User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
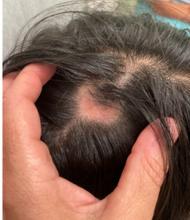
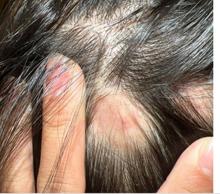
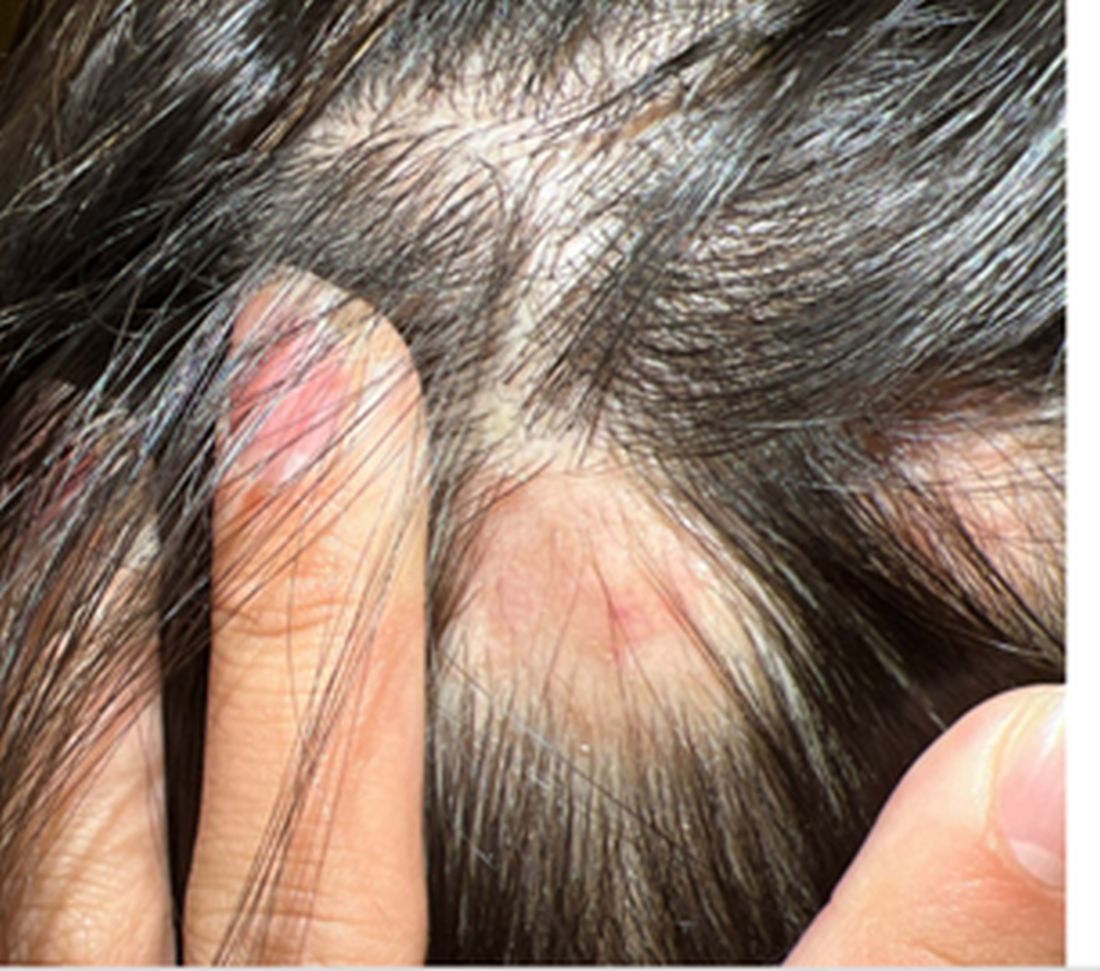
A 16-Year-Old Female Presents With Multiple Areas of Hair Loss on the Scalp
KOH analysis of the scales from the scalp areas revealed no fungal elements. Given the observed erythema and scaling, a punch biopsy was conducted. Histopathological examination of the biopsy sample displayed interface inflammation affecting both the infundibular and lower portions of hair follicles. The presence of folliculosebaceous units transitioning from intermediate to terminal size follicles was noted. A perifollicular, peri eccrine, superficial, and deep perivascular lymphoplasmacytic infiltrate was identified, alongside increased dermal mucin, findings consistent with a diagnosis of discoid lupus erythematosus.
Subsequent laboratory investigations were largely unremarkable, except for an elevated ANA titer (1:320, with a speckled pattern). The patient was initiated on a treatment regimen comprising intralesional triamcinolone and oral hydroxychloroquine (Plaquenil).
Discussion
It predominantly affects adults, yet pediatric cases account for 5%-7% of DLE diagnoses, with a significant predominance in females. Pediatric scalp DLE is particularly concerning due to its potential for causing scarring and permanent hair loss, which can significantly impact the psychological wellbeing of affected children.
The pathogenesis of DLE is multifactorial, involving genetic predispositions, environmental factors like UV light exposure, and immunological mechanisms leading to skin damage.
In children, DLE typically presents as well-demarcated, erythematous plaques with scale and follicular plugging, primarily affecting the scalp. Lesions may also exhibit changes in pigmentation, atrophy, and telangiectasia. The scalp involvement often leads to scarring alopecia, which can be distressing for pediatric patients. Unlike systemic lupus erythematosus (SLE), DLE is usually limited to the skin without systemic involvement. The progression of DLE to systemic lupus erythematosus in children has been previously described to be 22.2%. In a recent report of 201 pediatric cases of DLE, 12% of the cases progressed to systemic lupus erythematosus (SLE) and 14.5% had concurrent SLE. The onset of symptoms before the age of 10 years was the only statistically significant predictor for progression to SLE. Pruritus is a common symptom and may be correlated with disease activity.
The differential diagnosis for this patient encompassed a variety of conditions, including tinea capitis, alopecia areata, trichotillomania, and lichen planopilaris, each considered based on clinical presentation but ultimately excluded through clinical, microscopic, and biopsy findings.
Management strategies for pediatric scalp DLE aim at preventing disease progression, minimizing scarring, and addressing aesthetic concerns. These include the use of topical and intralesional corticosteroids, calcineurin inhibitors, and antimalarial agents like hydroxychloroquine, alongside stringent photoprotection to mitigate UV-triggered exacerbations.
Conclusion
The prognosis for pediatric scalp DLE can be favorable with timely and appropriate management, underscoring the importance of early diagnosis and intervention to prevent scarring and hair loss. However, ongoing surveillance is crucial for monitoring potential progression to systemic lupus erythematosus, albeit a low-risk transformation.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. George PM and Tunnessen WW. Childhood discoid lupus erythematosus. Arch Dermatol. 1993;129(5):613-617.
2. Hawat T et al. Pediatric discoid lupus erythematosus: Short report. Dermatol Ther. 2022 Jan;35(1):e15170. doi: 10.1111/dth.15170.
3. Arkin LM et al. Practice-based differences in paediatric discoid lupus erythematosus. Br J Dermatol. 2019 Oct;181(4):805-810. doi: 10.1111/bjd.17780.
KOH analysis of the scales from the scalp areas revealed no fungal elements. Given the observed erythema and scaling, a punch biopsy was conducted. Histopathological examination of the biopsy sample displayed interface inflammation affecting both the infundibular and lower portions of hair follicles. The presence of folliculosebaceous units transitioning from intermediate to terminal size follicles was noted. A perifollicular, peri eccrine, superficial, and deep perivascular lymphoplasmacytic infiltrate was identified, alongside increased dermal mucin, findings consistent with a diagnosis of discoid lupus erythematosus.
Subsequent laboratory investigations were largely unremarkable, except for an elevated ANA titer (1:320, with a speckled pattern). The patient was initiated on a treatment regimen comprising intralesional triamcinolone and oral hydroxychloroquine (Plaquenil).
Discussion
It predominantly affects adults, yet pediatric cases account for 5%-7% of DLE diagnoses, with a significant predominance in females. Pediatric scalp DLE is particularly concerning due to its potential for causing scarring and permanent hair loss, which can significantly impact the psychological wellbeing of affected children.
The pathogenesis of DLE is multifactorial, involving genetic predispositions, environmental factors like UV light exposure, and immunological mechanisms leading to skin damage.
In children, DLE typically presents as well-demarcated, erythematous plaques with scale and follicular plugging, primarily affecting the scalp. Lesions may also exhibit changes in pigmentation, atrophy, and telangiectasia. The scalp involvement often leads to scarring alopecia, which can be distressing for pediatric patients. Unlike systemic lupus erythematosus (SLE), DLE is usually limited to the skin without systemic involvement. The progression of DLE to systemic lupus erythematosus in children has been previously described to be 22.2%. In a recent report of 201 pediatric cases of DLE, 12% of the cases progressed to systemic lupus erythematosus (SLE) and 14.5% had concurrent SLE. The onset of symptoms before the age of 10 years was the only statistically significant predictor for progression to SLE. Pruritus is a common symptom and may be correlated with disease activity.
The differential diagnosis for this patient encompassed a variety of conditions, including tinea capitis, alopecia areata, trichotillomania, and lichen planopilaris, each considered based on clinical presentation but ultimately excluded through clinical, microscopic, and biopsy findings.
Management strategies for pediatric scalp DLE aim at preventing disease progression, minimizing scarring, and addressing aesthetic concerns. These include the use of topical and intralesional corticosteroids, calcineurin inhibitors, and antimalarial agents like hydroxychloroquine, alongside stringent photoprotection to mitigate UV-triggered exacerbations.
Conclusion
The prognosis for pediatric scalp DLE can be favorable with timely and appropriate management, underscoring the importance of early diagnosis and intervention to prevent scarring and hair loss. However, ongoing surveillance is crucial for monitoring potential progression to systemic lupus erythematosus, albeit a low-risk transformation.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. George PM and Tunnessen WW. Childhood discoid lupus erythematosus. Arch Dermatol. 1993;129(5):613-617.
2. Hawat T et al. Pediatric discoid lupus erythematosus: Short report. Dermatol Ther. 2022 Jan;35(1):e15170. doi: 10.1111/dth.15170.
3. Arkin LM et al. Practice-based differences in paediatric discoid lupus erythematosus. Br J Dermatol. 2019 Oct;181(4):805-810. doi: 10.1111/bjd.17780.
KOH analysis of the scales from the scalp areas revealed no fungal elements. Given the observed erythema and scaling, a punch biopsy was conducted. Histopathological examination of the biopsy sample displayed interface inflammation affecting both the infundibular and lower portions of hair follicles. The presence of folliculosebaceous units transitioning from intermediate to terminal size follicles was noted. A perifollicular, peri eccrine, superficial, and deep perivascular lymphoplasmacytic infiltrate was identified, alongside increased dermal mucin, findings consistent with a diagnosis of discoid lupus erythematosus.
Subsequent laboratory investigations were largely unremarkable, except for an elevated ANA titer (1:320, with a speckled pattern). The patient was initiated on a treatment regimen comprising intralesional triamcinolone and oral hydroxychloroquine (Plaquenil).
Discussion
It predominantly affects adults, yet pediatric cases account for 5%-7% of DLE diagnoses, with a significant predominance in females. Pediatric scalp DLE is particularly concerning due to its potential for causing scarring and permanent hair loss, which can significantly impact the psychological wellbeing of affected children.
The pathogenesis of DLE is multifactorial, involving genetic predispositions, environmental factors like UV light exposure, and immunological mechanisms leading to skin damage.
In children, DLE typically presents as well-demarcated, erythematous plaques with scale and follicular plugging, primarily affecting the scalp. Lesions may also exhibit changes in pigmentation, atrophy, and telangiectasia. The scalp involvement often leads to scarring alopecia, which can be distressing for pediatric patients. Unlike systemic lupus erythematosus (SLE), DLE is usually limited to the skin without systemic involvement. The progression of DLE to systemic lupus erythematosus in children has been previously described to be 22.2%. In a recent report of 201 pediatric cases of DLE, 12% of the cases progressed to systemic lupus erythematosus (SLE) and 14.5% had concurrent SLE. The onset of symptoms before the age of 10 years was the only statistically significant predictor for progression to SLE. Pruritus is a common symptom and may be correlated with disease activity.
The differential diagnosis for this patient encompassed a variety of conditions, including tinea capitis, alopecia areata, trichotillomania, and lichen planopilaris, each considered based on clinical presentation but ultimately excluded through clinical, microscopic, and biopsy findings.
Management strategies for pediatric scalp DLE aim at preventing disease progression, minimizing scarring, and addressing aesthetic concerns. These include the use of topical and intralesional corticosteroids, calcineurin inhibitors, and antimalarial agents like hydroxychloroquine, alongside stringent photoprotection to mitigate UV-triggered exacerbations.
Conclusion
The prognosis for pediatric scalp DLE can be favorable with timely and appropriate management, underscoring the importance of early diagnosis and intervention to prevent scarring and hair loss. However, ongoing surveillance is crucial for monitoring potential progression to systemic lupus erythematosus, albeit a low-risk transformation.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. George PM and Tunnessen WW. Childhood discoid lupus erythematosus. Arch Dermatol. 1993;129(5):613-617.
2. Hawat T et al. Pediatric discoid lupus erythematosus: Short report. Dermatol Ther. 2022 Jan;35(1):e15170. doi: 10.1111/dth.15170.
3. Arkin LM et al. Practice-based differences in paediatric discoid lupus erythematosus. Br J Dermatol. 2019 Oct;181(4):805-810. doi: 10.1111/bjd.17780.
Upon physical examination, the patient exhibited several patches of alopecia with accompanying perifollicular scaling, crusting, and erythema on the affected areas. Examination of her face revealed comedones and papules.
Mild Hidradenitis Suppurativa: Positive Results Reported for Topical Therapy
SAN DIEGO — cream, in a phase 2 trial.
“HS is a chronic, recurring inflammatory skin disease that is associated with painful inflammatory modules and abscesses,” said presenting author Martina J. Porter, MD, a dermatologist at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, Massachusetts. Dr. Porter presented the data during a late-breaking session at the annual meeting of the American Academy of Dermatology.
“Over time, these patients may progress to having tunnels, ulcerations, malodorous discharge, and permanent scarring,” she said. “Currently, there are no approved therapies for milder HS, and the standard treatments that we apply in clinical practice are often inadequate.”
Ruxolitinib is a selective Janus kinase (JAK) 1/JAK2 inhibitor that has demonstrated efficacy in other inflammatory and autoimmune skin diseases. Ruxolitinib cream, 1.5%, is approved for treating mild to moderate atopic dermatitis and nonsegmental vitiligo in patients ages 12 years and older.
The phase 2 double-blind, vehicle-controlled trial evaluated the efficacy and safety of ruxolitinib cream for mild HS. Researchers assigned 69 adults with Hurley stage I or II HS to receive 1.5% ruxolitinib cream or vehicle cream twice daily for 16 weeks. The primary endpoint was the change from baseline in AN count at week 16. To be eligible, patients had to have an AN count between 3 and 10.
“This is much more mild than what we have seen in any systemic therapy trials,” Dr. Porter said. “And, if patients had 3 lesions, they all needed to be in one anatomic area, but if they had 4-10 lesions, they had to have two anatomic areas involved. Also, no patients with active draining tunnels were allowed in the study.”
Of the 69 patients, 34 received ruxolitinib cream and 35 received vehicle. About 51% of patients in the vehicle arm were Black and 34% were White, while about 32% of patients in the ruxolitinib arm were Black and 56% were White.
The mean age of patients overall was 29 years, and about half the patients in both study arms had Hurley stage I disease, while the other half had Hurley stage II disease. Their average AN count ranged between 5.3 and 5.6 — mostly inflammatory nodules and few abscesses. Patients were not allowed to receive any type of intervention or rescue therapy during the study.
Dr. Porter reported that the least square mean change in AN count from baseline to week 16 was -2.42 in the vehicle arm vs -3.61 in the ruxolitinib cream arm (P <.05). The proportion of patients who achieved a 50% decrease in AN count was 79.2% in the ruxolitinib cream arm, compared with 56.5% of patients in the vehicle arm, respectively. More patients in the ruxolitinib cream arm achieved a 75% decrease in AN count (54.2% vs 25%), a 90% decrease in AN count (20.8 vs 12.5%), and a 100% decrease in AN count (20.8% vs 12.5%).
In other findings, 79.2% of patients in the ruxolitinib cream arm achieved a Hidradenitis Suppurativa Clinical Response score from baseline through week 16, compared with 50% of those in the vehicle group. The International Hidradenitis Suppurativa Severity Score System results favored the ruxolitinib cream arm (-4.46 vs -2.66 in the vehicle arm). Skin Pain and Itch numeric rating scale scores were moderate at baseline and improved similarly in both groups during the study.
Ruxolitinib cream was generally well tolerated over 16 weeks. No serious treatment-emergent adverse events were reported. The most common adverse event reported in the ruxolitinib cream group was COVID-19 and nasopharyngitis (two cases each) and one case of an application site reaction.
“Twice-daily 1.5% ruxolitinib cream was effective in patients with milder HS,” Dr. Porter concluded. “Modifications to our traditionally accepted clinical endpoints may be needed in studies of patients with milder HS.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the results, characterized the study as exciting for several reasons.
“First, with the global push in recent years to increase HS awareness, I am already seeing more patients earlier in their disease course with milder disease, and there is currently a gap in approved therapies for this patient population,” she told this news organization.
“Second, patients are very interested in topical therapies for HS and are thrilled whenever they learn that topical options are under investigation. This study had small patient numbers, but it was encouraging to see the positive results for ruxolitinib cream and that the treatment appeared well-tolerated.”
The trial was sponsored by the Incyte Corporation. Dr. Porter disclosed that she has received consulting fees from AbbVie, Alumis, Eli Lilly, Incyte, Janssen, Novartis, Pfizer, Prometheus Laboratories, Sanofi, Sonoma Biotherapeutics, Trifecta Clinical, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article appeared on Medscape.com .
SAN DIEGO — cream, in a phase 2 trial.
“HS is a chronic, recurring inflammatory skin disease that is associated with painful inflammatory modules and abscesses,” said presenting author Martina J. Porter, MD, a dermatologist at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, Massachusetts. Dr. Porter presented the data during a late-breaking session at the annual meeting of the American Academy of Dermatology.
“Over time, these patients may progress to having tunnels, ulcerations, malodorous discharge, and permanent scarring,” she said. “Currently, there are no approved therapies for milder HS, and the standard treatments that we apply in clinical practice are often inadequate.”
Ruxolitinib is a selective Janus kinase (JAK) 1/JAK2 inhibitor that has demonstrated efficacy in other inflammatory and autoimmune skin diseases. Ruxolitinib cream, 1.5%, is approved for treating mild to moderate atopic dermatitis and nonsegmental vitiligo in patients ages 12 years and older.
The phase 2 double-blind, vehicle-controlled trial evaluated the efficacy and safety of ruxolitinib cream for mild HS. Researchers assigned 69 adults with Hurley stage I or II HS to receive 1.5% ruxolitinib cream or vehicle cream twice daily for 16 weeks. The primary endpoint was the change from baseline in AN count at week 16. To be eligible, patients had to have an AN count between 3 and 10.
“This is much more mild than what we have seen in any systemic therapy trials,” Dr. Porter said. “And, if patients had 3 lesions, they all needed to be in one anatomic area, but if they had 4-10 lesions, they had to have two anatomic areas involved. Also, no patients with active draining tunnels were allowed in the study.”
Of the 69 patients, 34 received ruxolitinib cream and 35 received vehicle. About 51% of patients in the vehicle arm were Black and 34% were White, while about 32% of patients in the ruxolitinib arm were Black and 56% were White.
The mean age of patients overall was 29 years, and about half the patients in both study arms had Hurley stage I disease, while the other half had Hurley stage II disease. Their average AN count ranged between 5.3 and 5.6 — mostly inflammatory nodules and few abscesses. Patients were not allowed to receive any type of intervention or rescue therapy during the study.
Dr. Porter reported that the least square mean change in AN count from baseline to week 16 was -2.42 in the vehicle arm vs -3.61 in the ruxolitinib cream arm (P <.05). The proportion of patients who achieved a 50% decrease in AN count was 79.2% in the ruxolitinib cream arm, compared with 56.5% of patients in the vehicle arm, respectively. More patients in the ruxolitinib cream arm achieved a 75% decrease in AN count (54.2% vs 25%), a 90% decrease in AN count (20.8 vs 12.5%), and a 100% decrease in AN count (20.8% vs 12.5%).
In other findings, 79.2% of patients in the ruxolitinib cream arm achieved a Hidradenitis Suppurativa Clinical Response score from baseline through week 16, compared with 50% of those in the vehicle group. The International Hidradenitis Suppurativa Severity Score System results favored the ruxolitinib cream arm (-4.46 vs -2.66 in the vehicle arm). Skin Pain and Itch numeric rating scale scores were moderate at baseline and improved similarly in both groups during the study.
Ruxolitinib cream was generally well tolerated over 16 weeks. No serious treatment-emergent adverse events were reported. The most common adverse event reported in the ruxolitinib cream group was COVID-19 and nasopharyngitis (two cases each) and one case of an application site reaction.
“Twice-daily 1.5% ruxolitinib cream was effective in patients with milder HS,” Dr. Porter concluded. “Modifications to our traditionally accepted clinical endpoints may be needed in studies of patients with milder HS.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the results, characterized the study as exciting for several reasons.
“First, with the global push in recent years to increase HS awareness, I am already seeing more patients earlier in their disease course with milder disease, and there is currently a gap in approved therapies for this patient population,” she told this news organization.
“Second, patients are very interested in topical therapies for HS and are thrilled whenever they learn that topical options are under investigation. This study had small patient numbers, but it was encouraging to see the positive results for ruxolitinib cream and that the treatment appeared well-tolerated.”
The trial was sponsored by the Incyte Corporation. Dr. Porter disclosed that she has received consulting fees from AbbVie, Alumis, Eli Lilly, Incyte, Janssen, Novartis, Pfizer, Prometheus Laboratories, Sanofi, Sonoma Biotherapeutics, Trifecta Clinical, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article appeared on Medscape.com .
SAN DIEGO — cream, in a phase 2 trial.
“HS is a chronic, recurring inflammatory skin disease that is associated with painful inflammatory modules and abscesses,” said presenting author Martina J. Porter, MD, a dermatologist at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, Massachusetts. Dr. Porter presented the data during a late-breaking session at the annual meeting of the American Academy of Dermatology.
“Over time, these patients may progress to having tunnels, ulcerations, malodorous discharge, and permanent scarring,” she said. “Currently, there are no approved therapies for milder HS, and the standard treatments that we apply in clinical practice are often inadequate.”
Ruxolitinib is a selective Janus kinase (JAK) 1/JAK2 inhibitor that has demonstrated efficacy in other inflammatory and autoimmune skin diseases. Ruxolitinib cream, 1.5%, is approved for treating mild to moderate atopic dermatitis and nonsegmental vitiligo in patients ages 12 years and older.
The phase 2 double-blind, vehicle-controlled trial evaluated the efficacy and safety of ruxolitinib cream for mild HS. Researchers assigned 69 adults with Hurley stage I or II HS to receive 1.5% ruxolitinib cream or vehicle cream twice daily for 16 weeks. The primary endpoint was the change from baseline in AN count at week 16. To be eligible, patients had to have an AN count between 3 and 10.
“This is much more mild than what we have seen in any systemic therapy trials,” Dr. Porter said. “And, if patients had 3 lesions, they all needed to be in one anatomic area, but if they had 4-10 lesions, they had to have two anatomic areas involved. Also, no patients with active draining tunnels were allowed in the study.”
Of the 69 patients, 34 received ruxolitinib cream and 35 received vehicle. About 51% of patients in the vehicle arm were Black and 34% were White, while about 32% of patients in the ruxolitinib arm were Black and 56% were White.
The mean age of patients overall was 29 years, and about half the patients in both study arms had Hurley stage I disease, while the other half had Hurley stage II disease. Their average AN count ranged between 5.3 and 5.6 — mostly inflammatory nodules and few abscesses. Patients were not allowed to receive any type of intervention or rescue therapy during the study.
Dr. Porter reported that the least square mean change in AN count from baseline to week 16 was -2.42 in the vehicle arm vs -3.61 in the ruxolitinib cream arm (P <.05). The proportion of patients who achieved a 50% decrease in AN count was 79.2% in the ruxolitinib cream arm, compared with 56.5% of patients in the vehicle arm, respectively. More patients in the ruxolitinib cream arm achieved a 75% decrease in AN count (54.2% vs 25%), a 90% decrease in AN count (20.8 vs 12.5%), and a 100% decrease in AN count (20.8% vs 12.5%).
In other findings, 79.2% of patients in the ruxolitinib cream arm achieved a Hidradenitis Suppurativa Clinical Response score from baseline through week 16, compared with 50% of those in the vehicle group. The International Hidradenitis Suppurativa Severity Score System results favored the ruxolitinib cream arm (-4.46 vs -2.66 in the vehicle arm). Skin Pain and Itch numeric rating scale scores were moderate at baseline and improved similarly in both groups during the study.
Ruxolitinib cream was generally well tolerated over 16 weeks. No serious treatment-emergent adverse events were reported. The most common adverse event reported in the ruxolitinib cream group was COVID-19 and nasopharyngitis (two cases each) and one case of an application site reaction.
“Twice-daily 1.5% ruxolitinib cream was effective in patients with milder HS,” Dr. Porter concluded. “Modifications to our traditionally accepted clinical endpoints may be needed in studies of patients with milder HS.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the results, characterized the study as exciting for several reasons.
“First, with the global push in recent years to increase HS awareness, I am already seeing more patients earlier in their disease course with milder disease, and there is currently a gap in approved therapies for this patient population,” she told this news organization.
“Second, patients are very interested in topical therapies for HS and are thrilled whenever they learn that topical options are under investigation. This study had small patient numbers, but it was encouraging to see the positive results for ruxolitinib cream and that the treatment appeared well-tolerated.”
The trial was sponsored by the Incyte Corporation. Dr. Porter disclosed that she has received consulting fees from AbbVie, Alumis, Eli Lilly, Incyte, Janssen, Novartis, Pfizer, Prometheus Laboratories, Sanofi, Sonoma Biotherapeutics, Trifecta Clinical, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article appeared on Medscape.com .
FROM AAD 2024
Study Identifies Several Factors That Influence Longterm Antibiotic Prescribing for Acne
to follow them, according to the authors of a recently published study.
“This study explored why dermatologists still prescribe a good number of long-term antibiotics for people with acne,” the study’s senior author Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview. “And we found a lot of reasons.” The study was published online in JAMA Dermatology.
Using online surveys and semi-structured video interviews of 30 dermatologists, infectious disease physicians with expertise in antimicrobial stewardship, dermatology residents, and nonphysician clinicians, the investigators assessed respondents’ knowledge and attitudes regarding long-term antibiotics in acne. Salient themes impacting long-term antibiotic prescriptions included the following:
- A perceived dearth of evidence to justify changes in practice.
- Difficulties with iPLEDGE, the Risk Evaluation and Mitigation Strategy (REMS) for managing the teratogenic risks associated with isotretinoin, and with discussing oral contraceptives.
- “Navigating” discussions with about tapering-off of antibiotics.
- Challenging patient demands.
- A lack of effective tools for monitoring progress in antibiotic stewardship.
“It’s surprising there are so many barriers that make it difficult for dermatologists to stick with the guidelines even if they want to,” said Dr. Yeung, a coauthor of the recently released updated American Academy of Dermatology (AAD) acne management guidelines.
A dermatologist who wants to stop systemic antibiotics within 3 months may not know how to do so, he explained, or high demand for appointments may prevent timely follow-ups.
A major reason why dermatologists struggle to limit long-term antibiotic use is that there are very few substitutes that are perceived to work as well, said David J. Margolis, MD, PhD, who was not involved with the study and was asked to comment on the results. He is professor of epidemiology and dermatology at the University of Pennsylvania, Philadelphia.
“Part of the reason antibiotics are being used to treat acne is that they’re effective, and effective for severe disease,” he said. The alternatives, which are mostly topicals, said Dr. Margolis, do not work as well for moderate to severe disease or, with isotretinoin, involve time-consuming hurdles. Dr. Margolis said that he often hears such concerns from individual dermatologists. “But it’s helpful to see these in a well-organized, well-reported qualitative study.”
Infectious disease specialists surveyed considered limiting long-term antibiotic use as extremely important, while several dermatologists “argued that other specialties ‘underestimate the impact acne has on people’s lives,’ ” the authors wrote. Other respondents prioritized making the right choice for the patient at hand.
Although guidelines were never meant to be black and white, Dr. Yeung said, it is crucial to target the goal of tapering off after about 3-4 months — a cutoff with which guidelines from groups including the AAD, the Japanese Dermatological Association in guidelines from 2016, and 2017, respectively, and others concur.
He added, “Some folks believe that if the oral antibiotic is working, why stop? We need to develop evidence to show that reducing oral antibiotic use is important to our patients, not just to a theoretical problem of antibiotic resistance in society.” For example, in a study published in The Lancet in 2004, patients who used strictly topical regimens achieved efficacy similar to that of those who used only oral antibiotics.
In addition, some clinicians worried that limiting antibiotics could reduce patient satisfaction, spurring switches to other providers. However, he and the other authors of the JAMA Dermatology study noted that in a survey of patients with acne published in the Journal of Clinical and Aesthetic Dermatology in 2019, 76.9% said they would be “very or extremely likely” to use effective antibiotic-free treatments if offered.
Because most respondents were highly aware of the importance of antibiotic stewardship, Dr. Yeung said, additional passive education is not necessarily the answer. “It will take a concerted effort by our national societies to come up with resources and solutions for individual dermatologists to overcome some of these larger barriers.” Such solutions could range from training in communication and shared decision-making to implementing systems that provide individualized feedback to support antibiotic stewardship.
Many ongoing studies are examining antibiotic stewardship, Dr. Margolis said in the interview. However, he added, dermatologists’ idea of long-term use is 3 months, versus 1 month or less in other specialties. “Moreover, dermatology patients tend to be much healthier individuals and are rarely hospitalized, so there may be some issues comparing the ongoing studies to individuals with acne.” Future research will need to account for such differences, he said.
The study was funded by an American Acne & Rosacea Society Clinical Research Award. Dr. Yeung is associate editor of JAMA Dermatology. Dr. Margolis has received a National Institutes of Health grant to study doxycycline versus spironolactone in acne.
to follow them, according to the authors of a recently published study.
“This study explored why dermatologists still prescribe a good number of long-term antibiotics for people with acne,” the study’s senior author Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview. “And we found a lot of reasons.” The study was published online in JAMA Dermatology.
Using online surveys and semi-structured video interviews of 30 dermatologists, infectious disease physicians with expertise in antimicrobial stewardship, dermatology residents, and nonphysician clinicians, the investigators assessed respondents’ knowledge and attitudes regarding long-term antibiotics in acne. Salient themes impacting long-term antibiotic prescriptions included the following:
- A perceived dearth of evidence to justify changes in practice.
- Difficulties with iPLEDGE, the Risk Evaluation and Mitigation Strategy (REMS) for managing the teratogenic risks associated with isotretinoin, and with discussing oral contraceptives.
- “Navigating” discussions with about tapering-off of antibiotics.
- Challenging patient demands.
- A lack of effective tools for monitoring progress in antibiotic stewardship.
“It’s surprising there are so many barriers that make it difficult for dermatologists to stick with the guidelines even if they want to,” said Dr. Yeung, a coauthor of the recently released updated American Academy of Dermatology (AAD) acne management guidelines.
A dermatologist who wants to stop systemic antibiotics within 3 months may not know how to do so, he explained, or high demand for appointments may prevent timely follow-ups.
A major reason why dermatologists struggle to limit long-term antibiotic use is that there are very few substitutes that are perceived to work as well, said David J. Margolis, MD, PhD, who was not involved with the study and was asked to comment on the results. He is professor of epidemiology and dermatology at the University of Pennsylvania, Philadelphia.
“Part of the reason antibiotics are being used to treat acne is that they’re effective, and effective for severe disease,” he said. The alternatives, which are mostly topicals, said Dr. Margolis, do not work as well for moderate to severe disease or, with isotretinoin, involve time-consuming hurdles. Dr. Margolis said that he often hears such concerns from individual dermatologists. “But it’s helpful to see these in a well-organized, well-reported qualitative study.”
Infectious disease specialists surveyed considered limiting long-term antibiotic use as extremely important, while several dermatologists “argued that other specialties ‘underestimate the impact acne has on people’s lives,’ ” the authors wrote. Other respondents prioritized making the right choice for the patient at hand.
Although guidelines were never meant to be black and white, Dr. Yeung said, it is crucial to target the goal of tapering off after about 3-4 months — a cutoff with which guidelines from groups including the AAD, the Japanese Dermatological Association in guidelines from 2016, and 2017, respectively, and others concur.
He added, “Some folks believe that if the oral antibiotic is working, why stop? We need to develop evidence to show that reducing oral antibiotic use is important to our patients, not just to a theoretical problem of antibiotic resistance in society.” For example, in a study published in The Lancet in 2004, patients who used strictly topical regimens achieved efficacy similar to that of those who used only oral antibiotics.
In addition, some clinicians worried that limiting antibiotics could reduce patient satisfaction, spurring switches to other providers. However, he and the other authors of the JAMA Dermatology study noted that in a survey of patients with acne published in the Journal of Clinical and Aesthetic Dermatology in 2019, 76.9% said they would be “very or extremely likely” to use effective antibiotic-free treatments if offered.
Because most respondents were highly aware of the importance of antibiotic stewardship, Dr. Yeung said, additional passive education is not necessarily the answer. “It will take a concerted effort by our national societies to come up with resources and solutions for individual dermatologists to overcome some of these larger barriers.” Such solutions could range from training in communication and shared decision-making to implementing systems that provide individualized feedback to support antibiotic stewardship.
Many ongoing studies are examining antibiotic stewardship, Dr. Margolis said in the interview. However, he added, dermatologists’ idea of long-term use is 3 months, versus 1 month or less in other specialties. “Moreover, dermatology patients tend to be much healthier individuals and are rarely hospitalized, so there may be some issues comparing the ongoing studies to individuals with acne.” Future research will need to account for such differences, he said.
The study was funded by an American Acne & Rosacea Society Clinical Research Award. Dr. Yeung is associate editor of JAMA Dermatology. Dr. Margolis has received a National Institutes of Health grant to study doxycycline versus spironolactone in acne.
to follow them, according to the authors of a recently published study.
“This study explored why dermatologists still prescribe a good number of long-term antibiotics for people with acne,” the study’s senior author Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview. “And we found a lot of reasons.” The study was published online in JAMA Dermatology.
Using online surveys and semi-structured video interviews of 30 dermatologists, infectious disease physicians with expertise in antimicrobial stewardship, dermatology residents, and nonphysician clinicians, the investigators assessed respondents’ knowledge and attitudes regarding long-term antibiotics in acne. Salient themes impacting long-term antibiotic prescriptions included the following:
- A perceived dearth of evidence to justify changes in practice.
- Difficulties with iPLEDGE, the Risk Evaluation and Mitigation Strategy (REMS) for managing the teratogenic risks associated with isotretinoin, and with discussing oral contraceptives.
- “Navigating” discussions with about tapering-off of antibiotics.
- Challenging patient demands.
- A lack of effective tools for monitoring progress in antibiotic stewardship.
“It’s surprising there are so many barriers that make it difficult for dermatologists to stick with the guidelines even if they want to,” said Dr. Yeung, a coauthor of the recently released updated American Academy of Dermatology (AAD) acne management guidelines.
A dermatologist who wants to stop systemic antibiotics within 3 months may not know how to do so, he explained, or high demand for appointments may prevent timely follow-ups.
A major reason why dermatologists struggle to limit long-term antibiotic use is that there are very few substitutes that are perceived to work as well, said David J. Margolis, MD, PhD, who was not involved with the study and was asked to comment on the results. He is professor of epidemiology and dermatology at the University of Pennsylvania, Philadelphia.
“Part of the reason antibiotics are being used to treat acne is that they’re effective, and effective for severe disease,” he said. The alternatives, which are mostly topicals, said Dr. Margolis, do not work as well for moderate to severe disease or, with isotretinoin, involve time-consuming hurdles. Dr. Margolis said that he often hears such concerns from individual dermatologists. “But it’s helpful to see these in a well-organized, well-reported qualitative study.”
Infectious disease specialists surveyed considered limiting long-term antibiotic use as extremely important, while several dermatologists “argued that other specialties ‘underestimate the impact acne has on people’s lives,’ ” the authors wrote. Other respondents prioritized making the right choice for the patient at hand.
Although guidelines were never meant to be black and white, Dr. Yeung said, it is crucial to target the goal of tapering off after about 3-4 months — a cutoff with which guidelines from groups including the AAD, the Japanese Dermatological Association in guidelines from 2016, and 2017, respectively, and others concur.
He added, “Some folks believe that if the oral antibiotic is working, why stop? We need to develop evidence to show that reducing oral antibiotic use is important to our patients, not just to a theoretical problem of antibiotic resistance in society.” For example, in a study published in The Lancet in 2004, patients who used strictly topical regimens achieved efficacy similar to that of those who used only oral antibiotics.
In addition, some clinicians worried that limiting antibiotics could reduce patient satisfaction, spurring switches to other providers. However, he and the other authors of the JAMA Dermatology study noted that in a survey of patients with acne published in the Journal of Clinical and Aesthetic Dermatology in 2019, 76.9% said they would be “very or extremely likely” to use effective antibiotic-free treatments if offered.
Because most respondents were highly aware of the importance of antibiotic stewardship, Dr. Yeung said, additional passive education is not necessarily the answer. “It will take a concerted effort by our national societies to come up with resources and solutions for individual dermatologists to overcome some of these larger barriers.” Such solutions could range from training in communication and shared decision-making to implementing systems that provide individualized feedback to support antibiotic stewardship.
Many ongoing studies are examining antibiotic stewardship, Dr. Margolis said in the interview. However, he added, dermatologists’ idea of long-term use is 3 months, versus 1 month or less in other specialties. “Moreover, dermatology patients tend to be much healthier individuals and are rarely hospitalized, so there may be some issues comparing the ongoing studies to individuals with acne.” Future research will need to account for such differences, he said.
The study was funded by an American Acne & Rosacea Society Clinical Research Award. Dr. Yeung is associate editor of JAMA Dermatology. Dr. Margolis has received a National Institutes of Health grant to study doxycycline versus spironolactone in acne.
FROM JAMA DERMATOLOGY
Childhood Atopic Dermatitis Linked to IBD Risk
TOPLINE:
, but atopic manifestations are generally not associated with IBD.
METHODOLOGY:
- Studies examining the link between atopy and IBD have yielded inconsistent results. Many of these studies included adults, introducing recall bias, or relied on physician diagnoses that might have overlooked mild cases.
- Researchers analyzed prospectively collected data on 83,311 children from two cohort studies, ABIS (1997-1999) and MoBa (1999-2008), who were followed up from birth until 2021 or a diagnosis of IBD.
- Information on parents was collected prospectively via questionnaires on any atopy their children might have developed by the age of 3 years. Atopy included conditions such as AD, asthma, food allergy, or allergic rhinitis.
TAKEAWAY:
- A total of 301 participants were diagnosed with IBD over 1,174,756 person-years of follow-up. By the age of 3 years, 31,671 children (38%) were reported to have any atopic manifestation.
- Children with AD at the age of 3 years demonstrated a significantly higher risk for IBD (pooled adjusted hazard ratio [aHR], 1.46), Crohn’s disease (pooled aHR, 1.53), and ulcerative colitis (pooled aHR, 1.78).
- Any atopic manifestation by the age of 3 years was not associated with a subsequent risk for IBD, Crohn’s disease, or ulcerative colitis, nor were analyses focused on early-life food-related allergy, asthma, and allergic rhinitis.
IN PRACTICE:
According to the authors, these findings suggested potential shared underlying causes between AD and IBD, which could help identify individuals at risk, and “a deeper understanding could significantly benefit the development of novel treatment approaches capable of effectively addressing both conditions, consequently enhancing patient outcomes.”
SOURCE:
This study, led by Tereza Lerchova, MD, PhD, Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, was published online in The Journal of Pediatrics.
LIMITATIONS:
The findings of this study were mostly related to childhood-onset IBD instead of IBD in adult life. Lower participation in the MoBa study could limit generalizability to a broader population. In addition, there might have been lower participation from families without atopic manifestations.
DISCLOSURES:
The study was funded by the Swedish Society for Medical Research, Swedish Research Council, and ALF and supported by grants from the Swedish Child Diabetes Foundation, Swedish Council for Working Life and Social Research, Swedish Research Council, Medical Research Council of Southeast Sweden, JDRF Wallenberg Foundation, Linkoping University, and Joanna Cocozza Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, but atopic manifestations are generally not associated with IBD.
METHODOLOGY:
- Studies examining the link between atopy and IBD have yielded inconsistent results. Many of these studies included adults, introducing recall bias, or relied on physician diagnoses that might have overlooked mild cases.
- Researchers analyzed prospectively collected data on 83,311 children from two cohort studies, ABIS (1997-1999) and MoBa (1999-2008), who were followed up from birth until 2021 or a diagnosis of IBD.
- Information on parents was collected prospectively via questionnaires on any atopy their children might have developed by the age of 3 years. Atopy included conditions such as AD, asthma, food allergy, or allergic rhinitis.
TAKEAWAY:
- A total of 301 participants were diagnosed with IBD over 1,174,756 person-years of follow-up. By the age of 3 years, 31,671 children (38%) were reported to have any atopic manifestation.
- Children with AD at the age of 3 years demonstrated a significantly higher risk for IBD (pooled adjusted hazard ratio [aHR], 1.46), Crohn’s disease (pooled aHR, 1.53), and ulcerative colitis (pooled aHR, 1.78).
- Any atopic manifestation by the age of 3 years was not associated with a subsequent risk for IBD, Crohn’s disease, or ulcerative colitis, nor were analyses focused on early-life food-related allergy, asthma, and allergic rhinitis.
IN PRACTICE:
According to the authors, these findings suggested potential shared underlying causes between AD and IBD, which could help identify individuals at risk, and “a deeper understanding could significantly benefit the development of novel treatment approaches capable of effectively addressing both conditions, consequently enhancing patient outcomes.”
SOURCE:
This study, led by Tereza Lerchova, MD, PhD, Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, was published online in The Journal of Pediatrics.
LIMITATIONS:
The findings of this study were mostly related to childhood-onset IBD instead of IBD in adult life. Lower participation in the MoBa study could limit generalizability to a broader population. In addition, there might have been lower participation from families without atopic manifestations.
DISCLOSURES:
The study was funded by the Swedish Society for Medical Research, Swedish Research Council, and ALF and supported by grants from the Swedish Child Diabetes Foundation, Swedish Council for Working Life and Social Research, Swedish Research Council, Medical Research Council of Southeast Sweden, JDRF Wallenberg Foundation, Linkoping University, and Joanna Cocozza Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, but atopic manifestations are generally not associated with IBD.
METHODOLOGY:
- Studies examining the link between atopy and IBD have yielded inconsistent results. Many of these studies included adults, introducing recall bias, or relied on physician diagnoses that might have overlooked mild cases.
- Researchers analyzed prospectively collected data on 83,311 children from two cohort studies, ABIS (1997-1999) and MoBa (1999-2008), who were followed up from birth until 2021 or a diagnosis of IBD.
- Information on parents was collected prospectively via questionnaires on any atopy their children might have developed by the age of 3 years. Atopy included conditions such as AD, asthma, food allergy, or allergic rhinitis.
TAKEAWAY:
- A total of 301 participants were diagnosed with IBD over 1,174,756 person-years of follow-up. By the age of 3 years, 31,671 children (38%) were reported to have any atopic manifestation.
- Children with AD at the age of 3 years demonstrated a significantly higher risk for IBD (pooled adjusted hazard ratio [aHR], 1.46), Crohn’s disease (pooled aHR, 1.53), and ulcerative colitis (pooled aHR, 1.78).
- Any atopic manifestation by the age of 3 years was not associated with a subsequent risk for IBD, Crohn’s disease, or ulcerative colitis, nor were analyses focused on early-life food-related allergy, asthma, and allergic rhinitis.
IN PRACTICE:
According to the authors, these findings suggested potential shared underlying causes between AD and IBD, which could help identify individuals at risk, and “a deeper understanding could significantly benefit the development of novel treatment approaches capable of effectively addressing both conditions, consequently enhancing patient outcomes.”
SOURCE:
This study, led by Tereza Lerchova, MD, PhD, Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, was published online in The Journal of Pediatrics.
LIMITATIONS:
The findings of this study were mostly related to childhood-onset IBD instead of IBD in adult life. Lower participation in the MoBa study could limit generalizability to a broader population. In addition, there might have been lower participation from families without atopic manifestations.
DISCLOSURES:
The study was funded by the Swedish Society for Medical Research, Swedish Research Council, and ALF and supported by grants from the Swedish Child Diabetes Foundation, Swedish Council for Working Life and Social Research, Swedish Research Council, Medical Research Council of Southeast Sweden, JDRF Wallenberg Foundation, Linkoping University, and Joanna Cocozza Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Repeat MCED Testing May ID Early-Stage and Unscreened Cancers
This was the conclusion of recent data presented by Ora Karp Gordon, MD, MS, during a session at the American Association for Cancer Research annual meeting.
The MCED test, known as Galleri, was made clinically available in the United States in April 2021. Developed by GRAIL LLC, the test analyzes cell-free DNA in the blood using targeted methylation analysis and machine learning to detect the presence of a cancer signal and determine its organ of origin or cancer signal origin. The initial screening of over 53,000 individuals with the Galleri test detected a cancer signal in 1.1% of participants.
The new real-world analysis examines the outcomes of repeat MCED testing in 5,794 individuals.
The study looked at individuals who initially received a ‘no cancer signal detected’ result and then underwent a second Galleri test. Over 80% of participants received their follow-up test 10-18 months after the first, with a median interval between blood draws of 12.9 months.
“The repeat tests detect those cancer cases that have reached the detection threshold since their last MCED test, which should be less than one year of incidence,” Dr. Gordon, professor at Saint John’s Cancer Institute, Santa Monica, California, said in an interview. “We are just now starting to see results from patients who get their second and even third round of screening.”
“Galleri is recommended to be used annually in addition to USPSTF [US Preventive Services Task Force]–recommended cancer screening tests, like mammography and colonoscopy,” she said.
This recommendation is based on a modeling study suggesting that annual screening would improve stage shift, diagnostic yield, and potentially mortality when compared to biennial screening, although biennial screening was still favorable compared with no screening, she explained.
Early Real-World Evidence of Repeat Testing
Among the cohort of 5,794 individuals who received repeat testing, 26 received a positive cancer signal on their second test, yielding a cancer signal detection rate of 0.45% (95% CI: 0.31%-0.66%). The cancer signal detection rate was slightly higher in men. The rate was 0.50% (95% CI: 0.32%-0.81%; 17 of 3367) in men versus 0.37% (95% CI: 0.2%-0.7%; 9 of 2427) in women.
During her presentation, Dr. Gordon highlighted that the repeat testing signal detection rate was lower than the initial 0.95% rate (95% CI: 0.87-1.0; 510 of 53,744) seen in the previous larger cohort of patients who were retested at 1 year.
She acknowledged that the lower cancer signal detection rate of repeat testing may indicate some degree of ‘early adopter’ bias, where those who return for a second test are systematically different from the general screening population. This could suggest that broader population-level screening may yield different results, she continued.
Shift Toward Unscreened Cancers
The top cancer types identified in the second round of testing were lymphoid, head and neck, bladder/urothelial, colorectal, and anal cancers. Clinicians were able to confirm clinical outcomes in 12 of 26 cases, in which cancer signals were detected. Of those 12 cases, 8 individuals received a cancer diagnosis and 4 did not have cancer. The remaining 14 of 26 cases in which cancer signals were detected are still under investigation.
“We found a shift away from USPSTF screen-detected cancers, like breast, lung, and prostate, and relative increase in unscreened urinary, head and neck, and lymphoid cancers, with 75% of cancers being those without any screening guidelines,” Dr. Gordon said in an interview.
She added that patients who choose to retest may have different cancer rates for several reasons, including bias toward a population that is health conscious and adhered to all recommended cancer screening.
“So the shift toward unscreened cancers is not unexpected and highlights the value of Galleri,” she said, but also acknowledged that “continued monitoring is needed to see if this translates in a persistent finding over time and tests.”
Shift Toward Early-Stage Cancers
Staging information was available for five cases, and Dr. Gordon highlighted in her talk that four of these confirmed cancers were stage I, including cancers of the anus, head and neck, bladder, and lymphoma. The fifth confirmed cancer with staging information was stage IV ovarian cancer.
“It is still early, and the numbers are very small, but the detection of early-stage cancers with second annual testing is very encouraging as these are the cases where MCED testing could have the greatest impact in improving outcomes through earlier treatment,” Dr. Gordon told this publication.
During an interview after the talk, Kenneth L. Kehl, MD, MPH, echoed that data must be confirmed in larger cohorts.
“The shift toward earlier stage cancers that are less detectable by standard screening methods is an interesting result, but we need to be cautious since the numbers were relatively small, and we do not have data on cancers that were diagnosed among patients whose second MCED test was also negative,” said Dr. Kehl, a medical oncologist at Dana-Farber Cancer Institute, Boston.
MCED Results Could Help Direct Diagnostic Workup
The test’s ability to predict the organ of origin was highly accurate, correctly identifying the cancer type in all eight confirmed cases. Among the eight cases with a confirmed cancer diagnosis, the accuracy of the first prediction was 100%, and diagnoses included invasive cancers across multiple tissues and organs, including anus, colon, head and neck, urothelial tract, ovary, and the lymphatic system.
“The fact that the site of origin for 100% of confirmed cancers was accurately predicted with GRAIL’s CSO by Galleri test confirms the promise that this can guide workup when a cancer signal is detected,” Dr. Gordon noted in the interview.
Looking Ahead
Dr. Kehl, who was not involved in the MCED study, noted in an interview that “further data on test characteristics beyond positive predictive value, including the sensitivity, specificity, and negative predictive value, as well as demonstration of clinical benefit — ideally in a randomized trial — will likely be required for MCED testing to become a standard public health recommendation.”
He added that challenges associated with implementing annual screening with MCED tests include the risks of both false positives and false negatives as testing becomes more widely available.
“False positives cause anxiety and lead to additional testing that may carry its own risks, and we need to understand if potentially false negative tests will be associated with less uptake of established screening strategies,” Dr. Kehl said in an interview. However, he noted that serial testing could lead to more frequent diagnoses of early-stage cancers that may be less detectable by standard methods.
Dr. Gordon reported financial relationships with GRAIL LLC and Genetic Technologies Corporation. Dr. Kehl reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing healthcare products used by or on patients.
This was the conclusion of recent data presented by Ora Karp Gordon, MD, MS, during a session at the American Association for Cancer Research annual meeting.
The MCED test, known as Galleri, was made clinically available in the United States in April 2021. Developed by GRAIL LLC, the test analyzes cell-free DNA in the blood using targeted methylation analysis and machine learning to detect the presence of a cancer signal and determine its organ of origin or cancer signal origin. The initial screening of over 53,000 individuals with the Galleri test detected a cancer signal in 1.1% of participants.
The new real-world analysis examines the outcomes of repeat MCED testing in 5,794 individuals.
The study looked at individuals who initially received a ‘no cancer signal detected’ result and then underwent a second Galleri test. Over 80% of participants received their follow-up test 10-18 months after the first, with a median interval between blood draws of 12.9 months.
“The repeat tests detect those cancer cases that have reached the detection threshold since their last MCED test, which should be less than one year of incidence,” Dr. Gordon, professor at Saint John’s Cancer Institute, Santa Monica, California, said in an interview. “We are just now starting to see results from patients who get their second and even third round of screening.”
“Galleri is recommended to be used annually in addition to USPSTF [US Preventive Services Task Force]–recommended cancer screening tests, like mammography and colonoscopy,” she said.
This recommendation is based on a modeling study suggesting that annual screening would improve stage shift, diagnostic yield, and potentially mortality when compared to biennial screening, although biennial screening was still favorable compared with no screening, she explained.
Early Real-World Evidence of Repeat Testing
Among the cohort of 5,794 individuals who received repeat testing, 26 received a positive cancer signal on their second test, yielding a cancer signal detection rate of 0.45% (95% CI: 0.31%-0.66%). The cancer signal detection rate was slightly higher in men. The rate was 0.50% (95% CI: 0.32%-0.81%; 17 of 3367) in men versus 0.37% (95% CI: 0.2%-0.7%; 9 of 2427) in women.
During her presentation, Dr. Gordon highlighted that the repeat testing signal detection rate was lower than the initial 0.95% rate (95% CI: 0.87-1.0; 510 of 53,744) seen in the previous larger cohort of patients who were retested at 1 year.
She acknowledged that the lower cancer signal detection rate of repeat testing may indicate some degree of ‘early adopter’ bias, where those who return for a second test are systematically different from the general screening population. This could suggest that broader population-level screening may yield different results, she continued.
Shift Toward Unscreened Cancers
The top cancer types identified in the second round of testing were lymphoid, head and neck, bladder/urothelial, colorectal, and anal cancers. Clinicians were able to confirm clinical outcomes in 12 of 26 cases, in which cancer signals were detected. Of those 12 cases, 8 individuals received a cancer diagnosis and 4 did not have cancer. The remaining 14 of 26 cases in which cancer signals were detected are still under investigation.
“We found a shift away from USPSTF screen-detected cancers, like breast, lung, and prostate, and relative increase in unscreened urinary, head and neck, and lymphoid cancers, with 75% of cancers being those without any screening guidelines,” Dr. Gordon said in an interview.
She added that patients who choose to retest may have different cancer rates for several reasons, including bias toward a population that is health conscious and adhered to all recommended cancer screening.
“So the shift toward unscreened cancers is not unexpected and highlights the value of Galleri,” she said, but also acknowledged that “continued monitoring is needed to see if this translates in a persistent finding over time and tests.”
Shift Toward Early-Stage Cancers
Staging information was available for five cases, and Dr. Gordon highlighted in her talk that four of these confirmed cancers were stage I, including cancers of the anus, head and neck, bladder, and lymphoma. The fifth confirmed cancer with staging information was stage IV ovarian cancer.
“It is still early, and the numbers are very small, but the detection of early-stage cancers with second annual testing is very encouraging as these are the cases where MCED testing could have the greatest impact in improving outcomes through earlier treatment,” Dr. Gordon told this publication.
During an interview after the talk, Kenneth L. Kehl, MD, MPH, echoed that data must be confirmed in larger cohorts.
“The shift toward earlier stage cancers that are less detectable by standard screening methods is an interesting result, but we need to be cautious since the numbers were relatively small, and we do not have data on cancers that were diagnosed among patients whose second MCED test was also negative,” said Dr. Kehl, a medical oncologist at Dana-Farber Cancer Institute, Boston.
MCED Results Could Help Direct Diagnostic Workup
The test’s ability to predict the organ of origin was highly accurate, correctly identifying the cancer type in all eight confirmed cases. Among the eight cases with a confirmed cancer diagnosis, the accuracy of the first prediction was 100%, and diagnoses included invasive cancers across multiple tissues and organs, including anus, colon, head and neck, urothelial tract, ovary, and the lymphatic system.
“The fact that the site of origin for 100% of confirmed cancers was accurately predicted with GRAIL’s CSO by Galleri test confirms the promise that this can guide workup when a cancer signal is detected,” Dr. Gordon noted in the interview.
Looking Ahead
Dr. Kehl, who was not involved in the MCED study, noted in an interview that “further data on test characteristics beyond positive predictive value, including the sensitivity, specificity, and negative predictive value, as well as demonstration of clinical benefit — ideally in a randomized trial — will likely be required for MCED testing to become a standard public health recommendation.”
He added that challenges associated with implementing annual screening with MCED tests include the risks of both false positives and false negatives as testing becomes more widely available.
“False positives cause anxiety and lead to additional testing that may carry its own risks, and we need to understand if potentially false negative tests will be associated with less uptake of established screening strategies,” Dr. Kehl said in an interview. However, he noted that serial testing could lead to more frequent diagnoses of early-stage cancers that may be less detectable by standard methods.
Dr. Gordon reported financial relationships with GRAIL LLC and Genetic Technologies Corporation. Dr. Kehl reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing healthcare products used by or on patients.
This was the conclusion of recent data presented by Ora Karp Gordon, MD, MS, during a session at the American Association for Cancer Research annual meeting.
The MCED test, known as Galleri, was made clinically available in the United States in April 2021. Developed by GRAIL LLC, the test analyzes cell-free DNA in the blood using targeted methylation analysis and machine learning to detect the presence of a cancer signal and determine its organ of origin or cancer signal origin. The initial screening of over 53,000 individuals with the Galleri test detected a cancer signal in 1.1% of participants.
The new real-world analysis examines the outcomes of repeat MCED testing in 5,794 individuals.
The study looked at individuals who initially received a ‘no cancer signal detected’ result and then underwent a second Galleri test. Over 80% of participants received their follow-up test 10-18 months after the first, with a median interval between blood draws of 12.9 months.
“The repeat tests detect those cancer cases that have reached the detection threshold since their last MCED test, which should be less than one year of incidence,” Dr. Gordon, professor at Saint John’s Cancer Institute, Santa Monica, California, said in an interview. “We are just now starting to see results from patients who get their second and even third round of screening.”
“Galleri is recommended to be used annually in addition to USPSTF [US Preventive Services Task Force]–recommended cancer screening tests, like mammography and colonoscopy,” she said.
This recommendation is based on a modeling study suggesting that annual screening would improve stage shift, diagnostic yield, and potentially mortality when compared to biennial screening, although biennial screening was still favorable compared with no screening, she explained.
Early Real-World Evidence of Repeat Testing
Among the cohort of 5,794 individuals who received repeat testing, 26 received a positive cancer signal on their second test, yielding a cancer signal detection rate of 0.45% (95% CI: 0.31%-0.66%). The cancer signal detection rate was slightly higher in men. The rate was 0.50% (95% CI: 0.32%-0.81%; 17 of 3367) in men versus 0.37% (95% CI: 0.2%-0.7%; 9 of 2427) in women.
During her presentation, Dr. Gordon highlighted that the repeat testing signal detection rate was lower than the initial 0.95% rate (95% CI: 0.87-1.0; 510 of 53,744) seen in the previous larger cohort of patients who were retested at 1 year.
She acknowledged that the lower cancer signal detection rate of repeat testing may indicate some degree of ‘early adopter’ bias, where those who return for a second test are systematically different from the general screening population. This could suggest that broader population-level screening may yield different results, she continued.
Shift Toward Unscreened Cancers
The top cancer types identified in the second round of testing were lymphoid, head and neck, bladder/urothelial, colorectal, and anal cancers. Clinicians were able to confirm clinical outcomes in 12 of 26 cases, in which cancer signals were detected. Of those 12 cases, 8 individuals received a cancer diagnosis and 4 did not have cancer. The remaining 14 of 26 cases in which cancer signals were detected are still under investigation.
“We found a shift away from USPSTF screen-detected cancers, like breast, lung, and prostate, and relative increase in unscreened urinary, head and neck, and lymphoid cancers, with 75% of cancers being those without any screening guidelines,” Dr. Gordon said in an interview.
She added that patients who choose to retest may have different cancer rates for several reasons, including bias toward a population that is health conscious and adhered to all recommended cancer screening.
“So the shift toward unscreened cancers is not unexpected and highlights the value of Galleri,” she said, but also acknowledged that “continued monitoring is needed to see if this translates in a persistent finding over time and tests.”
Shift Toward Early-Stage Cancers
Staging information was available for five cases, and Dr. Gordon highlighted in her talk that four of these confirmed cancers were stage I, including cancers of the anus, head and neck, bladder, and lymphoma. The fifth confirmed cancer with staging information was stage IV ovarian cancer.
“It is still early, and the numbers are very small, but the detection of early-stage cancers with second annual testing is very encouraging as these are the cases where MCED testing could have the greatest impact in improving outcomes through earlier treatment,” Dr. Gordon told this publication.
During an interview after the talk, Kenneth L. Kehl, MD, MPH, echoed that data must be confirmed in larger cohorts.
“The shift toward earlier stage cancers that are less detectable by standard screening methods is an interesting result, but we need to be cautious since the numbers were relatively small, and we do not have data on cancers that were diagnosed among patients whose second MCED test was also negative,” said Dr. Kehl, a medical oncologist at Dana-Farber Cancer Institute, Boston.
MCED Results Could Help Direct Diagnostic Workup
The test’s ability to predict the organ of origin was highly accurate, correctly identifying the cancer type in all eight confirmed cases. Among the eight cases with a confirmed cancer diagnosis, the accuracy of the first prediction was 100%, and diagnoses included invasive cancers across multiple tissues and organs, including anus, colon, head and neck, urothelial tract, ovary, and the lymphatic system.
“The fact that the site of origin for 100% of confirmed cancers was accurately predicted with GRAIL’s CSO by Galleri test confirms the promise that this can guide workup when a cancer signal is detected,” Dr. Gordon noted in the interview.
Looking Ahead
Dr. Kehl, who was not involved in the MCED study, noted in an interview that “further data on test characteristics beyond positive predictive value, including the sensitivity, specificity, and negative predictive value, as well as demonstration of clinical benefit — ideally in a randomized trial — will likely be required for MCED testing to become a standard public health recommendation.”
He added that challenges associated with implementing annual screening with MCED tests include the risks of both false positives and false negatives as testing becomes more widely available.
“False positives cause anxiety and lead to additional testing that may carry its own risks, and we need to understand if potentially false negative tests will be associated with less uptake of established screening strategies,” Dr. Kehl said in an interview. However, he noted that serial testing could lead to more frequent diagnoses of early-stage cancers that may be less detectable by standard methods.
Dr. Gordon reported financial relationships with GRAIL LLC and Genetic Technologies Corporation. Dr. Kehl reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing healthcare products used by or on patients.
FROM AACR 2024
Medicine or Politics? Doctors Defend Their Social Activism
It should come as no surprise that when physicians speak out on social and political issues, there is sometimes a backlash. This can range from the typical trolling that occurs online to rarer cases of professional penalties. Two doctors were fired by NYU Langone Health late last year after they posted social media messages about the Israel-Hamas war. Still, many physicians are not only willing to stand up for what they believe in, but they see it as an essential part of their profession.
"We're now at a place where doctors need to engage in public advocacy as an urgent part of our job," wrote Rob Davidson, MD, an emergency department physician, at the onslaught of the COVID-19 pandemic. In an Op-Ed piece for The Guardian, Dr. Davidson noted how the virus forced many physicians into becoming "activist doctors," calling for adequate personal protective equipment and correcting misinformation. "What we want above all is for the administration to listen to doctors, nurses, and frontline health workers - and stop playing politics," he wrote.
'It's Not About Being Political'
The intersection of medicine and politics is hardly new. Doctors frequently testify before Congress, sharing their expertise on issues concerning public health. This, however, isn't the same as "playing politics."
"I'm not taking political stances," said Megan Ranney, MD, Dean of the Yale School of Public Health. "Rather, I'm using science to inform best practices, and I'm vocal around the area where I have expertise where we could do collectively better."
Dr. Ranney's work to end firearm injury and death garnered particular attention when she co-authored an open letter to the National Rifle Association (NRA) in 2018. She wrote the letter in response to a tweet by the organization, admonishing physicians to "stay in their lane" when it comes to gun control.
Dr. Ranney's letter discussed gun violence as a public health crisis and urged the NRA to "be part of the solution" by joining the collective effort to reduce firearm injury and death through research, education, and advocacy. "We are not anti-gun," she stated. "We are anti-bullet hole," adding that "almost half of doctors own guns."
The NRA disagreed. When Dr. Ranney testified before Congress during a hearing on gun violence in 2023, NRA spokesperson Billy McLaughlin condemned her testimony as an effort to "dismantle the Second Amendment," calling Dr. Ranney "a known gun control extremist."
"If you actually read what I write, or if you actually listen to what I say, I'm not saying things on behalf of one political party or another," said Dr. Ranney. "It's not about being political. It's about recognizing our role in describing what's happening and making it clear for the world to see. Showing where, based off of data, there may be a better path to improve health and wellbeing."
In spite of the backlash, Dr. Ranney has no regrets about being an activist. "In the current media landscape, folks love to slap labels on people that may or may not be accurate. To me, what matters isn't where I land with a particular politician or political party, but how the work that I do improves health for populations."
When the Need to Act Outweighs the Fear
Laura Andreson, DO, an ob.gyn, took activism a step further when she joined a group of women in Tennessee to file a suit against the state, the attorney general, and the state board of medical examiners. The issue was the Tennessee's abortion ban, which the suit claimed prevented women from getting "necessary and potentially life-saving medical care."
Dr. Andreson, who says she was "not at all" politically active in the past, began to realize how the abortion ban could drastically affect her profession and her patients. "I don't know what flipped in me, but I just felt like I could do this," she said.
Like Dr. Ranney, Dr. Andreson has been as visible as she has been vocal, giving press conferences and interviews, but she acknowledges she has some fears about safety. In fact, after filing the lawsuit, the Center for Reproductive Rights recommended that she go to a website, DeleteMe, that removes personal data from the internet, making it more difficult for people to find her information. "But my need to do this and my desire to do this is stronger than my fears," she added.
Dr. Andreson, who is part of a small practice, did check with both her coworkers and the hospital administration before moving forward with the lawsuit. She was relieved to find that she had the support of her practice and that there wasn't anything in the hospital bylaws to prevent her from filing the lawsuit. "But the people in the bigger institutions who probably have an even better expert base than I do, they are handcuffed," she said.
It has been, in Dr. Andreson's words, "a little uncomfortable" being on the board of the Tennessee Medical Association when the Tennessee Board of Medical Examiners is part of the lawsuit. "We're all members of the same group," she said. "But I'm not suing them as individuals; I'm suing them as an entity that is under our government."
Dr. Andreson said most people have been supportive of her activist work, though she admitted to feeling frustrated when she encounters apathy from fellow ob.gyns. She got little response when she circulated information explaining the abortion laws and trying to get others involved. But she still sees education as being a key part of making change happen.
"I think advocacy, as someone who is considered a responsible, trustworthy person by your community, is important, because you can sway some people just by educating them," she said.
Fighting Inequities in Medicine and Beyond
Christina Chen, MD, says she felt very supported by her medical community at the Mayo Clinic in Rochester, Minnesota, when she and 16 other Asian American physicians posted a video on Instagram in 2020 highlighting increased violence and harassment of Asian Americans during COVID-19. It soon went viral, and the Mayo Clinic distributed it across their social media channels. The only negative repercussions Mayo faced were a few posts on social media saying that politics should not be brought into the healthcare space. Dr. Chen disagrees.
"Social issues and political decisions have direct impact on the health of our communities," Dr. Chen said. "We know that we still have a long way to go to solve health inequities, which is a public health problem, and we all play a huge role in voicing our concerns."
Activism, however, seems to be more complicated when it involves physicians being critical of inequities within the medical field. Nephrologist, Vanessa Grubbs, MD, MPH, founded the nonprofit Black Doc Village in 2022 to raise awareness about the wrongful dismissal of Black residents and expand the Black physician workforce.
Dr. Grubbs said that the medical community has not been supportive of her activism. "The reason why I'm no longer in academia is in part because they got very upset with me tweeting about how some trainees are biased in their treatment of attendings," she said. "Senior White men attendings are often treated very differently than junior women of color faculty."
Dr. Grubbs also expressed her views in 2020 essay in the New England Journal of Medicine where she criticized academic medical institutions for ignoring systemic racism, paying lip service to diversity, equity, and inclusion, and staying "deafeningly silent" when issues of racism are raised.
Today, Black Doc Village is focused on conducting research that can be used to change policy. And Dr. Grubbs now has the full support of her colleagues at West Oakland Health, in Oakland, California, which aspires to advance the Bay Area Black community's health and dignity. "So, no one here has a problem with me speaking out," she added.
The emphasis on data-driven activism as opposed to "playing politics," is a recurring theme for many physicians who publicly engage with social issues.
"It's not partisan," Dr. Ranney said. "Rather, it's a commitment to translating science into actionable steps that can be used regardless of what political party you are in. My job is not to be on one side or the other, but to advance human health." These doctors challenge their critics to explain how such a goal is outside their purview.
A version of this article first appeared on Medscape.com.
It should come as no surprise that when physicians speak out on social and political issues, there is sometimes a backlash. This can range from the typical trolling that occurs online to rarer cases of professional penalties. Two doctors were fired by NYU Langone Health late last year after they posted social media messages about the Israel-Hamas war. Still, many physicians are not only willing to stand up for what they believe in, but they see it as an essential part of their profession.
"We're now at a place where doctors need to engage in public advocacy as an urgent part of our job," wrote Rob Davidson, MD, an emergency department physician, at the onslaught of the COVID-19 pandemic. In an Op-Ed piece for The Guardian, Dr. Davidson noted how the virus forced many physicians into becoming "activist doctors," calling for adequate personal protective equipment and correcting misinformation. "What we want above all is for the administration to listen to doctors, nurses, and frontline health workers - and stop playing politics," he wrote.
'It's Not About Being Political'
The intersection of medicine and politics is hardly new. Doctors frequently testify before Congress, sharing their expertise on issues concerning public health. This, however, isn't the same as "playing politics."
"I'm not taking political stances," said Megan Ranney, MD, Dean of the Yale School of Public Health. "Rather, I'm using science to inform best practices, and I'm vocal around the area where I have expertise where we could do collectively better."
Dr. Ranney's work to end firearm injury and death garnered particular attention when she co-authored an open letter to the National Rifle Association (NRA) in 2018. She wrote the letter in response to a tweet by the organization, admonishing physicians to "stay in their lane" when it comes to gun control.
Dr. Ranney's letter discussed gun violence as a public health crisis and urged the NRA to "be part of the solution" by joining the collective effort to reduce firearm injury and death through research, education, and advocacy. "We are not anti-gun," she stated. "We are anti-bullet hole," adding that "almost half of doctors own guns."
The NRA disagreed. When Dr. Ranney testified before Congress during a hearing on gun violence in 2023, NRA spokesperson Billy McLaughlin condemned her testimony as an effort to "dismantle the Second Amendment," calling Dr. Ranney "a known gun control extremist."
"If you actually read what I write, or if you actually listen to what I say, I'm not saying things on behalf of one political party or another," said Dr. Ranney. "It's not about being political. It's about recognizing our role in describing what's happening and making it clear for the world to see. Showing where, based off of data, there may be a better path to improve health and wellbeing."
In spite of the backlash, Dr. Ranney has no regrets about being an activist. "In the current media landscape, folks love to slap labels on people that may or may not be accurate. To me, what matters isn't where I land with a particular politician or political party, but how the work that I do improves health for populations."
When the Need to Act Outweighs the Fear
Laura Andreson, DO, an ob.gyn, took activism a step further when she joined a group of women in Tennessee to file a suit against the state, the attorney general, and the state board of medical examiners. The issue was the Tennessee's abortion ban, which the suit claimed prevented women from getting "necessary and potentially life-saving medical care."
Dr. Andreson, who says she was "not at all" politically active in the past, began to realize how the abortion ban could drastically affect her profession and her patients. "I don't know what flipped in me, but I just felt like I could do this," she said.
Like Dr. Ranney, Dr. Andreson has been as visible as she has been vocal, giving press conferences and interviews, but she acknowledges she has some fears about safety. In fact, after filing the lawsuit, the Center for Reproductive Rights recommended that she go to a website, DeleteMe, that removes personal data from the internet, making it more difficult for people to find her information. "But my need to do this and my desire to do this is stronger than my fears," she added.
Dr. Andreson, who is part of a small practice, did check with both her coworkers and the hospital administration before moving forward with the lawsuit. She was relieved to find that she had the support of her practice and that there wasn't anything in the hospital bylaws to prevent her from filing the lawsuit. "But the people in the bigger institutions who probably have an even better expert base than I do, they are handcuffed," she said.
It has been, in Dr. Andreson's words, "a little uncomfortable" being on the board of the Tennessee Medical Association when the Tennessee Board of Medical Examiners is part of the lawsuit. "We're all members of the same group," she said. "But I'm not suing them as individuals; I'm suing them as an entity that is under our government."
Dr. Andreson said most people have been supportive of her activist work, though she admitted to feeling frustrated when she encounters apathy from fellow ob.gyns. She got little response when she circulated information explaining the abortion laws and trying to get others involved. But she still sees education as being a key part of making change happen.
"I think advocacy, as someone who is considered a responsible, trustworthy person by your community, is important, because you can sway some people just by educating them," she said.
Fighting Inequities in Medicine and Beyond
Christina Chen, MD, says she felt very supported by her medical community at the Mayo Clinic in Rochester, Minnesota, when she and 16 other Asian American physicians posted a video on Instagram in 2020 highlighting increased violence and harassment of Asian Americans during COVID-19. It soon went viral, and the Mayo Clinic distributed it across their social media channels. The only negative repercussions Mayo faced were a few posts on social media saying that politics should not be brought into the healthcare space. Dr. Chen disagrees.
"Social issues and political decisions have direct impact on the health of our communities," Dr. Chen said. "We know that we still have a long way to go to solve health inequities, which is a public health problem, and we all play a huge role in voicing our concerns."
Activism, however, seems to be more complicated when it involves physicians being critical of inequities within the medical field. Nephrologist, Vanessa Grubbs, MD, MPH, founded the nonprofit Black Doc Village in 2022 to raise awareness about the wrongful dismissal of Black residents and expand the Black physician workforce.
Dr. Grubbs said that the medical community has not been supportive of her activism. "The reason why I'm no longer in academia is in part because they got very upset with me tweeting about how some trainees are biased in their treatment of attendings," she said. "Senior White men attendings are often treated very differently than junior women of color faculty."
Dr. Grubbs also expressed her views in 2020 essay in the New England Journal of Medicine where she criticized academic medical institutions for ignoring systemic racism, paying lip service to diversity, equity, and inclusion, and staying "deafeningly silent" when issues of racism are raised.
Today, Black Doc Village is focused on conducting research that can be used to change policy. And Dr. Grubbs now has the full support of her colleagues at West Oakland Health, in Oakland, California, which aspires to advance the Bay Area Black community's health and dignity. "So, no one here has a problem with me speaking out," she added.
The emphasis on data-driven activism as opposed to "playing politics," is a recurring theme for many physicians who publicly engage with social issues.
"It's not partisan," Dr. Ranney said. "Rather, it's a commitment to translating science into actionable steps that can be used regardless of what political party you are in. My job is not to be on one side or the other, but to advance human health." These doctors challenge their critics to explain how such a goal is outside their purview.
A version of this article first appeared on Medscape.com.
It should come as no surprise that when physicians speak out on social and political issues, there is sometimes a backlash. This can range from the typical trolling that occurs online to rarer cases of professional penalties. Two doctors were fired by NYU Langone Health late last year after they posted social media messages about the Israel-Hamas war. Still, many physicians are not only willing to stand up for what they believe in, but they see it as an essential part of their profession.
"We're now at a place where doctors need to engage in public advocacy as an urgent part of our job," wrote Rob Davidson, MD, an emergency department physician, at the onslaught of the COVID-19 pandemic. In an Op-Ed piece for The Guardian, Dr. Davidson noted how the virus forced many physicians into becoming "activist doctors," calling for adequate personal protective equipment and correcting misinformation. "What we want above all is for the administration to listen to doctors, nurses, and frontline health workers - and stop playing politics," he wrote.
'It's Not About Being Political'
The intersection of medicine and politics is hardly new. Doctors frequently testify before Congress, sharing their expertise on issues concerning public health. This, however, isn't the same as "playing politics."
"I'm not taking political stances," said Megan Ranney, MD, Dean of the Yale School of Public Health. "Rather, I'm using science to inform best practices, and I'm vocal around the area where I have expertise where we could do collectively better."
Dr. Ranney's work to end firearm injury and death garnered particular attention when she co-authored an open letter to the National Rifle Association (NRA) in 2018. She wrote the letter in response to a tweet by the organization, admonishing physicians to "stay in their lane" when it comes to gun control.
Dr. Ranney's letter discussed gun violence as a public health crisis and urged the NRA to "be part of the solution" by joining the collective effort to reduce firearm injury and death through research, education, and advocacy. "We are not anti-gun," she stated. "We are anti-bullet hole," adding that "almost half of doctors own guns."
The NRA disagreed. When Dr. Ranney testified before Congress during a hearing on gun violence in 2023, NRA spokesperson Billy McLaughlin condemned her testimony as an effort to "dismantle the Second Amendment," calling Dr. Ranney "a known gun control extremist."
"If you actually read what I write, or if you actually listen to what I say, I'm not saying things on behalf of one political party or another," said Dr. Ranney. "It's not about being political. It's about recognizing our role in describing what's happening and making it clear for the world to see. Showing where, based off of data, there may be a better path to improve health and wellbeing."
In spite of the backlash, Dr. Ranney has no regrets about being an activist. "In the current media landscape, folks love to slap labels on people that may or may not be accurate. To me, what matters isn't where I land with a particular politician or political party, but how the work that I do improves health for populations."
When the Need to Act Outweighs the Fear
Laura Andreson, DO, an ob.gyn, took activism a step further when she joined a group of women in Tennessee to file a suit against the state, the attorney general, and the state board of medical examiners. The issue was the Tennessee's abortion ban, which the suit claimed prevented women from getting "necessary and potentially life-saving medical care."
Dr. Andreson, who says she was "not at all" politically active in the past, began to realize how the abortion ban could drastically affect her profession and her patients. "I don't know what flipped in me, but I just felt like I could do this," she said.
Like Dr. Ranney, Dr. Andreson has been as visible as she has been vocal, giving press conferences and interviews, but she acknowledges she has some fears about safety. In fact, after filing the lawsuit, the Center for Reproductive Rights recommended that she go to a website, DeleteMe, that removes personal data from the internet, making it more difficult for people to find her information. "But my need to do this and my desire to do this is stronger than my fears," she added.
Dr. Andreson, who is part of a small practice, did check with both her coworkers and the hospital administration before moving forward with the lawsuit. She was relieved to find that she had the support of her practice and that there wasn't anything in the hospital bylaws to prevent her from filing the lawsuit. "But the people in the bigger institutions who probably have an even better expert base than I do, they are handcuffed," she said.
It has been, in Dr. Andreson's words, "a little uncomfortable" being on the board of the Tennessee Medical Association when the Tennessee Board of Medical Examiners is part of the lawsuit. "We're all members of the same group," she said. "But I'm not suing them as individuals; I'm suing them as an entity that is under our government."
Dr. Andreson said most people have been supportive of her activist work, though she admitted to feeling frustrated when she encounters apathy from fellow ob.gyns. She got little response when she circulated information explaining the abortion laws and trying to get others involved. But she still sees education as being a key part of making change happen.
"I think advocacy, as someone who is considered a responsible, trustworthy person by your community, is important, because you can sway some people just by educating them," she said.
Fighting Inequities in Medicine and Beyond
Christina Chen, MD, says she felt very supported by her medical community at the Mayo Clinic in Rochester, Minnesota, when she and 16 other Asian American physicians posted a video on Instagram in 2020 highlighting increased violence and harassment of Asian Americans during COVID-19. It soon went viral, and the Mayo Clinic distributed it across their social media channels. The only negative repercussions Mayo faced were a few posts on social media saying that politics should not be brought into the healthcare space. Dr. Chen disagrees.
"Social issues and political decisions have direct impact on the health of our communities," Dr. Chen said. "We know that we still have a long way to go to solve health inequities, which is a public health problem, and we all play a huge role in voicing our concerns."
Activism, however, seems to be more complicated when it involves physicians being critical of inequities within the medical field. Nephrologist, Vanessa Grubbs, MD, MPH, founded the nonprofit Black Doc Village in 2022 to raise awareness about the wrongful dismissal of Black residents and expand the Black physician workforce.
Dr. Grubbs said that the medical community has not been supportive of her activism. "The reason why I'm no longer in academia is in part because they got very upset with me tweeting about how some trainees are biased in their treatment of attendings," she said. "Senior White men attendings are often treated very differently than junior women of color faculty."
Dr. Grubbs also expressed her views in 2020 essay in the New England Journal of Medicine where she criticized academic medical institutions for ignoring systemic racism, paying lip service to diversity, equity, and inclusion, and staying "deafeningly silent" when issues of racism are raised.
Today, Black Doc Village is focused on conducting research that can be used to change policy. And Dr. Grubbs now has the full support of her colleagues at West Oakland Health, in Oakland, California, which aspires to advance the Bay Area Black community's health and dignity. "So, no one here has a problem with me speaking out," she added.
The emphasis on data-driven activism as opposed to "playing politics," is a recurring theme for many physicians who publicly engage with social issues.
"It's not partisan," Dr. Ranney said. "Rather, it's a commitment to translating science into actionable steps that can be used regardless of what political party you are in. My job is not to be on one side or the other, but to advance human health." These doctors challenge their critics to explain how such a goal is outside their purview.
A version of this article first appeared on Medscape.com.
Less Than 50% of Accelerated Approvals Show Clinical Benefit
despite being on the US market for more than 5 years, according to a new study.
Under the program, drugs are approved for marketing if they show benefit in surrogate markers thought to indicate efficacy. Progression-free survival, tumor response, and duration of response are the most used surrogate markers for accelerated approvals of cancer drugs. These are based largely on imaging studies that show either a stop in growth in the case of progression-free survival or tumor shrinkage in the case of tumor response.
Following accelerated approvals, companies are then supposed to show actual clinical benefit in confirmatory trials.
The problem with relying on surrogate markers for drug approvals is that they don’t always correlate with longer survival or improved quality of life, said Edward Cliff, MBBS, who presented the findings at the American Association for Cancer Research 2024 annual meeting (abstract 918). The study was also published in JAMA to coincide with the meeting presentation.
In some cancers, these markers work well, but in others they don’t, said Dr. Cliff, a hematology trainee at Brigham and Women’s Hospital, Boston, when the work was conducted, and now a hematology fellow at the Peter MacCallum Cancer Centre in Melbourne, Australia.
To determine whether cancer drugs granted accelerated approval ultimately show an overall survival or quality of life benefit, researchers reviewed 46 cancer drugs granted accelerated approvals between 2013 and 2017. Twenty (43%) were granted full approval after demonstrating survival or quality-of-life benefits.
Nine, however, were converted to full approvals on the basis of surrogate markers. These include a full approval for pembrolizumab in previously treated recurrent or refractory head and neck squamous cell carcinoma and a full approval for nivolumab for refractory locally advanced or metastatic urothelial carcinoma, both based on tumor response rate and duration of response.
Of the remaining 17 drugs evaluated in the trial, 10 have been withdrawn and seven do not yet have confirmatory trial results.
The reliance on surrogate markers means that these drugs are used for treatment, covered by insurance, and added to guidelines — all without solid evidence of real-world clinical benefit, said Dr. Cliff.
However, the goal should not be to do away with the accelerated approval process, because it sometimes does deliver powerful agents to patients quickly. Instead, Dr. Cliff told this news organization, the system needs to be improved so that “we keep the speed while getting certainty around clinical benefits” with robust and timely confirmatory trials.
In the meantime, “clinicians should communicate with patients about any residual uncertainty of clinical benefit when they offer novel therapies,” Dr. Cliff explained. “It’s important for them to have the information.”
There has been some progress on the issue. In December 2022, the US Congress passed the Food and Drug Administration Omnibus Reform Act. Among other things, the Act requires companies to have confirmation trials underway as a condition for accelerated approval, and to provide regular reports on their progress. The Act also expedites the withdrawal process for drugs that don’t show a benefit.
The Act has been put to the test twice recently. In February, FDA used the expedited process to remove the multiple myeloma drug melphalan flufenamide from the market. Melphalan flufenamide hadn’t been sold in the US for quite some time, so the process wasn’t contentious.
In March, Regeneron announced that accelerated approval for the follicular and diffuse B cell lymphoma drug odronextamab has been delayed pending enrollment in a confirmatory trial.
“There have been some promising steps,” Dr. Cliff said, but much work needs to be done.
Study moderator Shivaani Kummar, MD, agreed, noting that “the data is showing that the confirmatory trials aren’t happening at the pace which they should.”
But the solution is not to curtail approvals; it’s to make sure that accelerated approval commitments are met, said Dr. Kummar.
Still, “as a practicing oncologist, I welcome the accelerated pathway,” Dr. Kummar, a medical oncologist/hematologist at Oregon Health & Science University, Portland, told this news organization. “I want the availability to my patients.”
Having drugs approved on the basis of surrogate markers doesn’t necessarily mean patients are getting ineffective therapies, Dr. Kummar noted. For instance, if an agent just shrinks the tumor, it can sometimes still be “a huge clinical benefit because it can take the symptoms away.”
As for prescribing drugs based on accelerated approvals, she said she tells her patients that trials have been promising, but we don’t know what the long-term effects are. She and her patient then make a decision together.
The study was funded by Arnold Ventures. Dr. Kummar reported support from several companies, including Bayer, Gilead, and others. Dr. Cliff had no disclosures.
A version of this article appeared on Medscape.com.
despite being on the US market for more than 5 years, according to a new study.
Under the program, drugs are approved for marketing if they show benefit in surrogate markers thought to indicate efficacy. Progression-free survival, tumor response, and duration of response are the most used surrogate markers for accelerated approvals of cancer drugs. These are based largely on imaging studies that show either a stop in growth in the case of progression-free survival or tumor shrinkage in the case of tumor response.
Following accelerated approvals, companies are then supposed to show actual clinical benefit in confirmatory trials.
The problem with relying on surrogate markers for drug approvals is that they don’t always correlate with longer survival or improved quality of life, said Edward Cliff, MBBS, who presented the findings at the American Association for Cancer Research 2024 annual meeting (abstract 918). The study was also published in JAMA to coincide with the meeting presentation.
In some cancers, these markers work well, but in others they don’t, said Dr. Cliff, a hematology trainee at Brigham and Women’s Hospital, Boston, when the work was conducted, and now a hematology fellow at the Peter MacCallum Cancer Centre in Melbourne, Australia.
To determine whether cancer drugs granted accelerated approval ultimately show an overall survival or quality of life benefit, researchers reviewed 46 cancer drugs granted accelerated approvals between 2013 and 2017. Twenty (43%) were granted full approval after demonstrating survival or quality-of-life benefits.
Nine, however, were converted to full approvals on the basis of surrogate markers. These include a full approval for pembrolizumab in previously treated recurrent or refractory head and neck squamous cell carcinoma and a full approval for nivolumab for refractory locally advanced or metastatic urothelial carcinoma, both based on tumor response rate and duration of response.
Of the remaining 17 drugs evaluated in the trial, 10 have been withdrawn and seven do not yet have confirmatory trial results.
The reliance on surrogate markers means that these drugs are used for treatment, covered by insurance, and added to guidelines — all without solid evidence of real-world clinical benefit, said Dr. Cliff.
However, the goal should not be to do away with the accelerated approval process, because it sometimes does deliver powerful agents to patients quickly. Instead, Dr. Cliff told this news organization, the system needs to be improved so that “we keep the speed while getting certainty around clinical benefits” with robust and timely confirmatory trials.
In the meantime, “clinicians should communicate with patients about any residual uncertainty of clinical benefit when they offer novel therapies,” Dr. Cliff explained. “It’s important for them to have the information.”
There has been some progress on the issue. In December 2022, the US Congress passed the Food and Drug Administration Omnibus Reform Act. Among other things, the Act requires companies to have confirmation trials underway as a condition for accelerated approval, and to provide regular reports on their progress. The Act also expedites the withdrawal process for drugs that don’t show a benefit.
The Act has been put to the test twice recently. In February, FDA used the expedited process to remove the multiple myeloma drug melphalan flufenamide from the market. Melphalan flufenamide hadn’t been sold in the US for quite some time, so the process wasn’t contentious.
In March, Regeneron announced that accelerated approval for the follicular and diffuse B cell lymphoma drug odronextamab has been delayed pending enrollment in a confirmatory trial.
“There have been some promising steps,” Dr. Cliff said, but much work needs to be done.
Study moderator Shivaani Kummar, MD, agreed, noting that “the data is showing that the confirmatory trials aren’t happening at the pace which they should.”
But the solution is not to curtail approvals; it’s to make sure that accelerated approval commitments are met, said Dr. Kummar.
Still, “as a practicing oncologist, I welcome the accelerated pathway,” Dr. Kummar, a medical oncologist/hematologist at Oregon Health & Science University, Portland, told this news organization. “I want the availability to my patients.”
Having drugs approved on the basis of surrogate markers doesn’t necessarily mean patients are getting ineffective therapies, Dr. Kummar noted. For instance, if an agent just shrinks the tumor, it can sometimes still be “a huge clinical benefit because it can take the symptoms away.”
As for prescribing drugs based on accelerated approvals, she said she tells her patients that trials have been promising, but we don’t know what the long-term effects are. She and her patient then make a decision together.
The study was funded by Arnold Ventures. Dr. Kummar reported support from several companies, including Bayer, Gilead, and others. Dr. Cliff had no disclosures.
A version of this article appeared on Medscape.com.
despite being on the US market for more than 5 years, according to a new study.
Under the program, drugs are approved for marketing if they show benefit in surrogate markers thought to indicate efficacy. Progression-free survival, tumor response, and duration of response are the most used surrogate markers for accelerated approvals of cancer drugs. These are based largely on imaging studies that show either a stop in growth in the case of progression-free survival or tumor shrinkage in the case of tumor response.
Following accelerated approvals, companies are then supposed to show actual clinical benefit in confirmatory trials.
The problem with relying on surrogate markers for drug approvals is that they don’t always correlate with longer survival or improved quality of life, said Edward Cliff, MBBS, who presented the findings at the American Association for Cancer Research 2024 annual meeting (abstract 918). The study was also published in JAMA to coincide with the meeting presentation.
In some cancers, these markers work well, but in others they don’t, said Dr. Cliff, a hematology trainee at Brigham and Women’s Hospital, Boston, when the work was conducted, and now a hematology fellow at the Peter MacCallum Cancer Centre in Melbourne, Australia.
To determine whether cancer drugs granted accelerated approval ultimately show an overall survival or quality of life benefit, researchers reviewed 46 cancer drugs granted accelerated approvals between 2013 and 2017. Twenty (43%) were granted full approval after demonstrating survival or quality-of-life benefits.
Nine, however, were converted to full approvals on the basis of surrogate markers. These include a full approval for pembrolizumab in previously treated recurrent or refractory head and neck squamous cell carcinoma and a full approval for nivolumab for refractory locally advanced or metastatic urothelial carcinoma, both based on tumor response rate and duration of response.
Of the remaining 17 drugs evaluated in the trial, 10 have been withdrawn and seven do not yet have confirmatory trial results.
The reliance on surrogate markers means that these drugs are used for treatment, covered by insurance, and added to guidelines — all without solid evidence of real-world clinical benefit, said Dr. Cliff.
However, the goal should not be to do away with the accelerated approval process, because it sometimes does deliver powerful agents to patients quickly. Instead, Dr. Cliff told this news organization, the system needs to be improved so that “we keep the speed while getting certainty around clinical benefits” with robust and timely confirmatory trials.
In the meantime, “clinicians should communicate with patients about any residual uncertainty of clinical benefit when they offer novel therapies,” Dr. Cliff explained. “It’s important for them to have the information.”
There has been some progress on the issue. In December 2022, the US Congress passed the Food and Drug Administration Omnibus Reform Act. Among other things, the Act requires companies to have confirmation trials underway as a condition for accelerated approval, and to provide regular reports on their progress. The Act also expedites the withdrawal process for drugs that don’t show a benefit.
The Act has been put to the test twice recently. In February, FDA used the expedited process to remove the multiple myeloma drug melphalan flufenamide from the market. Melphalan flufenamide hadn’t been sold in the US for quite some time, so the process wasn’t contentious.
In March, Regeneron announced that accelerated approval for the follicular and diffuse B cell lymphoma drug odronextamab has been delayed pending enrollment in a confirmatory trial.
“There have been some promising steps,” Dr. Cliff said, but much work needs to be done.
Study moderator Shivaani Kummar, MD, agreed, noting that “the data is showing that the confirmatory trials aren’t happening at the pace which they should.”
But the solution is not to curtail approvals; it’s to make sure that accelerated approval commitments are met, said Dr. Kummar.
Still, “as a practicing oncologist, I welcome the accelerated pathway,” Dr. Kummar, a medical oncologist/hematologist at Oregon Health & Science University, Portland, told this news organization. “I want the availability to my patients.”
Having drugs approved on the basis of surrogate markers doesn’t necessarily mean patients are getting ineffective therapies, Dr. Kummar noted. For instance, if an agent just shrinks the tumor, it can sometimes still be “a huge clinical benefit because it can take the symptoms away.”
As for prescribing drugs based on accelerated approvals, she said she tells her patients that trials have been promising, but we don’t know what the long-term effects are. She and her patient then make a decision together.
The study was funded by Arnold Ventures. Dr. Kummar reported support from several companies, including Bayer, Gilead, and others. Dr. Cliff had no disclosures.
A version of this article appeared on Medscape.com.
Poop Doesn’t Lie: What Fecal ‘Forensics’ Tells Us About Diet
A lightbulb moment hit as Lawrence David was chatting one day with an ecologist who studies the microbiomes and diets of large herbivores in the African savanna. David was envious. He’d been studying the human microbiome, and this ecologist had tons of animal statistics that were way more specific than what David had obtained from people.
“How on earth do you get all these dietary data?” David recalled asking. “Obviously, he didn’t ask the animals what they ate.”
All those specific statistics came from DNA sequencing of animal scat scooped up from the savanna.
Indeed.
Depending on when you read this, you may have the DNA of more than a dozen plant species, plus another three or four animal species, gurgling through your gut. That’s the straight poop taken straight from, well, poop.
Diet, DNA, and Feces
Everything we eat (except vitamins, minerals, and salt) came from something that was living, and all living things have genomes.
“A decent fraction of that DNA” goes undigested and is then excreted, said David, a PhD and associate professor of molecular genetics and microbiology at Duke University, Durham, North Carolina.
“We are using DNA sequencing to reconstruct what people eat,” David said. “We try to see if there are patterns in what people eat and how we can measure them by DNA, or kind of genetic forensics.” Then they connect that data to health outcomes like obesity.
A typical person’s excrement probably contains the DNA of 10-20 plant species and three or four types of animal DNA. “And that’s the average person. Some people may have more like 40 types at any given time,” David said.
Studying DNA in human feces has potential applications in research and in clinical settings. For instance, it could help design personalized nutrition strategies for patients, something that’s already being tested. He hopes that DNA information will help “connect patterns in what people eat to their microbiomes.”
One big advantage: Feces don’t lie. In reconstructing someone’s diet, people either forget what they ate, fudge the truth, or can’t be bothered to keep track.
“Patients report the fruit they ate yesterday but not the M&Ms,” said Neil Stollman, MD, chief of the division of gastroenterology at Alta Bates Summit Medical Center in Oakland, California.
Some people can’t write it all down because they’re too old or too young — the very people at highest risk of nutrition-associated disease, said David.
Fetching and Figuring Out Feces
It’s a lot of work to collect and analyze fecal matter, for ethical, legal, and logistical reasons. “And then there’s sort of an ick factor to this kind of work,” David said.
To get samples, people place a plastic collection cup under the toilet seat to catch the stool. The person then swabs or scoops some of that into a tube, seals the top, and either brings it in or mails it to the lab.
In the lab, David said, “if the DNA is still inside the plant cells, we crack the cells open using a variety of methods. We use what’s called ‘a stomacher,’ which is like two big paddles, and we load the poop [which is in a plastic bag] into it and then squash it — mash it up. We also sometimes load small particles of what is basically glass into it and then shake really hard — it is another way you can physically break open the plant cells. This can also be done with chemicals. It’s like a chemistry lab,” he said, noting that this process takes about half a day to do.
There is much more bacterial DNA in stool than there is food DNA, and even a little human DNA and sometimes fungi, said David. “The concentration of bacteria in stool is amongst the highest concentrations of bacteria on the planet,” he said, but his lab focuses on the plant DNA they find.
They use a molecular process called polymerase chain reaction (PCR) that amplifies and selectively copies DNA from plants. (The scientists who invented this “ingenious” process won a Nobel Prize, David noted.) Like a COVID PCR test, the process only matches up for certain kinds of DNA and can be designed to be more specific or less specific. In David’s lab, they shoot for a middle ground of specificity, where the PCR process is targeting chloroplasts in plants.
Once they’ve detected all the different sequences of food species, they need to find the DNA code, a time-consuming step. His colleague Briana Petrone compiled a reference database of specific sequences of DNA that correspond to different species of plants. This work took more than a year, said David, noting that only a handful of other labs around the country are sequencing DNA in feces, most of them looking at it in animals, not humans.
There are 200,000 to 300,000 species of edible plants estimated to be on the planet, he said. “I think historically, humans have eaten about 7000 of them. We’re kind of like a walking repository of all this genetic material.”
What Scientists Learn from Fecal DNA
Tracking DNA in digested food can provide valuable data to researchers — information that could have a major impact on nutritional guidance for people with obesity and digestive diseases and other gastrointestinal and nutrition-related issues.
David and Petrone’s 2023 study analyzing DNA in stool samples, published in the Proceedings of the National Academy of Sciences (PNAS), showed what — and roughly how much — people ate.
They noticed that kids with obesity had a higher diversity of plants in them than kids without obesity. Sounds backward — wouldn’t a child who eats more plants be a healthier weight? “The more I dug into it, it turns out that foods that are more processed often tend to have more ingredients. So, a Big Mac and fries and a coffee have 19 different plant species,” said David.
Going forward, he said, researchers may have to be “more specific about how we think about dietary diversity. Maybe not all plant species count toward health in the same way.”
David’s work provides an innovative way to conduct nutrition research, said Jotham Suez, PhD, an assistant professor in the department of molecular microbiology and immunology at Johns Hopkins Bloomberg School of Public Health.
“We need to have some means of tracking what people actually ate during a study, whether it’s an intervention where we provide them with the food or an observational study where we let people eat their habitual diet and track it themselves,” said Suez, who studies the gut microbiome.
“Recall bias” makes food questionnaires and apps unreliable. And research suggests that some participants may underreport food intake, possibly because they don’t want to be judged or they misestimate how much they actually consumed.
“There’s huge promise” with a tool like the one described in the PNAS study for making connections between diet and disease, Suez said. But access may be an issue for many researchers. He expects techniques to improve and costs to go down, but there will be challenges. “This method is also almost exclusively looking at plant DNA material, Suez added, “and our diets contain multiple components that are not plants.”
And even if a person just eats an apple or a single cucumber, that food may be degraded somewhere else in the gut, and it may be digested differently in different people’s guts. “Metabolism, of course, can be different between people,” Suez said, so the amounts of data will vary. “In their study, the qualitative data is convincing. The quantitative is TBD [to be determined].”
But he said it might be “a perfect tool” for scientists who want to study indigestible fiber, which is an important area of science, too.
“I totally buy it as a potentially better way to do dietary analytics for disease associations,” said Stollman, an expert in fecal transplant and diverticulitis and a trustee of the American College of Gastroenterology. Stollman sees many patients with diverticular disease who could benefit.
“One of the core questions in the diverticular world is, what causes diverticular disease, so we can ideally prevent it? For decades, the theory has been that a low fiber diet contributes to it,” said Stollman, but testing DNA in patients’ stools could help researchers explore the question in a new and potentially more nuanced and accurate way. Findings might allow scientists to learn, “Do people who eat X get polyps? Is this diet a risk factor for X, Y, or Z disease?” said Stollman.
Future Clinical Applications
Brenda Davy, PhD, is a registered dietitian and professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech. She conducts research investigating the role of diet in the prevention and treatment of obesity and related conditions such as type 2 diabetes. She also develops dietary assessment methods. More than a decade ago, she developed one of the first rapid assessment tools for quantifying beverage intake — the Beverage Intake Questionnaire — an assessment that is still used today.
“Dietary assessment is necessary in both research and clinical settings,” Davy said. “If a physician diagnoses a patient with a certain condition, information about the patient’s usual dietary habits can help him or her prescribe dietary changes that may help treat that condition.”
Biospecimens, like fecal and urine samples, can be a safe, accurate way to collect that data, she said. Samples can be obtained easily and noninvasively “in a wide variety of populations such as children or older adults” and in clinical settings.
Davy and her team use David’s technology in their work — in particular, a tool called FoodSeq that applies DNA metabarcoding to human stool to collect information about food taxa consumed. Their two labs are now collaborating on a project investigating how ultraprocessed foods might impact type 2 diabetes risk and cardiovascular health.
There are many directions David’s lab would like to take their research, possibly partnering with epidemiologists on global studies that would help them expand their DNA database and better understand how, for example, climate change may be affecting diet diversity and to learn more about diet across different populations.
A version of this article appeared on Medscape.com.
A lightbulb moment hit as Lawrence David was chatting one day with an ecologist who studies the microbiomes and diets of large herbivores in the African savanna. David was envious. He’d been studying the human microbiome, and this ecologist had tons of animal statistics that were way more specific than what David had obtained from people.
“How on earth do you get all these dietary data?” David recalled asking. “Obviously, he didn’t ask the animals what they ate.”
All those specific statistics came from DNA sequencing of animal scat scooped up from the savanna.
Indeed.
Depending on when you read this, you may have the DNA of more than a dozen plant species, plus another three or four animal species, gurgling through your gut. That’s the straight poop taken straight from, well, poop.
Diet, DNA, and Feces
Everything we eat (except vitamins, minerals, and salt) came from something that was living, and all living things have genomes.
“A decent fraction of that DNA” goes undigested and is then excreted, said David, a PhD and associate professor of molecular genetics and microbiology at Duke University, Durham, North Carolina.
“We are using DNA sequencing to reconstruct what people eat,” David said. “We try to see if there are patterns in what people eat and how we can measure them by DNA, or kind of genetic forensics.” Then they connect that data to health outcomes like obesity.
A typical person’s excrement probably contains the DNA of 10-20 plant species and three or four types of animal DNA. “And that’s the average person. Some people may have more like 40 types at any given time,” David said.
Studying DNA in human feces has potential applications in research and in clinical settings. For instance, it could help design personalized nutrition strategies for patients, something that’s already being tested. He hopes that DNA information will help “connect patterns in what people eat to their microbiomes.”
One big advantage: Feces don’t lie. In reconstructing someone’s diet, people either forget what they ate, fudge the truth, or can’t be bothered to keep track.
“Patients report the fruit they ate yesterday but not the M&Ms,” said Neil Stollman, MD, chief of the division of gastroenterology at Alta Bates Summit Medical Center in Oakland, California.
Some people can’t write it all down because they’re too old or too young — the very people at highest risk of nutrition-associated disease, said David.
Fetching and Figuring Out Feces
It’s a lot of work to collect and analyze fecal matter, for ethical, legal, and logistical reasons. “And then there’s sort of an ick factor to this kind of work,” David said.
To get samples, people place a plastic collection cup under the toilet seat to catch the stool. The person then swabs or scoops some of that into a tube, seals the top, and either brings it in or mails it to the lab.
In the lab, David said, “if the DNA is still inside the plant cells, we crack the cells open using a variety of methods. We use what’s called ‘a stomacher,’ which is like two big paddles, and we load the poop [which is in a plastic bag] into it and then squash it — mash it up. We also sometimes load small particles of what is basically glass into it and then shake really hard — it is another way you can physically break open the plant cells. This can also be done with chemicals. It’s like a chemistry lab,” he said, noting that this process takes about half a day to do.
There is much more bacterial DNA in stool than there is food DNA, and even a little human DNA and sometimes fungi, said David. “The concentration of bacteria in stool is amongst the highest concentrations of bacteria on the planet,” he said, but his lab focuses on the plant DNA they find.
They use a molecular process called polymerase chain reaction (PCR) that amplifies and selectively copies DNA from plants. (The scientists who invented this “ingenious” process won a Nobel Prize, David noted.) Like a COVID PCR test, the process only matches up for certain kinds of DNA and can be designed to be more specific or less specific. In David’s lab, they shoot for a middle ground of specificity, where the PCR process is targeting chloroplasts in plants.
Once they’ve detected all the different sequences of food species, they need to find the DNA code, a time-consuming step. His colleague Briana Petrone compiled a reference database of specific sequences of DNA that correspond to different species of plants. This work took more than a year, said David, noting that only a handful of other labs around the country are sequencing DNA in feces, most of them looking at it in animals, not humans.
There are 200,000 to 300,000 species of edible plants estimated to be on the planet, he said. “I think historically, humans have eaten about 7000 of them. We’re kind of like a walking repository of all this genetic material.”
What Scientists Learn from Fecal DNA
Tracking DNA in digested food can provide valuable data to researchers — information that could have a major impact on nutritional guidance for people with obesity and digestive diseases and other gastrointestinal and nutrition-related issues.
David and Petrone’s 2023 study analyzing DNA in stool samples, published in the Proceedings of the National Academy of Sciences (PNAS), showed what — and roughly how much — people ate.
They noticed that kids with obesity had a higher diversity of plants in them than kids without obesity. Sounds backward — wouldn’t a child who eats more plants be a healthier weight? “The more I dug into it, it turns out that foods that are more processed often tend to have more ingredients. So, a Big Mac and fries and a coffee have 19 different plant species,” said David.
Going forward, he said, researchers may have to be “more specific about how we think about dietary diversity. Maybe not all plant species count toward health in the same way.”
David’s work provides an innovative way to conduct nutrition research, said Jotham Suez, PhD, an assistant professor in the department of molecular microbiology and immunology at Johns Hopkins Bloomberg School of Public Health.
“We need to have some means of tracking what people actually ate during a study, whether it’s an intervention where we provide them with the food or an observational study where we let people eat their habitual diet and track it themselves,” said Suez, who studies the gut microbiome.
“Recall bias” makes food questionnaires and apps unreliable. And research suggests that some participants may underreport food intake, possibly because they don’t want to be judged or they misestimate how much they actually consumed.
“There’s huge promise” with a tool like the one described in the PNAS study for making connections between diet and disease, Suez said. But access may be an issue for many researchers. He expects techniques to improve and costs to go down, but there will be challenges. “This method is also almost exclusively looking at plant DNA material, Suez added, “and our diets contain multiple components that are not plants.”
And even if a person just eats an apple or a single cucumber, that food may be degraded somewhere else in the gut, and it may be digested differently in different people’s guts. “Metabolism, of course, can be different between people,” Suez said, so the amounts of data will vary. “In their study, the qualitative data is convincing. The quantitative is TBD [to be determined].”
But he said it might be “a perfect tool” for scientists who want to study indigestible fiber, which is an important area of science, too.
“I totally buy it as a potentially better way to do dietary analytics for disease associations,” said Stollman, an expert in fecal transplant and diverticulitis and a trustee of the American College of Gastroenterology. Stollman sees many patients with diverticular disease who could benefit.
“One of the core questions in the diverticular world is, what causes diverticular disease, so we can ideally prevent it? For decades, the theory has been that a low fiber diet contributes to it,” said Stollman, but testing DNA in patients’ stools could help researchers explore the question in a new and potentially more nuanced and accurate way. Findings might allow scientists to learn, “Do people who eat X get polyps? Is this diet a risk factor for X, Y, or Z disease?” said Stollman.
Future Clinical Applications
Brenda Davy, PhD, is a registered dietitian and professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech. She conducts research investigating the role of diet in the prevention and treatment of obesity and related conditions such as type 2 diabetes. She also develops dietary assessment methods. More than a decade ago, she developed one of the first rapid assessment tools for quantifying beverage intake — the Beverage Intake Questionnaire — an assessment that is still used today.
“Dietary assessment is necessary in both research and clinical settings,” Davy said. “If a physician diagnoses a patient with a certain condition, information about the patient’s usual dietary habits can help him or her prescribe dietary changes that may help treat that condition.”
Biospecimens, like fecal and urine samples, can be a safe, accurate way to collect that data, she said. Samples can be obtained easily and noninvasively “in a wide variety of populations such as children or older adults” and in clinical settings.
Davy and her team use David’s technology in their work — in particular, a tool called FoodSeq that applies DNA metabarcoding to human stool to collect information about food taxa consumed. Their two labs are now collaborating on a project investigating how ultraprocessed foods might impact type 2 diabetes risk and cardiovascular health.
There are many directions David’s lab would like to take their research, possibly partnering with epidemiologists on global studies that would help them expand their DNA database and better understand how, for example, climate change may be affecting diet diversity and to learn more about diet across different populations.
A version of this article appeared on Medscape.com.
A lightbulb moment hit as Lawrence David was chatting one day with an ecologist who studies the microbiomes and diets of large herbivores in the African savanna. David was envious. He’d been studying the human microbiome, and this ecologist had tons of animal statistics that were way more specific than what David had obtained from people.
“How on earth do you get all these dietary data?” David recalled asking. “Obviously, he didn’t ask the animals what they ate.”
All those specific statistics came from DNA sequencing of animal scat scooped up from the savanna.
Indeed.
Depending on when you read this, you may have the DNA of more than a dozen plant species, plus another three or four animal species, gurgling through your gut. That’s the straight poop taken straight from, well, poop.
Diet, DNA, and Feces
Everything we eat (except vitamins, minerals, and salt) came from something that was living, and all living things have genomes.
“A decent fraction of that DNA” goes undigested and is then excreted, said David, a PhD and associate professor of molecular genetics and microbiology at Duke University, Durham, North Carolina.
“We are using DNA sequencing to reconstruct what people eat,” David said. “We try to see if there are patterns in what people eat and how we can measure them by DNA, or kind of genetic forensics.” Then they connect that data to health outcomes like obesity.
A typical person’s excrement probably contains the DNA of 10-20 plant species and three or four types of animal DNA. “And that’s the average person. Some people may have more like 40 types at any given time,” David said.
Studying DNA in human feces has potential applications in research and in clinical settings. For instance, it could help design personalized nutrition strategies for patients, something that’s already being tested. He hopes that DNA information will help “connect patterns in what people eat to their microbiomes.”
One big advantage: Feces don’t lie. In reconstructing someone’s diet, people either forget what they ate, fudge the truth, or can’t be bothered to keep track.
“Patients report the fruit they ate yesterday but not the M&Ms,” said Neil Stollman, MD, chief of the division of gastroenterology at Alta Bates Summit Medical Center in Oakland, California.
Some people can’t write it all down because they’re too old or too young — the very people at highest risk of nutrition-associated disease, said David.
Fetching and Figuring Out Feces
It’s a lot of work to collect and analyze fecal matter, for ethical, legal, and logistical reasons. “And then there’s sort of an ick factor to this kind of work,” David said.
To get samples, people place a plastic collection cup under the toilet seat to catch the stool. The person then swabs or scoops some of that into a tube, seals the top, and either brings it in or mails it to the lab.
In the lab, David said, “if the DNA is still inside the plant cells, we crack the cells open using a variety of methods. We use what’s called ‘a stomacher,’ which is like two big paddles, and we load the poop [which is in a plastic bag] into it and then squash it — mash it up. We also sometimes load small particles of what is basically glass into it and then shake really hard — it is another way you can physically break open the plant cells. This can also be done with chemicals. It’s like a chemistry lab,” he said, noting that this process takes about half a day to do.
There is much more bacterial DNA in stool than there is food DNA, and even a little human DNA and sometimes fungi, said David. “The concentration of bacteria in stool is amongst the highest concentrations of bacteria on the planet,” he said, but his lab focuses on the plant DNA they find.
They use a molecular process called polymerase chain reaction (PCR) that amplifies and selectively copies DNA from plants. (The scientists who invented this “ingenious” process won a Nobel Prize, David noted.) Like a COVID PCR test, the process only matches up for certain kinds of DNA and can be designed to be more specific or less specific. In David’s lab, they shoot for a middle ground of specificity, where the PCR process is targeting chloroplasts in plants.
Once they’ve detected all the different sequences of food species, they need to find the DNA code, a time-consuming step. His colleague Briana Petrone compiled a reference database of specific sequences of DNA that correspond to different species of plants. This work took more than a year, said David, noting that only a handful of other labs around the country are sequencing DNA in feces, most of them looking at it in animals, not humans.
There are 200,000 to 300,000 species of edible plants estimated to be on the planet, he said. “I think historically, humans have eaten about 7000 of them. We’re kind of like a walking repository of all this genetic material.”
What Scientists Learn from Fecal DNA
Tracking DNA in digested food can provide valuable data to researchers — information that could have a major impact on nutritional guidance for people with obesity and digestive diseases and other gastrointestinal and nutrition-related issues.
David and Petrone’s 2023 study analyzing DNA in stool samples, published in the Proceedings of the National Academy of Sciences (PNAS), showed what — and roughly how much — people ate.
They noticed that kids with obesity had a higher diversity of plants in them than kids without obesity. Sounds backward — wouldn’t a child who eats more plants be a healthier weight? “The more I dug into it, it turns out that foods that are more processed often tend to have more ingredients. So, a Big Mac and fries and a coffee have 19 different plant species,” said David.
Going forward, he said, researchers may have to be “more specific about how we think about dietary diversity. Maybe not all plant species count toward health in the same way.”
David’s work provides an innovative way to conduct nutrition research, said Jotham Suez, PhD, an assistant professor in the department of molecular microbiology and immunology at Johns Hopkins Bloomberg School of Public Health.
“We need to have some means of tracking what people actually ate during a study, whether it’s an intervention where we provide them with the food or an observational study where we let people eat their habitual diet and track it themselves,” said Suez, who studies the gut microbiome.
“Recall bias” makes food questionnaires and apps unreliable. And research suggests that some participants may underreport food intake, possibly because they don’t want to be judged or they misestimate how much they actually consumed.
“There’s huge promise” with a tool like the one described in the PNAS study for making connections between diet and disease, Suez said. But access may be an issue for many researchers. He expects techniques to improve and costs to go down, but there will be challenges. “This method is also almost exclusively looking at plant DNA material, Suez added, “and our diets contain multiple components that are not plants.”
And even if a person just eats an apple or a single cucumber, that food may be degraded somewhere else in the gut, and it may be digested differently in different people’s guts. “Metabolism, of course, can be different between people,” Suez said, so the amounts of data will vary. “In their study, the qualitative data is convincing. The quantitative is TBD [to be determined].”
But he said it might be “a perfect tool” for scientists who want to study indigestible fiber, which is an important area of science, too.
“I totally buy it as a potentially better way to do dietary analytics for disease associations,” said Stollman, an expert in fecal transplant and diverticulitis and a trustee of the American College of Gastroenterology. Stollman sees many patients with diverticular disease who could benefit.
“One of the core questions in the diverticular world is, what causes diverticular disease, so we can ideally prevent it? For decades, the theory has been that a low fiber diet contributes to it,” said Stollman, but testing DNA in patients’ stools could help researchers explore the question in a new and potentially more nuanced and accurate way. Findings might allow scientists to learn, “Do people who eat X get polyps? Is this diet a risk factor for X, Y, or Z disease?” said Stollman.
Future Clinical Applications
Brenda Davy, PhD, is a registered dietitian and professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech. She conducts research investigating the role of diet in the prevention and treatment of obesity and related conditions such as type 2 diabetes. She also develops dietary assessment methods. More than a decade ago, she developed one of the first rapid assessment tools for quantifying beverage intake — the Beverage Intake Questionnaire — an assessment that is still used today.
“Dietary assessment is necessary in both research and clinical settings,” Davy said. “If a physician diagnoses a patient with a certain condition, information about the patient’s usual dietary habits can help him or her prescribe dietary changes that may help treat that condition.”
Biospecimens, like fecal and urine samples, can be a safe, accurate way to collect that data, she said. Samples can be obtained easily and noninvasively “in a wide variety of populations such as children or older adults” and in clinical settings.
Davy and her team use David’s technology in their work — in particular, a tool called FoodSeq that applies DNA metabarcoding to human stool to collect information about food taxa consumed. Their two labs are now collaborating on a project investigating how ultraprocessed foods might impact type 2 diabetes risk and cardiovascular health.
There are many directions David’s lab would like to take their research, possibly partnering with epidemiologists on global studies that would help them expand their DNA database and better understand how, for example, climate change may be affecting diet diversity and to learn more about diet across different populations.
A version of this article appeared on Medscape.com.
Analysis Finds Low Malignancy Rate in Pediatric Longitudinal Melanonychia
TOPLINE:
METHODOLOGY:
- LM — a pigmented band in the nail plate caused by increased melanin deposition — occurs in children and adults, resulting from melanocytic activation or proliferation in response to infection, systemic disease, medication, trauma, and other factors.
- Clinical features of LM in children mimic red-flag signs of subungual melanoma in adults although rarely is subungual melanoma.
- A biopsy can confirm the diagnosis, but other considerations include the scar, cost and stress of a procedure, and possibly pain or deformity.
- The researchers conducted a systematic review and meta-analysis of the prevalence of clinical and dermoscopic features in 1391 pediatric patients with LM (diagnosed at a mean age of 5-13 years) from 24 studies published between 1996 and 2023.
TAKEAWAY:
- Of 731 lesions in which a diagnosis was provided, benign nail matrix nevus accounted for 86% of cases.
- Only eight cases of subungual melanoma in situ were diagnosed, with no cases of invasive melanoma identified.
- Most lesions occurred on the fingernails (76%), particularly in the first digits (45%), and the most frequent clinical features included dark-colored bands (70%), multicolored bands (48%), broad bandwidth (41%), and pseudo-Hutchinson sign (41%).
- During a median follow-up of 1-5.5 years, 30% of lesions continued to evolve with changes in width or color, while 23% remained stable and 20% underwent spontaneous regression.
IN PRACTICE:
“In the pivotal clinical decision of whether to biopsy a child with longitudinal melanonychia, perhaps with features that would require a prompt biopsy in an adult, this study provides data to support the option of clinical monitoring,” the authors wrote.
SOURCE:
The meta-analysis, led by Serena Yun-Chen Tsai, MD, in the Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, was published online in Pediatric Dermatology.
LIMITATIONS:
Most studies were conducted in Asia, and data stratified by skin type were limited. Inconsistent reporting and missing critical features could affect data quality. Also, certain features displayed high heterogeneity.
DISCLOSURES:
This meta-analysis was supported by the Pediatric Dermatology Research Alliance Career Bridge Research Grant. One co-author disclosed relationships with UpToDate (author, reviewer), Skin Analytics (consultant), and DermTech (research materials).
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- LM — a pigmented band in the nail plate caused by increased melanin deposition — occurs in children and adults, resulting from melanocytic activation or proliferation in response to infection, systemic disease, medication, trauma, and other factors.
- Clinical features of LM in children mimic red-flag signs of subungual melanoma in adults although rarely is subungual melanoma.
- A biopsy can confirm the diagnosis, but other considerations include the scar, cost and stress of a procedure, and possibly pain or deformity.
- The researchers conducted a systematic review and meta-analysis of the prevalence of clinical and dermoscopic features in 1391 pediatric patients with LM (diagnosed at a mean age of 5-13 years) from 24 studies published between 1996 and 2023.
TAKEAWAY:
- Of 731 lesions in which a diagnosis was provided, benign nail matrix nevus accounted for 86% of cases.
- Only eight cases of subungual melanoma in situ were diagnosed, with no cases of invasive melanoma identified.
- Most lesions occurred on the fingernails (76%), particularly in the first digits (45%), and the most frequent clinical features included dark-colored bands (70%), multicolored bands (48%), broad bandwidth (41%), and pseudo-Hutchinson sign (41%).
- During a median follow-up of 1-5.5 years, 30% of lesions continued to evolve with changes in width or color, while 23% remained stable and 20% underwent spontaneous regression.
IN PRACTICE:
“In the pivotal clinical decision of whether to biopsy a child with longitudinal melanonychia, perhaps with features that would require a prompt biopsy in an adult, this study provides data to support the option of clinical monitoring,” the authors wrote.
SOURCE:
The meta-analysis, led by Serena Yun-Chen Tsai, MD, in the Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, was published online in Pediatric Dermatology.
LIMITATIONS:
Most studies were conducted in Asia, and data stratified by skin type were limited. Inconsistent reporting and missing critical features could affect data quality. Also, certain features displayed high heterogeneity.
DISCLOSURES:
This meta-analysis was supported by the Pediatric Dermatology Research Alliance Career Bridge Research Grant. One co-author disclosed relationships with UpToDate (author, reviewer), Skin Analytics (consultant), and DermTech (research materials).
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- LM — a pigmented band in the nail plate caused by increased melanin deposition — occurs in children and adults, resulting from melanocytic activation or proliferation in response to infection, systemic disease, medication, trauma, and other factors.
- Clinical features of LM in children mimic red-flag signs of subungual melanoma in adults although rarely is subungual melanoma.
- A biopsy can confirm the diagnosis, but other considerations include the scar, cost and stress of a procedure, and possibly pain or deformity.
- The researchers conducted a systematic review and meta-analysis of the prevalence of clinical and dermoscopic features in 1391 pediatric patients with LM (diagnosed at a mean age of 5-13 years) from 24 studies published between 1996 and 2023.
TAKEAWAY:
- Of 731 lesions in which a diagnosis was provided, benign nail matrix nevus accounted for 86% of cases.
- Only eight cases of subungual melanoma in situ were diagnosed, with no cases of invasive melanoma identified.
- Most lesions occurred on the fingernails (76%), particularly in the first digits (45%), and the most frequent clinical features included dark-colored bands (70%), multicolored bands (48%), broad bandwidth (41%), and pseudo-Hutchinson sign (41%).
- During a median follow-up of 1-5.5 years, 30% of lesions continued to evolve with changes in width or color, while 23% remained stable and 20% underwent spontaneous regression.
IN PRACTICE:
“In the pivotal clinical decision of whether to biopsy a child with longitudinal melanonychia, perhaps with features that would require a prompt biopsy in an adult, this study provides data to support the option of clinical monitoring,” the authors wrote.
SOURCE:
The meta-analysis, led by Serena Yun-Chen Tsai, MD, in the Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, was published online in Pediatric Dermatology.
LIMITATIONS:
Most studies were conducted in Asia, and data stratified by skin type were limited. Inconsistent reporting and missing critical features could affect data quality. Also, certain features displayed high heterogeneity.
DISCLOSURES:
This meta-analysis was supported by the Pediatric Dermatology Research Alliance Career Bridge Research Grant. One co-author disclosed relationships with UpToDate (author, reviewer), Skin Analytics (consultant), and DermTech (research materials).
A version of this article appeared on Medscape.com.
Tooth Enamel Disorder Is a Feature of Kindler EB
TOPLINE:
METHODOLOGY:
- KEB or Kindler syndrome, a genetic skin-blistering disease associated with pathogenic variants in FERMT1, is the rarest type of EB. Early detection and preventive measures can minimize complications, such as gum disease and other oral health issues, that have been reported in patients with KEB.
- Amelogenesis imperfecta is a group of rare genetic developmental conditions characterized by tooth enamel defects and can be associated with hypersensitivity and eruption disturbances in teeth, as well as periodontal conditions.
- Researchers conducted a longitudinal study on 36 patients with KEB (age, 2 weeks to 70 years; 42% female) from two clinics in Germany and Chile from 2003 to 2023, with follow-up times of 1-24 years.
- The primary outcomes were presence of orofacial features, including amelogenesis imperfecta, intraoral wounds, and periodontal disease, and oral squamous cell carcinoma.
TAKEAWAY:
- All 11 patients with information on enamel structure in their records had pitted enamel anomalies (pitted amelogenesis imperfecta), with variable severity.
- Of patients whose enamel could not be analyzed, three had all teeth crowned in their 20s, suggesting enamel defects, and two had all teeth extracted in their teens or 20s, indicating severe periodontal disease.
- The most common orofacial features were periodontal disease (27 of 36 patients), intraoral lesions (16 of 22 patients), angular cheilitis (24 of 33 patients), and cheilitis (22 of 34 patients), gingival overgrowth (17 of 26 patients), microstomia (14 of 25 patients), and vestibular obliteration (8 of 16 patients).
- Oral squamous cell carcinoma was diagnosed at the site of chronic lip lesions in two patients, with lethal outcomes.
IN PRACTICE:
These findings highlight the extent and severity of oral manifestations in KEB, the authors concluded, adding that “oral care is mandatory” in patients with KEB.
SOURCE:
This report, led by Susanne Krämer, DDS, MSc, of Medical Faculty and Medical Center, University of Freiburg, Freiburg im Breisgau, Germany, was published online in JAMA Dermatology.
LIMITATIONS:
The small sample size and the retrospective nature of the study could limit its generalizability.
DISCLOSURES:
The authors did not disclose any source of funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- KEB or Kindler syndrome, a genetic skin-blistering disease associated with pathogenic variants in FERMT1, is the rarest type of EB. Early detection and preventive measures can minimize complications, such as gum disease and other oral health issues, that have been reported in patients with KEB.
- Amelogenesis imperfecta is a group of rare genetic developmental conditions characterized by tooth enamel defects and can be associated with hypersensitivity and eruption disturbances in teeth, as well as periodontal conditions.
- Researchers conducted a longitudinal study on 36 patients with KEB (age, 2 weeks to 70 years; 42% female) from two clinics in Germany and Chile from 2003 to 2023, with follow-up times of 1-24 years.
- The primary outcomes were presence of orofacial features, including amelogenesis imperfecta, intraoral wounds, and periodontal disease, and oral squamous cell carcinoma.
TAKEAWAY:
- All 11 patients with information on enamel structure in their records had pitted enamel anomalies (pitted amelogenesis imperfecta), with variable severity.
- Of patients whose enamel could not be analyzed, three had all teeth crowned in their 20s, suggesting enamel defects, and two had all teeth extracted in their teens or 20s, indicating severe periodontal disease.
- The most common orofacial features were periodontal disease (27 of 36 patients), intraoral lesions (16 of 22 patients), angular cheilitis (24 of 33 patients), and cheilitis (22 of 34 patients), gingival overgrowth (17 of 26 patients), microstomia (14 of 25 patients), and vestibular obliteration (8 of 16 patients).
- Oral squamous cell carcinoma was diagnosed at the site of chronic lip lesions in two patients, with lethal outcomes.
IN PRACTICE:
These findings highlight the extent and severity of oral manifestations in KEB, the authors concluded, adding that “oral care is mandatory” in patients with KEB.
SOURCE:
This report, led by Susanne Krämer, DDS, MSc, of Medical Faculty and Medical Center, University of Freiburg, Freiburg im Breisgau, Germany, was published online in JAMA Dermatology.
LIMITATIONS:
The small sample size and the retrospective nature of the study could limit its generalizability.
DISCLOSURES:
The authors did not disclose any source of funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- KEB or Kindler syndrome, a genetic skin-blistering disease associated with pathogenic variants in FERMT1, is the rarest type of EB. Early detection and preventive measures can minimize complications, such as gum disease and other oral health issues, that have been reported in patients with KEB.
- Amelogenesis imperfecta is a group of rare genetic developmental conditions characterized by tooth enamel defects and can be associated with hypersensitivity and eruption disturbances in teeth, as well as periodontal conditions.
- Researchers conducted a longitudinal study on 36 patients with KEB (age, 2 weeks to 70 years; 42% female) from two clinics in Germany and Chile from 2003 to 2023, with follow-up times of 1-24 years.
- The primary outcomes were presence of orofacial features, including amelogenesis imperfecta, intraoral wounds, and periodontal disease, and oral squamous cell carcinoma.
TAKEAWAY:
- All 11 patients with information on enamel structure in their records had pitted enamel anomalies (pitted amelogenesis imperfecta), with variable severity.
- Of patients whose enamel could not be analyzed, three had all teeth crowned in their 20s, suggesting enamel defects, and two had all teeth extracted in their teens or 20s, indicating severe periodontal disease.
- The most common orofacial features were periodontal disease (27 of 36 patients), intraoral lesions (16 of 22 patients), angular cheilitis (24 of 33 patients), and cheilitis (22 of 34 patients), gingival overgrowth (17 of 26 patients), microstomia (14 of 25 patients), and vestibular obliteration (8 of 16 patients).
- Oral squamous cell carcinoma was diagnosed at the site of chronic lip lesions in two patients, with lethal outcomes.
IN PRACTICE:
These findings highlight the extent and severity of oral manifestations in KEB, the authors concluded, adding that “oral care is mandatory” in patients with KEB.
SOURCE:
This report, led by Susanne Krämer, DDS, MSc, of Medical Faculty and Medical Center, University of Freiburg, Freiburg im Breisgau, Germany, was published online in JAMA Dermatology.
LIMITATIONS:
The small sample size and the retrospective nature of the study could limit its generalizability.
DISCLOSURES:
The authors did not disclose any source of funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.