User login
CV risk biomarkers tentatively identified in psoriatic disease
The risk of cardiovascular (CV) events in patients with psoriatic disease rises with higher levels of two cardiac biomarkers in a manner independent of risk calculated by the Framingham Risk Score (FRS), a longitudinal cohort study has shown. But researchers who conducted the study note that neither of the two biomarkers identified in the study – cardiac troponin I (cTnI) and N-terminal pro-brain-type natriuretic peptide (NT-proBNP) – led to an improvement in predictive performance when combined with the FRS, despite their association with carotid plaque burden.
Psoriasis and psoriatic arthritis are both associated with greater risk of CV morbidity and mortality, partly because of systemic inflammation that leads to atherogenesis. Measures of CV risk such as the FRS rely on traditional measures of CV risk and thus are likely to underestimate the CV event risk of people with psoriatic disease, according to the authors of the new study, published online in Arthritis & Rheumatology. The effort was led by Keith Colaço, MSc; Lihi Eder, MD, PhD; and other researchers affiliated with the University of Toronto.
“We are desperately in need of biomarker science advancement in psoriatic arthritis for a variety of places of guidance: How to choose a medication more accurately for the patient in front of us – that is, getting to be more like oncologists who use biomarkers to pick the best treatment or combination. That’s an important need. A second important need is how to guide clinicians regarding risk prediction for things like persistent, severe disease activity, progressive structural damage from disease, and, in this case, predicting a very common comorbidity that occurs in [psoriasis and] psoriatic arthritis patients,” Philip J. Mease, MD, told this news organization when asked to comment on the study.
Such biomarkers could assist with patient counseling, according to Dr. Mease, who is director of rheumatology research at Swedish Medical Center/Providence St. Joseph Health and is a clinical professor at the University of Washington, both in Seattle. Some patients may struggle with advice to lose weight or adopt lifestyle measures to limit CV risk, and more accurate predictions of risk may serve as further motivation. “It could well be that if you have a biomarker that accurately predicts a coming cataclysm, that it will lead you to redouble your efforts to do whatever it takes to reduce cardiovascular risk,” he said.
Both cTnI and NT-proBNP have been linked to increased CV risk in the general population, but little work has been done in the context of rheumatologic diseases.
The researchers analyzed data from 358 patients seen at the University of Toronto. The mean follow-up was 3.69 years. After adjustment for CV risk factors, lipid-lowering therapy, and creatinine levels, there was an association between cTnI levels and total carotid plaque area (adjusted beta coefficient, 0.21; 95% confidence interval, 0-0.41), but not for levels of NT-proBNP.
Atherosclerosis progressed in 89 participants overall, but multivariate adjustment revealed no significant relationship between progression and cTnI or NT-proBNP levels.
Separately, the researchers analyzed 1,000 individuals with psoriatic arthritis (n = 648) or with psoriasis and no arthritis (n = 352) whom they followed for a mean of 7.1 years after the patients underwent evaluation during 2002-2019. After adjustment for FRS, there was an association between the risk of a CV event and each 1–standard deviation increase in both cTnI (hazard ratio, 3.02; 95% CI, 1.12-8.16) and NT-proBNP (HR, 2.02; 95% CI, 1.28-3.18).
The combination of both biomarkers with the FRS predicted higher CV risk (HR, 1.91; 95% CI, 1.23-2.97). Neither biomarker made a statistically significant difference in changing CV risk prediction when added individually to FRS, although cTnI trended toward significance (HR, 2.60; 95% CI, 0.98-6.87).
Instead of the carotid plaque burden, Dr. Mease would have liked to have seen the authors evaluate calcium scores in coronary arteries as measured by CT. “I would have loved to have seen the researchers using that in addition to the carotid plaque assessment, to see what that would show us about these patients,” he said.
Only a small number of patients experienced CV events during the study period, which will likely make it necessary to conduct larger studies to identify a clear relationship. “You need a registry-type study with probably many hundreds if not thousands of patients in order to identify whether or not adding troponin could be useful to what we typically measure with patients when we’re trying to assess their risk,” Dr. Mease said.
The study was supported in part by the National Psoriasis Foundation and the Arthritis Society. Individual researchers have received support from a range of sources, including the Enid Walker Estate, the Women’s College Research Institute, the Arthritis Society, the National Psoriasis Foundation, the Edward Dunlop Foundation, the Ontario Ministry of Science and Innovation, and a Pfizer Chair Research Award. Some of the researchers have financial relationships with pharmaceutical companies that market drugs for psoriasis and psoriatic arthritis.
A version of this article first appeared on Medscape.com.
The risk of cardiovascular (CV) events in patients with psoriatic disease rises with higher levels of two cardiac biomarkers in a manner independent of risk calculated by the Framingham Risk Score (FRS), a longitudinal cohort study has shown. But researchers who conducted the study note that neither of the two biomarkers identified in the study – cardiac troponin I (cTnI) and N-terminal pro-brain-type natriuretic peptide (NT-proBNP) – led to an improvement in predictive performance when combined with the FRS, despite their association with carotid plaque burden.
Psoriasis and psoriatic arthritis are both associated with greater risk of CV morbidity and mortality, partly because of systemic inflammation that leads to atherogenesis. Measures of CV risk such as the FRS rely on traditional measures of CV risk and thus are likely to underestimate the CV event risk of people with psoriatic disease, according to the authors of the new study, published online in Arthritis & Rheumatology. The effort was led by Keith Colaço, MSc; Lihi Eder, MD, PhD; and other researchers affiliated with the University of Toronto.
“We are desperately in need of biomarker science advancement in psoriatic arthritis for a variety of places of guidance: How to choose a medication more accurately for the patient in front of us – that is, getting to be more like oncologists who use biomarkers to pick the best treatment or combination. That’s an important need. A second important need is how to guide clinicians regarding risk prediction for things like persistent, severe disease activity, progressive structural damage from disease, and, in this case, predicting a very common comorbidity that occurs in [psoriasis and] psoriatic arthritis patients,” Philip J. Mease, MD, told this news organization when asked to comment on the study.
Such biomarkers could assist with patient counseling, according to Dr. Mease, who is director of rheumatology research at Swedish Medical Center/Providence St. Joseph Health and is a clinical professor at the University of Washington, both in Seattle. Some patients may struggle with advice to lose weight or adopt lifestyle measures to limit CV risk, and more accurate predictions of risk may serve as further motivation. “It could well be that if you have a biomarker that accurately predicts a coming cataclysm, that it will lead you to redouble your efforts to do whatever it takes to reduce cardiovascular risk,” he said.
Both cTnI and NT-proBNP have been linked to increased CV risk in the general population, but little work has been done in the context of rheumatologic diseases.
The researchers analyzed data from 358 patients seen at the University of Toronto. The mean follow-up was 3.69 years. After adjustment for CV risk factors, lipid-lowering therapy, and creatinine levels, there was an association between cTnI levels and total carotid plaque area (adjusted beta coefficient, 0.21; 95% confidence interval, 0-0.41), but not for levels of NT-proBNP.
Atherosclerosis progressed in 89 participants overall, but multivariate adjustment revealed no significant relationship between progression and cTnI or NT-proBNP levels.
Separately, the researchers analyzed 1,000 individuals with psoriatic arthritis (n = 648) or with psoriasis and no arthritis (n = 352) whom they followed for a mean of 7.1 years after the patients underwent evaluation during 2002-2019. After adjustment for FRS, there was an association between the risk of a CV event and each 1–standard deviation increase in both cTnI (hazard ratio, 3.02; 95% CI, 1.12-8.16) and NT-proBNP (HR, 2.02; 95% CI, 1.28-3.18).
The combination of both biomarkers with the FRS predicted higher CV risk (HR, 1.91; 95% CI, 1.23-2.97). Neither biomarker made a statistically significant difference in changing CV risk prediction when added individually to FRS, although cTnI trended toward significance (HR, 2.60; 95% CI, 0.98-6.87).
Instead of the carotid plaque burden, Dr. Mease would have liked to have seen the authors evaluate calcium scores in coronary arteries as measured by CT. “I would have loved to have seen the researchers using that in addition to the carotid plaque assessment, to see what that would show us about these patients,” he said.
Only a small number of patients experienced CV events during the study period, which will likely make it necessary to conduct larger studies to identify a clear relationship. “You need a registry-type study with probably many hundreds if not thousands of patients in order to identify whether or not adding troponin could be useful to what we typically measure with patients when we’re trying to assess their risk,” Dr. Mease said.
The study was supported in part by the National Psoriasis Foundation and the Arthritis Society. Individual researchers have received support from a range of sources, including the Enid Walker Estate, the Women’s College Research Institute, the Arthritis Society, the National Psoriasis Foundation, the Edward Dunlop Foundation, the Ontario Ministry of Science and Innovation, and a Pfizer Chair Research Award. Some of the researchers have financial relationships with pharmaceutical companies that market drugs for psoriasis and psoriatic arthritis.
A version of this article first appeared on Medscape.com.
The risk of cardiovascular (CV) events in patients with psoriatic disease rises with higher levels of two cardiac biomarkers in a manner independent of risk calculated by the Framingham Risk Score (FRS), a longitudinal cohort study has shown. But researchers who conducted the study note that neither of the two biomarkers identified in the study – cardiac troponin I (cTnI) and N-terminal pro-brain-type natriuretic peptide (NT-proBNP) – led to an improvement in predictive performance when combined with the FRS, despite their association with carotid plaque burden.
Psoriasis and psoriatic arthritis are both associated with greater risk of CV morbidity and mortality, partly because of systemic inflammation that leads to atherogenesis. Measures of CV risk such as the FRS rely on traditional measures of CV risk and thus are likely to underestimate the CV event risk of people with psoriatic disease, according to the authors of the new study, published online in Arthritis & Rheumatology. The effort was led by Keith Colaço, MSc; Lihi Eder, MD, PhD; and other researchers affiliated with the University of Toronto.
“We are desperately in need of biomarker science advancement in psoriatic arthritis for a variety of places of guidance: How to choose a medication more accurately for the patient in front of us – that is, getting to be more like oncologists who use biomarkers to pick the best treatment or combination. That’s an important need. A second important need is how to guide clinicians regarding risk prediction for things like persistent, severe disease activity, progressive structural damage from disease, and, in this case, predicting a very common comorbidity that occurs in [psoriasis and] psoriatic arthritis patients,” Philip J. Mease, MD, told this news organization when asked to comment on the study.
Such biomarkers could assist with patient counseling, according to Dr. Mease, who is director of rheumatology research at Swedish Medical Center/Providence St. Joseph Health and is a clinical professor at the University of Washington, both in Seattle. Some patients may struggle with advice to lose weight or adopt lifestyle measures to limit CV risk, and more accurate predictions of risk may serve as further motivation. “It could well be that if you have a biomarker that accurately predicts a coming cataclysm, that it will lead you to redouble your efforts to do whatever it takes to reduce cardiovascular risk,” he said.
Both cTnI and NT-proBNP have been linked to increased CV risk in the general population, but little work has been done in the context of rheumatologic diseases.
The researchers analyzed data from 358 patients seen at the University of Toronto. The mean follow-up was 3.69 years. After adjustment for CV risk factors, lipid-lowering therapy, and creatinine levels, there was an association between cTnI levels and total carotid plaque area (adjusted beta coefficient, 0.21; 95% confidence interval, 0-0.41), but not for levels of NT-proBNP.
Atherosclerosis progressed in 89 participants overall, but multivariate adjustment revealed no significant relationship between progression and cTnI or NT-proBNP levels.
Separately, the researchers analyzed 1,000 individuals with psoriatic arthritis (n = 648) or with psoriasis and no arthritis (n = 352) whom they followed for a mean of 7.1 years after the patients underwent evaluation during 2002-2019. After adjustment for FRS, there was an association between the risk of a CV event and each 1–standard deviation increase in both cTnI (hazard ratio, 3.02; 95% CI, 1.12-8.16) and NT-proBNP (HR, 2.02; 95% CI, 1.28-3.18).
The combination of both biomarkers with the FRS predicted higher CV risk (HR, 1.91; 95% CI, 1.23-2.97). Neither biomarker made a statistically significant difference in changing CV risk prediction when added individually to FRS, although cTnI trended toward significance (HR, 2.60; 95% CI, 0.98-6.87).
Instead of the carotid plaque burden, Dr. Mease would have liked to have seen the authors evaluate calcium scores in coronary arteries as measured by CT. “I would have loved to have seen the researchers using that in addition to the carotid plaque assessment, to see what that would show us about these patients,” he said.
Only a small number of patients experienced CV events during the study period, which will likely make it necessary to conduct larger studies to identify a clear relationship. “You need a registry-type study with probably many hundreds if not thousands of patients in order to identify whether or not adding troponin could be useful to what we typically measure with patients when we’re trying to assess their risk,” Dr. Mease said.
The study was supported in part by the National Psoriasis Foundation and the Arthritis Society. Individual researchers have received support from a range of sources, including the Enid Walker Estate, the Women’s College Research Institute, the Arthritis Society, the National Psoriasis Foundation, the Edward Dunlop Foundation, the Ontario Ministry of Science and Innovation, and a Pfizer Chair Research Award. Some of the researchers have financial relationships with pharmaceutical companies that market drugs for psoriasis and psoriatic arthritis.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Drug survival study looks at what lasts longest in RA, axSpA, PsA, and psoriasis
Survival rates of biologics and other novel immunomodulatory drugs vary substantially across chronic inflammatory diseases, and rates are highest for rituximab in rheumatoid arthritis (RA) and golimumab in axial spondyloarthritis (axSpA), but with similar rates seen for most drugs used in the treatment of psoriasis and psoriatic arthritis (PsA), according to findings from a study of two Danish registries.
Drug survival refers to “the probability that patients will remain on a given drug, and is a proxy for efficacy as well as safety in daily clinical practice,” wrote Alexander Egeberg, MD, PhD, of the department of dermatology at Copenhagen University Hospital–Bispebjerg, and colleagues. Although the use of biologics has expanded for inflammatory diseases, real-world data on drug survival in newer agents such as interleukin (IL)-17, IL-23, and Janus kinase inhibitors are lacking, they said.
In a study published in Seminars in Arthritis and Rheumatism, the researchers reviewed data from the DANBIO and DERMBIO registries of patients in Denmark with inflammatory diseases including rheumatoid arthritis (RA), axial spondyloarthritis (AxSpA), psoriatic arthritis (PsA), and psoriasis.
The study population included 12,089 adults: 5,104 with RA, 2,157 with AxSpA, 2,251 with PsA, and 2,577 with psoriasis. Patients’ mean age at the time of first treatment for these conditions was 57.8 years, 42.3 years, 49 years, and 45 years, respectively. Participants were treated with biologics or novel small molecule therapies for RA, AxSpA, PsA, or psoriasis between January 2015 and May 2021 (from the DANBIO database) and November 2009 to November 2019 (DERMBIO database).
In adjusted models, drug survival in RA was highest for rituximab followed by baricitinib, etanercept, and tocilizumab. Drug survival in AxSpA was highest for golimumab, compared with all other drugs, followed by secukinumab and etanercept. Survival was lowest for infliximab. In PsA, drug survival was roughly equal for most drugs, including golimumab, secukinumab, and ixekizumab, with the lowest survival observed for tofacitinib and infliximab, compared with all other drugs. Drug survival in psoriasis was highest with guselkumab, followed by ustekinumab and IL-17 inhibitors.
However, the number of treatment series “was low for some drugs, and not all differences were statistically significant, which could influence the overall interpretability of these findings,” the researchers noted in their discussion.
Notably, the high treatment persistence for rituximab in RA patients needs further confirmation, the researchers said. “In Denmark, rituximab is often the biologic drug of choice in RA patients with a history of cancer while there is a reluctancy to use TNF [tumor necrosis factor] inhibitors in such patients; this may have prolonged the drug survival for rituximab treated patients due to limited treatment alternatives,” they said.
The findings were limited by several factors, including the observational study design and changes in guidelines over the course of the study, the researchers noted. Other limitations included the inability to adjust for certain variables, such as antibody status, body weight, and smoking, because of missing data, and a lack of data on the underlying reasons for drug discontinuation, they said.
However, the results were strengthened by the large number of patients and completeness of the registries, the researchers emphasized. The range in responses to different drug types across diseases supports the need for individualized treatments with attention to underlying disease, patient profile, and treatment history, they concluded.
The study received no outside funding. Eight coauthors reported financial ties to a number of pharmaceutical companies.
Survival rates of biologics and other novel immunomodulatory drugs vary substantially across chronic inflammatory diseases, and rates are highest for rituximab in rheumatoid arthritis (RA) and golimumab in axial spondyloarthritis (axSpA), but with similar rates seen for most drugs used in the treatment of psoriasis and psoriatic arthritis (PsA), according to findings from a study of two Danish registries.
Drug survival refers to “the probability that patients will remain on a given drug, and is a proxy for efficacy as well as safety in daily clinical practice,” wrote Alexander Egeberg, MD, PhD, of the department of dermatology at Copenhagen University Hospital–Bispebjerg, and colleagues. Although the use of biologics has expanded for inflammatory diseases, real-world data on drug survival in newer agents such as interleukin (IL)-17, IL-23, and Janus kinase inhibitors are lacking, they said.
In a study published in Seminars in Arthritis and Rheumatism, the researchers reviewed data from the DANBIO and DERMBIO registries of patients in Denmark with inflammatory diseases including rheumatoid arthritis (RA), axial spondyloarthritis (AxSpA), psoriatic arthritis (PsA), and psoriasis.
The study population included 12,089 adults: 5,104 with RA, 2,157 with AxSpA, 2,251 with PsA, and 2,577 with psoriasis. Patients’ mean age at the time of first treatment for these conditions was 57.8 years, 42.3 years, 49 years, and 45 years, respectively. Participants were treated with biologics or novel small molecule therapies for RA, AxSpA, PsA, or psoriasis between January 2015 and May 2021 (from the DANBIO database) and November 2009 to November 2019 (DERMBIO database).
In adjusted models, drug survival in RA was highest for rituximab followed by baricitinib, etanercept, and tocilizumab. Drug survival in AxSpA was highest for golimumab, compared with all other drugs, followed by secukinumab and etanercept. Survival was lowest for infliximab. In PsA, drug survival was roughly equal for most drugs, including golimumab, secukinumab, and ixekizumab, with the lowest survival observed for tofacitinib and infliximab, compared with all other drugs. Drug survival in psoriasis was highest with guselkumab, followed by ustekinumab and IL-17 inhibitors.
However, the number of treatment series “was low for some drugs, and not all differences were statistically significant, which could influence the overall interpretability of these findings,” the researchers noted in their discussion.
Notably, the high treatment persistence for rituximab in RA patients needs further confirmation, the researchers said. “In Denmark, rituximab is often the biologic drug of choice in RA patients with a history of cancer while there is a reluctancy to use TNF [tumor necrosis factor] inhibitors in such patients; this may have prolonged the drug survival for rituximab treated patients due to limited treatment alternatives,” they said.
The findings were limited by several factors, including the observational study design and changes in guidelines over the course of the study, the researchers noted. Other limitations included the inability to adjust for certain variables, such as antibody status, body weight, and smoking, because of missing data, and a lack of data on the underlying reasons for drug discontinuation, they said.
However, the results were strengthened by the large number of patients and completeness of the registries, the researchers emphasized. The range in responses to different drug types across diseases supports the need for individualized treatments with attention to underlying disease, patient profile, and treatment history, they concluded.
The study received no outside funding. Eight coauthors reported financial ties to a number of pharmaceutical companies.
Survival rates of biologics and other novel immunomodulatory drugs vary substantially across chronic inflammatory diseases, and rates are highest for rituximab in rheumatoid arthritis (RA) and golimumab in axial spondyloarthritis (axSpA), but with similar rates seen for most drugs used in the treatment of psoriasis and psoriatic arthritis (PsA), according to findings from a study of two Danish registries.
Drug survival refers to “the probability that patients will remain on a given drug, and is a proxy for efficacy as well as safety in daily clinical practice,” wrote Alexander Egeberg, MD, PhD, of the department of dermatology at Copenhagen University Hospital–Bispebjerg, and colleagues. Although the use of biologics has expanded for inflammatory diseases, real-world data on drug survival in newer agents such as interleukin (IL)-17, IL-23, and Janus kinase inhibitors are lacking, they said.
In a study published in Seminars in Arthritis and Rheumatism, the researchers reviewed data from the DANBIO and DERMBIO registries of patients in Denmark with inflammatory diseases including rheumatoid arthritis (RA), axial spondyloarthritis (AxSpA), psoriatic arthritis (PsA), and psoriasis.
The study population included 12,089 adults: 5,104 with RA, 2,157 with AxSpA, 2,251 with PsA, and 2,577 with psoriasis. Patients’ mean age at the time of first treatment for these conditions was 57.8 years, 42.3 years, 49 years, and 45 years, respectively. Participants were treated with biologics or novel small molecule therapies for RA, AxSpA, PsA, or psoriasis between January 2015 and May 2021 (from the DANBIO database) and November 2009 to November 2019 (DERMBIO database).
In adjusted models, drug survival in RA was highest for rituximab followed by baricitinib, etanercept, and tocilizumab. Drug survival in AxSpA was highest for golimumab, compared with all other drugs, followed by secukinumab and etanercept. Survival was lowest for infliximab. In PsA, drug survival was roughly equal for most drugs, including golimumab, secukinumab, and ixekizumab, with the lowest survival observed for tofacitinib and infliximab, compared with all other drugs. Drug survival in psoriasis was highest with guselkumab, followed by ustekinumab and IL-17 inhibitors.
However, the number of treatment series “was low for some drugs, and not all differences were statistically significant, which could influence the overall interpretability of these findings,” the researchers noted in their discussion.
Notably, the high treatment persistence for rituximab in RA patients needs further confirmation, the researchers said. “In Denmark, rituximab is often the biologic drug of choice in RA patients with a history of cancer while there is a reluctancy to use TNF [tumor necrosis factor] inhibitors in such patients; this may have prolonged the drug survival for rituximab treated patients due to limited treatment alternatives,” they said.
The findings were limited by several factors, including the observational study design and changes in guidelines over the course of the study, the researchers noted. Other limitations included the inability to adjust for certain variables, such as antibody status, body weight, and smoking, because of missing data, and a lack of data on the underlying reasons for drug discontinuation, they said.
However, the results were strengthened by the large number of patients and completeness of the registries, the researchers emphasized. The range in responses to different drug types across diseases supports the need for individualized treatments with attention to underlying disease, patient profile, and treatment history, they concluded.
The study received no outside funding. Eight coauthors reported financial ties to a number of pharmaceutical companies.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
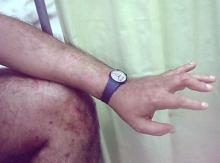
Painful swelling of fingers and toe
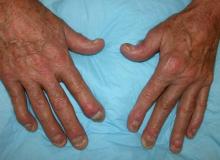
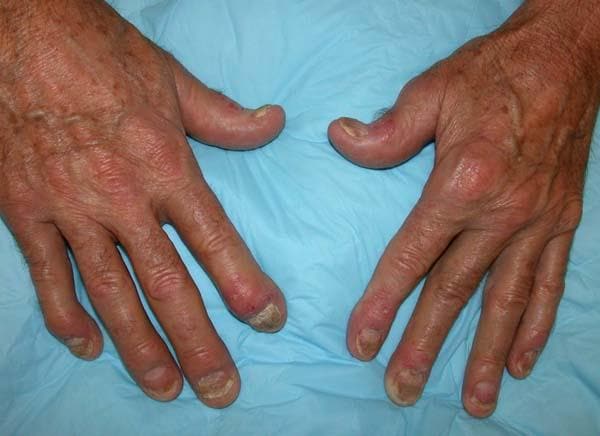
Although psoriatic arthritis is not the only disease associated with dactylitis — other culprits are sarcoidosis, septic arthritis, tuberculosis, and gout — dactylitis is one of the characteristic symptoms of psoriatic arthritis. Dactylitis is seen in as many as 35% of patients with psoriatic disease. Dactylitis clinically presents — as in this patient — with sausage-like swelling of the digits. It is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as one of the hallmarks of psoriatic arthritis.
Dactylitis has been thought to be a result of the concomitant swelling and inflammation of the flexor tendon sheaths of the metacarpophalangeal, metatarsophalangeal, or interphalangeal joints. Flexor tenosynovitis can be detected by examination with MRI and ultrasound. Dactylitis is associated with radiologically evident erosive damage to the joints.
Patients with psoriatic arthritis are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with psoriatic arthritis do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent, but rather indicates that different markers are needed that allow better quantification of systemic inflammation in psoriasis and psoriatic arthritis.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Although psoriatic arthritis is not the only disease associated with dactylitis — other culprits are sarcoidosis, septic arthritis, tuberculosis, and gout — dactylitis is one of the characteristic symptoms of psoriatic arthritis. Dactylitis is seen in as many as 35% of patients with psoriatic disease. Dactylitis clinically presents — as in this patient — with sausage-like swelling of the digits. It is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as one of the hallmarks of psoriatic arthritis.
Dactylitis has been thought to be a result of the concomitant swelling and inflammation of the flexor tendon sheaths of the metacarpophalangeal, metatarsophalangeal, or interphalangeal joints. Flexor tenosynovitis can be detected by examination with MRI and ultrasound. Dactylitis is associated with radiologically evident erosive damage to the joints.
Patients with psoriatic arthritis are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with psoriatic arthritis do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent, but rather indicates that different markers are needed that allow better quantification of systemic inflammation in psoriasis and psoriatic arthritis.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Although psoriatic arthritis is not the only disease associated with dactylitis — other culprits are sarcoidosis, septic arthritis, tuberculosis, and gout — dactylitis is one of the characteristic symptoms of psoriatic arthritis. Dactylitis is seen in as many as 35% of patients with psoriatic disease. Dactylitis clinically presents — as in this patient — with sausage-like swelling of the digits. It is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as one of the hallmarks of psoriatic arthritis.
Dactylitis has been thought to be a result of the concomitant swelling and inflammation of the flexor tendon sheaths of the metacarpophalangeal, metatarsophalangeal, or interphalangeal joints. Flexor tenosynovitis can be detected by examination with MRI and ultrasound. Dactylitis is associated with radiologically evident erosive damage to the joints.
Patients with psoriatic arthritis are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with psoriatic arthritis do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent, but rather indicates that different markers are needed that allow better quantification of systemic inflammation in psoriasis and psoriatic arthritis.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
A 35-year-old man presents with painful swelling of his right index and ring fingers as well as the fourth toe on his right foot, which has persisted for 5 days. He cannot perform his daily activities owing to severe pain in the affected fingers and toes. His medical history is unremarkable. His paternal uncle had psoriasis, which was successfully treated with adalimumab.
Physical assessment reveals tender, fusiform, swollen soft tissues in the affected fingertips, the fourth toe, and swollen palms. Nails are pitted. Hand radiography reveals mild edema of the soft tissue of the index and ring fingers but no significant joint abnormalities. Enthesitis is not present. Laboratory tests reveal a negative human leukocyte antigen B27 (HLA-B27) test, negative rheumatoid factor, negative antinuclear antibody, and normal C-reactive protein.
Dactylitis was diagnosed on the basis of clinical symptoms, radiographic results, and laboratory findings.
Intermittent joint aches
Fundamental changes in the initial pharmacologic approach to psoriatic arthritis were made in the 2018 American College Rheumatology/National Psoriasis (ACR/NPF) guidelines for the treatment of psoriatic arthritis. Previously, methotrexate had been widely used as the first-line agent. The 2018 guidelines recommend a tumor necrosis factor (TNF) inhibitor over methotrexate and other oral small molecules (leflunomide, cyclosporine, and apremilast).
Herein is a broad summary of the guidelines:
· Treat with TNF inhibitor over oral small molecule; may consider oral small molecule with mild psoriatic arthritis and psoriasis, patient preference, and/or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with oral small molecule over IL-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis and/or psoriatic arthritis
· Treat with oral small molecule over IL-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or psoriatic arthritis, or concomitant inflammatory bowel disease
· Treat with methotrexate over nonsteroidal anti-inflammatory drugs; may consider nonsteroidals for mild psoriatic arthritis and psoriasis
· Treat with IL-17 inhibitor over IL-12/23 inhibitor; may consider IL-12/23 inhibitor in a patient with concomitant inflammatory bowel disease
Note that these recommendations are based on conditional evidence (ie, low to very low quality). In fact, in the entire guideline document, only 6% of the recommendations are strong, whereas 96% are conditional. This emphasizes the importance of evaluating each patient individually and engaging in a discussion to choose optimal therapy.
Another set of guidelines, from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), was last updated in 2015. Since then, many of the agents above have been introduced. Updated GRAPPA guidelines are expected to be released later this year.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Fundamental changes in the initial pharmacologic approach to psoriatic arthritis were made in the 2018 American College Rheumatology/National Psoriasis (ACR/NPF) guidelines for the treatment of psoriatic arthritis. Previously, methotrexate had been widely used as the first-line agent. The 2018 guidelines recommend a tumor necrosis factor (TNF) inhibitor over methotrexate and other oral small molecules (leflunomide, cyclosporine, and apremilast).
Herein is a broad summary of the guidelines:
· Treat with TNF inhibitor over oral small molecule; may consider oral small molecule with mild psoriatic arthritis and psoriasis, patient preference, and/or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with oral small molecule over IL-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis and/or psoriatic arthritis
· Treat with oral small molecule over IL-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or psoriatic arthritis, or concomitant inflammatory bowel disease
· Treat with methotrexate over nonsteroidal anti-inflammatory drugs; may consider nonsteroidals for mild psoriatic arthritis and psoriasis
· Treat with IL-17 inhibitor over IL-12/23 inhibitor; may consider IL-12/23 inhibitor in a patient with concomitant inflammatory bowel disease
Note that these recommendations are based on conditional evidence (ie, low to very low quality). In fact, in the entire guideline document, only 6% of the recommendations are strong, whereas 96% are conditional. This emphasizes the importance of evaluating each patient individually and engaging in a discussion to choose optimal therapy.
Another set of guidelines, from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), was last updated in 2015. Since then, many of the agents above have been introduced. Updated GRAPPA guidelines are expected to be released later this year.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Fundamental changes in the initial pharmacologic approach to psoriatic arthritis were made in the 2018 American College Rheumatology/National Psoriasis (ACR/NPF) guidelines for the treatment of psoriatic arthritis. Previously, methotrexate had been widely used as the first-line agent. The 2018 guidelines recommend a tumor necrosis factor (TNF) inhibitor over methotrexate and other oral small molecules (leflunomide, cyclosporine, and apremilast).
Herein is a broad summary of the guidelines:
· Treat with TNF inhibitor over oral small molecule; may consider oral small molecule with mild psoriatic arthritis and psoriasis, patient preference, and/or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with oral small molecule over IL-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis and/or psoriatic arthritis
· Treat with oral small molecule over IL-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or psoriatic arthritis, or concomitant inflammatory bowel disease
· Treat with methotrexate over nonsteroidal anti-inflammatory drugs; may consider nonsteroidals for mild psoriatic arthritis and psoriasis
· Treat with IL-17 inhibitor over IL-12/23 inhibitor; may consider IL-12/23 inhibitor in a patient with concomitant inflammatory bowel disease
Note that these recommendations are based on conditional evidence (ie, low to very low quality). In fact, in the entire guideline document, only 6% of the recommendations are strong, whereas 96% are conditional. This emphasizes the importance of evaluating each patient individually and engaging in a discussion to choose optimal therapy.
Another set of guidelines, from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), was last updated in 2015. Since then, many of the agents above have been introduced. Updated GRAPPA guidelines are expected to be released later this year.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
A 56-year-old man presents because of intermittent joint aches and difficulty picking up his grandchild and cleaning his home. He has a 6-year history of scalp psoriasis that he has controlled with a salicylic acid shampoo. On physical examination, he has tenderness over both elbows and in his metacarpophalangeal and proximal interphalangeal (PIP) joints on both hands. Swollen joints are noted in the proximal and distal joints of the right hand. His fingernails show uniform pitting.
Neurologic examination shows no sensory deficits or hyperesthesia. Motor examination is unremarkable, and chest and abdominal findings are unremarkable. Blood pressure is 138/90 mm Hg. Radiographic imaging shows asymmetric erosive changes with very small areas of bony proliferation in the PIP joints.There is asymmetric narrowing of the joint space in the interphalangeal joints. Laboratory findings reveal an erythrocyte sedimentation rate of 35 mm/h, negative rheumatoid factor, negative antinuclear antibody, and C-reactive protein of 7 mg/dL.
These findings are consistent with a diagnosis of psoriatic arthritis. This patient has severe psoriatic arthritis based on radiographic evidence of erosive disease.
Methotrexate plus leflunomide proves effective for PsA
A new study has found that methotrexate plus leflunomide outperforms methotrexate alone as a treatment option for patients with psoriatic arthritis (PsA).
“We believe that prescribing this combination in routine practice is viable when combined with shared decision-making and strict monitoring of side effects,” write Michelle L.M. Mulder, MD, of the department of rheumatology at Sint Maartenskliniek in Nijmegen, the Netherlands, and her coauthors. Their findings were published in The Lancet Rheumatology.
The latest treatment guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European Alliance of Associations for Rheumatology recommend conventional synthetic disease-modifying antirheumatic drugs for patients with active PsA, but Dr. Mulder and her colleagues note a distinct lack of information on their effectiveness, especially this particular combination.
To assess the efficacy and safety of methotrexate plus leflunomide, they launched a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA. The majority of the participants in this trial – dubbed COMPLETE-PsA – were men (64%), and the median age of the patients was 55 years. All had active disease at baseline; the median swollen joint count (SJC) and tender joint count were 4.0 in both groups.
Participants were assigned to receive either methotrexate plus leflunomide (n = 39) or methotrexate plus placebo (n = 39). After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1; standard deviation, 1.4 vs. 3.7; SD, 1.3; treatment difference, –0.6; 90% confidence interval, –1.0 to –0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than did the monotherapy group (34%; P = .019).
Other notable differences after 16 weeks included improvements in SJC for 66 joints (–3.0 in the combination therapy group vs. –2.0 in the monotherapy group) and significantly better skin and nail measures – such as active psoriasis and change in body surface area – in the methotrexate plus leflunomide group.
When asked who should be prescribed the combination therapy and who should be prescribed methotrexate going forward, Dr. Mulder told this news organization, “At the moment, we have insufficient knowledge on who will benefit most or who will develop clinically relevant side effects. It seems warranted to discuss with every patient which approach they would prefer. This could be a step-down or -up approach.
“We hope to be able to better predict treatment response and side effects in the future via post hoc analysis of our study and via extensive flow-cytometric phenotyping of immune blood cells taken at baseline,” she added.
Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group. No participants died, and all patients with adverse events recovered completely.
“It appears good practice to do blood draws for laboratory tests on liver enzymes at least monthly for the first 4 months and every 4 months after that once stable dosing is achieved, as well as have a telephone consultation after 6-8 weeks to talk about possible side effects a patient might experience and change or add therapy if necessary,” Dr. Mulder added.
Study turns perception of combination therapy into reality
It had already been perceived by rheumatologists that methotrexate plus leflunomide was an effective combo for PsA, and this study reinforces those beliefs, Clementina López-Medina, MD, PhD, and colleagues from the University of Cordoba (Spain), write in an accompanying editorial.
They highlight this study’s notable strengths, one of which was defining “active disease” as two or more swollen joints, which opened the study up to a larger patient population. The editorialists also underline the confirmation that leflunomide plus methotrexate reduces both joint symptoms and skin involvement in this subset of patients, which had also been found in a previous study.
“Leflunomide is usually considered as a second-line option after methotrexate is unsuccessful,” they note, “despite the fact that methotrexate did not show superiority over placebo in previous trials.”
The editorialists were not surprised that the combination therapy was more toxic than the monotherapy. Rheumatologists could use these data to individualize treatment accordingly, they write, while keeping an eye on “gastrointestinal disturbances.”
Overall, Dr. López-Medina and colleagues say that the study results should “be considered not only in daily clinical practice but also in the development of future recommendations.”
Leflunomide: Forgotten no longer, at least for PsA
“I think we probably underutilize leflunomide,” Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, told this news organization. “Sometimes medicines get ‘old,’ for lack of a better term, and fall a little bit of out of favor, sometimes unnecessarily. Leflunomide falls into that category. Because it’s older, it doesn’t get as much buzz as what’s new and shiny.
“I was not surprised by the results on the joints,” he said, “because we know from previous studies that leflunomide works in that regard. What did surprise me is that the skin got better, especially with the combination.”
Regarding the side effects for the combination therapy, he commended the authors for limiting potential uncertainty by using such a high dose of methotrexate.
“By going with a dose of 25 mg [per week], no one can say, ‘They pulled their punches and methotrexate monotherapy would’ve been just as good if it was given at a higher dose,’ “ he said. “And they also used leflunomide at a high dose. It makes you wonder: Could you use lower doses, and do lower doses mean fewer lab test abnormalities? This positive study does lend itself to some other permutations in terms of study design.
“Even though this was a small study,” he added, “it brings us right back to: We should really consider leflunomide in the treatment of PsA.”
The authors acknowledge their study’s limitations, including the fact that it was conducted in a single country and the absence of a nontreatment placebo group. They also note the higher percentage of women in the methotrexate plus leflunomide group, “which might have lowered the treatment response and increased the adverse event rate, resulting in bias.”
The study was funded by a Regional Junior Researcher Grant from Sint Maartenskliniek. The authors reported numerous potential conflicts of interest, including receiving payment, research grants, and consulting and speaker fees from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new study has found that methotrexate plus leflunomide outperforms methotrexate alone as a treatment option for patients with psoriatic arthritis (PsA).
“We believe that prescribing this combination in routine practice is viable when combined with shared decision-making and strict monitoring of side effects,” write Michelle L.M. Mulder, MD, of the department of rheumatology at Sint Maartenskliniek in Nijmegen, the Netherlands, and her coauthors. Their findings were published in The Lancet Rheumatology.
The latest treatment guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European Alliance of Associations for Rheumatology recommend conventional synthetic disease-modifying antirheumatic drugs for patients with active PsA, but Dr. Mulder and her colleagues note a distinct lack of information on their effectiveness, especially this particular combination.
To assess the efficacy and safety of methotrexate plus leflunomide, they launched a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA. The majority of the participants in this trial – dubbed COMPLETE-PsA – were men (64%), and the median age of the patients was 55 years. All had active disease at baseline; the median swollen joint count (SJC) and tender joint count were 4.0 in both groups.
Participants were assigned to receive either methotrexate plus leflunomide (n = 39) or methotrexate plus placebo (n = 39). After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1; standard deviation, 1.4 vs. 3.7; SD, 1.3; treatment difference, –0.6; 90% confidence interval, –1.0 to –0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than did the monotherapy group (34%; P = .019).
Other notable differences after 16 weeks included improvements in SJC for 66 joints (–3.0 in the combination therapy group vs. –2.0 in the monotherapy group) and significantly better skin and nail measures – such as active psoriasis and change in body surface area – in the methotrexate plus leflunomide group.
When asked who should be prescribed the combination therapy and who should be prescribed methotrexate going forward, Dr. Mulder told this news organization, “At the moment, we have insufficient knowledge on who will benefit most or who will develop clinically relevant side effects. It seems warranted to discuss with every patient which approach they would prefer. This could be a step-down or -up approach.
“We hope to be able to better predict treatment response and side effects in the future via post hoc analysis of our study and via extensive flow-cytometric phenotyping of immune blood cells taken at baseline,” she added.
Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group. No participants died, and all patients with adverse events recovered completely.
“It appears good practice to do blood draws for laboratory tests on liver enzymes at least monthly for the first 4 months and every 4 months after that once stable dosing is achieved, as well as have a telephone consultation after 6-8 weeks to talk about possible side effects a patient might experience and change or add therapy if necessary,” Dr. Mulder added.
Study turns perception of combination therapy into reality
It had already been perceived by rheumatologists that methotrexate plus leflunomide was an effective combo for PsA, and this study reinforces those beliefs, Clementina López-Medina, MD, PhD, and colleagues from the University of Cordoba (Spain), write in an accompanying editorial.
They highlight this study’s notable strengths, one of which was defining “active disease” as two or more swollen joints, which opened the study up to a larger patient population. The editorialists also underline the confirmation that leflunomide plus methotrexate reduces both joint symptoms and skin involvement in this subset of patients, which had also been found in a previous study.
“Leflunomide is usually considered as a second-line option after methotrexate is unsuccessful,” they note, “despite the fact that methotrexate did not show superiority over placebo in previous trials.”
The editorialists were not surprised that the combination therapy was more toxic than the monotherapy. Rheumatologists could use these data to individualize treatment accordingly, they write, while keeping an eye on “gastrointestinal disturbances.”
Overall, Dr. López-Medina and colleagues say that the study results should “be considered not only in daily clinical practice but also in the development of future recommendations.”
Leflunomide: Forgotten no longer, at least for PsA
“I think we probably underutilize leflunomide,” Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, told this news organization. “Sometimes medicines get ‘old,’ for lack of a better term, and fall a little bit of out of favor, sometimes unnecessarily. Leflunomide falls into that category. Because it’s older, it doesn’t get as much buzz as what’s new and shiny.
“I was not surprised by the results on the joints,” he said, “because we know from previous studies that leflunomide works in that regard. What did surprise me is that the skin got better, especially with the combination.”
Regarding the side effects for the combination therapy, he commended the authors for limiting potential uncertainty by using such a high dose of methotrexate.
“By going with a dose of 25 mg [per week], no one can say, ‘They pulled their punches and methotrexate monotherapy would’ve been just as good if it was given at a higher dose,’ “ he said. “And they also used leflunomide at a high dose. It makes you wonder: Could you use lower doses, and do lower doses mean fewer lab test abnormalities? This positive study does lend itself to some other permutations in terms of study design.
“Even though this was a small study,” he added, “it brings us right back to: We should really consider leflunomide in the treatment of PsA.”
The authors acknowledge their study’s limitations, including the fact that it was conducted in a single country and the absence of a nontreatment placebo group. They also note the higher percentage of women in the methotrexate plus leflunomide group, “which might have lowered the treatment response and increased the adverse event rate, resulting in bias.”
The study was funded by a Regional Junior Researcher Grant from Sint Maartenskliniek. The authors reported numerous potential conflicts of interest, including receiving payment, research grants, and consulting and speaker fees from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new study has found that methotrexate plus leflunomide outperforms methotrexate alone as a treatment option for patients with psoriatic arthritis (PsA).
“We believe that prescribing this combination in routine practice is viable when combined with shared decision-making and strict monitoring of side effects,” write Michelle L.M. Mulder, MD, of the department of rheumatology at Sint Maartenskliniek in Nijmegen, the Netherlands, and her coauthors. Their findings were published in The Lancet Rheumatology.
The latest treatment guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European Alliance of Associations for Rheumatology recommend conventional synthetic disease-modifying antirheumatic drugs for patients with active PsA, but Dr. Mulder and her colleagues note a distinct lack of information on their effectiveness, especially this particular combination.
To assess the efficacy and safety of methotrexate plus leflunomide, they launched a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA. The majority of the participants in this trial – dubbed COMPLETE-PsA – were men (64%), and the median age of the patients was 55 years. All had active disease at baseline; the median swollen joint count (SJC) and tender joint count were 4.0 in both groups.
Participants were assigned to receive either methotrexate plus leflunomide (n = 39) or methotrexate plus placebo (n = 39). After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1; standard deviation, 1.4 vs. 3.7; SD, 1.3; treatment difference, –0.6; 90% confidence interval, –1.0 to –0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than did the monotherapy group (34%; P = .019).
Other notable differences after 16 weeks included improvements in SJC for 66 joints (–3.0 in the combination therapy group vs. –2.0 in the monotherapy group) and significantly better skin and nail measures – such as active psoriasis and change in body surface area – in the methotrexate plus leflunomide group.
When asked who should be prescribed the combination therapy and who should be prescribed methotrexate going forward, Dr. Mulder told this news organization, “At the moment, we have insufficient knowledge on who will benefit most or who will develop clinically relevant side effects. It seems warranted to discuss with every patient which approach they would prefer. This could be a step-down or -up approach.
“We hope to be able to better predict treatment response and side effects in the future via post hoc analysis of our study and via extensive flow-cytometric phenotyping of immune blood cells taken at baseline,” she added.
Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group. No participants died, and all patients with adverse events recovered completely.
“It appears good practice to do blood draws for laboratory tests on liver enzymes at least monthly for the first 4 months and every 4 months after that once stable dosing is achieved, as well as have a telephone consultation after 6-8 weeks to talk about possible side effects a patient might experience and change or add therapy if necessary,” Dr. Mulder added.
Study turns perception of combination therapy into reality
It had already been perceived by rheumatologists that methotrexate plus leflunomide was an effective combo for PsA, and this study reinforces those beliefs, Clementina López-Medina, MD, PhD, and colleagues from the University of Cordoba (Spain), write in an accompanying editorial.
They highlight this study’s notable strengths, one of which was defining “active disease” as two or more swollen joints, which opened the study up to a larger patient population. The editorialists also underline the confirmation that leflunomide plus methotrexate reduces both joint symptoms and skin involvement in this subset of patients, which had also been found in a previous study.
“Leflunomide is usually considered as a second-line option after methotrexate is unsuccessful,” they note, “despite the fact that methotrexate did not show superiority over placebo in previous trials.”
The editorialists were not surprised that the combination therapy was more toxic than the monotherapy. Rheumatologists could use these data to individualize treatment accordingly, they write, while keeping an eye on “gastrointestinal disturbances.”
Overall, Dr. López-Medina and colleagues say that the study results should “be considered not only in daily clinical practice but also in the development of future recommendations.”
Leflunomide: Forgotten no longer, at least for PsA
“I think we probably underutilize leflunomide,” Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, told this news organization. “Sometimes medicines get ‘old,’ for lack of a better term, and fall a little bit of out of favor, sometimes unnecessarily. Leflunomide falls into that category. Because it’s older, it doesn’t get as much buzz as what’s new and shiny.
“I was not surprised by the results on the joints,” he said, “because we know from previous studies that leflunomide works in that regard. What did surprise me is that the skin got better, especially with the combination.”
Regarding the side effects for the combination therapy, he commended the authors for limiting potential uncertainty by using such a high dose of methotrexate.
“By going with a dose of 25 mg [per week], no one can say, ‘They pulled their punches and methotrexate monotherapy would’ve been just as good if it was given at a higher dose,’ “ he said. “And they also used leflunomide at a high dose. It makes you wonder: Could you use lower doses, and do lower doses mean fewer lab test abnormalities? This positive study does lend itself to some other permutations in terms of study design.
“Even though this was a small study,” he added, “it brings us right back to: We should really consider leflunomide in the treatment of PsA.”
The authors acknowledge their study’s limitations, including the fact that it was conducted in a single country and the absence of a nontreatment placebo group. They also note the higher percentage of women in the methotrexate plus leflunomide group, “which might have lowered the treatment response and increased the adverse event rate, resulting in bias.”
The study was funded by a Regional Junior Researcher Grant from Sint Maartenskliniek. The authors reported numerous potential conflicts of interest, including receiving payment, research grants, and consulting and speaker fees from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Psoriatic Arthritis: Presentation and Diagnosis
Psoriatic Arthritis: The Basics
Updated perioperative guidance says when to hold antirheumatics
The American College of Rheumatology and the American Association of Hip and Knee Surgeons have released updated guidelines regarding whether to withhold drugs such as biologics and immunosuppressives for patients with inflammatory rheumatic disease who are scheduled to undergo elective total hip or knee replacement surgery.
The guidelines, published in a summary by the societies on Feb. 28, include revised and new recommendations about biologics and Janus kinase (JAK) inhibitors for patients with several types of inflammatory arthritis and systemic lupus erythematosus (SLE). In general, the guidelines recommend that the most powerful medications be withheld prior to surgery except for patients whose SLE is so severe that it threatens organs. They also recommend a shorter period of withholding drugs – 3 days instead of 7 – for JAK inhibitors.
The previous guidelines were published in 2017.
“These recommendations seek to balance flares of disease that are likely when medications are stopped vs. the risk of infection,” Susan M. Goodman, MD, a rheumatologist at the Hospital for Special Surgery, New York, and co–principal investigator of the guideline, told this news organization. “Patients and physicians may want to be either more conservative or more aggressive with their medications, depending on their personal priorities or specific medical history.”
According to Dr. Goodman, patients with inflammatory rheumatic diseases are especially likely to undergo joint replacement surgery because the conditions can damage the joints. “While the introduction of potent biologics has been linked to a decrease in surgery of soft tissues and small joints, there has been little impact on large-joint surgeries,” she said.
The risk of infection in these patients is about 50% higher than in the general population, she said. However, “it is hard to determine the magnitude of the effect of withholding medications, given the low rate of infection. In fact, using pharmaco-epidemiologic methods in large Medicare databases, no difference was seen in patients whose immunosuppressant medication infusions were close to the time of surgery compared to those patients whose medication infusions were months prior to surgery.”
The guidelines add a recommendation for the first time for apremilast (Otezla), saying that when it is administered twice daily it is okay to schedule surgery at any time.
Withholding drugs in patients with SLE
“We now recommend continuing biologics used to treat SLE – rituximab and belimumab – in patients with severe SLE but continue to recommend withholding them in less severe cases where there is little risk of organ damage,” Bryan D. Springer, MD, an orthopedic surgeon in Charlotte, N.C., first vice president of the AAHKS, and co–principal investigator of the new guidelines, told this news organization.
In severe SLE cases, the guidelines recommend timing total joint replacement surgery for 4-6 months after the latest IV dose of rituximab (Rituxan), which is given every 4-6 months. For patients taking belimumab (Benlysta), time surgery anytime when weekly subcutaneous doses are administered or at week 4 when monthly IV doses are given.
The guidelines also make recommendations regarding two new drugs for the treatment of severe SLE:
- Anifrolumab (Saphnelo): Time surgery at week 4 when IV treatment is given every 4 weeks.
- Voclosporin (Lupkynis): Continue doses when they’re given twice daily.
An ACR statement cautions that there are no published, peer-reviewed data regarding the use of these two drugs prior to total joint surgery. “The medications do increase the risk of infection,” the statement says, “and therefore their use in patients with severe SLE would merit review by the treating rheumatologist in consideration of surgery.”
Timing of stopping and restarting medication
The guidelines also recommend that certain drugs be withheld for patients with rheumatoid arthritis, ankylosing spondylitis, or any type of SLE and then “restarting the antirheumatic therapy once the wound shows evidence of healing, any sutures/staples are out, there is no significant swelling, erythema, or drainage, and there is no ongoing nonsurgical site infection, which is typically about 14 days.”
In regard to biologics, “we continue to recommend withholding biologic medications in patients with inflammatory arthritis, withholding the medication for a dosing cycle prior to surgery, and scheduling the surgery after that dose would be due,” Dr. Springer said. “For example, if a patient takes the medication every 4 weeks, the patient would withhold the dose of the medication and schedule surgery in the 5th week.”
The new recommendations for biologics suggest scheduling surgery at week 5 when the interleukin (IL)-17 inhibitor ixekizumab (Taltz) is given once every 4 weeks and at week 9 when the IL-23 inhibitor guselkumab (Tremfya) is given every 8 weeks.
The guidelines also revise the previous recommendation about tofacitinib (Xeljanz): Surgery should be scheduled on day 4 when the drug is given once or twice daily. New recommendations for fellow JAK inhibitors baricitinib (Olumiant, daily) and upadacitinib (Rinvoq, daily) are the same: Withhold for 3 days prior to surgery and perform surgery on the 4th day.
“We shortened the time between the last dose of JAK inhibitors and surgery to 3 days from 7 based on trial data demonstrating early flares when the drug was withheld, suggesting the immunosuppressant effect wears off sooner than we previously thought,” Dr. Springer said.
The guidelines caution that the recommendations for JAK inhibitors are for infection risk but do not consider the risk of cardiac events or venous thromboembolism.
In patients with nonsevere SLE, the guidelines revise the recommendations for mycophenolate mofetil (twice daily), cyclosporine (twice daily), and tacrolimus (twice daily, IV and oral). The new advice is to withhold the drugs for 1 week after last dose prior to surgery. New recommendations offer the same advice for belimumab, both IV and subcutaneous: Withhold for 1 week after last dose prior to surgery.
The board of the ACR approved the guidelines summary; the full manuscript has been submitted for peer review with an eye toward later publication in the journals Arthritis and Rheumatology and Arthritis Care and Research.
The ACR and AAHKS funded the guidelines. Dr. Goodman and Dr. Springer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The American College of Rheumatology and the American Association of Hip and Knee Surgeons have released updated guidelines regarding whether to withhold drugs such as biologics and immunosuppressives for patients with inflammatory rheumatic disease who are scheduled to undergo elective total hip or knee replacement surgery.
The guidelines, published in a summary by the societies on Feb. 28, include revised and new recommendations about biologics and Janus kinase (JAK) inhibitors for patients with several types of inflammatory arthritis and systemic lupus erythematosus (SLE). In general, the guidelines recommend that the most powerful medications be withheld prior to surgery except for patients whose SLE is so severe that it threatens organs. They also recommend a shorter period of withholding drugs – 3 days instead of 7 – for JAK inhibitors.
The previous guidelines were published in 2017.
“These recommendations seek to balance flares of disease that are likely when medications are stopped vs. the risk of infection,” Susan M. Goodman, MD, a rheumatologist at the Hospital for Special Surgery, New York, and co–principal investigator of the guideline, told this news organization. “Patients and physicians may want to be either more conservative or more aggressive with their medications, depending on their personal priorities or specific medical history.”
According to Dr. Goodman, patients with inflammatory rheumatic diseases are especially likely to undergo joint replacement surgery because the conditions can damage the joints. “While the introduction of potent biologics has been linked to a decrease in surgery of soft tissues and small joints, there has been little impact on large-joint surgeries,” she said.
The risk of infection in these patients is about 50% higher than in the general population, she said. However, “it is hard to determine the magnitude of the effect of withholding medications, given the low rate of infection. In fact, using pharmaco-epidemiologic methods in large Medicare databases, no difference was seen in patients whose immunosuppressant medication infusions were close to the time of surgery compared to those patients whose medication infusions were months prior to surgery.”
The guidelines add a recommendation for the first time for apremilast (Otezla), saying that when it is administered twice daily it is okay to schedule surgery at any time.
Withholding drugs in patients with SLE
“We now recommend continuing biologics used to treat SLE – rituximab and belimumab – in patients with severe SLE but continue to recommend withholding them in less severe cases where there is little risk of organ damage,” Bryan D. Springer, MD, an orthopedic surgeon in Charlotte, N.C., first vice president of the AAHKS, and co–principal investigator of the new guidelines, told this news organization.
In severe SLE cases, the guidelines recommend timing total joint replacement surgery for 4-6 months after the latest IV dose of rituximab (Rituxan), which is given every 4-6 months. For patients taking belimumab (Benlysta), time surgery anytime when weekly subcutaneous doses are administered or at week 4 when monthly IV doses are given.
The guidelines also make recommendations regarding two new drugs for the treatment of severe SLE:
- Anifrolumab (Saphnelo): Time surgery at week 4 when IV treatment is given every 4 weeks.
- Voclosporin (Lupkynis): Continue doses when they’re given twice daily.
An ACR statement cautions that there are no published, peer-reviewed data regarding the use of these two drugs prior to total joint surgery. “The medications do increase the risk of infection,” the statement says, “and therefore their use in patients with severe SLE would merit review by the treating rheumatologist in consideration of surgery.”
Timing of stopping and restarting medication
The guidelines also recommend that certain drugs be withheld for patients with rheumatoid arthritis, ankylosing spondylitis, or any type of SLE and then “restarting the antirheumatic therapy once the wound shows evidence of healing, any sutures/staples are out, there is no significant swelling, erythema, or drainage, and there is no ongoing nonsurgical site infection, which is typically about 14 days.”
In regard to biologics, “we continue to recommend withholding biologic medications in patients with inflammatory arthritis, withholding the medication for a dosing cycle prior to surgery, and scheduling the surgery after that dose would be due,” Dr. Springer said. “For example, if a patient takes the medication every 4 weeks, the patient would withhold the dose of the medication and schedule surgery in the 5th week.”
The new recommendations for biologics suggest scheduling surgery at week 5 when the interleukin (IL)-17 inhibitor ixekizumab (Taltz) is given once every 4 weeks and at week 9 when the IL-23 inhibitor guselkumab (Tremfya) is given every 8 weeks.
The guidelines also revise the previous recommendation about tofacitinib (Xeljanz): Surgery should be scheduled on day 4 when the drug is given once or twice daily. New recommendations for fellow JAK inhibitors baricitinib (Olumiant, daily) and upadacitinib (Rinvoq, daily) are the same: Withhold for 3 days prior to surgery and perform surgery on the 4th day.
“We shortened the time between the last dose of JAK inhibitors and surgery to 3 days from 7 based on trial data demonstrating early flares when the drug was withheld, suggesting the immunosuppressant effect wears off sooner than we previously thought,” Dr. Springer said.
The guidelines caution that the recommendations for JAK inhibitors are for infection risk but do not consider the risk of cardiac events or venous thromboembolism.
In patients with nonsevere SLE, the guidelines revise the recommendations for mycophenolate mofetil (twice daily), cyclosporine (twice daily), and tacrolimus (twice daily, IV and oral). The new advice is to withhold the drugs for 1 week after last dose prior to surgery. New recommendations offer the same advice for belimumab, both IV and subcutaneous: Withhold for 1 week after last dose prior to surgery.
The board of the ACR approved the guidelines summary; the full manuscript has been submitted for peer review with an eye toward later publication in the journals Arthritis and Rheumatology and Arthritis Care and Research.
The ACR and AAHKS funded the guidelines. Dr. Goodman and Dr. Springer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The American College of Rheumatology and the American Association of Hip and Knee Surgeons have released updated guidelines regarding whether to withhold drugs such as biologics and immunosuppressives for patients with inflammatory rheumatic disease who are scheduled to undergo elective total hip or knee replacement surgery.
The guidelines, published in a summary by the societies on Feb. 28, include revised and new recommendations about biologics and Janus kinase (JAK) inhibitors for patients with several types of inflammatory arthritis and systemic lupus erythematosus (SLE). In general, the guidelines recommend that the most powerful medications be withheld prior to surgery except for patients whose SLE is so severe that it threatens organs. They also recommend a shorter period of withholding drugs – 3 days instead of 7 – for JAK inhibitors.
The previous guidelines were published in 2017.
“These recommendations seek to balance flares of disease that are likely when medications are stopped vs. the risk of infection,” Susan M. Goodman, MD, a rheumatologist at the Hospital for Special Surgery, New York, and co–principal investigator of the guideline, told this news organization. “Patients and physicians may want to be either more conservative or more aggressive with their medications, depending on their personal priorities or specific medical history.”
According to Dr. Goodman, patients with inflammatory rheumatic diseases are especially likely to undergo joint replacement surgery because the conditions can damage the joints. “While the introduction of potent biologics has been linked to a decrease in surgery of soft tissues and small joints, there has been little impact on large-joint surgeries,” she said.
The risk of infection in these patients is about 50% higher than in the general population, she said. However, “it is hard to determine the magnitude of the effect of withholding medications, given the low rate of infection. In fact, using pharmaco-epidemiologic methods in large Medicare databases, no difference was seen in patients whose immunosuppressant medication infusions were close to the time of surgery compared to those patients whose medication infusions were months prior to surgery.”
The guidelines add a recommendation for the first time for apremilast (Otezla), saying that when it is administered twice daily it is okay to schedule surgery at any time.
Withholding drugs in patients with SLE
“We now recommend continuing biologics used to treat SLE – rituximab and belimumab – in patients with severe SLE but continue to recommend withholding them in less severe cases where there is little risk of organ damage,” Bryan D. Springer, MD, an orthopedic surgeon in Charlotte, N.C., first vice president of the AAHKS, and co–principal investigator of the new guidelines, told this news organization.
In severe SLE cases, the guidelines recommend timing total joint replacement surgery for 4-6 months after the latest IV dose of rituximab (Rituxan), which is given every 4-6 months. For patients taking belimumab (Benlysta), time surgery anytime when weekly subcutaneous doses are administered or at week 4 when monthly IV doses are given.
The guidelines also make recommendations regarding two new drugs for the treatment of severe SLE:
- Anifrolumab (Saphnelo): Time surgery at week 4 when IV treatment is given every 4 weeks.
- Voclosporin (Lupkynis): Continue doses when they’re given twice daily.
An ACR statement cautions that there are no published, peer-reviewed data regarding the use of these two drugs prior to total joint surgery. “The medications do increase the risk of infection,” the statement says, “and therefore their use in patients with severe SLE would merit review by the treating rheumatologist in consideration of surgery.”
Timing of stopping and restarting medication
The guidelines also recommend that certain drugs be withheld for patients with rheumatoid arthritis, ankylosing spondylitis, or any type of SLE and then “restarting the antirheumatic therapy once the wound shows evidence of healing, any sutures/staples are out, there is no significant swelling, erythema, or drainage, and there is no ongoing nonsurgical site infection, which is typically about 14 days.”
In regard to biologics, “we continue to recommend withholding biologic medications in patients with inflammatory arthritis, withholding the medication for a dosing cycle prior to surgery, and scheduling the surgery after that dose would be due,” Dr. Springer said. “For example, if a patient takes the medication every 4 weeks, the patient would withhold the dose of the medication and schedule surgery in the 5th week.”
The new recommendations for biologics suggest scheduling surgery at week 5 when the interleukin (IL)-17 inhibitor ixekizumab (Taltz) is given once every 4 weeks and at week 9 when the IL-23 inhibitor guselkumab (Tremfya) is given every 8 weeks.
The guidelines also revise the previous recommendation about tofacitinib (Xeljanz): Surgery should be scheduled on day 4 when the drug is given once or twice daily. New recommendations for fellow JAK inhibitors baricitinib (Olumiant, daily) and upadacitinib (Rinvoq, daily) are the same: Withhold for 3 days prior to surgery and perform surgery on the 4th day.
“We shortened the time between the last dose of JAK inhibitors and surgery to 3 days from 7 based on trial data demonstrating early flares when the drug was withheld, suggesting the immunosuppressant effect wears off sooner than we previously thought,” Dr. Springer said.
The guidelines caution that the recommendations for JAK inhibitors are for infection risk but do not consider the risk of cardiac events or venous thromboembolism.
In patients with nonsevere SLE, the guidelines revise the recommendations for mycophenolate mofetil (twice daily), cyclosporine (twice daily), and tacrolimus (twice daily, IV and oral). The new advice is to withhold the drugs for 1 week after last dose prior to surgery. New recommendations offer the same advice for belimumab, both IV and subcutaneous: Withhold for 1 week after last dose prior to surgery.
The board of the ACR approved the guidelines summary; the full manuscript has been submitted for peer review with an eye toward later publication in the journals Arthritis and Rheumatology and Arthritis Care and Research.
The ACR and AAHKS funded the guidelines. Dr. Goodman and Dr. Springer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One-third of psoriatic arthritis patients could have metabolic syndrome, data analysis finds
of 724 individuals, as did approximately 23%-63% of patients across multiple studies, investigators from Spain report.
Previous studies of people with PsA in particular suggest they are at an increased risk of cardiovascular disease and have a higher prevalence of metabolic syndrome, prompting recommendations on cardiovascular risk management for patients with PsA, wrote the authors, Ana Urruticoechea-Arana, MD, of the department of rheumatology, Hospital Can Misses, Ibiza, Spain, and colleagues.
However, assessing the prevalence of metabolic syndrome remains a challenge because the definition varies across studies, they noted.
For a more thorough assessment of the prevalence of metabolic syndrome in this population, the researchers conducted a study using two sources: a systematic literature review of 18 studies published up to March 2019, and data on patients with PsA enrolled in the CARMA (Spanish Cardiovascular in Rheumatology) project, a longitudinal cohort observational study of adults with inflammatory diseases in Spain. The findings were published March 1 in the Journal of Clinical Rheumatology.
The literature review included a total of a total of 2,452 patients with PsA, with a mean age between 42 and 59 years, and a mean disease duration ranging from 3 to 14 years.
The definitions of metabolic syndrome varied; the most common was the definition from the National Cholesterol Education Program (NECP ATP III). Other definitions used in the studies included those issued by the International Diabetes Federation, the World Health Organization, and the American Heart Association.
Across these studies, the rate of metabolic syndrome ranged from 23.5% to 62.9%. Prevalence was similar between men and women. One study that included patients with a PsA disease duration of only 3 years showed a prevalence of 38%, similar to the average prevalence overall. Another study showed a significantly higher prevalence of metabolic syndrome in patients with PsA and cutaneous psoriasis, compared with those without psoriasis (40.8% vs. 13.16%; P = .006).
The CARMA study included 724 patients with PsA; 45.4% were women and 21.8% were smokers. The mean age of the population in this study was 51 years, and the mean disease duration was 9 years. Overall, 222 patients (30.7%) met at least three criteria for metabolic syndrome, based on the NCEP ATP III definition. The most common abnormal findings for traditional cardiovascular risk factors in the CARMA cohort were high blood pressure (66.8%), hyperglycemia (42.6%), and hypertriglyceridemia (30.6%).
Despite the variation in prevalence of metabolic syndrome, depending on the definition used, the authors wrote, “It can be stated that the rate of [metabolic syndrome] in patients with PsA is in general very high, especially if we take into account the mean age of patients included in the studies.”
“These findings support the hypotheses that this increase in the inflammatory pathway in PsA may contribute a higher risk of cardiovascular events and [metabolic syndrome] in patients with PsA than patients with psoriasis alone, the risk being even higher in severe PsA,” and that insulin resistance, metabolic syndrome, and atherosclerotic events “may have a common inflammatory basis,” the researchers wrote in their discussion of the results.
The study findings were limited by several factors, most importantly the variation in definitions of metabolic syndrome in the literature review, which limits the generalizability of the results, the researchers said. Limitations of the CARMA study include the focus only on patients who were being cared for in hospitals, which might yield an overestimation of metabolic syndrome, they added.
However, the results support findings from previous studies and highlight the need for proper assessment of body weight and cardiovascular risk factors in patients with PsA at the onset of disease, they said.
“Furthermore, it is necessary to conduct more research to standardize (and modify as appropriate) the definition of [metabolic syndrome] and establish the best strategy for managing it in these patients,” they concluded.
The study was funded by an independent grant from UCB Pharma. One author disclosed receiving grants from Pfizer, Abbvie, Novartis, Roche, UCB, Sanofi, BMS, Lilly, MSD, and Janssen. Lead author Dr. Urruticoechea-Arana and the other authors had no disclosures.
of 724 individuals, as did approximately 23%-63% of patients across multiple studies, investigators from Spain report.
Previous studies of people with PsA in particular suggest they are at an increased risk of cardiovascular disease and have a higher prevalence of metabolic syndrome, prompting recommendations on cardiovascular risk management for patients with PsA, wrote the authors, Ana Urruticoechea-Arana, MD, of the department of rheumatology, Hospital Can Misses, Ibiza, Spain, and colleagues.
However, assessing the prevalence of metabolic syndrome remains a challenge because the definition varies across studies, they noted.
For a more thorough assessment of the prevalence of metabolic syndrome in this population, the researchers conducted a study using two sources: a systematic literature review of 18 studies published up to March 2019, and data on patients with PsA enrolled in the CARMA (Spanish Cardiovascular in Rheumatology) project, a longitudinal cohort observational study of adults with inflammatory diseases in Spain. The findings were published March 1 in the Journal of Clinical Rheumatology.
The literature review included a total of a total of 2,452 patients with PsA, with a mean age between 42 and 59 years, and a mean disease duration ranging from 3 to 14 years.
The definitions of metabolic syndrome varied; the most common was the definition from the National Cholesterol Education Program (NECP ATP III). Other definitions used in the studies included those issued by the International Diabetes Federation, the World Health Organization, and the American Heart Association.
Across these studies, the rate of metabolic syndrome ranged from 23.5% to 62.9%. Prevalence was similar between men and women. One study that included patients with a PsA disease duration of only 3 years showed a prevalence of 38%, similar to the average prevalence overall. Another study showed a significantly higher prevalence of metabolic syndrome in patients with PsA and cutaneous psoriasis, compared with those without psoriasis (40.8% vs. 13.16%; P = .006).
The CARMA study included 724 patients with PsA; 45.4% were women and 21.8% were smokers. The mean age of the population in this study was 51 years, and the mean disease duration was 9 years. Overall, 222 patients (30.7%) met at least three criteria for metabolic syndrome, based on the NCEP ATP III definition. The most common abnormal findings for traditional cardiovascular risk factors in the CARMA cohort were high blood pressure (66.8%), hyperglycemia (42.6%), and hypertriglyceridemia (30.6%).
Despite the variation in prevalence of metabolic syndrome, depending on the definition used, the authors wrote, “It can be stated that the rate of [metabolic syndrome] in patients with PsA is in general very high, especially if we take into account the mean age of patients included in the studies.”
“These findings support the hypotheses that this increase in the inflammatory pathway in PsA may contribute a higher risk of cardiovascular events and [metabolic syndrome] in patients with PsA than patients with psoriasis alone, the risk being even higher in severe PsA,” and that insulin resistance, metabolic syndrome, and atherosclerotic events “may have a common inflammatory basis,” the researchers wrote in their discussion of the results.
The study findings were limited by several factors, most importantly the variation in definitions of metabolic syndrome in the literature review, which limits the generalizability of the results, the researchers said. Limitations of the CARMA study include the focus only on patients who were being cared for in hospitals, which might yield an overestimation of metabolic syndrome, they added.
However, the results support findings from previous studies and highlight the need for proper assessment of body weight and cardiovascular risk factors in patients with PsA at the onset of disease, they said.
“Furthermore, it is necessary to conduct more research to standardize (and modify as appropriate) the definition of [metabolic syndrome] and establish the best strategy for managing it in these patients,” they concluded.
The study was funded by an independent grant from UCB Pharma. One author disclosed receiving grants from Pfizer, Abbvie, Novartis, Roche, UCB, Sanofi, BMS, Lilly, MSD, and Janssen. Lead author Dr. Urruticoechea-Arana and the other authors had no disclosures.
of 724 individuals, as did approximately 23%-63% of patients across multiple studies, investigators from Spain report.
Previous studies of people with PsA in particular suggest they are at an increased risk of cardiovascular disease and have a higher prevalence of metabolic syndrome, prompting recommendations on cardiovascular risk management for patients with PsA, wrote the authors, Ana Urruticoechea-Arana, MD, of the department of rheumatology, Hospital Can Misses, Ibiza, Spain, and colleagues.
However, assessing the prevalence of metabolic syndrome remains a challenge because the definition varies across studies, they noted.
For a more thorough assessment of the prevalence of metabolic syndrome in this population, the researchers conducted a study using two sources: a systematic literature review of 18 studies published up to March 2019, and data on patients with PsA enrolled in the CARMA (Spanish Cardiovascular in Rheumatology) project, a longitudinal cohort observational study of adults with inflammatory diseases in Spain. The findings were published March 1 in the Journal of Clinical Rheumatology.
The literature review included a total of a total of 2,452 patients with PsA, with a mean age between 42 and 59 years, and a mean disease duration ranging from 3 to 14 years.
The definitions of metabolic syndrome varied; the most common was the definition from the National Cholesterol Education Program (NECP ATP III). Other definitions used in the studies included those issued by the International Diabetes Federation, the World Health Organization, and the American Heart Association.
Across these studies, the rate of metabolic syndrome ranged from 23.5% to 62.9%. Prevalence was similar between men and women. One study that included patients with a PsA disease duration of only 3 years showed a prevalence of 38%, similar to the average prevalence overall. Another study showed a significantly higher prevalence of metabolic syndrome in patients with PsA and cutaneous psoriasis, compared with those without psoriasis (40.8% vs. 13.16%; P = .006).
The CARMA study included 724 patients with PsA; 45.4% were women and 21.8% were smokers. The mean age of the population in this study was 51 years, and the mean disease duration was 9 years. Overall, 222 patients (30.7%) met at least three criteria for metabolic syndrome, based on the NCEP ATP III definition. The most common abnormal findings for traditional cardiovascular risk factors in the CARMA cohort were high blood pressure (66.8%), hyperglycemia (42.6%), and hypertriglyceridemia (30.6%).
Despite the variation in prevalence of metabolic syndrome, depending on the definition used, the authors wrote, “It can be stated that the rate of [metabolic syndrome] in patients with PsA is in general very high, especially if we take into account the mean age of patients included in the studies.”
“These findings support the hypotheses that this increase in the inflammatory pathway in PsA may contribute a higher risk of cardiovascular events and [metabolic syndrome] in patients with PsA than patients with psoriasis alone, the risk being even higher in severe PsA,” and that insulin resistance, metabolic syndrome, and atherosclerotic events “may have a common inflammatory basis,” the researchers wrote in their discussion of the results.
The study findings were limited by several factors, most importantly the variation in definitions of metabolic syndrome in the literature review, which limits the generalizability of the results, the researchers said. Limitations of the CARMA study include the focus only on patients who were being cared for in hospitals, which might yield an overestimation of metabolic syndrome, they added.
However, the results support findings from previous studies and highlight the need for proper assessment of body weight and cardiovascular risk factors in patients with PsA at the onset of disease, they said.
“Furthermore, it is necessary to conduct more research to standardize (and modify as appropriate) the definition of [metabolic syndrome] and establish the best strategy for managing it in these patients,” they concluded.
The study was funded by an independent grant from UCB Pharma. One author disclosed receiving grants from Pfizer, Abbvie, Novartis, Roche, UCB, Sanofi, BMS, Lilly, MSD, and Janssen. Lead author Dr. Urruticoechea-Arana and the other authors had no disclosures.
FROM JOURNAL OF CLINICAL RHEUMATOLOGY
An Update on JAK Inhibitors in Skin Disease
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.