User login
Payment Reform Proposals Take Shape
Medicare’s experiment with bundling episodes of care is finding some encouraging signs of life after fee-for-service (see “A Bundle of Nerves” in the November issue of The Hospitalist). But beyond orthopedics, cardiology, and cardiovascular surgery, what diagnosis-related groups (DRGs) should be bundled, and how should such bundles be fairly divided?
Some healthcare administrators say the system might work best in areas with high device costs, such as spine surgery. SHM supports provisions in the Affordable Care Act establishing a voluntary national pilot program on bundling payments to healthcare providers, and in 2009 backed pilot programs for high-risk medical populations with COPD or congestive heart failure. Cynthia Mason, project manager with the CMS Medicare Demonstrations Group, says the latter is definitely on the list of resource-heavy conditions Medicare will be scrutinizing. “But, obviously, looking at chronic conditions is more challenging because the service is not as standardized as, say, a surgical procedure,” she adds.
That concern, in fact, is driving some of the pessimism from other healthcare experts.
“I think it’s not at all clear that there are very many conditions amenable to bundling,” says Robert Berenson, MD, a senior fellow in the Urban Institute’s Health Policy Center and vice chair of the Medicare Payment Advisory Commission (MedPAC). “Once you get down to the cases that everybody agrees lend themselves to bundling, it may be you're dealing with too small a percentage of spending to really want to go this route."
Emerging efforts to calculate how bundled payments should be fairly divided, however, also might provide more clarity on the best bundling candidates. The experimental PROMETHEUS payment model, developed by the Newton, Conn.-based Health Care Incentives Improvement Institute, is one example. It uses what are called evidence-informed case rates, or ECRs, to assign a budget for an entire episode of care. According to the nonprofit organization, ECRs are adjusted based on the severity and complexity of each patient’s condition, and an algorithm figures out how to divide the check.
There are limits, of course, in dealing with multiple comorbidities right off the bat. Even so, Stuart Guterman, vice president of the Washington, D.C.-based Commonwealth Fund's Program on Payment and System Reform, thinks a big chunk of our healthcare system's costs could be addressed with a limited number of well-defined but high-expense categories.
Click here to listen to Dr. Berenson and Guterman further discuss Medicare payment reform.
Medicare’s experiment with bundling episodes of care is finding some encouraging signs of life after fee-for-service (see “A Bundle of Nerves” in the November issue of The Hospitalist). But beyond orthopedics, cardiology, and cardiovascular surgery, what diagnosis-related groups (DRGs) should be bundled, and how should such bundles be fairly divided?
Some healthcare administrators say the system might work best in areas with high device costs, such as spine surgery. SHM supports provisions in the Affordable Care Act establishing a voluntary national pilot program on bundling payments to healthcare providers, and in 2009 backed pilot programs for high-risk medical populations with COPD or congestive heart failure. Cynthia Mason, project manager with the CMS Medicare Demonstrations Group, says the latter is definitely on the list of resource-heavy conditions Medicare will be scrutinizing. “But, obviously, looking at chronic conditions is more challenging because the service is not as standardized as, say, a surgical procedure,” she adds.
That concern, in fact, is driving some of the pessimism from other healthcare experts.
“I think it’s not at all clear that there are very many conditions amenable to bundling,” says Robert Berenson, MD, a senior fellow in the Urban Institute’s Health Policy Center and vice chair of the Medicare Payment Advisory Commission (MedPAC). “Once you get down to the cases that everybody agrees lend themselves to bundling, it may be you're dealing with too small a percentage of spending to really want to go this route."
Emerging efforts to calculate how bundled payments should be fairly divided, however, also might provide more clarity on the best bundling candidates. The experimental PROMETHEUS payment model, developed by the Newton, Conn.-based Health Care Incentives Improvement Institute, is one example. It uses what are called evidence-informed case rates, or ECRs, to assign a budget for an entire episode of care. According to the nonprofit organization, ECRs are adjusted based on the severity and complexity of each patient’s condition, and an algorithm figures out how to divide the check.
There are limits, of course, in dealing with multiple comorbidities right off the bat. Even so, Stuart Guterman, vice president of the Washington, D.C.-based Commonwealth Fund's Program on Payment and System Reform, thinks a big chunk of our healthcare system's costs could be addressed with a limited number of well-defined but high-expense categories.
Click here to listen to Dr. Berenson and Guterman further discuss Medicare payment reform.
Medicare’s experiment with bundling episodes of care is finding some encouraging signs of life after fee-for-service (see “A Bundle of Nerves” in the November issue of The Hospitalist). But beyond orthopedics, cardiology, and cardiovascular surgery, what diagnosis-related groups (DRGs) should be bundled, and how should such bundles be fairly divided?
Some healthcare administrators say the system might work best in areas with high device costs, such as spine surgery. SHM supports provisions in the Affordable Care Act establishing a voluntary national pilot program on bundling payments to healthcare providers, and in 2009 backed pilot programs for high-risk medical populations with COPD or congestive heart failure. Cynthia Mason, project manager with the CMS Medicare Demonstrations Group, says the latter is definitely on the list of resource-heavy conditions Medicare will be scrutinizing. “But, obviously, looking at chronic conditions is more challenging because the service is not as standardized as, say, a surgical procedure,” she adds.
That concern, in fact, is driving some of the pessimism from other healthcare experts.
“I think it’s not at all clear that there are very many conditions amenable to bundling,” says Robert Berenson, MD, a senior fellow in the Urban Institute’s Health Policy Center and vice chair of the Medicare Payment Advisory Commission (MedPAC). “Once you get down to the cases that everybody agrees lend themselves to bundling, it may be you're dealing with too small a percentage of spending to really want to go this route."
Emerging efforts to calculate how bundled payments should be fairly divided, however, also might provide more clarity on the best bundling candidates. The experimental PROMETHEUS payment model, developed by the Newton, Conn.-based Health Care Incentives Improvement Institute, is one example. It uses what are called evidence-informed case rates, or ECRs, to assign a budget for an entire episode of care. According to the nonprofit organization, ECRs are adjusted based on the severity and complexity of each patient’s condition, and an algorithm figures out how to divide the check.
There are limits, of course, in dealing with multiple comorbidities right off the bat. Even so, Stuart Guterman, vice president of the Washington, D.C.-based Commonwealth Fund's Program on Payment and System Reform, thinks a big chunk of our healthcare system's costs could be addressed with a limited number of well-defined but high-expense categories.
Click here to listen to Dr. Berenson and Guterman further discuss Medicare payment reform.
Treatment of CML continues to progress
NEW YORK—Even with a 10-year overall survival (OS) rate of 84%-90%, researchers continue to carry out trials on emerging drugs to try and find therapies that provide a better outlook for patients.
According to Susan O’Brien, MD, of MD Anderson Cancer Center in Houston, Texas, who presented at the NCCN 5th Annual Congress on Hematologic Malignancies, even if a better treatment is available, it will be tough to demonstrate survival benefits.
Dr O’Brien listed standard-dose imatinib, high-dose imatinib, imatinib-based combinations, second generation tyrosine kinase inhibitors (TKIs) and stem cell transplant as possible frontline therapies, noting that imatinib-based combinations are only used in clinical trials and second-generation TKIs are not available commercially.
At 8 years of follow-up, data on imatinib shows a survival rate of about 84%-90%, compared to about 50% for stem cell transplant, which continues to decrease over time. Dr O’Brien believes transplant is a viable option for some patients down the line, but at this point in time for chronic stage patients, transplant is not a reasonable option when one compares these two survival curves upfront.
Most recommendations for the use of imatinib come from follow-up on the IRIS trial, Hochhaus et al 2009, said Dr O’Brien. The trial compared patients on imatinib vs interferon (IFN-α) and low-dose Ara-C, and led to the approval of imatinib for frontline therapy.
Follow-up of the IRIS trial continues to be crucial because there are not 20- or 30-year data available. Now is the 8-year data will help physicians treat current patients.
Because imatinib is such a targeted therapy, investigators thought that if patients were on imatinib long enough they would develop a mutation or something that leads to resistance, said Dr O’Brien. And as time went on, they expected to see increasingly more episodes of transformation.
“When rate of transformation came out, people were surprised.... What you see is the opposite. If you see transformation, it happens early on,” she said
This finding led to the hypothesis that in some patients there is a very small resistant clone up front. When these patients are administered imatinib , it allows the resistant clone to emerge and form resistant disease. “There are no standard techniques that will pick up this clone, so there is no reason for testing,” she added.
She also pointed out that there has not been enough follow-up to form criteria for suboptimal response. Suboptimal response at 6 months may be more like failure than suboptimal response at 12 months. Dr O’Brien noted that if the response is not technically a failure, the guidelines say imatinib can be continued. But in fact, she said, many people on the NCCN guidelines committee felt that the imatinib dose should be increased in the case of suboptimal response.
Although imatinib has revolutionized the treatment of CML, the search continues for an even better option.
To this end, investigators have conducted 2 trials comparing imatinib head-to-head with nilotinib (Larson et al, 2010 ASCO abstract) or dasatinib (Kantarjian et al, NEJM 2010) in newly diagnosed patients.
At 12 months, nilotinib 300 mg and 400 mg twice a day produced a greater percentage of major molecular responses (MMR) than imatinib 400 mg once a day (44%, 43% vs 22%, respectively). More patients on either dose of nilotinib experienced complete cytogenetic response (CCyR) than with imatinib at 12 months (80%, 78% vs 65%, respectively) and overall response (85%, 82% vs 74%, respectively).
Dasatinib also proved to be more effective than imatinib. More patients randomized to 100mg of dasatinib once a day experienced CCyr by 12 months than patients randomized to 400mg of imatinib once a day (83% vs 72%) as frontline treatment. Percentages of confirmed CCyR were 77% for desatinib and 66% for imatinib. Also, 1.9% of patients receiving dasatinib progressed to accelerated phase or blast phase, compared to 3.5% of patients receiving imatinib.
Dasatinib and nilotonib both result in lower rates of anemia and neutropenia than imatinib. Imatinib therapy still has the lowest occurrence of thrombocytopenia.
With all these new developments and less than 10 years of data to go on, Dr O’Brien brought up some issues physicians should consider when choosing frontline therapy for CML, including the relevance of short-term endpoints, the difficulty of assessing survival differences among developing therapies, the cost of drugs, and the spectrum of toxicities. ![]()
NEW YORK—Even with a 10-year overall survival (OS) rate of 84%-90%, researchers continue to carry out trials on emerging drugs to try and find therapies that provide a better outlook for patients.
According to Susan O’Brien, MD, of MD Anderson Cancer Center in Houston, Texas, who presented at the NCCN 5th Annual Congress on Hematologic Malignancies, even if a better treatment is available, it will be tough to demonstrate survival benefits.
Dr O’Brien listed standard-dose imatinib, high-dose imatinib, imatinib-based combinations, second generation tyrosine kinase inhibitors (TKIs) and stem cell transplant as possible frontline therapies, noting that imatinib-based combinations are only used in clinical trials and second-generation TKIs are not available commercially.
At 8 years of follow-up, data on imatinib shows a survival rate of about 84%-90%, compared to about 50% for stem cell transplant, which continues to decrease over time. Dr O’Brien believes transplant is a viable option for some patients down the line, but at this point in time for chronic stage patients, transplant is not a reasonable option when one compares these two survival curves upfront.
Most recommendations for the use of imatinib come from follow-up on the IRIS trial, Hochhaus et al 2009, said Dr O’Brien. The trial compared patients on imatinib vs interferon (IFN-α) and low-dose Ara-C, and led to the approval of imatinib for frontline therapy.
Follow-up of the IRIS trial continues to be crucial because there are not 20- or 30-year data available. Now is the 8-year data will help physicians treat current patients.
Because imatinib is such a targeted therapy, investigators thought that if patients were on imatinib long enough they would develop a mutation or something that leads to resistance, said Dr O’Brien. And as time went on, they expected to see increasingly more episodes of transformation.
“When rate of transformation came out, people were surprised.... What you see is the opposite. If you see transformation, it happens early on,” she said
This finding led to the hypothesis that in some patients there is a very small resistant clone up front. When these patients are administered imatinib , it allows the resistant clone to emerge and form resistant disease. “There are no standard techniques that will pick up this clone, so there is no reason for testing,” she added.
She also pointed out that there has not been enough follow-up to form criteria for suboptimal response. Suboptimal response at 6 months may be more like failure than suboptimal response at 12 months. Dr O’Brien noted that if the response is not technically a failure, the guidelines say imatinib can be continued. But in fact, she said, many people on the NCCN guidelines committee felt that the imatinib dose should be increased in the case of suboptimal response.
Although imatinib has revolutionized the treatment of CML, the search continues for an even better option.
To this end, investigators have conducted 2 trials comparing imatinib head-to-head with nilotinib (Larson et al, 2010 ASCO abstract) or dasatinib (Kantarjian et al, NEJM 2010) in newly diagnosed patients.
At 12 months, nilotinib 300 mg and 400 mg twice a day produced a greater percentage of major molecular responses (MMR) than imatinib 400 mg once a day (44%, 43% vs 22%, respectively). More patients on either dose of nilotinib experienced complete cytogenetic response (CCyR) than with imatinib at 12 months (80%, 78% vs 65%, respectively) and overall response (85%, 82% vs 74%, respectively).
Dasatinib also proved to be more effective than imatinib. More patients randomized to 100mg of dasatinib once a day experienced CCyr by 12 months than patients randomized to 400mg of imatinib once a day (83% vs 72%) as frontline treatment. Percentages of confirmed CCyR were 77% for desatinib and 66% for imatinib. Also, 1.9% of patients receiving dasatinib progressed to accelerated phase or blast phase, compared to 3.5% of patients receiving imatinib.
Dasatinib and nilotonib both result in lower rates of anemia and neutropenia than imatinib. Imatinib therapy still has the lowest occurrence of thrombocytopenia.
With all these new developments and less than 10 years of data to go on, Dr O’Brien brought up some issues physicians should consider when choosing frontline therapy for CML, including the relevance of short-term endpoints, the difficulty of assessing survival differences among developing therapies, the cost of drugs, and the spectrum of toxicities. ![]()
NEW YORK—Even with a 10-year overall survival (OS) rate of 84%-90%, researchers continue to carry out trials on emerging drugs to try and find therapies that provide a better outlook for patients.
According to Susan O’Brien, MD, of MD Anderson Cancer Center in Houston, Texas, who presented at the NCCN 5th Annual Congress on Hematologic Malignancies, even if a better treatment is available, it will be tough to demonstrate survival benefits.
Dr O’Brien listed standard-dose imatinib, high-dose imatinib, imatinib-based combinations, second generation tyrosine kinase inhibitors (TKIs) and stem cell transplant as possible frontline therapies, noting that imatinib-based combinations are only used in clinical trials and second-generation TKIs are not available commercially.
At 8 years of follow-up, data on imatinib shows a survival rate of about 84%-90%, compared to about 50% for stem cell transplant, which continues to decrease over time. Dr O’Brien believes transplant is a viable option for some patients down the line, but at this point in time for chronic stage patients, transplant is not a reasonable option when one compares these two survival curves upfront.
Most recommendations for the use of imatinib come from follow-up on the IRIS trial, Hochhaus et al 2009, said Dr O’Brien. The trial compared patients on imatinib vs interferon (IFN-α) and low-dose Ara-C, and led to the approval of imatinib for frontline therapy.
Follow-up of the IRIS trial continues to be crucial because there are not 20- or 30-year data available. Now is the 8-year data will help physicians treat current patients.
Because imatinib is such a targeted therapy, investigators thought that if patients were on imatinib long enough they would develop a mutation or something that leads to resistance, said Dr O’Brien. And as time went on, they expected to see increasingly more episodes of transformation.
“When rate of transformation came out, people were surprised.... What you see is the opposite. If you see transformation, it happens early on,” she said
This finding led to the hypothesis that in some patients there is a very small resistant clone up front. When these patients are administered imatinib , it allows the resistant clone to emerge and form resistant disease. “There are no standard techniques that will pick up this clone, so there is no reason for testing,” she added.
She also pointed out that there has not been enough follow-up to form criteria for suboptimal response. Suboptimal response at 6 months may be more like failure than suboptimal response at 12 months. Dr O’Brien noted that if the response is not technically a failure, the guidelines say imatinib can be continued. But in fact, she said, many people on the NCCN guidelines committee felt that the imatinib dose should be increased in the case of suboptimal response.
Although imatinib has revolutionized the treatment of CML, the search continues for an even better option.
To this end, investigators have conducted 2 trials comparing imatinib head-to-head with nilotinib (Larson et al, 2010 ASCO abstract) or dasatinib (Kantarjian et al, NEJM 2010) in newly diagnosed patients.
At 12 months, nilotinib 300 mg and 400 mg twice a day produced a greater percentage of major molecular responses (MMR) than imatinib 400 mg once a day (44%, 43% vs 22%, respectively). More patients on either dose of nilotinib experienced complete cytogenetic response (CCyR) than with imatinib at 12 months (80%, 78% vs 65%, respectively) and overall response (85%, 82% vs 74%, respectively).
Dasatinib also proved to be more effective than imatinib. More patients randomized to 100mg of dasatinib once a day experienced CCyr by 12 months than patients randomized to 400mg of imatinib once a day (83% vs 72%) as frontline treatment. Percentages of confirmed CCyR were 77% for desatinib and 66% for imatinib. Also, 1.9% of patients receiving dasatinib progressed to accelerated phase or blast phase, compared to 3.5% of patients receiving imatinib.
Dasatinib and nilotonib both result in lower rates of anemia and neutropenia than imatinib. Imatinib therapy still has the lowest occurrence of thrombocytopenia.
With all these new developments and less than 10 years of data to go on, Dr O’Brien brought up some issues physicians should consider when choosing frontline therapy for CML, including the relevance of short-term endpoints, the difficulty of assessing survival differences among developing therapies, the cost of drugs, and the spectrum of toxicities. ![]()
Pending Tests at Discharge
The period following discharge is a vulnerable time for patientsthe prevalence of medical errors related to this transition is high and has important patient safety and medico‐legal ramifications.13 Factors contributing to this vulnerability include complexity of hospitalized patients, shorter lengths of stay, and increased discontinuity of care. Hospitalists have recognized this threat to patient safety and have worked toward improving information exchange between inpatient and outpatient providers at hospital discharge.46 Nonetheless, the evidence suggests that more work is necessary. A recent study found that discharge summaries are often incomplete, and do not contain important information requiring follow‐up, such as pending tests.7 Additionally, a review by Kripalani et al. characterizing information deficits at hospital discharge found few interventions which specifically improve communication of pending tests at hospital discharge.8
In a prior study we determined that 41% of patients left the hospital before all laboratory and radiology test results were finalized. Of these results, 9.4% were potentially actionable and could have altered management. Physicians were aware of only 38% of post‐discharge test results.9 This awareness gap is a consequence of several factors including the lack of systems to track and alert providers of test results finalized post discharge. Also, it is unclear who is responsible for pending tests at discharge, since these tests are ordered by the inpatient physicians but often reported in the time period between hospital discharge and the patient's first follow‐up appointment with the primary care physician (PCP). Because responsibility is not explicitly made in the final communication between physicians at discharge, such test results may not be reviewed in a timely manner, potentially resulting in delays in treatment, a need for readmission, or other unfavorable outcomes.
Even in integrated health systems with advanced electronic health records, missed test results which result in treatment delays remain prevalent.10, 11 Test result management applications aid clinicians in reviewing and acting upon results as they become available and such systems may provide solutions to this problem. At Partners Healthcare in Boston, the Results Manager (RM) application was developed to help clinicians in the ambulatory setting safely, reliably, and efficiently review and act upon test results. The application enables clinicians to prioritize test results, utilize guidelines, and generate letters to patients. This system also prompts physicians to set reminders for future testing.12 In a 2.5‐year study evaluating the impact of this intervention, PCPs at 26 adult primary care practices were able to expedite communication of outpatient laboratory and imaging test results to patients with the help of RM. Patients of physicians who participated in the project reported greater satisfaction with test result communication and with information provided about their condition than did a control group of similar patients.13 RM has not yet been studied in the inpatient setting or at care transitions. We describe an attempt at modifying the Partners RM application to help inpatient physicians manage pending tests at hospital discharge.
Methods
Study Setting and Participants
We piloted our application at 2 major academic medical centers (hospitals A and B) associated with Partners Healthcare, an integrated regional health delivery network in eastern Massachusetts, from October 2004 to March 2005. Both centers use the longitudinal medical record (LMR), the electronic medical record (EMR), for nearly all ambulatory practices. The LMR is an internally developed full‐featured EMR, including a repository of laboratory and radiology reports, discharge summaries, ambulatory care notes, medication lists, problem lists, coded allergies, and other patient data. Both centers also have their own inpatient results viewing and order entry systems which provide clinicians caring for patients in the hospital the ability to review results and write orders. Although possible, clinicians caring for patients in the inpatient setting do not routinely access LMR to view test results. Inpatient physician use of the LMR is generally limited to review of the outpatient record, medication lists, and ambulatory notes at admission.
At hospital A, the hospitalist attending physician is typically responsible for all communication to outpatient physicians at discharge, as well as for follow‐up on all test results that return after discharge. Hospital B has 2 types of hospitalist services. One is staffed only by hospitalist and nonhospitalist attending physicians. Nonhospitalist attending physicians were excluded because they care for their own patients in the inpatient and ambulatory setting and typically use RM to manage test results. The other hospitalist service at hospital B is a teaching service consisting of an attending physician, resident, and interns. For this service, the resident is responsible for communication at discharge and follow‐up on all pending tests. For purposes of this study inpatient physicians refers to those physicians responsible for communication with PCPs and follow‐up on pending tests. All inpatient physicians were eligible to participate during the study period.
Test Result Management Application
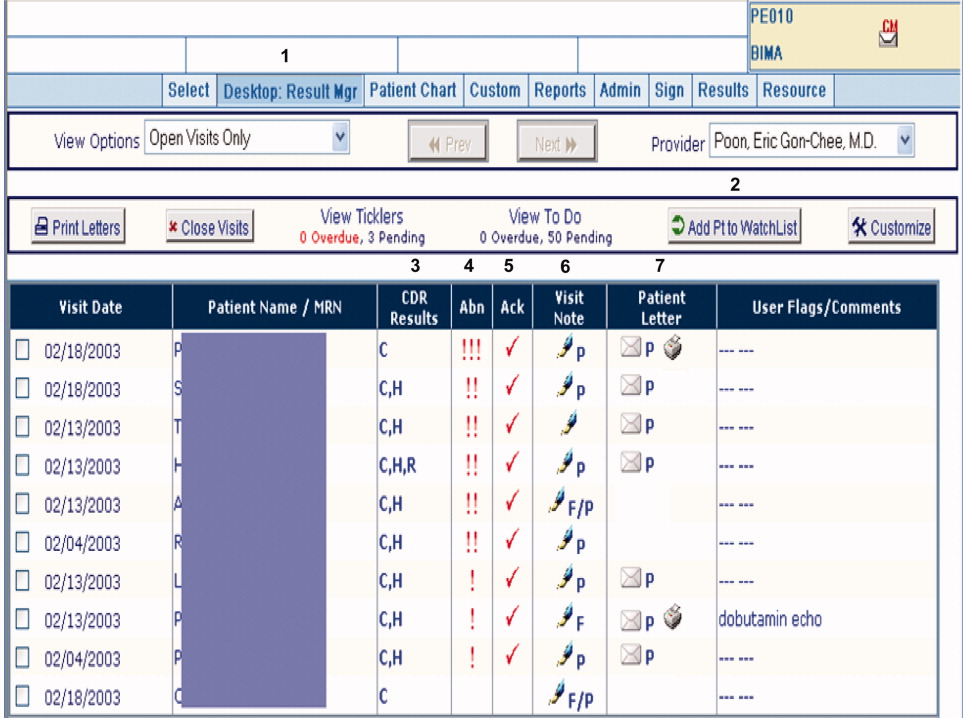
RM was originally developed by Partners Healthcare to improve timely review and appropriate management of test results in the ambulatory setting. RM was developed for and vetted by primarily ambulatory physicians. The application is browser‐based, provider‐centric, and embedded in the LMR to help ambulatory clinicians review and act upon test results in a safe, reliable, and efficient manner. Although RM has access to all inpatient and outpatient data in the Partners Clinical Data Repository (CDR), given the volume of inpatient tests ordered, hospital‐based results are suppressed by default to limit inundating ambulatory clinicians' queues. Therefore, users of RM only receive results of laboratory and radiology tests ordered in the ambulatory setting. They can track these tests for specific patients for a designated period of time by placing the patient on a watch list. Finally, RM incorporates extensive decision support features to classify the degree of abnormality for each result, presents guidelines to help clinicians manage abnormal results, allows clinicians to generate result letters to patients using predefined, context‐sensitive templates, and prompts physicians to set reminders for future testing. Because RM was developed from the ambulatory perspective, there was limited input from hospitalist physicians with regard to inpatient workflow in the original design of the module.12 See Figure 1 for a screen shot of RM and a description of its features.
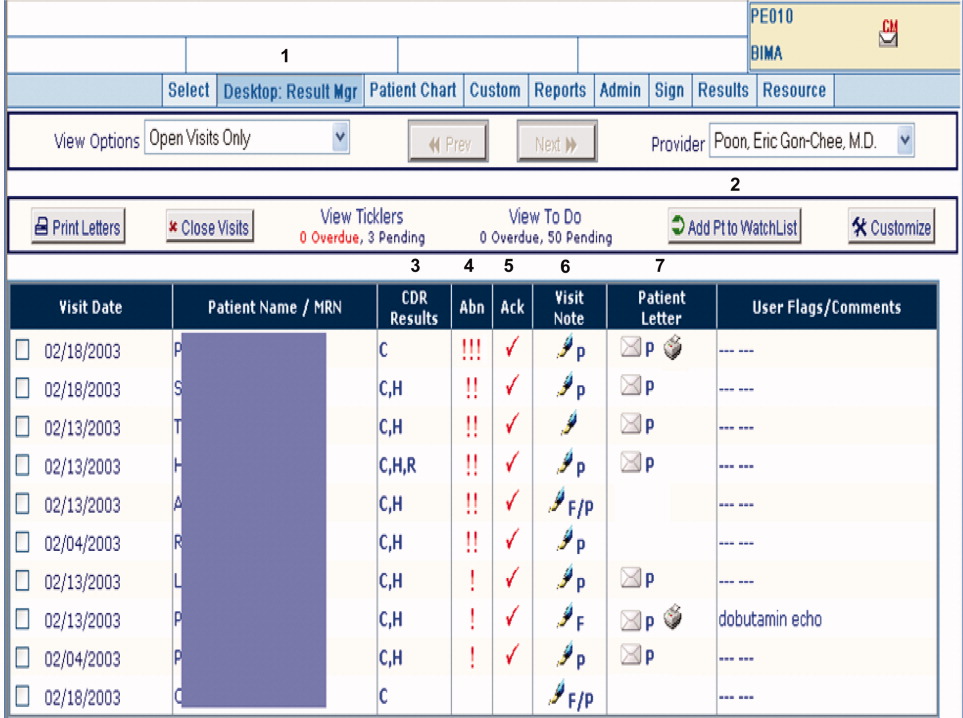
figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For purposes of this pilot, we modified RM to allow results of tests ordered in the inpatient setting to be available for viewing (Hospitalist Results Manager, HRM). This feature was turned on only for inpatient physicians as previously defined. Inpatient tests, including pending tests at discharge, continued to be suppressed from PCP's RM queue (however, any physician could access a patient's test result(s) directly from the Partners CDR). Inpatient physicians could track laboratory and radiology results finalized after discharge by keeping discharged patients on their HRM watch list for a designated period of time. The finalized results would become available for review in their HRM queue and abnormal results were displayed prominently at the top of this queue. Inpatient physicians were trained to use HRM in a series of meetings and demonstrations. Although HRM could be accessed from inpatient clinical workstations, it was not part of the inpatient clinical information system.
Surveys
Study surveys were developed and refined through an iterative process and pilot tested among inpatient physicians at both centers for clarity. We surveyed inpatient physicians five months after HRM implementation. Inpatient physicians were asked how often they used HRM, what barriers they faced (respondents asked to quantify agreement to statements on a 5‐point Likert scale), and which elements of an ideal system they would prefer. Finally, we solicited comments regarding perceived obstacles and suggestions for improvement. Because HRM was targeted to inpatient physicians, and because RM has been evaluated from the ambulatory perspective in a prior study,13 PCPs were not surveyed. See Supporting Information Appendix for the survey instrument used in the study.
Results
A total of 35 inpatient physicians participated in the pilot. Among 649 patients discharged during the study period, there were 1075 tests pending of which 555 were subsequently flagged as abnormal in HRM. Study surveys were sent to the 35 inpatient physician participants and 29 were completed, including partial responses (83% survey response rate). The 35 inpatient physician participants had the following characteristics: 22 were male, 13 were female; 21 were trainees and 14 were nontrainees/faculty. All 21 trainees were PGY2s. Nontrainees and faculty varied in experience level (PGY 15: 5, PGY 610: 7, PGY 1120: 1, PGY 21+: 1). Of 29 survey respondents, 7 were from hospital A and 22 were from hospital B; 19 were trainees and 10 were nontrainees/faculty. Of the 6 nonrespondents, 2 were from hospital A and 4 were from hospital B; 2 were trainees and 4 were nontrainees/faculty.
Table 1 shows the results of our survey of inpatient physicians regarding usage of HRM. Of 29 survey respondents, 14 (48%) reported never using HRM. Thirteen (45%) reported using HRM 1 to 2 times per week. None of the respondents used it more than 4 times per week. The frequency of usage was similar for hospitals A and B. Table 2 details barriers to using HRM. Twenty‐three inpatient physicians (79%) reported barriers. Seventeen (59%) thought that results in their HRM queue were not clinically relevant, 16 (55%) felt that HRM did not fit into their daily workflow, 14 (48%) had limited time to use HRM, and 12 (41%) noted that too many results in their HRM queue were on other physician's patients. Seven (24%) reported operational issues and 3 (10%) reported technical issues prohibiting use of HRM. With regard to preferred elements of an ideal results manager system, 21 (72%) inpatient physician respondents wanted to receive notification of abnormal and clinician‐designated pending test results. Four (14%) wanted to receive only abnormal results and 1 (3%) wanted to receive all results. Twenty‐seven (93%) physicians agreed that an ideally designed computerized test result management application would be valuable for managing pending tests at discharge.
| Frequency | Number of Inpatient Physicians Using HRM, n (%) | ||
|---|---|---|---|
| Overall | Hospital A | Hospital B | |
| |||
| Never | 14 (48) | 3 (43) | 11 (50) |
| 12 times per week | 13 (45) | 3 (43) | 10 (45.5) |
| 34 times per week | 2 (7) | 1 (14) | 1 (4.5) |
| 57 times per week | 0 | 0 | 0 |
| >7 times per week | 0 | 0 | 0 |
| Barrier | Overall, n (%) | Hospital A, n (%) | Hospital B, n (%) |
|---|---|---|---|
| |||
| Forgot to use HRM | 23 (79) | 7 (100) | 16 (73) |
| Results not clinically relevant | 17 (59) | 7 (100) | 10 (45) |
| Did not fit daily workflow | 16 (55) | 7 (100) | 9 (41) |
| Too little time to use HRM | 14 (48) | 6 (86) | 8 (46) |
| Results on others' patients | 12 (41) | 6 (86) | 6 (27) |
| HRM was difficult to use | 7 (24) | 2 (29) | 5 (23) |
| Had technical difficulties | 3 (10) | 0 (0) | 3 (14) |
Table 3 provides comments from inpatient physician respondents regarding obstacles prohibiting use of HRM and suggestions for future systems.
|
| Suggestions |
| Would be more useful if accessible from (the inpatient clinical information system). |
| Email notification (would have been useful). |
| At time of discharge, if there is a way to find pending labs at discharge, this would be of great utility. |
| Linking responsibility for follow‐up to test ordering (would have been useful). |
| Smarter system for filtering results so less important results are filtered out (is desirable). |
| Can the system be tied into PCP's email somehow? |
| Obstacles |
| Blood cultures, abnormal films can be difficult and time‐consuming to look up. |
| A big problem is results that automatically trigger even though they're not clinically relevant. |
| Keeping a record of patients that left with tests pending (is often difficult to do). |
| Addressing pending results is very time consuming. |
Discussion
We describe a pilot implementation of a computerized application for the management of pending tests at hospital discharge. From responses to post‐implementation surveys, we were able to identify multiple factors prohibiting successful implementation of the application. These observations may help inform future interventions and evaluations.
Almost half of inpatient physicians reported never using HRM despite training and reminders. The feedback provided by physicians in our study suggested that HRM was not ideally designed from an inpatient physician perspective. We discovered several barriers to its use: (1) HRM overburdened physicians with clinically irrelevant test results, suggesting that more robust filtering of abnormal but low importance test results may be required (eg, a borderline electrolyte abnormality or low but stable hematocrit); (2) HRM did not integrate well into inpatient workflowthe system was not integrated into the inpatient results viewing and computerized physician order entry (CPOE) applications, and therefore required an extra step to access; (3) there was no mechanism of alerting inpatient physicians that finalized test results were available for viewing in their HRM queues (eg, by email or by an alert in the inpatient computer system); (4) because responsibility for these results was unclear, most inpatient physicians had no formal method of managing them, and for many, using HRM represented an additional task; and finally (5) several physicians commented on finding results in their HRM queue that belonged to other physician's patients, implying that the hospital databases were inaccurate in identifying the discharging physician or that rotation schedules, and therefore patient responsibility, had changed in the intervening period. Table 4 summarizes the advantages and respective limitations of features of HRM available to inpatient physicians.
| Advantages | Limitations |
|---|---|
| |
| Creates a physician‐managed queue of pending test results by patient | Does not provide alert or push notification when new results available for patients |
| Filters test results by severity with most critical results appearing at the top of the queue | Severity filter set for outpatients; not restrictive enough for post‐discharge period, resulting in excessive alerting |
| Independent, voluntary acknowledgement of results by user | Active acknowledgment not required; no audit trail, feedback, or escalation if result not acknowledged |
| Embedded within LMR (the ambulatory EMR) | LMR not routinely used by many inpatient physicians |
| Offers patient communication tools (eg, pre‐populated patient results letter) | Tools not optimized for post‐discharge test result communication by inpatient physicians (eg, a tool for PCP result notification and acknowledgment) |
In the literature, there is little information regarding optimal features of a test result management system for transitions from the inpatient to ambulatory care setting. Prior studies outline important functions for results management systems developed for noninpatient sites of care, including the ambulatory and emergency room setting.12, 14, 15 These include a method of prioritizing by degree of abnormality, the ability to reliably and efficiently act upon results, and an automated alerting system for abnormal results. Findings from our study provide insight in defining core functions for result management systems which focus on transitions from the inpatient to ambulatory care setting. These functions include tight integration with applications used by inpatient physicians, clear assignment of responsibility for test results finalized after hospital discharge (as well as a mechanism to reassign responsibility), automated alerts to responsible providers of test results finalized post‐discharge, and ways to automatically filter test results to avoid over‐burdening physicians with clinically irrelevant results.
Almost all surveyed inpatient physicians agreed that an ideally designed electronic post‐discharge results management system would be valuable. For such systems to be successfully adopted, we offer several principles to help guide future work. These include: (1) clarifying responsibility at the time a test is ordered and again at discharge, (2) understanding workflow and communication patterns among inpatient and outpatient clinicians, and (3) integrating technological solutions into existing systems to minimize workflow disruptions. For example, if the primary responsibility for post‐discharge result follow‐up lies with the ordering physician, the system should be integrated within the EMR most often used by inpatient physicians and become part of inpatient physician workflow. If the system depends on administrative databases to identify the responsible providers, these must be accurate. Alternatively, in organizations with computerized provider order entry, responsibility for the result could be assigned when the test is ordered and confirmed at discharge (ie, the results management system would be integrated into the discharge order such that pending tests are reviewed at the time of discharge). The discharging physician should have the ability to assign responsibility for each pending test and select preferred mode(s) of notification once its result is finalized (eg, e‐mail, alphanumeric page, etc.). The system should have the ability to generate an automatic notification to the inpatient and PCP (and perhaps other designated providers involved in the patient's inpatient care), but it should not burden busy clinicians with unnecessary alerts and warnings. Finally, the rules by which results are prioritized must be robust enough to filter out less urgent results, and should be modified to reflect the severity of illness of recently discharged patients. In essence, in consideration of the time constraints of busy clinicians, an ideal results management system should achieve automated notification of test results while minimizing the risk of alert fatigue from the potentially large volume of alerts generated.
Our study has several important limitations. First, although our survey response rate was high, the sample size of actual participants was small. Second, because the study was conducted in 2 similar, tertiary care academic centers, it may not generalize to other settings (we note that hospital B included a nonteaching service similar to those in nonacademic medical centers). This may be particularly true in assessing the importance of specific barriers to use of results management systems, which may vary at different institutions. Third, the representation of survey respondents were skeweda majority of the responses were from trainees (all post‐graduate level [PGY] level 2) and from hospital B. Fourth, we did not actively monitor physician interaction with the test result management application, and therefore, we depended heavily on physician recollection of use of the system when responding to surveys. Finally, we did not convene focus groups of key individuals with regard to the factors facilitating or prohibiting adoption of the system. Use of semi‐structured, key informant interviews (ie, focus groups) before and after implementation of an electronic results management application, have been shown to be effective in evaluating potential barriers and facilitators of adoption.16 Focus groups of and/or interviews with inpatient and PCPs, physician extenders, and housestaff could have been useful to better characterize the potential barriers and facilitators of adoption noted by survey respondents in our study.
In summary, we offer several lessons from our attempt to implement a system to manage pending tests at hospital discharge. The success of implementing future systems to address this patient safety concern will rely on accurately assigning responsibility for these test results, integrating the system within clinical information systems commonly used by the inpatient physician, addressing workflow issues and time constraints, maximizing appropriateness of alerting, and minimizing alert fatigue.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- .Key legal principles for hospitalists.Am J Med.2001;111(9B):5S–9S.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,, et al.Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers.J Gen Intern Med.2009;24(9):1002–1006.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: Implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,.The continuing problem of missed test results in an integrated health system with an advanced electronic medical record.Jt Comm J Qual Patient Saf.2007;33(8):485–492.
- ,.The frequency of missed test results and associated treatment delays in a highly computerized health system.BMC Fam Pract.2007;8:32.
- ,,,,.Design and implementation of a comprehensive outpatient results manager.J Biomed Inform.2003;36(1–2):80–91.
- ,,, et al.Impact of an automated test results management system on patients' satisfaction about test result communication.Arch Intern Med.2007;167(20):2233–2239.
- ,,,,,.“I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care.Arch Intern Med.2004;164(20):2223–2228.
- ,,.Potential impact of a computerized system to report late‐arriving laboratory results in the emergency department.Pediatr Emerg Care.2000;16(5):313–315.
- ,,, et al.Electronic results management in pediatric ambulatory care: Qualitative assessment.Pediatrics.2009;123Suppl 2:S85–S91.
The period following discharge is a vulnerable time for patientsthe prevalence of medical errors related to this transition is high and has important patient safety and medico‐legal ramifications.13 Factors contributing to this vulnerability include complexity of hospitalized patients, shorter lengths of stay, and increased discontinuity of care. Hospitalists have recognized this threat to patient safety and have worked toward improving information exchange between inpatient and outpatient providers at hospital discharge.46 Nonetheless, the evidence suggests that more work is necessary. A recent study found that discharge summaries are often incomplete, and do not contain important information requiring follow‐up, such as pending tests.7 Additionally, a review by Kripalani et al. characterizing information deficits at hospital discharge found few interventions which specifically improve communication of pending tests at hospital discharge.8
In a prior study we determined that 41% of patients left the hospital before all laboratory and radiology test results were finalized. Of these results, 9.4% were potentially actionable and could have altered management. Physicians were aware of only 38% of post‐discharge test results.9 This awareness gap is a consequence of several factors including the lack of systems to track and alert providers of test results finalized post discharge. Also, it is unclear who is responsible for pending tests at discharge, since these tests are ordered by the inpatient physicians but often reported in the time period between hospital discharge and the patient's first follow‐up appointment with the primary care physician (PCP). Because responsibility is not explicitly made in the final communication between physicians at discharge, such test results may not be reviewed in a timely manner, potentially resulting in delays in treatment, a need for readmission, or other unfavorable outcomes.
Even in integrated health systems with advanced electronic health records, missed test results which result in treatment delays remain prevalent.10, 11 Test result management applications aid clinicians in reviewing and acting upon results as they become available and such systems may provide solutions to this problem. At Partners Healthcare in Boston, the Results Manager (RM) application was developed to help clinicians in the ambulatory setting safely, reliably, and efficiently review and act upon test results. The application enables clinicians to prioritize test results, utilize guidelines, and generate letters to patients. This system also prompts physicians to set reminders for future testing.12 In a 2.5‐year study evaluating the impact of this intervention, PCPs at 26 adult primary care practices were able to expedite communication of outpatient laboratory and imaging test results to patients with the help of RM. Patients of physicians who participated in the project reported greater satisfaction with test result communication and with information provided about their condition than did a control group of similar patients.13 RM has not yet been studied in the inpatient setting or at care transitions. We describe an attempt at modifying the Partners RM application to help inpatient physicians manage pending tests at hospital discharge.
Methods
Study Setting and Participants
We piloted our application at 2 major academic medical centers (hospitals A and B) associated with Partners Healthcare, an integrated regional health delivery network in eastern Massachusetts, from October 2004 to March 2005. Both centers use the longitudinal medical record (LMR), the electronic medical record (EMR), for nearly all ambulatory practices. The LMR is an internally developed full‐featured EMR, including a repository of laboratory and radiology reports, discharge summaries, ambulatory care notes, medication lists, problem lists, coded allergies, and other patient data. Both centers also have their own inpatient results viewing and order entry systems which provide clinicians caring for patients in the hospital the ability to review results and write orders. Although possible, clinicians caring for patients in the inpatient setting do not routinely access LMR to view test results. Inpatient physician use of the LMR is generally limited to review of the outpatient record, medication lists, and ambulatory notes at admission.
At hospital A, the hospitalist attending physician is typically responsible for all communication to outpatient physicians at discharge, as well as for follow‐up on all test results that return after discharge. Hospital B has 2 types of hospitalist services. One is staffed only by hospitalist and nonhospitalist attending physicians. Nonhospitalist attending physicians were excluded because they care for their own patients in the inpatient and ambulatory setting and typically use RM to manage test results. The other hospitalist service at hospital B is a teaching service consisting of an attending physician, resident, and interns. For this service, the resident is responsible for communication at discharge and follow‐up on all pending tests. For purposes of this study inpatient physicians refers to those physicians responsible for communication with PCPs and follow‐up on pending tests. All inpatient physicians were eligible to participate during the study period.
Test Result Management Application
RM was originally developed by Partners Healthcare to improve timely review and appropriate management of test results in the ambulatory setting. RM was developed for and vetted by primarily ambulatory physicians. The application is browser‐based, provider‐centric, and embedded in the LMR to help ambulatory clinicians review and act upon test results in a safe, reliable, and efficient manner. Although RM has access to all inpatient and outpatient data in the Partners Clinical Data Repository (CDR), given the volume of inpatient tests ordered, hospital‐based results are suppressed by default to limit inundating ambulatory clinicians' queues. Therefore, users of RM only receive results of laboratory and radiology tests ordered in the ambulatory setting. They can track these tests for specific patients for a designated period of time by placing the patient on a watch list. Finally, RM incorporates extensive decision support features to classify the degree of abnormality for each result, presents guidelines to help clinicians manage abnormal results, allows clinicians to generate result letters to patients using predefined, context‐sensitive templates, and prompts physicians to set reminders for future testing. Because RM was developed from the ambulatory perspective, there was limited input from hospitalist physicians with regard to inpatient workflow in the original design of the module.12 See Figure 1 for a screen shot of RM and a description of its features.

figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For purposes of this pilot, we modified RM to allow results of tests ordered in the inpatient setting to be available for viewing (Hospitalist Results Manager, HRM). This feature was turned on only for inpatient physicians as previously defined. Inpatient tests, including pending tests at discharge, continued to be suppressed from PCP's RM queue (however, any physician could access a patient's test result(s) directly from the Partners CDR). Inpatient physicians could track laboratory and radiology results finalized after discharge by keeping discharged patients on their HRM watch list for a designated period of time. The finalized results would become available for review in their HRM queue and abnormal results were displayed prominently at the top of this queue. Inpatient physicians were trained to use HRM in a series of meetings and demonstrations. Although HRM could be accessed from inpatient clinical workstations, it was not part of the inpatient clinical information system.
Surveys
Study surveys were developed and refined through an iterative process and pilot tested among inpatient physicians at both centers for clarity. We surveyed inpatient physicians five months after HRM implementation. Inpatient physicians were asked how often they used HRM, what barriers they faced (respondents asked to quantify agreement to statements on a 5‐point Likert scale), and which elements of an ideal system they would prefer. Finally, we solicited comments regarding perceived obstacles and suggestions for improvement. Because HRM was targeted to inpatient physicians, and because RM has been evaluated from the ambulatory perspective in a prior study,13 PCPs were not surveyed. See Supporting Information Appendix for the survey instrument used in the study.
Results
A total of 35 inpatient physicians participated in the pilot. Among 649 patients discharged during the study period, there were 1075 tests pending of which 555 were subsequently flagged as abnormal in HRM. Study surveys were sent to the 35 inpatient physician participants and 29 were completed, including partial responses (83% survey response rate). The 35 inpatient physician participants had the following characteristics: 22 were male, 13 were female; 21 were trainees and 14 were nontrainees/faculty. All 21 trainees were PGY2s. Nontrainees and faculty varied in experience level (PGY 15: 5, PGY 610: 7, PGY 1120: 1, PGY 21+: 1). Of 29 survey respondents, 7 were from hospital A and 22 were from hospital B; 19 were trainees and 10 were nontrainees/faculty. Of the 6 nonrespondents, 2 were from hospital A and 4 were from hospital B; 2 were trainees and 4 were nontrainees/faculty.
Table 1 shows the results of our survey of inpatient physicians regarding usage of HRM. Of 29 survey respondents, 14 (48%) reported never using HRM. Thirteen (45%) reported using HRM 1 to 2 times per week. None of the respondents used it more than 4 times per week. The frequency of usage was similar for hospitals A and B. Table 2 details barriers to using HRM. Twenty‐three inpatient physicians (79%) reported barriers. Seventeen (59%) thought that results in their HRM queue were not clinically relevant, 16 (55%) felt that HRM did not fit into their daily workflow, 14 (48%) had limited time to use HRM, and 12 (41%) noted that too many results in their HRM queue were on other physician's patients. Seven (24%) reported operational issues and 3 (10%) reported technical issues prohibiting use of HRM. With regard to preferred elements of an ideal results manager system, 21 (72%) inpatient physician respondents wanted to receive notification of abnormal and clinician‐designated pending test results. Four (14%) wanted to receive only abnormal results and 1 (3%) wanted to receive all results. Twenty‐seven (93%) physicians agreed that an ideally designed computerized test result management application would be valuable for managing pending tests at discharge.
| Frequency | Number of Inpatient Physicians Using HRM, n (%) | ||
|---|---|---|---|
| Overall | Hospital A | Hospital B | |
| |||
| Never | 14 (48) | 3 (43) | 11 (50) |
| 12 times per week | 13 (45) | 3 (43) | 10 (45.5) |
| 34 times per week | 2 (7) | 1 (14) | 1 (4.5) |
| 57 times per week | 0 | 0 | 0 |
| >7 times per week | 0 | 0 | 0 |
| Barrier | Overall, n (%) | Hospital A, n (%) | Hospital B, n (%) |
|---|---|---|---|
| |||
| Forgot to use HRM | 23 (79) | 7 (100) | 16 (73) |
| Results not clinically relevant | 17 (59) | 7 (100) | 10 (45) |
| Did not fit daily workflow | 16 (55) | 7 (100) | 9 (41) |
| Too little time to use HRM | 14 (48) | 6 (86) | 8 (46) |
| Results on others' patients | 12 (41) | 6 (86) | 6 (27) |
| HRM was difficult to use | 7 (24) | 2 (29) | 5 (23) |
| Had technical difficulties | 3 (10) | 0 (0) | 3 (14) |
Table 3 provides comments from inpatient physician respondents regarding obstacles prohibiting use of HRM and suggestions for future systems.
|
| Suggestions |
| Would be more useful if accessible from (the inpatient clinical information system). |
| Email notification (would have been useful). |
| At time of discharge, if there is a way to find pending labs at discharge, this would be of great utility. |
| Linking responsibility for follow‐up to test ordering (would have been useful). |
| Smarter system for filtering results so less important results are filtered out (is desirable). |
| Can the system be tied into PCP's email somehow? |
| Obstacles |
| Blood cultures, abnormal films can be difficult and time‐consuming to look up. |
| A big problem is results that automatically trigger even though they're not clinically relevant. |
| Keeping a record of patients that left with tests pending (is often difficult to do). |
| Addressing pending results is very time consuming. |
Discussion
We describe a pilot implementation of a computerized application for the management of pending tests at hospital discharge. From responses to post‐implementation surveys, we were able to identify multiple factors prohibiting successful implementation of the application. These observations may help inform future interventions and evaluations.
Almost half of inpatient physicians reported never using HRM despite training and reminders. The feedback provided by physicians in our study suggested that HRM was not ideally designed from an inpatient physician perspective. We discovered several barriers to its use: (1) HRM overburdened physicians with clinically irrelevant test results, suggesting that more robust filtering of abnormal but low importance test results may be required (eg, a borderline electrolyte abnormality or low but stable hematocrit); (2) HRM did not integrate well into inpatient workflowthe system was not integrated into the inpatient results viewing and computerized physician order entry (CPOE) applications, and therefore required an extra step to access; (3) there was no mechanism of alerting inpatient physicians that finalized test results were available for viewing in their HRM queues (eg, by email or by an alert in the inpatient computer system); (4) because responsibility for these results was unclear, most inpatient physicians had no formal method of managing them, and for many, using HRM represented an additional task; and finally (5) several physicians commented on finding results in their HRM queue that belonged to other physician's patients, implying that the hospital databases were inaccurate in identifying the discharging physician or that rotation schedules, and therefore patient responsibility, had changed in the intervening period. Table 4 summarizes the advantages and respective limitations of features of HRM available to inpatient physicians.
| Advantages | Limitations |
|---|---|
| |
| Creates a physician‐managed queue of pending test results by patient | Does not provide alert or push notification when new results available for patients |
| Filters test results by severity with most critical results appearing at the top of the queue | Severity filter set for outpatients; not restrictive enough for post‐discharge period, resulting in excessive alerting |
| Independent, voluntary acknowledgement of results by user | Active acknowledgment not required; no audit trail, feedback, or escalation if result not acknowledged |
| Embedded within LMR (the ambulatory EMR) | LMR not routinely used by many inpatient physicians |
| Offers patient communication tools (eg, pre‐populated patient results letter) | Tools not optimized for post‐discharge test result communication by inpatient physicians (eg, a tool for PCP result notification and acknowledgment) |
In the literature, there is little information regarding optimal features of a test result management system for transitions from the inpatient to ambulatory care setting. Prior studies outline important functions for results management systems developed for noninpatient sites of care, including the ambulatory and emergency room setting.12, 14, 15 These include a method of prioritizing by degree of abnormality, the ability to reliably and efficiently act upon results, and an automated alerting system for abnormal results. Findings from our study provide insight in defining core functions for result management systems which focus on transitions from the inpatient to ambulatory care setting. These functions include tight integration with applications used by inpatient physicians, clear assignment of responsibility for test results finalized after hospital discharge (as well as a mechanism to reassign responsibility), automated alerts to responsible providers of test results finalized post‐discharge, and ways to automatically filter test results to avoid over‐burdening physicians with clinically irrelevant results.
Almost all surveyed inpatient physicians agreed that an ideally designed electronic post‐discharge results management system would be valuable. For such systems to be successfully adopted, we offer several principles to help guide future work. These include: (1) clarifying responsibility at the time a test is ordered and again at discharge, (2) understanding workflow and communication patterns among inpatient and outpatient clinicians, and (3) integrating technological solutions into existing systems to minimize workflow disruptions. For example, if the primary responsibility for post‐discharge result follow‐up lies with the ordering physician, the system should be integrated within the EMR most often used by inpatient physicians and become part of inpatient physician workflow. If the system depends on administrative databases to identify the responsible providers, these must be accurate. Alternatively, in organizations with computerized provider order entry, responsibility for the result could be assigned when the test is ordered and confirmed at discharge (ie, the results management system would be integrated into the discharge order such that pending tests are reviewed at the time of discharge). The discharging physician should have the ability to assign responsibility for each pending test and select preferred mode(s) of notification once its result is finalized (eg, e‐mail, alphanumeric page, etc.). The system should have the ability to generate an automatic notification to the inpatient and PCP (and perhaps other designated providers involved in the patient's inpatient care), but it should not burden busy clinicians with unnecessary alerts and warnings. Finally, the rules by which results are prioritized must be robust enough to filter out less urgent results, and should be modified to reflect the severity of illness of recently discharged patients. In essence, in consideration of the time constraints of busy clinicians, an ideal results management system should achieve automated notification of test results while minimizing the risk of alert fatigue from the potentially large volume of alerts generated.
Our study has several important limitations. First, although our survey response rate was high, the sample size of actual participants was small. Second, because the study was conducted in 2 similar, tertiary care academic centers, it may not generalize to other settings (we note that hospital B included a nonteaching service similar to those in nonacademic medical centers). This may be particularly true in assessing the importance of specific barriers to use of results management systems, which may vary at different institutions. Third, the representation of survey respondents were skeweda majority of the responses were from trainees (all post‐graduate level [PGY] level 2) and from hospital B. Fourth, we did not actively monitor physician interaction with the test result management application, and therefore, we depended heavily on physician recollection of use of the system when responding to surveys. Finally, we did not convene focus groups of key individuals with regard to the factors facilitating or prohibiting adoption of the system. Use of semi‐structured, key informant interviews (ie, focus groups) before and after implementation of an electronic results management application, have been shown to be effective in evaluating potential barriers and facilitators of adoption.16 Focus groups of and/or interviews with inpatient and PCPs, physician extenders, and housestaff could have been useful to better characterize the potential barriers and facilitators of adoption noted by survey respondents in our study.
In summary, we offer several lessons from our attempt to implement a system to manage pending tests at hospital discharge. The success of implementing future systems to address this patient safety concern will rely on accurately assigning responsibility for these test results, integrating the system within clinical information systems commonly used by the inpatient physician, addressing workflow issues and time constraints, maximizing appropriateness of alerting, and minimizing alert fatigue.
The period following discharge is a vulnerable time for patientsthe prevalence of medical errors related to this transition is high and has important patient safety and medico‐legal ramifications.13 Factors contributing to this vulnerability include complexity of hospitalized patients, shorter lengths of stay, and increased discontinuity of care. Hospitalists have recognized this threat to patient safety and have worked toward improving information exchange between inpatient and outpatient providers at hospital discharge.46 Nonetheless, the evidence suggests that more work is necessary. A recent study found that discharge summaries are often incomplete, and do not contain important information requiring follow‐up, such as pending tests.7 Additionally, a review by Kripalani et al. characterizing information deficits at hospital discharge found few interventions which specifically improve communication of pending tests at hospital discharge.8
In a prior study we determined that 41% of patients left the hospital before all laboratory and radiology test results were finalized. Of these results, 9.4% were potentially actionable and could have altered management. Physicians were aware of only 38% of post‐discharge test results.9 This awareness gap is a consequence of several factors including the lack of systems to track and alert providers of test results finalized post discharge. Also, it is unclear who is responsible for pending tests at discharge, since these tests are ordered by the inpatient physicians but often reported in the time period between hospital discharge and the patient's first follow‐up appointment with the primary care physician (PCP). Because responsibility is not explicitly made in the final communication between physicians at discharge, such test results may not be reviewed in a timely manner, potentially resulting in delays in treatment, a need for readmission, or other unfavorable outcomes.
Even in integrated health systems with advanced electronic health records, missed test results which result in treatment delays remain prevalent.10, 11 Test result management applications aid clinicians in reviewing and acting upon results as they become available and such systems may provide solutions to this problem. At Partners Healthcare in Boston, the Results Manager (RM) application was developed to help clinicians in the ambulatory setting safely, reliably, and efficiently review and act upon test results. The application enables clinicians to prioritize test results, utilize guidelines, and generate letters to patients. This system also prompts physicians to set reminders for future testing.12 In a 2.5‐year study evaluating the impact of this intervention, PCPs at 26 adult primary care practices were able to expedite communication of outpatient laboratory and imaging test results to patients with the help of RM. Patients of physicians who participated in the project reported greater satisfaction with test result communication and with information provided about their condition than did a control group of similar patients.13 RM has not yet been studied in the inpatient setting or at care transitions. We describe an attempt at modifying the Partners RM application to help inpatient physicians manage pending tests at hospital discharge.
Methods
Study Setting and Participants
We piloted our application at 2 major academic medical centers (hospitals A and B) associated with Partners Healthcare, an integrated regional health delivery network in eastern Massachusetts, from October 2004 to March 2005. Both centers use the longitudinal medical record (LMR), the electronic medical record (EMR), for nearly all ambulatory practices. The LMR is an internally developed full‐featured EMR, including a repository of laboratory and radiology reports, discharge summaries, ambulatory care notes, medication lists, problem lists, coded allergies, and other patient data. Both centers also have their own inpatient results viewing and order entry systems which provide clinicians caring for patients in the hospital the ability to review results and write orders. Although possible, clinicians caring for patients in the inpatient setting do not routinely access LMR to view test results. Inpatient physician use of the LMR is generally limited to review of the outpatient record, medication lists, and ambulatory notes at admission.
At hospital A, the hospitalist attending physician is typically responsible for all communication to outpatient physicians at discharge, as well as for follow‐up on all test results that return after discharge. Hospital B has 2 types of hospitalist services. One is staffed only by hospitalist and nonhospitalist attending physicians. Nonhospitalist attending physicians were excluded because they care for their own patients in the inpatient and ambulatory setting and typically use RM to manage test results. The other hospitalist service at hospital B is a teaching service consisting of an attending physician, resident, and interns. For this service, the resident is responsible for communication at discharge and follow‐up on all pending tests. For purposes of this study inpatient physicians refers to those physicians responsible for communication with PCPs and follow‐up on pending tests. All inpatient physicians were eligible to participate during the study period.
Test Result Management Application
RM was originally developed by Partners Healthcare to improve timely review and appropriate management of test results in the ambulatory setting. RM was developed for and vetted by primarily ambulatory physicians. The application is browser‐based, provider‐centric, and embedded in the LMR to help ambulatory clinicians review and act upon test results in a safe, reliable, and efficient manner. Although RM has access to all inpatient and outpatient data in the Partners Clinical Data Repository (CDR), given the volume of inpatient tests ordered, hospital‐based results are suppressed by default to limit inundating ambulatory clinicians' queues. Therefore, users of RM only receive results of laboratory and radiology tests ordered in the ambulatory setting. They can track these tests for specific patients for a designated period of time by placing the patient on a watch list. Finally, RM incorporates extensive decision support features to classify the degree of abnormality for each result, presents guidelines to help clinicians manage abnormal results, allows clinicians to generate result letters to patients using predefined, context‐sensitive templates, and prompts physicians to set reminders for future testing. Because RM was developed from the ambulatory perspective, there was limited input from hospitalist physicians with regard to inpatient workflow in the original design of the module.12 See Figure 1 for a screen shot of RM and a description of its features.

figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For purposes of this pilot, we modified RM to allow results of tests ordered in the inpatient setting to be available for viewing (Hospitalist Results Manager, HRM). This feature was turned on only for inpatient physicians as previously defined. Inpatient tests, including pending tests at discharge, continued to be suppressed from PCP's RM queue (however, any physician could access a patient's test result(s) directly from the Partners CDR). Inpatient physicians could track laboratory and radiology results finalized after discharge by keeping discharged patients on their HRM watch list for a designated period of time. The finalized results would become available for review in their HRM queue and abnormal results were displayed prominently at the top of this queue. Inpatient physicians were trained to use HRM in a series of meetings and demonstrations. Although HRM could be accessed from inpatient clinical workstations, it was not part of the inpatient clinical information system.
Surveys
Study surveys were developed and refined through an iterative process and pilot tested among inpatient physicians at both centers for clarity. We surveyed inpatient physicians five months after HRM implementation. Inpatient physicians were asked how often they used HRM, what barriers they faced (respondents asked to quantify agreement to statements on a 5‐point Likert scale), and which elements of an ideal system they would prefer. Finally, we solicited comments regarding perceived obstacles and suggestions for improvement. Because HRM was targeted to inpatient physicians, and because RM has been evaluated from the ambulatory perspective in a prior study,13 PCPs were not surveyed. See Supporting Information Appendix for the survey instrument used in the study.
Results
A total of 35 inpatient physicians participated in the pilot. Among 649 patients discharged during the study period, there were 1075 tests pending of which 555 were subsequently flagged as abnormal in HRM. Study surveys were sent to the 35 inpatient physician participants and 29 were completed, including partial responses (83% survey response rate). The 35 inpatient physician participants had the following characteristics: 22 were male, 13 were female; 21 were trainees and 14 were nontrainees/faculty. All 21 trainees were PGY2s. Nontrainees and faculty varied in experience level (PGY 15: 5, PGY 610: 7, PGY 1120: 1, PGY 21+: 1). Of 29 survey respondents, 7 were from hospital A and 22 were from hospital B; 19 were trainees and 10 were nontrainees/faculty. Of the 6 nonrespondents, 2 were from hospital A and 4 were from hospital B; 2 were trainees and 4 were nontrainees/faculty.
Table 1 shows the results of our survey of inpatient physicians regarding usage of HRM. Of 29 survey respondents, 14 (48%) reported never using HRM. Thirteen (45%) reported using HRM 1 to 2 times per week. None of the respondents used it more than 4 times per week. The frequency of usage was similar for hospitals A and B. Table 2 details barriers to using HRM. Twenty‐three inpatient physicians (79%) reported barriers. Seventeen (59%) thought that results in their HRM queue were not clinically relevant, 16 (55%) felt that HRM did not fit into their daily workflow, 14 (48%) had limited time to use HRM, and 12 (41%) noted that too many results in their HRM queue were on other physician's patients. Seven (24%) reported operational issues and 3 (10%) reported technical issues prohibiting use of HRM. With regard to preferred elements of an ideal results manager system, 21 (72%) inpatient physician respondents wanted to receive notification of abnormal and clinician‐designated pending test results. Four (14%) wanted to receive only abnormal results and 1 (3%) wanted to receive all results. Twenty‐seven (93%) physicians agreed that an ideally designed computerized test result management application would be valuable for managing pending tests at discharge.
| Frequency | Number of Inpatient Physicians Using HRM, n (%) | ||
|---|---|---|---|
| Overall | Hospital A | Hospital B | |
| |||
| Never | 14 (48) | 3 (43) | 11 (50) |
| 12 times per week | 13 (45) | 3 (43) | 10 (45.5) |
| 34 times per week | 2 (7) | 1 (14) | 1 (4.5) |
| 57 times per week | 0 | 0 | 0 |
| >7 times per week | 0 | 0 | 0 |
| Barrier | Overall, n (%) | Hospital A, n (%) | Hospital B, n (%) |
|---|---|---|---|
| |||
| Forgot to use HRM | 23 (79) | 7 (100) | 16 (73) |
| Results not clinically relevant | 17 (59) | 7 (100) | 10 (45) |
| Did not fit daily workflow | 16 (55) | 7 (100) | 9 (41) |
| Too little time to use HRM | 14 (48) | 6 (86) | 8 (46) |
| Results on others' patients | 12 (41) | 6 (86) | 6 (27) |
| HRM was difficult to use | 7 (24) | 2 (29) | 5 (23) |
| Had technical difficulties | 3 (10) | 0 (0) | 3 (14) |
Table 3 provides comments from inpatient physician respondents regarding obstacles prohibiting use of HRM and suggestions for future systems.
|
| Suggestions |
| Would be more useful if accessible from (the inpatient clinical information system). |
| Email notification (would have been useful). |
| At time of discharge, if there is a way to find pending labs at discharge, this would be of great utility. |
| Linking responsibility for follow‐up to test ordering (would have been useful). |
| Smarter system for filtering results so less important results are filtered out (is desirable). |
| Can the system be tied into PCP's email somehow? |
| Obstacles |
| Blood cultures, abnormal films can be difficult and time‐consuming to look up. |
| A big problem is results that automatically trigger even though they're not clinically relevant. |
| Keeping a record of patients that left with tests pending (is often difficult to do). |
| Addressing pending results is very time consuming. |
Discussion
We describe a pilot implementation of a computerized application for the management of pending tests at hospital discharge. From responses to post‐implementation surveys, we were able to identify multiple factors prohibiting successful implementation of the application. These observations may help inform future interventions and evaluations.
Almost half of inpatient physicians reported never using HRM despite training and reminders. The feedback provided by physicians in our study suggested that HRM was not ideally designed from an inpatient physician perspective. We discovered several barriers to its use: (1) HRM overburdened physicians with clinically irrelevant test results, suggesting that more robust filtering of abnormal but low importance test results may be required (eg, a borderline electrolyte abnormality or low but stable hematocrit); (2) HRM did not integrate well into inpatient workflowthe system was not integrated into the inpatient results viewing and computerized physician order entry (CPOE) applications, and therefore required an extra step to access; (3) there was no mechanism of alerting inpatient physicians that finalized test results were available for viewing in their HRM queues (eg, by email or by an alert in the inpatient computer system); (4) because responsibility for these results was unclear, most inpatient physicians had no formal method of managing them, and for many, using HRM represented an additional task; and finally (5) several physicians commented on finding results in their HRM queue that belonged to other physician's patients, implying that the hospital databases were inaccurate in identifying the discharging physician or that rotation schedules, and therefore patient responsibility, had changed in the intervening period. Table 4 summarizes the advantages and respective limitations of features of HRM available to inpatient physicians.
| Advantages | Limitations |
|---|---|
| |
| Creates a physician‐managed queue of pending test results by patient | Does not provide alert or push notification when new results available for patients |
| Filters test results by severity with most critical results appearing at the top of the queue | Severity filter set for outpatients; not restrictive enough for post‐discharge period, resulting in excessive alerting |
| Independent, voluntary acknowledgement of results by user | Active acknowledgment not required; no audit trail, feedback, or escalation if result not acknowledged |
| Embedded within LMR (the ambulatory EMR) | LMR not routinely used by many inpatient physicians |
| Offers patient communication tools (eg, pre‐populated patient results letter) | Tools not optimized for post‐discharge test result communication by inpatient physicians (eg, a tool for PCP result notification and acknowledgment) |
In the literature, there is little information regarding optimal features of a test result management system for transitions from the inpatient to ambulatory care setting. Prior studies outline important functions for results management systems developed for noninpatient sites of care, including the ambulatory and emergency room setting.12, 14, 15 These include a method of prioritizing by degree of abnormality, the ability to reliably and efficiently act upon results, and an automated alerting system for abnormal results. Findings from our study provide insight in defining core functions for result management systems which focus on transitions from the inpatient to ambulatory care setting. These functions include tight integration with applications used by inpatient physicians, clear assignment of responsibility for test results finalized after hospital discharge (as well as a mechanism to reassign responsibility), automated alerts to responsible providers of test results finalized post‐discharge, and ways to automatically filter test results to avoid over‐burdening physicians with clinically irrelevant results.
Almost all surveyed inpatient physicians agreed that an ideally designed electronic post‐discharge results management system would be valuable. For such systems to be successfully adopted, we offer several principles to help guide future work. These include: (1) clarifying responsibility at the time a test is ordered and again at discharge, (2) understanding workflow and communication patterns among inpatient and outpatient clinicians, and (3) integrating technological solutions into existing systems to minimize workflow disruptions. For example, if the primary responsibility for post‐discharge result follow‐up lies with the ordering physician, the system should be integrated within the EMR most often used by inpatient physicians and become part of inpatient physician workflow. If the system depends on administrative databases to identify the responsible providers, these must be accurate. Alternatively, in organizations with computerized provider order entry, responsibility for the result could be assigned when the test is ordered and confirmed at discharge (ie, the results management system would be integrated into the discharge order such that pending tests are reviewed at the time of discharge). The discharging physician should have the ability to assign responsibility for each pending test and select preferred mode(s) of notification once its result is finalized (eg, e‐mail, alphanumeric page, etc.). The system should have the ability to generate an automatic notification to the inpatient and PCP (and perhaps other designated providers involved in the patient's inpatient care), but it should not burden busy clinicians with unnecessary alerts and warnings. Finally, the rules by which results are prioritized must be robust enough to filter out less urgent results, and should be modified to reflect the severity of illness of recently discharged patients. In essence, in consideration of the time constraints of busy clinicians, an ideal results management system should achieve automated notification of test results while minimizing the risk of alert fatigue from the potentially large volume of alerts generated.
Our study has several important limitations. First, although our survey response rate was high, the sample size of actual participants was small. Second, because the study was conducted in 2 similar, tertiary care academic centers, it may not generalize to other settings (we note that hospital B included a nonteaching service similar to those in nonacademic medical centers). This may be particularly true in assessing the importance of specific barriers to use of results management systems, which may vary at different institutions. Third, the representation of survey respondents were skeweda majority of the responses were from trainees (all post‐graduate level [PGY] level 2) and from hospital B. Fourth, we did not actively monitor physician interaction with the test result management application, and therefore, we depended heavily on physician recollection of use of the system when responding to surveys. Finally, we did not convene focus groups of key individuals with regard to the factors facilitating or prohibiting adoption of the system. Use of semi‐structured, key informant interviews (ie, focus groups) before and after implementation of an electronic results management application, have been shown to be effective in evaluating potential barriers and facilitators of adoption.16 Focus groups of and/or interviews with inpatient and PCPs, physician extenders, and housestaff could have been useful to better characterize the potential barriers and facilitators of adoption noted by survey respondents in our study.
In summary, we offer several lessons from our attempt to implement a system to manage pending tests at hospital discharge. The success of implementing future systems to address this patient safety concern will rely on accurately assigning responsibility for these test results, integrating the system within clinical information systems commonly used by the inpatient physician, addressing workflow issues and time constraints, maximizing appropriateness of alerting, and minimizing alert fatigue.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- .Key legal principles for hospitalists.Am J Med.2001;111(9B):5S–9S.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,, et al.Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers.J Gen Intern Med.2009;24(9):1002–1006.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: Implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,.The continuing problem of missed test results in an integrated health system with an advanced electronic medical record.Jt Comm J Qual Patient Saf.2007;33(8):485–492.
- ,.The frequency of missed test results and associated treatment delays in a highly computerized health system.BMC Fam Pract.2007;8:32.
- ,,,,.Design and implementation of a comprehensive outpatient results manager.J Biomed Inform.2003;36(1–2):80–91.
- ,,, et al.Impact of an automated test results management system on patients' satisfaction about test result communication.Arch Intern Med.2007;167(20):2233–2239.
- ,,,,,.“I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care.Arch Intern Med.2004;164(20):2223–2228.
- ,,.Potential impact of a computerized system to report late‐arriving laboratory results in the emergency department.Pediatr Emerg Care.2000;16(5):313–315.
- ,,, et al.Electronic results management in pediatric ambulatory care: Qualitative assessment.Pediatrics.2009;123Suppl 2:S85–S91.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- .Key legal principles for hospitalists.Am J Med.2001;111(9B):5S–9S.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,, et al.Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers.J Gen Intern Med.2009;24(9):1002–1006.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: Implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,.The continuing problem of missed test results in an integrated health system with an advanced electronic medical record.Jt Comm J Qual Patient Saf.2007;33(8):485–492.
- ,.The frequency of missed test results and associated treatment delays in a highly computerized health system.BMC Fam Pract.2007;8:32.
- ,,,,.Design and implementation of a comprehensive outpatient results manager.J Biomed Inform.2003;36(1–2):80–91.
- ,,, et al.Impact of an automated test results management system on patients' satisfaction about test result communication.Arch Intern Med.2007;167(20):2233–2239.
- ,,,,,.“I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care.Arch Intern Med.2004;164(20):2223–2228.
- ,,.Potential impact of a computerized system to report late‐arriving laboratory results in the emergency department.Pediatr Emerg Care.2000;16(5):313–315.
- ,,, et al.Electronic results management in pediatric ambulatory care: Qualitative assessment.Pediatrics.2009;123Suppl 2:S85–S91.
Copyright © 2010 Society of Hospital Medicine
BEST PRACTICES IN: Managing Eczema With Natural Ingredients
A supplement to Skin & Allergy News. This supplement was sponsored by Johnson & Johnson Consumer Products Company.
- What Role Should Colloidal Oatmeal Play?
- What Other Naturally Derived Ingredients May Benefit Patients With AD?
- The Value of 510(k) Products in Eczema
Faculty/Faculty Disclosure
Lawrence Eichenfield, MD
Chief of Pediatric and Adolescent Dermatology
Rady Children's Hospital and The University of California, San Diego School of Medicine
Professor of Clinical
Pediatrics and Medicine
(Dermatology)
The University of California
San Diego, CA
Dr. Eichenfield was a consultant to Johnson and Johnson.
Melissa Reyes Merin, MD
Clinical Fellow
Rady Children’s Hospital
San Diego, CA
Dr. Reyes Merin has nothing to disclose.
Copyright (c) 2010 Elsevier Inc.
A supplement to Skin & Allergy News. This supplement was sponsored by Johnson & Johnson Consumer Products Company.
- What Role Should Colloidal Oatmeal Play?
- What Other Naturally Derived Ingredients May Benefit Patients With AD?
- The Value of 510(k) Products in Eczema
Faculty/Faculty Disclosure
Lawrence Eichenfield, MD
Chief of Pediatric and Adolescent Dermatology
Rady Children's Hospital and The University of California, San Diego School of Medicine
Professor of Clinical
Pediatrics and Medicine
(Dermatology)
The University of California
San Diego, CA
Dr. Eichenfield was a consultant to Johnson and Johnson.
Melissa Reyes Merin, MD
Clinical Fellow
Rady Children’s Hospital
San Diego, CA
Dr. Reyes Merin has nothing to disclose.
Copyright (c) 2010 Elsevier Inc.
A supplement to Skin & Allergy News. This supplement was sponsored by Johnson & Johnson Consumer Products Company.
- What Role Should Colloidal Oatmeal Play?
- What Other Naturally Derived Ingredients May Benefit Patients With AD?
- The Value of 510(k) Products in Eczema
Faculty/Faculty Disclosure
Lawrence Eichenfield, MD
Chief of Pediatric and Adolescent Dermatology
Rady Children's Hospital and The University of California, San Diego School of Medicine
Professor of Clinical
Pediatrics and Medicine
(Dermatology)
The University of California
San Diego, CA
Dr. Eichenfield was a consultant to Johnson and Johnson.
Melissa Reyes Merin, MD
Clinical Fellow
Rady Children’s Hospital
San Diego, CA
Dr. Reyes Merin has nothing to disclose.
Copyright (c) 2010 Elsevier Inc.
New Treatment Option for AFib Patients
The FDA's approval of a new oral anticoagulant—the first in 56 years—has sparked conversation in internal-medicine circles, prompting the industry to wonder: Will the new drug unseat warfarin as the go-to therapy?
Warfarin is among the most common prescriptions written by hospitalists, but that is in part due to its status as the lone option for the prevention of strokes and embolisms in atrial fibrillation (AF) patients. But on Oct. 19, the FDA approved dabigatran etexilate (Pradaxa) for AF patients. Several other similar medications are under development.
An FDA announcement on the approval notes that in a trial of 18,000 patients, those taking dabigatran etexilate had fewer strokes than those who took warfarin. The study (N Engl J Med. 361;12:1139-1151) reported primary outcome rates were 1.69% per year in the warfarin group, compared with 1.53% per year in the group that received 110mg of dabigatran (P<0.001).
Marketers for the new drug have suggested that while physicians often are slow to accept new therapies, the elimination of blood monitoring that often increases length of stay could nudge hospitalists to adopt the treatment more quickly.
Kurt Pfeifer, MD, FACP, program director of the Internal Medicine Residency program at Medical College in Milwaukee and a bleeding-risk research follower, is not so sure. He says that until there is clinical evidence, it will be difficult to tout any potential long-term benefits of the new therapy.
"It's not time to have a funeral for warfarin," Dr. Pfeifer says.
He adds that dabigatran's initial trials have not showed him such a compelling efficacy that he would consider removing current warfarin patients from their therapy. He also says that the cost of the new medication—at least double that of warfarin, with no generics available—will be a stumbling block and could prevent it from hospital formularies.
"Even with all these alternative anticoagulants out there, there is still no doubt that warfarin will be a mainstay therapy," Dr. Pfeifer says. "There is a reason these drugs are around a long time. …They are effective and they are cheap."
The FDA's approval of a new oral anticoagulant—the first in 56 years—has sparked conversation in internal-medicine circles, prompting the industry to wonder: Will the new drug unseat warfarin as the go-to therapy?
Warfarin is among the most common prescriptions written by hospitalists, but that is in part due to its status as the lone option for the prevention of strokes and embolisms in atrial fibrillation (AF) patients. But on Oct. 19, the FDA approved dabigatran etexilate (Pradaxa) for AF patients. Several other similar medications are under development.
An FDA announcement on the approval notes that in a trial of 18,000 patients, those taking dabigatran etexilate had fewer strokes than those who took warfarin. The study (N Engl J Med. 361;12:1139-1151) reported primary outcome rates were 1.69% per year in the warfarin group, compared with 1.53% per year in the group that received 110mg of dabigatran (P<0.001).
Marketers for the new drug have suggested that while physicians often are slow to accept new therapies, the elimination of blood monitoring that often increases length of stay could nudge hospitalists to adopt the treatment more quickly.
Kurt Pfeifer, MD, FACP, program director of the Internal Medicine Residency program at Medical College in Milwaukee and a bleeding-risk research follower, is not so sure. He says that until there is clinical evidence, it will be difficult to tout any potential long-term benefits of the new therapy.
"It's not time to have a funeral for warfarin," Dr. Pfeifer says.
He adds that dabigatran's initial trials have not showed him such a compelling efficacy that he would consider removing current warfarin patients from their therapy. He also says that the cost of the new medication—at least double that of warfarin, with no generics available—will be a stumbling block and could prevent it from hospital formularies.
"Even with all these alternative anticoagulants out there, there is still no doubt that warfarin will be a mainstay therapy," Dr. Pfeifer says. "There is a reason these drugs are around a long time. …They are effective and they are cheap."
The FDA's approval of a new oral anticoagulant—the first in 56 years—has sparked conversation in internal-medicine circles, prompting the industry to wonder: Will the new drug unseat warfarin as the go-to therapy?
Warfarin is among the most common prescriptions written by hospitalists, but that is in part due to its status as the lone option for the prevention of strokes and embolisms in atrial fibrillation (AF) patients. But on Oct. 19, the FDA approved dabigatran etexilate (Pradaxa) for AF patients. Several other similar medications are under development.
An FDA announcement on the approval notes that in a trial of 18,000 patients, those taking dabigatran etexilate had fewer strokes than those who took warfarin. The study (N Engl J Med. 361;12:1139-1151) reported primary outcome rates were 1.69% per year in the warfarin group, compared with 1.53% per year in the group that received 110mg of dabigatran (P<0.001).
Marketers for the new drug have suggested that while physicians often are slow to accept new therapies, the elimination of blood monitoring that often increases length of stay could nudge hospitalists to adopt the treatment more quickly.
Kurt Pfeifer, MD, FACP, program director of the Internal Medicine Residency program at Medical College in Milwaukee and a bleeding-risk research follower, is not so sure. He says that until there is clinical evidence, it will be difficult to tout any potential long-term benefits of the new therapy.
"It's not time to have a funeral for warfarin," Dr. Pfeifer says.
He adds that dabigatran's initial trials have not showed him such a compelling efficacy that he would consider removing current warfarin patients from their therapy. He also says that the cost of the new medication—at least double that of warfarin, with no generics available—will be a stumbling block and could prevent it from hospital formularies.
"Even with all these alternative anticoagulants out there, there is still no doubt that warfarin will be a mainstay therapy," Dr. Pfeifer says. "There is a reason these drugs are around a long time. …They are effective and they are cheap."
A Boost for Pediatric Research
Pediatric Research in Inpatient Settings (PRIS), a network of pediatric hospitalists practicing at 150 academic and community hospitals, recently landed two federal stimulus grants totaling $12 million, far outstripping past support for research in the field, according to PRIS executive council chair Raj Srivastava, MD, MPH.
"Our mandate is to get federal dollars for multisite, transformative, clinical research for pediatric hospital medicine," says Dr. Srivastava, a pediatric hospitalist at Primary Children's Medical Center in Salt Lake City. "If we are going to be a real specialty, we have to conduct research to define best evidence and best practice: how to translate the evidence out into the field."
PRIS was formed in 2002 with sponsorship from SHM, the American Academy of Pediatrics, and the Academic Pediatric Association. Reorganized last year with a new executive council of active researchers, PRIS convened a strategic planning roundtable to reinvigorate the research agenda. In March, the Child Health Corporation of America, a business alliance of CEOs from 42 nonprofit children’s hospitals, awarded PRIS $1.4 million to support its infrastructure needs and a process that would prioritize research based on prevalence, cost, and variation in practice.
The federal stimulus monies, a pair of three-year grants awarded in September, will fund pediatric inpatient comparative effectiveness research. One of the grants, for $9 million, will support work to link clinical and administrative databases at six children’s hospitals. The second grant, worth $3 million, will be used to study the effectiveness of a resident handoff "bundle" of QI processes designed to enhance communication and improve signouts and transitions of care.
Dr. Srivastava says that, with the infrastructure now in place, more grant support will become possible. Adult hospitalists could use the PRIS experience as a model for building their own multisite research networks, he adds. “This kind of research matters because it is what we are all being asked to do in healthcare anyway,” he says.
Pediatric Research in Inpatient Settings (PRIS), a network of pediatric hospitalists practicing at 150 academic and community hospitals, recently landed two federal stimulus grants totaling $12 million, far outstripping past support for research in the field, according to PRIS executive council chair Raj Srivastava, MD, MPH.
"Our mandate is to get federal dollars for multisite, transformative, clinical research for pediatric hospital medicine," says Dr. Srivastava, a pediatric hospitalist at Primary Children's Medical Center in Salt Lake City. "If we are going to be a real specialty, we have to conduct research to define best evidence and best practice: how to translate the evidence out into the field."
PRIS was formed in 2002 with sponsorship from SHM, the American Academy of Pediatrics, and the Academic Pediatric Association. Reorganized last year with a new executive council of active researchers, PRIS convened a strategic planning roundtable to reinvigorate the research agenda. In March, the Child Health Corporation of America, a business alliance of CEOs from 42 nonprofit children’s hospitals, awarded PRIS $1.4 million to support its infrastructure needs and a process that would prioritize research based on prevalence, cost, and variation in practice.
The federal stimulus monies, a pair of three-year grants awarded in September, will fund pediatric inpatient comparative effectiveness research. One of the grants, for $9 million, will support work to link clinical and administrative databases at six children’s hospitals. The second grant, worth $3 million, will be used to study the effectiveness of a resident handoff "bundle" of QI processes designed to enhance communication and improve signouts and transitions of care.
Dr. Srivastava says that, with the infrastructure now in place, more grant support will become possible. Adult hospitalists could use the PRIS experience as a model for building their own multisite research networks, he adds. “This kind of research matters because it is what we are all being asked to do in healthcare anyway,” he says.
Pediatric Research in Inpatient Settings (PRIS), a network of pediatric hospitalists practicing at 150 academic and community hospitals, recently landed two federal stimulus grants totaling $12 million, far outstripping past support for research in the field, according to PRIS executive council chair Raj Srivastava, MD, MPH.
"Our mandate is to get federal dollars for multisite, transformative, clinical research for pediatric hospital medicine," says Dr. Srivastava, a pediatric hospitalist at Primary Children's Medical Center in Salt Lake City. "If we are going to be a real specialty, we have to conduct research to define best evidence and best practice: how to translate the evidence out into the field."
PRIS was formed in 2002 with sponsorship from SHM, the American Academy of Pediatrics, and the Academic Pediatric Association. Reorganized last year with a new executive council of active researchers, PRIS convened a strategic planning roundtable to reinvigorate the research agenda. In March, the Child Health Corporation of America, a business alliance of CEOs from 42 nonprofit children’s hospitals, awarded PRIS $1.4 million to support its infrastructure needs and a process that would prioritize research based on prevalence, cost, and variation in practice.
The federal stimulus monies, a pair of three-year grants awarded in September, will fund pediatric inpatient comparative effectiveness research. One of the grants, for $9 million, will support work to link clinical and administrative databases at six children’s hospitals. The second grant, worth $3 million, will be used to study the effectiveness of a resident handoff "bundle" of QI processes designed to enhance communication and improve signouts and transitions of care.
Dr. Srivastava says that, with the infrastructure now in place, more grant support will become possible. Adult hospitalists could use the PRIS experience as a model for building their own multisite research networks, he adds. “This kind of research matters because it is what we are all being asked to do in healthcare anyway,” he says.
Family CPR Training Before Discharge
Patients discharged from the hospital with coronary disease complications experience an increased risk of sudden cardiac arrest (SCA), which afflicts over 200,000 people in the United States each year with an 80% to 90% mortality rate.17 Prompt delivery of cardiopulmonary resuscitation (CPR) can triple the probability of survival from SCA, yet less than 25% of SCA victims receive bystander CPR.8 Given that 80% of SCA events occur in the home environment, hospitalization could serve as an important point of capture for family instruction in CPR. Prior investigations have suggested conducting conventional CPR training courses before discharge for family members. However, significant barriers exist to this approach, including the requirement for a certified instructor and a large time commitment for standard training.912
To address these resource and time barriers, the American Heart Association recently established a video self‐instruction (VSI) course in CPR, eliminating the need for an instructor and reducing the time requirement for training to 25 minutes. The course consists of a digital video disc (DVD) and low‐cost inflatable mannequin in a self‐contained kit.13 Several investigations have shown that CPR performance skills of students after VSI courses are similar to those of students after traditional CPR training programs.1417 This VSI program presents the unique opportunity for secondary training, given that the DVD and mannequin may be shared by primary trainees with family members or friends. This VSI approach has not been evaluated in the hospital setting or with family members of patients at risk for SCA. We sought to test the feasibility of an in‐hospital CPR training program using the VSI tool, with the hypothesis that VSI training would be well‐accepted by family members of hospitalized patients with known or suspected coronary disease. We further hypothesized that subjects would be able to perform skills adequately and would be motivated to subsequently share the VSI course with others after their family member's hospital discharge.
METHODS
This prospective, multicenter investigation was approved by the University of Pennsylvania Institutional Review Board (IRB) and represents the initial component of an ongoing longitudinal study testing different methods of CPR education in the hospital setting. Enrollment was conducted at 3 hospitals: The Hospital of the University of Pennsylvania (a 700‐bed tertiary‐care academic medical center), Penn Presbyterian Medical Center (a 300‐bed tertiary‐care and community hospital) and Pennsylvania Hospital (a 400‐bed community hospital).
Recruitment Strategy
Family members of hospitalized patients with known or suspected coronary disease were targeted in this investigation (eg, patients admitted with known myocardial infarction, or patients over 40 years old admitted with chest pain or shortness of breath who had a known history of coronary risk factors). Recruitment took place in the cardiology and telemetry wards of each hospital site by research assistants who were previously CPR trained, but not certified as CPR instructors. Subjects were considered eligible for participation if they were a family member of a current inpatient with known or suspected coronary disease and had not received CPR instruction within the past 2 years. Subjects were excluded if they were under age 18 years, felt unwell, or considered themselves physically unable to undergo CPR training. Eligible individuals were approached using an IRB‐approved recruitment script. If the family member declined participation, the research assistant collected the individual's demographic information and reason for nonparticipation.
If the targeted individual expressed willingness to undergo CPR training, the research assistant administered a pretraining questionnaire to obtain demographic information and history of prior CPR instruction. Subjects then underwent the VSI training program in a family consultation room within the hospital unit, proctored by the research assistant. The VSI program contains an instructional DVD that teaches standard CPR (30 compressions: 2 breaths), as well as the importance of recognizing a nonresponsive patient and calling 9‐1‐1. The training process, including set‐up, video review, and practice routinely took less than 45 minutes per subject. Upon completion of the VSI session, subjects were tested in their newly acquired CPR skills using a VSI or a standard CPR‐recording mannequin, with CPR data analyzed via commercial software (Skill‐Reporter ResuciAnne and Skill‐Reporter software, Laerdal Medical Corporation, Wappinger Falls, NY). Compression rate was calculated as compressions per minute, omitting pauses, with video‐recorded data abstracted and combined with objective CPR recordings. Subjects then completed a Likert scale semi‐quantitative self‐assessment to rate their perspectives on the CPR training experience. Subjects were not compensated financially, but were given the VSI kit to bring home with them at no cost, for the opportunity of performing secondary training.
Assessment of Secondary Training
In an effort to determine whether subjects shared the VSI kit with other family members, follow‐up telephone contact was made with enrollees approximately 1 month after initial CPR training. Subjects were asked to complete a brief survey that included self‐reporting of whether they shared the kit, and if so, how many individuals were trained by the subject (measurement of secondary training, defined as the mean number of people trained for each kit distributed).
Data Analysis and Statistical Calculations
All data, including compiled survey results and CPR quantitative data, were abstracted using a spreadsheet application (Excel, Microsoft Corporation, Redmond, WA). Descriptive statistics were used to compare demographics of enrolled vs. nonenrolled populations, using either student's t‐tests for continuous variables or chi square tests for categorical data. Data are presented as mean standard deviation (SD), with significance set at an alpha = 0.05.
RESULTS
Subject Characteristics and Demographics
Subjects were recruited at the 3 hospital sites between May 2009 and January 2010. A total of 756 eligible individuals were approached, and 280 accepted enrollment for CPR training, representing a 37% enrollment rate (Figure 1). Of the 280 enrolled, 136 underwent instruction using the VSI training program as described, and 144 were enrolled using an experimental method of VSI training in CPR; this second cohort will be described elsewhere. When comparing the eligible individuals who declined enrollment versus those who accepted (Table 1), no significant differences were observed with regard to age, gender or race (P = NS for each). Common reasons cited for nonparticipation included lack of interest or lack of time (data not shown).
| Enrolled (%) n = 136 | Screened/Not Enrolled (%) n = 476 | |
|---|---|---|
| ||
| Age, years | 52 15 | 46 26 |
| Female | 94 (69) | 326 (68) |
| Race | ||
| White | 101 (74) | 316 (66) |
| Black | 30 (22) | 90 (19) |
| Hispanic | 5 (4) | 8 (2) |
| Other/no response | 0 (0) | 59 (13) |
| Relationship to patient | ||
| Spouse | 49 (36) | 171 (36) |
| Immediate family* | 58 (43) | 168 (35) |
| Other | 28 (21) | 76 (16) |
| No response | 1 (1) | 61 (13) |
| Highest Education | ||
| Elementary | 1 (1) | 1 (1) |
| Middle schoo1 | 1 (1) | 7 (1) |
| High school | 46 (34) | 157 (33) |
| Some college/vocation | 36 (26) | 86 (18) |
| College | 30 (22) | 92 (19) |
| Graduate school | 22 (16) | 39 (8) |
| No response | 0 (0) | 94 (20) |
| Previous CPR training | ||
| No | 78 (57) | |
| Yes: within past 2 years | 0 (0) | |
| Yes: within past 25 years | 13 (10) | |
| Yes: within past 510 years | 5 (4) | |
| Yes: more than 10 years ago | 40 (29) | |
Demographics of the enrolled subject cohort are detailed in Table 1. The mean age of subjects was 52 15, and 94 of 136 (69%) were female. Enrolled subjects represented spouses or immediate family members of the hospitalized patient in 107 of 136 (79%) of cases, and the vast majority, 118 of 136 (87%), had either never received CPR training or had received it over 10 years prior to current enrollment.
Subject Perspectives
A posttraining survey revealed that most respondents, 101 of 136 (74%) felt comfortable or very comfortable learning CPR from the VSI kit, and 127 of 136 (93%) felt likely or very likely to share the VSI kit (Figure 2).
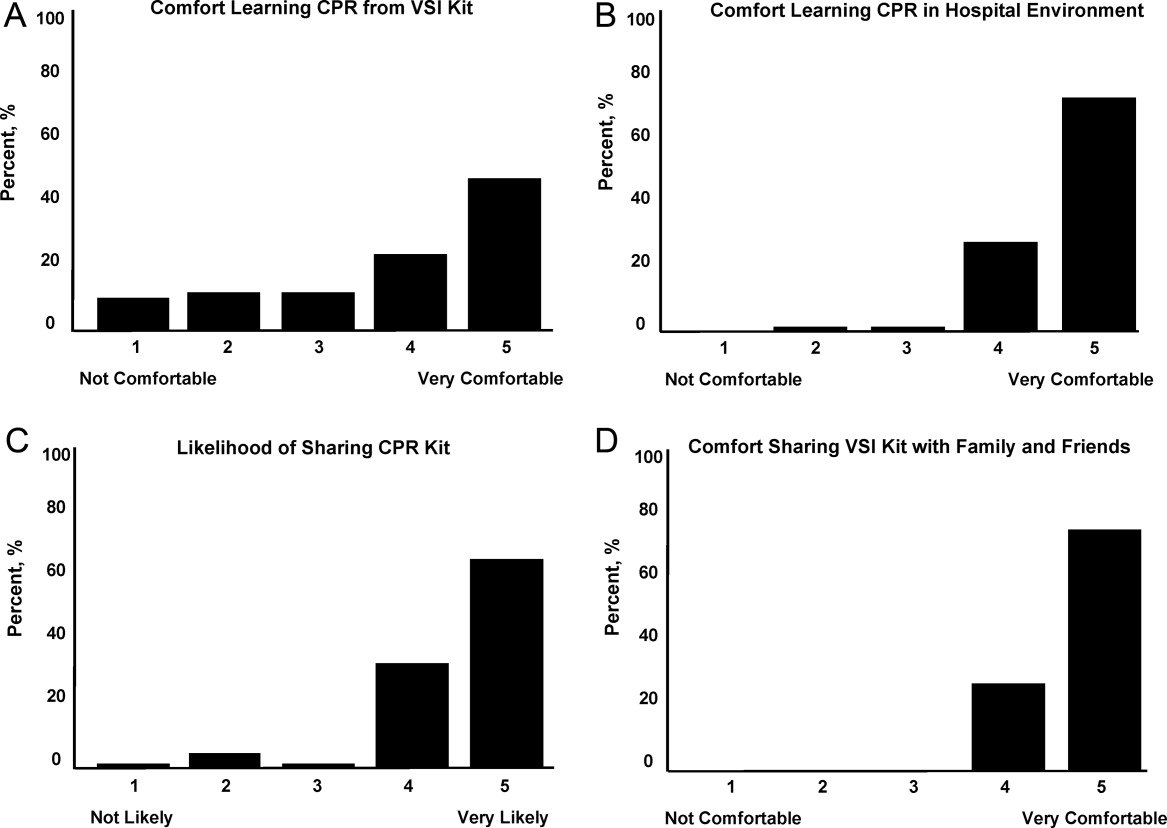
Resuscitation Skills Testing
After CPR training, subjects were asked to perform initial resuscitation actions including 2 minutes of CPR on a mannequin (Table 2). A total of 127 subjects completed these trainings, with 25 completing testing using a VSI kit and 102 using a depth‐recording mannequin; data from 9 subjects were excluded due to CPR recording technical problems. With regard to performance of initial resuscitation actions, 96 of 127 (76%) of subjects assessed responsiveness, 90 of 127 (71%) checked for breathing and 91 of 127 (72%) signaled the need to call for help. CPR was attempted by 127 of 127 (100%) subjects. The mean chest compression rate was 90 26 per minutes, and mean compression depth was 37 12 mm. The mean ventilation rate was 4 3 per minutes.
Secondary Training
Eligible subjects (n = 122) were surveyed via telephone 1 month after initial training, in which 95 individuals participated (78% response rate of those eligible for follow‐up). VSI kits were shared by 57 subjects, with a total of 132 additional individuals receiving VSI‐based CPR instruction. This represented a mean of 2.1 (median of 6) people trained per kit shared, with the actual number of people trained ranging from 1 to 15.
DISCUSSION
In the current work, we demonstrated the feasibility of using the hospital environment as a point of capture for training family members of at‐risk patients in CPR skills. Given that most SCA events occur in the home setting, family member training may hold greater potential for CPR delivery during actual events than training a similar number of younger laypersons at large. Other investigators have identified the focused identification and CPR training of populations at risk of SCA as an important and potentially efficient step to improve survival.10, 12, 18, 19 CPR education of family members before hospital discharge represents a logical extension of other cardiac risk factor‐focused health care education and services before patients are discharged home, including delivery of dietary counseling, diabetic teaching, and education regarding cardiac symptoms. To our knowledge, our work represents the first hospital‐based, adult, layperson, CPR training program using VSI as an instructional approach.
CPR training via a 25‐minute VSI program has been shown to yield CPR performance quality in trainees that is similar to that generated from formal CPR classes that require 3 hours to 4 hours.1416 While VSI training does not provide CPR certification, it is unlikely that the lack of testing and certification is a barrier to participation for the lay public. Indeed, the removal of the pressures of a formal class and testing may increase interest in CPR training through the VSI method.13, 20, 21
Several prior investigations have exploited VSI methodology as an outreach tool to teach CPR in various settings. A recent study in Norway used VSI CPR kits as refresher tools for hospital employees.22 Other work has focused on use of VSI implementation in schools.20, 21, 23 An example of this latter approach was a Danish initiative in which 35,000 VSI kits were distributed to seventh graders.20 Over 15,000 laypersons received secondary training at home by the initially trained students, highlighting a key advantage of the VSI kit approach. It has been argued that this secondary training phenomenon is among the reasons the VSI educational approach may offer a cost effective means for targeted family training.13, 20, 21
While participants in our program were able to adequately perform CPR skills and expressed self‐reported motivation and empowerment, it must be acknowledged that many trained laypersons still do not act when confronted with an actual arrest event.8 In addition, CPR quality at the time of actual performance may be variable, attenuating the survival benefit.2426 However, several population‐based observational studies have supported the notion that training more laypersons in CPR translates into improved overall survival rates from cardiac arrest.25, 27, 28 Further work will be required to follow newly trained, at‐risk family members over time to determine if SCA events occur, and if so, whether CPR was initiated.
Limitations
Willingness to undergo CPR training is likely to be confounded by cultural, regional, and educational factors. Therefore, the general applicability of this 3‐hospital program to other practice environments remains an open question. In our program, the majority of screened family members still refused participation; however, we did not discern a simple relationship between willingness to participate and age, gender, or race. Furthermore, we utilized paid research assistants as subject recruiters and proctors to the VSI training; from a broader implementation perspective, it would be important to determine whether hospital volunteers or staff could perform the training. In addition, while a VSI training kit currently costs $35 and a conventional CPR course could cost from $150 to $300, a formal cost‐effectiveness analysis of VSI training has yet to be performed. Another key limitation is that the secondary training effect was measured by participant self‐report, which may be prone to recall bias; however, no specific incentives or penalties were used to encourage over‐reporting of secondary training. Finally, in this short‐term feasibility study, no direct patient outcomes nor instances of CPR performance were measured.
CONCLUSIONS
In this prospective study of hospital‐based CPR training, we have shown that targeted training of families before hospital discharge is feasible, well received by trainees, and has the benefits of secondary training in the home environment, where most SCA events take place. This program could be easily implemented in other hospital or practice settings. Through targeted CPR training programs such as the one described in this investigation, at risk populations that are underrepresented in conventional CPR training classes can be equipped with important life‐saving skills. Further work on a larger scale will be required to measure the impact of such programs on patient outcomes.
Acknowledgements
The authors wish to thank Lori Albright, Matthew Buchwald, Laura Ebbeling, Emily Esposito, Lori Ingleton, Kristy Walsh, Benjamin Weisenthal, Julie Xu and Mariana Gonzalez for subject recruitment and data collection assistance.
- ,,, et al.Heart disease and stroke statistics–2010 update: a report from the American Heart Association.Circulation.2010;121(7):e46–e215.
- ,,, et al.Regional variation in out‐of‐hospital cardiac arrest incidence and outcome.JAMA.2008;300(12):1423–1431.
- ,,, et al.Prediction of sudden cardiac death after myocardial infarction in the beta‐blocking era.J Am Coll Cardiol.2003;42(4):652–658.
- ,,, et al.Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both.N Engl J Med.2005;352(25):2581–2588.
- ,,,.One‐year health status outcomes of unstable angina versus myocardial infarction: a prospective, observational cohort study of ACS survivors.BMC Cardiovasc Disord.2007;7:28.
- ,,,,.Sudden death after myocardial infarction.JAMA.2008;300(17):2022–2029.
- ,,.Elevated admission serum creatinine predicts poor myocardial blood flow and one‐year mortality in ST‐segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention.J Invasive Cardiol.2009;21(10):493–498.
- ,,,,,.CPR training and CPR performance: do CPR‐trained bystanders perform CPR?Acad Emerg Med.2006;13(6):596–601.
- ,,, et al.Reducing barriers for implementation of bystander‐initiated cardiopulmonary resuscitation: a scientific statement from the American Heart Association for healthcare providers, policymakers, and community leaders regarding the effectiveness of cardiopulmonary resuscitation.Circulation.2008;117(5):704–709.
- ,,.Understanding and improving low bystander CPR rates: a systematic review of the literature.CJEM.2008;10(1):51–65.
- ,.Are we training the right people yet? A survey of participants in public cardiopulmonary resuscitation classes.Resuscitation.1998;37(1):21–25.
- ,,, et al.Cardiac arrest in private locations: different strategies are needed to improve outcome.Resuscitation.2003;58(2):171–176.
- ,.The American Heart Association CPR Anytime Program: the potential impact of highly accessible training in cardiopulmonary resuscitation.J Cardiopulm Rehabil.2006;26(6):346–354.
- ,,,,.Laypersons may learn basic life support in 24 min using a personal resuscitation manikin.Resuscitation.2006;69(3):435–442.
- ,,, et al.Prospective, randomized trial of the effectiveness and retention of 30‐min layperson training for cardiopulmonary resuscitation and automated external defibrillators: The American Airlines Study.Resuscitation.2007;74(2):276–285.
- ,,,,,.Effectiveness of a 30‐min CPR self‐instruction program for lay responders: a controlled randomized study.Resuscitation.2005;67(1):31–43.
- ,,, et al.Randomized, controlled trial of video self‐instruction versus traditional CPR training.Ann Emerg Med.1998;31(3):364–369.
- ,,, et al.A randomized controlled trial of chest compression only CPR for older adults‐a pilot study.Resuscitation.2003;58(2):177–185.
- ,.Estimating cost‐effectiveness of mass cardiopulmonary resuscitation training strategies to improve survival from cardiac arrest in private locations.Prehosp Emerg Care.2004;8(4):420–423.
- ,,,.Disseminating cardiopulmonary resuscitation training by distributing 35,000 personal manikins among school children.Circulation.2007;116(12):1380–1385.
- ,,.Impact of a self‐instruction CPR kit on 7th graders' and adults' skills and CPR performance.Resuscitation.2008;79(1):103–108.
- ,,,,.Hospital employees improve basic life support skills and confidence with a personal resuscitation manikin and a 24‐min video instruction.Resuscitation.2009;80(8):898–902.
- ,,.High school students as ambassadors of CPR‐‐a model for reaching the most appropriate target population?Resuscitation.2010;81(1):78–81.
- ,,,.Three‐phase model of cardiac arrest: time‐dependent benefit of bystander cardiopulmonary resuscitation.Am J Cardiol.2006;98(4):497–499.
- ,,,.Performance of chest compressions by laypersons during the Public Access Defibrillation Trial.Resuscitation.2010;81(3):293–296.
- ,,, et al.Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in‐hospital cardiac arrest.Circulation.2005;111(4):428–434.
- ,,, et al.The Save Hearts in Arizona Registry and Education (SHARE) program: who is performing CPR and where are they doing it?Resuscitation.2007;75(1):68–75.
- ,,,,.Efficacy of bystander CPR: intervention by lay people and by health care professionals.Resuscitation.2005;66(3):291–295.
Patients discharged from the hospital with coronary disease complications experience an increased risk of sudden cardiac arrest (SCA), which afflicts over 200,000 people in the United States each year with an 80% to 90% mortality rate.17 Prompt delivery of cardiopulmonary resuscitation (CPR) can triple the probability of survival from SCA, yet less than 25% of SCA victims receive bystander CPR.8 Given that 80% of SCA events occur in the home environment, hospitalization could serve as an important point of capture for family instruction in CPR. Prior investigations have suggested conducting conventional CPR training courses before discharge for family members. However, significant barriers exist to this approach, including the requirement for a certified instructor and a large time commitment for standard training.912
To address these resource and time barriers, the American Heart Association recently established a video self‐instruction (VSI) course in CPR, eliminating the need for an instructor and reducing the time requirement for training to 25 minutes. The course consists of a digital video disc (DVD) and low‐cost inflatable mannequin in a self‐contained kit.13 Several investigations have shown that CPR performance skills of students after VSI courses are similar to those of students after traditional CPR training programs.1417 This VSI program presents the unique opportunity for secondary training, given that the DVD and mannequin may be shared by primary trainees with family members or friends. This VSI approach has not been evaluated in the hospital setting or with family members of patients at risk for SCA. We sought to test the feasibility of an in‐hospital CPR training program using the VSI tool, with the hypothesis that VSI training would be well‐accepted by family members of hospitalized patients with known or suspected coronary disease. We further hypothesized that subjects would be able to perform skills adequately and would be motivated to subsequently share the VSI course with others after their family member's hospital discharge.
METHODS
This prospective, multicenter investigation was approved by the University of Pennsylvania Institutional Review Board (IRB) and represents the initial component of an ongoing longitudinal study testing different methods of CPR education in the hospital setting. Enrollment was conducted at 3 hospitals: The Hospital of the University of Pennsylvania (a 700‐bed tertiary‐care academic medical center), Penn Presbyterian Medical Center (a 300‐bed tertiary‐care and community hospital) and Pennsylvania Hospital (a 400‐bed community hospital).
Recruitment Strategy
Family members of hospitalized patients with known or suspected coronary disease were targeted in this investigation (eg, patients admitted with known myocardial infarction, or patients over 40 years old admitted with chest pain or shortness of breath who had a known history of coronary risk factors). Recruitment took place in the cardiology and telemetry wards of each hospital site by research assistants who were previously CPR trained, but not certified as CPR instructors. Subjects were considered eligible for participation if they were a family member of a current inpatient with known or suspected coronary disease and had not received CPR instruction within the past 2 years. Subjects were excluded if they were under age 18 years, felt unwell, or considered themselves physically unable to undergo CPR training. Eligible individuals were approached using an IRB‐approved recruitment script. If the family member declined participation, the research assistant collected the individual's demographic information and reason for nonparticipation.
If the targeted individual expressed willingness to undergo CPR training, the research assistant administered a pretraining questionnaire to obtain demographic information and history of prior CPR instruction. Subjects then underwent the VSI training program in a family consultation room within the hospital unit, proctored by the research assistant. The VSI program contains an instructional DVD that teaches standard CPR (30 compressions: 2 breaths), as well as the importance of recognizing a nonresponsive patient and calling 9‐1‐1. The training process, including set‐up, video review, and practice routinely took less than 45 minutes per subject. Upon completion of the VSI session, subjects were tested in their newly acquired CPR skills using a VSI or a standard CPR‐recording mannequin, with CPR data analyzed via commercial software (Skill‐Reporter ResuciAnne and Skill‐Reporter software, Laerdal Medical Corporation, Wappinger Falls, NY). Compression rate was calculated as compressions per minute, omitting pauses, with video‐recorded data abstracted and combined with objective CPR recordings. Subjects then completed a Likert scale semi‐quantitative self‐assessment to rate their perspectives on the CPR training experience. Subjects were not compensated financially, but were given the VSI kit to bring home with them at no cost, for the opportunity of performing secondary training.
Assessment of Secondary Training
In an effort to determine whether subjects shared the VSI kit with other family members, follow‐up telephone contact was made with enrollees approximately 1 month after initial CPR training. Subjects were asked to complete a brief survey that included self‐reporting of whether they shared the kit, and if so, how many individuals were trained by the subject (measurement of secondary training, defined as the mean number of people trained for each kit distributed).
Data Analysis and Statistical Calculations
All data, including compiled survey results and CPR quantitative data, were abstracted using a spreadsheet application (Excel, Microsoft Corporation, Redmond, WA). Descriptive statistics were used to compare demographics of enrolled vs. nonenrolled populations, using either student's t‐tests for continuous variables or chi square tests for categorical data. Data are presented as mean standard deviation (SD), with significance set at an alpha = 0.05.
RESULTS
Subject Characteristics and Demographics
Subjects were recruited at the 3 hospital sites between May 2009 and January 2010. A total of 756 eligible individuals were approached, and 280 accepted enrollment for CPR training, representing a 37% enrollment rate (Figure 1). Of the 280 enrolled, 136 underwent instruction using the VSI training program as described, and 144 were enrolled using an experimental method of VSI training in CPR; this second cohort will be described elsewhere. When comparing the eligible individuals who declined enrollment versus those who accepted (Table 1), no significant differences were observed with regard to age, gender or race (P = NS for each). Common reasons cited for nonparticipation included lack of interest or lack of time (data not shown).

| Enrolled (%) n = 136 | Screened/Not Enrolled (%) n = 476 | |
|---|---|---|
| ||
| Age, years | 52 15 | 46 26 |
| Female | 94 (69) | 326 (68) |
| Race | ||
| White | 101 (74) | 316 (66) |
| Black | 30 (22) | 90 (19) |
| Hispanic | 5 (4) | 8 (2) |
| Other/no response | 0 (0) | 59 (13) |
| Relationship to patient | ||
| Spouse | 49 (36) | 171 (36) |
| Immediate family* | 58 (43) | 168 (35) |
| Other | 28 (21) | 76 (16) |
| No response | 1 (1) | 61 (13) |
| Highest Education | ||
| Elementary | 1 (1) | 1 (1) |
| Middle schoo1 | 1 (1) | 7 (1) |
| High school | 46 (34) | 157 (33) |
| Some college/vocation | 36 (26) | 86 (18) |
| College | 30 (22) | 92 (19) |
| Graduate school | 22 (16) | 39 (8) |
| No response | 0 (0) | 94 (20) |
| Previous CPR training | ||
| No | 78 (57) | |
| Yes: within past 2 years | 0 (0) | |
| Yes: within past 25 years | 13 (10) | |
| Yes: within past 510 years | 5 (4) | |
| Yes: more than 10 years ago | 40 (29) | |
Demographics of the enrolled subject cohort are detailed in Table 1. The mean age of subjects was 52 15, and 94 of 136 (69%) were female. Enrolled subjects represented spouses or immediate family members of the hospitalized patient in 107 of 136 (79%) of cases, and the vast majority, 118 of 136 (87%), had either never received CPR training or had received it over 10 years prior to current enrollment.
Subject Perspectives
A posttraining survey revealed that most respondents, 101 of 136 (74%) felt comfortable or very comfortable learning CPR from the VSI kit, and 127 of 136 (93%) felt likely or very likely to share the VSI kit (Figure 2).

Resuscitation Skills Testing
After CPR training, subjects were asked to perform initial resuscitation actions including 2 minutes of CPR on a mannequin (Table 2). A total of 127 subjects completed these trainings, with 25 completing testing using a VSI kit and 102 using a depth‐recording mannequin; data from 9 subjects were excluded due to CPR recording technical problems. With regard to performance of initial resuscitation actions, 96 of 127 (76%) of subjects assessed responsiveness, 90 of 127 (71%) checked for breathing and 91 of 127 (72%) signaled the need to call for help. CPR was attempted by 127 of 127 (100%) subjects. The mean chest compression rate was 90 26 per minutes, and mean compression depth was 37 12 mm. The mean ventilation rate was 4 3 per minutes.
Secondary Training
Eligible subjects (n = 122) were surveyed via telephone 1 month after initial training, in which 95 individuals participated (78% response rate of those eligible for follow‐up). VSI kits were shared by 57 subjects, with a total of 132 additional individuals receiving VSI‐based CPR instruction. This represented a mean of 2.1 (median of 6) people trained per kit shared, with the actual number of people trained ranging from 1 to 15.
DISCUSSION
In the current work, we demonstrated the feasibility of using the hospital environment as a point of capture for training family members of at‐risk patients in CPR skills. Given that most SCA events occur in the home setting, family member training may hold greater potential for CPR delivery during actual events than training a similar number of younger laypersons at large. Other investigators have identified the focused identification and CPR training of populations at risk of SCA as an important and potentially efficient step to improve survival.10, 12, 18, 19 CPR education of family members before hospital discharge represents a logical extension of other cardiac risk factor‐focused health care education and services before patients are discharged home, including delivery of dietary counseling, diabetic teaching, and education regarding cardiac symptoms. To our knowledge, our work represents the first hospital‐based, adult, layperson, CPR training program using VSI as an instructional approach.
CPR training via a 25‐minute VSI program has been shown to yield CPR performance quality in trainees that is similar to that generated from formal CPR classes that require 3 hours to 4 hours.1416 While VSI training does not provide CPR certification, it is unlikely that the lack of testing and certification is a barrier to participation for the lay public. Indeed, the removal of the pressures of a formal class and testing may increase interest in CPR training through the VSI method.13, 20, 21
Several prior investigations have exploited VSI methodology as an outreach tool to teach CPR in various settings. A recent study in Norway used VSI CPR kits as refresher tools for hospital employees.22 Other work has focused on use of VSI implementation in schools.20, 21, 23 An example of this latter approach was a Danish initiative in which 35,000 VSI kits were distributed to seventh graders.20 Over 15,000 laypersons received secondary training at home by the initially trained students, highlighting a key advantage of the VSI kit approach. It has been argued that this secondary training phenomenon is among the reasons the VSI educational approach may offer a cost effective means for targeted family training.13, 20, 21
While participants in our program were able to adequately perform CPR skills and expressed self‐reported motivation and empowerment, it must be acknowledged that many trained laypersons still do not act when confronted with an actual arrest event.8 In addition, CPR quality at the time of actual performance may be variable, attenuating the survival benefit.2426 However, several population‐based observational studies have supported the notion that training more laypersons in CPR translates into improved overall survival rates from cardiac arrest.25, 27, 28 Further work will be required to follow newly trained, at‐risk family members over time to determine if SCA events occur, and if so, whether CPR was initiated.
Limitations
Willingness to undergo CPR training is likely to be confounded by cultural, regional, and educational factors. Therefore, the general applicability of this 3‐hospital program to other practice environments remains an open question. In our program, the majority of screened family members still refused participation; however, we did not discern a simple relationship between willingness to participate and age, gender, or race. Furthermore, we utilized paid research assistants as subject recruiters and proctors to the VSI training; from a broader implementation perspective, it would be important to determine whether hospital volunteers or staff could perform the training. In addition, while a VSI training kit currently costs $35 and a conventional CPR course could cost from $150 to $300, a formal cost‐effectiveness analysis of VSI training has yet to be performed. Another key limitation is that the secondary training effect was measured by participant self‐report, which may be prone to recall bias; however, no specific incentives or penalties were used to encourage over‐reporting of secondary training. Finally, in this short‐term feasibility study, no direct patient outcomes nor instances of CPR performance were measured.
CONCLUSIONS
In this prospective study of hospital‐based CPR training, we have shown that targeted training of families before hospital discharge is feasible, well received by trainees, and has the benefits of secondary training in the home environment, where most SCA events take place. This program could be easily implemented in other hospital or practice settings. Through targeted CPR training programs such as the one described in this investigation, at risk populations that are underrepresented in conventional CPR training classes can be equipped with important life‐saving skills. Further work on a larger scale will be required to measure the impact of such programs on patient outcomes.
Acknowledgements
The authors wish to thank Lori Albright, Matthew Buchwald, Laura Ebbeling, Emily Esposito, Lori Ingleton, Kristy Walsh, Benjamin Weisenthal, Julie Xu and Mariana Gonzalez for subject recruitment and data collection assistance.
Patients discharged from the hospital with coronary disease complications experience an increased risk of sudden cardiac arrest (SCA), which afflicts over 200,000 people in the United States each year with an 80% to 90% mortality rate.17 Prompt delivery of cardiopulmonary resuscitation (CPR) can triple the probability of survival from SCA, yet less than 25% of SCA victims receive bystander CPR.8 Given that 80% of SCA events occur in the home environment, hospitalization could serve as an important point of capture for family instruction in CPR. Prior investigations have suggested conducting conventional CPR training courses before discharge for family members. However, significant barriers exist to this approach, including the requirement for a certified instructor and a large time commitment for standard training.912
To address these resource and time barriers, the American Heart Association recently established a video self‐instruction (VSI) course in CPR, eliminating the need for an instructor and reducing the time requirement for training to 25 minutes. The course consists of a digital video disc (DVD) and low‐cost inflatable mannequin in a self‐contained kit.13 Several investigations have shown that CPR performance skills of students after VSI courses are similar to those of students after traditional CPR training programs.1417 This VSI program presents the unique opportunity for secondary training, given that the DVD and mannequin may be shared by primary trainees with family members or friends. This VSI approach has not been evaluated in the hospital setting or with family members of patients at risk for SCA. We sought to test the feasibility of an in‐hospital CPR training program using the VSI tool, with the hypothesis that VSI training would be well‐accepted by family members of hospitalized patients with known or suspected coronary disease. We further hypothesized that subjects would be able to perform skills adequately and would be motivated to subsequently share the VSI course with others after their family member's hospital discharge.
METHODS
This prospective, multicenter investigation was approved by the University of Pennsylvania Institutional Review Board (IRB) and represents the initial component of an ongoing longitudinal study testing different methods of CPR education in the hospital setting. Enrollment was conducted at 3 hospitals: The Hospital of the University of Pennsylvania (a 700‐bed tertiary‐care academic medical center), Penn Presbyterian Medical Center (a 300‐bed tertiary‐care and community hospital) and Pennsylvania Hospital (a 400‐bed community hospital).
Recruitment Strategy
Family members of hospitalized patients with known or suspected coronary disease were targeted in this investigation (eg, patients admitted with known myocardial infarction, or patients over 40 years old admitted with chest pain or shortness of breath who had a known history of coronary risk factors). Recruitment took place in the cardiology and telemetry wards of each hospital site by research assistants who were previously CPR trained, but not certified as CPR instructors. Subjects were considered eligible for participation if they were a family member of a current inpatient with known or suspected coronary disease and had not received CPR instruction within the past 2 years. Subjects were excluded if they were under age 18 years, felt unwell, or considered themselves physically unable to undergo CPR training. Eligible individuals were approached using an IRB‐approved recruitment script. If the family member declined participation, the research assistant collected the individual's demographic information and reason for nonparticipation.
If the targeted individual expressed willingness to undergo CPR training, the research assistant administered a pretraining questionnaire to obtain demographic information and history of prior CPR instruction. Subjects then underwent the VSI training program in a family consultation room within the hospital unit, proctored by the research assistant. The VSI program contains an instructional DVD that teaches standard CPR (30 compressions: 2 breaths), as well as the importance of recognizing a nonresponsive patient and calling 9‐1‐1. The training process, including set‐up, video review, and practice routinely took less than 45 minutes per subject. Upon completion of the VSI session, subjects were tested in their newly acquired CPR skills using a VSI or a standard CPR‐recording mannequin, with CPR data analyzed via commercial software (Skill‐Reporter ResuciAnne and Skill‐Reporter software, Laerdal Medical Corporation, Wappinger Falls, NY). Compression rate was calculated as compressions per minute, omitting pauses, with video‐recorded data abstracted and combined with objective CPR recordings. Subjects then completed a Likert scale semi‐quantitative self‐assessment to rate their perspectives on the CPR training experience. Subjects were not compensated financially, but were given the VSI kit to bring home with them at no cost, for the opportunity of performing secondary training.
Assessment of Secondary Training
In an effort to determine whether subjects shared the VSI kit with other family members, follow‐up telephone contact was made with enrollees approximately 1 month after initial CPR training. Subjects were asked to complete a brief survey that included self‐reporting of whether they shared the kit, and if so, how many individuals were trained by the subject (measurement of secondary training, defined as the mean number of people trained for each kit distributed).
Data Analysis and Statistical Calculations
All data, including compiled survey results and CPR quantitative data, were abstracted using a spreadsheet application (Excel, Microsoft Corporation, Redmond, WA). Descriptive statistics were used to compare demographics of enrolled vs. nonenrolled populations, using either student's t‐tests for continuous variables or chi square tests for categorical data. Data are presented as mean standard deviation (SD), with significance set at an alpha = 0.05.
RESULTS
Subject Characteristics and Demographics
Subjects were recruited at the 3 hospital sites between May 2009 and January 2010. A total of 756 eligible individuals were approached, and 280 accepted enrollment for CPR training, representing a 37% enrollment rate (Figure 1). Of the 280 enrolled, 136 underwent instruction using the VSI training program as described, and 144 were enrolled using an experimental method of VSI training in CPR; this second cohort will be described elsewhere. When comparing the eligible individuals who declined enrollment versus those who accepted (Table 1), no significant differences were observed with regard to age, gender or race (P = NS for each). Common reasons cited for nonparticipation included lack of interest or lack of time (data not shown).

| Enrolled (%) n = 136 | Screened/Not Enrolled (%) n = 476 | |
|---|---|---|
| ||
| Age, years | 52 15 | 46 26 |
| Female | 94 (69) | 326 (68) |
| Race | ||
| White | 101 (74) | 316 (66) |
| Black | 30 (22) | 90 (19) |
| Hispanic | 5 (4) | 8 (2) |
| Other/no response | 0 (0) | 59 (13) |
| Relationship to patient | ||
| Spouse | 49 (36) | 171 (36) |
| Immediate family* | 58 (43) | 168 (35) |
| Other | 28 (21) | 76 (16) |
| No response | 1 (1) | 61 (13) |
| Highest Education | ||
| Elementary | 1 (1) | 1 (1) |
| Middle schoo1 | 1 (1) | 7 (1) |
| High school | 46 (34) | 157 (33) |
| Some college/vocation | 36 (26) | 86 (18) |
| College | 30 (22) | 92 (19) |
| Graduate school | 22 (16) | 39 (8) |
| No response | 0 (0) | 94 (20) |
| Previous CPR training | ||
| No | 78 (57) | |
| Yes: within past 2 years | 0 (0) | |
| Yes: within past 25 years | 13 (10) | |
| Yes: within past 510 years | 5 (4) | |
| Yes: more than 10 years ago | 40 (29) | |
Demographics of the enrolled subject cohort are detailed in Table 1. The mean age of subjects was 52 15, and 94 of 136 (69%) were female. Enrolled subjects represented spouses or immediate family members of the hospitalized patient in 107 of 136 (79%) of cases, and the vast majority, 118 of 136 (87%), had either never received CPR training or had received it over 10 years prior to current enrollment.
Subject Perspectives
A posttraining survey revealed that most respondents, 101 of 136 (74%) felt comfortable or very comfortable learning CPR from the VSI kit, and 127 of 136 (93%) felt likely or very likely to share the VSI kit (Figure 2).

Resuscitation Skills Testing
After CPR training, subjects were asked to perform initial resuscitation actions including 2 minutes of CPR on a mannequin (Table 2). A total of 127 subjects completed these trainings, with 25 completing testing using a VSI kit and 102 using a depth‐recording mannequin; data from 9 subjects were excluded due to CPR recording technical problems. With regard to performance of initial resuscitation actions, 96 of 127 (76%) of subjects assessed responsiveness, 90 of 127 (71%) checked for breathing and 91 of 127 (72%) signaled the need to call for help. CPR was attempted by 127 of 127 (100%) subjects. The mean chest compression rate was 90 26 per minutes, and mean compression depth was 37 12 mm. The mean ventilation rate was 4 3 per minutes.
Secondary Training
Eligible subjects (n = 122) were surveyed via telephone 1 month after initial training, in which 95 individuals participated (78% response rate of those eligible for follow‐up). VSI kits were shared by 57 subjects, with a total of 132 additional individuals receiving VSI‐based CPR instruction. This represented a mean of 2.1 (median of 6) people trained per kit shared, with the actual number of people trained ranging from 1 to 15.
DISCUSSION
In the current work, we demonstrated the feasibility of using the hospital environment as a point of capture for training family members of at‐risk patients in CPR skills. Given that most SCA events occur in the home setting, family member training may hold greater potential for CPR delivery during actual events than training a similar number of younger laypersons at large. Other investigators have identified the focused identification and CPR training of populations at risk of SCA as an important and potentially efficient step to improve survival.10, 12, 18, 19 CPR education of family members before hospital discharge represents a logical extension of other cardiac risk factor‐focused health care education and services before patients are discharged home, including delivery of dietary counseling, diabetic teaching, and education regarding cardiac symptoms. To our knowledge, our work represents the first hospital‐based, adult, layperson, CPR training program using VSI as an instructional approach.
CPR training via a 25‐minute VSI program has been shown to yield CPR performance quality in trainees that is similar to that generated from formal CPR classes that require 3 hours to 4 hours.1416 While VSI training does not provide CPR certification, it is unlikely that the lack of testing and certification is a barrier to participation for the lay public. Indeed, the removal of the pressures of a formal class and testing may increase interest in CPR training through the VSI method.13, 20, 21
Several prior investigations have exploited VSI methodology as an outreach tool to teach CPR in various settings. A recent study in Norway used VSI CPR kits as refresher tools for hospital employees.22 Other work has focused on use of VSI implementation in schools.20, 21, 23 An example of this latter approach was a Danish initiative in which 35,000 VSI kits were distributed to seventh graders.20 Over 15,000 laypersons received secondary training at home by the initially trained students, highlighting a key advantage of the VSI kit approach. It has been argued that this secondary training phenomenon is among the reasons the VSI educational approach may offer a cost effective means for targeted family training.13, 20, 21
While participants in our program were able to adequately perform CPR skills and expressed self‐reported motivation and empowerment, it must be acknowledged that many trained laypersons still do not act when confronted with an actual arrest event.8 In addition, CPR quality at the time of actual performance may be variable, attenuating the survival benefit.2426 However, several population‐based observational studies have supported the notion that training more laypersons in CPR translates into improved overall survival rates from cardiac arrest.25, 27, 28 Further work will be required to follow newly trained, at‐risk family members over time to determine if SCA events occur, and if so, whether CPR was initiated.
Limitations
Willingness to undergo CPR training is likely to be confounded by cultural, regional, and educational factors. Therefore, the general applicability of this 3‐hospital program to other practice environments remains an open question. In our program, the majority of screened family members still refused participation; however, we did not discern a simple relationship between willingness to participate and age, gender, or race. Furthermore, we utilized paid research assistants as subject recruiters and proctors to the VSI training; from a broader implementation perspective, it would be important to determine whether hospital volunteers or staff could perform the training. In addition, while a VSI training kit currently costs $35 and a conventional CPR course could cost from $150 to $300, a formal cost‐effectiveness analysis of VSI training has yet to be performed. Another key limitation is that the secondary training effect was measured by participant self‐report, which may be prone to recall bias; however, no specific incentives or penalties were used to encourage over‐reporting of secondary training. Finally, in this short‐term feasibility study, no direct patient outcomes nor instances of CPR performance were measured.
CONCLUSIONS
In this prospective study of hospital‐based CPR training, we have shown that targeted training of families before hospital discharge is feasible, well received by trainees, and has the benefits of secondary training in the home environment, where most SCA events take place. This program could be easily implemented in other hospital or practice settings. Through targeted CPR training programs such as the one described in this investigation, at risk populations that are underrepresented in conventional CPR training classes can be equipped with important life‐saving skills. Further work on a larger scale will be required to measure the impact of such programs on patient outcomes.
Acknowledgements
The authors wish to thank Lori Albright, Matthew Buchwald, Laura Ebbeling, Emily Esposito, Lori Ingleton, Kristy Walsh, Benjamin Weisenthal, Julie Xu and Mariana Gonzalez for subject recruitment and data collection assistance.
- ,,, et al.Heart disease and stroke statistics–2010 update: a report from the American Heart Association.Circulation.2010;121(7):e46–e215.
- ,,, et al.Regional variation in out‐of‐hospital cardiac arrest incidence and outcome.JAMA.2008;300(12):1423–1431.
- ,,, et al.Prediction of sudden cardiac death after myocardial infarction in the beta‐blocking era.J Am Coll Cardiol.2003;42(4):652–658.
- ,,, et al.Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both.N Engl J Med.2005;352(25):2581–2588.
- ,,,.One‐year health status outcomes of unstable angina versus myocardial infarction: a prospective, observational cohort study of ACS survivors.BMC Cardiovasc Disord.2007;7:28.
- ,,,,.Sudden death after myocardial infarction.JAMA.2008;300(17):2022–2029.
- ,,.Elevated admission serum creatinine predicts poor myocardial blood flow and one‐year mortality in ST‐segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention.J Invasive Cardiol.2009;21(10):493–498.
- ,,,,,.CPR training and CPR performance: do CPR‐trained bystanders perform CPR?Acad Emerg Med.2006;13(6):596–601.
- ,,, et al.Reducing barriers for implementation of bystander‐initiated cardiopulmonary resuscitation: a scientific statement from the American Heart Association for healthcare providers, policymakers, and community leaders regarding the effectiveness of cardiopulmonary resuscitation.Circulation.2008;117(5):704–709.
- ,,.Understanding and improving low bystander CPR rates: a systematic review of the literature.CJEM.2008;10(1):51–65.
- ,.Are we training the right people yet? A survey of participants in public cardiopulmonary resuscitation classes.Resuscitation.1998;37(1):21–25.
- ,,, et al.Cardiac arrest in private locations: different strategies are needed to improve outcome.Resuscitation.2003;58(2):171–176.
- ,.The American Heart Association CPR Anytime Program: the potential impact of highly accessible training in cardiopulmonary resuscitation.J Cardiopulm Rehabil.2006;26(6):346–354.
- ,,,,.Laypersons may learn basic life support in 24 min using a personal resuscitation manikin.Resuscitation.2006;69(3):435–442.
- ,,, et al.Prospective, randomized trial of the effectiveness and retention of 30‐min layperson training for cardiopulmonary resuscitation and automated external defibrillators: The American Airlines Study.Resuscitation.2007;74(2):276–285.
- ,,,,,.Effectiveness of a 30‐min CPR self‐instruction program for lay responders: a controlled randomized study.Resuscitation.2005;67(1):31–43.
- ,,, et al.Randomized, controlled trial of video self‐instruction versus traditional CPR training.Ann Emerg Med.1998;31(3):364–369.
- ,,, et al.A randomized controlled trial of chest compression only CPR for older adults‐a pilot study.Resuscitation.2003;58(2):177–185.
- ,.Estimating cost‐effectiveness of mass cardiopulmonary resuscitation training strategies to improve survival from cardiac arrest in private locations.Prehosp Emerg Care.2004;8(4):420–423.
- ,,,.Disseminating cardiopulmonary resuscitation training by distributing 35,000 personal manikins among school children.Circulation.2007;116(12):1380–1385.
- ,,.Impact of a self‐instruction CPR kit on 7th graders' and adults' skills and CPR performance.Resuscitation.2008;79(1):103–108.
- ,,,,.Hospital employees improve basic life support skills and confidence with a personal resuscitation manikin and a 24‐min video instruction.Resuscitation.2009;80(8):898–902.
- ,,.High school students as ambassadors of CPR‐‐a model for reaching the most appropriate target population?Resuscitation.2010;81(1):78–81.
- ,,,.Three‐phase model of cardiac arrest: time‐dependent benefit of bystander cardiopulmonary resuscitation.Am J Cardiol.2006;98(4):497–499.
- ,,,.Performance of chest compressions by laypersons during the Public Access Defibrillation Trial.Resuscitation.2010;81(3):293–296.
- ,,, et al.Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in‐hospital cardiac arrest.Circulation.2005;111(4):428–434.
- ,,, et al.The Save Hearts in Arizona Registry and Education (SHARE) program: who is performing CPR and where are they doing it?Resuscitation.2007;75(1):68–75.
- ,,,,.Efficacy of bystander CPR: intervention by lay people and by health care professionals.Resuscitation.2005;66(3):291–295.
- ,,, et al.Heart disease and stroke statistics–2010 update: a report from the American Heart Association.Circulation.2010;121(7):e46–e215.
- ,,, et al.Regional variation in out‐of‐hospital cardiac arrest incidence and outcome.JAMA.2008;300(12):1423–1431.
- ,,, et al.Prediction of sudden cardiac death after myocardial infarction in the beta‐blocking era.J Am Coll Cardiol.2003;42(4):652–658.
- ,,, et al.Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both.N Engl J Med.2005;352(25):2581–2588.
- ,,,.One‐year health status outcomes of unstable angina versus myocardial infarction: a prospective, observational cohort study of ACS survivors.BMC Cardiovasc Disord.2007;7:28.
- ,,,,.Sudden death after myocardial infarction.JAMA.2008;300(17):2022–2029.
- ,,.Elevated admission serum creatinine predicts poor myocardial blood flow and one‐year mortality in ST‐segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention.J Invasive Cardiol.2009;21(10):493–498.
- ,,,,,.CPR training and CPR performance: do CPR‐trained bystanders perform CPR?Acad Emerg Med.2006;13(6):596–601.
- ,,, et al.Reducing barriers for implementation of bystander‐initiated cardiopulmonary resuscitation: a scientific statement from the American Heart Association for healthcare providers, policymakers, and community leaders regarding the effectiveness of cardiopulmonary resuscitation.Circulation.2008;117(5):704–709.
- ,,.Understanding and improving low bystander CPR rates: a systematic review of the literature.CJEM.2008;10(1):51–65.
- ,.Are we training the right people yet? A survey of participants in public cardiopulmonary resuscitation classes.Resuscitation.1998;37(1):21–25.
- ,,, et al.Cardiac arrest in private locations: different strategies are needed to improve outcome.Resuscitation.2003;58(2):171–176.
- ,.The American Heart Association CPR Anytime Program: the potential impact of highly accessible training in cardiopulmonary resuscitation.J Cardiopulm Rehabil.2006;26(6):346–354.
- ,,,,.Laypersons may learn basic life support in 24 min using a personal resuscitation manikin.Resuscitation.2006;69(3):435–442.
- ,,, et al.Prospective, randomized trial of the effectiveness and retention of 30‐min layperson training for cardiopulmonary resuscitation and automated external defibrillators: The American Airlines Study.Resuscitation.2007;74(2):276–285.
- ,,,,,.Effectiveness of a 30‐min CPR self‐instruction program for lay responders: a controlled randomized study.Resuscitation.2005;67(1):31–43.
- ,,, et al.Randomized, controlled trial of video self‐instruction versus traditional CPR training.Ann Emerg Med.1998;31(3):364–369.
- ,,, et al.A randomized controlled trial of chest compression only CPR for older adults‐a pilot study.Resuscitation.2003;58(2):177–185.
- ,.Estimating cost‐effectiveness of mass cardiopulmonary resuscitation training strategies to improve survival from cardiac arrest in private locations.Prehosp Emerg Care.2004;8(4):420–423.
- ,,,.Disseminating cardiopulmonary resuscitation training by distributing 35,000 personal manikins among school children.Circulation.2007;116(12):1380–1385.
- ,,.Impact of a self‐instruction CPR kit on 7th graders' and adults' skills and CPR performance.Resuscitation.2008;79(1):103–108.
- ,,,,.Hospital employees improve basic life support skills and confidence with a personal resuscitation manikin and a 24‐min video instruction.Resuscitation.2009;80(8):898–902.
- ,,.High school students as ambassadors of CPR‐‐a model for reaching the most appropriate target population?Resuscitation.2010;81(1):78–81.
- ,,,.Three‐phase model of cardiac arrest: time‐dependent benefit of bystander cardiopulmonary resuscitation.Am J Cardiol.2006;98(4):497–499.
- ,,,.Performance of chest compressions by laypersons during the Public Access Defibrillation Trial.Resuscitation.2010;81(3):293–296.
- ,,, et al.Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in‐hospital cardiac arrest.Circulation.2005;111(4):428–434.
- ,,, et al.The Save Hearts in Arizona Registry and Education (SHARE) program: who is performing CPR and where are they doing it?Resuscitation.2007;75(1):68–75.
- ,,,,.Efficacy of bystander CPR: intervention by lay people and by health care professionals.Resuscitation.2005;66(3):291–295.
Greater Hospitalist Role Envisioned for Cancer Patients
Hospitalists who encounter the occasional late-stage colorectal cancer patient might be perplexed as to why the patient refuses to remove food from their in-room refrigerator and often are wearing mittens. But it would be immediately clear to them once they knew that the patient was on oxaliplatin—a less-than-decade-old medication delivered via the chemotherapy regimen known as FOLFOX—and that a common side effect is neuropathy resulting in extreme sensitivity to cold.
Why wouldn’t hospitalists know this? Because, according to a medical oncologist at Memorial Sloan-Kettering Cancer Center in New York City, oncologists traditionally have tried to holistically manage the care of cancer patients. In today’s age of new treatments and increased inpatient care for patients with aggressive cancers, though, Jason Konner, MD, says it’s time for hospitalists to take a greater role in the management of cancer patients.
The upshot: Dr. Konner envisions a new breed of oncologist-hospitalists. (Check out this in-depth look at specialty physicians adopting the HM model of care.)
“Universally, the hospitalist is going to have to be part of a team with the oncologist,” says Dr. Konner, assistant professor with the Gynecological Medical Oncology Service and Developmental Therapeutic Services. “We’re going to complement each other. There are definitely things that we can do that they can’t and definitely things they can do that we can’t. Right now, it’s just being part of the team to address the diverse medical complications of cancer. But I think that increasingly, [hospitalists] are going to be the primary caregivers, sometimes solely the caregivers, of patients with cancer complications.”
The concept, which was raised during an “Oncology for the Hospitalist” presentation at the fifth annual Mid-Atlantic Hospital Medicine Symposium at Mount Sinai School of Medicine in New York City, is not new, but it is particularly relevant as cancer mortality and incidence rates continue to drop. Dr. Konner counters that while improved screening techniques—mammographies and prostate-specific antigen (PSA) tests, to note a pair—have reduced incidences, the majority of “aggressive cancers and cancers that kill people” still require intensive inpatient care.
To wit, a pilot program at Mount Sinai several years ago dedicated a hospitalist to the oncology service in the hopes of developing a staffer with a new expertise. The brief program, which yielded little data because of its small sample size, was aimed at determining the efficacy of an oncology hospitalist.
Richard Quinn is a freelance writer based in New Jersey.
Hospitalists who encounter the occasional late-stage colorectal cancer patient might be perplexed as to why the patient refuses to remove food from their in-room refrigerator and often are wearing mittens. But it would be immediately clear to them once they knew that the patient was on oxaliplatin—a less-than-decade-old medication delivered via the chemotherapy regimen known as FOLFOX—and that a common side effect is neuropathy resulting in extreme sensitivity to cold.
Why wouldn’t hospitalists know this? Because, according to a medical oncologist at Memorial Sloan-Kettering Cancer Center in New York City, oncologists traditionally have tried to holistically manage the care of cancer patients. In today’s age of new treatments and increased inpatient care for patients with aggressive cancers, though, Jason Konner, MD, says it’s time for hospitalists to take a greater role in the management of cancer patients.
The upshot: Dr. Konner envisions a new breed of oncologist-hospitalists. (Check out this in-depth look at specialty physicians adopting the HM model of care.)
“Universally, the hospitalist is going to have to be part of a team with the oncologist,” says Dr. Konner, assistant professor with the Gynecological Medical Oncology Service and Developmental Therapeutic Services. “We’re going to complement each other. There are definitely things that we can do that they can’t and definitely things they can do that we can’t. Right now, it’s just being part of the team to address the diverse medical complications of cancer. But I think that increasingly, [hospitalists] are going to be the primary caregivers, sometimes solely the caregivers, of patients with cancer complications.”
The concept, which was raised during an “Oncology for the Hospitalist” presentation at the fifth annual Mid-Atlantic Hospital Medicine Symposium at Mount Sinai School of Medicine in New York City, is not new, but it is particularly relevant as cancer mortality and incidence rates continue to drop. Dr. Konner counters that while improved screening techniques—mammographies and prostate-specific antigen (PSA) tests, to note a pair—have reduced incidences, the majority of “aggressive cancers and cancers that kill people” still require intensive inpatient care.
To wit, a pilot program at Mount Sinai several years ago dedicated a hospitalist to the oncology service in the hopes of developing a staffer with a new expertise. The brief program, which yielded little data because of its small sample size, was aimed at determining the efficacy of an oncology hospitalist.
Richard Quinn is a freelance writer based in New Jersey.
Hospitalists who encounter the occasional late-stage colorectal cancer patient might be perplexed as to why the patient refuses to remove food from their in-room refrigerator and often are wearing mittens. But it would be immediately clear to them once they knew that the patient was on oxaliplatin—a less-than-decade-old medication delivered via the chemotherapy regimen known as FOLFOX—and that a common side effect is neuropathy resulting in extreme sensitivity to cold.
Why wouldn’t hospitalists know this? Because, according to a medical oncologist at Memorial Sloan-Kettering Cancer Center in New York City, oncologists traditionally have tried to holistically manage the care of cancer patients. In today’s age of new treatments and increased inpatient care for patients with aggressive cancers, though, Jason Konner, MD, says it’s time for hospitalists to take a greater role in the management of cancer patients.
The upshot: Dr. Konner envisions a new breed of oncologist-hospitalists. (Check out this in-depth look at specialty physicians adopting the HM model of care.)
“Universally, the hospitalist is going to have to be part of a team with the oncologist,” says Dr. Konner, assistant professor with the Gynecological Medical Oncology Service and Developmental Therapeutic Services. “We’re going to complement each other. There are definitely things that we can do that they can’t and definitely things they can do that we can’t. Right now, it’s just being part of the team to address the diverse medical complications of cancer. But I think that increasingly, [hospitalists] are going to be the primary caregivers, sometimes solely the caregivers, of patients with cancer complications.”
The concept, which was raised during an “Oncology for the Hospitalist” presentation at the fifth annual Mid-Atlantic Hospital Medicine Symposium at Mount Sinai School of Medicine in New York City, is not new, but it is particularly relevant as cancer mortality and incidence rates continue to drop. Dr. Konner counters that while improved screening techniques—mammographies and prostate-specific antigen (PSA) tests, to note a pair—have reduced incidences, the majority of “aggressive cancers and cancers that kill people” still require intensive inpatient care.
To wit, a pilot program at Mount Sinai several years ago dedicated a hospitalist to the oncology service in the hopes of developing a staffer with a new expertise. The brief program, which yielded little data because of its small sample size, was aimed at determining the efficacy of an oncology hospitalist.
Richard Quinn is a freelance writer based in New Jersey.
Diabetes Rates Expected to Double
Hospitalist Jeffrey Schnipper, MD, MPH, FHM, estimated last year that a full third of his current patient census was either diabetic or hypoglycemic, a figure that might seem out of place for someone who isn’t a diabetologist.
Last week, Dr. Schnipper's estimation made perfect sense with the release of a new report from the Centers for Disease Control and Prevention (CDC) that forecasts a near-doubling of diabetic incidences in the next 40 years.
"I won't be surprised when there's a day where half of my patients have diabetes or hypoglycemia," says Dr. Schnipper, director of clinical research and associate physician in the general medicine division at Brigham and Women's Hospitalist Service in Boston. "We all have to become experts in how to comanage these patients."
The CDC data, published in Population Health Metrics on Oct. 22, state that "annual diagnosed diabetes incidence [new cases] will increase from about 8 cases per 1,000 in 2008 to about 15 in 2050" (doi: 10.1186/1478-7954-8-29). The authors note that intervention can reduce that prevalence, but won't eliminate it.
Dr. Schnipper says the exponential growth of diabetes incidence will not directly correlate to growth in patient counts, as many diabetics will be able to control their disease without admission to the hospital. However, he says, a large percentage of new cases are likely to end up as hospitalized patients heaped on HM groups' already full plates.
He suggests one response to the looming surge in diabetics might be to administer an A1c test to nearly all of your admitted patients to determine blood-glucose levels, or develop new protocols for how, who, and when to screen for diabetes.
One obvious patient group to be concerned about is the obese population, which Dr. Schnipper says is a direct cause of the diabetic incidence increase. "What we're seeing is an epidemic of obesity causing an epidemic of diabetes," he adds. "We already need to know how to manage these patients."
Hospitalist Jeffrey Schnipper, MD, MPH, FHM, estimated last year that a full third of his current patient census was either diabetic or hypoglycemic, a figure that might seem out of place for someone who isn’t a diabetologist.
Last week, Dr. Schnipper's estimation made perfect sense with the release of a new report from the Centers for Disease Control and Prevention (CDC) that forecasts a near-doubling of diabetic incidences in the next 40 years.
"I won't be surprised when there's a day where half of my patients have diabetes or hypoglycemia," says Dr. Schnipper, director of clinical research and associate physician in the general medicine division at Brigham and Women's Hospitalist Service in Boston. "We all have to become experts in how to comanage these patients."
The CDC data, published in Population Health Metrics on Oct. 22, state that "annual diagnosed diabetes incidence [new cases] will increase from about 8 cases per 1,000 in 2008 to about 15 in 2050" (doi: 10.1186/1478-7954-8-29). The authors note that intervention can reduce that prevalence, but won't eliminate it.
Dr. Schnipper says the exponential growth of diabetes incidence will not directly correlate to growth in patient counts, as many diabetics will be able to control their disease without admission to the hospital. However, he says, a large percentage of new cases are likely to end up as hospitalized patients heaped on HM groups' already full plates.
He suggests one response to the looming surge in diabetics might be to administer an A1c test to nearly all of your admitted patients to determine blood-glucose levels, or develop new protocols for how, who, and when to screen for diabetes.
One obvious patient group to be concerned about is the obese population, which Dr. Schnipper says is a direct cause of the diabetic incidence increase. "What we're seeing is an epidemic of obesity causing an epidemic of diabetes," he adds. "We already need to know how to manage these patients."
Hospitalist Jeffrey Schnipper, MD, MPH, FHM, estimated last year that a full third of his current patient census was either diabetic or hypoglycemic, a figure that might seem out of place for someone who isn’t a diabetologist.
Last week, Dr. Schnipper's estimation made perfect sense with the release of a new report from the Centers for Disease Control and Prevention (CDC) that forecasts a near-doubling of diabetic incidences in the next 40 years.
"I won't be surprised when there's a day where half of my patients have diabetes or hypoglycemia," says Dr. Schnipper, director of clinical research and associate physician in the general medicine division at Brigham and Women's Hospitalist Service in Boston. "We all have to become experts in how to comanage these patients."
The CDC data, published in Population Health Metrics on Oct. 22, state that "annual diagnosed diabetes incidence [new cases] will increase from about 8 cases per 1,000 in 2008 to about 15 in 2050" (doi: 10.1186/1478-7954-8-29). The authors note that intervention can reduce that prevalence, but won't eliminate it.
Dr. Schnipper says the exponential growth of diabetes incidence will not directly correlate to growth in patient counts, as many diabetics will be able to control their disease without admission to the hospital. However, he says, a large percentage of new cases are likely to end up as hospitalized patients heaped on HM groups' already full plates.
He suggests one response to the looming surge in diabetics might be to administer an A1c test to nearly all of your admitted patients to determine blood-glucose levels, or develop new protocols for how, who, and when to screen for diabetes.
One obvious patient group to be concerned about is the obese population, which Dr. Schnipper says is a direct cause of the diabetic incidence increase. "What we're seeing is an epidemic of obesity causing an epidemic of diabetes," he adds. "We already need to know how to manage these patients."
In the Literature: Research You Need to Know
Clinical question: Are beta-blockers safe to use in patients with chest pain and recent cocaine use?
Background: Beta-blockers are known to improve outcomes after myocardial infarction, yet are contraindicated in chest pain associated with recent cocaine use. Recommendations against beta-blocker use in the setting of cocaine-induced chest pain are based on case reports, small human experiments, and the theoretical concern that beta-blockers may potentiate cocaine toxicity by creating unopposed alpha-adrenergic stimulation. Clinical outcomes of beta-blocker use in patients with cocaine use and chest pain are unknown.
Study design: Retrospective cohort study.
Setting: San Francisco General Hospital, San Francisco.
Synopsis: Three hundred thirty-one patients with chest pain and positive urine toxicologic screening for cocaine were admitted during the study period. One hundred fifty-one (46%) received a beta-blocker in the ED, per the discretion of the treating physicians. There were no differences in ECG abnormalities, troponin levels, length of stay, intubation, ventricular arrhythmias, use of vasopressors, or death in those patients who did and who did not receive a beta-blocker. Over a median follow-up of 972 days, patients who had been discharged on a beta-blocker did have a significant reduction in cardiovascular death (hazard ratio 0.29, 95% CI, 0.09-0.98, P=0.047).
Because this was an observational study and post-discharge data were limited only to vital status, definitive conclusions regarding the safety of beta-blockers in cocaine-associated chest pain cannot be made. The authors acknowledge that more rigorous study is indicated given the potential benefit of beta-blockers in this population.
Bottom line: Use of beta-blockers in patients with chest pain and positive urine drug screen for cocaine is not associated with immediate adverse outcomes and might actually reduce cardiovascular mortality over time.
Citation: Rangel C, Shu RG, Lazar LD, Vittinghoff E, Hsue PY, Marcus GM. Beta-blockers for chest pain associated with recent cocaine use. Arch Intern Med. 2010;170(10):874-879.
Reviewed for TH eWire by Kelly Cunningham MD, Joshua LaBrin, MD, Amanda Salanitro, MD, MSPH, Kelly Sopko, MD, Shelley Ellis, MD, MPH, and Elizabeth Rice MD, Section of Hospital Medicine, Vanderbilt University.
For more physician reviews of literature, visit our website.
Clinical question: Are beta-blockers safe to use in patients with chest pain and recent cocaine use?
Background: Beta-blockers are known to improve outcomes after myocardial infarction, yet are contraindicated in chest pain associated with recent cocaine use. Recommendations against beta-blocker use in the setting of cocaine-induced chest pain are based on case reports, small human experiments, and the theoretical concern that beta-blockers may potentiate cocaine toxicity by creating unopposed alpha-adrenergic stimulation. Clinical outcomes of beta-blocker use in patients with cocaine use and chest pain are unknown.
Study design: Retrospective cohort study.
Setting: San Francisco General Hospital, San Francisco.
Synopsis: Three hundred thirty-one patients with chest pain and positive urine toxicologic screening for cocaine were admitted during the study period. One hundred fifty-one (46%) received a beta-blocker in the ED, per the discretion of the treating physicians. There were no differences in ECG abnormalities, troponin levels, length of stay, intubation, ventricular arrhythmias, use of vasopressors, or death in those patients who did and who did not receive a beta-blocker. Over a median follow-up of 972 days, patients who had been discharged on a beta-blocker did have a significant reduction in cardiovascular death (hazard ratio 0.29, 95% CI, 0.09-0.98, P=0.047).
Because this was an observational study and post-discharge data were limited only to vital status, definitive conclusions regarding the safety of beta-blockers in cocaine-associated chest pain cannot be made. The authors acknowledge that more rigorous study is indicated given the potential benefit of beta-blockers in this population.
Bottom line: Use of beta-blockers in patients with chest pain and positive urine drug screen for cocaine is not associated with immediate adverse outcomes and might actually reduce cardiovascular mortality over time.
Citation: Rangel C, Shu RG, Lazar LD, Vittinghoff E, Hsue PY, Marcus GM. Beta-blockers for chest pain associated with recent cocaine use. Arch Intern Med. 2010;170(10):874-879.
Reviewed for TH eWire by Kelly Cunningham MD, Joshua LaBrin, MD, Amanda Salanitro, MD, MSPH, Kelly Sopko, MD, Shelley Ellis, MD, MPH, and Elizabeth Rice MD, Section of Hospital Medicine, Vanderbilt University.
For more physician reviews of literature, visit our website.
Clinical question: Are beta-blockers safe to use in patients with chest pain and recent cocaine use?
Background: Beta-blockers are known to improve outcomes after myocardial infarction, yet are contraindicated in chest pain associated with recent cocaine use. Recommendations against beta-blocker use in the setting of cocaine-induced chest pain are based on case reports, small human experiments, and the theoretical concern that beta-blockers may potentiate cocaine toxicity by creating unopposed alpha-adrenergic stimulation. Clinical outcomes of beta-blocker use in patients with cocaine use and chest pain are unknown.
Study design: Retrospective cohort study.
Setting: San Francisco General Hospital, San Francisco.
Synopsis: Three hundred thirty-one patients with chest pain and positive urine toxicologic screening for cocaine were admitted during the study period. One hundred fifty-one (46%) received a beta-blocker in the ED, per the discretion of the treating physicians. There were no differences in ECG abnormalities, troponin levels, length of stay, intubation, ventricular arrhythmias, use of vasopressors, or death in those patients who did and who did not receive a beta-blocker. Over a median follow-up of 972 days, patients who had been discharged on a beta-blocker did have a significant reduction in cardiovascular death (hazard ratio 0.29, 95% CI, 0.09-0.98, P=0.047).
Because this was an observational study and post-discharge data were limited only to vital status, definitive conclusions regarding the safety of beta-blockers in cocaine-associated chest pain cannot be made. The authors acknowledge that more rigorous study is indicated given the potential benefit of beta-blockers in this population.
Bottom line: Use of beta-blockers in patients with chest pain and positive urine drug screen for cocaine is not associated with immediate adverse outcomes and might actually reduce cardiovascular mortality over time.
Citation: Rangel C, Shu RG, Lazar LD, Vittinghoff E, Hsue PY, Marcus GM. Beta-blockers for chest pain associated with recent cocaine use. Arch Intern Med. 2010;170(10):874-879.
Reviewed for TH eWire by Kelly Cunningham MD, Joshua LaBrin, MD, Amanda Salanitro, MD, MSPH, Kelly Sopko, MD, Shelley Ellis, MD, MPH, and Elizabeth Rice MD, Section of Hospital Medicine, Vanderbilt University.
For more physician reviews of literature, visit our website.