User login
Documentation matters
Quality over quantity
Documentation has always been part of a physician’s job. Historically, in the days of paper records, physicians saw a patient on rounds and immediately following, while still on the unit, wrote a daily note detailing the events, test results, and plans since the last note. Addenda were written over the course of the day and night as needed.
The medical record was a chronological itemization of the encounter. The chart told the patient’s story, hopefully legibly and without excessive rehashing of previous material. The discharge summary then encapsulated the hospitalization in several coherent paragraphs.
In the current electronic records environment, we are inundated with excessive and repetitious information, data without interpretation, differentials without diagnoses. Prepopulation of templated notes, defaults without edit, and dictation without revision have degraded our documentation to the point of unintelligibility. The chronological storytelling and trustworthiness of the medical record has become suspect.
The Centers for Medicare & Medicaid Services is touting its “Patients over Paperwork” initiative. The solution is flawed (that is, future relaxation of documentation requirements for professional billing) because the premise is delusive. Documentation isn’t fundamentally the problem. Having clinicians jump through regulatory hoops which do not advance patients’ care, and providers misunderstanding the requirements for level-of-service billing are the essential issues. Getting no training on how to properly document in medical school/residency and receiving no formative feedback on documentation throughout one’s career compounds the problem. Having clinical documentation serve too many masters, including compliance, quality, medicolegal, utilization review, and reimbursement, is also to blame. The advent of the electronic medical record was just the straw that broke the camel’s back.
Many hospitals now have a clinical documentation integrity (CDI) team which is tasked with querying the provider when the health record documentation is conflicting, imprecise, incomplete, illegible, ambiguous, or inconsistent. They are charged with getting practitioners to associate clinical indicators with diagnoses and to consider removal of diagnoses which do not seem clinically valid from the existing documentation. From this explanation, you might well conclude that the CDI specialist could generate a query on every patient if they were so inclined, and you would be correct. But the goal isn’t to torture the physician – it is to ensure that the medical record is accurately depicting the encounter.
You are not being asked for more documentation by the CDI team; they are entreating you for higher-quality documentation. Let me give you some pointers to ward off queries.
- Tell the story. The most important goal of documentation is to clinically communicate to other caregivers. Think to yourself: “What would a fellow clinician need to know about this patient to understand why I drew those conclusions or to pick up where I left off?” At 2 a.m., that information, or lack thereof, could literally be a matter of life or death.
- Tell the truth. Embellishing the record or including invalid diagnoses with the intent to increase the severity of illness resulting in a more favorable diagnosis-related group – the inpatient risk-adjustment system – is considered fraud.
- You may like the convenience of copy forward, but do you relish reading other people’s copy and paste? Consider doing a documentation time-out. Before you copy and paste yesterday’s assessment and plan, stop and think: “Why is the patient still here? Why are we doing what we are doing?” If you choose to copy and paste, be certain to do mindful editing so the documentation represents the current situation and avoids redundancy. Appropriately editing copy and pasted documentation may prove more time consuming than generating a note de novo.
- Translate findings into diagnoses using your best medical judgment. One man’s hypotension may be another health care provider’s shock. Coders are not clinical and are not permitted to make inferences. A potassium of 6.7 may be hyperkalemia or it may be spurious – only a clinician may make that determination using their clinical expertise and experience. The coder is not allowed to read your mind. You must explicitly draw the conclusion that a febrile patient with bacteremia, encephalopathy, hypoxemia, and a blood pressure of 85/60 is in septic shock.
- Uncertain diagnoses (heralded by words such as: likely, possible, probable, suspected, rule out, etc.) which are not ruled out prior to discharge or demise are coded as if they were definitively present, for the inpatient technical side of hospital billing. This is distinctly different than the professional fee where you can only code definitive diagnoses. If you have a strong suspicion (not wild speculation) that a condition is present, best practice is to offer an uncertain diagnosis. Associate signs and symptoms with your most likely diagnosis: “Shortness of breath, pleuritic chest pain, and hypoxemia in the setting of cancer, probable pulmonary embolism.”
- Evolve, resolve, remove, and recap. If an uncertain diagnosis is ruled in, take away the uncertainty. If it is ruled out, don’t have 4 days of copy and pasted: “Possible eosinophilic pneumonia.” You do not have to maintain a resolved diagnosis ad infinitum. It can drop off the diagnosis list but be sure to have it reappear in the discharge summary.
- I know it can be a hASSLe to do excellent documentation, but it is critical for many reasons, most importantly for superlative patient care. More accurate coding and billing is an intended consequence. A: Acuity; S: Severity; S: Specificity (may affect the coding and the risk-adjustment implications. Acute systolic heart failure does not equal heart failure; type 2 diabetes mellitus with diabetic chronic kidney disease, stage 4 does not equal chronic kidney disease); and L: Linkage (of diagnosis with underlying cause or manifestation [e.g., because of, associated with, as a result of, secondary to, or from diabetic nephropathy, hypertensive encephalopathy]).
- If you have the capability to keep a running summary throughout the hospital stay, do so and keep it updated. A few moments of daily careful editing and composing can save time and effort at the back end creating the discharge summary. The follow-up care provider can reconstruct the hospital course and it is your last chance to spin the narrative for the lawyers.
- Read your documentation over. Ensure that it is clear, accurate, concise, and tells the story and the plans for the patient. Make sure that someone reading the note will know what you were thinking.
- Set up a program to self-audit documentation where monthly or quarterly, you and your partners mutually review a certain number of records and give each other feedback. Design an assessment tool which rates the quality of documentation elements which your hospital/network/service line values (clarity, copy and paste, complete and specific diagnoses, etc.). You know who the best documenters are. Why do you think their documentation is superior? How can you emulate them?
Finally, answer CDI queries. The CDI specialist is your ally, not your enemy. They want you to get credit for taking care of sick and complex patients. They are not permitted to lead the provider, so don’t ask them what they want you to write. But, if you don’t understand the query or issue, have a conversation and get it clarified. It is in everyone’s best interest to get this right.
Documentation improves patient care and demonstrates that you provided excellent patient care. Put mentation back into documentation.
Dr. Remer was a practicing emergency physician for 25 years and a physician advisor for 4 years. She is on the board of directors of the American College of Physician Advisors and the advisory board of the Association of Clinical Documentation Improvement Specialists. She currently provides consulting services for provider education on documentation, CDI, and ICD-10 coding. Dr. Remer can be reached at [email protected]
Quality over quantity
Quality over quantity
Documentation has always been part of a physician’s job. Historically, in the days of paper records, physicians saw a patient on rounds and immediately following, while still on the unit, wrote a daily note detailing the events, test results, and plans since the last note. Addenda were written over the course of the day and night as needed.
The medical record was a chronological itemization of the encounter. The chart told the patient’s story, hopefully legibly and without excessive rehashing of previous material. The discharge summary then encapsulated the hospitalization in several coherent paragraphs.
In the current electronic records environment, we are inundated with excessive and repetitious information, data without interpretation, differentials without diagnoses. Prepopulation of templated notes, defaults without edit, and dictation without revision have degraded our documentation to the point of unintelligibility. The chronological storytelling and trustworthiness of the medical record has become suspect.
The Centers for Medicare & Medicaid Services is touting its “Patients over Paperwork” initiative. The solution is flawed (that is, future relaxation of documentation requirements for professional billing) because the premise is delusive. Documentation isn’t fundamentally the problem. Having clinicians jump through regulatory hoops which do not advance patients’ care, and providers misunderstanding the requirements for level-of-service billing are the essential issues. Getting no training on how to properly document in medical school/residency and receiving no formative feedback on documentation throughout one’s career compounds the problem. Having clinical documentation serve too many masters, including compliance, quality, medicolegal, utilization review, and reimbursement, is also to blame. The advent of the electronic medical record was just the straw that broke the camel’s back.
Many hospitals now have a clinical documentation integrity (CDI) team which is tasked with querying the provider when the health record documentation is conflicting, imprecise, incomplete, illegible, ambiguous, or inconsistent. They are charged with getting practitioners to associate clinical indicators with diagnoses and to consider removal of diagnoses which do not seem clinically valid from the existing documentation. From this explanation, you might well conclude that the CDI specialist could generate a query on every patient if they were so inclined, and you would be correct. But the goal isn’t to torture the physician – it is to ensure that the medical record is accurately depicting the encounter.
You are not being asked for more documentation by the CDI team; they are entreating you for higher-quality documentation. Let me give you some pointers to ward off queries.
- Tell the story. The most important goal of documentation is to clinically communicate to other caregivers. Think to yourself: “What would a fellow clinician need to know about this patient to understand why I drew those conclusions or to pick up where I left off?” At 2 a.m., that information, or lack thereof, could literally be a matter of life or death.
- Tell the truth. Embellishing the record or including invalid diagnoses with the intent to increase the severity of illness resulting in a more favorable diagnosis-related group – the inpatient risk-adjustment system – is considered fraud.
- You may like the convenience of copy forward, but do you relish reading other people’s copy and paste? Consider doing a documentation time-out. Before you copy and paste yesterday’s assessment and plan, stop and think: “Why is the patient still here? Why are we doing what we are doing?” If you choose to copy and paste, be certain to do mindful editing so the documentation represents the current situation and avoids redundancy. Appropriately editing copy and pasted documentation may prove more time consuming than generating a note de novo.
- Translate findings into diagnoses using your best medical judgment. One man’s hypotension may be another health care provider’s shock. Coders are not clinical and are not permitted to make inferences. A potassium of 6.7 may be hyperkalemia or it may be spurious – only a clinician may make that determination using their clinical expertise and experience. The coder is not allowed to read your mind. You must explicitly draw the conclusion that a febrile patient with bacteremia, encephalopathy, hypoxemia, and a blood pressure of 85/60 is in septic shock.
- Uncertain diagnoses (heralded by words such as: likely, possible, probable, suspected, rule out, etc.) which are not ruled out prior to discharge or demise are coded as if they were definitively present, for the inpatient technical side of hospital billing. This is distinctly different than the professional fee where you can only code definitive diagnoses. If you have a strong suspicion (not wild speculation) that a condition is present, best practice is to offer an uncertain diagnosis. Associate signs and symptoms with your most likely diagnosis: “Shortness of breath, pleuritic chest pain, and hypoxemia in the setting of cancer, probable pulmonary embolism.”
- Evolve, resolve, remove, and recap. If an uncertain diagnosis is ruled in, take away the uncertainty. If it is ruled out, don’t have 4 days of copy and pasted: “Possible eosinophilic pneumonia.” You do not have to maintain a resolved diagnosis ad infinitum. It can drop off the diagnosis list but be sure to have it reappear in the discharge summary.
- I know it can be a hASSLe to do excellent documentation, but it is critical for many reasons, most importantly for superlative patient care. More accurate coding and billing is an intended consequence. A: Acuity; S: Severity; S: Specificity (may affect the coding and the risk-adjustment implications. Acute systolic heart failure does not equal heart failure; type 2 diabetes mellitus with diabetic chronic kidney disease, stage 4 does not equal chronic kidney disease); and L: Linkage (of diagnosis with underlying cause or manifestation [e.g., because of, associated with, as a result of, secondary to, or from diabetic nephropathy, hypertensive encephalopathy]).
- If you have the capability to keep a running summary throughout the hospital stay, do so and keep it updated. A few moments of daily careful editing and composing can save time and effort at the back end creating the discharge summary. The follow-up care provider can reconstruct the hospital course and it is your last chance to spin the narrative for the lawyers.
- Read your documentation over. Ensure that it is clear, accurate, concise, and tells the story and the plans for the patient. Make sure that someone reading the note will know what you were thinking.
- Set up a program to self-audit documentation where monthly or quarterly, you and your partners mutually review a certain number of records and give each other feedback. Design an assessment tool which rates the quality of documentation elements which your hospital/network/service line values (clarity, copy and paste, complete and specific diagnoses, etc.). You know who the best documenters are. Why do you think their documentation is superior? How can you emulate them?
Finally, answer CDI queries. The CDI specialist is your ally, not your enemy. They want you to get credit for taking care of sick and complex patients. They are not permitted to lead the provider, so don’t ask them what they want you to write. But, if you don’t understand the query or issue, have a conversation and get it clarified. It is in everyone’s best interest to get this right.
Documentation improves patient care and demonstrates that you provided excellent patient care. Put mentation back into documentation.
Dr. Remer was a practicing emergency physician for 25 years and a physician advisor for 4 years. She is on the board of directors of the American College of Physician Advisors and the advisory board of the Association of Clinical Documentation Improvement Specialists. She currently provides consulting services for provider education on documentation, CDI, and ICD-10 coding. Dr. Remer can be reached at [email protected]
Documentation has always been part of a physician’s job. Historically, in the days of paper records, physicians saw a patient on rounds and immediately following, while still on the unit, wrote a daily note detailing the events, test results, and plans since the last note. Addenda were written over the course of the day and night as needed.
The medical record was a chronological itemization of the encounter. The chart told the patient’s story, hopefully legibly and without excessive rehashing of previous material. The discharge summary then encapsulated the hospitalization in several coherent paragraphs.
In the current electronic records environment, we are inundated with excessive and repetitious information, data without interpretation, differentials without diagnoses. Prepopulation of templated notes, defaults without edit, and dictation without revision have degraded our documentation to the point of unintelligibility. The chronological storytelling and trustworthiness of the medical record has become suspect.
The Centers for Medicare & Medicaid Services is touting its “Patients over Paperwork” initiative. The solution is flawed (that is, future relaxation of documentation requirements for professional billing) because the premise is delusive. Documentation isn’t fundamentally the problem. Having clinicians jump through regulatory hoops which do not advance patients’ care, and providers misunderstanding the requirements for level-of-service billing are the essential issues. Getting no training on how to properly document in medical school/residency and receiving no formative feedback on documentation throughout one’s career compounds the problem. Having clinical documentation serve too many masters, including compliance, quality, medicolegal, utilization review, and reimbursement, is also to blame. The advent of the electronic medical record was just the straw that broke the camel’s back.
Many hospitals now have a clinical documentation integrity (CDI) team which is tasked with querying the provider when the health record documentation is conflicting, imprecise, incomplete, illegible, ambiguous, or inconsistent. They are charged with getting practitioners to associate clinical indicators with diagnoses and to consider removal of diagnoses which do not seem clinically valid from the existing documentation. From this explanation, you might well conclude that the CDI specialist could generate a query on every patient if they were so inclined, and you would be correct. But the goal isn’t to torture the physician – it is to ensure that the medical record is accurately depicting the encounter.
You are not being asked for more documentation by the CDI team; they are entreating you for higher-quality documentation. Let me give you some pointers to ward off queries.
- Tell the story. The most important goal of documentation is to clinically communicate to other caregivers. Think to yourself: “What would a fellow clinician need to know about this patient to understand why I drew those conclusions or to pick up where I left off?” At 2 a.m., that information, or lack thereof, could literally be a matter of life or death.
- Tell the truth. Embellishing the record or including invalid diagnoses with the intent to increase the severity of illness resulting in a more favorable diagnosis-related group – the inpatient risk-adjustment system – is considered fraud.
- You may like the convenience of copy forward, but do you relish reading other people’s copy and paste? Consider doing a documentation time-out. Before you copy and paste yesterday’s assessment and plan, stop and think: “Why is the patient still here? Why are we doing what we are doing?” If you choose to copy and paste, be certain to do mindful editing so the documentation represents the current situation and avoids redundancy. Appropriately editing copy and pasted documentation may prove more time consuming than generating a note de novo.
- Translate findings into diagnoses using your best medical judgment. One man’s hypotension may be another health care provider’s shock. Coders are not clinical and are not permitted to make inferences. A potassium of 6.7 may be hyperkalemia or it may be spurious – only a clinician may make that determination using their clinical expertise and experience. The coder is not allowed to read your mind. You must explicitly draw the conclusion that a febrile patient with bacteremia, encephalopathy, hypoxemia, and a blood pressure of 85/60 is in septic shock.
- Uncertain diagnoses (heralded by words such as: likely, possible, probable, suspected, rule out, etc.) which are not ruled out prior to discharge or demise are coded as if they were definitively present, for the inpatient technical side of hospital billing. This is distinctly different than the professional fee where you can only code definitive diagnoses. If you have a strong suspicion (not wild speculation) that a condition is present, best practice is to offer an uncertain diagnosis. Associate signs and symptoms with your most likely diagnosis: “Shortness of breath, pleuritic chest pain, and hypoxemia in the setting of cancer, probable pulmonary embolism.”
- Evolve, resolve, remove, and recap. If an uncertain diagnosis is ruled in, take away the uncertainty. If it is ruled out, don’t have 4 days of copy and pasted: “Possible eosinophilic pneumonia.” You do not have to maintain a resolved diagnosis ad infinitum. It can drop off the diagnosis list but be sure to have it reappear in the discharge summary.
- I know it can be a hASSLe to do excellent documentation, but it is critical for many reasons, most importantly for superlative patient care. More accurate coding and billing is an intended consequence. A: Acuity; S: Severity; S: Specificity (may affect the coding and the risk-adjustment implications. Acute systolic heart failure does not equal heart failure; type 2 diabetes mellitus with diabetic chronic kidney disease, stage 4 does not equal chronic kidney disease); and L: Linkage (of diagnosis with underlying cause or manifestation [e.g., because of, associated with, as a result of, secondary to, or from diabetic nephropathy, hypertensive encephalopathy]).
- If you have the capability to keep a running summary throughout the hospital stay, do so and keep it updated. A few moments of daily careful editing and composing can save time and effort at the back end creating the discharge summary. The follow-up care provider can reconstruct the hospital course and it is your last chance to spin the narrative for the lawyers.
- Read your documentation over. Ensure that it is clear, accurate, concise, and tells the story and the plans for the patient. Make sure that someone reading the note will know what you were thinking.
- Set up a program to self-audit documentation where monthly or quarterly, you and your partners mutually review a certain number of records and give each other feedback. Design an assessment tool which rates the quality of documentation elements which your hospital/network/service line values (clarity, copy and paste, complete and specific diagnoses, etc.). You know who the best documenters are. Why do you think their documentation is superior? How can you emulate them?
Finally, answer CDI queries. The CDI specialist is your ally, not your enemy. They want you to get credit for taking care of sick and complex patients. They are not permitted to lead the provider, so don’t ask them what they want you to write. But, if you don’t understand the query or issue, have a conversation and get it clarified. It is in everyone’s best interest to get this right.
Documentation improves patient care and demonstrates that you provided excellent patient care. Put mentation back into documentation.
Dr. Remer was a practicing emergency physician for 25 years and a physician advisor for 4 years. She is on the board of directors of the American College of Physician Advisors and the advisory board of the Association of Clinical Documentation Improvement Specialists. She currently provides consulting services for provider education on documentation, CDI, and ICD-10 coding. Dr. Remer can be reached at [email protected]
Novel coronavirus cases now at 11; entry ban and quarantine measures begin
, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a Centers for Disease Control and Prevention press briefing.
Four of the new cases are in California, and one in Massachusetts. Although four of the new cases have recent travel history to Wuhan, China, the epicenter of the 2019-nCoV outbreak, the fifth is a close household contact of one of the other California patients, said Dr. Messonnier. This last case is the second instance of person-to-person spread of 2019-nCoV in the United States.
“We expect to find additional cases of the novel coronavirus in the United States,” she said. “We expect to see more cases of person-to-person spread among close contacts. And we continue to expect this will happen given the explosive nature of this outbreak in China.”
As of the morning of Feb. 3, 167 persons under investigation, or PUIs, for possible 2019-nCoV have tested negative for the virus, and an additional 82 PUIs have testing pending – this latter figure includes some tests that are still in transit to the CDC, said Dr. Messonnier.
During the briefing, Dr. Messonnier emphasized both the aggressive nature of the U.S. public health response and the rationale for quick and assertive action. “The goal of our public health response is to protect and contain,” she said. “Strong measures now may blunt the impact of this virus on the United States.”
She cited the intensity of transmission in Hubei Province, the expansion of transmission to other provinces in China, the expansion of cases outside of China, and sporadic ongoing deaths from 2019-nCoV as drivers of the aggressive U.S. public health response.
A presidential proclamation is currently in place that bars U.S. entry to foreign nationals who have visited mainland China within the past 14 days; the ban does not apply to travelers from Hong Kong and Macao. Immediate family members of U.S. citizens and individuals who have U.S. permanent resident status are exempted from the entry ban and will be allowed entry into the United States.
However, explained Dr. Messonnier, those who have traveled to China recently and are permitted entry will be subject to screening. All passengers with such recent travel will be directed to one of 11 U.S. airports set up to perform additional screening.
As of Feb 3, the list of airports includes:
- San Francisco International Airport in California.
- Los Angeles International Airport in California.
- Hartsfield-Jackson Atlanta International Airport in Georgia.
- Daniel K. Inouye International Airport in Hawaii.
- O’Hare International Airport in Illinois.
- Detroit Metropolitan Airport in Michigan.
- Newark Liberty International Airport in New Jersey.
- John F. Kennedy International Airport in New York.
- Dallas/Fort Worth International Airport in Texas.
- Washington Dulles International Airport in Virginia.
- Seattle-Tacoma International Airport in Washington.
Travelers who have been to Hubei Province in the previous 14 days will have an additional health assessment at which they will be screened for fever, cough, or difficulty breathing. Any American citizens or exempt individuals who are symptomatic would then be transferred for further medical evaluation. Asymptomatic travelers in this category will be subject to a mandatory 14-day quarantine near their point of entry, rather than continuing on to their final destinations.
Dr. Messonnier emphasized that the mandatory 14-day quarantine is specifically for Americans or exempt individuals returning from Hubei Province, adding that the CDC is presently working with individual states to determine the exact venues for quarantine.
American citizens and exempt individuals returning from other parts of mainland China will be routed to one of the 11 airports and will also receive additional health screening. Symptomatic individuals in this travel category would be referred for further evaluation before being able to complete their itinerary.
Asymptomatic American citizens and exempt individuals who are returning from mainland China – but not Hubei Province – will be allowed to travel on to their final destinations, but will be asked to stay home as much as possible and to monitor their health during the 14 days after their return.
The U.S. Department of State is bringing back more Americans from Wuhan province this week, and these individuals will also be kept under federal quarantine for 14 days.
“There are likely to be confirmed infections among returning travelers,” said Dr. Messonnier. “It is important to note that this strategy is not meant to catch every single traveler returning from China with novel coronavirus; given the nature of this virus and how it’s spreading, that would be impossible, but working together we can catch the majority of them.
“The goal here is to slow the entry of this virus into the United States,” she said, adding that the nation’s health care and public health systems stand on high alert to detect the virus in community settings. In response to questioning from the press, Dr. Messonnier defended the stringent quarantine measures, noting that they are in line with those taken by some other nations, and with the aggressive action being taken by the Chinese government itself. “These actions are science based and aimed at protecting the health of all Americans,” she said.
The real-time reverse transcription polymerase chain reaction (rRT-PCR) assay that the CDC has developed detects 2019-nCoV in both respiratory and serum specimens. Dr. Messonnier reported that the CDC is today filing an emergency use authorization (EUA) application to the U.S. Food and Drug Administration to expedite access to the assay for public health laboratories across the country. “This will greatly enhance our capacity to test for this virus,” she said, noting that EUA approval may come as soon as the end of this week.
Although the CDC is poised to send an expert team to China, it’s still awaiting favorable results from the international negotiations currently underway. “This is a horrible situation in China,” said Dr. Messonnier. “Our presence on the ground in China would be a help to China. ... Science should trump everything else; that’s what we’re hoping – that the scientific expertise of the global community can be brought to bear on the incredibly complicated, difficult situation that our colleagues in China are dealing with.”
, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a Centers for Disease Control and Prevention press briefing.
Four of the new cases are in California, and one in Massachusetts. Although four of the new cases have recent travel history to Wuhan, China, the epicenter of the 2019-nCoV outbreak, the fifth is a close household contact of one of the other California patients, said Dr. Messonnier. This last case is the second instance of person-to-person spread of 2019-nCoV in the United States.
“We expect to find additional cases of the novel coronavirus in the United States,” she said. “We expect to see more cases of person-to-person spread among close contacts. And we continue to expect this will happen given the explosive nature of this outbreak in China.”
As of the morning of Feb. 3, 167 persons under investigation, or PUIs, for possible 2019-nCoV have tested negative for the virus, and an additional 82 PUIs have testing pending – this latter figure includes some tests that are still in transit to the CDC, said Dr. Messonnier.
During the briefing, Dr. Messonnier emphasized both the aggressive nature of the U.S. public health response and the rationale for quick and assertive action. “The goal of our public health response is to protect and contain,” she said. “Strong measures now may blunt the impact of this virus on the United States.”
She cited the intensity of transmission in Hubei Province, the expansion of transmission to other provinces in China, the expansion of cases outside of China, and sporadic ongoing deaths from 2019-nCoV as drivers of the aggressive U.S. public health response.
A presidential proclamation is currently in place that bars U.S. entry to foreign nationals who have visited mainland China within the past 14 days; the ban does not apply to travelers from Hong Kong and Macao. Immediate family members of U.S. citizens and individuals who have U.S. permanent resident status are exempted from the entry ban and will be allowed entry into the United States.
However, explained Dr. Messonnier, those who have traveled to China recently and are permitted entry will be subject to screening. All passengers with such recent travel will be directed to one of 11 U.S. airports set up to perform additional screening.
As of Feb 3, the list of airports includes:
- San Francisco International Airport in California.
- Los Angeles International Airport in California.
- Hartsfield-Jackson Atlanta International Airport in Georgia.
- Daniel K. Inouye International Airport in Hawaii.
- O’Hare International Airport in Illinois.
- Detroit Metropolitan Airport in Michigan.
- Newark Liberty International Airport in New Jersey.
- John F. Kennedy International Airport in New York.
- Dallas/Fort Worth International Airport in Texas.
- Washington Dulles International Airport in Virginia.
- Seattle-Tacoma International Airport in Washington.
Travelers who have been to Hubei Province in the previous 14 days will have an additional health assessment at which they will be screened for fever, cough, or difficulty breathing. Any American citizens or exempt individuals who are symptomatic would then be transferred for further medical evaluation. Asymptomatic travelers in this category will be subject to a mandatory 14-day quarantine near their point of entry, rather than continuing on to their final destinations.
Dr. Messonnier emphasized that the mandatory 14-day quarantine is specifically for Americans or exempt individuals returning from Hubei Province, adding that the CDC is presently working with individual states to determine the exact venues for quarantine.
American citizens and exempt individuals returning from other parts of mainland China will be routed to one of the 11 airports and will also receive additional health screening. Symptomatic individuals in this travel category would be referred for further evaluation before being able to complete their itinerary.
Asymptomatic American citizens and exempt individuals who are returning from mainland China – but not Hubei Province – will be allowed to travel on to their final destinations, but will be asked to stay home as much as possible and to monitor their health during the 14 days after their return.
The U.S. Department of State is bringing back more Americans from Wuhan province this week, and these individuals will also be kept under federal quarantine for 14 days.
“There are likely to be confirmed infections among returning travelers,” said Dr. Messonnier. “It is important to note that this strategy is not meant to catch every single traveler returning from China with novel coronavirus; given the nature of this virus and how it’s spreading, that would be impossible, but working together we can catch the majority of them.
“The goal here is to slow the entry of this virus into the United States,” she said, adding that the nation’s health care and public health systems stand on high alert to detect the virus in community settings. In response to questioning from the press, Dr. Messonnier defended the stringent quarantine measures, noting that they are in line with those taken by some other nations, and with the aggressive action being taken by the Chinese government itself. “These actions are science based and aimed at protecting the health of all Americans,” she said.
The real-time reverse transcription polymerase chain reaction (rRT-PCR) assay that the CDC has developed detects 2019-nCoV in both respiratory and serum specimens. Dr. Messonnier reported that the CDC is today filing an emergency use authorization (EUA) application to the U.S. Food and Drug Administration to expedite access to the assay for public health laboratories across the country. “This will greatly enhance our capacity to test for this virus,” she said, noting that EUA approval may come as soon as the end of this week.
Although the CDC is poised to send an expert team to China, it’s still awaiting favorable results from the international negotiations currently underway. “This is a horrible situation in China,” said Dr. Messonnier. “Our presence on the ground in China would be a help to China. ... Science should trump everything else; that’s what we’re hoping – that the scientific expertise of the global community can be brought to bear on the incredibly complicated, difficult situation that our colleagues in China are dealing with.”
, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a Centers for Disease Control and Prevention press briefing.
Four of the new cases are in California, and one in Massachusetts. Although four of the new cases have recent travel history to Wuhan, China, the epicenter of the 2019-nCoV outbreak, the fifth is a close household contact of one of the other California patients, said Dr. Messonnier. This last case is the second instance of person-to-person spread of 2019-nCoV in the United States.
“We expect to find additional cases of the novel coronavirus in the United States,” she said. “We expect to see more cases of person-to-person spread among close contacts. And we continue to expect this will happen given the explosive nature of this outbreak in China.”
As of the morning of Feb. 3, 167 persons under investigation, or PUIs, for possible 2019-nCoV have tested negative for the virus, and an additional 82 PUIs have testing pending – this latter figure includes some tests that are still in transit to the CDC, said Dr. Messonnier.
During the briefing, Dr. Messonnier emphasized both the aggressive nature of the U.S. public health response and the rationale for quick and assertive action. “The goal of our public health response is to protect and contain,” she said. “Strong measures now may blunt the impact of this virus on the United States.”
She cited the intensity of transmission in Hubei Province, the expansion of transmission to other provinces in China, the expansion of cases outside of China, and sporadic ongoing deaths from 2019-nCoV as drivers of the aggressive U.S. public health response.
A presidential proclamation is currently in place that bars U.S. entry to foreign nationals who have visited mainland China within the past 14 days; the ban does not apply to travelers from Hong Kong and Macao. Immediate family members of U.S. citizens and individuals who have U.S. permanent resident status are exempted from the entry ban and will be allowed entry into the United States.
However, explained Dr. Messonnier, those who have traveled to China recently and are permitted entry will be subject to screening. All passengers with such recent travel will be directed to one of 11 U.S. airports set up to perform additional screening.
As of Feb 3, the list of airports includes:
- San Francisco International Airport in California.
- Los Angeles International Airport in California.
- Hartsfield-Jackson Atlanta International Airport in Georgia.
- Daniel K. Inouye International Airport in Hawaii.
- O’Hare International Airport in Illinois.
- Detroit Metropolitan Airport in Michigan.
- Newark Liberty International Airport in New Jersey.
- John F. Kennedy International Airport in New York.
- Dallas/Fort Worth International Airport in Texas.
- Washington Dulles International Airport in Virginia.
- Seattle-Tacoma International Airport in Washington.
Travelers who have been to Hubei Province in the previous 14 days will have an additional health assessment at which they will be screened for fever, cough, or difficulty breathing. Any American citizens or exempt individuals who are symptomatic would then be transferred for further medical evaluation. Asymptomatic travelers in this category will be subject to a mandatory 14-day quarantine near their point of entry, rather than continuing on to their final destinations.
Dr. Messonnier emphasized that the mandatory 14-day quarantine is specifically for Americans or exempt individuals returning from Hubei Province, adding that the CDC is presently working with individual states to determine the exact venues for quarantine.
American citizens and exempt individuals returning from other parts of mainland China will be routed to one of the 11 airports and will also receive additional health screening. Symptomatic individuals in this travel category would be referred for further evaluation before being able to complete their itinerary.
Asymptomatic American citizens and exempt individuals who are returning from mainland China – but not Hubei Province – will be allowed to travel on to their final destinations, but will be asked to stay home as much as possible and to monitor their health during the 14 days after their return.
The U.S. Department of State is bringing back more Americans from Wuhan province this week, and these individuals will also be kept under federal quarantine for 14 days.
“There are likely to be confirmed infections among returning travelers,” said Dr. Messonnier. “It is important to note that this strategy is not meant to catch every single traveler returning from China with novel coronavirus; given the nature of this virus and how it’s spreading, that would be impossible, but working together we can catch the majority of them.
“The goal here is to slow the entry of this virus into the United States,” she said, adding that the nation’s health care and public health systems stand on high alert to detect the virus in community settings. In response to questioning from the press, Dr. Messonnier defended the stringent quarantine measures, noting that they are in line with those taken by some other nations, and with the aggressive action being taken by the Chinese government itself. “These actions are science based and aimed at protecting the health of all Americans,” she said.
The real-time reverse transcription polymerase chain reaction (rRT-PCR) assay that the CDC has developed detects 2019-nCoV in both respiratory and serum specimens. Dr. Messonnier reported that the CDC is today filing an emergency use authorization (EUA) application to the U.S. Food and Drug Administration to expedite access to the assay for public health laboratories across the country. “This will greatly enhance our capacity to test for this virus,” she said, noting that EUA approval may come as soon as the end of this week.
Although the CDC is poised to send an expert team to China, it’s still awaiting favorable results from the international negotiations currently underway. “This is a horrible situation in China,” said Dr. Messonnier. “Our presence on the ground in China would be a help to China. ... Science should trump everything else; that’s what we’re hoping – that the scientific expertise of the global community can be brought to bear on the incredibly complicated, difficult situation that our colleagues in China are dealing with.”
FROM A CDC PRESS BRIEFING
Don’t forget about the flu: 2019-2010 season is not over
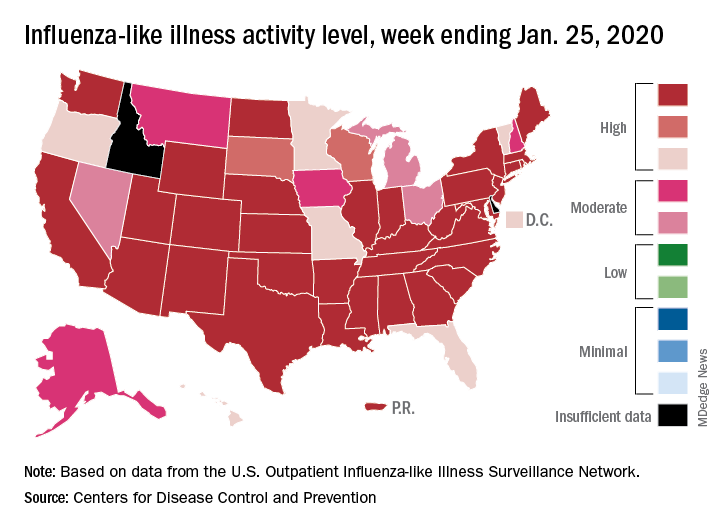
Nationally, an estimated 5.7% of all outpatients visiting health care providers had influenza-like illness (ILI) for the week ending Jan. 25, which was up from 5.1% the previous week but still lower than the current seasonal high of 7.1% recorded during the week of Dec. 22-28, the CDC’s influenza division reported.
Another key indicator of influenza activity, the percentage of respiratory specimens testing positive, also remains high as it rose from 25.7% the week before to 27.7% for the week ending Jan. 25, the influenza division said. That is the highest rate of the 2019-2020 season so far, surpassing the 26.8% reached during Dec. 22-28.
Another new seasonal high involves the number of states, 33 plus Puerto Rico, at the highest level of ILI activity on the CDC’s 1-10 scale for the latest reporting week, topping the 32 jurisdictions from the last full week of December. Another eight states and the District of Columbia were in the “high” range with activity levels of 8 and 9, and no state with available data was lower than level 6, the CDC data show.
Going along with the recent 2-week increase in activity is a large increase in the number of ILI-related pediatric deaths, which rose from 39 on Jan. 11 to the current count of 68, the CDC said. At the same point last year, there had been 36 pediatric deaths.
Other indicators of ILI severity, however, “are not high at this point in the season,” the influenza division noted. “Overall, hospitalization rates remain similar to what has been seen at this time during recent seasons, but rates among children and young adults are higher at this time than in recent seasons.” Overall pneumonia and influenza mortality is also low, the CDC added.

Nationally, an estimated 5.7% of all outpatients visiting health care providers had influenza-like illness (ILI) for the week ending Jan. 25, which was up from 5.1% the previous week but still lower than the current seasonal high of 7.1% recorded during the week of Dec. 22-28, the CDC’s influenza division reported.
Another key indicator of influenza activity, the percentage of respiratory specimens testing positive, also remains high as it rose from 25.7% the week before to 27.7% for the week ending Jan. 25, the influenza division said. That is the highest rate of the 2019-2020 season so far, surpassing the 26.8% reached during Dec. 22-28.
Another new seasonal high involves the number of states, 33 plus Puerto Rico, at the highest level of ILI activity on the CDC’s 1-10 scale for the latest reporting week, topping the 32 jurisdictions from the last full week of December. Another eight states and the District of Columbia were in the “high” range with activity levels of 8 and 9, and no state with available data was lower than level 6, the CDC data show.
Going along with the recent 2-week increase in activity is a large increase in the number of ILI-related pediatric deaths, which rose from 39 on Jan. 11 to the current count of 68, the CDC said. At the same point last year, there had been 36 pediatric deaths.
Other indicators of ILI severity, however, “are not high at this point in the season,” the influenza division noted. “Overall, hospitalization rates remain similar to what has been seen at this time during recent seasons, but rates among children and young adults are higher at this time than in recent seasons.” Overall pneumonia and influenza mortality is also low, the CDC added.

Nationally, an estimated 5.7% of all outpatients visiting health care providers had influenza-like illness (ILI) for the week ending Jan. 25, which was up from 5.1% the previous week but still lower than the current seasonal high of 7.1% recorded during the week of Dec. 22-28, the CDC’s influenza division reported.
Another key indicator of influenza activity, the percentage of respiratory specimens testing positive, also remains high as it rose from 25.7% the week before to 27.7% for the week ending Jan. 25, the influenza division said. That is the highest rate of the 2019-2020 season so far, surpassing the 26.8% reached during Dec. 22-28.
Another new seasonal high involves the number of states, 33 plus Puerto Rico, at the highest level of ILI activity on the CDC’s 1-10 scale for the latest reporting week, topping the 32 jurisdictions from the last full week of December. Another eight states and the District of Columbia were in the “high” range with activity levels of 8 and 9, and no state with available data was lower than level 6, the CDC data show.
Going along with the recent 2-week increase in activity is a large increase in the number of ILI-related pediatric deaths, which rose from 39 on Jan. 11 to the current count of 68, the CDC said. At the same point last year, there had been 36 pediatric deaths.
Other indicators of ILI severity, however, “are not high at this point in the season,” the influenza division noted. “Overall, hospitalization rates remain similar to what has been seen at this time during recent seasons, but rates among children and young adults are higher at this time than in recent seasons.” Overall pneumonia and influenza mortality is also low, the CDC added.
Initial ultrasound assessment of appendicitis curbs costs
Assessing appendicitis in children with initial ultrasound followed by computed tomography in the absence of appendix visualization and presence of secondary signs was the most cost-effective approach, according to data from a modeling study of 10 strategies.
Ultrasound is safer and less expensive than computed tomography and avoids radiation exposure; however, cost-effectiveness models of various approaches to imaging have not been well studied, wrote Rebecca Jennings, MD, of Seattle Children’s Hospital, Washington, and colleagues.
In a study published in Pediatrics, the researchers simulated a hypothetical patient population using a Markov cohort model and compared 10 different strategies including CT only, MRI only, and ultrasound followed by CT or MRI after ultrasounds that are negative or fail to visualize the appendix.
Overall, the most cost-effective strategy for moderate-risk patients was the use of ultrasound followed by CT or MRI if the ultrasound failed to visualize the appendix and secondary signs of inflammation were present in the right lower quadrant. The cost of this strategy was $4,815, with effectiveness of 0.99694 quality-adjusted life-years. “The most cost-effective strategy is highly dependent on a patient’s risk stratification,” the researchers noted. Based on their model, imaging was not cost effective for patients with a prevalence less than 16% or greater than 95%. However, those with appendicitis risk between 16% and 95% and no secondary signs of inflammation can forgo further imaging, even without visualization of the appendix for maximum cost-effectiveness, the researchers said.
The study was limited by several factors, including the inability to account for all potential costs related to imaging and outcomes, lack of accounting for the use of sedation when assessing costs, and inability to separate imaging costs from total hospital costs, the researchers noted. However, the results suggest that tailored imaging approaches based on patient risk are the most cost-effective strategies to assess appendicitis, they said.
“The diagnosis and exclusion of appendicitis continues to be one of the primary concerns of providers who care for children with abdominal pain,” wrote Rebecca M. Rentea, MD, and Charles L. Snyder, MD, of Children’s Mercy Hospital Kansas City, Mo., in an accompanying editorial (Pediatrics. 2020 Feb;145:e20193349).
“The best diagnostic and imaging approach to appendicitis has been a topic of interest for some time, and improvements such as appendicitis scoring systems, decreased use of ionized radiation, and adoption of clinical algorithms have been incremental but steady,” they said. Despite the potential of missed appendicitis, the use of an algorithm based on an initial ultrasound and previous possibility of appendicitis described in the study was the most cost effective, they said. In addition, “the ability to visualize the appendix did not alter the most cost-effective approach in those with a moderate risk of appendicitis (most patients),” they concluded.
The study was supported by the University of Washington and Seattle Children’s Hospital Quality Improvement Scholars Program. The researchers had no financial conflicts to disclose.
Dr. Rentea and Dr. Snyder had no financial conflicts to disclose.
SOURCE: Jennings R et al. Pediatrics. 2020. doi: 10.1542/peds.2019-1352.
Assessing appendicitis in children with initial ultrasound followed by computed tomography in the absence of appendix visualization and presence of secondary signs was the most cost-effective approach, according to data from a modeling study of 10 strategies.
Ultrasound is safer and less expensive than computed tomography and avoids radiation exposure; however, cost-effectiveness models of various approaches to imaging have not been well studied, wrote Rebecca Jennings, MD, of Seattle Children’s Hospital, Washington, and colleagues.
In a study published in Pediatrics, the researchers simulated a hypothetical patient population using a Markov cohort model and compared 10 different strategies including CT only, MRI only, and ultrasound followed by CT or MRI after ultrasounds that are negative or fail to visualize the appendix.
Overall, the most cost-effective strategy for moderate-risk patients was the use of ultrasound followed by CT or MRI if the ultrasound failed to visualize the appendix and secondary signs of inflammation were present in the right lower quadrant. The cost of this strategy was $4,815, with effectiveness of 0.99694 quality-adjusted life-years. “The most cost-effective strategy is highly dependent on a patient’s risk stratification,” the researchers noted. Based on their model, imaging was not cost effective for patients with a prevalence less than 16% or greater than 95%. However, those with appendicitis risk between 16% and 95% and no secondary signs of inflammation can forgo further imaging, even without visualization of the appendix for maximum cost-effectiveness, the researchers said.
The study was limited by several factors, including the inability to account for all potential costs related to imaging and outcomes, lack of accounting for the use of sedation when assessing costs, and inability to separate imaging costs from total hospital costs, the researchers noted. However, the results suggest that tailored imaging approaches based on patient risk are the most cost-effective strategies to assess appendicitis, they said.
“The diagnosis and exclusion of appendicitis continues to be one of the primary concerns of providers who care for children with abdominal pain,” wrote Rebecca M. Rentea, MD, and Charles L. Snyder, MD, of Children’s Mercy Hospital Kansas City, Mo., in an accompanying editorial (Pediatrics. 2020 Feb;145:e20193349).
“The best diagnostic and imaging approach to appendicitis has been a topic of interest for some time, and improvements such as appendicitis scoring systems, decreased use of ionized radiation, and adoption of clinical algorithms have been incremental but steady,” they said. Despite the potential of missed appendicitis, the use of an algorithm based on an initial ultrasound and previous possibility of appendicitis described in the study was the most cost effective, they said. In addition, “the ability to visualize the appendix did not alter the most cost-effective approach in those with a moderate risk of appendicitis (most patients),” they concluded.
The study was supported by the University of Washington and Seattle Children’s Hospital Quality Improvement Scholars Program. The researchers had no financial conflicts to disclose.
Dr. Rentea and Dr. Snyder had no financial conflicts to disclose.
SOURCE: Jennings R et al. Pediatrics. 2020. doi: 10.1542/peds.2019-1352.
Assessing appendicitis in children with initial ultrasound followed by computed tomography in the absence of appendix visualization and presence of secondary signs was the most cost-effective approach, according to data from a modeling study of 10 strategies.
Ultrasound is safer and less expensive than computed tomography and avoids radiation exposure; however, cost-effectiveness models of various approaches to imaging have not been well studied, wrote Rebecca Jennings, MD, of Seattle Children’s Hospital, Washington, and colleagues.
In a study published in Pediatrics, the researchers simulated a hypothetical patient population using a Markov cohort model and compared 10 different strategies including CT only, MRI only, and ultrasound followed by CT or MRI after ultrasounds that are negative or fail to visualize the appendix.
Overall, the most cost-effective strategy for moderate-risk patients was the use of ultrasound followed by CT or MRI if the ultrasound failed to visualize the appendix and secondary signs of inflammation were present in the right lower quadrant. The cost of this strategy was $4,815, with effectiveness of 0.99694 quality-adjusted life-years. “The most cost-effective strategy is highly dependent on a patient’s risk stratification,” the researchers noted. Based on their model, imaging was not cost effective for patients with a prevalence less than 16% or greater than 95%. However, those with appendicitis risk between 16% and 95% and no secondary signs of inflammation can forgo further imaging, even without visualization of the appendix for maximum cost-effectiveness, the researchers said.
The study was limited by several factors, including the inability to account for all potential costs related to imaging and outcomes, lack of accounting for the use of sedation when assessing costs, and inability to separate imaging costs from total hospital costs, the researchers noted. However, the results suggest that tailored imaging approaches based on patient risk are the most cost-effective strategies to assess appendicitis, they said.
“The diagnosis and exclusion of appendicitis continues to be one of the primary concerns of providers who care for children with abdominal pain,” wrote Rebecca M. Rentea, MD, and Charles L. Snyder, MD, of Children’s Mercy Hospital Kansas City, Mo., in an accompanying editorial (Pediatrics. 2020 Feb;145:e20193349).
“The best diagnostic and imaging approach to appendicitis has been a topic of interest for some time, and improvements such as appendicitis scoring systems, decreased use of ionized radiation, and adoption of clinical algorithms have been incremental but steady,” they said. Despite the potential of missed appendicitis, the use of an algorithm based on an initial ultrasound and previous possibility of appendicitis described in the study was the most cost effective, they said. In addition, “the ability to visualize the appendix did not alter the most cost-effective approach in those with a moderate risk of appendicitis (most patients),” they concluded.
The study was supported by the University of Washington and Seattle Children’s Hospital Quality Improvement Scholars Program. The researchers had no financial conflicts to disclose.
Dr. Rentea and Dr. Snyder had no financial conflicts to disclose.
SOURCE: Jennings R et al. Pediatrics. 2020. doi: 10.1542/peds.2019-1352.
FROM PEDIATRICS
Noninjectable modes of insulin delivery coming of age
LOS ANGELES – Injections may be the most common way for patients with diabetes to take insulin, but other modes of delivery are coming of age.
George Grunberger, MD, chairman of the Grunberger Diabetes Institute in Bloomfield Township, Mich., said that at least seven different agents that are being studied for the oral delivery of biologics for diabetes.
He outlined several at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
Oral insulin
ORMD-0801 from Oramed is an oral insulin capsule that prevents enzyme degradation and enhances intestinal absorption. Top-line, unpublished findings from a phase 2 study, which the company announced in November 2019, showed that ORMD-0801 significantly reduced hemoglobin A1c levels in patients with type 2 diabetes who were inadequately controlled on other standard-of-care drugs. ORMD-0801 dosed once daily reduced HbA1c by 0.60%, compared with 0.06% by placebo. “We’ll see when it’s going to wind up in the clinic,” Dr. Grunberger said. Oramed is also developing an oral glucagonlike peptide–1 analogue capsule, ORMD-0901, which has potential to be the first orally ingestible GLP-1 analogue.
Inhaled and absorbed insulin
Technosphere insulin (Affreza) is a novel inhalation powder for the treatment of diabetes that was developed by MannKind and approved by the Food and Drug Administration in 2014. Clinical studies have shown that Technosphere insulin delivers insulin with an ultrarapid pharmacokinetic profile that is different from all other insulin products, but similar to natural insulin release. “The idea was to develop a more patient-friendly device to deliver insulin directly into the lungs,” said Dr. Grunberger, who is also a clinical professor of internal medicine and molecular medicine and genetics at Wayne State University, Detroit. “When you inhale this into the lungs, there is one cell layer between the air sac and the circulation, so it works very quickly. The idea is to try to avoid injecting insulin to see if it helps. This is a prandial insulin – you inhale it before meals. The whole idea is that hopefully, you can reduce any fear of delayed postprandial hyperglycemia.”
In a randomized trial of 353 patients with inadequately controlled type 2 diabetes, those in the Technosphere insulin arm significantly reduced HbA1c by 0.8% from a baseline of 8.3%, compared with the placebo arm, which was reduced by 0.4% (P less than .0001; Diabetes Care. 2015;38[12]:2274-81). A greater number of patients treated with Technosphere insulin achieved an HbA1c of 7.0% or less, compared with placebo (38% vs. 19%; P = .0005). Dr. Grunberger noted that, in clinical trials lasting up to 2 years, patients treated with Technosphere insulin had a 40-mL greater decline from baseline in forced expiratory volume in 1 second (FEV1 ), compared with patients treated with comparator antidiabetes treatments. “But once you stop using the drug, FEV1 reverts to normal,” he said. “So, there does not appear to be lasting damage to your lungs and respiratory ability.”
In another development, Oral-Lyn from Generex Biotechnology, which delivers insulin through the oral mucosa, is being evaluated as a potential treatment option. In 2015, Generex partnered with the University of Toronto’s Center for Molecular Design and Preformulations to increase the bioavailability of insulin in the product and to reduce the number of sprays required to achieve effective prandial glucose control. In 2019, the company formed the NuGenerex Diabetes Research Center, which intended to accelerate the development of the reformulated Oral-Lyn-2, for type 2 diabetes, and Altsulin, for the treatment of type 1 diabetes. The programs are expected to initiate in the first quarter of 2020.
In the meantime, studies of intranasally delivered insulin continue to advance. “It works. It lowers glucose, but there is a whole slew of knowledge now about how it can also improve neurocognitive function,” Dr. Grunberger said.
Oral GLP-1 receptor agonists
Oral versions of glucagonlike peptide–1 (GLP-1) receptor agonists are also emerging as a treatment option. The FDA recently approved the first oral GLP-1 receptor agonist, semaglutide bound in the absorption enhancer sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate (SNAC). According to data from manufacturer Novo Nordisk, SNAC facilitates local increase of pH, which leads to a higher solubility. SNAC interacts with cell membranes of gastric mucosa, facilitating absorption within 30 minutes, “so the drug can penetrate the mucosa without lasting damage,” Dr. Grunberger said. The SNAC effect is size dependent and fully reversible.
In PIONEER 3, researchers found that, in adults with type 2 diabetes uncontrolled with metformin with or without sulfonylurea, oral semaglutide at dosages of 7 and 14 mg/day resulted in significantly greater reductions in HbA1c over 26 weeks, compared with sitagliptin, but there was no significant benefit with the 3-mg/d dosage (JAMA. 2019;321[15]:1466-80). In PIONEER 4, researchers compared the efficacy and safety of oral semaglutide with subcutaneous liraglutide (Lancet. 2019;394[10192]:P39-50). “There was no difference in HbA1c effect between the two groups, but oral semaglutide beat out sitagliptin in terms of weight loss,” Dr. Grunberger said. “It’s going to be interesting to see what’s going to happen in the marketplace as the drug gets widely launched.”
Nasal glucagon
He closed out his presentation by discussing the July 2019 FDA approval of Eli Lilly’s nasal glucagon for severe hypoglycemia – the first such treatment that can be administered without an injection. The nasally administered dry powder, known as Baqsimi, is a welcome alternative to current glucagon kits, “which contain multiple components,” said Dr. Grunberger, who is also a past president of the American Association of Clinical Endocrinologists. An adult pivotal study showed that supraphysiologic levels of glucagon were achieved within 5 minutes with both nasal and intramuscular glucagon (Diabetes Care. 2016;39[2]:264-70). Headache and nasal symptoms occurred more frequently with nasal glucagon, but most were resolved within 1 day. In addition, nausea and vomiting occurred at similar frequencies with nasal and intramuscular glucacon, and most cases were resolved within 1 day.
Similar results were observed in a pediatric study of 48 patients with type 1 diabetes who were older than 4 years, (Diabetes Care. 2016;39[4]:555-62).
Dr. Grunberger disclosed that has research contracts with Medtronic and Eli Lilly, and that he serves on speakers bureaus of Eli Lilly, Janssen, Novo Nordisk, and Sanofi.
LOS ANGELES – Injections may be the most common way for patients with diabetes to take insulin, but other modes of delivery are coming of age.
George Grunberger, MD, chairman of the Grunberger Diabetes Institute in Bloomfield Township, Mich., said that at least seven different agents that are being studied for the oral delivery of biologics for diabetes.
He outlined several at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
Oral insulin
ORMD-0801 from Oramed is an oral insulin capsule that prevents enzyme degradation and enhances intestinal absorption. Top-line, unpublished findings from a phase 2 study, which the company announced in November 2019, showed that ORMD-0801 significantly reduced hemoglobin A1c levels in patients with type 2 diabetes who were inadequately controlled on other standard-of-care drugs. ORMD-0801 dosed once daily reduced HbA1c by 0.60%, compared with 0.06% by placebo. “We’ll see when it’s going to wind up in the clinic,” Dr. Grunberger said. Oramed is also developing an oral glucagonlike peptide–1 analogue capsule, ORMD-0901, which has potential to be the first orally ingestible GLP-1 analogue.
Inhaled and absorbed insulin
Technosphere insulin (Affreza) is a novel inhalation powder for the treatment of diabetes that was developed by MannKind and approved by the Food and Drug Administration in 2014. Clinical studies have shown that Technosphere insulin delivers insulin with an ultrarapid pharmacokinetic profile that is different from all other insulin products, but similar to natural insulin release. “The idea was to develop a more patient-friendly device to deliver insulin directly into the lungs,” said Dr. Grunberger, who is also a clinical professor of internal medicine and molecular medicine and genetics at Wayne State University, Detroit. “When you inhale this into the lungs, there is one cell layer between the air sac and the circulation, so it works very quickly. The idea is to try to avoid injecting insulin to see if it helps. This is a prandial insulin – you inhale it before meals. The whole idea is that hopefully, you can reduce any fear of delayed postprandial hyperglycemia.”
In a randomized trial of 353 patients with inadequately controlled type 2 diabetes, those in the Technosphere insulin arm significantly reduced HbA1c by 0.8% from a baseline of 8.3%, compared with the placebo arm, which was reduced by 0.4% (P less than .0001; Diabetes Care. 2015;38[12]:2274-81). A greater number of patients treated with Technosphere insulin achieved an HbA1c of 7.0% or less, compared with placebo (38% vs. 19%; P = .0005). Dr. Grunberger noted that, in clinical trials lasting up to 2 years, patients treated with Technosphere insulin had a 40-mL greater decline from baseline in forced expiratory volume in 1 second (FEV1 ), compared with patients treated with comparator antidiabetes treatments. “But once you stop using the drug, FEV1 reverts to normal,” he said. “So, there does not appear to be lasting damage to your lungs and respiratory ability.”
In another development, Oral-Lyn from Generex Biotechnology, which delivers insulin through the oral mucosa, is being evaluated as a potential treatment option. In 2015, Generex partnered with the University of Toronto’s Center for Molecular Design and Preformulations to increase the bioavailability of insulin in the product and to reduce the number of sprays required to achieve effective prandial glucose control. In 2019, the company formed the NuGenerex Diabetes Research Center, which intended to accelerate the development of the reformulated Oral-Lyn-2, for type 2 diabetes, and Altsulin, for the treatment of type 1 diabetes. The programs are expected to initiate in the first quarter of 2020.
In the meantime, studies of intranasally delivered insulin continue to advance. “It works. It lowers glucose, but there is a whole slew of knowledge now about how it can also improve neurocognitive function,” Dr. Grunberger said.
Oral GLP-1 receptor agonists
Oral versions of glucagonlike peptide–1 (GLP-1) receptor agonists are also emerging as a treatment option. The FDA recently approved the first oral GLP-1 receptor agonist, semaglutide bound in the absorption enhancer sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate (SNAC). According to data from manufacturer Novo Nordisk, SNAC facilitates local increase of pH, which leads to a higher solubility. SNAC interacts with cell membranes of gastric mucosa, facilitating absorption within 30 minutes, “so the drug can penetrate the mucosa without lasting damage,” Dr. Grunberger said. The SNAC effect is size dependent and fully reversible.
In PIONEER 3, researchers found that, in adults with type 2 diabetes uncontrolled with metformin with or without sulfonylurea, oral semaglutide at dosages of 7 and 14 mg/day resulted in significantly greater reductions in HbA1c over 26 weeks, compared with sitagliptin, but there was no significant benefit with the 3-mg/d dosage (JAMA. 2019;321[15]:1466-80). In PIONEER 4, researchers compared the efficacy and safety of oral semaglutide with subcutaneous liraglutide (Lancet. 2019;394[10192]:P39-50). “There was no difference in HbA1c effect between the two groups, but oral semaglutide beat out sitagliptin in terms of weight loss,” Dr. Grunberger said. “It’s going to be interesting to see what’s going to happen in the marketplace as the drug gets widely launched.”
Nasal glucagon
He closed out his presentation by discussing the July 2019 FDA approval of Eli Lilly’s nasal glucagon for severe hypoglycemia – the first such treatment that can be administered without an injection. The nasally administered dry powder, known as Baqsimi, is a welcome alternative to current glucagon kits, “which contain multiple components,” said Dr. Grunberger, who is also a past president of the American Association of Clinical Endocrinologists. An adult pivotal study showed that supraphysiologic levels of glucagon were achieved within 5 minutes with both nasal and intramuscular glucagon (Diabetes Care. 2016;39[2]:264-70). Headache and nasal symptoms occurred more frequently with nasal glucagon, but most were resolved within 1 day. In addition, nausea and vomiting occurred at similar frequencies with nasal and intramuscular glucacon, and most cases were resolved within 1 day.
Similar results were observed in a pediatric study of 48 patients with type 1 diabetes who were older than 4 years, (Diabetes Care. 2016;39[4]:555-62).
Dr. Grunberger disclosed that has research contracts with Medtronic and Eli Lilly, and that he serves on speakers bureaus of Eli Lilly, Janssen, Novo Nordisk, and Sanofi.
LOS ANGELES – Injections may be the most common way for patients with diabetes to take insulin, but other modes of delivery are coming of age.
George Grunberger, MD, chairman of the Grunberger Diabetes Institute in Bloomfield Township, Mich., said that at least seven different agents that are being studied for the oral delivery of biologics for diabetes.
He outlined several at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
Oral insulin
ORMD-0801 from Oramed is an oral insulin capsule that prevents enzyme degradation and enhances intestinal absorption. Top-line, unpublished findings from a phase 2 study, which the company announced in November 2019, showed that ORMD-0801 significantly reduced hemoglobin A1c levels in patients with type 2 diabetes who were inadequately controlled on other standard-of-care drugs. ORMD-0801 dosed once daily reduced HbA1c by 0.60%, compared with 0.06% by placebo. “We’ll see when it’s going to wind up in the clinic,” Dr. Grunberger said. Oramed is also developing an oral glucagonlike peptide–1 analogue capsule, ORMD-0901, which has potential to be the first orally ingestible GLP-1 analogue.
Inhaled and absorbed insulin
Technosphere insulin (Affreza) is a novel inhalation powder for the treatment of diabetes that was developed by MannKind and approved by the Food and Drug Administration in 2014. Clinical studies have shown that Technosphere insulin delivers insulin with an ultrarapid pharmacokinetic profile that is different from all other insulin products, but similar to natural insulin release. “The idea was to develop a more patient-friendly device to deliver insulin directly into the lungs,” said Dr. Grunberger, who is also a clinical professor of internal medicine and molecular medicine and genetics at Wayne State University, Detroit. “When you inhale this into the lungs, there is one cell layer between the air sac and the circulation, so it works very quickly. The idea is to try to avoid injecting insulin to see if it helps. This is a prandial insulin – you inhale it before meals. The whole idea is that hopefully, you can reduce any fear of delayed postprandial hyperglycemia.”
In a randomized trial of 353 patients with inadequately controlled type 2 diabetes, those in the Technosphere insulin arm significantly reduced HbA1c by 0.8% from a baseline of 8.3%, compared with the placebo arm, which was reduced by 0.4% (P less than .0001; Diabetes Care. 2015;38[12]:2274-81). A greater number of patients treated with Technosphere insulin achieved an HbA1c of 7.0% or less, compared with placebo (38% vs. 19%; P = .0005). Dr. Grunberger noted that, in clinical trials lasting up to 2 years, patients treated with Technosphere insulin had a 40-mL greater decline from baseline in forced expiratory volume in 1 second (FEV1 ), compared with patients treated with comparator antidiabetes treatments. “But once you stop using the drug, FEV1 reverts to normal,” he said. “So, there does not appear to be lasting damage to your lungs and respiratory ability.”
In another development, Oral-Lyn from Generex Biotechnology, which delivers insulin through the oral mucosa, is being evaluated as a potential treatment option. In 2015, Generex partnered with the University of Toronto’s Center for Molecular Design and Preformulations to increase the bioavailability of insulin in the product and to reduce the number of sprays required to achieve effective prandial glucose control. In 2019, the company formed the NuGenerex Diabetes Research Center, which intended to accelerate the development of the reformulated Oral-Lyn-2, for type 2 diabetes, and Altsulin, for the treatment of type 1 diabetes. The programs are expected to initiate in the first quarter of 2020.
In the meantime, studies of intranasally delivered insulin continue to advance. “It works. It lowers glucose, but there is a whole slew of knowledge now about how it can also improve neurocognitive function,” Dr. Grunberger said.
Oral GLP-1 receptor agonists
Oral versions of glucagonlike peptide–1 (GLP-1) receptor agonists are also emerging as a treatment option. The FDA recently approved the first oral GLP-1 receptor agonist, semaglutide bound in the absorption enhancer sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate (SNAC). According to data from manufacturer Novo Nordisk, SNAC facilitates local increase of pH, which leads to a higher solubility. SNAC interacts with cell membranes of gastric mucosa, facilitating absorption within 30 minutes, “so the drug can penetrate the mucosa without lasting damage,” Dr. Grunberger said. The SNAC effect is size dependent and fully reversible.
In PIONEER 3, researchers found that, in adults with type 2 diabetes uncontrolled with metformin with or without sulfonylurea, oral semaglutide at dosages of 7 and 14 mg/day resulted in significantly greater reductions in HbA1c over 26 weeks, compared with sitagliptin, but there was no significant benefit with the 3-mg/d dosage (JAMA. 2019;321[15]:1466-80). In PIONEER 4, researchers compared the efficacy and safety of oral semaglutide with subcutaneous liraglutide (Lancet. 2019;394[10192]:P39-50). “There was no difference in HbA1c effect between the two groups, but oral semaglutide beat out sitagliptin in terms of weight loss,” Dr. Grunberger said. “It’s going to be interesting to see what’s going to happen in the marketplace as the drug gets widely launched.”
Nasal glucagon
He closed out his presentation by discussing the July 2019 FDA approval of Eli Lilly’s nasal glucagon for severe hypoglycemia – the first such treatment that can be administered without an injection. The nasally administered dry powder, known as Baqsimi, is a welcome alternative to current glucagon kits, “which contain multiple components,” said Dr. Grunberger, who is also a past president of the American Association of Clinical Endocrinologists. An adult pivotal study showed that supraphysiologic levels of glucagon were achieved within 5 minutes with both nasal and intramuscular glucagon (Diabetes Care. 2016;39[2]:264-70). Headache and nasal symptoms occurred more frequently with nasal glucagon, but most were resolved within 1 day. In addition, nausea and vomiting occurred at similar frequencies with nasal and intramuscular glucacon, and most cases were resolved within 1 day.
Similar results were observed in a pediatric study of 48 patients with type 1 diabetes who were older than 4 years, (Diabetes Care. 2016;39[4]:555-62).
Dr. Grunberger disclosed that has research contracts with Medtronic and Eli Lilly, and that he serves on speakers bureaus of Eli Lilly, Janssen, Novo Nordisk, and Sanofi.
EXPERT ANALYSIS FROM WCIRDC 2019
HHS declares coronavirus emergency, orders quarantine
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”

The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”

The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”

The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
Developing guidance for patient movement requests
Clear guidelines in policy needed
In hospital medicine, inpatients often request more freedom to move within the hospital complex for a wide range of both benign and potentially concerning reasons, says Sara Stream, MD.
“Hospitalists are often confronted with a dilemma when considering these patient requests: how to promote patient-centered care and autonomy while balancing patient safety, concerns for hospital liability, and the delivery of timely, efficient medical care,” said Dr. Stream, a hospitalist at the VA New York Harbor Healthcare System. Guidance from medical literature and institutional policies on inpatient movement are lacking, so Dr. Stream coauthored an article seeking to develop a framework with which hospitalists can approach patient requests for liberalized movement.
The authors concluded that for a small subset of patients, liberalized movement within the hospital may be clinically feasible: those who are medically, physically, and psychiatrically stable enough to move off their assigned floors without inordinate risk. “For the rest of inpatients, movement outside their monitored inpatient settings may interfere with appropriate medical care and undermine the indications for acute hospitalization,” Dr. Stream said.
Creating institutional policy that identifies relevant clinical, legal and ethical considerations, while incorporating the varied perspectives of physicians, patients, nurses, and hospital administration/risk management will allow requests for increased movement to be evaluated systematically and transparently.
“When patients request liberalized movement, hospitalists should consider the requests systematically: first to identify the intent behind requests, and then to follow a framework to determine whether increased movement would be safe and allow appropriate medical care without creating additional risks,” Dr. Stream said.
Hospitalists should assess and compile individual patient requests for liberalized movement and work with other physicians, nurses, hospital administration, and risk management to devise pertinent policy on this issue that is specific to their institutions. “By eventually creating clear guidelines in policy, health care providers will spend less time managing each individual request to leave the floor because they have a systematic strategy for making consistent decisions about patient movement,” the authors concluded.
Reference
1. Stream S, Alfandre D. “Just Getting a Cup of Coffee” – Considering Best Practices for Patients’ Movement off the Hospital Floor. J Hosp Med. 2019 Nov. doi: 10.12788/jhm.3227.
Clear guidelines in policy needed
Clear guidelines in policy needed
In hospital medicine, inpatients often request more freedom to move within the hospital complex for a wide range of both benign and potentially concerning reasons, says Sara Stream, MD.
“Hospitalists are often confronted with a dilemma when considering these patient requests: how to promote patient-centered care and autonomy while balancing patient safety, concerns for hospital liability, and the delivery of timely, efficient medical care,” said Dr. Stream, a hospitalist at the VA New York Harbor Healthcare System. Guidance from medical literature and institutional policies on inpatient movement are lacking, so Dr. Stream coauthored an article seeking to develop a framework with which hospitalists can approach patient requests for liberalized movement.
The authors concluded that for a small subset of patients, liberalized movement within the hospital may be clinically feasible: those who are medically, physically, and psychiatrically stable enough to move off their assigned floors without inordinate risk. “For the rest of inpatients, movement outside their monitored inpatient settings may interfere with appropriate medical care and undermine the indications for acute hospitalization,” Dr. Stream said.
Creating institutional policy that identifies relevant clinical, legal and ethical considerations, while incorporating the varied perspectives of physicians, patients, nurses, and hospital administration/risk management will allow requests for increased movement to be evaluated systematically and transparently.
“When patients request liberalized movement, hospitalists should consider the requests systematically: first to identify the intent behind requests, and then to follow a framework to determine whether increased movement would be safe and allow appropriate medical care without creating additional risks,” Dr. Stream said.
Hospitalists should assess and compile individual patient requests for liberalized movement and work with other physicians, nurses, hospital administration, and risk management to devise pertinent policy on this issue that is specific to their institutions. “By eventually creating clear guidelines in policy, health care providers will spend less time managing each individual request to leave the floor because they have a systematic strategy for making consistent decisions about patient movement,” the authors concluded.
Reference
1. Stream S, Alfandre D. “Just Getting a Cup of Coffee” – Considering Best Practices for Patients’ Movement off the Hospital Floor. J Hosp Med. 2019 Nov. doi: 10.12788/jhm.3227.
In hospital medicine, inpatients often request more freedom to move within the hospital complex for a wide range of both benign and potentially concerning reasons, says Sara Stream, MD.
“Hospitalists are often confronted with a dilemma when considering these patient requests: how to promote patient-centered care and autonomy while balancing patient safety, concerns for hospital liability, and the delivery of timely, efficient medical care,” said Dr. Stream, a hospitalist at the VA New York Harbor Healthcare System. Guidance from medical literature and institutional policies on inpatient movement are lacking, so Dr. Stream coauthored an article seeking to develop a framework with which hospitalists can approach patient requests for liberalized movement.
The authors concluded that for a small subset of patients, liberalized movement within the hospital may be clinically feasible: those who are medically, physically, and psychiatrically stable enough to move off their assigned floors without inordinate risk. “For the rest of inpatients, movement outside their monitored inpatient settings may interfere with appropriate medical care and undermine the indications for acute hospitalization,” Dr. Stream said.
Creating institutional policy that identifies relevant clinical, legal and ethical considerations, while incorporating the varied perspectives of physicians, patients, nurses, and hospital administration/risk management will allow requests for increased movement to be evaluated systematically and transparently.
“When patients request liberalized movement, hospitalists should consider the requests systematically: first to identify the intent behind requests, and then to follow a framework to determine whether increased movement would be safe and allow appropriate medical care without creating additional risks,” Dr. Stream said.
Hospitalists should assess and compile individual patient requests for liberalized movement and work with other physicians, nurses, hospital administration, and risk management to devise pertinent policy on this issue that is specific to their institutions. “By eventually creating clear guidelines in policy, health care providers will spend less time managing each individual request to leave the floor because they have a systematic strategy for making consistent decisions about patient movement,” the authors concluded.
Reference
1. Stream S, Alfandre D. “Just Getting a Cup of Coffee” – Considering Best Practices for Patients’ Movement off the Hospital Floor. J Hosp Med. 2019 Nov. doi: 10.12788/jhm.3227.
CDC: Opioid prescribing and use rates down since 2010
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
FROM MMWR SURVEILLANCE SUMMARIES
WHO declares public health emergency for novel coronavirus
Amid the rising spread of the 2019 Novel Coronavirus (2019-nCoV),
The declaration was made during a press briefing on Jan. 30 after a week of growing concern and pressure on WHO to designate the virus at a higher emergency level. WHO’s Emergency Committee made the nearly unanimous decision after considering the increasing number of coronavirus cases in China, the rising infections outside of China, and the questionable measures some countries are taking regarding travel, said committee chair Didier Houssin, MD, said during the press conference.
As of Jan. 30, there were 8,236 confirmed cases of the coronavirus in China and 171 deaths, with another 112 cases identified outside of China in 21 other countries.
“Declaring a Public Health Emergency of International Concern is likely to facilitate [WHO’s] leadership role for public health measures, holding countries to account concerning additional measures they may take regarding travel, trade, quarantine or screening, research efforts, global coordination and anticipation of economic impact [and] support to vulnerable states,” Dr. Houssin said during the press conference. “Declaring a PHEIC should certainly not be seen as manifestation of distrust in the Chinese authorities and people which are doing tremendous efforts on the frontlines of this outbreak, with transparency, and let us hope, with success.”
What happens next?
Once a PHEIC is declared, WHO launches a series of steps, including the release of temporary recommendations for the affected country on health measures to implement and guidance for other countries on preventing and reducing the international spread of the disease, WHO spokesman Tarik Jasarevic said in an interview.
“The purpose of declaring a PHEIC is to advise the world on what measures need to be taken to enhance global health security by preventing international transmission of an infectious hazard,” he said.
Following the Jan. 30 press conference, WHO released temporary guidance for China and for other countries regarding identifying, managing, containing, and preventing the virus. China is advised to continue updating the population about the outbreak, continue enhancing its public health measures for containment and surveillance of cases, and to continue collaboration with WHO and other partners to investigate the epidemiology and evolution of the outbreak and share data on all human cases.
Other countries should be prepared for containment, including the active surveillance, early detection, isolation, case management, and prevention of virus transmission and to share full data with WHO, according to the recommendations.
Under the International Health Regulations (IHR), countries are required to share information and data with WHO. Additionally, WHO leaders advised the global community to support low- and middle-income countries with their response to the coronavirus and to facilitate diagnostics, potential vaccines, and therapeutics in these areas.
The IHR requires that countries implementing health measures that go beyond what WHO recommends must send to WHO the public health rationale and justification within 48 hours of their implementation for WHO review, Mr. Jasarevic noted.
“WHO is obliged to share the information about measures and the justification received with other countries involved,” he said.
PHEIC travel and resource impact
Declaration of a PHEIC means WHO will now oversee any travel restrictions made by other countries in response to 2019-nCoV. The agency recommends that countries conduct a risk and cost-benefit analysis before enacting travel restrictions and other countries are required to inform WHO about any travel measures taken.
“Countries will be asked to provide public health justification for any travel or trade measures that are not scientifically based, such as refusal of entry of suspect cases or unaffected persons to affected areas,” Mr. Jasarevic said in an interview.
As far as resources, the PHEIC mechanism is not a fundraising mechanism, but some donors might consider a PHEIC declaration as a trigger for releasing additional funding to respond to the health threat, he said.
Allison T. Chamberlain, PhD, acting director for the Emory Center for Public Health Preparedness and Research at the Emory Rollins School of Public Health in Atlanta, said national governments and nongovernmental aid organizations are among the most affected by a PHEIC because they are looked at to provide assistance to the most heavily affected areas and to bolster public health preparedness within their own borders.
“In terms of resources that are deployed, a Public Health Emergency of International Concern raises levels of international support and commitment to stopping the emergency,” Dr. Chamberlain said in an interview. “By doing so, it gives countries the needed flexibility to release financial resources of their own accord to support things like response teams that might go into heavily affected areas to assist, for instance.”
WHO Director-General Dr. Tedros Adhanom Ghebreyesus stressed that cooperation among countries is key during the PHEIC.
“We can only stop it together,” he said during the press conference. “This is the time for facts, not fear. This is the time for science, not rumors. This is the time for solidarity, not stigma.”
This is the sixth PHEIC declared by WHO in the last 10 years. Such declarations were made for the 2009 H1NI influenza pandemic, the 2014 polio resurgence, the 2014 Ebola outbreak in West Africa, the 2016 Zika virus, and the 2019 Kivu Ebola outbreak in the Democratic Republic of Congo.
Amid the rising spread of the 2019 Novel Coronavirus (2019-nCoV),
The declaration was made during a press briefing on Jan. 30 after a week of growing concern and pressure on WHO to designate the virus at a higher emergency level. WHO’s Emergency Committee made the nearly unanimous decision after considering the increasing number of coronavirus cases in China, the rising infections outside of China, and the questionable measures some countries are taking regarding travel, said committee chair Didier Houssin, MD, said during the press conference.
As of Jan. 30, there were 8,236 confirmed cases of the coronavirus in China and 171 deaths, with another 112 cases identified outside of China in 21 other countries.
“Declaring a Public Health Emergency of International Concern is likely to facilitate [WHO’s] leadership role for public health measures, holding countries to account concerning additional measures they may take regarding travel, trade, quarantine or screening, research efforts, global coordination and anticipation of economic impact [and] support to vulnerable states,” Dr. Houssin said during the press conference. “Declaring a PHEIC should certainly not be seen as manifestation of distrust in the Chinese authorities and people which are doing tremendous efforts on the frontlines of this outbreak, with transparency, and let us hope, with success.”
What happens next?
Once a PHEIC is declared, WHO launches a series of steps, including the release of temporary recommendations for the affected country on health measures to implement and guidance for other countries on preventing and reducing the international spread of the disease, WHO spokesman Tarik Jasarevic said in an interview.
“The purpose of declaring a PHEIC is to advise the world on what measures need to be taken to enhance global health security by preventing international transmission of an infectious hazard,” he said.
Following the Jan. 30 press conference, WHO released temporary guidance for China and for other countries regarding identifying, managing, containing, and preventing the virus. China is advised to continue updating the population about the outbreak, continue enhancing its public health measures for containment and surveillance of cases, and to continue collaboration with WHO and other partners to investigate the epidemiology and evolution of the outbreak and share data on all human cases.
Other countries should be prepared for containment, including the active surveillance, early detection, isolation, case management, and prevention of virus transmission and to share full data with WHO, according to the recommendations.
Under the International Health Regulations (IHR), countries are required to share information and data with WHO. Additionally, WHO leaders advised the global community to support low- and middle-income countries with their response to the coronavirus and to facilitate diagnostics, potential vaccines, and therapeutics in these areas.
The IHR requires that countries implementing health measures that go beyond what WHO recommends must send to WHO the public health rationale and justification within 48 hours of their implementation for WHO review, Mr. Jasarevic noted.
“WHO is obliged to share the information about measures and the justification received with other countries involved,” he said.
PHEIC travel and resource impact
Declaration of a PHEIC means WHO will now oversee any travel restrictions made by other countries in response to 2019-nCoV. The agency recommends that countries conduct a risk and cost-benefit analysis before enacting travel restrictions and other countries are required to inform WHO about any travel measures taken.
“Countries will be asked to provide public health justification for any travel or trade measures that are not scientifically based, such as refusal of entry of suspect cases or unaffected persons to affected areas,” Mr. Jasarevic said in an interview.
As far as resources, the PHEIC mechanism is not a fundraising mechanism, but some donors might consider a PHEIC declaration as a trigger for releasing additional funding to respond to the health threat, he said.
Allison T. Chamberlain, PhD, acting director for the Emory Center for Public Health Preparedness and Research at the Emory Rollins School of Public Health in Atlanta, said national governments and nongovernmental aid organizations are among the most affected by a PHEIC because they are looked at to provide assistance to the most heavily affected areas and to bolster public health preparedness within their own borders.
“In terms of resources that are deployed, a Public Health Emergency of International Concern raises levels of international support and commitment to stopping the emergency,” Dr. Chamberlain said in an interview. “By doing so, it gives countries the needed flexibility to release financial resources of their own accord to support things like response teams that might go into heavily affected areas to assist, for instance.”
WHO Director-General Dr. Tedros Adhanom Ghebreyesus stressed that cooperation among countries is key during the PHEIC.
“We can only stop it together,” he said during the press conference. “This is the time for facts, not fear. This is the time for science, not rumors. This is the time for solidarity, not stigma.”
This is the sixth PHEIC declared by WHO in the last 10 years. Such declarations were made for the 2009 H1NI influenza pandemic, the 2014 polio resurgence, the 2014 Ebola outbreak in West Africa, the 2016 Zika virus, and the 2019 Kivu Ebola outbreak in the Democratic Republic of Congo.
Amid the rising spread of the 2019 Novel Coronavirus (2019-nCoV),
The declaration was made during a press briefing on Jan. 30 after a week of growing concern and pressure on WHO to designate the virus at a higher emergency level. WHO’s Emergency Committee made the nearly unanimous decision after considering the increasing number of coronavirus cases in China, the rising infections outside of China, and the questionable measures some countries are taking regarding travel, said committee chair Didier Houssin, MD, said during the press conference.
As of Jan. 30, there were 8,236 confirmed cases of the coronavirus in China and 171 deaths, with another 112 cases identified outside of China in 21 other countries.
“Declaring a Public Health Emergency of International Concern is likely to facilitate [WHO’s] leadership role for public health measures, holding countries to account concerning additional measures they may take regarding travel, trade, quarantine or screening, research efforts, global coordination and anticipation of economic impact [and] support to vulnerable states,” Dr. Houssin said during the press conference. “Declaring a PHEIC should certainly not be seen as manifestation of distrust in the Chinese authorities and people which are doing tremendous efforts on the frontlines of this outbreak, with transparency, and let us hope, with success.”
What happens next?
Once a PHEIC is declared, WHO launches a series of steps, including the release of temporary recommendations for the affected country on health measures to implement and guidance for other countries on preventing and reducing the international spread of the disease, WHO spokesman Tarik Jasarevic said in an interview.
“The purpose of declaring a PHEIC is to advise the world on what measures need to be taken to enhance global health security by preventing international transmission of an infectious hazard,” he said.
Following the Jan. 30 press conference, WHO released temporary guidance for China and for other countries regarding identifying, managing, containing, and preventing the virus. China is advised to continue updating the population about the outbreak, continue enhancing its public health measures for containment and surveillance of cases, and to continue collaboration with WHO and other partners to investigate the epidemiology and evolution of the outbreak and share data on all human cases.
Other countries should be prepared for containment, including the active surveillance, early detection, isolation, case management, and prevention of virus transmission and to share full data with WHO, according to the recommendations.
Under the International Health Regulations (IHR), countries are required to share information and data with WHO. Additionally, WHO leaders advised the global community to support low- and middle-income countries with their response to the coronavirus and to facilitate diagnostics, potential vaccines, and therapeutics in these areas.
The IHR requires that countries implementing health measures that go beyond what WHO recommends must send to WHO the public health rationale and justification within 48 hours of their implementation for WHO review, Mr. Jasarevic noted.
“WHO is obliged to share the information about measures and the justification received with other countries involved,” he said.
PHEIC travel and resource impact
Declaration of a PHEIC means WHO will now oversee any travel restrictions made by other countries in response to 2019-nCoV. The agency recommends that countries conduct a risk and cost-benefit analysis before enacting travel restrictions and other countries are required to inform WHO about any travel measures taken.
“Countries will be asked to provide public health justification for any travel or trade measures that are not scientifically based, such as refusal of entry of suspect cases or unaffected persons to affected areas,” Mr. Jasarevic said in an interview.
As far as resources, the PHEIC mechanism is not a fundraising mechanism, but some donors might consider a PHEIC declaration as a trigger for releasing additional funding to respond to the health threat, he said.
Allison T. Chamberlain, PhD, acting director for the Emory Center for Public Health Preparedness and Research at the Emory Rollins School of Public Health in Atlanta, said national governments and nongovernmental aid organizations are among the most affected by a PHEIC because they are looked at to provide assistance to the most heavily affected areas and to bolster public health preparedness within their own borders.
“In terms of resources that are deployed, a Public Health Emergency of International Concern raises levels of international support and commitment to stopping the emergency,” Dr. Chamberlain said in an interview. “By doing so, it gives countries the needed flexibility to release financial resources of their own accord to support things like response teams that might go into heavily affected areas to assist, for instance.”
WHO Director-General Dr. Tedros Adhanom Ghebreyesus stressed that cooperation among countries is key during the PHEIC.
“We can only stop it together,” he said during the press conference. “This is the time for facts, not fear. This is the time for science, not rumors. This is the time for solidarity, not stigma.”
This is the sixth PHEIC declared by WHO in the last 10 years. Such declarations were made for the 2009 H1NI influenza pandemic, the 2014 polio resurgence, the 2014 Ebola outbreak in West Africa, the 2016 Zika virus, and the 2019 Kivu Ebola outbreak in the Democratic Republic of Congo.
2019 Novel Coronavirus: Frequently asked questions for clinicians
The 2019 Novel Coronavirus (2019-nCoV) outbreak has unfolded so rapidly that many clinicians are scrambling to stay on top of it. Here are the answers to some frequently asked questions about how to prepare your clinic to respond to this outbreak.
Keep in mind that the outbreak is moving rapidly. Though scientific and epidemiologic knowledge has increased at unprecedented speed, there is much we don’t know, and some of what we think we know will change. Follow the links for the most up-to-date information.
What should our clinic do first?
Plan ahead with the following:
- Develop a plan for office staff to take travel histories from anyone with a respiratory illness and provide training for those who need it. Travel history at present should include asking about travel to China in the past 14 days, specifically Wuhan city or Hubei province.
- Review up-to-date infection control practices with all office staff and provide training for those who need it.
- Take an inventory of supplies of personal protective equipment (PPE), such as gowns, gloves, masks, eye protection, and N95 respirators or powered air-purifying respirators (PAPRs), and order items that are missing or low in stock.
- Fit-test users of N95 masks for maximal effectiveness.
- Plan where a potential patient would be isolated while obtaining expert advice.
- Know whom to contact at the state or local health department if you have a patient with the appropriate travel history.
The Centers for Disease Control and Prevention has prepared a toolkit to help frontline health care professionals prepare for this virus. Providers need to stay up to date on the latest recommendations, as the situation is changing rapidly.
When should I suspect 2019-nCoV illness, and what should I do?
Take the following steps to assess the concern and respond:
- If a patient with respiratory illness has traveled to China in the past 14 days, immediately put a mask on the patient and move the individual to a private room. Use a negative-pressure room if available.
- Put on appropriate PPE (including gloves, gown, eye protection, and mask) for contact, droplet, and airborne precautions. CDC recommends an N95 respirator mask if available, although we don’t know yet if there is true airborne spread.
- Obtain an accurate travel history, including dates and cities. (Tip: Get the correct spelling, as the English spelling of cities in China can cause confusion.)
- If the patient meets the current CDC definition of “person under investigation” or PUI, or if you need guidance on how to proceed, notify infection control (if you are in a facility that has it) and call your state or local health department immediately.
- Contact public health authorities who can help decide whether the patient should be admitted to airborne isolation or monitored at home with appropriate precautions.
What is the definition of a PUI?
The current definition of a PUI is a person who has fever and symptoms of a respiratory infection (cough, shortness of breath) AND who has EITHER been in Wuhan city or Hubei province in the past 14 days OR had close contact with a person either under investigation for 2019-nCoV infection or with confirmed infection. The definition of a PUI will change over time, so check this link.
How can I test for 2019-nCoV?
As of Jan. 30, 2020, testing is by polymerase chain reaction (PCR) and is available in the United States only through the CDC in Atlanta. Testing should soon be available in state health department laboratories. If public health authorities decide that your patient should be tested, they will instruct you on which samples to obtain.
The full sequence of 2019-nCoV has been shared, so some reference laboratories may develop and validate tests, ideally with assistance from CDC. If testing becomes available, make certain that it is a reputable lab that has carefully validated the test.
Should I test for other viruses?
Because the symptoms of 2019-nCoV infection overlap with those of influenza and other respiratory viruses, PCR testing for other viruses should be considered if it will change management (i.e., change the decision to provide influenza antivirals). Use appropriate PPE while collecting specimens, including eye protection. If 2019-nCoV is a consideration, you may want to send the specimen to a hospital lab for testing, where the sample will be processed under a biosafety hood, rather than doing point-of-care testing in the office.
How dangerous is 2019-nCoV?
The current estimated mortality rate is 2%-3%. That is probably an overestimate, as those with severe disease and those who die are more likely to be tested and reported early in an epidemic.
Our current knowledge is based on preliminary reports from hospitalized patients and will probably change. From the speed of spread and a single family cluster, it seems likely that there are milder cases and perhaps asymptomatic infection.
What else do I need to know about coronaviruses?
Coronaviruses are a large and diverse group of viruses, many of which are animal viruses. Before the discovery of the 2019-nCoV, six coronaviruses were known to infect humans. Four of these (HKU1, NL63, OC43, and 229E) predominantly caused mild to moderate upper respiratory illness, and they are thought to be responsible for 10%-30% of colds. They occasionally cause viral pneumonia and can be detected by some commercial multiplex panels.
Two other coronaviruses have caused outbreaks of severe respiratory illness in people: SARS, which emerged in Southern China in 2002, and MERS in the Middle East, in 2012. Unlike SARS, sporadic cases of MERS continue to occur.
The current outbreak is caused by 2019-nCoV, a previously unknown beta coronavirus. It is most closely related (~96%) to a bat virus and shares about 80% sequence homology with SARS CoV.
Andrew T. Pavia, MD, is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious disease in the department of pediatrics at the University of Utah, Salt Lake City. He is also director of hospital epidemiology and associate director of antimicrobial stewardship at Primary Children’s Hospital, Salt Lake City. Dr. Pavia has disclosed that he has served as a consultant for Genentech, Merck, and Seqirus and that he has served as associate editor for The Sanford Guide.
This article first appeared on Medscape.com.
The 2019 Novel Coronavirus (2019-nCoV) outbreak has unfolded so rapidly that many clinicians are scrambling to stay on top of it. Here are the answers to some frequently asked questions about how to prepare your clinic to respond to this outbreak.
Keep in mind that the outbreak is moving rapidly. Though scientific and epidemiologic knowledge has increased at unprecedented speed, there is much we don’t know, and some of what we think we know will change. Follow the links for the most up-to-date information.
What should our clinic do first?
Plan ahead with the following:
- Develop a plan for office staff to take travel histories from anyone with a respiratory illness and provide training for those who need it. Travel history at present should include asking about travel to China in the past 14 days, specifically Wuhan city or Hubei province.
- Review up-to-date infection control practices with all office staff and provide training for those who need it.
- Take an inventory of supplies of personal protective equipment (PPE), such as gowns, gloves, masks, eye protection, and N95 respirators or powered air-purifying respirators (PAPRs), and order items that are missing or low in stock.
- Fit-test users of N95 masks for maximal effectiveness.
- Plan where a potential patient would be isolated while obtaining expert advice.
- Know whom to contact at the state or local health department if you have a patient with the appropriate travel history.
The Centers for Disease Control and Prevention has prepared a toolkit to help frontline health care professionals prepare for this virus. Providers need to stay up to date on the latest recommendations, as the situation is changing rapidly.
When should I suspect 2019-nCoV illness, and what should I do?
Take the following steps to assess the concern and respond:
- If a patient with respiratory illness has traveled to China in the past 14 days, immediately put a mask on the patient and move the individual to a private room. Use a negative-pressure room if available.
- Put on appropriate PPE (including gloves, gown, eye protection, and mask) for contact, droplet, and airborne precautions. CDC recommends an N95 respirator mask if available, although we don’t know yet if there is true airborne spread.
- Obtain an accurate travel history, including dates and cities. (Tip: Get the correct spelling, as the English spelling of cities in China can cause confusion.)
- If the patient meets the current CDC definition of “person under investigation” or PUI, or if you need guidance on how to proceed, notify infection control (if you are in a facility that has it) and call your state or local health department immediately.
- Contact public health authorities who can help decide whether the patient should be admitted to airborne isolation or monitored at home with appropriate precautions.
What is the definition of a PUI?
The current definition of a PUI is a person who has fever and symptoms of a respiratory infection (cough, shortness of breath) AND who has EITHER been in Wuhan city or Hubei province in the past 14 days OR had close contact with a person either under investigation for 2019-nCoV infection or with confirmed infection. The definition of a PUI will change over time, so check this link.
How can I test for 2019-nCoV?
As of Jan. 30, 2020, testing is by polymerase chain reaction (PCR) and is available in the United States only through the CDC in Atlanta. Testing should soon be available in state health department laboratories. If public health authorities decide that your patient should be tested, they will instruct you on which samples to obtain.
The full sequence of 2019-nCoV has been shared, so some reference laboratories may develop and validate tests, ideally with assistance from CDC. If testing becomes available, make certain that it is a reputable lab that has carefully validated the test.
Should I test for other viruses?
Because the symptoms of 2019-nCoV infection overlap with those of influenza and other respiratory viruses, PCR testing for other viruses should be considered if it will change management (i.e., change the decision to provide influenza antivirals). Use appropriate PPE while collecting specimens, including eye protection. If 2019-nCoV is a consideration, you may want to send the specimen to a hospital lab for testing, where the sample will be processed under a biosafety hood, rather than doing point-of-care testing in the office.
How dangerous is 2019-nCoV?
The current estimated mortality rate is 2%-3%. That is probably an overestimate, as those with severe disease and those who die are more likely to be tested and reported early in an epidemic.
Our current knowledge is based on preliminary reports from hospitalized patients and will probably change. From the speed of spread and a single family cluster, it seems likely that there are milder cases and perhaps asymptomatic infection.
What else do I need to know about coronaviruses?
Coronaviruses are a large and diverse group of viruses, many of which are animal viruses. Before the discovery of the 2019-nCoV, six coronaviruses were known to infect humans. Four of these (HKU1, NL63, OC43, and 229E) predominantly caused mild to moderate upper respiratory illness, and they are thought to be responsible for 10%-30% of colds. They occasionally cause viral pneumonia and can be detected by some commercial multiplex panels.
Two other coronaviruses have caused outbreaks of severe respiratory illness in people: SARS, which emerged in Southern China in 2002, and MERS in the Middle East, in 2012. Unlike SARS, sporadic cases of MERS continue to occur.
The current outbreak is caused by 2019-nCoV, a previously unknown beta coronavirus. It is most closely related (~96%) to a bat virus and shares about 80% sequence homology with SARS CoV.
Andrew T. Pavia, MD, is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious disease in the department of pediatrics at the University of Utah, Salt Lake City. He is also director of hospital epidemiology and associate director of antimicrobial stewardship at Primary Children’s Hospital, Salt Lake City. Dr. Pavia has disclosed that he has served as a consultant for Genentech, Merck, and Seqirus and that he has served as associate editor for The Sanford Guide.
This article first appeared on Medscape.com.
The 2019 Novel Coronavirus (2019-nCoV) outbreak has unfolded so rapidly that many clinicians are scrambling to stay on top of it. Here are the answers to some frequently asked questions about how to prepare your clinic to respond to this outbreak.
Keep in mind that the outbreak is moving rapidly. Though scientific and epidemiologic knowledge has increased at unprecedented speed, there is much we don’t know, and some of what we think we know will change. Follow the links for the most up-to-date information.
What should our clinic do first?
Plan ahead with the following:
- Develop a plan for office staff to take travel histories from anyone with a respiratory illness and provide training for those who need it. Travel history at present should include asking about travel to China in the past 14 days, specifically Wuhan city or Hubei province.
- Review up-to-date infection control practices with all office staff and provide training for those who need it.
- Take an inventory of supplies of personal protective equipment (PPE), such as gowns, gloves, masks, eye protection, and N95 respirators or powered air-purifying respirators (PAPRs), and order items that are missing or low in stock.
- Fit-test users of N95 masks for maximal effectiveness.
- Plan where a potential patient would be isolated while obtaining expert advice.
- Know whom to contact at the state or local health department if you have a patient with the appropriate travel history.
The Centers for Disease Control and Prevention has prepared a toolkit to help frontline health care professionals prepare for this virus. Providers need to stay up to date on the latest recommendations, as the situation is changing rapidly.
When should I suspect 2019-nCoV illness, and what should I do?
Take the following steps to assess the concern and respond:
- If a patient with respiratory illness has traveled to China in the past 14 days, immediately put a mask on the patient and move the individual to a private room. Use a negative-pressure room if available.
- Put on appropriate PPE (including gloves, gown, eye protection, and mask) for contact, droplet, and airborne precautions. CDC recommends an N95 respirator mask if available, although we don’t know yet if there is true airborne spread.
- Obtain an accurate travel history, including dates and cities. (Tip: Get the correct spelling, as the English spelling of cities in China can cause confusion.)
- If the patient meets the current CDC definition of “person under investigation” or PUI, or if you need guidance on how to proceed, notify infection control (if you are in a facility that has it) and call your state or local health department immediately.
- Contact public health authorities who can help decide whether the patient should be admitted to airborne isolation or monitored at home with appropriate precautions.
What is the definition of a PUI?
The current definition of a PUI is a person who has fever and symptoms of a respiratory infection (cough, shortness of breath) AND who has EITHER been in Wuhan city or Hubei province in the past 14 days OR had close contact with a person either under investigation for 2019-nCoV infection or with confirmed infection. The definition of a PUI will change over time, so check this link.
How can I test for 2019-nCoV?
As of Jan. 30, 2020, testing is by polymerase chain reaction (PCR) and is available in the United States only through the CDC in Atlanta. Testing should soon be available in state health department laboratories. If public health authorities decide that your patient should be tested, they will instruct you on which samples to obtain.
The full sequence of 2019-nCoV has been shared, so some reference laboratories may develop and validate tests, ideally with assistance from CDC. If testing becomes available, make certain that it is a reputable lab that has carefully validated the test.
Should I test for other viruses?
Because the symptoms of 2019-nCoV infection overlap with those of influenza and other respiratory viruses, PCR testing for other viruses should be considered if it will change management (i.e., change the decision to provide influenza antivirals). Use appropriate PPE while collecting specimens, including eye protection. If 2019-nCoV is a consideration, you may want to send the specimen to a hospital lab for testing, where the sample will be processed under a biosafety hood, rather than doing point-of-care testing in the office.
How dangerous is 2019-nCoV?
The current estimated mortality rate is 2%-3%. That is probably an overestimate, as those with severe disease and those who die are more likely to be tested and reported early in an epidemic.
Our current knowledge is based on preliminary reports from hospitalized patients and will probably change. From the speed of spread and a single family cluster, it seems likely that there are milder cases and perhaps asymptomatic infection.
What else do I need to know about coronaviruses?
Coronaviruses are a large and diverse group of viruses, many of which are animal viruses. Before the discovery of the 2019-nCoV, six coronaviruses were known to infect humans. Four of these (HKU1, NL63, OC43, and 229E) predominantly caused mild to moderate upper respiratory illness, and they are thought to be responsible for 10%-30% of colds. They occasionally cause viral pneumonia and can be detected by some commercial multiplex panels.
Two other coronaviruses have caused outbreaks of severe respiratory illness in people: SARS, which emerged in Southern China in 2002, and MERS in the Middle East, in 2012. Unlike SARS, sporadic cases of MERS continue to occur.
The current outbreak is caused by 2019-nCoV, a previously unknown beta coronavirus. It is most closely related (~96%) to a bat virus and shares about 80% sequence homology with SARS CoV.
Andrew T. Pavia, MD, is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious disease in the department of pediatrics at the University of Utah, Salt Lake City. He is also director of hospital epidemiology and associate director of antimicrobial stewardship at Primary Children’s Hospital, Salt Lake City. Dr. Pavia has disclosed that he has served as a consultant for Genentech, Merck, and Seqirus and that he has served as associate editor for The Sanford Guide.
This article first appeared on Medscape.com.








