User login
Lack of paid sick leave is a barrier to cancer screening
“Our results provide evidence for policymakers considering legislative or regulatory solutions to address insufficient screening adherence and highlight an understudied benefit of expanding paid sick leave coverage,” wrote authors who were led by Kevin Callison, PhD, of the Tulane University School of Public Health and Tropical Medicine, New Orleans.
The findings were published earlier this year in the New England Journal of Medicine.
Despite an Affordable Care Act provision eliminating most cost-sharing for cancer screening, the rate for recommended breast and colorectal cancer screening among U.S. adults is lower than 70%. Work commitments, time constraints, and the prospect of lost wages are frequently cited as contributing factors to this underuse of preventive care. Researchers hypothesized that having paid sick leave coverage for the use of preventive services could improve adherence to cancer screening guidelines. With continued failure to pass a bill mandating federal paid sick leave legislation, nearly 30% of the nation’s workforce lacks this coverage. Rates are lower for low-income workers, women, and underserved racial and ethnic groups, the authors write.
Coverage mandates have become politically contentious, as evidenced by the fact that their passage by some states (n = 17), counties (n = 4) and cities (n = 18) has been met by many states (n = 18) passing preemption laws banning municipalities from adopting the laws.
In this study, researchers examined the rate of colorectal and breast cancer screening at 12- and 24-month intervals among people living in one of 61 cities. Before paid sick leave mandates were put in place, cancer screening rates were similar across the board. But once mandates were put in place, cancer screening rates were higher among workers affected by the mandate by 1.31% (95% confidence interval, 0.28-2.34) for 12-month colorectal cancer screening, 1.56% (95% CI, 0.33-2.79) for 24-month colorectal cancer screening, 1.22% (95% CI, −0.20 to 2.64) for 12-month mammography, and 2.07% (95% CI, 0.15-3.99) for 24-month mammography.
“Although these appear to be modest effects, spread across a large population, these indicate a fairly substantial gain in cancer screenings,” Dr. Callison said.
Prior studies showing positive associations between having paid sick leave coverage and whether someone receives cancer screenings are likely confounded by selection bias because they compare workers who have such coverage to those who do not, Dr. Callison and colleagues state in their paper.
“Although the lack of paid sick leave coverage may hinder access to preventive care, current evidence is insufficient to draw meaningful conclusions about its relationship to cancer screening,” the authors write, citing that particularly health conscious workers may take jobs offering sick leave coverage.
Through quasi-experimental design, the present study aimed to overcome such confounding issues. Its analytic sample, using administrative data from the Merative MarketScan Research Databases, encompassed approximately 2.5 million person-specific records per year for the colorectal cancer screening sample. The researchers’ mammography sample included 1.3 million person-specific records per year of the period examined.
The associations cited above translate into relative colorectal cancer screening increases of 8.1% in the 12-month adjusted model and a 5.9% relative increase from the premandate rate in the 24-month adjusted model. The rate was 1.56 percentage points (95% CI, 0.33-2.79) higher in the cities subject to the paid sick leave mandates (a 5.9% relative increase from the premandate rate). For screening mammography in the cities subject to the mandates, the 12-month adjusted 1.22% increase (95% CI, –0.20 to 2.64) represented a 2.5% relative increase from the premandate level. The adjusted 24-month rate increase of 2.07% (95% CI, 0.15-4.00) represented a 3.3% relative increase from premandate rates.
“However, these estimates are averages across all workers in our sample, many of whom likely already had paid sick leave coverage prior to the enactment of a mandate,” Dr. Callison said in the interview. “In fact, in other work related to this project, we estimated that about 28% of private sector workers gain paid sick leave when a mandate is enacted. So then, if we scale our findings by the share of workers actually gaining paid sick leave coverage, our estimates are much larger – a 9%-12% increase in screening mammography and a 21%-29% increase in colorectal cancer screening.”
Dr. Callison and his team are in the process of developing a follow-up proposal that would examine the effects of paid sick leave on downstream outcomes of the cancer care continuum, such as timing from diagnosis to treatment initiation. “We also hope to examine who benefits from these additional screens and what they mean for health equity. Data limitations prevented us from exploring that issue in the current study,” he said.
Dr. Callison had no conflicts associated with this study.
“Our results provide evidence for policymakers considering legislative or regulatory solutions to address insufficient screening adherence and highlight an understudied benefit of expanding paid sick leave coverage,” wrote authors who were led by Kevin Callison, PhD, of the Tulane University School of Public Health and Tropical Medicine, New Orleans.
The findings were published earlier this year in the New England Journal of Medicine.
Despite an Affordable Care Act provision eliminating most cost-sharing for cancer screening, the rate for recommended breast and colorectal cancer screening among U.S. adults is lower than 70%. Work commitments, time constraints, and the prospect of lost wages are frequently cited as contributing factors to this underuse of preventive care. Researchers hypothesized that having paid sick leave coverage for the use of preventive services could improve adherence to cancer screening guidelines. With continued failure to pass a bill mandating federal paid sick leave legislation, nearly 30% of the nation’s workforce lacks this coverage. Rates are lower for low-income workers, women, and underserved racial and ethnic groups, the authors write.
Coverage mandates have become politically contentious, as evidenced by the fact that their passage by some states (n = 17), counties (n = 4) and cities (n = 18) has been met by many states (n = 18) passing preemption laws banning municipalities from adopting the laws.
In this study, researchers examined the rate of colorectal and breast cancer screening at 12- and 24-month intervals among people living in one of 61 cities. Before paid sick leave mandates were put in place, cancer screening rates were similar across the board. But once mandates were put in place, cancer screening rates were higher among workers affected by the mandate by 1.31% (95% confidence interval, 0.28-2.34) for 12-month colorectal cancer screening, 1.56% (95% CI, 0.33-2.79) for 24-month colorectal cancer screening, 1.22% (95% CI, −0.20 to 2.64) for 12-month mammography, and 2.07% (95% CI, 0.15-3.99) for 24-month mammography.
“Although these appear to be modest effects, spread across a large population, these indicate a fairly substantial gain in cancer screenings,” Dr. Callison said.
Prior studies showing positive associations between having paid sick leave coverage and whether someone receives cancer screenings are likely confounded by selection bias because they compare workers who have such coverage to those who do not, Dr. Callison and colleagues state in their paper.
“Although the lack of paid sick leave coverage may hinder access to preventive care, current evidence is insufficient to draw meaningful conclusions about its relationship to cancer screening,” the authors write, citing that particularly health conscious workers may take jobs offering sick leave coverage.
Through quasi-experimental design, the present study aimed to overcome such confounding issues. Its analytic sample, using administrative data from the Merative MarketScan Research Databases, encompassed approximately 2.5 million person-specific records per year for the colorectal cancer screening sample. The researchers’ mammography sample included 1.3 million person-specific records per year of the period examined.
The associations cited above translate into relative colorectal cancer screening increases of 8.1% in the 12-month adjusted model and a 5.9% relative increase from the premandate rate in the 24-month adjusted model. The rate was 1.56 percentage points (95% CI, 0.33-2.79) higher in the cities subject to the paid sick leave mandates (a 5.9% relative increase from the premandate rate). For screening mammography in the cities subject to the mandates, the 12-month adjusted 1.22% increase (95% CI, –0.20 to 2.64) represented a 2.5% relative increase from the premandate level. The adjusted 24-month rate increase of 2.07% (95% CI, 0.15-4.00) represented a 3.3% relative increase from premandate rates.
“However, these estimates are averages across all workers in our sample, many of whom likely already had paid sick leave coverage prior to the enactment of a mandate,” Dr. Callison said in the interview. “In fact, in other work related to this project, we estimated that about 28% of private sector workers gain paid sick leave when a mandate is enacted. So then, if we scale our findings by the share of workers actually gaining paid sick leave coverage, our estimates are much larger – a 9%-12% increase in screening mammography and a 21%-29% increase in colorectal cancer screening.”
Dr. Callison and his team are in the process of developing a follow-up proposal that would examine the effects of paid sick leave on downstream outcomes of the cancer care continuum, such as timing from diagnosis to treatment initiation. “We also hope to examine who benefits from these additional screens and what they mean for health equity. Data limitations prevented us from exploring that issue in the current study,” he said.
Dr. Callison had no conflicts associated with this study.
“Our results provide evidence for policymakers considering legislative or regulatory solutions to address insufficient screening adherence and highlight an understudied benefit of expanding paid sick leave coverage,” wrote authors who were led by Kevin Callison, PhD, of the Tulane University School of Public Health and Tropical Medicine, New Orleans.
The findings were published earlier this year in the New England Journal of Medicine.
Despite an Affordable Care Act provision eliminating most cost-sharing for cancer screening, the rate for recommended breast and colorectal cancer screening among U.S. adults is lower than 70%. Work commitments, time constraints, and the prospect of lost wages are frequently cited as contributing factors to this underuse of preventive care. Researchers hypothesized that having paid sick leave coverage for the use of preventive services could improve adherence to cancer screening guidelines. With continued failure to pass a bill mandating federal paid sick leave legislation, nearly 30% of the nation’s workforce lacks this coverage. Rates are lower for low-income workers, women, and underserved racial and ethnic groups, the authors write.
Coverage mandates have become politically contentious, as evidenced by the fact that their passage by some states (n = 17), counties (n = 4) and cities (n = 18) has been met by many states (n = 18) passing preemption laws banning municipalities from adopting the laws.
In this study, researchers examined the rate of colorectal and breast cancer screening at 12- and 24-month intervals among people living in one of 61 cities. Before paid sick leave mandates were put in place, cancer screening rates were similar across the board. But once mandates were put in place, cancer screening rates were higher among workers affected by the mandate by 1.31% (95% confidence interval, 0.28-2.34) for 12-month colorectal cancer screening, 1.56% (95% CI, 0.33-2.79) for 24-month colorectal cancer screening, 1.22% (95% CI, −0.20 to 2.64) for 12-month mammography, and 2.07% (95% CI, 0.15-3.99) for 24-month mammography.
“Although these appear to be modest effects, spread across a large population, these indicate a fairly substantial gain in cancer screenings,” Dr. Callison said.
Prior studies showing positive associations between having paid sick leave coverage and whether someone receives cancer screenings are likely confounded by selection bias because they compare workers who have such coverage to those who do not, Dr. Callison and colleagues state in their paper.
“Although the lack of paid sick leave coverage may hinder access to preventive care, current evidence is insufficient to draw meaningful conclusions about its relationship to cancer screening,” the authors write, citing that particularly health conscious workers may take jobs offering sick leave coverage.
Through quasi-experimental design, the present study aimed to overcome such confounding issues. Its analytic sample, using administrative data from the Merative MarketScan Research Databases, encompassed approximately 2.5 million person-specific records per year for the colorectal cancer screening sample. The researchers’ mammography sample included 1.3 million person-specific records per year of the period examined.
The associations cited above translate into relative colorectal cancer screening increases of 8.1% in the 12-month adjusted model and a 5.9% relative increase from the premandate rate in the 24-month adjusted model. The rate was 1.56 percentage points (95% CI, 0.33-2.79) higher in the cities subject to the paid sick leave mandates (a 5.9% relative increase from the premandate rate). For screening mammography in the cities subject to the mandates, the 12-month adjusted 1.22% increase (95% CI, –0.20 to 2.64) represented a 2.5% relative increase from the premandate level. The adjusted 24-month rate increase of 2.07% (95% CI, 0.15-4.00) represented a 3.3% relative increase from premandate rates.
“However, these estimates are averages across all workers in our sample, many of whom likely already had paid sick leave coverage prior to the enactment of a mandate,” Dr. Callison said in the interview. “In fact, in other work related to this project, we estimated that about 28% of private sector workers gain paid sick leave when a mandate is enacted. So then, if we scale our findings by the share of workers actually gaining paid sick leave coverage, our estimates are much larger – a 9%-12% increase in screening mammography and a 21%-29% increase in colorectal cancer screening.”
Dr. Callison and his team are in the process of developing a follow-up proposal that would examine the effects of paid sick leave on downstream outcomes of the cancer care continuum, such as timing from diagnosis to treatment initiation. “We also hope to examine who benefits from these additional screens and what they mean for health equity. Data limitations prevented us from exploring that issue in the current study,” he said.
Dr. Callison had no conflicts associated with this study.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Unprecedented drop seen in early colorectal cancer cases due to aspirin use
CHICAGO – The authors say that aspirin could prove to be an effective strategy in preventing early-onset colorectal cancer cases.
“What we have here is a 15% reduction for all adenomas and 33% for those with advanced histology, which to us is quite substantial. We have not seen that much [33%] in previous studies so I would think it definitely needs more study,” said Cassandra D. Fritz, MD, MPHS, a gastroenterologist with Washington University, St. Louis, in an oral presentation given at the annual Digestive Disease Week®.
“This finding is important given the alarming rise in the incidence and mortality of early-onset colorectal cancer (age < 50 years), and our limited understanding of the underlying drivers to direct prevention efforts,” Dr. Fritz said. Early-onset colorectal cancer cases have doubled since 1995, she said.
The study confirms evidence from 30 years of research that suggests regular aspirin use reduces cancer risk. In patients with Lynch syndrome, the CAPP2 study showed that aspirin has a protective effect against colorectal cancer at 20 years follow-up.
While emerging data have suggested that aspirin use may reduce later-onset colorectal cancer, it was not known if regular aspirin and NSAID use are associated with diminished risk of early-onset conventional adenomas, and especially the high-risk adenomas conferring greater malignant potential known to be the major precursor of early-onset colorectal cancer. An unpublished analysis of molecular markers by the study’s senior author, Yin Cao, ScD, MPH, also of Washington University, found that at least 57% of early-onset colorectal cancers developed from the conventional adenoma-carcinoma pathway.
The objective of the new study was to assess the association between regular aspirin or NSAID use at least twice weekly, with the risk of developing early-onset adenoma. The analysis is based on an evaluation of data from the Nurses’ Health Study II of 32,058 women who had at least one colonoscopy before age 50 (1991-2015). High-risk adenomas included those that were at least 1 cm with tubulovillous/villous histology or high-grade dysplasia, or the presence of at least three adenomas.
There were 1,247 early-onset adenomas, among which 290 were considered high risk. The risk of adenomas among patients who took aspirin or NSAIDs regularly for cardiovascular protection or for inflammatory conditions, was lower than in those who did not take aspirin and/or NSAIDs regularly. While the association was similar for high-risk vs. low-risk adenomas, the benefit was more pronounced for adenomas of tubulovillous/villous histology or with high-grade dysplasia (odds ratio, 0.67; 95% confidence interval, 0.51-0.89), a 33% reduction, compared with tubular adenomas (OR, 0.90; 95% CI, 0.79-1.0; P for heterogeneity = .02).
With later-onset adenomas, risk reduction was confined primarily to large (OR, 0.76; 95% CI, 0.62-0.93) or multiple adenomas (OR, 0.57; 95% CI, 0.40-0.83), but not adenomas of advanced histology (OR, 0.92; 95% CI, 0.73-1.17).
“With colorectal cancer rates increasing, we still don’t have any preventative strategies beyond screening. With this 15% reduction with aspirin/NSAIDS in early-onset adenoma – and particularly for the quite substantial 33% benefit in advanced adenoma with advanced histology, we need to think about a precision-based chemoprevention strategy for early-onset precursors of colorectal cancer,” Dr. Cao said.
The U.S. Preventive Services Task Force issued a new recommendation in 2021 stating that colorectal cancer screening for people with average risk should start 5 years sooner at age 45. “As we know,” Dr. Yin said, “many younger adults are not screened. That’s why we’re looking into potential early-onset colorectal cancer chemopreventative agents.”
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Dr. Fritz had no disclosures and Dr. Cao listed consulting for Geneoscopy.
CHICAGO – The authors say that aspirin could prove to be an effective strategy in preventing early-onset colorectal cancer cases.
“What we have here is a 15% reduction for all adenomas and 33% for those with advanced histology, which to us is quite substantial. We have not seen that much [33%] in previous studies so I would think it definitely needs more study,” said Cassandra D. Fritz, MD, MPHS, a gastroenterologist with Washington University, St. Louis, in an oral presentation given at the annual Digestive Disease Week®.
“This finding is important given the alarming rise in the incidence and mortality of early-onset colorectal cancer (age < 50 years), and our limited understanding of the underlying drivers to direct prevention efforts,” Dr. Fritz said. Early-onset colorectal cancer cases have doubled since 1995, she said.
The study confirms evidence from 30 years of research that suggests regular aspirin use reduces cancer risk. In patients with Lynch syndrome, the CAPP2 study showed that aspirin has a protective effect against colorectal cancer at 20 years follow-up.
While emerging data have suggested that aspirin use may reduce later-onset colorectal cancer, it was not known if regular aspirin and NSAID use are associated with diminished risk of early-onset conventional adenomas, and especially the high-risk adenomas conferring greater malignant potential known to be the major precursor of early-onset colorectal cancer. An unpublished analysis of molecular markers by the study’s senior author, Yin Cao, ScD, MPH, also of Washington University, found that at least 57% of early-onset colorectal cancers developed from the conventional adenoma-carcinoma pathway.
The objective of the new study was to assess the association between regular aspirin or NSAID use at least twice weekly, with the risk of developing early-onset adenoma. The analysis is based on an evaluation of data from the Nurses’ Health Study II of 32,058 women who had at least one colonoscopy before age 50 (1991-2015). High-risk adenomas included those that were at least 1 cm with tubulovillous/villous histology or high-grade dysplasia, or the presence of at least three adenomas.
There were 1,247 early-onset adenomas, among which 290 were considered high risk. The risk of adenomas among patients who took aspirin or NSAIDs regularly for cardiovascular protection or for inflammatory conditions, was lower than in those who did not take aspirin and/or NSAIDs regularly. While the association was similar for high-risk vs. low-risk adenomas, the benefit was more pronounced for adenomas of tubulovillous/villous histology or with high-grade dysplasia (odds ratio, 0.67; 95% confidence interval, 0.51-0.89), a 33% reduction, compared with tubular adenomas (OR, 0.90; 95% CI, 0.79-1.0; P for heterogeneity = .02).
With later-onset adenomas, risk reduction was confined primarily to large (OR, 0.76; 95% CI, 0.62-0.93) or multiple adenomas (OR, 0.57; 95% CI, 0.40-0.83), but not adenomas of advanced histology (OR, 0.92; 95% CI, 0.73-1.17).
“With colorectal cancer rates increasing, we still don’t have any preventative strategies beyond screening. With this 15% reduction with aspirin/NSAIDS in early-onset adenoma – and particularly for the quite substantial 33% benefit in advanced adenoma with advanced histology, we need to think about a precision-based chemoprevention strategy for early-onset precursors of colorectal cancer,” Dr. Cao said.
The U.S. Preventive Services Task Force issued a new recommendation in 2021 stating that colorectal cancer screening for people with average risk should start 5 years sooner at age 45. “As we know,” Dr. Yin said, “many younger adults are not screened. That’s why we’re looking into potential early-onset colorectal cancer chemopreventative agents.”
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Dr. Fritz had no disclosures and Dr. Cao listed consulting for Geneoscopy.
CHICAGO – The authors say that aspirin could prove to be an effective strategy in preventing early-onset colorectal cancer cases.
“What we have here is a 15% reduction for all adenomas and 33% for those with advanced histology, which to us is quite substantial. We have not seen that much [33%] in previous studies so I would think it definitely needs more study,” said Cassandra D. Fritz, MD, MPHS, a gastroenterologist with Washington University, St. Louis, in an oral presentation given at the annual Digestive Disease Week®.
“This finding is important given the alarming rise in the incidence and mortality of early-onset colorectal cancer (age < 50 years), and our limited understanding of the underlying drivers to direct prevention efforts,” Dr. Fritz said. Early-onset colorectal cancer cases have doubled since 1995, she said.
The study confirms evidence from 30 years of research that suggests regular aspirin use reduces cancer risk. In patients with Lynch syndrome, the CAPP2 study showed that aspirin has a protective effect against colorectal cancer at 20 years follow-up.
While emerging data have suggested that aspirin use may reduce later-onset colorectal cancer, it was not known if regular aspirin and NSAID use are associated with diminished risk of early-onset conventional adenomas, and especially the high-risk adenomas conferring greater malignant potential known to be the major precursor of early-onset colorectal cancer. An unpublished analysis of molecular markers by the study’s senior author, Yin Cao, ScD, MPH, also of Washington University, found that at least 57% of early-onset colorectal cancers developed from the conventional adenoma-carcinoma pathway.
The objective of the new study was to assess the association between regular aspirin or NSAID use at least twice weekly, with the risk of developing early-onset adenoma. The analysis is based on an evaluation of data from the Nurses’ Health Study II of 32,058 women who had at least one colonoscopy before age 50 (1991-2015). High-risk adenomas included those that were at least 1 cm with tubulovillous/villous histology or high-grade dysplasia, or the presence of at least three adenomas.
There were 1,247 early-onset adenomas, among which 290 were considered high risk. The risk of adenomas among patients who took aspirin or NSAIDs regularly for cardiovascular protection or for inflammatory conditions, was lower than in those who did not take aspirin and/or NSAIDs regularly. While the association was similar for high-risk vs. low-risk adenomas, the benefit was more pronounced for adenomas of tubulovillous/villous histology or with high-grade dysplasia (odds ratio, 0.67; 95% confidence interval, 0.51-0.89), a 33% reduction, compared with tubular adenomas (OR, 0.90; 95% CI, 0.79-1.0; P for heterogeneity = .02).
With later-onset adenomas, risk reduction was confined primarily to large (OR, 0.76; 95% CI, 0.62-0.93) or multiple adenomas (OR, 0.57; 95% CI, 0.40-0.83), but not adenomas of advanced histology (OR, 0.92; 95% CI, 0.73-1.17).
“With colorectal cancer rates increasing, we still don’t have any preventative strategies beyond screening. With this 15% reduction with aspirin/NSAIDS in early-onset adenoma – and particularly for the quite substantial 33% benefit in advanced adenoma with advanced histology, we need to think about a precision-based chemoprevention strategy for early-onset precursors of colorectal cancer,” Dr. Cao said.
The U.S. Preventive Services Task Force issued a new recommendation in 2021 stating that colorectal cancer screening for people with average risk should start 5 years sooner at age 45. “As we know,” Dr. Yin said, “many younger adults are not screened. That’s why we’re looking into potential early-onset colorectal cancer chemopreventative agents.”
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Dr. Fritz had no disclosures and Dr. Cao listed consulting for Geneoscopy.
AT DDW 2023
Cardiopathy no basis for choosing anticoagulation in ESUS
MUNICH, GERMANY – suggest findings from the ARCADIA trial.
The trial, which was halted early, randomized more than 1,000 ESUS patients with atrial cardiomyopathy to apixaban or placebo. Results showed that apixaban did not improve rates of recurrent stroke of any kind nor safety outcomes such as major hemorrhage and all-cause mortality.
The results were presented at the annual meeting of the European Stroke Organisation Conference.
“We found no benefit of apixaban over aspirin in patients with ESUS who had evidence of atrial cardiopathy, at least based on the criteria in our trial,” said study presenter Hooman Kamel, MD, MS, vice chair for research and chief of neurocritical care in the department of neurology, Weill Cornell Medicine, New York.
“It could be that this concept of thrombogenic atrial cardiopathy really isn’t present unless there is also atrial fibrillation,” he continued, suggesting alternatively that results may be caused by the “incorrect choice of atrial cardiopathy biomarkers or thresholds.”
“We chose these because they were clinically scalable and usable in a multicenter design,” Dr. Kamel explained, adding that there are a number of different proposed biomarkers that could be used in a future study.
The team will now perform secondary analyses over the coming months to “try to help sort out some of these potential explanations.”
Dr. Kamel concluded, however, that, “as of now, no strategy of anticoagulation has been found to be better than antiplatelet therapy for secondary stroke prevention after ESUS.”
Similar results
Approached for comment, session cochair Robin Lemmens, MD, PhD, a neurologist in the department of neurosciences, UZ Leuven (Belgium), noted that this is the third ESUS trial, after the NAVIGATE and RE-SPECT trials, and they have all showed “similar results.”
He said, however, that there “could be various reasons for that, and it’s good that they mentioned looking into the subgroups,” as has been done for those other studies.
“Most of these trials were initiated under the concept that most of these patients would have had underlying atrial fibrillation, and then of course there would have been a benefit for anticoagulation.”
“It turns out that that’s not the case,” Dr. Lemmens said, “probably because there’s a lot of heterogeneity in these patients,” with different reasons for developing stroke, “not just only potentially underlying atrial fibrillation.”
Session cochair Arthur Liesz, MD, PhD, Institute for Stroke and Dementia Research, University Hospital, LMU Munich, added that it is important to consider the definition of atrial cardiopathy in this context.
If this was limited only to structural cardiopathy, then this “was a rather small subpopulation in this study,” he said in an interview.
Dr. Liesz said that it could instead have been conducted with “more stringent cutoffs,” and could have considered blood biomarkers, “which then would have delivered more overlap with structural cardiopathy,” and allowed those patients to be analyzed separately.
Heterogeneous etiologies?
Dr. Kamel began by noting that the failure of NAVIGATE and RESPECT to show a benefit from anticoagulation in the prevention of recurrent stroke in patients with ESUS led to the hypothesis that this is “perhaps due to heterogeneous underlying etiologies.”
Moreover, these etiologies “may require different types of antithrombotic therapy to best prevent recurrence, and one such underlying etiology may be atrial cardiopathy.”
He explained that several observational studies have found, in the absence of atrial fibrillation, associations between stroke and different markers of atrial cardiopathy and, “given the proven benefit of anticoagulation in preventing strokes in patients with atrial fibrillation, it seems plausible” that they may also benefit.
To investigate further, the team conducted ARCADIA, an investigator-initiated, multicenter, randomized trial involving patients aged 45 years and older from 185 sites in the United States and Canada with a clinical diagnosis of stroke that met the consensus criteria for ESUS.
They also were required to have undergone brain imaging to rule out hemorrhagic stroke, and to have a modified Rankin Scale score of 4 or less, indicating up to a moderately severe degree of disability.
They also had atrial cardiopathy, as determined by P-wave terminal force in V1 greater than 5,000mcV*ms on electrocardiography, serum N-terminal prohormone of brain natriuretic peptide levels greater than 250 pg/mL, or a left atrium diameter of at least 3 cm/m2.
The patients were randomly assigned to apixaban 5 mg or 2.5 mg twice daily plus aspirin placebo, or apixaban placebo plus aspirin 81 mg daily. Those diagnosed with atrial fibrillation after randomization crossed over to open-label anticoagulant therapy at physician discretion.
Dr. Kamel reported that, in 2022, after enrollment of 1015 patients with a mean follow-up of 1.8 years, the trial was halted at the planned interim efficacy/futility analysis, adding that there were “no safety concerns.”
The apixaban and aspirin groups were well balanced in terms of their baseline characteristics. The mean age was 68 years, and 54% were female. Three-quarters of the participants were White; 21.1% were Black.
Prior stroke was reported in 19% of patients. Hypertension was common, in about 77%, and type 2 diabetes was seen in 31%. There were relatively few cases of ischemic heart disease, heart failure, and peripheral arterial disease.
The primary efficacy outcome of recurrent stroke of any type occurred in 4.4% of both patients treated with apixaban and those given aspirin, at a hazard ratio of 1.00 (95% confidence interval, 0.64-1.55). Similar findings were seen when looking individually at ischemic and hemorrhagic stroke, and stroke of undetermined type.
There was also no significant difference in the secondary outcomes of recurrent ischemic stroke or systemic embolism, at 4.1% versus 4.4% (HR, 0.92; 95% CI, 0.59-1.44), and recurrent stroke of any type or death from any cause, at 7.3% versus 6.8% (HR, 1.08; 95% CI, 0.76-1.52).
In terms of safety, rates of major hemorrhage were low and almost identical between the groups, at 0.7% with apixaban and 0.8% for aspirin (HR, 1.02; 95% CI, 0.29-3.51), and were similar for all-cause mortality, at 1.8% versus 1.2% (HR, 1.53; 95% CI, 0.63-3.74).
Proportionately more patients treated with aspirin experienced symptomatic intracranial hemorrhage, at 1.1% versus 0%.
The trial results generated a flurry of interest on Twitter.
Thomas Ford, MD, a vascular neurology fellow from Boston Medical Center, described the results as “disappointing,” although he was “curious to see if there was any signal of benefit in subgroup analyses.”
Shadi Yaghi, codirector of the Comprehensive Stroke Center at Brown University, Providence, R.I., added that the trial “begs the question [as to] whether all device-detected atrial fibrillation warrants anticoagulation.”
Replying, Mitchell Elkind, MD, MPhil, professor of neurology and epidemiology at Columbia University Irving Medical Center, New York, said that he agrees with this interpretation.
“Maybe the issue is not with the concept of atrial cardiopathy but with the need to [anticoagulate] all patients with low [atrial fibrillation] burden or incidental [atrial fibrillation] after stroke.”
The study was funded by the National Institutes of Health and the National Institute of Neurological Disorders and Stroke. The study drug was provided in kind by BMS-Pfizer, and ancillary funding for the NTproBNP assays was provided by Roche. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
MUNICH, GERMANY – suggest findings from the ARCADIA trial.
The trial, which was halted early, randomized more than 1,000 ESUS patients with atrial cardiomyopathy to apixaban or placebo. Results showed that apixaban did not improve rates of recurrent stroke of any kind nor safety outcomes such as major hemorrhage and all-cause mortality.
The results were presented at the annual meeting of the European Stroke Organisation Conference.
“We found no benefit of apixaban over aspirin in patients with ESUS who had evidence of atrial cardiopathy, at least based on the criteria in our trial,” said study presenter Hooman Kamel, MD, MS, vice chair for research and chief of neurocritical care in the department of neurology, Weill Cornell Medicine, New York.
“It could be that this concept of thrombogenic atrial cardiopathy really isn’t present unless there is also atrial fibrillation,” he continued, suggesting alternatively that results may be caused by the “incorrect choice of atrial cardiopathy biomarkers or thresholds.”
“We chose these because they were clinically scalable and usable in a multicenter design,” Dr. Kamel explained, adding that there are a number of different proposed biomarkers that could be used in a future study.
The team will now perform secondary analyses over the coming months to “try to help sort out some of these potential explanations.”
Dr. Kamel concluded, however, that, “as of now, no strategy of anticoagulation has been found to be better than antiplatelet therapy for secondary stroke prevention after ESUS.”
Similar results
Approached for comment, session cochair Robin Lemmens, MD, PhD, a neurologist in the department of neurosciences, UZ Leuven (Belgium), noted that this is the third ESUS trial, after the NAVIGATE and RE-SPECT trials, and they have all showed “similar results.”
He said, however, that there “could be various reasons for that, and it’s good that they mentioned looking into the subgroups,” as has been done for those other studies.
“Most of these trials were initiated under the concept that most of these patients would have had underlying atrial fibrillation, and then of course there would have been a benefit for anticoagulation.”
“It turns out that that’s not the case,” Dr. Lemmens said, “probably because there’s a lot of heterogeneity in these patients,” with different reasons for developing stroke, “not just only potentially underlying atrial fibrillation.”
Session cochair Arthur Liesz, MD, PhD, Institute for Stroke and Dementia Research, University Hospital, LMU Munich, added that it is important to consider the definition of atrial cardiopathy in this context.
If this was limited only to structural cardiopathy, then this “was a rather small subpopulation in this study,” he said in an interview.
Dr. Liesz said that it could instead have been conducted with “more stringent cutoffs,” and could have considered blood biomarkers, “which then would have delivered more overlap with structural cardiopathy,” and allowed those patients to be analyzed separately.
Heterogeneous etiologies?
Dr. Kamel began by noting that the failure of NAVIGATE and RESPECT to show a benefit from anticoagulation in the prevention of recurrent stroke in patients with ESUS led to the hypothesis that this is “perhaps due to heterogeneous underlying etiologies.”
Moreover, these etiologies “may require different types of antithrombotic therapy to best prevent recurrence, and one such underlying etiology may be atrial cardiopathy.”
He explained that several observational studies have found, in the absence of atrial fibrillation, associations between stroke and different markers of atrial cardiopathy and, “given the proven benefit of anticoagulation in preventing strokes in patients with atrial fibrillation, it seems plausible” that they may also benefit.
To investigate further, the team conducted ARCADIA, an investigator-initiated, multicenter, randomized trial involving patients aged 45 years and older from 185 sites in the United States and Canada with a clinical diagnosis of stroke that met the consensus criteria for ESUS.
They also were required to have undergone brain imaging to rule out hemorrhagic stroke, and to have a modified Rankin Scale score of 4 or less, indicating up to a moderately severe degree of disability.
They also had atrial cardiopathy, as determined by P-wave terminal force in V1 greater than 5,000mcV*ms on electrocardiography, serum N-terminal prohormone of brain natriuretic peptide levels greater than 250 pg/mL, or a left atrium diameter of at least 3 cm/m2.
The patients were randomly assigned to apixaban 5 mg or 2.5 mg twice daily plus aspirin placebo, or apixaban placebo plus aspirin 81 mg daily. Those diagnosed with atrial fibrillation after randomization crossed over to open-label anticoagulant therapy at physician discretion.
Dr. Kamel reported that, in 2022, after enrollment of 1015 patients with a mean follow-up of 1.8 years, the trial was halted at the planned interim efficacy/futility analysis, adding that there were “no safety concerns.”
The apixaban and aspirin groups were well balanced in terms of their baseline characteristics. The mean age was 68 years, and 54% were female. Three-quarters of the participants were White; 21.1% were Black.
Prior stroke was reported in 19% of patients. Hypertension was common, in about 77%, and type 2 diabetes was seen in 31%. There were relatively few cases of ischemic heart disease, heart failure, and peripheral arterial disease.
The primary efficacy outcome of recurrent stroke of any type occurred in 4.4% of both patients treated with apixaban and those given aspirin, at a hazard ratio of 1.00 (95% confidence interval, 0.64-1.55). Similar findings were seen when looking individually at ischemic and hemorrhagic stroke, and stroke of undetermined type.
There was also no significant difference in the secondary outcomes of recurrent ischemic stroke or systemic embolism, at 4.1% versus 4.4% (HR, 0.92; 95% CI, 0.59-1.44), and recurrent stroke of any type or death from any cause, at 7.3% versus 6.8% (HR, 1.08; 95% CI, 0.76-1.52).
In terms of safety, rates of major hemorrhage were low and almost identical between the groups, at 0.7% with apixaban and 0.8% for aspirin (HR, 1.02; 95% CI, 0.29-3.51), and were similar for all-cause mortality, at 1.8% versus 1.2% (HR, 1.53; 95% CI, 0.63-3.74).
Proportionately more patients treated with aspirin experienced symptomatic intracranial hemorrhage, at 1.1% versus 0%.
The trial results generated a flurry of interest on Twitter.
Thomas Ford, MD, a vascular neurology fellow from Boston Medical Center, described the results as “disappointing,” although he was “curious to see if there was any signal of benefit in subgroup analyses.”
Shadi Yaghi, codirector of the Comprehensive Stroke Center at Brown University, Providence, R.I., added that the trial “begs the question [as to] whether all device-detected atrial fibrillation warrants anticoagulation.”
Replying, Mitchell Elkind, MD, MPhil, professor of neurology and epidemiology at Columbia University Irving Medical Center, New York, said that he agrees with this interpretation.
“Maybe the issue is not with the concept of atrial cardiopathy but with the need to [anticoagulate] all patients with low [atrial fibrillation] burden or incidental [atrial fibrillation] after stroke.”
The study was funded by the National Institutes of Health and the National Institute of Neurological Disorders and Stroke. The study drug was provided in kind by BMS-Pfizer, and ancillary funding for the NTproBNP assays was provided by Roche. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
MUNICH, GERMANY – suggest findings from the ARCADIA trial.
The trial, which was halted early, randomized more than 1,000 ESUS patients with atrial cardiomyopathy to apixaban or placebo. Results showed that apixaban did not improve rates of recurrent stroke of any kind nor safety outcomes such as major hemorrhage and all-cause mortality.
The results were presented at the annual meeting of the European Stroke Organisation Conference.
“We found no benefit of apixaban over aspirin in patients with ESUS who had evidence of atrial cardiopathy, at least based on the criteria in our trial,” said study presenter Hooman Kamel, MD, MS, vice chair for research and chief of neurocritical care in the department of neurology, Weill Cornell Medicine, New York.
“It could be that this concept of thrombogenic atrial cardiopathy really isn’t present unless there is also atrial fibrillation,” he continued, suggesting alternatively that results may be caused by the “incorrect choice of atrial cardiopathy biomarkers or thresholds.”
“We chose these because they were clinically scalable and usable in a multicenter design,” Dr. Kamel explained, adding that there are a number of different proposed biomarkers that could be used in a future study.
The team will now perform secondary analyses over the coming months to “try to help sort out some of these potential explanations.”
Dr. Kamel concluded, however, that, “as of now, no strategy of anticoagulation has been found to be better than antiplatelet therapy for secondary stroke prevention after ESUS.”
Similar results
Approached for comment, session cochair Robin Lemmens, MD, PhD, a neurologist in the department of neurosciences, UZ Leuven (Belgium), noted that this is the third ESUS trial, after the NAVIGATE and RE-SPECT trials, and they have all showed “similar results.”
He said, however, that there “could be various reasons for that, and it’s good that they mentioned looking into the subgroups,” as has been done for those other studies.
“Most of these trials were initiated under the concept that most of these patients would have had underlying atrial fibrillation, and then of course there would have been a benefit for anticoagulation.”
“It turns out that that’s not the case,” Dr. Lemmens said, “probably because there’s a lot of heterogeneity in these patients,” with different reasons for developing stroke, “not just only potentially underlying atrial fibrillation.”
Session cochair Arthur Liesz, MD, PhD, Institute for Stroke and Dementia Research, University Hospital, LMU Munich, added that it is important to consider the definition of atrial cardiopathy in this context.
If this was limited only to structural cardiopathy, then this “was a rather small subpopulation in this study,” he said in an interview.
Dr. Liesz said that it could instead have been conducted with “more stringent cutoffs,” and could have considered blood biomarkers, “which then would have delivered more overlap with structural cardiopathy,” and allowed those patients to be analyzed separately.
Heterogeneous etiologies?
Dr. Kamel began by noting that the failure of NAVIGATE and RESPECT to show a benefit from anticoagulation in the prevention of recurrent stroke in patients with ESUS led to the hypothesis that this is “perhaps due to heterogeneous underlying etiologies.”
Moreover, these etiologies “may require different types of antithrombotic therapy to best prevent recurrence, and one such underlying etiology may be atrial cardiopathy.”
He explained that several observational studies have found, in the absence of atrial fibrillation, associations between stroke and different markers of atrial cardiopathy and, “given the proven benefit of anticoagulation in preventing strokes in patients with atrial fibrillation, it seems plausible” that they may also benefit.
To investigate further, the team conducted ARCADIA, an investigator-initiated, multicenter, randomized trial involving patients aged 45 years and older from 185 sites in the United States and Canada with a clinical diagnosis of stroke that met the consensus criteria for ESUS.
They also were required to have undergone brain imaging to rule out hemorrhagic stroke, and to have a modified Rankin Scale score of 4 or less, indicating up to a moderately severe degree of disability.
They also had atrial cardiopathy, as determined by P-wave terminal force in V1 greater than 5,000mcV*ms on electrocardiography, serum N-terminal prohormone of brain natriuretic peptide levels greater than 250 pg/mL, or a left atrium diameter of at least 3 cm/m2.
The patients were randomly assigned to apixaban 5 mg or 2.5 mg twice daily plus aspirin placebo, or apixaban placebo plus aspirin 81 mg daily. Those diagnosed with atrial fibrillation after randomization crossed over to open-label anticoagulant therapy at physician discretion.
Dr. Kamel reported that, in 2022, after enrollment of 1015 patients with a mean follow-up of 1.8 years, the trial was halted at the planned interim efficacy/futility analysis, adding that there were “no safety concerns.”
The apixaban and aspirin groups were well balanced in terms of their baseline characteristics. The mean age was 68 years, and 54% were female. Three-quarters of the participants were White; 21.1% were Black.
Prior stroke was reported in 19% of patients. Hypertension was common, in about 77%, and type 2 diabetes was seen in 31%. There were relatively few cases of ischemic heart disease, heart failure, and peripheral arterial disease.
The primary efficacy outcome of recurrent stroke of any type occurred in 4.4% of both patients treated with apixaban and those given aspirin, at a hazard ratio of 1.00 (95% confidence interval, 0.64-1.55). Similar findings were seen when looking individually at ischemic and hemorrhagic stroke, and stroke of undetermined type.
There was also no significant difference in the secondary outcomes of recurrent ischemic stroke or systemic embolism, at 4.1% versus 4.4% (HR, 0.92; 95% CI, 0.59-1.44), and recurrent stroke of any type or death from any cause, at 7.3% versus 6.8% (HR, 1.08; 95% CI, 0.76-1.52).
In terms of safety, rates of major hemorrhage were low and almost identical between the groups, at 0.7% with apixaban and 0.8% for aspirin (HR, 1.02; 95% CI, 0.29-3.51), and were similar for all-cause mortality, at 1.8% versus 1.2% (HR, 1.53; 95% CI, 0.63-3.74).
Proportionately more patients treated with aspirin experienced symptomatic intracranial hemorrhage, at 1.1% versus 0%.
The trial results generated a flurry of interest on Twitter.
Thomas Ford, MD, a vascular neurology fellow from Boston Medical Center, described the results as “disappointing,” although he was “curious to see if there was any signal of benefit in subgroup analyses.”
Shadi Yaghi, codirector of the Comprehensive Stroke Center at Brown University, Providence, R.I., added that the trial “begs the question [as to] whether all device-detected atrial fibrillation warrants anticoagulation.”
Replying, Mitchell Elkind, MD, MPhil, professor of neurology and epidemiology at Columbia University Irving Medical Center, New York, said that he agrees with this interpretation.
“Maybe the issue is not with the concept of atrial cardiopathy but with the need to [anticoagulate] all patients with low [atrial fibrillation] burden or incidental [atrial fibrillation] after stroke.”
The study was funded by the National Institutes of Health and the National Institute of Neurological Disorders and Stroke. The study drug was provided in kind by BMS-Pfizer, and ancillary funding for the NTproBNP assays was provided by Roche. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
FROM ESOC 2023
Commentary: AD, RA, Probiotics, and a New JAK inhibitor, June 2023
The most common adverse events were acne, headache, upper respiratory tract infection, creatine phosphokinase (CPK) level elevations, and nasopharyngitis. I'm confident that none of my patients will have CPK elevations detected, because I won't be testing for it. I suspect that CPK elevations come from people being more physically active when their skin clears up.
I have the sense that our comfort with Janus kinase (JAK) inhibition will grow rapidly as we use the drug for patients with vitiligo, alopecia areata, resistant atopic dermatitis, and other diseases. Many of us are comfortable with methotrexate, dapsone, cyclosporine, and mycophenolate; our comfort with JAK inhibitors for our patients who need it will almost surely follow.
Williams and colleagues did a meta-analysis looking at the relationship between AD and rheumatoid arthritis (RA). They claim that “patients with AD had significantly increased odds of comorbid RA.” This claim should not be taken at face value. What they found was that the observed rate of RA was higher in patients with AD than in controls, and the difference was not something you would see by chance very often. But that does not make something significant. To truly be significant, you'd expect it to be clinically meaningful. The relationship they found — even if not due to chance or to some unmeasured bias — wasn't clinically relevant, in my opinion. RA is a relatively rare phenomenon. If it is barely more common in patients with AD than in controls, it is still rare in AD patients. We don't need to screen for RA in AD patients. We don't need to do anything with this apparent association.
The bottom line is to be wary when you see an article that reports a “significant” finding, especially when it is based on a higher relative risk, like an odds ratio. What we need to know is what the absolute magnitude of the risk is. We need to know how many patients with AD you'd have to see before you'd see one more case of RA due to AD. Williams and colleagues' study doesn't report the information we need as clinicians.
Fijan and colleagues did a meta-analysis to assess the effects of single-strain probiotic lactobacilli on atopic dermatitis. The found a “significant” (meaning, statistically significant) reduction with the lactobacilli treatment compared with placebo. They used the SCORing Atopic Dermatitis (SCORAD) index as the outcome. There was a mean 4.5-unit improvement.
For comparison, Wollenberg and colleagues1 reported the SCORAD improvement seen with dupilumab: 49- and 46-unit improvements in children and adolescents, respectively.
While I'm sure that patients would love a safe, effective, “all natural” probiotic option for atopic dermatitis, I'm not optimistic that this gut magic is going to work.
Additional Reference
- Wollenberg A, Marcoux D, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORing Atopic Dermatitis outcomes in paediatric patients with atopic dermatitis. Acta Derm Venereol. 2022;102:adv00726. doi: 10.2340/actadv.v102.854
The most common adverse events were acne, headache, upper respiratory tract infection, creatine phosphokinase (CPK) level elevations, and nasopharyngitis. I'm confident that none of my patients will have CPK elevations detected, because I won't be testing for it. I suspect that CPK elevations come from people being more physically active when their skin clears up.
I have the sense that our comfort with Janus kinase (JAK) inhibition will grow rapidly as we use the drug for patients with vitiligo, alopecia areata, resistant atopic dermatitis, and other diseases. Many of us are comfortable with methotrexate, dapsone, cyclosporine, and mycophenolate; our comfort with JAK inhibitors for our patients who need it will almost surely follow.
Williams and colleagues did a meta-analysis looking at the relationship between AD and rheumatoid arthritis (RA). They claim that “patients with AD had significantly increased odds of comorbid RA.” This claim should not be taken at face value. What they found was that the observed rate of RA was higher in patients with AD than in controls, and the difference was not something you would see by chance very often. But that does not make something significant. To truly be significant, you'd expect it to be clinically meaningful. The relationship they found — even if not due to chance or to some unmeasured bias — wasn't clinically relevant, in my opinion. RA is a relatively rare phenomenon. If it is barely more common in patients with AD than in controls, it is still rare in AD patients. We don't need to screen for RA in AD patients. We don't need to do anything with this apparent association.
The bottom line is to be wary when you see an article that reports a “significant” finding, especially when it is based on a higher relative risk, like an odds ratio. What we need to know is what the absolute magnitude of the risk is. We need to know how many patients with AD you'd have to see before you'd see one more case of RA due to AD. Williams and colleagues' study doesn't report the information we need as clinicians.
Fijan and colleagues did a meta-analysis to assess the effects of single-strain probiotic lactobacilli on atopic dermatitis. The found a “significant” (meaning, statistically significant) reduction with the lactobacilli treatment compared with placebo. They used the SCORing Atopic Dermatitis (SCORAD) index as the outcome. There was a mean 4.5-unit improvement.
For comparison, Wollenberg and colleagues1 reported the SCORAD improvement seen with dupilumab: 49- and 46-unit improvements in children and adolescents, respectively.
While I'm sure that patients would love a safe, effective, “all natural” probiotic option for atopic dermatitis, I'm not optimistic that this gut magic is going to work.
Additional Reference
- Wollenberg A, Marcoux D, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORing Atopic Dermatitis outcomes in paediatric patients with atopic dermatitis. Acta Derm Venereol. 2022;102:adv00726. doi: 10.2340/actadv.v102.854
The most common adverse events were acne, headache, upper respiratory tract infection, creatine phosphokinase (CPK) level elevations, and nasopharyngitis. I'm confident that none of my patients will have CPK elevations detected, because I won't be testing for it. I suspect that CPK elevations come from people being more physically active when their skin clears up.
I have the sense that our comfort with Janus kinase (JAK) inhibition will grow rapidly as we use the drug for patients with vitiligo, alopecia areata, resistant atopic dermatitis, and other diseases. Many of us are comfortable with methotrexate, dapsone, cyclosporine, and mycophenolate; our comfort with JAK inhibitors for our patients who need it will almost surely follow.
Williams and colleagues did a meta-analysis looking at the relationship between AD and rheumatoid arthritis (RA). They claim that “patients with AD had significantly increased odds of comorbid RA.” This claim should not be taken at face value. What they found was that the observed rate of RA was higher in patients with AD than in controls, and the difference was not something you would see by chance very often. But that does not make something significant. To truly be significant, you'd expect it to be clinically meaningful. The relationship they found — even if not due to chance or to some unmeasured bias — wasn't clinically relevant, in my opinion. RA is a relatively rare phenomenon. If it is barely more common in patients with AD than in controls, it is still rare in AD patients. We don't need to screen for RA in AD patients. We don't need to do anything with this apparent association.
The bottom line is to be wary when you see an article that reports a “significant” finding, especially when it is based on a higher relative risk, like an odds ratio. What we need to know is what the absolute magnitude of the risk is. We need to know how many patients with AD you'd have to see before you'd see one more case of RA due to AD. Williams and colleagues' study doesn't report the information we need as clinicians.
Fijan and colleagues did a meta-analysis to assess the effects of single-strain probiotic lactobacilli on atopic dermatitis. The found a “significant” (meaning, statistically significant) reduction with the lactobacilli treatment compared with placebo. They used the SCORing Atopic Dermatitis (SCORAD) index as the outcome. There was a mean 4.5-unit improvement.
For comparison, Wollenberg and colleagues1 reported the SCORAD improvement seen with dupilumab: 49- and 46-unit improvements in children and adolescents, respectively.
While I'm sure that patients would love a safe, effective, “all natural” probiotic option for atopic dermatitis, I'm not optimistic that this gut magic is going to work.
Additional Reference
- Wollenberg A, Marcoux D, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORing Atopic Dermatitis outcomes in paediatric patients with atopic dermatitis. Acta Derm Venereol. 2022;102:adv00726. doi: 10.2340/actadv.v102.854
No added benefit from revascularization in low-risk CAS
MUNICH – , suggests a planned interim analysis of ECST-2.
Almost 430 patients with symptomatic and asymptomatic atherosclerotic carotid stenosis greater than or equal to 50% and a Carotid Artery Risk (CAR) score less than 20% were randomly assigned to OMT alone or OMT plus revascularization with carotid endarterectomy (CEA) or carotid artery stenting.
The study, which was presented at the annual European Stroke Organisation Conference, was stopped early because of slow recruitment.
Nevertheless, the current results showed that there was no significant difference at 2 years between the treatment groups in the rate of a composite endpoint, as well as the occurrence of any stroke, myocardial infarction, and periprocedural death.
In other words, “there was no evidence of benefit at 2 years from additional carotid revascularization” in patients with carotid stenosis who had a low to intermediate predicted stroke risk, said study presenter Paul Nederkoorn, MD, PhD, department of neurology, Amsterdam UMC, University of Amsterdam.
He added, however, that the complete 2 years will include additional analyses, including an analysis of silent infarcts on MRI, which may affect the results, and that longer clinical follow-up is required.
Future work will include the design and validation of a novel stroke risk prediction tool that will include MRI plaque imaging and will allow individualized patient selection for revascularization, as well as a cost-effectiveness analysis, he noted.
Conclusions ‘difficult’
Session co-chair Peter Kelly, MD, professor of neurology at Mater University Hospital/University College Dublin, and president-elect of the European Stroke Association, described the findings as “interesting” and that it was “great to see them.”
“I’m sure we’ll be discussing these results for a while,” he added.
But co-chair Else Charlotte Sandset, MD, PhD, a consultant neurologist in the Stroke Unit, department of neurology, Oslo University Hospital, said that it’s “difficult to draw firm conclusions from the trial.”
The patients were highly selected, recruitment was “perhaps a bit too slow,” and the study was probably conducted over too many sites, she said in an interview.
Dr. Sandset also noted that the options available for OMT have changed over the course of the study, as well as the overall approach to management.
“We are more aware of how we should treat” these patients, and “we’re probably a bit more aggressive,” which will have shifted the outcomes in the comparator arm as the study progressed.
“That is the challenge of doing these trials that take many years to run – our practice changes.”
‘Old evidence’
In his presentation, Dr. Nederkoorn pointed out that, while the current guidelines for CEA are “robust,” they are based on “old evidence” from trials conducted 20-30 years ago.
During that time, he said, medical treatment has improved significantly, and the risk for stroke has approximately halved. Yet the decision to perform CEA is still largely based on the degree of stenosis and the patient’s symptom status.
Dr. Nederkoorn suggested, however, that factors such as plaque ulceration and patient characteristics and comorbidities might influence the risk-benefit ratio for revascularization.
The current trial was therefore established to test the hypothesis that patients with carotid stenosis greater than or equal to 50% and a low to intermediate risk of stroke will not benefit from additional carotid revascularization on top of optimized medical therapy.
The team conducted a prospective, multicenter, open clinical trial in which patients with both symptomatic and asymptomatic atherosclerotic carotid stenosis were randomly assigned to revascularization plus OMT or OMT alone.
Dr. Nederkoorn explained that a low to intermediate 5-year risk for stroke was established using the CAR score less than 20%.
This is based on a range of parameters, including the sex and age of the patient, degree of stenosis, the type of and time since the event, and the presence of comorbidities, among other factors.
He said that the data was originally derived from the NASCET trial, which was published in 1998, and the first ECST trial, published in the same year.
Since then, the risk of ipsilateral stroke has “strongly declined,” Dr. Nederkoorn said, and so the CAR score was recalibrated to reflect the likely benefit of current OMT.
For the trial, OMT included antihypertensive and cholesterol-lowering medications, and dietary changes, alongside antiplatelet agents and anticoagulation, if indicated, to achieve predefined, guideline-led lipid and blood pressure targets.
Revascularization included CEA and coronary artery stenting in selected patients and was recommended to be performed within 2 weeks of randomization in symptomatic patients and within 4 weeks in asymptomatic patients.
When the trial started in 2012, the intention was to recruit 2,000 patients, with a planned interim analysis after enrollment of 320 patients.
However, recruitment was suspended in 2019, with 429 patients having been enrolled, as it was clear that achieving a cohort of 2,000 patients was “not practical without a change in the trial design” to include MRI plaque imaging and without further funding.
Dr. Nederkoorn showed that the baseline characteristics of the OMT and revascularization plus OMT groups were comparable. The average age of the patients in the groups was 71-72 years, and 31% were female.
Symptomatic disease was present in about 40% of patients, and about 76% had hypertension. Type 2 diabetes was reported in roughly one-quarter of the patients.
There was no difference in the time from randomization to the revascularization procedure between patients with asymptomatic and symptomatic disease.
Moving to the primary outcome, which was a composite of periprocedural death within 90 days of randomization and clinically manifest stroke or myocardial infarction at 2 years, Dr. Nederkoorn showed that there was no significant difference between the treatment groups.
Despite a suggestion that patients undergoing revascularization experienced “more harm” in the initial follow-up period, particularly in patients with a CAR score greater than 10%, the event curves met at around 18 months.
Overall, the hazard ratio between revascularization plus OMT versus OMT alone was 0.96 (95% confidence interval, 0.53-1.76, P = .90).
Breaking down the composite endpoint, there was a numerically lower rate of any stroke with OMT alone, compared with revascularization plus OMT over the study period, but again the difference was not significant at 2 years, at a hazard ratio of 0.68 (95% CI, 0.32-1.42, P = .30).
There was only one case of periprocedural death, in the revascularization arm. Although myocardial infarction was numerically twice as likely with OMT alone, compared with the combined intervention arm, the difference was not significant, at a hazard ratio of 2.00 (95% CI, 0.68-5.84, P = .21).
The study was funded by the National Institute for Health and Care Research, the Swiss National Science Foundation, The Netherlands Organisation of Scientific Research, and the Leeds Neurology Foundation. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MUNICH – , suggests a planned interim analysis of ECST-2.
Almost 430 patients with symptomatic and asymptomatic atherosclerotic carotid stenosis greater than or equal to 50% and a Carotid Artery Risk (CAR) score less than 20% were randomly assigned to OMT alone or OMT plus revascularization with carotid endarterectomy (CEA) or carotid artery stenting.
The study, which was presented at the annual European Stroke Organisation Conference, was stopped early because of slow recruitment.
Nevertheless, the current results showed that there was no significant difference at 2 years between the treatment groups in the rate of a composite endpoint, as well as the occurrence of any stroke, myocardial infarction, and periprocedural death.
In other words, “there was no evidence of benefit at 2 years from additional carotid revascularization” in patients with carotid stenosis who had a low to intermediate predicted stroke risk, said study presenter Paul Nederkoorn, MD, PhD, department of neurology, Amsterdam UMC, University of Amsterdam.
He added, however, that the complete 2 years will include additional analyses, including an analysis of silent infarcts on MRI, which may affect the results, and that longer clinical follow-up is required.
Future work will include the design and validation of a novel stroke risk prediction tool that will include MRI plaque imaging and will allow individualized patient selection for revascularization, as well as a cost-effectiveness analysis, he noted.
Conclusions ‘difficult’
Session co-chair Peter Kelly, MD, professor of neurology at Mater University Hospital/University College Dublin, and president-elect of the European Stroke Association, described the findings as “interesting” and that it was “great to see them.”
“I’m sure we’ll be discussing these results for a while,” he added.
But co-chair Else Charlotte Sandset, MD, PhD, a consultant neurologist in the Stroke Unit, department of neurology, Oslo University Hospital, said that it’s “difficult to draw firm conclusions from the trial.”
The patients were highly selected, recruitment was “perhaps a bit too slow,” and the study was probably conducted over too many sites, she said in an interview.
Dr. Sandset also noted that the options available for OMT have changed over the course of the study, as well as the overall approach to management.
“We are more aware of how we should treat” these patients, and “we’re probably a bit more aggressive,” which will have shifted the outcomes in the comparator arm as the study progressed.
“That is the challenge of doing these trials that take many years to run – our practice changes.”
‘Old evidence’
In his presentation, Dr. Nederkoorn pointed out that, while the current guidelines for CEA are “robust,” they are based on “old evidence” from trials conducted 20-30 years ago.
During that time, he said, medical treatment has improved significantly, and the risk for stroke has approximately halved. Yet the decision to perform CEA is still largely based on the degree of stenosis and the patient’s symptom status.
Dr. Nederkoorn suggested, however, that factors such as plaque ulceration and patient characteristics and comorbidities might influence the risk-benefit ratio for revascularization.
The current trial was therefore established to test the hypothesis that patients with carotid stenosis greater than or equal to 50% and a low to intermediate risk of stroke will not benefit from additional carotid revascularization on top of optimized medical therapy.
The team conducted a prospective, multicenter, open clinical trial in which patients with both symptomatic and asymptomatic atherosclerotic carotid stenosis were randomly assigned to revascularization plus OMT or OMT alone.
Dr. Nederkoorn explained that a low to intermediate 5-year risk for stroke was established using the CAR score less than 20%.
This is based on a range of parameters, including the sex and age of the patient, degree of stenosis, the type of and time since the event, and the presence of comorbidities, among other factors.
He said that the data was originally derived from the NASCET trial, which was published in 1998, and the first ECST trial, published in the same year.
Since then, the risk of ipsilateral stroke has “strongly declined,” Dr. Nederkoorn said, and so the CAR score was recalibrated to reflect the likely benefit of current OMT.
For the trial, OMT included antihypertensive and cholesterol-lowering medications, and dietary changes, alongside antiplatelet agents and anticoagulation, if indicated, to achieve predefined, guideline-led lipid and blood pressure targets.
Revascularization included CEA and coronary artery stenting in selected patients and was recommended to be performed within 2 weeks of randomization in symptomatic patients and within 4 weeks in asymptomatic patients.
When the trial started in 2012, the intention was to recruit 2,000 patients, with a planned interim analysis after enrollment of 320 patients.
However, recruitment was suspended in 2019, with 429 patients having been enrolled, as it was clear that achieving a cohort of 2,000 patients was “not practical without a change in the trial design” to include MRI plaque imaging and without further funding.
Dr. Nederkoorn showed that the baseline characteristics of the OMT and revascularization plus OMT groups were comparable. The average age of the patients in the groups was 71-72 years, and 31% were female.
Symptomatic disease was present in about 40% of patients, and about 76% had hypertension. Type 2 diabetes was reported in roughly one-quarter of the patients.
There was no difference in the time from randomization to the revascularization procedure between patients with asymptomatic and symptomatic disease.
Moving to the primary outcome, which was a composite of periprocedural death within 90 days of randomization and clinically manifest stroke or myocardial infarction at 2 years, Dr. Nederkoorn showed that there was no significant difference between the treatment groups.
Despite a suggestion that patients undergoing revascularization experienced “more harm” in the initial follow-up period, particularly in patients with a CAR score greater than 10%, the event curves met at around 18 months.
Overall, the hazard ratio between revascularization plus OMT versus OMT alone was 0.96 (95% confidence interval, 0.53-1.76, P = .90).
Breaking down the composite endpoint, there was a numerically lower rate of any stroke with OMT alone, compared with revascularization plus OMT over the study period, but again the difference was not significant at 2 years, at a hazard ratio of 0.68 (95% CI, 0.32-1.42, P = .30).
There was only one case of periprocedural death, in the revascularization arm. Although myocardial infarction was numerically twice as likely with OMT alone, compared with the combined intervention arm, the difference was not significant, at a hazard ratio of 2.00 (95% CI, 0.68-5.84, P = .21).
The study was funded by the National Institute for Health and Care Research, the Swiss National Science Foundation, The Netherlands Organisation of Scientific Research, and the Leeds Neurology Foundation. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MUNICH – , suggests a planned interim analysis of ECST-2.
Almost 430 patients with symptomatic and asymptomatic atherosclerotic carotid stenosis greater than or equal to 50% and a Carotid Artery Risk (CAR) score less than 20% were randomly assigned to OMT alone or OMT plus revascularization with carotid endarterectomy (CEA) or carotid artery stenting.
The study, which was presented at the annual European Stroke Organisation Conference, was stopped early because of slow recruitment.
Nevertheless, the current results showed that there was no significant difference at 2 years between the treatment groups in the rate of a composite endpoint, as well as the occurrence of any stroke, myocardial infarction, and periprocedural death.
In other words, “there was no evidence of benefit at 2 years from additional carotid revascularization” in patients with carotid stenosis who had a low to intermediate predicted stroke risk, said study presenter Paul Nederkoorn, MD, PhD, department of neurology, Amsterdam UMC, University of Amsterdam.
He added, however, that the complete 2 years will include additional analyses, including an analysis of silent infarcts on MRI, which may affect the results, and that longer clinical follow-up is required.
Future work will include the design and validation of a novel stroke risk prediction tool that will include MRI plaque imaging and will allow individualized patient selection for revascularization, as well as a cost-effectiveness analysis, he noted.
Conclusions ‘difficult’
Session co-chair Peter Kelly, MD, professor of neurology at Mater University Hospital/University College Dublin, and president-elect of the European Stroke Association, described the findings as “interesting” and that it was “great to see them.”
“I’m sure we’ll be discussing these results for a while,” he added.
But co-chair Else Charlotte Sandset, MD, PhD, a consultant neurologist in the Stroke Unit, department of neurology, Oslo University Hospital, said that it’s “difficult to draw firm conclusions from the trial.”
The patients were highly selected, recruitment was “perhaps a bit too slow,” and the study was probably conducted over too many sites, she said in an interview.
Dr. Sandset also noted that the options available for OMT have changed over the course of the study, as well as the overall approach to management.
“We are more aware of how we should treat” these patients, and “we’re probably a bit more aggressive,” which will have shifted the outcomes in the comparator arm as the study progressed.
“That is the challenge of doing these trials that take many years to run – our practice changes.”
‘Old evidence’
In his presentation, Dr. Nederkoorn pointed out that, while the current guidelines for CEA are “robust,” they are based on “old evidence” from trials conducted 20-30 years ago.
During that time, he said, medical treatment has improved significantly, and the risk for stroke has approximately halved. Yet the decision to perform CEA is still largely based on the degree of stenosis and the patient’s symptom status.
Dr. Nederkoorn suggested, however, that factors such as plaque ulceration and patient characteristics and comorbidities might influence the risk-benefit ratio for revascularization.
The current trial was therefore established to test the hypothesis that patients with carotid stenosis greater than or equal to 50% and a low to intermediate risk of stroke will not benefit from additional carotid revascularization on top of optimized medical therapy.
The team conducted a prospective, multicenter, open clinical trial in which patients with both symptomatic and asymptomatic atherosclerotic carotid stenosis were randomly assigned to revascularization plus OMT or OMT alone.
Dr. Nederkoorn explained that a low to intermediate 5-year risk for stroke was established using the CAR score less than 20%.
This is based on a range of parameters, including the sex and age of the patient, degree of stenosis, the type of and time since the event, and the presence of comorbidities, among other factors.
He said that the data was originally derived from the NASCET trial, which was published in 1998, and the first ECST trial, published in the same year.
Since then, the risk of ipsilateral stroke has “strongly declined,” Dr. Nederkoorn said, and so the CAR score was recalibrated to reflect the likely benefit of current OMT.
For the trial, OMT included antihypertensive and cholesterol-lowering medications, and dietary changes, alongside antiplatelet agents and anticoagulation, if indicated, to achieve predefined, guideline-led lipid and blood pressure targets.
Revascularization included CEA and coronary artery stenting in selected patients and was recommended to be performed within 2 weeks of randomization in symptomatic patients and within 4 weeks in asymptomatic patients.
When the trial started in 2012, the intention was to recruit 2,000 patients, with a planned interim analysis after enrollment of 320 patients.
However, recruitment was suspended in 2019, with 429 patients having been enrolled, as it was clear that achieving a cohort of 2,000 patients was “not practical without a change in the trial design” to include MRI plaque imaging and without further funding.
Dr. Nederkoorn showed that the baseline characteristics of the OMT and revascularization plus OMT groups were comparable. The average age of the patients in the groups was 71-72 years, and 31% were female.
Symptomatic disease was present in about 40% of patients, and about 76% had hypertension. Type 2 diabetes was reported in roughly one-quarter of the patients.
There was no difference in the time from randomization to the revascularization procedure between patients with asymptomatic and symptomatic disease.
Moving to the primary outcome, which was a composite of periprocedural death within 90 days of randomization and clinically manifest stroke or myocardial infarction at 2 years, Dr. Nederkoorn showed that there was no significant difference between the treatment groups.
Despite a suggestion that patients undergoing revascularization experienced “more harm” in the initial follow-up period, particularly in patients with a CAR score greater than 10%, the event curves met at around 18 months.
Overall, the hazard ratio between revascularization plus OMT versus OMT alone was 0.96 (95% confidence interval, 0.53-1.76, P = .90).
Breaking down the composite endpoint, there was a numerically lower rate of any stroke with OMT alone, compared with revascularization plus OMT over the study period, but again the difference was not significant at 2 years, at a hazard ratio of 0.68 (95% CI, 0.32-1.42, P = .30).
There was only one case of periprocedural death, in the revascularization arm. Although myocardial infarction was numerically twice as likely with OMT alone, compared with the combined intervention arm, the difference was not significant, at a hazard ratio of 2.00 (95% CI, 0.68-5.84, P = .21).
The study was funded by the National Institute for Health and Care Research, the Swiss National Science Foundation, The Netherlands Organisation of Scientific Research, and the Leeds Neurology Foundation. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM ESOC 2023
Itchy scaling rash
A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013

A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013
Advances in Microbiome Therapeutics From DDW 2023
Study results of microbiotic therapeutics for Clostridioides difficile infection (CDI) and a microbial dietary score that points to increased cancer risk are the microbiome advances from Digestive Disease Week 2023, as selected by Dr Purna Kashyap, from the Mayo Clinic in Rochester, Minnesota.
Dr Kashyap starts with four studies examining microbiotic therapeutics for patients with CDI; the first two looked at RBX2660, which was recently approved by the US Food and Drug Administration (FDA).
The first study showed that clonal engraftment of RBX2660 microbiota was associated with clinical response to the treatment, while the second indicated that the therapy is safe and effective in immunocompromised patients.
Next, Dr Kashyap discusses a study of SER-109, also recently approved by the FDA. ESOSPOR IV revealed that the oral microbiome therapeutic achieved durable responses, even in patients with two or more CDI recurrences.
After discussing a final CDI study that may provide a mechanism for the effectiveness of the live biotherapeutic VE303, he moves on to colon cancer.
Dr Kashyap explains that a microbial dietary score was found to be associated not only with low-quality diets but also with an increased risk for colorectal cancer.
--
Purna C. Kashyap, MBBS, Professor of Medicine and Physiology; Consultant, Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota
Purna C. Kashyap, MBBS, has disclosed no relevant financial relationships.
Digestive Disease Week® was sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Study results of microbiotic therapeutics for Clostridioides difficile infection (CDI) and a microbial dietary score that points to increased cancer risk are the microbiome advances from Digestive Disease Week 2023, as selected by Dr Purna Kashyap, from the Mayo Clinic in Rochester, Minnesota.
Dr Kashyap starts with four studies examining microbiotic therapeutics for patients with CDI; the first two looked at RBX2660, which was recently approved by the US Food and Drug Administration (FDA).
The first study showed that clonal engraftment of RBX2660 microbiota was associated with clinical response to the treatment, while the second indicated that the therapy is safe and effective in immunocompromised patients.
Next, Dr Kashyap discusses a study of SER-109, also recently approved by the FDA. ESOSPOR IV revealed that the oral microbiome therapeutic achieved durable responses, even in patients with two or more CDI recurrences.
After discussing a final CDI study that may provide a mechanism for the effectiveness of the live biotherapeutic VE303, he moves on to colon cancer.
Dr Kashyap explains that a microbial dietary score was found to be associated not only with low-quality diets but also with an increased risk for colorectal cancer.
--
Purna C. Kashyap, MBBS, Professor of Medicine and Physiology; Consultant, Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota
Purna C. Kashyap, MBBS, has disclosed no relevant financial relationships.
Digestive Disease Week® was sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Study results of microbiotic therapeutics for Clostridioides difficile infection (CDI) and a microbial dietary score that points to increased cancer risk are the microbiome advances from Digestive Disease Week 2023, as selected by Dr Purna Kashyap, from the Mayo Clinic in Rochester, Minnesota.
Dr Kashyap starts with four studies examining microbiotic therapeutics for patients with CDI; the first two looked at RBX2660, which was recently approved by the US Food and Drug Administration (FDA).
The first study showed that clonal engraftment of RBX2660 microbiota was associated with clinical response to the treatment, while the second indicated that the therapy is safe and effective in immunocompromised patients.
Next, Dr Kashyap discusses a study of SER-109, also recently approved by the FDA. ESOSPOR IV revealed that the oral microbiome therapeutic achieved durable responses, even in patients with two or more CDI recurrences.
After discussing a final CDI study that may provide a mechanism for the effectiveness of the live biotherapeutic VE303, he moves on to colon cancer.
Dr Kashyap explains that a microbial dietary score was found to be associated not only with low-quality diets but also with an increased risk for colorectal cancer.
--
Purna C. Kashyap, MBBS, Professor of Medicine and Physiology; Consultant, Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota
Purna C. Kashyap, MBBS, has disclosed no relevant financial relationships.
Digestive Disease Week® was sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.

Internists in 2022: Increased earnings can’t stop rising dissatisfaction
Internists experienced many of the usual ups and downs regarding nonclinical matters in 2022: Compensation was up, but satisfaction with compensation was down; the percentage of internists who would choose another specialty was up and time spent on paperwork and administration was down only slightly.
A year that began with the COVID-19 Omicron surge ended with many of the same old issues regaining the attention of physicians, according to those who responded to Medscape’s annual compensation survey, which was conducted from Oct. 2, 2022, to Jan. 17, 2023.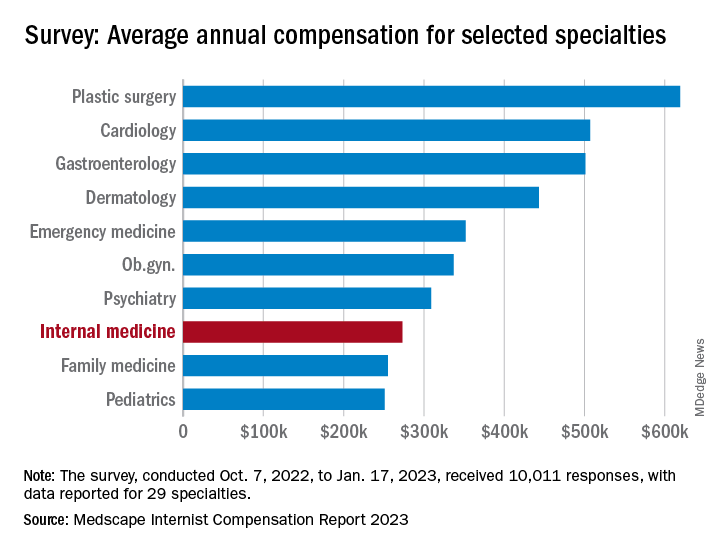
“Decreasing Medicare reimbursement and poor payor mix destroy our income,” one physician wrote, and another said that “patients have become rude and come with poor information from social media.” One respondent described the situation this way: “Overwhelming burnout. I had to reduce my hours to keep myself from quitting medicine completely.”
For internists at least, some of the survey results were positive. For the 13% of the 10,011 respondents who practice internal medicine, average compensation went from $264,000 in 2021 to $273,000 in 2022, an increase of almost 4% that matched the average for all physicians. Among the other primary care specialists, pediatricians did almost as well with a 3% increase, but ob.gyns. and family physicians only managed to keep their 2022 earnings at 2021 levels.
Overall physician compensation for 2022 was $352,000, an increase of almost 18% since 2018. “Supply and demand is the biggest driver,” Mike Belkin, JD, of physician recruitment firm Merritt Hawkins, said in an interview. “Organizations understand it’s not getting any easier to get good candidates, and so for the most part, physicians are getting good offers.”
The latest increase in earnings among internists also included a decline: The disparity between mens’ and womens’ compensation dropped from 24% in 2021 to 16% in 2022. The gap was slightly larger for all physicians in 2022, with men earning about 19% more than women, and larger again among specialists at 27%, but both of those figures are lower than in recent years, Medscape said.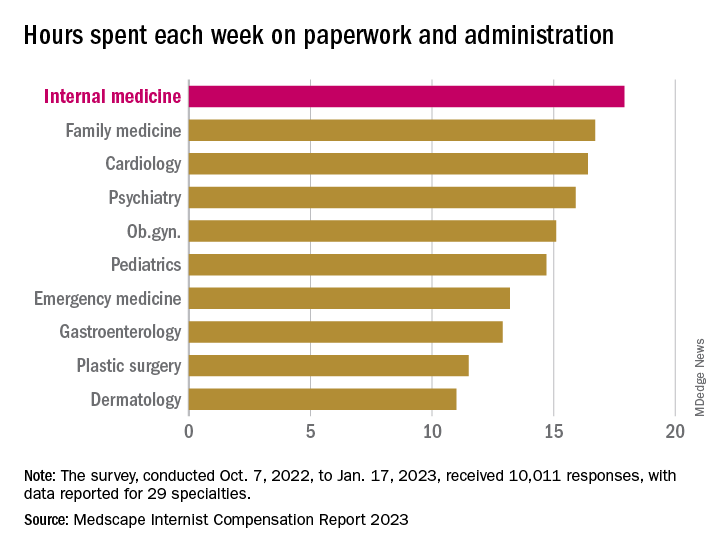
Satisfaction with their compensation, however, was not high for internists: Only 43% feel that they are fairly paid, coming in above only ophthalmology (42%) and infectious diseases (35%) and well below psychiatry (68%) at the top of the list, the Medscape data show. In the 2022 report, 49% of internists said that they had been fairly paid.
In another source of potential dissatisfaction, internist respondents reported spending an average of 17.9 hours each week on paperwork and administration, just below the survey leaders, physical medicine and rehabilitation (18.5 hours) and nephrology (18.1 hours) and well above anesthesiology, which was the lowest of the 29 specialties at 9.0 hours, and the 2022 average of 15.5 hours for all physicians, Medscape said. A small bright spot comes in the form of a decline from the internists’ time of 18.7 hours per week in 2021.
When asked if they would choose medicine again, 72% of internist respondents and 73% of all physicians said yes, with emergency medicine (65%) and dermatology (86%) representing the two extremes. A question about specialty choice showed internists to be the least likely of the 29 included specialties to follow the same path, with 61% (down from 63% in 2022) approving their initial selection, versus 97% for plastic surgeons, Medscape reported.
Commenters among the survey respondents were not identified by specialty, but dissatisfaction on many fronts was a definite theme:
- “Our costs go up, and our reimbursement does not.”
- “Our practice was acquired by venture capital firms; they slashed costs.”
- “My productivity bonus should have come to $45,000. Instead I was paid only $15,000. Yet cardiologists and administrators who were working from home part of the year received their full bonus.”
- “I will no longer practice cookbook mediocrity.”
Internists experienced many of the usual ups and downs regarding nonclinical matters in 2022: Compensation was up, but satisfaction with compensation was down; the percentage of internists who would choose another specialty was up and time spent on paperwork and administration was down only slightly.
A year that began with the COVID-19 Omicron surge ended with many of the same old issues regaining the attention of physicians, according to those who responded to Medscape’s annual compensation survey, which was conducted from Oct. 2, 2022, to Jan. 17, 2023.
“Decreasing Medicare reimbursement and poor payor mix destroy our income,” one physician wrote, and another said that “patients have become rude and come with poor information from social media.” One respondent described the situation this way: “Overwhelming burnout. I had to reduce my hours to keep myself from quitting medicine completely.”
For internists at least, some of the survey results were positive. For the 13% of the 10,011 respondents who practice internal medicine, average compensation went from $264,000 in 2021 to $273,000 in 2022, an increase of almost 4% that matched the average for all physicians. Among the other primary care specialists, pediatricians did almost as well with a 3% increase, but ob.gyns. and family physicians only managed to keep their 2022 earnings at 2021 levels.
Overall physician compensation for 2022 was $352,000, an increase of almost 18% since 2018. “Supply and demand is the biggest driver,” Mike Belkin, JD, of physician recruitment firm Merritt Hawkins, said in an interview. “Organizations understand it’s not getting any easier to get good candidates, and so for the most part, physicians are getting good offers.”
The latest increase in earnings among internists also included a decline: The disparity between mens’ and womens’ compensation dropped from 24% in 2021 to 16% in 2022. The gap was slightly larger for all physicians in 2022, with men earning about 19% more than women, and larger again among specialists at 27%, but both of those figures are lower than in recent years, Medscape said.
Satisfaction with their compensation, however, was not high for internists: Only 43% feel that they are fairly paid, coming in above only ophthalmology (42%) and infectious diseases (35%) and well below psychiatry (68%) at the top of the list, the Medscape data show. In the 2022 report, 49% of internists said that they had been fairly paid.
In another source of potential dissatisfaction, internist respondents reported spending an average of 17.9 hours each week on paperwork and administration, just below the survey leaders, physical medicine and rehabilitation (18.5 hours) and nephrology (18.1 hours) and well above anesthesiology, which was the lowest of the 29 specialties at 9.0 hours, and the 2022 average of 15.5 hours for all physicians, Medscape said. A small bright spot comes in the form of a decline from the internists’ time of 18.7 hours per week in 2021.
When asked if they would choose medicine again, 72% of internist respondents and 73% of all physicians said yes, with emergency medicine (65%) and dermatology (86%) representing the two extremes. A question about specialty choice showed internists to be the least likely of the 29 included specialties to follow the same path, with 61% (down from 63% in 2022) approving their initial selection, versus 97% for plastic surgeons, Medscape reported.
Commenters among the survey respondents were not identified by specialty, but dissatisfaction on many fronts was a definite theme:
- “Our costs go up, and our reimbursement does not.”
- “Our practice was acquired by venture capital firms; they slashed costs.”
- “My productivity bonus should have come to $45,000. Instead I was paid only $15,000. Yet cardiologists and administrators who were working from home part of the year received their full bonus.”
- “I will no longer practice cookbook mediocrity.”
Internists experienced many of the usual ups and downs regarding nonclinical matters in 2022: Compensation was up, but satisfaction with compensation was down; the percentage of internists who would choose another specialty was up and time spent on paperwork and administration was down only slightly.
A year that began with the COVID-19 Omicron surge ended with many of the same old issues regaining the attention of physicians, according to those who responded to Medscape’s annual compensation survey, which was conducted from Oct. 2, 2022, to Jan. 17, 2023.
“Decreasing Medicare reimbursement and poor payor mix destroy our income,” one physician wrote, and another said that “patients have become rude and come with poor information from social media.” One respondent described the situation this way: “Overwhelming burnout. I had to reduce my hours to keep myself from quitting medicine completely.”
For internists at least, some of the survey results were positive. For the 13% of the 10,011 respondents who practice internal medicine, average compensation went from $264,000 in 2021 to $273,000 in 2022, an increase of almost 4% that matched the average for all physicians. Among the other primary care specialists, pediatricians did almost as well with a 3% increase, but ob.gyns. and family physicians only managed to keep their 2022 earnings at 2021 levels.
Overall physician compensation for 2022 was $352,000, an increase of almost 18% since 2018. “Supply and demand is the biggest driver,” Mike Belkin, JD, of physician recruitment firm Merritt Hawkins, said in an interview. “Organizations understand it’s not getting any easier to get good candidates, and so for the most part, physicians are getting good offers.”
The latest increase in earnings among internists also included a decline: The disparity between mens’ and womens’ compensation dropped from 24% in 2021 to 16% in 2022. The gap was slightly larger for all physicians in 2022, with men earning about 19% more than women, and larger again among specialists at 27%, but both of those figures are lower than in recent years, Medscape said.
Satisfaction with their compensation, however, was not high for internists: Only 43% feel that they are fairly paid, coming in above only ophthalmology (42%) and infectious diseases (35%) and well below psychiatry (68%) at the top of the list, the Medscape data show. In the 2022 report, 49% of internists said that they had been fairly paid.
In another source of potential dissatisfaction, internist respondents reported spending an average of 17.9 hours each week on paperwork and administration, just below the survey leaders, physical medicine and rehabilitation (18.5 hours) and nephrology (18.1 hours) and well above anesthesiology, which was the lowest of the 29 specialties at 9.0 hours, and the 2022 average of 15.5 hours for all physicians, Medscape said. A small bright spot comes in the form of a decline from the internists’ time of 18.7 hours per week in 2021.
When asked if they would choose medicine again, 72% of internist respondents and 73% of all physicians said yes, with emergency medicine (65%) and dermatology (86%) representing the two extremes. A question about specialty choice showed internists to be the least likely of the 29 included specialties to follow the same path, with 61% (down from 63% in 2022) approving their initial selection, versus 97% for plastic surgeons, Medscape reported.
Commenters among the survey respondents were not identified by specialty, but dissatisfaction on many fronts was a definite theme:
- “Our costs go up, and our reimbursement does not.”
- “Our practice was acquired by venture capital firms; they slashed costs.”
- “My productivity bonus should have come to $45,000. Instead I was paid only $15,000. Yet cardiologists and administrators who were working from home part of the year received their full bonus.”
- “I will no longer practice cookbook mediocrity.”
Researchers discover brain abnormalities in babies who had SIDS
For decades, researchers have been trying to understand why some otherwise healthy babies under 1 year old mysteriously die during their sleep. SIDS is the leading cause of infant death in the U.S., affecting 103 out of every 100,000 babies.
The new study found that babies who died of SIDS had abnormalities in certain brain receptors responsible for waking and restoring breathing. The scientists decided to look at the babies’ brains at the molecular level because previous research showed that the same kind of brain receptors in rodents are responsible for protective breathing functions during sleep.
The study was published in the Journal of Neuropathology & Experimental Neurology. The researchers compared brain stems from 70 babies, some of whom died of SIDS and some who died of other causes.
Despite discovering the differences in the babies’ brains, the lead author of the paper said more study is needed.
Robin Haynes, PhD, who studies SIDS at Boston Children’s Hospital, said in a statement that “the relationship between the abnormalities and cause of death remains unknown.”
She said there is no way to identify babies with the brain abnormalities, and “thus, adherence to safe-sleep practices remains critical.”
The American Academy of Pediatrics recommends numerous steps for creating a safe sleeping environment for babies, including placing babies on their backs on a firm surface. Education campaigns targeting parents and caregivers in the 1990s are largely considered successful, but SIDS rates have remained steady since the practices became widely used.
A version of this article first appeared on WebMD.com.
For decades, researchers have been trying to understand why some otherwise healthy babies under 1 year old mysteriously die during their sleep. SIDS is the leading cause of infant death in the U.S., affecting 103 out of every 100,000 babies.
The new study found that babies who died of SIDS had abnormalities in certain brain receptors responsible for waking and restoring breathing. The scientists decided to look at the babies’ brains at the molecular level because previous research showed that the same kind of brain receptors in rodents are responsible for protective breathing functions during sleep.
The study was published in the Journal of Neuropathology & Experimental Neurology. The researchers compared brain stems from 70 babies, some of whom died of SIDS and some who died of other causes.
Despite discovering the differences in the babies’ brains, the lead author of the paper said more study is needed.
Robin Haynes, PhD, who studies SIDS at Boston Children’s Hospital, said in a statement that “the relationship between the abnormalities and cause of death remains unknown.”
She said there is no way to identify babies with the brain abnormalities, and “thus, adherence to safe-sleep practices remains critical.”
The American Academy of Pediatrics recommends numerous steps for creating a safe sleeping environment for babies, including placing babies on their backs on a firm surface. Education campaigns targeting parents and caregivers in the 1990s are largely considered successful, but SIDS rates have remained steady since the practices became widely used.
A version of this article first appeared on WebMD.com.
For decades, researchers have been trying to understand why some otherwise healthy babies under 1 year old mysteriously die during their sleep. SIDS is the leading cause of infant death in the U.S., affecting 103 out of every 100,000 babies.
The new study found that babies who died of SIDS had abnormalities in certain brain receptors responsible for waking and restoring breathing. The scientists decided to look at the babies’ brains at the molecular level because previous research showed that the same kind of brain receptors in rodents are responsible for protective breathing functions during sleep.
The study was published in the Journal of Neuropathology & Experimental Neurology. The researchers compared brain stems from 70 babies, some of whom died of SIDS and some who died of other causes.
Despite discovering the differences in the babies’ brains, the lead author of the paper said more study is needed.
Robin Haynes, PhD, who studies SIDS at Boston Children’s Hospital, said in a statement that “the relationship between the abnormalities and cause of death remains unknown.”
She said there is no way to identify babies with the brain abnormalities, and “thus, adherence to safe-sleep practices remains critical.”
The American Academy of Pediatrics recommends numerous steps for creating a safe sleeping environment for babies, including placing babies on their backs on a firm surface. Education campaigns targeting parents and caregivers in the 1990s are largely considered successful, but SIDS rates have remained steady since the practices became widely used.
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF NEUROPATHY & EXPERIMENTAL NEUROLOGY
Key Takeaways in Ulcerative Colitis From DDW 2023
Efficacy and long-term safety data of novel drugs, thiopurine withdrawal, and the effect of high-dose opioid use on outcomes are among the key takeaways in ulcerative colitis from Digestive Disease Week (DDW) 2023, as reported by Dr Joseph Feuerstein, from Harvard Medical School, in Boston, Massachusetts.
Dr Feuerstein starts with the QUASAR study of the IL-13 inhibitor guselkumab, which showed that the drug was associated with significantly improved clinical remission over placebo.
Next, an open-label extension of the True North study demonstrated that the oral S1P receptor modulator ozanimod proved to be safe over a 3-year follow-up period. Another trial examining safety found that withdrawal from thiopurine and vedolizumab combination therapy may not be a viable strategy.
Dr Feuerstein then turns to a retrospective analysis of older patients who underwent segmental colectomy in which the procedure was associated with low rates of complications and postoperative flares.
Finally, another retrospective study suggested that, contrary to expectations, high-dose opioid use does not appear to worsen clinical outcomes in acute severe ulcerative colitis.
--
Joseph D. Feuerstein, MD, Associate Professor of Medicine, Department of Gastroenterology, Harvard Medical School; Attending in Gastroenterology, Center for Inflammatory Bowel Disease, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Joseph D. Feuerstein, MD, has disclosed no relevant financial relationships.
Digestive Disease Week® was sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Efficacy and long-term safety data of novel drugs, thiopurine withdrawal, and the effect of high-dose opioid use on outcomes are among the key takeaways in ulcerative colitis from Digestive Disease Week (DDW) 2023, as reported by Dr Joseph Feuerstein, from Harvard Medical School, in Boston, Massachusetts.
Dr Feuerstein starts with the QUASAR study of the IL-13 inhibitor guselkumab, which showed that the drug was associated with significantly improved clinical remission over placebo.
Next, an open-label extension of the True North study demonstrated that the oral S1P receptor modulator ozanimod proved to be safe over a 3-year follow-up period. Another trial examining safety found that withdrawal from thiopurine and vedolizumab combination therapy may not be a viable strategy.
Dr Feuerstein then turns to a retrospective analysis of older patients who underwent segmental colectomy in which the procedure was associated with low rates of complications and postoperative flares.
Finally, another retrospective study suggested that, contrary to expectations, high-dose opioid use does not appear to worsen clinical outcomes in acute severe ulcerative colitis.
--
Joseph D. Feuerstein, MD, Associate Professor of Medicine, Department of Gastroenterology, Harvard Medical School; Attending in Gastroenterology, Center for Inflammatory Bowel Disease, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Joseph D. Feuerstein, MD, has disclosed no relevant financial relationships.
Digestive Disease Week® was sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Efficacy and long-term safety data of novel drugs, thiopurine withdrawal, and the effect of high-dose opioid use on outcomes are among the key takeaways in ulcerative colitis from Digestive Disease Week (DDW) 2023, as reported by Dr Joseph Feuerstein, from Harvard Medical School, in Boston, Massachusetts.
Dr Feuerstein starts with the QUASAR study of the IL-13 inhibitor guselkumab, which showed that the drug was associated with significantly improved clinical remission over placebo.
Next, an open-label extension of the True North study demonstrated that the oral S1P receptor modulator ozanimod proved to be safe over a 3-year follow-up period. Another trial examining safety found that withdrawal from thiopurine and vedolizumab combination therapy may not be a viable strategy.
Dr Feuerstein then turns to a retrospective analysis of older patients who underwent segmental colectomy in which the procedure was associated with low rates of complications and postoperative flares.
Finally, another retrospective study suggested that, contrary to expectations, high-dose opioid use does not appear to worsen clinical outcomes in acute severe ulcerative colitis.
--
Joseph D. Feuerstein, MD, Associate Professor of Medicine, Department of Gastroenterology, Harvard Medical School; Attending in Gastroenterology, Center for Inflammatory Bowel Disease, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Joseph D. Feuerstein, MD, has disclosed no relevant financial relationships.
Digestive Disease Week® was sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.