User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Is the presence of enanthem a clue for COVID-19?
Larger studies should explore and confirm this association, the study’s authors and other experts suggested.
Dermatologists are already aware of the connection between enanthem and viral etiology. “As seen with other viral infections, we wondered if COVID-19 could produce enanthem in addition to skin rash exanthem,” one of the study author’s, Juan Jiménez-Cauhe, MD, a dermatologist with Hospital Universitario Ramon y Cajal, Madrid, said in an interview. He and his colleagues summarized their findings in a research letter in JAMA Dermatology.
They examined the oral cavity of 21 COVID-19 patients at a tertiary care hospital who also had a skin rash from March 30 to April 8. They classified enanthems into four categories: petechial, macular, macular with petechiae, or erythematovesicular. Six of the patients presented with oral lesions, all of them located in the palate; in one patient, the enanthem was macular, it was petechial in two patients and was macular with petechiae in three patients. The six patients ranged between the ages of 40 and 69 years; four were women.
Petechial or vesicular patterns are often associated with viral infections. In this particular study, the investigators did not observe vesicular lesions.
On average, mucocutaneous lesions appeared about 12 days after the onset of COVID-19 symptoms. “Interestingly, this latency was shorter in patients with petechial enanthem, compared with those with a macular lesion with petechiae appearance,” the authors wrote.
This shorter time might suggest an association for SARS-CoV-2, said Dr. Jiménez-Cauhe. Strong cough may have also caused petechial lesions on the palate, but it’s unlikely, as they appeared close in time to COVID-19 symptoms. It’s also unlikely that any drugs caused the lesions, as drug rashes can take 2-3 weeks to appear.
This fits in line with other evidence of broader skin manifestations appearing at the same time or after COVID-19, Esther Freeman, MD, said in an interview. Dr. Freeman, director of global health dermatology at Massachusetts General Hospital, Boston, is the principal investigator of the COVID-19 Dermatology Registry, a collaboration of the American Academy of Dermatology and International League of Dermatological Societies.
The study’s small cohort made it difficult to establish a solid association between the oral lesions and SARS-CoV-2. “However, the presence of enanthem in a patient with a skin rash is a useful finding that suggests a viral etiology rather than a drug reaction. This is particularly useful in COVID-19 patients, who were receiving many drugs as part of the treatment,” Dr. Jimenez-Cauhe said. Future studies should assess whether the presence of enanthem and exanthem lead physicians to consider SARS-CoV-2 as possible agents, ruling out infection with a blood or nasopharyngeal test.
This study adds to the growing body of knowledge on cutaneous and mucocutaneous findings associated with SARS-CoV-2 infection, Jules Lipoff, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, said in an interview. “One challenge in evaluating these findings is that these findings are nonspecific, and medication reactions can often cause similar rashes, such as morbilliform eruptions that can be associated with both viruses and medications.”
Enanthems, as the study authors noted, are more specific to viral infections and are less commonly associated with medication reactions. “So, even though this is a small case series with significant limitations, it does add more evidence that COVID-19 is directly responsible for findings in the skin and mucous membranes,” said Dr. Lipoff.
Dr. Freeman noted that the study may also encourage clinicians to look in a patient’s mouth when assessing for SARS-CoV-2. Additional research should examine these data in a larger population.
Several studies by Dr. Freeman, Dr. Lipoff, and others strongly suggest that SARS-CoV-2 has a spectrum of associated dermatologic manifestations. One evaluated perniolike skin lesions (J Am Acad Dermatol. 2020 Aug; 83[2]:486-92). The other was a case series from the COVID-19 registry that examined 716 cases of new-onset dermatologic symptoms in patients from 31 countries with confirmed/suspected SARS-CoV-2 (J Am Acad Dermatol. 2020 Jul 2;S0190-9622[20]32126-5.).
The authors of the report had no disclosures.
SOURCE: Jimenez-Cauhe J et al. JAMA Dermatol. 2020 Jul 15. doi: 10.1001/jamadermatol.2020.2550.
Larger studies should explore and confirm this association, the study’s authors and other experts suggested.
Dermatologists are already aware of the connection between enanthem and viral etiology. “As seen with other viral infections, we wondered if COVID-19 could produce enanthem in addition to skin rash exanthem,” one of the study author’s, Juan Jiménez-Cauhe, MD, a dermatologist with Hospital Universitario Ramon y Cajal, Madrid, said in an interview. He and his colleagues summarized their findings in a research letter in JAMA Dermatology.
They examined the oral cavity of 21 COVID-19 patients at a tertiary care hospital who also had a skin rash from March 30 to April 8. They classified enanthems into four categories: petechial, macular, macular with petechiae, or erythematovesicular. Six of the patients presented with oral lesions, all of them located in the palate; in one patient, the enanthem was macular, it was petechial in two patients and was macular with petechiae in three patients. The six patients ranged between the ages of 40 and 69 years; four were women.
Petechial or vesicular patterns are often associated with viral infections. In this particular study, the investigators did not observe vesicular lesions.
On average, mucocutaneous lesions appeared about 12 days after the onset of COVID-19 symptoms. “Interestingly, this latency was shorter in patients with petechial enanthem, compared with those with a macular lesion with petechiae appearance,” the authors wrote.
This shorter time might suggest an association for SARS-CoV-2, said Dr. Jiménez-Cauhe. Strong cough may have also caused petechial lesions on the palate, but it’s unlikely, as they appeared close in time to COVID-19 symptoms. It’s also unlikely that any drugs caused the lesions, as drug rashes can take 2-3 weeks to appear.
This fits in line with other evidence of broader skin manifestations appearing at the same time or after COVID-19, Esther Freeman, MD, said in an interview. Dr. Freeman, director of global health dermatology at Massachusetts General Hospital, Boston, is the principal investigator of the COVID-19 Dermatology Registry, a collaboration of the American Academy of Dermatology and International League of Dermatological Societies.
The study’s small cohort made it difficult to establish a solid association between the oral lesions and SARS-CoV-2. “However, the presence of enanthem in a patient with a skin rash is a useful finding that suggests a viral etiology rather than a drug reaction. This is particularly useful in COVID-19 patients, who were receiving many drugs as part of the treatment,” Dr. Jimenez-Cauhe said. Future studies should assess whether the presence of enanthem and exanthem lead physicians to consider SARS-CoV-2 as possible agents, ruling out infection with a blood or nasopharyngeal test.
This study adds to the growing body of knowledge on cutaneous and mucocutaneous findings associated with SARS-CoV-2 infection, Jules Lipoff, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, said in an interview. “One challenge in evaluating these findings is that these findings are nonspecific, and medication reactions can often cause similar rashes, such as morbilliform eruptions that can be associated with both viruses and medications.”
Enanthems, as the study authors noted, are more specific to viral infections and are less commonly associated with medication reactions. “So, even though this is a small case series with significant limitations, it does add more evidence that COVID-19 is directly responsible for findings in the skin and mucous membranes,” said Dr. Lipoff.
Dr. Freeman noted that the study may also encourage clinicians to look in a patient’s mouth when assessing for SARS-CoV-2. Additional research should examine these data in a larger population.
Several studies by Dr. Freeman, Dr. Lipoff, and others strongly suggest that SARS-CoV-2 has a spectrum of associated dermatologic manifestations. One evaluated perniolike skin lesions (J Am Acad Dermatol. 2020 Aug; 83[2]:486-92). The other was a case series from the COVID-19 registry that examined 716 cases of new-onset dermatologic symptoms in patients from 31 countries with confirmed/suspected SARS-CoV-2 (J Am Acad Dermatol. 2020 Jul 2;S0190-9622[20]32126-5.).
The authors of the report had no disclosures.
SOURCE: Jimenez-Cauhe J et al. JAMA Dermatol. 2020 Jul 15. doi: 10.1001/jamadermatol.2020.2550.
Larger studies should explore and confirm this association, the study’s authors and other experts suggested.
Dermatologists are already aware of the connection between enanthem and viral etiology. “As seen with other viral infections, we wondered if COVID-19 could produce enanthem in addition to skin rash exanthem,” one of the study author’s, Juan Jiménez-Cauhe, MD, a dermatologist with Hospital Universitario Ramon y Cajal, Madrid, said in an interview. He and his colleagues summarized their findings in a research letter in JAMA Dermatology.
They examined the oral cavity of 21 COVID-19 patients at a tertiary care hospital who also had a skin rash from March 30 to April 8. They classified enanthems into four categories: petechial, macular, macular with petechiae, or erythematovesicular. Six of the patients presented with oral lesions, all of them located in the palate; in one patient, the enanthem was macular, it was petechial in two patients and was macular with petechiae in three patients. The six patients ranged between the ages of 40 and 69 years; four were women.
Petechial or vesicular patterns are often associated with viral infections. In this particular study, the investigators did not observe vesicular lesions.
On average, mucocutaneous lesions appeared about 12 days after the onset of COVID-19 symptoms. “Interestingly, this latency was shorter in patients with petechial enanthem, compared with those with a macular lesion with petechiae appearance,” the authors wrote.
This shorter time might suggest an association for SARS-CoV-2, said Dr. Jiménez-Cauhe. Strong cough may have also caused petechial lesions on the palate, but it’s unlikely, as they appeared close in time to COVID-19 symptoms. It’s also unlikely that any drugs caused the lesions, as drug rashes can take 2-3 weeks to appear.
This fits in line with other evidence of broader skin manifestations appearing at the same time or after COVID-19, Esther Freeman, MD, said in an interview. Dr. Freeman, director of global health dermatology at Massachusetts General Hospital, Boston, is the principal investigator of the COVID-19 Dermatology Registry, a collaboration of the American Academy of Dermatology and International League of Dermatological Societies.
The study’s small cohort made it difficult to establish a solid association between the oral lesions and SARS-CoV-2. “However, the presence of enanthem in a patient with a skin rash is a useful finding that suggests a viral etiology rather than a drug reaction. This is particularly useful in COVID-19 patients, who were receiving many drugs as part of the treatment,” Dr. Jimenez-Cauhe said. Future studies should assess whether the presence of enanthem and exanthem lead physicians to consider SARS-CoV-2 as possible agents, ruling out infection with a blood or nasopharyngeal test.
This study adds to the growing body of knowledge on cutaneous and mucocutaneous findings associated with SARS-CoV-2 infection, Jules Lipoff, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, said in an interview. “One challenge in evaluating these findings is that these findings are nonspecific, and medication reactions can often cause similar rashes, such as morbilliform eruptions that can be associated with both viruses and medications.”
Enanthems, as the study authors noted, are more specific to viral infections and are less commonly associated with medication reactions. “So, even though this is a small case series with significant limitations, it does add more evidence that COVID-19 is directly responsible for findings in the skin and mucous membranes,” said Dr. Lipoff.
Dr. Freeman noted that the study may also encourage clinicians to look in a patient’s mouth when assessing for SARS-CoV-2. Additional research should examine these data in a larger population.
Several studies by Dr. Freeman, Dr. Lipoff, and others strongly suggest that SARS-CoV-2 has a spectrum of associated dermatologic manifestations. One evaluated perniolike skin lesions (J Am Acad Dermatol. 2020 Aug; 83[2]:486-92). The other was a case series from the COVID-19 registry that examined 716 cases of new-onset dermatologic symptoms in patients from 31 countries with confirmed/suspected SARS-CoV-2 (J Am Acad Dermatol. 2020 Jul 2;S0190-9622[20]32126-5.).
The authors of the report had no disclosures.
SOURCE: Jimenez-Cauhe J et al. JAMA Dermatol. 2020 Jul 15. doi: 10.1001/jamadermatol.2020.2550.
FROM JAMA DERMATOLOGY
Internists’ use of ultrasound can reduce radiology referrals
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.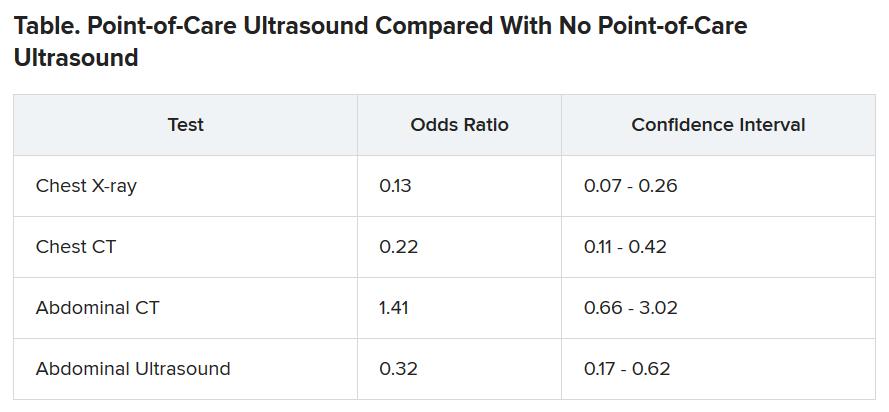
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Ob.gyns. struggle to keep pace with changing COVID-19 knowledge
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
OSHA in the COVID-19 era
As more and more reopened medical practices ramp up toward normal activity, the safety of patients and health care workers alike remains paramount. As always,
Most of the modified guidelines are already familiar: wear masks (and other personal protective equipment as necessary); maintain social distancing; have hand cleaner, soap, and water readily available; and sanitize between patient examinations.
It is also important to remember that COVID-19 is now a reportable disease; check with your local health authorities as to where and how. Also remember that, if you decide to screen employees and/or patients for fevers and other symptoms of COVID-19, those data are subject to HIPAA rules and must be kept confidential.
Now might be a good time to confirm that you remain in compliance with both the new and old regulations. Even if you hold regular safety meetings – which often is not the case – it is always a good idea to occasionally conduct a comprehensive review, which could save you a lot in fines.
So get your OSHA logs out, and walk through your office. Start by making sure you have an official OSHA poster, which enumerates employee rights and explains how to file complaints. Every office must have one posted in plain site, and is what an OSHA inspector will look for first. They are available for free at OSHA’s website or you can order one by calling 800-321-OSHA.
How long have you had your written exposure control plan for blood-borne pathogens? This plan should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, as well as your implementation of universal precautions. It should be updated annually to reflect changes in technology – and new threats, such as COVID-19.
Review your list of hazardous substances, which all employees have a right to know about. OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are classified as “hazardous.” Also remember that you’re probably using new disinfectants, which may need to be added to your list. For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
It is not necessary to adopt every new safety device as it comes on the market, but you should document which ones you are using and which ones you decide not to use – and why. For example, if you and your employees decide against buying a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it is worth, you still should document how you made that decision and why you believe that your current protocol is as good or better.
All at-risk employees should be provided with hepatitis B vaccine at no cost to them. And after any exposure to dangerous pathogens – which now include COVID-19 – you also must provide and pay for appropriate medical treatment and follow-up.
Another important consideration in your review: Electrical devices and their power sources in the office. All electrically powered equipment – medical or clerical – must operate safely and should all be examined. It is particularly important to check how wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Medical and dental offices are not required to keep an injury and illness log under federal OSHA regulations, which other businesses must. However, your state may have a requirement that supersedes the federal law so you should check with your state, or with your local OSHA office, regarding any such requirements.
It is important to take OSHA regulations seriously because failure to comply with them can result in stiff penalties running into many thousands of dollars.
To be certain you are complying with all the rules, you can call your local OSHA office and request an inspection. This is the easiest and cheapest way because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they discover.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
As more and more reopened medical practices ramp up toward normal activity, the safety of patients and health care workers alike remains paramount. As always,
Most of the modified guidelines are already familiar: wear masks (and other personal protective equipment as necessary); maintain social distancing; have hand cleaner, soap, and water readily available; and sanitize between patient examinations.
It is also important to remember that COVID-19 is now a reportable disease; check with your local health authorities as to where and how. Also remember that, if you decide to screen employees and/or patients for fevers and other symptoms of COVID-19, those data are subject to HIPAA rules and must be kept confidential.
Now might be a good time to confirm that you remain in compliance with both the new and old regulations. Even if you hold regular safety meetings – which often is not the case – it is always a good idea to occasionally conduct a comprehensive review, which could save you a lot in fines.
So get your OSHA logs out, and walk through your office. Start by making sure you have an official OSHA poster, which enumerates employee rights and explains how to file complaints. Every office must have one posted in plain site, and is what an OSHA inspector will look for first. They are available for free at OSHA’s website or you can order one by calling 800-321-OSHA.
How long have you had your written exposure control plan for blood-borne pathogens? This plan should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, as well as your implementation of universal precautions. It should be updated annually to reflect changes in technology – and new threats, such as COVID-19.
Review your list of hazardous substances, which all employees have a right to know about. OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are classified as “hazardous.” Also remember that you’re probably using new disinfectants, which may need to be added to your list. For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
It is not necessary to adopt every new safety device as it comes on the market, but you should document which ones you are using and which ones you decide not to use – and why. For example, if you and your employees decide against buying a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it is worth, you still should document how you made that decision and why you believe that your current protocol is as good or better.
All at-risk employees should be provided with hepatitis B vaccine at no cost to them. And after any exposure to dangerous pathogens – which now include COVID-19 – you also must provide and pay for appropriate medical treatment and follow-up.
Another important consideration in your review: Electrical devices and their power sources in the office. All electrically powered equipment – medical or clerical – must operate safely and should all be examined. It is particularly important to check how wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Medical and dental offices are not required to keep an injury and illness log under federal OSHA regulations, which other businesses must. However, your state may have a requirement that supersedes the federal law so you should check with your state, or with your local OSHA office, regarding any such requirements.
It is important to take OSHA regulations seriously because failure to comply with them can result in stiff penalties running into many thousands of dollars.
To be certain you are complying with all the rules, you can call your local OSHA office and request an inspection. This is the easiest and cheapest way because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they discover.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
As more and more reopened medical practices ramp up toward normal activity, the safety of patients and health care workers alike remains paramount. As always,
Most of the modified guidelines are already familiar: wear masks (and other personal protective equipment as necessary); maintain social distancing; have hand cleaner, soap, and water readily available; and sanitize between patient examinations.
It is also important to remember that COVID-19 is now a reportable disease; check with your local health authorities as to where and how. Also remember that, if you decide to screen employees and/or patients for fevers and other symptoms of COVID-19, those data are subject to HIPAA rules and must be kept confidential.
Now might be a good time to confirm that you remain in compliance with both the new and old regulations. Even if you hold regular safety meetings – which often is not the case – it is always a good idea to occasionally conduct a comprehensive review, which could save you a lot in fines.
So get your OSHA logs out, and walk through your office. Start by making sure you have an official OSHA poster, which enumerates employee rights and explains how to file complaints. Every office must have one posted in plain site, and is what an OSHA inspector will look for first. They are available for free at OSHA’s website or you can order one by calling 800-321-OSHA.
How long have you had your written exposure control plan for blood-borne pathogens? This plan should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, as well as your implementation of universal precautions. It should be updated annually to reflect changes in technology – and new threats, such as COVID-19.
Review your list of hazardous substances, which all employees have a right to know about. OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are classified as “hazardous.” Also remember that you’re probably using new disinfectants, which may need to be added to your list. For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
It is not necessary to adopt every new safety device as it comes on the market, but you should document which ones you are using and which ones you decide not to use – and why. For example, if you and your employees decide against buying a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it is worth, you still should document how you made that decision and why you believe that your current protocol is as good or better.
All at-risk employees should be provided with hepatitis B vaccine at no cost to them. And after any exposure to dangerous pathogens – which now include COVID-19 – you also must provide and pay for appropriate medical treatment and follow-up.
Another important consideration in your review: Electrical devices and their power sources in the office. All electrically powered equipment – medical or clerical – must operate safely and should all be examined. It is particularly important to check how wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Medical and dental offices are not required to keep an injury and illness log under federal OSHA regulations, which other businesses must. However, your state may have a requirement that supersedes the federal law so you should check with your state, or with your local OSHA office, regarding any such requirements.
It is important to take OSHA regulations seriously because failure to comply with them can result in stiff penalties running into many thousands of dollars.
To be certain you are complying with all the rules, you can call your local OSHA office and request an inspection. This is the easiest and cheapest way because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they discover.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Medics with ‘long COVID’ call for clinical recognition
Thousands of coronavirus patients risk going without treatment and support for debilitating symptoms lasting months because of a lack of awareness of ‘long COVID’, according to a group formed by clinicians with extended serious after-effects of the virus.
Many members of the 100-strong Facebook group UK doctors: COVID “Long tail” have been unable to work for weeks after failing to recover from an episode of COVID-19. They warn of the need for clinical recognition of “long COVID,” along with systems to log symptoms and manage patients in the community. Without this, there could be major consequences for return to work across all professions, as well as implications for disease prevention.
‘Weird symptoms’
Three of the group: Dr Amali Lokugamage, consultant obstetrician at the Whittington Hospital; Dr Sharon Taylor, child psychiatrist at St Mary’s Hospital London, and Dr Clare Rayner, a retired occupational health physician and lecturer at the University of Manchester, have highlighted their concerns in The BMJ and on social media groups. They say colleagues are observing a range of symptoms of long COVID in their practices.
These include cardiac, gut and respiratory symptoms, skin manifestations, neurological and psychiatric symptoms, severe fatigue, and relapsing fevers, sometimes continuing for more than 16 weeks, and which they say go well beyond definitions of chronic fatigue. The authors are also aware of a pattern of symptom clusters recurring every third or fourth day, which in some cases are so severe that people are having to take extended periods of sick leave.
Writing in The BMJ the authors say: “Concerns have been raised about the lack of awareness among NHS doctors, nurses, paramedics, and other healthcare professionals with regard to the prolonged, varied, and weird symptoms [of COVID-19].”
Speaking to Medscape News UK, Dr. Clare Rayner said: “We see a huge need that is not being met, because these cases are just not being seen in hospital. All the attention has been on the acute illness.”
She pointed to the urgent need for government planning for a surge in people requiring support to return to work following long-term COVID-19 symptoms. According to occupational health research, only 10-40% of people who take 6 weeks off work return to work, dropping to 5%-10% after an absence of 6 months.
In her own case, she is recovering after 4 months of illness, including a hospital admission with gut symptoms and dehydration, and 2 weeks of social service home support. She has experienced a range of relapsing and remitting symptoms, which she describes as ‘bizarre and coming in phases’.
Stimulating recovery
The recently-announced NHS portal for COVID-19 patients has been welcomed by the authors as an opportunity for long-standing symptoms to reach the medical and Government radar. But Dr Taylor believes it should have been set up from the start with input from patients with symptoms, to make sure that any support provided reflects the nature of the problems experienced.
In her case, as a previously regular gym attender with a resting heart rate in the 50s, she has now been diagnosed as having multi-organ disease affecting her heart, spleen, lung, and autonomic system. She has fluid on the lungs and heart, and suffers from continuous chest pain and oxygen desaturation when lying down. She has not been able to work since she contracted COVID-19 in March.
“COVID patients with the chronic form of the disease need to be involved in research right from the start to ensure the right questions are asked - not just those who have had acute disease,” she insists to Medscape News UK. “We need to gather evidence, to inform the development of a multi-disciplinary approach and a range of rehabilitation options depending on the organs involved.
“The focus needs to be on stimulating recovery and preventing development of chronic problems. We still don’t know if those with chronic COVID disease are infectious, how long their prolonged cardio-respiratory and neurological complications will last, and crucially whether treatment will reduce the duration of their problems. The worry is that left unattended, these patients may develop irreversible damage leading to chronic illness.”
General practice
GPs have been at the forefront of management of the long-standing consequences of COVID-19. In its recent report General practice in the post-COVID world, the Royal College of General Practitioners highlights the need for urgent government planning and funding to prepare general practice services for facilitating the recovery of local communities.
The report calls on the four governments of the UK each to produce a comprehensive plan to support GPs in managing the longer-term effects of COVID-19 in the community, including costed proposals for additional funding for general practice; workforce solutions; reductions in regulatory burdens and ‘red tape’; a systematic approach for identifying patients most likely to need primary care support, and proposals for how health inequalities will be minimized to ensure all patients have access to the necessary post-COVID-19 care.
RCGP Chair Professor Martin Marshall said: “COVID-19 will leave a lingering and difficult legacy and it is GPs working with patients in their communities who will be picking up the pieces.”
One issue is the lack of a reliable estimate of the prevalence of post viral symptoms for other viruses, let alone for COVID-19. Even a 1% chance of long-term problems amongst survivors would suggest 2500 with a need for extra support, but experience with post-viral syndrome generally suggests the prevalence may be more like 3%.
The BMA has been carrying out tracker surveys of its own members at 2-week intervals since March. The most recent, involving more than 5000 doctors, indicated that around 30% of doctors who believed they’d had COVID-19 were still experiencing physical symptoms they thought were caused by the virus, 21% had taken sick leave, and a further 9% had taken annual leave to deal with ongoing symptoms.
Dr David Strain, chair of the BMA medical academic staff committee and clinical senior lecturer at the University of Exeter Medical School, has a particular interest in the after-effects of COVID-19. He said it was becoming evident that the virus was leaving a lasting legacy with a significant number of people, even younger ones.
He told Medscape News UK: “Once COVID-19 enters the nervous system, the lasting symptoms on people can range from a mild loss of sense of smell or taste, to more severe symptoms such as difficulties in concentration. A small number have also been left with chronic fatigue syndrome, which is poorly understood, and can be difficult to treat. This does not appear to be dependent on the initial severity of COVID-19 symptoms.
“Currently, it is impossible to predict the prevalence of longer-lasting effects. A full assessment of COVID-19’s impact will only be possible once people return to work on a regular basis and the effect on their physical health becomes evident. Of the doctors in the BMA survey who had experienced COVID-19, 15% took sick leave beyond their acute illness, and another 6% used annual leave allowance to extend their recovery time.
“Clearly, more research will be needed into the long-term consequences of COVID-19 and the future treatments needed to deal with them.”
Further research
The National Institute for Health Research (NIHR) has called for applications for research to enhance understanding and management of the health and social care consequences of the global COVID-19 pandemic beyond the acute phase, with a particular focus on ‘health outcomes, public health, social care and health service delivery and to mitigate the impact of subsequent phases and aftermath’.
The authors of The BMJ article stress the wide-ranging nature of ‘long COVID’ symptoms and warn of the dangers of treating them for research purposes under the banner of chronic fatigue. They say: “These wide-ranging, unusual, and potentially very serious symptoms can be anxiety-provoking, particularly secondary to a virus that has only been known to the world for 8 months and which we have barely begun to understand. However, it is dismissive solely to attribute such symptoms to anxiety in the thousands of patients like ourselves who have attended hospital or general practice with chronic COVID-19.”
This article first appeared on Medscape.com.
Thousands of coronavirus patients risk going without treatment and support for debilitating symptoms lasting months because of a lack of awareness of ‘long COVID’, according to a group formed by clinicians with extended serious after-effects of the virus.
Many members of the 100-strong Facebook group UK doctors: COVID “Long tail” have been unable to work for weeks after failing to recover from an episode of COVID-19. They warn of the need for clinical recognition of “long COVID,” along with systems to log symptoms and manage patients in the community. Without this, there could be major consequences for return to work across all professions, as well as implications for disease prevention.
‘Weird symptoms’
Three of the group: Dr Amali Lokugamage, consultant obstetrician at the Whittington Hospital; Dr Sharon Taylor, child psychiatrist at St Mary’s Hospital London, and Dr Clare Rayner, a retired occupational health physician and lecturer at the University of Manchester, have highlighted their concerns in The BMJ and on social media groups. They say colleagues are observing a range of symptoms of long COVID in their practices.
These include cardiac, gut and respiratory symptoms, skin manifestations, neurological and psychiatric symptoms, severe fatigue, and relapsing fevers, sometimes continuing for more than 16 weeks, and which they say go well beyond definitions of chronic fatigue. The authors are also aware of a pattern of symptom clusters recurring every third or fourth day, which in some cases are so severe that people are having to take extended periods of sick leave.
Writing in The BMJ the authors say: “Concerns have been raised about the lack of awareness among NHS doctors, nurses, paramedics, and other healthcare professionals with regard to the prolonged, varied, and weird symptoms [of COVID-19].”
Speaking to Medscape News UK, Dr. Clare Rayner said: “We see a huge need that is not being met, because these cases are just not being seen in hospital. All the attention has been on the acute illness.”
She pointed to the urgent need for government planning for a surge in people requiring support to return to work following long-term COVID-19 symptoms. According to occupational health research, only 10-40% of people who take 6 weeks off work return to work, dropping to 5%-10% after an absence of 6 months.
In her own case, she is recovering after 4 months of illness, including a hospital admission with gut symptoms and dehydration, and 2 weeks of social service home support. She has experienced a range of relapsing and remitting symptoms, which she describes as ‘bizarre and coming in phases’.
Stimulating recovery
The recently-announced NHS portal for COVID-19 patients has been welcomed by the authors as an opportunity for long-standing symptoms to reach the medical and Government radar. But Dr Taylor believes it should have been set up from the start with input from patients with symptoms, to make sure that any support provided reflects the nature of the problems experienced.
In her case, as a previously regular gym attender with a resting heart rate in the 50s, she has now been diagnosed as having multi-organ disease affecting her heart, spleen, lung, and autonomic system. She has fluid on the lungs and heart, and suffers from continuous chest pain and oxygen desaturation when lying down. She has not been able to work since she contracted COVID-19 in March.
“COVID patients with the chronic form of the disease need to be involved in research right from the start to ensure the right questions are asked - not just those who have had acute disease,” she insists to Medscape News UK. “We need to gather evidence, to inform the development of a multi-disciplinary approach and a range of rehabilitation options depending on the organs involved.
“The focus needs to be on stimulating recovery and preventing development of chronic problems. We still don’t know if those with chronic COVID disease are infectious, how long their prolonged cardio-respiratory and neurological complications will last, and crucially whether treatment will reduce the duration of their problems. The worry is that left unattended, these patients may develop irreversible damage leading to chronic illness.”
General practice
GPs have been at the forefront of management of the long-standing consequences of COVID-19. In its recent report General practice in the post-COVID world, the Royal College of General Practitioners highlights the need for urgent government planning and funding to prepare general practice services for facilitating the recovery of local communities.
The report calls on the four governments of the UK each to produce a comprehensive plan to support GPs in managing the longer-term effects of COVID-19 in the community, including costed proposals for additional funding for general practice; workforce solutions; reductions in regulatory burdens and ‘red tape’; a systematic approach for identifying patients most likely to need primary care support, and proposals for how health inequalities will be minimized to ensure all patients have access to the necessary post-COVID-19 care.
RCGP Chair Professor Martin Marshall said: “COVID-19 will leave a lingering and difficult legacy and it is GPs working with patients in their communities who will be picking up the pieces.”
One issue is the lack of a reliable estimate of the prevalence of post viral symptoms for other viruses, let alone for COVID-19. Even a 1% chance of long-term problems amongst survivors would suggest 2500 with a need for extra support, but experience with post-viral syndrome generally suggests the prevalence may be more like 3%.
The BMA has been carrying out tracker surveys of its own members at 2-week intervals since March. The most recent, involving more than 5000 doctors, indicated that around 30% of doctors who believed they’d had COVID-19 were still experiencing physical symptoms they thought were caused by the virus, 21% had taken sick leave, and a further 9% had taken annual leave to deal with ongoing symptoms.
Dr David Strain, chair of the BMA medical academic staff committee and clinical senior lecturer at the University of Exeter Medical School, has a particular interest in the after-effects of COVID-19. He said it was becoming evident that the virus was leaving a lasting legacy with a significant number of people, even younger ones.
He told Medscape News UK: “Once COVID-19 enters the nervous system, the lasting symptoms on people can range from a mild loss of sense of smell or taste, to more severe symptoms such as difficulties in concentration. A small number have also been left with chronic fatigue syndrome, which is poorly understood, and can be difficult to treat. This does not appear to be dependent on the initial severity of COVID-19 symptoms.
“Currently, it is impossible to predict the prevalence of longer-lasting effects. A full assessment of COVID-19’s impact will only be possible once people return to work on a regular basis and the effect on their physical health becomes evident. Of the doctors in the BMA survey who had experienced COVID-19, 15% took sick leave beyond their acute illness, and another 6% used annual leave allowance to extend their recovery time.
“Clearly, more research will be needed into the long-term consequences of COVID-19 and the future treatments needed to deal with them.”
Further research
The National Institute for Health Research (NIHR) has called for applications for research to enhance understanding and management of the health and social care consequences of the global COVID-19 pandemic beyond the acute phase, with a particular focus on ‘health outcomes, public health, social care and health service delivery and to mitigate the impact of subsequent phases and aftermath’.
The authors of The BMJ article stress the wide-ranging nature of ‘long COVID’ symptoms and warn of the dangers of treating them for research purposes under the banner of chronic fatigue. They say: “These wide-ranging, unusual, and potentially very serious symptoms can be anxiety-provoking, particularly secondary to a virus that has only been known to the world for 8 months and which we have barely begun to understand. However, it is dismissive solely to attribute such symptoms to anxiety in the thousands of patients like ourselves who have attended hospital or general practice with chronic COVID-19.”
This article first appeared on Medscape.com.
Thousands of coronavirus patients risk going without treatment and support for debilitating symptoms lasting months because of a lack of awareness of ‘long COVID’, according to a group formed by clinicians with extended serious after-effects of the virus.
Many members of the 100-strong Facebook group UK doctors: COVID “Long tail” have been unable to work for weeks after failing to recover from an episode of COVID-19. They warn of the need for clinical recognition of “long COVID,” along with systems to log symptoms and manage patients in the community. Without this, there could be major consequences for return to work across all professions, as well as implications for disease prevention.
‘Weird symptoms’
Three of the group: Dr Amali Lokugamage, consultant obstetrician at the Whittington Hospital; Dr Sharon Taylor, child psychiatrist at St Mary’s Hospital London, and Dr Clare Rayner, a retired occupational health physician and lecturer at the University of Manchester, have highlighted their concerns in The BMJ and on social media groups. They say colleagues are observing a range of symptoms of long COVID in their practices.
These include cardiac, gut and respiratory symptoms, skin manifestations, neurological and psychiatric symptoms, severe fatigue, and relapsing fevers, sometimes continuing for more than 16 weeks, and which they say go well beyond definitions of chronic fatigue. The authors are also aware of a pattern of symptom clusters recurring every third or fourth day, which in some cases are so severe that people are having to take extended periods of sick leave.
Writing in The BMJ the authors say: “Concerns have been raised about the lack of awareness among NHS doctors, nurses, paramedics, and other healthcare professionals with regard to the prolonged, varied, and weird symptoms [of COVID-19].”
Speaking to Medscape News UK, Dr. Clare Rayner said: “We see a huge need that is not being met, because these cases are just not being seen in hospital. All the attention has been on the acute illness.”
She pointed to the urgent need for government planning for a surge in people requiring support to return to work following long-term COVID-19 symptoms. According to occupational health research, only 10-40% of people who take 6 weeks off work return to work, dropping to 5%-10% after an absence of 6 months.
In her own case, she is recovering after 4 months of illness, including a hospital admission with gut symptoms and dehydration, and 2 weeks of social service home support. She has experienced a range of relapsing and remitting symptoms, which she describes as ‘bizarre and coming in phases’.
Stimulating recovery
The recently-announced NHS portal for COVID-19 patients has been welcomed by the authors as an opportunity for long-standing symptoms to reach the medical and Government radar. But Dr Taylor believes it should have been set up from the start with input from patients with symptoms, to make sure that any support provided reflects the nature of the problems experienced.
In her case, as a previously regular gym attender with a resting heart rate in the 50s, she has now been diagnosed as having multi-organ disease affecting her heart, spleen, lung, and autonomic system. She has fluid on the lungs and heart, and suffers from continuous chest pain and oxygen desaturation when lying down. She has not been able to work since she contracted COVID-19 in March.
“COVID patients with the chronic form of the disease need to be involved in research right from the start to ensure the right questions are asked - not just those who have had acute disease,” she insists to Medscape News UK. “We need to gather evidence, to inform the development of a multi-disciplinary approach and a range of rehabilitation options depending on the organs involved.
“The focus needs to be on stimulating recovery and preventing development of chronic problems. We still don’t know if those with chronic COVID disease are infectious, how long their prolonged cardio-respiratory and neurological complications will last, and crucially whether treatment will reduce the duration of their problems. The worry is that left unattended, these patients may develop irreversible damage leading to chronic illness.”
General practice
GPs have been at the forefront of management of the long-standing consequences of COVID-19. In its recent report General practice in the post-COVID world, the Royal College of General Practitioners highlights the need for urgent government planning and funding to prepare general practice services for facilitating the recovery of local communities.
The report calls on the four governments of the UK each to produce a comprehensive plan to support GPs in managing the longer-term effects of COVID-19 in the community, including costed proposals for additional funding for general practice; workforce solutions; reductions in regulatory burdens and ‘red tape’; a systematic approach for identifying patients most likely to need primary care support, and proposals for how health inequalities will be minimized to ensure all patients have access to the necessary post-COVID-19 care.
RCGP Chair Professor Martin Marshall said: “COVID-19 will leave a lingering and difficult legacy and it is GPs working with patients in their communities who will be picking up the pieces.”
One issue is the lack of a reliable estimate of the prevalence of post viral symptoms for other viruses, let alone for COVID-19. Even a 1% chance of long-term problems amongst survivors would suggest 2500 with a need for extra support, but experience with post-viral syndrome generally suggests the prevalence may be more like 3%.
The BMA has been carrying out tracker surveys of its own members at 2-week intervals since March. The most recent, involving more than 5000 doctors, indicated that around 30% of doctors who believed they’d had COVID-19 were still experiencing physical symptoms they thought were caused by the virus, 21% had taken sick leave, and a further 9% had taken annual leave to deal with ongoing symptoms.
Dr David Strain, chair of the BMA medical academic staff committee and clinical senior lecturer at the University of Exeter Medical School, has a particular interest in the after-effects of COVID-19. He said it was becoming evident that the virus was leaving a lasting legacy with a significant number of people, even younger ones.
He told Medscape News UK: “Once COVID-19 enters the nervous system, the lasting symptoms on people can range from a mild loss of sense of smell or taste, to more severe symptoms such as difficulties in concentration. A small number have also been left with chronic fatigue syndrome, which is poorly understood, and can be difficult to treat. This does not appear to be dependent on the initial severity of COVID-19 symptoms.
“Currently, it is impossible to predict the prevalence of longer-lasting effects. A full assessment of COVID-19’s impact will only be possible once people return to work on a regular basis and the effect on their physical health becomes evident. Of the doctors in the BMA survey who had experienced COVID-19, 15% took sick leave beyond their acute illness, and another 6% used annual leave allowance to extend their recovery time.
“Clearly, more research will be needed into the long-term consequences of COVID-19 and the future treatments needed to deal with them.”
Further research
The National Institute for Health Research (NIHR) has called for applications for research to enhance understanding and management of the health and social care consequences of the global COVID-19 pandemic beyond the acute phase, with a particular focus on ‘health outcomes, public health, social care and health service delivery and to mitigate the impact of subsequent phases and aftermath’.
The authors of The BMJ article stress the wide-ranging nature of ‘long COVID’ symptoms and warn of the dangers of treating them for research purposes under the banner of chronic fatigue. They say: “These wide-ranging, unusual, and potentially very serious symptoms can be anxiety-provoking, particularly secondary to a virus that has only been known to the world for 8 months and which we have barely begun to understand. However, it is dismissive solely to attribute such symptoms to anxiety in the thousands of patients like ourselves who have attended hospital or general practice with chronic COVID-19.”
This article first appeared on Medscape.com.
Quitting smoking after MI has huge benefits in young adults
Young adult smokers who stop smoking in the first year after an initial myocardial infarction are far less likely to die over the next 10 years than their peers who continue to smoke. Yet nearly two-thirds keep smoking after the event, according to new data from the Partners YOUNG-MI Registry.
“Smoking is one of the most common risk factors for developing an MI at a young age. ... This reinforces the need to have more young individuals avoid, or quit, the use of tobacco,” Ron Blankstein, MD, Brigham and Women’s Hospital and Harvard Medical School, Boston, said in an interview.
Yet, the finding that 62% of young adults continue to smoke 1 year after MI points to an “enormous need for better smoking cessation efforts following a heart attack,” he said.
“Powerful” message for clinicians
“This study joins an incredibly powerful body of evidence that says if you quit smoking, you’re going to live longer,” said Michael Fiore, MD, MPH, MBA, director of the University of Wisconsin Center for Tobacco Research and Intervention, Madison, who wasn’t involved in the study.
“As physicians, there is nothing we can do that will have a greater impact for our patients than quitting smoking. The study is a powerful call for clinicians to intervene with their patients that smoke – both if you have an MI or if you don’t,” Dr. Fiore told this news organization.
The study involved 2,072 individuals 50 years or younger (median age, 45 years; 81% male) who were hospitalized for an initial MI at two large academic medical centers in Boston. Of these, 33.9% were never-smokers, 13.6% were former smokers, and 52.5% were smokers at the time of their MI.
During a median follow-up of 10.2 years, those who quit smoking had a significantly lower rate of death from any cause (unadjusted hazard ratio, 0.35; 95% confidence interval, 0.19-0.63; P < .001) and a cardiovascular cause (HR, 0.29; 95% CI, 0.11-0.79; P = .02), relative to those who continued to smoke.
The results remained statistically significant in a propensity-matched analysis for both all-cause (HR, 0.30; 95% CI, 0.16-0.56; P < .001) and CV mortality (HR, 0.19; 95% CI, 0.06-0.56; P = .003).
“Although patients who quit smoking were similar to those who continued to smoke with respect to their baseline characteristics, smoking cessation was associated with an approximate 70%-80% reduction in all-cause and CV mortality,” the authors note in their article, published online July 8 in JAMA Network Open.
They say it’s also noteworthy that long-term death rates of never-smokers and former smokers who quit before the MI were nearly identical.
‘A failure of our health care system’
The bottom line, said Dr. Blankstein, is that it is “never too late to quit, and those who experience an MI should do so right away. Our health care system must help promote such efforts, as there is immense room for improvement.”
Dr. Fiore said: “When I see an article like this, it just reminds me that, if you’re really thinking about staying healthy, there is nothing better you can do to improve the quality and longevity of your life than quitting smoking.”
The observation that many patients continue to smoke after MI is a “failure of our health care system, and it’s an individual failure in that these individuals are not able to overcome their powerful nicotine dependence. It’s an unfortunate occurrence that’s resulting in unnecessary deaths,” said Dr. Fiore.
There is no “magic bullet” to overcome nicotine addiction, but there are approved treatments that can “substantially boost quit rates,” he noted.
The two most effective smoking-cessation treatments are varenicline (Chantix) and combination nicotine replacement therapy, a patch combined ideally with nicotine mini lozenges, particularly when combined with some brief counseling, said Fiore.
He encourages cardiologists to get their patients to commit to quitting and then link them to resources such as 1-800-QUIT-NOW or SmokeFree.gov.
Funding for the study was provided by grants from the National Heart, Lung, and Blood Institute. Dr. Blankstein reported receiving research support from Amgen and Astellas. Dr. Fiore had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Young adult smokers who stop smoking in the first year after an initial myocardial infarction are far less likely to die over the next 10 years than their peers who continue to smoke. Yet nearly two-thirds keep smoking after the event, according to new data from the Partners YOUNG-MI Registry.
“Smoking is one of the most common risk factors for developing an MI at a young age. ... This reinforces the need to have more young individuals avoid, or quit, the use of tobacco,” Ron Blankstein, MD, Brigham and Women’s Hospital and Harvard Medical School, Boston, said in an interview.
Yet, the finding that 62% of young adults continue to smoke 1 year after MI points to an “enormous need for better smoking cessation efforts following a heart attack,” he said.
“Powerful” message for clinicians
“This study joins an incredibly powerful body of evidence that says if you quit smoking, you’re going to live longer,” said Michael Fiore, MD, MPH, MBA, director of the University of Wisconsin Center for Tobacco Research and Intervention, Madison, who wasn’t involved in the study.
“As physicians, there is nothing we can do that will have a greater impact for our patients than quitting smoking. The study is a powerful call for clinicians to intervene with their patients that smoke – both if you have an MI or if you don’t,” Dr. Fiore told this news organization.
The study involved 2,072 individuals 50 years or younger (median age, 45 years; 81% male) who were hospitalized for an initial MI at two large academic medical centers in Boston. Of these, 33.9% were never-smokers, 13.6% were former smokers, and 52.5% were smokers at the time of their MI.
During a median follow-up of 10.2 years, those who quit smoking had a significantly lower rate of death from any cause (unadjusted hazard ratio, 0.35; 95% confidence interval, 0.19-0.63; P < .001) and a cardiovascular cause (HR, 0.29; 95% CI, 0.11-0.79; P = .02), relative to those who continued to smoke.
The results remained statistically significant in a propensity-matched analysis for both all-cause (HR, 0.30; 95% CI, 0.16-0.56; P < .001) and CV mortality (HR, 0.19; 95% CI, 0.06-0.56; P = .003).
“Although patients who quit smoking were similar to those who continued to smoke with respect to their baseline characteristics, smoking cessation was associated with an approximate 70%-80% reduction in all-cause and CV mortality,” the authors note in their article, published online July 8 in JAMA Network Open.
They say it’s also noteworthy that long-term death rates of never-smokers and former smokers who quit before the MI were nearly identical.
‘A failure of our health care system’
The bottom line, said Dr. Blankstein, is that it is “never too late to quit, and those who experience an MI should do so right away. Our health care system must help promote such efforts, as there is immense room for improvement.”
Dr. Fiore said: “When I see an article like this, it just reminds me that, if you’re really thinking about staying healthy, there is nothing better you can do to improve the quality and longevity of your life than quitting smoking.”
The observation that many patients continue to smoke after MI is a “failure of our health care system, and it’s an individual failure in that these individuals are not able to overcome their powerful nicotine dependence. It’s an unfortunate occurrence that’s resulting in unnecessary deaths,” said Dr. Fiore.
There is no “magic bullet” to overcome nicotine addiction, but there are approved treatments that can “substantially boost quit rates,” he noted.
The two most effective smoking-cessation treatments are varenicline (Chantix) and combination nicotine replacement therapy, a patch combined ideally with nicotine mini lozenges, particularly when combined with some brief counseling, said Fiore.
He encourages cardiologists to get their patients to commit to quitting and then link them to resources such as 1-800-QUIT-NOW or SmokeFree.gov.
Funding for the study was provided by grants from the National Heart, Lung, and Blood Institute. Dr. Blankstein reported receiving research support from Amgen and Astellas. Dr. Fiore had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Young adult smokers who stop smoking in the first year after an initial myocardial infarction are far less likely to die over the next 10 years than their peers who continue to smoke. Yet nearly two-thirds keep smoking after the event, according to new data from the Partners YOUNG-MI Registry.
“Smoking is one of the most common risk factors for developing an MI at a young age. ... This reinforces the need to have more young individuals avoid, or quit, the use of tobacco,” Ron Blankstein, MD, Brigham and Women’s Hospital and Harvard Medical School, Boston, said in an interview.
Yet, the finding that 62% of young adults continue to smoke 1 year after MI points to an “enormous need for better smoking cessation efforts following a heart attack,” he said.
“Powerful” message for clinicians
“This study joins an incredibly powerful body of evidence that says if you quit smoking, you’re going to live longer,” said Michael Fiore, MD, MPH, MBA, director of the University of Wisconsin Center for Tobacco Research and Intervention, Madison, who wasn’t involved in the study.
“As physicians, there is nothing we can do that will have a greater impact for our patients than quitting smoking. The study is a powerful call for clinicians to intervene with their patients that smoke – both if you have an MI or if you don’t,” Dr. Fiore told this news organization.
The study involved 2,072 individuals 50 years or younger (median age, 45 years; 81% male) who were hospitalized for an initial MI at two large academic medical centers in Boston. Of these, 33.9% were never-smokers, 13.6% were former smokers, and 52.5% were smokers at the time of their MI.
During a median follow-up of 10.2 years, those who quit smoking had a significantly lower rate of death from any cause (unadjusted hazard ratio, 0.35; 95% confidence interval, 0.19-0.63; P < .001) and a cardiovascular cause (HR, 0.29; 95% CI, 0.11-0.79; P = .02), relative to those who continued to smoke.
The results remained statistically significant in a propensity-matched analysis for both all-cause (HR, 0.30; 95% CI, 0.16-0.56; P < .001) and CV mortality (HR, 0.19; 95% CI, 0.06-0.56; P = .003).
“Although patients who quit smoking were similar to those who continued to smoke with respect to their baseline characteristics, smoking cessation was associated with an approximate 70%-80% reduction in all-cause and CV mortality,” the authors note in their article, published online July 8 in JAMA Network Open.
They say it’s also noteworthy that long-term death rates of never-smokers and former smokers who quit before the MI were nearly identical.
‘A failure of our health care system’
The bottom line, said Dr. Blankstein, is that it is “never too late to quit, and those who experience an MI should do so right away. Our health care system must help promote such efforts, as there is immense room for improvement.”
Dr. Fiore said: “When I see an article like this, it just reminds me that, if you’re really thinking about staying healthy, there is nothing better you can do to improve the quality and longevity of your life than quitting smoking.”
The observation that many patients continue to smoke after MI is a “failure of our health care system, and it’s an individual failure in that these individuals are not able to overcome their powerful nicotine dependence. It’s an unfortunate occurrence that’s resulting in unnecessary deaths,” said Dr. Fiore.
There is no “magic bullet” to overcome nicotine addiction, but there are approved treatments that can “substantially boost quit rates,” he noted.
The two most effective smoking-cessation treatments are varenicline (Chantix) and combination nicotine replacement therapy, a patch combined ideally with nicotine mini lozenges, particularly when combined with some brief counseling, said Fiore.
He encourages cardiologists to get their patients to commit to quitting and then link them to resources such as 1-800-QUIT-NOW or SmokeFree.gov.
Funding for the study was provided by grants from the National Heart, Lung, and Blood Institute. Dr. Blankstein reported receiving research support from Amgen and Astellas. Dr. Fiore had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Socioeconomic status key factor in CPAP adherence in older adults
The benefits of continuous positive airway pressure therapy for patients with obstructive sleep apnea are well documented, but it only works if patients can adhere to the therapy.
A large national study of older Medicare patients with obstructive sleep apnea (OSA) has identified lower socioeconomic status and comorbidities as independent risk factors for nonadherence to continuous positive airway pressure (CPAP) therapy.
“[The] present results represent the largest study to date of rates and predictors of CPAP adherence among older adults in the United States. In our national sample of Medicare beneficiaries, adherence rates were generally lower than previously reported in smaller, clinic-based studies,” Emerson M. Wickwire, PhD, of the Sleep Disorders Center and division of pulmonary and critical care medicine at the University of Maryland, Baltimore, and colleagues wrote in Sleep.
Dr. Wickwire and colleagues estimated CPAP machine adherence using a 5% sample of Medicare claims data, identifying 3,229 Medicare beneficiaries with OSA who began CPAP therapy between 2009 and 2011. Individuals in the sample were aged at least 65 years with a new diagnosis of OSA, 88.1% of beneficiaries were white, and 52.3% were male.
The researchers applied objective adherence criteria set by the Centers for Medicare & Medicaid Services, which defines CPAP adherence as a patient using CPAP for at least 4 hours on 70% of nights, or CPAP use for 21 of 30 consecutive days within 90 days after beginning therapy.
Using CPAP machine charges as a measure of who adhered to therapy, they found 1,420 of 3,229 individuals (44%) achieved adherence under these criteria, which included making 13 monthly payments during their CPAP machine’s “rent-to-own” period. Partial adherence was found in 997 individuals (30.9%) who made between 4-12 payments on their CPAP machine, while 812 individuals (25.2%) made 4 payments or fewer on their CPAP machines, which the researchers classified as nonadherence. Nonadherers tended to be slightly younger (mean, 72.5 years vs. 79.2 years; P < .001) and had a higher number of comorbidities (35.2% vs. 30.4%; P = .002), compared with individuals with high adherence. Anxiety (odds ratio, 1.34; 95% confidence interval, 1.12-1.61), anemia (OR, 1.16; 95% CI, 1.02-1.32), fibromyalgia (OR, 1.19; 95% CI, 1.03-1.38), traumatic brain injury (OR, 1.58; 95% CI, 1.21-2.07), and Medicaid eligibility (OR, 1.48; 95% CI, 1.24-1.75) were all independently associated with lower CPAP adherence. Medicaid eligibility was considered an indicator of lower socioeconomic status.
Krishna M. Sundar, MD, FCCP, director at the Sleep-Wake Center in the University of Utah pulmonary division in Salt Lake City and CHEST Physician editorial board member, said in an interview that studies have shown early signs of adherence within the first few weeks are an important indicator of overall adherence to CPAP therapy. However, the use of CPAP machine payments in the study by Dr. Wickwire and colleagues was a novel way to track adherence.
Some of the issues with nonadherence may be related to challenges in using the technology, but it is the clinician’s role to communicate with patients about the effectiveness of CPAP and identifying reasons for nonadherence while also attempting to tease out the subtle socioeconomic factors related to nonadherence, Dr. Sundar noted. “We need to alter our practice to make sure that we communicate with these patients and better understand what are the social factors in getting the CPAP or utilizing CPAP, and also following these patients more closely, especially in the first month of starting CPAP therapy.
“Just because somebody has severe sleep apnea and other comorbid conditions does not mean that they’re going to wear the CPAP,” he said. “So, the fact that socioeconomic factors play an equal if not more important role in terms of predicting CPAP adherence. That is an important takeaway.”
Octavian C. Ioachimescu, MD, FCCP, of Emory University, Atlanta, and the Atlanta Veteran Affairs Administration and CHEST Physician editorial board member, said in an interview that the study raises a major question of what is next. “What can we offer to these patients, and what is the real-world compliance to that ‘next-best’ modality?” Dr. Ioachimescu said. “What are the outcomes of these individuals in the point-of-care environment, or ‘real world?’ ”
The analysis by the authors adds the perspective of a “real-world depiction of clinical care for patients with OSA,” Dr. Ioachimescu said. “One major lesson of such an analysis is that the health care goal setting that is referential to initial, randomized, well-controlled studies on highly selected patient populations need to be reassessed periodically from the point of view of actual results in the clinics.”
Clinicians may need to borrow ideas from other therapeutic fields to help improve patient adherence, he said. “[W]e may be able to develop and implement in the future peer involvement, behavioral and cognitive approaches, motivational enhancement interventions, as well as elements of acceptance and commitment techniques, all in the larger context of more integrated and in the same time individualized approaches to therapy.”
The investigators concluded that, “relative to Medicare-only beneficiaries, those eligible for both Medicare and Medicaid were significantly less likely to adhere to CPAP. Future research should seek to develop a deeper understanding of the mechanisms through which [socioeconomic status] and other social determinants impact patient experience throughout the OSA diagnostic and treatment process, including receiving, acclimating, and adhering to CPAP therapy.”
Dr. Sundar concurred with this assessment and said more research is needed on factors impacting adherence such as poverty, homelessness, and home support systems. “It’s not just coordinating with the patient. Clearly, more work is needed in understanding the social aspects of CPAP adherence.”
This study was funded in part by an investigator-initiated grant provided by ResMed to Dr. Wickmire’s institution, the University of Maryland, Baltimore. Dr. Wickmire reported being a scientific consultant to DayZz, Eisai, Merck, and Purdue and holds shares in WellTap. Dr. Oldstone is a ResMed employee and shareholder. Dr. Sundar reported being a cofounder of Hypnoscure, which creates software for population management of sleep apnea, and an investigator in trials where ResMed and Respironics devices were used. Dr. Ioachimescu reported no relevant financial disclosures.
SOURCE: Wickwire EM et al. Sleep. 2020 Jun 23. doi: 10.1093/sleep/zsaa122.
The benefits of continuous positive airway pressure therapy for patients with obstructive sleep apnea are well documented, but it only works if patients can adhere to the therapy.
A large national study of older Medicare patients with obstructive sleep apnea (OSA) has identified lower socioeconomic status and comorbidities as independent risk factors for nonadherence to continuous positive airway pressure (CPAP) therapy.
“[The] present results represent the largest study to date of rates and predictors of CPAP adherence among older adults in the United States. In our national sample of Medicare beneficiaries, adherence rates were generally lower than previously reported in smaller, clinic-based studies,” Emerson M. Wickwire, PhD, of the Sleep Disorders Center and division of pulmonary and critical care medicine at the University of Maryland, Baltimore, and colleagues wrote in Sleep.
Dr. Wickwire and colleagues estimated CPAP machine adherence using a 5% sample of Medicare claims data, identifying 3,229 Medicare beneficiaries with OSA who began CPAP therapy between 2009 and 2011. Individuals in the sample were aged at least 65 years with a new diagnosis of OSA, 88.1% of beneficiaries were white, and 52.3% were male.
The researchers applied objective adherence criteria set by the Centers for Medicare & Medicaid Services, which defines CPAP adherence as a patient using CPAP for at least 4 hours on 70% of nights, or CPAP use for 21 of 30 consecutive days within 90 days after beginning therapy.
Using CPAP machine charges as a measure of who adhered to therapy, they found 1,420 of 3,229 individuals (44%) achieved adherence under these criteria, which included making 13 monthly payments during their CPAP machine’s “rent-to-own” period. Partial adherence was found in 997 individuals (30.9%) who made between 4-12 payments on their CPAP machine, while 812 individuals (25.2%) made 4 payments or fewer on their CPAP machines, which the researchers classified as nonadherence. Nonadherers tended to be slightly younger (mean, 72.5 years vs. 79.2 years; P < .001) and had a higher number of comorbidities (35.2% vs. 30.4%; P = .002), compared with individuals with high adherence. Anxiety (odds ratio, 1.34; 95% confidence interval, 1.12-1.61), anemia (OR, 1.16; 95% CI, 1.02-1.32), fibromyalgia (OR, 1.19; 95% CI, 1.03-1.38), traumatic brain injury (OR, 1.58; 95% CI, 1.21-2.07), and Medicaid eligibility (OR, 1.48; 95% CI, 1.24-1.75) were all independently associated with lower CPAP adherence. Medicaid eligibility was considered an indicator of lower socioeconomic status.
Krishna M. Sundar, MD, FCCP, director at the Sleep-Wake Center in the University of Utah pulmonary division in Salt Lake City and CHEST Physician editorial board member, said in an interview that studies have shown early signs of adherence within the first few weeks are an important indicator of overall adherence to CPAP therapy. However, the use of CPAP machine payments in the study by Dr. Wickwire and colleagues was a novel way to track adherence.
Some of the issues with nonadherence may be related to challenges in using the technology, but it is the clinician’s role to communicate with patients about the effectiveness of CPAP and identifying reasons for nonadherence while also attempting to tease out the subtle socioeconomic factors related to nonadherence, Dr. Sundar noted. “We need to alter our practice to make sure that we communicate with these patients and better understand what are the social factors in getting the CPAP or utilizing CPAP, and also following these patients more closely, especially in the first month of starting CPAP therapy.
“Just because somebody has severe sleep apnea and other comorbid conditions does not mean that they’re going to wear the CPAP,” he said. “So, the fact that socioeconomic factors play an equal if not more important role in terms of predicting CPAP adherence. That is an important takeaway.”
Octavian C. Ioachimescu, MD, FCCP, of Emory University, Atlanta, and the Atlanta Veteran Affairs Administration and CHEST Physician editorial board member, said in an interview that the study raises a major question of what is next. “What can we offer to these patients, and what is the real-world compliance to that ‘next-best’ modality?” Dr. Ioachimescu said. “What are the outcomes of these individuals in the point-of-care environment, or ‘real world?’ ”
The analysis by the authors adds the perspective of a “real-world depiction of clinical care for patients with OSA,” Dr. Ioachimescu said. “One major lesson of such an analysis is that the health care goal setting that is referential to initial, randomized, well-controlled studies on highly selected patient populations need to be reassessed periodically from the point of view of actual results in the clinics.”
Clinicians may need to borrow ideas from other therapeutic fields to help improve patient adherence, he said. “[W]e may be able to develop and implement in the future peer involvement, behavioral and cognitive approaches, motivational enhancement interventions, as well as elements of acceptance and commitment techniques, all in the larger context of more integrated and in the same time individualized approaches to therapy.”
The investigators concluded that, “relative to Medicare-only beneficiaries, those eligible for both Medicare and Medicaid were significantly less likely to adhere to CPAP. Future research should seek to develop a deeper understanding of the mechanisms through which [socioeconomic status] and other social determinants impact patient experience throughout the OSA diagnostic and treatment process, including receiving, acclimating, and adhering to CPAP therapy.”
Dr. Sundar concurred with this assessment and said more research is needed on factors impacting adherence such as poverty, homelessness, and home support systems. “It’s not just coordinating with the patient. Clearly, more work is needed in understanding the social aspects of CPAP adherence.”
This study was funded in part by an investigator-initiated grant provided by ResMed to Dr. Wickmire’s institution, the University of Maryland, Baltimore. Dr. Wickmire reported being a scientific consultant to DayZz, Eisai, Merck, and Purdue and holds shares in WellTap. Dr. Oldstone is a ResMed employee and shareholder. Dr. Sundar reported being a cofounder of Hypnoscure, which creates software for population management of sleep apnea, and an investigator in trials where ResMed and Respironics devices were used. Dr. Ioachimescu reported no relevant financial disclosures.
SOURCE: Wickwire EM et al. Sleep. 2020 Jun 23. doi: 10.1093/sleep/zsaa122.
The benefits of continuous positive airway pressure therapy for patients with obstructive sleep apnea are well documented, but it only works if patients can adhere to the therapy.
A large national study of older Medicare patients with obstructive sleep apnea (OSA) has identified lower socioeconomic status and comorbidities as independent risk factors for nonadherence to continuous positive airway pressure (CPAP) therapy.
“[The] present results represent the largest study to date of rates and predictors of CPAP adherence among older adults in the United States. In our national sample of Medicare beneficiaries, adherence rates were generally lower than previously reported in smaller, clinic-based studies,” Emerson M. Wickwire, PhD, of the Sleep Disorders Center and division of pulmonary and critical care medicine at the University of Maryland, Baltimore, and colleagues wrote in Sleep.
Dr. Wickwire and colleagues estimated CPAP machine adherence using a 5% sample of Medicare claims data, identifying 3,229 Medicare beneficiaries with OSA who began CPAP therapy between 2009 and 2011. Individuals in the sample were aged at least 65 years with a new diagnosis of OSA, 88.1% of beneficiaries were white, and 52.3% were male.
The researchers applied objective adherence criteria set by the Centers for Medicare & Medicaid Services, which defines CPAP adherence as a patient using CPAP for at least 4 hours on 70% of nights, or CPAP use for 21 of 30 consecutive days within 90 days after beginning therapy.
Using CPAP machine charges as a measure of who adhered to therapy, they found 1,420 of 3,229 individuals (44%) achieved adherence under these criteria, which included making 13 monthly payments during their CPAP machine’s “rent-to-own” period. Partial adherence was found in 997 individuals (30.9%) who made between 4-12 payments on their CPAP machine, while 812 individuals (25.2%) made 4 payments or fewer on their CPAP machines, which the researchers classified as nonadherence. Nonadherers tended to be slightly younger (mean, 72.5 years vs. 79.2 years; P < .001) and had a higher number of comorbidities (35.2% vs. 30.4%; P = .002), compared with individuals with high adherence. Anxiety (odds ratio, 1.34; 95% confidence interval, 1.12-1.61), anemia (OR, 1.16; 95% CI, 1.02-1.32), fibromyalgia (OR, 1.19; 95% CI, 1.03-1.38), traumatic brain injury (OR, 1.58; 95% CI, 1.21-2.07), and Medicaid eligibility (OR, 1.48; 95% CI, 1.24-1.75) were all independently associated with lower CPAP adherence. Medicaid eligibility was considered an indicator of lower socioeconomic status.
Krishna M. Sundar, MD, FCCP, director at the Sleep-Wake Center in the University of Utah pulmonary division in Salt Lake City and CHEST Physician editorial board member, said in an interview that studies have shown early signs of adherence within the first few weeks are an important indicator of overall adherence to CPAP therapy. However, the use of CPAP machine payments in the study by Dr. Wickwire and colleagues was a novel way to track adherence.
Some of the issues with nonadherence may be related to challenges in using the technology, but it is the clinician’s role to communicate with patients about the effectiveness of CPAP and identifying reasons for nonadherence while also attempting to tease out the subtle socioeconomic factors related to nonadherence, Dr. Sundar noted. “We need to alter our practice to make sure that we communicate with these patients and better understand what are the social factors in getting the CPAP or utilizing CPAP, and also following these patients more closely, especially in the first month of starting CPAP therapy.
“Just because somebody has severe sleep apnea and other comorbid conditions does not mean that they’re going to wear the CPAP,” he said. “So, the fact that socioeconomic factors play an equal if not more important role in terms of predicting CPAP adherence. That is an important takeaway.”
Octavian C. Ioachimescu, MD, FCCP, of Emory University, Atlanta, and the Atlanta Veteran Affairs Administration and CHEST Physician editorial board member, said in an interview that the study raises a major question of what is next. “What can we offer to these patients, and what is the real-world compliance to that ‘next-best’ modality?” Dr. Ioachimescu said. “What are the outcomes of these individuals in the point-of-care environment, or ‘real world?’ ”
The analysis by the authors adds the perspective of a “real-world depiction of clinical care for patients with OSA,” Dr. Ioachimescu said. “One major lesson of such an analysis is that the health care goal setting that is referential to initial, randomized, well-controlled studies on highly selected patient populations need to be reassessed periodically from the point of view of actual results in the clinics.”
Clinicians may need to borrow ideas from other therapeutic fields to help improve patient adherence, he said. “[W]e may be able to develop and implement in the future peer involvement, behavioral and cognitive approaches, motivational enhancement interventions, as well as elements of acceptance and commitment techniques, all in the larger context of more integrated and in the same time individualized approaches to therapy.”
The investigators concluded that, “relative to Medicare-only beneficiaries, those eligible for both Medicare and Medicaid were significantly less likely to adhere to CPAP. Future research should seek to develop a deeper understanding of the mechanisms through which [socioeconomic status] and other social determinants impact patient experience throughout the OSA diagnostic and treatment process, including receiving, acclimating, and adhering to CPAP therapy.”
Dr. Sundar concurred with this assessment and said more research is needed on factors impacting adherence such as poverty, homelessness, and home support systems. “It’s not just coordinating with the patient. Clearly, more work is needed in understanding the social aspects of CPAP adherence.”
This study was funded in part by an investigator-initiated grant provided by ResMed to Dr. Wickmire’s institution, the University of Maryland, Baltimore. Dr. Wickmire reported being a scientific consultant to DayZz, Eisai, Merck, and Purdue and holds shares in WellTap. Dr. Oldstone is a ResMed employee and shareholder. Dr. Sundar reported being a cofounder of Hypnoscure, which creates software for population management of sleep apnea, and an investigator in trials where ResMed and Respironics devices were used. Dr. Ioachimescu reported no relevant financial disclosures.
SOURCE: Wickwire EM et al. Sleep. 2020 Jun 23. doi: 10.1093/sleep/zsaa122.
FROM SLEEP
Oxford coronavirus vaccine ‘triggers immune response’
The early stage results, published in The Lancet, found that the candidate vaccine, known as ChAdOx1 nCoV-19, provoked a T-cell response peaking 14 days after vaccination, and an antibody response within 28 days.
Andrew Pollard, chief investigator on the study, and professor of pediatric infection and immunity at Oxford University, described the results as “encouraging”. He told a briefing convened by the Science Media Centre on Monday that it was “a really important milestone on the path to the development of the vaccine”.
In the Commons, the Health Secretary, Matt Hancock, hailed the results for taking us “one step closer to finding a vaccine that can potentially save lives, all around the world”.
The trial, which has so far involved 1,077 healthy adults, caused minor side effects when compared with a control group given a meningitis vaccine. Fatigue and headache were the most commonly reported reactions.
However, there were no serious adverse events from the vaccine, the researchers said.
‘Still a long way to go’
Sarah Gilbert, lead researcher of the vaccine development program, and professor of vaccinology at Oxford, cautioned that there was still a long way to go before the team could confirm that the vaccine could protect against developing COVID-19.
“The difficulty that we have, and that all vaccine developers have in trying to make a vaccine against this particular virus, is that we don’t know how strong that immune response needs to be,” she said.
“So, we can’t say just by looking at immune responses whether this is going to protect people or not. And the only way we’re going to find out is by doing the large phase 3 trials and wait for people to be infected as part of that trial before we know if the vaccine can work.”
The authors noted some limitations to their findings. They said more research was needed to confirm their results in different groups of people – including older age groups, those with other health conditions, and in ethnically and geographically diverse populations.
A notable result of the trial was that participants given a second dose of the vaccine appeared to display a stronger immune response, a finding that had influenced plans to “look at two dose regimes as well as one dose regimes in the phase 3 trial”, Prof Adrian Hill, director of Oxford’s Jenner Institute, confirmed.
ChAdOx1 nCoV-19 is made from a weakened version of an adenovirus that causes infections in chimpanzees. The virus has been genetically modified so that it cannot grow in humans.
On Monday, the government announced that it had struck a deal with AstraZeneca for access to 100 million doses of the Oxford vaccine, in addition to millions of doses of other promising candidate vaccines.
Expert reaction to the findings
The Medical Research Council helped to fund the trial. Executive Chair Professor Fiona Watt commented: “It is truly remarkable how fast this vaccine has progressed, with our support, through early clinical trials, and it is very encouraging that it shows no safety concerns and evokes strong immune responses.
“There is a lot that we don’t yet know about immunity to the virus that causes COVID-19. However, it seems that both antibody and T cell immunity are important, and this vaccine triggers both responses. The much anticipated next milestone will be the results of the larger trials that are happening now to find out if the vaccine will protect people from the virus.”
Jonathan Ball, professor of molecular virology at the University of Nottingham, told the SMC: “The results of the Oxford chimp adenovirus vaccine candidate show that the vaccine is able to generate antibodies and T cells in humans and these persisted for several weeks. Whilst encouraging there is still a long way to go before we can herald the arrival of a successful coronavirus vaccine.
“It is unclear whether the levels of immunity can protect against infection – that’s what the larger ongoing phase III trials are designed to test. Nor do we know if this vaccine can protect those most vulnerable to severe COVID-19 disease.”
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene and Tropical Medicine, commented: “For the vaccine to be really useful, we not only need the larger studies conducted where COVID-19 is still occurring at a high rate, but we need to be reasonably sure that the protection lasts for a considerable time.”
He said it was also vital that people older than 55 were included in later trials.
Richard Torbett, chief executive of the Association of the British Pharmaceutical Industry, said: “Developing a vaccine is an incredibly difficult challenge; the fact that there are multiple candidates in development is hopefully a sign that the hard work will ultimately pay off.
“But we must be patient. Proving that a vaccine is safe and effective is a long process and we could still be many months away.”
This article first appeared on Medscape.com.
The early stage results, published in The Lancet, found that the candidate vaccine, known as ChAdOx1 nCoV-19, provoked a T-cell response peaking 14 days after vaccination, and an antibody response within 28 days.
Andrew Pollard, chief investigator on the study, and professor of pediatric infection and immunity at Oxford University, described the results as “encouraging”. He told a briefing convened by the Science Media Centre on Monday that it was “a really important milestone on the path to the development of the vaccine”.
In the Commons, the Health Secretary, Matt Hancock, hailed the results for taking us “one step closer to finding a vaccine that can potentially save lives, all around the world”.
The trial, which has so far involved 1,077 healthy adults, caused minor side effects when compared with a control group given a meningitis vaccine. Fatigue and headache were the most commonly reported reactions.
However, there were no serious adverse events from the vaccine, the researchers said.
‘Still a long way to go’
Sarah Gilbert, lead researcher of the vaccine development program, and professor of vaccinology at Oxford, cautioned that there was still a long way to go before the team could confirm that the vaccine could protect against developing COVID-19.
“The difficulty that we have, and that all vaccine developers have in trying to make a vaccine against this particular virus, is that we don’t know how strong that immune response needs to be,” she said.
“So, we can’t say just by looking at immune responses whether this is going to protect people or not. And the only way we’re going to find out is by doing the large phase 3 trials and wait for people to be infected as part of that trial before we know if the vaccine can work.”
The authors noted some limitations to their findings. They said more research was needed to confirm their results in different groups of people – including older age groups, those with other health conditions, and in ethnically and geographically diverse populations.
A notable result of the trial was that participants given a second dose of the vaccine appeared to display a stronger immune response, a finding that had influenced plans to “look at two dose regimes as well as one dose regimes in the phase 3 trial”, Prof Adrian Hill, director of Oxford’s Jenner Institute, confirmed.
ChAdOx1 nCoV-19 is made from a weakened version of an adenovirus that causes infections in chimpanzees. The virus has been genetically modified so that it cannot grow in humans.
On Monday, the government announced that it had struck a deal with AstraZeneca for access to 100 million doses of the Oxford vaccine, in addition to millions of doses of other promising candidate vaccines.
Expert reaction to the findings
The Medical Research Council helped to fund the trial. Executive Chair Professor Fiona Watt commented: “It is truly remarkable how fast this vaccine has progressed, with our support, through early clinical trials, and it is very encouraging that it shows no safety concerns and evokes strong immune responses.
“There is a lot that we don’t yet know about immunity to the virus that causes COVID-19. However, it seems that both antibody and T cell immunity are important, and this vaccine triggers both responses. The much anticipated next milestone will be the results of the larger trials that are happening now to find out if the vaccine will protect people from the virus.”
Jonathan Ball, professor of molecular virology at the University of Nottingham, told the SMC: “The results of the Oxford chimp adenovirus vaccine candidate show that the vaccine is able to generate antibodies and T cells in humans and these persisted for several weeks. Whilst encouraging there is still a long way to go before we can herald the arrival of a successful coronavirus vaccine.
“It is unclear whether the levels of immunity can protect against infection – that’s what the larger ongoing phase III trials are designed to test. Nor do we know if this vaccine can protect those most vulnerable to severe COVID-19 disease.”
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene and Tropical Medicine, commented: “For the vaccine to be really useful, we not only need the larger studies conducted where COVID-19 is still occurring at a high rate, but we need to be reasonably sure that the protection lasts for a considerable time.”
He said it was also vital that people older than 55 were included in later trials.
Richard Torbett, chief executive of the Association of the British Pharmaceutical Industry, said: “Developing a vaccine is an incredibly difficult challenge; the fact that there are multiple candidates in development is hopefully a sign that the hard work will ultimately pay off.
“But we must be patient. Proving that a vaccine is safe and effective is a long process and we could still be many months away.”
This article first appeared on Medscape.com.
The early stage results, published in The Lancet, found that the candidate vaccine, known as ChAdOx1 nCoV-19, provoked a T-cell response peaking 14 days after vaccination, and an antibody response within 28 days.
Andrew Pollard, chief investigator on the study, and professor of pediatric infection and immunity at Oxford University, described the results as “encouraging”. He told a briefing convened by the Science Media Centre on Monday that it was “a really important milestone on the path to the development of the vaccine”.
In the Commons, the Health Secretary, Matt Hancock, hailed the results for taking us “one step closer to finding a vaccine that can potentially save lives, all around the world”.
The trial, which has so far involved 1,077 healthy adults, caused minor side effects when compared with a control group given a meningitis vaccine. Fatigue and headache were the most commonly reported reactions.
However, there were no serious adverse events from the vaccine, the researchers said.
‘Still a long way to go’
Sarah Gilbert, lead researcher of the vaccine development program, and professor of vaccinology at Oxford, cautioned that there was still a long way to go before the team could confirm that the vaccine could protect against developing COVID-19.
“The difficulty that we have, and that all vaccine developers have in trying to make a vaccine against this particular virus, is that we don’t know how strong that immune response needs to be,” she said.
“So, we can’t say just by looking at immune responses whether this is going to protect people or not. And the only way we’re going to find out is by doing the large phase 3 trials and wait for people to be infected as part of that trial before we know if the vaccine can work.”
The authors noted some limitations to their findings. They said more research was needed to confirm their results in different groups of people – including older age groups, those with other health conditions, and in ethnically and geographically diverse populations.
A notable result of the trial was that participants given a second dose of the vaccine appeared to display a stronger immune response, a finding that had influenced plans to “look at two dose regimes as well as one dose regimes in the phase 3 trial”, Prof Adrian Hill, director of Oxford’s Jenner Institute, confirmed.
ChAdOx1 nCoV-19 is made from a weakened version of an adenovirus that causes infections in chimpanzees. The virus has been genetically modified so that it cannot grow in humans.
On Monday, the government announced that it had struck a deal with AstraZeneca for access to 100 million doses of the Oxford vaccine, in addition to millions of doses of other promising candidate vaccines.
Expert reaction to the findings
The Medical Research Council helped to fund the trial. Executive Chair Professor Fiona Watt commented: “It is truly remarkable how fast this vaccine has progressed, with our support, through early clinical trials, and it is very encouraging that it shows no safety concerns and evokes strong immune responses.
“There is a lot that we don’t yet know about immunity to the virus that causes COVID-19. However, it seems that both antibody and T cell immunity are important, and this vaccine triggers both responses. The much anticipated next milestone will be the results of the larger trials that are happening now to find out if the vaccine will protect people from the virus.”
Jonathan Ball, professor of molecular virology at the University of Nottingham, told the SMC: “The results of the Oxford chimp adenovirus vaccine candidate show that the vaccine is able to generate antibodies and T cells in humans and these persisted for several weeks. Whilst encouraging there is still a long way to go before we can herald the arrival of a successful coronavirus vaccine.
“It is unclear whether the levels of immunity can protect against infection – that’s what the larger ongoing phase III trials are designed to test. Nor do we know if this vaccine can protect those most vulnerable to severe COVID-19 disease.”
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene and Tropical Medicine, commented: “For the vaccine to be really useful, we not only need the larger studies conducted where COVID-19 is still occurring at a high rate, but we need to be reasonably sure that the protection lasts for a considerable time.”
He said it was also vital that people older than 55 were included in later trials.
Richard Torbett, chief executive of the Association of the British Pharmaceutical Industry, said: “Developing a vaccine is an incredibly difficult challenge; the fact that there are multiple candidates in development is hopefully a sign that the hard work will ultimately pay off.
“But we must be patient. Proving that a vaccine is safe and effective is a long process and we could still be many months away.”
This article first appeared on Medscape.com.
COVID vaccine tested in people shows early promise
the company says in a news release.
Researchers also reported some side effects in the 45 people in the phase I study, but no significant safety issues, the news release says.
The vaccine is among hundreds being tested worldwide in an effort to halt the pandemic that has killed nearly 600,000 worldwide.
A researcher testing the vaccine called the results encouraging but cautioned more study is needed. “Importantly, the vaccine resulted in a robust immune response,” Evan Anderson, MD, principal investigator for the trial at Emory University, says in a news release. Emory and Kaiser Permanente Washington Health Research Institute were the two sites for the study.
The company is already testing the vaccine in a larger group of people, known as a phase II trial. It plans to begin phase III trials in late July. Phase III trials involve testing the vaccine on an even larger group and are the final step before FDA approval.
The study results are published in The New England Journal of Medicine. The study was led by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Moderna’s vaccine uses messenger RNA, also called mRNA. It carries the instruction for making the spike protein, a key protein on the surface of the virus that allows it to enter cells when a person is infected. After it’s injected, it goes to the immune cells and instructs them to make copies of the spike protein, acting as if the cells have been infected with the actual coronavirus. This allows other immune cells to develop immunity.
In the study, participants were divided into three groups of 15 people each. All groups received two vaccinations 28 days apart. Each group received a different strength of the vaccine – either 25, 100, or 250 micrograms.
Every person in the study developed antibodies that can block the infection. Most commonly reported side effects after the second vaccination in the 100-microgram group were fatigue, chills, headache, and muscle pains, ranging from mild to moderately severe.
The phase II study has 300 heathy adults ages 18-55, along with another 300 ages 55 and older
Moderna says it hopes to include about 30,000 participants at the 100-microgram dose level in the U.S. for the phase III trial. The estimated start date is July 27.
This article first appeared on WebMD.com.
the company says in a news release.
Researchers also reported some side effects in the 45 people in the phase I study, but no significant safety issues, the news release says.
The vaccine is among hundreds being tested worldwide in an effort to halt the pandemic that has killed nearly 600,000 worldwide.
A researcher testing the vaccine called the results encouraging but cautioned more study is needed. “Importantly, the vaccine resulted in a robust immune response,” Evan Anderson, MD, principal investigator for the trial at Emory University, says in a news release. Emory and Kaiser Permanente Washington Health Research Institute were the two sites for the study.
The company is already testing the vaccine in a larger group of people, known as a phase II trial. It plans to begin phase III trials in late July. Phase III trials involve testing the vaccine on an even larger group and are the final step before FDA approval.
The study results are published in The New England Journal of Medicine. The study was led by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Moderna’s vaccine uses messenger RNA, also called mRNA. It carries the instruction for making the spike protein, a key protein on the surface of the virus that allows it to enter cells when a person is infected. After it’s injected, it goes to the immune cells and instructs them to make copies of the spike protein, acting as if the cells have been infected with the actual coronavirus. This allows other immune cells to develop immunity.
In the study, participants were divided into three groups of 15 people each. All groups received two vaccinations 28 days apart. Each group received a different strength of the vaccine – either 25, 100, or 250 micrograms.
Every person in the study developed antibodies that can block the infection. Most commonly reported side effects after the second vaccination in the 100-microgram group were fatigue, chills, headache, and muscle pains, ranging from mild to moderately severe.
The phase II study has 300 heathy adults ages 18-55, along with another 300 ages 55 and older
Moderna says it hopes to include about 30,000 participants at the 100-microgram dose level in the U.S. for the phase III trial. The estimated start date is July 27.
This article first appeared on WebMD.com.
the company says in a news release.
Researchers also reported some side effects in the 45 people in the phase I study, but no significant safety issues, the news release says.
The vaccine is among hundreds being tested worldwide in an effort to halt the pandemic that has killed nearly 600,000 worldwide.
A researcher testing the vaccine called the results encouraging but cautioned more study is needed. “Importantly, the vaccine resulted in a robust immune response,” Evan Anderson, MD, principal investigator for the trial at Emory University, says in a news release. Emory and Kaiser Permanente Washington Health Research Institute were the two sites for the study.
The company is already testing the vaccine in a larger group of people, known as a phase II trial. It plans to begin phase III trials in late July. Phase III trials involve testing the vaccine on an even larger group and are the final step before FDA approval.
The study results are published in The New England Journal of Medicine. The study was led by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Moderna’s vaccine uses messenger RNA, also called mRNA. It carries the instruction for making the spike protein, a key protein on the surface of the virus that allows it to enter cells when a person is infected. After it’s injected, it goes to the immune cells and instructs them to make copies of the spike protein, acting as if the cells have been infected with the actual coronavirus. This allows other immune cells to develop immunity.
In the study, participants were divided into three groups of 15 people each. All groups received two vaccinations 28 days apart. Each group received a different strength of the vaccine – either 25, 100, or 250 micrograms.
Every person in the study developed antibodies that can block the infection. Most commonly reported side effects after the second vaccination in the 100-microgram group were fatigue, chills, headache, and muscle pains, ranging from mild to moderately severe.
The phase II study has 300 heathy adults ages 18-55, along with another 300 ages 55 and older
Moderna says it hopes to include about 30,000 participants at the 100-microgram dose level in the U.S. for the phase III trial. The estimated start date is July 27.
This article first appeared on WebMD.com.
Consider adverse childhood experiences during the pandemic
We live in historic times. A worldwide pandemic is surging in the United States, with millions infected and the world’s highest death rate. Many of our hospitals are overwhelmed. Schools have been closed for months. Businesses are struggling, and unemployment is at record levels. The murder of George Floyd unleashed an outpouring of grief and rage over police brutality and structural racism.
It is ironic that this age of adversity emerged at the same time that efforts to assess and address childhood adversity are gaining momentum. The effects of adverse childhood experiences (ACEs) have been well known for decades, but only recently have efforts at universal screening been initiated in primary care offices around the country. The multiple crises we face have made this work more pressing than ever. And
While there has long been awareness, especially among pediatricians, of the social determinants of health, it was only 1995 when Robert F. Anda, MD, and Vincent J. Felitti, MD, set about studying over 13,000 adult patients at Kaiser Permanente to understand the relationship between childhood trauma and chronic health problems in adulthood. In 1998 they published the results of this landmark study, establishing that childhood trauma was common and that it predicted chronic diseases and psychosocial problems in adulthood1.
They detailed 10 specific ACEs, and a patient’s ACE score was determined by how many of these experiences they had before they turned 18 years: neglect (emotional or physical), abuse (emotional, physical or sexual), and household dysfunction (parental divorce, incarceration of a parent, domestic violence, parental mental illness, or parental substance abuse). They found that more than half of adults studied had a score of at least 1, and 6% had scores of 4 or more. Those adults with an ACE score of 4 or more are twice as likely to be obese, twice as likely to smoke, and seven times as likely to abuse alcohol as the rest of the population. They are 4 times as likely to have emphysema, 5 times as likely to have depression, and 12 times as likely to attempt suicide. They have higher rates of heart disease, autoimmune disorders, and cancer. Those with ACE scores of 6 or more have their life expectancy shortened by an average of 20 years.
The value of knowing about these risk factors would seem self-evident; it would inform a patient’s health care from screening for cancer or heart disease, referral for mild depressive symptoms, and counseling about alcohol consumption. But this research did not lead to the establishment of routine screening for childhood adversity in primary care practices. There are multiple reasons for this, including growing pressure on physician time and discomfort with starting conversations about potentially traumatic material. But perhaps the greatest obstacle has been uncertainty about what to offer patients who screened in. What is the treatment for a high ACE score?
Even without treatments, we have learned much about childhood adversity since Dr. Anda and Dr. Felitti published their landmark study. Other more chronic adverse childhood experiences also contribute to adult health risk, such as poverty, homelessness, discrimination, community violence, parental chronic illness, or disability or placement in foster care. Having a high ACE score does not only affect health in adulthood. Children with an ACE score of 4 are 2 times as likely to have asthma2,3 and allergies3, 2 times as likely to be obese4, 3 times as likely to have headaches3 and dental problems5,6, 4 times as likely to have depression7,8, 5 times as likely to have ADHD8,9, 7 times as likely to have high rates of school absenteeism3 and aggression10, and over 30 times as likely to have learning or behavioral problems at school4. There is a growing body of knowledge about how chronic, severe stress in childhood affects can lead to pathological alterations in neuroendocrine and immune function. But this has not led to any concrete treatments that may be preventive or reparative.
Movement toward expanding screening nonetheless has accelerated. In California, Nadine Burke-Harris, MD, a pediatrician who studied ACEs and children’s health was named the state’s first Surgeon General in 2019 and spearheaded an effort to make screening for ACEs easier. Starting in 2020, MediCal will pay for annual screenings, and the state is offering training and resources on how to screen and what to do with the information to help patients and families.
The coronavirus pandemic has only highlighted the risks of childhood adversity. The burden of infection and mortality has been borne disproportionately by people of color and those with multiple chronic medical conditions (obesity, cardiovascular disease, diabetes, etc.). While viruses do not discriminate, they are more likely to infect those with higher risk of exposure and to kill those who are physiologically vulnerable.
And the pandemic increases the risk for adversity for today’s children and families. When children cannot attend school, financially vulnerable parents may have to choose between supervising them or feeding them. Families who suddenly are all in a small apartment together without school or other outside supports may be at higher risk for domestic violence and child abuse. Unemployment and financial uncertainty will increase the rates of substance abuse and depression amongst parents. And the serious illness or death of a parent will be a more common event for children in the year ahead. One of these risk factors may increase the likelihood of others.
Beyond the obvious need for substantial policy changes focused on housing, education, and health care, And resilience can build on itself, as children face subsequent challenges with the support of caring connected adults.
The critical first step is asking. Then listen calmly and supportively, normalizing for parents and children how common these experiences are. Explain how they affect health and well-being. Explain that adversity and its consequences are not their fault. Then educate them about what is in their control: the skills they can practice to buffer against the consequences of adversity and build resilience. They sound simple, but still require effort and work. And the pandemic has created some difficulty (social distancing) and opportunity (more family time, fewer school demands).
Sleep
Help parents establish and protect consistent, restful sleep for their children. They can set a consistent bedtime and a calm routine, with screens all off at least 30 minutes before sleep and reading before sleep. Restful sleep is physiologically and psychologically protective to everyone in a family.
Movement
Beyond directly improving physical health, establishing habits of exercise – especially outside – every day can effectively manage ongoing stress, build skills of self-regulation, and help with sleep.
Find out what parents and their children like to do together (walking the dog, shooting hoops, even dancing) and help them devise ways to create family routines around exercise.
Nutrition
Food should be a source of pleasure, but stress can make food into a source of comfort or escape. Help parents to create realistic ways to consistently offer healthy family meals and discourage unhealthy habits.
Even small changes like water instead of soda can help, and there are nutritional and emotional benefits to eating a healthy breakfast or dinner together as a family.
Connections
Nourishing social connections are protective. Help parents think about protecting time to spend with their children for talking, playing games, or even singing.
They should support their children’s connections to other caring adults, through community organizations (church, community centers, or sports), and they should know who their children’s reliable friends are. Parents will benefit from these supports for themselves, which in turn will benefit the full family.
Self-awareness
Activities that cultivate mindfulness are protective. Parents can simply ask how their children are feeling, physically or emotionally, and be able to bear it when it is uncomfortable. Work towards nonjudgmental awareness of how they are feeling. Learning what is relaxing or recharging for them (exercise, music, a hot bath, a good book, time with a friend) will protect against defaulting into maladaptive coping such as escape, numbing, or avoidance.
Of course, if you learn about symptoms that suggest PTSD, depression, or addiction, you should help your patient connect with effective treatment. The difficulty of referring to a mental health provider does not mean you should not try and bring as many people onto the team and into the orbit of the child and family at risk. It may be easier to access some therapy given the new availability of telemedicine visits across many more systems of care. Although the heaviest burdens of adversity are not being borne equally, the fact that adversity is currently a shared experience makes this a moment of promise.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Dr. Swick and Dr. Jellinek had no relevant financial disclosures. Email them at [email protected].
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Ann Allergy Asthma Immunol. 2015;114: 379-84.
3. BMC Public Health. 2018. doi: 10.1186/s12889-018-5699-8.
4. Child Abuse Negl. 2011 Jun;35(6):408-13.
5. Community Dent Oral Epidemiol. 2015;43:193-9.
6. Community Dent Oral Epidemiol. 2018 Oct;46(5): 442-8.
7. Pediatrics 2016 Apr. doi: 10.1542/peds.2015-4016.
8. Matern Child Health J. 2016 Apr. doi: 10.1007/s10995-015-1915-7.
9. Acad Pediatr. 2017 May-Jun. doi: 10.1016/j.acap.2016.08.013.
10. Pediatrics. 2010 Apr. doi: 10.1542/peds.2009-0597.
This article was updated 7/27/2020.
We live in historic times. A worldwide pandemic is surging in the United States, with millions infected and the world’s highest death rate. Many of our hospitals are overwhelmed. Schools have been closed for months. Businesses are struggling, and unemployment is at record levels. The murder of George Floyd unleashed an outpouring of grief and rage over police brutality and structural racism.
It is ironic that this age of adversity emerged at the same time that efforts to assess and address childhood adversity are gaining momentum. The effects of adverse childhood experiences (ACEs) have been well known for decades, but only recently have efforts at universal screening been initiated in primary care offices around the country. The multiple crises we face have made this work more pressing than ever. And
While there has long been awareness, especially among pediatricians, of the social determinants of health, it was only 1995 when Robert F. Anda, MD, and Vincent J. Felitti, MD, set about studying over 13,000 adult patients at Kaiser Permanente to understand the relationship between childhood trauma and chronic health problems in adulthood. In 1998 they published the results of this landmark study, establishing that childhood trauma was common and that it predicted chronic diseases and psychosocial problems in adulthood1.
They detailed 10 specific ACEs, and a patient’s ACE score was determined by how many of these experiences they had before they turned 18 years: neglect (emotional or physical), abuse (emotional, physical or sexual), and household dysfunction (parental divorce, incarceration of a parent, domestic violence, parental mental illness, or parental substance abuse). They found that more than half of adults studied had a score of at least 1, and 6% had scores of 4 or more. Those adults with an ACE score of 4 or more are twice as likely to be obese, twice as likely to smoke, and seven times as likely to abuse alcohol as the rest of the population. They are 4 times as likely to have emphysema, 5 times as likely to have depression, and 12 times as likely to attempt suicide. They have higher rates of heart disease, autoimmune disorders, and cancer. Those with ACE scores of 6 or more have their life expectancy shortened by an average of 20 years.
The value of knowing about these risk factors would seem self-evident; it would inform a patient’s health care from screening for cancer or heart disease, referral for mild depressive symptoms, and counseling about alcohol consumption. But this research did not lead to the establishment of routine screening for childhood adversity in primary care practices. There are multiple reasons for this, including growing pressure on physician time and discomfort with starting conversations about potentially traumatic material. But perhaps the greatest obstacle has been uncertainty about what to offer patients who screened in. What is the treatment for a high ACE score?
Even without treatments, we have learned much about childhood adversity since Dr. Anda and Dr. Felitti published their landmark study. Other more chronic adverse childhood experiences also contribute to adult health risk, such as poverty, homelessness, discrimination, community violence, parental chronic illness, or disability or placement in foster care. Having a high ACE score does not only affect health in adulthood. Children with an ACE score of 4 are 2 times as likely to have asthma2,3 and allergies3, 2 times as likely to be obese4, 3 times as likely to have headaches3 and dental problems5,6, 4 times as likely to have depression7,8, 5 times as likely to have ADHD8,9, 7 times as likely to have high rates of school absenteeism3 and aggression10, and over 30 times as likely to have learning or behavioral problems at school4. There is a growing body of knowledge about how chronic, severe stress in childhood affects can lead to pathological alterations in neuroendocrine and immune function. But this has not led to any concrete treatments that may be preventive or reparative.
Movement toward expanding screening nonetheless has accelerated. In California, Nadine Burke-Harris, MD, a pediatrician who studied ACEs and children’s health was named the state’s first Surgeon General in 2019 and spearheaded an effort to make screening for ACEs easier. Starting in 2020, MediCal will pay for annual screenings, and the state is offering training and resources on how to screen and what to do with the information to help patients and families.
The coronavirus pandemic has only highlighted the risks of childhood adversity. The burden of infection and mortality has been borne disproportionately by people of color and those with multiple chronic medical conditions (obesity, cardiovascular disease, diabetes, etc.). While viruses do not discriminate, they are more likely to infect those with higher risk of exposure and to kill those who are physiologically vulnerable.
And the pandemic increases the risk for adversity for today’s children and families. When children cannot attend school, financially vulnerable parents may have to choose between supervising them or feeding them. Families who suddenly are all in a small apartment together without school or other outside supports may be at higher risk for domestic violence and child abuse. Unemployment and financial uncertainty will increase the rates of substance abuse and depression amongst parents. And the serious illness or death of a parent will be a more common event for children in the year ahead. One of these risk factors may increase the likelihood of others.
Beyond the obvious need for substantial policy changes focused on housing, education, and health care, And resilience can build on itself, as children face subsequent challenges with the support of caring connected adults.
The critical first step is asking. Then listen calmly and supportively, normalizing for parents and children how common these experiences are. Explain how they affect health and well-being. Explain that adversity and its consequences are not their fault. Then educate them about what is in their control: the skills they can practice to buffer against the consequences of adversity and build resilience. They sound simple, but still require effort and work. And the pandemic has created some difficulty (social distancing) and opportunity (more family time, fewer school demands).
Sleep
Help parents establish and protect consistent, restful sleep for their children. They can set a consistent bedtime and a calm routine, with screens all off at least 30 minutes before sleep and reading before sleep. Restful sleep is physiologically and psychologically protective to everyone in a family.
Movement
Beyond directly improving physical health, establishing habits of exercise – especially outside – every day can effectively manage ongoing stress, build skills of self-regulation, and help with sleep.
Find out what parents and their children like to do together (walking the dog, shooting hoops, even dancing) and help them devise ways to create family routines around exercise.
Nutrition
Food should be a source of pleasure, but stress can make food into a source of comfort or escape. Help parents to create realistic ways to consistently offer healthy family meals and discourage unhealthy habits.
Even small changes like water instead of soda can help, and there are nutritional and emotional benefits to eating a healthy breakfast or dinner together as a family.
Connections
Nourishing social connections are protective. Help parents think about protecting time to spend with their children for talking, playing games, or even singing.
They should support their children’s connections to other caring adults, through community organizations (church, community centers, or sports), and they should know who their children’s reliable friends are. Parents will benefit from these supports for themselves, which in turn will benefit the full family.
Self-awareness
Activities that cultivate mindfulness are protective. Parents can simply ask how their children are feeling, physically or emotionally, and be able to bear it when it is uncomfortable. Work towards nonjudgmental awareness of how they are feeling. Learning what is relaxing or recharging for them (exercise, music, a hot bath, a good book, time with a friend) will protect against defaulting into maladaptive coping such as escape, numbing, or avoidance.
Of course, if you learn about symptoms that suggest PTSD, depression, or addiction, you should help your patient connect with effective treatment. The difficulty of referring to a mental health provider does not mean you should not try and bring as many people onto the team and into the orbit of the child and family at risk. It may be easier to access some therapy given the new availability of telemedicine visits across many more systems of care. Although the heaviest burdens of adversity are not being borne equally, the fact that adversity is currently a shared experience makes this a moment of promise.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Dr. Swick and Dr. Jellinek had no relevant financial disclosures. Email them at [email protected].
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Ann Allergy Asthma Immunol. 2015;114: 379-84.
3. BMC Public Health. 2018. doi: 10.1186/s12889-018-5699-8.
4. Child Abuse Negl. 2011 Jun;35(6):408-13.
5. Community Dent Oral Epidemiol. 2015;43:193-9.
6. Community Dent Oral Epidemiol. 2018 Oct;46(5): 442-8.
7. Pediatrics 2016 Apr. doi: 10.1542/peds.2015-4016.
8. Matern Child Health J. 2016 Apr. doi: 10.1007/s10995-015-1915-7.
9. Acad Pediatr. 2017 May-Jun. doi: 10.1016/j.acap.2016.08.013.
10. Pediatrics. 2010 Apr. doi: 10.1542/peds.2009-0597.
This article was updated 7/27/2020.
We live in historic times. A worldwide pandemic is surging in the United States, with millions infected and the world’s highest death rate. Many of our hospitals are overwhelmed. Schools have been closed for months. Businesses are struggling, and unemployment is at record levels. The murder of George Floyd unleashed an outpouring of grief and rage over police brutality and structural racism.
It is ironic that this age of adversity emerged at the same time that efforts to assess and address childhood adversity are gaining momentum. The effects of adverse childhood experiences (ACEs) have been well known for decades, but only recently have efforts at universal screening been initiated in primary care offices around the country. The multiple crises we face have made this work more pressing than ever. And
While there has long been awareness, especially among pediatricians, of the social determinants of health, it was only 1995 when Robert F. Anda, MD, and Vincent J. Felitti, MD, set about studying over 13,000 adult patients at Kaiser Permanente to understand the relationship between childhood trauma and chronic health problems in adulthood. In 1998 they published the results of this landmark study, establishing that childhood trauma was common and that it predicted chronic diseases and psychosocial problems in adulthood1.
They detailed 10 specific ACEs, and a patient’s ACE score was determined by how many of these experiences they had before they turned 18 years: neglect (emotional or physical), abuse (emotional, physical or sexual), and household dysfunction (parental divorce, incarceration of a parent, domestic violence, parental mental illness, or parental substance abuse). They found that more than half of adults studied had a score of at least 1, and 6% had scores of 4 or more. Those adults with an ACE score of 4 or more are twice as likely to be obese, twice as likely to smoke, and seven times as likely to abuse alcohol as the rest of the population. They are 4 times as likely to have emphysema, 5 times as likely to have depression, and 12 times as likely to attempt suicide. They have higher rates of heart disease, autoimmune disorders, and cancer. Those with ACE scores of 6 or more have their life expectancy shortened by an average of 20 years.
The value of knowing about these risk factors would seem self-evident; it would inform a patient’s health care from screening for cancer or heart disease, referral for mild depressive symptoms, and counseling about alcohol consumption. But this research did not lead to the establishment of routine screening for childhood adversity in primary care practices. There are multiple reasons for this, including growing pressure on physician time and discomfort with starting conversations about potentially traumatic material. But perhaps the greatest obstacle has been uncertainty about what to offer patients who screened in. What is the treatment for a high ACE score?
Even without treatments, we have learned much about childhood adversity since Dr. Anda and Dr. Felitti published their landmark study. Other more chronic adverse childhood experiences also contribute to adult health risk, such as poverty, homelessness, discrimination, community violence, parental chronic illness, or disability or placement in foster care. Having a high ACE score does not only affect health in adulthood. Children with an ACE score of 4 are 2 times as likely to have asthma2,3 and allergies3, 2 times as likely to be obese4, 3 times as likely to have headaches3 and dental problems5,6, 4 times as likely to have depression7,8, 5 times as likely to have ADHD8,9, 7 times as likely to have high rates of school absenteeism3 and aggression10, and over 30 times as likely to have learning or behavioral problems at school4. There is a growing body of knowledge about how chronic, severe stress in childhood affects can lead to pathological alterations in neuroendocrine and immune function. But this has not led to any concrete treatments that may be preventive or reparative.
Movement toward expanding screening nonetheless has accelerated. In California, Nadine Burke-Harris, MD, a pediatrician who studied ACEs and children’s health was named the state’s first Surgeon General in 2019 and spearheaded an effort to make screening for ACEs easier. Starting in 2020, MediCal will pay for annual screenings, and the state is offering training and resources on how to screen and what to do with the information to help patients and families.
The coronavirus pandemic has only highlighted the risks of childhood adversity. The burden of infection and mortality has been borne disproportionately by people of color and those with multiple chronic medical conditions (obesity, cardiovascular disease, diabetes, etc.). While viruses do not discriminate, they are more likely to infect those with higher risk of exposure and to kill those who are physiologically vulnerable.
And the pandemic increases the risk for adversity for today’s children and families. When children cannot attend school, financially vulnerable parents may have to choose between supervising them or feeding them. Families who suddenly are all in a small apartment together without school or other outside supports may be at higher risk for domestic violence and child abuse. Unemployment and financial uncertainty will increase the rates of substance abuse and depression amongst parents. And the serious illness or death of a parent will be a more common event for children in the year ahead. One of these risk factors may increase the likelihood of others.
Beyond the obvious need for substantial policy changes focused on housing, education, and health care, And resilience can build on itself, as children face subsequent challenges with the support of caring connected adults.
The critical first step is asking. Then listen calmly and supportively, normalizing for parents and children how common these experiences are. Explain how they affect health and well-being. Explain that adversity and its consequences are not their fault. Then educate them about what is in their control: the skills they can practice to buffer against the consequences of adversity and build resilience. They sound simple, but still require effort and work. And the pandemic has created some difficulty (social distancing) and opportunity (more family time, fewer school demands).
Sleep
Help parents establish and protect consistent, restful sleep for their children. They can set a consistent bedtime and a calm routine, with screens all off at least 30 minutes before sleep and reading before sleep. Restful sleep is physiologically and psychologically protective to everyone in a family.
Movement
Beyond directly improving physical health, establishing habits of exercise – especially outside – every day can effectively manage ongoing stress, build skills of self-regulation, and help with sleep.
Find out what parents and their children like to do together (walking the dog, shooting hoops, even dancing) and help them devise ways to create family routines around exercise.
Nutrition
Food should be a source of pleasure, but stress can make food into a source of comfort or escape. Help parents to create realistic ways to consistently offer healthy family meals and discourage unhealthy habits.
Even small changes like water instead of soda can help, and there are nutritional and emotional benefits to eating a healthy breakfast or dinner together as a family.
Connections
Nourishing social connections are protective. Help parents think about protecting time to spend with their children for talking, playing games, or even singing.
They should support their children’s connections to other caring adults, through community organizations (church, community centers, or sports), and they should know who their children’s reliable friends are. Parents will benefit from these supports for themselves, which in turn will benefit the full family.
Self-awareness
Activities that cultivate mindfulness are protective. Parents can simply ask how their children are feeling, physically or emotionally, and be able to bear it when it is uncomfortable. Work towards nonjudgmental awareness of how they are feeling. Learning what is relaxing or recharging for them (exercise, music, a hot bath, a good book, time with a friend) will protect against defaulting into maladaptive coping such as escape, numbing, or avoidance.
Of course, if you learn about symptoms that suggest PTSD, depression, or addiction, you should help your patient connect with effective treatment. The difficulty of referring to a mental health provider does not mean you should not try and bring as many people onto the team and into the orbit of the child and family at risk. It may be easier to access some therapy given the new availability of telemedicine visits across many more systems of care. Although the heaviest burdens of adversity are not being borne equally, the fact that adversity is currently a shared experience makes this a moment of promise.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Dr. Swick and Dr. Jellinek had no relevant financial disclosures. Email them at [email protected].
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Ann Allergy Asthma Immunol. 2015;114: 379-84.
3. BMC Public Health. 2018. doi: 10.1186/s12889-018-5699-8.
4. Child Abuse Negl. 2011 Jun;35(6):408-13.
5. Community Dent Oral Epidemiol. 2015;43:193-9.
6. Community Dent Oral Epidemiol. 2018 Oct;46(5): 442-8.
7. Pediatrics 2016 Apr. doi: 10.1542/peds.2015-4016.
8. Matern Child Health J. 2016 Apr. doi: 10.1007/s10995-015-1915-7.
9. Acad Pediatr. 2017 May-Jun. doi: 10.1016/j.acap.2016.08.013.
10. Pediatrics. 2010 Apr. doi: 10.1542/peds.2009-0597.
This article was updated 7/27/2020.













