in systolic pressure to less than 140 mm Hg in the denervation group.
A subset of patients (20 in the denervation group and 25 in the control group) underwent 24-hour ambulatory blood pressure monitoring at 6 months. This showed a similar though less pronounced fall in blood pressure in the denervation group and no change in the controls. A subanalysis that censored all data for patients whose medication was increased during the follow-up period showed a blood pressure reduction of −31/−12 mm Hg (SD 22/11 mm Hg) in the renal denervation group.
Adverse events. Procedure-related adverse events included a single femoral artery pseudoaneurysm, one case of postprocedural hypotension requiring a reduction in antihypertensive medications, and 7 (13%) of 52 patients who experienced intraprocedural bradycardia requiring atropine.
Effect on renal function. No significant difference was noted between groups in the mean change in renal function at 6 months, whether assessed by eGFR, serum creatinine level, or cystatin C level. At 6 months, no patient had a decrease of more than 50% in eGFR, although two patients who underwent renal denervation and three controls had more than a 25% decrease in eGFR.
At 6 months, the urine albumin-to-creatinine ratio had changed by a median of −3 mg/g (range −1,089 to 76) in 38 patients in the treatment group and by 1 mg/g (range −538 to 227) in 37 controls.
Most patients (88%) undergoing renal denervation underwent renal arterial imaging at 6 months, on which a single patient showed possible progression of an underlying atherosclerotic lesion that was unrelated to the procedure and that did not require intervention.
Denervation and the normal stress response. Whether renal denervation negatively affects the body’s physiologic response to stress that is normally mediated by sympathetic nerve activity was addressed in an extended investigation of Symplicity HTN-2 using cardiopulmonary exercise tests at baseline and 3 months after renal denervation.18 In the denervation group, blood pressure during exercise was significantly lower at 3 months than at baseline, but the heart rate increase at different levels of exercise was not affected. Additionally, the resting heart rate was lower and heart rate recovery after exercise improved after the procedure, particularly in patients without diabetes.
Comments. The Symplicity HTN-2 trial benefited from a randomized trial design and strict inclusion criteria of treatment resistance, but it still had notable limitations. A pretrial evaluation for causes of secondary hypertension or white-coat hypertension was not explicitly described. The control group did not undergo a sham procedure, and data analyzers were not masked to treatment assignment. Although not analyzed as a primary end point, the use of home-based and 24-hour ambulatory blood pressure assessment—measures important for determining white-coat hypertension—revealed substantial differences in blood pressure changes relative to office measurements. Because nearly all the patients (97%) were white, the generalizability of treatment results to black patients with resistant hypertension may be limited. Isolated diastolic hypertension (defined as diastolic pressure ≥ 90 mm Hg with systolic pressure < 140 mm Hg), which is more common in younger patients, was not studied.
DOES RENAL DENERVATION REDUCE SYMPATHETIC TONE?
A subgroup of 10 patients in the Symplicity HTN-1 trial whose mean 6-month office blood pressure was reduced by 22/12 mm Hg underwent assessment of renal norepinephrine spillover. A substantial (47%) reduction in renal norepinephrine spillover was noted 1 month after the procedure.14
The investigators additionally described a marked reduction in renal norepinephrine spillover from both kidneys in one patient, with a reduction of 48% from the left kidney and 75% from the right kidney 1 month after the procedure. Whole-body norepinephrine spillover in this patient was reduced by 42%. This effect was accompanied by a 50% decrease in plasma renin activity and by an increase in renal plasma flow. Aldosterone levels were not reported.19
Thus, the decrease in renal norepinephrine spillover suggests a reduction of renal efferent activity, and the decrease in total body norepinephrine spillover suggests a reduction in central sympathetic drive via the renal afferent pathway.
Microneurography in this same patient showed a gradual reduction in muscle sympathetic nerve activity to normal levels, from 56 bursts per minute at baseline to 41 at 30 days and 19 at 12 months).19 Decreased renin secretion, via circulating angiotensin II, may affect central sympathetic outflow as well.
Comments. While these findings address some of the underlying mechanisms, the small number of patients in whom these studies were done limits the generalizability of the results. The impact of the procedure on renal hemodynamics will need to be studied, including possible direct effects of the procedure, and whether there are differences in different study populations or differences based on blood pressure levels.
WHICH PATIENTS RESPOND BEST TO THIS PROCEDURE?
Although the Symplicity HTN-2 investigators report some predictors of increased reduction in blood pressure on multivariate analysis, including increased blood pressure at baseline and reduced heart rate at baseline, these are not specific enough to enable patient selection.
Interestingly, results from the expanded cohort of the Symplicity HTN-1 study found that patients on central sympatholytic agents such as clonidine had a greater reduction in blood pressure, although the reason for this is unclear.16 Identifying specific predictors of treatment success at baseline will be essential in future studies.
The earlier Symplicity trials and the ongoing Symplicity HTN-3 trial are in patients who have high blood pressure not responding to three or more antihypertensive drugs. The mean baseline systolic blood pressure in the Symplicity HTN-1 and HTN-2 trials was 178 mm Hg, and patients were taking an average of five antihypertensive drugs (Table 1). It is not known whether denervation will produce similar blood-pressure-lowering results across the spectrum of hypertension severity.
WHAT ARE THE LONG-TERM RESULTS OF DENERVATION?
Enthusiasm for the results from the Symplicity trials is tempered by concerns about the durability of the effects of the procedure, the need for better understanding of the impact of renal denervation on a wide array of pathophysiologic cascades leading to hypertension, and the effect on renal hemodynamics.
Antihypertensive efficacy has been reported to persist up to 2 years after the procedure,16 with recent unpublished data suggesting efficacy up to 3 years, but longer follow-up is needed to address whether these effects are finite.
Although reinnervation of afferent renal nerves has not been described, transplant models have shown anatomic regrowth of efferent nerves; the impact of this efferent reinnervation on blood pressure remains unclear. Experience from renal transplantation also shows that implanted kidneys that are “denervated” can still maintain fluid and electrolyte regulation.
Follow-up renal imaging in the Symplicity trials did not indicate renal artery stenosis at the sites of denervation in patients who underwent the procedure. Animal studies using the Symplicity catheter system showed renal nerve injury as evidenced by nerve fibrosis and thickened epineurium and perineurium, but no significant smooth muscle hyperplasia, arterial stenosis, or thrombosis by angiography or histology at 6 months.20
WHAT ARE THE RISKS?
Adverse effects that were noted in the short term are detailed under discussion of the trials and in Table 2.
Long-term adverse events in the Symplicity HTN-2 trial that required hospitalization were reported in five patients in the denervation group and three patients in the control group (Table 2). These included transient ischemic attacks, hypertensive crises, hypotensive episodes, angina, and nausea.
Renal function was maintained for the duration of both trials, and details regarding eGFR change have been described above under the discussion of the trials.
Diffuse visceral pain at the time of the procedure is reported as an expected occurrence, managed with intravenous analgesic medications.
DOES SYMPATHETIC DENERVATION HAVE A ROLE IN OTHER CONDITIONS?
Interestingly, other sympathetically driven diseases, such as diabetes mellitus and polycystic ovary syndrome, may prove to be targets for this therapy in the future.21
Mahfoud et al22 conducted a pilot study in 37 patients with resistant hypertension undergoing renal denervation and 13 control patients. Fasting glucose levels declined from 118 ± 3.4 mg/dL to 108 ± 3.8 mg/dL after 3 months in the intervention group (P = .039), compared with no change in the control group. Insulin and C-peptide levels were also lower in the intervention group. The reported improvement in glucose metabolism and insulin sensitivity suggests that the beneficial effects of this procedure may extend beyond blood pressure reduction.
Brandt et al23 reported regression of left ventricular hypertrophy and significantly improved cardiac functional parameters, including increase in ejection fraction and improved diastolic dysfunction, in a study of 46 patients who underwent renal denervation. This findings suggests a potential beneficial effect on cardiac remodeling.
Witkowski et al24 reported lowering of blood pressure in 10 patients with refractory hypertension and obstructive sleep apnea who underwent renal denervation, which was accompanied by improvement of sleep apnea severity.
Ukena et al25 reported reduction in ventricular tachyarrhythmias in two patients with congestive heart failure who had therapy-resistant electrical storm.
A recent pilot study in 15 patients with stage 3 and 4 chronic kidney disease (mean eGFR 31 mL/min/1.73 m2) showed significantly improved office blood pressure control up to 1 year, restoration of nocturnal dipping on 24-hour monitoring, as well as a nonsignificant trend towards increased hemoglobin levels and decreased proteinuria. No additional deterioration of renal function was reported in these patients (2 patients had renal function assessed up to 1 year).26
Thus, the benefits of this procedure may extend to other diseases that have a common underlying thread of elevated sympathetic activity, by targeting the “sympathorenal” axis.27
GUARDED OPTIMISM AND FUTURE DIRECTIONS
Given the well-known cardiovascular risks and health care costs associated with uncontrolled hypertension and the continued challenge that physicians face in managing it, novel therapies such as renal denervation may provide an adjunct to existing pharmacologic approaches.
While there is certainly cause for guarded optimism, especially with the striking blood pressure-lowering results seen in trials so far, it should be kept in mind that the mechanisms leading to the hypertensive response are complex and multifactorial, and further understanding of this therapy with long-term follow-up is needed. A comparison study with spironolactone, which is increasingly being used to treat resistant hypertension (in the absence of a diagnosis of primary aldosteronism)28,29 would help to further establish the role of this procedure.
Studies of carotid baroreceptor stimulation via an implantable device have shown sustained reduction in blood pressure in patients with resistant hypertension. A study comparing this technique with renal denervation for efficacy and safety end points could be considered in the future.30,31
The planned Symplicity HTN-3 study in the United States will be the largest trial to date, with a targeted randomization of more than 500 patients using strict enrollment criteria, including the use of maximally tolerated doses of diuretics and more focus on the use of ambulatory blood pressure monitoring and on the blinding of participants. This study will help further analysis of this technology in a more diverse population.32,33
Future studies should be designed to clarify pathophysiologic mechanisms, patient selection criteria, effects on target organ damage, and efficacy in patients with chronic kidney disease, obesity, congestive heart failure, and in less severe forms of hypertension.
A CALL FOR PARTICIPANTS IN A CLINICAL TRIAL
The Departments of Cardiology and Nephrology and Hypertension at Cleveland Clinic are currently enrolling patients in the Symplicity HTN-3 trial. For more information, please contact George Thomas, MD ([email protected]), or Mehdi Shishehbor, DO, MPH ([email protected]), or visit www.symplifybptrial.com.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Vital Health Stat 13( 169) 2011. http://www.cdc.gov/nchs/data/series/sr_13/sr13_169.pdf. Accessed April 24, 2012.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc 1953; 152:1501–1504.
- Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 2009; 54:1195–1201.
- Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation 1977; 56:691–698.
- Kon V. Neural control of renal circulation. Miner Electrolyte Metab 1989; 15:33–43.
- Campese VM. Neurogenic factors and hypertension in renal disease. Kidney Int Suppl 2000; 75:S2–S6.
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34:724–728.
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 2004; 287:H695–H703.
- Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol 1983; 245:F1–F14.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatmentresistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Petidis K, Anyfanti P, Doumas M. Renal sympathetic denervation: renal function concerns. Hypertension 2011; 58:e19; author replye20.
- Ukena C, Mahfoud F, Kindermann I, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol 2011; 58:1176–1182.
- Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension (letter). N Engl J Med 2009; 361:932–934.
- Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol 2011; 100:1095–1101.
- Schlaich MP, Straznicky N, Grima M, et al. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens 2011; 29:991–996.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
- Ukena C, Bauer A, Mahfoud F, et al. Renal sympathetic denervation for treatment of electrical storm: first-inman experience. Clin Res Cardiol 2012; 101:63–67.
- Herring D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012; May 17[Epub ahead of print]
- Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Böhm M, Krum H. Sympatho-renal axis in chronic disease. Clin Res Cardiol 2011; 100:1049–1057.
- Chapman N, Dobson J, Wilson S, et al; Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003; 16:925–930.
- Papademetriou V, Doumas M, Faselis C, et al. Carotid baroreceptor stimulation for the treatment of resistant hypertension. Int J Hypertens 2011; 2011:964394.
- Ng MM, Sica DA, Frishman WH. Rheos: an implantable carotid sinus stimulation device for the nonpharmacologic treatment of resistant hypertension. Cardiol Rev 2011; 19:52–57.
- US National Institutes of Health. Renal denervation in patients with uncontrolled hypertension (SYMPLICITY HTN-3). http://www.clinicaltrials.gov/ct2/show/NCT01418261. Accessed June 7, 2012.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the Symplicity HTN-3 trial. Clin Cardiol 2012 May 9. [Epub ahead of print]
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Vital Health Stat 13( 169) 2011. http://www.cdc.gov/nchs/data/series/sr_13/sr13_169.pdf. Accessed April 24, 2012.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc 1953; 152:1501–1504.
- Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 2009; 54:1195–1201.
- Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation 1977; 56:691–698.
- Kon V. Neural control of renal circulation. Miner Electrolyte Metab 1989; 15:33–43.
- Campese VM. Neurogenic factors and hypertension in renal disease. Kidney Int Suppl 2000; 75:S2–S6.
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34:724–728.
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 2004; 287:H695–H703.
- Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol 1983; 245:F1–F14.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatmentresistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Petidis K, Anyfanti P, Doumas M. Renal sympathetic denervation: renal function concerns. Hypertension 2011; 58:e19; author replye20.
- Ukena C, Mahfoud F, Kindermann I, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol 2011; 58:1176–1182.
- Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension (letter). N Engl J Med 2009; 361:932–934.
- Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol 2011; 100:1095–1101.
- Schlaich MP, Straznicky N, Grima M, et al. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens 2011; 29:991–996.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
- Ukena C, Bauer A, Mahfoud F, et al. Renal sympathetic denervation for treatment of electrical storm: first-inman experience. Clin Res Cardiol 2012; 101:63–67.
- Herring D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012; May 17[Epub ahead of print]
- Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Böhm M, Krum H. Sympatho-renal axis in chronic disease. Clin Res Cardiol 2011; 100:1049–1057.
- Chapman N, Dobson J, Wilson S, et al; Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003; 16:925–930.
- Papademetriou V, Doumas M, Faselis C, et al. Carotid baroreceptor stimulation for the treatment of resistant hypertension. Int J Hypertens 2011; 2011:964394.
- Ng MM, Sica DA, Frishman WH. Rheos: an implantable carotid sinus stimulation device for the nonpharmacologic treatment of resistant hypertension. Cardiol Rev 2011; 19:52–57.
- US National Institutes of Health. Renal denervation in patients with uncontrolled hypertension (SYMPLICITY HTN-3). http://www.clinicaltrials.gov/ct2/show/NCT01418261. Accessed June 7, 2012.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the Symplicity HTN-3 trial. Clin Cardiol 2012 May 9. [Epub ahead of print]
KEY POINTS
- Renal sympathetic nerves help regulate volume and blood pressure as they innervate the renal tubules, blood vessels, and juxtaglomerular apparatus. They carry both afferent and efferent signals between the central nervous system and the kidneys.
- Surgical sympathectomy was done in the 1950s for malignant hypertension. It had lasting antihypertensive results but also caused severe procedure-related morbidity. A new percutaneous procedure for selective renal denervation offers the advantage of causing few major procedure-related adverse effects.
- Selective renal denervation decreases norepinephrine spillover and muscle sympathetic nerve activity, evidence that the procedure reduces sympathetic tone.
- The major clinical trials done so far have found that renal denervation lowers blood pressure significantly, and the reduction is sustained for at least 3 years.
Correction: Anemia, leukocytosis, abdominal pain, flushing, and bone and skin lesion
In the June 2012 issue, on page 384 of the Clinical Picture article by Álvarez-Twose et al (Álvarez-Twose I, Vañó-Galván S, Sanchez-Muñoz L, Fernandez-Zapardiel S, Escribano L. The Clinical Picture: anemia, leukocytosis, abdominal pain, flushing, and bone and skin lesions. Cleve Clin J Med 2012; 79:384–386), Dr. Alvarez-Twose’s first name was spelled incorrectly. The correct spelling is Iván. This error has been corrected in the online version.
In the June 2012 issue, on page 384 of the Clinical Picture article by Álvarez-Twose et al (Álvarez-Twose I, Vañó-Galván S, Sanchez-Muñoz L, Fernandez-Zapardiel S, Escribano L. The Clinical Picture: anemia, leukocytosis, abdominal pain, flushing, and bone and skin lesions. Cleve Clin J Med 2012; 79:384–386), Dr. Alvarez-Twose’s first name was spelled incorrectly. The correct spelling is Iván. This error has been corrected in the online version.
In the June 2012 issue, on page 384 of the Clinical Picture article by Álvarez-Twose et al (Álvarez-Twose I, Vañó-Galván S, Sanchez-Muñoz L, Fernandez-Zapardiel S, Escribano L. The Clinical Picture: anemia, leukocytosis, abdominal pain, flushing, and bone and skin lesions. Cleve Clin J Med 2012; 79:384–386), Dr. Alvarez-Twose’s first name was spelled incorrectly. The correct spelling is Iván. This error has been corrected in the online version.
Septic Arthritis and Osteomyelitis Due to the Chromoblastomycosis Agent Fonsecaea pedrosoi
Lichen Planus: An Update and Review
A perplexing case of altered mental status
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Agitated and paranoid
Mr. E, age 55, presents to the emergency department (ED) with a 2-week history of altered mental status (AMS). His wife reports, “He was normal one day and the next day he was not.” Mr. E also presents with sleep disturbance, decreased appetite and speech, and a 20-lb weight loss. His family reports no recent stressors or head trauma. Mr. E is agitated in the ED and receives a single dose of IV haloperidol, 5 mg. He exhibits paranoia and is afraid to get a CT scan. The medical team attempts a lumbar puncture (LP), but Mr. E does not cooperate.
His laboratory values are: potassium, 3.0 mEq/L; creatinine, 1.60 mg/dL; calcium, 10.6 mg/dL; thyroid-stimulating hormone, 0.177 IU/L; vitamin B12, >1500 pg/mL; folate, >20 ng/mL; and creatine kinase, 281 U/L. Urine drug screen is positive for benzodiazepines and opiates, neither of which was prescribed, and blood alcohol is negative.
Mr. E is admitted for further workup of AMS. His daughter-in-law states that Mr. E is an alcoholic and she is concerned that he may have mixed drugs and alcohol. The medical service starts Mr. E on empiric antimicrobials—vancomycin, ceftriaxone, and acyclovir—because of his AMS, and performs an LP to rule out intracranial pathology. His LP results are unremarkable.
Mr. E appears to be confused during psychiatric evaluation. He requests to be “hypnotized on a helicopter to find out what is wrong with me.” His wife states that Mr. E drank vodka daily but decreased his alcohol consumption after surgery 5 months ago. Before his current admission, he was drinking approximately half a glass of vodka every few days, according to his wife. Mr. E says he has no prior psychiatric admissions. During the mental status exam, his eye contact is poor, with latency of response to questions, thought blocking, and psychomotor retardation. He is alert, oriented to time, place, and person, and cooperative. He cannot concentrate or focus during the interview. He denies suicidal or homicidal ideation.
The authors’ observations
Mr. E appeared to be delirious, as evidenced by the sudden onset of waxing and waning changes in consciousness, attention deficits, and cognition. He also had a history of daily alcohol use and decreased his alcohol intake after a surgery 5 months ago, which puts him at risk for Wernicke’s encephalopathy.1-3 The type of surgery and whether he received adequate thiamine supplementation at that time was unclear. Because Mr. E is older, he has a higher risk of mortality and morbidity from delirium.4,5 We started Mr. E on quetiapine, 50 mg/d, for delirium and an IV lorazepam taper, starting at 2 mg every 8 hours, because the extent of his alcohol and benzodiazepine use was unclear—we weren’t sure how forthcoming he was about his alcohol use. He received IV thiamine supplementation followed by oral thiamine, 100 mg/d.
The authors’ recommendations
We requested a neurology consult, EEG, CSF cultures, and brain MRI (Table 1).6 EEG, chest radiography, thyroid scan, and CT scan were normal and MRI showed no acute intracranial process. However, there was a redemonstration of increased T1 signal seen within the bilateral basal ganglia and relative diminutive appearance to the bilateral mamillary bodies, which suggests possible liver disease and/or alcohol abuse. These findings were unchanged from an MRI Mr. E received 10 years ago, were consistent with his history of alcohol abuse, and may indicate an underlying predisposition to delirium. A CT scan of the abdomen showed hepatic cirrhosis with prominent, tortuous vessels of the upper abdomen, subtle ill-defined hypodensity of the anterior aspect of the liver, and an enlarged spleen.
Mr. E’s mental functioning did not improve with quetiapine and lorazepam. Further evaluation revealed a negative human immunodeficiency virus test and normal heavy metals, ammonia, ceruloplasmin, and thiamine. We suspected limbic encephalitis because of Mr. E’s memory problems and behavioral and psychiatric manifestations,7 but CSF was unremarkable and limbic encephalitis workup of CSF and paraneoplastic antibody panel were negative.
Mr. E’s primary care physician stated that at an appointment 1 month ago, Mr. E was alert, oriented, and conversational with normal thought processing. At that time he had presented with rectal bleeding, occult blood in his stool, and an unintentional 25-lb weight loss over 2 months. It was not clear if his weight loss was caused by poor nutrition—which is common among chronic alcoholics—or an occult disease process.
After 10 days, Mr. E was discharged home from the medicine service with no clear cause of his AMS.
Table 1
Suggested workup for altered mental status
| Complete blood count, basic metabolic profile, creatine kinase |
| Thyroid-stimulating hormone, thyroid scan |
| Vitamin B12, folate, thiamine |
| Blood alcohol, urine drug screen |
| Urine analysis and cultures |
| Lumbar puncture—CSF staining and cultures |
| Chest radiography |
| CT and MRI scan of brain |
| Electroencephalography |
| Neuropsychiatric testing |
| CSF: cerebrospinal fluid Source: Reference 6 |
EVALUATION: Worsening behavior
One week later, Mr. E presents to the ED with continued AMS and worsening behavior at home. Two days ago, he attempted to strangle his dog and cut himself with a knife. His paranoia was worsening and his oral intake continued to decrease. In the ED, Mr. E does not want a chest radiograph because, “I don’t like radiation contaminating my body”; his family stated that he had been suspicious of radiography all of his life. He receives empiric ceftriaxone because of a possible urinary tract infection. Urine culture is positive for Pseudomonas aeruginosa and he is switched to ciprofloxacin. Mr. E is admitted for further workup.
Mr. E’s mother states, “I think this change in behavior is related to my son drinking alcohol for 20 years. This is exactly how he acted when he was on drugs. I think he is having a flashback.” She also reports her son purchased multiple chemicals—the details of which are unclear—that he left lying around the house.
His wife says that after discharge a week ago, Mr. E was stable for 1 or 2 days and then “he started going downhill.” He became more paranoid and he started talking about cameras watching him in his house. Mr. E took quetiapine, 50 mg/d, for a few days, then refused because he thought there was something in the medication. Mr. E’s family feels that at times he is responding to internal stimuli. He makes statements about his DNA being changed and reports that he has 2 wives and the wife in the room was not the real one, which suggests Capgras syndrome. His wife provides a home medication list that includes vitamin B complex, vitamins B12, E, and C, a multivitamin, zinc, magnesium, fish oil, garlic, calcium, glucosamine, chondroitin, herbal supplements, and gingko. The psychiatry team recommends switching from quetiapine to olanzapine, 15 mg/d, because Mr. E was paranoid about taking quetiapine.
We determine that Mr. E does not have medical decision-making capacity.
Because his symptoms do not improve, Mr. E is transferred to the psychiatric intensive care unit. His mental status shows little change while there. Neuropsychiatric testing shows only “cognitive deficits.” He shows signs consistent with neurologic dysfunction in terms of stimulus-bound responding and perseveration, which is compatible with the bilateral basal ganglia lesion seen on MRI. However, some of his behaviors suggest psychiatric and motivationally driven or manipulative etiology. During this testing he was difficult to evaluate and needed to be convinced to engage. At times he was illogical and at other times he showed good focus and recall. It is difficult to draw more definitive conclusions and Mr. E is discharged home with minor improvement in his symptoms. He didn’t attend follow-up appointments. During a courtesy call a few months after his admission, his wife revealed that Mr. E had died after shooting himself. It is unclear if it was an accident or suicide.
The authors’ observations
Mr. E’s diagnosis remains unclear (for a summary of his clinical course, see Table 2). Although his initial presentation was consistent with delirium, the lack of an identifiable medical cause, prolonged time course, and lack of improvement with dopamine blocking agents suggest additional diagnoses such as Wernicke-Korsakoff syndrome, rapidly progressive dementia, or a substance-induced disorder. He displayed paranoia and bizarre delusions, which would suggest a thought disorder. However, he also had a history of substance use. A few months after we saw Mr. E, “bath salt” (methylenedioxypyrovalerone) abuse gained national attention. Patients with bath salt intoxication present with confusion, paranoia, and behavioral disturbances as well as a prolonged course.8
Mr. E’s CT and MRI scans, history of alcohol use, and cirrhosis also point to Wernicke-Korsakoff syndrome as an underlying diagnosis. It is unclear whether Mr. E experienced alcohol withdrawal and IV glucose without adequate thiamine replacement during a prior surgery. However, MRI findings were unchanged from a previous study 10 years ago.
It is puzzling whether Mr. E’s AMS was a first psychotic break, a result of drug and alcohol use, rapidly progressing dementia, or another neurologic problem that we have not identified. Our tentative diagnosis was Wernicke-Korsakoff syndrome because of his history of alcohol use and imaging findings.
Although we used a multidisciplinary team approach that included psychiatry, internal medicine, neurology, neuropsychology, and an aggressive and thorough workup, we could not establish a definitive diagnosis. Unsolved cases such as this can leave patients and clinicians frustrated and may lead to unfavorable outcomes. Additional resources such as a telephone call after the first missed appointment may have been warranted.
Table 2
Mr. E’s clinical course
| Symptoms | Treatment | |
|---|---|---|
| First ED visit | Agitation Confusion Sleep disturbance Decreased appetite and speech 20-lb weight loss | Empiric antimicrobials for possible meningitis Haloperidol for agitation Quetiapine for delirium Lorazepam taper Thiamine supplementation |
| Second ED visit | Violent behavior Worsening paranoia Responding to internal stimuli Mr. E believes he has 2 wives, but the wife in the room is not the real one, which suggests possible Capgras syndrome Cognitive deficits on mental status exam | Switch from ceftriaxone to ciprofloxacin for Pseudomonas aeruginosa Switch from quetiapine to olanzapine |
| ED: emergency department | ||
Related Resources
- Kaufman DM. Clinical neurology for psychiatrists. 6th ed. Philadelphia, PA: Saunders Elsevier; 2007.
- Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
Drug Brand Names
- Acyclovir • Zovirax
- Ceftriaxone • Rocephin
- Ciprofloxacin • Cipro
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Thiamine • Betaxin
- Vancomycin • Vancocin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jiang W, Gagliardi JP, Raj YP, et al. Acute psychotic disorder after gastric bypass surgery: differential diagnosis and treatment. Am J Psychiatry. 2006;163(1):15-19.
2. Harrison RA, Vu T, Hunter AJ. Wernicke’s encephalopathy in a patient with schizophrenia. J Gen Intern Med. 2006;21(12):C8-C11.
3. Sechi GP, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and treatment. Lancet Neurol. 2007;6(5):442-455.
4. American Psychiatric Association. Practice guidelines for the treatment of patients with delirium. Am J Psychiatry. 1999;156(5 suppl):1-20.
5. Sharon KI, Fearing MA, Marcantonio RA. Delirium. In: Halter JB Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill Medical; 2009:647–658.
6. Sadock BJ, Sadock VA. Delirium dementia, and amnestic and other cognitive disorders. In: Sadock BJ, Kaplan HI, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. Philadelphia, PA: Lippincott Williams and Wilkins; 2007:319–372.
7. Ahmad SA, Archer HA, Rice CM, et al. Seronegative limbic encephalitis: case report, literature review and proposed treatment algorithm. Pract Neurol. 2011;11(6):355-361.
8. Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967-968.
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Agitated and paranoid
Mr. E, age 55, presents to the emergency department (ED) with a 2-week history of altered mental status (AMS). His wife reports, “He was normal one day and the next day he was not.” Mr. E also presents with sleep disturbance, decreased appetite and speech, and a 20-lb weight loss. His family reports no recent stressors or head trauma. Mr. E is agitated in the ED and receives a single dose of IV haloperidol, 5 mg. He exhibits paranoia and is afraid to get a CT scan. The medical team attempts a lumbar puncture (LP), but Mr. E does not cooperate.
His laboratory values are: potassium, 3.0 mEq/L; creatinine, 1.60 mg/dL; calcium, 10.6 mg/dL; thyroid-stimulating hormone, 0.177 IU/L; vitamin B12, >1500 pg/mL; folate, >20 ng/mL; and creatine kinase, 281 U/L. Urine drug screen is positive for benzodiazepines and opiates, neither of which was prescribed, and blood alcohol is negative.
Mr. E is admitted for further workup of AMS. His daughter-in-law states that Mr. E is an alcoholic and she is concerned that he may have mixed drugs and alcohol. The medical service starts Mr. E on empiric antimicrobials—vancomycin, ceftriaxone, and acyclovir—because of his AMS, and performs an LP to rule out intracranial pathology. His LP results are unremarkable.
Mr. E appears to be confused during psychiatric evaluation. He requests to be “hypnotized on a helicopter to find out what is wrong with me.” His wife states that Mr. E drank vodka daily but decreased his alcohol consumption after surgery 5 months ago. Before his current admission, he was drinking approximately half a glass of vodka every few days, according to his wife. Mr. E says he has no prior psychiatric admissions. During the mental status exam, his eye contact is poor, with latency of response to questions, thought blocking, and psychomotor retardation. He is alert, oriented to time, place, and person, and cooperative. He cannot concentrate or focus during the interview. He denies suicidal or homicidal ideation.
The authors’ observations
Mr. E appeared to be delirious, as evidenced by the sudden onset of waxing and waning changes in consciousness, attention deficits, and cognition. He also had a history of daily alcohol use and decreased his alcohol intake after a surgery 5 months ago, which puts him at risk for Wernicke’s encephalopathy.1-3 The type of surgery and whether he received adequate thiamine supplementation at that time was unclear. Because Mr. E is older, he has a higher risk of mortality and morbidity from delirium.4,5 We started Mr. E on quetiapine, 50 mg/d, for delirium and an IV lorazepam taper, starting at 2 mg every 8 hours, because the extent of his alcohol and benzodiazepine use was unclear—we weren’t sure how forthcoming he was about his alcohol use. He received IV thiamine supplementation followed by oral thiamine, 100 mg/d.
The authors’ recommendations
We requested a neurology consult, EEG, CSF cultures, and brain MRI (Table 1).6 EEG, chest radiography, thyroid scan, and CT scan were normal and MRI showed no acute intracranial process. However, there was a redemonstration of increased T1 signal seen within the bilateral basal ganglia and relative diminutive appearance to the bilateral mamillary bodies, which suggests possible liver disease and/or alcohol abuse. These findings were unchanged from an MRI Mr. E received 10 years ago, were consistent with his history of alcohol abuse, and may indicate an underlying predisposition to delirium. A CT scan of the abdomen showed hepatic cirrhosis with prominent, tortuous vessels of the upper abdomen, subtle ill-defined hypodensity of the anterior aspect of the liver, and an enlarged spleen.
Mr. E’s mental functioning did not improve with quetiapine and lorazepam. Further evaluation revealed a negative human immunodeficiency virus test and normal heavy metals, ammonia, ceruloplasmin, and thiamine. We suspected limbic encephalitis because of Mr. E’s memory problems and behavioral and psychiatric manifestations,7 but CSF was unremarkable and limbic encephalitis workup of CSF and paraneoplastic antibody panel were negative.
Mr. E’s primary care physician stated that at an appointment 1 month ago, Mr. E was alert, oriented, and conversational with normal thought processing. At that time he had presented with rectal bleeding, occult blood in his stool, and an unintentional 25-lb weight loss over 2 months. It was not clear if his weight loss was caused by poor nutrition—which is common among chronic alcoholics—or an occult disease process.
After 10 days, Mr. E was discharged home from the medicine service with no clear cause of his AMS.
Table 1
Suggested workup for altered mental status
| Complete blood count, basic metabolic profile, creatine kinase |
| Thyroid-stimulating hormone, thyroid scan |
| Vitamin B12, folate, thiamine |
| Blood alcohol, urine drug screen |
| Urine analysis and cultures |
| Lumbar puncture—CSF staining and cultures |
| Chest radiography |
| CT and MRI scan of brain |
| Electroencephalography |
| Neuropsychiatric testing |
| CSF: cerebrospinal fluid Source: Reference 6 |
EVALUATION: Worsening behavior
One week later, Mr. E presents to the ED with continued AMS and worsening behavior at home. Two days ago, he attempted to strangle his dog and cut himself with a knife. His paranoia was worsening and his oral intake continued to decrease. In the ED, Mr. E does not want a chest radiograph because, “I don’t like radiation contaminating my body”; his family stated that he had been suspicious of radiography all of his life. He receives empiric ceftriaxone because of a possible urinary tract infection. Urine culture is positive for Pseudomonas aeruginosa and he is switched to ciprofloxacin. Mr. E is admitted for further workup.
Mr. E’s mother states, “I think this change in behavior is related to my son drinking alcohol for 20 years. This is exactly how he acted when he was on drugs. I think he is having a flashback.” She also reports her son purchased multiple chemicals—the details of which are unclear—that he left lying around the house.
His wife says that after discharge a week ago, Mr. E was stable for 1 or 2 days and then “he started going downhill.” He became more paranoid and he started talking about cameras watching him in his house. Mr. E took quetiapine, 50 mg/d, for a few days, then refused because he thought there was something in the medication. Mr. E’s family feels that at times he is responding to internal stimuli. He makes statements about his DNA being changed and reports that he has 2 wives and the wife in the room was not the real one, which suggests Capgras syndrome. His wife provides a home medication list that includes vitamin B complex, vitamins B12, E, and C, a multivitamin, zinc, magnesium, fish oil, garlic, calcium, glucosamine, chondroitin, herbal supplements, and gingko. The psychiatry team recommends switching from quetiapine to olanzapine, 15 mg/d, because Mr. E was paranoid about taking quetiapine.
We determine that Mr. E does not have medical decision-making capacity.
Because his symptoms do not improve, Mr. E is transferred to the psychiatric intensive care unit. His mental status shows little change while there. Neuropsychiatric testing shows only “cognitive deficits.” He shows signs consistent with neurologic dysfunction in terms of stimulus-bound responding and perseveration, which is compatible with the bilateral basal ganglia lesion seen on MRI. However, some of his behaviors suggest psychiatric and motivationally driven or manipulative etiology. During this testing he was difficult to evaluate and needed to be convinced to engage. At times he was illogical and at other times he showed good focus and recall. It is difficult to draw more definitive conclusions and Mr. E is discharged home with minor improvement in his symptoms. He didn’t attend follow-up appointments. During a courtesy call a few months after his admission, his wife revealed that Mr. E had died after shooting himself. It is unclear if it was an accident or suicide.
The authors’ observations
Mr. E’s diagnosis remains unclear (for a summary of his clinical course, see Table 2). Although his initial presentation was consistent with delirium, the lack of an identifiable medical cause, prolonged time course, and lack of improvement with dopamine blocking agents suggest additional diagnoses such as Wernicke-Korsakoff syndrome, rapidly progressive dementia, or a substance-induced disorder. He displayed paranoia and bizarre delusions, which would suggest a thought disorder. However, he also had a history of substance use. A few months after we saw Mr. E, “bath salt” (methylenedioxypyrovalerone) abuse gained national attention. Patients with bath salt intoxication present with confusion, paranoia, and behavioral disturbances as well as a prolonged course.8
Mr. E’s CT and MRI scans, history of alcohol use, and cirrhosis also point to Wernicke-Korsakoff syndrome as an underlying diagnosis. It is unclear whether Mr. E experienced alcohol withdrawal and IV glucose without adequate thiamine replacement during a prior surgery. However, MRI findings were unchanged from a previous study 10 years ago.
It is puzzling whether Mr. E’s AMS was a first psychotic break, a result of drug and alcohol use, rapidly progressing dementia, or another neurologic problem that we have not identified. Our tentative diagnosis was Wernicke-Korsakoff syndrome because of his history of alcohol use and imaging findings.
Although we used a multidisciplinary team approach that included psychiatry, internal medicine, neurology, neuropsychology, and an aggressive and thorough workup, we could not establish a definitive diagnosis. Unsolved cases such as this can leave patients and clinicians frustrated and may lead to unfavorable outcomes. Additional resources such as a telephone call after the first missed appointment may have been warranted.
Table 2
Mr. E’s clinical course
| Symptoms | Treatment | |
|---|---|---|
| First ED visit | Agitation Confusion Sleep disturbance Decreased appetite and speech 20-lb weight loss | Empiric antimicrobials for possible meningitis Haloperidol for agitation Quetiapine for delirium Lorazepam taper Thiamine supplementation |
| Second ED visit | Violent behavior Worsening paranoia Responding to internal stimuli Mr. E believes he has 2 wives, but the wife in the room is not the real one, which suggests possible Capgras syndrome Cognitive deficits on mental status exam | Switch from ceftriaxone to ciprofloxacin for Pseudomonas aeruginosa Switch from quetiapine to olanzapine |
| ED: emergency department | ||
Related Resources
- Kaufman DM. Clinical neurology for psychiatrists. 6th ed. Philadelphia, PA: Saunders Elsevier; 2007.
- Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
Drug Brand Names
- Acyclovir • Zovirax
- Ceftriaxone • Rocephin
- Ciprofloxacin • Cipro
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Thiamine • Betaxin
- Vancomycin • Vancocin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Agitated and paranoid
Mr. E, age 55, presents to the emergency department (ED) with a 2-week history of altered mental status (AMS). His wife reports, “He was normal one day and the next day he was not.” Mr. E also presents with sleep disturbance, decreased appetite and speech, and a 20-lb weight loss. His family reports no recent stressors or head trauma. Mr. E is agitated in the ED and receives a single dose of IV haloperidol, 5 mg. He exhibits paranoia and is afraid to get a CT scan. The medical team attempts a lumbar puncture (LP), but Mr. E does not cooperate.
His laboratory values are: potassium, 3.0 mEq/L; creatinine, 1.60 mg/dL; calcium, 10.6 mg/dL; thyroid-stimulating hormone, 0.177 IU/L; vitamin B12, >1500 pg/mL; folate, >20 ng/mL; and creatine kinase, 281 U/L. Urine drug screen is positive for benzodiazepines and opiates, neither of which was prescribed, and blood alcohol is negative.
Mr. E is admitted for further workup of AMS. His daughter-in-law states that Mr. E is an alcoholic and she is concerned that he may have mixed drugs and alcohol. The medical service starts Mr. E on empiric antimicrobials—vancomycin, ceftriaxone, and acyclovir—because of his AMS, and performs an LP to rule out intracranial pathology. His LP results are unremarkable.
Mr. E appears to be confused during psychiatric evaluation. He requests to be “hypnotized on a helicopter to find out what is wrong with me.” His wife states that Mr. E drank vodka daily but decreased his alcohol consumption after surgery 5 months ago. Before his current admission, he was drinking approximately half a glass of vodka every few days, according to his wife. Mr. E says he has no prior psychiatric admissions. During the mental status exam, his eye contact is poor, with latency of response to questions, thought blocking, and psychomotor retardation. He is alert, oriented to time, place, and person, and cooperative. He cannot concentrate or focus during the interview. He denies suicidal or homicidal ideation.
The authors’ observations
Mr. E appeared to be delirious, as evidenced by the sudden onset of waxing and waning changes in consciousness, attention deficits, and cognition. He also had a history of daily alcohol use and decreased his alcohol intake after a surgery 5 months ago, which puts him at risk for Wernicke’s encephalopathy.1-3 The type of surgery and whether he received adequate thiamine supplementation at that time was unclear. Because Mr. E is older, he has a higher risk of mortality and morbidity from delirium.4,5 We started Mr. E on quetiapine, 50 mg/d, for delirium and an IV lorazepam taper, starting at 2 mg every 8 hours, because the extent of his alcohol and benzodiazepine use was unclear—we weren’t sure how forthcoming he was about his alcohol use. He received IV thiamine supplementation followed by oral thiamine, 100 mg/d.
The authors’ recommendations
We requested a neurology consult, EEG, CSF cultures, and brain MRI (Table 1).6 EEG, chest radiography, thyroid scan, and CT scan were normal and MRI showed no acute intracranial process. However, there was a redemonstration of increased T1 signal seen within the bilateral basal ganglia and relative diminutive appearance to the bilateral mamillary bodies, which suggests possible liver disease and/or alcohol abuse. These findings were unchanged from an MRI Mr. E received 10 years ago, were consistent with his history of alcohol abuse, and may indicate an underlying predisposition to delirium. A CT scan of the abdomen showed hepatic cirrhosis with prominent, tortuous vessels of the upper abdomen, subtle ill-defined hypodensity of the anterior aspect of the liver, and an enlarged spleen.
Mr. E’s mental functioning did not improve with quetiapine and lorazepam. Further evaluation revealed a negative human immunodeficiency virus test and normal heavy metals, ammonia, ceruloplasmin, and thiamine. We suspected limbic encephalitis because of Mr. E’s memory problems and behavioral and psychiatric manifestations,7 but CSF was unremarkable and limbic encephalitis workup of CSF and paraneoplastic antibody panel were negative.
Mr. E’s primary care physician stated that at an appointment 1 month ago, Mr. E was alert, oriented, and conversational with normal thought processing. At that time he had presented with rectal bleeding, occult blood in his stool, and an unintentional 25-lb weight loss over 2 months. It was not clear if his weight loss was caused by poor nutrition—which is common among chronic alcoholics—or an occult disease process.
After 10 days, Mr. E was discharged home from the medicine service with no clear cause of his AMS.
Table 1
Suggested workup for altered mental status
| Complete blood count, basic metabolic profile, creatine kinase |
| Thyroid-stimulating hormone, thyroid scan |
| Vitamin B12, folate, thiamine |
| Blood alcohol, urine drug screen |
| Urine analysis and cultures |
| Lumbar puncture—CSF staining and cultures |
| Chest radiography |
| CT and MRI scan of brain |
| Electroencephalography |
| Neuropsychiatric testing |
| CSF: cerebrospinal fluid Source: Reference 6 |
EVALUATION: Worsening behavior
One week later, Mr. E presents to the ED with continued AMS and worsening behavior at home. Two days ago, he attempted to strangle his dog and cut himself with a knife. His paranoia was worsening and his oral intake continued to decrease. In the ED, Mr. E does not want a chest radiograph because, “I don’t like radiation contaminating my body”; his family stated that he had been suspicious of radiography all of his life. He receives empiric ceftriaxone because of a possible urinary tract infection. Urine culture is positive for Pseudomonas aeruginosa and he is switched to ciprofloxacin. Mr. E is admitted for further workup.
Mr. E’s mother states, “I think this change in behavior is related to my son drinking alcohol for 20 years. This is exactly how he acted when he was on drugs. I think he is having a flashback.” She also reports her son purchased multiple chemicals—the details of which are unclear—that he left lying around the house.
His wife says that after discharge a week ago, Mr. E was stable for 1 or 2 days and then “he started going downhill.” He became more paranoid and he started talking about cameras watching him in his house. Mr. E took quetiapine, 50 mg/d, for a few days, then refused because he thought there was something in the medication. Mr. E’s family feels that at times he is responding to internal stimuli. He makes statements about his DNA being changed and reports that he has 2 wives and the wife in the room was not the real one, which suggests Capgras syndrome. His wife provides a home medication list that includes vitamin B complex, vitamins B12, E, and C, a multivitamin, zinc, magnesium, fish oil, garlic, calcium, glucosamine, chondroitin, herbal supplements, and gingko. The psychiatry team recommends switching from quetiapine to olanzapine, 15 mg/d, because Mr. E was paranoid about taking quetiapine.
We determine that Mr. E does not have medical decision-making capacity.
Because his symptoms do not improve, Mr. E is transferred to the psychiatric intensive care unit. His mental status shows little change while there. Neuropsychiatric testing shows only “cognitive deficits.” He shows signs consistent with neurologic dysfunction in terms of stimulus-bound responding and perseveration, which is compatible with the bilateral basal ganglia lesion seen on MRI. However, some of his behaviors suggest psychiatric and motivationally driven or manipulative etiology. During this testing he was difficult to evaluate and needed to be convinced to engage. At times he was illogical and at other times he showed good focus and recall. It is difficult to draw more definitive conclusions and Mr. E is discharged home with minor improvement in his symptoms. He didn’t attend follow-up appointments. During a courtesy call a few months after his admission, his wife revealed that Mr. E had died after shooting himself. It is unclear if it was an accident or suicide.
The authors’ observations
Mr. E’s diagnosis remains unclear (for a summary of his clinical course, see Table 2). Although his initial presentation was consistent with delirium, the lack of an identifiable medical cause, prolonged time course, and lack of improvement with dopamine blocking agents suggest additional diagnoses such as Wernicke-Korsakoff syndrome, rapidly progressive dementia, or a substance-induced disorder. He displayed paranoia and bizarre delusions, which would suggest a thought disorder. However, he also had a history of substance use. A few months after we saw Mr. E, “bath salt” (methylenedioxypyrovalerone) abuse gained national attention. Patients with bath salt intoxication present with confusion, paranoia, and behavioral disturbances as well as a prolonged course.8
Mr. E’s CT and MRI scans, history of alcohol use, and cirrhosis also point to Wernicke-Korsakoff syndrome as an underlying diagnosis. It is unclear whether Mr. E experienced alcohol withdrawal and IV glucose without adequate thiamine replacement during a prior surgery. However, MRI findings were unchanged from a previous study 10 years ago.
It is puzzling whether Mr. E’s AMS was a first psychotic break, a result of drug and alcohol use, rapidly progressing dementia, or another neurologic problem that we have not identified. Our tentative diagnosis was Wernicke-Korsakoff syndrome because of his history of alcohol use and imaging findings.
Although we used a multidisciplinary team approach that included psychiatry, internal medicine, neurology, neuropsychology, and an aggressive and thorough workup, we could not establish a definitive diagnosis. Unsolved cases such as this can leave patients and clinicians frustrated and may lead to unfavorable outcomes. Additional resources such as a telephone call after the first missed appointment may have been warranted.
Table 2
Mr. E’s clinical course
| Symptoms | Treatment | |
|---|---|---|
| First ED visit | Agitation Confusion Sleep disturbance Decreased appetite and speech 20-lb weight loss | Empiric antimicrobials for possible meningitis Haloperidol for agitation Quetiapine for delirium Lorazepam taper Thiamine supplementation |
| Second ED visit | Violent behavior Worsening paranoia Responding to internal stimuli Mr. E believes he has 2 wives, but the wife in the room is not the real one, which suggests possible Capgras syndrome Cognitive deficits on mental status exam | Switch from ceftriaxone to ciprofloxacin for Pseudomonas aeruginosa Switch from quetiapine to olanzapine |
| ED: emergency department | ||
Related Resources
- Kaufman DM. Clinical neurology for psychiatrists. 6th ed. Philadelphia, PA: Saunders Elsevier; 2007.
- Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
Drug Brand Names
- Acyclovir • Zovirax
- Ceftriaxone • Rocephin
- Ciprofloxacin • Cipro
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Thiamine • Betaxin
- Vancomycin • Vancocin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jiang W, Gagliardi JP, Raj YP, et al. Acute psychotic disorder after gastric bypass surgery: differential diagnosis and treatment. Am J Psychiatry. 2006;163(1):15-19.
2. Harrison RA, Vu T, Hunter AJ. Wernicke’s encephalopathy in a patient with schizophrenia. J Gen Intern Med. 2006;21(12):C8-C11.
3. Sechi GP, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and treatment. Lancet Neurol. 2007;6(5):442-455.
4. American Psychiatric Association. Practice guidelines for the treatment of patients with delirium. Am J Psychiatry. 1999;156(5 suppl):1-20.
5. Sharon KI, Fearing MA, Marcantonio RA. Delirium. In: Halter JB Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill Medical; 2009:647–658.
6. Sadock BJ, Sadock VA. Delirium dementia, and amnestic and other cognitive disorders. In: Sadock BJ, Kaplan HI, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. Philadelphia, PA: Lippincott Williams and Wilkins; 2007:319–372.
7. Ahmad SA, Archer HA, Rice CM, et al. Seronegative limbic encephalitis: case report, literature review and proposed treatment algorithm. Pract Neurol. 2011;11(6):355-361.
8. Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967-968.
1. Jiang W, Gagliardi JP, Raj YP, et al. Acute psychotic disorder after gastric bypass surgery: differential diagnosis and treatment. Am J Psychiatry. 2006;163(1):15-19.
2. Harrison RA, Vu T, Hunter AJ. Wernicke’s encephalopathy in a patient with schizophrenia. J Gen Intern Med. 2006;21(12):C8-C11.
3. Sechi GP, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and treatment. Lancet Neurol. 2007;6(5):442-455.
4. American Psychiatric Association. Practice guidelines for the treatment of patients with delirium. Am J Psychiatry. 1999;156(5 suppl):1-20.
5. Sharon KI, Fearing MA, Marcantonio RA. Delirium. In: Halter JB Ouslander JG, Tinetti ME, et al, eds. Hazzard’s geriatric medicine and gerontology. 6th ed. New York, NY: McGraw-Hill Medical; 2009:647–658.
6. Sadock BJ, Sadock VA. Delirium dementia, and amnestic and other cognitive disorders. In: Sadock BJ, Kaplan HI, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. Philadelphia, PA: Lippincott Williams and Wilkins; 2007:319–372.
7. Ahmad SA, Archer HA, Rice CM, et al. Seronegative limbic encephalitis: case report, literature review and proposed treatment algorithm. Pract Neurol. 2011;11(6):355-361.
8. Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967-968.