User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
One in five men carries high-risk HPV in international study
Findings from a meta-analysis of 65 studies conducted in 35 countries indicate that These estimates provide further weight to arguments in favor of vaccinating boys against HPV to prevent certain types of cancer.
“Our results support that sexually active men, regardless of age, are an important reservoir of HPV genital infection,” wrote the authors in The Lancet Global Health . “These estimates emphasize the importance of incorporating men into comprehensive HPV prevention strategies to reduce HPV-related morbidity and mortality in men and ultimately achieve elimination of cervical cancer and other HPV-related diseases.”
Literature review
HPV infection is the most common sexually transmitted viral infection worldwide. More than 200 HPV types can be transmitted sexually, and at least 12 types are oncogenic. Previous studies have shown that most sexually active men and women acquire at least one genital HPV infection during their lifetime.
Although most HPV infections are asymptomatic, they can lead to cancer. Indeed, HPV is involved in the development of cervical, vulval, and vaginal cancers, as well as oropharyngeal and anal cancers, which also affect the male population. More than 25% of cancers caused by HPV occur in men.
Despite these observations, fewer epidemiologic studies have assessed HPV infection in men than in women. To determine the prevalence of HPV infection in the male population, Laia Bruni, MD, MPH, PhD, an epidemiologist at the Catalan Institute of Oncology in Barcelona, and her colleagues collated data from 65 studies conducted in 35 countries pertaining to males older than 15 years.
In this literature review, the researchers selected studies that reported infection rates in males without HPV-related symptoms. Studies conducted exclusively in populations that were considered at increased risk for sexually transmitted infections (STIs) were excluded. Overall, the analysis included close to 45,000 men.
Prevalent HPV genotype
Testing for HPV was conducted on samples collected from the anus and genitals. The results show a global pooled prevalence of HPV infection in males older than 15 years of 31% for any HPV and 21% for HR-HPV. One of these viruses, HPV-16, was the most prevalent HPV genotype (5% prevalence).
HPV prevalence was highest among young adults. It stabilized and decreased from age 50 years. Between ages 25 and 29 years, 35% of men are infected with HPV. It should be noted that prevalence is already high in the youngest group, reaching 28% in males between the ages of 15 and 19 years. The variations are similar for HR-HPV infections.
This age-related change is different from rates in women. Among the female population, HPV prevalence peaks soon after first sexual activity and declines with age, with a slight rebound after ages 50–55 years (i.e., often after or around the time of menopause), wrote the researchers.
The results also show country- and region-based disparities. The pooled prevalence for any HPV was highest in Sub-Saharan Africa (37%), followed by Europe and Northern America (36%). The lowest prevalence was in East and Southeast Asia (15%). Here again, the trends are similar with high-risk HPV.
Preventive measures
“Our study draws attention to the high prevalence, ranging from 20% to 30% for HR-HPV in men across most regions, and the need for strengthening HPV prevention within overall STI control efforts,” wrote the authors.
“Future epidemiological studies are needed to monitor trends in prevalence in men, especially considering the roll-out of HPV vaccination in girls and young women and that many countries are beginning to vaccinate boys.”
In France, the HPV vaccination program was extended in 2021 to include all boys between the ages of 11 and 14 years (two-dose schedule), with a catch-up course in males up to age 19 years (three-dose schedule). This is the same vaccine program as for girls. It is also recommended for men up to age 26 years who have sex with other men.
The 2023 return to school will see the launch of a general vaccination campaign aimed at seventh-grade students, both boys and girls, with parental consent, to increase vaccine coverage. In 2021, vaccine uptake was 43.6% in girls between the ages of 15 and 18 years and scarcely 6% in boys, according to Public Health France.
Two vaccines are in use: the bivalent Cervarix vaccine, which is effective against HPV-16 and HPV-18, and the nonavalent Gardasil 9, which is effective against types 16, 18, 31, 33, 45, 52, and 58. Both provide protection against HPV-16, the type most common in men, which is responsible for more than half of cases of cervical cancer.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Findings from a meta-analysis of 65 studies conducted in 35 countries indicate that These estimates provide further weight to arguments in favor of vaccinating boys against HPV to prevent certain types of cancer.
“Our results support that sexually active men, regardless of age, are an important reservoir of HPV genital infection,” wrote the authors in The Lancet Global Health . “These estimates emphasize the importance of incorporating men into comprehensive HPV prevention strategies to reduce HPV-related morbidity and mortality in men and ultimately achieve elimination of cervical cancer and other HPV-related diseases.”
Literature review
HPV infection is the most common sexually transmitted viral infection worldwide. More than 200 HPV types can be transmitted sexually, and at least 12 types are oncogenic. Previous studies have shown that most sexually active men and women acquire at least one genital HPV infection during their lifetime.
Although most HPV infections are asymptomatic, they can lead to cancer. Indeed, HPV is involved in the development of cervical, vulval, and vaginal cancers, as well as oropharyngeal and anal cancers, which also affect the male population. More than 25% of cancers caused by HPV occur in men.
Despite these observations, fewer epidemiologic studies have assessed HPV infection in men than in women. To determine the prevalence of HPV infection in the male population, Laia Bruni, MD, MPH, PhD, an epidemiologist at the Catalan Institute of Oncology in Barcelona, and her colleagues collated data from 65 studies conducted in 35 countries pertaining to males older than 15 years.
In this literature review, the researchers selected studies that reported infection rates in males without HPV-related symptoms. Studies conducted exclusively in populations that were considered at increased risk for sexually transmitted infections (STIs) were excluded. Overall, the analysis included close to 45,000 men.
Prevalent HPV genotype
Testing for HPV was conducted on samples collected from the anus and genitals. The results show a global pooled prevalence of HPV infection in males older than 15 years of 31% for any HPV and 21% for HR-HPV. One of these viruses, HPV-16, was the most prevalent HPV genotype (5% prevalence).
HPV prevalence was highest among young adults. It stabilized and decreased from age 50 years. Between ages 25 and 29 years, 35% of men are infected with HPV. It should be noted that prevalence is already high in the youngest group, reaching 28% in males between the ages of 15 and 19 years. The variations are similar for HR-HPV infections.
This age-related change is different from rates in women. Among the female population, HPV prevalence peaks soon after first sexual activity and declines with age, with a slight rebound after ages 50–55 years (i.e., often after or around the time of menopause), wrote the researchers.
The results also show country- and region-based disparities. The pooled prevalence for any HPV was highest in Sub-Saharan Africa (37%), followed by Europe and Northern America (36%). The lowest prevalence was in East and Southeast Asia (15%). Here again, the trends are similar with high-risk HPV.
Preventive measures
“Our study draws attention to the high prevalence, ranging from 20% to 30% for HR-HPV in men across most regions, and the need for strengthening HPV prevention within overall STI control efforts,” wrote the authors.
“Future epidemiological studies are needed to monitor trends in prevalence in men, especially considering the roll-out of HPV vaccination in girls and young women and that many countries are beginning to vaccinate boys.”
In France, the HPV vaccination program was extended in 2021 to include all boys between the ages of 11 and 14 years (two-dose schedule), with a catch-up course in males up to age 19 years (three-dose schedule). This is the same vaccine program as for girls. It is also recommended for men up to age 26 years who have sex with other men.
The 2023 return to school will see the launch of a general vaccination campaign aimed at seventh-grade students, both boys and girls, with parental consent, to increase vaccine coverage. In 2021, vaccine uptake was 43.6% in girls between the ages of 15 and 18 years and scarcely 6% in boys, according to Public Health France.
Two vaccines are in use: the bivalent Cervarix vaccine, which is effective against HPV-16 and HPV-18, and the nonavalent Gardasil 9, which is effective against types 16, 18, 31, 33, 45, 52, and 58. Both provide protection against HPV-16, the type most common in men, which is responsible for more than half of cases of cervical cancer.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Findings from a meta-analysis of 65 studies conducted in 35 countries indicate that These estimates provide further weight to arguments in favor of vaccinating boys against HPV to prevent certain types of cancer.
“Our results support that sexually active men, regardless of age, are an important reservoir of HPV genital infection,” wrote the authors in The Lancet Global Health . “These estimates emphasize the importance of incorporating men into comprehensive HPV prevention strategies to reduce HPV-related morbidity and mortality in men and ultimately achieve elimination of cervical cancer and other HPV-related diseases.”
Literature review
HPV infection is the most common sexually transmitted viral infection worldwide. More than 200 HPV types can be transmitted sexually, and at least 12 types are oncogenic. Previous studies have shown that most sexually active men and women acquire at least one genital HPV infection during their lifetime.
Although most HPV infections are asymptomatic, they can lead to cancer. Indeed, HPV is involved in the development of cervical, vulval, and vaginal cancers, as well as oropharyngeal and anal cancers, which also affect the male population. More than 25% of cancers caused by HPV occur in men.
Despite these observations, fewer epidemiologic studies have assessed HPV infection in men than in women. To determine the prevalence of HPV infection in the male population, Laia Bruni, MD, MPH, PhD, an epidemiologist at the Catalan Institute of Oncology in Barcelona, and her colleagues collated data from 65 studies conducted in 35 countries pertaining to males older than 15 years.
In this literature review, the researchers selected studies that reported infection rates in males without HPV-related symptoms. Studies conducted exclusively in populations that were considered at increased risk for sexually transmitted infections (STIs) were excluded. Overall, the analysis included close to 45,000 men.
Prevalent HPV genotype
Testing for HPV was conducted on samples collected from the anus and genitals. The results show a global pooled prevalence of HPV infection in males older than 15 years of 31% for any HPV and 21% for HR-HPV. One of these viruses, HPV-16, was the most prevalent HPV genotype (5% prevalence).
HPV prevalence was highest among young adults. It stabilized and decreased from age 50 years. Between ages 25 and 29 years, 35% of men are infected with HPV. It should be noted that prevalence is already high in the youngest group, reaching 28% in males between the ages of 15 and 19 years. The variations are similar for HR-HPV infections.
This age-related change is different from rates in women. Among the female population, HPV prevalence peaks soon after first sexual activity and declines with age, with a slight rebound after ages 50–55 years (i.e., often after or around the time of menopause), wrote the researchers.
The results also show country- and region-based disparities. The pooled prevalence for any HPV was highest in Sub-Saharan Africa (37%), followed by Europe and Northern America (36%). The lowest prevalence was in East and Southeast Asia (15%). Here again, the trends are similar with high-risk HPV.
Preventive measures
“Our study draws attention to the high prevalence, ranging from 20% to 30% for HR-HPV in men across most regions, and the need for strengthening HPV prevention within overall STI control efforts,” wrote the authors.
“Future epidemiological studies are needed to monitor trends in prevalence in men, especially considering the roll-out of HPV vaccination in girls and young women and that many countries are beginning to vaccinate boys.”
In France, the HPV vaccination program was extended in 2021 to include all boys between the ages of 11 and 14 years (two-dose schedule), with a catch-up course in males up to age 19 years (three-dose schedule). This is the same vaccine program as for girls. It is also recommended for men up to age 26 years who have sex with other men.
The 2023 return to school will see the launch of a general vaccination campaign aimed at seventh-grade students, both boys and girls, with parental consent, to increase vaccine coverage. In 2021, vaccine uptake was 43.6% in girls between the ages of 15 and 18 years and scarcely 6% in boys, according to Public Health France.
Two vaccines are in use: the bivalent Cervarix vaccine, which is effective against HPV-16 and HPV-18, and the nonavalent Gardasil 9, which is effective against types 16, 18, 31, 33, 45, 52, and 58. Both provide protection against HPV-16, the type most common in men, which is responsible for more than half of cases of cervical cancer.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
FROM THE LANCET GLOBAL HEALTH
Five questions for COVID experts: How concerned should we be?
COVID-19 hospitalizations have been on the rise for weeks as summer nears its end, but how concerned should you be? SARS-CoV-2, the virus behind COVID, continues to evolve and surprise us. So COVID transmission, hospitalization, and death rates can be difficult to predict.
, especially now that testing and vaccinations are no longer free of charge.
Question 1: Are you expecting an end-of-summer COVID wave to be substantial?
Eric Topol, MD: “This wave won’t likely be substantial and could be more of a ‘wavelet.’ I’m not thinking that physicians are too concerned,” said Dr. Topol, founder and director of Scripps Research Translational Institute in La Jolla, Calif.
Thomas Gut, DO: “It’s always impossible to predict the severity of COVID waves. Although the virus has generally mutated in ways that favor easier transmission and milder illness, there have been a handful of surprising mutations that were more dangerous and deadly than the preceding strain,” said Dr. Gut, associate chair of medicine at Staten Island University Hospital/Northwell Health in New York.
Robert Atmar, MD: “I’ll start with the caveat that prognosticating for SARS-CoV-2 is a bit hazardous as we remain in unknown territory for some aspects of its epidemiology and evolution,” said Dr. Atmar, a professor of infectious diseases at Baylor College of Medicine in Houston. “It depends on your definition of substantial. We, at least in Houston, are already in the midst of a substantial surge in the burden of infection, at least as monitored through wastewater surveillance. The amount of virus in the wastewater already exceeds the peak level we saw last winter. That said, the increased infection burden has not translated into large increases in hospitalizations for COVID-19. Most persons hospitalized in our hospital are admitted with infection, not for the consequences of infection.”
Stuart Campbell Ray, MD: “It looks like there is a rise in infections, but the proportional rise in hospitalizations from severe cases is lower than in the past, suggesting that folks are protected by the immunity we’ve gained over the past few years through vaccination and prior infections. Of course, we should be thinking about how that applies to each of us – how recently we had a vaccine or COVID-19, and whether we might see more severe infections as immunity wanes,” said Dr. Ray, who is a professor of medicine in the division of infectious diseases at Johns Hopkins University in Baltimore.
Question 2: Is a return to masks or mask mandates coming this fall or winter?
Dr. Topol: “Mandating masks doesn’t work very well, but we may see wide use again if a descendant of [variant] BA.2.86 takes off.”
Dr. Gut: “It’s difficult to predict if there are any mask mandates returning at any point. Ever since the Omicron strains emerged, COVID has been relatively mild, compared to previous strains, so there probably won’t be any plan to start masking in public unless a more deadly strain appears.”
Dr. Atmar: “I do not think we will see a return to mask mandates this fall or winter for a variety of reasons. The primary one is that I don’t think the public will accept mask mandates. However, I think masking can continue to be an adjunctive measure to enhance protection from infection, along with booster vaccination.”
Dr. Ray: “Some people will choose to wear masks during a surge, particularly in situations like commuting where they don’t interfere with what they’re doing. They will wear masks particularly if they want to avoid infection due to concerns about others they care about, disruption of work or travel plans, or concerns about long-term consequences of repeated COVID-19.”
Question 3: Now that COVID testing and vaccinations are no longer free of charge, how might that affect their use?
Dr. Topol: “It was already low, and this will undoubtedly further compromise their uptake.”
Dr. Gut: “I do expect that testing will become less common now that tests are no longer free. I’m sure there will be a lower amount of detection in patients with milder or asymptomatic disease compared to what we had previously.”
Dr. Atmar: “If there are out-of-pocket costs for the SARS-CoV-2 vaccine, or if the administrative paperwork attached to getting a vaccine is increased, the uptake of SARS-CoV-2 vaccines will likely decrease. It will be important to communicate to the populations targeted for vaccination the potential benefits of such vaccination.”
Dr. Ray: “A challenge with COVID-19, all along, has been disparities in access to care, and this will be worse without public support for prevention and testing. This applies to everyone but is especially burdensome for those who are often marginalized in our health care system and society in general. I hope that we’ll find ways to ensure that people who need tests and vaccinations are able to access them, as good health is in everyone’s interest.”
Question 4: Will the new vaccines against COVID work for the currently circulating variants?
Dr. Topol: “The XBB.1.5 boosters will be out Sept. 14. They should help versus EG.5.1 and FL.1.5.1. The FL.1.5.1 variant is gaining now.”
Dr. Gut: “In the next several weeks, we expect the newer monovalent XBB-based vaccines to be offered that offer good protection against current circulating COVID variants along with the new Eris variant.”
Dr. Atmar: “The vaccines are expected to induce immune responses to the currently circulating variants, most of which are strains that evolved from the vaccine strain. The vaccine is expected to be most effective in preventing severe illness and will likely be less effective in preventing infection and mild illness.”
Dr. Ray: “Yes, the updated vaccine design has a spike antigen (XBB.1.5) nearly identical to the current dominant variant (EG.5). Even as variants change, the boosters stimulate B cells and T cells to help protect in a way that is safer than getting COVID-19 infection.”
Question 5: Is there anything we should watch out for regarding the BA.2.86 variant in particular?
Dr. Topol: “The scenario could change if there are new functional mutations added to it.”
Dr. Gut: “BA.2.86 is still fairly uncommon and does not have much data to directly make any informed guesses. However, in general, people that have been exposed to more recent mutations of the COVID virus have been shown to have more protection from newer upcoming mutations. It’s fair to guess that people that have not had recent infection from COVID, or have not had a recent booster, are at higher risk for being infected by any XBB- or BA.2-based strains.”
Dr. Atmar: BA.2.86 has been designated as a variant under monitoring. We will want to see whether it becomes more common and if there are any unexpected characteristics associated with infection by this variant.”
Dr. Ray: “It’s still rare, but it’s been seen in geographically dispersed places, so it’s got legs. The question is how effectively it will bypass some of the immunity we’ve gained. T cells are likely to remain protective, because they target so many parts of the virus that change more slowly, but antibodies from B cells to spike protein may have more trouble recognizing BA.2.86, whether those antibodies were made to a vaccine or a prior variant.”
A version of this article first appeared on WebMD.com.
COVID-19 hospitalizations have been on the rise for weeks as summer nears its end, but how concerned should you be? SARS-CoV-2, the virus behind COVID, continues to evolve and surprise us. So COVID transmission, hospitalization, and death rates can be difficult to predict.
, especially now that testing and vaccinations are no longer free of charge.
Question 1: Are you expecting an end-of-summer COVID wave to be substantial?
Eric Topol, MD: “This wave won’t likely be substantial and could be more of a ‘wavelet.’ I’m not thinking that physicians are too concerned,” said Dr. Topol, founder and director of Scripps Research Translational Institute in La Jolla, Calif.
Thomas Gut, DO: “It’s always impossible to predict the severity of COVID waves. Although the virus has generally mutated in ways that favor easier transmission and milder illness, there have been a handful of surprising mutations that were more dangerous and deadly than the preceding strain,” said Dr. Gut, associate chair of medicine at Staten Island University Hospital/Northwell Health in New York.
Robert Atmar, MD: “I’ll start with the caveat that prognosticating for SARS-CoV-2 is a bit hazardous as we remain in unknown territory for some aspects of its epidemiology and evolution,” said Dr. Atmar, a professor of infectious diseases at Baylor College of Medicine in Houston. “It depends on your definition of substantial. We, at least in Houston, are already in the midst of a substantial surge in the burden of infection, at least as monitored through wastewater surveillance. The amount of virus in the wastewater already exceeds the peak level we saw last winter. That said, the increased infection burden has not translated into large increases in hospitalizations for COVID-19. Most persons hospitalized in our hospital are admitted with infection, not for the consequences of infection.”
Stuart Campbell Ray, MD: “It looks like there is a rise in infections, but the proportional rise in hospitalizations from severe cases is lower than in the past, suggesting that folks are protected by the immunity we’ve gained over the past few years through vaccination and prior infections. Of course, we should be thinking about how that applies to each of us – how recently we had a vaccine or COVID-19, and whether we might see more severe infections as immunity wanes,” said Dr. Ray, who is a professor of medicine in the division of infectious diseases at Johns Hopkins University in Baltimore.
Question 2: Is a return to masks or mask mandates coming this fall or winter?
Dr. Topol: “Mandating masks doesn’t work very well, but we may see wide use again if a descendant of [variant] BA.2.86 takes off.”
Dr. Gut: “It’s difficult to predict if there are any mask mandates returning at any point. Ever since the Omicron strains emerged, COVID has been relatively mild, compared to previous strains, so there probably won’t be any plan to start masking in public unless a more deadly strain appears.”
Dr. Atmar: “I do not think we will see a return to mask mandates this fall or winter for a variety of reasons. The primary one is that I don’t think the public will accept mask mandates. However, I think masking can continue to be an adjunctive measure to enhance protection from infection, along with booster vaccination.”
Dr. Ray: “Some people will choose to wear masks during a surge, particularly in situations like commuting where they don’t interfere with what they’re doing. They will wear masks particularly if they want to avoid infection due to concerns about others they care about, disruption of work or travel plans, or concerns about long-term consequences of repeated COVID-19.”
Question 3: Now that COVID testing and vaccinations are no longer free of charge, how might that affect their use?
Dr. Topol: “It was already low, and this will undoubtedly further compromise their uptake.”
Dr. Gut: “I do expect that testing will become less common now that tests are no longer free. I’m sure there will be a lower amount of detection in patients with milder or asymptomatic disease compared to what we had previously.”
Dr. Atmar: “If there are out-of-pocket costs for the SARS-CoV-2 vaccine, or if the administrative paperwork attached to getting a vaccine is increased, the uptake of SARS-CoV-2 vaccines will likely decrease. It will be important to communicate to the populations targeted for vaccination the potential benefits of such vaccination.”
Dr. Ray: “A challenge with COVID-19, all along, has been disparities in access to care, and this will be worse without public support for prevention and testing. This applies to everyone but is especially burdensome for those who are often marginalized in our health care system and society in general. I hope that we’ll find ways to ensure that people who need tests and vaccinations are able to access them, as good health is in everyone’s interest.”
Question 4: Will the new vaccines against COVID work for the currently circulating variants?
Dr. Topol: “The XBB.1.5 boosters will be out Sept. 14. They should help versus EG.5.1 and FL.1.5.1. The FL.1.5.1 variant is gaining now.”
Dr. Gut: “In the next several weeks, we expect the newer monovalent XBB-based vaccines to be offered that offer good protection against current circulating COVID variants along with the new Eris variant.”
Dr. Atmar: “The vaccines are expected to induce immune responses to the currently circulating variants, most of which are strains that evolved from the vaccine strain. The vaccine is expected to be most effective in preventing severe illness and will likely be less effective in preventing infection and mild illness.”
Dr. Ray: “Yes, the updated vaccine design has a spike antigen (XBB.1.5) nearly identical to the current dominant variant (EG.5). Even as variants change, the boosters stimulate B cells and T cells to help protect in a way that is safer than getting COVID-19 infection.”
Question 5: Is there anything we should watch out for regarding the BA.2.86 variant in particular?
Dr. Topol: “The scenario could change if there are new functional mutations added to it.”
Dr. Gut: “BA.2.86 is still fairly uncommon and does not have much data to directly make any informed guesses. However, in general, people that have been exposed to more recent mutations of the COVID virus have been shown to have more protection from newer upcoming mutations. It’s fair to guess that people that have not had recent infection from COVID, or have not had a recent booster, are at higher risk for being infected by any XBB- or BA.2-based strains.”
Dr. Atmar: BA.2.86 has been designated as a variant under monitoring. We will want to see whether it becomes more common and if there are any unexpected characteristics associated with infection by this variant.”
Dr. Ray: “It’s still rare, but it’s been seen in geographically dispersed places, so it’s got legs. The question is how effectively it will bypass some of the immunity we’ve gained. T cells are likely to remain protective, because they target so many parts of the virus that change more slowly, but antibodies from B cells to spike protein may have more trouble recognizing BA.2.86, whether those antibodies were made to a vaccine or a prior variant.”
A version of this article first appeared on WebMD.com.
COVID-19 hospitalizations have been on the rise for weeks as summer nears its end, but how concerned should you be? SARS-CoV-2, the virus behind COVID, continues to evolve and surprise us. So COVID transmission, hospitalization, and death rates can be difficult to predict.
, especially now that testing and vaccinations are no longer free of charge.
Question 1: Are you expecting an end-of-summer COVID wave to be substantial?
Eric Topol, MD: “This wave won’t likely be substantial and could be more of a ‘wavelet.’ I’m not thinking that physicians are too concerned,” said Dr. Topol, founder and director of Scripps Research Translational Institute in La Jolla, Calif.
Thomas Gut, DO: “It’s always impossible to predict the severity of COVID waves. Although the virus has generally mutated in ways that favor easier transmission and milder illness, there have been a handful of surprising mutations that were more dangerous and deadly than the preceding strain,” said Dr. Gut, associate chair of medicine at Staten Island University Hospital/Northwell Health in New York.
Robert Atmar, MD: “I’ll start with the caveat that prognosticating for SARS-CoV-2 is a bit hazardous as we remain in unknown territory for some aspects of its epidemiology and evolution,” said Dr. Atmar, a professor of infectious diseases at Baylor College of Medicine in Houston. “It depends on your definition of substantial. We, at least in Houston, are already in the midst of a substantial surge in the burden of infection, at least as monitored through wastewater surveillance. The amount of virus in the wastewater already exceeds the peak level we saw last winter. That said, the increased infection burden has not translated into large increases in hospitalizations for COVID-19. Most persons hospitalized in our hospital are admitted with infection, not for the consequences of infection.”
Stuart Campbell Ray, MD: “It looks like there is a rise in infections, but the proportional rise in hospitalizations from severe cases is lower than in the past, suggesting that folks are protected by the immunity we’ve gained over the past few years through vaccination and prior infections. Of course, we should be thinking about how that applies to each of us – how recently we had a vaccine or COVID-19, and whether we might see more severe infections as immunity wanes,” said Dr. Ray, who is a professor of medicine in the division of infectious diseases at Johns Hopkins University in Baltimore.
Question 2: Is a return to masks or mask mandates coming this fall or winter?
Dr. Topol: “Mandating masks doesn’t work very well, but we may see wide use again if a descendant of [variant] BA.2.86 takes off.”
Dr. Gut: “It’s difficult to predict if there are any mask mandates returning at any point. Ever since the Omicron strains emerged, COVID has been relatively mild, compared to previous strains, so there probably won’t be any plan to start masking in public unless a more deadly strain appears.”
Dr. Atmar: “I do not think we will see a return to mask mandates this fall or winter for a variety of reasons. The primary one is that I don’t think the public will accept mask mandates. However, I think masking can continue to be an adjunctive measure to enhance protection from infection, along with booster vaccination.”
Dr. Ray: “Some people will choose to wear masks during a surge, particularly in situations like commuting where they don’t interfere with what they’re doing. They will wear masks particularly if they want to avoid infection due to concerns about others they care about, disruption of work or travel plans, or concerns about long-term consequences of repeated COVID-19.”
Question 3: Now that COVID testing and vaccinations are no longer free of charge, how might that affect their use?
Dr. Topol: “It was already low, and this will undoubtedly further compromise their uptake.”
Dr. Gut: “I do expect that testing will become less common now that tests are no longer free. I’m sure there will be a lower amount of detection in patients with milder or asymptomatic disease compared to what we had previously.”
Dr. Atmar: “If there are out-of-pocket costs for the SARS-CoV-2 vaccine, or if the administrative paperwork attached to getting a vaccine is increased, the uptake of SARS-CoV-2 vaccines will likely decrease. It will be important to communicate to the populations targeted for vaccination the potential benefits of such vaccination.”
Dr. Ray: “A challenge with COVID-19, all along, has been disparities in access to care, and this will be worse without public support for prevention and testing. This applies to everyone but is especially burdensome for those who are often marginalized in our health care system and society in general. I hope that we’ll find ways to ensure that people who need tests and vaccinations are able to access them, as good health is in everyone’s interest.”
Question 4: Will the new vaccines against COVID work for the currently circulating variants?
Dr. Topol: “The XBB.1.5 boosters will be out Sept. 14. They should help versus EG.5.1 and FL.1.5.1. The FL.1.5.1 variant is gaining now.”
Dr. Gut: “In the next several weeks, we expect the newer monovalent XBB-based vaccines to be offered that offer good protection against current circulating COVID variants along with the new Eris variant.”
Dr. Atmar: “The vaccines are expected to induce immune responses to the currently circulating variants, most of which are strains that evolved from the vaccine strain. The vaccine is expected to be most effective in preventing severe illness and will likely be less effective in preventing infection and mild illness.”
Dr. Ray: “Yes, the updated vaccine design has a spike antigen (XBB.1.5) nearly identical to the current dominant variant (EG.5). Even as variants change, the boosters stimulate B cells and T cells to help protect in a way that is safer than getting COVID-19 infection.”
Question 5: Is there anything we should watch out for regarding the BA.2.86 variant in particular?
Dr. Topol: “The scenario could change if there are new functional mutations added to it.”
Dr. Gut: “BA.2.86 is still fairly uncommon and does not have much data to directly make any informed guesses. However, in general, people that have been exposed to more recent mutations of the COVID virus have been shown to have more protection from newer upcoming mutations. It’s fair to guess that people that have not had recent infection from COVID, or have not had a recent booster, are at higher risk for being infected by any XBB- or BA.2-based strains.”
Dr. Atmar: BA.2.86 has been designated as a variant under monitoring. We will want to see whether it becomes more common and if there are any unexpected characteristics associated with infection by this variant.”
Dr. Ray: “It’s still rare, but it’s been seen in geographically dispersed places, so it’s got legs. The question is how effectively it will bypass some of the immunity we’ve gained. T cells are likely to remain protective, because they target so many parts of the virus that change more slowly, but antibodies from B cells to spike protein may have more trouble recognizing BA.2.86, whether those antibodies were made to a vaccine or a prior variant.”
A version of this article first appeared on WebMD.com.
How to optimize in-hospital antimicrobial prescribing?
Variability in antimicrobial prescribing among hospital-based physicians is not associated with patient characteristics or clinical outcomes, data suggest. The lowest level of such prescribing within each hospital could be considered a target for antimicrobial stewardship, according to the researchers.
In a multicenter study of 124 physicians responsible for more than 124,000 hospitalized patients, the difference in mean prescribing between the highest and lowest quartiles of prescription volume was 15.8 days of treatment per 100 patient-days.
Baseline patient characteristics were similar across the quartiles, and there were no differences in patient outcomes, including in-hospital deaths, hospital length of stay, intensive care unit transfer, and hospital readmission.
Although the investigators expected variation in prescribing, “what surprised us most was the limited association with any differences in clinical outcomes, particularly when it came to the amount of antimicrobials used,” study author Mark T. McIntyre, PharmD, pharmacotherapy specialist at the Sinai Health System in Toronto, told this news organization.
“Importantly, this is not a study that defines quality of care,” he said. “We looked at natural variation in practice and association with outcomes. So, I don’t want clinicians to think, ‘Well, I’m high, therefore I’m bad,’ or, ‘I’m low, therefore I’m good.’
“This is an early explanatory analysis that asks whether this is an opportunity to optimize prescribing in ways we hadn’t thought of before,” he said. “Now that we don’t have an association with higher or lower prescribing and outcomes, we can look at what else is driving that antimicrobial prescribing and what we can do about it. Comfort level, risk tolerance, and social, cultural, and contextual factors all likely play a role.”
The study was published online in the Canadian Medical Association Journal.
Antimicrobial reductions possible
The investigators conducted a retrospective cohort study using the General Medicine Inpatient Initiative database to assess physician-level volume and spectrum of antimicrobial prescribing in adult general medical wards. Four academic hospitals in Toronto were evaluated for the period 2010 to 2019.
The investigators stratified physicians into quartiles by hospital site on the basis of volume of antimicrobial prescribing (specifically, days of therapy per 100 patient-days and antimicrobial-free days) and antibacterial spectrum (modified spectrum score, which assigns a value to each antibacterial agent on the basis of its breadth of coverage).
They also examined potential differences between physician quartiles in patient characteristics, such as age, sex, the Laboratory-Based Acute Physiology Score, discharge diagnosis, and the Charlson Comorbidity Index.
Multilevel modeling allowed the investigators to evaluate the association between clinical outcomes and antimicrobial volume and spectrum.
The primary measure was days of therapy per 100 patient-days.
As noted, the cohort included 124 physicians who were responsible for 124,158 hospital admissions. The median physician-level volume of antimicrobial prescribing was 56.1 days of therapy per 100 patient-days. Patient characteristics were balanced across the quartiles of physician prescribing.
The difference in mean prescribing between physician quartile 4 and quartile 1 was 15.8 days of therapy per 100 patient-days, meaning the median physician in quartile 4 prescribed antimicrobials at a volume that was 30% higher than that of the median physician in quartile 1.
No significant differences were noted for any clinical outcome with regard to quartile of days of therapy, antimicrobial-free days, or modified spectrum score after adjustment for patient-level characteristics.
In addition, no significant differences in the case mix between quartile 4 and quartile 1 were found when the cohort was restricted to patients admitted and discharged by the same most responsible person, nor were differences found in an analysis that was restricted to those without a discharge diagnosis code of palliative care.
In-hospital mortality was higher among patients cared for by prescribers with higher modified spectrum scores (odds ratio, 1.13). “We still can’t fully explain this finding,” Dr. McIntyre acknowledged. “We only saw that in our primary analysis. When we did several sensitivity analyses, that finding didn’t appear.”
The authors concluded, “Ultimately, without discernible benefit in outcomes of patients of physicians who prescribe more frequently, less antimicrobial exposure may be possible, leading to lower risk of antimicrobial resistance.”
Decision-making support
Commenting on the study, Lawrence I. Kaplan, MD, section chief of general internal medicine and associate dean for interprofessional education at the Lewis Katz School of Medicine at Temple University in Philadelphia, said, “Trying to get to the lowest quartile would be a goal, and given that physician characteristics are involved, I think there needs to be much better training in clinical management decision-making: how you come about making a decision based on a diagnosis for a particular patient, in or out of the hospital.” Dr. Kaplan was not involved in the research.
“Clinical decision-making tools that can be plugged into the electronic health record can help,” he suggested. “The tools basically ask if a patient meets certain criteria and then might give a prompt that says, for example, ‘These symptoms are not consistent with bacterial sinusitis. The patient should be treated with decongestants, nasal steroids, et cetera, because antibiotics aren’t appropriate.’
“It’s a bit like checkbox medicine, which a lot of physicians bridle at,” he said. “But if it’s really based on evidence, I think that’s an appropriate use of evidence-based medicine.”
Dr. Kaplan said that more research is needed into the best way to get a physician or any provider to step back and say, “Is this the right decision?” or, “I’m doing this but I’m really on shaky ground. What am I missing?’” He noted that the Society for Medical Decision Making publishes research and resources in this area.
“I love the fact that the paper was authored by an interdisciplinary group,” Dr. Kaplan added. “A pharmacist embedded in the team can, for example, help with treatment decision-making and point out potential drug interactions that prescribers might not be aware of.
“We need to stop practicing medicine siloed, which is what we do a lot of ways, both in the hospital and out of the hospital, because it’s the path of least resistance,” Dr. Kaplan added. “But when we can say, ‘Hey, I have a question about this,’ be it to a computer or a colleague, I would argue that we come up with better care.”
No funding was provided for the study. Dr. McIntyre and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Variability in antimicrobial prescribing among hospital-based physicians is not associated with patient characteristics or clinical outcomes, data suggest. The lowest level of such prescribing within each hospital could be considered a target for antimicrobial stewardship, according to the researchers.
In a multicenter study of 124 physicians responsible for more than 124,000 hospitalized patients, the difference in mean prescribing between the highest and lowest quartiles of prescription volume was 15.8 days of treatment per 100 patient-days.
Baseline patient characteristics were similar across the quartiles, and there were no differences in patient outcomes, including in-hospital deaths, hospital length of stay, intensive care unit transfer, and hospital readmission.
Although the investigators expected variation in prescribing, “what surprised us most was the limited association with any differences in clinical outcomes, particularly when it came to the amount of antimicrobials used,” study author Mark T. McIntyre, PharmD, pharmacotherapy specialist at the Sinai Health System in Toronto, told this news organization.
“Importantly, this is not a study that defines quality of care,” he said. “We looked at natural variation in practice and association with outcomes. So, I don’t want clinicians to think, ‘Well, I’m high, therefore I’m bad,’ or, ‘I’m low, therefore I’m good.’
“This is an early explanatory analysis that asks whether this is an opportunity to optimize prescribing in ways we hadn’t thought of before,” he said. “Now that we don’t have an association with higher or lower prescribing and outcomes, we can look at what else is driving that antimicrobial prescribing and what we can do about it. Comfort level, risk tolerance, and social, cultural, and contextual factors all likely play a role.”
The study was published online in the Canadian Medical Association Journal.
Antimicrobial reductions possible
The investigators conducted a retrospective cohort study using the General Medicine Inpatient Initiative database to assess physician-level volume and spectrum of antimicrobial prescribing in adult general medical wards. Four academic hospitals in Toronto were evaluated for the period 2010 to 2019.
The investigators stratified physicians into quartiles by hospital site on the basis of volume of antimicrobial prescribing (specifically, days of therapy per 100 patient-days and antimicrobial-free days) and antibacterial spectrum (modified spectrum score, which assigns a value to each antibacterial agent on the basis of its breadth of coverage).
They also examined potential differences between physician quartiles in patient characteristics, such as age, sex, the Laboratory-Based Acute Physiology Score, discharge diagnosis, and the Charlson Comorbidity Index.
Multilevel modeling allowed the investigators to evaluate the association between clinical outcomes and antimicrobial volume and spectrum.
The primary measure was days of therapy per 100 patient-days.
As noted, the cohort included 124 physicians who were responsible for 124,158 hospital admissions. The median physician-level volume of antimicrobial prescribing was 56.1 days of therapy per 100 patient-days. Patient characteristics were balanced across the quartiles of physician prescribing.
The difference in mean prescribing between physician quartile 4 and quartile 1 was 15.8 days of therapy per 100 patient-days, meaning the median physician in quartile 4 prescribed antimicrobials at a volume that was 30% higher than that of the median physician in quartile 1.
No significant differences were noted for any clinical outcome with regard to quartile of days of therapy, antimicrobial-free days, or modified spectrum score after adjustment for patient-level characteristics.
In addition, no significant differences in the case mix between quartile 4 and quartile 1 were found when the cohort was restricted to patients admitted and discharged by the same most responsible person, nor were differences found in an analysis that was restricted to those without a discharge diagnosis code of palliative care.
In-hospital mortality was higher among patients cared for by prescribers with higher modified spectrum scores (odds ratio, 1.13). “We still can’t fully explain this finding,” Dr. McIntyre acknowledged. “We only saw that in our primary analysis. When we did several sensitivity analyses, that finding didn’t appear.”
The authors concluded, “Ultimately, without discernible benefit in outcomes of patients of physicians who prescribe more frequently, less antimicrobial exposure may be possible, leading to lower risk of antimicrobial resistance.”
Decision-making support
Commenting on the study, Lawrence I. Kaplan, MD, section chief of general internal medicine and associate dean for interprofessional education at the Lewis Katz School of Medicine at Temple University in Philadelphia, said, “Trying to get to the lowest quartile would be a goal, and given that physician characteristics are involved, I think there needs to be much better training in clinical management decision-making: how you come about making a decision based on a diagnosis for a particular patient, in or out of the hospital.” Dr. Kaplan was not involved in the research.
“Clinical decision-making tools that can be plugged into the electronic health record can help,” he suggested. “The tools basically ask if a patient meets certain criteria and then might give a prompt that says, for example, ‘These symptoms are not consistent with bacterial sinusitis. The patient should be treated with decongestants, nasal steroids, et cetera, because antibiotics aren’t appropriate.’
“It’s a bit like checkbox medicine, which a lot of physicians bridle at,” he said. “But if it’s really based on evidence, I think that’s an appropriate use of evidence-based medicine.”
Dr. Kaplan said that more research is needed into the best way to get a physician or any provider to step back and say, “Is this the right decision?” or, “I’m doing this but I’m really on shaky ground. What am I missing?’” He noted that the Society for Medical Decision Making publishes research and resources in this area.
“I love the fact that the paper was authored by an interdisciplinary group,” Dr. Kaplan added. “A pharmacist embedded in the team can, for example, help with treatment decision-making and point out potential drug interactions that prescribers might not be aware of.
“We need to stop practicing medicine siloed, which is what we do a lot of ways, both in the hospital and out of the hospital, because it’s the path of least resistance,” Dr. Kaplan added. “But when we can say, ‘Hey, I have a question about this,’ be it to a computer or a colleague, I would argue that we come up with better care.”
No funding was provided for the study. Dr. McIntyre and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Variability in antimicrobial prescribing among hospital-based physicians is not associated with patient characteristics or clinical outcomes, data suggest. The lowest level of such prescribing within each hospital could be considered a target for antimicrobial stewardship, according to the researchers.
In a multicenter study of 124 physicians responsible for more than 124,000 hospitalized patients, the difference in mean prescribing between the highest and lowest quartiles of prescription volume was 15.8 days of treatment per 100 patient-days.
Baseline patient characteristics were similar across the quartiles, and there were no differences in patient outcomes, including in-hospital deaths, hospital length of stay, intensive care unit transfer, and hospital readmission.
Although the investigators expected variation in prescribing, “what surprised us most was the limited association with any differences in clinical outcomes, particularly when it came to the amount of antimicrobials used,” study author Mark T. McIntyre, PharmD, pharmacotherapy specialist at the Sinai Health System in Toronto, told this news organization.
“Importantly, this is not a study that defines quality of care,” he said. “We looked at natural variation in practice and association with outcomes. So, I don’t want clinicians to think, ‘Well, I’m high, therefore I’m bad,’ or, ‘I’m low, therefore I’m good.’
“This is an early explanatory analysis that asks whether this is an opportunity to optimize prescribing in ways we hadn’t thought of before,” he said. “Now that we don’t have an association with higher or lower prescribing and outcomes, we can look at what else is driving that antimicrobial prescribing and what we can do about it. Comfort level, risk tolerance, and social, cultural, and contextual factors all likely play a role.”
The study was published online in the Canadian Medical Association Journal.
Antimicrobial reductions possible
The investigators conducted a retrospective cohort study using the General Medicine Inpatient Initiative database to assess physician-level volume and spectrum of antimicrobial prescribing in adult general medical wards. Four academic hospitals in Toronto were evaluated for the period 2010 to 2019.
The investigators stratified physicians into quartiles by hospital site on the basis of volume of antimicrobial prescribing (specifically, days of therapy per 100 patient-days and antimicrobial-free days) and antibacterial spectrum (modified spectrum score, which assigns a value to each antibacterial agent on the basis of its breadth of coverage).
They also examined potential differences between physician quartiles in patient characteristics, such as age, sex, the Laboratory-Based Acute Physiology Score, discharge diagnosis, and the Charlson Comorbidity Index.
Multilevel modeling allowed the investigators to evaluate the association between clinical outcomes and antimicrobial volume and spectrum.
The primary measure was days of therapy per 100 patient-days.
As noted, the cohort included 124 physicians who were responsible for 124,158 hospital admissions. The median physician-level volume of antimicrobial prescribing was 56.1 days of therapy per 100 patient-days. Patient characteristics were balanced across the quartiles of physician prescribing.
The difference in mean prescribing between physician quartile 4 and quartile 1 was 15.8 days of therapy per 100 patient-days, meaning the median physician in quartile 4 prescribed antimicrobials at a volume that was 30% higher than that of the median physician in quartile 1.
No significant differences were noted for any clinical outcome with regard to quartile of days of therapy, antimicrobial-free days, or modified spectrum score after adjustment for patient-level characteristics.
In addition, no significant differences in the case mix between quartile 4 and quartile 1 were found when the cohort was restricted to patients admitted and discharged by the same most responsible person, nor were differences found in an analysis that was restricted to those without a discharge diagnosis code of palliative care.
In-hospital mortality was higher among patients cared for by prescribers with higher modified spectrum scores (odds ratio, 1.13). “We still can’t fully explain this finding,” Dr. McIntyre acknowledged. “We only saw that in our primary analysis. When we did several sensitivity analyses, that finding didn’t appear.”
The authors concluded, “Ultimately, without discernible benefit in outcomes of patients of physicians who prescribe more frequently, less antimicrobial exposure may be possible, leading to lower risk of antimicrobial resistance.”
Decision-making support
Commenting on the study, Lawrence I. Kaplan, MD, section chief of general internal medicine and associate dean for interprofessional education at the Lewis Katz School of Medicine at Temple University in Philadelphia, said, “Trying to get to the lowest quartile would be a goal, and given that physician characteristics are involved, I think there needs to be much better training in clinical management decision-making: how you come about making a decision based on a diagnosis for a particular patient, in or out of the hospital.” Dr. Kaplan was not involved in the research.
“Clinical decision-making tools that can be plugged into the electronic health record can help,” he suggested. “The tools basically ask if a patient meets certain criteria and then might give a prompt that says, for example, ‘These symptoms are not consistent with bacterial sinusitis. The patient should be treated with decongestants, nasal steroids, et cetera, because antibiotics aren’t appropriate.’
“It’s a bit like checkbox medicine, which a lot of physicians bridle at,” he said. “But if it’s really based on evidence, I think that’s an appropriate use of evidence-based medicine.”
Dr. Kaplan said that more research is needed into the best way to get a physician or any provider to step back and say, “Is this the right decision?” or, “I’m doing this but I’m really on shaky ground. What am I missing?’” He noted that the Society for Medical Decision Making publishes research and resources in this area.
“I love the fact that the paper was authored by an interdisciplinary group,” Dr. Kaplan added. “A pharmacist embedded in the team can, for example, help with treatment decision-making and point out potential drug interactions that prescribers might not be aware of.
“We need to stop practicing medicine siloed, which is what we do a lot of ways, both in the hospital and out of the hospital, because it’s the path of least resistance,” Dr. Kaplan added. “But when we can say, ‘Hey, I have a question about this,’ be it to a computer or a colleague, I would argue that we come up with better care.”
No funding was provided for the study. Dr. McIntyre and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FDA to step up oversight of cosmetics, assess ‘forever chemicals’
They are also preparing to assess potential risks of so-called forever chemicals in these products.
The Food and Drug Administration last year gained new authority over cosmetics when Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by adding this bill to a December budget package.
“On average, consumers in the U.S. use six to 12 cosmetics products daily. But, until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced,” Namandjé Bumpus, PhD, FDA’s chief scientist, said in a press release.
In the statement, the FDA announced the release of a draft guidance document that is intended to help companies comply with the transparency requirements slated to kick in this December. The agency is accepting comments on this draft guidance through Sept. 7.
“Later this year, registration and listing of cosmetic product facilities and products will become a requirement, making information about cosmetic products, including the ingredients used in products and the facilities where they are produced, readily available to the agency,” Dr. Bumpus said.
The products, according to the FDA statement, include makeup, nail polishes, shaving creams, other grooming products, perfumes, face and body cleansers, hair products, moisturizers, and other skin care items.
MoCRA “represents a sea change in how FDA regulates the cosmetics industry,” attorneys Frederick R. Ball, Alyson Walker Lotman, and Kelly A. Bonner, wrote in an article for the Food and Drug Law Institute published in spring 2023.
The FDA has called the MoCRA law “the most significant expansion” of its authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic Act was passed in 1938.
The agency is in the process of expanding its staff to carry out newly authorized duties, including the tracking of adverse events. The FDA budget request for fiscal 2024, which begins Oct. 1, seeks $5 million for work needed to implement MoCRA.
PFAS, or ‘forever chemicals’
Some of the requested FDA funding is intended to prepare the agency to assess the use of per-and polyfluoroalkyl substances (PFAS) in cosmetics.
MoCRA sets a 3-year deadline for the FDA to issue an assessment of the use and potential risks of PFAS in cosmetics products. PFAS are sometimes added as ingredients in some cosmetic products, including lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara, according to the FDA. Sometimes the presence of PFAS in cosmetics is unintentional and is the result of impurities in raw materials or is due to the breakdown of ingredients, the FDA said.
The FDA’s website says that so far, the available research doesn’t allow for “definitive conclusions about the potential health risks of PFAS in cosmetics.”
The Centers for Disease Control and Prevention has stated that research has suggested potential links between high levels of certain PFAS, in general, with increased cholesterol levels, changes in liver enzyme levels, increased risk of hypertension or preeclampsia in pregnant women, and increased risk of kidney or testicular cancer.
PFAS compounds often are used to resist grease, oil, water, and heat in industrial settings. They are used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they can reduce friction, according to a National Academies of Sciences, Engineering, and Medicine report on PFAS that was issued last year.
PFAS are known as “forever chemicals” because they contain a carbon-fluorine bond, which does not break naturally. Even when PFAS are transformed in the body, they can assume other forms of PFAS that preserve the troublesome carbon-fluorine bond. With PFAS, the human body is confronted with a substance it doesn’t have the tools to process.
This is in contrast to proteins and carbohydrates, which are in a sense prepackaged for relatively easy disassembly in the human body. Many of these compounds have weak links that enzymes and stomach acid can take apart, such as sulfur-to-sulfur (disulfide) bonds. That’s why protein-based biotech drugs are injected instead of administered as pills. The ultimate goal of this digestion is for the body to gain energy from these compounds.
But with PFAS, the body faces the challenge of carbon-fluorine bonds that are very hard to break down, and there is no payoff for these efforts, Graham F. Peaslee, PhD, professor of physics at the University of Notre Dame (Indiana), told this news organization.
“Nothing will naturally eat it because when you break the bond, it’s like eating celery,” he said. “You use more calories to eat the celery than you gain back from it.”
Interest from a U.S. senator
Dr. Peaslee was one of the authors of a 2021 article about PFAS in cosmetics that appeared in the journal Environmental Science and Technology Letters.
In the article, Dr. Peaslee and colleagues reported on their screening of 231 cosmetic products purchased in the United States and Canada using particle-induced gamma-ray emission spectroscopy. They found cases of undisclosed PFAS in cosmetic products. Foundations, mascaras, and lip products were noted as being especially problematic.
Sen. Susan Collins (R-ME) cited Dr. Peaslee’s article in a 2021 floor speech as she argued for having the FDA ban the intentional addition of PFAS to cosmetics.
“The findings of this study are particularly alarming, as many of these products are subject to direct human exposure,” Sen. Collins said. “For example, lipstick is often inadvertently ingested, and mascara is sometimes absorbed through tear ducts.”
In addition, workers at cosmetics plants may be exposed to PFAS and discarded cosmetics that have these compounds, which could potentially contaminate drinking water, Sen. Collins said. In 2021, she introduced legislation seeking a ban on PFAS that are intentionally added to cosmetics. That legislation did not advance through the Senate.
But the Senate Appropriations Committee, on which Sen. Collins is the ranking Republican, wants the FDA to keep a ban on PFAS in mind.
The Senate Agriculture Appropriations subcommittee, which oversees the FDA’s budget, raised the issue of PFAS and cosmetics in a June report. The FDA should develop a plan outlining research needed to inform “regulatory decision making, including potential development of a proposed rule to ban intentionally added PFAS substances in cosmetics,” the subcommittee said.
A version of this article first appeared on Medscape.com.
They are also preparing to assess potential risks of so-called forever chemicals in these products.
The Food and Drug Administration last year gained new authority over cosmetics when Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by adding this bill to a December budget package.
“On average, consumers in the U.S. use six to 12 cosmetics products daily. But, until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced,” Namandjé Bumpus, PhD, FDA’s chief scientist, said in a press release.
In the statement, the FDA announced the release of a draft guidance document that is intended to help companies comply with the transparency requirements slated to kick in this December. The agency is accepting comments on this draft guidance through Sept. 7.
“Later this year, registration and listing of cosmetic product facilities and products will become a requirement, making information about cosmetic products, including the ingredients used in products and the facilities where they are produced, readily available to the agency,” Dr. Bumpus said.
The products, according to the FDA statement, include makeup, nail polishes, shaving creams, other grooming products, perfumes, face and body cleansers, hair products, moisturizers, and other skin care items.
MoCRA “represents a sea change in how FDA regulates the cosmetics industry,” attorneys Frederick R. Ball, Alyson Walker Lotman, and Kelly A. Bonner, wrote in an article for the Food and Drug Law Institute published in spring 2023.
The FDA has called the MoCRA law “the most significant expansion” of its authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic Act was passed in 1938.
The agency is in the process of expanding its staff to carry out newly authorized duties, including the tracking of adverse events. The FDA budget request for fiscal 2024, which begins Oct. 1, seeks $5 million for work needed to implement MoCRA.
PFAS, or ‘forever chemicals’
Some of the requested FDA funding is intended to prepare the agency to assess the use of per-and polyfluoroalkyl substances (PFAS) in cosmetics.
MoCRA sets a 3-year deadline for the FDA to issue an assessment of the use and potential risks of PFAS in cosmetics products. PFAS are sometimes added as ingredients in some cosmetic products, including lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara, according to the FDA. Sometimes the presence of PFAS in cosmetics is unintentional and is the result of impurities in raw materials or is due to the breakdown of ingredients, the FDA said.
The FDA’s website says that so far, the available research doesn’t allow for “definitive conclusions about the potential health risks of PFAS in cosmetics.”
The Centers for Disease Control and Prevention has stated that research has suggested potential links between high levels of certain PFAS, in general, with increased cholesterol levels, changes in liver enzyme levels, increased risk of hypertension or preeclampsia in pregnant women, and increased risk of kidney or testicular cancer.
PFAS compounds often are used to resist grease, oil, water, and heat in industrial settings. They are used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they can reduce friction, according to a National Academies of Sciences, Engineering, and Medicine report on PFAS that was issued last year.
PFAS are known as “forever chemicals” because they contain a carbon-fluorine bond, which does not break naturally. Even when PFAS are transformed in the body, they can assume other forms of PFAS that preserve the troublesome carbon-fluorine bond. With PFAS, the human body is confronted with a substance it doesn’t have the tools to process.
This is in contrast to proteins and carbohydrates, which are in a sense prepackaged for relatively easy disassembly in the human body. Many of these compounds have weak links that enzymes and stomach acid can take apart, such as sulfur-to-sulfur (disulfide) bonds. That’s why protein-based biotech drugs are injected instead of administered as pills. The ultimate goal of this digestion is for the body to gain energy from these compounds.
But with PFAS, the body faces the challenge of carbon-fluorine bonds that are very hard to break down, and there is no payoff for these efforts, Graham F. Peaslee, PhD, professor of physics at the University of Notre Dame (Indiana), told this news organization.
“Nothing will naturally eat it because when you break the bond, it’s like eating celery,” he said. “You use more calories to eat the celery than you gain back from it.”
Interest from a U.S. senator
Dr. Peaslee was one of the authors of a 2021 article about PFAS in cosmetics that appeared in the journal Environmental Science and Technology Letters.
In the article, Dr. Peaslee and colleagues reported on their screening of 231 cosmetic products purchased in the United States and Canada using particle-induced gamma-ray emission spectroscopy. They found cases of undisclosed PFAS in cosmetic products. Foundations, mascaras, and lip products were noted as being especially problematic.
Sen. Susan Collins (R-ME) cited Dr. Peaslee’s article in a 2021 floor speech as she argued for having the FDA ban the intentional addition of PFAS to cosmetics.
“The findings of this study are particularly alarming, as many of these products are subject to direct human exposure,” Sen. Collins said. “For example, lipstick is often inadvertently ingested, and mascara is sometimes absorbed through tear ducts.”
In addition, workers at cosmetics plants may be exposed to PFAS and discarded cosmetics that have these compounds, which could potentially contaminate drinking water, Sen. Collins said. In 2021, she introduced legislation seeking a ban on PFAS that are intentionally added to cosmetics. That legislation did not advance through the Senate.
But the Senate Appropriations Committee, on which Sen. Collins is the ranking Republican, wants the FDA to keep a ban on PFAS in mind.
The Senate Agriculture Appropriations subcommittee, which oversees the FDA’s budget, raised the issue of PFAS and cosmetics in a June report. The FDA should develop a plan outlining research needed to inform “regulatory decision making, including potential development of a proposed rule to ban intentionally added PFAS substances in cosmetics,” the subcommittee said.
A version of this article first appeared on Medscape.com.
They are also preparing to assess potential risks of so-called forever chemicals in these products.
The Food and Drug Administration last year gained new authority over cosmetics when Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by adding this bill to a December budget package.
“On average, consumers in the U.S. use six to 12 cosmetics products daily. But, until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced,” Namandjé Bumpus, PhD, FDA’s chief scientist, said in a press release.
In the statement, the FDA announced the release of a draft guidance document that is intended to help companies comply with the transparency requirements slated to kick in this December. The agency is accepting comments on this draft guidance through Sept. 7.
“Later this year, registration and listing of cosmetic product facilities and products will become a requirement, making information about cosmetic products, including the ingredients used in products and the facilities where they are produced, readily available to the agency,” Dr. Bumpus said.
The products, according to the FDA statement, include makeup, nail polishes, shaving creams, other grooming products, perfumes, face and body cleansers, hair products, moisturizers, and other skin care items.
MoCRA “represents a sea change in how FDA regulates the cosmetics industry,” attorneys Frederick R. Ball, Alyson Walker Lotman, and Kelly A. Bonner, wrote in an article for the Food and Drug Law Institute published in spring 2023.
The FDA has called the MoCRA law “the most significant expansion” of its authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic Act was passed in 1938.
The agency is in the process of expanding its staff to carry out newly authorized duties, including the tracking of adverse events. The FDA budget request for fiscal 2024, which begins Oct. 1, seeks $5 million for work needed to implement MoCRA.
PFAS, or ‘forever chemicals’
Some of the requested FDA funding is intended to prepare the agency to assess the use of per-and polyfluoroalkyl substances (PFAS) in cosmetics.
MoCRA sets a 3-year deadline for the FDA to issue an assessment of the use and potential risks of PFAS in cosmetics products. PFAS are sometimes added as ingredients in some cosmetic products, including lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara, according to the FDA. Sometimes the presence of PFAS in cosmetics is unintentional and is the result of impurities in raw materials or is due to the breakdown of ingredients, the FDA said.
The FDA’s website says that so far, the available research doesn’t allow for “definitive conclusions about the potential health risks of PFAS in cosmetics.”
The Centers for Disease Control and Prevention has stated that research has suggested potential links between high levels of certain PFAS, in general, with increased cholesterol levels, changes in liver enzyme levels, increased risk of hypertension or preeclampsia in pregnant women, and increased risk of kidney or testicular cancer.
PFAS compounds often are used to resist grease, oil, water, and heat in industrial settings. They are used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they can reduce friction, according to a National Academies of Sciences, Engineering, and Medicine report on PFAS that was issued last year.
PFAS are known as “forever chemicals” because they contain a carbon-fluorine bond, which does not break naturally. Even when PFAS are transformed in the body, they can assume other forms of PFAS that preserve the troublesome carbon-fluorine bond. With PFAS, the human body is confronted with a substance it doesn’t have the tools to process.
This is in contrast to proteins and carbohydrates, which are in a sense prepackaged for relatively easy disassembly in the human body. Many of these compounds have weak links that enzymes and stomach acid can take apart, such as sulfur-to-sulfur (disulfide) bonds. That’s why protein-based biotech drugs are injected instead of administered as pills. The ultimate goal of this digestion is for the body to gain energy from these compounds.
But with PFAS, the body faces the challenge of carbon-fluorine bonds that are very hard to break down, and there is no payoff for these efforts, Graham F. Peaslee, PhD, professor of physics at the University of Notre Dame (Indiana), told this news organization.
“Nothing will naturally eat it because when you break the bond, it’s like eating celery,” he said. “You use more calories to eat the celery than you gain back from it.”
Interest from a U.S. senator
Dr. Peaslee was one of the authors of a 2021 article about PFAS in cosmetics that appeared in the journal Environmental Science and Technology Letters.
In the article, Dr. Peaslee and colleagues reported on their screening of 231 cosmetic products purchased in the United States and Canada using particle-induced gamma-ray emission spectroscopy. They found cases of undisclosed PFAS in cosmetic products. Foundations, mascaras, and lip products were noted as being especially problematic.
Sen. Susan Collins (R-ME) cited Dr. Peaslee’s article in a 2021 floor speech as she argued for having the FDA ban the intentional addition of PFAS to cosmetics.
“The findings of this study are particularly alarming, as many of these products are subject to direct human exposure,” Sen. Collins said. “For example, lipstick is often inadvertently ingested, and mascara is sometimes absorbed through tear ducts.”
In addition, workers at cosmetics plants may be exposed to PFAS and discarded cosmetics that have these compounds, which could potentially contaminate drinking water, Sen. Collins said. In 2021, she introduced legislation seeking a ban on PFAS that are intentionally added to cosmetics. That legislation did not advance through the Senate.
But the Senate Appropriations Committee, on which Sen. Collins is the ranking Republican, wants the FDA to keep a ban on PFAS in mind.
The Senate Agriculture Appropriations subcommittee, which oversees the FDA’s budget, raised the issue of PFAS and cosmetics in a June report. The FDA should develop a plan outlining research needed to inform “regulatory decision making, including potential development of a proposed rule to ban intentionally added PFAS substances in cosmetics,” the subcommittee said.
A version of this article first appeared on Medscape.com.
Gender-affirming surgeries nearly tripled between 2016 and 2019: Study
a new study published in JAMA Network Open found.
Breast and chest surgeries were the most common procedures performed, and the number of surgical procedures carried out increased with age. The researchers said that, in addition to legal shifts, the established safety of the surgeries and resulting increase in quality of life may also help explain the increase.
“The point of this is to raise awareness and to really document the patterns of care in the United States,” said Jason Wright, MD, an associate professor at Columbia University, New York. “We hope that people understand that these procedures are being performed more commonly and they’re out there.”
A study published in 2022 in JAMA Pediatrics found that the number of chest reconstruction surgeries among U.S. adolescents rose fourfold between 2016 and 2019.
The new study included data from 2016 to 2020 in the Nationwide Ambulatory Surgery Sample and the National Inpatient Sample. More than 48,000 patients with diagnosis codes for gender identity disorder, transsexualism, or a personal history of sex reassignment were identified. Age ranges were grouped as 12-18 (7.7%), 19-30 (52.3%), and 31-40 (21.8%).
The number of gender-affirming procedures rose from 4,552 in 2016 to a peak of 13,011 in 2019. (A slight decline to 12,818 procedures in 2020 was attributed to the COVID-19 pandemic.) The surgeries were grouped into three categories: breast and chest procedures, which occurred in 56.6% of patients; genital reconstructive surgeries (35.1%), and other facial cosmetic procedures (13.9%).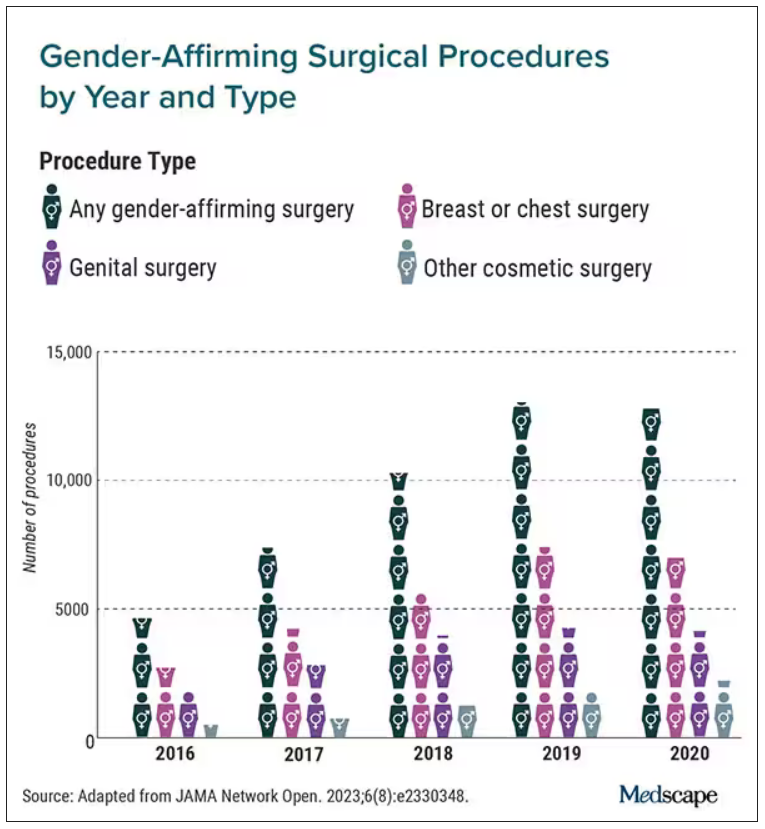
“We really wanted to try to make this as representative as we could,” Dr. Wright said. “I think this is really the best estimates that are available to date.”
Chest and breast procedures made up a higher percentage of surgeries in younger patients, while genital surgical procedures made up a higher percentage in older patients. For example, patients aged 19-30 made up 59.1% of breast or chest surgeries and 44.2% of genital surgeries. However, those aged 31-40 accounted for 26.2% of genital surgeries and 18.1% of breast or chest surgeries. For ages 41-50, the spread was more than double, accounting for 12.8% of genital surgeries and only 6.1% of breast or chest surgeries, according to the researchers.
Undocumented uptick
In addition to more inclusive health insurance, Dr. Wright said the increase in these procedures can also be attributed to studies showing their safety and the long-term association with high patient satisfaction.
Kevin Wang, MD, medical director of Providence–Swedish Health Services’ LGBTQIA+ program in Seattle, agreed that changes in health insurance coverage for gender-affirming surgery likely account in part for their increase. But he added that more clinicians are performing these procedures.
He said gender-affirming surgeries improve quality of life for the people who undergo them. The American Academy of Pediatrics has said it would be conducting a thorough review of the effects of transgender care on youth. A 2018 policy statement from the group said transgender youth should “have access to comprehensive, gender-affirming, and developmentally appropriate health care that is provided in a safe and inclusive clinical space.”
Dr. Wright cited several limitations to his group’s study that may result in the undercapture of transgender individuals and gender-affirming surgery; in particular, while the study captured inpatient and ambulatory surgical procedures in large, nationwide datasets, a small number of the procedures could have been performed in other settings.
Guiding a patient through gender-affirming care and surgical procedures can be an arduous process, including understanding their goals, using hormone therapy, and making referrals to specialists. Dr. Wang said he works to maximize his patients’ physical, mental, and emotional health, and helps them understand the risks.
He cited the double standard of a cisgender woman wanting breast augmentation without justification, but someone who identifies as transgender has many more boxes to check – for example, seeing a behavior health specialist to demonstrate they understand the risks and securing a letter of support from their primary care physician to undergo a similar procedure.
“It’s just interesting how the transgender community has to jump through so many more barriers and hoops for affirming, lifesaving procedures where you have other people who are doing it for aesthetic purposes and do not require any type of authorization,” Dr. Wang said.
Dr. Wright said he hopes the findings call attention to the need for more professionals working in the gender-affirming care field.
“I think for the medical community, it’s important to raise the idea that these procedures are becoming more common,” Dr. Wright said. “We are going to need specialists who have expertise in transgender care and surgeons who have the ability to perform these operations. Hopefully, this sheds light on the resources that are going to be required to care for these patients going forward.”
Dr. Wright reported receiving grants from Merck and personal fees from UpToDate outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
a new study published in JAMA Network Open found.
Breast and chest surgeries were the most common procedures performed, and the number of surgical procedures carried out increased with age. The researchers said that, in addition to legal shifts, the established safety of the surgeries and resulting increase in quality of life may also help explain the increase.
“The point of this is to raise awareness and to really document the patterns of care in the United States,” said Jason Wright, MD, an associate professor at Columbia University, New York. “We hope that people understand that these procedures are being performed more commonly and they’re out there.”
A study published in 2022 in JAMA Pediatrics found that the number of chest reconstruction surgeries among U.S. adolescents rose fourfold between 2016 and 2019.
The new study included data from 2016 to 2020 in the Nationwide Ambulatory Surgery Sample and the National Inpatient Sample. More than 48,000 patients with diagnosis codes for gender identity disorder, transsexualism, or a personal history of sex reassignment were identified. Age ranges were grouped as 12-18 (7.7%), 19-30 (52.3%), and 31-40 (21.8%).
The number of gender-affirming procedures rose from 4,552 in 2016 to a peak of 13,011 in 2019. (A slight decline to 12,818 procedures in 2020 was attributed to the COVID-19 pandemic.) The surgeries were grouped into three categories: breast and chest procedures, which occurred in 56.6% of patients; genital reconstructive surgeries (35.1%), and other facial cosmetic procedures (13.9%).
“We really wanted to try to make this as representative as we could,” Dr. Wright said. “I think this is really the best estimates that are available to date.”
Chest and breast procedures made up a higher percentage of surgeries in younger patients, while genital surgical procedures made up a higher percentage in older patients. For example, patients aged 19-30 made up 59.1% of breast or chest surgeries and 44.2% of genital surgeries. However, those aged 31-40 accounted for 26.2% of genital surgeries and 18.1% of breast or chest surgeries. For ages 41-50, the spread was more than double, accounting for 12.8% of genital surgeries and only 6.1% of breast or chest surgeries, according to the researchers.
Undocumented uptick
In addition to more inclusive health insurance, Dr. Wright said the increase in these procedures can also be attributed to studies showing their safety and the long-term association with high patient satisfaction.
Kevin Wang, MD, medical director of Providence–Swedish Health Services’ LGBTQIA+ program in Seattle, agreed that changes in health insurance coverage for gender-affirming surgery likely account in part for their increase. But he added that more clinicians are performing these procedures.
He said gender-affirming surgeries improve quality of life for the people who undergo them. The American Academy of Pediatrics has said it would be conducting a thorough review of the effects of transgender care on youth. A 2018 policy statement from the group said transgender youth should “have access to comprehensive, gender-affirming, and developmentally appropriate health care that is provided in a safe and inclusive clinical space.”
Dr. Wright cited several limitations to his group’s study that may result in the undercapture of transgender individuals and gender-affirming surgery; in particular, while the study captured inpatient and ambulatory surgical procedures in large, nationwide datasets, a small number of the procedures could have been performed in other settings.
Guiding a patient through gender-affirming care and surgical procedures can be an arduous process, including understanding their goals, using hormone therapy, and making referrals to specialists. Dr. Wang said he works to maximize his patients’ physical, mental, and emotional health, and helps them understand the risks.
He cited the double standard of a cisgender woman wanting breast augmentation without justification, but someone who identifies as transgender has many more boxes to check – for example, seeing a behavior health specialist to demonstrate they understand the risks and securing a letter of support from their primary care physician to undergo a similar procedure.
“It’s just interesting how the transgender community has to jump through so many more barriers and hoops for affirming, lifesaving procedures where you have other people who are doing it for aesthetic purposes and do not require any type of authorization,” Dr. Wang said.
Dr. Wright said he hopes the findings call attention to the need for more professionals working in the gender-affirming care field.
“I think for the medical community, it’s important to raise the idea that these procedures are becoming more common,” Dr. Wright said. “We are going to need specialists who have expertise in transgender care and surgeons who have the ability to perform these operations. Hopefully, this sheds light on the resources that are going to be required to care for these patients going forward.”
Dr. Wright reported receiving grants from Merck and personal fees from UpToDate outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
a new study published in JAMA Network Open found.
Breast and chest surgeries were the most common procedures performed, and the number of surgical procedures carried out increased with age. The researchers said that, in addition to legal shifts, the established safety of the surgeries and resulting increase in quality of life may also help explain the increase.
“The point of this is to raise awareness and to really document the patterns of care in the United States,” said Jason Wright, MD, an associate professor at Columbia University, New York. “We hope that people understand that these procedures are being performed more commonly and they’re out there.”
A study published in 2022 in JAMA Pediatrics found that the number of chest reconstruction surgeries among U.S. adolescents rose fourfold between 2016 and 2019.
The new study included data from 2016 to 2020 in the Nationwide Ambulatory Surgery Sample and the National Inpatient Sample. More than 48,000 patients with diagnosis codes for gender identity disorder, transsexualism, or a personal history of sex reassignment were identified. Age ranges were grouped as 12-18 (7.7%), 19-30 (52.3%), and 31-40 (21.8%).
The number of gender-affirming procedures rose from 4,552 in 2016 to a peak of 13,011 in 2019. (A slight decline to 12,818 procedures in 2020 was attributed to the COVID-19 pandemic.) The surgeries were grouped into three categories: breast and chest procedures, which occurred in 56.6% of patients; genital reconstructive surgeries (35.1%), and other facial cosmetic procedures (13.9%).
“We really wanted to try to make this as representative as we could,” Dr. Wright said. “I think this is really the best estimates that are available to date.”
Chest and breast procedures made up a higher percentage of surgeries in younger patients, while genital surgical procedures made up a higher percentage in older patients. For example, patients aged 19-30 made up 59.1% of breast or chest surgeries and 44.2% of genital surgeries. However, those aged 31-40 accounted for 26.2% of genital surgeries and 18.1% of breast or chest surgeries. For ages 41-50, the spread was more than double, accounting for 12.8% of genital surgeries and only 6.1% of breast or chest surgeries, according to the researchers.
Undocumented uptick
In addition to more inclusive health insurance, Dr. Wright said the increase in these procedures can also be attributed to studies showing their safety and the long-term association with high patient satisfaction.
Kevin Wang, MD, medical director of Providence–Swedish Health Services’ LGBTQIA+ program in Seattle, agreed that changes in health insurance coverage for gender-affirming surgery likely account in part for their increase. But he added that more clinicians are performing these procedures.
He said gender-affirming surgeries improve quality of life for the people who undergo them. The American Academy of Pediatrics has said it would be conducting a thorough review of the effects of transgender care on youth. A 2018 policy statement from the group said transgender youth should “have access to comprehensive, gender-affirming, and developmentally appropriate health care that is provided in a safe and inclusive clinical space.”
Dr. Wright cited several limitations to his group’s study that may result in the undercapture of transgender individuals and gender-affirming surgery; in particular, while the study captured inpatient and ambulatory surgical procedures in large, nationwide datasets, a small number of the procedures could have been performed in other settings.
Guiding a patient through gender-affirming care and surgical procedures can be an arduous process, including understanding their goals, using hormone therapy, and making referrals to specialists. Dr. Wang said he works to maximize his patients’ physical, mental, and emotional health, and helps them understand the risks.
He cited the double standard of a cisgender woman wanting breast augmentation without justification, but someone who identifies as transgender has many more boxes to check – for example, seeing a behavior health specialist to demonstrate they understand the risks and securing a letter of support from their primary care physician to undergo a similar procedure.
“It’s just interesting how the transgender community has to jump through so many more barriers and hoops for affirming, lifesaving procedures where you have other people who are doing it for aesthetic purposes and do not require any type of authorization,” Dr. Wang said.
Dr. Wright said he hopes the findings call attention to the need for more professionals working in the gender-affirming care field.
“I think for the medical community, it’s important to raise the idea that these procedures are becoming more common,” Dr. Wright said. “We are going to need specialists who have expertise in transgender care and surgeons who have the ability to perform these operations. Hopefully, this sheds light on the resources that are going to be required to care for these patients going forward.”
Dr. Wright reported receiving grants from Merck and personal fees from UpToDate outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Medicare announces 10 drugs targeted for price cuts in 2026
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
CTE common among young athletes in largest brain donor study
Analysis of brain tissue from athletes who were exposed to RHIs and died before the age of 30 revealed neuropathological evidence of shrinkage of the brain and microscopic changes that indicate a breach of the blood-brain barrier. The case series also identified the first known American female athlete with CTE.
Nearly all of those with CTE had a mild form of the disease and 71% played only at the amateur level in youth, high school, or college sports.
“A lot of people think CTE is a result of high-level, professional play such as football, ice hockey, and boxing, but it can affect amateur athletes and can affect people at a young age,” lead author Ann McKee, MD, professor of neurology and pathology and director of the Chronic Traumatic Encephalopathy Center at Boston University, said in an interview.
The findings were published online in JAMA Neurology.
A rare look
Brain donation at younger ages is rare, so most of what is known about CTE comes from studies in older athletes.
“We’ve always known that young people could develop this disease early after just amateur high school, youth, and college exposure, but this is the largest study of donor brains at this age,” Dr. McKee said.
The case series included 152 brains of athletes who played contact sports, experienced RHIs, and died before age 30. The tissues are part of the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Brain Bank and were donated between February 2008 and September 2022.
Researchers reviewed the donors’ medical records and conducted retrospective interviews with the donors’ next of kin to assess cognitive symptoms, mood disturbances, and neurobehavioral issues.
Donors died between the ages of 13 and 29 years, 92.8% were male and 73% were White. In 57.2% of the cases, suicide was the cause of death, with no difference between those with or without CTE.
CTE was neuropathologically diagnosed in 41.4% of athletes, using diagnostic criteria developed by the National Institute of Neurological Disorders and Stroke.
More than 95% had mild CTE. Diagnosis was associated with older age (mean difference, 3.92 years; P < .001) and significantly more years of exposure to contact sports (11.6 vs. 8.8 years).
Among those with CTE, 71.4% played amateur sports, including football (60.9%), soccer (17.2%), hockey (7.8%), and wrestling (7%).
The cohort includes the first known American female athlete with CTE. Recruiting female brain donors has always been a challenge, Dr. McKee said. In this study, females comprised about 7% of the entire cohort and tended to be younger and play fewer years of a sport, compared with their male counterparts. All of that could lower their risk for CTE, Dr. McKee said.
“We don’t have enough brain donations to make any comments about differences between the genders, but we’ve always known that women can develop CTE,” she said. “It’s been reported after domestic violence and in an autistic woman who was a headbanger, so it was just a matter of time before we found our first case.”
Early stage of CTE?
Neuropathological analysis revealed neuronal p-tau aggregates in all CTE cases, a hallmark of the disease.
Young athletes with CTE had significantly more ventricular dilatation, suggesting atrophy or shrinkage of the brain, and more cavum septum pellucidum.
“I was surprised that even at this very young age group we could see structural changes to the gross pathology,” Dr. McKee said.
Investigators also found evidence of perivascular macrophages in the deep white matter, a microscopic change that correlated with CTE and years of play and indicates a breach of the blood-brain barrier that could allow pro-inflammatory molecules to enter the brain, setting up a neuroinflammatory response.
“Neuroinflammation is a very early change after repetitive head impacts, as well as in CTE,” Dr. McKee said. “This may be one of the mechanisms by which the inflammation starts, meaning microvascular injury might be an integral part of the pathogenesis of CTE.”
A message for clinicians
All athletes had symptoms of mood and neurobehavioral dysfunction common in people with RHIs. There were no significant differences in those clinical symptoms based on CTE diagnosis, which is likely related to the retrospective nature of the clinical evaluations, Dr. McKee said.
While the study leaves many questions about CTE in younger athletes unanswered, there is a message for clinicians and for patients in the findings, she said.
For clinicians, it’s important to note that “this young population of amateur athletes can be very symptomatic, and in all likelihood, a lot of these symptoms are reversible with proper care and management,” Dr. McKee said.
“For individual athletes, it’s important to note that 58% of this cohort did not have CTE, so just because you have these symptoms is not an indication that you have a neurodegenerative disease,” she added.
The study was funded by Andlinger Foundation, the National Football League, Mac Parkman Foundation, National Operating Committee on Standards for Athletic Equipment, and the Nick and Lynn Buoniconti Foundation, World Wrestling Entertainment, Alzheimer’s Association, National Institutes of Health, Concussion Legacy Foundation, U.S. Department of Defense and the U.S. Department of Veterans Affairs. Dr. McKee is a member of the Mackey-White Health and Safety Committee of the National Football League Players Association and reported receiving grants from the NIH and Department of Veteran Affairs and other funding from the Buoniconti Foundation and Mac Parkman Foundation during the conduct of the study.
A version of this article appeared on Medscape.com.
Analysis of brain tissue from athletes who were exposed to RHIs and died before the age of 30 revealed neuropathological evidence of shrinkage of the brain and microscopic changes that indicate a breach of the blood-brain barrier. The case series also identified the first known American female athlete with CTE.
Nearly all of those with CTE had a mild form of the disease and 71% played only at the amateur level in youth, high school, or college sports.
“A lot of people think CTE is a result of high-level, professional play such as football, ice hockey, and boxing, but it can affect amateur athletes and can affect people at a young age,” lead author Ann McKee, MD, professor of neurology and pathology and director of the Chronic Traumatic Encephalopathy Center at Boston University, said in an interview.
The findings were published online in JAMA Neurology.
A rare look
Brain donation at younger ages is rare, so most of what is known about CTE comes from studies in older athletes.
“We’ve always known that young people could develop this disease early after just amateur high school, youth, and college exposure, but this is the largest study of donor brains at this age,” Dr. McKee said.
The case series included 152 brains of athletes who played contact sports, experienced RHIs, and died before age 30. The tissues are part of the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Brain Bank and were donated between February 2008 and September 2022.
Researchers reviewed the donors’ medical records and conducted retrospective interviews with the donors’ next of kin to assess cognitive symptoms, mood disturbances, and neurobehavioral issues.
Donors died between the ages of 13 and 29 years, 92.8% were male and 73% were White. In 57.2% of the cases, suicide was the cause of death, with no difference between those with or without CTE.
CTE was neuropathologically diagnosed in 41.4% of athletes, using diagnostic criteria developed by the National Institute of Neurological Disorders and Stroke.
More than 95% had mild CTE. Diagnosis was associated with older age (mean difference, 3.92 years; P < .001) and significantly more years of exposure to contact sports (11.6 vs. 8.8 years).
Among those with CTE, 71.4% played amateur sports, including football (60.9%), soccer (17.2%), hockey (7.8%), and wrestling (7%).
The cohort includes the first known American female athlete with CTE. Recruiting female brain donors has always been a challenge, Dr. McKee said. In this study, females comprised about 7% of the entire cohort and tended to be younger and play fewer years of a sport, compared with their male counterparts. All of that could lower their risk for CTE, Dr. McKee said.
“We don’t have enough brain donations to make any comments about differences between the genders, but we’ve always known that women can develop CTE,” she said. “It’s been reported after domestic violence and in an autistic woman who was a headbanger, so it was just a matter of time before we found our first case.”
Early stage of CTE?
Neuropathological analysis revealed neuronal p-tau aggregates in all CTE cases, a hallmark of the disease.
Young athletes with CTE had significantly more ventricular dilatation, suggesting atrophy or shrinkage of the brain, and more cavum septum pellucidum.
“I was surprised that even at this very young age group we could see structural changes to the gross pathology,” Dr. McKee said.
Investigators also found evidence of perivascular macrophages in the deep white matter, a microscopic change that correlated with CTE and years of play and indicates a breach of the blood-brain barrier that could allow pro-inflammatory molecules to enter the brain, setting up a neuroinflammatory response.
“Neuroinflammation is a very early change after repetitive head impacts, as well as in CTE,” Dr. McKee said. “This may be one of the mechanisms by which the inflammation starts, meaning microvascular injury might be an integral part of the pathogenesis of CTE.”
A message for clinicians
All athletes had symptoms of mood and neurobehavioral dysfunction common in people with RHIs. There were no significant differences in those clinical symptoms based on CTE diagnosis, which is likely related to the retrospective nature of the clinical evaluations, Dr. McKee said.
While the study leaves many questions about CTE in younger athletes unanswered, there is a message for clinicians and for patients in the findings, she said.
For clinicians, it’s important to note that “this young population of amateur athletes can be very symptomatic, and in all likelihood, a lot of these symptoms are reversible with proper care and management,” Dr. McKee said.
“For individual athletes, it’s important to note that 58% of this cohort did not have CTE, so just because you have these symptoms is not an indication that you have a neurodegenerative disease,” she added.
The study was funded by Andlinger Foundation, the National Football League, Mac Parkman Foundation, National Operating Committee on Standards for Athletic Equipment, and the Nick and Lynn Buoniconti Foundation, World Wrestling Entertainment, Alzheimer’s Association, National Institutes of Health, Concussion Legacy Foundation, U.S. Department of Defense and the U.S. Department of Veterans Affairs. Dr. McKee is a member of the Mackey-White Health and Safety Committee of the National Football League Players Association and reported receiving grants from the NIH and Department of Veteran Affairs and other funding from the Buoniconti Foundation and Mac Parkman Foundation during the conduct of the study.
A version of this article appeared on Medscape.com.
Analysis of brain tissue from athletes who were exposed to RHIs and died before the age of 30 revealed neuropathological evidence of shrinkage of the brain and microscopic changes that indicate a breach of the blood-brain barrier. The case series also identified the first known American female athlete with CTE.
Nearly all of those with CTE had a mild form of the disease and 71% played only at the amateur level in youth, high school, or college sports.
“A lot of people think CTE is a result of high-level, professional play such as football, ice hockey, and boxing, but it can affect amateur athletes and can affect people at a young age,” lead author Ann McKee, MD, professor of neurology and pathology and director of the Chronic Traumatic Encephalopathy Center at Boston University, said in an interview.
The findings were published online in JAMA Neurology.
A rare look
Brain donation at younger ages is rare, so most of what is known about CTE comes from studies in older athletes.
“We’ve always known that young people could develop this disease early after just amateur high school, youth, and college exposure, but this is the largest study of donor brains at this age,” Dr. McKee said.
The case series included 152 brains of athletes who played contact sports, experienced RHIs, and died before age 30. The tissues are part of the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Brain Bank and were donated between February 2008 and September 2022.
Researchers reviewed the donors’ medical records and conducted retrospective interviews with the donors’ next of kin to assess cognitive symptoms, mood disturbances, and neurobehavioral issues.
Donors died between the ages of 13 and 29 years, 92.8% were male and 73% were White. In 57.2% of the cases, suicide was the cause of death, with no difference between those with or without CTE.
CTE was neuropathologically diagnosed in 41.4% of athletes, using diagnostic criteria developed by the National Institute of Neurological Disorders and Stroke.
More than 95% had mild CTE. Diagnosis was associated with older age (mean difference, 3.92 years; P < .001) and significantly more years of exposure to contact sports (11.6 vs. 8.8 years).
Among those with CTE, 71.4% played amateur sports, including football (60.9%), soccer (17.2%), hockey (7.8%), and wrestling (7%).
The cohort includes the first known American female athlete with CTE. Recruiting female brain donors has always been a challenge, Dr. McKee said. In this study, females comprised about 7% of the entire cohort and tended to be younger and play fewer years of a sport, compared with their male counterparts. All of that could lower their risk for CTE, Dr. McKee said.
“We don’t have enough brain donations to make any comments about differences between the genders, but we’ve always known that women can develop CTE,” she said. “It’s been reported after domestic violence and in an autistic woman who was a headbanger, so it was just a matter of time before we found our first case.”
Early stage of CTE?
Neuropathological analysis revealed neuronal p-tau aggregates in all CTE cases, a hallmark of the disease.
Young athletes with CTE had significantly more ventricular dilatation, suggesting atrophy or shrinkage of the brain, and more cavum septum pellucidum.
“I was surprised that even at this very young age group we could see structural changes to the gross pathology,” Dr. McKee said.
Investigators also found evidence of perivascular macrophages in the deep white matter, a microscopic change that correlated with CTE and years of play and indicates a breach of the blood-brain barrier that could allow pro-inflammatory molecules to enter the brain, setting up a neuroinflammatory response.
“Neuroinflammation is a very early change after repetitive head impacts, as well as in CTE,” Dr. McKee said. “This may be one of the mechanisms by which the inflammation starts, meaning microvascular injury might be an integral part of the pathogenesis of CTE.”
A message for clinicians
All athletes had symptoms of mood and neurobehavioral dysfunction common in people with RHIs. There were no significant differences in those clinical symptoms based on CTE diagnosis, which is likely related to the retrospective nature of the clinical evaluations, Dr. McKee said.
While the study leaves many questions about CTE in younger athletes unanswered, there is a message for clinicians and for patients in the findings, she said.
For clinicians, it’s important to note that “this young population of amateur athletes can be very symptomatic, and in all likelihood, a lot of these symptoms are reversible with proper care and management,” Dr. McKee said.
“For individual athletes, it’s important to note that 58% of this cohort did not have CTE, so just because you have these symptoms is not an indication that you have a neurodegenerative disease,” she added.
The study was funded by Andlinger Foundation, the National Football League, Mac Parkman Foundation, National Operating Committee on Standards for Athletic Equipment, and the Nick and Lynn Buoniconti Foundation, World Wrestling Entertainment, Alzheimer’s Association, National Institutes of Health, Concussion Legacy Foundation, U.S. Department of Defense and the U.S. Department of Veterans Affairs. Dr. McKee is a member of the Mackey-White Health and Safety Committee of the National Football League Players Association and reported receiving grants from the NIH and Department of Veteran Affairs and other funding from the Buoniconti Foundation and Mac Parkman Foundation during the conduct of the study.
A version of this article appeared on Medscape.com.
Severe COVID may cause long-term cellular changes: Study
The small study, published in Cell and funded by the National Institutes of Health, details how immune cells were analyzed through blood samples collected from 38 patients recovering from severe COVID and other critical illnesses, and from 19 healthy people. Researchers from Weill Cornell Medicine, New York, and The Jackson Laboratory for Genomic Medicine, Farmington, Conn., found through isolating hematopoietic stem cells that people recovering from severe bouts of COVID had changes to their DNA that were passed down to offspring cells.
The research team, led by Steven Josefowicz, PhD, of Weill Cornell’s pathology department, and Duygu Ucar, PhD, associate professor at The Jackson Laboratory for Genomic Medicine, discovered that this chain reaction of stem cell changes caused a boost in the production of monocytes. The authors found that, due to the innate cellular changes from a severe case of COVID, patients in recovery ended up producing a larger amount of inflammatory cytokines, rather than monocytes – distinct from samples collected from healthy patients and those recovering from other critical illnesses.
These changes to patients’ epigenetic landscapes were observed even a year after the initial COVID-19 infection. While the small participant pool meant that the research team could not establish a direct line between these innate changes and any ensuing health outcomes, the research provides us with clues as to why patients continue to struggle with inflammation and long COVID symptoms well after they recover.
While the authors reiterate the study’s limitations and hesitate to make any clear-cut associations between the results and long-term health outcomes, Wolfgang Leitner, PhD, from the NIH’s National Institute of Allergy and Infectious Diseases, predicts that long COVID can, at least in part, be explained by the changes in innate immune responses.
“Ideally, the authors would have had cells from each patient before they got infected, as a comparator, to see what the epigenetic landscape was before COVID changed it,” said Dr. Leitner. “Clear links between the severity of COVID and genetics were discovered already early in the pandemic and this paper should prompt follow-up studies that link mutations in immune genes with the epigenetic changes described here.”
Dr. Leitner said he had some initial predictions about the long-term impact of COVID-19, but he had not anticipated some of what the study’s findings now show.
“Unlike in the case of, for example, influenza, where the lungs go into ‘repair mode’ after the infection has been resolved – which leaves people susceptible to secondary infections for up to several months – this study shows that after severe COVID, the immune system remains in ‘emergency mode’ and in a heightened state of inflammation,” said Dr. Leitner.
“That further aggravates the problem the initial strong inflammation causes: even higher risk of autoimmune disease, but also, cancer.”
Commenting on the findings, Eric Topol, MD, editor-in-chief of Medscape Medical News, said the study presents “evidence that a key line of immune cells are essentially irrevocably, epigenetically altered and activated.
“You do not want to have this [COVID],” he added.
The study also highlights the researchers’ novel approach to isolating hematopoietic stem cells, found largely in bone marrow. This type of research has been limited in the past because of how costly and invasive it can be to analyze cells in bone marrow. But, by isolating and enriching hematopoietic stem cells, the team can decipher the full cellular diversity of the cells’ bone marrow counterparts.
“This revelation opened the doors to study, at single-cell resolution, how stem cells are affected upon infection and vaccination with a simple blood draw,” representatives from the Jackson lab said in a press release.
A version of this article appeared on Medscape.com.
The small study, published in Cell and funded by the National Institutes of Health, details how immune cells were analyzed through blood samples collected from 38 patients recovering from severe COVID and other critical illnesses, and from 19 healthy people. Researchers from Weill Cornell Medicine, New York, and The Jackson Laboratory for Genomic Medicine, Farmington, Conn., found through isolating hematopoietic stem cells that people recovering from severe bouts of COVID had changes to their DNA that were passed down to offspring cells.
The research team, led by Steven Josefowicz, PhD, of Weill Cornell’s pathology department, and Duygu Ucar, PhD, associate professor at The Jackson Laboratory for Genomic Medicine, discovered that this chain reaction of stem cell changes caused a boost in the production of monocytes. The authors found that, due to the innate cellular changes from a severe case of COVID, patients in recovery ended up producing a larger amount of inflammatory cytokines, rather than monocytes – distinct from samples collected from healthy patients and those recovering from other critical illnesses.
These changes to patients’ epigenetic landscapes were observed even a year after the initial COVID-19 infection. While the small participant pool meant that the research team could not establish a direct line between these innate changes and any ensuing health outcomes, the research provides us with clues as to why patients continue to struggle with inflammation and long COVID symptoms well after they recover.
While the authors reiterate the study’s limitations and hesitate to make any clear-cut associations between the results and long-term health outcomes, Wolfgang Leitner, PhD, from the NIH’s National Institute of Allergy and Infectious Diseases, predicts that long COVID can, at least in part, be explained by the changes in innate immune responses.
“Ideally, the authors would have had cells from each patient before they got infected, as a comparator, to see what the epigenetic landscape was before COVID changed it,” said Dr. Leitner. “Clear links between the severity of COVID and genetics were discovered already early in the pandemic and this paper should prompt follow-up studies that link mutations in immune genes with the epigenetic changes described here.”
Dr. Leitner said he had some initial predictions about the long-term impact of COVID-19, but he had not anticipated some of what the study’s findings now show.
“Unlike in the case of, for example, influenza, where the lungs go into ‘repair mode’ after the infection has been resolved – which leaves people susceptible to secondary infections for up to several months – this study shows that after severe COVID, the immune system remains in ‘emergency mode’ and in a heightened state of inflammation,” said Dr. Leitner.
“That further aggravates the problem the initial strong inflammation causes: even higher risk of autoimmune disease, but also, cancer.”
Commenting on the findings, Eric Topol, MD, editor-in-chief of Medscape Medical News, said the study presents “evidence that a key line of immune cells are essentially irrevocably, epigenetically altered and activated.
“You do not want to have this [COVID],” he added.
The study also highlights the researchers’ novel approach to isolating hematopoietic stem cells, found largely in bone marrow. This type of research has been limited in the past because of how costly and invasive it can be to analyze cells in bone marrow. But, by isolating and enriching hematopoietic stem cells, the team can decipher the full cellular diversity of the cells’ bone marrow counterparts.
“This revelation opened the doors to study, at single-cell resolution, how stem cells are affected upon infection and vaccination with a simple blood draw,” representatives from the Jackson lab said in a press release.
A version of this article appeared on Medscape.com.
The small study, published in Cell and funded by the National Institutes of Health, details how immune cells were analyzed through blood samples collected from 38 patients recovering from severe COVID and other critical illnesses, and from 19 healthy people. Researchers from Weill Cornell Medicine, New York, and The Jackson Laboratory for Genomic Medicine, Farmington, Conn., found through isolating hematopoietic stem cells that people recovering from severe bouts of COVID had changes to their DNA that were passed down to offspring cells.
The research team, led by Steven Josefowicz, PhD, of Weill Cornell’s pathology department, and Duygu Ucar, PhD, associate professor at The Jackson Laboratory for Genomic Medicine, discovered that this chain reaction of stem cell changes caused a boost in the production of monocytes. The authors found that, due to the innate cellular changes from a severe case of COVID, patients in recovery ended up producing a larger amount of inflammatory cytokines, rather than monocytes – distinct from samples collected from healthy patients and those recovering from other critical illnesses.
These changes to patients’ epigenetic landscapes were observed even a year after the initial COVID-19 infection. While the small participant pool meant that the research team could not establish a direct line between these innate changes and any ensuing health outcomes, the research provides us with clues as to why patients continue to struggle with inflammation and long COVID symptoms well after they recover.
While the authors reiterate the study’s limitations and hesitate to make any clear-cut associations between the results and long-term health outcomes, Wolfgang Leitner, PhD, from the NIH’s National Institute of Allergy and Infectious Diseases, predicts that long COVID can, at least in part, be explained by the changes in innate immune responses.
“Ideally, the authors would have had cells from each patient before they got infected, as a comparator, to see what the epigenetic landscape was before COVID changed it,” said Dr. Leitner. “Clear links between the severity of COVID and genetics were discovered already early in the pandemic and this paper should prompt follow-up studies that link mutations in immune genes with the epigenetic changes described here.”
Dr. Leitner said he had some initial predictions about the long-term impact of COVID-19, but he had not anticipated some of what the study’s findings now show.
“Unlike in the case of, for example, influenza, where the lungs go into ‘repair mode’ after the infection has been resolved – which leaves people susceptible to secondary infections for up to several months – this study shows that after severe COVID, the immune system remains in ‘emergency mode’ and in a heightened state of inflammation,” said Dr. Leitner.
“That further aggravates the problem the initial strong inflammation causes: even higher risk of autoimmune disease, but also, cancer.”
Commenting on the findings, Eric Topol, MD, editor-in-chief of Medscape Medical News, said the study presents “evidence that a key line of immune cells are essentially irrevocably, epigenetically altered and activated.
“You do not want to have this [COVID],” he added.
The study also highlights the researchers’ novel approach to isolating hematopoietic stem cells, found largely in bone marrow. This type of research has been limited in the past because of how costly and invasive it can be to analyze cells in bone marrow. But, by isolating and enriching hematopoietic stem cells, the team can decipher the full cellular diversity of the cells’ bone marrow counterparts.
“This revelation opened the doors to study, at single-cell resolution, how stem cells are affected upon infection and vaccination with a simple blood draw,” representatives from the Jackson lab said in a press release.
A version of this article appeared on Medscape.com.
FROM CELL
Cruel summer for medical students and Taylor Swift fans
Most medical students won’t see Taylor Swift perform her hit song “Cruel Summer,” but they will spend thousands of dollars on ERAS as they prepare for the 2024 residency match. Medical students applying for residency tend to be as stressed out as Swifties trying to score concert tickets. Aside from the expenses of residency applications, students also face an increasingly complex application process: a match algorithm many of them do not understand and major changes to the application process that most learn about right before the application cycle begins.
I have gone through two matches myself, one for internal medicine and one for neurology, and I have also guided students through the process for almost a decade as a dean of student affairs at a medical school. Every summer, the application process is filled with numerous changes, often with little, if any, warning for the students. One year, for example, a specialty required additional essays tailored to each program. Though this requirement may have helped programs discern which students are most enthusiastic about their programs, it also disadvantaged students working on busier rotations, strapped for time to write as many as 70 additional essays in a matter of weeks.
Other recent changes have included “signaling” programs, selecting preferred regions, and preinterview recordings for some specialties. In 2023, students cannot include more than 10 activities on their ERAS application. I have spoken to students at numerous medical schools concerned about the difficulty of selecting 10 activities out of dozens of meaningful pursuits throughout their journeys; this challenge is particularly acute for students who had other careers before entering medical school.
The stress continues to mount even after residency applications have been submitted. Students often feel tied to their phones because offers for residency interviews roll in day and night by email, and if they wait more than a few hours to respond, they’re often moved to a waiting list for their preferred interview date. One year, while we were rounding on patients, a student stepped away to schedule an interview; while doing so, he missed out on managing a patient who developed a neurologic emergency. Thankfully, many but not all specialties have put rules in place to allow students more time to think through interview offers. Having more time to think, even if it’s just 48 hours, may decrease stress, limit the negative impacts on medical education, and promote informed decisions during interview season.
To be sure, most changes are being made in an effort to improve the experience of the students and programs. But as with anything, the result has been a mix of good and bad. The transition to virtual interviews allowed students to apply more broadly to programs without worrying about travel costs. The move also benefits students with disabilities who face accessibility and other challenges with traveling. However, virtual interviews came with several downsides, including but not limited to an increased number of applications submitted (recall that this was also a benefit), interview hoarding, and challenges of connecting personally via virtual platform. Despite the virtual format, applicants increasingly are doing in-person second looks, which some worry may give those applicants an additional advantage over applicants who do not have the time or financial resources to travel for a second look. Despite these shortcomings, it is important that virtual interviews remain an option for those applicants who need it.
Another change, which has been extensively debated in medical education in recent years, was the switch to pass/fail on the USMLE Step 1 exam. Though this move decreased the stress students experienced in the first 2 years of medical school, it has resulted in a new challenge as many residency programs put more emphasis on USMLE Step 2. Many medical students feel they do not have a good gauge of their competitiveness until a few weeks before they submit their application, particularly those applicants attending medical schools that do not provide them with information regarding their class standing until right before they submit their applications.
By the time Swift’s Eras Tour ends in the summer of 2024, medical students will already have matched and started their residency programs. At the same time, a new batch of students will be entering the next year’s match. Though the number of anticipated changes may not reach the level of seismic activity caused by the Swifties at her Seattle concert, many medical students fear that the changes may be just like tectonic plates shifting the match process away from its original purpose: to provide an orderly and fair mechanism for matching the preferences of applicants for U.S. residency positions with the preferences of residency program directors.
Dr. Etienne is with WMCHealth Good Samaritan Hospital, New York, and New York Medical College. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Most medical students won’t see Taylor Swift perform her hit song “Cruel Summer,” but they will spend thousands of dollars on ERAS as they prepare for the 2024 residency match. Medical students applying for residency tend to be as stressed out as Swifties trying to score concert tickets. Aside from the expenses of residency applications, students also face an increasingly complex application process: a match algorithm many of them do not understand and major changes to the application process that most learn about right before the application cycle begins.
I have gone through two matches myself, one for internal medicine and one for neurology, and I have also guided students through the process for almost a decade as a dean of student affairs at a medical school. Every summer, the application process is filled with numerous changes, often with little, if any, warning for the students. One year, for example, a specialty required additional essays tailored to each program. Though this requirement may have helped programs discern which students are most enthusiastic about their programs, it also disadvantaged students working on busier rotations, strapped for time to write as many as 70 additional essays in a matter of weeks.
Other recent changes have included “signaling” programs, selecting preferred regions, and preinterview recordings for some specialties. In 2023, students cannot include more than 10 activities on their ERAS application. I have spoken to students at numerous medical schools concerned about the difficulty of selecting 10 activities out of dozens of meaningful pursuits throughout their journeys; this challenge is particularly acute for students who had other careers before entering medical school.
The stress continues to mount even after residency applications have been submitted. Students often feel tied to their phones because offers for residency interviews roll in day and night by email, and if they wait more than a few hours to respond, they’re often moved to a waiting list for their preferred interview date. One year, while we were rounding on patients, a student stepped away to schedule an interview; while doing so, he missed out on managing a patient who developed a neurologic emergency. Thankfully, many but not all specialties have put rules in place to allow students more time to think through interview offers. Having more time to think, even if it’s just 48 hours, may decrease stress, limit the negative impacts on medical education, and promote informed decisions during interview season.
To be sure, most changes are being made in an effort to improve the experience of the students and programs. But as with anything, the result has been a mix of good and bad. The transition to virtual interviews allowed students to apply more broadly to programs without worrying about travel costs. The move also benefits students with disabilities who face accessibility and other challenges with traveling. However, virtual interviews came with several downsides, including but not limited to an increased number of applications submitted (recall that this was also a benefit), interview hoarding, and challenges of connecting personally via virtual platform. Despite the virtual format, applicants increasingly are doing in-person second looks, which some worry may give those applicants an additional advantage over applicants who do not have the time or financial resources to travel for a second look. Despite these shortcomings, it is important that virtual interviews remain an option for those applicants who need it.
Another change, which has been extensively debated in medical education in recent years, was the switch to pass/fail on the USMLE Step 1 exam. Though this move decreased the stress students experienced in the first 2 years of medical school, it has resulted in a new challenge as many residency programs put more emphasis on USMLE Step 2. Many medical students feel they do not have a good gauge of their competitiveness until a few weeks before they submit their application, particularly those applicants attending medical schools that do not provide them with information regarding their class standing until right before they submit their applications.
By the time Swift’s Eras Tour ends in the summer of 2024, medical students will already have matched and started their residency programs. At the same time, a new batch of students will be entering the next year’s match. Though the number of anticipated changes may not reach the level of seismic activity caused by the Swifties at her Seattle concert, many medical students fear that the changes may be just like tectonic plates shifting the match process away from its original purpose: to provide an orderly and fair mechanism for matching the preferences of applicants for U.S. residency positions with the preferences of residency program directors.
Dr. Etienne is with WMCHealth Good Samaritan Hospital, New York, and New York Medical College. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Most medical students won’t see Taylor Swift perform her hit song “Cruel Summer,” but they will spend thousands of dollars on ERAS as they prepare for the 2024 residency match. Medical students applying for residency tend to be as stressed out as Swifties trying to score concert tickets. Aside from the expenses of residency applications, students also face an increasingly complex application process: a match algorithm many of them do not understand and major changes to the application process that most learn about right before the application cycle begins.
I have gone through two matches myself, one for internal medicine and one for neurology, and I have also guided students through the process for almost a decade as a dean of student affairs at a medical school. Every summer, the application process is filled with numerous changes, often with little, if any, warning for the students. One year, for example, a specialty required additional essays tailored to each program. Though this requirement may have helped programs discern which students are most enthusiastic about their programs, it also disadvantaged students working on busier rotations, strapped for time to write as many as 70 additional essays in a matter of weeks.
Other recent changes have included “signaling” programs, selecting preferred regions, and preinterview recordings for some specialties. In 2023, students cannot include more than 10 activities on their ERAS application. I have spoken to students at numerous medical schools concerned about the difficulty of selecting 10 activities out of dozens of meaningful pursuits throughout their journeys; this challenge is particularly acute for students who had other careers before entering medical school.
The stress continues to mount even after residency applications have been submitted. Students often feel tied to their phones because offers for residency interviews roll in day and night by email, and if they wait more than a few hours to respond, they’re often moved to a waiting list for their preferred interview date. One year, while we were rounding on patients, a student stepped away to schedule an interview; while doing so, he missed out on managing a patient who developed a neurologic emergency. Thankfully, many but not all specialties have put rules in place to allow students more time to think through interview offers. Having more time to think, even if it’s just 48 hours, may decrease stress, limit the negative impacts on medical education, and promote informed decisions during interview season.
To be sure, most changes are being made in an effort to improve the experience of the students and programs. But as with anything, the result has been a mix of good and bad. The transition to virtual interviews allowed students to apply more broadly to programs without worrying about travel costs. The move also benefits students with disabilities who face accessibility and other challenges with traveling. However, virtual interviews came with several downsides, including but not limited to an increased number of applications submitted (recall that this was also a benefit), interview hoarding, and challenges of connecting personally via virtual platform. Despite the virtual format, applicants increasingly are doing in-person second looks, which some worry may give those applicants an additional advantage over applicants who do not have the time or financial resources to travel for a second look. Despite these shortcomings, it is important that virtual interviews remain an option for those applicants who need it.
Another change, which has been extensively debated in medical education in recent years, was the switch to pass/fail on the USMLE Step 1 exam. Though this move decreased the stress students experienced in the first 2 years of medical school, it has resulted in a new challenge as many residency programs put more emphasis on USMLE Step 2. Many medical students feel they do not have a good gauge of their competitiveness until a few weeks before they submit their application, particularly those applicants attending medical schools that do not provide them with information regarding their class standing until right before they submit their applications.
By the time Swift’s Eras Tour ends in the summer of 2024, medical students will already have matched and started their residency programs. At the same time, a new batch of students will be entering the next year’s match. Though the number of anticipated changes may not reach the level of seismic activity caused by the Swifties at her Seattle concert, many medical students fear that the changes may be just like tectonic plates shifting the match process away from its original purpose: to provide an orderly and fair mechanism for matching the preferences of applicants for U.S. residency positions with the preferences of residency program directors.
Dr. Etienne is with WMCHealth Good Samaritan Hospital, New York, and New York Medical College. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Number of people with long COVID could be vastly underestimated
It’s been estimated that up to one-third of people who survive acute SARS-CoV-2 infection will suffer a post-viral syndrome with lingering neurologic and other symptoms – now known as long COVID or neurological postacute sequelae of SARS-CoV-2 infection (Neuro-PASC).
However, Researchers found a significant proportion of patients in their small study who had never tested positive for COVID-19 but who were having symptoms of long COVID nevertheless showed evidence of immune responses consistent with previous exposure.
“We estimate that millions of people got COVID in the U.S. during the first year of the pandemic and then developed long COVID, yet they did not get a positive COVID diagnosis because of testing limitations,” Igor J. Koralnik, MD, of Northwestern Medicine Comprehensive COVID-19 Center in Chicago, said in an interview.
He noted that many post-COVID-19 clinics in the United States don’t accept people with long COVID symptoms who do not have a positive test result.
Patients with long COVID symptoms but without laboratory evidence of prior infection, “who have often been rejected and stigmatized, should feel vindicated by the results of our study,” Dr. Koralnik said.
“We think that those patients deserve the same clinical care as those with a positive test, as well as inclusion in research studies. This is what we are doing at Northwestern Medicine’s Comprehensive COVID[-19] Center,” Dr. Koralnik added.
The study was published online in the journal Neurology: Neuroimmunology & Neuroinflammation.
Delayed care
The researchers measured SARS-CoV-2-specific humoral and cell-mediated immune responses against nucleocapsid protein and spike proteins, which indicate a prior COVID-19 infection, in 29 patients with post-viral syndrome after suspected COVID-19, including neurologic symptoms such as cognitive impairment, headache, and fatigue, but who did not have a confirmed positive COVID-19 test.
They did the same in 32 age- and sex-matched COVID long haulers with confirmed Neuro-PASC and 18 healthy controls with none of the symptoms of long COVID and no known exposure to SARS-CoV-2 or positive test result.
They found that 12 of the 29 patients (41%) with post-viral syndrome (but no positive COVID-19 test) had detectable humoral and cellular immune responses consistent with prior exposure to SARS-CoV-2. Three-quarters harbored antinucleocapsid and 50% harbored antispike responses.
“Our data suggest that at least 4 million people with post-viral syndrome similar to long COVID may indeed have detectable immune responses to support a COVID diagnosis,” Dr. Koralnik said in a news release.
The 12 patients with post-viral syndrome but without a confirmed COVID-19 test had neurologic symptoms similar to those of patients with confirmed Neuro-PASC.
However, lack of a confirmed COVID-19 diagnosis likely contributed to the 5-month delay in the median time from symptom onset to clinic visit, the researchers said. They were evaluated at a median of 10.7 months vs. 5.4 months for Neuro-PASC patients.
Dr. Koralnik said in an interview that the “most important take-home message” of the study is that patients with post-viral syndrome often present with clinical manifestations similar to those of confirmed patients with Neuro-PASC, suggesting that a positive result by commercially available SARS-CoV-2 diagnostic test should not be a prerequisite for accessing care.
Patients with post-viral syndrome may benefit from the same clinical care as confirmed patients with Neuro-PASC, and the absence of a positive SARS-CoV-2 test should not preclude or delay treatment, he added.
A version of this article first appeared on Medscape.com .
This article was updated 8/28/23.
It’s been estimated that up to one-third of people who survive acute SARS-CoV-2 infection will suffer a post-viral syndrome with lingering neurologic and other symptoms – now known as long COVID or neurological postacute sequelae of SARS-CoV-2 infection (Neuro-PASC).
However, Researchers found a significant proportion of patients in their small study who had never tested positive for COVID-19 but who were having symptoms of long COVID nevertheless showed evidence of immune responses consistent with previous exposure.
“We estimate that millions of people got COVID in the U.S. during the first year of the pandemic and then developed long COVID, yet they did not get a positive COVID diagnosis because of testing limitations,” Igor J. Koralnik, MD, of Northwestern Medicine Comprehensive COVID-19 Center in Chicago, said in an interview.
He noted that many post-COVID-19 clinics in the United States don’t accept people with long COVID symptoms who do not have a positive test result.
Patients with long COVID symptoms but without laboratory evidence of prior infection, “who have often been rejected and stigmatized, should feel vindicated by the results of our study,” Dr. Koralnik said.
“We think that those patients deserve the same clinical care as those with a positive test, as well as inclusion in research studies. This is what we are doing at Northwestern Medicine’s Comprehensive COVID[-19] Center,” Dr. Koralnik added.
The study was published online in the journal Neurology: Neuroimmunology & Neuroinflammation.
Delayed care
The researchers measured SARS-CoV-2-specific humoral and cell-mediated immune responses against nucleocapsid protein and spike proteins, which indicate a prior COVID-19 infection, in 29 patients with post-viral syndrome after suspected COVID-19, including neurologic symptoms such as cognitive impairment, headache, and fatigue, but who did not have a confirmed positive COVID-19 test.
They did the same in 32 age- and sex-matched COVID long haulers with confirmed Neuro-PASC and 18 healthy controls with none of the symptoms of long COVID and no known exposure to SARS-CoV-2 or positive test result.
They found that 12 of the 29 patients (41%) with post-viral syndrome (but no positive COVID-19 test) had detectable humoral and cellular immune responses consistent with prior exposure to SARS-CoV-2. Three-quarters harbored antinucleocapsid and 50% harbored antispike responses.
“Our data suggest that at least 4 million people with post-viral syndrome similar to long COVID may indeed have detectable immune responses to support a COVID diagnosis,” Dr. Koralnik said in a news release.
The 12 patients with post-viral syndrome but without a confirmed COVID-19 test had neurologic symptoms similar to those of patients with confirmed Neuro-PASC.
However, lack of a confirmed COVID-19 diagnosis likely contributed to the 5-month delay in the median time from symptom onset to clinic visit, the researchers said. They were evaluated at a median of 10.7 months vs. 5.4 months for Neuro-PASC patients.
Dr. Koralnik said in an interview that the “most important take-home message” of the study is that patients with post-viral syndrome often present with clinical manifestations similar to those of confirmed patients with Neuro-PASC, suggesting that a positive result by commercially available SARS-CoV-2 diagnostic test should not be a prerequisite for accessing care.
Patients with post-viral syndrome may benefit from the same clinical care as confirmed patients with Neuro-PASC, and the absence of a positive SARS-CoV-2 test should not preclude or delay treatment, he added.
A version of this article first appeared on Medscape.com .
This article was updated 8/28/23.
It’s been estimated that up to one-third of people who survive acute SARS-CoV-2 infection will suffer a post-viral syndrome with lingering neurologic and other symptoms – now known as long COVID or neurological postacute sequelae of SARS-CoV-2 infection (Neuro-PASC).
However, Researchers found a significant proportion of patients in their small study who had never tested positive for COVID-19 but who were having symptoms of long COVID nevertheless showed evidence of immune responses consistent with previous exposure.
“We estimate that millions of people got COVID in the U.S. during the first year of the pandemic and then developed long COVID, yet they did not get a positive COVID diagnosis because of testing limitations,” Igor J. Koralnik, MD, of Northwestern Medicine Comprehensive COVID-19 Center in Chicago, said in an interview.
He noted that many post-COVID-19 clinics in the United States don’t accept people with long COVID symptoms who do not have a positive test result.
Patients with long COVID symptoms but without laboratory evidence of prior infection, “who have often been rejected and stigmatized, should feel vindicated by the results of our study,” Dr. Koralnik said.
“We think that those patients deserve the same clinical care as those with a positive test, as well as inclusion in research studies. This is what we are doing at Northwestern Medicine’s Comprehensive COVID[-19] Center,” Dr. Koralnik added.
The study was published online in the journal Neurology: Neuroimmunology & Neuroinflammation.
Delayed care
The researchers measured SARS-CoV-2-specific humoral and cell-mediated immune responses against nucleocapsid protein and spike proteins, which indicate a prior COVID-19 infection, in 29 patients with post-viral syndrome after suspected COVID-19, including neurologic symptoms such as cognitive impairment, headache, and fatigue, but who did not have a confirmed positive COVID-19 test.
They did the same in 32 age- and sex-matched COVID long haulers with confirmed Neuro-PASC and 18 healthy controls with none of the symptoms of long COVID and no known exposure to SARS-CoV-2 or positive test result.
They found that 12 of the 29 patients (41%) with post-viral syndrome (but no positive COVID-19 test) had detectable humoral and cellular immune responses consistent with prior exposure to SARS-CoV-2. Three-quarters harbored antinucleocapsid and 50% harbored antispike responses.
“Our data suggest that at least 4 million people with post-viral syndrome similar to long COVID may indeed have detectable immune responses to support a COVID diagnosis,” Dr. Koralnik said in a news release.
The 12 patients with post-viral syndrome but without a confirmed COVID-19 test had neurologic symptoms similar to those of patients with confirmed Neuro-PASC.
However, lack of a confirmed COVID-19 diagnosis likely contributed to the 5-month delay in the median time from symptom onset to clinic visit, the researchers said. They were evaluated at a median of 10.7 months vs. 5.4 months for Neuro-PASC patients.
Dr. Koralnik said in an interview that the “most important take-home message” of the study is that patients with post-viral syndrome often present with clinical manifestations similar to those of confirmed patients with Neuro-PASC, suggesting that a positive result by commercially available SARS-CoV-2 diagnostic test should not be a prerequisite for accessing care.
Patients with post-viral syndrome may benefit from the same clinical care as confirmed patients with Neuro-PASC, and the absence of a positive SARS-CoV-2 test should not preclude or delay treatment, he added.
A version of this article first appeared on Medscape.com .
This article was updated 8/28/23.
FROM NEUROLOGY, NEUROIMMUNOLOGY & NEUROINFLAMMATION

