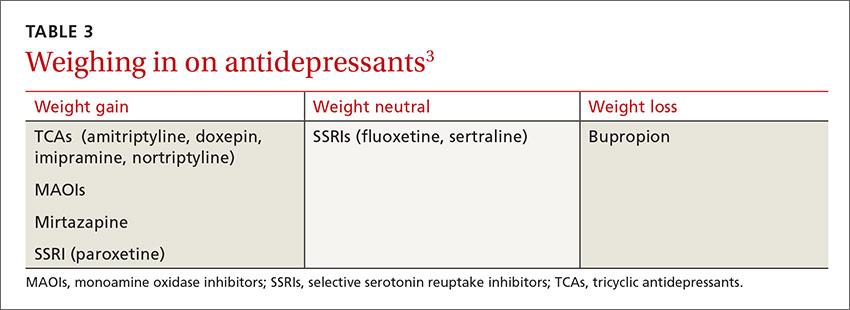
User login
Is diabetes distress on your radar screen?
Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of those with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder;13 rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.14,15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings,19 we wrote this review to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.
Diabetes distress: What it is, what it’s not
For patients with type 2 diabetes, diabetes distress centers around 4 main issues:
- frustration with the demands of self-care;
- apprehension about the future and the possibility of developing serious complications;
- concern about both the quality and the cost of required medical care; and
- perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least 5 of the 9 symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least 2 weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes,24 and the co-occurrence of a psychiatric disorder and diabetes distress is high.25 Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that individuals diagnosed with both diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
Screening for diabetes distress
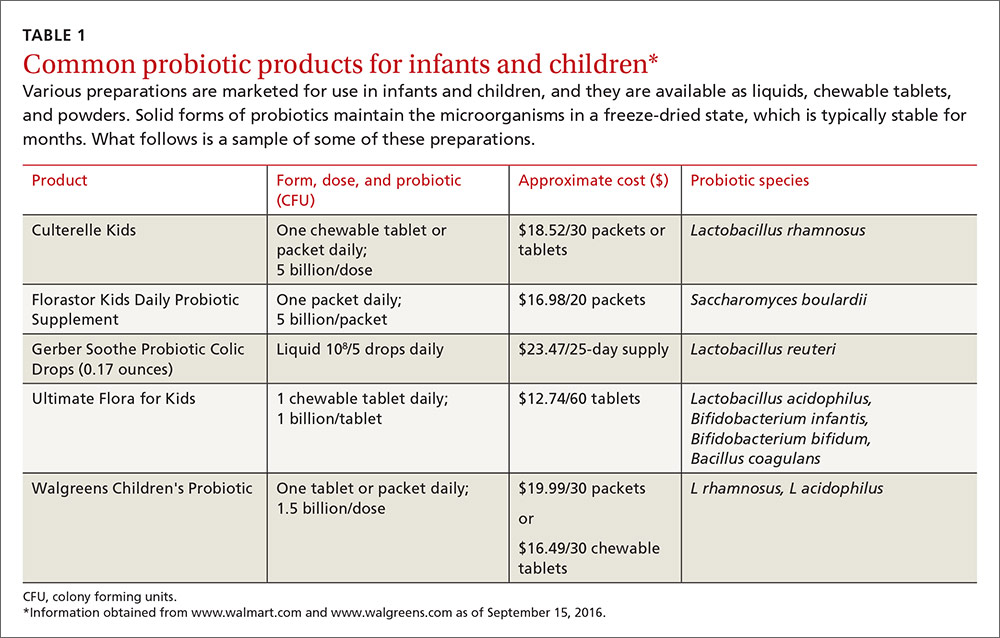
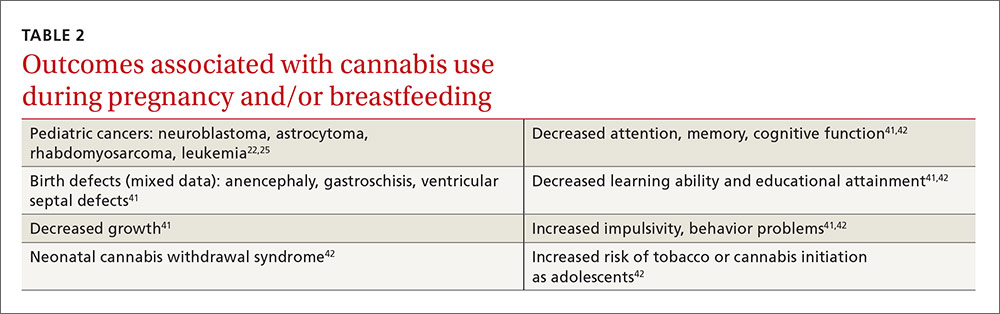
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (TABLE).26-30 Some of the measures are easily accessible online; others require subscription to MEDLINE.
Problem Areas in Diabetes (PAID): There are 3 versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a 5-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS): This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with 2 items29 and a 28-item scale specifically for patients with type 1 diabetes.30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the 7 sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
How to respond to diabetes distress
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only 2 studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial31 recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT). The trial had 3 arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress-specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), along with self-management behaviors and HbA1c.
Participants in all 3 arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in HbA1c were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other 2 groups.31
The DIAMOS trial32 recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a 2-arm RCT. One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, HbA1c, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, HbA1c, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function,36-38 the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors. Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care physicians without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care physicians to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Physicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussion. (See “Directing help where it’s most needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46
SIDEBAR
Directing help where it's most needed
CASE 1 ›
Conduct a behavioral experiment
Fred J, a 67-year-old diagnosed with type 2 diabetes 6 years ago, comes in for a diabetes check-up. He is a new patient who recently retired from his job as a contractor and was referred by a colleague. In response to a question about his diabetes management, Mr. J tells you he’s having a hard time.
“I get down on myself,” the patient says. “I take my medications every day at the exact same time, but when I test my sugar, it’s 260 or 280. I know I did this to myself. If only I weighed less, ate better, or exercised more.”
At other times, “I think, 'Why bother?'” Mr. J adds. “I feel like there’s nothing I can do to make it better.”
The DDS-2 screen you gave Mr. J bears out his high level of distress and his fear of complications. He tells you about an aunt who “had diabetes like me and had to go on dialysis, then died 2 years later.” When you ask what he fears most, Mr. J says he worries about kidney failure. “I don’t want to go on dialysis,” he insists.
You take the opportunity to point out that nephropathy is not inevitable and that he can perform self-care behaviors now that will prevent or delay kidney complications.
You also decide to try a cognitive behavioral technique in an attempt to change his thought process. You ask Mr. J to agree to a week-long behavioral experiment to examine the effect of walking for 30 minutes each day.
He agrees. You advise him to write down his predictions before he begins the experiment and then to keep a log, checking and recording his glucose levels before and after each walk. You schedule a follow-up visit to discuss the results, hoping that a reduction in blood glucose levels will convince Mr. J that exercise is beneficial to his diabetes.
CASE 2 ›
Identify the problem; brainstorm with the patient
Susan T, a 46-year-old with a husband and 2 teenage children, comes in for her 3-month diabetes check-up. At her last visit, she expressed concerns about her family’s lack of cooperation as she struggled to change her diet. This time, she appears frustrated and distraught.
Your nurse administered the PAID-5 while Ms. T was in the waiting room and entered her score—8, indicating high diabetes distress—in the electronic medical record. You ask Ms. T what’s happening, knowing that encouraging her to verbalize her feelings is a way to increase her trust and help alleviate her concerns.
You also try the following problem-solving technique:
Define the problem. Ms. T is having a hard time maintaining a healthy diet. Her husband and children refuse to eat the healthy meals she prepares and want her to cook separate dinners for them.
Identify challenges. The patient works full-time and does not have the time or energy to cook separate meals. In addition, she is upset by her family’s lack of support in her efforts to control her disease.
Brainstorm multiple solutions:
1) Ms. T can prepare all of her own meals for the work week on Sunday, then cook for the others when she returns from work.
2) Her husband and children can make their own dinner if they do not want to eat the healthier meals she prepares.
3) The patient can join a diabetes support group where she will meet, and possibly learn from, other patients who may be struggling with diabetes self-care.
4) Ms. T can ask her husband and children to come to her next diabetes check-up so they can learn about the importance of family support in diabetes management directly from you.
5) The patient’s family can receive information about a healthy diabetes diet from a certified diabetes educator.
Decide on appropriate solutions. The patient agrees to try and prepare her weekday meals on Sunday so that she is not tempted to eat less healthy options. She also agrees to bring her family to her next diabetes check-up and to diabetes education classes.
CORRESPONDENCE
Elizabeth A. Beverly, PhD, Department of Family Medicine, Ohio University Heritage College of Osteopathic Medicine, 35 W. Green Drive, Athens, OH 45701; [email protected].
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educator. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educator. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psych. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. New Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectrum. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl): S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Education Counseling. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabetic Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabetic Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with Type 2 diabetes: a longitudinal study. Diabetic Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:2822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educator. 2007;33:1080-1103; 1104-1086.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatr. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychologist. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabetic Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diabetes Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PloS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Education Counseling. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for Type 2 diabetes care: a qualitative study. Diabetes Spectrum. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. Available at: https://www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed August 15, 2016.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of those with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder;13 rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.14,15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings,19 we wrote this review to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.
Diabetes distress: What it is, what it’s not
For patients with type 2 diabetes, diabetes distress centers around 4 main issues:
- frustration with the demands of self-care;
- apprehension about the future and the possibility of developing serious complications;
- concern about both the quality and the cost of required medical care; and
- perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least 5 of the 9 symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least 2 weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes,24 and the co-occurrence of a psychiatric disorder and diabetes distress is high.25 Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that individuals diagnosed with both diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
Screening for diabetes distress
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (TABLE).26-30 Some of the measures are easily accessible online; others require subscription to MEDLINE.
Problem Areas in Diabetes (PAID): There are 3 versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a 5-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS): This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with 2 items29 and a 28-item scale specifically for patients with type 1 diabetes.30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the 7 sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
How to respond to diabetes distress
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only 2 studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial31 recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT). The trial had 3 arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress-specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), along with self-management behaviors and HbA1c.
Participants in all 3 arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in HbA1c were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other 2 groups.31
The DIAMOS trial32 recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a 2-arm RCT. One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, HbA1c, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, HbA1c, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function,36-38 the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors. Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care physicians without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care physicians to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Physicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussion. (See “Directing help where it’s most needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46
SIDEBAR
Directing help where it's most needed
CASE 1 ›
Conduct a behavioral experiment
Fred J, a 67-year-old diagnosed with type 2 diabetes 6 years ago, comes in for a diabetes check-up. He is a new patient who recently retired from his job as a contractor and was referred by a colleague. In response to a question about his diabetes management, Mr. J tells you he’s having a hard time.
“I get down on myself,” the patient says. “I take my medications every day at the exact same time, but when I test my sugar, it’s 260 or 280. I know I did this to myself. If only I weighed less, ate better, or exercised more.”
At other times, “I think, 'Why bother?'” Mr. J adds. “I feel like there’s nothing I can do to make it better.”
The DDS-2 screen you gave Mr. J bears out his high level of distress and his fear of complications. He tells you about an aunt who “had diabetes like me and had to go on dialysis, then died 2 years later.” When you ask what he fears most, Mr. J says he worries about kidney failure. “I don’t want to go on dialysis,” he insists.
You take the opportunity to point out that nephropathy is not inevitable and that he can perform self-care behaviors now that will prevent or delay kidney complications.
You also decide to try a cognitive behavioral technique in an attempt to change his thought process. You ask Mr. J to agree to a week-long behavioral experiment to examine the effect of walking for 30 minutes each day.
He agrees. You advise him to write down his predictions before he begins the experiment and then to keep a log, checking and recording his glucose levels before and after each walk. You schedule a follow-up visit to discuss the results, hoping that a reduction in blood glucose levels will convince Mr. J that exercise is beneficial to his diabetes.
CASE 2 ›
Identify the problem; brainstorm with the patient
Susan T, a 46-year-old with a husband and 2 teenage children, comes in for her 3-month diabetes check-up. At her last visit, she expressed concerns about her family’s lack of cooperation as she struggled to change her diet. This time, she appears frustrated and distraught.
Your nurse administered the PAID-5 while Ms. T was in the waiting room and entered her score—8, indicating high diabetes distress—in the electronic medical record. You ask Ms. T what’s happening, knowing that encouraging her to verbalize her feelings is a way to increase her trust and help alleviate her concerns.
You also try the following problem-solving technique:
Define the problem. Ms. T is having a hard time maintaining a healthy diet. Her husband and children refuse to eat the healthy meals she prepares and want her to cook separate dinners for them.
Identify challenges. The patient works full-time and does not have the time or energy to cook separate meals. In addition, she is upset by her family’s lack of support in her efforts to control her disease.
Brainstorm multiple solutions:
1) Ms. T can prepare all of her own meals for the work week on Sunday, then cook for the others when she returns from work.
2) Her husband and children can make their own dinner if they do not want to eat the healthier meals she prepares.
3) The patient can join a diabetes support group where she will meet, and possibly learn from, other patients who may be struggling with diabetes self-care.
4) Ms. T can ask her husband and children to come to her next diabetes check-up so they can learn about the importance of family support in diabetes management directly from you.
5) The patient’s family can receive information about a healthy diabetes diet from a certified diabetes educator.
Decide on appropriate solutions. The patient agrees to try and prepare her weekday meals on Sunday so that she is not tempted to eat less healthy options. She also agrees to bring her family to her next diabetes check-up and to diabetes education classes.
CORRESPONDENCE
Elizabeth A. Beverly, PhD, Department of Family Medicine, Ohio University Heritage College of Osteopathic Medicine, 35 W. Green Drive, Athens, OH 45701; [email protected].
Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of those with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder;13 rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.14,15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings,19 we wrote this review to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.
Diabetes distress: What it is, what it’s not
For patients with type 2 diabetes, diabetes distress centers around 4 main issues:
- frustration with the demands of self-care;
- apprehension about the future and the possibility of developing serious complications;
- concern about both the quality and the cost of required medical care; and
- perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least 5 of the 9 symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least 2 weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes,24 and the co-occurrence of a psychiatric disorder and diabetes distress is high.25 Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that individuals diagnosed with both diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
Screening for diabetes distress
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (TABLE).26-30 Some of the measures are easily accessible online; others require subscription to MEDLINE.
Problem Areas in Diabetes (PAID): There are 3 versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a 5-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS): This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with 2 items29 and a 28-item scale specifically for patients with type 1 diabetes.30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the 7 sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
How to respond to diabetes distress
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only 2 studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial31 recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT). The trial had 3 arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress-specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), along with self-management behaviors and HbA1c.
Participants in all 3 arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in HbA1c were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other 2 groups.31
The DIAMOS trial32 recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a 2-arm RCT. One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, HbA1c, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, HbA1c, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function,36-38 the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors. Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care physicians without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care physicians to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Physicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussion. (See “Directing help where it’s most needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46
SIDEBAR
Directing help where it's most needed
CASE 1 ›
Conduct a behavioral experiment
Fred J, a 67-year-old diagnosed with type 2 diabetes 6 years ago, comes in for a diabetes check-up. He is a new patient who recently retired from his job as a contractor and was referred by a colleague. In response to a question about his diabetes management, Mr. J tells you he’s having a hard time.
“I get down on myself,” the patient says. “I take my medications every day at the exact same time, but when I test my sugar, it’s 260 or 280. I know I did this to myself. If only I weighed less, ate better, or exercised more.”
At other times, “I think, 'Why bother?'” Mr. J adds. “I feel like there’s nothing I can do to make it better.”
The DDS-2 screen you gave Mr. J bears out his high level of distress and his fear of complications. He tells you about an aunt who “had diabetes like me and had to go on dialysis, then died 2 years later.” When you ask what he fears most, Mr. J says he worries about kidney failure. “I don’t want to go on dialysis,” he insists.
You take the opportunity to point out that nephropathy is not inevitable and that he can perform self-care behaviors now that will prevent or delay kidney complications.
You also decide to try a cognitive behavioral technique in an attempt to change his thought process. You ask Mr. J to agree to a week-long behavioral experiment to examine the effect of walking for 30 minutes each day.
He agrees. You advise him to write down his predictions before he begins the experiment and then to keep a log, checking and recording his glucose levels before and after each walk. You schedule a follow-up visit to discuss the results, hoping that a reduction in blood glucose levels will convince Mr. J that exercise is beneficial to his diabetes.
CASE 2 ›
Identify the problem; brainstorm with the patient
Susan T, a 46-year-old with a husband and 2 teenage children, comes in for her 3-month diabetes check-up. At her last visit, she expressed concerns about her family’s lack of cooperation as she struggled to change her diet. This time, she appears frustrated and distraught.
Your nurse administered the PAID-5 while Ms. T was in the waiting room and entered her score—8, indicating high diabetes distress—in the electronic medical record. You ask Ms. T what’s happening, knowing that encouraging her to verbalize her feelings is a way to increase her trust and help alleviate her concerns.
You also try the following problem-solving technique:
Define the problem. Ms. T is having a hard time maintaining a healthy diet. Her husband and children refuse to eat the healthy meals she prepares and want her to cook separate dinners for them.
Identify challenges. The patient works full-time and does not have the time or energy to cook separate meals. In addition, she is upset by her family’s lack of support in her efforts to control her disease.
Brainstorm multiple solutions:
1) Ms. T can prepare all of her own meals for the work week on Sunday, then cook for the others when she returns from work.
2) Her husband and children can make their own dinner if they do not want to eat the healthier meals she prepares.
3) The patient can join a diabetes support group where she will meet, and possibly learn from, other patients who may be struggling with diabetes self-care.
4) Ms. T can ask her husband and children to come to her next diabetes check-up so they can learn about the importance of family support in diabetes management directly from you.
5) The patient’s family can receive information about a healthy diabetes diet from a certified diabetes educator.
Decide on appropriate solutions. The patient agrees to try and prepare her weekday meals on Sunday so that she is not tempted to eat less healthy options. She also agrees to bring her family to her next diabetes check-up and to diabetes education classes.
CORRESPONDENCE
Elizabeth A. Beverly, PhD, Department of Family Medicine, Ohio University Heritage College of Osteopathic Medicine, 35 W. Green Drive, Athens, OH 45701; [email protected].
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educator. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educator. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psych. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. New Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectrum. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl): S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Education Counseling. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabetic Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabetic Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with Type 2 diabetes: a longitudinal study. Diabetic Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:2822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educator. 2007;33:1080-1103; 1104-1086.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatr. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychologist. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabetic Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diabetes Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PloS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Education Counseling. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for Type 2 diabetes care: a qualitative study. Diabetes Spectrum. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. Available at: https://www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed August 15, 2016.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educator. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educator. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psych. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. New Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectrum. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl): S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Education Counseling. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabetic Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabetic Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with Type 2 diabetes: a longitudinal study. Diabetic Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:2822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educator. 2007;33:1080-1103; 1104-1086.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatr. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychologist. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabetic Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diabetes Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PloS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Education Counseling. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for Type 2 diabetes care: a qualitative study. Diabetes Spectrum. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. Available at: https://www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed August 15, 2016.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
PRACTICE RECOMMENDATIONS
› Educate patients about diabetes distress, explaining that diabetes is manageable and that neither complications nor diabetes distress is inevitable. C
› Empower patients to take an active role in self-management of diabetes, encouraging them to express their concerns and ask open-ended questions. A
› Support shared decision-making by inquiring about patients’ values and treatment preferences, presenting options, and reviewing the risks and benefits of each. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
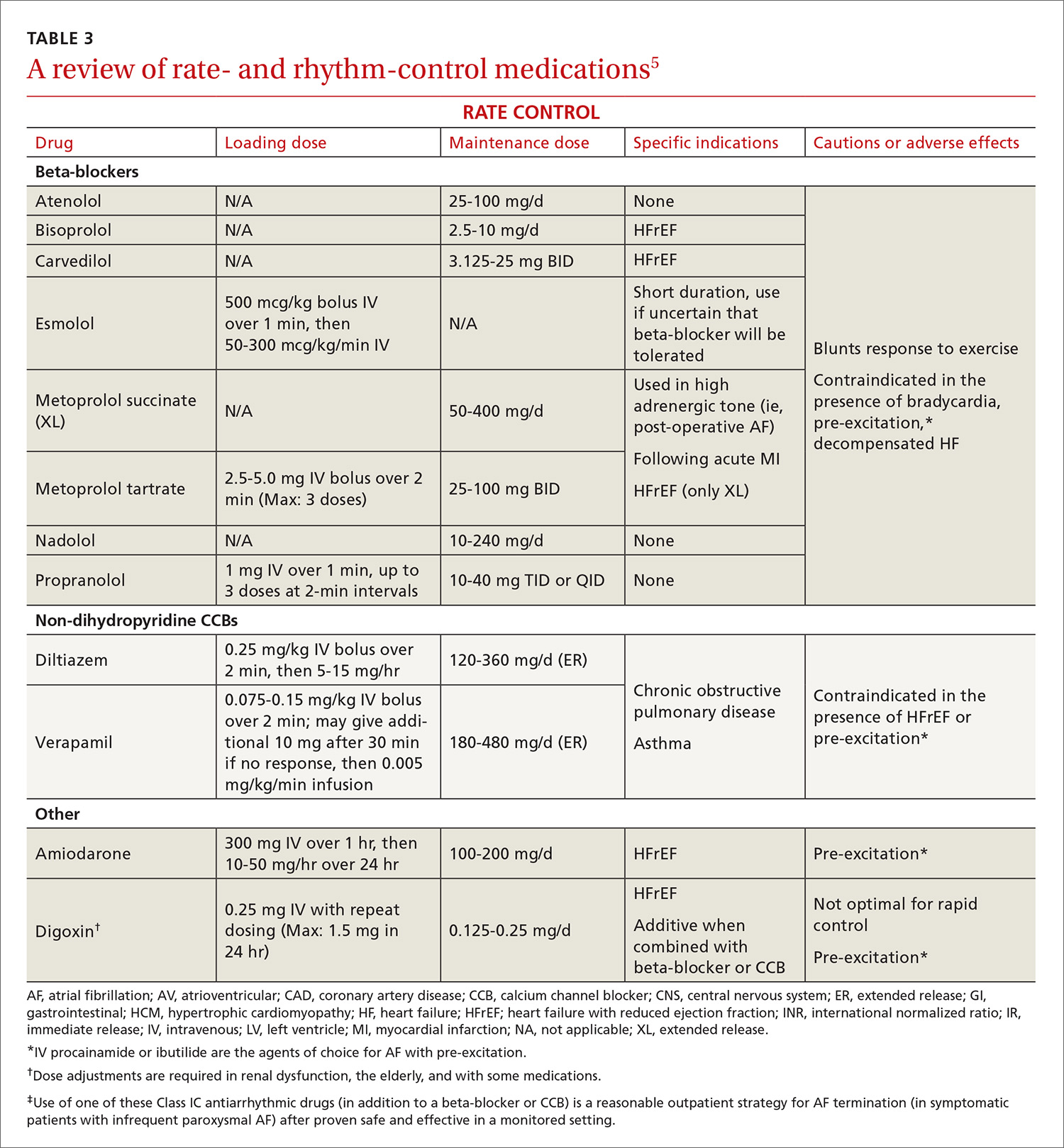
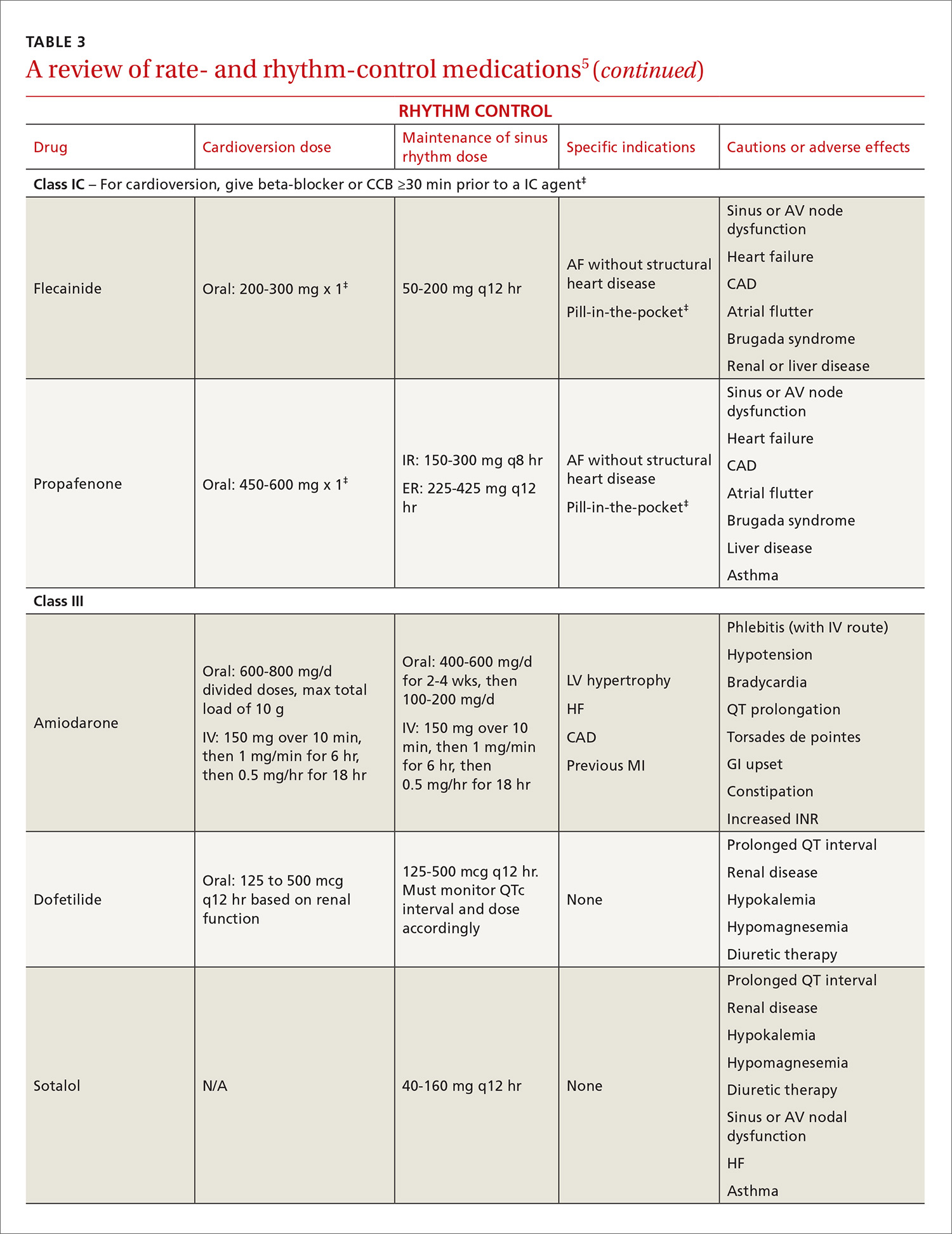
Atrial fibrillation: Effective strategies using the latest tools
Atrial fibrillation (AF)—the most common supraventricular tachycardia—affects as many as 6.1 million adults in the United States.1 It is associated with a 5-fold increased risk of stroke,2 a 3-fold increased risk of heart failure (HF),3 and about a 2-fold increased risk of dementia4 and mortality.2 The prevalence of AF increases with maturity, from 2% in people <65 years of age to 9% in those ≥65 years,5 and that prevalence is expected to double over the next 25 years as the population ages.1
The primary goals of treatment are to alleviate symptoms and prevent thromboembolism. Strokes related to AF are more likely to result in severe disability or death when compared with those unrelated to AF.6 And yet anticoagulation remains underutilized.7
The net clinical benefit of oral anticoagulation appears to be greatest in patients with the highest risk of bleeding, since these patients are also at the highest risk for stroke.8 Patients at increased risk of stroke are more likely to receive oral anticoagulation; however, for unknown reasons, more than half of people with the highest risk of stroke are not prescribed these important anti-blood-clotting medications.7 One theory is that physicians may be relying on their gut rather than objective risk scores, and underuse of validated schemata leads to poor estimation of risk.
For example, results from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) trial, which involved over 10,000 people with AF, found that although 72% (n=7251) had high-risk CHADS2 scores (≥2), only 16% were assessed as having a high risk of stroke by physicians.9 Along the same lines, a recent study of Canadian primary care physicians showed that stroke risk and bleeding risk were not evaluated with validated tools in 58% and 81% of patients, respectively, leading to both significant underestimation and overestimation of risk.10
This review provides the tools to identify when anticoagulation is indicated, reports the advantages and disadvantages of the currently available anticoagulants, and discusses the selection and implementation of rate- vs rhythm-control strategies. But first, a word about the etiology, classification, and diagnosis of AF.
AF: The result of any number of cardiac and non-cardiac causes
AF is characterized by uncoordinated activation of the atria, which results in ineffective atrial contractions and an irregular, often rapid, ventricular response. It is the ultimate clinical manifestation of multiple diseases that alter atrial tissue through inflammation, fibrosis, or hypertrophy.5 The most common causes are hypertension, coronary artery disease, HF, cardiomyopathies, and valvular heart disease, all of which stimulate the renin-angiotensin-aldosterone system, leading to increased susceptibility to arrhythmia.5 Atrial ectopic tachycardia, Wolff-Parkinson-White (WPW) syndrome, and atrioventricular (AV) nodal reentrant tachycardia also may precipitate AF.5 In these cases, AF usually resolves after catheter ablation (CA) of the primary arrhythmia.11 Unrecognized AF may trigger atrial flutter, and more than 80% of patients who undergo radiofrequency ablation for atrial flutter experience AF at some point in the subsequent 5 years.12
Non-cardiac causes of AF include sleep apnea, obesity, hyperthyroidism, drugs, electrocution, pneumonia, and pulmonary embolism.5 An association between binge drinking and AF (“holiday heart syndrome”) has long been recognized. The evidence now suggests that alcohol increases the risk of AF in a dose-dependent manner with intakes of ≥1 drink per day (12 g per drink).13
Classification schema no longer includes “lone AF”
AF is classified in terms of the duration of episodes:5
- Paroxysmal AF is characterized by brief episodes that terminate spontaneously or with intervention within 7 days of onset. These episodes recur with variable frequency.
- Persistent AF refers to AF that is continuously sustained for more than 7 days.
- Longstanding persistent AF refers to continuous AF that lasts longer than 12 months.
- Permanent AF is not an inherent pathophysiologic attribute of AF, but rather an acceptance of AF where the patient and physician abandon further efforts to restore and/or maintain sinus rhythm.
- Nonvalvular AF occurs in the absence of a valve replacement (mechanical or bioprosthetic), rheumatic mitral stenosis, or mitral valve repair.
Although paroxysmal and persistent AF may occur in the same individual, the distinction is still clinically relevant, as outcomes of certain therapies, such as CA, are superior in patients with paroxysmal AF.14 With a more complete understanding of AF pathophysiology, guidelines now discourage use of the potentially confusing term “lone AF,” which has historically been applied to younger patients with no known clinical risk factors or echocardiographic abnormalities. As a result, therapeutic decisions are no longer based on this nomenclature, according to the 2014 AF practice guideline from the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS).5
Patient complaints—or incidental findings—can prompt a Dx
Fatigue is the most common symptom of AF. Other signs and symptoms include palpitations, dyspnea, HF, hypotension, syncope, chest pain, and stroke. Some patients are asymptomatic, and AF is an incidental finding when an irregular pulse is discovered during a physical examination. The diagnosis is confirmed by electrocardiogram (EKG), telemetry, Holter monitor, event recorder, or an implanted electrocardiographic recording device. A chest x-ray, serum electrolyte levels, a complete blood count, thyroid testing, and renal and hepatic function testing are recommended. Transthoracic echocardiography to measure cardiac function, detect underlying structural heart disease, and evaluate atrial size is essential.5
An electrophysiologic (EP) study may be needed for diagnosis or treatment if another arrhythmia is present. Aberrant conduction may cause AF to present as a wide complex tachycardia and be mislabeled as ventricular tachycardia. The presence of delta waves is an indication for an EP study targeting the WPW accessory pathway. Transesophageal echocardiography (TEE) is the most sensitive and specific test for left atrial thrombi. If you are considering a TEE for a patient with AF of unknown, or >48 hours’, duration who has not been anticoagulated in the preceding 3 weeks, obtain it before performing cardioversion because of the risk of embolism.5
Stroke prevention
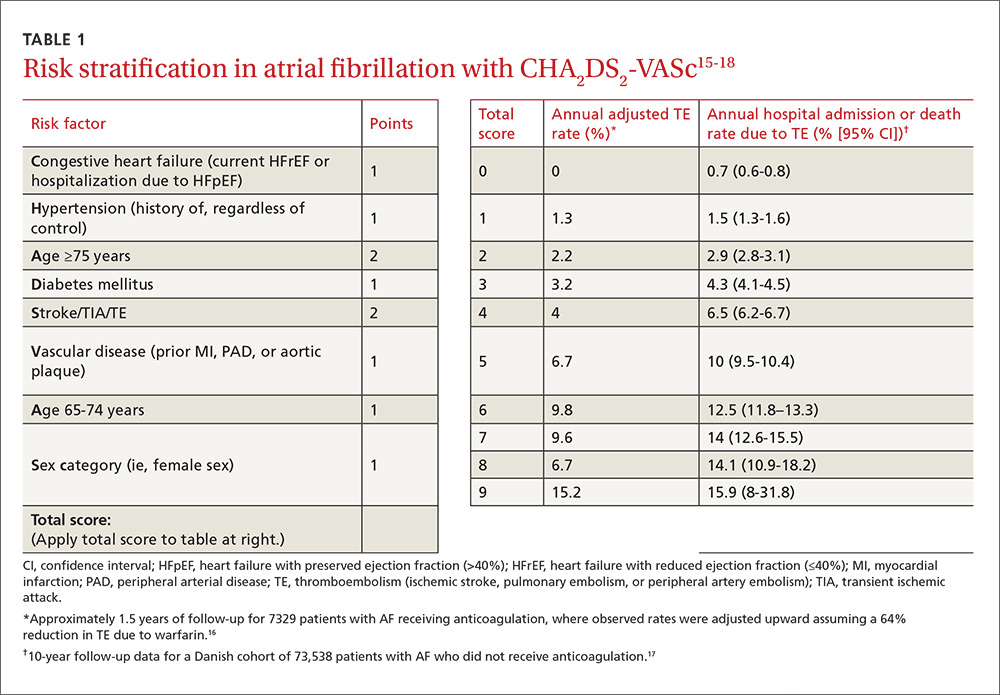
The ACC/AHA/HRS AF guideline recommends basing anticoagulation decisions on thromboembolic risk, regardless of AF pattern (paroxysmal, persistent, or permanent) (Class I recommendation).5 For patients with nonvalvular AF and atrial flutter, the guideline recommends using the Birmingham 2009 schema (CHA2DS2-VASc score) (TABLE 115-18) to estimate thromboembolic risk.5,15 CHA2DS2-VASc improves on the older CHADS2 score by significantly reducing the number of patients categorized as having intermediate risk and better identifying truly low-risk patients who are unlikely to benefit from anticoagulation.16,17,19
Men with a CHA2DS2-VASc score of zero and women with a score of one do not need anticoagulation.5,20 Discuss the risks and benefits of oral anticoagulation with men who have a score of one. In these intermediate-risk men, antiplatelet therapy with aspirin and/or clopidogrel may be reasonable, especially if there is an indication other than stroke prevention (eg, post-myocardial infarction). Oral anticoagulation is strongly recommended for all patients with a CHA2DS2-VASc score of 2 or higher.5,18,21,22
Anticoagulant considerations: Warfarin vs DOACs
Warfarin was the gold standard for stroke prevention in nonvalvular AF until the direct oral anticoagulants (DOACs) became available in 2010. Guidelines in the United States and the United Kingdom recommend shared decision-making to help patients with AF who do not have a specific indication for warfarin choose between warfarin and the DOACs.5,21 Canadian and European guidelines recommend DOACs as the first-line option for anticoagulation and reserve warfarin for patients who have contraindications to, or are unable to afford, DOACs.18,22 All current guidelines recommend continuing warfarin in patients who are stable, well controlled, and satisfied with warfarin therapy and the monitoring and dietary restrictions it entails.
DOACs are as effective as warfarin. All of the DOACs are approved for stroke prevention based on individual phase III non-inferiority trials in which they were compared to warfarin.23-26 In addition, a meta-analysis of these 4 trials involving a total of 71,683 patients (mean age 70-73 years; median follow-up, 1.8-2.8 years) evaluated the benefits and risks of the 4 DOACs against the former gold standard.27
Higher doses of the DOACs (dabigatran 150 mg BID, rivaroxaban 20 mg/d, edoxaban 60 mg/d, and apixaban 5 mg BID) reduced the rates of stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; P<.0001; number needed to treat [NNT]=147), hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; P<.0001; NNT=219), and all-cause mortality (RR=0.90; 95% CI, 0.85-0.95; P=.0003; NNT=128), compared with warfarin.27 It is important to note that while lower doses of some DOACs (dabigatran 110 mg BID and edoxaban 30 mg/d) were not as effective at preventing ischemic stroke when compared with warfarin (RR=1.3; 95% CI, 1-1.6; P=.045), they still significantly reduced hemorrhagic stroke (RR=0.33; 95% CI, 0.23-0.46; P<.0001) and all-cause mortality (RR=0.89; 95% CI, 0.83-0.96; P=.003).
Of course, the biggest concern is bleeding. In that same meta-analysis, the difference in major bleeding events with DOACs vs warfarin was not statistically significant (RR=0.86; 95% CI, 0.73-1; P=.06). While DOACs likely lower rates of intracranial hemorrhage (RR=0.48; 95% CI, 0.39-0.59; P<.0001; NNT=132), they seem to increase the risk of gastrointestinal (GI) bleeding (RR=1.3; 95% CI, 1-1.6; P=.043; number needed to harm [NNH]=185).27
There was significant heterogeneity in the GI bleeding outcome, however. When compared with warfarin, GI bleeding was increased by dabigatran 150 mg BID (RR=1.5; 95% CI, 1.2-1.9; P<.001) and edoxaban 60 mg/d (HR=1.2; 95% CI, 1.02-1.5; P=.03), but there were no significant differences for dabigatran 110 mg BID or apixaban 5 mg BID.23,25,26
On the other hand, edoxaban 30 mg/d had a lower risk of GI bleeding when compared with warfarin (HR=0.67; 95% CI, 0.53-0.83; P<.001).25 Without head-to-head trials, it is impossible to know if one DOAC is superior to another. Apixaban 5 mg BID appears to offer the best overall balance between efficacy and safety. Other DOACs may be better options for patients who have specific concerns regarding efficacy or safety.28,29
Convenience, interactions, and cost may be the deciding factors. Since all DOACs are fairly comparable in efficacy and safety, other factors such as convenience, interactions with other medications, and cost should be considered when deciding on a medication for an individual patient (TABLE 230,31). The DOACs require no lab monitoring or dose titration, and all 4 have fewer potential drug interactions than warfarin.30 Due to their relatively short half-lives, strict adherence is critical; DOACs are not suitable for patients who frequently miss doses.5 (For more information on starting or switching to DOACs, see, “Is a novel anticoagulant right for your patient?” J Fam Pract. 2014;63:22-28.)
A word about DOACs and renal impairment. Another concern with DOACs is their reliance on renal metabolism and excretion. A meta-analysis of the 4 phase III trials of the DOACs, this time involving 58,338 patients, evaluated DOAC efficacy and safety compared to warfarin in the presence of kidney dysfunction.32 Renal function was categorized as normal (estimated glomerular filtration rate [eGFR] >80 mL/min/1.73 m2), mildly impaired (eGFR 50-80 mL/min/1.73 m2), or moderately impaired (eGFR <50 mL/min/1.73m2). Compared with warfarin, DOACs lowered stroke risk in patients with mild (RR=0.71; 95% CI, 0.62-0.81) or moderate (RR=0.79; 95% CI, 0.66-0.94) renal impairment. DOACs also reduced major bleeding compared to warfarin in patients with mild (RR=0.88; 95% CI, 0.80-0.97) or moderate (RR=0.80; 95% CI, 0.66-0.94) renal impairment. How the DOACs fare in patients with severe renal dysfunction could not be determined because such patients were excluded from the trials.
Keep in mind that the DOACs require dose adjustment at different levels of renal impairment (TABLE 230,31), and warfarin remains the only recommended treatment for patients with severe renal impairment, according to both AHA/ACC/HRS and European Society of Cardiology guidelines.5,18
Tools to help assess patients’ bleeding risk
Of the available scoring mechanisms to identify risk factors for bleeding, 3 have been specifically validated in AF populations (ie, ATRIA,33 HEMORR2HAGES,34 and HAS-BLED35). Of the 3, HAS-BLED is superior,36 the most practical, and recommended by expert guidelines.18,21,22 Additionally, HAS-BLED has good correlation with intracranial hemorrhage risk. The HAS-BLED score ranges from 0 to 9 points with one point assigned for each of the following:35
- Hypertension–uncontrolled with systolic BP >160 mm Hg
- Abnormal liver function–cirrhosis, bilirubin >2× normal, or liver enzymes >3× normal
- Abnormal renal function–dialysis, transplant, or serum creatinine >2.26 mg/dL
- Stroke history–including lacunar infarcts
- Bleeding predisposition–history of major bleeding due to any cause
- Labile international normalized ratio (INR)–time in therapeutic range <60%
- Elderly–age >65 years
- Drug–antiplatelet agents, including nonsteroidal anti-inflammatory drugs
- Alcohol usage–>8 drinks per week.
Patients with a HAS-BLED score ≥3 warrant additional monitoring and attempts to reduce bleeding risk by addressing modifiable risk factors. Bleeding risk scores should not be used to exclude patients from anticoagulation therapy.5 In fact, the British National Institute for Health and Clinical Excellence (NICE) guidelines state that anticoagulation should not be withheld solely due to fall risk.21
Also, anticoagulation with warfarin should not be permanently discontinued because of a single GI bleed, since restarting warfarin is associated with decreased risks of thromboembolism and mortality and a statistically insignificant increase in recurrent GI bleeding.37 Restarting DOAC therapy following a GI bleed has not been evaluated in clinical trials; however, it may be reasonable to use one of the DOAC doses with a lower risk of GI bleeding (dabigatran 110 mg BID, apixaban 5 mg BID, or edoxaban 30 mg/d) in patients who have experienced a GI bleed on warfarin or another DOAC.18,22
An online calculator is available that uses CHA2DS2-VASc and HAS-BLED scores to determine an individual’s risk/benefit profile with the various anticoagulation strategies available (http://www.sparctool.com). Consider percutaneous left atrial appendage occlusion if the risks of anticoagulation truly exceed the benefits.38
Rate control vs rhythm control
Most patients who present with AF require immediate ventricular rate control to reduce symptoms. In the acute setting, this can be accomplished with intravenous (IV) beta-blockers or IV calcium channel antagonists.5,39 If the patient is hemodynamically unstable, urgent direct-current cardioversion is the preferred treatment strategy and should not be delayed pending anticoagulation. IV amiodarone can be used in the ICU patient who does not require cardioversion, but is unable to tolerate beta-blockers or calcium channel antagonists.40 Once the patient is stable, long-term treatment focuses on ventricular rate control or restoration and maintenance of sinus rhythm.
The AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial enrolled 4060 patients (mean age 70 years, mean follow-up 3.5 years) with paroxysmal and persistent AF and randomized them to either pharmacologic rate control or rhythm control.41 No significant differences were found in all-cause mortality or in the composite secondary endpoint of death, ischemic stroke, anoxic encephalopathy, major bleeding, or cardiac arrest. In addition, no significant differences emerged in quality of life or global functional status. The number of patients requiring hospitalization during follow-up was significantly lower in the rate-control group vs the rhythm-control group (73% vs 80%; P<.001). Anticoagulation was encouraged but not mandated in the rhythm-control group after 4 weeks in sinus rhythm, and there was a trend toward higher mortality in the rhythm-control group (27% vs 26%; P=.08).
Patients <65 years were excluded from the AFFIRM trial. When younger patients experience significant symptoms, early referral to Cardiology should be considered to discuss the long-term benefits and risks of a rhythm-control strategy. Regardless of age, when patients remain symptomatic despite rate- or rhythm-control management, the strategy should be changed.5
Rate-control targets and options
Target heart rates should be individualized. The 2014 ACC/AHA/HRS guideline recommends a resting target heart rate <80 beats per minute (bpm) in symptomatic patients.5 In patients with permanent AF who remain asymptomatic at higher resting heart rates, a more lenient rate-control strategy (resting heart rate <110 bpm) has demonstrated outcomes equivalent to those of a more strict approach (resting heart rate <80 bpm and heart rate during moderate exercise <110 bpm).42 Pharmacologic rate-control options include beta-blockers, non-dihydropyridine calcium channel antagonists, and digoxin (TABLE 35). Digoxin is associated with increased all-cause mortality in patients with AF regardless of HF status (HR=1.4; 95% CI, 1.2-1.6, P=.0001).43 Digoxin should be reserved for patients who are sedentary or have inadequate control with first-line medications.5
Indications for rhythm control
The NICE guidelines, which are consistent with the ACC/AHA/HRS guidelines, recommend rate control as the first-line strategy for AF management, except in people:21
- whose AF has a reversible cause
- who have HF believed to be primarily caused by AF
- with new-onset AF
- with atrial flutter that is considered suitable for an ablation strategy to restore sinus rhythm
- for whom a rhythm-control strategy would be more suitable based on clinical judgment.
In addition, patients who continue to experience symptomatic AF despite an adequate trial of rate control should be offered rhythm control.5
Pharmacologic rhythm-control strategies. Antiarrhythmic drugs can be used for chemical cardioversion, reduction of paroxysms, and long-term maintenance of sinus rhythm. The most commonly used antiarrhythmic drugs are Class IC and Class III agents (TABLE 3).5 Tailored drug selection for each patient is key. Patients with left atrial diameters >4.5 cm are less likely to remain in sinus rhythm, and patients with left ventricular hypertrophy are at increased risk for proarrhythmic adverse effects.44 Patients with paroxysmal AF may be candidates for a “pill-in-the-pocket” strategy using propafenone or flecainide.5
AF frequently progresses from paroxysmal to persistent and can subsequently result in electrical and structural remodeling that becomes irreversible over time.45 The patient with uncontrolled symptoms despite attempts at rate control and rhythm control should be promptly referred to an electrophysiologist.
Surgical interventions for rate or rhythm control
Electrophysiology interventions include AV nodal ablation with pacemaker placement for rate control, or catheter-directed ablation (radiofrequency or cryotherapy) for rhythm control. CA appears to be more effective than pharmacologic rhythm control.46,47 Treatment with CA is indicated for symptomatic paroxysmal AF when a rhythm-control strategy is desired and the AF is refractory to, or the patient is intolerant of, at least one class I or III antiarrhythmic medication.5 With these same caveats, CA is a reasonable strategy for symptomatic persistent AF.
Consider more invasive interventions, such as an atrial maze procedure, when patients require cardiac surgery for another indication. Patients with an increased risk of thromboembolism (based on CHA2DS2-VASc) remain at high risk even after successful ablation.48 As a result, some guidelines recommend continued long-term anticoagulation following CA.18,22
CORRESPONDENCE
Philip Dooley, MD, University of Kansas School of Medicine–Wichita Family Medicine Residency at Via Christi, 707 North Emporia, Wichita, KS 67207; [email protected].
ACKNOWLEDGMENTS
We thank Professor Anne Walling, MB, ChB, FFPHM, Department of Family and Community Medicine, University of Kansas School of Medicine–Wichita for her suggestions and critical review of an earlier version of this manuscript.
1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. National implications for Rhythm Management and Stroke Prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375.
2. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N-9N.
3. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476-484.
4. Ott A, Breteler MMB, de Bruyne MC, et al. Atrial fibrillation and dementia in a population-based study: The Rotterdam Study. Stroke. 1997;28:316-321.
5. January CT, Wann L, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-e76.
6. Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. Stroke. 1996;27:1760-1764.
7. Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016;1:55-62.
8. Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106:739-749.
9. Steinberg BA, Kim S, Thomas L, et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2014;129:2005-2012.
10. Angaran P, Dorian P, Tan MK, et al. The risk stratification and stroke prevention therapy care gap in Canadian atrial fibrillation patients. Can J Cardiol. 2016;32:336-343.
11. Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779-786.
12. Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799-802.
13. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281-289.
14. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171-257.
15. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263-272.
16. Lip GYH, Frison L, Halperin JL, et al. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731-2738.
17. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124.
18. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719-2747.
19. Olesen JB, Torp-Pedersen C, Hansen ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172-1179.
20. Friberg L, Benson L, Rosenqvist M, et al. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522.
21. National Institute for Health and Clinical Excellence (NICE). Atrial fibrillation: the management of atrial fibrillation [CG180]. 2014. Available at: https://www.nice.org.uk/guidance/cg180. Accessed July 31, 2016.
22. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30:1114-1130.
23. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
24. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
25. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104.
26. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
27. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
28. Morimoto T, Crawford B, Wada K, et al. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66:466-474.
29. Verdecchia P, Angeli F, Bartolini C, et al. Safety and efficacy of non-vitamin K oral anticoagulants in non-valvular atrial fibrillation: a Bayesian meta-analysis approach. Expert Opin Drug Saf. 2015;14:7-20.
30. Micromedex® 2.0 (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com. Accessed August 18, 2016.
31. GoodRx. Available at: https://www.goodrx.com. Accessed August 18, 2016.
32. Del-Carpio Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016;117:69-75.
33. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395-401.
34. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713-719.
35. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100.
36. Zhu W, He W, Guo L, et al. The HAS-BLED Score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561.
37. Chai-Adisaksopha C, Hillis C, Monreal M, et al. Thromboembolic events, recurrent bleeding and mortality after resuming anticoagulant following gastrointestinal bleeding. A meta-analysis. Thromb Haemost. 2015;114:819-825.
38. Xu H, Xie X, Wang B, et al. Efficacy and safety of percutaneous left atrial appendage occlusion for stroke prevention in nonvalvular atrial fibrillation: a meta-analysis of contemporary studies. Heart Lung Circ. 2016;25:1107-1117.
39. Siu CW, Lau CP, Lee WL, et al. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37:2174-2179.
40. Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594-598.
41. The Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
42. Van Gelder IC, Groenveld HF, Crijns HJGM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
43. Wang ZQ, Zhang R, Chen MT, et al. Digoxin is associated with increased all-cause mortality in patients with atrial fibrillation regardless of concomitant heart failure: a meta-analysis. J Cardiovasc Pharmacol. 2015;66:270-275.
44. Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026-2033.
45. de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation: clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725-731.
46. Cheng X, Li X, He Y, et al. Catheter ablation versus anti-arrhythmic drug therapy for the management of atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol. 2014;41:267-272.
47. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637-1644.
48.
Atrial fibrillation (AF)—the most common supraventricular tachycardia—affects as many as 6.1 million adults in the United States.1 It is associated with a 5-fold increased risk of stroke,2 a 3-fold increased risk of heart failure (HF),3 and about a 2-fold increased risk of dementia4 and mortality.2 The prevalence of AF increases with maturity, from 2% in people <65 years of age to 9% in those ≥65 years,5 and that prevalence is expected to double over the next 25 years as the population ages.1
The primary goals of treatment are to alleviate symptoms and prevent thromboembolism. Strokes related to AF are more likely to result in severe disability or death when compared with those unrelated to AF.6 And yet anticoagulation remains underutilized.7
The net clinical benefit of oral anticoagulation appears to be greatest in patients with the highest risk of bleeding, since these patients are also at the highest risk for stroke.8 Patients at increased risk of stroke are more likely to receive oral anticoagulation; however, for unknown reasons, more than half of people with the highest risk of stroke are not prescribed these important anti-blood-clotting medications.7 One theory is that physicians may be relying on their gut rather than objective risk scores, and underuse of validated schemata leads to poor estimation of risk.
For example, results from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) trial, which involved over 10,000 people with AF, found that although 72% (n=7251) had high-risk CHADS2 scores (≥2), only 16% were assessed as having a high risk of stroke by physicians.9 Along the same lines, a recent study of Canadian primary care physicians showed that stroke risk and bleeding risk were not evaluated with validated tools in 58% and 81% of patients, respectively, leading to both significant underestimation and overestimation of risk.10
This review provides the tools to identify when anticoagulation is indicated, reports the advantages and disadvantages of the currently available anticoagulants, and discusses the selection and implementation of rate- vs rhythm-control strategies. But first, a word about the etiology, classification, and diagnosis of AF.
AF: The result of any number of cardiac and non-cardiac causes
AF is characterized by uncoordinated activation of the atria, which results in ineffective atrial contractions and an irregular, often rapid, ventricular response. It is the ultimate clinical manifestation of multiple diseases that alter atrial tissue through inflammation, fibrosis, or hypertrophy.5 The most common causes are hypertension, coronary artery disease, HF, cardiomyopathies, and valvular heart disease, all of which stimulate the renin-angiotensin-aldosterone system, leading to increased susceptibility to arrhythmia.5 Atrial ectopic tachycardia, Wolff-Parkinson-White (WPW) syndrome, and atrioventricular (AV) nodal reentrant tachycardia also may precipitate AF.5 In these cases, AF usually resolves after catheter ablation (CA) of the primary arrhythmia.11 Unrecognized AF may trigger atrial flutter, and more than 80% of patients who undergo radiofrequency ablation for atrial flutter experience AF at some point in the subsequent 5 years.12
Non-cardiac causes of AF include sleep apnea, obesity, hyperthyroidism, drugs, electrocution, pneumonia, and pulmonary embolism.5 An association between binge drinking and AF (“holiday heart syndrome”) has long been recognized. The evidence now suggests that alcohol increases the risk of AF in a dose-dependent manner with intakes of ≥1 drink per day (12 g per drink).13
Classification schema no longer includes “lone AF”
AF is classified in terms of the duration of episodes:5
- Paroxysmal AF is characterized by brief episodes that terminate spontaneously or with intervention within 7 days of onset. These episodes recur with variable frequency.
- Persistent AF refers to AF that is continuously sustained for more than 7 days.
- Longstanding persistent AF refers to continuous AF that lasts longer than 12 months.
- Permanent AF is not an inherent pathophysiologic attribute of AF, but rather an acceptance of AF where the patient and physician abandon further efforts to restore and/or maintain sinus rhythm.
- Nonvalvular AF occurs in the absence of a valve replacement (mechanical or bioprosthetic), rheumatic mitral stenosis, or mitral valve repair.
Although paroxysmal and persistent AF may occur in the same individual, the distinction is still clinically relevant, as outcomes of certain therapies, such as CA, are superior in patients with paroxysmal AF.14 With a more complete understanding of AF pathophysiology, guidelines now discourage use of the potentially confusing term “lone AF,” which has historically been applied to younger patients with no known clinical risk factors or echocardiographic abnormalities. As a result, therapeutic decisions are no longer based on this nomenclature, according to the 2014 AF practice guideline from the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS).5
Patient complaints—or incidental findings—can prompt a Dx
Fatigue is the most common symptom of AF. Other signs and symptoms include palpitations, dyspnea, HF, hypotension, syncope, chest pain, and stroke. Some patients are asymptomatic, and AF is an incidental finding when an irregular pulse is discovered during a physical examination. The diagnosis is confirmed by electrocardiogram (EKG), telemetry, Holter monitor, event recorder, or an implanted electrocardiographic recording device. A chest x-ray, serum electrolyte levels, a complete blood count, thyroid testing, and renal and hepatic function testing are recommended. Transthoracic echocardiography to measure cardiac function, detect underlying structural heart disease, and evaluate atrial size is essential.5
An electrophysiologic (EP) study may be needed for diagnosis or treatment if another arrhythmia is present. Aberrant conduction may cause AF to present as a wide complex tachycardia and be mislabeled as ventricular tachycardia. The presence of delta waves is an indication for an EP study targeting the WPW accessory pathway. Transesophageal echocardiography (TEE) is the most sensitive and specific test for left atrial thrombi. If you are considering a TEE for a patient with AF of unknown, or >48 hours’, duration who has not been anticoagulated in the preceding 3 weeks, obtain it before performing cardioversion because of the risk of embolism.5
Stroke prevention
The ACC/AHA/HRS AF guideline recommends basing anticoagulation decisions on thromboembolic risk, regardless of AF pattern (paroxysmal, persistent, or permanent) (Class I recommendation).5 For patients with nonvalvular AF and atrial flutter, the guideline recommends using the Birmingham 2009 schema (CHA2DS2-VASc score) (TABLE 115-18) to estimate thromboembolic risk.5,15 CHA2DS2-VASc improves on the older CHADS2 score by significantly reducing the number of patients categorized as having intermediate risk and better identifying truly low-risk patients who are unlikely to benefit from anticoagulation.16,17,19
Men with a CHA2DS2-VASc score of zero and women with a score of one do not need anticoagulation.5,20 Discuss the risks and benefits of oral anticoagulation with men who have a score of one. In these intermediate-risk men, antiplatelet therapy with aspirin and/or clopidogrel may be reasonable, especially if there is an indication other than stroke prevention (eg, post-myocardial infarction). Oral anticoagulation is strongly recommended for all patients with a CHA2DS2-VASc score of 2 or higher.5,18,21,22
Anticoagulant considerations: Warfarin vs DOACs
Warfarin was the gold standard for stroke prevention in nonvalvular AF until the direct oral anticoagulants (DOACs) became available in 2010. Guidelines in the United States and the United Kingdom recommend shared decision-making to help patients with AF who do not have a specific indication for warfarin choose between warfarin and the DOACs.5,21 Canadian and European guidelines recommend DOACs as the first-line option for anticoagulation and reserve warfarin for patients who have contraindications to, or are unable to afford, DOACs.18,22 All current guidelines recommend continuing warfarin in patients who are stable, well controlled, and satisfied with warfarin therapy and the monitoring and dietary restrictions it entails.
DOACs are as effective as warfarin. All of the DOACs are approved for stroke prevention based on individual phase III non-inferiority trials in which they were compared to warfarin.23-26 In addition, a meta-analysis of these 4 trials involving a total of 71,683 patients (mean age 70-73 years; median follow-up, 1.8-2.8 years) evaluated the benefits and risks of the 4 DOACs against the former gold standard.27
Higher doses of the DOACs (dabigatran 150 mg BID, rivaroxaban 20 mg/d, edoxaban 60 mg/d, and apixaban 5 mg BID) reduced the rates of stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; P<.0001; number needed to treat [NNT]=147), hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; P<.0001; NNT=219), and all-cause mortality (RR=0.90; 95% CI, 0.85-0.95; P=.0003; NNT=128), compared with warfarin.27 It is important to note that while lower doses of some DOACs (dabigatran 110 mg BID and edoxaban 30 mg/d) were not as effective at preventing ischemic stroke when compared with warfarin (RR=1.3; 95% CI, 1-1.6; P=.045), they still significantly reduced hemorrhagic stroke (RR=0.33; 95% CI, 0.23-0.46; P<.0001) and all-cause mortality (RR=0.89; 95% CI, 0.83-0.96; P=.003).
Of course, the biggest concern is bleeding. In that same meta-analysis, the difference in major bleeding events with DOACs vs warfarin was not statistically significant (RR=0.86; 95% CI, 0.73-1; P=.06). While DOACs likely lower rates of intracranial hemorrhage (RR=0.48; 95% CI, 0.39-0.59; P<.0001; NNT=132), they seem to increase the risk of gastrointestinal (GI) bleeding (RR=1.3; 95% CI, 1-1.6; P=.043; number needed to harm [NNH]=185).27
There was significant heterogeneity in the GI bleeding outcome, however. When compared with warfarin, GI bleeding was increased by dabigatran 150 mg BID (RR=1.5; 95% CI, 1.2-1.9; P<.001) and edoxaban 60 mg/d (HR=1.2; 95% CI, 1.02-1.5; P=.03), but there were no significant differences for dabigatran 110 mg BID or apixaban 5 mg BID.23,25,26
On the other hand, edoxaban 30 mg/d had a lower risk of GI bleeding when compared with warfarin (HR=0.67; 95% CI, 0.53-0.83; P<.001).25 Without head-to-head trials, it is impossible to know if one DOAC is superior to another. Apixaban 5 mg BID appears to offer the best overall balance between efficacy and safety. Other DOACs may be better options for patients who have specific concerns regarding efficacy or safety.28,29
Convenience, interactions, and cost may be the deciding factors. Since all DOACs are fairly comparable in efficacy and safety, other factors such as convenience, interactions with other medications, and cost should be considered when deciding on a medication for an individual patient (TABLE 230,31). The DOACs require no lab monitoring or dose titration, and all 4 have fewer potential drug interactions than warfarin.30 Due to their relatively short half-lives, strict adherence is critical; DOACs are not suitable for patients who frequently miss doses.5 (For more information on starting or switching to DOACs, see, “Is a novel anticoagulant right for your patient?” J Fam Pract. 2014;63:22-28.)
A word about DOACs and renal impairment. Another concern with DOACs is their reliance on renal metabolism and excretion. A meta-analysis of the 4 phase III trials of the DOACs, this time involving 58,338 patients, evaluated DOAC efficacy and safety compared to warfarin in the presence of kidney dysfunction.32 Renal function was categorized as normal (estimated glomerular filtration rate [eGFR] >80 mL/min/1.73 m2), mildly impaired (eGFR 50-80 mL/min/1.73 m2), or moderately impaired (eGFR <50 mL/min/1.73m2). Compared with warfarin, DOACs lowered stroke risk in patients with mild (RR=0.71; 95% CI, 0.62-0.81) or moderate (RR=0.79; 95% CI, 0.66-0.94) renal impairment. DOACs also reduced major bleeding compared to warfarin in patients with mild (RR=0.88; 95% CI, 0.80-0.97) or moderate (RR=0.80; 95% CI, 0.66-0.94) renal impairment. How the DOACs fare in patients with severe renal dysfunction could not be determined because such patients were excluded from the trials.
Keep in mind that the DOACs require dose adjustment at different levels of renal impairment (TABLE 230,31), and warfarin remains the only recommended treatment for patients with severe renal impairment, according to both AHA/ACC/HRS and European Society of Cardiology guidelines.5,18
Tools to help assess patients’ bleeding risk
Of the available scoring mechanisms to identify risk factors for bleeding, 3 have been specifically validated in AF populations (ie, ATRIA,33 HEMORR2HAGES,34 and HAS-BLED35). Of the 3, HAS-BLED is superior,36 the most practical, and recommended by expert guidelines.18,21,22 Additionally, HAS-BLED has good correlation with intracranial hemorrhage risk. The HAS-BLED score ranges from 0 to 9 points with one point assigned for each of the following:35
- Hypertension–uncontrolled with systolic BP >160 mm Hg
- Abnormal liver function–cirrhosis, bilirubin >2× normal, or liver enzymes >3× normal
- Abnormal renal function–dialysis, transplant, or serum creatinine >2.26 mg/dL
- Stroke history–including lacunar infarcts
- Bleeding predisposition–history of major bleeding due to any cause
- Labile international normalized ratio (INR)–time in therapeutic range <60%
- Elderly–age >65 years
- Drug–antiplatelet agents, including nonsteroidal anti-inflammatory drugs
- Alcohol usage–>8 drinks per week.
Patients with a HAS-BLED score ≥3 warrant additional monitoring and attempts to reduce bleeding risk by addressing modifiable risk factors. Bleeding risk scores should not be used to exclude patients from anticoagulation therapy.5 In fact, the British National Institute for Health and Clinical Excellence (NICE) guidelines state that anticoagulation should not be withheld solely due to fall risk.21
Also, anticoagulation with warfarin should not be permanently discontinued because of a single GI bleed, since restarting warfarin is associated with decreased risks of thromboembolism and mortality and a statistically insignificant increase in recurrent GI bleeding.37 Restarting DOAC therapy following a GI bleed has not been evaluated in clinical trials; however, it may be reasonable to use one of the DOAC doses with a lower risk of GI bleeding (dabigatran 110 mg BID, apixaban 5 mg BID, or edoxaban 30 mg/d) in patients who have experienced a GI bleed on warfarin or another DOAC.18,22
An online calculator is available that uses CHA2DS2-VASc and HAS-BLED scores to determine an individual’s risk/benefit profile with the various anticoagulation strategies available (http://www.sparctool.com). Consider percutaneous left atrial appendage occlusion if the risks of anticoagulation truly exceed the benefits.38
Rate control vs rhythm control
Most patients who present with AF require immediate ventricular rate control to reduce symptoms. In the acute setting, this can be accomplished with intravenous (IV) beta-blockers or IV calcium channel antagonists.5,39 If the patient is hemodynamically unstable, urgent direct-current cardioversion is the preferred treatment strategy and should not be delayed pending anticoagulation. IV amiodarone can be used in the ICU patient who does not require cardioversion, but is unable to tolerate beta-blockers or calcium channel antagonists.40 Once the patient is stable, long-term treatment focuses on ventricular rate control or restoration and maintenance of sinus rhythm.
The AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial enrolled 4060 patients (mean age 70 years, mean follow-up 3.5 years) with paroxysmal and persistent AF and randomized them to either pharmacologic rate control or rhythm control.41 No significant differences were found in all-cause mortality or in the composite secondary endpoint of death, ischemic stroke, anoxic encephalopathy, major bleeding, or cardiac arrest. In addition, no significant differences emerged in quality of life or global functional status. The number of patients requiring hospitalization during follow-up was significantly lower in the rate-control group vs the rhythm-control group (73% vs 80%; P<.001). Anticoagulation was encouraged but not mandated in the rhythm-control group after 4 weeks in sinus rhythm, and there was a trend toward higher mortality in the rhythm-control group (27% vs 26%; P=.08).
Patients <65 years were excluded from the AFFIRM trial. When younger patients experience significant symptoms, early referral to Cardiology should be considered to discuss the long-term benefits and risks of a rhythm-control strategy. Regardless of age, when patients remain symptomatic despite rate- or rhythm-control management, the strategy should be changed.5
Rate-control targets and options
Target heart rates should be individualized. The 2014 ACC/AHA/HRS guideline recommends a resting target heart rate <80 beats per minute (bpm) in symptomatic patients.5 In patients with permanent AF who remain asymptomatic at higher resting heart rates, a more lenient rate-control strategy (resting heart rate <110 bpm) has demonstrated outcomes equivalent to those of a more strict approach (resting heart rate <80 bpm and heart rate during moderate exercise <110 bpm).42 Pharmacologic rate-control options include beta-blockers, non-dihydropyridine calcium channel antagonists, and digoxin (TABLE 35). Digoxin is associated with increased all-cause mortality in patients with AF regardless of HF status (HR=1.4; 95% CI, 1.2-1.6, P=.0001).43 Digoxin should be reserved for patients who are sedentary or have inadequate control with first-line medications.5
Indications for rhythm control
The NICE guidelines, which are consistent with the ACC/AHA/HRS guidelines, recommend rate control as the first-line strategy for AF management, except in people:21
- whose AF has a reversible cause
- who have HF believed to be primarily caused by AF
- with new-onset AF
- with atrial flutter that is considered suitable for an ablation strategy to restore sinus rhythm
- for whom a rhythm-control strategy would be more suitable based on clinical judgment.
In addition, patients who continue to experience symptomatic AF despite an adequate trial of rate control should be offered rhythm control.5
Pharmacologic rhythm-control strategies. Antiarrhythmic drugs can be used for chemical cardioversion, reduction of paroxysms, and long-term maintenance of sinus rhythm. The most commonly used antiarrhythmic drugs are Class IC and Class III agents (TABLE 3).5 Tailored drug selection for each patient is key. Patients with left atrial diameters >4.5 cm are less likely to remain in sinus rhythm, and patients with left ventricular hypertrophy are at increased risk for proarrhythmic adverse effects.44 Patients with paroxysmal AF may be candidates for a “pill-in-the-pocket” strategy using propafenone or flecainide.5
AF frequently progresses from paroxysmal to persistent and can subsequently result in electrical and structural remodeling that becomes irreversible over time.45 The patient with uncontrolled symptoms despite attempts at rate control and rhythm control should be promptly referred to an electrophysiologist.
Surgical interventions for rate or rhythm control
Electrophysiology interventions include AV nodal ablation with pacemaker placement for rate control, or catheter-directed ablation (radiofrequency or cryotherapy) for rhythm control. CA appears to be more effective than pharmacologic rhythm control.46,47 Treatment with CA is indicated for symptomatic paroxysmal AF when a rhythm-control strategy is desired and the AF is refractory to, or the patient is intolerant of, at least one class I or III antiarrhythmic medication.5 With these same caveats, CA is a reasonable strategy for symptomatic persistent AF.
Consider more invasive interventions, such as an atrial maze procedure, when patients require cardiac surgery for another indication. Patients with an increased risk of thromboembolism (based on CHA2DS2-VASc) remain at high risk even after successful ablation.48 As a result, some guidelines recommend continued long-term anticoagulation following CA.18,22
CORRESPONDENCE
Philip Dooley, MD, University of Kansas School of Medicine–Wichita Family Medicine Residency at Via Christi, 707 North Emporia, Wichita, KS 67207; [email protected].
ACKNOWLEDGMENTS
We thank Professor Anne Walling, MB, ChB, FFPHM, Department of Family and Community Medicine, University of Kansas School of Medicine–Wichita for her suggestions and critical review of an earlier version of this manuscript.
Atrial fibrillation (AF)—the most common supraventricular tachycardia—affects as many as 6.1 million adults in the United States.1 It is associated with a 5-fold increased risk of stroke,2 a 3-fold increased risk of heart failure (HF),3 and about a 2-fold increased risk of dementia4 and mortality.2 The prevalence of AF increases with maturity, from 2% in people <65 years of age to 9% in those ≥65 years,5 and that prevalence is expected to double over the next 25 years as the population ages.1
The primary goals of treatment are to alleviate symptoms and prevent thromboembolism. Strokes related to AF are more likely to result in severe disability or death when compared with those unrelated to AF.6 And yet anticoagulation remains underutilized.7
The net clinical benefit of oral anticoagulation appears to be greatest in patients with the highest risk of bleeding, since these patients are also at the highest risk for stroke.8 Patients at increased risk of stroke are more likely to receive oral anticoagulation; however, for unknown reasons, more than half of people with the highest risk of stroke are not prescribed these important anti-blood-clotting medications.7 One theory is that physicians may be relying on their gut rather than objective risk scores, and underuse of validated schemata leads to poor estimation of risk.
For example, results from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) trial, which involved over 10,000 people with AF, found that although 72% (n=7251) had high-risk CHADS2 scores (≥2), only 16% were assessed as having a high risk of stroke by physicians.9 Along the same lines, a recent study of Canadian primary care physicians showed that stroke risk and bleeding risk were not evaluated with validated tools in 58% and 81% of patients, respectively, leading to both significant underestimation and overestimation of risk.10
This review provides the tools to identify when anticoagulation is indicated, reports the advantages and disadvantages of the currently available anticoagulants, and discusses the selection and implementation of rate- vs rhythm-control strategies. But first, a word about the etiology, classification, and diagnosis of AF.
AF: The result of any number of cardiac and non-cardiac causes
AF is characterized by uncoordinated activation of the atria, which results in ineffective atrial contractions and an irregular, often rapid, ventricular response. It is the ultimate clinical manifestation of multiple diseases that alter atrial tissue through inflammation, fibrosis, or hypertrophy.5 The most common causes are hypertension, coronary artery disease, HF, cardiomyopathies, and valvular heart disease, all of which stimulate the renin-angiotensin-aldosterone system, leading to increased susceptibility to arrhythmia.5 Atrial ectopic tachycardia, Wolff-Parkinson-White (WPW) syndrome, and atrioventricular (AV) nodal reentrant tachycardia also may precipitate AF.5 In these cases, AF usually resolves after catheter ablation (CA) of the primary arrhythmia.11 Unrecognized AF may trigger atrial flutter, and more than 80% of patients who undergo radiofrequency ablation for atrial flutter experience AF at some point in the subsequent 5 years.12
Non-cardiac causes of AF include sleep apnea, obesity, hyperthyroidism, drugs, electrocution, pneumonia, and pulmonary embolism.5 An association between binge drinking and AF (“holiday heart syndrome”) has long been recognized. The evidence now suggests that alcohol increases the risk of AF in a dose-dependent manner with intakes of ≥1 drink per day (12 g per drink).13
Classification schema no longer includes “lone AF”
AF is classified in terms of the duration of episodes:5
- Paroxysmal AF is characterized by brief episodes that terminate spontaneously or with intervention within 7 days of onset. These episodes recur with variable frequency.
- Persistent AF refers to AF that is continuously sustained for more than 7 days.
- Longstanding persistent AF refers to continuous AF that lasts longer than 12 months.
- Permanent AF is not an inherent pathophysiologic attribute of AF, but rather an acceptance of AF where the patient and physician abandon further efforts to restore and/or maintain sinus rhythm.
- Nonvalvular AF occurs in the absence of a valve replacement (mechanical or bioprosthetic), rheumatic mitral stenosis, or mitral valve repair.
Although paroxysmal and persistent AF may occur in the same individual, the distinction is still clinically relevant, as outcomes of certain therapies, such as CA, are superior in patients with paroxysmal AF.14 With a more complete understanding of AF pathophysiology, guidelines now discourage use of the potentially confusing term “lone AF,” which has historically been applied to younger patients with no known clinical risk factors or echocardiographic abnormalities. As a result, therapeutic decisions are no longer based on this nomenclature, according to the 2014 AF practice guideline from the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS).5
Patient complaints—or incidental findings—can prompt a Dx
Fatigue is the most common symptom of AF. Other signs and symptoms include palpitations, dyspnea, HF, hypotension, syncope, chest pain, and stroke. Some patients are asymptomatic, and AF is an incidental finding when an irregular pulse is discovered during a physical examination. The diagnosis is confirmed by electrocardiogram (EKG), telemetry, Holter monitor, event recorder, or an implanted electrocardiographic recording device. A chest x-ray, serum electrolyte levels, a complete blood count, thyroid testing, and renal and hepatic function testing are recommended. Transthoracic echocardiography to measure cardiac function, detect underlying structural heart disease, and evaluate atrial size is essential.5
An electrophysiologic (EP) study may be needed for diagnosis or treatment if another arrhythmia is present. Aberrant conduction may cause AF to present as a wide complex tachycardia and be mislabeled as ventricular tachycardia. The presence of delta waves is an indication for an EP study targeting the WPW accessory pathway. Transesophageal echocardiography (TEE) is the most sensitive and specific test for left atrial thrombi. If you are considering a TEE for a patient with AF of unknown, or >48 hours’, duration who has not been anticoagulated in the preceding 3 weeks, obtain it before performing cardioversion because of the risk of embolism.5
Stroke prevention
The ACC/AHA/HRS AF guideline recommends basing anticoagulation decisions on thromboembolic risk, regardless of AF pattern (paroxysmal, persistent, or permanent) (Class I recommendation).5 For patients with nonvalvular AF and atrial flutter, the guideline recommends using the Birmingham 2009 schema (CHA2DS2-VASc score) (TABLE 115-18) to estimate thromboembolic risk.5,15 CHA2DS2-VASc improves on the older CHADS2 score by significantly reducing the number of patients categorized as having intermediate risk and better identifying truly low-risk patients who are unlikely to benefit from anticoagulation.16,17,19
Men with a CHA2DS2-VASc score of zero and women with a score of one do not need anticoagulation.5,20 Discuss the risks and benefits of oral anticoagulation with men who have a score of one. In these intermediate-risk men, antiplatelet therapy with aspirin and/or clopidogrel may be reasonable, especially if there is an indication other than stroke prevention (eg, post-myocardial infarction). Oral anticoagulation is strongly recommended for all patients with a CHA2DS2-VASc score of 2 or higher.5,18,21,22
Anticoagulant considerations: Warfarin vs DOACs
Warfarin was the gold standard for stroke prevention in nonvalvular AF until the direct oral anticoagulants (DOACs) became available in 2010. Guidelines in the United States and the United Kingdom recommend shared decision-making to help patients with AF who do not have a specific indication for warfarin choose between warfarin and the DOACs.5,21 Canadian and European guidelines recommend DOACs as the first-line option for anticoagulation and reserve warfarin for patients who have contraindications to, or are unable to afford, DOACs.18,22 All current guidelines recommend continuing warfarin in patients who are stable, well controlled, and satisfied with warfarin therapy and the monitoring and dietary restrictions it entails.
DOACs are as effective as warfarin. All of the DOACs are approved for stroke prevention based on individual phase III non-inferiority trials in which they were compared to warfarin.23-26 In addition, a meta-analysis of these 4 trials involving a total of 71,683 patients (mean age 70-73 years; median follow-up, 1.8-2.8 years) evaluated the benefits and risks of the 4 DOACs against the former gold standard.27
Higher doses of the DOACs (dabigatran 150 mg BID, rivaroxaban 20 mg/d, edoxaban 60 mg/d, and apixaban 5 mg BID) reduced the rates of stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; P<.0001; number needed to treat [NNT]=147), hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; P<.0001; NNT=219), and all-cause mortality (RR=0.90; 95% CI, 0.85-0.95; P=.0003; NNT=128), compared with warfarin.27 It is important to note that while lower doses of some DOACs (dabigatran 110 mg BID and edoxaban 30 mg/d) were not as effective at preventing ischemic stroke when compared with warfarin (RR=1.3; 95% CI, 1-1.6; P=.045), they still significantly reduced hemorrhagic stroke (RR=0.33; 95% CI, 0.23-0.46; P<.0001) and all-cause mortality (RR=0.89; 95% CI, 0.83-0.96; P=.003).
Of course, the biggest concern is bleeding. In that same meta-analysis, the difference in major bleeding events with DOACs vs warfarin was not statistically significant (RR=0.86; 95% CI, 0.73-1; P=.06). While DOACs likely lower rates of intracranial hemorrhage (RR=0.48; 95% CI, 0.39-0.59; P<.0001; NNT=132), they seem to increase the risk of gastrointestinal (GI) bleeding (RR=1.3; 95% CI, 1-1.6; P=.043; number needed to harm [NNH]=185).27
There was significant heterogeneity in the GI bleeding outcome, however. When compared with warfarin, GI bleeding was increased by dabigatran 150 mg BID (RR=1.5; 95% CI, 1.2-1.9; P<.001) and edoxaban 60 mg/d (HR=1.2; 95% CI, 1.02-1.5; P=.03), but there were no significant differences for dabigatran 110 mg BID or apixaban 5 mg BID.23,25,26
On the other hand, edoxaban 30 mg/d had a lower risk of GI bleeding when compared with warfarin (HR=0.67; 95% CI, 0.53-0.83; P<.001).25 Without head-to-head trials, it is impossible to know if one DOAC is superior to another. Apixaban 5 mg BID appears to offer the best overall balance between efficacy and safety. Other DOACs may be better options for patients who have specific concerns regarding efficacy or safety.28,29
Convenience, interactions, and cost may be the deciding factors. Since all DOACs are fairly comparable in efficacy and safety, other factors such as convenience, interactions with other medications, and cost should be considered when deciding on a medication for an individual patient (TABLE 230,31). The DOACs require no lab monitoring or dose titration, and all 4 have fewer potential drug interactions than warfarin.30 Due to their relatively short half-lives, strict adherence is critical; DOACs are not suitable for patients who frequently miss doses.5 (For more information on starting or switching to DOACs, see, “Is a novel anticoagulant right for your patient?” J Fam Pract. 2014;63:22-28.)
A word about DOACs and renal impairment. Another concern with DOACs is their reliance on renal metabolism and excretion. A meta-analysis of the 4 phase III trials of the DOACs, this time involving 58,338 patients, evaluated DOAC efficacy and safety compared to warfarin in the presence of kidney dysfunction.32 Renal function was categorized as normal (estimated glomerular filtration rate [eGFR] >80 mL/min/1.73 m2), mildly impaired (eGFR 50-80 mL/min/1.73 m2), or moderately impaired (eGFR <50 mL/min/1.73m2). Compared with warfarin, DOACs lowered stroke risk in patients with mild (RR=0.71; 95% CI, 0.62-0.81) or moderate (RR=0.79; 95% CI, 0.66-0.94) renal impairment. DOACs also reduced major bleeding compared to warfarin in patients with mild (RR=0.88; 95% CI, 0.80-0.97) or moderate (RR=0.80; 95% CI, 0.66-0.94) renal impairment. How the DOACs fare in patients with severe renal dysfunction could not be determined because such patients were excluded from the trials.
Keep in mind that the DOACs require dose adjustment at different levels of renal impairment (TABLE 230,31), and warfarin remains the only recommended treatment for patients with severe renal impairment, according to both AHA/ACC/HRS and European Society of Cardiology guidelines.5,18
Tools to help assess patients’ bleeding risk
Of the available scoring mechanisms to identify risk factors for bleeding, 3 have been specifically validated in AF populations (ie, ATRIA,33 HEMORR2HAGES,34 and HAS-BLED35). Of the 3, HAS-BLED is superior,36 the most practical, and recommended by expert guidelines.18,21,22 Additionally, HAS-BLED has good correlation with intracranial hemorrhage risk. The HAS-BLED score ranges from 0 to 9 points with one point assigned for each of the following:35
- Hypertension–uncontrolled with systolic BP >160 mm Hg
- Abnormal liver function–cirrhosis, bilirubin >2× normal, or liver enzymes >3× normal
- Abnormal renal function–dialysis, transplant, or serum creatinine >2.26 mg/dL
- Stroke history–including lacunar infarcts
- Bleeding predisposition–history of major bleeding due to any cause
- Labile international normalized ratio (INR)–time in therapeutic range <60%
- Elderly–age >65 years
- Drug–antiplatelet agents, including nonsteroidal anti-inflammatory drugs
- Alcohol usage–>8 drinks per week.
Patients with a HAS-BLED score ≥3 warrant additional monitoring and attempts to reduce bleeding risk by addressing modifiable risk factors. Bleeding risk scores should not be used to exclude patients from anticoagulation therapy.5 In fact, the British National Institute for Health and Clinical Excellence (NICE) guidelines state that anticoagulation should not be withheld solely due to fall risk.21
Also, anticoagulation with warfarin should not be permanently discontinued because of a single GI bleed, since restarting warfarin is associated with decreased risks of thromboembolism and mortality and a statistically insignificant increase in recurrent GI bleeding.37 Restarting DOAC therapy following a GI bleed has not been evaluated in clinical trials; however, it may be reasonable to use one of the DOAC doses with a lower risk of GI bleeding (dabigatran 110 mg BID, apixaban 5 mg BID, or edoxaban 30 mg/d) in patients who have experienced a GI bleed on warfarin or another DOAC.18,22
An online calculator is available that uses CHA2DS2-VASc and HAS-BLED scores to determine an individual’s risk/benefit profile with the various anticoagulation strategies available (http://www.sparctool.com). Consider percutaneous left atrial appendage occlusion if the risks of anticoagulation truly exceed the benefits.38
Rate control vs rhythm control
Most patients who present with AF require immediate ventricular rate control to reduce symptoms. In the acute setting, this can be accomplished with intravenous (IV) beta-blockers or IV calcium channel antagonists.5,39 If the patient is hemodynamically unstable, urgent direct-current cardioversion is the preferred treatment strategy and should not be delayed pending anticoagulation. IV amiodarone can be used in the ICU patient who does not require cardioversion, but is unable to tolerate beta-blockers or calcium channel antagonists.40 Once the patient is stable, long-term treatment focuses on ventricular rate control or restoration and maintenance of sinus rhythm.
The AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial enrolled 4060 patients (mean age 70 years, mean follow-up 3.5 years) with paroxysmal and persistent AF and randomized them to either pharmacologic rate control or rhythm control.41 No significant differences were found in all-cause mortality or in the composite secondary endpoint of death, ischemic stroke, anoxic encephalopathy, major bleeding, or cardiac arrest. In addition, no significant differences emerged in quality of life or global functional status. The number of patients requiring hospitalization during follow-up was significantly lower in the rate-control group vs the rhythm-control group (73% vs 80%; P<.001). Anticoagulation was encouraged but not mandated in the rhythm-control group after 4 weeks in sinus rhythm, and there was a trend toward higher mortality in the rhythm-control group (27% vs 26%; P=.08).
Patients <65 years were excluded from the AFFIRM trial. When younger patients experience significant symptoms, early referral to Cardiology should be considered to discuss the long-term benefits and risks of a rhythm-control strategy. Regardless of age, when patients remain symptomatic despite rate- or rhythm-control management, the strategy should be changed.5
Rate-control targets and options
Target heart rates should be individualized. The 2014 ACC/AHA/HRS guideline recommends a resting target heart rate <80 beats per minute (bpm) in symptomatic patients.5 In patients with permanent AF who remain asymptomatic at higher resting heart rates, a more lenient rate-control strategy (resting heart rate <110 bpm) has demonstrated outcomes equivalent to those of a more strict approach (resting heart rate <80 bpm and heart rate during moderate exercise <110 bpm).42 Pharmacologic rate-control options include beta-blockers, non-dihydropyridine calcium channel antagonists, and digoxin (TABLE 35). Digoxin is associated with increased all-cause mortality in patients with AF regardless of HF status (HR=1.4; 95% CI, 1.2-1.6, P=.0001).43 Digoxin should be reserved for patients who are sedentary or have inadequate control with first-line medications.5
Indications for rhythm control
The NICE guidelines, which are consistent with the ACC/AHA/HRS guidelines, recommend rate control as the first-line strategy for AF management, except in people:21
- whose AF has a reversible cause
- who have HF believed to be primarily caused by AF
- with new-onset AF
- with atrial flutter that is considered suitable for an ablation strategy to restore sinus rhythm
- for whom a rhythm-control strategy would be more suitable based on clinical judgment.
In addition, patients who continue to experience symptomatic AF despite an adequate trial of rate control should be offered rhythm control.5
Pharmacologic rhythm-control strategies. Antiarrhythmic drugs can be used for chemical cardioversion, reduction of paroxysms, and long-term maintenance of sinus rhythm. The most commonly used antiarrhythmic drugs are Class IC and Class III agents (TABLE 3).5 Tailored drug selection for each patient is key. Patients with left atrial diameters >4.5 cm are less likely to remain in sinus rhythm, and patients with left ventricular hypertrophy are at increased risk for proarrhythmic adverse effects.44 Patients with paroxysmal AF may be candidates for a “pill-in-the-pocket” strategy using propafenone or flecainide.5
AF frequently progresses from paroxysmal to persistent and can subsequently result in electrical and structural remodeling that becomes irreversible over time.45 The patient with uncontrolled symptoms despite attempts at rate control and rhythm control should be promptly referred to an electrophysiologist.
Surgical interventions for rate or rhythm control
Electrophysiology interventions include AV nodal ablation with pacemaker placement for rate control, or catheter-directed ablation (radiofrequency or cryotherapy) for rhythm control. CA appears to be more effective than pharmacologic rhythm control.46,47 Treatment with CA is indicated for symptomatic paroxysmal AF when a rhythm-control strategy is desired and the AF is refractory to, or the patient is intolerant of, at least one class I or III antiarrhythmic medication.5 With these same caveats, CA is a reasonable strategy for symptomatic persistent AF.
Consider more invasive interventions, such as an atrial maze procedure, when patients require cardiac surgery for another indication. Patients with an increased risk of thromboembolism (based on CHA2DS2-VASc) remain at high risk even after successful ablation.48 As a result, some guidelines recommend continued long-term anticoagulation following CA.18,22
CORRESPONDENCE
Philip Dooley, MD, University of Kansas School of Medicine–Wichita Family Medicine Residency at Via Christi, 707 North Emporia, Wichita, KS 67207; [email protected].
ACKNOWLEDGMENTS
We thank Professor Anne Walling, MB, ChB, FFPHM, Department of Family and Community Medicine, University of Kansas School of Medicine–Wichita for her suggestions and critical review of an earlier version of this manuscript.
1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. National implications for Rhythm Management and Stroke Prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375.
2. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N-9N.
3. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476-484.
4. Ott A, Breteler MMB, de Bruyne MC, et al. Atrial fibrillation and dementia in a population-based study: The Rotterdam Study. Stroke. 1997;28:316-321.
5. January CT, Wann L, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-e76.
6. Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. Stroke. 1996;27:1760-1764.
7. Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016;1:55-62.
8. Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106:739-749.
9. Steinberg BA, Kim S, Thomas L, et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2014;129:2005-2012.
10. Angaran P, Dorian P, Tan MK, et al. The risk stratification and stroke prevention therapy care gap in Canadian atrial fibrillation patients. Can J Cardiol. 2016;32:336-343.
11. Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779-786.
12. Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799-802.
13. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281-289.
14. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171-257.
15. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263-272.
16. Lip GYH, Frison L, Halperin JL, et al. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731-2738.
17. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124.
18. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719-2747.
19. Olesen JB, Torp-Pedersen C, Hansen ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172-1179.
20. Friberg L, Benson L, Rosenqvist M, et al. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522.
21. National Institute for Health and Clinical Excellence (NICE). Atrial fibrillation: the management of atrial fibrillation [CG180]. 2014. Available at: https://www.nice.org.uk/guidance/cg180. Accessed July 31, 2016.
22. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30:1114-1130.
23. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
24. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
25. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104.
26. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
27. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
28. Morimoto T, Crawford B, Wada K, et al. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66:466-474.
29. Verdecchia P, Angeli F, Bartolini C, et al. Safety and efficacy of non-vitamin K oral anticoagulants in non-valvular atrial fibrillation: a Bayesian meta-analysis approach. Expert Opin Drug Saf. 2015;14:7-20.
30. Micromedex® 2.0 (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com. Accessed August 18, 2016.
31. GoodRx. Available at: https://www.goodrx.com. Accessed August 18, 2016.
32. Del-Carpio Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016;117:69-75.
33. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395-401.
34. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713-719.
35. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100.
36. Zhu W, He W, Guo L, et al. The HAS-BLED Score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561.
37. Chai-Adisaksopha C, Hillis C, Monreal M, et al. Thromboembolic events, recurrent bleeding and mortality after resuming anticoagulant following gastrointestinal bleeding. A meta-analysis. Thromb Haemost. 2015;114:819-825.
38. Xu H, Xie X, Wang B, et al. Efficacy and safety of percutaneous left atrial appendage occlusion for stroke prevention in nonvalvular atrial fibrillation: a meta-analysis of contemporary studies. Heart Lung Circ. 2016;25:1107-1117.
39. Siu CW, Lau CP, Lee WL, et al. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37:2174-2179.
40. Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594-598.
41. The Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
42. Van Gelder IC, Groenveld HF, Crijns HJGM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
43. Wang ZQ, Zhang R, Chen MT, et al. Digoxin is associated with increased all-cause mortality in patients with atrial fibrillation regardless of concomitant heart failure: a meta-analysis. J Cardiovasc Pharmacol. 2015;66:270-275.
44. Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026-2033.
45. de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation: clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725-731.
46. Cheng X, Li X, He Y, et al. Catheter ablation versus anti-arrhythmic drug therapy for the management of atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol. 2014;41:267-272.
47. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637-1644.
48.
1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. National implications for Rhythm Management and Stroke Prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375.
2. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N-9N.
3. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476-484.
4. Ott A, Breteler MMB, de Bruyne MC, et al. Atrial fibrillation and dementia in a population-based study: The Rotterdam Study. Stroke. 1997;28:316-321.
5. January CT, Wann L, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-e76.
6. Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. Stroke. 1996;27:1760-1764.
7. Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016;1:55-62.
8. Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106:739-749.
9. Steinberg BA, Kim S, Thomas L, et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2014;129:2005-2012.
10. Angaran P, Dorian P, Tan MK, et al. The risk stratification and stroke prevention therapy care gap in Canadian atrial fibrillation patients. Can J Cardiol. 2016;32:336-343.
11. Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779-786.
12. Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799-802.
13. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281-289.
14. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171-257.
15. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263-272.
16. Lip GYH, Frison L, Halperin JL, et al. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731-2738.
17. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124.
18. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719-2747.
19. Olesen JB, Torp-Pedersen C, Hansen ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172-1179.
20. Friberg L, Benson L, Rosenqvist M, et al. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522.
21. National Institute for Health and Clinical Excellence (NICE). Atrial fibrillation: the management of atrial fibrillation [CG180]. 2014. Available at: https://www.nice.org.uk/guidance/cg180. Accessed July 31, 2016.
22. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30:1114-1130.
23. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
24. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
25. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104.
26. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
27. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
28. Morimoto T, Crawford B, Wada K, et al. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66:466-474.
29. Verdecchia P, Angeli F, Bartolini C, et al. Safety and efficacy of non-vitamin K oral anticoagulants in non-valvular atrial fibrillation: a Bayesian meta-analysis approach. Expert Opin Drug Saf. 2015;14:7-20.
30. Micromedex® 2.0 (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com. Accessed August 18, 2016.
31. GoodRx. Available at: https://www.goodrx.com. Accessed August 18, 2016.
32. Del-Carpio Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016;117:69-75.
33. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395-401.
34. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713-719.
35. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100.
36. Zhu W, He W, Guo L, et al. The HAS-BLED Score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561.
37. Chai-Adisaksopha C, Hillis C, Monreal M, et al. Thromboembolic events, recurrent bleeding and mortality after resuming anticoagulant following gastrointestinal bleeding. A meta-analysis. Thromb Haemost. 2015;114:819-825.
38. Xu H, Xie X, Wang B, et al. Efficacy and safety of percutaneous left atrial appendage occlusion for stroke prevention in nonvalvular atrial fibrillation: a meta-analysis of contemporary studies. Heart Lung Circ. 2016;25:1107-1117.
39. Siu CW, Lau CP, Lee WL, et al. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37:2174-2179.
40. Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594-598.
41. The Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
42. Van Gelder IC, Groenveld HF, Crijns HJGM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
43. Wang ZQ, Zhang R, Chen MT, et al. Digoxin is associated with increased all-cause mortality in patients with atrial fibrillation regardless of concomitant heart failure: a meta-analysis. J Cardiovasc Pharmacol. 2015;66:270-275.
44. Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026-2033.
45. de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation: clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725-731.
46. Cheng X, Li X, He Y, et al. Catheter ablation versus anti-arrhythmic drug therapy for the management of atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol. 2014;41:267-272.
47. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637-1644.
48.
PRACTICE RECOMMENDATIONS
› Use the CHA2DS2-VASc score to assess the risk of thromboembolism, including ischemic stroke. A
› Consider prescribing a direct oral anticoagulant (DOAC) instead of warfarin for patients with nonvalvular atrial fibrillation (AF) because they are superior at preventing strokes and lowering all-cause mortality in this population. B
› Do not use a DOAC in patients with mechanical heart valves, hemodynamically significant mitral stenosis, or severe chronic kidney disease (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2). A
› Pursue a rate-control strategy for most patients with AF, although rhythm control may be preferable for younger (<65 years) symptomatic patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Improving your approach to nasal obstruction
Nasal obstruction is one of the most common reasons that patients visit their primary care providers.1,2 Often described by patients as nasal congestion or the inability to adequately breathe out of one or both nostrils during the day and/or night, nasal obstruction commonly interferes with a patient’s ability to eat, sleep, and function, thereby significantly impacting quality of life. Overlapping presentations can make discerning the exact cause of nasal obstruction difficult.
To improve diagnosis and treatment, we review here the evidence-based recommendations for the most common causes of nasal obstruction: rhinitis, rhinosinusitis (RS), drug-induced nasal obstruction, and mechanical/structural abnormalities (TABLE 13-14).
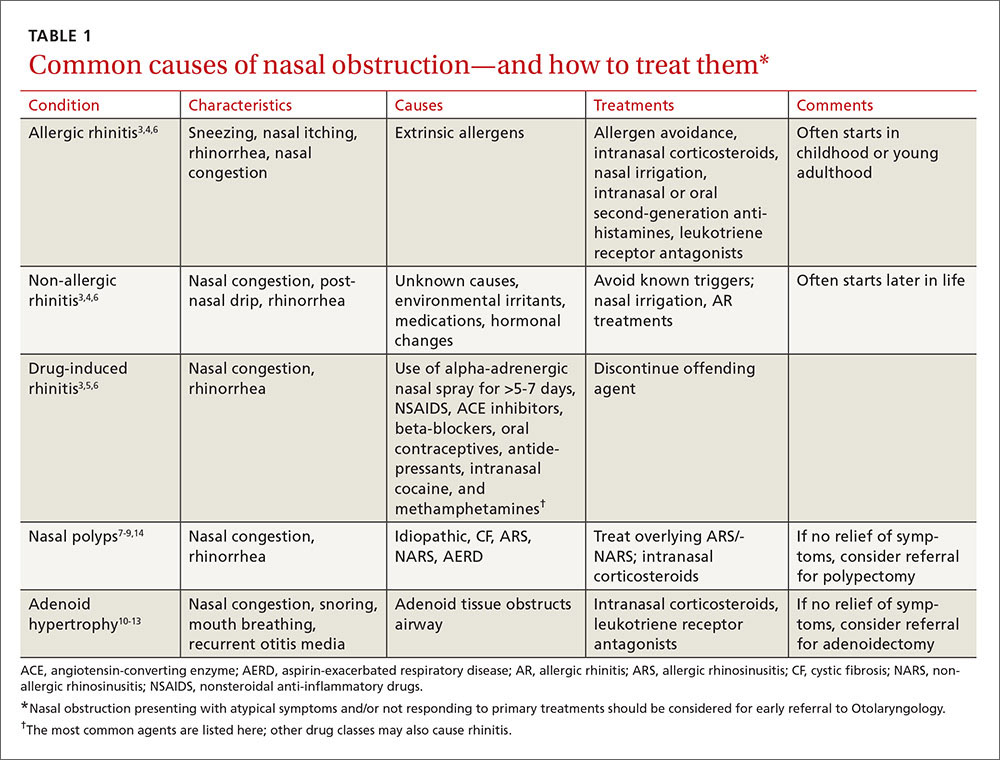
Rhinitis/rhinosinusitis: It all begins with inflammation
Sneezing, rhinorrhea, nasal congestion, and nasal itching are complaints that signal rhinitis, which affects 30 to 60 million people in the United States annually.3 Rhinitis can be allergic, non-allergic, infectious, hormonal, or occupational in nature. All forms of rhinitis share inflammation as the cause of the nasal obstruction. The most common form is allergic rhinitis (AR), which includes seasonal AR and perennial AR. Seasonal AR is typically caused by outdoor allergens and waxes and wanes with pollen seasons. Perennial AR is caused mostly by indoor allergens, such as dust mites, molds, cockroaches, and pet dander; it persists all or most of the year.6 Causes of non-allergic rhinitis (NAR) include environmental irritants such as cigarette smoke, perfume, and car exhaust; medications; and hormonal changes,6 but most causes of NAR are unknown.3,6
While AR can begin at any age, most people develop symptoms in childhood or as young adults, whereas NAR tends to begin later in life. Nasal itching can help to distinguish AR from NAR. NAR symptoms tend to be perennial and include postnasal drainage. If symptoms persist longer than 12 weeks despite treatment, the condition becomes known as chronic rhinosinusitis (CRS).
Treatment of rhinitis: Tiered and often continuous
Treatment of AR and NAR is similar and multitiered beginning with the avoidance of irritants and/or allergens whenever possible, moving on to pharmacotherapy, and, at least for AR, ending with allergen immunotherapy. Treatment is often an ongoing process and typically requires continuous therapy as opposed to treatment on an as-needed basis.3 It is unnecessary to perform allergy testing before making a presumed diagnosis of NAR and starting treatment.6
Intranasal corticosteroids. Currently, intranasal glucocorticosteroids (INGCs) are the most effective monotherapy for AR and NAR and have few adverse effects when used at prescribed doses.3,4 For mild to intermittent symptoms, begin with the maximum dosage of an INGC for the patient’s age and proceed with incremental reductions to identify the lowest effective dose.3 If INGCs alone are ineffective, studies have shown that the addition of an intranasal second-generation antihistamine can be of some benefit.3,4 In fact, an INGC and an intranasal antihistamine—along with saline nasal irrigation—is recommended for both AR and NAR resistant to single therapy.3,6,15 If intranasal antihistamines are not an option, oral therapy can be initiated.
Start with second-generation antihistamines and consider LRAs. For oral therapy, start with second-generation antihistamines (loratadine, cetirizine, fexofenadine). First-generation antihistamines (diphenhydramine, hydroxyzine, chlorpheniramine), although widely available at relatively low cost, can cause several significant adverse effects including sedation, impaired cognitive function, and agitation in children.3,4 Because second-generation antihistamines have fewer adverse effects, they are recommended as first-line therapy when oral antihistamine therapy is desired, such as for nasal congestion, sneezing, and itchy, watery eyes.
Of note: A 2014 meta-analysis found that a leukotriene receptor antagonist (LRA) (montelukast) had efficacy similar to oral antihistamines for symptom relief in AR, and that LRAs may be better suited to nighttime symptoms (difficulty falling asleep, nighttime awakenings, congestion on awakening), while antihistamines may provide better relief of daytime symptoms (pruritus, rhinorrhea, sneezing).16 Although further head-to-head, double-blind randomized controlled trials (RCTs) are needed to confirm the results and investigate possible gender differences in symptom response, consider an LRA for first-line therapy in patients with AR who have predominantly nighttime symptoms.
What about pregnant women and the elderly?
It is important to consider teratogenicity when selecting medications for pregnant patients, especially during the first trimester.3 Nasal cromolyn has the most reassuring safety profile in pregnancy. Cetirizine, chlorpheniramine, loratadine, diphenhydramine, and tripelennamine may be used in pregnancy. The US Food and Drug Administration considers them to have a low risk of fetal harm, based on human data, whereas it views many other antihistamines as probably safe, based on limited or no human data. Most INGCs are not expected to cause fetal harm, but limited human data are available. Avoid prescribing oral decongestants to women who are in the first trimester of pregnancy due to the risk of gastroschisis in newborns.17
Elderly patients represent another population for which adverse effects must be carefully considered. Allergies in individuals >65 years of age are uncommon. Rhinitis in this age group is often secondary to cholinergic hyperactivity, alpha-adrenergic hyperactivity, or rhinosinusitis. Given elderly patients’ increased susceptibility to the potential adverse central nervous system (CNS) and anticholinergic effects of antihistamines, non-sedating medications are recommended. Oral decongestants also should be used with caution in this population, not only because of CNS effects, but also because of heart and bladder effects3 (TABLE 218).
For drug-induced rhinitis, stop the offending drug and consider an INGC
Several types of medications, both oral and inhaled, are known to cause rhinitis. The use of alpha-adrenergic decongestant sprays for more than 5 to 7 days can induce rebound congestion on withdrawal, known as rhinitis medicamentosa.3 Repeated use of intranasal cocaine and methamphetamines can also result in rebound congestion. Oral medications that can result in rhinitis or congestion include angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, nonsteroidal anti-inflammatory drugs (NSAIDS), oral contraceptives, and even antidepressants.3
The treatment for drug-induced rhinitis is termination of the offending agent. INGCs can be used to help decrease inflammation and control symptoms once the offending agent is discontinued.
Mechanical/structural causes of obstruction are wide-ranging
Mechanical/structural causes of nasal obstruction range from foreign bodies to anatomical variations including nasal polyps, a deviated septum, adenoidal hypertrophy, foreign bodies, and tumors. Because more than one etiology may be at work, it is best to first treat any non-mechanical causes of obstruction, such as ARS or NARS.
Nasal polyposis often requires both a medical and surgical approach
Nasal polyps are benign growths arising from the mucosa of the nasal sinuses and nasal cavities and affecting up to 4% of the population.7 Their etiology is unclear, but we do know that nasal polyps result from underlying inflammation.7 Uncommon in children outside of those affected by cystic fibrosis,7 nasal polyposis can be associated with disease processes such as AR and sinusitis. Polyps are also associated with clinical syndromes such as aspirin-exacerbated respiratory disease (AERD) syndrome, which involves upper and lower respiratory tract symptoms in patients with asthma who have taken aspirin or other NSAIDs.9
Symptoms vary with the location and size of the polyps, but generally include nasal congestion, alteration in smell, and rhinorrhea. The goals of treatment are to restore or improve nasal breathing and olfaction and prevent recurrence.8 This often requires both a medical and surgical approach.
Topical corticosteroids are effective at reducing both the size of polyps and associated symptoms (rhinorrhea, rhinitis).8 And research has shown that steroids reduce the need for both primary and repeat surgical polypectomies.4 Other treatments to consider prior to surgery (if no symptom reduction occurs with INGCs) include systemic (oral) corticosteroids, intra-polyp steroid injections, macrolide antibiotics, and nasal washes.7,14
When symptoms of polyposis are refractory to medical management, functional endoscopic sinus surgery (FESS) is the surgical procedure of choice.3 In addition to refractory symptoms, indications for FESS include the need to correct anatomic deformities believed to be contributing to the persistence of disease and the need to debulk advanced nasal polyposis.3 The principal goal is to restore patency to the ostiomeatal unit.3
Several studies have reported a high success rate for FESS in improving the symptoms of CRS.3,19-23 In a 1992 study, for example, 98% of patients reported improvement following surgery,19 and in a follow-up report approximately 6 years later, 98% of patients continued to report subjective improvement.22
For septal etiologies, consider septoplasty
Deviation of the nasal septum is a common structural etiology for nasal obstruction arising primarily from congenital, genetic, or traumatic causes.24 Turbulent airflow from the septal deviation often causes turbinate hypertrophy, which creates (or exacerbates) the obstructive symptoms from the septal deviation.25
Septoplasty is the most common ear, nose, and throat operation in adults.26 Reduction of nasal symptoms has been reported in up to 89% of patients who receive this surgery, according to one single-center, non-randomized trial.27 Currently, at least one multicenter, randomized trial is underway that aims to develop evidence-based guidelines for septoplasty.26
Septal perforation is another etiology that can present with nasal obstruction symptoms. Causes include traumatic perforation, inflammatory or collagen vascular diseases, infections, overuse of vasoconstrictive medications, and malignancy.28,29 A careful inspection of the nasal septum is necessary to identify a perforation; this may require nasal endoscopy.
Anterior, rather than posterior, perforations are more likely to cause symptoms of nasal obstruction. Posterior perforations rarely require treatment unless malignancy is suspected, in which case referral for biopsy is recommended. Anterior perforations are treated initially with avoidance of any causative agent if, for example, the problem is drug- or medication-induced, and then with humidification and emollients.28,29
For anterior perforations, septal silicone buttons can be used for recalcitrant symptoms. However, observational studies indicate that for long-term symptom resolution, silicone buttons are effective in only about one-third of patients.29
For patients with persistent symptoms despite the above measures, surgical repair with various flap techniques is an option. A meta-analysis of case studies involving various techniques concluded that there is a wide variety of options, and that surgeons must weigh factors such as the characteristics and etiology of the perforation and their own experience and expertise when choosing from among available methods.30 Additional good quality research is necessary before clear recommendations regarding technique can be made.
Adenoid hypertrophy: Consider corticosteroid nasal drops
Adenoid hypertrophy is a common cause of chronic nasal obstruction in children. Although adenoidectomy is commonly performed to correct the problem, current evidence regarding the efficacy of the procedure is inconclusive.10 Evidence demonstrates corticosteroid nasal drops significantly reduce symptoms of nasal obstruction in children and may provide an effective alternative to surgical resection.18 Studies have also demonstrated that treatment with oral LRAs significantly reduces adenoid size and nasal obstruction symptoms.12,13
Foreign bodies: Don’t forget “a mother’s kiss”
Foreign bodies are the most common cause of nasal obstruction in the pediatric population. There is a paucity of high-quality evidence on removal of these objects; however, a number of retrospective reviews and case series support that most objects can be removed in the office or emergency department without otolaryngologic referral.31,32
Techniques for removal include positive pressure, which is best used for smooth or soft objects. Positive pressure techniques include having the patient blow their own nose or having a parent use a mouth-to-mouth–type blowing technique (ie, the “mother’s kiss” method).32 Refer patients to Otolaryngology if the obstruction involves:31
- objects not easily visualized by anterior rhinoscopy
- chronic or impacted objects
- button batteries or magnets
- penetrating or hooked objects
- any object that cannot be removed during an initial attempt.
Nasal tumors: More common in older men
Nasal tumors occur most often in the nasal cavity itself and are more common in men ≥60 years.33 There is no notable racial predominance.33 Other risk factors include human papillomavirus (HPV) infection, tobacco smoke, and occupational exposure to inhaled wood dust, glues, and adhesives.34-37
Benign tumors occurring in the nasal cavity are a diverse group of disorders, including inverted papillomas, squamous papillomas, pyogenic granulomas, and other less common lesions, all of which typically present with nasal obstruction as a symptom. Many of these lesions cause local tissue destruction or have a high incidence of recurrence. These tumors are treated universally with nasoendoscopic resection.38
Malignant nasal tumors are rare but serious causes of nasal obstruction, making up 3% of all head and neck cancers.39 Most nasal cancers present when they are locally advanced and cause unilateral nasal obstruction, lacrimation, and epistaxis. These symptoms are typically refractory to initial medical management and present as CRS. This diagnosis should be suspected in certain patient groups, such as those who have been exposed to wood dust (eg, construction workers or those who work in wood mills).36
Computed tomography is the gold standard imaging method for CRS; however, if nasal cancer is suspected, referral for biopsy and histopathologic examination is necessary for a final diagnosis.39 Because of the nonspecific nature of their initial presentation, many nasal tumors are at an advanced stage and carry a poor prognosis by the time they are diagnosed.39
CORRESPONDENCE
Margaret A. Bayard, MD, MPH, FAAFP, Naval Hospital Camp Pendleton, 200 Mercy Circle, Camp Pendleton, CA 92005; [email protected].
1. Centers for Disease Control and Prevention. CDC’s FastStats on Ambulatory Care Use and Physician Office Visits. Selected patient and provider characteristics for ambulatory care visits to physician offices and hospital outpatient and emergency departments: United States, 2009-2010. Available at: http://www.cdc.gov/nchs/data/ahcd/combined_tables/AMC_2009-2010_combined_web_table01.pdf. Accessed November 9, 2016.
2. US Census Bureau. Table 158. Visits to office-based physician and hospital outpatient departments, 2006. Available at: http://www2.census.gov/library/publications/2006/compendia/statab/126ed/tables/07s0158.xls. Accessed November 13, 2016.
3. Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103-S115.
4. Wallace DV, Dykewicz MS. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1-S84.
5. Krouse J, Lund V, Fokkens W, et al. Diagnostic strategies in nasal congestion. Int J Gen Med. 2010;3:59-67.
6. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2-S8.
7. Newton JR, Ah-See KW. A review of nasal polyposis. Ther Clin Risk Manag. 2008;4:507-512.
8. Badia L, Lund V. Topical corticosteroids in nasal polyposis. Drugs. 2001;61:573-578.
9. Fahrenholz JM. Natural history and clinical features of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol. 2003;24:113-124.
10. van den Aardweg MT, Schilder AG, Herket E, et al. Adenoidectomy for recurrent or chronic nasal symptoms in children. Cochrane Database Syst Rev. 2010;CD008282.
11. Demirhan H, Aksoy F, Ozturan O, et al. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int J Pediatr Otorhinolaryngol. 2010;74:773-776.
12. Shokouhi F, Jahromi AM, Majidi MR, et al. Montelukast in adenoid hypertrophy: its effect on size and symptoms. Iran J Otorhinolaryngol. 2015;27:433-448.
13. Goldbart AD, Greenberg-Dotan S, Tai A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130:e575-e580.
14. Moss WJ, Kjos KB, Karnezis TT, et al. Intranasal steroid injections and blindness: our personal experience and a review of the past 60 years. Laryngoscope. 2015;125:796-800.
15. Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis. Laryngoscope. 2013;123:53-56.
16. Xu Y, Zhang J, Wang J. The efficacy and safety of selective H1-antihistamine versus leukotriene receptor antagonist for seasonal allergic rhinitis: a meta-analysis. PLoS One. 2014;9:e112815.
17. Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
18. Lexi-Comp Online. Available at: online.lexi.com/crlsql/servlet/crlonline. Accessed November 9, 2016.
19. Kennedy DW. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope.1992;102:1-18.
20. Khalil HS, Nunez DA. Functional endoscopic sinus surgery for chronic rhinosinusitis. Cochrane Database Syst Rev. 2006;CD004458.
21. Chambers DW, Davis WE, Cook PR, et al. Long-term outcome analysis of functional endoscopic sinus surgery: correlation of symptoms with endoscopic examination findings and potential prognostic variables. Laryngoscope. 1997;107:504-510.
22. Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151-157.
23. Jakobsen J, Svendstrup F. Functional endoscopic sinus surgery in chronic sinusitis—a series of 237 consecutively operated patients. Acta Otolaryngol Suppl. 2000;543:158-161.
24. Aziz T, Biron VL, Ansari K, et al. Measurement tools for the diagnosis of nasal septal deviation: a systematic review. J Otolaryngol Head Neck Surg. 2014;43:11.
25. Grutzenmacher S, Robinson DM, Grafe K, et al. First findings concerning airflow in noses with septal deviation and compensatory turbinate hypertrophy—a model study. ORL J Otorhinolaryngol Relat Spec. 2006;68:199-205.
26. van Egmond MMHT, Rovers MM, Hendriks CTM, et al. Effectiveness of septoplasty versus non-surgical management for nasal obstruction due to deviated nasal septum in adults: study protocol for a randomized controlled trial. Trials. 2015;16:500.
27. Gandomi B, Bayat A, Kazemei T. Outcomes of septoplasty in young adults: the Nasal Obstruction Septoplasty Effectiveness study. Am J Otolaryngol. 2010;31:189-192.
28. Døsen L, Have R. Silicone button in nasal septal perforation. Long term observations. Rhinology. 2008;46:324-327.
29. Kridel RW. Considerations in the etiology, treatment and repair of septal perforations. Facial Plast Surg Clin North Am. 2004;12:435-450.
30. Goh AY, Hussain SS. Different surgical treatments for nasal septal perforation and their outcomes. J Laryngol Otol. 2007;121:419-426.
31. Mackle T, Conlon B. Foreign bodies of the nose and ears in children. Should these be managed in the accident and emergency setting? Int J Pediatr Otorhinolaryngol. 2006;70:425-428.
32. Cook S, Burton M, Glasziou P. Efficacy and safety of the “mother’s kiss” technique: a systematic review of case reports and case series. CMAJ. 2012;184:E904-E912.
33. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
34. Benninger MS. The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999:13:435-438.
35. Luce D, Gerin M, Leclerc A, et al. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int J Cancer. 1993;53:224-231.
36. Mayr SI, Hafizovic K, Waldfahrer F, et al. Characterization of initial clinical symptoms and risk factors for sinonasal adenocarcinomas: results of a case-control study. Int Arch Occup Environ Health. 2010;83;631-638.
37. Syrjänen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174-181.
38. Wood JW, Casiano RR. Inverted papillomas and benign nonneoplastic lesions of the nasal cavity. Am J Rhinol Allergy. 2012;26:157-163.
39. Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging. 2012;12:136-152.
Nasal obstruction is one of the most common reasons that patients visit their primary care providers.1,2 Often described by patients as nasal congestion or the inability to adequately breathe out of one or both nostrils during the day and/or night, nasal obstruction commonly interferes with a patient’s ability to eat, sleep, and function, thereby significantly impacting quality of life. Overlapping presentations can make discerning the exact cause of nasal obstruction difficult.
To improve diagnosis and treatment, we review here the evidence-based recommendations for the most common causes of nasal obstruction: rhinitis, rhinosinusitis (RS), drug-induced nasal obstruction, and mechanical/structural abnormalities (TABLE 13-14).

Rhinitis/rhinosinusitis: It all begins with inflammation
Sneezing, rhinorrhea, nasal congestion, and nasal itching are complaints that signal rhinitis, which affects 30 to 60 million people in the United States annually.3 Rhinitis can be allergic, non-allergic, infectious, hormonal, or occupational in nature. All forms of rhinitis share inflammation as the cause of the nasal obstruction. The most common form is allergic rhinitis (AR), which includes seasonal AR and perennial AR. Seasonal AR is typically caused by outdoor allergens and waxes and wanes with pollen seasons. Perennial AR is caused mostly by indoor allergens, such as dust mites, molds, cockroaches, and pet dander; it persists all or most of the year.6 Causes of non-allergic rhinitis (NAR) include environmental irritants such as cigarette smoke, perfume, and car exhaust; medications; and hormonal changes,6 but most causes of NAR are unknown.3,6
While AR can begin at any age, most people develop symptoms in childhood or as young adults, whereas NAR tends to begin later in life. Nasal itching can help to distinguish AR from NAR. NAR symptoms tend to be perennial and include postnasal drainage. If symptoms persist longer than 12 weeks despite treatment, the condition becomes known as chronic rhinosinusitis (CRS).
Treatment of rhinitis: Tiered and often continuous
Treatment of AR and NAR is similar and multitiered beginning with the avoidance of irritants and/or allergens whenever possible, moving on to pharmacotherapy, and, at least for AR, ending with allergen immunotherapy. Treatment is often an ongoing process and typically requires continuous therapy as opposed to treatment on an as-needed basis.3 It is unnecessary to perform allergy testing before making a presumed diagnosis of NAR and starting treatment.6
Intranasal corticosteroids. Currently, intranasal glucocorticosteroids (INGCs) are the most effective monotherapy for AR and NAR and have few adverse effects when used at prescribed doses.3,4 For mild to intermittent symptoms, begin with the maximum dosage of an INGC for the patient’s age and proceed with incremental reductions to identify the lowest effective dose.3 If INGCs alone are ineffective, studies have shown that the addition of an intranasal second-generation antihistamine can be of some benefit.3,4 In fact, an INGC and an intranasal antihistamine—along with saline nasal irrigation—is recommended for both AR and NAR resistant to single therapy.3,6,15 If intranasal antihistamines are not an option, oral therapy can be initiated.
Start with second-generation antihistamines and consider LRAs. For oral therapy, start with second-generation antihistamines (loratadine, cetirizine, fexofenadine). First-generation antihistamines (diphenhydramine, hydroxyzine, chlorpheniramine), although widely available at relatively low cost, can cause several significant adverse effects including sedation, impaired cognitive function, and agitation in children.3,4 Because second-generation antihistamines have fewer adverse effects, they are recommended as first-line therapy when oral antihistamine therapy is desired, such as for nasal congestion, sneezing, and itchy, watery eyes.
Of note: A 2014 meta-analysis found that a leukotriene receptor antagonist (LRA) (montelukast) had efficacy similar to oral antihistamines for symptom relief in AR, and that LRAs may be better suited to nighttime symptoms (difficulty falling asleep, nighttime awakenings, congestion on awakening), while antihistamines may provide better relief of daytime symptoms (pruritus, rhinorrhea, sneezing).16 Although further head-to-head, double-blind randomized controlled trials (RCTs) are needed to confirm the results and investigate possible gender differences in symptom response, consider an LRA for first-line therapy in patients with AR who have predominantly nighttime symptoms.
What about pregnant women and the elderly?
It is important to consider teratogenicity when selecting medications for pregnant patients, especially during the first trimester.3 Nasal cromolyn has the most reassuring safety profile in pregnancy. Cetirizine, chlorpheniramine, loratadine, diphenhydramine, and tripelennamine may be used in pregnancy. The US Food and Drug Administration considers them to have a low risk of fetal harm, based on human data, whereas it views many other antihistamines as probably safe, based on limited or no human data. Most INGCs are not expected to cause fetal harm, but limited human data are available. Avoid prescribing oral decongestants to women who are in the first trimester of pregnancy due to the risk of gastroschisis in newborns.17
Elderly patients represent another population for which adverse effects must be carefully considered. Allergies in individuals >65 years of age are uncommon. Rhinitis in this age group is often secondary to cholinergic hyperactivity, alpha-adrenergic hyperactivity, or rhinosinusitis. Given elderly patients’ increased susceptibility to the potential adverse central nervous system (CNS) and anticholinergic effects of antihistamines, non-sedating medications are recommended. Oral decongestants also should be used with caution in this population, not only because of CNS effects, but also because of heart and bladder effects3 (TABLE 218).
For drug-induced rhinitis, stop the offending drug and consider an INGC
Several types of medications, both oral and inhaled, are known to cause rhinitis. The use of alpha-adrenergic decongestant sprays for more than 5 to 7 days can induce rebound congestion on withdrawal, known as rhinitis medicamentosa.3 Repeated use of intranasal cocaine and methamphetamines can also result in rebound congestion. Oral medications that can result in rhinitis or congestion include angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, nonsteroidal anti-inflammatory drugs (NSAIDS), oral contraceptives, and even antidepressants.3
The treatment for drug-induced rhinitis is termination of the offending agent. INGCs can be used to help decrease inflammation and control symptoms once the offending agent is discontinued.
Mechanical/structural causes of obstruction are wide-ranging
Mechanical/structural causes of nasal obstruction range from foreign bodies to anatomical variations including nasal polyps, a deviated septum, adenoidal hypertrophy, foreign bodies, and tumors. Because more than one etiology may be at work, it is best to first treat any non-mechanical causes of obstruction, such as ARS or NARS.
Nasal polyposis often requires both a medical and surgical approach
Nasal polyps are benign growths arising from the mucosa of the nasal sinuses and nasal cavities and affecting up to 4% of the population.7 Their etiology is unclear, but we do know that nasal polyps result from underlying inflammation.7 Uncommon in children outside of those affected by cystic fibrosis,7 nasal polyposis can be associated with disease processes such as AR and sinusitis. Polyps are also associated with clinical syndromes such as aspirin-exacerbated respiratory disease (AERD) syndrome, which involves upper and lower respiratory tract symptoms in patients with asthma who have taken aspirin or other NSAIDs.9
Symptoms vary with the location and size of the polyps, but generally include nasal congestion, alteration in smell, and rhinorrhea. The goals of treatment are to restore or improve nasal breathing and olfaction and prevent recurrence.8 This often requires both a medical and surgical approach.
Topical corticosteroids are effective at reducing both the size of polyps and associated symptoms (rhinorrhea, rhinitis).8 And research has shown that steroids reduce the need for both primary and repeat surgical polypectomies.4 Other treatments to consider prior to surgery (if no symptom reduction occurs with INGCs) include systemic (oral) corticosteroids, intra-polyp steroid injections, macrolide antibiotics, and nasal washes.7,14
When symptoms of polyposis are refractory to medical management, functional endoscopic sinus surgery (FESS) is the surgical procedure of choice.3 In addition to refractory symptoms, indications for FESS include the need to correct anatomic deformities believed to be contributing to the persistence of disease and the need to debulk advanced nasal polyposis.3 The principal goal is to restore patency to the ostiomeatal unit.3
Several studies have reported a high success rate for FESS in improving the symptoms of CRS.3,19-23 In a 1992 study, for example, 98% of patients reported improvement following surgery,19 and in a follow-up report approximately 6 years later, 98% of patients continued to report subjective improvement.22
For septal etiologies, consider septoplasty
Deviation of the nasal septum is a common structural etiology for nasal obstruction arising primarily from congenital, genetic, or traumatic causes.24 Turbulent airflow from the septal deviation often causes turbinate hypertrophy, which creates (or exacerbates) the obstructive symptoms from the septal deviation.25
Septoplasty is the most common ear, nose, and throat operation in adults.26 Reduction of nasal symptoms has been reported in up to 89% of patients who receive this surgery, according to one single-center, non-randomized trial.27 Currently, at least one multicenter, randomized trial is underway that aims to develop evidence-based guidelines for septoplasty.26
Septal perforation is another etiology that can present with nasal obstruction symptoms. Causes include traumatic perforation, inflammatory or collagen vascular diseases, infections, overuse of vasoconstrictive medications, and malignancy.28,29 A careful inspection of the nasal septum is necessary to identify a perforation; this may require nasal endoscopy.
Anterior, rather than posterior, perforations are more likely to cause symptoms of nasal obstruction. Posterior perforations rarely require treatment unless malignancy is suspected, in which case referral for biopsy is recommended. Anterior perforations are treated initially with avoidance of any causative agent if, for example, the problem is drug- or medication-induced, and then with humidification and emollients.28,29
For anterior perforations, septal silicone buttons can be used for recalcitrant symptoms. However, observational studies indicate that for long-term symptom resolution, silicone buttons are effective in only about one-third of patients.29
For patients with persistent symptoms despite the above measures, surgical repair with various flap techniques is an option. A meta-analysis of case studies involving various techniques concluded that there is a wide variety of options, and that surgeons must weigh factors such as the characteristics and etiology of the perforation and their own experience and expertise when choosing from among available methods.30 Additional good quality research is necessary before clear recommendations regarding technique can be made.
Adenoid hypertrophy: Consider corticosteroid nasal drops
Adenoid hypertrophy is a common cause of chronic nasal obstruction in children. Although adenoidectomy is commonly performed to correct the problem, current evidence regarding the efficacy of the procedure is inconclusive.10 Evidence demonstrates corticosteroid nasal drops significantly reduce symptoms of nasal obstruction in children and may provide an effective alternative to surgical resection.18 Studies have also demonstrated that treatment with oral LRAs significantly reduces adenoid size and nasal obstruction symptoms.12,13
Foreign bodies: Don’t forget “a mother’s kiss”
Foreign bodies are the most common cause of nasal obstruction in the pediatric population. There is a paucity of high-quality evidence on removal of these objects; however, a number of retrospective reviews and case series support that most objects can be removed in the office or emergency department without otolaryngologic referral.31,32
Techniques for removal include positive pressure, which is best used for smooth or soft objects. Positive pressure techniques include having the patient blow their own nose or having a parent use a mouth-to-mouth–type blowing technique (ie, the “mother’s kiss” method).32 Refer patients to Otolaryngology if the obstruction involves:31
- objects not easily visualized by anterior rhinoscopy
- chronic or impacted objects
- button batteries or magnets
- penetrating or hooked objects
- any object that cannot be removed during an initial attempt.
Nasal tumors: More common in older men
Nasal tumors occur most often in the nasal cavity itself and are more common in men ≥60 years.33 There is no notable racial predominance.33 Other risk factors include human papillomavirus (HPV) infection, tobacco smoke, and occupational exposure to inhaled wood dust, glues, and adhesives.34-37
Benign tumors occurring in the nasal cavity are a diverse group of disorders, including inverted papillomas, squamous papillomas, pyogenic granulomas, and other less common lesions, all of which typically present with nasal obstruction as a symptom. Many of these lesions cause local tissue destruction or have a high incidence of recurrence. These tumors are treated universally with nasoendoscopic resection.38
Malignant nasal tumors are rare but serious causes of nasal obstruction, making up 3% of all head and neck cancers.39 Most nasal cancers present when they are locally advanced and cause unilateral nasal obstruction, lacrimation, and epistaxis. These symptoms are typically refractory to initial medical management and present as CRS. This diagnosis should be suspected in certain patient groups, such as those who have been exposed to wood dust (eg, construction workers or those who work in wood mills).36
Computed tomography is the gold standard imaging method for CRS; however, if nasal cancer is suspected, referral for biopsy and histopathologic examination is necessary for a final diagnosis.39 Because of the nonspecific nature of their initial presentation, many nasal tumors are at an advanced stage and carry a poor prognosis by the time they are diagnosed.39
CORRESPONDENCE
Margaret A. Bayard, MD, MPH, FAAFP, Naval Hospital Camp Pendleton, 200 Mercy Circle, Camp Pendleton, CA 92005; [email protected].
Nasal obstruction is one of the most common reasons that patients visit their primary care providers.1,2 Often described by patients as nasal congestion or the inability to adequately breathe out of one or both nostrils during the day and/or night, nasal obstruction commonly interferes with a patient’s ability to eat, sleep, and function, thereby significantly impacting quality of life. Overlapping presentations can make discerning the exact cause of nasal obstruction difficult.
To improve diagnosis and treatment, we review here the evidence-based recommendations for the most common causes of nasal obstruction: rhinitis, rhinosinusitis (RS), drug-induced nasal obstruction, and mechanical/structural abnormalities (TABLE 13-14).

Rhinitis/rhinosinusitis: It all begins with inflammation
Sneezing, rhinorrhea, nasal congestion, and nasal itching are complaints that signal rhinitis, which affects 30 to 60 million people in the United States annually.3 Rhinitis can be allergic, non-allergic, infectious, hormonal, or occupational in nature. All forms of rhinitis share inflammation as the cause of the nasal obstruction. The most common form is allergic rhinitis (AR), which includes seasonal AR and perennial AR. Seasonal AR is typically caused by outdoor allergens and waxes and wanes with pollen seasons. Perennial AR is caused mostly by indoor allergens, such as dust mites, molds, cockroaches, and pet dander; it persists all or most of the year.6 Causes of non-allergic rhinitis (NAR) include environmental irritants such as cigarette smoke, perfume, and car exhaust; medications; and hormonal changes,6 but most causes of NAR are unknown.3,6
While AR can begin at any age, most people develop symptoms in childhood or as young adults, whereas NAR tends to begin later in life. Nasal itching can help to distinguish AR from NAR. NAR symptoms tend to be perennial and include postnasal drainage. If symptoms persist longer than 12 weeks despite treatment, the condition becomes known as chronic rhinosinusitis (CRS).
Treatment of rhinitis: Tiered and often continuous
Treatment of AR and NAR is similar and multitiered beginning with the avoidance of irritants and/or allergens whenever possible, moving on to pharmacotherapy, and, at least for AR, ending with allergen immunotherapy. Treatment is often an ongoing process and typically requires continuous therapy as opposed to treatment on an as-needed basis.3 It is unnecessary to perform allergy testing before making a presumed diagnosis of NAR and starting treatment.6
Intranasal corticosteroids. Currently, intranasal glucocorticosteroids (INGCs) are the most effective monotherapy for AR and NAR and have few adverse effects when used at prescribed doses.3,4 For mild to intermittent symptoms, begin with the maximum dosage of an INGC for the patient’s age and proceed with incremental reductions to identify the lowest effective dose.3 If INGCs alone are ineffective, studies have shown that the addition of an intranasal second-generation antihistamine can be of some benefit.3,4 In fact, an INGC and an intranasal antihistamine—along with saline nasal irrigation—is recommended for both AR and NAR resistant to single therapy.3,6,15 If intranasal antihistamines are not an option, oral therapy can be initiated.
Start with second-generation antihistamines and consider LRAs. For oral therapy, start with second-generation antihistamines (loratadine, cetirizine, fexofenadine). First-generation antihistamines (diphenhydramine, hydroxyzine, chlorpheniramine), although widely available at relatively low cost, can cause several significant adverse effects including sedation, impaired cognitive function, and agitation in children.3,4 Because second-generation antihistamines have fewer adverse effects, they are recommended as first-line therapy when oral antihistamine therapy is desired, such as for nasal congestion, sneezing, and itchy, watery eyes.
Of note: A 2014 meta-analysis found that a leukotriene receptor antagonist (LRA) (montelukast) had efficacy similar to oral antihistamines for symptom relief in AR, and that LRAs may be better suited to nighttime symptoms (difficulty falling asleep, nighttime awakenings, congestion on awakening), while antihistamines may provide better relief of daytime symptoms (pruritus, rhinorrhea, sneezing).16 Although further head-to-head, double-blind randomized controlled trials (RCTs) are needed to confirm the results and investigate possible gender differences in symptom response, consider an LRA for first-line therapy in patients with AR who have predominantly nighttime symptoms.
What about pregnant women and the elderly?
It is important to consider teratogenicity when selecting medications for pregnant patients, especially during the first trimester.3 Nasal cromolyn has the most reassuring safety profile in pregnancy. Cetirizine, chlorpheniramine, loratadine, diphenhydramine, and tripelennamine may be used in pregnancy. The US Food and Drug Administration considers them to have a low risk of fetal harm, based on human data, whereas it views many other antihistamines as probably safe, based on limited or no human data. Most INGCs are not expected to cause fetal harm, but limited human data are available. Avoid prescribing oral decongestants to women who are in the first trimester of pregnancy due to the risk of gastroschisis in newborns.17
Elderly patients represent another population for which adverse effects must be carefully considered. Allergies in individuals >65 years of age are uncommon. Rhinitis in this age group is often secondary to cholinergic hyperactivity, alpha-adrenergic hyperactivity, or rhinosinusitis. Given elderly patients’ increased susceptibility to the potential adverse central nervous system (CNS) and anticholinergic effects of antihistamines, non-sedating medications are recommended. Oral decongestants also should be used with caution in this population, not only because of CNS effects, but also because of heart and bladder effects3 (TABLE 218).
For drug-induced rhinitis, stop the offending drug and consider an INGC
Several types of medications, both oral and inhaled, are known to cause rhinitis. The use of alpha-adrenergic decongestant sprays for more than 5 to 7 days can induce rebound congestion on withdrawal, known as rhinitis medicamentosa.3 Repeated use of intranasal cocaine and methamphetamines can also result in rebound congestion. Oral medications that can result in rhinitis or congestion include angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, nonsteroidal anti-inflammatory drugs (NSAIDS), oral contraceptives, and even antidepressants.3
The treatment for drug-induced rhinitis is termination of the offending agent. INGCs can be used to help decrease inflammation and control symptoms once the offending agent is discontinued.
Mechanical/structural causes of obstruction are wide-ranging
Mechanical/structural causes of nasal obstruction range from foreign bodies to anatomical variations including nasal polyps, a deviated septum, adenoidal hypertrophy, foreign bodies, and tumors. Because more than one etiology may be at work, it is best to first treat any non-mechanical causes of obstruction, such as ARS or NARS.
Nasal polyposis often requires both a medical and surgical approach
Nasal polyps are benign growths arising from the mucosa of the nasal sinuses and nasal cavities and affecting up to 4% of the population.7 Their etiology is unclear, but we do know that nasal polyps result from underlying inflammation.7 Uncommon in children outside of those affected by cystic fibrosis,7 nasal polyposis can be associated with disease processes such as AR and sinusitis. Polyps are also associated with clinical syndromes such as aspirin-exacerbated respiratory disease (AERD) syndrome, which involves upper and lower respiratory tract symptoms in patients with asthma who have taken aspirin or other NSAIDs.9
Symptoms vary with the location and size of the polyps, but generally include nasal congestion, alteration in smell, and rhinorrhea. The goals of treatment are to restore or improve nasal breathing and olfaction and prevent recurrence.8 This often requires both a medical and surgical approach.
Topical corticosteroids are effective at reducing both the size of polyps and associated symptoms (rhinorrhea, rhinitis).8 And research has shown that steroids reduce the need for both primary and repeat surgical polypectomies.4 Other treatments to consider prior to surgery (if no symptom reduction occurs with INGCs) include systemic (oral) corticosteroids, intra-polyp steroid injections, macrolide antibiotics, and nasal washes.7,14
When symptoms of polyposis are refractory to medical management, functional endoscopic sinus surgery (FESS) is the surgical procedure of choice.3 In addition to refractory symptoms, indications for FESS include the need to correct anatomic deformities believed to be contributing to the persistence of disease and the need to debulk advanced nasal polyposis.3 The principal goal is to restore patency to the ostiomeatal unit.3
Several studies have reported a high success rate for FESS in improving the symptoms of CRS.3,19-23 In a 1992 study, for example, 98% of patients reported improvement following surgery,19 and in a follow-up report approximately 6 years later, 98% of patients continued to report subjective improvement.22
For septal etiologies, consider septoplasty
Deviation of the nasal septum is a common structural etiology for nasal obstruction arising primarily from congenital, genetic, or traumatic causes.24 Turbulent airflow from the septal deviation often causes turbinate hypertrophy, which creates (or exacerbates) the obstructive symptoms from the septal deviation.25
Septoplasty is the most common ear, nose, and throat operation in adults.26 Reduction of nasal symptoms has been reported in up to 89% of patients who receive this surgery, according to one single-center, non-randomized trial.27 Currently, at least one multicenter, randomized trial is underway that aims to develop evidence-based guidelines for septoplasty.26
Septal perforation is another etiology that can present with nasal obstruction symptoms. Causes include traumatic perforation, inflammatory or collagen vascular diseases, infections, overuse of vasoconstrictive medications, and malignancy.28,29 A careful inspection of the nasal septum is necessary to identify a perforation; this may require nasal endoscopy.
Anterior, rather than posterior, perforations are more likely to cause symptoms of nasal obstruction. Posterior perforations rarely require treatment unless malignancy is suspected, in which case referral for biopsy is recommended. Anterior perforations are treated initially with avoidance of any causative agent if, for example, the problem is drug- or medication-induced, and then with humidification and emollients.28,29
For anterior perforations, septal silicone buttons can be used for recalcitrant symptoms. However, observational studies indicate that for long-term symptom resolution, silicone buttons are effective in only about one-third of patients.29
For patients with persistent symptoms despite the above measures, surgical repair with various flap techniques is an option. A meta-analysis of case studies involving various techniques concluded that there is a wide variety of options, and that surgeons must weigh factors such as the characteristics and etiology of the perforation and their own experience and expertise when choosing from among available methods.30 Additional good quality research is necessary before clear recommendations regarding technique can be made.
Adenoid hypertrophy: Consider corticosteroid nasal drops
Adenoid hypertrophy is a common cause of chronic nasal obstruction in children. Although adenoidectomy is commonly performed to correct the problem, current evidence regarding the efficacy of the procedure is inconclusive.10 Evidence demonstrates corticosteroid nasal drops significantly reduce symptoms of nasal obstruction in children and may provide an effective alternative to surgical resection.18 Studies have also demonstrated that treatment with oral LRAs significantly reduces adenoid size and nasal obstruction symptoms.12,13
Foreign bodies: Don’t forget “a mother’s kiss”
Foreign bodies are the most common cause of nasal obstruction in the pediatric population. There is a paucity of high-quality evidence on removal of these objects; however, a number of retrospective reviews and case series support that most objects can be removed in the office or emergency department without otolaryngologic referral.31,32
Techniques for removal include positive pressure, which is best used for smooth or soft objects. Positive pressure techniques include having the patient blow their own nose or having a parent use a mouth-to-mouth–type blowing technique (ie, the “mother’s kiss” method).32 Refer patients to Otolaryngology if the obstruction involves:31
- objects not easily visualized by anterior rhinoscopy
- chronic or impacted objects
- button batteries or magnets
- penetrating or hooked objects
- any object that cannot be removed during an initial attempt.
Nasal tumors: More common in older men
Nasal tumors occur most often in the nasal cavity itself and are more common in men ≥60 years.33 There is no notable racial predominance.33 Other risk factors include human papillomavirus (HPV) infection, tobacco smoke, and occupational exposure to inhaled wood dust, glues, and adhesives.34-37
Benign tumors occurring in the nasal cavity are a diverse group of disorders, including inverted papillomas, squamous papillomas, pyogenic granulomas, and other less common lesions, all of which typically present with nasal obstruction as a symptom. Many of these lesions cause local tissue destruction or have a high incidence of recurrence. These tumors are treated universally with nasoendoscopic resection.38
Malignant nasal tumors are rare but serious causes of nasal obstruction, making up 3% of all head and neck cancers.39 Most nasal cancers present when they are locally advanced and cause unilateral nasal obstruction, lacrimation, and epistaxis. These symptoms are typically refractory to initial medical management and present as CRS. This diagnosis should be suspected in certain patient groups, such as those who have been exposed to wood dust (eg, construction workers or those who work in wood mills).36
Computed tomography is the gold standard imaging method for CRS; however, if nasal cancer is suspected, referral for biopsy and histopathologic examination is necessary for a final diagnosis.39 Because of the nonspecific nature of their initial presentation, many nasal tumors are at an advanced stage and carry a poor prognosis by the time they are diagnosed.39
CORRESPONDENCE
Margaret A. Bayard, MD, MPH, FAAFP, Naval Hospital Camp Pendleton, 200 Mercy Circle, Camp Pendleton, CA 92005; [email protected].
1. Centers for Disease Control and Prevention. CDC’s FastStats on Ambulatory Care Use and Physician Office Visits. Selected patient and provider characteristics for ambulatory care visits to physician offices and hospital outpatient and emergency departments: United States, 2009-2010. Available at: http://www.cdc.gov/nchs/data/ahcd/combined_tables/AMC_2009-2010_combined_web_table01.pdf. Accessed November 9, 2016.
2. US Census Bureau. Table 158. Visits to office-based physician and hospital outpatient departments, 2006. Available at: http://www2.census.gov/library/publications/2006/compendia/statab/126ed/tables/07s0158.xls. Accessed November 13, 2016.
3. Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103-S115.
4. Wallace DV, Dykewicz MS. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1-S84.
5. Krouse J, Lund V, Fokkens W, et al. Diagnostic strategies in nasal congestion. Int J Gen Med. 2010;3:59-67.
6. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2-S8.
7. Newton JR, Ah-See KW. A review of nasal polyposis. Ther Clin Risk Manag. 2008;4:507-512.
8. Badia L, Lund V. Topical corticosteroids in nasal polyposis. Drugs. 2001;61:573-578.
9. Fahrenholz JM. Natural history and clinical features of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol. 2003;24:113-124.
10. van den Aardweg MT, Schilder AG, Herket E, et al. Adenoidectomy for recurrent or chronic nasal symptoms in children. Cochrane Database Syst Rev. 2010;CD008282.
11. Demirhan H, Aksoy F, Ozturan O, et al. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int J Pediatr Otorhinolaryngol. 2010;74:773-776.
12. Shokouhi F, Jahromi AM, Majidi MR, et al. Montelukast in adenoid hypertrophy: its effect on size and symptoms. Iran J Otorhinolaryngol. 2015;27:433-448.
13. Goldbart AD, Greenberg-Dotan S, Tai A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130:e575-e580.
14. Moss WJ, Kjos KB, Karnezis TT, et al. Intranasal steroid injections and blindness: our personal experience and a review of the past 60 years. Laryngoscope. 2015;125:796-800.
15. Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis. Laryngoscope. 2013;123:53-56.
16. Xu Y, Zhang J, Wang J. The efficacy and safety of selective H1-antihistamine versus leukotriene receptor antagonist for seasonal allergic rhinitis: a meta-analysis. PLoS One. 2014;9:e112815.
17. Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
18. Lexi-Comp Online. Available at: online.lexi.com/crlsql/servlet/crlonline. Accessed November 9, 2016.
19. Kennedy DW. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope.1992;102:1-18.
20. Khalil HS, Nunez DA. Functional endoscopic sinus surgery for chronic rhinosinusitis. Cochrane Database Syst Rev. 2006;CD004458.
21. Chambers DW, Davis WE, Cook PR, et al. Long-term outcome analysis of functional endoscopic sinus surgery: correlation of symptoms with endoscopic examination findings and potential prognostic variables. Laryngoscope. 1997;107:504-510.
22. Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151-157.
23. Jakobsen J, Svendstrup F. Functional endoscopic sinus surgery in chronic sinusitis—a series of 237 consecutively operated patients. Acta Otolaryngol Suppl. 2000;543:158-161.
24. Aziz T, Biron VL, Ansari K, et al. Measurement tools for the diagnosis of nasal septal deviation: a systematic review. J Otolaryngol Head Neck Surg. 2014;43:11.
25. Grutzenmacher S, Robinson DM, Grafe K, et al. First findings concerning airflow in noses with septal deviation and compensatory turbinate hypertrophy—a model study. ORL J Otorhinolaryngol Relat Spec. 2006;68:199-205.
26. van Egmond MMHT, Rovers MM, Hendriks CTM, et al. Effectiveness of septoplasty versus non-surgical management for nasal obstruction due to deviated nasal septum in adults: study protocol for a randomized controlled trial. Trials. 2015;16:500.
27. Gandomi B, Bayat A, Kazemei T. Outcomes of septoplasty in young adults: the Nasal Obstruction Septoplasty Effectiveness study. Am J Otolaryngol. 2010;31:189-192.
28. Døsen L, Have R. Silicone button in nasal septal perforation. Long term observations. Rhinology. 2008;46:324-327.
29. Kridel RW. Considerations in the etiology, treatment and repair of septal perforations. Facial Plast Surg Clin North Am. 2004;12:435-450.
30. Goh AY, Hussain SS. Different surgical treatments for nasal septal perforation and their outcomes. J Laryngol Otol. 2007;121:419-426.
31. Mackle T, Conlon B. Foreign bodies of the nose and ears in children. Should these be managed in the accident and emergency setting? Int J Pediatr Otorhinolaryngol. 2006;70:425-428.
32. Cook S, Burton M, Glasziou P. Efficacy and safety of the “mother’s kiss” technique: a systematic review of case reports and case series. CMAJ. 2012;184:E904-E912.
33. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
34. Benninger MS. The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999:13:435-438.
35. Luce D, Gerin M, Leclerc A, et al. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int J Cancer. 1993;53:224-231.
36. Mayr SI, Hafizovic K, Waldfahrer F, et al. Characterization of initial clinical symptoms and risk factors for sinonasal adenocarcinomas: results of a case-control study. Int Arch Occup Environ Health. 2010;83;631-638.
37. Syrjänen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174-181.
38. Wood JW, Casiano RR. Inverted papillomas and benign nonneoplastic lesions of the nasal cavity. Am J Rhinol Allergy. 2012;26:157-163.
39. Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging. 2012;12:136-152.
1. Centers for Disease Control and Prevention. CDC’s FastStats on Ambulatory Care Use and Physician Office Visits. Selected patient and provider characteristics for ambulatory care visits to physician offices and hospital outpatient and emergency departments: United States, 2009-2010. Available at: http://www.cdc.gov/nchs/data/ahcd/combined_tables/AMC_2009-2010_combined_web_table01.pdf. Accessed November 9, 2016.
2. US Census Bureau. Table 158. Visits to office-based physician and hospital outpatient departments, 2006. Available at: http://www2.census.gov/library/publications/2006/compendia/statab/126ed/tables/07s0158.xls. Accessed November 13, 2016.
3. Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103-S115.
4. Wallace DV, Dykewicz MS. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1-S84.
5. Krouse J, Lund V, Fokkens W, et al. Diagnostic strategies in nasal congestion. Int J Gen Med. 2010;3:59-67.
6. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2-S8.
7. Newton JR, Ah-See KW. A review of nasal polyposis. Ther Clin Risk Manag. 2008;4:507-512.
8. Badia L, Lund V. Topical corticosteroids in nasal polyposis. Drugs. 2001;61:573-578.
9. Fahrenholz JM. Natural history and clinical features of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol. 2003;24:113-124.
10. van den Aardweg MT, Schilder AG, Herket E, et al. Adenoidectomy for recurrent or chronic nasal symptoms in children. Cochrane Database Syst Rev. 2010;CD008282.
11. Demirhan H, Aksoy F, Ozturan O, et al. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int J Pediatr Otorhinolaryngol. 2010;74:773-776.
12. Shokouhi F, Jahromi AM, Majidi MR, et al. Montelukast in adenoid hypertrophy: its effect on size and symptoms. Iran J Otorhinolaryngol. 2015;27:433-448.
13. Goldbart AD, Greenberg-Dotan S, Tai A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130:e575-e580.
14. Moss WJ, Kjos KB, Karnezis TT, et al. Intranasal steroid injections and blindness: our personal experience and a review of the past 60 years. Laryngoscope. 2015;125:796-800.
15. Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis. Laryngoscope. 2013;123:53-56.
16. Xu Y, Zhang J, Wang J. The efficacy and safety of selective H1-antihistamine versus leukotriene receptor antagonist for seasonal allergic rhinitis: a meta-analysis. PLoS One. 2014;9:e112815.
17. Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
18. Lexi-Comp Online. Available at: online.lexi.com/crlsql/servlet/crlonline. Accessed November 9, 2016.
19. Kennedy DW. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope.1992;102:1-18.
20. Khalil HS, Nunez DA. Functional endoscopic sinus surgery for chronic rhinosinusitis. Cochrane Database Syst Rev. 2006;CD004458.
21. Chambers DW, Davis WE, Cook PR, et al. Long-term outcome analysis of functional endoscopic sinus surgery: correlation of symptoms with endoscopic examination findings and potential prognostic variables. Laryngoscope. 1997;107:504-510.
22. Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151-157.
23. Jakobsen J, Svendstrup F. Functional endoscopic sinus surgery in chronic sinusitis—a series of 237 consecutively operated patients. Acta Otolaryngol Suppl. 2000;543:158-161.
24. Aziz T, Biron VL, Ansari K, et al. Measurement tools for the diagnosis of nasal septal deviation: a systematic review. J Otolaryngol Head Neck Surg. 2014;43:11.
25. Grutzenmacher S, Robinson DM, Grafe K, et al. First findings concerning airflow in noses with septal deviation and compensatory turbinate hypertrophy—a model study. ORL J Otorhinolaryngol Relat Spec. 2006;68:199-205.
26. van Egmond MMHT, Rovers MM, Hendriks CTM, et al. Effectiveness of septoplasty versus non-surgical management for nasal obstruction due to deviated nasal septum in adults: study protocol for a randomized controlled trial. Trials. 2015;16:500.
27. Gandomi B, Bayat A, Kazemei T. Outcomes of septoplasty in young adults: the Nasal Obstruction Septoplasty Effectiveness study. Am J Otolaryngol. 2010;31:189-192.
28. Døsen L, Have R. Silicone button in nasal septal perforation. Long term observations. Rhinology. 2008;46:324-327.
29. Kridel RW. Considerations in the etiology, treatment and repair of septal perforations. Facial Plast Surg Clin North Am. 2004;12:435-450.
30. Goh AY, Hussain SS. Different surgical treatments for nasal septal perforation and their outcomes. J Laryngol Otol. 2007;121:419-426.
31. Mackle T, Conlon B. Foreign bodies of the nose and ears in children. Should these be managed in the accident and emergency setting? Int J Pediatr Otorhinolaryngol. 2006;70:425-428.
32. Cook S, Burton M, Glasziou P. Efficacy and safety of the “mother’s kiss” technique: a systematic review of case reports and case series. CMAJ. 2012;184:E904-E912.
33. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
34. Benninger MS. The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999:13:435-438.
35. Luce D, Gerin M, Leclerc A, et al. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int J Cancer. 1993;53:224-231.
36. Mayr SI, Hafizovic K, Waldfahrer F, et al. Characterization of initial clinical symptoms and risk factors for sinonasal adenocarcinomas: results of a case-control study. Int Arch Occup Environ Health. 2010;83;631-638.
37. Syrjänen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174-181.
38. Wood JW, Casiano RR. Inverted papillomas and benign nonneoplastic lesions of the nasal cavity. Am J Rhinol Allergy. 2012;26:157-163.
39. Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging. 2012;12:136-152.
PRACTICE RECOMMENDATIONS
› Consider intranasal corticosteroids for patients with nasal polyps, as they are effective at reducing the size of the polyps and associated symptoms of obstruction, rhinorrhea, and rhinitis. A
› Prescribe intranasal corticosteroids for patients with adenoid hypertrophy. A
› Refer patients with chronic refractory rhinosinusitis for functional endoscopic sinus surgery. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Does your patient really need testosterone replacement?
Over the past decade, androgen replacement prescriptions for men ≥40 years of age have increased 3-fold, according to one study.1 While one could argue this trend represents greater attention to an underdiagnosed problem, the study of prescription claims for almost 11 million men found that a quarter of them did not have a testosterone level documented in the 12 months prior to receiving treatment.1
At the same time, sales of testosterone products totaled about $2.4 billion dollars in 2013, a number projected to top $4 billion by 2017.2 The increase in prescribing is thought to be due, at least in part, to direct-to-consumer marketing techniques encouraging patients to seek medical attention if they are experiencing non-specific symptoms, such as fatigue and lack of energy, because their “problem” could be due to low testosterone.
Testosterone begins to decrease after age 40
The Endocrine Society defines “androgen deficiency” as low serum testosterone (generally <280 ng/dL for healthy young men) along with signs and symptoms of hypogonadism, including decreased sexual function; loss of axillary and/or pubic hair; low bone mineral density; loss of motivation and/or concentration; poor mood or depression; decline in cognitive function; and loss of muscle strength and work capacity (TABLE 1).3
Primary vs secondary hypogonadism. Primary (or hypogonadotropic) hypogonadism results when the testes fail to produce adequate testosterone in the presence of normal serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Secondary hypogonadism is pituitary or hypothalamic in origin. Patients with primary hypogonadism will have high LH and FSH levels, whereas patients with secondary hypogonadism will have low or normal LH and FSH levels.4 The Endocrine Society recommends checking LH and FSH levels in all patients with hypogonadism to differentiate the primary from the secondary type.3 Patients with late onset primary hypogonadism do not require any further evaluation. In young men, it is important to consider Klinefelter syndrome. This diagnosis can be determined with a karyotype. In patients with secondary hypogonadism, checking serum iron, prolactin, and other pituitary hormones, and getting a magnetic resonance imaging scan of the sella turcica may be indicated. This will rule out infiltrative diseases, such as hemochromatosis, prolactinoma, and hypothalamic or pituitary neoplasm.
Testosterone is present in the body in 3 forms: free testosterone, albumin-bound testosterone, and testosterone bound to sex hormone-binding globulin (SHBG). In young healthy men, only 1% to 2% of testosterone is free, about 40% is albumin-bound and readily dissociates to free testosterone, and the remainder is tightly bound to SHBG, which does not readily dissociate and is therefore biologically unavailable.5 The amount of SHBG increases with age, decreasing the amount of bioavailable testosterone.
Serum levels of testosterone remain approximately stable until about age 40. After age 40, total levels of testosterone decrease by 1% to 2% annually, and serum free testosterone levels decrease by 2% to 3% annually.6 Testing of free testosterone levels is recommended when a patient falls in the low normal range of total testosterone (see below).
Testosterone screening: How and for whom?
The Endocrine Society, consistent with the American Urological Association and the European Association of Urology, recommends against screening the general population for testosterone deficiency, fearing overdiagnosis and treatment of asymptomatic men.3,7,8
The Endocrine Society’s recommendation for targeted screening states that for men with chronic diseases (eg, diabetes mellitus, end-stage renal disease, and chronic obstructive lung disease), measurement of testosterone may be indicated by symptoms such as sexual dysfunction, unexplained weight loss, weakness, or mobility limitation. The recommendation also states that in men with other conditions (eg, pituitary mass, human immunodeficiency virus (HIV)-associated weight loss, low-trauma fracture, or treatment with medications that affect testosterone production), measurement of testosterone may be indicated, regardless of symptoms.3 The United States Preventive Services Task Force does not have any specific recommendations regarding screening for hypogonadism in men.
Start with total serum testosterone
Measuring total serum testosterone should be the initial test for suspected testosterone deficiency. Testosterone levels vary throughout the day, peaking in the morning. As a result, levels should generally be measured before 10 am.
Lab values to watch for. Again, the lower limit of the normal testosterone range in healthy young men is 280 to 300 ng/dL, but may vary depending on the laboratory or assay used.3 If the level is abnormal (<280 ng/dL), repeat the test at least a month later prior to initiating testosterone replacement.3 For men with values in the low normal range and clinical symptoms, obtain levels of free testosterone to confirm the diagnosis.
Patients with chronic diseases, such as obesity, diabetes mellitus, liver disease, nephrotic syndrome, or thyroid disease, are more likely to have an increase in SHBG. For these patients, check free testosterone levels in the setting of symptoms and a low-to-normal total testosterone level.9 If a patient has symptoms of hypogonadism and a total testosterone level in the low normal range, as well as a free testosterone level that is less than the lower limit of normal for a laboratory (typically around 50 ng/dL), it is reasonable to offer testosterone replacement.
Medications such as glucocorticoids and opioids can affect testosterone levels, as can acute or subacute illness.10 Therefore, do not measure testosterone levels while a patient is receiving these medications, and wait until a patient has recovered from being ill before doing any testing.
Temper your response with older men. Many men >65 years old may have testosterone levels below the normal range for healthy, young counterparts. This decline is of uncertain clinical significance; it remains unclear if lower levels in older men result in health problems. Some have suggested establishing age-adjusted normal values, and recommend not initiating testosterone replacement therapy in older men until serum levels are below 200 ng/dL, rather than 280 ng/dL, which is the generally accepted lower limit for younger populations.3,11,12
Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.
Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17
Testosterone replacement raises issues of abuse and CV risk
On October 25, 2016, the US Food and Drug Administration (FDA) approved class-wide labeling changes for all prescription testosterone products, alerting prescribers to the agent’s abuse potential and the serious cardiac and mental health adverse outcomes that have been reported as a result of such abuse. In addition, the FDA is revising the Abuse and Dependence section to include new safety information regarding the risks associated with abuse of testosterone and other anabolic androgenic steroids.18
Prior to this announcement, the FDA had mandated in 2015 that product labels include information about a possible increased risk of myocardial infarction (MI) and stroke in people using testosterone. This warning was based on 2 published studies that showed increased cardiovascular risk.19,20 However, a third larger study showed no increase in risk.21 All 3 of these studies were retrospective and had methodologic limitations, including differing baseline testosterone levels, insufficient documentation of baseline levels, and inadequate monitoring of response to therapy.
A recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology in response to the older FDA warning cites the need for randomized controlled trials (RCTs) to elucidate whether an association exists between testosterone replacement and cardiovascular risk.22
Of note, researchers have shown that androgen deprivation therapy (ADT) in patients with prostate cancer impacts cardiovascular risk factors (ie, it increases body fat and decreases lean body mass, increases total cholesterol, and increases insulin resistance and risk of diabetes). ADT may also be associated with increased cardiovascular mortality, although data are conflicting.23
Investigators have shown that testosterone replacement positively affects certain risk factors for cardiovascular disease (CVD) including increasing lean muscle mass and improving laboratory values associated with the metabolic syndrome.24 A large retrospective cohort study of male veterans with documented low total testosterone levels who received their medical care at the Veterans Health Administration (VHA) found that those who received testosterone replacement and achieved normal testosterone levels had lower all-cause, cardiovascular, and stroke mortality than controls.21 The men who did not achieve normal testosterone levels also had lower all-cause mortality (but significantly less than those with normalization of serum testosterone levels), but no change in stroke or cardiovascular mortality.
Since this study was retrospective, there were significant limitations, including unknown baseline characteristics of patients in each group. The CVD risks associated with testosterone therapy in middle-aged and older men should be discussed by physicians and their patients on an individual basis. Some experts believe that patients who have had an MI, revascularization, or a stroke within the past 6 months are not good candidates for replacement therapy.25
Until there are better data from prospective RCTs, it may be prudent to make sure that traditional CVD risk factors including smoking, hypertension, hyperlipidemia, and diabetes have been assessed and are appropriately managed in men prescribed testosterone replacement.
Testosterone helps with ED in certain cases
Testosterone deficiency is associated with sexual dysfunction in men, including decreased libido and erectile dysfunction (ED). About 20% to 40% of men with ED will have low testosterone, although replacement does not always improve the condition.2
Current guidelines do not recommend testosterone replacement to treat ED or sexual dysfunction in the absence of a low serum testosterone level and recommend evaluating for other causes of sexual problems in men.3 In one study, men who did not have documented hypogonadism received testosterone replacement therapy for sexual dysfunction including ED or ejaculator dysfunction. These patients saw no improvement in symptoms.26
CORRESPONDENCE
J. Andrew Hoover, MD, Department of Family and Community Medicine, Lancaster General Hospital, 540 North Duke Street, Lancaster, PA 17604; [email protected].
1. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466.
2. PL Detail-Document #311005. The use of testosterone and the aging male. Pharmacist’s Letter/Prescriber’s Letter. October 2015.
3. Bhasin S, Cunningham GR, Hayes FJ, et al. Therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536-2559.
4. Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-1818.
5. Kaufman J, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876.
6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598.
7. American Urological Association. AUA position statement on testosterone therapy. Available at: https://www.auanet.org/education/testosterone-therapy.cfm. Accessed October 24, 2016.
8. Dohle GR, Arver S, Bettocchi C, et al. European Association of Urology. Guidelines on male hypogonadism. 2015. Available at: http://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR1.pdf. Accessed October 24, 2016.
9. Tanna MS, Schwartzbard A, Berger JS, et al. Management of hypogonadism in cardiovascular patients: what are the implications of testosterone therapy on cardiovascular morbidity? Urol Clin North Am. 2016;43:247-260.
10. Matsumoto AM. The testis. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. 4th ed. New York, NY: McGraw-Hill; 2001:635-705.
11. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016:374:611-624.
12. Loughlin KR, Klap J. Selective use of testosterone replacement therapy. J Urol. 2016;196:1340-1341.
13. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650.
14. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500-4510.
16. PL Detail-Document #311005. Comparison of testosterone products. Pharmacists’s Letter/Prescriber’s Letter. October 2015.
17. Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol. 2015;7:378-387.
18. US Food and Drug Administration. Testosterone and other anabolic androgenic steroids (AAS): FDA statement - Risks associated with abuse and dependence. Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm526151.htm. Accessed October 26, 2016.
19. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
20. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone-therapy prescription in men. PLoS One. 2014;9:e85805.
21. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715.
22. Goodman N, Guay A, Dandona P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21:1066-1073.
23. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85:436-443.
24. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-840.
25. Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545-577.
26. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146-151.
Over the past decade, androgen replacement prescriptions for men ≥40 years of age have increased 3-fold, according to one study.1 While one could argue this trend represents greater attention to an underdiagnosed problem, the study of prescription claims for almost 11 million men found that a quarter of them did not have a testosterone level documented in the 12 months prior to receiving treatment.1
At the same time, sales of testosterone products totaled about $2.4 billion dollars in 2013, a number projected to top $4 billion by 2017.2 The increase in prescribing is thought to be due, at least in part, to direct-to-consumer marketing techniques encouraging patients to seek medical attention if they are experiencing non-specific symptoms, such as fatigue and lack of energy, because their “problem” could be due to low testosterone.
Testosterone begins to decrease after age 40
The Endocrine Society defines “androgen deficiency” as low serum testosterone (generally <280 ng/dL for healthy young men) along with signs and symptoms of hypogonadism, including decreased sexual function; loss of axillary and/or pubic hair; low bone mineral density; loss of motivation and/or concentration; poor mood or depression; decline in cognitive function; and loss of muscle strength and work capacity (TABLE 1).3
Primary vs secondary hypogonadism. Primary (or hypogonadotropic) hypogonadism results when the testes fail to produce adequate testosterone in the presence of normal serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Secondary hypogonadism is pituitary or hypothalamic in origin. Patients with primary hypogonadism will have high LH and FSH levels, whereas patients with secondary hypogonadism will have low or normal LH and FSH levels.4 The Endocrine Society recommends checking LH and FSH levels in all patients with hypogonadism to differentiate the primary from the secondary type.3 Patients with late onset primary hypogonadism do not require any further evaluation. In young men, it is important to consider Klinefelter syndrome. This diagnosis can be determined with a karyotype. In patients with secondary hypogonadism, checking serum iron, prolactin, and other pituitary hormones, and getting a magnetic resonance imaging scan of the sella turcica may be indicated. This will rule out infiltrative diseases, such as hemochromatosis, prolactinoma, and hypothalamic or pituitary neoplasm.
Testosterone is present in the body in 3 forms: free testosterone, albumin-bound testosterone, and testosterone bound to sex hormone-binding globulin (SHBG). In young healthy men, only 1% to 2% of testosterone is free, about 40% is albumin-bound and readily dissociates to free testosterone, and the remainder is tightly bound to SHBG, which does not readily dissociate and is therefore biologically unavailable.5 The amount of SHBG increases with age, decreasing the amount of bioavailable testosterone.
Serum levels of testosterone remain approximately stable until about age 40. After age 40, total levels of testosterone decrease by 1% to 2% annually, and serum free testosterone levels decrease by 2% to 3% annually.6 Testing of free testosterone levels is recommended when a patient falls in the low normal range of total testosterone (see below).
Testosterone screening: How and for whom?
The Endocrine Society, consistent with the American Urological Association and the European Association of Urology, recommends against screening the general population for testosterone deficiency, fearing overdiagnosis and treatment of asymptomatic men.3,7,8
The Endocrine Society’s recommendation for targeted screening states that for men with chronic diseases (eg, diabetes mellitus, end-stage renal disease, and chronic obstructive lung disease), measurement of testosterone may be indicated by symptoms such as sexual dysfunction, unexplained weight loss, weakness, or mobility limitation. The recommendation also states that in men with other conditions (eg, pituitary mass, human immunodeficiency virus (HIV)-associated weight loss, low-trauma fracture, or treatment with medications that affect testosterone production), measurement of testosterone may be indicated, regardless of symptoms.3 The United States Preventive Services Task Force does not have any specific recommendations regarding screening for hypogonadism in men.
Start with total serum testosterone
Measuring total serum testosterone should be the initial test for suspected testosterone deficiency. Testosterone levels vary throughout the day, peaking in the morning. As a result, levels should generally be measured before 10 am.
Lab values to watch for. Again, the lower limit of the normal testosterone range in healthy young men is 280 to 300 ng/dL, but may vary depending on the laboratory or assay used.3 If the level is abnormal (<280 ng/dL), repeat the test at least a month later prior to initiating testosterone replacement.3 For men with values in the low normal range and clinical symptoms, obtain levels of free testosterone to confirm the diagnosis.
Patients with chronic diseases, such as obesity, diabetes mellitus, liver disease, nephrotic syndrome, or thyroid disease, are more likely to have an increase in SHBG. For these patients, check free testosterone levels in the setting of symptoms and a low-to-normal total testosterone level.9 If a patient has symptoms of hypogonadism and a total testosterone level in the low normal range, as well as a free testosterone level that is less than the lower limit of normal for a laboratory (typically around 50 ng/dL), it is reasonable to offer testosterone replacement.
Medications such as glucocorticoids and opioids can affect testosterone levels, as can acute or subacute illness.10 Therefore, do not measure testosterone levels while a patient is receiving these medications, and wait until a patient has recovered from being ill before doing any testing.
Temper your response with older men. Many men >65 years old may have testosterone levels below the normal range for healthy, young counterparts. This decline is of uncertain clinical significance; it remains unclear if lower levels in older men result in health problems. Some have suggested establishing age-adjusted normal values, and recommend not initiating testosterone replacement therapy in older men until serum levels are below 200 ng/dL, rather than 280 ng/dL, which is the generally accepted lower limit for younger populations.3,11,12
Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.
Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17
Testosterone replacement raises issues of abuse and CV risk
On October 25, 2016, the US Food and Drug Administration (FDA) approved class-wide labeling changes for all prescription testosterone products, alerting prescribers to the agent’s abuse potential and the serious cardiac and mental health adverse outcomes that have been reported as a result of such abuse. In addition, the FDA is revising the Abuse and Dependence section to include new safety information regarding the risks associated with abuse of testosterone and other anabolic androgenic steroids.18
Prior to this announcement, the FDA had mandated in 2015 that product labels include information about a possible increased risk of myocardial infarction (MI) and stroke in people using testosterone. This warning was based on 2 published studies that showed increased cardiovascular risk.19,20 However, a third larger study showed no increase in risk.21 All 3 of these studies were retrospective and had methodologic limitations, including differing baseline testosterone levels, insufficient documentation of baseline levels, and inadequate monitoring of response to therapy.
A recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology in response to the older FDA warning cites the need for randomized controlled trials (RCTs) to elucidate whether an association exists between testosterone replacement and cardiovascular risk.22
Of note, researchers have shown that androgen deprivation therapy (ADT) in patients with prostate cancer impacts cardiovascular risk factors (ie, it increases body fat and decreases lean body mass, increases total cholesterol, and increases insulin resistance and risk of diabetes). ADT may also be associated with increased cardiovascular mortality, although data are conflicting.23
Investigators have shown that testosterone replacement positively affects certain risk factors for cardiovascular disease (CVD) including increasing lean muscle mass and improving laboratory values associated with the metabolic syndrome.24 A large retrospective cohort study of male veterans with documented low total testosterone levels who received their medical care at the Veterans Health Administration (VHA) found that those who received testosterone replacement and achieved normal testosterone levels had lower all-cause, cardiovascular, and stroke mortality than controls.21 The men who did not achieve normal testosterone levels also had lower all-cause mortality (but significantly less than those with normalization of serum testosterone levels), but no change in stroke or cardiovascular mortality.
Since this study was retrospective, there were significant limitations, including unknown baseline characteristics of patients in each group. The CVD risks associated with testosterone therapy in middle-aged and older men should be discussed by physicians and their patients on an individual basis. Some experts believe that patients who have had an MI, revascularization, or a stroke within the past 6 months are not good candidates for replacement therapy.25
Until there are better data from prospective RCTs, it may be prudent to make sure that traditional CVD risk factors including smoking, hypertension, hyperlipidemia, and diabetes have been assessed and are appropriately managed in men prescribed testosterone replacement.
Testosterone helps with ED in certain cases
Testosterone deficiency is associated with sexual dysfunction in men, including decreased libido and erectile dysfunction (ED). About 20% to 40% of men with ED will have low testosterone, although replacement does not always improve the condition.2
Current guidelines do not recommend testosterone replacement to treat ED or sexual dysfunction in the absence of a low serum testosterone level and recommend evaluating for other causes of sexual problems in men.3 In one study, men who did not have documented hypogonadism received testosterone replacement therapy for sexual dysfunction including ED or ejaculator dysfunction. These patients saw no improvement in symptoms.26
CORRESPONDENCE
J. Andrew Hoover, MD, Department of Family and Community Medicine, Lancaster General Hospital, 540 North Duke Street, Lancaster, PA 17604; [email protected].
Over the past decade, androgen replacement prescriptions for men ≥40 years of age have increased 3-fold, according to one study.1 While one could argue this trend represents greater attention to an underdiagnosed problem, the study of prescription claims for almost 11 million men found that a quarter of them did not have a testosterone level documented in the 12 months prior to receiving treatment.1
At the same time, sales of testosterone products totaled about $2.4 billion dollars in 2013, a number projected to top $4 billion by 2017.2 The increase in prescribing is thought to be due, at least in part, to direct-to-consumer marketing techniques encouraging patients to seek medical attention if they are experiencing non-specific symptoms, such as fatigue and lack of energy, because their “problem” could be due to low testosterone.
Testosterone begins to decrease after age 40
The Endocrine Society defines “androgen deficiency” as low serum testosterone (generally <280 ng/dL for healthy young men) along with signs and symptoms of hypogonadism, including decreased sexual function; loss of axillary and/or pubic hair; low bone mineral density; loss of motivation and/or concentration; poor mood or depression; decline in cognitive function; and loss of muscle strength and work capacity (TABLE 1).3
Primary vs secondary hypogonadism. Primary (or hypogonadotropic) hypogonadism results when the testes fail to produce adequate testosterone in the presence of normal serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Secondary hypogonadism is pituitary or hypothalamic in origin. Patients with primary hypogonadism will have high LH and FSH levels, whereas patients with secondary hypogonadism will have low or normal LH and FSH levels.4 The Endocrine Society recommends checking LH and FSH levels in all patients with hypogonadism to differentiate the primary from the secondary type.3 Patients with late onset primary hypogonadism do not require any further evaluation. In young men, it is important to consider Klinefelter syndrome. This diagnosis can be determined with a karyotype. In patients with secondary hypogonadism, checking serum iron, prolactin, and other pituitary hormones, and getting a magnetic resonance imaging scan of the sella turcica may be indicated. This will rule out infiltrative diseases, such as hemochromatosis, prolactinoma, and hypothalamic or pituitary neoplasm.
Testosterone is present in the body in 3 forms: free testosterone, albumin-bound testosterone, and testosterone bound to sex hormone-binding globulin (SHBG). In young healthy men, only 1% to 2% of testosterone is free, about 40% is albumin-bound and readily dissociates to free testosterone, and the remainder is tightly bound to SHBG, which does not readily dissociate and is therefore biologically unavailable.5 The amount of SHBG increases with age, decreasing the amount of bioavailable testosterone.
Serum levels of testosterone remain approximately stable until about age 40. After age 40, total levels of testosterone decrease by 1% to 2% annually, and serum free testosterone levels decrease by 2% to 3% annually.6 Testing of free testosterone levels is recommended when a patient falls in the low normal range of total testosterone (see below).
Testosterone screening: How and for whom?
The Endocrine Society, consistent with the American Urological Association and the European Association of Urology, recommends against screening the general population for testosterone deficiency, fearing overdiagnosis and treatment of asymptomatic men.3,7,8
The Endocrine Society’s recommendation for targeted screening states that for men with chronic diseases (eg, diabetes mellitus, end-stage renal disease, and chronic obstructive lung disease), measurement of testosterone may be indicated by symptoms such as sexual dysfunction, unexplained weight loss, weakness, or mobility limitation. The recommendation also states that in men with other conditions (eg, pituitary mass, human immunodeficiency virus (HIV)-associated weight loss, low-trauma fracture, or treatment with medications that affect testosterone production), measurement of testosterone may be indicated, regardless of symptoms.3 The United States Preventive Services Task Force does not have any specific recommendations regarding screening for hypogonadism in men.
Start with total serum testosterone
Measuring total serum testosterone should be the initial test for suspected testosterone deficiency. Testosterone levels vary throughout the day, peaking in the morning. As a result, levels should generally be measured before 10 am.
Lab values to watch for. Again, the lower limit of the normal testosterone range in healthy young men is 280 to 300 ng/dL, but may vary depending on the laboratory or assay used.3 If the level is abnormal (<280 ng/dL), repeat the test at least a month later prior to initiating testosterone replacement.3 For men with values in the low normal range and clinical symptoms, obtain levels of free testosterone to confirm the diagnosis.
Patients with chronic diseases, such as obesity, diabetes mellitus, liver disease, nephrotic syndrome, or thyroid disease, are more likely to have an increase in SHBG. For these patients, check free testosterone levels in the setting of symptoms and a low-to-normal total testosterone level.9 If a patient has symptoms of hypogonadism and a total testosterone level in the low normal range, as well as a free testosterone level that is less than the lower limit of normal for a laboratory (typically around 50 ng/dL), it is reasonable to offer testosterone replacement.
Medications such as glucocorticoids and opioids can affect testosterone levels, as can acute or subacute illness.10 Therefore, do not measure testosterone levels while a patient is receiving these medications, and wait until a patient has recovered from being ill before doing any testing.
Temper your response with older men. Many men >65 years old may have testosterone levels below the normal range for healthy, young counterparts. This decline is of uncertain clinical significance; it remains unclear if lower levels in older men result in health problems. Some have suggested establishing age-adjusted normal values, and recommend not initiating testosterone replacement therapy in older men until serum levels are below 200 ng/dL, rather than 280 ng/dL, which is the generally accepted lower limit for younger populations.3,11,12
Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.
Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17
Testosterone replacement raises issues of abuse and CV risk
On October 25, 2016, the US Food and Drug Administration (FDA) approved class-wide labeling changes for all prescription testosterone products, alerting prescribers to the agent’s abuse potential and the serious cardiac and mental health adverse outcomes that have been reported as a result of such abuse. In addition, the FDA is revising the Abuse and Dependence section to include new safety information regarding the risks associated with abuse of testosterone and other anabolic androgenic steroids.18
Prior to this announcement, the FDA had mandated in 2015 that product labels include information about a possible increased risk of myocardial infarction (MI) and stroke in people using testosterone. This warning was based on 2 published studies that showed increased cardiovascular risk.19,20 However, a third larger study showed no increase in risk.21 All 3 of these studies were retrospective and had methodologic limitations, including differing baseline testosterone levels, insufficient documentation of baseline levels, and inadequate monitoring of response to therapy.
A recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology in response to the older FDA warning cites the need for randomized controlled trials (RCTs) to elucidate whether an association exists between testosterone replacement and cardiovascular risk.22
Of note, researchers have shown that androgen deprivation therapy (ADT) in patients with prostate cancer impacts cardiovascular risk factors (ie, it increases body fat and decreases lean body mass, increases total cholesterol, and increases insulin resistance and risk of diabetes). ADT may also be associated with increased cardiovascular mortality, although data are conflicting.23
Investigators have shown that testosterone replacement positively affects certain risk factors for cardiovascular disease (CVD) including increasing lean muscle mass and improving laboratory values associated with the metabolic syndrome.24 A large retrospective cohort study of male veterans with documented low total testosterone levels who received their medical care at the Veterans Health Administration (VHA) found that those who received testosterone replacement and achieved normal testosterone levels had lower all-cause, cardiovascular, and stroke mortality than controls.21 The men who did not achieve normal testosterone levels also had lower all-cause mortality (but significantly less than those with normalization of serum testosterone levels), but no change in stroke or cardiovascular mortality.
Since this study was retrospective, there were significant limitations, including unknown baseline characteristics of patients in each group. The CVD risks associated with testosterone therapy in middle-aged and older men should be discussed by physicians and their patients on an individual basis. Some experts believe that patients who have had an MI, revascularization, or a stroke within the past 6 months are not good candidates for replacement therapy.25
Until there are better data from prospective RCTs, it may be prudent to make sure that traditional CVD risk factors including smoking, hypertension, hyperlipidemia, and diabetes have been assessed and are appropriately managed in men prescribed testosterone replacement.
Testosterone helps with ED in certain cases
Testosterone deficiency is associated with sexual dysfunction in men, including decreased libido and erectile dysfunction (ED). About 20% to 40% of men with ED will have low testosterone, although replacement does not always improve the condition.2
Current guidelines do not recommend testosterone replacement to treat ED or sexual dysfunction in the absence of a low serum testosterone level and recommend evaluating for other causes of sexual problems in men.3 In one study, men who did not have documented hypogonadism received testosterone replacement therapy for sexual dysfunction including ED or ejaculator dysfunction. These patients saw no improvement in symptoms.26
CORRESPONDENCE
J. Andrew Hoover, MD, Department of Family and Community Medicine, Lancaster General Hospital, 540 North Duke Street, Lancaster, PA 17604; [email protected].
1. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466.
2. PL Detail-Document #311005. The use of testosterone and the aging male. Pharmacist’s Letter/Prescriber’s Letter. October 2015.
3. Bhasin S, Cunningham GR, Hayes FJ, et al. Therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536-2559.
4. Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-1818.
5. Kaufman J, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876.
6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598.
7. American Urological Association. AUA position statement on testosterone therapy. Available at: https://www.auanet.org/education/testosterone-therapy.cfm. Accessed October 24, 2016.
8. Dohle GR, Arver S, Bettocchi C, et al. European Association of Urology. Guidelines on male hypogonadism. 2015. Available at: http://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR1.pdf. Accessed October 24, 2016.
9. Tanna MS, Schwartzbard A, Berger JS, et al. Management of hypogonadism in cardiovascular patients: what are the implications of testosterone therapy on cardiovascular morbidity? Urol Clin North Am. 2016;43:247-260.
10. Matsumoto AM. The testis. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. 4th ed. New York, NY: McGraw-Hill; 2001:635-705.
11. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016:374:611-624.
12. Loughlin KR, Klap J. Selective use of testosterone replacement therapy. J Urol. 2016;196:1340-1341.
13. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650.
14. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500-4510.
16. PL Detail-Document #311005. Comparison of testosterone products. Pharmacists’s Letter/Prescriber’s Letter. October 2015.
17. Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol. 2015;7:378-387.
18. US Food and Drug Administration. Testosterone and other anabolic androgenic steroids (AAS): FDA statement - Risks associated with abuse and dependence. Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm526151.htm. Accessed October 26, 2016.
19. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
20. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone-therapy prescription in men. PLoS One. 2014;9:e85805.
21. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715.
22. Goodman N, Guay A, Dandona P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21:1066-1073.
23. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85:436-443.
24. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-840.
25. Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545-577.
26. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146-151.
1. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466.
2. PL Detail-Document #311005. The use of testosterone and the aging male. Pharmacist’s Letter/Prescriber’s Letter. October 2015.
3. Bhasin S, Cunningham GR, Hayes FJ, et al. Therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536-2559.
4. Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-1818.
5. Kaufman J, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876.
6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598.
7. American Urological Association. AUA position statement on testosterone therapy. Available at: https://www.auanet.org/education/testosterone-therapy.cfm. Accessed October 24, 2016.
8. Dohle GR, Arver S, Bettocchi C, et al. European Association of Urology. Guidelines on male hypogonadism. 2015. Available at: http://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR1.pdf. Accessed October 24, 2016.
9. Tanna MS, Schwartzbard A, Berger JS, et al. Management of hypogonadism in cardiovascular patients: what are the implications of testosterone therapy on cardiovascular morbidity? Urol Clin North Am. 2016;43:247-260.
10. Matsumoto AM. The testis. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. 4th ed. New York, NY: McGraw-Hill; 2001:635-705.
11. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016:374:611-624.
12. Loughlin KR, Klap J. Selective use of testosterone replacement therapy. J Urol. 2016;196:1340-1341.
13. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650.
14. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500-4510.
16. PL Detail-Document #311005. Comparison of testosterone products. Pharmacists’s Letter/Prescriber’s Letter. October 2015.
17. Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol. 2015;7:378-387.
18. US Food and Drug Administration. Testosterone and other anabolic androgenic steroids (AAS): FDA statement - Risks associated with abuse and dependence. Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm526151.htm. Accessed October 26, 2016.
19. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
20. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone-therapy prescription in men. PLoS One. 2014;9:e85805.
21. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715.
22. Goodman N, Guay A, Dandona P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21:1066-1073.
23. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85:436-443.
24. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-840.
25. Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545-577.
26. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146-151.
PRACTICE RECOMMENDATIONS
› Confirm suspected hypogonadism by getting 2 serum testosterone levels at least one month apart prior to initiating testosterone replacement therapy. B
› Consider testosterone replacement therapy when there is both laboratory and clinical evidence of hypogonadism. B
› Offer testosterone replacement to older men (≥65 years) with hypogonadism only after talking to them about the risks and benefits. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Antibiotic stewardship: The FP’s role
Any use of antibiotics can promote the development of drug resistance, but antibiotic misuse is far more likely to lead to superinfections, allergic reactions, and adverse events. These are pressing concerns in ambulatory care, as well as in inpatient settings, but efforts to address unnecessary and inappropriate use of antibiotics have been more focused on the latter.1
A US study published in 2013 found that, in one year alone, nearly 260 million courses of antibiotics were prescribed to patients in ambulatory care. Patients <2 years or >65 years of age had the highest antibiotic prescription rates, and practitioners in family medicine were the most likely to prescribe them.2 Yet evidence suggests that more than half of all community-based antibiotic prescriptions may be unnecessary or inappropriate—the combined result of patient, physician, and health system factors.3 The continuing shift of medical services from acute care to community-based settings has contributed to an increase in antibiotic-resistant infections in the community, as well.
Skin infections caused by Staphylococcus aureus, respiratory infections caused by Streptococcus pneumoniae, urinary tract infections (UTIs) caused by Escherichia coli and other Enterobacteriaceae, and sexually transmitted diseases such as gonorrhea are recent examples of community-transmitted infections for which increases in antibiotic resistance rates have been reported.4 It is crucial for primary care physicians to know when watchful waiting is appropriate, when antibiotics are indicated, and, when needed, which antimicrobials are most likely to be effective. The case studies, text, and tables that follow can be used as a guide.
CASE 1 ›
Ear tugging, fever, and upper respiratory symptoms
Two-year-old Daniel is brought in to see his family physician. His mother reports that he has been tugging at his right ear for the past 24 hours. Daniel also has upper respiratory symptoms and an axial temperature of 101.9°F. He had one episode of vomiting, which kept him out of day care today. The patient’s past medical history is significant for surgery to repair an atrial septal defect 15 months ago.
Daniel takes no medication, has no drug allergies, and his immunizations are up to date. He was given a course of antibiotics to treat otitis media 2 months ago. A physical exam reveals an irritable but normally developing child. An otoscopic exam reveals reddened tympanic membranes bilaterally with normal mobility.
CASE 2 ›
Dysuria and urethral discharge
Twenty-year-old Jon F visits his FP and reports painful urination and purulent urethral discharge of 3 days’ duration. He denies having flank pain. When asked about his sexual history, Mr. F acknowledges having had unprotected intercourse with a sex worker less than 2 weeks ago.
The patient’s past medical history is unremarkable. He reports smoking marijuana occasionally, but denies other recreational drug use.
He tested negative for human immunodeficiency virus (HIV) 18 months ago, but says he has used condoms inconsistently since then. A physical exam reveals normal vital signs, with no sores or rashes. His chest, heart, musculoskeletal, abdominal, and rectal exams are normal. A genital exam reveals a normal circumcised penis, with reddened meatus and purulent discharge; the scrotum and testes are normal and without lesions, tenderness, or masses.
If Daniel and Jon F were your patients, would you prescribe antibiotics for them—and if so, what drugs would you select?
How to manage community-acquired MRSA
Methicillin-resistant S aureus (MRSA) is a common antimicrobial-resistant pathogen found in health care settings and in the community. While most community-acquired infections are minor and involve the skin and soft tissues, community-acquired (CA)-MRSA can cause pneumonia, thrombophlebitis, and necrotizing fasciitis.5
Identifying patients at risk
Individuals who share personal items, such as toothbrushes or razors, or have physical contact with anyone who is infected with or who carries the multidrug-resistant pathogen (eg, those spending time in crowded spaces like prisons, schools, or day care centers) are at increased risk for CA-MRSA. So, too, are intravenous (IV) drug users, men who have sex with men, individuals who have repeated skin trauma or contact with animals, and those of Native American, African-American, and Pacific Islander descent.5
MRSA resistance mechanisms include expression of altered penicillin-binding proteins, which have a reduced affinity for beta-lactam antibiotics. MRSA isolates may also be resistant to macrolides, aminoglycosides, fluoroquinolones, and clindamycin. CA-MRSA is not typically associated with the same degree of multidrug resistance as hospital-acquired strains. Instead, CA-MRSA usually produces a toxin known as Panton-Valentine leukocidin (PVL), which leads to leukocyte destruction and local tissue necrosis.6
Uncomplicated CA-MRSA skin and soft-tissue infections usually respond to incision and drainage, provided the patient (or parent) is instructed in and adheres to the recommended hygiene and wound care provisions. Antimicrobial agents are generally reserved for patients who have extensive disease involving multiple infection sites and/or systemic symptoms, purulent cellulitis without drainable foci, or septic phlebitis, or failed to respond to incision and drainage alone. Extreme age is a reasonable indication for antibiotics as well. Patients with more serious conditions, such as pneumonia, thrombophlebitis, or necrotizing fasciitis, require high acuity care and IV antibiotics.7
First-line empiric therapy in the outpatient setting should be either trimethoprim/sulfamethoxazole (TMP/SMX) or a long-acting tetracycline (TABLE 1).7 The ideal adult dose of TMP/SMX is 2 double-strength tablets (160/800 mg) twice daily for 5 to 10 days, as treatment failures are more prevalent with lower doses. Tetracyclines should be avoided in patients younger than 8 years.7
Clindamycin is an option in settings in which isolates can be tested to ensure a lack of inducible resistance; it is also the preferred agent for pregnant women.7 Topical mupirocin may be suitable for children with minor skin infections or secondarily infected skin lesions. Oral linezolid and tedizolid can also be used to treat CA-MRSA, but cost and the potential for drug interaction may prohibit their use. Linezolid inhibits PVL toxin production, however, and may be useful in more serious infections, such as necrotizing fasciitis.7
Strategies for preventing recurrent infection include personal and environmental hygiene measures for patients and close contacts. Decolonization strategies such as application of intranasal mupirocin 2% and washing with chlorhexidine soap may also be considered.7
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13
Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19
High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1
How best to manage uncomplicated cystitis
Treating uncomplicated cystitis is challenging for a number of reasons, including increasing gram-negative resistance, a lack of surveillance data describing local outpatient resistance rates, and limited reliable oral options. UTIs caused by resistant organisms, such as ESBL-producing Enterobacteriaceae, are associated with recent antibiotic use, recurrent UTIs, recent hospitalization, advanced age, multiple comorbidities, hemodialysis, recent international travel, and urinary catheterization.23,24 Urine cultures and susceptibilities should be included in an assessment of patients with any of these risk factors and used to inform antibiotic selection.24 First-line treatment options for uncomplicated cystitis include nitrofurantoin, TMP/SMX (in regions where the uropathogen resistance is <20%), and fosfomycin.25
Multidrug-resistant pathogens
Nitrofurantoin, fosfomycin, and in some instances, fluoroquinolones, are options for treating multidrug-resistant uropathogens, as guided by susceptibility results (TABLE 4).24,26-28 IV antibiotics may be necessary for patients who have severe infections or live in long-term care facilities.24,29 UTIs caused by carbapenem-resistant Enterobacteriaceae are fortunately still rare in outpatient settings.30 There is a lack of high-quality evidence describing best practices for treating infections caused by ESBL-producing bacteria in the community; therefore, antimicrobial selection should be based on infection severity and patient-specific factors.1
Neisseria gonorrhoeae is multidrug-resistant
CASE 2 › Jon F
Mr. F’s physician suspects gonorrhea, although chlamydia cannot be ruled out, and orders a urethral culture, first-catch urine test using nucleic acid amplification, syphilis, and HIV tests. The physician administers ceftriaxone 250 mg IM and azithromycin 1 g PO, instructs the patient to contact all sex partners within the last 60 days, and emphasizes the importance of using latex condoms consistently.
Three days later, Jon returns, complaining that his symptoms have not improved. The urethral culture shows a gram-negative oxidase-positive diplococcus and testing confirms N gonorrhoeae. The lab results also reveal a high cephalosporin minimal inhibitory concentration, negative tests for chlamydia and HIV, and a non-reactive rapid plasma reagin.
Gonococcal infections are the second most common communicable disease reported in the United States, with some 820,000 new cases annually.31 Sites of infection include the cervix, urethra, and rectum, and less commonly, the pharynx, conjunctiva, joints, meninges, and endocardium. Those at risk for gonorrhea are: 31
- women who are sexually active and <25 years of age
- any woman with a new sex partner
- any individual with multiple sex partners or a partner with concurrent partners and/or a known sexually transmitted infection (STI)
- an individual who has a history of inconsistent condom use in non-monogamous relationships
- sex workers.
SIDEBAR
Combatting antibiotic resistance: A call to action for FPs
- Use the best available evidence to determine when antibiotics are indicated.
- Develop and use “antibiograms”—tools that use data from patient specimens to identify patterns of antibiotic susceptibility and guide appropriate empiric antibiotic selection.
- Use rapid diagnostic assays to differentiate between viral and bacterial infections and identify resistant bacteria.
- Add antibiotic use to the list of metrics your practice uses to assess clinician performance.
- Adopt standard, clear language to explain to patients and parents when—and why—antibiotics are not indicated.
- Support public health surveillance efforts by learning and adhering to reporting requirements in your community.
The difficulty of treating gonorrhea
Cephalosporins are now the only class of antimicrobials with reliable activity against N gonorrhoeae. The Centers for Disease Control and Prevention (CDC) no longer recommends fluoroquinolones due to increasing resistance. However, cefixime-resistant strains of gonorrhea and treatment failures have been reported, and the drug is no longer recommended for gonorrhea treatment.31
Combination therapy with one dose of ceftriaxone 250 mg IM and one dose of azithromycin 1 g orally is recommended by the CDC,32 as combination therapy improves efficacy and delays the development of resistance. Azithromycin is preferred over doxycycline as the second agent. That’s both because of a higher prevalence of tetracycline resistance among circulating gonococci and azithromycin’s activity against Chlamydia trachomatis, as patients with gonococcal infections are frequently co-infected with this organism.31
Suspected treatment failures are more likely caused by re-infection than drug resistance. If resistance is suspected, however, physicians should seek guidance from an infectious diseases specialist or the CDC on repeat cultures, susceptibility testing, and antimicrobial therapy.
Two treatment regimens have demonstrated efficacy against cephalosporin-resistant N gonorrhoeae:31,32
- A single dose of gemifloxacin 320 mg PO plus azithromycin 2 g PO or
- a single dose of gentamicin 240 mg IM plus azithromycin 2 g PO.
CASE 2 › Jon F
Mr. F’s physician consults with an infectious disease specialist at the local hospital, who confirms his suspicion that the patient has been infected with antibiotic-resistant N gonorrhoeae. The physician administers gentamicin 240 mg IM plus azithromycin 2 g orally, and warns the patient that he may experience gastrointestinal adverse effects. The physician reports the case, as required, to the local health department.
Antibiotic stewardship: What’s being done…
Efforts to improve antibiotic use in the community setting include a variety of strategies, including academic detailing (ie, evidence-based promotion of drug therapies rather than promotion by manufacturer representatives), patient education, clinical decision support, multi-faceted programs using a combination of interventions, and local and national campaigns.33-36 Recent examples of successful campaigns include the American Board of Internal Medicine Foundation’s Choosing Wisely initiative (choosingwisely.org) and the CDC’s Get Smart program (cdc.gov/getsmart/community/index.html).
Get Smart promotes appropriate antibiotic use by prescribers and aims to decrease demand for antibiotics by patients and promote adherence to prescribed antibiotic regimens. Studies have found that incorporating treatment algorithms and clinical decision support systems into existing electronic medical record systems has led to more appropriate prescribing.37-39
The most effective interventions target both patients/parents and prescribers, provide evidence-based prescribing prompts, require prescribers to justify antibiotic use, and involve clinicians in their design. Future directions should include collaboration with municipal or regional public health organizations to identify community-wide critical infections and resistance trends and strategies that use behavioral interventions to address inappropriate prescribing.40
…and what you can do
Family physicians are key to determining the outcome of the war against antibiotic resistance. See “Combatting antibiotic resistance: A call to action for FPs” on the previous page for specific interventions you can initiate without delay.
The tide will turn only through modification of both prescriber and patient behavior and formalized programs in our communities. Education about appropriate use needs to be included in medical school curricula and continue in the clinic setting through education of physicians in training, medical students, and office staff.41 Become an advocate by promoting the principles of optimal antibiotic stewardship as outlined by the recent IDSA Guidelines for Implementing an Antibiotic Stewardship Program.42 Go to http://cid.oxfordjournals.org/content/62/10/1197.long to learn more.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy Practice & Administration, School of Pharmacy, University of Saint Joseph, 229 Trumbull Street, Hartford, CT 06103; [email protected].
1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
2. Hicks DO, Taylor TH. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461-1462.
3. Fleming-Dutra KE, Hersh, A, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 31, 2016.
5. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
6. Chambers HF. Methicillin-resistant Staphylococcus aureus: mechanisms of resistance and implications for treatment. Postgrad Med. 2001;109:43-50.
7. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;5:285-292.
8. Couch KA, Geide T. ASHP therapeutic position statement on strategies for identifying and preventing pneumococcal resistance. Am J Health-Syst Pharm. 2014;71:417-424.
9. Centers for Disease Control and Prevention (2010) ABCs report: Streptococcus pneumoniae. Available at: www.cdc.gov/abcs/reports-findings/survreports/spneu10.html. Accessed October 28, 2016.
10. Pallares R, Fenoll A, Linares J for the Spanish Pneumococcal Infection Study Network. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides, and fluoroquinolones. Int J Antimicrob Agents. 2003;22(suppl):S15-S24.
11. US Food and Drug Administration. The benefits and risks of systemic fluoroquinolone antibacterial drugs for the treatment of acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in patients who have chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTI). 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 28, 2016.
12. Nuorti JP, Butler JC, Farley NM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl):S27-S72.
14. Gwaltney JM Jr, Wiesinger BA, Patrieb JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233.
15. Diekema DJ, Brueggemann AB, Doern GV. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg Infect Dis. 2000;6:552-556.
16. Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631-639.
17. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e1-e41.
18. Lieberthal AS, Caroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
19. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
20. Sanchez GV, Adams SJ, Baird AM, et al. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother. 2013:68:1838-1841.
21. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738.
22. Gupta K, Hooten TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
23. Ben-Ami R, Rodrıguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690.
24. Walker E, Lyman A, Gupta K, et al. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960-965.
25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
26. Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin N Am. 2014;28:49-59.
27. Neuner EA, Sekeres J, Hall GS, et al. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56:5744-5748.
28. Tasbakan MI, Pullukcu H, Sipahi OR, et al. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554-556.
29. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;268:1897-1902.
30. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479-1487.
31. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64:1-137.
32. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
33. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
34. Belongia EA, Sullivan BJ, Chyou, PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108;575-583.
35. Gjelstad S, Hoye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ. 2013;347:f4403.
36. Huttner B, Harbarth S. ‘Antibiotics are not automatic anymore’’—the French national campaign to cut antibiotic overuse. PLoS Med. 2009;6: e1000080.
37. Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
38. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.
40. Meeker D, Linder JA, Fox CR, et al
41. Schwartz BS, Armstrong WS, Ohl CA, et al. Create allies, IDSA stewardship commitments should prioritize health professions learners. Clin Infect Dis. 2015; 61:1626-1627.
42. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program:guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:1-27.
Any use of antibiotics can promote the development of drug resistance, but antibiotic misuse is far more likely to lead to superinfections, allergic reactions, and adverse events. These are pressing concerns in ambulatory care, as well as in inpatient settings, but efforts to address unnecessary and inappropriate use of antibiotics have been more focused on the latter.1
A US study published in 2013 found that, in one year alone, nearly 260 million courses of antibiotics were prescribed to patients in ambulatory care. Patients <2 years or >65 years of age had the highest antibiotic prescription rates, and practitioners in family medicine were the most likely to prescribe them.2 Yet evidence suggests that more than half of all community-based antibiotic prescriptions may be unnecessary or inappropriate—the combined result of patient, physician, and health system factors.3 The continuing shift of medical services from acute care to community-based settings has contributed to an increase in antibiotic-resistant infections in the community, as well.
Skin infections caused by Staphylococcus aureus, respiratory infections caused by Streptococcus pneumoniae, urinary tract infections (UTIs) caused by Escherichia coli and other Enterobacteriaceae, and sexually transmitted diseases such as gonorrhea are recent examples of community-transmitted infections for which increases in antibiotic resistance rates have been reported.4 It is crucial for primary care physicians to know when watchful waiting is appropriate, when antibiotics are indicated, and, when needed, which antimicrobials are most likely to be effective. The case studies, text, and tables that follow can be used as a guide.
CASE 1 ›
Ear tugging, fever, and upper respiratory symptoms
Two-year-old Daniel is brought in to see his family physician. His mother reports that he has been tugging at his right ear for the past 24 hours. Daniel also has upper respiratory symptoms and an axial temperature of 101.9°F. He had one episode of vomiting, which kept him out of day care today. The patient’s past medical history is significant for surgery to repair an atrial septal defect 15 months ago.
Daniel takes no medication, has no drug allergies, and his immunizations are up to date. He was given a course of antibiotics to treat otitis media 2 months ago. A physical exam reveals an irritable but normally developing child. An otoscopic exam reveals reddened tympanic membranes bilaterally with normal mobility.
CASE 2 ›
Dysuria and urethral discharge
Twenty-year-old Jon F visits his FP and reports painful urination and purulent urethral discharge of 3 days’ duration. He denies having flank pain. When asked about his sexual history, Mr. F acknowledges having had unprotected intercourse with a sex worker less than 2 weeks ago.
The patient’s past medical history is unremarkable. He reports smoking marijuana occasionally, but denies other recreational drug use.
He tested negative for human immunodeficiency virus (HIV) 18 months ago, but says he has used condoms inconsistently since then. A physical exam reveals normal vital signs, with no sores or rashes. His chest, heart, musculoskeletal, abdominal, and rectal exams are normal. A genital exam reveals a normal circumcised penis, with reddened meatus and purulent discharge; the scrotum and testes are normal and without lesions, tenderness, or masses.
If Daniel and Jon F were your patients, would you prescribe antibiotics for them—and if so, what drugs would you select?
How to manage community-acquired MRSA
Methicillin-resistant S aureus (MRSA) is a common antimicrobial-resistant pathogen found in health care settings and in the community. While most community-acquired infections are minor and involve the skin and soft tissues, community-acquired (CA)-MRSA can cause pneumonia, thrombophlebitis, and necrotizing fasciitis.5
Identifying patients at risk
Individuals who share personal items, such as toothbrushes or razors, or have physical contact with anyone who is infected with or who carries the multidrug-resistant pathogen (eg, those spending time in crowded spaces like prisons, schools, or day care centers) are at increased risk for CA-MRSA. So, too, are intravenous (IV) drug users, men who have sex with men, individuals who have repeated skin trauma or contact with animals, and those of Native American, African-American, and Pacific Islander descent.5
MRSA resistance mechanisms include expression of altered penicillin-binding proteins, which have a reduced affinity for beta-lactam antibiotics. MRSA isolates may also be resistant to macrolides, aminoglycosides, fluoroquinolones, and clindamycin. CA-MRSA is not typically associated with the same degree of multidrug resistance as hospital-acquired strains. Instead, CA-MRSA usually produces a toxin known as Panton-Valentine leukocidin (PVL), which leads to leukocyte destruction and local tissue necrosis.6
Uncomplicated CA-MRSA skin and soft-tissue infections usually respond to incision and drainage, provided the patient (or parent) is instructed in and adheres to the recommended hygiene and wound care provisions. Antimicrobial agents are generally reserved for patients who have extensive disease involving multiple infection sites and/or systemic symptoms, purulent cellulitis without drainable foci, or septic phlebitis, or failed to respond to incision and drainage alone. Extreme age is a reasonable indication for antibiotics as well. Patients with more serious conditions, such as pneumonia, thrombophlebitis, or necrotizing fasciitis, require high acuity care and IV antibiotics.7
First-line empiric therapy in the outpatient setting should be either trimethoprim/sulfamethoxazole (TMP/SMX) or a long-acting tetracycline (TABLE 1).7 The ideal adult dose of TMP/SMX is 2 double-strength tablets (160/800 mg) twice daily for 5 to 10 days, as treatment failures are more prevalent with lower doses. Tetracyclines should be avoided in patients younger than 8 years.7
Clindamycin is an option in settings in which isolates can be tested to ensure a lack of inducible resistance; it is also the preferred agent for pregnant women.7 Topical mupirocin may be suitable for children with minor skin infections or secondarily infected skin lesions. Oral linezolid and tedizolid can also be used to treat CA-MRSA, but cost and the potential for drug interaction may prohibit their use. Linezolid inhibits PVL toxin production, however, and may be useful in more serious infections, such as necrotizing fasciitis.7
Strategies for preventing recurrent infection include personal and environmental hygiene measures for patients and close contacts. Decolonization strategies such as application of intranasal mupirocin 2% and washing with chlorhexidine soap may also be considered.7
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13
Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19
High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1
How best to manage uncomplicated cystitis
Treating uncomplicated cystitis is challenging for a number of reasons, including increasing gram-negative resistance, a lack of surveillance data describing local outpatient resistance rates, and limited reliable oral options. UTIs caused by resistant organisms, such as ESBL-producing Enterobacteriaceae, are associated with recent antibiotic use, recurrent UTIs, recent hospitalization, advanced age, multiple comorbidities, hemodialysis, recent international travel, and urinary catheterization.23,24 Urine cultures and susceptibilities should be included in an assessment of patients with any of these risk factors and used to inform antibiotic selection.24 First-line treatment options for uncomplicated cystitis include nitrofurantoin, TMP/SMX (in regions where the uropathogen resistance is <20%), and fosfomycin.25
Multidrug-resistant pathogens
Nitrofurantoin, fosfomycin, and in some instances, fluoroquinolones, are options for treating multidrug-resistant uropathogens, as guided by susceptibility results (TABLE 4).24,26-28 IV antibiotics may be necessary for patients who have severe infections or live in long-term care facilities.24,29 UTIs caused by carbapenem-resistant Enterobacteriaceae are fortunately still rare in outpatient settings.30 There is a lack of high-quality evidence describing best practices for treating infections caused by ESBL-producing bacteria in the community; therefore, antimicrobial selection should be based on infection severity and patient-specific factors.1
Neisseria gonorrhoeae is multidrug-resistant
CASE 2 › Jon F
Mr. F’s physician suspects gonorrhea, although chlamydia cannot be ruled out, and orders a urethral culture, first-catch urine test using nucleic acid amplification, syphilis, and HIV tests. The physician administers ceftriaxone 250 mg IM and azithromycin 1 g PO, instructs the patient to contact all sex partners within the last 60 days, and emphasizes the importance of using latex condoms consistently.
Three days later, Jon returns, complaining that his symptoms have not improved. The urethral culture shows a gram-negative oxidase-positive diplococcus and testing confirms N gonorrhoeae. The lab results also reveal a high cephalosporin minimal inhibitory concentration, negative tests for chlamydia and HIV, and a non-reactive rapid plasma reagin.
Gonococcal infections are the second most common communicable disease reported in the United States, with some 820,000 new cases annually.31 Sites of infection include the cervix, urethra, and rectum, and less commonly, the pharynx, conjunctiva, joints, meninges, and endocardium. Those at risk for gonorrhea are: 31
- women who are sexually active and <25 years of age
- any woman with a new sex partner
- any individual with multiple sex partners or a partner with concurrent partners and/or a known sexually transmitted infection (STI)
- an individual who has a history of inconsistent condom use in non-monogamous relationships
- sex workers.
SIDEBAR
Combatting antibiotic resistance: A call to action for FPs
- Use the best available evidence to determine when antibiotics are indicated.
- Develop and use “antibiograms”—tools that use data from patient specimens to identify patterns of antibiotic susceptibility and guide appropriate empiric antibiotic selection.
- Use rapid diagnostic assays to differentiate between viral and bacterial infections and identify resistant bacteria.
- Add antibiotic use to the list of metrics your practice uses to assess clinician performance.
- Adopt standard, clear language to explain to patients and parents when—and why—antibiotics are not indicated.
- Support public health surveillance efforts by learning and adhering to reporting requirements in your community.
The difficulty of treating gonorrhea
Cephalosporins are now the only class of antimicrobials with reliable activity against N gonorrhoeae. The Centers for Disease Control and Prevention (CDC) no longer recommends fluoroquinolones due to increasing resistance. However, cefixime-resistant strains of gonorrhea and treatment failures have been reported, and the drug is no longer recommended for gonorrhea treatment.31
Combination therapy with one dose of ceftriaxone 250 mg IM and one dose of azithromycin 1 g orally is recommended by the CDC,32 as combination therapy improves efficacy and delays the development of resistance. Azithromycin is preferred over doxycycline as the second agent. That’s both because of a higher prevalence of tetracycline resistance among circulating gonococci and azithromycin’s activity against Chlamydia trachomatis, as patients with gonococcal infections are frequently co-infected with this organism.31
Suspected treatment failures are more likely caused by re-infection than drug resistance. If resistance is suspected, however, physicians should seek guidance from an infectious diseases specialist or the CDC on repeat cultures, susceptibility testing, and antimicrobial therapy.
Two treatment regimens have demonstrated efficacy against cephalosporin-resistant N gonorrhoeae:31,32
- A single dose of gemifloxacin 320 mg PO plus azithromycin 2 g PO or
- a single dose of gentamicin 240 mg IM plus azithromycin 2 g PO.
CASE 2 › Jon F
Mr. F’s physician consults with an infectious disease specialist at the local hospital, who confirms his suspicion that the patient has been infected with antibiotic-resistant N gonorrhoeae. The physician administers gentamicin 240 mg IM plus azithromycin 2 g orally, and warns the patient that he may experience gastrointestinal adverse effects. The physician reports the case, as required, to the local health department.
Antibiotic stewardship: What’s being done…
Efforts to improve antibiotic use in the community setting include a variety of strategies, including academic detailing (ie, evidence-based promotion of drug therapies rather than promotion by manufacturer representatives), patient education, clinical decision support, multi-faceted programs using a combination of interventions, and local and national campaigns.33-36 Recent examples of successful campaigns include the American Board of Internal Medicine Foundation’s Choosing Wisely initiative (choosingwisely.org) and the CDC’s Get Smart program (cdc.gov/getsmart/community/index.html).
Get Smart promotes appropriate antibiotic use by prescribers and aims to decrease demand for antibiotics by patients and promote adherence to prescribed antibiotic regimens. Studies have found that incorporating treatment algorithms and clinical decision support systems into existing electronic medical record systems has led to more appropriate prescribing.37-39
The most effective interventions target both patients/parents and prescribers, provide evidence-based prescribing prompts, require prescribers to justify antibiotic use, and involve clinicians in their design. Future directions should include collaboration with municipal or regional public health organizations to identify community-wide critical infections and resistance trends and strategies that use behavioral interventions to address inappropriate prescribing.40
…and what you can do
Family physicians are key to determining the outcome of the war against antibiotic resistance. See “Combatting antibiotic resistance: A call to action for FPs” on the previous page for specific interventions you can initiate without delay.
The tide will turn only through modification of both prescriber and patient behavior and formalized programs in our communities. Education about appropriate use needs to be included in medical school curricula and continue in the clinic setting through education of physicians in training, medical students, and office staff.41 Become an advocate by promoting the principles of optimal antibiotic stewardship as outlined by the recent IDSA Guidelines for Implementing an Antibiotic Stewardship Program.42 Go to http://cid.oxfordjournals.org/content/62/10/1197.long to learn more.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy Practice & Administration, School of Pharmacy, University of Saint Joseph, 229 Trumbull Street, Hartford, CT 06103; [email protected].
Any use of antibiotics can promote the development of drug resistance, but antibiotic misuse is far more likely to lead to superinfections, allergic reactions, and adverse events. These are pressing concerns in ambulatory care, as well as in inpatient settings, but efforts to address unnecessary and inappropriate use of antibiotics have been more focused on the latter.1
A US study published in 2013 found that, in one year alone, nearly 260 million courses of antibiotics were prescribed to patients in ambulatory care. Patients <2 years or >65 years of age had the highest antibiotic prescription rates, and practitioners in family medicine were the most likely to prescribe them.2 Yet evidence suggests that more than half of all community-based antibiotic prescriptions may be unnecessary or inappropriate—the combined result of patient, physician, and health system factors.3 The continuing shift of medical services from acute care to community-based settings has contributed to an increase in antibiotic-resistant infections in the community, as well.
Skin infections caused by Staphylococcus aureus, respiratory infections caused by Streptococcus pneumoniae, urinary tract infections (UTIs) caused by Escherichia coli and other Enterobacteriaceae, and sexually transmitted diseases such as gonorrhea are recent examples of community-transmitted infections for which increases in antibiotic resistance rates have been reported.4 It is crucial for primary care physicians to know when watchful waiting is appropriate, when antibiotics are indicated, and, when needed, which antimicrobials are most likely to be effective. The case studies, text, and tables that follow can be used as a guide.
CASE 1 ›
Ear tugging, fever, and upper respiratory symptoms
Two-year-old Daniel is brought in to see his family physician. His mother reports that he has been tugging at his right ear for the past 24 hours. Daniel also has upper respiratory symptoms and an axial temperature of 101.9°F. He had one episode of vomiting, which kept him out of day care today. The patient’s past medical history is significant for surgery to repair an atrial septal defect 15 months ago.
Daniel takes no medication, has no drug allergies, and his immunizations are up to date. He was given a course of antibiotics to treat otitis media 2 months ago. A physical exam reveals an irritable but normally developing child. An otoscopic exam reveals reddened tympanic membranes bilaterally with normal mobility.
CASE 2 ›
Dysuria and urethral discharge
Twenty-year-old Jon F visits his FP and reports painful urination and purulent urethral discharge of 3 days’ duration. He denies having flank pain. When asked about his sexual history, Mr. F acknowledges having had unprotected intercourse with a sex worker less than 2 weeks ago.
The patient’s past medical history is unremarkable. He reports smoking marijuana occasionally, but denies other recreational drug use.
He tested negative for human immunodeficiency virus (HIV) 18 months ago, but says he has used condoms inconsistently since then. A physical exam reveals normal vital signs, with no sores or rashes. His chest, heart, musculoskeletal, abdominal, and rectal exams are normal. A genital exam reveals a normal circumcised penis, with reddened meatus and purulent discharge; the scrotum and testes are normal and without lesions, tenderness, or masses.
If Daniel and Jon F were your patients, would you prescribe antibiotics for them—and if so, what drugs would you select?
How to manage community-acquired MRSA
Methicillin-resistant S aureus (MRSA) is a common antimicrobial-resistant pathogen found in health care settings and in the community. While most community-acquired infections are minor and involve the skin and soft tissues, community-acquired (CA)-MRSA can cause pneumonia, thrombophlebitis, and necrotizing fasciitis.5
Identifying patients at risk
Individuals who share personal items, such as toothbrushes or razors, or have physical contact with anyone who is infected with or who carries the multidrug-resistant pathogen (eg, those spending time in crowded spaces like prisons, schools, or day care centers) are at increased risk for CA-MRSA. So, too, are intravenous (IV) drug users, men who have sex with men, individuals who have repeated skin trauma or contact with animals, and those of Native American, African-American, and Pacific Islander descent.5
MRSA resistance mechanisms include expression of altered penicillin-binding proteins, which have a reduced affinity for beta-lactam antibiotics. MRSA isolates may also be resistant to macrolides, aminoglycosides, fluoroquinolones, and clindamycin. CA-MRSA is not typically associated with the same degree of multidrug resistance as hospital-acquired strains. Instead, CA-MRSA usually produces a toxin known as Panton-Valentine leukocidin (PVL), which leads to leukocyte destruction and local tissue necrosis.6
Uncomplicated CA-MRSA skin and soft-tissue infections usually respond to incision and drainage, provided the patient (or parent) is instructed in and adheres to the recommended hygiene and wound care provisions. Antimicrobial agents are generally reserved for patients who have extensive disease involving multiple infection sites and/or systemic symptoms, purulent cellulitis without drainable foci, or septic phlebitis, or failed to respond to incision and drainage alone. Extreme age is a reasonable indication for antibiotics as well. Patients with more serious conditions, such as pneumonia, thrombophlebitis, or necrotizing fasciitis, require high acuity care and IV antibiotics.7
First-line empiric therapy in the outpatient setting should be either trimethoprim/sulfamethoxazole (TMP/SMX) or a long-acting tetracycline (TABLE 1).7 The ideal adult dose of TMP/SMX is 2 double-strength tablets (160/800 mg) twice daily for 5 to 10 days, as treatment failures are more prevalent with lower doses. Tetracyclines should be avoided in patients younger than 8 years.7
Clindamycin is an option in settings in which isolates can be tested to ensure a lack of inducible resistance; it is also the preferred agent for pregnant women.7 Topical mupirocin may be suitable for children with minor skin infections or secondarily infected skin lesions. Oral linezolid and tedizolid can also be used to treat CA-MRSA, but cost and the potential for drug interaction may prohibit their use. Linezolid inhibits PVL toxin production, however, and may be useful in more serious infections, such as necrotizing fasciitis.7
Strategies for preventing recurrent infection include personal and environmental hygiene measures for patients and close contacts. Decolonization strategies such as application of intranasal mupirocin 2% and washing with chlorhexidine soap may also be considered.7
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13
Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19
High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1
How best to manage uncomplicated cystitis
Treating uncomplicated cystitis is challenging for a number of reasons, including increasing gram-negative resistance, a lack of surveillance data describing local outpatient resistance rates, and limited reliable oral options. UTIs caused by resistant organisms, such as ESBL-producing Enterobacteriaceae, are associated with recent antibiotic use, recurrent UTIs, recent hospitalization, advanced age, multiple comorbidities, hemodialysis, recent international travel, and urinary catheterization.23,24 Urine cultures and susceptibilities should be included in an assessment of patients with any of these risk factors and used to inform antibiotic selection.24 First-line treatment options for uncomplicated cystitis include nitrofurantoin, TMP/SMX (in regions where the uropathogen resistance is <20%), and fosfomycin.25
Multidrug-resistant pathogens
Nitrofurantoin, fosfomycin, and in some instances, fluoroquinolones, are options for treating multidrug-resistant uropathogens, as guided by susceptibility results (TABLE 4).24,26-28 IV antibiotics may be necessary for patients who have severe infections or live in long-term care facilities.24,29 UTIs caused by carbapenem-resistant Enterobacteriaceae are fortunately still rare in outpatient settings.30 There is a lack of high-quality evidence describing best practices for treating infections caused by ESBL-producing bacteria in the community; therefore, antimicrobial selection should be based on infection severity and patient-specific factors.1
Neisseria gonorrhoeae is multidrug-resistant
CASE 2 › Jon F
Mr. F’s physician suspects gonorrhea, although chlamydia cannot be ruled out, and orders a urethral culture, first-catch urine test using nucleic acid amplification, syphilis, and HIV tests. The physician administers ceftriaxone 250 mg IM and azithromycin 1 g PO, instructs the patient to contact all sex partners within the last 60 days, and emphasizes the importance of using latex condoms consistently.
Three days later, Jon returns, complaining that his symptoms have not improved. The urethral culture shows a gram-negative oxidase-positive diplococcus and testing confirms N gonorrhoeae. The lab results also reveal a high cephalosporin minimal inhibitory concentration, negative tests for chlamydia and HIV, and a non-reactive rapid plasma reagin.
Gonococcal infections are the second most common communicable disease reported in the United States, with some 820,000 new cases annually.31 Sites of infection include the cervix, urethra, and rectum, and less commonly, the pharynx, conjunctiva, joints, meninges, and endocardium. Those at risk for gonorrhea are: 31
- women who are sexually active and <25 years of age
- any woman with a new sex partner
- any individual with multiple sex partners or a partner with concurrent partners and/or a known sexually transmitted infection (STI)
- an individual who has a history of inconsistent condom use in non-monogamous relationships
- sex workers.
SIDEBAR
Combatting antibiotic resistance: A call to action for FPs
- Use the best available evidence to determine when antibiotics are indicated.
- Develop and use “antibiograms”—tools that use data from patient specimens to identify patterns of antibiotic susceptibility and guide appropriate empiric antibiotic selection.
- Use rapid diagnostic assays to differentiate between viral and bacterial infections and identify resistant bacteria.
- Add antibiotic use to the list of metrics your practice uses to assess clinician performance.
- Adopt standard, clear language to explain to patients and parents when—and why—antibiotics are not indicated.
- Support public health surveillance efforts by learning and adhering to reporting requirements in your community.
The difficulty of treating gonorrhea
Cephalosporins are now the only class of antimicrobials with reliable activity against N gonorrhoeae. The Centers for Disease Control and Prevention (CDC) no longer recommends fluoroquinolones due to increasing resistance. However, cefixime-resistant strains of gonorrhea and treatment failures have been reported, and the drug is no longer recommended for gonorrhea treatment.31
Combination therapy with one dose of ceftriaxone 250 mg IM and one dose of azithromycin 1 g orally is recommended by the CDC,32 as combination therapy improves efficacy and delays the development of resistance. Azithromycin is preferred over doxycycline as the second agent. That’s both because of a higher prevalence of tetracycline resistance among circulating gonococci and azithromycin’s activity against Chlamydia trachomatis, as patients with gonococcal infections are frequently co-infected with this organism.31
Suspected treatment failures are more likely caused by re-infection than drug resistance. If resistance is suspected, however, physicians should seek guidance from an infectious diseases specialist or the CDC on repeat cultures, susceptibility testing, and antimicrobial therapy.
Two treatment regimens have demonstrated efficacy against cephalosporin-resistant N gonorrhoeae:31,32
- A single dose of gemifloxacin 320 mg PO plus azithromycin 2 g PO or
- a single dose of gentamicin 240 mg IM plus azithromycin 2 g PO.
CASE 2 › Jon F
Mr. F’s physician consults with an infectious disease specialist at the local hospital, who confirms his suspicion that the patient has been infected with antibiotic-resistant N gonorrhoeae. The physician administers gentamicin 240 mg IM plus azithromycin 2 g orally, and warns the patient that he may experience gastrointestinal adverse effects. The physician reports the case, as required, to the local health department.
Antibiotic stewardship: What’s being done…
Efforts to improve antibiotic use in the community setting include a variety of strategies, including academic detailing (ie, evidence-based promotion of drug therapies rather than promotion by manufacturer representatives), patient education, clinical decision support, multi-faceted programs using a combination of interventions, and local and national campaigns.33-36 Recent examples of successful campaigns include the American Board of Internal Medicine Foundation’s Choosing Wisely initiative (choosingwisely.org) and the CDC’s Get Smart program (cdc.gov/getsmart/community/index.html).
Get Smart promotes appropriate antibiotic use by prescribers and aims to decrease demand for antibiotics by patients and promote adherence to prescribed antibiotic regimens. Studies have found that incorporating treatment algorithms and clinical decision support systems into existing electronic medical record systems has led to more appropriate prescribing.37-39
The most effective interventions target both patients/parents and prescribers, provide evidence-based prescribing prompts, require prescribers to justify antibiotic use, and involve clinicians in their design. Future directions should include collaboration with municipal or regional public health organizations to identify community-wide critical infections and resistance trends and strategies that use behavioral interventions to address inappropriate prescribing.40
…and what you can do
Family physicians are key to determining the outcome of the war against antibiotic resistance. See “Combatting antibiotic resistance: A call to action for FPs” on the previous page for specific interventions you can initiate without delay.
The tide will turn only through modification of both prescriber and patient behavior and formalized programs in our communities. Education about appropriate use needs to be included in medical school curricula and continue in the clinic setting through education of physicians in training, medical students, and office staff.41 Become an advocate by promoting the principles of optimal antibiotic stewardship as outlined by the recent IDSA Guidelines for Implementing an Antibiotic Stewardship Program.42 Go to http://cid.oxfordjournals.org/content/62/10/1197.long to learn more.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy Practice & Administration, School of Pharmacy, University of Saint Joseph, 229 Trumbull Street, Hartford, CT 06103; [email protected].
1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
2. Hicks DO, Taylor TH. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461-1462.
3. Fleming-Dutra KE, Hersh, A, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 31, 2016.
5. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
6. Chambers HF. Methicillin-resistant Staphylococcus aureus: mechanisms of resistance and implications for treatment. Postgrad Med. 2001;109:43-50.
7. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;5:285-292.
8. Couch KA, Geide T. ASHP therapeutic position statement on strategies for identifying and preventing pneumococcal resistance. Am J Health-Syst Pharm. 2014;71:417-424.
9. Centers for Disease Control and Prevention (2010) ABCs report: Streptococcus pneumoniae. Available at: www.cdc.gov/abcs/reports-findings/survreports/spneu10.html. Accessed October 28, 2016.
10. Pallares R, Fenoll A, Linares J for the Spanish Pneumococcal Infection Study Network. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides, and fluoroquinolones. Int J Antimicrob Agents. 2003;22(suppl):S15-S24.
11. US Food and Drug Administration. The benefits and risks of systemic fluoroquinolone antibacterial drugs for the treatment of acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in patients who have chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTI). 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 28, 2016.
12. Nuorti JP, Butler JC, Farley NM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl):S27-S72.
14. Gwaltney JM Jr, Wiesinger BA, Patrieb JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233.
15. Diekema DJ, Brueggemann AB, Doern GV. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg Infect Dis. 2000;6:552-556.
16. Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631-639.
17. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e1-e41.
18. Lieberthal AS, Caroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
19. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
20. Sanchez GV, Adams SJ, Baird AM, et al. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother. 2013:68:1838-1841.
21. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738.
22. Gupta K, Hooten TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
23. Ben-Ami R, Rodrıguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690.
24. Walker E, Lyman A, Gupta K, et al. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960-965.
25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
26. Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin N Am. 2014;28:49-59.
27. Neuner EA, Sekeres J, Hall GS, et al. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56:5744-5748.
28. Tasbakan MI, Pullukcu H, Sipahi OR, et al. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554-556.
29. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;268:1897-1902.
30. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479-1487.
31. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64:1-137.
32. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
33. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
34. Belongia EA, Sullivan BJ, Chyou, PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108;575-583.
35. Gjelstad S, Hoye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ. 2013;347:f4403.
36. Huttner B, Harbarth S. ‘Antibiotics are not automatic anymore’’—the French national campaign to cut antibiotic overuse. PLoS Med. 2009;6: e1000080.
37. Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
38. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.
40. Meeker D, Linder JA, Fox CR, et al
41. Schwartz BS, Armstrong WS, Ohl CA, et al. Create allies, IDSA stewardship commitments should prioritize health professions learners. Clin Infect Dis. 2015; 61:1626-1627.
42. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program:guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:1-27.
1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
2. Hicks DO, Taylor TH. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461-1462.
3. Fleming-Dutra KE, Hersh, A, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 31, 2016.
5. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
6. Chambers HF. Methicillin-resistant Staphylococcus aureus: mechanisms of resistance and implications for treatment. Postgrad Med. 2001;109:43-50.
7. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;5:285-292.
8. Couch KA, Geide T. ASHP therapeutic position statement on strategies for identifying and preventing pneumococcal resistance. Am J Health-Syst Pharm. 2014;71:417-424.
9. Centers for Disease Control and Prevention (2010) ABCs report: Streptococcus pneumoniae. Available at: www.cdc.gov/abcs/reports-findings/survreports/spneu10.html. Accessed October 28, 2016.
10. Pallares R, Fenoll A, Linares J for the Spanish Pneumococcal Infection Study Network. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides, and fluoroquinolones. Int J Antimicrob Agents. 2003;22(suppl):S15-S24.
11. US Food and Drug Administration. The benefits and risks of systemic fluoroquinolone antibacterial drugs for the treatment of acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in patients who have chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTI). 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 28, 2016.
12. Nuorti JP, Butler JC, Farley NM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl):S27-S72.
14. Gwaltney JM Jr, Wiesinger BA, Patrieb JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233.
15. Diekema DJ, Brueggemann AB, Doern GV. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg Infect Dis. 2000;6:552-556.
16. Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631-639.
17. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e1-e41.
18. Lieberthal AS, Caroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
19. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
20. Sanchez GV, Adams SJ, Baird AM, et al. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother. 2013:68:1838-1841.
21. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738.
22. Gupta K, Hooten TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
23. Ben-Ami R, Rodrıguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690.
24. Walker E, Lyman A, Gupta K, et al. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960-965.
25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
26. Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin N Am. 2014;28:49-59.
27. Neuner EA, Sekeres J, Hall GS, et al. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56:5744-5748.
28. Tasbakan MI, Pullukcu H, Sipahi OR, et al. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554-556.
29. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;268:1897-1902.
30. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479-1487.
31. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64:1-137.
32. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
33. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
34. Belongia EA, Sullivan BJ, Chyou, PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108;575-583.
35. Gjelstad S, Hoye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ. 2013;347:f4403.
36. Huttner B, Harbarth S. ‘Antibiotics are not automatic anymore’’—the French national campaign to cut antibiotic overuse. PLoS Med. 2009;6: e1000080.
37. Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
38. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.
40. Meeker D, Linder JA, Fox CR, et al
41. Schwartz BS, Armstrong WS, Ohl CA, et al. Create allies, IDSA stewardship commitments should prioritize health professions learners. Clin Infect Dis. 2015; 61:1626-1627.
42. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program:guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:1-27.
PRACTICE RECOMMENDATIONS
› Manage uncomplicated cutaneous abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus with incision and drainage alone. A
› Treat upper respiratory infections associated with drug-resistant Streptococcus pneumoniae with high-dose amoxicillin, which has been found to overcome penicillin resistance. A
› Administer dual therapy with ceftriaxone and azithromycin to patients with gonococcal infections. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
When can infants and children benefit from probiotics?
PRACTICE RECOMMENDATIONS
› Recommend a trial of Lactobacillus reuteri for breastfed infants with colic. A
› Consider Lactobacillus and Bifidobacterium species for the prevention of upper respiratory infections (URIs) and to shorten the course of URI illness. B
› Do not recommend probiotics for the prevention of respiratory or gastrointestinal allergies. A
› Consider probiotics for the reduction of abdominal pain in pediatric irritable bowel syndrome, as well as to reduce diarrhea associated with antibiotic use and acute gastroenteritis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Ms. B, a 26-year-old woman, presents to your office with her 3-year-old son for a well-child examination. During the course of the conversation, she asks you if she should be giving her child probiotics to improve his general health. Many of her friends, who also have their children in day care, have told her that probiotics, “are nature’s way of fighting infection.” Her son currently takes no medications, and has no history of asthma or recent gastrointestinal disturbances. He was treated for 2 ear infections last winter, approximately 3 months apart. His physical exam is normal and, after today, his immunizations will be up to date. How should you respond?
The use of probiotics as over-the-counter treatments for a variety of conditions continues to grow, with retail sales of functional probiotic foods and supplements topping $35 billion worldwide in 2014.1 In children, claims of benefit for gastrointestinal (GI) disorders, colic, and allergy prevention, as well as prevention and treatment of upper respiratory infections (URIs) have existed for over 10 years.2-4 The human gut flora develops rapidly after birth and is known to be influenced by route of delivery (vaginal vs cesarean), type of feeding (breast vs formula), and other environmental factors.5 The use of probiotics to influence the types of bacteria in a child’s intestinal tract continues to be an area of active research. (For more on probiotic formulations, see TABLE 1.)
This article summarizes recent research on probiotic use in infants and children. New data support the use of probiotics for the treatment of colic and atopic eczema; however, the data on using probiotics in the management of URIs is less robust and mixed. And while probiotics improve irritable bowel syndrome (IBS) stomach pain, they do not help with related diarrhea or constipation. All of these data are summarized in TABLE 2.6-29
L reuteri improves symptoms in breastfed infants with colic
Infant colic is a relatively common condition known to negatively impact maternal mental health and the mother/child relationship.6 Numerous randomized controlled trials (RCTs) over the years have demonstrated mixed results with using probiotics to decrease crying times, with differences noted between infants who are solely breastfed and those who are not.7
In the most recent meta-analysis of 6 studies (n=427) that focused only on the probiotic Lactobacillus reuteri, breastfed infants with colic receiving a daily dose of 108 colony forming units (CFU) cried an average of 56 fewer minutes/day than those in the control group (95% confidence interval [CI], -64.4 to -47.3; P=.001) at day 21 of treatment.8 Although 2 studies in this meta-analysis included a small number of mixed-fed and formula-fed infants, the majority of trials do not show benefit for these infants. Trials assessing the use of L reuteri for prevention of colic have not shown positive results.7
Probiotics may help p
The mechanisms by which probiotics may prevent or shorten the course of URIs are not obvious. Current theories include boosting the immune function of the respiratory mucosa, acting as a competitive inhibitor for viruses, and secreting antiviral compounds.9 Multiple reviews published in the last 3 years, however, add to the evidence that the apparent benefit is real.
A 2013 meta-analysis assessed data from 4 RCTs (N=1805), which used Lactobacillus rhamnosus as the sole probiotic for prevention of URIs. In treated children, otitis media incidence was reduced by 24% (relative risk [RR] 0.76; 95% CI, 0.64-0.91) and risk of URI was reduced by 38% (RR 0.62; 95% CI, 0.50-0.78).10 The number needed to treat (NNT) was 4 for URI prevention, and the authors noted that adverse events were similar in the treatment and control groups.
A 2014 systematic review and meta-analysis of 20 RCTs examining duration of illness included 10 studies dedicated to pediatric subjects (age 12 months to 12 years).11 There were significantly fewer days of illness per person (standardized mean difference -0.31; 95% CI, -0.41 to -0.11) and each illness episode was shorter by three-quarters of a day (weighted mean difference -0.77; 95% CI, -1.5 to -0.04) in participants who received a probiotic vs those who received a placebo. Probiotics used in these studies belonged to the Lactobacillus and Bifidobacterium genera.
A 2015 systematic review of 14 RCTs assessing the benefits of probiotics, particularly Lactobacillus and Bifidobacterium strains, on URI occurrence and symptoms, showed mixed results.12 Seven of 12 studies found lowered rates of URI and otitis media incidence, 7 of 11 RCTs reported a significant reduction in severity scores for URI, and 4 of 8 RCTs reported significant reductions in school absenteeism between the probiotic and control groups. In a summary statement, the authors noted that “at least one beneficial effect of prophylactic probiotics was observed in the majority of RCTs,” and that “none of the studies reported any serious adverse events.”
Perinatal probiotics: No benefit for allergic conditions—except eczema
Allergic disease is on the rise and continues to plague children with reduced quality of life, potentially life-threatening reactions, and missed activities, including school. The gut microbiome likely influences a child’s allergic propensity through its effects on T-helper cells, transforming growth factor (TGF), and immunoglobulin A (IgA)—all known components of the allergic response. As the hygiene hypothesis suggests, the quantity and types of bacteria that inhabit the GI tract early in life play a significant role in determining a person’s later allergic responses.13
In a 2013 meta-analysis of 20 trials (N=4866), researchers looked specifically at probiotic use and the diagnosis of asthma and incident wheezing. Single and combination products of Lactobacillus and Bifidobacterium given prenatally and/or postnatally were included in the studies. The authors found no evidence to support a protective association between perinatal use of probiotics and diagnosed asthma (RR=0.99; 95% CI, 0.81-0.21) or childhood incident wheezing (RR=0.97; 95% CI, 0.87-1.09; 9 trials, 1949 infants).14
In a more recent meta-analysis (2015) conducted to inform the World Allergy Organization, 29 studies were evaluated to assess the impact of probiotics on allergic symptoms of the skin, respiratory system, and GI tract.15 No significant benefit was noted for any allergic condition except for eczema. Probiotics reduced the risk of eczema when given during the last trimester of pregnancy (RR=0.71; 95% CI, 0.60-0.84), when used by breastfeeding mothers (RR=0.57; 95% CI, 0.47-0.69), and when given to infants (RR=0.80; 95% CI, 0.68-0.94).
A 2014 systematic review and meta-analysis (N=2797) explored probiotic use specifically for the prevention of eczema.16 The pooled relative risk for all the studies was 0.74 (95% CI, 0.67-0.82). Evidence was strongest for probiotics containing the Lactobacillus species rhamnosus and paracasei, as well as for Bifidobacterium lactis. No benefit was noted with Lactobacillus acidophilus or other Bifidobacterium species. These newer reviews on eczema prevention contrast with an older Cochrane review published in 2008 (12 RCTs, N=781), which did not show significant benefit for the treatment of eczema.17
Probiotics improve IBS stomach pain, but not diarrhea or constipation
IBS is a functional disorder of the GI tract that affects up to 20% of children and teenagers and leads to a significant decrease in quality of life.18 Current theories of causation include bacterial overgrowth and neuronal hyperactivity, which may be amenable to change with supplemental probiotics.
A 2015 systematic review of non-pharmacological treatments for functional abdominal pain disorders identified 4 studies dedicated to IBS in children.19 A subgroup analysis of 3 RCTs (n=309) that looked at giving L rhamnosus to 5- to 17-year-olds with IBS showed improved abdominal pain (according to various pain scales) compared to the placebo group. Study participants received at least 3 x 109 CFU twice a day for 4 to 8 weeks. Relative risk for improvement was 1.7 (95% CI, 1.27-2.27) with an NNT of 4. None of these studies showed significant improvement in either frequency or severity of diarrhea or constipation.
A separate crossover RCT (N=59) compared placebo to VSL#3, a product containing 8 probiotics (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, L acidophilus, Lactobacillus plantarum, L paracasei, Lactobacillus bulgaricus, and Streptococcus hermophiles), given in age-dependent doses for 6 weeks to children aged 4 to 18 years.20 The frequency and intensity of abdominal pain were measured on a 5-point Likert scale. The group treated with VSL#3 dropped 1.0 ± 0.2 points vs 0.5 ± 0.2 points in the control group (P<.05) and reported an improved quality of life.
These agents reduce antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) occurs in 5% to 30% of children who receive antibiotic therapy.21 It occurs most frequently with the use of cephalosporins, penicillin, fluoroquinolones, and clindamycin, and is likely caused by an alteration of the normal gut flora. Colitis caused by Clostridium difficile remains the most serious antibiotic-associated GI complication.
A systematic review of the specific probiotic Saccharomyces boulardii conducted in 2015 analyzed data from 6 RCTs (n=1653) to determine the effect of co-administration of this probiotic with antibiotics.22 The pooled relative risk for AAD in children receiving the probiotic was 0.43 (95% CI, 0.3-0.6) compared to antibiotics alone. The absolute risk of AAD dropped from 20.9% to 8.8%, translating to a NNT of 8. Two of the RCTs specifically looked at rates of C difficile infection (n=579). C difficile infection rates dropped by 75% (RR=.25; 95% CI, 0.08-0.73) in the treatment group. This dramatic treatment effect was not seen in studies involving adults.
A similar systematic review focusing on L rhamnosus conducted in 2015 pooled data from 5 RCTs (n=445) to see if the probiotic would decrease AAD in children if it was co-administered with antibiotics.23 The relative risk for AAD in this treatment group was 0.48 (95% CI, 0.26-0.89) with an absolute risk reduction of 13.4% (23% compared to 9.6%), translating to an NNT of 7.
A Cochrane review published in 2015 included 23 studies (N=3938) and found similar results with an RR for AAD of 0.46 for treated children (95% CI, 0.35-0.61).24 Doses of probiotics ranged from 5 to 40 billion CFU/day. Although many probiotic species were used in these studies, S boulardii and L rhamnosus were cited as having the strongest data to support use in this context.
Probiotics reduce the duration, frequency of acute infectious diarrhea
Diarrhea remains the second leading cause of death among children one to 59 months of age worldwide.25 Current World Health Organization recommendations include oral rehydration salts, continued feeding to avoid dehydration, and zinc to decrease the duration and severity of illness.26 Multiple studies in adults confirm that a variety of probiotics decrease both the duration and severity of diarrhea in acute gastroenteritis.27
The authors of a 2013 systematic review of probiotics for the treatment of community-acquired acute diarrhea in children less than 5 years of age analyzed data from 8 RCTs (N=1755).28 Various probiotics were used including Lactobacillus species, Streptococcus thermophilus, Bifidobacterium species, and Saccharomyces boulardii for between 4 and 10 days. Six of these studies (n=1164) measured diarrhea duration and found a 14% reduction (95% CI, 3.8%-24.2%) in days of illness for those children treated vs those receiving placebo. Five studies (n=925) measured the difference in stool frequency on Day 2 of illness and reported a reduction of 13.1% (95% CI, 0.8%-5.3%) in the number of stools in the treated group vs the placebo group.
This review augments a Cochrane meta-analysis of 63 studies (N=8014) published in 2010.27 Fifty-six of these studies included infants and children. Pooled analysis of the varied probiotic treatments showed a mean reduction in duration of diarrhea of just over a day (24.76 hours; 95% CI, 15.9-33.6 hours; n=4555, trials=35) and decreased stool frequency on Day 2 of treatment (mean difference 0.80; 95% CI, 0.45-1.14; n=2751, trials=20). The authors concluded that probiotics “have clear beneficial effects in shortening the duration and reducing stool frequency in acute infectious diarrhea.”
Pediatric society weighs in. In 2014, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition issued guidelines regarding probiotic use for the treatment of acute gastroenteritis.29 In addition to rehydration therapy, these guidelines recommend the use of L rhamnosus and/or S boulardii as first-line treatments. Lower quality evidence is available for the use of L reuteri.
CASE › In response to Ms. B’s query about starting her young son on probiotics, you tell her that studies have shown that probiotics are safe for children when given in appropriate doses. They have been shown to help children recover from diarrheal illnesses and can help reduce the number of colds and ear infections when taken regularly. The reason you are giving them determines which strains you should use. You recommend giving her child a formulation of probiotic that contains Lactobacillus or Bifidobacterium with a dose range of 2 to 10 billion CFUs taken daily to reduce the risk of her child getting another ear infection.
CORRESPONDENCE
Paul Dassow, MD, MSPH, 1100 E. 3rd St, Chattanooga, TN 37403; [email protected].
1. Euromonitor International. Global and regional trends of the probiotics and omega fatty acids market. June 23, 2015. Available at: http://uschinahpa.org/wp-content/uploads/2015/07/EMI-US-China-HPA-Probiotic-and-Omega-2015-Final.pdf. Accessed September 9, 2016.
2. Du Toit G, Lack G. Can food allergy be prevented? The current evidence. Pediatr Clin North Am. 2011;58:481-509.
3. Gerritsen J, Smidt H, Rijkers GT, et al. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209-240.
4. Versalovic J. The human microbiome and probiotics: implications for pediatrics. Ann Nutr Metab. 2013;63:42-52.
5. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
6. Akman I, Kușçu K, Özdemir N, et al. Mothers’ postpartum psychological adjustment and infantile colic. Arch Dis Child. 2006;91:417-419.
7. Sung V, Collett S, de Gooyer T, et al. Probiotics to prevent or treat excessive infant crying systematic review and meta-analysis. JAMA Pediatr. 2013:167:1150-1157.
8. Harb T, Matsuyama M, David M, et al. Infant colic—what works: a systematic review of interventions for breastfed infants. J Pediatr Gastroenterol Nutr. 2016;62:668-686.
9. Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
10. Liu S, Hu P, Du X, et al. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013;50:377-381.
11. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
12. Ozen M, Kocabas Sandal G, Dinleyici EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. 2015;15:9-20.
13. Azad MB, Konya T, Maughan H, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15.
14. Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. Brit Med J. 2013;347:f6471.
15. Cuello-Garcia CA, Bro˙zek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952-961.
16. Mansfield JA, Bergin SW, Cooper JR, et al. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. 2014;179:580-592.
17. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
18. Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Expert Rev Gastroenterol Hepatol. 2010;4:293-304.
19. Rutten JMTM, Korterink JL, Venmans LMAJ, et al. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics. 2015;135:522-535.
20. Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24-30.
21. Turck D, Bernet JP, Marx J, et al. Incidence and risk factors of oral antibiotic associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37:22-26.
22. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
23. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149-1157.
24. Goldenberg JZ, Lytvyn L, Steurich J, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2015;12:CD004827.
25. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151-2161.
26. WHO/UNICEF Joint Statement: Clinical Management of Acute Diarrhea. August 2004. Available at: http://www.unicef.org/publications/files/ENAcute_Diarrhoea_reprint.pdf. Accessed September 9, 2016.
27. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst Rev. 2010;(11):CD003048.
28. Applegate JA, Fischer Walker CL, Ambikapathi R, et al. Systematic review of probiotics for the treatment of community-acquired acute diarrhea in children. BMC Public Health. 2013;13:S16.
29. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132-152.
PRACTICE RECOMMENDATIONS
› Recommend a trial of Lactobacillus reuteri for breastfed infants with colic. A
› Consider Lactobacillus and Bifidobacterium species for the prevention of upper respiratory infections (URIs) and to shorten the course of URI illness. B
› Do not recommend probiotics for the prevention of respiratory or gastrointestinal allergies. A
› Consider probiotics for the reduction of abdominal pain in pediatric irritable bowel syndrome, as well as to reduce diarrhea associated with antibiotic use and acute gastroenteritis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Ms. B, a 26-year-old woman, presents to your office with her 3-year-old son for a well-child examination. During the course of the conversation, she asks you if she should be giving her child probiotics to improve his general health. Many of her friends, who also have their children in day care, have told her that probiotics, “are nature’s way of fighting infection.” Her son currently takes no medications, and has no history of asthma or recent gastrointestinal disturbances. He was treated for 2 ear infections last winter, approximately 3 months apart. His physical exam is normal and, after today, his immunizations will be up to date. How should you respond?
The use of probiotics as over-the-counter treatments for a variety of conditions continues to grow, with retail sales of functional probiotic foods and supplements topping $35 billion worldwide in 2014.1 In children, claims of benefit for gastrointestinal (GI) disorders, colic, and allergy prevention, as well as prevention and treatment of upper respiratory infections (URIs) have existed for over 10 years.2-4 The human gut flora develops rapidly after birth and is known to be influenced by route of delivery (vaginal vs cesarean), type of feeding (breast vs formula), and other environmental factors.5 The use of probiotics to influence the types of bacteria in a child’s intestinal tract continues to be an area of active research. (For more on probiotic formulations, see TABLE 1.)
This article summarizes recent research on probiotic use in infants and children. New data support the use of probiotics for the treatment of colic and atopic eczema; however, the data on using probiotics in the management of URIs is less robust and mixed. And while probiotics improve irritable bowel syndrome (IBS) stomach pain, they do not help with related diarrhea or constipation. All of these data are summarized in TABLE 2.6-29
L reuteri improves symptoms in breastfed infants with colic
Infant colic is a relatively common condition known to negatively impact maternal mental health and the mother/child relationship.6 Numerous randomized controlled trials (RCTs) over the years have demonstrated mixed results with using probiotics to decrease crying times, with differences noted between infants who are solely breastfed and those who are not.7
In the most recent meta-analysis of 6 studies (n=427) that focused only on the probiotic Lactobacillus reuteri, breastfed infants with colic receiving a daily dose of 108 colony forming units (CFU) cried an average of 56 fewer minutes/day than those in the control group (95% confidence interval [CI], -64.4 to -47.3; P=.001) at day 21 of treatment.8 Although 2 studies in this meta-analysis included a small number of mixed-fed and formula-fed infants, the majority of trials do not show benefit for these infants. Trials assessing the use of L reuteri for prevention of colic have not shown positive results.7
Probiotics may help p
The mechanisms by which probiotics may prevent or shorten the course of URIs are not obvious. Current theories include boosting the immune function of the respiratory mucosa, acting as a competitive inhibitor for viruses, and secreting antiviral compounds.9 Multiple reviews published in the last 3 years, however, add to the evidence that the apparent benefit is real.
A 2013 meta-analysis assessed data from 4 RCTs (N=1805), which used Lactobacillus rhamnosus as the sole probiotic for prevention of URIs. In treated children, otitis media incidence was reduced by 24% (relative risk [RR] 0.76; 95% CI, 0.64-0.91) and risk of URI was reduced by 38% (RR 0.62; 95% CI, 0.50-0.78).10 The number needed to treat (NNT) was 4 for URI prevention, and the authors noted that adverse events were similar in the treatment and control groups.
A 2014 systematic review and meta-analysis of 20 RCTs examining duration of illness included 10 studies dedicated to pediatric subjects (age 12 months to 12 years).11 There were significantly fewer days of illness per person (standardized mean difference -0.31; 95% CI, -0.41 to -0.11) and each illness episode was shorter by three-quarters of a day (weighted mean difference -0.77; 95% CI, -1.5 to -0.04) in participants who received a probiotic vs those who received a placebo. Probiotics used in these studies belonged to the Lactobacillus and Bifidobacterium genera.
A 2015 systematic review of 14 RCTs assessing the benefits of probiotics, particularly Lactobacillus and Bifidobacterium strains, on URI occurrence and symptoms, showed mixed results.12 Seven of 12 studies found lowered rates of URI and otitis media incidence, 7 of 11 RCTs reported a significant reduction in severity scores for URI, and 4 of 8 RCTs reported significant reductions in school absenteeism between the probiotic and control groups. In a summary statement, the authors noted that “at least one beneficial effect of prophylactic probiotics was observed in the majority of RCTs,” and that “none of the studies reported any serious adverse events.”
Perinatal probiotics: No benefit for allergic conditions—except eczema
Allergic disease is on the rise and continues to plague children with reduced quality of life, potentially life-threatening reactions, and missed activities, including school. The gut microbiome likely influences a child’s allergic propensity through its effects on T-helper cells, transforming growth factor (TGF), and immunoglobulin A (IgA)—all known components of the allergic response. As the hygiene hypothesis suggests, the quantity and types of bacteria that inhabit the GI tract early in life play a significant role in determining a person’s later allergic responses.13
In a 2013 meta-analysis of 20 trials (N=4866), researchers looked specifically at probiotic use and the diagnosis of asthma and incident wheezing. Single and combination products of Lactobacillus and Bifidobacterium given prenatally and/or postnatally were included in the studies. The authors found no evidence to support a protective association between perinatal use of probiotics and diagnosed asthma (RR=0.99; 95% CI, 0.81-0.21) or childhood incident wheezing (RR=0.97; 95% CI, 0.87-1.09; 9 trials, 1949 infants).14
In a more recent meta-analysis (2015) conducted to inform the World Allergy Organization, 29 studies were evaluated to assess the impact of probiotics on allergic symptoms of the skin, respiratory system, and GI tract.15 No significant benefit was noted for any allergic condition except for eczema. Probiotics reduced the risk of eczema when given during the last trimester of pregnancy (RR=0.71; 95% CI, 0.60-0.84), when used by breastfeeding mothers (RR=0.57; 95% CI, 0.47-0.69), and when given to infants (RR=0.80; 95% CI, 0.68-0.94).
A 2014 systematic review and meta-analysis (N=2797) explored probiotic use specifically for the prevention of eczema.16 The pooled relative risk for all the studies was 0.74 (95% CI, 0.67-0.82). Evidence was strongest for probiotics containing the Lactobacillus species rhamnosus and paracasei, as well as for Bifidobacterium lactis. No benefit was noted with Lactobacillus acidophilus or other Bifidobacterium species. These newer reviews on eczema prevention contrast with an older Cochrane review published in 2008 (12 RCTs, N=781), which did not show significant benefit for the treatment of eczema.17
Probiotics improve IBS stomach pain, but not diarrhea or constipation
IBS is a functional disorder of the GI tract that affects up to 20% of children and teenagers and leads to a significant decrease in quality of life.18 Current theories of causation include bacterial overgrowth and neuronal hyperactivity, which may be amenable to change with supplemental probiotics.
A 2015 systematic review of non-pharmacological treatments for functional abdominal pain disorders identified 4 studies dedicated to IBS in children.19 A subgroup analysis of 3 RCTs (n=309) that looked at giving L rhamnosus to 5- to 17-year-olds with IBS showed improved abdominal pain (according to various pain scales) compared to the placebo group. Study participants received at least 3 x 109 CFU twice a day for 4 to 8 weeks. Relative risk for improvement was 1.7 (95% CI, 1.27-2.27) with an NNT of 4. None of these studies showed significant improvement in either frequency or severity of diarrhea or constipation.
A separate crossover RCT (N=59) compared placebo to VSL#3, a product containing 8 probiotics (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, L acidophilus, Lactobacillus plantarum, L paracasei, Lactobacillus bulgaricus, and Streptococcus hermophiles), given in age-dependent doses for 6 weeks to children aged 4 to 18 years.20 The frequency and intensity of abdominal pain were measured on a 5-point Likert scale. The group treated with VSL#3 dropped 1.0 ± 0.2 points vs 0.5 ± 0.2 points in the control group (P<.05) and reported an improved quality of life.
These agents reduce antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) occurs in 5% to 30% of children who receive antibiotic therapy.21 It occurs most frequently with the use of cephalosporins, penicillin, fluoroquinolones, and clindamycin, and is likely caused by an alteration of the normal gut flora. Colitis caused by Clostridium difficile remains the most serious antibiotic-associated GI complication.
A systematic review of the specific probiotic Saccharomyces boulardii conducted in 2015 analyzed data from 6 RCTs (n=1653) to determine the effect of co-administration of this probiotic with antibiotics.22 The pooled relative risk for AAD in children receiving the probiotic was 0.43 (95% CI, 0.3-0.6) compared to antibiotics alone. The absolute risk of AAD dropped from 20.9% to 8.8%, translating to a NNT of 8. Two of the RCTs specifically looked at rates of C difficile infection (n=579). C difficile infection rates dropped by 75% (RR=.25; 95% CI, 0.08-0.73) in the treatment group. This dramatic treatment effect was not seen in studies involving adults.
A similar systematic review focusing on L rhamnosus conducted in 2015 pooled data from 5 RCTs (n=445) to see if the probiotic would decrease AAD in children if it was co-administered with antibiotics.23 The relative risk for AAD in this treatment group was 0.48 (95% CI, 0.26-0.89) with an absolute risk reduction of 13.4% (23% compared to 9.6%), translating to an NNT of 7.
A Cochrane review published in 2015 included 23 studies (N=3938) and found similar results with an RR for AAD of 0.46 for treated children (95% CI, 0.35-0.61).24 Doses of probiotics ranged from 5 to 40 billion CFU/day. Although many probiotic species were used in these studies, S boulardii and L rhamnosus were cited as having the strongest data to support use in this context.
Probiotics reduce the duration, frequency of acute infectious diarrhea
Diarrhea remains the second leading cause of death among children one to 59 months of age worldwide.25 Current World Health Organization recommendations include oral rehydration salts, continued feeding to avoid dehydration, and zinc to decrease the duration and severity of illness.26 Multiple studies in adults confirm that a variety of probiotics decrease both the duration and severity of diarrhea in acute gastroenteritis.27
The authors of a 2013 systematic review of probiotics for the treatment of community-acquired acute diarrhea in children less than 5 years of age analyzed data from 8 RCTs (N=1755).28 Various probiotics were used including Lactobacillus species, Streptococcus thermophilus, Bifidobacterium species, and Saccharomyces boulardii for between 4 and 10 days. Six of these studies (n=1164) measured diarrhea duration and found a 14% reduction (95% CI, 3.8%-24.2%) in days of illness for those children treated vs those receiving placebo. Five studies (n=925) measured the difference in stool frequency on Day 2 of illness and reported a reduction of 13.1% (95% CI, 0.8%-5.3%) in the number of stools in the treated group vs the placebo group.
This review augments a Cochrane meta-analysis of 63 studies (N=8014) published in 2010.27 Fifty-six of these studies included infants and children. Pooled analysis of the varied probiotic treatments showed a mean reduction in duration of diarrhea of just over a day (24.76 hours; 95% CI, 15.9-33.6 hours; n=4555, trials=35) and decreased stool frequency on Day 2 of treatment (mean difference 0.80; 95% CI, 0.45-1.14; n=2751, trials=20). The authors concluded that probiotics “have clear beneficial effects in shortening the duration and reducing stool frequency in acute infectious diarrhea.”
Pediatric society weighs in. In 2014, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition issued guidelines regarding probiotic use for the treatment of acute gastroenteritis.29 In addition to rehydration therapy, these guidelines recommend the use of L rhamnosus and/or S boulardii as first-line treatments. Lower quality evidence is available for the use of L reuteri.
CASE › In response to Ms. B’s query about starting her young son on probiotics, you tell her that studies have shown that probiotics are safe for children when given in appropriate doses. They have been shown to help children recover from diarrheal illnesses and can help reduce the number of colds and ear infections when taken regularly. The reason you are giving them determines which strains you should use. You recommend giving her child a formulation of probiotic that contains Lactobacillus or Bifidobacterium with a dose range of 2 to 10 billion CFUs taken daily to reduce the risk of her child getting another ear infection.
CORRESPONDENCE
Paul Dassow, MD, MSPH, 1100 E. 3rd St, Chattanooga, TN 37403; [email protected].
PRACTICE RECOMMENDATIONS
› Recommend a trial of Lactobacillus reuteri for breastfed infants with colic. A
› Consider Lactobacillus and Bifidobacterium species for the prevention of upper respiratory infections (URIs) and to shorten the course of URI illness. B
› Do not recommend probiotics for the prevention of respiratory or gastrointestinal allergies. A
› Consider probiotics for the reduction of abdominal pain in pediatric irritable bowel syndrome, as well as to reduce diarrhea associated with antibiotic use and acute gastroenteritis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Ms. B, a 26-year-old woman, presents to your office with her 3-year-old son for a well-child examination. During the course of the conversation, she asks you if she should be giving her child probiotics to improve his general health. Many of her friends, who also have their children in day care, have told her that probiotics, “are nature’s way of fighting infection.” Her son currently takes no medications, and has no history of asthma or recent gastrointestinal disturbances. He was treated for 2 ear infections last winter, approximately 3 months apart. His physical exam is normal and, after today, his immunizations will be up to date. How should you respond?
The use of probiotics as over-the-counter treatments for a variety of conditions continues to grow, with retail sales of functional probiotic foods and supplements topping $35 billion worldwide in 2014.1 In children, claims of benefit for gastrointestinal (GI) disorders, colic, and allergy prevention, as well as prevention and treatment of upper respiratory infections (URIs) have existed for over 10 years.2-4 The human gut flora develops rapidly after birth and is known to be influenced by route of delivery (vaginal vs cesarean), type of feeding (breast vs formula), and other environmental factors.5 The use of probiotics to influence the types of bacteria in a child’s intestinal tract continues to be an area of active research. (For more on probiotic formulations, see TABLE 1.)
This article summarizes recent research on probiotic use in infants and children. New data support the use of probiotics for the treatment of colic and atopic eczema; however, the data on using probiotics in the management of URIs is less robust and mixed. And while probiotics improve irritable bowel syndrome (IBS) stomach pain, they do not help with related diarrhea or constipation. All of these data are summarized in TABLE 2.6-29
L reuteri improves symptoms in breastfed infants with colic
Infant colic is a relatively common condition known to negatively impact maternal mental health and the mother/child relationship.6 Numerous randomized controlled trials (RCTs) over the years have demonstrated mixed results with using probiotics to decrease crying times, with differences noted between infants who are solely breastfed and those who are not.7
In the most recent meta-analysis of 6 studies (n=427) that focused only on the probiotic Lactobacillus reuteri, breastfed infants with colic receiving a daily dose of 108 colony forming units (CFU) cried an average of 56 fewer minutes/day than those in the control group (95% confidence interval [CI], -64.4 to -47.3; P=.001) at day 21 of treatment.8 Although 2 studies in this meta-analysis included a small number of mixed-fed and formula-fed infants, the majority of trials do not show benefit for these infants. Trials assessing the use of L reuteri for prevention of colic have not shown positive results.7
Probiotics may help p
The mechanisms by which probiotics may prevent or shorten the course of URIs are not obvious. Current theories include boosting the immune function of the respiratory mucosa, acting as a competitive inhibitor for viruses, and secreting antiviral compounds.9 Multiple reviews published in the last 3 years, however, add to the evidence that the apparent benefit is real.
A 2013 meta-analysis assessed data from 4 RCTs (N=1805), which used Lactobacillus rhamnosus as the sole probiotic for prevention of URIs. In treated children, otitis media incidence was reduced by 24% (relative risk [RR] 0.76; 95% CI, 0.64-0.91) and risk of URI was reduced by 38% (RR 0.62; 95% CI, 0.50-0.78).10 The number needed to treat (NNT) was 4 for URI prevention, and the authors noted that adverse events were similar in the treatment and control groups.
A 2014 systematic review and meta-analysis of 20 RCTs examining duration of illness included 10 studies dedicated to pediatric subjects (age 12 months to 12 years).11 There were significantly fewer days of illness per person (standardized mean difference -0.31; 95% CI, -0.41 to -0.11) and each illness episode was shorter by three-quarters of a day (weighted mean difference -0.77; 95% CI, -1.5 to -0.04) in participants who received a probiotic vs those who received a placebo. Probiotics used in these studies belonged to the Lactobacillus and Bifidobacterium genera.
A 2015 systematic review of 14 RCTs assessing the benefits of probiotics, particularly Lactobacillus and Bifidobacterium strains, on URI occurrence and symptoms, showed mixed results.12 Seven of 12 studies found lowered rates of URI and otitis media incidence, 7 of 11 RCTs reported a significant reduction in severity scores for URI, and 4 of 8 RCTs reported significant reductions in school absenteeism between the probiotic and control groups. In a summary statement, the authors noted that “at least one beneficial effect of prophylactic probiotics was observed in the majority of RCTs,” and that “none of the studies reported any serious adverse events.”
Perinatal probiotics: No benefit for allergic conditions—except eczema
Allergic disease is on the rise and continues to plague children with reduced quality of life, potentially life-threatening reactions, and missed activities, including school. The gut microbiome likely influences a child’s allergic propensity through its effects on T-helper cells, transforming growth factor (TGF), and immunoglobulin A (IgA)—all known components of the allergic response. As the hygiene hypothesis suggests, the quantity and types of bacteria that inhabit the GI tract early in life play a significant role in determining a person’s later allergic responses.13
In a 2013 meta-analysis of 20 trials (N=4866), researchers looked specifically at probiotic use and the diagnosis of asthma and incident wheezing. Single and combination products of Lactobacillus and Bifidobacterium given prenatally and/or postnatally were included in the studies. The authors found no evidence to support a protective association between perinatal use of probiotics and diagnosed asthma (RR=0.99; 95% CI, 0.81-0.21) or childhood incident wheezing (RR=0.97; 95% CI, 0.87-1.09; 9 trials, 1949 infants).14
In a more recent meta-analysis (2015) conducted to inform the World Allergy Organization, 29 studies were evaluated to assess the impact of probiotics on allergic symptoms of the skin, respiratory system, and GI tract.15 No significant benefit was noted for any allergic condition except for eczema. Probiotics reduced the risk of eczema when given during the last trimester of pregnancy (RR=0.71; 95% CI, 0.60-0.84), when used by breastfeeding mothers (RR=0.57; 95% CI, 0.47-0.69), and when given to infants (RR=0.80; 95% CI, 0.68-0.94).
A 2014 systematic review and meta-analysis (N=2797) explored probiotic use specifically for the prevention of eczema.16 The pooled relative risk for all the studies was 0.74 (95% CI, 0.67-0.82). Evidence was strongest for probiotics containing the Lactobacillus species rhamnosus and paracasei, as well as for Bifidobacterium lactis. No benefit was noted with Lactobacillus acidophilus or other Bifidobacterium species. These newer reviews on eczema prevention contrast with an older Cochrane review published in 2008 (12 RCTs, N=781), which did not show significant benefit for the treatment of eczema.17
Probiotics improve IBS stomach pain, but not diarrhea or constipation
IBS is a functional disorder of the GI tract that affects up to 20% of children and teenagers and leads to a significant decrease in quality of life.18 Current theories of causation include bacterial overgrowth and neuronal hyperactivity, which may be amenable to change with supplemental probiotics.
A 2015 systematic review of non-pharmacological treatments for functional abdominal pain disorders identified 4 studies dedicated to IBS in children.19 A subgroup analysis of 3 RCTs (n=309) that looked at giving L rhamnosus to 5- to 17-year-olds with IBS showed improved abdominal pain (according to various pain scales) compared to the placebo group. Study participants received at least 3 x 109 CFU twice a day for 4 to 8 weeks. Relative risk for improvement was 1.7 (95% CI, 1.27-2.27) with an NNT of 4. None of these studies showed significant improvement in either frequency or severity of diarrhea or constipation.
A separate crossover RCT (N=59) compared placebo to VSL#3, a product containing 8 probiotics (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, L acidophilus, Lactobacillus plantarum, L paracasei, Lactobacillus bulgaricus, and Streptococcus hermophiles), given in age-dependent doses for 6 weeks to children aged 4 to 18 years.20 The frequency and intensity of abdominal pain were measured on a 5-point Likert scale. The group treated with VSL#3 dropped 1.0 ± 0.2 points vs 0.5 ± 0.2 points in the control group (P<.05) and reported an improved quality of life.
These agents reduce antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) occurs in 5% to 30% of children who receive antibiotic therapy.21 It occurs most frequently with the use of cephalosporins, penicillin, fluoroquinolones, and clindamycin, and is likely caused by an alteration of the normal gut flora. Colitis caused by Clostridium difficile remains the most serious antibiotic-associated GI complication.
A systematic review of the specific probiotic Saccharomyces boulardii conducted in 2015 analyzed data from 6 RCTs (n=1653) to determine the effect of co-administration of this probiotic with antibiotics.22 The pooled relative risk for AAD in children receiving the probiotic was 0.43 (95% CI, 0.3-0.6) compared to antibiotics alone. The absolute risk of AAD dropped from 20.9% to 8.8%, translating to a NNT of 8. Two of the RCTs specifically looked at rates of C difficile infection (n=579). C difficile infection rates dropped by 75% (RR=.25; 95% CI, 0.08-0.73) in the treatment group. This dramatic treatment effect was not seen in studies involving adults.
A similar systematic review focusing on L rhamnosus conducted in 2015 pooled data from 5 RCTs (n=445) to see if the probiotic would decrease AAD in children if it was co-administered with antibiotics.23 The relative risk for AAD in this treatment group was 0.48 (95% CI, 0.26-0.89) with an absolute risk reduction of 13.4% (23% compared to 9.6%), translating to an NNT of 7.
A Cochrane review published in 2015 included 23 studies (N=3938) and found similar results with an RR for AAD of 0.46 for treated children (95% CI, 0.35-0.61).24 Doses of probiotics ranged from 5 to 40 billion CFU/day. Although many probiotic species were used in these studies, S boulardii and L rhamnosus were cited as having the strongest data to support use in this context.
Probiotics reduce the duration, frequency of acute infectious diarrhea
Diarrhea remains the second leading cause of death among children one to 59 months of age worldwide.25 Current World Health Organization recommendations include oral rehydration salts, continued feeding to avoid dehydration, and zinc to decrease the duration and severity of illness.26 Multiple studies in adults confirm that a variety of probiotics decrease both the duration and severity of diarrhea in acute gastroenteritis.27
The authors of a 2013 systematic review of probiotics for the treatment of community-acquired acute diarrhea in children less than 5 years of age analyzed data from 8 RCTs (N=1755).28 Various probiotics were used including Lactobacillus species, Streptococcus thermophilus, Bifidobacterium species, and Saccharomyces boulardii for between 4 and 10 days. Six of these studies (n=1164) measured diarrhea duration and found a 14% reduction (95% CI, 3.8%-24.2%) in days of illness for those children treated vs those receiving placebo. Five studies (n=925) measured the difference in stool frequency on Day 2 of illness and reported a reduction of 13.1% (95% CI, 0.8%-5.3%) in the number of stools in the treated group vs the placebo group.
This review augments a Cochrane meta-analysis of 63 studies (N=8014) published in 2010.27 Fifty-six of these studies included infants and children. Pooled analysis of the varied probiotic treatments showed a mean reduction in duration of diarrhea of just over a day (24.76 hours; 95% CI, 15.9-33.6 hours; n=4555, trials=35) and decreased stool frequency on Day 2 of treatment (mean difference 0.80; 95% CI, 0.45-1.14; n=2751, trials=20). The authors concluded that probiotics “have clear beneficial effects in shortening the duration and reducing stool frequency in acute infectious diarrhea.”
Pediatric society weighs in. In 2014, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition issued guidelines regarding probiotic use for the treatment of acute gastroenteritis.29 In addition to rehydration therapy, these guidelines recommend the use of L rhamnosus and/or S boulardii as first-line treatments. Lower quality evidence is available for the use of L reuteri.
CASE › In response to Ms. B’s query about starting her young son on probiotics, you tell her that studies have shown that probiotics are safe for children when given in appropriate doses. They have been shown to help children recover from diarrheal illnesses and can help reduce the number of colds and ear infections when taken regularly. The reason you are giving them determines which strains you should use. You recommend giving her child a formulation of probiotic that contains Lactobacillus or Bifidobacterium with a dose range of 2 to 10 billion CFUs taken daily to reduce the risk of her child getting another ear infection.
CORRESPONDENCE
Paul Dassow, MD, MSPH, 1100 E. 3rd St, Chattanooga, TN 37403; [email protected].
1. Euromonitor International. Global and regional trends of the probiotics and omega fatty acids market. June 23, 2015. Available at: http://uschinahpa.org/wp-content/uploads/2015/07/EMI-US-China-HPA-Probiotic-and-Omega-2015-Final.pdf. Accessed September 9, 2016.
2. Du Toit G, Lack G. Can food allergy be prevented? The current evidence. Pediatr Clin North Am. 2011;58:481-509.
3. Gerritsen J, Smidt H, Rijkers GT, et al. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209-240.
4. Versalovic J. The human microbiome and probiotics: implications for pediatrics. Ann Nutr Metab. 2013;63:42-52.
5. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
6. Akman I, Kușçu K, Özdemir N, et al. Mothers’ postpartum psychological adjustment and infantile colic. Arch Dis Child. 2006;91:417-419.
7. Sung V, Collett S, de Gooyer T, et al. Probiotics to prevent or treat excessive infant crying systematic review and meta-analysis. JAMA Pediatr. 2013:167:1150-1157.
8. Harb T, Matsuyama M, David M, et al. Infant colic—what works: a systematic review of interventions for breastfed infants. J Pediatr Gastroenterol Nutr. 2016;62:668-686.
9. Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
10. Liu S, Hu P, Du X, et al. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013;50:377-381.
11. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
12. Ozen M, Kocabas Sandal G, Dinleyici EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. 2015;15:9-20.
13. Azad MB, Konya T, Maughan H, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15.
14. Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. Brit Med J. 2013;347:f6471.
15. Cuello-Garcia CA, Bro˙zek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952-961.
16. Mansfield JA, Bergin SW, Cooper JR, et al. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. 2014;179:580-592.
17. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
18. Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Expert Rev Gastroenterol Hepatol. 2010;4:293-304.
19. Rutten JMTM, Korterink JL, Venmans LMAJ, et al. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics. 2015;135:522-535.
20. Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24-30.
21. Turck D, Bernet JP, Marx J, et al. Incidence and risk factors of oral antibiotic associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37:22-26.
22. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
23. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149-1157.
24. Goldenberg JZ, Lytvyn L, Steurich J, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2015;12:CD004827.
25. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151-2161.
26. WHO/UNICEF Joint Statement: Clinical Management of Acute Diarrhea. August 2004. Available at: http://www.unicef.org/publications/files/ENAcute_Diarrhoea_reprint.pdf. Accessed September 9, 2016.
27. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst Rev. 2010;(11):CD003048.
28. Applegate JA, Fischer Walker CL, Ambikapathi R, et al. Systematic review of probiotics for the treatment of community-acquired acute diarrhea in children. BMC Public Health. 2013;13:S16.
29. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132-152.
1. Euromonitor International. Global and regional trends of the probiotics and omega fatty acids market. June 23, 2015. Available at: http://uschinahpa.org/wp-content/uploads/2015/07/EMI-US-China-HPA-Probiotic-and-Omega-2015-Final.pdf. Accessed September 9, 2016.
2. Du Toit G, Lack G. Can food allergy be prevented? The current evidence. Pediatr Clin North Am. 2011;58:481-509.
3. Gerritsen J, Smidt H, Rijkers GT, et al. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209-240.
4. Versalovic J. The human microbiome and probiotics: implications for pediatrics. Ann Nutr Metab. 2013;63:42-52.
5. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
6. Akman I, Kușçu K, Özdemir N, et al. Mothers’ postpartum psychological adjustment and infantile colic. Arch Dis Child. 2006;91:417-419.
7. Sung V, Collett S, de Gooyer T, et al. Probiotics to prevent or treat excessive infant crying systematic review and meta-analysis. JAMA Pediatr. 2013:167:1150-1157.
8. Harb T, Matsuyama M, David M, et al. Infant colic—what works: a systematic review of interventions for breastfed infants. J Pediatr Gastroenterol Nutr. 2016;62:668-686.
9. Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
10. Liu S, Hu P, Du X, et al. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013;50:377-381.
11. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
12. Ozen M, Kocabas Sandal G, Dinleyici EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. 2015;15:9-20.
13. Azad MB, Konya T, Maughan H, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15.
14. Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. Brit Med J. 2013;347:f6471.
15. Cuello-Garcia CA, Bro˙zek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952-961.
16. Mansfield JA, Bergin SW, Cooper JR, et al. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. 2014;179:580-592.
17. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
18. Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Expert Rev Gastroenterol Hepatol. 2010;4:293-304.
19. Rutten JMTM, Korterink JL, Venmans LMAJ, et al. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics. 2015;135:522-535.
20. Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24-30.
21. Turck D, Bernet JP, Marx J, et al. Incidence and risk factors of oral antibiotic associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37:22-26.
22. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
23. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149-1157.
24. Goldenberg JZ, Lytvyn L, Steurich J, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2015;12:CD004827.
25. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151-2161.
26. WHO/UNICEF Joint Statement: Clinical Management of Acute Diarrhea. August 2004. Available at: http://www.unicef.org/publications/files/ENAcute_Diarrhoea_reprint.pdf. Accessed September 9, 2016.
27. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst Rev. 2010;(11):CD003048.
28. Applegate JA, Fischer Walker CL, Ambikapathi R, et al. Systematic review of probiotics for the treatment of community-acquired acute diarrhea in children. BMC Public Health. 2013;13:S16.
29. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132-152.
Recreational cannabinoid use: The hazards behind the “high”
PRACTICE RECOMMENDATIONS
› Screen all patients for use of addiction-prone substances. A
› Screen cannabis users with a validated secondary screen for problematic use. A
› Counsel patients that there is no evidence that use of recreational cannabis is safe; advise them that it can cause numerous physical, psychomotor, cognitive, and psychiatric effects. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Approximately 156 million Americans (49% of the population) have tried cannabis.1 About 5.7 million people ages 12 years and older use it daily or almost daily, a number that has nearly doubled since 2006.2 There are 6600 new users in the United States every day,2 and almost half of all high school students will have tried it by graduation.3
There is limited evidence that cannabis may have medical benefit in some circumstances.4 (See “Medical marijuana: A treatment worth trying?” J Fam Pract. 2016;65:178-185 or http://www.mdedge.com/jfponline/article/106836/medical-marijuana-treatment-worth-trying.) As a result, it is now legal for medical purposes in 25 states. Recreational use by adults is also legal in 4 states and the District of Columbia.5 The US Food and Drug Administration, however, has reaffirmed its stance that marijuana is a Schedule I drug on the basis of its “high potential for abuse” and the absence of “currently accepted medical uses.”6
The effects of legalizing the medical and recreational use of cannabis for individuals—and society as a whole—are uncertain. Debate is ongoing about the risks, benefits, and rights of individuals. Some argue it is safer than alcohol or that criminalization has been ineffective and even harmful. Others make the case for personal liberty and autonomy. Still, others are convinced legalization is a misdirected experiment that will result in diverse adverse outcomes. Regardless, it is important that primary care providers understand the ramifications of marijuana use. This evidence-based narrative highlights major negative consequences of non-medical cannabinoid use.
Potential adverse consequences of cannabis use
Although the potential adverse consequences are vast, the literature on this subject is limited for various reasons:
- Many studies are observational with a small sample size.
- Most studies examine smoked cannabis—not other routes of delivery.
- When smoked, the dose, frequency, duration, and smoking technique are variable.
- The quantity of Δ-9-tetrahydrocannabinol (THC), the primary psychoactive component in cannabis, is variable. (For more on the chemical properties of the marijuana plant, see “Cannabinoids: A diverse group of chemicals.”7)
- Most studies do not examine medical users, who are expected to use less cannabis or lower doses of THC.
- There are confounding effects of other drugs, notably tobacco, which is used by up to 90% of cannabis users.8
Lower quality of life. In general, regular non-medical cannabis use is associated with a lower quality of life and poorer socioeconomic outcomes (TABLE 1).9-12 Physical and mental health is ranked lower by heavy users as compared to extremely low users.9 Some who attempt butane extraction of THC from the plant have experienced explosions and severe burns.13
Studies regarding cannabis use and weight are conflicting. Appetite and weight may increase initially, and young adults who increase their use of the drug are more likely to find themselves on an increasing obesity trajectory.14 However, in an observational study of nearly 11,000 participants ages 20 to 59 years, cannabis users had a lower body mass index, better lipid parameters, and were less likely to have diabetes than non-using counterparts.15
Elevated rates of MI. Chronic effects may include oral health problems,16 gynecomastia, and changes in sexual function.17 Elevated rates of myocardial infarction, cardiomyopathy, limb arteritis, and stroke have been observed.18 Synthetic cannabinoids have been associated with heart attacks and acute renal injury in youth;19,20 however, plant-based marijuana does not affect the kidneys. In addition, high doses of plant-based marijuana can result in cannabinoid hyperemesis syndrome, characterized by cyclic vomiting and compulsive bathing that resolves with cessation of the drug.21
No major pulmonary effects. Interestingly, cannabis does not appear to have major negative pulmonary effects. Acutely, smoking marijuana causes bronchodilation.22 Chronic, low-level use over 20 years is associated with an increase in forced expiratory volume in one second (FEV1), but this upward trend diminishes and may reverse in high-level users.23 Although higher lung volumes are observed, cannabis does not appear to contribute to the development of chronic obstructive pulmonary disease, but can cause chronic bronchitis that resolves with smoking cessation.22 Chronic use has also been tied to airway infection. Lastly, fungal growth has been found on marijuana plants, which is concerning because of the potential to expose people to Aspergillus.22,24
Cannabis and cancer? The jury is out. Cannabis contains at least 33 carcinogens25 and may be contaminated with pesticides,26 but research about its relationship with cancer is incomplete. Although smoking results in histopathologic changes of the bronchial mucosa, evidence of lung cancer is mixed.22,25,27 Some studies have suggested associations with cancers of the brain, testis, prostate, and cervix,25,27 as well as certain rare cancers in children due to parental exposure.25,27
There are conflicting data about associations with head and neck squamous cell carcinoma,25,27,28 bladder cancer,25,29 and non-Hodgkin’s lymphoma.25,30 Some studies suggest marijuana offers protection against certain types of cancer. In fact, it appears that some cannabinoids found in marijuana, such as cannabidiol (CBD), may be antineoplastic.31 The potential oncogenic effects of edible and topical cannabinoid products have not been investigated.
Use linked to car accidents. More recent work indicates cannabis use is associated with injuries in motor vehicle,32 non-traffic,33 and workplace34 settings. In fact, a meta-analysis found a near-doubling of motor vehicle accidents with recent use.32 Risk is dose-dependent and heightened with alcohol.35-37 Psychomotor impairment persists for at least 6 hours after smoking cannabis,38 at least 10 hours after ingesting it,37 and may last up to 24 hours, as indicated by a study involving pilots using a flight simulator.39
In contrast to alcohol, there is a greater decrement in routine vs complex driving tasks in experimental studies.35,36 Behavioral strategies, like driving slowly, are employed to compensate for impairment, but the ability to do so is lost with alcohol co-ingestion.35 Importantly, individuals using marijuana may not recognize the presence or extent of the impairment they are experiencing,37,39 placing themselves and others in danger.
Data are insufficient to ascribe to marijuana an increase in overall mortality,40 and there have been no reported overdose deaths from respiratory depression. However, a few deaths and a greater number of hospitalizations, due mainly to central nervous system effects including agitation, depression, coma, delirium, and toxic psychosis, have been attributed to the use of synthetic cannabinoids.20
Cannabis use can pose a risk to the fetus. About 5% of pregnant women report recent marijuana use2 for recreational or medical reasons (eg, morning sickness), and there is concern about its effects on the developing fetus. Certain rare pediatric cancers22,25 and birth defects41 have been reported with cannabis use (TABLE 222,25,41,42). Neonatal withdrawal is minor, if present at all.42 Moderate evidence indicates prenatal and breastfeeding exposure can result in multiple developmental problems, as well as an increased likelihood of initiating tobacco and marijuana use as teens.41,42
Cognitive effects of cannabis are a concern. The central nervous system is not fully myelinated until the age of 18, and complete maturation continues beyond that. Due to neuroplasticity, life experiences and exogenous agents may result in further changes. Cannabis produces changes in brain structure and function that are evident on neuroimaging.43 Although accidental pediatric intoxication is alarming, negative consequences are likely to be of short duration.
Regular use by youth, on the other hand, negatively affects cognition and delays brain maturation, especially for younger initiates.9,38,44 With abstinence, deficits tend to normalize, but they may last indefinitely among young people who continue to use marijuana.44
Dyscognition is less severe and is more likely to resolve with abstinence in adults,44 which may tip the scale for adults weighing whether to use cannabis for a medical purpose.45 Keep in mind that individuals may not be aware of their cognitive deficits,46 even though nearly all domains (from basic motor coordination to more complex executive function tasks, such as the ability to control emotions and behavior) are affected.44 A possible exception may be improvement in attention with acute use in daily, but not occasional, users.44 Highly focused attention, however, is not always beneficial if it delays redirection toward a new urgent stimulus.
Mood benefit? Research suggests otherwise. The psychiatric effects of cannabis are not fully understood. Users may claim mood benefit, but research suggests marijuana prompts the development or worsening of anxiety, depression, and suicidality.12,47 Violence, paranoia, and borderline personality features have also been associated with use.38,47 Amotivational syndrome, a disorder that includes apathy, callousness, and antisocial behavior, has been described, but the interplay between cannabis and motivation beyond recent use is unclear.48
Lifetime cannabis use is related to panic,49 yet correlational studies suggest both benefit and problems for individuals who use cannabis for posttraumatic stress disorder.50 It is now well established that marijuana use is an independent causal risk factor for the development of psychosis, particularly in vulnerable youth, and that it worsens schizophrenia in those who suffer from it.51 Human experimental studies suggest this may be because the effect of THC is counteracted by CBD.52 Synthetic cannabinoids are even more potent anxiogenic and psychogenic agents than plant-based marijuana.19,20
Cannabis Use Disorder
About 9% of those who try cannabis develop Cannabis Use Disorder, which is characterized by continued use of the substance despite significant distress or impairment.53 Cannabis Use Disorder is essentially an addiction. Primary risk factors include male gender, younger age at marijuana initiation, and personal or family history of other substance or psychiatric problems.53
Although cannabis use often precedes use of other addiction-prone substances, it remains unclear if it is a “gateway” to the use of other illicit drugs.54 Marijuana withdrawal is relatively minor and is comparable to that for tobacco.55 While there are no known effective pharmacotherapies for discontinuing cannabis use, addiction therapy—including cognitive behavioral therapy and trigger management—is effective.56
SIDEBAR
Cannabinoids: A diverse group of chemicalsCannabis, the genus name for 3 species of marijuana plant (sativa, indica, ruderalis), has come to mean any psychoactive part of the plant and is used interchangeably with “marijuana.” There are at least 85 different cannabinoids in the native plant.7
Cannabinoids are a diverse group of chemicals that have activity at cannabinoid receptors. Δ-9-tetrahydrocannabinol (THC), a partial agonist of the CB1 receptor, is the primary psychoactive component and is found in larger quantities in Cannabis sativa, which is preferred by non-medical users. Cannabidiol (CBD), a weak partial CB1 antagonist, exhibits few, if any, psychotropic properties and is more plentiful in Cannabis indica.
Synthetic cannabinioids are a heterogeneous group of manufactured drugs that are full CB1 agonists and that are more potent than THC, yet are often assumed to be safe by users. Typically, they are dissolved in solvents, sprayed onto inert plant materials, and marketed as herbal products like “K2” and “spice.”
So how should the evidence inform your care?
Screen all patients for use of cannabinoids and other addiction-prone substances.57 Follow any affirmative answers to your questions about cannabis use by asking about potential negative consequences of use. For example, ask patients:
- How often during the past 6 months did you find that you were unable to stop using cannabis once you started?
- How often during the past 6 months did you fail to do what was expected of you because of using cannabis? (For more questions, see the Cannabis Use Disorder Identification Test available at: http://www.otago.ac.nz/nationaladdictioncentre/pdfs/cudit-r.pdf.)
Other validated screening tools include the Severity of Dependence Scale, the Cannabis Abuse Screening Test, and the Problematic Use of Marijuana.58
Counsel patients about possible adverse effects and inform them there is no evidence that recreational marijuana or synthetic cannabinoids can be used safely over time. Consider medical use requests only if there is a favorable risk/benefit balance, other recognized treatment options have been exhausted, and you have a strong understanding of the use of cannabis in the medical condition being considered.4
Since brief interventions using motivational interviewing to reduce or eliminate recreational use have not been found to be effective,59 referral to an addiction specialist may be indicated. If a diagnosis of cannabis use disorder is established, then abstinence from addiction-prone substances including marijuana, programs like Marijuana Anonymous (Available at: https://www.marijuana-anonymous.org/), and individualized addiction therapy scaled to the severity of the condition can be effective.56 Because psychiatric conditions frequently co-occur and complicate addiction,53 they should be screened for and managed, as well.
Drug testing. Cannabis Use Disorder has significant relapse potential.60 Abstinence and treatment adherence should be ascertained through regular follow-up that includes a clinical interview, exam, and body fluid drug testing. Point-of-care urine analysis for substances of potential addiction has limited utility. Definitive testing of urine with gas chromotography/mass spectrometry (GC/MS) or liquid chromatography (LC/MS-MS) can eliminate THC false-positives and false-negatives that can occur with point-of-care urine immunoassays. In addition, GCMS and LC/MS-MS can identify synthetic cannabinoids; in-office immunoassays cannot.
If the patient relapses, subsequent medical care should be coordinated with an addiction specialist with the goal of helping the patient to abstain from cannabis.
CORRESPONDENCE
Steven Wright, MD, FAAFP, 5325 Ridge Trail, Littleton, CO 80123; [email protected].
1. Pew Research Center. 6 facts about marijuana. Available at: http://www.pewresearch.org/fact-tank/2015/04/14/6-facts-about-marijuana/. Accessed September 27, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Pub # (SMA) 14-4863. 2014. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf. Accessed September 27, 2015.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future National Survey on Drug Use 1975-2015. Available at: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Accessed September 23, 2015.
4. Metts J, Wright S, Sundaram J, et al. Medical marijuana: a treatment worth trying? J Fam Pract. 2016;65:178-185.
5. Governing the states and localities. State marijuana laws map. Available at: http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html. Accessed October 12, 2016.
6. US Drug Enforcement Administration. Drug scheduling. Available at: https://www.dea.gov/druginfo/ds.shtml. Accessed October 12, 2016.
7. El-Alfy AT, Ivey K, Robinson K, et al. Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav. 2010;95:434-442.
8. Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107:1404-1417.
9. Gruber AJ, Pope HG, Hudson JI, et al. Attributes of long-term heavy cannabis users: a case-control study. Psychol Med. 2003;33:1415-1422.
10. Palamar JJ, Fenstermaker M, Kamboukos D, et al. Adverse psychosocial outcomes associated with drug use among US high school seniors: a comparison of alcohol and marijuana. Am J Drug Alcohol Abuse. 2014;40:438-446.
11. Zwerling C, Ryan J, Orav EJ. The efficacy of preemployment drug screening for marijuana and cocaine in predicting employment outcome. JAMA. 1990;264:2639-2643.
12. Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123-1135.
13. Bell C, Slim J, Flaten HK, et al. Butane hash oil burns associated with marijuana liberalization in Colorado. J Med Toxicol. 2015;11:422-425.
14. Huang DYC, Lanza HI, Anglin MD. Association between adolescent substance use and obesity in young adulthood: a group-based dual trajectory analysis. Addict Behav. 2013;38:2653-2660.
15. Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494.
16. Cho CM, Hirsch R, Johnstone S. General and oral health implications of cannabis use. Aust Dent J. 2005;50:70-74.
17. Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav. 2010;58:91-99.
18. Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg. 2013;27:996-1005.
19. Mills B, Yepes A, Nugent K. Synthetic cannabinoids. Am J Med Sci. 2015;350:59-62.
20. Tuv SS, Strand MC, Karinen R, et al. Effect and occurrence of synthetic cannabinoids. Tidsskr Nor Laegeforen. 2012;132:2285-2288.
21. Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011;104:659-964.
22. Gates P, Jaffe A, Copeland J. Cannabis smoking and respiratory health: considerations of the literature. Respirology. 2014;19:655-662.
23. Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years: The Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2012;307:173-181.
24. Verweij PE, Kerremans JJ, Vos A, et al. Fungal contamination of tobacco and marijuana. JAMA. 2000;284:2875.
25. Office of Environmental Health Hazard Assessment. Evidence on the carcinogenicity of marijuana smoke. August 2009. Available at: http://oehha.ca.gov/media/downloads/crnr/finalmjsmokehid.pdf. Accessed September 5, 2015.
26. Stone D. Cannabis, pesticides and conflicting laws: the dilemma for legalized States and implications for public health. Regul Toxicol Pharmacol. 2014;69:284-288.
27. Hashibe M, Straif K, Tashkin DP, et al. Epidemiologic review of marijuana and cancer risk. Alcohol. 2005;35:265-275.
28. Liang C, McClean MD, Marsit C, et al. A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prev Res (Phila). 2009;2:759-768.
29. Thomas AA, Wallner LP, Quinn VP, et al. Association between cannabis use and the risk of bladder cancer: results from the California Men’s Health Study. Urology. 2015;85:388-392.
30. Holly EA, Lele C, Bracci PM, et al. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Francisco Bay area, California. Am J Epidemiol. 1999;150:375-389.
31. Massi P, Solinas M, Cinquina V, et al. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303-312.
32. Ashbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536.
33.Barrio G, Jimenez-Mejias E, Pulido J, et al. Association between cannabis use and non-traffic injuries. Accid Anal Prev. 2012;47:172-176.
34. MacDonald S, Hall W, Roman P, et al. Testing for cannabis in the work-place: a review of the evidence. Addiction. 2010;105:408-416.
35. Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict. 2009;18:185-193.
36. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109-119.
37. Menetrey A, Augsburger M, Favrat B, et al. Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoid levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Δ9-THC. J Anal Toxicol. 2005;29:327-338.
38. Raemakers JG, Kaurert G, van Ruitenbeek P, et al. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296-2303.
39. Leirer VO, Yesavage JA, Morrow DG. Marijuana carry-over effects on aircraft pilot performance. Aviat Space Environ Med. 1991;62:221-227.
40. Calabria B, Degenhardt L, Hall W, et al. Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010;29:318-330.
41. Colorado Department of Public Health and Environment. Monitoring health concerns related to marijuana in Colorado: 2014. Changes in marijuana use patterns, systematic literature review, and possible marijuana-related health effects. Available at: http://www2.cde.state.co.us/artemis/hemonos/he1282m332015internet/he1282m332015internet01.pdf. Accessed September 5, 2015.
42. Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn. Perinatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009-1024.
43. Batalla A, Bhattacharyya S, Yücel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821.
44. Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1-8.
45. Pavisian B, MacIntosh BJ, Szilagyi G, et al. Effects of cannabis on cognition in patients with multiple sclerosis: a psychometric and MRI study. Neurology. 2014;82:1879-1887.
46. Bartholomew J, Holroyd S, Heffernan TM. Does cannabis use affect prospective memory in young adults? J Psychopharmacol. 2010;24:241-246.
47. Copeland J, Rooke S, Swift W. Changes in cannabis use among young people: impact on mental health. Curr Opin Psychiatry. 2013;26:325-329.
48. Ari M, Sahpolat M, Kokacya H, et al. Amotivational syndrome: less known and diagnosed as a clinical. J Mood Disord. 2015;5:31-35.
49. Zvolensky MJ, Cougle JR, Johnson KA, et al. Marijuana use and panic psychopathology among a representative sample of adults. Exp Clin Psychopharmacol. 2010;18(2):129-134.
50. Yarnell S. The use of medicinal marijuana for posttraumatic stress disorder: a review of the current literature. Prim Care Companion CNS Disord. 2015;17(3).
51. Le Bec PY, Fatséas M, Denis C, et al. Cannabis and psychosis: search of a causal link through a critical and systematic review. Encephale. 2009;35:377-385.
52. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19-27.
53. Lopez-Quintero C, Perez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
54. Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108:84-97.
55. Vandrey RG, Budney AJ, Hughes JR, et al. A within subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92:48-54.
56.Budney AJ, Roffman R, Stephens RS, et al. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4-16.
57. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Phys. 2014;60:801-808.
58. Piontek D, Kraus L, Klempova D. Short scales to assess cannabis-related problems: a review of psychometric properties. Subst Abuse Treat Prev Policy. 2008;3:25.
59. Saitz R, Palfai TPA, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312:502-513.
60. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689-1695.
PRACTICE RECOMMENDATIONS
› Screen all patients for use of addiction-prone substances. A
› Screen cannabis users with a validated secondary screen for problematic use. A
› Counsel patients that there is no evidence that use of recreational cannabis is safe; advise them that it can cause numerous physical, psychomotor, cognitive, and psychiatric effects. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Approximately 156 million Americans (49% of the population) have tried cannabis.1 About 5.7 million people ages 12 years and older use it daily or almost daily, a number that has nearly doubled since 2006.2 There are 6600 new users in the United States every day,2 and almost half of all high school students will have tried it by graduation.3
There is limited evidence that cannabis may have medical benefit in some circumstances.4 (See “Medical marijuana: A treatment worth trying?” J Fam Pract. 2016;65:178-185 or http://www.mdedge.com/jfponline/article/106836/medical-marijuana-treatment-worth-trying.) As a result, it is now legal for medical purposes in 25 states. Recreational use by adults is also legal in 4 states and the District of Columbia.5 The US Food and Drug Administration, however, has reaffirmed its stance that marijuana is a Schedule I drug on the basis of its “high potential for abuse” and the absence of “currently accepted medical uses.”6
The effects of legalizing the medical and recreational use of cannabis for individuals—and society as a whole—are uncertain. Debate is ongoing about the risks, benefits, and rights of individuals. Some argue it is safer than alcohol or that criminalization has been ineffective and even harmful. Others make the case for personal liberty and autonomy. Still, others are convinced legalization is a misdirected experiment that will result in diverse adverse outcomes. Regardless, it is important that primary care providers understand the ramifications of marijuana use. This evidence-based narrative highlights major negative consequences of non-medical cannabinoid use.
Potential adverse consequences of cannabis use
Although the potential adverse consequences are vast, the literature on this subject is limited for various reasons:
- Many studies are observational with a small sample size.
- Most studies examine smoked cannabis—not other routes of delivery.
- When smoked, the dose, frequency, duration, and smoking technique are variable.
- The quantity of Δ-9-tetrahydrocannabinol (THC), the primary psychoactive component in cannabis, is variable. (For more on the chemical properties of the marijuana plant, see “Cannabinoids: A diverse group of chemicals.”7)
- Most studies do not examine medical users, who are expected to use less cannabis or lower doses of THC.
- There are confounding effects of other drugs, notably tobacco, which is used by up to 90% of cannabis users.8
Lower quality of life. In general, regular non-medical cannabis use is associated with a lower quality of life and poorer socioeconomic outcomes (TABLE 1).9-12 Physical and mental health is ranked lower by heavy users as compared to extremely low users.9 Some who attempt butane extraction of THC from the plant have experienced explosions and severe burns.13
Studies regarding cannabis use and weight are conflicting. Appetite and weight may increase initially, and young adults who increase their use of the drug are more likely to find themselves on an increasing obesity trajectory.14 However, in an observational study of nearly 11,000 participants ages 20 to 59 years, cannabis users had a lower body mass index, better lipid parameters, and were less likely to have diabetes than non-using counterparts.15
Elevated rates of MI. Chronic effects may include oral health problems,16 gynecomastia, and changes in sexual function.17 Elevated rates of myocardial infarction, cardiomyopathy, limb arteritis, and stroke have been observed.18 Synthetic cannabinoids have been associated with heart attacks and acute renal injury in youth;19,20 however, plant-based marijuana does not affect the kidneys. In addition, high doses of plant-based marijuana can result in cannabinoid hyperemesis syndrome, characterized by cyclic vomiting and compulsive bathing that resolves with cessation of the drug.21
No major pulmonary effects. Interestingly, cannabis does not appear to have major negative pulmonary effects. Acutely, smoking marijuana causes bronchodilation.22 Chronic, low-level use over 20 years is associated with an increase in forced expiratory volume in one second (FEV1), but this upward trend diminishes and may reverse in high-level users.23 Although higher lung volumes are observed, cannabis does not appear to contribute to the development of chronic obstructive pulmonary disease, but can cause chronic bronchitis that resolves with smoking cessation.22 Chronic use has also been tied to airway infection. Lastly, fungal growth has been found on marijuana plants, which is concerning because of the potential to expose people to Aspergillus.22,24
Cannabis and cancer? The jury is out. Cannabis contains at least 33 carcinogens25 and may be contaminated with pesticides,26 but research about its relationship with cancer is incomplete. Although smoking results in histopathologic changes of the bronchial mucosa, evidence of lung cancer is mixed.22,25,27 Some studies have suggested associations with cancers of the brain, testis, prostate, and cervix,25,27 as well as certain rare cancers in children due to parental exposure.25,27
There are conflicting data about associations with head and neck squamous cell carcinoma,25,27,28 bladder cancer,25,29 and non-Hodgkin’s lymphoma.25,30 Some studies suggest marijuana offers protection against certain types of cancer. In fact, it appears that some cannabinoids found in marijuana, such as cannabidiol (CBD), may be antineoplastic.31 The potential oncogenic effects of edible and topical cannabinoid products have not been investigated.
Use linked to car accidents. More recent work indicates cannabis use is associated with injuries in motor vehicle,32 non-traffic,33 and workplace34 settings. In fact, a meta-analysis found a near-doubling of motor vehicle accidents with recent use.32 Risk is dose-dependent and heightened with alcohol.35-37 Psychomotor impairment persists for at least 6 hours after smoking cannabis,38 at least 10 hours after ingesting it,37 and may last up to 24 hours, as indicated by a study involving pilots using a flight simulator.39
In contrast to alcohol, there is a greater decrement in routine vs complex driving tasks in experimental studies.35,36 Behavioral strategies, like driving slowly, are employed to compensate for impairment, but the ability to do so is lost with alcohol co-ingestion.35 Importantly, individuals using marijuana may not recognize the presence or extent of the impairment they are experiencing,37,39 placing themselves and others in danger.
Data are insufficient to ascribe to marijuana an increase in overall mortality,40 and there have been no reported overdose deaths from respiratory depression. However, a few deaths and a greater number of hospitalizations, due mainly to central nervous system effects including agitation, depression, coma, delirium, and toxic psychosis, have been attributed to the use of synthetic cannabinoids.20
Cannabis use can pose a risk to the fetus. About 5% of pregnant women report recent marijuana use2 for recreational or medical reasons (eg, morning sickness), and there is concern about its effects on the developing fetus. Certain rare pediatric cancers22,25 and birth defects41 have been reported with cannabis use (TABLE 222,25,41,42). Neonatal withdrawal is minor, if present at all.42 Moderate evidence indicates prenatal and breastfeeding exposure can result in multiple developmental problems, as well as an increased likelihood of initiating tobacco and marijuana use as teens.41,42
Cognitive effects of cannabis are a concern. The central nervous system is not fully myelinated until the age of 18, and complete maturation continues beyond that. Due to neuroplasticity, life experiences and exogenous agents may result in further changes. Cannabis produces changes in brain structure and function that are evident on neuroimaging.43 Although accidental pediatric intoxication is alarming, negative consequences are likely to be of short duration.
Regular use by youth, on the other hand, negatively affects cognition and delays brain maturation, especially for younger initiates.9,38,44 With abstinence, deficits tend to normalize, but they may last indefinitely among young people who continue to use marijuana.44
Dyscognition is less severe and is more likely to resolve with abstinence in adults,44 which may tip the scale for adults weighing whether to use cannabis for a medical purpose.45 Keep in mind that individuals may not be aware of their cognitive deficits,46 even though nearly all domains (from basic motor coordination to more complex executive function tasks, such as the ability to control emotions and behavior) are affected.44 A possible exception may be improvement in attention with acute use in daily, but not occasional, users.44 Highly focused attention, however, is not always beneficial if it delays redirection toward a new urgent stimulus.
Mood benefit? Research suggests otherwise. The psychiatric effects of cannabis are not fully understood. Users may claim mood benefit, but research suggests marijuana prompts the development or worsening of anxiety, depression, and suicidality.12,47 Violence, paranoia, and borderline personality features have also been associated with use.38,47 Amotivational syndrome, a disorder that includes apathy, callousness, and antisocial behavior, has been described, but the interplay between cannabis and motivation beyond recent use is unclear.48
Lifetime cannabis use is related to panic,49 yet correlational studies suggest both benefit and problems for individuals who use cannabis for posttraumatic stress disorder.50 It is now well established that marijuana use is an independent causal risk factor for the development of psychosis, particularly in vulnerable youth, and that it worsens schizophrenia in those who suffer from it.51 Human experimental studies suggest this may be because the effect of THC is counteracted by CBD.52 Synthetic cannabinoids are even more potent anxiogenic and psychogenic agents than plant-based marijuana.19,20
Cannabis Use Disorder
About 9% of those who try cannabis develop Cannabis Use Disorder, which is characterized by continued use of the substance despite significant distress or impairment.53 Cannabis Use Disorder is essentially an addiction. Primary risk factors include male gender, younger age at marijuana initiation, and personal or family history of other substance or psychiatric problems.53
Although cannabis use often precedes use of other addiction-prone substances, it remains unclear if it is a “gateway” to the use of other illicit drugs.54 Marijuana withdrawal is relatively minor and is comparable to that for tobacco.55 While there are no known effective pharmacotherapies for discontinuing cannabis use, addiction therapy—including cognitive behavioral therapy and trigger management—is effective.56
SIDEBAR
Cannabinoids: A diverse group of chemicalsCannabis, the genus name for 3 species of marijuana plant (sativa, indica, ruderalis), has come to mean any psychoactive part of the plant and is used interchangeably with “marijuana.” There are at least 85 different cannabinoids in the native plant.7
Cannabinoids are a diverse group of chemicals that have activity at cannabinoid receptors. Δ-9-tetrahydrocannabinol (THC), a partial agonist of the CB1 receptor, is the primary psychoactive component and is found in larger quantities in Cannabis sativa, which is preferred by non-medical users. Cannabidiol (CBD), a weak partial CB1 antagonist, exhibits few, if any, psychotropic properties and is more plentiful in Cannabis indica.
Synthetic cannabinioids are a heterogeneous group of manufactured drugs that are full CB1 agonists and that are more potent than THC, yet are often assumed to be safe by users. Typically, they are dissolved in solvents, sprayed onto inert plant materials, and marketed as herbal products like “K2” and “spice.”
So how should the evidence inform your care?
Screen all patients for use of cannabinoids and other addiction-prone substances.57 Follow any affirmative answers to your questions about cannabis use by asking about potential negative consequences of use. For example, ask patients:
- How often during the past 6 months did you find that you were unable to stop using cannabis once you started?
- How often during the past 6 months did you fail to do what was expected of you because of using cannabis? (For more questions, see the Cannabis Use Disorder Identification Test available at: http://www.otago.ac.nz/nationaladdictioncentre/pdfs/cudit-r.pdf.)
Other validated screening tools include the Severity of Dependence Scale, the Cannabis Abuse Screening Test, and the Problematic Use of Marijuana.58
Counsel patients about possible adverse effects and inform them there is no evidence that recreational marijuana or synthetic cannabinoids can be used safely over time. Consider medical use requests only if there is a favorable risk/benefit balance, other recognized treatment options have been exhausted, and you have a strong understanding of the use of cannabis in the medical condition being considered.4
Since brief interventions using motivational interviewing to reduce or eliminate recreational use have not been found to be effective,59 referral to an addiction specialist may be indicated. If a diagnosis of cannabis use disorder is established, then abstinence from addiction-prone substances including marijuana, programs like Marijuana Anonymous (Available at: https://www.marijuana-anonymous.org/), and individualized addiction therapy scaled to the severity of the condition can be effective.56 Because psychiatric conditions frequently co-occur and complicate addiction,53 they should be screened for and managed, as well.
Drug testing. Cannabis Use Disorder has significant relapse potential.60 Abstinence and treatment adherence should be ascertained through regular follow-up that includes a clinical interview, exam, and body fluid drug testing. Point-of-care urine analysis for substances of potential addiction has limited utility. Definitive testing of urine with gas chromotography/mass spectrometry (GC/MS) or liquid chromatography (LC/MS-MS) can eliminate THC false-positives and false-negatives that can occur with point-of-care urine immunoassays. In addition, GCMS and LC/MS-MS can identify synthetic cannabinoids; in-office immunoassays cannot.
If the patient relapses, subsequent medical care should be coordinated with an addiction specialist with the goal of helping the patient to abstain from cannabis.
CORRESPONDENCE
Steven Wright, MD, FAAFP, 5325 Ridge Trail, Littleton, CO 80123; [email protected].
PRACTICE RECOMMENDATIONS
› Screen all patients for use of addiction-prone substances. A
› Screen cannabis users with a validated secondary screen for problematic use. A
› Counsel patients that there is no evidence that use of recreational cannabis is safe; advise them that it can cause numerous physical, psychomotor, cognitive, and psychiatric effects. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Approximately 156 million Americans (49% of the population) have tried cannabis.1 About 5.7 million people ages 12 years and older use it daily or almost daily, a number that has nearly doubled since 2006.2 There are 6600 new users in the United States every day,2 and almost half of all high school students will have tried it by graduation.3
There is limited evidence that cannabis may have medical benefit in some circumstances.4 (See “Medical marijuana: A treatment worth trying?” J Fam Pract. 2016;65:178-185 or http://www.mdedge.com/jfponline/article/106836/medical-marijuana-treatment-worth-trying.) As a result, it is now legal for medical purposes in 25 states. Recreational use by adults is also legal in 4 states and the District of Columbia.5 The US Food and Drug Administration, however, has reaffirmed its stance that marijuana is a Schedule I drug on the basis of its “high potential for abuse” and the absence of “currently accepted medical uses.”6
The effects of legalizing the medical and recreational use of cannabis for individuals—and society as a whole—are uncertain. Debate is ongoing about the risks, benefits, and rights of individuals. Some argue it is safer than alcohol or that criminalization has been ineffective and even harmful. Others make the case for personal liberty and autonomy. Still, others are convinced legalization is a misdirected experiment that will result in diverse adverse outcomes. Regardless, it is important that primary care providers understand the ramifications of marijuana use. This evidence-based narrative highlights major negative consequences of non-medical cannabinoid use.
Potential adverse consequences of cannabis use
Although the potential adverse consequences are vast, the literature on this subject is limited for various reasons:
- Many studies are observational with a small sample size.
- Most studies examine smoked cannabis—not other routes of delivery.
- When smoked, the dose, frequency, duration, and smoking technique are variable.
- The quantity of Δ-9-tetrahydrocannabinol (THC), the primary psychoactive component in cannabis, is variable. (For more on the chemical properties of the marijuana plant, see “Cannabinoids: A diverse group of chemicals.”7)
- Most studies do not examine medical users, who are expected to use less cannabis or lower doses of THC.
- There are confounding effects of other drugs, notably tobacco, which is used by up to 90% of cannabis users.8
Lower quality of life. In general, regular non-medical cannabis use is associated with a lower quality of life and poorer socioeconomic outcomes (TABLE 1).9-12 Physical and mental health is ranked lower by heavy users as compared to extremely low users.9 Some who attempt butane extraction of THC from the plant have experienced explosions and severe burns.13
Studies regarding cannabis use and weight are conflicting. Appetite and weight may increase initially, and young adults who increase their use of the drug are more likely to find themselves on an increasing obesity trajectory.14 However, in an observational study of nearly 11,000 participants ages 20 to 59 years, cannabis users had a lower body mass index, better lipid parameters, and were less likely to have diabetes than non-using counterparts.15
Elevated rates of MI. Chronic effects may include oral health problems,16 gynecomastia, and changes in sexual function.17 Elevated rates of myocardial infarction, cardiomyopathy, limb arteritis, and stroke have been observed.18 Synthetic cannabinoids have been associated with heart attacks and acute renal injury in youth;19,20 however, plant-based marijuana does not affect the kidneys. In addition, high doses of plant-based marijuana can result in cannabinoid hyperemesis syndrome, characterized by cyclic vomiting and compulsive bathing that resolves with cessation of the drug.21
No major pulmonary effects. Interestingly, cannabis does not appear to have major negative pulmonary effects. Acutely, smoking marijuana causes bronchodilation.22 Chronic, low-level use over 20 years is associated with an increase in forced expiratory volume in one second (FEV1), but this upward trend diminishes and may reverse in high-level users.23 Although higher lung volumes are observed, cannabis does not appear to contribute to the development of chronic obstructive pulmonary disease, but can cause chronic bronchitis that resolves with smoking cessation.22 Chronic use has also been tied to airway infection. Lastly, fungal growth has been found on marijuana plants, which is concerning because of the potential to expose people to Aspergillus.22,24
Cannabis and cancer? The jury is out. Cannabis contains at least 33 carcinogens25 and may be contaminated with pesticides,26 but research about its relationship with cancer is incomplete. Although smoking results in histopathologic changes of the bronchial mucosa, evidence of lung cancer is mixed.22,25,27 Some studies have suggested associations with cancers of the brain, testis, prostate, and cervix,25,27 as well as certain rare cancers in children due to parental exposure.25,27
There are conflicting data about associations with head and neck squamous cell carcinoma,25,27,28 bladder cancer,25,29 and non-Hodgkin’s lymphoma.25,30 Some studies suggest marijuana offers protection against certain types of cancer. In fact, it appears that some cannabinoids found in marijuana, such as cannabidiol (CBD), may be antineoplastic.31 The potential oncogenic effects of edible and topical cannabinoid products have not been investigated.
Use linked to car accidents. More recent work indicates cannabis use is associated with injuries in motor vehicle,32 non-traffic,33 and workplace34 settings. In fact, a meta-analysis found a near-doubling of motor vehicle accidents with recent use.32 Risk is dose-dependent and heightened with alcohol.35-37 Psychomotor impairment persists for at least 6 hours after smoking cannabis,38 at least 10 hours after ingesting it,37 and may last up to 24 hours, as indicated by a study involving pilots using a flight simulator.39
In contrast to alcohol, there is a greater decrement in routine vs complex driving tasks in experimental studies.35,36 Behavioral strategies, like driving slowly, are employed to compensate for impairment, but the ability to do so is lost with alcohol co-ingestion.35 Importantly, individuals using marijuana may not recognize the presence or extent of the impairment they are experiencing,37,39 placing themselves and others in danger.
Data are insufficient to ascribe to marijuana an increase in overall mortality,40 and there have been no reported overdose deaths from respiratory depression. However, a few deaths and a greater number of hospitalizations, due mainly to central nervous system effects including agitation, depression, coma, delirium, and toxic psychosis, have been attributed to the use of synthetic cannabinoids.20
Cannabis use can pose a risk to the fetus. About 5% of pregnant women report recent marijuana use2 for recreational or medical reasons (eg, morning sickness), and there is concern about its effects on the developing fetus. Certain rare pediatric cancers22,25 and birth defects41 have been reported with cannabis use (TABLE 222,25,41,42). Neonatal withdrawal is minor, if present at all.42 Moderate evidence indicates prenatal and breastfeeding exposure can result in multiple developmental problems, as well as an increased likelihood of initiating tobacco and marijuana use as teens.41,42
Cognitive effects of cannabis are a concern. The central nervous system is not fully myelinated until the age of 18, and complete maturation continues beyond that. Due to neuroplasticity, life experiences and exogenous agents may result in further changes. Cannabis produces changes in brain structure and function that are evident on neuroimaging.43 Although accidental pediatric intoxication is alarming, negative consequences are likely to be of short duration.
Regular use by youth, on the other hand, negatively affects cognition and delays brain maturation, especially for younger initiates.9,38,44 With abstinence, deficits tend to normalize, but they may last indefinitely among young people who continue to use marijuana.44
Dyscognition is less severe and is more likely to resolve with abstinence in adults,44 which may tip the scale for adults weighing whether to use cannabis for a medical purpose.45 Keep in mind that individuals may not be aware of their cognitive deficits,46 even though nearly all domains (from basic motor coordination to more complex executive function tasks, such as the ability to control emotions and behavior) are affected.44 A possible exception may be improvement in attention with acute use in daily, but not occasional, users.44 Highly focused attention, however, is not always beneficial if it delays redirection toward a new urgent stimulus.
Mood benefit? Research suggests otherwise. The psychiatric effects of cannabis are not fully understood. Users may claim mood benefit, but research suggests marijuana prompts the development or worsening of anxiety, depression, and suicidality.12,47 Violence, paranoia, and borderline personality features have also been associated with use.38,47 Amotivational syndrome, a disorder that includes apathy, callousness, and antisocial behavior, has been described, but the interplay between cannabis and motivation beyond recent use is unclear.48
Lifetime cannabis use is related to panic,49 yet correlational studies suggest both benefit and problems for individuals who use cannabis for posttraumatic stress disorder.50 It is now well established that marijuana use is an independent causal risk factor for the development of psychosis, particularly in vulnerable youth, and that it worsens schizophrenia in those who suffer from it.51 Human experimental studies suggest this may be because the effect of THC is counteracted by CBD.52 Synthetic cannabinoids are even more potent anxiogenic and psychogenic agents than plant-based marijuana.19,20
Cannabis Use Disorder
About 9% of those who try cannabis develop Cannabis Use Disorder, which is characterized by continued use of the substance despite significant distress or impairment.53 Cannabis Use Disorder is essentially an addiction. Primary risk factors include male gender, younger age at marijuana initiation, and personal or family history of other substance or psychiatric problems.53
Although cannabis use often precedes use of other addiction-prone substances, it remains unclear if it is a “gateway” to the use of other illicit drugs.54 Marijuana withdrawal is relatively minor and is comparable to that for tobacco.55 While there are no known effective pharmacotherapies for discontinuing cannabis use, addiction therapy—including cognitive behavioral therapy and trigger management—is effective.56
SIDEBAR
Cannabinoids: A diverse group of chemicalsCannabis, the genus name for 3 species of marijuana plant (sativa, indica, ruderalis), has come to mean any psychoactive part of the plant and is used interchangeably with “marijuana.” There are at least 85 different cannabinoids in the native plant.7
Cannabinoids are a diverse group of chemicals that have activity at cannabinoid receptors. Δ-9-tetrahydrocannabinol (THC), a partial agonist of the CB1 receptor, is the primary psychoactive component and is found in larger quantities in Cannabis sativa, which is preferred by non-medical users. Cannabidiol (CBD), a weak partial CB1 antagonist, exhibits few, if any, psychotropic properties and is more plentiful in Cannabis indica.
Synthetic cannabinioids are a heterogeneous group of manufactured drugs that are full CB1 agonists and that are more potent than THC, yet are often assumed to be safe by users. Typically, they are dissolved in solvents, sprayed onto inert plant materials, and marketed as herbal products like “K2” and “spice.”
So how should the evidence inform your care?
Screen all patients for use of cannabinoids and other addiction-prone substances.57 Follow any affirmative answers to your questions about cannabis use by asking about potential negative consequences of use. For example, ask patients:
- How often during the past 6 months did you find that you were unable to stop using cannabis once you started?
- How often during the past 6 months did you fail to do what was expected of you because of using cannabis? (For more questions, see the Cannabis Use Disorder Identification Test available at: http://www.otago.ac.nz/nationaladdictioncentre/pdfs/cudit-r.pdf.)
Other validated screening tools include the Severity of Dependence Scale, the Cannabis Abuse Screening Test, and the Problematic Use of Marijuana.58
Counsel patients about possible adverse effects and inform them there is no evidence that recreational marijuana or synthetic cannabinoids can be used safely over time. Consider medical use requests only if there is a favorable risk/benefit balance, other recognized treatment options have been exhausted, and you have a strong understanding of the use of cannabis in the medical condition being considered.4
Since brief interventions using motivational interviewing to reduce or eliminate recreational use have not been found to be effective,59 referral to an addiction specialist may be indicated. If a diagnosis of cannabis use disorder is established, then abstinence from addiction-prone substances including marijuana, programs like Marijuana Anonymous (Available at: https://www.marijuana-anonymous.org/), and individualized addiction therapy scaled to the severity of the condition can be effective.56 Because psychiatric conditions frequently co-occur and complicate addiction,53 they should be screened for and managed, as well.
Drug testing. Cannabis Use Disorder has significant relapse potential.60 Abstinence and treatment adherence should be ascertained through regular follow-up that includes a clinical interview, exam, and body fluid drug testing. Point-of-care urine analysis for substances of potential addiction has limited utility. Definitive testing of urine with gas chromotography/mass spectrometry (GC/MS) or liquid chromatography (LC/MS-MS) can eliminate THC false-positives and false-negatives that can occur with point-of-care urine immunoassays. In addition, GCMS and LC/MS-MS can identify synthetic cannabinoids; in-office immunoassays cannot.
If the patient relapses, subsequent medical care should be coordinated with an addiction specialist with the goal of helping the patient to abstain from cannabis.
CORRESPONDENCE
Steven Wright, MD, FAAFP, 5325 Ridge Trail, Littleton, CO 80123; [email protected].
1. Pew Research Center. 6 facts about marijuana. Available at: http://www.pewresearch.org/fact-tank/2015/04/14/6-facts-about-marijuana/. Accessed September 27, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Pub # (SMA) 14-4863. 2014. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf. Accessed September 27, 2015.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future National Survey on Drug Use 1975-2015. Available at: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Accessed September 23, 2015.
4. Metts J, Wright S, Sundaram J, et al. Medical marijuana: a treatment worth trying? J Fam Pract. 2016;65:178-185.
5. Governing the states and localities. State marijuana laws map. Available at: http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html. Accessed October 12, 2016.
6. US Drug Enforcement Administration. Drug scheduling. Available at: https://www.dea.gov/druginfo/ds.shtml. Accessed October 12, 2016.
7. El-Alfy AT, Ivey K, Robinson K, et al. Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav. 2010;95:434-442.
8. Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107:1404-1417.
9. Gruber AJ, Pope HG, Hudson JI, et al. Attributes of long-term heavy cannabis users: a case-control study. Psychol Med. 2003;33:1415-1422.
10. Palamar JJ, Fenstermaker M, Kamboukos D, et al. Adverse psychosocial outcomes associated with drug use among US high school seniors: a comparison of alcohol and marijuana. Am J Drug Alcohol Abuse. 2014;40:438-446.
11. Zwerling C, Ryan J, Orav EJ. The efficacy of preemployment drug screening for marijuana and cocaine in predicting employment outcome. JAMA. 1990;264:2639-2643.
12. Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123-1135.
13. Bell C, Slim J, Flaten HK, et al. Butane hash oil burns associated with marijuana liberalization in Colorado. J Med Toxicol. 2015;11:422-425.
14. Huang DYC, Lanza HI, Anglin MD. Association between adolescent substance use and obesity in young adulthood: a group-based dual trajectory analysis. Addict Behav. 2013;38:2653-2660.
15. Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494.
16. Cho CM, Hirsch R, Johnstone S. General and oral health implications of cannabis use. Aust Dent J. 2005;50:70-74.
17. Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav. 2010;58:91-99.
18. Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg. 2013;27:996-1005.
19. Mills B, Yepes A, Nugent K. Synthetic cannabinoids. Am J Med Sci. 2015;350:59-62.
20. Tuv SS, Strand MC, Karinen R, et al. Effect and occurrence of synthetic cannabinoids. Tidsskr Nor Laegeforen. 2012;132:2285-2288.
21. Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011;104:659-964.
22. Gates P, Jaffe A, Copeland J. Cannabis smoking and respiratory health: considerations of the literature. Respirology. 2014;19:655-662.
23. Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years: The Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2012;307:173-181.
24. Verweij PE, Kerremans JJ, Vos A, et al. Fungal contamination of tobacco and marijuana. JAMA. 2000;284:2875.
25. Office of Environmental Health Hazard Assessment. Evidence on the carcinogenicity of marijuana smoke. August 2009. Available at: http://oehha.ca.gov/media/downloads/crnr/finalmjsmokehid.pdf. Accessed September 5, 2015.
26. Stone D. Cannabis, pesticides and conflicting laws: the dilemma for legalized States and implications for public health. Regul Toxicol Pharmacol. 2014;69:284-288.
27. Hashibe M, Straif K, Tashkin DP, et al. Epidemiologic review of marijuana and cancer risk. Alcohol. 2005;35:265-275.
28. Liang C, McClean MD, Marsit C, et al. A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prev Res (Phila). 2009;2:759-768.
29. Thomas AA, Wallner LP, Quinn VP, et al. Association between cannabis use and the risk of bladder cancer: results from the California Men’s Health Study. Urology. 2015;85:388-392.
30. Holly EA, Lele C, Bracci PM, et al. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Francisco Bay area, California. Am J Epidemiol. 1999;150:375-389.
31. Massi P, Solinas M, Cinquina V, et al. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303-312.
32. Ashbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536.
33.Barrio G, Jimenez-Mejias E, Pulido J, et al. Association between cannabis use and non-traffic injuries. Accid Anal Prev. 2012;47:172-176.
34. MacDonald S, Hall W, Roman P, et al. Testing for cannabis in the work-place: a review of the evidence. Addiction. 2010;105:408-416.
35. Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict. 2009;18:185-193.
36. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109-119.
37. Menetrey A, Augsburger M, Favrat B, et al. Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoid levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Δ9-THC. J Anal Toxicol. 2005;29:327-338.
38. Raemakers JG, Kaurert G, van Ruitenbeek P, et al. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296-2303.
39. Leirer VO, Yesavage JA, Morrow DG. Marijuana carry-over effects on aircraft pilot performance. Aviat Space Environ Med. 1991;62:221-227.
40. Calabria B, Degenhardt L, Hall W, et al. Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010;29:318-330.
41. Colorado Department of Public Health and Environment. Monitoring health concerns related to marijuana in Colorado: 2014. Changes in marijuana use patterns, systematic literature review, and possible marijuana-related health effects. Available at: http://www2.cde.state.co.us/artemis/hemonos/he1282m332015internet/he1282m332015internet01.pdf. Accessed September 5, 2015.
42. Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn. Perinatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009-1024.
43. Batalla A, Bhattacharyya S, Yücel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821.
44. Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1-8.
45. Pavisian B, MacIntosh BJ, Szilagyi G, et al. Effects of cannabis on cognition in patients with multiple sclerosis: a psychometric and MRI study. Neurology. 2014;82:1879-1887.
46. Bartholomew J, Holroyd S, Heffernan TM. Does cannabis use affect prospective memory in young adults? J Psychopharmacol. 2010;24:241-246.
47. Copeland J, Rooke S, Swift W. Changes in cannabis use among young people: impact on mental health. Curr Opin Psychiatry. 2013;26:325-329.
48. Ari M, Sahpolat M, Kokacya H, et al. Amotivational syndrome: less known and diagnosed as a clinical. J Mood Disord. 2015;5:31-35.
49. Zvolensky MJ, Cougle JR, Johnson KA, et al. Marijuana use and panic psychopathology among a representative sample of adults. Exp Clin Psychopharmacol. 2010;18(2):129-134.
50. Yarnell S. The use of medicinal marijuana for posttraumatic stress disorder: a review of the current literature. Prim Care Companion CNS Disord. 2015;17(3).
51. Le Bec PY, Fatséas M, Denis C, et al. Cannabis and psychosis: search of a causal link through a critical and systematic review. Encephale. 2009;35:377-385.
52. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19-27.
53. Lopez-Quintero C, Perez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
54. Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108:84-97.
55. Vandrey RG, Budney AJ, Hughes JR, et al. A within subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92:48-54.
56.Budney AJ, Roffman R, Stephens RS, et al. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4-16.
57. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Phys. 2014;60:801-808.
58. Piontek D, Kraus L, Klempova D. Short scales to assess cannabis-related problems: a review of psychometric properties. Subst Abuse Treat Prev Policy. 2008;3:25.
59. Saitz R, Palfai TPA, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312:502-513.
60. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689-1695.
1. Pew Research Center. 6 facts about marijuana. Available at: http://www.pewresearch.org/fact-tank/2015/04/14/6-facts-about-marijuana/. Accessed September 27, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Pub # (SMA) 14-4863. 2014. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf. Accessed September 27, 2015.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future National Survey on Drug Use 1975-2015. Available at: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Accessed September 23, 2015.
4. Metts J, Wright S, Sundaram J, et al. Medical marijuana: a treatment worth trying? J Fam Pract. 2016;65:178-185.
5. Governing the states and localities. State marijuana laws map. Available at: http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html. Accessed October 12, 2016.
6. US Drug Enforcement Administration. Drug scheduling. Available at: https://www.dea.gov/druginfo/ds.shtml. Accessed October 12, 2016.
7. El-Alfy AT, Ivey K, Robinson K, et al. Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav. 2010;95:434-442.
8. Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107:1404-1417.
9. Gruber AJ, Pope HG, Hudson JI, et al. Attributes of long-term heavy cannabis users: a case-control study. Psychol Med. 2003;33:1415-1422.
10. Palamar JJ, Fenstermaker M, Kamboukos D, et al. Adverse psychosocial outcomes associated with drug use among US high school seniors: a comparison of alcohol and marijuana. Am J Drug Alcohol Abuse. 2014;40:438-446.
11. Zwerling C, Ryan J, Orav EJ. The efficacy of preemployment drug screening for marijuana and cocaine in predicting employment outcome. JAMA. 1990;264:2639-2643.
12. Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123-1135.
13. Bell C, Slim J, Flaten HK, et al. Butane hash oil burns associated with marijuana liberalization in Colorado. J Med Toxicol. 2015;11:422-425.
14. Huang DYC, Lanza HI, Anglin MD. Association between adolescent substance use and obesity in young adulthood: a group-based dual trajectory analysis. Addict Behav. 2013;38:2653-2660.
15. Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494.
16. Cho CM, Hirsch R, Johnstone S. General and oral health implications of cannabis use. Aust Dent J. 2005;50:70-74.
17. Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav. 2010;58:91-99.
18. Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg. 2013;27:996-1005.
19. Mills B, Yepes A, Nugent K. Synthetic cannabinoids. Am J Med Sci. 2015;350:59-62.
20. Tuv SS, Strand MC, Karinen R, et al. Effect and occurrence of synthetic cannabinoids. Tidsskr Nor Laegeforen. 2012;132:2285-2288.
21. Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011;104:659-964.
22. Gates P, Jaffe A, Copeland J. Cannabis smoking and respiratory health: considerations of the literature. Respirology. 2014;19:655-662.
23. Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years: The Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2012;307:173-181.
24. Verweij PE, Kerremans JJ, Vos A, et al. Fungal contamination of tobacco and marijuana. JAMA. 2000;284:2875.
25. Office of Environmental Health Hazard Assessment. Evidence on the carcinogenicity of marijuana smoke. August 2009. Available at: http://oehha.ca.gov/media/downloads/crnr/finalmjsmokehid.pdf. Accessed September 5, 2015.
26. Stone D. Cannabis, pesticides and conflicting laws: the dilemma for legalized States and implications for public health. Regul Toxicol Pharmacol. 2014;69:284-288.
27. Hashibe M, Straif K, Tashkin DP, et al. Epidemiologic review of marijuana and cancer risk. Alcohol. 2005;35:265-275.
28. Liang C, McClean MD, Marsit C, et al. A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prev Res (Phila). 2009;2:759-768.
29. Thomas AA, Wallner LP, Quinn VP, et al. Association between cannabis use and the risk of bladder cancer: results from the California Men’s Health Study. Urology. 2015;85:388-392.
30. Holly EA, Lele C, Bracci PM, et al. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Francisco Bay area, California. Am J Epidemiol. 1999;150:375-389.
31. Massi P, Solinas M, Cinquina V, et al. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303-312.
32. Ashbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536.
33.Barrio G, Jimenez-Mejias E, Pulido J, et al. Association between cannabis use and non-traffic injuries. Accid Anal Prev. 2012;47:172-176.
34. MacDonald S, Hall W, Roman P, et al. Testing for cannabis in the work-place: a review of the evidence. Addiction. 2010;105:408-416.
35. Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict. 2009;18:185-193.
36. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109-119.
37. Menetrey A, Augsburger M, Favrat B, et al. Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoid levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Δ9-THC. J Anal Toxicol. 2005;29:327-338.
38. Raemakers JG, Kaurert G, van Ruitenbeek P, et al. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296-2303.
39. Leirer VO, Yesavage JA, Morrow DG. Marijuana carry-over effects on aircraft pilot performance. Aviat Space Environ Med. 1991;62:221-227.
40. Calabria B, Degenhardt L, Hall W, et al. Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010;29:318-330.
41. Colorado Department of Public Health and Environment. Monitoring health concerns related to marijuana in Colorado: 2014. Changes in marijuana use patterns, systematic literature review, and possible marijuana-related health effects. Available at: http://www2.cde.state.co.us/artemis/hemonos/he1282m332015internet/he1282m332015internet01.pdf. Accessed September 5, 2015.
42. Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn. Perinatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009-1024.
43. Batalla A, Bhattacharyya S, Yücel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821.
44. Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1-8.
45. Pavisian B, MacIntosh BJ, Szilagyi G, et al. Effects of cannabis on cognition in patients with multiple sclerosis: a psychometric and MRI study. Neurology. 2014;82:1879-1887.
46. Bartholomew J, Holroyd S, Heffernan TM. Does cannabis use affect prospective memory in young adults? J Psychopharmacol. 2010;24:241-246.
47. Copeland J, Rooke S, Swift W. Changes in cannabis use among young people: impact on mental health. Curr Opin Psychiatry. 2013;26:325-329.
48. Ari M, Sahpolat M, Kokacya H, et al. Amotivational syndrome: less known and diagnosed as a clinical. J Mood Disord. 2015;5:31-35.
49. Zvolensky MJ, Cougle JR, Johnson KA, et al. Marijuana use and panic psychopathology among a representative sample of adults. Exp Clin Psychopharmacol. 2010;18(2):129-134.
50. Yarnell S. The use of medicinal marijuana for posttraumatic stress disorder: a review of the current literature. Prim Care Companion CNS Disord. 2015;17(3).
51. Le Bec PY, Fatséas M, Denis C, et al. Cannabis and psychosis: search of a causal link through a critical and systematic review. Encephale. 2009;35:377-385.
52. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19-27.
53. Lopez-Quintero C, Perez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
54. Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108:84-97.
55. Vandrey RG, Budney AJ, Hughes JR, et al. A within subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92:48-54.
56.Budney AJ, Roffman R, Stephens RS, et al. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4-16.
57. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Phys. 2014;60:801-808.
58. Piontek D, Kraus L, Klempova D. Short scales to assess cannabis-related problems: a review of psychometric properties. Subst Abuse Treat Prev Policy. 2008;3:25.
59. Saitz R, Palfai TPA, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312:502-513.
60. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689-1695.
Drug-induced weight gain: Rethinking our choices
PRACTICE RECOMMENDATIONS
› Choose weight-loss-promoting medications, such as metformin, sodium-glucose co-transporter 2 inhibitors, and glucagon-like peptide-1 agonists, and weight-neutral medications, such as DPP-4 inhibitors, as first- and second-line agents for patients with type 2 diabetes who are overweight or obese. A
› Prescribe angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium channel blockers as first- and second-line antihypertensive therapy for patients who are overweight or obese. A
› Select antidepressants that promote weight loss, such as bupropion, or weight-neutral agents, such as fluoxetine and sertraline, for patients who are overweight or obese and require treatment for depression. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Medications can have an unpredictable and variable effect on weight. Some drugs trigger weight gain in one patient while inducing weight loss in another. Others may lead to weight loss initially but cause weight gain when taken long term.1 Often, a drug’s effect on a patient’s weight depends on his or her medical history and lifestyle, including factors like insulin resistance, diet, and exercise level.
To make matters worse, clinical studies of drug-related effects on weight can be misleading. Because researchers often report a mean weight change—an average of those who had little or no change in weight when taking the drug and individuals who may have gained a significant amount of weight—a drug’s potential to cause weight gain may be underestimated. Few studies include an analysis of the range—eg, how many participants gained or lost various percentages of body weight. What’s more, pharmacology studies typically follow participants for a few months to a few years, whereas weight changes can be cumulative when a medication is taken for many years.
The nation’s continually growing obesity epidemic makes it crucial for physicians to consider the weight effects of medications being prescribed and to balance the benefits of treatment with the potential for weight gain. Until recently, the medical literature offered little guidance.
In 2015, the Endocrine Society published clinical practice guidelines for pharmacologic management of obesity, including data on medications that cause weight gain and suggesting alternatives that are weight-neutral or promote weight loss.2
In the pages that follow, we present case studies, tables, and a review of the latest evidence to highlight optimal drug treatment for patients who are overweight or obese, and are also being treated for diabetes, hypertension, and depression. You’ll find a brief discussion of weight management strategies related to other drugs and conditions in the sidebar.2-5
CASE 1 › 40-year-old man with diabetes and hyperlipidemia
Brian P, who has come in for an annual checkup, has a body mass index (BMI) of 30 kg/m2. He also has hyperlipidemia and type 2 diabetes, for which he has been taking metformin for several years. A year ago, his hemoglobin A1c (HbA1c) was 7.3%, so his physician added glyburide to his regimen.
In the year since, Mr. P has gained 12 lbs (5.4 kg) but achieved only a minimal reduction in HbA1c (to 6.8%). He expresses concern about the cardiovascular effects of the extra weight and says that diet and exercise have not helped him control his weight.
CASE 2 › Older woman with hypertension and hypothyroidism
Addie K, age 64, is obese (BMI, 37 kg/m2) and has hypertension and hypothyroidism, for which she takes metoprolol and levothyroxine. Ms. K says that she is careful about what she eats and exercises several times a week, but still has seen her weight increase steadily for the past several years.
CASE 3 › Young man with depression
Charlie D, a 21-year-old college student, is a new patient. He has depression and is obese (BMI, 34 kg/m2). The patient says he was diagnosed with depression by his former primary care physician, who prescribed paroxetine a year ago. He requests a refill of the paroxetine, which he reports has successfully boosted his mood. When asked about his weight, he admits that he has gained 8 lbs (3.6 kg) since he began taking the drug.
If these were your patients, what weight management steps would you take? Before we provide some recommendations, let’s review the evidence.
Antidiabetic agents and weight
While some antidiabetic agents are weight-neutral and others promote weight loss, several therapies are associated with weight gain6 (TABLE 13). Patients like Mr. P can gain as much as 10 kg in 3 to 6 months after beginning treatment with insulin, thiazolidinediones (TZDs), sulfonylureas, and other insulin secretagogues.2,7
A recent systematic review and meta-analysis of 257 randomized controlled trials (RCTs) found weight gain to be associated with the use of pioglitazone (2.6 kg), glimepiride (2.1 kg), glyburide (2.6 kg), glipizide (2.2 kg), and sitagliptin (0.55 kg). A modest weight loss was associated with acarbose, exenatide, liraglutide, metformin, miglitol, and pramlintide.8
Sulfonylureas are generally associated with a 1.5 to 2.5 kg weight gain.9-11 In an analysis of 27 RCTs of noninsulin antidiabetic drugs in patients whose disease was not controlled by metformin alone, TZDs, sulfonylureas, and meglitinides were associated with a 1.77 to 2.08 kg weight gain.9 Furthermore, those taking sulfonylureas and meglitinides had higher rates of hypoglycemia compared with patients taking placebo (relative risk, 4.50-7.50). In fact, sulfonylureas have the highest risk of serious hypoglycemia of any noninsulin therapy.6
In contrast, metformin—the most commonly prescribed oral agent for type 2 diabetes—promotes mild weight loss by multiple mechanisms and has a good safety profile.12,13 Thus, some physicians use metformin off label for weight loss and diabetes prevention and have suggested that it be approved for these indications.13
Glycemic control and weight loss
Glucagon-like peptide-1 (GLP-1) agonists lead to weight loss by decreasing appetite and enhancing satiety, as well as improving glycemic control. Liraglutide received Food and Drug Administration (FDA) approval in 2014 as a treatment for chronic weight management at a higher dose (3 mg/d) than that used to treat diabetes (1.8 mg/d).14
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a relatively new class of antidiabetic medication that reduce glucose reabsorption by the kidneys, leading to increased urinary glucose excretion.15 The associated weight loss, in addition to a reduction in hyperglycemia, may be due to the subsequent calorie loss through glycosuria.
Both dipeptidyl peptidase-4 (DPP-4) inhibitors and alpha-glucosidase inhibitors (AGIs) appear to be weight-neutral or to induce minimal changes in weight.16 Although the systematic review mentioned earlier found a 0.55 kg weight gain associated with sitagliptin,8 most studies of DPP-4 inhibitors report weight neutrality.17-19 Pramlintide, the amylin analogue that has FDA approval for use in combination with existing insulin treatment, can prevent weight gain or lead to weight loss.20,21
The Endocrine Society Clinical Practice Guideline recommends concomitantly prescribing at least one weight loss-promoting medication (such as metformin, a GLP-1 agonist, or pramlintide) to patients with obesity and type 2 diabetes who require insulin to mitigate weight gain due to insulin.2
The 2016 Comprehensive Type 2 Diabetes Management Algorithm published by the American Association of Clinical Endocrinologists and American College of Endocrinology recommends that the initiation of diabetes therapies be based on the risks of weight gain and hypoglycemia, among other factors. The algorithm calls for metformin as first-line therapy, followed by a GLP-1 agonist as a second-line therapy, and an SGLT2 inhibitor as a third-line therapy.6
Finally, FDA-approved anti-obesity medications may be appropriate for patients with diabetes who are unable to lose weight with lifestyle interventions alone.22 Each medication is associated with improvements in glucose in addition to other metabolic parameters.
CASE 1 › A better choice for Mr. P
Because Mr. P has gained weight—and, indeed, developed obesity—since he started taking glyburide, it is clear that a sulfonylurea is not the best choice for this patient. An antidiabetic agent that is weight-neutral or that promotes weight loss, such as an SGLT2 inhibitor or a GLP-1 agonist, would be more suitable. The drug should be prescribed in conjunction with his metformin, which has a favorable weight profile and helps reduce HbA1c, as both SGLT2 inhibitors and GLP-1 agonists also do.
If Mr. P were to switch to an SGLT2 inhibitor, a combination pill containing metformin would be an effective way to limit the patient’s pill burden.
Treating hypertension without weight gain
Thiazide diuretics are often recommended as first-line agents for the treatment of hypertension, but their dose-related adverse effects, including dyslipidemia and insulin resistance, are undesirable for patients who are overweight or obese and at risk for metabolic syndrome and type 2 diabetes.23 Beta-adrenergic blockers have been shown to promote weight gain and prevent weight loss, especially in patients who have both hypertension and diabetes.24 In addition to having potential adverse metabolic effects on lipids and/or insulin sensitivity, beta-blockers can decrease metabolic rate by 10% and they may have other negative effects on energy metabolism, as well.25
In a meta-analysis of 8 RCTs that lasted ≥6 months, changes in body weight were higher in participants on beta-blockers, with a median difference of 1.2 kg (−0.4 to 3.5 kg) between those on beta-blockers and the control group.26 The evidence suggests that beta-blockers should not necessarily be first-line treatment for hypertension in patients who are overweight or obese and that obesity management in patients with hypertension may be harder if they are being treated with a beta-blocker.
When a different drug in the same class will do
There are exceptions, however. When beta-blockers are required—for patients with coronary artery disease, heart failure, or an arrhythmia, for example—a selective agent with a vasodilating component, such as carvedilol or nebivolol, is recommended.2 These drugs appear to have less potential for weight gain and to have minimal effect on lipid and glucose metabolism.26,27
In a study of 1106 patients with hypertension, those taking metoprolol had a statistically significant mean weight gain of 1.19 kg (P<.001) compared with patients taking carvedilol (mean weight gain, 0.17 kg; P=.36).24 While 4.5% of those in the metoprolol group gained ≥7% of their body weight, that was true of only 1.1% of those taking carvedilol. Thus, weight gain can sometimes be minimized by choosing a different medication within the same drug class.
ACE inhibitors, ARBs, and calcium channel blockers
Antihypertensive medications that are not associated with weight gain or insulin resistance include angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs) (TABLE 2).3 Angiotensin contributes to obesity-related hypertension, as it is overexpressed in obesity, making ACE inhibitors and ARBs desirable options for the treatment of patients who are obese. And, because many patients who are obese also suffer from type 2 diabetes or prediabetes, they’re likely to benefit from the renal protection provided by ACE inhibitors and ARBs, as well.
CASE 2 › Switching antihypertensives
Switching Ms. K from metoprolol, a beta-blocker, to an ACE inhibitor, ARB, or CCB may help prevent further weight gain, and possibly even lead to weight loss. Any drug in any of these 3 classes of medications would be a reasonable choice. However, if the patient had a condition that warranted use of a beta-blocker, a selective agent with a vasodilating component such as carvedilol or nebivolol might be helpful.
SIDEBAR
Weight management strategies for several other conditionsIn addition to medications for common conditions such as diabetes, hypertension, and depression, there are numerous other drugs that can cause unwanted weight gain. These include some antiseizure agents, antipsychotics, contraceptives, hormones, and migraine therapies, as well as corticosteroids. In view of both the nation’s obesity epidemic and the many drugs that are known to adversely affect weight maintenance, it is crucial to do a careful risk-benefit analysis and a search for alternatives whenever you prescribe a new medication for a patient who is overweight or obese or has metabolic risk factors.2-5
When weight-neutral substitutes exist, such medications should be considered, if appropriate, to prevent or lessen pharmacologic weight gain. For example, topiramate and zonisamide are preferable to other antiepileptics, such as valproic acid and gabapentin when it comes to weight management.2-4 It is essential to keep in mind, however, that medications in the same class are not always interchangeable.
For patients with inflammatory conditions such as rheumatoid arthritis, disease-modifying antirheumatic drugs (DMARDs) are preferable to corticosteroids whenever possible.2-4 For the many patients for whom steroids or other drugs known to cause weight gain are necessary, however, dietary and lifestyle counseling—advising patients to eat a healthful diet and maintain adequate activity levels, among other interventions—may help to mitigate the effects.
And when there are no alternative medications available, use the lowest possible dose for the shortest duration necessary.
Choosing an antidepressant when weight is an issue
For patients with psychiatric conditions, weight gain is often multifactorial. One key issue: Weight gain is a common adverse effect of many antidepressants (TABLE 3).3 Within classes of antidepressants, there is a range of weight gain potential, which can vary depending on the duration of therapy.2
In a meta-analysis of 116 studies, selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine and sertraline were associated with weight loss in short-term use (4-12 weeks) and weight neutrality when used for >4 months.1 Patients who had type 2 diabetes as well as depression had an average weight loss from fluoxetine of 5.1 kg (3.3–6.9 kg) at 24- to 26-week follow up.28
Among SSRI and tricyclic (TCA) antidepressants, paroxetine and amitriptyline, respectively, had the greatest risk for weight gain.1,29 No significant weight effect was observed for either citalopram or escitalopram. Keep in mind, however, that the effect of each antidepressant on weight may vary greatly from one patient to another.1 For example, while Mr. D gained 3.6 kg on paroxetine, some patients gain no weight at all.
In the systematic review and meta-analysis of 257 RCTs, weight gain was associated with the use of amitriptyline (1.8 kg) and mirtazapine (1.5 kg), while weight loss was associated with bupropion and fluoxetine (-1.3 kg for each).8
This antidepressant can decrease cravings
Bupropion, a norepinephrine and dopamine reuptake inhibitor, is the only antidepressant that has been consistently shown to cause weight loss.30,31 Clinical trials have found that it decreases body weight by suppressing appetite and reducing food cravings.30 Bupropion is approved for the treatment of depression and as a smoking cessation aide. And, in 2014, a bupropion-naltrexone combination received FDA approval for chronic weight management, sold under the brand name Contrave.32
As different classes of antidepressants are often prescribed for different types of depression, it is important to be aware that the few that are weight-neutral and weight-loss-promoting are not appropriate for all patients with depression. For example, bupropion is an activating agent and can exacerbate anxiety. Thus, a patient with concomitant depression and anxiety might be a better candidate for another antidepressant, which could lead to some weight gain but would better manage the individual’s symptoms. In such cases, the rule of thumb should be to prescribe the lowest dose required for clinical efficacy for the shortest duration necessary.
CASE 3 › Change antidepressants— and keep a close watch
Depending on the nature of Mr. D’s depression, bupropion, fluoxetine, or sertraline might be a reasonable alternative to paroxetine to prevent or reduce further drug-induced weight gain.
Frequent follow-up visits should be scheduled until the transition has been completed and his condition stabilized. If Mr. D’s new antidepressant is bupropion, monitoring him for signs of anxiety would be required.
CORRESPONDENCE
Katherine H. Saunders, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; [email protected].
1. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259-1272.
2. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100:342-362.
3. Apovian CM, Aronne L, Powell AG. Clinical Management of Obesity. West Islip, NY: Professional Communications, Inc., 2015.
4. Aronne LJ. A Practical Guide to Drug-induced Weight Gain. Minneapolis, Minn: McGraw-Hill; 2002.
5. Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs: a systematic review. QJM. 2007;100:395-404.
6. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2016 executive summary. Endocr Pract. 2016;22:84-113.
7. Aronne LJ. Drug-induced weight gain: non-CNS medications. In: A Practical Guide to Drug-Induced Weight Gain. Minneapolis, Minn: McGraw-Hill: 2002:77-91.
8. Domecq JP, Prutsky G, Leppin A, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:363-370.
9. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
10. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
11. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481.
12. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21:323-329.
13. Igel LI, Sinha A, Saunders KH, et al. Metformin: an old therapy that deserves a new indication for the treatment of obesity. Curr Atheroscler Rep. 2016;18:16.
14. US Food and Drug Administration. FDA approves weight-management drug Saxenda. December 23, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed October 1, 2016.
15. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495-502.
16. van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154-163.
17. Hong ES, Khang AR, Yoon JW, et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795-802.
18. Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509-1515.
19. Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38:89-101.
20. Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784-790.
21. Aronne L, Fujioka K, Aroda V, et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab. 2007;92:2977-2983.
22. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
23. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the Obesity Society and the American Society of Hypertension. Obesity (Silver Spring). 2013;21:8-24.
24. Messerli FH, Bell DS, Fonseca V, et al. Body weight changes with beta-blocker use: results from GEMINI. Am J Med. 2007;120:610-615.
25. Pischon T, Sharma AM. Use of beta-blockers in obesity hypertension: potential role of weight gain. Obes Rev. 2001;2:275-280.
26. Sharma AM, Pischon T, Hardt S, et al. Hypothesis: beta-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37:250-254.
27. Manrique C, Whaley-Connell A, Sowers JR. Nebivolol in obese and non-obese hypertensive patients. J Clin Hypertens (Greenwich). 2009;11:309-315.
28. Norris SL, Zhang X, Avenell A, et al. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(1):CD004096.
29. Rosenzweig-Lipson S, Beyer CE, Hughes ZA, et al. Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther. 2007;113:134-153.
30. Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother. 2007;7:17-24.
31. Arterburn D, Sofer T, Boudreau DM, et al. Long-term weight change after initiating second-generation antidepressants. J Clin Med. 2016;5:piiE48.
32. US Food and Drug Administration. FDA approves weight-management drug Contrave. September 10, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm413896.htm. Accessed October 1, 2016.
PRACTICE RECOMMENDATIONS
› Choose weight-loss-promoting medications, such as metformin, sodium-glucose co-transporter 2 inhibitors, and glucagon-like peptide-1 agonists, and weight-neutral medications, such as DPP-4 inhibitors, as first- and second-line agents for patients with type 2 diabetes who are overweight or obese. A
› Prescribe angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium channel blockers as first- and second-line antihypertensive therapy for patients who are overweight or obese. A
› Select antidepressants that promote weight loss, such as bupropion, or weight-neutral agents, such as fluoxetine and sertraline, for patients who are overweight or obese and require treatment for depression. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Medications can have an unpredictable and variable effect on weight. Some drugs trigger weight gain in one patient while inducing weight loss in another. Others may lead to weight loss initially but cause weight gain when taken long term.1 Often, a drug’s effect on a patient’s weight depends on his or her medical history and lifestyle, including factors like insulin resistance, diet, and exercise level.
To make matters worse, clinical studies of drug-related effects on weight can be misleading. Because researchers often report a mean weight change—an average of those who had little or no change in weight when taking the drug and individuals who may have gained a significant amount of weight—a drug’s potential to cause weight gain may be underestimated. Few studies include an analysis of the range—eg, how many participants gained or lost various percentages of body weight. What’s more, pharmacology studies typically follow participants for a few months to a few years, whereas weight changes can be cumulative when a medication is taken for many years.
The nation’s continually growing obesity epidemic makes it crucial for physicians to consider the weight effects of medications being prescribed and to balance the benefits of treatment with the potential for weight gain. Until recently, the medical literature offered little guidance.
In 2015, the Endocrine Society published clinical practice guidelines for pharmacologic management of obesity, including data on medications that cause weight gain and suggesting alternatives that are weight-neutral or promote weight loss.2
In the pages that follow, we present case studies, tables, and a review of the latest evidence to highlight optimal drug treatment for patients who are overweight or obese, and are also being treated for diabetes, hypertension, and depression. You’ll find a brief discussion of weight management strategies related to other drugs and conditions in the sidebar.2-5
CASE 1 › 40-year-old man with diabetes and hyperlipidemia
Brian P, who has come in for an annual checkup, has a body mass index (BMI) of 30 kg/m2. He also has hyperlipidemia and type 2 diabetes, for which he has been taking metformin for several years. A year ago, his hemoglobin A1c (HbA1c) was 7.3%, so his physician added glyburide to his regimen.
In the year since, Mr. P has gained 12 lbs (5.4 kg) but achieved only a minimal reduction in HbA1c (to 6.8%). He expresses concern about the cardiovascular effects of the extra weight and says that diet and exercise have not helped him control his weight.
CASE 2 › Older woman with hypertension and hypothyroidism
Addie K, age 64, is obese (BMI, 37 kg/m2) and has hypertension and hypothyroidism, for which she takes metoprolol and levothyroxine. Ms. K says that she is careful about what she eats and exercises several times a week, but still has seen her weight increase steadily for the past several years.
CASE 3 › Young man with depression
Charlie D, a 21-year-old college student, is a new patient. He has depression and is obese (BMI, 34 kg/m2). The patient says he was diagnosed with depression by his former primary care physician, who prescribed paroxetine a year ago. He requests a refill of the paroxetine, which he reports has successfully boosted his mood. When asked about his weight, he admits that he has gained 8 lbs (3.6 kg) since he began taking the drug.
If these were your patients, what weight management steps would you take? Before we provide some recommendations, let’s review the evidence.
Antidiabetic agents and weight
While some antidiabetic agents are weight-neutral and others promote weight loss, several therapies are associated with weight gain6 (TABLE 13). Patients like Mr. P can gain as much as 10 kg in 3 to 6 months after beginning treatment with insulin, thiazolidinediones (TZDs), sulfonylureas, and other insulin secretagogues.2,7
A recent systematic review and meta-analysis of 257 randomized controlled trials (RCTs) found weight gain to be associated with the use of pioglitazone (2.6 kg), glimepiride (2.1 kg), glyburide (2.6 kg), glipizide (2.2 kg), and sitagliptin (0.55 kg). A modest weight loss was associated with acarbose, exenatide, liraglutide, metformin, miglitol, and pramlintide.8
Sulfonylureas are generally associated with a 1.5 to 2.5 kg weight gain.9-11 In an analysis of 27 RCTs of noninsulin antidiabetic drugs in patients whose disease was not controlled by metformin alone, TZDs, sulfonylureas, and meglitinides were associated with a 1.77 to 2.08 kg weight gain.9 Furthermore, those taking sulfonylureas and meglitinides had higher rates of hypoglycemia compared with patients taking placebo (relative risk, 4.50-7.50). In fact, sulfonylureas have the highest risk of serious hypoglycemia of any noninsulin therapy.6
In contrast, metformin—the most commonly prescribed oral agent for type 2 diabetes—promotes mild weight loss by multiple mechanisms and has a good safety profile.12,13 Thus, some physicians use metformin off label for weight loss and diabetes prevention and have suggested that it be approved for these indications.13
Glycemic control and weight loss
Glucagon-like peptide-1 (GLP-1) agonists lead to weight loss by decreasing appetite and enhancing satiety, as well as improving glycemic control. Liraglutide received Food and Drug Administration (FDA) approval in 2014 as a treatment for chronic weight management at a higher dose (3 mg/d) than that used to treat diabetes (1.8 mg/d).14
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a relatively new class of antidiabetic medication that reduce glucose reabsorption by the kidneys, leading to increased urinary glucose excretion.15 The associated weight loss, in addition to a reduction in hyperglycemia, may be due to the subsequent calorie loss through glycosuria.
Both dipeptidyl peptidase-4 (DPP-4) inhibitors and alpha-glucosidase inhibitors (AGIs) appear to be weight-neutral or to induce minimal changes in weight.16 Although the systematic review mentioned earlier found a 0.55 kg weight gain associated with sitagliptin,8 most studies of DPP-4 inhibitors report weight neutrality.17-19 Pramlintide, the amylin analogue that has FDA approval for use in combination with existing insulin treatment, can prevent weight gain or lead to weight loss.20,21
The Endocrine Society Clinical Practice Guideline recommends concomitantly prescribing at least one weight loss-promoting medication (such as metformin, a GLP-1 agonist, or pramlintide) to patients with obesity and type 2 diabetes who require insulin to mitigate weight gain due to insulin.2
The 2016 Comprehensive Type 2 Diabetes Management Algorithm published by the American Association of Clinical Endocrinologists and American College of Endocrinology recommends that the initiation of diabetes therapies be based on the risks of weight gain and hypoglycemia, among other factors. The algorithm calls for metformin as first-line therapy, followed by a GLP-1 agonist as a second-line therapy, and an SGLT2 inhibitor as a third-line therapy.6
Finally, FDA-approved anti-obesity medications may be appropriate for patients with diabetes who are unable to lose weight with lifestyle interventions alone.22 Each medication is associated with improvements in glucose in addition to other metabolic parameters.
CASE 1 › A better choice for Mr. P
Because Mr. P has gained weight—and, indeed, developed obesity—since he started taking glyburide, it is clear that a sulfonylurea is not the best choice for this patient. An antidiabetic agent that is weight-neutral or that promotes weight loss, such as an SGLT2 inhibitor or a GLP-1 agonist, would be more suitable. The drug should be prescribed in conjunction with his metformin, which has a favorable weight profile and helps reduce HbA1c, as both SGLT2 inhibitors and GLP-1 agonists also do.
If Mr. P were to switch to an SGLT2 inhibitor, a combination pill containing metformin would be an effective way to limit the patient’s pill burden.
Treating hypertension without weight gain
Thiazide diuretics are often recommended as first-line agents for the treatment of hypertension, but their dose-related adverse effects, including dyslipidemia and insulin resistance, are undesirable for patients who are overweight or obese and at risk for metabolic syndrome and type 2 diabetes.23 Beta-adrenergic blockers have been shown to promote weight gain and prevent weight loss, especially in patients who have both hypertension and diabetes.24 In addition to having potential adverse metabolic effects on lipids and/or insulin sensitivity, beta-blockers can decrease metabolic rate by 10% and they may have other negative effects on energy metabolism, as well.25
In a meta-analysis of 8 RCTs that lasted ≥6 months, changes in body weight were higher in participants on beta-blockers, with a median difference of 1.2 kg (−0.4 to 3.5 kg) between those on beta-blockers and the control group.26 The evidence suggests that beta-blockers should not necessarily be first-line treatment for hypertension in patients who are overweight or obese and that obesity management in patients with hypertension may be harder if they are being treated with a beta-blocker.
When a different drug in the same class will do
There are exceptions, however. When beta-blockers are required—for patients with coronary artery disease, heart failure, or an arrhythmia, for example—a selective agent with a vasodilating component, such as carvedilol or nebivolol, is recommended.2 These drugs appear to have less potential for weight gain and to have minimal effect on lipid and glucose metabolism.26,27
In a study of 1106 patients with hypertension, those taking metoprolol had a statistically significant mean weight gain of 1.19 kg (P<.001) compared with patients taking carvedilol (mean weight gain, 0.17 kg; P=.36).24 While 4.5% of those in the metoprolol group gained ≥7% of their body weight, that was true of only 1.1% of those taking carvedilol. Thus, weight gain can sometimes be minimized by choosing a different medication within the same drug class.
ACE inhibitors, ARBs, and calcium channel blockers
Antihypertensive medications that are not associated with weight gain or insulin resistance include angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs) (TABLE 2).3 Angiotensin contributes to obesity-related hypertension, as it is overexpressed in obesity, making ACE inhibitors and ARBs desirable options for the treatment of patients who are obese. And, because many patients who are obese also suffer from type 2 diabetes or prediabetes, they’re likely to benefit from the renal protection provided by ACE inhibitors and ARBs, as well.
CASE 2 › Switching antihypertensives
Switching Ms. K from metoprolol, a beta-blocker, to an ACE inhibitor, ARB, or CCB may help prevent further weight gain, and possibly even lead to weight loss. Any drug in any of these 3 classes of medications would be a reasonable choice. However, if the patient had a condition that warranted use of a beta-blocker, a selective agent with a vasodilating component such as carvedilol or nebivolol might be helpful.
SIDEBAR
Weight management strategies for several other conditionsIn addition to medications for common conditions such as diabetes, hypertension, and depression, there are numerous other drugs that can cause unwanted weight gain. These include some antiseizure agents, antipsychotics, contraceptives, hormones, and migraine therapies, as well as corticosteroids. In view of both the nation’s obesity epidemic and the many drugs that are known to adversely affect weight maintenance, it is crucial to do a careful risk-benefit analysis and a search for alternatives whenever you prescribe a new medication for a patient who is overweight or obese or has metabolic risk factors.2-5
When weight-neutral substitutes exist, such medications should be considered, if appropriate, to prevent or lessen pharmacologic weight gain. For example, topiramate and zonisamide are preferable to other antiepileptics, such as valproic acid and gabapentin when it comes to weight management.2-4 It is essential to keep in mind, however, that medications in the same class are not always interchangeable.
For patients with inflammatory conditions such as rheumatoid arthritis, disease-modifying antirheumatic drugs (DMARDs) are preferable to corticosteroids whenever possible.2-4 For the many patients for whom steroids or other drugs known to cause weight gain are necessary, however, dietary and lifestyle counseling—advising patients to eat a healthful diet and maintain adequate activity levels, among other interventions—may help to mitigate the effects.
And when there are no alternative medications available, use the lowest possible dose for the shortest duration necessary.
Choosing an antidepressant when weight is an issue
For patients with psychiatric conditions, weight gain is often multifactorial. One key issue: Weight gain is a common adverse effect of many antidepressants (TABLE 3).3 Within classes of antidepressants, there is a range of weight gain potential, which can vary depending on the duration of therapy.2
In a meta-analysis of 116 studies, selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine and sertraline were associated with weight loss in short-term use (4-12 weeks) and weight neutrality when used for >4 months.1 Patients who had type 2 diabetes as well as depression had an average weight loss from fluoxetine of 5.1 kg (3.3–6.9 kg) at 24- to 26-week follow up.28
Among SSRI and tricyclic (TCA) antidepressants, paroxetine and amitriptyline, respectively, had the greatest risk for weight gain.1,29 No significant weight effect was observed for either citalopram or escitalopram. Keep in mind, however, that the effect of each antidepressant on weight may vary greatly from one patient to another.1 For example, while Mr. D gained 3.6 kg on paroxetine, some patients gain no weight at all.
In the systematic review and meta-analysis of 257 RCTs, weight gain was associated with the use of amitriptyline (1.8 kg) and mirtazapine (1.5 kg), while weight loss was associated with bupropion and fluoxetine (-1.3 kg for each).8
This antidepressant can decrease cravings
Bupropion, a norepinephrine and dopamine reuptake inhibitor, is the only antidepressant that has been consistently shown to cause weight loss.30,31 Clinical trials have found that it decreases body weight by suppressing appetite and reducing food cravings.30 Bupropion is approved for the treatment of depression and as a smoking cessation aide. And, in 2014, a bupropion-naltrexone combination received FDA approval for chronic weight management, sold under the brand name Contrave.32
As different classes of antidepressants are often prescribed for different types of depression, it is important to be aware that the few that are weight-neutral and weight-loss-promoting are not appropriate for all patients with depression. For example, bupropion is an activating agent and can exacerbate anxiety. Thus, a patient with concomitant depression and anxiety might be a better candidate for another antidepressant, which could lead to some weight gain but would better manage the individual’s symptoms. In such cases, the rule of thumb should be to prescribe the lowest dose required for clinical efficacy for the shortest duration necessary.
CASE 3 › Change antidepressants— and keep a close watch
Depending on the nature of Mr. D’s depression, bupropion, fluoxetine, or sertraline might be a reasonable alternative to paroxetine to prevent or reduce further drug-induced weight gain.
Frequent follow-up visits should be scheduled until the transition has been completed and his condition stabilized. If Mr. D’s new antidepressant is bupropion, monitoring him for signs of anxiety would be required.
CORRESPONDENCE
Katherine H. Saunders, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; [email protected].
PRACTICE RECOMMENDATIONS
› Choose weight-loss-promoting medications, such as metformin, sodium-glucose co-transporter 2 inhibitors, and glucagon-like peptide-1 agonists, and weight-neutral medications, such as DPP-4 inhibitors, as first- and second-line agents for patients with type 2 diabetes who are overweight or obese. A
› Prescribe angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium channel blockers as first- and second-line antihypertensive therapy for patients who are overweight or obese. A
› Select antidepressants that promote weight loss, such as bupropion, or weight-neutral agents, such as fluoxetine and sertraline, for patients who are overweight or obese and require treatment for depression. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Medications can have an unpredictable and variable effect on weight. Some drugs trigger weight gain in one patient while inducing weight loss in another. Others may lead to weight loss initially but cause weight gain when taken long term.1 Often, a drug’s effect on a patient’s weight depends on his or her medical history and lifestyle, including factors like insulin resistance, diet, and exercise level.
To make matters worse, clinical studies of drug-related effects on weight can be misleading. Because researchers often report a mean weight change—an average of those who had little or no change in weight when taking the drug and individuals who may have gained a significant amount of weight—a drug’s potential to cause weight gain may be underestimated. Few studies include an analysis of the range—eg, how many participants gained or lost various percentages of body weight. What’s more, pharmacology studies typically follow participants for a few months to a few years, whereas weight changes can be cumulative when a medication is taken for many years.
The nation’s continually growing obesity epidemic makes it crucial for physicians to consider the weight effects of medications being prescribed and to balance the benefits of treatment with the potential for weight gain. Until recently, the medical literature offered little guidance.
In 2015, the Endocrine Society published clinical practice guidelines for pharmacologic management of obesity, including data on medications that cause weight gain and suggesting alternatives that are weight-neutral or promote weight loss.2
In the pages that follow, we present case studies, tables, and a review of the latest evidence to highlight optimal drug treatment for patients who are overweight or obese, and are also being treated for diabetes, hypertension, and depression. You’ll find a brief discussion of weight management strategies related to other drugs and conditions in the sidebar.2-5
CASE 1 › 40-year-old man with diabetes and hyperlipidemia
Brian P, who has come in for an annual checkup, has a body mass index (BMI) of 30 kg/m2. He also has hyperlipidemia and type 2 diabetes, for which he has been taking metformin for several years. A year ago, his hemoglobin A1c (HbA1c) was 7.3%, so his physician added glyburide to his regimen.
In the year since, Mr. P has gained 12 lbs (5.4 kg) but achieved only a minimal reduction in HbA1c (to 6.8%). He expresses concern about the cardiovascular effects of the extra weight and says that diet and exercise have not helped him control his weight.
CASE 2 › Older woman with hypertension and hypothyroidism
Addie K, age 64, is obese (BMI, 37 kg/m2) and has hypertension and hypothyroidism, for which she takes metoprolol and levothyroxine. Ms. K says that she is careful about what she eats and exercises several times a week, but still has seen her weight increase steadily for the past several years.
CASE 3 › Young man with depression
Charlie D, a 21-year-old college student, is a new patient. He has depression and is obese (BMI, 34 kg/m2). The patient says he was diagnosed with depression by his former primary care physician, who prescribed paroxetine a year ago. He requests a refill of the paroxetine, which he reports has successfully boosted his mood. When asked about his weight, he admits that he has gained 8 lbs (3.6 kg) since he began taking the drug.
If these were your patients, what weight management steps would you take? Before we provide some recommendations, let’s review the evidence.
Antidiabetic agents and weight
While some antidiabetic agents are weight-neutral and others promote weight loss, several therapies are associated with weight gain6 (TABLE 13). Patients like Mr. P can gain as much as 10 kg in 3 to 6 months after beginning treatment with insulin, thiazolidinediones (TZDs), sulfonylureas, and other insulin secretagogues.2,7
A recent systematic review and meta-analysis of 257 randomized controlled trials (RCTs) found weight gain to be associated with the use of pioglitazone (2.6 kg), glimepiride (2.1 kg), glyburide (2.6 kg), glipizide (2.2 kg), and sitagliptin (0.55 kg). A modest weight loss was associated with acarbose, exenatide, liraglutide, metformin, miglitol, and pramlintide.8
Sulfonylureas are generally associated with a 1.5 to 2.5 kg weight gain.9-11 In an analysis of 27 RCTs of noninsulin antidiabetic drugs in patients whose disease was not controlled by metformin alone, TZDs, sulfonylureas, and meglitinides were associated with a 1.77 to 2.08 kg weight gain.9 Furthermore, those taking sulfonylureas and meglitinides had higher rates of hypoglycemia compared with patients taking placebo (relative risk, 4.50-7.50). In fact, sulfonylureas have the highest risk of serious hypoglycemia of any noninsulin therapy.6
In contrast, metformin—the most commonly prescribed oral agent for type 2 diabetes—promotes mild weight loss by multiple mechanisms and has a good safety profile.12,13 Thus, some physicians use metformin off label for weight loss and diabetes prevention and have suggested that it be approved for these indications.13
Glycemic control and weight loss
Glucagon-like peptide-1 (GLP-1) agonists lead to weight loss by decreasing appetite and enhancing satiety, as well as improving glycemic control. Liraglutide received Food and Drug Administration (FDA) approval in 2014 as a treatment for chronic weight management at a higher dose (3 mg/d) than that used to treat diabetes (1.8 mg/d).14
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a relatively new class of antidiabetic medication that reduce glucose reabsorption by the kidneys, leading to increased urinary glucose excretion.15 The associated weight loss, in addition to a reduction in hyperglycemia, may be due to the subsequent calorie loss through glycosuria.
Both dipeptidyl peptidase-4 (DPP-4) inhibitors and alpha-glucosidase inhibitors (AGIs) appear to be weight-neutral or to induce minimal changes in weight.16 Although the systematic review mentioned earlier found a 0.55 kg weight gain associated with sitagliptin,8 most studies of DPP-4 inhibitors report weight neutrality.17-19 Pramlintide, the amylin analogue that has FDA approval for use in combination with existing insulin treatment, can prevent weight gain or lead to weight loss.20,21
The Endocrine Society Clinical Practice Guideline recommends concomitantly prescribing at least one weight loss-promoting medication (such as metformin, a GLP-1 agonist, or pramlintide) to patients with obesity and type 2 diabetes who require insulin to mitigate weight gain due to insulin.2
The 2016 Comprehensive Type 2 Diabetes Management Algorithm published by the American Association of Clinical Endocrinologists and American College of Endocrinology recommends that the initiation of diabetes therapies be based on the risks of weight gain and hypoglycemia, among other factors. The algorithm calls for metformin as first-line therapy, followed by a GLP-1 agonist as a second-line therapy, and an SGLT2 inhibitor as a third-line therapy.6
Finally, FDA-approved anti-obesity medications may be appropriate for patients with diabetes who are unable to lose weight with lifestyle interventions alone.22 Each medication is associated with improvements in glucose in addition to other metabolic parameters.
CASE 1 › A better choice for Mr. P
Because Mr. P has gained weight—and, indeed, developed obesity—since he started taking glyburide, it is clear that a sulfonylurea is not the best choice for this patient. An antidiabetic agent that is weight-neutral or that promotes weight loss, such as an SGLT2 inhibitor or a GLP-1 agonist, would be more suitable. The drug should be prescribed in conjunction with his metformin, which has a favorable weight profile and helps reduce HbA1c, as both SGLT2 inhibitors and GLP-1 agonists also do.
If Mr. P were to switch to an SGLT2 inhibitor, a combination pill containing metformin would be an effective way to limit the patient’s pill burden.
Treating hypertension without weight gain
Thiazide diuretics are often recommended as first-line agents for the treatment of hypertension, but their dose-related adverse effects, including dyslipidemia and insulin resistance, are undesirable for patients who are overweight or obese and at risk for metabolic syndrome and type 2 diabetes.23 Beta-adrenergic blockers have been shown to promote weight gain and prevent weight loss, especially in patients who have both hypertension and diabetes.24 In addition to having potential adverse metabolic effects on lipids and/or insulin sensitivity, beta-blockers can decrease metabolic rate by 10% and they may have other negative effects on energy metabolism, as well.25
In a meta-analysis of 8 RCTs that lasted ≥6 months, changes in body weight were higher in participants on beta-blockers, with a median difference of 1.2 kg (−0.4 to 3.5 kg) between those on beta-blockers and the control group.26 The evidence suggests that beta-blockers should not necessarily be first-line treatment for hypertension in patients who are overweight or obese and that obesity management in patients with hypertension may be harder if they are being treated with a beta-blocker.
When a different drug in the same class will do
There are exceptions, however. When beta-blockers are required—for patients with coronary artery disease, heart failure, or an arrhythmia, for example—a selective agent with a vasodilating component, such as carvedilol or nebivolol, is recommended.2 These drugs appear to have less potential for weight gain and to have minimal effect on lipid and glucose metabolism.26,27
In a study of 1106 patients with hypertension, those taking metoprolol had a statistically significant mean weight gain of 1.19 kg (P<.001) compared with patients taking carvedilol (mean weight gain, 0.17 kg; P=.36).24 While 4.5% of those in the metoprolol group gained ≥7% of their body weight, that was true of only 1.1% of those taking carvedilol. Thus, weight gain can sometimes be minimized by choosing a different medication within the same drug class.
ACE inhibitors, ARBs, and calcium channel blockers
Antihypertensive medications that are not associated with weight gain or insulin resistance include angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs) (TABLE 2).3 Angiotensin contributes to obesity-related hypertension, as it is overexpressed in obesity, making ACE inhibitors and ARBs desirable options for the treatment of patients who are obese. And, because many patients who are obese also suffer from type 2 diabetes or prediabetes, they’re likely to benefit from the renal protection provided by ACE inhibitors and ARBs, as well.
CASE 2 › Switching antihypertensives
Switching Ms. K from metoprolol, a beta-blocker, to an ACE inhibitor, ARB, or CCB may help prevent further weight gain, and possibly even lead to weight loss. Any drug in any of these 3 classes of medications would be a reasonable choice. However, if the patient had a condition that warranted use of a beta-blocker, a selective agent with a vasodilating component such as carvedilol or nebivolol might be helpful.
SIDEBAR
Weight management strategies for several other conditionsIn addition to medications for common conditions such as diabetes, hypertension, and depression, there are numerous other drugs that can cause unwanted weight gain. These include some antiseizure agents, antipsychotics, contraceptives, hormones, and migraine therapies, as well as corticosteroids. In view of both the nation’s obesity epidemic and the many drugs that are known to adversely affect weight maintenance, it is crucial to do a careful risk-benefit analysis and a search for alternatives whenever you prescribe a new medication for a patient who is overweight or obese or has metabolic risk factors.2-5
When weight-neutral substitutes exist, such medications should be considered, if appropriate, to prevent or lessen pharmacologic weight gain. For example, topiramate and zonisamide are preferable to other antiepileptics, such as valproic acid and gabapentin when it comes to weight management.2-4 It is essential to keep in mind, however, that medications in the same class are not always interchangeable.
For patients with inflammatory conditions such as rheumatoid arthritis, disease-modifying antirheumatic drugs (DMARDs) are preferable to corticosteroids whenever possible.2-4 For the many patients for whom steroids or other drugs known to cause weight gain are necessary, however, dietary and lifestyle counseling—advising patients to eat a healthful diet and maintain adequate activity levels, among other interventions—may help to mitigate the effects.
And when there are no alternative medications available, use the lowest possible dose for the shortest duration necessary.
Choosing an antidepressant when weight is an issue
For patients with psychiatric conditions, weight gain is often multifactorial. One key issue: Weight gain is a common adverse effect of many antidepressants (TABLE 3).3 Within classes of antidepressants, there is a range of weight gain potential, which can vary depending on the duration of therapy.2
In a meta-analysis of 116 studies, selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine and sertraline were associated with weight loss in short-term use (4-12 weeks) and weight neutrality when used for >4 months.1 Patients who had type 2 diabetes as well as depression had an average weight loss from fluoxetine of 5.1 kg (3.3–6.9 kg) at 24- to 26-week follow up.28
Among SSRI and tricyclic (TCA) antidepressants, paroxetine and amitriptyline, respectively, had the greatest risk for weight gain.1,29 No significant weight effect was observed for either citalopram or escitalopram. Keep in mind, however, that the effect of each antidepressant on weight may vary greatly from one patient to another.1 For example, while Mr. D gained 3.6 kg on paroxetine, some patients gain no weight at all.
In the systematic review and meta-analysis of 257 RCTs, weight gain was associated with the use of amitriptyline (1.8 kg) and mirtazapine (1.5 kg), while weight loss was associated with bupropion and fluoxetine (-1.3 kg for each).8
This antidepressant can decrease cravings
Bupropion, a norepinephrine and dopamine reuptake inhibitor, is the only antidepressant that has been consistently shown to cause weight loss.30,31 Clinical trials have found that it decreases body weight by suppressing appetite and reducing food cravings.30 Bupropion is approved for the treatment of depression and as a smoking cessation aide. And, in 2014, a bupropion-naltrexone combination received FDA approval for chronic weight management, sold under the brand name Contrave.32
As different classes of antidepressants are often prescribed for different types of depression, it is important to be aware that the few that are weight-neutral and weight-loss-promoting are not appropriate for all patients with depression. For example, bupropion is an activating agent and can exacerbate anxiety. Thus, a patient with concomitant depression and anxiety might be a better candidate for another antidepressant, which could lead to some weight gain but would better manage the individual’s symptoms. In such cases, the rule of thumb should be to prescribe the lowest dose required for clinical efficacy for the shortest duration necessary.
CASE 3 › Change antidepressants— and keep a close watch
Depending on the nature of Mr. D’s depression, bupropion, fluoxetine, or sertraline might be a reasonable alternative to paroxetine to prevent or reduce further drug-induced weight gain.
Frequent follow-up visits should be scheduled until the transition has been completed and his condition stabilized. If Mr. D’s new antidepressant is bupropion, monitoring him for signs of anxiety would be required.
CORRESPONDENCE
Katherine H. Saunders, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; [email protected].
1. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259-1272.
2. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100:342-362.
3. Apovian CM, Aronne L, Powell AG. Clinical Management of Obesity. West Islip, NY: Professional Communications, Inc., 2015.
4. Aronne LJ. A Practical Guide to Drug-induced Weight Gain. Minneapolis, Minn: McGraw-Hill; 2002.
5. Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs: a systematic review. QJM. 2007;100:395-404.
6. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2016 executive summary. Endocr Pract. 2016;22:84-113.
7. Aronne LJ. Drug-induced weight gain: non-CNS medications. In: A Practical Guide to Drug-Induced Weight Gain. Minneapolis, Minn: McGraw-Hill: 2002:77-91.
8. Domecq JP, Prutsky G, Leppin A, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:363-370.
9. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
10. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
11. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481.
12. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21:323-329.
13. Igel LI, Sinha A, Saunders KH, et al. Metformin: an old therapy that deserves a new indication for the treatment of obesity. Curr Atheroscler Rep. 2016;18:16.
14. US Food and Drug Administration. FDA approves weight-management drug Saxenda. December 23, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed October 1, 2016.
15. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495-502.
16. van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154-163.
17. Hong ES, Khang AR, Yoon JW, et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795-802.
18. Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509-1515.
19. Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38:89-101.
20. Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784-790.
21. Aronne L, Fujioka K, Aroda V, et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab. 2007;92:2977-2983.
22. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
23. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the Obesity Society and the American Society of Hypertension. Obesity (Silver Spring). 2013;21:8-24.
24. Messerli FH, Bell DS, Fonseca V, et al. Body weight changes with beta-blocker use: results from GEMINI. Am J Med. 2007;120:610-615.
25. Pischon T, Sharma AM. Use of beta-blockers in obesity hypertension: potential role of weight gain. Obes Rev. 2001;2:275-280.
26. Sharma AM, Pischon T, Hardt S, et al. Hypothesis: beta-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37:250-254.
27. Manrique C, Whaley-Connell A, Sowers JR. Nebivolol in obese and non-obese hypertensive patients. J Clin Hypertens (Greenwich). 2009;11:309-315.
28. Norris SL, Zhang X, Avenell A, et al. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(1):CD004096.
29. Rosenzweig-Lipson S, Beyer CE, Hughes ZA, et al. Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther. 2007;113:134-153.
30. Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother. 2007;7:17-24.
31. Arterburn D, Sofer T, Boudreau DM, et al. Long-term weight change after initiating second-generation antidepressants. J Clin Med. 2016;5:piiE48.
32. US Food and Drug Administration. FDA approves weight-management drug Contrave. September 10, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm413896.htm. Accessed October 1, 2016.
1. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259-1272.
2. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100:342-362.
3. Apovian CM, Aronne L, Powell AG. Clinical Management of Obesity. West Islip, NY: Professional Communications, Inc., 2015.
4. Aronne LJ. A Practical Guide to Drug-induced Weight Gain. Minneapolis, Minn: McGraw-Hill; 2002.
5. Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs: a systematic review. QJM. 2007;100:395-404.
6. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2016 executive summary. Endocr Pract. 2016;22:84-113.
7. Aronne LJ. Drug-induced weight gain: non-CNS medications. In: A Practical Guide to Drug-Induced Weight Gain. Minneapolis, Minn: McGraw-Hill: 2002:77-91.
8. Domecq JP, Prutsky G, Leppin A, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:363-370.
9. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
10. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
11. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481.
12. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21:323-329.
13. Igel LI, Sinha A, Saunders KH, et al. Metformin: an old therapy that deserves a new indication for the treatment of obesity. Curr Atheroscler Rep. 2016;18:16.
14. US Food and Drug Administration. FDA approves weight-management drug Saxenda. December 23, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed October 1, 2016.
15. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495-502.
16. van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154-163.
17. Hong ES, Khang AR, Yoon JW, et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795-802.
18. Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509-1515.
19. Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38:89-101.
20. Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784-790.
21. Aronne L, Fujioka K, Aroda V, et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab. 2007;92:2977-2983.
22. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
23. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the Obesity Society and the American Society of Hypertension. Obesity (Silver Spring). 2013;21:8-24.
24. Messerli FH, Bell DS, Fonseca V, et al. Body weight changes with beta-blocker use: results from GEMINI. Am J Med. 2007;120:610-615.
25. Pischon T, Sharma AM. Use of beta-blockers in obesity hypertension: potential role of weight gain. Obes Rev. 2001;2:275-280.
26. Sharma AM, Pischon T, Hardt S, et al. Hypothesis: beta-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37:250-254.
27. Manrique C, Whaley-Connell A, Sowers JR. Nebivolol in obese and non-obese hypertensive patients. J Clin Hypertens (Greenwich). 2009;11:309-315.
28. Norris SL, Zhang X, Avenell A, et al. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(1):CD004096.
29. Rosenzweig-Lipson S, Beyer CE, Hughes ZA, et al. Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther. 2007;113:134-153.
30. Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother. 2007;7:17-24.
31. Arterburn D, Sofer T, Boudreau DM, et al. Long-term weight change after initiating second-generation antidepressants. J Clin Med. 2016;5:piiE48.
32. US Food and Drug Administration. FDA approves weight-management drug Contrave. September 10, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm413896.htm. Accessed October 1, 2016.