User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Impaired vision an overlooked dementia risk factor
Investigators analyzed estimated population attributable fractions (PAFs) associated with dementia in more than 16,000 older adults. A PAF represents the number of dementia cases that could be prevented if a given risk factor were eliminated.
Results showed the PAF of vision impairment was 1.8%, suggesting that healthy vision had the potential to prevent more than 100,000 cases of dementia in the United States.
“Vision impairment and blindness disproportionately impact older adults, yet vision impairment is often preventable or even correctable,” study investigator Joshua Ehrlich MD, assistant professor of ophthalmology and visual sciences, University of Michigan, Ann Arbor, said in an interview.
Poor vision affects not only how individuals see the world, but also their systemic health and well-being, Dr. Ehrlich said.
“Accordingly, ensuring that older adults receive appropriate eye care is vital to promoting health, independence, and optimal aging,” he added.
The findings were published online in JAMA Neurology.
A surprising omission
There is an “urgent need to identify modifiable risk factors for dementia that can be targeted with interventions to slow cognitive decline and prevent dementia,” the investigators wrote.
In 2020, the Lancet Commission report on dementia prevention, intervention, and care proposed a life-course model of 12 potentially modifiable dementia risk factors. This included lower educational level, hearing loss, traumatic brain injury, hypertension, excessive alcohol consumption, obesity, smoking, depression, social isolation, physical inactivity, diabetes, and air pollution.
Together, these factors are associated with about 40% of dementia cases worldwide, the report notes.
Vision impairment was not included in this model, “despite considerable evidence that it is associated with an elevated risk of incident dementia and that it may operate through the same pathways as hearing loss,” the current researchers wrote.
“We have known for some time that vision impairment is a risk factor for dementia [and] we also know that a very large fraction of vision impairment, possibly in excess of 80%, is avoidable or has simply yet to be addressed,” Dr. Ehrlich said.
He and his colleagues found it “surprising that vision impairment had been ignored in key models of modifiable dementia risk factors that are used to shape health policy and resource allocation.” They set out to demonstrate that, “in fact, vision impairment is just as influential as a number of other long accepted modifiable dementia risk factors.”
The investigators assessed data from the Health and Retirement Study (HRS), a panel study that surveys more than 20,000 U.S. adults aged 50 years or older every 2 years.
The investigators applied the same methods used by the Lancet Commission to the HRS dataset and added vision impairment to the Lancet life-course model. Air pollution was excluded in their model “because those data were not readily available in the HRS,” the researchers wrote.
They noted the PAF is “based on the population prevalence and relative risk of dementia for each risk factor” and is “weighted, based on a principal components analysis, to account for communality (clustering of risk factors).”
A missed prevention opportunity
The sample included 16,690 participants (54% were women, 51.5% were at least age 65, 80.2% were White, 10.6% were Black, 9.2% were other).
In total, the 12 potentially modifiable risk factors used in the researchers’ model were associated with an estimated 62.4% of dementia cases in the United States, with hypertension as the most prevalent risk factor with the highest weighted PAF.
A new focus for prevention
Commenting for this article, Suzann Pershing, MD, associate professor of ophthalmology, Stanford (Calif.) University, called the study “particularly important because, despite growing recognition of its importance in relation to cognition, visual impairment is often an underrecognized risk factor.”
The current research “builds on increasingly robust medical literature linking visual impairment and dementia, applying analogous methods to those used for the life course model recently presented by the Lancet Commission to evaluate potentially modifiable dementia risk factors,” said Dr. Pershing, who was not involved with the study.
The investigators “make a compelling argument for inclusion of visual impairment as one of the potentially modifiable risk factors; practicing clinicians and health care systems may consider screening and targeted therapies to address visual impairment, with a goal of population health and contributing to a reduction in future dementia disease burden,” she added.
In an accompanying editorial), Jennifer Deal, PhD, department of epidemiology and Cochlear Center for Hearing and Public Health, Baltimore, and Julio Rojas, MD, PhD, Memory and Aging Center, department of neurology, Weill Institute for Neurosciences, University of California, San Francisco, call the findings “an important reminder that dementia is a social problem in which potentially treatable risk factors, including visual impairment, are highly prevalent in disadvantaged populations.”
The editorialists noted that 90% of cases of vision impairment are “preventable or have yet to be treated. The two “highly cost-effective interventions” of eyeglasses and/or cataract surgery “remain underused both in the U.S. and globally, especially in disadvantaged communities,” they wrote.
They added that more research is needed to “test the effectiveness of interventions to preserve cognitive health by promoting healthy vision.”
The study was supported by grants from the National Institute on Aging, the National Institutes of Health, and Research to Prevent Blindness. The investigators reported no relevant financial relationships. Dr. Deal reported having received grants from the National Institute on Aging. Dr. Rojas reported serving as site principal investigator on clinical trials for Eli Lilly and Eisai and receiving grants from the National Institute on Aging. Dr. Pershing is a consultant for Acumen, and Verana Health (as DigiSight Technologies).
A version of this article first appeared on Medscape.com.
Investigators analyzed estimated population attributable fractions (PAFs) associated with dementia in more than 16,000 older adults. A PAF represents the number of dementia cases that could be prevented if a given risk factor were eliminated.
Results showed the PAF of vision impairment was 1.8%, suggesting that healthy vision had the potential to prevent more than 100,000 cases of dementia in the United States.
“Vision impairment and blindness disproportionately impact older adults, yet vision impairment is often preventable or even correctable,” study investigator Joshua Ehrlich MD, assistant professor of ophthalmology and visual sciences, University of Michigan, Ann Arbor, said in an interview.
Poor vision affects not only how individuals see the world, but also their systemic health and well-being, Dr. Ehrlich said.
“Accordingly, ensuring that older adults receive appropriate eye care is vital to promoting health, independence, and optimal aging,” he added.
The findings were published online in JAMA Neurology.
A surprising omission
There is an “urgent need to identify modifiable risk factors for dementia that can be targeted with interventions to slow cognitive decline and prevent dementia,” the investigators wrote.
In 2020, the Lancet Commission report on dementia prevention, intervention, and care proposed a life-course model of 12 potentially modifiable dementia risk factors. This included lower educational level, hearing loss, traumatic brain injury, hypertension, excessive alcohol consumption, obesity, smoking, depression, social isolation, physical inactivity, diabetes, and air pollution.
Together, these factors are associated with about 40% of dementia cases worldwide, the report notes.
Vision impairment was not included in this model, “despite considerable evidence that it is associated with an elevated risk of incident dementia and that it may operate through the same pathways as hearing loss,” the current researchers wrote.
“We have known for some time that vision impairment is a risk factor for dementia [and] we also know that a very large fraction of vision impairment, possibly in excess of 80%, is avoidable or has simply yet to be addressed,” Dr. Ehrlich said.
He and his colleagues found it “surprising that vision impairment had been ignored in key models of modifiable dementia risk factors that are used to shape health policy and resource allocation.” They set out to demonstrate that, “in fact, vision impairment is just as influential as a number of other long accepted modifiable dementia risk factors.”
The investigators assessed data from the Health and Retirement Study (HRS), a panel study that surveys more than 20,000 U.S. adults aged 50 years or older every 2 years.
The investigators applied the same methods used by the Lancet Commission to the HRS dataset and added vision impairment to the Lancet life-course model. Air pollution was excluded in their model “because those data were not readily available in the HRS,” the researchers wrote.
They noted the PAF is “based on the population prevalence and relative risk of dementia for each risk factor” and is “weighted, based on a principal components analysis, to account for communality (clustering of risk factors).”
A missed prevention opportunity
The sample included 16,690 participants (54% were women, 51.5% were at least age 65, 80.2% were White, 10.6% were Black, 9.2% were other).
In total, the 12 potentially modifiable risk factors used in the researchers’ model were associated with an estimated 62.4% of dementia cases in the United States, with hypertension as the most prevalent risk factor with the highest weighted PAF.
A new focus for prevention
Commenting for this article, Suzann Pershing, MD, associate professor of ophthalmology, Stanford (Calif.) University, called the study “particularly important because, despite growing recognition of its importance in relation to cognition, visual impairment is often an underrecognized risk factor.”
The current research “builds on increasingly robust medical literature linking visual impairment and dementia, applying analogous methods to those used for the life course model recently presented by the Lancet Commission to evaluate potentially modifiable dementia risk factors,” said Dr. Pershing, who was not involved with the study.
The investigators “make a compelling argument for inclusion of visual impairment as one of the potentially modifiable risk factors; practicing clinicians and health care systems may consider screening and targeted therapies to address visual impairment, with a goal of population health and contributing to a reduction in future dementia disease burden,” she added.
In an accompanying editorial), Jennifer Deal, PhD, department of epidemiology and Cochlear Center for Hearing and Public Health, Baltimore, and Julio Rojas, MD, PhD, Memory and Aging Center, department of neurology, Weill Institute for Neurosciences, University of California, San Francisco, call the findings “an important reminder that dementia is a social problem in which potentially treatable risk factors, including visual impairment, are highly prevalent in disadvantaged populations.”
The editorialists noted that 90% of cases of vision impairment are “preventable or have yet to be treated. The two “highly cost-effective interventions” of eyeglasses and/or cataract surgery “remain underused both in the U.S. and globally, especially in disadvantaged communities,” they wrote.
They added that more research is needed to “test the effectiveness of interventions to preserve cognitive health by promoting healthy vision.”
The study was supported by grants from the National Institute on Aging, the National Institutes of Health, and Research to Prevent Blindness. The investigators reported no relevant financial relationships. Dr. Deal reported having received grants from the National Institute on Aging. Dr. Rojas reported serving as site principal investigator on clinical trials for Eli Lilly and Eisai and receiving grants from the National Institute on Aging. Dr. Pershing is a consultant for Acumen, and Verana Health (as DigiSight Technologies).
A version of this article first appeared on Medscape.com.
Investigators analyzed estimated population attributable fractions (PAFs) associated with dementia in more than 16,000 older adults. A PAF represents the number of dementia cases that could be prevented if a given risk factor were eliminated.
Results showed the PAF of vision impairment was 1.8%, suggesting that healthy vision had the potential to prevent more than 100,000 cases of dementia in the United States.
“Vision impairment and blindness disproportionately impact older adults, yet vision impairment is often preventable or even correctable,” study investigator Joshua Ehrlich MD, assistant professor of ophthalmology and visual sciences, University of Michigan, Ann Arbor, said in an interview.
Poor vision affects not only how individuals see the world, but also their systemic health and well-being, Dr. Ehrlich said.
“Accordingly, ensuring that older adults receive appropriate eye care is vital to promoting health, independence, and optimal aging,” he added.
The findings were published online in JAMA Neurology.
A surprising omission
There is an “urgent need to identify modifiable risk factors for dementia that can be targeted with interventions to slow cognitive decline and prevent dementia,” the investigators wrote.
In 2020, the Lancet Commission report on dementia prevention, intervention, and care proposed a life-course model of 12 potentially modifiable dementia risk factors. This included lower educational level, hearing loss, traumatic brain injury, hypertension, excessive alcohol consumption, obesity, smoking, depression, social isolation, physical inactivity, diabetes, and air pollution.
Together, these factors are associated with about 40% of dementia cases worldwide, the report notes.
Vision impairment was not included in this model, “despite considerable evidence that it is associated with an elevated risk of incident dementia and that it may operate through the same pathways as hearing loss,” the current researchers wrote.
“We have known for some time that vision impairment is a risk factor for dementia [and] we also know that a very large fraction of vision impairment, possibly in excess of 80%, is avoidable or has simply yet to be addressed,” Dr. Ehrlich said.
He and his colleagues found it “surprising that vision impairment had been ignored in key models of modifiable dementia risk factors that are used to shape health policy and resource allocation.” They set out to demonstrate that, “in fact, vision impairment is just as influential as a number of other long accepted modifiable dementia risk factors.”
The investigators assessed data from the Health and Retirement Study (HRS), a panel study that surveys more than 20,000 U.S. adults aged 50 years or older every 2 years.
The investigators applied the same methods used by the Lancet Commission to the HRS dataset and added vision impairment to the Lancet life-course model. Air pollution was excluded in their model “because those data were not readily available in the HRS,” the researchers wrote.
They noted the PAF is “based on the population prevalence and relative risk of dementia for each risk factor” and is “weighted, based on a principal components analysis, to account for communality (clustering of risk factors).”
A missed prevention opportunity
The sample included 16,690 participants (54% were women, 51.5% were at least age 65, 80.2% were White, 10.6% were Black, 9.2% were other).
In total, the 12 potentially modifiable risk factors used in the researchers’ model were associated with an estimated 62.4% of dementia cases in the United States, with hypertension as the most prevalent risk factor with the highest weighted PAF.
A new focus for prevention
Commenting for this article, Suzann Pershing, MD, associate professor of ophthalmology, Stanford (Calif.) University, called the study “particularly important because, despite growing recognition of its importance in relation to cognition, visual impairment is often an underrecognized risk factor.”
The current research “builds on increasingly robust medical literature linking visual impairment and dementia, applying analogous methods to those used for the life course model recently presented by the Lancet Commission to evaluate potentially modifiable dementia risk factors,” said Dr. Pershing, who was not involved with the study.
The investigators “make a compelling argument for inclusion of visual impairment as one of the potentially modifiable risk factors; practicing clinicians and health care systems may consider screening and targeted therapies to address visual impairment, with a goal of population health and contributing to a reduction in future dementia disease burden,” she added.
In an accompanying editorial), Jennifer Deal, PhD, department of epidemiology and Cochlear Center for Hearing and Public Health, Baltimore, and Julio Rojas, MD, PhD, Memory and Aging Center, department of neurology, Weill Institute for Neurosciences, University of California, San Francisco, call the findings “an important reminder that dementia is a social problem in which potentially treatable risk factors, including visual impairment, are highly prevalent in disadvantaged populations.”
The editorialists noted that 90% of cases of vision impairment are “preventable or have yet to be treated. The two “highly cost-effective interventions” of eyeglasses and/or cataract surgery “remain underused both in the U.S. and globally, especially in disadvantaged communities,” they wrote.
They added that more research is needed to “test the effectiveness of interventions to preserve cognitive health by promoting healthy vision.”
The study was supported by grants from the National Institute on Aging, the National Institutes of Health, and Research to Prevent Blindness. The investigators reported no relevant financial relationships. Dr. Deal reported having received grants from the National Institute on Aging. Dr. Rojas reported serving as site principal investigator on clinical trials for Eli Lilly and Eisai and receiving grants from the National Institute on Aging. Dr. Pershing is a consultant for Acumen, and Verana Health (as DigiSight Technologies).
A version of this article first appeared on Medscape.com.
Three symptoms suggest higher risk for self-injury in cancer
, according to a Canadian study.
In a population-based, case-control study, each of these symptoms was associated with an increase of at least 60% in the risk for NFSI in the following 180 days, the investigators report.
“Clinicians should know that self-injury is a real problem after a cancer diagnosis,” lead investigator Julie Hallet, MD, an associate scientist at Sunnybrook Health Sciences Centre in Toronto, told this news organization.
Self-injury “does not necessarily represent an attempted suicide,” she added. “While our data do not allow us to know what the intent was, we know from other work that the repercussions of distress in patients with cancer are much broader than suicide. Self-injury can be a means to cope with psychological difficulties for some patients, without intent for suicide.”
The study was published online in JAMA Oncology.
Nine common symptoms
The study included adults who were diagnosed with cancer between Jan. 1, 2007, and March 31, 2019, and had completed the Edmonton Symptom Assessment System (ESAS) evaluation within 36 months of their index cancer diagnosis. ESAS evaluates nine common cancer-associated symptoms, including pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath, on a patient-reported scale of 0 (absence of symptom) to 10 (worst possible symptom).
The analysis included 406 patients who had visited an emergency department for an NFSI within 180 days of their ESAS evaluation, as well as 1,624 matched control patients with cancer who did not have an NFSI. Case patients and control patients were matched according to age at cancer diagnosis, sex, prior self-injury within 5 years of being diagnosed with cancer, and cancer type. Nonmatched covariates included psychiatric illness and therapy received before NFSI, comorbidity burden, material deprivation, and cancer stage.
Toward tailored intervention
A higher proportion of case patients than control patients reported moderate to severe scores for all nine ESAS symptoms. In an adjusted analysis, moderate to severe anxiety (odds ratio, 1.61), depression (OR, 1.66), and shortness of breath (OR, 1.65) were independently associated with higher odds of subsequent NFSI. Each 10-point increase in total ESAS score also was associated with increased risk (OR, 1.51).
“These findings are important to enhance the use of screening ESAS scores to better support patients,” say the authors. “Scores from ESAS assessments can be used to identify patients at higher risk of NFSI, indicating higher level of distress, and help direct tailored assessment and intervention.”
In prior work, Dr. Hallet’s group showed that NFSI occurs in 3 of every 1,000 patients with cancer. NFSI is more frequent among younger patients and those with a history of prior mental illness. “Identifying patients at risk in clinical practice requires you to inquire about a patient’s prior history, identify high symptom scores and ask about them, and trigger intervention pathways when risk is identified,” said Dr. Hallet.
“For example, a young patient with head and neck cancer and a prior history of mental illness who reports high scores for anxiety and drowsiness would be at high risk of self-injury,” she added. Such a patient should be referred to psycho-oncology, psychiatry, or social work. “To facilitate this, we are working on prognostic scores that can be integrated in clinical practice, such as an electronic medical record, to flag patients at risk,” said Dr. Hallet. “Future work will also need to identify the optimal care pathways for at-risk patients.”
Self-injury vs. suicidality
Commenting on the study for this news organization, Madeline Li, MD, PhD, a psychiatrist and clinician-scientist at Toronto’s Princess Margaret Cancer Centre, said that the findings are “underwhelming” because they tell us what is already known – that “NFSI is associated with distress, and cancer is a stressor.” It would have been more interesting to ask how to distinguish patients at risk for suicide from those at risk for self-harm without suicide, she added.
“The way these authors formulated NFSI included both self-harm intent and suicidal intent,” she explained. The researchers compared patients who were at risk for these two types of events with patients without NFSI. “When we see self-harm without suicidal intent in the emergency room, it’s mostly people making cries for help,” said Dr. Li. “These are people who cut their wrists or take small overdoses on purpose without the intent to die. It would have been more interesting to see if there are different risk factors for people who are just going to self-harm vs. those who are actually going to attempt suicide.”
The study’s identification of risk factors for NSFI is important because “it does tell us that when there’s anxiety, depression, and shortness of breath, we should pay attention to these patients and do something about it,” said Dr. Li. Still, research in cancer psychiatry needs to shift its focus from identifying and addressing existing risk factors to preventing them from developing, she added.
“We need to move earlier and provide emotional and mental health support to cancer patients to prevent them from becoming suicidal, rather than intervening when somebody already is,” Dr. Li concluded.
The study was funded by the Hanna Research Award from the division of surgical oncology at the Odette Cancer Centre–Sunnybrook Health Sciences Centre and by a Sunnybrook Health Sciences Centre Alternate Funding Plan Innovation grant. It was also supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Hallet has received personal fees from Ipsen Biopharmaceuticals Canada and AAA outside the submitted work. Dr. Li reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a Canadian study.
In a population-based, case-control study, each of these symptoms was associated with an increase of at least 60% in the risk for NFSI in the following 180 days, the investigators report.
“Clinicians should know that self-injury is a real problem after a cancer diagnosis,” lead investigator Julie Hallet, MD, an associate scientist at Sunnybrook Health Sciences Centre in Toronto, told this news organization.
Self-injury “does not necessarily represent an attempted suicide,” she added. “While our data do not allow us to know what the intent was, we know from other work that the repercussions of distress in patients with cancer are much broader than suicide. Self-injury can be a means to cope with psychological difficulties for some patients, without intent for suicide.”
The study was published online in JAMA Oncology.
Nine common symptoms
The study included adults who were diagnosed with cancer between Jan. 1, 2007, and March 31, 2019, and had completed the Edmonton Symptom Assessment System (ESAS) evaluation within 36 months of their index cancer diagnosis. ESAS evaluates nine common cancer-associated symptoms, including pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath, on a patient-reported scale of 0 (absence of symptom) to 10 (worst possible symptom).
The analysis included 406 patients who had visited an emergency department for an NFSI within 180 days of their ESAS evaluation, as well as 1,624 matched control patients with cancer who did not have an NFSI. Case patients and control patients were matched according to age at cancer diagnosis, sex, prior self-injury within 5 years of being diagnosed with cancer, and cancer type. Nonmatched covariates included psychiatric illness and therapy received before NFSI, comorbidity burden, material deprivation, and cancer stage.
Toward tailored intervention
A higher proportion of case patients than control patients reported moderate to severe scores for all nine ESAS symptoms. In an adjusted analysis, moderate to severe anxiety (odds ratio, 1.61), depression (OR, 1.66), and shortness of breath (OR, 1.65) were independently associated with higher odds of subsequent NFSI. Each 10-point increase in total ESAS score also was associated with increased risk (OR, 1.51).
“These findings are important to enhance the use of screening ESAS scores to better support patients,” say the authors. “Scores from ESAS assessments can be used to identify patients at higher risk of NFSI, indicating higher level of distress, and help direct tailored assessment and intervention.”
In prior work, Dr. Hallet’s group showed that NFSI occurs in 3 of every 1,000 patients with cancer. NFSI is more frequent among younger patients and those with a history of prior mental illness. “Identifying patients at risk in clinical practice requires you to inquire about a patient’s prior history, identify high symptom scores and ask about them, and trigger intervention pathways when risk is identified,” said Dr. Hallet.
“For example, a young patient with head and neck cancer and a prior history of mental illness who reports high scores for anxiety and drowsiness would be at high risk of self-injury,” she added. Such a patient should be referred to psycho-oncology, psychiatry, or social work. “To facilitate this, we are working on prognostic scores that can be integrated in clinical practice, such as an electronic medical record, to flag patients at risk,” said Dr. Hallet. “Future work will also need to identify the optimal care pathways for at-risk patients.”
Self-injury vs. suicidality
Commenting on the study for this news organization, Madeline Li, MD, PhD, a psychiatrist and clinician-scientist at Toronto’s Princess Margaret Cancer Centre, said that the findings are “underwhelming” because they tell us what is already known – that “NFSI is associated with distress, and cancer is a stressor.” It would have been more interesting to ask how to distinguish patients at risk for suicide from those at risk for self-harm without suicide, she added.
“The way these authors formulated NFSI included both self-harm intent and suicidal intent,” she explained. The researchers compared patients who were at risk for these two types of events with patients without NFSI. “When we see self-harm without suicidal intent in the emergency room, it’s mostly people making cries for help,” said Dr. Li. “These are people who cut their wrists or take small overdoses on purpose without the intent to die. It would have been more interesting to see if there are different risk factors for people who are just going to self-harm vs. those who are actually going to attempt suicide.”
The study’s identification of risk factors for NSFI is important because “it does tell us that when there’s anxiety, depression, and shortness of breath, we should pay attention to these patients and do something about it,” said Dr. Li. Still, research in cancer psychiatry needs to shift its focus from identifying and addressing existing risk factors to preventing them from developing, she added.
“We need to move earlier and provide emotional and mental health support to cancer patients to prevent them from becoming suicidal, rather than intervening when somebody already is,” Dr. Li concluded.
The study was funded by the Hanna Research Award from the division of surgical oncology at the Odette Cancer Centre–Sunnybrook Health Sciences Centre and by a Sunnybrook Health Sciences Centre Alternate Funding Plan Innovation grant. It was also supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Hallet has received personal fees from Ipsen Biopharmaceuticals Canada and AAA outside the submitted work. Dr. Li reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a Canadian study.
In a population-based, case-control study, each of these symptoms was associated with an increase of at least 60% in the risk for NFSI in the following 180 days, the investigators report.
“Clinicians should know that self-injury is a real problem after a cancer diagnosis,” lead investigator Julie Hallet, MD, an associate scientist at Sunnybrook Health Sciences Centre in Toronto, told this news organization.
Self-injury “does not necessarily represent an attempted suicide,” she added. “While our data do not allow us to know what the intent was, we know from other work that the repercussions of distress in patients with cancer are much broader than suicide. Self-injury can be a means to cope with psychological difficulties for some patients, without intent for suicide.”
The study was published online in JAMA Oncology.
Nine common symptoms
The study included adults who were diagnosed with cancer between Jan. 1, 2007, and March 31, 2019, and had completed the Edmonton Symptom Assessment System (ESAS) evaluation within 36 months of their index cancer diagnosis. ESAS evaluates nine common cancer-associated symptoms, including pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath, on a patient-reported scale of 0 (absence of symptom) to 10 (worst possible symptom).
The analysis included 406 patients who had visited an emergency department for an NFSI within 180 days of their ESAS evaluation, as well as 1,624 matched control patients with cancer who did not have an NFSI. Case patients and control patients were matched according to age at cancer diagnosis, sex, prior self-injury within 5 years of being diagnosed with cancer, and cancer type. Nonmatched covariates included psychiatric illness and therapy received before NFSI, comorbidity burden, material deprivation, and cancer stage.
Toward tailored intervention
A higher proportion of case patients than control patients reported moderate to severe scores for all nine ESAS symptoms. In an adjusted analysis, moderate to severe anxiety (odds ratio, 1.61), depression (OR, 1.66), and shortness of breath (OR, 1.65) were independently associated with higher odds of subsequent NFSI. Each 10-point increase in total ESAS score also was associated with increased risk (OR, 1.51).
“These findings are important to enhance the use of screening ESAS scores to better support patients,” say the authors. “Scores from ESAS assessments can be used to identify patients at higher risk of NFSI, indicating higher level of distress, and help direct tailored assessment and intervention.”
In prior work, Dr. Hallet’s group showed that NFSI occurs in 3 of every 1,000 patients with cancer. NFSI is more frequent among younger patients and those with a history of prior mental illness. “Identifying patients at risk in clinical practice requires you to inquire about a patient’s prior history, identify high symptom scores and ask about them, and trigger intervention pathways when risk is identified,” said Dr. Hallet.
“For example, a young patient with head and neck cancer and a prior history of mental illness who reports high scores for anxiety and drowsiness would be at high risk of self-injury,” she added. Such a patient should be referred to psycho-oncology, psychiatry, or social work. “To facilitate this, we are working on prognostic scores that can be integrated in clinical practice, such as an electronic medical record, to flag patients at risk,” said Dr. Hallet. “Future work will also need to identify the optimal care pathways for at-risk patients.”
Self-injury vs. suicidality
Commenting on the study for this news organization, Madeline Li, MD, PhD, a psychiatrist and clinician-scientist at Toronto’s Princess Margaret Cancer Centre, said that the findings are “underwhelming” because they tell us what is already known – that “NFSI is associated with distress, and cancer is a stressor.” It would have been more interesting to ask how to distinguish patients at risk for suicide from those at risk for self-harm without suicide, she added.
“The way these authors formulated NFSI included both self-harm intent and suicidal intent,” she explained. The researchers compared patients who were at risk for these two types of events with patients without NFSI. “When we see self-harm without suicidal intent in the emergency room, it’s mostly people making cries for help,” said Dr. Li. “These are people who cut their wrists or take small overdoses on purpose without the intent to die. It would have been more interesting to see if there are different risk factors for people who are just going to self-harm vs. those who are actually going to attempt suicide.”
The study’s identification of risk factors for NSFI is important because “it does tell us that when there’s anxiety, depression, and shortness of breath, we should pay attention to these patients and do something about it,” said Dr. Li. Still, research in cancer psychiatry needs to shift its focus from identifying and addressing existing risk factors to preventing them from developing, she added.
“We need to move earlier and provide emotional and mental health support to cancer patients to prevent them from becoming suicidal, rather than intervening when somebody already is,” Dr. Li concluded.
The study was funded by the Hanna Research Award from the division of surgical oncology at the Odette Cancer Centre–Sunnybrook Health Sciences Centre and by a Sunnybrook Health Sciences Centre Alternate Funding Plan Innovation grant. It was also supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Hallet has received personal fees from Ipsen Biopharmaceuticals Canada and AAA outside the submitted work. Dr. Li reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
It’s time to shame the fat shamers
Fat shaming doesn’t work. If it did, obesity as we know it wouldn’t exist because if the one thing society ensures isn’t lacking for people with obesity, it’s shame. We know that fat shaming doesn’t lead to weight loss and that it’s actually correlated with weight gain: More shame leads to more gain (Puhl and Suh; Sutin and Terracciano; Tomiyama et al).
Shaming and weight stigma have far more concerning associations than weight gain. People who report experiencing more weight stigma have an increased risk for depression, anxiety, low self-esteem, poor body image, substance abuse, suicidality, unhealthy eating behaviors, disordered eating, increased caloric intake, exercise avoidance, decreased exercise motivation potentially due to heightened cortisol reactivity, elevated C-reactive protein, and elevated blood pressure.
Meanwhile, people with obesity – likely in part owing to negative weight-biased experiences in health care – are reluctant to discuss weight with their health care providers and are less likely to seek care at all for any conditions. When care is sought, people with obesity are more likely to receive substandard treatment, including receiving fewer preventive health screenings, decreased health education, and decreased time spent in appointments.
Remember that obesity is not a conscious choice
A fact that is conveniently forgotten by those who are most prone to fat shaming is that obesity, like every chronic noncommunicable disease, isn’t a choice that is consciously made by patients.
And yes, though there are lifestyle means that might affect weight, there are lifestyle means that might affect all chronic diseases – yet obesity is the only one we seem to moralize about. It’s also worth noting that other chronic diseases’ lifestyle levers tend not to be governed by thousands of genes and dozens of hormones; those trying to “lifestyle” their way out of obesity are swimming against strong physiologic currents that influence our most seminally important survival drive: eating.
But forgetting about physiologic currents, there is also staggering privilege associated with intentional perpetual behavior change around food and fitness in the name of health.
Whereas medicine and the world are right and quick to embrace the fights against racism, sexism, and homophobia, the push to confront weight bias is far rarer, despite the fact that it’s been shown to be rampant among health care professionals.
Protecting the rights of people with obesity
Perhaps though, times are changing. Movements are popping up to protect the rights of people with obesity while combating hate.
Of note, Brazil seems to have embraced a campaign to fight gordofobia — the Portuguese term used to describe weight-based discrimination. For instance, laws are being passed to ensure appropriate seating is supplied in schools for children with obesity, an annual day was formalized to promote the rights of people with obesity, preferential seating is provided on subways for people with obesity, and fines have been levied against at least one comedian for making fat jokes on the grounds of the state’s duty to protect minorities.
We need to take this fight to medicine. Given the incredibly depressing prevalence of weight bias among trainees, medical schools and residency programs should ensure countering weight bias is not only part of the curriculum but that it’s explicitly examined. National medical licensing examinations should include weight bias as well.
Though we’re closer than ever before to widely effective treatment options for obesity, it’s likely to still be decades before pharmaceutical options to treat obesity are as effective, accepted, and encouraged as medications to treat hypertension, dyslipidemia, diabetes, and more are today.
If you’re curious about your own implicit weight biases, consider taking Harvard’s Implicit Association Test for Weight. You might also want to take a few moments and review the Strategies to Overcome and Prevent Obesity Alliances’ Weight Can’t Wait guide for advice on the management of obesity in primary care.
Treat patients with obesity the same as you would those with any chronic condition.
Also, consider your physical office space. Do you have chairs suitable for patients with obesity (wide base and with arms to help patients rise)? A scale that measures up to high weights that’s in a private location? Appropriately sized blood pressure cuffs?
If not,
Examples include the family doctor who hadn’t checked my patient’s blood pressure in over a decade because he couldn’t be bothered buying an appropriately sized blood pressure cuff. Or the fertility doctor who told one of my patients that perhaps her weight reflected God’s will that she does not have children.
Finally, if reading this article about treating people with obesity the same as you would patients with other chronic, noncommunicable, lifestyle responsive diseases made you angry, there’s a great chance that you’re part of the problem.
Dr. Freedhoff, is associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute, a nonsurgical weight management center. He is one of Canada’s most outspoken obesity experts and the author of The Diet Fix: Why Diets Fail and How to Make Yours Work. He has disclosed the following: He served as a director, officer, partner, employee, adviser, consultant, or trustee for Bariatric Medical Institute and Constant Health; has received research grant from Novo Nordisk, and has publicly shared opinions via Weighty Matters and social media. A version of this article first appeared on Medscape.com.
Fat shaming doesn’t work. If it did, obesity as we know it wouldn’t exist because if the one thing society ensures isn’t lacking for people with obesity, it’s shame. We know that fat shaming doesn’t lead to weight loss and that it’s actually correlated with weight gain: More shame leads to more gain (Puhl and Suh; Sutin and Terracciano; Tomiyama et al).
Shaming and weight stigma have far more concerning associations than weight gain. People who report experiencing more weight stigma have an increased risk for depression, anxiety, low self-esteem, poor body image, substance abuse, suicidality, unhealthy eating behaviors, disordered eating, increased caloric intake, exercise avoidance, decreased exercise motivation potentially due to heightened cortisol reactivity, elevated C-reactive protein, and elevated blood pressure.
Meanwhile, people with obesity – likely in part owing to negative weight-biased experiences in health care – are reluctant to discuss weight with their health care providers and are less likely to seek care at all for any conditions. When care is sought, people with obesity are more likely to receive substandard treatment, including receiving fewer preventive health screenings, decreased health education, and decreased time spent in appointments.
Remember that obesity is not a conscious choice
A fact that is conveniently forgotten by those who are most prone to fat shaming is that obesity, like every chronic noncommunicable disease, isn’t a choice that is consciously made by patients.
And yes, though there are lifestyle means that might affect weight, there are lifestyle means that might affect all chronic diseases – yet obesity is the only one we seem to moralize about. It’s also worth noting that other chronic diseases’ lifestyle levers tend not to be governed by thousands of genes and dozens of hormones; those trying to “lifestyle” their way out of obesity are swimming against strong physiologic currents that influence our most seminally important survival drive: eating.
But forgetting about physiologic currents, there is also staggering privilege associated with intentional perpetual behavior change around food and fitness in the name of health.
Whereas medicine and the world are right and quick to embrace the fights against racism, sexism, and homophobia, the push to confront weight bias is far rarer, despite the fact that it’s been shown to be rampant among health care professionals.
Protecting the rights of people with obesity
Perhaps though, times are changing. Movements are popping up to protect the rights of people with obesity while combating hate.
Of note, Brazil seems to have embraced a campaign to fight gordofobia — the Portuguese term used to describe weight-based discrimination. For instance, laws are being passed to ensure appropriate seating is supplied in schools for children with obesity, an annual day was formalized to promote the rights of people with obesity, preferential seating is provided on subways for people with obesity, and fines have been levied against at least one comedian for making fat jokes on the grounds of the state’s duty to protect minorities.
We need to take this fight to medicine. Given the incredibly depressing prevalence of weight bias among trainees, medical schools and residency programs should ensure countering weight bias is not only part of the curriculum but that it’s explicitly examined. National medical licensing examinations should include weight bias as well.
Though we’re closer than ever before to widely effective treatment options for obesity, it’s likely to still be decades before pharmaceutical options to treat obesity are as effective, accepted, and encouraged as medications to treat hypertension, dyslipidemia, diabetes, and more are today.
If you’re curious about your own implicit weight biases, consider taking Harvard’s Implicit Association Test for Weight. You might also want to take a few moments and review the Strategies to Overcome and Prevent Obesity Alliances’ Weight Can’t Wait guide for advice on the management of obesity in primary care.
Treat patients with obesity the same as you would those with any chronic condition.
Also, consider your physical office space. Do you have chairs suitable for patients with obesity (wide base and with arms to help patients rise)? A scale that measures up to high weights that’s in a private location? Appropriately sized blood pressure cuffs?
If not,
Examples include the family doctor who hadn’t checked my patient’s blood pressure in over a decade because he couldn’t be bothered buying an appropriately sized blood pressure cuff. Or the fertility doctor who told one of my patients that perhaps her weight reflected God’s will that she does not have children.
Finally, if reading this article about treating people with obesity the same as you would patients with other chronic, noncommunicable, lifestyle responsive diseases made you angry, there’s a great chance that you’re part of the problem.
Dr. Freedhoff, is associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute, a nonsurgical weight management center. He is one of Canada’s most outspoken obesity experts and the author of The Diet Fix: Why Diets Fail and How to Make Yours Work. He has disclosed the following: He served as a director, officer, partner, employee, adviser, consultant, or trustee for Bariatric Medical Institute and Constant Health; has received research grant from Novo Nordisk, and has publicly shared opinions via Weighty Matters and social media. A version of this article first appeared on Medscape.com.
Fat shaming doesn’t work. If it did, obesity as we know it wouldn’t exist because if the one thing society ensures isn’t lacking for people with obesity, it’s shame. We know that fat shaming doesn’t lead to weight loss and that it’s actually correlated with weight gain: More shame leads to more gain (Puhl and Suh; Sutin and Terracciano; Tomiyama et al).
Shaming and weight stigma have far more concerning associations than weight gain. People who report experiencing more weight stigma have an increased risk for depression, anxiety, low self-esteem, poor body image, substance abuse, suicidality, unhealthy eating behaviors, disordered eating, increased caloric intake, exercise avoidance, decreased exercise motivation potentially due to heightened cortisol reactivity, elevated C-reactive protein, and elevated blood pressure.
Meanwhile, people with obesity – likely in part owing to negative weight-biased experiences in health care – are reluctant to discuss weight with their health care providers and are less likely to seek care at all for any conditions. When care is sought, people with obesity are more likely to receive substandard treatment, including receiving fewer preventive health screenings, decreased health education, and decreased time spent in appointments.
Remember that obesity is not a conscious choice
A fact that is conveniently forgotten by those who are most prone to fat shaming is that obesity, like every chronic noncommunicable disease, isn’t a choice that is consciously made by patients.
And yes, though there are lifestyle means that might affect weight, there are lifestyle means that might affect all chronic diseases – yet obesity is the only one we seem to moralize about. It’s also worth noting that other chronic diseases’ lifestyle levers tend not to be governed by thousands of genes and dozens of hormones; those trying to “lifestyle” their way out of obesity are swimming against strong physiologic currents that influence our most seminally important survival drive: eating.
But forgetting about physiologic currents, there is also staggering privilege associated with intentional perpetual behavior change around food and fitness in the name of health.
Whereas medicine and the world are right and quick to embrace the fights against racism, sexism, and homophobia, the push to confront weight bias is far rarer, despite the fact that it’s been shown to be rampant among health care professionals.
Protecting the rights of people with obesity
Perhaps though, times are changing. Movements are popping up to protect the rights of people with obesity while combating hate.
Of note, Brazil seems to have embraced a campaign to fight gordofobia — the Portuguese term used to describe weight-based discrimination. For instance, laws are being passed to ensure appropriate seating is supplied in schools for children with obesity, an annual day was formalized to promote the rights of people with obesity, preferential seating is provided on subways for people with obesity, and fines have been levied against at least one comedian for making fat jokes on the grounds of the state’s duty to protect minorities.
We need to take this fight to medicine. Given the incredibly depressing prevalence of weight bias among trainees, medical schools and residency programs should ensure countering weight bias is not only part of the curriculum but that it’s explicitly examined. National medical licensing examinations should include weight bias as well.
Though we’re closer than ever before to widely effective treatment options for obesity, it’s likely to still be decades before pharmaceutical options to treat obesity are as effective, accepted, and encouraged as medications to treat hypertension, dyslipidemia, diabetes, and more are today.
If you’re curious about your own implicit weight biases, consider taking Harvard’s Implicit Association Test for Weight. You might also want to take a few moments and review the Strategies to Overcome and Prevent Obesity Alliances’ Weight Can’t Wait guide for advice on the management of obesity in primary care.
Treat patients with obesity the same as you would those with any chronic condition.
Also, consider your physical office space. Do you have chairs suitable for patients with obesity (wide base and with arms to help patients rise)? A scale that measures up to high weights that’s in a private location? Appropriately sized blood pressure cuffs?
If not,
Examples include the family doctor who hadn’t checked my patient’s blood pressure in over a decade because he couldn’t be bothered buying an appropriately sized blood pressure cuff. Or the fertility doctor who told one of my patients that perhaps her weight reflected God’s will that she does not have children.
Finally, if reading this article about treating people with obesity the same as you would patients with other chronic, noncommunicable, lifestyle responsive diseases made you angry, there’s a great chance that you’re part of the problem.
Dr. Freedhoff, is associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute, a nonsurgical weight management center. He is one of Canada’s most outspoken obesity experts and the author of The Diet Fix: Why Diets Fail and How to Make Yours Work. He has disclosed the following: He served as a director, officer, partner, employee, adviser, consultant, or trustee for Bariatric Medical Institute and Constant Health; has received research grant from Novo Nordisk, and has publicly shared opinions via Weighty Matters and social media. A version of this article first appeared on Medscape.com.
Career pivots: A new perspective on psychiatry
Psychiatrists practice a field of medicine that relies on one’s clinical perspective to interpret observable behaviors originating from the brains of others. In this manner, psychiatry and photography are similar. And digital technology has changed them both.
In photography, there are many technical aspects for one to master when framing and capturing a shot. The length of exposure. The amount of light needed. The speed of the film, which is its sensitivity to light. The aperture that controls how much light falls on the film. The movement of the subject across the film during the exposure. Despite the fact that physical film has mostly yielded to electronic sensors over the past couple decades, these basic aspects of photography remain.
But perspective is the critical ingredient. This is what brings the greatest impact to photography. The composition, or the subject of the photograph and how its elements – foreground, background, shapes, patterns, texture, shadow, motion, leading lines, and focal points – are arranged. The most powerful way to improve the composition – more powerful than fancy camera bells and whistles – is to move. One step to the left or right, one step forward or back. Stand on your toes, or crouch to your knees. Pivot this way or that. A simple change in perspective dramatically changes the nature and the energy of the captured image.
In fact, many physicians are changing what they actually do for a living. Pivoting their clinical perspectives. And applying those perspectives to other areas. The latest catalyst fueling these career pivots, these changes in perspectives, has been the incredible global impact of the tiny little coronavirus known as SARS-CoV-2. The COVID-19 pandemic that began two years ago has disrupted the entire planet. The virus has caused us all to change our perspective, to see our world differently, and our place in it.
The virus has exposed defects in our health care delivery system. And physicians have necessarily reacted, injecting changes in what they do and how they do it. Many of these changes rely on digital technology, building upon the groundwork laid over the past couple of decades to convert our paper processes into electronic processes, and our manual work flows into digital work flows. This groundwork is no small thing, as it relies on conventions and standards, such as DICOM, LOINC, AES, CDA, UMLS, FHIR, ICD, NDC, USCDI, and SNOMED-CT. Establishing, maintaining, and evolving health care standards requires organized groups of people to come together to share their diverse perspectives. This is but one of many places where physicians are using their unique clinical perspective to share what they see with others.
This column will focus on these professional pivots that physicians make when they take a step to the left or right to change their perspective and share their viewpoints in different settings with diverse groups of people. Some of these pivots are small, while others are career changing. But the theme that knits them together is about taking what one has learned while helping others achieve better health, and using that perspective to make a difference.
Dr. Daviss is chief medical officer for Optum Maryland and immediate past president of the Maryland-DC Society of Addiction Medicine, and former medical director and senior medical advisor at SAMHSA. He is coauthor of the 2011 book, Shrink Rap: Three Psychiatrists Explain Their Work. Psychiatrists and other physicians may share their own experience with pivots they have made with Dr. Daviss via email ([email protected]) or Twitter (@HITshrink). The opinions expressed are solely those of the author and do not necessarily reflect those of his employer or organizations with which he is associated.
Psychiatrists practice a field of medicine that relies on one’s clinical perspective to interpret observable behaviors originating from the brains of others. In this manner, psychiatry and photography are similar. And digital technology has changed them both.
In photography, there are many technical aspects for one to master when framing and capturing a shot. The length of exposure. The amount of light needed. The speed of the film, which is its sensitivity to light. The aperture that controls how much light falls on the film. The movement of the subject across the film during the exposure. Despite the fact that physical film has mostly yielded to electronic sensors over the past couple decades, these basic aspects of photography remain.
But perspective is the critical ingredient. This is what brings the greatest impact to photography. The composition, or the subject of the photograph and how its elements – foreground, background, shapes, patterns, texture, shadow, motion, leading lines, and focal points – are arranged. The most powerful way to improve the composition – more powerful than fancy camera bells and whistles – is to move. One step to the left or right, one step forward or back. Stand on your toes, or crouch to your knees. Pivot this way or that. A simple change in perspective dramatically changes the nature and the energy of the captured image.
In fact, many physicians are changing what they actually do for a living. Pivoting their clinical perspectives. And applying those perspectives to other areas. The latest catalyst fueling these career pivots, these changes in perspectives, has been the incredible global impact of the tiny little coronavirus known as SARS-CoV-2. The COVID-19 pandemic that began two years ago has disrupted the entire planet. The virus has caused us all to change our perspective, to see our world differently, and our place in it.
The virus has exposed defects in our health care delivery system. And physicians have necessarily reacted, injecting changes in what they do and how they do it. Many of these changes rely on digital technology, building upon the groundwork laid over the past couple of decades to convert our paper processes into electronic processes, and our manual work flows into digital work flows. This groundwork is no small thing, as it relies on conventions and standards, such as DICOM, LOINC, AES, CDA, UMLS, FHIR, ICD, NDC, USCDI, and SNOMED-CT. Establishing, maintaining, and evolving health care standards requires organized groups of people to come together to share their diverse perspectives. This is but one of many places where physicians are using their unique clinical perspective to share what they see with others.
This column will focus on these professional pivots that physicians make when they take a step to the left or right to change their perspective and share their viewpoints in different settings with diverse groups of people. Some of these pivots are small, while others are career changing. But the theme that knits them together is about taking what one has learned while helping others achieve better health, and using that perspective to make a difference.
Dr. Daviss is chief medical officer for Optum Maryland and immediate past president of the Maryland-DC Society of Addiction Medicine, and former medical director and senior medical advisor at SAMHSA. He is coauthor of the 2011 book, Shrink Rap: Three Psychiatrists Explain Their Work. Psychiatrists and other physicians may share their own experience with pivots they have made with Dr. Daviss via email ([email protected]) or Twitter (@HITshrink). The opinions expressed are solely those of the author and do not necessarily reflect those of his employer or organizations with which he is associated.
Psychiatrists practice a field of medicine that relies on one’s clinical perspective to interpret observable behaviors originating from the brains of others. In this manner, psychiatry and photography are similar. And digital technology has changed them both.
In photography, there are many technical aspects for one to master when framing and capturing a shot. The length of exposure. The amount of light needed. The speed of the film, which is its sensitivity to light. The aperture that controls how much light falls on the film. The movement of the subject across the film during the exposure. Despite the fact that physical film has mostly yielded to electronic sensors over the past couple decades, these basic aspects of photography remain.
But perspective is the critical ingredient. This is what brings the greatest impact to photography. The composition, or the subject of the photograph and how its elements – foreground, background, shapes, patterns, texture, shadow, motion, leading lines, and focal points – are arranged. The most powerful way to improve the composition – more powerful than fancy camera bells and whistles – is to move. One step to the left or right, one step forward or back. Stand on your toes, or crouch to your knees. Pivot this way or that. A simple change in perspective dramatically changes the nature and the energy of the captured image.
In fact, many physicians are changing what they actually do for a living. Pivoting their clinical perspectives. And applying those perspectives to other areas. The latest catalyst fueling these career pivots, these changes in perspectives, has been the incredible global impact of the tiny little coronavirus known as SARS-CoV-2. The COVID-19 pandemic that began two years ago has disrupted the entire planet. The virus has caused us all to change our perspective, to see our world differently, and our place in it.
The virus has exposed defects in our health care delivery system. And physicians have necessarily reacted, injecting changes in what they do and how they do it. Many of these changes rely on digital technology, building upon the groundwork laid over the past couple of decades to convert our paper processes into electronic processes, and our manual work flows into digital work flows. This groundwork is no small thing, as it relies on conventions and standards, such as DICOM, LOINC, AES, CDA, UMLS, FHIR, ICD, NDC, USCDI, and SNOMED-CT. Establishing, maintaining, and evolving health care standards requires organized groups of people to come together to share their diverse perspectives. This is but one of many places where physicians are using their unique clinical perspective to share what they see with others.
This column will focus on these professional pivots that physicians make when they take a step to the left or right to change their perspective and share their viewpoints in different settings with diverse groups of people. Some of these pivots are small, while others are career changing. But the theme that knits them together is about taking what one has learned while helping others achieve better health, and using that perspective to make a difference.
Dr. Daviss is chief medical officer for Optum Maryland and immediate past president of the Maryland-DC Society of Addiction Medicine, and former medical director and senior medical advisor at SAMHSA. He is coauthor of the 2011 book, Shrink Rap: Three Psychiatrists Explain Their Work. Psychiatrists and other physicians may share their own experience with pivots they have made with Dr. Daviss via email ([email protected]) or Twitter (@HITshrink). The opinions expressed are solely those of the author and do not necessarily reflect those of his employer or organizations with which he is associated.
Virtual reality an ‘exciting opportunity’ for geriatric psychiatry
Researchers are increasingly turning their attention to virtual reality (VR) for the treatment of psychiatric disorders in older adults.
Recent studies have highlighted the usefulness of VR in treating depression and loneliness in older patients who may be socially isolated because of their age, comorbidities, or the COVID-19 pandemic.
“The unique capability of virtual reality to create an immersive and engaging setting is an exciting opportunity for geriatric psychiatry,” Harmehr Sekhon, PhD, postdoctoral research fellow, Lady Davis Institute/Jewish General Hospital, McGill University, Montreal, and McLean Hospital, Harvard Medical School, Boston, told this news organization.
, Dr. Sekhon said.
One novel approach involves using VR to administer a mindfulness intervention in older adults. Dr. Sekhon shared information on her own mindfulness study and on other developments in VR and telemedicine at the American Association for Geriatric Psychiatry annual meeting.
Potential bridging tool
As the population ages, the prevalence of mental health disorders increases. Telemedicine has proved to be a potential “bridge” to address the health care needs of older adults, Dr. Sekhon noted.
She cited her systematic review of telemedicine for older adults with dementia during COVID-19. Results showed that telemedicine was a “beneficial approach” to assisting these individuals and that it increased accessibility, said Dr. Sekhon.
In addition, a survey published last year showed that 87% of Americans in general want to continue using telehealth services after the pandemic. Most respondents agreed that telehealth had made it easier to get the care they needed. They also reported having received the same level of care via telehealth as with in-person care.
A growing body of research shows that VR has “positive influences on mood and well-being, cognition, pain management, [and] treatment of phobias in younger adults,” Dr. Sekhon said. She added that there is evidence that VR is feasible for older adults, with applications in cognitive disorders.
She cited a recent systematic review of 55 studies that assessed the impact of different types of VR on mental health in older adults. The results showed that VR could be helpful in screening for cognitive impairment – and it was comparable to some paper-based assessment. It was also useful as a training tool for those with cognitive impairment.
Examples of VR interventions that can be used to treat cognitive impairment include “virtual cities, kitchens, supermarkets,” Dr. Sekhon noted.
The technology is increasingly being used as a tool to deliver psychotherapy, in which patient engagement is “a key determinant” of outcomes, she added. “Virtual reality is a cutting-edge, engaging, and immersive technique to administer psychotherapy,” she said.
Such VR approaches are proving successful in older patients. Dr. Sekhon highlighted the case of an 85-year-old woman who engaged in ten sessions of psychodynamic psychotherapy that targeted persistent dysthymia and negativistic mood. The case was part of a proof-of-concept study published in the May issue of the American Journal of Geriatric Psychiatry.
Dr. Sekhon noted the intervention was well tolerated and was associated with minimal side effects.
VR-based meditation
Dr. Sekhon and her colleagues are now conducting a randomized controlled trial of VR meditation in older adults. VR-based meditation has been shown to increase relaxation and to decrease anxiety, sadness, and anger in younger adults. However, it has not been studied in the geriatric population.
The pilot study is assessing the feasibility and tolerability of VR meditation for older adults and its effects on stress, anxiety, depression, sleep, and quality of life. The study involves 30 adults aged 60 years and older.
Participants receive either 15-minute VR mindfulness meditation sessions twice a week for 4 weeks or are on a control wait list. The meditation sessions are user friendly and focus on breath meditation and body scans, Dr. Sekhon reported.
Because participants are older and balance is a concern, safety steps are incorporated into the sessions. “We ensure they’re doing this in a seated position, in a chair with arm rests, so that they’re very stable and there’s no risk of falls,” said Dr. Sekhon.
Another concern with VR is motion sickness, she noted. “It’s pretty minimal, but the best way we found so far is giving older adults time to adapt and feel comfortable with the VR,” she said. From the first session, participants learn how to put on the device and are checked to make sure they are comfortable with the process. To help them get used to everything, video and audio are not included during the first session.
Dr. Sekhon noted that results from the study are expected later this year.
In addition to mindfulness, researchers are using VR to deliver other established interventions, such as exposure therapy – and are implementing these approaches in varied environments, including long-term and palliative care settings.
VR-related technology is constantly improving and is becoming easier to use and more affordable, said Dr. Sekhon. She noted that the simplest devices that rely on smartphones cost as little as $15.
Although VR in older adults is promising, there are barriers to its adoption and use in research, she noted. For example, older adults may have cognitive, visual, or hearing impairments. They may have limited digital literacy, and/or they may not have access to the required technology.
These barriers can be overcome through workarounds, including providing instructional videos and digital literacy assistance via Zoom and working with community partners to facilitate study recruitment of older patients, Dr. Sekhon said.
Dr. Sekhon’s research is funded by the Canadian Institutes of Health Research and the Fonds de recherche du Quebec Sante.
A version of this article first appeared on Medscape.com.
Researchers are increasingly turning their attention to virtual reality (VR) for the treatment of psychiatric disorders in older adults.
Recent studies have highlighted the usefulness of VR in treating depression and loneliness in older patients who may be socially isolated because of their age, comorbidities, or the COVID-19 pandemic.
“The unique capability of virtual reality to create an immersive and engaging setting is an exciting opportunity for geriatric psychiatry,” Harmehr Sekhon, PhD, postdoctoral research fellow, Lady Davis Institute/Jewish General Hospital, McGill University, Montreal, and McLean Hospital, Harvard Medical School, Boston, told this news organization.
, Dr. Sekhon said.
One novel approach involves using VR to administer a mindfulness intervention in older adults. Dr. Sekhon shared information on her own mindfulness study and on other developments in VR and telemedicine at the American Association for Geriatric Psychiatry annual meeting.
Potential bridging tool
As the population ages, the prevalence of mental health disorders increases. Telemedicine has proved to be a potential “bridge” to address the health care needs of older adults, Dr. Sekhon noted.
She cited her systematic review of telemedicine for older adults with dementia during COVID-19. Results showed that telemedicine was a “beneficial approach” to assisting these individuals and that it increased accessibility, said Dr. Sekhon.
In addition, a survey published last year showed that 87% of Americans in general want to continue using telehealth services after the pandemic. Most respondents agreed that telehealth had made it easier to get the care they needed. They also reported having received the same level of care via telehealth as with in-person care.
A growing body of research shows that VR has “positive influences on mood and well-being, cognition, pain management, [and] treatment of phobias in younger adults,” Dr. Sekhon said. She added that there is evidence that VR is feasible for older adults, with applications in cognitive disorders.
She cited a recent systematic review of 55 studies that assessed the impact of different types of VR on mental health in older adults. The results showed that VR could be helpful in screening for cognitive impairment – and it was comparable to some paper-based assessment. It was also useful as a training tool for those with cognitive impairment.
Examples of VR interventions that can be used to treat cognitive impairment include “virtual cities, kitchens, supermarkets,” Dr. Sekhon noted.
The technology is increasingly being used as a tool to deliver psychotherapy, in which patient engagement is “a key determinant” of outcomes, she added. “Virtual reality is a cutting-edge, engaging, and immersive technique to administer psychotherapy,” she said.
Such VR approaches are proving successful in older patients. Dr. Sekhon highlighted the case of an 85-year-old woman who engaged in ten sessions of psychodynamic psychotherapy that targeted persistent dysthymia and negativistic mood. The case was part of a proof-of-concept study published in the May issue of the American Journal of Geriatric Psychiatry.
Dr. Sekhon noted the intervention was well tolerated and was associated with minimal side effects.
VR-based meditation
Dr. Sekhon and her colleagues are now conducting a randomized controlled trial of VR meditation in older adults. VR-based meditation has been shown to increase relaxation and to decrease anxiety, sadness, and anger in younger adults. However, it has not been studied in the geriatric population.
The pilot study is assessing the feasibility and tolerability of VR meditation for older adults and its effects on stress, anxiety, depression, sleep, and quality of life. The study involves 30 adults aged 60 years and older.
Participants receive either 15-minute VR mindfulness meditation sessions twice a week for 4 weeks or are on a control wait list. The meditation sessions are user friendly and focus on breath meditation and body scans, Dr. Sekhon reported.
Because participants are older and balance is a concern, safety steps are incorporated into the sessions. “We ensure they’re doing this in a seated position, in a chair with arm rests, so that they’re very stable and there’s no risk of falls,” said Dr. Sekhon.
Another concern with VR is motion sickness, she noted. “It’s pretty minimal, but the best way we found so far is giving older adults time to adapt and feel comfortable with the VR,” she said. From the first session, participants learn how to put on the device and are checked to make sure they are comfortable with the process. To help them get used to everything, video and audio are not included during the first session.
Dr. Sekhon noted that results from the study are expected later this year.
In addition to mindfulness, researchers are using VR to deliver other established interventions, such as exposure therapy – and are implementing these approaches in varied environments, including long-term and palliative care settings.
VR-related technology is constantly improving and is becoming easier to use and more affordable, said Dr. Sekhon. She noted that the simplest devices that rely on smartphones cost as little as $15.
Although VR in older adults is promising, there are barriers to its adoption and use in research, she noted. For example, older adults may have cognitive, visual, or hearing impairments. They may have limited digital literacy, and/or they may not have access to the required technology.
These barriers can be overcome through workarounds, including providing instructional videos and digital literacy assistance via Zoom and working with community partners to facilitate study recruitment of older patients, Dr. Sekhon said.
Dr. Sekhon’s research is funded by the Canadian Institutes of Health Research and the Fonds de recherche du Quebec Sante.
A version of this article first appeared on Medscape.com.
Researchers are increasingly turning their attention to virtual reality (VR) for the treatment of psychiatric disorders in older adults.
Recent studies have highlighted the usefulness of VR in treating depression and loneliness in older patients who may be socially isolated because of their age, comorbidities, or the COVID-19 pandemic.
“The unique capability of virtual reality to create an immersive and engaging setting is an exciting opportunity for geriatric psychiatry,” Harmehr Sekhon, PhD, postdoctoral research fellow, Lady Davis Institute/Jewish General Hospital, McGill University, Montreal, and McLean Hospital, Harvard Medical School, Boston, told this news organization.
, Dr. Sekhon said.
One novel approach involves using VR to administer a mindfulness intervention in older adults. Dr. Sekhon shared information on her own mindfulness study and on other developments in VR and telemedicine at the American Association for Geriatric Psychiatry annual meeting.
Potential bridging tool
As the population ages, the prevalence of mental health disorders increases. Telemedicine has proved to be a potential “bridge” to address the health care needs of older adults, Dr. Sekhon noted.
She cited her systematic review of telemedicine for older adults with dementia during COVID-19. Results showed that telemedicine was a “beneficial approach” to assisting these individuals and that it increased accessibility, said Dr. Sekhon.
In addition, a survey published last year showed that 87% of Americans in general want to continue using telehealth services after the pandemic. Most respondents agreed that telehealth had made it easier to get the care they needed. They also reported having received the same level of care via telehealth as with in-person care.
A growing body of research shows that VR has “positive influences on mood and well-being, cognition, pain management, [and] treatment of phobias in younger adults,” Dr. Sekhon said. She added that there is evidence that VR is feasible for older adults, with applications in cognitive disorders.
She cited a recent systematic review of 55 studies that assessed the impact of different types of VR on mental health in older adults. The results showed that VR could be helpful in screening for cognitive impairment – and it was comparable to some paper-based assessment. It was also useful as a training tool for those with cognitive impairment.
Examples of VR interventions that can be used to treat cognitive impairment include “virtual cities, kitchens, supermarkets,” Dr. Sekhon noted.
The technology is increasingly being used as a tool to deliver psychotherapy, in which patient engagement is “a key determinant” of outcomes, she added. “Virtual reality is a cutting-edge, engaging, and immersive technique to administer psychotherapy,” she said.
Such VR approaches are proving successful in older patients. Dr. Sekhon highlighted the case of an 85-year-old woman who engaged in ten sessions of psychodynamic psychotherapy that targeted persistent dysthymia and negativistic mood. The case was part of a proof-of-concept study published in the May issue of the American Journal of Geriatric Psychiatry.
Dr. Sekhon noted the intervention was well tolerated and was associated with minimal side effects.
VR-based meditation
Dr. Sekhon and her colleagues are now conducting a randomized controlled trial of VR meditation in older adults. VR-based meditation has been shown to increase relaxation and to decrease anxiety, sadness, and anger in younger adults. However, it has not been studied in the geriatric population.
The pilot study is assessing the feasibility and tolerability of VR meditation for older adults and its effects on stress, anxiety, depression, sleep, and quality of life. The study involves 30 adults aged 60 years and older.
Participants receive either 15-minute VR mindfulness meditation sessions twice a week for 4 weeks or are on a control wait list. The meditation sessions are user friendly and focus on breath meditation and body scans, Dr. Sekhon reported.
Because participants are older and balance is a concern, safety steps are incorporated into the sessions. “We ensure they’re doing this in a seated position, in a chair with arm rests, so that they’re very stable and there’s no risk of falls,” said Dr. Sekhon.
Another concern with VR is motion sickness, she noted. “It’s pretty minimal, but the best way we found so far is giving older adults time to adapt and feel comfortable with the VR,” she said. From the first session, participants learn how to put on the device and are checked to make sure they are comfortable with the process. To help them get used to everything, video and audio are not included during the first session.
Dr. Sekhon noted that results from the study are expected later this year.
In addition to mindfulness, researchers are using VR to deliver other established interventions, such as exposure therapy – and are implementing these approaches in varied environments, including long-term and palliative care settings.
VR-related technology is constantly improving and is becoming easier to use and more affordable, said Dr. Sekhon. She noted that the simplest devices that rely on smartphones cost as little as $15.
Although VR in older adults is promising, there are barriers to its adoption and use in research, she noted. For example, older adults may have cognitive, visual, or hearing impairments. They may have limited digital literacy, and/or they may not have access to the required technology.
These barriers can be overcome through workarounds, including providing instructional videos and digital literacy assistance via Zoom and working with community partners to facilitate study recruitment of older patients, Dr. Sekhon said.
Dr. Sekhon’s research is funded by the Canadian Institutes of Health Research and the Fonds de recherche du Quebec Sante.
A version of this article first appeared on Medscape.com.
FROM AAGP 2022
How old is too old to work as a doctor?
Air traffic controllers face mandatory retirement at age 56, with exceptions up to 61. Commercial airline pilots must bow out at 65; same for foreign service employees. Physicians, however, have no age limit, regardless of specialty.
As the profession rapidly ages – some 30% of the physician workforce is currently a senior, according to the American Medical Association – the topic of whether or not there should be a standard measure or age for retirement is front and center. The AMA’s Council on Medical Education formed a workgroup to look into the issue in 2015 and 2018, and in 2021, delegates adopted a set of guidelines for screening and assessing physicians, but stopped short of a mandate.
Mark Katlic, MD, chair of surgery at Lifebridge Health System, Baltimore, has devoted a decade to studying this topic. “I’m a bit of an outlier looking into this,” he says. “The public is unaware and seemingly unconcerned about the issue. Even among the medical profession, there’s been a series of fits and starts to develop a cohesive approach.”
One of the reasons guidelines – mandatory or otherwise – have been tough to come by is that aging brings with it a huge degree of variability. “If you look at a group of 80-year-olds, there will be much more variability than within a group of 40-year-olds,” Dr. Katlic pointed out.
Indeed, some 80-year-olds can easily continue to teach college courses, keep up in 10K running races, or perform delicate surgeries. Yet others in their peer group might struggle to properly button a shirt, walk a flight of stairs, or remember yesterday’s meals. Functional age is not the same as chronological age.
Frank Stockdale, MD, PhD, an 86-year-old practicing oncologist at Stanford (Calif.) University Health, counts himself in the camp opposed to age-based assessments. “It’s age discrimination,” he says. “Physicians receive assessments throughout their careers as part of the accreditation process – there’s no need to change that as doctors reach a certain age.”
Dr. Stockdale suggests that in many cases, malpractice suits are filed against mid-career doctors, not those of advanced age. “If you’re using the argument that there is an accumulation of deficits with age, the fact is that those deficits begin well before your 70s,” he said. “It’s better to have a uniform screening policy and begin at a much younger age.”
At Stanford, in fact, there was a former assessment policy that included cognitive testing, but physicians were successful in seeing that portion of testing eliminated. “It is a physical examination, by a physician of choice, certifying that for the privileges requested there is no physical or mental reason the candidate cannot safely perform them,” Dr. Stockdale explained.
In some cases, medical staffs have filed lawsuits to fight age-related testing. In New Haven, Conn., for instance, the U.S. Equal Employment Opportunity Commission (EEOC) filed a suit in 2020 on behalf of the Yale New Haven Hospital staff, alleging a discriminatory “late career practitioner policy.”
A similar case in Minnesota reached a settlement in 2021, providing monetary relief to staff impacted by out-of-pocket costs for the assessment, in addition to requiring that the hospital in question report to the EEOC any complaints related to age discrimination.
James Ellison, MD, MPH, chair in Memory Care and Geriatrics with ChristianaCare in Wilmington, Del., points out that aging can bring benefits for practicing physicians. “Age is very individualized and there are good and bad consequences,” he said. “Experience can build knowledge and confidence and expertise, and it does improve diagnostic accuracy.”
On the flip side, however, age-related brain changes include loss of volume and lower levels of some neurotransmitters, resulting in cognitive changes. “Functional changes occur too,” Dr. Ellison said.
“Just as some aging athletes may lose a degree of speed, strength, and flexibility, and some aging scientists may lose a part of their former cognitive speed, flexibility, and mental strength, aging health care providers can lose some of the physical coordination, strength, and visual acuity necessary to perform demanding surgical operations. They can also lose some of the processing speed, working memory, and executive function that allows them to excel in cognitive professional tasks.”
An estimated 5.8 million Americans age 65 and older have Alzheimer’s dementia, according to the Alzheimer’s Association.
Picking an arbitrary age for mandatory retirement isn’t the right approach for physicians, said Dr. Katlic. Rather, he said, the answer is to establish late-practitioner screening programs. “Very few hospitals have them, however,” he pointed out. “We do [at Lifebridge Health], and so do a few dozen others, but that’s out of hundreds.”
Instead, what typically plays out is that hospital staff might begin to notice a decline in a colleague. Things like a disheveled appearance or lack of hygiene, or trouble with memory, such as getting lost en route back to his or her office. Even dangerous behaviors such as nodding off during a procedure are not unheard of.
There are many examples of physician decline that fly under the radar. “Unfortunately, it’s unusual for cognitively impaired health care providers to recognize and report their own difficulties,” said Dr. Ellison. “Although peers are expected to report cognitively impaired colleagues, they often fail to do so. In some other countries, age-based assessment is an accepted policy. In the U.S., this is not a uniform policy.”
Sometimes physicians can remain on the job in spite of decline thanks to certain “props,” according to Dr. Ellison. “Good procedures, efficient supports, and various workarounds compensate,” he said, “but often are not sufficient to maintain high-quality practice.”
Most often, these situations play out slowly, until the problem becomes glaringly obvious and potentially dangerous, and someone in a position of power must step in.
“Often, it’s hearsay from a nurse or another staff member, and then a hospital president or chief of staff must make a career-affecting decision for the doctor in question,” said Dr. Katlic.
Because there is little self- or colleague policing – and barring official or binding guidelines on the aging physician issue – both Dr. Katlic and Dr. Ellison are proponents of late-career screening.
How screening can help
As it stands, Dr. Katlic maintains that the profession isn’t doing enough to ensure public safety. “We have peer review and recertification processes, but when you get down to it, we don’t police ourselves well,” he said. “All physicians are assessed throughout their careers as part of the hospital accreditation process, which is fair and adequate.”
Dr. Katlic said that there are three main benchmarks that physicians should be able to meet at an agreed upon age: a physical exam, a neurocognitive screening, and an eye exam. “At some reasonable age, I personally believe these exams should take place,” he said. “We can allow doctors to pick their own practitioners for the eye and physical exams, but the neurocognitive exam should be completed by a PhD neuropsychologist.”
At Lifebridge, for instance, these screenings begin at age 75 and take place every 2 years, during the recredentialing process. It applies to all specialties, not just surgeons. “Surgery is a little different in that it requires fine motor skills in addition to the others we test, but you want any physician to be cognitively intact,” Dr. Katlic pointed out. “All doctors need the ability to make decisions quickly, often under noisy, distracting conditions.”
Dr. Ellison supports applying the screenings to all specialties. “Let’s not forget that all physicians must be alert to the many ways in which their patients reveal what needs attention, evaluation, and treatment,” he said. “Some health care tasks could be performed without visual input; for example, perhaps psychotherapy could be provided competently by a clinician who lacks visual acuity. Auditory input might not be necessary for reading x-rays – but the information a health care provider gets from their eyes and ears is important, not just for surgeons.”
University of California San Diego has established what it calls its Physician Assessment and Clinical Education (PACE) program. One of the nation’s oldest and largest such programs, the hospital founded PACE in 1996. Most physicians taking part arrive as a requirement of disciplinary action from the state medical board, but a small percentage self-refers.
PACE involves two phases. The first is a 2-day set of tests and measures core competency knowledge. Phase 2 is more comprehensive and lasts 5 days. Here, within their specialty, physicians participate in the activities of the corresponding residency program. Faculty evaluates the physician, and a multidisciplinary team meets to review all the findings of the combined phases.
Depending on the results, doctors may face remediation steps that range from programs to address performance deficiencies to residency-level clinical experiences. According to a paper on the program published by the institution, “most physicians referred to the PACE program are found to have mild to moderate performance dyscompetence.”
In the case of the 2021 guidelines adopted by AMA delegates, there are nine principles for assessment. They should be evidence-based, ethical, relevant, accountable, fair and equitable, transparent, supportive, and nonburdensome, and should afford physicians due process protections.
Looking ahead
Even Dr. Katlic worries about the possibility of Congress intervening to establish federal-level, mandatory retirement age. “This just doesn’t make sense for our profession given the great variability we see,” he said. “My biggest hope is that more individual hospitals will institute these screenings.”
As the physician population ages – and the influx of new doctors shrinks – the slope becomes even more slippery. The AMA is predicting a physician shortage of nearly 40,000 by the year 2034. This strengthens arguments to keep existing physicians practicing for as long as possible and might make institutions less likely to screen.
It’s all a delicate balancing act and a continuing work in progress, said Dr. Ellison. “Ultimately, I believe we need to find a way to understand and address the possible implications for public safety, while at the same time protecting the privacy and dignity of our valued older physicians and other health care providers.”
A version of this article first appeared on Medscape.com.
Air traffic controllers face mandatory retirement at age 56, with exceptions up to 61. Commercial airline pilots must bow out at 65; same for foreign service employees. Physicians, however, have no age limit, regardless of specialty.
As the profession rapidly ages – some 30% of the physician workforce is currently a senior, according to the American Medical Association – the topic of whether or not there should be a standard measure or age for retirement is front and center. The AMA’s Council on Medical Education formed a workgroup to look into the issue in 2015 and 2018, and in 2021, delegates adopted a set of guidelines for screening and assessing physicians, but stopped short of a mandate.
Mark Katlic, MD, chair of surgery at Lifebridge Health System, Baltimore, has devoted a decade to studying this topic. “I’m a bit of an outlier looking into this,” he says. “The public is unaware and seemingly unconcerned about the issue. Even among the medical profession, there’s been a series of fits and starts to develop a cohesive approach.”
One of the reasons guidelines – mandatory or otherwise – have been tough to come by is that aging brings with it a huge degree of variability. “If you look at a group of 80-year-olds, there will be much more variability than within a group of 40-year-olds,” Dr. Katlic pointed out.
Indeed, some 80-year-olds can easily continue to teach college courses, keep up in 10K running races, or perform delicate surgeries. Yet others in their peer group might struggle to properly button a shirt, walk a flight of stairs, or remember yesterday’s meals. Functional age is not the same as chronological age.
Frank Stockdale, MD, PhD, an 86-year-old practicing oncologist at Stanford (Calif.) University Health, counts himself in the camp opposed to age-based assessments. “It’s age discrimination,” he says. “Physicians receive assessments throughout their careers as part of the accreditation process – there’s no need to change that as doctors reach a certain age.”
Dr. Stockdale suggests that in many cases, malpractice suits are filed against mid-career doctors, not those of advanced age. “If you’re using the argument that there is an accumulation of deficits with age, the fact is that those deficits begin well before your 70s,” he said. “It’s better to have a uniform screening policy and begin at a much younger age.”
At Stanford, in fact, there was a former assessment policy that included cognitive testing, but physicians were successful in seeing that portion of testing eliminated. “It is a physical examination, by a physician of choice, certifying that for the privileges requested there is no physical or mental reason the candidate cannot safely perform them,” Dr. Stockdale explained.
In some cases, medical staffs have filed lawsuits to fight age-related testing. In New Haven, Conn., for instance, the U.S. Equal Employment Opportunity Commission (EEOC) filed a suit in 2020 on behalf of the Yale New Haven Hospital staff, alleging a discriminatory “late career practitioner policy.”
A similar case in Minnesota reached a settlement in 2021, providing monetary relief to staff impacted by out-of-pocket costs for the assessment, in addition to requiring that the hospital in question report to the EEOC any complaints related to age discrimination.
James Ellison, MD, MPH, chair in Memory Care and Geriatrics with ChristianaCare in Wilmington, Del., points out that aging can bring benefits for practicing physicians. “Age is very individualized and there are good and bad consequences,” he said. “Experience can build knowledge and confidence and expertise, and it does improve diagnostic accuracy.”
On the flip side, however, age-related brain changes include loss of volume and lower levels of some neurotransmitters, resulting in cognitive changes. “Functional changes occur too,” Dr. Ellison said.
“Just as some aging athletes may lose a degree of speed, strength, and flexibility, and some aging scientists may lose a part of their former cognitive speed, flexibility, and mental strength, aging health care providers can lose some of the physical coordination, strength, and visual acuity necessary to perform demanding surgical operations. They can also lose some of the processing speed, working memory, and executive function that allows them to excel in cognitive professional tasks.”
An estimated 5.8 million Americans age 65 and older have Alzheimer’s dementia, according to the Alzheimer’s Association.
Picking an arbitrary age for mandatory retirement isn’t the right approach for physicians, said Dr. Katlic. Rather, he said, the answer is to establish late-practitioner screening programs. “Very few hospitals have them, however,” he pointed out. “We do [at Lifebridge Health], and so do a few dozen others, but that’s out of hundreds.”
Instead, what typically plays out is that hospital staff might begin to notice a decline in a colleague. Things like a disheveled appearance or lack of hygiene, or trouble with memory, such as getting lost en route back to his or her office. Even dangerous behaviors such as nodding off during a procedure are not unheard of.
There are many examples of physician decline that fly under the radar. “Unfortunately, it’s unusual for cognitively impaired health care providers to recognize and report their own difficulties,” said Dr. Ellison. “Although peers are expected to report cognitively impaired colleagues, they often fail to do so. In some other countries, age-based assessment is an accepted policy. In the U.S., this is not a uniform policy.”
Sometimes physicians can remain on the job in spite of decline thanks to certain “props,” according to Dr. Ellison. “Good procedures, efficient supports, and various workarounds compensate,” he said, “but often are not sufficient to maintain high-quality practice.”
Most often, these situations play out slowly, until the problem becomes glaringly obvious and potentially dangerous, and someone in a position of power must step in.
“Often, it’s hearsay from a nurse or another staff member, and then a hospital president or chief of staff must make a career-affecting decision for the doctor in question,” said Dr. Katlic.
Because there is little self- or colleague policing – and barring official or binding guidelines on the aging physician issue – both Dr. Katlic and Dr. Ellison are proponents of late-career screening.
How screening can help
As it stands, Dr. Katlic maintains that the profession isn’t doing enough to ensure public safety. “We have peer review and recertification processes, but when you get down to it, we don’t police ourselves well,” he said. “All physicians are assessed throughout their careers as part of the hospital accreditation process, which is fair and adequate.”
Dr. Katlic said that there are three main benchmarks that physicians should be able to meet at an agreed upon age: a physical exam, a neurocognitive screening, and an eye exam. “At some reasonable age, I personally believe these exams should take place,” he said. “We can allow doctors to pick their own practitioners for the eye and physical exams, but the neurocognitive exam should be completed by a PhD neuropsychologist.”
At Lifebridge, for instance, these screenings begin at age 75 and take place every 2 years, during the recredentialing process. It applies to all specialties, not just surgeons. “Surgery is a little different in that it requires fine motor skills in addition to the others we test, but you want any physician to be cognitively intact,” Dr. Katlic pointed out. “All doctors need the ability to make decisions quickly, often under noisy, distracting conditions.”
Dr. Ellison supports applying the screenings to all specialties. “Let’s not forget that all physicians must be alert to the many ways in which their patients reveal what needs attention, evaluation, and treatment,” he said. “Some health care tasks could be performed without visual input; for example, perhaps psychotherapy could be provided competently by a clinician who lacks visual acuity. Auditory input might not be necessary for reading x-rays – but the information a health care provider gets from their eyes and ears is important, not just for surgeons.”
University of California San Diego has established what it calls its Physician Assessment and Clinical Education (PACE) program. One of the nation’s oldest and largest such programs, the hospital founded PACE in 1996. Most physicians taking part arrive as a requirement of disciplinary action from the state medical board, but a small percentage self-refers.
PACE involves two phases. The first is a 2-day set of tests and measures core competency knowledge. Phase 2 is more comprehensive and lasts 5 days. Here, within their specialty, physicians participate in the activities of the corresponding residency program. Faculty evaluates the physician, and a multidisciplinary team meets to review all the findings of the combined phases.
Depending on the results, doctors may face remediation steps that range from programs to address performance deficiencies to residency-level clinical experiences. According to a paper on the program published by the institution, “most physicians referred to the PACE program are found to have mild to moderate performance dyscompetence.”
In the case of the 2021 guidelines adopted by AMA delegates, there are nine principles for assessment. They should be evidence-based, ethical, relevant, accountable, fair and equitable, transparent, supportive, and nonburdensome, and should afford physicians due process protections.
Looking ahead
Even Dr. Katlic worries about the possibility of Congress intervening to establish federal-level, mandatory retirement age. “This just doesn’t make sense for our profession given the great variability we see,” he said. “My biggest hope is that more individual hospitals will institute these screenings.”
As the physician population ages – and the influx of new doctors shrinks – the slope becomes even more slippery. The AMA is predicting a physician shortage of nearly 40,000 by the year 2034. This strengthens arguments to keep existing physicians practicing for as long as possible and might make institutions less likely to screen.
It’s all a delicate balancing act and a continuing work in progress, said Dr. Ellison. “Ultimately, I believe we need to find a way to understand and address the possible implications for public safety, while at the same time protecting the privacy and dignity of our valued older physicians and other health care providers.”
A version of this article first appeared on Medscape.com.
Air traffic controllers face mandatory retirement at age 56, with exceptions up to 61. Commercial airline pilots must bow out at 65; same for foreign service employees. Physicians, however, have no age limit, regardless of specialty.
As the profession rapidly ages – some 30% of the physician workforce is currently a senior, according to the American Medical Association – the topic of whether or not there should be a standard measure or age for retirement is front and center. The AMA’s Council on Medical Education formed a workgroup to look into the issue in 2015 and 2018, and in 2021, delegates adopted a set of guidelines for screening and assessing physicians, but stopped short of a mandate.
Mark Katlic, MD, chair of surgery at Lifebridge Health System, Baltimore, has devoted a decade to studying this topic. “I’m a bit of an outlier looking into this,” he says. “The public is unaware and seemingly unconcerned about the issue. Even among the medical profession, there’s been a series of fits and starts to develop a cohesive approach.”
One of the reasons guidelines – mandatory or otherwise – have been tough to come by is that aging brings with it a huge degree of variability. “If you look at a group of 80-year-olds, there will be much more variability than within a group of 40-year-olds,” Dr. Katlic pointed out.
Indeed, some 80-year-olds can easily continue to teach college courses, keep up in 10K running races, or perform delicate surgeries. Yet others in their peer group might struggle to properly button a shirt, walk a flight of stairs, or remember yesterday’s meals. Functional age is not the same as chronological age.
Frank Stockdale, MD, PhD, an 86-year-old practicing oncologist at Stanford (Calif.) University Health, counts himself in the camp opposed to age-based assessments. “It’s age discrimination,” he says. “Physicians receive assessments throughout their careers as part of the accreditation process – there’s no need to change that as doctors reach a certain age.”
Dr. Stockdale suggests that in many cases, malpractice suits are filed against mid-career doctors, not those of advanced age. “If you’re using the argument that there is an accumulation of deficits with age, the fact is that those deficits begin well before your 70s,” he said. “It’s better to have a uniform screening policy and begin at a much younger age.”
At Stanford, in fact, there was a former assessment policy that included cognitive testing, but physicians were successful in seeing that portion of testing eliminated. “It is a physical examination, by a physician of choice, certifying that for the privileges requested there is no physical or mental reason the candidate cannot safely perform them,” Dr. Stockdale explained.
In some cases, medical staffs have filed lawsuits to fight age-related testing. In New Haven, Conn., for instance, the U.S. Equal Employment Opportunity Commission (EEOC) filed a suit in 2020 on behalf of the Yale New Haven Hospital staff, alleging a discriminatory “late career practitioner policy.”
A similar case in Minnesota reached a settlement in 2021, providing monetary relief to staff impacted by out-of-pocket costs for the assessment, in addition to requiring that the hospital in question report to the EEOC any complaints related to age discrimination.
James Ellison, MD, MPH, chair in Memory Care and Geriatrics with ChristianaCare in Wilmington, Del., points out that aging can bring benefits for practicing physicians. “Age is very individualized and there are good and bad consequences,” he said. “Experience can build knowledge and confidence and expertise, and it does improve diagnostic accuracy.”
On the flip side, however, age-related brain changes include loss of volume and lower levels of some neurotransmitters, resulting in cognitive changes. “Functional changes occur too,” Dr. Ellison said.
“Just as some aging athletes may lose a degree of speed, strength, and flexibility, and some aging scientists may lose a part of their former cognitive speed, flexibility, and mental strength, aging health care providers can lose some of the physical coordination, strength, and visual acuity necessary to perform demanding surgical operations. They can also lose some of the processing speed, working memory, and executive function that allows them to excel in cognitive professional tasks.”
An estimated 5.8 million Americans age 65 and older have Alzheimer’s dementia, according to the Alzheimer’s Association.
Picking an arbitrary age for mandatory retirement isn’t the right approach for physicians, said Dr. Katlic. Rather, he said, the answer is to establish late-practitioner screening programs. “Very few hospitals have them, however,” he pointed out. “We do [at Lifebridge Health], and so do a few dozen others, but that’s out of hundreds.”
Instead, what typically plays out is that hospital staff might begin to notice a decline in a colleague. Things like a disheveled appearance or lack of hygiene, or trouble with memory, such as getting lost en route back to his or her office. Even dangerous behaviors such as nodding off during a procedure are not unheard of.
There are many examples of physician decline that fly under the radar. “Unfortunately, it’s unusual for cognitively impaired health care providers to recognize and report their own difficulties,” said Dr. Ellison. “Although peers are expected to report cognitively impaired colleagues, they often fail to do so. In some other countries, age-based assessment is an accepted policy. In the U.S., this is not a uniform policy.”
Sometimes physicians can remain on the job in spite of decline thanks to certain “props,” according to Dr. Ellison. “Good procedures, efficient supports, and various workarounds compensate,” he said, “but often are not sufficient to maintain high-quality practice.”
Most often, these situations play out slowly, until the problem becomes glaringly obvious and potentially dangerous, and someone in a position of power must step in.
“Often, it’s hearsay from a nurse or another staff member, and then a hospital president or chief of staff must make a career-affecting decision for the doctor in question,” said Dr. Katlic.
Because there is little self- or colleague policing – and barring official or binding guidelines on the aging physician issue – both Dr. Katlic and Dr. Ellison are proponents of late-career screening.
How screening can help
As it stands, Dr. Katlic maintains that the profession isn’t doing enough to ensure public safety. “We have peer review and recertification processes, but when you get down to it, we don’t police ourselves well,” he said. “All physicians are assessed throughout their careers as part of the hospital accreditation process, which is fair and adequate.”
Dr. Katlic said that there are three main benchmarks that physicians should be able to meet at an agreed upon age: a physical exam, a neurocognitive screening, and an eye exam. “At some reasonable age, I personally believe these exams should take place,” he said. “We can allow doctors to pick their own practitioners for the eye and physical exams, but the neurocognitive exam should be completed by a PhD neuropsychologist.”
At Lifebridge, for instance, these screenings begin at age 75 and take place every 2 years, during the recredentialing process. It applies to all specialties, not just surgeons. “Surgery is a little different in that it requires fine motor skills in addition to the others we test, but you want any physician to be cognitively intact,” Dr. Katlic pointed out. “All doctors need the ability to make decisions quickly, often under noisy, distracting conditions.”
Dr. Ellison supports applying the screenings to all specialties. “Let’s not forget that all physicians must be alert to the many ways in which their patients reveal what needs attention, evaluation, and treatment,” he said. “Some health care tasks could be performed without visual input; for example, perhaps psychotherapy could be provided competently by a clinician who lacks visual acuity. Auditory input might not be necessary for reading x-rays – but the information a health care provider gets from their eyes and ears is important, not just for surgeons.”
University of California San Diego has established what it calls its Physician Assessment and Clinical Education (PACE) program. One of the nation’s oldest and largest such programs, the hospital founded PACE in 1996. Most physicians taking part arrive as a requirement of disciplinary action from the state medical board, but a small percentage self-refers.
PACE involves two phases. The first is a 2-day set of tests and measures core competency knowledge. Phase 2 is more comprehensive and lasts 5 days. Here, within their specialty, physicians participate in the activities of the corresponding residency program. Faculty evaluates the physician, and a multidisciplinary team meets to review all the findings of the combined phases.
Depending on the results, doctors may face remediation steps that range from programs to address performance deficiencies to residency-level clinical experiences. According to a paper on the program published by the institution, “most physicians referred to the PACE program are found to have mild to moderate performance dyscompetence.”
In the case of the 2021 guidelines adopted by AMA delegates, there are nine principles for assessment. They should be evidence-based, ethical, relevant, accountable, fair and equitable, transparent, supportive, and nonburdensome, and should afford physicians due process protections.
Looking ahead
Even Dr. Katlic worries about the possibility of Congress intervening to establish federal-level, mandatory retirement age. “This just doesn’t make sense for our profession given the great variability we see,” he said. “My biggest hope is that more individual hospitals will institute these screenings.”
As the physician population ages – and the influx of new doctors shrinks – the slope becomes even more slippery. The AMA is predicting a physician shortage of nearly 40,000 by the year 2034. This strengthens arguments to keep existing physicians practicing for as long as possible and might make institutions less likely to screen.
It’s all a delicate balancing act and a continuing work in progress, said Dr. Ellison. “Ultimately, I believe we need to find a way to understand and address the possible implications for public safety, while at the same time protecting the privacy and dignity of our valued older physicians and other health care providers.”
A version of this article first appeared on Medscape.com.
Neurotransmitter-based diagnosis and treatment: A hypothesis (Part 1)
It is unfortunate that, in some clinical areas, medical conditions are still treated by name and not based on the underlying pathological process. It would be odd in 2022 to treat “dropsy” instead of heart or kidney disease (2 very different causes of edema). Similarly, if the FDA had been approving drugs 150 years ago, we would have medications on label for “dementia praecox,” not schizophrenia or Alzheimer disease. With the help of DSM-5, psychiatry still resides in the descriptive symptomatic world of disorders.
In the United States, thanks to Freud, psychiatric symptoms became separated from medical symptoms, which made it more difficult to associate psychiatric manifestations with the underlying pathophysiology. Though the physical manifestations that parallel emotional symptoms—such as the dry mouth of anxiety, the tremor and leg weakness of fear, the constipation and blurry vision of depression, the breathing difficulty of anger, the abdominal pain of stress, the blushing of shyness, the palpitations of flashbacks, and endless others—are well known, the present classification of psychiatric disorders is blind to it. Neurochemical causes of gastrointestinal spasm or muscle tension are better researched than underlying central neurochemistry, though the same neurotransmitters drive them.
Can the biochemistry of psychiatric symptoms be judged on the basis of peripheral symptoms? Can the mental manifestations be connected to biological causation, and vice versa? Would psychiatrists be better off selecting treatments by recognizing involved neurotransmitters instead of addressing descriptive “depression, anxiety, and psychosis”? Each of these clinical syndromes may be caused by entirely different underlying neuronal mechanisms. Such mechanisms could be suggested if medical symptoms (which are measurable and objective) would become part of the psychiatric diagnosis. Is treating the “cough” sufficient, or would recognition that tuberculosis caused the cough guide better treatment? Is it time to abandon descriptive conditions and replace them with a specific “mechanism-based” viewpoint?
Ample research has shown that serotonin, dopamine, norepinephrine, endorphins, glutamate, and gamma aminobutyric acid (GABA) are the neurotransmitters most responsible in the process of both psychiatric disorders and chronic pain. These neurotransmitters are involved in much more than emotions (including the feeling of pain). An abundance of medical symptom clusters point toward which neurotransmitter dysfunction may be leading in specific cases of distinct types of depression, psychosis, anxiety, or “chronic pain.” Even presently, there are medications available (both for FDA-approved indications and off-label) that can be used to regulate these neurotransmitters, allowing practitioners to target the possible biological underlining of psychiatric or pain pathology. Hopefully, in the not-so-distant future, there will be specific medications for serotonin, dopamine, and noradrenergic depression as well as for GABAergic anxiety, endorphin psychosis, noradrenergic insomnia, and similar conditions.
Numerous neurotransmitters may be connected to both depression and pain in all their forms. These include (but are not limited to) prostaglandins, bradykinins, substance P, potassium, magnesium, calcium, histamine, adenosine triphosphate, calcitonin gene-related peptide (CGRP), nitric oxide (NO), cholecystokinin 7 (CCK7), neurotrophic growth factor (NGF), neurotensin, acetylcholine (Ach), oxytocin, cannabinoids, and others. These have not been researched sufficiently to identify their clinical presentation of excessive or insufficient availability at the sites of neurotransmission. It is difficult to draw conclusions about what kind of clinical symptoms they may cause (outside of pain), and therefore, they are not addressed in this article.
Both high and low levels of certain neurotransmitters may be associated with psychiatric conditions and chronic pain. Too much is as bad as too little.1 This applies to both quantity of neurotransmitters as well as quality of the corresponding receptor activity. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation. Reading indirect signs of bodily functions is a basic clinical skill that should not be forgotten, even in the time of advanced technology.
A different way of viewing psychiatric disorders
In this article, we present 4 hypothetical clinical cases to emphasize a possible way of analyzing symptoms to identify underlying pathology and guide more effective treatment. In no way do these descriptions reflect the entire set of symptoms caused by neurotransmitters; we created them based on what is presently known or suspected, and extensive research is required to confirm or disprove what we describe here.
Continue to: There are no well-recognized...
There are no well-recognized, well-established, reliable, or validated syndromes described in our work. Our goal is to suggest an alternative way of looking at psychiatric disorders by viewing syndromal presentation through the lens of specific neurotransmitters. The collection of symptoms associated with various neurotransmitters as presented in this hypothesis is not complete. We have assembled what is described in the literature as a suggestion for specific future research. We simplified these clinical presentations by omitting scenarios in which a specific neurotransmitter increases in one area but not another. For example, all the symptoms of dopamine excess we describe would not have to occur concurrently in the same patient, but they may develop in certain patients depending on which dopaminergic pathway is exhibiting excess activity. Such distinctions may be established only by exhaustive research not yet conducted.
Our proposal may seem radical, but it truly is not. For example, if we know that dopamine excess may cause seizures, psychosis, and blood pressure elevation, why not consider dopamine excess as an underlying cause in a patient with depression who exhibits these symptoms simultaneously? And why not call it “dopamine excess syndrome”? We already have “serotonin syndrome” for a patient experiencing a serotonin storm. However, using the same logic, it should be called “serotonin excess syndrome.” And if we know of “serotonin excess syndrome,” why not consider “serotonin deficiency syndrome”?
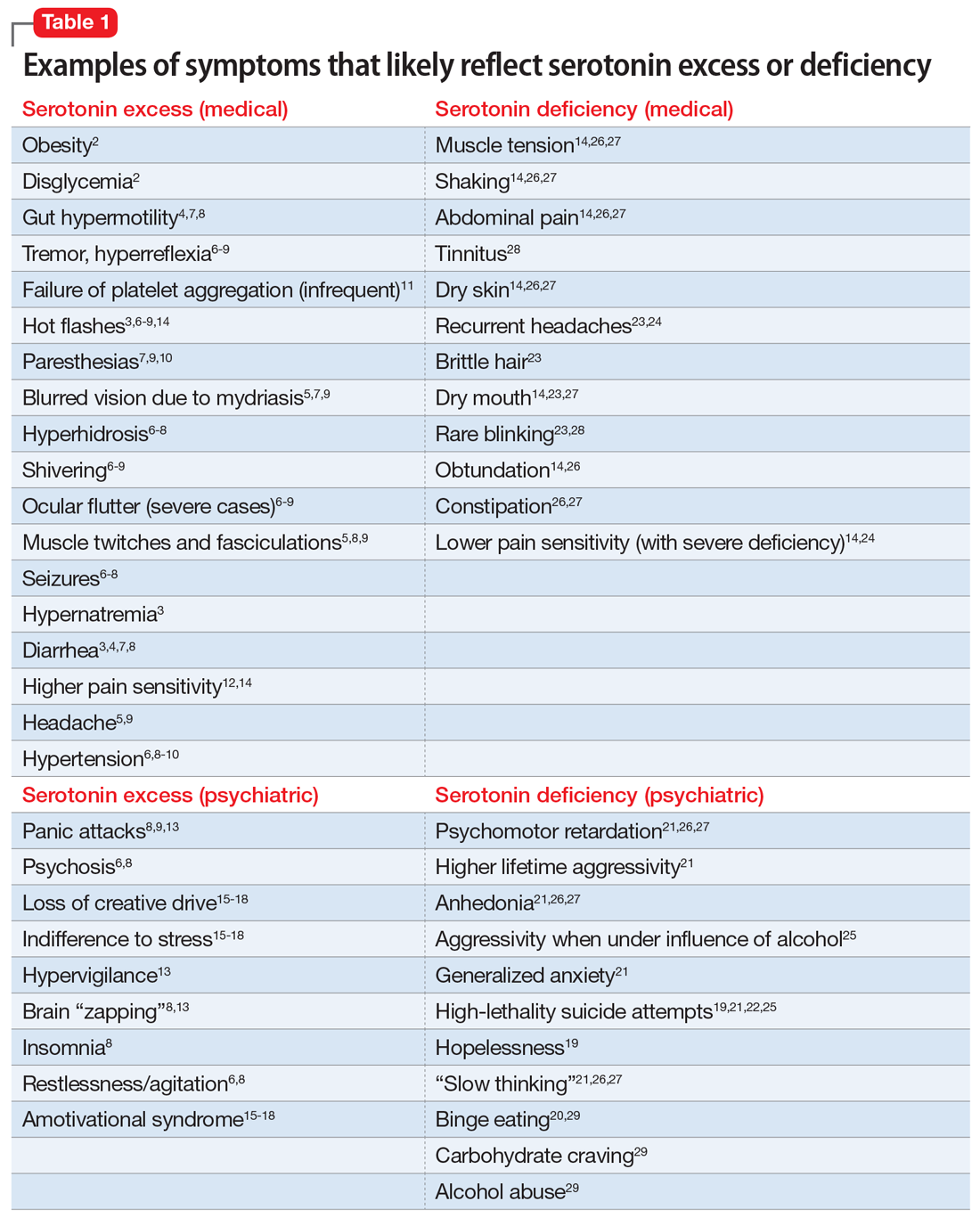
In Part 1 of this article, we discuss serotonin and dopamine. Table 1 outlines medical and psychiatric symptoms that likely reflect serotonin excess2-18 and deficiency,14,19-29 and Table 2 lists symptoms that likely reflect dopamine excess14,30-41 and deficiency.4,14,20,38,40-43 In Part 2 we will touch on endorphins and norepinephrine, and in Part 3 we will conclude by looking at GABA and glutamate.
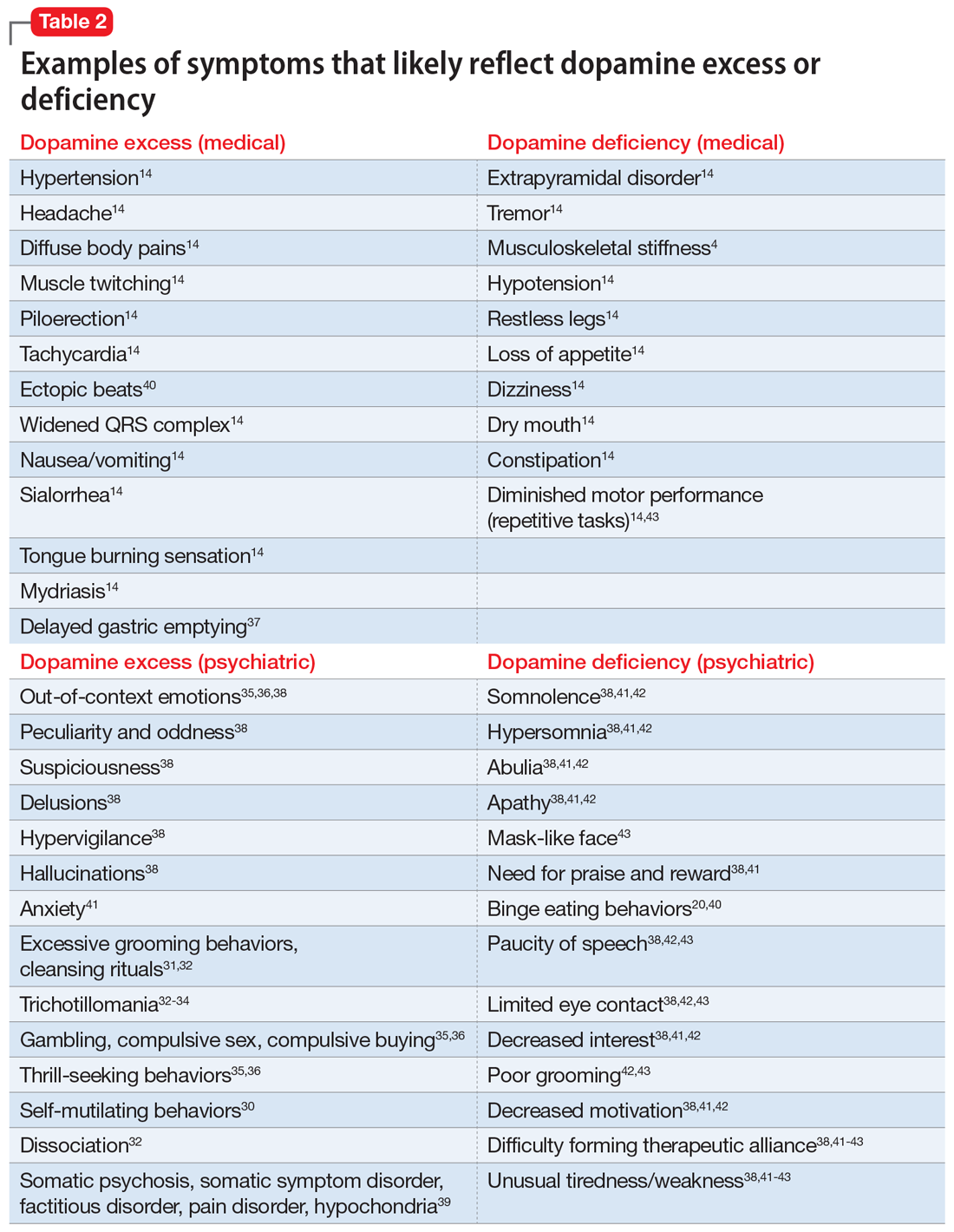
Serotonin excess (Table 12-18)
On a recent office visit, Ms. H reports that most of the time she does not feel much of anything, but she still experiences panic attacks8,9,13,15 and is easily agitated.6,8 Her mother died recently, and Ms. H is concerned that she did not grieve.15-18 She failed her last semester in college and was indifferent to her failure.18 She sleeps poorly,8 is failing her creative classes, and wonders why she has lost her artistic inclination.16-18 Ms. H has difficulty with amotivation, planning, social interactions, and speech.16,17 All of those symptoms worsened after she was prescribed fluoxetine approximately 1 year ago for her “blues.” Ms. H is obese and continues to gain weight,2 though she frequently has diarrhea,3,4,7,8 loud peristalsis, and abdominal cramps.4,7,8 She sweats easily6-8 and her heart frequently races.8,9 Additionally, Ms. H’s primary care physician told her that she has “borderline diabetes.”2 She is prone to frequent bruising11 and is easy to shake, even when she is experiencing minimal anxiety.6-9 Ms. H had consulted with a neurologist because of unusual electrical “zapping” in her brain and muscle twitches.5,8,9,13 She had experienced a seizure as a child, but this was possibly related to hypernatremia,2 and she has not taken any anticonvulsant medication for several years.8 She exhibits hyperactive deep tendon reflexes and tremors5,7,9 and blinks frequently.6,9 She experiences hot flashes,3,6-8,14 does not tolerate heat, and prefers cooler weather.8,9 Her pains and aches,12,14 to which she has been prone all of her life, have recently become much worse, and she was diagnosed with fibromyalgia in part because she frequently feels stiff all over.10 She complains of strange tingling and prickling sensations in her hands and feet, especially when anxious.7,9,10 Her headaches also worsened and may be precipitated by bright light, as her pupils are usually dilated.5,7,9 Her hypertension is fairly controlled with medication.6,8-10 Ms. H says she experienced a psychotic episode when she was in her mid-teens,6,8 but reassures you that “she is not that bad now,” although she remains hypervigilant.13 Also while in her teens, Ms. H was treated with paroxetine and experienced restlessness, agitation, delirium, tachycardia, fluctuating blood pressure, diaphoresis, diarrhea, and neuromuscular excitation, which prompted discontinuation of the antidepressant.5-7,9,10
Impression. Ms. H exhibits symptoms associated with serotonin hyperactivity. Discontinuing and avoiding selective serotonin reuptake inhibitors (SSRIs) would be prudent; prescribing an anticonvulsant would be reasonable. Using a GABAergic medication to suppress serotonin (eg, baclofen) is likely to help. Avoiding dopaminergic medications is a must. Antidepressive antipsychotics would be logical to use. The use of serotonin-suppressing medications may be considered. One may argue for the use of beta-blockers in such a patient.
Continue to: Serotonin deficiency
Serotonin deficiency (Table 114,19-29)
Mr. A is chronically depressed, hopeless,19 and easily angered.21 He does not believe anyone can help him.19 You are concerned for his safety because he had attempted to end his life by shooting himself in the chest.19,21,22,25 Even when he’s not particularly depressed, Mr. A does not enjoy much of anything.21,26,27 He becomes particularly agitated when he drinks alcohol,25 which unfortunately is common for him.29 He engages in binge eating to feel better; he knows this is not healthy but he cannot control his behavior.20,29 Mr. A is poorly compliant with his medications, even with a blood thinner, which he was prescribed due to an episode of deep vein thrombosis. He complains of chronic daily headaches and episodic migraines.23,24 He rarely blinks,23,28 his skin is dry and cool, his hair is brittle,23 his mouth is dry,14,23,27 and he constantly licks his chapped lips.14,26,27 Mr. A frequently has general body pain26,31 but is dismissive of his body aches and completely stops reporting pain when his depression gets particularly severe. When depressed, he is slow in movement and thinking.14,21,26,27 He is more concerned with anxiety than depression.21 Mr. A is plagued by constipation, abdominal pain, muscle tension, and episodes of shaking.14,26,27 He also frequently complains about chronic tinnitus.28
Impression. Mr. A shows symptoms associated with serotonin hypoactivity. SSRIs and any other antidepressants with serotonin activity would be an obvious choice for treatment. A mood-stabilizing antipsychotic with serotonin activity would be welcome in treatment. Thyroid hormone supplementation may be of value, especially if thyroid stimulating hormone level is high. Light therapy, a diet with food that contains tryptophan, psychotherapy, and exercise are desirable. Avoiding benzodiazepines would be a good idea.
Dopamine excess (Table 214,30-41)
Ms. L presents with complaints of “fibromyalgia” and “daily headaches,”14 and also dissociation (finding herself in places when she does not know how she got there) and “out-of-body experiences.”32 She is odd, and states that people do not understand her and that she is “different.”38 Her friend, who is present at the appointment, elaborates on Ms. L’s bizarreness and oddness in behavior, out-of-context emotions, suspiciousness, paranoia, and possible hallucinations.35,36,38 Ms. L discloses frequent diffuse body pains, headaches, nausea, excessive salivation, and tongue burning, as well as muscle twitching.14 Sex worsens her headaches and body pain. She reports seizures that are not registered on EEG. In the office, she is suspicious, exhibits odd posturing, tends to misinterpret your words, and makes you feel uncomfortable. Anxiety38 and multiple obsessive-compulsive symptoms, especially excessive cleaning and grooming, complicate Ms. L’s life.31,32,34 On examination, she is hypertensive, and she has scars caused by self-cutting and skin picking on her arms.30-32 An electrocardiogram shows an elevated heart rate, widened QRS complex, and ectopic heartbeats.14 Ms. L has experienced trichotillomania since adolescence32-34 and her fingernails are bitten to the skin.34 She has difficulty with impulse control, and thrill-seeking is a prominent part of her life, mainly via gambling, compulsive sex, and compulsive buying.35,36 She also says she experiences indigestion and delayed gastric emptying.37
Impression. Ms. L exhibits multiple symptoms associated with dopamine excess. Dopamine antagonists should be considered and may help not only with her psychiatric symptoms but also with her pain symptoms. Bupropion (as a dopamine agonist), caffeine, and stimulants should be avoided.
Excessive dopamine is, in extreme cases, associated with somatic psychosis, somatic symptom disorder, factitious disorder, pain disorder, and hypochondria.39 It may come with odd and bizarre/peculiar symptoms out of proportion with objectively identified pathology. These symptoms are common in chronic pain and headache patients, and need to be addressed by appropriate use of dopamine antagonizing medications.39
Continue to: Dopamine deficiency
Dopamine deficiency (Table 24,14,20,38,40-43)
Mr. W experiences widespread pain, including chronic back pain, headaches, and abdominal pain. He also has substantial anhedonia, lack of interest, procrastination, and hypersomnia.41,42 He is apathetic and has difficulty getting up in the morning.41,42 Unusual tiredness and weakness drive him to overuse caffeine; he states that 5 Mountain Dews and 4 cups of regular coffee a day make his headaches bearable.38,41-43 Sex also improves his headaches. Since childhood, he has taken stimulants for attention-deficit/hyperactivity disorder. He reports that occasional use of cocaine helps ease his pain and depression. Mr. W’s wife is concerned with her husband’s low sexual drive and alcohol consumption, and discloses that he has periodic trouble with gambling. Mr. W was forced into psychotherapy but never was able to work productively with his therapist.38,41-43 He loves eating and cannot control his weight.40 This contrasts with episodic anorexia he experienced when he was younger.20 His face is usually emotionless.43 Mr. W is prone to constipation.14 His restless leg syndrome and periodic limb movement disorder are so bad that his wife refuses to share a bed with him.14 He is clumsy and has a problem with repetitive motor tasks.43 A paucity of speech, limited eye contact, poor grooming, and difficulty forming therapeutic alliances have long been part of Mr. W’s history.38,42,43 On physical examination, he has a dry mouth; he is stiff, tremulous, and hypotensive.14
Impression. Mr. W shows multiple symptoms associated with dopamine deficiency. Bupropion may be reasonable to consider. Dopamine augmentation via the use of stimulants is warranted in such patients, especially if stimulants had not been tried before (lisdexamfetamine would be a good choice to minimize addictive potential). For a patient with dopamine deficiency, levodopa may improve more than just restless legs. Amantadine may improve dopaminergic signaling through the accelerated dopamine release and decrease in presynaptic uptake, so this medication may be carefully tried.44 Pain treatment would not be successful for Mr. W without simultaneous treatment for his substance use.
Bottom Line
Both high and low levels of serotonin and dopamine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: How to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Amantadine • Gocovri
Baclofen • Ozobax
Bupropion • Wellbutrin
Fluoxetine • Prozac
Lisdexamfetamine • Vyvanse
Paroxetine • Paxil
1. Stahl SM. Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors. CNS Spectr. 2017;22(4):305-311.
2. Young RL, Lumsden AL, Martin AM, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42(11):1880-1889.
3. Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79-S85.
4. Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319-342.
5. Prakash S, Belani P, Trivedi A. Headache as a presenting feature in patients with serotonin syndrome: a case series. Cephalalgia. 2014;34(2):148-153.
6. van Ewijk CE, Jacobs GE, Girbes ARJ. Unsuspected serotonin toxicity in the ICU. Ann Intensive Care. 2016;6(1):85.
7. Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care. 2014;21(1):108-113.
8. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
9. Ansari H, Kouti L. Drug interaction and serotonin toxicity with opioid use: another reason to avoid opioids in headache and migraine treatment. Curr Pain Headache Rep. 2016;20(8):50.
10. Ott M, Mannchen JK, Jamshidi F, et al. Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol. 2019;9:2045125318818814. doi:10.1177/2045125318818814
11. Cerrito F, Lazzaro MP, Gaudio E, et al. 5HT2-receptors and serotonin release: their role in human platelet aggregation. Life Sci. 1993;53(3):209-215.
12. Ohayon MM. Pain sensitivity, depression, and sleep deprivation: links with serotoninergic dysfunction. J Psychiatr Res. 2009;43(16):1243-1245.
13. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
14. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:120,199,201-204,730-740.
15. Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11(2):181-186.
16. George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette’s syndrome and obsessive compulsive disorder. J Clin Psychiatry. 1992;53(10):379-380.
17. Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52(3):131-133.
18. Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10(5):343-345.
19. Samuelsson M, Jokinen J, Nordström AL, et al. CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatr Scand. 2006;113(1):44-47.
20. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
21. Mann JJ, Oquendo M, Underwood MD, et al. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60 Suppl 2:7-116.
22. Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2):162-171.
23. Joseph R, Welch KM, D’Andrea G. Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia. 1989;9(4):293-299.
24. Pakalnis A, Splaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49(10):1486-1492.
25. Virkkunen M, Goldman D, Nielsen DA, et al. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271-275.
26. Liu Y, Zhao J, Fan X, et al. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front Psychiatry. 2019;10:286.
27. Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179-1187.
28. O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999;48(12):980-990.
29. Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42(2):147-151.
30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
31. Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33(1):1-2.
32. Tse W, Hälbig TD. Skin picking in Parkinson’s disease: a behavioral side-effect of dopaminergic treatment? Psychiatry Clin Neurosci. 2010;64(2):214.
33. Ayaydın H. Probable emergence of symptoms of trichotillomania by atomoxetine: a case report. Psychiatry and Clinical Psychopharmacology. 2019;29(2)220-222.
34. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702. doi:10.1155/2016/9782702
35. Clark CA, Dagher A. The role of dopamine in risk taking: a specific look at Parkinson’s disease and gambling. Front Behav Neurosci. 2014;8:196.
36. Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79-93.
37. Chen TS, Chang FY. Elevated serum dopamine increases while coffee consumption decreases the occurrence of reddish streaks in the intact stomach. J Gastroenterol Hepatol. 2013;28(12):1810-1814.
38. Wong-Riley MT. Neuroscience Secrets. 1st edition. Spanish version. Hanley & Belfus; 1999:420-429.
39. Arbuck DM. Antipsychotics, dopamine, and pain. Current Psychiatry. 2020;19(1):25-29,31.
40. Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25-33.
41. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46.
42. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506.
43. Gepshtein S, Li X, Snider J, et al. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26(3):645-657.
44. Scarff JR. The ABCDs of treating tardive dyskinesia. Current Psychiatry. 2020;19(4):21,55.
It is unfortunate that, in some clinical areas, medical conditions are still treated by name and not based on the underlying pathological process. It would be odd in 2022 to treat “dropsy” instead of heart or kidney disease (2 very different causes of edema). Similarly, if the FDA had been approving drugs 150 years ago, we would have medications on label for “dementia praecox,” not schizophrenia or Alzheimer disease. With the help of DSM-5, psychiatry still resides in the descriptive symptomatic world of disorders.
In the United States, thanks to Freud, psychiatric symptoms became separated from medical symptoms, which made it more difficult to associate psychiatric manifestations with the underlying pathophysiology. Though the physical manifestations that parallel emotional symptoms—such as the dry mouth of anxiety, the tremor and leg weakness of fear, the constipation and blurry vision of depression, the breathing difficulty of anger, the abdominal pain of stress, the blushing of shyness, the palpitations of flashbacks, and endless others—are well known, the present classification of psychiatric disorders is blind to it. Neurochemical causes of gastrointestinal spasm or muscle tension are better researched than underlying central neurochemistry, though the same neurotransmitters drive them.
Can the biochemistry of psychiatric symptoms be judged on the basis of peripheral symptoms? Can the mental manifestations be connected to biological causation, and vice versa? Would psychiatrists be better off selecting treatments by recognizing involved neurotransmitters instead of addressing descriptive “depression, anxiety, and psychosis”? Each of these clinical syndromes may be caused by entirely different underlying neuronal mechanisms. Such mechanisms could be suggested if medical symptoms (which are measurable and objective) would become part of the psychiatric diagnosis. Is treating the “cough” sufficient, or would recognition that tuberculosis caused the cough guide better treatment? Is it time to abandon descriptive conditions and replace them with a specific “mechanism-based” viewpoint?
Ample research has shown that serotonin, dopamine, norepinephrine, endorphins, glutamate, and gamma aminobutyric acid (GABA) are the neurotransmitters most responsible in the process of both psychiatric disorders and chronic pain. These neurotransmitters are involved in much more than emotions (including the feeling of pain). An abundance of medical symptom clusters point toward which neurotransmitter dysfunction may be leading in specific cases of distinct types of depression, psychosis, anxiety, or “chronic pain.” Even presently, there are medications available (both for FDA-approved indications and off-label) that can be used to regulate these neurotransmitters, allowing practitioners to target the possible biological underlining of psychiatric or pain pathology. Hopefully, in the not-so-distant future, there will be specific medications for serotonin, dopamine, and noradrenergic depression as well as for GABAergic anxiety, endorphin psychosis, noradrenergic insomnia, and similar conditions.
Numerous neurotransmitters may be connected to both depression and pain in all their forms. These include (but are not limited to) prostaglandins, bradykinins, substance P, potassium, magnesium, calcium, histamine, adenosine triphosphate, calcitonin gene-related peptide (CGRP), nitric oxide (NO), cholecystokinin 7 (CCK7), neurotrophic growth factor (NGF), neurotensin, acetylcholine (Ach), oxytocin, cannabinoids, and others. These have not been researched sufficiently to identify their clinical presentation of excessive or insufficient availability at the sites of neurotransmission. It is difficult to draw conclusions about what kind of clinical symptoms they may cause (outside of pain), and therefore, they are not addressed in this article.
Both high and low levels of certain neurotransmitters may be associated with psychiatric conditions and chronic pain. Too much is as bad as too little.1 This applies to both quantity of neurotransmitters as well as quality of the corresponding receptor activity. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation. Reading indirect signs of bodily functions is a basic clinical skill that should not be forgotten, even in the time of advanced technology.
A different way of viewing psychiatric disorders
In this article, we present 4 hypothetical clinical cases to emphasize a possible way of analyzing symptoms to identify underlying pathology and guide more effective treatment. In no way do these descriptions reflect the entire set of symptoms caused by neurotransmitters; we created them based on what is presently known or suspected, and extensive research is required to confirm or disprove what we describe here.
Continue to: There are no well-recognized...
There are no well-recognized, well-established, reliable, or validated syndromes described in our work. Our goal is to suggest an alternative way of looking at psychiatric disorders by viewing syndromal presentation through the lens of specific neurotransmitters. The collection of symptoms associated with various neurotransmitters as presented in this hypothesis is not complete. We have assembled what is described in the literature as a suggestion for specific future research. We simplified these clinical presentations by omitting scenarios in which a specific neurotransmitter increases in one area but not another. For example, all the symptoms of dopamine excess we describe would not have to occur concurrently in the same patient, but they may develop in certain patients depending on which dopaminergic pathway is exhibiting excess activity. Such distinctions may be established only by exhaustive research not yet conducted.
Our proposal may seem radical, but it truly is not. For example, if we know that dopamine excess may cause seizures, psychosis, and blood pressure elevation, why not consider dopamine excess as an underlying cause in a patient with depression who exhibits these symptoms simultaneously? And why not call it “dopamine excess syndrome”? We already have “serotonin syndrome” for a patient experiencing a serotonin storm. However, using the same logic, it should be called “serotonin excess syndrome.” And if we know of “serotonin excess syndrome,” why not consider “serotonin deficiency syndrome”?

In Part 1 of this article, we discuss serotonin and dopamine. Table 1 outlines medical and psychiatric symptoms that likely reflect serotonin excess2-18 and deficiency,14,19-29 and Table 2 lists symptoms that likely reflect dopamine excess14,30-41 and deficiency.4,14,20,38,40-43 In Part 2 we will touch on endorphins and norepinephrine, and in Part 3 we will conclude by looking at GABA and glutamate.

Serotonin excess (Table 12-18)
On a recent office visit, Ms. H reports that most of the time she does not feel much of anything, but she still experiences panic attacks8,9,13,15 and is easily agitated.6,8 Her mother died recently, and Ms. H is concerned that she did not grieve.15-18 She failed her last semester in college and was indifferent to her failure.18 She sleeps poorly,8 is failing her creative classes, and wonders why she has lost her artistic inclination.16-18 Ms. H has difficulty with amotivation, planning, social interactions, and speech.16,17 All of those symptoms worsened after she was prescribed fluoxetine approximately 1 year ago for her “blues.” Ms. H is obese and continues to gain weight,2 though she frequently has diarrhea,3,4,7,8 loud peristalsis, and abdominal cramps.4,7,8 She sweats easily6-8 and her heart frequently races.8,9 Additionally, Ms. H’s primary care physician told her that she has “borderline diabetes.”2 She is prone to frequent bruising11 and is easy to shake, even when she is experiencing minimal anxiety.6-9 Ms. H had consulted with a neurologist because of unusual electrical “zapping” in her brain and muscle twitches.5,8,9,13 She had experienced a seizure as a child, but this was possibly related to hypernatremia,2 and she has not taken any anticonvulsant medication for several years.8 She exhibits hyperactive deep tendon reflexes and tremors5,7,9 and blinks frequently.6,9 She experiences hot flashes,3,6-8,14 does not tolerate heat, and prefers cooler weather.8,9 Her pains and aches,12,14 to which she has been prone all of her life, have recently become much worse, and she was diagnosed with fibromyalgia in part because she frequently feels stiff all over.10 She complains of strange tingling and prickling sensations in her hands and feet, especially when anxious.7,9,10 Her headaches also worsened and may be precipitated by bright light, as her pupils are usually dilated.5,7,9 Her hypertension is fairly controlled with medication.6,8-10 Ms. H says she experienced a psychotic episode when she was in her mid-teens,6,8 but reassures you that “she is not that bad now,” although she remains hypervigilant.13 Also while in her teens, Ms. H was treated with paroxetine and experienced restlessness, agitation, delirium, tachycardia, fluctuating blood pressure, diaphoresis, diarrhea, and neuromuscular excitation, which prompted discontinuation of the antidepressant.5-7,9,10
Impression. Ms. H exhibits symptoms associated with serotonin hyperactivity. Discontinuing and avoiding selective serotonin reuptake inhibitors (SSRIs) would be prudent; prescribing an anticonvulsant would be reasonable. Using a GABAergic medication to suppress serotonin (eg, baclofen) is likely to help. Avoiding dopaminergic medications is a must. Antidepressive antipsychotics would be logical to use. The use of serotonin-suppressing medications may be considered. One may argue for the use of beta-blockers in such a patient.
Continue to: Serotonin deficiency
Serotonin deficiency (Table 114,19-29)
Mr. A is chronically depressed, hopeless,19 and easily angered.21 He does not believe anyone can help him.19 You are concerned for his safety because he had attempted to end his life by shooting himself in the chest.19,21,22,25 Even when he’s not particularly depressed, Mr. A does not enjoy much of anything.21,26,27 He becomes particularly agitated when he drinks alcohol,25 which unfortunately is common for him.29 He engages in binge eating to feel better; he knows this is not healthy but he cannot control his behavior.20,29 Mr. A is poorly compliant with his medications, even with a blood thinner, which he was prescribed due to an episode of deep vein thrombosis. He complains of chronic daily headaches and episodic migraines.23,24 He rarely blinks,23,28 his skin is dry and cool, his hair is brittle,23 his mouth is dry,14,23,27 and he constantly licks his chapped lips.14,26,27 Mr. A frequently has general body pain26,31 but is dismissive of his body aches and completely stops reporting pain when his depression gets particularly severe. When depressed, he is slow in movement and thinking.14,21,26,27 He is more concerned with anxiety than depression.21 Mr. A is plagued by constipation, abdominal pain, muscle tension, and episodes of shaking.14,26,27 He also frequently complains about chronic tinnitus.28
Impression. Mr. A shows symptoms associated with serotonin hypoactivity. SSRIs and any other antidepressants with serotonin activity would be an obvious choice for treatment. A mood-stabilizing antipsychotic with serotonin activity would be welcome in treatment. Thyroid hormone supplementation may be of value, especially if thyroid stimulating hormone level is high. Light therapy, a diet with food that contains tryptophan, psychotherapy, and exercise are desirable. Avoiding benzodiazepines would be a good idea.
Dopamine excess (Table 214,30-41)
Ms. L presents with complaints of “fibromyalgia” and “daily headaches,”14 and also dissociation (finding herself in places when she does not know how she got there) and “out-of-body experiences.”32 She is odd, and states that people do not understand her and that she is “different.”38 Her friend, who is present at the appointment, elaborates on Ms. L’s bizarreness and oddness in behavior, out-of-context emotions, suspiciousness, paranoia, and possible hallucinations.35,36,38 Ms. L discloses frequent diffuse body pains, headaches, nausea, excessive salivation, and tongue burning, as well as muscle twitching.14 Sex worsens her headaches and body pain. She reports seizures that are not registered on EEG. In the office, she is suspicious, exhibits odd posturing, tends to misinterpret your words, and makes you feel uncomfortable. Anxiety38 and multiple obsessive-compulsive symptoms, especially excessive cleaning and grooming, complicate Ms. L’s life.31,32,34 On examination, she is hypertensive, and she has scars caused by self-cutting and skin picking on her arms.30-32 An electrocardiogram shows an elevated heart rate, widened QRS complex, and ectopic heartbeats.14 Ms. L has experienced trichotillomania since adolescence32-34 and her fingernails are bitten to the skin.34 She has difficulty with impulse control, and thrill-seeking is a prominent part of her life, mainly via gambling, compulsive sex, and compulsive buying.35,36 She also says she experiences indigestion and delayed gastric emptying.37
Impression. Ms. L exhibits multiple symptoms associated with dopamine excess. Dopamine antagonists should be considered and may help not only with her psychiatric symptoms but also with her pain symptoms. Bupropion (as a dopamine agonist), caffeine, and stimulants should be avoided.
Excessive dopamine is, in extreme cases, associated with somatic psychosis, somatic symptom disorder, factitious disorder, pain disorder, and hypochondria.39 It may come with odd and bizarre/peculiar symptoms out of proportion with objectively identified pathology. These symptoms are common in chronic pain and headache patients, and need to be addressed by appropriate use of dopamine antagonizing medications.39
Continue to: Dopamine deficiency
Dopamine deficiency (Table 24,14,20,38,40-43)
Mr. W experiences widespread pain, including chronic back pain, headaches, and abdominal pain. He also has substantial anhedonia, lack of interest, procrastination, and hypersomnia.41,42 He is apathetic and has difficulty getting up in the morning.41,42 Unusual tiredness and weakness drive him to overuse caffeine; he states that 5 Mountain Dews and 4 cups of regular coffee a day make his headaches bearable.38,41-43 Sex also improves his headaches. Since childhood, he has taken stimulants for attention-deficit/hyperactivity disorder. He reports that occasional use of cocaine helps ease his pain and depression. Mr. W’s wife is concerned with her husband’s low sexual drive and alcohol consumption, and discloses that he has periodic trouble with gambling. Mr. W was forced into psychotherapy but never was able to work productively with his therapist.38,41-43 He loves eating and cannot control his weight.40 This contrasts with episodic anorexia he experienced when he was younger.20 His face is usually emotionless.43 Mr. W is prone to constipation.14 His restless leg syndrome and periodic limb movement disorder are so bad that his wife refuses to share a bed with him.14 He is clumsy and has a problem with repetitive motor tasks.43 A paucity of speech, limited eye contact, poor grooming, and difficulty forming therapeutic alliances have long been part of Mr. W’s history.38,42,43 On physical examination, he has a dry mouth; he is stiff, tremulous, and hypotensive.14
Impression. Mr. W shows multiple symptoms associated with dopamine deficiency. Bupropion may be reasonable to consider. Dopamine augmentation via the use of stimulants is warranted in such patients, especially if stimulants had not been tried before (lisdexamfetamine would be a good choice to minimize addictive potential). For a patient with dopamine deficiency, levodopa may improve more than just restless legs. Amantadine may improve dopaminergic signaling through the accelerated dopamine release and decrease in presynaptic uptake, so this medication may be carefully tried.44 Pain treatment would not be successful for Mr. W without simultaneous treatment for his substance use.
Bottom Line
Both high and low levels of serotonin and dopamine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: How to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Amantadine • Gocovri
Baclofen • Ozobax
Bupropion • Wellbutrin
Fluoxetine • Prozac
Lisdexamfetamine • Vyvanse
Paroxetine • Paxil
It is unfortunate that, in some clinical areas, medical conditions are still treated by name and not based on the underlying pathological process. It would be odd in 2022 to treat “dropsy” instead of heart or kidney disease (2 very different causes of edema). Similarly, if the FDA had been approving drugs 150 years ago, we would have medications on label for “dementia praecox,” not schizophrenia or Alzheimer disease. With the help of DSM-5, psychiatry still resides in the descriptive symptomatic world of disorders.
In the United States, thanks to Freud, psychiatric symptoms became separated from medical symptoms, which made it more difficult to associate psychiatric manifestations with the underlying pathophysiology. Though the physical manifestations that parallel emotional symptoms—such as the dry mouth of anxiety, the tremor and leg weakness of fear, the constipation and blurry vision of depression, the breathing difficulty of anger, the abdominal pain of stress, the blushing of shyness, the palpitations of flashbacks, and endless others—are well known, the present classification of psychiatric disorders is blind to it. Neurochemical causes of gastrointestinal spasm or muscle tension are better researched than underlying central neurochemistry, though the same neurotransmitters drive them.
Can the biochemistry of psychiatric symptoms be judged on the basis of peripheral symptoms? Can the mental manifestations be connected to biological causation, and vice versa? Would psychiatrists be better off selecting treatments by recognizing involved neurotransmitters instead of addressing descriptive “depression, anxiety, and psychosis”? Each of these clinical syndromes may be caused by entirely different underlying neuronal mechanisms. Such mechanisms could be suggested if medical symptoms (which are measurable and objective) would become part of the psychiatric diagnosis. Is treating the “cough” sufficient, or would recognition that tuberculosis caused the cough guide better treatment? Is it time to abandon descriptive conditions and replace them with a specific “mechanism-based” viewpoint?
Ample research has shown that serotonin, dopamine, norepinephrine, endorphins, glutamate, and gamma aminobutyric acid (GABA) are the neurotransmitters most responsible in the process of both psychiatric disorders and chronic pain. These neurotransmitters are involved in much more than emotions (including the feeling of pain). An abundance of medical symptom clusters point toward which neurotransmitter dysfunction may be leading in specific cases of distinct types of depression, psychosis, anxiety, or “chronic pain.” Even presently, there are medications available (both for FDA-approved indications and off-label) that can be used to regulate these neurotransmitters, allowing practitioners to target the possible biological underlining of psychiatric or pain pathology. Hopefully, in the not-so-distant future, there will be specific medications for serotonin, dopamine, and noradrenergic depression as well as for GABAergic anxiety, endorphin psychosis, noradrenergic insomnia, and similar conditions.
Numerous neurotransmitters may be connected to both depression and pain in all their forms. These include (but are not limited to) prostaglandins, bradykinins, substance P, potassium, magnesium, calcium, histamine, adenosine triphosphate, calcitonin gene-related peptide (CGRP), nitric oxide (NO), cholecystokinin 7 (CCK7), neurotrophic growth factor (NGF), neurotensin, acetylcholine (Ach), oxytocin, cannabinoids, and others. These have not been researched sufficiently to identify their clinical presentation of excessive or insufficient availability at the sites of neurotransmission. It is difficult to draw conclusions about what kind of clinical symptoms they may cause (outside of pain), and therefore, they are not addressed in this article.
Both high and low levels of certain neurotransmitters may be associated with psychiatric conditions and chronic pain. Too much is as bad as too little.1 This applies to both quantity of neurotransmitters as well as quality of the corresponding receptor activity. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation. Reading indirect signs of bodily functions is a basic clinical skill that should not be forgotten, even in the time of advanced technology.
A different way of viewing psychiatric disorders
In this article, we present 4 hypothetical clinical cases to emphasize a possible way of analyzing symptoms to identify underlying pathology and guide more effective treatment. In no way do these descriptions reflect the entire set of symptoms caused by neurotransmitters; we created them based on what is presently known or suspected, and extensive research is required to confirm or disprove what we describe here.
Continue to: There are no well-recognized...
There are no well-recognized, well-established, reliable, or validated syndromes described in our work. Our goal is to suggest an alternative way of looking at psychiatric disorders by viewing syndromal presentation through the lens of specific neurotransmitters. The collection of symptoms associated with various neurotransmitters as presented in this hypothesis is not complete. We have assembled what is described in the literature as a suggestion for specific future research. We simplified these clinical presentations by omitting scenarios in which a specific neurotransmitter increases in one area but not another. For example, all the symptoms of dopamine excess we describe would not have to occur concurrently in the same patient, but they may develop in certain patients depending on which dopaminergic pathway is exhibiting excess activity. Such distinctions may be established only by exhaustive research not yet conducted.
Our proposal may seem radical, but it truly is not. For example, if we know that dopamine excess may cause seizures, psychosis, and blood pressure elevation, why not consider dopamine excess as an underlying cause in a patient with depression who exhibits these symptoms simultaneously? And why not call it “dopamine excess syndrome”? We already have “serotonin syndrome” for a patient experiencing a serotonin storm. However, using the same logic, it should be called “serotonin excess syndrome.” And if we know of “serotonin excess syndrome,” why not consider “serotonin deficiency syndrome”?

In Part 1 of this article, we discuss serotonin and dopamine. Table 1 outlines medical and psychiatric symptoms that likely reflect serotonin excess2-18 and deficiency,14,19-29 and Table 2 lists symptoms that likely reflect dopamine excess14,30-41 and deficiency.4,14,20,38,40-43 In Part 2 we will touch on endorphins and norepinephrine, and in Part 3 we will conclude by looking at GABA and glutamate.

Serotonin excess (Table 12-18)
On a recent office visit, Ms. H reports that most of the time she does not feel much of anything, but she still experiences panic attacks8,9,13,15 and is easily agitated.6,8 Her mother died recently, and Ms. H is concerned that she did not grieve.15-18 She failed her last semester in college and was indifferent to her failure.18 She sleeps poorly,8 is failing her creative classes, and wonders why she has lost her artistic inclination.16-18 Ms. H has difficulty with amotivation, planning, social interactions, and speech.16,17 All of those symptoms worsened after she was prescribed fluoxetine approximately 1 year ago for her “blues.” Ms. H is obese and continues to gain weight,2 though she frequently has diarrhea,3,4,7,8 loud peristalsis, and abdominal cramps.4,7,8 She sweats easily6-8 and her heart frequently races.8,9 Additionally, Ms. H’s primary care physician told her that she has “borderline diabetes.”2 She is prone to frequent bruising11 and is easy to shake, even when she is experiencing minimal anxiety.6-9 Ms. H had consulted with a neurologist because of unusual electrical “zapping” in her brain and muscle twitches.5,8,9,13 She had experienced a seizure as a child, but this was possibly related to hypernatremia,2 and she has not taken any anticonvulsant medication for several years.8 She exhibits hyperactive deep tendon reflexes and tremors5,7,9 and blinks frequently.6,9 She experiences hot flashes,3,6-8,14 does not tolerate heat, and prefers cooler weather.8,9 Her pains and aches,12,14 to which she has been prone all of her life, have recently become much worse, and she was diagnosed with fibromyalgia in part because she frequently feels stiff all over.10 She complains of strange tingling and prickling sensations in her hands and feet, especially when anxious.7,9,10 Her headaches also worsened and may be precipitated by bright light, as her pupils are usually dilated.5,7,9 Her hypertension is fairly controlled with medication.6,8-10 Ms. H says she experienced a psychotic episode when she was in her mid-teens,6,8 but reassures you that “she is not that bad now,” although she remains hypervigilant.13 Also while in her teens, Ms. H was treated with paroxetine and experienced restlessness, agitation, delirium, tachycardia, fluctuating blood pressure, diaphoresis, diarrhea, and neuromuscular excitation, which prompted discontinuation of the antidepressant.5-7,9,10
Impression. Ms. H exhibits symptoms associated with serotonin hyperactivity. Discontinuing and avoiding selective serotonin reuptake inhibitors (SSRIs) would be prudent; prescribing an anticonvulsant would be reasonable. Using a GABAergic medication to suppress serotonin (eg, baclofen) is likely to help. Avoiding dopaminergic medications is a must. Antidepressive antipsychotics would be logical to use. The use of serotonin-suppressing medications may be considered. One may argue for the use of beta-blockers in such a patient.
Continue to: Serotonin deficiency
Serotonin deficiency (Table 114,19-29)
Mr. A is chronically depressed, hopeless,19 and easily angered.21 He does not believe anyone can help him.19 You are concerned for his safety because he had attempted to end his life by shooting himself in the chest.19,21,22,25 Even when he’s not particularly depressed, Mr. A does not enjoy much of anything.21,26,27 He becomes particularly agitated when he drinks alcohol,25 which unfortunately is common for him.29 He engages in binge eating to feel better; he knows this is not healthy but he cannot control his behavior.20,29 Mr. A is poorly compliant with his medications, even with a blood thinner, which he was prescribed due to an episode of deep vein thrombosis. He complains of chronic daily headaches and episodic migraines.23,24 He rarely blinks,23,28 his skin is dry and cool, his hair is brittle,23 his mouth is dry,14,23,27 and he constantly licks his chapped lips.14,26,27 Mr. A frequently has general body pain26,31 but is dismissive of his body aches and completely stops reporting pain when his depression gets particularly severe. When depressed, he is slow in movement and thinking.14,21,26,27 He is more concerned with anxiety than depression.21 Mr. A is plagued by constipation, abdominal pain, muscle tension, and episodes of shaking.14,26,27 He also frequently complains about chronic tinnitus.28
Impression. Mr. A shows symptoms associated with serotonin hypoactivity. SSRIs and any other antidepressants with serotonin activity would be an obvious choice for treatment. A mood-stabilizing antipsychotic with serotonin activity would be welcome in treatment. Thyroid hormone supplementation may be of value, especially if thyroid stimulating hormone level is high. Light therapy, a diet with food that contains tryptophan, psychotherapy, and exercise are desirable. Avoiding benzodiazepines would be a good idea.
Dopamine excess (Table 214,30-41)
Ms. L presents with complaints of “fibromyalgia” and “daily headaches,”14 and also dissociation (finding herself in places when she does not know how she got there) and “out-of-body experiences.”32 She is odd, and states that people do not understand her and that she is “different.”38 Her friend, who is present at the appointment, elaborates on Ms. L’s bizarreness and oddness in behavior, out-of-context emotions, suspiciousness, paranoia, and possible hallucinations.35,36,38 Ms. L discloses frequent diffuse body pains, headaches, nausea, excessive salivation, and tongue burning, as well as muscle twitching.14 Sex worsens her headaches and body pain. She reports seizures that are not registered on EEG. In the office, she is suspicious, exhibits odd posturing, tends to misinterpret your words, and makes you feel uncomfortable. Anxiety38 and multiple obsessive-compulsive symptoms, especially excessive cleaning and grooming, complicate Ms. L’s life.31,32,34 On examination, she is hypertensive, and she has scars caused by self-cutting and skin picking on her arms.30-32 An electrocardiogram shows an elevated heart rate, widened QRS complex, and ectopic heartbeats.14 Ms. L has experienced trichotillomania since adolescence32-34 and her fingernails are bitten to the skin.34 She has difficulty with impulse control, and thrill-seeking is a prominent part of her life, mainly via gambling, compulsive sex, and compulsive buying.35,36 She also says she experiences indigestion and delayed gastric emptying.37
Impression. Ms. L exhibits multiple symptoms associated with dopamine excess. Dopamine antagonists should be considered and may help not only with her psychiatric symptoms but also with her pain symptoms. Bupropion (as a dopamine agonist), caffeine, and stimulants should be avoided.
Excessive dopamine is, in extreme cases, associated with somatic psychosis, somatic symptom disorder, factitious disorder, pain disorder, and hypochondria.39 It may come with odd and bizarre/peculiar symptoms out of proportion with objectively identified pathology. These symptoms are common in chronic pain and headache patients, and need to be addressed by appropriate use of dopamine antagonizing medications.39
Continue to: Dopamine deficiency
Dopamine deficiency (Table 24,14,20,38,40-43)
Mr. W experiences widespread pain, including chronic back pain, headaches, and abdominal pain. He also has substantial anhedonia, lack of interest, procrastination, and hypersomnia.41,42 He is apathetic and has difficulty getting up in the morning.41,42 Unusual tiredness and weakness drive him to overuse caffeine; he states that 5 Mountain Dews and 4 cups of regular coffee a day make his headaches bearable.38,41-43 Sex also improves his headaches. Since childhood, he has taken stimulants for attention-deficit/hyperactivity disorder. He reports that occasional use of cocaine helps ease his pain and depression. Mr. W’s wife is concerned with her husband’s low sexual drive and alcohol consumption, and discloses that he has periodic trouble with gambling. Mr. W was forced into psychotherapy but never was able to work productively with his therapist.38,41-43 He loves eating and cannot control his weight.40 This contrasts with episodic anorexia he experienced when he was younger.20 His face is usually emotionless.43 Mr. W is prone to constipation.14 His restless leg syndrome and periodic limb movement disorder are so bad that his wife refuses to share a bed with him.14 He is clumsy and has a problem with repetitive motor tasks.43 A paucity of speech, limited eye contact, poor grooming, and difficulty forming therapeutic alliances have long been part of Mr. W’s history.38,42,43 On physical examination, he has a dry mouth; he is stiff, tremulous, and hypotensive.14
Impression. Mr. W shows multiple symptoms associated with dopamine deficiency. Bupropion may be reasonable to consider. Dopamine augmentation via the use of stimulants is warranted in such patients, especially if stimulants had not been tried before (lisdexamfetamine would be a good choice to minimize addictive potential). For a patient with dopamine deficiency, levodopa may improve more than just restless legs. Amantadine may improve dopaminergic signaling through the accelerated dopamine release and decrease in presynaptic uptake, so this medication may be carefully tried.44 Pain treatment would not be successful for Mr. W without simultaneous treatment for his substance use.
Bottom Line
Both high and low levels of serotonin and dopamine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: How to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Amantadine • Gocovri
Baclofen • Ozobax
Bupropion • Wellbutrin
Fluoxetine • Prozac
Lisdexamfetamine • Vyvanse
Paroxetine • Paxil
1. Stahl SM. Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors. CNS Spectr. 2017;22(4):305-311.
2. Young RL, Lumsden AL, Martin AM, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42(11):1880-1889.
3. Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79-S85.
4. Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319-342.
5. Prakash S, Belani P, Trivedi A. Headache as a presenting feature in patients with serotonin syndrome: a case series. Cephalalgia. 2014;34(2):148-153.
6. van Ewijk CE, Jacobs GE, Girbes ARJ. Unsuspected serotonin toxicity in the ICU. Ann Intensive Care. 2016;6(1):85.
7. Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care. 2014;21(1):108-113.
8. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
9. Ansari H, Kouti L. Drug interaction and serotonin toxicity with opioid use: another reason to avoid opioids in headache and migraine treatment. Curr Pain Headache Rep. 2016;20(8):50.
10. Ott M, Mannchen JK, Jamshidi F, et al. Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol. 2019;9:2045125318818814. doi:10.1177/2045125318818814
11. Cerrito F, Lazzaro MP, Gaudio E, et al. 5HT2-receptors and serotonin release: their role in human platelet aggregation. Life Sci. 1993;53(3):209-215.
12. Ohayon MM. Pain sensitivity, depression, and sleep deprivation: links with serotoninergic dysfunction. J Psychiatr Res. 2009;43(16):1243-1245.
13. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
14. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:120,199,201-204,730-740.
15. Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11(2):181-186.
16. George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette’s syndrome and obsessive compulsive disorder. J Clin Psychiatry. 1992;53(10):379-380.
17. Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52(3):131-133.
18. Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10(5):343-345.
19. Samuelsson M, Jokinen J, Nordström AL, et al. CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatr Scand. 2006;113(1):44-47.
20. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
21. Mann JJ, Oquendo M, Underwood MD, et al. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60 Suppl 2:7-116.
22. Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2):162-171.
23. Joseph R, Welch KM, D’Andrea G. Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia. 1989;9(4):293-299.
24. Pakalnis A, Splaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49(10):1486-1492.
25. Virkkunen M, Goldman D, Nielsen DA, et al. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271-275.
26. Liu Y, Zhao J, Fan X, et al. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front Psychiatry. 2019;10:286.
27. Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179-1187.
28. O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999;48(12):980-990.
29. Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42(2):147-151.
30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
31. Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33(1):1-2.
32. Tse W, Hälbig TD. Skin picking in Parkinson’s disease: a behavioral side-effect of dopaminergic treatment? Psychiatry Clin Neurosci. 2010;64(2):214.
33. Ayaydın H. Probable emergence of symptoms of trichotillomania by atomoxetine: a case report. Psychiatry and Clinical Psychopharmacology. 2019;29(2)220-222.
34. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702. doi:10.1155/2016/9782702
35. Clark CA, Dagher A. The role of dopamine in risk taking: a specific look at Parkinson’s disease and gambling. Front Behav Neurosci. 2014;8:196.
36. Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79-93.
37. Chen TS, Chang FY. Elevated serum dopamine increases while coffee consumption decreases the occurrence of reddish streaks in the intact stomach. J Gastroenterol Hepatol. 2013;28(12):1810-1814.
38. Wong-Riley MT. Neuroscience Secrets. 1st edition. Spanish version. Hanley & Belfus; 1999:420-429.
39. Arbuck DM. Antipsychotics, dopamine, and pain. Current Psychiatry. 2020;19(1):25-29,31.
40. Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25-33.
41. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46.
42. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506.
43. Gepshtein S, Li X, Snider J, et al. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26(3):645-657.
44. Scarff JR. The ABCDs of treating tardive dyskinesia. Current Psychiatry. 2020;19(4):21,55.
1. Stahl SM. Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors. CNS Spectr. 2017;22(4):305-311.
2. Young RL, Lumsden AL, Martin AM, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42(11):1880-1889.
3. Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79-S85.
4. Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319-342.
5. Prakash S, Belani P, Trivedi A. Headache as a presenting feature in patients with serotonin syndrome: a case series. Cephalalgia. 2014;34(2):148-153.
6. van Ewijk CE, Jacobs GE, Girbes ARJ. Unsuspected serotonin toxicity in the ICU. Ann Intensive Care. 2016;6(1):85.
7. Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care. 2014;21(1):108-113.
8. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
9. Ansari H, Kouti L. Drug interaction and serotonin toxicity with opioid use: another reason to avoid opioids in headache and migraine treatment. Curr Pain Headache Rep. 2016;20(8):50.
10. Ott M, Mannchen JK, Jamshidi F, et al. Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol. 2019;9:2045125318818814. doi:10.1177/2045125318818814
11. Cerrito F, Lazzaro MP, Gaudio E, et al. 5HT2-receptors and serotonin release: their role in human platelet aggregation. Life Sci. 1993;53(3):209-215.
12. Ohayon MM. Pain sensitivity, depression, and sleep deprivation: links with serotoninergic dysfunction. J Psychiatr Res. 2009;43(16):1243-1245.
13. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
14. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:120,199,201-204,730-740.
15. Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11(2):181-186.
16. George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette’s syndrome and obsessive compulsive disorder. J Clin Psychiatry. 1992;53(10):379-380.
17. Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52(3):131-133.
18. Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10(5):343-345.
19. Samuelsson M, Jokinen J, Nordström AL, et al. CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatr Scand. 2006;113(1):44-47.
20. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
21. Mann JJ, Oquendo M, Underwood MD, et al. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60 Suppl 2:7-116.
22. Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2):162-171.
23. Joseph R, Welch KM, D’Andrea G. Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia. 1989;9(4):293-299.
24. Pakalnis A, Splaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49(10):1486-1492.
25. Virkkunen M, Goldman D, Nielsen DA, et al. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271-275.
26. Liu Y, Zhao J, Fan X, et al. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front Psychiatry. 2019;10:286.
27. Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179-1187.
28. O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999;48(12):980-990.
29. Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42(2):147-151.
30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
31. Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33(1):1-2.
32. Tse W, Hälbig TD. Skin picking in Parkinson’s disease: a behavioral side-effect of dopaminergic treatment? Psychiatry Clin Neurosci. 2010;64(2):214.
33. Ayaydın H. Probable emergence of symptoms of trichotillomania by atomoxetine: a case report. Psychiatry and Clinical Psychopharmacology. 2019;29(2)220-222.
34. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702. doi:10.1155/2016/9782702
35. Clark CA, Dagher A. The role of dopamine in risk taking: a specific look at Parkinson’s disease and gambling. Front Behav Neurosci. 2014;8:196.
36. Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79-93.
37. Chen TS, Chang FY. Elevated serum dopamine increases while coffee consumption decreases the occurrence of reddish streaks in the intact stomach. J Gastroenterol Hepatol. 2013;28(12):1810-1814.
38. Wong-Riley MT. Neuroscience Secrets. 1st edition. Spanish version. Hanley & Belfus; 1999:420-429.
39. Arbuck DM. Antipsychotics, dopamine, and pain. Current Psychiatry. 2020;19(1):25-29,31.
40. Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25-33.
41. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46.
42. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506.
43. Gepshtein S, Li X, Snider J, et al. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26(3):645-657.
44. Scarff JR. The ABCDs of treating tardive dyskinesia. Current Psychiatry. 2020;19(4):21,55.
30 years of fake nursing ends with 7-year prison sentence
A Canadian woman who officials allege faked being a registered nurse for some 30 years in Canada and the United States is scheduled to appear in court next month after being sentenced to 7 years in prison.
She was previously sentenced April 22 in an Ontario court after she pled guilty in January to seven offenses, including impersonation, assault with a weapon, and assault, according to CBC Radio-Canada.
Ms. Cleroux, who uses several aliases, had a long history of deception in three provinces in Canada, as well as in Colorado and Florida. The sentencing in Ontario stemmed from incidents at a medical and dental clinic in Ottawa last year, which included administration of medications to patients through needle injections, Ottawa Police reported in a press statement obtained by this news organization.
Authorities charged Ms. Cleroux in September with assault with a weapon and criminal negligence causing bodily harm, along with “personation to gain advantage,” obtaining by false pretense, and using a forged document, this news organization reported.
Ms. Cleroux has been in custody since her arrest by Ottawa Police in August.
The Vancouver Police Department (VPD) charged Ms. Cleroux last year with fraud of over $5,000 and personation with intent. VPD investigated claims that an employee at BC Women’s Hospital fraudulently identified herself as a nurse while working there between June 2020 and June 2021, according to a VPD press release.
Nursing colleges in British Columbia and Ontario issued warnings that she had used aliases and purported to be a registered nurse to gain employment. The aliases included Melanie Thompson, Melanie Smith, and Melanie Cleroux.
Ms. Cleroux was believed to be a student in a nursing school in Colorado, but she only completed 2 years of a 4-year nursing course and was never certified as a nurse, according to CBC. Her criminal record dates back 30 years and includes 67 adult convictions and other convictions in her youth, CBC reported.
A version of this article first appeared on Medscape.com.
A Canadian woman who officials allege faked being a registered nurse for some 30 years in Canada and the United States is scheduled to appear in court next month after being sentenced to 7 years in prison.
She was previously sentenced April 22 in an Ontario court after she pled guilty in January to seven offenses, including impersonation, assault with a weapon, and assault, according to CBC Radio-Canada.
Ms. Cleroux, who uses several aliases, had a long history of deception in three provinces in Canada, as well as in Colorado and Florida. The sentencing in Ontario stemmed from incidents at a medical and dental clinic in Ottawa last year, which included administration of medications to patients through needle injections, Ottawa Police reported in a press statement obtained by this news organization.
Authorities charged Ms. Cleroux in September with assault with a weapon and criminal negligence causing bodily harm, along with “personation to gain advantage,” obtaining by false pretense, and using a forged document, this news organization reported.
Ms. Cleroux has been in custody since her arrest by Ottawa Police in August.
The Vancouver Police Department (VPD) charged Ms. Cleroux last year with fraud of over $5,000 and personation with intent. VPD investigated claims that an employee at BC Women’s Hospital fraudulently identified herself as a nurse while working there between June 2020 and June 2021, according to a VPD press release.
Nursing colleges in British Columbia and Ontario issued warnings that she had used aliases and purported to be a registered nurse to gain employment. The aliases included Melanie Thompson, Melanie Smith, and Melanie Cleroux.
Ms. Cleroux was believed to be a student in a nursing school in Colorado, but she only completed 2 years of a 4-year nursing course and was never certified as a nurse, according to CBC. Her criminal record dates back 30 years and includes 67 adult convictions and other convictions in her youth, CBC reported.
A version of this article first appeared on Medscape.com.
A Canadian woman who officials allege faked being a registered nurse for some 30 years in Canada and the United States is scheduled to appear in court next month after being sentenced to 7 years in prison.
She was previously sentenced April 22 in an Ontario court after she pled guilty in January to seven offenses, including impersonation, assault with a weapon, and assault, according to CBC Radio-Canada.
Ms. Cleroux, who uses several aliases, had a long history of deception in three provinces in Canada, as well as in Colorado and Florida. The sentencing in Ontario stemmed from incidents at a medical and dental clinic in Ottawa last year, which included administration of medications to patients through needle injections, Ottawa Police reported in a press statement obtained by this news organization.
Authorities charged Ms. Cleroux in September with assault with a weapon and criminal negligence causing bodily harm, along with “personation to gain advantage,” obtaining by false pretense, and using a forged document, this news organization reported.
Ms. Cleroux has been in custody since her arrest by Ottawa Police in August.
The Vancouver Police Department (VPD) charged Ms. Cleroux last year with fraud of over $5,000 and personation with intent. VPD investigated claims that an employee at BC Women’s Hospital fraudulently identified herself as a nurse while working there between June 2020 and June 2021, according to a VPD press release.
Nursing colleges in British Columbia and Ontario issued warnings that she had used aliases and purported to be a registered nurse to gain employment. The aliases included Melanie Thompson, Melanie Smith, and Melanie Cleroux.
Ms. Cleroux was believed to be a student in a nursing school in Colorado, but she only completed 2 years of a 4-year nursing course and was never certified as a nurse, according to CBC. Her criminal record dates back 30 years and includes 67 adult convictions and other convictions in her youth, CBC reported.
A version of this article first appeared on Medscape.com.
Hospital readmission remains common for teens with nonfatal drug overdose
Approximately 1 in 5 adolescents hospitalized for nonfatal drug overdoses were readmitted within 6 months, based on data from more than 12,000 individuals.
Previous studies suggest that many adolescents fail to receive timely treatment for addiction after a nonfatal overdose, but the rates of hospital readmission in this population have not been examined, according to Julie Gaither, PhD, of Yale University, New Haven, Conn.
In a study presented at the annual meeting of the Pediatric Academic Societies, Dr. Gaither and her colleague, John M. Leventhal, MD, also of Yale University, used data from the 2016 Nationwide Readmissions Database to examine incidence and recurrent hospitalizations for nonfatal drug overdoses in adolescents. The study population included 12,952 patients aged 11-21 years who were admitted to a hospital after a nonfatal drug overdose in 2016. Of these, 15% were younger than 15 years, and 52.1% were females.
Overall, 76.2% of the overdoses involved opioids; 77.9% involved a prescription opioid, 15.3% involved heroin, and 7.9% involved fentanyl.
Across all drug overdoses, the majority (86.5%) were attributed to accidental intent and 11.8% were attributed to self-harm. Notably, females were nearly four times more likely than males to attempt suicide (odds ratio, 3.57). After the initial hospitalization, 79.3% of the patients were discharged home, and 11.5% went to a short-term care facility.
The 6-month hospital readmission rate was 21.4%. Of the patients readmitted for any cause, 18.2% of readmissions were for recurrent overdoses, and 92.1% of these were attributed to opioids.
The median cost of the initial hospital admission was $23,705 (ranging from $11,902 to $54,682) and the median cost of the first readmission was $25,416 (ranging from $13,905 to $48,810). In 42.1% of all hospitalizations, Medicaid was the primary payer.
The study findings were limited by the relatively high number of Medicaid patients, which may limit generalizability, but is strengthened by the large sample size.
The findings highlight not only the need for prevention efforts to limit opioid use among adolescents, but also “speak to the need for timely evidenced-based addiction treatment and appropriate follow-up care for teens following hospitalization for a nonfatal drug overdose,” the researchers wrote in their abstract.
Potential for postpandemic surge in drug use
Interestingly, some recent research has shown a decline in teens’ substance use during the pandemic, Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“However, as the world begins ‘opening up’ again, I suspect rates of drug use will rise – especially with the significant burden of mental health issues adolescents have struggled with during the last few years,” said Dr. Curran, who was not involved with the current study.
“Sadly, I am not surprised by this study’s findings. Too often, teens with substance abuse issues are not connected to effective, evidenced-based treatment, and for those who are, the wait list can be long,” she said.
“Teens who are misusing drugs – either to get high or to attempt suicide – who are admitted for nonfatal overdose have a high rate of readmission for recurrent drug overdose,” Dr. Curran said. “This high rate of readmission has serious social and financial implications,” she added. “This study is part of a growing body of literature that supports the importance of getting adolescents into effective, evidence-based substance abuse treatment, such as medication-assisted treatment in opioid abuse. However, we also should be advocating for improved funding for and access to these treatments for all individuals.”
The study received no outside funding. Dr. Gaither had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Approximately 1 in 5 adolescents hospitalized for nonfatal drug overdoses were readmitted within 6 months, based on data from more than 12,000 individuals.
Previous studies suggest that many adolescents fail to receive timely treatment for addiction after a nonfatal overdose, but the rates of hospital readmission in this population have not been examined, according to Julie Gaither, PhD, of Yale University, New Haven, Conn.
In a study presented at the annual meeting of the Pediatric Academic Societies, Dr. Gaither and her colleague, John M. Leventhal, MD, also of Yale University, used data from the 2016 Nationwide Readmissions Database to examine incidence and recurrent hospitalizations for nonfatal drug overdoses in adolescents. The study population included 12,952 patients aged 11-21 years who were admitted to a hospital after a nonfatal drug overdose in 2016. Of these, 15% were younger than 15 years, and 52.1% were females.
Overall, 76.2% of the overdoses involved opioids; 77.9% involved a prescription opioid, 15.3% involved heroin, and 7.9% involved fentanyl.
Across all drug overdoses, the majority (86.5%) were attributed to accidental intent and 11.8% were attributed to self-harm. Notably, females were nearly four times more likely than males to attempt suicide (odds ratio, 3.57). After the initial hospitalization, 79.3% of the patients were discharged home, and 11.5% went to a short-term care facility.
The 6-month hospital readmission rate was 21.4%. Of the patients readmitted for any cause, 18.2% of readmissions were for recurrent overdoses, and 92.1% of these were attributed to opioids.
The median cost of the initial hospital admission was $23,705 (ranging from $11,902 to $54,682) and the median cost of the first readmission was $25,416 (ranging from $13,905 to $48,810). In 42.1% of all hospitalizations, Medicaid was the primary payer.
The study findings were limited by the relatively high number of Medicaid patients, which may limit generalizability, but is strengthened by the large sample size.
The findings highlight not only the need for prevention efforts to limit opioid use among adolescents, but also “speak to the need for timely evidenced-based addiction treatment and appropriate follow-up care for teens following hospitalization for a nonfatal drug overdose,” the researchers wrote in their abstract.
Potential for postpandemic surge in drug use
Interestingly, some recent research has shown a decline in teens’ substance use during the pandemic, Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“However, as the world begins ‘opening up’ again, I suspect rates of drug use will rise – especially with the significant burden of mental health issues adolescents have struggled with during the last few years,” said Dr. Curran, who was not involved with the current study.
“Sadly, I am not surprised by this study’s findings. Too often, teens with substance abuse issues are not connected to effective, evidenced-based treatment, and for those who are, the wait list can be long,” she said.
“Teens who are misusing drugs – either to get high or to attempt suicide – who are admitted for nonfatal overdose have a high rate of readmission for recurrent drug overdose,” Dr. Curran said. “This high rate of readmission has serious social and financial implications,” she added. “This study is part of a growing body of literature that supports the importance of getting adolescents into effective, evidence-based substance abuse treatment, such as medication-assisted treatment in opioid abuse. However, we also should be advocating for improved funding for and access to these treatments for all individuals.”
The study received no outside funding. Dr. Gaither had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Approximately 1 in 5 adolescents hospitalized for nonfatal drug overdoses were readmitted within 6 months, based on data from more than 12,000 individuals.
Previous studies suggest that many adolescents fail to receive timely treatment for addiction after a nonfatal overdose, but the rates of hospital readmission in this population have not been examined, according to Julie Gaither, PhD, of Yale University, New Haven, Conn.
In a study presented at the annual meeting of the Pediatric Academic Societies, Dr. Gaither and her colleague, John M. Leventhal, MD, also of Yale University, used data from the 2016 Nationwide Readmissions Database to examine incidence and recurrent hospitalizations for nonfatal drug overdoses in adolescents. The study population included 12,952 patients aged 11-21 years who were admitted to a hospital after a nonfatal drug overdose in 2016. Of these, 15% were younger than 15 years, and 52.1% were females.
Overall, 76.2% of the overdoses involved opioids; 77.9% involved a prescription opioid, 15.3% involved heroin, and 7.9% involved fentanyl.
Across all drug overdoses, the majority (86.5%) were attributed to accidental intent and 11.8% were attributed to self-harm. Notably, females were nearly four times more likely than males to attempt suicide (odds ratio, 3.57). After the initial hospitalization, 79.3% of the patients were discharged home, and 11.5% went to a short-term care facility.
The 6-month hospital readmission rate was 21.4%. Of the patients readmitted for any cause, 18.2% of readmissions were for recurrent overdoses, and 92.1% of these were attributed to opioids.
The median cost of the initial hospital admission was $23,705 (ranging from $11,902 to $54,682) and the median cost of the first readmission was $25,416 (ranging from $13,905 to $48,810). In 42.1% of all hospitalizations, Medicaid was the primary payer.
The study findings were limited by the relatively high number of Medicaid patients, which may limit generalizability, but is strengthened by the large sample size.
The findings highlight not only the need for prevention efforts to limit opioid use among adolescents, but also “speak to the need for timely evidenced-based addiction treatment and appropriate follow-up care for teens following hospitalization for a nonfatal drug overdose,” the researchers wrote in their abstract.
Potential for postpandemic surge in drug use
Interestingly, some recent research has shown a decline in teens’ substance use during the pandemic, Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“However, as the world begins ‘opening up’ again, I suspect rates of drug use will rise – especially with the significant burden of mental health issues adolescents have struggled with during the last few years,” said Dr. Curran, who was not involved with the current study.
“Sadly, I am not surprised by this study’s findings. Too often, teens with substance abuse issues are not connected to effective, evidenced-based treatment, and for those who are, the wait list can be long,” she said.
“Teens who are misusing drugs – either to get high or to attempt suicide – who are admitted for nonfatal overdose have a high rate of readmission for recurrent drug overdose,” Dr. Curran said. “This high rate of readmission has serious social and financial implications,” she added. “This study is part of a growing body of literature that supports the importance of getting adolescents into effective, evidence-based substance abuse treatment, such as medication-assisted treatment in opioid abuse. However, we also should be advocating for improved funding for and access to these treatments for all individuals.”
The study received no outside funding. Dr. Gaither had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM PAS 2022
Nap length linked to cognitive changes
No wonder we feel worse after naps
Some of us have hectic schedules that may make a nap feel more necessary. It’s common knowledge that naps shouldn’t be too long – maybe 20 minutes or so – but if you frequently take 3-hour naps and wake up thinking you’re late for school even though you’re 47 and have your PhD, this LOTME is for you.
Studies have shown that there is a link between napping during the day and Alzheimer’s/cognitive decline, but now we’ve got a double whammy for you: Longer and more frequent napping is linked to worse cognition after a year, and in turn, those with cognitive decline and Alzheimer’s are known to nap longer and more frequently during the day.
“We now know that the pathology related to cognitive decline can cause other changes in function,” he said. “It’s really a multisystem disorder, also including difficulty sleeping, changes in movement, changes in body composition, depression symptoms, behavioral changes, etc.,” coauthor Aron Buchman, MD, said in a statement from Rush University Medical Center.
The investigators monitored 1,400 patients over the course of 14 years with wrist bracelets that recorded when a person was not active during the day and considered that a nap.
At the beginning of the study, 75% of the study subjects had no cognitive impairment, 19.5% had some cognitive impairment, and approximately 4% had Alzheimer’s. Napping during the day only increased about 11 minutes a year for those with no signs of cognitive impairment, but those who showed significantly more signs of cognitive decline doubled their nap time and those actually diagnosed with Alzheimer’s tripled theirs.
The investigators did not imply that napping causes Alzheimer’s, but they noted that people who are older and nap more than an hour a day are 40% more likely to be at risk. It is something to consider and monitor.
Sometimes, after all, a nap seems like the best idea ever, but more often than not we wake up feeling 10 times worse. Our bodies may be giving us a heads up.
Pokemon Go away depression
The summer of 2016 was a great time if you happened to be a fan of Pokemon. Which is quite a lot of people. For almost 20 years millions have enjoyed the games and animated series, but Pokemon Go brought the thrill of catching Pokemon to life in a whole new way. For the first time, you could go out into the world and pretend you were a real Pokemon trainer, and everywhere you went, there would be others like you.
The ability to chase after Pikachu and Charizard in real life (well, augmented reality, but close enough) seemed to bring people a lot of joy, but seemed is never good enough for science. Can’t have anecdotes, we need data! So researchers at the London School of Economics and Political Science conducted a study into how Pokemon Go affected local Internet search rates of depression as the game was released slowly around the world.
Through analyzing Google Trend data of words like “depression,” “anxiety,” and “stress,” the researchers found that the release of Pokemon Go was significantly associated with a noticeable, though short-term, drop in depression-related Internet searches. Location-based augmented reality games may alleviate symptoms of mild depression, the researchers said, as they encourage physical activity, face-to-face socialization, and exposure to nature, though they added that simply going outside is likely not enough to combat clinical cases of severe depression.
Still, augmented reality games represent a viable target for public health investment, since they’re easy to use and inexpensive to make. That said, we’re not sure we want the FDA or CDC making a new Pokemon Go game. They’d probably end up filling the streets with Mr. Mime. And no one would leave their house for that.
And now a word from our sponsor
How many times has this happened to you? You need to repair a jet engine, inspect a nuclear reactor cooling system, AND perform bowel surgery, but you can’t carry around all the heavy, old-fashioned tools needed for those jobs.
Well, we’ve got one tool that can do it all! And that tool is a snake. No, it’s a robot.
It’s both! It’s the COntinuum roBot for Remote Applications. COBRA is the robot that looks like a snake! A snake that’s 5 meters long but only as thick as a pencil (about 9 mm in diameter). A robot with “extraordinary manoeuvrability and responsiveness due to … a compliant-joint structure and multiple continuous sections that enable it to bend at around 90 degrees,” according to the team at the University of Nottingham (England) that developed it.
COBRA comes equipped with a stereovision camera and a miniature cutting tool to perform complex industrial repair, but other devices can be interchanged for possible medical use.
COBRA and its joystick-like controller were designed to be easy to use. Dr. Oladejo Olaleye, the ear, nose, and throat and robotic surgeon at University Hospitals of Leicester who is directing its surgical development, was able to use COBRA on a dummy after just 5 minutes of training. He called it “the future of diagnostic endoscopy and therapeutic surgery.”
Don’t be the last aircraft engineer/nuclear technician/surgeon on your block to have this ultraslender, ultramaneuverable reptilian repair robot. Get your COBRA now! Operators are standing by.
Disclaimer: Robot is still under development and not yet on sale.
Rule, (worm) Britannia!
As long as there have been people, there have been parasitic worms living in their guts. Helminth infection is a continuing and largely ignored crisis in poor, tropical nations, though worm-based diseases have been basically eliminated from wealthier countries.
This wasn’t always the case, however, as a study published in PLOS Neglected Tropical Diseases (now there’s a specific topic) has found. The researchers detail the glorious history of helminth infestation in the United Kingdom from the Victorian era all the way back to prehistory, scouring hundreds of skeletons found in 17 sites across the country for eggs, which can remain intact for thousands of years.
The researchers found that two eras in particular had very high rates of infection. Unsurprisingly, the late medieval era was one of them, but the other is less obvious. The Romans were famous for their hygiene, their baths, and their plumbing, but maybe they also should be famous for the abundance of worms in their bellies. That doesn’t make sense at first: Shouldn’t good hygiene lower infection? The benefits of a good sewer system, however, are lessened when the waste containing said infectious organisms is used to fertilize crops. Recycling is generally a good thing, but less so when you’re recycling parasitic worms.
Curiously, of the three sites from the industrial age, only the one in London had high levels of worm infestation. Considering how dirty and cramped 19th-century British cities were, one might expect disease to run rampant (tuberculosis certainly did), but the sites in Oxford and Birmingham were almost devoid of worms. The researchers theorized that this was because of access to clean well water. Or maybe worms just have a thing for London. [Editor’s note: It’s probably not that.]
No wonder we feel worse after naps
Some of us have hectic schedules that may make a nap feel more necessary. It’s common knowledge that naps shouldn’t be too long – maybe 20 minutes or so – but if you frequently take 3-hour naps and wake up thinking you’re late for school even though you’re 47 and have your PhD, this LOTME is for you.
Studies have shown that there is a link between napping during the day and Alzheimer’s/cognitive decline, but now we’ve got a double whammy for you: Longer and more frequent napping is linked to worse cognition after a year, and in turn, those with cognitive decline and Alzheimer’s are known to nap longer and more frequently during the day.
“We now know that the pathology related to cognitive decline can cause other changes in function,” he said. “It’s really a multisystem disorder, also including difficulty sleeping, changes in movement, changes in body composition, depression symptoms, behavioral changes, etc.,” coauthor Aron Buchman, MD, said in a statement from Rush University Medical Center.
The investigators monitored 1,400 patients over the course of 14 years with wrist bracelets that recorded when a person was not active during the day and considered that a nap.
At the beginning of the study, 75% of the study subjects had no cognitive impairment, 19.5% had some cognitive impairment, and approximately 4% had Alzheimer’s. Napping during the day only increased about 11 minutes a year for those with no signs of cognitive impairment, but those who showed significantly more signs of cognitive decline doubled their nap time and those actually diagnosed with Alzheimer’s tripled theirs.
The investigators did not imply that napping causes Alzheimer’s, but they noted that people who are older and nap more than an hour a day are 40% more likely to be at risk. It is something to consider and monitor.
Sometimes, after all, a nap seems like the best idea ever, but more often than not we wake up feeling 10 times worse. Our bodies may be giving us a heads up.
Pokemon Go away depression
The summer of 2016 was a great time if you happened to be a fan of Pokemon. Which is quite a lot of people. For almost 20 years millions have enjoyed the games and animated series, but Pokemon Go brought the thrill of catching Pokemon to life in a whole new way. For the first time, you could go out into the world and pretend you were a real Pokemon trainer, and everywhere you went, there would be others like you.
The ability to chase after Pikachu and Charizard in real life (well, augmented reality, but close enough) seemed to bring people a lot of joy, but seemed is never good enough for science. Can’t have anecdotes, we need data! So researchers at the London School of Economics and Political Science conducted a study into how Pokemon Go affected local Internet search rates of depression as the game was released slowly around the world.
Through analyzing Google Trend data of words like “depression,” “anxiety,” and “stress,” the researchers found that the release of Pokemon Go was significantly associated with a noticeable, though short-term, drop in depression-related Internet searches. Location-based augmented reality games may alleviate symptoms of mild depression, the researchers said, as they encourage physical activity, face-to-face socialization, and exposure to nature, though they added that simply going outside is likely not enough to combat clinical cases of severe depression.
Still, augmented reality games represent a viable target for public health investment, since they’re easy to use and inexpensive to make. That said, we’re not sure we want the FDA or CDC making a new Pokemon Go game. They’d probably end up filling the streets with Mr. Mime. And no one would leave their house for that.
And now a word from our sponsor
How many times has this happened to you? You need to repair a jet engine, inspect a nuclear reactor cooling system, AND perform bowel surgery, but you can’t carry around all the heavy, old-fashioned tools needed for those jobs.
Well, we’ve got one tool that can do it all! And that tool is a snake. No, it’s a robot.
It’s both! It’s the COntinuum roBot for Remote Applications. COBRA is the robot that looks like a snake! A snake that’s 5 meters long but only as thick as a pencil (about 9 mm in diameter). A robot with “extraordinary manoeuvrability and responsiveness due to … a compliant-joint structure and multiple continuous sections that enable it to bend at around 90 degrees,” according to the team at the University of Nottingham (England) that developed it.
COBRA comes equipped with a stereovision camera and a miniature cutting tool to perform complex industrial repair, but other devices can be interchanged for possible medical use.
COBRA and its joystick-like controller were designed to be easy to use. Dr. Oladejo Olaleye, the ear, nose, and throat and robotic surgeon at University Hospitals of Leicester who is directing its surgical development, was able to use COBRA on a dummy after just 5 minutes of training. He called it “the future of diagnostic endoscopy and therapeutic surgery.”
Don’t be the last aircraft engineer/nuclear technician/surgeon on your block to have this ultraslender, ultramaneuverable reptilian repair robot. Get your COBRA now! Operators are standing by.
Disclaimer: Robot is still under development and not yet on sale.
Rule, (worm) Britannia!
As long as there have been people, there have been parasitic worms living in their guts. Helminth infection is a continuing and largely ignored crisis in poor, tropical nations, though worm-based diseases have been basically eliminated from wealthier countries.
This wasn’t always the case, however, as a study published in PLOS Neglected Tropical Diseases (now there’s a specific topic) has found. The researchers detail the glorious history of helminth infestation in the United Kingdom from the Victorian era all the way back to prehistory, scouring hundreds of skeletons found in 17 sites across the country for eggs, which can remain intact for thousands of years.
The researchers found that two eras in particular had very high rates of infection. Unsurprisingly, the late medieval era was one of them, but the other is less obvious. The Romans were famous for their hygiene, their baths, and their plumbing, but maybe they also should be famous for the abundance of worms in their bellies. That doesn’t make sense at first: Shouldn’t good hygiene lower infection? The benefits of a good sewer system, however, are lessened when the waste containing said infectious organisms is used to fertilize crops. Recycling is generally a good thing, but less so when you’re recycling parasitic worms.
Curiously, of the three sites from the industrial age, only the one in London had high levels of worm infestation. Considering how dirty and cramped 19th-century British cities were, one might expect disease to run rampant (tuberculosis certainly did), but the sites in Oxford and Birmingham were almost devoid of worms. The researchers theorized that this was because of access to clean well water. Or maybe worms just have a thing for London. [Editor’s note: It’s probably not that.]
No wonder we feel worse after naps
Some of us have hectic schedules that may make a nap feel more necessary. It’s common knowledge that naps shouldn’t be too long – maybe 20 minutes or so – but if you frequently take 3-hour naps and wake up thinking you’re late for school even though you’re 47 and have your PhD, this LOTME is for you.
Studies have shown that there is a link between napping during the day and Alzheimer’s/cognitive decline, but now we’ve got a double whammy for you: Longer and more frequent napping is linked to worse cognition after a year, and in turn, those with cognitive decline and Alzheimer’s are known to nap longer and more frequently during the day.
“We now know that the pathology related to cognitive decline can cause other changes in function,” he said. “It’s really a multisystem disorder, also including difficulty sleeping, changes in movement, changes in body composition, depression symptoms, behavioral changes, etc.,” coauthor Aron Buchman, MD, said in a statement from Rush University Medical Center.
The investigators monitored 1,400 patients over the course of 14 years with wrist bracelets that recorded when a person was not active during the day and considered that a nap.
At the beginning of the study, 75% of the study subjects had no cognitive impairment, 19.5% had some cognitive impairment, and approximately 4% had Alzheimer’s. Napping during the day only increased about 11 minutes a year for those with no signs of cognitive impairment, but those who showed significantly more signs of cognitive decline doubled their nap time and those actually diagnosed with Alzheimer’s tripled theirs.
The investigators did not imply that napping causes Alzheimer’s, but they noted that people who are older and nap more than an hour a day are 40% more likely to be at risk. It is something to consider and monitor.
Sometimes, after all, a nap seems like the best idea ever, but more often than not we wake up feeling 10 times worse. Our bodies may be giving us a heads up.
Pokemon Go away depression
The summer of 2016 was a great time if you happened to be a fan of Pokemon. Which is quite a lot of people. For almost 20 years millions have enjoyed the games and animated series, but Pokemon Go brought the thrill of catching Pokemon to life in a whole new way. For the first time, you could go out into the world and pretend you were a real Pokemon trainer, and everywhere you went, there would be others like you.
The ability to chase after Pikachu and Charizard in real life (well, augmented reality, but close enough) seemed to bring people a lot of joy, but seemed is never good enough for science. Can’t have anecdotes, we need data! So researchers at the London School of Economics and Political Science conducted a study into how Pokemon Go affected local Internet search rates of depression as the game was released slowly around the world.
Through analyzing Google Trend data of words like “depression,” “anxiety,” and “stress,” the researchers found that the release of Pokemon Go was significantly associated with a noticeable, though short-term, drop in depression-related Internet searches. Location-based augmented reality games may alleviate symptoms of mild depression, the researchers said, as they encourage physical activity, face-to-face socialization, and exposure to nature, though they added that simply going outside is likely not enough to combat clinical cases of severe depression.
Still, augmented reality games represent a viable target for public health investment, since they’re easy to use and inexpensive to make. That said, we’re not sure we want the FDA or CDC making a new Pokemon Go game. They’d probably end up filling the streets with Mr. Mime. And no one would leave their house for that.
And now a word from our sponsor
How many times has this happened to you? You need to repair a jet engine, inspect a nuclear reactor cooling system, AND perform bowel surgery, but you can’t carry around all the heavy, old-fashioned tools needed for those jobs.
Well, we’ve got one tool that can do it all! And that tool is a snake. No, it’s a robot.
It’s both! It’s the COntinuum roBot for Remote Applications. COBRA is the robot that looks like a snake! A snake that’s 5 meters long but only as thick as a pencil (about 9 mm in diameter). A robot with “extraordinary manoeuvrability and responsiveness due to … a compliant-joint structure and multiple continuous sections that enable it to bend at around 90 degrees,” according to the team at the University of Nottingham (England) that developed it.
COBRA comes equipped with a stereovision camera and a miniature cutting tool to perform complex industrial repair, but other devices can be interchanged for possible medical use.
COBRA and its joystick-like controller were designed to be easy to use. Dr. Oladejo Olaleye, the ear, nose, and throat and robotic surgeon at University Hospitals of Leicester who is directing its surgical development, was able to use COBRA on a dummy after just 5 minutes of training. He called it “the future of diagnostic endoscopy and therapeutic surgery.”
Don’t be the last aircraft engineer/nuclear technician/surgeon on your block to have this ultraslender, ultramaneuverable reptilian repair robot. Get your COBRA now! Operators are standing by.
Disclaimer: Robot is still under development and not yet on sale.
Rule, (worm) Britannia!
As long as there have been people, there have been parasitic worms living in their guts. Helminth infection is a continuing and largely ignored crisis in poor, tropical nations, though worm-based diseases have been basically eliminated from wealthier countries.
This wasn’t always the case, however, as a study published in PLOS Neglected Tropical Diseases (now there’s a specific topic) has found. The researchers detail the glorious history of helminth infestation in the United Kingdom from the Victorian era all the way back to prehistory, scouring hundreds of skeletons found in 17 sites across the country for eggs, which can remain intact for thousands of years.
The researchers found that two eras in particular had very high rates of infection. Unsurprisingly, the late medieval era was one of them, but the other is less obvious. The Romans were famous for their hygiene, their baths, and their plumbing, but maybe they also should be famous for the abundance of worms in their bellies. That doesn’t make sense at first: Shouldn’t good hygiene lower infection? The benefits of a good sewer system, however, are lessened when the waste containing said infectious organisms is used to fertilize crops. Recycling is generally a good thing, but less so when you’re recycling parasitic worms.
Curiously, of the three sites from the industrial age, only the one in London had high levels of worm infestation. Considering how dirty and cramped 19th-century British cities were, one might expect disease to run rampant (tuberculosis certainly did), but the sites in Oxford and Birmingham were almost devoid of worms. The researchers theorized that this was because of access to clean well water. Or maybe worms just have a thing for London. [Editor’s note: It’s probably not that.]