User login
Buprenorphine update: Looser rules and a helpful injectable
SAN FRANCISCO – As the opioid epidemic continues to grow and evolve, the federal government is trying to make it easier for clinicians to treat abusers with the drug buprenorphine, psychiatrists told colleagues at the annual meeting of the American Psychiatric Association. And an injectable version of the drug is making a big difference.
John A. Renner Jr., MD, of Boston University, said in a presentation at the APA meeting.
As Dr. Renner explained, the United States is now in the fourth wave of nearly a quarter-century of opioid overdose-related deaths. The outbreak began in 1999 as prescription opioids spurred deaths, and heroin overdoses began to rise in 2010. The third wave brought rises in deaths from synthetic opioids such as fentanyl in 2013. In 2015, the fourth wave – driven by deaths from combinations of synthetic opioids and psychostimulants like methamphetamines – started in 2015.
COVID-19 seems to have played a role too: In 2020, opioid overdose deaths spiked during the early months of the pandemic. In 2021, drug-related overdose deaths overall hit a high of 106,889, including 80,411 linked to opioids. In contrast, fewer than 20,000 drug-related overdose deaths were reported in 1999.
On the other hand, deaths from prescription drug overdoses are falling, Dr. Renner said, suggesting “improvement in terms of how clinicians are handling medications and our prescribing practices. But that’s being masked by what’s happened with fentanyl and methamphetamine.”
Buprenorphine (Subutex), used to treat opioid use withdrawal, is itself an opioid and can cause addiction and death in some cases. However, Dr. Renner highlighted a 2023 study that determined that efforts to increase its use from 2019 to 2021 didn’t appear to boost buprenorphine-related overdose deaths in the United States.
New federal regulations aim to make it easier to prescribe buprenorphine. Thanks to Congressional legislation, the Drug Enforcement Administration in January 2023 eliminated regulations requiring clinicians to undergo special training to get an “X-waiver” to be able to prescribe buprenorphine. But they’re not off the hook entirely: As of June 27, 2023, providers must have undergone training in order to apply for – or renew – a DEA license to prescribe certain controlled substances like buprenorphine.
“I’m afraid that people will be able to meet that requirement easily, and they’re not going to get good coordinated teaching,” Dr. Renner said. “I’m not sure that’s really going to improve the quality of care that we’re delivering.”
In regard to treatment, psychiatrist Dong Chan Park, MD, of Boston University, touted a long-acting injectable form of buprenorphine known by the brand name Sublocade. The FDA approved Sublocade in 2017 for patients who’ve been taking sublingual buprenorphine for at least 7 days, although Dr. Park said research suggests the 7-day period may not be necessary.
“We’ve utilized this about 2.5-plus years in my hospital, and it’s really been a game changer for some of our sickest, most challenging patients,” he said at the APA presentation. As he explained, one benefit is that patients can’t repeatedly avoid doses depending on how they feel, as they may do with the sublingual version. “On the first day of injection, you can actually stop the sublingual buprenorphine.”
Dr. Renner emphasized the importance of getting users on buprenorphine as fast as possible. If the treatment begins in the ED, he said, “they need to have a system that is going to be able to pick them up and continue the care.”
Otherwise, the risk is high. “We’re in a very dangerous era,” he said, “where the patient walks out the door, and then they die.”
Dr. Park had no disclosures, and Dr. Renner disclosed royalties from the APA.
SAN FRANCISCO – As the opioid epidemic continues to grow and evolve, the federal government is trying to make it easier for clinicians to treat abusers with the drug buprenorphine, psychiatrists told colleagues at the annual meeting of the American Psychiatric Association. And an injectable version of the drug is making a big difference.
John A. Renner Jr., MD, of Boston University, said in a presentation at the APA meeting.
As Dr. Renner explained, the United States is now in the fourth wave of nearly a quarter-century of opioid overdose-related deaths. The outbreak began in 1999 as prescription opioids spurred deaths, and heroin overdoses began to rise in 2010. The third wave brought rises in deaths from synthetic opioids such as fentanyl in 2013. In 2015, the fourth wave – driven by deaths from combinations of synthetic opioids and psychostimulants like methamphetamines – started in 2015.
COVID-19 seems to have played a role too: In 2020, opioid overdose deaths spiked during the early months of the pandemic. In 2021, drug-related overdose deaths overall hit a high of 106,889, including 80,411 linked to opioids. In contrast, fewer than 20,000 drug-related overdose deaths were reported in 1999.
On the other hand, deaths from prescription drug overdoses are falling, Dr. Renner said, suggesting “improvement in terms of how clinicians are handling medications and our prescribing practices. But that’s being masked by what’s happened with fentanyl and methamphetamine.”
Buprenorphine (Subutex), used to treat opioid use withdrawal, is itself an opioid and can cause addiction and death in some cases. However, Dr. Renner highlighted a 2023 study that determined that efforts to increase its use from 2019 to 2021 didn’t appear to boost buprenorphine-related overdose deaths in the United States.
New federal regulations aim to make it easier to prescribe buprenorphine. Thanks to Congressional legislation, the Drug Enforcement Administration in January 2023 eliminated regulations requiring clinicians to undergo special training to get an “X-waiver” to be able to prescribe buprenorphine. But they’re not off the hook entirely: As of June 27, 2023, providers must have undergone training in order to apply for – or renew – a DEA license to prescribe certain controlled substances like buprenorphine.
“I’m afraid that people will be able to meet that requirement easily, and they’re not going to get good coordinated teaching,” Dr. Renner said. “I’m not sure that’s really going to improve the quality of care that we’re delivering.”
In regard to treatment, psychiatrist Dong Chan Park, MD, of Boston University, touted a long-acting injectable form of buprenorphine known by the brand name Sublocade. The FDA approved Sublocade in 2017 for patients who’ve been taking sublingual buprenorphine for at least 7 days, although Dr. Park said research suggests the 7-day period may not be necessary.
“We’ve utilized this about 2.5-plus years in my hospital, and it’s really been a game changer for some of our sickest, most challenging patients,” he said at the APA presentation. As he explained, one benefit is that patients can’t repeatedly avoid doses depending on how they feel, as they may do with the sublingual version. “On the first day of injection, you can actually stop the sublingual buprenorphine.”
Dr. Renner emphasized the importance of getting users on buprenorphine as fast as possible. If the treatment begins in the ED, he said, “they need to have a system that is going to be able to pick them up and continue the care.”
Otherwise, the risk is high. “We’re in a very dangerous era,” he said, “where the patient walks out the door, and then they die.”
Dr. Park had no disclosures, and Dr. Renner disclosed royalties from the APA.
SAN FRANCISCO – As the opioid epidemic continues to grow and evolve, the federal government is trying to make it easier for clinicians to treat abusers with the drug buprenorphine, psychiatrists told colleagues at the annual meeting of the American Psychiatric Association. And an injectable version of the drug is making a big difference.
John A. Renner Jr., MD, of Boston University, said in a presentation at the APA meeting.
As Dr. Renner explained, the United States is now in the fourth wave of nearly a quarter-century of opioid overdose-related deaths. The outbreak began in 1999 as prescription opioids spurred deaths, and heroin overdoses began to rise in 2010. The third wave brought rises in deaths from synthetic opioids such as fentanyl in 2013. In 2015, the fourth wave – driven by deaths from combinations of synthetic opioids and psychostimulants like methamphetamines – started in 2015.
COVID-19 seems to have played a role too: In 2020, opioid overdose deaths spiked during the early months of the pandemic. In 2021, drug-related overdose deaths overall hit a high of 106,889, including 80,411 linked to opioids. In contrast, fewer than 20,000 drug-related overdose deaths were reported in 1999.
On the other hand, deaths from prescription drug overdoses are falling, Dr. Renner said, suggesting “improvement in terms of how clinicians are handling medications and our prescribing practices. But that’s being masked by what’s happened with fentanyl and methamphetamine.”
Buprenorphine (Subutex), used to treat opioid use withdrawal, is itself an opioid and can cause addiction and death in some cases. However, Dr. Renner highlighted a 2023 study that determined that efforts to increase its use from 2019 to 2021 didn’t appear to boost buprenorphine-related overdose deaths in the United States.
New federal regulations aim to make it easier to prescribe buprenorphine. Thanks to Congressional legislation, the Drug Enforcement Administration in January 2023 eliminated regulations requiring clinicians to undergo special training to get an “X-waiver” to be able to prescribe buprenorphine. But they’re not off the hook entirely: As of June 27, 2023, providers must have undergone training in order to apply for – or renew – a DEA license to prescribe certain controlled substances like buprenorphine.
“I’m afraid that people will be able to meet that requirement easily, and they’re not going to get good coordinated teaching,” Dr. Renner said. “I’m not sure that’s really going to improve the quality of care that we’re delivering.”
In regard to treatment, psychiatrist Dong Chan Park, MD, of Boston University, touted a long-acting injectable form of buprenorphine known by the brand name Sublocade. The FDA approved Sublocade in 2017 for patients who’ve been taking sublingual buprenorphine for at least 7 days, although Dr. Park said research suggests the 7-day period may not be necessary.
“We’ve utilized this about 2.5-plus years in my hospital, and it’s really been a game changer for some of our sickest, most challenging patients,” he said at the APA presentation. As he explained, one benefit is that patients can’t repeatedly avoid doses depending on how they feel, as they may do with the sublingual version. “On the first day of injection, you can actually stop the sublingual buprenorphine.”
Dr. Renner emphasized the importance of getting users on buprenorphine as fast as possible. If the treatment begins in the ED, he said, “they need to have a system that is going to be able to pick them up and continue the care.”
Otherwise, the risk is high. “We’re in a very dangerous era,” he said, “where the patient walks out the door, and then they die.”
Dr. Park had no disclosures, and Dr. Renner disclosed royalties from the APA.
AT APA 2023
Crusted Scabies Presenting as Erythroderma in a Patient With Iatrogenic Immunosuppression for Treatment of Granulomatosis With Polyangiitis
Scabies is caused by cutaneous ectoparasitic infection by the mite Sarcoptes scabiei var hominis. The infection is highly contagious via direct skin-to-skin contact or indirectly through infested bedding, clothing or fomites.1,2 Scabies occurs at all ages, in all ethnic groups, and at all socioeconomic levels.1 Analysis by the Global Burden of Disease estimates that 200 million individuals have been infected with scabies worldwide. The World Health Organization has declared scabies a neglected tropical disease.3
Crusted scabies is a severe and rare form of scabies, with hyperinfestation of thousands to millions of mites, and more commonly is associated with immunosuppressed states, including HIV and hematologic malignancies.1,2,4 Crusted scabies has a high mortality rate due to sepsis when left untreated.3,5
Occasionally, iatrogenic immunosuppression contributes to the development of crusted scabies.1,2 Iatrogenic immunosuppression leading to crusted scabies most commonly occurs secondary to immunosuppression after bone marrow or solid organ transplantation.6 Less often, crusted scabies is caused by iatrogenic immunosuppression from other clinical scenarios.1,2
We describe a patient with iatrogenic immunosuppression due to azathioprine-induced myelosuppression for the treatment of granulomatosis with polyangiitis (GPA) who developed crusted scabies that clinically presented as erythroderma. Crusted scabies should be included in the differential diagnosis of erythroderma, especially in the setting of iatrogenic immunosuppression, for timely and appropriate management.
Case Report
An 84-year-old man presented with worsening pruritus, erythema, and thick yellow scale that progressed to erythroderma over the last 2 weeks. He was diagnosed with GPA 6 months prior to presentation and was treated with azathioprine 150 mg/d, prednisone 10 mg/d, and sulfamethoxazole 800 mg plus trimethoprim 160 mg twice weekly for prophylaxis against Pneumocystis jirovecii pneumonia.
Three weeks prior to presentation, the patient was hospitalized for pancytopenia attributed to azathioprine-induced myelosuppression (hemoglobin, 6.1 g/dL [reference range, 13.5–18.0 g/dL]; hematocrit, 17.5% [reference range, 42%–52%]; white blood cell count, 1.66×103/μL [reference range, 4.0–10.5×103/μL]; platelet count, 146×103/μL [reference range, 150–450×103/μL]; absolute neutrophil count, 1.29×103/μL [reference range, 1.4–6.5×103/μL]). He was transferred to a skilled nursing facility after discharge and referred to dermatology for evaluation of the worsening pruritic rash.
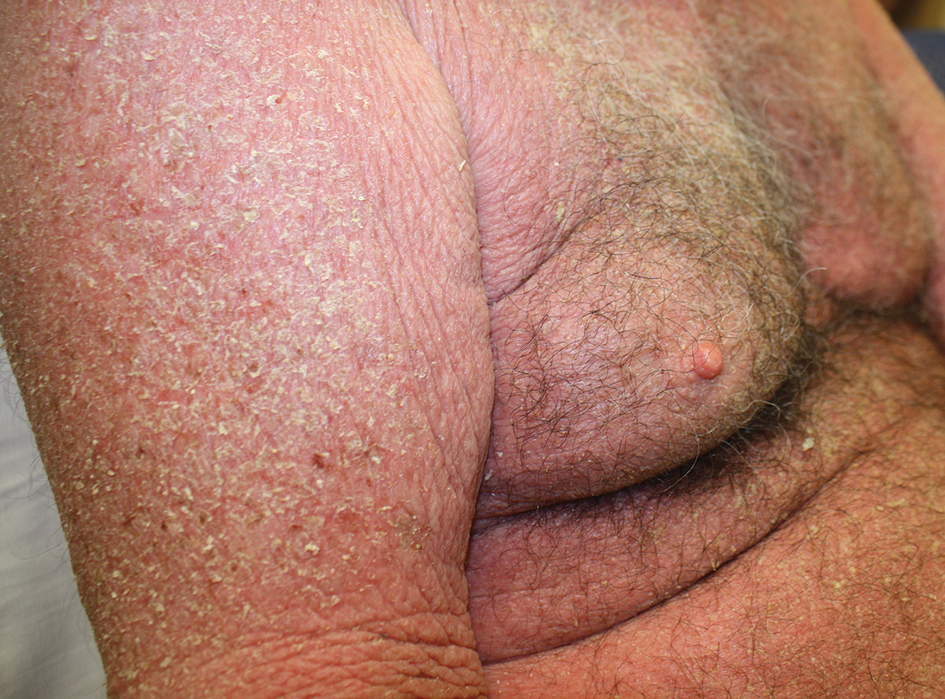
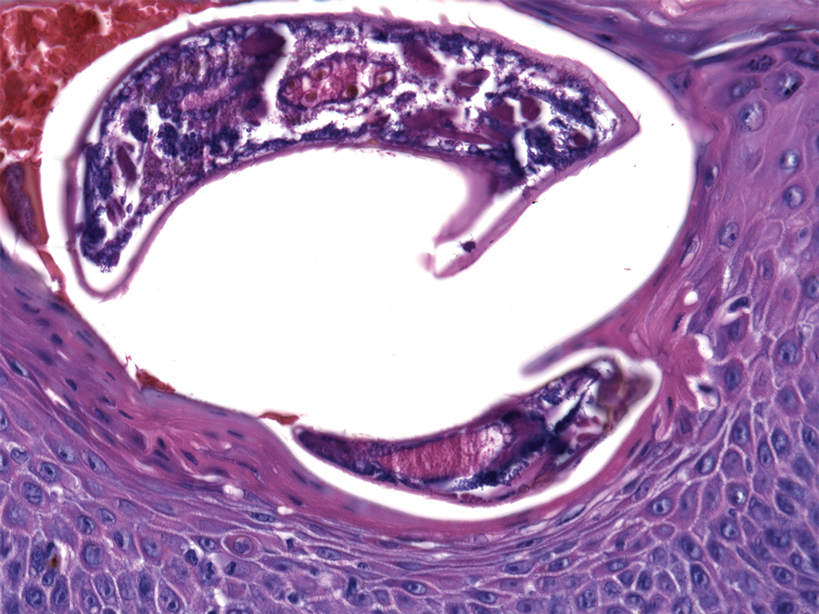
At the current presentation, the patient denied close contact with anyone who had a similar rash at home or at the skilled nursing facility. Physical examination revealed diffuse erythroderma with yellow scale on the scalp, trunk, arms, and legs (Figure 1). The palms showed scattered 2- to 3-mm pustules. The mucosal surfaces did not have lesions. A punch biopsy of a pustule from the right arm revealed focal spongiosis, parakeratosis, and acanthosis, as well as a perivascular and interstitial mixed inflammatory infiltrate with lymphocytes and eosinophils. Organisms morphologically compatible with scabies were found in the stratum corneum (Figure 2). Another punch biopsy of a pustule from the right arm was performed for direct immunofluorescence (DIF) and was negative for immunoglobulin deposition. Mineral oil preparation from pustules on the palm was positive for mites.
The patient was treated with permethrin cream 5% and oral ivermectin 200 μg/kg on day 1 and day 10. The prednisone dosage was increased from 10 mg/d to 50 mg/d and tapered over 2 weeks to treat the symptomatic rash and GPA. He remains on maintenance rituximab for GPA, without recurrence of scabies.
Comment
Pathogenesis—As an obligate parasite, S scabiei spends its entire life cycle within the host. Impregnated female mites burrow into the epidermis after mating and lay eggs daily for 1 to 2 months. Eggs hatch 2 or 3 days later. Larvae then migrate to the skin surface; burrow into the stratum corneum, where they mature into adults; and then mate on the skin surface.1,4
Clinical Presentation and Sequelae—Typically, scabies presents 2 to 6 weeks after initial exposure with generalized and intense itching and inflammatory pruritic papules on the finger webs, wrists, elbows, axillae, buttocks, umbilicus, genitalia, and areolae.1 Burrows are specific for scabies but may not always be present. Often, there are nonspecific secondary lesions, including excoriations, dermatitis, and impetiginization.
Complications of scabies can be severe, with initial colonization and infection of the skin resulting in impetigo and cellulitis. Systematic sequelae from local skin infection include post-streptococcal glomerulonephritis, rheumatic fever, and sepsis. Mortality from sepsis in scabies can be high.3,5
Classic Crusted Scabies and Other Variants—Crusted scabies presents with psoriasiform hyperkeratotic plaques involving the hands and feet with potential nail involvement that can become more generalized.1 Alterations in CD4+ T-cell function have been implicated in the development of crusted scabies, in which an excessive helper T cell (TH2) response is elicited against the ectoparasite, which may help explain the intense pruritus of scabies.6 Occasionally, iatrogenic immunosuppression contributes to development of crusted scabies,1 as was the case with our patient. However, it is rare for crusted scabies to present with erythroderma.7
Other atypical presentations of scabies include a seborrheic dermatitis–like presentation in infants, nodular lesions in the groin and axillae in more chronic scabies, and vesicles or bullous lesions.1
Diagnosis—Identification of mites, eggs, or feces is necessary for definitive diagnosis of scabies.8 These materials can be obtained through skin scrapings with mineral oil and observed under light microscopy or direct dermoscopy. Multiple scrapings on many lesions should be performed because failure to identify mites can be common and does not rule out scabies. Dermoscopic examination of active lesions under low power also can be helpful, given that identification of dark brown triangular structures can correspond to visualization of the pigmented anterior section of the mite.9-11 A skin biopsy can help identify mites, but histopathology often shows a nonspecific hypersensitivity reaction.12 Therefore, empiric treatment often is necessary.
Differential Diagnosis—The differential diagnosis of erythroderma is broad and includes a drug eruption; Sézary syndrome; and pre-existing skin diseases, including psoriasis, atopic dermatitis, pityriasis rubra pilaris, pemphigus foliaceus, and bullous pemphigoid. Histopathology is critical to differentiate these diagnoses. Bullous pemphigoid and pemphigus foliaceus are immunobullous diseases that typically are positive for immunoglobulin deposition on DIF. In rare cases, scabies also can present with bullae and positive DIF test results.13
Treatment—First-line treatment of crusted scabies in the United States is permethrin cream 5%, followed by oral ivermectin 200 μg/kg.4,5,14,15 Other scabicides include topicals such as benzyl benzoate 10% to 25%; precipitated sulfur 2% to 10%; crotamiton 10%; malathion 0.5%; and lindane 1%.5 The association of neurotoxicity with lindane has considerably reduced the drug’s use.1
During treatment of scabies, it is important to isolate patients to mitigate the possibility of spread.4 Pruritus can persist for a few weeks after completion of therapy.5 Patients should be closely monitored to ensure that this symptom is secondary to skin inflammation and not incomplete treatment.
Treatment of crusted scabies may require repeated treatments to decrease the notable mite burden as well as the associated crusting and scale. Adding a keratolytic such as 5% to 10% salicylic acid in petrolatum to the treatment regimen may be useful for breaking up thick scale.5
Immunosuppression—With numerous immunomodulatory drugs for treating autoimmunity comes an increased risk for iatrogenic immunosuppression that may contribute to the development of crusted scabies.16 In a number of autoimmune diseases such as rheumatoid arthritis,17-19 psoriasis,20,21 pemphigus vulgaris,22 systemic lupus erythematosus,23 systemic sclerosis,22,24 bullous pemphigoid,25,26 and dermatomyositis,27 patients have developed crusted scabies secondary to treatment-related immunosuppression. These immunosuppressive therapies include systemic steroids,22-24,26-31 methotrexate,23 infliximab,18 adalimumab,21 toclizumab,19 and etanercept.20 In a case of drug-induced Stevens-Johnson syndrome, the patient developed crusted scabies during long-term use of oral steroids.22
Patients with a malignancy who are being treated with chemotherapy also can develop crusted scabies.28 Crusted scabies has even been associated with long-term topical steroid32-34 and topical calcineurin inhibitor use.16
Iatrogenic immunosuppression in our patient resulted from treatment of GPA with azathioprine, an immunosuppressive drug that acts as an antagonist of the breakdown of purines, leading to inhibition of DNA, RNA, and protein synthesis.35 On occasion, azathioprine can induce immunosuppression in the form of myelosuppression and resulting pancytopenia, as was the case with our patient.
Conclusion
Although scabies is designated as a neglected tropical disease by the World Health Organization, it still causes a notable burden worldwide, regardless of the economics. Our case highlights an unusual presentation of scabies as erythroderma in the setting of iatrogenic immunosuppression from azathioprine use. Dermatologists should consider crusted scabies in the differential diagnosis of erythroderma, especially in immunocompromised patients, to avoid delays in diagnosis and treatment. Immunosuppressive therapy is an important mainstay in the treatment of many conditions, but it is important to consider that these medications can place patients at an increased risk for rare opportunistic infections. Therefore, patients receiving such treatment should be closely monitored.
- Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006;354:1718-1727. doi:10.1056/NEJMcp052784
- Salgado F, Elston DM. What’s eating you? scabies in the developing world. Cutis. 2017;100:287-289.
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Wang X-D, Shen H, Liu Z-H. Contagious erythroderma. J Emerg Med. 2016;51:180-181. doi:10.1016/j.jemermed.2016.05.027
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146. doi:10.1016/j.jaad.2010.03.010
- Bollea Garlatti LA, Torre AC, Bollea Garlatti ML, et al.. Dermoscopy aids the diagnosis of crusted scabies in an erythrodermic patient. J Am Acad Dermatol. 2015;73:E93-E95. doi:10.1016/j.jaad.2015.04.061
- Tang J, You Z, Ran Y. Simple methods to enhance the diagnosis of scabies. J Am Acad Dermatol. 2019;80:E99-E100. doi:10.1016/j.jaad.2017.07.038
- Falk ES, Eide TJ. Histologic and clinical findings in human scabies. Int J Dermatol. 1981;20:600-605. doi:10.1111/j.1365-4362.1981.tb00844.x
- Shahab RKA, Loo DS. Bullous scabies. J Am Acad Dermatol. 2003;49:346-350. doi:10.1067/s0190-9622(03)00876-4
- Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007:CD000320. doi:10.1002/14651858.CD000320.pub2
- Rosumeck S, Nast A, Dressler C. Evaluation of ivermectin vs permethrin for treating scabies—summary of a Cochrane Review. JAMA Dermatol. 2019;155:730-732. doi:10.1001/jamadermatol.2019.0279
- Ruiz-Maldonado R. Pimecrolimus related crusted scabies in an infant. Pediatr Dermatol. 2006;23:299-300. doi:10.1111/j.1525-1470.2006.00241.x
- Bu X, Fan J, Hu X, et al. Norwegian scabies in a patient treated with Tripterygium glycoside for rheumatoid arthritis. An Bras Dermatol. 2017;92:556-558. doi:10.1590/abd1806-4841.20174946
- Pipitone MA, Adams B, Sheth A, et al. Crusted scabies in a patient being treated with infliximab for juvenile rheumatoid arthritis. J Am Acad Dermatol. 2005;52:719-720. doi:10.1016/j.jaad.2004.12.039
- Baccouche K, Sellam J, Guegan S, et al. Crusted Norwegian scabies, an opportunistic infection, with tocilizumab in rheumatoid arthritis. Joint Bone Spine. 2011;78:402-404. doi:10.1016/j.jbspin.2011.02.008
- Saillard C, Darrieux L, Safa G. Crusted scabies complicates etanercept therapy in a patient with severe psoriasis. J Am Acad Dermatol. 2013;68:E138-E139. doi:10.1016/j.jaad.2012.09.049
- Belvisi V, Orsi GB, Del Borgo C, et al. Large nosocomial outbreakassociated with a Norwegian scabies index case undergoing TNF-α inhibitor treatment: management and control. Infect Control Hosp Epidemiol. 2015;36:1358-1360. doi:10.1017/ice.2015.188
- Nofal A. Variable response of crusted scabies to oral ivermectin: report on eight Egyptian patients. J Eur Acad Dermatol Venereol. 2009;23:793-797. doi:10.1111/j.1468-3083.2009.03177.x
- Yee BE, Carlos CA, Hata T. Crusted scabies of the scalp in a patient with systemic lupus erythematosus. Dermatol Online J. 2014;20:13030/qt9dm891gd.
- Bumb RA, Mehta RD. Crusted scabies in a patient of systemic sclerosis. Indian J Dermatol Venereol Leprol. 2000;66:143-144.
- Hylwa SA, Loss L, Grassi M. Crusted scabies and tinea corporis after treatment of presumed bullous pemphigoid. Cutis. 2013;92:193-198.
- Svecova D, Chmurova N, Pallova A, et al. Norwegian scabies in immunosuppressed patient misdiagnosed as an adverse drug reaction. Epidemiol Mikrobiol Imunol. 2009;58:121-123.
- Dourmishev AL, Serafimova DK, Dourmishev LA, et al. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin. Int J Dermatol. 1998;37:231-234. doi:10.1046/j.1365-4362.1998.00330.x
- Mortazavi H, Abedini R, Sadri F, et al. Crusted scabies in a patient with brain astrocytoma: report of a case. Int J Infect Dis. 2010;14:E526-E527. doi:10.1016/j.ijid.2009.06.011
- Lima FCDR, Cerqueira AMM, MBS, et al. Crusted scabies due to indiscriminate use of glucocorticoid therapy in infant. An Bras Dermatol. 2017;92:383-385. doi:10.1590/abd1806-4841.20174433
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2010;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Ohtaki N, Taniguchi H, Ohtomo H. Oral ivermectin treatment in two cases of scabies: effective in crusted scabies induced by corticosteroid but ineffective in nail scabies. J Dermatol. 2003;30:411-416. doi:10.1111/j.1346-8138.2003.tb00408.x
- Bilan P, Colin-Gorski AM, Chapelon E, et al. Crusted scabies induced by topical corticosteroids: a case report [in French]. Arch Pediatr. 2015;22:1292-1294. doi:10.1016/j.arcped.2015.09.004
- Marlière V, Roul S, C, et al. Crusted (Norwegian) scabies induced by use of topical corticosteroids and treated successfully with ivermectin. J Pediatr. 1999;135:122-124. doi:10.1016/s0022-3476(99)70342-2
- Jaramillo-Ayerbe F, J. Ivermectin for crusted Norwegian scabies induced by use of topical steroids. Arch Dermatol. 1998;134:143-145. doi:10.1001/archderm.134.2.143
- Elion GB. The purine path to chemotherapy. Science. 1989;244:41-47. doi:10.1126/science.2649979
Scabies is caused by cutaneous ectoparasitic infection by the mite Sarcoptes scabiei var hominis. The infection is highly contagious via direct skin-to-skin contact or indirectly through infested bedding, clothing or fomites.1,2 Scabies occurs at all ages, in all ethnic groups, and at all socioeconomic levels.1 Analysis by the Global Burden of Disease estimates that 200 million individuals have been infected with scabies worldwide. The World Health Organization has declared scabies a neglected tropical disease.3
Crusted scabies is a severe and rare form of scabies, with hyperinfestation of thousands to millions of mites, and more commonly is associated with immunosuppressed states, including HIV and hematologic malignancies.1,2,4 Crusted scabies has a high mortality rate due to sepsis when left untreated.3,5
Occasionally, iatrogenic immunosuppression contributes to the development of crusted scabies.1,2 Iatrogenic immunosuppression leading to crusted scabies most commonly occurs secondary to immunosuppression after bone marrow or solid organ transplantation.6 Less often, crusted scabies is caused by iatrogenic immunosuppression from other clinical scenarios.1,2
We describe a patient with iatrogenic immunosuppression due to azathioprine-induced myelosuppression for the treatment of granulomatosis with polyangiitis (GPA) who developed crusted scabies that clinically presented as erythroderma. Crusted scabies should be included in the differential diagnosis of erythroderma, especially in the setting of iatrogenic immunosuppression, for timely and appropriate management.
Case Report
An 84-year-old man presented with worsening pruritus, erythema, and thick yellow scale that progressed to erythroderma over the last 2 weeks. He was diagnosed with GPA 6 months prior to presentation and was treated with azathioprine 150 mg/d, prednisone 10 mg/d, and sulfamethoxazole 800 mg plus trimethoprim 160 mg twice weekly for prophylaxis against Pneumocystis jirovecii pneumonia.
Three weeks prior to presentation, the patient was hospitalized for pancytopenia attributed to azathioprine-induced myelosuppression (hemoglobin, 6.1 g/dL [reference range, 13.5–18.0 g/dL]; hematocrit, 17.5% [reference range, 42%–52%]; white blood cell count, 1.66×103/μL [reference range, 4.0–10.5×103/μL]; platelet count, 146×103/μL [reference range, 150–450×103/μL]; absolute neutrophil count, 1.29×103/μL [reference range, 1.4–6.5×103/μL]). He was transferred to a skilled nursing facility after discharge and referred to dermatology for evaluation of the worsening pruritic rash.

At the current presentation, the patient denied close contact with anyone who had a similar rash at home or at the skilled nursing facility. Physical examination revealed diffuse erythroderma with yellow scale on the scalp, trunk, arms, and legs (Figure 1). The palms showed scattered 2- to 3-mm pustules. The mucosal surfaces did not have lesions. A punch biopsy of a pustule from the right arm revealed focal spongiosis, parakeratosis, and acanthosis, as well as a perivascular and interstitial mixed inflammatory infiltrate with lymphocytes and eosinophils. Organisms morphologically compatible with scabies were found in the stratum corneum (Figure 2). Another punch biopsy of a pustule from the right arm was performed for direct immunofluorescence (DIF) and was negative for immunoglobulin deposition. Mineral oil preparation from pustules on the palm was positive for mites.

The patient was treated with permethrin cream 5% and oral ivermectin 200 μg/kg on day 1 and day 10. The prednisone dosage was increased from 10 mg/d to 50 mg/d and tapered over 2 weeks to treat the symptomatic rash and GPA. He remains on maintenance rituximab for GPA, without recurrence of scabies.
Comment
Pathogenesis—As an obligate parasite, S scabiei spends its entire life cycle within the host. Impregnated female mites burrow into the epidermis after mating and lay eggs daily for 1 to 2 months. Eggs hatch 2 or 3 days later. Larvae then migrate to the skin surface; burrow into the stratum corneum, where they mature into adults; and then mate on the skin surface.1,4
Clinical Presentation and Sequelae—Typically, scabies presents 2 to 6 weeks after initial exposure with generalized and intense itching and inflammatory pruritic papules on the finger webs, wrists, elbows, axillae, buttocks, umbilicus, genitalia, and areolae.1 Burrows are specific for scabies but may not always be present. Often, there are nonspecific secondary lesions, including excoriations, dermatitis, and impetiginization.
Complications of scabies can be severe, with initial colonization and infection of the skin resulting in impetigo and cellulitis. Systematic sequelae from local skin infection include post-streptococcal glomerulonephritis, rheumatic fever, and sepsis. Mortality from sepsis in scabies can be high.3,5
Classic Crusted Scabies and Other Variants—Crusted scabies presents with psoriasiform hyperkeratotic plaques involving the hands and feet with potential nail involvement that can become more generalized.1 Alterations in CD4+ T-cell function have been implicated in the development of crusted scabies, in which an excessive helper T cell (TH2) response is elicited against the ectoparasite, which may help explain the intense pruritus of scabies.6 Occasionally, iatrogenic immunosuppression contributes to development of crusted scabies,1 as was the case with our patient. However, it is rare for crusted scabies to present with erythroderma.7
Other atypical presentations of scabies include a seborrheic dermatitis–like presentation in infants, nodular lesions in the groin and axillae in more chronic scabies, and vesicles or bullous lesions.1
Diagnosis—Identification of mites, eggs, or feces is necessary for definitive diagnosis of scabies.8 These materials can be obtained through skin scrapings with mineral oil and observed under light microscopy or direct dermoscopy. Multiple scrapings on many lesions should be performed because failure to identify mites can be common and does not rule out scabies. Dermoscopic examination of active lesions under low power also can be helpful, given that identification of dark brown triangular structures can correspond to visualization of the pigmented anterior section of the mite.9-11 A skin biopsy can help identify mites, but histopathology often shows a nonspecific hypersensitivity reaction.12 Therefore, empiric treatment often is necessary.
Differential Diagnosis—The differential diagnosis of erythroderma is broad and includes a drug eruption; Sézary syndrome; and pre-existing skin diseases, including psoriasis, atopic dermatitis, pityriasis rubra pilaris, pemphigus foliaceus, and bullous pemphigoid. Histopathology is critical to differentiate these diagnoses. Bullous pemphigoid and pemphigus foliaceus are immunobullous diseases that typically are positive for immunoglobulin deposition on DIF. In rare cases, scabies also can present with bullae and positive DIF test results.13
Treatment—First-line treatment of crusted scabies in the United States is permethrin cream 5%, followed by oral ivermectin 200 μg/kg.4,5,14,15 Other scabicides include topicals such as benzyl benzoate 10% to 25%; precipitated sulfur 2% to 10%; crotamiton 10%; malathion 0.5%; and lindane 1%.5 The association of neurotoxicity with lindane has considerably reduced the drug’s use.1
During treatment of scabies, it is important to isolate patients to mitigate the possibility of spread.4 Pruritus can persist for a few weeks after completion of therapy.5 Patients should be closely monitored to ensure that this symptom is secondary to skin inflammation and not incomplete treatment.
Treatment of crusted scabies may require repeated treatments to decrease the notable mite burden as well as the associated crusting and scale. Adding a keratolytic such as 5% to 10% salicylic acid in petrolatum to the treatment regimen may be useful for breaking up thick scale.5
Immunosuppression—With numerous immunomodulatory drugs for treating autoimmunity comes an increased risk for iatrogenic immunosuppression that may contribute to the development of crusted scabies.16 In a number of autoimmune diseases such as rheumatoid arthritis,17-19 psoriasis,20,21 pemphigus vulgaris,22 systemic lupus erythematosus,23 systemic sclerosis,22,24 bullous pemphigoid,25,26 and dermatomyositis,27 patients have developed crusted scabies secondary to treatment-related immunosuppression. These immunosuppressive therapies include systemic steroids,22-24,26-31 methotrexate,23 infliximab,18 adalimumab,21 toclizumab,19 and etanercept.20 In a case of drug-induced Stevens-Johnson syndrome, the patient developed crusted scabies during long-term use of oral steroids.22
Patients with a malignancy who are being treated with chemotherapy also can develop crusted scabies.28 Crusted scabies has even been associated with long-term topical steroid32-34 and topical calcineurin inhibitor use.16
Iatrogenic immunosuppression in our patient resulted from treatment of GPA with azathioprine, an immunosuppressive drug that acts as an antagonist of the breakdown of purines, leading to inhibition of DNA, RNA, and protein synthesis.35 On occasion, azathioprine can induce immunosuppression in the form of myelosuppression and resulting pancytopenia, as was the case with our patient.
Conclusion
Although scabies is designated as a neglected tropical disease by the World Health Organization, it still causes a notable burden worldwide, regardless of the economics. Our case highlights an unusual presentation of scabies as erythroderma in the setting of iatrogenic immunosuppression from azathioprine use. Dermatologists should consider crusted scabies in the differential diagnosis of erythroderma, especially in immunocompromised patients, to avoid delays in diagnosis and treatment. Immunosuppressive therapy is an important mainstay in the treatment of many conditions, but it is important to consider that these medications can place patients at an increased risk for rare opportunistic infections. Therefore, patients receiving such treatment should be closely monitored.
Scabies is caused by cutaneous ectoparasitic infection by the mite Sarcoptes scabiei var hominis. The infection is highly contagious via direct skin-to-skin contact or indirectly through infested bedding, clothing or fomites.1,2 Scabies occurs at all ages, in all ethnic groups, and at all socioeconomic levels.1 Analysis by the Global Burden of Disease estimates that 200 million individuals have been infected with scabies worldwide. The World Health Organization has declared scabies a neglected tropical disease.3
Crusted scabies is a severe and rare form of scabies, with hyperinfestation of thousands to millions of mites, and more commonly is associated with immunosuppressed states, including HIV and hematologic malignancies.1,2,4 Crusted scabies has a high mortality rate due to sepsis when left untreated.3,5
Occasionally, iatrogenic immunosuppression contributes to the development of crusted scabies.1,2 Iatrogenic immunosuppression leading to crusted scabies most commonly occurs secondary to immunosuppression after bone marrow or solid organ transplantation.6 Less often, crusted scabies is caused by iatrogenic immunosuppression from other clinical scenarios.1,2
We describe a patient with iatrogenic immunosuppression due to azathioprine-induced myelosuppression for the treatment of granulomatosis with polyangiitis (GPA) who developed crusted scabies that clinically presented as erythroderma. Crusted scabies should be included in the differential diagnosis of erythroderma, especially in the setting of iatrogenic immunosuppression, for timely and appropriate management.
Case Report
An 84-year-old man presented with worsening pruritus, erythema, and thick yellow scale that progressed to erythroderma over the last 2 weeks. He was diagnosed with GPA 6 months prior to presentation and was treated with azathioprine 150 mg/d, prednisone 10 mg/d, and sulfamethoxazole 800 mg plus trimethoprim 160 mg twice weekly for prophylaxis against Pneumocystis jirovecii pneumonia.
Three weeks prior to presentation, the patient was hospitalized for pancytopenia attributed to azathioprine-induced myelosuppression (hemoglobin, 6.1 g/dL [reference range, 13.5–18.0 g/dL]; hematocrit, 17.5% [reference range, 42%–52%]; white blood cell count, 1.66×103/μL [reference range, 4.0–10.5×103/μL]; platelet count, 146×103/μL [reference range, 150–450×103/μL]; absolute neutrophil count, 1.29×103/μL [reference range, 1.4–6.5×103/μL]). He was transferred to a skilled nursing facility after discharge and referred to dermatology for evaluation of the worsening pruritic rash.

At the current presentation, the patient denied close contact with anyone who had a similar rash at home or at the skilled nursing facility. Physical examination revealed diffuse erythroderma with yellow scale on the scalp, trunk, arms, and legs (Figure 1). The palms showed scattered 2- to 3-mm pustules. The mucosal surfaces did not have lesions. A punch biopsy of a pustule from the right arm revealed focal spongiosis, parakeratosis, and acanthosis, as well as a perivascular and interstitial mixed inflammatory infiltrate with lymphocytes and eosinophils. Organisms morphologically compatible with scabies were found in the stratum corneum (Figure 2). Another punch biopsy of a pustule from the right arm was performed for direct immunofluorescence (DIF) and was negative for immunoglobulin deposition. Mineral oil preparation from pustules on the palm was positive for mites.

The patient was treated with permethrin cream 5% and oral ivermectin 200 μg/kg on day 1 and day 10. The prednisone dosage was increased from 10 mg/d to 50 mg/d and tapered over 2 weeks to treat the symptomatic rash and GPA. He remains on maintenance rituximab for GPA, without recurrence of scabies.
Comment
Pathogenesis—As an obligate parasite, S scabiei spends its entire life cycle within the host. Impregnated female mites burrow into the epidermis after mating and lay eggs daily for 1 to 2 months. Eggs hatch 2 or 3 days later. Larvae then migrate to the skin surface; burrow into the stratum corneum, where they mature into adults; and then mate on the skin surface.1,4
Clinical Presentation and Sequelae—Typically, scabies presents 2 to 6 weeks after initial exposure with generalized and intense itching and inflammatory pruritic papules on the finger webs, wrists, elbows, axillae, buttocks, umbilicus, genitalia, and areolae.1 Burrows are specific for scabies but may not always be present. Often, there are nonspecific secondary lesions, including excoriations, dermatitis, and impetiginization.
Complications of scabies can be severe, with initial colonization and infection of the skin resulting in impetigo and cellulitis. Systematic sequelae from local skin infection include post-streptococcal glomerulonephritis, rheumatic fever, and sepsis. Mortality from sepsis in scabies can be high.3,5
Classic Crusted Scabies and Other Variants—Crusted scabies presents with psoriasiform hyperkeratotic plaques involving the hands and feet with potential nail involvement that can become more generalized.1 Alterations in CD4+ T-cell function have been implicated in the development of crusted scabies, in which an excessive helper T cell (TH2) response is elicited against the ectoparasite, which may help explain the intense pruritus of scabies.6 Occasionally, iatrogenic immunosuppression contributes to development of crusted scabies,1 as was the case with our patient. However, it is rare for crusted scabies to present with erythroderma.7
Other atypical presentations of scabies include a seborrheic dermatitis–like presentation in infants, nodular lesions in the groin and axillae in more chronic scabies, and vesicles or bullous lesions.1
Diagnosis—Identification of mites, eggs, or feces is necessary for definitive diagnosis of scabies.8 These materials can be obtained through skin scrapings with mineral oil and observed under light microscopy or direct dermoscopy. Multiple scrapings on many lesions should be performed because failure to identify mites can be common and does not rule out scabies. Dermoscopic examination of active lesions under low power also can be helpful, given that identification of dark brown triangular structures can correspond to visualization of the pigmented anterior section of the mite.9-11 A skin biopsy can help identify mites, but histopathology often shows a nonspecific hypersensitivity reaction.12 Therefore, empiric treatment often is necessary.
Differential Diagnosis—The differential diagnosis of erythroderma is broad and includes a drug eruption; Sézary syndrome; and pre-existing skin diseases, including psoriasis, atopic dermatitis, pityriasis rubra pilaris, pemphigus foliaceus, and bullous pemphigoid. Histopathology is critical to differentiate these diagnoses. Bullous pemphigoid and pemphigus foliaceus are immunobullous diseases that typically are positive for immunoglobulin deposition on DIF. In rare cases, scabies also can present with bullae and positive DIF test results.13
Treatment—First-line treatment of crusted scabies in the United States is permethrin cream 5%, followed by oral ivermectin 200 μg/kg.4,5,14,15 Other scabicides include topicals such as benzyl benzoate 10% to 25%; precipitated sulfur 2% to 10%; crotamiton 10%; malathion 0.5%; and lindane 1%.5 The association of neurotoxicity with lindane has considerably reduced the drug’s use.1
During treatment of scabies, it is important to isolate patients to mitigate the possibility of spread.4 Pruritus can persist for a few weeks after completion of therapy.5 Patients should be closely monitored to ensure that this symptom is secondary to skin inflammation and not incomplete treatment.
Treatment of crusted scabies may require repeated treatments to decrease the notable mite burden as well as the associated crusting and scale. Adding a keratolytic such as 5% to 10% salicylic acid in petrolatum to the treatment regimen may be useful for breaking up thick scale.5
Immunosuppression—With numerous immunomodulatory drugs for treating autoimmunity comes an increased risk for iatrogenic immunosuppression that may contribute to the development of crusted scabies.16 In a number of autoimmune diseases such as rheumatoid arthritis,17-19 psoriasis,20,21 pemphigus vulgaris,22 systemic lupus erythematosus,23 systemic sclerosis,22,24 bullous pemphigoid,25,26 and dermatomyositis,27 patients have developed crusted scabies secondary to treatment-related immunosuppression. These immunosuppressive therapies include systemic steroids,22-24,26-31 methotrexate,23 infliximab,18 adalimumab,21 toclizumab,19 and etanercept.20 In a case of drug-induced Stevens-Johnson syndrome, the patient developed crusted scabies during long-term use of oral steroids.22
Patients with a malignancy who are being treated with chemotherapy also can develop crusted scabies.28 Crusted scabies has even been associated with long-term topical steroid32-34 and topical calcineurin inhibitor use.16
Iatrogenic immunosuppression in our patient resulted from treatment of GPA with azathioprine, an immunosuppressive drug that acts as an antagonist of the breakdown of purines, leading to inhibition of DNA, RNA, and protein synthesis.35 On occasion, azathioprine can induce immunosuppression in the form of myelosuppression and resulting pancytopenia, as was the case with our patient.
Conclusion
Although scabies is designated as a neglected tropical disease by the World Health Organization, it still causes a notable burden worldwide, regardless of the economics. Our case highlights an unusual presentation of scabies as erythroderma in the setting of iatrogenic immunosuppression from azathioprine use. Dermatologists should consider crusted scabies in the differential diagnosis of erythroderma, especially in immunocompromised patients, to avoid delays in diagnosis and treatment. Immunosuppressive therapy is an important mainstay in the treatment of many conditions, but it is important to consider that these medications can place patients at an increased risk for rare opportunistic infections. Therefore, patients receiving such treatment should be closely monitored.
- Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006;354:1718-1727. doi:10.1056/NEJMcp052784
- Salgado F, Elston DM. What’s eating you? scabies in the developing world. Cutis. 2017;100:287-289.
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Wang X-D, Shen H, Liu Z-H. Contagious erythroderma. J Emerg Med. 2016;51:180-181. doi:10.1016/j.jemermed.2016.05.027
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146. doi:10.1016/j.jaad.2010.03.010
- Bollea Garlatti LA, Torre AC, Bollea Garlatti ML, et al.. Dermoscopy aids the diagnosis of crusted scabies in an erythrodermic patient. J Am Acad Dermatol. 2015;73:E93-E95. doi:10.1016/j.jaad.2015.04.061
- Tang J, You Z, Ran Y. Simple methods to enhance the diagnosis of scabies. J Am Acad Dermatol. 2019;80:E99-E100. doi:10.1016/j.jaad.2017.07.038
- Falk ES, Eide TJ. Histologic and clinical findings in human scabies. Int J Dermatol. 1981;20:600-605. doi:10.1111/j.1365-4362.1981.tb00844.x
- Shahab RKA, Loo DS. Bullous scabies. J Am Acad Dermatol. 2003;49:346-350. doi:10.1067/s0190-9622(03)00876-4
- Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007:CD000320. doi:10.1002/14651858.CD000320.pub2
- Rosumeck S, Nast A, Dressler C. Evaluation of ivermectin vs permethrin for treating scabies—summary of a Cochrane Review. JAMA Dermatol. 2019;155:730-732. doi:10.1001/jamadermatol.2019.0279
- Ruiz-Maldonado R. Pimecrolimus related crusted scabies in an infant. Pediatr Dermatol. 2006;23:299-300. doi:10.1111/j.1525-1470.2006.00241.x
- Bu X, Fan J, Hu X, et al. Norwegian scabies in a patient treated with Tripterygium glycoside for rheumatoid arthritis. An Bras Dermatol. 2017;92:556-558. doi:10.1590/abd1806-4841.20174946
- Pipitone MA, Adams B, Sheth A, et al. Crusted scabies in a patient being treated with infliximab for juvenile rheumatoid arthritis. J Am Acad Dermatol. 2005;52:719-720. doi:10.1016/j.jaad.2004.12.039
- Baccouche K, Sellam J, Guegan S, et al. Crusted Norwegian scabies, an opportunistic infection, with tocilizumab in rheumatoid arthritis. Joint Bone Spine. 2011;78:402-404. doi:10.1016/j.jbspin.2011.02.008
- Saillard C, Darrieux L, Safa G. Crusted scabies complicates etanercept therapy in a patient with severe psoriasis. J Am Acad Dermatol. 2013;68:E138-E139. doi:10.1016/j.jaad.2012.09.049
- Belvisi V, Orsi GB, Del Borgo C, et al. Large nosocomial outbreakassociated with a Norwegian scabies index case undergoing TNF-α inhibitor treatment: management and control. Infect Control Hosp Epidemiol. 2015;36:1358-1360. doi:10.1017/ice.2015.188
- Nofal A. Variable response of crusted scabies to oral ivermectin: report on eight Egyptian patients. J Eur Acad Dermatol Venereol. 2009;23:793-797. doi:10.1111/j.1468-3083.2009.03177.x
- Yee BE, Carlos CA, Hata T. Crusted scabies of the scalp in a patient with systemic lupus erythematosus. Dermatol Online J. 2014;20:13030/qt9dm891gd.
- Bumb RA, Mehta RD. Crusted scabies in a patient of systemic sclerosis. Indian J Dermatol Venereol Leprol. 2000;66:143-144.
- Hylwa SA, Loss L, Grassi M. Crusted scabies and tinea corporis after treatment of presumed bullous pemphigoid. Cutis. 2013;92:193-198.
- Svecova D, Chmurova N, Pallova A, et al. Norwegian scabies in immunosuppressed patient misdiagnosed as an adverse drug reaction. Epidemiol Mikrobiol Imunol. 2009;58:121-123.
- Dourmishev AL, Serafimova DK, Dourmishev LA, et al. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin. Int J Dermatol. 1998;37:231-234. doi:10.1046/j.1365-4362.1998.00330.x
- Mortazavi H, Abedini R, Sadri F, et al. Crusted scabies in a patient with brain astrocytoma: report of a case. Int J Infect Dis. 2010;14:E526-E527. doi:10.1016/j.ijid.2009.06.011
- Lima FCDR, Cerqueira AMM, MBS, et al. Crusted scabies due to indiscriminate use of glucocorticoid therapy in infant. An Bras Dermatol. 2017;92:383-385. doi:10.1590/abd1806-4841.20174433
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2010;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Ohtaki N, Taniguchi H, Ohtomo H. Oral ivermectin treatment in two cases of scabies: effective in crusted scabies induced by corticosteroid but ineffective in nail scabies. J Dermatol. 2003;30:411-416. doi:10.1111/j.1346-8138.2003.tb00408.x
- Bilan P, Colin-Gorski AM, Chapelon E, et al. Crusted scabies induced by topical corticosteroids: a case report [in French]. Arch Pediatr. 2015;22:1292-1294. doi:10.1016/j.arcped.2015.09.004
- Marlière V, Roul S, C, et al. Crusted (Norwegian) scabies induced by use of topical corticosteroids and treated successfully with ivermectin. J Pediatr. 1999;135:122-124. doi:10.1016/s0022-3476(99)70342-2
- Jaramillo-Ayerbe F, J. Ivermectin for crusted Norwegian scabies induced by use of topical steroids. Arch Dermatol. 1998;134:143-145. doi:10.1001/archderm.134.2.143
- Elion GB. The purine path to chemotherapy. Science. 1989;244:41-47. doi:10.1126/science.2649979
- Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006;354:1718-1727. doi:10.1056/NEJMcp052784
- Salgado F, Elston DM. What’s eating you? scabies in the developing world. Cutis. 2017;100:287-289.
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Wang X-D, Shen H, Liu Z-H. Contagious erythroderma. J Emerg Med. 2016;51:180-181. doi:10.1016/j.jemermed.2016.05.027
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146. doi:10.1016/j.jaad.2010.03.010
- Bollea Garlatti LA, Torre AC, Bollea Garlatti ML, et al.. Dermoscopy aids the diagnosis of crusted scabies in an erythrodermic patient. J Am Acad Dermatol. 2015;73:E93-E95. doi:10.1016/j.jaad.2015.04.061
- Tang J, You Z, Ran Y. Simple methods to enhance the diagnosis of scabies. J Am Acad Dermatol. 2019;80:E99-E100. doi:10.1016/j.jaad.2017.07.038
- Falk ES, Eide TJ. Histologic and clinical findings in human scabies. Int J Dermatol. 1981;20:600-605. doi:10.1111/j.1365-4362.1981.tb00844.x
- Shahab RKA, Loo DS. Bullous scabies. J Am Acad Dermatol. 2003;49:346-350. doi:10.1067/s0190-9622(03)00876-4
- Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007:CD000320. doi:10.1002/14651858.CD000320.pub2
- Rosumeck S, Nast A, Dressler C. Evaluation of ivermectin vs permethrin for treating scabies—summary of a Cochrane Review. JAMA Dermatol. 2019;155:730-732. doi:10.1001/jamadermatol.2019.0279
- Ruiz-Maldonado R. Pimecrolimus related crusted scabies in an infant. Pediatr Dermatol. 2006;23:299-300. doi:10.1111/j.1525-1470.2006.00241.x
- Bu X, Fan J, Hu X, et al. Norwegian scabies in a patient treated with Tripterygium glycoside for rheumatoid arthritis. An Bras Dermatol. 2017;92:556-558. doi:10.1590/abd1806-4841.20174946
- Pipitone MA, Adams B, Sheth A, et al. Crusted scabies in a patient being treated with infliximab for juvenile rheumatoid arthritis. J Am Acad Dermatol. 2005;52:719-720. doi:10.1016/j.jaad.2004.12.039
- Baccouche K, Sellam J, Guegan S, et al. Crusted Norwegian scabies, an opportunistic infection, with tocilizumab in rheumatoid arthritis. Joint Bone Spine. 2011;78:402-404. doi:10.1016/j.jbspin.2011.02.008
- Saillard C, Darrieux L, Safa G. Crusted scabies complicates etanercept therapy in a patient with severe psoriasis. J Am Acad Dermatol. 2013;68:E138-E139. doi:10.1016/j.jaad.2012.09.049
- Belvisi V, Orsi GB, Del Borgo C, et al. Large nosocomial outbreakassociated with a Norwegian scabies index case undergoing TNF-α inhibitor treatment: management and control. Infect Control Hosp Epidemiol. 2015;36:1358-1360. doi:10.1017/ice.2015.188
- Nofal A. Variable response of crusted scabies to oral ivermectin: report on eight Egyptian patients. J Eur Acad Dermatol Venereol. 2009;23:793-797. doi:10.1111/j.1468-3083.2009.03177.x
- Yee BE, Carlos CA, Hata T. Crusted scabies of the scalp in a patient with systemic lupus erythematosus. Dermatol Online J. 2014;20:13030/qt9dm891gd.
- Bumb RA, Mehta RD. Crusted scabies in a patient of systemic sclerosis. Indian J Dermatol Venereol Leprol. 2000;66:143-144.
- Hylwa SA, Loss L, Grassi M. Crusted scabies and tinea corporis after treatment of presumed bullous pemphigoid. Cutis. 2013;92:193-198.
- Svecova D, Chmurova N, Pallova A, et al. Norwegian scabies in immunosuppressed patient misdiagnosed as an adverse drug reaction. Epidemiol Mikrobiol Imunol. 2009;58:121-123.
- Dourmishev AL, Serafimova DK, Dourmishev LA, et al. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin. Int J Dermatol. 1998;37:231-234. doi:10.1046/j.1365-4362.1998.00330.x
- Mortazavi H, Abedini R, Sadri F, et al. Crusted scabies in a patient with brain astrocytoma: report of a case. Int J Infect Dis. 2010;14:E526-E527. doi:10.1016/j.ijid.2009.06.011
- Lima FCDR, Cerqueira AMM, MBS, et al. Crusted scabies due to indiscriminate use of glucocorticoid therapy in infant. An Bras Dermatol. 2017;92:383-385. doi:10.1590/abd1806-4841.20174433
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2010;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Ohtaki N, Taniguchi H, Ohtomo H. Oral ivermectin treatment in two cases of scabies: effective in crusted scabies induced by corticosteroid but ineffective in nail scabies. J Dermatol. 2003;30:411-416. doi:10.1111/j.1346-8138.2003.tb00408.x
- Bilan P, Colin-Gorski AM, Chapelon E, et al. Crusted scabies induced by topical corticosteroids: a case report [in French]. Arch Pediatr. 2015;22:1292-1294. doi:10.1016/j.arcped.2015.09.004
- Marlière V, Roul S, C, et al. Crusted (Norwegian) scabies induced by use of topical corticosteroids and treated successfully with ivermectin. J Pediatr. 1999;135:122-124. doi:10.1016/s0022-3476(99)70342-2
- Jaramillo-Ayerbe F, J. Ivermectin for crusted Norwegian scabies induced by use of topical steroids. Arch Dermatol. 1998;134:143-145. doi:10.1001/archderm.134.2.143
- Elion GB. The purine path to chemotherapy. Science. 1989;244:41-47. doi:10.1126/science.2649979
Practice Points
- Crusted scabies is a highly contagious, severe cutaneous ectoparasitic infection that can present atypically in the form of erythroderma.
- Immunomodulatory drugs for the treatment of autoimmune disease can predispose patients to infection, including ectoparasitic infection.
- Dermatologists should be familiar with the full scope of the clinical presentations of scabies and should especially consider this condition in the differential diagnosis of patients who present in an immunosuppressed state.
Primary Effusion Lymphoma: An Infiltrative Plaque in a Patient With HIV
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.
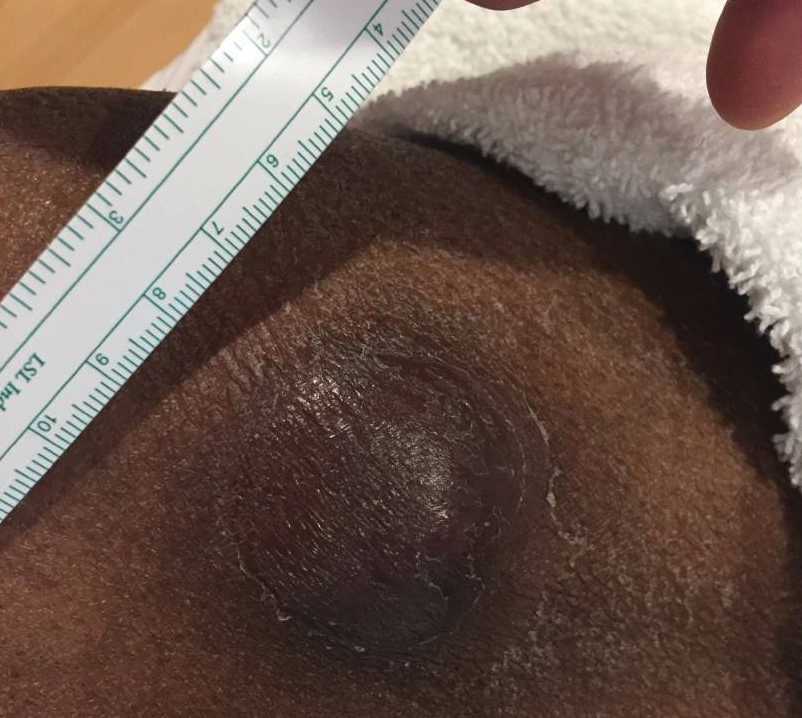
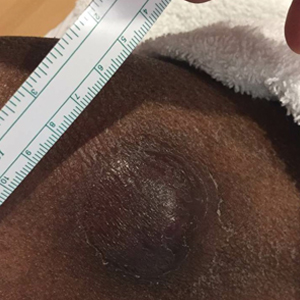
At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.
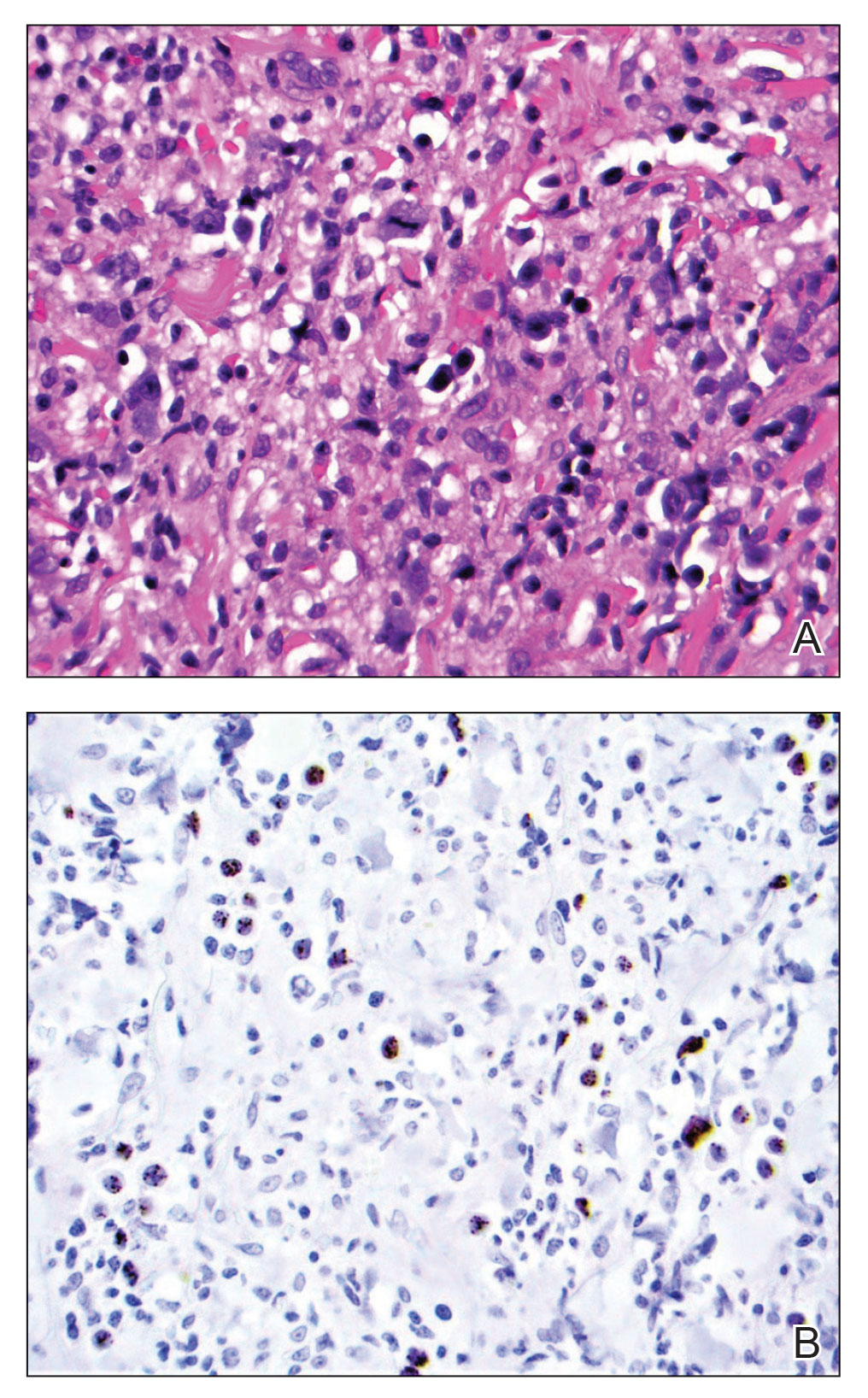
Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
Practice Points
- Extracavitary primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that occurs solely in the presence of human herpesvirus 8 infection and typically is associated with HIV/AIDS.
- Diagnosis necessitates a thorough workup and correlation of histologic, molecular, and immunophenotypic analysis.
- Antiretroviral therapy in HIV-positive patients and intensive chemotherapy regimens are the current recommended treatments. Despite newer targeted agents, the prognosis remains poor.
ILD risk elevated in RA, PsA after starting biologic or targeted synthetic DMARDs
MILAN – Patients with psoriatic arthritis (PsA) who are using biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) have fivefold higher risk for interstitial lung disease (ILD) than does the general population, according to the first study to explore risk of ILD in this particular patient group.
The study also found 10-fold higher risk of ILD in patients with RA who were starting a b/tsDMARD, compared with the general population, while the addition of methotrexate did not appear to be associated with increased risk for ILD in either RA nor PsA.
Sella Aarrestad Provan, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital, Oslo, presented the results at the annual European Congress of Rheumatology.
Explaining the motivation for the study, Dr. Aarrestad Provan said that, in RA, methotrexate’s role in ILD development remained unclear, while some small studies linked b/tsDMARDs with risk for ILD. “In PsA, very few studies have explored the risk of ILD, and no systematic studies have looked at ILD risk factors in this disease.”
The researchers analyzed patient data from hospital and death registries across five Nordic countries (Denmark, Norway, Finland, Iceland, and Sweden) and compared them with general population controls. They calculated risk ratios for people who developed ILD within 5 years of starting a b/tsDMARD (with or without methotrexate).
A total of 37,010 patients with RA, 12,341 with PsA, and 569,451 members of the general population were included in the analysis, with respective disease durations of 10 and 8.9 years. Methotrexate was used along with b/tsDMARDs in 49% of patients with RA and 41% with PsA, and most patients were already on methotrexate when b/tsDMARDs were started. The tumor necrosis factor inhibitor etanercept (Enbrel) was the most commonly used b/tsDMARD in both RA and PsA, followed by infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars).
The incidence of ILD within 5 years of starting a b/tsDMARD was 0.8% in patients with RA, 0.2% with PsA, and 0.1% in the general population, and these findings generated hazard ratios of 10.1 (95% confidence interval, 8.6-11.9) for RA and 5.0 (95% CI, 3.4-7.4) for PsA, compared with the general population as reference.
When the risk for ILD was explored according to methotrexate use in RA patients, “there was no signal of increased risk across patients using methotrexate,” Dr. Aarrestad Provan reported. When risk of ILD was explored according to b/tsDMARD use in RA patients, a signal of increased risk was observed with rituximab, she noted, “but upon adjusting for age, sex, and comorbidities, this association was no longer significant, but was still numerically increased.”
Iain McInnes, MD, PhD, vice principal, professor of rheumatology, and head of the College of Medical, Veterinary and Life Sciences at the University of Glasgow, remarked that he “loves results that are unexpected” and thanked the researcher for such an “important study.”
“For years, we’ve been interested in the potential for DMARDs to impact interstitial lung disease, with potential that drugs could make it worse, or better,” he said. “This study is wonderful and novel because first of all, there hasn’t, until now, been a direct comparison between RA and PsA in quite this way, and secondly, we haven’t really assessed whether there is a drug-related risk in PsA. Note that drug related does not necessarily imply causality.”
Regarding mechanisms, Dr. McInnes added that “epidemiologic studies suggest that PsA often coexists with the presence of cardiometabolic syndrome and obesity, which has a higher prevalence in PsA than in RA. Obesity is also related to ILD. As such, it begs the question of whether cardiometabolic, diabetes, or obesity-related features may give us a clue as to what is going on in these PsA patients.”
The research was supported by NordForsk and FOREUM. Dr. Aarrestad Provan reported serving as a consultant to Boehringer Ingelheim and Novartis and receiving grant/research support from Boehringer Ingelheim. Dr. McInnes declared no disclosures relevant to this study.
MILAN – Patients with psoriatic arthritis (PsA) who are using biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) have fivefold higher risk for interstitial lung disease (ILD) than does the general population, according to the first study to explore risk of ILD in this particular patient group.
The study also found 10-fold higher risk of ILD in patients with RA who were starting a b/tsDMARD, compared with the general population, while the addition of methotrexate did not appear to be associated with increased risk for ILD in either RA nor PsA.
Sella Aarrestad Provan, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital, Oslo, presented the results at the annual European Congress of Rheumatology.
Explaining the motivation for the study, Dr. Aarrestad Provan said that, in RA, methotrexate’s role in ILD development remained unclear, while some small studies linked b/tsDMARDs with risk for ILD. “In PsA, very few studies have explored the risk of ILD, and no systematic studies have looked at ILD risk factors in this disease.”
The researchers analyzed patient data from hospital and death registries across five Nordic countries (Denmark, Norway, Finland, Iceland, and Sweden) and compared them with general population controls. They calculated risk ratios for people who developed ILD within 5 years of starting a b/tsDMARD (with or without methotrexate).
A total of 37,010 patients with RA, 12,341 with PsA, and 569,451 members of the general population were included in the analysis, with respective disease durations of 10 and 8.9 years. Methotrexate was used along with b/tsDMARDs in 49% of patients with RA and 41% with PsA, and most patients were already on methotrexate when b/tsDMARDs were started. The tumor necrosis factor inhibitor etanercept (Enbrel) was the most commonly used b/tsDMARD in both RA and PsA, followed by infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars).
The incidence of ILD within 5 years of starting a b/tsDMARD was 0.8% in patients with RA, 0.2% with PsA, and 0.1% in the general population, and these findings generated hazard ratios of 10.1 (95% confidence interval, 8.6-11.9) for RA and 5.0 (95% CI, 3.4-7.4) for PsA, compared with the general population as reference.
When the risk for ILD was explored according to methotrexate use in RA patients, “there was no signal of increased risk across patients using methotrexate,” Dr. Aarrestad Provan reported. When risk of ILD was explored according to b/tsDMARD use in RA patients, a signal of increased risk was observed with rituximab, she noted, “but upon adjusting for age, sex, and comorbidities, this association was no longer significant, but was still numerically increased.”
Iain McInnes, MD, PhD, vice principal, professor of rheumatology, and head of the College of Medical, Veterinary and Life Sciences at the University of Glasgow, remarked that he “loves results that are unexpected” and thanked the researcher for such an “important study.”
“For years, we’ve been interested in the potential for DMARDs to impact interstitial lung disease, with potential that drugs could make it worse, or better,” he said. “This study is wonderful and novel because first of all, there hasn’t, until now, been a direct comparison between RA and PsA in quite this way, and secondly, we haven’t really assessed whether there is a drug-related risk in PsA. Note that drug related does not necessarily imply causality.”
Regarding mechanisms, Dr. McInnes added that “epidemiologic studies suggest that PsA often coexists with the presence of cardiometabolic syndrome and obesity, which has a higher prevalence in PsA than in RA. Obesity is also related to ILD. As such, it begs the question of whether cardiometabolic, diabetes, or obesity-related features may give us a clue as to what is going on in these PsA patients.”
The research was supported by NordForsk and FOREUM. Dr. Aarrestad Provan reported serving as a consultant to Boehringer Ingelheim and Novartis and receiving grant/research support from Boehringer Ingelheim. Dr. McInnes declared no disclosures relevant to this study.
MILAN – Patients with psoriatic arthritis (PsA) who are using biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) have fivefold higher risk for interstitial lung disease (ILD) than does the general population, according to the first study to explore risk of ILD in this particular patient group.
The study also found 10-fold higher risk of ILD in patients with RA who were starting a b/tsDMARD, compared with the general population, while the addition of methotrexate did not appear to be associated with increased risk for ILD in either RA nor PsA.
Sella Aarrestad Provan, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital, Oslo, presented the results at the annual European Congress of Rheumatology.
Explaining the motivation for the study, Dr. Aarrestad Provan said that, in RA, methotrexate’s role in ILD development remained unclear, while some small studies linked b/tsDMARDs with risk for ILD. “In PsA, very few studies have explored the risk of ILD, and no systematic studies have looked at ILD risk factors in this disease.”
The researchers analyzed patient data from hospital and death registries across five Nordic countries (Denmark, Norway, Finland, Iceland, and Sweden) and compared them with general population controls. They calculated risk ratios for people who developed ILD within 5 years of starting a b/tsDMARD (with or without methotrexate).
A total of 37,010 patients with RA, 12,341 with PsA, and 569,451 members of the general population were included in the analysis, with respective disease durations of 10 and 8.9 years. Methotrexate was used along with b/tsDMARDs in 49% of patients with RA and 41% with PsA, and most patients were already on methotrexate when b/tsDMARDs were started. The tumor necrosis factor inhibitor etanercept (Enbrel) was the most commonly used b/tsDMARD in both RA and PsA, followed by infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars).
The incidence of ILD within 5 years of starting a b/tsDMARD was 0.8% in patients with RA, 0.2% with PsA, and 0.1% in the general population, and these findings generated hazard ratios of 10.1 (95% confidence interval, 8.6-11.9) for RA and 5.0 (95% CI, 3.4-7.4) for PsA, compared with the general population as reference.
When the risk for ILD was explored according to methotrexate use in RA patients, “there was no signal of increased risk across patients using methotrexate,” Dr. Aarrestad Provan reported. When risk of ILD was explored according to b/tsDMARD use in RA patients, a signal of increased risk was observed with rituximab, she noted, “but upon adjusting for age, sex, and comorbidities, this association was no longer significant, but was still numerically increased.”
Iain McInnes, MD, PhD, vice principal, professor of rheumatology, and head of the College of Medical, Veterinary and Life Sciences at the University of Glasgow, remarked that he “loves results that are unexpected” and thanked the researcher for such an “important study.”
“For years, we’ve been interested in the potential for DMARDs to impact interstitial lung disease, with potential that drugs could make it worse, or better,” he said. “This study is wonderful and novel because first of all, there hasn’t, until now, been a direct comparison between RA and PsA in quite this way, and secondly, we haven’t really assessed whether there is a drug-related risk in PsA. Note that drug related does not necessarily imply causality.”
Regarding mechanisms, Dr. McInnes added that “epidemiologic studies suggest that PsA often coexists with the presence of cardiometabolic syndrome and obesity, which has a higher prevalence in PsA than in RA. Obesity is also related to ILD. As such, it begs the question of whether cardiometabolic, diabetes, or obesity-related features may give us a clue as to what is going on in these PsA patients.”
The research was supported by NordForsk and FOREUM. Dr. Aarrestad Provan reported serving as a consultant to Boehringer Ingelheim and Novartis and receiving grant/research support from Boehringer Ingelheim. Dr. McInnes declared no disclosures relevant to this study.
AT EULAR 2023
Minimally invasive vs. open surgery in pancreatic cancer
, suggest results from the international DIPLOMA study.
In the trial, around 260 patients were randomly assigned to undergo either open surgery or minimally invasive laparoscopic or robot-assisted surgery. Rates of complete tumor removal were comparable between the groups.
In addition, the disease-free and overall survival rates at 3 years were nearly identical.
“For pancreatic cancer, we have proven for the first time that minimally invasive distal pancreatectomy is as good as open surgery,” commented principal investigator Mohammad Abu Hilal, MD, PhD, surgical director at the Instituto Ospedaliero Fondazione Poliambulanza in Brescia, Italy.
“Our research provides reassurance for surgeons and can help patients by giving them the information they need to have a conversation with their doctor about how they want to be treated,” he added.
Dr. Hilal was speaking at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology, where the study will be presented (abstract 4163) on June 5.
The study was not able to show that there was a benefit in terms of shorter hospital stays or greater functional recovery with the minimally invasive approach, Dr. Hilal noted, but he suggested that this could be because of differences in postoperative procedures between the participating centers.
He said in an interview that minimally invasive surgery is becoming “very common all over the world,” particularly in the United States, and that randomized controlled trials are “always the last step” in convincing people to use the technique.
He also emphasized that the “best results are obtained in high-volume centers where surgeons do more than at least 50 pancreatic resections a year,” because the minimally invasive approach is “quite complex and difficult,” more so than open surgery.
“This confirmatory study proves that minimally invasive surgical techniques are a safe and effective option for resectable pancreatic cancer,” commented ASCO expert Jennifer F. Tseng, MD, chair of surgery at Boston University and surgeon-in-chief at the Boston Medical Center. It may also “provide benefits like faster recovery time and less infection risk, without increasing cancer risk.”
The results from this trial “will help both surgeons and patients feel comfortable that minimally invasive surgery, in expert hands, is not inferior to open surgery,” she commented in a statement.
Minimally invasive surgery
Only around 12% of patients with pancreatic cancer are diagnosed when the disease is at an early enough stage for surgical resection to be a possibility, Dr. Hilal noted. Minimally invasive pancreatectomy, particularly the distal procedure, was introduced around 25 years ago, but it was initially used only for benign tumors or borderline malignancies.
It took another 10 years before it was considered in cases of confirmed malignancies, “and the main reason for this delay was concerns about the oncological efficiency” of MIDP in terms of its ability to achieve radical resection and an adequate lymph node yield. At the same time, some concerns about minimally invasive surgery for cancer were raised because of results from randomized trials in other cancer types, such as hysterectomy for cervical cancer. Some studies showed worse survival after minimally invasive surgery than after open surgery.
In recent years, use of minimally invasive techniques for pancreatic cancer has become an increasingly “hot topic in many surgical forums,” Dr. Hilal said.
So his team set out to investigate the approach in a phase 3 noninferiority trial. The investigators focused on patients who had an indication for elective distal pancreatectomy plus splenectomy because of proven or highly suspected pancreatic ductal adenocarcinoma in the pancreatic body or tail.
Patients from 35 centers in 12 countries were recruited between May 2018 and May 2021 and were randomly assigned to undergo either MIDP or open distal pancreatectomy.
Patients, nurses, and pathologists were blinded to the surgical procedure by covering of the abdominal wall.
None of the patients underwent adjuvant or neoadjuvant chemotherapy.
Following the procedure, the patients were followed up at 2 weeks and at 1, 3, 6, and 12 months, and a CT scan was performed at 12 months. A range of assessments was performed at each visit, including quality of life measures.
From 1,146 patients initially screened, 261 patients were included.
A few patients withdrew; 131 patients underwent MIDP, and 127 underwent open surgery and were included in the intention-to-treat analysis. Of those, 129 and 125, respectively, were included in the follow-up analysis.
The results confirmed the noninferiority of MIDP, compared with open surgery, with a rate of R0 radical resection (defined as ≥ 1 mm distance between the tumor and the surgical margin) of 73% vs. 69% (P = .039).
In addition, the lymph node yield was comparable between the two approaches, at an average of 22 nodes for MIDP vs. 23 for open surgery (P = .89), and the time to functional recovery was identical, at 5 days for both (P = .22).
The rate of intraperitoneal recurrence was found to be 41% with MIDP, compared with 38% for patients who underwent open surgery.
Dr. Hilal also showed that the rate of serious adverse events, such as bleeding or organ damage, was similar between the two procedures, at 18% with minimally invasive surgery vs. 22% for the open procedure.
Turning to the survival curves, he noted that it is “very clear” that the two procedures achieved near-identical results, at a hazard ratio of 0.99 (P = .94) for overall survival and 0.97 (P = .88) for disease-free survival when comparing MIDP with open surgery.
The researchers will continue to follow up the patients for 3-5 years and will analyze the lymph nodes retrieved to determine whether removal of the spleen is necessary.
The study was funded by Medtronic and Ethicon. Dr. Hilal has relationships with Ethicon and Medtronic. Dr. Tseng has relationships with Aegerion, Amgen, AstraZeneca, Bristol-Myers Squibb, Cubist, Curadel Surgical Innovations, Daiichi Sankyo/Lilly, GlaxoSmithKline, Intarcia Therapeutics, Merck, MyoKardia, PanTher Therapeutics, Pfizer, Quest Diagnostics, Sanofi, Vertex, and Zeus.
A version of this article first appeared on Medscape.com.
, suggest results from the international DIPLOMA study.
In the trial, around 260 patients were randomly assigned to undergo either open surgery or minimally invasive laparoscopic or robot-assisted surgery. Rates of complete tumor removal were comparable between the groups.
In addition, the disease-free and overall survival rates at 3 years were nearly identical.
“For pancreatic cancer, we have proven for the first time that minimally invasive distal pancreatectomy is as good as open surgery,” commented principal investigator Mohammad Abu Hilal, MD, PhD, surgical director at the Instituto Ospedaliero Fondazione Poliambulanza in Brescia, Italy.
“Our research provides reassurance for surgeons and can help patients by giving them the information they need to have a conversation with their doctor about how they want to be treated,” he added.
Dr. Hilal was speaking at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology, where the study will be presented (abstract 4163) on June 5.
The study was not able to show that there was a benefit in terms of shorter hospital stays or greater functional recovery with the minimally invasive approach, Dr. Hilal noted, but he suggested that this could be because of differences in postoperative procedures between the participating centers.
He said in an interview that minimally invasive surgery is becoming “very common all over the world,” particularly in the United States, and that randomized controlled trials are “always the last step” in convincing people to use the technique.
He also emphasized that the “best results are obtained in high-volume centers where surgeons do more than at least 50 pancreatic resections a year,” because the minimally invasive approach is “quite complex and difficult,” more so than open surgery.
“This confirmatory study proves that minimally invasive surgical techniques are a safe and effective option for resectable pancreatic cancer,” commented ASCO expert Jennifer F. Tseng, MD, chair of surgery at Boston University and surgeon-in-chief at the Boston Medical Center. It may also “provide benefits like faster recovery time and less infection risk, without increasing cancer risk.”
The results from this trial “will help both surgeons and patients feel comfortable that minimally invasive surgery, in expert hands, is not inferior to open surgery,” she commented in a statement.
Minimally invasive surgery
Only around 12% of patients with pancreatic cancer are diagnosed when the disease is at an early enough stage for surgical resection to be a possibility, Dr. Hilal noted. Minimally invasive pancreatectomy, particularly the distal procedure, was introduced around 25 years ago, but it was initially used only for benign tumors or borderline malignancies.
It took another 10 years before it was considered in cases of confirmed malignancies, “and the main reason for this delay was concerns about the oncological efficiency” of MIDP in terms of its ability to achieve radical resection and an adequate lymph node yield. At the same time, some concerns about minimally invasive surgery for cancer were raised because of results from randomized trials in other cancer types, such as hysterectomy for cervical cancer. Some studies showed worse survival after minimally invasive surgery than after open surgery.
In recent years, use of minimally invasive techniques for pancreatic cancer has become an increasingly “hot topic in many surgical forums,” Dr. Hilal said.
So his team set out to investigate the approach in a phase 3 noninferiority trial. The investigators focused on patients who had an indication for elective distal pancreatectomy plus splenectomy because of proven or highly suspected pancreatic ductal adenocarcinoma in the pancreatic body or tail.
Patients from 35 centers in 12 countries were recruited between May 2018 and May 2021 and were randomly assigned to undergo either MIDP or open distal pancreatectomy.
Patients, nurses, and pathologists were blinded to the surgical procedure by covering of the abdominal wall.
None of the patients underwent adjuvant or neoadjuvant chemotherapy.
Following the procedure, the patients were followed up at 2 weeks and at 1, 3, 6, and 12 months, and a CT scan was performed at 12 months. A range of assessments was performed at each visit, including quality of life measures.
From 1,146 patients initially screened, 261 patients were included.
A few patients withdrew; 131 patients underwent MIDP, and 127 underwent open surgery and were included in the intention-to-treat analysis. Of those, 129 and 125, respectively, were included in the follow-up analysis.
The results confirmed the noninferiority of MIDP, compared with open surgery, with a rate of R0 radical resection (defined as ≥ 1 mm distance between the tumor and the surgical margin) of 73% vs. 69% (P = .039).
In addition, the lymph node yield was comparable between the two approaches, at an average of 22 nodes for MIDP vs. 23 for open surgery (P = .89), and the time to functional recovery was identical, at 5 days for both (P = .22).
The rate of intraperitoneal recurrence was found to be 41% with MIDP, compared with 38% for patients who underwent open surgery.
Dr. Hilal also showed that the rate of serious adverse events, such as bleeding or organ damage, was similar between the two procedures, at 18% with minimally invasive surgery vs. 22% for the open procedure.
Turning to the survival curves, he noted that it is “very clear” that the two procedures achieved near-identical results, at a hazard ratio of 0.99 (P = .94) for overall survival and 0.97 (P = .88) for disease-free survival when comparing MIDP with open surgery.
The researchers will continue to follow up the patients for 3-5 years and will analyze the lymph nodes retrieved to determine whether removal of the spleen is necessary.
The study was funded by Medtronic and Ethicon. Dr. Hilal has relationships with Ethicon and Medtronic. Dr. Tseng has relationships with Aegerion, Amgen, AstraZeneca, Bristol-Myers Squibb, Cubist, Curadel Surgical Innovations, Daiichi Sankyo/Lilly, GlaxoSmithKline, Intarcia Therapeutics, Merck, MyoKardia, PanTher Therapeutics, Pfizer, Quest Diagnostics, Sanofi, Vertex, and Zeus.
A version of this article first appeared on Medscape.com.
, suggest results from the international DIPLOMA study.
In the trial, around 260 patients were randomly assigned to undergo either open surgery or minimally invasive laparoscopic or robot-assisted surgery. Rates of complete tumor removal were comparable between the groups.
In addition, the disease-free and overall survival rates at 3 years were nearly identical.
“For pancreatic cancer, we have proven for the first time that minimally invasive distal pancreatectomy is as good as open surgery,” commented principal investigator Mohammad Abu Hilal, MD, PhD, surgical director at the Instituto Ospedaliero Fondazione Poliambulanza in Brescia, Italy.
“Our research provides reassurance for surgeons and can help patients by giving them the information they need to have a conversation with their doctor about how they want to be treated,” he added.
Dr. Hilal was speaking at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology, where the study will be presented (abstract 4163) on June 5.
The study was not able to show that there was a benefit in terms of shorter hospital stays or greater functional recovery with the minimally invasive approach, Dr. Hilal noted, but he suggested that this could be because of differences in postoperative procedures between the participating centers.
He said in an interview that minimally invasive surgery is becoming “very common all over the world,” particularly in the United States, and that randomized controlled trials are “always the last step” in convincing people to use the technique.
He also emphasized that the “best results are obtained in high-volume centers where surgeons do more than at least 50 pancreatic resections a year,” because the minimally invasive approach is “quite complex and difficult,” more so than open surgery.
“This confirmatory study proves that minimally invasive surgical techniques are a safe and effective option for resectable pancreatic cancer,” commented ASCO expert Jennifer F. Tseng, MD, chair of surgery at Boston University and surgeon-in-chief at the Boston Medical Center. It may also “provide benefits like faster recovery time and less infection risk, without increasing cancer risk.”
The results from this trial “will help both surgeons and patients feel comfortable that minimally invasive surgery, in expert hands, is not inferior to open surgery,” she commented in a statement.
Minimally invasive surgery
Only around 12% of patients with pancreatic cancer are diagnosed when the disease is at an early enough stage for surgical resection to be a possibility, Dr. Hilal noted. Minimally invasive pancreatectomy, particularly the distal procedure, was introduced around 25 years ago, but it was initially used only for benign tumors or borderline malignancies.
It took another 10 years before it was considered in cases of confirmed malignancies, “and the main reason for this delay was concerns about the oncological efficiency” of MIDP in terms of its ability to achieve radical resection and an adequate lymph node yield. At the same time, some concerns about minimally invasive surgery for cancer were raised because of results from randomized trials in other cancer types, such as hysterectomy for cervical cancer. Some studies showed worse survival after minimally invasive surgery than after open surgery.
In recent years, use of minimally invasive techniques for pancreatic cancer has become an increasingly “hot topic in many surgical forums,” Dr. Hilal said.
So his team set out to investigate the approach in a phase 3 noninferiority trial. The investigators focused on patients who had an indication for elective distal pancreatectomy plus splenectomy because of proven or highly suspected pancreatic ductal adenocarcinoma in the pancreatic body or tail.
Patients from 35 centers in 12 countries were recruited between May 2018 and May 2021 and were randomly assigned to undergo either MIDP or open distal pancreatectomy.
Patients, nurses, and pathologists were blinded to the surgical procedure by covering of the abdominal wall.
None of the patients underwent adjuvant or neoadjuvant chemotherapy.
Following the procedure, the patients were followed up at 2 weeks and at 1, 3, 6, and 12 months, and a CT scan was performed at 12 months. A range of assessments was performed at each visit, including quality of life measures.
From 1,146 patients initially screened, 261 patients were included.
A few patients withdrew; 131 patients underwent MIDP, and 127 underwent open surgery and were included in the intention-to-treat analysis. Of those, 129 and 125, respectively, were included in the follow-up analysis.
The results confirmed the noninferiority of MIDP, compared with open surgery, with a rate of R0 radical resection (defined as ≥ 1 mm distance between the tumor and the surgical margin) of 73% vs. 69% (P = .039).
In addition, the lymph node yield was comparable between the two approaches, at an average of 22 nodes for MIDP vs. 23 for open surgery (P = .89), and the time to functional recovery was identical, at 5 days for both (P = .22).
The rate of intraperitoneal recurrence was found to be 41% with MIDP, compared with 38% for patients who underwent open surgery.
Dr. Hilal also showed that the rate of serious adverse events, such as bleeding or organ damage, was similar between the two procedures, at 18% with minimally invasive surgery vs. 22% for the open procedure.
Turning to the survival curves, he noted that it is “very clear” that the two procedures achieved near-identical results, at a hazard ratio of 0.99 (P = .94) for overall survival and 0.97 (P = .88) for disease-free survival when comparing MIDP with open surgery.
The researchers will continue to follow up the patients for 3-5 years and will analyze the lymph nodes retrieved to determine whether removal of the spleen is necessary.
The study was funded by Medtronic and Ethicon. Dr. Hilal has relationships with Ethicon and Medtronic. Dr. Tseng has relationships with Aegerion, Amgen, AstraZeneca, Bristol-Myers Squibb, Cubist, Curadel Surgical Innovations, Daiichi Sankyo/Lilly, GlaxoSmithKline, Intarcia Therapeutics, Merck, MyoKardia, PanTher Therapeutics, Pfizer, Quest Diagnostics, Sanofi, Vertex, and Zeus.
A version of this article first appeared on Medscape.com.
FROM ASCO 2023
Warning on use of sotorasib after ICI in lung cancer
because of the risk of increased toxicity.
Sotorasib is indicated for adults with locally advanced or metastatic NSCLC who carry a KRASG12C mutation, which occurs in about 13% of cases.
Since its approval in 2021, sotorasib has emerged as “a new standard of care” for such patients after chemotherapy and anti–PD-L1 failure, the investigators say.
The new warning comes after the team compared 48 patients who received an anti–PD-L1 – most often pembrolizumab alone or in combination with platinum-based chemotherapy – before sotorasib with a control group of 54 patients who either didn’t receive an anti–PD-L1 before sotorasib or had at least one other treatment in between.
The team found that sequential anti–PD-L1 and sotorasib therapy significantly increased the risk of severe sotorasib-related hepatotoxicity and also the risk of non-liver adverse events, typically in patients who received sotorasib within 30 days of an anti–PD-L1.
“We suggest avoiding starting sotorasib within 30 days from the last anti–PD-(L)1 infusion,” say senior author Michaël Duruisseaux, MD, PhD, Louis Pradel Hospital, Bron, France, and collegues.
The findings should also “prompt a close monitoring for the development of hepatotoxicity and non-liver AEs [in] patients who receive sotorasib after anti–PD-(L)1,” they add.
The study was published in the Journal of Thoracic Oncology.
Actionable findings
“I consider the results to be highly credible and informative to my own practice,” said Jack West, MD, a thoracic medical oncologist at the City of Hope outside of Los Angeles, said in an interview.
The findings “may lead me to favor a trial of docetaxel as an intervening therapy for patients who have very recently discontinued immunotherapy, deferring sotorasib at least a few weeks and ideally several months,” Dr. West commented. “I think this is a particularly reasonable approach when we remember that sotorasib conferred no improvement in overall survival at all over docetaxel in the CodeBreaK 200 trial in KRASG12C-mutated NSCLC.”
Overall, the study “corroborates what we’ve seen in the limited first-line experience of sotorasib combined with immunotherapy and also echoes our experience of other targeted therapies, such as osimertinib administered in the weeks just after patients received immunotherapy, which is known to be associated with life-threatening pneumonitis,” he said.
Jared Weiss, MD, a thoracic medical oncologist at the University of North Carolina, Chapel Hill, said that given the long half-life of immune checkpoint inhibitors, “it is quite understandable that the toxicity challenges we previously saw with concurrent administration of immunotherapy and certain targeted therapies would be recapitulated in patients who had a relatively short interval between prior checkpoint inhibitor therapy and sotorasib.”
Even so, because of the aggressiveness of NSCLC, long treatment delays between immunotherapy and sotorasib therapy are “not a favored option.”
Like Dr. West, Dr. Weiss said docetaxel (with or without ramucirumab) is a sound intervening alternative.
Another option is to use adagrasib in the second line instead of sotorasib, Dr. Weiss suggested. It’s also a KRASG12C inhibitor but hasn’t so far been associated with severe hepatotoxicity, he said.
Hossein Borghaei, DO, a thoracic medical oncologist at Fox Chase Cancer Center, Philadelphia, agrees with his colleagues and thinks that what the French team found “is real.”
As the investigators suggest, “it might be that sotorasib leads to an inflammatory microenvironment that causes hepatotoxicity in the presence of a checkpoint inhibitor. In that case,” a lower dose of sotorasib might help reduce toxicity while remaining effective, Dr. Broghaei suggested.
Study details
The French team was prompted to investigate the issue by a report of life-threatening hepatitis in a patient with NSCLC for whom sotorasib therapy was initiated 14 weeks after treatment with pembrolizumab, as well as by “the long story of adverse events ... observed with sequential use of [immune checkpoint inhibitors] and targeted therapy.”
Like Dr. Weiss, they note that severe hepatotoxicity after anti–PD-L1 therapy has not, to date, been reported for other KRASG12C inhibitors.
Patients in the study were treated outside of clinical trials at 16 medical centers in France.
Half of the patients (24/48) who were treated immediately with an anti–PD-L1 after sotorasib therapy developed grade 3 or higher sotorasib-related adverse events, including 16 (33%) with severe sotorasib-related hepatotoxicity. Severe diarrhea and fatigue were also more frequent with sequential therapy.
Severe events typically occurred within 30 days of the last anti–PD-L1 infusion and to a lesser extent within 31-60 days.
In the control arm, the rate of severe sotorasib-related adverse events was 13% (7/54). Six patients (11%) experienced severe hepatotoxicity. There was one sotorasib-related death in the sequential therapy arm, which was due to toxic epidermal necrosis. No deaths occurred in the control group.
The two groups were balanced with respect to history of daily alcohol consumption and the presence of liver metastasis. More patients in the control arm had a history of hepatobiliary disease.
The study received no outside funding. Many of the authors report ties with pharmaceutical companies, including to Amgen, the maker of sotorasib, and Mirati Therapeutics, the maker of adagrasib. Dr. Weiss was an adagrasib investigator for Mirati. Dr. West is a regular contribiutor to Medscape and is an adviser for Amgen and Mirati as well as a speaker for Amgen. Dr. Borghaei reported extensive company ties. He has received research support, travel funding, and consulting fees from Amgen as well as consulting fees from Mirati.
A version of this article first appeared on Medscape.com.
because of the risk of increased toxicity.
Sotorasib is indicated for adults with locally advanced or metastatic NSCLC who carry a KRASG12C mutation, which occurs in about 13% of cases.
Since its approval in 2021, sotorasib has emerged as “a new standard of care” for such patients after chemotherapy and anti–PD-L1 failure, the investigators say.
The new warning comes after the team compared 48 patients who received an anti–PD-L1 – most often pembrolizumab alone or in combination with platinum-based chemotherapy – before sotorasib with a control group of 54 patients who either didn’t receive an anti–PD-L1 before sotorasib or had at least one other treatment in between.
The team found that sequential anti–PD-L1 and sotorasib therapy significantly increased the risk of severe sotorasib-related hepatotoxicity and also the risk of non-liver adverse events, typically in patients who received sotorasib within 30 days of an anti–PD-L1.
“We suggest avoiding starting sotorasib within 30 days from the last anti–PD-(L)1 infusion,” say senior author Michaël Duruisseaux, MD, PhD, Louis Pradel Hospital, Bron, France, and collegues.
The findings should also “prompt a close monitoring for the development of hepatotoxicity and non-liver AEs [in] patients who receive sotorasib after anti–PD-(L)1,” they add.
The study was published in the Journal of Thoracic Oncology.
Actionable findings
“I consider the results to be highly credible and informative to my own practice,” said Jack West, MD, a thoracic medical oncologist at the City of Hope outside of Los Angeles, said in an interview.
The findings “may lead me to favor a trial of docetaxel as an intervening therapy for patients who have very recently discontinued immunotherapy, deferring sotorasib at least a few weeks and ideally several months,” Dr. West commented. “I think this is a particularly reasonable approach when we remember that sotorasib conferred no improvement in overall survival at all over docetaxel in the CodeBreaK 200 trial in KRASG12C-mutated NSCLC.”
Overall, the study “corroborates what we’ve seen in the limited first-line experience of sotorasib combined with immunotherapy and also echoes our experience of other targeted therapies, such as osimertinib administered in the weeks just after patients received immunotherapy, which is known to be associated with life-threatening pneumonitis,” he said.
Jared Weiss, MD, a thoracic medical oncologist at the University of North Carolina, Chapel Hill, said that given the long half-life of immune checkpoint inhibitors, “it is quite understandable that the toxicity challenges we previously saw with concurrent administration of immunotherapy and certain targeted therapies would be recapitulated in patients who had a relatively short interval between prior checkpoint inhibitor therapy and sotorasib.”
Even so, because of the aggressiveness of NSCLC, long treatment delays between immunotherapy and sotorasib therapy are “not a favored option.”
Like Dr. West, Dr. Weiss said docetaxel (with or without ramucirumab) is a sound intervening alternative.
Another option is to use adagrasib in the second line instead of sotorasib, Dr. Weiss suggested. It’s also a KRASG12C inhibitor but hasn’t so far been associated with severe hepatotoxicity, he said.
Hossein Borghaei, DO, a thoracic medical oncologist at Fox Chase Cancer Center, Philadelphia, agrees with his colleagues and thinks that what the French team found “is real.”
As the investigators suggest, “it might be that sotorasib leads to an inflammatory microenvironment that causes hepatotoxicity in the presence of a checkpoint inhibitor. In that case,” a lower dose of sotorasib might help reduce toxicity while remaining effective, Dr. Broghaei suggested.
Study details
The French team was prompted to investigate the issue by a report of life-threatening hepatitis in a patient with NSCLC for whom sotorasib therapy was initiated 14 weeks after treatment with pembrolizumab, as well as by “the long story of adverse events ... observed with sequential use of [immune checkpoint inhibitors] and targeted therapy.”
Like Dr. Weiss, they note that severe hepatotoxicity after anti–PD-L1 therapy has not, to date, been reported for other KRASG12C inhibitors.
Patients in the study were treated outside of clinical trials at 16 medical centers in France.
Half of the patients (24/48) who were treated immediately with an anti–PD-L1 after sotorasib therapy developed grade 3 or higher sotorasib-related adverse events, including 16 (33%) with severe sotorasib-related hepatotoxicity. Severe diarrhea and fatigue were also more frequent with sequential therapy.
Severe events typically occurred within 30 days of the last anti–PD-L1 infusion and to a lesser extent within 31-60 days.
In the control arm, the rate of severe sotorasib-related adverse events was 13% (7/54). Six patients (11%) experienced severe hepatotoxicity. There was one sotorasib-related death in the sequential therapy arm, which was due to toxic epidermal necrosis. No deaths occurred in the control group.
The two groups were balanced with respect to history of daily alcohol consumption and the presence of liver metastasis. More patients in the control arm had a history of hepatobiliary disease.
The study received no outside funding. Many of the authors report ties with pharmaceutical companies, including to Amgen, the maker of sotorasib, and Mirati Therapeutics, the maker of adagrasib. Dr. Weiss was an adagrasib investigator for Mirati. Dr. West is a regular contribiutor to Medscape and is an adviser for Amgen and Mirati as well as a speaker for Amgen. Dr. Borghaei reported extensive company ties. He has received research support, travel funding, and consulting fees from Amgen as well as consulting fees from Mirati.
A version of this article first appeared on Medscape.com.
because of the risk of increased toxicity.
Sotorasib is indicated for adults with locally advanced or metastatic NSCLC who carry a KRASG12C mutation, which occurs in about 13% of cases.
Since its approval in 2021, sotorasib has emerged as “a new standard of care” for such patients after chemotherapy and anti–PD-L1 failure, the investigators say.
The new warning comes after the team compared 48 patients who received an anti–PD-L1 – most often pembrolizumab alone or in combination with platinum-based chemotherapy – before sotorasib with a control group of 54 patients who either didn’t receive an anti–PD-L1 before sotorasib or had at least one other treatment in between.
The team found that sequential anti–PD-L1 and sotorasib therapy significantly increased the risk of severe sotorasib-related hepatotoxicity and also the risk of non-liver adverse events, typically in patients who received sotorasib within 30 days of an anti–PD-L1.
“We suggest avoiding starting sotorasib within 30 days from the last anti–PD-(L)1 infusion,” say senior author Michaël Duruisseaux, MD, PhD, Louis Pradel Hospital, Bron, France, and collegues.
The findings should also “prompt a close monitoring for the development of hepatotoxicity and non-liver AEs [in] patients who receive sotorasib after anti–PD-(L)1,” they add.
The study was published in the Journal of Thoracic Oncology.
Actionable findings
“I consider the results to be highly credible and informative to my own practice,” said Jack West, MD, a thoracic medical oncologist at the City of Hope outside of Los Angeles, said in an interview.
The findings “may lead me to favor a trial of docetaxel as an intervening therapy for patients who have very recently discontinued immunotherapy, deferring sotorasib at least a few weeks and ideally several months,” Dr. West commented. “I think this is a particularly reasonable approach when we remember that sotorasib conferred no improvement in overall survival at all over docetaxel in the CodeBreaK 200 trial in KRASG12C-mutated NSCLC.”
Overall, the study “corroborates what we’ve seen in the limited first-line experience of sotorasib combined with immunotherapy and also echoes our experience of other targeted therapies, such as osimertinib administered in the weeks just after patients received immunotherapy, which is known to be associated with life-threatening pneumonitis,” he said.
Jared Weiss, MD, a thoracic medical oncologist at the University of North Carolina, Chapel Hill, said that given the long half-life of immune checkpoint inhibitors, “it is quite understandable that the toxicity challenges we previously saw with concurrent administration of immunotherapy and certain targeted therapies would be recapitulated in patients who had a relatively short interval between prior checkpoint inhibitor therapy and sotorasib.”
Even so, because of the aggressiveness of NSCLC, long treatment delays between immunotherapy and sotorasib therapy are “not a favored option.”
Like Dr. West, Dr. Weiss said docetaxel (with or without ramucirumab) is a sound intervening alternative.
Another option is to use adagrasib in the second line instead of sotorasib, Dr. Weiss suggested. It’s also a KRASG12C inhibitor but hasn’t so far been associated with severe hepatotoxicity, he said.
Hossein Borghaei, DO, a thoracic medical oncologist at Fox Chase Cancer Center, Philadelphia, agrees with his colleagues and thinks that what the French team found “is real.”
As the investigators suggest, “it might be that sotorasib leads to an inflammatory microenvironment that causes hepatotoxicity in the presence of a checkpoint inhibitor. In that case,” a lower dose of sotorasib might help reduce toxicity while remaining effective, Dr. Broghaei suggested.
Study details
The French team was prompted to investigate the issue by a report of life-threatening hepatitis in a patient with NSCLC for whom sotorasib therapy was initiated 14 weeks after treatment with pembrolizumab, as well as by “the long story of adverse events ... observed with sequential use of [immune checkpoint inhibitors] and targeted therapy.”
Like Dr. Weiss, they note that severe hepatotoxicity after anti–PD-L1 therapy has not, to date, been reported for other KRASG12C inhibitors.
Patients in the study were treated outside of clinical trials at 16 medical centers in France.
Half of the patients (24/48) who were treated immediately with an anti–PD-L1 after sotorasib therapy developed grade 3 or higher sotorasib-related adverse events, including 16 (33%) with severe sotorasib-related hepatotoxicity. Severe diarrhea and fatigue were also more frequent with sequential therapy.
Severe events typically occurred within 30 days of the last anti–PD-L1 infusion and to a lesser extent within 31-60 days.
In the control arm, the rate of severe sotorasib-related adverse events was 13% (7/54). Six patients (11%) experienced severe hepatotoxicity. There was one sotorasib-related death in the sequential therapy arm, which was due to toxic epidermal necrosis. No deaths occurred in the control group.
The two groups were balanced with respect to history of daily alcohol consumption and the presence of liver metastasis. More patients in the control arm had a history of hepatobiliary disease.
The study received no outside funding. Many of the authors report ties with pharmaceutical companies, including to Amgen, the maker of sotorasib, and Mirati Therapeutics, the maker of adagrasib. Dr. Weiss was an adagrasib investigator for Mirati. Dr. West is a regular contribiutor to Medscape and is an adviser for Amgen and Mirati as well as a speaker for Amgen. Dr. Borghaei reported extensive company ties. He has received research support, travel funding, and consulting fees from Amgen as well as consulting fees from Mirati.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THORACIC ONCOLOGY
Posluma approved for PET imaging in prostate cancer
The product is approved for use in men with suspected metastasis who are candidates for definitive therapy and for men with suspected recurrence, as evidenced by elevations in serum prostate-specific antigen (PSA) level, according to a press release from marketer Blue Earth Diagnostics.
Posluma binds prostate-specific membrane antigen (PSMA), which is usually overexpressed on prostate cancer cells, and tags the cells with fluorine-18 (F18), a positron emitter. Because of the radiolabeling, PET imaging can be used to gauge the extent of disease.
Posluma will be available in the United States in June 2023 from Blue Earth’s U.S. manufacturer and distributor, PETNET Solutions.
Blue Earth says that its new agent, which was known as 18F-rhPSMA-7.3 PET during trials, “is the first and only FDA-approved, PSMA-targeted imaging agent developed with proprietary radiohybrid technology.”
However, a similar product is currently on the U.S. market – the PSMA PET imaging radiopharmaceutical gallium-68 gozetotide (Illuccix, Locometz), which has the same two indications. Gozetotide is also indicated for metastatic prostate cancer amenable to lutetium Lu 177 vipivotide tetraxetan PSMA-directed therapy.
Approval based on two single-arm trials
Posluma’s approval was based on two single-arm trials from Blue Earth.
In the LIGHTHOUSE trial, 296 men underwent Posluma PET imaging before radical prostatectomy with pelvic lymph node dissection. About a quarter turned out to have positive nodes on pathology.
Posluma’s sensitivity for predicting positive nodes was low, ranging from 23% to 30% among three readers who were blinded to clinical information, but its specificity was high, ranging from 93% to 97%, according to the product labeling.
“The study showed that Posluma PET provided clinically valuable information prior to surgery that would likely result in management changes for these patients,” said investigator Brian Chapin, MD, a urologist at the University of Texas MD Anderson Cancer Center, Houston, in the company press release.
The second trial, SPOTLIGHT, included 389 men suspected of experiencing recurrence on the basis of elevations in PSA.
Posluma PET’s ability to detect true recurrence was compared with use of histology or other imaging techniques, including CT, MRI, technetium-99m bone scan, and fluciclovine F18 PET. In regions deemed positive for recurrence on Posluma PET by three readers, 46%-60% were positive by the other techniques, the labeling says.
Overall, the “results demonstrated high detection rates ... even at low PSA levels,” Blue Earth said.
Adverse events were minimal in the trials. The most frequent were diarrhea (0.7%), increases in blood pressure (0.5%), and injection-site pain (0.4%).
The product labeling warns that Posluma PET contributes to patients’ overall long-term cumulative radiation exposure and that interpretation with respect to recurrence may differ among readers.
The labeling also cautions that “a negative image does not rule out the presence of prostate cancer and a positive image does not confirm the presence of prostate cancer. ... Uptake is not specific for prostate cancer and may occur in other types of cancer, in nonmalignant processes, and in normal tissues.”
In addition, it notes that androgen deprivation therapy “and other therapies targeting the androgen pathway, such as androgen receptor antagonists, may result in changes in uptake of flotufolastat F18 in prostate cancer.”
The labeling for gozetotide carries the same warnings and precautions.
A version of this article first appeared on Medscape.com.
The product is approved for use in men with suspected metastasis who are candidates for definitive therapy and for men with suspected recurrence, as evidenced by elevations in serum prostate-specific antigen (PSA) level, according to a press release from marketer Blue Earth Diagnostics.
Posluma binds prostate-specific membrane antigen (PSMA), which is usually overexpressed on prostate cancer cells, and tags the cells with fluorine-18 (F18), a positron emitter. Because of the radiolabeling, PET imaging can be used to gauge the extent of disease.
Posluma will be available in the United States in June 2023 from Blue Earth’s U.S. manufacturer and distributor, PETNET Solutions.
Blue Earth says that its new agent, which was known as 18F-rhPSMA-7.3 PET during trials, “is the first and only FDA-approved, PSMA-targeted imaging agent developed with proprietary radiohybrid technology.”
However, a similar product is currently on the U.S. market – the PSMA PET imaging radiopharmaceutical gallium-68 gozetotide (Illuccix, Locometz), which has the same two indications. Gozetotide is also indicated for metastatic prostate cancer amenable to lutetium Lu 177 vipivotide tetraxetan PSMA-directed therapy.
Approval based on two single-arm trials
Posluma’s approval was based on two single-arm trials from Blue Earth.
In the LIGHTHOUSE trial, 296 men underwent Posluma PET imaging before radical prostatectomy with pelvic lymph node dissection. About a quarter turned out to have positive nodes on pathology.
Posluma’s sensitivity for predicting positive nodes was low, ranging from 23% to 30% among three readers who were blinded to clinical information, but its specificity was high, ranging from 93% to 97%, according to the product labeling.
“The study showed that Posluma PET provided clinically valuable information prior to surgery that would likely result in management changes for these patients,” said investigator Brian Chapin, MD, a urologist at the University of Texas MD Anderson Cancer Center, Houston, in the company press release.
The second trial, SPOTLIGHT, included 389 men suspected of experiencing recurrence on the basis of elevations in PSA.
Posluma PET’s ability to detect true recurrence was compared with use of histology or other imaging techniques, including CT, MRI, technetium-99m bone scan, and fluciclovine F18 PET. In regions deemed positive for recurrence on Posluma PET by three readers, 46%-60% were positive by the other techniques, the labeling says.
Overall, the “results demonstrated high detection rates ... even at low PSA levels,” Blue Earth said.
Adverse events were minimal in the trials. The most frequent were diarrhea (0.7%), increases in blood pressure (0.5%), and injection-site pain (0.4%).
The product labeling warns that Posluma PET contributes to patients’ overall long-term cumulative radiation exposure and that interpretation with respect to recurrence may differ among readers.
The labeling also cautions that “a negative image does not rule out the presence of prostate cancer and a positive image does not confirm the presence of prostate cancer. ... Uptake is not specific for prostate cancer and may occur in other types of cancer, in nonmalignant processes, and in normal tissues.”
In addition, it notes that androgen deprivation therapy “and other therapies targeting the androgen pathway, such as androgen receptor antagonists, may result in changes in uptake of flotufolastat F18 in prostate cancer.”
The labeling for gozetotide carries the same warnings and precautions.
A version of this article first appeared on Medscape.com.
The product is approved for use in men with suspected metastasis who are candidates for definitive therapy and for men with suspected recurrence, as evidenced by elevations in serum prostate-specific antigen (PSA) level, according to a press release from marketer Blue Earth Diagnostics.
Posluma binds prostate-specific membrane antigen (PSMA), which is usually overexpressed on prostate cancer cells, and tags the cells with fluorine-18 (F18), a positron emitter. Because of the radiolabeling, PET imaging can be used to gauge the extent of disease.
Posluma will be available in the United States in June 2023 from Blue Earth’s U.S. manufacturer and distributor, PETNET Solutions.
Blue Earth says that its new agent, which was known as 18F-rhPSMA-7.3 PET during trials, “is the first and only FDA-approved, PSMA-targeted imaging agent developed with proprietary radiohybrid technology.”
However, a similar product is currently on the U.S. market – the PSMA PET imaging radiopharmaceutical gallium-68 gozetotide (Illuccix, Locometz), which has the same two indications. Gozetotide is also indicated for metastatic prostate cancer amenable to lutetium Lu 177 vipivotide tetraxetan PSMA-directed therapy.
Approval based on two single-arm trials
Posluma’s approval was based on two single-arm trials from Blue Earth.
In the LIGHTHOUSE trial, 296 men underwent Posluma PET imaging before radical prostatectomy with pelvic lymph node dissection. About a quarter turned out to have positive nodes on pathology.
Posluma’s sensitivity for predicting positive nodes was low, ranging from 23% to 30% among three readers who were blinded to clinical information, but its specificity was high, ranging from 93% to 97%, according to the product labeling.
“The study showed that Posluma PET provided clinically valuable information prior to surgery that would likely result in management changes for these patients,” said investigator Brian Chapin, MD, a urologist at the University of Texas MD Anderson Cancer Center, Houston, in the company press release.
The second trial, SPOTLIGHT, included 389 men suspected of experiencing recurrence on the basis of elevations in PSA.
Posluma PET’s ability to detect true recurrence was compared with use of histology or other imaging techniques, including CT, MRI, technetium-99m bone scan, and fluciclovine F18 PET. In regions deemed positive for recurrence on Posluma PET by three readers, 46%-60% were positive by the other techniques, the labeling says.
Overall, the “results demonstrated high detection rates ... even at low PSA levels,” Blue Earth said.
Adverse events were minimal in the trials. The most frequent were diarrhea (0.7%), increases in blood pressure (0.5%), and injection-site pain (0.4%).
The product labeling warns that Posluma PET contributes to patients’ overall long-term cumulative radiation exposure and that interpretation with respect to recurrence may differ among readers.
The labeling also cautions that “a negative image does not rule out the presence of prostate cancer and a positive image does not confirm the presence of prostate cancer. ... Uptake is not specific for prostate cancer and may occur in other types of cancer, in nonmalignant processes, and in normal tissues.”
In addition, it notes that androgen deprivation therapy “and other therapies targeting the androgen pathway, such as androgen receptor antagonists, may result in changes in uptake of flotufolastat F18 in prostate cancer.”
The labeling for gozetotide carries the same warnings and precautions.
A version of this article first appeared on Medscape.com.
Regular exercise may boost pain tolerance
new research suggests.
In a large observational study of more than 10,000 adults, researchers found those who consistently engage in moderate to vigorous physical activity over the 7- to 8-year study period reported the highest pain tolerance. However, the results also showed that even light exercise was associated with greater pain tolerance.
“There were indications that both total amount of physical activity over time, as well as the direction of change in activity level over time matters to how high your pain tolerance is,” lead investigator Anders Pedersen Årnes, PT, MPH, research fellow and adviser at the University Hospital of North Norway, affiliated with the University of Tromsø, said in an interview. “As an observational study, this points toward the possibility that increased physical activity might increase pain tolerance.”
The findings were published online in PLOS One.
Anything is better than nothing
The researchers drew from the prospective, population-based Tromsø health study, a health survey that draws on surveys conducted periodically since 1974 among residents in northern Norway.
The study included 10,732 participants who completed surveys in 2007-2008 and again in 2015-2016.
Data on physical activity, experimental pain tolerance, sex, sociodemographic covariates, and chronic pain was collected through questionnaires, biological samples and clinical examination.
Pain tolerance was measured using the cold-pressor test (CPT), in which participants submerge their hand in icy water for as long as possible.
CPT tolerance was 7%, 14%, and 16% higher respectively for light, moderate, and vigorous consistent exercise across the two surveys versus the sedentary group.
“Engaging in habitual physical activity in leisure time is associated with higher pain tolerance,” Mr. Årnes said. “Any kind of activity over time is better than being sedentary.”
The researchers also found that people who were sedentary at baseline who reported greater physical activity at follow-up also had higher pain tolerance than those who remained sedentary, although this finding was not statistically significant.
This highest pain tolerance was noted in people who engaged in moderate to vigorous exercise over time, with a 20.4-second longer performance in the CPT than those who were consistently sedentary (P < .001; 95% confidence interval, 13.7-27.1).
There was no significant difference in pain tolerance between men and women and all participants experienced a decline in tolerance over time.
“Results indicate that a positive change in physical activity level over time was associated with higher pain tolerance,” Mr. Årnes said. “Your total activity level might decide how much, as more seems to be better.”
More work needed
The long follow-up and large number of patients are two strengths of the study, Steven Cohen, MD, chief of pain medicine and professor of anesthesiology, neurology, physical medicine & rehabilitation and psychiatry at Johns Hopkins University, Baltimore, said in an interview.
“This study explored the relationship between general physical activity levels and one form of acute pain, but data from other studies show a benefit for other forms of pain,” said Dr. Cohen, who was not part of the research. “Taken together, this suggests that exercise is beneficial for individuals living with pain.”
The findings demonstrate an association between exercise and pain tolerance and other research has shown evidence of a cause-and-effect relationship, Dr. Cohen said. However, “more work is needed to determine what mediates these effects.”
Questions also remain about how exercise might impact tolerance or risk for chronic pain, he added.
Investigators are now working on a follow-up study of how the effect of exercise on pain tolerance might influence chronic pain risk, Mr. Årnes said.
The study received no specific funding. Mr. Årnes and Dr. Cohen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In a large observational study of more than 10,000 adults, researchers found those who consistently engage in moderate to vigorous physical activity over the 7- to 8-year study period reported the highest pain tolerance. However, the results also showed that even light exercise was associated with greater pain tolerance.
“There were indications that both total amount of physical activity over time, as well as the direction of change in activity level over time matters to how high your pain tolerance is,” lead investigator Anders Pedersen Årnes, PT, MPH, research fellow and adviser at the University Hospital of North Norway, affiliated with the University of Tromsø, said in an interview. “As an observational study, this points toward the possibility that increased physical activity might increase pain tolerance.”
The findings were published online in PLOS One.
Anything is better than nothing
The researchers drew from the prospective, population-based Tromsø health study, a health survey that draws on surveys conducted periodically since 1974 among residents in northern Norway.
The study included 10,732 participants who completed surveys in 2007-2008 and again in 2015-2016.
Data on physical activity, experimental pain tolerance, sex, sociodemographic covariates, and chronic pain was collected through questionnaires, biological samples and clinical examination.
Pain tolerance was measured using the cold-pressor test (CPT), in which participants submerge their hand in icy water for as long as possible.
CPT tolerance was 7%, 14%, and 16% higher respectively for light, moderate, and vigorous consistent exercise across the two surveys versus the sedentary group.
“Engaging in habitual physical activity in leisure time is associated with higher pain tolerance,” Mr. Årnes said. “Any kind of activity over time is better than being sedentary.”
The researchers also found that people who were sedentary at baseline who reported greater physical activity at follow-up also had higher pain tolerance than those who remained sedentary, although this finding was not statistically significant.
This highest pain tolerance was noted in people who engaged in moderate to vigorous exercise over time, with a 20.4-second longer performance in the CPT than those who were consistently sedentary (P < .001; 95% confidence interval, 13.7-27.1).
There was no significant difference in pain tolerance between men and women and all participants experienced a decline in tolerance over time.
“Results indicate that a positive change in physical activity level over time was associated with higher pain tolerance,” Mr. Årnes said. “Your total activity level might decide how much, as more seems to be better.”
More work needed
The long follow-up and large number of patients are two strengths of the study, Steven Cohen, MD, chief of pain medicine and professor of anesthesiology, neurology, physical medicine & rehabilitation and psychiatry at Johns Hopkins University, Baltimore, said in an interview.
“This study explored the relationship between general physical activity levels and one form of acute pain, but data from other studies show a benefit for other forms of pain,” said Dr. Cohen, who was not part of the research. “Taken together, this suggests that exercise is beneficial for individuals living with pain.”
The findings demonstrate an association between exercise and pain tolerance and other research has shown evidence of a cause-and-effect relationship, Dr. Cohen said. However, “more work is needed to determine what mediates these effects.”
Questions also remain about how exercise might impact tolerance or risk for chronic pain, he added.
Investigators are now working on a follow-up study of how the effect of exercise on pain tolerance might influence chronic pain risk, Mr. Årnes said.
The study received no specific funding. Mr. Årnes and Dr. Cohen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In a large observational study of more than 10,000 adults, researchers found those who consistently engage in moderate to vigorous physical activity over the 7- to 8-year study period reported the highest pain tolerance. However, the results also showed that even light exercise was associated with greater pain tolerance.
“There were indications that both total amount of physical activity over time, as well as the direction of change in activity level over time matters to how high your pain tolerance is,” lead investigator Anders Pedersen Årnes, PT, MPH, research fellow and adviser at the University Hospital of North Norway, affiliated with the University of Tromsø, said in an interview. “As an observational study, this points toward the possibility that increased physical activity might increase pain tolerance.”
The findings were published online in PLOS One.
Anything is better than nothing
The researchers drew from the prospective, population-based Tromsø health study, a health survey that draws on surveys conducted periodically since 1974 among residents in northern Norway.
The study included 10,732 participants who completed surveys in 2007-2008 and again in 2015-2016.
Data on physical activity, experimental pain tolerance, sex, sociodemographic covariates, and chronic pain was collected through questionnaires, biological samples and clinical examination.
Pain tolerance was measured using the cold-pressor test (CPT), in which participants submerge their hand in icy water for as long as possible.
CPT tolerance was 7%, 14%, and 16% higher respectively for light, moderate, and vigorous consistent exercise across the two surveys versus the sedentary group.
“Engaging in habitual physical activity in leisure time is associated with higher pain tolerance,” Mr. Årnes said. “Any kind of activity over time is better than being sedentary.”
The researchers also found that people who were sedentary at baseline who reported greater physical activity at follow-up also had higher pain tolerance than those who remained sedentary, although this finding was not statistically significant.
This highest pain tolerance was noted in people who engaged in moderate to vigorous exercise over time, with a 20.4-second longer performance in the CPT than those who were consistently sedentary (P < .001; 95% confidence interval, 13.7-27.1).
There was no significant difference in pain tolerance between men and women and all participants experienced a decline in tolerance over time.
“Results indicate that a positive change in physical activity level over time was associated with higher pain tolerance,” Mr. Årnes said. “Your total activity level might decide how much, as more seems to be better.”
More work needed
The long follow-up and large number of patients are two strengths of the study, Steven Cohen, MD, chief of pain medicine and professor of anesthesiology, neurology, physical medicine & rehabilitation and psychiatry at Johns Hopkins University, Baltimore, said in an interview.
“This study explored the relationship between general physical activity levels and one form of acute pain, but data from other studies show a benefit for other forms of pain,” said Dr. Cohen, who was not part of the research. “Taken together, this suggests that exercise is beneficial for individuals living with pain.”
The findings demonstrate an association between exercise and pain tolerance and other research has shown evidence of a cause-and-effect relationship, Dr. Cohen said. However, “more work is needed to determine what mediates these effects.”
Questions also remain about how exercise might impact tolerance or risk for chronic pain, he added.
Investigators are now working on a follow-up study of how the effect of exercise on pain tolerance might influence chronic pain risk, Mr. Årnes said.
The study received no specific funding. Mr. Årnes and Dr. Cohen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
Weight-control surgery surging among children and teens, report says
.
Metabolic and bariatric surgeries have been on the rise among youths aged 10 to 19 since 2016, the report says. From 2020 to 2021, the number shot up by 19%.
The procedures change parts of the digestive system, helping the person feel more full and less hungry – thereby contributing to weight loss.
Among American children, obesity affects 20%, or 15 million people between 2 and 19. In addition, more are becoming afflicted with severe obesity, with a body mass index 20% higher than the marker for obesity.
“Behavioral lifestyle interventions alone do not result in long-term, clinically important weight loss among youth with severe obesity,” the study’s authors wrote. “Metabolic and bariatric surgery (MBS) is a safe and effective treatment.”
The American Academy of Pediatrics updated its guidelines for the treatment of obesity this year for the first time in 15 years, CNN reported. “The new guidelines urge prompt use of behavior therapy and lifestyle changes and, for the first time, recommend surgery and medications for some young people,” CNN wrote.
Black and Hispanic children have higher rates of childhood obesity, the CDC says. Use of surgeries rose 42% among Black youths and 53% among Hispanic youths between 2020 and 2021.
A version of this article first appeared on WebMD.com.
.
Metabolic and bariatric surgeries have been on the rise among youths aged 10 to 19 since 2016, the report says. From 2020 to 2021, the number shot up by 19%.
The procedures change parts of the digestive system, helping the person feel more full and less hungry – thereby contributing to weight loss.
Among American children, obesity affects 20%, or 15 million people between 2 and 19. In addition, more are becoming afflicted with severe obesity, with a body mass index 20% higher than the marker for obesity.
“Behavioral lifestyle interventions alone do not result in long-term, clinically important weight loss among youth with severe obesity,” the study’s authors wrote. “Metabolic and bariatric surgery (MBS) is a safe and effective treatment.”
The American Academy of Pediatrics updated its guidelines for the treatment of obesity this year for the first time in 15 years, CNN reported. “The new guidelines urge prompt use of behavior therapy and lifestyle changes and, for the first time, recommend surgery and medications for some young people,” CNN wrote.
Black and Hispanic children have higher rates of childhood obesity, the CDC says. Use of surgeries rose 42% among Black youths and 53% among Hispanic youths between 2020 and 2021.
A version of this article first appeared on WebMD.com.
.
Metabolic and bariatric surgeries have been on the rise among youths aged 10 to 19 since 2016, the report says. From 2020 to 2021, the number shot up by 19%.
The procedures change parts of the digestive system, helping the person feel more full and less hungry – thereby contributing to weight loss.
Among American children, obesity affects 20%, or 15 million people between 2 and 19. In addition, more are becoming afflicted with severe obesity, with a body mass index 20% higher than the marker for obesity.
“Behavioral lifestyle interventions alone do not result in long-term, clinically important weight loss among youth with severe obesity,” the study’s authors wrote. “Metabolic and bariatric surgery (MBS) is a safe and effective treatment.”
The American Academy of Pediatrics updated its guidelines for the treatment of obesity this year for the first time in 15 years, CNN reported. “The new guidelines urge prompt use of behavior therapy and lifestyle changes and, for the first time, recommend surgery and medications for some young people,” CNN wrote.
Black and Hispanic children have higher rates of childhood obesity, the CDC says. Use of surgeries rose 42% among Black youths and 53% among Hispanic youths between 2020 and 2021.
A version of this article first appeared on WebMD.com.
FROM JAMA PEDIATRICS
What's your diagnosis?
Answer to ‘What’s your diagnosis?’: Gastric adenocarcinoma and proximal polyposis of the stomach syndrome.
Fundic gland polyps (FGPs) are the most common gastric polyps and when occurring in the sporadic setting are typically benign; however, FGPs that occur in gastrointestinal polyposis syndromes such as familial adenomatosis polyposis can progress to adenocarcinoma and require surveillance. Therefore, it is important to distinguish sporadic versus syndromic fundic gland polyposis. Gastric adenocarcinoma and proximal polyposis of the stomach is a recently described condition that significantly increases the risk of developing invasive gastric adenocarcinoma from FGPs. Diagnostic criteria include (1) gastric polyposis restricted to the body and fundus with no small bowel or colonic involvement, (2) >100 gastric polyps or >30 polyps in a first-degree relative, (3) histology consistent with FGP with areas of dysplasia, (4) a family history consistent with an autosomal-dominant pattern of inheritance, and (5) exclusion of other syndromes and proton pump inhibitor use.1 Unlike familial adenomatosis polyposis, the polyposis is restricted to the oxyntic mucosa of the gastric body and fundus with sparing of the gastric antrum, small bowel, and colon. The genetic basis of the disease has been attributed to a point mutation in the APC gene promotor IB region leading to a loss of tumor suppressor function.2 Typical histology shows large FGPs with areas of low-grade and high-grade dysplasia, as seen in our patient.
There are few data on the natural history of gastric adenocarcinoma and proximal polyposis of the stomach, but effective surveillance is limited by the degree of polyposis. There are multiple reports of hidden adenocarcinoma on surgically resected specimens, as well as rapid progression to metastatic adenocarcinoma despite adequate diagnosis and surveillance.1,3 Therefore, total gastrectomy should be offered to patients who are surgical candidates. Our patient underwent genetic testing that revealed a point mutation in the APC promotor IB. He declined surgical intervention and opted for surveillance endoscopy every 6 months.
References
1. Worthley D.L. et al. Gut. 2012;61:774-9
2. Li J et al. Am J Hum Genet. 2016;98:830-42
3. Rudloff U. Clin Exp Gastroenterol. 2018;11:447-59
Answer to ‘What’s your diagnosis?’: Gastric adenocarcinoma and proximal polyposis of the stomach syndrome.
Fundic gland polyps (FGPs) are the most common gastric polyps and when occurring in the sporadic setting are typically benign; however, FGPs that occur in gastrointestinal polyposis syndromes such as familial adenomatosis polyposis can progress to adenocarcinoma and require surveillance. Therefore, it is important to distinguish sporadic versus syndromic fundic gland polyposis. Gastric adenocarcinoma and proximal polyposis of the stomach is a recently described condition that significantly increases the risk of developing invasive gastric adenocarcinoma from FGPs. Diagnostic criteria include (1) gastric polyposis restricted to the body and fundus with no small bowel or colonic involvement, (2) >100 gastric polyps or >30 polyps in a first-degree relative, (3) histology consistent with FGP with areas of dysplasia, (4) a family history consistent with an autosomal-dominant pattern of inheritance, and (5) exclusion of other syndromes and proton pump inhibitor use.1 Unlike familial adenomatosis polyposis, the polyposis is restricted to the oxyntic mucosa of the gastric body and fundus with sparing of the gastric antrum, small bowel, and colon. The genetic basis of the disease has been attributed to a point mutation in the APC gene promotor IB region leading to a loss of tumor suppressor function.2 Typical histology shows large FGPs with areas of low-grade and high-grade dysplasia, as seen in our patient.
There are few data on the natural history of gastric adenocarcinoma and proximal polyposis of the stomach, but effective surveillance is limited by the degree of polyposis. There are multiple reports of hidden adenocarcinoma on surgically resected specimens, as well as rapid progression to metastatic adenocarcinoma despite adequate diagnosis and surveillance.1,3 Therefore, total gastrectomy should be offered to patients who are surgical candidates. Our patient underwent genetic testing that revealed a point mutation in the APC promotor IB. He declined surgical intervention and opted for surveillance endoscopy every 6 months.
References
1. Worthley D.L. et al. Gut. 2012;61:774-9
2. Li J et al. Am J Hum Genet. 2016;98:830-42
3. Rudloff U. Clin Exp Gastroenterol. 2018;11:447-59
Answer to ‘What’s your diagnosis?’: Gastric adenocarcinoma and proximal polyposis of the stomach syndrome.
Fundic gland polyps (FGPs) are the most common gastric polyps and when occurring in the sporadic setting are typically benign; however, FGPs that occur in gastrointestinal polyposis syndromes such as familial adenomatosis polyposis can progress to adenocarcinoma and require surveillance. Therefore, it is important to distinguish sporadic versus syndromic fundic gland polyposis. Gastric adenocarcinoma and proximal polyposis of the stomach is a recently described condition that significantly increases the risk of developing invasive gastric adenocarcinoma from FGPs. Diagnostic criteria include (1) gastric polyposis restricted to the body and fundus with no small bowel or colonic involvement, (2) >100 gastric polyps or >30 polyps in a first-degree relative, (3) histology consistent with FGP with areas of dysplasia, (4) a family history consistent with an autosomal-dominant pattern of inheritance, and (5) exclusion of other syndromes and proton pump inhibitor use.1 Unlike familial adenomatosis polyposis, the polyposis is restricted to the oxyntic mucosa of the gastric body and fundus with sparing of the gastric antrum, small bowel, and colon. The genetic basis of the disease has been attributed to a point mutation in the APC gene promotor IB region leading to a loss of tumor suppressor function.2 Typical histology shows large FGPs with areas of low-grade and high-grade dysplasia, as seen in our patient.
There are few data on the natural history of gastric adenocarcinoma and proximal polyposis of the stomach, but effective surveillance is limited by the degree of polyposis. There are multiple reports of hidden adenocarcinoma on surgically resected specimens, as well as rapid progression to metastatic adenocarcinoma despite adequate diagnosis and surveillance.1,3 Therefore, total gastrectomy should be offered to patients who are surgical candidates. Our patient underwent genetic testing that revealed a point mutation in the APC promotor IB. He declined surgical intervention and opted for surveillance endoscopy every 6 months.
References
1. Worthley D.L. et al. Gut. 2012;61:774-9
2. Li J et al. Am J Hum Genet. 2016;98:830-42
3. Rudloff U. Clin Exp Gastroenterol. 2018;11:447-59
A 72-year-old man with compensated cirrhosis owing to autoimmune hepatitis presented for evaluation of an indeterminate gastric lesion found during an otherwise normal endoscopic retrograde cholangiopancreatography performed for incidental ductal dilation seen on cross-sectional imaging. He did not endorse any abdominal pain, dyspepsia, or weight loss and was not on a proton pump inhibitor. Family history was notable for a daughter diagnosed with metastatic gastric adenocarcinoma at the age of 44 years.
Upper endoscopy showed innumerable sessile polyps of variable size carpeting the gastric body and fundus (Figure A) with a large, mound-like mass lesion in the fundus (Figure A, arrow and inset). Echoendoscopy revealed a hypoechoic, noncircumferential mass restricted to the mucosal surface with well-defined borders (Figure B, arrow). A technically challenging, piecemeal endoscopic mucosal resection was performed. The patient also underwent a colonoscopy that was unremarkable. Pathology of the gastric lesion was consistent with a fundic gland polyp (Figure C, arrowheads) containing low-grade and high-grade dysplasia (Figure C, arrows).