User login
Helping alleviate hospitalist burnout
Focus on systemic factors
For hospitalists, burnout is a widespread and ongoing problem. In 2011, a Mayo Clinic study found that 45% of U.S. physicians had at least one symptom of professional burnout; by 2014, that number had risen to 54%.

“Burnout among physicians has been shown to be linked to quality of care, impacting medical errors, mortality ratios in hospitalized patients, and lower patient satisfaction,” said Ingrid T. Katz, MD, MHS, assistant professor of medicine at Harvard Medical School, Boston, and coauthor of a recent column on the subject published in the New England Journal of Medicine.
Widespread burnout is caused by systemic factors, not individual failures. “These systemic factors range from excessive clerical burden to ‘work beyond work,’ where people end up taking work home at night and are often found interfacing with the EHR well after their normal work day,” Dr. Katz said. “Many also express their disdain for the model of practice that no longer values autonomy, which was seen as inherent in the profession prior to the current model of care.”
Moving towards a better framework would require an inherent trust in physicians, limiting unnecessary intrusions into a physician’s practice that do not impact medical care. “It would remove the burden of excessive documentation and allow for physicians to get reinspired by the practice of medicine, an inherently altruistic profession,” Dr. Katz said.
Changes might include eliminating excessive clerical demands and improving EHRs to allow physicians to return to the bedside. Workloads would be geared towards quality in care and not focused on improving the bottom line of a health care system. One health system Dr. Katz wrote about instituted a team-based model; under this system medical assistants gather data and reconcile medications, allowing physicians to focus on performing physical exams and making medical decisions.
“Burnout will diminish when physicians are empowered to be part of the solution and hospital systems make changes that recognize the totality of the challenges that physicians face,” Dr. Katz said, adding that hospitalists are in a unique position to promote such changes on a systemic level. “Leadership needs to be willing to inform and engage their physicians, monitor well-being of physicians as closely as they monitor quality in care, and implement changes when needed.”
Reference
1. Katz IT et al. Beyond Burnout – Redesigning Care to Restore Meaning and Sanity for Physicians. N Engl J Med. 2018 Jan 25. doi: 10.1056/NEJMp1716845.
Focus on systemic factors
Focus on systemic factors
For hospitalists, burnout is a widespread and ongoing problem. In 2011, a Mayo Clinic study found that 45% of U.S. physicians had at least one symptom of professional burnout; by 2014, that number had risen to 54%.

“Burnout among physicians has been shown to be linked to quality of care, impacting medical errors, mortality ratios in hospitalized patients, and lower patient satisfaction,” said Ingrid T. Katz, MD, MHS, assistant professor of medicine at Harvard Medical School, Boston, and coauthor of a recent column on the subject published in the New England Journal of Medicine.
Widespread burnout is caused by systemic factors, not individual failures. “These systemic factors range from excessive clerical burden to ‘work beyond work,’ where people end up taking work home at night and are often found interfacing with the EHR well after their normal work day,” Dr. Katz said. “Many also express their disdain for the model of practice that no longer values autonomy, which was seen as inherent in the profession prior to the current model of care.”
Moving towards a better framework would require an inherent trust in physicians, limiting unnecessary intrusions into a physician’s practice that do not impact medical care. “It would remove the burden of excessive documentation and allow for physicians to get reinspired by the practice of medicine, an inherently altruistic profession,” Dr. Katz said.
Changes might include eliminating excessive clerical demands and improving EHRs to allow physicians to return to the bedside. Workloads would be geared towards quality in care and not focused on improving the bottom line of a health care system. One health system Dr. Katz wrote about instituted a team-based model; under this system medical assistants gather data and reconcile medications, allowing physicians to focus on performing physical exams and making medical decisions.
“Burnout will diminish when physicians are empowered to be part of the solution and hospital systems make changes that recognize the totality of the challenges that physicians face,” Dr. Katz said, adding that hospitalists are in a unique position to promote such changes on a systemic level. “Leadership needs to be willing to inform and engage their physicians, monitor well-being of physicians as closely as they monitor quality in care, and implement changes when needed.”
Reference
1. Katz IT et al. Beyond Burnout – Redesigning Care to Restore Meaning and Sanity for Physicians. N Engl J Med. 2018 Jan 25. doi: 10.1056/NEJMp1716845.
For hospitalists, burnout is a widespread and ongoing problem. In 2011, a Mayo Clinic study found that 45% of U.S. physicians had at least one symptom of professional burnout; by 2014, that number had risen to 54%.

“Burnout among physicians has been shown to be linked to quality of care, impacting medical errors, mortality ratios in hospitalized patients, and lower patient satisfaction,” said Ingrid T. Katz, MD, MHS, assistant professor of medicine at Harvard Medical School, Boston, and coauthor of a recent column on the subject published in the New England Journal of Medicine.
Widespread burnout is caused by systemic factors, not individual failures. “These systemic factors range from excessive clerical burden to ‘work beyond work,’ where people end up taking work home at night and are often found interfacing with the EHR well after their normal work day,” Dr. Katz said. “Many also express their disdain for the model of practice that no longer values autonomy, which was seen as inherent in the profession prior to the current model of care.”
Moving towards a better framework would require an inherent trust in physicians, limiting unnecessary intrusions into a physician’s practice that do not impact medical care. “It would remove the burden of excessive documentation and allow for physicians to get reinspired by the practice of medicine, an inherently altruistic profession,” Dr. Katz said.
Changes might include eliminating excessive clerical demands and improving EHRs to allow physicians to return to the bedside. Workloads would be geared towards quality in care and not focused on improving the bottom line of a health care system. One health system Dr. Katz wrote about instituted a team-based model; under this system medical assistants gather data and reconcile medications, allowing physicians to focus on performing physical exams and making medical decisions.
“Burnout will diminish when physicians are empowered to be part of the solution and hospital systems make changes that recognize the totality of the challenges that physicians face,” Dr. Katz said, adding that hospitalists are in a unique position to promote such changes on a systemic level. “Leadership needs to be willing to inform and engage their physicians, monitor well-being of physicians as closely as they monitor quality in care, and implement changes when needed.”
Reference
1. Katz IT et al. Beyond Burnout – Redesigning Care to Restore Meaning and Sanity for Physicians. N Engl J Med. 2018 Jan 25. doi: 10.1056/NEJMp1716845.
FDA approves Xofluza for treatment of influenza
The Food and Drug Administration has approved Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for 48 hours or less.
The FDA approval is based on results from two randomized, clinical trials. In both trials, patients who received Xofluza experienced a shorter duration until alleviation of symptoms, compared with patients who received a placebo. In the second trial, patients who received Xofluza and patients who received another approved antiviral influenza medication experienced similar durations until symptom alleviation.
“When treatment is started within 48 hours of becoming sick with flu symptoms, antiviral drugs can lessen symptoms and shorten the time patients feel sick. Having more treatment options that work in different ways to attack the virus is important because flu viruses can become resistant to antiviral drugs,” Debra Birnkrant, MD, director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
The most common adverse events associated with Xofluza were diarrhea and bronchitis.
“This is the first new antiviral flu treatment with a novel mechanism of action approved by the FDA in nearly 20 years,” FDA Commissioner Scott Gottlieb, MD, added. “With thousands of people getting the flu every year, and many people becoming seriously ill, having safe and effective treatment alternatives is critical. This novel drug provides an important, additional treatment option.”
Find the full press release on the FDA website.
The Food and Drug Administration has approved Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for 48 hours or less.
The FDA approval is based on results from two randomized, clinical trials. In both trials, patients who received Xofluza experienced a shorter duration until alleviation of symptoms, compared with patients who received a placebo. In the second trial, patients who received Xofluza and patients who received another approved antiviral influenza medication experienced similar durations until symptom alleviation.
“When treatment is started within 48 hours of becoming sick with flu symptoms, antiviral drugs can lessen symptoms and shorten the time patients feel sick. Having more treatment options that work in different ways to attack the virus is important because flu viruses can become resistant to antiviral drugs,” Debra Birnkrant, MD, director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
The most common adverse events associated with Xofluza were diarrhea and bronchitis.
“This is the first new antiviral flu treatment with a novel mechanism of action approved by the FDA in nearly 20 years,” FDA Commissioner Scott Gottlieb, MD, added. “With thousands of people getting the flu every year, and many people becoming seriously ill, having safe and effective treatment alternatives is critical. This novel drug provides an important, additional treatment option.”
Find the full press release on the FDA website.
The Food and Drug Administration has approved Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for 48 hours or less.
The FDA approval is based on results from two randomized, clinical trials. In both trials, patients who received Xofluza experienced a shorter duration until alleviation of symptoms, compared with patients who received a placebo. In the second trial, patients who received Xofluza and patients who received another approved antiviral influenza medication experienced similar durations until symptom alleviation.
“When treatment is started within 48 hours of becoming sick with flu symptoms, antiviral drugs can lessen symptoms and shorten the time patients feel sick. Having more treatment options that work in different ways to attack the virus is important because flu viruses can become resistant to antiviral drugs,” Debra Birnkrant, MD, director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
The most common adverse events associated with Xofluza were diarrhea and bronchitis.
“This is the first new antiviral flu treatment with a novel mechanism of action approved by the FDA in nearly 20 years,” FDA Commissioner Scott Gottlieb, MD, added. “With thousands of people getting the flu every year, and many people becoming seriously ill, having safe and effective treatment alternatives is critical. This novel drug provides an important, additional treatment option.”
Find the full press release on the FDA website.
Study: Problems persist with APMs
Physicians continue to support advanced alternative payment models despite the fact that operational issues have not improved over the last 4 years and new ones have cropped up, according to a follow-up survey conducted by the RAND Corporation for the American Medical Association.
“All the things we heard in 2014 were still present in 2018. Both the challenges that practices had experienced back in 2014 having to do with data timeliness, data completeness and accuracy, payment model execution, all those challenges persisted,” Mark W. Friedberg, MD, senior physician policy researcher at RAND, said in an interview.
RAND surveyed 31 practices of varying practice size and specialty across six geographic regions, some of which participated in the 2014 survey. Supplemental information was provided by interviews with 32 market observers, 8 health plan leaders, 10 hospital and hospital system leaders, 10 state and local medical society leaders, and 4 chapter leaders with MGMA (formerly the Medical Group Management Association).
“We had thought we would hear that the problem had gotten a little bit better since there has been some investment in trying to tamp down the wide range of measures that are involved in these alternative payment models,” Dr. Friedberg said. “We did not see any evidence of that having any effect on the practices that participated in this study this time around.”
Indeed, concerns reported in 2014 were again reported in 2018, along with a new set of concerns, including the perceived pace of change in alternative payment models (APMs), the complexity of APMs, and physician concerns over two-sided risk models.
“Practices, especially those that participated both times, said in 2014 we had these challenges [of rapid changes in APM models] and since then, things have just gotten a lot faster,” he said, noting that doctors are complaining of models that are going through changes, sometimes without much warning. “They are changing quite rapidly from year to year. If you look at the MACRA QPP [Quality Payment Program] for example, that model changes every year to some extent and those things are hard for them to keep up with.”
Running hand in hand with the change is the complexity of the changes, a result of expanding performance measures and uncertainty with thresholds for penalties and rewards and in some ways has had little impact on improving care.
Dr. Friedberg noted that some practices are hiring people to examine APMs to devise strategic ways to choose and report data for maximum return.
“In a practice, for example, if their quality of care was already very good, what these folks ended up doing was help them choose measures and work the attribution algorithms in a strategic way to either guarantee a bonus or minimize the risk of incurring a penalty,” he said.
He also noted that practices appear to becoming more risk averse.
“We heard a lot more of the following thing, which is that if [practices] were in a two-sided risk model, several of them reported trying and succeeding in some cases offloading the downside risk to partners,” Dr. Friedberg reported. “And what this resulted in was that the practice, even though from the payer’s perspective they are in a two-sided model, the practice was actually in a one-sided model with a partner who is taking all of the downside risk and a portion of the upside risk, leaving a small upside risk proposition that remained for the practice.”
He said the range of partners that were absorbing the downside risk included hospitals, device manufacturers, consulting companies, or private equity firms.
Despite the concerns surrounding APMs, Dr. Friedberg said that “we did not hear practices broadly saying that they just weren’t interested in alternative payment models. In general, practices still remained pretty enthusiastic about these alternative payment models in theory. If they could be made simpler, if the pace of change weren’t quite so fast, that they would have a chance to really do some important care improvements in alternative payment models.”
He noted some of the surveyed practices were able to make investments in care as a direct result of participating in APMs, such as in behavioral health capabilities in primary care, for example, leading to quality of care improvements.
However, these issues could reveal a future unwillingness to participate in APMs, especially two-sided risk models, something at least the Centers for Medicare & Medicaid Services are pushing for as a stated goal of the QPP is to get practices to participate in APMs and take on more risk.
The growing aversion to taking on downside risk could lead practices to simply stay in fee for service and simply take the payment penalty because it is a fixed amount that can be planned for, as opposed to the fluctuations of bonuses and penalties that comes with a rapidly changing APM environment, Dr. Friedberg said.
Going forward, the report makes a number of recommendations to help create an environment that would potentially make APMs more successful, including simplifying the models; creating stable, predictable, and moderately paced pathways to APM participation; making data available in a more timely fashion; minimizing downside risk or helping practices better manage it; and designing APMs that will encourage clinical changes to help improve the effectiveness of care delivered.
Physicians continue to support advanced alternative payment models despite the fact that operational issues have not improved over the last 4 years and new ones have cropped up, according to a follow-up survey conducted by the RAND Corporation for the American Medical Association.
“All the things we heard in 2014 were still present in 2018. Both the challenges that practices had experienced back in 2014 having to do with data timeliness, data completeness and accuracy, payment model execution, all those challenges persisted,” Mark W. Friedberg, MD, senior physician policy researcher at RAND, said in an interview.
RAND surveyed 31 practices of varying practice size and specialty across six geographic regions, some of which participated in the 2014 survey. Supplemental information was provided by interviews with 32 market observers, 8 health plan leaders, 10 hospital and hospital system leaders, 10 state and local medical society leaders, and 4 chapter leaders with MGMA (formerly the Medical Group Management Association).
“We had thought we would hear that the problem had gotten a little bit better since there has been some investment in trying to tamp down the wide range of measures that are involved in these alternative payment models,” Dr. Friedberg said. “We did not see any evidence of that having any effect on the practices that participated in this study this time around.”
Indeed, concerns reported in 2014 were again reported in 2018, along with a new set of concerns, including the perceived pace of change in alternative payment models (APMs), the complexity of APMs, and physician concerns over two-sided risk models.
“Practices, especially those that participated both times, said in 2014 we had these challenges [of rapid changes in APM models] and since then, things have just gotten a lot faster,” he said, noting that doctors are complaining of models that are going through changes, sometimes without much warning. “They are changing quite rapidly from year to year. If you look at the MACRA QPP [Quality Payment Program] for example, that model changes every year to some extent and those things are hard for them to keep up with.”
Running hand in hand with the change is the complexity of the changes, a result of expanding performance measures and uncertainty with thresholds for penalties and rewards and in some ways has had little impact on improving care.
Dr. Friedberg noted that some practices are hiring people to examine APMs to devise strategic ways to choose and report data for maximum return.
“In a practice, for example, if their quality of care was already very good, what these folks ended up doing was help them choose measures and work the attribution algorithms in a strategic way to either guarantee a bonus or minimize the risk of incurring a penalty,” he said.
He also noted that practices appear to becoming more risk averse.
“We heard a lot more of the following thing, which is that if [practices] were in a two-sided risk model, several of them reported trying and succeeding in some cases offloading the downside risk to partners,” Dr. Friedberg reported. “And what this resulted in was that the practice, even though from the payer’s perspective they are in a two-sided model, the practice was actually in a one-sided model with a partner who is taking all of the downside risk and a portion of the upside risk, leaving a small upside risk proposition that remained for the practice.”
He said the range of partners that were absorbing the downside risk included hospitals, device manufacturers, consulting companies, or private equity firms.
Despite the concerns surrounding APMs, Dr. Friedberg said that “we did not hear practices broadly saying that they just weren’t interested in alternative payment models. In general, practices still remained pretty enthusiastic about these alternative payment models in theory. If they could be made simpler, if the pace of change weren’t quite so fast, that they would have a chance to really do some important care improvements in alternative payment models.”
He noted some of the surveyed practices were able to make investments in care as a direct result of participating in APMs, such as in behavioral health capabilities in primary care, for example, leading to quality of care improvements.
However, these issues could reveal a future unwillingness to participate in APMs, especially two-sided risk models, something at least the Centers for Medicare & Medicaid Services are pushing for as a stated goal of the QPP is to get practices to participate in APMs and take on more risk.
The growing aversion to taking on downside risk could lead practices to simply stay in fee for service and simply take the payment penalty because it is a fixed amount that can be planned for, as opposed to the fluctuations of bonuses and penalties that comes with a rapidly changing APM environment, Dr. Friedberg said.
Going forward, the report makes a number of recommendations to help create an environment that would potentially make APMs more successful, including simplifying the models; creating stable, predictable, and moderately paced pathways to APM participation; making data available in a more timely fashion; minimizing downside risk or helping practices better manage it; and designing APMs that will encourage clinical changes to help improve the effectiveness of care delivered.
Physicians continue to support advanced alternative payment models despite the fact that operational issues have not improved over the last 4 years and new ones have cropped up, according to a follow-up survey conducted by the RAND Corporation for the American Medical Association.
“All the things we heard in 2014 were still present in 2018. Both the challenges that practices had experienced back in 2014 having to do with data timeliness, data completeness and accuracy, payment model execution, all those challenges persisted,” Mark W. Friedberg, MD, senior physician policy researcher at RAND, said in an interview.
RAND surveyed 31 practices of varying practice size and specialty across six geographic regions, some of which participated in the 2014 survey. Supplemental information was provided by interviews with 32 market observers, 8 health plan leaders, 10 hospital and hospital system leaders, 10 state and local medical society leaders, and 4 chapter leaders with MGMA (formerly the Medical Group Management Association).
“We had thought we would hear that the problem had gotten a little bit better since there has been some investment in trying to tamp down the wide range of measures that are involved in these alternative payment models,” Dr. Friedberg said. “We did not see any evidence of that having any effect on the practices that participated in this study this time around.”
Indeed, concerns reported in 2014 were again reported in 2018, along with a new set of concerns, including the perceived pace of change in alternative payment models (APMs), the complexity of APMs, and physician concerns over two-sided risk models.
“Practices, especially those that participated both times, said in 2014 we had these challenges [of rapid changes in APM models] and since then, things have just gotten a lot faster,” he said, noting that doctors are complaining of models that are going through changes, sometimes without much warning. “They are changing quite rapidly from year to year. If you look at the MACRA QPP [Quality Payment Program] for example, that model changes every year to some extent and those things are hard for them to keep up with.”
Running hand in hand with the change is the complexity of the changes, a result of expanding performance measures and uncertainty with thresholds for penalties and rewards and in some ways has had little impact on improving care.
Dr. Friedberg noted that some practices are hiring people to examine APMs to devise strategic ways to choose and report data for maximum return.
“In a practice, for example, if their quality of care was already very good, what these folks ended up doing was help them choose measures and work the attribution algorithms in a strategic way to either guarantee a bonus or minimize the risk of incurring a penalty,” he said.
He also noted that practices appear to becoming more risk averse.
“We heard a lot more of the following thing, which is that if [practices] were in a two-sided risk model, several of them reported trying and succeeding in some cases offloading the downside risk to partners,” Dr. Friedberg reported. “And what this resulted in was that the practice, even though from the payer’s perspective they are in a two-sided model, the practice was actually in a one-sided model with a partner who is taking all of the downside risk and a portion of the upside risk, leaving a small upside risk proposition that remained for the practice.”
He said the range of partners that were absorbing the downside risk included hospitals, device manufacturers, consulting companies, or private equity firms.
Despite the concerns surrounding APMs, Dr. Friedberg said that “we did not hear practices broadly saying that they just weren’t interested in alternative payment models. In general, practices still remained pretty enthusiastic about these alternative payment models in theory. If they could be made simpler, if the pace of change weren’t quite so fast, that they would have a chance to really do some important care improvements in alternative payment models.”
He noted some of the surveyed practices were able to make investments in care as a direct result of participating in APMs, such as in behavioral health capabilities in primary care, for example, leading to quality of care improvements.
However, these issues could reveal a future unwillingness to participate in APMs, especially two-sided risk models, something at least the Centers for Medicare & Medicaid Services are pushing for as a stated goal of the QPP is to get practices to participate in APMs and take on more risk.
The growing aversion to taking on downside risk could lead practices to simply stay in fee for service and simply take the payment penalty because it is a fixed amount that can be planned for, as opposed to the fluctuations of bonuses and penalties that comes with a rapidly changing APM environment, Dr. Friedberg said.
Going forward, the report makes a number of recommendations to help create an environment that would potentially make APMs more successful, including simplifying the models; creating stable, predictable, and moderately paced pathways to APM participation; making data available in a more timely fashion; minimizing downside risk or helping practices better manage it; and designing APMs that will encourage clinical changes to help improve the effectiveness of care delivered.
Stroke risk in elderly following AMI extends to 12 weeks
ATLANTA – Acute myocardial infarction is associated with a risk of stroke that extends beyond the 1-month time window currently considered the at-risk period, according to an analysis of Medicare data.
“The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiology classification systems and clinical trial selection criteria,” lead study author Alexander E. Merkler, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In an effort to better understand the duration of heightened stroke risk after acute myocardial infarction, Dr. Merkler, a neurologist at New York–based Weill Cornell Medicine, and his colleagues conducted a retrospective cohort study using inpatient and outpatient claims during 2008-2015 from a nationally representative 5% sample of Medicare beneficiaries who were at least 66 years old. They used previously validated ICD-9-CM diagnosis codes to ascertain the exposure variable of acute MI and the outcome of ischemic stroke but excluded strokes that occurred during an acute MI hospitalization.
Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or by Sept. 30, 2015. The researchers fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjusting for demographics, stroke risk factors, and Charlson comorbidities. Next, they used the corresponding survival probabilities to compute the hazard ratio (HR) in each 4-week interval after discharge, up to week 12. They also conducted a subgroup analysis to evaluate the duration of heightened ischemic stroke risk by MI type: ST-segment elevation MI (STEMI) versus non-STEMI (NSTEMI).
Dr. Merkler and his colleagues drew from data on 1.7 million eligible beneficiaries. Of these, 46,182 were hospitalized for acute MI and 80,466 for ischemic stroke. After they adjusted for demographics, stroke risk factors, and Charlson comorbidities, the researchers found that the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6). It was no longer significantly elevated afterward. The prolonged period of heightened ischemic stroke risk was evident in patients with both STEMI and NSTEMI.
“We were surprised by how long the risk of stroke lasts after MI,” Dr. Merkler said. He acknowledged certain limitations of the analysis, including the fact that patients were all over the age of 65 years. “In addition, we lack granular detail such as severity of MI [and] the extent of stroke work-up,” he said.
Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
[email protected]
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
ATLANTA – Acute myocardial infarction is associated with a risk of stroke that extends beyond the 1-month time window currently considered the at-risk period, according to an analysis of Medicare data.
“The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiology classification systems and clinical trial selection criteria,” lead study author Alexander E. Merkler, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In an effort to better understand the duration of heightened stroke risk after acute myocardial infarction, Dr. Merkler, a neurologist at New York–based Weill Cornell Medicine, and his colleagues conducted a retrospective cohort study using inpatient and outpatient claims during 2008-2015 from a nationally representative 5% sample of Medicare beneficiaries who were at least 66 years old. They used previously validated ICD-9-CM diagnosis codes to ascertain the exposure variable of acute MI and the outcome of ischemic stroke but excluded strokes that occurred during an acute MI hospitalization.
Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or by Sept. 30, 2015. The researchers fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjusting for demographics, stroke risk factors, and Charlson comorbidities. Next, they used the corresponding survival probabilities to compute the hazard ratio (HR) in each 4-week interval after discharge, up to week 12. They also conducted a subgroup analysis to evaluate the duration of heightened ischemic stroke risk by MI type: ST-segment elevation MI (STEMI) versus non-STEMI (NSTEMI).
Dr. Merkler and his colleagues drew from data on 1.7 million eligible beneficiaries. Of these, 46,182 were hospitalized for acute MI and 80,466 for ischemic stroke. After they adjusted for demographics, stroke risk factors, and Charlson comorbidities, the researchers found that the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6). It was no longer significantly elevated afterward. The prolonged period of heightened ischemic stroke risk was evident in patients with both STEMI and NSTEMI.
“We were surprised by how long the risk of stroke lasts after MI,” Dr. Merkler said. He acknowledged certain limitations of the analysis, including the fact that patients were all over the age of 65 years. “In addition, we lack granular detail such as severity of MI [and] the extent of stroke work-up,” he said.
Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
[email protected]
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
ATLANTA – Acute myocardial infarction is associated with a risk of stroke that extends beyond the 1-month time window currently considered the at-risk period, according to an analysis of Medicare data.
“The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiology classification systems and clinical trial selection criteria,” lead study author Alexander E. Merkler, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In an effort to better understand the duration of heightened stroke risk after acute myocardial infarction, Dr. Merkler, a neurologist at New York–based Weill Cornell Medicine, and his colleagues conducted a retrospective cohort study using inpatient and outpatient claims during 2008-2015 from a nationally representative 5% sample of Medicare beneficiaries who were at least 66 years old. They used previously validated ICD-9-CM diagnosis codes to ascertain the exposure variable of acute MI and the outcome of ischemic stroke but excluded strokes that occurred during an acute MI hospitalization.
Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or by Sept. 30, 2015. The researchers fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjusting for demographics, stroke risk factors, and Charlson comorbidities. Next, they used the corresponding survival probabilities to compute the hazard ratio (HR) in each 4-week interval after discharge, up to week 12. They also conducted a subgroup analysis to evaluate the duration of heightened ischemic stroke risk by MI type: ST-segment elevation MI (STEMI) versus non-STEMI (NSTEMI).
Dr. Merkler and his colleagues drew from data on 1.7 million eligible beneficiaries. Of these, 46,182 were hospitalized for acute MI and 80,466 for ischemic stroke. After they adjusted for demographics, stroke risk factors, and Charlson comorbidities, the researchers found that the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6). It was no longer significantly elevated afterward. The prolonged period of heightened ischemic stroke risk was evident in patients with both STEMI and NSTEMI.
“We were surprised by how long the risk of stroke lasts after MI,” Dr. Merkler said. He acknowledged certain limitations of the analysis, including the fact that patients were all over the age of 65 years. “In addition, we lack granular detail such as severity of MI [and] the extent of stroke work-up,” he said.
Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
[email protected]
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
AT ANA 2018
Key clinical point: .
Major finding: The risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6).
Study details: An analysis of 46,182 Medicare beneficiaries who were hospitalized for acute MI and 80,466 who were hospitalized for ischemic stroke.
Disclosures: Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
Drug overdose deaths down since late 2017
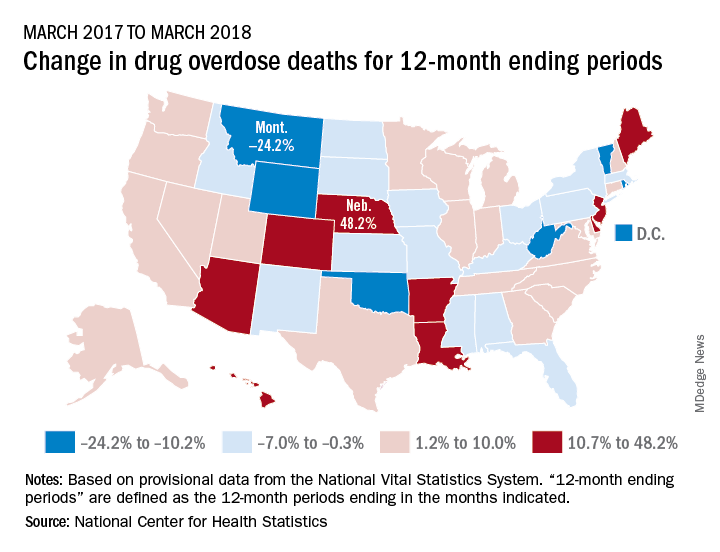
Longer-term data, however, show an increase over the year from March 2017 to March 2018, as the short-term decrease was not enough to overcome the previous year’s increase. The provisional 12-month ending count – deaths during the 12-month period ending in the month indicated – went from 66,859 in March 2017 to 68,690 in March 2018, an increase of 2.7%, the NCHS reported.
That year-long increase was not spread evenly among the states. Nebraska’s 12-month ending count jumped over 48% from March 2017 to March 2018, more than twice as much as second-place Hawaii’s 20.9%. Montana had the largest drop over that year, –24.2%, with Wyoming next at –20.7% and the District of Columbia third at –14.8%, data from the National Vital Statistics System show.
“Provisional drug overdose death data are often incomplete,” the NCHS noted, “and the degree of completeness varies by jurisdiction and 12-month ending period. Consequently, the numbers of drug overdose deaths are underestimated, based on provisional data relative to final data and are subject to random variation.”

Longer-term data, however, show an increase over the year from March 2017 to March 2018, as the short-term decrease was not enough to overcome the previous year’s increase. The provisional 12-month ending count – deaths during the 12-month period ending in the month indicated – went from 66,859 in March 2017 to 68,690 in March 2018, an increase of 2.7%, the NCHS reported.
That year-long increase was not spread evenly among the states. Nebraska’s 12-month ending count jumped over 48% from March 2017 to March 2018, more than twice as much as second-place Hawaii’s 20.9%. Montana had the largest drop over that year, –24.2%, with Wyoming next at –20.7% and the District of Columbia third at –14.8%, data from the National Vital Statistics System show.
“Provisional drug overdose death data are often incomplete,” the NCHS noted, “and the degree of completeness varies by jurisdiction and 12-month ending period. Consequently, the numbers of drug overdose deaths are underestimated, based on provisional data relative to final data and are subject to random variation.”

Longer-term data, however, show an increase over the year from March 2017 to March 2018, as the short-term decrease was not enough to overcome the previous year’s increase. The provisional 12-month ending count – deaths during the 12-month period ending in the month indicated – went from 66,859 in March 2017 to 68,690 in March 2018, an increase of 2.7%, the NCHS reported.
That year-long increase was not spread evenly among the states. Nebraska’s 12-month ending count jumped over 48% from March 2017 to March 2018, more than twice as much as second-place Hawaii’s 20.9%. Montana had the largest drop over that year, –24.2%, with Wyoming next at –20.7% and the District of Columbia third at –14.8%, data from the National Vital Statistics System show.
“Provisional drug overdose death data are often incomplete,” the NCHS noted, “and the degree of completeness varies by jurisdiction and 12-month ending period. Consequently, the numbers of drug overdose deaths are underestimated, based on provisional data relative to final data and are subject to random variation.”
Hospital medicine and palliative care: Wearing both hats
Dr. Barbara Egan leads SHM’s Palliative Care Work Group
Editor’s note: Each month, the Society of Hospitalist Medicine puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Visit www.hospitalmedicine.org for more information on how you can lend your expertise to help improve the care of hospitalized patients.
This month, The Hospitalist spotlights Barbara Egan, MD, FACP, SFHM, chief of the hospital medicine service in the department of medicine at Memorial Sloan Kettering Cancer Center in New York. Barbara has been a member of SHM since 2005, is dual certified in hospital medicine and palliative care, and is the chair of SHM’s Palliative Care Work Group.
When did you first hear about SHM, and why did you decide to become a member?
I first learned about SHM when I was an internal medicine resident at Brigham and Women’s Hospital, Boston, in the early 2000s. BWH had an extremely strong hospitalist group; the staff I worked with served as powerful role models for me and inspired my interest in becoming a hospitalist. One of my attendings suggested that I join SHM, which I did right after I graduated from residency. I attended my first SHM Annual Conference in 2005. By then, I was working as a hospitalist at Memorial Sloan Kettering Cancer Center. SHM and the field of hospital medicine have exploded since my career first began, and I am happy to have grown alongside them. Similarly, our hospital medicine group here at MSKCC has dramatically grown, from 1 hospitalist (me) to more than 30!
How did you get involved with SHM’s Palliative Care Work Group, and what has the work group accomplished since you joined?
I was honored to be invited to join SHM’s Palliative Care Work Group in 2017 by Wendy Anderson, MD, a colleague and now a friend from University of California, San Francisco. Wendy is a visionary leader who practices and researches at the intersection of palliative care and hospital medicine. She and I met during 2015, when we were both invited to join a collaboration between SHM and the Hastings Center in Garrison, N.Y., which was aimed at improving hospitalists’ ability to provide outstanding care to hospitalized patients with life-limiting illnesses. That collaboration resulted in the Improving Communication about Serious Illness–Implementation Guide, a compilation of resources and best practices.
Wendy was chairing the SHM Palliative Care Work Group and invited me to join, which I did with great enthusiasm. This group consists of several passionate and brilliant hospitalists whose practices, in a variety of ways, involve both hospital medicine and palliative medicine. I was so honored when Wendy passed the baton to me last spring and invited me to chair the Work Group. I am lucky to have the opportunity to collaborate with this group of dynamic individuals, and we are well supported by an outstanding SHM staff member, Nick Marzano.
Are there any new projects that the work group is currently focusing on?
The primary focus of SHM’s Palliative Care Work Group is educational. That is, we aim to assess and help meet the educational needs of hospitalists, thereby helping to empower them to be outstanding providers of primary palliative care to seriously ill, hospitalized patients. To that end, we were very proud to orchestrate a palliative care mini-track for the first time at HM18. To our group’s delight, the attendance and reviews of that track were great. Thus, we were invited to further expand the palliative care offerings at HM19. We are busy planning for HM19: a full-day pre-course in palliative medicine; several podium presentations which will touch on ethical challenges, symptom management, prognostication, and other important topics; and a workshop in communication skills.
What led to your dual certification and how do your two specialties overlap?
I am board certified in internal medicine with Focused Practice in Hospital Medicine by virtue of my clinical training and my primary clinical practice as a hospitalist. As a hospitalist in a cancer center, I spend most of my time caring for patients with late- and end-stage malignancy. As such, early in my career, I had to develop a broad base of palliative medical skills, such as pain and symptom management and communication skills. I find this work extremely rewarding, albeit emotionally taxing. I have learned to redefine what clinical “success” looks like – my patients often have unfixable medical problems, but I can always strive to improve their lives in some way, even if that means helping to provide them with a painless, dignified death as opposed to curing them.
When the American Board of Medical Specialties established a board certification in Hospice and Palliative Medicine, there briefly existed a pathway to be “grandfathered” in, i.e., to qualify for board certification through an examination and clinical experience, as opposed to a fellowship. I jumped at the chance to formalize my palliative care skills and experience, and I attained board certification in 2012. This allowed me to further diversify my clinical practice here at MSKCC.
Hospital medicine is still my first love, and I spend most of my time practicing as a hospitalist on our solid tumor services. But now I also spend several weeks each year attending as a consultant on our inpatient supportive care service. In that role, I am able to collaborate with a fantastic multidisciplinary team consisting of MDs, NPs, a chaplain, a pharmacist, a social worker, and integrative medicine practitioners. I also love the opportunity to teach and mentor our palliative medicine fellows.
To me, the opportunity to marry hospital medicine and palliative medicine in my career was a natural fit. Hospitalists, particularly those caring exclusively for cancer patients like I do, need to provide excellent palliative care to our patients every day. The opportunity to further my training and to obtain board certification was a golden one, and I love being able to wear both hats here at MSKCC.
Ms. Steele is a marketing communications specialist at the Society of Hospital Medicine.
Dr. Barbara Egan leads SHM’s Palliative Care Work Group
Dr. Barbara Egan leads SHM’s Palliative Care Work Group
Editor’s note: Each month, the Society of Hospitalist Medicine puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Visit www.hospitalmedicine.org for more information on how you can lend your expertise to help improve the care of hospitalized patients.
This month, The Hospitalist spotlights Barbara Egan, MD, FACP, SFHM, chief of the hospital medicine service in the department of medicine at Memorial Sloan Kettering Cancer Center in New York. Barbara has been a member of SHM since 2005, is dual certified in hospital medicine and palliative care, and is the chair of SHM’s Palliative Care Work Group.
When did you first hear about SHM, and why did you decide to become a member?
I first learned about SHM when I was an internal medicine resident at Brigham and Women’s Hospital, Boston, in the early 2000s. BWH had an extremely strong hospitalist group; the staff I worked with served as powerful role models for me and inspired my interest in becoming a hospitalist. One of my attendings suggested that I join SHM, which I did right after I graduated from residency. I attended my first SHM Annual Conference in 2005. By then, I was working as a hospitalist at Memorial Sloan Kettering Cancer Center. SHM and the field of hospital medicine have exploded since my career first began, and I am happy to have grown alongside them. Similarly, our hospital medicine group here at MSKCC has dramatically grown, from 1 hospitalist (me) to more than 30!
How did you get involved with SHM’s Palliative Care Work Group, and what has the work group accomplished since you joined?
I was honored to be invited to join SHM’s Palliative Care Work Group in 2017 by Wendy Anderson, MD, a colleague and now a friend from University of California, San Francisco. Wendy is a visionary leader who practices and researches at the intersection of palliative care and hospital medicine. She and I met during 2015, when we were both invited to join a collaboration between SHM and the Hastings Center in Garrison, N.Y., which was aimed at improving hospitalists’ ability to provide outstanding care to hospitalized patients with life-limiting illnesses. That collaboration resulted in the Improving Communication about Serious Illness–Implementation Guide, a compilation of resources and best practices.
Wendy was chairing the SHM Palliative Care Work Group and invited me to join, which I did with great enthusiasm. This group consists of several passionate and brilliant hospitalists whose practices, in a variety of ways, involve both hospital medicine and palliative medicine. I was so honored when Wendy passed the baton to me last spring and invited me to chair the Work Group. I am lucky to have the opportunity to collaborate with this group of dynamic individuals, and we are well supported by an outstanding SHM staff member, Nick Marzano.
Are there any new projects that the work group is currently focusing on?
The primary focus of SHM’s Palliative Care Work Group is educational. That is, we aim to assess and help meet the educational needs of hospitalists, thereby helping to empower them to be outstanding providers of primary palliative care to seriously ill, hospitalized patients. To that end, we were very proud to orchestrate a palliative care mini-track for the first time at HM18. To our group’s delight, the attendance and reviews of that track were great. Thus, we were invited to further expand the palliative care offerings at HM19. We are busy planning for HM19: a full-day pre-course in palliative medicine; several podium presentations which will touch on ethical challenges, symptom management, prognostication, and other important topics; and a workshop in communication skills.
What led to your dual certification and how do your two specialties overlap?
I am board certified in internal medicine with Focused Practice in Hospital Medicine by virtue of my clinical training and my primary clinical practice as a hospitalist. As a hospitalist in a cancer center, I spend most of my time caring for patients with late- and end-stage malignancy. As such, early in my career, I had to develop a broad base of palliative medical skills, such as pain and symptom management and communication skills. I find this work extremely rewarding, albeit emotionally taxing. I have learned to redefine what clinical “success” looks like – my patients often have unfixable medical problems, but I can always strive to improve their lives in some way, even if that means helping to provide them with a painless, dignified death as opposed to curing them.
When the American Board of Medical Specialties established a board certification in Hospice and Palliative Medicine, there briefly existed a pathway to be “grandfathered” in, i.e., to qualify for board certification through an examination and clinical experience, as opposed to a fellowship. I jumped at the chance to formalize my palliative care skills and experience, and I attained board certification in 2012. This allowed me to further diversify my clinical practice here at MSKCC.
Hospital medicine is still my first love, and I spend most of my time practicing as a hospitalist on our solid tumor services. But now I also spend several weeks each year attending as a consultant on our inpatient supportive care service. In that role, I am able to collaborate with a fantastic multidisciplinary team consisting of MDs, NPs, a chaplain, a pharmacist, a social worker, and integrative medicine practitioners. I also love the opportunity to teach and mentor our palliative medicine fellows.
To me, the opportunity to marry hospital medicine and palliative medicine in my career was a natural fit. Hospitalists, particularly those caring exclusively for cancer patients like I do, need to provide excellent palliative care to our patients every day. The opportunity to further my training and to obtain board certification was a golden one, and I love being able to wear both hats here at MSKCC.
Ms. Steele is a marketing communications specialist at the Society of Hospital Medicine.
Editor’s note: Each month, the Society of Hospitalist Medicine puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Visit www.hospitalmedicine.org for more information on how you can lend your expertise to help improve the care of hospitalized patients.
This month, The Hospitalist spotlights Barbara Egan, MD, FACP, SFHM, chief of the hospital medicine service in the department of medicine at Memorial Sloan Kettering Cancer Center in New York. Barbara has been a member of SHM since 2005, is dual certified in hospital medicine and palliative care, and is the chair of SHM’s Palliative Care Work Group.
When did you first hear about SHM, and why did you decide to become a member?
I first learned about SHM when I was an internal medicine resident at Brigham and Women’s Hospital, Boston, in the early 2000s. BWH had an extremely strong hospitalist group; the staff I worked with served as powerful role models for me and inspired my interest in becoming a hospitalist. One of my attendings suggested that I join SHM, which I did right after I graduated from residency. I attended my first SHM Annual Conference in 2005. By then, I was working as a hospitalist at Memorial Sloan Kettering Cancer Center. SHM and the field of hospital medicine have exploded since my career first began, and I am happy to have grown alongside them. Similarly, our hospital medicine group here at MSKCC has dramatically grown, from 1 hospitalist (me) to more than 30!
How did you get involved with SHM’s Palliative Care Work Group, and what has the work group accomplished since you joined?
I was honored to be invited to join SHM’s Palliative Care Work Group in 2017 by Wendy Anderson, MD, a colleague and now a friend from University of California, San Francisco. Wendy is a visionary leader who practices and researches at the intersection of palliative care and hospital medicine. She and I met during 2015, when we were both invited to join a collaboration between SHM and the Hastings Center in Garrison, N.Y., which was aimed at improving hospitalists’ ability to provide outstanding care to hospitalized patients with life-limiting illnesses. That collaboration resulted in the Improving Communication about Serious Illness–Implementation Guide, a compilation of resources and best practices.
Wendy was chairing the SHM Palliative Care Work Group and invited me to join, which I did with great enthusiasm. This group consists of several passionate and brilliant hospitalists whose practices, in a variety of ways, involve both hospital medicine and palliative medicine. I was so honored when Wendy passed the baton to me last spring and invited me to chair the Work Group. I am lucky to have the opportunity to collaborate with this group of dynamic individuals, and we are well supported by an outstanding SHM staff member, Nick Marzano.
Are there any new projects that the work group is currently focusing on?
The primary focus of SHM’s Palliative Care Work Group is educational. That is, we aim to assess and help meet the educational needs of hospitalists, thereby helping to empower them to be outstanding providers of primary palliative care to seriously ill, hospitalized patients. To that end, we were very proud to orchestrate a palliative care mini-track for the first time at HM18. To our group’s delight, the attendance and reviews of that track were great. Thus, we were invited to further expand the palliative care offerings at HM19. We are busy planning for HM19: a full-day pre-course in palliative medicine; several podium presentations which will touch on ethical challenges, symptom management, prognostication, and other important topics; and a workshop in communication skills.
What led to your dual certification and how do your two specialties overlap?
I am board certified in internal medicine with Focused Practice in Hospital Medicine by virtue of my clinical training and my primary clinical practice as a hospitalist. As a hospitalist in a cancer center, I spend most of my time caring for patients with late- and end-stage malignancy. As such, early in my career, I had to develop a broad base of palliative medical skills, such as pain and symptom management and communication skills. I find this work extremely rewarding, albeit emotionally taxing. I have learned to redefine what clinical “success” looks like – my patients often have unfixable medical problems, but I can always strive to improve their lives in some way, even if that means helping to provide them with a painless, dignified death as opposed to curing them.
When the American Board of Medical Specialties established a board certification in Hospice and Palliative Medicine, there briefly existed a pathway to be “grandfathered” in, i.e., to qualify for board certification through an examination and clinical experience, as opposed to a fellowship. I jumped at the chance to formalize my palliative care skills and experience, and I attained board certification in 2012. This allowed me to further diversify my clinical practice here at MSKCC.
Hospital medicine is still my first love, and I spend most of my time practicing as a hospitalist on our solid tumor services. But now I also spend several weeks each year attending as a consultant on our inpatient supportive care service. In that role, I am able to collaborate with a fantastic multidisciplinary team consisting of MDs, NPs, a chaplain, a pharmacist, a social worker, and integrative medicine practitioners. I also love the opportunity to teach and mentor our palliative medicine fellows.
To me, the opportunity to marry hospital medicine and palliative medicine in my career was a natural fit. Hospitalists, particularly those caring exclusively for cancer patients like I do, need to provide excellent palliative care to our patients every day. The opportunity to further my training and to obtain board certification was a golden one, and I love being able to wear both hats here at MSKCC.
Ms. Steele is a marketing communications specialist at the Society of Hospital Medicine.
Reducing alarm fatigue
Monitoring from a centralized location
Hospitalists hearing the constant noise from cardiac telemetry monitoring systems can experience alarm fatigue – a nationwide phenomenon that can lead to an increase in patient deaths.
The American Heart Association reports that fewer than one in four adults survived an in-hospital cardiac arrest in 2013; other studies showed that up to 44% of inpatient cardiac arrests were not detected appropriately, according to the Cleveland Clinic.
Clinicians at the Cleveland Clinic have tried centralized monitoring to address the problem. They’ve established a “mission control” center, where off-site personnel monitor sensors and high-definition cameras and vital signs such as blood pressure, heart rate, and respiration. On-site action is requested when appropriate; unimportant alarms are dismissed.
In August 2016, results from the first 13 months of the Cleveland Clinic program were published in JAMA. They revealed that the monitoring system could help reduce rates of unimportant alarms with no increase in cardiopulmonary arrest events. The centralized unit monitored 99,048 patient orders, and ultimately detected serious problems and accurately notified on-site staff for 79% of 3,243 events, which included a rhythm and/or rate change within 1 hour or less of the event. Accurate notification to on-site hospital staff was more than 84%.
Since then, improvements to the system have continued, according to Cleveland Clinic, and include doubling the number of monitored patients per technician and improved clinical outcomes.
Reference
Cleveland Clinic: Consult QD – An update on the centralized cardiac telemetry monitoring unit
Monitoring from a centralized location
Monitoring from a centralized location
Hospitalists hearing the constant noise from cardiac telemetry monitoring systems can experience alarm fatigue – a nationwide phenomenon that can lead to an increase in patient deaths.
The American Heart Association reports that fewer than one in four adults survived an in-hospital cardiac arrest in 2013; other studies showed that up to 44% of inpatient cardiac arrests were not detected appropriately, according to the Cleveland Clinic.
Clinicians at the Cleveland Clinic have tried centralized monitoring to address the problem. They’ve established a “mission control” center, where off-site personnel monitor sensors and high-definition cameras and vital signs such as blood pressure, heart rate, and respiration. On-site action is requested when appropriate; unimportant alarms are dismissed.
In August 2016, results from the first 13 months of the Cleveland Clinic program were published in JAMA. They revealed that the monitoring system could help reduce rates of unimportant alarms with no increase in cardiopulmonary arrest events. The centralized unit monitored 99,048 patient orders, and ultimately detected serious problems and accurately notified on-site staff for 79% of 3,243 events, which included a rhythm and/or rate change within 1 hour or less of the event. Accurate notification to on-site hospital staff was more than 84%.
Since then, improvements to the system have continued, according to Cleveland Clinic, and include doubling the number of monitored patients per technician and improved clinical outcomes.
Reference
Cleveland Clinic: Consult QD – An update on the centralized cardiac telemetry monitoring unit
Hospitalists hearing the constant noise from cardiac telemetry monitoring systems can experience alarm fatigue – a nationwide phenomenon that can lead to an increase in patient deaths.
The American Heart Association reports that fewer than one in four adults survived an in-hospital cardiac arrest in 2013; other studies showed that up to 44% of inpatient cardiac arrests were not detected appropriately, according to the Cleveland Clinic.
Clinicians at the Cleveland Clinic have tried centralized monitoring to address the problem. They’ve established a “mission control” center, where off-site personnel monitor sensors and high-definition cameras and vital signs such as blood pressure, heart rate, and respiration. On-site action is requested when appropriate; unimportant alarms are dismissed.
In August 2016, results from the first 13 months of the Cleveland Clinic program were published in JAMA. They revealed that the monitoring system could help reduce rates of unimportant alarms with no increase in cardiopulmonary arrest events. The centralized unit monitored 99,048 patient orders, and ultimately detected serious problems and accurately notified on-site staff for 79% of 3,243 events, which included a rhythm and/or rate change within 1 hour or less of the event. Accurate notification to on-site hospital staff was more than 84%.
Since then, improvements to the system have continued, according to Cleveland Clinic, and include doubling the number of monitored patients per technician and improved clinical outcomes.
Reference
Cleveland Clinic: Consult QD – An update on the centralized cardiac telemetry monitoring unit
Readmission to non-index hospital following acute stroke linked to worse outcomes
ATLANTA – Following an acute stroke, optimizing stroke secondary prevention measures, medical complications, and transitions of care is essential to reducing 30-day readmissions and improving patient outcomes, a large analysis of national data showed.
“Care that is fragmented with readmissions to other hospitals results not only in more expensive care and longer length of stay but also increased mortality for our acute stroke patients,” lead study author Laura K. Stein, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In 2017, a study of the Nationwide Readmissions Database demonstrated that 12.1% of patients with acute ischemic stroke were readmitted within 30 days (Stroke 2017;48:1386-8). It cited that 89.6% were unplanned and 12.9% were preventable. “However, this study did not examine whether patients were admitted to the discharging hospital or a different hospital,” said Dr. Stein, a neurologist at the Icahn School of Medicine at Mount Sinai, New York. “Furthermore, it did not include metrics such as cost, length of stay, and mortality with 30-day readmissions. Hospitals are increasingly held accountable and penalized for metrics such as length of stay and 30-day readmissions.”
In 2010, the Centers for Medicare & Medicaid Services introduced the Hospital Readmissions Reduction Program in an attempt to decrease readmissions following hospitalizations for acute myocardial infarction, heart failure, and pneumonia. “In 2012, CMS started reducing Medicare payments for hospitals with excess readmissions,” said Dr. Stein, who is a fellowship-trained stroke specialist. “While readmission to the same hospital has great implications for hospital systems, any readmission has great implications for patients.”
In what is believed to be the first study of its kind, Dr. Stein and her colleagues drew from the 2013 Nationwide Readmissions Database to examine in-hospital outcomes associated with 30-day readmission to a different hospital for acute ischemic stroke. They used ICD-9 codes to identify index stroke admissions and all-cause readmissions. Outcomes of interest were length of stay, total charges, and in-hospital mortality during the 30-day readmission. The main predictor was readmission to another hospital, compared with readmission to the same hospital as the index acute stroke admission. The researchers used linear regression for the outcomes of length of stay and charges, and logistic regression for in-hospital mortality. They adjusted for several variables during the index admission, including age, sex, vascular risk factors, hospital bed size, teaching hospital status, insurance status, discharge destination, National Center for Health Statistics urban-rural location classification, length of stay, and total charges.
Of 24,545 acute stroke patients readmitted within 30 days, 7,274 (30%) were readmitted to a different hospital. The top three reasons for readmission were acute cerebrovascular disease, septicemia, and renal failure. In fully adjusted models, readmission to a different hospital was associated with an increased length of stay of 0.97 days (P less than .0001) and a mean of $7,677.28 greater total charges, compared with readmission to the same hospital (P less than .0001). The fully adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
“While it is conceivable that cost and length of stay could be higher with readmission to a different hospital because of a need for additional testing with a lack of familiarity with the patient, it is concerning that mortality is higher,” Dr. Stein said. “These findings emphasize the importance of optimizing secondary stroke prevention and medical complications following acute stroke before discharge. Additionally, they emphasize the importance of good transitions of care from the inpatient to outpatient setting (whether that’s to a rehabilitation facility, skilled nursing facility, or home) and accessibility of the discharging stroke team after discharge.”
She acknowledged certain limitations of the analysis, including its reliance of administrative data, which could include misclassification of diagnoses and comorbidities based on ICD-9 codes. “However, we have chosen ICD-9 codes for stroke that have been previously validated in the literature,” Dr. Stein said. “For instance, the validated codes for stroke as the primary discharge diagnosis have a sensitivity of 74%, specificity of 95%, and positive predictive value of 88%. Second, we do not know stroke subtype or severity of stroke. Third, we do not know what the transitions of care plan were when the patients left the hospital following index acute ischemic stroke admission and why these patients ended up being readmitted to a different hospital rather than the one that treated them for their acute stroke.”
The researchers reported having no financial disclosures.
SOURCE: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
ATLANTA – Following an acute stroke, optimizing stroke secondary prevention measures, medical complications, and transitions of care is essential to reducing 30-day readmissions and improving patient outcomes, a large analysis of national data showed.
“Care that is fragmented with readmissions to other hospitals results not only in more expensive care and longer length of stay but also increased mortality for our acute stroke patients,” lead study author Laura K. Stein, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In 2017, a study of the Nationwide Readmissions Database demonstrated that 12.1% of patients with acute ischemic stroke were readmitted within 30 days (Stroke 2017;48:1386-8). It cited that 89.6% were unplanned and 12.9% were preventable. “However, this study did not examine whether patients were admitted to the discharging hospital or a different hospital,” said Dr. Stein, a neurologist at the Icahn School of Medicine at Mount Sinai, New York. “Furthermore, it did not include metrics such as cost, length of stay, and mortality with 30-day readmissions. Hospitals are increasingly held accountable and penalized for metrics such as length of stay and 30-day readmissions.”
In 2010, the Centers for Medicare & Medicaid Services introduced the Hospital Readmissions Reduction Program in an attempt to decrease readmissions following hospitalizations for acute myocardial infarction, heart failure, and pneumonia. “In 2012, CMS started reducing Medicare payments for hospitals with excess readmissions,” said Dr. Stein, who is a fellowship-trained stroke specialist. “While readmission to the same hospital has great implications for hospital systems, any readmission has great implications for patients.”
In what is believed to be the first study of its kind, Dr. Stein and her colleagues drew from the 2013 Nationwide Readmissions Database to examine in-hospital outcomes associated with 30-day readmission to a different hospital for acute ischemic stroke. They used ICD-9 codes to identify index stroke admissions and all-cause readmissions. Outcomes of interest were length of stay, total charges, and in-hospital mortality during the 30-day readmission. The main predictor was readmission to another hospital, compared with readmission to the same hospital as the index acute stroke admission. The researchers used linear regression for the outcomes of length of stay and charges, and logistic regression for in-hospital mortality. They adjusted for several variables during the index admission, including age, sex, vascular risk factors, hospital bed size, teaching hospital status, insurance status, discharge destination, National Center for Health Statistics urban-rural location classification, length of stay, and total charges.
Of 24,545 acute stroke patients readmitted within 30 days, 7,274 (30%) were readmitted to a different hospital. The top three reasons for readmission were acute cerebrovascular disease, septicemia, and renal failure. In fully adjusted models, readmission to a different hospital was associated with an increased length of stay of 0.97 days (P less than .0001) and a mean of $7,677.28 greater total charges, compared with readmission to the same hospital (P less than .0001). The fully adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
“While it is conceivable that cost and length of stay could be higher with readmission to a different hospital because of a need for additional testing with a lack of familiarity with the patient, it is concerning that mortality is higher,” Dr. Stein said. “These findings emphasize the importance of optimizing secondary stroke prevention and medical complications following acute stroke before discharge. Additionally, they emphasize the importance of good transitions of care from the inpatient to outpatient setting (whether that’s to a rehabilitation facility, skilled nursing facility, or home) and accessibility of the discharging stroke team after discharge.”
She acknowledged certain limitations of the analysis, including its reliance of administrative data, which could include misclassification of diagnoses and comorbidities based on ICD-9 codes. “However, we have chosen ICD-9 codes for stroke that have been previously validated in the literature,” Dr. Stein said. “For instance, the validated codes for stroke as the primary discharge diagnosis have a sensitivity of 74%, specificity of 95%, and positive predictive value of 88%. Second, we do not know stroke subtype or severity of stroke. Third, we do not know what the transitions of care plan were when the patients left the hospital following index acute ischemic stroke admission and why these patients ended up being readmitted to a different hospital rather than the one that treated them for their acute stroke.”
The researchers reported having no financial disclosures.
SOURCE: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
ATLANTA – Following an acute stroke, optimizing stroke secondary prevention measures, medical complications, and transitions of care is essential to reducing 30-day readmissions and improving patient outcomes, a large analysis of national data showed.
“Care that is fragmented with readmissions to other hospitals results not only in more expensive care and longer length of stay but also increased mortality for our acute stroke patients,” lead study author Laura K. Stein, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In 2017, a study of the Nationwide Readmissions Database demonstrated that 12.1% of patients with acute ischemic stroke were readmitted within 30 days (Stroke 2017;48:1386-8). It cited that 89.6% were unplanned and 12.9% were preventable. “However, this study did not examine whether patients were admitted to the discharging hospital or a different hospital,” said Dr. Stein, a neurologist at the Icahn School of Medicine at Mount Sinai, New York. “Furthermore, it did not include metrics such as cost, length of stay, and mortality with 30-day readmissions. Hospitals are increasingly held accountable and penalized for metrics such as length of stay and 30-day readmissions.”
In 2010, the Centers for Medicare & Medicaid Services introduced the Hospital Readmissions Reduction Program in an attempt to decrease readmissions following hospitalizations for acute myocardial infarction, heart failure, and pneumonia. “In 2012, CMS started reducing Medicare payments for hospitals with excess readmissions,” said Dr. Stein, who is a fellowship-trained stroke specialist. “While readmission to the same hospital has great implications for hospital systems, any readmission has great implications for patients.”
In what is believed to be the first study of its kind, Dr. Stein and her colleagues drew from the 2013 Nationwide Readmissions Database to examine in-hospital outcomes associated with 30-day readmission to a different hospital for acute ischemic stroke. They used ICD-9 codes to identify index stroke admissions and all-cause readmissions. Outcomes of interest were length of stay, total charges, and in-hospital mortality during the 30-day readmission. The main predictor was readmission to another hospital, compared with readmission to the same hospital as the index acute stroke admission. The researchers used linear regression for the outcomes of length of stay and charges, and logistic regression for in-hospital mortality. They adjusted for several variables during the index admission, including age, sex, vascular risk factors, hospital bed size, teaching hospital status, insurance status, discharge destination, National Center for Health Statistics urban-rural location classification, length of stay, and total charges.
Of 24,545 acute stroke patients readmitted within 30 days, 7,274 (30%) were readmitted to a different hospital. The top three reasons for readmission were acute cerebrovascular disease, septicemia, and renal failure. In fully adjusted models, readmission to a different hospital was associated with an increased length of stay of 0.97 days (P less than .0001) and a mean of $7,677.28 greater total charges, compared with readmission to the same hospital (P less than .0001). The fully adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
“While it is conceivable that cost and length of stay could be higher with readmission to a different hospital because of a need for additional testing with a lack of familiarity with the patient, it is concerning that mortality is higher,” Dr. Stein said. “These findings emphasize the importance of optimizing secondary stroke prevention and medical complications following acute stroke before discharge. Additionally, they emphasize the importance of good transitions of care from the inpatient to outpatient setting (whether that’s to a rehabilitation facility, skilled nursing facility, or home) and accessibility of the discharging stroke team after discharge.”
She acknowledged certain limitations of the analysis, including its reliance of administrative data, which could include misclassification of diagnoses and comorbidities based on ICD-9 codes. “However, we have chosen ICD-9 codes for stroke that have been previously validated in the literature,” Dr. Stein said. “For instance, the validated codes for stroke as the primary discharge diagnosis have a sensitivity of 74%, specificity of 95%, and positive predictive value of 88%. Second, we do not know stroke subtype or severity of stroke. Third, we do not know what the transitions of care plan were when the patients left the hospital following index acute ischemic stroke admission and why these patients ended up being readmitted to a different hospital rather than the one that treated them for their acute stroke.”
The researchers reported having no financial disclosures.
SOURCE: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
REPORTING FROM ANA 2018
Key clinical point:
Major finding: The adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
Study details: A review of 24,545 acute stroke patients 2013 from the Nationwide Readmissions Database.
Disclosures: The researchers reported having no financial disclosures.
Source: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
Blood test may obviate need for head CTs in brain trauma evaluation
SAN DIEGO – A biomarker test based on the presence of two proteins in the blood appears to be suitable for ruling out significant intracranial injuries in patients with a history of mild traumatic brain injury (TBI) without the need for a CT head scan, according to data presented at the annual meeting of the American College of Emergency Physicians.
according to Jeffrey J. Bazarian, MD, professor of emergency medicine, University of Rochester (New York).
In the ALERT-TBI study, which evaluated the biomarker test, 1,959 patients with suspected TBI at 22 participating EDs in the United States and Europe were enrolled and available for analysis. All had mild TBI as defined as a Glasgow Coma Scale (GCS) score of 13-15.
The treating ED physician’s decision to order a head CT scan was the major criterion for study entry. All enrolled patients had their blood drawn within 12 hours in order to quantify two biomarkers, C-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP).
The biomarker test for TBI was negative when the UCH-L1 value was less than 327 pg/mL and the GFAP was less than 22 pg/mL; the test was positive if either value was at this threshold or higher. To evaluate the sensitivity and specificity of this dual-biomarker test, results were correlated with head CT scans read by two neurologists blinded to the biomarker values.
The mean age of the study population was 48.8 years and slightly more than half were male. About half of the suspected TBI in these patients was attributed to falls and about one third to motor vehicle accidents.
Typical of TBI with GCS scores in the mild range, only 6% of the patients had a positive CT head scan. Of the 125 positive CT scans, the most common injury detected on CT scan was subarachnoid hemorrhage followed by subdural hematoma.
Of the 671 negative biomarker tests, 668 had normal head CT scans. Of the three false positives, one included a cavernous malformation that may have been present prior to the TBI. The others were a small subarachnoid hemorrhage and a small subdural hematoma. Overall the negative predictive value was 99.6% and the sensitivity was 97.6%.
Although the biomarker specificity was only 36% with an even-lower positive predictive value, the goal of the test was to rule out significant TBI to avoid the need for CT scan. On this basis, the biomarker test, which is being developed under the proprietary name Banyan BTI, appears to be promising. The data, according to Dr. Bazarian, have been submitted to the Food and Drug Administration.
“Head CT scans are the current standard for evaluating intracranial injuries after TBI, but they are overused, based on the high proportion that do not show an injury,” said Dr. Bazarian. Although he does not know the disposition of the FDA application, he said, based on these data, “I would definitely be using this test if it were available.”
SAN DIEGO – A biomarker test based on the presence of two proteins in the blood appears to be suitable for ruling out significant intracranial injuries in patients with a history of mild traumatic brain injury (TBI) without the need for a CT head scan, according to data presented at the annual meeting of the American College of Emergency Physicians.
according to Jeffrey J. Bazarian, MD, professor of emergency medicine, University of Rochester (New York).
In the ALERT-TBI study, which evaluated the biomarker test, 1,959 patients with suspected TBI at 22 participating EDs in the United States and Europe were enrolled and available for analysis. All had mild TBI as defined as a Glasgow Coma Scale (GCS) score of 13-15.
The treating ED physician’s decision to order a head CT scan was the major criterion for study entry. All enrolled patients had their blood drawn within 12 hours in order to quantify two biomarkers, C-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP).
The biomarker test for TBI was negative when the UCH-L1 value was less than 327 pg/mL and the GFAP was less than 22 pg/mL; the test was positive if either value was at this threshold or higher. To evaluate the sensitivity and specificity of this dual-biomarker test, results were correlated with head CT scans read by two neurologists blinded to the biomarker values.
The mean age of the study population was 48.8 years and slightly more than half were male. About half of the suspected TBI in these patients was attributed to falls and about one third to motor vehicle accidents.
Typical of TBI with GCS scores in the mild range, only 6% of the patients had a positive CT head scan. Of the 125 positive CT scans, the most common injury detected on CT scan was subarachnoid hemorrhage followed by subdural hematoma.
Of the 671 negative biomarker tests, 668 had normal head CT scans. Of the three false positives, one included a cavernous malformation that may have been present prior to the TBI. The others were a small subarachnoid hemorrhage and a small subdural hematoma. Overall the negative predictive value was 99.6% and the sensitivity was 97.6%.
Although the biomarker specificity was only 36% with an even-lower positive predictive value, the goal of the test was to rule out significant TBI to avoid the need for CT scan. On this basis, the biomarker test, which is being developed under the proprietary name Banyan BTI, appears to be promising. The data, according to Dr. Bazarian, have been submitted to the Food and Drug Administration.
“Head CT scans are the current standard for evaluating intracranial injuries after TBI, but they are overused, based on the high proportion that do not show an injury,” said Dr. Bazarian. Although he does not know the disposition of the FDA application, he said, based on these data, “I would definitely be using this test if it were available.”
SAN DIEGO – A biomarker test based on the presence of two proteins in the blood appears to be suitable for ruling out significant intracranial injuries in patients with a history of mild traumatic brain injury (TBI) without the need for a CT head scan, according to data presented at the annual meeting of the American College of Emergency Physicians.
according to Jeffrey J. Bazarian, MD, professor of emergency medicine, University of Rochester (New York).
In the ALERT-TBI study, which evaluated the biomarker test, 1,959 patients with suspected TBI at 22 participating EDs in the United States and Europe were enrolled and available for analysis. All had mild TBI as defined as a Glasgow Coma Scale (GCS) score of 13-15.
The treating ED physician’s decision to order a head CT scan was the major criterion for study entry. All enrolled patients had their blood drawn within 12 hours in order to quantify two biomarkers, C-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP).
The biomarker test for TBI was negative when the UCH-L1 value was less than 327 pg/mL and the GFAP was less than 22 pg/mL; the test was positive if either value was at this threshold or higher. To evaluate the sensitivity and specificity of this dual-biomarker test, results were correlated with head CT scans read by two neurologists blinded to the biomarker values.
The mean age of the study population was 48.8 years and slightly more than half were male. About half of the suspected TBI in these patients was attributed to falls and about one third to motor vehicle accidents.
Typical of TBI with GCS scores in the mild range, only 6% of the patients had a positive CT head scan. Of the 125 positive CT scans, the most common injury detected on CT scan was subarachnoid hemorrhage followed by subdural hematoma.
Of the 671 negative biomarker tests, 668 had normal head CT scans. Of the three false positives, one included a cavernous malformation that may have been present prior to the TBI. The others were a small subarachnoid hemorrhage and a small subdural hematoma. Overall the negative predictive value was 99.6% and the sensitivity was 97.6%.
Although the biomarker specificity was only 36% with an even-lower positive predictive value, the goal of the test was to rule out significant TBI to avoid the need for CT scan. On this basis, the biomarker test, which is being developed under the proprietary name Banyan BTI, appears to be promising. The data, according to Dr. Bazarian, have been submitted to the Food and Drug Administration.
“Head CT scans are the current standard for evaluating intracranial injuries after TBI, but they are overused, based on the high proportion that do not show an injury,” said Dr. Bazarian. Although he does not know the disposition of the FDA application, he said, based on these data, “I would definitely be using this test if it were available.”
FROM ACEP 2018
Key clinical point: In patients with mild head trauma, a simple blood test may eliminate need and cost for routine CT scans.
Major finding: In patients a history of head trauma, the biomarker test had a 99.6% negative predictive value in ruling out injury.
Study details: Prospective, controlled registration study.
Disclosures: Dr. Bazarian reported no financial relationships relevant to this study, which was in part funded by Banyan Biomarkers.
DAPT’s benefit after stroke or TIA clusters in first 21 days
MONTREAL – The optimal length for dual antiplatelet therapy in patients who have just had a mild stroke or transient ischemic attack is 21 days, a duration of combined treatment that maximized protection against major ischemic events while minimizing the extra risk for a major hemorrhage, according to a prespecified analysis of data from the POINT trial.
The POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial randomized 4,881 patients with a very recent mild stroke or transient ischemic attack and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, dual antiplatelet therapy (DAPT) cut the incidence of a major ischemic event by a relative 25% but also more than doubled the rate of major hemorrhage (New Engl J Med. 2018 Jul 19;377[3]:215-25).
The new, prespecified analysis looked at outcomes on a week-by-week basis over the course of 90 days of treatment, and showed that during the first 21 days the rate of major ischemic events was 5.6% among patients on aspirin only and 3.6% among those on DAPT, a statistically significant 35% relative cut in these adverse outcomes by using DAPT, Jordan J. Elm, PhD, reported at the World Stroke Congress. During the subsequent 69 days on treatment, the incidence of major ischemic events was roughly 1% in both arms of the study, showing that after 3 weeks the incremental benefit from DAPT disappeared, said Dr. Elm, a biostatistician at the Medical University of South Carolina, Charleston.
In contrast, the doubled rate of major hemorrhages (mostly reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment, meaning that limiting DAPT to just 21 days could prevent many of the excess hemorrhages.
“These results suggest that limiting clopidogrel plus aspirin use to 21 days may maximize benefit and reduce risk,” Dr. Elm said, especially in light of the findings confirming the efficacy of 21 days of DAPT following a minor stroke or TIA that had been reported several years ago in the CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial (New Engl J Med. 2013 Jul 4;369[1]:11-9).
Although the new finding from the POINT results came in a secondary analysis, it’s statistically legitimate and should be taken into account when writing treatment guidelines, she said, emphasizing that “this is a very important analysis that is not just hypothesis generating.”
Another finding from the new analysis was that a large number of major ischemic events, and hence a large number of the events prevented by DAPT, occurred in the first 2 days following the index event, a finding made possible because the POINT investigators enrolled patients and started treatment within 12 hours of the qualifying events.
“It’s better to start treatment early,” Dr. Elm noted, but she also highlighted that major ischemic events continued to accumulate during days 3-21, suggesting that patients could still benefit from DAPT even if treatment did not start until 24 or 48 hours after their index event.
POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm reported no disclosures.
SOURCE: Elm JJ et al. World Stroke Congress, Late-breaking session.
The new model using data from the POINT trial confirms what had been previously shown in the CHANCE trial – that 21 days is a sensible cutoff for dual antiplatelet treatment for patients immediately following a mild stroke or transient ischemic attack. Treatment with dual antiplatelet therapy for 21 days provides the same added benefit as 90 days of treatment but with less excess bleeding. The new findings confirm that the CHANCE results were not specific to a Chinese population.

For the time being, clopidogrel is the evidence-based antiplatelet drug to pair with aspirin for this indication. Clopidogrel has the advantages of being generic, cheap, available, and familiar. It’s possible that another P2Y12 inhibitor, such as ticagrelor (Brilinta), might work even better, but that needs to be proven to justify the added expense of a brand-name antiplatelet drug.
Mike Sharma, MD , is a stroke neurologist at McMaster University, Hamilton, Ont. He has been an advisor to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. He made these comments in an interview.
The new model using data from the POINT trial confirms what had been previously shown in the CHANCE trial – that 21 days is a sensible cutoff for dual antiplatelet treatment for patients immediately following a mild stroke or transient ischemic attack. Treatment with dual antiplatelet therapy for 21 days provides the same added benefit as 90 days of treatment but with less excess bleeding. The new findings confirm that the CHANCE results were not specific to a Chinese population.

For the time being, clopidogrel is the evidence-based antiplatelet drug to pair with aspirin for this indication. Clopidogrel has the advantages of being generic, cheap, available, and familiar. It’s possible that another P2Y12 inhibitor, such as ticagrelor (Brilinta), might work even better, but that needs to be proven to justify the added expense of a brand-name antiplatelet drug.
Mike Sharma, MD , is a stroke neurologist at McMaster University, Hamilton, Ont. He has been an advisor to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. He made these comments in an interview.
The new model using data from the POINT trial confirms what had been previously shown in the CHANCE trial – that 21 days is a sensible cutoff for dual antiplatelet treatment for patients immediately following a mild stroke or transient ischemic attack. Treatment with dual antiplatelet therapy for 21 days provides the same added benefit as 90 days of treatment but with less excess bleeding. The new findings confirm that the CHANCE results were not specific to a Chinese population.

For the time being, clopidogrel is the evidence-based antiplatelet drug to pair with aspirin for this indication. Clopidogrel has the advantages of being generic, cheap, available, and familiar. It’s possible that another P2Y12 inhibitor, such as ticagrelor (Brilinta), might work even better, but that needs to be proven to justify the added expense of a brand-name antiplatelet drug.
Mike Sharma, MD , is a stroke neurologist at McMaster University, Hamilton, Ont. He has been an advisor to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. He made these comments in an interview.
MONTREAL – The optimal length for dual antiplatelet therapy in patients who have just had a mild stroke or transient ischemic attack is 21 days, a duration of combined treatment that maximized protection against major ischemic events while minimizing the extra risk for a major hemorrhage, according to a prespecified analysis of data from the POINT trial.
The POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial randomized 4,881 patients with a very recent mild stroke or transient ischemic attack and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, dual antiplatelet therapy (DAPT) cut the incidence of a major ischemic event by a relative 25% but also more than doubled the rate of major hemorrhage (New Engl J Med. 2018 Jul 19;377[3]:215-25).
The new, prespecified analysis looked at outcomes on a week-by-week basis over the course of 90 days of treatment, and showed that during the first 21 days the rate of major ischemic events was 5.6% among patients on aspirin only and 3.6% among those on DAPT, a statistically significant 35% relative cut in these adverse outcomes by using DAPT, Jordan J. Elm, PhD, reported at the World Stroke Congress. During the subsequent 69 days on treatment, the incidence of major ischemic events was roughly 1% in both arms of the study, showing that after 3 weeks the incremental benefit from DAPT disappeared, said Dr. Elm, a biostatistician at the Medical University of South Carolina, Charleston.
In contrast, the doubled rate of major hemorrhages (mostly reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment, meaning that limiting DAPT to just 21 days could prevent many of the excess hemorrhages.
“These results suggest that limiting clopidogrel plus aspirin use to 21 days may maximize benefit and reduce risk,” Dr. Elm said, especially in light of the findings confirming the efficacy of 21 days of DAPT following a minor stroke or TIA that had been reported several years ago in the CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial (New Engl J Med. 2013 Jul 4;369[1]:11-9).
Although the new finding from the POINT results came in a secondary analysis, it’s statistically legitimate and should be taken into account when writing treatment guidelines, she said, emphasizing that “this is a very important analysis that is not just hypothesis generating.”
Another finding from the new analysis was that a large number of major ischemic events, and hence a large number of the events prevented by DAPT, occurred in the first 2 days following the index event, a finding made possible because the POINT investigators enrolled patients and started treatment within 12 hours of the qualifying events.
“It’s better to start treatment early,” Dr. Elm noted, but she also highlighted that major ischemic events continued to accumulate during days 3-21, suggesting that patients could still benefit from DAPT even if treatment did not start until 24 or 48 hours after their index event.
POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm reported no disclosures.
SOURCE: Elm JJ et al. World Stroke Congress, Late-breaking session.
MONTREAL – The optimal length for dual antiplatelet therapy in patients who have just had a mild stroke or transient ischemic attack is 21 days, a duration of combined treatment that maximized protection against major ischemic events while minimizing the extra risk for a major hemorrhage, according to a prespecified analysis of data from the POINT trial.
The POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial randomized 4,881 patients with a very recent mild stroke or transient ischemic attack and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, dual antiplatelet therapy (DAPT) cut the incidence of a major ischemic event by a relative 25% but also more than doubled the rate of major hemorrhage (New Engl J Med. 2018 Jul 19;377[3]:215-25).
The new, prespecified analysis looked at outcomes on a week-by-week basis over the course of 90 days of treatment, and showed that during the first 21 days the rate of major ischemic events was 5.6% among patients on aspirin only and 3.6% among those on DAPT, a statistically significant 35% relative cut in these adverse outcomes by using DAPT, Jordan J. Elm, PhD, reported at the World Stroke Congress. During the subsequent 69 days on treatment, the incidence of major ischemic events was roughly 1% in both arms of the study, showing that after 3 weeks the incremental benefit from DAPT disappeared, said Dr. Elm, a biostatistician at the Medical University of South Carolina, Charleston.
In contrast, the doubled rate of major hemorrhages (mostly reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment, meaning that limiting DAPT to just 21 days could prevent many of the excess hemorrhages.
“These results suggest that limiting clopidogrel plus aspirin use to 21 days may maximize benefit and reduce risk,” Dr. Elm said, especially in light of the findings confirming the efficacy of 21 days of DAPT following a minor stroke or TIA that had been reported several years ago in the CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial (New Engl J Med. 2013 Jul 4;369[1]:11-9).
Although the new finding from the POINT results came in a secondary analysis, it’s statistically legitimate and should be taken into account when writing treatment guidelines, she said, emphasizing that “this is a very important analysis that is not just hypothesis generating.”
Another finding from the new analysis was that a large number of major ischemic events, and hence a large number of the events prevented by DAPT, occurred in the first 2 days following the index event, a finding made possible because the POINT investigators enrolled patients and started treatment within 12 hours of the qualifying events.
“It’s better to start treatment early,” Dr. Elm noted, but she also highlighted that major ischemic events continued to accumulate during days 3-21, suggesting that patients could still benefit from DAPT even if treatment did not start until 24 or 48 hours after their index event.
POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm reported no disclosures.
SOURCE: Elm JJ et al. World Stroke Congress, Late-breaking session.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point: All of DAPT’s extra benefit over aspirin alone in recent stroke or transient ischemic attack patients happened during the first 21 days.
Major finding: During the first 21 days, DAPT cut major ischemic events by 35%, compared with aspirin only.
Study details: A prespecified, secondary analysis from POINT, a multicenter, randomized trial with 4,881 patients.
Disclosures: POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm had no disclosures.
Source: Elm JJ et al. World Stroke Congress, Late-breaking session.