User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Clinical Utility of Methicillin-Resistant Staphylococcus aureus Polymerase Chain Reaction Nasal Swab Testing in Lower Respiratory Tract Infections
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).
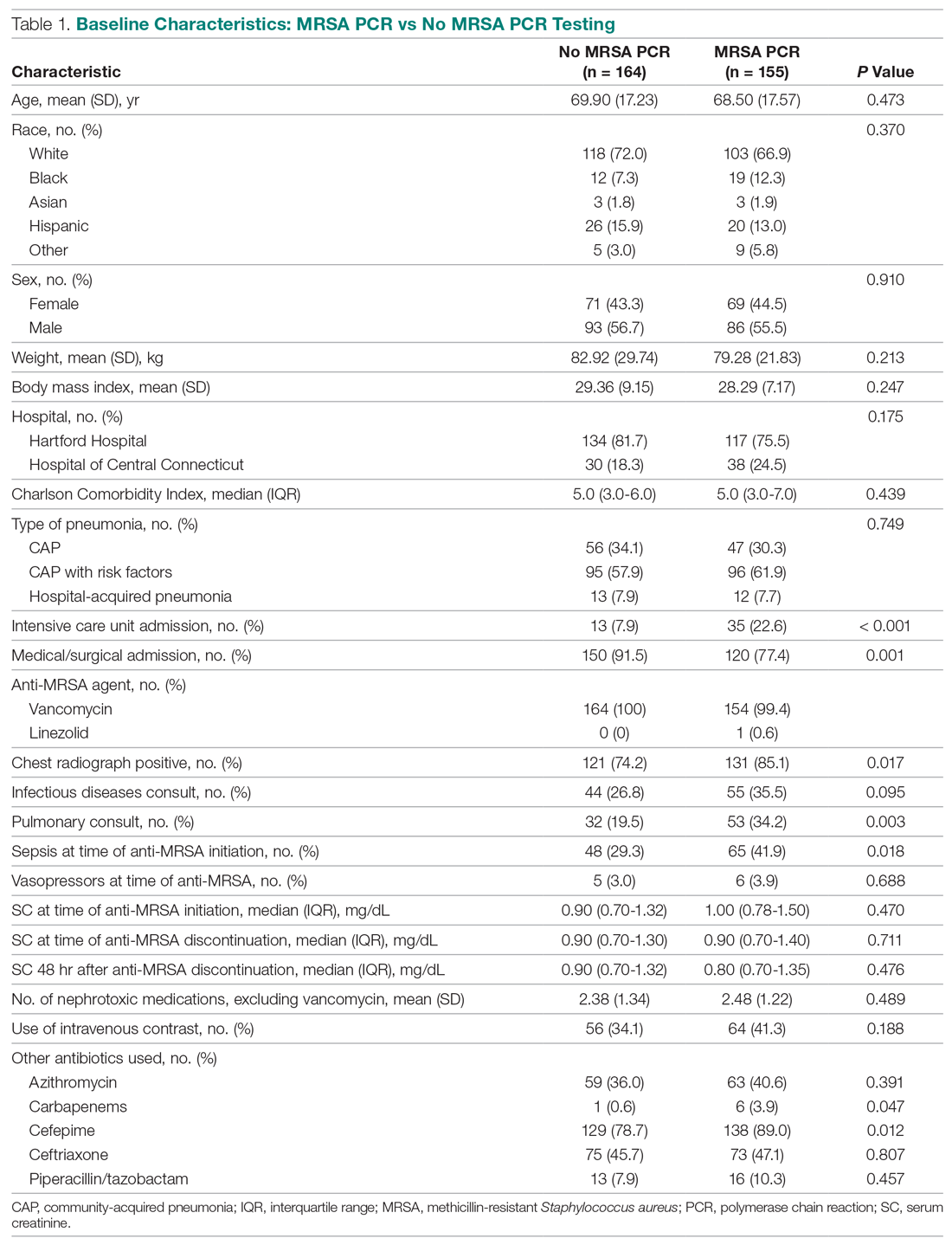
In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.
Outcomes
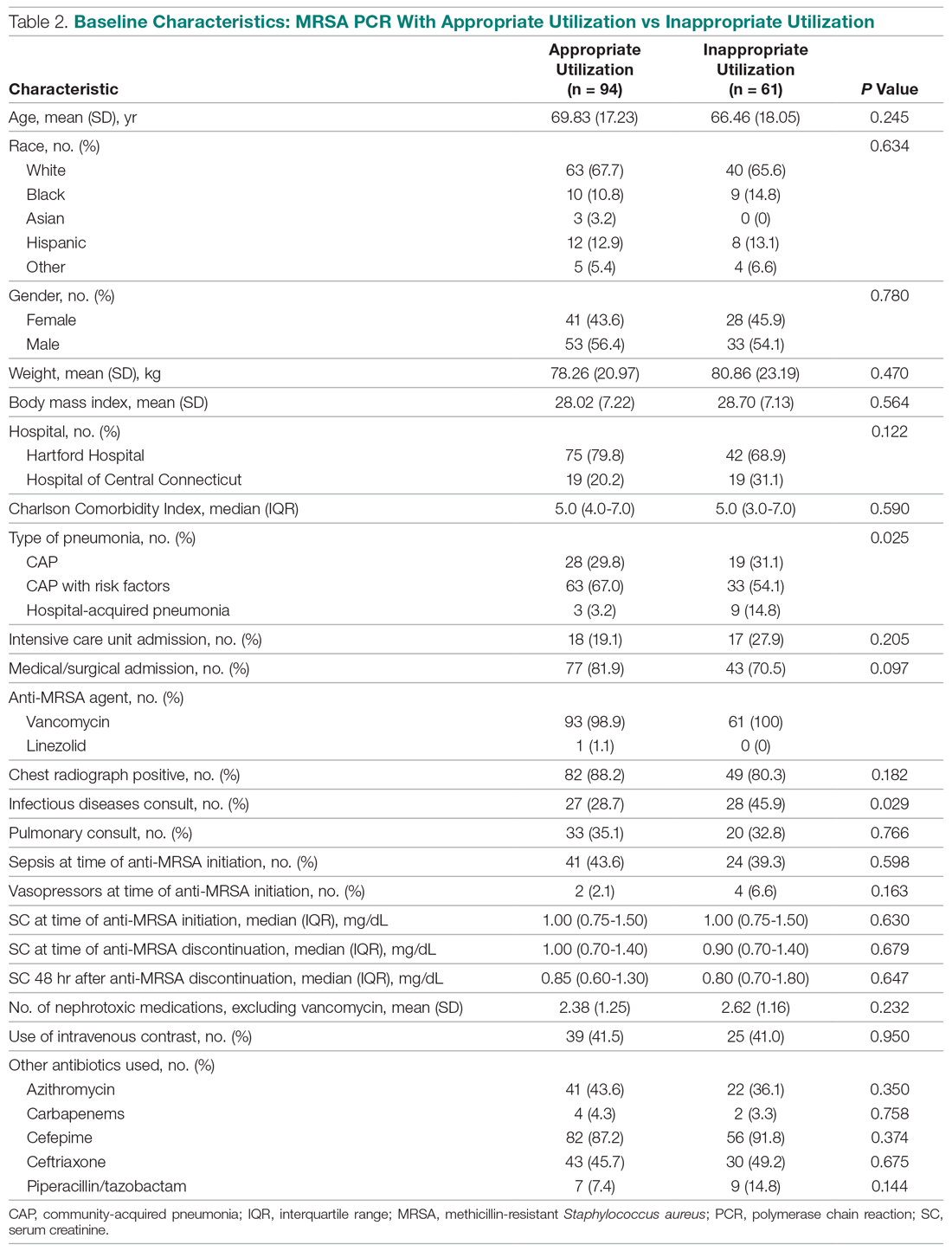
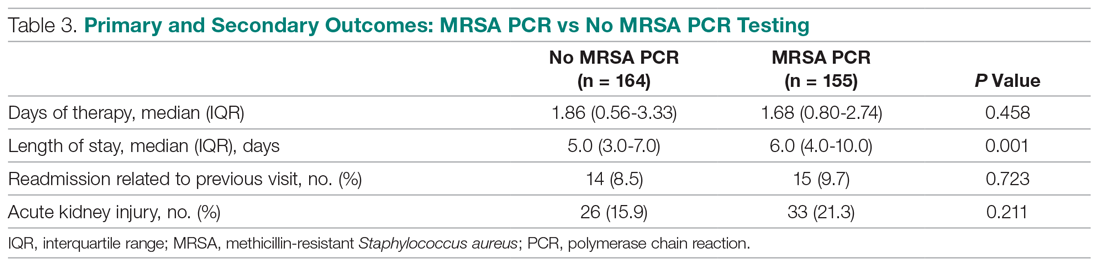
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.
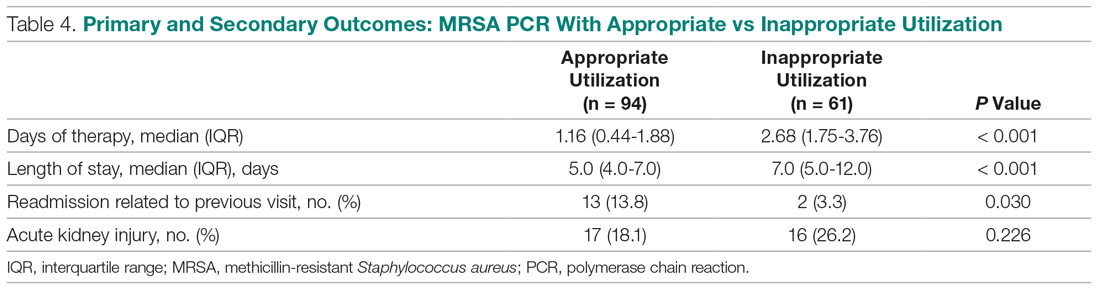
In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.
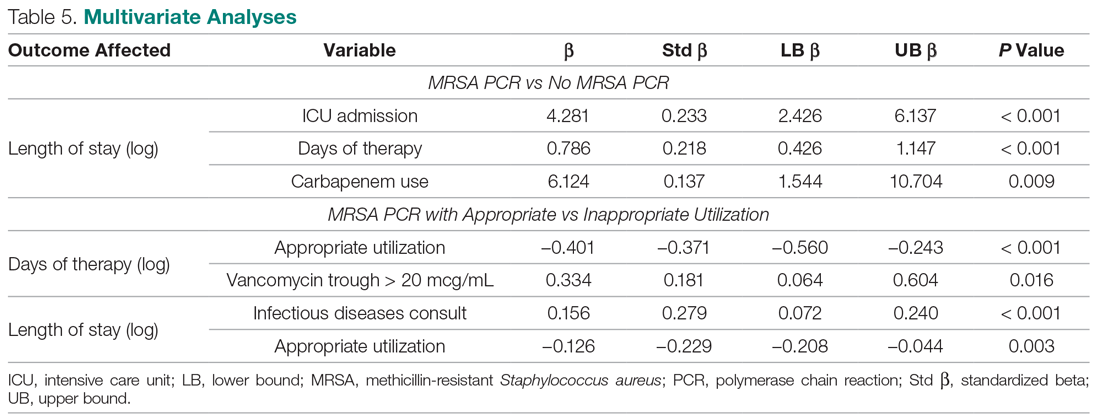
A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.
Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).

In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.

Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.

In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.

A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.

Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).

In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.

Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.

In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.

A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.

Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
Observational study again suggests lasting impact of COVID-19 on heart
A new study using cardiac magnetic resonance (CMR) imaging to examine the effects of novel coronavirus infection on the heart showed signs suggestive of myocarditis in 4 out of 26 competitive athletes who recovered from asymptomatic or mild cases of COVID-19.
While these and other similar findings are concerning, commentators are saying the results are preliminary and do not indicate widespread CMR screening is appropriate.
Two of the 4 patients showing signs of myocarditis in this series had no symptoms of COVID-19 but tested positive on routine testing. An additional 12 student athletes (46%) showed late gadolinium enhancement (LGE), of whom 8 (30.8%) had LGE without T2 elevation suggestive of prior myocardial injury.
An additional 12 student athletes (46%) showed late gadolinium enhancement (LGE), of whom 8 (31%) had LGE without T2 elevation suggestive of prior myocardial injury.
This finding, said Saurabh Rajpal, MBBS, MD, the study’s lead author, “could suggest prior myocardial injury or it could suggest athletic myocardial adaptation.”
In a research letter published in JAMA Cardiology, Rajpal and colleagues at Ohio State University in Columbus, described the findings of comprehensive CMR examinations in competitive athletes referred to the sport medicine clinic after testing positive for COVID-19 on reverse transcriptase-polymerase chain reaction between June and August 2020.
The university had made the decision in the spring to use CMR imaging as a screening tool for return to play, said Dr. Rajpal. While CMR is being used for research purposes, the American College of Cardiology’s recent “consensus expert opinion” statement on resumption of sport and exercise after COVID-19 infection does not require CMR imaging for resumption of competitive activity (JAMA Cardiol. 2020 May 13. doi:10.1001/jamacardio.2020.2136).
None of the athletes required hospitalization for their illness, and only 27% reported mild symptoms during the short-term infection, including sore throat, shortness of breath, myalgia, and fever.
On the day of CMR imaging, ECG and transthoracic echocardiography were performed, and serum troponin I was measured. There were no diagnostic ST/T wave changes, ventricular function and volumes were normal, and no athletes showed elevated serum troponin levels.
The updated Lake Louise Criteria were used to assess CMR findings consistent with myocarditis.
“I don’t think this is a COVID-specific issue. We have seen myocarditis after other viral infections; it’s just that COVID-19 is the most studied thus far, and with strenuous activity, inflammation in the heart can be risky,” Dr. Rajpal said in an interview. He added that more long-term and larger studies with control populations are needed.
His group is continuing to follow these athletes and has suggested that CMR “may provide an excellent risk-stratification assessment for myocarditis in athletes who have recovered from COVID-19 to guide safe competitive sports participation.”
Significance still unknown
Matthew Martinez, MD, the director of sports cardiology at Atlantic Health – Morristown (N.J.) Medical Center and the Gagnon Cardiovascular Institute, urged caution in making too much of the findings of this small study.
“We know that viruses cause myocardial damage and myocarditis. What we don’t know is how important these findings are. And in terms of risk, would we find the same phenomenon if we did this, say, in flu patients or in other age groups?” Dr. Martinez said in an interview.
“I haven’t seen all the images, but what I’d want to know is are these very subtle findings? Are these overt findings? Is this part of an active individual with symptoms? I need to know a little more data before I can tell if this influences the increased risk of sudden cardiac death that we often associate with myocarditis. I’m not sure how this should influence making decisions with regards to return to play.”
Dr. Martinez, who is the ACC’s chair of Sports and Exercise but was not an author of their recent guidance on return to sport, said that he is not routinely using CMR to assess athletes post-infection, as per the ACC’s recommendations.
“My approach is to evaluate anybody with a history of COVID infection and, first, determine whether it was an important infection with significant symptoms or not. And then, if they’re participating at a high level or are professional athletes, I would suggest an ECG, echo, and troponin. That’s our recommendation for the last several months and is still an appropriate way to evaluate that group.”
“In the presence of an abnormality or ongoing symptoms, I would ask for an MRI at that point,” said Dr. Martinez.
“We just don’t have much data on athletes with no symptoms to use to interpret these CMR findings and the study didn’t offer any controls. We don’t even know if these findings are new findings or old findings that have just been identified now,” he added.
New, updated recommendations from the ACC are coming soon, said Dr. Martinez. “I do not expect them to include CMR as first line.”
Cardiologists concerned about misinformation
This is at least the fourth study showing myocardial damage post-COVID-19 infection and there is concern in the medical community that the media has overstated the risks of heart damage, especially in athletes, and at the same time overstated the benefits of CMR.
In particular, Puntmann et al reported in July a 100-patient study that showed evidence of myocardial inflammation by CMR in 78% of patients recently recovered from a bout of COVID-19 (JAMA Cardiol. 2020 Jul 27; doi:10.1001/jamacardio.2020.3557).
“That paper is completely problematic,” John Mandrola, MD, of Baptists Medical Associates, Louisville, Ky., said in an interview. “It has the same overarching weaknesses [of other studies] that it’s observational and retrospective, but there were also numerical issues. So to me that paper is an interesting observation, but utterly unconvincing and preliminary,” said Dr. Mandrola.
Those limitations didn’t stop the study from garnering media attention, however. The Altmetric score (an attention score that tracks all mentions of an article in the media and on social media) for the Puntmann et al paper is approaching 13,000, including coverage from 276 news outlets and more than 19,000 tweets, putting it in the 99th percentile of all research outputs tracked by Altmetric to date.
To counter this, an “open letter” posted online just days before the Rajpal study published urging professional societies to “offer clear guidance discouraging CMR screening for COVID-19 related heart abnormalities in asymptomatic members of the general public.” The letter was signed by 51 clinicians, researchers, and imaging specialists from around the world.
Dr. Mandrola, one of the signatories, said: “This topic really scares people, and when it gets in the media like this, I think the leaders of these societies need to come out and say something really clear on major news networks letting people know that it’s just way too premature to start doing CMRs on every athlete that’s gotten this virus.”
“I understand that the current guidelines may be clear that CMR is not a first-line test for this indication, but when the media coverage is so extensive and so overblown, I wonder how much impact the guidelines will have in countering this fear that’s in the community,” he added.
Asked to comment on the letter, Dr. Rajpal said he agrees with the signatories that asymptomatic people from general population do not need routine cardiac MRI. “However, competitive athletes are a different story. Testing depends on risk assessment in specific population and competitive athletes as per our protocol will get enhanced cardiac workup including CMR for responsible and safe start of competitive sports. ... In the present scenario, while we get more data including control data, we will continue with our current protocol.”
Dr. Mandrola is Medscape Cardiology’s Chief Cardiology Consultant. MDedge is part of the Medscape Professional Network.
This article first appeared on Medscape.com.
A new study using cardiac magnetic resonance (CMR) imaging to examine the effects of novel coronavirus infection on the heart showed signs suggestive of myocarditis in 4 out of 26 competitive athletes who recovered from asymptomatic or mild cases of COVID-19.
While these and other similar findings are concerning, commentators are saying the results are preliminary and do not indicate widespread CMR screening is appropriate.
Two of the 4 patients showing signs of myocarditis in this series had no symptoms of COVID-19 but tested positive on routine testing. An additional 12 student athletes (46%) showed late gadolinium enhancement (LGE), of whom 8 (30.8%) had LGE without T2 elevation suggestive of prior myocardial injury.
An additional 12 student athletes (46%) showed late gadolinium enhancement (LGE), of whom 8 (31%) had LGE without T2 elevation suggestive of prior myocardial injury.
This finding, said Saurabh Rajpal, MBBS, MD, the study’s lead author, “could suggest prior myocardial injury or it could suggest athletic myocardial adaptation.”
In a research letter published in JAMA Cardiology, Rajpal and colleagues at Ohio State University in Columbus, described the findings of comprehensive CMR examinations in competitive athletes referred to the sport medicine clinic after testing positive for COVID-19 on reverse transcriptase-polymerase chain reaction between June and August 2020.
The university had made the decision in the spring to use CMR imaging as a screening tool for return to play, said Dr. Rajpal. While CMR is being used for research purposes, the American College of Cardiology’s recent “consensus expert opinion” statement on resumption of sport and exercise after COVID-19 infection does not require CMR imaging for resumption of competitive activity (JAMA Cardiol. 2020 May 13. doi:10.1001/jamacardio.2020.2136).
None of the athletes required hospitalization for their illness, and only 27% reported mild symptoms during the short-term infection, including sore throat, shortness of breath, myalgia, and fever.
On the day of CMR imaging, ECG and transthoracic echocardiography were performed, and serum troponin I was measured. There were no diagnostic ST/T wave changes, ventricular function and volumes were normal, and no athletes showed elevated serum troponin levels.
The updated Lake Louise Criteria were used to assess CMR findings consistent with myocarditis.
“I don’t think this is a COVID-specific issue. We have seen myocarditis after other viral infections; it’s just that COVID-19 is the most studied thus far, and with strenuous activity, inflammation in the heart can be risky,” Dr. Rajpal said in an interview. He added that more long-term and larger studies with control populations are needed.
His group is continuing to follow these athletes and has suggested that CMR “may provide an excellent risk-stratification assessment for myocarditis in athletes who have recovered from COVID-19 to guide safe competitive sports participation.”
Significance still unknown
Matthew Martinez, MD, the director of sports cardiology at Atlantic Health – Morristown (N.J.) Medical Center and the Gagnon Cardiovascular Institute, urged caution in making too much of the findings of this small study.
“We know that viruses cause myocardial damage and myocarditis. What we don’t know is how important these findings are. And in terms of risk, would we find the same phenomenon if we did this, say, in flu patients or in other age groups?” Dr. Martinez said in an interview.
“I haven’t seen all the images, but what I’d want to know is are these very subtle findings? Are these overt findings? Is this part of an active individual with symptoms? I need to know a little more data before I can tell if this influences the increased risk of sudden cardiac death that we often associate with myocarditis. I’m not sure how this should influence making decisions with regards to return to play.”
Dr. Martinez, who is the ACC’s chair of Sports and Exercise but was not an author of their recent guidance on return to sport, said that he is not routinely using CMR to assess athletes post-infection, as per the ACC’s recommendations.
“My approach is to evaluate anybody with a history of COVID infection and, first, determine whether it was an important infection with significant symptoms or not. And then, if they’re participating at a high level or are professional athletes, I would suggest an ECG, echo, and troponin. That’s our recommendation for the last several months and is still an appropriate way to evaluate that group.”
“In the presence of an abnormality or ongoing symptoms, I would ask for an MRI at that point,” said Dr. Martinez.
“We just don’t have much data on athletes with no symptoms to use to interpret these CMR findings and the study didn’t offer any controls. We don’t even know if these findings are new findings or old findings that have just been identified now,” he added.
New, updated recommendations from the ACC are coming soon, said Dr. Martinez. “I do not expect them to include CMR as first line.”
Cardiologists concerned about misinformation
This is at least the fourth study showing myocardial damage post-COVID-19 infection and there is concern in the medical community that the media has overstated the risks of heart damage, especially in athletes, and at the same time overstated the benefits of CMR.
In particular, Puntmann et al reported in July a 100-patient study that showed evidence of myocardial inflammation by CMR in 78% of patients recently recovered from a bout of COVID-19 (JAMA Cardiol. 2020 Jul 27; doi:10.1001/jamacardio.2020.3557).
“That paper is completely problematic,” John Mandrola, MD, of Baptists Medical Associates, Louisville, Ky., said in an interview. “It has the same overarching weaknesses [of other studies] that it’s observational and retrospective, but there were also numerical issues. So to me that paper is an interesting observation, but utterly unconvincing and preliminary,” said Dr. Mandrola.
Those limitations didn’t stop the study from garnering media attention, however. The Altmetric score (an attention score that tracks all mentions of an article in the media and on social media) for the Puntmann et al paper is approaching 13,000, including coverage from 276 news outlets and more than 19,000 tweets, putting it in the 99th percentile of all research outputs tracked by Altmetric to date.
To counter this, an “open letter” posted online just days before the Rajpal study published urging professional societies to “offer clear guidance discouraging CMR screening for COVID-19 related heart abnormalities in asymptomatic members of the general public.” The letter was signed by 51 clinicians, researchers, and imaging specialists from around the world.
Dr. Mandrola, one of the signatories, said: “This topic really scares people, and when it gets in the media like this, I think the leaders of these societies need to come out and say something really clear on major news networks letting people know that it’s just way too premature to start doing CMRs on every athlete that’s gotten this virus.”
“I understand that the current guidelines may be clear that CMR is not a first-line test for this indication, but when the media coverage is so extensive and so overblown, I wonder how much impact the guidelines will have in countering this fear that’s in the community,” he added.
Asked to comment on the letter, Dr. Rajpal said he agrees with the signatories that asymptomatic people from general population do not need routine cardiac MRI. “However, competitive athletes are a different story. Testing depends on risk assessment in specific population and competitive athletes as per our protocol will get enhanced cardiac workup including CMR for responsible and safe start of competitive sports. ... In the present scenario, while we get more data including control data, we will continue with our current protocol.”
Dr. Mandrola is Medscape Cardiology’s Chief Cardiology Consultant. MDedge is part of the Medscape Professional Network.
This article first appeared on Medscape.com.
A new study using cardiac magnetic resonance (CMR) imaging to examine the effects of novel coronavirus infection on the heart showed signs suggestive of myocarditis in 4 out of 26 competitive athletes who recovered from asymptomatic or mild cases of COVID-19.
While these and other similar findings are concerning, commentators are saying the results are preliminary and do not indicate widespread CMR screening is appropriate.
Two of the 4 patients showing signs of myocarditis in this series had no symptoms of COVID-19 but tested positive on routine testing. An additional 12 student athletes (46%) showed late gadolinium enhancement (LGE), of whom 8 (30.8%) had LGE without T2 elevation suggestive of prior myocardial injury.
An additional 12 student athletes (46%) showed late gadolinium enhancement (LGE), of whom 8 (31%) had LGE without T2 elevation suggestive of prior myocardial injury.
This finding, said Saurabh Rajpal, MBBS, MD, the study’s lead author, “could suggest prior myocardial injury or it could suggest athletic myocardial adaptation.”
In a research letter published in JAMA Cardiology, Rajpal and colleagues at Ohio State University in Columbus, described the findings of comprehensive CMR examinations in competitive athletes referred to the sport medicine clinic after testing positive for COVID-19 on reverse transcriptase-polymerase chain reaction between June and August 2020.
The university had made the decision in the spring to use CMR imaging as a screening tool for return to play, said Dr. Rajpal. While CMR is being used for research purposes, the American College of Cardiology’s recent “consensus expert opinion” statement on resumption of sport and exercise after COVID-19 infection does not require CMR imaging for resumption of competitive activity (JAMA Cardiol. 2020 May 13. doi:10.1001/jamacardio.2020.2136).
None of the athletes required hospitalization for their illness, and only 27% reported mild symptoms during the short-term infection, including sore throat, shortness of breath, myalgia, and fever.
On the day of CMR imaging, ECG and transthoracic echocardiography were performed, and serum troponin I was measured. There were no diagnostic ST/T wave changes, ventricular function and volumes were normal, and no athletes showed elevated serum troponin levels.
The updated Lake Louise Criteria were used to assess CMR findings consistent with myocarditis.
“I don’t think this is a COVID-specific issue. We have seen myocarditis after other viral infections; it’s just that COVID-19 is the most studied thus far, and with strenuous activity, inflammation in the heart can be risky,” Dr. Rajpal said in an interview. He added that more long-term and larger studies with control populations are needed.
His group is continuing to follow these athletes and has suggested that CMR “may provide an excellent risk-stratification assessment for myocarditis in athletes who have recovered from COVID-19 to guide safe competitive sports participation.”
Significance still unknown
Matthew Martinez, MD, the director of sports cardiology at Atlantic Health – Morristown (N.J.) Medical Center and the Gagnon Cardiovascular Institute, urged caution in making too much of the findings of this small study.
“We know that viruses cause myocardial damage and myocarditis. What we don’t know is how important these findings are. And in terms of risk, would we find the same phenomenon if we did this, say, in flu patients or in other age groups?” Dr. Martinez said in an interview.
“I haven’t seen all the images, but what I’d want to know is are these very subtle findings? Are these overt findings? Is this part of an active individual with symptoms? I need to know a little more data before I can tell if this influences the increased risk of sudden cardiac death that we often associate with myocarditis. I’m not sure how this should influence making decisions with regards to return to play.”
Dr. Martinez, who is the ACC’s chair of Sports and Exercise but was not an author of their recent guidance on return to sport, said that he is not routinely using CMR to assess athletes post-infection, as per the ACC’s recommendations.
“My approach is to evaluate anybody with a history of COVID infection and, first, determine whether it was an important infection with significant symptoms or not. And then, if they’re participating at a high level or are professional athletes, I would suggest an ECG, echo, and troponin. That’s our recommendation for the last several months and is still an appropriate way to evaluate that group.”
“In the presence of an abnormality or ongoing symptoms, I would ask for an MRI at that point,” said Dr. Martinez.
“We just don’t have much data on athletes with no symptoms to use to interpret these CMR findings and the study didn’t offer any controls. We don’t even know if these findings are new findings or old findings that have just been identified now,” he added.
New, updated recommendations from the ACC are coming soon, said Dr. Martinez. “I do not expect them to include CMR as first line.”
Cardiologists concerned about misinformation
This is at least the fourth study showing myocardial damage post-COVID-19 infection and there is concern in the medical community that the media has overstated the risks of heart damage, especially in athletes, and at the same time overstated the benefits of CMR.
In particular, Puntmann et al reported in July a 100-patient study that showed evidence of myocardial inflammation by CMR in 78% of patients recently recovered from a bout of COVID-19 (JAMA Cardiol. 2020 Jul 27; doi:10.1001/jamacardio.2020.3557).
“That paper is completely problematic,” John Mandrola, MD, of Baptists Medical Associates, Louisville, Ky., said in an interview. “It has the same overarching weaknesses [of other studies] that it’s observational and retrospective, but there were also numerical issues. So to me that paper is an interesting observation, but utterly unconvincing and preliminary,” said Dr. Mandrola.
Those limitations didn’t stop the study from garnering media attention, however. The Altmetric score (an attention score that tracks all mentions of an article in the media and on social media) for the Puntmann et al paper is approaching 13,000, including coverage from 276 news outlets and more than 19,000 tweets, putting it in the 99th percentile of all research outputs tracked by Altmetric to date.
To counter this, an “open letter” posted online just days before the Rajpal study published urging professional societies to “offer clear guidance discouraging CMR screening for COVID-19 related heart abnormalities in asymptomatic members of the general public.” The letter was signed by 51 clinicians, researchers, and imaging specialists from around the world.
Dr. Mandrola, one of the signatories, said: “This topic really scares people, and when it gets in the media like this, I think the leaders of these societies need to come out and say something really clear on major news networks letting people know that it’s just way too premature to start doing CMRs on every athlete that’s gotten this virus.”
“I understand that the current guidelines may be clear that CMR is not a first-line test for this indication, but when the media coverage is so extensive and so overblown, I wonder how much impact the guidelines will have in countering this fear that’s in the community,” he added.
Asked to comment on the letter, Dr. Rajpal said he agrees with the signatories that asymptomatic people from general population do not need routine cardiac MRI. “However, competitive athletes are a different story. Testing depends on risk assessment in specific population and competitive athletes as per our protocol will get enhanced cardiac workup including CMR for responsible and safe start of competitive sports. ... In the present scenario, while we get more data including control data, we will continue with our current protocol.”
Dr. Mandrola is Medscape Cardiology’s Chief Cardiology Consultant. MDedge is part of the Medscape Professional Network.
This article first appeared on Medscape.com.
Wildfires’ toxic air leaves damage long after the smoke clears
When researchers arrived in Seeley Lake, Mont., a town tucked in the northern Rockies, 3 years ago, they could still smell the smoke a day after it cleared from devastating wildfires. Their plan was to chart how long it took for people to recover from living for 7 weeks surrounded by relentless smoke.
They still don’t know, because most residents haven’t recovered. In fact, they’ve gotten worse.
Forest fires had funneled hazardous air into Seeley Lake, a town of fewer than 2,000 people, for 49 days. The air quality was so bad that on some days the monitoring stations couldn’t measure the extent of the pollution. The intensity of the smoke and the length of time residents had been trapped in it were unprecedented, prompting county officials to issue their first evacuation orders because of smoke, not fire risk.
Many people stayed. That made Seeley Lake an ideal place to track the long-term health of people inundated by wildfire pollution.
So far, researchers have found that people’s lung capacity declined in the first 2 years after the smoke cleared. Chris Migliaccio, PhD, an immunologist with the University of Montana, Missoula, and associates found the percentage of residents whose lung function sank below normal thresholds more than doubled in the first year after the fire and remained low a year after that.
“There’s something wrong there,” Dr. Migliaccio said.
While it’s long been known that smoke can be dangerous when in the thick of it – triggering asthma attacks, cardiac arrests, hospitalizations and more – the Seeley Lake research confirmed what public health experts feared: Wildfire haze can have consequences long after it’s gone.
That doesn’t bode well for the 78 million people in the western United States now confronting historic wildfires.
Toxic air from fires has blanketed California and the Pacific Northwest for weeks now, causing some of the world’s worst air quality. California fires have burned roughly 2.3 million acres so far this year, and the wildfire season isn’t over yet. Oregon estimates 500,000 people in the state have been under a notice to either prepare to evacuate or leave. Smoke from the West Coast blazes has drifted as far away as Europe.
Extreme wildfires are predicted to become a regular occurrence because of climate change. And, as more people increasingly settle in fire-prone places, the risks increase. That’s shifted wildfires from being a perennial reality for rural mountain towns to becoming an annual threat for areas across the West.
Perry Hystad, PhD, an associate professor at Oregon State University, Corvallis, said the Seeley Lake research offers unique insights into wildfire smoke’s impact, which until recently had largely been unexplored. He said similar studies are likely to follow because of this fire season.
“This is the question that everybody is asking,” Dr. Hystad said. “‘I’ve been sitting in smoke for 2 weeks, how concerned should I be?’”
Dr. Migliaccio wants to know whether the lung damage he saw in Seeley Lake is reversible – or even treatable. (Think of an inhaler for asthma or other medication that prevents swollen airways.)
But those discoveries will have to wait. The team hasn’t been able to return to Seeley Lake this year because of the coronavirus pandemic.
Dr. Migliaccio said more research is needed on whether wildfire smoke damages organs besides the lungs, and whether routine exposure makes people more susceptible to diseases.
The combination of the fire season and the pandemic has spurred other questions as well, like whether heavy smoke exposure could lead to more COVID-19 deaths. A recent study showed a spike in influenza cases following major fire seasons.
“Now you have the combination of flu season and COVID and the wildfires,” Dr. Migliaccio said. “How are all these things going to interact come late fall or winter?”
A case study
Seeley Lake has long known smoke. It sits in a narrow valley between vast stretches of thick forests.
On a recent September day, Boyd Gossard stood on his back porch and pointed toward the mountains that were ablaze in 2017.
Mr. Gossard, 80, expects to have some summer days veiled in haze. But that year, he said, he could hardly see his neighbor’s house a few hundred feet away.
“I’ve seen a lot of smoke in my career,” said Mr. Gossard, who worked in timber management and served as a wildland firefighter. “But having to just live in it like this was very different. It got to you after a while.”
When Missoula County health officials urged people to leave town and flee the hazardous smoke, many residents stayed close to home. Some said their jobs wouldn’t let them leave. Others didn’t have a place to go – or the money to get there.
Health officials warned those who stayed to avoid exercising and breathing too hard, to remain inside, and to follow steps to make their homes as smoke free as possible. The health department also worked to get air filters to those who needed them most.
But when flames got too close, some people had to sleep outside in campsites on the other side of town.
Understanding the science of smoke
One of the known dangers of smoke is particulate matter. Smaller than the width of a human hair, it can bypass a body’s defenses, lodging deep into lungs. Lu Hu, PhD, an atmospheric chemist with the University of Montana, said air quality reports are based on how much of that pollution is in the air.
“It’s like lead; there’s no safe level, but still we have a safety measure for what’s allowable,” Dr. Hu said. “Some things kill you fast and some things kill you slowly.”
While air quality measurements can gauge the overall amount of pollution, they can’t assess which specific toxins people are inhaling. Dr. Hu is collaborating with other scientists to better predict how smoke travels and what pollutants people actually breathe.
He said smoke’s chemistry changes based on how far it travels and what’s burning, among other factors.
Over the past few years, teams of researchers drove trucks along fire lines to collect smoke samples. Other scientists boarded cargo planes and flew into smoke plumes to take samples right from a fire’s source. Still others stationed at a mountain lookout captured smoke drifting in from nearby fires. And ground-level machines at a Missoula site logged data over 2 summers.
Bob Yokelson, PhD, a longtime smoke researcher with the University of Montana, said scientists are getting closer to understanding its contents. And, he said, “it’s not all bad news.”
Temperature and sunlight can change some pollutants over time. Some dangerous particles seem to disappear. But others, such as ozone, can increase as smoke ages.
Dr. Yokelson said scientists are still a long way from determining a safe level of exposure to the hundred-odd pollutants in smoke.
“We can complete the circle by measuring not only what’s in smoke, but measuring what’s happening to the people who breathe it,” Dr. Yokelson said. “That’s where the future of health research on smoke is going to go.”
Coping with nowhere to flee
In the meantime, those studying wildland smoke hope what they’ve learned so far can better prepare people to live in the haze when evacuation isn’t an option.
Joan Wollan, 82, was one of the Seeley Lake study participants. She stayed put during the 2017 fire because her house at the time sat on a border of the evacuation zone. The air made her eyes burn and her husband cough. She ordered air filters to create cleaner air inside her home, which helped.
On a recent day, the air in Mrs. Wollan’s new neighborhood in Missoula turned that familiar gray-orange as traces of fires from elsewhere appeared. Local health officials warned that western Montana could get hit by some of the worst air quality the state had seen since those 2017 fires.
If it got bad enough, Mrs. Wollan said, she’d get the filters out of storage or look for a way to get to cleaner air – “if there is someplace in Montana that isn’t smoky.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
When researchers arrived in Seeley Lake, Mont., a town tucked in the northern Rockies, 3 years ago, they could still smell the smoke a day after it cleared from devastating wildfires. Their plan was to chart how long it took for people to recover from living for 7 weeks surrounded by relentless smoke.
They still don’t know, because most residents haven’t recovered. In fact, they’ve gotten worse.
Forest fires had funneled hazardous air into Seeley Lake, a town of fewer than 2,000 people, for 49 days. The air quality was so bad that on some days the monitoring stations couldn’t measure the extent of the pollution. The intensity of the smoke and the length of time residents had been trapped in it were unprecedented, prompting county officials to issue their first evacuation orders because of smoke, not fire risk.
Many people stayed. That made Seeley Lake an ideal place to track the long-term health of people inundated by wildfire pollution.
So far, researchers have found that people’s lung capacity declined in the first 2 years after the smoke cleared. Chris Migliaccio, PhD, an immunologist with the University of Montana, Missoula, and associates found the percentage of residents whose lung function sank below normal thresholds more than doubled in the first year after the fire and remained low a year after that.
“There’s something wrong there,” Dr. Migliaccio said.
While it’s long been known that smoke can be dangerous when in the thick of it – triggering asthma attacks, cardiac arrests, hospitalizations and more – the Seeley Lake research confirmed what public health experts feared: Wildfire haze can have consequences long after it’s gone.
That doesn’t bode well for the 78 million people in the western United States now confronting historic wildfires.
Toxic air from fires has blanketed California and the Pacific Northwest for weeks now, causing some of the world’s worst air quality. California fires have burned roughly 2.3 million acres so far this year, and the wildfire season isn’t over yet. Oregon estimates 500,000 people in the state have been under a notice to either prepare to evacuate or leave. Smoke from the West Coast blazes has drifted as far away as Europe.
Extreme wildfires are predicted to become a regular occurrence because of climate change. And, as more people increasingly settle in fire-prone places, the risks increase. That’s shifted wildfires from being a perennial reality for rural mountain towns to becoming an annual threat for areas across the West.
Perry Hystad, PhD, an associate professor at Oregon State University, Corvallis, said the Seeley Lake research offers unique insights into wildfire smoke’s impact, which until recently had largely been unexplored. He said similar studies are likely to follow because of this fire season.
“This is the question that everybody is asking,” Dr. Hystad said. “‘I’ve been sitting in smoke for 2 weeks, how concerned should I be?’”
Dr. Migliaccio wants to know whether the lung damage he saw in Seeley Lake is reversible – or even treatable. (Think of an inhaler for asthma or other medication that prevents swollen airways.)
But those discoveries will have to wait. The team hasn’t been able to return to Seeley Lake this year because of the coronavirus pandemic.
Dr. Migliaccio said more research is needed on whether wildfire smoke damages organs besides the lungs, and whether routine exposure makes people more susceptible to diseases.
The combination of the fire season and the pandemic has spurred other questions as well, like whether heavy smoke exposure could lead to more COVID-19 deaths. A recent study showed a spike in influenza cases following major fire seasons.
“Now you have the combination of flu season and COVID and the wildfires,” Dr. Migliaccio said. “How are all these things going to interact come late fall or winter?”
A case study
Seeley Lake has long known smoke. It sits in a narrow valley between vast stretches of thick forests.
On a recent September day, Boyd Gossard stood on his back porch and pointed toward the mountains that were ablaze in 2017.
Mr. Gossard, 80, expects to have some summer days veiled in haze. But that year, he said, he could hardly see his neighbor’s house a few hundred feet away.
“I’ve seen a lot of smoke in my career,” said Mr. Gossard, who worked in timber management and served as a wildland firefighter. “But having to just live in it like this was very different. It got to you after a while.”
When Missoula County health officials urged people to leave town and flee the hazardous smoke, many residents stayed close to home. Some said their jobs wouldn’t let them leave. Others didn’t have a place to go – or the money to get there.
Health officials warned those who stayed to avoid exercising and breathing too hard, to remain inside, and to follow steps to make their homes as smoke free as possible. The health department also worked to get air filters to those who needed them most.
But when flames got too close, some people had to sleep outside in campsites on the other side of town.
Understanding the science of smoke
One of the known dangers of smoke is particulate matter. Smaller than the width of a human hair, it can bypass a body’s defenses, lodging deep into lungs. Lu Hu, PhD, an atmospheric chemist with the University of Montana, said air quality reports are based on how much of that pollution is in the air.
“It’s like lead; there’s no safe level, but still we have a safety measure for what’s allowable,” Dr. Hu said. “Some things kill you fast and some things kill you slowly.”
While air quality measurements can gauge the overall amount of pollution, they can’t assess which specific toxins people are inhaling. Dr. Hu is collaborating with other scientists to better predict how smoke travels and what pollutants people actually breathe.
He said smoke’s chemistry changes based on how far it travels and what’s burning, among other factors.
Over the past few years, teams of researchers drove trucks along fire lines to collect smoke samples. Other scientists boarded cargo planes and flew into smoke plumes to take samples right from a fire’s source. Still others stationed at a mountain lookout captured smoke drifting in from nearby fires. And ground-level machines at a Missoula site logged data over 2 summers.
Bob Yokelson, PhD, a longtime smoke researcher with the University of Montana, said scientists are getting closer to understanding its contents. And, he said, “it’s not all bad news.”
Temperature and sunlight can change some pollutants over time. Some dangerous particles seem to disappear. But others, such as ozone, can increase as smoke ages.
Dr. Yokelson said scientists are still a long way from determining a safe level of exposure to the hundred-odd pollutants in smoke.
“We can complete the circle by measuring not only what’s in smoke, but measuring what’s happening to the people who breathe it,” Dr. Yokelson said. “That’s where the future of health research on smoke is going to go.”
Coping with nowhere to flee
In the meantime, those studying wildland smoke hope what they’ve learned so far can better prepare people to live in the haze when evacuation isn’t an option.
Joan Wollan, 82, was one of the Seeley Lake study participants. She stayed put during the 2017 fire because her house at the time sat on a border of the evacuation zone. The air made her eyes burn and her husband cough. She ordered air filters to create cleaner air inside her home, which helped.
On a recent day, the air in Mrs. Wollan’s new neighborhood in Missoula turned that familiar gray-orange as traces of fires from elsewhere appeared. Local health officials warned that western Montana could get hit by some of the worst air quality the state had seen since those 2017 fires.
If it got bad enough, Mrs. Wollan said, she’d get the filters out of storage or look for a way to get to cleaner air – “if there is someplace in Montana that isn’t smoky.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
When researchers arrived in Seeley Lake, Mont., a town tucked in the northern Rockies, 3 years ago, they could still smell the smoke a day after it cleared from devastating wildfires. Their plan was to chart how long it took for people to recover from living for 7 weeks surrounded by relentless smoke.
They still don’t know, because most residents haven’t recovered. In fact, they’ve gotten worse.
Forest fires had funneled hazardous air into Seeley Lake, a town of fewer than 2,000 people, for 49 days. The air quality was so bad that on some days the monitoring stations couldn’t measure the extent of the pollution. The intensity of the smoke and the length of time residents had been trapped in it were unprecedented, prompting county officials to issue their first evacuation orders because of smoke, not fire risk.
Many people stayed. That made Seeley Lake an ideal place to track the long-term health of people inundated by wildfire pollution.
So far, researchers have found that people’s lung capacity declined in the first 2 years after the smoke cleared. Chris Migliaccio, PhD, an immunologist with the University of Montana, Missoula, and associates found the percentage of residents whose lung function sank below normal thresholds more than doubled in the first year after the fire and remained low a year after that.
“There’s something wrong there,” Dr. Migliaccio said.
While it’s long been known that smoke can be dangerous when in the thick of it – triggering asthma attacks, cardiac arrests, hospitalizations and more – the Seeley Lake research confirmed what public health experts feared: Wildfire haze can have consequences long after it’s gone.
That doesn’t bode well for the 78 million people in the western United States now confronting historic wildfires.
Toxic air from fires has blanketed California and the Pacific Northwest for weeks now, causing some of the world’s worst air quality. California fires have burned roughly 2.3 million acres so far this year, and the wildfire season isn’t over yet. Oregon estimates 500,000 people in the state have been under a notice to either prepare to evacuate or leave. Smoke from the West Coast blazes has drifted as far away as Europe.
Extreme wildfires are predicted to become a regular occurrence because of climate change. And, as more people increasingly settle in fire-prone places, the risks increase. That’s shifted wildfires from being a perennial reality for rural mountain towns to becoming an annual threat for areas across the West.
Perry Hystad, PhD, an associate professor at Oregon State University, Corvallis, said the Seeley Lake research offers unique insights into wildfire smoke’s impact, which until recently had largely been unexplored. He said similar studies are likely to follow because of this fire season.
“This is the question that everybody is asking,” Dr. Hystad said. “‘I’ve been sitting in smoke for 2 weeks, how concerned should I be?’”
Dr. Migliaccio wants to know whether the lung damage he saw in Seeley Lake is reversible – or even treatable. (Think of an inhaler for asthma or other medication that prevents swollen airways.)
But those discoveries will have to wait. The team hasn’t been able to return to Seeley Lake this year because of the coronavirus pandemic.
Dr. Migliaccio said more research is needed on whether wildfire smoke damages organs besides the lungs, and whether routine exposure makes people more susceptible to diseases.
The combination of the fire season and the pandemic has spurred other questions as well, like whether heavy smoke exposure could lead to more COVID-19 deaths. A recent study showed a spike in influenza cases following major fire seasons.
“Now you have the combination of flu season and COVID and the wildfires,” Dr. Migliaccio said. “How are all these things going to interact come late fall or winter?”
A case study
Seeley Lake has long known smoke. It sits in a narrow valley between vast stretches of thick forests.
On a recent September day, Boyd Gossard stood on his back porch and pointed toward the mountains that were ablaze in 2017.
Mr. Gossard, 80, expects to have some summer days veiled in haze. But that year, he said, he could hardly see his neighbor’s house a few hundred feet away.
“I’ve seen a lot of smoke in my career,” said Mr. Gossard, who worked in timber management and served as a wildland firefighter. “But having to just live in it like this was very different. It got to you after a while.”
When Missoula County health officials urged people to leave town and flee the hazardous smoke, many residents stayed close to home. Some said their jobs wouldn’t let them leave. Others didn’t have a place to go – or the money to get there.
Health officials warned those who stayed to avoid exercising and breathing too hard, to remain inside, and to follow steps to make their homes as smoke free as possible. The health department also worked to get air filters to those who needed them most.
But when flames got too close, some people had to sleep outside in campsites on the other side of town.
Understanding the science of smoke
One of the known dangers of smoke is particulate matter. Smaller than the width of a human hair, it can bypass a body’s defenses, lodging deep into lungs. Lu Hu, PhD, an atmospheric chemist with the University of Montana, said air quality reports are based on how much of that pollution is in the air.
“It’s like lead; there’s no safe level, but still we have a safety measure for what’s allowable,” Dr. Hu said. “Some things kill you fast and some things kill you slowly.”
While air quality measurements can gauge the overall amount of pollution, they can’t assess which specific toxins people are inhaling. Dr. Hu is collaborating with other scientists to better predict how smoke travels and what pollutants people actually breathe.
He said smoke’s chemistry changes based on how far it travels and what’s burning, among other factors.
Over the past few years, teams of researchers drove trucks along fire lines to collect smoke samples. Other scientists boarded cargo planes and flew into smoke plumes to take samples right from a fire’s source. Still others stationed at a mountain lookout captured smoke drifting in from nearby fires. And ground-level machines at a Missoula site logged data over 2 summers.
Bob Yokelson, PhD, a longtime smoke researcher with the University of Montana, said scientists are getting closer to understanding its contents. And, he said, “it’s not all bad news.”
Temperature and sunlight can change some pollutants over time. Some dangerous particles seem to disappear. But others, such as ozone, can increase as smoke ages.
Dr. Yokelson said scientists are still a long way from determining a safe level of exposure to the hundred-odd pollutants in smoke.
“We can complete the circle by measuring not only what’s in smoke, but measuring what’s happening to the people who breathe it,” Dr. Yokelson said. “That’s where the future of health research on smoke is going to go.”
Coping with nowhere to flee
In the meantime, those studying wildland smoke hope what they’ve learned so far can better prepare people to live in the haze when evacuation isn’t an option.
Joan Wollan, 82, was one of the Seeley Lake study participants. She stayed put during the 2017 fire because her house at the time sat on a border of the evacuation zone. The air made her eyes burn and her husband cough. She ordered air filters to create cleaner air inside her home, which helped.
On a recent day, the air in Mrs. Wollan’s new neighborhood in Missoula turned that familiar gray-orange as traces of fires from elsewhere appeared. Local health officials warned that western Montana could get hit by some of the worst air quality the state had seen since those 2017 fires.
If it got bad enough, Mrs. Wollan said, she’d get the filters out of storage or look for a way to get to cleaner air – “if there is someplace in Montana that isn’t smoky.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Noninvasive ventilation: Options and cautions for patients with COVID-19
Early on in the COVID-19 pandemic,
“We were concerned that, if we put them on high-flow nasal cannula or a noninvasive ventilation, that we would create aerosols that would then be a risk to clinicians,” Meghan Lane-Fall, MD, MSHP, FCCM, said at a Society for Critical Care Medicine virtual meeting called COVID-19: What’s Next. “However, we’ve gotten much more comfortable with infection control. We’ve gotten much more comfortable with controlling these aerosols, with making sure that our clinicians are protected with the appropriate protective equipment. We’ve also realized that patients who end up becoming intubated have really poor outcomes, so we’ve looked at our practice critically and tried to figure out how to support patients noninvasively when that’s possible.”
Respiratory support options
According to Dr. Lane-Fall, an associate professor of anesthesiology and critical care at the University of Pennsylvania, Philadelphia, there are two basic types of respiratory support in patients with moderate, severe, or critical COVID-19: noninvasive and invasive. Noninvasive options include CPAP or BiPAP which can be delivered through nasal pillows, masks, and helmets, as well as high-flow nasal oxygen. Invasive options include endotracheal intubation, tracheostomy, and extracorporeal membrane oxygenation (ECMO), usually the veno-venous (VV) form. “But it’s uncommon to need VV ECMO, even in patients who have critical COVID-19,” she said.
Factors that favor noninvasive ventilation include stably high oxygen requirements, normal mental status, ward location of care, and moderate to severe COVID-19. Factors that favor invasive ventilation include someone who’s deteriorating rapidly, “whose oxygen requirements aren’t stable or who is cardiopulmonary compromised,” said Dr. Lane-Fall, who is also co–medical director of the Trauma Surgery Intensive Care Unit at Penn Presbyterian Medical Center, also in Philadelphia. Other factors include the need for other invasive procedures such as surgery or if they have severe to critical COVID-19, “not just pneumonia, but [illness that’s] progressing into [acute respiratory distress syndrome],” she said.
Indications for urgent endotracheal intubation as opposed to giving a trial of noninvasive ventilation or high-flow nasal oxygen include altered mental status, inability to protect airway, copious amounts of secretions, a Glasgow Coma Scale score of less than 8, severe respiratory acidosis, hypopnea or apnea, shock, or an inability to tolerate noninvasive support. “This is a relative contraindication,” Dr. Lane-Fall said. “I’ve certainly talked people through the BiPAP mask or the helmet. If you tell a patient, ‘I don’t want to have to put in a breathing tube; I want to maintain you on this,’ often they’ll be able to work through it.”
Safety precautions
Aerosolizing procedures require attention to location, personnel, and equipment, including personal protective equipment (PPE), said Dr. Lane-Fall, who is an anesthesiologist by training. “When you are intubating someone, whether they have COVID-19 or not, you are sort of in the belly of the beast,” she said. “You are very exposed to secretions that occur at the time of endotracheal intubation. That’s why it’s important for us to have PPE and barriers to protect ourselves from potential exposure to aerosols during the care of patients with COVID-19.”
In February 2020, the non-for-profit Anesthesia Patient Safety Foundation published recommendations for airway management in patients with suspected COVID-19. A separate guidance was published the British Journal of Anaesthesiology based on emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China. “The idea here is that you want to intubate under controlled conditions,” said Dr. Lane-Fall, who is an author of the guidance. “You want to use the most experienced operator. You want to have full PPE, including an N95 mask, or something more protective like a powered air purifying respirator or an N95 mask with a face shield. You want the eyes, nose, and mouth of the operator covered completely.”
CPR, another aerosolizing procedure, requires vigilant safety precautions as well. “We struggled with this a little bit at our institution, because our inclination as intensivists when someone is pulseless is to run into the room and start chest compressions and to start resuscitation,” Dr. Lane-Fall said. “But the act of chest compression itself can create aerosols that can present risk to clinicians. We had to tell our clinicians that they have to put on PPE before they do CPR. The buzz phrase here is that there is no emergency in a pandemic. The idea here is that the good of that one patient is outweighed by the good of all the other patients that you could care for if you didn’t have COVID-19 as a clinician. So we have had to encourage our staff to put on PPE first before attending to patients first, even if it delays patient care. Once you have donned PPE, when you’re administering CPR, the number of staff should be minimized. You should have a compressor, and someone to relieve the compressor, and a code leader, someone tending to the airway. But in general, anyone who’s not actively involved should not be in the room.”
Risks during extubation
Extubation of COVID-19 patients is also an aerosolizing procedure not just because you’re pulling an endotracheal tube out of the airway but because coughing is a normal part of extubation. “We’ve had to be careful with how we approach extubation in COVID-19 patients,” Dr. Lane-Fall said. “Ideally you’re doing this in a negative pressure environment. We have also had to use full PPE, covering the eyes and face, and putting on a gown for precaution.”
Reintubation of COVID-19 patients is not uncommon. She and her colleagues at Penn Medicine created procedures for having intubators at the ready outside the room in case the patient were to decompensate clinically. “Another thing we learned is that it’s useful to do a leak test prior to extubation, because there may be airway edema related to prolonged intubation in these patients,” Dr. Lane-Fall said. “We found that, if a leak is absent on checking the cuff leak, the use of steroids for a day or 2 may help decrease airway edema. That improves the chances of extubation success.”
Strategies for aerosol containment
She concluded her remarks by reviewing airway control adjuncts and clinician safety. This includes physically isolating COVID-19 patients in negative pressure rooms and avoiding and minimizing aerosols, including the use of rapid intubation, “where we induce anesthesia for intubation but we don’t bag-mask the patient because that creates aerosols,” she said. The Anesthesia Patient Safety Foundation guidelines advocate for the use of video laryngoscopy so that you can visualize the glottis easily “and make sure that you successfully intubate the glottis and not the esophagus,” she said.
A smart strategy for aerosol containment is to use the most experienced laryngoscopist available. “If you are in a teaching program, ideally you’re using your most experienced resident, or you’re using fellows or attending physicians,” Dr. Lane-Fall said. “This is not the space for an inexperienced learner.”
Another way to make intubation faster and easier in COVID-19 patients is to use an intubation box, which features a plexiglass shield that enables the intubator to use their hands to get in the patient’s airway while being protected from viral droplets generated during intubation. The box can be cleaned after each use. Blueprints for an open source intubation box can be found at http://www.intubationbox.com.
Expert view on aerosol containment in COVID-19
“While there is a dearth of evidence from controlled trials, recommendations mentioned in this story are based on the best available evidence and are in agreement with guidelines from several expert groups,” said David L. Bowton, MD, FCCP, FCCM, of the department of anesthesiology at Wake Forest Baptist Health in Winston-Salem, NC. “The recommendation of Dr. Lane-Fall’s that is perhaps most controversial is the use of an intubation box. Multiple designs for these intubation/aerosol containment devices have been proposed, and the data supporting their ease of use and efficacy has been mixed [See Anaesthesia 2020;75(8):1014-21 and Anaesthesia. 2020. doi: 10.1111/anae.15188]. While bag valve mask ventilation should be avoided if possible, it may be a valuable rescue tool in the severely hypoxemic patient when used with two-person technique to achieve a tight seal and a PEEP valve and an HME over the exhalation port to minimize aerosol spread.
“It cannot be stressed enough that the most skilled individual should be tasked with intubating the patient and as few providers as possible [usually three] should be in the room and have donned full PPE. Negative pressure rooms should be used whenever feasible. Noninvasive ventilation appears safer from an infection control standpoint than initially feared and its use has become more widespread. However, noninvasive ventilation is not without its hazards, and Dr. Lane-Fall’s enumeration of the patient characteristics applicable to the selection of patients for noninvasive ventilation are extremely important. At our institution, the use of noninvasive ventilation and especially high-flow oxygen therapy has increased. Staff have become more comfortable with the donning and doffing of PPE.”
Dr. Lane-Fall reported having no financial disclosures.
Early on in the COVID-19 pandemic,
“We were concerned that, if we put them on high-flow nasal cannula or a noninvasive ventilation, that we would create aerosols that would then be a risk to clinicians,” Meghan Lane-Fall, MD, MSHP, FCCM, said at a Society for Critical Care Medicine virtual meeting called COVID-19: What’s Next. “However, we’ve gotten much more comfortable with infection control. We’ve gotten much more comfortable with controlling these aerosols, with making sure that our clinicians are protected with the appropriate protective equipment. We’ve also realized that patients who end up becoming intubated have really poor outcomes, so we’ve looked at our practice critically and tried to figure out how to support patients noninvasively when that’s possible.”
Respiratory support options
According to Dr. Lane-Fall, an associate professor of anesthesiology and critical care at the University of Pennsylvania, Philadelphia, there are two basic types of respiratory support in patients with moderate, severe, or critical COVID-19: noninvasive and invasive. Noninvasive options include CPAP or BiPAP which can be delivered through nasal pillows, masks, and helmets, as well as high-flow nasal oxygen. Invasive options include endotracheal intubation, tracheostomy, and extracorporeal membrane oxygenation (ECMO), usually the veno-venous (VV) form. “But it’s uncommon to need VV ECMO, even in patients who have critical COVID-19,” she said.
Factors that favor noninvasive ventilation include stably high oxygen requirements, normal mental status, ward location of care, and moderate to severe COVID-19. Factors that favor invasive ventilation include someone who’s deteriorating rapidly, “whose oxygen requirements aren’t stable or who is cardiopulmonary compromised,” said Dr. Lane-Fall, who is also co–medical director of the Trauma Surgery Intensive Care Unit at Penn Presbyterian Medical Center, also in Philadelphia. Other factors include the need for other invasive procedures such as surgery or if they have severe to critical COVID-19, “not just pneumonia, but [illness that’s] progressing into [acute respiratory distress syndrome],” she said.
Indications for urgent endotracheal intubation as opposed to giving a trial of noninvasive ventilation or high-flow nasal oxygen include altered mental status, inability to protect airway, copious amounts of secretions, a Glasgow Coma Scale score of less than 8, severe respiratory acidosis, hypopnea or apnea, shock, or an inability to tolerate noninvasive support. “This is a relative contraindication,” Dr. Lane-Fall said. “I’ve certainly talked people through the BiPAP mask or the helmet. If you tell a patient, ‘I don’t want to have to put in a breathing tube; I want to maintain you on this,’ often they’ll be able to work through it.”
Safety precautions
Aerosolizing procedures require attention to location, personnel, and equipment, including personal protective equipment (PPE), said Dr. Lane-Fall, who is an anesthesiologist by training. “When you are intubating someone, whether they have COVID-19 or not, you are sort of in the belly of the beast,” she said. “You are very exposed to secretions that occur at the time of endotracheal intubation. That’s why it’s important for us to have PPE and barriers to protect ourselves from potential exposure to aerosols during the care of patients with COVID-19.”
In February 2020, the non-for-profit Anesthesia Patient Safety Foundation published recommendations for airway management in patients with suspected COVID-19. A separate guidance was published the British Journal of Anaesthesiology based on emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China. “The idea here is that you want to intubate under controlled conditions,” said Dr. Lane-Fall, who is an author of the guidance. “You want to use the most experienced operator. You want to have full PPE, including an N95 mask, or something more protective like a powered air purifying respirator or an N95 mask with a face shield. You want the eyes, nose, and mouth of the operator covered completely.”
CPR, another aerosolizing procedure, requires vigilant safety precautions as well. “We struggled with this a little bit at our institution, because our inclination as intensivists when someone is pulseless is to run into the room and start chest compressions and to start resuscitation,” Dr. Lane-Fall said. “But the act of chest compression itself can create aerosols that can present risk to clinicians. We had to tell our clinicians that they have to put on PPE before they do CPR. The buzz phrase here is that there is no emergency in a pandemic. The idea here is that the good of that one patient is outweighed by the good of all the other patients that you could care for if you didn’t have COVID-19 as a clinician. So we have had to encourage our staff to put on PPE first before attending to patients first, even if it delays patient care. Once you have donned PPE, when you’re administering CPR, the number of staff should be minimized. You should have a compressor, and someone to relieve the compressor, and a code leader, someone tending to the airway. But in general, anyone who’s not actively involved should not be in the room.”
Risks during extubation
Extubation of COVID-19 patients is also an aerosolizing procedure not just because you’re pulling an endotracheal tube out of the airway but because coughing is a normal part of extubation. “We’ve had to be careful with how we approach extubation in COVID-19 patients,” Dr. Lane-Fall said. “Ideally you’re doing this in a negative pressure environment. We have also had to use full PPE, covering the eyes and face, and putting on a gown for precaution.”
Reintubation of COVID-19 patients is not uncommon. She and her colleagues at Penn Medicine created procedures for having intubators at the ready outside the room in case the patient were to decompensate clinically. “Another thing we learned is that it’s useful to do a leak test prior to extubation, because there may be airway edema related to prolonged intubation in these patients,” Dr. Lane-Fall said. “We found that, if a leak is absent on checking the cuff leak, the use of steroids for a day or 2 may help decrease airway edema. That improves the chances of extubation success.”
Strategies for aerosol containment
She concluded her remarks by reviewing airway control adjuncts and clinician safety. This includes physically isolating COVID-19 patients in negative pressure rooms and avoiding and minimizing aerosols, including the use of rapid intubation, “where we induce anesthesia for intubation but we don’t bag-mask the patient because that creates aerosols,” she said. The Anesthesia Patient Safety Foundation guidelines advocate for the use of video laryngoscopy so that you can visualize the glottis easily “and make sure that you successfully intubate the glottis and not the esophagus,” she said.
A smart strategy for aerosol containment is to use the most experienced laryngoscopist available. “If you are in a teaching program, ideally you’re using your most experienced resident, or you’re using fellows or attending physicians,” Dr. Lane-Fall said. “This is not the space for an inexperienced learner.”
Another way to make intubation faster and easier in COVID-19 patients is to use an intubation box, which features a plexiglass shield that enables the intubator to use their hands to get in the patient’s airway while being protected from viral droplets generated during intubation. The box can be cleaned after each use. Blueprints for an open source intubation box can be found at http://www.intubationbox.com.
Expert view on aerosol containment in COVID-19
“While there is a dearth of evidence from controlled trials, recommendations mentioned in this story are based on the best available evidence and are in agreement with guidelines from several expert groups,” said David L. Bowton, MD, FCCP, FCCM, of the department of anesthesiology at Wake Forest Baptist Health in Winston-Salem, NC. “The recommendation of Dr. Lane-Fall’s that is perhaps most controversial is the use of an intubation box. Multiple designs for these intubation/aerosol containment devices have been proposed, and the data supporting their ease of use and efficacy has been mixed [See Anaesthesia 2020;75(8):1014-21 and Anaesthesia. 2020. doi: 10.1111/anae.15188]. While bag valve mask ventilation should be avoided if possible, it may be a valuable rescue tool in the severely hypoxemic patient when used with two-person technique to achieve a tight seal and a PEEP valve and an HME over the exhalation port to minimize aerosol spread.
“It cannot be stressed enough that the most skilled individual should be tasked with intubating the patient and as few providers as possible [usually three] should be in the room and have donned full PPE. Negative pressure rooms should be used whenever feasible. Noninvasive ventilation appears safer from an infection control standpoint than initially feared and its use has become more widespread. However, noninvasive ventilation is not without its hazards, and Dr. Lane-Fall’s enumeration of the patient characteristics applicable to the selection of patients for noninvasive ventilation are extremely important. At our institution, the use of noninvasive ventilation and especially high-flow oxygen therapy has increased. Staff have become more comfortable with the donning and doffing of PPE.”
Dr. Lane-Fall reported having no financial disclosures.
Early on in the COVID-19 pandemic,
“We were concerned that, if we put them on high-flow nasal cannula or a noninvasive ventilation, that we would create aerosols that would then be a risk to clinicians,” Meghan Lane-Fall, MD, MSHP, FCCM, said at a Society for Critical Care Medicine virtual meeting called COVID-19: What’s Next. “However, we’ve gotten much more comfortable with infection control. We’ve gotten much more comfortable with controlling these aerosols, with making sure that our clinicians are protected with the appropriate protective equipment. We’ve also realized that patients who end up becoming intubated have really poor outcomes, so we’ve looked at our practice critically and tried to figure out how to support patients noninvasively when that’s possible.”
Respiratory support options
According to Dr. Lane-Fall, an associate professor of anesthesiology and critical care at the University of Pennsylvania, Philadelphia, there are two basic types of respiratory support in patients with moderate, severe, or critical COVID-19: noninvasive and invasive. Noninvasive options include CPAP or BiPAP which can be delivered through nasal pillows, masks, and helmets, as well as high-flow nasal oxygen. Invasive options include endotracheal intubation, tracheostomy, and extracorporeal membrane oxygenation (ECMO), usually the veno-venous (VV) form. “But it’s uncommon to need VV ECMO, even in patients who have critical COVID-19,” she said.
Factors that favor noninvasive ventilation include stably high oxygen requirements, normal mental status, ward location of care, and moderate to severe COVID-19. Factors that favor invasive ventilation include someone who’s deteriorating rapidly, “whose oxygen requirements aren’t stable or who is cardiopulmonary compromised,” said Dr. Lane-Fall, who is also co–medical director of the Trauma Surgery Intensive Care Unit at Penn Presbyterian Medical Center, also in Philadelphia. Other factors include the need for other invasive procedures such as surgery or if they have severe to critical COVID-19, “not just pneumonia, but [illness that’s] progressing into [acute respiratory distress syndrome],” she said.
Indications for urgent endotracheal intubation as opposed to giving a trial of noninvasive ventilation or high-flow nasal oxygen include altered mental status, inability to protect airway, copious amounts of secretions, a Glasgow Coma Scale score of less than 8, severe respiratory acidosis, hypopnea or apnea, shock, or an inability to tolerate noninvasive support. “This is a relative contraindication,” Dr. Lane-Fall said. “I’ve certainly talked people through the BiPAP mask or the helmet. If you tell a patient, ‘I don’t want to have to put in a breathing tube; I want to maintain you on this,’ often they’ll be able to work through it.”
Safety precautions
Aerosolizing procedures require attention to location, personnel, and equipment, including personal protective equipment (PPE), said Dr. Lane-Fall, who is an anesthesiologist by training. “When you are intubating someone, whether they have COVID-19 or not, you are sort of in the belly of the beast,” she said. “You are very exposed to secretions that occur at the time of endotracheal intubation. That’s why it’s important for us to have PPE and barriers to protect ourselves from potential exposure to aerosols during the care of patients with COVID-19.”
In February 2020, the non-for-profit Anesthesia Patient Safety Foundation published recommendations for airway management in patients with suspected COVID-19. A separate guidance was published the British Journal of Anaesthesiology based on emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China. “The idea here is that you want to intubate under controlled conditions,” said Dr. Lane-Fall, who is an author of the guidance. “You want to use the most experienced operator. You want to have full PPE, including an N95 mask, or something more protective like a powered air purifying respirator or an N95 mask with a face shield. You want the eyes, nose, and mouth of the operator covered completely.”
CPR, another aerosolizing procedure, requires vigilant safety precautions as well. “We struggled with this a little bit at our institution, because our inclination as intensivists when someone is pulseless is to run into the room and start chest compressions and to start resuscitation,” Dr. Lane-Fall said. “But the act of chest compression itself can create aerosols that can present risk to clinicians. We had to tell our clinicians that they have to put on PPE before they do CPR. The buzz phrase here is that there is no emergency in a pandemic. The idea here is that the good of that one patient is outweighed by the good of all the other patients that you could care for if you didn’t have COVID-19 as a clinician. So we have had to encourage our staff to put on PPE first before attending to patients first, even if it delays patient care. Once you have donned PPE, when you’re administering CPR, the number of staff should be minimized. You should have a compressor, and someone to relieve the compressor, and a code leader, someone tending to the airway. But in general, anyone who’s not actively involved should not be in the room.”
Risks during extubation
Extubation of COVID-19 patients is also an aerosolizing procedure not just because you’re pulling an endotracheal tube out of the airway but because coughing is a normal part of extubation. “We’ve had to be careful with how we approach extubation in COVID-19 patients,” Dr. Lane-Fall said. “Ideally you’re doing this in a negative pressure environment. We have also had to use full PPE, covering the eyes and face, and putting on a gown for precaution.”
Reintubation of COVID-19 patients is not uncommon. She and her colleagues at Penn Medicine created procedures for having intubators at the ready outside the room in case the patient were to decompensate clinically. “Another thing we learned is that it’s useful to do a leak test prior to extubation, because there may be airway edema related to prolonged intubation in these patients,” Dr. Lane-Fall said. “We found that, if a leak is absent on checking the cuff leak, the use of steroids for a day or 2 may help decrease airway edema. That improves the chances of extubation success.”
Strategies for aerosol containment
She concluded her remarks by reviewing airway control adjuncts and clinician safety. This includes physically isolating COVID-19 patients in negative pressure rooms and avoiding and minimizing aerosols, including the use of rapid intubation, “where we induce anesthesia for intubation but we don’t bag-mask the patient because that creates aerosols,” she said. The Anesthesia Patient Safety Foundation guidelines advocate for the use of video laryngoscopy so that you can visualize the glottis easily “and make sure that you successfully intubate the glottis and not the esophagus,” she said.
A smart strategy for aerosol containment is to use the most experienced laryngoscopist available. “If you are in a teaching program, ideally you’re using your most experienced resident, or you’re using fellows or attending physicians,” Dr. Lane-Fall said. “This is not the space for an inexperienced learner.”
Another way to make intubation faster and easier in COVID-19 patients is to use an intubation box, which features a plexiglass shield that enables the intubator to use their hands to get in the patient’s airway while being protected from viral droplets generated during intubation. The box can be cleaned after each use. Blueprints for an open source intubation box can be found at http://www.intubationbox.com.
Expert view on aerosol containment in COVID-19
“While there is a dearth of evidence from controlled trials, recommendations mentioned in this story are based on the best available evidence and are in agreement with guidelines from several expert groups,” said David L. Bowton, MD, FCCP, FCCM, of the department of anesthesiology at Wake Forest Baptist Health in Winston-Salem, NC. “The recommendation of Dr. Lane-Fall’s that is perhaps most controversial is the use of an intubation box. Multiple designs for these intubation/aerosol containment devices have been proposed, and the data supporting their ease of use and efficacy has been mixed [See Anaesthesia 2020;75(8):1014-21 and Anaesthesia. 2020. doi: 10.1111/anae.15188]. While bag valve mask ventilation should be avoided if possible, it may be a valuable rescue tool in the severely hypoxemic patient when used with two-person technique to achieve a tight seal and a PEEP valve and an HME over the exhalation port to minimize aerosol spread.
“It cannot be stressed enough that the most skilled individual should be tasked with intubating the patient and as few providers as possible [usually three] should be in the room and have donned full PPE. Negative pressure rooms should be used whenever feasible. Noninvasive ventilation appears safer from an infection control standpoint than initially feared and its use has become more widespread. However, noninvasive ventilation is not without its hazards, and Dr. Lane-Fall’s enumeration of the patient characteristics applicable to the selection of patients for noninvasive ventilation are extremely important. At our institution, the use of noninvasive ventilation and especially high-flow oxygen therapy has increased. Staff have become more comfortable with the donning and doffing of PPE.”
Dr. Lane-Fall reported having no financial disclosures.
FROM AN SCCM VIRTUAL MEETING
Nocturnal oxygen no help for isolated desaturation in COPD
Nocturnal oxygen therapy for patients with COPD and isolated nocturnal oxygen desaturation does not improve survival or delay disease progression, according to findings published Sept. 17 in The New England Journal of Medicine. The new report adds to evidence that the widely implemented and costly practice may be unnecessary.
Patients with COPD who do not qualify for long-term oxygen therapy (LTOT) are commonly prescribed nocturnal oxygen in the belief that it can delay disease progression, possibly by decreasing alveolar hypoventilation and ventilation-perfusion mismatch.
But investigations so far and the new study from the International Nocturnal Oxygen (INOX) Trial have not borne this out.
“There is no indication that nocturnal oxygen has a positive or negative effect on survival or progression to long-term oxygen therapy in patients with nocturnal hypoxemia in COPD. Consequently, there is no reason for physicians to screen for nocturnal hypoxemia in COPD,” study leader Yves Lacasse, MD, told Medscape Medical News.
Lacasse is from the Institut Universitaire de Cardiologie et de Pneumologie de Québec–Université Laval, Quebec, Canada.
The idea that the therapy helps is firmly entrenched.
In the early 1980s, two trials indicated that patients who had COPD and severe chronic daytime hypoxemia benefit from LTOT (15-18 hours a day or longer).
A decade later, two landmark trials (the Nocturnal Oxygen Therapy Trial and the British Medical Research Council Trial) added to evidence that LTOT may prolong life for patients with COPD and severe daytime hypoxemia.
“The good news from both trials was that oxygen saves lives. From this moment, oxygen therapy became a standard of care, and confirmatory trials would be considered unethical,” Lacasse explained.
“Oxygen therapy gained widespread acceptance by official organizations for treatment of most chronic cardiorespiratory conditions complicated by severe hypoxemia, even if proof of efficacy is lacking. New indications emerged, such as isolated nocturnal oxygen desaturation. Even in COPD, inappropriate prescriptions of home oxygen therapy are not unusual. Oxygen is everywhere,” Lacasse continued.
A meta-analysis from 2005 identified two trials that evaluated home oxygen therapy specifically for isolated nocturnal desaturation. Both found no survival benefit from nocturnal oxygen.
The study by Lacasse and colleagues assessed effects on mortality or worsening of disease (progression to LTOT) with 3-4 years of nocturnal oxygen supplementation.
Participants, whose oxygen saturation was less than 90% for at least 30% of the recording time on nocturnal oximetry, received oxygen or ambient air from a sham device as a placebo for at least 4 hours per session. The goal of treatment was nocturnal oxygen saturation exceeding 90% for at least 90% of the recorded time.
The trial protocol excluded patients with severe obesity, apnea, lung cancer, left heart failure, interstitial lung disease, or bronchiectasis.
The study was initially powered in 2010 to include 600 participants, with half to receive placebo. The study assumed mortality of 20% among control patients over 3 years; 20% of patients progressed to LTOT.
When recruiting lagged, the data safety monitoring board and steering committee extended follow-up to 4 years. In 2014, they requested an interim analysis, and recruitment ceased. Overall, 243 patients participated.
Lacasse cited several reasons for the difficulty with recruitment as well as retention: unwillingness to take the risk of receiving placebo instead of a readily available treatment, fading interest over time, and frailty that affects compliance.
Patients in the study came from 28 community or university-affiliated hospitals in Canada, Portugal, Spain, and France. At the 3-year mark, 39% of patients (48 of 123) who were assigned to nocturnal oxygen therapy and 42% (50 of 119) of those taking placebo had met criteria for LTOT or had died (difference, −3.0 percentage points; P = .64). The groups did not differ appreciably in rates of exacerbation and hospitalization.
The researchers could not analyze subgroups because the patients were very similar with regard to the severity of nocturnal oxygen desaturation, Lacasse said.
Economics enters into the picture – home oxygen therapy is second only to hospitalization as the most expensive healthcare expenditure associated with clinical care for COPD in developed countries. “The math is simple. There is enormous potential for saving money if the results of our clinical trial are applied appropriately,” said Lacasse.
William Bailey, MD, professor emeritus of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, agrees that the practice is overused.
“There is a built-in bias in the medical community. Most believe that anyone with lung disease benefits from oxygen. Even some of our investigators had a hard time believing the results. The study was well designed, carefully carried out, and I feel confident that the results are reliable,” he said.
Shawn P. E. Nishi, MD, director of bronchoscopy and advanced pulmonary procedures, division of pulmonary and critical care medicine, the University of Texas Medical Branch, Galveston, Texas, mentioned the study’s main limitation, which the authors readily acknowledge.
“Unfortunately, the trial had difficulty recruiting subjects, with less than half of expected enrollment achieved, and was underpowered to make any conclusions. Other studies have examined nocturnal oxygen use and have not shown a mortality benefit,” Nishi explained.
She added that the study did not evaluate use of LTOT for improving outcomes other than mortality, including quality of life, cardiovascular morbidity, depression, cognitive function, exercise capacity, and frequency of COPD exacerbations or hospitalization.
Other limitations of the study include suboptimal adherence to the therapy and interpretation of the clinical significance on the basis of a survey of Canadian pulmonologists.
This article first appeared on Medscape.com.
Nocturnal oxygen therapy for patients with COPD and isolated nocturnal oxygen desaturation does not improve survival or delay disease progression, according to findings published Sept. 17 in The New England Journal of Medicine. The new report adds to evidence that the widely implemented and costly practice may be unnecessary.
Patients with COPD who do not qualify for long-term oxygen therapy (LTOT) are commonly prescribed nocturnal oxygen in the belief that it can delay disease progression, possibly by decreasing alveolar hypoventilation and ventilation-perfusion mismatch.
But investigations so far and the new study from the International Nocturnal Oxygen (INOX) Trial have not borne this out.
“There is no indication that nocturnal oxygen has a positive or negative effect on survival or progression to long-term oxygen therapy in patients with nocturnal hypoxemia in COPD. Consequently, there is no reason for physicians to screen for nocturnal hypoxemia in COPD,” study leader Yves Lacasse, MD, told Medscape Medical News.
Lacasse is from the Institut Universitaire de Cardiologie et de Pneumologie de Québec–Université Laval, Quebec, Canada.
The idea that the therapy helps is firmly entrenched.
In the early 1980s, two trials indicated that patients who had COPD and severe chronic daytime hypoxemia benefit from LTOT (15-18 hours a day or longer).
A decade later, two landmark trials (the Nocturnal Oxygen Therapy Trial and the British Medical Research Council Trial) added to evidence that LTOT may prolong life for patients with COPD and severe daytime hypoxemia.
“The good news from both trials was that oxygen saves lives. From this moment, oxygen therapy became a standard of care, and confirmatory trials would be considered unethical,” Lacasse explained.
“Oxygen therapy gained widespread acceptance by official organizations for treatment of most chronic cardiorespiratory conditions complicated by severe hypoxemia, even if proof of efficacy is lacking. New indications emerged, such as isolated nocturnal oxygen desaturation. Even in COPD, inappropriate prescriptions of home oxygen therapy are not unusual. Oxygen is everywhere,” Lacasse continued.
A meta-analysis from 2005 identified two trials that evaluated home oxygen therapy specifically for isolated nocturnal desaturation. Both found no survival benefit from nocturnal oxygen.
The study by Lacasse and colleagues assessed effects on mortality or worsening of disease (progression to LTOT) with 3-4 years of nocturnal oxygen supplementation.
Participants, whose oxygen saturation was less than 90% for at least 30% of the recording time on nocturnal oximetry, received oxygen or ambient air from a sham device as a placebo for at least 4 hours per session. The goal of treatment was nocturnal oxygen saturation exceeding 90% for at least 90% of the recorded time.
The trial protocol excluded patients with severe obesity, apnea, lung cancer, left heart failure, interstitial lung disease, or bronchiectasis.
The study was initially powered in 2010 to include 600 participants, with half to receive placebo. The study assumed mortality of 20% among control patients over 3 years; 20% of patients progressed to LTOT.
When recruiting lagged, the data safety monitoring board and steering committee extended follow-up to 4 years. In 2014, they requested an interim analysis, and recruitment ceased. Overall, 243 patients participated.
Lacasse cited several reasons for the difficulty with recruitment as well as retention: unwillingness to take the risk of receiving placebo instead of a readily available treatment, fading interest over time, and frailty that affects compliance.
Patients in the study came from 28 community or university-affiliated hospitals in Canada, Portugal, Spain, and France. At the 3-year mark, 39% of patients (48 of 123) who were assigned to nocturnal oxygen therapy and 42% (50 of 119) of those taking placebo had met criteria for LTOT or had died (difference, −3.0 percentage points; P = .64). The groups did not differ appreciably in rates of exacerbation and hospitalization.
The researchers could not analyze subgroups because the patients were very similar with regard to the severity of nocturnal oxygen desaturation, Lacasse said.
Economics enters into the picture – home oxygen therapy is second only to hospitalization as the most expensive healthcare expenditure associated with clinical care for COPD in developed countries. “The math is simple. There is enormous potential for saving money if the results of our clinical trial are applied appropriately,” said Lacasse.
William Bailey, MD, professor emeritus of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, agrees that the practice is overused.
“There is a built-in bias in the medical community. Most believe that anyone with lung disease benefits from oxygen. Even some of our investigators had a hard time believing the results. The study was well designed, carefully carried out, and I feel confident that the results are reliable,” he said.
Shawn P. E. Nishi, MD, director of bronchoscopy and advanced pulmonary procedures, division of pulmonary and critical care medicine, the University of Texas Medical Branch, Galveston, Texas, mentioned the study’s main limitation, which the authors readily acknowledge.
“Unfortunately, the trial had difficulty recruiting subjects, with less than half of expected enrollment achieved, and was underpowered to make any conclusions. Other studies have examined nocturnal oxygen use and have not shown a mortality benefit,” Nishi explained.
She added that the study did not evaluate use of LTOT for improving outcomes other than mortality, including quality of life, cardiovascular morbidity, depression, cognitive function, exercise capacity, and frequency of COPD exacerbations or hospitalization.
Other limitations of the study include suboptimal adherence to the therapy and interpretation of the clinical significance on the basis of a survey of Canadian pulmonologists.
This article first appeared on Medscape.com.
Nocturnal oxygen therapy for patients with COPD and isolated nocturnal oxygen desaturation does not improve survival or delay disease progression, according to findings published Sept. 17 in The New England Journal of Medicine. The new report adds to evidence that the widely implemented and costly practice may be unnecessary.
Patients with COPD who do not qualify for long-term oxygen therapy (LTOT) are commonly prescribed nocturnal oxygen in the belief that it can delay disease progression, possibly by decreasing alveolar hypoventilation and ventilation-perfusion mismatch.
But investigations so far and the new study from the International Nocturnal Oxygen (INOX) Trial have not borne this out.
“There is no indication that nocturnal oxygen has a positive or negative effect on survival or progression to long-term oxygen therapy in patients with nocturnal hypoxemia in COPD. Consequently, there is no reason for physicians to screen for nocturnal hypoxemia in COPD,” study leader Yves Lacasse, MD, told Medscape Medical News.
Lacasse is from the Institut Universitaire de Cardiologie et de Pneumologie de Québec–Université Laval, Quebec, Canada.
The idea that the therapy helps is firmly entrenched.
In the early 1980s, two trials indicated that patients who had COPD and severe chronic daytime hypoxemia benefit from LTOT (15-18 hours a day or longer).
A decade later, two landmark trials (the Nocturnal Oxygen Therapy Trial and the British Medical Research Council Trial) added to evidence that LTOT may prolong life for patients with COPD and severe daytime hypoxemia.
“The good news from both trials was that oxygen saves lives. From this moment, oxygen therapy became a standard of care, and confirmatory trials would be considered unethical,” Lacasse explained.
“Oxygen therapy gained widespread acceptance by official organizations for treatment of most chronic cardiorespiratory conditions complicated by severe hypoxemia, even if proof of efficacy is lacking. New indications emerged, such as isolated nocturnal oxygen desaturation. Even in COPD, inappropriate prescriptions of home oxygen therapy are not unusual. Oxygen is everywhere,” Lacasse continued.
A meta-analysis from 2005 identified two trials that evaluated home oxygen therapy specifically for isolated nocturnal desaturation. Both found no survival benefit from nocturnal oxygen.
The study by Lacasse and colleagues assessed effects on mortality or worsening of disease (progression to LTOT) with 3-4 years of nocturnal oxygen supplementation.
Participants, whose oxygen saturation was less than 90% for at least 30% of the recording time on nocturnal oximetry, received oxygen or ambient air from a sham device as a placebo for at least 4 hours per session. The goal of treatment was nocturnal oxygen saturation exceeding 90% for at least 90% of the recorded time.
The trial protocol excluded patients with severe obesity, apnea, lung cancer, left heart failure, interstitial lung disease, or bronchiectasis.
The study was initially powered in 2010 to include 600 participants, with half to receive placebo. The study assumed mortality of 20% among control patients over 3 years; 20% of patients progressed to LTOT.
When recruiting lagged, the data safety monitoring board and steering committee extended follow-up to 4 years. In 2014, they requested an interim analysis, and recruitment ceased. Overall, 243 patients participated.
Lacasse cited several reasons for the difficulty with recruitment as well as retention: unwillingness to take the risk of receiving placebo instead of a readily available treatment, fading interest over time, and frailty that affects compliance.
Patients in the study came from 28 community or university-affiliated hospitals in Canada, Portugal, Spain, and France. At the 3-year mark, 39% of patients (48 of 123) who were assigned to nocturnal oxygen therapy and 42% (50 of 119) of those taking placebo had met criteria for LTOT or had died (difference, −3.0 percentage points; P = .64). The groups did not differ appreciably in rates of exacerbation and hospitalization.
The researchers could not analyze subgroups because the patients were very similar with regard to the severity of nocturnal oxygen desaturation, Lacasse said.
Economics enters into the picture – home oxygen therapy is second only to hospitalization as the most expensive healthcare expenditure associated with clinical care for COPD in developed countries. “The math is simple. There is enormous potential for saving money if the results of our clinical trial are applied appropriately,” said Lacasse.
William Bailey, MD, professor emeritus of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, agrees that the practice is overused.
“There is a built-in bias in the medical community. Most believe that anyone with lung disease benefits from oxygen. Even some of our investigators had a hard time believing the results. The study was well designed, carefully carried out, and I feel confident that the results are reliable,” he said.
Shawn P. E. Nishi, MD, director of bronchoscopy and advanced pulmonary procedures, division of pulmonary and critical care medicine, the University of Texas Medical Branch, Galveston, Texas, mentioned the study’s main limitation, which the authors readily acknowledge.
“Unfortunately, the trial had difficulty recruiting subjects, with less than half of expected enrollment achieved, and was underpowered to make any conclusions. Other studies have examined nocturnal oxygen use and have not shown a mortality benefit,” Nishi explained.
She added that the study did not evaluate use of LTOT for improving outcomes other than mortality, including quality of life, cardiovascular morbidity, depression, cognitive function, exercise capacity, and frequency of COPD exacerbations or hospitalization.
Other limitations of the study include suboptimal adherence to the therapy and interpretation of the clinical significance on the basis of a survey of Canadian pulmonologists.
This article first appeared on Medscape.com.
Many Americans still concerned about access to health care
according to the results of a survey conducted Aug. 7-26.
Nationally, 23.8% of respondents said that they were very concerned about being able to receive care during the pandemic, and another 27.4% said that they were somewhat concerned. Just under a quarter, 24.3%, said they were not very concerned, while 20.4% were not at all concerned, the COVID-19 Consortium for Understanding the Public’s Policy Preferences Across States reported after surveying 21,196 adults.
At the state level, Mississippi had the most adults (35.5%) who were very concerned about their access to care, followed by Texas (32.7%) and Nevada (32.4%). The residents of Montana were least likely (10.5%) to be very concerned, with Vermont next at 11.6% and Wyoming slightly higher at 13.8%. Montana also had the highest proportion of adults, 30.2%, who were not at all concerned, the consortium’s data show.
When asked about getting the coronavirus themselves, 67.8% of U.S. adults came down on the concerned side (33.3% somewhat and 34.5% very concerned) versus 30.8% who were not concerned (18.6% were not very concerned; 12.2% were not concerned at all.). Respondents’ concern was higher for their family members’ risk of getting coronavirus: 30.2% were somewhat concerned and 47.6% were very concerned, the consortium said.
Among many other topics, respondents were asked how closely they had followed recommended health guidelines in the last week, with the two extremes shown here:
- Avoiding contact with other people: 49.3% very closely, 4.8% not at all closely.
- Frequently washing hands: 74.7% very, 1.6% not at all.
- Disinfecting often-touched surfaces: 54.4% very, 4.3% not at all.
- Wearing a face mask in public: 75.7% very, 3.5% not at all.
The consortium is a joint project of the Network Science Institute of Northeastern University; the Shorenstein Center on Media, Politics, and Public Policy of Harvard University; Harvard Medical School; the School of Communication and Information at Rutgers University; and the department of political science at Northwestern University. The project is supported by grants from the National Science Foundation.
according to the results of a survey conducted Aug. 7-26.
Nationally, 23.8% of respondents said that they were very concerned about being able to receive care during the pandemic, and another 27.4% said that they were somewhat concerned. Just under a quarter, 24.3%, said they were not very concerned, while 20.4% were not at all concerned, the COVID-19 Consortium for Understanding the Public’s Policy Preferences Across States reported after surveying 21,196 adults.
At the state level, Mississippi had the most adults (35.5%) who were very concerned about their access to care, followed by Texas (32.7%) and Nevada (32.4%). The residents of Montana were least likely (10.5%) to be very concerned, with Vermont next at 11.6% and Wyoming slightly higher at 13.8%. Montana also had the highest proportion of adults, 30.2%, who were not at all concerned, the consortium’s data show.
When asked about getting the coronavirus themselves, 67.8% of U.S. adults came down on the concerned side (33.3% somewhat and 34.5% very concerned) versus 30.8% who were not concerned (18.6% were not very concerned; 12.2% were not concerned at all.). Respondents’ concern was higher for their family members’ risk of getting coronavirus: 30.2% were somewhat concerned and 47.6% were very concerned, the consortium said.
Among many other topics, respondents were asked how closely they had followed recommended health guidelines in the last week, with the two extremes shown here:
- Avoiding contact with other people: 49.3% very closely, 4.8% not at all closely.
- Frequently washing hands: 74.7% very, 1.6% not at all.
- Disinfecting often-touched surfaces: 54.4% very, 4.3% not at all.
- Wearing a face mask in public: 75.7% very, 3.5% not at all.
The consortium is a joint project of the Network Science Institute of Northeastern University; the Shorenstein Center on Media, Politics, and Public Policy of Harvard University; Harvard Medical School; the School of Communication and Information at Rutgers University; and the department of political science at Northwestern University. The project is supported by grants from the National Science Foundation.
according to the results of a survey conducted Aug. 7-26.
Nationally, 23.8% of respondents said that they were very concerned about being able to receive care during the pandemic, and another 27.4% said that they were somewhat concerned. Just under a quarter, 24.3%, said they were not very concerned, while 20.4% were not at all concerned, the COVID-19 Consortium for Understanding the Public’s Policy Preferences Across States reported after surveying 21,196 adults.
At the state level, Mississippi had the most adults (35.5%) who were very concerned about their access to care, followed by Texas (32.7%) and Nevada (32.4%). The residents of Montana were least likely (10.5%) to be very concerned, with Vermont next at 11.6% and Wyoming slightly higher at 13.8%. Montana also had the highest proportion of adults, 30.2%, who were not at all concerned, the consortium’s data show.
When asked about getting the coronavirus themselves, 67.8% of U.S. adults came down on the concerned side (33.3% somewhat and 34.5% very concerned) versus 30.8% who were not concerned (18.6% were not very concerned; 12.2% were not concerned at all.). Respondents’ concern was higher for their family members’ risk of getting coronavirus: 30.2% were somewhat concerned and 47.6% were very concerned, the consortium said.
Among many other topics, respondents were asked how closely they had followed recommended health guidelines in the last week, with the two extremes shown here:
- Avoiding contact with other people: 49.3% very closely, 4.8% not at all closely.
- Frequently washing hands: 74.7% very, 1.6% not at all.
- Disinfecting often-touched surfaces: 54.4% very, 4.3% not at all.
- Wearing a face mask in public: 75.7% very, 3.5% not at all.
The consortium is a joint project of the Network Science Institute of Northeastern University; the Shorenstein Center on Media, Politics, and Public Policy of Harvard University; Harvard Medical School; the School of Communication and Information at Rutgers University; and the department of political science at Northwestern University. The project is supported by grants from the National Science Foundation.
Study validates OSA phenotypes in Latinos
Three previously described clinical phenotypes of obstructive sleep apnea (OSA) have been validated in a large and diverse Hispanic/Latino community-based population for the first time, according to findings presented at the virtual annual meeting of the Associated Professional Sleep Societies.
The three OSA symptom profiles present in this population – labeled “minimally symptomatic,” “disturbed sleep,” and “daytime sleepiness” – are consistent with recent findings from the Sleep Apnea Global Interdisciplinary Consortium, which were published in Sleep, but there are notable differences in the prevalence of these clusters, with the minimally symptomatic cluster much more prevalent than in prior research, reported Kevin Gonzalez, of the University of California, San Diego.
“Other biopsychosocial factors may be contributing to OSA phenotypes among Hispanics and Latinos,” Mr. Gonzalez said in his presentation. Prior research to characterize the heterogeneity of sleep apnea has not included a diverse Latino population, he emphasized.
The adults studied were aged 18-74 years and participants in the multisite Hispanic Community Health Study/Study of Latinos (HCHS/SOL), a comprehensive study of Hispanic/Latino health and disease in the United States. Their respiratory events were measured overnight in HCHS/SOL sleep reading centers with an ARES Unicorder 5.2, B-Alert. Sleep patterns and risk factors were assessed using the Sleep Heart Health Study Sleep Habits Questionnaire and the Epworth Sleepiness Scale.
Participants meeting the criteria for moderate to severe OSA (with an Apnea Hypopnea Index of 15 or above) were included in the analysis (n = 1,623). Their average age was 52.4 ± 13.9 years, and 34.1% were female.
To identify phenotype clusters, investigators performed a latent class analysis using 15 common OSA symptoms and a survey weighted to adjust for selection bias. The three clusters offering the “best” fit for the data aligned with the previously reported phenotypes and identified daytime sleepiness in 15.3%, disturbed sleep (insomnia-like symptoms) in 37.7%, and minimally symptomatic (a low symptom profile) in 46.9%.
These phenotypes were reported in the European Respiratory Journal in 2014 in a cluster analysis of data from a sleep apnea cohort in Iceland and later replicated in the analysis of data from the Sleep Apnea Global Interdisciplinary Consortium published in Sleep in 2018. The consortium study also added two additional phenotypes, labeled “upper airway symptoms dominant” and “sleepiness dominant.”
The prevalence of a “minimally symptomatic group” in the new analysis of the Hispanics/Latinos in the United States is much higher than reported in these prior studies, at least partly, the investigators believed, because the “prior studies were clinical samples, and the people who were minimally symptomatic didn’t get to the sleep centers,” Mr. Gonzalez said in an interview after the meeting.
Patients with a phenotype of daytime sleepiness – the most common phenotype in prior research – constituted only a minority in the Hispanic/Latino population, he said.
Alberto Ramos, MD, of the University of Miami and the principal investigator, said in an interview that the research team is currently analyzing “if and how these different [phenotypic] clusters could affect the incidence of comorbidities” recorded in the HCHS/SOL study, such as hypertension, diabetes, cardiovascular disease, and cognitive decline.
For now, he said, the findings suggest that OSA may be especially underrecognized in Hispanics and Latinos and that there is more research to be done to better identify and stratify patients with varying symptomatology for more personalized treatment and for clinical trial selection. “Maybe we should expand our criteria ... broaden our [recognition] of the presentation of sleep apnea and the symptoms associated with it, not only in Hispanics but maybe in the general population,” Dr. Ramos said.
In commenting on the study, Krishna M. Sundar, MD, FCCP, director of the Sleep-Wake Center at the University of Utah, Salt Lake City, said that insomnia and daytime sleepiness are “key associations with obstructive sleep apnea and may predict different outcomes with untreated OSA.” Such heterogeneity is “only beginning to be appreciated,” he said. “The expression of OSA with these symptoms points to how OSA impacts quality of life” and how symptomatology in addition to Apnea Hypopnea Index “may be an important determinant of treatment benefit and compliance.”
The investigators reported no relevant disclosures. Dr. Sundar said that he is cofounder of Hypnoscure, software for population management of sleep apnea, but with no monies received.
Three previously described clinical phenotypes of obstructive sleep apnea (OSA) have been validated in a large and diverse Hispanic/Latino community-based population for the first time, according to findings presented at the virtual annual meeting of the Associated Professional Sleep Societies.
The three OSA symptom profiles present in this population – labeled “minimally symptomatic,” “disturbed sleep,” and “daytime sleepiness” – are consistent with recent findings from the Sleep Apnea Global Interdisciplinary Consortium, which were published in Sleep, but there are notable differences in the prevalence of these clusters, with the minimally symptomatic cluster much more prevalent than in prior research, reported Kevin Gonzalez, of the University of California, San Diego.
“Other biopsychosocial factors may be contributing to OSA phenotypes among Hispanics and Latinos,” Mr. Gonzalez said in his presentation. Prior research to characterize the heterogeneity of sleep apnea has not included a diverse Latino population, he emphasized.
The adults studied were aged 18-74 years and participants in the multisite Hispanic Community Health Study/Study of Latinos (HCHS/SOL), a comprehensive study of Hispanic/Latino health and disease in the United States. Their respiratory events were measured overnight in HCHS/SOL sleep reading centers with an ARES Unicorder 5.2, B-Alert. Sleep patterns and risk factors were assessed using the Sleep Heart Health Study Sleep Habits Questionnaire and the Epworth Sleepiness Scale.
Participants meeting the criteria for moderate to severe OSA (with an Apnea Hypopnea Index of 15 or above) were included in the analysis (n = 1,623). Their average age was 52.4 ± 13.9 years, and 34.1% were female.
To identify phenotype clusters, investigators performed a latent class analysis using 15 common OSA symptoms and a survey weighted to adjust for selection bias. The three clusters offering the “best” fit for the data aligned with the previously reported phenotypes and identified daytime sleepiness in 15.3%, disturbed sleep (insomnia-like symptoms) in 37.7%, and minimally symptomatic (a low symptom profile) in 46.9%.
These phenotypes were reported in the European Respiratory Journal in 2014 in a cluster analysis of data from a sleep apnea cohort in Iceland and later replicated in the analysis of data from the Sleep Apnea Global Interdisciplinary Consortium published in Sleep in 2018. The consortium study also added two additional phenotypes, labeled “upper airway symptoms dominant” and “sleepiness dominant.”
The prevalence of a “minimally symptomatic group” in the new analysis of the Hispanics/Latinos in the United States is much higher than reported in these prior studies, at least partly, the investigators believed, because the “prior studies were clinical samples, and the people who were minimally symptomatic didn’t get to the sleep centers,” Mr. Gonzalez said in an interview after the meeting.
Patients with a phenotype of daytime sleepiness – the most common phenotype in prior research – constituted only a minority in the Hispanic/Latino population, he said.
Alberto Ramos, MD, of the University of Miami and the principal investigator, said in an interview that the research team is currently analyzing “if and how these different [phenotypic] clusters could affect the incidence of comorbidities” recorded in the HCHS/SOL study, such as hypertension, diabetes, cardiovascular disease, and cognitive decline.
For now, he said, the findings suggest that OSA may be especially underrecognized in Hispanics and Latinos and that there is more research to be done to better identify and stratify patients with varying symptomatology for more personalized treatment and for clinical trial selection. “Maybe we should expand our criteria ... broaden our [recognition] of the presentation of sleep apnea and the symptoms associated with it, not only in Hispanics but maybe in the general population,” Dr. Ramos said.
In commenting on the study, Krishna M. Sundar, MD, FCCP, director of the Sleep-Wake Center at the University of Utah, Salt Lake City, said that insomnia and daytime sleepiness are “key associations with obstructive sleep apnea and may predict different outcomes with untreated OSA.” Such heterogeneity is “only beginning to be appreciated,” he said. “The expression of OSA with these symptoms points to how OSA impacts quality of life” and how symptomatology in addition to Apnea Hypopnea Index “may be an important determinant of treatment benefit and compliance.”
The investigators reported no relevant disclosures. Dr. Sundar said that he is cofounder of Hypnoscure, software for population management of sleep apnea, but with no monies received.
Three previously described clinical phenotypes of obstructive sleep apnea (OSA) have been validated in a large and diverse Hispanic/Latino community-based population for the first time, according to findings presented at the virtual annual meeting of the Associated Professional Sleep Societies.
The three OSA symptom profiles present in this population – labeled “minimally symptomatic,” “disturbed sleep,” and “daytime sleepiness” – are consistent with recent findings from the Sleep Apnea Global Interdisciplinary Consortium, which were published in Sleep, but there are notable differences in the prevalence of these clusters, with the minimally symptomatic cluster much more prevalent than in prior research, reported Kevin Gonzalez, of the University of California, San Diego.
“Other biopsychosocial factors may be contributing to OSA phenotypes among Hispanics and Latinos,” Mr. Gonzalez said in his presentation. Prior research to characterize the heterogeneity of sleep apnea has not included a diverse Latino population, he emphasized.
The adults studied were aged 18-74 years and participants in the multisite Hispanic Community Health Study/Study of Latinos (HCHS/SOL), a comprehensive study of Hispanic/Latino health and disease in the United States. Their respiratory events were measured overnight in HCHS/SOL sleep reading centers with an ARES Unicorder 5.2, B-Alert. Sleep patterns and risk factors were assessed using the Sleep Heart Health Study Sleep Habits Questionnaire and the Epworth Sleepiness Scale.
Participants meeting the criteria for moderate to severe OSA (with an Apnea Hypopnea Index of 15 or above) were included in the analysis (n = 1,623). Their average age was 52.4 ± 13.9 years, and 34.1% were female.
To identify phenotype clusters, investigators performed a latent class analysis using 15 common OSA symptoms and a survey weighted to adjust for selection bias. The three clusters offering the “best” fit for the data aligned with the previously reported phenotypes and identified daytime sleepiness in 15.3%, disturbed sleep (insomnia-like symptoms) in 37.7%, and minimally symptomatic (a low symptom profile) in 46.9%.
These phenotypes were reported in the European Respiratory Journal in 2014 in a cluster analysis of data from a sleep apnea cohort in Iceland and later replicated in the analysis of data from the Sleep Apnea Global Interdisciplinary Consortium published in Sleep in 2018. The consortium study also added two additional phenotypes, labeled “upper airway symptoms dominant” and “sleepiness dominant.”
The prevalence of a “minimally symptomatic group” in the new analysis of the Hispanics/Latinos in the United States is much higher than reported in these prior studies, at least partly, the investigators believed, because the “prior studies were clinical samples, and the people who were minimally symptomatic didn’t get to the sleep centers,” Mr. Gonzalez said in an interview after the meeting.
Patients with a phenotype of daytime sleepiness – the most common phenotype in prior research – constituted only a minority in the Hispanic/Latino population, he said.
Alberto Ramos, MD, of the University of Miami and the principal investigator, said in an interview that the research team is currently analyzing “if and how these different [phenotypic] clusters could affect the incidence of comorbidities” recorded in the HCHS/SOL study, such as hypertension, diabetes, cardiovascular disease, and cognitive decline.
For now, he said, the findings suggest that OSA may be especially underrecognized in Hispanics and Latinos and that there is more research to be done to better identify and stratify patients with varying symptomatology for more personalized treatment and for clinical trial selection. “Maybe we should expand our criteria ... broaden our [recognition] of the presentation of sleep apnea and the symptoms associated with it, not only in Hispanics but maybe in the general population,” Dr. Ramos said.
In commenting on the study, Krishna M. Sundar, MD, FCCP, director of the Sleep-Wake Center at the University of Utah, Salt Lake City, said that insomnia and daytime sleepiness are “key associations with obstructive sleep apnea and may predict different outcomes with untreated OSA.” Such heterogeneity is “only beginning to be appreciated,” he said. “The expression of OSA with these symptoms points to how OSA impacts quality of life” and how symptomatology in addition to Apnea Hypopnea Index “may be an important determinant of treatment benefit and compliance.”
The investigators reported no relevant disclosures. Dr. Sundar said that he is cofounder of Hypnoscure, software for population management of sleep apnea, but with no monies received.
REPORTING FROM SLEEP 2020
2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic
Respiratory virus seasons usually follow a fairly well-known pattern. Enterovirus 68 (EV-D68) is a summer-to-early fall virus with biennial peak years. Rhinovirus (HRv) and adenovirus (Adv) occur nearly year-round but may have small upticks in the first month or so that children return to school. Early in the school year, upper respiratory infections from both HRv and Adv and viral sore throats from Adv are common, with conjunctivitis from Adv outbreaks in some years. October to November is human parainfluenza (HPiV) 1 and 2 season, often presenting as croup. Human metapneumovirus infections span October through April. In late November to December, influenza begins, usually with an A type, later transitioning to a B type in February through April. Also in December, respiratory syncytial virus (RSV) starts, characteristically with bronchiolitis presentations, peaking in February to March and tapering off in May. In late March to April, HPiV 3 also appears for 4-6 weeks.

Will 2020-2021 be different?
Summer was remarkably free of expected enterovirus activity, suggesting that the seasonal parade may differ this year. Remember that the 2019-2020 respiratory season suddenly and nearly completely stopped in March because of social distancing and lockdowns needed to address the SARS-CoV-2 pandemic.
The mild influenza season in the southern hemisphere suggests that our influenza season also could be mild. But perhaps not – most southern hemisphere countries that are surveyed for influenza activities had the most intense SARS-CoV-2 mitigations, making the observed mildness potentially related more to social mitigation than less virulent influenza strains. If so, southern hemisphere influenza data may not apply to the United States, where social distancing and masks are ignored or used inconsistently by almost half the population.
Further, the stop-and-go pattern of in-person school/college attendance adds to uncertainties for the usual orderly virus-specific seasonality. The result may be multiple stop-and-go “pop-up” or “mini” outbreaks for any given virus potentially reflected as exaggerated local or regional differences in circulation of various viruses. The erratic seasonality also would increase coinfections, which could present with more severe or different symptoms.
SARS-CoV-2’s potential interaction
Will the relatively mild presentations for most children with SARS-CoV-2 hold up in the setting of coinfections or sequential respiratory viral infections? Could SARS-CoV-2 cause worse/more prolonged symptoms or more sequelae if paired simultaneously or in tandem with a traditional respiratory virus? To date, data on the frequency and severity of SARS-CoV-2 coinfections are conflicting and sparse, but it appears that non-SARS-CoV-2 viruses can be involved in 15%-50% pediatric acute respiratory infections.1,2
However, it may not be important to know about coinfecting viruses other than influenza (can be treated) or SARS-CoV-2 (needs quarantine and contact tracing), unless symptoms are atypical or more severe than usual. For example, a young child with bronchiolitis is most likely infected with RSV, but HPiV, influenza, metapneumovirus, HRv, and even SARS-CoV-2 can cause bronchiolitis. Even so, testing outpatients for RSV or non-influenza is not routine or even clinically helpful. Supportive treatment and restriction from daycare attendance are sufficient management for outpatient ARIs whether presenting as bronchiolitis or not.
Considerations for SARS-CoV-2 testing: Outpatient bronchiolitis
If a child presents with classic bronchiolitis but has above moderate to severe symptoms, is SARS-CoV-2 a consideration? Perhaps, if SARS-CoV-2 acts similarly to non-SARS-CoV-2s.
A recent report from the 30th Multicenter Airway Research Collaboration (MARC-30) surveillance study (2007-2014) of children hospitalized with clinical bronchiolitis evaluated respiratory viruses, including RSV and the four common non-SARS coronaviruses using molecular testing.3 Among 1,880 subjects, a CoV (alpha CoV: NL63 or 229E, or beta CoV: KKU1 or OC43) was detected in 12%. Yet most had only RSV (n = 1,661); 32 had only CoV (n = 32). But note that 219 had both.
Bronchiolitis subjects with CoV were older – median 3.7 (1.4-5.8) vs. 2.8 (1.9-7.2) years – and more likely male than were RSV subjects (68% vs. 58%). OC43 was most frequent followed by equal numbers of HKU1 and NL63, while 229E was the least frequent. Medical utilization and severity did not differ among the CoVs, or between RSV+CoV vs. RSV alone, unless one considered CoV viral load as a variable. ICU use increased when the polymerase chain reaction cycle threshold result indicated a high CoV viral load.
These data suggest CoVs are not infrequent coinfectors with RSV in bronchiolitis – and that SARS-CoV-2 is the same. Therefore, a bronchiolitis presentation doesn’t necessarily take us off the hook for the need to consider SARS-CoV-2 testing, particularly in the somewhat older bronchiolitis patient with more than mild symptoms.
Considerations for SARS-CoV-2 testing: Outpatient influenza-like illness
In 2020-2021, the Centers for Disease Control and Prevention recommends considering empiric antiviral treatment for ILIs (fever plus either cough or sore throat) based upon our clinical judgement, even in non-high-risk children.4
While pediatric COVID-19 illnesses are predominantly asymptomatic or mild, a febrile ARI is also a SARS-CoV-2 compatible presentation. So, if all we use is our clinical judgment, how do we know if the febrile ARI is due to influenza or SARS-CoV-2 or both? At least one study used a highly sensitive and specific molecular influenza test to show that the accuracy of clinically diagnosing influenza in children is not much better than flipping a coin and would lead to potential antiviral overuse.5
So, it seems ideal to test for influenza when possible. Point-of-care (POC) tests are frequently used for outpatients. Eight POC Clinical Laboratory Improvement Amendments (CLIA)–waived kits, some also detecting RSV, are available but most have modest sensitivity (60%-80%) compared with lab-based molecular tests.6 That said, if supplies and kits for one of the POC tests are available to us during these SARS-CoV-2 stressed times (back orders seem more common this year), a positive influenza test in the first 48 hours of symptoms confirms the option to prescribe an antiviral. Yet how will we have confidence that the febrile ARI is not also partly due to SARS-CoV-2? Currently febrile ARIs usually are considered SARS-CoV-2 and the children are sent for SARS-CoV-2 testing. During influenza season, it seems we will need to continue to send febrile outpatients for SARS-CoV-2 testing, even if POC influenza positive, via whatever mechanisms are available as time goes on.
We expect more rapid pediatric testing modalities for SARS-CoV-2 (maybe even saliva tests) to become available over the next months. Indeed, rapid antigen tests and rapid molecular tests are being evaluated in adults and seem destined for CLIA waivers as POC tests, and even home testing kits. Pediatric approvals hopefully also will occur. So, the pathways for SARS-CoV-2 testing available now will likely change over this winter. But be aware that supplies/kits will be prioritized to locations within high need areas and bulk purchase contracts. So POC kits may remain scarce for practices, meaning a reference laboratory still could be the way to go for SARS-CoV-2 for at least the rest of 2020. Reference labs are becoming creative as well; one combined detection of influenza A, influenza B, RSV, and SARS-CoV-2 into one test, and hopes to get approval for swab collection that can be done by families at home and mailed in.
Summary
Expect variations on the traditional parade of seasonal respiratory viruses, with increased numbers of coinfections. Choosing the outpatient who needs influenza testing is the same as in past years, although we have CDC permissive recommendations to prescribe antivirals for any outpatient ILI within the first 48 hours of symptoms. Still, POC testing for influenza remains potentially valuable in the ILI patient. The choice of whether and how to test for SARS-CoV-2 given its potential to be a primary or coinfecting agent in presentations linked more closely to a traditional virus (e.g. RSV bronchiolitis) will be a test of our clinical judgement until more data and easier testing are available. Further complicating coinfection recognition is the fact that many sick visits occur by telehealth and much testing is done at drive-through SARS-CoV-2 testing facilities with no clinician exam. Unless we are liberal in SARS-CoV-2 testing, detecting SARS-CoV-2 coinfections is easier said than done given its usually mild presentation being overshadowed by any coinfecting virus.
But understanding who has SARS-CoV-2, even as a coinfection, still is essential in controlling the pandemic. We will need to be vigilant for evolving approaches to SARS-CoV-2 testing in the context of symptomatic ARI presentations, knowing this will likely remain a moving target for the foreseeable future.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatrics. 2020;146(1):e20200961.
2. JAMA. 2020 May 26;323(20):2085-6.
3. Pediatrics. 2020. doi: 10.1542/peds.2020-1267.
4. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
5. J. Pediatr. 2020. doi: 10.1016/j.jpeds.2020.08.007.
6. www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html.
Respiratory virus seasons usually follow a fairly well-known pattern. Enterovirus 68 (EV-D68) is a summer-to-early fall virus with biennial peak years. Rhinovirus (HRv) and adenovirus (Adv) occur nearly year-round but may have small upticks in the first month or so that children return to school. Early in the school year, upper respiratory infections from both HRv and Adv and viral sore throats from Adv are common, with conjunctivitis from Adv outbreaks in some years. October to November is human parainfluenza (HPiV) 1 and 2 season, often presenting as croup. Human metapneumovirus infections span October through April. In late November to December, influenza begins, usually with an A type, later transitioning to a B type in February through April. Also in December, respiratory syncytial virus (RSV) starts, characteristically with bronchiolitis presentations, peaking in February to March and tapering off in May. In late March to April, HPiV 3 also appears for 4-6 weeks.

Will 2020-2021 be different?
Summer was remarkably free of expected enterovirus activity, suggesting that the seasonal parade may differ this year. Remember that the 2019-2020 respiratory season suddenly and nearly completely stopped in March because of social distancing and lockdowns needed to address the SARS-CoV-2 pandemic.
The mild influenza season in the southern hemisphere suggests that our influenza season also could be mild. But perhaps not – most southern hemisphere countries that are surveyed for influenza activities had the most intense SARS-CoV-2 mitigations, making the observed mildness potentially related more to social mitigation than less virulent influenza strains. If so, southern hemisphere influenza data may not apply to the United States, where social distancing and masks are ignored or used inconsistently by almost half the population.
Further, the stop-and-go pattern of in-person school/college attendance adds to uncertainties for the usual orderly virus-specific seasonality. The result may be multiple stop-and-go “pop-up” or “mini” outbreaks for any given virus potentially reflected as exaggerated local or regional differences in circulation of various viruses. The erratic seasonality also would increase coinfections, which could present with more severe or different symptoms.
SARS-CoV-2’s potential interaction
Will the relatively mild presentations for most children with SARS-CoV-2 hold up in the setting of coinfections or sequential respiratory viral infections? Could SARS-CoV-2 cause worse/more prolonged symptoms or more sequelae if paired simultaneously or in tandem with a traditional respiratory virus? To date, data on the frequency and severity of SARS-CoV-2 coinfections are conflicting and sparse, but it appears that non-SARS-CoV-2 viruses can be involved in 15%-50% pediatric acute respiratory infections.1,2
However, it may not be important to know about coinfecting viruses other than influenza (can be treated) or SARS-CoV-2 (needs quarantine and contact tracing), unless symptoms are atypical or more severe than usual. For example, a young child with bronchiolitis is most likely infected with RSV, but HPiV, influenza, metapneumovirus, HRv, and even SARS-CoV-2 can cause bronchiolitis. Even so, testing outpatients for RSV or non-influenza is not routine or even clinically helpful. Supportive treatment and restriction from daycare attendance are sufficient management for outpatient ARIs whether presenting as bronchiolitis or not.
Considerations for SARS-CoV-2 testing: Outpatient bronchiolitis
If a child presents with classic bronchiolitis but has above moderate to severe symptoms, is SARS-CoV-2 a consideration? Perhaps, if SARS-CoV-2 acts similarly to non-SARS-CoV-2s.
A recent report from the 30th Multicenter Airway Research Collaboration (MARC-30) surveillance study (2007-2014) of children hospitalized with clinical bronchiolitis evaluated respiratory viruses, including RSV and the four common non-SARS coronaviruses using molecular testing.3 Among 1,880 subjects, a CoV (alpha CoV: NL63 or 229E, or beta CoV: KKU1 or OC43) was detected in 12%. Yet most had only RSV (n = 1,661); 32 had only CoV (n = 32). But note that 219 had both.
Bronchiolitis subjects with CoV were older – median 3.7 (1.4-5.8) vs. 2.8 (1.9-7.2) years – and more likely male than were RSV subjects (68% vs. 58%). OC43 was most frequent followed by equal numbers of HKU1 and NL63, while 229E was the least frequent. Medical utilization and severity did not differ among the CoVs, or between RSV+CoV vs. RSV alone, unless one considered CoV viral load as a variable. ICU use increased when the polymerase chain reaction cycle threshold result indicated a high CoV viral load.
These data suggest CoVs are not infrequent coinfectors with RSV in bronchiolitis – and that SARS-CoV-2 is the same. Therefore, a bronchiolitis presentation doesn’t necessarily take us off the hook for the need to consider SARS-CoV-2 testing, particularly in the somewhat older bronchiolitis patient with more than mild symptoms.
Considerations for SARS-CoV-2 testing: Outpatient influenza-like illness
In 2020-2021, the Centers for Disease Control and Prevention recommends considering empiric antiviral treatment for ILIs (fever plus either cough or sore throat) based upon our clinical judgement, even in non-high-risk children.4
While pediatric COVID-19 illnesses are predominantly asymptomatic or mild, a febrile ARI is also a SARS-CoV-2 compatible presentation. So, if all we use is our clinical judgment, how do we know if the febrile ARI is due to influenza or SARS-CoV-2 or both? At least one study used a highly sensitive and specific molecular influenza test to show that the accuracy of clinically diagnosing influenza in children is not much better than flipping a coin and would lead to potential antiviral overuse.5
So, it seems ideal to test for influenza when possible. Point-of-care (POC) tests are frequently used for outpatients. Eight POC Clinical Laboratory Improvement Amendments (CLIA)–waived kits, some also detecting RSV, are available but most have modest sensitivity (60%-80%) compared with lab-based molecular tests.6 That said, if supplies and kits for one of the POC tests are available to us during these SARS-CoV-2 stressed times (back orders seem more common this year), a positive influenza test in the first 48 hours of symptoms confirms the option to prescribe an antiviral. Yet how will we have confidence that the febrile ARI is not also partly due to SARS-CoV-2? Currently febrile ARIs usually are considered SARS-CoV-2 and the children are sent for SARS-CoV-2 testing. During influenza season, it seems we will need to continue to send febrile outpatients for SARS-CoV-2 testing, even if POC influenza positive, via whatever mechanisms are available as time goes on.
We expect more rapid pediatric testing modalities for SARS-CoV-2 (maybe even saliva tests) to become available over the next months. Indeed, rapid antigen tests and rapid molecular tests are being evaluated in adults and seem destined for CLIA waivers as POC tests, and even home testing kits. Pediatric approvals hopefully also will occur. So, the pathways for SARS-CoV-2 testing available now will likely change over this winter. But be aware that supplies/kits will be prioritized to locations within high need areas and bulk purchase contracts. So POC kits may remain scarce for practices, meaning a reference laboratory still could be the way to go for SARS-CoV-2 for at least the rest of 2020. Reference labs are becoming creative as well; one combined detection of influenza A, influenza B, RSV, and SARS-CoV-2 into one test, and hopes to get approval for swab collection that can be done by families at home and mailed in.
Summary
Expect variations on the traditional parade of seasonal respiratory viruses, with increased numbers of coinfections. Choosing the outpatient who needs influenza testing is the same as in past years, although we have CDC permissive recommendations to prescribe antivirals for any outpatient ILI within the first 48 hours of symptoms. Still, POC testing for influenza remains potentially valuable in the ILI patient. The choice of whether and how to test for SARS-CoV-2 given its potential to be a primary or coinfecting agent in presentations linked more closely to a traditional virus (e.g. RSV bronchiolitis) will be a test of our clinical judgement until more data and easier testing are available. Further complicating coinfection recognition is the fact that many sick visits occur by telehealth and much testing is done at drive-through SARS-CoV-2 testing facilities with no clinician exam. Unless we are liberal in SARS-CoV-2 testing, detecting SARS-CoV-2 coinfections is easier said than done given its usually mild presentation being overshadowed by any coinfecting virus.
But understanding who has SARS-CoV-2, even as a coinfection, still is essential in controlling the pandemic. We will need to be vigilant for evolving approaches to SARS-CoV-2 testing in the context of symptomatic ARI presentations, knowing this will likely remain a moving target for the foreseeable future.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatrics. 2020;146(1):e20200961.
2. JAMA. 2020 May 26;323(20):2085-6.
3. Pediatrics. 2020. doi: 10.1542/peds.2020-1267.
4. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
5. J. Pediatr. 2020. doi: 10.1016/j.jpeds.2020.08.007.
6. www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html.
Respiratory virus seasons usually follow a fairly well-known pattern. Enterovirus 68 (EV-D68) is a summer-to-early fall virus with biennial peak years. Rhinovirus (HRv) and adenovirus (Adv) occur nearly year-round but may have small upticks in the first month or so that children return to school. Early in the school year, upper respiratory infections from both HRv and Adv and viral sore throats from Adv are common, with conjunctivitis from Adv outbreaks in some years. October to November is human parainfluenza (HPiV) 1 and 2 season, often presenting as croup. Human metapneumovirus infections span October through April. In late November to December, influenza begins, usually with an A type, later transitioning to a B type in February through April. Also in December, respiratory syncytial virus (RSV) starts, characteristically with bronchiolitis presentations, peaking in February to March and tapering off in May. In late March to April, HPiV 3 also appears for 4-6 weeks.

Will 2020-2021 be different?
Summer was remarkably free of expected enterovirus activity, suggesting that the seasonal parade may differ this year. Remember that the 2019-2020 respiratory season suddenly and nearly completely stopped in March because of social distancing and lockdowns needed to address the SARS-CoV-2 pandemic.
The mild influenza season in the southern hemisphere suggests that our influenza season also could be mild. But perhaps not – most southern hemisphere countries that are surveyed for influenza activities had the most intense SARS-CoV-2 mitigations, making the observed mildness potentially related more to social mitigation than less virulent influenza strains. If so, southern hemisphere influenza data may not apply to the United States, where social distancing and masks are ignored or used inconsistently by almost half the population.
Further, the stop-and-go pattern of in-person school/college attendance adds to uncertainties for the usual orderly virus-specific seasonality. The result may be multiple stop-and-go “pop-up” or “mini” outbreaks for any given virus potentially reflected as exaggerated local or regional differences in circulation of various viruses. The erratic seasonality also would increase coinfections, which could present with more severe or different symptoms.
SARS-CoV-2’s potential interaction
Will the relatively mild presentations for most children with SARS-CoV-2 hold up in the setting of coinfections or sequential respiratory viral infections? Could SARS-CoV-2 cause worse/more prolonged symptoms or more sequelae if paired simultaneously or in tandem with a traditional respiratory virus? To date, data on the frequency and severity of SARS-CoV-2 coinfections are conflicting and sparse, but it appears that non-SARS-CoV-2 viruses can be involved in 15%-50% pediatric acute respiratory infections.1,2
However, it may not be important to know about coinfecting viruses other than influenza (can be treated) or SARS-CoV-2 (needs quarantine and contact tracing), unless symptoms are atypical or more severe than usual. For example, a young child with bronchiolitis is most likely infected with RSV, but HPiV, influenza, metapneumovirus, HRv, and even SARS-CoV-2 can cause bronchiolitis. Even so, testing outpatients for RSV or non-influenza is not routine or even clinically helpful. Supportive treatment and restriction from daycare attendance are sufficient management for outpatient ARIs whether presenting as bronchiolitis or not.
Considerations for SARS-CoV-2 testing: Outpatient bronchiolitis
If a child presents with classic bronchiolitis but has above moderate to severe symptoms, is SARS-CoV-2 a consideration? Perhaps, if SARS-CoV-2 acts similarly to non-SARS-CoV-2s.
A recent report from the 30th Multicenter Airway Research Collaboration (MARC-30) surveillance study (2007-2014) of children hospitalized with clinical bronchiolitis evaluated respiratory viruses, including RSV and the four common non-SARS coronaviruses using molecular testing.3 Among 1,880 subjects, a CoV (alpha CoV: NL63 or 229E, or beta CoV: KKU1 or OC43) was detected in 12%. Yet most had only RSV (n = 1,661); 32 had only CoV (n = 32). But note that 219 had both.
Bronchiolitis subjects with CoV were older – median 3.7 (1.4-5.8) vs. 2.8 (1.9-7.2) years – and more likely male than were RSV subjects (68% vs. 58%). OC43 was most frequent followed by equal numbers of HKU1 and NL63, while 229E was the least frequent. Medical utilization and severity did not differ among the CoVs, or between RSV+CoV vs. RSV alone, unless one considered CoV viral load as a variable. ICU use increased when the polymerase chain reaction cycle threshold result indicated a high CoV viral load.
These data suggest CoVs are not infrequent coinfectors with RSV in bronchiolitis – and that SARS-CoV-2 is the same. Therefore, a bronchiolitis presentation doesn’t necessarily take us off the hook for the need to consider SARS-CoV-2 testing, particularly in the somewhat older bronchiolitis patient with more than mild symptoms.
Considerations for SARS-CoV-2 testing: Outpatient influenza-like illness
In 2020-2021, the Centers for Disease Control and Prevention recommends considering empiric antiviral treatment for ILIs (fever plus either cough or sore throat) based upon our clinical judgement, even in non-high-risk children.4
While pediatric COVID-19 illnesses are predominantly asymptomatic or mild, a febrile ARI is also a SARS-CoV-2 compatible presentation. So, if all we use is our clinical judgment, how do we know if the febrile ARI is due to influenza or SARS-CoV-2 or both? At least one study used a highly sensitive and specific molecular influenza test to show that the accuracy of clinically diagnosing influenza in children is not much better than flipping a coin and would lead to potential antiviral overuse.5
So, it seems ideal to test for influenza when possible. Point-of-care (POC) tests are frequently used for outpatients. Eight POC Clinical Laboratory Improvement Amendments (CLIA)–waived kits, some also detecting RSV, are available but most have modest sensitivity (60%-80%) compared with lab-based molecular tests.6 That said, if supplies and kits for one of the POC tests are available to us during these SARS-CoV-2 stressed times (back orders seem more common this year), a positive influenza test in the first 48 hours of symptoms confirms the option to prescribe an antiviral. Yet how will we have confidence that the febrile ARI is not also partly due to SARS-CoV-2? Currently febrile ARIs usually are considered SARS-CoV-2 and the children are sent for SARS-CoV-2 testing. During influenza season, it seems we will need to continue to send febrile outpatients for SARS-CoV-2 testing, even if POC influenza positive, via whatever mechanisms are available as time goes on.
We expect more rapid pediatric testing modalities for SARS-CoV-2 (maybe even saliva tests) to become available over the next months. Indeed, rapid antigen tests and rapid molecular tests are being evaluated in adults and seem destined for CLIA waivers as POC tests, and even home testing kits. Pediatric approvals hopefully also will occur. So, the pathways for SARS-CoV-2 testing available now will likely change over this winter. But be aware that supplies/kits will be prioritized to locations within high need areas and bulk purchase contracts. So POC kits may remain scarce for practices, meaning a reference laboratory still could be the way to go for SARS-CoV-2 for at least the rest of 2020. Reference labs are becoming creative as well; one combined detection of influenza A, influenza B, RSV, and SARS-CoV-2 into one test, and hopes to get approval for swab collection that can be done by families at home and mailed in.
Summary
Expect variations on the traditional parade of seasonal respiratory viruses, with increased numbers of coinfections. Choosing the outpatient who needs influenza testing is the same as in past years, although we have CDC permissive recommendations to prescribe antivirals for any outpatient ILI within the first 48 hours of symptoms. Still, POC testing for influenza remains potentially valuable in the ILI patient. The choice of whether and how to test for SARS-CoV-2 given its potential to be a primary or coinfecting agent in presentations linked more closely to a traditional virus (e.g. RSV bronchiolitis) will be a test of our clinical judgement until more data and easier testing are available. Further complicating coinfection recognition is the fact that many sick visits occur by telehealth and much testing is done at drive-through SARS-CoV-2 testing facilities with no clinician exam. Unless we are liberal in SARS-CoV-2 testing, detecting SARS-CoV-2 coinfections is easier said than done given its usually mild presentation being overshadowed by any coinfecting virus.
But understanding who has SARS-CoV-2, even as a coinfection, still is essential in controlling the pandemic. We will need to be vigilant for evolving approaches to SARS-CoV-2 testing in the context of symptomatic ARI presentations, knowing this will likely remain a moving target for the foreseeable future.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatrics. 2020;146(1):e20200961.
2. JAMA. 2020 May 26;323(20):2085-6.
3. Pediatrics. 2020. doi: 10.1542/peds.2020-1267.
4. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
5. J. Pediatr. 2020. doi: 10.1016/j.jpeds.2020.08.007.
6. www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html.
Dr. Fauci: ‘About 40%-45% of infections are asymptomatic’
Anthony Fauci, MD, highlighting the latest COVID-19 developments on Friday, said, “It is now clear that about 40%-45% of infections are asymptomatic.”
Asymptomatic carriers can account for a large proportion — up to 50% — of virus transmissions, Fauci, director of the National Institute of Allergy and Infectious Diseases, told a virtual crowd of critical care clinicians gathered by the Society of Critical Care Medicine.
Such transmissions have made response strategies, such as contact tracing, extremely difficult, he said.
Lew Kaplan, MD, president of SCCM, told Medscape Medical News after the presentation: “That really supports the universal wearing of masks and the capstone message from that – you should protect one another.
“That kind of social responsibility that sits within the public health domain to me is as important as the vaccine candidates and the science behind the receptors. It underpins the necessary relationship and the interdependence of the medical community with the public,” Kaplan added.
Fauci’s plenary led the SCCM’s conference, “COVID-19: What’s Next/Preparing for the Second Wave,” running today and Saturday.
Why U.S. response lags behind Spain and Italy
“This virus has literally exploded upon the planet in a pandemic manner which is unparalleled to anything we’ve seen in the last 102 years since the pandemic of 1918,” Fauci said.
“Unfortunately, the United States has been hit harder than any other country in the world, with 6 million reported cases.”
He explained that in the European Union countries the disease spiked early on and returned to a low baseline. “Unfortunately for them,” Fauci said, “as they’re trying to open up their economy, it’s coming back up.”
The United States, he explained, plateaued at about 20,000 cases a day, then a surge of cases in Florida, California, Texas, and Arizona brought the cases to 70,000 a day. Now cases have returned to 35,000-40,000 a day.
The difference in the trajectory of the response, he said, is that, compared with Spain and Italy for example, the United States has not shut down mobility in parks, outdoor spaces, and grocery stores nearly as much as some European countries did.
He pointed to numerous clusters of cases, spread from social or work gatherings, including the well-known Skagit County Washington state choir practice in March, in which a symptomatic choir member infected 87% of the 61 people rehearsing.
Vaccine by end of the year
As for a vaccine timeline, Fauci told SCCM members, “We project that by the end of this year, namely November/December, we will know if we have a safe and effective vaccine and we are cautiously optimistic that we will be successful, based on promising data in the animal model as well as good immunological data that we see from the phase 1 and phase 2 trials.”
However, also on Friday, Fauci told MSNBC’s Andrea Mitchell that a sense of normalcy is not likely before the middle of next year.
“By the time you mobilize the distribution of the vaccinations, and you get the majority, or more, of the population vaccinated and protected, that’s likely not going to happen [until] the mid- or end of 2021,” he said.
According to the Centers for Disease Control and Prevention (CDC) case tracker, as of Thursday, COVID-19 had resulted in more than 190,000 deaths overall and more than 256,000 new cases in the United States in the past 7 days.
Fauci has warned that the next few months will be critical in the virus’ trajectory, with the double onslaught of COVID-19 and the flu season.
On Thursday, Fauci said, “We need to hunker down and get through this fall and winter because it’s not going to be easy.”
Fauci remains a top trusted source in COVID-19 information, poll numbers show.
A Kaiser Family Foundation poll released Thursday found that 68% of US adults had a fair amount or a great deal of trust that Fauci would provide reliable information on COVID-19, just slightly more that the 67% who said they trust the CDC information. About half (53%) say they trust Deborah Birx, MD, the coordinator for the White House Coronavirus Task Force, as a reliable source of information.
The poll also found that 54% of Americans said they would not get a COVID-19 vaccine if one was approved by the US Food and Drug Administration before the November election and was made available and free to all who wanted it.
Kaplan and Fauci report no relevant financial relationships.
This article first appeared on Medscape.com.
Anthony Fauci, MD, highlighting the latest COVID-19 developments on Friday, said, “It is now clear that about 40%-45% of infections are asymptomatic.”
Asymptomatic carriers can account for a large proportion — up to 50% — of virus transmissions, Fauci, director of the National Institute of Allergy and Infectious Diseases, told a virtual crowd of critical care clinicians gathered by the Society of Critical Care Medicine.
Such transmissions have made response strategies, such as contact tracing, extremely difficult, he said.
Lew Kaplan, MD, president of SCCM, told Medscape Medical News after the presentation: “That really supports the universal wearing of masks and the capstone message from that – you should protect one another.
“That kind of social responsibility that sits within the public health domain to me is as important as the vaccine candidates and the science behind the receptors. It underpins the necessary relationship and the interdependence of the medical community with the public,” Kaplan added.
Fauci’s plenary led the SCCM’s conference, “COVID-19: What’s Next/Preparing for the Second Wave,” running today and Saturday.
Why U.S. response lags behind Spain and Italy
“This virus has literally exploded upon the planet in a pandemic manner which is unparalleled to anything we’ve seen in the last 102 years since the pandemic of 1918,” Fauci said.
“Unfortunately, the United States has been hit harder than any other country in the world, with 6 million reported cases.”
He explained that in the European Union countries the disease spiked early on and returned to a low baseline. “Unfortunately for them,” Fauci said, “as they’re trying to open up their economy, it’s coming back up.”
The United States, he explained, plateaued at about 20,000 cases a day, then a surge of cases in Florida, California, Texas, and Arizona brought the cases to 70,000 a day. Now cases have returned to 35,000-40,000 a day.
The difference in the trajectory of the response, he said, is that, compared with Spain and Italy for example, the United States has not shut down mobility in parks, outdoor spaces, and grocery stores nearly as much as some European countries did.
He pointed to numerous clusters of cases, spread from social or work gatherings, including the well-known Skagit County Washington state choir practice in March, in which a symptomatic choir member infected 87% of the 61 people rehearsing.
Vaccine by end of the year
As for a vaccine timeline, Fauci told SCCM members, “We project that by the end of this year, namely November/December, we will know if we have a safe and effective vaccine and we are cautiously optimistic that we will be successful, based on promising data in the animal model as well as good immunological data that we see from the phase 1 and phase 2 trials.”
However, also on Friday, Fauci told MSNBC’s Andrea Mitchell that a sense of normalcy is not likely before the middle of next year.
“By the time you mobilize the distribution of the vaccinations, and you get the majority, or more, of the population vaccinated and protected, that’s likely not going to happen [until] the mid- or end of 2021,” he said.
According to the Centers for Disease Control and Prevention (CDC) case tracker, as of Thursday, COVID-19 had resulted in more than 190,000 deaths overall and more than 256,000 new cases in the United States in the past 7 days.
Fauci has warned that the next few months will be critical in the virus’ trajectory, with the double onslaught of COVID-19 and the flu season.
On Thursday, Fauci said, “We need to hunker down and get through this fall and winter because it’s not going to be easy.”
Fauci remains a top trusted source in COVID-19 information, poll numbers show.
A Kaiser Family Foundation poll released Thursday found that 68% of US adults had a fair amount or a great deal of trust that Fauci would provide reliable information on COVID-19, just slightly more that the 67% who said they trust the CDC information. About half (53%) say they trust Deborah Birx, MD, the coordinator for the White House Coronavirus Task Force, as a reliable source of information.
The poll also found that 54% of Americans said they would not get a COVID-19 vaccine if one was approved by the US Food and Drug Administration before the November election and was made available and free to all who wanted it.
Kaplan and Fauci report no relevant financial relationships.
This article first appeared on Medscape.com.
Anthony Fauci, MD, highlighting the latest COVID-19 developments on Friday, said, “It is now clear that about 40%-45% of infections are asymptomatic.”
Asymptomatic carriers can account for a large proportion — up to 50% — of virus transmissions, Fauci, director of the National Institute of Allergy and Infectious Diseases, told a virtual crowd of critical care clinicians gathered by the Society of Critical Care Medicine.
Such transmissions have made response strategies, such as contact tracing, extremely difficult, he said.
Lew Kaplan, MD, president of SCCM, told Medscape Medical News after the presentation: “That really supports the universal wearing of masks and the capstone message from that – you should protect one another.
“That kind of social responsibility that sits within the public health domain to me is as important as the vaccine candidates and the science behind the receptors. It underpins the necessary relationship and the interdependence of the medical community with the public,” Kaplan added.
Fauci’s plenary led the SCCM’s conference, “COVID-19: What’s Next/Preparing for the Second Wave,” running today and Saturday.
Why U.S. response lags behind Spain and Italy
“This virus has literally exploded upon the planet in a pandemic manner which is unparalleled to anything we’ve seen in the last 102 years since the pandemic of 1918,” Fauci said.
“Unfortunately, the United States has been hit harder than any other country in the world, with 6 million reported cases.”
He explained that in the European Union countries the disease spiked early on and returned to a low baseline. “Unfortunately for them,” Fauci said, “as they’re trying to open up their economy, it’s coming back up.”
The United States, he explained, plateaued at about 20,000 cases a day, then a surge of cases in Florida, California, Texas, and Arizona brought the cases to 70,000 a day. Now cases have returned to 35,000-40,000 a day.
The difference in the trajectory of the response, he said, is that, compared with Spain and Italy for example, the United States has not shut down mobility in parks, outdoor spaces, and grocery stores nearly as much as some European countries did.
He pointed to numerous clusters of cases, spread from social or work gatherings, including the well-known Skagit County Washington state choir practice in March, in which a symptomatic choir member infected 87% of the 61 people rehearsing.
Vaccine by end of the year
As for a vaccine timeline, Fauci told SCCM members, “We project that by the end of this year, namely November/December, we will know if we have a safe and effective vaccine and we are cautiously optimistic that we will be successful, based on promising data in the animal model as well as good immunological data that we see from the phase 1 and phase 2 trials.”
However, also on Friday, Fauci told MSNBC’s Andrea Mitchell that a sense of normalcy is not likely before the middle of next year.
“By the time you mobilize the distribution of the vaccinations, and you get the majority, or more, of the population vaccinated and protected, that’s likely not going to happen [until] the mid- or end of 2021,” he said.
According to the Centers for Disease Control and Prevention (CDC) case tracker, as of Thursday, COVID-19 had resulted in more than 190,000 deaths overall and more than 256,000 new cases in the United States in the past 7 days.
Fauci has warned that the next few months will be critical in the virus’ trajectory, with the double onslaught of COVID-19 and the flu season.
On Thursday, Fauci said, “We need to hunker down and get through this fall and winter because it’s not going to be easy.”
Fauci remains a top trusted source in COVID-19 information, poll numbers show.
A Kaiser Family Foundation poll released Thursday found that 68% of US adults had a fair amount or a great deal of trust that Fauci would provide reliable information on COVID-19, just slightly more that the 67% who said they trust the CDC information. About half (53%) say they trust Deborah Birx, MD, the coordinator for the White House Coronavirus Task Force, as a reliable source of information.
The poll also found that 54% of Americans said they would not get a COVID-19 vaccine if one was approved by the US Food and Drug Administration before the November election and was made available and free to all who wanted it.
Kaplan and Fauci report no relevant financial relationships.
This article first appeared on Medscape.com.
Conspiracy theories
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].















