User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
COVID-19 in 2020: Deaths and disparities
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
FROM MMWR
Nonfatal opioid overdose rises in teen girls
More adolescent girls than boys experienced nonfatal opioid overdose and reported baseline levels of anxiety, depression, and self-harm, according to data from a retrospective cohort study of more than 20,000 youth in the United States.
Previous studies have identified sex-based differences in opioid overdose such as a higher prevalence of co-occurring psychiatric disorders in women compared with men, wrote Sarah M. Bagley, MD, of Boston University, and colleagues. “However, few studies have examined whether such sex-based differences in opioid overdose risk extend to the population of adolescents and young adults,” they said.
In a retrospective cohort study published in JAMA Network Open, the researchers identified 20,312 commercially insured youth aged 11-24 years who experienced a nonfatal opioid overdose between Jan. 1, 2006, and Dec. 31, 2017, and reviewed data using the IBM MarketScan Commercial Database. The average age of the study population was 20 years and approximately 42% were female.
Females aged 11-16 years had a significantly higher incidence of nonfatal opioid overdose (60%) compared with males, but this trend reversed at age 17 years, after which the incidence of nonfatal opioid overdose became significantly higher in males. “Our finding that females younger than 17 years had a higher incidence of NFOD is consistent with epidemiologic data that have indicated changes in alcohol and drug prevalence among female youths,” the researchers wrote.
Overall, 57.8% of the cohort had mood and anxiety disorders, 12.8% had trauma- or stress-related disorders, and 11.7% had attention-deficit/hyperactivity disorder.
When analyzed by sex, females had a significantly higher prevalence than that of males of mood or anxiety disorders (65.5% vs. 51.9%) trauma or stress-related disorders (16.4% vs. 10.1%) and attempts at suicide or self-harm (14.6% vs. 9.9%). Males had significantly higher prevalence than that of females of opioid use disorder (44.7% vs. 29.2%), cannabis use disorder (18.3% vs. 11.3%), and alcohol use disorder (20.3% vs. 14.4%).
“Although in our study, female youths had a lower prevalence of all substance use disorders, including OUD [opioid use disorder], and a higher prevalence of mood and trauma-associated disorders, both male and female youths had a higher prevalence of psychiatric illness and substance use disorder than youths in the general population,” the researchers noted.
The study findings were limited by several factors including the inclusion only of youth with commercial insurance, with no uninsured or publicly insured youth, and only those youth who sought health care after a nonfatal opioid overdose, the researchers noted. The prevalence of substance use and mental health disorders may be over- or underdiagnosed, and race was not included as a variable because of unreliable data, they added. The database also did not allow for gender identity beyond sex as listed by the insurance carrier, they said.
However, the results indicate significant differences in the incidence of nonfatal opioid overdose and accompanying mental health and substance use disorders based on age and sex, they said.
“These differences may have important implications for developing effective interventions to prevent first-time NFOD and to engage youths in care after an NFOD,” they concluded.
The study was supported by grants to several researchers from the National Institute on Drug Abuse, National Institutes of Health, and the Charles A. King Trust. The researchers had no financial conflicts to disclose.
More adolescent girls than boys experienced nonfatal opioid overdose and reported baseline levels of anxiety, depression, and self-harm, according to data from a retrospective cohort study of more than 20,000 youth in the United States.
Previous studies have identified sex-based differences in opioid overdose such as a higher prevalence of co-occurring psychiatric disorders in women compared with men, wrote Sarah M. Bagley, MD, of Boston University, and colleagues. “However, few studies have examined whether such sex-based differences in opioid overdose risk extend to the population of adolescents and young adults,” they said.
In a retrospective cohort study published in JAMA Network Open, the researchers identified 20,312 commercially insured youth aged 11-24 years who experienced a nonfatal opioid overdose between Jan. 1, 2006, and Dec. 31, 2017, and reviewed data using the IBM MarketScan Commercial Database. The average age of the study population was 20 years and approximately 42% were female.
Females aged 11-16 years had a significantly higher incidence of nonfatal opioid overdose (60%) compared with males, but this trend reversed at age 17 years, after which the incidence of nonfatal opioid overdose became significantly higher in males. “Our finding that females younger than 17 years had a higher incidence of NFOD is consistent with epidemiologic data that have indicated changes in alcohol and drug prevalence among female youths,” the researchers wrote.
Overall, 57.8% of the cohort had mood and anxiety disorders, 12.8% had trauma- or stress-related disorders, and 11.7% had attention-deficit/hyperactivity disorder.
When analyzed by sex, females had a significantly higher prevalence than that of males of mood or anxiety disorders (65.5% vs. 51.9%) trauma or stress-related disorders (16.4% vs. 10.1%) and attempts at suicide or self-harm (14.6% vs. 9.9%). Males had significantly higher prevalence than that of females of opioid use disorder (44.7% vs. 29.2%), cannabis use disorder (18.3% vs. 11.3%), and alcohol use disorder (20.3% vs. 14.4%).
“Although in our study, female youths had a lower prevalence of all substance use disorders, including OUD [opioid use disorder], and a higher prevalence of mood and trauma-associated disorders, both male and female youths had a higher prevalence of psychiatric illness and substance use disorder than youths in the general population,” the researchers noted.
The study findings were limited by several factors including the inclusion only of youth with commercial insurance, with no uninsured or publicly insured youth, and only those youth who sought health care after a nonfatal opioid overdose, the researchers noted. The prevalence of substance use and mental health disorders may be over- or underdiagnosed, and race was not included as a variable because of unreliable data, they added. The database also did not allow for gender identity beyond sex as listed by the insurance carrier, they said.
However, the results indicate significant differences in the incidence of nonfatal opioid overdose and accompanying mental health and substance use disorders based on age and sex, they said.
“These differences may have important implications for developing effective interventions to prevent first-time NFOD and to engage youths in care after an NFOD,” they concluded.
The study was supported by grants to several researchers from the National Institute on Drug Abuse, National Institutes of Health, and the Charles A. King Trust. The researchers had no financial conflicts to disclose.
More adolescent girls than boys experienced nonfatal opioid overdose and reported baseline levels of anxiety, depression, and self-harm, according to data from a retrospective cohort study of more than 20,000 youth in the United States.
Previous studies have identified sex-based differences in opioid overdose such as a higher prevalence of co-occurring psychiatric disorders in women compared with men, wrote Sarah M. Bagley, MD, of Boston University, and colleagues. “However, few studies have examined whether such sex-based differences in opioid overdose risk extend to the population of adolescents and young adults,” they said.
In a retrospective cohort study published in JAMA Network Open, the researchers identified 20,312 commercially insured youth aged 11-24 years who experienced a nonfatal opioid overdose between Jan. 1, 2006, and Dec. 31, 2017, and reviewed data using the IBM MarketScan Commercial Database. The average age of the study population was 20 years and approximately 42% were female.
Females aged 11-16 years had a significantly higher incidence of nonfatal opioid overdose (60%) compared with males, but this trend reversed at age 17 years, after which the incidence of nonfatal opioid overdose became significantly higher in males. “Our finding that females younger than 17 years had a higher incidence of NFOD is consistent with epidemiologic data that have indicated changes in alcohol and drug prevalence among female youths,” the researchers wrote.
Overall, 57.8% of the cohort had mood and anxiety disorders, 12.8% had trauma- or stress-related disorders, and 11.7% had attention-deficit/hyperactivity disorder.
When analyzed by sex, females had a significantly higher prevalence than that of males of mood or anxiety disorders (65.5% vs. 51.9%) trauma or stress-related disorders (16.4% vs. 10.1%) and attempts at suicide or self-harm (14.6% vs. 9.9%). Males had significantly higher prevalence than that of females of opioid use disorder (44.7% vs. 29.2%), cannabis use disorder (18.3% vs. 11.3%), and alcohol use disorder (20.3% vs. 14.4%).
“Although in our study, female youths had a lower prevalence of all substance use disorders, including OUD [opioid use disorder], and a higher prevalence of mood and trauma-associated disorders, both male and female youths had a higher prevalence of psychiatric illness and substance use disorder than youths in the general population,” the researchers noted.
The study findings were limited by several factors including the inclusion only of youth with commercial insurance, with no uninsured or publicly insured youth, and only those youth who sought health care after a nonfatal opioid overdose, the researchers noted. The prevalence of substance use and mental health disorders may be over- or underdiagnosed, and race was not included as a variable because of unreliable data, they added. The database also did not allow for gender identity beyond sex as listed by the insurance carrier, they said.
However, the results indicate significant differences in the incidence of nonfatal opioid overdose and accompanying mental health and substance use disorders based on age and sex, they said.
“These differences may have important implications for developing effective interventions to prevent first-time NFOD and to engage youths in care after an NFOD,” they concluded.
The study was supported by grants to several researchers from the National Institute on Drug Abuse, National Institutes of Health, and the Charles A. King Trust. The researchers had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Autism Acceptance Month: Raising awareness and closing the diagnosis gap
April is National Autism Awareness Month, and April 2 is World Autism Awareness Day. In the United States, there appears to be a heightened level of awareness of this condition over the past 10-15 years that has helped reduced its stigma, improve early identification, and (most importantly) increase access to early interventions for children and families.
The most recent prevalence estimates of autism in children in the United States is 1 in 54. This is a 10% increase since 2014 (1 in 59). Those most recent Centers for Disease Control and Prevention surveillance reports also point to a reduction in the racial gap between Black and White children when it comes to diagnosis.1 Across the globe, there are more than 100 autism societies, and research designed to improve prevalence data in lower- to middle-income countries has also increased.2
Even with these recent encouraging numbers regarding identification of autism in historically underrepresented groups in the United States, there are still differences among those groups, compared with children who are socioeconomically well-off, White, or live in large urban areas.3 Specifically, Latinx children were documented to be identified with autism at lower rates, compared with Whites and Blacks. In addition, Black and Latinx children are still diagnosed at a later age, compared with White children. This is important to note because historically, Black and Latinx children have been diagnosed with severe forms of autism or co-occurring intellectual disability at a higher rate, compared with their White counterparts.4 Thus, it would not be inappropriate to infer that Black and Latinx children with “milder” presenting autism symptoms or without co-occurring ID are not identified at the same rates, compared with their White peers. Furthermore, when peering into the international data, epidemiologic studies regarding prevalence, clinical course, and outcomes is skewed heavily toward a few Western industrialized nations, Japan, and South Korea.5
In all, when observing Autism Awareness Month, we should continue to recognize that these aforementioned epidemiologic disparities still exist – both locally and globally. because both clinical and research work have consequences that are not yet fully known. As long as these trends remain, racial and socioeconomic differences in access to treatment in the United States will remain. From an international perspective, we may never appreciate the true extent of the cultural variability within autism symptoms and so may never appreciate the full spectrum of ways the condition can present.
References
1. MMWR Surveill Summ. 2020 Mar 27;69(4):1-12. Erratum in: MMWR Morb Mortal Wkly Rep. 2020 Apr 24;69(16):503.
2. Lancet Glob Health. 2018 Oct;6(10):e1100-21.
3. Am J Public Health. 2009;99(3):493-8.
4. J Dev Behav Pediatr. 2011 Apr;32(3):179-87 and MMWR Surveill Summ. 2019;68(2):1-19.
5. Brain Sci. 2020;10(5):274. doi: 10.3390/brainsci10050274.
Dr. Emejuru is a child and adolescent psychiatrist with Community Hospital of Monterey Peninsula (CHOMP) and its Ohana Center for Child and Adolescent Behavioral Health in Monterey, Calif. His expertise is specific to conducting evaluations for autism spectrum disorder and evaluating, diagnosing, and treating co-occurring psychiatric disorders after training at the Johns Hopkins Hospital/Kennedy Krieger Institute’s Center for Autism and Related Disorders in Baltimore. He has no conflicts of interest.
April is National Autism Awareness Month, and April 2 is World Autism Awareness Day. In the United States, there appears to be a heightened level of awareness of this condition over the past 10-15 years that has helped reduced its stigma, improve early identification, and (most importantly) increase access to early interventions for children and families.
The most recent prevalence estimates of autism in children in the United States is 1 in 54. This is a 10% increase since 2014 (1 in 59). Those most recent Centers for Disease Control and Prevention surveillance reports also point to a reduction in the racial gap between Black and White children when it comes to diagnosis.1 Across the globe, there are more than 100 autism societies, and research designed to improve prevalence data in lower- to middle-income countries has also increased.2
Even with these recent encouraging numbers regarding identification of autism in historically underrepresented groups in the United States, there are still differences among those groups, compared with children who are socioeconomically well-off, White, or live in large urban areas.3 Specifically, Latinx children were documented to be identified with autism at lower rates, compared with Whites and Blacks. In addition, Black and Latinx children are still diagnosed at a later age, compared with White children. This is important to note because historically, Black and Latinx children have been diagnosed with severe forms of autism or co-occurring intellectual disability at a higher rate, compared with their White counterparts.4 Thus, it would not be inappropriate to infer that Black and Latinx children with “milder” presenting autism symptoms or without co-occurring ID are not identified at the same rates, compared with their White peers. Furthermore, when peering into the international data, epidemiologic studies regarding prevalence, clinical course, and outcomes is skewed heavily toward a few Western industrialized nations, Japan, and South Korea.5
In all, when observing Autism Awareness Month, we should continue to recognize that these aforementioned epidemiologic disparities still exist – both locally and globally. because both clinical and research work have consequences that are not yet fully known. As long as these trends remain, racial and socioeconomic differences in access to treatment in the United States will remain. From an international perspective, we may never appreciate the true extent of the cultural variability within autism symptoms and so may never appreciate the full spectrum of ways the condition can present.
References
1. MMWR Surveill Summ. 2020 Mar 27;69(4):1-12. Erratum in: MMWR Morb Mortal Wkly Rep. 2020 Apr 24;69(16):503.
2. Lancet Glob Health. 2018 Oct;6(10):e1100-21.
3. Am J Public Health. 2009;99(3):493-8.
4. J Dev Behav Pediatr. 2011 Apr;32(3):179-87 and MMWR Surveill Summ. 2019;68(2):1-19.
5. Brain Sci. 2020;10(5):274. doi: 10.3390/brainsci10050274.
Dr. Emejuru is a child and adolescent psychiatrist with Community Hospital of Monterey Peninsula (CHOMP) and its Ohana Center for Child and Adolescent Behavioral Health in Monterey, Calif. His expertise is specific to conducting evaluations for autism spectrum disorder and evaluating, diagnosing, and treating co-occurring psychiatric disorders after training at the Johns Hopkins Hospital/Kennedy Krieger Institute’s Center for Autism and Related Disorders in Baltimore. He has no conflicts of interest.
April is National Autism Awareness Month, and April 2 is World Autism Awareness Day. In the United States, there appears to be a heightened level of awareness of this condition over the past 10-15 years that has helped reduced its stigma, improve early identification, and (most importantly) increase access to early interventions for children and families.
The most recent prevalence estimates of autism in children in the United States is 1 in 54. This is a 10% increase since 2014 (1 in 59). Those most recent Centers for Disease Control and Prevention surveillance reports also point to a reduction in the racial gap between Black and White children when it comes to diagnosis.1 Across the globe, there are more than 100 autism societies, and research designed to improve prevalence data in lower- to middle-income countries has also increased.2
Even with these recent encouraging numbers regarding identification of autism in historically underrepresented groups in the United States, there are still differences among those groups, compared with children who are socioeconomically well-off, White, or live in large urban areas.3 Specifically, Latinx children were documented to be identified with autism at lower rates, compared with Whites and Blacks. In addition, Black and Latinx children are still diagnosed at a later age, compared with White children. This is important to note because historically, Black and Latinx children have been diagnosed with severe forms of autism or co-occurring intellectual disability at a higher rate, compared with their White counterparts.4 Thus, it would not be inappropriate to infer that Black and Latinx children with “milder” presenting autism symptoms or without co-occurring ID are not identified at the same rates, compared with their White peers. Furthermore, when peering into the international data, epidemiologic studies regarding prevalence, clinical course, and outcomes is skewed heavily toward a few Western industrialized nations, Japan, and South Korea.5
In all, when observing Autism Awareness Month, we should continue to recognize that these aforementioned epidemiologic disparities still exist – both locally and globally. because both clinical and research work have consequences that are not yet fully known. As long as these trends remain, racial and socioeconomic differences in access to treatment in the United States will remain. From an international perspective, we may never appreciate the true extent of the cultural variability within autism symptoms and so may never appreciate the full spectrum of ways the condition can present.
References
1. MMWR Surveill Summ. 2020 Mar 27;69(4):1-12. Erratum in: MMWR Morb Mortal Wkly Rep. 2020 Apr 24;69(16):503.
2. Lancet Glob Health. 2018 Oct;6(10):e1100-21.
3. Am J Public Health. 2009;99(3):493-8.
4. J Dev Behav Pediatr. 2011 Apr;32(3):179-87 and MMWR Surveill Summ. 2019;68(2):1-19.
5. Brain Sci. 2020;10(5):274. doi: 10.3390/brainsci10050274.
Dr. Emejuru is a child and adolescent psychiatrist with Community Hospital of Monterey Peninsula (CHOMP) and its Ohana Center for Child and Adolescent Behavioral Health in Monterey, Calif. His expertise is specific to conducting evaluations for autism spectrum disorder and evaluating, diagnosing, and treating co-occurring psychiatric disorders after training at the Johns Hopkins Hospital/Kennedy Krieger Institute’s Center for Autism and Related Disorders in Baltimore. He has no conflicts of interest.
A clinical approach to pharmacotherapy for personality disorders
DSM-5 defines personality disorders (PDs) as the presence of an enduring pattern of inner experience and behavior that “deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adulthood, is stable over time, and leads to distress or impairment.”1 As a general rule, PDs are not limited to episodes of illness, but reflect an individual’s long-term adjustment. These disorders occur in 10% to 15% of the general population; the rates are especially high in health care settings, in criminal offenders, and in those with a substance use disorder (SUD).2 PDs nearly always have an onset in adolescence or early adulthood and tend to diminish in severity with advancing age. They are associated with high rates of unemployment, homelessness, divorce and separation, domestic violence, substance misuse, and suicide.3
Psychotherapy is the first-line treatment for PDs, but there has been growing interest in using pharmacotherapy to treat PDs. While much of the PD treatment literature focuses on borderline PD,4-9 this article describes diagnosis, potential pharmacotherapy strategies, and methods to assess response to treatment for patients with all types of PDs.
Recognizing and diagnosing personality disorders
The diagnosis of a PD requires an understanding of DSM-5 criteria combined with a comprehensive psychiatric history and mental status examination. The patient’s history is the most important basis for diagnosing a PD.2 Collateral information from relatives or friends can help confirm the severity and pervasiveness of the individual’s personality problems. In some patients, long-term observation might be necessary to confirm the presence of a PD. Some clinicians are reluctant to diagnose PDs because of stigma, a problem common among patients with borderline PD.10,11
To screen for PDs, a clinician might ask the patient about problems with interpersonal relationships, sense of self, work, affect, impulse control, and reality testing. Table 112 lists general screening questions for the presence of a PD from the Iowa Personality Disorders Screen. Structured diagnostic interviews and self-report assessments could boost recognition of PDs, but these tools are rarely used outside of research settings.13,14
The PD clusters
DSM-5 divides 10 PDs into 3 clusters based on shared phenomenology and diagnostic criteria. Few patients have a “pure” case in which they meet criteria for only a single personality disorder.1
Cluster A. “Eccentric cluster” disorders are united by social aversion, a failure to form close attachments, or paranoia and suspiciousness.15 These include paranoid, schizoid, and schizotypal PD. Low self-awareness is typical. There are no treatment guidelines for these disorders, although there is some clinical trial data for schizotypal PD.
Cluster B. “Dramatic cluster” disorders share dramatic, emotional, and erratic characteristics.14 These include narcissistic, antisocial, borderline, and histrionic PD. Antisocial and narcissistic patients have low self-awareness. There are treatment guidelines for antisocial and borderline PD, and a variety of clinical trial data is available for the latter.15
Continue to: Cluster C
Cluster C. “Anxious cluster” disorders are united by anxiousness, fearfulness, and poor self-esteem. Many of these patients also display interpersonal rigidity.15 These disorders include avoidant, dependent, and obsessive-compulsive PD. There are no treatment guidelines or clinical trial data for these disorders.
Why consider pharmacotherapy for personality disorders?
The consensus among experts is that psychotherapy is the treatment of choice for PDs.15 Despite significant gaps in the evidence base, there has been a growing interest in using psychotropic medication to treat PDs. For example, research shows that >90% of patients with borderline PD are prescribed medication, most typically antidepressants, antipsychotics, mood stabilizers, stimulants, or sedative-hypnotics.16,17
Increased interest in pharmacotherapy for PDs could be related to research showing the importance of underlying neurobiology, particularly for antisocial and borderline PD.18,19 This work is complemented by genetic research showing the heritability of PD traits and disorders.20,21 Another factor could be renewed interest in dimensional approaches to the classification of PDs, as exemplified by DSM-5’s alternative model for PDs.1 This approach aligns with some expert recommendations to focus on treating PD symptom dimensions, rather than the syndrome itself.22
Importantly, no psychotropic medication is FDA-approved for the treatment of any PD. For that reason, prescribing medication for a PD is “off-label,” although prescribing a medication for a comorbid disorder for which the drug has an FDA-approved indication is not (eg, prescribing an antidepressant for major depressive disorder [MDD]).
Principles for prescribing
Despite gaps in research data, general principles for using medication to treat PDs have emerged from treatment guidelines for antisocial and borderline PD, clinical trial data, reviews and meta-analyses, and expert opinion. Clinicians should address the following considerations before prescribing medication to a patient with a PD.
Continue to: PD diagnosis
PD diagnosis. Has the patient been properly assessed and diagnosed? While history is the most important basis for diagnosis, the clinician should be familiar with the PDs and DSM-5 criteria. Has the patient been informed of the diagnosis and its implications for treatment?
Patient interest in medication. Is the patient interested in taking medication? Patients with borderline PD are often prescribed medication, but there are sparse data for the other PDs. The patient might have little interest in the PD diagnosis or its treatment.
Comorbidity. Has the patient been assessed for comorbid psychiatric disorders that could interfere with medication use (ie, an SUD) or might be a focus of treatment (eg, MDD)? Patients with PDs typically have significant comorbidity that a thorough evaluation will uncover.
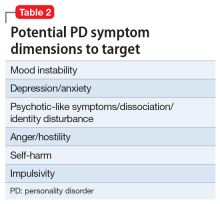
PD symptom dimensions. Has the patient been assessed to determine cognitive or behavioral symptom dimensions of their PD? One or more symptom dimension(s) could be the focus of treatment. Table 2 lists examples of PD symptom dimensions.
Strategies to guide prescribing
Strategies to help guide prescribing include targeting any comorbid disorder(s), targeting important PD symptom dimensions (eg, impulsive aggression), choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself.
Continue to: Targeting comorbid disorders
Targeting comorbid disorders. National Institute for Health and Care Excellence guidelines for antisocial and borderline PD recommend that clinicians focus on treating comorbid disorders, a position echoed in Cochrane and other reviews.4,9,22-26 For example, a patient with borderline PD experiencing a major depressive episode could be treated with an antidepressant. Targeting the depressive symptoms could boost the patient’s mood, perhaps lessening the individual’s PD symptoms or reducing their severity.
Targeting important symptom dimensions. For patients with borderline PD, several guidelines and reviews have suggested that treatment should focus on emotional dysregulation and impulsive aggression (mood stabilizers, antipsychotics), or cognitive-perceptual symptoms (antipsychotics).4-6,15 There is some evidence that mood stabilizers or second-generation antipsychotics could help reduce impulsive aggression in patients with antisocial PD.27
Choosing medication based on similarity to another disorder known to respond to medication. Avoidant PD overlaps with social anxiety disorder and can be conceptualized as a chronic, pervasive social phobia. Avoidant PD might respond to a medication known to be effective for treating social anxiety disorder, such as a selective serotonin reuptake inhibitor (SSRI) or venlafaxine.28 Treating obsessive-compulsive PD with an SSRI is another example of this strategy, as 1 small study of fluvoxamine suggests.29 Obsessive-compulsive PD is common in persons with obsessive-compulsive disorder, and overlap includes preoccupation with orders, rules, and lists, and an inability to throw things out.
Targeting the PD syndrome. Another strategy is to target the PD itself. Clinical trial data suggest the antipsychotic risperidone can reduce the symptoms of schizotypal PD.30 Considering that this PD has a genetic association with schizophrenia, it is not surprising that the patient’s ideas of reference, odd communication, or transient paranoia might respond to an antipsychotic. Data from randomized controlled trials (RCTs) support the use of the second-generation antipsychotics aripiprazole and quetiapine to treat BPD.31,32 While older guidelines4,5 supported the use of the mood stabilizer lamotrigine, a recent RCT found that it was no more effective than placebo for borderline PD or its symptom dimensions.33
What to do before prescribing
Before writing a prescription, the clinician and patient should discuss the presence of a PD and the desirability of treatment. The patient should understand the limited evidence base and know that medication prescribed for a PD is off-label. The clinician should discuss medication selection and its rationale, and whether the medication is targeting a comorbid disorder, symptom dimension(s), or the PD itself. Additional considerations for prescribing for patients with PDs are listed in Table 3.34

Continue to: Avoid polypharmacy
Avoid polypharmacy. Many patients with borderline PD are prescribed multiple psychotropic medications.16,17 This approach leads to greater expense and more adverse effects, and is not evidence-based.
Avoid benzodiazepines. Many patients with borderline PD are prescribed benzodiazepines, often as part of a polypharmacy regimen. These drugs can cause disinhibition, thereby increasing acting-out behaviors and self-harm.35 Also, patients with PDs often have SUDs, which is a contraindication for benzodiazepine use.
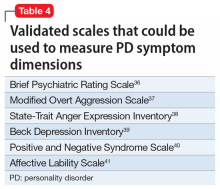
Rate the patient’s improvement. Both the patient and clinician can benefit from monitoring symptomatic improvement. Several validated scales can be used to rate depression, anxiety, impulsivity, mood lability, anger, and aggression (Table 436-41).Some validated scales for borderline PD align with DSM-5 criteria. Two such widely used instruments are the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD)42 and the self-rated Borderline Evaluation of Severity Over Time (BEST).43 Each has questions that could be pulled to rate a symptom dimension of interest, such as affective instability, anger dyscontrol, or abandonment fears (Table 542,43).
A visual analog scale is easy to use and can target symptom dimensions of interest.44 For example, a clinician could use a visual analog scale to rate mood instability by asking a patient to rate their mood severity by making a mark along a 10-cm line (0 = “Most erratic emotions I have experienced,” 10 = “Most stable I have ever experienced my emotions to be”). This score can be recorded at baseline and subsequent visits.
Take-home points
PDs are common in the general population and health care settings. They are underrecognized by the general public and mental health professionals, often because of stigma. Clinicians could boost their recognition of these disorders by embedding simple screening questions in their patient assessments. Many patients with PDs will be interested in pharmacotherapy for their disorder or symptoms. Treatment strategies include targeting the comorbid disorder(s), targeting important PD symptom dimensions, choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself. Each strategy has its limitations and varying degrees of empirical support. Treatment response can be monitored using validated scales or a visual analog scale.
Continue to: Bottom Line
Bottom Line
Although psychotherapy is the first-line treatment and no medications are FDAapproved for treating personality disorders (PDs), there has been growing interest in using psychotropic medication to treat PDs. Strategies for pharmacotherapy include targeting comorbid disorders, PD symptom dimensions, or the PD itself. Choice of medication can be based on the similarity of the PD with another disorder known to respond to medication.
Related Resources
- Correa Da Costa S, Sanches M, Soares JC. Bipolar disorder or borderline personality disorder? Current Psychiatry. 2019;18(11):26-29,35-39.
- Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
Drug Brand Names
Aripiprazole • Abilify
Fluvoxamine • Luvox
Lamotrigine • Lamictal
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Black DW, Andreasen N. Personality disorders. In: Black DW, Andreasen N. Introductory textbook of psychiatry, 7th edition. American Psychiatric Publishing; 2020:410-423.
3. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord 2004;18(3):226-239.
4. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
5. Vita A, De Peri L, Sacchetti E. Antipsychotics, antidepressants, anticonvulsants, and placebo on the symptom dimensions of borderline personality disorder – a meta-analysis of randomized controlled and open-label trials. J Clin Psychopharmacol. 2011;31(5):613-624.
6. Stoffers JM, Lieb K. Pharmacotherapy for borderline personality disorder – current evidence and recent trends. Curr Psychiatry Rep. 2015;17(1):534.
7. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31(5):345-356.
8. Black DW, Paris J, Schulz SC. Personality disorders: evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M, ed. Cognitive behavioral psychopharmacology: the clinical practice of evidence-based biopsychosocial integration. John Wiley & Sons; 2018:137-165.
9. Stoffers-Winterling J, Sorebø OJ, Lieb K. Pharmacotherapy for borderline personality disorder: an update of published, unpublished and ongoing studies. Curr Psychiatry Rep. 2020;22(8):37.
10. Lewis G, Appleby L. Personality disorder: the patients psychiatrists dislike. Br J Psychiatry. 1988;153:44-49.
11. Black DW, Pfohl B, Blum N, et al. Attitudes toward borderline personality disorder: a survey of 706 mental health clinicians. CNS Spectr. 2011;16(3):67-74.
12. Langbehn DR, Pfohl BM, Reynolds S, et al. The Iowa Personality Disorder Screen: development and preliminary validation of a brief screening interview. J Pers Disord. 1999;13(1):75-89.
13. Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV). American Psychiatric Press; 1997.
14. First MB, Spitzer RL, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part II: multisite test-retest reliability study. J Pers Disord. 1995;9(2):92-104.
15. Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
16. Zanarini MC, Frankenburg FR, Reich DB, et al. Treatment rates for patients with borderline personality disorder and other personality disorders: a 16-year study. Psychiatr Serv. 2015;66(1):15-20.
17. Black DW, Allen J, McCormick B, et al. Treatment received by persons with BPD participating in a randomized clinical trial of the Systems Training for Emotional Predictability and Problem Solving programme. Person Ment Health. 2011;5(3):159-168.
18. Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. Behav Sci Law. 2008;26(1):65-83.
19. Ruocco AC, Amirthavasagam S, Choi-Kain LW, et al. Neural correlates of negative emotionality in BPD: an activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73(2):153-160.
20. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831.
21. Slutske WS. The genetics of antisocial behavior. Curr Psychiatry Rep. 2001;3(2):158-162.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
23. National Institute for Health and Care Excellence (NICE). Borderline personality disorder: recognition and management. Clinical guideline [CG78]. Published January 2009. https://www.nice.org.uk/guidance/cg78
24. National Institute for Health and Care Excellence (NICE). Antisocial personality disorder: prevention and management. Clinical guideline [CG77]. Published January 2009. Updated March 27, 2013. https://www.nice.org.uk/guidance/cg77
25. Khalifa N, Duggan C, Stoffers J, et al. Pharmacologic interventions for antisocial personality disorder. Cochrane Database Syst Rep. 2010;(8):CD007667.
26. Stoffers JM, Völlm BA, Rücker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;2012(8):CD005652.
27. Black DW. The treatment of antisocial personality disorder. Current Treatment Options in Psychiatry. 2017. https://doi.org/10.1007/s40501-017-0123-z
28. Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280(8):708-713.
29. Ansseau M. The obsessive-compulsive personality: diagnostic aspects and treatment possibilities. In: Den Boer JA, Westenberg HGM, eds. Focus on obsessive-compulsive spectrum disorders. Syn-Thesis; 1997:61-73.
30. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64(6):628-634.
31. Black DW, Zanarini MC, Romine A, et al. Comparison of low and moderate dosages of extended-release quetiapine in borderline personality disorder: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2014;171(11):1174-1182.
32. Nickel MK, Muelbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2006;163(5):833-838.
33. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764.
34. Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry. 2004;65(12)1660-1665.
35. Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder. Alprazolam, carbamazepine, trifluoperazine, and tranylcypromine. Arch Gen Psychiatry. 1988;45(2):111-119.
36. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812.
37. Ratey JJ, Gutheil CM. The measurement of aggressive behavior: reflections on the use of the Overt Aggression Scale and the Modified Overt Aggression Scale. J Neuropsychiatr Clin Neurosci. 1991;3(2):S57-S60.
38. Spielberger CD, Sydeman SJ, Owen AE, et al. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In: Maruish ME, ed. The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers; 1999:993-1021.
39. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. Psychological Corp; 1996.
40. Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule – Expanded Form. The University of Iowa; 1999.
41. Harvey D, Greenberg BR, Serper MR, et al. The affective lability scales: development, reliability, and validity. J Clin Psychol. 1989;45(5):786-793.
42. Zanarini MC, Vujanovic AA, Parachini EA, et al. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. J Person Disord. 2003:17(3):233-242.
43. Pfohl B, Blum N, St John D, et al. Reliability and validity of the Borderline Evaluation of Severity Over Time (BEST): a new scale to measure severity and change in borderline personality disorder. J Person Disord. 2009;23(3):281-293.
44. Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiatr Res. 1997;31(5):569-579.
DSM-5 defines personality disorders (PDs) as the presence of an enduring pattern of inner experience and behavior that “deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adulthood, is stable over time, and leads to distress or impairment.”1 As a general rule, PDs are not limited to episodes of illness, but reflect an individual’s long-term adjustment. These disorders occur in 10% to 15% of the general population; the rates are especially high in health care settings, in criminal offenders, and in those with a substance use disorder (SUD).2 PDs nearly always have an onset in adolescence or early adulthood and tend to diminish in severity with advancing age. They are associated with high rates of unemployment, homelessness, divorce and separation, domestic violence, substance misuse, and suicide.3
Psychotherapy is the first-line treatment for PDs, but there has been growing interest in using pharmacotherapy to treat PDs. While much of the PD treatment literature focuses on borderline PD,4-9 this article describes diagnosis, potential pharmacotherapy strategies, and methods to assess response to treatment for patients with all types of PDs.
Recognizing and diagnosing personality disorders
The diagnosis of a PD requires an understanding of DSM-5 criteria combined with a comprehensive psychiatric history and mental status examination. The patient’s history is the most important basis for diagnosing a PD.2 Collateral information from relatives or friends can help confirm the severity and pervasiveness of the individual’s personality problems. In some patients, long-term observation might be necessary to confirm the presence of a PD. Some clinicians are reluctant to diagnose PDs because of stigma, a problem common among patients with borderline PD.10,11
To screen for PDs, a clinician might ask the patient about problems with interpersonal relationships, sense of self, work, affect, impulse control, and reality testing. Table 112 lists general screening questions for the presence of a PD from the Iowa Personality Disorders Screen. Structured diagnostic interviews and self-report assessments could boost recognition of PDs, but these tools are rarely used outside of research settings.13,14
The PD clusters
DSM-5 divides 10 PDs into 3 clusters based on shared phenomenology and diagnostic criteria. Few patients have a “pure” case in which they meet criteria for only a single personality disorder.1
Cluster A. “Eccentric cluster” disorders are united by social aversion, a failure to form close attachments, or paranoia and suspiciousness.15 These include paranoid, schizoid, and schizotypal PD. Low self-awareness is typical. There are no treatment guidelines for these disorders, although there is some clinical trial data for schizotypal PD.
Cluster B. “Dramatic cluster” disorders share dramatic, emotional, and erratic characteristics.14 These include narcissistic, antisocial, borderline, and histrionic PD. Antisocial and narcissistic patients have low self-awareness. There are treatment guidelines for antisocial and borderline PD, and a variety of clinical trial data is available for the latter.15
Continue to: Cluster C
Cluster C. “Anxious cluster” disorders are united by anxiousness, fearfulness, and poor self-esteem. Many of these patients also display interpersonal rigidity.15 These disorders include avoidant, dependent, and obsessive-compulsive PD. There are no treatment guidelines or clinical trial data for these disorders.
Why consider pharmacotherapy for personality disorders?
The consensus among experts is that psychotherapy is the treatment of choice for PDs.15 Despite significant gaps in the evidence base, there has been a growing interest in using psychotropic medication to treat PDs. For example, research shows that >90% of patients with borderline PD are prescribed medication, most typically antidepressants, antipsychotics, mood stabilizers, stimulants, or sedative-hypnotics.16,17
Increased interest in pharmacotherapy for PDs could be related to research showing the importance of underlying neurobiology, particularly for antisocial and borderline PD.18,19 This work is complemented by genetic research showing the heritability of PD traits and disorders.20,21 Another factor could be renewed interest in dimensional approaches to the classification of PDs, as exemplified by DSM-5’s alternative model for PDs.1 This approach aligns with some expert recommendations to focus on treating PD symptom dimensions, rather than the syndrome itself.22
Importantly, no psychotropic medication is FDA-approved for the treatment of any PD. For that reason, prescribing medication for a PD is “off-label,” although prescribing a medication for a comorbid disorder for which the drug has an FDA-approved indication is not (eg, prescribing an antidepressant for major depressive disorder [MDD]).
Principles for prescribing
Despite gaps in research data, general principles for using medication to treat PDs have emerged from treatment guidelines for antisocial and borderline PD, clinical trial data, reviews and meta-analyses, and expert opinion. Clinicians should address the following considerations before prescribing medication to a patient with a PD.
Continue to: PD diagnosis
PD diagnosis. Has the patient been properly assessed and diagnosed? While history is the most important basis for diagnosis, the clinician should be familiar with the PDs and DSM-5 criteria. Has the patient been informed of the diagnosis and its implications for treatment?
Patient interest in medication. Is the patient interested in taking medication? Patients with borderline PD are often prescribed medication, but there are sparse data for the other PDs. The patient might have little interest in the PD diagnosis or its treatment.
Comorbidity. Has the patient been assessed for comorbid psychiatric disorders that could interfere with medication use (ie, an SUD) or might be a focus of treatment (eg, MDD)? Patients with PDs typically have significant comorbidity that a thorough evaluation will uncover.
PD symptom dimensions. Has the patient been assessed to determine cognitive or behavioral symptom dimensions of their PD? One or more symptom dimension(s) could be the focus of treatment. Table 2 lists examples of PD symptom dimensions.
Strategies to guide prescribing
Strategies to help guide prescribing include targeting any comorbid disorder(s), targeting important PD symptom dimensions (eg, impulsive aggression), choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself.
Continue to: Targeting comorbid disorders
Targeting comorbid disorders. National Institute for Health and Care Excellence guidelines for antisocial and borderline PD recommend that clinicians focus on treating comorbid disorders, a position echoed in Cochrane and other reviews.4,9,22-26 For example, a patient with borderline PD experiencing a major depressive episode could be treated with an antidepressant. Targeting the depressive symptoms could boost the patient’s mood, perhaps lessening the individual’s PD symptoms or reducing their severity.
Targeting important symptom dimensions. For patients with borderline PD, several guidelines and reviews have suggested that treatment should focus on emotional dysregulation and impulsive aggression (mood stabilizers, antipsychotics), or cognitive-perceptual symptoms (antipsychotics).4-6,15 There is some evidence that mood stabilizers or second-generation antipsychotics could help reduce impulsive aggression in patients with antisocial PD.27
Choosing medication based on similarity to another disorder known to respond to medication. Avoidant PD overlaps with social anxiety disorder and can be conceptualized as a chronic, pervasive social phobia. Avoidant PD might respond to a medication known to be effective for treating social anxiety disorder, such as a selective serotonin reuptake inhibitor (SSRI) or venlafaxine.28 Treating obsessive-compulsive PD with an SSRI is another example of this strategy, as 1 small study of fluvoxamine suggests.29 Obsessive-compulsive PD is common in persons with obsessive-compulsive disorder, and overlap includes preoccupation with orders, rules, and lists, and an inability to throw things out.
Targeting the PD syndrome. Another strategy is to target the PD itself. Clinical trial data suggest the antipsychotic risperidone can reduce the symptoms of schizotypal PD.30 Considering that this PD has a genetic association with schizophrenia, it is not surprising that the patient’s ideas of reference, odd communication, or transient paranoia might respond to an antipsychotic. Data from randomized controlled trials (RCTs) support the use of the second-generation antipsychotics aripiprazole and quetiapine to treat BPD.31,32 While older guidelines4,5 supported the use of the mood stabilizer lamotrigine, a recent RCT found that it was no more effective than placebo for borderline PD or its symptom dimensions.33
What to do before prescribing
Before writing a prescription, the clinician and patient should discuss the presence of a PD and the desirability of treatment. The patient should understand the limited evidence base and know that medication prescribed for a PD is off-label. The clinician should discuss medication selection and its rationale, and whether the medication is targeting a comorbid disorder, symptom dimension(s), or the PD itself. Additional considerations for prescribing for patients with PDs are listed in Table 3.34

Continue to: Avoid polypharmacy
Avoid polypharmacy. Many patients with borderline PD are prescribed multiple psychotropic medications.16,17 This approach leads to greater expense and more adverse effects, and is not evidence-based.
Avoid benzodiazepines. Many patients with borderline PD are prescribed benzodiazepines, often as part of a polypharmacy regimen. These drugs can cause disinhibition, thereby increasing acting-out behaviors and self-harm.35 Also, patients with PDs often have SUDs, which is a contraindication for benzodiazepine use.
Rate the patient’s improvement. Both the patient and clinician can benefit from monitoring symptomatic improvement. Several validated scales can be used to rate depression, anxiety, impulsivity, mood lability, anger, and aggression (Table 436-41).Some validated scales for borderline PD align with DSM-5 criteria. Two such widely used instruments are the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD)42 and the self-rated Borderline Evaluation of Severity Over Time (BEST).43 Each has questions that could be pulled to rate a symptom dimension of interest, such as affective instability, anger dyscontrol, or abandonment fears (Table 542,43).
A visual analog scale is easy to use and can target symptom dimensions of interest.44 For example, a clinician could use a visual analog scale to rate mood instability by asking a patient to rate their mood severity by making a mark along a 10-cm line (0 = “Most erratic emotions I have experienced,” 10 = “Most stable I have ever experienced my emotions to be”). This score can be recorded at baseline and subsequent visits.
Take-home points
PDs are common in the general population and health care settings. They are underrecognized by the general public and mental health professionals, often because of stigma. Clinicians could boost their recognition of these disorders by embedding simple screening questions in their patient assessments. Many patients with PDs will be interested in pharmacotherapy for their disorder or symptoms. Treatment strategies include targeting the comorbid disorder(s), targeting important PD symptom dimensions, choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself. Each strategy has its limitations and varying degrees of empirical support. Treatment response can be monitored using validated scales or a visual analog scale.
Continue to: Bottom Line
Bottom Line
Although psychotherapy is the first-line treatment and no medications are FDAapproved for treating personality disorders (PDs), there has been growing interest in using psychotropic medication to treat PDs. Strategies for pharmacotherapy include targeting comorbid disorders, PD symptom dimensions, or the PD itself. Choice of medication can be based on the similarity of the PD with another disorder known to respond to medication.
Related Resources
- Correa Da Costa S, Sanches M, Soares JC. Bipolar disorder or borderline personality disorder? Current Psychiatry. 2019;18(11):26-29,35-39.
- Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
Drug Brand Names
Aripiprazole • Abilify
Fluvoxamine • Luvox
Lamotrigine • Lamictal
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
DSM-5 defines personality disorders (PDs) as the presence of an enduring pattern of inner experience and behavior that “deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adulthood, is stable over time, and leads to distress or impairment.”1 As a general rule, PDs are not limited to episodes of illness, but reflect an individual’s long-term adjustment. These disorders occur in 10% to 15% of the general population; the rates are especially high in health care settings, in criminal offenders, and in those with a substance use disorder (SUD).2 PDs nearly always have an onset in adolescence or early adulthood and tend to diminish in severity with advancing age. They are associated with high rates of unemployment, homelessness, divorce and separation, domestic violence, substance misuse, and suicide.3
Psychotherapy is the first-line treatment for PDs, but there has been growing interest in using pharmacotherapy to treat PDs. While much of the PD treatment literature focuses on borderline PD,4-9 this article describes diagnosis, potential pharmacotherapy strategies, and methods to assess response to treatment for patients with all types of PDs.
Recognizing and diagnosing personality disorders
The diagnosis of a PD requires an understanding of DSM-5 criteria combined with a comprehensive psychiatric history and mental status examination. The patient’s history is the most important basis for diagnosing a PD.2 Collateral information from relatives or friends can help confirm the severity and pervasiveness of the individual’s personality problems. In some patients, long-term observation might be necessary to confirm the presence of a PD. Some clinicians are reluctant to diagnose PDs because of stigma, a problem common among patients with borderline PD.10,11
To screen for PDs, a clinician might ask the patient about problems with interpersonal relationships, sense of self, work, affect, impulse control, and reality testing. Table 112 lists general screening questions for the presence of a PD from the Iowa Personality Disorders Screen. Structured diagnostic interviews and self-report assessments could boost recognition of PDs, but these tools are rarely used outside of research settings.13,14
The PD clusters
DSM-5 divides 10 PDs into 3 clusters based on shared phenomenology and diagnostic criteria. Few patients have a “pure” case in which they meet criteria for only a single personality disorder.1
Cluster A. “Eccentric cluster” disorders are united by social aversion, a failure to form close attachments, or paranoia and suspiciousness.15 These include paranoid, schizoid, and schizotypal PD. Low self-awareness is typical. There are no treatment guidelines for these disorders, although there is some clinical trial data for schizotypal PD.
Cluster B. “Dramatic cluster” disorders share dramatic, emotional, and erratic characteristics.14 These include narcissistic, antisocial, borderline, and histrionic PD. Antisocial and narcissistic patients have low self-awareness. There are treatment guidelines for antisocial and borderline PD, and a variety of clinical trial data is available for the latter.15
Continue to: Cluster C
Cluster C. “Anxious cluster” disorders are united by anxiousness, fearfulness, and poor self-esteem. Many of these patients also display interpersonal rigidity.15 These disorders include avoidant, dependent, and obsessive-compulsive PD. There are no treatment guidelines or clinical trial data for these disorders.
Why consider pharmacotherapy for personality disorders?
The consensus among experts is that psychotherapy is the treatment of choice for PDs.15 Despite significant gaps in the evidence base, there has been a growing interest in using psychotropic medication to treat PDs. For example, research shows that >90% of patients with borderline PD are prescribed medication, most typically antidepressants, antipsychotics, mood stabilizers, stimulants, or sedative-hypnotics.16,17
Increased interest in pharmacotherapy for PDs could be related to research showing the importance of underlying neurobiology, particularly for antisocial and borderline PD.18,19 This work is complemented by genetic research showing the heritability of PD traits and disorders.20,21 Another factor could be renewed interest in dimensional approaches to the classification of PDs, as exemplified by DSM-5’s alternative model for PDs.1 This approach aligns with some expert recommendations to focus on treating PD symptom dimensions, rather than the syndrome itself.22
Importantly, no psychotropic medication is FDA-approved for the treatment of any PD. For that reason, prescribing medication for a PD is “off-label,” although prescribing a medication for a comorbid disorder for which the drug has an FDA-approved indication is not (eg, prescribing an antidepressant for major depressive disorder [MDD]).
Principles for prescribing
Despite gaps in research data, general principles for using medication to treat PDs have emerged from treatment guidelines for antisocial and borderline PD, clinical trial data, reviews and meta-analyses, and expert opinion. Clinicians should address the following considerations before prescribing medication to a patient with a PD.
Continue to: PD diagnosis
PD diagnosis. Has the patient been properly assessed and diagnosed? While history is the most important basis for diagnosis, the clinician should be familiar with the PDs and DSM-5 criteria. Has the patient been informed of the diagnosis and its implications for treatment?
Patient interest in medication. Is the patient interested in taking medication? Patients with borderline PD are often prescribed medication, but there are sparse data for the other PDs. The patient might have little interest in the PD diagnosis or its treatment.
Comorbidity. Has the patient been assessed for comorbid psychiatric disorders that could interfere with medication use (ie, an SUD) or might be a focus of treatment (eg, MDD)? Patients with PDs typically have significant comorbidity that a thorough evaluation will uncover.
PD symptom dimensions. Has the patient been assessed to determine cognitive or behavioral symptom dimensions of their PD? One or more symptom dimension(s) could be the focus of treatment. Table 2 lists examples of PD symptom dimensions.
Strategies to guide prescribing
Strategies to help guide prescribing include targeting any comorbid disorder(s), targeting important PD symptom dimensions (eg, impulsive aggression), choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself.
Continue to: Targeting comorbid disorders
Targeting comorbid disorders. National Institute for Health and Care Excellence guidelines for antisocial and borderline PD recommend that clinicians focus on treating comorbid disorders, a position echoed in Cochrane and other reviews.4,9,22-26 For example, a patient with borderline PD experiencing a major depressive episode could be treated with an antidepressant. Targeting the depressive symptoms could boost the patient’s mood, perhaps lessening the individual’s PD symptoms or reducing their severity.
Targeting important symptom dimensions. For patients with borderline PD, several guidelines and reviews have suggested that treatment should focus on emotional dysregulation and impulsive aggression (mood stabilizers, antipsychotics), or cognitive-perceptual symptoms (antipsychotics).4-6,15 There is some evidence that mood stabilizers or second-generation antipsychotics could help reduce impulsive aggression in patients with antisocial PD.27
Choosing medication based on similarity to another disorder known to respond to medication. Avoidant PD overlaps with social anxiety disorder and can be conceptualized as a chronic, pervasive social phobia. Avoidant PD might respond to a medication known to be effective for treating social anxiety disorder, such as a selective serotonin reuptake inhibitor (SSRI) or venlafaxine.28 Treating obsessive-compulsive PD with an SSRI is another example of this strategy, as 1 small study of fluvoxamine suggests.29 Obsessive-compulsive PD is common in persons with obsessive-compulsive disorder, and overlap includes preoccupation with orders, rules, and lists, and an inability to throw things out.
Targeting the PD syndrome. Another strategy is to target the PD itself. Clinical trial data suggest the antipsychotic risperidone can reduce the symptoms of schizotypal PD.30 Considering that this PD has a genetic association with schizophrenia, it is not surprising that the patient’s ideas of reference, odd communication, or transient paranoia might respond to an antipsychotic. Data from randomized controlled trials (RCTs) support the use of the second-generation antipsychotics aripiprazole and quetiapine to treat BPD.31,32 While older guidelines4,5 supported the use of the mood stabilizer lamotrigine, a recent RCT found that it was no more effective than placebo for borderline PD or its symptom dimensions.33
What to do before prescribing
Before writing a prescription, the clinician and patient should discuss the presence of a PD and the desirability of treatment. The patient should understand the limited evidence base and know that medication prescribed for a PD is off-label. The clinician should discuss medication selection and its rationale, and whether the medication is targeting a comorbid disorder, symptom dimension(s), or the PD itself. Additional considerations for prescribing for patients with PDs are listed in Table 3.34

Continue to: Avoid polypharmacy
Avoid polypharmacy. Many patients with borderline PD are prescribed multiple psychotropic medications.16,17 This approach leads to greater expense and more adverse effects, and is not evidence-based.
Avoid benzodiazepines. Many patients with borderline PD are prescribed benzodiazepines, often as part of a polypharmacy regimen. These drugs can cause disinhibition, thereby increasing acting-out behaviors and self-harm.35 Also, patients with PDs often have SUDs, which is a contraindication for benzodiazepine use.
Rate the patient’s improvement. Both the patient and clinician can benefit from monitoring symptomatic improvement. Several validated scales can be used to rate depression, anxiety, impulsivity, mood lability, anger, and aggression (Table 436-41).Some validated scales for borderline PD align with DSM-5 criteria. Two such widely used instruments are the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD)42 and the self-rated Borderline Evaluation of Severity Over Time (BEST).43 Each has questions that could be pulled to rate a symptom dimension of interest, such as affective instability, anger dyscontrol, or abandonment fears (Table 542,43).
A visual analog scale is easy to use and can target symptom dimensions of interest.44 For example, a clinician could use a visual analog scale to rate mood instability by asking a patient to rate their mood severity by making a mark along a 10-cm line (0 = “Most erratic emotions I have experienced,” 10 = “Most stable I have ever experienced my emotions to be”). This score can be recorded at baseline and subsequent visits.
Take-home points
PDs are common in the general population and health care settings. They are underrecognized by the general public and mental health professionals, often because of stigma. Clinicians could boost their recognition of these disorders by embedding simple screening questions in their patient assessments. Many patients with PDs will be interested in pharmacotherapy for their disorder or symptoms. Treatment strategies include targeting the comorbid disorder(s), targeting important PD symptom dimensions, choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself. Each strategy has its limitations and varying degrees of empirical support. Treatment response can be monitored using validated scales or a visual analog scale.
Continue to: Bottom Line
Bottom Line
Although psychotherapy is the first-line treatment and no medications are FDAapproved for treating personality disorders (PDs), there has been growing interest in using psychotropic medication to treat PDs. Strategies for pharmacotherapy include targeting comorbid disorders, PD symptom dimensions, or the PD itself. Choice of medication can be based on the similarity of the PD with another disorder known to respond to medication.
Related Resources
- Correa Da Costa S, Sanches M, Soares JC. Bipolar disorder or borderline personality disorder? Current Psychiatry. 2019;18(11):26-29,35-39.
- Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
Drug Brand Names
Aripiprazole • Abilify
Fluvoxamine • Luvox
Lamotrigine • Lamictal
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Black DW, Andreasen N. Personality disorders. In: Black DW, Andreasen N. Introductory textbook of psychiatry, 7th edition. American Psychiatric Publishing; 2020:410-423.
3. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord 2004;18(3):226-239.
4. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
5. Vita A, De Peri L, Sacchetti E. Antipsychotics, antidepressants, anticonvulsants, and placebo on the symptom dimensions of borderline personality disorder – a meta-analysis of randomized controlled and open-label trials. J Clin Psychopharmacol. 2011;31(5):613-624.
6. Stoffers JM, Lieb K. Pharmacotherapy for borderline personality disorder – current evidence and recent trends. Curr Psychiatry Rep. 2015;17(1):534.
7. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31(5):345-356.
8. Black DW, Paris J, Schulz SC. Personality disorders: evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M, ed. Cognitive behavioral psychopharmacology: the clinical practice of evidence-based biopsychosocial integration. John Wiley & Sons; 2018:137-165.
9. Stoffers-Winterling J, Sorebø OJ, Lieb K. Pharmacotherapy for borderline personality disorder: an update of published, unpublished and ongoing studies. Curr Psychiatry Rep. 2020;22(8):37.
10. Lewis G, Appleby L. Personality disorder: the patients psychiatrists dislike. Br J Psychiatry. 1988;153:44-49.
11. Black DW, Pfohl B, Blum N, et al. Attitudes toward borderline personality disorder: a survey of 706 mental health clinicians. CNS Spectr. 2011;16(3):67-74.
12. Langbehn DR, Pfohl BM, Reynolds S, et al. The Iowa Personality Disorder Screen: development and preliminary validation of a brief screening interview. J Pers Disord. 1999;13(1):75-89.
13. Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV). American Psychiatric Press; 1997.
14. First MB, Spitzer RL, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part II: multisite test-retest reliability study. J Pers Disord. 1995;9(2):92-104.
15. Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
16. Zanarini MC, Frankenburg FR, Reich DB, et al. Treatment rates for patients with borderline personality disorder and other personality disorders: a 16-year study. Psychiatr Serv. 2015;66(1):15-20.
17. Black DW, Allen J, McCormick B, et al. Treatment received by persons with BPD participating in a randomized clinical trial of the Systems Training for Emotional Predictability and Problem Solving programme. Person Ment Health. 2011;5(3):159-168.
18. Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. Behav Sci Law. 2008;26(1):65-83.
19. Ruocco AC, Amirthavasagam S, Choi-Kain LW, et al. Neural correlates of negative emotionality in BPD: an activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73(2):153-160.
20. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831.
21. Slutske WS. The genetics of antisocial behavior. Curr Psychiatry Rep. 2001;3(2):158-162.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
23. National Institute for Health and Care Excellence (NICE). Borderline personality disorder: recognition and management. Clinical guideline [CG78]. Published January 2009. https://www.nice.org.uk/guidance/cg78
24. National Institute for Health and Care Excellence (NICE). Antisocial personality disorder: prevention and management. Clinical guideline [CG77]. Published January 2009. Updated March 27, 2013. https://www.nice.org.uk/guidance/cg77
25. Khalifa N, Duggan C, Stoffers J, et al. Pharmacologic interventions for antisocial personality disorder. Cochrane Database Syst Rep. 2010;(8):CD007667.
26. Stoffers JM, Völlm BA, Rücker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;2012(8):CD005652.
27. Black DW. The treatment of antisocial personality disorder. Current Treatment Options in Psychiatry. 2017. https://doi.org/10.1007/s40501-017-0123-z
28. Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280(8):708-713.
29. Ansseau M. The obsessive-compulsive personality: diagnostic aspects and treatment possibilities. In: Den Boer JA, Westenberg HGM, eds. Focus on obsessive-compulsive spectrum disorders. Syn-Thesis; 1997:61-73.
30. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64(6):628-634.
31. Black DW, Zanarini MC, Romine A, et al. Comparison of low and moderate dosages of extended-release quetiapine in borderline personality disorder: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2014;171(11):1174-1182.
32. Nickel MK, Muelbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2006;163(5):833-838.
33. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764.
34. Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry. 2004;65(12)1660-1665.
35. Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder. Alprazolam, carbamazepine, trifluoperazine, and tranylcypromine. Arch Gen Psychiatry. 1988;45(2):111-119.
36. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812.
37. Ratey JJ, Gutheil CM. The measurement of aggressive behavior: reflections on the use of the Overt Aggression Scale and the Modified Overt Aggression Scale. J Neuropsychiatr Clin Neurosci. 1991;3(2):S57-S60.
38. Spielberger CD, Sydeman SJ, Owen AE, et al. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In: Maruish ME, ed. The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers; 1999:993-1021.
39. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. Psychological Corp; 1996.
40. Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule – Expanded Form. The University of Iowa; 1999.
41. Harvey D, Greenberg BR, Serper MR, et al. The affective lability scales: development, reliability, and validity. J Clin Psychol. 1989;45(5):786-793.
42. Zanarini MC, Vujanovic AA, Parachini EA, et al. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. J Person Disord. 2003:17(3):233-242.
43. Pfohl B, Blum N, St John D, et al. Reliability and validity of the Borderline Evaluation of Severity Over Time (BEST): a new scale to measure severity and change in borderline personality disorder. J Person Disord. 2009;23(3):281-293.
44. Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiatr Res. 1997;31(5):569-579.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Black DW, Andreasen N. Personality disorders. In: Black DW, Andreasen N. Introductory textbook of psychiatry, 7th edition. American Psychiatric Publishing; 2020:410-423.
3. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord 2004;18(3):226-239.
4. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
5. Vita A, De Peri L, Sacchetti E. Antipsychotics, antidepressants, anticonvulsants, and placebo on the symptom dimensions of borderline personality disorder – a meta-analysis of randomized controlled and open-label trials. J Clin Psychopharmacol. 2011;31(5):613-624.
6. Stoffers JM, Lieb K. Pharmacotherapy for borderline personality disorder – current evidence and recent trends. Curr Psychiatry Rep. 2015;17(1):534.
7. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31(5):345-356.
8. Black DW, Paris J, Schulz SC. Personality disorders: evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M, ed. Cognitive behavioral psychopharmacology: the clinical practice of evidence-based biopsychosocial integration. John Wiley & Sons; 2018:137-165.
9. Stoffers-Winterling J, Sorebø OJ, Lieb K. Pharmacotherapy for borderline personality disorder: an update of published, unpublished and ongoing studies. Curr Psychiatry Rep. 2020;22(8):37.
10. Lewis G, Appleby L. Personality disorder: the patients psychiatrists dislike. Br J Psychiatry. 1988;153:44-49.
11. Black DW, Pfohl B, Blum N, et al. Attitudes toward borderline personality disorder: a survey of 706 mental health clinicians. CNS Spectr. 2011;16(3):67-74.
12. Langbehn DR, Pfohl BM, Reynolds S, et al. The Iowa Personality Disorder Screen: development and preliminary validation of a brief screening interview. J Pers Disord. 1999;13(1):75-89.
13. Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV). American Psychiatric Press; 1997.
14. First MB, Spitzer RL, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part II: multisite test-retest reliability study. J Pers Disord. 1995;9(2):92-104.
15. Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
16. Zanarini MC, Frankenburg FR, Reich DB, et al. Treatment rates for patients with borderline personality disorder and other personality disorders: a 16-year study. Psychiatr Serv. 2015;66(1):15-20.
17. Black DW, Allen J, McCormick B, et al. Treatment received by persons with BPD participating in a randomized clinical trial of the Systems Training for Emotional Predictability and Problem Solving programme. Person Ment Health. 2011;5(3):159-168.
18. Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. Behav Sci Law. 2008;26(1):65-83.
19. Ruocco AC, Amirthavasagam S, Choi-Kain LW, et al. Neural correlates of negative emotionality in BPD: an activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73(2):153-160.
20. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831.
21. Slutske WS. The genetics of antisocial behavior. Curr Psychiatry Rep. 2001;3(2):158-162.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
23. National Institute for Health and Care Excellence (NICE). Borderline personality disorder: recognition and management. Clinical guideline [CG78]. Published January 2009. https://www.nice.org.uk/guidance/cg78
24. National Institute for Health and Care Excellence (NICE). Antisocial personality disorder: prevention and management. Clinical guideline [CG77]. Published January 2009. Updated March 27, 2013. https://www.nice.org.uk/guidance/cg77
25. Khalifa N, Duggan C, Stoffers J, et al. Pharmacologic interventions for antisocial personality disorder. Cochrane Database Syst Rep. 2010;(8):CD007667.
26. Stoffers JM, Völlm BA, Rücker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;2012(8):CD005652.
27. Black DW. The treatment of antisocial personality disorder. Current Treatment Options in Psychiatry. 2017. https://doi.org/10.1007/s40501-017-0123-z
28. Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280(8):708-713.
29. Ansseau M. The obsessive-compulsive personality: diagnostic aspects and treatment possibilities. In: Den Boer JA, Westenberg HGM, eds. Focus on obsessive-compulsive spectrum disorders. Syn-Thesis; 1997:61-73.
30. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64(6):628-634.
31. Black DW, Zanarini MC, Romine A, et al. Comparison of low and moderate dosages of extended-release quetiapine in borderline personality disorder: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2014;171(11):1174-1182.
32. Nickel MK, Muelbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2006;163(5):833-838.
33. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764.
34. Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry. 2004;65(12)1660-1665.
35. Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder. Alprazolam, carbamazepine, trifluoperazine, and tranylcypromine. Arch Gen Psychiatry. 1988;45(2):111-119.
36. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812.
37. Ratey JJ, Gutheil CM. The measurement of aggressive behavior: reflections on the use of the Overt Aggression Scale and the Modified Overt Aggression Scale. J Neuropsychiatr Clin Neurosci. 1991;3(2):S57-S60.
38. Spielberger CD, Sydeman SJ, Owen AE, et al. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In: Maruish ME, ed. The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers; 1999:993-1021.
39. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. Psychological Corp; 1996.
40. Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule – Expanded Form. The University of Iowa; 1999.
41. Harvey D, Greenberg BR, Serper MR, et al. The affective lability scales: development, reliability, and validity. J Clin Psychol. 1989;45(5):786-793.
42. Zanarini MC, Vujanovic AA, Parachini EA, et al. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. J Person Disord. 2003:17(3):233-242.
43. Pfohl B, Blum N, St John D, et al. Reliability and validity of the Borderline Evaluation of Severity Over Time (BEST): a new scale to measure severity and change in borderline personality disorder. J Person Disord. 2009;23(3):281-293.
44. Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiatr Res. 1997;31(5):569-579.
Antidepressants, TMS, and the risk of affective switch in bipolar depression
Because treatment resistance is a pervasive problem in bipolar depression, the use of neuromodulation treatments such as transcranial magnetic stimulation (TMS) is increasing for patients with this disorder.1-7 Patients with bipolar disorder tend to spend the majority of the time with depressive symptoms, which underscores the importance of providing effective treatment for bipolar depression, especially given the chronicity of this disease.2,3,5 Only a few medications are FDA-approved for treating bipolar depression (Table).
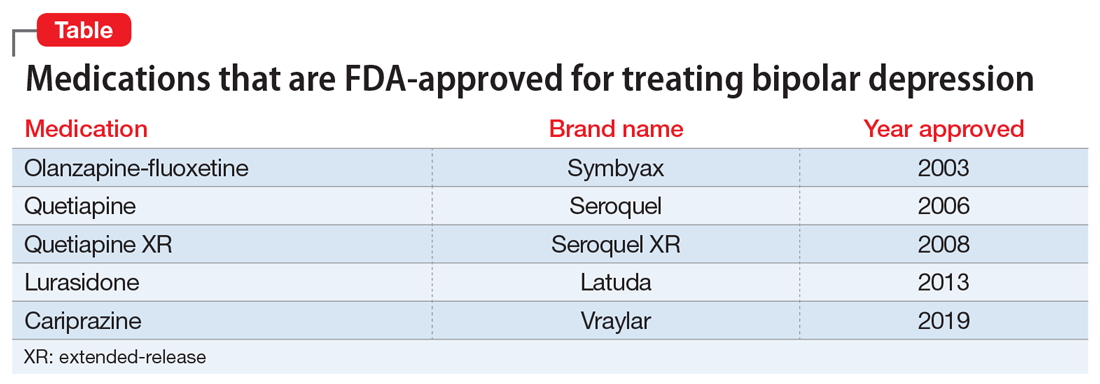
In this article, we describe the case of a patient with treatment-resistant bipolar depression undergoing adjunctive TMS treatment who experienced an affective switch from depression to mania. We also discuss evidence regarding the likelihood of treatment-emergent mania for antidepressants vs TMS in bipolar depression.
CASE
Ms. W, a 60-year-old White female with a history of bipolar I disorder and attention-deficit/hyperactivity disorder (ADHD), presented for TMS evaluation during a depressive episode. Throughout her life, she had experienced numerous manic episodes, but as she got older she noted an increasing frequency of depressive episodes. Over the course of her illness, she had completed adequate trials at therapeutic doses of many medications, including second-generation antipsychotics (SGAs) (aripiprazole, lurasidone, olanzapine, quetiapine), mood stabilizers (lamotrigine, lithium), and antidepressants (bupropion, venlafaxine, fluoxetine, mirtazapine, trazodone). A course of electroconvulsive therapy was not effective. Ms. W had a long-standing diagnosis of ADHD and had been treated with stimulants for >10 years, although it was unclear whether formal neuropsychological testing had been conducted to confirm this diagnosis. She had >10 suicide attempts and multiple psychiatric hospitalizations.
At her initial evaluation for TMS, Ms. W said she had depressive symptoms predominating for the past 2 years, including low mood, hopelessness, poor sleep, poor appetite, anhedonia, and suicidal ideation without a plan. At the time, she was taking clonazepam, 0.5 mg twice a day; lurasidone, 40 mg/d at bedtime; fluoxetine, 60 mg/d; trazodone, 50 mg/d at bedtime; and methylphenidate, 40 mg/d, and was participating in psychotherapy consistently.
After Ms. W and her clinicians discussed alternatives, risks, benefits, and adverse effects, she consented to adjunctive TMS treatment and provided written informed consent. The treatment plan was outlined as 6 weeks of daily TMS therapy (NeuroStar; Neuronetics, Malvern, PA), 1 treatment per day, 5 days a week. Her clinical status was assessed weekly using the Quick Inventory of Depressive Symptomatology (QIDS) for depression, Generalized Anxiety Disorder 7-item scale (GAD-7) for anxiety, and Young Mania Rating Scale (YMRS) for mania. The Figure shows the trends in Ms. W’s QIDS, GAD-7, and YMRS scores over the course of TMS treatment.
Prior to initiating TMS, her baseline scores were QIDS: 25, GAD-7: 9, and YMRS: 7, indicating very severe depression, mild anxiety, and the absence of mania. Ms. W’s psychotropic regimen remained unchanged throughout the course of her TMS treatment. After her motor threshold was determined, her TMS treatment began at 80% of motor threshold and was titrated up to 95% at the first treatment. By the second treatment, it was titrated up to 110%. By the third treatment, it was titrated up to 120% of motor threshold, which is the percentage used for the remaining treatments.
Initially, Ms. W reported some improvement in her depression, but this improvement was short-lived, and she continued to have elevated QIDS scores throughout treatment. By treatment #21, her QIDS and GAD-7 scores remained elevated, and her YMRS score had increased to 12. Due to this increase in YMRS score, the YMRS was repeated on the next 2 treatment days (#22 and #23), and her score was 6 on both days. When Ms. W presented for treatment #25, she was disorganized, irritable, and endorsed racing thoughts and decreased sleep. She was involuntarily hospitalized for mania, and TMS was discontinued. Unfortunately, she did not complete any clinical scales on that day. Upon admission to the hospital, Ms. W reported that at approximately the time of treatment #21, she had a fluctuation in her mood that consisted of increased goal-directed activity, decreased need for sleep, racing thoughts, and increased frivolous spending. She was treated with lithium, 300 mg twice a day. Lurasidone was increased to 80 mg/d at bedtime, and she continued clonazepam, trazodone, and methylphenidate at the previous doses. Over 14 days, Ms. W’s mania gradually resolved, and she was discharged home.
Continue to: Mixed evidence on the risk of switching
Mixed evidence on the risk of switching
Currently, several TMS devices are FDA-cleared for treating unipolar major depressive disorder, obsessive-compulsive disorder, and certain types of migraine. In March 2020, the FDA granted Breakthrough Device Designation for one TMS device, the NeuroStar Advanced Therapy System, for the treatment of bipolar depression.8 This designation created an expedited pathway for prioritized FDA review of the NeuroStar Advanced Therapy clinical trial program.
Few published clinical studies have evaluated using TMS to treat patients with bipolar depression.9-15 As with any antidepressant treatment for bipolar depression, there is a risk of affective switch from depression to mania when using TMS. Most of the literature available regarding the treatment of bipolar depression focuses on the risk of antidepressant medications to induce an affective switch. This risk depends on the class of the antidepressant,16 and there is a paucity of studies examining the risk of switch with TMS.
Interpretation of available literature is limited due to inconsistencies in the definition of an affective switch, the variable length of treatment with antidepressants, the use of concurrent medications such as mood stabilizers, and confounders such as the natural course of switching in bipolar disorder.17 Overall, the evidence for treatment-emergent mania related to antidepressant use is mixed, and the reported rate of treatment-emergent mania varies. In a systematic review and meta-analysis of >20 randomized controlled trials that included 1,316 patients with bipolar disorder who received antidepressants, Fornaro et al18 found that the incidence of treatment-emergent mania was 11.8%. It is generally recommended that if antidepressants are used to treat patients with bipolar disorder, they should be given with a traditional mood stabilizer to prevent affective switches, although whether mood stabilizers can prevent such switches is unproven.19
In a literature review by Xia et al,20 the affective switch rate in patients with bipolar depression who were treated with TMS was 3.1%, which was not statistically different from the affective switch rate with sham treatment.However, most of the patients included in this analysis were receiving other medications concurrently, and the length of treatment was 2 weeks, which is shorter than the average length of TMS treatment in clinical practice. In a recent literature review by Rachid,21 TMS was found to possibly induce manic episodes when used as monotherapy or in combination with antidepressants in patients with bipolar depression. To reduce the risk of treatment-emergent mania, current recommendations advise the use of a mood stabilizer for a minimum of 2 weeks before initiating TMS.1
In our case, Ms. W was receiving antidepressants (fluoxetine and trazodone), lurasidone (an SGA that is FDA-approved for bipolar depression), and methylphenidate before starting TMS treatment. Fluoxetine, trazodone, and methylphenidate may possibly contribute to an increased risk of an affective switch.1,22 Further studies are needed to clarify whether mood stabilizers or SGAs can prevent the development of mania in patients with bipolar depression who undergo TMS treatment.20
Continue to: Because bipolar depression poses...
Because bipolar depression poses a major clinical challenge,23,24 it is imperative to consider alternate treatments. When evaluating alternative treatment strategies, one may consider TMS in conjunction with a traditional mood stabilizer because this regimen may have a lower risk of treatment-emergent mania compared with antidepressants.1,25
Acknowledgment
The authors thank Dr. Sy Saeed for his expertise and guidance on this article.
Bottom Line
For patients with bipolar depression, treatment with transcranial magnetic stimulation in conjunction with a mood stabilizer may have lower rates of treatment-emergent mania than treatment with antidepressants.
Related Resources
- Transcranial magnetic stimulation: clinical applications for psychiatric practice. Bermudes RA, Lanocha K, Janicak PG, eds. American Psychiatric Association Publishing; 2017.
- Gold AK, Ornelas AC, Cirillo P, et al. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav. 2019;9(10):e01419. doi: 10.1002/brb3.1419
Drug Brand Names
Aripiprazole • Abilify
Bupropion • Wellbutrin
Cariprazine • Vraylar
Clonazepam • Klonopin
Fluoxetine • Prozac
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin, Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Olanzapine-fluoxetine • Symbyax
Quetiapine • Seroquel
Trazodone • Desyrel
Venlafaxine • Effexor
1. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes RA, Lanocha K, Janicak PG, eds. Transcranial magnetic stimulation: clinical applications for psychiatric practice. American Psychiatric Association Publishing; 2017:127-156.
2. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
3. Gitlin M. Treatment-resistant bipolar disorder. Molecular Psychiatry. 2006;11(3):227-240.
4. Harrison PJ, Geddes JR, Tunbridge EM. The emerging neurobiology of bipolar disorder. Trends Neurosci. 2018;41(1):18-30.
5. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68(3):241-251.
6. Myczkowski ML, Fernandes A, Moreno M, et al. Cognitive outcomes of TMS treatment in bipolar depression: safety data from a randomized controlled trial. J Affect Disord. 2018;235: 20-26.
7. Tavares DF, Myczkowski ML, Alberto RL, et al. Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42(13):2593-2601.
8. Neuronetics. FDA grants NeuroStar® Advanced Therapy System Breakthrough Device Designation to treat bipolar depression. Accessed February 2, 2021. https://www.globenewswire.com/news-release/2020/03/06/1996447/0/en/FDA-Grants-NeuroStar-Advanced-Therapy-System-Breakthrough-Device-Designation-to-Treat-Bipolar-Depression.html
9. Cohen RB, Brunoni AR, Boggio PS, et al. Clinical predictors associated with duration of repetitive transcranial magnetic stimulation treatment for remission in bipolar depression: a naturalistic study. J Nerv Ment Dis. 2010;198(9):679-681.
10. Connolly KR, Helmer A, Cristancho MA, et al. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73(4):e567-e573.
11. Dell’osso B, D’Urso N, Castellano F, et al. Long-term efficacy after acute augmentative repetitive transcranial magnetic stimulation in bipolar depression: a 1-year follow-up study. J ECT. 2011;27(2):141-144.
12. Dell’Osso B, Mundo E, D’Urso N, et al. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disord. 2009;11(1):76-81.
13. Harel EV, Zangen A, Roth Y, et al. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12(2):119-126.
14. Nahas Z, Kozel FA, Li X, et al. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord. 2003;5(1):40-47.
15. Tamas RL, Menkes D, El-Mallakh RS. Stimulating research: a prospective, randomized, double-blind, sham-controlled study of slow transcranial magnetic stimulation in depressed bipolar patients. J Neuropsychiatry Clin Neurosci. 2007;19(2):198-199.
16. Tundo A, Cavalieri P, Navari S, et al. Treating bipolar depression - antidepressants and alternatives: a critical review of the literature. Acta Neuropsychiatrica. 2011:23(3):94-105.
17. Gijsman HJ, Geddes JR, Rendell JM, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry. 2004;161(9):1537-1547.
18. Fornaro M, Anastasia A, Novello S, et al. Incidence, prevalence and clinical correlates of antidepressant‐emergent mania in bipolar depression: a systematic review and meta‐analysis. Bipolar Disord. 2018;20(3):195-227.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
20. Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119-130.
21. Rachid F. Repetitive transcranial magnetic stimulation and treatment-emergent mania and hypomania: a review of the literature. J Psychiatr Pract. 2017;23(2):150-159.
22. Victorin A, Rydén E, Thase M, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
23. Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214(1):27-35.
24. Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1.
25. Phillips AL, Burr RL, Dunner DL. Repetitive transcranial magnetic stimulation in the treatment of bipolar depression: Experience from a clinical setting. J Psychiatr Pract. 2020;26(1):37-45.
Because treatment resistance is a pervasive problem in bipolar depression, the use of neuromodulation treatments such as transcranial magnetic stimulation (TMS) is increasing for patients with this disorder.1-7 Patients with bipolar disorder tend to spend the majority of the time with depressive symptoms, which underscores the importance of providing effective treatment for bipolar depression, especially given the chronicity of this disease.2,3,5 Only a few medications are FDA-approved for treating bipolar depression (Table).

In this article, we describe the case of a patient with treatment-resistant bipolar depression undergoing adjunctive TMS treatment who experienced an affective switch from depression to mania. We also discuss evidence regarding the likelihood of treatment-emergent mania for antidepressants vs TMS in bipolar depression.
CASE
Ms. W, a 60-year-old White female with a history of bipolar I disorder and attention-deficit/hyperactivity disorder (ADHD), presented for TMS evaluation during a depressive episode. Throughout her life, she had experienced numerous manic episodes, but as she got older she noted an increasing frequency of depressive episodes. Over the course of her illness, she had completed adequate trials at therapeutic doses of many medications, including second-generation antipsychotics (SGAs) (aripiprazole, lurasidone, olanzapine, quetiapine), mood stabilizers (lamotrigine, lithium), and antidepressants (bupropion, venlafaxine, fluoxetine, mirtazapine, trazodone). A course of electroconvulsive therapy was not effective. Ms. W had a long-standing diagnosis of ADHD and had been treated with stimulants for >10 years, although it was unclear whether formal neuropsychological testing had been conducted to confirm this diagnosis. She had >10 suicide attempts and multiple psychiatric hospitalizations.
At her initial evaluation for TMS, Ms. W said she had depressive symptoms predominating for the past 2 years, including low mood, hopelessness, poor sleep, poor appetite, anhedonia, and suicidal ideation without a plan. At the time, she was taking clonazepam, 0.5 mg twice a day; lurasidone, 40 mg/d at bedtime; fluoxetine, 60 mg/d; trazodone, 50 mg/d at bedtime; and methylphenidate, 40 mg/d, and was participating in psychotherapy consistently.
After Ms. W and her clinicians discussed alternatives, risks, benefits, and adverse effects, she consented to adjunctive TMS treatment and provided written informed consent. The treatment plan was outlined as 6 weeks of daily TMS therapy (NeuroStar; Neuronetics, Malvern, PA), 1 treatment per day, 5 days a week. Her clinical status was assessed weekly using the Quick Inventory of Depressive Symptomatology (QIDS) for depression, Generalized Anxiety Disorder 7-item scale (GAD-7) for anxiety, and Young Mania Rating Scale (YMRS) for mania. The Figure shows the trends in Ms. W’s QIDS, GAD-7, and YMRS scores over the course of TMS treatment.

Prior to initiating TMS, her baseline scores were QIDS: 25, GAD-7: 9, and YMRS: 7, indicating very severe depression, mild anxiety, and the absence of mania. Ms. W’s psychotropic regimen remained unchanged throughout the course of her TMS treatment. After her motor threshold was determined, her TMS treatment began at 80% of motor threshold and was titrated up to 95% at the first treatment. By the second treatment, it was titrated up to 110%. By the third treatment, it was titrated up to 120% of motor threshold, which is the percentage used for the remaining treatments.
Initially, Ms. W reported some improvement in her depression, but this improvement was short-lived, and she continued to have elevated QIDS scores throughout treatment. By treatment #21, her QIDS and GAD-7 scores remained elevated, and her YMRS score had increased to 12. Due to this increase in YMRS score, the YMRS was repeated on the next 2 treatment days (#22 and #23), and her score was 6 on both days. When Ms. W presented for treatment #25, she was disorganized, irritable, and endorsed racing thoughts and decreased sleep. She was involuntarily hospitalized for mania, and TMS was discontinued. Unfortunately, she did not complete any clinical scales on that day. Upon admission to the hospital, Ms. W reported that at approximately the time of treatment #21, she had a fluctuation in her mood that consisted of increased goal-directed activity, decreased need for sleep, racing thoughts, and increased frivolous spending. She was treated with lithium, 300 mg twice a day. Lurasidone was increased to 80 mg/d at bedtime, and she continued clonazepam, trazodone, and methylphenidate at the previous doses. Over 14 days, Ms. W’s mania gradually resolved, and she was discharged home.
Continue to: Mixed evidence on the risk of switching
Mixed evidence on the risk of switching
Currently, several TMS devices are FDA-cleared for treating unipolar major depressive disorder, obsessive-compulsive disorder, and certain types of migraine. In March 2020, the FDA granted Breakthrough Device Designation for one TMS device, the NeuroStar Advanced Therapy System, for the treatment of bipolar depression.8 This designation created an expedited pathway for prioritized FDA review of the NeuroStar Advanced Therapy clinical trial program.
Few published clinical studies have evaluated using TMS to treat patients with bipolar depression.9-15 As with any antidepressant treatment for bipolar depression, there is a risk of affective switch from depression to mania when using TMS. Most of the literature available regarding the treatment of bipolar depression focuses on the risk of antidepressant medications to induce an affective switch. This risk depends on the class of the antidepressant,16 and there is a paucity of studies examining the risk of switch with TMS.
Interpretation of available literature is limited due to inconsistencies in the definition of an affective switch, the variable length of treatment with antidepressants, the use of concurrent medications such as mood stabilizers, and confounders such as the natural course of switching in bipolar disorder.17 Overall, the evidence for treatment-emergent mania related to antidepressant use is mixed, and the reported rate of treatment-emergent mania varies. In a systematic review and meta-analysis of >20 randomized controlled trials that included 1,316 patients with bipolar disorder who received antidepressants, Fornaro et al18 found that the incidence of treatment-emergent mania was 11.8%. It is generally recommended that if antidepressants are used to treat patients with bipolar disorder, they should be given with a traditional mood stabilizer to prevent affective switches, although whether mood stabilizers can prevent such switches is unproven.19
In a literature review by Xia et al,20 the affective switch rate in patients with bipolar depression who were treated with TMS was 3.1%, which was not statistically different from the affective switch rate with sham treatment.However, most of the patients included in this analysis were receiving other medications concurrently, and the length of treatment was 2 weeks, which is shorter than the average length of TMS treatment in clinical practice. In a recent literature review by Rachid,21 TMS was found to possibly induce manic episodes when used as monotherapy or in combination with antidepressants in patients with bipolar depression. To reduce the risk of treatment-emergent mania, current recommendations advise the use of a mood stabilizer for a minimum of 2 weeks before initiating TMS.1
In our case, Ms. W was receiving antidepressants (fluoxetine and trazodone), lurasidone (an SGA that is FDA-approved for bipolar depression), and methylphenidate before starting TMS treatment. Fluoxetine, trazodone, and methylphenidate may possibly contribute to an increased risk of an affective switch.1,22 Further studies are needed to clarify whether mood stabilizers or SGAs can prevent the development of mania in patients with bipolar depression who undergo TMS treatment.20
Continue to: Because bipolar depression poses...
Because bipolar depression poses a major clinical challenge,23,24 it is imperative to consider alternate treatments. When evaluating alternative treatment strategies, one may consider TMS in conjunction with a traditional mood stabilizer because this regimen may have a lower risk of treatment-emergent mania compared with antidepressants.1,25
Acknowledgment
The authors thank Dr. Sy Saeed for his expertise and guidance on this article.
Bottom Line
For patients with bipolar depression, treatment with transcranial magnetic stimulation in conjunction with a mood stabilizer may have lower rates of treatment-emergent mania than treatment with antidepressants.
Related Resources
- Transcranial magnetic stimulation: clinical applications for psychiatric practice. Bermudes RA, Lanocha K, Janicak PG, eds. American Psychiatric Association Publishing; 2017.
- Gold AK, Ornelas AC, Cirillo P, et al. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav. 2019;9(10):e01419. doi: 10.1002/brb3.1419
Drug Brand Names
Aripiprazole • Abilify
Bupropion • Wellbutrin
Cariprazine • Vraylar
Clonazepam • Klonopin
Fluoxetine • Prozac
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin, Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Olanzapine-fluoxetine • Symbyax
Quetiapine • Seroquel
Trazodone • Desyrel
Venlafaxine • Effexor
Because treatment resistance is a pervasive problem in bipolar depression, the use of neuromodulation treatments such as transcranial magnetic stimulation (TMS) is increasing for patients with this disorder.1-7 Patients with bipolar disorder tend to spend the majority of the time with depressive symptoms, which underscores the importance of providing effective treatment for bipolar depression, especially given the chronicity of this disease.2,3,5 Only a few medications are FDA-approved for treating bipolar depression (Table).

In this article, we describe the case of a patient with treatment-resistant bipolar depression undergoing adjunctive TMS treatment who experienced an affective switch from depression to mania. We also discuss evidence regarding the likelihood of treatment-emergent mania for antidepressants vs TMS in bipolar depression.
CASE
Ms. W, a 60-year-old White female with a history of bipolar I disorder and attention-deficit/hyperactivity disorder (ADHD), presented for TMS evaluation during a depressive episode. Throughout her life, she had experienced numerous manic episodes, but as she got older she noted an increasing frequency of depressive episodes. Over the course of her illness, she had completed adequate trials at therapeutic doses of many medications, including second-generation antipsychotics (SGAs) (aripiprazole, lurasidone, olanzapine, quetiapine), mood stabilizers (lamotrigine, lithium), and antidepressants (bupropion, venlafaxine, fluoxetine, mirtazapine, trazodone). A course of electroconvulsive therapy was not effective. Ms. W had a long-standing diagnosis of ADHD and had been treated with stimulants for >10 years, although it was unclear whether formal neuropsychological testing had been conducted to confirm this diagnosis. She had >10 suicide attempts and multiple psychiatric hospitalizations.
At her initial evaluation for TMS, Ms. W said she had depressive symptoms predominating for the past 2 years, including low mood, hopelessness, poor sleep, poor appetite, anhedonia, and suicidal ideation without a plan. At the time, she was taking clonazepam, 0.5 mg twice a day; lurasidone, 40 mg/d at bedtime; fluoxetine, 60 mg/d; trazodone, 50 mg/d at bedtime; and methylphenidate, 40 mg/d, and was participating in psychotherapy consistently.
After Ms. W and her clinicians discussed alternatives, risks, benefits, and adverse effects, she consented to adjunctive TMS treatment and provided written informed consent. The treatment plan was outlined as 6 weeks of daily TMS therapy (NeuroStar; Neuronetics, Malvern, PA), 1 treatment per day, 5 days a week. Her clinical status was assessed weekly using the Quick Inventory of Depressive Symptomatology (QIDS) for depression, Generalized Anxiety Disorder 7-item scale (GAD-7) for anxiety, and Young Mania Rating Scale (YMRS) for mania. The Figure shows the trends in Ms. W’s QIDS, GAD-7, and YMRS scores over the course of TMS treatment.

Prior to initiating TMS, her baseline scores were QIDS: 25, GAD-7: 9, and YMRS: 7, indicating very severe depression, mild anxiety, and the absence of mania. Ms. W’s psychotropic regimen remained unchanged throughout the course of her TMS treatment. After her motor threshold was determined, her TMS treatment began at 80% of motor threshold and was titrated up to 95% at the first treatment. By the second treatment, it was titrated up to 110%. By the third treatment, it was titrated up to 120% of motor threshold, which is the percentage used for the remaining treatments.
Initially, Ms. W reported some improvement in her depression, but this improvement was short-lived, and she continued to have elevated QIDS scores throughout treatment. By treatment #21, her QIDS and GAD-7 scores remained elevated, and her YMRS score had increased to 12. Due to this increase in YMRS score, the YMRS was repeated on the next 2 treatment days (#22 and #23), and her score was 6 on both days. When Ms. W presented for treatment #25, she was disorganized, irritable, and endorsed racing thoughts and decreased sleep. She was involuntarily hospitalized for mania, and TMS was discontinued. Unfortunately, she did not complete any clinical scales on that day. Upon admission to the hospital, Ms. W reported that at approximately the time of treatment #21, she had a fluctuation in her mood that consisted of increased goal-directed activity, decreased need for sleep, racing thoughts, and increased frivolous spending. She was treated with lithium, 300 mg twice a day. Lurasidone was increased to 80 mg/d at bedtime, and she continued clonazepam, trazodone, and methylphenidate at the previous doses. Over 14 days, Ms. W’s mania gradually resolved, and she was discharged home.
Continue to: Mixed evidence on the risk of switching
Mixed evidence on the risk of switching
Currently, several TMS devices are FDA-cleared for treating unipolar major depressive disorder, obsessive-compulsive disorder, and certain types of migraine. In March 2020, the FDA granted Breakthrough Device Designation for one TMS device, the NeuroStar Advanced Therapy System, for the treatment of bipolar depression.8 This designation created an expedited pathway for prioritized FDA review of the NeuroStar Advanced Therapy clinical trial program.
Few published clinical studies have evaluated using TMS to treat patients with bipolar depression.9-15 As with any antidepressant treatment for bipolar depression, there is a risk of affective switch from depression to mania when using TMS. Most of the literature available regarding the treatment of bipolar depression focuses on the risk of antidepressant medications to induce an affective switch. This risk depends on the class of the antidepressant,16 and there is a paucity of studies examining the risk of switch with TMS.
Interpretation of available literature is limited due to inconsistencies in the definition of an affective switch, the variable length of treatment with antidepressants, the use of concurrent medications such as mood stabilizers, and confounders such as the natural course of switching in bipolar disorder.17 Overall, the evidence for treatment-emergent mania related to antidepressant use is mixed, and the reported rate of treatment-emergent mania varies. In a systematic review and meta-analysis of >20 randomized controlled trials that included 1,316 patients with bipolar disorder who received antidepressants, Fornaro et al18 found that the incidence of treatment-emergent mania was 11.8%. It is generally recommended that if antidepressants are used to treat patients with bipolar disorder, they should be given with a traditional mood stabilizer to prevent affective switches, although whether mood stabilizers can prevent such switches is unproven.19
In a literature review by Xia et al,20 the affective switch rate in patients with bipolar depression who were treated with TMS was 3.1%, which was not statistically different from the affective switch rate with sham treatment.However, most of the patients included in this analysis were receiving other medications concurrently, and the length of treatment was 2 weeks, which is shorter than the average length of TMS treatment in clinical practice. In a recent literature review by Rachid,21 TMS was found to possibly induce manic episodes when used as monotherapy or in combination with antidepressants in patients with bipolar depression. To reduce the risk of treatment-emergent mania, current recommendations advise the use of a mood stabilizer for a minimum of 2 weeks before initiating TMS.1
In our case, Ms. W was receiving antidepressants (fluoxetine and trazodone), lurasidone (an SGA that is FDA-approved for bipolar depression), and methylphenidate before starting TMS treatment. Fluoxetine, trazodone, and methylphenidate may possibly contribute to an increased risk of an affective switch.1,22 Further studies are needed to clarify whether mood stabilizers or SGAs can prevent the development of mania in patients with bipolar depression who undergo TMS treatment.20
Continue to: Because bipolar depression poses...
Because bipolar depression poses a major clinical challenge,23,24 it is imperative to consider alternate treatments. When evaluating alternative treatment strategies, one may consider TMS in conjunction with a traditional mood stabilizer because this regimen may have a lower risk of treatment-emergent mania compared with antidepressants.1,25
Acknowledgment
The authors thank Dr. Sy Saeed for his expertise and guidance on this article.
Bottom Line
For patients with bipolar depression, treatment with transcranial magnetic stimulation in conjunction with a mood stabilizer may have lower rates of treatment-emergent mania than treatment with antidepressants.
Related Resources
- Transcranial magnetic stimulation: clinical applications for psychiatric practice. Bermudes RA, Lanocha K, Janicak PG, eds. American Psychiatric Association Publishing; 2017.
- Gold AK, Ornelas AC, Cirillo P, et al. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav. 2019;9(10):e01419. doi: 10.1002/brb3.1419
Drug Brand Names
Aripiprazole • Abilify
Bupropion • Wellbutrin
Cariprazine • Vraylar
Clonazepam • Klonopin
Fluoxetine • Prozac
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin, Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Olanzapine-fluoxetine • Symbyax
Quetiapine • Seroquel
Trazodone • Desyrel
Venlafaxine • Effexor
1. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes RA, Lanocha K, Janicak PG, eds. Transcranial magnetic stimulation: clinical applications for psychiatric practice. American Psychiatric Association Publishing; 2017:127-156.
2. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
3. Gitlin M. Treatment-resistant bipolar disorder. Molecular Psychiatry. 2006;11(3):227-240.
4. Harrison PJ, Geddes JR, Tunbridge EM. The emerging neurobiology of bipolar disorder. Trends Neurosci. 2018;41(1):18-30.
5. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68(3):241-251.
6. Myczkowski ML, Fernandes A, Moreno M, et al. Cognitive outcomes of TMS treatment in bipolar depression: safety data from a randomized controlled trial. J Affect Disord. 2018;235: 20-26.
7. Tavares DF, Myczkowski ML, Alberto RL, et al. Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42(13):2593-2601.
8. Neuronetics. FDA grants NeuroStar® Advanced Therapy System Breakthrough Device Designation to treat bipolar depression. Accessed February 2, 2021. https://www.globenewswire.com/news-release/2020/03/06/1996447/0/en/FDA-Grants-NeuroStar-Advanced-Therapy-System-Breakthrough-Device-Designation-to-Treat-Bipolar-Depression.html
9. Cohen RB, Brunoni AR, Boggio PS, et al. Clinical predictors associated with duration of repetitive transcranial magnetic stimulation treatment for remission in bipolar depression: a naturalistic study. J Nerv Ment Dis. 2010;198(9):679-681.
10. Connolly KR, Helmer A, Cristancho MA, et al. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73(4):e567-e573.
11. Dell’osso B, D’Urso N, Castellano F, et al. Long-term efficacy after acute augmentative repetitive transcranial magnetic stimulation in bipolar depression: a 1-year follow-up study. J ECT. 2011;27(2):141-144.
12. Dell’Osso B, Mundo E, D’Urso N, et al. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disord. 2009;11(1):76-81.
13. Harel EV, Zangen A, Roth Y, et al. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12(2):119-126.
14. Nahas Z, Kozel FA, Li X, et al. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord. 2003;5(1):40-47.
15. Tamas RL, Menkes D, El-Mallakh RS. Stimulating research: a prospective, randomized, double-blind, sham-controlled study of slow transcranial magnetic stimulation in depressed bipolar patients. J Neuropsychiatry Clin Neurosci. 2007;19(2):198-199.
16. Tundo A, Cavalieri P, Navari S, et al. Treating bipolar depression - antidepressants and alternatives: a critical review of the literature. Acta Neuropsychiatrica. 2011:23(3):94-105.
17. Gijsman HJ, Geddes JR, Rendell JM, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry. 2004;161(9):1537-1547.
18. Fornaro M, Anastasia A, Novello S, et al. Incidence, prevalence and clinical correlates of antidepressant‐emergent mania in bipolar depression: a systematic review and meta‐analysis. Bipolar Disord. 2018;20(3):195-227.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
20. Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119-130.
21. Rachid F. Repetitive transcranial magnetic stimulation and treatment-emergent mania and hypomania: a review of the literature. J Psychiatr Pract. 2017;23(2):150-159.
22. Victorin A, Rydén E, Thase M, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
23. Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214(1):27-35.
24. Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1.
25. Phillips AL, Burr RL, Dunner DL. Repetitive transcranial magnetic stimulation in the treatment of bipolar depression: Experience from a clinical setting. J Psychiatr Pract. 2020;26(1):37-45.
1. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes RA, Lanocha K, Janicak PG, eds. Transcranial magnetic stimulation: clinical applications for psychiatric practice. American Psychiatric Association Publishing; 2017:127-156.
2. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
3. Gitlin M. Treatment-resistant bipolar disorder. Molecular Psychiatry. 2006;11(3):227-240.
4. Harrison PJ, Geddes JR, Tunbridge EM. The emerging neurobiology of bipolar disorder. Trends Neurosci. 2018;41(1):18-30.
5. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68(3):241-251.
6. Myczkowski ML, Fernandes A, Moreno M, et al. Cognitive outcomes of TMS treatment in bipolar depression: safety data from a randomized controlled trial. J Affect Disord. 2018;235: 20-26.
7. Tavares DF, Myczkowski ML, Alberto RL, et al. Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42(13):2593-2601.
8. Neuronetics. FDA grants NeuroStar® Advanced Therapy System Breakthrough Device Designation to treat bipolar depression. Accessed February 2, 2021. https://www.globenewswire.com/news-release/2020/03/06/1996447/0/en/FDA-Grants-NeuroStar-Advanced-Therapy-System-Breakthrough-Device-Designation-to-Treat-Bipolar-Depression.html
9. Cohen RB, Brunoni AR, Boggio PS, et al. Clinical predictors associated with duration of repetitive transcranial magnetic stimulation treatment for remission in bipolar depression: a naturalistic study. J Nerv Ment Dis. 2010;198(9):679-681.
10. Connolly KR, Helmer A, Cristancho MA, et al. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73(4):e567-e573.
11. Dell’osso B, D’Urso N, Castellano F, et al. Long-term efficacy after acute augmentative repetitive transcranial magnetic stimulation in bipolar depression: a 1-year follow-up study. J ECT. 2011;27(2):141-144.
12. Dell’Osso B, Mundo E, D’Urso N, et al. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disord. 2009;11(1):76-81.
13. Harel EV, Zangen A, Roth Y, et al. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12(2):119-126.
14. Nahas Z, Kozel FA, Li X, et al. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord. 2003;5(1):40-47.
15. Tamas RL, Menkes D, El-Mallakh RS. Stimulating research: a prospective, randomized, double-blind, sham-controlled study of slow transcranial magnetic stimulation in depressed bipolar patients. J Neuropsychiatry Clin Neurosci. 2007;19(2):198-199.
16. Tundo A, Cavalieri P, Navari S, et al. Treating bipolar depression - antidepressants and alternatives: a critical review of the literature. Acta Neuropsychiatrica. 2011:23(3):94-105.
17. Gijsman HJ, Geddes JR, Rendell JM, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry. 2004;161(9):1537-1547.
18. Fornaro M, Anastasia A, Novello S, et al. Incidence, prevalence and clinical correlates of antidepressant‐emergent mania in bipolar depression: a systematic review and meta‐analysis. Bipolar Disord. 2018;20(3):195-227.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
20. Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119-130.
21. Rachid F. Repetitive transcranial magnetic stimulation and treatment-emergent mania and hypomania: a review of the literature. J Psychiatr Pract. 2017;23(2):150-159.
22. Victorin A, Rydén E, Thase M, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
23. Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214(1):27-35.
24. Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1.
25. Phillips AL, Burr RL, Dunner DL. Repetitive transcranial magnetic stimulation in the treatment of bipolar depression: Experience from a clinical setting. J Psychiatr Pract. 2020;26(1):37-45.
More than just 3 dogs: Is burnout getting in the way of knowing our patients?
Do you ever leave work thinking “Why do I always feel so tired after my shift?” “How can I overcome this fatigue?” “Is this what I expected?” “How can I get over the dread of so much administrative work when I want more time for my patients?” As clinicians, we face these and many other questions every day. These questions are the result of feeling entrapped in a health care system that has forgotten that clinicians need enough time to get to know and connect with their patients. Burnout is real, and relying on wellness activities is not sufficient to overcome it. Instead, taking the time for some introspection and self-reflection can help to overcome these difficulties.
A valuable lesson
Ten months into my intern year as a psychiatry resident, while on a busy night shift at the psychiatry emergency unit, an 86-year-old man arrived alone, hopeless, and with persistent death wishes. He needed to be heard and comforted by someone. Although he understood the nonnegotiable need to be hospitalized, he was extremely hesitant. But why? After all, he expressed wanting to get better and feared going back home alone, yet he was unwilling to be admitted to the hospital for acute care.
I knew I had to address the reason behind my patient’s ambivalence by further exploring his history. Nonetheless, my physician-in-training mind was battling feelings of stress secondary to what at the time seemed to be a never-ending to-do list full of nurses’ requests and patient-related tasks. Despite an unconscious temptation to rush through the history to please my go, go, go! trainee mind, I do not regret having taken the time to ask and address the often-feared “why.” Why was my patient reluctant to accept my recommendation?
To my surprise, it turned out to be an important matter. He said, “I have 3 dogs back home I don’t want to leave alone. They are the only living memory of my wife, who passed away 5 months ago. They help me stay alive.” I was struck by a feeling of empathy, but also guilt for having almost rushed through the history and not being thorough enough to ask why.
Take time to explore ‘why’
Do we really recognize the importance of being inquisitive in our history-taking? What might seem a simple matter to us (in my patient’s case, his 3 dogs were his main support system) can be a significant cause of a patient’s distress. A patients’ hesitancy to accept our recommendations can be secondary to reasons that unfortunately at times we only partially explore, or do not explore at all. Asking why can open Pandora’s box. It can uncover feelings and emotions such as frustration, anger, anxiety, and sorrow. It can also reveal uncertainties regarding topics such as race, gender identity, sexual orientation, socioeconomic status, and religion. We should be driven by humble curiosity, and tailor the interview by purposefully asking questions with the goal of learning and understanding our patients’ concerns. This practice serves to cultivate honest and trustworthy physician-patient relationships founded on empathy and respect.
If we know that obtaining an in-depth history is crucial for formulating a patient’s treatment plan, why do we sometimes fall in the trap of obtaining superficial ones, at times limiting ourselves to checklists? Reasons for not delving into our patients’ histories include (but are not limited to) an overload of patients, time constraints, a physician’s personal style, unconscious bias, suboptimal mentoring, and burnout. Of all these reasons, I worry the most about burnout. Physicians face insurmountable academic, institutional, and administrative demands. These constraints inarguably contribute to feeling rushed, and eventually possibly burned out.
Using self-reflection to prevent burnout
Physician burnout—as well as attempts to define, identify, target, and prevent it—has been on the rise in the past decades. If burnout affects the physician-patient relationship, we should make efforts to mitigate it. One should try to rely on internal, rather than external, influences to positively influence our delivery of care. One way to do this is by really getting to know the patient in front of us: a father, mother, brother, sister, member of the community, etc. Understanding our patient’s needs and concerns promotes empathy and connectedness. Another way is to exercise self-reflection by asking ourselves: How do I feel about the care I delivered today? Did I make an effort to fully understand my patients’ concerns? Did I make each patient feel understood? Was I rushing through the day, or was I mindful of the person in front of me? Did I deliver the care I wish I had received?
Although there are innumerable ways to target physician burnout, these self-reflections are quick, simple exercises that easily can be woven into a clinician’s busy schedule. The goal is to be mindful of improving the quality of our interactions with patients to ultimately cultivate our own well-being by potentiating a sense of fulfilment and satisfaction with our profession. I encourage clinicians to always go after the “why.” After all, why not? Thankfully, after some persuasion, my patient accepted voluntary admission, and arranged with neighbors to take care of his 3 dogs.
Do you ever leave work thinking “Why do I always feel so tired after my shift?” “How can I overcome this fatigue?” “Is this what I expected?” “How can I get over the dread of so much administrative work when I want more time for my patients?” As clinicians, we face these and many other questions every day. These questions are the result of feeling entrapped in a health care system that has forgotten that clinicians need enough time to get to know and connect with their patients. Burnout is real, and relying on wellness activities is not sufficient to overcome it. Instead, taking the time for some introspection and self-reflection can help to overcome these difficulties.
A valuable lesson
Ten months into my intern year as a psychiatry resident, while on a busy night shift at the psychiatry emergency unit, an 86-year-old man arrived alone, hopeless, and with persistent death wishes. He needed to be heard and comforted by someone. Although he understood the nonnegotiable need to be hospitalized, he was extremely hesitant. But why? After all, he expressed wanting to get better and feared going back home alone, yet he was unwilling to be admitted to the hospital for acute care.
I knew I had to address the reason behind my patient’s ambivalence by further exploring his history. Nonetheless, my physician-in-training mind was battling feelings of stress secondary to what at the time seemed to be a never-ending to-do list full of nurses’ requests and patient-related tasks. Despite an unconscious temptation to rush through the history to please my go, go, go! trainee mind, I do not regret having taken the time to ask and address the often-feared “why.” Why was my patient reluctant to accept my recommendation?
To my surprise, it turned out to be an important matter. He said, “I have 3 dogs back home I don’t want to leave alone. They are the only living memory of my wife, who passed away 5 months ago. They help me stay alive.” I was struck by a feeling of empathy, but also guilt for having almost rushed through the history and not being thorough enough to ask why.
Take time to explore ‘why’
Do we really recognize the importance of being inquisitive in our history-taking? What might seem a simple matter to us (in my patient’s case, his 3 dogs were his main support system) can be a significant cause of a patient’s distress. A patients’ hesitancy to accept our recommendations can be secondary to reasons that unfortunately at times we only partially explore, or do not explore at all. Asking why can open Pandora’s box. It can uncover feelings and emotions such as frustration, anger, anxiety, and sorrow. It can also reveal uncertainties regarding topics such as race, gender identity, sexual orientation, socioeconomic status, and religion. We should be driven by humble curiosity, and tailor the interview by purposefully asking questions with the goal of learning and understanding our patients’ concerns. This practice serves to cultivate honest and trustworthy physician-patient relationships founded on empathy and respect.
If we know that obtaining an in-depth history is crucial for formulating a patient’s treatment plan, why do we sometimes fall in the trap of obtaining superficial ones, at times limiting ourselves to checklists? Reasons for not delving into our patients’ histories include (but are not limited to) an overload of patients, time constraints, a physician’s personal style, unconscious bias, suboptimal mentoring, and burnout. Of all these reasons, I worry the most about burnout. Physicians face insurmountable academic, institutional, and administrative demands. These constraints inarguably contribute to feeling rushed, and eventually possibly burned out.
Using self-reflection to prevent burnout
Physician burnout—as well as attempts to define, identify, target, and prevent it—has been on the rise in the past decades. If burnout affects the physician-patient relationship, we should make efforts to mitigate it. One should try to rely on internal, rather than external, influences to positively influence our delivery of care. One way to do this is by really getting to know the patient in front of us: a father, mother, brother, sister, member of the community, etc. Understanding our patient’s needs and concerns promotes empathy and connectedness. Another way is to exercise self-reflection by asking ourselves: How do I feel about the care I delivered today? Did I make an effort to fully understand my patients’ concerns? Did I make each patient feel understood? Was I rushing through the day, or was I mindful of the person in front of me? Did I deliver the care I wish I had received?
Although there are innumerable ways to target physician burnout, these self-reflections are quick, simple exercises that easily can be woven into a clinician’s busy schedule. The goal is to be mindful of improving the quality of our interactions with patients to ultimately cultivate our own well-being by potentiating a sense of fulfilment and satisfaction with our profession. I encourage clinicians to always go after the “why.” After all, why not? Thankfully, after some persuasion, my patient accepted voluntary admission, and arranged with neighbors to take care of his 3 dogs.
Do you ever leave work thinking “Why do I always feel so tired after my shift?” “How can I overcome this fatigue?” “Is this what I expected?” “How can I get over the dread of so much administrative work when I want more time for my patients?” As clinicians, we face these and many other questions every day. These questions are the result of feeling entrapped in a health care system that has forgotten that clinicians need enough time to get to know and connect with their patients. Burnout is real, and relying on wellness activities is not sufficient to overcome it. Instead, taking the time for some introspection and self-reflection can help to overcome these difficulties.
A valuable lesson
Ten months into my intern year as a psychiatry resident, while on a busy night shift at the psychiatry emergency unit, an 86-year-old man arrived alone, hopeless, and with persistent death wishes. He needed to be heard and comforted by someone. Although he understood the nonnegotiable need to be hospitalized, he was extremely hesitant. But why? After all, he expressed wanting to get better and feared going back home alone, yet he was unwilling to be admitted to the hospital for acute care.
I knew I had to address the reason behind my patient’s ambivalence by further exploring his history. Nonetheless, my physician-in-training mind was battling feelings of stress secondary to what at the time seemed to be a never-ending to-do list full of nurses’ requests and patient-related tasks. Despite an unconscious temptation to rush through the history to please my go, go, go! trainee mind, I do not regret having taken the time to ask and address the often-feared “why.” Why was my patient reluctant to accept my recommendation?
To my surprise, it turned out to be an important matter. He said, “I have 3 dogs back home I don’t want to leave alone. They are the only living memory of my wife, who passed away 5 months ago. They help me stay alive.” I was struck by a feeling of empathy, but also guilt for having almost rushed through the history and not being thorough enough to ask why.
Take time to explore ‘why’
Do we really recognize the importance of being inquisitive in our history-taking? What might seem a simple matter to us (in my patient’s case, his 3 dogs were his main support system) can be a significant cause of a patient’s distress. A patients’ hesitancy to accept our recommendations can be secondary to reasons that unfortunately at times we only partially explore, or do not explore at all. Asking why can open Pandora’s box. It can uncover feelings and emotions such as frustration, anger, anxiety, and sorrow. It can also reveal uncertainties regarding topics such as race, gender identity, sexual orientation, socioeconomic status, and religion. We should be driven by humble curiosity, and tailor the interview by purposefully asking questions with the goal of learning and understanding our patients’ concerns. This practice serves to cultivate honest and trustworthy physician-patient relationships founded on empathy and respect.
If we know that obtaining an in-depth history is crucial for formulating a patient’s treatment plan, why do we sometimes fall in the trap of obtaining superficial ones, at times limiting ourselves to checklists? Reasons for not delving into our patients’ histories include (but are not limited to) an overload of patients, time constraints, a physician’s personal style, unconscious bias, suboptimal mentoring, and burnout. Of all these reasons, I worry the most about burnout. Physicians face insurmountable academic, institutional, and administrative demands. These constraints inarguably contribute to feeling rushed, and eventually possibly burned out.
Using self-reflection to prevent burnout
Physician burnout—as well as attempts to define, identify, target, and prevent it—has been on the rise in the past decades. If burnout affects the physician-patient relationship, we should make efforts to mitigate it. One should try to rely on internal, rather than external, influences to positively influence our delivery of care. One way to do this is by really getting to know the patient in front of us: a father, mother, brother, sister, member of the community, etc. Understanding our patient’s needs and concerns promotes empathy and connectedness. Another way is to exercise self-reflection by asking ourselves: How do I feel about the care I delivered today? Did I make an effort to fully understand my patients’ concerns? Did I make each patient feel understood? Was I rushing through the day, or was I mindful of the person in front of me? Did I deliver the care I wish I had received?
Although there are innumerable ways to target physician burnout, these self-reflections are quick, simple exercises that easily can be woven into a clinician’s busy schedule. The goal is to be mindful of improving the quality of our interactions with patients to ultimately cultivate our own well-being by potentiating a sense of fulfilment and satisfaction with our profession. I encourage clinicians to always go after the “why.” After all, why not? Thankfully, after some persuasion, my patient accepted voluntary admission, and arranged with neighbors to take care of his 3 dogs.
A resident’s guide to lithium
Lithium has been used in psychiatry for more than half a century and is considered the gold standard for treating acute mania and maintenance treatment of bipolar disorder.1 Evidence supports its use to reduce suicidal behavior and as an adjunctive treatment for major depressive disorder.2 However, lithium has fallen out of favor because of its narrow therapeutic index as well as the introduction of newer psychotropic medications that have a quicker onset of action and do not require strict blood monitoring. For residents early in their training, keeping track of the laboratory monitoring and medical screening can be confusing. Different institutions and countries have specific guidelines and recommendations for monitoring patients receiving lithium, which adds to the confusion.
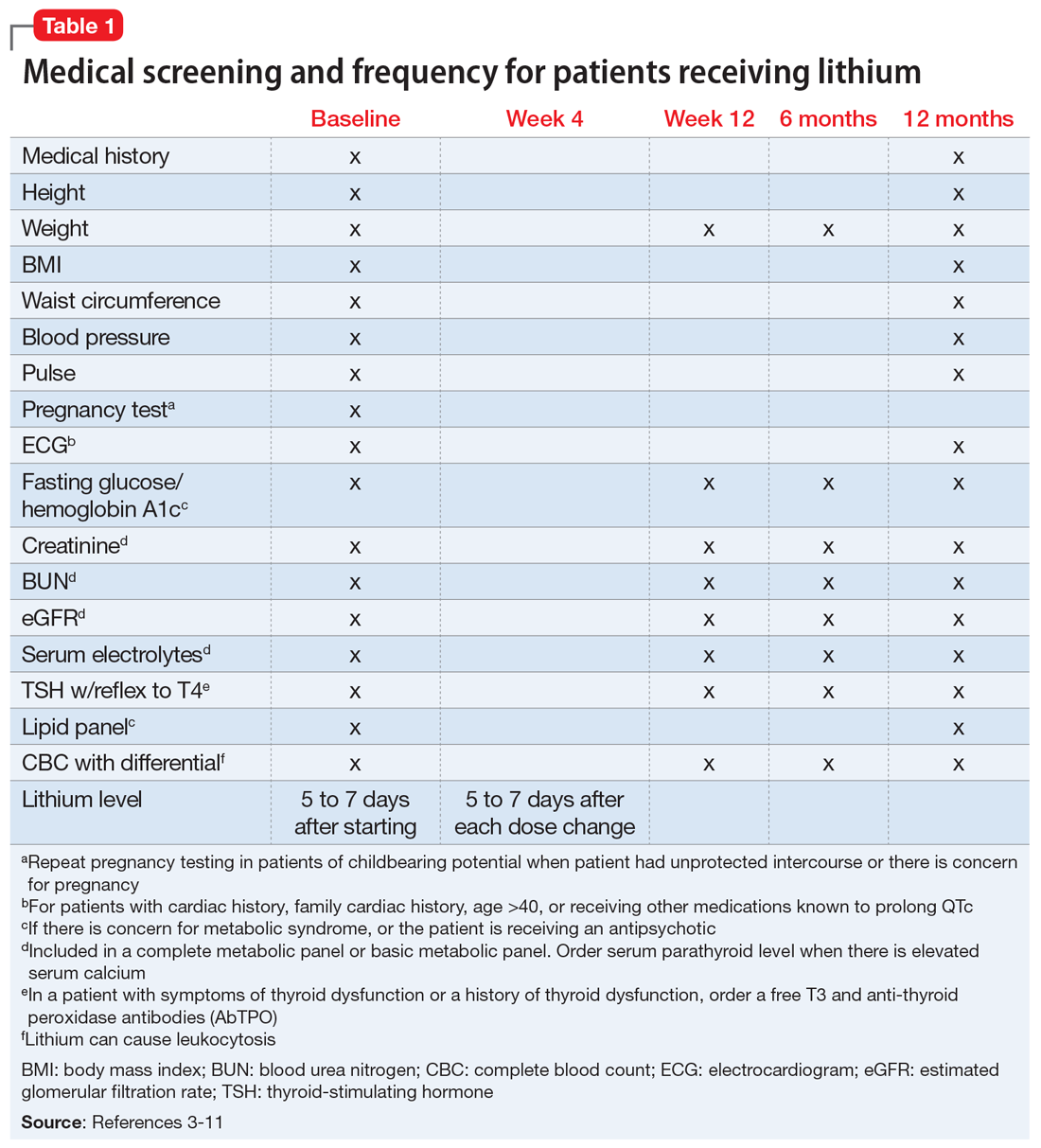
We completed a literature review to develop clear and concise recommendations for lithium monitoring for residents in our psychiatry residency program. These recommendations outline screening at baseline and after patients treated with lithium achieve stability. Table 13-11 outlines medical screening parameters, including bloodwork, that should be completed before initiating treatment, and how often such screening should be repeated. Table 2 incorporates these parameters into progress notes in the electronic medical record to keep track of the laboratory values and when they were last drawn. Our aim is to help residents stay organized and prevent missed screenings.
How often should lithium levels be monitored?
After starting a patient on lithium, check the level within 5 to 7 days, and 5 to 7 days after each dose change. Draw the lithium level 10 to 14 hours after the patient’s last dose (12 hours is best).1 Because of dosage changes, lithium levels usually are monitored more frequently during the first 3 months of treatment until therapeutic levels are reached or symptoms are controlled. It is recommended to monitor lithium levels every 3 months for the first year and every 6 months after the first year of treatment once the patient is stable and considering age, medical health, and how consistently a patient reports symptoms/adverse effects.3,5 Continue monitoring levels every 3 months in older adults; in patients with renal dysfunction, thyroid dysfunction, hypercalcemia, or other significant medical comorbidities; and in those who are taking medications that affect lithium, such as pain medications (nonsteroidal anti-inflammatory drugs can raise lithium levels), certain antihypertensives (angiotensin-converting-enzyme inhibitors can raise lithium levels), and diuretics (thiazide diuretics can raise lithium levels; osmotic diuretics and carbonic anhydrase inhibitors can reduce lithium levels).1,3,5
Lithium levels could vary by up to 0.5 mEq/L during transition between manic, euthymic, and depressive states.12 On a consistent dosage, lithium levels decrease during mania because of hemodilution, and increase during depression secondary to physiological effects specific to these episodes.13,14
Recommendations for plasma lithium levels (trough levels)
Mania. Lithium levels of 0.8 to 1.2 mEq/L often are needed to achieve symptom control during manic episodes.15 As levels approach 1.5 mEq/L, patients are at increased risk for intolerable adverse effects (eg, nausea and vomiting) and toxicity.16,17 Adverse effects at higher levels may result in patients abruptly discontinuing lithium. Patients who experience mania before a depressive episode at illness onsettend to have a better treatment response with lithium.18 Lithium monotherapy has been shown to be less effective for acute mania than antipsychotics or combination therapies.19 Consider combining lithium with valproate or antipsychotics for patients who have tolerated lithium in the past and plan to use lithium for maintenance treatment.20
Maintenance. In adults, the lithium level should be 0.60 to 80mEq/L, but consider levels of 0.40 to 0.60 mEq/L in patients who have a good response to lithium but develop adverse effects at higher levels.21 For patients who do not respond to treatment, such as those with severe mania, maintenance levels can be increased to 0.76 to 0.90 mEq/L.22 These same recommendations for maintenance levels can be used for children and adolescents. In older adults, aim for maintenance levels of 0.4 to 0.6 mEq/L. For patients age 65 to 79, the maximum level is 0.7 to 0.8 mEq/L, and should not exceed 0.7 mEq/L in patients age >80. Lithium levels <0.4 mEq/L do not appear to be effective.21
Depression. Aim for a lithium level of 0.6 to 1.0 mEq/L for patients with depression.11
Continue to: Renal function monitoring frequency
Renal function monitoring frequency
Obtain a basic metabolic panel or comprehensive metabolic panel to establish baseline levels of creatinine, blood urea nitrogen (BUN), and estimated glomerular filtration rate (eGFR). Repeat testing at Week 12 and at 6 months to detect any changes. Renal function can be monitored every 6 to 12 months in stable patients, but should be closely watched when a patient’s clinical status changes.3 A new lower eGFR value after starting lithium therapy should be investigated with a repeat test in 2 weeks.23 Mild elevations in creatinine should be monitored, and further medical workup with a nephrologist is recommended for patients with a creatinine level ≥1.6 mg/dL.24 It is important to note that creatinine might remain within normal limits if there is considerable reduction in glomerular function. Creatinine levels could vary because of body mass and diet. Creatinine levels can be low in nonmuscular patients and elevated in patients who consume large amounts of protein.23,25
Ordering a basic metabolic panel also allows electrolyte monitoring. Hyponatremia and dehydration can lead to elevated lithium levels and result in toxicity; hypokalemia might increase the risk of lithium-induced cardiac toxicity. Monitor calcium (corrected serum calcium) because hypercalcemia has been seen in patients treated with lithium.
Thyroid function monitoring frequency
Obtain levels of thyroid-stimulating hormone with reflex to free T4 at baseline, 12 weeks, and 6 months. Monitor thyroid function every 6 to 12 months in stable patients and when a patient’s clinical status changes, such as with new reports of medical or psychiatric symptoms and when there is concern for thyroid dysfunction.3
Lithium and neurotoxicity
Lithium is known to have neurotoxic effects, such as effects on fast-acting neurons leading to dyscoordination or tremor, even at therapeutic levels.26 This is especially the case when lithium is combined with an antipsychotic,26,27 a combination that is used to treat bipolar I disorder with psychotic features. Older adults are at greater risk for neurotoxicity because of physiological changes associated with increasing age.28
Educate patients about adherence, diet, and exercise
Patients might stop taking their psychotropic medications when they start feeling better. Instruct patients to discuss discontinuation with the prescribing clinician before they stop any medication. Educate patients that rapidly discontinuing lithium therapy puts them at high risk of relapse29 and increases the risk of developing treatment-refractory symptoms.23,30 Emphasize the importance of staying hydrated and maintaining adequate sodium in their diet.17,31 Consuming excessive sodium can reduce lithium levels.17,32 Lithium levels could increase when patients experience excessive sweating, such as during exercise or being outside on warm days, because of sodium and volume loss.17,33
1. Tondo L, Alda M, Bauer M, et al. Clinical use of lithium salts: guide for users and prescribers. Int J Bipolar Disord. 2019;7(1):16. doi:10.1186/s40345-019-0151-2
2. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28. doi:10.1186/s40345-015-0028-y
3. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159:1-50.
4. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15:1‐44. doi:10.1111/bdi.12025
5. National Collaborating Center for Mental Health (UK). Bipolar disorder: the NICE guideline on the assessment and management of bipolar disorder in adults, children and young people in primary and secondary care. The British Psychological Society and The Royal College of Psychiatrists; 2014.
6. Kupka R, Goossens P, van Bendegem M, et al. Multidisciplinaire richtlijn bipolaire stoornissen. Nederlandse Vereniging voor Psychiatrie (NVvP); 2015. Accessed August 10, 2020. http://www.nvvp.net/stream/richtlijn-bipolaire-stoornissen-2015
7. Malhi GS, Bassett D, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2015;49:1087‐1206. doi:10.1177/0004867415617657
8. Nederlof M, Heerdink ER, Egberts ACG, et al. Monitoring of patients treated with lithium for bipolar disorder: an international survey. Int J Bipolar Disord. 2018;6(1):12. doi:10.1186/s40345-018-0120-1
9. Leo RJ, Sharma M, Chrostowski DA. A case of lithium-induced symptomatic hypercalcemia. Prim Care Companion J Clin Psychiatry. 2010;12(4):PCC.09l00917. doi:10.4088/PCC.09l00917yel
10. McHenry CR, Lee K. Lithium therapy and disorders of the parathyroid glands. Endocr Pract. 1996;2(2):103-109. doi:10.4158/EP.2.2.103
11. Stahl SM. The prescribers guide: Stahl’s essential psychopharmacology. 6th ed. Cambridge University Press; 2017.
12. Kukopulos A, Reginaldi D. Variations of serum lithium concentrations correlated with the phases of manic-depressive psychosis. Agressologie. 1978;19(D):219-222.
13. Rittmannsberger H, Malsiner-Walli G. Mood-dependent changes of serum lithium concentration in a rapid cycling patient maintained on stable doses of lithium carbonate. Bipolar Disord. 2013;15(3):333-337. doi:10.1111/bdi.12066
14. Hochman E, Weizman A, Valevski A, et al. Association between bipolar episodes and fluid and electrolyte homeostasis: a retrospective longitudinal study. Bipolar Disord. 2014;16(8):781-789. doi:10.1111/bdi.12248
15. Volkmann C, Bschor T, Köhler S. Lithium treatment over the lifespan in bipolar disorders. Front Psychiatry. 2020;11:377. doi: 10.3389/fpsyt.2020.00377
16. Boltan DD, Fenves AZ. Effectiveness of normal saline diuresis in treating lithium overdose. Proc (Bayl Univ Med Cent). 2008;21(3):261-263. doi:10.1080/08998280.2008.11928407
17. Sadock BJ, Saddock VA, Ruiz P. Kaplan and Sadock’s synopsis of psychiatry. 11th ed. Wolters Kluwer; 2014.
18. Tighe SK, Mahon PB, Potash JB. Predictors of lithium response in bipolar disorder. Ther Adv Chronic Dis. 2011;2(3):209-226. doi:10.1177/2040622311399173
19. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. doi:10.1016/S0140-6736(11)60873-8
20. Smith LA, Cornelius V, Tacchi MJ, et al. Acute bipolar mania: a systematic review and meta-analysis of co-therapy vs monotherapy. Acta Psychiatr Scand. 2016;115(1):12-20. doi:10.1111/j.1600-0447.2006.00912.x
21. Nolen WA, Licht RW, Young AH, et al; ISBD/IGSLI Task Force on the treatment with lithium. What is the optimal serum level for lithium in the maintenance treatment of bipolar disorder? A systematic review and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Bipolar Disord. 2019;21(5):394-409. doi:10.1111/bdi.12805
22. Maj M, Starace F, Nolfe G, et al. Minimum plasma lithium levels required for effective prophylaxis in DSM III bipolar disorder: a prospective study. Pharmacopsychiatry. 1986;19(6):420-423. doi:10.1055/s-2007-1017280
23. Gupta S, Kripalani M, Khastgir U, et al. Management of the renal adverse effects of lithium. Advances in Psychiatric Treatment. 2013;19(6):457-466. doi:10.1192/apt.bp.112.010306
24. Gitlin M. Lithium and the kidney: an updated review. Drug Saf. 1999;20(3):231-243. doi:10.2165/00002018-199920030-00004
25. Jefferson JW. A clinician’s guide to monitoring kidney function in lithium-treated patients. J Clin Psychiatry. 2010;71(9):1153-1157. doi:10.4088/JCP.09m05917yel
26. Shah VC, Kayathi P, Singh G, et al. Enhance your understanding of lithium neurotoxicity. Prim Care Companion CNS Disord. 2015;17(3):10.4088/PCC.14l01767. doi:10.4088/PCC.14l01767
27. Netto I, Phutane VH. Reversible lithium neurotoxicity: review of the literature. Prim Care Companion CNS Disord. 2012;14(1):PCC.11r01197. doi:10.4088/PCC.11r01197
28. Mohandas E, Rajmohan V. Lithium use in special populations. Indian J Psychiatry. 2007;49(3):211-218. doi:10.4103/0019-5545.37325
29. Gupta S, Khastgir U. Drug information update. Lithium and chronic kidney disease: debates and dilemmas. BJPsych Bull. 2017;41(4):216-220. doi:10.1192/pb.bp.116.054031
30. Post RM. Preventing the malignant transformation of bipolar disorder. JAMA. 2018;319(12):1197-1198. doi:10.1001/jama.2018.0322
31. Timmer RT, Sands JM. Lithium intoxication. J Am Soc Nephrol. 1999;10(3):666-674.
32. Demers RG, Heninger GR. Sodium intake and lithium treatment in mania. Am J Psychiatry. 1971;128(1):100-104. doi:10.1176/ajp.128.1.100
33. Hedya SA, Avula A, Swoboda HD. Lithium toxicity. In: StatPearls. StatPearls Publishing; 2020.
Lithium has been used in psychiatry for more than half a century and is considered the gold standard for treating acute mania and maintenance treatment of bipolar disorder.1 Evidence supports its use to reduce suicidal behavior and as an adjunctive treatment for major depressive disorder.2 However, lithium has fallen out of favor because of its narrow therapeutic index as well as the introduction of newer psychotropic medications that have a quicker onset of action and do not require strict blood monitoring. For residents early in their training, keeping track of the laboratory monitoring and medical screening can be confusing. Different institutions and countries have specific guidelines and recommendations for monitoring patients receiving lithium, which adds to the confusion.

We completed a literature review to develop clear and concise recommendations for lithium monitoring for residents in our psychiatry residency program. These recommendations outline screening at baseline and after patients treated with lithium achieve stability. Table 13-11 outlines medical screening parameters, including bloodwork, that should be completed before initiating treatment, and how often such screening should be repeated. Table 2 incorporates these parameters into progress notes in the electronic medical record to keep track of the laboratory values and when they were last drawn. Our aim is to help residents stay organized and prevent missed screenings.
How often should lithium levels be monitored?
After starting a patient on lithium, check the level within 5 to 7 days, and 5 to 7 days after each dose change. Draw the lithium level 10 to 14 hours after the patient’s last dose (12 hours is best).1 Because of dosage changes, lithium levels usually are monitored more frequently during the first 3 months of treatment until therapeutic levels are reached or symptoms are controlled. It is recommended to monitor lithium levels every 3 months for the first year and every 6 months after the first year of treatment once the patient is stable and considering age, medical health, and how consistently a patient reports symptoms/adverse effects.3,5 Continue monitoring levels every 3 months in older adults; in patients with renal dysfunction, thyroid dysfunction, hypercalcemia, or other significant medical comorbidities; and in those who are taking medications that affect lithium, such as pain medications (nonsteroidal anti-inflammatory drugs can raise lithium levels), certain antihypertensives (angiotensin-converting-enzyme inhibitors can raise lithium levels), and diuretics (thiazide diuretics can raise lithium levels; osmotic diuretics and carbonic anhydrase inhibitors can reduce lithium levels).1,3,5
Lithium levels could vary by up to 0.5 mEq/L during transition between manic, euthymic, and depressive states.12 On a consistent dosage, lithium levels decrease during mania because of hemodilution, and increase during depression secondary to physiological effects specific to these episodes.13,14
Recommendations for plasma lithium levels (trough levels)
Mania. Lithium levels of 0.8 to 1.2 mEq/L often are needed to achieve symptom control during manic episodes.15 As levels approach 1.5 mEq/L, patients are at increased risk for intolerable adverse effects (eg, nausea and vomiting) and toxicity.16,17 Adverse effects at higher levels may result in patients abruptly discontinuing lithium. Patients who experience mania before a depressive episode at illness onsettend to have a better treatment response with lithium.18 Lithium monotherapy has been shown to be less effective for acute mania than antipsychotics or combination therapies.19 Consider combining lithium with valproate or antipsychotics for patients who have tolerated lithium in the past and plan to use lithium for maintenance treatment.20
Maintenance. In adults, the lithium level should be 0.60 to 80mEq/L, but consider levels of 0.40 to 0.60 mEq/L in patients who have a good response to lithium but develop adverse effects at higher levels.21 For patients who do not respond to treatment, such as those with severe mania, maintenance levels can be increased to 0.76 to 0.90 mEq/L.22 These same recommendations for maintenance levels can be used for children and adolescents. In older adults, aim for maintenance levels of 0.4 to 0.6 mEq/L. For patients age 65 to 79, the maximum level is 0.7 to 0.8 mEq/L, and should not exceed 0.7 mEq/L in patients age >80. Lithium levels <0.4 mEq/L do not appear to be effective.21
Depression. Aim for a lithium level of 0.6 to 1.0 mEq/L for patients with depression.11
Continue to: Renal function monitoring frequency
Renal function monitoring frequency
Obtain a basic metabolic panel or comprehensive metabolic panel to establish baseline levels of creatinine, blood urea nitrogen (BUN), and estimated glomerular filtration rate (eGFR). Repeat testing at Week 12 and at 6 months to detect any changes. Renal function can be monitored every 6 to 12 months in stable patients, but should be closely watched when a patient’s clinical status changes.3 A new lower eGFR value after starting lithium therapy should be investigated with a repeat test in 2 weeks.23 Mild elevations in creatinine should be monitored, and further medical workup with a nephrologist is recommended for patients with a creatinine level ≥1.6 mg/dL.24 It is important to note that creatinine might remain within normal limits if there is considerable reduction in glomerular function. Creatinine levels could vary because of body mass and diet. Creatinine levels can be low in nonmuscular patients and elevated in patients who consume large amounts of protein.23,25
Ordering a basic metabolic panel also allows electrolyte monitoring. Hyponatremia and dehydration can lead to elevated lithium levels and result in toxicity; hypokalemia might increase the risk of lithium-induced cardiac toxicity. Monitor calcium (corrected serum calcium) because hypercalcemia has been seen in patients treated with lithium.
Thyroid function monitoring frequency
Obtain levels of thyroid-stimulating hormone with reflex to free T4 at baseline, 12 weeks, and 6 months. Monitor thyroid function every 6 to 12 months in stable patients and when a patient’s clinical status changes, such as with new reports of medical or psychiatric symptoms and when there is concern for thyroid dysfunction.3
Lithium and neurotoxicity
Lithium is known to have neurotoxic effects, such as effects on fast-acting neurons leading to dyscoordination or tremor, even at therapeutic levels.26 This is especially the case when lithium is combined with an antipsychotic,26,27 a combination that is used to treat bipolar I disorder with psychotic features. Older adults are at greater risk for neurotoxicity because of physiological changes associated with increasing age.28
Educate patients about adherence, diet, and exercise
Patients might stop taking their psychotropic medications when they start feeling better. Instruct patients to discuss discontinuation with the prescribing clinician before they stop any medication. Educate patients that rapidly discontinuing lithium therapy puts them at high risk of relapse29 and increases the risk of developing treatment-refractory symptoms.23,30 Emphasize the importance of staying hydrated and maintaining adequate sodium in their diet.17,31 Consuming excessive sodium can reduce lithium levels.17,32 Lithium levels could increase when patients experience excessive sweating, such as during exercise or being outside on warm days, because of sodium and volume loss.17,33
Lithium has been used in psychiatry for more than half a century and is considered the gold standard for treating acute mania and maintenance treatment of bipolar disorder.1 Evidence supports its use to reduce suicidal behavior and as an adjunctive treatment for major depressive disorder.2 However, lithium has fallen out of favor because of its narrow therapeutic index as well as the introduction of newer psychotropic medications that have a quicker onset of action and do not require strict blood monitoring. For residents early in their training, keeping track of the laboratory monitoring and medical screening can be confusing. Different institutions and countries have specific guidelines and recommendations for monitoring patients receiving lithium, which adds to the confusion.

We completed a literature review to develop clear and concise recommendations for lithium monitoring for residents in our psychiatry residency program. These recommendations outline screening at baseline and after patients treated with lithium achieve stability. Table 13-11 outlines medical screening parameters, including bloodwork, that should be completed before initiating treatment, and how often such screening should be repeated. Table 2 incorporates these parameters into progress notes in the electronic medical record to keep track of the laboratory values and when they were last drawn. Our aim is to help residents stay organized and prevent missed screenings.
How often should lithium levels be monitored?
After starting a patient on lithium, check the level within 5 to 7 days, and 5 to 7 days after each dose change. Draw the lithium level 10 to 14 hours after the patient’s last dose (12 hours is best).1 Because of dosage changes, lithium levels usually are monitored more frequently during the first 3 months of treatment until therapeutic levels are reached or symptoms are controlled. It is recommended to monitor lithium levels every 3 months for the first year and every 6 months after the first year of treatment once the patient is stable and considering age, medical health, and how consistently a patient reports symptoms/adverse effects.3,5 Continue monitoring levels every 3 months in older adults; in patients with renal dysfunction, thyroid dysfunction, hypercalcemia, or other significant medical comorbidities; and in those who are taking medications that affect lithium, such as pain medications (nonsteroidal anti-inflammatory drugs can raise lithium levels), certain antihypertensives (angiotensin-converting-enzyme inhibitors can raise lithium levels), and diuretics (thiazide diuretics can raise lithium levels; osmotic diuretics and carbonic anhydrase inhibitors can reduce lithium levels).1,3,5
Lithium levels could vary by up to 0.5 mEq/L during transition between manic, euthymic, and depressive states.12 On a consistent dosage, lithium levels decrease during mania because of hemodilution, and increase during depression secondary to physiological effects specific to these episodes.13,14
Recommendations for plasma lithium levels (trough levels)
Mania. Lithium levels of 0.8 to 1.2 mEq/L often are needed to achieve symptom control during manic episodes.15 As levels approach 1.5 mEq/L, patients are at increased risk for intolerable adverse effects (eg, nausea and vomiting) and toxicity.16,17 Adverse effects at higher levels may result in patients abruptly discontinuing lithium. Patients who experience mania before a depressive episode at illness onsettend to have a better treatment response with lithium.18 Lithium monotherapy has been shown to be less effective for acute mania than antipsychotics or combination therapies.19 Consider combining lithium with valproate or antipsychotics for patients who have tolerated lithium in the past and plan to use lithium for maintenance treatment.20
Maintenance. In adults, the lithium level should be 0.60 to 80mEq/L, but consider levels of 0.40 to 0.60 mEq/L in patients who have a good response to lithium but develop adverse effects at higher levels.21 For patients who do not respond to treatment, such as those with severe mania, maintenance levels can be increased to 0.76 to 0.90 mEq/L.22 These same recommendations for maintenance levels can be used for children and adolescents. In older adults, aim for maintenance levels of 0.4 to 0.6 mEq/L. For patients age 65 to 79, the maximum level is 0.7 to 0.8 mEq/L, and should not exceed 0.7 mEq/L in patients age >80. Lithium levels <0.4 mEq/L do not appear to be effective.21
Depression. Aim for a lithium level of 0.6 to 1.0 mEq/L for patients with depression.11
Continue to: Renal function monitoring frequency
Renal function monitoring frequency
Obtain a basic metabolic panel or comprehensive metabolic panel to establish baseline levels of creatinine, blood urea nitrogen (BUN), and estimated glomerular filtration rate (eGFR). Repeat testing at Week 12 and at 6 months to detect any changes. Renal function can be monitored every 6 to 12 months in stable patients, but should be closely watched when a patient’s clinical status changes.3 A new lower eGFR value after starting lithium therapy should be investigated with a repeat test in 2 weeks.23 Mild elevations in creatinine should be monitored, and further medical workup with a nephrologist is recommended for patients with a creatinine level ≥1.6 mg/dL.24 It is important to note that creatinine might remain within normal limits if there is considerable reduction in glomerular function. Creatinine levels could vary because of body mass and diet. Creatinine levels can be low in nonmuscular patients and elevated in patients who consume large amounts of protein.23,25
Ordering a basic metabolic panel also allows electrolyte monitoring. Hyponatremia and dehydration can lead to elevated lithium levels and result in toxicity; hypokalemia might increase the risk of lithium-induced cardiac toxicity. Monitor calcium (corrected serum calcium) because hypercalcemia has been seen in patients treated with lithium.
Thyroid function monitoring frequency
Obtain levels of thyroid-stimulating hormone with reflex to free T4 at baseline, 12 weeks, and 6 months. Monitor thyroid function every 6 to 12 months in stable patients and when a patient’s clinical status changes, such as with new reports of medical or psychiatric symptoms and when there is concern for thyroid dysfunction.3
Lithium and neurotoxicity
Lithium is known to have neurotoxic effects, such as effects on fast-acting neurons leading to dyscoordination or tremor, even at therapeutic levels.26 This is especially the case when lithium is combined with an antipsychotic,26,27 a combination that is used to treat bipolar I disorder with psychotic features. Older adults are at greater risk for neurotoxicity because of physiological changes associated with increasing age.28
Educate patients about adherence, diet, and exercise
Patients might stop taking their psychotropic medications when they start feeling better. Instruct patients to discuss discontinuation with the prescribing clinician before they stop any medication. Educate patients that rapidly discontinuing lithium therapy puts them at high risk of relapse29 and increases the risk of developing treatment-refractory symptoms.23,30 Emphasize the importance of staying hydrated and maintaining adequate sodium in their diet.17,31 Consuming excessive sodium can reduce lithium levels.17,32 Lithium levels could increase when patients experience excessive sweating, such as during exercise or being outside on warm days, because of sodium and volume loss.17,33
1. Tondo L, Alda M, Bauer M, et al. Clinical use of lithium salts: guide for users and prescribers. Int J Bipolar Disord. 2019;7(1):16. doi:10.1186/s40345-019-0151-2
2. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28. doi:10.1186/s40345-015-0028-y
3. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159:1-50.
4. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15:1‐44. doi:10.1111/bdi.12025
5. National Collaborating Center for Mental Health (UK). Bipolar disorder: the NICE guideline on the assessment and management of bipolar disorder in adults, children and young people in primary and secondary care. The British Psychological Society and The Royal College of Psychiatrists; 2014.
6. Kupka R, Goossens P, van Bendegem M, et al. Multidisciplinaire richtlijn bipolaire stoornissen. Nederlandse Vereniging voor Psychiatrie (NVvP); 2015. Accessed August 10, 2020. http://www.nvvp.net/stream/richtlijn-bipolaire-stoornissen-2015
7. Malhi GS, Bassett D, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2015;49:1087‐1206. doi:10.1177/0004867415617657
8. Nederlof M, Heerdink ER, Egberts ACG, et al. Monitoring of patients treated with lithium for bipolar disorder: an international survey. Int J Bipolar Disord. 2018;6(1):12. doi:10.1186/s40345-018-0120-1
9. Leo RJ, Sharma M, Chrostowski DA. A case of lithium-induced symptomatic hypercalcemia. Prim Care Companion J Clin Psychiatry. 2010;12(4):PCC.09l00917. doi:10.4088/PCC.09l00917yel
10. McHenry CR, Lee K. Lithium therapy and disorders of the parathyroid glands. Endocr Pract. 1996;2(2):103-109. doi:10.4158/EP.2.2.103
11. Stahl SM. The prescribers guide: Stahl’s essential psychopharmacology. 6th ed. Cambridge University Press; 2017.
12. Kukopulos A, Reginaldi D. Variations of serum lithium concentrations correlated with the phases of manic-depressive psychosis. Agressologie. 1978;19(D):219-222.
13. Rittmannsberger H, Malsiner-Walli G. Mood-dependent changes of serum lithium concentration in a rapid cycling patient maintained on stable doses of lithium carbonate. Bipolar Disord. 2013;15(3):333-337. doi:10.1111/bdi.12066
14. Hochman E, Weizman A, Valevski A, et al. Association between bipolar episodes and fluid and electrolyte homeostasis: a retrospective longitudinal study. Bipolar Disord. 2014;16(8):781-789. doi:10.1111/bdi.12248
15. Volkmann C, Bschor T, Köhler S. Lithium treatment over the lifespan in bipolar disorders. Front Psychiatry. 2020;11:377. doi: 10.3389/fpsyt.2020.00377
16. Boltan DD, Fenves AZ. Effectiveness of normal saline diuresis in treating lithium overdose. Proc (Bayl Univ Med Cent). 2008;21(3):261-263. doi:10.1080/08998280.2008.11928407
17. Sadock BJ, Saddock VA, Ruiz P. Kaplan and Sadock’s synopsis of psychiatry. 11th ed. Wolters Kluwer; 2014.
18. Tighe SK, Mahon PB, Potash JB. Predictors of lithium response in bipolar disorder. Ther Adv Chronic Dis. 2011;2(3):209-226. doi:10.1177/2040622311399173
19. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. doi:10.1016/S0140-6736(11)60873-8
20. Smith LA, Cornelius V, Tacchi MJ, et al. Acute bipolar mania: a systematic review and meta-analysis of co-therapy vs monotherapy. Acta Psychiatr Scand. 2016;115(1):12-20. doi:10.1111/j.1600-0447.2006.00912.x
21. Nolen WA, Licht RW, Young AH, et al; ISBD/IGSLI Task Force on the treatment with lithium. What is the optimal serum level for lithium in the maintenance treatment of bipolar disorder? A systematic review and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Bipolar Disord. 2019;21(5):394-409. doi:10.1111/bdi.12805
22. Maj M, Starace F, Nolfe G, et al. Minimum plasma lithium levels required for effective prophylaxis in DSM III bipolar disorder: a prospective study. Pharmacopsychiatry. 1986;19(6):420-423. doi:10.1055/s-2007-1017280
23. Gupta S, Kripalani M, Khastgir U, et al. Management of the renal adverse effects of lithium. Advances in Psychiatric Treatment. 2013;19(6):457-466. doi:10.1192/apt.bp.112.010306
24. Gitlin M. Lithium and the kidney: an updated review. Drug Saf. 1999;20(3):231-243. doi:10.2165/00002018-199920030-00004
25. Jefferson JW. A clinician’s guide to monitoring kidney function in lithium-treated patients. J Clin Psychiatry. 2010;71(9):1153-1157. doi:10.4088/JCP.09m05917yel
26. Shah VC, Kayathi P, Singh G, et al. Enhance your understanding of lithium neurotoxicity. Prim Care Companion CNS Disord. 2015;17(3):10.4088/PCC.14l01767. doi:10.4088/PCC.14l01767
27. Netto I, Phutane VH. Reversible lithium neurotoxicity: review of the literature. Prim Care Companion CNS Disord. 2012;14(1):PCC.11r01197. doi:10.4088/PCC.11r01197
28. Mohandas E, Rajmohan V. Lithium use in special populations. Indian J Psychiatry. 2007;49(3):211-218. doi:10.4103/0019-5545.37325
29. Gupta S, Khastgir U. Drug information update. Lithium and chronic kidney disease: debates and dilemmas. BJPsych Bull. 2017;41(4):216-220. doi:10.1192/pb.bp.116.054031
30. Post RM. Preventing the malignant transformation of bipolar disorder. JAMA. 2018;319(12):1197-1198. doi:10.1001/jama.2018.0322
31. Timmer RT, Sands JM. Lithium intoxication. J Am Soc Nephrol. 1999;10(3):666-674.
32. Demers RG, Heninger GR. Sodium intake and lithium treatment in mania. Am J Psychiatry. 1971;128(1):100-104. doi:10.1176/ajp.128.1.100
33. Hedya SA, Avula A, Swoboda HD. Lithium toxicity. In: StatPearls. StatPearls Publishing; 2020.
1. Tondo L, Alda M, Bauer M, et al. Clinical use of lithium salts: guide for users and prescribers. Int J Bipolar Disord. 2019;7(1):16. doi:10.1186/s40345-019-0151-2
2. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28. doi:10.1186/s40345-015-0028-y
3. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159:1-50.
4. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15:1‐44. doi:10.1111/bdi.12025
5. National Collaborating Center for Mental Health (UK). Bipolar disorder: the NICE guideline on the assessment and management of bipolar disorder in adults, children and young people in primary and secondary care. The British Psychological Society and The Royal College of Psychiatrists; 2014.
6. Kupka R, Goossens P, van Bendegem M, et al. Multidisciplinaire richtlijn bipolaire stoornissen. Nederlandse Vereniging voor Psychiatrie (NVvP); 2015. Accessed August 10, 2020. http://www.nvvp.net/stream/richtlijn-bipolaire-stoornissen-2015
7. Malhi GS, Bassett D, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2015;49:1087‐1206. doi:10.1177/0004867415617657
8. Nederlof M, Heerdink ER, Egberts ACG, et al. Monitoring of patients treated with lithium for bipolar disorder: an international survey. Int J Bipolar Disord. 2018;6(1):12. doi:10.1186/s40345-018-0120-1
9. Leo RJ, Sharma M, Chrostowski DA. A case of lithium-induced symptomatic hypercalcemia. Prim Care Companion J Clin Psychiatry. 2010;12(4):PCC.09l00917. doi:10.4088/PCC.09l00917yel
10. McHenry CR, Lee K. Lithium therapy and disorders of the parathyroid glands. Endocr Pract. 1996;2(2):103-109. doi:10.4158/EP.2.2.103
11. Stahl SM. The prescribers guide: Stahl’s essential psychopharmacology. 6th ed. Cambridge University Press; 2017.
12. Kukopulos A, Reginaldi D. Variations of serum lithium concentrations correlated with the phases of manic-depressive psychosis. Agressologie. 1978;19(D):219-222.
13. Rittmannsberger H, Malsiner-Walli G. Mood-dependent changes of serum lithium concentration in a rapid cycling patient maintained on stable doses of lithium carbonate. Bipolar Disord. 2013;15(3):333-337. doi:10.1111/bdi.12066
14. Hochman E, Weizman A, Valevski A, et al. Association between bipolar episodes and fluid and electrolyte homeostasis: a retrospective longitudinal study. Bipolar Disord. 2014;16(8):781-789. doi:10.1111/bdi.12248
15. Volkmann C, Bschor T, Köhler S. Lithium treatment over the lifespan in bipolar disorders. Front Psychiatry. 2020;11:377. doi: 10.3389/fpsyt.2020.00377
16. Boltan DD, Fenves AZ. Effectiveness of normal saline diuresis in treating lithium overdose. Proc (Bayl Univ Med Cent). 2008;21(3):261-263. doi:10.1080/08998280.2008.11928407
17. Sadock BJ, Saddock VA, Ruiz P. Kaplan and Sadock’s synopsis of psychiatry. 11th ed. Wolters Kluwer; 2014.
18. Tighe SK, Mahon PB, Potash JB. Predictors of lithium response in bipolar disorder. Ther Adv Chronic Dis. 2011;2(3):209-226. doi:10.1177/2040622311399173
19. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. doi:10.1016/S0140-6736(11)60873-8
20. Smith LA, Cornelius V, Tacchi MJ, et al. Acute bipolar mania: a systematic review and meta-analysis of co-therapy vs monotherapy. Acta Psychiatr Scand. 2016;115(1):12-20. doi:10.1111/j.1600-0447.2006.00912.x
21. Nolen WA, Licht RW, Young AH, et al; ISBD/IGSLI Task Force on the treatment with lithium. What is the optimal serum level for lithium in the maintenance treatment of bipolar disorder? A systematic review and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Bipolar Disord. 2019;21(5):394-409. doi:10.1111/bdi.12805
22. Maj M, Starace F, Nolfe G, et al. Minimum plasma lithium levels required for effective prophylaxis in DSM III bipolar disorder: a prospective study. Pharmacopsychiatry. 1986;19(6):420-423. doi:10.1055/s-2007-1017280
23. Gupta S, Kripalani M, Khastgir U, et al. Management of the renal adverse effects of lithium. Advances in Psychiatric Treatment. 2013;19(6):457-466. doi:10.1192/apt.bp.112.010306
24. Gitlin M. Lithium and the kidney: an updated review. Drug Saf. 1999;20(3):231-243. doi:10.2165/00002018-199920030-00004
25. Jefferson JW. A clinician’s guide to monitoring kidney function in lithium-treated patients. J Clin Psychiatry. 2010;71(9):1153-1157. doi:10.4088/JCP.09m05917yel
26. Shah VC, Kayathi P, Singh G, et al. Enhance your understanding of lithium neurotoxicity. Prim Care Companion CNS Disord. 2015;17(3):10.4088/PCC.14l01767. doi:10.4088/PCC.14l01767
27. Netto I, Phutane VH. Reversible lithium neurotoxicity: review of the literature. Prim Care Companion CNS Disord. 2012;14(1):PCC.11r01197. doi:10.4088/PCC.11r01197
28. Mohandas E, Rajmohan V. Lithium use in special populations. Indian J Psychiatry. 2007;49(3):211-218. doi:10.4103/0019-5545.37325
29. Gupta S, Khastgir U. Drug information update. Lithium and chronic kidney disease: debates and dilemmas. BJPsych Bull. 2017;41(4):216-220. doi:10.1192/pb.bp.116.054031
30. Post RM. Preventing the malignant transformation of bipolar disorder. JAMA. 2018;319(12):1197-1198. doi:10.1001/jama.2018.0322
31. Timmer RT, Sands JM. Lithium intoxication. J Am Soc Nephrol. 1999;10(3):666-674.
32. Demers RG, Heninger GR. Sodium intake and lithium treatment in mania. Am J Psychiatry. 1971;128(1):100-104. doi:10.1176/ajp.128.1.100
33. Hedya SA, Avula A, Swoboda HD. Lithium toxicity. In: StatPearls. StatPearls Publishing; 2020.
Today’s psychiatric neuroscience advances were science fiction during my residency
During my residency training years, I had many rosy and bold dreams about the future of psychiatry, hoping for many breakthroughs.
Early on, I decided to pursue an academic career, and specifically to focus on the neurobiology of schizophrenia, bipolar disorder, and other psychoses. I secured a neuroscience mentor, conducted a research project, and presented my findings at the American Psychiatric Association Annual Meeting. Although at the time everyone used the term “functional” to describe mental illnesses, I was convinced that they were all neurologic conditions, with prominent psychiatric manifestations. And I have been proven right.
After my residency, I eagerly pursued a neuroscience fellowship at the National Institutes of Health. My fantasy was that during my career as a psychiatric neuroscientist, brain exploration would uncover the many mysteries of psychiatric disorders. I was insightful enough to recognize that what I envisioned for the future of psychiatry qualified as science fiction, but I never stopped dreaming.
Today, the advances in psychiatric neuroscience that were unimaginable during my residency have become dazzling discoveries. My journey as a psychiatric neuroscientist has been more thrilling than I ever imagined. I recall doing postmortem research on the brains of hundreds of deceased psychiatric patients, noticing sulci widening and ventricular dilatation, and wondering whether one day we would be able to detect those atrophic changes while the patients were alive. Although I measured those changes in postmortem brains, I was cognizant that due to preservation artifacts, such measurements were less reliable than measurements of living brains.
And then the advent of neuroimaging fulfilled my fantasies. This began towards the end of my fellowship, and has exploded with neurobiologic findings throughout my academic career. Then came dramatic methodologies to probe brain molecular and cellular pathologies, followed by breakthrough clinical advances. Entirely new vistas of research into psychiatric brain disorders are opening every day. The exhilaration will never end!
From science fiction to clinical reality
Here is a quick outline of some of the “science fiction” of psychiatry that has come true since my training days. Back then, these discoveries were completely absent from the radar screen of psychiatry, when it was still a fledgling medical specialty struggling to emerge from the dominant yet nonempirical era of psychoanalysis.
Brain exploration methods. Unprecedented breakthroughs in computer technology have allowed psychiatric neuroscientists to create a new field of neuroimaging research that includes:
- cerebral blood flow (CBF)
- position emission tomography (PET)
- single photon emission computed tomography (SPECT).
Continue to: These functional neuroimaging...
These functional neuroimaging methods (using ionizing radiation) have enabled clinicians to see abnormal blood flow patterns in the brains of living patients. One of the earliest findings was hypofrontality in patients with schizophrenia, implicating frontal pathology in this severe brain disorder. PET was also used for dopamine and serotonin receptor imaging.
Computerized axia tomography. Compared with skull X-rays, CT (“CAT”) scans provided a more detailed view of brain tissue, and began a structural neuroimaging revolution that enriched psychiatric research, but also was applied to organs other than the brain.
Magnetic resonance imaging (MRI) became the “big kahuna” of neuroimaging when arrived in the early 1980s and quickly supplanted CT research because it is safer (no ionizing radiation, and it can be repeated multiple times with or without tasks). It also provided exquisite neuroanatomical details of brain tissue with stunning fidelity. Subsequently, several MRI techniques/software programs were developed that advanced research in psychiatry to multiple new frontiers, including:
- Morphological neuroimaging with MRI
- Magnetic resonance spectroscopy (MRS), which acts like a living, noninvasive biopsy of several chemicals (such as choline, lactate, glutamine, adenosine triphosphate, and the neuronal marker N-acetylcysteine) in a small volume (≤1 cc) of neural tissue in various regions
- Functional MRI (fMRI), which measures blood flow changes during actual or imagined tasks in the brains of patients vs healthy controls
- Diffusion tensor imaging (DTI), which evaluates the integrity of white matter (60% of brain volume, including 137,000 miles of myelinated fibers) by measuring the flow of water inside myelinated fibers (anisotropy and diffusivity). DTI of the corpus callosum, the largest brain commissure that is comprised of 200 million interhemispheric fibers, has revealed many abnormalities. This was one of the structures I investigated during my fellowship, including a histopathological study.1
All 4 of these neuroimaging techniques continue to generate a wealth of data about brain structure and function in psychosis, mood disorders, anxiety disorders, borderline personality disorder, obsessive-compulsive disorder, eating disorders, and substance use disorders. All these discoveries were utterly impossible to predict during my residency. I am proud to have published the first reports in the literature of ventricular enlargement in patients with bipolar disorder,2 cortical atrophy in schizophrenia and mania,3 reductions of hippocampal volume in patients with schizophrenia using MRS,4 and progressive brain atrophy in patients with schizophrenia.5 It is especially gratifying that I played a small role in translating my science fiction fantasies into clinical reality!
Other breakthrough methodologies that are advancing psychiatric neuroscience today but were science fiction during my residency days include:
- Pluripotent stem cells, which enable the de-differentiation of adult skin cells and then re-differentiating them into any type of cell, including neurons. This allows researchers to conduct studies on any patient’s brain cells without needing to do an invasive, high-risk brain biopsy. As a young resident, I would never have predicted that this virtual brain biopsy would be possible!
- Optogenetics, which enables controlling cell behavior using light and genetically encoded light-sensitive proteins. This triggered a cornucopia of neuroscience discoveries by using optogenetics to modulate cell-signaling cascades to understand cellular biology. Halorhodopsin and bacteriorhodopsin are used as tools to turn neurons off or on rapidly and safely.
- Genome-wide association studies (GWAS) have revolutionized the field of molecular neurogenetics and are enabling clinicians to detect risk genes by comparing the DNA samples of thousands of psychiatric patients with thousands of healthy controls. This is how several hundred risk genes have been identified for schizophrenia, bipolar disorder, autism spectrum disorder, and more to come.
- Clustered regularly interspaced short palindromic repeats (CRISPR) is a remarkable genetic “scissors” (that earned its inventors the 2020 Nobel Prize) that allows splicing out a disease gene and splicing in a normal gene. This will have an enormous future application in preventing an adulthood illness at its roots during fetal life. The future medical implications for psychiatric disorders are prodigious!
Continue to: Clinical advances
Clinical advances. Many therapies or approaches that did not exist during my residency (and how I dreamed about them back then!) are available to today’s clinicians. These include:
- Rapid-acting antidepressants that reverse severe and chronic depression and suicidal urges within a few hours or a couple of days. As a resident, I waited for weeks or months to see patients with depression reach the full remission that is now achieved practically the same day with IV ketamine, intranasal esketamine, IV scopolamine, and inhalable nitrous oxide. During my residency, the closest thing we had to a rapid-acting treatment for depression was electroconvulsive therapy (ECT), but that usually took 2 to 3 weeks. Psychiatric clinicians should never cease to appreciate how an intractable, treatment-refractory depression can rapidly be turned off like a light switch, restoring normal mood to desperately ill persons.
- Neuromodulation techniques are flourishing. Beyond ECT, transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), transcranial direct current stimulation (tDCS), deep brain stimulation (DBS), low field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near-infrared radiation (NIR), and focused ultrasound (FUS) are approved or under development, offering millions of patients with various neuropsychiatric disorders potential recovery not with pharmacotherapy, but via a brain-targeted approach.
- Telepsychiatry. Now taken for granted during the COVID-19 pandemic, telepsychiatry was completely unimaginable during my residency. Yes, we had phones, but not smartphones! The only “zoom” we knew was the furious sound of a sports car engine! To be able to see and evaluate a patient literally anywhere in the world was science fiction personified! Increased remote access to psychiatric care by patients everywhere is a truly remarkable advance that helped avoid a disastrous lack of psychiatric treatment during the current pandemic that brought in-person interactions between psychiatric physicians and their patients to a screeching halt.
- Neurobiologic effects of psychotherapy. Viewing psychotherapy as a neurobiologic treatment was totally unknown and unimaginable during my residency. I was heavily trained in various types of psychotherapies, but not once did any of my supervisors mention experiential neuroplasticity as a brain-altering process, or that psychotherapy changes brain structure, induces experimental neuroplasticity, and induces billions of dendritic spines in patients’ cortex and limbic structures, helping them connect the dots and develop new insights. No one knew that psychotherapy can mimic the neural effects of pharmacotherapy.
- Immunomodulatory effects of psychotherapy. It was completely unknown that psychotherapies such as cognitive-behavioral therapy can lower levels of inflammatory biomarkers in patients’ CSF and serum. Back then, no one imagined that psychotherapy had immunomodulatory effects. These discoveries are revolutionary for us psychiatrists and confirm the neurobiologic mechanisms of psychotherapy for every patient we treat.
- Epigenetics. This was rarely, if ever, mentioned when I was a resident. We knew from clinical studies that children who were abused or neglected often develop severe mood or psychotic disorders in adulthood. But we did not know that trauma modifies some genes via under- or overexpression, and that such epigenetic changes alter brain development towards psychopathology. The mysteries of psychiatric brain disorders generated by childhood trauma have been clarified by advances in epigenetics.
Aspirational, futuristic therapies. Even now, as a seasoned psychiatric neuroscientist, I continue to dream. Research is providing many clues for potentially radical psychiatric treatments that go beyond standard antipsychotics, antidepressants, mood stabilizers, or anxiolytics. But today, I fully expect that scientific dreams eventually come true through research. For example, the following neuroscientific therapeutics strategies may someday become routine in clinical practice:
- microglia inhibition
- mitochondria repair
- anti-apoptotic therapy
- white matter connectivity restoration
- neuroprotection (enhancing neurogenesis, increasing neurotropic factors, and enhancing synaptogenesis)
- reverse glutamate N-methyl-
d -aspartate hypofunction - prevent amyloid formation.
Data analysis breakthroughs. Side-by-side with the explosion of new findings and amassing mountains of data in psychiatric neuroscience, unprecedented and revolutionary data-management techniques have emerged to facilitate the herculean task of data analysis to extract the mythical needle in a haystack and derive the overall impact of masses of data. These techniques, whose names were not in our vocabulary during my residency days, include:
- machine learning
- artificial intelligence
- deep learning
- big data.
With the help of powerful computers and ingenious software, discovering critical nuggets of knowledge about the brain and predicting the best approaches to healing dysfunctional brains are now possible. Those powerful methods of analyzing massive data are the vehicles for transforming science fiction to reality by assembling the jigsaw puzzle(s) of the human brain, arguably the last frontier in medical science.
My life experiences as a psychiatric neuroscientist have convinced me that nothing is beyond the reach of scientific research. Unraveling the divine brain’s complexities will eventually become reality. So, let us never stop dreaming and fantasizing!
1. Nasrallah HA, McCalley-Whitters M, Bigelow LB, et al. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res. 1983;8(4):251-260.
2. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affect Disord. 1982;4(1):15-19.
3. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cortical atrophy in schizophrenia and mania: a comparative CT study. J Clin Psychiatry. 1982;43(11):439-441.
4. Nasrallah HA, Skinner TE, Schmalbrock P, et al. Proton magnetic resonance spectroscopy (1H MRS) of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry. 1994;165(4):481-485.
5. Nasrallah HA, Olson SC, McCalley-Whitters M, et al. Cerebral ventricular enlargement in schizophrenia. A preliminary follow-up study. Arch Gen Psychiatry. 1986;43(2):157-159.
During my residency training years, I had many rosy and bold dreams about the future of psychiatry, hoping for many breakthroughs.
Early on, I decided to pursue an academic career, and specifically to focus on the neurobiology of schizophrenia, bipolar disorder, and other psychoses. I secured a neuroscience mentor, conducted a research project, and presented my findings at the American Psychiatric Association Annual Meeting. Although at the time everyone used the term “functional” to describe mental illnesses, I was convinced that they were all neurologic conditions, with prominent psychiatric manifestations. And I have been proven right.
After my residency, I eagerly pursued a neuroscience fellowship at the National Institutes of Health. My fantasy was that during my career as a psychiatric neuroscientist, brain exploration would uncover the many mysteries of psychiatric disorders. I was insightful enough to recognize that what I envisioned for the future of psychiatry qualified as science fiction, but I never stopped dreaming.
Today, the advances in psychiatric neuroscience that were unimaginable during my residency have become dazzling discoveries. My journey as a psychiatric neuroscientist has been more thrilling than I ever imagined. I recall doing postmortem research on the brains of hundreds of deceased psychiatric patients, noticing sulci widening and ventricular dilatation, and wondering whether one day we would be able to detect those atrophic changes while the patients were alive. Although I measured those changes in postmortem brains, I was cognizant that due to preservation artifacts, such measurements were less reliable than measurements of living brains.
And then the advent of neuroimaging fulfilled my fantasies. This began towards the end of my fellowship, and has exploded with neurobiologic findings throughout my academic career. Then came dramatic methodologies to probe brain molecular and cellular pathologies, followed by breakthrough clinical advances. Entirely new vistas of research into psychiatric brain disorders are opening every day. The exhilaration will never end!
From science fiction to clinical reality
Here is a quick outline of some of the “science fiction” of psychiatry that has come true since my training days. Back then, these discoveries were completely absent from the radar screen of psychiatry, when it was still a fledgling medical specialty struggling to emerge from the dominant yet nonempirical era of psychoanalysis.
Brain exploration methods. Unprecedented breakthroughs in computer technology have allowed psychiatric neuroscientists to create a new field of neuroimaging research that includes:
- cerebral blood flow (CBF)
- position emission tomography (PET)
- single photon emission computed tomography (SPECT).
Continue to: These functional neuroimaging...
These functional neuroimaging methods (using ionizing radiation) have enabled clinicians to see abnormal blood flow patterns in the brains of living patients. One of the earliest findings was hypofrontality in patients with schizophrenia, implicating frontal pathology in this severe brain disorder. PET was also used for dopamine and serotonin receptor imaging.
Computerized axia tomography. Compared with skull X-rays, CT (“CAT”) scans provided a more detailed view of brain tissue, and began a structural neuroimaging revolution that enriched psychiatric research, but also was applied to organs other than the brain.
Magnetic resonance imaging (MRI) became the “big kahuna” of neuroimaging when arrived in the early 1980s and quickly supplanted CT research because it is safer (no ionizing radiation, and it can be repeated multiple times with or without tasks). It also provided exquisite neuroanatomical details of brain tissue with stunning fidelity. Subsequently, several MRI techniques/software programs were developed that advanced research in psychiatry to multiple new frontiers, including:
- Morphological neuroimaging with MRI
- Magnetic resonance spectroscopy (MRS), which acts like a living, noninvasive biopsy of several chemicals (such as choline, lactate, glutamine, adenosine triphosphate, and the neuronal marker N-acetylcysteine) in a small volume (≤1 cc) of neural tissue in various regions
- Functional MRI (fMRI), which measures blood flow changes during actual or imagined tasks in the brains of patients vs healthy controls
- Diffusion tensor imaging (DTI), which evaluates the integrity of white matter (60% of brain volume, including 137,000 miles of myelinated fibers) by measuring the flow of water inside myelinated fibers (anisotropy and diffusivity). DTI of the corpus callosum, the largest brain commissure that is comprised of 200 million interhemispheric fibers, has revealed many abnormalities. This was one of the structures I investigated during my fellowship, including a histopathological study.1
All 4 of these neuroimaging techniques continue to generate a wealth of data about brain structure and function in psychosis, mood disorders, anxiety disorders, borderline personality disorder, obsessive-compulsive disorder, eating disorders, and substance use disorders. All these discoveries were utterly impossible to predict during my residency. I am proud to have published the first reports in the literature of ventricular enlargement in patients with bipolar disorder,2 cortical atrophy in schizophrenia and mania,3 reductions of hippocampal volume in patients with schizophrenia using MRS,4 and progressive brain atrophy in patients with schizophrenia.5 It is especially gratifying that I played a small role in translating my science fiction fantasies into clinical reality!
Other breakthrough methodologies that are advancing psychiatric neuroscience today but were science fiction during my residency days include:
- Pluripotent stem cells, which enable the de-differentiation of adult skin cells and then re-differentiating them into any type of cell, including neurons. This allows researchers to conduct studies on any patient’s brain cells without needing to do an invasive, high-risk brain biopsy. As a young resident, I would never have predicted that this virtual brain biopsy would be possible!
- Optogenetics, which enables controlling cell behavior using light and genetically encoded light-sensitive proteins. This triggered a cornucopia of neuroscience discoveries by using optogenetics to modulate cell-signaling cascades to understand cellular biology. Halorhodopsin and bacteriorhodopsin are used as tools to turn neurons off or on rapidly and safely.
- Genome-wide association studies (GWAS) have revolutionized the field of molecular neurogenetics and are enabling clinicians to detect risk genes by comparing the DNA samples of thousands of psychiatric patients with thousands of healthy controls. This is how several hundred risk genes have been identified for schizophrenia, bipolar disorder, autism spectrum disorder, and more to come.
- Clustered regularly interspaced short palindromic repeats (CRISPR) is a remarkable genetic “scissors” (that earned its inventors the 2020 Nobel Prize) that allows splicing out a disease gene and splicing in a normal gene. This will have an enormous future application in preventing an adulthood illness at its roots during fetal life. The future medical implications for psychiatric disorders are prodigious!
Continue to: Clinical advances
Clinical advances. Many therapies or approaches that did not exist during my residency (and how I dreamed about them back then!) are available to today’s clinicians. These include:
- Rapid-acting antidepressants that reverse severe and chronic depression and suicidal urges within a few hours or a couple of days. As a resident, I waited for weeks or months to see patients with depression reach the full remission that is now achieved practically the same day with IV ketamine, intranasal esketamine, IV scopolamine, and inhalable nitrous oxide. During my residency, the closest thing we had to a rapid-acting treatment for depression was electroconvulsive therapy (ECT), but that usually took 2 to 3 weeks. Psychiatric clinicians should never cease to appreciate how an intractable, treatment-refractory depression can rapidly be turned off like a light switch, restoring normal mood to desperately ill persons.
- Neuromodulation techniques are flourishing. Beyond ECT, transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), transcranial direct current stimulation (tDCS), deep brain stimulation (DBS), low field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near-infrared radiation (NIR), and focused ultrasound (FUS) are approved or under development, offering millions of patients with various neuropsychiatric disorders potential recovery not with pharmacotherapy, but via a brain-targeted approach.
- Telepsychiatry. Now taken for granted during the COVID-19 pandemic, telepsychiatry was completely unimaginable during my residency. Yes, we had phones, but not smartphones! The only “zoom” we knew was the furious sound of a sports car engine! To be able to see and evaluate a patient literally anywhere in the world was science fiction personified! Increased remote access to psychiatric care by patients everywhere is a truly remarkable advance that helped avoid a disastrous lack of psychiatric treatment during the current pandemic that brought in-person interactions between psychiatric physicians and their patients to a screeching halt.
- Neurobiologic effects of psychotherapy. Viewing psychotherapy as a neurobiologic treatment was totally unknown and unimaginable during my residency. I was heavily trained in various types of psychotherapies, but not once did any of my supervisors mention experiential neuroplasticity as a brain-altering process, or that psychotherapy changes brain structure, induces experimental neuroplasticity, and induces billions of dendritic spines in patients’ cortex and limbic structures, helping them connect the dots and develop new insights. No one knew that psychotherapy can mimic the neural effects of pharmacotherapy.
- Immunomodulatory effects of psychotherapy. It was completely unknown that psychotherapies such as cognitive-behavioral therapy can lower levels of inflammatory biomarkers in patients’ CSF and serum. Back then, no one imagined that psychotherapy had immunomodulatory effects. These discoveries are revolutionary for us psychiatrists and confirm the neurobiologic mechanisms of psychotherapy for every patient we treat.
- Epigenetics. This was rarely, if ever, mentioned when I was a resident. We knew from clinical studies that children who were abused or neglected often develop severe mood or psychotic disorders in adulthood. But we did not know that trauma modifies some genes via under- or overexpression, and that such epigenetic changes alter brain development towards psychopathology. The mysteries of psychiatric brain disorders generated by childhood trauma have been clarified by advances in epigenetics.
Aspirational, futuristic therapies. Even now, as a seasoned psychiatric neuroscientist, I continue to dream. Research is providing many clues for potentially radical psychiatric treatments that go beyond standard antipsychotics, antidepressants, mood stabilizers, or anxiolytics. But today, I fully expect that scientific dreams eventually come true through research. For example, the following neuroscientific therapeutics strategies may someday become routine in clinical practice:
- microglia inhibition
- mitochondria repair
- anti-apoptotic therapy
- white matter connectivity restoration
- neuroprotection (enhancing neurogenesis, increasing neurotropic factors, and enhancing synaptogenesis)
- reverse glutamate N-methyl-
d -aspartate hypofunction - prevent amyloid formation.
Data analysis breakthroughs. Side-by-side with the explosion of new findings and amassing mountains of data in psychiatric neuroscience, unprecedented and revolutionary data-management techniques have emerged to facilitate the herculean task of data analysis to extract the mythical needle in a haystack and derive the overall impact of masses of data. These techniques, whose names were not in our vocabulary during my residency days, include:
- machine learning
- artificial intelligence
- deep learning
- big data.
With the help of powerful computers and ingenious software, discovering critical nuggets of knowledge about the brain and predicting the best approaches to healing dysfunctional brains are now possible. Those powerful methods of analyzing massive data are the vehicles for transforming science fiction to reality by assembling the jigsaw puzzle(s) of the human brain, arguably the last frontier in medical science.
My life experiences as a psychiatric neuroscientist have convinced me that nothing is beyond the reach of scientific research. Unraveling the divine brain’s complexities will eventually become reality. So, let us never stop dreaming and fantasizing!
During my residency training years, I had many rosy and bold dreams about the future of psychiatry, hoping for many breakthroughs.
Early on, I decided to pursue an academic career, and specifically to focus on the neurobiology of schizophrenia, bipolar disorder, and other psychoses. I secured a neuroscience mentor, conducted a research project, and presented my findings at the American Psychiatric Association Annual Meeting. Although at the time everyone used the term “functional” to describe mental illnesses, I was convinced that they were all neurologic conditions, with prominent psychiatric manifestations. And I have been proven right.
After my residency, I eagerly pursued a neuroscience fellowship at the National Institutes of Health. My fantasy was that during my career as a psychiatric neuroscientist, brain exploration would uncover the many mysteries of psychiatric disorders. I was insightful enough to recognize that what I envisioned for the future of psychiatry qualified as science fiction, but I never stopped dreaming.
Today, the advances in psychiatric neuroscience that were unimaginable during my residency have become dazzling discoveries. My journey as a psychiatric neuroscientist has been more thrilling than I ever imagined. I recall doing postmortem research on the brains of hundreds of deceased psychiatric patients, noticing sulci widening and ventricular dilatation, and wondering whether one day we would be able to detect those atrophic changes while the patients were alive. Although I measured those changes in postmortem brains, I was cognizant that due to preservation artifacts, such measurements were less reliable than measurements of living brains.
And then the advent of neuroimaging fulfilled my fantasies. This began towards the end of my fellowship, and has exploded with neurobiologic findings throughout my academic career. Then came dramatic methodologies to probe brain molecular and cellular pathologies, followed by breakthrough clinical advances. Entirely new vistas of research into psychiatric brain disorders are opening every day. The exhilaration will never end!
From science fiction to clinical reality
Here is a quick outline of some of the “science fiction” of psychiatry that has come true since my training days. Back then, these discoveries were completely absent from the radar screen of psychiatry, when it was still a fledgling medical specialty struggling to emerge from the dominant yet nonempirical era of psychoanalysis.
Brain exploration methods. Unprecedented breakthroughs in computer technology have allowed psychiatric neuroscientists to create a new field of neuroimaging research that includes:
- cerebral blood flow (CBF)
- position emission tomography (PET)
- single photon emission computed tomography (SPECT).
Continue to: These functional neuroimaging...
These functional neuroimaging methods (using ionizing radiation) have enabled clinicians to see abnormal blood flow patterns in the brains of living patients. One of the earliest findings was hypofrontality in patients with schizophrenia, implicating frontal pathology in this severe brain disorder. PET was also used for dopamine and serotonin receptor imaging.
Computerized axia tomography. Compared with skull X-rays, CT (“CAT”) scans provided a more detailed view of brain tissue, and began a structural neuroimaging revolution that enriched psychiatric research, but also was applied to organs other than the brain.
Magnetic resonance imaging (MRI) became the “big kahuna” of neuroimaging when arrived in the early 1980s and quickly supplanted CT research because it is safer (no ionizing radiation, and it can be repeated multiple times with or without tasks). It also provided exquisite neuroanatomical details of brain tissue with stunning fidelity. Subsequently, several MRI techniques/software programs were developed that advanced research in psychiatry to multiple new frontiers, including:
- Morphological neuroimaging with MRI
- Magnetic resonance spectroscopy (MRS), which acts like a living, noninvasive biopsy of several chemicals (such as choline, lactate, glutamine, adenosine triphosphate, and the neuronal marker N-acetylcysteine) in a small volume (≤1 cc) of neural tissue in various regions
- Functional MRI (fMRI), which measures blood flow changes during actual or imagined tasks in the brains of patients vs healthy controls
- Diffusion tensor imaging (DTI), which evaluates the integrity of white matter (60% of brain volume, including 137,000 miles of myelinated fibers) by measuring the flow of water inside myelinated fibers (anisotropy and diffusivity). DTI of the corpus callosum, the largest brain commissure that is comprised of 200 million interhemispheric fibers, has revealed many abnormalities. This was one of the structures I investigated during my fellowship, including a histopathological study.1
All 4 of these neuroimaging techniques continue to generate a wealth of data about brain structure and function in psychosis, mood disorders, anxiety disorders, borderline personality disorder, obsessive-compulsive disorder, eating disorders, and substance use disorders. All these discoveries were utterly impossible to predict during my residency. I am proud to have published the first reports in the literature of ventricular enlargement in patients with bipolar disorder,2 cortical atrophy in schizophrenia and mania,3 reductions of hippocampal volume in patients with schizophrenia using MRS,4 and progressive brain atrophy in patients with schizophrenia.5 It is especially gratifying that I played a small role in translating my science fiction fantasies into clinical reality!
Other breakthrough methodologies that are advancing psychiatric neuroscience today but were science fiction during my residency days include:
- Pluripotent stem cells, which enable the de-differentiation of adult skin cells and then re-differentiating them into any type of cell, including neurons. This allows researchers to conduct studies on any patient’s brain cells without needing to do an invasive, high-risk brain biopsy. As a young resident, I would never have predicted that this virtual brain biopsy would be possible!
- Optogenetics, which enables controlling cell behavior using light and genetically encoded light-sensitive proteins. This triggered a cornucopia of neuroscience discoveries by using optogenetics to modulate cell-signaling cascades to understand cellular biology. Halorhodopsin and bacteriorhodopsin are used as tools to turn neurons off or on rapidly and safely.
- Genome-wide association studies (GWAS) have revolutionized the field of molecular neurogenetics and are enabling clinicians to detect risk genes by comparing the DNA samples of thousands of psychiatric patients with thousands of healthy controls. This is how several hundred risk genes have been identified for schizophrenia, bipolar disorder, autism spectrum disorder, and more to come.
- Clustered regularly interspaced short palindromic repeats (CRISPR) is a remarkable genetic “scissors” (that earned its inventors the 2020 Nobel Prize) that allows splicing out a disease gene and splicing in a normal gene. This will have an enormous future application in preventing an adulthood illness at its roots during fetal life. The future medical implications for psychiatric disorders are prodigious!
Continue to: Clinical advances
Clinical advances. Many therapies or approaches that did not exist during my residency (and how I dreamed about them back then!) are available to today’s clinicians. These include:
- Rapid-acting antidepressants that reverse severe and chronic depression and suicidal urges within a few hours or a couple of days. As a resident, I waited for weeks or months to see patients with depression reach the full remission that is now achieved practically the same day with IV ketamine, intranasal esketamine, IV scopolamine, and inhalable nitrous oxide. During my residency, the closest thing we had to a rapid-acting treatment for depression was electroconvulsive therapy (ECT), but that usually took 2 to 3 weeks. Psychiatric clinicians should never cease to appreciate how an intractable, treatment-refractory depression can rapidly be turned off like a light switch, restoring normal mood to desperately ill persons.
- Neuromodulation techniques are flourishing. Beyond ECT, transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), transcranial direct current stimulation (tDCS), deep brain stimulation (DBS), low field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near-infrared radiation (NIR), and focused ultrasound (FUS) are approved or under development, offering millions of patients with various neuropsychiatric disorders potential recovery not with pharmacotherapy, but via a brain-targeted approach.
- Telepsychiatry. Now taken for granted during the COVID-19 pandemic, telepsychiatry was completely unimaginable during my residency. Yes, we had phones, but not smartphones! The only “zoom” we knew was the furious sound of a sports car engine! To be able to see and evaluate a patient literally anywhere in the world was science fiction personified! Increased remote access to psychiatric care by patients everywhere is a truly remarkable advance that helped avoid a disastrous lack of psychiatric treatment during the current pandemic that brought in-person interactions between psychiatric physicians and their patients to a screeching halt.
- Neurobiologic effects of psychotherapy. Viewing psychotherapy as a neurobiologic treatment was totally unknown and unimaginable during my residency. I was heavily trained in various types of psychotherapies, but not once did any of my supervisors mention experiential neuroplasticity as a brain-altering process, or that psychotherapy changes brain structure, induces experimental neuroplasticity, and induces billions of dendritic spines in patients’ cortex and limbic structures, helping them connect the dots and develop new insights. No one knew that psychotherapy can mimic the neural effects of pharmacotherapy.
- Immunomodulatory effects of psychotherapy. It was completely unknown that psychotherapies such as cognitive-behavioral therapy can lower levels of inflammatory biomarkers in patients’ CSF and serum. Back then, no one imagined that psychotherapy had immunomodulatory effects. These discoveries are revolutionary for us psychiatrists and confirm the neurobiologic mechanisms of psychotherapy for every patient we treat.
- Epigenetics. This was rarely, if ever, mentioned when I was a resident. We knew from clinical studies that children who were abused or neglected often develop severe mood or psychotic disorders in adulthood. But we did not know that trauma modifies some genes via under- or overexpression, and that such epigenetic changes alter brain development towards psychopathology. The mysteries of psychiatric brain disorders generated by childhood trauma have been clarified by advances in epigenetics.
Aspirational, futuristic therapies. Even now, as a seasoned psychiatric neuroscientist, I continue to dream. Research is providing many clues for potentially radical psychiatric treatments that go beyond standard antipsychotics, antidepressants, mood stabilizers, or anxiolytics. But today, I fully expect that scientific dreams eventually come true through research. For example, the following neuroscientific therapeutics strategies may someday become routine in clinical practice:
- microglia inhibition
- mitochondria repair
- anti-apoptotic therapy
- white matter connectivity restoration
- neuroprotection (enhancing neurogenesis, increasing neurotropic factors, and enhancing synaptogenesis)
- reverse glutamate N-methyl-
d -aspartate hypofunction - prevent amyloid formation.
Data analysis breakthroughs. Side-by-side with the explosion of new findings and amassing mountains of data in psychiatric neuroscience, unprecedented and revolutionary data-management techniques have emerged to facilitate the herculean task of data analysis to extract the mythical needle in a haystack and derive the overall impact of masses of data. These techniques, whose names were not in our vocabulary during my residency days, include:
- machine learning
- artificial intelligence
- deep learning
- big data.
With the help of powerful computers and ingenious software, discovering critical nuggets of knowledge about the brain and predicting the best approaches to healing dysfunctional brains are now possible. Those powerful methods of analyzing massive data are the vehicles for transforming science fiction to reality by assembling the jigsaw puzzle(s) of the human brain, arguably the last frontier in medical science.
My life experiences as a psychiatric neuroscientist have convinced me that nothing is beyond the reach of scientific research. Unraveling the divine brain’s complexities will eventually become reality. So, let us never stop dreaming and fantasizing!
1. Nasrallah HA, McCalley-Whitters M, Bigelow LB, et al. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res. 1983;8(4):251-260.
2. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affect Disord. 1982;4(1):15-19.
3. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cortical atrophy in schizophrenia and mania: a comparative CT study. J Clin Psychiatry. 1982;43(11):439-441.
4. Nasrallah HA, Skinner TE, Schmalbrock P, et al. Proton magnetic resonance spectroscopy (1H MRS) of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry. 1994;165(4):481-485.
5. Nasrallah HA, Olson SC, McCalley-Whitters M, et al. Cerebral ventricular enlargement in schizophrenia. A preliminary follow-up study. Arch Gen Psychiatry. 1986;43(2):157-159.
1. Nasrallah HA, McCalley-Whitters M, Bigelow LB, et al. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res. 1983;8(4):251-260.
2. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affect Disord. 1982;4(1):15-19.
3. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cortical atrophy in schizophrenia and mania: a comparative CT study. J Clin Psychiatry. 1982;43(11):439-441.
4. Nasrallah HA, Skinner TE, Schmalbrock P, et al. Proton magnetic resonance spectroscopy (1H MRS) of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry. 1994;165(4):481-485.
5. Nasrallah HA, Olson SC, McCalley-Whitters M, et al. Cerebral ventricular enlargement in schizophrenia. A preliminary follow-up study. Arch Gen Psychiatry. 1986;43(2):157-159.
Nothing up his sleeve: Decompensation after bariatric surgery
CASE Sudden-onset low mood
Mr. G, age 64, is obese (body mass index [BMI] 37 kg/m2) and has a history of schizoaffective disorder. He is recovering from a sleeve gastrectomy, a surgical weight-loss procedure in which a large portion of the stomach is removed. Seven weeks after his surgery, he experiences a sudden onset of “low mood” and fears that he will become suicidal; he has a history of suicide attempts. Mr. G calls his long-term outpatient clinic and is advised to go to the emergency department (ED).
For years, Mr. G had been stable in a group home setting, and had always been adherent to treatment and forthcoming about his medications with both his bariatric surgeon and psychiatrist. Within the last month, he had been seen at the clinic, had no psychiatric symptoms, and was recovering well from the sleeve gastrectomy.
HISTORY A stable regimen
Mr. G’s psychiatric symptoms initially developed when he was in his 20s, during a time in which he reported using “a lot of drugs.” He had multiple suicide attempts, and multiple inpatient and outpatient treatments. He was diagnosed with schizoaffective disorder.
Mr. G has been stable on medications for the last 2 years. His outpatient psychotropic regimen is divalproex sodium extended-release (ER), 2,500 mg every night at bedtime; iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime.
In the group home, Mr. G spends his days socializing, studying philosophy, and writing essays. He hopes to find a job in the craftsman industry.
Mr. G’s medical history includes obesity (BMI: 37 kg/m2). Since the surgery, he has been receiving omeprazole, 40 mg/d, a proton pump inhibitor (PPI), to decrease the amount of acid in his stomach. Three weeks after surgery, he had an unremarkable postoperative outpatient psychiatry visit. Divalproex sodium ER was maintained at the pre-surgical dose of 2,500 mg/d.
EVALUATION Depressed and frightened
In the ED, Mr. G’s vitals are normal, but his serum valproic acid (VPA) level is 33.68 µg/mL (therapeutic range: 50 to 125 µg/mL), despite being compliant with treatment. Mr. G is discharged from the ED and told to follow up with his outpatient psychiatrist the next day.
Continue to: At his outpatient psychiatry appointment...
At his outpatient psychiatry appointment, Mr. G’s vital signs are normal, but he reports increasing depression and worsened mood. On mental status examination, Mr. G’s appearance is well groomed, and no agitation nor fidgeting are observed. His behavior is cooperative but somewhat disorganized. He is perseverative on “feeling so low.” He has poor eye contact, which is unusual for him. Mr. G’s speech is loud compared with his baseline. Affect is congruent to mood, which he describes as “depressed and frightened.” He is also noted to be irritable. His thought process is abstract and tangential, which is within his baseline. Mr. G’s thought content is fearful and negativistic, despite his usual positivity and optimism. He denies hallucinations and is oriented to time, place, and person. His judgment, attention, and memory are all within normal limits.
[polldaddy:10790537]
The authors’ observations
The psychiatrist rules out malingering/nonadherence due to Mr. G’s long history of treatment compliance, as evidenced by his past symptom control and therapeutic serum VPA levels. Mr. G was compliant with his postoperative appointments and has been healing well. Therefore, the treatment team believed that Mr. G’s intense and acute decompensation had to be related to a recent change. The notable changes in Mr. G’s case included his sleeve gastrectomy, and the addition of omeprazole to his medication regimen.
The treatment team observed that Mr. G had a long history of compliance with his medications and his symptoms were consistent with a low serum VPA level, which led to the conclusion that the low serum VPA level measured while he was in the ED was likely accurate. This prompted the team to consider Mr. G’s recent surgery. It is well documented that some bariatric surgeries can cause poor absorption of certain vitamins, minerals, and medications. However, Mr. G had a sleeve gastrectomy, which preserves absorption. At this point, the team considered if the patient’s recent medication change was the source of his low VPA level.
The psychiatrist concluded that the issue must have been with the addition of omeprazole because Mr. G’s sleeve gastrectomy was noneventful, he was healing well and being closely monitored by his bariatric surgeon, and this type of surgery preserves absorption. Fortunately, Mr. G was a good historian and had informed his psychiatrist about the addition of omeprazole after his sleeve gastrectomy. The psychiatrist knew acidity was important for the absorption of some medications. Although she was unsure as to whether the problem was a lack of absorption or lack of delivery, the psychiatrist knew a medication change was necessary to raise Mr. G’s serum VPA levels.
TREATMENT A change in divalproex formulation
The psychiatrist switches Mr. G’s formulation of divalproex sodium ER, 2,500 mg/d, to valproic acid immediate-release (IR) liquid capsules. He receives a total daily dose of 2,500 mg, but the dosage is split into 3 times a day. The omeprazole is continued to maintain the postoperative healing process, and he receives his other medications as well (iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime).
[polldaddy:10790540]
Continue to: The authors' observations
The authors’ observations
The key component to creating a treatment plan for Mr. G centered on understanding drug metabolism and delivery. Acidity plays a role in dissolution of many medications, which led the team to surmise that the PPI, omeprazole, was the culprit. Through research, they understood that the divalproex sodium ER formulation needed a more acidic environment to dissolve, and therefore, an IR formulation was needed.
Different formulations, different characteristics
Medications can be produced in different formulations such as IR, delayed-release (DR), and ER formulations. Different formulations may contain the same medication at identical strengths; however, they may not be bioequivalent and should be titrated based on both the properties of the medication and the release type.1
Immediate-release formulations are developed to dissolve without delaying or prolonging absorption of the medication. These formulations typically include “superdisintegrants” containing croscarmellose sodium2 so that they disintegrate, de-aggregate, and/or dissolve when they come into contact with water or the gastrointestinal tract.3-7
Delayed-release formulations rely on the gastrointestinal pH to release the medication after a certain amount of time has elapsed due to the enteric coating surrounding the tablet. This enteric coating prevents gastric mucosa/gastric juices from inactivating an acid-labile medication.8
Extended-release formulations, such as the divalproex sodium ER that was originally prescribed to Mr. G, are designed to release the medication in a controlled manner over an extended period of time, and at a predetermined rate and location following administration.8-9 The advantage of this type of formulation is that it can be used to reduce dose frequency and improve adherence.10 Extended-release formulations are designed to minimize fluctuations in serum drug concentration between doses,11 thereby reducing the risk of adverse effects.12,13 A list of some common extended-release psychiatric medications is shown in the Table.
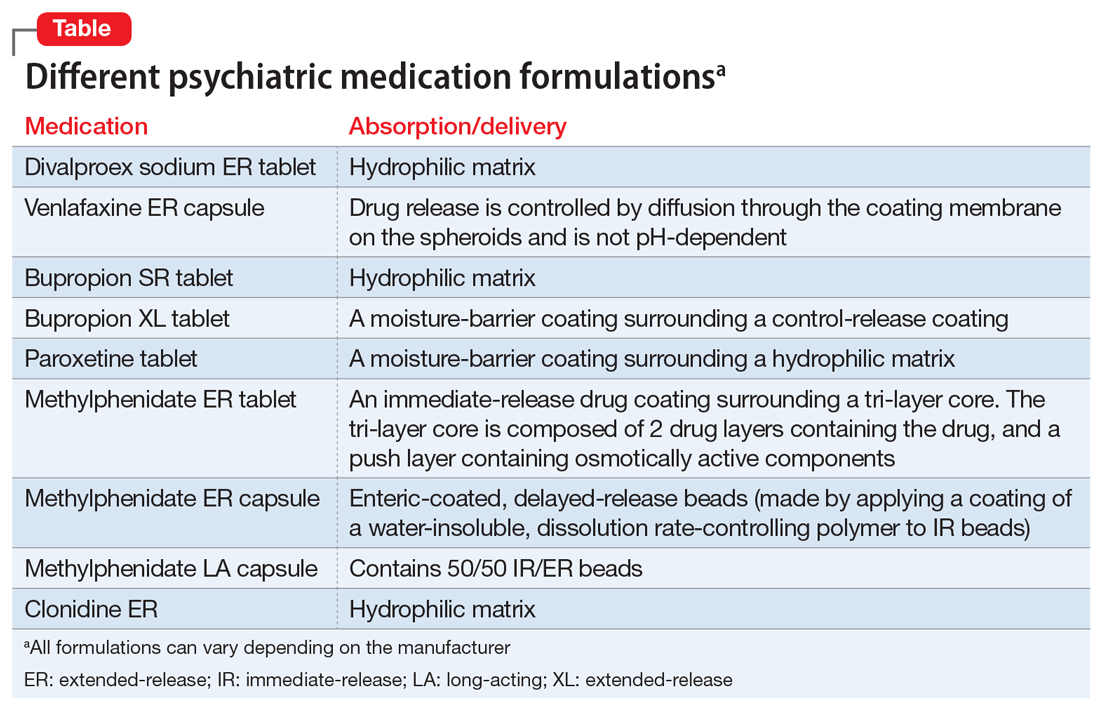
Continue to: The 5 oral formulations...
The 5 oral formulations of medications that contain valproic acid include:
- syrup
- capsule
- sprinkle
- enteric-coated delayed-release and extended-release
A parenteral form via IV is available for patients who are unable to swallow.
Absorption vs delivery
Any gastric bypass surgery can have postoperative complications, one of which can include absorption deficiencies of vitamins and minerals. Sleeve gastrectomy has the least amount of absorption-related nutritional deficiencies.14 Additionally, this procedure preserves the stomach’s ability to produce gastric acid. Therefore, regardless of formulation, there should be no initial postsurgical need to change psychotropic medication formulations. However, because VPA is related to B-vitamin deficiency, supplementation can be considered.
Omeprazole is a PPI that increases pH in the stomach and is often prescribed to promote healing of gastric surgery. However, in Mr. G’s case, omeprazole created a non-acidic environment in his stomach, which prevented the divalproex sodium ER formulation from being dissolved and the medication from being delivered. Mr. G’s absorption ability was preserved, which was confirmed by his rapid recovery and increased serum VPA levels once he was switched to the IR formulation. There is no literature supporting a recommended length of time a patient can receive omeprazole therapy for sleeve gastrectomy; this is at the surgeon’s discretion. Mr. G’s prescription for omeprazole was for 3 months.
Proper valproate dosing
In Mr. G’s case, it could be hypothesized that the VPA dosing was incorrect. For mood disorders, oral VPA dosing is 25 mg/kg/d. Mr. G lost 40 pounds, which would translate to a 450-mg reduction in dose. Despite maintaining his original dose, his serum VPA levels decreased by almost 50% and could not be attributed to trough measurement. In this case, Mr. G was prescribed a higher dose than needed given his weight loss.
Continue to: Divalproex sodium ER...
Divalproex sodium ER is a hydrophilic matrix tablet that requires a low pH to dissolve. Switching to an IR formulation bypassed the need for a low pH and the VPA was delivered and absorbed. Mr. G was always able to absorb the medication, but only when delivered. The Table lists other psychiatric medications that clinicians should be aware of that utilize similar hydrophilic matrix technology to slowly release medications through the gastrointestinal tract and also require low pH to release the medication from the tablet.
OUTCOME Stable once again
Two and a half weeks after his medication formulation is changed from divalproex sodium ER to IR, Mr. G shows improvement in his symptoms. His serum VPA level is 52 µg/mL, which is within therapeutic limits. He continues receiving his previous medications as well. He reports “feeling much better” and denies having any depressive symptoms nor anxiety. Mr. G is able to maintain eye contact, and has positive thought content, improved organization of thinking, and retained abstraction.
Bottom Line
All medication changes should be identified at each visit. Many extended-release psychiatric medications require lower pH to release the medication from the tablet. When evaluating nonresponse to psychotropic medications, anything that affects pH in the stomach should be considered.
Related Resources
- Monte SV, Russo KM, Mustafa E. Impact of sleeve gastrectomy on psychiatric medication use and symptoms. J Obes. 2018; 2018:8532602. doi: 10.1155/2018/8532602
- Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
- ObesityHelp, Inc. https://www.obesityhelp.com/medications-after-bariatric-surgery-wls/
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Clonidine ER • Kapvay
Divalproex sodium extended- release tablets • Depakote ER
Escitalopram • Lexapro
Iloperidone • Fanapt
Methylphenidate ER tablet • Concerta
Methylphenidate ER capsule • Metadate, Jornay
Methylphenidate LA capsule • Ritalin LA
Mirtazapine • Remeron
Omeprazole • Prilosec, Zegerid
Paroxetine • Paxil
Valproic acid immediate- release capsules and solution • Depakene
Valproate sodium IV • Depacon
Venlafaxine • Effexor
1. Wheless JW, Phelps SJ. A clinician’s guide to oral extended-release drug delivery systems in epilepsy. J Pediatr Pharmacol Ther. 2018;23(4):277-292.
2. Jaimini M, Ranga S, Kumar A, et al. A review on immediate release drug delivery system by using design of experiment. J Drug Discov Therap. 2013;1(12):21-27.
3. Bhandari N, Kumar A, Choudhary A, et al. A review on immediate release drug delivery system. Int Res J Pharm App Sci. 2014;49(1):78-87.
4. Eatock J, Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatr Dis Treat. 2007;3(1):117-131.
5. Manjunath R, Davis KL, Candrilli SD, et al. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372-378.
6. Samsonsen C, Reimers A, Bråthen G, et al. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia. 2014;55(11):e125-e128. doi: 10.1111/epi.12801
7. Mangal M, Thakral S, Goswami M, et al. Superdisintegrants: an updated review. Int Pharmacy Pharmaceut Sci Res. 2012;2(2):26-35.
8. Tablets. United States Pharmacopeia. Accessed January 21, 2021. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1151s87.html
9. Holquist C, Fava W. FDA safety page: delayed- vs. extended-release Rxs. Drug Topics. Published July 23, 2007. Accessed January 21, 2021. https://www.drugtopics.com/view/fda-safety-page-delayed-release-vs-extended-release-rxs
10. Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
11. Perucca E. Extended-release formulations of antiepileptic drugs: rationale and comparative value. Epilepsy Curr. 2009;9(6):153-157.
12. Bialer M. Extended-release formulations for the treatment of epilepsy. CNS Drugs. 2007;21(9):765-774.
13. Pellock JM, Smith MC, Cloyd JC, et al. Extended-release formulations: simplifying strategies in the management of antiepileptic drug therapy. Epilepsy Behav. 2004;5(3):301-307.
14. Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg 2013;56(5):347-352.
CASE Sudden-onset low mood
Mr. G, age 64, is obese (body mass index [BMI] 37 kg/m2) and has a history of schizoaffective disorder. He is recovering from a sleeve gastrectomy, a surgical weight-loss procedure in which a large portion of the stomach is removed. Seven weeks after his surgery, he experiences a sudden onset of “low mood” and fears that he will become suicidal; he has a history of suicide attempts. Mr. G calls his long-term outpatient clinic and is advised to go to the emergency department (ED).
For years, Mr. G had been stable in a group home setting, and had always been adherent to treatment and forthcoming about his medications with both his bariatric surgeon and psychiatrist. Within the last month, he had been seen at the clinic, had no psychiatric symptoms, and was recovering well from the sleeve gastrectomy.
HISTORY A stable regimen
Mr. G’s psychiatric symptoms initially developed when he was in his 20s, during a time in which he reported using “a lot of drugs.” He had multiple suicide attempts, and multiple inpatient and outpatient treatments. He was diagnosed with schizoaffective disorder.
Mr. G has been stable on medications for the last 2 years. His outpatient psychotropic regimen is divalproex sodium extended-release (ER), 2,500 mg every night at bedtime; iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime.
In the group home, Mr. G spends his days socializing, studying philosophy, and writing essays. He hopes to find a job in the craftsman industry.
Mr. G’s medical history includes obesity (BMI: 37 kg/m2). Since the surgery, he has been receiving omeprazole, 40 mg/d, a proton pump inhibitor (PPI), to decrease the amount of acid in his stomach. Three weeks after surgery, he had an unremarkable postoperative outpatient psychiatry visit. Divalproex sodium ER was maintained at the pre-surgical dose of 2,500 mg/d.
EVALUATION Depressed and frightened
In the ED, Mr. G’s vitals are normal, but his serum valproic acid (VPA) level is 33.68 µg/mL (therapeutic range: 50 to 125 µg/mL), despite being compliant with treatment. Mr. G is discharged from the ED and told to follow up with his outpatient psychiatrist the next day.
Continue to: At his outpatient psychiatry appointment...
At his outpatient psychiatry appointment, Mr. G’s vital signs are normal, but he reports increasing depression and worsened mood. On mental status examination, Mr. G’s appearance is well groomed, and no agitation nor fidgeting are observed. His behavior is cooperative but somewhat disorganized. He is perseverative on “feeling so low.” He has poor eye contact, which is unusual for him. Mr. G’s speech is loud compared with his baseline. Affect is congruent to mood, which he describes as “depressed and frightened.” He is also noted to be irritable. His thought process is abstract and tangential, which is within his baseline. Mr. G’s thought content is fearful and negativistic, despite his usual positivity and optimism. He denies hallucinations and is oriented to time, place, and person. His judgment, attention, and memory are all within normal limits.
[polldaddy:10790537]
The authors’ observations
The psychiatrist rules out malingering/nonadherence due to Mr. G’s long history of treatment compliance, as evidenced by his past symptom control and therapeutic serum VPA levels. Mr. G was compliant with his postoperative appointments and has been healing well. Therefore, the treatment team believed that Mr. G’s intense and acute decompensation had to be related to a recent change. The notable changes in Mr. G’s case included his sleeve gastrectomy, and the addition of omeprazole to his medication regimen.
The treatment team observed that Mr. G had a long history of compliance with his medications and his symptoms were consistent with a low serum VPA level, which led to the conclusion that the low serum VPA level measured while he was in the ED was likely accurate. This prompted the team to consider Mr. G’s recent surgery. It is well documented that some bariatric surgeries can cause poor absorption of certain vitamins, minerals, and medications. However, Mr. G had a sleeve gastrectomy, which preserves absorption. At this point, the team considered if the patient’s recent medication change was the source of his low VPA level.
The psychiatrist concluded that the issue must have been with the addition of omeprazole because Mr. G’s sleeve gastrectomy was noneventful, he was healing well and being closely monitored by his bariatric surgeon, and this type of surgery preserves absorption. Fortunately, Mr. G was a good historian and had informed his psychiatrist about the addition of omeprazole after his sleeve gastrectomy. The psychiatrist knew acidity was important for the absorption of some medications. Although she was unsure as to whether the problem was a lack of absorption or lack of delivery, the psychiatrist knew a medication change was necessary to raise Mr. G’s serum VPA levels.
TREATMENT A change in divalproex formulation
The psychiatrist switches Mr. G’s formulation of divalproex sodium ER, 2,500 mg/d, to valproic acid immediate-release (IR) liquid capsules. He receives a total daily dose of 2,500 mg, but the dosage is split into 3 times a day. The omeprazole is continued to maintain the postoperative healing process, and he receives his other medications as well (iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime).
[polldaddy:10790540]
Continue to: The authors' observations
The authors’ observations
The key component to creating a treatment plan for Mr. G centered on understanding drug metabolism and delivery. Acidity plays a role in dissolution of many medications, which led the team to surmise that the PPI, omeprazole, was the culprit. Through research, they understood that the divalproex sodium ER formulation needed a more acidic environment to dissolve, and therefore, an IR formulation was needed.
Different formulations, different characteristics
Medications can be produced in different formulations such as IR, delayed-release (DR), and ER formulations. Different formulations may contain the same medication at identical strengths; however, they may not be bioequivalent and should be titrated based on both the properties of the medication and the release type.1
Immediate-release formulations are developed to dissolve without delaying or prolonging absorption of the medication. These formulations typically include “superdisintegrants” containing croscarmellose sodium2 so that they disintegrate, de-aggregate, and/or dissolve when they come into contact with water or the gastrointestinal tract.3-7
Delayed-release formulations rely on the gastrointestinal pH to release the medication after a certain amount of time has elapsed due to the enteric coating surrounding the tablet. This enteric coating prevents gastric mucosa/gastric juices from inactivating an acid-labile medication.8
Extended-release formulations, such as the divalproex sodium ER that was originally prescribed to Mr. G, are designed to release the medication in a controlled manner over an extended period of time, and at a predetermined rate and location following administration.8-9 The advantage of this type of formulation is that it can be used to reduce dose frequency and improve adherence.10 Extended-release formulations are designed to minimize fluctuations in serum drug concentration between doses,11 thereby reducing the risk of adverse effects.12,13 A list of some common extended-release psychiatric medications is shown in the Table.

Continue to: The 5 oral formulations...
The 5 oral formulations of medications that contain valproic acid include:
- syrup
- capsule
- sprinkle
- enteric-coated delayed-release and extended-release
A parenteral form via IV is available for patients who are unable to swallow.
Absorption vs delivery
Any gastric bypass surgery can have postoperative complications, one of which can include absorption deficiencies of vitamins and minerals. Sleeve gastrectomy has the least amount of absorption-related nutritional deficiencies.14 Additionally, this procedure preserves the stomach’s ability to produce gastric acid. Therefore, regardless of formulation, there should be no initial postsurgical need to change psychotropic medication formulations. However, because VPA is related to B-vitamin deficiency, supplementation can be considered.
Omeprazole is a PPI that increases pH in the stomach and is often prescribed to promote healing of gastric surgery. However, in Mr. G’s case, omeprazole created a non-acidic environment in his stomach, which prevented the divalproex sodium ER formulation from being dissolved and the medication from being delivered. Mr. G’s absorption ability was preserved, which was confirmed by his rapid recovery and increased serum VPA levels once he was switched to the IR formulation. There is no literature supporting a recommended length of time a patient can receive omeprazole therapy for sleeve gastrectomy; this is at the surgeon’s discretion. Mr. G’s prescription for omeprazole was for 3 months.
Proper valproate dosing
In Mr. G’s case, it could be hypothesized that the VPA dosing was incorrect. For mood disorders, oral VPA dosing is 25 mg/kg/d. Mr. G lost 40 pounds, which would translate to a 450-mg reduction in dose. Despite maintaining his original dose, his serum VPA levels decreased by almost 50% and could not be attributed to trough measurement. In this case, Mr. G was prescribed a higher dose than needed given his weight loss.
Continue to: Divalproex sodium ER...
Divalproex sodium ER is a hydrophilic matrix tablet that requires a low pH to dissolve. Switching to an IR formulation bypassed the need for a low pH and the VPA was delivered and absorbed. Mr. G was always able to absorb the medication, but only when delivered. The Table lists other psychiatric medications that clinicians should be aware of that utilize similar hydrophilic matrix technology to slowly release medications through the gastrointestinal tract and also require low pH to release the medication from the tablet.
OUTCOME Stable once again
Two and a half weeks after his medication formulation is changed from divalproex sodium ER to IR, Mr. G shows improvement in his symptoms. His serum VPA level is 52 µg/mL, which is within therapeutic limits. He continues receiving his previous medications as well. He reports “feeling much better” and denies having any depressive symptoms nor anxiety. Mr. G is able to maintain eye contact, and has positive thought content, improved organization of thinking, and retained abstraction.
Bottom Line
All medication changes should be identified at each visit. Many extended-release psychiatric medications require lower pH to release the medication from the tablet. When evaluating nonresponse to psychotropic medications, anything that affects pH in the stomach should be considered.
Related Resources
- Monte SV, Russo KM, Mustafa E. Impact of sleeve gastrectomy on psychiatric medication use and symptoms. J Obes. 2018; 2018:8532602. doi: 10.1155/2018/8532602
- Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
- ObesityHelp, Inc. https://www.obesityhelp.com/medications-after-bariatric-surgery-wls/
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Clonidine ER • Kapvay
Divalproex sodium extended- release tablets • Depakote ER
Escitalopram • Lexapro
Iloperidone • Fanapt
Methylphenidate ER tablet • Concerta
Methylphenidate ER capsule • Metadate, Jornay
Methylphenidate LA capsule • Ritalin LA
Mirtazapine • Remeron
Omeprazole • Prilosec, Zegerid
Paroxetine • Paxil
Valproic acid immediate- release capsules and solution • Depakene
Valproate sodium IV • Depacon
Venlafaxine • Effexor
CASE Sudden-onset low mood
Mr. G, age 64, is obese (body mass index [BMI] 37 kg/m2) and has a history of schizoaffective disorder. He is recovering from a sleeve gastrectomy, a surgical weight-loss procedure in which a large portion of the stomach is removed. Seven weeks after his surgery, he experiences a sudden onset of “low mood” and fears that he will become suicidal; he has a history of suicide attempts. Mr. G calls his long-term outpatient clinic and is advised to go to the emergency department (ED).
For years, Mr. G had been stable in a group home setting, and had always been adherent to treatment and forthcoming about his medications with both his bariatric surgeon and psychiatrist. Within the last month, he had been seen at the clinic, had no psychiatric symptoms, and was recovering well from the sleeve gastrectomy.
HISTORY A stable regimen
Mr. G’s psychiatric symptoms initially developed when he was in his 20s, during a time in which he reported using “a lot of drugs.” He had multiple suicide attempts, and multiple inpatient and outpatient treatments. He was diagnosed with schizoaffective disorder.
Mr. G has been stable on medications for the last 2 years. His outpatient psychotropic regimen is divalproex sodium extended-release (ER), 2,500 mg every night at bedtime; iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime.
In the group home, Mr. G spends his days socializing, studying philosophy, and writing essays. He hopes to find a job in the craftsman industry.
Mr. G’s medical history includes obesity (BMI: 37 kg/m2). Since the surgery, he has been receiving omeprazole, 40 mg/d, a proton pump inhibitor (PPI), to decrease the amount of acid in his stomach. Three weeks after surgery, he had an unremarkable postoperative outpatient psychiatry visit. Divalproex sodium ER was maintained at the pre-surgical dose of 2,500 mg/d.
EVALUATION Depressed and frightened
In the ED, Mr. G’s vitals are normal, but his serum valproic acid (VPA) level is 33.68 µg/mL (therapeutic range: 50 to 125 µg/mL), despite being compliant with treatment. Mr. G is discharged from the ED and told to follow up with his outpatient psychiatrist the next day.
Continue to: At his outpatient psychiatry appointment...
At his outpatient psychiatry appointment, Mr. G’s vital signs are normal, but he reports increasing depression and worsened mood. On mental status examination, Mr. G’s appearance is well groomed, and no agitation nor fidgeting are observed. His behavior is cooperative but somewhat disorganized. He is perseverative on “feeling so low.” He has poor eye contact, which is unusual for him. Mr. G’s speech is loud compared with his baseline. Affect is congruent to mood, which he describes as “depressed and frightened.” He is also noted to be irritable. His thought process is abstract and tangential, which is within his baseline. Mr. G’s thought content is fearful and negativistic, despite his usual positivity and optimism. He denies hallucinations and is oriented to time, place, and person. His judgment, attention, and memory are all within normal limits.
[polldaddy:10790537]
The authors’ observations
The psychiatrist rules out malingering/nonadherence due to Mr. G’s long history of treatment compliance, as evidenced by his past symptom control and therapeutic serum VPA levels. Mr. G was compliant with his postoperative appointments and has been healing well. Therefore, the treatment team believed that Mr. G’s intense and acute decompensation had to be related to a recent change. The notable changes in Mr. G’s case included his sleeve gastrectomy, and the addition of omeprazole to his medication regimen.
The treatment team observed that Mr. G had a long history of compliance with his medications and his symptoms were consistent with a low serum VPA level, which led to the conclusion that the low serum VPA level measured while he was in the ED was likely accurate. This prompted the team to consider Mr. G’s recent surgery. It is well documented that some bariatric surgeries can cause poor absorption of certain vitamins, minerals, and medications. However, Mr. G had a sleeve gastrectomy, which preserves absorption. At this point, the team considered if the patient’s recent medication change was the source of his low VPA level.
The psychiatrist concluded that the issue must have been with the addition of omeprazole because Mr. G’s sleeve gastrectomy was noneventful, he was healing well and being closely monitored by his bariatric surgeon, and this type of surgery preserves absorption. Fortunately, Mr. G was a good historian and had informed his psychiatrist about the addition of omeprazole after his sleeve gastrectomy. The psychiatrist knew acidity was important for the absorption of some medications. Although she was unsure as to whether the problem was a lack of absorption or lack of delivery, the psychiatrist knew a medication change was necessary to raise Mr. G’s serum VPA levels.
TREATMENT A change in divalproex formulation
The psychiatrist switches Mr. G’s formulation of divalproex sodium ER, 2,500 mg/d, to valproic acid immediate-release (IR) liquid capsules. He receives a total daily dose of 2,500 mg, but the dosage is split into 3 times a day. The omeprazole is continued to maintain the postoperative healing process, and he receives his other medications as well (iloperidone, 8 mg twice a day; escitalopram, 10 mg/d; and mirtazapine, 30 mg every night at bedtime).
[polldaddy:10790540]
Continue to: The authors' observations
The authors’ observations
The key component to creating a treatment plan for Mr. G centered on understanding drug metabolism and delivery. Acidity plays a role in dissolution of many medications, which led the team to surmise that the PPI, omeprazole, was the culprit. Through research, they understood that the divalproex sodium ER formulation needed a more acidic environment to dissolve, and therefore, an IR formulation was needed.
Different formulations, different characteristics
Medications can be produced in different formulations such as IR, delayed-release (DR), and ER formulations. Different formulations may contain the same medication at identical strengths; however, they may not be bioequivalent and should be titrated based on both the properties of the medication and the release type.1
Immediate-release formulations are developed to dissolve without delaying or prolonging absorption of the medication. These formulations typically include “superdisintegrants” containing croscarmellose sodium2 so that they disintegrate, de-aggregate, and/or dissolve when they come into contact with water or the gastrointestinal tract.3-7
Delayed-release formulations rely on the gastrointestinal pH to release the medication after a certain amount of time has elapsed due to the enteric coating surrounding the tablet. This enteric coating prevents gastric mucosa/gastric juices from inactivating an acid-labile medication.8
Extended-release formulations, such as the divalproex sodium ER that was originally prescribed to Mr. G, are designed to release the medication in a controlled manner over an extended period of time, and at a predetermined rate and location following administration.8-9 The advantage of this type of formulation is that it can be used to reduce dose frequency and improve adherence.10 Extended-release formulations are designed to minimize fluctuations in serum drug concentration between doses,11 thereby reducing the risk of adverse effects.12,13 A list of some common extended-release psychiatric medications is shown in the Table.

Continue to: The 5 oral formulations...
The 5 oral formulations of medications that contain valproic acid include:
- syrup
- capsule
- sprinkle
- enteric-coated delayed-release and extended-release
A parenteral form via IV is available for patients who are unable to swallow.
Absorption vs delivery
Any gastric bypass surgery can have postoperative complications, one of which can include absorption deficiencies of vitamins and minerals. Sleeve gastrectomy has the least amount of absorption-related nutritional deficiencies.14 Additionally, this procedure preserves the stomach’s ability to produce gastric acid. Therefore, regardless of formulation, there should be no initial postsurgical need to change psychotropic medication formulations. However, because VPA is related to B-vitamin deficiency, supplementation can be considered.
Omeprazole is a PPI that increases pH in the stomach and is often prescribed to promote healing of gastric surgery. However, in Mr. G’s case, omeprazole created a non-acidic environment in his stomach, which prevented the divalproex sodium ER formulation from being dissolved and the medication from being delivered. Mr. G’s absorption ability was preserved, which was confirmed by his rapid recovery and increased serum VPA levels once he was switched to the IR formulation. There is no literature supporting a recommended length of time a patient can receive omeprazole therapy for sleeve gastrectomy; this is at the surgeon’s discretion. Mr. G’s prescription for omeprazole was for 3 months.
Proper valproate dosing
In Mr. G’s case, it could be hypothesized that the VPA dosing was incorrect. For mood disorders, oral VPA dosing is 25 mg/kg/d. Mr. G lost 40 pounds, which would translate to a 450-mg reduction in dose. Despite maintaining his original dose, his serum VPA levels decreased by almost 50% and could not be attributed to trough measurement. In this case, Mr. G was prescribed a higher dose than needed given his weight loss.
Continue to: Divalproex sodium ER...
Divalproex sodium ER is a hydrophilic matrix tablet that requires a low pH to dissolve. Switching to an IR formulation bypassed the need for a low pH and the VPA was delivered and absorbed. Mr. G was always able to absorb the medication, but only when delivered. The Table lists other psychiatric medications that clinicians should be aware of that utilize similar hydrophilic matrix technology to slowly release medications through the gastrointestinal tract and also require low pH to release the medication from the tablet.
OUTCOME Stable once again
Two and a half weeks after his medication formulation is changed from divalproex sodium ER to IR, Mr. G shows improvement in his symptoms. His serum VPA level is 52 µg/mL, which is within therapeutic limits. He continues receiving his previous medications as well. He reports “feeling much better” and denies having any depressive symptoms nor anxiety. Mr. G is able to maintain eye contact, and has positive thought content, improved organization of thinking, and retained abstraction.
Bottom Line
All medication changes should be identified at each visit. Many extended-release psychiatric medications require lower pH to release the medication from the tablet. When evaluating nonresponse to psychotropic medications, anything that affects pH in the stomach should be considered.
Related Resources
- Monte SV, Russo KM, Mustafa E. Impact of sleeve gastrectomy on psychiatric medication use and symptoms. J Obes. 2018; 2018:8532602. doi: 10.1155/2018/8532602
- Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
- ObesityHelp, Inc. https://www.obesityhelp.com/medications-after-bariatric-surgery-wls/
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Clonidine ER • Kapvay
Divalproex sodium extended- release tablets • Depakote ER
Escitalopram • Lexapro
Iloperidone • Fanapt
Methylphenidate ER tablet • Concerta
Methylphenidate ER capsule • Metadate, Jornay
Methylphenidate LA capsule • Ritalin LA
Mirtazapine • Remeron
Omeprazole • Prilosec, Zegerid
Paroxetine • Paxil
Valproic acid immediate- release capsules and solution • Depakene
Valproate sodium IV • Depacon
Venlafaxine • Effexor
1. Wheless JW, Phelps SJ. A clinician’s guide to oral extended-release drug delivery systems in epilepsy. J Pediatr Pharmacol Ther. 2018;23(4):277-292.
2. Jaimini M, Ranga S, Kumar A, et al. A review on immediate release drug delivery system by using design of experiment. J Drug Discov Therap. 2013;1(12):21-27.
3. Bhandari N, Kumar A, Choudhary A, et al. A review on immediate release drug delivery system. Int Res J Pharm App Sci. 2014;49(1):78-87.
4. Eatock J, Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatr Dis Treat. 2007;3(1):117-131.
5. Manjunath R, Davis KL, Candrilli SD, et al. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372-378.
6. Samsonsen C, Reimers A, Bråthen G, et al. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia. 2014;55(11):e125-e128. doi: 10.1111/epi.12801
7. Mangal M, Thakral S, Goswami M, et al. Superdisintegrants: an updated review. Int Pharmacy Pharmaceut Sci Res. 2012;2(2):26-35.
8. Tablets. United States Pharmacopeia. Accessed January 21, 2021. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1151s87.html
9. Holquist C, Fava W. FDA safety page: delayed- vs. extended-release Rxs. Drug Topics. Published July 23, 2007. Accessed January 21, 2021. https://www.drugtopics.com/view/fda-safety-page-delayed-release-vs-extended-release-rxs
10. Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
11. Perucca E. Extended-release formulations of antiepileptic drugs: rationale and comparative value. Epilepsy Curr. 2009;9(6):153-157.
12. Bialer M. Extended-release formulations for the treatment of epilepsy. CNS Drugs. 2007;21(9):765-774.
13. Pellock JM, Smith MC, Cloyd JC, et al. Extended-release formulations: simplifying strategies in the management of antiepileptic drug therapy. Epilepsy Behav. 2004;5(3):301-307.
14. Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg 2013;56(5):347-352.
1. Wheless JW, Phelps SJ. A clinician’s guide to oral extended-release drug delivery systems in epilepsy. J Pediatr Pharmacol Ther. 2018;23(4):277-292.
2. Jaimini M, Ranga S, Kumar A, et al. A review on immediate release drug delivery system by using design of experiment. J Drug Discov Therap. 2013;1(12):21-27.
3. Bhandari N, Kumar A, Choudhary A, et al. A review on immediate release drug delivery system. Int Res J Pharm App Sci. 2014;49(1):78-87.
4. Eatock J, Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatr Dis Treat. 2007;3(1):117-131.
5. Manjunath R, Davis KL, Candrilli SD, et al. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372-378.
6. Samsonsen C, Reimers A, Bråthen G, et al. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia. 2014;55(11):e125-e128. doi: 10.1111/epi.12801
7. Mangal M, Thakral S, Goswami M, et al. Superdisintegrants: an updated review. Int Pharmacy Pharmaceut Sci Res. 2012;2(2):26-35.
8. Tablets. United States Pharmacopeia. Accessed January 21, 2021. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1151s87.html
9. Holquist C, Fava W. FDA safety page: delayed- vs. extended-release Rxs. Drug Topics. Published July 23, 2007. Accessed January 21, 2021. https://www.drugtopics.com/view/fda-safety-page-delayed-release-vs-extended-release-rxs
10. Qiu Y, Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23-32.
11. Perucca E. Extended-release formulations of antiepileptic drugs: rationale and comparative value. Epilepsy Curr. 2009;9(6):153-157.
12. Bialer M. Extended-release formulations for the treatment of epilepsy. CNS Drugs. 2007;21(9):765-774.
13. Pellock JM, Smith MC, Cloyd JC, et al. Extended-release formulations: simplifying strategies in the management of antiepileptic drug therapy. Epilepsy Behav. 2004;5(3):301-307.
14. Sarkhosh K, Birch DW, Sharma A, et al. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon’s guide. Can J Surg 2013;56(5):347-352.
Steroid-induced psychiatric symptoms: What you need to know
Ms. N, age 30, presents to the emergency department for altered mental status, insomnia, and behavioral changes, which she has experienced for 1 week. On evaluation, she grabs a clinician’s hand and details her business ideas and life story with no prompting. Ms. N’s mental status examination is significant for hyperverbal speech with increased rate and volume; tangential thought process; and bright, expanded affect.
One week earlier, Ms. N was hospitalized for sudden-onset chest pain, weakness, and dizziness. She received 45 minutes of cardiopulmonary resuscitation prior to presentation and was found to have a ST-segment elevation myocardial infarction that required emergent left anterior descending coronary artery and right coronary artery percutaneous coronary intervention to place drug-eluting stents. Her recovery was complicated by acute cardiogenic shock, pulmonary edema, and hypoxic respiratory failure. Subsequently, she was intubated, admitted to the ICU, and received high-dose corticosteroids, including IV methylprednisolone, 40 mg every 12 hours, which was tapered prior to discharge. Her husband reports that since Ms. N came home, she has been more talkative and irritable, ruminating about past events, unable to sleep (<1 hour/night), and crying frequently. She has also been endorsing visual and auditory hallucinations, with increased praying and listening to religious music.
The frequent clinical use of steroids necessitates an understanding of these medications’ various adverse effects. The manifestations of steroid-induced psychiatric symptoms are broad and can involve affective, behavioral, and cognitive domains. While the current mechanism is unknown, this phenomenon may be related to decreased levels of corticotropin, norepinephrine, and beta-endorphin immunoreactivity, as well as effects on brain regions such as the hippocampus and amygdala. The best interventions for steroid-induced psychiatric symptoms are awareness and early diagnosis. There are no FDA-approved treatments for steroid-induced psychiatric symptoms; initial measures should include tapering or discontinuing corticosteroids.
In this article, we review the literature on the incidence, characteristics, differential diagnoses, proposed mechanism, risk factors, and proposed treatments of steroid-induced psychiatric symptoms.
A wide range of presentations
Steroid use has increased over the past 2 decades, with 10% of medical and surgical inpatients and 1% to 3% of the general population taking long-term glucocorticoids.1 Even with topical application, steroid therapy is often systemically absorbed, and thus may lead to steroid-induced psychiatric symptoms. The incidence of steroid-induced psychiatric symptoms is difficult to assess because there can be a wide range of reactions that are dose- and time-related. Three reviews of a total of 122 cases reports found that an estimated 5% of patients treated with steroids experience severe psychiatric reactions.1-3
Steroid-induced psychopathology can include mood, behavioral, and/or cognitive impairments. Mania/hypomania is the most common overall psychiatric symptom; the most common mood manifestations are anxiety and depression.4,5 Other possible steroid-induced symptoms include psychosis, dementia, panic disorder, delirium, suicidal thinking and behavior, aggressive behavior, insomnia, agitation, depersonalization, and euphoria.5 The most common cognitive impairment is verbal or declarative memory deficit; others include distractibility and deficits in attention and psychomotor speed.5 These psychiatric symptoms can have a rapid onset, possibly within hours of starting steroids.1 However, studies have reported a median time to onset of 11.5 days; 39% of cases had onset during the first week and 62% within 2 weeks.3,6 After reducing or stopping the steroid, it may take days to weeks before symptoms start to subside.2
What to consider in the differential Dx
Psychiatric symptoms that are induced by steroids can mimic metabolic, neurologic, or toxic disorders. Other factors to consider include drug withdrawal/intoxication, infections, and paraneoplastic syndromes.4,5 Although there is no reported correlation between the location of neurologic lesions and the development of specific psychiatric symptoms, manic symptoms appear most commonly with lesions in the right frontal lobe. 4 Other factors to note include the presence of new-onset psychiatric illnesses such as bipolar, mood, or thought disorders,4 as well as psychosocial stressors that might be contributing to the patient’s presentation.5
Continue to: Proposed mechanisms
Proposed mechanisms
Although the exact mechanism by which steroids induce psychiatric symptoms is unknown, several mechanisms have been proposed. One hypothesis is that steroid-induced psychopathology is related to decreased levels of corticotropin, norepinephrine, and beta-endorphin immunoreactivity.4,5,7 This may explain why many patients with major depressive disorder have elevated cortisol production and/or lack of suppression of cortisol secretion during a dexamethasone stimulation test, and why approximately one-half of patients with Cushing’s disease experience depressive symptoms.8 This is also likely why antipsychotics, which typically reduce cortisol, are efficacious treatments for some steroid-induced psychiatric symptoms.9
Cognitive impairments from steroid use may be related to these agents’ effects on certain brain regions. One such area is the hippocampus, an important mediator in the creation and maintenance of episodic and declarative memories.5,8,9 Acute glucocorticoid use is associated with decreased activity in the left hippocampus, reduced hippocampal glucose metabolism, and reduced cerebral blood flow in the posterior medial temporal lobe.10 Long-term glucocorticoid exposure is associated with smaller hippocampal volume and lower levels of temporal lobe N-acetylaspartate, a marker of neuronal viability.10 Because working memory depends on the prefrontal cortex and declarative memory relies on the hippocampus, deficits in these functions can be attributed to the effect of prolonged glucocorticoid exposure on glucocorticoid or mineralocorticoid receptors in the hippocampus, reduction of hippocampal volume, or elevated glutamate accumulation in that area.11 In addition, high cortisol levels inhibit brain-derived neurotrophic factor, which plays a crucial role in maintaining neural architecture in key brain regions such as the hippocampus and prefrontal cortex.11 There is also a correlation between the duration of prednisone treatment and atrophy of the right amygdala, which is an important regulator of mood and anxiety.11 Both the hippocampus and amygdala have dense collections of glucocorticoid receptors. This may explain why patients who receive high-dose corticosteroids can have reversible atrophy in the hypothalamus and amygdala, leading to deficits in emotional learning and the stress response.
Factors that increase risk
Several factors can increase the risk of steroid-induced psychopathology. The most significant is the dose; higher doses are more likely to produce psychiatric symptoms.1,5 Concurrent use of drugs that increase circulating levels of corticosteroids, such as inhibitors of the cytochrome P450 (CYP) enzyme (eg, clarithromycin), also increases the likelihood of developing psychiatric symptoms.1,5 Risk is also increased in patients with liver or renal dysfunction.1 Cerebral spinal fluid/serum albumin ratio, a marker of blood-brain barrier damage, and low serum complement levels were also reported to be independent risk factors,12 with the thought that increased permeability of the blood-brain barrier may allow hydrophobic steroid molecules to more easily penetrate the CNS, leading to increased neuropsychiatric effects. Hypoalbuminemia is another reported risk factor, perhaps because lower levels of serum albumin are related to higher levels of free and active glucocorticoids, which are normally inactive when bound to albumin.13 There also appears to be an increased prevalence of steroid-induced psychopathology in women, perhaps due to greater propensity in women to seek medical care or a higher prevalence of women with medical disorders that are treated with steroids.5 A previous history of psychiatric disorders may not increase risk.5
Several methods for reducing risk have been proposed, including using a divided-dosing regimens that may lower peak steroid plasma concentrations.13,14 However, the best prevention of steroid-induced psychiatric symptoms are awareness, early diagnosis, and intervention. Studies have suggested that N-methyl-
Treatment options
There are no FDA-approved medications for managing steroid-induced psychiatric symptoms.1,16 Treatment is based on evidence from case reports and a few small case series (Table2-5,17,18).
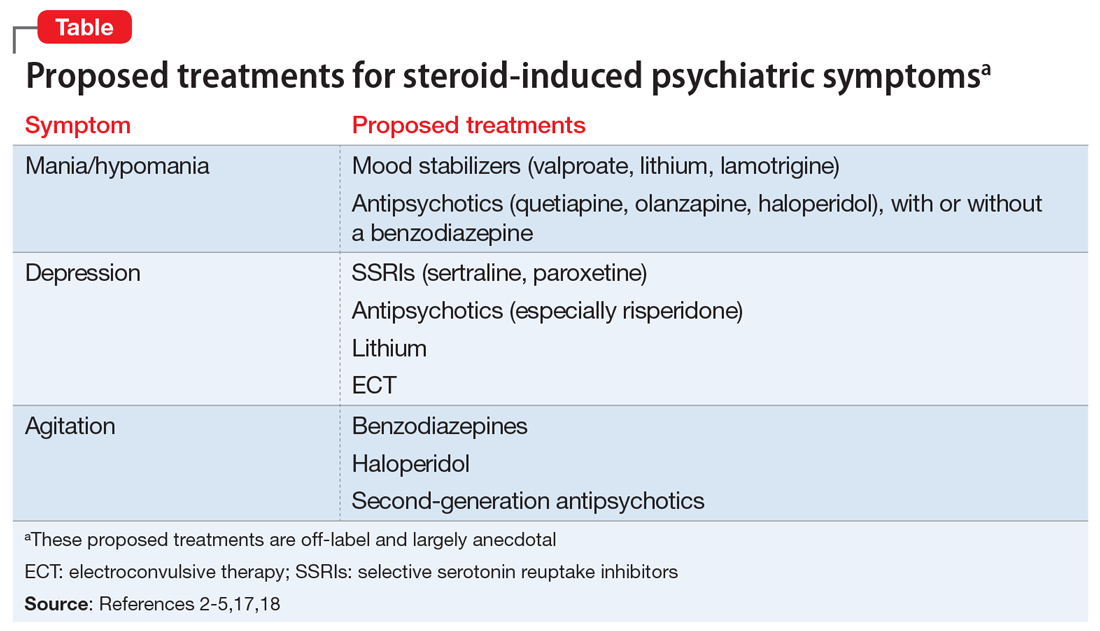
Continue to: When possible, initial treatment...
When possible, initial treatment should include discontinuing or tapering corticosteroids to <40 mg/d of prednisone-equivalent.1,4,10,18 Most studies have reported rapid reversal of deficits in declarative memory and of hippocampal volume loss once corticosteroids were tapered and discontinued.1,18 One study reported that >90% of patients recovered within 6 weeks, with patients with delirium recovering more quickly (mean: 5.4 days) than those with depression, mania, or psychosis (mean: 19.3 days).3 Another found that the vast majority (92%) of patients treated only with a steroid taper achieved clinical recovery, and 84% recovered with administration of antipsychotics without a steroid taper.3 In this study, all patients who received electroconvulsive therapy (ECT) recovered, as did those who received a steroid taper plus lithium or antipsychotics. Steroid tapering regimens are especially important for patients who have received long-term glucocorticoid treatment. Patients need to be closely monitored for signs of new or increased depression, delirium, or confusion during the taper. If these symptoms occur, the patient should be checked for adrenocortical insufficiency, which can be resolved by re-administering or increasing the dosage of the glucocorticoid.10
Mania. The treatment of mania/hypomania includes mood stabilizers (valproate, lithium, lamotrigine) and antipsychotics (quetiapine, olanzapine, haloperidol).2,4,5,10,14,18 Valproate has been reported to be an effective prophylactic of corticosteroid-induced mania,2 perhaps because it dampens neuronal hyperexcitability by attenuating NMDA receptors, blocking voltage-dependent sodium channels, and inhibiting the synthesis of cortical GABAergic steroids. Starting valproate while continuing corticosteroids (if necessary) may help lessen mania.2 Benzodiazepines also may be useful on a short-term basis.
Depression. Steroid-induced depression may be treated with sertraline or other first-line antidepressants.5,14 Consider ECT for patients with severe depression. Support for the use of antipsychotic medications stems from studies that reported steroids’ role in disrupting dopamine and 5HT2 activity. Lithium also has been used successfully to manage and prevent glucocorticoid-associated affective disorder.10,18 It can be used alone or in combination with selective serotonin reuptake inhibitors to alleviate depressive symptoms.10 Tricyclic antidepressants are generally avoided because their anticholinergic effects can exacerbate or worsen delirium.18 In general, ECT is an effective treatment for persistent and/or unresponsive steroid-induced depression,2,10 but may be difficult to use in patients with serious medical illnesses.
Agitation. Medications that have been proposed for treating steroid-induced agitation include benzodiazepines, haloperidol, and second-generation antipsychotics.5,17
Other considerations. Clinicians, patients, and families should discuss in detail the risks of steroid-induced psychiatric symptoms so an early diagnosis and appropriate intervention can be implemented. Before starting steroids, it is important to review the patient’s current medication list to ensure that steroid treatment is indicated, and to check for potential drug–drug interactions. In addition, the medical condition that is being treated with steroids also needs to be carefully reviewed, because certain illnesses are associated with the development of psychiatric symptoms. 5,10
Continue to: Young children...
Young children (age <6) and older adults appear to be at greater risk for cognitive and memory disturbances from steroid use.10 In addition, patients have individual levels of susceptibility to steroid-induced psychiatric symptoms that can vary over time. The risk for adverse effects may be elevated based on response to previous courses of glucocorticoid treatment.10 While gender, age, dosage, and duration of treatment influence risk, it is not possible to predict which patients will experience psychiatric effects during a given course of glucocorticoid therapy. Therefore, all patients should be considered to have the potential of developing such effects, and should be monitored during glucocorticoid treatment and withdrawal.
Goals for future research
To help reduce the severity of and cost associated with steroid-induced psychiatric symptoms,5,14 future studies should focus on controlled trials of preventative strategies. In particular, recent advances in genetic mapping may help identify involvement of certain genes or polymorphisms.5 Because current guidelines for the prevention and treatment of steroid-induced psychiatric symptoms are not evidence-based, controlled clinical trials are needed to elucidate the optimal management of such symptoms. There is much interplay between many of the proposed mechanisms of steroid-induced psychiatric symptoms, and future studies can help uncover a deeper understanding of the intricacies of this phenomenon.
CASE CONTINUED
Mrs. N is admitted for altered mental status. Medical workup includes MRI of the brain, MRI of the neck, cardiac echocardiogram, and EEG. There is no evidence of acute structural pathology. She is started on olanzapine, 10 mg/d at bedtime for manic and psychotic symptoms, and is discharged after 5 days. After 1 month, the outpatient psychiatrist gradually decreases and discontinues olanzapine as Mrs. N steadily returns to baseline. One year after discharge, Mrs. N continues to report resolution of her manic and psychotic symptoms.
Bottom Line
Steroids can induce a wide range of psychiatric symptoms, including mania/ hypomania, anxiety, and depression. Initial treatment typically includes tapering or discontinuing the steroid when possible. Other proposed treatments include certain antipsychotics, antidepressants, and other psychotropics, but the supporting evidence is largely anecdotal or based on case studies. Additional research is needed to elucidate the mechanism and treatment recommendations.
Related Resources
- Janes M, Kuster S, Goldson TM, et al. Steroid-induced psychosis. Proc (Bayl Univ Med Cent). 2019;32(4):614-615.
- Mayo Clinic. Prednisone and other corticosteroids. https://www.mayoclinic.org/steroids/art-20045692
Drug Brand Names
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Methylprednisolone injection • Solu-Medrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproate • Depakote
1. Dubovsky AN, Arvikar S, Stern TA, et al. The neuropsychiatric complications of glucocorticoid use: steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
2. Roxanas MG, Hunt GE. Rapid reversal of corticosteroid-induced mania with sodium valproate: a case series of 20 patients. Psychosomatics. 2012;53(6):575-581.
3. Lewis DA, Smith RE. Steroid‐induced psychiatric syndromes. A report of 14 cases and a review of the literature. J Affect Disord. 1983;5(4):319-332.
4. Warren KN, Katakam J, Espiridion ED. Acute-onset mania in a patient with non-small cell lung cancer. Cureus. 2019;11(8):e5436.
5. Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
6. Ling MH, Perry PJ, Tsuang MT. Side effects of corticosteroid therapy. Psychiatric aspects. Arch Gen. Psychiatry. 1981;38(4):471-477.
7. Ularntinon S, Tzuang D, Dahl G, et al. Concurrent treatment of steroid-related mood and psychotic symptoms with risperidone. Pediatrics. 2010;125(5):e1241-e1245.
8. Pokladinkova J, Meyboom RH, Vlcek J, et al. Intranasally administered corticosteroids and neuropsychiatric disturbances: a review of the international pharmacovigilance programme of the World Health Organization. Ann Allergy Asthma Immunol. 2008;101(1):67-73.
9. Walker EF, Trotman HD, Pearce BD, et al. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatry. 2013;74(6):410-417.
10. Wolkowitz OM, Reus UI. Treatment of depression with antiglucocorticoid drugs. Psychosom Med. 1999;61(5):698-711.
11. Judd LL, Schettler PJ, Brown ES, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry. 2014;171(10):1045-1051.
12. Appenzeller S, Cendes F, Costallat LT. Acute psychosis in systemic lupus erythematosus. Rheumatol Int. 2008;28(3):237-243.
13. Glynne-Jones R, Vernon CC, Bell G. Is steroid psychosis preventable by divided doses? Lancet. 1986;2(8520):1404.
14. Ismail MF, Lavelle C, Cassidy EM. Steroid-induced mental disorders in cancer patients: a systematic review. Future Oncol. 2017;13(29):2719-2731.
15. Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89-98.
16. Brown BS, Stuard G, Liggin JDM, et al. Effect of phenytoin on mood and declarative memory during prescription corticosteroid therapy. Biol Psychiatry. 2005;57(5):543-548.
17. Desai S, Khanani S, Shad MU, et al. Attenutation of amygdala atrophy with lamotrigine in patients receiving corticosteroid therapy. J Clin Psychopharmacol. 2009;29(3):284-287.
18. Gable M, Depry D. Sustained corticosteroid-induced mania and psychosis despite cessation: a case study and brief literature review. Int J Psychiatry Med. 2015;50(4):398-404.
Ms. N, age 30, presents to the emergency department for altered mental status, insomnia, and behavioral changes, which she has experienced for 1 week. On evaluation, she grabs a clinician’s hand and details her business ideas and life story with no prompting. Ms. N’s mental status examination is significant for hyperverbal speech with increased rate and volume; tangential thought process; and bright, expanded affect.
One week earlier, Ms. N was hospitalized for sudden-onset chest pain, weakness, and dizziness. She received 45 minutes of cardiopulmonary resuscitation prior to presentation and was found to have a ST-segment elevation myocardial infarction that required emergent left anterior descending coronary artery and right coronary artery percutaneous coronary intervention to place drug-eluting stents. Her recovery was complicated by acute cardiogenic shock, pulmonary edema, and hypoxic respiratory failure. Subsequently, she was intubated, admitted to the ICU, and received high-dose corticosteroids, including IV methylprednisolone, 40 mg every 12 hours, which was tapered prior to discharge. Her husband reports that since Ms. N came home, she has been more talkative and irritable, ruminating about past events, unable to sleep (<1 hour/night), and crying frequently. She has also been endorsing visual and auditory hallucinations, with increased praying and listening to religious music.
The frequent clinical use of steroids necessitates an understanding of these medications’ various adverse effects. The manifestations of steroid-induced psychiatric symptoms are broad and can involve affective, behavioral, and cognitive domains. While the current mechanism is unknown, this phenomenon may be related to decreased levels of corticotropin, norepinephrine, and beta-endorphin immunoreactivity, as well as effects on brain regions such as the hippocampus and amygdala. The best interventions for steroid-induced psychiatric symptoms are awareness and early diagnosis. There are no FDA-approved treatments for steroid-induced psychiatric symptoms; initial measures should include tapering or discontinuing corticosteroids.
In this article, we review the literature on the incidence, characteristics, differential diagnoses, proposed mechanism, risk factors, and proposed treatments of steroid-induced psychiatric symptoms.
A wide range of presentations
Steroid use has increased over the past 2 decades, with 10% of medical and surgical inpatients and 1% to 3% of the general population taking long-term glucocorticoids.1 Even with topical application, steroid therapy is often systemically absorbed, and thus may lead to steroid-induced psychiatric symptoms. The incidence of steroid-induced psychiatric symptoms is difficult to assess because there can be a wide range of reactions that are dose- and time-related. Three reviews of a total of 122 cases reports found that an estimated 5% of patients treated with steroids experience severe psychiatric reactions.1-3
Steroid-induced psychopathology can include mood, behavioral, and/or cognitive impairments. Mania/hypomania is the most common overall psychiatric symptom; the most common mood manifestations are anxiety and depression.4,5 Other possible steroid-induced symptoms include psychosis, dementia, panic disorder, delirium, suicidal thinking and behavior, aggressive behavior, insomnia, agitation, depersonalization, and euphoria.5 The most common cognitive impairment is verbal or declarative memory deficit; others include distractibility and deficits in attention and psychomotor speed.5 These psychiatric symptoms can have a rapid onset, possibly within hours of starting steroids.1 However, studies have reported a median time to onset of 11.5 days; 39% of cases had onset during the first week and 62% within 2 weeks.3,6 After reducing or stopping the steroid, it may take days to weeks before symptoms start to subside.2
What to consider in the differential Dx
Psychiatric symptoms that are induced by steroids can mimic metabolic, neurologic, or toxic disorders. Other factors to consider include drug withdrawal/intoxication, infections, and paraneoplastic syndromes.4,5 Although there is no reported correlation between the location of neurologic lesions and the development of specific psychiatric symptoms, manic symptoms appear most commonly with lesions in the right frontal lobe. 4 Other factors to note include the presence of new-onset psychiatric illnesses such as bipolar, mood, or thought disorders,4 as well as psychosocial stressors that might be contributing to the patient’s presentation.5
Continue to: Proposed mechanisms
Proposed mechanisms
Although the exact mechanism by which steroids induce psychiatric symptoms is unknown, several mechanisms have been proposed. One hypothesis is that steroid-induced psychopathology is related to decreased levels of corticotropin, norepinephrine, and beta-endorphin immunoreactivity.4,5,7 This may explain why many patients with major depressive disorder have elevated cortisol production and/or lack of suppression of cortisol secretion during a dexamethasone stimulation test, and why approximately one-half of patients with Cushing’s disease experience depressive symptoms.8 This is also likely why antipsychotics, which typically reduce cortisol, are efficacious treatments for some steroid-induced psychiatric symptoms.9
Cognitive impairments from steroid use may be related to these agents’ effects on certain brain regions. One such area is the hippocampus, an important mediator in the creation and maintenance of episodic and declarative memories.5,8,9 Acute glucocorticoid use is associated with decreased activity in the left hippocampus, reduced hippocampal glucose metabolism, and reduced cerebral blood flow in the posterior medial temporal lobe.10 Long-term glucocorticoid exposure is associated with smaller hippocampal volume and lower levels of temporal lobe N-acetylaspartate, a marker of neuronal viability.10 Because working memory depends on the prefrontal cortex and declarative memory relies on the hippocampus, deficits in these functions can be attributed to the effect of prolonged glucocorticoid exposure on glucocorticoid or mineralocorticoid receptors in the hippocampus, reduction of hippocampal volume, or elevated glutamate accumulation in that area.11 In addition, high cortisol levels inhibit brain-derived neurotrophic factor, which plays a crucial role in maintaining neural architecture in key brain regions such as the hippocampus and prefrontal cortex.11 There is also a correlation between the duration of prednisone treatment and atrophy of the right amygdala, which is an important regulator of mood and anxiety.11 Both the hippocampus and amygdala have dense collections of glucocorticoid receptors. This may explain why patients who receive high-dose corticosteroids can have reversible atrophy in the hypothalamus and amygdala, leading to deficits in emotional learning and the stress response.
Factors that increase risk
Several factors can increase the risk of steroid-induced psychopathology. The most significant is the dose; higher doses are more likely to produce psychiatric symptoms.1,5 Concurrent use of drugs that increase circulating levels of corticosteroids, such as inhibitors of the cytochrome P450 (CYP) enzyme (eg, clarithromycin), also increases the likelihood of developing psychiatric symptoms.1,5 Risk is also increased in patients with liver or renal dysfunction.1 Cerebral spinal fluid/serum albumin ratio, a marker of blood-brain barrier damage, and low serum complement levels were also reported to be independent risk factors,12 with the thought that increased permeability of the blood-brain barrier may allow hydrophobic steroid molecules to more easily penetrate the CNS, leading to increased neuropsychiatric effects. Hypoalbuminemia is another reported risk factor, perhaps because lower levels of serum albumin are related to higher levels of free and active glucocorticoids, which are normally inactive when bound to albumin.13 There also appears to be an increased prevalence of steroid-induced psychopathology in women, perhaps due to greater propensity in women to seek medical care or a higher prevalence of women with medical disorders that are treated with steroids.5 A previous history of psychiatric disorders may not increase risk.5
Several methods for reducing risk have been proposed, including using a divided-dosing regimens that may lower peak steroid plasma concentrations.13,14 However, the best prevention of steroid-induced psychiatric symptoms are awareness, early diagnosis, and intervention. Studies have suggested that N-methyl-
Treatment options
There are no FDA-approved medications for managing steroid-induced psychiatric symptoms.1,16 Treatment is based on evidence from case reports and a few small case series (Table2-5,17,18).

Continue to: When possible, initial treatment...
When possible, initial treatment should include discontinuing or tapering corticosteroids to <40 mg/d of prednisone-equivalent.1,4,10,18 Most studies have reported rapid reversal of deficits in declarative memory and of hippocampal volume loss once corticosteroids were tapered and discontinued.1,18 One study reported that >90% of patients recovered within 6 weeks, with patients with delirium recovering more quickly (mean: 5.4 days) than those with depression, mania, or psychosis (mean: 19.3 days).3 Another found that the vast majority (92%) of patients treated only with a steroid taper achieved clinical recovery, and 84% recovered with administration of antipsychotics without a steroid taper.3 In this study, all patients who received electroconvulsive therapy (ECT) recovered, as did those who received a steroid taper plus lithium or antipsychotics. Steroid tapering regimens are especially important for patients who have received long-term glucocorticoid treatment. Patients need to be closely monitored for signs of new or increased depression, delirium, or confusion during the taper. If these symptoms occur, the patient should be checked for adrenocortical insufficiency, which can be resolved by re-administering or increasing the dosage of the glucocorticoid.10
Mania. The treatment of mania/hypomania includes mood stabilizers (valproate, lithium, lamotrigine) and antipsychotics (quetiapine, olanzapine, haloperidol).2,4,5,10,14,18 Valproate has been reported to be an effective prophylactic of corticosteroid-induced mania,2 perhaps because it dampens neuronal hyperexcitability by attenuating NMDA receptors, blocking voltage-dependent sodium channels, and inhibiting the synthesis of cortical GABAergic steroids. Starting valproate while continuing corticosteroids (if necessary) may help lessen mania.2 Benzodiazepines also may be useful on a short-term basis.
Depression. Steroid-induced depression may be treated with sertraline or other first-line antidepressants.5,14 Consider ECT for patients with severe depression. Support for the use of antipsychotic medications stems from studies that reported steroids’ role in disrupting dopamine and 5HT2 activity. Lithium also has been used successfully to manage and prevent glucocorticoid-associated affective disorder.10,18 It can be used alone or in combination with selective serotonin reuptake inhibitors to alleviate depressive symptoms.10 Tricyclic antidepressants are generally avoided because their anticholinergic effects can exacerbate or worsen delirium.18 In general, ECT is an effective treatment for persistent and/or unresponsive steroid-induced depression,2,10 but may be difficult to use in patients with serious medical illnesses.
Agitation. Medications that have been proposed for treating steroid-induced agitation include benzodiazepines, haloperidol, and second-generation antipsychotics.5,17
Other considerations. Clinicians, patients, and families should discuss in detail the risks of steroid-induced psychiatric symptoms so an early diagnosis and appropriate intervention can be implemented. Before starting steroids, it is important to review the patient’s current medication list to ensure that steroid treatment is indicated, and to check for potential drug–drug interactions. In addition, the medical condition that is being treated with steroids also needs to be carefully reviewed, because certain illnesses are associated with the development of psychiatric symptoms. 5,10
Continue to: Young children...
Young children (age <6) and older adults appear to be at greater risk for cognitive and memory disturbances from steroid use.10 In addition, patients have individual levels of susceptibility to steroid-induced psychiatric symptoms that can vary over time. The risk for adverse effects may be elevated based on response to previous courses of glucocorticoid treatment.10 While gender, age, dosage, and duration of treatment influence risk, it is not possible to predict which patients will experience psychiatric effects during a given course of glucocorticoid therapy. Therefore, all patients should be considered to have the potential of developing such effects, and should be monitored during glucocorticoid treatment and withdrawal.
Goals for future research
To help reduce the severity of and cost associated with steroid-induced psychiatric symptoms,5,14 future studies should focus on controlled trials of preventative strategies. In particular, recent advances in genetic mapping may help identify involvement of certain genes or polymorphisms.5 Because current guidelines for the prevention and treatment of steroid-induced psychiatric symptoms are not evidence-based, controlled clinical trials are needed to elucidate the optimal management of such symptoms. There is much interplay between many of the proposed mechanisms of steroid-induced psychiatric symptoms, and future studies can help uncover a deeper understanding of the intricacies of this phenomenon.
CASE CONTINUED
Mrs. N is admitted for altered mental status. Medical workup includes MRI of the brain, MRI of the neck, cardiac echocardiogram, and EEG. There is no evidence of acute structural pathology. She is started on olanzapine, 10 mg/d at bedtime for manic and psychotic symptoms, and is discharged after 5 days. After 1 month, the outpatient psychiatrist gradually decreases and discontinues olanzapine as Mrs. N steadily returns to baseline. One year after discharge, Mrs. N continues to report resolution of her manic and psychotic symptoms.
Bottom Line
Steroids can induce a wide range of psychiatric symptoms, including mania/ hypomania, anxiety, and depression. Initial treatment typically includes tapering or discontinuing the steroid when possible. Other proposed treatments include certain antipsychotics, antidepressants, and other psychotropics, but the supporting evidence is largely anecdotal or based on case studies. Additional research is needed to elucidate the mechanism and treatment recommendations.
Related Resources
- Janes M, Kuster S, Goldson TM, et al. Steroid-induced psychosis. Proc (Bayl Univ Med Cent). 2019;32(4):614-615.
- Mayo Clinic. Prednisone and other corticosteroids. https://www.mayoclinic.org/steroids/art-20045692
Drug Brand Names
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Methylprednisolone injection • Solu-Medrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproate • Depakote
Ms. N, age 30, presents to the emergency department for altered mental status, insomnia, and behavioral changes, which she has experienced for 1 week. On evaluation, she grabs a clinician’s hand and details her business ideas and life story with no prompting. Ms. N’s mental status examination is significant for hyperverbal speech with increased rate and volume; tangential thought process; and bright, expanded affect.
One week earlier, Ms. N was hospitalized for sudden-onset chest pain, weakness, and dizziness. She received 45 minutes of cardiopulmonary resuscitation prior to presentation and was found to have a ST-segment elevation myocardial infarction that required emergent left anterior descending coronary artery and right coronary artery percutaneous coronary intervention to place drug-eluting stents. Her recovery was complicated by acute cardiogenic shock, pulmonary edema, and hypoxic respiratory failure. Subsequently, she was intubated, admitted to the ICU, and received high-dose corticosteroids, including IV methylprednisolone, 40 mg every 12 hours, which was tapered prior to discharge. Her husband reports that since Ms. N came home, she has been more talkative and irritable, ruminating about past events, unable to sleep (<1 hour/night), and crying frequently. She has also been endorsing visual and auditory hallucinations, with increased praying and listening to religious music.
The frequent clinical use of steroids necessitates an understanding of these medications’ various adverse effects. The manifestations of steroid-induced psychiatric symptoms are broad and can involve affective, behavioral, and cognitive domains. While the current mechanism is unknown, this phenomenon may be related to decreased levels of corticotropin, norepinephrine, and beta-endorphin immunoreactivity, as well as effects on brain regions such as the hippocampus and amygdala. The best interventions for steroid-induced psychiatric symptoms are awareness and early diagnosis. There are no FDA-approved treatments for steroid-induced psychiatric symptoms; initial measures should include tapering or discontinuing corticosteroids.
In this article, we review the literature on the incidence, characteristics, differential diagnoses, proposed mechanism, risk factors, and proposed treatments of steroid-induced psychiatric symptoms.
A wide range of presentations
Steroid use has increased over the past 2 decades, with 10% of medical and surgical inpatients and 1% to 3% of the general population taking long-term glucocorticoids.1 Even with topical application, steroid therapy is often systemically absorbed, and thus may lead to steroid-induced psychiatric symptoms. The incidence of steroid-induced psychiatric symptoms is difficult to assess because there can be a wide range of reactions that are dose- and time-related. Three reviews of a total of 122 cases reports found that an estimated 5% of patients treated with steroids experience severe psychiatric reactions.1-3
Steroid-induced psychopathology can include mood, behavioral, and/or cognitive impairments. Mania/hypomania is the most common overall psychiatric symptom; the most common mood manifestations are anxiety and depression.4,5 Other possible steroid-induced symptoms include psychosis, dementia, panic disorder, delirium, suicidal thinking and behavior, aggressive behavior, insomnia, agitation, depersonalization, and euphoria.5 The most common cognitive impairment is verbal or declarative memory deficit; others include distractibility and deficits in attention and psychomotor speed.5 These psychiatric symptoms can have a rapid onset, possibly within hours of starting steroids.1 However, studies have reported a median time to onset of 11.5 days; 39% of cases had onset during the first week and 62% within 2 weeks.3,6 After reducing or stopping the steroid, it may take days to weeks before symptoms start to subside.2
What to consider in the differential Dx
Psychiatric symptoms that are induced by steroids can mimic metabolic, neurologic, or toxic disorders. Other factors to consider include drug withdrawal/intoxication, infections, and paraneoplastic syndromes.4,5 Although there is no reported correlation between the location of neurologic lesions and the development of specific psychiatric symptoms, manic symptoms appear most commonly with lesions in the right frontal lobe. 4 Other factors to note include the presence of new-onset psychiatric illnesses such as bipolar, mood, or thought disorders,4 as well as psychosocial stressors that might be contributing to the patient’s presentation.5
Continue to: Proposed mechanisms
Proposed mechanisms
Although the exact mechanism by which steroids induce psychiatric symptoms is unknown, several mechanisms have been proposed. One hypothesis is that steroid-induced psychopathology is related to decreased levels of corticotropin, norepinephrine, and beta-endorphin immunoreactivity.4,5,7 This may explain why many patients with major depressive disorder have elevated cortisol production and/or lack of suppression of cortisol secretion during a dexamethasone stimulation test, and why approximately one-half of patients with Cushing’s disease experience depressive symptoms.8 This is also likely why antipsychotics, which typically reduce cortisol, are efficacious treatments for some steroid-induced psychiatric symptoms.9
Cognitive impairments from steroid use may be related to these agents’ effects on certain brain regions. One such area is the hippocampus, an important mediator in the creation and maintenance of episodic and declarative memories.5,8,9 Acute glucocorticoid use is associated with decreased activity in the left hippocampus, reduced hippocampal glucose metabolism, and reduced cerebral blood flow in the posterior medial temporal lobe.10 Long-term glucocorticoid exposure is associated with smaller hippocampal volume and lower levels of temporal lobe N-acetylaspartate, a marker of neuronal viability.10 Because working memory depends on the prefrontal cortex and declarative memory relies on the hippocampus, deficits in these functions can be attributed to the effect of prolonged glucocorticoid exposure on glucocorticoid or mineralocorticoid receptors in the hippocampus, reduction of hippocampal volume, or elevated glutamate accumulation in that area.11 In addition, high cortisol levels inhibit brain-derived neurotrophic factor, which plays a crucial role in maintaining neural architecture in key brain regions such as the hippocampus and prefrontal cortex.11 There is also a correlation between the duration of prednisone treatment and atrophy of the right amygdala, which is an important regulator of mood and anxiety.11 Both the hippocampus and amygdala have dense collections of glucocorticoid receptors. This may explain why patients who receive high-dose corticosteroids can have reversible atrophy in the hypothalamus and amygdala, leading to deficits in emotional learning and the stress response.
Factors that increase risk
Several factors can increase the risk of steroid-induced psychopathology. The most significant is the dose; higher doses are more likely to produce psychiatric symptoms.1,5 Concurrent use of drugs that increase circulating levels of corticosteroids, such as inhibitors of the cytochrome P450 (CYP) enzyme (eg, clarithromycin), also increases the likelihood of developing psychiatric symptoms.1,5 Risk is also increased in patients with liver or renal dysfunction.1 Cerebral spinal fluid/serum albumin ratio, a marker of blood-brain barrier damage, and low serum complement levels were also reported to be independent risk factors,12 with the thought that increased permeability of the blood-brain barrier may allow hydrophobic steroid molecules to more easily penetrate the CNS, leading to increased neuropsychiatric effects. Hypoalbuminemia is another reported risk factor, perhaps because lower levels of serum albumin are related to higher levels of free and active glucocorticoids, which are normally inactive when bound to albumin.13 There also appears to be an increased prevalence of steroid-induced psychopathology in women, perhaps due to greater propensity in women to seek medical care or a higher prevalence of women with medical disorders that are treated with steroids.5 A previous history of psychiatric disorders may not increase risk.5
Several methods for reducing risk have been proposed, including using a divided-dosing regimens that may lower peak steroid plasma concentrations.13,14 However, the best prevention of steroid-induced psychiatric symptoms are awareness, early diagnosis, and intervention. Studies have suggested that N-methyl-
Treatment options
There are no FDA-approved medications for managing steroid-induced psychiatric symptoms.1,16 Treatment is based on evidence from case reports and a few small case series (Table2-5,17,18).

Continue to: When possible, initial treatment...
When possible, initial treatment should include discontinuing or tapering corticosteroids to <40 mg/d of prednisone-equivalent.1,4,10,18 Most studies have reported rapid reversal of deficits in declarative memory and of hippocampal volume loss once corticosteroids were tapered and discontinued.1,18 One study reported that >90% of patients recovered within 6 weeks, with patients with delirium recovering more quickly (mean: 5.4 days) than those with depression, mania, or psychosis (mean: 19.3 days).3 Another found that the vast majority (92%) of patients treated only with a steroid taper achieved clinical recovery, and 84% recovered with administration of antipsychotics without a steroid taper.3 In this study, all patients who received electroconvulsive therapy (ECT) recovered, as did those who received a steroid taper plus lithium or antipsychotics. Steroid tapering regimens are especially important for patients who have received long-term glucocorticoid treatment. Patients need to be closely monitored for signs of new or increased depression, delirium, or confusion during the taper. If these symptoms occur, the patient should be checked for adrenocortical insufficiency, which can be resolved by re-administering or increasing the dosage of the glucocorticoid.10
Mania. The treatment of mania/hypomania includes mood stabilizers (valproate, lithium, lamotrigine) and antipsychotics (quetiapine, olanzapine, haloperidol).2,4,5,10,14,18 Valproate has been reported to be an effective prophylactic of corticosteroid-induced mania,2 perhaps because it dampens neuronal hyperexcitability by attenuating NMDA receptors, blocking voltage-dependent sodium channels, and inhibiting the synthesis of cortical GABAergic steroids. Starting valproate while continuing corticosteroids (if necessary) may help lessen mania.2 Benzodiazepines also may be useful on a short-term basis.
Depression. Steroid-induced depression may be treated with sertraline or other first-line antidepressants.5,14 Consider ECT for patients with severe depression. Support for the use of antipsychotic medications stems from studies that reported steroids’ role in disrupting dopamine and 5HT2 activity. Lithium also has been used successfully to manage and prevent glucocorticoid-associated affective disorder.10,18 It can be used alone or in combination with selective serotonin reuptake inhibitors to alleviate depressive symptoms.10 Tricyclic antidepressants are generally avoided because their anticholinergic effects can exacerbate or worsen delirium.18 In general, ECT is an effective treatment for persistent and/or unresponsive steroid-induced depression,2,10 but may be difficult to use in patients with serious medical illnesses.
Agitation. Medications that have been proposed for treating steroid-induced agitation include benzodiazepines, haloperidol, and second-generation antipsychotics.5,17
Other considerations. Clinicians, patients, and families should discuss in detail the risks of steroid-induced psychiatric symptoms so an early diagnosis and appropriate intervention can be implemented. Before starting steroids, it is important to review the patient’s current medication list to ensure that steroid treatment is indicated, and to check for potential drug–drug interactions. In addition, the medical condition that is being treated with steroids also needs to be carefully reviewed, because certain illnesses are associated with the development of psychiatric symptoms. 5,10
Continue to: Young children...
Young children (age <6) and older adults appear to be at greater risk for cognitive and memory disturbances from steroid use.10 In addition, patients have individual levels of susceptibility to steroid-induced psychiatric symptoms that can vary over time. The risk for adverse effects may be elevated based on response to previous courses of glucocorticoid treatment.10 While gender, age, dosage, and duration of treatment influence risk, it is not possible to predict which patients will experience psychiatric effects during a given course of glucocorticoid therapy. Therefore, all patients should be considered to have the potential of developing such effects, and should be monitored during glucocorticoid treatment and withdrawal.
Goals for future research
To help reduce the severity of and cost associated with steroid-induced psychiatric symptoms,5,14 future studies should focus on controlled trials of preventative strategies. In particular, recent advances in genetic mapping may help identify involvement of certain genes or polymorphisms.5 Because current guidelines for the prevention and treatment of steroid-induced psychiatric symptoms are not evidence-based, controlled clinical trials are needed to elucidate the optimal management of such symptoms. There is much interplay between many of the proposed mechanisms of steroid-induced psychiatric symptoms, and future studies can help uncover a deeper understanding of the intricacies of this phenomenon.
CASE CONTINUED
Mrs. N is admitted for altered mental status. Medical workup includes MRI of the brain, MRI of the neck, cardiac echocardiogram, and EEG. There is no evidence of acute structural pathology. She is started on olanzapine, 10 mg/d at bedtime for manic and psychotic symptoms, and is discharged after 5 days. After 1 month, the outpatient psychiatrist gradually decreases and discontinues olanzapine as Mrs. N steadily returns to baseline. One year after discharge, Mrs. N continues to report resolution of her manic and psychotic symptoms.
Bottom Line
Steroids can induce a wide range of psychiatric symptoms, including mania/ hypomania, anxiety, and depression. Initial treatment typically includes tapering or discontinuing the steroid when possible. Other proposed treatments include certain antipsychotics, antidepressants, and other psychotropics, but the supporting evidence is largely anecdotal or based on case studies. Additional research is needed to elucidate the mechanism and treatment recommendations.
Related Resources
- Janes M, Kuster S, Goldson TM, et al. Steroid-induced psychosis. Proc (Bayl Univ Med Cent). 2019;32(4):614-615.
- Mayo Clinic. Prednisone and other corticosteroids. https://www.mayoclinic.org/steroids/art-20045692
Drug Brand Names
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Methylprednisolone injection • Solu-Medrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproate • Depakote
1. Dubovsky AN, Arvikar S, Stern TA, et al. The neuropsychiatric complications of glucocorticoid use: steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
2. Roxanas MG, Hunt GE. Rapid reversal of corticosteroid-induced mania with sodium valproate: a case series of 20 patients. Psychosomatics. 2012;53(6):575-581.
3. Lewis DA, Smith RE. Steroid‐induced psychiatric syndromes. A report of 14 cases and a review of the literature. J Affect Disord. 1983;5(4):319-332.
4. Warren KN, Katakam J, Espiridion ED. Acute-onset mania in a patient with non-small cell lung cancer. Cureus. 2019;11(8):e5436.
5. Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
6. Ling MH, Perry PJ, Tsuang MT. Side effects of corticosteroid therapy. Psychiatric aspects. Arch Gen. Psychiatry. 1981;38(4):471-477.
7. Ularntinon S, Tzuang D, Dahl G, et al. Concurrent treatment of steroid-related mood and psychotic symptoms with risperidone. Pediatrics. 2010;125(5):e1241-e1245.
8. Pokladinkova J, Meyboom RH, Vlcek J, et al. Intranasally administered corticosteroids and neuropsychiatric disturbances: a review of the international pharmacovigilance programme of the World Health Organization. Ann Allergy Asthma Immunol. 2008;101(1):67-73.
9. Walker EF, Trotman HD, Pearce BD, et al. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatry. 2013;74(6):410-417.
10. Wolkowitz OM, Reus UI. Treatment of depression with antiglucocorticoid drugs. Psychosom Med. 1999;61(5):698-711.
11. Judd LL, Schettler PJ, Brown ES, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry. 2014;171(10):1045-1051.
12. Appenzeller S, Cendes F, Costallat LT. Acute psychosis in systemic lupus erythematosus. Rheumatol Int. 2008;28(3):237-243.
13. Glynne-Jones R, Vernon CC, Bell G. Is steroid psychosis preventable by divided doses? Lancet. 1986;2(8520):1404.
14. Ismail MF, Lavelle C, Cassidy EM. Steroid-induced mental disorders in cancer patients: a systematic review. Future Oncol. 2017;13(29):2719-2731.
15. Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89-98.
16. Brown BS, Stuard G, Liggin JDM, et al. Effect of phenytoin on mood and declarative memory during prescription corticosteroid therapy. Biol Psychiatry. 2005;57(5):543-548.
17. Desai S, Khanani S, Shad MU, et al. Attenutation of amygdala atrophy with lamotrigine in patients receiving corticosteroid therapy. J Clin Psychopharmacol. 2009;29(3):284-287.
18. Gable M, Depry D. Sustained corticosteroid-induced mania and psychosis despite cessation: a case study and brief literature review. Int J Psychiatry Med. 2015;50(4):398-404.
1. Dubovsky AN, Arvikar S, Stern TA, et al. The neuropsychiatric complications of glucocorticoid use: steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
2. Roxanas MG, Hunt GE. Rapid reversal of corticosteroid-induced mania with sodium valproate: a case series of 20 patients. Psychosomatics. 2012;53(6):575-581.
3. Lewis DA, Smith RE. Steroid‐induced psychiatric syndromes. A report of 14 cases and a review of the literature. J Affect Disord. 1983;5(4):319-332.
4. Warren KN, Katakam J, Espiridion ED. Acute-onset mania in a patient with non-small cell lung cancer. Cureus. 2019;11(8):e5436.
5. Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
6. Ling MH, Perry PJ, Tsuang MT. Side effects of corticosteroid therapy. Psychiatric aspects. Arch Gen. Psychiatry. 1981;38(4):471-477.
7. Ularntinon S, Tzuang D, Dahl G, et al. Concurrent treatment of steroid-related mood and psychotic symptoms with risperidone. Pediatrics. 2010;125(5):e1241-e1245.
8. Pokladinkova J, Meyboom RH, Vlcek J, et al. Intranasally administered corticosteroids and neuropsychiatric disturbances: a review of the international pharmacovigilance programme of the World Health Organization. Ann Allergy Asthma Immunol. 2008;101(1):67-73.
9. Walker EF, Trotman HD, Pearce BD, et al. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatry. 2013;74(6):410-417.
10. Wolkowitz OM, Reus UI. Treatment of depression with antiglucocorticoid drugs. Psychosom Med. 1999;61(5):698-711.
11. Judd LL, Schettler PJ, Brown ES, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry. 2014;171(10):1045-1051.
12. Appenzeller S, Cendes F, Costallat LT. Acute psychosis in systemic lupus erythematosus. Rheumatol Int. 2008;28(3):237-243.
13. Glynne-Jones R, Vernon CC, Bell G. Is steroid psychosis preventable by divided doses? Lancet. 1986;2(8520):1404.
14. Ismail MF, Lavelle C, Cassidy EM. Steroid-induced mental disorders in cancer patients: a systematic review. Future Oncol. 2017;13(29):2719-2731.
15. Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89-98.
16. Brown BS, Stuard G, Liggin JDM, et al. Effect of phenytoin on mood and declarative memory during prescription corticosteroid therapy. Biol Psychiatry. 2005;57(5):543-548.
17. Desai S, Khanani S, Shad MU, et al. Attenutation of amygdala atrophy with lamotrigine in patients receiving corticosteroid therapy. J Clin Psychopharmacol. 2009;29(3):284-287.
18. Gable M, Depry D. Sustained corticosteroid-induced mania and psychosis despite cessation: a case study and brief literature review. Int J Psychiatry Med. 2015;50(4):398-404.