User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Nurses under fire: The stress of medical malpractice
Just because nurses are sued less often than doctors doesn’t mean that their actions aren’t a focus of a large number of medical malpractice lawsuits. A condition known as medical malpractice stress syndrome (MMSS) is increasingly being recognized as affecting medical professionals who are subjected to litigation.
According to a 2019 report by CRICO, the risk management arm of Harvard’s medical facilities, nursing was a “primary service” in 34% of cases with a high-severity injury and in 44% of cases that were closed with a payment. And even though nurses were named as defendants only 14% of the time, likely because many nurses don’t have their own personal malpractice coverage, their hospitals or facilities were sued in most of these cases – making the nurses important witnesses for the defense.
We have every reason to believe that things have gotten worse since the CRICO study was published. Chronic nursing shortages were exacerbated during the COVID pandemic, and we have seen a large number of nurses leave the workforce altogether. In a recent survey of nurses by Hospital IQ, 90% of respondents said they were considering leaving the nursing profession in the next year, with 71% of nurses who have more than 15 years of nursing experience thinking about leaving within the next few months.
Those remaining are faced with increased workloads and extra shifts – often mandated – and working with too little sleep. Their commitment to their mission is heroic, but they are only human; it’s hard to imagine the number of errors, the number of bad outcomes, and the number of lawsuits going anywhere but up.
And of course, the entire profession has been fixated on the recent case of the Tennessee nurse who was prosecuted criminally and convicted in connection with a fatal medication error.
These are all reasons to expect that an increasing number of nurses are going to be trying to cope with symptoms of MMSS. Too many of them will initially be viewed by lawyers or claims professionals as simply defensive, arrogant, or difficult to work with. In fact, it’s impossible to know how many cases are settled just to avoid the risk of such a “difficult client” being deposed.
These caring, hard-working, and committed individuals have had their lives shaken in ways that they never expected. Nurses with MMSS need support, but traditional psychotherapy, with a diffuse focus and long-time horizon, is not the most effective option. What’s necessary is practical support that is short term, goal oriented, and tailored to the specifics of the pending litigation process.
Most important, they need to know that they are not experiencing this alone, that MMSS is a common phenomenon, and that a productive coaching relationship can be highly effective.
When approached and supported effectively, nurses – and indeed all medical professionals – can regain their confidence and focus, continue having productive professional and personal lives, and reduce the likelihood of a downhill spiral. And it makes it more likely that they’ll remain in the profession rather than becoming just another statistic in the ever-worsening shortage of nurses in the United States.
Signs of MMSS in nurses
Mixed with their feelings of anxiety and depression, nurses with MMSS often have thoughts such as:
- Am I going to lose my license?
- Am I going to lose my job?
- Will my reputation be destroyed? Will I ever be able to work as a nurse again?
- What am I going to do for a living?
- If I lose everything, will my spouse divorce me? Will I lose my kids?
- I don’t think I did anything wrong, but what if I’m still found to be at fault?
- Did I miss something? Did I make a mistake? Was there something more that I should have done?
- What’s going to happen next? What else could go wrong?
- Are there more people out there who are going to sue me?
- Everything feels overwhelming and out of control.
- My entire identity is now in question.
- How do I get this case out of my head? I can’t focus on anything else.
- I’m developing medical problems of my own.
- I’m having difficulty focusing at work and relating to patients; how do I know who’s going to sue me next?
- I wish that I could escape it all; I feel like killing myself.
Gail Fiore is president of The Winning Focus, which works with physicians and other professionals involved in litigation who are having difficulty coping with stress, anxiety, and other emotional issues. A version of this article first appeared on Medscape.com.
Just because nurses are sued less often than doctors doesn’t mean that their actions aren’t a focus of a large number of medical malpractice lawsuits. A condition known as medical malpractice stress syndrome (MMSS) is increasingly being recognized as affecting medical professionals who are subjected to litigation.
According to a 2019 report by CRICO, the risk management arm of Harvard’s medical facilities, nursing was a “primary service” in 34% of cases with a high-severity injury and in 44% of cases that were closed with a payment. And even though nurses were named as defendants only 14% of the time, likely because many nurses don’t have their own personal malpractice coverage, their hospitals or facilities were sued in most of these cases – making the nurses important witnesses for the defense.
We have every reason to believe that things have gotten worse since the CRICO study was published. Chronic nursing shortages were exacerbated during the COVID pandemic, and we have seen a large number of nurses leave the workforce altogether. In a recent survey of nurses by Hospital IQ, 90% of respondents said they were considering leaving the nursing profession in the next year, with 71% of nurses who have more than 15 years of nursing experience thinking about leaving within the next few months.
Those remaining are faced with increased workloads and extra shifts – often mandated – and working with too little sleep. Their commitment to their mission is heroic, but they are only human; it’s hard to imagine the number of errors, the number of bad outcomes, and the number of lawsuits going anywhere but up.
And of course, the entire profession has been fixated on the recent case of the Tennessee nurse who was prosecuted criminally and convicted in connection with a fatal medication error.
These are all reasons to expect that an increasing number of nurses are going to be trying to cope with symptoms of MMSS. Too many of them will initially be viewed by lawyers or claims professionals as simply defensive, arrogant, or difficult to work with. In fact, it’s impossible to know how many cases are settled just to avoid the risk of such a “difficult client” being deposed.
These caring, hard-working, and committed individuals have had their lives shaken in ways that they never expected. Nurses with MMSS need support, but traditional psychotherapy, with a diffuse focus and long-time horizon, is not the most effective option. What’s necessary is practical support that is short term, goal oriented, and tailored to the specifics of the pending litigation process.
Most important, they need to know that they are not experiencing this alone, that MMSS is a common phenomenon, and that a productive coaching relationship can be highly effective.
When approached and supported effectively, nurses – and indeed all medical professionals – can regain their confidence and focus, continue having productive professional and personal lives, and reduce the likelihood of a downhill spiral. And it makes it more likely that they’ll remain in the profession rather than becoming just another statistic in the ever-worsening shortage of nurses in the United States.
Signs of MMSS in nurses
Mixed with their feelings of anxiety and depression, nurses with MMSS often have thoughts such as:
- Am I going to lose my license?
- Am I going to lose my job?
- Will my reputation be destroyed? Will I ever be able to work as a nurse again?
- What am I going to do for a living?
- If I lose everything, will my spouse divorce me? Will I lose my kids?
- I don’t think I did anything wrong, but what if I’m still found to be at fault?
- Did I miss something? Did I make a mistake? Was there something more that I should have done?
- What’s going to happen next? What else could go wrong?
- Are there more people out there who are going to sue me?
- Everything feels overwhelming and out of control.
- My entire identity is now in question.
- How do I get this case out of my head? I can’t focus on anything else.
- I’m developing medical problems of my own.
- I’m having difficulty focusing at work and relating to patients; how do I know who’s going to sue me next?
- I wish that I could escape it all; I feel like killing myself.
Gail Fiore is president of The Winning Focus, which works with physicians and other professionals involved in litigation who are having difficulty coping with stress, anxiety, and other emotional issues. A version of this article first appeared on Medscape.com.
Just because nurses are sued less often than doctors doesn’t mean that their actions aren’t a focus of a large number of medical malpractice lawsuits. A condition known as medical malpractice stress syndrome (MMSS) is increasingly being recognized as affecting medical professionals who are subjected to litigation.
According to a 2019 report by CRICO, the risk management arm of Harvard’s medical facilities, nursing was a “primary service” in 34% of cases with a high-severity injury and in 44% of cases that were closed with a payment. And even though nurses were named as defendants only 14% of the time, likely because many nurses don’t have their own personal malpractice coverage, their hospitals or facilities were sued in most of these cases – making the nurses important witnesses for the defense.
We have every reason to believe that things have gotten worse since the CRICO study was published. Chronic nursing shortages were exacerbated during the COVID pandemic, and we have seen a large number of nurses leave the workforce altogether. In a recent survey of nurses by Hospital IQ, 90% of respondents said they were considering leaving the nursing profession in the next year, with 71% of nurses who have more than 15 years of nursing experience thinking about leaving within the next few months.
Those remaining are faced with increased workloads and extra shifts – often mandated – and working with too little sleep. Their commitment to their mission is heroic, but they are only human; it’s hard to imagine the number of errors, the number of bad outcomes, and the number of lawsuits going anywhere but up.
And of course, the entire profession has been fixated on the recent case of the Tennessee nurse who was prosecuted criminally and convicted in connection with a fatal medication error.
These are all reasons to expect that an increasing number of nurses are going to be trying to cope with symptoms of MMSS. Too many of them will initially be viewed by lawyers or claims professionals as simply defensive, arrogant, or difficult to work with. In fact, it’s impossible to know how many cases are settled just to avoid the risk of such a “difficult client” being deposed.
These caring, hard-working, and committed individuals have had their lives shaken in ways that they never expected. Nurses with MMSS need support, but traditional psychotherapy, with a diffuse focus and long-time horizon, is not the most effective option. What’s necessary is practical support that is short term, goal oriented, and tailored to the specifics of the pending litigation process.
Most important, they need to know that they are not experiencing this alone, that MMSS is a common phenomenon, and that a productive coaching relationship can be highly effective.
When approached and supported effectively, nurses – and indeed all medical professionals – can regain their confidence and focus, continue having productive professional and personal lives, and reduce the likelihood of a downhill spiral. And it makes it more likely that they’ll remain in the profession rather than becoming just another statistic in the ever-worsening shortage of nurses in the United States.
Signs of MMSS in nurses
Mixed with their feelings of anxiety and depression, nurses with MMSS often have thoughts such as:
- Am I going to lose my license?
- Am I going to lose my job?
- Will my reputation be destroyed? Will I ever be able to work as a nurse again?
- What am I going to do for a living?
- If I lose everything, will my spouse divorce me? Will I lose my kids?
- I don’t think I did anything wrong, but what if I’m still found to be at fault?
- Did I miss something? Did I make a mistake? Was there something more that I should have done?
- What’s going to happen next? What else could go wrong?
- Are there more people out there who are going to sue me?
- Everything feels overwhelming and out of control.
- My entire identity is now in question.
- How do I get this case out of my head? I can’t focus on anything else.
- I’m developing medical problems of my own.
- I’m having difficulty focusing at work and relating to patients; how do I know who’s going to sue me next?
- I wish that I could escape it all; I feel like killing myself.
Gail Fiore is president of The Winning Focus, which works with physicians and other professionals involved in litigation who are having difficulty coping with stress, anxiety, and other emotional issues. A version of this article first appeared on Medscape.com.
Are physician white coats becoming obsolete? How docs dress for work now
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
How to make visits run more smoothly and be more productive
We all feel pressure from not having enough time to care for our patients the way we want to.
Organ recital
Some of our patients need to share an update on all their symptoms each visit, old and new, including those that are minor or possibly concerning. I have learned over the years that, for many patients, this allows them to release the worry about symptoms.
Some symptoms are so distressing and severe that symptomatic treatment is needed, but most aren’t.
I am very honest with patients when I have no idea what is causing their symptoms. I tell them, we will watch for other clues to see if the symptom needs a workup.
One thing I don’t do, and I strongly recommend against, is doing a review of systems. This leads a patient to believe you are concerned about exploring each possible symptom, ones that they didn’t even bring up! The yield is very low, and the time commitment is great.
The angry patient
Imagine a scenario when you are running 15 minutes behind and, when you step into the room, your patient is angry. You are already behind, and helping the patient navigate their anger will be part of your clinic visit.
In these situations, I always address the patient’s anger immediately. Problems with getting appointments with specialists, delays in diagnostic tests, or a broken entry to the parking garage have all been sources of my patients’ frustrations.
When we have limited time, using much of the clinic visit to process frustration leads to empty clinic visits. I listen and work to empathize with the patient, often agreeing that there are so many messed up aspects of the health care system. I do not like to use the corporate “I am sad you feel that way” response, because I feel it is not helpful. Instead, I tell them how much I want to help them today in any way I can at this visit.
The Internet sleuth
When our patients have new symptoms, some of them will go to the Internet to try to self-diagnose. Sometimes they make a correct diagnosis, but other times consider scary diagnoses we would not consider based on their symptoms and risk factors.
In these scenarios, I always ask the patient why they think their diagnosis is accurate. Their response to this question gives me insight into where their beliefs come from and helps me understand what information I need to provide.
McMullan said physicians can be defensive, collaborative, and informative when they interact with patients about information they have found on the Internet. In the first model, the physician is authoritative. The second involves working with the patient and obtaining and analyzing information. In the third model, the physician provides reputable internet sites to patients for obtaining information.
‘Oh, by the way’
Patients frequently bring up sensitive topics or complicated requests after the visit has wrapped up. Topics such as insomnia, erectile dysfunction, and anxiety are often brought up with the assumption that a quick prescription is the answer. For many years, I would add time to the appointment and try to address these issues as quickly as I could. But I invariably did a poor job at helping with these problems. Now, I offer to see the patient back soon to spend an entire visit discussing the newly brought up concern. I tell them that the problem is too important to not have my full attention and focus.
Pearls
- Empathetically listen to descriptions of symptoms, but don’t focus on fixing them.
- Empathize with the angry patient, and move on to taking care of their medical problems.
- Avoid the urge to address newly raised problems at the end of the visit.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose.
We all feel pressure from not having enough time to care for our patients the way we want to.
Organ recital
Some of our patients need to share an update on all their symptoms each visit, old and new, including those that are minor or possibly concerning. I have learned over the years that, for many patients, this allows them to release the worry about symptoms.
Some symptoms are so distressing and severe that symptomatic treatment is needed, but most aren’t.
I am very honest with patients when I have no idea what is causing their symptoms. I tell them, we will watch for other clues to see if the symptom needs a workup.
One thing I don’t do, and I strongly recommend against, is doing a review of systems. This leads a patient to believe you are concerned about exploring each possible symptom, ones that they didn’t even bring up! The yield is very low, and the time commitment is great.
The angry patient
Imagine a scenario when you are running 15 minutes behind and, when you step into the room, your patient is angry. You are already behind, and helping the patient navigate their anger will be part of your clinic visit.
In these situations, I always address the patient’s anger immediately. Problems with getting appointments with specialists, delays in diagnostic tests, or a broken entry to the parking garage have all been sources of my patients’ frustrations.
When we have limited time, using much of the clinic visit to process frustration leads to empty clinic visits. I listen and work to empathize with the patient, often agreeing that there are so many messed up aspects of the health care system. I do not like to use the corporate “I am sad you feel that way” response, because I feel it is not helpful. Instead, I tell them how much I want to help them today in any way I can at this visit.
The Internet sleuth
When our patients have new symptoms, some of them will go to the Internet to try to self-diagnose. Sometimes they make a correct diagnosis, but other times consider scary diagnoses we would not consider based on their symptoms and risk factors.
In these scenarios, I always ask the patient why they think their diagnosis is accurate. Their response to this question gives me insight into where their beliefs come from and helps me understand what information I need to provide.
McMullan said physicians can be defensive, collaborative, and informative when they interact with patients about information they have found on the Internet. In the first model, the physician is authoritative. The second involves working with the patient and obtaining and analyzing information. In the third model, the physician provides reputable internet sites to patients for obtaining information.
‘Oh, by the way’
Patients frequently bring up sensitive topics or complicated requests after the visit has wrapped up. Topics such as insomnia, erectile dysfunction, and anxiety are often brought up with the assumption that a quick prescription is the answer. For many years, I would add time to the appointment and try to address these issues as quickly as I could. But I invariably did a poor job at helping with these problems. Now, I offer to see the patient back soon to spend an entire visit discussing the newly brought up concern. I tell them that the problem is too important to not have my full attention and focus.
Pearls
- Empathetically listen to descriptions of symptoms, but don’t focus on fixing them.
- Empathize with the angry patient, and move on to taking care of their medical problems.
- Avoid the urge to address newly raised problems at the end of the visit.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose.
We all feel pressure from not having enough time to care for our patients the way we want to.
Organ recital
Some of our patients need to share an update on all their symptoms each visit, old and new, including those that are minor or possibly concerning. I have learned over the years that, for many patients, this allows them to release the worry about symptoms.
Some symptoms are so distressing and severe that symptomatic treatment is needed, but most aren’t.
I am very honest with patients when I have no idea what is causing their symptoms. I tell them, we will watch for other clues to see if the symptom needs a workup.
One thing I don’t do, and I strongly recommend against, is doing a review of systems. This leads a patient to believe you are concerned about exploring each possible symptom, ones that they didn’t even bring up! The yield is very low, and the time commitment is great.
The angry patient
Imagine a scenario when you are running 15 minutes behind and, when you step into the room, your patient is angry. You are already behind, and helping the patient navigate their anger will be part of your clinic visit.
In these situations, I always address the patient’s anger immediately. Problems with getting appointments with specialists, delays in diagnostic tests, or a broken entry to the parking garage have all been sources of my patients’ frustrations.
When we have limited time, using much of the clinic visit to process frustration leads to empty clinic visits. I listen and work to empathize with the patient, often agreeing that there are so many messed up aspects of the health care system. I do not like to use the corporate “I am sad you feel that way” response, because I feel it is not helpful. Instead, I tell them how much I want to help them today in any way I can at this visit.
The Internet sleuth
When our patients have new symptoms, some of them will go to the Internet to try to self-diagnose. Sometimes they make a correct diagnosis, but other times consider scary diagnoses we would not consider based on their symptoms and risk factors.
In these scenarios, I always ask the patient why they think their diagnosis is accurate. Their response to this question gives me insight into where their beliefs come from and helps me understand what information I need to provide.
McMullan said physicians can be defensive, collaborative, and informative when they interact with patients about information they have found on the Internet. In the first model, the physician is authoritative. The second involves working with the patient and obtaining and analyzing information. In the third model, the physician provides reputable internet sites to patients for obtaining information.
‘Oh, by the way’
Patients frequently bring up sensitive topics or complicated requests after the visit has wrapped up. Topics such as insomnia, erectile dysfunction, and anxiety are often brought up with the assumption that a quick prescription is the answer. For many years, I would add time to the appointment and try to address these issues as quickly as I could. But I invariably did a poor job at helping with these problems. Now, I offer to see the patient back soon to spend an entire visit discussing the newly brought up concern. I tell them that the problem is too important to not have my full attention and focus.
Pearls
- Empathetically listen to descriptions of symptoms, but don’t focus on fixing them.
- Empathize with the angry patient, and move on to taking care of their medical problems.
- Avoid the urge to address newly raised problems at the end of the visit.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose.
Sex toys for science
California researchers are seeking women willing to use sex toys for science.
“We have not had good-quality studies with the use of modern vibrators,” Alexandra Dubinskaya, MD, an obstetrician who is leading the study, said in an interview.
Vibrators of various kinds have been used by women for centuries if not millennia. More than half of women in the United States have at least some experience with the devices.
Victorian-era physicians are said to have routinely prescribed multiple types of vibrators to treat “female hysteria,” although the frequency with which vibrators were recommended for therapeutic purposes has been questioned.
Still, Dr. Dubinskaya said vibrators have a long history of use as therapy – with some evidence of success.
She and her colleagues reviewed the medical literature and found that studies generally supported the use of vibrators for increased blood flow in pelvic tissues, improved sexual function, including orgasms, and possibly urinary incontinence by helping to strengthen the pelvic floor. They also appear to boost desire, arousal, and genital sensation.
For the new study, Dr. Dubinskaya and her colleagues hope to eventually include 100 women between the ages of 18 and 99 years. Each will receive a commercially available genital vibrator and instructions to use the device to reach orgasm three times per week for 3 to 4 months. The researchers will track any changes in sexual function, pelvic prolapse, urinary continence, and other measures of pelvic and sexual health.
The goal of the study, Dr. Dubinskaya said, is to provide prospective data for clinicians who might consider recommending vibrators to their patients – a list that includes urologists, gynecologists, and experts in sexual medicine.
These clinicians “are frequently the first to encounter questions on women’s sexual function, pelvic floor problems, and vulvar health,” Dr. Dubinskaya said. She noted that such questions are common.
Asking women to consider using vibrators might seem too sensitive a subject in a clinical setting, but Dr. Dubinskaya said data indicate that women are receptive to the suggestion.
Debra Lynne Herbenick, PhD, director of the Center for Sexual Health Promotion and a professor of public health at Indiana University, Indianapolis, who has studied vibrator use in the United States, said the research could make a valuable contribution to sexual health.
“This study is an important next step because it is a prospective study and will be able to assess changes in sexual and pelvic floor function over time in relation to vibrator use,” Dr. Herbenick said. Owing to the limited quality of the currently available evidence, these data have the potential “to support clinicians’ recommendations and also their communication with patients.”
Dr. Dubinskaya and Dr. Herbenick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
California researchers are seeking women willing to use sex toys for science.
“We have not had good-quality studies with the use of modern vibrators,” Alexandra Dubinskaya, MD, an obstetrician who is leading the study, said in an interview.
Vibrators of various kinds have been used by women for centuries if not millennia. More than half of women in the United States have at least some experience with the devices.
Victorian-era physicians are said to have routinely prescribed multiple types of vibrators to treat “female hysteria,” although the frequency with which vibrators were recommended for therapeutic purposes has been questioned.
Still, Dr. Dubinskaya said vibrators have a long history of use as therapy – with some evidence of success.
She and her colleagues reviewed the medical literature and found that studies generally supported the use of vibrators for increased blood flow in pelvic tissues, improved sexual function, including orgasms, and possibly urinary incontinence by helping to strengthen the pelvic floor. They also appear to boost desire, arousal, and genital sensation.
For the new study, Dr. Dubinskaya and her colleagues hope to eventually include 100 women between the ages of 18 and 99 years. Each will receive a commercially available genital vibrator and instructions to use the device to reach orgasm three times per week for 3 to 4 months. The researchers will track any changes in sexual function, pelvic prolapse, urinary continence, and other measures of pelvic and sexual health.
The goal of the study, Dr. Dubinskaya said, is to provide prospective data for clinicians who might consider recommending vibrators to their patients – a list that includes urologists, gynecologists, and experts in sexual medicine.
These clinicians “are frequently the first to encounter questions on women’s sexual function, pelvic floor problems, and vulvar health,” Dr. Dubinskaya said. She noted that such questions are common.
Asking women to consider using vibrators might seem too sensitive a subject in a clinical setting, but Dr. Dubinskaya said data indicate that women are receptive to the suggestion.
Debra Lynne Herbenick, PhD, director of the Center for Sexual Health Promotion and a professor of public health at Indiana University, Indianapolis, who has studied vibrator use in the United States, said the research could make a valuable contribution to sexual health.
“This study is an important next step because it is a prospective study and will be able to assess changes in sexual and pelvic floor function over time in relation to vibrator use,” Dr. Herbenick said. Owing to the limited quality of the currently available evidence, these data have the potential “to support clinicians’ recommendations and also their communication with patients.”
Dr. Dubinskaya and Dr. Herbenick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
California researchers are seeking women willing to use sex toys for science.
“We have not had good-quality studies with the use of modern vibrators,” Alexandra Dubinskaya, MD, an obstetrician who is leading the study, said in an interview.
Vibrators of various kinds have been used by women for centuries if not millennia. More than half of women in the United States have at least some experience with the devices.
Victorian-era physicians are said to have routinely prescribed multiple types of vibrators to treat “female hysteria,” although the frequency with which vibrators were recommended for therapeutic purposes has been questioned.
Still, Dr. Dubinskaya said vibrators have a long history of use as therapy – with some evidence of success.
She and her colleagues reviewed the medical literature and found that studies generally supported the use of vibrators for increased blood flow in pelvic tissues, improved sexual function, including orgasms, and possibly urinary incontinence by helping to strengthen the pelvic floor. They also appear to boost desire, arousal, and genital sensation.
For the new study, Dr. Dubinskaya and her colleagues hope to eventually include 100 women between the ages of 18 and 99 years. Each will receive a commercially available genital vibrator and instructions to use the device to reach orgasm three times per week for 3 to 4 months. The researchers will track any changes in sexual function, pelvic prolapse, urinary continence, and other measures of pelvic and sexual health.
The goal of the study, Dr. Dubinskaya said, is to provide prospective data for clinicians who might consider recommending vibrators to their patients – a list that includes urologists, gynecologists, and experts in sexual medicine.
These clinicians “are frequently the first to encounter questions on women’s sexual function, pelvic floor problems, and vulvar health,” Dr. Dubinskaya said. She noted that such questions are common.
Asking women to consider using vibrators might seem too sensitive a subject in a clinical setting, but Dr. Dubinskaya said data indicate that women are receptive to the suggestion.
Debra Lynne Herbenick, PhD, director of the Center for Sexual Health Promotion and a professor of public health at Indiana University, Indianapolis, who has studied vibrator use in the United States, said the research could make a valuable contribution to sexual health.
“This study is an important next step because it is a prospective study and will be able to assess changes in sexual and pelvic floor function over time in relation to vibrator use,” Dr. Herbenick said. Owing to the limited quality of the currently available evidence, these data have the potential “to support clinicians’ recommendations and also their communication with patients.”
Dr. Dubinskaya and Dr. Herbenick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study: Uterine polyp removal in office possible via ultrasound
Ultrasound-guided endometrial polypectomy could be a lower-cost, easily accessible alternative to hysteroscopy for women with abnormal uterine bleeding and polyps, researchers reported at the 2022 annual meeting of the American College of Obstetricians and Gynecologists.
The prospective study of 30 patients who underwent the experimental procedure showed that clinicians were able to remove all the polyps they identified quickly and without sedation.
The technique is a “clever way to address endometrial polyps,” said Lara Harvey, MD, MPH, a minimally invasive gynecologic surgeon at Vanderbilt University Medical Center, Nashville, Tenn., who was not involved in the study.
“If you’re a physician with access to in-office ultrasound and you’re familiar with saline infusion sonohysterogram, then this might be a useful approach without a lot of added expense, but more research is needed to validate the technique,” Dr. Harvey said in an interview.
The new technique was initially developed at the University of South Florida as an alternative to surgery for patients with medical comorbidities that placed them at an increased risk of complications with general anesthesia, according to Lauri Hochberg, MD, director of gynecologic imaging at the University of South Florida, Tampa.
However, “we found that it was effective and well-tolerated in general and began offering it to all patients with endometrial polyps, even if they were healthy and at low risk for surgical complications,” Dr. Hochberg told this news organization.
The procedure is performed by introducing pediatric grasping forceps into the uterus with ultrasound guidance. Doctors direct patients to take ibuprofen prior to the procedure, in addition to administering misoprostol intravaginally the night prior in cases of cervical stenosis. Lidocaine is also injected into the cervix and uterine cavity prior to polyp removal, both for anesthesia and to help visualize polyps on an ultrasound.
The 30 patients included in the study had polyps 5 cm or smaller in size and abnormal uterine bleeding. Dr. Hochberg said she chose 5 cm as a cut-off because larger lesions require more procedure time over potentially two visits to remove using the new approach. Patients were mean age 55 years, mean body mass index of 31, and 70% had postmenopausal bleeding.
According to Dr. Hochberg and Papri Sarkar, MD, a 4th-year resident working with her, procedures lasted an average of 12 minutes and allowed for complete polypectomy in all cases. The average polyp volume was 1.26 cm3 and pathologists found two cancerous lesions.
Patients reported median pain and satisfaction scores of 5 and 10 on 10-point scales, respectively. In addition, 13 of 16 patients who returned 3 months later for a saline infusion sonography showed no evidence of polyp recurrence and 14 patients reported complete resolution of symptoms.
Although a direct comparison of the in-office procedure and conventional hysteroscopy would help better define the role of the procedure, the findings indicate it is “safe and effective” and “would be a great tool to help patients” with abnormal uterine bleeding, Dr. Hochberg said.
“Physicians would need to learn the skill of ultrasound-guided removal, but this can be accomplished with study,” she added.
Dr. Harvey also expressed concern that because the new procedure does not allow for direct visualization of the base of the polyp, physicians may not excise the entire lesion. Providers interested in the procedure should “proceed with caution” until there are larger studies published, she said.
“I think widely deploying this technique for postmenopausal bleeding in particular, where there is a higher chance of endometrial cancer, would require really good data comparing it to the gold standard of hysteroscopy and showing that, yes, it is as good at removing polyps and also at diagnosing cancer,” Dr. Harvey said.
Dr. Harvey, Dr. Hochberg, and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ultrasound-guided endometrial polypectomy could be a lower-cost, easily accessible alternative to hysteroscopy for women with abnormal uterine bleeding and polyps, researchers reported at the 2022 annual meeting of the American College of Obstetricians and Gynecologists.
The prospective study of 30 patients who underwent the experimental procedure showed that clinicians were able to remove all the polyps they identified quickly and without sedation.
The technique is a “clever way to address endometrial polyps,” said Lara Harvey, MD, MPH, a minimally invasive gynecologic surgeon at Vanderbilt University Medical Center, Nashville, Tenn., who was not involved in the study.
“If you’re a physician with access to in-office ultrasound and you’re familiar with saline infusion sonohysterogram, then this might be a useful approach without a lot of added expense, but more research is needed to validate the technique,” Dr. Harvey said in an interview.
The new technique was initially developed at the University of South Florida as an alternative to surgery for patients with medical comorbidities that placed them at an increased risk of complications with general anesthesia, according to Lauri Hochberg, MD, director of gynecologic imaging at the University of South Florida, Tampa.
However, “we found that it was effective and well-tolerated in general and began offering it to all patients with endometrial polyps, even if they were healthy and at low risk for surgical complications,” Dr. Hochberg told this news organization.
The procedure is performed by introducing pediatric grasping forceps into the uterus with ultrasound guidance. Doctors direct patients to take ibuprofen prior to the procedure, in addition to administering misoprostol intravaginally the night prior in cases of cervical stenosis. Lidocaine is also injected into the cervix and uterine cavity prior to polyp removal, both for anesthesia and to help visualize polyps on an ultrasound.
The 30 patients included in the study had polyps 5 cm or smaller in size and abnormal uterine bleeding. Dr. Hochberg said she chose 5 cm as a cut-off because larger lesions require more procedure time over potentially two visits to remove using the new approach. Patients were mean age 55 years, mean body mass index of 31, and 70% had postmenopausal bleeding.
According to Dr. Hochberg and Papri Sarkar, MD, a 4th-year resident working with her, procedures lasted an average of 12 minutes and allowed for complete polypectomy in all cases. The average polyp volume was 1.26 cm3 and pathologists found two cancerous lesions.
Patients reported median pain and satisfaction scores of 5 and 10 on 10-point scales, respectively. In addition, 13 of 16 patients who returned 3 months later for a saline infusion sonography showed no evidence of polyp recurrence and 14 patients reported complete resolution of symptoms.
Although a direct comparison of the in-office procedure and conventional hysteroscopy would help better define the role of the procedure, the findings indicate it is “safe and effective” and “would be a great tool to help patients” with abnormal uterine bleeding, Dr. Hochberg said.
“Physicians would need to learn the skill of ultrasound-guided removal, but this can be accomplished with study,” she added.
Dr. Harvey also expressed concern that because the new procedure does not allow for direct visualization of the base of the polyp, physicians may not excise the entire lesion. Providers interested in the procedure should “proceed with caution” until there are larger studies published, she said.
“I think widely deploying this technique for postmenopausal bleeding in particular, where there is a higher chance of endometrial cancer, would require really good data comparing it to the gold standard of hysteroscopy and showing that, yes, it is as good at removing polyps and also at diagnosing cancer,” Dr. Harvey said.
Dr. Harvey, Dr. Hochberg, and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ultrasound-guided endometrial polypectomy could be a lower-cost, easily accessible alternative to hysteroscopy for women with abnormal uterine bleeding and polyps, researchers reported at the 2022 annual meeting of the American College of Obstetricians and Gynecologists.
The prospective study of 30 patients who underwent the experimental procedure showed that clinicians were able to remove all the polyps they identified quickly and without sedation.
The technique is a “clever way to address endometrial polyps,” said Lara Harvey, MD, MPH, a minimally invasive gynecologic surgeon at Vanderbilt University Medical Center, Nashville, Tenn., who was not involved in the study.
“If you’re a physician with access to in-office ultrasound and you’re familiar with saline infusion sonohysterogram, then this might be a useful approach without a lot of added expense, but more research is needed to validate the technique,” Dr. Harvey said in an interview.
The new technique was initially developed at the University of South Florida as an alternative to surgery for patients with medical comorbidities that placed them at an increased risk of complications with general anesthesia, according to Lauri Hochberg, MD, director of gynecologic imaging at the University of South Florida, Tampa.
However, “we found that it was effective and well-tolerated in general and began offering it to all patients with endometrial polyps, even if they were healthy and at low risk for surgical complications,” Dr. Hochberg told this news organization.
The procedure is performed by introducing pediatric grasping forceps into the uterus with ultrasound guidance. Doctors direct patients to take ibuprofen prior to the procedure, in addition to administering misoprostol intravaginally the night prior in cases of cervical stenosis. Lidocaine is also injected into the cervix and uterine cavity prior to polyp removal, both for anesthesia and to help visualize polyps on an ultrasound.
The 30 patients included in the study had polyps 5 cm or smaller in size and abnormal uterine bleeding. Dr. Hochberg said she chose 5 cm as a cut-off because larger lesions require more procedure time over potentially two visits to remove using the new approach. Patients were mean age 55 years, mean body mass index of 31, and 70% had postmenopausal bleeding.
According to Dr. Hochberg and Papri Sarkar, MD, a 4th-year resident working with her, procedures lasted an average of 12 minutes and allowed for complete polypectomy in all cases. The average polyp volume was 1.26 cm3 and pathologists found two cancerous lesions.
Patients reported median pain and satisfaction scores of 5 and 10 on 10-point scales, respectively. In addition, 13 of 16 patients who returned 3 months later for a saline infusion sonography showed no evidence of polyp recurrence and 14 patients reported complete resolution of symptoms.
Although a direct comparison of the in-office procedure and conventional hysteroscopy would help better define the role of the procedure, the findings indicate it is “safe and effective” and “would be a great tool to help patients” with abnormal uterine bleeding, Dr. Hochberg said.
“Physicians would need to learn the skill of ultrasound-guided removal, but this can be accomplished with study,” she added.
Dr. Harvey also expressed concern that because the new procedure does not allow for direct visualization of the base of the polyp, physicians may not excise the entire lesion. Providers interested in the procedure should “proceed with caution” until there are larger studies published, she said.
“I think widely deploying this technique for postmenopausal bleeding in particular, where there is a higher chance of endometrial cancer, would require really good data comparing it to the gold standard of hysteroscopy and showing that, yes, it is as good at removing polyps and also at diagnosing cancer,” Dr. Harvey said.
Dr. Harvey, Dr. Hochberg, and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
IUD cuts heavy menses in nulliparous patients with obesity
New phase 3 data support the use of the levonorgestrel 52-mg intrauterine device in nulliparous women with obesity and heavy menstrual bleeding. The findings, presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, showed a 97% reduction in blood loss 6 months after placement of the device, which is sold as the contraceptive Liletta by Medicines360 and AbbVie.
Experts say the results fill a gap in research because prior clinical trials of the IUD and a competitor, Mirena (Bayer), excluded significantly obese as well as nulliparous populations.
William Schlaff, MD, professor and chairman of the department of obstetrics & gynecology, Thomas Jefferson University, Philadelphia, said the absence of confirmatory evidence in these women has meant that, although use of the IUD has been “pretty widespread,” clinicians have been uncertain about the efficacy of the approach.
“Now we have objective data from a well-designed study that supports a practice that many of us have felt is probably a good one,” Dr. Schlaff, who was not involved in the new study, said in an interview.
Lead researcher Mitchell Creinin, MD, professor of obstetrics and gynecology at UC Davis Health, Sacramento, and colleagues at several centers across the country provided treatment with Liletta to 105 individuals with proven heavy menstrual bleeding. The patients’ median blood loss during two menses prior to placement of the device was 165 mL (range, 73-520 mL).
Participant demographics were: 65% White, 24% Black, 10% Hispanic, 4% Asian, and 7% who identified with other racial groups. Mean body mass index was 30.9 kg/m2, and 45% of individuals met the criteria for obesity (BMI > 30), including 13% who had a BMI of at least 40. Nearly 30% of participants in the study had never given birth and none had known medical, anatomic, infectious, or neoplastic causes of bleeding.
According to Dr. Creinin, 86 women were assessed 3 months after device placement, and their median blood loss at the time was 9.5 mL (interquartile range, 2.5-22.9 mL), representing a median 93% decrease from baseline. Median blood loss 6 months after placement of the IUD was 3.8 mL (IQR, 0-10.1 mL), a 97% reduction from baseline.
Regardless of parity or BMI, blood loss at 6 months was 97%-97.5% lower than baseline, Dr. Creinin reported.
Among the 23% of participants who did not complete the study, 4% experienced expulsions of the device, which Dr. Creinin said is a rate twice as high as that seen in women using hormone-releasing IUDs for contraception. However, he said it “is consistent with other studies among patients with quantitatively proven heavy menstrual bleeding.”
Another 6% of women who did not complete the study removed the device owing to bleeding and cramping complaints, 9% were lost to follow-up or withdrew consent, and 5% discontinued treatment for unspecified reasons, Dr. Creinin said.
“Etiologies for heavy menstrual bleeding may be different in the individuals we studied, so our findings provide assurance that these populations with heavy menstrual bleeding are equally well treated” with the IUD, Dr. Creinin said.
Dr. Creinin reported study funding from Medicines360. Dr. Schlaff reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
New phase 3 data support the use of the levonorgestrel 52-mg intrauterine device in nulliparous women with obesity and heavy menstrual bleeding. The findings, presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, showed a 97% reduction in blood loss 6 months after placement of the device, which is sold as the contraceptive Liletta by Medicines360 and AbbVie.
Experts say the results fill a gap in research because prior clinical trials of the IUD and a competitor, Mirena (Bayer), excluded significantly obese as well as nulliparous populations.
William Schlaff, MD, professor and chairman of the department of obstetrics & gynecology, Thomas Jefferson University, Philadelphia, said the absence of confirmatory evidence in these women has meant that, although use of the IUD has been “pretty widespread,” clinicians have been uncertain about the efficacy of the approach.
“Now we have objective data from a well-designed study that supports a practice that many of us have felt is probably a good one,” Dr. Schlaff, who was not involved in the new study, said in an interview.
Lead researcher Mitchell Creinin, MD, professor of obstetrics and gynecology at UC Davis Health, Sacramento, and colleagues at several centers across the country provided treatment with Liletta to 105 individuals with proven heavy menstrual bleeding. The patients’ median blood loss during two menses prior to placement of the device was 165 mL (range, 73-520 mL).
Participant demographics were: 65% White, 24% Black, 10% Hispanic, 4% Asian, and 7% who identified with other racial groups. Mean body mass index was 30.9 kg/m2, and 45% of individuals met the criteria for obesity (BMI > 30), including 13% who had a BMI of at least 40. Nearly 30% of participants in the study had never given birth and none had known medical, anatomic, infectious, or neoplastic causes of bleeding.
According to Dr. Creinin, 86 women were assessed 3 months after device placement, and their median blood loss at the time was 9.5 mL (interquartile range, 2.5-22.9 mL), representing a median 93% decrease from baseline. Median blood loss 6 months after placement of the IUD was 3.8 mL (IQR, 0-10.1 mL), a 97% reduction from baseline.
Regardless of parity or BMI, blood loss at 6 months was 97%-97.5% lower than baseline, Dr. Creinin reported.
Among the 23% of participants who did not complete the study, 4% experienced expulsions of the device, which Dr. Creinin said is a rate twice as high as that seen in women using hormone-releasing IUDs for contraception. However, he said it “is consistent with other studies among patients with quantitatively proven heavy menstrual bleeding.”
Another 6% of women who did not complete the study removed the device owing to bleeding and cramping complaints, 9% were lost to follow-up or withdrew consent, and 5% discontinued treatment for unspecified reasons, Dr. Creinin said.
“Etiologies for heavy menstrual bleeding may be different in the individuals we studied, so our findings provide assurance that these populations with heavy menstrual bleeding are equally well treated” with the IUD, Dr. Creinin said.
Dr. Creinin reported study funding from Medicines360. Dr. Schlaff reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
New phase 3 data support the use of the levonorgestrel 52-mg intrauterine device in nulliparous women with obesity and heavy menstrual bleeding. The findings, presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, showed a 97% reduction in blood loss 6 months after placement of the device, which is sold as the contraceptive Liletta by Medicines360 and AbbVie.
Experts say the results fill a gap in research because prior clinical trials of the IUD and a competitor, Mirena (Bayer), excluded significantly obese as well as nulliparous populations.
William Schlaff, MD, professor and chairman of the department of obstetrics & gynecology, Thomas Jefferson University, Philadelphia, said the absence of confirmatory evidence in these women has meant that, although use of the IUD has been “pretty widespread,” clinicians have been uncertain about the efficacy of the approach.
“Now we have objective data from a well-designed study that supports a practice that many of us have felt is probably a good one,” Dr. Schlaff, who was not involved in the new study, said in an interview.
Lead researcher Mitchell Creinin, MD, professor of obstetrics and gynecology at UC Davis Health, Sacramento, and colleagues at several centers across the country provided treatment with Liletta to 105 individuals with proven heavy menstrual bleeding. The patients’ median blood loss during two menses prior to placement of the device was 165 mL (range, 73-520 mL).
Participant demographics were: 65% White, 24% Black, 10% Hispanic, 4% Asian, and 7% who identified with other racial groups. Mean body mass index was 30.9 kg/m2, and 45% of individuals met the criteria for obesity (BMI > 30), including 13% who had a BMI of at least 40. Nearly 30% of participants in the study had never given birth and none had known medical, anatomic, infectious, or neoplastic causes of bleeding.
According to Dr. Creinin, 86 women were assessed 3 months after device placement, and their median blood loss at the time was 9.5 mL (interquartile range, 2.5-22.9 mL), representing a median 93% decrease from baseline. Median blood loss 6 months after placement of the IUD was 3.8 mL (IQR, 0-10.1 mL), a 97% reduction from baseline.
Regardless of parity or BMI, blood loss at 6 months was 97%-97.5% lower than baseline, Dr. Creinin reported.
Among the 23% of participants who did not complete the study, 4% experienced expulsions of the device, which Dr. Creinin said is a rate twice as high as that seen in women using hormone-releasing IUDs for contraception. However, he said it “is consistent with other studies among patients with quantitatively proven heavy menstrual bleeding.”
Another 6% of women who did not complete the study removed the device owing to bleeding and cramping complaints, 9% were lost to follow-up or withdrew consent, and 5% discontinued treatment for unspecified reasons, Dr. Creinin said.
“Etiologies for heavy menstrual bleeding may be different in the individuals we studied, so our findings provide assurance that these populations with heavy menstrual bleeding are equally well treated” with the IUD, Dr. Creinin said.
Dr. Creinin reported study funding from Medicines360. Dr. Schlaff reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ACOG 2022
Society of Gynecologic Surgeons meeting champions training of future gynecologic surgeons
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
How to teach vaginal surgery through simulation
Vaginal surgery, including vaginal hysterectomy, is slowly becoming a dying art. According to the National Inpatient Sample and the Nationwide Ambulatory Surgery Sample from 2018, only 11.8% of all hysterectomies were performed vaginally.1 The combination of uterine-sparing surgeries, advances in conservative therapies for benign uterine conditions, and the diversification of minimally invasive routes (laparoscopic and robotic) has resulted in a continued downtrend in vaginal surgical volumes. This shift has led to fewer operative learning opportunities and declining graduating resident surgical volume.2 According to the Accreditation Council for Graduate Medical Education (ACGME), the minimum number of vaginal hysterectomies is 15, which represents only the minimum accepted exposure and does not imply competency.
In response, surgical simulation has been used for skill acquisition and maintenance outside of the operating room in a learning environment that is safe for the learners and does not expose patients to additional risk. Educators are uniquely poised to use simulation to teach residents and to evaluate their procedural competency. Although vaginal surgery, specifically vaginal hysterectomy, continues to decline, it can be resuscitated with the assistance of surgical simulation.
In this article, we provide a broad overview of vaginal surgical simulation. We discuss the basic tenets of simulation, review how to teach and evaluate vaginal surgical skills, and present some of the commonly available vaginal surgery simulation models and their associated resources, cost, setup time, fidelity, and limitations.
Simulation principles relevant for vaginal hysterectomy simulation
Here, we review simulation-based learning principles that will help place specific simulation models into perspective.
One size does not fit all
Simulation, like many educational interventions, does not work via a “one-size-fits-all” approach. While the American College of Obstetricians and Gynecologists (ACOG) Simulations Working Group (SWG) has created a toolkit (available online at https://www.acog.org/education-and-events/simulations/about/curriculum) with many ready-to-use how-to simulation descriptions and lesson plans that cover common topics, what works in one setting may not work in another. The SWG created those modules to help educators save time and resources and to avoid reinventing the wheel for each simulation session. However, these simulations need to be adapted to the local needs of trainees and resources, such as faculty time, space, models, and funding.
Cost vs fidelity
It is important to distinguish between cost and fidelity. “Low cost” is often incorrectly used interchangeably with “low fidelity” when referring to models and simulations. The most basic principle of fidelity is that it is associated with situational realism that in turn, drives learning.3,4 For example, the term high fidelity does apply to a virtual reality robotic surgery simulator, which also is high cost. However, a low-cost beef tongue model of fourth-degree laceration5 is high fidelity, while more expensive commercial models are less realistic, which makes them high cost and low fidelity.6 When selecting simulation models, educators need to consider cost based on their available resources and the level of fidelity needed for their learners.
Continue to: Task breakdown...
Task breakdown
As surgeon-educators, we love to teach! And while educators are passionate about imparting vaginal hysterectomy skills to the next generation of surgeons, it is important to assess where the learners are technically. Vaginal hysterectomy is a high-complexity procedure, with each step involving a unique skill set that is new to residents as learners; this is where the science of learning can help us teach more effectively.7 Focusing on doing the entire procedure all at once is more likely to result in cognitive overload, while a better approach is to break the procedure down into several components and practice those parts until goal proficiency is reached.
Deliberate practice
The idea of deliberate practice was popularized by Malcolm Gladwell in his book titled Outliers, in which he gives examples of how 10,000 hours of practice leads to mastery of complex skills. This concept was deepened by the work of cognitive psychologist Anders Ericsson, who emphasized that not only the duration but also the quality of practice—which involves concentration, analysis, and problem-solving—leads to the most effective training.8
In surgical education, this concept translates into many domains. For example, an individualized learning plan includes frequent low-stakes assessments, video recording for later viewing and analysis, surgical coaching, and detailed planning of future training sessions to incorporate past performance. “Just doing” surgery on a simulator (or in the operating room) results in missed learning opportunities.
Logistics and implementation: Who, where, when
The simulation “formula” takes into account multiple factors but should start with learning objectives and then an assessment of what resources are available to address them. For example, if one surgeon-educator and one resident-learner are available for 30 minutes in between cases in the operating room, and the goal is to teach the resident clamp-and-tie technique on pedicles, the “milk carton” model9 and a few instruments from the vaginal hysterectomy tray are ideal for this training. On the other hand, if it is important to achieve competency for an entire procedure prior to operating room debut and a group of surgeon-educators is available to share the time commitment of 2-hour sessions per each resident, then the PROMPT (PRactical Obstetric Multi-Professional Training) shoulder dystocia model could be used (TABLE).10-14
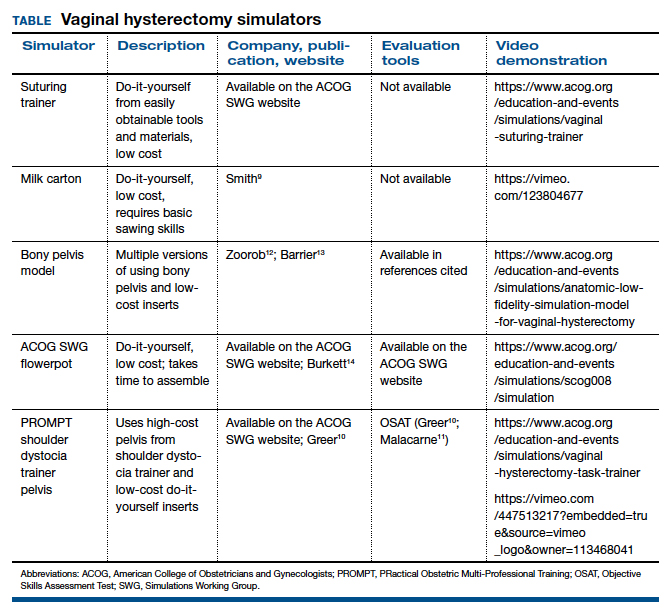
Learning curves
Ideally, educators would like to know how many simulated training sessions are needed for a learner to reach a proficiency level and become operating room ready. Such information about learning curves, unfortunately, is not available yet for vaginal hysterectomies. The first step in the process is to establish a baseline for performance to know a starting point, with assessment tools specific to each simulator; the next step is to study how many “takes” are needed for learners to move through their learning curve.15 The use of assessment tools can help assess each learner’s progression.
Continue to: Evaluation, assessment, and feedback...
Evaluation, assessment, and feedback
With more emphasis being placed on patient safety and transparency in every aspect of health care, including surgical training, graduate medical education leaders increasingly highlight the importance of objective assessment tools and outcome-based standards for certification of competency in surgery.16,17 Commonly used assessment tools that have reliability and validity evidence include surgical checklists and global rating scales. Checklists for common gynecologic procedures, including vaginal hysterectomy, as well as a global rating scale specifically developed for vaginal surgery (Vaginal Surgical Skills Index, VSSI)18 are accessible on the ACOG Simulations Working Group Surgical Curriculum in Obstetrics and Gynecology website.19
While checklists contain the main steps of each procedure, these lists do not assess for how well each step of the procedure is performed. By contrast, global rating scales, such as the VSSI, can discriminate between surgeons with different skill levels both in the simulation and operating room settings; each metric within the global rating scale (for example, time and motion) does not pertain to the performance of a procedure’s specific step but rather to the overall performance of the entire procedure.18,20 Hence, to provide detailed feedback, especially for formative assessment, both checklists and global rating scales often are used together.
Although standardized, checklists and global rating scales ultimately are still subjective (not objective) assessment tools. Recently, more attention has been to use surgical data science, particularly artificial intelligence methods, to objectively assess surgical performance by analyzing data generated during the performance of surgery, such as instrumental motion and video.21 These methods have been applied to a wide range of surgical techniques, including open, laparoscopic, robotic, endoscopic, and microsurgical approaches. Most of these types of studies have used assessment of surgical skill as the main outcome, with fewer studies correlating skill with clinically relevant metrics, such as patient outcomes.22-25 Although this is an area of active research, these methods are still being developed, and their validity and utility are not well established. For now, educators should continue to use validated checklists and global rating skills to help assess any type of surgical performance, particularly vaginal surgery.
Vaginal surgical simulation models
Vaginal surgery requires a surgeon to operate in a narrow, deep space. This requires ambidexterity, accurate depth perception, understanding of how to handle tissues, and use of movements that are efficient, fluid, and rhythmic. Multiple proposed simulation models are relevant to vaginal surgery, and these vary based on level of fidelity, cost, feasibility, ability to maintain standardization, ease of construction (if required), and generalizability to all of pelvic surgery (that is, procedure specific vs basic skills focused).10,11,13,26-31
Below, we describe various simulation models that are available for teaching vaginal surgical skills.
Vaginal hysterectomy simulation model
One commercially available simulation model for vaginal hysterectomy (as well as other vaginal surgical procedures, such as midurethral sling and anterior and posterior colporrhaphy) is the Miya Model (Miyazaki Enterprises) (FIGURE 1) and its accompanying MiyaMODEL App. In a multi-institutional study funded by the National Institutes of Health (NIH), the Miya Model, when used with the VSSI, was shown to be a valid assessment tool in terms of ability to differentiate a competent from a noncompetent surgeon.20 Currently, an ongoing NIH-sponsored multi-institutional study is assessing the Miya Model as a teaching tool and whether skills acquired on the Miya Model are transferable to the operating room.
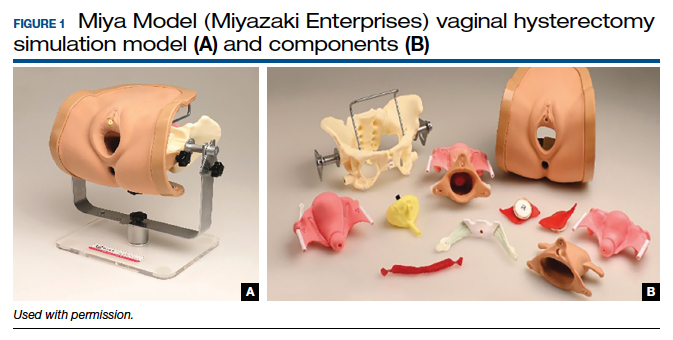
Continue to: Low-cost vaginal hysterectomy models...
Low-cost vaginal hysterectomy models
Multiple low-cost vaginal hysterectomy simulation models are described. Two models developed many years ago include the ACOG SWG flowerpot model14 and the PROMPT shoulder dystocia pelvic trainer model.10,11,14 The former model is low cost as it can be constructed from easily obtained household materials, but its downside is that it takes time and effort to obtain the materials and to assemble them. The latter model is faster to assemble but requires one to use a PROMPT pelvis for shoulder dystocia training, which has a considerable upfront cost. However, it is available in most hospitals with considerable obstetrical volume, and it allows for the most realistic perineum, which is helpful in recreating the feel of vaginal surgery, including retraction and exposure.
Many models created and described in the literature are variations of the models mentioned above, and many use commercially available low-cost bony pelvis models and polyvinyl chloride (PVC) pipes as a foundation for the soft tissue inserts to attach.12,13,31-33 Each model varies on what it “teaches best” regarding realism—for example, teaching anatomy, working in a tight space, dissection, or clamp placement and suture ligature.
Furthermore, since vaginal hysterectomy is a high-complexity procedure in terms of skills (working in confined space, limited view, “upside-down” anatomy, and need to direct assistants for retraction and exposure), task breakdown is important for simulation learning, as it is not efficient to repeat the entire procedure until proficiency is reached. Two trainers have been described to address that need: the milk carton and the vaginal suturing trainer. The latter allows learners to practice clamp placement and pedicle ligation multiple times, including in confined space (FIGURE 2), and the former allows them to do the same in a procedural matter as the clamp placement moves caudad to cephalad during the procedure (FIGURE 3).
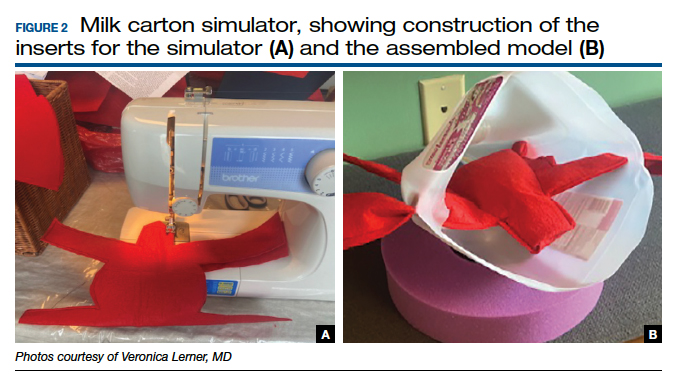

Native tissue pelvic floor surgery simulation
While there are few publications regarding surgical simulation models for native tissue pelvic floor surgeries, a low-cost anterior and posterior repair model was developed for the ACOG SWG Simulation Toolkit and published online in 2017, after their peer-review process. The fidelity is moderate for this low-cost model, which costs less than $5 per use. The simulation model requires a new vaginal insert for each learner, which is fast and easy to make and requires only a few components; however, the bony pelvis (for example, the flowerpot model) needs to be purchased or created. The stage of the anterior wall prolapse can be adjusted by the amount of fluid placed in the balloon, which is used to simulate the bladder. The more fluid that is placed in the “bladder,” the more severe the anterior wall prolapse appears. The vaginal caliber can be adjusted, if needed, by increasing or decreasing the size of the components to create the vagina, but the suggested sizes simulate a significantly widened vaginal caliber that would benefit from a posterior repair with perineorrhaphy. Although there is no validity evidence for this model, a skills assessment is available through the ACOG Simulation Surgical Curriculum. Of note, native tissue colpopexy repairs are also possible with this model (or another high-fidelity model, such as the Miya Model), if the sacrospinous ligaments and/or uterosacral ligaments are available on the pelvic model in use. This model’s limitations include the absence of a high-fidelity plane of dissection of the vaginal muscularis, and that no bleeding is encountered, which is the case for many low-cost models.19,34
Fundamentals of Vaginal Surgery (FVS) basic surgical skills simulation
The FVS simulation system, consisting of a task trainer paired with 6 selected surgical tasks, was developed to teach basic skills used in vaginal surgery.35 The FVS task trainer is 3D printed and has 3 main components: a base piece that allows for different surgical materials to be secured, a depth extender, and a width reducer. In addition, it has a mobile phone mount and a window into the system to enable video capture of skills exercises.
The FVS simulator is designed to enable 6 surgical tasks, including one-handed knot tying, two-handed knot tying, running suturing, plication suturing, Heaney transfixion pedicle ligation, and free pedicle ligation (FIGURE 4). In a pilot study, the FVS simulation system was deemed representative of the intended surgical field, useful for inclusion in a training program, and favored as a tool for both training and testing. Additionally, an initial proficiency score of 400 was set, which discriminated between novice and expert surgeons.35
An advantage of this simulation system is that it allows learners to focus on basic skills, rather than on an entire specific procedure. Further, the system is standardized, as it is commercially manufactured; this also allows for easy assembly. The disadvantage of this model is that it cannot be modified to teach specific vaginal procedures, and it must be purchased, rather than constructed on site. Further studies are needed to create generalizable proficiency scores and to assess its use in training and testing. For more information on the FVS simulation model, visit the Arbor Simulation website (http://arborsim.com).
Surgical simulation’s important role
Surgical skills can be learned and improved in the simulation setting in a low-stakes, low-pressure environment. Simulation can enable basic skills development and then higher-level learning of complex procedures. Skill assessment is important to aid in learning (via formative assessments) and for examination or certification (summative assessments).
With decreasing vaginal surgical volumes occurring nationally, it is becoming even more important to use surgical simulation to teach and maintain vaginal surgical skills. In this article, we reviewed various different simulation models that can be used for developing vaginal surgical skills and presented the advantages, limitations, and resources relevant for each simulation model. ●
- Wright JD, Huang Y, Li AH, et al. Nationwide estimates of annual inpatient and outpatient hysterectomies performed in the United States. Obstet Gynecol. 2022;139:446-448.
- Gressel GM, Potts JR 3rd, Cha S, et al. Hysterectomy route and numbers reported by graduating residents in obstetrics and gynecology training programs. Obstet Gynecol. 2020;135:268-273.
- Lioce L, ed. Healthcare Simulation Dictionary. 2nd ed. Rockville, MD; Agency for Healthcare Research and Quality: 2020. AHRQ Publication No. 20-0019.
- Norman G, Dore K, Grierson L. The minimal relationship between simulation fidelity and transfer of learning. Med Educ. 2012;46:636-647.
- Illston JD, Ballard AC, Ellington DR, et al. Modified beef tongue model for fourth-degree laceration repair simulation. Obstet Gynecol. 2017;129:491-496.
- WorldPoint website. 3B Scientific Episiotomy and Suturing Trainer. https://www.worldpoint.com/3b-episiotomy-and-suturing-sim. Accessed April 20, 2022.
- Balafoutas D, Joukhadar R, Kiesel M, et al. The role of deconstructive teaching in the training of laparoscopy. JSLS. 2019;23:e2019.00020.
- Ericsson KA, Harwell KW. Deliberate practice and proposed limits on the effects of practice on the acquisition of expert performance: why the original definition matters and recommendations for future research. Front Psychol. 2019;10:2396.
- Smith TM, Fenner DE. Vaginal hysterectomy teaching model—an educational video. Female Pelvic Med Reconstr Surg. 2012;18:S43. Abstract.
- Greer JA, Segal S, Salva CR, et al. Development and validation of simulation training for vaginal hysterectomy. J Minim Invasive Gynecol. 2014;21:74-82.
- Malacarne DR, Escobar CM, Lam CJ, et al. Teaching vaginal hysterectomy via simulation: creation and validation of the objective skills assessment tool for simulated vaginal hysterectomy on a task trainer and performance among different levels of trainees. Female Pelvic Med Reconstr Surg. 2019;25:298-304.
- Zoorob D, Frenn R, Moffitt M, et al. Multi-institutional validation of a vaginal hysterectomy simulation model for resident training. J Minim Invasive Gynecol. 2021;28:1490-1496.e1.
- Barrier BF, Thompson AB, McCullough MW, et al. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7:374-379.
- Burkett LS, Makin J, Ackenbom M, et al. Validation of transvaginal hysterectomy surgical model—modification of the flowerpot model to improve vesicovaginal plane simulation. J Minim Invasive Gynecol. 2021;28:1526-1530.
- Escobar C, Malacarne Pape D, Ferrante KL, et al. Who should be teaching vaginal hysterectomy on a task trainer? A multicenter randomized trial of peer versus expert coaching. J Surg Simul. 2020;7:63-72.
- The obstetrics and gynecology milestone project. J Grad Med Educ. 2014;6(1 suppl 1):129-143.
- Nasca TJ, Philibert I, Brigham T, et al. The next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366:1051-1056.
- Chen CCG, Korn A, Klingele C, et al. Objective assessment of vaginal surgical skills. Am J Obstet Gynecol. 2010;203:79.e1-8.
- American College of Obstetricians and Gynecologists. Surgical curriculum in obstetrics and gynecology. https://www.acog.org /education-and-events/simulations/surgical-curriculum-in-ob-gyn.
- Chen CCG, Lockrow EG, DeStephano CC, et al. Establishing validity for a vaginal hysterectomy simulation model for surgical skills assessment. Obstet Gynecol. 2020;136:942-949.
- Vedula SS, Hager GD. Surgical data science: the new knowledge domain. Innov Surg Sci. 2017;2:109-121.
- Witthaus MW, Farooq S, Melnyk R, et al. Incorporation and validation of clinically relevant performance metrics of simulation (CRPMS) into a novel full-immersion simulation platform for nerve-sparing robot-assisted radical prostatectomy (NS-RARP) utilizing three-dimensional printing and hydrogel casting technology. BJU Int. 2020;125:322-332.
- Vedula SS, Malpani A, Ahmidi N, et al. Task-level vs segment-level quantitative metrics for surgical skill assessment. J Surg Educ. 2016;73:482-489.
- Maier-Hein L, Eisenmann M, Sarikaya D, et al. Surgical data science—from concepts toward clinical translation. Med Image Anal. 2022;76:102306.
- Hung AJ, Chen J, Gill IS. Automated performance metrics and machine learning algorithms to measure surgeon performance and anticipate clinical outcomes in robotic surgery. JAMA Surg. 2018;153:770-771.
- Altman K, Chen G, Chou B, et al. Surgical curriculum in obstetrics and gynecology: vaginal hysterectomy simulation. https://cfweb.acog. org/scog/scog008/Simulation.cfm.
- DeLancey JOL. Basic Exercises: Surgical Technique. Davis + Geck; Brooklyn, NY: 1987.
- Geoffrion R, Suen MW, Koenig NA, et al. Teaching vaginal surgery to junior residents: initial validation of 3 novel procedure-specific low-fidelity models. J Surg Educ. 2016;73:157-161.
- Pandey VA, Wolfe JHN, Lindhal AK, et al. Validity of an exam assessment in surgical skill: EBSQ-VASC pilot study. Eur J Vasc Endovasc Surg. 2004;27:341-348.
- Limbs&Things website. Knot Tying Trainer. https://limbsandthings. com/us/products/50050/50050-knot-tying-trainer. Accessed April 20, 2022.
- Vaughan MH, Kim-Fine S, Hullfish KL, et al. Validation of the simulated vaginal hysterectomy trainer. J Minim Invasive Gynecol. 2018;25:1101-1106.
- Braun K, Henley B, Ray C, et al. Teaching vaginal hysterectomy: low fidelity trainer provides effective simulation at low cost. Obstet Gynecol. 2017;130:44S.
- Anand M, Duffy CP, Vragovic O, et al. Surgical anatomy of vaginal hysterectomy—impact of a resident-constructed simulation model. Female Pelvic Med Reconstr Surg. 2018;24:176-182.
- Chen CC, Vaccaro CM. ACOG Simulation Consortium Surgical Curriculum: anterior and posterior repair. 2017. https://cfweb.acog. org/scog/.
- Schmidt PC, Fairchild PS, Fenner DE, et al. The Fundamentals of Vaginal Surgery pilot study: developing, validating, and setting proficiency scores for a vaginal surgical skills simulation system. Am J Obstet Gynecol. 2021;225:558.e1-558.e11.
Vaginal surgery, including vaginal hysterectomy, is slowly becoming a dying art. According to the National Inpatient Sample and the Nationwide Ambulatory Surgery Sample from 2018, only 11.8% of all hysterectomies were performed vaginally.1 The combination of uterine-sparing surgeries, advances in conservative therapies for benign uterine conditions, and the diversification of minimally invasive routes (laparoscopic and robotic) has resulted in a continued downtrend in vaginal surgical volumes. This shift has led to fewer operative learning opportunities and declining graduating resident surgical volume.2 According to the Accreditation Council for Graduate Medical Education (ACGME), the minimum number of vaginal hysterectomies is 15, which represents only the minimum accepted exposure and does not imply competency.
In response, surgical simulation has been used for skill acquisition and maintenance outside of the operating room in a learning environment that is safe for the learners and does not expose patients to additional risk. Educators are uniquely poised to use simulation to teach residents and to evaluate their procedural competency. Although vaginal surgery, specifically vaginal hysterectomy, continues to decline, it can be resuscitated with the assistance of surgical simulation.
In this article, we provide a broad overview of vaginal surgical simulation. We discuss the basic tenets of simulation, review how to teach and evaluate vaginal surgical skills, and present some of the commonly available vaginal surgery simulation models and their associated resources, cost, setup time, fidelity, and limitations.
Simulation principles relevant for vaginal hysterectomy simulation
Here, we review simulation-based learning principles that will help place specific simulation models into perspective.
One size does not fit all
Simulation, like many educational interventions, does not work via a “one-size-fits-all” approach. While the American College of Obstetricians and Gynecologists (ACOG) Simulations Working Group (SWG) has created a toolkit (available online at https://www.acog.org/education-and-events/simulations/about/curriculum) with many ready-to-use how-to simulation descriptions and lesson plans that cover common topics, what works in one setting may not work in another. The SWG created those modules to help educators save time and resources and to avoid reinventing the wheel for each simulation session. However, these simulations need to be adapted to the local needs of trainees and resources, such as faculty time, space, models, and funding.
Cost vs fidelity
It is important to distinguish between cost and fidelity. “Low cost” is often incorrectly used interchangeably with “low fidelity” when referring to models and simulations. The most basic principle of fidelity is that it is associated with situational realism that in turn, drives learning.3,4 For example, the term high fidelity does apply to a virtual reality robotic surgery simulator, which also is high cost. However, a low-cost beef tongue model of fourth-degree laceration5 is high fidelity, while more expensive commercial models are less realistic, which makes them high cost and low fidelity.6 When selecting simulation models, educators need to consider cost based on their available resources and the level of fidelity needed for their learners.
Continue to: Task breakdown...
Task breakdown
As surgeon-educators, we love to teach! And while educators are passionate about imparting vaginal hysterectomy skills to the next generation of surgeons, it is important to assess where the learners are technically. Vaginal hysterectomy is a high-complexity procedure, with each step involving a unique skill set that is new to residents as learners; this is where the science of learning can help us teach more effectively.7 Focusing on doing the entire procedure all at once is more likely to result in cognitive overload, while a better approach is to break the procedure down into several components and practice those parts until goal proficiency is reached.
Deliberate practice
The idea of deliberate practice was popularized by Malcolm Gladwell in his book titled Outliers, in which he gives examples of how 10,000 hours of practice leads to mastery of complex skills. This concept was deepened by the work of cognitive psychologist Anders Ericsson, who emphasized that not only the duration but also the quality of practice—which involves concentration, analysis, and problem-solving—leads to the most effective training.8
In surgical education, this concept translates into many domains. For example, an individualized learning plan includes frequent low-stakes assessments, video recording for later viewing and analysis, surgical coaching, and detailed planning of future training sessions to incorporate past performance. “Just doing” surgery on a simulator (or in the operating room) results in missed learning opportunities.
Logistics and implementation: Who, where, when
The simulation “formula” takes into account multiple factors but should start with learning objectives and then an assessment of what resources are available to address them. For example, if one surgeon-educator and one resident-learner are available for 30 minutes in between cases in the operating room, and the goal is to teach the resident clamp-and-tie technique on pedicles, the “milk carton” model9 and a few instruments from the vaginal hysterectomy tray are ideal for this training. On the other hand, if it is important to achieve competency for an entire procedure prior to operating room debut and a group of surgeon-educators is available to share the time commitment of 2-hour sessions per each resident, then the PROMPT (PRactical Obstetric Multi-Professional Training) shoulder dystocia model could be used (TABLE).10-14

Learning curves
Ideally, educators would like to know how many simulated training sessions are needed for a learner to reach a proficiency level and become operating room ready. Such information about learning curves, unfortunately, is not available yet for vaginal hysterectomies. The first step in the process is to establish a baseline for performance to know a starting point, with assessment tools specific to each simulator; the next step is to study how many “takes” are needed for learners to move through their learning curve.15 The use of assessment tools can help assess each learner’s progression.
Continue to: Evaluation, assessment, and feedback...
Evaluation, assessment, and feedback
With more emphasis being placed on patient safety and transparency in every aspect of health care, including surgical training, graduate medical education leaders increasingly highlight the importance of objective assessment tools and outcome-based standards for certification of competency in surgery.16,17 Commonly used assessment tools that have reliability and validity evidence include surgical checklists and global rating scales. Checklists for common gynecologic procedures, including vaginal hysterectomy, as well as a global rating scale specifically developed for vaginal surgery (Vaginal Surgical Skills Index, VSSI)18 are accessible on the ACOG Simulations Working Group Surgical Curriculum in Obstetrics and Gynecology website.19
While checklists contain the main steps of each procedure, these lists do not assess for how well each step of the procedure is performed. By contrast, global rating scales, such as the VSSI, can discriminate between surgeons with different skill levels both in the simulation and operating room settings; each metric within the global rating scale (for example, time and motion) does not pertain to the performance of a procedure’s specific step but rather to the overall performance of the entire procedure.18,20 Hence, to provide detailed feedback, especially for formative assessment, both checklists and global rating scales often are used together.
Although standardized, checklists and global rating scales ultimately are still subjective (not objective) assessment tools. Recently, more attention has been to use surgical data science, particularly artificial intelligence methods, to objectively assess surgical performance by analyzing data generated during the performance of surgery, such as instrumental motion and video.21 These methods have been applied to a wide range of surgical techniques, including open, laparoscopic, robotic, endoscopic, and microsurgical approaches. Most of these types of studies have used assessment of surgical skill as the main outcome, with fewer studies correlating skill with clinically relevant metrics, such as patient outcomes.22-25 Although this is an area of active research, these methods are still being developed, and their validity and utility are not well established. For now, educators should continue to use validated checklists and global rating skills to help assess any type of surgical performance, particularly vaginal surgery.
Vaginal surgical simulation models
Vaginal surgery requires a surgeon to operate in a narrow, deep space. This requires ambidexterity, accurate depth perception, understanding of how to handle tissues, and use of movements that are efficient, fluid, and rhythmic. Multiple proposed simulation models are relevant to vaginal surgery, and these vary based on level of fidelity, cost, feasibility, ability to maintain standardization, ease of construction (if required), and generalizability to all of pelvic surgery (that is, procedure specific vs basic skills focused).10,11,13,26-31
Below, we describe various simulation models that are available for teaching vaginal surgical skills.
Vaginal hysterectomy simulation model
One commercially available simulation model for vaginal hysterectomy (as well as other vaginal surgical procedures, such as midurethral sling and anterior and posterior colporrhaphy) is the Miya Model (Miyazaki Enterprises) (FIGURE 1) and its accompanying MiyaMODEL App. In a multi-institutional study funded by the National Institutes of Health (NIH), the Miya Model, when used with the VSSI, was shown to be a valid assessment tool in terms of ability to differentiate a competent from a noncompetent surgeon.20 Currently, an ongoing NIH-sponsored multi-institutional study is assessing the Miya Model as a teaching tool and whether skills acquired on the Miya Model are transferable to the operating room.

Continue to: Low-cost vaginal hysterectomy models...
Low-cost vaginal hysterectomy models
Multiple low-cost vaginal hysterectomy simulation models are described. Two models developed many years ago include the ACOG SWG flowerpot model14 and the PROMPT shoulder dystocia pelvic trainer model.10,11,14 The former model is low cost as it can be constructed from easily obtained household materials, but its downside is that it takes time and effort to obtain the materials and to assemble them. The latter model is faster to assemble but requires one to use a PROMPT pelvis for shoulder dystocia training, which has a considerable upfront cost. However, it is available in most hospitals with considerable obstetrical volume, and it allows for the most realistic perineum, which is helpful in recreating the feel of vaginal surgery, including retraction and exposure.
Many models created and described in the literature are variations of the models mentioned above, and many use commercially available low-cost bony pelvis models and polyvinyl chloride (PVC) pipes as a foundation for the soft tissue inserts to attach.12,13,31-33 Each model varies on what it “teaches best” regarding realism—for example, teaching anatomy, working in a tight space, dissection, or clamp placement and suture ligature.
Furthermore, since vaginal hysterectomy is a high-complexity procedure in terms of skills (working in confined space, limited view, “upside-down” anatomy, and need to direct assistants for retraction and exposure), task breakdown is important for simulation learning, as it is not efficient to repeat the entire procedure until proficiency is reached. Two trainers have been described to address that need: the milk carton and the vaginal suturing trainer. The latter allows learners to practice clamp placement and pedicle ligation multiple times, including in confined space (FIGURE 2), and the former allows them to do the same in a procedural matter as the clamp placement moves caudad to cephalad during the procedure (FIGURE 3).


Native tissue pelvic floor surgery simulation
While there are few publications regarding surgical simulation models for native tissue pelvic floor surgeries, a low-cost anterior and posterior repair model was developed for the ACOG SWG Simulation Toolkit and published online in 2017, after their peer-review process. The fidelity is moderate for this low-cost model, which costs less than $5 per use. The simulation model requires a new vaginal insert for each learner, which is fast and easy to make and requires only a few components; however, the bony pelvis (for example, the flowerpot model) needs to be purchased or created. The stage of the anterior wall prolapse can be adjusted by the amount of fluid placed in the balloon, which is used to simulate the bladder. The more fluid that is placed in the “bladder,” the more severe the anterior wall prolapse appears. The vaginal caliber can be adjusted, if needed, by increasing or decreasing the size of the components to create the vagina, but the suggested sizes simulate a significantly widened vaginal caliber that would benefit from a posterior repair with perineorrhaphy. Although there is no validity evidence for this model, a skills assessment is available through the ACOG Simulation Surgical Curriculum. Of note, native tissue colpopexy repairs are also possible with this model (or another high-fidelity model, such as the Miya Model), if the sacrospinous ligaments and/or uterosacral ligaments are available on the pelvic model in use. This model’s limitations include the absence of a high-fidelity plane of dissection of the vaginal muscularis, and that no bleeding is encountered, which is the case for many low-cost models.19,34
Fundamentals of Vaginal Surgery (FVS) basic surgical skills simulation
The FVS simulation system, consisting of a task trainer paired with 6 selected surgical tasks, was developed to teach basic skills used in vaginal surgery.35 The FVS task trainer is 3D printed and has 3 main components: a base piece that allows for different surgical materials to be secured, a depth extender, and a width reducer. In addition, it has a mobile phone mount and a window into the system to enable video capture of skills exercises.
The FVS simulator is designed to enable 6 surgical tasks, including one-handed knot tying, two-handed knot tying, running suturing, plication suturing, Heaney transfixion pedicle ligation, and free pedicle ligation (FIGURE 4). In a pilot study, the FVS simulation system was deemed representative of the intended surgical field, useful for inclusion in a training program, and favored as a tool for both training and testing. Additionally, an initial proficiency score of 400 was set, which discriminated between novice and expert surgeons.35
An advantage of this simulation system is that it allows learners to focus on basic skills, rather than on an entire specific procedure. Further, the system is standardized, as it is commercially manufactured; this also allows for easy assembly. The disadvantage of this model is that it cannot be modified to teach specific vaginal procedures, and it must be purchased, rather than constructed on site. Further studies are needed to create generalizable proficiency scores and to assess its use in training and testing. For more information on the FVS simulation model, visit the Arbor Simulation website (http://arborsim.com).
Surgical simulation’s important role
Surgical skills can be learned and improved in the simulation setting in a low-stakes, low-pressure environment. Simulation can enable basic skills development and then higher-level learning of complex procedures. Skill assessment is important to aid in learning (via formative assessments) and for examination or certification (summative assessments).
With decreasing vaginal surgical volumes occurring nationally, it is becoming even more important to use surgical simulation to teach and maintain vaginal surgical skills. In this article, we reviewed various different simulation models that can be used for developing vaginal surgical skills and presented the advantages, limitations, and resources relevant for each simulation model. ●
Vaginal surgery, including vaginal hysterectomy, is slowly becoming a dying art. According to the National Inpatient Sample and the Nationwide Ambulatory Surgery Sample from 2018, only 11.8% of all hysterectomies were performed vaginally.1 The combination of uterine-sparing surgeries, advances in conservative therapies for benign uterine conditions, and the diversification of minimally invasive routes (laparoscopic and robotic) has resulted in a continued downtrend in vaginal surgical volumes. This shift has led to fewer operative learning opportunities and declining graduating resident surgical volume.2 According to the Accreditation Council for Graduate Medical Education (ACGME), the minimum number of vaginal hysterectomies is 15, which represents only the minimum accepted exposure and does not imply competency.
In response, surgical simulation has been used for skill acquisition and maintenance outside of the operating room in a learning environment that is safe for the learners and does not expose patients to additional risk. Educators are uniquely poised to use simulation to teach residents and to evaluate their procedural competency. Although vaginal surgery, specifically vaginal hysterectomy, continues to decline, it can be resuscitated with the assistance of surgical simulation.
In this article, we provide a broad overview of vaginal surgical simulation. We discuss the basic tenets of simulation, review how to teach and evaluate vaginal surgical skills, and present some of the commonly available vaginal surgery simulation models and their associated resources, cost, setup time, fidelity, and limitations.
Simulation principles relevant for vaginal hysterectomy simulation
Here, we review simulation-based learning principles that will help place specific simulation models into perspective.
One size does not fit all
Simulation, like many educational interventions, does not work via a “one-size-fits-all” approach. While the American College of Obstetricians and Gynecologists (ACOG) Simulations Working Group (SWG) has created a toolkit (available online at https://www.acog.org/education-and-events/simulations/about/curriculum) with many ready-to-use how-to simulation descriptions and lesson plans that cover common topics, what works in one setting may not work in another. The SWG created those modules to help educators save time and resources and to avoid reinventing the wheel for each simulation session. However, these simulations need to be adapted to the local needs of trainees and resources, such as faculty time, space, models, and funding.
Cost vs fidelity
It is important to distinguish between cost and fidelity. “Low cost” is often incorrectly used interchangeably with “low fidelity” when referring to models and simulations. The most basic principle of fidelity is that it is associated with situational realism that in turn, drives learning.3,4 For example, the term high fidelity does apply to a virtual reality robotic surgery simulator, which also is high cost. However, a low-cost beef tongue model of fourth-degree laceration5 is high fidelity, while more expensive commercial models are less realistic, which makes them high cost and low fidelity.6 When selecting simulation models, educators need to consider cost based on their available resources and the level of fidelity needed for their learners.
Continue to: Task breakdown...
Task breakdown
As surgeon-educators, we love to teach! And while educators are passionate about imparting vaginal hysterectomy skills to the next generation of surgeons, it is important to assess where the learners are technically. Vaginal hysterectomy is a high-complexity procedure, with each step involving a unique skill set that is new to residents as learners; this is where the science of learning can help us teach more effectively.7 Focusing on doing the entire procedure all at once is more likely to result in cognitive overload, while a better approach is to break the procedure down into several components and practice those parts until goal proficiency is reached.
Deliberate practice
The idea of deliberate practice was popularized by Malcolm Gladwell in his book titled Outliers, in which he gives examples of how 10,000 hours of practice leads to mastery of complex skills. This concept was deepened by the work of cognitive psychologist Anders Ericsson, who emphasized that not only the duration but also the quality of practice—which involves concentration, analysis, and problem-solving—leads to the most effective training.8
In surgical education, this concept translates into many domains. For example, an individualized learning plan includes frequent low-stakes assessments, video recording for later viewing and analysis, surgical coaching, and detailed planning of future training sessions to incorporate past performance. “Just doing” surgery on a simulator (or in the operating room) results in missed learning opportunities.
Logistics and implementation: Who, where, when
The simulation “formula” takes into account multiple factors but should start with learning objectives and then an assessment of what resources are available to address them. For example, if one surgeon-educator and one resident-learner are available for 30 minutes in between cases in the operating room, and the goal is to teach the resident clamp-and-tie technique on pedicles, the “milk carton” model9 and a few instruments from the vaginal hysterectomy tray are ideal for this training. On the other hand, if it is important to achieve competency for an entire procedure prior to operating room debut and a group of surgeon-educators is available to share the time commitment of 2-hour sessions per each resident, then the PROMPT (PRactical Obstetric Multi-Professional Training) shoulder dystocia model could be used (TABLE).10-14

Learning curves
Ideally, educators would like to know how many simulated training sessions are needed for a learner to reach a proficiency level and become operating room ready. Such information about learning curves, unfortunately, is not available yet for vaginal hysterectomies. The first step in the process is to establish a baseline for performance to know a starting point, with assessment tools specific to each simulator; the next step is to study how many “takes” are needed for learners to move through their learning curve.15 The use of assessment tools can help assess each learner’s progression.
Continue to: Evaluation, assessment, and feedback...
Evaluation, assessment, and feedback
With more emphasis being placed on patient safety and transparency in every aspect of health care, including surgical training, graduate medical education leaders increasingly highlight the importance of objective assessment tools and outcome-based standards for certification of competency in surgery.16,17 Commonly used assessment tools that have reliability and validity evidence include surgical checklists and global rating scales. Checklists for common gynecologic procedures, including vaginal hysterectomy, as well as a global rating scale specifically developed for vaginal surgery (Vaginal Surgical Skills Index, VSSI)18 are accessible on the ACOG Simulations Working Group Surgical Curriculum in Obstetrics and Gynecology website.19
While checklists contain the main steps of each procedure, these lists do not assess for how well each step of the procedure is performed. By contrast, global rating scales, such as the VSSI, can discriminate between surgeons with different skill levels both in the simulation and operating room settings; each metric within the global rating scale (for example, time and motion) does not pertain to the performance of a procedure’s specific step but rather to the overall performance of the entire procedure.18,20 Hence, to provide detailed feedback, especially for formative assessment, both checklists and global rating scales often are used together.
Although standardized, checklists and global rating scales ultimately are still subjective (not objective) assessment tools. Recently, more attention has been to use surgical data science, particularly artificial intelligence methods, to objectively assess surgical performance by analyzing data generated during the performance of surgery, such as instrumental motion and video.21 These methods have been applied to a wide range of surgical techniques, including open, laparoscopic, robotic, endoscopic, and microsurgical approaches. Most of these types of studies have used assessment of surgical skill as the main outcome, with fewer studies correlating skill with clinically relevant metrics, such as patient outcomes.22-25 Although this is an area of active research, these methods are still being developed, and their validity and utility are not well established. For now, educators should continue to use validated checklists and global rating skills to help assess any type of surgical performance, particularly vaginal surgery.
Vaginal surgical simulation models
Vaginal surgery requires a surgeon to operate in a narrow, deep space. This requires ambidexterity, accurate depth perception, understanding of how to handle tissues, and use of movements that are efficient, fluid, and rhythmic. Multiple proposed simulation models are relevant to vaginal surgery, and these vary based on level of fidelity, cost, feasibility, ability to maintain standardization, ease of construction (if required), and generalizability to all of pelvic surgery (that is, procedure specific vs basic skills focused).10,11,13,26-31
Below, we describe various simulation models that are available for teaching vaginal surgical skills.
Vaginal hysterectomy simulation model
One commercially available simulation model for vaginal hysterectomy (as well as other vaginal surgical procedures, such as midurethral sling and anterior and posterior colporrhaphy) is the Miya Model (Miyazaki Enterprises) (FIGURE 1) and its accompanying MiyaMODEL App. In a multi-institutional study funded by the National Institutes of Health (NIH), the Miya Model, when used with the VSSI, was shown to be a valid assessment tool in terms of ability to differentiate a competent from a noncompetent surgeon.20 Currently, an ongoing NIH-sponsored multi-institutional study is assessing the Miya Model as a teaching tool and whether skills acquired on the Miya Model are transferable to the operating room.

Continue to: Low-cost vaginal hysterectomy models...
Low-cost vaginal hysterectomy models
Multiple low-cost vaginal hysterectomy simulation models are described. Two models developed many years ago include the ACOG SWG flowerpot model14 and the PROMPT shoulder dystocia pelvic trainer model.10,11,14 The former model is low cost as it can be constructed from easily obtained household materials, but its downside is that it takes time and effort to obtain the materials and to assemble them. The latter model is faster to assemble but requires one to use a PROMPT pelvis for shoulder dystocia training, which has a considerable upfront cost. However, it is available in most hospitals with considerable obstetrical volume, and it allows for the most realistic perineum, which is helpful in recreating the feel of vaginal surgery, including retraction and exposure.
Many models created and described in the literature are variations of the models mentioned above, and many use commercially available low-cost bony pelvis models and polyvinyl chloride (PVC) pipes as a foundation for the soft tissue inserts to attach.12,13,31-33 Each model varies on what it “teaches best” regarding realism—for example, teaching anatomy, working in a tight space, dissection, or clamp placement and suture ligature.
Furthermore, since vaginal hysterectomy is a high-complexity procedure in terms of skills (working in confined space, limited view, “upside-down” anatomy, and need to direct assistants for retraction and exposure), task breakdown is important for simulation learning, as it is not efficient to repeat the entire procedure until proficiency is reached. Two trainers have been described to address that need: the milk carton and the vaginal suturing trainer. The latter allows learners to practice clamp placement and pedicle ligation multiple times, including in confined space (FIGURE 2), and the former allows them to do the same in a procedural matter as the clamp placement moves caudad to cephalad during the procedure (FIGURE 3).


Native tissue pelvic floor surgery simulation
While there are few publications regarding surgical simulation models for native tissue pelvic floor surgeries, a low-cost anterior and posterior repair model was developed for the ACOG SWG Simulation Toolkit and published online in 2017, after their peer-review process. The fidelity is moderate for this low-cost model, which costs less than $5 per use. The simulation model requires a new vaginal insert for each learner, which is fast and easy to make and requires only a few components; however, the bony pelvis (for example, the flowerpot model) needs to be purchased or created. The stage of the anterior wall prolapse can be adjusted by the amount of fluid placed in the balloon, which is used to simulate the bladder. The more fluid that is placed in the “bladder,” the more severe the anterior wall prolapse appears. The vaginal caliber can be adjusted, if needed, by increasing or decreasing the size of the components to create the vagina, but the suggested sizes simulate a significantly widened vaginal caliber that would benefit from a posterior repair with perineorrhaphy. Although there is no validity evidence for this model, a skills assessment is available through the ACOG Simulation Surgical Curriculum. Of note, native tissue colpopexy repairs are also possible with this model (or another high-fidelity model, such as the Miya Model), if the sacrospinous ligaments and/or uterosacral ligaments are available on the pelvic model in use. This model’s limitations include the absence of a high-fidelity plane of dissection of the vaginal muscularis, and that no bleeding is encountered, which is the case for many low-cost models.19,34
Fundamentals of Vaginal Surgery (FVS) basic surgical skills simulation
The FVS simulation system, consisting of a task trainer paired with 6 selected surgical tasks, was developed to teach basic skills used in vaginal surgery.35 The FVS task trainer is 3D printed and has 3 main components: a base piece that allows for different surgical materials to be secured, a depth extender, and a width reducer. In addition, it has a mobile phone mount and a window into the system to enable video capture of skills exercises.
The FVS simulator is designed to enable 6 surgical tasks, including one-handed knot tying, two-handed knot tying, running suturing, plication suturing, Heaney transfixion pedicle ligation, and free pedicle ligation (FIGURE 4). In a pilot study, the FVS simulation system was deemed representative of the intended surgical field, useful for inclusion in a training program, and favored as a tool for both training and testing. Additionally, an initial proficiency score of 400 was set, which discriminated between novice and expert surgeons.35
An advantage of this simulation system is that it allows learners to focus on basic skills, rather than on an entire specific procedure. Further, the system is standardized, as it is commercially manufactured; this also allows for easy assembly. The disadvantage of this model is that it cannot be modified to teach specific vaginal procedures, and it must be purchased, rather than constructed on site. Further studies are needed to create generalizable proficiency scores and to assess its use in training and testing. For more information on the FVS simulation model, visit the Arbor Simulation website (http://arborsim.com).
Surgical simulation’s important role
Surgical skills can be learned and improved in the simulation setting in a low-stakes, low-pressure environment. Simulation can enable basic skills development and then higher-level learning of complex procedures. Skill assessment is important to aid in learning (via formative assessments) and for examination or certification (summative assessments).
With decreasing vaginal surgical volumes occurring nationally, it is becoming even more important to use surgical simulation to teach and maintain vaginal surgical skills. In this article, we reviewed various different simulation models that can be used for developing vaginal surgical skills and presented the advantages, limitations, and resources relevant for each simulation model. ●
- Wright JD, Huang Y, Li AH, et al. Nationwide estimates of annual inpatient and outpatient hysterectomies performed in the United States. Obstet Gynecol. 2022;139:446-448.
- Gressel GM, Potts JR 3rd, Cha S, et al. Hysterectomy route and numbers reported by graduating residents in obstetrics and gynecology training programs. Obstet Gynecol. 2020;135:268-273.
- Lioce L, ed. Healthcare Simulation Dictionary. 2nd ed. Rockville, MD; Agency for Healthcare Research and Quality: 2020. AHRQ Publication No. 20-0019.
- Norman G, Dore K, Grierson L. The minimal relationship between simulation fidelity and transfer of learning. Med Educ. 2012;46:636-647.
- Illston JD, Ballard AC, Ellington DR, et al. Modified beef tongue model for fourth-degree laceration repair simulation. Obstet Gynecol. 2017;129:491-496.
- WorldPoint website. 3B Scientific Episiotomy and Suturing Trainer. https://www.worldpoint.com/3b-episiotomy-and-suturing-sim. Accessed April 20, 2022.
- Balafoutas D, Joukhadar R, Kiesel M, et al. The role of deconstructive teaching in the training of laparoscopy. JSLS. 2019;23:e2019.00020.
- Ericsson KA, Harwell KW. Deliberate practice and proposed limits on the effects of practice on the acquisition of expert performance: why the original definition matters and recommendations for future research. Front Psychol. 2019;10:2396.
- Smith TM, Fenner DE. Vaginal hysterectomy teaching model—an educational video. Female Pelvic Med Reconstr Surg. 2012;18:S43. Abstract.
- Greer JA, Segal S, Salva CR, et al. Development and validation of simulation training for vaginal hysterectomy. J Minim Invasive Gynecol. 2014;21:74-82.
- Malacarne DR, Escobar CM, Lam CJ, et al. Teaching vaginal hysterectomy via simulation: creation and validation of the objective skills assessment tool for simulated vaginal hysterectomy on a task trainer and performance among different levels of trainees. Female Pelvic Med Reconstr Surg. 2019;25:298-304.
- Zoorob D, Frenn R, Moffitt M, et al. Multi-institutional validation of a vaginal hysterectomy simulation model for resident training. J Minim Invasive Gynecol. 2021;28:1490-1496.e1.
- Barrier BF, Thompson AB, McCullough MW, et al. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7:374-379.
- Burkett LS, Makin J, Ackenbom M, et al. Validation of transvaginal hysterectomy surgical model—modification of the flowerpot model to improve vesicovaginal plane simulation. J Minim Invasive Gynecol. 2021;28:1526-1530.
- Escobar C, Malacarne Pape D, Ferrante KL, et al. Who should be teaching vaginal hysterectomy on a task trainer? A multicenter randomized trial of peer versus expert coaching. J Surg Simul. 2020;7:63-72.
- The obstetrics and gynecology milestone project. J Grad Med Educ. 2014;6(1 suppl 1):129-143.
- Nasca TJ, Philibert I, Brigham T, et al. The next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366:1051-1056.
- Chen CCG, Korn A, Klingele C, et al. Objective assessment of vaginal surgical skills. Am J Obstet Gynecol. 2010;203:79.e1-8.
- American College of Obstetricians and Gynecologists. Surgical curriculum in obstetrics and gynecology. https://www.acog.org /education-and-events/simulations/surgical-curriculum-in-ob-gyn.
- Chen CCG, Lockrow EG, DeStephano CC, et al. Establishing validity for a vaginal hysterectomy simulation model for surgical skills assessment. Obstet Gynecol. 2020;136:942-949.
- Vedula SS, Hager GD. Surgical data science: the new knowledge domain. Innov Surg Sci. 2017;2:109-121.
- Witthaus MW, Farooq S, Melnyk R, et al. Incorporation and validation of clinically relevant performance metrics of simulation (CRPMS) into a novel full-immersion simulation platform for nerve-sparing robot-assisted radical prostatectomy (NS-RARP) utilizing three-dimensional printing and hydrogel casting technology. BJU Int. 2020;125:322-332.
- Vedula SS, Malpani A, Ahmidi N, et al. Task-level vs segment-level quantitative metrics for surgical skill assessment. J Surg Educ. 2016;73:482-489.
- Maier-Hein L, Eisenmann M, Sarikaya D, et al. Surgical data science—from concepts toward clinical translation. Med Image Anal. 2022;76:102306.
- Hung AJ, Chen J, Gill IS. Automated performance metrics and machine learning algorithms to measure surgeon performance and anticipate clinical outcomes in robotic surgery. JAMA Surg. 2018;153:770-771.
- Altman K, Chen G, Chou B, et al. Surgical curriculum in obstetrics and gynecology: vaginal hysterectomy simulation. https://cfweb.acog. org/scog/scog008/Simulation.cfm.
- DeLancey JOL. Basic Exercises: Surgical Technique. Davis + Geck; Brooklyn, NY: 1987.
- Geoffrion R, Suen MW, Koenig NA, et al. Teaching vaginal surgery to junior residents: initial validation of 3 novel procedure-specific low-fidelity models. J Surg Educ. 2016;73:157-161.
- Pandey VA, Wolfe JHN, Lindhal AK, et al. Validity of an exam assessment in surgical skill: EBSQ-VASC pilot study. Eur J Vasc Endovasc Surg. 2004;27:341-348.
- Limbs&Things website. Knot Tying Trainer. https://limbsandthings. com/us/products/50050/50050-knot-tying-trainer. Accessed April 20, 2022.
- Vaughan MH, Kim-Fine S, Hullfish KL, et al. Validation of the simulated vaginal hysterectomy trainer. J Minim Invasive Gynecol. 2018;25:1101-1106.
- Braun K, Henley B, Ray C, et al. Teaching vaginal hysterectomy: low fidelity trainer provides effective simulation at low cost. Obstet Gynecol. 2017;130:44S.
- Anand M, Duffy CP, Vragovic O, et al. Surgical anatomy of vaginal hysterectomy—impact of a resident-constructed simulation model. Female Pelvic Med Reconstr Surg. 2018;24:176-182.
- Chen CC, Vaccaro CM. ACOG Simulation Consortium Surgical Curriculum: anterior and posterior repair. 2017. https://cfweb.acog. org/scog/.
- Schmidt PC, Fairchild PS, Fenner DE, et al. The Fundamentals of Vaginal Surgery pilot study: developing, validating, and setting proficiency scores for a vaginal surgical skills simulation system. Am J Obstet Gynecol. 2021;225:558.e1-558.e11.
- Wright JD, Huang Y, Li AH, et al. Nationwide estimates of annual inpatient and outpatient hysterectomies performed in the United States. Obstet Gynecol. 2022;139:446-448.
- Gressel GM, Potts JR 3rd, Cha S, et al. Hysterectomy route and numbers reported by graduating residents in obstetrics and gynecology training programs. Obstet Gynecol. 2020;135:268-273.
- Lioce L, ed. Healthcare Simulation Dictionary. 2nd ed. Rockville, MD; Agency for Healthcare Research and Quality: 2020. AHRQ Publication No. 20-0019.
- Norman G, Dore K, Grierson L. The minimal relationship between simulation fidelity and transfer of learning. Med Educ. 2012;46:636-647.
- Illston JD, Ballard AC, Ellington DR, et al. Modified beef tongue model for fourth-degree laceration repair simulation. Obstet Gynecol. 2017;129:491-496.
- WorldPoint website. 3B Scientific Episiotomy and Suturing Trainer. https://www.worldpoint.com/3b-episiotomy-and-suturing-sim. Accessed April 20, 2022.
- Balafoutas D, Joukhadar R, Kiesel M, et al. The role of deconstructive teaching in the training of laparoscopy. JSLS. 2019;23:e2019.00020.
- Ericsson KA, Harwell KW. Deliberate practice and proposed limits on the effects of practice on the acquisition of expert performance: why the original definition matters and recommendations for future research. Front Psychol. 2019;10:2396.
- Smith TM, Fenner DE. Vaginal hysterectomy teaching model—an educational video. Female Pelvic Med Reconstr Surg. 2012;18:S43. Abstract.
- Greer JA, Segal S, Salva CR, et al. Development and validation of simulation training for vaginal hysterectomy. J Minim Invasive Gynecol. 2014;21:74-82.
- Malacarne DR, Escobar CM, Lam CJ, et al. Teaching vaginal hysterectomy via simulation: creation and validation of the objective skills assessment tool for simulated vaginal hysterectomy on a task trainer and performance among different levels of trainees. Female Pelvic Med Reconstr Surg. 2019;25:298-304.
- Zoorob D, Frenn R, Moffitt M, et al. Multi-institutional validation of a vaginal hysterectomy simulation model for resident training. J Minim Invasive Gynecol. 2021;28:1490-1496.e1.
- Barrier BF, Thompson AB, McCullough MW, et al. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7:374-379.
- Burkett LS, Makin J, Ackenbom M, et al. Validation of transvaginal hysterectomy surgical model—modification of the flowerpot model to improve vesicovaginal plane simulation. J Minim Invasive Gynecol. 2021;28:1526-1530.
- Escobar C, Malacarne Pape D, Ferrante KL, et al. Who should be teaching vaginal hysterectomy on a task trainer? A multicenter randomized trial of peer versus expert coaching. J Surg Simul. 2020;7:63-72.
- The obstetrics and gynecology milestone project. J Grad Med Educ. 2014;6(1 suppl 1):129-143.
- Nasca TJ, Philibert I, Brigham T, et al. The next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366:1051-1056.
- Chen CCG, Korn A, Klingele C, et al. Objective assessment of vaginal surgical skills. Am J Obstet Gynecol. 2010;203:79.e1-8.
- American College of Obstetricians and Gynecologists. Surgical curriculum in obstetrics and gynecology. https://www.acog.org /education-and-events/simulations/surgical-curriculum-in-ob-gyn.
- Chen CCG, Lockrow EG, DeStephano CC, et al. Establishing validity for a vaginal hysterectomy simulation model for surgical skills assessment. Obstet Gynecol. 2020;136:942-949.
- Vedula SS, Hager GD. Surgical data science: the new knowledge domain. Innov Surg Sci. 2017;2:109-121.
- Witthaus MW, Farooq S, Melnyk R, et al. Incorporation and validation of clinically relevant performance metrics of simulation (CRPMS) into a novel full-immersion simulation platform for nerve-sparing robot-assisted radical prostatectomy (NS-RARP) utilizing three-dimensional printing and hydrogel casting technology. BJU Int. 2020;125:322-332.
- Vedula SS, Malpani A, Ahmidi N, et al. Task-level vs segment-level quantitative metrics for surgical skill assessment. J Surg Educ. 2016;73:482-489.
- Maier-Hein L, Eisenmann M, Sarikaya D, et al. Surgical data science—from concepts toward clinical translation. Med Image Anal. 2022;76:102306.
- Hung AJ, Chen J, Gill IS. Automated performance metrics and machine learning algorithms to measure surgeon performance and anticipate clinical outcomes in robotic surgery. JAMA Surg. 2018;153:770-771.
- Altman K, Chen G, Chou B, et al. Surgical curriculum in obstetrics and gynecology: vaginal hysterectomy simulation. https://cfweb.acog. org/scog/scog008/Simulation.cfm.
- DeLancey JOL. Basic Exercises: Surgical Technique. Davis + Geck; Brooklyn, NY: 1987.
- Geoffrion R, Suen MW, Koenig NA, et al. Teaching vaginal surgery to junior residents: initial validation of 3 novel procedure-specific low-fidelity models. J Surg Educ. 2016;73:157-161.
- Pandey VA, Wolfe JHN, Lindhal AK, et al. Validity of an exam assessment in surgical skill: EBSQ-VASC pilot study. Eur J Vasc Endovasc Surg. 2004;27:341-348.
- Limbs&Things website. Knot Tying Trainer. https://limbsandthings. com/us/products/50050/50050-knot-tying-trainer. Accessed April 20, 2022.
- Vaughan MH, Kim-Fine S, Hullfish KL, et al. Validation of the simulated vaginal hysterectomy trainer. J Minim Invasive Gynecol. 2018;25:1101-1106.
- Braun K, Henley B, Ray C, et al. Teaching vaginal hysterectomy: low fidelity trainer provides effective simulation at low cost. Obstet Gynecol. 2017;130:44S.
- Anand M, Duffy CP, Vragovic O, et al. Surgical anatomy of vaginal hysterectomy—impact of a resident-constructed simulation model. Female Pelvic Med Reconstr Surg. 2018;24:176-182.
- Chen CC, Vaccaro CM. ACOG Simulation Consortium Surgical Curriculum: anterior and posterior repair. 2017. https://cfweb.acog. org/scog/.
- Schmidt PC, Fairchild PS, Fenner DE, et al. The Fundamentals of Vaginal Surgery pilot study: developing, validating, and setting proficiency scores for a vaginal surgical skills simulation system. Am J Obstet Gynecol. 2021;225:558.e1-558.e11.
2022 Update on cervical disease
Cervical cancer is an important global health problem with an estimated 604,127 new cases and 341,831 deaths in 2020.1 Nearly 85% of the disease burden affects individuals from low and middle-income countries. The World Health Organization (WHO) set forth the goal for all countries to reach and maintain an incidence rate of below 4 per 100,000 women by 2030 as part of the Global Strategy to Accelerate the Elimination of Cervical Cancer.
Although traditional Pap cytology has been the cornerstone of screening programs, its poor sensitivity of approximately 50% and limitations in accessibility require new strategies to achieve the elimination of cervical cancer.2 The discovery that persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of cervical cancer led to the development of diagnostic HPV tests, which have higher sensitivity than cytology (96.1% vs 53.0%) but somewhat lower specificity (90.7% vs 96.3%) for the detection of cervical intraepithelial neoplasia (CIN) 2 or worse lesions.2 Initially, HPV testing was incorporated as a method to triage atypical squamous cells of undetermined significance (ASCUS) cytology results.3 Later, the concept of cotesting with cytology emerged,4,5 and since then, several clinical trials have demonstrated the effectiveness of primary HPV screening.6-9
In 2020, the WHO recommended HPV DNA testing as the primary screening method starting at the age of 30 years, with regular testing every 5 to 10 years, for the general population.10 Currently, primary HPV has been adopted in multiple countries, including Australia, the Netherlands, Turkey, England, and Argentina.
In the United States, there are 3 currently acceptable screening strategies: cytology, cytology plus HPV (cotesting), and primary HPV testing (TABLE). The American Cancer Society (ACS) specifically states that HPV testing alone every 5 years is preferred starting at age 25 years; cotesting every 5 years or cytology alone every 3 years are also acceptable.11 The US Preventive Services Task Force (USPSTF) states that cytology alone every 3 years starting at 21 years and then HPV testing alone or cotesting every 5 years or cytology every 3 years starting at age 30 are all acceptable strategies.12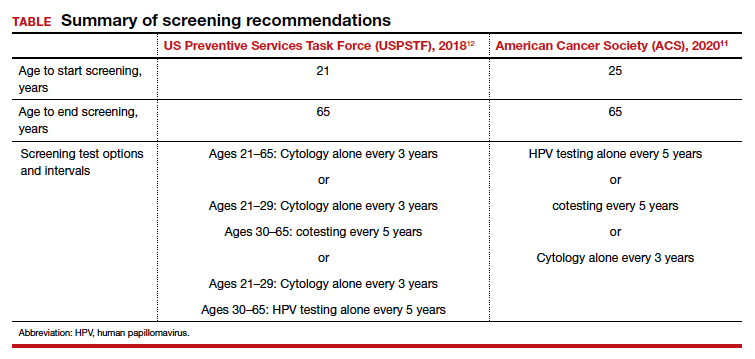
When applying these guidelines, it is important to note that they are intended for the screening of patients with all prior normal results with no symptoms. These routine screening guidelines do not apply to special populations, such as those with a history of abnormal results or treatment, a history of immunosuppression,13 a history of HPV-related vulvar or vaginal dysplasia,14-16 or a history of hysterectomy with removal of the cervix and no prior history of cervical dysplasia.17,18 By contrast, surveillance is interval testing for those who have either an abnormal prior test result or treatment; these may be managed per risk-based estimates provided by the American Society for Colposcopy and Cervical Pathology (ASCCP).18,19 Finally, diagnosis is evaluation (which may include diagnostic cytology) of a patient with abnormal signs and/or symptoms (such as bleeding, pain, discharge, or cervical mass).
In this Update, we present the evidence for primary HPV testing, the management options for a positive result in the United States, and research that will improve uptake of primary HPV testing as well as accessibility.
Change in screening paradigm: Evidence for primary HPV testing
HPV DNA tests are multiplex assays that detect the DNA of targeted high-risk HPV types, using multiple probes, either by direct genomic detection or by amplification of a viral DNA fragment using polymerase chain reaction (PCR).20,21 Alternatively, HPV mRNA-based tests detect the expression of E6 and E7 oncoproteins, a marker of viral integration.20 In examining the data from well-conducted clinical trials, 2 important observations are that different HPV assays were used and that direct comparison may not be valid. In addition, not all tests used in the studies are approved by the US Food and Drug Administration (FDA) for primary HPV testing.
Continue to: FDA-approved HPV tests...
FDA-approved HPV tests
Currently, 2 tests are FDA approved for primary HPV screening. The Cobas HPV test (Roche Molecular Diagnostics) was the first FDA-approved test for primary HPV screening in women aged 25 years and older.6 This test reports pooled results from 12 high-risk (hr) HPV types (31/33/35/39/45/51/52/56/58/59/66/68) with reflex genotyping for HPV 16/18, and thus it provides an immediate triage option for HPV-positive women. Of note, it is also approved for cotesting. The second FDA-approved test is the BD Onclarity HPV assay (Becton, Dickinson and Company) for primary HPV screening.22 It detects 14 hrHPV types, types 16/18/45 specifically as well as types 31/33/35/39/51/52/56/58/59/66/68.
Other HPV tests are FDA approved for cotesting and reflex testing but not for primary HPV testing. The Hybrid Capture test, or HC2 (Qiagen Inc), was the first HPV test to be approved by the FDA in 1997 for reflex testing of women with ASCUS cytology. In 2003, it was approved for cotesting along with cytology in women aged 30 years and older.20,21 In 2009, the Cervista HPV HR test (Hologic Inc) was approved for cotesting. The Aptima HPV assay (Hologic Inc), which is also approved for cotesting, is an RNA-based assay that allows detection of E6/E7 mRNA transcripts of 14 HPV types.23
Comparing HPV testing with cytology
Ronco and colleagues pooled data from 4 European randomized controlled trials (RCTs)—Swedescreen, POBASCAM, NTCC, ARTISTIC—with a total of 176,464 participants randomly assigned to HPV or cytology screening.24 Swedescreen and POBASCAM used GP5/GP6 PCR, while ARTISTIC and NTCC used HC2 for primary HPV screening. The screening interval was 3 years in all except 5 years in POBASCAM. The pooled detection rate of invasive disease was similar in the 2 arms, with pooled rate ratio for cancer detection being 0.79 (95% confidence interval [CI], 0.46–1.36) in the first 2.5 years, but was 0.45 (95% CI, 0.25–0.81), favoring the HPV arm, after 2.5 years. HPV testing was more effective in preventing cases of adenocarcinoma than squamous cell carcinoma (0.31 [95% CI, 0.14–0.69] vs 0.78 [95% CI, 0.49–1.25]). The authors concluded that HPV-based screening from age 30 years provided 60% to 70% better protection than cytology.
The result of the above meta-analysis was confirmed by the HPV FOCAL RCT that investigated the efficacy of HPV testing (HC2) in comparison with cytology.25 The detection rates for CIN 3 lesions supported primary HPV screening, with an absolute difference in incidence rate of 2.67/1,000 (95% CI, 0.53–4.88) at study randomization and 3.22/1,000 (95% CI, 5.12–1.48) at study exit 4 years later.
Cotesting using HPV and cytology: Marginal benefit
Dillner and colleagues were one of the first groups to report on the risk of CIN 3 based on both HPV and cytology status.26 Using pooled analysis of data from multiple countries, these investigators reported that the cumulative incidence rates (CIR) of CIN 3 after 6 years of follow-up increased consistently in HPV-positive subjects, and an HPV-positive result more accurately predicted CIN 3+ at 5 years than cytology alone. Furthermore, HPV negativity provided greater reassurance than cytology alone. At 5 years of follow-up, the rates of CIN 3+ were 0.25% (0.12%–0.41%) for women negative for HPV compared with 0.83% (0.50%–1.13%) for women with negative cytology results. There was little difference in rates for CIN 3+ between women with negative results on both tests and women who were negative for HPV.
The important question is then the marginal benefit of cotesting, which is the most costly screening option. A study of 331,818 women enrolled for cotesting at Kaiser Permanente found that the risk of CIN 3+ predicted by HPV testing alone when compared with cytology was significantly higher at both 3 years (5.0% vs 3.8%; P = .046) and 5 years (7.6% vs 4.7%; P = .001).27 A negative cytology result did not decrease the risk of CIN 3 further for HPV-negative patients (3 years: 0.047% vs 0.063%, P = .6; 5 years: 0.16% vs 0.17%, P = .8). They concluded that a negative HPV test was enough reassurance for low risk of CIN 3+ and that an additional negative cytology result does not provide extra reassurance.
Furthermore, a systematic meta-analysis of 48 studies, including 8 RCTs, found that the addition of cytology to HPV testing raised the sensitivity by 2% for CIN 3 compared with HPV testing alone. This improvement in sensitivity was at the expense of considerable loss of specificity, with a ratio of 0.93 (95% CI, 0.92–0.95) for CIN 3.28 Schiffman and colleagues also assessed the relative contribution of HPV testing and cytology in detection of CIN 3 and cancer.29 The HPV component alone identified a significantly higher proportion of preinvasive and invasive disease than cytology. Only 3.5% of precancers and 5.9% of cancers were preceded by HPV-negative, cytology-positive results. Thus, cytology contributed only 5 cases per million women per year to the sensitivity of the combined test, at the cost of significantly more colposcopies. Hence, the evidence suggests that there is limited benefit of adding cytology to HPV testing.30
Continue to: Triage of a positive HPV result...
Triage of a positive HPV result
An important limitation of HPV testing is its inability to discriminate between transient and persistent infections. Referral of all HPV-positive cases to colposcopy would overburden the system with associated unnecessary procedures. Hence, a triage strategy is essential to identify clinically important infections that truly require colposcopic evaluation. The FIGURE illustrates the management of a primary HPV test result performed for screening.
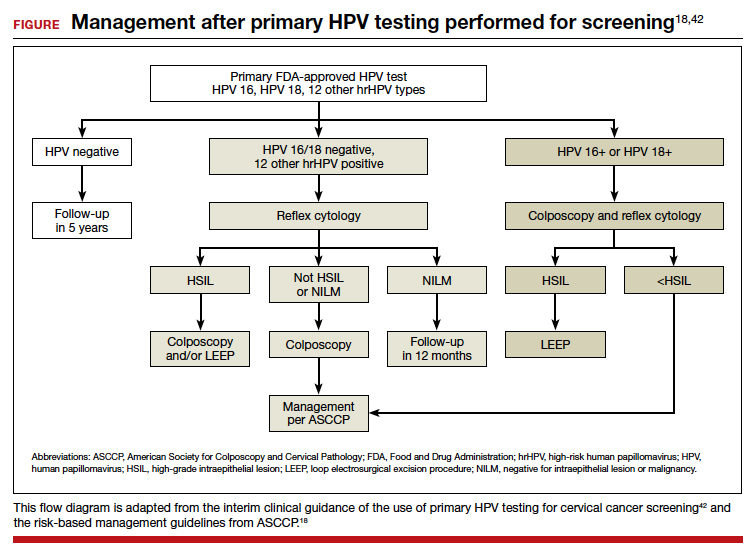
HPV genotyping
One strategy for triaging a positive HPV test result is genotyping. HPV 16 and 18 have the highest risk of persistence and progression and merit immediate referral to colposcopy. In the ATHENA trial, CIN 3 was identified in 17.8% (95% CI, 14.8–20.7%) of HPV 16 positive women at baseline, and the CIR increased to 25.2% (95% CI, 21.7–28.7%) after 3 years. The 3-year CIR of CIN 3 was only 5.4% (95% CI, 4.5–6.3%) in women with HPV genotypes other than 16/18. HPV 18–positive women had a 3-year CIR that was intermediate between women with HPV 16 and women with the 12 other genotypes.6 Hence, HPV 16/18–positive cases should be referred for immediate colposcopy, and negative cases should be followed up with cytology and referred for colposcopy if the cytology is ASCUS or worse.31
In July 2020, extended genotyping was approved by the FDA with individual detection of HPV 31, 51, 52 (in addition to 16, 18, and 45) and pooled detection of 33/58, 35/39/68, and 56/59/66. One study found that individual genotypes HPV 16 and 31 carry baseline risk values for CIN 3+ (8.1% and 7.5%, respectively) that are above the 5-year risk threshold for referral to colposcopy following the ASCCP risk-based management guideline.32
Cytology
The higher specificity of cytology makes it an option for triaging HPV-positive cases, and current management guidelines recommend triage to both genotyping and cytology for all patients who are HPV positive, and especially if they are HPV positive but HPV 16/18 negative. Of note, cytology results remain more subjective than those of primary HPV testing, but the combination of initial HPV testing with reflex to cytology is a reasonable and cost effective next step.18 The VASCAR trial found higher colposcopy referrals in the HPV screening and cytology triage group compared with the cytology alone group (19.36 vs 14.54 per 1,000 women).33 The ATHENA trial investigated various triage strategies for HPV-positive cases and its impact on colposcopy referrals.6 Using HPV genotyping and reflex cytology, if HPV 16/18 was positive, colposcopy was advised, but if any of the other 12 HPV types were positive, reflex cytology was done. If reported as ASCUS or worse, colposcopy was performed; conversely, if it was normal, women were rescreened with cotesting after 1 year. Although this strategy led to a reduction in the number of colposcopies, referrals were still higher in the primary HPV arm (3,769 colposcopies per 294 cases) compared with cytology (1,934 colposcopies per 179 cases) or cotesting (3,097 colposcopies per 240 cases) in women aged 25 years.14
p16/Ki-67 Dual-Stain
Diffused p16 immunohistochemical staining, as opposed to focal staining, is associated with active HPV infection but can be present in low-grade as well as high-grade lesions.34 Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, these tests are supportive of active HPV infection and of a high-grade lesion. Incorporation of these stains to cytology alone provides additional objective reassurance to cytology, where there is much inter- and intra-observer variability. These stains can be done by laboratories using the stains alone or they can use the FDA-approved p16/Ki-67 Dual-Stain immunohistochemistry (DS), CINtec PLUS Cytology (Roche Diagnostics). However, DS is not yet formally incorporated into triage algorithms by national guidelines.
The IMPACT trial assessed the performance of DS compared with cytology in the triage of HPV-positive results, with or without HPV 16/18 genotyping.35 This was a prospective observational screening study of 35,263 women aged 25 to 65 years across 32 sites in the United States. Of the 4,927 HPV-positive patients with DS results, the sensitivity of DS for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) and 86.0% (95% CI, 77.5%–91.6%) in HPV 16/18–positive and in the 12 other genotypes, respectively. Using DS alone to triage HPV-positive results showed significantly higher sensitivity and specificity than HPV 16/18 genotyping with cytology triage of 12 “other” genotypes, and substantially higher sensitivity but lower specificity than using cytology alone. Of note, triage with DS alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Similarly, a retrospective analysis of the ATHENA trial cohort of HPV-positive results of 7,727 patients aged 25 years or older also demonstrated increased sensitivity of DS compared with cytology (74.9% vs 51.9%; P<.0001) and similar specificities (74.1% vs 75%; P = .3198).36 The European PALMS study, which included 27,349 women aged 18 years or older across 5 countries who underwent routine screening with HPV testing, cytology, and DS, confirmed these findings.37 The sensitivity of DS was higher than that of cytology (86.7% vs 68.5%; P<.001) for CIN 3+ with comparable specificities (95.2% vs 95.4%; P = .15).
Challenges and opportunities to improve access to primary HPV screening
The historical success of the Pap test in reducing the incidence of cervical cancer relied on individuals having access to the test. This remains true as screening transitions to primary HPV testing. Limitations of HPV-based screening include provider and patient knowledge; access to tests; cost; need for new laboratory infrastructure; need to leverage the electronic health record to record results, calculate a patient’s risk and determine next steps; and the need to re-educate patients and providers about this new model of care. The American Cancer Society and the Centers for Disease Control and Prevention are currently leading initiatives to help adopt primary HPV screening in the United States and to facilitate new care approaches.
Self-collection and independence from subjective cytology would further improve access. Multiple effectiveness studies and patient acceptability studies have shown that primary HPV screening via self-collection is effective, cost effective, and acceptable to women, especially among underscreened populations.38 Sensitivity is comparable to clinician-obtained samples with polymerase chain reaction–based HPV tests. Furthermore, newer molecular tests that detect methylated target host genes or methylated viral genome can be used to triage HPV-positive cases. Several host methylation markers that identify the specific host genes (for example, CADM1, MAL, and miR-124-2) have been shown to be more specific, reproducible, and can be used in self-collected samples as they are based on molecular methylation analysis.39 The ASCCP monitors these new developments and will incorporate promising tests and approaches once validated and FDA approved into the risk-based management guidelines. An erratum was recently published, and the risk-calculator is also available on the ASCCP website free of charge (https://app.asccp.org).40
In conclusion, transition to primary HPV testing from Pap cytology in cervical cancer screening has many challenges but also opportunities. Learning from the experience of countries that have already adopted primary HPV testing is crucial to successful implementation of this new screening paradigm.41 The evidence supporting primary HPV screening with its improved sensitivity is clear, and the existing triage options and innovations will continue to improve triage of patients with clinically important lesions as well as accessibility. With strong advocacy and sound implementation, the WHO goal of cervical cancer elimination and 70% of women being screened with a high-performance test by age 35 and again by age 45 is achievable. ●
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71: 209-249.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5-14.
- Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Prac Res Clin Obstet Gynaecol. 2018;47:107-118.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94-101.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd edition. Geneva: 2021. https://www .who.int/publications/i/item/9789240030824. Accessed April 28, 2022.
- American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer. American Cancer Society; 2020. https://www.cancer .org/cancer/cervical-cancer/detection-diagnosis-staging /cervical-cancer-screening-guidelines.html. Accessed April 28, 2022.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens KD, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Gen Tract Dis. 2019;23:87-101.
- Committee opinion no. 675. Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol. 2018;148:126-131.
- Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:10511062.
- Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-370.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2020;24:102-131.
- Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Gen Tract Dis. 2020;24:132-143.
- Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:209-220.
- Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520.
- Ejegod D, Bottari F, Pedersen H, et al. The BD Onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54:2267-2272.
- Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343-353.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: followup of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88-99.
- Schiffman M, Kinney WK, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Nat Cancer Inst. 2018;110:501-508.
- Jin XW, Lipold L, Foucher J, et al. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J Gen Intern Med. 2016;31:1338-1344.
- Tota JE, Bentley J, Blake J, et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev Med. 2017;98:15-20.
- Stoler MH, Wright TC Jr, Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol. 2019;153:26-33.
- Louvanto K, Chevarie-Davis M, Ramanakumar AV, et al. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210:474.e1-7.
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884-891.
- Wright TC Jr, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471.
- Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51-56.
- Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Nat Cancer Inst. 2013;105:15501557.
- Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315-322.
- Perkins RB, Guido RS, Castle PE, et al. Erratum: 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2021;25:330-331.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19-e27.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-182.
Cervical cancer is an important global health problem with an estimated 604,127 new cases and 341,831 deaths in 2020.1 Nearly 85% of the disease burden affects individuals from low and middle-income countries. The World Health Organization (WHO) set forth the goal for all countries to reach and maintain an incidence rate of below 4 per 100,000 women by 2030 as part of the Global Strategy to Accelerate the Elimination of Cervical Cancer.
Although traditional Pap cytology has been the cornerstone of screening programs, its poor sensitivity of approximately 50% and limitations in accessibility require new strategies to achieve the elimination of cervical cancer.2 The discovery that persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of cervical cancer led to the development of diagnostic HPV tests, which have higher sensitivity than cytology (96.1% vs 53.0%) but somewhat lower specificity (90.7% vs 96.3%) for the detection of cervical intraepithelial neoplasia (CIN) 2 or worse lesions.2 Initially, HPV testing was incorporated as a method to triage atypical squamous cells of undetermined significance (ASCUS) cytology results.3 Later, the concept of cotesting with cytology emerged,4,5 and since then, several clinical trials have demonstrated the effectiveness of primary HPV screening.6-9
In 2020, the WHO recommended HPV DNA testing as the primary screening method starting at the age of 30 years, with regular testing every 5 to 10 years, for the general population.10 Currently, primary HPV has been adopted in multiple countries, including Australia, the Netherlands, Turkey, England, and Argentina.
In the United States, there are 3 currently acceptable screening strategies: cytology, cytology plus HPV (cotesting), and primary HPV testing (TABLE). The American Cancer Society (ACS) specifically states that HPV testing alone every 5 years is preferred starting at age 25 years; cotesting every 5 years or cytology alone every 3 years are also acceptable.11 The US Preventive Services Task Force (USPSTF) states that cytology alone every 3 years starting at 21 years and then HPV testing alone or cotesting every 5 years or cytology every 3 years starting at age 30 are all acceptable strategies.12
When applying these guidelines, it is important to note that they are intended for the screening of patients with all prior normal results with no symptoms. These routine screening guidelines do not apply to special populations, such as those with a history of abnormal results or treatment, a history of immunosuppression,13 a history of HPV-related vulvar or vaginal dysplasia,14-16 or a history of hysterectomy with removal of the cervix and no prior history of cervical dysplasia.17,18 By contrast, surveillance is interval testing for those who have either an abnormal prior test result or treatment; these may be managed per risk-based estimates provided by the American Society for Colposcopy and Cervical Pathology (ASCCP).18,19 Finally, diagnosis is evaluation (which may include diagnostic cytology) of a patient with abnormal signs and/or symptoms (such as bleeding, pain, discharge, or cervical mass).
In this Update, we present the evidence for primary HPV testing, the management options for a positive result in the United States, and research that will improve uptake of primary HPV testing as well as accessibility.
Change in screening paradigm: Evidence for primary HPV testing
HPV DNA tests are multiplex assays that detect the DNA of targeted high-risk HPV types, using multiple probes, either by direct genomic detection or by amplification of a viral DNA fragment using polymerase chain reaction (PCR).20,21 Alternatively, HPV mRNA-based tests detect the expression of E6 and E7 oncoproteins, a marker of viral integration.20 In examining the data from well-conducted clinical trials, 2 important observations are that different HPV assays were used and that direct comparison may not be valid. In addition, not all tests used in the studies are approved by the US Food and Drug Administration (FDA) for primary HPV testing.
Continue to: FDA-approved HPV tests...
FDA-approved HPV tests
Currently, 2 tests are FDA approved for primary HPV screening. The Cobas HPV test (Roche Molecular Diagnostics) was the first FDA-approved test for primary HPV screening in women aged 25 years and older.6 This test reports pooled results from 12 high-risk (hr) HPV types (31/33/35/39/45/51/52/56/58/59/66/68) with reflex genotyping for HPV 16/18, and thus it provides an immediate triage option for HPV-positive women. Of note, it is also approved for cotesting. The second FDA-approved test is the BD Onclarity HPV assay (Becton, Dickinson and Company) for primary HPV screening.22 It detects 14 hrHPV types, types 16/18/45 specifically as well as types 31/33/35/39/51/52/56/58/59/66/68.
Other HPV tests are FDA approved for cotesting and reflex testing but not for primary HPV testing. The Hybrid Capture test, or HC2 (Qiagen Inc), was the first HPV test to be approved by the FDA in 1997 for reflex testing of women with ASCUS cytology. In 2003, it was approved for cotesting along with cytology in women aged 30 years and older.20,21 In 2009, the Cervista HPV HR test (Hologic Inc) was approved for cotesting. The Aptima HPV assay (Hologic Inc), which is also approved for cotesting, is an RNA-based assay that allows detection of E6/E7 mRNA transcripts of 14 HPV types.23
Comparing HPV testing with cytology
Ronco and colleagues pooled data from 4 European randomized controlled trials (RCTs)—Swedescreen, POBASCAM, NTCC, ARTISTIC—with a total of 176,464 participants randomly assigned to HPV or cytology screening.24 Swedescreen and POBASCAM used GP5/GP6 PCR, while ARTISTIC and NTCC used HC2 for primary HPV screening. The screening interval was 3 years in all except 5 years in POBASCAM. The pooled detection rate of invasive disease was similar in the 2 arms, with pooled rate ratio for cancer detection being 0.79 (95% confidence interval [CI], 0.46–1.36) in the first 2.5 years, but was 0.45 (95% CI, 0.25–0.81), favoring the HPV arm, after 2.5 years. HPV testing was more effective in preventing cases of adenocarcinoma than squamous cell carcinoma (0.31 [95% CI, 0.14–0.69] vs 0.78 [95% CI, 0.49–1.25]). The authors concluded that HPV-based screening from age 30 years provided 60% to 70% better protection than cytology.
The result of the above meta-analysis was confirmed by the HPV FOCAL RCT that investigated the efficacy of HPV testing (HC2) in comparison with cytology.25 The detection rates for CIN 3 lesions supported primary HPV screening, with an absolute difference in incidence rate of 2.67/1,000 (95% CI, 0.53–4.88) at study randomization and 3.22/1,000 (95% CI, 5.12–1.48) at study exit 4 years later.
Cotesting using HPV and cytology: Marginal benefit
Dillner and colleagues were one of the first groups to report on the risk of CIN 3 based on both HPV and cytology status.26 Using pooled analysis of data from multiple countries, these investigators reported that the cumulative incidence rates (CIR) of CIN 3 after 6 years of follow-up increased consistently in HPV-positive subjects, and an HPV-positive result more accurately predicted CIN 3+ at 5 years than cytology alone. Furthermore, HPV negativity provided greater reassurance than cytology alone. At 5 years of follow-up, the rates of CIN 3+ were 0.25% (0.12%–0.41%) for women negative for HPV compared with 0.83% (0.50%–1.13%) for women with negative cytology results. There was little difference in rates for CIN 3+ between women with negative results on both tests and women who were negative for HPV.
The important question is then the marginal benefit of cotesting, which is the most costly screening option. A study of 331,818 women enrolled for cotesting at Kaiser Permanente found that the risk of CIN 3+ predicted by HPV testing alone when compared with cytology was significantly higher at both 3 years (5.0% vs 3.8%; P = .046) and 5 years (7.6% vs 4.7%; P = .001).27 A negative cytology result did not decrease the risk of CIN 3 further for HPV-negative patients (3 years: 0.047% vs 0.063%, P = .6; 5 years: 0.16% vs 0.17%, P = .8). They concluded that a negative HPV test was enough reassurance for low risk of CIN 3+ and that an additional negative cytology result does not provide extra reassurance.
Furthermore, a systematic meta-analysis of 48 studies, including 8 RCTs, found that the addition of cytology to HPV testing raised the sensitivity by 2% for CIN 3 compared with HPV testing alone. This improvement in sensitivity was at the expense of considerable loss of specificity, with a ratio of 0.93 (95% CI, 0.92–0.95) for CIN 3.28 Schiffman and colleagues also assessed the relative contribution of HPV testing and cytology in detection of CIN 3 and cancer.29 The HPV component alone identified a significantly higher proportion of preinvasive and invasive disease than cytology. Only 3.5% of precancers and 5.9% of cancers were preceded by HPV-negative, cytology-positive results. Thus, cytology contributed only 5 cases per million women per year to the sensitivity of the combined test, at the cost of significantly more colposcopies. Hence, the evidence suggests that there is limited benefit of adding cytology to HPV testing.30
Continue to: Triage of a positive HPV result...
Triage of a positive HPV result
An important limitation of HPV testing is its inability to discriminate between transient and persistent infections. Referral of all HPV-positive cases to colposcopy would overburden the system with associated unnecessary procedures. Hence, a triage strategy is essential to identify clinically important infections that truly require colposcopic evaluation. The FIGURE illustrates the management of a primary HPV test result performed for screening.

HPV genotyping
One strategy for triaging a positive HPV test result is genotyping. HPV 16 and 18 have the highest risk of persistence and progression and merit immediate referral to colposcopy. In the ATHENA trial, CIN 3 was identified in 17.8% (95% CI, 14.8–20.7%) of HPV 16 positive women at baseline, and the CIR increased to 25.2% (95% CI, 21.7–28.7%) after 3 years. The 3-year CIR of CIN 3 was only 5.4% (95% CI, 4.5–6.3%) in women with HPV genotypes other than 16/18. HPV 18–positive women had a 3-year CIR that was intermediate between women with HPV 16 and women with the 12 other genotypes.6 Hence, HPV 16/18–positive cases should be referred for immediate colposcopy, and negative cases should be followed up with cytology and referred for colposcopy if the cytology is ASCUS or worse.31
In July 2020, extended genotyping was approved by the FDA with individual detection of HPV 31, 51, 52 (in addition to 16, 18, and 45) and pooled detection of 33/58, 35/39/68, and 56/59/66. One study found that individual genotypes HPV 16 and 31 carry baseline risk values for CIN 3+ (8.1% and 7.5%, respectively) that are above the 5-year risk threshold for referral to colposcopy following the ASCCP risk-based management guideline.32
Cytology
The higher specificity of cytology makes it an option for triaging HPV-positive cases, and current management guidelines recommend triage to both genotyping and cytology for all patients who are HPV positive, and especially if they are HPV positive but HPV 16/18 negative. Of note, cytology results remain more subjective than those of primary HPV testing, but the combination of initial HPV testing with reflex to cytology is a reasonable and cost effective next step.18 The VASCAR trial found higher colposcopy referrals in the HPV screening and cytology triage group compared with the cytology alone group (19.36 vs 14.54 per 1,000 women).33 The ATHENA trial investigated various triage strategies for HPV-positive cases and its impact on colposcopy referrals.6 Using HPV genotyping and reflex cytology, if HPV 16/18 was positive, colposcopy was advised, but if any of the other 12 HPV types were positive, reflex cytology was done. If reported as ASCUS or worse, colposcopy was performed; conversely, if it was normal, women were rescreened with cotesting after 1 year. Although this strategy led to a reduction in the number of colposcopies, referrals were still higher in the primary HPV arm (3,769 colposcopies per 294 cases) compared with cytology (1,934 colposcopies per 179 cases) or cotesting (3,097 colposcopies per 240 cases) in women aged 25 years.14
p16/Ki-67 Dual-Stain
Diffused p16 immunohistochemical staining, as opposed to focal staining, is associated with active HPV infection but can be present in low-grade as well as high-grade lesions.34 Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, these tests are supportive of active HPV infection and of a high-grade lesion. Incorporation of these stains to cytology alone provides additional objective reassurance to cytology, where there is much inter- and intra-observer variability. These stains can be done by laboratories using the stains alone or they can use the FDA-approved p16/Ki-67 Dual-Stain immunohistochemistry (DS), CINtec PLUS Cytology (Roche Diagnostics). However, DS is not yet formally incorporated into triage algorithms by national guidelines.
The IMPACT trial assessed the performance of DS compared with cytology in the triage of HPV-positive results, with or without HPV 16/18 genotyping.35 This was a prospective observational screening study of 35,263 women aged 25 to 65 years across 32 sites in the United States. Of the 4,927 HPV-positive patients with DS results, the sensitivity of DS for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) and 86.0% (95% CI, 77.5%–91.6%) in HPV 16/18–positive and in the 12 other genotypes, respectively. Using DS alone to triage HPV-positive results showed significantly higher sensitivity and specificity than HPV 16/18 genotyping with cytology triage of 12 “other” genotypes, and substantially higher sensitivity but lower specificity than using cytology alone. Of note, triage with DS alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Similarly, a retrospective analysis of the ATHENA trial cohort of HPV-positive results of 7,727 patients aged 25 years or older also demonstrated increased sensitivity of DS compared with cytology (74.9% vs 51.9%; P<.0001) and similar specificities (74.1% vs 75%; P = .3198).36 The European PALMS study, which included 27,349 women aged 18 years or older across 5 countries who underwent routine screening with HPV testing, cytology, and DS, confirmed these findings.37 The sensitivity of DS was higher than that of cytology (86.7% vs 68.5%; P<.001) for CIN 3+ with comparable specificities (95.2% vs 95.4%; P = .15).
Challenges and opportunities to improve access to primary HPV screening
The historical success of the Pap test in reducing the incidence of cervical cancer relied on individuals having access to the test. This remains true as screening transitions to primary HPV testing. Limitations of HPV-based screening include provider and patient knowledge; access to tests; cost; need for new laboratory infrastructure; need to leverage the electronic health record to record results, calculate a patient’s risk and determine next steps; and the need to re-educate patients and providers about this new model of care. The American Cancer Society and the Centers for Disease Control and Prevention are currently leading initiatives to help adopt primary HPV screening in the United States and to facilitate new care approaches.
Self-collection and independence from subjective cytology would further improve access. Multiple effectiveness studies and patient acceptability studies have shown that primary HPV screening via self-collection is effective, cost effective, and acceptable to women, especially among underscreened populations.38 Sensitivity is comparable to clinician-obtained samples with polymerase chain reaction–based HPV tests. Furthermore, newer molecular tests that detect methylated target host genes or methylated viral genome can be used to triage HPV-positive cases. Several host methylation markers that identify the specific host genes (for example, CADM1, MAL, and miR-124-2) have been shown to be more specific, reproducible, and can be used in self-collected samples as they are based on molecular methylation analysis.39 The ASCCP monitors these new developments and will incorporate promising tests and approaches once validated and FDA approved into the risk-based management guidelines. An erratum was recently published, and the risk-calculator is also available on the ASCCP website free of charge (https://app.asccp.org).40
In conclusion, transition to primary HPV testing from Pap cytology in cervical cancer screening has many challenges but also opportunities. Learning from the experience of countries that have already adopted primary HPV testing is crucial to successful implementation of this new screening paradigm.41 The evidence supporting primary HPV screening with its improved sensitivity is clear, and the existing triage options and innovations will continue to improve triage of patients with clinically important lesions as well as accessibility. With strong advocacy and sound implementation, the WHO goal of cervical cancer elimination and 70% of women being screened with a high-performance test by age 35 and again by age 45 is achievable. ●
Cervical cancer is an important global health problem with an estimated 604,127 new cases and 341,831 deaths in 2020.1 Nearly 85% of the disease burden affects individuals from low and middle-income countries. The World Health Organization (WHO) set forth the goal for all countries to reach and maintain an incidence rate of below 4 per 100,000 women by 2030 as part of the Global Strategy to Accelerate the Elimination of Cervical Cancer.
Although traditional Pap cytology has been the cornerstone of screening programs, its poor sensitivity of approximately 50% and limitations in accessibility require new strategies to achieve the elimination of cervical cancer.2 The discovery that persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of cervical cancer led to the development of diagnostic HPV tests, which have higher sensitivity than cytology (96.1% vs 53.0%) but somewhat lower specificity (90.7% vs 96.3%) for the detection of cervical intraepithelial neoplasia (CIN) 2 or worse lesions.2 Initially, HPV testing was incorporated as a method to triage atypical squamous cells of undetermined significance (ASCUS) cytology results.3 Later, the concept of cotesting with cytology emerged,4,5 and since then, several clinical trials have demonstrated the effectiveness of primary HPV screening.6-9
In 2020, the WHO recommended HPV DNA testing as the primary screening method starting at the age of 30 years, with regular testing every 5 to 10 years, for the general population.10 Currently, primary HPV has been adopted in multiple countries, including Australia, the Netherlands, Turkey, England, and Argentina.
In the United States, there are 3 currently acceptable screening strategies: cytology, cytology plus HPV (cotesting), and primary HPV testing (TABLE). The American Cancer Society (ACS) specifically states that HPV testing alone every 5 years is preferred starting at age 25 years; cotesting every 5 years or cytology alone every 3 years are also acceptable.11 The US Preventive Services Task Force (USPSTF) states that cytology alone every 3 years starting at 21 years and then HPV testing alone or cotesting every 5 years or cytology every 3 years starting at age 30 are all acceptable strategies.12
When applying these guidelines, it is important to note that they are intended for the screening of patients with all prior normal results with no symptoms. These routine screening guidelines do not apply to special populations, such as those with a history of abnormal results or treatment, a history of immunosuppression,13 a history of HPV-related vulvar or vaginal dysplasia,14-16 or a history of hysterectomy with removal of the cervix and no prior history of cervical dysplasia.17,18 By contrast, surveillance is interval testing for those who have either an abnormal prior test result or treatment; these may be managed per risk-based estimates provided by the American Society for Colposcopy and Cervical Pathology (ASCCP).18,19 Finally, diagnosis is evaluation (which may include diagnostic cytology) of a patient with abnormal signs and/or symptoms (such as bleeding, pain, discharge, or cervical mass).
In this Update, we present the evidence for primary HPV testing, the management options for a positive result in the United States, and research that will improve uptake of primary HPV testing as well as accessibility.
Change in screening paradigm: Evidence for primary HPV testing
HPV DNA tests are multiplex assays that detect the DNA of targeted high-risk HPV types, using multiple probes, either by direct genomic detection or by amplification of a viral DNA fragment using polymerase chain reaction (PCR).20,21 Alternatively, HPV mRNA-based tests detect the expression of E6 and E7 oncoproteins, a marker of viral integration.20 In examining the data from well-conducted clinical trials, 2 important observations are that different HPV assays were used and that direct comparison may not be valid. In addition, not all tests used in the studies are approved by the US Food and Drug Administration (FDA) for primary HPV testing.
Continue to: FDA-approved HPV tests...
FDA-approved HPV tests
Currently, 2 tests are FDA approved for primary HPV screening. The Cobas HPV test (Roche Molecular Diagnostics) was the first FDA-approved test for primary HPV screening in women aged 25 years and older.6 This test reports pooled results from 12 high-risk (hr) HPV types (31/33/35/39/45/51/52/56/58/59/66/68) with reflex genotyping for HPV 16/18, and thus it provides an immediate triage option for HPV-positive women. Of note, it is also approved for cotesting. The second FDA-approved test is the BD Onclarity HPV assay (Becton, Dickinson and Company) for primary HPV screening.22 It detects 14 hrHPV types, types 16/18/45 specifically as well as types 31/33/35/39/51/52/56/58/59/66/68.
Other HPV tests are FDA approved for cotesting and reflex testing but not for primary HPV testing. The Hybrid Capture test, or HC2 (Qiagen Inc), was the first HPV test to be approved by the FDA in 1997 for reflex testing of women with ASCUS cytology. In 2003, it was approved for cotesting along with cytology in women aged 30 years and older.20,21 In 2009, the Cervista HPV HR test (Hologic Inc) was approved for cotesting. The Aptima HPV assay (Hologic Inc), which is also approved for cotesting, is an RNA-based assay that allows detection of E6/E7 mRNA transcripts of 14 HPV types.23
Comparing HPV testing with cytology
Ronco and colleagues pooled data from 4 European randomized controlled trials (RCTs)—Swedescreen, POBASCAM, NTCC, ARTISTIC—with a total of 176,464 participants randomly assigned to HPV or cytology screening.24 Swedescreen and POBASCAM used GP5/GP6 PCR, while ARTISTIC and NTCC used HC2 for primary HPV screening. The screening interval was 3 years in all except 5 years in POBASCAM. The pooled detection rate of invasive disease was similar in the 2 arms, with pooled rate ratio for cancer detection being 0.79 (95% confidence interval [CI], 0.46–1.36) in the first 2.5 years, but was 0.45 (95% CI, 0.25–0.81), favoring the HPV arm, after 2.5 years. HPV testing was more effective in preventing cases of adenocarcinoma than squamous cell carcinoma (0.31 [95% CI, 0.14–0.69] vs 0.78 [95% CI, 0.49–1.25]). The authors concluded that HPV-based screening from age 30 years provided 60% to 70% better protection than cytology.
The result of the above meta-analysis was confirmed by the HPV FOCAL RCT that investigated the efficacy of HPV testing (HC2) in comparison with cytology.25 The detection rates for CIN 3 lesions supported primary HPV screening, with an absolute difference in incidence rate of 2.67/1,000 (95% CI, 0.53–4.88) at study randomization and 3.22/1,000 (95% CI, 5.12–1.48) at study exit 4 years later.
Cotesting using HPV and cytology: Marginal benefit
Dillner and colleagues were one of the first groups to report on the risk of CIN 3 based on both HPV and cytology status.26 Using pooled analysis of data from multiple countries, these investigators reported that the cumulative incidence rates (CIR) of CIN 3 after 6 years of follow-up increased consistently in HPV-positive subjects, and an HPV-positive result more accurately predicted CIN 3+ at 5 years than cytology alone. Furthermore, HPV negativity provided greater reassurance than cytology alone. At 5 years of follow-up, the rates of CIN 3+ were 0.25% (0.12%–0.41%) for women negative for HPV compared with 0.83% (0.50%–1.13%) for women with negative cytology results. There was little difference in rates for CIN 3+ between women with negative results on both tests and women who were negative for HPV.
The important question is then the marginal benefit of cotesting, which is the most costly screening option. A study of 331,818 women enrolled for cotesting at Kaiser Permanente found that the risk of CIN 3+ predicted by HPV testing alone when compared with cytology was significantly higher at both 3 years (5.0% vs 3.8%; P = .046) and 5 years (7.6% vs 4.7%; P = .001).27 A negative cytology result did not decrease the risk of CIN 3 further for HPV-negative patients (3 years: 0.047% vs 0.063%, P = .6; 5 years: 0.16% vs 0.17%, P = .8). They concluded that a negative HPV test was enough reassurance for low risk of CIN 3+ and that an additional negative cytology result does not provide extra reassurance.
Furthermore, a systematic meta-analysis of 48 studies, including 8 RCTs, found that the addition of cytology to HPV testing raised the sensitivity by 2% for CIN 3 compared with HPV testing alone. This improvement in sensitivity was at the expense of considerable loss of specificity, with a ratio of 0.93 (95% CI, 0.92–0.95) for CIN 3.28 Schiffman and colleagues also assessed the relative contribution of HPV testing and cytology in detection of CIN 3 and cancer.29 The HPV component alone identified a significantly higher proportion of preinvasive and invasive disease than cytology. Only 3.5% of precancers and 5.9% of cancers were preceded by HPV-negative, cytology-positive results. Thus, cytology contributed only 5 cases per million women per year to the sensitivity of the combined test, at the cost of significantly more colposcopies. Hence, the evidence suggests that there is limited benefit of adding cytology to HPV testing.30
Continue to: Triage of a positive HPV result...
Triage of a positive HPV result
An important limitation of HPV testing is its inability to discriminate between transient and persistent infections. Referral of all HPV-positive cases to colposcopy would overburden the system with associated unnecessary procedures. Hence, a triage strategy is essential to identify clinically important infections that truly require colposcopic evaluation. The FIGURE illustrates the management of a primary HPV test result performed for screening.

HPV genotyping
One strategy for triaging a positive HPV test result is genotyping. HPV 16 and 18 have the highest risk of persistence and progression and merit immediate referral to colposcopy. In the ATHENA trial, CIN 3 was identified in 17.8% (95% CI, 14.8–20.7%) of HPV 16 positive women at baseline, and the CIR increased to 25.2% (95% CI, 21.7–28.7%) after 3 years. The 3-year CIR of CIN 3 was only 5.4% (95% CI, 4.5–6.3%) in women with HPV genotypes other than 16/18. HPV 18–positive women had a 3-year CIR that was intermediate between women with HPV 16 and women with the 12 other genotypes.6 Hence, HPV 16/18–positive cases should be referred for immediate colposcopy, and negative cases should be followed up with cytology and referred for colposcopy if the cytology is ASCUS or worse.31
In July 2020, extended genotyping was approved by the FDA with individual detection of HPV 31, 51, 52 (in addition to 16, 18, and 45) and pooled detection of 33/58, 35/39/68, and 56/59/66. One study found that individual genotypes HPV 16 and 31 carry baseline risk values for CIN 3+ (8.1% and 7.5%, respectively) that are above the 5-year risk threshold for referral to colposcopy following the ASCCP risk-based management guideline.32
Cytology
The higher specificity of cytology makes it an option for triaging HPV-positive cases, and current management guidelines recommend triage to both genotyping and cytology for all patients who are HPV positive, and especially if they are HPV positive but HPV 16/18 negative. Of note, cytology results remain more subjective than those of primary HPV testing, but the combination of initial HPV testing with reflex to cytology is a reasonable and cost effective next step.18 The VASCAR trial found higher colposcopy referrals in the HPV screening and cytology triage group compared with the cytology alone group (19.36 vs 14.54 per 1,000 women).33 The ATHENA trial investigated various triage strategies for HPV-positive cases and its impact on colposcopy referrals.6 Using HPV genotyping and reflex cytology, if HPV 16/18 was positive, colposcopy was advised, but if any of the other 12 HPV types were positive, reflex cytology was done. If reported as ASCUS or worse, colposcopy was performed; conversely, if it was normal, women were rescreened with cotesting after 1 year. Although this strategy led to a reduction in the number of colposcopies, referrals were still higher in the primary HPV arm (3,769 colposcopies per 294 cases) compared with cytology (1,934 colposcopies per 179 cases) or cotesting (3,097 colposcopies per 240 cases) in women aged 25 years.14
p16/Ki-67 Dual-Stain
Diffused p16 immunohistochemical staining, as opposed to focal staining, is associated with active HPV infection but can be present in low-grade as well as high-grade lesions.34 Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, these tests are supportive of active HPV infection and of a high-grade lesion. Incorporation of these stains to cytology alone provides additional objective reassurance to cytology, where there is much inter- and intra-observer variability. These stains can be done by laboratories using the stains alone or they can use the FDA-approved p16/Ki-67 Dual-Stain immunohistochemistry (DS), CINtec PLUS Cytology (Roche Diagnostics). However, DS is not yet formally incorporated into triage algorithms by national guidelines.
The IMPACT trial assessed the performance of DS compared with cytology in the triage of HPV-positive results, with or without HPV 16/18 genotyping.35 This was a prospective observational screening study of 35,263 women aged 25 to 65 years across 32 sites in the United States. Of the 4,927 HPV-positive patients with DS results, the sensitivity of DS for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) and 86.0% (95% CI, 77.5%–91.6%) in HPV 16/18–positive and in the 12 other genotypes, respectively. Using DS alone to triage HPV-positive results showed significantly higher sensitivity and specificity than HPV 16/18 genotyping with cytology triage of 12 “other” genotypes, and substantially higher sensitivity but lower specificity than using cytology alone. Of note, triage with DS alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Similarly, a retrospective analysis of the ATHENA trial cohort of HPV-positive results of 7,727 patients aged 25 years or older also demonstrated increased sensitivity of DS compared with cytology (74.9% vs 51.9%; P<.0001) and similar specificities (74.1% vs 75%; P = .3198).36 The European PALMS study, which included 27,349 women aged 18 years or older across 5 countries who underwent routine screening with HPV testing, cytology, and DS, confirmed these findings.37 The sensitivity of DS was higher than that of cytology (86.7% vs 68.5%; P<.001) for CIN 3+ with comparable specificities (95.2% vs 95.4%; P = .15).
Challenges and opportunities to improve access to primary HPV screening
The historical success of the Pap test in reducing the incidence of cervical cancer relied on individuals having access to the test. This remains true as screening transitions to primary HPV testing. Limitations of HPV-based screening include provider and patient knowledge; access to tests; cost; need for new laboratory infrastructure; need to leverage the electronic health record to record results, calculate a patient’s risk and determine next steps; and the need to re-educate patients and providers about this new model of care. The American Cancer Society and the Centers for Disease Control and Prevention are currently leading initiatives to help adopt primary HPV screening in the United States and to facilitate new care approaches.
Self-collection and independence from subjective cytology would further improve access. Multiple effectiveness studies and patient acceptability studies have shown that primary HPV screening via self-collection is effective, cost effective, and acceptable to women, especially among underscreened populations.38 Sensitivity is comparable to clinician-obtained samples with polymerase chain reaction–based HPV tests. Furthermore, newer molecular tests that detect methylated target host genes or methylated viral genome can be used to triage HPV-positive cases. Several host methylation markers that identify the specific host genes (for example, CADM1, MAL, and miR-124-2) have been shown to be more specific, reproducible, and can be used in self-collected samples as they are based on molecular methylation analysis.39 The ASCCP monitors these new developments and will incorporate promising tests and approaches once validated and FDA approved into the risk-based management guidelines. An erratum was recently published, and the risk-calculator is also available on the ASCCP website free of charge (https://app.asccp.org).40
In conclusion, transition to primary HPV testing from Pap cytology in cervical cancer screening has many challenges but also opportunities. Learning from the experience of countries that have already adopted primary HPV testing is crucial to successful implementation of this new screening paradigm.41 The evidence supporting primary HPV screening with its improved sensitivity is clear, and the existing triage options and innovations will continue to improve triage of patients with clinically important lesions as well as accessibility. With strong advocacy and sound implementation, the WHO goal of cervical cancer elimination and 70% of women being screened with a high-performance test by age 35 and again by age 45 is achievable. ●
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71: 209-249.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5-14.
- Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Prac Res Clin Obstet Gynaecol. 2018;47:107-118.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94-101.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd edition. Geneva: 2021. https://www .who.int/publications/i/item/9789240030824. Accessed April 28, 2022.
- American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer. American Cancer Society; 2020. https://www.cancer .org/cancer/cervical-cancer/detection-diagnosis-staging /cervical-cancer-screening-guidelines.html. Accessed April 28, 2022.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens KD, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Gen Tract Dis. 2019;23:87-101.
- Committee opinion no. 675. Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol. 2018;148:126-131.
- Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:10511062.
- Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-370.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2020;24:102-131.
- Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Gen Tract Dis. 2020;24:132-143.
- Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:209-220.
- Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520.
- Ejegod D, Bottari F, Pedersen H, et al. The BD Onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54:2267-2272.
- Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343-353.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: followup of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88-99.
- Schiffman M, Kinney WK, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Nat Cancer Inst. 2018;110:501-508.
- Jin XW, Lipold L, Foucher J, et al. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J Gen Intern Med. 2016;31:1338-1344.
- Tota JE, Bentley J, Blake J, et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev Med. 2017;98:15-20.
- Stoler MH, Wright TC Jr, Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol. 2019;153:26-33.
- Louvanto K, Chevarie-Davis M, Ramanakumar AV, et al. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210:474.e1-7.
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884-891.
- Wright TC Jr, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471.
- Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51-56.
- Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Nat Cancer Inst. 2013;105:15501557.
- Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315-322.
- Perkins RB, Guido RS, Castle PE, et al. Erratum: 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2021;25:330-331.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19-e27.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-182.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71: 209-249.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5-14.
- Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Prac Res Clin Obstet Gynaecol. 2018;47:107-118.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94-101.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd edition. Geneva: 2021. https://www .who.int/publications/i/item/9789240030824. Accessed April 28, 2022.
- American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer. American Cancer Society; 2020. https://www.cancer .org/cancer/cervical-cancer/detection-diagnosis-staging /cervical-cancer-screening-guidelines.html. Accessed April 28, 2022.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens KD, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Gen Tract Dis. 2019;23:87-101.
- Committee opinion no. 675. Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol. 2018;148:126-131.
- Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:10511062.
- Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-370.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2020;24:102-131.
- Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Gen Tract Dis. 2020;24:132-143.
- Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:209-220.
- Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520.
- Ejegod D, Bottari F, Pedersen H, et al. The BD Onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54:2267-2272.
- Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343-353.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: followup of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88-99.
- Schiffman M, Kinney WK, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Nat Cancer Inst. 2018;110:501-508.
- Jin XW, Lipold L, Foucher J, et al. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J Gen Intern Med. 2016;31:1338-1344.
- Tota JE, Bentley J, Blake J, et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev Med. 2017;98:15-20.
- Stoler MH, Wright TC Jr, Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol. 2019;153:26-33.
- Louvanto K, Chevarie-Davis M, Ramanakumar AV, et al. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210:474.e1-7.
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884-891.
- Wright TC Jr, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471.
- Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51-56.
- Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Nat Cancer Inst. 2013;105:15501557.
- Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315-322.
- Perkins RB, Guido RS, Castle PE, et al. Erratum: 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2021;25:330-331.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19-e27.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-182.
Cervical cancer: A path to eradication
David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.

David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●

David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.