User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
HPV Vax Tied to Lower Odds of Cervical Lesion Progression
TOPLINE:
Among women with cervical intraepithelial neoplasia grade 2 (CIN2), vaccination against human papillomavirus (HPV) before age 20 is associated with lower odds of progression.
METHODOLOGY:
- Researchers analyzed data from 7904 women in Denmark who were undergoing active surveillance for CIN2 between 2007 and 2020.
- CIN2 lesions on their own. Removing them can increase the risk for during subsequent pregnancies, the researchers noted.
- Nearly half of the women had received at least one dose of an HPV vaccine at least 1 year before the diagnosis of cervical dysplasia.
TAKEAWAY:
- During 28 months of follow-up, the risk for progression was 22.9% for women vaccinated before age 15, 31.5% for women vaccinated between ages 15 and 20, and 37.6% for women who were not vaccinated.
- Women vaccinated before age 15 had a 35% lower risk for progression than unvaccinated women, after adjusting for cytology, income, and education (adjusted relative risk, 0.65; 95% CI, 0.57-0.75).
- Cervical cancer developed in 0.37% of the unvaccinated women and 0.13% of the vaccinated women.
- All cases of cervical cancer in the vaccinated group occurred in women who received the vaccine after age 20.
IN PRACTICE:
“These findings suggest that HPV vaccination status may be used to identify women at higher risk for progression, thereby enabling risk stratification at the time of CIN2 diagnosis,” the researchers wrote.
SOURCE:
Louise Krog, BscMed, with Aarhus University, Aarhus, Denmark, was the corresponding author of the study. The research was published online in the American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study authors had limited information about potential confounders such as smoking, immunosuppressive conditions, and the age at which patients became sexually active.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Carpenter Axel Kastrup-Nielsen’s Memorial Fund, and the Dagmar Marshall’s Fund. Co-authors disclosed ties to AstraZeneca, Roche, and Hologic.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women with cervical intraepithelial neoplasia grade 2 (CIN2), vaccination against human papillomavirus (HPV) before age 20 is associated with lower odds of progression.
METHODOLOGY:
- Researchers analyzed data from 7904 women in Denmark who were undergoing active surveillance for CIN2 between 2007 and 2020.
- CIN2 lesions on their own. Removing them can increase the risk for during subsequent pregnancies, the researchers noted.
- Nearly half of the women had received at least one dose of an HPV vaccine at least 1 year before the diagnosis of cervical dysplasia.
TAKEAWAY:
- During 28 months of follow-up, the risk for progression was 22.9% for women vaccinated before age 15, 31.5% for women vaccinated between ages 15 and 20, and 37.6% for women who were not vaccinated.
- Women vaccinated before age 15 had a 35% lower risk for progression than unvaccinated women, after adjusting for cytology, income, and education (adjusted relative risk, 0.65; 95% CI, 0.57-0.75).
- Cervical cancer developed in 0.37% of the unvaccinated women and 0.13% of the vaccinated women.
- All cases of cervical cancer in the vaccinated group occurred in women who received the vaccine after age 20.
IN PRACTICE:
“These findings suggest that HPV vaccination status may be used to identify women at higher risk for progression, thereby enabling risk stratification at the time of CIN2 diagnosis,” the researchers wrote.
SOURCE:
Louise Krog, BscMed, with Aarhus University, Aarhus, Denmark, was the corresponding author of the study. The research was published online in the American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study authors had limited information about potential confounders such as smoking, immunosuppressive conditions, and the age at which patients became sexually active.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Carpenter Axel Kastrup-Nielsen’s Memorial Fund, and the Dagmar Marshall’s Fund. Co-authors disclosed ties to AstraZeneca, Roche, and Hologic.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women with cervical intraepithelial neoplasia grade 2 (CIN2), vaccination against human papillomavirus (HPV) before age 20 is associated with lower odds of progression.
METHODOLOGY:
- Researchers analyzed data from 7904 women in Denmark who were undergoing active surveillance for CIN2 between 2007 and 2020.
- CIN2 lesions on their own. Removing them can increase the risk for during subsequent pregnancies, the researchers noted.
- Nearly half of the women had received at least one dose of an HPV vaccine at least 1 year before the diagnosis of cervical dysplasia.
TAKEAWAY:
- During 28 months of follow-up, the risk for progression was 22.9% for women vaccinated before age 15, 31.5% for women vaccinated between ages 15 and 20, and 37.6% for women who were not vaccinated.
- Women vaccinated before age 15 had a 35% lower risk for progression than unvaccinated women, after adjusting for cytology, income, and education (adjusted relative risk, 0.65; 95% CI, 0.57-0.75).
- Cervical cancer developed in 0.37% of the unvaccinated women and 0.13% of the vaccinated women.
- All cases of cervical cancer in the vaccinated group occurred in women who received the vaccine after age 20.
IN PRACTICE:
“These findings suggest that HPV vaccination status may be used to identify women at higher risk for progression, thereby enabling risk stratification at the time of CIN2 diagnosis,” the researchers wrote.
SOURCE:
Louise Krog, BscMed, with Aarhus University, Aarhus, Denmark, was the corresponding author of the study. The research was published online in the American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study authors had limited information about potential confounders such as smoking, immunosuppressive conditions, and the age at which patients became sexually active.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Carpenter Axel Kastrup-Nielsen’s Memorial Fund, and the Dagmar Marshall’s Fund. Co-authors disclosed ties to AstraZeneca, Roche, and Hologic.
A version of this article appeared on Medscape.com.
Analysis Finds Risk of Alopecia Areata After COVID-19 Infection
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
FROM JAMA DERMATOLOGY
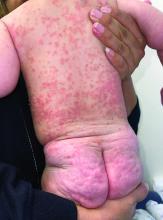
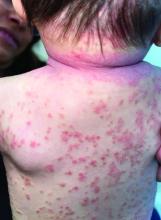
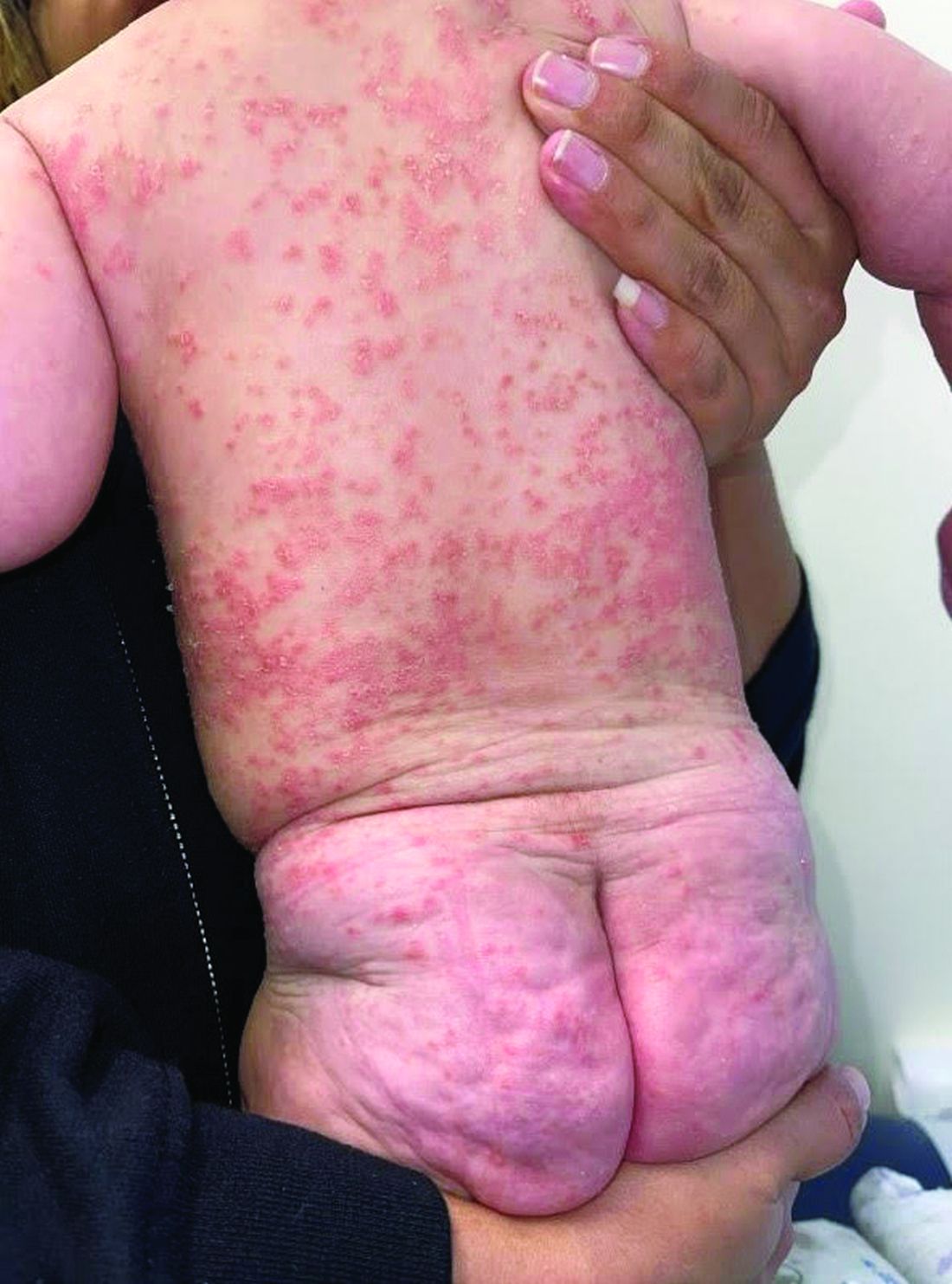
A 4-month-old male was referred for a 3-week history of an itchy generalized rash that started on the neck
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
On physical exam, there was an erythematous patch with overlying areas of macerations on the neck and axilla. The trunk, extremities, and diaper area had multiple psoriasiform erythematous thin plaques with overlying scales.
What’s the Disease Burden From Plastic Exposure?
Exposure to endocrine-disrupting chemicals (EDCs) via daily use of plastics is a major contributor to the overall disease burden in the United States and the associated costs to society amount to more than 1% of the gross domestic product, revealed a large-scale analysis.
The research, published in the Journal of the Endocrine Society, indicated that taken together, the disease burden attributable to EDCs used in the manufacture of plastics added up to almost $250 billion in 2018 alone.
“The diseases due to plastics run the entire life course from preterm birth to obesity, heart disease, and cancers,” commented lead author Leonardo Trasande, MD, MPP, Jim G. Hendrick, MD Professor of Pediatrics, Department of Pediatrics, NYU Langone Medical Center, New York, in a release.
“Our study drives home the need to address chemicals used in plastic materials” through global treaties and other policy initiatives, he said, so as to “reduce these costs” in line with reductions in exposure to the chemicals.
Co-author Michael Belliveau, Executive Director at Defend Our Health in Portland, ME, agreed, saying: “We can reduce these health costs and the prevalence of chronic endocrine diseases such as diabetes and obesity if governments and companies enact policies that minimize exposure to EDCs to protect public health and the environment.”
Plastics may contain any one of a number of EDCs, such as polybrominated diphenylethers in flame retardant additives, phthalates in food packaging, bisphenols in can linings, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in nonstick cooking utensils.
in developing fetuses and children, and even death.
In March 2022, the United Nations Environment Assembly committed to a global plastics treaty to “end plastic pollution and forge an international legally binding agreement by 2024” that “addresses the full life cycle of plastic, including its production, design and disposal.”
Minimizing EDC Exposure
But what can doctors tell their patients today to help them reduce their exposure to EDCs?
“There are safe and simple steps that people can take to limit their exposure to the chemicals of greatest concern,” Dr. Trasande told this news organization.
This can be partly achieved by reducing plastic use down to its essentials. “To use an example, when you are flying, fill up a stainless steel container after clearing security. At home, use glass or stainless steel” rather than plastic bottles or containers.
In particular, “avoiding microwaving plastic is important,” Dr. Trasande said, “even if a container says it’s microwave-safe.”
He warned that “many chemicals used in plastic are not covalently bound, and heat facilitates leaching into food. Microscopic contaminants can also get into food when you microwave plastic.”
Dr. Trasande also suggests limiting canned food consumption and avoiding cleaning plastic food containers in machine dishwashers.
Calculating the Disease Burden
To accurately assess the “the tradeoffs involved in the ongoing reliance on plastic production as a source of economic productivity,” the current researchers calculated the attributable disease burden and cost related to EDCs used in plastic materials in the United States in 2018.
Building on previously published analyses, they used industry reports, publications by national and international governing bodies, and peer-reviewed publications to determine the usage of each type of EDC and its attributable disease and disability burden.
This plastic-related fraction (PRF) of disease burden was then used to calculate an updated cost estimate for each EDC, based on the assumption that the disease burden is directly proportional to its exposure.
They found that for bisphenol A, 97.5% of its use, and therefore its estimated PRF of disease burden, was related to the manufacture of plastics, while this figure was 98%-100% for phthalates. For PDBE, 98% of its use was in plastics vs 93% for PFAS.
The researchers then estimated that the total plastic-attributable disease burden in the United States in 2018 cost the nation $249 billion, or 1.22% of the gross domestic product. Of this, $159 billion was linked to PDBE exposure, which is associated with diseases such as cancer.
Moreover, $1.02 billion plastic-attributable disease burden was associated with bisphenol A exposure, which can have potentially harmful health effects on the immune system; followed by $66.7 billion due to phthalates, which are linked to preterm birth, reduced sperm count, and childhood obesity; and $22.4 billion due to PFAS, which are associated with kidney failure and gestational diabetes.
The study was supported by the National Institutes of Health and the Passport Foundation.
Dr. Trasande declared relationships with Audible, Houghton Mifflin, Paidos, and Kobunsha, none of which relate to the present manuscript.
No other financial relationships were declared.
A version of this article appeared on Medscape.com.
Exposure to endocrine-disrupting chemicals (EDCs) via daily use of plastics is a major contributor to the overall disease burden in the United States and the associated costs to society amount to more than 1% of the gross domestic product, revealed a large-scale analysis.
The research, published in the Journal of the Endocrine Society, indicated that taken together, the disease burden attributable to EDCs used in the manufacture of plastics added up to almost $250 billion in 2018 alone.
“The diseases due to plastics run the entire life course from preterm birth to obesity, heart disease, and cancers,” commented lead author Leonardo Trasande, MD, MPP, Jim G. Hendrick, MD Professor of Pediatrics, Department of Pediatrics, NYU Langone Medical Center, New York, in a release.
“Our study drives home the need to address chemicals used in plastic materials” through global treaties and other policy initiatives, he said, so as to “reduce these costs” in line with reductions in exposure to the chemicals.
Co-author Michael Belliveau, Executive Director at Defend Our Health in Portland, ME, agreed, saying: “We can reduce these health costs and the prevalence of chronic endocrine diseases such as diabetes and obesity if governments and companies enact policies that minimize exposure to EDCs to protect public health and the environment.”
Plastics may contain any one of a number of EDCs, such as polybrominated diphenylethers in flame retardant additives, phthalates in food packaging, bisphenols in can linings, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in nonstick cooking utensils.
in developing fetuses and children, and even death.
In March 2022, the United Nations Environment Assembly committed to a global plastics treaty to “end plastic pollution and forge an international legally binding agreement by 2024” that “addresses the full life cycle of plastic, including its production, design and disposal.”
Minimizing EDC Exposure
But what can doctors tell their patients today to help them reduce their exposure to EDCs?
“There are safe and simple steps that people can take to limit their exposure to the chemicals of greatest concern,” Dr. Trasande told this news organization.
This can be partly achieved by reducing plastic use down to its essentials. “To use an example, when you are flying, fill up a stainless steel container after clearing security. At home, use glass or stainless steel” rather than plastic bottles or containers.
In particular, “avoiding microwaving plastic is important,” Dr. Trasande said, “even if a container says it’s microwave-safe.”
He warned that “many chemicals used in plastic are not covalently bound, and heat facilitates leaching into food. Microscopic contaminants can also get into food when you microwave plastic.”
Dr. Trasande also suggests limiting canned food consumption and avoiding cleaning plastic food containers in machine dishwashers.
Calculating the Disease Burden
To accurately assess the “the tradeoffs involved in the ongoing reliance on plastic production as a source of economic productivity,” the current researchers calculated the attributable disease burden and cost related to EDCs used in plastic materials in the United States in 2018.
Building on previously published analyses, they used industry reports, publications by national and international governing bodies, and peer-reviewed publications to determine the usage of each type of EDC and its attributable disease and disability burden.
This plastic-related fraction (PRF) of disease burden was then used to calculate an updated cost estimate for each EDC, based on the assumption that the disease burden is directly proportional to its exposure.
They found that for bisphenol A, 97.5% of its use, and therefore its estimated PRF of disease burden, was related to the manufacture of plastics, while this figure was 98%-100% for phthalates. For PDBE, 98% of its use was in plastics vs 93% for PFAS.
The researchers then estimated that the total plastic-attributable disease burden in the United States in 2018 cost the nation $249 billion, or 1.22% of the gross domestic product. Of this, $159 billion was linked to PDBE exposure, which is associated with diseases such as cancer.
Moreover, $1.02 billion plastic-attributable disease burden was associated with bisphenol A exposure, which can have potentially harmful health effects on the immune system; followed by $66.7 billion due to phthalates, which are linked to preterm birth, reduced sperm count, and childhood obesity; and $22.4 billion due to PFAS, which are associated with kidney failure and gestational diabetes.
The study was supported by the National Institutes of Health and the Passport Foundation.
Dr. Trasande declared relationships with Audible, Houghton Mifflin, Paidos, and Kobunsha, none of which relate to the present manuscript.
No other financial relationships were declared.
A version of this article appeared on Medscape.com.
Exposure to endocrine-disrupting chemicals (EDCs) via daily use of plastics is a major contributor to the overall disease burden in the United States and the associated costs to society amount to more than 1% of the gross domestic product, revealed a large-scale analysis.
The research, published in the Journal of the Endocrine Society, indicated that taken together, the disease burden attributable to EDCs used in the manufacture of plastics added up to almost $250 billion in 2018 alone.
“The diseases due to plastics run the entire life course from preterm birth to obesity, heart disease, and cancers,” commented lead author Leonardo Trasande, MD, MPP, Jim G. Hendrick, MD Professor of Pediatrics, Department of Pediatrics, NYU Langone Medical Center, New York, in a release.
“Our study drives home the need to address chemicals used in plastic materials” through global treaties and other policy initiatives, he said, so as to “reduce these costs” in line with reductions in exposure to the chemicals.
Co-author Michael Belliveau, Executive Director at Defend Our Health in Portland, ME, agreed, saying: “We can reduce these health costs and the prevalence of chronic endocrine diseases such as diabetes and obesity if governments and companies enact policies that minimize exposure to EDCs to protect public health and the environment.”
Plastics may contain any one of a number of EDCs, such as polybrominated diphenylethers in flame retardant additives, phthalates in food packaging, bisphenols in can linings, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in nonstick cooking utensils.
in developing fetuses and children, and even death.
In March 2022, the United Nations Environment Assembly committed to a global plastics treaty to “end plastic pollution and forge an international legally binding agreement by 2024” that “addresses the full life cycle of plastic, including its production, design and disposal.”
Minimizing EDC Exposure
But what can doctors tell their patients today to help them reduce their exposure to EDCs?
“There are safe and simple steps that people can take to limit their exposure to the chemicals of greatest concern,” Dr. Trasande told this news organization.
This can be partly achieved by reducing plastic use down to its essentials. “To use an example, when you are flying, fill up a stainless steel container after clearing security. At home, use glass or stainless steel” rather than plastic bottles or containers.
In particular, “avoiding microwaving plastic is important,” Dr. Trasande said, “even if a container says it’s microwave-safe.”
He warned that “many chemicals used in plastic are not covalently bound, and heat facilitates leaching into food. Microscopic contaminants can also get into food when you microwave plastic.”
Dr. Trasande also suggests limiting canned food consumption and avoiding cleaning plastic food containers in machine dishwashers.
Calculating the Disease Burden
To accurately assess the “the tradeoffs involved in the ongoing reliance on plastic production as a source of economic productivity,” the current researchers calculated the attributable disease burden and cost related to EDCs used in plastic materials in the United States in 2018.
Building on previously published analyses, they used industry reports, publications by national and international governing bodies, and peer-reviewed publications to determine the usage of each type of EDC and its attributable disease and disability burden.
This plastic-related fraction (PRF) of disease burden was then used to calculate an updated cost estimate for each EDC, based on the assumption that the disease burden is directly proportional to its exposure.
They found that for bisphenol A, 97.5% of its use, and therefore its estimated PRF of disease burden, was related to the manufacture of plastics, while this figure was 98%-100% for phthalates. For PDBE, 98% of its use was in plastics vs 93% for PFAS.
The researchers then estimated that the total plastic-attributable disease burden in the United States in 2018 cost the nation $249 billion, or 1.22% of the gross domestic product. Of this, $159 billion was linked to PDBE exposure, which is associated with diseases such as cancer.
Moreover, $1.02 billion plastic-attributable disease burden was associated with bisphenol A exposure, which can have potentially harmful health effects on the immune system; followed by $66.7 billion due to phthalates, which are linked to preterm birth, reduced sperm count, and childhood obesity; and $22.4 billion due to PFAS, which are associated with kidney failure and gestational diabetes.
The study was supported by the National Institutes of Health and the Passport Foundation.
Dr. Trasande declared relationships with Audible, Houghton Mifflin, Paidos, and Kobunsha, none of which relate to the present manuscript.
No other financial relationships were declared.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF THE ENDOCRINE SOCIETY
More Evidence Suggests That ‘Long Flu’ Is a Thing
You may have never heard of it, but you may have had it. More evidence points to “long flu” being a real phenomenon, with a large study showing symptoms persist at least 4 weeks or more after some people are hospitalized for the flu.
Researchers compared long flu to long COVID-19 and found long flu happened less often and was less severe overall. This difference could be because the flu mostly affects the lungs whereas COVID can affect any number of organ systems in the body.
The investigators were surprised that both long flu and long COVID were linked to a greater burden of health loss, compared to either initial infection.
“I think COVID and long COVID made us realize that infections have long-term consequences, and often the toll of those long-term consequences is much larger than the toll of acute disease,” said Ziyad Al-Aly, MD, senior author of the study and chief of research and development at the VA St. Louis Health Care System.
“I know, having studied long COVID for the past 4 years, I should not be surprised. But I am in awe of what these infections can do to the long-term health of affected individuals,” said Dr. Al-Aly, who is also a clinical epidemiologist at Washington University in St. Louis.
Dr. Al-Aly and colleagues Yan Xie, PhD, and Taeyoung Choi, MS, analyzed US Department of Veterans Affairs medical records. They compared 81,280 people hospitalized with COVID to 10,985 people hospitalized with the flu before the COVID pandemic. They checked up to 18 months after initial infections to see who developed long flu or long COVID symptoms.
The study was published online in The Lancet Infectious Diseases.
It’s an interesting study, said Aaron E. Glatt, MD, chairman of the Department of Medicine and a hospital epidemiologist at Mount Sinai South Nassau in Oceanside, NY, who was not part of the research.
“There is a concern with many viruses that you can have long-term consequences,” said Dr. Glatt, who is also a fellow of the Infectious Diseases Society of America. He said the possibility of long-term symptoms with the flu is not new, “but it’s nice to have more data.”
People hospitalized with COVID had a 50% higher risk of death during the study period than people hospitalized with the flu. Put another way, for every 100 people admitted to the hospital with COVID, about eight more died than those hospitalized with the flu over the following 18 months. Hospital admissions and admissions to the intensive care unit were also higher in the long COVID group — 20 more people and nine more people, respectively, for every 100 people admitted to the hospital with COVID.
More research is needed, Dr. Glatt said. “With many of these viruses, we don’t understand what they do to the body.” A prospective study to see if antiviral treatments make a difference, for example, would be useful, he noted.
Dr. Al-Aly and colleagues would like to do more studies.
“We need to more deeply understand how and why acute infections cause long-term illness,” he said, noting that he also wants to investigate ways to prevent and treat the long-term effects.
“Much remains to be done, and we are deeply committed to doing our best to develop those answers.”
A version of this article first appeared on WebMD.com.
You may have never heard of it, but you may have had it. More evidence points to “long flu” being a real phenomenon, with a large study showing symptoms persist at least 4 weeks or more after some people are hospitalized for the flu.
Researchers compared long flu to long COVID-19 and found long flu happened less often and was less severe overall. This difference could be because the flu mostly affects the lungs whereas COVID can affect any number of organ systems in the body.
The investigators were surprised that both long flu and long COVID were linked to a greater burden of health loss, compared to either initial infection.
“I think COVID and long COVID made us realize that infections have long-term consequences, and often the toll of those long-term consequences is much larger than the toll of acute disease,” said Ziyad Al-Aly, MD, senior author of the study and chief of research and development at the VA St. Louis Health Care System.
“I know, having studied long COVID for the past 4 years, I should not be surprised. But I am in awe of what these infections can do to the long-term health of affected individuals,” said Dr. Al-Aly, who is also a clinical epidemiologist at Washington University in St. Louis.
Dr. Al-Aly and colleagues Yan Xie, PhD, and Taeyoung Choi, MS, analyzed US Department of Veterans Affairs medical records. They compared 81,280 people hospitalized with COVID to 10,985 people hospitalized with the flu before the COVID pandemic. They checked up to 18 months after initial infections to see who developed long flu or long COVID symptoms.
The study was published online in The Lancet Infectious Diseases.
It’s an interesting study, said Aaron E. Glatt, MD, chairman of the Department of Medicine and a hospital epidemiologist at Mount Sinai South Nassau in Oceanside, NY, who was not part of the research.
“There is a concern with many viruses that you can have long-term consequences,” said Dr. Glatt, who is also a fellow of the Infectious Diseases Society of America. He said the possibility of long-term symptoms with the flu is not new, “but it’s nice to have more data.”
People hospitalized with COVID had a 50% higher risk of death during the study period than people hospitalized with the flu. Put another way, for every 100 people admitted to the hospital with COVID, about eight more died than those hospitalized with the flu over the following 18 months. Hospital admissions and admissions to the intensive care unit were also higher in the long COVID group — 20 more people and nine more people, respectively, for every 100 people admitted to the hospital with COVID.
More research is needed, Dr. Glatt said. “With many of these viruses, we don’t understand what they do to the body.” A prospective study to see if antiviral treatments make a difference, for example, would be useful, he noted.
Dr. Al-Aly and colleagues would like to do more studies.
“We need to more deeply understand how and why acute infections cause long-term illness,” he said, noting that he also wants to investigate ways to prevent and treat the long-term effects.
“Much remains to be done, and we are deeply committed to doing our best to develop those answers.”
A version of this article first appeared on WebMD.com.
You may have never heard of it, but you may have had it. More evidence points to “long flu” being a real phenomenon, with a large study showing symptoms persist at least 4 weeks or more after some people are hospitalized for the flu.
Researchers compared long flu to long COVID-19 and found long flu happened less often and was less severe overall. This difference could be because the flu mostly affects the lungs whereas COVID can affect any number of organ systems in the body.
The investigators were surprised that both long flu and long COVID were linked to a greater burden of health loss, compared to either initial infection.
“I think COVID and long COVID made us realize that infections have long-term consequences, and often the toll of those long-term consequences is much larger than the toll of acute disease,” said Ziyad Al-Aly, MD, senior author of the study and chief of research and development at the VA St. Louis Health Care System.
“I know, having studied long COVID for the past 4 years, I should not be surprised. But I am in awe of what these infections can do to the long-term health of affected individuals,” said Dr. Al-Aly, who is also a clinical epidemiologist at Washington University in St. Louis.
Dr. Al-Aly and colleagues Yan Xie, PhD, and Taeyoung Choi, MS, analyzed US Department of Veterans Affairs medical records. They compared 81,280 people hospitalized with COVID to 10,985 people hospitalized with the flu before the COVID pandemic. They checked up to 18 months after initial infections to see who developed long flu or long COVID symptoms.
The study was published online in The Lancet Infectious Diseases.
It’s an interesting study, said Aaron E. Glatt, MD, chairman of the Department of Medicine and a hospital epidemiologist at Mount Sinai South Nassau in Oceanside, NY, who was not part of the research.
“There is a concern with many viruses that you can have long-term consequences,” said Dr. Glatt, who is also a fellow of the Infectious Diseases Society of America. He said the possibility of long-term symptoms with the flu is not new, “but it’s nice to have more data.”
People hospitalized with COVID had a 50% higher risk of death during the study period than people hospitalized with the flu. Put another way, for every 100 people admitted to the hospital with COVID, about eight more died than those hospitalized with the flu over the following 18 months. Hospital admissions and admissions to the intensive care unit were also higher in the long COVID group — 20 more people and nine more people, respectively, for every 100 people admitted to the hospital with COVID.
More research is needed, Dr. Glatt said. “With many of these viruses, we don’t understand what they do to the body.” A prospective study to see if antiviral treatments make a difference, for example, would be useful, he noted.
Dr. Al-Aly and colleagues would like to do more studies.
“We need to more deeply understand how and why acute infections cause long-term illness,” he said, noting that he also wants to investigate ways to prevent and treat the long-term effects.
“Much remains to be done, and we are deeply committed to doing our best to develop those answers.”
A version of this article first appeared on WebMD.com.
FROM THE LANCET INFECTIOUS DISEASES
Suicide II
How might you discuss a suicidal ideation, an anxiety-provoking topic, with your patients and their parents? After a positive screen, there will be times when you decide your patient should go to an emergency department for an urgent evaluation. However, most of the time you will be able to help the family identify strategies to lower risk and improve safety and resilience, while waiting for a thorough psychiatric evaluation.
Bring in the Parents: Modeling Validation, Structure, and Optimism
If you have identified some degree of suicide risk in your patient, either with a screening instrument or in your clinical interview, ask your patient if you can bring their parents into the conversation. They may resist, and if so, find out why they are hesitant. Are they worried about causing their parents some distress? Are they concerned their parents will be surprised? Disappointed? Scared? Angry? Acknowledge how hard it can be to find a way to talk about such emotional material with parents. What is their communication like with their parents usually? Do they talk every night at dinner or rarely? Are their interactions usually lighthearted or playful? Brief? Irritable and angry? Have they talked about or managed difficult times before as a family? How did that go? Did they feel they ended up supporting an anxious or depressed single parent? Was their parent harsh and punitive? Since involving the parent is essential, if you become concerned that a conversation with the parent would truly increase the risk of suicide, perhaps because of reports of violence at home, then you may need to send your patient to the emergency department so they can be assessed in a safe setting where a clinical team can evaluate your patient while involving more (or different) members of the family.
Most of the time, your patient will describe a situation that will simply be uncomfortable or stressful for their parent. Don’t be dismissive of their concerns. Instead, acknowledge that talking about their inner life will feel hard. Validate that their parents will be sad, worried, and stressed to hear about what they are feeling. Then offer that parents always prefer to know what is happening with their child so they can help, even if that means only being present to bear it alongside them. You can remind them that you will be there, too, to reassure their parents that this is a common problem and that you can face it and help it to get better together. Find out if they would like you to take the lead in speaking about it, but do not let them wait in the waiting room. Discussing the topic with you with both parents and patient in the room will help even those families that are not great communicators to begin to be more connected, even if you do most of the talking. While you need to bring their symptoms and suicidality to their parents’ attention, find out if there are any details they would rather not share. Perhaps they are struggling with questions of gender identity or sexual orientation, or are thinking of giving up an activity their parents may be very invested in. While any future treatment will prioritize honest communication within the family, communication about their emerging identity should not be rushed, and especially not in the setting of concerns about suicide risk.
With the information you do gather, there are often steps you can take to lower the stress level. The parents’ awareness of their suffering, perhaps acknowledging a broken heart, excessive academic pressure, or a major disappointment may suggest steps to lower the stress level. A mental health referral might introduce a sense of hope. A reminder of their meaningful connection to a parent, a team, a religion, or an activity may also remind the adolescent of a positive view of their future.
Introducing the Topic
When you bring parents into the room, let them know that there is something important and difficult that you need to discuss with them together. Ask if they have noted any changes in their child’s behavior, school performance, or demeanor. Have they had any worries about their teenager? If they have, affirm that they are picking up on something real, and ask more about it. If they have not, offer that their child has been doing a valiant job of soldiering through their days while managing some strong and difficult thoughts and feelings. Walk them through some of what you have learned from your patient, always inviting your patient to affirm or add to what you are detailing. Most parents are keenly aware of the prevalence of suicidal thoughts during adolescence. Bring it into the open, and offer that the next steps are going to be to add more adults to their child’s orbit to help diagnose and treat any underlying psychiatric illness. Reassure them that you are confident that psychiatric illnesses are treatable, even curable. Reassure them that one of the best safety measures is good communication and connectedness with parents.
Help Parents to Be Good Listeners
Some parents may respond with heightened anxiety and need for reassurance from their child. Others may try to talk their child out of their suicidal thoughts. But your year is going so well! You got a great grade in calculus! Gently model validation: Acknowledge to the parents that it is understandable to feel worried or to look for a rational argument against suicide. Offer that feelings don’t usually respond to logic, but do improve with support and time. It may be better for everyone to treat this topic more like the weather so it is easier to talk about and manage. No one gets defensive or distressed if it’s raining, they just put on the right gear. Has the parent ever felt depressed? Did they ever have suicidal ideation growing up? Can they agree to check in at regular times? Could the child speak up if they are feeling badly? Can all agree that parents should check in if their child seems more down? Help them to acknowledge how hard it is to bear strong feelings, but that it is always better together.
Identify Coping Strategies
In front of parents, ask your patient if anything helps when they are feeling at their worst. If they can’t identify anything, offer some possibilities: a walk outside together? making art or music? being out in nature? snuggling with a beloved pet? a set of jumping jacks to get their heart rate up? a favorite playlist? Talking to a particular friend or relative? Make a list. Prioritize activities that are healthy and connect them to others when they are feeling their worst.
Focus on the Basics
Make a concrete and practical plan for steps they can all take to improve well-being. Start with strategies to ensure restful sleep at night, regular exercise, and healthy nutrition. Depression and anxiety often interfere with these functions, so families can work together to support them even while waiting for assessment by a psychiatrist. Help them identify modest rules or routines (consistent bedtime, no screens in the bedroom, a daily walk after dinner) that parents can set that will make a difference.
Set Up Speed Bumps
Talk together about setting up some speed bumps to support their child’s safety. Find out if there are firearms in the home. Be crystal clear that they should be locked, preferably with ammunition, in a separate secure place. Their child should have no knowledge of how to access them, or they should be stored out of the home for the time being.
Parents should lock any medications that could be dangerous in overdose (including in homes if the adolescent will be visiting). Educate them about Tylenol and any prescription medications in their home that should be locked. This part of a conversation is always stressful. Acknowledge that, and remind everyone that, these are important strategies. It should be always be easier to ask their parent for help if they are feeling terrible than it is to access something dangerous.
Acknowledge the Strain
Finally, it is important to acknowledge how hard it is for your patient to bear these feelings, and that speaking up about them may feel like the last thing they want to do. Applaud them for their strength while reminding them that they need to share if they feel worse. Likewise, model for parents that feeling stressed and worried in this circumstance is normal. They should think about how to take good care of themselves. The same well-being strategies you reviewed for their child can work for them too! They may want to focus on sleep or exercise, enhance their nourishing social connections, protect time for beloved hobbies. Everyone should hear that they should never worry alone. If someone feels more worried, bring it to their parent, therapist, psychiatrist, spouse, or to you. They should trust their instincts if they think it is time to go to the emergency department. With supportive open communication, they will strengthen the protective connections which in turn will see the family through the course of the treatable illnesses that cause suicidal thoughts.
Lastly, this is difficult work for any physician. As psychiatrists, we worry about higher-risk teenagers when we decide that hospitalization carries a bigger risk than benefit. Pediatricians see many more teenagers with suicidal ideation and even though the statistical risk is very low, no one knows how to predict any individual teenager’s behavior. Therefore, pediatricians face the direct stress of the clinical work and the deeper stress of knowing there is always some uncertainty in medicine.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
How might you discuss a suicidal ideation, an anxiety-provoking topic, with your patients and their parents? After a positive screen, there will be times when you decide your patient should go to an emergency department for an urgent evaluation. However, most of the time you will be able to help the family identify strategies to lower risk and improve safety and resilience, while waiting for a thorough psychiatric evaluation.
Bring in the Parents: Modeling Validation, Structure, and Optimism
If you have identified some degree of suicide risk in your patient, either with a screening instrument or in your clinical interview, ask your patient if you can bring their parents into the conversation. They may resist, and if so, find out why they are hesitant. Are they worried about causing their parents some distress? Are they concerned their parents will be surprised? Disappointed? Scared? Angry? Acknowledge how hard it can be to find a way to talk about such emotional material with parents. What is their communication like with their parents usually? Do they talk every night at dinner or rarely? Are their interactions usually lighthearted or playful? Brief? Irritable and angry? Have they talked about or managed difficult times before as a family? How did that go? Did they feel they ended up supporting an anxious or depressed single parent? Was their parent harsh and punitive? Since involving the parent is essential, if you become concerned that a conversation with the parent would truly increase the risk of suicide, perhaps because of reports of violence at home, then you may need to send your patient to the emergency department so they can be assessed in a safe setting where a clinical team can evaluate your patient while involving more (or different) members of the family.
Most of the time, your patient will describe a situation that will simply be uncomfortable or stressful for their parent. Don’t be dismissive of their concerns. Instead, acknowledge that talking about their inner life will feel hard. Validate that their parents will be sad, worried, and stressed to hear about what they are feeling. Then offer that parents always prefer to know what is happening with their child so they can help, even if that means only being present to bear it alongside them. You can remind them that you will be there, too, to reassure their parents that this is a common problem and that you can face it and help it to get better together. Find out if they would like you to take the lead in speaking about it, but do not let them wait in the waiting room. Discussing the topic with you with both parents and patient in the room will help even those families that are not great communicators to begin to be more connected, even if you do most of the talking. While you need to bring their symptoms and suicidality to their parents’ attention, find out if there are any details they would rather not share. Perhaps they are struggling with questions of gender identity or sexual orientation, or are thinking of giving up an activity their parents may be very invested in. While any future treatment will prioritize honest communication within the family, communication about their emerging identity should not be rushed, and especially not in the setting of concerns about suicide risk.
With the information you do gather, there are often steps you can take to lower the stress level. The parents’ awareness of their suffering, perhaps acknowledging a broken heart, excessive academic pressure, or a major disappointment may suggest steps to lower the stress level. A mental health referral might introduce a sense of hope. A reminder of their meaningful connection to a parent, a team, a religion, or an activity may also remind the adolescent of a positive view of their future.
Introducing the Topic
When you bring parents into the room, let them know that there is something important and difficult that you need to discuss with them together. Ask if they have noted any changes in their child’s behavior, school performance, or demeanor. Have they had any worries about their teenager? If they have, affirm that they are picking up on something real, and ask more about it. If they have not, offer that their child has been doing a valiant job of soldiering through their days while managing some strong and difficult thoughts and feelings. Walk them through some of what you have learned from your patient, always inviting your patient to affirm or add to what you are detailing. Most parents are keenly aware of the prevalence of suicidal thoughts during adolescence. Bring it into the open, and offer that the next steps are going to be to add more adults to their child’s orbit to help diagnose and treat any underlying psychiatric illness. Reassure them that you are confident that psychiatric illnesses are treatable, even curable. Reassure them that one of the best safety measures is good communication and connectedness with parents.
Help Parents to Be Good Listeners
Some parents may respond with heightened anxiety and need for reassurance from their child. Others may try to talk their child out of their suicidal thoughts. But your year is going so well! You got a great grade in calculus! Gently model validation: Acknowledge to the parents that it is understandable to feel worried or to look for a rational argument against suicide. Offer that feelings don’t usually respond to logic, but do improve with support and time. It may be better for everyone to treat this topic more like the weather so it is easier to talk about and manage. No one gets defensive or distressed if it’s raining, they just put on the right gear. Has the parent ever felt depressed? Did they ever have suicidal ideation growing up? Can they agree to check in at regular times? Could the child speak up if they are feeling badly? Can all agree that parents should check in if their child seems more down? Help them to acknowledge how hard it is to bear strong feelings, but that it is always better together.
Identify Coping Strategies
In front of parents, ask your patient if anything helps when they are feeling at their worst. If they can’t identify anything, offer some possibilities: a walk outside together? making art or music? being out in nature? snuggling with a beloved pet? a set of jumping jacks to get their heart rate up? a favorite playlist? Talking to a particular friend or relative? Make a list. Prioritize activities that are healthy and connect them to others when they are feeling their worst.
Focus on the Basics
Make a concrete and practical plan for steps they can all take to improve well-being. Start with strategies to ensure restful sleep at night, regular exercise, and healthy nutrition. Depression and anxiety often interfere with these functions, so families can work together to support them even while waiting for assessment by a psychiatrist. Help them identify modest rules or routines (consistent bedtime, no screens in the bedroom, a daily walk after dinner) that parents can set that will make a difference.
Set Up Speed Bumps
Talk together about setting up some speed bumps to support their child’s safety. Find out if there are firearms in the home. Be crystal clear that they should be locked, preferably with ammunition, in a separate secure place. Their child should have no knowledge of how to access them, or they should be stored out of the home for the time being.
Parents should lock any medications that could be dangerous in overdose (including in homes if the adolescent will be visiting). Educate them about Tylenol and any prescription medications in their home that should be locked. This part of a conversation is always stressful. Acknowledge that, and remind everyone that, these are important strategies. It should be always be easier to ask their parent for help if they are feeling terrible than it is to access something dangerous.
Acknowledge the Strain
Finally, it is important to acknowledge how hard it is for your patient to bear these feelings, and that speaking up about them may feel like the last thing they want to do. Applaud them for their strength while reminding them that they need to share if they feel worse. Likewise, model for parents that feeling stressed and worried in this circumstance is normal. They should think about how to take good care of themselves. The same well-being strategies you reviewed for their child can work for them too! They may want to focus on sleep or exercise, enhance their nourishing social connections, protect time for beloved hobbies. Everyone should hear that they should never worry alone. If someone feels more worried, bring it to their parent, therapist, psychiatrist, spouse, or to you. They should trust their instincts if they think it is time to go to the emergency department. With supportive open communication, they will strengthen the protective connections which in turn will see the family through the course of the treatable illnesses that cause suicidal thoughts.
Lastly, this is difficult work for any physician. As psychiatrists, we worry about higher-risk teenagers when we decide that hospitalization carries a bigger risk than benefit. Pediatricians see many more teenagers with suicidal ideation and even though the statistical risk is very low, no one knows how to predict any individual teenager’s behavior. Therefore, pediatricians face the direct stress of the clinical work and the deeper stress of knowing there is always some uncertainty in medicine.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
How might you discuss a suicidal ideation, an anxiety-provoking topic, with your patients and their parents? After a positive screen, there will be times when you decide your patient should go to an emergency department for an urgent evaluation. However, most of the time you will be able to help the family identify strategies to lower risk and improve safety and resilience, while waiting for a thorough psychiatric evaluation.
Bring in the Parents: Modeling Validation, Structure, and Optimism
If you have identified some degree of suicide risk in your patient, either with a screening instrument or in your clinical interview, ask your patient if you can bring their parents into the conversation. They may resist, and if so, find out why they are hesitant. Are they worried about causing their parents some distress? Are they concerned their parents will be surprised? Disappointed? Scared? Angry? Acknowledge how hard it can be to find a way to talk about such emotional material with parents. What is their communication like with their parents usually? Do they talk every night at dinner or rarely? Are their interactions usually lighthearted or playful? Brief? Irritable and angry? Have they talked about or managed difficult times before as a family? How did that go? Did they feel they ended up supporting an anxious or depressed single parent? Was their parent harsh and punitive? Since involving the parent is essential, if you become concerned that a conversation with the parent would truly increase the risk of suicide, perhaps because of reports of violence at home, then you may need to send your patient to the emergency department so they can be assessed in a safe setting where a clinical team can evaluate your patient while involving more (or different) members of the family.
Most of the time, your patient will describe a situation that will simply be uncomfortable or stressful for their parent. Don’t be dismissive of their concerns. Instead, acknowledge that talking about their inner life will feel hard. Validate that their parents will be sad, worried, and stressed to hear about what they are feeling. Then offer that parents always prefer to know what is happening with their child so they can help, even if that means only being present to bear it alongside them. You can remind them that you will be there, too, to reassure their parents that this is a common problem and that you can face it and help it to get better together. Find out if they would like you to take the lead in speaking about it, but do not let them wait in the waiting room. Discussing the topic with you with both parents and patient in the room will help even those families that are not great communicators to begin to be more connected, even if you do most of the talking. While you need to bring their symptoms and suicidality to their parents’ attention, find out if there are any details they would rather not share. Perhaps they are struggling with questions of gender identity or sexual orientation, or are thinking of giving up an activity their parents may be very invested in. While any future treatment will prioritize honest communication within the family, communication about their emerging identity should not be rushed, and especially not in the setting of concerns about suicide risk.
With the information you do gather, there are often steps you can take to lower the stress level. The parents’ awareness of their suffering, perhaps acknowledging a broken heart, excessive academic pressure, or a major disappointment may suggest steps to lower the stress level. A mental health referral might introduce a sense of hope. A reminder of their meaningful connection to a parent, a team, a religion, or an activity may also remind the adolescent of a positive view of their future.
Introducing the Topic
When you bring parents into the room, let them know that there is something important and difficult that you need to discuss with them together. Ask if they have noted any changes in their child’s behavior, school performance, or demeanor. Have they had any worries about their teenager? If they have, affirm that they are picking up on something real, and ask more about it. If they have not, offer that their child has been doing a valiant job of soldiering through their days while managing some strong and difficult thoughts and feelings. Walk them through some of what you have learned from your patient, always inviting your patient to affirm or add to what you are detailing. Most parents are keenly aware of the prevalence of suicidal thoughts during adolescence. Bring it into the open, and offer that the next steps are going to be to add more adults to their child’s orbit to help diagnose and treat any underlying psychiatric illness. Reassure them that you are confident that psychiatric illnesses are treatable, even curable. Reassure them that one of the best safety measures is good communication and connectedness with parents.
Help Parents to Be Good Listeners
Some parents may respond with heightened anxiety and need for reassurance from their child. Others may try to talk their child out of their suicidal thoughts. But your year is going so well! You got a great grade in calculus! Gently model validation: Acknowledge to the parents that it is understandable to feel worried or to look for a rational argument against suicide. Offer that feelings don’t usually respond to logic, but do improve with support and time. It may be better for everyone to treat this topic more like the weather so it is easier to talk about and manage. No one gets defensive or distressed if it’s raining, they just put on the right gear. Has the parent ever felt depressed? Did they ever have suicidal ideation growing up? Can they agree to check in at regular times? Could the child speak up if they are feeling badly? Can all agree that parents should check in if their child seems more down? Help them to acknowledge how hard it is to bear strong feelings, but that it is always better together.
Identify Coping Strategies
In front of parents, ask your patient if anything helps when they are feeling at their worst. If they can’t identify anything, offer some possibilities: a walk outside together? making art or music? being out in nature? snuggling with a beloved pet? a set of jumping jacks to get their heart rate up? a favorite playlist? Talking to a particular friend or relative? Make a list. Prioritize activities that are healthy and connect them to others when they are feeling their worst.
Focus on the Basics
Make a concrete and practical plan for steps they can all take to improve well-being. Start with strategies to ensure restful sleep at night, regular exercise, and healthy nutrition. Depression and anxiety often interfere with these functions, so families can work together to support them even while waiting for assessment by a psychiatrist. Help them identify modest rules or routines (consistent bedtime, no screens in the bedroom, a daily walk after dinner) that parents can set that will make a difference.
Set Up Speed Bumps
Talk together about setting up some speed bumps to support their child’s safety. Find out if there are firearms in the home. Be crystal clear that they should be locked, preferably with ammunition, in a separate secure place. Their child should have no knowledge of how to access them, or they should be stored out of the home for the time being.
Parents should lock any medications that could be dangerous in overdose (including in homes if the adolescent will be visiting). Educate them about Tylenol and any prescription medications in their home that should be locked. This part of a conversation is always stressful. Acknowledge that, and remind everyone that, these are important strategies. It should be always be easier to ask their parent for help if they are feeling terrible than it is to access something dangerous.
Acknowledge the Strain
Finally, it is important to acknowledge how hard it is for your patient to bear these feelings, and that speaking up about them may feel like the last thing they want to do. Applaud them for their strength while reminding them that they need to share if they feel worse. Likewise, model for parents that feeling stressed and worried in this circumstance is normal. They should think about how to take good care of themselves. The same well-being strategies you reviewed for their child can work for them too! They may want to focus on sleep or exercise, enhance their nourishing social connections, protect time for beloved hobbies. Everyone should hear that they should never worry alone. If someone feels more worried, bring it to their parent, therapist, psychiatrist, spouse, or to you. They should trust their instincts if they think it is time to go to the emergency department. With supportive open communication, they will strengthen the protective connections which in turn will see the family through the course of the treatable illnesses that cause suicidal thoughts.
Lastly, this is difficult work for any physician. As psychiatrists, we worry about higher-risk teenagers when we decide that hospitalization carries a bigger risk than benefit. Pediatricians see many more teenagers with suicidal ideation and even though the statistical risk is very low, no one knows how to predict any individual teenager’s behavior. Therefore, pediatricians face the direct stress of the clinical work and the deeper stress of knowing there is always some uncertainty in medicine.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
A Tale of Two Babies and the ‘Family Tragedy’ of Congenital Syphilis
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected]. (Also [email protected].)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected]. (Also [email protected].)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected]. (Also [email protected].)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
AI Aids in Monitoring Asthma in Young Children
Can asthma symptoms be monitored reliably at home? Until now, the answer would have been yes, but not in preschool-age patients. Recent findings in Annals of Family Medicine suggest that this limitation can be overcome with the assistance of artificial intelligence (AI).
Objectivity Challenge
A timely diagnosis of asthma exacerbations, which is crucial for proper disease management, requires effective home monitoring. While some lung function parameters, like peak expiratory flow (PEF), can be measured by patients at home, tools for this purpose are not designed for very young children.
“To achieve effective asthma management, patients should be given the necessary tools to allow them to recognize and respond to worsening asthma,” wrote the study authors. Despite the Global Initiative for Asthma identifying respiratory sounds as a fundamental parameter for exacerbation recognition, these are almost exclusively evaluated during doctor visits. Recognizing respiratory sounds and judging whether there has been a change can be challenging for those outside the medical profession.
To enhance home monitoring, researchers from the Department of Pediatric Pneumology and Rheumatology at the University of Lublin, Poland, experimented with the StethoMe stethoscope, which enables the recognition of pathologic signs, including continuous and transient noises. This AI-assisted stethoscope, trained on over 10,000 respiratory sound recordings, is certified as a Class IIa medical device in Europe.
The ‘Smart’ Stethoscope
The 6-month study enlisted 149 patients with asthma (90 children and 59 adults). Participants self-monitored (but parents or caregivers managed for children) once daily in the first 2 weeks and at least once weekly thereafter using three tools. The first was the StethoMe stethoscope, which was used for detecting respiratory sounds, respiratory rate (RR), heart rate (HR), and inspiration/expiration ratio (I/E). Patients were provided a “map” of chest points at which to position the stethoscope. The second was a pulse oximeter, which was used to measure oxygen saturation. The third was a peak flow meter for quantifying PEF. Simultaneously, a health questionnaire was completed.
Data from 6029 completed self-monitoring sessions were used to determine the most effective parameter for exacerbation recognition, quantified by the area under the receiver operating characteristic curve (AUC). The researchers concluded that the parameter with the best performance was wheeze intensity in young children (AUC 84%, 95% CI, 82%-85%), wheeze intensity in older children (AUC, 81%; 95% CI, 79%-84%), and questionnaire response for adults (AUC, 92%; 95% CI, 89%-95%). Combining multiple parameters increased effectiveness.
“The present results clearly show that a set of parameters (wheezes, rhonchi, coarse and fine crackles, HR, RR, and I/E) measured by a device such as an AI-aided home stethoscope allows for the detection of exacerbations without the need for performing PEF measurements, which can be equivocal,” the study authors concluded. “In addition, in the case of younger children (age, < 5 years), when introduced on a large scale, the analyzed home stethoscope appears to be a promising tool that might make asthma diagnosis more straightforward and substantially facilitate asthma monitoring.”
A version of this article first appeared on Medscape.com. This article was translated from Univadis Italy, which is part of the Medscape professional network.
Can asthma symptoms be monitored reliably at home? Until now, the answer would have been yes, but not in preschool-age patients. Recent findings in Annals of Family Medicine suggest that this limitation can be overcome with the assistance of artificial intelligence (AI).
Objectivity Challenge
A timely diagnosis of asthma exacerbations, which is crucial for proper disease management, requires effective home monitoring. While some lung function parameters, like peak expiratory flow (PEF), can be measured by patients at home, tools for this purpose are not designed for very young children.
“To achieve effective asthma management, patients should be given the necessary tools to allow them to recognize and respond to worsening asthma,” wrote the study authors. Despite the Global Initiative for Asthma identifying respiratory sounds as a fundamental parameter for exacerbation recognition, these are almost exclusively evaluated during doctor visits. Recognizing respiratory sounds and judging whether there has been a change can be challenging for those outside the medical profession.
To enhance home monitoring, researchers from the Department of Pediatric Pneumology and Rheumatology at the University of Lublin, Poland, experimented with the StethoMe stethoscope, which enables the recognition of pathologic signs, including continuous and transient noises. This AI-assisted stethoscope, trained on over 10,000 respiratory sound recordings, is certified as a Class IIa medical device in Europe.
The ‘Smart’ Stethoscope
The 6-month study enlisted 149 patients with asthma (90 children and 59 adults). Participants self-monitored (but parents or caregivers managed for children) once daily in the first 2 weeks and at least once weekly thereafter using three tools. The first was the StethoMe stethoscope, which was used for detecting respiratory sounds, respiratory rate (RR), heart rate (HR), and inspiration/expiration ratio (I/E). Patients were provided a “map” of chest points at which to position the stethoscope. The second was a pulse oximeter, which was used to measure oxygen saturation. The third was a peak flow meter for quantifying PEF. Simultaneously, a health questionnaire was completed.
Data from 6029 completed self-monitoring sessions were used to determine the most effective parameter for exacerbation recognition, quantified by the area under the receiver operating characteristic curve (AUC). The researchers concluded that the parameter with the best performance was wheeze intensity in young children (AUC 84%, 95% CI, 82%-85%), wheeze intensity in older children (AUC, 81%; 95% CI, 79%-84%), and questionnaire response for adults (AUC, 92%; 95% CI, 89%-95%). Combining multiple parameters increased effectiveness.
“The present results clearly show that a set of parameters (wheezes, rhonchi, coarse and fine crackles, HR, RR, and I/E) measured by a device such as an AI-aided home stethoscope allows for the detection of exacerbations without the need for performing PEF measurements, which can be equivocal,” the study authors concluded. “In addition, in the case of younger children (age, < 5 years), when introduced on a large scale, the analyzed home stethoscope appears to be a promising tool that might make asthma diagnosis more straightforward and substantially facilitate asthma monitoring.”
A version of this article first appeared on Medscape.com. This article was translated from Univadis Italy, which is part of the Medscape professional network.
Can asthma symptoms be monitored reliably at home? Until now, the answer would have been yes, but not in preschool-age patients. Recent findings in Annals of Family Medicine suggest that this limitation can be overcome with the assistance of artificial intelligence (AI).
Objectivity Challenge
A timely diagnosis of asthma exacerbations, which is crucial for proper disease management, requires effective home monitoring. While some lung function parameters, like peak expiratory flow (PEF), can be measured by patients at home, tools for this purpose are not designed for very young children.
“To achieve effective asthma management, patients should be given the necessary tools to allow them to recognize and respond to worsening asthma,” wrote the study authors. Despite the Global Initiative for Asthma identifying respiratory sounds as a fundamental parameter for exacerbation recognition, these are almost exclusively evaluated during doctor visits. Recognizing respiratory sounds and judging whether there has been a change can be challenging for those outside the medical profession.
To enhance home monitoring, researchers from the Department of Pediatric Pneumology and Rheumatology at the University of Lublin, Poland, experimented with the StethoMe stethoscope, which enables the recognition of pathologic signs, including continuous and transient noises. This AI-assisted stethoscope, trained on over 10,000 respiratory sound recordings, is certified as a Class IIa medical device in Europe.
The ‘Smart’ Stethoscope
The 6-month study enlisted 149 patients with asthma (90 children and 59 adults). Participants self-monitored (but parents or caregivers managed for children) once daily in the first 2 weeks and at least once weekly thereafter using three tools. The first was the StethoMe stethoscope, which was used for detecting respiratory sounds, respiratory rate (RR), heart rate (HR), and inspiration/expiration ratio (I/E). Patients were provided a “map” of chest points at which to position the stethoscope. The second was a pulse oximeter, which was used to measure oxygen saturation. The third was a peak flow meter for quantifying PEF. Simultaneously, a health questionnaire was completed.
Data from 6029 completed self-monitoring sessions were used to determine the most effective parameter for exacerbation recognition, quantified by the area under the receiver operating characteristic curve (AUC). The researchers concluded that the parameter with the best performance was wheeze intensity in young children (AUC 84%, 95% CI, 82%-85%), wheeze intensity in older children (AUC, 81%; 95% CI, 79%-84%), and questionnaire response for adults (AUC, 92%; 95% CI, 89%-95%). Combining multiple parameters increased effectiveness.
“The present results clearly show that a set of parameters (wheezes, rhonchi, coarse and fine crackles, HR, RR, and I/E) measured by a device such as an AI-aided home stethoscope allows for the detection of exacerbations without the need for performing PEF measurements, which can be equivocal,” the study authors concluded. “In addition, in the case of younger children (age, < 5 years), when introduced on a large scale, the analyzed home stethoscope appears to be a promising tool that might make asthma diagnosis more straightforward and substantially facilitate asthma monitoring.”
A version of this article first appeared on Medscape.com. This article was translated from Univadis Italy, which is part of the Medscape professional network.
FROM ANNALS OF FAMILY MEDICINE
Higher-Dose Atypical Antipsychotics Risky in Young Adults
High doses of a second-generation antipsychotic are associated with a significantly increased risk for death in young adults, adding to longstanding safety concerns regarding the use of higher doses of antipsychotic medication in this age group.
In a large cohort study, people aged 18-24 years had a significantly higher risk for death when starting a second-generation antipsychotic at doses greater than 100-mg chlorpromazine equivalents, but no increased mortality risk with lower doses.
There was no association with mortality risk in children aged 5-17 years with either dose.
“This finding suggests that antipsychotic medication–related fatalities are rare in healthy children without psychosis,” lead investigator Wayne Ray, PhD, from Vanderbilt University School of Medicine in Nashville, Tennessee, and colleagues wrote in a recent study that was published online in JAMA Psychiatry.
“In contrast, young adults aged 18-24 years treated with doses greater than 100-mg chlorpromazine equivalents had 127.5 additional deaths for every 100,000 person-years of exposure, suggesting further investigations of antipsychotic medication safety in this population are needed.”
Large, Retrospective Study
The researchers compared mortality for more than 2 million Medicaid patients aged 5-24 years (mean age, 13 years; 51% men) starting treatment with a second-generation antipsychotic vs control psychiatric medications. None of them had a diagnosis of severe somatic illness, schizophrenia, or related psychosis.
From January 2004 through September 2013, more than 21 million prescriptions were filled — roughly 5.4 million for antipsychotic doses of 100 mg or less, 2.8 million for doses greater than 100 mg, and 13.5 million for control medications.
The most commonly prescribed antipsychotic medication was risperidone, followed by aripiprazole, quetiapine, ziprasidone, and olanzapine. The most commonly prescribed control medication was clonidine, followed by atomoxetine, guanfacine, and sertraline.
In the overall study population, there was no significant association with risk for death for antipsychotic doses less than or equal to 100-mg chlorpromazine equivalents (hazard ratio [HR], 1.08; 95% CI, 0.89-1.32). However, mortality risk was increased at doses greater than 100 mg (HR, 1.37; 95% CI, 1.11-1.70).
Looking at mortality risk by age, for children aged 5-17 years, there was no significant association with either antipsychotic dose, whereas young adults aged 18-24 years had increased risk for doses greater than 100 mg (HR, 1.68; 95% CI, 1.23-2.29).
Start Low, Go Slow
“Start low and go slow is always a good rule of thumb when it comes to the use of these and any medicines, especially among especially among children and adolescents,” Caleb Alexander, MD, codirector of the Center for Drug Safety and Effectiveness at Johns Hopkins University in Baltimore, Maryland, who wasn’t involved in the study, told this news organization.
Higher-dose antipsychotic treatment was significantly associated with overdose deaths (HR, 1.57; 95% CI, 1.02-2.42) and other unintentional injury deaths (HR, 1.57; 95% CI, 1.12-2.22), but not with nonoverdose suicide deaths or cardiovascular/metabolic deaths.
Death certificates listed opioid involvement in more than half of overdose deaths in those taking higher antipsychotic doses as well as those taking control medications.
“That’s a good reminder that the risk of these medicines may increase markedly when they’re combined with other treatments, such as prescription opioids,” Dr. Alexander said.
Also weighing in on the research, Anish Dube, MD, chair of the American Psychiatric Association’s Council on Children, Adolescents, and their Families, said the study is “notable for both the increased risk of death among young adults 18-24 prescribed treatment with antipsychotics at doses greater than 100-mg chlorpromazine equivalents, but also for the absence of such a finding with antipsychotic use in younger age groups,” he said.
“This suggests an interaction between other factors more common to young adults, such as substance use as mentioned by the authors, and concurrent treatment with antipsychotic medications at doses greater than 100-mg chlorpromazine equivalents,” said Dr. Dube.
“As the authors point out, additional research is needed to help clarify the observed increased risk of death at this developmental juncture so as to allow us to better predict which young adults may be especially vulnerable,” Dr. Dube said.
The findings also point to a need for caution when prescribing any antipsychotic medications off label, Dr. Dube added, especially among people aged 18-24 years, and other treatments should be considered when possible.
“Thankfully, with greater awareness and increased scrutiny, overall prescriptions for antipsychotic medications in the pediatric and young adult populations have likely decreased since the study period,” he said.
Limitations of the study include potential residual confounding, confining the study population to Medicaid recipients, restriction to second-generation antipsychotics, and exclusion of individuals with psychoses or severe somatic illness. Also, insufficient numbers of deaths from specific causes precluded an examination of individual antipsychotics or more detailed dose categories.
“No study is perfect,” said Dr. Alexander, “and some of the findings may be due to unmeasured differences between the groups that were being compared. That’s the elephant in the room.”
The study was funded by a grant from the National Institute for Child Health and Human Development. Dr. Ray, Dr. Alexander, and Dr. Dube have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
High doses of a second-generation antipsychotic are associated with a significantly increased risk for death in young adults, adding to longstanding safety concerns regarding the use of higher doses of antipsychotic medication in this age group.
In a large cohort study, people aged 18-24 years had a significantly higher risk for death when starting a second-generation antipsychotic at doses greater than 100-mg chlorpromazine equivalents, but no increased mortality risk with lower doses.
There was no association with mortality risk in children aged 5-17 years with either dose.
“This finding suggests that antipsychotic medication–related fatalities are rare in healthy children without psychosis,” lead investigator Wayne Ray, PhD, from Vanderbilt University School of Medicine in Nashville, Tennessee, and colleagues wrote in a recent study that was published online in JAMA Psychiatry.
“In contrast, young adults aged 18-24 years treated with doses greater than 100-mg chlorpromazine equivalents had 127.5 additional deaths for every 100,000 person-years of exposure, suggesting further investigations of antipsychotic medication safety in this population are needed.”
Large, Retrospective Study
The researchers compared mortality for more than 2 million Medicaid patients aged 5-24 years (mean age, 13 years; 51% men) starting treatment with a second-generation antipsychotic vs control psychiatric medications. None of them had a diagnosis of severe somatic illness, schizophrenia, or related psychosis.
From January 2004 through September 2013, more than 21 million prescriptions were filled — roughly 5.4 million for antipsychotic doses of 100 mg or less, 2.8 million for doses greater than 100 mg, and 13.5 million for control medications.
The most commonly prescribed antipsychotic medication was risperidone, followed by aripiprazole, quetiapine, ziprasidone, and olanzapine. The most commonly prescribed control medication was clonidine, followed by atomoxetine, guanfacine, and sertraline.
In the overall study population, there was no significant association with risk for death for antipsychotic doses less than or equal to 100-mg chlorpromazine equivalents (hazard ratio [HR], 1.08; 95% CI, 0.89-1.32). However, mortality risk was increased at doses greater than 100 mg (HR, 1.37; 95% CI, 1.11-1.70).
Looking at mortality risk by age, for children aged 5-17 years, there was no significant association with either antipsychotic dose, whereas young adults aged 18-24 years had increased risk for doses greater than 100 mg (HR, 1.68; 95% CI, 1.23-2.29).
Start Low, Go Slow
“Start low and go slow is always a good rule of thumb when it comes to the use of these and any medicines, especially among especially among children and adolescents,” Caleb Alexander, MD, codirector of the Center for Drug Safety and Effectiveness at Johns Hopkins University in Baltimore, Maryland, who wasn’t involved in the study, told this news organization.
Higher-dose antipsychotic treatment was significantly associated with overdose deaths (HR, 1.57; 95% CI, 1.02-2.42) and other unintentional injury deaths (HR, 1.57; 95% CI, 1.12-2.22), but not with nonoverdose suicide deaths or cardiovascular/metabolic deaths.
Death certificates listed opioid involvement in more than half of overdose deaths in those taking higher antipsychotic doses as well as those taking control medications.
“That’s a good reminder that the risk of these medicines may increase markedly when they’re combined with other treatments, such as prescription opioids,” Dr. Alexander said.
Also weighing in on the research, Anish Dube, MD, chair of the American Psychiatric Association’s Council on Children, Adolescents, and their Families, said the study is “notable for both the increased risk of death among young adults 18-24 prescribed treatment with antipsychotics at doses greater than 100-mg chlorpromazine equivalents, but also for the absence of such a finding with antipsychotic use in younger age groups,” he said.
“This suggests an interaction between other factors more common to young adults, such as substance use as mentioned by the authors, and concurrent treatment with antipsychotic medications at doses greater than 100-mg chlorpromazine equivalents,” said Dr. Dube.
“As the authors point out, additional research is needed to help clarify the observed increased risk of death at this developmental juncture so as to allow us to better predict which young adults may be especially vulnerable,” Dr. Dube said.
The findings also point to a need for caution when prescribing any antipsychotic medications off label, Dr. Dube added, especially among people aged 18-24 years, and other treatments should be considered when possible.
“Thankfully, with greater awareness and increased scrutiny, overall prescriptions for antipsychotic medications in the pediatric and young adult populations have likely decreased since the study period,” he said.
Limitations of the study include potential residual confounding, confining the study population to Medicaid recipients, restriction to second-generation antipsychotics, and exclusion of individuals with psychoses or severe somatic illness. Also, insufficient numbers of deaths from specific causes precluded an examination of individual antipsychotics or more detailed dose categories.
“No study is perfect,” said Dr. Alexander, “and some of the findings may be due to unmeasured differences between the groups that were being compared. That’s the elephant in the room.”
The study was funded by a grant from the National Institute for Child Health and Human Development. Dr. Ray, Dr. Alexander, and Dr. Dube have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
High doses of a second-generation antipsychotic are associated with a significantly increased risk for death in young adults, adding to longstanding safety concerns regarding the use of higher doses of antipsychotic medication in this age group.
In a large cohort study, people aged 18-24 years had a significantly higher risk for death when starting a second-generation antipsychotic at doses greater than 100-mg chlorpromazine equivalents, but no increased mortality risk with lower doses.
There was no association with mortality risk in children aged 5-17 years with either dose.
“This finding suggests that antipsychotic medication–related fatalities are rare in healthy children without psychosis,” lead investigator Wayne Ray, PhD, from Vanderbilt University School of Medicine in Nashville, Tennessee, and colleagues wrote in a recent study that was published online in JAMA Psychiatry.
“In contrast, young adults aged 18-24 years treated with doses greater than 100-mg chlorpromazine equivalents had 127.5 additional deaths for every 100,000 person-years of exposure, suggesting further investigations of antipsychotic medication safety in this population are needed.”
Large, Retrospective Study
The researchers compared mortality for more than 2 million Medicaid patients aged 5-24 years (mean age, 13 years; 51% men) starting treatment with a second-generation antipsychotic vs control psychiatric medications. None of them had a diagnosis of severe somatic illness, schizophrenia, or related psychosis.
From January 2004 through September 2013, more than 21 million prescriptions were filled — roughly 5.4 million for antipsychotic doses of 100 mg or less, 2.8 million for doses greater than 100 mg, and 13.5 million for control medications.
The most commonly prescribed antipsychotic medication was risperidone, followed by aripiprazole, quetiapine, ziprasidone, and olanzapine. The most commonly prescribed control medication was clonidine, followed by atomoxetine, guanfacine, and sertraline.
In the overall study population, there was no significant association with risk for death for antipsychotic doses less than or equal to 100-mg chlorpromazine equivalents (hazard ratio [HR], 1.08; 95% CI, 0.89-1.32). However, mortality risk was increased at doses greater than 100 mg (HR, 1.37; 95% CI, 1.11-1.70).
Looking at mortality risk by age, for children aged 5-17 years, there was no significant association with either antipsychotic dose, whereas young adults aged 18-24 years had increased risk for doses greater than 100 mg (HR, 1.68; 95% CI, 1.23-2.29).
Start Low, Go Slow
“Start low and go slow is always a good rule of thumb when it comes to the use of these and any medicines, especially among especially among children and adolescents,” Caleb Alexander, MD, codirector of the Center for Drug Safety and Effectiveness at Johns Hopkins University in Baltimore, Maryland, who wasn’t involved in the study, told this news organization.
Higher-dose antipsychotic treatment was significantly associated with overdose deaths (HR, 1.57; 95% CI, 1.02-2.42) and other unintentional injury deaths (HR, 1.57; 95% CI, 1.12-2.22), but not with nonoverdose suicide deaths or cardiovascular/metabolic deaths.
Death certificates listed opioid involvement in more than half of overdose deaths in those taking higher antipsychotic doses as well as those taking control medications.
“That’s a good reminder that the risk of these medicines may increase markedly when they’re combined with other treatments, such as prescription opioids,” Dr. Alexander said.
Also weighing in on the research, Anish Dube, MD, chair of the American Psychiatric Association’s Council on Children, Adolescents, and their Families, said the study is “notable for both the increased risk of death among young adults 18-24 prescribed treatment with antipsychotics at doses greater than 100-mg chlorpromazine equivalents, but also for the absence of such a finding with antipsychotic use in younger age groups,” he said.
“This suggests an interaction between other factors more common to young adults, such as substance use as mentioned by the authors, and concurrent treatment with antipsychotic medications at doses greater than 100-mg chlorpromazine equivalents,” said Dr. Dube.
“As the authors point out, additional research is needed to help clarify the observed increased risk of death at this developmental juncture so as to allow us to better predict which young adults may be especially vulnerable,” Dr. Dube said.
The findings also point to a need for caution when prescribing any antipsychotic medications off label, Dr. Dube added, especially among people aged 18-24 years, and other treatments should be considered when possible.
“Thankfully, with greater awareness and increased scrutiny, overall prescriptions for antipsychotic medications in the pediatric and young adult populations have likely decreased since the study period,” he said.
Limitations of the study include potential residual confounding, confining the study population to Medicaid recipients, restriction to second-generation antipsychotics, and exclusion of individuals with psychoses or severe somatic illness. Also, insufficient numbers of deaths from specific causes precluded an examination of individual antipsychotics or more detailed dose categories.
“No study is perfect,” said Dr. Alexander, “and some of the findings may be due to unmeasured differences between the groups that were being compared. That’s the elephant in the room.”
The study was funded by a grant from the National Institute for Child Health and Human Development. Dr. Ray, Dr. Alexander, and Dr. Dube have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Myo-inositol is one of the components of an integrative approach to acne
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
FROM IDS 2023