User login
Is the contemporary mental health crisis among youth due to DMN disruption?
The advent of unprecedented technologies drastically altering the behavior of children and adolescents, compounded by prolonged isolation from a once-in-a-century pandemic, may have negatively impacted the normal connectivity of the human brain among youth, leading to the current alarming increase of depression, anxiety, and suicidality among this population.
The human brain is comprised of multiple large-scale networks that are functionally connected and control feelings, thoughts, and behaviors. As clinical neuroscientists, psychiatrists must consider the profound impact of a massive societal shift in human behavior on the functional connectivity of brain networks in health and disease. The advent of smartphones, social media, and video game addiction may have disrupted the developing brain networks in children and adolescents, leading to the current escalating epidemic of mental disorders in youth.
The major networks in the human brain include the default mode network (DMN), the salience network, the limbic system, the dorsal attention network, the central executive network, and the visual system.1 Each network connects several brain regions. Researchers can use functional MRI to detect the connectivity of those networks. When blood flow increases concurrently across 2 or 3 networks, this indicates those networks are functionally connected.
There was an old “dogma” that brain regions use energy only when activated and being used. Hans Berger, who developed the EEG in 1929, noticed electrical activity at rest and proposed that the brain is constantly busy, but his neurology peers did not take him seriously.2 In the 1950s, Louis Sokoloff noticed that brain metabolism was the same whether a person is at rest or doing math. In the 1970s, David Ingvar discovered that the highest blood flow in the frontal lobe occurred when a person was at rest.3 Finally, in 2007, Raichle et al4 used positron emission tomography scans to confirm that the frontal lobe is most active when a person is not doing anything. He labeled this phenomenon the DMN, comprising the medial fronto-parietal cortex, the posterior cingulate gyrus, the precuneus, and the angular gyrus. Interestingly, the number of publications about the DMN has skyrocketed since 2007.
The many roles of the DMN
Ongoing research has revealed that the DMN is most active at rest, and its anatomical hubs mediate several key functions5:
- Posterior cingulate gyrus (the central core of the DMN): remembering the past and thinking about the future
- Medial prefrontal cortex: autobiographical memories, future goals and events, reflecting on one’s emotional self, and considering decisions about family members
- Dorsal medial subsystem: thinking about others, determining and inferring the purpose of other people’s actions
- Temporo-parietal junction: reflecting on the beliefs and emotions of others (known as “theory of mind”6)
- Lateral parietal junction: retrieval of social and conceptual knowledge
- Hippocampus: forming new memories, remembering the past, imagining the future
- Posterior-inferior parietal lobe: junction of auditory, visual, and somatic sensory information and attention
- Precuneus: Visual, sensory-motor, and attention.
Many terms have been used to describe the function of the DMN, including “daydreaming,” “auto-pilot,” “mind-wondering,” “reminiscing,” “contemplating,” “self-reflection,” “the neurological basis of the self,” and “seat of literary creativity.”
Psychiatric consequences of DMN deactivation
When another brain network, the attention network (which is also referred to as the task-positive network), is activated consciously and volitionally to perform a task that demands focus (such as text messaging, playing video games, or continuously interacting with social media sites), DMN activity declines.
Continue to: The DMN does not exist...
The DMN does not exist in infants, but starts to develop in childhood.7 It is enhanced by exercise, daydreaming, and sleep, activities that are common in childhood but have declined drastically with the widespread use of smartphones, video games, and social media, which for many youth occupy the bulk of their waking hours. Those tasks, which require continuous attention, deactivate the DMN. In fact, research has shown that addictive behavior decreases the connectivity of the DMN and suppresses its activity.8 Most children and adolescents can be regarded as essentially addicted to social media, text messaging, and video games. Unsurprisingly, serious psychiatric consequences follow.9
DMN dysfunction has been reported in several psychiatric conditions, including depression, posttraumatic stress disorder, autism, schizophrenia, anxiety, obsessive-compulsive disorder, and substance use.10-12 Impaired social interactions and communications, negative ruminations, suicidal ideas, and impaired encoding of long-term memories are some of the adverse effects of DMN dysfunction. The good news is that the DMN’s connectivity and functioning can be modulated and restored by meditation, mentalizing, exercise, psychotherapy, antidepressants, and psychedelics.13,14
The lockdown and stress of the COVID-19 pandemic added insult to injury and exacerbated mental illness in children by isolating them from each other and intensifying their technological addiction to fill the void of isolation. This crisis in youth mental health continues unabated, and calls for action to prevent grim outcomes. DMN dysfunction in youth can be reversed with treatment, but access to mental health care has become more challenging due to workforce shortages and insurance restrictions. Psychiatrists and parents must work diligently to treat psychiatrically affected youth, which has become a DaMN serious problem…
1. Yao Z, Hu B, Xie Y, et al. A review of structural and functional brain networks: small world and atlas. Brain Inform. 2015;2(1):45-52. doi:10.1007/s40708-015-0009-z
2. Raichle ME. The brain’s dark energy. Sci Am. 2010;302(3):44-49. doi:10.1038/scientific american0310-44
3. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi:10.1196/annals.1440.011
4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083-1090; discussion 1097-1099. doi:10.1016/j.neuroimage.2007.02.041
5. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251-270. doi:10.1177/1073858411403316
6. Tsoukalas I. Theory of mind: towards an evolutionary theory. Evolutionary Psychological Science. 2018;4(1):38-66. https://doi.org/10.1007/s40806-017-0112-x
7. Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279-296. doi:10.1016/j.neubiorev.2008.09.002
8. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313-331. doi:10.1016/j.neuroimage.2019.06.036
9. Bommersbach TJ, McKean AJ, Olfson M, et al. National trends in mental health-related emergency department visits among youth, 2011-2020. JAMA. 2023;329(17):1469-1477. doi:10.1001/jama.2023.4809
10. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi:10.1146/annurev-clinpsy-032511-143049
11. Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489-498. doi:10.1016/j.neuroimage.2018.05.005
12. Nagata JM, Chu J, Zamora G, et al. Screen time and obsessive-compulsive disorder among children 9-10 years old: a prospective cohort study. J Adolesc Health. 2023;72(3):390-396. doi:10.1016/j.jadohealth.2022.10.023
13. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48-73. doi:10.1016/j.neubiorev.2014.03.016
14. Gattuso JJ, Perkins D, Ruffell S, et al. Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol. 2023;26(3):155-188. doi:10.1093/ijnp/pyac074
The advent of unprecedented technologies drastically altering the behavior of children and adolescents, compounded by prolonged isolation from a once-in-a-century pandemic, may have negatively impacted the normal connectivity of the human brain among youth, leading to the current alarming increase of depression, anxiety, and suicidality among this population.
The human brain is comprised of multiple large-scale networks that are functionally connected and control feelings, thoughts, and behaviors. As clinical neuroscientists, psychiatrists must consider the profound impact of a massive societal shift in human behavior on the functional connectivity of brain networks in health and disease. The advent of smartphones, social media, and video game addiction may have disrupted the developing brain networks in children and adolescents, leading to the current escalating epidemic of mental disorders in youth.
The major networks in the human brain include the default mode network (DMN), the salience network, the limbic system, the dorsal attention network, the central executive network, and the visual system.1 Each network connects several brain regions. Researchers can use functional MRI to detect the connectivity of those networks. When blood flow increases concurrently across 2 or 3 networks, this indicates those networks are functionally connected.
There was an old “dogma” that brain regions use energy only when activated and being used. Hans Berger, who developed the EEG in 1929, noticed electrical activity at rest and proposed that the brain is constantly busy, but his neurology peers did not take him seriously.2 In the 1950s, Louis Sokoloff noticed that brain metabolism was the same whether a person is at rest or doing math. In the 1970s, David Ingvar discovered that the highest blood flow in the frontal lobe occurred when a person was at rest.3 Finally, in 2007, Raichle et al4 used positron emission tomography scans to confirm that the frontal lobe is most active when a person is not doing anything. He labeled this phenomenon the DMN, comprising the medial fronto-parietal cortex, the posterior cingulate gyrus, the precuneus, and the angular gyrus. Interestingly, the number of publications about the DMN has skyrocketed since 2007.
The many roles of the DMN
Ongoing research has revealed that the DMN is most active at rest, and its anatomical hubs mediate several key functions5:
- Posterior cingulate gyrus (the central core of the DMN): remembering the past and thinking about the future
- Medial prefrontal cortex: autobiographical memories, future goals and events, reflecting on one’s emotional self, and considering decisions about family members
- Dorsal medial subsystem: thinking about others, determining and inferring the purpose of other people’s actions
- Temporo-parietal junction: reflecting on the beliefs and emotions of others (known as “theory of mind”6)
- Lateral parietal junction: retrieval of social and conceptual knowledge
- Hippocampus: forming new memories, remembering the past, imagining the future
- Posterior-inferior parietal lobe: junction of auditory, visual, and somatic sensory information and attention
- Precuneus: Visual, sensory-motor, and attention.
Many terms have been used to describe the function of the DMN, including “daydreaming,” “auto-pilot,” “mind-wondering,” “reminiscing,” “contemplating,” “self-reflection,” “the neurological basis of the self,” and “seat of literary creativity.”
Psychiatric consequences of DMN deactivation
When another brain network, the attention network (which is also referred to as the task-positive network), is activated consciously and volitionally to perform a task that demands focus (such as text messaging, playing video games, or continuously interacting with social media sites), DMN activity declines.
Continue to: The DMN does not exist...
The DMN does not exist in infants, but starts to develop in childhood.7 It is enhanced by exercise, daydreaming, and sleep, activities that are common in childhood but have declined drastically with the widespread use of smartphones, video games, and social media, which for many youth occupy the bulk of their waking hours. Those tasks, which require continuous attention, deactivate the DMN. In fact, research has shown that addictive behavior decreases the connectivity of the DMN and suppresses its activity.8 Most children and adolescents can be regarded as essentially addicted to social media, text messaging, and video games. Unsurprisingly, serious psychiatric consequences follow.9
DMN dysfunction has been reported in several psychiatric conditions, including depression, posttraumatic stress disorder, autism, schizophrenia, anxiety, obsessive-compulsive disorder, and substance use.10-12 Impaired social interactions and communications, negative ruminations, suicidal ideas, and impaired encoding of long-term memories are some of the adverse effects of DMN dysfunction. The good news is that the DMN’s connectivity and functioning can be modulated and restored by meditation, mentalizing, exercise, psychotherapy, antidepressants, and psychedelics.13,14
The lockdown and stress of the COVID-19 pandemic added insult to injury and exacerbated mental illness in children by isolating them from each other and intensifying their technological addiction to fill the void of isolation. This crisis in youth mental health continues unabated, and calls for action to prevent grim outcomes. DMN dysfunction in youth can be reversed with treatment, but access to mental health care has become more challenging due to workforce shortages and insurance restrictions. Psychiatrists and parents must work diligently to treat psychiatrically affected youth, which has become a DaMN serious problem…
The advent of unprecedented technologies drastically altering the behavior of children and adolescents, compounded by prolonged isolation from a once-in-a-century pandemic, may have negatively impacted the normal connectivity of the human brain among youth, leading to the current alarming increase of depression, anxiety, and suicidality among this population.
The human brain is comprised of multiple large-scale networks that are functionally connected and control feelings, thoughts, and behaviors. As clinical neuroscientists, psychiatrists must consider the profound impact of a massive societal shift in human behavior on the functional connectivity of brain networks in health and disease. The advent of smartphones, social media, and video game addiction may have disrupted the developing brain networks in children and adolescents, leading to the current escalating epidemic of mental disorders in youth.
The major networks in the human brain include the default mode network (DMN), the salience network, the limbic system, the dorsal attention network, the central executive network, and the visual system.1 Each network connects several brain regions. Researchers can use functional MRI to detect the connectivity of those networks. When blood flow increases concurrently across 2 or 3 networks, this indicates those networks are functionally connected.
There was an old “dogma” that brain regions use energy only when activated and being used. Hans Berger, who developed the EEG in 1929, noticed electrical activity at rest and proposed that the brain is constantly busy, but his neurology peers did not take him seriously.2 In the 1950s, Louis Sokoloff noticed that brain metabolism was the same whether a person is at rest or doing math. In the 1970s, David Ingvar discovered that the highest blood flow in the frontal lobe occurred when a person was at rest.3 Finally, in 2007, Raichle et al4 used positron emission tomography scans to confirm that the frontal lobe is most active when a person is not doing anything. He labeled this phenomenon the DMN, comprising the medial fronto-parietal cortex, the posterior cingulate gyrus, the precuneus, and the angular gyrus. Interestingly, the number of publications about the DMN has skyrocketed since 2007.
The many roles of the DMN
Ongoing research has revealed that the DMN is most active at rest, and its anatomical hubs mediate several key functions5:
- Posterior cingulate gyrus (the central core of the DMN): remembering the past and thinking about the future
- Medial prefrontal cortex: autobiographical memories, future goals and events, reflecting on one’s emotional self, and considering decisions about family members
- Dorsal medial subsystem: thinking about others, determining and inferring the purpose of other people’s actions
- Temporo-parietal junction: reflecting on the beliefs and emotions of others (known as “theory of mind”6)
- Lateral parietal junction: retrieval of social and conceptual knowledge
- Hippocampus: forming new memories, remembering the past, imagining the future
- Posterior-inferior parietal lobe: junction of auditory, visual, and somatic sensory information and attention
- Precuneus: Visual, sensory-motor, and attention.
Many terms have been used to describe the function of the DMN, including “daydreaming,” “auto-pilot,” “mind-wondering,” “reminiscing,” “contemplating,” “self-reflection,” “the neurological basis of the self,” and “seat of literary creativity.”
Psychiatric consequences of DMN deactivation
When another brain network, the attention network (which is also referred to as the task-positive network), is activated consciously and volitionally to perform a task that demands focus (such as text messaging, playing video games, or continuously interacting with social media sites), DMN activity declines.
Continue to: The DMN does not exist...
The DMN does not exist in infants, but starts to develop in childhood.7 It is enhanced by exercise, daydreaming, and sleep, activities that are common in childhood but have declined drastically with the widespread use of smartphones, video games, and social media, which for many youth occupy the bulk of their waking hours. Those tasks, which require continuous attention, deactivate the DMN. In fact, research has shown that addictive behavior decreases the connectivity of the DMN and suppresses its activity.8 Most children and adolescents can be regarded as essentially addicted to social media, text messaging, and video games. Unsurprisingly, serious psychiatric consequences follow.9
DMN dysfunction has been reported in several psychiatric conditions, including depression, posttraumatic stress disorder, autism, schizophrenia, anxiety, obsessive-compulsive disorder, and substance use.10-12 Impaired social interactions and communications, negative ruminations, suicidal ideas, and impaired encoding of long-term memories are some of the adverse effects of DMN dysfunction. The good news is that the DMN’s connectivity and functioning can be modulated and restored by meditation, mentalizing, exercise, psychotherapy, antidepressants, and psychedelics.13,14
The lockdown and stress of the COVID-19 pandemic added insult to injury and exacerbated mental illness in children by isolating them from each other and intensifying their technological addiction to fill the void of isolation. This crisis in youth mental health continues unabated, and calls for action to prevent grim outcomes. DMN dysfunction in youth can be reversed with treatment, but access to mental health care has become more challenging due to workforce shortages and insurance restrictions. Psychiatrists and parents must work diligently to treat psychiatrically affected youth, which has become a DaMN serious problem…
1. Yao Z, Hu B, Xie Y, et al. A review of structural and functional brain networks: small world and atlas. Brain Inform. 2015;2(1):45-52. doi:10.1007/s40708-015-0009-z
2. Raichle ME. The brain’s dark energy. Sci Am. 2010;302(3):44-49. doi:10.1038/scientific american0310-44
3. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi:10.1196/annals.1440.011
4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083-1090; discussion 1097-1099. doi:10.1016/j.neuroimage.2007.02.041
5. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251-270. doi:10.1177/1073858411403316
6. Tsoukalas I. Theory of mind: towards an evolutionary theory. Evolutionary Psychological Science. 2018;4(1):38-66. https://doi.org/10.1007/s40806-017-0112-x
7. Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279-296. doi:10.1016/j.neubiorev.2008.09.002
8. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313-331. doi:10.1016/j.neuroimage.2019.06.036
9. Bommersbach TJ, McKean AJ, Olfson M, et al. National trends in mental health-related emergency department visits among youth, 2011-2020. JAMA. 2023;329(17):1469-1477. doi:10.1001/jama.2023.4809
10. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi:10.1146/annurev-clinpsy-032511-143049
11. Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489-498. doi:10.1016/j.neuroimage.2018.05.005
12. Nagata JM, Chu J, Zamora G, et al. Screen time and obsessive-compulsive disorder among children 9-10 years old: a prospective cohort study. J Adolesc Health. 2023;72(3):390-396. doi:10.1016/j.jadohealth.2022.10.023
13. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48-73. doi:10.1016/j.neubiorev.2014.03.016
14. Gattuso JJ, Perkins D, Ruffell S, et al. Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol. 2023;26(3):155-188. doi:10.1093/ijnp/pyac074
1. Yao Z, Hu B, Xie Y, et al. A review of structural and functional brain networks: small world and atlas. Brain Inform. 2015;2(1):45-52. doi:10.1007/s40708-015-0009-z
2. Raichle ME. The brain’s dark energy. Sci Am. 2010;302(3):44-49. doi:10.1038/scientific american0310-44
3. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi:10.1196/annals.1440.011
4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083-1090; discussion 1097-1099. doi:10.1016/j.neuroimage.2007.02.041
5. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251-270. doi:10.1177/1073858411403316
6. Tsoukalas I. Theory of mind: towards an evolutionary theory. Evolutionary Psychological Science. 2018;4(1):38-66. https://doi.org/10.1007/s40806-017-0112-x
7. Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279-296. doi:10.1016/j.neubiorev.2008.09.002
8. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313-331. doi:10.1016/j.neuroimage.2019.06.036
9. Bommersbach TJ, McKean AJ, Olfson M, et al. National trends in mental health-related emergency department visits among youth, 2011-2020. JAMA. 2023;329(17):1469-1477. doi:10.1001/jama.2023.4809
10. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi:10.1146/annurev-clinpsy-032511-143049
11. Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489-498. doi:10.1016/j.neuroimage.2018.05.005
12. Nagata JM, Chu J, Zamora G, et al. Screen time and obsessive-compulsive disorder among children 9-10 years old: a prospective cohort study. J Adolesc Health. 2023;72(3):390-396. doi:10.1016/j.jadohealth.2022.10.023
13. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48-73. doi:10.1016/j.neubiorev.2014.03.016
14. Gattuso JJ, Perkins D, Ruffell S, et al. Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol. 2023;26(3):155-188. doi:10.1093/ijnp/pyac074
High-dose stimulants for adult ADHD
Ms. H, age 30, presents to the outpatient clinic for a follow-up visit, where she reports difficulty paying attention to conversations, starting and completing tasks, and meeting deadlines. These challenges occur at work and home. Her psychiatric history includes attention-deficit/hyperactivity disorder (ADHD), major depressive disorder, and generalized anxiety disorder. Approximately 10 years ago, she underwent Roux-en-Y gastric bypass surgery. Following surgery, Ms. H’s care team prescribed liquid formulations of medications whenever possible to minimize malabsorption. Ms. H may be a rapid metabolizer; she says the effects of her prescribed stimulants only last briefly, so she has to frequently redose. As a result, she often runs out of her monthly stimulant allotment earlier than expected.
Ms. H’s current medications include dextroamphetamine/amphetamine immediate-release (IR) 30 mg 3 times daily, atenolol 50 mg/d, and escitalopram oral solution 10 mg/d. Previous unsuccessful medication trials for her ADHD include methylphenidate IR 20 mg 3 times daily and lisdexamfetamine 70 mg/d. Ms. H reports that when her responsibilities increased at work or home, she took methylphenidate IR 20 mg up to 6 times daily to relieve her symptoms.
In the United States, ADHD affects an estimated 4.4% of adults age 18 to 44.1 The actual rate may be higher, however, as recent research has called into question the hypothesis that approximately 50% of cases of childhood ADHD remit by adulthood.2 Prevalence estimates relying on DSM-IV criteria (which were designed with children in mind) can underestimate this condition in adults. Newer data suggest that up to 90% of individuals with ADHD in childhood continue to experience significant ADHD symptoms into adulthood.2
Unless contraindications are present, methylphenidate or amphetamine-based stimulants are the medications of choice for treating adult ADHD.3 Many formulations of both medications are available,4 which allows clinicians to better tailor therapy to each patient’s pharmacokinetics and daily schedule. Although there can be differences in response and tolerability, methylphenidate and amphetamine offer comparable efficacy and a similar adverse effect profile.5
Because amphetamine is more potent than methylphenidate, clinicians commonly start treatment with an amphetamine dose that is one-half to two-thirds the dose of methylphenidate.6 While both classes of stimulants inhibit the reuptake of dopamine and norepinephrine into presynaptic neurons, amphetamines also promote the release of dopamine and norepinephrine from their storage sites in presynaptic nerve terminals.3
Methylphenidate
Methylphenidate IR has an average onset of action of 30 to 45 minutes and its effects last approximately 3 to 4 hours. The extended-release (XR) formulations have varying onsets of action, with durations of action up to 12 hours (Table 13,7).4 The XR products usually immediately release a certain percentage of the medication, eliminating the need for an additional IR tablet. One methylphenidate XR product (Jornay) as well as serdexmethylphenidate/dexmethylphenidate (Azstarys) offer durations of action of 24 to 36 hours. Methylphenidate is primarily metabolized by carboxylesterase 1 (CES1) to the inactive metabolite ritalinic acid. Most of the medication (60% to 80%) is excreted in the urine as ritalinic acid.4 Theoretically, genetic variations in the CES1 and concomitant use of medications that compete with or alter this pathway may impact methylphenidate pharmacokinetics.8 However, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.4

Amphetamine
Dextroamphetamine/amphetamine IR has an average onset of action of 30 to 45 minutes and its effects last approximately 4 to 6 hours. XR formulations have varying onsets of action, with durations of action up to 13 hours (Table 23,7,9).4 One XR product, mixed salts of single amphetamine entity (Mydayis), has a duration of action of 16 hours. In XR formulations, a certain percentage of the medication is typically released immediately, eliminating the need for an additional IR tablet. Amphetamine is primarily metabolized by cytochrome P450 (CYP) 2D6 hydroxylation and oxidative deamination. Genetic variability in amphetamine metabolism may be relevant due to CYP2D6 polymorphisms. Ultra-rapid metabolizers might need higher doses, while poor metabolizers might require smaller amounts and may be more susceptible to adverse effects.4 However, there is currently insufficient data supporting gene/medication concentration relationships. As is the case with methylphenidate, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.6

Continue to: Impaired medication absorption after bariatric surgery
Impaired medication absorption after bariatric surgery
Medication malabsorption following bariatric surgery is a significant concern. In a systematic review of 22 studies, Padwal et al10 found that in one-third of these studies, decreased absorption following bariatric surgery may be present in patients taking medications that have poor absorption, high lipophilicity, or enterohepatic recirculation. Childress et al11 found that methylphenidate IR and dextroamphetamine/amphetamine are both well absorbed, with bioavailability percentages of 100% and 90%, respectively. Additional research shows both stimulants have rapid absorption rates but relatively poor bioavailability.12 In one study analyzing the dissolution of common psychiatric medications, methylphenidate was shown to dissolve slightly more in the Roux-en-Y gastric bypass surgery model (80 mg) compared to controls (70 mg).13 One case indicated potential methylphenidate toxicity following Roux-en-Y gastric bypass surgery,14 while another suggested impaired absorption following the same procedure.15 A case-control design study assessing the impact of Roux-en-Y gastric bypass surgery on the pharmacokinetic properties of lisdexamfetamine found no significant differences between the Roux-en-Y group (n = 10) and nonsurgical controls (n = 10). The investigators concluded that while data suggest adjusting lisdexamfetamine dosing following Roux-en-Y gastric bypass surgery is unnecessary, there may be interindividual differences, and individualized dosing regimens may be needed.16
When managing patients who might be experiencing medication malabsorption, it may be helpful to use dosage forms that avoid disintegration, acidic environments, and slow dissolution. Because they are more rapidly absorbed and not susceptible to disintegration and dissolution, liquid formulations are recommended.17 For medications that are not available as a liquid, an IR formulation is recommended.18
Using nonoral routes of administration that avoid the anatomical changes of the gastrointestinal tract should be considered for patients who have undergone Roux-en-Y gastric bypass surgery.17 The methylphenidate transdermal patch, a medication delivery system that avoids gut and hepatic first-pass metabolism, can improve medication bioavailability, reduce dose frequency, and stabilize medication delivery. It is available in 4 sizes/dosages: 10 mg/9 hours, 15 mg/9 hours, 20 mg/9 hours, and 30 mg/9 hours. Methylphenidate is delivered at a steady rate based upon patch size. The onset of action of the patch is approximately 2 hours, and patients should wear the patch for 9 hours, then remove it. Methylphenidate will still be absorbed up to 2 to 3 hours after patch removal. Appropriate application and removal of the patch is important for optimal effectiveness and to avoid adverse effects.4
In March 2022, t
CASE CONTINUED
Ms. H emphasizes her desire to maintain functionality in all areas of life, while her care team reiterates the risks of continuing to take high-dose stimulants. Both Ms. H and her care team acknowledge that stimulant usage could be worsening her anxiety, and that Roux-en-Y gastric bypass surgery may be a possible explanation for her dosing challenges.
Continue to: Following consultation with the pharmacist...
Following consultation with the pharmacist, the care team explains the possible pharmacokinetic benefits of using the methylphenidate transdermal patch. After completing the prior authorization paperwork, Ms. H is started on the 30 mg/d patch. This dose was selected because she previously tolerated high-dose stimulants, including methylphenidate IR 20 mg up to 6 times daily. At a follow-up visit 1 month after starting the patch, Ms. H reports an improvement in her ADHD symptoms and says she is not experiencing any adverse effects.
Related Resources
- DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
- Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):16-27. doi:10.12788/cp.0344
Drug Brand Names
Amphetamine sulfate • Adzenys ER, Adzenys XR-ODT, Dyanavel XR, Evekeo
Atenolol • Tenormin
Dexmethylphenidate • Focalin, Focalin XR
Dextroamphetamine transdermal • Xelstrym
Dextroamphetamine • Dexedrine, Dexedrine Spansule, ProCentra, Zenzedi
Escitalopram • Lexapro
Lisdexamfetamine • Vyvanse
Methylphenidate • Aptensio XR, Adhansia XR, Concerta, Cotempla, Jornay PM, Metadate CD, Metadate ER, Methylin, Qullichew ER, Quillivant XR, Relexxii, Ritalin, Ritalin LA
Methylphenidate transdermal • Daytrana
Mixed amphetamine salts • Adderall, Adderall XR
Mixed salts of a single-entity amphetamine • Mydayis
Serdexmethylphenidate and dexmethylphenidate • Azstarys
1. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723. doi:10.1176/ajp.2006.163.4.716
2. Sibley MH, Arnold LE, Swanson JM, et al. Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am J Psychiatry. 2022;179(2):142-151. doi:10.1176/appi.ajp.2021.21010032
3. Cleveland KW, Boyle J, Robinson RF. Attention-deficit/hyperactivity disorder. In: Chisholm-Burns MA, Schwinghammer TL, Malone PM, et al, eds. Pharmacotherapy Principles & Practice. 6th ed. McGraw Hill; 2022. Accessed December 1, 2022. https://ppp.mhmedical.com/content.aspx?bookid=3114§ionid=261474885
4. Steingard R, Taskiran S, Connor DF, et al. New formulations of stimulants: an update for clinicians. J Child Adolesc Psychopharmacol. 2019;29(5):324-339. doi:10.1089/cap.2019.0043
5. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270. doi:10.1016/j.neubiorev.2018.02.001
6. Markowitz JS, Patrick KS. The clinical pharmacokinetics of amphetamines utilized in the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017;27(8):678-689. doi:10.1089/cap.2017.0071
7. Mullen S. Medication Table 2: Attention Deficit Hyperactivity Disorder. In: English C, ed. CPNP Psychiatric Pharmacotherapy Review Course. 2022-2023 ed. College of Psychiatric and Neurologic Pharmacists; 2022.
8. Zhu HJ, Patrick KS, Yuan HJ, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82(6):1241-1248. doi:10.1016/j.ajhg.2008.04.015
9. Xelstrym [package insert]. Miami, FL: Noven Pharmaceuticals, Inc.; 2022.
10. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789X.2009.00614.x
11. Childress AC, Komolova M, Sallee FR. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019;15(11):937-974. doi:10.1080/17425255.2019.1675636
12. Markowitz JS, Melchert PW. The pharmacokinetics and pharmacogenomics of psychostimulants. Child Adolesc Psychiatr Clin N Am. 2022;31(3):393-416. doi:10.1016/j.chc.2022.03.003
13. Seaman JS, Bowers SP, Dixon P, et al. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46(3):250-253. doi:10.1176/appi.psy.46.3.250
14. Ludvigsson M, Haenni A. Methylphenidate toxicity after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(5):e55-e57. doi:10.1016/j.soard.2016.03.015
15. Azran C, Langguth P, Dahan A. Impaired oral absorption of methylphenidate after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2017;13(7):1245-1247. doi:10.1016/j.soard.2017.03.003
16. Steffen KJ, Mohammad AS, Roerig JL, et al. Lisdexamfetamine pharmacokinetic comparison between patients who underwent Roux-en-Y gastric bypass and nonsurgical controls. Obes Surg. 2021;31(10):4289-4294. doi:10.1007/s11695-020-04969-4
17. Buxton ILO. Pharmacokinetics: the dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton LL, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 14th ed. McGraw Hill; 2023. Accessed December 1, 2022. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2189§ionid=166182905
18. DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
Ms. H, age 30, presents to the outpatient clinic for a follow-up visit, where she reports difficulty paying attention to conversations, starting and completing tasks, and meeting deadlines. These challenges occur at work and home. Her psychiatric history includes attention-deficit/hyperactivity disorder (ADHD), major depressive disorder, and generalized anxiety disorder. Approximately 10 years ago, she underwent Roux-en-Y gastric bypass surgery. Following surgery, Ms. H’s care team prescribed liquid formulations of medications whenever possible to minimize malabsorption. Ms. H may be a rapid metabolizer; she says the effects of her prescribed stimulants only last briefly, so she has to frequently redose. As a result, she often runs out of her monthly stimulant allotment earlier than expected.
Ms. H’s current medications include dextroamphetamine/amphetamine immediate-release (IR) 30 mg 3 times daily, atenolol 50 mg/d, and escitalopram oral solution 10 mg/d. Previous unsuccessful medication trials for her ADHD include methylphenidate IR 20 mg 3 times daily and lisdexamfetamine 70 mg/d. Ms. H reports that when her responsibilities increased at work or home, she took methylphenidate IR 20 mg up to 6 times daily to relieve her symptoms.
In the United States, ADHD affects an estimated 4.4% of adults age 18 to 44.1 The actual rate may be higher, however, as recent research has called into question the hypothesis that approximately 50% of cases of childhood ADHD remit by adulthood.2 Prevalence estimates relying on DSM-IV criteria (which were designed with children in mind) can underestimate this condition in adults. Newer data suggest that up to 90% of individuals with ADHD in childhood continue to experience significant ADHD symptoms into adulthood.2
Unless contraindications are present, methylphenidate or amphetamine-based stimulants are the medications of choice for treating adult ADHD.3 Many formulations of both medications are available,4 which allows clinicians to better tailor therapy to each patient’s pharmacokinetics and daily schedule. Although there can be differences in response and tolerability, methylphenidate and amphetamine offer comparable efficacy and a similar adverse effect profile.5
Because amphetamine is more potent than methylphenidate, clinicians commonly start treatment with an amphetamine dose that is one-half to two-thirds the dose of methylphenidate.6 While both classes of stimulants inhibit the reuptake of dopamine and norepinephrine into presynaptic neurons, amphetamines also promote the release of dopamine and norepinephrine from their storage sites in presynaptic nerve terminals.3
Methylphenidate
Methylphenidate IR has an average onset of action of 30 to 45 minutes and its effects last approximately 3 to 4 hours. The extended-release (XR) formulations have varying onsets of action, with durations of action up to 12 hours (Table 13,7).4 The XR products usually immediately release a certain percentage of the medication, eliminating the need for an additional IR tablet. One methylphenidate XR product (Jornay) as well as serdexmethylphenidate/dexmethylphenidate (Azstarys) offer durations of action of 24 to 36 hours. Methylphenidate is primarily metabolized by carboxylesterase 1 (CES1) to the inactive metabolite ritalinic acid. Most of the medication (60% to 80%) is excreted in the urine as ritalinic acid.4 Theoretically, genetic variations in the CES1 and concomitant use of medications that compete with or alter this pathway may impact methylphenidate pharmacokinetics.8 However, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.4

Amphetamine
Dextroamphetamine/amphetamine IR has an average onset of action of 30 to 45 minutes and its effects last approximately 4 to 6 hours. XR formulations have varying onsets of action, with durations of action up to 13 hours (Table 23,7,9).4 One XR product, mixed salts of single amphetamine entity (Mydayis), has a duration of action of 16 hours. In XR formulations, a certain percentage of the medication is typically released immediately, eliminating the need for an additional IR tablet. Amphetamine is primarily metabolized by cytochrome P450 (CYP) 2D6 hydroxylation and oxidative deamination. Genetic variability in amphetamine metabolism may be relevant due to CYP2D6 polymorphisms. Ultra-rapid metabolizers might need higher doses, while poor metabolizers might require smaller amounts and may be more susceptible to adverse effects.4 However, there is currently insufficient data supporting gene/medication concentration relationships. As is the case with methylphenidate, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.6

Continue to: Impaired medication absorption after bariatric surgery
Impaired medication absorption after bariatric surgery
Medication malabsorption following bariatric surgery is a significant concern. In a systematic review of 22 studies, Padwal et al10 found that in one-third of these studies, decreased absorption following bariatric surgery may be present in patients taking medications that have poor absorption, high lipophilicity, or enterohepatic recirculation. Childress et al11 found that methylphenidate IR and dextroamphetamine/amphetamine are both well absorbed, with bioavailability percentages of 100% and 90%, respectively. Additional research shows both stimulants have rapid absorption rates but relatively poor bioavailability.12 In one study analyzing the dissolution of common psychiatric medications, methylphenidate was shown to dissolve slightly more in the Roux-en-Y gastric bypass surgery model (80 mg) compared to controls (70 mg).13 One case indicated potential methylphenidate toxicity following Roux-en-Y gastric bypass surgery,14 while another suggested impaired absorption following the same procedure.15 A case-control design study assessing the impact of Roux-en-Y gastric bypass surgery on the pharmacokinetic properties of lisdexamfetamine found no significant differences between the Roux-en-Y group (n = 10) and nonsurgical controls (n = 10). The investigators concluded that while data suggest adjusting lisdexamfetamine dosing following Roux-en-Y gastric bypass surgery is unnecessary, there may be interindividual differences, and individualized dosing regimens may be needed.16
When managing patients who might be experiencing medication malabsorption, it may be helpful to use dosage forms that avoid disintegration, acidic environments, and slow dissolution. Because they are more rapidly absorbed and not susceptible to disintegration and dissolution, liquid formulations are recommended.17 For medications that are not available as a liquid, an IR formulation is recommended.18
Using nonoral routes of administration that avoid the anatomical changes of the gastrointestinal tract should be considered for patients who have undergone Roux-en-Y gastric bypass surgery.17 The methylphenidate transdermal patch, a medication delivery system that avoids gut and hepatic first-pass metabolism, can improve medication bioavailability, reduce dose frequency, and stabilize medication delivery. It is available in 4 sizes/dosages: 10 mg/9 hours, 15 mg/9 hours, 20 mg/9 hours, and 30 mg/9 hours. Methylphenidate is delivered at a steady rate based upon patch size. The onset of action of the patch is approximately 2 hours, and patients should wear the patch for 9 hours, then remove it. Methylphenidate will still be absorbed up to 2 to 3 hours after patch removal. Appropriate application and removal of the patch is important for optimal effectiveness and to avoid adverse effects.4
In March 2022, t
CASE CONTINUED
Ms. H emphasizes her desire to maintain functionality in all areas of life, while her care team reiterates the risks of continuing to take high-dose stimulants. Both Ms. H and her care team acknowledge that stimulant usage could be worsening her anxiety, and that Roux-en-Y gastric bypass surgery may be a possible explanation for her dosing challenges.
Continue to: Following consultation with the pharmacist...
Following consultation with the pharmacist, the care team explains the possible pharmacokinetic benefits of using the methylphenidate transdermal patch. After completing the prior authorization paperwork, Ms. H is started on the 30 mg/d patch. This dose was selected because she previously tolerated high-dose stimulants, including methylphenidate IR 20 mg up to 6 times daily. At a follow-up visit 1 month after starting the patch, Ms. H reports an improvement in her ADHD symptoms and says she is not experiencing any adverse effects.
Related Resources
- DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
- Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):16-27. doi:10.12788/cp.0344
Drug Brand Names
Amphetamine sulfate • Adzenys ER, Adzenys XR-ODT, Dyanavel XR, Evekeo
Atenolol • Tenormin
Dexmethylphenidate • Focalin, Focalin XR
Dextroamphetamine transdermal • Xelstrym
Dextroamphetamine • Dexedrine, Dexedrine Spansule, ProCentra, Zenzedi
Escitalopram • Lexapro
Lisdexamfetamine • Vyvanse
Methylphenidate • Aptensio XR, Adhansia XR, Concerta, Cotempla, Jornay PM, Metadate CD, Metadate ER, Methylin, Qullichew ER, Quillivant XR, Relexxii, Ritalin, Ritalin LA
Methylphenidate transdermal • Daytrana
Mixed amphetamine salts • Adderall, Adderall XR
Mixed salts of a single-entity amphetamine • Mydayis
Serdexmethylphenidate and dexmethylphenidate • Azstarys
Ms. H, age 30, presents to the outpatient clinic for a follow-up visit, where she reports difficulty paying attention to conversations, starting and completing tasks, and meeting deadlines. These challenges occur at work and home. Her psychiatric history includes attention-deficit/hyperactivity disorder (ADHD), major depressive disorder, and generalized anxiety disorder. Approximately 10 years ago, she underwent Roux-en-Y gastric bypass surgery. Following surgery, Ms. H’s care team prescribed liquid formulations of medications whenever possible to minimize malabsorption. Ms. H may be a rapid metabolizer; she says the effects of her prescribed stimulants only last briefly, so she has to frequently redose. As a result, she often runs out of her monthly stimulant allotment earlier than expected.
Ms. H’s current medications include dextroamphetamine/amphetamine immediate-release (IR) 30 mg 3 times daily, atenolol 50 mg/d, and escitalopram oral solution 10 mg/d. Previous unsuccessful medication trials for her ADHD include methylphenidate IR 20 mg 3 times daily and lisdexamfetamine 70 mg/d. Ms. H reports that when her responsibilities increased at work or home, she took methylphenidate IR 20 mg up to 6 times daily to relieve her symptoms.
In the United States, ADHD affects an estimated 4.4% of adults age 18 to 44.1 The actual rate may be higher, however, as recent research has called into question the hypothesis that approximately 50% of cases of childhood ADHD remit by adulthood.2 Prevalence estimates relying on DSM-IV criteria (which were designed with children in mind) can underestimate this condition in adults. Newer data suggest that up to 90% of individuals with ADHD in childhood continue to experience significant ADHD symptoms into adulthood.2
Unless contraindications are present, methylphenidate or amphetamine-based stimulants are the medications of choice for treating adult ADHD.3 Many formulations of both medications are available,4 which allows clinicians to better tailor therapy to each patient’s pharmacokinetics and daily schedule. Although there can be differences in response and tolerability, methylphenidate and amphetamine offer comparable efficacy and a similar adverse effect profile.5
Because amphetamine is more potent than methylphenidate, clinicians commonly start treatment with an amphetamine dose that is one-half to two-thirds the dose of methylphenidate.6 While both classes of stimulants inhibit the reuptake of dopamine and norepinephrine into presynaptic neurons, amphetamines also promote the release of dopamine and norepinephrine from their storage sites in presynaptic nerve terminals.3
Methylphenidate
Methylphenidate IR has an average onset of action of 30 to 45 minutes and its effects last approximately 3 to 4 hours. The extended-release (XR) formulations have varying onsets of action, with durations of action up to 12 hours (Table 13,7).4 The XR products usually immediately release a certain percentage of the medication, eliminating the need for an additional IR tablet. One methylphenidate XR product (Jornay) as well as serdexmethylphenidate/dexmethylphenidate (Azstarys) offer durations of action of 24 to 36 hours. Methylphenidate is primarily metabolized by carboxylesterase 1 (CES1) to the inactive metabolite ritalinic acid. Most of the medication (60% to 80%) is excreted in the urine as ritalinic acid.4 Theoretically, genetic variations in the CES1 and concomitant use of medications that compete with or alter this pathway may impact methylphenidate pharmacokinetics.8 However, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.4

Amphetamine
Dextroamphetamine/amphetamine IR has an average onset of action of 30 to 45 minutes and its effects last approximately 4 to 6 hours. XR formulations have varying onsets of action, with durations of action up to 13 hours (Table 23,7,9).4 One XR product, mixed salts of single amphetamine entity (Mydayis), has a duration of action of 16 hours. In XR formulations, a certain percentage of the medication is typically released immediately, eliminating the need for an additional IR tablet. Amphetamine is primarily metabolized by cytochrome P450 (CYP) 2D6 hydroxylation and oxidative deamination. Genetic variability in amphetamine metabolism may be relevant due to CYP2D6 polymorphisms. Ultra-rapid metabolizers might need higher doses, while poor metabolizers might require smaller amounts and may be more susceptible to adverse effects.4 However, there is currently insufficient data supporting gene/medication concentration relationships. As is the case with methylphenidate, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.6

Continue to: Impaired medication absorption after bariatric surgery
Impaired medication absorption after bariatric surgery
Medication malabsorption following bariatric surgery is a significant concern. In a systematic review of 22 studies, Padwal et al10 found that in one-third of these studies, decreased absorption following bariatric surgery may be present in patients taking medications that have poor absorption, high lipophilicity, or enterohepatic recirculation. Childress et al11 found that methylphenidate IR and dextroamphetamine/amphetamine are both well absorbed, with bioavailability percentages of 100% and 90%, respectively. Additional research shows both stimulants have rapid absorption rates but relatively poor bioavailability.12 In one study analyzing the dissolution of common psychiatric medications, methylphenidate was shown to dissolve slightly more in the Roux-en-Y gastric bypass surgery model (80 mg) compared to controls (70 mg).13 One case indicated potential methylphenidate toxicity following Roux-en-Y gastric bypass surgery,14 while another suggested impaired absorption following the same procedure.15 A case-control design study assessing the impact of Roux-en-Y gastric bypass surgery on the pharmacokinetic properties of lisdexamfetamine found no significant differences between the Roux-en-Y group (n = 10) and nonsurgical controls (n = 10). The investigators concluded that while data suggest adjusting lisdexamfetamine dosing following Roux-en-Y gastric bypass surgery is unnecessary, there may be interindividual differences, and individualized dosing regimens may be needed.16
When managing patients who might be experiencing medication malabsorption, it may be helpful to use dosage forms that avoid disintegration, acidic environments, and slow dissolution. Because they are more rapidly absorbed and not susceptible to disintegration and dissolution, liquid formulations are recommended.17 For medications that are not available as a liquid, an IR formulation is recommended.18
Using nonoral routes of administration that avoid the anatomical changes of the gastrointestinal tract should be considered for patients who have undergone Roux-en-Y gastric bypass surgery.17 The methylphenidate transdermal patch, a medication delivery system that avoids gut and hepatic first-pass metabolism, can improve medication bioavailability, reduce dose frequency, and stabilize medication delivery. It is available in 4 sizes/dosages: 10 mg/9 hours, 15 mg/9 hours, 20 mg/9 hours, and 30 mg/9 hours. Methylphenidate is delivered at a steady rate based upon patch size. The onset of action of the patch is approximately 2 hours, and patients should wear the patch for 9 hours, then remove it. Methylphenidate will still be absorbed up to 2 to 3 hours after patch removal. Appropriate application and removal of the patch is important for optimal effectiveness and to avoid adverse effects.4
In March 2022, t
CASE CONTINUED
Ms. H emphasizes her desire to maintain functionality in all areas of life, while her care team reiterates the risks of continuing to take high-dose stimulants. Both Ms. H and her care team acknowledge that stimulant usage could be worsening her anxiety, and that Roux-en-Y gastric bypass surgery may be a possible explanation for her dosing challenges.
Continue to: Following consultation with the pharmacist...
Following consultation with the pharmacist, the care team explains the possible pharmacokinetic benefits of using the methylphenidate transdermal patch. After completing the prior authorization paperwork, Ms. H is started on the 30 mg/d patch. This dose was selected because she previously tolerated high-dose stimulants, including methylphenidate IR 20 mg up to 6 times daily. At a follow-up visit 1 month after starting the patch, Ms. H reports an improvement in her ADHD symptoms and says she is not experiencing any adverse effects.
Related Resources
- DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
- Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):16-27. doi:10.12788/cp.0344
Drug Brand Names
Amphetamine sulfate • Adzenys ER, Adzenys XR-ODT, Dyanavel XR, Evekeo
Atenolol • Tenormin
Dexmethylphenidate • Focalin, Focalin XR
Dextroamphetamine transdermal • Xelstrym
Dextroamphetamine • Dexedrine, Dexedrine Spansule, ProCentra, Zenzedi
Escitalopram • Lexapro
Lisdexamfetamine • Vyvanse
Methylphenidate • Aptensio XR, Adhansia XR, Concerta, Cotempla, Jornay PM, Metadate CD, Metadate ER, Methylin, Qullichew ER, Quillivant XR, Relexxii, Ritalin, Ritalin LA
Methylphenidate transdermal • Daytrana
Mixed amphetamine salts • Adderall, Adderall XR
Mixed salts of a single-entity amphetamine • Mydayis
Serdexmethylphenidate and dexmethylphenidate • Azstarys
1. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723. doi:10.1176/ajp.2006.163.4.716
2. Sibley MH, Arnold LE, Swanson JM, et al. Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am J Psychiatry. 2022;179(2):142-151. doi:10.1176/appi.ajp.2021.21010032
3. Cleveland KW, Boyle J, Robinson RF. Attention-deficit/hyperactivity disorder. In: Chisholm-Burns MA, Schwinghammer TL, Malone PM, et al, eds. Pharmacotherapy Principles & Practice. 6th ed. McGraw Hill; 2022. Accessed December 1, 2022. https://ppp.mhmedical.com/content.aspx?bookid=3114§ionid=261474885
4. Steingard R, Taskiran S, Connor DF, et al. New formulations of stimulants: an update for clinicians. J Child Adolesc Psychopharmacol. 2019;29(5):324-339. doi:10.1089/cap.2019.0043
5. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270. doi:10.1016/j.neubiorev.2018.02.001
6. Markowitz JS, Patrick KS. The clinical pharmacokinetics of amphetamines utilized in the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017;27(8):678-689. doi:10.1089/cap.2017.0071
7. Mullen S. Medication Table 2: Attention Deficit Hyperactivity Disorder. In: English C, ed. CPNP Psychiatric Pharmacotherapy Review Course. 2022-2023 ed. College of Psychiatric and Neurologic Pharmacists; 2022.
8. Zhu HJ, Patrick KS, Yuan HJ, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82(6):1241-1248. doi:10.1016/j.ajhg.2008.04.015
9. Xelstrym [package insert]. Miami, FL: Noven Pharmaceuticals, Inc.; 2022.
10. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789X.2009.00614.x
11. Childress AC, Komolova M, Sallee FR. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019;15(11):937-974. doi:10.1080/17425255.2019.1675636
12. Markowitz JS, Melchert PW. The pharmacokinetics and pharmacogenomics of psychostimulants. Child Adolesc Psychiatr Clin N Am. 2022;31(3):393-416. doi:10.1016/j.chc.2022.03.003
13. Seaman JS, Bowers SP, Dixon P, et al. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46(3):250-253. doi:10.1176/appi.psy.46.3.250
14. Ludvigsson M, Haenni A. Methylphenidate toxicity after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(5):e55-e57. doi:10.1016/j.soard.2016.03.015
15. Azran C, Langguth P, Dahan A. Impaired oral absorption of methylphenidate after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2017;13(7):1245-1247. doi:10.1016/j.soard.2017.03.003
16. Steffen KJ, Mohammad AS, Roerig JL, et al. Lisdexamfetamine pharmacokinetic comparison between patients who underwent Roux-en-Y gastric bypass and nonsurgical controls. Obes Surg. 2021;31(10):4289-4294. doi:10.1007/s11695-020-04969-4
17. Buxton ILO. Pharmacokinetics: the dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton LL, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 14th ed. McGraw Hill; 2023. Accessed December 1, 2022. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2189§ionid=166182905
18. DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
1. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723. doi:10.1176/ajp.2006.163.4.716
2. Sibley MH, Arnold LE, Swanson JM, et al. Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am J Psychiatry. 2022;179(2):142-151. doi:10.1176/appi.ajp.2021.21010032
3. Cleveland KW, Boyle J, Robinson RF. Attention-deficit/hyperactivity disorder. In: Chisholm-Burns MA, Schwinghammer TL, Malone PM, et al, eds. Pharmacotherapy Principles & Practice. 6th ed. McGraw Hill; 2022. Accessed December 1, 2022. https://ppp.mhmedical.com/content.aspx?bookid=3114§ionid=261474885
4. Steingard R, Taskiran S, Connor DF, et al. New formulations of stimulants: an update for clinicians. J Child Adolesc Psychopharmacol. 2019;29(5):324-339. doi:10.1089/cap.2019.0043
5. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270. doi:10.1016/j.neubiorev.2018.02.001
6. Markowitz JS, Patrick KS. The clinical pharmacokinetics of amphetamines utilized in the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017;27(8):678-689. doi:10.1089/cap.2017.0071
7. Mullen S. Medication Table 2: Attention Deficit Hyperactivity Disorder. In: English C, ed. CPNP Psychiatric Pharmacotherapy Review Course. 2022-2023 ed. College of Psychiatric and Neurologic Pharmacists; 2022.
8. Zhu HJ, Patrick KS, Yuan HJ, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82(6):1241-1248. doi:10.1016/j.ajhg.2008.04.015
9. Xelstrym [package insert]. Miami, FL: Noven Pharmaceuticals, Inc.; 2022.
10. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789X.2009.00614.x
11. Childress AC, Komolova M, Sallee FR. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019;15(11):937-974. doi:10.1080/17425255.2019.1675636
12. Markowitz JS, Melchert PW. The pharmacokinetics and pharmacogenomics of psychostimulants. Child Adolesc Psychiatr Clin N Am. 2022;31(3):393-416. doi:10.1016/j.chc.2022.03.003
13. Seaman JS, Bowers SP, Dixon P, et al. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46(3):250-253. doi:10.1176/appi.psy.46.3.250
14. Ludvigsson M, Haenni A. Methylphenidate toxicity after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(5):e55-e57. doi:10.1016/j.soard.2016.03.015
15. Azran C, Langguth P, Dahan A. Impaired oral absorption of methylphenidate after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2017;13(7):1245-1247. doi:10.1016/j.soard.2017.03.003
16. Steffen KJ, Mohammad AS, Roerig JL, et al. Lisdexamfetamine pharmacokinetic comparison between patients who underwent Roux-en-Y gastric bypass and nonsurgical controls. Obes Surg. 2021;31(10):4289-4294. doi:10.1007/s11695-020-04969-4
17. Buxton ILO. Pharmacokinetics: the dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton LL, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 14th ed. McGraw Hill; 2023. Accessed December 1, 2022. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2189§ionid=166182905
18. DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
Serious complications due to ‘huffing’
CASE A relapse and crisis
Ms. G, age 32, is brought to the emergency department (ED) by police after being found in a stupor-like state in a public restroom. The consultation-liaison (CL) psychiatry team assesses her for concerns of self-harm and suicide behavior. Ms. G discloses that she “huffs” an average of 4 canisters of air dusters daily to cope with psychosocial stressors and achieve a euphoric state. She recently lost her job, which led to homelessness, financial difficulties, a relapse to aerosol use after 2 years of abstinence, and stealing aerosol cans. The latest incident follows 2 prior arrests, which led officers to bring her to the ED for medical evaluation. Ms. G has a history of bipolar disorder (BD), generalized anxiety disorder (GAD), insomnia, and inhalant use disorder.
HISTORY Inhalant abuse and suicide attempt
Ms. G reports a longstanding history of severe inhalant abuse, primarily with air dusters due to their accessibility and low cost. She previously underwent inpatient rehab for inhalant abuse, and received inpatient psychiatry treatment 5 years ago for a suicide attempt by overdose linked to psychosocial stressors. In addition to BD, GAD, insomnia, and inhalant use disorder, Ms. G has a history of neuropathy, seizures, and recurrent hypokalemia. She is single and does not have insurance.
[polldaddy:12318871]
The authors’ observations
Inhalant abuse is the intentional inhalation of volatile substances to achieve an altered mental state. Inhalants are commercially available products that can produce intoxication if inhaled, such as glue, toluene, spray paint, gasoline, and lighter fluid (Table 11).
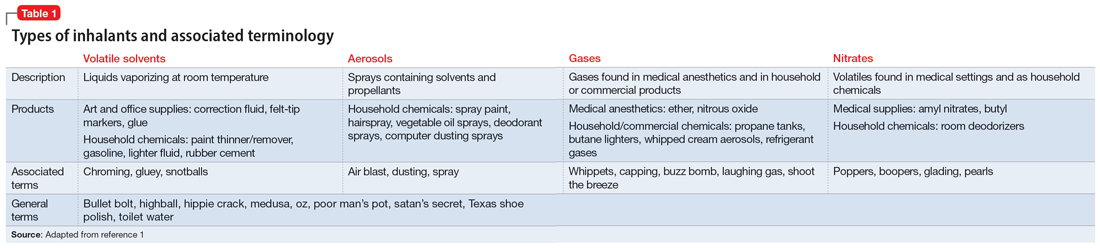
The epidemiology of inhalant abuse is difficult to accurately report due to a lack of recognition and social stigma. Due to inhalants’ ease of access and low cost, this form of substance abuse is popular among adolescents, adults of low socioeconomic status, individuals who live in rural areas, and those living in institutions. Inhalants act as reinforcers, producing a euphoric state. Rapid pulmonary absorption and lipid solubility of the substance rapidly alters the brain. Inhalant abuse can result in chemical and thermal burns, withdrawal symptoms, persistent mental illness, and catastrophic medical emergencies such as ventricular arrhythmias leading to disruptive myocardial electrical propagation. Chronic abuse can cause irreversible neurological and neuropsychological effects, cardiomyopathy, rapid airway compromise, pulmonary debilitations, renal tubular acidosis, bone marrow toxicity, reduced immunity, and peripheral neuropathy.2 Ms. G’s diagnosis of inhalant use disorder was based on her mental state and history of severe inhalant misuse, specifically with air dusters. Several additional factors further support this diagnosis, including the fact she survived a suicide attempt by overdose 5 years ago, had an inpatient rehabilitation placement for inhalant abuse, experiences insomnia, and was attempting to self-treat a depressive episode relapse with inhalants.
EVALUATION Depressed but cooperative
After being monitored in the ED for several hours, Ms. G is no longer in a stupor-like state. She has poor body habitus, appears older than her stated age, and is unkempt in appearance/attire. She is mildly distressed but relatively cooperative and engaged during the interview. Ms. G has a depressed mood and is anxious, with mood-congruent affect, and is tearful at times, especially when discussing recent stressors. She denies suicidality, homicidality, paranoia, delusions, and hallucinations. Her thought process is linear, goal-directed, and logical. She has fair insight, but relatively poor and impulsive judgment. The nursing staff expresses concerns that Ms. G was possibly responding to internal stimuli and behaving bizarrely during her initial presentation; this was not evident upon examination.
Ms. G reports having acute-on-chronic headaches, intermittent myalgias and weakness in her lower extremities (acute), and polyneuropathy (chronic). She denies a history of manic episodes or psychosis but reports previous relative hypomanic episodes that vacillated with periods of recurrent depressive episodes. Ms. G denies using illicit substances other than tobacco and inhalants. She says she had adhered to her outpatient psychiatric management services and medication regimen (duloxetine 60 mg/d at bedtime for mood/migraines, trazodone 150 mg/d at bedtime for insomnia, ziprasidone 40 mg/d at bedtime for BD, carbamazepine 200 mg twice daily for neuropathy/migraines, gabapentin 400 mg 3 times daily for neuropathy migraines/anxiety, and propranolol 10 mg 3 times daily for anxiety/tremors/migraine prophylaxis) until 4 days before her current presentation to the ED, when she used inhalants and was arrested.
Ms. G’s vitals are mostly unremarkable, but her heart rate is 116 beats per minute. There are no acute findings on physical examination. She is not pregnant, and her creatinine, glomerular filtration rate, complete blood count, and thyroid-stimulating hormone are all within normal limits. Her blood sugar is high (120 mg/dL; reference range 70 to 100 mg/dL). She has slight transaminitis with high aspartate aminotransferase (93 U/L; reference range 17 to 59 U/L) and high alanine aminotransferase (69 U/L; reference range 20 to 35 U/L); chronic hypokalemia (2.4 mmol/L; reference range 3.5 to 5.2 mmol/L), which leads the primary team to initiate a potassium replacement protocol; lactic acidosis (2.2 mmol/L; normal levels <2 mmol/L); and creatine kinase (CK) 5,930 U/L.
[polldaddy:12318873]
Continue to: The authors' observations
The authors’ observations
Efforts to improve the laboratory diagnosis of inhalant abuse are ongoing, but they have not yet been widely implemented. Systemic screening and assessment of inhalant use can help prevent and treat complications. For Ms. G, we considered several possible complications, including hypoglycemia. Although the classic triad of myalgia, weakness, and myoglobinuria (tea-colored urine) was not present, elevated CK levels in the context of Ms. G’s intermittent myalgia and lower extremity weakness led us to suspect she was experiencing moderate rhabdomyolysis (Table 23).
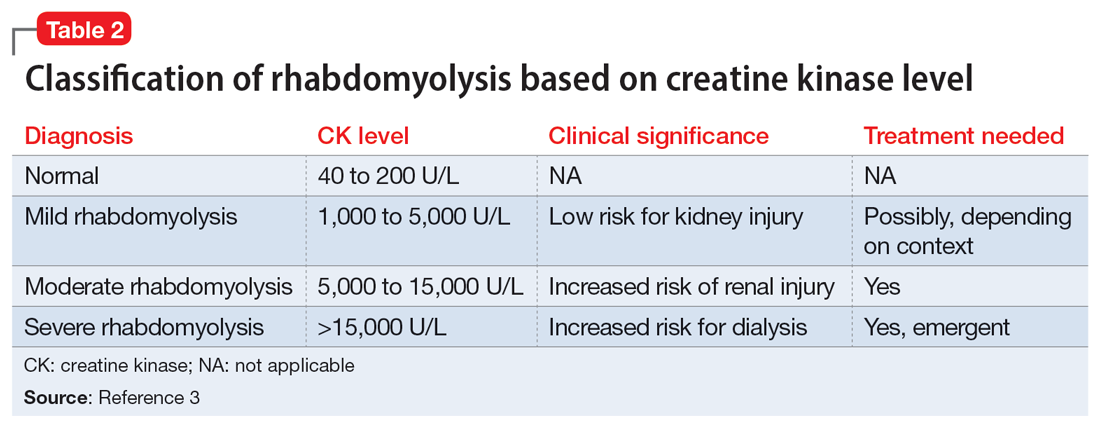
Rhabdomyolysis can be caused by several factors, including drug abuse, trauma, neuromuscular syndrome, and immobility. Treatment is mainly supportive, with a focus on preserving the ABCs (airway, breathing, circulation) and renal function through vigorous rehydration.4 We postulated Ms. G’s rhabdomyolysis was caused by muscle damage directly resulting from inhalant abuse and compounded by her remaining in prolonged fixed position on the ground after overdosing on inhalants.
TREATMENT Rehydration and psychotropics
The treatment team initiates IV fluid hydration of chloride 0.9% 150 mL/h and monitors Ms. G until she is stable and the trajectory of her CK levels begins to decline. On hospital Day 2, Ms. G’s CK decreases to 2,475 U/L and her lactic acid levels normalize. Ms. G restarts her regimen of duloxetine 60 mg/d, trazodone 150 mg/d, ziprasidone 40 mg/d, carbamazepine 200 mg twice daily, gabapentin 400 mg 3 times daily, and propranolol 10 mg 3 times daily. The team adds quetiapine 25 mg as needed for hallucinations, paranoia, and/or anxiety. Ms. G is closely monitored due to the potential risk of toxicity-induced or withdrawal-induced psychotic symptoms.
[polldaddy:12318869]
The authors’ observations
Presently, there are no effective treatments for acute inhalant intoxication or withdrawal, which makes supportive care and vigilant monitoring the only options.5 Although clinical research has not led to any FDA-approved treatments for chronic inhalant use disorder, a multipronged biopsychosocial treatment approach is critical in light of the negative consequences of inhalant abuse, including poor academic performance, criminal behavior, abuse of other substances, social maladjustment, low self-esteem, and suicidality.6
Ms. G had a moderate form of rhabdomyolysis, which was managed with IV fluid rehydration. Education and counseling were crucial to help Ms. G understand the unintended complications and potentially life-threatening consequences of inhalant abuse, with rehabilitation services to encourage abstinence. Ms. G had previously undergone successful inpatient rehabilitation and was willing to start such services again. She reported success with gabapentin for her polyneuropathy and migraines, which may be long-term consequences of prolonged inhalant abuse with neurological lesions. Ziprasidone may have mitigated some of the impulsivity and hypomanic symptoms of her BD that could make her more likely to engage in risky self-harm behaviors.
Continue to: After extensive discussion...
After extensive discussion on the long-term complications of inhalant abuse, Ms. G was motivated, cooperative, and sought care to return to rehabilitation services. The CL psychiatry team collaborated with the social work team to address the psychosocial components of Ms. G’s homelessness and facilitated an application for a local resource to obtain rehabilitation placement and living assistance. Her years of abstinence from inhalant use and success with rehabilitation demonstrate the need for a multimodal approach to manage and treat inhalant use disorder. Outpatient follow-up arrangements were made with local mental health resources.
OUTCOME Improved outlook and discharge
Ms. G reports improved mood and willingness to change her substance use habits. The treatment team counsels her on the acute risk of fatal arrhythmias and end-organ complications of inhalant abuse. They warn her about the potential long-term effects of mood alterations, neurological lesions, and polyneuropathy that could possibly worsen with substance abuse. Ms. G expresses appreciation for this counseling, the help associated with her aftercare, and the referral to restart the 30-day inpatient rehabilitation services. The team arranges follow-up with outpatient psychiatry and outpatient therapy services to enhance Ms. G’s coping skills and mitigate her reliance on inhalants to regulate her mood.
Bottom Line
Inhalant use is a poorly understood form of substance abuse that disproportionately affects vulnerable populations. It can lead to life-threatening medical emergencies such as rhabdomyolysis. Clinicians need to be able to identify and manage inhalant abuse and associated complications, as well as provide appropriate education and counseling to prevent further misuse.
Related Resources
- Gude J, Bisen V, Fujii K. Medication-induced rhabdomyolysis. Current Psychiatry. 2023;22(2):39-40. doi:10.12788/cp.0332
- Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity--a Euro-DEN study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal. pone.0246297
Drug Brand Names
Carbamazepine • Tegretol
Duloxetine • Cymbalta
Gabapentin • Neurontin
Propranolol • Inderal
Quetiapine • Seroquel
Trazodone • Oleptro
Ziprasidone • Geodon
1. Ahern NR, Falsafi N. Inhalant abuse: youth at risk. J Psychosoc Nurs Ment Health Serv. 2013;51(8):19-24. doi:10.3928/02793695-20130612-02
2. Howard MO, Bowen SE, Garland EL, et al. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Prac. 2011;6(1):18-31.
3. Farkas J. Rhabdomyolysis. Internet Book of Critical Care. June 25, 2021. Accessed February 24, 2023. https://emcrit.org/ibcc/rhabdo/
4. Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
5. Muller AA, Muller GF. Inhalant abuse. J Emerg Nurs. 2006;32(5):447-448. doi:10.1016/j.jen.2006.05.018
6. Kozel N, Sloboda Z, De La Rosa M, eds. Epidemiology of Inhalant Abuse: An International Perspective; Nida Research Monograph 148. National Institute on Drug Abuse Research, US Dept of Health and Human Services; 1995. Accessed April 20, 2023. https://archives.nida.nih.gov/sites/default/files/monograph148.pdf
CASE A relapse and crisis
Ms. G, age 32, is brought to the emergency department (ED) by police after being found in a stupor-like state in a public restroom. The consultation-liaison (CL) psychiatry team assesses her for concerns of self-harm and suicide behavior. Ms. G discloses that she “huffs” an average of 4 canisters of air dusters daily to cope with psychosocial stressors and achieve a euphoric state. She recently lost her job, which led to homelessness, financial difficulties, a relapse to aerosol use after 2 years of abstinence, and stealing aerosol cans. The latest incident follows 2 prior arrests, which led officers to bring her to the ED for medical evaluation. Ms. G has a history of bipolar disorder (BD), generalized anxiety disorder (GAD), insomnia, and inhalant use disorder.
HISTORY Inhalant abuse and suicide attempt
Ms. G reports a longstanding history of severe inhalant abuse, primarily with air dusters due to their accessibility and low cost. She previously underwent inpatient rehab for inhalant abuse, and received inpatient psychiatry treatment 5 years ago for a suicide attempt by overdose linked to psychosocial stressors. In addition to BD, GAD, insomnia, and inhalant use disorder, Ms. G has a history of neuropathy, seizures, and recurrent hypokalemia. She is single and does not have insurance.
[polldaddy:12318871]
The authors’ observations
Inhalant abuse is the intentional inhalation of volatile substances to achieve an altered mental state. Inhalants are commercially available products that can produce intoxication if inhaled, such as glue, toluene, spray paint, gasoline, and lighter fluid (Table 11).

The epidemiology of inhalant abuse is difficult to accurately report due to a lack of recognition and social stigma. Due to inhalants’ ease of access and low cost, this form of substance abuse is popular among adolescents, adults of low socioeconomic status, individuals who live in rural areas, and those living in institutions. Inhalants act as reinforcers, producing a euphoric state. Rapid pulmonary absorption and lipid solubility of the substance rapidly alters the brain. Inhalant abuse can result in chemical and thermal burns, withdrawal symptoms, persistent mental illness, and catastrophic medical emergencies such as ventricular arrhythmias leading to disruptive myocardial electrical propagation. Chronic abuse can cause irreversible neurological and neuropsychological effects, cardiomyopathy, rapid airway compromise, pulmonary debilitations, renal tubular acidosis, bone marrow toxicity, reduced immunity, and peripheral neuropathy.2 Ms. G’s diagnosis of inhalant use disorder was based on her mental state and history of severe inhalant misuse, specifically with air dusters. Several additional factors further support this diagnosis, including the fact she survived a suicide attempt by overdose 5 years ago, had an inpatient rehabilitation placement for inhalant abuse, experiences insomnia, and was attempting to self-treat a depressive episode relapse with inhalants.
EVALUATION Depressed but cooperative
After being monitored in the ED for several hours, Ms. G is no longer in a stupor-like state. She has poor body habitus, appears older than her stated age, and is unkempt in appearance/attire. She is mildly distressed but relatively cooperative and engaged during the interview. Ms. G has a depressed mood and is anxious, with mood-congruent affect, and is tearful at times, especially when discussing recent stressors. She denies suicidality, homicidality, paranoia, delusions, and hallucinations. Her thought process is linear, goal-directed, and logical. She has fair insight, but relatively poor and impulsive judgment. The nursing staff expresses concerns that Ms. G was possibly responding to internal stimuli and behaving bizarrely during her initial presentation; this was not evident upon examination.
Ms. G reports having acute-on-chronic headaches, intermittent myalgias and weakness in her lower extremities (acute), and polyneuropathy (chronic). She denies a history of manic episodes or psychosis but reports previous relative hypomanic episodes that vacillated with periods of recurrent depressive episodes. Ms. G denies using illicit substances other than tobacco and inhalants. She says she had adhered to her outpatient psychiatric management services and medication regimen (duloxetine 60 mg/d at bedtime for mood/migraines, trazodone 150 mg/d at bedtime for insomnia, ziprasidone 40 mg/d at bedtime for BD, carbamazepine 200 mg twice daily for neuropathy/migraines, gabapentin 400 mg 3 times daily for neuropathy migraines/anxiety, and propranolol 10 mg 3 times daily for anxiety/tremors/migraine prophylaxis) until 4 days before her current presentation to the ED, when she used inhalants and was arrested.
Ms. G’s vitals are mostly unremarkable, but her heart rate is 116 beats per minute. There are no acute findings on physical examination. She is not pregnant, and her creatinine, glomerular filtration rate, complete blood count, and thyroid-stimulating hormone are all within normal limits. Her blood sugar is high (120 mg/dL; reference range 70 to 100 mg/dL). She has slight transaminitis with high aspartate aminotransferase (93 U/L; reference range 17 to 59 U/L) and high alanine aminotransferase (69 U/L; reference range 20 to 35 U/L); chronic hypokalemia (2.4 mmol/L; reference range 3.5 to 5.2 mmol/L), which leads the primary team to initiate a potassium replacement protocol; lactic acidosis (2.2 mmol/L; normal levels <2 mmol/L); and creatine kinase (CK) 5,930 U/L.
[polldaddy:12318873]
Continue to: The authors' observations
The authors’ observations
Efforts to improve the laboratory diagnosis of inhalant abuse are ongoing, but they have not yet been widely implemented. Systemic screening and assessment of inhalant use can help prevent and treat complications. For Ms. G, we considered several possible complications, including hypoglycemia. Although the classic triad of myalgia, weakness, and myoglobinuria (tea-colored urine) was not present, elevated CK levels in the context of Ms. G’s intermittent myalgia and lower extremity weakness led us to suspect she was experiencing moderate rhabdomyolysis (Table 23).

Rhabdomyolysis can be caused by several factors, including drug abuse, trauma, neuromuscular syndrome, and immobility. Treatment is mainly supportive, with a focus on preserving the ABCs (airway, breathing, circulation) and renal function through vigorous rehydration.4 We postulated Ms. G’s rhabdomyolysis was caused by muscle damage directly resulting from inhalant abuse and compounded by her remaining in prolonged fixed position on the ground after overdosing on inhalants.
TREATMENT Rehydration and psychotropics
The treatment team initiates IV fluid hydration of chloride 0.9% 150 mL/h and monitors Ms. G until she is stable and the trajectory of her CK levels begins to decline. On hospital Day 2, Ms. G’s CK decreases to 2,475 U/L and her lactic acid levels normalize. Ms. G restarts her regimen of duloxetine 60 mg/d, trazodone 150 mg/d, ziprasidone 40 mg/d, carbamazepine 200 mg twice daily, gabapentin 400 mg 3 times daily, and propranolol 10 mg 3 times daily. The team adds quetiapine 25 mg as needed for hallucinations, paranoia, and/or anxiety. Ms. G is closely monitored due to the potential risk of toxicity-induced or withdrawal-induced psychotic symptoms.
[polldaddy:12318869]
The authors’ observations
Presently, there are no effective treatments for acute inhalant intoxication or withdrawal, which makes supportive care and vigilant monitoring the only options.5 Although clinical research has not led to any FDA-approved treatments for chronic inhalant use disorder, a multipronged biopsychosocial treatment approach is critical in light of the negative consequences of inhalant abuse, including poor academic performance, criminal behavior, abuse of other substances, social maladjustment, low self-esteem, and suicidality.6
Ms. G had a moderate form of rhabdomyolysis, which was managed with IV fluid rehydration. Education and counseling were crucial to help Ms. G understand the unintended complications and potentially life-threatening consequences of inhalant abuse, with rehabilitation services to encourage abstinence. Ms. G had previously undergone successful inpatient rehabilitation and was willing to start such services again. She reported success with gabapentin for her polyneuropathy and migraines, which may be long-term consequences of prolonged inhalant abuse with neurological lesions. Ziprasidone may have mitigated some of the impulsivity and hypomanic symptoms of her BD that could make her more likely to engage in risky self-harm behaviors.
Continue to: After extensive discussion...
After extensive discussion on the long-term complications of inhalant abuse, Ms. G was motivated, cooperative, and sought care to return to rehabilitation services. The CL psychiatry team collaborated with the social work team to address the psychosocial components of Ms. G’s homelessness and facilitated an application for a local resource to obtain rehabilitation placement and living assistance. Her years of abstinence from inhalant use and success with rehabilitation demonstrate the need for a multimodal approach to manage and treat inhalant use disorder. Outpatient follow-up arrangements were made with local mental health resources.
OUTCOME Improved outlook and discharge
Ms. G reports improved mood and willingness to change her substance use habits. The treatment team counsels her on the acute risk of fatal arrhythmias and end-organ complications of inhalant abuse. They warn her about the potential long-term effects of mood alterations, neurological lesions, and polyneuropathy that could possibly worsen with substance abuse. Ms. G expresses appreciation for this counseling, the help associated with her aftercare, and the referral to restart the 30-day inpatient rehabilitation services. The team arranges follow-up with outpatient psychiatry and outpatient therapy services to enhance Ms. G’s coping skills and mitigate her reliance on inhalants to regulate her mood.
Bottom Line
Inhalant use is a poorly understood form of substance abuse that disproportionately affects vulnerable populations. It can lead to life-threatening medical emergencies such as rhabdomyolysis. Clinicians need to be able to identify and manage inhalant abuse and associated complications, as well as provide appropriate education and counseling to prevent further misuse.
Related Resources
- Gude J, Bisen V, Fujii K. Medication-induced rhabdomyolysis. Current Psychiatry. 2023;22(2):39-40. doi:10.12788/cp.0332
- Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity--a Euro-DEN study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal. pone.0246297
Drug Brand Names
Carbamazepine • Tegretol
Duloxetine • Cymbalta
Gabapentin • Neurontin
Propranolol • Inderal
Quetiapine • Seroquel
Trazodone • Oleptro
Ziprasidone • Geodon
CASE A relapse and crisis
Ms. G, age 32, is brought to the emergency department (ED) by police after being found in a stupor-like state in a public restroom. The consultation-liaison (CL) psychiatry team assesses her for concerns of self-harm and suicide behavior. Ms. G discloses that she “huffs” an average of 4 canisters of air dusters daily to cope with psychosocial stressors and achieve a euphoric state. She recently lost her job, which led to homelessness, financial difficulties, a relapse to aerosol use after 2 years of abstinence, and stealing aerosol cans. The latest incident follows 2 prior arrests, which led officers to bring her to the ED for medical evaluation. Ms. G has a history of bipolar disorder (BD), generalized anxiety disorder (GAD), insomnia, and inhalant use disorder.
HISTORY Inhalant abuse and suicide attempt
Ms. G reports a longstanding history of severe inhalant abuse, primarily with air dusters due to their accessibility and low cost. She previously underwent inpatient rehab for inhalant abuse, and received inpatient psychiatry treatment 5 years ago for a suicide attempt by overdose linked to psychosocial stressors. In addition to BD, GAD, insomnia, and inhalant use disorder, Ms. G has a history of neuropathy, seizures, and recurrent hypokalemia. She is single and does not have insurance.
[polldaddy:12318871]
The authors’ observations
Inhalant abuse is the intentional inhalation of volatile substances to achieve an altered mental state. Inhalants are commercially available products that can produce intoxication if inhaled, such as glue, toluene, spray paint, gasoline, and lighter fluid (Table 11).

The epidemiology of inhalant abuse is difficult to accurately report due to a lack of recognition and social stigma. Due to inhalants’ ease of access and low cost, this form of substance abuse is popular among adolescents, adults of low socioeconomic status, individuals who live in rural areas, and those living in institutions. Inhalants act as reinforcers, producing a euphoric state. Rapid pulmonary absorption and lipid solubility of the substance rapidly alters the brain. Inhalant abuse can result in chemical and thermal burns, withdrawal symptoms, persistent mental illness, and catastrophic medical emergencies such as ventricular arrhythmias leading to disruptive myocardial electrical propagation. Chronic abuse can cause irreversible neurological and neuropsychological effects, cardiomyopathy, rapid airway compromise, pulmonary debilitations, renal tubular acidosis, bone marrow toxicity, reduced immunity, and peripheral neuropathy.2 Ms. G’s diagnosis of inhalant use disorder was based on her mental state and history of severe inhalant misuse, specifically with air dusters. Several additional factors further support this diagnosis, including the fact she survived a suicide attempt by overdose 5 years ago, had an inpatient rehabilitation placement for inhalant abuse, experiences insomnia, and was attempting to self-treat a depressive episode relapse with inhalants.
EVALUATION Depressed but cooperative
After being monitored in the ED for several hours, Ms. G is no longer in a stupor-like state. She has poor body habitus, appears older than her stated age, and is unkempt in appearance/attire. She is mildly distressed but relatively cooperative and engaged during the interview. Ms. G has a depressed mood and is anxious, with mood-congruent affect, and is tearful at times, especially when discussing recent stressors. She denies suicidality, homicidality, paranoia, delusions, and hallucinations. Her thought process is linear, goal-directed, and logical. She has fair insight, but relatively poor and impulsive judgment. The nursing staff expresses concerns that Ms. G was possibly responding to internal stimuli and behaving bizarrely during her initial presentation; this was not evident upon examination.
Ms. G reports having acute-on-chronic headaches, intermittent myalgias and weakness in her lower extremities (acute), and polyneuropathy (chronic). She denies a history of manic episodes or psychosis but reports previous relative hypomanic episodes that vacillated with periods of recurrent depressive episodes. Ms. G denies using illicit substances other than tobacco and inhalants. She says she had adhered to her outpatient psychiatric management services and medication regimen (duloxetine 60 mg/d at bedtime for mood/migraines, trazodone 150 mg/d at bedtime for insomnia, ziprasidone 40 mg/d at bedtime for BD, carbamazepine 200 mg twice daily for neuropathy/migraines, gabapentin 400 mg 3 times daily for neuropathy migraines/anxiety, and propranolol 10 mg 3 times daily for anxiety/tremors/migraine prophylaxis) until 4 days before her current presentation to the ED, when she used inhalants and was arrested.
Ms. G’s vitals are mostly unremarkable, but her heart rate is 116 beats per minute. There are no acute findings on physical examination. She is not pregnant, and her creatinine, glomerular filtration rate, complete blood count, and thyroid-stimulating hormone are all within normal limits. Her blood sugar is high (120 mg/dL; reference range 70 to 100 mg/dL). She has slight transaminitis with high aspartate aminotransferase (93 U/L; reference range 17 to 59 U/L) and high alanine aminotransferase (69 U/L; reference range 20 to 35 U/L); chronic hypokalemia (2.4 mmol/L; reference range 3.5 to 5.2 mmol/L), which leads the primary team to initiate a potassium replacement protocol; lactic acidosis (2.2 mmol/L; normal levels <2 mmol/L); and creatine kinase (CK) 5,930 U/L.
[polldaddy:12318873]
Continue to: The authors' observations
The authors’ observations
Efforts to improve the laboratory diagnosis of inhalant abuse are ongoing, but they have not yet been widely implemented. Systemic screening and assessment of inhalant use can help prevent and treat complications. For Ms. G, we considered several possible complications, including hypoglycemia. Although the classic triad of myalgia, weakness, and myoglobinuria (tea-colored urine) was not present, elevated CK levels in the context of Ms. G’s intermittent myalgia and lower extremity weakness led us to suspect she was experiencing moderate rhabdomyolysis (Table 23).

Rhabdomyolysis can be caused by several factors, including drug abuse, trauma, neuromuscular syndrome, and immobility. Treatment is mainly supportive, with a focus on preserving the ABCs (airway, breathing, circulation) and renal function through vigorous rehydration.4 We postulated Ms. G’s rhabdomyolysis was caused by muscle damage directly resulting from inhalant abuse and compounded by her remaining in prolonged fixed position on the ground after overdosing on inhalants.
TREATMENT Rehydration and psychotropics
The treatment team initiates IV fluid hydration of chloride 0.9% 150 mL/h and monitors Ms. G until she is stable and the trajectory of her CK levels begins to decline. On hospital Day 2, Ms. G’s CK decreases to 2,475 U/L and her lactic acid levels normalize. Ms. G restarts her regimen of duloxetine 60 mg/d, trazodone 150 mg/d, ziprasidone 40 mg/d, carbamazepine 200 mg twice daily, gabapentin 400 mg 3 times daily, and propranolol 10 mg 3 times daily. The team adds quetiapine 25 mg as needed for hallucinations, paranoia, and/or anxiety. Ms. G is closely monitored due to the potential risk of toxicity-induced or withdrawal-induced psychotic symptoms.
[polldaddy:12318869]
The authors’ observations
Presently, there are no effective treatments for acute inhalant intoxication or withdrawal, which makes supportive care and vigilant monitoring the only options.5 Although clinical research has not led to any FDA-approved treatments for chronic inhalant use disorder, a multipronged biopsychosocial treatment approach is critical in light of the negative consequences of inhalant abuse, including poor academic performance, criminal behavior, abuse of other substances, social maladjustment, low self-esteem, and suicidality.6
Ms. G had a moderate form of rhabdomyolysis, which was managed with IV fluid rehydration. Education and counseling were crucial to help Ms. G understand the unintended complications and potentially life-threatening consequences of inhalant abuse, with rehabilitation services to encourage abstinence. Ms. G had previously undergone successful inpatient rehabilitation and was willing to start such services again. She reported success with gabapentin for her polyneuropathy and migraines, which may be long-term consequences of prolonged inhalant abuse with neurological lesions. Ziprasidone may have mitigated some of the impulsivity and hypomanic symptoms of her BD that could make her more likely to engage in risky self-harm behaviors.
Continue to: After extensive discussion...
After extensive discussion on the long-term complications of inhalant abuse, Ms. G was motivated, cooperative, and sought care to return to rehabilitation services. The CL psychiatry team collaborated with the social work team to address the psychosocial components of Ms. G’s homelessness and facilitated an application for a local resource to obtain rehabilitation placement and living assistance. Her years of abstinence from inhalant use and success with rehabilitation demonstrate the need for a multimodal approach to manage and treat inhalant use disorder. Outpatient follow-up arrangements were made with local mental health resources.
OUTCOME Improved outlook and discharge
Ms. G reports improved mood and willingness to change her substance use habits. The treatment team counsels her on the acute risk of fatal arrhythmias and end-organ complications of inhalant abuse. They warn her about the potential long-term effects of mood alterations, neurological lesions, and polyneuropathy that could possibly worsen with substance abuse. Ms. G expresses appreciation for this counseling, the help associated with her aftercare, and the referral to restart the 30-day inpatient rehabilitation services. The team arranges follow-up with outpatient psychiatry and outpatient therapy services to enhance Ms. G’s coping skills and mitigate her reliance on inhalants to regulate her mood.
Bottom Line
Inhalant use is a poorly understood form of substance abuse that disproportionately affects vulnerable populations. It can lead to life-threatening medical emergencies such as rhabdomyolysis. Clinicians need to be able to identify and manage inhalant abuse and associated complications, as well as provide appropriate education and counseling to prevent further misuse.
Related Resources
- Gude J, Bisen V, Fujii K. Medication-induced rhabdomyolysis. Current Psychiatry. 2023;22(2):39-40. doi:10.12788/cp.0332
- Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity--a Euro-DEN study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal. pone.0246297
Drug Brand Names
Carbamazepine • Tegretol
Duloxetine • Cymbalta
Gabapentin • Neurontin
Propranolol • Inderal
Quetiapine • Seroquel
Trazodone • Oleptro
Ziprasidone • Geodon
1. Ahern NR, Falsafi N. Inhalant abuse: youth at risk. J Psychosoc Nurs Ment Health Serv. 2013;51(8):19-24. doi:10.3928/02793695-20130612-02
2. Howard MO, Bowen SE, Garland EL, et al. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Prac. 2011;6(1):18-31.
3. Farkas J. Rhabdomyolysis. Internet Book of Critical Care. June 25, 2021. Accessed February 24, 2023. https://emcrit.org/ibcc/rhabdo/
4. Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
5. Muller AA, Muller GF. Inhalant abuse. J Emerg Nurs. 2006;32(5):447-448. doi:10.1016/j.jen.2006.05.018
6. Kozel N, Sloboda Z, De La Rosa M, eds. Epidemiology of Inhalant Abuse: An International Perspective; Nida Research Monograph 148. National Institute on Drug Abuse Research, US Dept of Health and Human Services; 1995. Accessed April 20, 2023. https://archives.nida.nih.gov/sites/default/files/monograph148.pdf
1. Ahern NR, Falsafi N. Inhalant abuse: youth at risk. J Psychosoc Nurs Ment Health Serv. 2013;51(8):19-24. doi:10.3928/02793695-20130612-02
2. Howard MO, Bowen SE, Garland EL, et al. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Prac. 2011;6(1):18-31.
3. Farkas J. Rhabdomyolysis. Internet Book of Critical Care. June 25, 2021. Accessed February 24, 2023. https://emcrit.org/ibcc/rhabdo/
4. Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
5. Muller AA, Muller GF. Inhalant abuse. J Emerg Nurs. 2006;32(5):447-448. doi:10.1016/j.jen.2006.05.018
6. Kozel N, Sloboda Z, De La Rosa M, eds. Epidemiology of Inhalant Abuse: An International Perspective; Nida Research Monograph 148. National Institute on Drug Abuse Research, US Dept of Health and Human Services; 1995. Accessed April 20, 2023. https://archives.nida.nih.gov/sites/default/files/monograph148.pdf
When a patient wants to stop taking their antipsychotic: Be ‘A SPORT’
For patients with schizophrenia, adherence to antipsychotic treatment reduces the rate of relapse of psychosis, lowers the rate of rehospitalization, and reduces the severity of illness.1 Despite this, patients may want to discontinue their medications for multiple reasons, including limited insight, adverse effects, or a negative attitude toward medication.1 Understanding a patient’s reason for wanting to discontinue their antipsychotic is critical to providing patient-centered care, building the therapeutic alliance, and offering potential solutions.
Clinicians can recall the mnemonic “A SPORT” (Table) to help ensure they have a thorough discussion with patients about the risks of discontinuation and potential solutions.

Points to cover
First, explore and acknowledge if a patient is experiencing adverse effects from their antipsychotic, which may be causing them to have a negative attitude toward medications. If a patient is experiencing adverse effects from their antipsychotic, offer interventions to mitigate those effects, such as adding an anticholinergic agent to address extrapyramidal symptoms. Decreasing the antipsychotic dosage might reduce the adverse effects burden while still optimizing the benefits from the antipsychotic. Additionally, switching to an alternate medication with a more favorable adverse effect profile may be an option. Whether the patient is experiencing intolerable adverse effects or just has a negative view of their prescribed antipsychotic, it is important to discuss switching medications.
Identifying patient attitudes and their general perspective toward their medication and illness is key. Similarly, a patient’s impaired insight into their mental illness has been associated with treatment discontinuation.2 A strong therapeutic alliance with your patient is of the utmost importance in these situations.
Long-acting injectable antipsychotics (LAIs) are useful clinical tools for patients who struggle to adhere to oral medications. Educating patients and caregivers about other formulations—namely LAIs—can help clarify any misconceptions they may have. One study found that patients who were prescribed oral antipsychotics thought LAIs would be painful, have worse adverse effects, and would not be beneficial in preventing relapse.3 In addition to LAIs, other formulations of antipsychotic medications, such as patches, sublingual tablets, or liquids, may be an option.
For patients to be able to provide informed consent regarding the decision to discontinue their antipsychotic, it is important to educate them about the risks of not taking an antipsychotic, such as an increased risk of relapse, hospitalization, and poor outcomes. Explain that patients with first-episode psychosis who achieve remission of symptoms while taking an antipsychotic can remain in remission with continued treatment, but there is a 5-fold increased risk of relapse when discontinuing an antipsychotic during first-episode psychosis.4
Lastly, despite discussing the risks and benefits, if a patient is determined to discontinue their antipsychotic, we recommend a slow taper of medication rather than abrupt discontinuation. Research has shown that more than one-half of patients who abruptly discontinue an antipsychotic experience withdrawal symptoms, including (but not limited to) nausea, vomiting, abdominal pain, and headaches, as well as anxiety, restlessness, and insomnia.5 These symptoms may occur within 4 weeks after discontinuation.5 While there are no clear guidelines on deprescribing antipsychotics, it is best to individualize the taper based on patient response. Family and caregiver involvement, close follow-up, and symptom monitoring should be integrated into the tapering process.6
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherenc. 2017;11:449-468. doi:10.2147/PPA.S124658
2. Kim J, Ozzoude M, Nakajima S, et al. Insight and medication adherence in schizophrenia: an analysis of the CATIE trial. Neuropharmacology. 2020;168:107634. doi:10.1016/j.neuropharm.2019.05.011
3. Sugawara N, Kudo S, Ishioka M, et al. Attitudes toward long-acting injectable antipsychotics among patients with schizophrenia in Japan. Neuropsychiatr Dis Treat. 2019;15:205-211. doi:10.2147/NDT.S188337
4. Winton-Brown TT, Elanjithara T, Power P, et al. Five-fold increased risk of relapse following breaks in antipsychotic treatment of first episode psychosis. Schizophr Res. 2017;179:50-56. doi:10.1016/j.schres.2016.09.029
5. Brandt L, Bschor T, Henssler J, et al. Antipsychotic withdrawal symptoms: a systematic review and meta-analysis. Front Psychiatry. 2020;11:569912. doi:10.3389/fpsyt.2020.569912
6. Gupta S, Cahill JD, Miller R. Deprescribing antipsychotics: a guide for clinicians. BJPsych Advances. 2018;24(5):295-302. doi:10.1192/bja.2018.2
For patients with schizophrenia, adherence to antipsychotic treatment reduces the rate of relapse of psychosis, lowers the rate of rehospitalization, and reduces the severity of illness.1 Despite this, patients may want to discontinue their medications for multiple reasons, including limited insight, adverse effects, or a negative attitude toward medication.1 Understanding a patient’s reason for wanting to discontinue their antipsychotic is critical to providing patient-centered care, building the therapeutic alliance, and offering potential solutions.
Clinicians can recall the mnemonic “A SPORT” (Table) to help ensure they have a thorough discussion with patients about the risks of discontinuation and potential solutions.

Points to cover
First, explore and acknowledge if a patient is experiencing adverse effects from their antipsychotic, which may be causing them to have a negative attitude toward medications. If a patient is experiencing adverse effects from their antipsychotic, offer interventions to mitigate those effects, such as adding an anticholinergic agent to address extrapyramidal symptoms. Decreasing the antipsychotic dosage might reduce the adverse effects burden while still optimizing the benefits from the antipsychotic. Additionally, switching to an alternate medication with a more favorable adverse effect profile may be an option. Whether the patient is experiencing intolerable adverse effects or just has a negative view of their prescribed antipsychotic, it is important to discuss switching medications.
Identifying patient attitudes and their general perspective toward their medication and illness is key. Similarly, a patient’s impaired insight into their mental illness has been associated with treatment discontinuation.2 A strong therapeutic alliance with your patient is of the utmost importance in these situations.
Long-acting injectable antipsychotics (LAIs) are useful clinical tools for patients who struggle to adhere to oral medications. Educating patients and caregivers about other formulations—namely LAIs—can help clarify any misconceptions they may have. One study found that patients who were prescribed oral antipsychotics thought LAIs would be painful, have worse adverse effects, and would not be beneficial in preventing relapse.3 In addition to LAIs, other formulations of antipsychotic medications, such as patches, sublingual tablets, or liquids, may be an option.
For patients to be able to provide informed consent regarding the decision to discontinue their antipsychotic, it is important to educate them about the risks of not taking an antipsychotic, such as an increased risk of relapse, hospitalization, and poor outcomes. Explain that patients with first-episode psychosis who achieve remission of symptoms while taking an antipsychotic can remain in remission with continued treatment, but there is a 5-fold increased risk of relapse when discontinuing an antipsychotic during first-episode psychosis.4
Lastly, despite discussing the risks and benefits, if a patient is determined to discontinue their antipsychotic, we recommend a slow taper of medication rather than abrupt discontinuation. Research has shown that more than one-half of patients who abruptly discontinue an antipsychotic experience withdrawal symptoms, including (but not limited to) nausea, vomiting, abdominal pain, and headaches, as well as anxiety, restlessness, and insomnia.5 These symptoms may occur within 4 weeks after discontinuation.5 While there are no clear guidelines on deprescribing antipsychotics, it is best to individualize the taper based on patient response. Family and caregiver involvement, close follow-up, and symptom monitoring should be integrated into the tapering process.6
For patients with schizophrenia, adherence to antipsychotic treatment reduces the rate of relapse of psychosis, lowers the rate of rehospitalization, and reduces the severity of illness.1 Despite this, patients may want to discontinue their medications for multiple reasons, including limited insight, adverse effects, or a negative attitude toward medication.1 Understanding a patient’s reason for wanting to discontinue their antipsychotic is critical to providing patient-centered care, building the therapeutic alliance, and offering potential solutions.
Clinicians can recall the mnemonic “A SPORT” (Table) to help ensure they have a thorough discussion with patients about the risks of discontinuation and potential solutions.

Points to cover
First, explore and acknowledge if a patient is experiencing adverse effects from their antipsychotic, which may be causing them to have a negative attitude toward medications. If a patient is experiencing adverse effects from their antipsychotic, offer interventions to mitigate those effects, such as adding an anticholinergic agent to address extrapyramidal symptoms. Decreasing the antipsychotic dosage might reduce the adverse effects burden while still optimizing the benefits from the antipsychotic. Additionally, switching to an alternate medication with a more favorable adverse effect profile may be an option. Whether the patient is experiencing intolerable adverse effects or just has a negative view of their prescribed antipsychotic, it is important to discuss switching medications.
Identifying patient attitudes and their general perspective toward their medication and illness is key. Similarly, a patient’s impaired insight into their mental illness has been associated with treatment discontinuation.2 A strong therapeutic alliance with your patient is of the utmost importance in these situations.
Long-acting injectable antipsychotics (LAIs) are useful clinical tools for patients who struggle to adhere to oral medications. Educating patients and caregivers about other formulations—namely LAIs—can help clarify any misconceptions they may have. One study found that patients who were prescribed oral antipsychotics thought LAIs would be painful, have worse adverse effects, and would not be beneficial in preventing relapse.3 In addition to LAIs, other formulations of antipsychotic medications, such as patches, sublingual tablets, or liquids, may be an option.
For patients to be able to provide informed consent regarding the decision to discontinue their antipsychotic, it is important to educate them about the risks of not taking an antipsychotic, such as an increased risk of relapse, hospitalization, and poor outcomes. Explain that patients with first-episode psychosis who achieve remission of symptoms while taking an antipsychotic can remain in remission with continued treatment, but there is a 5-fold increased risk of relapse when discontinuing an antipsychotic during first-episode psychosis.4
Lastly, despite discussing the risks and benefits, if a patient is determined to discontinue their antipsychotic, we recommend a slow taper of medication rather than abrupt discontinuation. Research has shown that more than one-half of patients who abruptly discontinue an antipsychotic experience withdrawal symptoms, including (but not limited to) nausea, vomiting, abdominal pain, and headaches, as well as anxiety, restlessness, and insomnia.5 These symptoms may occur within 4 weeks after discontinuation.5 While there are no clear guidelines on deprescribing antipsychotics, it is best to individualize the taper based on patient response. Family and caregiver involvement, close follow-up, and symptom monitoring should be integrated into the tapering process.6
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherenc. 2017;11:449-468. doi:10.2147/PPA.S124658
2. Kim J, Ozzoude M, Nakajima S, et al. Insight and medication adherence in schizophrenia: an analysis of the CATIE trial. Neuropharmacology. 2020;168:107634. doi:10.1016/j.neuropharm.2019.05.011
3. Sugawara N, Kudo S, Ishioka M, et al. Attitudes toward long-acting injectable antipsychotics among patients with schizophrenia in Japan. Neuropsychiatr Dis Treat. 2019;15:205-211. doi:10.2147/NDT.S188337
4. Winton-Brown TT, Elanjithara T, Power P, et al. Five-fold increased risk of relapse following breaks in antipsychotic treatment of first episode psychosis. Schizophr Res. 2017;179:50-56. doi:10.1016/j.schres.2016.09.029
5. Brandt L, Bschor T, Henssler J, et al. Antipsychotic withdrawal symptoms: a systematic review and meta-analysis. Front Psychiatry. 2020;11:569912. doi:10.3389/fpsyt.2020.569912
6. Gupta S, Cahill JD, Miller R. Deprescribing antipsychotics: a guide for clinicians. BJPsych Advances. 2018;24(5):295-302. doi:10.1192/bja.2018.2
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherenc. 2017;11:449-468. doi:10.2147/PPA.S124658
2. Kim J, Ozzoude M, Nakajima S, et al. Insight and medication adherence in schizophrenia: an analysis of the CATIE trial. Neuropharmacology. 2020;168:107634. doi:10.1016/j.neuropharm.2019.05.011
3. Sugawara N, Kudo S, Ishioka M, et al. Attitudes toward long-acting injectable antipsychotics among patients with schizophrenia in Japan. Neuropsychiatr Dis Treat. 2019;15:205-211. doi:10.2147/NDT.S188337
4. Winton-Brown TT, Elanjithara T, Power P, et al. Five-fold increased risk of relapse following breaks in antipsychotic treatment of first episode psychosis. Schizophr Res. 2017;179:50-56. doi:10.1016/j.schres.2016.09.029
5. Brandt L, Bschor T, Henssler J, et al. Antipsychotic withdrawal symptoms: a systematic review and meta-analysis. Front Psychiatry. 2020;11:569912. doi:10.3389/fpsyt.2020.569912
6. Gupta S, Cahill JD, Miller R. Deprescribing antipsychotics: a guide for clinicians. BJPsych Advances. 2018;24(5):295-302. doi:10.1192/bja.2018.2
Dysphagia in a patient with schizophrenia: Is the antipsychotic the culprit?
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Mr. N, age 58, has a history of schizophrenia, tobacco use disorder, and alcohol use disorder. For many years, Mr. N has been receiving IM olanzapine 2.5 mg/d to treat his schizophrenia. He lives in a psychiatric hospital but was sent to our hospital after being found to have severe oropharyngeal dysphasia on a modified barium swallow study. There was concern for aspiration due to a history of choking episodes, which had been occurring for almost 1 month. During the modified barium swallow study, Mr. N was noted to have aspiration with deep laryngeal penetration during the pharyngeal stages of swallowing to all consistencies; this did not improve with the chin-tuck maneuver. In addition, during a CT scan of the cervical spine, an osteophyte was noted at the C5-C6 level, with possible impingement of the cervical esophagus and decreased upper esophageal sphincter opening.
Due to these findings, Mr. N was sent to our emergency department (ED) for further evaluation. In the ED, his vital signs were stable. He endorsed having a cough after eating, a sensation of having food stuck in his throat, and some hoarseness. His physical examination was notable for poor dentition. Results of a standard laboratory workup were all within normal limits. X-ray was notable for hazy opacities in the right upper to mid lung zones. Mr. N was admitted to the medical unit for further evaluation and management.
Narrowing the diagnosis
Because Mr. N was aspirating both liquids and solids, it was imperative that we identify the cause as soon as possible. The consultations that followed slowly guided the treatment team toward a diagnosis of antipsychotic-induced dysphagia. Otolaryngology identified insensate larynx during a flexible fiberoptic laryngoscopy exam, which was highly suggestive of a neurological dysfunction such as dystonia. Furthermore, an esophagogastroduodenoscopy found no structural abnormalities to explain Mr. N’s dysphagia, which ruled out impingement of the cervical esophagus by the osteophyte. An MRI of the brain ruled out structural abnormalities or evidence of stroke. Finally, a speech and language pathologist confirmed decreased laryngeal closure and airway protection with a repeat modified barium swallow, which led to aspiration during swallowing. Psychiatry recommended starting diphenhydramine to treat Mr. N’s extrapyramidal symptoms (EPS). A 6-day trial was initiated, with a single 50 mg IV dose on the first day followed by 25 mL oral twice daily for the remaining 5 days. In addition, olanzapine was discontinued.
Switching to a different diet and antipsychotic
Two days after starting diphenhydramine, Mr. N was switched to a puree diet. His ability to swallow improved, and he no longer coughed. However, on repeat modified barium swallow, aspiration was still noted for all types of liquids and solids. No structural improvements were seen.
Mr. N was discharged back to his psychiatric hospital, and his antipsychotic was changed from olanzapine to oral aripiprazole 2 mg/d. The aripiprazole dose was kept low to prevent the recurrence of dystonia and because at the time, his schizophrenia was asymptomatic. Mr. N was also prescribed oral diphenhydramine 25 mL twice daily.
At a 2-week follow-up appointment, Mr. N continued to show clinical improvement on the puree diet with thin liquids and continued the prescribed medication regimen.
Dysphagia as a manifestation of EPS
All antipsychotics, and particularly first-generation agents, are associated with EPS.1 These symptoms may be the result of antagonistic binding of dopaminergic D2 receptors within mesolimbic and mesocortical pathways of the brain, as well as parts of basal ganglia such as the caudate nucleus.2
In addition to the examples listed in the Table,2 EPS can present as dysphagia, esophageal dysmotility, or aspiration, none of which may be recognized as EPS. Research has found haloperidol, loxapine, trifluoperazine, olanzapine, risperidone, quetiapine, clozapine, and aripiprazole are associated with dysphagia.3-6 Strategies to treat antipsychotic-induced dysphagia include discontinuing the antipsychotic, lowering the dose, and changing to another medication.7
1. Crouse EL, Alastanos JN, Bozymski KM, et al. Dysphagia with second-generation antipsychotics: a case report and review of the literature. Ment Health Clin. 2018;7(2):56-64. doi:10.9740/mhc.2017.03.056
2. D’Souza RS, Hooten WM. Extrapyramidal symptoms. StatPearls Publishing; 2022. Updated January 8, 2023. Accessed April 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK534115/
3. Dziewas R, Warnecke T, Schnabel M, et al. Neuroleptic-induced dysphagia: case report and literature review. Dysphagia. 2007;22(1):63-67. doi:10.1007/s00455-006-9032-9
4. Kalf JG, de Swart BJ, Bloem BR, et al. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18(4):311-315. doi:10.1016/j.parkreldis.2011.11.006
5. Lin TW, Lee BS, Liao YC, et al. High dosage of aripiprazole-induced dysphagia. Int J Eat Disord. 2012;45(2):305-306. doi:10.1002/eat.20934
6. Stewart JT. Dysphagia associated with risperidone therapy. Dysphagia. 2003;18(4):274-275. doi:10.1007/s00455-003-0006-x
7. Lee JC, Takeshita J. Antipsychotic-induced dysphagia: a case report. Prim Care Companion CNS Disord. 2015;17(5):10.4088/PCC.15I01792. doi:10.4088/PCC.15I01792
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Mr. N, age 58, has a history of schizophrenia, tobacco use disorder, and alcohol use disorder. For many years, Mr. N has been receiving IM olanzapine 2.5 mg/d to treat his schizophrenia. He lives in a psychiatric hospital but was sent to our hospital after being found to have severe oropharyngeal dysphasia on a modified barium swallow study. There was concern for aspiration due to a history of choking episodes, which had been occurring for almost 1 month. During the modified barium swallow study, Mr. N was noted to have aspiration with deep laryngeal penetration during the pharyngeal stages of swallowing to all consistencies; this did not improve with the chin-tuck maneuver. In addition, during a CT scan of the cervical spine, an osteophyte was noted at the C5-C6 level, with possible impingement of the cervical esophagus and decreased upper esophageal sphincter opening.
Due to these findings, Mr. N was sent to our emergency department (ED) for further evaluation. In the ED, his vital signs were stable. He endorsed having a cough after eating, a sensation of having food stuck in his throat, and some hoarseness. His physical examination was notable for poor dentition. Results of a standard laboratory workup were all within normal limits. X-ray was notable for hazy opacities in the right upper to mid lung zones. Mr. N was admitted to the medical unit for further evaluation and management.
Narrowing the diagnosis
Because Mr. N was aspirating both liquids and solids, it was imperative that we identify the cause as soon as possible. The consultations that followed slowly guided the treatment team toward a diagnosis of antipsychotic-induced dysphagia. Otolaryngology identified insensate larynx during a flexible fiberoptic laryngoscopy exam, which was highly suggestive of a neurological dysfunction such as dystonia. Furthermore, an esophagogastroduodenoscopy found no structural abnormalities to explain Mr. N’s dysphagia, which ruled out impingement of the cervical esophagus by the osteophyte. An MRI of the brain ruled out structural abnormalities or evidence of stroke. Finally, a speech and language pathologist confirmed decreased laryngeal closure and airway protection with a repeat modified barium swallow, which led to aspiration during swallowing. Psychiatry recommended starting diphenhydramine to treat Mr. N’s extrapyramidal symptoms (EPS). A 6-day trial was initiated, with a single 50 mg IV dose on the first day followed by 25 mL oral twice daily for the remaining 5 days. In addition, olanzapine was discontinued.
Switching to a different diet and antipsychotic
Two days after starting diphenhydramine, Mr. N was switched to a puree diet. His ability to swallow improved, and he no longer coughed. However, on repeat modified barium swallow, aspiration was still noted for all types of liquids and solids. No structural improvements were seen.
Mr. N was discharged back to his psychiatric hospital, and his antipsychotic was changed from olanzapine to oral aripiprazole 2 mg/d. The aripiprazole dose was kept low to prevent the recurrence of dystonia and because at the time, his schizophrenia was asymptomatic. Mr. N was also prescribed oral diphenhydramine 25 mL twice daily.
At a 2-week follow-up appointment, Mr. N continued to show clinical improvement on the puree diet with thin liquids and continued the prescribed medication regimen.
Dysphagia as a manifestation of EPS
All antipsychotics, and particularly first-generation agents, are associated with EPS.1 These symptoms may be the result of antagonistic binding of dopaminergic D2 receptors within mesolimbic and mesocortical pathways of the brain, as well as parts of basal ganglia such as the caudate nucleus.2
In addition to the examples listed in the Table,2 EPS can present as dysphagia, esophageal dysmotility, or aspiration, none of which may be recognized as EPS. Research has found haloperidol, loxapine, trifluoperazine, olanzapine, risperidone, quetiapine, clozapine, and aripiprazole are associated with dysphagia.3-6 Strategies to treat antipsychotic-induced dysphagia include discontinuing the antipsychotic, lowering the dose, and changing to another medication.7

Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Mr. N, age 58, has a history of schizophrenia, tobacco use disorder, and alcohol use disorder. For many years, Mr. N has been receiving IM olanzapine 2.5 mg/d to treat his schizophrenia. He lives in a psychiatric hospital but was sent to our hospital after being found to have severe oropharyngeal dysphasia on a modified barium swallow study. There was concern for aspiration due to a history of choking episodes, which had been occurring for almost 1 month. During the modified barium swallow study, Mr. N was noted to have aspiration with deep laryngeal penetration during the pharyngeal stages of swallowing to all consistencies; this did not improve with the chin-tuck maneuver. In addition, during a CT scan of the cervical spine, an osteophyte was noted at the C5-C6 level, with possible impingement of the cervical esophagus and decreased upper esophageal sphincter opening.
Due to these findings, Mr. N was sent to our emergency department (ED) for further evaluation. In the ED, his vital signs were stable. He endorsed having a cough after eating, a sensation of having food stuck in his throat, and some hoarseness. His physical examination was notable for poor dentition. Results of a standard laboratory workup were all within normal limits. X-ray was notable for hazy opacities in the right upper to mid lung zones. Mr. N was admitted to the medical unit for further evaluation and management.
Narrowing the diagnosis
Because Mr. N was aspirating both liquids and solids, it was imperative that we identify the cause as soon as possible. The consultations that followed slowly guided the treatment team toward a diagnosis of antipsychotic-induced dysphagia. Otolaryngology identified insensate larynx during a flexible fiberoptic laryngoscopy exam, which was highly suggestive of a neurological dysfunction such as dystonia. Furthermore, an esophagogastroduodenoscopy found no structural abnormalities to explain Mr. N’s dysphagia, which ruled out impingement of the cervical esophagus by the osteophyte. An MRI of the brain ruled out structural abnormalities or evidence of stroke. Finally, a speech and language pathologist confirmed decreased laryngeal closure and airway protection with a repeat modified barium swallow, which led to aspiration during swallowing. Psychiatry recommended starting diphenhydramine to treat Mr. N’s extrapyramidal symptoms (EPS). A 6-day trial was initiated, with a single 50 mg IV dose on the first day followed by 25 mL oral twice daily for the remaining 5 days. In addition, olanzapine was discontinued.
Switching to a different diet and antipsychotic
Two days after starting diphenhydramine, Mr. N was switched to a puree diet. His ability to swallow improved, and he no longer coughed. However, on repeat modified barium swallow, aspiration was still noted for all types of liquids and solids. No structural improvements were seen.
Mr. N was discharged back to his psychiatric hospital, and his antipsychotic was changed from olanzapine to oral aripiprazole 2 mg/d. The aripiprazole dose was kept low to prevent the recurrence of dystonia and because at the time, his schizophrenia was asymptomatic. Mr. N was also prescribed oral diphenhydramine 25 mL twice daily.
At a 2-week follow-up appointment, Mr. N continued to show clinical improvement on the puree diet with thin liquids and continued the prescribed medication regimen.
Dysphagia as a manifestation of EPS
All antipsychotics, and particularly first-generation agents, are associated with EPS.1 These symptoms may be the result of antagonistic binding of dopaminergic D2 receptors within mesolimbic and mesocortical pathways of the brain, as well as parts of basal ganglia such as the caudate nucleus.2
In addition to the examples listed in the Table,2 EPS can present as dysphagia, esophageal dysmotility, or aspiration, none of which may be recognized as EPS. Research has found haloperidol, loxapine, trifluoperazine, olanzapine, risperidone, quetiapine, clozapine, and aripiprazole are associated with dysphagia.3-6 Strategies to treat antipsychotic-induced dysphagia include discontinuing the antipsychotic, lowering the dose, and changing to another medication.7

1. Crouse EL, Alastanos JN, Bozymski KM, et al. Dysphagia with second-generation antipsychotics: a case report and review of the literature. Ment Health Clin. 2018;7(2):56-64. doi:10.9740/mhc.2017.03.056
2. D’Souza RS, Hooten WM. Extrapyramidal symptoms. StatPearls Publishing; 2022. Updated January 8, 2023. Accessed April 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK534115/
3. Dziewas R, Warnecke T, Schnabel M, et al. Neuroleptic-induced dysphagia: case report and literature review. Dysphagia. 2007;22(1):63-67. doi:10.1007/s00455-006-9032-9
4. Kalf JG, de Swart BJ, Bloem BR, et al. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18(4):311-315. doi:10.1016/j.parkreldis.2011.11.006
5. Lin TW, Lee BS, Liao YC, et al. High dosage of aripiprazole-induced dysphagia. Int J Eat Disord. 2012;45(2):305-306. doi:10.1002/eat.20934
6. Stewart JT. Dysphagia associated with risperidone therapy. Dysphagia. 2003;18(4):274-275. doi:10.1007/s00455-003-0006-x
7. Lee JC, Takeshita J. Antipsychotic-induced dysphagia: a case report. Prim Care Companion CNS Disord. 2015;17(5):10.4088/PCC.15I01792. doi:10.4088/PCC.15I01792
1. Crouse EL, Alastanos JN, Bozymski KM, et al. Dysphagia with second-generation antipsychotics: a case report and review of the literature. Ment Health Clin. 2018;7(2):56-64. doi:10.9740/mhc.2017.03.056
2. D’Souza RS, Hooten WM. Extrapyramidal symptoms. StatPearls Publishing; 2022. Updated January 8, 2023. Accessed April 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK534115/
3. Dziewas R, Warnecke T, Schnabel M, et al. Neuroleptic-induced dysphagia: case report and literature review. Dysphagia. 2007;22(1):63-67. doi:10.1007/s00455-006-9032-9
4. Kalf JG, de Swart BJ, Bloem BR, et al. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18(4):311-315. doi:10.1016/j.parkreldis.2011.11.006
5. Lin TW, Lee BS, Liao YC, et al. High dosage of aripiprazole-induced dysphagia. Int J Eat Disord. 2012;45(2):305-306. doi:10.1002/eat.20934
6. Stewart JT. Dysphagia associated with risperidone therapy. Dysphagia. 2003;18(4):274-275. doi:10.1007/s00455-003-0006-x
7. Lee JC, Takeshita J. Antipsychotic-induced dysphagia: a case report. Prim Care Companion CNS Disord. 2015;17(5):10.4088/PCC.15I01792. doi:10.4088/PCC.15I01792
More on AI-generated content
In his recent editorial (“A ‘guest editorial’ … generated by ChatGPT?”
Sara Hartley, MD
Berkeley, California
I just read the “guest editorial” generated by ChatGPT. Thank you for this article. Although this is truly an amazing advancement in artificial intelligence (AI), I feel this guest editorial was very basic. It did not read like scientific writing. It read more like it was written at an 11th- or 12th-grade level, though I am fully aware that the question was simple, and thus the answer was not very deep. I can’t deny that if I had been tested, chances are good I would have fallen among the 32% of my peers who would not have recognized it as AI. I appreciate that you (and your team) are working on a protocol regarding how to include content generated by or with the help of AI. God knows if (most likely, when) people with evil minds will use AI to spread false information that may dispute the accredited scientific data and research that guide the medical world and many other fields. I wonder if AI can serve as a search engine that is better or easier to use than PubMed (for example) and the other services we use for research and learning.
Alex Mustachi, PMHNP-BC
Suffern, New York
I wanted to let you know how much I enjoyed reading your recent editorial on AI and scientific writing. Sharing the 4 AI-generated “articles” with readers (“For artificial intelligence, the future is finally here,”
Martha Sajatovic, MD
Cleveland, Ohio
Continue to: The AI-generated samples...
The Al-generated samples were fascinating. As far as I superficially noted, the spelling, grammar, and punctuation were correct. That is better than one gets from most student compositions. However, the articles were completely lacking in depth or apparent insight. The article on anosognosia mentioned it can be present in up to 50% of cases of schizophrenia. In my experience, it is present in approximately 99.9% of cases. It clearly did not consider if anosognosia is also present in alcoholics, codependents, abusers, or people with bizarre political beliefs. But I guess the “intelligence” wasn’t asked that. The other samples also show shallow thinking and repetitive wording—pretty much like my high school junior compositions.
Maybe an appropriate use for AI is a task such as evaluating suicide notes. AI’s success causes one to feel nonplussed. Much more disconcerting was a recent news article that reported AI made up nonexistent references to a professor’s alleged sexual harassment, and then generated citations to its own made-up reference.1 That is indeed frightening new territory. How does one fight against a machine to clear their own name?
Linda Miller, NP
Harrisonburg, Virginia
References
1. Verma P, Oremus W. ChatGPT invented a sexual harassment scandal and named a real law prof as the accused. The Washington Post. April 5, 2023. Accessed May 8, 2023. https://www.washingtonpost.com/technology/2023/04/05/chatgpt-lies/
Thank you, Dr. Nasrallah, for your latest thought-provoking articles on AI. Time and again you provide the profession with cutting-edge, relevant food for thought. Caveat emptor, indeed.
Lawrence E. Cormier, MD
Denver, Colorado
Continue to: We read with interest...
We read with interest Dr. Nasrallah’s editorial that invited readers to share their take on the quality of an AI-generated writing sample. I (MZP) was a computational neuroscience major at Columbia University and was accepted to medical school in 2022 at age 19. I identify with the character traits common among many young tech entrepreneurs driving the AI revolution—social awkwardness; discomfort with subjective emotions; restricted areas of interest; algorithmic thinking; strict, naive idealism; and an obsession with data. To gain a deeper understanding of Sam Altman, the CEO of OpenAI (the company that created ChatGPT), we analyzed a 2.5-hour interview that MIT research scientist Lex Fridman conducted with Altman.1 As a result, we began to discern why AI-generated text feels so stiff and bland compared to the superior fluidity and expressiveness of human communication. As of now, the creation is a reflection of its creator.
Generally speaking, computer scientists are not warm and fuzzy types. Hence, ChatGPT strives to be neutral, accurate, and objective compared to more biased and fallible humans, and, consequently, its language lacks the emotive flair we have come to relish in normal human interactions. In the interview, Altman discusses several solutions that will soon raise the quality of ChatGPT’s currently deficient emotional quotient to approximate its superior IQ. Altruistically, Altman has opened ChatGPT to all, so we can freely interact and utilize its potential to increase our productivity exponentially. As a result, ChatGPT interfaces with millions of humans through RLHF (reinforcement learning from human feedback), which makes each iteration more in tune with our sensibilities.2 Another initiative Altman is undertaking is to depart his Silicon Valley bubble for a road trip to interact with “regular people” and gain a better sense of how to make ChatGPT more user-friendly.1
What’s so saddening about Dr. Nasrallah’s homework assignment is that he is asking us to evaluate with our mature adult standards an article that was written at the emotional stage of a child in early high school. But our hubris and complacency are entirely unfounded because ChatGPT is learning much faster than we ever could, and it will quickly surpass us all as it continues to evolve.
It is also quite disconcerting to hear how Altman is naively relying upon governmental regulation and corporate responsibility to manage the potential misuse of future artificial general intelligence for social, economic, and political control and upheaval. We know well the harmful effects of the internet and social media, particularly on our youth, yet our laws still lag far behind the fact that these technological innovations are simultaneously enhancing our knowledge while destroying our souls. As custodians of our world, dedicated to promoting and preserving mental well-being, we cannot wait much longer to intervene in properly parenting AI along its wisest developmental trajectory before it is too late.
Maxwell Zachary Price, BA
Nutley, New Jersey
Richard Louis Price, MD
New York, New York
References
1. Sam Altman: OpenAI CEO on GPT-4, ChatGPT, and the Future of AI. Lex Fridman Podcast #367. March 25, 2023. Accessed April 5, 2023. https://www.youtube.com/watch?v=L_Guz73e6fw
2. Heikkilä M. How OpenAI is trying to make ChatGPT safer and less biased. MIT Technology Review. Published February 21, 2023. Accessed April 5, 2023. https://www.technologyreview.com/2023/02/21/1068893/how-openai-is-trying-to-make-chatgpt-safer-and-less-biased/
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
In his recent editorial (“A ‘guest editorial’ … generated by ChatGPT?”
Sara Hartley, MD
Berkeley, California
I just read the “guest editorial” generated by ChatGPT. Thank you for this article. Although this is truly an amazing advancement in artificial intelligence (AI), I feel this guest editorial was very basic. It did not read like scientific writing. It read more like it was written at an 11th- or 12th-grade level, though I am fully aware that the question was simple, and thus the answer was not very deep. I can’t deny that if I had been tested, chances are good I would have fallen among the 32% of my peers who would not have recognized it as AI. I appreciate that you (and your team) are working on a protocol regarding how to include content generated by or with the help of AI. God knows if (most likely, when) people with evil minds will use AI to spread false information that may dispute the accredited scientific data and research that guide the medical world and many other fields. I wonder if AI can serve as a search engine that is better or easier to use than PubMed (for example) and the other services we use for research and learning.
Alex Mustachi, PMHNP-BC
Suffern, New York
I wanted to let you know how much I enjoyed reading your recent editorial on AI and scientific writing. Sharing the 4 AI-generated “articles” with readers (“For artificial intelligence, the future is finally here,”
Martha Sajatovic, MD
Cleveland, Ohio
Continue to: The AI-generated samples...
The Al-generated samples were fascinating. As far as I superficially noted, the spelling, grammar, and punctuation were correct. That is better than one gets from most student compositions. However, the articles were completely lacking in depth or apparent insight. The article on anosognosia mentioned it can be present in up to 50% of cases of schizophrenia. In my experience, it is present in approximately 99.9% of cases. It clearly did not consider if anosognosia is also present in alcoholics, codependents, abusers, or people with bizarre political beliefs. But I guess the “intelligence” wasn’t asked that. The other samples also show shallow thinking and repetitive wording—pretty much like my high school junior compositions.
Maybe an appropriate use for AI is a task such as evaluating suicide notes. AI’s success causes one to feel nonplussed. Much more disconcerting was a recent news article that reported AI made up nonexistent references to a professor’s alleged sexual harassment, and then generated citations to its own made-up reference.1 That is indeed frightening new territory. How does one fight against a machine to clear their own name?
Linda Miller, NP
Harrisonburg, Virginia
References
1. Verma P, Oremus W. ChatGPT invented a sexual harassment scandal and named a real law prof as the accused. The Washington Post. April 5, 2023. Accessed May 8, 2023. https://www.washingtonpost.com/technology/2023/04/05/chatgpt-lies/
Thank you, Dr. Nasrallah, for your latest thought-provoking articles on AI. Time and again you provide the profession with cutting-edge, relevant food for thought. Caveat emptor, indeed.
Lawrence E. Cormier, MD
Denver, Colorado
Continue to: We read with interest...
We read with interest Dr. Nasrallah’s editorial that invited readers to share their take on the quality of an AI-generated writing sample. I (MZP) was a computational neuroscience major at Columbia University and was accepted to medical school in 2022 at age 19. I identify with the character traits common among many young tech entrepreneurs driving the AI revolution—social awkwardness; discomfort with subjective emotions; restricted areas of interest; algorithmic thinking; strict, naive idealism; and an obsession with data. To gain a deeper understanding of Sam Altman, the CEO of OpenAI (the company that created ChatGPT), we analyzed a 2.5-hour interview that MIT research scientist Lex Fridman conducted with Altman.1 As a result, we began to discern why AI-generated text feels so stiff and bland compared to the superior fluidity and expressiveness of human communication. As of now, the creation is a reflection of its creator.
Generally speaking, computer scientists are not warm and fuzzy types. Hence, ChatGPT strives to be neutral, accurate, and objective compared to more biased and fallible humans, and, consequently, its language lacks the emotive flair we have come to relish in normal human interactions. In the interview, Altman discusses several solutions that will soon raise the quality of ChatGPT’s currently deficient emotional quotient to approximate its superior IQ. Altruistically, Altman has opened ChatGPT to all, so we can freely interact and utilize its potential to increase our productivity exponentially. As a result, ChatGPT interfaces with millions of humans through RLHF (reinforcement learning from human feedback), which makes each iteration more in tune with our sensibilities.2 Another initiative Altman is undertaking is to depart his Silicon Valley bubble for a road trip to interact with “regular people” and gain a better sense of how to make ChatGPT more user-friendly.1
What’s so saddening about Dr. Nasrallah’s homework assignment is that he is asking us to evaluate with our mature adult standards an article that was written at the emotional stage of a child in early high school. But our hubris and complacency are entirely unfounded because ChatGPT is learning much faster than we ever could, and it will quickly surpass us all as it continues to evolve.
It is also quite disconcerting to hear how Altman is naively relying upon governmental regulation and corporate responsibility to manage the potential misuse of future artificial general intelligence for social, economic, and political control and upheaval. We know well the harmful effects of the internet and social media, particularly on our youth, yet our laws still lag far behind the fact that these technological innovations are simultaneously enhancing our knowledge while destroying our souls. As custodians of our world, dedicated to promoting and preserving mental well-being, we cannot wait much longer to intervene in properly parenting AI along its wisest developmental trajectory before it is too late.
Maxwell Zachary Price, BA
Nutley, New Jersey
Richard Louis Price, MD
New York, New York
References
1. Sam Altman: OpenAI CEO on GPT-4, ChatGPT, and the Future of AI. Lex Fridman Podcast #367. March 25, 2023. Accessed April 5, 2023. https://www.youtube.com/watch?v=L_Guz73e6fw
2. Heikkilä M. How OpenAI is trying to make ChatGPT safer and less biased. MIT Technology Review. Published February 21, 2023. Accessed April 5, 2023. https://www.technologyreview.com/2023/02/21/1068893/how-openai-is-trying-to-make-chatgpt-safer-and-less-biased/
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
In his recent editorial (“A ‘guest editorial’ … generated by ChatGPT?”
Sara Hartley, MD
Berkeley, California
I just read the “guest editorial” generated by ChatGPT. Thank you for this article. Although this is truly an amazing advancement in artificial intelligence (AI), I feel this guest editorial was very basic. It did not read like scientific writing. It read more like it was written at an 11th- or 12th-grade level, though I am fully aware that the question was simple, and thus the answer was not very deep. I can’t deny that if I had been tested, chances are good I would have fallen among the 32% of my peers who would not have recognized it as AI. I appreciate that you (and your team) are working on a protocol regarding how to include content generated by or with the help of AI. God knows if (most likely, when) people with evil minds will use AI to spread false information that may dispute the accredited scientific data and research that guide the medical world and many other fields. I wonder if AI can serve as a search engine that is better or easier to use than PubMed (for example) and the other services we use for research and learning.
Alex Mustachi, PMHNP-BC
Suffern, New York
I wanted to let you know how much I enjoyed reading your recent editorial on AI and scientific writing. Sharing the 4 AI-generated “articles” with readers (“For artificial intelligence, the future is finally here,”
Martha Sajatovic, MD
Cleveland, Ohio
Continue to: The AI-generated samples...
The Al-generated samples were fascinating. As far as I superficially noted, the spelling, grammar, and punctuation were correct. That is better than one gets from most student compositions. However, the articles were completely lacking in depth or apparent insight. The article on anosognosia mentioned it can be present in up to 50% of cases of schizophrenia. In my experience, it is present in approximately 99.9% of cases. It clearly did not consider if anosognosia is also present in alcoholics, codependents, abusers, or people with bizarre political beliefs. But I guess the “intelligence” wasn’t asked that. The other samples also show shallow thinking and repetitive wording—pretty much like my high school junior compositions.
Maybe an appropriate use for AI is a task such as evaluating suicide notes. AI’s success causes one to feel nonplussed. Much more disconcerting was a recent news article that reported AI made up nonexistent references to a professor’s alleged sexual harassment, and then generated citations to its own made-up reference.1 That is indeed frightening new territory. How does one fight against a machine to clear their own name?
Linda Miller, NP
Harrisonburg, Virginia
References
1. Verma P, Oremus W. ChatGPT invented a sexual harassment scandal and named a real law prof as the accused. The Washington Post. April 5, 2023. Accessed May 8, 2023. https://www.washingtonpost.com/technology/2023/04/05/chatgpt-lies/
Thank you, Dr. Nasrallah, for your latest thought-provoking articles on AI. Time and again you provide the profession with cutting-edge, relevant food for thought. Caveat emptor, indeed.
Lawrence E. Cormier, MD
Denver, Colorado
Continue to: We read with interest...
We read with interest Dr. Nasrallah’s editorial that invited readers to share their take on the quality of an AI-generated writing sample. I (MZP) was a computational neuroscience major at Columbia University and was accepted to medical school in 2022 at age 19. I identify with the character traits common among many young tech entrepreneurs driving the AI revolution—social awkwardness; discomfort with subjective emotions; restricted areas of interest; algorithmic thinking; strict, naive idealism; and an obsession with data. To gain a deeper understanding of Sam Altman, the CEO of OpenAI (the company that created ChatGPT), we analyzed a 2.5-hour interview that MIT research scientist Lex Fridman conducted with Altman.1 As a result, we began to discern why AI-generated text feels so stiff and bland compared to the superior fluidity and expressiveness of human communication. As of now, the creation is a reflection of its creator.
Generally speaking, computer scientists are not warm and fuzzy types. Hence, ChatGPT strives to be neutral, accurate, and objective compared to more biased and fallible humans, and, consequently, its language lacks the emotive flair we have come to relish in normal human interactions. In the interview, Altman discusses several solutions that will soon raise the quality of ChatGPT’s currently deficient emotional quotient to approximate its superior IQ. Altruistically, Altman has opened ChatGPT to all, so we can freely interact and utilize its potential to increase our productivity exponentially. As a result, ChatGPT interfaces with millions of humans through RLHF (reinforcement learning from human feedback), which makes each iteration more in tune with our sensibilities.2 Another initiative Altman is undertaking is to depart his Silicon Valley bubble for a road trip to interact with “regular people” and gain a better sense of how to make ChatGPT more user-friendly.1
What’s so saddening about Dr. Nasrallah’s homework assignment is that he is asking us to evaluate with our mature adult standards an article that was written at the emotional stage of a child in early high school. But our hubris and complacency are entirely unfounded because ChatGPT is learning much faster than we ever could, and it will quickly surpass us all as it continues to evolve.
It is also quite disconcerting to hear how Altman is naively relying upon governmental regulation and corporate responsibility to manage the potential misuse of future artificial general intelligence for social, economic, and political control and upheaval. We know well the harmful effects of the internet and social media, particularly on our youth, yet our laws still lag far behind the fact that these technological innovations are simultaneously enhancing our knowledge while destroying our souls. As custodians of our world, dedicated to promoting and preserving mental well-being, we cannot wait much longer to intervene in properly parenting AI along its wisest developmental trajectory before it is too late.
Maxwell Zachary Price, BA
Nutley, New Jersey
Richard Louis Price, MD
New York, New York
References
1. Sam Altman: OpenAI CEO on GPT-4, ChatGPT, and the Future of AI. Lex Fridman Podcast #367. March 25, 2023. Accessed April 5, 2023. https://www.youtube.com/watch?v=L_Guz73e6fw
2. Heikkilä M. How OpenAI is trying to make ChatGPT safer and less biased. MIT Technology Review. Published February 21, 2023. Accessed April 5, 2023. https://www.technologyreview.com/2023/02/21/1068893/how-openai-is-trying-to-make-chatgpt-safer-and-less-biased/
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
Brooklyn gastroenterologist: Good listening skills make a doctor a better teacher, person
After the 2004 Indian Ocean earthquake and tsunami, he traveled to his home country of Sri Lanka to help people who were in need and establish an orphanage. He has applied his skills as a gastroenterologist in the U.S. military and the New York City Police Department.
He was in New York during the 9-11 terrorist attacks. To this day, he treats patients with residual GI problems and precancerous changes associated with 9-11. “I’m involved in screening those people who were the first responders referred by the NYPD,” he says.
This year Dr. Iswara earned the Distinguished Clinician Award in Private Practice from the American Gastroenterological Association. “He puts his patients first in every endeavor – and every question that he asks with regards to research and education is linked to the ultimate measuring stick of improving patient care,” according to an AGA announcement of the award.
When dealing with patients and colleagues, he offers this simple pearl of advice: Listen and then listen some more.
“Once you listen more, you can find out their issues much more in depth, and you can give a satisfying answer to them and their problems. Listening is a kindness and a compassionate thing. It not only makes you become a better teacher, but a better person,” said Dr. Iswara, attending gastroenterologist at Maimonides Medical Center in New York.
In an interview, he talked more in depth about his GI beginnings, his role as a mentor, and why he always starts the day with a prayer. He also confided about the useful time management habit kept from his military days that gives him energy.
Question: What gives you joy in day-to-day practice?
Dr. Iswara: One of the main joys is my colleagues, coworkers, fellows, and my patients. The patients come No. 1. As I walk into my practice area or in the hospital, there is a sense of inner happiness in my mind to see the smiles of the patients and the greetings I get from the patients and all the coworkers. I also see smiling patients with anxiety in their face, trying to get my attention to take care of them.
After I see the patient, I change to a different mode, a kind of a professional mode to give the best to the people whom I’m caring for, who are trusting me with their lives.
One thing I do in my mind before I even start the day, I do a silent prayer to guide me, to give compassionate care and safe care. I will not harm anyone who is depending on my care.
Q: Who was your mentor?
Dr. Iswara: I was lucky enough to have been trained by Baroukh El Kodsi, MD, at Maimonides Medical Center. He recently passed away and was a legend in Brooklyn. I was his first-generation trainee, and I was able to pass on my skills to my trainees. Now so many people who are in Brooklyn; they were trained by me, so it’s kind of growth by generations.
When I finished the training with Dr. Kodsi, he hired me as an associate director of the GI department at Maimonides. I became the program director, then division chief, then I became a director of advanced endoscopy. All these gastroenterology procedures started after 1975 while I was doing the training, so I was one of the pioneers to bring all this new technology to our hospital. I’m still involved in fellowship education.
Q: Can we talk more about your accomplishments? Perhaps you can discuss your AGA award and what you received it for.
Dr. Iswara: I’m humbled and honored by this role, and I’ll be forever grateful to AGA for this prestigious honor at the late stage of my career.
I have been a continuous AGA member for the last 45 years. I probably have one of the longest durations of being an actively practicing gastroenterologist in Brooklyn. I’ve also done academic work, teaching so many young gastroenterologists, motivating several of them to become leading gastroenterologists.
Q: If you could describe a scene of your vision for the future, what it would it be in terms of how gastroenterology is practiced?
Dr. Iswara: I’d like to see the newer generation practice more of a clinical medicine than technical medicine. Sometimes when I see the young people, they sit in front of the computer more than talking and touching the patient. There has to be some sort of a balance where the newer people should be taught more bedside personal care, touching the patient, looking at the patient’s face. They are kind of under pressure to write longer notes than to examine the patient, so I think this has to change.
Q: Describe how you would spend a free Saturday afternoon.
Dr. Iswara: When I was in the military, I was told that to prevent battle fatigue you had to take a rest. I really try to take a rest almost 2 hours every day in the daytime. This rejuvenates me.
We live in New York, and I love to go to shows, especially magic shows. I love magic and illusion.
On free Saturday evenings, I also spend time with my grandchildren in the city, watching them in their baseball, soccer, swimming, and other activities. I love to spend time with them.
Lightning round
Texting or talking?
Texting
Favorite city in the U.S. besides the one you live?
Naples, Fla.
Favorite breakfast?
Pancakes
Dark Chocolate or milk chocolate?
Cadbury from England
Last movie you watched?
“To Sir, With Love”
After the 2004 Indian Ocean earthquake and tsunami, he traveled to his home country of Sri Lanka to help people who were in need and establish an orphanage. He has applied his skills as a gastroenterologist in the U.S. military and the New York City Police Department.
He was in New York during the 9-11 terrorist attacks. To this day, he treats patients with residual GI problems and precancerous changes associated with 9-11. “I’m involved in screening those people who were the first responders referred by the NYPD,” he says.
This year Dr. Iswara earned the Distinguished Clinician Award in Private Practice from the American Gastroenterological Association. “He puts his patients first in every endeavor – and every question that he asks with regards to research and education is linked to the ultimate measuring stick of improving patient care,” according to an AGA announcement of the award.
When dealing with patients and colleagues, he offers this simple pearl of advice: Listen and then listen some more.
“Once you listen more, you can find out their issues much more in depth, and you can give a satisfying answer to them and their problems. Listening is a kindness and a compassionate thing. It not only makes you become a better teacher, but a better person,” said Dr. Iswara, attending gastroenterologist at Maimonides Medical Center in New York.
In an interview, he talked more in depth about his GI beginnings, his role as a mentor, and why he always starts the day with a prayer. He also confided about the useful time management habit kept from his military days that gives him energy.
Question: What gives you joy in day-to-day practice?
Dr. Iswara: One of the main joys is my colleagues, coworkers, fellows, and my patients. The patients come No. 1. As I walk into my practice area or in the hospital, there is a sense of inner happiness in my mind to see the smiles of the patients and the greetings I get from the patients and all the coworkers. I also see smiling patients with anxiety in their face, trying to get my attention to take care of them.
After I see the patient, I change to a different mode, a kind of a professional mode to give the best to the people whom I’m caring for, who are trusting me with their lives.
One thing I do in my mind before I even start the day, I do a silent prayer to guide me, to give compassionate care and safe care. I will not harm anyone who is depending on my care.
Q: Who was your mentor?
Dr. Iswara: I was lucky enough to have been trained by Baroukh El Kodsi, MD, at Maimonides Medical Center. He recently passed away and was a legend in Brooklyn. I was his first-generation trainee, and I was able to pass on my skills to my trainees. Now so many people who are in Brooklyn; they were trained by me, so it’s kind of growth by generations.
When I finished the training with Dr. Kodsi, he hired me as an associate director of the GI department at Maimonides. I became the program director, then division chief, then I became a director of advanced endoscopy. All these gastroenterology procedures started after 1975 while I was doing the training, so I was one of the pioneers to bring all this new technology to our hospital. I’m still involved in fellowship education.
Q: Can we talk more about your accomplishments? Perhaps you can discuss your AGA award and what you received it for.
Dr. Iswara: I’m humbled and honored by this role, and I’ll be forever grateful to AGA for this prestigious honor at the late stage of my career.
I have been a continuous AGA member for the last 45 years. I probably have one of the longest durations of being an actively practicing gastroenterologist in Brooklyn. I’ve also done academic work, teaching so many young gastroenterologists, motivating several of them to become leading gastroenterologists.
Q: If you could describe a scene of your vision for the future, what it would it be in terms of how gastroenterology is practiced?
Dr. Iswara: I’d like to see the newer generation practice more of a clinical medicine than technical medicine. Sometimes when I see the young people, they sit in front of the computer more than talking and touching the patient. There has to be some sort of a balance where the newer people should be taught more bedside personal care, touching the patient, looking at the patient’s face. They are kind of under pressure to write longer notes than to examine the patient, so I think this has to change.
Q: Describe how you would spend a free Saturday afternoon.
Dr. Iswara: When I was in the military, I was told that to prevent battle fatigue you had to take a rest. I really try to take a rest almost 2 hours every day in the daytime. This rejuvenates me.
We live in New York, and I love to go to shows, especially magic shows. I love magic and illusion.
On free Saturday evenings, I also spend time with my grandchildren in the city, watching them in their baseball, soccer, swimming, and other activities. I love to spend time with them.
Lightning round
Texting or talking?
Texting
Favorite city in the U.S. besides the one you live?
Naples, Fla.
Favorite breakfast?
Pancakes
Dark Chocolate or milk chocolate?
Cadbury from England
Last movie you watched?
“To Sir, With Love”
After the 2004 Indian Ocean earthquake and tsunami, he traveled to his home country of Sri Lanka to help people who were in need and establish an orphanage. He has applied his skills as a gastroenterologist in the U.S. military and the New York City Police Department.
He was in New York during the 9-11 terrorist attacks. To this day, he treats patients with residual GI problems and precancerous changes associated with 9-11. “I’m involved in screening those people who were the first responders referred by the NYPD,” he says.
This year Dr. Iswara earned the Distinguished Clinician Award in Private Practice from the American Gastroenterological Association. “He puts his patients first in every endeavor – and every question that he asks with regards to research and education is linked to the ultimate measuring stick of improving patient care,” according to an AGA announcement of the award.
When dealing with patients and colleagues, he offers this simple pearl of advice: Listen and then listen some more.
“Once you listen more, you can find out their issues much more in depth, and you can give a satisfying answer to them and their problems. Listening is a kindness and a compassionate thing. It not only makes you become a better teacher, but a better person,” said Dr. Iswara, attending gastroenterologist at Maimonides Medical Center in New York.
In an interview, he talked more in depth about his GI beginnings, his role as a mentor, and why he always starts the day with a prayer. He also confided about the useful time management habit kept from his military days that gives him energy.
Question: What gives you joy in day-to-day practice?
Dr. Iswara: One of the main joys is my colleagues, coworkers, fellows, and my patients. The patients come No. 1. As I walk into my practice area or in the hospital, there is a sense of inner happiness in my mind to see the smiles of the patients and the greetings I get from the patients and all the coworkers. I also see smiling patients with anxiety in their face, trying to get my attention to take care of them.
After I see the patient, I change to a different mode, a kind of a professional mode to give the best to the people whom I’m caring for, who are trusting me with their lives.
One thing I do in my mind before I even start the day, I do a silent prayer to guide me, to give compassionate care and safe care. I will not harm anyone who is depending on my care.
Q: Who was your mentor?
Dr. Iswara: I was lucky enough to have been trained by Baroukh El Kodsi, MD, at Maimonides Medical Center. He recently passed away and was a legend in Brooklyn. I was his first-generation trainee, and I was able to pass on my skills to my trainees. Now so many people who are in Brooklyn; they were trained by me, so it’s kind of growth by generations.
When I finished the training with Dr. Kodsi, he hired me as an associate director of the GI department at Maimonides. I became the program director, then division chief, then I became a director of advanced endoscopy. All these gastroenterology procedures started after 1975 while I was doing the training, so I was one of the pioneers to bring all this new technology to our hospital. I’m still involved in fellowship education.
Q: Can we talk more about your accomplishments? Perhaps you can discuss your AGA award and what you received it for.
Dr. Iswara: I’m humbled and honored by this role, and I’ll be forever grateful to AGA for this prestigious honor at the late stage of my career.
I have been a continuous AGA member for the last 45 years. I probably have one of the longest durations of being an actively practicing gastroenterologist in Brooklyn. I’ve also done academic work, teaching so many young gastroenterologists, motivating several of them to become leading gastroenterologists.
Q: If you could describe a scene of your vision for the future, what it would it be in terms of how gastroenterology is practiced?
Dr. Iswara: I’d like to see the newer generation practice more of a clinical medicine than technical medicine. Sometimes when I see the young people, they sit in front of the computer more than talking and touching the patient. There has to be some sort of a balance where the newer people should be taught more bedside personal care, touching the patient, looking at the patient’s face. They are kind of under pressure to write longer notes than to examine the patient, so I think this has to change.
Q: Describe how you would spend a free Saturday afternoon.
Dr. Iswara: When I was in the military, I was told that to prevent battle fatigue you had to take a rest. I really try to take a rest almost 2 hours every day in the daytime. This rejuvenates me.
We live in New York, and I love to go to shows, especially magic shows. I love magic and illusion.
On free Saturday evenings, I also spend time with my grandchildren in the city, watching them in their baseball, soccer, swimming, and other activities. I love to spend time with them.
Lightning round
Texting or talking?
Texting
Favorite city in the U.S. besides the one you live?
Naples, Fla.
Favorite breakfast?
Pancakes
Dark Chocolate or milk chocolate?
Cadbury from England
Last movie you watched?
“To Sir, With Love”
Noninvasive Methods for the Diagnosis of Endometriosis
What is the value of considering noninvasive methods for the diagnosis of endometriosis?
Dr. Flores: There is great value in noninvasive diagnostics for endometriosis. This is because while surgical diagnosis is the “gold standard,” surgery is invasive, and waiting until a surgical diagnosis can be made further contributes to delays in diagnosis. However, more recently there has been a shift toward utilizing noninvasive approaches to the diagnosis of endometriosis, with the primary one focusing on clinically diagnosing endometriosis.
One of the first things to remember is the importance of gathering a patient history and conducting a physical exam. We've all learned this in medical school, and it comes into play even more so with a condition such as endometriosis. Endometriosis is defined as a benign gynecologic disease characterized by endometrial-like tissue outside of the uterus, but this definition does not reflect the true scope and manifestations of endometriosis. Research over the years has demonstrated that endometriosis has systemic effects—affecting regions of the brain associated with anxiety/depression, altering pain sensitization, and having inflammatory effects that can not only affect the reproductive organs but also other organ systems. As such, our questions when evaluating patients for endometriosis need to focus on these various aspects of the disease.
Endometriosis usually leads to cyclic pain. This is because just as the lining of the uterus (the endometrium) grows and sheds every month in response to hormones, endometriotic lesions—which are endometrial-like tissue outside of the uterus—also grow and shed each month. However, there is no outflow for this shed tissue and, as a result, there is an inflammatory response as well as pain. Depending on where those lesions implant, symptoms can include not only cyclic pelvic pain but also cyclic bowel/bladder pain. I’ve also had patients complain of cyclic sharp/shooting leg pain.
Many times, patients present to us after having seen several different types of providers and having been diagnosed with conditions such as irritable bowel syndrome or painful bladder syndrome. However, if you talk to patients and ask them to tell you a little bit more about this bowel or bladder pain, they will frequently endorse that their symptoms are cyclic/most severe during their menses. With respect to pelvic pain, endometriosis-related pelvic pain is usually progressive—becoming progressively more painful over the years. These symptoms are strong indicators that endometriosis is the cause. A pelvic exam is also helpful as findings of nodularity or a fixed uterus may lend further support for endometriosis; a normal exam, however, does not rule out endometriosis.
What are the primary imaging techniques used to diagnose endometriosis?
Dr. Flores: While history and physical exam are the primary components of the clinical diagnosis, imaging can also be helpful. The 2 techniques most often used are pelvic ultrasound and magnetic resonance imaging (MRI).
While transvaginal ultrasound is sensitive and specific for diagnosing endometriomas (ovarian cysts of endometriotic tissue) and may also be able to accurately identify deep-infiltrating endometriosis, it is limited in its ability to visualize peritoneal disease. MRI can improve diagnosis of endometriosis and better estimate the depth of invasion of deep-infiltrating disease, as well as confirm diagnosis of an endometrioma. While MRI is an option for peritoneal endometriosis, superficial disease is usually not detected. Lastly, computed tomography imaging of the chest can be used when thoracic endometriosis is suspected but is otherwise not routinely recommended. Imaging is also helpful in ruling in/out other potential etiologies of pelvic pain such as fibroids and adenomyosis. It is important to recognize, however, that the absence of any findings of endometriosis on imaging does not rule out the disease.
What other best practices do you implement in your day-to-day to aid in diagnosis?
Dr. Flores: Take the time to listen to your patient. Often, they’ve seen several providers before ultimately seeing a provider who can diagnosis their endometriosis without the need for surgical evaluation. We have to ask questions related to their pain and when the pain occurs, and we can’t forget to also ask about pain during intercourse, as well as non-menstrual pelvic pain. Additionally, it is important to recognize that, for patients who may have been suffering from endometriosis for several years before reaching a diagnosis, they may present with chronic pelvic pain. In this case, it is important to ask what their menstrual cycles were like before the pelvic pain became chronic, and usually patients note cyclic pelvic pain that became progressive. We also know that patients who have a first-degree relative with endometriosis are 7 times more likely to be affected by the disease, so asking about a family history of endometriosis is important.
We have to think about endometriosis as a systemic disease. Previously, endometriosis was incorrectly thought of as solely a pelvic disease, but we've been learning more and more through research that it truly is a chronic, systemic disease with multifactorial effects throughout the body. For example, we have found that endometriosis affects regions of the brain associated with anxiety and depression, as well as causing changes in metabolism. For example, a common misconception is that women with a low body mass index (BMI) were at risk for endometriosis, when in fact it's just the opposite—it is the endometriosis that is causing changes in metabolism that lead to a decreased BMI. Patients with endometriosis also frequently struggle with mood disorders; therefore, we cannot dismiss this aspect of the disease process. It is imperative that we help patients feel heard and let them know that some of the mood symptoms they are experiencing may be related to their endometriosis. Expanding our view of endometriosis as a disease that extends beyond the pelvis and thinking about the systemic effects of endometriosis is key.
We have also identified small molecules (microRNAs) that are predictive of endometriosis. They are continuing to be investigated as a noninvasive biomarker of endometriosis.
Can you talk a little more about these biomarkers?
Dr. Flores: In terms of biomarkers, this is actually some exciting work I was fortunate to be involved in with Dr. Hugh Taylor at Yale. We studied circulating molecules known as microRNAs—these are small, noncoding RNAs that can modify gene expression. In endometriosis, we've identified several that, when combined, have a high sensitivity and specificity for diagnosing endometriosis. These specific microRNAs are undergoing continued studies to ensure that they are reliable in predicting endometriosis. Hopefully they will be available soon for clinical use, as this would be of great value to help shorten the time to diagnosis of endometriosis and ultimately avoid delays in endometriosis treatment.
What is the value of considering noninvasive methods for the diagnosis of endometriosis?
Dr. Flores: There is great value in noninvasive diagnostics for endometriosis. This is because while surgical diagnosis is the “gold standard,” surgery is invasive, and waiting until a surgical diagnosis can be made further contributes to delays in diagnosis. However, more recently there has been a shift toward utilizing noninvasive approaches to the diagnosis of endometriosis, with the primary one focusing on clinically diagnosing endometriosis.
One of the first things to remember is the importance of gathering a patient history and conducting a physical exam. We've all learned this in medical school, and it comes into play even more so with a condition such as endometriosis. Endometriosis is defined as a benign gynecologic disease characterized by endometrial-like tissue outside of the uterus, but this definition does not reflect the true scope and manifestations of endometriosis. Research over the years has demonstrated that endometriosis has systemic effects—affecting regions of the brain associated with anxiety/depression, altering pain sensitization, and having inflammatory effects that can not only affect the reproductive organs but also other organ systems. As such, our questions when evaluating patients for endometriosis need to focus on these various aspects of the disease.
Endometriosis usually leads to cyclic pain. This is because just as the lining of the uterus (the endometrium) grows and sheds every month in response to hormones, endometriotic lesions—which are endometrial-like tissue outside of the uterus—also grow and shed each month. However, there is no outflow for this shed tissue and, as a result, there is an inflammatory response as well as pain. Depending on where those lesions implant, symptoms can include not only cyclic pelvic pain but also cyclic bowel/bladder pain. I’ve also had patients complain of cyclic sharp/shooting leg pain.
Many times, patients present to us after having seen several different types of providers and having been diagnosed with conditions such as irritable bowel syndrome or painful bladder syndrome. However, if you talk to patients and ask them to tell you a little bit more about this bowel or bladder pain, they will frequently endorse that their symptoms are cyclic/most severe during their menses. With respect to pelvic pain, endometriosis-related pelvic pain is usually progressive—becoming progressively more painful over the years. These symptoms are strong indicators that endometriosis is the cause. A pelvic exam is also helpful as findings of nodularity or a fixed uterus may lend further support for endometriosis; a normal exam, however, does not rule out endometriosis.
What are the primary imaging techniques used to diagnose endometriosis?
Dr. Flores: While history and physical exam are the primary components of the clinical diagnosis, imaging can also be helpful. The 2 techniques most often used are pelvic ultrasound and magnetic resonance imaging (MRI).
While transvaginal ultrasound is sensitive and specific for diagnosing endometriomas (ovarian cysts of endometriotic tissue) and may also be able to accurately identify deep-infiltrating endometriosis, it is limited in its ability to visualize peritoneal disease. MRI can improve diagnosis of endometriosis and better estimate the depth of invasion of deep-infiltrating disease, as well as confirm diagnosis of an endometrioma. While MRI is an option for peritoneal endometriosis, superficial disease is usually not detected. Lastly, computed tomography imaging of the chest can be used when thoracic endometriosis is suspected but is otherwise not routinely recommended. Imaging is also helpful in ruling in/out other potential etiologies of pelvic pain such as fibroids and adenomyosis. It is important to recognize, however, that the absence of any findings of endometriosis on imaging does not rule out the disease.
What other best practices do you implement in your day-to-day to aid in diagnosis?
Dr. Flores: Take the time to listen to your patient. Often, they’ve seen several providers before ultimately seeing a provider who can diagnosis their endometriosis without the need for surgical evaluation. We have to ask questions related to their pain and when the pain occurs, and we can’t forget to also ask about pain during intercourse, as well as non-menstrual pelvic pain. Additionally, it is important to recognize that, for patients who may have been suffering from endometriosis for several years before reaching a diagnosis, they may present with chronic pelvic pain. In this case, it is important to ask what their menstrual cycles were like before the pelvic pain became chronic, and usually patients note cyclic pelvic pain that became progressive. We also know that patients who have a first-degree relative with endometriosis are 7 times more likely to be affected by the disease, so asking about a family history of endometriosis is important.
We have to think about endometriosis as a systemic disease. Previously, endometriosis was incorrectly thought of as solely a pelvic disease, but we've been learning more and more through research that it truly is a chronic, systemic disease with multifactorial effects throughout the body. For example, we have found that endometriosis affects regions of the brain associated with anxiety and depression, as well as causing changes in metabolism. For example, a common misconception is that women with a low body mass index (BMI) were at risk for endometriosis, when in fact it's just the opposite—it is the endometriosis that is causing changes in metabolism that lead to a decreased BMI. Patients with endometriosis also frequently struggle with mood disorders; therefore, we cannot dismiss this aspect of the disease process. It is imperative that we help patients feel heard and let them know that some of the mood symptoms they are experiencing may be related to their endometriosis. Expanding our view of endometriosis as a disease that extends beyond the pelvis and thinking about the systemic effects of endometriosis is key.
We have also identified small molecules (microRNAs) that are predictive of endometriosis. They are continuing to be investigated as a noninvasive biomarker of endometriosis.
Can you talk a little more about these biomarkers?
Dr. Flores: In terms of biomarkers, this is actually some exciting work I was fortunate to be involved in with Dr. Hugh Taylor at Yale. We studied circulating molecules known as microRNAs—these are small, noncoding RNAs that can modify gene expression. In endometriosis, we've identified several that, when combined, have a high sensitivity and specificity for diagnosing endometriosis. These specific microRNAs are undergoing continued studies to ensure that they are reliable in predicting endometriosis. Hopefully they will be available soon for clinical use, as this would be of great value to help shorten the time to diagnosis of endometriosis and ultimately avoid delays in endometriosis treatment.
What is the value of considering noninvasive methods for the diagnosis of endometriosis?
Dr. Flores: There is great value in noninvasive diagnostics for endometriosis. This is because while surgical diagnosis is the “gold standard,” surgery is invasive, and waiting until a surgical diagnosis can be made further contributes to delays in diagnosis. However, more recently there has been a shift toward utilizing noninvasive approaches to the diagnosis of endometriosis, with the primary one focusing on clinically diagnosing endometriosis.
One of the first things to remember is the importance of gathering a patient history and conducting a physical exam. We've all learned this in medical school, and it comes into play even more so with a condition such as endometriosis. Endometriosis is defined as a benign gynecologic disease characterized by endometrial-like tissue outside of the uterus, but this definition does not reflect the true scope and manifestations of endometriosis. Research over the years has demonstrated that endometriosis has systemic effects—affecting regions of the brain associated with anxiety/depression, altering pain sensitization, and having inflammatory effects that can not only affect the reproductive organs but also other organ systems. As such, our questions when evaluating patients for endometriosis need to focus on these various aspects of the disease.
Endometriosis usually leads to cyclic pain. This is because just as the lining of the uterus (the endometrium) grows and sheds every month in response to hormones, endometriotic lesions—which are endometrial-like tissue outside of the uterus—also grow and shed each month. However, there is no outflow for this shed tissue and, as a result, there is an inflammatory response as well as pain. Depending on where those lesions implant, symptoms can include not only cyclic pelvic pain but also cyclic bowel/bladder pain. I’ve also had patients complain of cyclic sharp/shooting leg pain.
Many times, patients present to us after having seen several different types of providers and having been diagnosed with conditions such as irritable bowel syndrome or painful bladder syndrome. However, if you talk to patients and ask them to tell you a little bit more about this bowel or bladder pain, they will frequently endorse that their symptoms are cyclic/most severe during their menses. With respect to pelvic pain, endometriosis-related pelvic pain is usually progressive—becoming progressively more painful over the years. These symptoms are strong indicators that endometriosis is the cause. A pelvic exam is also helpful as findings of nodularity or a fixed uterus may lend further support for endometriosis; a normal exam, however, does not rule out endometriosis.
What are the primary imaging techniques used to diagnose endometriosis?
Dr. Flores: While history and physical exam are the primary components of the clinical diagnosis, imaging can also be helpful. The 2 techniques most often used are pelvic ultrasound and magnetic resonance imaging (MRI).
While transvaginal ultrasound is sensitive and specific for diagnosing endometriomas (ovarian cysts of endometriotic tissue) and may also be able to accurately identify deep-infiltrating endometriosis, it is limited in its ability to visualize peritoneal disease. MRI can improve diagnosis of endometriosis and better estimate the depth of invasion of deep-infiltrating disease, as well as confirm diagnosis of an endometrioma. While MRI is an option for peritoneal endometriosis, superficial disease is usually not detected. Lastly, computed tomography imaging of the chest can be used when thoracic endometriosis is suspected but is otherwise not routinely recommended. Imaging is also helpful in ruling in/out other potential etiologies of pelvic pain such as fibroids and adenomyosis. It is important to recognize, however, that the absence of any findings of endometriosis on imaging does not rule out the disease.
What other best practices do you implement in your day-to-day to aid in diagnosis?
Dr. Flores: Take the time to listen to your patient. Often, they’ve seen several providers before ultimately seeing a provider who can diagnosis their endometriosis without the need for surgical evaluation. We have to ask questions related to their pain and when the pain occurs, and we can’t forget to also ask about pain during intercourse, as well as non-menstrual pelvic pain. Additionally, it is important to recognize that, for patients who may have been suffering from endometriosis for several years before reaching a diagnosis, they may present with chronic pelvic pain. In this case, it is important to ask what their menstrual cycles were like before the pelvic pain became chronic, and usually patients note cyclic pelvic pain that became progressive. We also know that patients who have a first-degree relative with endometriosis are 7 times more likely to be affected by the disease, so asking about a family history of endometriosis is important.
We have to think about endometriosis as a systemic disease. Previously, endometriosis was incorrectly thought of as solely a pelvic disease, but we've been learning more and more through research that it truly is a chronic, systemic disease with multifactorial effects throughout the body. For example, we have found that endometriosis affects regions of the brain associated with anxiety and depression, as well as causing changes in metabolism. For example, a common misconception is that women with a low body mass index (BMI) were at risk for endometriosis, when in fact it's just the opposite—it is the endometriosis that is causing changes in metabolism that lead to a decreased BMI. Patients with endometriosis also frequently struggle with mood disorders; therefore, we cannot dismiss this aspect of the disease process. It is imperative that we help patients feel heard and let them know that some of the mood symptoms they are experiencing may be related to their endometriosis. Expanding our view of endometriosis as a disease that extends beyond the pelvis and thinking about the systemic effects of endometriosis is key.
We have also identified small molecules (microRNAs) that are predictive of endometriosis. They are continuing to be investigated as a noninvasive biomarker of endometriosis.
Can you talk a little more about these biomarkers?
Dr. Flores: In terms of biomarkers, this is actually some exciting work I was fortunate to be involved in with Dr. Hugh Taylor at Yale. We studied circulating molecules known as microRNAs—these are small, noncoding RNAs that can modify gene expression. In endometriosis, we've identified several that, when combined, have a high sensitivity and specificity for diagnosing endometriosis. These specific microRNAs are undergoing continued studies to ensure that they are reliable in predicting endometriosis. Hopefully they will be available soon for clinical use, as this would be of great value to help shorten the time to diagnosis of endometriosis and ultimately avoid delays in endometriosis treatment.
The AGA Research Foundation awards $2.66 million in research funding
The American Gastroenterological Association (AGA) is proud to announce the 71 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“This year’s recipients are determined to make an impact on digestive health care through their research,” said Michael Camilleri, MD, AGAF, chair, AGA Research Foundation. “We are honored to support these talented individuals at a critical stage in their careers and research projects. We look forward to seeing their great accomplishments.”

Treatment options for digestive diseases begin with vigorous research. The AGA Research Foundation supports medical investigators as they advance our understanding of gastrointestinal and liver conditions. The AGA Research Awards Program is made possible thanks to generous donors and funders. Learn more about the AGA Research Foundation at foundation.gastro.org.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Alexander Nguyen, MD, PhD, The Regent of the University of California, Los Angeles
Jeffrey W. Patterson-Fortin, MD, PhD, Dana-Farber Cancer Institute, Boston, Massachusetts
Sean Spencer, MD, PhD, Stanford Medicine, California
Ken Y. Hui, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, Maryland
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
Martina Molgora, PhD, Washington University School of Medicine, St. Louis, Missouri
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Brooke R. Druliner, PhD, Mayo Clinic, Rochester, Minnesota
Specialty Awards
AGA-Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Simon Schwörer, PhD, University of Chicago, Illinois
AGA-R. Robert & Sally Funderburg Research Award in Gastric Cancer
Bryson W. Katona, MD, PhD, University of Pennsylvania Perelman School of Medicine, Philadelphia
AGA-Amgen Fellowship-to-Faculty Transition Award
Cynthia Hsu, MD, PhD, University of California, San Diego
AGA-Bristol Myers Squibb Fellowship-to-Faculty Transition Award
Siyan Cao, MD, PhD, Washington University in St. Louis
Amit Ringel, MD, Brigham and Women’s Hospital, Boston, Massachusetts
Pilot Awards
AGA Pilot Research Award In Digestive Disease Health Disparities
Sharad Wadhwani, MD, MPH, University of California, San Francisco
AGA Pilot Research Award in Health Disparities
Enrique Soto Pérez de Celis, MD, PhD, MS, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán
AGA Pilot Research Award
Diana L. Snyder, MD, Mayo Clinic, Rochester, Minnesota
Michael Li, MD, MPH, University of California, San Francisco
Patricia Bloom, MD, University of Michigan, Ann Arbor
Edward Barnes, MD, MPH, University of North Carolina School of Medicine, Chapel Hill
AGA-Amgen Pilot Research Award In Digestive Disease Health Disparities
Laura Targownik, MD, MSHS, University of Toronto/Mount Sinai Hospital, Toronto, ON
Undergraduate Research Awards
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gwyneth Garramone, Loyola Marymount University, Los Angeles, California
Ella McLaren, University of California, San Diego
Nathan Moy, University of Southern California, Los Angeles
Hussein Elfayoumy, Johns Hopkins University, Baltimore, Maryland
Isabelle Garcia-Fischer, Tufts University, Medford, Massachusetts
Lidia Appell, University of New Mexico, Albuquerque
Katherine Burkman, Duke University, Durham, North Carolina
Alexa Boylan, Spelman College, Atlanta, Georgia
AGA-Dr. Harvey Young Education and Development Foundation’s Young Guts Scholar Program
Lucy Zhao, Massachusetts Institute of Technology Koch Institute for Integrative Cancer Research, Cambridge
Andrew Tran, Duke University, Durham, North Carolina
Sohaib Hassan, Rutgers University – Verzi Lab, New Brunswick, New Jersey
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor
Daniella Montalvo, University of Miami, Coral Gables, Florida
Sara Chough, Columbia University Irving Medical Center, New York, New York
Abstract Awards
Fellow Abstract Awards
David Flores Marin, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Jesse Platt, MD, PhD, Massachusetts General Hospital, Boston
Devika Gandhi, MD, Loma Linda University, California
Amanda Krause, MD, University of California, San Diego
Cynthia Tsay, MD, Mphil, Johns Hopkins Hospital, Baltimore, Maryland
Suha Abushamma, MD, Cleveland Clinic Foundation, Ohio
Md Obaidul Islam, PhD, University of Miami, Coral Gables, Florida
Sakteesh Gurunathan, MD, New York University School of Medicine, New York
Aaron Yeoh, MD, Stanford Hospital & Clinics, California
Yang Xiao, PhD, Mayo Clinic, Rochester, Minnesota
Jacques Gonzales, PhD, MS, Michigan State University, East Lansing
Kai Wang, MD, PhD, Harvard T.H. Chan School of Public Health, Cambridge, Massachusetts
Hoyeol Kim, PhD, Cedars Sinai Medical Center, New York, New York
Babajide Ojo, PhD, MS, Stanford University, California
AGA Fellow Abstract of the Year Award
Stefania Tocci, PhD, MS, University of Massachusetts, Cambridge
Student Abstract Awards
Pritha Chatterjee, MS, University of California, Riverside
Ela Contreras Panta, Vanderbilt University, Nashville, Tennessee
Mihir Shah, MD, MBBS, John H. Stroger Hospital of Cook County, Chicago, Illinois
Yuhan Fu, DO, Metrohealth Medical Center, Cleveland, Ohio
Raissa Nana Sede Mbakop, MD, Piedmont Athens Regional Medical Center, Athens, Georgia
Eleazar Montalvan-Sanchez, MD, Indiana University School of Medicine, Bloomington
Sarang Gupta, MD, St. Michael’s Hospital, Toronto, Ontario
Daniel Kim, Harvard Medical School, Cambridge, Massachusetts
Hannah Hrncir, Emory University, Decatur, Georgia
Zarwa Saqib, McMaster University, Hamilton, Ontario
Ying Zhu, MD, PhD, University of Michigan, Ann Arbor
Lizeth Cifuentes, MD, University of Pittsburgh Medical Center, Pennsylvania
Sharvani Dhandibhotla, MBBS, MS, Massachusetts General Hospital, Boston
Lauren Lynch, Baylor College of Medicine, Houston, Texas
AGA Student Abstract of The Year Award
Gabrielle Waclawik, MD, MPH, University of Wisconsin, Madison
AGA Abstract Award for Health Disparities Research
Soyoun Min, PhD, Lerner Research Institute (fellow), Cleveland, Ohio
Xiaobei Zhang, PhD , David Geffen School of Medicine at University of California, Los Angeles (fellow)
Matthew Zhao, David Geffen School of Medicine at University of California, Los Angeles (student)
Hannah Fiske, MD, Brown University/Rhode Island Hospital (student), Providence
AGA-APFED Abstract Award in Eosinophilic GI Diseases
Matthew Buendia, MD, Vanderbilt University Medical Center – Monroe Carell Jr. Children’s Hospital, Nashville, Tennessee
Alexandra L. Strauss, MD, University of Pennsylvania Health System, Philadelphia
Mira Yang, Northwestern Feinberg School of Medicine, Chicago, Illinois
AGA-Moti L. & Kamla Rustgi International Travel Award
Aviv Pudipeddi, MBBS, Concord Repatriation General Hospital, Sydney, Australia
Dianqin Sun, MBBS, Mmed, Erasmus University Medical Center, Rotterdam, Netherlands
The American Gastroenterological Association (AGA) is proud to announce the 71 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“This year’s recipients are determined to make an impact on digestive health care through their research,” said Michael Camilleri, MD, AGAF, chair, AGA Research Foundation. “We are honored to support these talented individuals at a critical stage in their careers and research projects. We look forward to seeing their great accomplishments.”

Treatment options for digestive diseases begin with vigorous research. The AGA Research Foundation supports medical investigators as they advance our understanding of gastrointestinal and liver conditions. The AGA Research Awards Program is made possible thanks to generous donors and funders. Learn more about the AGA Research Foundation at foundation.gastro.org.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Alexander Nguyen, MD, PhD, The Regent of the University of California, Los Angeles
Jeffrey W. Patterson-Fortin, MD, PhD, Dana-Farber Cancer Institute, Boston, Massachusetts
Sean Spencer, MD, PhD, Stanford Medicine, California
Ken Y. Hui, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, Maryland
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
Martina Molgora, PhD, Washington University School of Medicine, St. Louis, Missouri
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Brooke R. Druliner, PhD, Mayo Clinic, Rochester, Minnesota
Specialty Awards
AGA-Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Simon Schwörer, PhD, University of Chicago, Illinois
AGA-R. Robert & Sally Funderburg Research Award in Gastric Cancer
Bryson W. Katona, MD, PhD, University of Pennsylvania Perelman School of Medicine, Philadelphia
AGA-Amgen Fellowship-to-Faculty Transition Award
Cynthia Hsu, MD, PhD, University of California, San Diego
AGA-Bristol Myers Squibb Fellowship-to-Faculty Transition Award
Siyan Cao, MD, PhD, Washington University in St. Louis
Amit Ringel, MD, Brigham and Women’s Hospital, Boston, Massachusetts
Pilot Awards
AGA Pilot Research Award In Digestive Disease Health Disparities
Sharad Wadhwani, MD, MPH, University of California, San Francisco
AGA Pilot Research Award in Health Disparities
Enrique Soto Pérez de Celis, MD, PhD, MS, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán
AGA Pilot Research Award
Diana L. Snyder, MD, Mayo Clinic, Rochester, Minnesota
Michael Li, MD, MPH, University of California, San Francisco
Patricia Bloom, MD, University of Michigan, Ann Arbor
Edward Barnes, MD, MPH, University of North Carolina School of Medicine, Chapel Hill
AGA-Amgen Pilot Research Award In Digestive Disease Health Disparities
Laura Targownik, MD, MSHS, University of Toronto/Mount Sinai Hospital, Toronto, ON
Undergraduate Research Awards
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gwyneth Garramone, Loyola Marymount University, Los Angeles, California
Ella McLaren, University of California, San Diego
Nathan Moy, University of Southern California, Los Angeles
Hussein Elfayoumy, Johns Hopkins University, Baltimore, Maryland
Isabelle Garcia-Fischer, Tufts University, Medford, Massachusetts
Lidia Appell, University of New Mexico, Albuquerque
Katherine Burkman, Duke University, Durham, North Carolina
Alexa Boylan, Spelman College, Atlanta, Georgia
AGA-Dr. Harvey Young Education and Development Foundation’s Young Guts Scholar Program
Lucy Zhao, Massachusetts Institute of Technology Koch Institute for Integrative Cancer Research, Cambridge
Andrew Tran, Duke University, Durham, North Carolina
Sohaib Hassan, Rutgers University – Verzi Lab, New Brunswick, New Jersey
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor
Daniella Montalvo, University of Miami, Coral Gables, Florida
Sara Chough, Columbia University Irving Medical Center, New York, New York
Abstract Awards
Fellow Abstract Awards
David Flores Marin, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Jesse Platt, MD, PhD, Massachusetts General Hospital, Boston
Devika Gandhi, MD, Loma Linda University, California
Amanda Krause, MD, University of California, San Diego
Cynthia Tsay, MD, Mphil, Johns Hopkins Hospital, Baltimore, Maryland
Suha Abushamma, MD, Cleveland Clinic Foundation, Ohio
Md Obaidul Islam, PhD, University of Miami, Coral Gables, Florida
Sakteesh Gurunathan, MD, New York University School of Medicine, New York
Aaron Yeoh, MD, Stanford Hospital & Clinics, California
Yang Xiao, PhD, Mayo Clinic, Rochester, Minnesota
Jacques Gonzales, PhD, MS, Michigan State University, East Lansing
Kai Wang, MD, PhD, Harvard T.H. Chan School of Public Health, Cambridge, Massachusetts
Hoyeol Kim, PhD, Cedars Sinai Medical Center, New York, New York
Babajide Ojo, PhD, MS, Stanford University, California
AGA Fellow Abstract of the Year Award
Stefania Tocci, PhD, MS, University of Massachusetts, Cambridge
Student Abstract Awards
Pritha Chatterjee, MS, University of California, Riverside
Ela Contreras Panta, Vanderbilt University, Nashville, Tennessee
Mihir Shah, MD, MBBS, John H. Stroger Hospital of Cook County, Chicago, Illinois
Yuhan Fu, DO, Metrohealth Medical Center, Cleveland, Ohio
Raissa Nana Sede Mbakop, MD, Piedmont Athens Regional Medical Center, Athens, Georgia
Eleazar Montalvan-Sanchez, MD, Indiana University School of Medicine, Bloomington
Sarang Gupta, MD, St. Michael’s Hospital, Toronto, Ontario
Daniel Kim, Harvard Medical School, Cambridge, Massachusetts
Hannah Hrncir, Emory University, Decatur, Georgia
Zarwa Saqib, McMaster University, Hamilton, Ontario
Ying Zhu, MD, PhD, University of Michigan, Ann Arbor
Lizeth Cifuentes, MD, University of Pittsburgh Medical Center, Pennsylvania
Sharvani Dhandibhotla, MBBS, MS, Massachusetts General Hospital, Boston
Lauren Lynch, Baylor College of Medicine, Houston, Texas
AGA Student Abstract of The Year Award
Gabrielle Waclawik, MD, MPH, University of Wisconsin, Madison
AGA Abstract Award for Health Disparities Research
Soyoun Min, PhD, Lerner Research Institute (fellow), Cleveland, Ohio
Xiaobei Zhang, PhD , David Geffen School of Medicine at University of California, Los Angeles (fellow)
Matthew Zhao, David Geffen School of Medicine at University of California, Los Angeles (student)
Hannah Fiske, MD, Brown University/Rhode Island Hospital (student), Providence
AGA-APFED Abstract Award in Eosinophilic GI Diseases
Matthew Buendia, MD, Vanderbilt University Medical Center – Monroe Carell Jr. Children’s Hospital, Nashville, Tennessee
Alexandra L. Strauss, MD, University of Pennsylvania Health System, Philadelphia
Mira Yang, Northwestern Feinberg School of Medicine, Chicago, Illinois
AGA-Moti L. & Kamla Rustgi International Travel Award
Aviv Pudipeddi, MBBS, Concord Repatriation General Hospital, Sydney, Australia
Dianqin Sun, MBBS, Mmed, Erasmus University Medical Center, Rotterdam, Netherlands
The American Gastroenterological Association (AGA) is proud to announce the 71 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“This year’s recipients are determined to make an impact on digestive health care through their research,” said Michael Camilleri, MD, AGAF, chair, AGA Research Foundation. “We are honored to support these talented individuals at a critical stage in their careers and research projects. We look forward to seeing their great accomplishments.”

Treatment options for digestive diseases begin with vigorous research. The AGA Research Foundation supports medical investigators as they advance our understanding of gastrointestinal and liver conditions. The AGA Research Awards Program is made possible thanks to generous donors and funders. Learn more about the AGA Research Foundation at foundation.gastro.org.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Alexander Nguyen, MD, PhD, The Regent of the University of California, Los Angeles
Jeffrey W. Patterson-Fortin, MD, PhD, Dana-Farber Cancer Institute, Boston, Massachusetts
Sean Spencer, MD, PhD, Stanford Medicine, California
Ken Y. Hui, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, Maryland
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
Martina Molgora, PhD, Washington University School of Medicine, St. Louis, Missouri
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Brooke R. Druliner, PhD, Mayo Clinic, Rochester, Minnesota
Specialty Awards
AGA-Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Simon Schwörer, PhD, University of Chicago, Illinois
AGA-R. Robert & Sally Funderburg Research Award in Gastric Cancer
Bryson W. Katona, MD, PhD, University of Pennsylvania Perelman School of Medicine, Philadelphia
AGA-Amgen Fellowship-to-Faculty Transition Award
Cynthia Hsu, MD, PhD, University of California, San Diego
AGA-Bristol Myers Squibb Fellowship-to-Faculty Transition Award
Siyan Cao, MD, PhD, Washington University in St. Louis
Amit Ringel, MD, Brigham and Women’s Hospital, Boston, Massachusetts
Pilot Awards
AGA Pilot Research Award In Digestive Disease Health Disparities
Sharad Wadhwani, MD, MPH, University of California, San Francisco
AGA Pilot Research Award in Health Disparities
Enrique Soto Pérez de Celis, MD, PhD, MS, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán
AGA Pilot Research Award
Diana L. Snyder, MD, Mayo Clinic, Rochester, Minnesota
Michael Li, MD, MPH, University of California, San Francisco
Patricia Bloom, MD, University of Michigan, Ann Arbor
Edward Barnes, MD, MPH, University of North Carolina School of Medicine, Chapel Hill
AGA-Amgen Pilot Research Award In Digestive Disease Health Disparities
Laura Targownik, MD, MSHS, University of Toronto/Mount Sinai Hospital, Toronto, ON
Undergraduate Research Awards
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gwyneth Garramone, Loyola Marymount University, Los Angeles, California
Ella McLaren, University of California, San Diego
Nathan Moy, University of Southern California, Los Angeles
Hussein Elfayoumy, Johns Hopkins University, Baltimore, Maryland
Isabelle Garcia-Fischer, Tufts University, Medford, Massachusetts
Lidia Appell, University of New Mexico, Albuquerque
Katherine Burkman, Duke University, Durham, North Carolina
Alexa Boylan, Spelman College, Atlanta, Georgia
AGA-Dr. Harvey Young Education and Development Foundation’s Young Guts Scholar Program
Lucy Zhao, Massachusetts Institute of Technology Koch Institute for Integrative Cancer Research, Cambridge
Andrew Tran, Duke University, Durham, North Carolina
Sohaib Hassan, Rutgers University – Verzi Lab, New Brunswick, New Jersey
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor
Daniella Montalvo, University of Miami, Coral Gables, Florida
Sara Chough, Columbia University Irving Medical Center, New York, New York
Abstract Awards
Fellow Abstract Awards
David Flores Marin, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Jesse Platt, MD, PhD, Massachusetts General Hospital, Boston
Devika Gandhi, MD, Loma Linda University, California
Amanda Krause, MD, University of California, San Diego
Cynthia Tsay, MD, Mphil, Johns Hopkins Hospital, Baltimore, Maryland
Suha Abushamma, MD, Cleveland Clinic Foundation, Ohio
Md Obaidul Islam, PhD, University of Miami, Coral Gables, Florida
Sakteesh Gurunathan, MD, New York University School of Medicine, New York
Aaron Yeoh, MD, Stanford Hospital & Clinics, California
Yang Xiao, PhD, Mayo Clinic, Rochester, Minnesota
Jacques Gonzales, PhD, MS, Michigan State University, East Lansing
Kai Wang, MD, PhD, Harvard T.H. Chan School of Public Health, Cambridge, Massachusetts
Hoyeol Kim, PhD, Cedars Sinai Medical Center, New York, New York
Babajide Ojo, PhD, MS, Stanford University, California
AGA Fellow Abstract of the Year Award
Stefania Tocci, PhD, MS, University of Massachusetts, Cambridge
Student Abstract Awards
Pritha Chatterjee, MS, University of California, Riverside
Ela Contreras Panta, Vanderbilt University, Nashville, Tennessee
Mihir Shah, MD, MBBS, John H. Stroger Hospital of Cook County, Chicago, Illinois
Yuhan Fu, DO, Metrohealth Medical Center, Cleveland, Ohio
Raissa Nana Sede Mbakop, MD, Piedmont Athens Regional Medical Center, Athens, Georgia
Eleazar Montalvan-Sanchez, MD, Indiana University School of Medicine, Bloomington
Sarang Gupta, MD, St. Michael’s Hospital, Toronto, Ontario
Daniel Kim, Harvard Medical School, Cambridge, Massachusetts
Hannah Hrncir, Emory University, Decatur, Georgia
Zarwa Saqib, McMaster University, Hamilton, Ontario
Ying Zhu, MD, PhD, University of Michigan, Ann Arbor
Lizeth Cifuentes, MD, University of Pittsburgh Medical Center, Pennsylvania
Sharvani Dhandibhotla, MBBS, MS, Massachusetts General Hospital, Boston
Lauren Lynch, Baylor College of Medicine, Houston, Texas
AGA Student Abstract of The Year Award
Gabrielle Waclawik, MD, MPH, University of Wisconsin, Madison
AGA Abstract Award for Health Disparities Research
Soyoun Min, PhD, Lerner Research Institute (fellow), Cleveland, Ohio
Xiaobei Zhang, PhD , David Geffen School of Medicine at University of California, Los Angeles (fellow)
Matthew Zhao, David Geffen School of Medicine at University of California, Los Angeles (student)
Hannah Fiske, MD, Brown University/Rhode Island Hospital (student), Providence
AGA-APFED Abstract Award in Eosinophilic GI Diseases
Matthew Buendia, MD, Vanderbilt University Medical Center – Monroe Carell Jr. Children’s Hospital, Nashville, Tennessee
Alexandra L. Strauss, MD, University of Pennsylvania Health System, Philadelphia
Mira Yang, Northwestern Feinberg School of Medicine, Chicago, Illinois
AGA-Moti L. & Kamla Rustgi International Travel Award
Aviv Pudipeddi, MBBS, Concord Repatriation General Hospital, Sydney, Australia
Dianqin Sun, MBBS, Mmed, Erasmus University Medical Center, Rotterdam, Netherlands
Membership priorities shape the AGA advocacy agenda
Here, we present key highlights from the survey findings and share opportunities for members to engage in GI advocacy.
AGA advocacy has contributed to significant recent successes that include lowering the average-risk of colorectal cancer screening age from 50 to 45 years, phasing out cost-sharing burdens associated with polypectomy at screening colonoscopy, encouraging federal support to focus on GI cancer disparities, ensuring coverage for telehealth services, expanding colonoscopy coverage after positive noninvasive colorectal cancer screening tests, and mitigating scheduled cuts in Medicare reimbursement for GI services.
Despite these important successes, the GI community faces significant challenges that include persisting GI health disparities; declines in reimbursement and increased prior authorization burdens for GI procedures and clinic visits, limited research funding to address the burden of GI disease, climate change, provider burnout, and increasing administrative burdens (such as insurance prior authorizations and step therapy policies.
The AGA sought to better understand policy priorities of the GI community by disseminating a 34-question policy priority survey to AGA members in December 2022. A total of 251 members responded to the survey with career stage and primary practice setting varying among respondents (Figure 1). The AGA vetted and selected 10 health policy issues of highest interest with 95% of survey respondents agreeing these 10 selected topics covered the top priority issues impacting gastroenterology (Figure 2).
From these 10 policy issues, members were asked to identify the top 5 issues that AGA advocacy efforts should address.
The issues most frequently identified included reducing administrative burdens and patient delays in care because of increased prior authorizations (78%), ensuring fair reimbursement for GI providers (68%), reducing insurance-initiated switching of patient treatments for nonmedical reasons (58%), maintaining coverage of video and telephone evaluation and management visits (55%), and reducing delays in clinical care resulting from step therapy protocols (53%).
Other important issues included ensuring patients with pre-existing conditions have access to essential benefits and quality specialty care (43%); protecting providers from medical licensing restrictions and liability to deliver care across state lines (35%); addressing Medicare Quality Payment Program reporting requirements and lack of specialty advanced payment models (27%); increasing funding for GI health disparities (24%); and, increasing federal research funding to ensure greater opportunities for diverse early career investigators (20%).
Most problematic burdens
Survey respondents identified insurer prior authorization and step therapy burdens as especially problematic. 93% of respondents described the impact of prior authorization on their practices as “significantly burdensome” (61%) or “somewhat burdensome” (32%).
About 95% noted that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes “significantly” (56%) or “somewhat” (39%) negatively. 84% described the burdens associated with prior authorization policies as having increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
Likewise, step therapy protocols were perceived by 84% of respondents as burdensome; by 88% as negatively impactful on patient access to clinically appropriate treatments; and, by 88% as negatively impactful on patient clinical outcomes.
About 84% of respondents noted increases in the frequency of nonmedical switching and dosing restrictions over the last 5 years, with 90% perceiving negative impacts on patient clinical outcomes. 73% of respondents reported increased burdens associated with compliance in the Medicare QPP over the last 5 years.
AGA’s advocacy work
About 76% of respondents were interested in learning more about the AGA’s advocacy work. We presented some of the various opportunities and resources for members to engage with and contribute to AGA advocacy efforts (see pie chart). Based on the tremendous efforts and dedication of AGA staff, some of these opportunities include educational modules on AGA University, DDW programming, the AGA Washington Insider monthly policy newsletter, preformatted communications available through the AGA Advocacy Action Center, participation in AGA Advocacy Days or the AGA Congressional Advocates Program, service on the AGA Government Affairs Committee, and/or contributing to the AGA Political Action Committee.
Overall, the survey respondents illustrate the diversity and enthusiasm of AGA membership. Importantly, 95% of AGA members responding to the survey agreed these 10 selected policy issues are inclusive of the current top priority issues of the GI community. Amidst an ever-shifting health care landscape, we – the AGA community – must remain vigilant and adaptable to best address expected and unexpected changes and challenges to our patients and colleagues. In this respect, we should encourage constructive communication and dialogue between AGA membership, leadership, other issue stakeholders, government representatives and entities, and payers.
Amit Patel, MD, is a gastroenterologist and associate professor of medicine at Duke University and the Durham Veterans Affairs Medical Center, both in Durham, N.C. He serves on the editorial review board of Gastroenterology. Rotonya McCants Carr, MD, is the Cyrus E. Rubin Chair and division head of gastroenterology at the University of Washington, Seattle. Both Dr. Patel and Dr. Carr serve on the AGA Government Affairs Committee. The contents of this article do not represent the views of the Department of Veterans Affairs.
Reference
Patel A et al. Gastroenterology. 2023 May;164[6]:847-50.
Here, we present key highlights from the survey findings and share opportunities for members to engage in GI advocacy.
AGA advocacy has contributed to significant recent successes that include lowering the average-risk of colorectal cancer screening age from 50 to 45 years, phasing out cost-sharing burdens associated with polypectomy at screening colonoscopy, encouraging federal support to focus on GI cancer disparities, ensuring coverage for telehealth services, expanding colonoscopy coverage after positive noninvasive colorectal cancer screening tests, and mitigating scheduled cuts in Medicare reimbursement for GI services.
Despite these important successes, the GI community faces significant challenges that include persisting GI health disparities; declines in reimbursement and increased prior authorization burdens for GI procedures and clinic visits, limited research funding to address the burden of GI disease, climate change, provider burnout, and increasing administrative burdens (such as insurance prior authorizations and step therapy policies.
The AGA sought to better understand policy priorities of the GI community by disseminating a 34-question policy priority survey to AGA members in December 2022. A total of 251 members responded to the survey with career stage and primary practice setting varying among respondents (Figure 1). The AGA vetted and selected 10 health policy issues of highest interest with 95% of survey respondents agreeing these 10 selected topics covered the top priority issues impacting gastroenterology (Figure 2).
From these 10 policy issues, members were asked to identify the top 5 issues that AGA advocacy efforts should address.
The issues most frequently identified included reducing administrative burdens and patient delays in care because of increased prior authorizations (78%), ensuring fair reimbursement for GI providers (68%), reducing insurance-initiated switching of patient treatments for nonmedical reasons (58%), maintaining coverage of video and telephone evaluation and management visits (55%), and reducing delays in clinical care resulting from step therapy protocols (53%).
Other important issues included ensuring patients with pre-existing conditions have access to essential benefits and quality specialty care (43%); protecting providers from medical licensing restrictions and liability to deliver care across state lines (35%); addressing Medicare Quality Payment Program reporting requirements and lack of specialty advanced payment models (27%); increasing funding for GI health disparities (24%); and, increasing federal research funding to ensure greater opportunities for diverse early career investigators (20%).
Most problematic burdens
Survey respondents identified insurer prior authorization and step therapy burdens as especially problematic. 93% of respondents described the impact of prior authorization on their practices as “significantly burdensome” (61%) or “somewhat burdensome” (32%).
About 95% noted that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes “significantly” (56%) or “somewhat” (39%) negatively. 84% described the burdens associated with prior authorization policies as having increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
Likewise, step therapy protocols were perceived by 84% of respondents as burdensome; by 88% as negatively impactful on patient access to clinically appropriate treatments; and, by 88% as negatively impactful on patient clinical outcomes.
About 84% of respondents noted increases in the frequency of nonmedical switching and dosing restrictions over the last 5 years, with 90% perceiving negative impacts on patient clinical outcomes. 73% of respondents reported increased burdens associated with compliance in the Medicare QPP over the last 5 years.
AGA’s advocacy work
About 76% of respondents were interested in learning more about the AGA’s advocacy work. We presented some of the various opportunities and resources for members to engage with and contribute to AGA advocacy efforts (see pie chart). Based on the tremendous efforts and dedication of AGA staff, some of these opportunities include educational modules on AGA University, DDW programming, the AGA Washington Insider monthly policy newsletter, preformatted communications available through the AGA Advocacy Action Center, participation in AGA Advocacy Days or the AGA Congressional Advocates Program, service on the AGA Government Affairs Committee, and/or contributing to the AGA Political Action Committee.
Overall, the survey respondents illustrate the diversity and enthusiasm of AGA membership. Importantly, 95% of AGA members responding to the survey agreed these 10 selected policy issues are inclusive of the current top priority issues of the GI community. Amidst an ever-shifting health care landscape, we – the AGA community – must remain vigilant and adaptable to best address expected and unexpected changes and challenges to our patients and colleagues. In this respect, we should encourage constructive communication and dialogue between AGA membership, leadership, other issue stakeholders, government representatives and entities, and payers.
Amit Patel, MD, is a gastroenterologist and associate professor of medicine at Duke University and the Durham Veterans Affairs Medical Center, both in Durham, N.C. He serves on the editorial review board of Gastroenterology. Rotonya McCants Carr, MD, is the Cyrus E. Rubin Chair and division head of gastroenterology at the University of Washington, Seattle. Both Dr. Patel and Dr. Carr serve on the AGA Government Affairs Committee. The contents of this article do not represent the views of the Department of Veterans Affairs.
Reference
Patel A et al. Gastroenterology. 2023 May;164[6]:847-50.
Here, we present key highlights from the survey findings and share opportunities for members to engage in GI advocacy.
AGA advocacy has contributed to significant recent successes that include lowering the average-risk of colorectal cancer screening age from 50 to 45 years, phasing out cost-sharing burdens associated with polypectomy at screening colonoscopy, encouraging federal support to focus on GI cancer disparities, ensuring coverage for telehealth services, expanding colonoscopy coverage after positive noninvasive colorectal cancer screening tests, and mitigating scheduled cuts in Medicare reimbursement for GI services.
Despite these important successes, the GI community faces significant challenges that include persisting GI health disparities; declines in reimbursement and increased prior authorization burdens for GI procedures and clinic visits, limited research funding to address the burden of GI disease, climate change, provider burnout, and increasing administrative burdens (such as insurance prior authorizations and step therapy policies.
The AGA sought to better understand policy priorities of the GI community by disseminating a 34-question policy priority survey to AGA members in December 2022. A total of 251 members responded to the survey with career stage and primary practice setting varying among respondents (Figure 1). The AGA vetted and selected 10 health policy issues of highest interest with 95% of survey respondents agreeing these 10 selected topics covered the top priority issues impacting gastroenterology (Figure 2).
From these 10 policy issues, members were asked to identify the top 5 issues that AGA advocacy efforts should address.
The issues most frequently identified included reducing administrative burdens and patient delays in care because of increased prior authorizations (78%), ensuring fair reimbursement for GI providers (68%), reducing insurance-initiated switching of patient treatments for nonmedical reasons (58%), maintaining coverage of video and telephone evaluation and management visits (55%), and reducing delays in clinical care resulting from step therapy protocols (53%).
Other important issues included ensuring patients with pre-existing conditions have access to essential benefits and quality specialty care (43%); protecting providers from medical licensing restrictions and liability to deliver care across state lines (35%); addressing Medicare Quality Payment Program reporting requirements and lack of specialty advanced payment models (27%); increasing funding for GI health disparities (24%); and, increasing federal research funding to ensure greater opportunities for diverse early career investigators (20%).
Most problematic burdens
Survey respondents identified insurer prior authorization and step therapy burdens as especially problematic. 93% of respondents described the impact of prior authorization on their practices as “significantly burdensome” (61%) or “somewhat burdensome” (32%).
About 95% noted that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes “significantly” (56%) or “somewhat” (39%) negatively. 84% described the burdens associated with prior authorization policies as having increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
Likewise, step therapy protocols were perceived by 84% of respondents as burdensome; by 88% as negatively impactful on patient access to clinically appropriate treatments; and, by 88% as negatively impactful on patient clinical outcomes.
About 84% of respondents noted increases in the frequency of nonmedical switching and dosing restrictions over the last 5 years, with 90% perceiving negative impacts on patient clinical outcomes. 73% of respondents reported increased burdens associated with compliance in the Medicare QPP over the last 5 years.
AGA’s advocacy work
About 76% of respondents were interested in learning more about the AGA’s advocacy work. We presented some of the various opportunities and resources for members to engage with and contribute to AGA advocacy efforts (see pie chart). Based on the tremendous efforts and dedication of AGA staff, some of these opportunities include educational modules on AGA University, DDW programming, the AGA Washington Insider monthly policy newsletter, preformatted communications available through the AGA Advocacy Action Center, participation in AGA Advocacy Days or the AGA Congressional Advocates Program, service on the AGA Government Affairs Committee, and/or contributing to the AGA Political Action Committee.
Overall, the survey respondents illustrate the diversity and enthusiasm of AGA membership. Importantly, 95% of AGA members responding to the survey agreed these 10 selected policy issues are inclusive of the current top priority issues of the GI community. Amidst an ever-shifting health care landscape, we – the AGA community – must remain vigilant and adaptable to best address expected and unexpected changes and challenges to our patients and colleagues. In this respect, we should encourage constructive communication and dialogue between AGA membership, leadership, other issue stakeholders, government representatives and entities, and payers.
Amit Patel, MD, is a gastroenterologist and associate professor of medicine at Duke University and the Durham Veterans Affairs Medical Center, both in Durham, N.C. He serves on the editorial review board of Gastroenterology. Rotonya McCants Carr, MD, is the Cyrus E. Rubin Chair and division head of gastroenterology at the University of Washington, Seattle. Both Dr. Patel and Dr. Carr serve on the AGA Government Affairs Committee. The contents of this article do not represent the views of the Department of Veterans Affairs.
Reference
Patel A et al. Gastroenterology. 2023 May;164[6]:847-50.











