User login
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
.1
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively.2 Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope.3 Further, infection rates and vasovagal events were far lower with hysteroscopy.1
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity.4 Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
How should physicians perform office hysteroscopy to minimize patient discomfort?
The classic paradigm has been to focus on paracervical blocks, anxiolytics, and a supportive environment (such as mood music). However, those are far more important when your hysteroscope is larger than the natural cervical lumen. If you can use small hysteroscopes (< 3 mm for the nulliparous cervix, < 4 mm for the parous cervix), most women will not require cervical dilation, which further enhances the patient experience.
Using a flexible hysteroscope for suspected pathology, making sure not to overdistend the uterus (particularly in high-risk patients such as those with tubal occlusion and cervical stenosis), and vaginoscopy can all minimize patient discomfort. We have published data showing that by using a 2.8-mm flexible diagnostic hysteroscope in a group of mostly nulliparous women, greater than 50% have no discomfort, and more than 90% will have mild to no discomfort.3
What operative hysteroscopy procedures can be performed safely in a physician’s office, and what equipment is required?
Though highly dependent on experience and resources, reproductive endocrinology and infertility specialists (REIs) arguably have the easiest transition to operative office hysteroscopy by utilizing the analgesia and procedure room that is standard for oocyte retrieval and simply adding hysteroscopic procedures. The accompanying table stratifies general hysteroscopic procedures by difficulty.
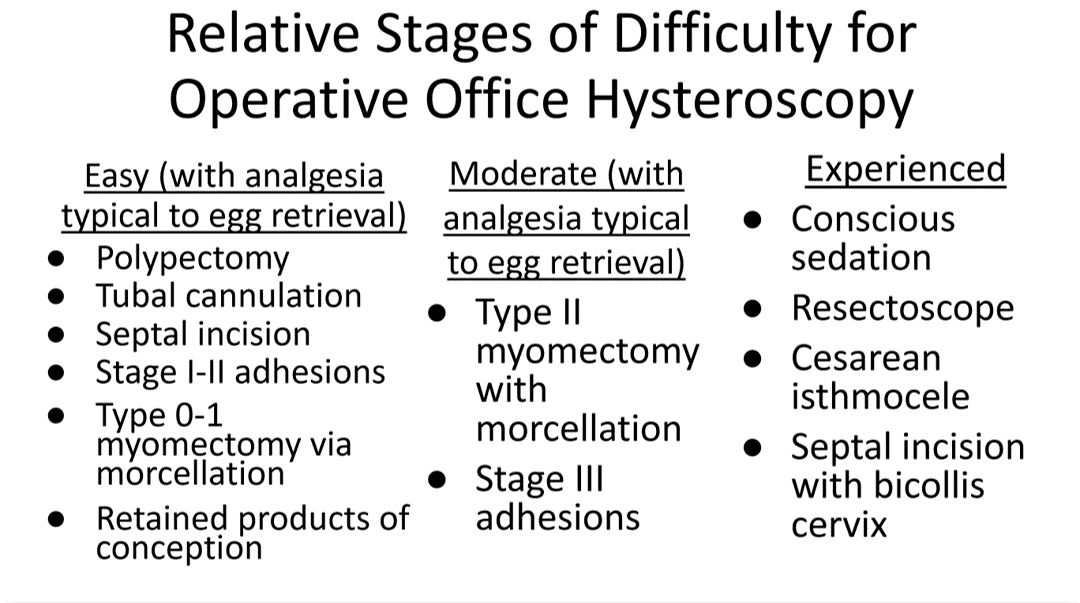
If one can use propofol or a similar level of sedation (which is routinely utilized for oocyte aspiration), there are few hysteroscopies that cannot be accomplished in the office. However, the less sedation and analgesia, the more judicious one must be in patient selection. Moreover, there are trade-offs between visualization, comfort, and instrumentation.
The greater the uterine distention and diameter of the hysteroscope, the more patients experience pain. One-third of patients (especially nulliparous) will discontinue a procedure with a 5-mm hysteroscope because of discomfort.5 However, as one drops to 4.5 mm and smaller operative hysteroscopes, instruments often occupy the inflow channel, limiting distention and visualization, which also can affect completion rates and safety.
When is operative hysteroscopy best suited for the OR?
In addition to physician experience and clinical resources, the critical factors guiding our choices for selecting the OR rather than the office, include:
- Loss of landmarks. Though Dr. Parry now does most severe intrauterine adhesion cases in the office with ultrasound guidance, when neither ostia can be visualized there is meaningful risk for perforation. Preoperative estrogen, development of planes with the diagnostic hysteroscope prior, and preparing the patient for a possible multistage procedure are all important.
- Use of energy. There are many excellent hysteroscopic surgeons who use the resectoscope well in the office. However, with possible patient movement and potential perforation with energy leading to a bowel injury, there can be greater risk when using energy relative to other methods (such as forceps, scissors, and mechanical morcellation).
- Deeper fibroids. Fibroids displace rather than invade the myometrium, and one can sonographically visualize the myometrium reapproximate over a fibroid as it herniates more into the uterine cavity. Nevertheless, the closer a fibroid comes to the serosa, the more mindful one should be of risks and balances for hysteroscopic removal.
In a patient with a severely stenotic cervix or tortuous endocervical canal, what preprocedure methods do you find helpful, and do you utilize abdominal ultrasound guidance?
If using a 2.8-mm flexible diagnostic hysteroscope, we find 99.8%-99.9% of cervices can be successfully cannulated in the office, with rare exception, that is, following cryotherapy or chlamydia cervicitis. This is the equivalent of your dilator having a camera on the tip and fully articulating to adjust to the cervical path.
Transvaginal sonography prior to hysteroscopy where one maps the cervical lumen helps anticipate problems (along with being familiar with the patient’s history). For the rare dilation under anesthesia, concurrent sonography with a 2.8-mm flexible hysteroscope and intermittent dilator use has been sufficient for our exceptions without the need for lacrimal dilators, vasopressin, misoprostol, and other adjuncts. Of note, we use a 1080p flexible endoscope, as lower resolution would make this more challenging.
In patients with recurrent implantation failure following IVF, is hysteroscopy superior to 3D saline infusion sonogram?
At an American Society of Reproductive Medicine 2021 session, Ilan Tur-Kaspa, MD, and Dr. Parry debated the topic of 2D ultrasound combined with hysteroscopy vs. 3D saline infusion sonography. Core areas of agreement were that expert hands for any approach are better than nonexpert, and high-resolution technology is better than lower resolution. There was also agreement that extrauterine and myometrial disease, such as intramural fibroids and adenomyosis, are contributory factors.
So, sonography will always have a role. However, existing and forthcoming data show hysteroscopy to improve live birth rates for patients with recurrent implantation failure after IVF. Dr. Parry finds diagnostic hysteroscopy easier for identifying endometritis, sessile and cornual polyps, retained products of conception (which are often isoechogenic with the endometrium) and lateral adhesions.
The reality is that there is variability among physicians and midlevel providers in both sonographic and diagnostic hysteroscopic skill. If one wants to verify findings with another team member, acknowledging that there can be nuances to identifying these pathologies by sonography, it is easier to share and discuss findings through hysteroscopic video than sonographic records.
When is endometrial biopsy indicated during office hysteroscopy?
The patients of an REI are very unlikely to have endometrial cancer (or even hyperplasia) outside of polyps (or arguably hypervascular areas of overgrowth), so the focus is on resecting visualized pathology relative to random biopsy.
However, the threshold for biopsy should be adjusted to the patient population, as well as to individual findings and risk. RVUs are greatly increased (11.1 > 41.57) with biopsy, helping sustainability. Additionally, if one places the hysteroscope on endometrium and applies suction through the inflow channel, one can obtain a sample with small-caliber diagnostic hysteroscopes and without having to use forceps.
What is your threshold for fluid deficit in hysteroscopy?
We follow AAGL guidelines, which for operative hysteroscopy are 2,500 mL of isotonic fluids or 1,000 mL of hypotonic fluids in low-risk patients. This should be further reduced to 500 mL of isotonic fluids in the elderly and even 300 mL in those with cardiovascular compromise.6
For patients who request sedation for office hysteroscopy, which option do you recommend – paracervical block alone, nitrous oxide, or the combination?
For diagnostic, greater than 95% of our patients do not require even over-the-counter analgesic medications. For operative, we consider all permissible resources that allow for a safe combination that is appropriate to the pathology and clinical setting, such as paracervical blocks, nitrous oxide, NSAIDs such as ketorolac, anxiolytics, and more.
The goal is to optimize the patient experience. However, the top three criteria that influence successful operative office hysteroscopy for a conscious patient are a parous cervix, judicious patient selection, and pre- and intraoperative verbal analgesia. Informed consent and engagement improve the experience of both the patient and physician.
Dr. Parry is the founder of Positive Steps Fertility in Madison, Miss. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Parry JP et al. J Minim Invasive Gynecol. 2017 May-Jun. doi: 10.1016/j.jmig.2017.02.010.
2. Wadhwa L et al. 2017 Apr-Jun. doi: 10.4103/jhrs.JHRS_123_16.
3. Parry JP et al. Fertil Steril. 2017 Oct. doi: 10.1016/j.fertnstert.2017.07.1159.
4. Penzias A et al. Fertil Steril. 2021 Nov. doi: 10.1016/j.fertnstert.2021.08.038.
5. Campo R et al. Hum Reprod. 2005 Jan;20(1):258-63. doi: 10.1093/humrep/deh559.
6. AAGL AAGL practice report: Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013 Mar-Apr. doi: 10.1016/j.jmig.2012.12.002.
What role does diagnostic office hysteroscopy play in an infertility evaluation?
.1
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively.2 Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope.3 Further, infection rates and vasovagal events were far lower with hysteroscopy.1
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity.4 Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
How should physicians perform office hysteroscopy to minimize patient discomfort?
The classic paradigm has been to focus on paracervical blocks, anxiolytics, and a supportive environment (such as mood music). However, those are far more important when your hysteroscope is larger than the natural cervical lumen. If you can use small hysteroscopes (< 3 mm for the nulliparous cervix, < 4 mm for the parous cervix), most women will not require cervical dilation, which further enhances the patient experience.
Using a flexible hysteroscope for suspected pathology, making sure not to overdistend the uterus (particularly in high-risk patients such as those with tubal occlusion and cervical stenosis), and vaginoscopy can all minimize patient discomfort. We have published data showing that by using a 2.8-mm flexible diagnostic hysteroscope in a group of mostly nulliparous women, greater than 50% have no discomfort, and more than 90% will have mild to no discomfort.3
What operative hysteroscopy procedures can be performed safely in a physician’s office, and what equipment is required?
Though highly dependent on experience and resources, reproductive endocrinology and infertility specialists (REIs) arguably have the easiest transition to operative office hysteroscopy by utilizing the analgesia and procedure room that is standard for oocyte retrieval and simply adding hysteroscopic procedures. The accompanying table stratifies general hysteroscopic procedures by difficulty.

If one can use propofol or a similar level of sedation (which is routinely utilized for oocyte aspiration), there are few hysteroscopies that cannot be accomplished in the office. However, the less sedation and analgesia, the more judicious one must be in patient selection. Moreover, there are trade-offs between visualization, comfort, and instrumentation.
The greater the uterine distention and diameter of the hysteroscope, the more patients experience pain. One-third of patients (especially nulliparous) will discontinue a procedure with a 5-mm hysteroscope because of discomfort.5 However, as one drops to 4.5 mm and smaller operative hysteroscopes, instruments often occupy the inflow channel, limiting distention and visualization, which also can affect completion rates and safety.
When is operative hysteroscopy best suited for the OR?
In addition to physician experience and clinical resources, the critical factors guiding our choices for selecting the OR rather than the office, include:
- Loss of landmarks. Though Dr. Parry now does most severe intrauterine adhesion cases in the office with ultrasound guidance, when neither ostia can be visualized there is meaningful risk for perforation. Preoperative estrogen, development of planes with the diagnostic hysteroscope prior, and preparing the patient for a possible multistage procedure are all important.
- Use of energy. There are many excellent hysteroscopic surgeons who use the resectoscope well in the office. However, with possible patient movement and potential perforation with energy leading to a bowel injury, there can be greater risk when using energy relative to other methods (such as forceps, scissors, and mechanical morcellation).
- Deeper fibroids. Fibroids displace rather than invade the myometrium, and one can sonographically visualize the myometrium reapproximate over a fibroid as it herniates more into the uterine cavity. Nevertheless, the closer a fibroid comes to the serosa, the more mindful one should be of risks and balances for hysteroscopic removal.
In a patient with a severely stenotic cervix or tortuous endocervical canal, what preprocedure methods do you find helpful, and do you utilize abdominal ultrasound guidance?
If using a 2.8-mm flexible diagnostic hysteroscope, we find 99.8%-99.9% of cervices can be successfully cannulated in the office, with rare exception, that is, following cryotherapy or chlamydia cervicitis. This is the equivalent of your dilator having a camera on the tip and fully articulating to adjust to the cervical path.
Transvaginal sonography prior to hysteroscopy where one maps the cervical lumen helps anticipate problems (along with being familiar with the patient’s history). For the rare dilation under anesthesia, concurrent sonography with a 2.8-mm flexible hysteroscope and intermittent dilator use has been sufficient for our exceptions without the need for lacrimal dilators, vasopressin, misoprostol, and other adjuncts. Of note, we use a 1080p flexible endoscope, as lower resolution would make this more challenging.
In patients with recurrent implantation failure following IVF, is hysteroscopy superior to 3D saline infusion sonogram?
At an American Society of Reproductive Medicine 2021 session, Ilan Tur-Kaspa, MD, and Dr. Parry debated the topic of 2D ultrasound combined with hysteroscopy vs. 3D saline infusion sonography. Core areas of agreement were that expert hands for any approach are better than nonexpert, and high-resolution technology is better than lower resolution. There was also agreement that extrauterine and myometrial disease, such as intramural fibroids and adenomyosis, are contributory factors.
So, sonography will always have a role. However, existing and forthcoming data show hysteroscopy to improve live birth rates for patients with recurrent implantation failure after IVF. Dr. Parry finds diagnostic hysteroscopy easier for identifying endometritis, sessile and cornual polyps, retained products of conception (which are often isoechogenic with the endometrium) and lateral adhesions.
The reality is that there is variability among physicians and midlevel providers in both sonographic and diagnostic hysteroscopic skill. If one wants to verify findings with another team member, acknowledging that there can be nuances to identifying these pathologies by sonography, it is easier to share and discuss findings through hysteroscopic video than sonographic records.
When is endometrial biopsy indicated during office hysteroscopy?
The patients of an REI are very unlikely to have endometrial cancer (or even hyperplasia) outside of polyps (or arguably hypervascular areas of overgrowth), so the focus is on resecting visualized pathology relative to random biopsy.
However, the threshold for biopsy should be adjusted to the patient population, as well as to individual findings and risk. RVUs are greatly increased (11.1 > 41.57) with biopsy, helping sustainability. Additionally, if one places the hysteroscope on endometrium and applies suction through the inflow channel, one can obtain a sample with small-caliber diagnostic hysteroscopes and without having to use forceps.
What is your threshold for fluid deficit in hysteroscopy?
We follow AAGL guidelines, which for operative hysteroscopy are 2,500 mL of isotonic fluids or 1,000 mL of hypotonic fluids in low-risk patients. This should be further reduced to 500 mL of isotonic fluids in the elderly and even 300 mL in those with cardiovascular compromise.6
For patients who request sedation for office hysteroscopy, which option do you recommend – paracervical block alone, nitrous oxide, or the combination?
For diagnostic, greater than 95% of our patients do not require even over-the-counter analgesic medications. For operative, we consider all permissible resources that allow for a safe combination that is appropriate to the pathology and clinical setting, such as paracervical blocks, nitrous oxide, NSAIDs such as ketorolac, anxiolytics, and more.
The goal is to optimize the patient experience. However, the top three criteria that influence successful operative office hysteroscopy for a conscious patient are a parous cervix, judicious patient selection, and pre- and intraoperative verbal analgesia. Informed consent and engagement improve the experience of both the patient and physician.
Dr. Parry is the founder of Positive Steps Fertility in Madison, Miss. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Parry JP et al. J Minim Invasive Gynecol. 2017 May-Jun. doi: 10.1016/j.jmig.2017.02.010.
2. Wadhwa L et al. 2017 Apr-Jun. doi: 10.4103/jhrs.JHRS_123_16.
3. Parry JP et al. Fertil Steril. 2017 Oct. doi: 10.1016/j.fertnstert.2017.07.1159.
4. Penzias A et al. Fertil Steril. 2021 Nov. doi: 10.1016/j.fertnstert.2021.08.038.
5. Campo R et al. Hum Reprod. 2005 Jan;20(1):258-63. doi: 10.1093/humrep/deh559.
6. AAGL AAGL practice report: Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013 Mar-Apr. doi: 10.1016/j.jmig.2012.12.002.
What role does diagnostic office hysteroscopy play in an infertility evaluation?
.1
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively.2 Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope.3 Further, infection rates and vasovagal events were far lower with hysteroscopy.1
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity.4 Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
How should physicians perform office hysteroscopy to minimize patient discomfort?
The classic paradigm has been to focus on paracervical blocks, anxiolytics, and a supportive environment (such as mood music). However, those are far more important when your hysteroscope is larger than the natural cervical lumen. If you can use small hysteroscopes (< 3 mm for the nulliparous cervix, < 4 mm for the parous cervix), most women will not require cervical dilation, which further enhances the patient experience.
Using a flexible hysteroscope for suspected pathology, making sure not to overdistend the uterus (particularly in high-risk patients such as those with tubal occlusion and cervical stenosis), and vaginoscopy can all minimize patient discomfort. We have published data showing that by using a 2.8-mm flexible diagnostic hysteroscope in a group of mostly nulliparous women, greater than 50% have no discomfort, and more than 90% will have mild to no discomfort.3
What operative hysteroscopy procedures can be performed safely in a physician’s office, and what equipment is required?
Though highly dependent on experience and resources, reproductive endocrinology and infertility specialists (REIs) arguably have the easiest transition to operative office hysteroscopy by utilizing the analgesia and procedure room that is standard for oocyte retrieval and simply adding hysteroscopic procedures. The accompanying table stratifies general hysteroscopic procedures by difficulty.

If one can use propofol or a similar level of sedation (which is routinely utilized for oocyte aspiration), there are few hysteroscopies that cannot be accomplished in the office. However, the less sedation and analgesia, the more judicious one must be in patient selection. Moreover, there are trade-offs between visualization, comfort, and instrumentation.
The greater the uterine distention and diameter of the hysteroscope, the more patients experience pain. One-third of patients (especially nulliparous) will discontinue a procedure with a 5-mm hysteroscope because of discomfort.5 However, as one drops to 4.5 mm and smaller operative hysteroscopes, instruments often occupy the inflow channel, limiting distention and visualization, which also can affect completion rates and safety.
When is operative hysteroscopy best suited for the OR?
In addition to physician experience and clinical resources, the critical factors guiding our choices for selecting the OR rather than the office, include:
- Loss of landmarks. Though Dr. Parry now does most severe intrauterine adhesion cases in the office with ultrasound guidance, when neither ostia can be visualized there is meaningful risk for perforation. Preoperative estrogen, development of planes with the diagnostic hysteroscope prior, and preparing the patient for a possible multistage procedure are all important.
- Use of energy. There are many excellent hysteroscopic surgeons who use the resectoscope well in the office. However, with possible patient movement and potential perforation with energy leading to a bowel injury, there can be greater risk when using energy relative to other methods (such as forceps, scissors, and mechanical morcellation).
- Deeper fibroids. Fibroids displace rather than invade the myometrium, and one can sonographically visualize the myometrium reapproximate over a fibroid as it herniates more into the uterine cavity. Nevertheless, the closer a fibroid comes to the serosa, the more mindful one should be of risks and balances for hysteroscopic removal.
In a patient with a severely stenotic cervix or tortuous endocervical canal, what preprocedure methods do you find helpful, and do you utilize abdominal ultrasound guidance?
If using a 2.8-mm flexible diagnostic hysteroscope, we find 99.8%-99.9% of cervices can be successfully cannulated in the office, with rare exception, that is, following cryotherapy or chlamydia cervicitis. This is the equivalent of your dilator having a camera on the tip and fully articulating to adjust to the cervical path.
Transvaginal sonography prior to hysteroscopy where one maps the cervical lumen helps anticipate problems (along with being familiar with the patient’s history). For the rare dilation under anesthesia, concurrent sonography with a 2.8-mm flexible hysteroscope and intermittent dilator use has been sufficient for our exceptions without the need for lacrimal dilators, vasopressin, misoprostol, and other adjuncts. Of note, we use a 1080p flexible endoscope, as lower resolution would make this more challenging.
In patients with recurrent implantation failure following IVF, is hysteroscopy superior to 3D saline infusion sonogram?
At an American Society of Reproductive Medicine 2021 session, Ilan Tur-Kaspa, MD, and Dr. Parry debated the topic of 2D ultrasound combined with hysteroscopy vs. 3D saline infusion sonography. Core areas of agreement were that expert hands for any approach are better than nonexpert, and high-resolution technology is better than lower resolution. There was also agreement that extrauterine and myometrial disease, such as intramural fibroids and adenomyosis, are contributory factors.
So, sonography will always have a role. However, existing and forthcoming data show hysteroscopy to improve live birth rates for patients with recurrent implantation failure after IVF. Dr. Parry finds diagnostic hysteroscopy easier for identifying endometritis, sessile and cornual polyps, retained products of conception (which are often isoechogenic with the endometrium) and lateral adhesions.
The reality is that there is variability among physicians and midlevel providers in both sonographic and diagnostic hysteroscopic skill. If one wants to verify findings with another team member, acknowledging that there can be nuances to identifying these pathologies by sonography, it is easier to share and discuss findings through hysteroscopic video than sonographic records.
When is endometrial biopsy indicated during office hysteroscopy?
The patients of an REI are very unlikely to have endometrial cancer (or even hyperplasia) outside of polyps (or arguably hypervascular areas of overgrowth), so the focus is on resecting visualized pathology relative to random biopsy.
However, the threshold for biopsy should be adjusted to the patient population, as well as to individual findings and risk. RVUs are greatly increased (11.1 > 41.57) with biopsy, helping sustainability. Additionally, if one places the hysteroscope on endometrium and applies suction through the inflow channel, one can obtain a sample with small-caliber diagnostic hysteroscopes and without having to use forceps.
What is your threshold for fluid deficit in hysteroscopy?
We follow AAGL guidelines, which for operative hysteroscopy are 2,500 mL of isotonic fluids or 1,000 mL of hypotonic fluids in low-risk patients. This should be further reduced to 500 mL of isotonic fluids in the elderly and even 300 mL in those with cardiovascular compromise.6
For patients who request sedation for office hysteroscopy, which option do you recommend – paracervical block alone, nitrous oxide, or the combination?
For diagnostic, greater than 95% of our patients do not require even over-the-counter analgesic medications. For operative, we consider all permissible resources that allow for a safe combination that is appropriate to the pathology and clinical setting, such as paracervical blocks, nitrous oxide, NSAIDs such as ketorolac, anxiolytics, and more.
The goal is to optimize the patient experience. However, the top three criteria that influence successful operative office hysteroscopy for a conscious patient are a parous cervix, judicious patient selection, and pre- and intraoperative verbal analgesia. Informed consent and engagement improve the experience of both the patient and physician.
Dr. Parry is the founder of Positive Steps Fertility in Madison, Miss. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Parry JP et al. J Minim Invasive Gynecol. 2017 May-Jun. doi: 10.1016/j.jmig.2017.02.010.
2. Wadhwa L et al. 2017 Apr-Jun. doi: 10.4103/jhrs.JHRS_123_16.
3. Parry JP et al. Fertil Steril. 2017 Oct. doi: 10.1016/j.fertnstert.2017.07.1159.
4. Penzias A et al. Fertil Steril. 2021 Nov. doi: 10.1016/j.fertnstert.2021.08.038.
5. Campo R et al. Hum Reprod. 2005 Jan;20(1):258-63. doi: 10.1093/humrep/deh559.
6. AAGL AAGL practice report: Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013 Mar-Apr. doi: 10.1016/j.jmig.2012.12.002.
The diagnostic and therapeutic challenges of syringoma
Pain and pruritus are the most common complaints in patients who present to vulvar clinics.1 These symptoms can be related to a variety of conditions, including vulvar lesions. There are both common and uncommon vulvar lesions. Vulvar lesions can be skin colored, yellow, and red. Certain lesions can be diagnosed with history and physical examination alone. Some more common lesions include acrochordons (skin tags), benign growths that are common in patients with diabetes, obesity, and pregnancy.2,3 Other common vulvar lesions are papillomatosis, lichen simplex chronicus, and epidermoid cysts. Other lesions include low- and high-grade squamous intraepithelial lesions (HSIL).4 These lesions require biopsy for diagnosis as high-grade lesions require treatment. HSIL of the vulva is considered a premalignancy that necessitates treatment.5 Other lesions that can present with vulvar complaints are molluscum contagiosum, Bartholin gland duct cyst, intradermal melanocytic nevus, and squamous cell carcinoma.
Rarely, other less common conditions can present as vulvar lesions. Syringomas are benign eccrine sweat gland neoplasms. They are more commonly found on the face, neck, or chest.6 On the vulva they are generally small subcutaneous skin-colored papules.7 They may be asymptomatic and noted only on routine examination.
Vulvar syringomas also may present with symptoms. On the vulva, syringomas often present as pruritic papules that can be isolated or multifocal. Often on the labia majora they range in size from 2 to 20 mm.8
They can coalesce to form a larger lesion. They also may be described as painful. When syringomas are pruritic, the overlying skin may appear thickened from rubbing or scratching, and excoriations may be present.
Since vulvar syringomas are rare, there is no standard treatment. Biopsy is necessary for definitive diagnosis. For asymptomatic cases, expectant management is warranted. In symptomatic cases treatment can be considered. Treatment options include cryotherapy, laser ablation, and intralesional electrodissection.8 Intralesional electrodissection and curettage also has been described as treatment.9 Other treatment options include surgical excision of individual lesions or larger excisions if multifocal.
The case study described in "Case letter: Vulvar syringoma" highlights the diagnostic and therapeutic challenges associated with rare lesions of the vulva. Referral to a specialty clinic may be warranted in these challenging cases. ●
- Hansen A, Carr K, Jensen JT. Characteristics and initial diagnoses in women presenting to a referral center for vulvovaginal disorders in 1996–2000. J Reprod Med. 2002; 47: 854-860.
- Boza JC, Trindade EN, Peruzzo J, et al. Skin manifestations of obesity: a comparative study. J Eur Acad Dermatol Venereol. 2012;26:1220-1223.
- Winton GB, Lewis CW. Dermatoses of pregnancy. J Am Acad Dermatol. 1982;6:977-998.
- Bornstein J, Bogliatto F, Haefner HK, et al; ISSVD Terminology Committee. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) terminology of vulvar squamous intraepithelial lesions. J Low Genit Tract Dis. 2016;20:11-14.
- American College of Obstetricians and Gynecologists. Committee opinion no. 675: management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Heller DS. Benign tumors and tumor-like lesions of the vulva. Clin Obstet Gynecol. 2015;58:526-535.
- Shalabi MMK, Homan K, Bicknell L. Vulvar syringomas. Proc (Bayl Univer Med Cent). 2022;35:113-114.
- Ozdemir O, Sari ME, Sen E, et al. Vulvar syringoma in a postmenopausal woman: a case report. J Reprod Med. 2015;60:452-454.
- Stevenson TR, Swanson NA. Syringoma: removal by electrodesiccation and curettage. Ann Plast Surg. 1985;15:151-154.
Pain and pruritus are the most common complaints in patients who present to vulvar clinics.1 These symptoms can be related to a variety of conditions, including vulvar lesions. There are both common and uncommon vulvar lesions. Vulvar lesions can be skin colored, yellow, and red. Certain lesions can be diagnosed with history and physical examination alone. Some more common lesions include acrochordons (skin tags), benign growths that are common in patients with diabetes, obesity, and pregnancy.2,3 Other common vulvar lesions are papillomatosis, lichen simplex chronicus, and epidermoid cysts. Other lesions include low- and high-grade squamous intraepithelial lesions (HSIL).4 These lesions require biopsy for diagnosis as high-grade lesions require treatment. HSIL of the vulva is considered a premalignancy that necessitates treatment.5 Other lesions that can present with vulvar complaints are molluscum contagiosum, Bartholin gland duct cyst, intradermal melanocytic nevus, and squamous cell carcinoma.
Rarely, other less common conditions can present as vulvar lesions. Syringomas are benign eccrine sweat gland neoplasms. They are more commonly found on the face, neck, or chest.6 On the vulva they are generally small subcutaneous skin-colored papules.7 They may be asymptomatic and noted only on routine examination.
Vulvar syringomas also may present with symptoms. On the vulva, syringomas often present as pruritic papules that can be isolated or multifocal. Often on the labia majora they range in size from 2 to 20 mm.8
They can coalesce to form a larger lesion. They also may be described as painful. When syringomas are pruritic, the overlying skin may appear thickened from rubbing or scratching, and excoriations may be present.
Since vulvar syringomas are rare, there is no standard treatment. Biopsy is necessary for definitive diagnosis. For asymptomatic cases, expectant management is warranted. In symptomatic cases treatment can be considered. Treatment options include cryotherapy, laser ablation, and intralesional electrodissection.8 Intralesional electrodissection and curettage also has been described as treatment.9 Other treatment options include surgical excision of individual lesions or larger excisions if multifocal.
The case study described in "Case letter: Vulvar syringoma" highlights the diagnostic and therapeutic challenges associated with rare lesions of the vulva. Referral to a specialty clinic may be warranted in these challenging cases. ●
Pain and pruritus are the most common complaints in patients who present to vulvar clinics.1 These symptoms can be related to a variety of conditions, including vulvar lesions. There are both common and uncommon vulvar lesions. Vulvar lesions can be skin colored, yellow, and red. Certain lesions can be diagnosed with history and physical examination alone. Some more common lesions include acrochordons (skin tags), benign growths that are common in patients with diabetes, obesity, and pregnancy.2,3 Other common vulvar lesions are papillomatosis, lichen simplex chronicus, and epidermoid cysts. Other lesions include low- and high-grade squamous intraepithelial lesions (HSIL).4 These lesions require biopsy for diagnosis as high-grade lesions require treatment. HSIL of the vulva is considered a premalignancy that necessitates treatment.5 Other lesions that can present with vulvar complaints are molluscum contagiosum, Bartholin gland duct cyst, intradermal melanocytic nevus, and squamous cell carcinoma.
Rarely, other less common conditions can present as vulvar lesions. Syringomas are benign eccrine sweat gland neoplasms. They are more commonly found on the face, neck, or chest.6 On the vulva they are generally small subcutaneous skin-colored papules.7 They may be asymptomatic and noted only on routine examination.
Vulvar syringomas also may present with symptoms. On the vulva, syringomas often present as pruritic papules that can be isolated or multifocal. Often on the labia majora they range in size from 2 to 20 mm.8
They can coalesce to form a larger lesion. They also may be described as painful. When syringomas are pruritic, the overlying skin may appear thickened from rubbing or scratching, and excoriations may be present.
Since vulvar syringomas are rare, there is no standard treatment. Biopsy is necessary for definitive diagnosis. For asymptomatic cases, expectant management is warranted. In symptomatic cases treatment can be considered. Treatment options include cryotherapy, laser ablation, and intralesional electrodissection.8 Intralesional electrodissection and curettage also has been described as treatment.9 Other treatment options include surgical excision of individual lesions or larger excisions if multifocal.
The case study described in "Case letter: Vulvar syringoma" highlights the diagnostic and therapeutic challenges associated with rare lesions of the vulva. Referral to a specialty clinic may be warranted in these challenging cases. ●
- Hansen A, Carr K, Jensen JT. Characteristics and initial diagnoses in women presenting to a referral center for vulvovaginal disorders in 1996–2000. J Reprod Med. 2002; 47: 854-860.
- Boza JC, Trindade EN, Peruzzo J, et al. Skin manifestations of obesity: a comparative study. J Eur Acad Dermatol Venereol. 2012;26:1220-1223.
- Winton GB, Lewis CW. Dermatoses of pregnancy. J Am Acad Dermatol. 1982;6:977-998.
- Bornstein J, Bogliatto F, Haefner HK, et al; ISSVD Terminology Committee. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) terminology of vulvar squamous intraepithelial lesions. J Low Genit Tract Dis. 2016;20:11-14.
- American College of Obstetricians and Gynecologists. Committee opinion no. 675: management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Heller DS. Benign tumors and tumor-like lesions of the vulva. Clin Obstet Gynecol. 2015;58:526-535.
- Shalabi MMK, Homan K, Bicknell L. Vulvar syringomas. Proc (Bayl Univer Med Cent). 2022;35:113-114.
- Ozdemir O, Sari ME, Sen E, et al. Vulvar syringoma in a postmenopausal woman: a case report. J Reprod Med. 2015;60:452-454.
- Stevenson TR, Swanson NA. Syringoma: removal by electrodesiccation and curettage. Ann Plast Surg. 1985;15:151-154.
- Hansen A, Carr K, Jensen JT. Characteristics and initial diagnoses in women presenting to a referral center for vulvovaginal disorders in 1996–2000. J Reprod Med. 2002; 47: 854-860.
- Boza JC, Trindade EN, Peruzzo J, et al. Skin manifestations of obesity: a comparative study. J Eur Acad Dermatol Venereol. 2012;26:1220-1223.
- Winton GB, Lewis CW. Dermatoses of pregnancy. J Am Acad Dermatol. 1982;6:977-998.
- Bornstein J, Bogliatto F, Haefner HK, et al; ISSVD Terminology Committee. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) terminology of vulvar squamous intraepithelial lesions. J Low Genit Tract Dis. 2016;20:11-14.
- American College of Obstetricians and Gynecologists. Committee opinion no. 675: management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Heller DS. Benign tumors and tumor-like lesions of the vulva. Clin Obstet Gynecol. 2015;58:526-535.
- Shalabi MMK, Homan K, Bicknell L. Vulvar syringomas. Proc (Bayl Univer Med Cent). 2022;35:113-114.
- Ozdemir O, Sari ME, Sen E, et al. Vulvar syringoma in a postmenopausal woman: a case report. J Reprod Med. 2015;60:452-454.
- Stevenson TR, Swanson NA. Syringoma: removal by electrodesiccation and curettage. Ann Plast Surg. 1985;15:151-154.
Preventive antipyretics, antibiotics not needed in stroke
“The results of PRECIOUS do not support preventive use of antiemetic, antipyretic, or antibiotic drugs in older patients with acute stroke,” senior study author Bart van der Worp, MD, professor of acute neurology at University Medical Center, Utrecht, the Netherlands, concluded.
“This trial was all about prevention,” trial co-investigator, Philip Bath, MD, professor of stroke medicine at the University of Nottingham (England), said in an interview.
“It was trying to improve outcomes by preventing infection, fever, and aspiration pneumonia, but the message from these results is that while we should be on the lookout for these complications and treat them early when they occur, we don’t need to give these medications on a prophylactic basis.”
The PREvention of Complications to Improve OUtcome in elderly patients with acute Stroke (PRECIOUS) trial was presented at the annual European Stroke Organisation Conference, held in Munich.
Dr. Van der Worp explained that infections, fever, and aspiration pneumonia frequently occur following stroke, particularly in older patients, and these poststroke complications are associated with an increased risk of death and poor functional outcome.
“We assessed whether a pharmacological strategy to reduce the risk of infections and fever improves outcomes of elderly patients with moderately severe or severe stroke,” he said.
Previous studies looking at this approach have been performed in broad populations of stroke patients who had a relatively low risk of poststroke complications, thereby reducing the potential for benefit from these interventions.
The current PRECIOUS trial was therefore conducted in a more selective elderly population with more severe strokes, a group believed to be at higher risk of infection and fever.
The trial included patients aged 66 years or older with moderately severe to severe ischemic stroke (National Institutes of Health Stroke Scale score ≥ 6) or intracerebral hemorrhage.
They were randomized in a 3 x 2 factorial design to oral, rectal, or intravenous metoclopramide (10 mg three times a day); intravenous ceftriaxone (2,000 mg once daily); oral, rectal, or intravenous paracetamol (1,000 mg four times daily); or usual care.
Medications were started within 24 hours after symptom onset and continued for 4 days or until complete recovery or discharge from hospital, if earlier.
“We assessed these three simple, safe, and inexpensive therapies – paracetamol to prevent fever; the antiemetic, metoclopramide, to prevent aspiration; and ceftriaxone, which is the preferred antibiotic for post-stroke pneumonia in the Netherlands,” Dr. van der Worp said.
The primary outcome was modified Rankin Scale (mRS) score at 90 days.
The trial was aiming to enroll 3,800 patients from 67 European sites but was stopped after 1,493 patients had been included because of lack of funding. After excluding patients who withdrew consent or were lost to follow-up, 1,471 patients were included in the intention-to-treat analysis.
Results showed no effect on the primary outcome of any of the prophylactic treatments.
“None of the medications had any effect on the functional outcome at 90 days. This was a surprise to me,” Dr. Van der Worp commented. “I had expected that at least one of the medications would have been of benefit.”
A secondary outcome was the diagnosis of pneumonia, which again was not reduced by any of the medications.
“Remarkably, neither ceftriaxone nor metoclopramide had any effect on the risk of developing pneumonia. It was all quite disappointing,” van der Worp said.
There was, however, a reduction in the incidence of urinary tract infections in the ceftriaxone group.
Trying to explain why there was a reduction in urinary tract infections but not pneumonia with the antibiotic, Dr. Van der Worp pointed out that poststroke pneumonia is to a large extent caused by a mechanical process (aspiration), and bacteria may only play a minor role in its development.
He said he was therefore surprised that metoclopramide, which should prevent the mechanical process of aspiration, did not reduce the development of pneumonia.
He suggested that some patients may have already experienced aspiration before the metoclopramide was started, noting that many patients with acute stroke already have signs of pneumonia on CT scan in the first few hours after symptom onset.
A previous smaller study (MAPS) had shown a lower rate of pneumonia in stroke patients given metoclopramide, but in this study the drug was given for 3 weeks.
Discussing the PRECIOUS trial at the ESOC meeting, Christine Roffe, MD, professor of stroke medicine at Keele (England) University, and senior investigator of the MAPS study, suggested that a longer period of metoclopramide treatment may be needed than the 4 days given in the PRECIOUS study, as the risk of pneumonia persists for longer than just a few days.
She noted that another trial (MAPS-2) is now underway in the United Kingdom to try and confirm the first MAPS result with longer duration metoclopramide.
Dr. Van der Worp responded: “Certainly, I think that the MAPS-2 study should be continued. It is investigating a much longer duration of treatment, which may be beneficial, especially in patients with more severe strokes.”
On the reason for the disappointing results with paracetamol, Dr. Van der Worp elaborated: “We found that only a very few of these older patients developed a fever – only about 5% in the control group. Paracetamol did reduce the risk of fever, but because the proportion of patients who developed fever was so small, this may have been why it didn’t translate into any effect on the functional outcome.”
Dr. Roffe concluded that PRECIOUS was an important study. “There is also a positive message here. We have all been worried about using too many antibiotics. We need to make sure we use these drugs responsibly. I think this trial has told us there is little point in using antibiotics in a preventative way in these patients.”
She added that although the trial was stopped prematurely, it had produced decisive results.
“Yes, I believe that even if the trial was much larger, we still would not have shown an effect,” Dr. Van der Worp agreed.
A version of this article first appeared on Medscape.com.
“The results of PRECIOUS do not support preventive use of antiemetic, antipyretic, or antibiotic drugs in older patients with acute stroke,” senior study author Bart van der Worp, MD, professor of acute neurology at University Medical Center, Utrecht, the Netherlands, concluded.
“This trial was all about prevention,” trial co-investigator, Philip Bath, MD, professor of stroke medicine at the University of Nottingham (England), said in an interview.
“It was trying to improve outcomes by preventing infection, fever, and aspiration pneumonia, but the message from these results is that while we should be on the lookout for these complications and treat them early when they occur, we don’t need to give these medications on a prophylactic basis.”
The PREvention of Complications to Improve OUtcome in elderly patients with acute Stroke (PRECIOUS) trial was presented at the annual European Stroke Organisation Conference, held in Munich.
Dr. Van der Worp explained that infections, fever, and aspiration pneumonia frequently occur following stroke, particularly in older patients, and these poststroke complications are associated with an increased risk of death and poor functional outcome.
“We assessed whether a pharmacological strategy to reduce the risk of infections and fever improves outcomes of elderly patients with moderately severe or severe stroke,” he said.
Previous studies looking at this approach have been performed in broad populations of stroke patients who had a relatively low risk of poststroke complications, thereby reducing the potential for benefit from these interventions.
The current PRECIOUS trial was therefore conducted in a more selective elderly population with more severe strokes, a group believed to be at higher risk of infection and fever.
The trial included patients aged 66 years or older with moderately severe to severe ischemic stroke (National Institutes of Health Stroke Scale score ≥ 6) or intracerebral hemorrhage.
They were randomized in a 3 x 2 factorial design to oral, rectal, or intravenous metoclopramide (10 mg three times a day); intravenous ceftriaxone (2,000 mg once daily); oral, rectal, or intravenous paracetamol (1,000 mg four times daily); or usual care.
Medications were started within 24 hours after symptom onset and continued for 4 days or until complete recovery or discharge from hospital, if earlier.
“We assessed these three simple, safe, and inexpensive therapies – paracetamol to prevent fever; the antiemetic, metoclopramide, to prevent aspiration; and ceftriaxone, which is the preferred antibiotic for post-stroke pneumonia in the Netherlands,” Dr. van der Worp said.
The primary outcome was modified Rankin Scale (mRS) score at 90 days.
The trial was aiming to enroll 3,800 patients from 67 European sites but was stopped after 1,493 patients had been included because of lack of funding. After excluding patients who withdrew consent or were lost to follow-up, 1,471 patients were included in the intention-to-treat analysis.
Results showed no effect on the primary outcome of any of the prophylactic treatments.
“None of the medications had any effect on the functional outcome at 90 days. This was a surprise to me,” Dr. Van der Worp commented. “I had expected that at least one of the medications would have been of benefit.”
A secondary outcome was the diagnosis of pneumonia, which again was not reduced by any of the medications.
“Remarkably, neither ceftriaxone nor metoclopramide had any effect on the risk of developing pneumonia. It was all quite disappointing,” van der Worp said.
There was, however, a reduction in the incidence of urinary tract infections in the ceftriaxone group.
Trying to explain why there was a reduction in urinary tract infections but not pneumonia with the antibiotic, Dr. Van der Worp pointed out that poststroke pneumonia is to a large extent caused by a mechanical process (aspiration), and bacteria may only play a minor role in its development.
He said he was therefore surprised that metoclopramide, which should prevent the mechanical process of aspiration, did not reduce the development of pneumonia.
He suggested that some patients may have already experienced aspiration before the metoclopramide was started, noting that many patients with acute stroke already have signs of pneumonia on CT scan in the first few hours after symptom onset.
A previous smaller study (MAPS) had shown a lower rate of pneumonia in stroke patients given metoclopramide, but in this study the drug was given for 3 weeks.
Discussing the PRECIOUS trial at the ESOC meeting, Christine Roffe, MD, professor of stroke medicine at Keele (England) University, and senior investigator of the MAPS study, suggested that a longer period of metoclopramide treatment may be needed than the 4 days given in the PRECIOUS study, as the risk of pneumonia persists for longer than just a few days.
She noted that another trial (MAPS-2) is now underway in the United Kingdom to try and confirm the first MAPS result with longer duration metoclopramide.
Dr. Van der Worp responded: “Certainly, I think that the MAPS-2 study should be continued. It is investigating a much longer duration of treatment, which may be beneficial, especially in patients with more severe strokes.”
On the reason for the disappointing results with paracetamol, Dr. Van der Worp elaborated: “We found that only a very few of these older patients developed a fever – only about 5% in the control group. Paracetamol did reduce the risk of fever, but because the proportion of patients who developed fever was so small, this may have been why it didn’t translate into any effect on the functional outcome.”
Dr. Roffe concluded that PRECIOUS was an important study. “There is also a positive message here. We have all been worried about using too many antibiotics. We need to make sure we use these drugs responsibly. I think this trial has told us there is little point in using antibiotics in a preventative way in these patients.”
She added that although the trial was stopped prematurely, it had produced decisive results.
“Yes, I believe that even if the trial was much larger, we still would not have shown an effect,” Dr. Van der Worp agreed.
A version of this article first appeared on Medscape.com.
“The results of PRECIOUS do not support preventive use of antiemetic, antipyretic, or antibiotic drugs in older patients with acute stroke,” senior study author Bart van der Worp, MD, professor of acute neurology at University Medical Center, Utrecht, the Netherlands, concluded.
“This trial was all about prevention,” trial co-investigator, Philip Bath, MD, professor of stroke medicine at the University of Nottingham (England), said in an interview.
“It was trying to improve outcomes by preventing infection, fever, and aspiration pneumonia, but the message from these results is that while we should be on the lookout for these complications and treat them early when they occur, we don’t need to give these medications on a prophylactic basis.”
The PREvention of Complications to Improve OUtcome in elderly patients with acute Stroke (PRECIOUS) trial was presented at the annual European Stroke Organisation Conference, held in Munich.
Dr. Van der Worp explained that infections, fever, and aspiration pneumonia frequently occur following stroke, particularly in older patients, and these poststroke complications are associated with an increased risk of death and poor functional outcome.
“We assessed whether a pharmacological strategy to reduce the risk of infections and fever improves outcomes of elderly patients with moderately severe or severe stroke,” he said.
Previous studies looking at this approach have been performed in broad populations of stroke patients who had a relatively low risk of poststroke complications, thereby reducing the potential for benefit from these interventions.
The current PRECIOUS trial was therefore conducted in a more selective elderly population with more severe strokes, a group believed to be at higher risk of infection and fever.
The trial included patients aged 66 years or older with moderately severe to severe ischemic stroke (National Institutes of Health Stroke Scale score ≥ 6) or intracerebral hemorrhage.
They were randomized in a 3 x 2 factorial design to oral, rectal, or intravenous metoclopramide (10 mg three times a day); intravenous ceftriaxone (2,000 mg once daily); oral, rectal, or intravenous paracetamol (1,000 mg four times daily); or usual care.
Medications were started within 24 hours after symptom onset and continued for 4 days or until complete recovery or discharge from hospital, if earlier.
“We assessed these three simple, safe, and inexpensive therapies – paracetamol to prevent fever; the antiemetic, metoclopramide, to prevent aspiration; and ceftriaxone, which is the preferred antibiotic for post-stroke pneumonia in the Netherlands,” Dr. van der Worp said.
The primary outcome was modified Rankin Scale (mRS) score at 90 days.
The trial was aiming to enroll 3,800 patients from 67 European sites but was stopped after 1,493 patients had been included because of lack of funding. After excluding patients who withdrew consent or were lost to follow-up, 1,471 patients were included in the intention-to-treat analysis.
Results showed no effect on the primary outcome of any of the prophylactic treatments.
“None of the medications had any effect on the functional outcome at 90 days. This was a surprise to me,” Dr. Van der Worp commented. “I had expected that at least one of the medications would have been of benefit.”
A secondary outcome was the diagnosis of pneumonia, which again was not reduced by any of the medications.
“Remarkably, neither ceftriaxone nor metoclopramide had any effect on the risk of developing pneumonia. It was all quite disappointing,” van der Worp said.
There was, however, a reduction in the incidence of urinary tract infections in the ceftriaxone group.
Trying to explain why there was a reduction in urinary tract infections but not pneumonia with the antibiotic, Dr. Van der Worp pointed out that poststroke pneumonia is to a large extent caused by a mechanical process (aspiration), and bacteria may only play a minor role in its development.
He said he was therefore surprised that metoclopramide, which should prevent the mechanical process of aspiration, did not reduce the development of pneumonia.
He suggested that some patients may have already experienced aspiration before the metoclopramide was started, noting that many patients with acute stroke already have signs of pneumonia on CT scan in the first few hours after symptom onset.
A previous smaller study (MAPS) had shown a lower rate of pneumonia in stroke patients given metoclopramide, but in this study the drug was given for 3 weeks.
Discussing the PRECIOUS trial at the ESOC meeting, Christine Roffe, MD, professor of stroke medicine at Keele (England) University, and senior investigator of the MAPS study, suggested that a longer period of metoclopramide treatment may be needed than the 4 days given in the PRECIOUS study, as the risk of pneumonia persists for longer than just a few days.
She noted that another trial (MAPS-2) is now underway in the United Kingdom to try and confirm the first MAPS result with longer duration metoclopramide.
Dr. Van der Worp responded: “Certainly, I think that the MAPS-2 study should be continued. It is investigating a much longer duration of treatment, which may be beneficial, especially in patients with more severe strokes.”
On the reason for the disappointing results with paracetamol, Dr. Van der Worp elaborated: “We found that only a very few of these older patients developed a fever – only about 5% in the control group. Paracetamol did reduce the risk of fever, but because the proportion of patients who developed fever was so small, this may have been why it didn’t translate into any effect on the functional outcome.”
Dr. Roffe concluded that PRECIOUS was an important study. “There is also a positive message here. We have all been worried about using too many antibiotics. We need to make sure we use these drugs responsibly. I think this trial has told us there is little point in using antibiotics in a preventative way in these patients.”
She added that although the trial was stopped prematurely, it had produced decisive results.
“Yes, I believe that even if the trial was much larger, we still would not have shown an effect,” Dr. Van der Worp agreed.
A version of this article first appeared on Medscape.com.
FROM ESOC 2023
Endocrinology pay steadily climbs, gender gap closes
Endocrinologists report steady increases in pay in the Medscape Endocrinologist Compensation Report 2023, but more doctors dropped insurers that pay the least, compared with last year, and only about two-thirds of respondents say they would choose medicine again as a career if given the chance.
In the survey of more than 10,000 physicians in over 29 specialties,
Those earnings still place them in the lowest five specialties in terms of pay, above infectious diseases, family medicine, pediatrics, and public health and preventive medicine. The latter is at the bottom of the list, with average annual earnings of $249,000.
Conversely, the top three specialties were plastic surgery, at an average of $619,000 per annum, followed by orthopedics, at $573,000, and cardiology, at $507,000.
Specialties in which the most significant changes in annual compensation occurred were led by oncology, with a 13% increase from 2022, followed by gastroenterology, with an 11% increase. On the opposite end, ophthalmologists experienced a 7% decline in earnings, while emergency medicine had a 6% decrease from 2022.
Since Medscape’s 2015 report, annual salaries for endocrinologists have increased by 36%. Similar patterns in compensation increases since 2015 occurred across all specialties. In contrast to some other specialties, endocrinologists did not experience a significant decline in earnings during the pandemic.
Across all specialties, men still earned more than women in the 2023 report – with a gap of 19% ($386,000 vs. $300,000). However, there appears to be progress, as the difference represents the lowest gender pay gap in 5 years.
This gradual improvement should likely continue as awareness of pay discrepancies grows and new generations emerge, said Theresa Rohr-Kirchgraber, MD, president of the American Medical Women’s Association and professor of medicine at AU/USA Medical Partnership, Athens, Ga., in the report.
“Due to efforts by many, some institutions and health care organizations have reviewed their salary lines and recognized the discrepancies not only between the sexes but also between new hires” and more established workers, she explained in the report.
“[The new hires] can be offered significantly more than those more senior physicians who have been working there for years and hired under a different pay structure,” she noted.
Nearly half of endocrinologists (45%) reported taking on extra work outside of their profession, up from 39% in the 2022 report. Among them, 31% reported other medical-related work, 8% reported “medical moonlighting,” 7% reported non–medical-related work, and 2% added more hours to their primary job as a physician.
Endocrinologists were in the lowest third of specialties in terms of their impressions of fair compensation, with only 45% reporting that they felt adequately paid. On the lowest end was infectious disease, with only 35% feeling their compensation is fair. By contrast, the highest response, 68%, was among psychiatrists.
Nevertheless, 85% of endocrinologists report that they would choose the same specialty again if given the chance. Responses ranged from 61% in internal medicine to 97% in plastic surgery.
Of note, fewer – 71% of endocrinologists – responded that they would choose medicine again, down from the 76% of endocrinologists who answered yes to the same question in 2022. At the bottom of the list was emergency medicine, with only 61% saying they would choose medicine again. The highest rates were in dermatology, at 86%, and allergy and immunology, at 84%.
In terms of time spent seeing patients, endocrinologists are more likely to see patients less than 30 hours per week, at 24%, compared with physicians overall, at 19%; 61% of endocrinologists report seeing patients 30-40 hours per week, versus 53% of all physicians.
Only 12% report seeing patients 41-50 hours per week, compared with 16% of all physicians. And 4% reported seeing patients 51 hours or more weekly, versus 11% of physicians overall.
The proportion of endocrinologists who reported that they would drop insurers that pay the least was notably up in the current report, at 25%, versus just 15% in the 2022 report; 22% indicated they would not drop insurers because “I need all payers”; 16% said no because “it’s inappropriate”; and the remainder responded no for other reasons.
Overall, the leading response by physicians for the most rewarding aspects of their job were “being good at what I am doing/finding answers, diagnoses,” reported by 32%, followed by “gratitude from/relationships with patients” (24%) and “making the world a better place (for example, helping others),” at 22%.
Conversely, the most challenging aspect, described by 20%, is “having so many rules and regulations,” followed by “difficulties getting fair reimbursement from or dealing with Medicare and/or other insurers (17%).”
A version of this article first appeared on Medscape.com.
Endocrinologists report steady increases in pay in the Medscape Endocrinologist Compensation Report 2023, but more doctors dropped insurers that pay the least, compared with last year, and only about two-thirds of respondents say they would choose medicine again as a career if given the chance.
In the survey of more than 10,000 physicians in over 29 specialties,
Those earnings still place them in the lowest five specialties in terms of pay, above infectious diseases, family medicine, pediatrics, and public health and preventive medicine. The latter is at the bottom of the list, with average annual earnings of $249,000.
Conversely, the top three specialties were plastic surgery, at an average of $619,000 per annum, followed by orthopedics, at $573,000, and cardiology, at $507,000.
Specialties in which the most significant changes in annual compensation occurred were led by oncology, with a 13% increase from 2022, followed by gastroenterology, with an 11% increase. On the opposite end, ophthalmologists experienced a 7% decline in earnings, while emergency medicine had a 6% decrease from 2022.
Since Medscape’s 2015 report, annual salaries for endocrinologists have increased by 36%. Similar patterns in compensation increases since 2015 occurred across all specialties. In contrast to some other specialties, endocrinologists did not experience a significant decline in earnings during the pandemic.
Across all specialties, men still earned more than women in the 2023 report – with a gap of 19% ($386,000 vs. $300,000). However, there appears to be progress, as the difference represents the lowest gender pay gap in 5 years.
This gradual improvement should likely continue as awareness of pay discrepancies grows and new generations emerge, said Theresa Rohr-Kirchgraber, MD, president of the American Medical Women’s Association and professor of medicine at AU/USA Medical Partnership, Athens, Ga., in the report.
“Due to efforts by many, some institutions and health care organizations have reviewed their salary lines and recognized the discrepancies not only between the sexes but also between new hires” and more established workers, she explained in the report.
“[The new hires] can be offered significantly more than those more senior physicians who have been working there for years and hired under a different pay structure,” she noted.
Nearly half of endocrinologists (45%) reported taking on extra work outside of their profession, up from 39% in the 2022 report. Among them, 31% reported other medical-related work, 8% reported “medical moonlighting,” 7% reported non–medical-related work, and 2% added more hours to their primary job as a physician.
Endocrinologists were in the lowest third of specialties in terms of their impressions of fair compensation, with only 45% reporting that they felt adequately paid. On the lowest end was infectious disease, with only 35% feeling their compensation is fair. By contrast, the highest response, 68%, was among psychiatrists.
Nevertheless, 85% of endocrinologists report that they would choose the same specialty again if given the chance. Responses ranged from 61% in internal medicine to 97% in plastic surgery.
Of note, fewer – 71% of endocrinologists – responded that they would choose medicine again, down from the 76% of endocrinologists who answered yes to the same question in 2022. At the bottom of the list was emergency medicine, with only 61% saying they would choose medicine again. The highest rates were in dermatology, at 86%, and allergy and immunology, at 84%.
In terms of time spent seeing patients, endocrinologists are more likely to see patients less than 30 hours per week, at 24%, compared with physicians overall, at 19%; 61% of endocrinologists report seeing patients 30-40 hours per week, versus 53% of all physicians.
Only 12% report seeing patients 41-50 hours per week, compared with 16% of all physicians. And 4% reported seeing patients 51 hours or more weekly, versus 11% of physicians overall.
The proportion of endocrinologists who reported that they would drop insurers that pay the least was notably up in the current report, at 25%, versus just 15% in the 2022 report; 22% indicated they would not drop insurers because “I need all payers”; 16% said no because “it’s inappropriate”; and the remainder responded no for other reasons.
Overall, the leading response by physicians for the most rewarding aspects of their job were “being good at what I am doing/finding answers, diagnoses,” reported by 32%, followed by “gratitude from/relationships with patients” (24%) and “making the world a better place (for example, helping others),” at 22%.
Conversely, the most challenging aspect, described by 20%, is “having so many rules and regulations,” followed by “difficulties getting fair reimbursement from or dealing with Medicare and/or other insurers (17%).”
A version of this article first appeared on Medscape.com.
Endocrinologists report steady increases in pay in the Medscape Endocrinologist Compensation Report 2023, but more doctors dropped insurers that pay the least, compared with last year, and only about two-thirds of respondents say they would choose medicine again as a career if given the chance.
In the survey of more than 10,000 physicians in over 29 specialties,
Those earnings still place them in the lowest five specialties in terms of pay, above infectious diseases, family medicine, pediatrics, and public health and preventive medicine. The latter is at the bottom of the list, with average annual earnings of $249,000.
Conversely, the top three specialties were plastic surgery, at an average of $619,000 per annum, followed by orthopedics, at $573,000, and cardiology, at $507,000.
Specialties in which the most significant changes in annual compensation occurred were led by oncology, with a 13% increase from 2022, followed by gastroenterology, with an 11% increase. On the opposite end, ophthalmologists experienced a 7% decline in earnings, while emergency medicine had a 6% decrease from 2022.
Since Medscape’s 2015 report, annual salaries for endocrinologists have increased by 36%. Similar patterns in compensation increases since 2015 occurred across all specialties. In contrast to some other specialties, endocrinologists did not experience a significant decline in earnings during the pandemic.
Across all specialties, men still earned more than women in the 2023 report – with a gap of 19% ($386,000 vs. $300,000). However, there appears to be progress, as the difference represents the lowest gender pay gap in 5 years.
This gradual improvement should likely continue as awareness of pay discrepancies grows and new generations emerge, said Theresa Rohr-Kirchgraber, MD, president of the American Medical Women’s Association and professor of medicine at AU/USA Medical Partnership, Athens, Ga., in the report.
“Due to efforts by many, some institutions and health care organizations have reviewed their salary lines and recognized the discrepancies not only between the sexes but also between new hires” and more established workers, she explained in the report.
“[The new hires] can be offered significantly more than those more senior physicians who have been working there for years and hired under a different pay structure,” she noted.
Nearly half of endocrinologists (45%) reported taking on extra work outside of their profession, up from 39% in the 2022 report. Among them, 31% reported other medical-related work, 8% reported “medical moonlighting,” 7% reported non–medical-related work, and 2% added more hours to their primary job as a physician.
Endocrinologists were in the lowest third of specialties in terms of their impressions of fair compensation, with only 45% reporting that they felt adequately paid. On the lowest end was infectious disease, with only 35% feeling their compensation is fair. By contrast, the highest response, 68%, was among psychiatrists.
Nevertheless, 85% of endocrinologists report that they would choose the same specialty again if given the chance. Responses ranged from 61% in internal medicine to 97% in plastic surgery.
Of note, fewer – 71% of endocrinologists – responded that they would choose medicine again, down from the 76% of endocrinologists who answered yes to the same question in 2022. At the bottom of the list was emergency medicine, with only 61% saying they would choose medicine again. The highest rates were in dermatology, at 86%, and allergy and immunology, at 84%.
In terms of time spent seeing patients, endocrinologists are more likely to see patients less than 30 hours per week, at 24%, compared with physicians overall, at 19%; 61% of endocrinologists report seeing patients 30-40 hours per week, versus 53% of all physicians.
Only 12% report seeing patients 41-50 hours per week, compared with 16% of all physicians. And 4% reported seeing patients 51 hours or more weekly, versus 11% of physicians overall.
The proportion of endocrinologists who reported that they would drop insurers that pay the least was notably up in the current report, at 25%, versus just 15% in the 2022 report; 22% indicated they would not drop insurers because “I need all payers”; 16% said no because “it’s inappropriate”; and the remainder responded no for other reasons.
Overall, the leading response by physicians for the most rewarding aspects of their job were “being good at what I am doing/finding answers, diagnoses,” reported by 32%, followed by “gratitude from/relationships with patients” (24%) and “making the world a better place (for example, helping others),” at 22%.
Conversely, the most challenging aspect, described by 20%, is “having so many rules and regulations,” followed by “difficulties getting fair reimbursement from or dealing with Medicare and/or other insurers (17%).”
A version of this article first appeared on Medscape.com.
Traditional Mexican food has health benefits
, according to researchers from the Institute of Sciences at Benemérita Autonomous University of Puebla, Puebla, Mexico.
Their study, published in the journal Foods, is the first to produce tables showing the phenolic content of Mexican dishes. Physicians and nutritionists can use this information as a reference tool when drawing up diet recommendations for patients who could benefit from a higher intake of phenolic compounds (PC).
“Up until now, there hasn’t been a table that we – nutritionists and physicians – could look at and see exactly which foods were richest in these compounds. In the United States, European countries, Asian countries – they’ve all had food tables; in Mexico, we didn’t,” said lead author Julia Alatorre-Cruz, PhD, a biological scientist and postdoctoral researcher at BUAP. “So, it’s a fairly innovative contribution. As a bonus, the information can be used to analyze the relationship between diet and noncommunicable diseases in the Mexican population.”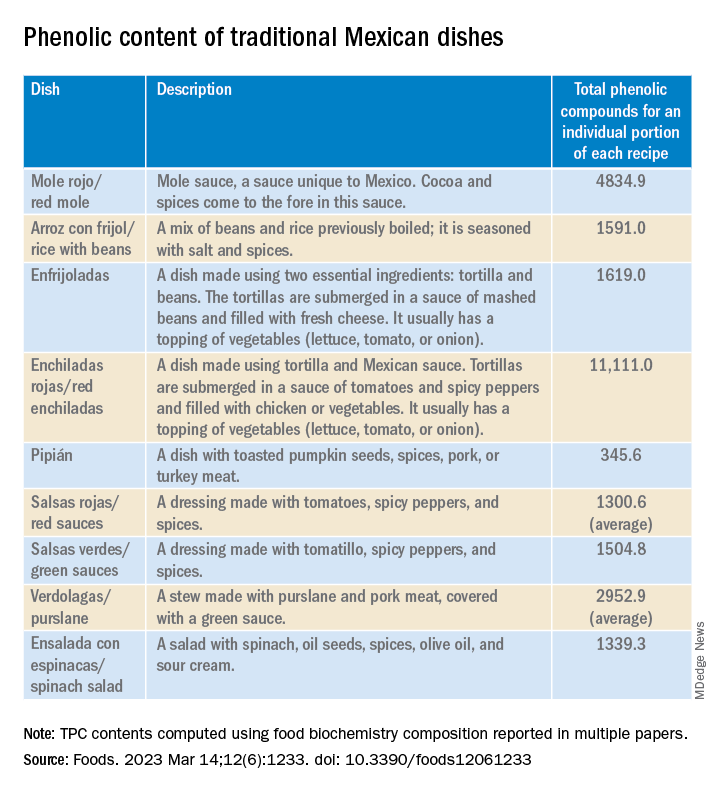
In recent years, nutrition science has focused on counteracting nutrient deficiency and some diseases by identifying active-food components. Diet offers the possibility to improve the patient’s health conditions by using these components or functional food.
PCs are a diverse group of plant micronutrients, some of which modulate physiologic and molecular pathways involved in energy metabolism. They can act by different mechanisms; the most important of them are conducted by anti-inflammatory, antioxidant activities, and are antiallergic.
Moreover, recent studies explain how PCs positively affect certain illnesses, such as obesity, diabetes, cardiovascular diseases, thrombocytopenia, and metabolic syndrome. Several common features characterize these pathologies – among them are the redox balance and a notable inflammatory response that strongly alters the biochemical and functional characteristics of the affected tissues.
Traditional Mexican food is characterized by grains, tubers, legumes, vegetables, and spices, most of which are rich in PCs. However, the Mexican diet has changed over the past decades because traditional food has been replaced with ultraprocessed food with high-caloric values. Moreover, some vegetables and fruits are preferably consumed after processing, which affects the quantity, quality, and bioavailability of the PCs. In addition, diseases associated with eating habits have increased by more than 27% in the Mexican population.
The objective of the study was to determine whether participants with a higher PC intake from beverages or Mexican dishes have better health conditions than those with a lower intake.
A total of 973 adults (798 females, 175 males) aged 18-79 years were enrolled in this cross-sectional study. The data were obtained from a validated, self-administered food consumption survey that was posted on social media (Facebook) or sent via WhatsApp or email. In one section, there was a list of fruits, vegetables, cereals, legumes, seeds, spices, beverages, and Mexican dishes. The participants were asked to indicate how often in the past month they had consumed these items. There were also sections for providing identification data (for example, age, sex, marital status), height and weight information, and medical history.
“The study was carried out during the pandemic, so that limited contact with the participants,” said Dr. Alatorre-Cruz. “Not being able to directly interact with them was a challenge. For example, we would have liked to have taken those anthropometric measurements ourselves.”
The researchers performed K-means clustering to determine the participant’s health-condition level, resulting in two groups: those with less diseases (LD, n = 649) and those with more diseases (MD, n = 324).
Using the food biochemistry composition reported in multiple papers, the researchers computed the average total phenolic compounds (TPC) for each item listed in the survey. For Mexican dishes, they added the TPC of each recipe’s ingredient, then recalculated to come up with TPC for an individual portion of each recipe (TPCr). To analyze the results of the LD group and of the MD group, phenolic compounds intake of recipe was calculated for each participant.
To Dr. Alatorre-Cruz, the biggest challenge was determining the content of compounds in traditional dishes. “Extensive, in-depth research was done to gather as much information as possible about all of the foods – especially about local and regional ones, because we have such a wide variety – looking to see where that information would match up with the exact method with which the compounds of interest were extracted.”
As expected, the team found that food with high PC was associated with a better health condition. However, the consumption of beverages and Mexican dishes was lower than their expectations. As noted in the article, the Mexican diet has changed over the last decades because traditional food has been replaced with ultraprocessed food with high-caloric values.
The authors suggest that their data confirm the alarming changes previously reported in the Mexican diet – changes possibly attributable to the increased influence of other countries via social media and economic globalization. However, they also found that beans, corn (mainly tortilla), and nopal intake remained preserved in the Mexican eating habits.
Their statistical analyses revealed that sex, age, and education seem to play a role in the presence or absence of diseases in the Mexican cohort. Men, participants over age 29 years, and those with lower levels of education had more diseases.
Dr. Alatorre-Cruz told this news organization that the Foods article opens new lines of research for the group to pursue. “One future proposal looks to enroll patients with conditions where there’s an increase in oxidative stress – which we know has quite a detrimental effect – and look into possible links with their diet. But now, maybe the focus can be on patients with a single condition and using these traditional-food tables to find links. We also want to know more about the molecular or biochemical mechanisms that are modulating the association that we saw first in animal models.”
Laura Álvarez, MD, a specialist in clinical nutrition, is the founder of the NUTRIENT project, which focuses on nutrition and strength training. In her opinion, the study extols the benefits of Mexican food. “I’ve always thought that people have this idea that Mexican food isn’t good. For example, thinking that it’s very high in fat. But in reality, Mexican food is very rich in nutrients.”
She added that research should be broadened, by enrolling a larger group of participants and evaluating not only a greater number and a greater variety of Mexican dishes, and new variables as well. “I think that body composition could be taken into account. In this study, they took the BMI into account to assess overweight and obesity, but ... the BMI doesn’t tell us much. Overweight can be due to fat or due to muscle. If we could get even more specific and find out what type of fat, that would give us more information about other [possible] diseases that ... need to be taken into account.”
This research was funded by the Institute of Sciences, Benemérita Autonomous University of Puebla. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results. Dr. Alatorre-Cruz and Dr. Álvarez have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to researchers from the Institute of Sciences at Benemérita Autonomous University of Puebla, Puebla, Mexico.
Their study, published in the journal Foods, is the first to produce tables showing the phenolic content of Mexican dishes. Physicians and nutritionists can use this information as a reference tool when drawing up diet recommendations for patients who could benefit from a higher intake of phenolic compounds (PC).
“Up until now, there hasn’t been a table that we – nutritionists and physicians – could look at and see exactly which foods were richest in these compounds. In the United States, European countries, Asian countries – they’ve all had food tables; in Mexico, we didn’t,” said lead author Julia Alatorre-Cruz, PhD, a biological scientist and postdoctoral researcher at BUAP. “So, it’s a fairly innovative contribution. As a bonus, the information can be used to analyze the relationship between diet and noncommunicable diseases in the Mexican population.”
In recent years, nutrition science has focused on counteracting nutrient deficiency and some diseases by identifying active-food components. Diet offers the possibility to improve the patient’s health conditions by using these components or functional food.
PCs are a diverse group of plant micronutrients, some of which modulate physiologic and molecular pathways involved in energy metabolism. They can act by different mechanisms; the most important of them are conducted by anti-inflammatory, antioxidant activities, and are antiallergic.
Moreover, recent studies explain how PCs positively affect certain illnesses, such as obesity, diabetes, cardiovascular diseases, thrombocytopenia, and metabolic syndrome. Several common features characterize these pathologies – among them are the redox balance and a notable inflammatory response that strongly alters the biochemical and functional characteristics of the affected tissues.
Traditional Mexican food is characterized by grains, tubers, legumes, vegetables, and spices, most of which are rich in PCs. However, the Mexican diet has changed over the past decades because traditional food has been replaced with ultraprocessed food with high-caloric values. Moreover, some vegetables and fruits are preferably consumed after processing, which affects the quantity, quality, and bioavailability of the PCs. In addition, diseases associated with eating habits have increased by more than 27% in the Mexican population.
The objective of the study was to determine whether participants with a higher PC intake from beverages or Mexican dishes have better health conditions than those with a lower intake.
A total of 973 adults (798 females, 175 males) aged 18-79 years were enrolled in this cross-sectional study. The data were obtained from a validated, self-administered food consumption survey that was posted on social media (Facebook) or sent via WhatsApp or email. In one section, there was a list of fruits, vegetables, cereals, legumes, seeds, spices, beverages, and Mexican dishes. The participants were asked to indicate how often in the past month they had consumed these items. There were also sections for providing identification data (for example, age, sex, marital status), height and weight information, and medical history.
“The study was carried out during the pandemic, so that limited contact with the participants,” said Dr. Alatorre-Cruz. “Not being able to directly interact with them was a challenge. For example, we would have liked to have taken those anthropometric measurements ourselves.”
The researchers performed K-means clustering to determine the participant’s health-condition level, resulting in two groups: those with less diseases (LD, n = 649) and those with more diseases (MD, n = 324).
Using the food biochemistry composition reported in multiple papers, the researchers computed the average total phenolic compounds (TPC) for each item listed in the survey. For Mexican dishes, they added the TPC of each recipe’s ingredient, then recalculated to come up with TPC for an individual portion of each recipe (TPCr). To analyze the results of the LD group and of the MD group, phenolic compounds intake of recipe was calculated for each participant.
To Dr. Alatorre-Cruz, the biggest challenge was determining the content of compounds in traditional dishes. “Extensive, in-depth research was done to gather as much information as possible about all of the foods – especially about local and regional ones, because we have such a wide variety – looking to see where that information would match up with the exact method with which the compounds of interest were extracted.”
As expected, the team found that food with high PC was associated with a better health condition. However, the consumption of beverages and Mexican dishes was lower than their expectations. As noted in the article, the Mexican diet has changed over the last decades because traditional food has been replaced with ultraprocessed food with high-caloric values.
The authors suggest that their data confirm the alarming changes previously reported in the Mexican diet – changes possibly attributable to the increased influence of other countries via social media and economic globalization. However, they also found that beans, corn (mainly tortilla), and nopal intake remained preserved in the Mexican eating habits.
Their statistical analyses revealed that sex, age, and education seem to play a role in the presence or absence of diseases in the Mexican cohort. Men, participants over age 29 years, and those with lower levels of education had more diseases.
Dr. Alatorre-Cruz told this news organization that the Foods article opens new lines of research for the group to pursue. “One future proposal looks to enroll patients with conditions where there’s an increase in oxidative stress – which we know has quite a detrimental effect – and look into possible links with their diet. But now, maybe the focus can be on patients with a single condition and using these traditional-food tables to find links. We also want to know more about the molecular or biochemical mechanisms that are modulating the association that we saw first in animal models.”
Laura Álvarez, MD, a specialist in clinical nutrition, is the founder of the NUTRIENT project, which focuses on nutrition and strength training. In her opinion, the study extols the benefits of Mexican food. “I’ve always thought that people have this idea that Mexican food isn’t good. For example, thinking that it’s very high in fat. But in reality, Mexican food is very rich in nutrients.”
She added that research should be broadened, by enrolling a larger group of participants and evaluating not only a greater number and a greater variety of Mexican dishes, and new variables as well. “I think that body composition could be taken into account. In this study, they took the BMI into account to assess overweight and obesity, but ... the BMI doesn’t tell us much. Overweight can be due to fat or due to muscle. If we could get even more specific and find out what type of fat, that would give us more information about other [possible] diseases that ... need to be taken into account.”
This research was funded by the Institute of Sciences, Benemérita Autonomous University of Puebla. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results. Dr. Alatorre-Cruz and Dr. Álvarez have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to researchers from the Institute of Sciences at Benemérita Autonomous University of Puebla, Puebla, Mexico.
Their study, published in the journal Foods, is the first to produce tables showing the phenolic content of Mexican dishes. Physicians and nutritionists can use this information as a reference tool when drawing up diet recommendations for patients who could benefit from a higher intake of phenolic compounds (PC).
“Up until now, there hasn’t been a table that we – nutritionists and physicians – could look at and see exactly which foods were richest in these compounds. In the United States, European countries, Asian countries – they’ve all had food tables; in Mexico, we didn’t,” said lead author Julia Alatorre-Cruz, PhD, a biological scientist and postdoctoral researcher at BUAP. “So, it’s a fairly innovative contribution. As a bonus, the information can be used to analyze the relationship between diet and noncommunicable diseases in the Mexican population.”
In recent years, nutrition science has focused on counteracting nutrient deficiency and some diseases by identifying active-food components. Diet offers the possibility to improve the patient’s health conditions by using these components or functional food.
PCs are a diverse group of plant micronutrients, some of which modulate physiologic and molecular pathways involved in energy metabolism. They can act by different mechanisms; the most important of them are conducted by anti-inflammatory, antioxidant activities, and are antiallergic.
Moreover, recent studies explain how PCs positively affect certain illnesses, such as obesity, diabetes, cardiovascular diseases, thrombocytopenia, and metabolic syndrome. Several common features characterize these pathologies – among them are the redox balance and a notable inflammatory response that strongly alters the biochemical and functional characteristics of the affected tissues.
Traditional Mexican food is characterized by grains, tubers, legumes, vegetables, and spices, most of which are rich in PCs. However, the Mexican diet has changed over the past decades because traditional food has been replaced with ultraprocessed food with high-caloric values. Moreover, some vegetables and fruits are preferably consumed after processing, which affects the quantity, quality, and bioavailability of the PCs. In addition, diseases associated with eating habits have increased by more than 27% in the Mexican population.
The objective of the study was to determine whether participants with a higher PC intake from beverages or Mexican dishes have better health conditions than those with a lower intake.
A total of 973 adults (798 females, 175 males) aged 18-79 years were enrolled in this cross-sectional study. The data were obtained from a validated, self-administered food consumption survey that was posted on social media (Facebook) or sent via WhatsApp or email. In one section, there was a list of fruits, vegetables, cereals, legumes, seeds, spices, beverages, and Mexican dishes. The participants were asked to indicate how often in the past month they had consumed these items. There were also sections for providing identification data (for example, age, sex, marital status), height and weight information, and medical history.
“The study was carried out during the pandemic, so that limited contact with the participants,” said Dr. Alatorre-Cruz. “Not being able to directly interact with them was a challenge. For example, we would have liked to have taken those anthropometric measurements ourselves.”
The researchers performed K-means clustering to determine the participant’s health-condition level, resulting in two groups: those with less diseases (LD, n = 649) and those with more diseases (MD, n = 324).
Using the food biochemistry composition reported in multiple papers, the researchers computed the average total phenolic compounds (TPC) for each item listed in the survey. For Mexican dishes, they added the TPC of each recipe’s ingredient, then recalculated to come up with TPC for an individual portion of each recipe (TPCr). To analyze the results of the LD group and of the MD group, phenolic compounds intake of recipe was calculated for each participant.
To Dr. Alatorre-Cruz, the biggest challenge was determining the content of compounds in traditional dishes. “Extensive, in-depth research was done to gather as much information as possible about all of the foods – especially about local and regional ones, because we have such a wide variety – looking to see where that information would match up with the exact method with which the compounds of interest were extracted.”
As expected, the team found that food with high PC was associated with a better health condition. However, the consumption of beverages and Mexican dishes was lower than their expectations. As noted in the article, the Mexican diet has changed over the last decades because traditional food has been replaced with ultraprocessed food with high-caloric values.
The authors suggest that their data confirm the alarming changes previously reported in the Mexican diet – changes possibly attributable to the increased influence of other countries via social media and economic globalization. However, they also found that beans, corn (mainly tortilla), and nopal intake remained preserved in the Mexican eating habits.
Their statistical analyses revealed that sex, age, and education seem to play a role in the presence or absence of diseases in the Mexican cohort. Men, participants over age 29 years, and those with lower levels of education had more diseases.
Dr. Alatorre-Cruz told this news organization that the Foods article opens new lines of research for the group to pursue. “One future proposal looks to enroll patients with conditions where there’s an increase in oxidative stress – which we know has quite a detrimental effect – and look into possible links with their diet. But now, maybe the focus can be on patients with a single condition and using these traditional-food tables to find links. We also want to know more about the molecular or biochemical mechanisms that are modulating the association that we saw first in animal models.”
Laura Álvarez, MD, a specialist in clinical nutrition, is the founder of the NUTRIENT project, which focuses on nutrition and strength training. In her opinion, the study extols the benefits of Mexican food. “I’ve always thought that people have this idea that Mexican food isn’t good. For example, thinking that it’s very high in fat. But in reality, Mexican food is very rich in nutrients.”
She added that research should be broadened, by enrolling a larger group of participants and evaluating not only a greater number and a greater variety of Mexican dishes, and new variables as well. “I think that body composition could be taken into account. In this study, they took the BMI into account to assess overweight and obesity, but ... the BMI doesn’t tell us much. Overweight can be due to fat or due to muscle. If we could get even more specific and find out what type of fat, that would give us more information about other [possible] diseases that ... need to be taken into account.”
This research was funded by the Institute of Sciences, Benemérita Autonomous University of Puebla. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results. Dr. Alatorre-Cruz and Dr. Álvarez have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FOODS
Intensive BP reduction after stroke recanalization harmful
MUNICH, GERMANY – suggests results from the OPTIMAL-BP trial.
The research, presented at the annual European Stroke Organisation Conference, supports the latest U.S. and European guidelines, which recommend a relatively high upper SBP limit.
For the trial, which was halted early, more than 300 patients who successfully underwent IAT for acute ischemic stroke were randomly assigned to intensive or conventional BP management within 2 hours of recanalization.
Patients in the intensive group were 44% less likely than those assigned to conventional management to have a favorable outcome of a modified Rankin Scale (mRS) score of 0-2 at 3 months, while having similar rates of adverse outcomes.
The results suggest that intensive BP lowering in the 24 hours after recanalization leads to an increased risk of disability without decreasing the risk of intracerebral hemorrhage (ICH) or death, said study presenter Hyo Suk Nam, MD, PhD, department of neurology, Yonsei (South Korea) University.
Consequently, the trial “does not support intensive blood pressure management” in that early post-IAT period, although the “optimal blood pressure range remains unclear and requires more investigation,” he said.
Dr. Nam added that the results suggest, “despite recanalization, some areas in the ischemic brain may have already been damaged,” or that surrounding areas continue to have reduced blood circulation.
He believes that these areas may have reduced capacity for autoregulation and so “may not effectively counteract sudden drops in blood pressure.
“Thus, intensive blood pressure lowering may further reduce blood flow ... and exacerbate ischemic injury.”
On the other hand, the conventional group confirmed prior studies indicating that high SBP is associated with poor outcomes.
Dr. Nam suggested that increased BP “may be a physiological response to the acute stress of stroke,” but that the adverse outcomes in some patients “might reflect stroke severity rather than being a direct effect of raised blood pressure.”
Session cochair Carlos Molina, MD, director of the stroke unit and brain hemodynamics at Vall d’Hebron Hospital, Barcelona, commented that “it’s very important to remember that the guidances are endorsed by the results of this study.
He said in an interview that “intensive blood pressure lowering harms the brain, especially just after reperfusion.
“So, the results are in line with the previous concept that we need to be careful, as intensive blood pressure lowering is associated with clinical deterioration and poor outcomes.”
He agreed with Dr. Nam that, with high BP also being harmful, the optimal range is currently unclear.
Dr. Molina underlined, however, that, in the absence of further studies, “we have to stick to the guidelines.”
Dr. Nam pointed out that, while high BP can result in reperfusion injury or ICH, “too low blood pressure can worsen cerebral ischemia.”
Yet the management of BP after successful recanalization with IAT is “largely unknown.”
He noted that, while both the European Stroke Organisation and American Heart Association/American Stroke Association guidelines recommend that BP should be kept below 180/105 mm Hg in patients who have undergone successful recanalization, the evidence class for this recommendation is “weak.”
Furthermore, observational studies have indicated that higher maximum or average SBP is associated with poor outcomes, but two multicenter clinical trials of intensive BP lowering after IAT, BP-TARGET and ENCHANTED2/MT, had conflicting results.
The researchers therefore investigated whether intensive BP management would result in better clinical outcomes in the 24 hours after successful recanalization with IAT.
They conducted a multicenter, open-label trial in which patients aged 20 years and older who underwent IAT for acute ischemic stroke with large cerebrovascular occlusion and had an SBP of at least 140 mm Hg were recruited from 19 centers in South Korea between June 2020 and November 2022.
The patients were randomly assigned within 2 hours of successful recanalization to intensive BP management, targeting an SBP less than 140 mm Hg, or conventional management, targeting an SBP of 140-180 mm Hg.
Clinicians could use local treatment protocols based on available intravenous BP-lowering drugs. BP was measured every 15 minutes for the first hour after randomization and then hourly for 24 hours.
The trial was terminated early because of safety concerns after the ENCHANTED2/MT trial revealed a negative impact on mRS scores at 3 months with intensive BP management.
Of 1,606 potentially eligible patients with acute ischemic stroke treated with IAT, 306 were randomly assigned, with 155 in the intensive group and 150 in the conventional group included in the primary analysis.
The mean age was 73.1 years, and 40.3% were women. The average National Institutes of Health Stroke Scale (NIHSS) score prior to IAT was 13. The mean time from stroke onset to randomization was 480 minutes (interquartile range, 320-820 minutes).
At 24 hours, the mean SBP in the intensive group was 129.2 mm Hg versus 138.0 mm Hg in the conventional group, for a between-group difference of 9.6 mm Hg (95% confidence interval, –12.2 to –6.9, P < .001).
Patients in the intensive group spent 80.3% of the first 24 hours with SBP less than 140 mm Hg versus 54.2% in the conventional group (P < .001). In contrast, conventional group patients spent 42.1% of the first 24 hours with SBP 140-180 mm Hg versus 14.2% in the intensive group.
Crucially, Dr. Nam showed that patients in the intensive BP-lowering group were significantly less likely than those in the conventional group to have a favorable outcome, defined as an mRS score of 0-2, at 3 months, at 39.4% versus 54.4%, or an adjusted odds ratio of 0.56 (95% CI, 0.33-0.96, P = .034).
Moreover, a poor outcome was 1.84 (95% CI, 1.17-2.91) times more common in the intervention group than the conventional group, Dr. Nam reported, with a number needed to harm of 6.6.
In terms of safety, there was no significant difference in rates of symptomatic ICH between the groups, at 9% in the intensive versus 8.1% in the conventional groups, or an aOR of 1.10 (95% CI, 0.48-2.53, P = .816).
There was also no difference in the rate of death related to the index stroke within 90 days, at 7.7% versus 5.4% (AOR, 1.73; 95% CI, 0.61-4.92, P = .307).
There were also no significant differences between the groups in key secondary outcomes, such as NIHSS score at 24 hours, recanalization at 24 hours, favorable outcome on the mRS at 1 month, and the EQ-5D-3L quality of life score.
However, patients in the intensive group were substantially more likely to experience malignant brain edema, at 7.7% versus 1.3% in the conventional group (aOR, 7.88; 95% CI, 1.57-39.39, P = .012).
Restricted cubic spline regression analysis indicated that there was a U-shaped relationship between mean SBP during the 24 hours following IAT and the odds ratio of a poor outcome, in which both a low and a high BPe were unfavorable.
Dr. Nam cautioned that, when interpreting the results, the early termination of the study may have reduced its statistical power and increased the likelihood of random and exaggerated treatment effects.
He also noted that the study was conducted in South Korea, and so the results may not be generalizable to other populations.
The study received a grant from the Patient-Centered Clinical Research Coordinating Center, funded by the Ministry of Health and Welfare. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MUNICH, GERMANY – suggests results from the OPTIMAL-BP trial.
The research, presented at the annual European Stroke Organisation Conference, supports the latest U.S. and European guidelines, which recommend a relatively high upper SBP limit.
For the trial, which was halted early, more than 300 patients who successfully underwent IAT for acute ischemic stroke were randomly assigned to intensive or conventional BP management within 2 hours of recanalization.
Patients in the intensive group were 44% less likely than those assigned to conventional management to have a favorable outcome of a modified Rankin Scale (mRS) score of 0-2 at 3 months, while having similar rates of adverse outcomes.
The results suggest that intensive BP lowering in the 24 hours after recanalization leads to an increased risk of disability without decreasing the risk of intracerebral hemorrhage (ICH) or death, said study presenter Hyo Suk Nam, MD, PhD, department of neurology, Yonsei (South Korea) University.
Consequently, the trial “does not support intensive blood pressure management” in that early post-IAT period, although the “optimal blood pressure range remains unclear and requires more investigation,” he said.
Dr. Nam added that the results suggest, “despite recanalization, some areas in the ischemic brain may have already been damaged,” or that surrounding areas continue to have reduced blood circulation.
He believes that these areas may have reduced capacity for autoregulation and so “may not effectively counteract sudden drops in blood pressure.
“Thus, intensive blood pressure lowering may further reduce blood flow ... and exacerbate ischemic injury.”
On the other hand, the conventional group confirmed prior studies indicating that high SBP is associated with poor outcomes.
Dr. Nam suggested that increased BP “may be a physiological response to the acute stress of stroke,” but that the adverse outcomes in some patients “might reflect stroke severity rather than being a direct effect of raised blood pressure.”
Session cochair Carlos Molina, MD, director of the stroke unit and brain hemodynamics at Vall d’Hebron Hospital, Barcelona, commented that “it’s very important to remember that the guidances are endorsed by the results of this study.
He said in an interview that “intensive blood pressure lowering harms the brain, especially just after reperfusion.
“So, the results are in line with the previous concept that we need to be careful, as intensive blood pressure lowering is associated with clinical deterioration and poor outcomes.”
He agreed with Dr. Nam that, with high BP also being harmful, the optimal range is currently unclear.
Dr. Molina underlined, however, that, in the absence of further studies, “we have to stick to the guidelines.”
Dr. Nam pointed out that, while high BP can result in reperfusion injury or ICH, “too low blood pressure can worsen cerebral ischemia.”
Yet the management of BP after successful recanalization with IAT is “largely unknown.”
He noted that, while both the European Stroke Organisation and American Heart Association/American Stroke Association guidelines recommend that BP should be kept below 180/105 mm Hg in patients who have undergone successful recanalization, the evidence class for this recommendation is “weak.”
Furthermore, observational studies have indicated that higher maximum or average SBP is associated with poor outcomes, but two multicenter clinical trials of intensive BP lowering after IAT, BP-TARGET and ENCHANTED2/MT, had conflicting results.
The researchers therefore investigated whether intensive BP management would result in better clinical outcomes in the 24 hours after successful recanalization with IAT.
They conducted a multicenter, open-label trial in which patients aged 20 years and older who underwent IAT for acute ischemic stroke with large cerebrovascular occlusion and had an SBP of at least 140 mm Hg were recruited from 19 centers in South Korea between June 2020 and November 2022.
The patients were randomly assigned within 2 hours of successful recanalization to intensive BP management, targeting an SBP less than 140 mm Hg, or conventional management, targeting an SBP of 140-180 mm Hg.
Clinicians could use local treatment protocols based on available intravenous BP-lowering drugs. BP was measured every 15 minutes for the first hour after randomization and then hourly for 24 hours.
The trial was terminated early because of safety concerns after the ENCHANTED2/MT trial revealed a negative impact on mRS scores at 3 months with intensive BP management.
Of 1,606 potentially eligible patients with acute ischemic stroke treated with IAT, 306 were randomly assigned, with 155 in the intensive group and 150 in the conventional group included in the primary analysis.
The mean age was 73.1 years, and 40.3% were women. The average National Institutes of Health Stroke Scale (NIHSS) score prior to IAT was 13. The mean time from stroke onset to randomization was 480 minutes (interquartile range, 320-820 minutes).
At 24 hours, the mean SBP in the intensive group was 129.2 mm Hg versus 138.0 mm Hg in the conventional group, for a between-group difference of 9.6 mm Hg (95% confidence interval, –12.2 to –6.9, P < .001).
Patients in the intensive group spent 80.3% of the first 24 hours with SBP less than 140 mm Hg versus 54.2% in the conventional group (P < .001). In contrast, conventional group patients spent 42.1% of the first 24 hours with SBP 140-180 mm Hg versus 14.2% in the intensive group.
Crucially, Dr. Nam showed that patients in the intensive BP-lowering group were significantly less likely than those in the conventional group to have a favorable outcome, defined as an mRS score of 0-2, at 3 months, at 39.4% versus 54.4%, or an adjusted odds ratio of 0.56 (95% CI, 0.33-0.96, P = .034).
Moreover, a poor outcome was 1.84 (95% CI, 1.17-2.91) times more common in the intervention group than the conventional group, Dr. Nam reported, with a number needed to harm of 6.6.
In terms of safety, there was no significant difference in rates of symptomatic ICH between the groups, at 9% in the intensive versus 8.1% in the conventional groups, or an aOR of 1.10 (95% CI, 0.48-2.53, P = .816).
There was also no difference in the rate of death related to the index stroke within 90 days, at 7.7% versus 5.4% (AOR, 1.73; 95% CI, 0.61-4.92, P = .307).
There were also no significant differences between the groups in key secondary outcomes, such as NIHSS score at 24 hours, recanalization at 24 hours, favorable outcome on the mRS at 1 month, and the EQ-5D-3L quality of life score.
However, patients in the intensive group were substantially more likely to experience malignant brain edema, at 7.7% versus 1.3% in the conventional group (aOR, 7.88; 95% CI, 1.57-39.39, P = .012).
Restricted cubic spline regression analysis indicated that there was a U-shaped relationship between mean SBP during the 24 hours following IAT and the odds ratio of a poor outcome, in which both a low and a high BPe were unfavorable.
Dr. Nam cautioned that, when interpreting the results, the early termination of the study may have reduced its statistical power and increased the likelihood of random and exaggerated treatment effects.
He also noted that the study was conducted in South Korea, and so the results may not be generalizable to other populations.
The study received a grant from the Patient-Centered Clinical Research Coordinating Center, funded by the Ministry of Health and Welfare. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MUNICH, GERMANY – suggests results from the OPTIMAL-BP trial.
The research, presented at the annual European Stroke Organisation Conference, supports the latest U.S. and European guidelines, which recommend a relatively high upper SBP limit.
For the trial, which was halted early, more than 300 patients who successfully underwent IAT for acute ischemic stroke were randomly assigned to intensive or conventional BP management within 2 hours of recanalization.
Patients in the intensive group were 44% less likely than those assigned to conventional management to have a favorable outcome of a modified Rankin Scale (mRS) score of 0-2 at 3 months, while having similar rates of adverse outcomes.
The results suggest that intensive BP lowering in the 24 hours after recanalization leads to an increased risk of disability without decreasing the risk of intracerebral hemorrhage (ICH) or death, said study presenter Hyo Suk Nam, MD, PhD, department of neurology, Yonsei (South Korea) University.
Consequently, the trial “does not support intensive blood pressure management” in that early post-IAT period, although the “optimal blood pressure range remains unclear and requires more investigation,” he said.
Dr. Nam added that the results suggest, “despite recanalization, some areas in the ischemic brain may have already been damaged,” or that surrounding areas continue to have reduced blood circulation.
He believes that these areas may have reduced capacity for autoregulation and so “may not effectively counteract sudden drops in blood pressure.
“Thus, intensive blood pressure lowering may further reduce blood flow ... and exacerbate ischemic injury.”
On the other hand, the conventional group confirmed prior studies indicating that high SBP is associated with poor outcomes.
Dr. Nam suggested that increased BP “may be a physiological response to the acute stress of stroke,” but that the adverse outcomes in some patients “might reflect stroke severity rather than being a direct effect of raised blood pressure.”
Session cochair Carlos Molina, MD, director of the stroke unit and brain hemodynamics at Vall d’Hebron Hospital, Barcelona, commented that “it’s very important to remember that the guidances are endorsed by the results of this study.
He said in an interview that “intensive blood pressure lowering harms the brain, especially just after reperfusion.
“So, the results are in line with the previous concept that we need to be careful, as intensive blood pressure lowering is associated with clinical deterioration and poor outcomes.”
He agreed with Dr. Nam that, with high BP also being harmful, the optimal range is currently unclear.
Dr. Molina underlined, however, that, in the absence of further studies, “we have to stick to the guidelines.”
Dr. Nam pointed out that, while high BP can result in reperfusion injury or ICH, “too low blood pressure can worsen cerebral ischemia.”
Yet the management of BP after successful recanalization with IAT is “largely unknown.”
He noted that, while both the European Stroke Organisation and American Heart Association/American Stroke Association guidelines recommend that BP should be kept below 180/105 mm Hg in patients who have undergone successful recanalization, the evidence class for this recommendation is “weak.”
Furthermore, observational studies have indicated that higher maximum or average SBP is associated with poor outcomes, but two multicenter clinical trials of intensive BP lowering after IAT, BP-TARGET and ENCHANTED2/MT, had conflicting results.
The researchers therefore investigated whether intensive BP management would result in better clinical outcomes in the 24 hours after successful recanalization with IAT.
They conducted a multicenter, open-label trial in which patients aged 20 years and older who underwent IAT for acute ischemic stroke with large cerebrovascular occlusion and had an SBP of at least 140 mm Hg were recruited from 19 centers in South Korea between June 2020 and November 2022.
The patients were randomly assigned within 2 hours of successful recanalization to intensive BP management, targeting an SBP less than 140 mm Hg, or conventional management, targeting an SBP of 140-180 mm Hg.
Clinicians could use local treatment protocols based on available intravenous BP-lowering drugs. BP was measured every 15 minutes for the first hour after randomization and then hourly for 24 hours.
The trial was terminated early because of safety concerns after the ENCHANTED2/MT trial revealed a negative impact on mRS scores at 3 months with intensive BP management.
Of 1,606 potentially eligible patients with acute ischemic stroke treated with IAT, 306 were randomly assigned, with 155 in the intensive group and 150 in the conventional group included in the primary analysis.
The mean age was 73.1 years, and 40.3% were women. The average National Institutes of Health Stroke Scale (NIHSS) score prior to IAT was 13. The mean time from stroke onset to randomization was 480 minutes (interquartile range, 320-820 minutes).
At 24 hours, the mean SBP in the intensive group was 129.2 mm Hg versus 138.0 mm Hg in the conventional group, for a between-group difference of 9.6 mm Hg (95% confidence interval, –12.2 to –6.9, P < .001).
Patients in the intensive group spent 80.3% of the first 24 hours with SBP less than 140 mm Hg versus 54.2% in the conventional group (P < .001). In contrast, conventional group patients spent 42.1% of the first 24 hours with SBP 140-180 mm Hg versus 14.2% in the intensive group.
Crucially, Dr. Nam showed that patients in the intensive BP-lowering group were significantly less likely than those in the conventional group to have a favorable outcome, defined as an mRS score of 0-2, at 3 months, at 39.4% versus 54.4%, or an adjusted odds ratio of 0.56 (95% CI, 0.33-0.96, P = .034).
Moreover, a poor outcome was 1.84 (95% CI, 1.17-2.91) times more common in the intervention group than the conventional group, Dr. Nam reported, with a number needed to harm of 6.6.
In terms of safety, there was no significant difference in rates of symptomatic ICH between the groups, at 9% in the intensive versus 8.1% in the conventional groups, or an aOR of 1.10 (95% CI, 0.48-2.53, P = .816).
There was also no difference in the rate of death related to the index stroke within 90 days, at 7.7% versus 5.4% (AOR, 1.73; 95% CI, 0.61-4.92, P = .307).
There were also no significant differences between the groups in key secondary outcomes, such as NIHSS score at 24 hours, recanalization at 24 hours, favorable outcome on the mRS at 1 month, and the EQ-5D-3L quality of life score.
However, patients in the intensive group were substantially more likely to experience malignant brain edema, at 7.7% versus 1.3% in the conventional group (aOR, 7.88; 95% CI, 1.57-39.39, P = .012).
Restricted cubic spline regression analysis indicated that there was a U-shaped relationship between mean SBP during the 24 hours following IAT and the odds ratio of a poor outcome, in which both a low and a high BPe were unfavorable.
Dr. Nam cautioned that, when interpreting the results, the early termination of the study may have reduced its statistical power and increased the likelihood of random and exaggerated treatment effects.
He also noted that the study was conducted in South Korea, and so the results may not be generalizable to other populations.
The study received a grant from the Patient-Centered Clinical Research Coordinating Center, funded by the Ministry of Health and Welfare. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
AT ESOC 2023
Tenecteplase late after stroke misses endpoint: TIMELESS
However, there were some encouraging trends, and there did not appear to be an increase in intracranial hemorrhage (ICH), leading to hope that the option of late thrombolysis in this group of patients may still have potential.
The TIMELESS study tested the approach of giving thrombolysis with tenecteplase (TNK) to patients with a large-vessel occlusion stroke up to 24 hours after symptom onset. Patients were selected by perfusion imaging, and those who had a stroke with a small core and large amount of salvageable brain tissue were included in the placebo-controlled study.
“This is first trial to try giving a thrombolytic so late – up to 24 hours after last known well. While we did not meet the primary outcome, there were some promising findings,” lead author, Gregory Albers, MD, director of the Stanford (Calif.) Stroke Center and professor of neurology at Stanford University, said in an interview.
“The most encouraging observation was that we did not show any safety issues with giving TNK to this population at such a late time. Many people thought this would be too high risk but there was no increase in ICH, which was very low and the same in both groups,” Dr. Albers said.
“And we saw some evidence of drug effect. There appeared to be a benefit in patients with M1 occlusions, the most common type of large-vessel occlusion, who represented half the patients in the study,” he added.
The researchers also gained information on the logistics and timing of TNK administration in this late period which they hope can guide the design of a future trial.
Dr. Albers presented the TIMELESS trial at the annual European Stroke Organisation Conference.
He explained that there is increasing evidence that intravenous thrombolysis can improve outcome in selected patients even beyond the traditional 4.5-hour time window.
The phase 3, double-blind, randomized, placebo-controlled TIMELESS study sought to investigate whether tenecteplase administered to patients with ischemic stroke with large-vessel occlusion presenting between 4.5 and 24 hours after last known well would improve clinical outcome as measured by modified Rankin Scale (mRS) at day 90.
The trial included 458 patients with an internal carotid artery occlusion or middle cerebral artery segment 1 or 2 occlusion and presenting with salvageable tissue on imaging. They were randomly assigned 1:1 to either intravenous tenecteplase (0.25 mg/kg; maximum, 25 mg) or placebo.
The proportion of patients treated with mechanical thrombectomy were similar between the two treatment arms (around 77%). The study completion rate was higher than 96% in both treatment arms.
The primary endpoint analyses showed no significant difference in the odds of a lower mRS score at day 90, but there was a slight trend toward benefit in the TNK group in the shift analysis, with a common odds ratio of 1.13 (95% confidence interval, 0.81-1.56; P = .48).
The percentage of patients achieving a favorable outcome, defined as an mRS of 0-2, was not significantly different between the treatment groups: 46% in the TNK group versus 42% in the placebo group (nominal P = .41).
Promising safety data
There were no significant safety issues, and the risk for bleeding was not significantly increased in the tenecteplase group. Symptomatic ICH occurred in 3.2% of the TNK group versus 2.3% of the placebo group, a nonsignificant difference.
“The low rate of ICH with TNK at this late time point is very reassuring,” Dr. Albers said. “We believe the reason for the low ICH rate is probably because these patients were selected for small core strokes. We also found that there was a trend towards the most benefit from TNK in patients with the smallest cores, supporting the use of imaging to select patients.”
The secondary endpoint of complete recanalization at 24-hours post randomization was higher in the TNK group at 76.7%, compared with 63.9% in the placebo group (P = .006).
Benefit in M1 occlusions?
Subgroup analysis showed that there appeared to be a benefit of TNK in the 227 patients included who had an M1 occlusion. In this group, the common odds ratio for a more favorable outcome in the mRS shift analysis with TNK was 1.59 (95% CI, 1.00-2.52; adjusted nominal P = .051).
The percentage of patients with a favorable outcome (mRS, 0-2) at 90 days in the M1 occlusion subgroup was 45.9% for TNK versus 31.4% for placebo, giving an adjusted odds ratio of 2.03 (95% CI, 1.14-3.66; nominal P = .017).
But Dr. Albers cautioned that this was an exploratory analysis, and no formal conclusions should be drawn from these data.
“We saw very strong results in favor of giving thrombolysis in the patients with M1 occlusions. We had preliminary pilot data suggesting this approach may work in these patients,” he commented.
“But we included the smaller M2 occlusions as well, because we thought that as there should be less clot in an M2 occlusion it might be easier to dissolve with thrombolysis,” he added. “But surprisingly, the M2 occlusion patients seemed to do worse with TNK than placebo, and the M1 patients did better.”
Timing of TNK
Dr. Albers said that there was also information from in the study on the timing of TNK administration.
In patients who also received thrombectomy, who made up of the majority of those in the study, the average time of TNK administration was only 20 minutes before the thrombectomy procedure.
“We had hoped to have a longer time between thrombolysis and thrombectomy so the drug would have more time to work. The idea was that patients would be given TNK at the primary stroke center before being transferred for thrombectomy, but actually only a few patients received TNK at the primary stroke center,” Dr. Albers explained.
“But, again surprisingly, we found that patients given TNK right at the time of the thrombectomy procedure seemed to show a trend toward benefit over placebo,” he reported.
He suggested that this may be caused by the thrombolytic dissolving the small fragments that can sometimes break off and cause further occlusions when the clot is removed by thrombectomy.
“We have learned a lot from this study, and we are planning to go forward with the information gained to plan a second study, in which we will focus on patients with M1 occlusions and try to get the drug on board at primary stroke centers, so it has more time to work before thrombectomy,” he added.
Commenting on the TIMELESS study at the ESOC meeting, Georgios Tsivgoulis, MD, professor of neurology, University of Athens, said that he thought the trial had shown three important results: “Firstly, TNK appeared to be safe in this late window in these selected patients – that is a very important observation. Secondly, reperfusion rates at 24 hours were increased with TNK and we know that this translates into clinical benefit. And thirdly, there was a neutral effect on primary outcomes, but I think the sample size of 438 patients was not large enough to show efficacy.”
Dr. Tsivgoulis concluded that these points need to be addressed in future trials.
The TIMELESS trial was funded by Genentech.
A version of this article first appeared on Medscape.com.
However, there were some encouraging trends, and there did not appear to be an increase in intracranial hemorrhage (ICH), leading to hope that the option of late thrombolysis in this group of patients may still have potential.
The TIMELESS study tested the approach of giving thrombolysis with tenecteplase (TNK) to patients with a large-vessel occlusion stroke up to 24 hours after symptom onset. Patients were selected by perfusion imaging, and those who had a stroke with a small core and large amount of salvageable brain tissue were included in the placebo-controlled study.
“This is first trial to try giving a thrombolytic so late – up to 24 hours after last known well. While we did not meet the primary outcome, there were some promising findings,” lead author, Gregory Albers, MD, director of the Stanford (Calif.) Stroke Center and professor of neurology at Stanford University, said in an interview.
“The most encouraging observation was that we did not show any safety issues with giving TNK to this population at such a late time. Many people thought this would be too high risk but there was no increase in ICH, which was very low and the same in both groups,” Dr. Albers said.
“And we saw some evidence of drug effect. There appeared to be a benefit in patients with M1 occlusions, the most common type of large-vessel occlusion, who represented half the patients in the study,” he added.
The researchers also gained information on the logistics and timing of TNK administration in this late period which they hope can guide the design of a future trial.
Dr. Albers presented the TIMELESS trial at the annual European Stroke Organisation Conference.
He explained that there is increasing evidence that intravenous thrombolysis can improve outcome in selected patients even beyond the traditional 4.5-hour time window.
The phase 3, double-blind, randomized, placebo-controlled TIMELESS study sought to investigate whether tenecteplase administered to patients with ischemic stroke with large-vessel occlusion presenting between 4.5 and 24 hours after last known well would improve clinical outcome as measured by modified Rankin Scale (mRS) at day 90.
The trial included 458 patients with an internal carotid artery occlusion or middle cerebral artery segment 1 or 2 occlusion and presenting with salvageable tissue on imaging. They were randomly assigned 1:1 to either intravenous tenecteplase (0.25 mg/kg; maximum, 25 mg) or placebo.
The proportion of patients treated with mechanical thrombectomy were similar between the two treatment arms (around 77%). The study completion rate was higher than 96% in both treatment arms.
The primary endpoint analyses showed no significant difference in the odds of a lower mRS score at day 90, but there was a slight trend toward benefit in the TNK group in the shift analysis, with a common odds ratio of 1.13 (95% confidence interval, 0.81-1.56; P = .48).
The percentage of patients achieving a favorable outcome, defined as an mRS of 0-2, was not significantly different between the treatment groups: 46% in the TNK group versus 42% in the placebo group (nominal P = .41).
Promising safety data
There were no significant safety issues, and the risk for bleeding was not significantly increased in the tenecteplase group. Symptomatic ICH occurred in 3.2% of the TNK group versus 2.3% of the placebo group, a nonsignificant difference.
“The low rate of ICH with TNK at this late time point is very reassuring,” Dr. Albers said. “We believe the reason for the low ICH rate is probably because these patients were selected for small core strokes. We also found that there was a trend towards the most benefit from TNK in patients with the smallest cores, supporting the use of imaging to select patients.”
The secondary endpoint of complete recanalization at 24-hours post randomization was higher in the TNK group at 76.7%, compared with 63.9% in the placebo group (P = .006).
Benefit in M1 occlusions?
Subgroup analysis showed that there appeared to be a benefit of TNK in the 227 patients included who had an M1 occlusion. In this group, the common odds ratio for a more favorable outcome in the mRS shift analysis with TNK was 1.59 (95% CI, 1.00-2.52; adjusted nominal P = .051).
The percentage of patients with a favorable outcome (mRS, 0-2) at 90 days in the M1 occlusion subgroup was 45.9% for TNK versus 31.4% for placebo, giving an adjusted odds ratio of 2.03 (95% CI, 1.14-3.66; nominal P = .017).
But Dr. Albers cautioned that this was an exploratory analysis, and no formal conclusions should be drawn from these data.
“We saw very strong results in favor of giving thrombolysis in the patients with M1 occlusions. We had preliminary pilot data suggesting this approach may work in these patients,” he commented.
“But we included the smaller M2 occlusions as well, because we thought that as there should be less clot in an M2 occlusion it might be easier to dissolve with thrombolysis,” he added. “But surprisingly, the M2 occlusion patients seemed to do worse with TNK than placebo, and the M1 patients did better.”
Timing of TNK
Dr. Albers said that there was also information from in the study on the timing of TNK administration.
In patients who also received thrombectomy, who made up of the majority of those in the study, the average time of TNK administration was only 20 minutes before the thrombectomy procedure.
“We had hoped to have a longer time between thrombolysis and thrombectomy so the drug would have more time to work. The idea was that patients would be given TNK at the primary stroke center before being transferred for thrombectomy, but actually only a few patients received TNK at the primary stroke center,” Dr. Albers explained.
“But, again surprisingly, we found that patients given TNK right at the time of the thrombectomy procedure seemed to show a trend toward benefit over placebo,” he reported.
He suggested that this may be caused by the thrombolytic dissolving the small fragments that can sometimes break off and cause further occlusions when the clot is removed by thrombectomy.
“We have learned a lot from this study, and we are planning to go forward with the information gained to plan a second study, in which we will focus on patients with M1 occlusions and try to get the drug on board at primary stroke centers, so it has more time to work before thrombectomy,” he added.
Commenting on the TIMELESS study at the ESOC meeting, Georgios Tsivgoulis, MD, professor of neurology, University of Athens, said that he thought the trial had shown three important results: “Firstly, TNK appeared to be safe in this late window in these selected patients – that is a very important observation. Secondly, reperfusion rates at 24 hours were increased with TNK and we know that this translates into clinical benefit. And thirdly, there was a neutral effect on primary outcomes, but I think the sample size of 438 patients was not large enough to show efficacy.”
Dr. Tsivgoulis concluded that these points need to be addressed in future trials.
The TIMELESS trial was funded by Genentech.
A version of this article first appeared on Medscape.com.
However, there were some encouraging trends, and there did not appear to be an increase in intracranial hemorrhage (ICH), leading to hope that the option of late thrombolysis in this group of patients may still have potential.
The TIMELESS study tested the approach of giving thrombolysis with tenecteplase (TNK) to patients with a large-vessel occlusion stroke up to 24 hours after symptom onset. Patients were selected by perfusion imaging, and those who had a stroke with a small core and large amount of salvageable brain tissue were included in the placebo-controlled study.
“This is first trial to try giving a thrombolytic so late – up to 24 hours after last known well. While we did not meet the primary outcome, there were some promising findings,” lead author, Gregory Albers, MD, director of the Stanford (Calif.) Stroke Center and professor of neurology at Stanford University, said in an interview.
“The most encouraging observation was that we did not show any safety issues with giving TNK to this population at such a late time. Many people thought this would be too high risk but there was no increase in ICH, which was very low and the same in both groups,” Dr. Albers said.
“And we saw some evidence of drug effect. There appeared to be a benefit in patients with M1 occlusions, the most common type of large-vessel occlusion, who represented half the patients in the study,” he added.
The researchers also gained information on the logistics and timing of TNK administration in this late period which they hope can guide the design of a future trial.
Dr. Albers presented the TIMELESS trial at the annual European Stroke Organisation Conference.
He explained that there is increasing evidence that intravenous thrombolysis can improve outcome in selected patients even beyond the traditional 4.5-hour time window.
The phase 3, double-blind, randomized, placebo-controlled TIMELESS study sought to investigate whether tenecteplase administered to patients with ischemic stroke with large-vessel occlusion presenting between 4.5 and 24 hours after last known well would improve clinical outcome as measured by modified Rankin Scale (mRS) at day 90.
The trial included 458 patients with an internal carotid artery occlusion or middle cerebral artery segment 1 or 2 occlusion and presenting with salvageable tissue on imaging. They were randomly assigned 1:1 to either intravenous tenecteplase (0.25 mg/kg; maximum, 25 mg) or placebo.
The proportion of patients treated with mechanical thrombectomy were similar between the two treatment arms (around 77%). The study completion rate was higher than 96% in both treatment arms.
The primary endpoint analyses showed no significant difference in the odds of a lower mRS score at day 90, but there was a slight trend toward benefit in the TNK group in the shift analysis, with a common odds ratio of 1.13 (95% confidence interval, 0.81-1.56; P = .48).
The percentage of patients achieving a favorable outcome, defined as an mRS of 0-2, was not significantly different between the treatment groups: 46% in the TNK group versus 42% in the placebo group (nominal P = .41).
Promising safety data
There were no significant safety issues, and the risk for bleeding was not significantly increased in the tenecteplase group. Symptomatic ICH occurred in 3.2% of the TNK group versus 2.3% of the placebo group, a nonsignificant difference.
“The low rate of ICH with TNK at this late time point is very reassuring,” Dr. Albers said. “We believe the reason for the low ICH rate is probably because these patients were selected for small core strokes. We also found that there was a trend towards the most benefit from TNK in patients with the smallest cores, supporting the use of imaging to select patients.”
The secondary endpoint of complete recanalization at 24-hours post randomization was higher in the TNK group at 76.7%, compared with 63.9% in the placebo group (P = .006).
Benefit in M1 occlusions?
Subgroup analysis showed that there appeared to be a benefit of TNK in the 227 patients included who had an M1 occlusion. In this group, the common odds ratio for a more favorable outcome in the mRS shift analysis with TNK was 1.59 (95% CI, 1.00-2.52; adjusted nominal P = .051).
The percentage of patients with a favorable outcome (mRS, 0-2) at 90 days in the M1 occlusion subgroup was 45.9% for TNK versus 31.4% for placebo, giving an adjusted odds ratio of 2.03 (95% CI, 1.14-3.66; nominal P = .017).
But Dr. Albers cautioned that this was an exploratory analysis, and no formal conclusions should be drawn from these data.
“We saw very strong results in favor of giving thrombolysis in the patients with M1 occlusions. We had preliminary pilot data suggesting this approach may work in these patients,” he commented.
“But we included the smaller M2 occlusions as well, because we thought that as there should be less clot in an M2 occlusion it might be easier to dissolve with thrombolysis,” he added. “But surprisingly, the M2 occlusion patients seemed to do worse with TNK than placebo, and the M1 patients did better.”
Timing of TNK
Dr. Albers said that there was also information from in the study on the timing of TNK administration.
In patients who also received thrombectomy, who made up of the majority of those in the study, the average time of TNK administration was only 20 minutes before the thrombectomy procedure.
“We had hoped to have a longer time between thrombolysis and thrombectomy so the drug would have more time to work. The idea was that patients would be given TNK at the primary stroke center before being transferred for thrombectomy, but actually only a few patients received TNK at the primary stroke center,” Dr. Albers explained.
“But, again surprisingly, we found that patients given TNK right at the time of the thrombectomy procedure seemed to show a trend toward benefit over placebo,” he reported.
He suggested that this may be caused by the thrombolytic dissolving the small fragments that can sometimes break off and cause further occlusions when the clot is removed by thrombectomy.
“We have learned a lot from this study, and we are planning to go forward with the information gained to plan a second study, in which we will focus on patients with M1 occlusions and try to get the drug on board at primary stroke centers, so it has more time to work before thrombectomy,” he added.
Commenting on the TIMELESS study at the ESOC meeting, Georgios Tsivgoulis, MD, professor of neurology, University of Athens, said that he thought the trial had shown three important results: “Firstly, TNK appeared to be safe in this late window in these selected patients – that is a very important observation. Secondly, reperfusion rates at 24 hours were increased with TNK and we know that this translates into clinical benefit. And thirdly, there was a neutral effect on primary outcomes, but I think the sample size of 438 patients was not large enough to show efficacy.”
Dr. Tsivgoulis concluded that these points need to be addressed in future trials.
The TIMELESS trial was funded by Genentech.
A version of this article first appeared on Medscape.com.
FROM ESOC 2023
Enthesitis, arthritis, tenosynovitis linked to dupilumab use for atopic dermatitis
Around 5% of patients treated with dupilumab (Dupixent) for moderate-to-severe atopic dermatitis experience musculoskeletal (MSK) symptoms, according to the results of a descriptive study.
The main MSK symptom seen in the observational cohort was enthesitis, but some patients also experienced arthritis and tenosynovitis a median of 17 weeks after starting dupilumab treatment. Together these symptoms represent a new MSK syndrome, say researchers from the United Kingdom.
“The pattern of MSK symptoms and signs is characteristic of psoriatic arthritis/peripheral spondyloarthritis,” Bruce Kirkham, MD, and collaborators report in Arthritis & Rheumatology.
“We started a few years ago and have been following the patients for quite a long time,” Dr. Kirkham, a consultant rheumatologist at Guy’s and St. Thomas’ NHS Foundation Trust, London, told this news organization.
“We’re still seeing patients with the same type of syndrome presenting occasionally. It’s not a very common adverse event, but we think it continues,” he observed.
“Most of them don’t have very severe problems, and a lot of them can be treated with quite simple drugs or, alternatively, reducing the frequency of the injection,” Dr. Kirkham added.
Characterizing the MSK symptoms
Of 470 patients with atopic dermatitis who started treatment with dupilumab at Guy’s and St. Thomas’ NHS Foundation Trust between October 2018 and February 2021, 36 (7.65%) developed rheumatic symptoms and were referred to the rheumatology department. These individuals had their family history assessed and thorough MSK evaluations, which included antibody and inflammatory markers, ultrasound of the peripheral small joints, and MRI of the large joints and spine.
A total of 26 (5.5%) patients – 14 of whom were male – had inflammatory enthesitis, arthritis, and/or tenosynovitis. Of the others, seven had osteoarthritis and three had degenerative spine disease.
Enthesitis was the most common finding in those with rheumatic symptoms, occurring on its own in 11 patients, with arthritis in three patients, and tenosynovitis in two patients.
These symptoms appeared 2-48 weeks after starting dupilumab treatment and were categorized as mild in 16 (61%) cases, moderate in six cases, and severe in four cases.
No specific predictors of the MSK symptoms seen were noted. Patient age, sex, duration of their atopic dermatitis, or how their skin condition had been previously treated did not help identify those who might develop rheumatic problems.
Conservative management approach
All patients had “outstanding” responses to treatment, Dr. Kirkham noted: The mean Eczema Area and Severity Index score before dupilumab treatment was 21, falling to 4.2 with treatment, indicating a mean 80% improvement.
Co-author Joseph Nathan, MBChB, of London North West Healthcare NHS Trust, who collaborated on the research while working within Dr. Kirkham’s group, said separately: “The concern that patients have is that when they start a medication and develop a side effect is that the medication is going to be stopped.”
Clinicians treating the patients took a conservative approach, prescribing NSAIDs such as cyclooxygenase-2 inhibitors or altering the frequency with which dupilumab was given.
With this approach, MSK symptoms resolved in 15 patients who remained on treatment and in seven who had to stop dupilumab. There were four patients, however, who had unresolved symptoms even once dupilumab treatment had been stopped.
Altering the local cytokine balance
Dupilumab is a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor. This results in blocking the function of not only IL-4 but also IL-13.
Dr. Kirkham and colleagues think this might not only alter the balance of cytokines in the skin but also in the joints and entheses with IL-17, IL-23, or even tumor necrosis factor playing a possible role. Another thought is that many circulating T-cells in the skin move to the joints and entheses to trigger symptoms.
IL-13 inhibition does seem to be important, as another British research team, from the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), has found.
At the recent annual meeting of the British Society for Rheumatology, Sizheng Steven Zhao, MBChB, PhD, and colleagues reported that among people who carried a genetic variant predisposing them to having low IL-13 function, there was a higher risk for inflammatory diseases such as psoriatic arthritis and other spondyloarthropathy-related diseases.
Indeed, when the single nucleotide polymorphism rs20541 was present, the odds for having psoriatic arthritis and psoriasis were higher than when it was not.
The findings are consistent with the idea that IL-4 and IL-13 may be acting as a restraint towards MSK diseases in some patients, Dr. Zhao and co-authors suggest.
“The genetic data supports what [Dr. Kirkham and team] have said from a mechanistic point of view,” Dr. Zhao said in an interview. “What you’re observing has a genetic basis.”
Dermatology perspective
Approved by the U.S. Food and Drug Administration in 2017, dupilumab has since been hailed as a “breakthrough” in atopic dermatitis treatment. Given as a subcutaneous injection every 2 weeks, it provides a much-needed option for people who have moderate-to-severe disease and have tried other available treatments, including corticosteroids.
Dupilumab has since also been approved for asthma, chronic sinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis and is used off-label for other skin conditions such as contact dermatitis, chronic spontaneous urticaria, and alopecia areata.
“Dupilumab, like a lot of medications for atopic dermatitis, is a relatively new drug, and we are still learning about its safety,” Joel M. Gelfand, MD, MSCE, of the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
“Inflammatory arthritis has been reported in patients treated with dupilumab, and this new study provides some useful estimates,” added Dr. Gelfand, who is a professor of dermatology and epidemiology and directs the Psoriasis and Phototherapy Treatment Center, Philadelphia.
“There was no control group,” Dr. Gelfand said, so “a causal relationship cannot be well established based on these data alone. The mechanism is not known but may result from a shifting of the immune system.”
Dr. Zhao observed: “We don’t know what the natural history of these adverse events is. We don’t know if stopping the drug early will prevent long-term adverse events. So, we don’t know if people will ultimately develop permanent psoriatic arthritis if we don’t intervene quick enough when we observe an adverse event.”
Being aware of the possibility of rheumatic side effects occurring with dupilumab and similar agents is key, Dr. Gelfand and Dr. Kirkham both said independently.
“I have personally seen this entity in my practice,” Dr. Gelfand said. “It is important to clinicians prescribing dupilumab to alert patients about this potential side effect and ask about joint symptoms in follow-up.”
Dr. Kirkham said: “Prescribers need to be aware of it, because up until now it’s been just very vaguely discussed as sort of aches and pains, arthralgias, and it’s a much more specific of a kind of syndrome of enthesitis, arthritis, tenosynovitis – a little like psoriatic arthritis.”
Not everyone has come across these side effects, however, as Steven Daveluy, MD, associate professor and dermatology program director at Wayne State University, Detroit, said in an interview.
“This article and the other case series both noted the musculoskeletal symptoms occurred in about 5% of patients, which surprised me since I haven’t seen it in my practice and have enough patients being treated with dupilumab that I would expect to see a case at that rate,” Dr. Daveluy said.
“The majority of cases are mild and respond to treatment with anti-inflammatories like naproxen, which is available over the counter. It’s likely that patients with a mild case could simply treat their pain with naproxen that’s already in their medicine cabinet until it resolves, never bringing it to the doctor’s attention,” he suggested.
“Dupilumab is still a safe and effective medication that can change the lives of patients suffering from atopic dermatitis,” he said.
“Awareness of this potential side effect can help dermatologists recognize it early and work together with patients to determine the best course of action.”
All research mentioned in this article was independently supported. Dr. Kirkham, Mr. Nathan, Dr. Zhao, and Dr. Daveluy report no relevant financial relationships. Dr. Gelfand has served as a consultant for numerous pharmaceutical companies and receives research grants from Amgen, Boehringer Ingelheim, and Pfizer. He is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
A version of this article first appeared on Medscape.com.
Around 5% of patients treated with dupilumab (Dupixent) for moderate-to-severe atopic dermatitis experience musculoskeletal (MSK) symptoms, according to the results of a descriptive study.
The main MSK symptom seen in the observational cohort was enthesitis, but some patients also experienced arthritis and tenosynovitis a median of 17 weeks after starting dupilumab treatment. Together these symptoms represent a new MSK syndrome, say researchers from the United Kingdom.
“The pattern of MSK symptoms and signs is characteristic of psoriatic arthritis/peripheral spondyloarthritis,” Bruce Kirkham, MD, and collaborators report in Arthritis & Rheumatology.
“We started a few years ago and have been following the patients for quite a long time,” Dr. Kirkham, a consultant rheumatologist at Guy’s and St. Thomas’ NHS Foundation Trust, London, told this news organization.
“We’re still seeing patients with the same type of syndrome presenting occasionally. It’s not a very common adverse event, but we think it continues,” he observed.
“Most of them don’t have very severe problems, and a lot of them can be treated with quite simple drugs or, alternatively, reducing the frequency of the injection,” Dr. Kirkham added.
Characterizing the MSK symptoms
Of 470 patients with atopic dermatitis who started treatment with dupilumab at Guy’s and St. Thomas’ NHS Foundation Trust between October 2018 and February 2021, 36 (7.65%) developed rheumatic symptoms and were referred to the rheumatology department. These individuals had their family history assessed and thorough MSK evaluations, which included antibody and inflammatory markers, ultrasound of the peripheral small joints, and MRI of the large joints and spine.
A total of 26 (5.5%) patients – 14 of whom were male – had inflammatory enthesitis, arthritis, and/or tenosynovitis. Of the others, seven had osteoarthritis and three had degenerative spine disease.
Enthesitis was the most common finding in those with rheumatic symptoms, occurring on its own in 11 patients, with arthritis in three patients, and tenosynovitis in two patients.
These symptoms appeared 2-48 weeks after starting dupilumab treatment and were categorized as mild in 16 (61%) cases, moderate in six cases, and severe in four cases.
No specific predictors of the MSK symptoms seen were noted. Patient age, sex, duration of their atopic dermatitis, or how their skin condition had been previously treated did not help identify those who might develop rheumatic problems.
Conservative management approach
All patients had “outstanding” responses to treatment, Dr. Kirkham noted: The mean Eczema Area and Severity Index score before dupilumab treatment was 21, falling to 4.2 with treatment, indicating a mean 80% improvement.
Co-author Joseph Nathan, MBChB, of London North West Healthcare NHS Trust, who collaborated on the research while working within Dr. Kirkham’s group, said separately: “The concern that patients have is that when they start a medication and develop a side effect is that the medication is going to be stopped.”
Clinicians treating the patients took a conservative approach, prescribing NSAIDs such as cyclooxygenase-2 inhibitors or altering the frequency with which dupilumab was given.
With this approach, MSK symptoms resolved in 15 patients who remained on treatment and in seven who had to stop dupilumab. There were four patients, however, who had unresolved symptoms even once dupilumab treatment had been stopped.
Altering the local cytokine balance
Dupilumab is a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor. This results in blocking the function of not only IL-4 but also IL-13.
Dr. Kirkham and colleagues think this might not only alter the balance of cytokines in the skin but also in the joints and entheses with IL-17, IL-23, or even tumor necrosis factor playing a possible role. Another thought is that many circulating T-cells in the skin move to the joints and entheses to trigger symptoms.
IL-13 inhibition does seem to be important, as another British research team, from the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), has found.
At the recent annual meeting of the British Society for Rheumatology, Sizheng Steven Zhao, MBChB, PhD, and colleagues reported that among people who carried a genetic variant predisposing them to having low IL-13 function, there was a higher risk for inflammatory diseases such as psoriatic arthritis and other spondyloarthropathy-related diseases.
Indeed, when the single nucleotide polymorphism rs20541 was present, the odds for having psoriatic arthritis and psoriasis were higher than when it was not.
The findings are consistent with the idea that IL-4 and IL-13 may be acting as a restraint towards MSK diseases in some patients, Dr. Zhao and co-authors suggest.
“The genetic data supports what [Dr. Kirkham and team] have said from a mechanistic point of view,” Dr. Zhao said in an interview. “What you’re observing has a genetic basis.”
Dermatology perspective
Approved by the U.S. Food and Drug Administration in 2017, dupilumab has since been hailed as a “breakthrough” in atopic dermatitis treatment. Given as a subcutaneous injection every 2 weeks, it provides a much-needed option for people who have moderate-to-severe disease and have tried other available treatments, including corticosteroids.
Dupilumab has since also been approved for asthma, chronic sinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis and is used off-label for other skin conditions such as contact dermatitis, chronic spontaneous urticaria, and alopecia areata.
“Dupilumab, like a lot of medications for atopic dermatitis, is a relatively new drug, and we are still learning about its safety,” Joel M. Gelfand, MD, MSCE, of the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
“Inflammatory arthritis has been reported in patients treated with dupilumab, and this new study provides some useful estimates,” added Dr. Gelfand, who is a professor of dermatology and epidemiology and directs the Psoriasis and Phototherapy Treatment Center, Philadelphia.
“There was no control group,” Dr. Gelfand said, so “a causal relationship cannot be well established based on these data alone. The mechanism is not known but may result from a shifting of the immune system.”
Dr. Zhao observed: “We don’t know what the natural history of these adverse events is. We don’t know if stopping the drug early will prevent long-term adverse events. So, we don’t know if people will ultimately develop permanent psoriatic arthritis if we don’t intervene quick enough when we observe an adverse event.”
Being aware of the possibility of rheumatic side effects occurring with dupilumab and similar agents is key, Dr. Gelfand and Dr. Kirkham both said independently.
“I have personally seen this entity in my practice,” Dr. Gelfand said. “It is important to clinicians prescribing dupilumab to alert patients about this potential side effect and ask about joint symptoms in follow-up.”
Dr. Kirkham said: “Prescribers need to be aware of it, because up until now it’s been just very vaguely discussed as sort of aches and pains, arthralgias, and it’s a much more specific of a kind of syndrome of enthesitis, arthritis, tenosynovitis – a little like psoriatic arthritis.”
Not everyone has come across these side effects, however, as Steven Daveluy, MD, associate professor and dermatology program director at Wayne State University, Detroit, said in an interview.
“This article and the other case series both noted the musculoskeletal symptoms occurred in about 5% of patients, which surprised me since I haven’t seen it in my practice and have enough patients being treated with dupilumab that I would expect to see a case at that rate,” Dr. Daveluy said.
“The majority of cases are mild and respond to treatment with anti-inflammatories like naproxen, which is available over the counter. It’s likely that patients with a mild case could simply treat their pain with naproxen that’s already in their medicine cabinet until it resolves, never bringing it to the doctor’s attention,” he suggested.
“Dupilumab is still a safe and effective medication that can change the lives of patients suffering from atopic dermatitis,” he said.
“Awareness of this potential side effect can help dermatologists recognize it early and work together with patients to determine the best course of action.”
All research mentioned in this article was independently supported. Dr. Kirkham, Mr. Nathan, Dr. Zhao, and Dr. Daveluy report no relevant financial relationships. Dr. Gelfand has served as a consultant for numerous pharmaceutical companies and receives research grants from Amgen, Boehringer Ingelheim, and Pfizer. He is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
A version of this article first appeared on Medscape.com.
Around 5% of patients treated with dupilumab (Dupixent) for moderate-to-severe atopic dermatitis experience musculoskeletal (MSK) symptoms, according to the results of a descriptive study.
The main MSK symptom seen in the observational cohort was enthesitis, but some patients also experienced arthritis and tenosynovitis a median of 17 weeks after starting dupilumab treatment. Together these symptoms represent a new MSK syndrome, say researchers from the United Kingdom.
“The pattern of MSK symptoms and signs is characteristic of psoriatic arthritis/peripheral spondyloarthritis,” Bruce Kirkham, MD, and collaborators report in Arthritis & Rheumatology.
“We started a few years ago and have been following the patients for quite a long time,” Dr. Kirkham, a consultant rheumatologist at Guy’s and St. Thomas’ NHS Foundation Trust, London, told this news organization.
“We’re still seeing patients with the same type of syndrome presenting occasionally. It’s not a very common adverse event, but we think it continues,” he observed.
“Most of them don’t have very severe problems, and a lot of them can be treated with quite simple drugs or, alternatively, reducing the frequency of the injection,” Dr. Kirkham added.
Characterizing the MSK symptoms
Of 470 patients with atopic dermatitis who started treatment with dupilumab at Guy’s and St. Thomas’ NHS Foundation Trust between October 2018 and February 2021, 36 (7.65%) developed rheumatic symptoms and were referred to the rheumatology department. These individuals had their family history assessed and thorough MSK evaluations, which included antibody and inflammatory markers, ultrasound of the peripheral small joints, and MRI of the large joints and spine.
A total of 26 (5.5%) patients – 14 of whom were male – had inflammatory enthesitis, arthritis, and/or tenosynovitis. Of the others, seven had osteoarthritis and three had degenerative spine disease.
Enthesitis was the most common finding in those with rheumatic symptoms, occurring on its own in 11 patients, with arthritis in three patients, and tenosynovitis in two patients.
These symptoms appeared 2-48 weeks after starting dupilumab treatment and were categorized as mild in 16 (61%) cases, moderate in six cases, and severe in four cases.
No specific predictors of the MSK symptoms seen were noted. Patient age, sex, duration of their atopic dermatitis, or how their skin condition had been previously treated did not help identify those who might develop rheumatic problems.
Conservative management approach
All patients had “outstanding” responses to treatment, Dr. Kirkham noted: The mean Eczema Area and Severity Index score before dupilumab treatment was 21, falling to 4.2 with treatment, indicating a mean 80% improvement.
Co-author Joseph Nathan, MBChB, of London North West Healthcare NHS Trust, who collaborated on the research while working within Dr. Kirkham’s group, said separately: “The concern that patients have is that when they start a medication and develop a side effect is that the medication is going to be stopped.”
Clinicians treating the patients took a conservative approach, prescribing NSAIDs such as cyclooxygenase-2 inhibitors or altering the frequency with which dupilumab was given.
With this approach, MSK symptoms resolved in 15 patients who remained on treatment and in seven who had to stop dupilumab. There were four patients, however, who had unresolved symptoms even once dupilumab treatment had been stopped.
Altering the local cytokine balance
Dupilumab is a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor. This results in blocking the function of not only IL-4 but also IL-13.
Dr. Kirkham and colleagues think this might not only alter the balance of cytokines in the skin but also in the joints and entheses with IL-17, IL-23, or even tumor necrosis factor playing a possible role. Another thought is that many circulating T-cells in the skin move to the joints and entheses to trigger symptoms.
IL-13 inhibition does seem to be important, as another British research team, from the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), has found.
At the recent annual meeting of the British Society for Rheumatology, Sizheng Steven Zhao, MBChB, PhD, and colleagues reported that among people who carried a genetic variant predisposing them to having low IL-13 function, there was a higher risk for inflammatory diseases such as psoriatic arthritis and other spondyloarthropathy-related diseases.
Indeed, when the single nucleotide polymorphism rs20541 was present, the odds for having psoriatic arthritis and psoriasis were higher than when it was not.
The findings are consistent with the idea that IL-4 and IL-13 may be acting as a restraint towards MSK diseases in some patients, Dr. Zhao and co-authors suggest.
“The genetic data supports what [Dr. Kirkham and team] have said from a mechanistic point of view,” Dr. Zhao said in an interview. “What you’re observing has a genetic basis.”
Dermatology perspective
Approved by the U.S. Food and Drug Administration in 2017, dupilumab has since been hailed as a “breakthrough” in atopic dermatitis treatment. Given as a subcutaneous injection every 2 weeks, it provides a much-needed option for people who have moderate-to-severe disease and have tried other available treatments, including corticosteroids.
Dupilumab has since also been approved for asthma, chronic sinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis and is used off-label for other skin conditions such as contact dermatitis, chronic spontaneous urticaria, and alopecia areata.
“Dupilumab, like a lot of medications for atopic dermatitis, is a relatively new drug, and we are still learning about its safety,” Joel M. Gelfand, MD, MSCE, of the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
“Inflammatory arthritis has been reported in patients treated with dupilumab, and this new study provides some useful estimates,” added Dr. Gelfand, who is a professor of dermatology and epidemiology and directs the Psoriasis and Phototherapy Treatment Center, Philadelphia.
“There was no control group,” Dr. Gelfand said, so “a causal relationship cannot be well established based on these data alone. The mechanism is not known but may result from a shifting of the immune system.”
Dr. Zhao observed: “We don’t know what the natural history of these adverse events is. We don’t know if stopping the drug early will prevent long-term adverse events. So, we don’t know if people will ultimately develop permanent psoriatic arthritis if we don’t intervene quick enough when we observe an adverse event.”
Being aware of the possibility of rheumatic side effects occurring with dupilumab and similar agents is key, Dr. Gelfand and Dr. Kirkham both said independently.
“I have personally seen this entity in my practice,” Dr. Gelfand said. “It is important to clinicians prescribing dupilumab to alert patients about this potential side effect and ask about joint symptoms in follow-up.”
Dr. Kirkham said: “Prescribers need to be aware of it, because up until now it’s been just very vaguely discussed as sort of aches and pains, arthralgias, and it’s a much more specific of a kind of syndrome of enthesitis, arthritis, tenosynovitis – a little like psoriatic arthritis.”
Not everyone has come across these side effects, however, as Steven Daveluy, MD, associate professor and dermatology program director at Wayne State University, Detroit, said in an interview.
“This article and the other case series both noted the musculoskeletal symptoms occurred in about 5% of patients, which surprised me since I haven’t seen it in my practice and have enough patients being treated with dupilumab that I would expect to see a case at that rate,” Dr. Daveluy said.
“The majority of cases are mild and respond to treatment with anti-inflammatories like naproxen, which is available over the counter. It’s likely that patients with a mild case could simply treat their pain with naproxen that’s already in their medicine cabinet until it resolves, never bringing it to the doctor’s attention,” he suggested.
“Dupilumab is still a safe and effective medication that can change the lives of patients suffering from atopic dermatitis,” he said.
“Awareness of this potential side effect can help dermatologists recognize it early and work together with patients to determine the best course of action.”
All research mentioned in this article was independently supported. Dr. Kirkham, Mr. Nathan, Dr. Zhao, and Dr. Daveluy report no relevant financial relationships. Dr. Gelfand has served as a consultant for numerous pharmaceutical companies and receives research grants from Amgen, Boehringer Ingelheim, and Pfizer. He is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Two biologics show no difference in axial spondyloarthritis radiographic progression over 2 years
MILAN – Secukinumab (Cosentyx) and biosimilar adalimumab-adaz (Hyrimoz) injection proved to have similar efficacy for limiting spinal radiographic progression over a 2-year period in patients with radiographic axial spondyloarthritis (r-axSpA) in the SURPASS study, a phase 3b, randomized controlled trial.
The study, presented at the annual European Congress of Rheumatology, represents the first head-to-head trial comparing the effects of two biologic disease-modifying antirheumatic drugs (bDMARDs) in axSpA. Notably, secukinumab and adalimumab-adaz target different pathways as an interleukin-17A inhibitor and a tumor necrosis factor (TNF) inhibitor, respectively.
Both TNF and IL-17A have been implicated in the pathogenesis of axSpA. Anti-TNF agents and the IL-17A inhibitor secukinumab have demonstrated effectiveness in improving symptoms, signs, and physical function in patients with axSpA and are approved therapies for the disease. However, limited data exist regarding the effect of bDMARDs in slowing radiographic progression, which is a key therapeutic goal in axSpA to prevent irreversible structural damage.
The SURPASS trial, funded by Novartis, enrolled 859 biologic-naive adult patients with moderate to severe r-axSpA. Participants were randomly assigned (1:1:1) to receive secukinumab 150 mg (n = 287), secukinumab 300 mg (n = 286), or adalimumab-adaz 40 mg (n = 286). The primary endpoint was the proportion of patients with no radiographic progression at the 2-year mark (week 104). Radiographic progression was defined as a change from baseline in modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS; range, 0-72) of 0.5 or less. The radiographic assessments were conducted by three independent evaluators who were blinded to treatment and the chronology of images.
Baseline characteristics indicated that the study population (78.5% male; mean age, 42.1 years) had a high risk of radiographic progression. The proportion of patients with no radiographic progression at week 104 was 66.1% in the secukinumab 150-mg arm, 66.9% in the secukinumab 300-mg arm, and 65.6% in the adalimumab-adaz arm. The mean change from baseline in mSASSS was 0.54, 0.55, and 0.72, respectively.
Notably, more than half of the patients (56.9%, 53.8%, and 53.3%, respectively) with at least one syndesmophyte at baseline did not develop new syndesmophytes over the 2-year period. The observed reductions in sacroiliac joint and spinal edema were comparable across all treatment groups. The safety profile of secukinumab and adalimumab-adaz was consistent with their well-established profiles.
No significant differences were observed between the treatment groups in terms of the primary and secondary endpoints. Study presenter and lead author Xenofon Baraliakos, MD, PhD, medical director of the Rheumatism Centre and professor of internal medicine and rheumatology at Ruhr University Bochum (Germany), stated: “Anti-TNF therapy has been considered the gold-standard treatment for axial spondyloarthritis in terms of slowing or halting radiographic progression. Our aim was to investigate whether other modes of action, such as IL-17 inhibition, achieve the same results. The primary hypothesis was that IL-17 inhibition could be even more effective than TNF blockade. However, our data indicate that secukinumab is at least as good as TNF blockers.
“Several risk factors, including high C-reactive protein [CRP] levels, male gender, high disease activity, and baseline radiographic damage (e.g., presence of syndesmophytes), are associated with structural progression,” Dr. Baraliakos explained. “We performed subgroup analyses and found no differences. This is a positive outcome as it suggests that there is no need to select patients based on either secukinumab or anti-TNF agents.”
When making treatment decisions, other factors must be taken into consideration. “Our study specifically examined radiographic progression. The clinical outcomes, indications, and contraindications for anti-TNF agents and secukinumab differ,” Dr. Baraliakos explained. “For instance, secukinumab may be preferred for patients with psoriasis, while adalimumab is more suitable for those with inflammatory bowel disease. Although these bDMARDs are not interchangeable, they have the same positive effect on radiographic progression.”
Not a definitive answer about structural progression
An open question remains. Alexandre Sepriano, MD, PhD, a rheumatologist at Hospital Egas Moniz and researcher at NOVA Medical School, both in Lisbon, commented: “The study was designed to maximize the chances of detecting a difference, if any, in spinal radiographic progression between secukinumab 150 mg and 300 mg and adalimumab. The included patients had a high risk of progression at baseline; in addition to back pain, they either had elevated CRP or at least one syndesmophyte on spine radiographs. Consequently, baseline structural damage was high [mean mSASSS, 17].”
“After 2 years, no difference was observed in the percentage of patients with no progression across the study arms. This finding does not definitively answer whether bDMARDs can modify structural progression or if secukinumab and adalimumab are equally effective in this regard,” explained Dr. Sepriano, who was not involved in the study. “However, there is good news for patients. Both secukinumab and adalimumab are potent anti-inflammatory drugs that effectively alleviate axial inflammation caused by the disease. This was demonstrated by the reduction in inflammatory scores on MRI in the SURPASS study. It aligns with robust evidence that both IL-17 inhibitors and TNF inhibitors are effective in improving symptoms in individuals with axSpA.
“Researchers continue to make significant efforts to understand how axial inflammation contributes to pathological new bone formation in axSpA,” Dr. Sepriano continued. “Understanding these mechanisms can guide future research aimed at interfering with disease progression. Furthermore, the use of new methods to quantify structural progression in axSpA, such as low-dose CT, which has shown greater sensitivity to change than traditional methods, can pave the way for new studies with fewer patients and shorter follow-up periods, thereby increasing the likelihood of detecting treatment effects.”
Dr. Baraliakos has received speaking and consulting fees and grant/research support from AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Merck Sharp & Dohme, Novartis, Pfizer, and UCB. Dr. Sepriano has received speaking and/or consulting fees from AbbVie, Novartis, UCB, and Lilly. The trial was sponsored by Novartis.
A version of this article first appeared on Medscape.com.
MILAN – Secukinumab (Cosentyx) and biosimilar adalimumab-adaz (Hyrimoz) injection proved to have similar efficacy for limiting spinal radiographic progression over a 2-year period in patients with radiographic axial spondyloarthritis (r-axSpA) in the SURPASS study, a phase 3b, randomized controlled trial.
The study, presented at the annual European Congress of Rheumatology, represents the first head-to-head trial comparing the effects of two biologic disease-modifying antirheumatic drugs (bDMARDs) in axSpA. Notably, secukinumab and adalimumab-adaz target different pathways as an interleukin-17A inhibitor and a tumor necrosis factor (TNF) inhibitor, respectively.
Both TNF and IL-17A have been implicated in the pathogenesis of axSpA. Anti-TNF agents and the IL-17A inhibitor secukinumab have demonstrated effectiveness in improving symptoms, signs, and physical function in patients with axSpA and are approved therapies for the disease. However, limited data exist regarding the effect of bDMARDs in slowing radiographic progression, which is a key therapeutic goal in axSpA to prevent irreversible structural damage.
The SURPASS trial, funded by Novartis, enrolled 859 biologic-naive adult patients with moderate to severe r-axSpA. Participants were randomly assigned (1:1:1) to receive secukinumab 150 mg (n = 287), secukinumab 300 mg (n = 286), or adalimumab-adaz 40 mg (n = 286). The primary endpoint was the proportion of patients with no radiographic progression at the 2-year mark (week 104). Radiographic progression was defined as a change from baseline in modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS; range, 0-72) of 0.5 or less. The radiographic assessments were conducted by three independent evaluators who were blinded to treatment and the chronology of images.
Baseline characteristics indicated that the study population (78.5% male; mean age, 42.1 years) had a high risk of radiographic progression. The proportion of patients with no radiographic progression at week 104 was 66.1% in the secukinumab 150-mg arm, 66.9% in the secukinumab 300-mg arm, and 65.6% in the adalimumab-adaz arm. The mean change from baseline in mSASSS was 0.54, 0.55, and 0.72, respectively.
Notably, more than half of the patients (56.9%, 53.8%, and 53.3%, respectively) with at least one syndesmophyte at baseline did not develop new syndesmophytes over the 2-year period. The observed reductions in sacroiliac joint and spinal edema were comparable across all treatment groups. The safety profile of secukinumab and adalimumab-adaz was consistent with their well-established profiles.
No significant differences were observed between the treatment groups in terms of the primary and secondary endpoints. Study presenter and lead author Xenofon Baraliakos, MD, PhD, medical director of the Rheumatism Centre and professor of internal medicine and rheumatology at Ruhr University Bochum (Germany), stated: “Anti-TNF therapy has been considered the gold-standard treatment for axial spondyloarthritis in terms of slowing or halting radiographic progression. Our aim was to investigate whether other modes of action, such as IL-17 inhibition, achieve the same results. The primary hypothesis was that IL-17 inhibition could be even more effective than TNF blockade. However, our data indicate that secukinumab is at least as good as TNF blockers.
“Several risk factors, including high C-reactive protein [CRP] levels, male gender, high disease activity, and baseline radiographic damage (e.g., presence of syndesmophytes), are associated with structural progression,” Dr. Baraliakos explained. “We performed subgroup analyses and found no differences. This is a positive outcome as it suggests that there is no need to select patients based on either secukinumab or anti-TNF agents.”
When making treatment decisions, other factors must be taken into consideration. “Our study specifically examined radiographic progression. The clinical outcomes, indications, and contraindications for anti-TNF agents and secukinumab differ,” Dr. Baraliakos explained. “For instance, secukinumab may be preferred for patients with psoriasis, while adalimumab is more suitable for those with inflammatory bowel disease. Although these bDMARDs are not interchangeable, they have the same positive effect on radiographic progression.”
Not a definitive answer about structural progression
An open question remains. Alexandre Sepriano, MD, PhD, a rheumatologist at Hospital Egas Moniz and researcher at NOVA Medical School, both in Lisbon, commented: “The study was designed to maximize the chances of detecting a difference, if any, in spinal radiographic progression between secukinumab 150 mg and 300 mg and adalimumab. The included patients had a high risk of progression at baseline; in addition to back pain, they either had elevated CRP or at least one syndesmophyte on spine radiographs. Consequently, baseline structural damage was high [mean mSASSS, 17].”
“After 2 years, no difference was observed in the percentage of patients with no progression across the study arms. This finding does not definitively answer whether bDMARDs can modify structural progression or if secukinumab and adalimumab are equally effective in this regard,” explained Dr. Sepriano, who was not involved in the study. “However, there is good news for patients. Both secukinumab and adalimumab are potent anti-inflammatory drugs that effectively alleviate axial inflammation caused by the disease. This was demonstrated by the reduction in inflammatory scores on MRI in the SURPASS study. It aligns with robust evidence that both IL-17 inhibitors and TNF inhibitors are effective in improving symptoms in individuals with axSpA.
“Researchers continue to make significant efforts to understand how axial inflammation contributes to pathological new bone formation in axSpA,” Dr. Sepriano continued. “Understanding these mechanisms can guide future research aimed at interfering with disease progression. Furthermore, the use of new methods to quantify structural progression in axSpA, such as low-dose CT, which has shown greater sensitivity to change than traditional methods, can pave the way for new studies with fewer patients and shorter follow-up periods, thereby increasing the likelihood of detecting treatment effects.”
Dr. Baraliakos has received speaking and consulting fees and grant/research support from AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Merck Sharp & Dohme, Novartis, Pfizer, and UCB. Dr. Sepriano has received speaking and/or consulting fees from AbbVie, Novartis, UCB, and Lilly. The trial was sponsored by Novartis.
A version of this article first appeared on Medscape.com.
MILAN – Secukinumab (Cosentyx) and biosimilar adalimumab-adaz (Hyrimoz) injection proved to have similar efficacy for limiting spinal radiographic progression over a 2-year period in patients with radiographic axial spondyloarthritis (r-axSpA) in the SURPASS study, a phase 3b, randomized controlled trial.
The study, presented at the annual European Congress of Rheumatology, represents the first head-to-head trial comparing the effects of two biologic disease-modifying antirheumatic drugs (bDMARDs) in axSpA. Notably, secukinumab and adalimumab-adaz target different pathways as an interleukin-17A inhibitor and a tumor necrosis factor (TNF) inhibitor, respectively.
Both TNF and IL-17A have been implicated in the pathogenesis of axSpA. Anti-TNF agents and the IL-17A inhibitor secukinumab have demonstrated effectiveness in improving symptoms, signs, and physical function in patients with axSpA and are approved therapies for the disease. However, limited data exist regarding the effect of bDMARDs in slowing radiographic progression, which is a key therapeutic goal in axSpA to prevent irreversible structural damage.
The SURPASS trial, funded by Novartis, enrolled 859 biologic-naive adult patients with moderate to severe r-axSpA. Participants were randomly assigned (1:1:1) to receive secukinumab 150 mg (n = 287), secukinumab 300 mg (n = 286), or adalimumab-adaz 40 mg (n = 286). The primary endpoint was the proportion of patients with no radiographic progression at the 2-year mark (week 104). Radiographic progression was defined as a change from baseline in modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS; range, 0-72) of 0.5 or less. The radiographic assessments were conducted by three independent evaluators who were blinded to treatment and the chronology of images.
Baseline characteristics indicated that the study population (78.5% male; mean age, 42.1 years) had a high risk of radiographic progression. The proportion of patients with no radiographic progression at week 104 was 66.1% in the secukinumab 150-mg arm, 66.9% in the secukinumab 300-mg arm, and 65.6% in the adalimumab-adaz arm. The mean change from baseline in mSASSS was 0.54, 0.55, and 0.72, respectively.
Notably, more than half of the patients (56.9%, 53.8%, and 53.3%, respectively) with at least one syndesmophyte at baseline did not develop new syndesmophytes over the 2-year period. The observed reductions in sacroiliac joint and spinal edema were comparable across all treatment groups. The safety profile of secukinumab and adalimumab-adaz was consistent with their well-established profiles.
No significant differences were observed between the treatment groups in terms of the primary and secondary endpoints. Study presenter and lead author Xenofon Baraliakos, MD, PhD, medical director of the Rheumatism Centre and professor of internal medicine and rheumatology at Ruhr University Bochum (Germany), stated: “Anti-TNF therapy has been considered the gold-standard treatment for axial spondyloarthritis in terms of slowing or halting radiographic progression. Our aim was to investigate whether other modes of action, such as IL-17 inhibition, achieve the same results. The primary hypothesis was that IL-17 inhibition could be even more effective than TNF blockade. However, our data indicate that secukinumab is at least as good as TNF blockers.
“Several risk factors, including high C-reactive protein [CRP] levels, male gender, high disease activity, and baseline radiographic damage (e.g., presence of syndesmophytes), are associated with structural progression,” Dr. Baraliakos explained. “We performed subgroup analyses and found no differences. This is a positive outcome as it suggests that there is no need to select patients based on either secukinumab or anti-TNF agents.”
When making treatment decisions, other factors must be taken into consideration. “Our study specifically examined radiographic progression. The clinical outcomes, indications, and contraindications for anti-TNF agents and secukinumab differ,” Dr. Baraliakos explained. “For instance, secukinumab may be preferred for patients with psoriasis, while adalimumab is more suitable for those with inflammatory bowel disease. Although these bDMARDs are not interchangeable, they have the same positive effect on radiographic progression.”
Not a definitive answer about structural progression
An open question remains. Alexandre Sepriano, MD, PhD, a rheumatologist at Hospital Egas Moniz and researcher at NOVA Medical School, both in Lisbon, commented: “The study was designed to maximize the chances of detecting a difference, if any, in spinal radiographic progression between secukinumab 150 mg and 300 mg and adalimumab. The included patients had a high risk of progression at baseline; in addition to back pain, they either had elevated CRP or at least one syndesmophyte on spine radiographs. Consequently, baseline structural damage was high [mean mSASSS, 17].”
“After 2 years, no difference was observed in the percentage of patients with no progression across the study arms. This finding does not definitively answer whether bDMARDs can modify structural progression or if secukinumab and adalimumab are equally effective in this regard,” explained Dr. Sepriano, who was not involved in the study. “However, there is good news for patients. Both secukinumab and adalimumab are potent anti-inflammatory drugs that effectively alleviate axial inflammation caused by the disease. This was demonstrated by the reduction in inflammatory scores on MRI in the SURPASS study. It aligns with robust evidence that both IL-17 inhibitors and TNF inhibitors are effective in improving symptoms in individuals with axSpA.
“Researchers continue to make significant efforts to understand how axial inflammation contributes to pathological new bone formation in axSpA,” Dr. Sepriano continued. “Understanding these mechanisms can guide future research aimed at interfering with disease progression. Furthermore, the use of new methods to quantify structural progression in axSpA, such as low-dose CT, which has shown greater sensitivity to change than traditional methods, can pave the way for new studies with fewer patients and shorter follow-up periods, thereby increasing the likelihood of detecting treatment effects.”
Dr. Baraliakos has received speaking and consulting fees and grant/research support from AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Merck Sharp & Dohme, Novartis, Pfizer, and UCB. Dr. Sepriano has received speaking and/or consulting fees from AbbVie, Novartis, UCB, and Lilly. The trial was sponsored by Novartis.
A version of this article first appeared on Medscape.com.
AT EULAR 2023
Lupus nephritis: Hopes, questions arise for baricitinib
MILAN – The oral Janus kinase (JAK) 1/2 inhibitor baricitinib (Olumiant) demonstrated significantly better efficacy than cyclophosphamide infusions in the treatment of lupus nephritis in a small, independently funded, phase 3, double-blind clinical trial, Manal Hassanien, MD, reported at the annual European Congress of Rheumatology.
Baricitinib, licensed by Eli Lilly, has been recognized as a potential therapeutic option in systemic lupus, and is approved in the United States to treat RA, alopecia areata, and COVID-19 in certain hospitalized adults. It is also approved to treat atopic dermatitis in Europe. However, it previously yielded disappointing results in phase 3 clinical trials SLE-BRAVE-I and SLE-BRAVE-II for systemic lupus erythematosus. The trial results presented at EULAR suggest that baricitinib could be beneficial in the treatment of lupus nephritis, further establishing the role of JAK inhibitors in autoimmune disease therapy.
“Lupus nephritis typically develops within 5 years of initial lupus symptoms,” said Dr. Hassanien, of the rheumatology research and advanced therapeutics department at Assiut (Egypt) University. “Research has shown that up to 60% of lupus patients will eventually develop lupus nephritis. The management of proliferative lupus nephritis usually involves an initial phase focused on preventing the development of irreversible damage, followed by a maintenance phase to control lupus activity. Despite significant progress, lupus nephritis still carries an increased risk of end-stage renal disease and mortality.”
The study’s primary endpoint of 24-hour proteinuria response rate (≥ 50% reduction from baseline) at week 12 was significantly greater with baricitinib 4 mg daily, compared with monthly cyclophosphamide infusions at 0.7 mg/m2 (70% vs. 43%; P < .0001). At week 24, 76.6% of the baricitinib group met the primary endpoint, compared with 50% in the cyclophosphamide group. Two multiplicity-controlled secondary endpoints, C3 serum level and the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), also showed statistical significance at 12 weeks (P < .01).
The 6-month trial included 60 adult patients (age 18 years and older) with a clinical diagnosis of lupus nephritis fulfilling classification criteria for LN grade III and IV. Patients needed to demonstrate objective signs of active nephritis consistent with persistent proteinuria greater than 0.5 g/day and/or cellular casts at screening to be included. Additional inclusion criteria were SLEDAI-2K greater than 4 and assessment of anti–double-stranded DNA and C3 serum levels at study entry. The patients were randomly assigned to two equal-sized groups, with one group receiving baricitinib 4 mg daily and a monthly placebo saline infusion, and the other group receiving monthly cyclophosphamide infusions and oral placebo tablets.
The incidence of adverse events was comparable between the two treatment groups, with 48% of patients in the baricitinib group and 46% in the cyclophosphamide group experiencing adverse events. Only three serious adverse events, specifically serious infection or herpes zoster, were recorded, leading to treatment discontinuation.
Two patients (6.6%) in the baricitinib group and one patient (3.3%) in the cyclophosphamide group were affected. The researchers recorded no major adverse cardiovascular or venous thromboembolic events, which are known to occur at higher rates among some users of baricitinib and other JAK inhibitors. The safety profile of baricitinib was consistent with observations made in other inflammatory musculoskeletal diseases, and no new risks were identified.
However, there were some concerns expressed by audience members during the presentation.
“The primary endpoint is limited at proteinuria, while biopsy is considered the gold standard for measuring efficacy,” said Eric F. Morand, MD, head of the Monash Health rheumatology unit, Melbourne. This was not the only critical comment regarding the study that emerged during the discussion. The use of a 4-mg dosage regimen throughout the entire study duration (despite official recommendations suggesting a 2-mg dosage in the long run) and the positive outcomes observed in the control group treated with cyclophosphamide were also mentioned.
Dr. Hassanien acknowledged that this is a small and relatively short study and disclosed plans to extend the follow-up period to 1 year and conduct a renal biopsy.
Dr. Hassanien reported no relevant financial relationships. Assiut University funded the trial.
A version of this article first appeared on Medscape.com.
MILAN – The oral Janus kinase (JAK) 1/2 inhibitor baricitinib (Olumiant) demonstrated significantly better efficacy than cyclophosphamide infusions in the treatment of lupus nephritis in a small, independently funded, phase 3, double-blind clinical trial, Manal Hassanien, MD, reported at the annual European Congress of Rheumatology.
Baricitinib, licensed by Eli Lilly, has been recognized as a potential therapeutic option in systemic lupus, and is approved in the United States to treat RA, alopecia areata, and COVID-19 in certain hospitalized adults. It is also approved to treat atopic dermatitis in Europe. However, it previously yielded disappointing results in phase 3 clinical trials SLE-BRAVE-I and SLE-BRAVE-II for systemic lupus erythematosus. The trial results presented at EULAR suggest that baricitinib could be beneficial in the treatment of lupus nephritis, further establishing the role of JAK inhibitors in autoimmune disease therapy.
“Lupus nephritis typically develops within 5 years of initial lupus symptoms,” said Dr. Hassanien, of the rheumatology research and advanced therapeutics department at Assiut (Egypt) University. “Research has shown that up to 60% of lupus patients will eventually develop lupus nephritis. The management of proliferative lupus nephritis usually involves an initial phase focused on preventing the development of irreversible damage, followed by a maintenance phase to control lupus activity. Despite significant progress, lupus nephritis still carries an increased risk of end-stage renal disease and mortality.”
The study’s primary endpoint of 24-hour proteinuria response rate (≥ 50% reduction from baseline) at week 12 was significantly greater with baricitinib 4 mg daily, compared with monthly cyclophosphamide infusions at 0.7 mg/m2 (70% vs. 43%; P < .0001). At week 24, 76.6% of the baricitinib group met the primary endpoint, compared with 50% in the cyclophosphamide group. Two multiplicity-controlled secondary endpoints, C3 serum level and the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), also showed statistical significance at 12 weeks (P < .01).
The 6-month trial included 60 adult patients (age 18 years and older) with a clinical diagnosis of lupus nephritis fulfilling classification criteria for LN grade III and IV. Patients needed to demonstrate objective signs of active nephritis consistent with persistent proteinuria greater than 0.5 g/day and/or cellular casts at screening to be included. Additional inclusion criteria were SLEDAI-2K greater than 4 and assessment of anti–double-stranded DNA and C3 serum levels at study entry. The patients were randomly assigned to two equal-sized groups, with one group receiving baricitinib 4 mg daily and a monthly placebo saline infusion, and the other group receiving monthly cyclophosphamide infusions and oral placebo tablets.
The incidence of adverse events was comparable between the two treatment groups, with 48% of patients in the baricitinib group and 46% in the cyclophosphamide group experiencing adverse events. Only three serious adverse events, specifically serious infection or herpes zoster, were recorded, leading to treatment discontinuation.
Two patients (6.6%) in the baricitinib group and one patient (3.3%) in the cyclophosphamide group were affected. The researchers recorded no major adverse cardiovascular or venous thromboembolic events, which are known to occur at higher rates among some users of baricitinib and other JAK inhibitors. The safety profile of baricitinib was consistent with observations made in other inflammatory musculoskeletal diseases, and no new risks were identified.
However, there were some concerns expressed by audience members during the presentation.
“The primary endpoint is limited at proteinuria, while biopsy is considered the gold standard for measuring efficacy,” said Eric F. Morand, MD, head of the Monash Health rheumatology unit, Melbourne. This was not the only critical comment regarding the study that emerged during the discussion. The use of a 4-mg dosage regimen throughout the entire study duration (despite official recommendations suggesting a 2-mg dosage in the long run) and the positive outcomes observed in the control group treated with cyclophosphamide were also mentioned.
Dr. Hassanien acknowledged that this is a small and relatively short study and disclosed plans to extend the follow-up period to 1 year and conduct a renal biopsy.
Dr. Hassanien reported no relevant financial relationships. Assiut University funded the trial.
A version of this article first appeared on Medscape.com.
MILAN – The oral Janus kinase (JAK) 1/2 inhibitor baricitinib (Olumiant) demonstrated significantly better efficacy than cyclophosphamide infusions in the treatment of lupus nephritis in a small, independently funded, phase 3, double-blind clinical trial, Manal Hassanien, MD, reported at the annual European Congress of Rheumatology.
Baricitinib, licensed by Eli Lilly, has been recognized as a potential therapeutic option in systemic lupus, and is approved in the United States to treat RA, alopecia areata, and COVID-19 in certain hospitalized adults. It is also approved to treat atopic dermatitis in Europe. However, it previously yielded disappointing results in phase 3 clinical trials SLE-BRAVE-I and SLE-BRAVE-II for systemic lupus erythematosus. The trial results presented at EULAR suggest that baricitinib could be beneficial in the treatment of lupus nephritis, further establishing the role of JAK inhibitors in autoimmune disease therapy.
“Lupus nephritis typically develops within 5 years of initial lupus symptoms,” said Dr. Hassanien, of the rheumatology research and advanced therapeutics department at Assiut (Egypt) University. “Research has shown that up to 60% of lupus patients will eventually develop lupus nephritis. The management of proliferative lupus nephritis usually involves an initial phase focused on preventing the development of irreversible damage, followed by a maintenance phase to control lupus activity. Despite significant progress, lupus nephritis still carries an increased risk of end-stage renal disease and mortality.”
The study’s primary endpoint of 24-hour proteinuria response rate (≥ 50% reduction from baseline) at week 12 was significantly greater with baricitinib 4 mg daily, compared with monthly cyclophosphamide infusions at 0.7 mg/m2 (70% vs. 43%; P < .0001). At week 24, 76.6% of the baricitinib group met the primary endpoint, compared with 50% in the cyclophosphamide group. Two multiplicity-controlled secondary endpoints, C3 serum level and the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), also showed statistical significance at 12 weeks (P < .01).
The 6-month trial included 60 adult patients (age 18 years and older) with a clinical diagnosis of lupus nephritis fulfilling classification criteria for LN grade III and IV. Patients needed to demonstrate objective signs of active nephritis consistent with persistent proteinuria greater than 0.5 g/day and/or cellular casts at screening to be included. Additional inclusion criteria were SLEDAI-2K greater than 4 and assessment of anti–double-stranded DNA and C3 serum levels at study entry. The patients were randomly assigned to two equal-sized groups, with one group receiving baricitinib 4 mg daily and a monthly placebo saline infusion, and the other group receiving monthly cyclophosphamide infusions and oral placebo tablets.
The incidence of adverse events was comparable between the two treatment groups, with 48% of patients in the baricitinib group and 46% in the cyclophosphamide group experiencing adverse events. Only three serious adverse events, specifically serious infection or herpes zoster, were recorded, leading to treatment discontinuation.
Two patients (6.6%) in the baricitinib group and one patient (3.3%) in the cyclophosphamide group were affected. The researchers recorded no major adverse cardiovascular or venous thromboembolic events, which are known to occur at higher rates among some users of baricitinib and other JAK inhibitors. The safety profile of baricitinib was consistent with observations made in other inflammatory musculoskeletal diseases, and no new risks were identified.
However, there were some concerns expressed by audience members during the presentation.
“The primary endpoint is limited at proteinuria, while biopsy is considered the gold standard for measuring efficacy,” said Eric F. Morand, MD, head of the Monash Health rheumatology unit, Melbourne. This was not the only critical comment regarding the study that emerged during the discussion. The use of a 4-mg dosage regimen throughout the entire study duration (despite official recommendations suggesting a 2-mg dosage in the long run) and the positive outcomes observed in the control group treated with cyclophosphamide were also mentioned.
Dr. Hassanien acknowledged that this is a small and relatively short study and disclosed plans to extend the follow-up period to 1 year and conduct a renal biopsy.
Dr. Hassanien reported no relevant financial relationships. Assiut University funded the trial.
A version of this article first appeared on Medscape.com.
AT EULAR 2023











