User login
Partnering to optimize care of childhood cancer survivors
The number of childhood cancer survivors (CCSs) entering the adult health care system is increasing, a not-so-surprising trend when you consider that more than 80% of children and adolescents given a cancer diagnosis become long-term survivors.1 This patient population has a heightened risk for developing at least one chronic health problem, resulting from therapy. By the fourth decade of life, 88% of all CCSs will have a chronic condition,2 and about one-third develop a late effect that is either severe or life-threatening.3 In contrast to patients with many other pediatric chronic diseases that manifest at an early age and are progressive, CCSs are often physically well for many years, or decades, prior to their manifestation of late effects.4
Cancer survivorship has varying definitions; however, we define cancer survivorship as the phase of cancer care for individuals who have been diagnosed with cancer and have completed primary treatment for their disease.5 Cancer survivorship, which is becoming more widely acknowledged as a distinct and critically important phase of cancer care, includes:6
- “surveillance for recurrence,
- evaluation … and treatment of medical and psychosocial consequences of treatment,
- recommendations for screening for new primary cancers,
- health promotion recommendations, and
- provision of a written treatment summary and care plan to the patient and other health professionals.”
Although models of survivorship care vary, their common goal is to promote optimal health and well-being in cancer survivors, and to prevent and detect any health concerns that may be related to prior cancer diagnosis or treatment.
Some pediatric cancer survivors have not received recommended survivorship care because of a lack of insurance or limitations from pre-existing conditions.4,7 The Affordable Care Act may remove these barriers for many.8 Others, however, fail to receive such recommendations because national models of transition are lacking. Unique considerations for this population include their need to establish age appropriate, lifelong follow-up care (and education) from a primary care provider (PCP). Unfortunately, many CCSs become lost to follow-up and fail to receive recommended survivorship care when they discontinue the relationship with their pediatrician or family practitioner and their pediatric oncologist. Fewer than 25% of CCSs who have been successfully treated for cancer during childhood continue to be followed by a cancer center and are at risk for missing survivorship-focused care or recommended screening.4,9
PCPs are an invaluable link in helping CCSs to continue to receive recommended care and surveillance. However, PCPs experience barriers in providing cancer care because of a lack of timely and specific communication from oncologists and limited knowledge of guidelines and resources available to them.10 The purpose of this article is to share information with you, the family physician, about childhood cancer survivorship needs, available resources, and how partnering with pediatric oncologists may improve treatment and health outcomes for CCSs.
Providing for the future health of childhood cancer survivors
Numerous studies have outlined the myriad of potential late effects that CCSs may experience from disease and treatment.11,12 These effects can manifest at any time and can appear in virtually every body system from the central nervous system, to the lungs, heart, bones, and endocrine systems. CCSs' particular risk for late effects may result from many factors including cancer diagnosis, types of treatments (eg, surgery, chemotherapy, radiation, and stem-cell transplant), and dosages of medications, gender, and age at diagnosis.
Determining individual risk for late effects
The Children’s Oncology Group (COG) is the world’s largest organization devoted exclusively to childhood and adolescent cancer research, including the long-term health of cancer survivors. To help provide more individualized recommendations, COG has set forth risk-based, evidence-based, exposure-related clinical practice guidelines to offer recommendations for screening and management of late effects in survivors of childhood and adolescent cancers.13 (These guidelines, Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, are available at http://www.survivorshipguidelines.org.) The purpose of the guidelines is to standardize and enhance follow-up care for CCSs throughout their lifespan.13 To remain current, a multidisciplinary task force reviews and incorporates findings from the medical literature—including evaluations of the cost-effectiveness of recommended testing—into guideline revisions at least every 5 years.
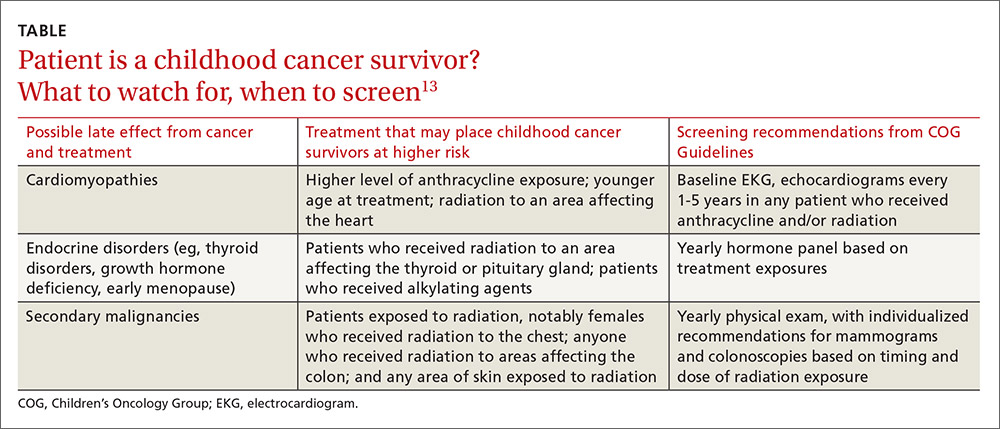
Some of the most severe or life-threatening late effects include cardiomyopathies, endocrine disorders, and secondary malignancies (TABLE).13 Ongoing follow-up care is based on a survivor’s individual risk level and the frequency of lifelong recommended screening. The majority of patients will require yearly follow-up with additional testing, such as echocardiograms occurring as infrequently as every 2 to 5 years. Patients who received more intense therapy, such as hematopoietic stem-cell transplants, will require follow-up (often including annual echocardiograms, blood work, and a thorough physical exam) every 6 months to one year. Common testing and surveillance include blood pressure checks, urinalyses, thyroid function tests, lipid panels, echocardiograms, and electrocardiograms.
After treatment, patients should receive survivorship care plans
For health care providers to use COG Guidelines effectively across medical disciplines, it is important to know critical pieces of the patient’s cancer diagnosis and treatment history. In 2006, the Institute of Medicine released a report14 recommending that all cancer survivors be given a comprehensive care summary and follow-up plan when they complete their primary cancer care. More recently, the Commission on Cancer of the American College of Surgeons has mandated that, in order to be a cancer program accredited by the Commission, all cancer patients must be given a survivorship care plan after completing treatment.15 Generated by the treating cancer center, these care plans are meant to concisely communicate a patient’s cancer diagnosis, treatment, and long-term risks to other health care providers (across disciplines and institutions).
What’s included in a survivorship care plan?
The survivorship care plan is a paper or electronic document created by the treating institution that contains 2 components: a treatment summary and a long-term care plan based on medical/treatment history. The treatment summary includes, at a minimum, general background information (eg, demographics, pertinent medical history, diagnostic details, and significant treatment complications) and a therapeutic summary (such as dates of treatment, protocol, and details of chemotherapy, radiation, hematopoietic stem-cell transplant, and/or surgery).
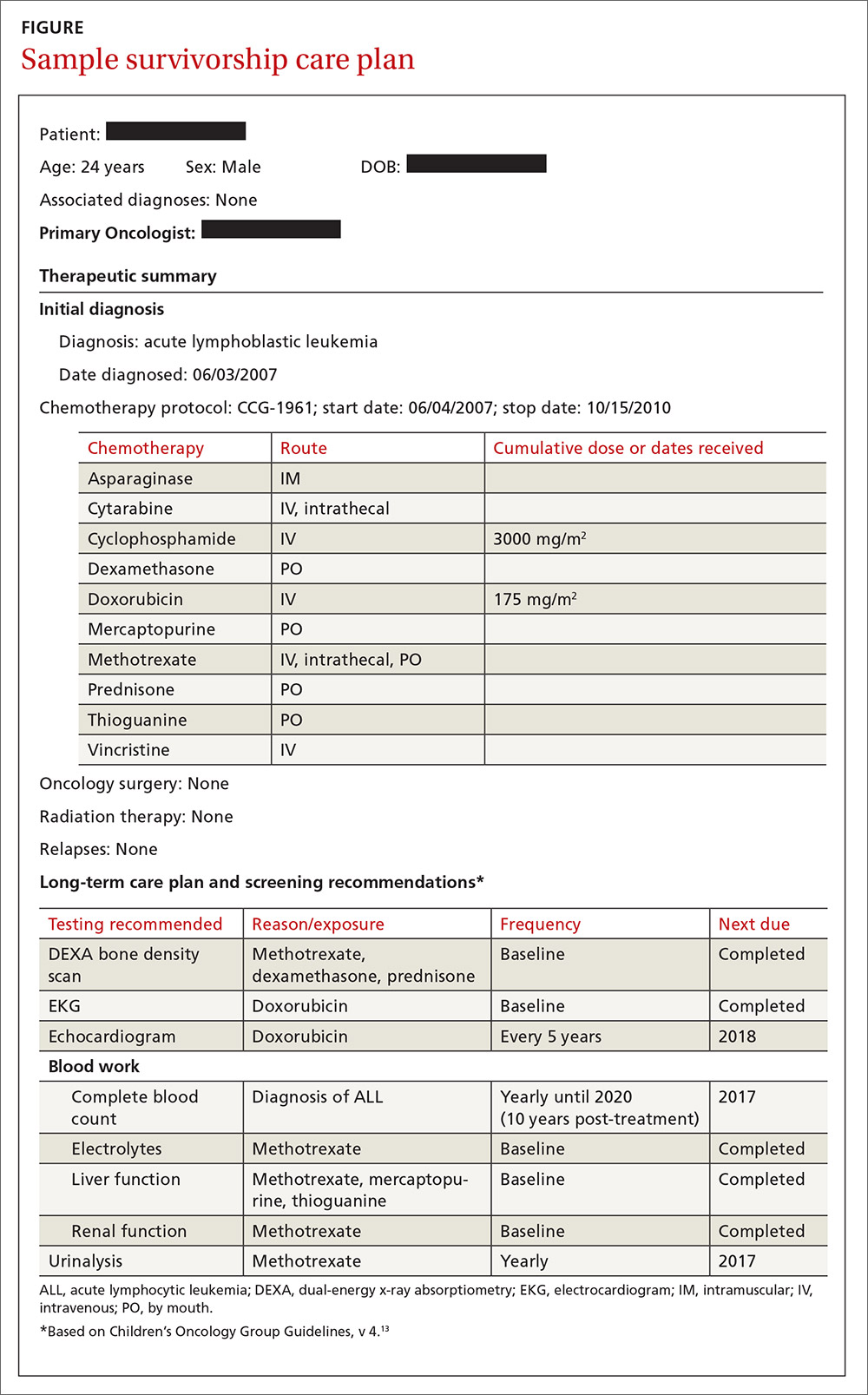
The second component, the long-term care plan, details potential long-term effects specific to the treatment received, and recommendations for ongoing follow-up related to long-term risk (FIGURE). The post-treatment plan is primarily based on COG Guideline recommendations. Many institutions are introducing an electronic-based survivorship care plan, either in addition to or in replacement of a paper-based care plan. Electronic-based care plans have several benefits for patients and providers, including increased accessibility, and some offer the ability to easily update follow-up recommendations, as guidelines change, without the need for manual entry.
Shared care for cancer survivors: Oncology and primary care
Numerous models of cancer survivorship care have been described, including care by the treating oncologist, a dedicated cancer survivorship program, or follow-up completed by PCPs. There is no consensus on the best model, although many have noted that shared care is a critically important component of successful cancer survivorship care,6,16–18 and appears to be the preferred model of PCPs.19
Shared care, as the name implies, involves care that is coordinated between 2 or more health providers across specialties or locations.20 This model has shown improved outcomes in other chronic disease-management models, such as those for diabetes21 and chronic renal disease.22 One study23 found that colorectal cancer survivors who were seen by both an oncologist and a PCP were significantly more likely to receive recommended testing and follow-up to promote overall health than when they were followed by either physician alone. Information sharing between oncology and PCPs is critical to maintaining and promoting optimal health and well-being in cancer survivors, and requires ongoing communication and a concerted effort to facilitate and maintain collaboration between oncology specialists and other health care providers.6,17
Role of the cancer center in survivorship care
Although every cancer center has a slightly different timeline and structure in terms of survivorship care, there are common themes across programs regarding the type of care provided. Immediately following treatment, care is focused on surveillance for recurrence, with appointments ranging from monthly to a few times a year. This care is most often provided by the primary oncologist.
The next phase of care is reached 2 to 5 years after treatment, when recurrence is no longer a significant risk, and care is focused on monitoring and treating late effects. Depending on the center, this care may be coordinated by a dedicated survivorship clinic, the primary oncologist, or the PCP. In some models,6 the survivorship team is integrated into the patient’s care from the beginning of treatment, while others do not become active in care until the patient is considered cured of disease. In all models, a survivorship care plan should be completed after treatment has ended and before transitioning care to a PCP.
In our institution’s model, we have a survivorship program that serves patients who are more than 5 years from the completion of their treatment. Our survivorship team is comprised of a pediatric oncologist, advanced practice practitioner (APP) coordinator, a project coordinator, a clinical social worker, and a research staff member. Patients are seen every one to 2 years, depending on their overall risk for late effects. For those who are seen every other year, we are available to the PCP for questions or concerns, and the survivorship team connects with the CCS by phone to screen for any change in health status that would alter recommendations for an earlier follow-up at the oncology center.
A typical visit to our survivorship clinic includes completion of an annual health questionnaire, which addresses current health issues, as well as screening for anxiety, depression, nicotine, alcohol, and drug use. This questionnaire is reviewed by the pediatric oncologist and is used to tailor screening, referrals, and patient education based on current complaints. The oncologist also performs a thorough physical exam with special attention to areas in which late effects may occur (eg, skin exam in areas of previous radiation). In addition, each patient receives an individualized treatment summary based on COG guidelines, which is updated before each visit by the APP coordinator. The APP coordinator reviews the document at each visit and offers patient education and health maintenance counseling.
Ensuring patients aren’t lost to follow-up. In our experience, numerous patients become lost to follow-up as they age, enter college or the workforce, or move away. So, rather than attempting to follow these patients for life, we work to transition patient care to a PCP of their choice, particularly if they are at least 21 years old and more than 10 years post-diagnosis. However, we will work to transition at any time at the request of the CCS. Even when a patient’s ongoing care is transitioned to a PCP, we will remain as a continuing resource to PCPs and CCSs on an as-needed basis.
Role of primary care providers in survivorship care
Every health care provider caring for a CCS should have a copy of the patient’s survivorship care plan. This document should be provided by the treating institution, but research has shown that as many as 86% of PCPs fail to receive this critical information.24 Any PCP who treats a patient with a history of cancer and has not received a survivorship care plan should contact the treating cancer center to request a copy. A properly prepared survivorship care plan summarizes the patient’s disease and treatment history, and provides a road map of the patient’s risk for long-term effects from disease and treatment.
The most important sections of the survivorship care plan for use in primary care will be the list of potential late effects and ongoing recommended testing. This list will help to guide the PCP’s differential and work-up for specific complaints. For example, knowing that a patient is at risk for a second malignancy because of radiation therapy may result in earlier diagnostic imaging, leading to a timelier diagnosis.
The COG screening recommendations that are generally included in a survivorship care plan are appropriate for survivors who are asymptomatic and presenting for routine, exposure-based medical follow-up. More extensive work-ups are presumed to be completed as clinically indicated. Consultation with a pediatric long-term follow-up clinic is also encouraged, particularly if a concern arises.
A complementary set of patient education materials, known as “Health Links,” accompany the COG guidelines to broaden their application and enhance patient follow-up visits. A survivorship care plan and the COG Guidelines help ensure that CCSs receive appropriate ongoing follow-up based on their history. A collaborative approach between Oncology and PCPs is essential to improve the quality of care for CCSs and to maintain the long-term health of this vulnerable population.
CORRESPONDENCE
Jean M. Tersak, Children’s Hospital of Pittsburgh of UPMC, 4401 Penn Avenue, 5th Floor Plaza Building, Pittsburgh, PA 15224; [email protected].
1. Ries LAG, Eisner MP, Kosary CL, et al, eds. SEER Cancer Statistics Review, 1975-2002. National Cancer Institute. Bethesda, MD. Available at: http://seer.cancer.gov/csr/1975_2002/. Accessed May 26, 2016.
2. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653-663.
3. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572-1582.
4. Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4401-4409.
5. Feuerstein M. Defining cancer survivorship. J Cancer Surviv. 2007;1:5-7.
6. McCabe MS, Jacobs LA. Clinical update: survivorship care—models and programs. Semin Oncol Nurs. 2012;28:e1-e8.
7. Oeffinger K, Mertens A, Hudson M, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2:61-70.
8. Mueller EL, Park ER, Davis MM. What the affordable care act means for survivors of pediatric cancer. J Clin Oncol. 2014;32:615-617.
9. Oeffinger KC. Longitudinal risk-based health care for adult survivors of childhood cancer. Curr Probl Cancer. 2003;27:143-167.
10. Lawrence RA, McLoone JK, Wakefield CE, et al. Primary care physicians’ perspectives of their role in cancer care: a systematic review. J Gen Intern Med. 2016:1-15.
11. Schwartz CL. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4:45-54.
12. Late Effects of Treatment for Childhood Cancer (PDQ(R)): Health Professional Version [Internet]. Bethesda, MD: National Cancer Institute. Updated March 31, 2016. Available at: www.cancer.gov/types/childhood-cancers/late-effects-hp-pdq. Accessed June 2, 2016.
13. Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer, Version 4.0. Monrovia CA: Children’s Oncology Group. 2013. Available at: www.survivorshipguidelines.org. Accessed June 2, 2016.
14. Hewitt M, Greenfield S, Stovall E, Committee on Cancer Survivorship: Improving Care and Quality of Life. National Cancer Policy Board, Institute of Medicine, National Research Council, eds. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press; 2005.
15. Commission on Cancer [Internet]. Cancer Program Standards: Ensuring Patient-Centered Care. Chicago, IL: American College of Surgeons; 2015. Available at: https://www.facs.org/quality%20programs/cancer/coc/standards. Accessed June 2, 2016.
16. Askins MA, Moore BD. Preventing neurocognitive late effects in childhood cancer survivors. J Child Neurol. 2008;23:1160-1171.
17. McCabe MS, Jacobs L. Survivorship care: models and programs. Semin Oncol Nurs. 2008;24:202-207.
18. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117-5124.
19. Potosky AL, Han PKJ, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26:1403-1410.
20. Gilbert SM, Miller DC, Hollenbeck BK, et al. Cancer survivorship: challenges and changing paradigms. J Urol. 2008;179:431-438.
21. Renders CM, Valk GD, de Sonnaville JJ, et al. Quality of care for patients with Type 2 diabetes mellitus—a long-term comparison of two quality improvement programmes in the Netherlands. Diabet Med. 2003;20:846-852.
22. Jones C, Roderick P, Harris S, et al. An evaluation of a shared primary and secondary care nephrology service for managing patients with moderate to advanced CKD. Am J Kidney Dis. 2006;47:103-114.
23. Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101:1712-1719.
24. Sima JL, Perkins SM, Haggstrom DA. Primary care physician perceptions of adult survivors of childhood cancer. J Pediatr Hematol Oncol. 2014;36:118-124.
The number of childhood cancer survivors (CCSs) entering the adult health care system is increasing, a not-so-surprising trend when you consider that more than 80% of children and adolescents given a cancer diagnosis become long-term survivors.1 This patient population has a heightened risk for developing at least one chronic health problem, resulting from therapy. By the fourth decade of life, 88% of all CCSs will have a chronic condition,2 and about one-third develop a late effect that is either severe or life-threatening.3 In contrast to patients with many other pediatric chronic diseases that manifest at an early age and are progressive, CCSs are often physically well for many years, or decades, prior to their manifestation of late effects.4
Cancer survivorship has varying definitions; however, we define cancer survivorship as the phase of cancer care for individuals who have been diagnosed with cancer and have completed primary treatment for their disease.5 Cancer survivorship, which is becoming more widely acknowledged as a distinct and critically important phase of cancer care, includes:6
- “surveillance for recurrence,
- evaluation … and treatment of medical and psychosocial consequences of treatment,
- recommendations for screening for new primary cancers,
- health promotion recommendations, and
- provision of a written treatment summary and care plan to the patient and other health professionals.”
Although models of survivorship care vary, their common goal is to promote optimal health and well-being in cancer survivors, and to prevent and detect any health concerns that may be related to prior cancer diagnosis or treatment.
Some pediatric cancer survivors have not received recommended survivorship care because of a lack of insurance or limitations from pre-existing conditions.4,7 The Affordable Care Act may remove these barriers for many.8 Others, however, fail to receive such recommendations because national models of transition are lacking. Unique considerations for this population include their need to establish age appropriate, lifelong follow-up care (and education) from a primary care provider (PCP). Unfortunately, many CCSs become lost to follow-up and fail to receive recommended survivorship care when they discontinue the relationship with their pediatrician or family practitioner and their pediatric oncologist. Fewer than 25% of CCSs who have been successfully treated for cancer during childhood continue to be followed by a cancer center and are at risk for missing survivorship-focused care or recommended screening.4,9
PCPs are an invaluable link in helping CCSs to continue to receive recommended care and surveillance. However, PCPs experience barriers in providing cancer care because of a lack of timely and specific communication from oncologists and limited knowledge of guidelines and resources available to them.10 The purpose of this article is to share information with you, the family physician, about childhood cancer survivorship needs, available resources, and how partnering with pediatric oncologists may improve treatment and health outcomes for CCSs.
Providing for the future health of childhood cancer survivors
Numerous studies have outlined the myriad of potential late effects that CCSs may experience from disease and treatment.11,12 These effects can manifest at any time and can appear in virtually every body system from the central nervous system, to the lungs, heart, bones, and endocrine systems. CCSs' particular risk for late effects may result from many factors including cancer diagnosis, types of treatments (eg, surgery, chemotherapy, radiation, and stem-cell transplant), and dosages of medications, gender, and age at diagnosis.
Determining individual risk for late effects
The Children’s Oncology Group (COG) is the world’s largest organization devoted exclusively to childhood and adolescent cancer research, including the long-term health of cancer survivors. To help provide more individualized recommendations, COG has set forth risk-based, evidence-based, exposure-related clinical practice guidelines to offer recommendations for screening and management of late effects in survivors of childhood and adolescent cancers.13 (These guidelines, Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, are available at http://www.survivorshipguidelines.org.) The purpose of the guidelines is to standardize and enhance follow-up care for CCSs throughout their lifespan.13 To remain current, a multidisciplinary task force reviews and incorporates findings from the medical literature—including evaluations of the cost-effectiveness of recommended testing—into guideline revisions at least every 5 years.
Some of the most severe or life-threatening late effects include cardiomyopathies, endocrine disorders, and secondary malignancies (TABLE).13 Ongoing follow-up care is based on a survivor’s individual risk level and the frequency of lifelong recommended screening. The majority of patients will require yearly follow-up with additional testing, such as echocardiograms occurring as infrequently as every 2 to 5 years. Patients who received more intense therapy, such as hematopoietic stem-cell transplants, will require follow-up (often including annual echocardiograms, blood work, and a thorough physical exam) every 6 months to one year. Common testing and surveillance include blood pressure checks, urinalyses, thyroid function tests, lipid panels, echocardiograms, and electrocardiograms.
After treatment, patients should receive survivorship care plans
For health care providers to use COG Guidelines effectively across medical disciplines, it is important to know critical pieces of the patient’s cancer diagnosis and treatment history. In 2006, the Institute of Medicine released a report14 recommending that all cancer survivors be given a comprehensive care summary and follow-up plan when they complete their primary cancer care. More recently, the Commission on Cancer of the American College of Surgeons has mandated that, in order to be a cancer program accredited by the Commission, all cancer patients must be given a survivorship care plan after completing treatment.15 Generated by the treating cancer center, these care plans are meant to concisely communicate a patient’s cancer diagnosis, treatment, and long-term risks to other health care providers (across disciplines and institutions).
What’s included in a survivorship care plan?
The survivorship care plan is a paper or electronic document created by the treating institution that contains 2 components: a treatment summary and a long-term care plan based on medical/treatment history. The treatment summary includes, at a minimum, general background information (eg, demographics, pertinent medical history, diagnostic details, and significant treatment complications) and a therapeutic summary (such as dates of treatment, protocol, and details of chemotherapy, radiation, hematopoietic stem-cell transplant, and/or surgery).
The second component, the long-term care plan, details potential long-term effects specific to the treatment received, and recommendations for ongoing follow-up related to long-term risk (FIGURE). The post-treatment plan is primarily based on COG Guideline recommendations. Many institutions are introducing an electronic-based survivorship care plan, either in addition to or in replacement of a paper-based care plan. Electronic-based care plans have several benefits for patients and providers, including increased accessibility, and some offer the ability to easily update follow-up recommendations, as guidelines change, without the need for manual entry.
Shared care for cancer survivors: Oncology and primary care
Numerous models of cancer survivorship care have been described, including care by the treating oncologist, a dedicated cancer survivorship program, or follow-up completed by PCPs. There is no consensus on the best model, although many have noted that shared care is a critically important component of successful cancer survivorship care,6,16–18 and appears to be the preferred model of PCPs.19
Shared care, as the name implies, involves care that is coordinated between 2 or more health providers across specialties or locations.20 This model has shown improved outcomes in other chronic disease-management models, such as those for diabetes21 and chronic renal disease.22 One study23 found that colorectal cancer survivors who were seen by both an oncologist and a PCP were significantly more likely to receive recommended testing and follow-up to promote overall health than when they were followed by either physician alone. Information sharing between oncology and PCPs is critical to maintaining and promoting optimal health and well-being in cancer survivors, and requires ongoing communication and a concerted effort to facilitate and maintain collaboration between oncology specialists and other health care providers.6,17
Role of the cancer center in survivorship care
Although every cancer center has a slightly different timeline and structure in terms of survivorship care, there are common themes across programs regarding the type of care provided. Immediately following treatment, care is focused on surveillance for recurrence, with appointments ranging from monthly to a few times a year. This care is most often provided by the primary oncologist.
The next phase of care is reached 2 to 5 years after treatment, when recurrence is no longer a significant risk, and care is focused on monitoring and treating late effects. Depending on the center, this care may be coordinated by a dedicated survivorship clinic, the primary oncologist, or the PCP. In some models,6 the survivorship team is integrated into the patient’s care from the beginning of treatment, while others do not become active in care until the patient is considered cured of disease. In all models, a survivorship care plan should be completed after treatment has ended and before transitioning care to a PCP.
In our institution’s model, we have a survivorship program that serves patients who are more than 5 years from the completion of their treatment. Our survivorship team is comprised of a pediatric oncologist, advanced practice practitioner (APP) coordinator, a project coordinator, a clinical social worker, and a research staff member. Patients are seen every one to 2 years, depending on their overall risk for late effects. For those who are seen every other year, we are available to the PCP for questions or concerns, and the survivorship team connects with the CCS by phone to screen for any change in health status that would alter recommendations for an earlier follow-up at the oncology center.
A typical visit to our survivorship clinic includes completion of an annual health questionnaire, which addresses current health issues, as well as screening for anxiety, depression, nicotine, alcohol, and drug use. This questionnaire is reviewed by the pediatric oncologist and is used to tailor screening, referrals, and patient education based on current complaints. The oncologist also performs a thorough physical exam with special attention to areas in which late effects may occur (eg, skin exam in areas of previous radiation). In addition, each patient receives an individualized treatment summary based on COG guidelines, which is updated before each visit by the APP coordinator. The APP coordinator reviews the document at each visit and offers patient education and health maintenance counseling.
Ensuring patients aren’t lost to follow-up. In our experience, numerous patients become lost to follow-up as they age, enter college or the workforce, or move away. So, rather than attempting to follow these patients for life, we work to transition patient care to a PCP of their choice, particularly if they are at least 21 years old and more than 10 years post-diagnosis. However, we will work to transition at any time at the request of the CCS. Even when a patient’s ongoing care is transitioned to a PCP, we will remain as a continuing resource to PCPs and CCSs on an as-needed basis.
Role of primary care providers in survivorship care
Every health care provider caring for a CCS should have a copy of the patient’s survivorship care plan. This document should be provided by the treating institution, but research has shown that as many as 86% of PCPs fail to receive this critical information.24 Any PCP who treats a patient with a history of cancer and has not received a survivorship care plan should contact the treating cancer center to request a copy. A properly prepared survivorship care plan summarizes the patient’s disease and treatment history, and provides a road map of the patient’s risk for long-term effects from disease and treatment.
The most important sections of the survivorship care plan for use in primary care will be the list of potential late effects and ongoing recommended testing. This list will help to guide the PCP’s differential and work-up for specific complaints. For example, knowing that a patient is at risk for a second malignancy because of radiation therapy may result in earlier diagnostic imaging, leading to a timelier diagnosis.
The COG screening recommendations that are generally included in a survivorship care plan are appropriate for survivors who are asymptomatic and presenting for routine, exposure-based medical follow-up. More extensive work-ups are presumed to be completed as clinically indicated. Consultation with a pediatric long-term follow-up clinic is also encouraged, particularly if a concern arises.
A complementary set of patient education materials, known as “Health Links,” accompany the COG guidelines to broaden their application and enhance patient follow-up visits. A survivorship care plan and the COG Guidelines help ensure that CCSs receive appropriate ongoing follow-up based on their history. A collaborative approach between Oncology and PCPs is essential to improve the quality of care for CCSs and to maintain the long-term health of this vulnerable population.
CORRESPONDENCE
Jean M. Tersak, Children’s Hospital of Pittsburgh of UPMC, 4401 Penn Avenue, 5th Floor Plaza Building, Pittsburgh, PA 15224; [email protected].
The number of childhood cancer survivors (CCSs) entering the adult health care system is increasing, a not-so-surprising trend when you consider that more than 80% of children and adolescents given a cancer diagnosis become long-term survivors.1 This patient population has a heightened risk for developing at least one chronic health problem, resulting from therapy. By the fourth decade of life, 88% of all CCSs will have a chronic condition,2 and about one-third develop a late effect that is either severe or life-threatening.3 In contrast to patients with many other pediatric chronic diseases that manifest at an early age and are progressive, CCSs are often physically well for many years, or decades, prior to their manifestation of late effects.4
Cancer survivorship has varying definitions; however, we define cancer survivorship as the phase of cancer care for individuals who have been diagnosed with cancer and have completed primary treatment for their disease.5 Cancer survivorship, which is becoming more widely acknowledged as a distinct and critically important phase of cancer care, includes:6
- “surveillance for recurrence,
- evaluation … and treatment of medical and psychosocial consequences of treatment,
- recommendations for screening for new primary cancers,
- health promotion recommendations, and
- provision of a written treatment summary and care plan to the patient and other health professionals.”
Although models of survivorship care vary, their common goal is to promote optimal health and well-being in cancer survivors, and to prevent and detect any health concerns that may be related to prior cancer diagnosis or treatment.
Some pediatric cancer survivors have not received recommended survivorship care because of a lack of insurance or limitations from pre-existing conditions.4,7 The Affordable Care Act may remove these barriers for many.8 Others, however, fail to receive such recommendations because national models of transition are lacking. Unique considerations for this population include their need to establish age appropriate, lifelong follow-up care (and education) from a primary care provider (PCP). Unfortunately, many CCSs become lost to follow-up and fail to receive recommended survivorship care when they discontinue the relationship with their pediatrician or family practitioner and their pediatric oncologist. Fewer than 25% of CCSs who have been successfully treated for cancer during childhood continue to be followed by a cancer center and are at risk for missing survivorship-focused care or recommended screening.4,9
PCPs are an invaluable link in helping CCSs to continue to receive recommended care and surveillance. However, PCPs experience barriers in providing cancer care because of a lack of timely and specific communication from oncologists and limited knowledge of guidelines and resources available to them.10 The purpose of this article is to share information with you, the family physician, about childhood cancer survivorship needs, available resources, and how partnering with pediatric oncologists may improve treatment and health outcomes for CCSs.
Providing for the future health of childhood cancer survivors
Numerous studies have outlined the myriad of potential late effects that CCSs may experience from disease and treatment.11,12 These effects can manifest at any time and can appear in virtually every body system from the central nervous system, to the lungs, heart, bones, and endocrine systems. CCSs' particular risk for late effects may result from many factors including cancer diagnosis, types of treatments (eg, surgery, chemotherapy, radiation, and stem-cell transplant), and dosages of medications, gender, and age at diagnosis.
Determining individual risk for late effects
The Children’s Oncology Group (COG) is the world’s largest organization devoted exclusively to childhood and adolescent cancer research, including the long-term health of cancer survivors. To help provide more individualized recommendations, COG has set forth risk-based, evidence-based, exposure-related clinical practice guidelines to offer recommendations for screening and management of late effects in survivors of childhood and adolescent cancers.13 (These guidelines, Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, are available at http://www.survivorshipguidelines.org.) The purpose of the guidelines is to standardize and enhance follow-up care for CCSs throughout their lifespan.13 To remain current, a multidisciplinary task force reviews and incorporates findings from the medical literature—including evaluations of the cost-effectiveness of recommended testing—into guideline revisions at least every 5 years.
Some of the most severe or life-threatening late effects include cardiomyopathies, endocrine disorders, and secondary malignancies (TABLE).13 Ongoing follow-up care is based on a survivor’s individual risk level and the frequency of lifelong recommended screening. The majority of patients will require yearly follow-up with additional testing, such as echocardiograms occurring as infrequently as every 2 to 5 years. Patients who received more intense therapy, such as hematopoietic stem-cell transplants, will require follow-up (often including annual echocardiograms, blood work, and a thorough physical exam) every 6 months to one year. Common testing and surveillance include blood pressure checks, urinalyses, thyroid function tests, lipid panels, echocardiograms, and electrocardiograms.
After treatment, patients should receive survivorship care plans
For health care providers to use COG Guidelines effectively across medical disciplines, it is important to know critical pieces of the patient’s cancer diagnosis and treatment history. In 2006, the Institute of Medicine released a report14 recommending that all cancer survivors be given a comprehensive care summary and follow-up plan when they complete their primary cancer care. More recently, the Commission on Cancer of the American College of Surgeons has mandated that, in order to be a cancer program accredited by the Commission, all cancer patients must be given a survivorship care plan after completing treatment.15 Generated by the treating cancer center, these care plans are meant to concisely communicate a patient’s cancer diagnosis, treatment, and long-term risks to other health care providers (across disciplines and institutions).
What’s included in a survivorship care plan?
The survivorship care plan is a paper or electronic document created by the treating institution that contains 2 components: a treatment summary and a long-term care plan based on medical/treatment history. The treatment summary includes, at a minimum, general background information (eg, demographics, pertinent medical history, diagnostic details, and significant treatment complications) and a therapeutic summary (such as dates of treatment, protocol, and details of chemotherapy, radiation, hematopoietic stem-cell transplant, and/or surgery).
The second component, the long-term care plan, details potential long-term effects specific to the treatment received, and recommendations for ongoing follow-up related to long-term risk (FIGURE). The post-treatment plan is primarily based on COG Guideline recommendations. Many institutions are introducing an electronic-based survivorship care plan, either in addition to or in replacement of a paper-based care plan. Electronic-based care plans have several benefits for patients and providers, including increased accessibility, and some offer the ability to easily update follow-up recommendations, as guidelines change, without the need for manual entry.
Shared care for cancer survivors: Oncology and primary care
Numerous models of cancer survivorship care have been described, including care by the treating oncologist, a dedicated cancer survivorship program, or follow-up completed by PCPs. There is no consensus on the best model, although many have noted that shared care is a critically important component of successful cancer survivorship care,6,16–18 and appears to be the preferred model of PCPs.19
Shared care, as the name implies, involves care that is coordinated between 2 or more health providers across specialties or locations.20 This model has shown improved outcomes in other chronic disease-management models, such as those for diabetes21 and chronic renal disease.22 One study23 found that colorectal cancer survivors who were seen by both an oncologist and a PCP were significantly more likely to receive recommended testing and follow-up to promote overall health than when they were followed by either physician alone. Information sharing between oncology and PCPs is critical to maintaining and promoting optimal health and well-being in cancer survivors, and requires ongoing communication and a concerted effort to facilitate and maintain collaboration between oncology specialists and other health care providers.6,17
Role of the cancer center in survivorship care
Although every cancer center has a slightly different timeline and structure in terms of survivorship care, there are common themes across programs regarding the type of care provided. Immediately following treatment, care is focused on surveillance for recurrence, with appointments ranging from monthly to a few times a year. This care is most often provided by the primary oncologist.
The next phase of care is reached 2 to 5 years after treatment, when recurrence is no longer a significant risk, and care is focused on monitoring and treating late effects. Depending on the center, this care may be coordinated by a dedicated survivorship clinic, the primary oncologist, or the PCP. In some models,6 the survivorship team is integrated into the patient’s care from the beginning of treatment, while others do not become active in care until the patient is considered cured of disease. In all models, a survivorship care plan should be completed after treatment has ended and before transitioning care to a PCP.
In our institution’s model, we have a survivorship program that serves patients who are more than 5 years from the completion of their treatment. Our survivorship team is comprised of a pediatric oncologist, advanced practice practitioner (APP) coordinator, a project coordinator, a clinical social worker, and a research staff member. Patients are seen every one to 2 years, depending on their overall risk for late effects. For those who are seen every other year, we are available to the PCP for questions or concerns, and the survivorship team connects with the CCS by phone to screen for any change in health status that would alter recommendations for an earlier follow-up at the oncology center.
A typical visit to our survivorship clinic includes completion of an annual health questionnaire, which addresses current health issues, as well as screening for anxiety, depression, nicotine, alcohol, and drug use. This questionnaire is reviewed by the pediatric oncologist and is used to tailor screening, referrals, and patient education based on current complaints. The oncologist also performs a thorough physical exam with special attention to areas in which late effects may occur (eg, skin exam in areas of previous radiation). In addition, each patient receives an individualized treatment summary based on COG guidelines, which is updated before each visit by the APP coordinator. The APP coordinator reviews the document at each visit and offers patient education and health maintenance counseling.
Ensuring patients aren’t lost to follow-up. In our experience, numerous patients become lost to follow-up as they age, enter college or the workforce, or move away. So, rather than attempting to follow these patients for life, we work to transition patient care to a PCP of their choice, particularly if they are at least 21 years old and more than 10 years post-diagnosis. However, we will work to transition at any time at the request of the CCS. Even when a patient’s ongoing care is transitioned to a PCP, we will remain as a continuing resource to PCPs and CCSs on an as-needed basis.
Role of primary care providers in survivorship care
Every health care provider caring for a CCS should have a copy of the patient’s survivorship care plan. This document should be provided by the treating institution, but research has shown that as many as 86% of PCPs fail to receive this critical information.24 Any PCP who treats a patient with a history of cancer and has not received a survivorship care plan should contact the treating cancer center to request a copy. A properly prepared survivorship care plan summarizes the patient’s disease and treatment history, and provides a road map of the patient’s risk for long-term effects from disease and treatment.
The most important sections of the survivorship care plan for use in primary care will be the list of potential late effects and ongoing recommended testing. This list will help to guide the PCP’s differential and work-up for specific complaints. For example, knowing that a patient is at risk for a second malignancy because of radiation therapy may result in earlier diagnostic imaging, leading to a timelier diagnosis.
The COG screening recommendations that are generally included in a survivorship care plan are appropriate for survivors who are asymptomatic and presenting for routine, exposure-based medical follow-up. More extensive work-ups are presumed to be completed as clinically indicated. Consultation with a pediatric long-term follow-up clinic is also encouraged, particularly if a concern arises.
A complementary set of patient education materials, known as “Health Links,” accompany the COG guidelines to broaden their application and enhance patient follow-up visits. A survivorship care plan and the COG Guidelines help ensure that CCSs receive appropriate ongoing follow-up based on their history. A collaborative approach between Oncology and PCPs is essential to improve the quality of care for CCSs and to maintain the long-term health of this vulnerable population.
CORRESPONDENCE
Jean M. Tersak, Children’s Hospital of Pittsburgh of UPMC, 4401 Penn Avenue, 5th Floor Plaza Building, Pittsburgh, PA 15224; [email protected].
1. Ries LAG, Eisner MP, Kosary CL, et al, eds. SEER Cancer Statistics Review, 1975-2002. National Cancer Institute. Bethesda, MD. Available at: http://seer.cancer.gov/csr/1975_2002/. Accessed May 26, 2016.
2. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653-663.
3. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572-1582.
4. Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4401-4409.
5. Feuerstein M. Defining cancer survivorship. J Cancer Surviv. 2007;1:5-7.
6. McCabe MS, Jacobs LA. Clinical update: survivorship care—models and programs. Semin Oncol Nurs. 2012;28:e1-e8.
7. Oeffinger K, Mertens A, Hudson M, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2:61-70.
8. Mueller EL, Park ER, Davis MM. What the affordable care act means for survivors of pediatric cancer. J Clin Oncol. 2014;32:615-617.
9. Oeffinger KC. Longitudinal risk-based health care for adult survivors of childhood cancer. Curr Probl Cancer. 2003;27:143-167.
10. Lawrence RA, McLoone JK, Wakefield CE, et al. Primary care physicians’ perspectives of their role in cancer care: a systematic review. J Gen Intern Med. 2016:1-15.
11. Schwartz CL. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4:45-54.
12. Late Effects of Treatment for Childhood Cancer (PDQ(R)): Health Professional Version [Internet]. Bethesda, MD: National Cancer Institute. Updated March 31, 2016. Available at: www.cancer.gov/types/childhood-cancers/late-effects-hp-pdq. Accessed June 2, 2016.
13. Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer, Version 4.0. Monrovia CA: Children’s Oncology Group. 2013. Available at: www.survivorshipguidelines.org. Accessed June 2, 2016.
14. Hewitt M, Greenfield S, Stovall E, Committee on Cancer Survivorship: Improving Care and Quality of Life. National Cancer Policy Board, Institute of Medicine, National Research Council, eds. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press; 2005.
15. Commission on Cancer [Internet]. Cancer Program Standards: Ensuring Patient-Centered Care. Chicago, IL: American College of Surgeons; 2015. Available at: https://www.facs.org/quality%20programs/cancer/coc/standards. Accessed June 2, 2016.
16. Askins MA, Moore BD. Preventing neurocognitive late effects in childhood cancer survivors. J Child Neurol. 2008;23:1160-1171.
17. McCabe MS, Jacobs L. Survivorship care: models and programs. Semin Oncol Nurs. 2008;24:202-207.
18. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117-5124.
19. Potosky AL, Han PKJ, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26:1403-1410.
20. Gilbert SM, Miller DC, Hollenbeck BK, et al. Cancer survivorship: challenges and changing paradigms. J Urol. 2008;179:431-438.
21. Renders CM, Valk GD, de Sonnaville JJ, et al. Quality of care for patients with Type 2 diabetes mellitus—a long-term comparison of two quality improvement programmes in the Netherlands. Diabet Med. 2003;20:846-852.
22. Jones C, Roderick P, Harris S, et al. An evaluation of a shared primary and secondary care nephrology service for managing patients with moderate to advanced CKD. Am J Kidney Dis. 2006;47:103-114.
23. Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101:1712-1719.
24. Sima JL, Perkins SM, Haggstrom DA. Primary care physician perceptions of adult survivors of childhood cancer. J Pediatr Hematol Oncol. 2014;36:118-124.
1. Ries LAG, Eisner MP, Kosary CL, et al, eds. SEER Cancer Statistics Review, 1975-2002. National Cancer Institute. Bethesda, MD. Available at: http://seer.cancer.gov/csr/1975_2002/. Accessed May 26, 2016.
2. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653-663.
3. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572-1582.
4. Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4401-4409.
5. Feuerstein M. Defining cancer survivorship. J Cancer Surviv. 2007;1:5-7.
6. McCabe MS, Jacobs LA. Clinical update: survivorship care—models and programs. Semin Oncol Nurs. 2012;28:e1-e8.
7. Oeffinger K, Mertens A, Hudson M, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2:61-70.
8. Mueller EL, Park ER, Davis MM. What the affordable care act means for survivors of pediatric cancer. J Clin Oncol. 2014;32:615-617.
9. Oeffinger KC. Longitudinal risk-based health care for adult survivors of childhood cancer. Curr Probl Cancer. 2003;27:143-167.
10. Lawrence RA, McLoone JK, Wakefield CE, et al. Primary care physicians’ perspectives of their role in cancer care: a systematic review. J Gen Intern Med. 2016:1-15.
11. Schwartz CL. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4:45-54.
12. Late Effects of Treatment for Childhood Cancer (PDQ(R)): Health Professional Version [Internet]. Bethesda, MD: National Cancer Institute. Updated March 31, 2016. Available at: www.cancer.gov/types/childhood-cancers/late-effects-hp-pdq. Accessed June 2, 2016.
13. Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer, Version 4.0. Monrovia CA: Children’s Oncology Group. 2013. Available at: www.survivorshipguidelines.org. Accessed June 2, 2016.
14. Hewitt M, Greenfield S, Stovall E, Committee on Cancer Survivorship: Improving Care and Quality of Life. National Cancer Policy Board, Institute of Medicine, National Research Council, eds. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press; 2005.
15. Commission on Cancer [Internet]. Cancer Program Standards: Ensuring Patient-Centered Care. Chicago, IL: American College of Surgeons; 2015. Available at: https://www.facs.org/quality%20programs/cancer/coc/standards. Accessed June 2, 2016.
16. Askins MA, Moore BD. Preventing neurocognitive late effects in childhood cancer survivors. J Child Neurol. 2008;23:1160-1171.
17. McCabe MS, Jacobs L. Survivorship care: models and programs. Semin Oncol Nurs. 2008;24:202-207.
18. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117-5124.
19. Potosky AL, Han PKJ, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26:1403-1410.
20. Gilbert SM, Miller DC, Hollenbeck BK, et al. Cancer survivorship: challenges and changing paradigms. J Urol. 2008;179:431-438.
21. Renders CM, Valk GD, de Sonnaville JJ, et al. Quality of care for patients with Type 2 diabetes mellitus—a long-term comparison of two quality improvement programmes in the Netherlands. Diabet Med. 2003;20:846-852.
22. Jones C, Roderick P, Harris S, et al. An evaluation of a shared primary and secondary care nephrology service for managing patients with moderate to advanced CKD. Am J Kidney Dis. 2006;47:103-114.
23. Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101:1712-1719.
24. Sima JL, Perkins SM, Haggstrom DA. Primary care physician perceptions of adult survivors of childhood cancer. J Pediatr Hematol Oncol. 2014;36:118-124.
PRACTICE RECOMMENDATIONS
› Use the survivorship care plan from the patient’s primary oncologist to guide your screening and management of late effects. C
› Apply the Children’s Oncology Group Guidelines, which are risk-based, exposure-related, clinical practice guidelines, to direct screening and management of late effects in survivors of pediatric malignancies. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hepatitis C: Screening changes, treatment advances
Several recent developments have prompted a renewed focus on the way we screen for, and manage the treatment of, hepatitis C virus (HCV) infection. In 2013, the United States Preventive Services Task Force expanded its HCV screening guidelines to include baby boomers born between 1945 and 1965, regardless of apparent risk factors (TABLE 11).2 The recommendation is based on the high prevalence of chronic HCV in this cohort, estimated to be 4.3%, which is about 4 times higher than that of the general US population.3 It is believed that 75% of chronic HCV infections in the United States are in this cohort. After decades of infection, many in this age group are now presenting with advanced disease, leading to 19,659 HCV-related deaths in America in 2014.4
In addition, while HCV incidence in America had been steadily declining, it is now once again on the rise among young, non-urban whites, mainly because of increasing intravenous drug use in this population.5 On a positive note, new highly-effective and better-tolerated treatments are greatly improving the care we can provide.
In light of these factors, family physicians (FPs) are likely to be screening for HCV more than ever before and must be prepared to provide appropriate counseling and initial clinical management for those with positive test results. This article reviews the evaluation and primary care management of HCV-infected patients, as well as approaches to treatment with the newest direct-acting antivirals (DAAs).
The natural history of hepatitis C (and what we’re seeing as boomers age)
Acute HCV infection is rarely symptomatic, but results in chronic infection approximately 75% of the time.6 While some chronically infected individuals remain unaffected, most develop some degree of hepatic fibrosis, and 20% will develop cirrhosis within 20 years of diagnosis.7-9
The rate of progression is variable; factors that result in more rapid progression of liver disease include coinfection with HIV or HBV, overweight or obesity, insulin resistance, male gender, and use of alcohol.7 As the baby boomer cohort has aged, patients infected in their youth are now presenting with the sequelae of decompensated cirrhosis, including ascites, portal vein thrombosis, and thrombocytopenia.
Extrahepatic manifestations of chronic hepatitis C can include fatigue, membranoproliferative glomerulonephritis, porphyria cutanea tarda, cryoglobulinemia, a higher likelihood of insulin resistance, and possibly lymphoma.10-12
Chronic HCV is also the major contributor to the increased incidence of hepatocellular carcinoma (HCC), which has tripled in the past 2 decades in the United States.13
Although results are inconsistent, studies suggest 5% to 10% of HCV-infected patients will succumb to liver-related death.7
Who you’ll screen
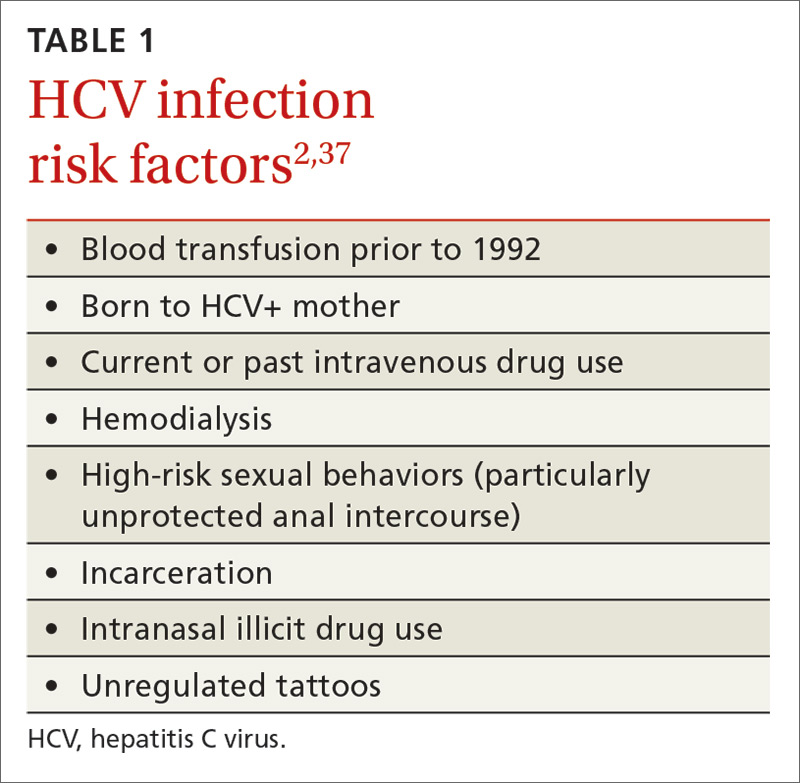
If your patient is at heightened risk of contracting HCV infection (TABLE 11) or was born between 1945 and 1965, you’ll want to screen for infection with an HCV antibody test. A positive antibody test must be followed by testing for hepatitis C viral RNA to confirm whether the patient is chronically infected or is among the approximately 25% of patients who spontaneously clear the virus.6
For the patient with no detectable HCV RNA, no further evaluation or treatment is necessary. HCV viral load itself provides little insight into the rate of progression of the illness, but does correlate with risk of transmission.14 Counseling patients about the full testing protocol before screening can help to reduce anxiety and confusion.
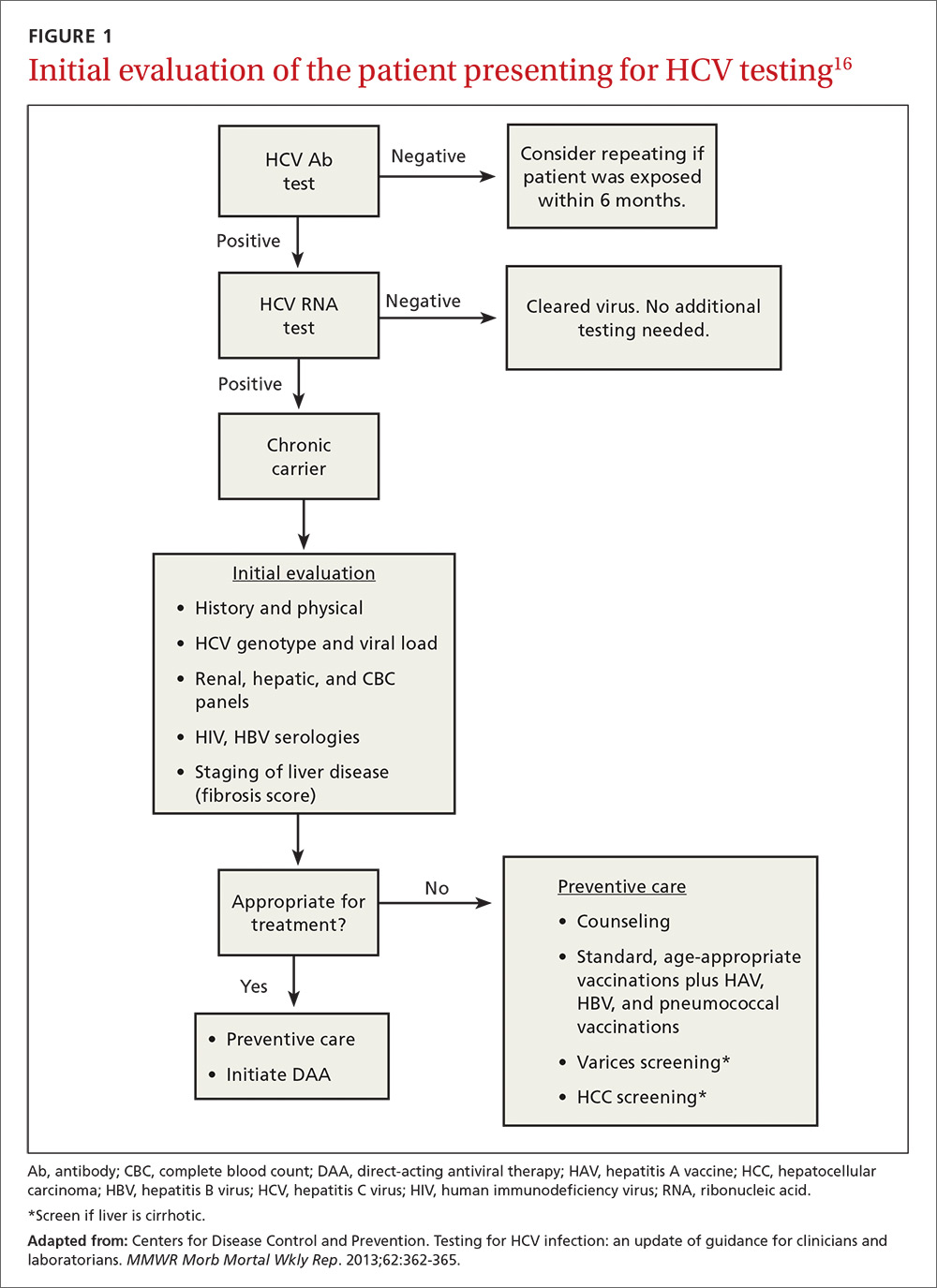
At present, 6 genotypes and multiple subtypes of HCV have been identified; these have important implications for prognosis and treatment.15 HCV genotyping is frequently ordered along with a test for HCV viral load, but may be deferred until after fibrosis staging is performed (more on that in a bit). It may also be deferred if treatment is not planned within the next 12 weeks, as its main clinical use is to guide choice of treatment. Once chronic infection has been confirmed by the presence of HCV viremia, further work-up focuses on evaluating the effects on the host, which, in turn, helps the provider finalize a treatment plan (FIGURE 116).
Follow initial screening with an evaluation including liver disease staging
Following a screen that comes back positive for HCV, you’ll conduct a more thorough history including questioning about previous or ongoing risk factors for HCV, perform a physical examination that includes looking for signs of liver failure or extrahepatic disease, and order more lab work. Laboratory investigations include a complete blood count; renal and hepatic panels; and testing for human immunodeficiency virus (HIV) antibody, hepatitis B virus (HBV) surface antigen, HBV surface antibody, and HBV core antibody.1,17 Finally, the patient must be evaluated for hepatic fibrosis and cirrhosis to quantify the likelihood of developing liver failure and HCC.
Staging liver disease is a prerequisite to treating HCV infection because the extent of liver fibrosis impacts not only prognosis, but also the choice of the treatment regimen and the duration of therapy. The traditional gold standard for diagnosing hepatic fibrosis and cirrhosis has been a liver biopsy; however, a single 1.6-mm biopsy evaluates only a small portion of the liver and can miss affected liver parenchyma. In addition, a liver biopsy carries a small, but not inconsequential, risk of morbidity, and can be costly and complex to arrange.
Several noninvasive options are now available and are typically the preferred methods for staging liver disease. FibroSURE (LabCorp), for example, uses a peripheral venous blood sample and combines the patient’s age, gender, and 6 biochemical markers to generate a range of scores that correspond to the fibrosis component of the well-known METAVIR scoring system and correlate with results of liver biopsies.18,19 (The METAVIR system is a histology-based scoring system that grades fibrosis from F0 [no fibrosis] to F4 [cirrhosis].)
Noninvasive imaging studies assess for fibrosis more directly by assessing liver elasticity, either by ultrasound or magnetic resonance (MR) technology. The ultrasound modality FibroScan (Echosens) is currently the most widely available, although some data suggest the more expensive MR elastography has higher sensitivity (94% sensitive for METAVIR F2 or higher compared to 79% by FibroScan).20,21 While each of these modalities has limitations (eg, body habitus, availability), these tests allow stratification of patients into categories of low, moderate, and high risk for cirrhosis without the risks of biopsy.
A curable viral infection
HCV is one of the few curable chronic viral infections; unlike HBV and HIV, HCV does not create a long-term intra-nuclear reservoir. DAAs have cure rates of more than 95% for many HCV genotypes,22-24 allowing the possibility for dramatic reductions in prevalence in the decades to come.
Cure is defined by reaching a sustained virologic response (SVR), or absence of detectable viral load, 12 weeks after completion of therapy. Patients with HCV infection with advanced fibrosis who achieve SVR have shown benefits beyond improvement in liver function and histology. One large, multicenter, prospective study of 530 patients with chronic HCV, for example, found that those who achieved SVR experienced a 76% reduction in the risk of HCC and a 66% reduction in all-cause mortality (number needed to treat [NNT] was 5.8 to prevent one death or 6 to prevent one case of HCC in 10 years) compared to those without SVR.25 Other extrahepatic manifestations that impair quality of life, such as renal disease, autoimmune disease, and circulatory problems, are likewise reduced.25
Guidelines now recommend treating most patients with HCV infection
Until 2011, HCV treatment included the injectable immune-activating agent interferon and the non–HCV-specific antiviral ribavirin. This regimen had low SVR rates of 40% to 60% and adverse effects that were often intolerable.26 The advent of the first-generation HCV protease inhibitors in 2011 improved SVR rates, which have continued to improve exponentially with the development of combination therapy using DAAs (TABLE 227-29). (In order to stay up to date with the latest options for the treatment of HCV, see The American Association for the Study of Liver Diseases treatment guidelines at: http://hcvguidelines.org.)
What the guidelines say. Due to the tolerability and efficacy of the new DAAs, current guidelines state that HCV treatment should be recommended to most patients with HCV infection—not just those with advanced disease.30 This is a major change from prior guidelines, which were based on more toxic and less effective regimens. Limited data from long-term cohort studies of patients using interferon-based regimens suggest that the benefits of SVR are greatest for those treated at early stages before significant fibrosis develops. At least one analysis involving over 4000 patients found, however, that this approach may be less cost-effective, with an NNT of 20 to prevent one death in 20 years.31
In practice, the decision to treat requires a discussion between the patient and provider, weighing the risks and benefits of treatment in the context of the patients’ comorbidities and overall life expectancy. Such a discussion must also include cost. Many insurance companies will still only cover antiviral therapy for patients with advanced fibrosis, but these restrictions are slowly lifting and are having significant implications for our health care system. By one estimate, treating all patients with HCV at current drug prices would cost approximately $250 billion—about one-tenth of the total annual health care costs in this country.32 As policies change and the cost of drug regimens decreases from increasing competition, access is likely to improve for the majority of Americans.
Which regimen is most likely to be successful?
Many factors influence the choice of regimen and likelihood for SVR. These factors include whether the patient has cirrhosis and any comorbidities, the hepatitis C genotype involved, and any prior treatment the patient may have received. (See TABLE 37,12,18,28,30,33 for a comprehensive list.)
The easiest patients to treat are treatment-naive, with minimal liver disease and a favorable genotype. For example, combination therapy with the NS5B inhibitor sofosbuvir and an NS5A inhibitor (ledipasvir, daclatasvir, or velpatasvir) administered for 12 weeks has an SVR rate of >95% in genotype-1, treatment-naive, non-cirrhotic patients.22-24 Patients with prior treatment failure, especially failure on DAA therapy, or who have genotype 3, may be less responsive to standard therapies and may require more complex regimens or a longer duration of therapy.
Patients requiring special attention. It’s preferable to manage patients with decompensated liver disease in a specialized hepatology center due to the possibility of further decline and need for transplant prior to completion of therapy. Patients with HIV are another population that requires special attention. As many as 25% of HIV-infected patients are co-infected with HCV; their treatment follows the same principles as that in non-HIV patients, with extra attention paid to avoiding drug-drug interactions. Elbasvir/grazoprevir, for example, should not be used with any protease inhibitors, with nevirapine, or with efavirenz, and sofosbuvir should not be used with efavirenz, nevirapine, or tipranavir.34
Beyond medication regimens: The advice you’ll offer
In addition to counseling about antiviral therapy, patients with HCV infection require other types of advice and care that are often best administered by a primary care physician who is familiar with the patient and his or her family and community.
Prevention of transmission
Many patients have concerns about transmission of the virus to family members, co-workers, and sexual partners. You can assure patients that they are not likely to spread the virus in the workplace, even in health care environments.
Close contacts are also not at risk as long as basic prevention measures, such as not sharing toothbrushes or razors, are established to avoid transmission of blood and bodily fluids. Patient handouts can be found at the Centers for Disease Control and Prevention Web site (http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc).35
Patients and their sexual partners, however, must be counseled about the risk of sexual transmission. In monogamous relationships between serodiscordant partners who practice vaginal intercourse, there is a low, but clinically important, risk of transmission of HCV—up to 0.6% per year.36 Anal intercourse and co-infection with HIV increase this risk significantly.37 Pregnant women must be advised on the currently non-modifiable risk of transmission to newborns, which is approximately 6% in mono-infected women, but may be at least twice as likely in HIV/HCV co-infected women.38,39
Staying healthy. In addition to pneumococcal and standard age-appropriate vaccines, vaccination against hepatitis A and HBV is recommended for all HCV-infected patients to reduce the risk of a severe acute hepatitis.40,41 Advise patients to avoid alcohol, to consume a healthy diet, and to participate in regular activity and exercise. Review the patient’s medication list for hepatotoxic drugs and counsel the patient on the risks of excessive use of acetaminophen, non-steroidal anti-inflammatory drugs, and herbal medicines such as kava kava. Because obesity and metabolic syndrome are known risk factors for hepatic steatosis, which hastens the progression to cirrhosis and liver failure, counsel overweight and obese patients on the importance of healthy weight loss.42,43
Disease-related screenings. Consider screening all HCV patients for diabetes mellitus (DM) because people with chronic HCV infection have a higher prevalence of insulin resistance than those who are HCV-negative, and patients with type 2 DM are at higher risk for worse outcomes of their HCV infection.44 In addition, screen all patients with a METAVIR score of F3 or higher every 6 months for HCC using liver ultrasound, and recommend upper endoscopy to patients with cirrhosis to screen for esophageal varices.45,46
Health maintenance after treatment
Once patients have achieved SVR 12 weeks after completion of therapy, they are deemed cured. However, those patients who were already METAVIR F3 or higher maintain sufficient risk of HCC to recommend ongoing screening with ultrasound.47,48
CORRESPONDENCE
Mark Shaffer, MD, 3209 Colonial Drive, Columbia, SC 29206; [email protected].
1. AASLD-IDSA. HCV testing and linkage to care. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. Accessed August 22, 2016.
2. US Preventive Services Task Force. Hepatitis C: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-C-screening. Accessed August 28, 2016.
3. Denniston MM, Jiles RN, Brobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300.
4. Centers for Disease Control and Prevention. Surveillance for viral hepatitis-United States, 2014. Available at: https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm. Accessed February 6, 2017.
5. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mort Wkly Rep. 2015;64:453-458.
6. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41.
7. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46.
8. Klevens M, Huang X, Yeo AE, et al. The burden of liver disease among persons with hepatitis C in the United States. Conference on Retroviruses and Opportunistic Infections. Seattle, February 23-24, 2015. Abstract 145.
9. Zarski JP, McHutchison J, Bronowicki JP, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003;38:307-314.
10. Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore). 2000;79:47-56.
11. Vannata B, Arcaini L, Zucca E. Hepatitis C virus-associated B-cell non-Hodgkin’s lymphomas: what do we know? Ther Adv Hematol. 2016;7:94-107.
12. Gastaldi G, Goossens N, Clément S, et al. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: a review. J Adv Res. 2017;8:149-159.
13. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127.
14. Elrazek AE, Amer M, Hawary B, et al. Prediction of HCV vertical transmission: What are factors should be optimized using data mining computational analysis. Liver Int. 2016.
15. Wang LS, D’Souza LS, Jacobson IM. Hepatitis C-A clinical review. J Med Virol. 2016;88:1844-1855.
16. Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-365.
17. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm522932.htm. Accessed December 15, 2016.
18. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
19. Patel K, Friedrich-Rust M, Lurie Y, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17:4581-4589.
20. Shiraishi A, Hiraoka A, Aibiki T, et al. Real-time tissue elastography: non-invasive evaluation of liver fibrosis in chronic liver disease due to HCV. Hepatogastroenterology. 2014;61:2084-2090.
21. Yoon JH, Lee JM, Joo I, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772-782.
22. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898.
23. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373:714-725.
24. Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599-2607.
25. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593.
26. NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46.
27. AASLD-IDSA. Initial treatment of HCV infection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/initial-treatment-hcv-infection. Accessed August 24, 2016.
28. Lexicomp. Wolters Kluwer. Clinical Drug Information, Inc. Available at: http://online.lexi.com/action/home.
29. GoodRx. Available at: https//www.goodrx.com. Accessed January 25, 2017.
30. AASLD-IDSA. When and in whom to initiate HCV therapy. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. Accessed August 31, 2016.
31. Jezequel C, Bardou-Jacquet E, Desille Y, et al. Survival of patients infected by chronic hepatitis C and F0F1 fibrosis at baseline after a 15-years follow-up. Poster presented at: 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015; Vienna, Austria.
32. Lin KW. Should family physicians routinely screen patients for hepatitis C? Am Fam Physician. 2016;93:17-18.
33. Center for Medicare and Medicaid Services. Center for Medicaid and CHIP Services. Medicaid drug rebate program notice. Release no. 172. Available at: https://www.medicaid.gov/medicaid-chip-program-information/by-topics/prescription-drugs/downloads/rx-releases/state-releases/state-rel-172.pdf. Accessed August 24, 2016.
34. AASLD-IDSA. Unique patient populations: patients with HIV/HCV coinfection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/unique-patient-populations-patients-hivhcv-coinfection. Accessed February 6, 2017.
35. Centers for Disease Control and Prevention. Viral hepatitis-hepatitis C information. Patient education resources. Available at: http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc. Accessed June 15, 2016.
36. Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:S99-S105.
37. Chan DP, Sun HY, Wong HT, et al. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis. 2016;49:47-58.
38. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
39. European Paediatric Hepatitis C Virus Network. A significant sex—but not elective cesarean section—effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005;192:1872-1879.
40. Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102-1106.
41. Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci. 2005;50:1525-1531.
42. Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555-561.
43. Hu KQ, Kyulo NL, Esrailian E, et al. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J Hepatol. 2004;40:147-154.
44. Hammerstad SS, Grock SF, Lee HJ, et al. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134.
45. Lok AS, Seeff LV, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138-148.
46. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5-10.
47. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337.
Several recent developments have prompted a renewed focus on the way we screen for, and manage the treatment of, hepatitis C virus (HCV) infection. In 2013, the United States Preventive Services Task Force expanded its HCV screening guidelines to include baby boomers born between 1945 and 1965, regardless of apparent risk factors (TABLE 11).2 The recommendation is based on the high prevalence of chronic HCV in this cohort, estimated to be 4.3%, which is about 4 times higher than that of the general US population.3 It is believed that 75% of chronic HCV infections in the United States are in this cohort. After decades of infection, many in this age group are now presenting with advanced disease, leading to 19,659 HCV-related deaths in America in 2014.4
In addition, while HCV incidence in America had been steadily declining, it is now once again on the rise among young, non-urban whites, mainly because of increasing intravenous drug use in this population.5 On a positive note, new highly-effective and better-tolerated treatments are greatly improving the care we can provide.
In light of these factors, family physicians (FPs) are likely to be screening for HCV more than ever before and must be prepared to provide appropriate counseling and initial clinical management for those with positive test results. This article reviews the evaluation and primary care management of HCV-infected patients, as well as approaches to treatment with the newest direct-acting antivirals (DAAs).
The natural history of hepatitis C (and what we’re seeing as boomers age)
Acute HCV infection is rarely symptomatic, but results in chronic infection approximately 75% of the time.6 While some chronically infected individuals remain unaffected, most develop some degree of hepatic fibrosis, and 20% will develop cirrhosis within 20 years of diagnosis.7-9
The rate of progression is variable; factors that result in more rapid progression of liver disease include coinfection with HIV or HBV, overweight or obesity, insulin resistance, male gender, and use of alcohol.7 As the baby boomer cohort has aged, patients infected in their youth are now presenting with the sequelae of decompensated cirrhosis, including ascites, portal vein thrombosis, and thrombocytopenia.
Extrahepatic manifestations of chronic hepatitis C can include fatigue, membranoproliferative glomerulonephritis, porphyria cutanea tarda, cryoglobulinemia, a higher likelihood of insulin resistance, and possibly lymphoma.10-12
Chronic HCV is also the major contributor to the increased incidence of hepatocellular carcinoma (HCC), which has tripled in the past 2 decades in the United States.13
Although results are inconsistent, studies suggest 5% to 10% of HCV-infected patients will succumb to liver-related death.7
Who you’ll screen
If your patient is at heightened risk of contracting HCV infection (TABLE 11) or was born between 1945 and 1965, you’ll want to screen for infection with an HCV antibody test. A positive antibody test must be followed by testing for hepatitis C viral RNA to confirm whether the patient is chronically infected or is among the approximately 25% of patients who spontaneously clear the virus.6
For the patient with no detectable HCV RNA, no further evaluation or treatment is necessary. HCV viral load itself provides little insight into the rate of progression of the illness, but does correlate with risk of transmission.14 Counseling patients about the full testing protocol before screening can help to reduce anxiety and confusion.
At present, 6 genotypes and multiple subtypes of HCV have been identified; these have important implications for prognosis and treatment.15 HCV genotyping is frequently ordered along with a test for HCV viral load, but may be deferred until after fibrosis staging is performed (more on that in a bit). It may also be deferred if treatment is not planned within the next 12 weeks, as its main clinical use is to guide choice of treatment. Once chronic infection has been confirmed by the presence of HCV viremia, further work-up focuses on evaluating the effects on the host, which, in turn, helps the provider finalize a treatment plan (FIGURE 116).
Follow initial screening with an evaluation including liver disease staging
Following a screen that comes back positive for HCV, you’ll conduct a more thorough history including questioning about previous or ongoing risk factors for HCV, perform a physical examination that includes looking for signs of liver failure or extrahepatic disease, and order more lab work. Laboratory investigations include a complete blood count; renal and hepatic panels; and testing for human immunodeficiency virus (HIV) antibody, hepatitis B virus (HBV) surface antigen, HBV surface antibody, and HBV core antibody.1,17 Finally, the patient must be evaluated for hepatic fibrosis and cirrhosis to quantify the likelihood of developing liver failure and HCC.
Staging liver disease is a prerequisite to treating HCV infection because the extent of liver fibrosis impacts not only prognosis, but also the choice of the treatment regimen and the duration of therapy. The traditional gold standard for diagnosing hepatic fibrosis and cirrhosis has been a liver biopsy; however, a single 1.6-mm biopsy evaluates only a small portion of the liver and can miss affected liver parenchyma. In addition, a liver biopsy carries a small, but not inconsequential, risk of morbidity, and can be costly and complex to arrange.
Several noninvasive options are now available and are typically the preferred methods for staging liver disease. FibroSURE (LabCorp), for example, uses a peripheral venous blood sample and combines the patient’s age, gender, and 6 biochemical markers to generate a range of scores that correspond to the fibrosis component of the well-known METAVIR scoring system and correlate with results of liver biopsies.18,19 (The METAVIR system is a histology-based scoring system that grades fibrosis from F0 [no fibrosis] to F4 [cirrhosis].)
Noninvasive imaging studies assess for fibrosis more directly by assessing liver elasticity, either by ultrasound or magnetic resonance (MR) technology. The ultrasound modality FibroScan (Echosens) is currently the most widely available, although some data suggest the more expensive MR elastography has higher sensitivity (94% sensitive for METAVIR F2 or higher compared to 79% by FibroScan).20,21 While each of these modalities has limitations (eg, body habitus, availability), these tests allow stratification of patients into categories of low, moderate, and high risk for cirrhosis without the risks of biopsy.
A curable viral infection
HCV is one of the few curable chronic viral infections; unlike HBV and HIV, HCV does not create a long-term intra-nuclear reservoir. DAAs have cure rates of more than 95% for many HCV genotypes,22-24 allowing the possibility for dramatic reductions in prevalence in the decades to come.
Cure is defined by reaching a sustained virologic response (SVR), or absence of detectable viral load, 12 weeks after completion of therapy. Patients with HCV infection with advanced fibrosis who achieve SVR have shown benefits beyond improvement in liver function and histology. One large, multicenter, prospective study of 530 patients with chronic HCV, for example, found that those who achieved SVR experienced a 76% reduction in the risk of HCC and a 66% reduction in all-cause mortality (number needed to treat [NNT] was 5.8 to prevent one death or 6 to prevent one case of HCC in 10 years) compared to those without SVR.25 Other extrahepatic manifestations that impair quality of life, such as renal disease, autoimmune disease, and circulatory problems, are likewise reduced.25
Guidelines now recommend treating most patients with HCV infection
Until 2011, HCV treatment included the injectable immune-activating agent interferon and the non–HCV-specific antiviral ribavirin. This regimen had low SVR rates of 40% to 60% and adverse effects that were often intolerable.26 The advent of the first-generation HCV protease inhibitors in 2011 improved SVR rates, which have continued to improve exponentially with the development of combination therapy using DAAs (TABLE 227-29). (In order to stay up to date with the latest options for the treatment of HCV, see The American Association for the Study of Liver Diseases treatment guidelines at: http://hcvguidelines.org.)
What the guidelines say. Due to the tolerability and efficacy of the new DAAs, current guidelines state that HCV treatment should be recommended to most patients with HCV infection—not just those with advanced disease.30 This is a major change from prior guidelines, which were based on more toxic and less effective regimens. Limited data from long-term cohort studies of patients using interferon-based regimens suggest that the benefits of SVR are greatest for those treated at early stages before significant fibrosis develops. At least one analysis involving over 4000 patients found, however, that this approach may be less cost-effective, with an NNT of 20 to prevent one death in 20 years.31
In practice, the decision to treat requires a discussion between the patient and provider, weighing the risks and benefits of treatment in the context of the patients’ comorbidities and overall life expectancy. Such a discussion must also include cost. Many insurance companies will still only cover antiviral therapy for patients with advanced fibrosis, but these restrictions are slowly lifting and are having significant implications for our health care system. By one estimate, treating all patients with HCV at current drug prices would cost approximately $250 billion—about one-tenth of the total annual health care costs in this country.32 As policies change and the cost of drug regimens decreases from increasing competition, access is likely to improve for the majority of Americans.
Which regimen is most likely to be successful?
Many factors influence the choice of regimen and likelihood for SVR. These factors include whether the patient has cirrhosis and any comorbidities, the hepatitis C genotype involved, and any prior treatment the patient may have received. (See TABLE 37,12,18,28,30,33 for a comprehensive list.)
The easiest patients to treat are treatment-naive, with minimal liver disease and a favorable genotype. For example, combination therapy with the NS5B inhibitor sofosbuvir and an NS5A inhibitor (ledipasvir, daclatasvir, or velpatasvir) administered for 12 weeks has an SVR rate of >95% in genotype-1, treatment-naive, non-cirrhotic patients.22-24 Patients with prior treatment failure, especially failure on DAA therapy, or who have genotype 3, may be less responsive to standard therapies and may require more complex regimens or a longer duration of therapy.
Patients requiring special attention. It’s preferable to manage patients with decompensated liver disease in a specialized hepatology center due to the possibility of further decline and need for transplant prior to completion of therapy. Patients with HIV are another population that requires special attention. As many as 25% of HIV-infected patients are co-infected with HCV; their treatment follows the same principles as that in non-HIV patients, with extra attention paid to avoiding drug-drug interactions. Elbasvir/grazoprevir, for example, should not be used with any protease inhibitors, with nevirapine, or with efavirenz, and sofosbuvir should not be used with efavirenz, nevirapine, or tipranavir.34
Beyond medication regimens: The advice you’ll offer
In addition to counseling about antiviral therapy, patients with HCV infection require other types of advice and care that are often best administered by a primary care physician who is familiar with the patient and his or her family and community.
Prevention of transmission
Many patients have concerns about transmission of the virus to family members, co-workers, and sexual partners. You can assure patients that they are not likely to spread the virus in the workplace, even in health care environments.
Close contacts are also not at risk as long as basic prevention measures, such as not sharing toothbrushes or razors, are established to avoid transmission of blood and bodily fluids. Patient handouts can be found at the Centers for Disease Control and Prevention Web site (http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc).35
Patients and their sexual partners, however, must be counseled about the risk of sexual transmission. In monogamous relationships between serodiscordant partners who practice vaginal intercourse, there is a low, but clinically important, risk of transmission of HCV—up to 0.6% per year.36 Anal intercourse and co-infection with HIV increase this risk significantly.37 Pregnant women must be advised on the currently non-modifiable risk of transmission to newborns, which is approximately 6% in mono-infected women, but may be at least twice as likely in HIV/HCV co-infected women.38,39
Staying healthy. In addition to pneumococcal and standard age-appropriate vaccines, vaccination against hepatitis A and HBV is recommended for all HCV-infected patients to reduce the risk of a severe acute hepatitis.40,41 Advise patients to avoid alcohol, to consume a healthy diet, and to participate in regular activity and exercise. Review the patient’s medication list for hepatotoxic drugs and counsel the patient on the risks of excessive use of acetaminophen, non-steroidal anti-inflammatory drugs, and herbal medicines such as kava kava. Because obesity and metabolic syndrome are known risk factors for hepatic steatosis, which hastens the progression to cirrhosis and liver failure, counsel overweight and obese patients on the importance of healthy weight loss.42,43
Disease-related screenings. Consider screening all HCV patients for diabetes mellitus (DM) because people with chronic HCV infection have a higher prevalence of insulin resistance than those who are HCV-negative, and patients with type 2 DM are at higher risk for worse outcomes of their HCV infection.44 In addition, screen all patients with a METAVIR score of F3 or higher every 6 months for HCC using liver ultrasound, and recommend upper endoscopy to patients with cirrhosis to screen for esophageal varices.45,46
Health maintenance after treatment
Once patients have achieved SVR 12 weeks after completion of therapy, they are deemed cured. However, those patients who were already METAVIR F3 or higher maintain sufficient risk of HCC to recommend ongoing screening with ultrasound.47,48
CORRESPONDENCE
Mark Shaffer, MD, 3209 Colonial Drive, Columbia, SC 29206; [email protected].
Several recent developments have prompted a renewed focus on the way we screen for, and manage the treatment of, hepatitis C virus (HCV) infection. In 2013, the United States Preventive Services Task Force expanded its HCV screening guidelines to include baby boomers born between 1945 and 1965, regardless of apparent risk factors (TABLE 11).2 The recommendation is based on the high prevalence of chronic HCV in this cohort, estimated to be 4.3%, which is about 4 times higher than that of the general US population.3 It is believed that 75% of chronic HCV infections in the United States are in this cohort. After decades of infection, many in this age group are now presenting with advanced disease, leading to 19,659 HCV-related deaths in America in 2014.4
In addition, while HCV incidence in America had been steadily declining, it is now once again on the rise among young, non-urban whites, mainly because of increasing intravenous drug use in this population.5 On a positive note, new highly-effective and better-tolerated treatments are greatly improving the care we can provide.
In light of these factors, family physicians (FPs) are likely to be screening for HCV more than ever before and must be prepared to provide appropriate counseling and initial clinical management for those with positive test results. This article reviews the evaluation and primary care management of HCV-infected patients, as well as approaches to treatment with the newest direct-acting antivirals (DAAs).
The natural history of hepatitis C (and what we’re seeing as boomers age)
Acute HCV infection is rarely symptomatic, but results in chronic infection approximately 75% of the time.6 While some chronically infected individuals remain unaffected, most develop some degree of hepatic fibrosis, and 20% will develop cirrhosis within 20 years of diagnosis.7-9
The rate of progression is variable; factors that result in more rapid progression of liver disease include coinfection with HIV or HBV, overweight or obesity, insulin resistance, male gender, and use of alcohol.7 As the baby boomer cohort has aged, patients infected in their youth are now presenting with the sequelae of decompensated cirrhosis, including ascites, portal vein thrombosis, and thrombocytopenia.
Extrahepatic manifestations of chronic hepatitis C can include fatigue, membranoproliferative glomerulonephritis, porphyria cutanea tarda, cryoglobulinemia, a higher likelihood of insulin resistance, and possibly lymphoma.10-12
Chronic HCV is also the major contributor to the increased incidence of hepatocellular carcinoma (HCC), which has tripled in the past 2 decades in the United States.13
Although results are inconsistent, studies suggest 5% to 10% of HCV-infected patients will succumb to liver-related death.7
Who you’ll screen
If your patient is at heightened risk of contracting HCV infection (TABLE 11) or was born between 1945 and 1965, you’ll want to screen for infection with an HCV antibody test. A positive antibody test must be followed by testing for hepatitis C viral RNA to confirm whether the patient is chronically infected or is among the approximately 25% of patients who spontaneously clear the virus.6
For the patient with no detectable HCV RNA, no further evaluation or treatment is necessary. HCV viral load itself provides little insight into the rate of progression of the illness, but does correlate with risk of transmission.14 Counseling patients about the full testing protocol before screening can help to reduce anxiety and confusion.
At present, 6 genotypes and multiple subtypes of HCV have been identified; these have important implications for prognosis and treatment.15 HCV genotyping is frequently ordered along with a test for HCV viral load, but may be deferred until after fibrosis staging is performed (more on that in a bit). It may also be deferred if treatment is not planned within the next 12 weeks, as its main clinical use is to guide choice of treatment. Once chronic infection has been confirmed by the presence of HCV viremia, further work-up focuses on evaluating the effects on the host, which, in turn, helps the provider finalize a treatment plan (FIGURE 116).
Follow initial screening with an evaluation including liver disease staging
Following a screen that comes back positive for HCV, you’ll conduct a more thorough history including questioning about previous or ongoing risk factors for HCV, perform a physical examination that includes looking for signs of liver failure or extrahepatic disease, and order more lab work. Laboratory investigations include a complete blood count; renal and hepatic panels; and testing for human immunodeficiency virus (HIV) antibody, hepatitis B virus (HBV) surface antigen, HBV surface antibody, and HBV core antibody.1,17 Finally, the patient must be evaluated for hepatic fibrosis and cirrhosis to quantify the likelihood of developing liver failure and HCC.
Staging liver disease is a prerequisite to treating HCV infection because the extent of liver fibrosis impacts not only prognosis, but also the choice of the treatment regimen and the duration of therapy. The traditional gold standard for diagnosing hepatic fibrosis and cirrhosis has been a liver biopsy; however, a single 1.6-mm biopsy evaluates only a small portion of the liver and can miss affected liver parenchyma. In addition, a liver biopsy carries a small, but not inconsequential, risk of morbidity, and can be costly and complex to arrange.
Several noninvasive options are now available and are typically the preferred methods for staging liver disease. FibroSURE (LabCorp), for example, uses a peripheral venous blood sample and combines the patient’s age, gender, and 6 biochemical markers to generate a range of scores that correspond to the fibrosis component of the well-known METAVIR scoring system and correlate with results of liver biopsies.18,19 (The METAVIR system is a histology-based scoring system that grades fibrosis from F0 [no fibrosis] to F4 [cirrhosis].)
Noninvasive imaging studies assess for fibrosis more directly by assessing liver elasticity, either by ultrasound or magnetic resonance (MR) technology. The ultrasound modality FibroScan (Echosens) is currently the most widely available, although some data suggest the more expensive MR elastography has higher sensitivity (94% sensitive for METAVIR F2 or higher compared to 79% by FibroScan).20,21 While each of these modalities has limitations (eg, body habitus, availability), these tests allow stratification of patients into categories of low, moderate, and high risk for cirrhosis without the risks of biopsy.
A curable viral infection
HCV is one of the few curable chronic viral infections; unlike HBV and HIV, HCV does not create a long-term intra-nuclear reservoir. DAAs have cure rates of more than 95% for many HCV genotypes,22-24 allowing the possibility for dramatic reductions in prevalence in the decades to come.
Cure is defined by reaching a sustained virologic response (SVR), or absence of detectable viral load, 12 weeks after completion of therapy. Patients with HCV infection with advanced fibrosis who achieve SVR have shown benefits beyond improvement in liver function and histology. One large, multicenter, prospective study of 530 patients with chronic HCV, for example, found that those who achieved SVR experienced a 76% reduction in the risk of HCC and a 66% reduction in all-cause mortality (number needed to treat [NNT] was 5.8 to prevent one death or 6 to prevent one case of HCC in 10 years) compared to those without SVR.25 Other extrahepatic manifestations that impair quality of life, such as renal disease, autoimmune disease, and circulatory problems, are likewise reduced.25
Guidelines now recommend treating most patients with HCV infection
Until 2011, HCV treatment included the injectable immune-activating agent interferon and the non–HCV-specific antiviral ribavirin. This regimen had low SVR rates of 40% to 60% and adverse effects that were often intolerable.26 The advent of the first-generation HCV protease inhibitors in 2011 improved SVR rates, which have continued to improve exponentially with the development of combination therapy using DAAs (TABLE 227-29). (In order to stay up to date with the latest options for the treatment of HCV, see The American Association for the Study of Liver Diseases treatment guidelines at: http://hcvguidelines.org.)
What the guidelines say. Due to the tolerability and efficacy of the new DAAs, current guidelines state that HCV treatment should be recommended to most patients with HCV infection—not just those with advanced disease.30 This is a major change from prior guidelines, which were based on more toxic and less effective regimens. Limited data from long-term cohort studies of patients using interferon-based regimens suggest that the benefits of SVR are greatest for those treated at early stages before significant fibrosis develops. At least one analysis involving over 4000 patients found, however, that this approach may be less cost-effective, with an NNT of 20 to prevent one death in 20 years.31
In practice, the decision to treat requires a discussion between the patient and provider, weighing the risks and benefits of treatment in the context of the patients’ comorbidities and overall life expectancy. Such a discussion must also include cost. Many insurance companies will still only cover antiviral therapy for patients with advanced fibrosis, but these restrictions are slowly lifting and are having significant implications for our health care system. By one estimate, treating all patients with HCV at current drug prices would cost approximately $250 billion—about one-tenth of the total annual health care costs in this country.32 As policies change and the cost of drug regimens decreases from increasing competition, access is likely to improve for the majority of Americans.
Which regimen is most likely to be successful?
Many factors influence the choice of regimen and likelihood for SVR. These factors include whether the patient has cirrhosis and any comorbidities, the hepatitis C genotype involved, and any prior treatment the patient may have received. (See TABLE 37,12,18,28,30,33 for a comprehensive list.)
The easiest patients to treat are treatment-naive, with minimal liver disease and a favorable genotype. For example, combination therapy with the NS5B inhibitor sofosbuvir and an NS5A inhibitor (ledipasvir, daclatasvir, or velpatasvir) administered for 12 weeks has an SVR rate of >95% in genotype-1, treatment-naive, non-cirrhotic patients.22-24 Patients with prior treatment failure, especially failure on DAA therapy, or who have genotype 3, may be less responsive to standard therapies and may require more complex regimens or a longer duration of therapy.
Patients requiring special attention. It’s preferable to manage patients with decompensated liver disease in a specialized hepatology center due to the possibility of further decline and need for transplant prior to completion of therapy. Patients with HIV are another population that requires special attention. As many as 25% of HIV-infected patients are co-infected with HCV; their treatment follows the same principles as that in non-HIV patients, with extra attention paid to avoiding drug-drug interactions. Elbasvir/grazoprevir, for example, should not be used with any protease inhibitors, with nevirapine, or with efavirenz, and sofosbuvir should not be used with efavirenz, nevirapine, or tipranavir.34
Beyond medication regimens: The advice you’ll offer
In addition to counseling about antiviral therapy, patients with HCV infection require other types of advice and care that are often best administered by a primary care physician who is familiar with the patient and his or her family and community.
Prevention of transmission
Many patients have concerns about transmission of the virus to family members, co-workers, and sexual partners. You can assure patients that they are not likely to spread the virus in the workplace, even in health care environments.
Close contacts are also not at risk as long as basic prevention measures, such as not sharing toothbrushes or razors, are established to avoid transmission of blood and bodily fluids. Patient handouts can be found at the Centers for Disease Control and Prevention Web site (http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc).35
Patients and their sexual partners, however, must be counseled about the risk of sexual transmission. In monogamous relationships between serodiscordant partners who practice vaginal intercourse, there is a low, but clinically important, risk of transmission of HCV—up to 0.6% per year.36 Anal intercourse and co-infection with HIV increase this risk significantly.37 Pregnant women must be advised on the currently non-modifiable risk of transmission to newborns, which is approximately 6% in mono-infected women, but may be at least twice as likely in HIV/HCV co-infected women.38,39
Staying healthy. In addition to pneumococcal and standard age-appropriate vaccines, vaccination against hepatitis A and HBV is recommended for all HCV-infected patients to reduce the risk of a severe acute hepatitis.40,41 Advise patients to avoid alcohol, to consume a healthy diet, and to participate in regular activity and exercise. Review the patient’s medication list for hepatotoxic drugs and counsel the patient on the risks of excessive use of acetaminophen, non-steroidal anti-inflammatory drugs, and herbal medicines such as kava kava. Because obesity and metabolic syndrome are known risk factors for hepatic steatosis, which hastens the progression to cirrhosis and liver failure, counsel overweight and obese patients on the importance of healthy weight loss.42,43
Disease-related screenings. Consider screening all HCV patients for diabetes mellitus (DM) because people with chronic HCV infection have a higher prevalence of insulin resistance than those who are HCV-negative, and patients with type 2 DM are at higher risk for worse outcomes of their HCV infection.44 In addition, screen all patients with a METAVIR score of F3 or higher every 6 months for HCC using liver ultrasound, and recommend upper endoscopy to patients with cirrhosis to screen for esophageal varices.45,46
Health maintenance after treatment
Once patients have achieved SVR 12 weeks after completion of therapy, they are deemed cured. However, those patients who were already METAVIR F3 or higher maintain sufficient risk of HCC to recommend ongoing screening with ultrasound.47,48
CORRESPONDENCE
Mark Shaffer, MD, 3209 Colonial Drive, Columbia, SC 29206; [email protected].
1. AASLD-IDSA. HCV testing and linkage to care. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. Accessed August 22, 2016.
2. US Preventive Services Task Force. Hepatitis C: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-C-screening. Accessed August 28, 2016.
3. Denniston MM, Jiles RN, Brobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300.
4. Centers for Disease Control and Prevention. Surveillance for viral hepatitis-United States, 2014. Available at: https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm. Accessed February 6, 2017.
5. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mort Wkly Rep. 2015;64:453-458.
6. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41.
7. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46.
8. Klevens M, Huang X, Yeo AE, et al. The burden of liver disease among persons with hepatitis C in the United States. Conference on Retroviruses and Opportunistic Infections. Seattle, February 23-24, 2015. Abstract 145.
9. Zarski JP, McHutchison J, Bronowicki JP, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003;38:307-314.
10. Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore). 2000;79:47-56.
11. Vannata B, Arcaini L, Zucca E. Hepatitis C virus-associated B-cell non-Hodgkin’s lymphomas: what do we know? Ther Adv Hematol. 2016;7:94-107.
12. Gastaldi G, Goossens N, Clément S, et al. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: a review. J Adv Res. 2017;8:149-159.
13. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127.
14. Elrazek AE, Amer M, Hawary B, et al. Prediction of HCV vertical transmission: What are factors should be optimized using data mining computational analysis. Liver Int. 2016.
15. Wang LS, D’Souza LS, Jacobson IM. Hepatitis C-A clinical review. J Med Virol. 2016;88:1844-1855.
16. Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-365.
17. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm522932.htm. Accessed December 15, 2016.
18. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
19. Patel K, Friedrich-Rust M, Lurie Y, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17:4581-4589.
20. Shiraishi A, Hiraoka A, Aibiki T, et al. Real-time tissue elastography: non-invasive evaluation of liver fibrosis in chronic liver disease due to HCV. Hepatogastroenterology. 2014;61:2084-2090.
21. Yoon JH, Lee JM, Joo I, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772-782.
22. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898.
23. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373:714-725.
24. Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599-2607.
25. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593.
26. NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46.
27. AASLD-IDSA. Initial treatment of HCV infection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/initial-treatment-hcv-infection. Accessed August 24, 2016.
28. Lexicomp. Wolters Kluwer. Clinical Drug Information, Inc. Available at: http://online.lexi.com/action/home.
29. GoodRx. Available at: https//www.goodrx.com. Accessed January 25, 2017.
30. AASLD-IDSA. When and in whom to initiate HCV therapy. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. Accessed August 31, 2016.
31. Jezequel C, Bardou-Jacquet E, Desille Y, et al. Survival of patients infected by chronic hepatitis C and F0F1 fibrosis at baseline after a 15-years follow-up. Poster presented at: 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015; Vienna, Austria.
32. Lin KW. Should family physicians routinely screen patients for hepatitis C? Am Fam Physician. 2016;93:17-18.
33. Center for Medicare and Medicaid Services. Center for Medicaid and CHIP Services. Medicaid drug rebate program notice. Release no. 172. Available at: https://www.medicaid.gov/medicaid-chip-program-information/by-topics/prescription-drugs/downloads/rx-releases/state-releases/state-rel-172.pdf. Accessed August 24, 2016.
34. AASLD-IDSA. Unique patient populations: patients with HIV/HCV coinfection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/unique-patient-populations-patients-hivhcv-coinfection. Accessed February 6, 2017.
35. Centers for Disease Control and Prevention. Viral hepatitis-hepatitis C information. Patient education resources. Available at: http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc. Accessed June 15, 2016.
36. Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:S99-S105.
37. Chan DP, Sun HY, Wong HT, et al. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis. 2016;49:47-58.
38. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
39. European Paediatric Hepatitis C Virus Network. A significant sex—but not elective cesarean section—effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005;192:1872-1879.
40. Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102-1106.
41. Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci. 2005;50:1525-1531.
42. Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555-561.
43. Hu KQ, Kyulo NL, Esrailian E, et al. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J Hepatol. 2004;40:147-154.
44. Hammerstad SS, Grock SF, Lee HJ, et al. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134.
45. Lok AS, Seeff LV, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138-148.
46. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5-10.
47. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337.
1. AASLD-IDSA. HCV testing and linkage to care. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. Accessed August 22, 2016.
2. US Preventive Services Task Force. Hepatitis C: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-C-screening. Accessed August 28, 2016.
3. Denniston MM, Jiles RN, Brobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300.
4. Centers for Disease Control and Prevention. Surveillance for viral hepatitis-United States, 2014. Available at: https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm. Accessed February 6, 2017.
5. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mort Wkly Rep. 2015;64:453-458.
6. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41.
7. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46.
8. Klevens M, Huang X, Yeo AE, et al. The burden of liver disease among persons with hepatitis C in the United States. Conference on Retroviruses and Opportunistic Infections. Seattle, February 23-24, 2015. Abstract 145.
9. Zarski JP, McHutchison J, Bronowicki JP, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003;38:307-314.
10. Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore). 2000;79:47-56.
11. Vannata B, Arcaini L, Zucca E. Hepatitis C virus-associated B-cell non-Hodgkin’s lymphomas: what do we know? Ther Adv Hematol. 2016;7:94-107.
12. Gastaldi G, Goossens N, Clément S, et al. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: a review. J Adv Res. 2017;8:149-159.
13. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127.
14. Elrazek AE, Amer M, Hawary B, et al. Prediction of HCV vertical transmission: What are factors should be optimized using data mining computational analysis. Liver Int. 2016.
15. Wang LS, D’Souza LS, Jacobson IM. Hepatitis C-A clinical review. J Med Virol. 2016;88:1844-1855.
16. Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-365.
17. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm522932.htm. Accessed December 15, 2016.
18. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
19. Patel K, Friedrich-Rust M, Lurie Y, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17:4581-4589.
20. Shiraishi A, Hiraoka A, Aibiki T, et al. Real-time tissue elastography: non-invasive evaluation of liver fibrosis in chronic liver disease due to HCV. Hepatogastroenterology. 2014;61:2084-2090.
21. Yoon JH, Lee JM, Joo I, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772-782.
22. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898.
23. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373:714-725.
24. Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599-2607.
25. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593.
26. NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46.
27. AASLD-IDSA. Initial treatment of HCV infection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/initial-treatment-hcv-infection. Accessed August 24, 2016.
28. Lexicomp. Wolters Kluwer. Clinical Drug Information, Inc. Available at: http://online.lexi.com/action/home.
29. GoodRx. Available at: https//www.goodrx.com. Accessed January 25, 2017.
30. AASLD-IDSA. When and in whom to initiate HCV therapy. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. Accessed August 31, 2016.
31. Jezequel C, Bardou-Jacquet E, Desille Y, et al. Survival of patients infected by chronic hepatitis C and F0F1 fibrosis at baseline after a 15-years follow-up. Poster presented at: 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015; Vienna, Austria.
32. Lin KW. Should family physicians routinely screen patients for hepatitis C? Am Fam Physician. 2016;93:17-18.
33. Center for Medicare and Medicaid Services. Center for Medicaid and CHIP Services. Medicaid drug rebate program notice. Release no. 172. Available at: https://www.medicaid.gov/medicaid-chip-program-information/by-topics/prescription-drugs/downloads/rx-releases/state-releases/state-rel-172.pdf. Accessed August 24, 2016.
34. AASLD-IDSA. Unique patient populations: patients with HIV/HCV coinfection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/unique-patient-populations-patients-hivhcv-coinfection. Accessed February 6, 2017.
35. Centers for Disease Control and Prevention. Viral hepatitis-hepatitis C information. Patient education resources. Available at: http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc. Accessed June 15, 2016.
36. Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:S99-S105.
37. Chan DP, Sun HY, Wong HT, et al. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis. 2016;49:47-58.
38. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
39. European Paediatric Hepatitis C Virus Network. A significant sex—but not elective cesarean section—effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005;192:1872-1879.
40. Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102-1106.
41. Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci. 2005;50:1525-1531.
42. Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555-561.
43. Hu KQ, Kyulo NL, Esrailian E, et al. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J Hepatol. 2004;40:147-154.
44. Hammerstad SS, Grock SF, Lee HJ, et al. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134.
45. Lok AS, Seeff LV, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138-148.
46. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5-10.
47. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337.
PRACTICE RECOMMENDATIONS
› Offer hepatitis C virus (HCV) screening to all patients with identified risk factors, as well as anyone born between 1945 and 1965, regardless of risk factors. B
› Offer human immunodeficiency virus and hepatitis B testing, as well as hepatitis A, hepatitis B, and pneumococcal vaccinations, to all patients with chronic HCV infection. C
› Consider treatment with interferon-free direct-acting antiviral therapies for all patients with chronic HCV infection to reduce liver-related and all-cause morbidity and mortality. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Dupuytren’s disease: How to recognize its early signs
CASE › A 52-year-old right-hand-dominant white man arrived at our clinic complaining that he was unable to straighten his right ring finger. He had no associated pain or numbness, and had not injured his hand. The patient had type 2 diabetes that was controlled with metformin. He had no history of surgery or drug allergies, did not smoke, and said he drank 2 to 3 alcoholic beverages per day. He was a car salesman and was self-conscious when shaking hands with customers. On physical examination, we noted that he held his right ring finger at roughly 45 degrees of flexion at the metacarpophalangeal joint; a painless cord-like structure was palpable on the palmar surface of that joint. His left hand had no abnormalities.
If this were your patient, how would you proceed?
Dupuytren’s disease (DD) is a disabling fibroproliferative disorder of the hand for which there is no cure. While the exact cause of DD is unknown, it has been linked to a number of risk factors, including smoking, alcohol consumption, and diabetes. It affects about 5% of the US population, and up to 70% of affected individuals may initially seek treatment from a primary care physician.1 The disease is also referred to as Dupuytren’s contracture, which describes the flexion contractures of fingers at the end stage of the disease. Palmar fibromatosis is yet another name for the disorder.
DD refers to a spectrum of presentations ranging from nodules to cords to discernible contractures, and it is not known which patients with early Dupuytren changes will progress to severe contracture. With recognition of early changes, nonsurgical intervention is possible, such as collagenase injection or percutaneous fasciotomy, and can slow the progression of DD, restore function, and avoid or delay surgical intervention. DD is a clinically challenging disorder. Treatment for an affected area may resolve symptoms, only to have them recur in that location or another.
How underlying pathology correlates with clinical findings
DD affects the palmar fascia, a thick triangular-shaped sheet of dense fibrous collagenous connective tissue that lies deep to the dermis and superficial to the flexor tendons of the hand with fibers extending both into the skin and into the deep tissue. The palmar fascia secures the skin during gripping and twisting motions, and it bifurcates into distal extensions, called pretendinous bands, that overlay and mimic the flexor tendons.
Clinical findings reflect the progression of underlying pathology. The earliest manifestation of DD is painless dimpling of the skin on the palmar surface of the hand.2 Over time, the underlying fibrosis with increased collagen deposition can progress, leading to development of nodules and eventually, cords, which are sometimes mistaken for flexor tendons. Dupuytren-like fibrotic tissue can occur on the sole of the foot (Ledderhose disease) and penis (Peyronie’s disease).2,3 In patients with these coexisting conditions, prognosis is generally worse.4 With the hands, bilateral involvement is common, although it is usually more severe in one hand. The ring finger is the digit most frequently involved, followed by the little finger, middle finger, and index finger. The thumb is rarely affected.3
As the disease progresses and cords contract, the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints develop flexion contractures. The distal interphalangeal (DIP) joint, rarely involved, instead exhibits a hyperextension contracture. Digital flexion contractures are often disabling, interfering with daily activities such as picking up a glass, shaking hands, or putting one's hand in a pants pocket. Many patients seek medical attention only after a palpable nodule, cord, or flexion contracture becomes apparent (FIGURE 1).
In a study of 326 patients who reported Dupuytren’s symptoms, the most common symptoms that led them to seek treatment were, in descending order, a hard bump (48%), a ropelike growth (12%), dimpling (11%), and finger contractures (10%). Only 9% of patients seeking treatment for hand symptoms associated with DD had received a diagnosis of DD from their initial medical encounter, causing an unnecessary delay in treatment.1
Who is at increased risk for DD?
DD is most often seen in elderly white men of European descent.5 In the United States, the prevalence of the disease is roughly 5%,1 compared with 4% to 39% in northwestern Europe (eg, Iceland).6 The male-to-female ratio of DD ranges from 6:1 to 15:1.7 The prevalence of DD appears to increase with age. The prevalence in men and women is similar up to age 45 years, after which the rate is much greater in males.7
DD is associated with many risk factors including smoking, alcohol consumption, vascular insufficiency, epilepsy, hyperlipidemia, manual labor, occupations with exposure to vibration, hand trauma, and even hand surgery such as carpal tunnel release or trigger finger release.8-11 It is also associated with diabetes; particularly type 1 insulin-dependent diabetes.12 There may also be an association with frozen shoulder.12,13
The need for surgical treatment of DD becomes more likely with a history of cigarette smoking and heavy alcohol consumption. There seems to be an association with epilepsy, most likely from anti-epileptic drugs.14 Rheumatoid arthritis is the only condition that has been associated with a lower incidence of DD, possibly because of the use of anti-inflammatory drugs.15 There are genetic differences between patients with and without DD, although a “Dupuytren’s gene” has not been identified.
Rule out possible DD mimics
The differential diagnosis for flexion contracture of the MCP or PIP joints seen in Dupuytren’s disease includes stenosing flexor tenosynovitis, or trigger finger. Other conditions that can mimic DD are ulnar claw hand, trauma scars, intrinsic joint diseases such as degenerative or rheumatoid arthritis (RA), diabetic cheiroarthropathy, camptodactyly, and Volkmann’s contracture. Trauma scars—especially longitudinal scars—have a tendency to contract and develop keloid formation.
Stenosing flexor tenosynovitis and ulnar claw hand are distinguishable from DD by full or nearly full active or passive extension of the affected digit, whereas DD is a true contracture of the joint that does not allow full extension. A careful history can rule out previous injury to the area. Although intrinsic joint disease, such as RA, can cause finger contractures, the joints are usually enlarged, painful, and associated with characteristic radiologic findings.
Unlike DD, diabetic cheiroarthropathy often involves all of the digits except for the thumb, and is often associated with a waxy appearance of the skin. Camptodactyly is an autosomal dominant disorder that more often presents in childhood and can be caused by a number of congenital syndromes. Volkmann’s contracture can manifest as a claw-like deformity of the hand caused by undiagnosed compartment syndrome of the forearm.
Assess the severity of DD
In your evaluation, first identify palmar or digital fibromatosis presenting as a nodule or a cord. Second, estimate the degree of MCP and PIP joint contractures. A common measure of contracture is the Hueston tabletop test. Ask the patient to place the palm of the hand on a flat surface. If the patient is unable to completely flatten the hand against the surface, presume a positive result (FIGURE 2).16
An accurate measure of the degree of flexion can be accomplished with a goniometer. For a simple assessment of severity, have the patient place each affected finger along the convexity of a spoon. If adjoining surfaces are flush, assume that the contracture is at least 30 degrees (FIGURE 3). The severity of DD can also be graded according to the Tubiana classification system (TABLE17), wherein the total deformity or contracture score is the sum of the angles of all 3 digital joints of the finger.
A look at the treatment options
Once the diagnosis of DD is made, reassure the patient that the disease is not cancerous, although it may be progressive. Advise patients, too, that several treatment options (detailed below) are available, but that surgery is the mainstay of treatment. (Of note: Despite what would seem logical, stretching a cord to straighten the finger is not recommended, as it may actually worsen the condition.18)
Options for early DD. Corticosteroids such as triamcinolone may have a role as an adjunct for early DD by reducing the size and firmness of the Dupuytren’s nodules and possibly slowing the progression of the disease. However, in one study of 63 patients, half of the individuals experienced reactivation of disease between one and 3 years.19
Radiotherapy has been studied as a potential treatment for early DD and for patients unable to undergo surgery. Radiation does have a biologic effect on nodular DD, but has no effect on the cords that cause the joint contracture. In a long-term follow-up study of 135 patients treated with radiotherapy, disease remained stable in 59% of patients, improved in 10%, and progressed in 31%.20 Results were better for early stage disease. This modality is not used for more advanced cases.
Surgery is the primary method by which to restore function and minimize complications. In the past, referral was recommended for MCP joint contractures >30 degrees or for any involvment of the PIP joint, because involvement of the latter carries a worse prognosis and may not be fully correctable, even with surgery.21 However, treatment may also be warranted in cases of functional disability—regardless of the degree of contracture (eg, for pain associated with a prominent nodule or cord when gripping objects).
Depending on disease progression and the surgeon’s preference, the most popular procedures are fasciotomies and fasciectomies. Surgical options for contractures—in increasing order of invasiveness and amounts of fascia removed—are fasciotomy (needle, open), fasciectomy (partial, radical), and dermofasciectomy.
Needle fasciotomy is an outpatient procedure performed under local block. The surgeon uses a needle bevel to transect the diseased cord at multiple puncture sites.
Open fasciotomy is also an outpatient procedure involving an incision that allows direct visualization of the cord and internal structures before dividing the fascia. Percutaneous and open fasciotomies are more successful for MCP joint contractures and less successful for PIP joint contractures.
With partial fasciectomy, only the grossly affected fascia is removed.
With radical fasciectomy, the entire palmar fascia is removed, regardless of which areas are grossly diseased.
Dermofasciectomy removes diseased fascia and the overlying skin, with a skin graft applied to heal the wound.
Another option: Collagenase injection. In February 2010, the US Food and Drug Administration approved collagenase clostridium histolyticum (Xiaflex) as the first drug specifically indicated for the treatment of Dupuytren’s contracture. Collagenase is injected directly into the fascial cord, cleaving its collagen component and progressively softening and breaking down the cord. If not properly injected, collagenase may injure neurovascular structures and may rupture tendons.
So how do the treatment options compare?
We found no randomized prospective trials comparing treatment options for DD, but there are a number of trials that shed light on a variety of the available options.
Treatment success. In general, partial fasciectomies have shown the greatest success in reducing contractures and maintaining the lowest recurrence rates. Correction of contracture to ≤5 degrees was seen in 94% of MCP joint contractures treated with partial fasciectomy, in 77% of MCP joints treated with collagenase, and in 55% of MCP joints treated with percutaneous needle fasciotomies.22,23 PIP joint contracture correction was not as successful: Correction of contracture to ≤5 degrees was seen in 47% of those treated with partial fasciectomy, 40% treated with collagenase, and 26% treated with needle fasciotomy.22,23
Recurrence rates. When recurrence was defined as loss of passive extension >30 degrees, the recurrence rate for MCP joint contractures with partial fasciectomy was 21%, compared with 85% for needle fasciotomy.22 In a similar review, recurrence rates for partial fasciectomy ranged from 12% to 39% compared with 50% to 58% for needle fasciotomy.24 With collagenase injection, 5-year data have shown an overall recurrence rate of 32%, with a recurrence of 26% at the MCP joint and 46% at the PIP joint.25 In this trial, recurrence was
Complications. Although fasciectomies have shown the best efficacy and lowest recurrence rates, complications such as infection, neurapraxia, and digital nerve injury are more likely with these procedures.26,27
Early recognition and referral to a hand specialist for the treatment of DD may allow the use of less invasive techniques and improved functional results.
CASE › Absent a history of trauma and features typical of other hand/digit disorders, we diagnosed Dupuytren’s disease in this patient. Potential treatments included partial fasciectomy and collagenase injection. After discussing the risks and benefits of each of these procedures, the patient elected to undergo a partial fasciectomy. The surgery was uncomplicated and the abnormality was corrected to within 5 degrees of full extension. At the patient’s one-year follow-up visit, there was no evidence of recurrence.
1. DiBenedetti DB, Nguyen D, Zografos L, et al. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand. 2011;6:149-158.
2. Townley WA, Baker R, Sheppard N, et al. Dupuytren’s contracture unfolded. BMJ. 2006;332:397-400.
3. Rayan GM. Clinical presentation and types of Dupuytren’s disease. Hand Clin. 1999;15:87-96.
4. Hindocha S, Stanley JK, Watson S, et al. Dupuytren’s diathesis revisited: Evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am. 2006;31:1626-1634.
5. Bayat A, McGrouther DA. Management of Dupuytren’s disease—clear advice for an elusive condition. Ann R Coll Surg Engl. 2006;88:3-8.
6. Gudmundsson KG, Arngrimsson R, Sigfússon M, et al. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;53:291-296.
7. Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren’s contracture in men and women. J Hand Surg Br. 1999;24:456-459.
8. Noble J, Arafa M, Royle SG, et al. The association between alcohol, hepatic pathology and Dupuytren’s disease. J Hand Surg Br. 1992;17:71–74.
9. Mikkelsen OA. Dupuytren’s disease—initial symptoms, age of onset and spontaneous course. Hand. 1977;9:11-15.
10. Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol. 1997;24:153-159.
11. Liss GM, Stock SR. Can Dupuytren’s contracture be work related? review of the evidence. Am J Ind Med. 1996;29:521-532.
12. Geoghegan JM, Forbes J, Clark DI, et al. Dupuytren’s disease risk factors. J Hand Surg Br. 2004;29:423-426.
13. Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg. 2001;10:149-151.
14. Nunn AC, Schreuder FB. Dupuytren’s contracture: emerging insight into a Viking disease. J Hand Surg. 2014;19:481-490.
15. Arafa M, Steingold R F, Noble J. The incidence of Dupuytren’s disease in patients with rheumatoid arthritis. J Hand Surg Br. 1984;9:165-166.
16. Hueston J. Lessons in Dupuytren’s disease. Ann Chir Main Memb Super. 1992;11:349-354.
17. Tubiana R. Prognosis and treatment of Dupuytren’s contracture. J Bone and Joint Surg AM. 1955;37:1155-1168.
18. Howard JC, Varallo VM, Ross DC, et al. Elevated levels of ß-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren’s disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16.
19. Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000;25:1157-1162.
20. Betz N, Ott OJ, Adamietz B, et al. Radiotherapy in early-stage Dupuytren’s contracture. Long-term results after 13 years. Strahlenther Onkol. 2010;186:82-90.
21. Smith AC. Diagnosis and indications for surgical treatment. Hand Clin. 1991;7:635-642.
22. van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469-477.
23. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968-979.
24. Chen NC, Srinivasan RC, Shauver MJ, et al. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand. 2011;6:250-255.
25. Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-year data. J Hand Surg Am. 2015;40:1597-1605.
26. Toppi JT, Trompf L, Smoll NR, et al. Dupuytren’s contracture: an analysis of outcomes of percutaneous needle fasciotomy versus open fasciectomy. ANZ J Surg. 2015;85:639-643.
27. Cheung K, Walley KC, Rozental TD. Management of complications of Dupuytren contracture. Hand Clini. 2015;31:345-354.
CASE › A 52-year-old right-hand-dominant white man arrived at our clinic complaining that he was unable to straighten his right ring finger. He had no associated pain or numbness, and had not injured his hand. The patient had type 2 diabetes that was controlled with metformin. He had no history of surgery or drug allergies, did not smoke, and said he drank 2 to 3 alcoholic beverages per day. He was a car salesman and was self-conscious when shaking hands with customers. On physical examination, we noted that he held his right ring finger at roughly 45 degrees of flexion at the metacarpophalangeal joint; a painless cord-like structure was palpable on the palmar surface of that joint. His left hand had no abnormalities.
If this were your patient, how would you proceed?
Dupuytren’s disease (DD) is a disabling fibroproliferative disorder of the hand for which there is no cure. While the exact cause of DD is unknown, it has been linked to a number of risk factors, including smoking, alcohol consumption, and diabetes. It affects about 5% of the US population, and up to 70% of affected individuals may initially seek treatment from a primary care physician.1 The disease is also referred to as Dupuytren’s contracture, which describes the flexion contractures of fingers at the end stage of the disease. Palmar fibromatosis is yet another name for the disorder.
DD refers to a spectrum of presentations ranging from nodules to cords to discernible contractures, and it is not known which patients with early Dupuytren changes will progress to severe contracture. With recognition of early changes, nonsurgical intervention is possible, such as collagenase injection or percutaneous fasciotomy, and can slow the progression of DD, restore function, and avoid or delay surgical intervention. DD is a clinically challenging disorder. Treatment for an affected area may resolve symptoms, only to have them recur in that location or another.
How underlying pathology correlates with clinical findings
DD affects the palmar fascia, a thick triangular-shaped sheet of dense fibrous collagenous connective tissue that lies deep to the dermis and superficial to the flexor tendons of the hand with fibers extending both into the skin and into the deep tissue. The palmar fascia secures the skin during gripping and twisting motions, and it bifurcates into distal extensions, called pretendinous bands, that overlay and mimic the flexor tendons.
Clinical findings reflect the progression of underlying pathology. The earliest manifestation of DD is painless dimpling of the skin on the palmar surface of the hand.2 Over time, the underlying fibrosis with increased collagen deposition can progress, leading to development of nodules and eventually, cords, which are sometimes mistaken for flexor tendons. Dupuytren-like fibrotic tissue can occur on the sole of the foot (Ledderhose disease) and penis (Peyronie’s disease).2,3 In patients with these coexisting conditions, prognosis is generally worse.4 With the hands, bilateral involvement is common, although it is usually more severe in one hand. The ring finger is the digit most frequently involved, followed by the little finger, middle finger, and index finger. The thumb is rarely affected.3
As the disease progresses and cords contract, the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints develop flexion contractures. The distal interphalangeal (DIP) joint, rarely involved, instead exhibits a hyperextension contracture. Digital flexion contractures are often disabling, interfering with daily activities such as picking up a glass, shaking hands, or putting one's hand in a pants pocket. Many patients seek medical attention only after a palpable nodule, cord, or flexion contracture becomes apparent (FIGURE 1).
In a study of 326 patients who reported Dupuytren’s symptoms, the most common symptoms that led them to seek treatment were, in descending order, a hard bump (48%), a ropelike growth (12%), dimpling (11%), and finger contractures (10%). Only 9% of patients seeking treatment for hand symptoms associated with DD had received a diagnosis of DD from their initial medical encounter, causing an unnecessary delay in treatment.1
Who is at increased risk for DD?
DD is most often seen in elderly white men of European descent.5 In the United States, the prevalence of the disease is roughly 5%,1 compared with 4% to 39% in northwestern Europe (eg, Iceland).6 The male-to-female ratio of DD ranges from 6:1 to 15:1.7 The prevalence of DD appears to increase with age. The prevalence in men and women is similar up to age 45 years, after which the rate is much greater in males.7
DD is associated with many risk factors including smoking, alcohol consumption, vascular insufficiency, epilepsy, hyperlipidemia, manual labor, occupations with exposure to vibration, hand trauma, and even hand surgery such as carpal tunnel release or trigger finger release.8-11 It is also associated with diabetes; particularly type 1 insulin-dependent diabetes.12 There may also be an association with frozen shoulder.12,13
The need for surgical treatment of DD becomes more likely with a history of cigarette smoking and heavy alcohol consumption. There seems to be an association with epilepsy, most likely from anti-epileptic drugs.14 Rheumatoid arthritis is the only condition that has been associated with a lower incidence of DD, possibly because of the use of anti-inflammatory drugs.15 There are genetic differences between patients with and without DD, although a “Dupuytren’s gene” has not been identified.
Rule out possible DD mimics
The differential diagnosis for flexion contracture of the MCP or PIP joints seen in Dupuytren’s disease includes stenosing flexor tenosynovitis, or trigger finger. Other conditions that can mimic DD are ulnar claw hand, trauma scars, intrinsic joint diseases such as degenerative or rheumatoid arthritis (RA), diabetic cheiroarthropathy, camptodactyly, and Volkmann’s contracture. Trauma scars—especially longitudinal scars—have a tendency to contract and develop keloid formation.
Stenosing flexor tenosynovitis and ulnar claw hand are distinguishable from DD by full or nearly full active or passive extension of the affected digit, whereas DD is a true contracture of the joint that does not allow full extension. A careful history can rule out previous injury to the area. Although intrinsic joint disease, such as RA, can cause finger contractures, the joints are usually enlarged, painful, and associated with characteristic radiologic findings.
Unlike DD, diabetic cheiroarthropathy often involves all of the digits except for the thumb, and is often associated with a waxy appearance of the skin. Camptodactyly is an autosomal dominant disorder that more often presents in childhood and can be caused by a number of congenital syndromes. Volkmann’s contracture can manifest as a claw-like deformity of the hand caused by undiagnosed compartment syndrome of the forearm.
Assess the severity of DD
In your evaluation, first identify palmar or digital fibromatosis presenting as a nodule or a cord. Second, estimate the degree of MCP and PIP joint contractures. A common measure of contracture is the Hueston tabletop test. Ask the patient to place the palm of the hand on a flat surface. If the patient is unable to completely flatten the hand against the surface, presume a positive result (FIGURE 2).16
An accurate measure of the degree of flexion can be accomplished with a goniometer. For a simple assessment of severity, have the patient place each affected finger along the convexity of a spoon. If adjoining surfaces are flush, assume that the contracture is at least 30 degrees (FIGURE 3). The severity of DD can also be graded according to the Tubiana classification system (TABLE17), wherein the total deformity or contracture score is the sum of the angles of all 3 digital joints of the finger.
A look at the treatment options
Once the diagnosis of DD is made, reassure the patient that the disease is not cancerous, although it may be progressive. Advise patients, too, that several treatment options (detailed below) are available, but that surgery is the mainstay of treatment. (Of note: Despite what would seem logical, stretching a cord to straighten the finger is not recommended, as it may actually worsen the condition.18)
Options for early DD. Corticosteroids such as triamcinolone may have a role as an adjunct for early DD by reducing the size and firmness of the Dupuytren’s nodules and possibly slowing the progression of the disease. However, in one study of 63 patients, half of the individuals experienced reactivation of disease between one and 3 years.19
Radiotherapy has been studied as a potential treatment for early DD and for patients unable to undergo surgery. Radiation does have a biologic effect on nodular DD, but has no effect on the cords that cause the joint contracture. In a long-term follow-up study of 135 patients treated with radiotherapy, disease remained stable in 59% of patients, improved in 10%, and progressed in 31%.20 Results were better for early stage disease. This modality is not used for more advanced cases.
Surgery is the primary method by which to restore function and minimize complications. In the past, referral was recommended for MCP joint contractures >30 degrees or for any involvment of the PIP joint, because involvement of the latter carries a worse prognosis and may not be fully correctable, even with surgery.21 However, treatment may also be warranted in cases of functional disability—regardless of the degree of contracture (eg, for pain associated with a prominent nodule or cord when gripping objects).
Depending on disease progression and the surgeon’s preference, the most popular procedures are fasciotomies and fasciectomies. Surgical options for contractures—in increasing order of invasiveness and amounts of fascia removed—are fasciotomy (needle, open), fasciectomy (partial, radical), and dermofasciectomy.
Needle fasciotomy is an outpatient procedure performed under local block. The surgeon uses a needle bevel to transect the diseased cord at multiple puncture sites.
Open fasciotomy is also an outpatient procedure involving an incision that allows direct visualization of the cord and internal structures before dividing the fascia. Percutaneous and open fasciotomies are more successful for MCP joint contractures and less successful for PIP joint contractures.
With partial fasciectomy, only the grossly affected fascia is removed.
With radical fasciectomy, the entire palmar fascia is removed, regardless of which areas are grossly diseased.
Dermofasciectomy removes diseased fascia and the overlying skin, with a skin graft applied to heal the wound.
Another option: Collagenase injection. In February 2010, the US Food and Drug Administration approved collagenase clostridium histolyticum (Xiaflex) as the first drug specifically indicated for the treatment of Dupuytren’s contracture. Collagenase is injected directly into the fascial cord, cleaving its collagen component and progressively softening and breaking down the cord. If not properly injected, collagenase may injure neurovascular structures and may rupture tendons.
So how do the treatment options compare?
We found no randomized prospective trials comparing treatment options for DD, but there are a number of trials that shed light on a variety of the available options.
Treatment success. In general, partial fasciectomies have shown the greatest success in reducing contractures and maintaining the lowest recurrence rates. Correction of contracture to ≤5 degrees was seen in 94% of MCP joint contractures treated with partial fasciectomy, in 77% of MCP joints treated with collagenase, and in 55% of MCP joints treated with percutaneous needle fasciotomies.22,23 PIP joint contracture correction was not as successful: Correction of contracture to ≤5 degrees was seen in 47% of those treated with partial fasciectomy, 40% treated with collagenase, and 26% treated with needle fasciotomy.22,23
Recurrence rates. When recurrence was defined as loss of passive extension >30 degrees, the recurrence rate for MCP joint contractures with partial fasciectomy was 21%, compared with 85% for needle fasciotomy.22 In a similar review, recurrence rates for partial fasciectomy ranged from 12% to 39% compared with 50% to 58% for needle fasciotomy.24 With collagenase injection, 5-year data have shown an overall recurrence rate of 32%, with a recurrence of 26% at the MCP joint and 46% at the PIP joint.25 In this trial, recurrence was
Complications. Although fasciectomies have shown the best efficacy and lowest recurrence rates, complications such as infection, neurapraxia, and digital nerve injury are more likely with these procedures.26,27
Early recognition and referral to a hand specialist for the treatment of DD may allow the use of less invasive techniques and improved functional results.
CASE › Absent a history of trauma and features typical of other hand/digit disorders, we diagnosed Dupuytren’s disease in this patient. Potential treatments included partial fasciectomy and collagenase injection. After discussing the risks and benefits of each of these procedures, the patient elected to undergo a partial fasciectomy. The surgery was uncomplicated and the abnormality was corrected to within 5 degrees of full extension. At the patient’s one-year follow-up visit, there was no evidence of recurrence.
CASE › A 52-year-old right-hand-dominant white man arrived at our clinic complaining that he was unable to straighten his right ring finger. He had no associated pain or numbness, and had not injured his hand. The patient had type 2 diabetes that was controlled with metformin. He had no history of surgery or drug allergies, did not smoke, and said he drank 2 to 3 alcoholic beverages per day. He was a car salesman and was self-conscious when shaking hands with customers. On physical examination, we noted that he held his right ring finger at roughly 45 degrees of flexion at the metacarpophalangeal joint; a painless cord-like structure was palpable on the palmar surface of that joint. His left hand had no abnormalities.
If this were your patient, how would you proceed?
Dupuytren’s disease (DD) is a disabling fibroproliferative disorder of the hand for which there is no cure. While the exact cause of DD is unknown, it has been linked to a number of risk factors, including smoking, alcohol consumption, and diabetes. It affects about 5% of the US population, and up to 70% of affected individuals may initially seek treatment from a primary care physician.1 The disease is also referred to as Dupuytren’s contracture, which describes the flexion contractures of fingers at the end stage of the disease. Palmar fibromatosis is yet another name for the disorder.
DD refers to a spectrum of presentations ranging from nodules to cords to discernible contractures, and it is not known which patients with early Dupuytren changes will progress to severe contracture. With recognition of early changes, nonsurgical intervention is possible, such as collagenase injection or percutaneous fasciotomy, and can slow the progression of DD, restore function, and avoid or delay surgical intervention. DD is a clinically challenging disorder. Treatment for an affected area may resolve symptoms, only to have them recur in that location or another.
How underlying pathology correlates with clinical findings
DD affects the palmar fascia, a thick triangular-shaped sheet of dense fibrous collagenous connective tissue that lies deep to the dermis and superficial to the flexor tendons of the hand with fibers extending both into the skin and into the deep tissue. The palmar fascia secures the skin during gripping and twisting motions, and it bifurcates into distal extensions, called pretendinous bands, that overlay and mimic the flexor tendons.
Clinical findings reflect the progression of underlying pathology. The earliest manifestation of DD is painless dimpling of the skin on the palmar surface of the hand.2 Over time, the underlying fibrosis with increased collagen deposition can progress, leading to development of nodules and eventually, cords, which are sometimes mistaken for flexor tendons. Dupuytren-like fibrotic tissue can occur on the sole of the foot (Ledderhose disease) and penis (Peyronie’s disease).2,3 In patients with these coexisting conditions, prognosis is generally worse.4 With the hands, bilateral involvement is common, although it is usually more severe in one hand. The ring finger is the digit most frequently involved, followed by the little finger, middle finger, and index finger. The thumb is rarely affected.3
As the disease progresses and cords contract, the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints develop flexion contractures. The distal interphalangeal (DIP) joint, rarely involved, instead exhibits a hyperextension contracture. Digital flexion contractures are often disabling, interfering with daily activities such as picking up a glass, shaking hands, or putting one's hand in a pants pocket. Many patients seek medical attention only after a palpable nodule, cord, or flexion contracture becomes apparent (FIGURE 1).
In a study of 326 patients who reported Dupuytren’s symptoms, the most common symptoms that led them to seek treatment were, in descending order, a hard bump (48%), a ropelike growth (12%), dimpling (11%), and finger contractures (10%). Only 9% of patients seeking treatment for hand symptoms associated with DD had received a diagnosis of DD from their initial medical encounter, causing an unnecessary delay in treatment.1
Who is at increased risk for DD?
DD is most often seen in elderly white men of European descent.5 In the United States, the prevalence of the disease is roughly 5%,1 compared with 4% to 39% in northwestern Europe (eg, Iceland).6 The male-to-female ratio of DD ranges from 6:1 to 15:1.7 The prevalence of DD appears to increase with age. The prevalence in men and women is similar up to age 45 years, after which the rate is much greater in males.7
DD is associated with many risk factors including smoking, alcohol consumption, vascular insufficiency, epilepsy, hyperlipidemia, manual labor, occupations with exposure to vibration, hand trauma, and even hand surgery such as carpal tunnel release or trigger finger release.8-11 It is also associated with diabetes; particularly type 1 insulin-dependent diabetes.12 There may also be an association with frozen shoulder.12,13
The need for surgical treatment of DD becomes more likely with a history of cigarette smoking and heavy alcohol consumption. There seems to be an association with epilepsy, most likely from anti-epileptic drugs.14 Rheumatoid arthritis is the only condition that has been associated with a lower incidence of DD, possibly because of the use of anti-inflammatory drugs.15 There are genetic differences between patients with and without DD, although a “Dupuytren’s gene” has not been identified.
Rule out possible DD mimics
The differential diagnosis for flexion contracture of the MCP or PIP joints seen in Dupuytren’s disease includes stenosing flexor tenosynovitis, or trigger finger. Other conditions that can mimic DD are ulnar claw hand, trauma scars, intrinsic joint diseases such as degenerative or rheumatoid arthritis (RA), diabetic cheiroarthropathy, camptodactyly, and Volkmann’s contracture. Trauma scars—especially longitudinal scars—have a tendency to contract and develop keloid formation.
Stenosing flexor tenosynovitis and ulnar claw hand are distinguishable from DD by full or nearly full active or passive extension of the affected digit, whereas DD is a true contracture of the joint that does not allow full extension. A careful history can rule out previous injury to the area. Although intrinsic joint disease, such as RA, can cause finger contractures, the joints are usually enlarged, painful, and associated with characteristic radiologic findings.
Unlike DD, diabetic cheiroarthropathy often involves all of the digits except for the thumb, and is often associated with a waxy appearance of the skin. Camptodactyly is an autosomal dominant disorder that more often presents in childhood and can be caused by a number of congenital syndromes. Volkmann’s contracture can manifest as a claw-like deformity of the hand caused by undiagnosed compartment syndrome of the forearm.
Assess the severity of DD
In your evaluation, first identify palmar or digital fibromatosis presenting as a nodule or a cord. Second, estimate the degree of MCP and PIP joint contractures. A common measure of contracture is the Hueston tabletop test. Ask the patient to place the palm of the hand on a flat surface. If the patient is unable to completely flatten the hand against the surface, presume a positive result (FIGURE 2).16
An accurate measure of the degree of flexion can be accomplished with a goniometer. For a simple assessment of severity, have the patient place each affected finger along the convexity of a spoon. If adjoining surfaces are flush, assume that the contracture is at least 30 degrees (FIGURE 3). The severity of DD can also be graded according to the Tubiana classification system (TABLE17), wherein the total deformity or contracture score is the sum of the angles of all 3 digital joints of the finger.
A look at the treatment options
Once the diagnosis of DD is made, reassure the patient that the disease is not cancerous, although it may be progressive. Advise patients, too, that several treatment options (detailed below) are available, but that surgery is the mainstay of treatment. (Of note: Despite what would seem logical, stretching a cord to straighten the finger is not recommended, as it may actually worsen the condition.18)
Options for early DD. Corticosteroids such as triamcinolone may have a role as an adjunct for early DD by reducing the size and firmness of the Dupuytren’s nodules and possibly slowing the progression of the disease. However, in one study of 63 patients, half of the individuals experienced reactivation of disease between one and 3 years.19
Radiotherapy has been studied as a potential treatment for early DD and for patients unable to undergo surgery. Radiation does have a biologic effect on nodular DD, but has no effect on the cords that cause the joint contracture. In a long-term follow-up study of 135 patients treated with radiotherapy, disease remained stable in 59% of patients, improved in 10%, and progressed in 31%.20 Results were better for early stage disease. This modality is not used for more advanced cases.
Surgery is the primary method by which to restore function and minimize complications. In the past, referral was recommended for MCP joint contractures >30 degrees or for any involvment of the PIP joint, because involvement of the latter carries a worse prognosis and may not be fully correctable, even with surgery.21 However, treatment may also be warranted in cases of functional disability—regardless of the degree of contracture (eg, for pain associated with a prominent nodule or cord when gripping objects).
Depending on disease progression and the surgeon’s preference, the most popular procedures are fasciotomies and fasciectomies. Surgical options for contractures—in increasing order of invasiveness and amounts of fascia removed—are fasciotomy (needle, open), fasciectomy (partial, radical), and dermofasciectomy.
Needle fasciotomy is an outpatient procedure performed under local block. The surgeon uses a needle bevel to transect the diseased cord at multiple puncture sites.
Open fasciotomy is also an outpatient procedure involving an incision that allows direct visualization of the cord and internal structures before dividing the fascia. Percutaneous and open fasciotomies are more successful for MCP joint contractures and less successful for PIP joint contractures.
With partial fasciectomy, only the grossly affected fascia is removed.
With radical fasciectomy, the entire palmar fascia is removed, regardless of which areas are grossly diseased.
Dermofasciectomy removes diseased fascia and the overlying skin, with a skin graft applied to heal the wound.
Another option: Collagenase injection. In February 2010, the US Food and Drug Administration approved collagenase clostridium histolyticum (Xiaflex) as the first drug specifically indicated for the treatment of Dupuytren’s contracture. Collagenase is injected directly into the fascial cord, cleaving its collagen component and progressively softening and breaking down the cord. If not properly injected, collagenase may injure neurovascular structures and may rupture tendons.
So how do the treatment options compare?
We found no randomized prospective trials comparing treatment options for DD, but there are a number of trials that shed light on a variety of the available options.
Treatment success. In general, partial fasciectomies have shown the greatest success in reducing contractures and maintaining the lowest recurrence rates. Correction of contracture to ≤5 degrees was seen in 94% of MCP joint contractures treated with partial fasciectomy, in 77% of MCP joints treated with collagenase, and in 55% of MCP joints treated with percutaneous needle fasciotomies.22,23 PIP joint contracture correction was not as successful: Correction of contracture to ≤5 degrees was seen in 47% of those treated with partial fasciectomy, 40% treated with collagenase, and 26% treated with needle fasciotomy.22,23
Recurrence rates. When recurrence was defined as loss of passive extension >30 degrees, the recurrence rate for MCP joint contractures with partial fasciectomy was 21%, compared with 85% for needle fasciotomy.22 In a similar review, recurrence rates for partial fasciectomy ranged from 12% to 39% compared with 50% to 58% for needle fasciotomy.24 With collagenase injection, 5-year data have shown an overall recurrence rate of 32%, with a recurrence of 26% at the MCP joint and 46% at the PIP joint.25 In this trial, recurrence was
Complications. Although fasciectomies have shown the best efficacy and lowest recurrence rates, complications such as infection, neurapraxia, and digital nerve injury are more likely with these procedures.26,27
Early recognition and referral to a hand specialist for the treatment of DD may allow the use of less invasive techniques and improved functional results.
CASE › Absent a history of trauma and features typical of other hand/digit disorders, we diagnosed Dupuytren’s disease in this patient. Potential treatments included partial fasciectomy and collagenase injection. After discussing the risks and benefits of each of these procedures, the patient elected to undergo a partial fasciectomy. The surgery was uncomplicated and the abnormality was corrected to within 5 degrees of full extension. At the patient’s one-year follow-up visit, there was no evidence of recurrence.
1. DiBenedetti DB, Nguyen D, Zografos L, et al. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand. 2011;6:149-158.
2. Townley WA, Baker R, Sheppard N, et al. Dupuytren’s contracture unfolded. BMJ. 2006;332:397-400.
3. Rayan GM. Clinical presentation and types of Dupuytren’s disease. Hand Clin. 1999;15:87-96.
4. Hindocha S, Stanley JK, Watson S, et al. Dupuytren’s diathesis revisited: Evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am. 2006;31:1626-1634.
5. Bayat A, McGrouther DA. Management of Dupuytren’s disease—clear advice for an elusive condition. Ann R Coll Surg Engl. 2006;88:3-8.
6. Gudmundsson KG, Arngrimsson R, Sigfússon M, et al. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;53:291-296.
7. Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren’s contracture in men and women. J Hand Surg Br. 1999;24:456-459.
8. Noble J, Arafa M, Royle SG, et al. The association between alcohol, hepatic pathology and Dupuytren’s disease. J Hand Surg Br. 1992;17:71–74.
9. Mikkelsen OA. Dupuytren’s disease—initial symptoms, age of onset and spontaneous course. Hand. 1977;9:11-15.
10. Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol. 1997;24:153-159.
11. Liss GM, Stock SR. Can Dupuytren’s contracture be work related? review of the evidence. Am J Ind Med. 1996;29:521-532.
12. Geoghegan JM, Forbes J, Clark DI, et al. Dupuytren’s disease risk factors. J Hand Surg Br. 2004;29:423-426.
13. Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg. 2001;10:149-151.
14. Nunn AC, Schreuder FB. Dupuytren’s contracture: emerging insight into a Viking disease. J Hand Surg. 2014;19:481-490.
15. Arafa M, Steingold R F, Noble J. The incidence of Dupuytren’s disease in patients with rheumatoid arthritis. J Hand Surg Br. 1984;9:165-166.
16. Hueston J. Lessons in Dupuytren’s disease. Ann Chir Main Memb Super. 1992;11:349-354.
17. Tubiana R. Prognosis and treatment of Dupuytren’s contracture. J Bone and Joint Surg AM. 1955;37:1155-1168.
18. Howard JC, Varallo VM, Ross DC, et al. Elevated levels of ß-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren’s disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16.
19. Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000;25:1157-1162.
20. Betz N, Ott OJ, Adamietz B, et al. Radiotherapy in early-stage Dupuytren’s contracture. Long-term results after 13 years. Strahlenther Onkol. 2010;186:82-90.
21. Smith AC. Diagnosis and indications for surgical treatment. Hand Clin. 1991;7:635-642.
22. van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469-477.
23. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968-979.
24. Chen NC, Srinivasan RC, Shauver MJ, et al. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand. 2011;6:250-255.
25. Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-year data. J Hand Surg Am. 2015;40:1597-1605.
26. Toppi JT, Trompf L, Smoll NR, et al. Dupuytren’s contracture: an analysis of outcomes of percutaneous needle fasciotomy versus open fasciectomy. ANZ J Surg. 2015;85:639-643.
27. Cheung K, Walley KC, Rozental TD. Management of complications of Dupuytren contracture. Hand Clini. 2015;31:345-354.
1. DiBenedetti DB, Nguyen D, Zografos L, et al. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand. 2011;6:149-158.
2. Townley WA, Baker R, Sheppard N, et al. Dupuytren’s contracture unfolded. BMJ. 2006;332:397-400.
3. Rayan GM. Clinical presentation and types of Dupuytren’s disease. Hand Clin. 1999;15:87-96.
4. Hindocha S, Stanley JK, Watson S, et al. Dupuytren’s diathesis revisited: Evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am. 2006;31:1626-1634.
5. Bayat A, McGrouther DA. Management of Dupuytren’s disease—clear advice for an elusive condition. Ann R Coll Surg Engl. 2006;88:3-8.
6. Gudmundsson KG, Arngrimsson R, Sigfússon M, et al. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;53:291-296.
7. Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren’s contracture in men and women. J Hand Surg Br. 1999;24:456-459.
8. Noble J, Arafa M, Royle SG, et al. The association between alcohol, hepatic pathology and Dupuytren’s disease. J Hand Surg Br. 1992;17:71–74.
9. Mikkelsen OA. Dupuytren’s disease—initial symptoms, age of onset and spontaneous course. Hand. 1977;9:11-15.
10. Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol. 1997;24:153-159.
11. Liss GM, Stock SR. Can Dupuytren’s contracture be work related? review of the evidence. Am J Ind Med. 1996;29:521-532.
12. Geoghegan JM, Forbes J, Clark DI, et al. Dupuytren’s disease risk factors. J Hand Surg Br. 2004;29:423-426.
13. Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg. 2001;10:149-151.
14. Nunn AC, Schreuder FB. Dupuytren’s contracture: emerging insight into a Viking disease. J Hand Surg. 2014;19:481-490.
15. Arafa M, Steingold R F, Noble J. The incidence of Dupuytren’s disease in patients with rheumatoid arthritis. J Hand Surg Br. 1984;9:165-166.
16. Hueston J. Lessons in Dupuytren’s disease. Ann Chir Main Memb Super. 1992;11:349-354.
17. Tubiana R. Prognosis and treatment of Dupuytren’s contracture. J Bone and Joint Surg AM. 1955;37:1155-1168.
18. Howard JC, Varallo VM, Ross DC, et al. Elevated levels of ß-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren’s disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16.
19. Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000;25:1157-1162.
20. Betz N, Ott OJ, Adamietz B, et al. Radiotherapy in early-stage Dupuytren’s contracture. Long-term results after 13 years. Strahlenther Onkol. 2010;186:82-90.
21. Smith AC. Diagnosis and indications for surgical treatment. Hand Clin. 1991;7:635-642.
22. van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469-477.
23. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968-979.
24. Chen NC, Srinivasan RC, Shauver MJ, et al. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand. 2011;6:250-255.
25. Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-year data. J Hand Surg Am. 2015;40:1597-1605.
26. Toppi JT, Trompf L, Smoll NR, et al. Dupuytren’s contracture: an analysis of outcomes of percutaneous needle fasciotomy versus open fasciectomy. ANZ J Surg. 2015;85:639-643.
27. Cheung K, Walley KC, Rozental TD. Management of complications of Dupuytren contracture. Hand Clini. 2015;31:345-354.
PRACTICE RECOMMENDATIONS
› Evaluate patients with suspected Dupuytren’s disease (DD) for coexisting conditions such as Ledderhose disease and Peyronie’s disease. C
› Use the Hueston tabletop test to assess severity of DD. C
› Do not recommend stretching exercises for DD; they can hasten disease progression. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic pain: How to approach these 3 common conditions
CASE 1 › Lola A is a 28-year-old woman with a history of muscular aches and joint pain throughout her body, fatigue, and mental fogginess. She has been seen by a rheumatologist and has been given a diagnosis of fibromyalgia, but just moved to your town and is establishing care. She is feeling desperate because her pain has worsened and the medication previously prescribed (gabapentin 300 mg tid) is no longer working. She asks to try oxycodone.
CASE 2 › Matt P is a 59-year-old truck driver with severe hip osteoarthritis (OA). His orthopedist recommended against hip replacement at this time because of his young age and a heart condition that makes him high risk. His pain makes sitting for long periods very difficult. He presents to you for help because he is worried he will be unable to continue working.
CASE 3 › Keith B is a 56-year-old construction worker who has been suffering from bouts of back pain for many years. The pain has become more debilitating over time; currently, it is constant, and Mr. B can hardly make it through his work day. He has been getting hydrocodone/acetaminophen from urgent care centers and emergency rooms, but he isn’t sure it is helping and is coming to you to assume his pain management.
Chronic pain (defined as pain >3 months in duration), is a complex, heterogeneous condition affecting an estimated 116 million US adults.1 Much of the management of chronic pain occurs in primary care settings, placing family physicians (FPs) on the front lines of 2 epidemics: that of chronic pain itself and that of the abuse and misuse of opioid pain medications.
In an effort to improve communication about the risks and benefits of opioid therapy and the safety and effectiveness of pain treatments in general, many professional organizations, health care institutions, and recently the Centers for Disease Control and Prevention,2 have published guidelines on the use of opioids for non-malignant chronic pain. With these guidelines in mind—and in light of the latest evidence—we propose the paradigm that follows for the treatment of chronic pain. A critical aspect of this paradigm is determining the pathophysiology underlying a patient’s pain in order to develop a well-rounded, multimodal, evidence-based treatment plan. Detailed here is the application of this approach to the treatment of 3 common chronic pain diagnoses: fibromyalgia, osteoarthritis, and low back pain.
Look to the central and peripheral nervous system
Acute pain begins with activation of peripheral nociceptors at the site of injury. This causes depolarization up the spinal cord and through the brain stem to higher cortical centers where the pain is perceived and localized. Descending neural pathways transport both excitatory and inhibitory information from the brain to the periphery via the spinal cord, which either increases or decreases the perception of pain.3
When damage/injury doesn’t correlate with the perception of pain
Until recently, it was assumed that chronic pain worked much the same way as acute pain and was caused by ongoing nociceptive input in the periphery, but research has shown us that the central nervous system can play a large role in the modulation of nociception. This new understanding comes from the lack of evidence pointing to any pain state in which the degree of nociceptive input correlates with the degree of pain experienced.
For most patients with chronic pain, regardless of their diagnosis, there is some degree of alteration in the processing of nociceptive signals by the central nervous system contributing to the experience of pain.4 This alteration is thought to be the result of peripheral nociceptive signaling persisting past the point of tissue healing, leading to a hypersensitivity of nerve fibers; the fibers then continue to respond to low, or even absent, sensory stimuli.
Central sensitization is the term used when this hypersensitivity develops in the superficial, deep, and ventral cord nerves. When this happens, pain is often accompanied by other systemic symptoms such as fatigue and slowed cognitive processing, often in the setting of little to no actual stimulation of the peripheral nociceptors.3 (For more on this, see “A new paradigm for pain?” J Fam Pract. 2016;65:598-605 or go to http://www.mdedge.com/jfponline/article/111257/pain/new-paradigm-pain.)
TABLE 14 lists the possible mechanisms of pain, which can be broken down into 4 categories: peripheral nociceptive (inflammatory or mechanical), peripheral neuropathic (underlying damage to a peripheral nerve), central (referring to when the central nervous system is the primary entity involved in maintaining the pain), or any combination of the 3.
As pain becomes chronic, multiple mechanisms overlap
It is important to remember that for any single pain diagnosis, there is likely to be—at least initially—a principle underlying mechanism generating the pain. But as the pain becomes chronic, an overlap of multiple mechanisms develops, with central sensitization often playing a more dominant role than peripheral stimulation (regardless of the diagnosis).
For example, in a patient with rheumatoid arthritis (RA), peripheral nociceptive input (in the form of inflammation) is likely the initial mechanism at work, but as time goes on, central processing becomes more involved. The patient may then begin to experience pain that is disproportional to what is generally expected with RA and may develop other somatic symptoms. The diagnosis then becomes pain primarily related to RA with central sensitization, and both need to be addressed in a treatment plan. In rheumatic conditions, comorbid fibromyalgia (indicative of central sensitization) is thought to occur in 15% to 30% of patients.5
FPs can utilize the underlying mechanisms to cut across diagnostic labels and tailor treatments to those that are most likely to be effective. For a patient with more prominent peripheral involvement, a procedural intervention such as injections or surgery alone may suffice, whereas a broader approach including psychotherapy, medications, exercise, and other lifestyle interventions may be necessary for a patient with pain caused predominantly by central sensitization.
Addressing both peripheral and central components is essential. One prospective, observational cohort study of more than 600 patients scheduled for unilateral total knee or total hip arthroplasty found that those patients with a higher degree of centralization of pain (as measured by widespread pain index and modified fibromyalgia screening scales6) were less likely to report improvement in the affected body part and in overall body pain following the surgery.7
There is a high degree of overlap among many of the chronic pain syndromes (fibromyalgia, irritable bowel syndrome, interstitial cystitis, chronic headaches) that have been found to have a central sensitization component.8 Providers of primary care are aptly positioned to recognize central sensitization as the underlying pathology and target treatment effectively.
Tailor the treatment plan to the underlying mechanisms of pain
As with any chronic condition, a thorough work-up (complete with history, physical exam, and diagnostic testing, as appropriate) is indicated. In the setting of chronic pain, it’s important to identify both the primary mechanism, as well as secondary factors that may be contributing to the patient’s pain, before developing your treatment plan. These secondary factors may include co-occurring affect disorders,9 a history of trauma,10 poor sleep,11 and tobacco use,12 among others. A history of trauma, for example, co-exists with many pain syndromes. For these patients, central sensitization is responsible for much of their pain. As a result, traditional cognitive behavioral therapy (CBT) may not be the best option because of its focus on accepting pain as a chronic diagnosis; more trauma-focused treatments such as those dealing in emotional awareness and understanding of the central nervous system’s role in chronic pain need to be considered.13
3 common conditions. Below we present evidence-based treatment approaches for 3 conditions that are typically associated with each of the major mechanisms of chronic pain generation: fibromyalgia (a central sensitization cause), OA (a peripheral nociceptive cause), and low back pain (a mixed pain state).
Fibromyalgia: A case of central sensitization
Fibromyalgia is a hallmark diagnosis for those patients in whom central sensitization is the dominant cause of pain. These patients usually present with widespread, diffuse pain, as well as somatic symptoms such as fatigue, memory difficulties, and poor sleep quality.8 When explaining the pain mechanism (ie, central sensitization) to patients, it may be useful to use the analogy of a volume control dial that is stuck in the “high” position and can’t be turned down.
Genes, the environment, and neurotransmitters play a role. The origin of the pain amplification process is believed to be multifactorial.
- Genetic factors are thought to contribute to a predisposition for amplification. To date, 5 sets of genes have been implicated in increased sensitivity to pain leading to increased risk of the development of chronic pain during a patient’s lifetime.14-19
- Environmental factors (eg, early life trauma, physical trauma especially to the trunk, certain infections such as Lyme disease and Epstein-Barr virus, and emotional stress) may trigger or exacerbate symptoms.8 Of note: Only about 5% to 10% of people who experience these triggers actually develop a chronic pain state, while the rest regain their baseline health.4 This raises the question of whether there is a point during an acute pain episode in which one can intervene and prevent the acute pain from becoming chronic in those at higher risk.4
- Imbalances of neurotransmitters (high glutamate;20 low norepinephrine, serotonin,21 and gamma-aminobutyric acid [GABA]22) play a role in central amplification. These substances not only affect sensory transmission, but also control levels of alertness, sleep, mood, and memory.
The diagnostic criteria for fibromyalgia were modified in 2011 to remove the tender point examination and to add in somatic symptoms.6 These criteria can be useful in the clinical setting in identifying not only fibromyalgia itself but also the degree of “fibromyalgianess” a patient has, which is an indicator of how large a role the centralization process plays in the maintenance of chronic pain.23,24
Treatment: Multimodal and patient empowering. Evidence-based treatment options for fibromyalgia, as well as other conditions for which there is a high degree of centralized pain, can be found in TABLE 2.25-36 Multimodal treatment, with an emphasis on patient knowledge and empowerment, is generally thought to be the most beneficial.25,37 Treatment should almost always include CBT and exercise/activity therapies,26,29 which have high degrees of efficacy with few adverse effects.
In terms of medication, centrally-acting agents (tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors [SNRIs], and alpha 2 delta ligands) are the most effective. There is little to no data showing benefit from anti-inflammatories or opioids in the setting of fibromyalgia. There is some data to suggest that combination therapy, for example with an SNRI (milnacipran) and an alpha 2 delta ligand (pregabalin), may provide more benefit than treating with pregabalin alone.38
Complementary and alternative therapies (eg, yoga, chiropractic care, acupuncture, massage) are being studied more, and while evidence is only preliminary in terms of efficacy, there is increasing emphasis being placed on the need for patients with chronic pain to shift their treatment expectations to greater acceptance of pain and the need for ongoing self-care.28 (For more advice on managing fibromyalgia, see the related videos at http://bit.ly/2lPEt0f and http://bit.ly/2lmjEcn.)
Osteoarthritis: An example of peripheral nociceptive pain
OA is a condition long thought to be characterized by damage to the cartilage and bone; however, as with many other pain diagnoses, there is frequently little correlation between damage seen on radiographs and the amount of pain that patients experience.
One study analyzed data on almost 7000 patients from the National Health and Nutrition Examination Survey (NHANES I) and found that between 30% and 50% of OA patients with moderate to severe radiographic changes were asymptomatic, and 10% of those with moderate to severe pain had normal radiographs or only mild changes.39 Research is showing that many factors may contribute to this discrepancy, including the typical “wear and tear” of the disease, subacute levels of inflammation that can lead to peripheral sensitization,40 and, in some patients, a centralized pain component. The patients with more centralized pain often have pain that is disproportionate to radiographic evidence, as well as more somatic symptoms such as fatigue, sleep disturbance, and memory issues.41
Treatment should be multimodal and include interventions targeted at halting the progression of damage as well as palliation of pain. All treatment plans for OA should also include exercise, weight reduction, and self-management, in addition to pharmacologic interventions, to reduce both the micro-inflammation and the centralized pain component (when present). Intra-articular injections of various types have been studied with some having more efficacy in pain reduction and functional improvement than others.42-45 See TABLE 342-61 for a summary of evidence-based treatment options.
Low back pain—a mixed pain state
Low back pain (LBP) has been recognized as a mixed pain state for quite some time. While some patients may experience purely nociceptive and/or neuropathic pain, most cases are nonspecific with patients experiencing varying degrees of nociceptive (myofascial low back pain), neuropathic (lumbar radiculopathy), and central sensitization pain.62,63 Evidence for centralized pain is demonstrated in studies showing hyperalgesia,64 augmented central pain processing,65 involvement of the emotional brain,66 and delayed recovery influenced by poor coping strategies.67
When developing a treatment plan for a patient with chronic low back pain, remember that the pain derives from a complex combination of pathophysiologic contributors. Identifying where a patient lies on the pain centralization spectrum can help you tailor treatment.
In one study of 548 patients presenting to a tertiary pain clinic with primary spine pain diagnoses, 42% met diagnostic criteria for fibromyalgia.68 Compared to criteria-negative patients, these patients tended to be younger, unemployed, and receiving compensation; they had greater pain intensity, pain interference, and used stronger words to describe their neuropathic pain; they also had higher levels of depression/anxiety and a lower level of physical function.
Because low back pain is a condition with high prevalence and associated disability, many clinical boards have created guidelines for management. These guidelines tend to vary in the strength of evidence used, and the extent to which they are followed in clinical practice remains largely unknown. Recommendations frequently discourage the use of ultrasound/electrotherapy, but many encourage short-term use of medications (see “How effective are opioids for chronic low back pain?” J Fam Pract. 2015;64:584-584), supervised exercise therapy, CBT, and multidisciplinary treatment.
Guidelines tend to differ most widely with regard to recommendations for spinal manipulation and specific drug therapies.69 The classes of drugs that may be most useful when centralized pain is present include the SNRIs and the alpha 2 delta calcium channel ligands.4 See TABLE 470-89 for a summary of evidence-based treatment options.
CASE 1 › Ms. A is started on amitriptyline 25 mg at bedtime, which improves her fatigue and cognitive symptoms. During monthly office visits, her FP educates her about the pathophysiology of fibromyalgia and uses motivational interviewing to get her slowly moving and increasing her activity level. She is weaned off the gabapentin previously prescribed, as her symptoms stabilize and improve.
CASE 2 › Mr. P is sent for a steroid injection, which decreases his pain temporarily. During this time, he begins physical therapy; slowly, with increased movement, his function improves. A trial of duloxetine provides pain relief; that combined with intermittent nonsteroidal anti-inflammatory drugs (NSAIDs) has allowed Mr. P to maintain his function and his job.
CASE 3 › Because Mr. B was only taking the narcotics intermittently and wasn’t certain they were helping, CBT was sufficient to wean Mr. B off the medication without any worsening of his pain in the process. By participating in physical therapy, he has learned how to perform certain tasks at his job without pain or injury. He uses NSAIDs as needed for pain.
CORRESPONDENCE
Jill Schneiderhan, MD, 24 Frank Lloyd Wright Dr., Lobby H, Suite 2300, Ann Arbor, MI 48105; [email protected].
ACKNOWLEDGEMENTS
We thank Drs. Daniel Clauw (University of Michigan, Ann Arbor) and Martha Rumschlag (Providence Family Medicine Residency Program, Southfield, Mich), for their valuable contributions to this article.
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US); 2011.
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
3. Aronoff GM. What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am. 2016;100:31-42.
4. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6-19.
5. Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335-342.
6. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113-1122.
7. Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386-1394.
8. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233-251.
9. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
10. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157:1799-1809.
11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539-1552.
12. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977-992.
13. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
14. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240-1243.
15. van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628-629.
16. McLean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101-107.
17. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519-2527.
18. Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269-1277.
19. Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852-12860.
20. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies abberrant brain chemistry, connectivity, and functional responses in chronic pain patients. Anesthesiology. 2013;119:1453-1464.
21. Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550-556.
22. Foerster BR, Petrou M, Edden RAE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579-583.
23. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
26. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
27. Porter NS, Jason LA, Boulton A, et al. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010;16:235-249.
28. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12.
29. Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991-2005.
30. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104-113.
31. Moldofsky H, Harris HW, Archambault WT, et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653-2663.
32. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;Sep 8 Suppl 2:S63-S74.
33. Häuser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69-81.
34. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;Jun 23:CD010692.
35. MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15:469-475.
36. Younger J, Noor N, McCue R, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529-538.
37. Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337-344.
38. Mease PJ, Farmer MV, Palmer RH, et al. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskeletal Dis. 2013;5:113-126.
39. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
40. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890-1896.
41. Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043-1056.
42. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
43. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328.
44. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505.
45. Wu T, Song HX, Dong Y, et al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil. 2016.
46. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956.
48. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:CD007912.
49. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.
50. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105.
51. Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:76.
52. Berthelot JM, Darrieutort-Lafitte C, Le Goff B, et al. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397-401.
53. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
54. Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
55. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
56. Wu D, Huang Y, Gu Y, et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013;67:585-594.
57. Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
58. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156-165.
59. Clinton CM, O’Brien S, Law J, et al. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152.
60. Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312.
61. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
62. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333-E346.
63. Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15:4-15.
64. Hübscher M, Moloney N, Rebbeck T, et al. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain. 2014;30:886-893.
65. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
66. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165-12173.
68. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285-3292.
69. Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075-2094.
70. Oliveira VC, Ferreira PH, Maher CG, et al. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res. 2012;64:1739-1748.
71. Engers A, Jellema P, Wensing M, et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008:CD004057.
72. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335.
73. French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006:CD004750.
74. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskeletal Disord. 2014;15:286.
75. Franke H, Fryer G, Ostelo RW, et al. Muscle energy technique for non-specific low back pain. Cochrane Database Syst Rev. 2015:CD009852.
76. Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004:197-210.
77. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015:CD001929.
78. Khadilkar A, Odebiyi DO, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low back pain. Cochrane Database Syst Rev. 2008:CD003008.
79. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014:CD009169.
80. Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005:CD001351.
81. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
82. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
83. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
84. Staal JB, de Bie R, de Vet HC, et al. Injection therapy for subacute and chronic low back pain. Cochrane Database Syst Rev. 2008:CD001824.
85. Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34:1094-1109.
86. Felson D. Paracetamol is ineffective for spinal pain and knee and hip osteoarthritis. Evid Based Med. 2015;20:205.
87. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
88. Enthoven WT, Roelofs PD, Deyo RA, et al. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087.
89. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143.
CASE 1 › Lola A is a 28-year-old woman with a history of muscular aches and joint pain throughout her body, fatigue, and mental fogginess. She has been seen by a rheumatologist and has been given a diagnosis of fibromyalgia, but just moved to your town and is establishing care. She is feeling desperate because her pain has worsened and the medication previously prescribed (gabapentin 300 mg tid) is no longer working. She asks to try oxycodone.
CASE 2 › Matt P is a 59-year-old truck driver with severe hip osteoarthritis (OA). His orthopedist recommended against hip replacement at this time because of his young age and a heart condition that makes him high risk. His pain makes sitting for long periods very difficult. He presents to you for help because he is worried he will be unable to continue working.
CASE 3 › Keith B is a 56-year-old construction worker who has been suffering from bouts of back pain for many years. The pain has become more debilitating over time; currently, it is constant, and Mr. B can hardly make it through his work day. He has been getting hydrocodone/acetaminophen from urgent care centers and emergency rooms, but he isn’t sure it is helping and is coming to you to assume his pain management.
Chronic pain (defined as pain >3 months in duration), is a complex, heterogeneous condition affecting an estimated 116 million US adults.1 Much of the management of chronic pain occurs in primary care settings, placing family physicians (FPs) on the front lines of 2 epidemics: that of chronic pain itself and that of the abuse and misuse of opioid pain medications.
In an effort to improve communication about the risks and benefits of opioid therapy and the safety and effectiveness of pain treatments in general, many professional organizations, health care institutions, and recently the Centers for Disease Control and Prevention,2 have published guidelines on the use of opioids for non-malignant chronic pain. With these guidelines in mind—and in light of the latest evidence—we propose the paradigm that follows for the treatment of chronic pain. A critical aspect of this paradigm is determining the pathophysiology underlying a patient’s pain in order to develop a well-rounded, multimodal, evidence-based treatment plan. Detailed here is the application of this approach to the treatment of 3 common chronic pain diagnoses: fibromyalgia, osteoarthritis, and low back pain.
Look to the central and peripheral nervous system
Acute pain begins with activation of peripheral nociceptors at the site of injury. This causes depolarization up the spinal cord and through the brain stem to higher cortical centers where the pain is perceived and localized. Descending neural pathways transport both excitatory and inhibitory information from the brain to the periphery via the spinal cord, which either increases or decreases the perception of pain.3
When damage/injury doesn’t correlate with the perception of pain
Until recently, it was assumed that chronic pain worked much the same way as acute pain and was caused by ongoing nociceptive input in the periphery, but research has shown us that the central nervous system can play a large role in the modulation of nociception. This new understanding comes from the lack of evidence pointing to any pain state in which the degree of nociceptive input correlates with the degree of pain experienced.
For most patients with chronic pain, regardless of their diagnosis, there is some degree of alteration in the processing of nociceptive signals by the central nervous system contributing to the experience of pain.4 This alteration is thought to be the result of peripheral nociceptive signaling persisting past the point of tissue healing, leading to a hypersensitivity of nerve fibers; the fibers then continue to respond to low, or even absent, sensory stimuli.
Central sensitization is the term used when this hypersensitivity develops in the superficial, deep, and ventral cord nerves. When this happens, pain is often accompanied by other systemic symptoms such as fatigue and slowed cognitive processing, often in the setting of little to no actual stimulation of the peripheral nociceptors.3 (For more on this, see “A new paradigm for pain?” J Fam Pract. 2016;65:598-605 or go to http://www.mdedge.com/jfponline/article/111257/pain/new-paradigm-pain.)
TABLE 14 lists the possible mechanisms of pain, which can be broken down into 4 categories: peripheral nociceptive (inflammatory or mechanical), peripheral neuropathic (underlying damage to a peripheral nerve), central (referring to when the central nervous system is the primary entity involved in maintaining the pain), or any combination of the 3.
As pain becomes chronic, multiple mechanisms overlap
It is important to remember that for any single pain diagnosis, there is likely to be—at least initially—a principle underlying mechanism generating the pain. But as the pain becomes chronic, an overlap of multiple mechanisms develops, with central sensitization often playing a more dominant role than peripheral stimulation (regardless of the diagnosis).
For example, in a patient with rheumatoid arthritis (RA), peripheral nociceptive input (in the form of inflammation) is likely the initial mechanism at work, but as time goes on, central processing becomes more involved. The patient may then begin to experience pain that is disproportional to what is generally expected with RA and may develop other somatic symptoms. The diagnosis then becomes pain primarily related to RA with central sensitization, and both need to be addressed in a treatment plan. In rheumatic conditions, comorbid fibromyalgia (indicative of central sensitization) is thought to occur in 15% to 30% of patients.5
FPs can utilize the underlying mechanisms to cut across diagnostic labels and tailor treatments to those that are most likely to be effective. For a patient with more prominent peripheral involvement, a procedural intervention such as injections or surgery alone may suffice, whereas a broader approach including psychotherapy, medications, exercise, and other lifestyle interventions may be necessary for a patient with pain caused predominantly by central sensitization.
Addressing both peripheral and central components is essential. One prospective, observational cohort study of more than 600 patients scheduled for unilateral total knee or total hip arthroplasty found that those patients with a higher degree of centralization of pain (as measured by widespread pain index and modified fibromyalgia screening scales6) were less likely to report improvement in the affected body part and in overall body pain following the surgery.7
There is a high degree of overlap among many of the chronic pain syndromes (fibromyalgia, irritable bowel syndrome, interstitial cystitis, chronic headaches) that have been found to have a central sensitization component.8 Providers of primary care are aptly positioned to recognize central sensitization as the underlying pathology and target treatment effectively.
Tailor the treatment plan to the underlying mechanisms of pain
As with any chronic condition, a thorough work-up (complete with history, physical exam, and diagnostic testing, as appropriate) is indicated. In the setting of chronic pain, it’s important to identify both the primary mechanism, as well as secondary factors that may be contributing to the patient’s pain, before developing your treatment plan. These secondary factors may include co-occurring affect disorders,9 a history of trauma,10 poor sleep,11 and tobacco use,12 among others. A history of trauma, for example, co-exists with many pain syndromes. For these patients, central sensitization is responsible for much of their pain. As a result, traditional cognitive behavioral therapy (CBT) may not be the best option because of its focus on accepting pain as a chronic diagnosis; more trauma-focused treatments such as those dealing in emotional awareness and understanding of the central nervous system’s role in chronic pain need to be considered.13
3 common conditions. Below we present evidence-based treatment approaches for 3 conditions that are typically associated with each of the major mechanisms of chronic pain generation: fibromyalgia (a central sensitization cause), OA (a peripheral nociceptive cause), and low back pain (a mixed pain state).
Fibromyalgia: A case of central sensitization
Fibromyalgia is a hallmark diagnosis for those patients in whom central sensitization is the dominant cause of pain. These patients usually present with widespread, diffuse pain, as well as somatic symptoms such as fatigue, memory difficulties, and poor sleep quality.8 When explaining the pain mechanism (ie, central sensitization) to patients, it may be useful to use the analogy of a volume control dial that is stuck in the “high” position and can’t be turned down.
Genes, the environment, and neurotransmitters play a role. The origin of the pain amplification process is believed to be multifactorial.
- Genetic factors are thought to contribute to a predisposition for amplification. To date, 5 sets of genes have been implicated in increased sensitivity to pain leading to increased risk of the development of chronic pain during a patient’s lifetime.14-19
- Environmental factors (eg, early life trauma, physical trauma especially to the trunk, certain infections such as Lyme disease and Epstein-Barr virus, and emotional stress) may trigger or exacerbate symptoms.8 Of note: Only about 5% to 10% of people who experience these triggers actually develop a chronic pain state, while the rest regain their baseline health.4 This raises the question of whether there is a point during an acute pain episode in which one can intervene and prevent the acute pain from becoming chronic in those at higher risk.4
- Imbalances of neurotransmitters (high glutamate;20 low norepinephrine, serotonin,21 and gamma-aminobutyric acid [GABA]22) play a role in central amplification. These substances not only affect sensory transmission, but also control levels of alertness, sleep, mood, and memory.
The diagnostic criteria for fibromyalgia were modified in 2011 to remove the tender point examination and to add in somatic symptoms.6 These criteria can be useful in the clinical setting in identifying not only fibromyalgia itself but also the degree of “fibromyalgianess” a patient has, which is an indicator of how large a role the centralization process plays in the maintenance of chronic pain.23,24
Treatment: Multimodal and patient empowering. Evidence-based treatment options for fibromyalgia, as well as other conditions for which there is a high degree of centralized pain, can be found in TABLE 2.25-36 Multimodal treatment, with an emphasis on patient knowledge and empowerment, is generally thought to be the most beneficial.25,37 Treatment should almost always include CBT and exercise/activity therapies,26,29 which have high degrees of efficacy with few adverse effects.
In terms of medication, centrally-acting agents (tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors [SNRIs], and alpha 2 delta ligands) are the most effective. There is little to no data showing benefit from anti-inflammatories or opioids in the setting of fibromyalgia. There is some data to suggest that combination therapy, for example with an SNRI (milnacipran) and an alpha 2 delta ligand (pregabalin), may provide more benefit than treating with pregabalin alone.38
Complementary and alternative therapies (eg, yoga, chiropractic care, acupuncture, massage) are being studied more, and while evidence is only preliminary in terms of efficacy, there is increasing emphasis being placed on the need for patients with chronic pain to shift their treatment expectations to greater acceptance of pain and the need for ongoing self-care.28 (For more advice on managing fibromyalgia, see the related videos at http://bit.ly/2lPEt0f and http://bit.ly/2lmjEcn.)
Osteoarthritis: An example of peripheral nociceptive pain
OA is a condition long thought to be characterized by damage to the cartilage and bone; however, as with many other pain diagnoses, there is frequently little correlation between damage seen on radiographs and the amount of pain that patients experience.
One study analyzed data on almost 7000 patients from the National Health and Nutrition Examination Survey (NHANES I) and found that between 30% and 50% of OA patients with moderate to severe radiographic changes were asymptomatic, and 10% of those with moderate to severe pain had normal radiographs or only mild changes.39 Research is showing that many factors may contribute to this discrepancy, including the typical “wear and tear” of the disease, subacute levels of inflammation that can lead to peripheral sensitization,40 and, in some patients, a centralized pain component. The patients with more centralized pain often have pain that is disproportionate to radiographic evidence, as well as more somatic symptoms such as fatigue, sleep disturbance, and memory issues.41
Treatment should be multimodal and include interventions targeted at halting the progression of damage as well as palliation of pain. All treatment plans for OA should also include exercise, weight reduction, and self-management, in addition to pharmacologic interventions, to reduce both the micro-inflammation and the centralized pain component (when present). Intra-articular injections of various types have been studied with some having more efficacy in pain reduction and functional improvement than others.42-45 See TABLE 342-61 for a summary of evidence-based treatment options.
Low back pain—a mixed pain state
Low back pain (LBP) has been recognized as a mixed pain state for quite some time. While some patients may experience purely nociceptive and/or neuropathic pain, most cases are nonspecific with patients experiencing varying degrees of nociceptive (myofascial low back pain), neuropathic (lumbar radiculopathy), and central sensitization pain.62,63 Evidence for centralized pain is demonstrated in studies showing hyperalgesia,64 augmented central pain processing,65 involvement of the emotional brain,66 and delayed recovery influenced by poor coping strategies.67
When developing a treatment plan for a patient with chronic low back pain, remember that the pain derives from a complex combination of pathophysiologic contributors. Identifying where a patient lies on the pain centralization spectrum can help you tailor treatment.
In one study of 548 patients presenting to a tertiary pain clinic with primary spine pain diagnoses, 42% met diagnostic criteria for fibromyalgia.68 Compared to criteria-negative patients, these patients tended to be younger, unemployed, and receiving compensation; they had greater pain intensity, pain interference, and used stronger words to describe their neuropathic pain; they also had higher levels of depression/anxiety and a lower level of physical function.
Because low back pain is a condition with high prevalence and associated disability, many clinical boards have created guidelines for management. These guidelines tend to vary in the strength of evidence used, and the extent to which they are followed in clinical practice remains largely unknown. Recommendations frequently discourage the use of ultrasound/electrotherapy, but many encourage short-term use of medications (see “How effective are opioids for chronic low back pain?” J Fam Pract. 2015;64:584-584), supervised exercise therapy, CBT, and multidisciplinary treatment.
Guidelines tend to differ most widely with regard to recommendations for spinal manipulation and specific drug therapies.69 The classes of drugs that may be most useful when centralized pain is present include the SNRIs and the alpha 2 delta calcium channel ligands.4 See TABLE 470-89 for a summary of evidence-based treatment options.
CASE 1 › Ms. A is started on amitriptyline 25 mg at bedtime, which improves her fatigue and cognitive symptoms. During monthly office visits, her FP educates her about the pathophysiology of fibromyalgia and uses motivational interviewing to get her slowly moving and increasing her activity level. She is weaned off the gabapentin previously prescribed, as her symptoms stabilize and improve.
CASE 2 › Mr. P is sent for a steroid injection, which decreases his pain temporarily. During this time, he begins physical therapy; slowly, with increased movement, his function improves. A trial of duloxetine provides pain relief; that combined with intermittent nonsteroidal anti-inflammatory drugs (NSAIDs) has allowed Mr. P to maintain his function and his job.
CASE 3 › Because Mr. B was only taking the narcotics intermittently and wasn’t certain they were helping, CBT was sufficient to wean Mr. B off the medication without any worsening of his pain in the process. By participating in physical therapy, he has learned how to perform certain tasks at his job without pain or injury. He uses NSAIDs as needed for pain.
CORRESPONDENCE
Jill Schneiderhan, MD, 24 Frank Lloyd Wright Dr., Lobby H, Suite 2300, Ann Arbor, MI 48105; [email protected].
ACKNOWLEDGEMENTS
We thank Drs. Daniel Clauw (University of Michigan, Ann Arbor) and Martha Rumschlag (Providence Family Medicine Residency Program, Southfield, Mich), for their valuable contributions to this article.
CASE 1 › Lola A is a 28-year-old woman with a history of muscular aches and joint pain throughout her body, fatigue, and mental fogginess. She has been seen by a rheumatologist and has been given a diagnosis of fibromyalgia, but just moved to your town and is establishing care. She is feeling desperate because her pain has worsened and the medication previously prescribed (gabapentin 300 mg tid) is no longer working. She asks to try oxycodone.
CASE 2 › Matt P is a 59-year-old truck driver with severe hip osteoarthritis (OA). His orthopedist recommended against hip replacement at this time because of his young age and a heart condition that makes him high risk. His pain makes sitting for long periods very difficult. He presents to you for help because he is worried he will be unable to continue working.
CASE 3 › Keith B is a 56-year-old construction worker who has been suffering from bouts of back pain for many years. The pain has become more debilitating over time; currently, it is constant, and Mr. B can hardly make it through his work day. He has been getting hydrocodone/acetaminophen from urgent care centers and emergency rooms, but he isn’t sure it is helping and is coming to you to assume his pain management.
Chronic pain (defined as pain >3 months in duration), is a complex, heterogeneous condition affecting an estimated 116 million US adults.1 Much of the management of chronic pain occurs in primary care settings, placing family physicians (FPs) on the front lines of 2 epidemics: that of chronic pain itself and that of the abuse and misuse of opioid pain medications.
In an effort to improve communication about the risks and benefits of opioid therapy and the safety and effectiveness of pain treatments in general, many professional organizations, health care institutions, and recently the Centers for Disease Control and Prevention,2 have published guidelines on the use of opioids for non-malignant chronic pain. With these guidelines in mind—and in light of the latest evidence—we propose the paradigm that follows for the treatment of chronic pain. A critical aspect of this paradigm is determining the pathophysiology underlying a patient’s pain in order to develop a well-rounded, multimodal, evidence-based treatment plan. Detailed here is the application of this approach to the treatment of 3 common chronic pain diagnoses: fibromyalgia, osteoarthritis, and low back pain.
Look to the central and peripheral nervous system
Acute pain begins with activation of peripheral nociceptors at the site of injury. This causes depolarization up the spinal cord and through the brain stem to higher cortical centers where the pain is perceived and localized. Descending neural pathways transport both excitatory and inhibitory information from the brain to the periphery via the spinal cord, which either increases or decreases the perception of pain.3
When damage/injury doesn’t correlate with the perception of pain
Until recently, it was assumed that chronic pain worked much the same way as acute pain and was caused by ongoing nociceptive input in the periphery, but research has shown us that the central nervous system can play a large role in the modulation of nociception. This new understanding comes from the lack of evidence pointing to any pain state in which the degree of nociceptive input correlates with the degree of pain experienced.
For most patients with chronic pain, regardless of their diagnosis, there is some degree of alteration in the processing of nociceptive signals by the central nervous system contributing to the experience of pain.4 This alteration is thought to be the result of peripheral nociceptive signaling persisting past the point of tissue healing, leading to a hypersensitivity of nerve fibers; the fibers then continue to respond to low, or even absent, sensory stimuli.
Central sensitization is the term used when this hypersensitivity develops in the superficial, deep, and ventral cord nerves. When this happens, pain is often accompanied by other systemic symptoms such as fatigue and slowed cognitive processing, often in the setting of little to no actual stimulation of the peripheral nociceptors.3 (For more on this, see “A new paradigm for pain?” J Fam Pract. 2016;65:598-605 or go to http://www.mdedge.com/jfponline/article/111257/pain/new-paradigm-pain.)
TABLE 14 lists the possible mechanisms of pain, which can be broken down into 4 categories: peripheral nociceptive (inflammatory or mechanical), peripheral neuropathic (underlying damage to a peripheral nerve), central (referring to when the central nervous system is the primary entity involved in maintaining the pain), or any combination of the 3.
As pain becomes chronic, multiple mechanisms overlap
It is important to remember that for any single pain diagnosis, there is likely to be—at least initially—a principle underlying mechanism generating the pain. But as the pain becomes chronic, an overlap of multiple mechanisms develops, with central sensitization often playing a more dominant role than peripheral stimulation (regardless of the diagnosis).
For example, in a patient with rheumatoid arthritis (RA), peripheral nociceptive input (in the form of inflammation) is likely the initial mechanism at work, but as time goes on, central processing becomes more involved. The patient may then begin to experience pain that is disproportional to what is generally expected with RA and may develop other somatic symptoms. The diagnosis then becomes pain primarily related to RA with central sensitization, and both need to be addressed in a treatment plan. In rheumatic conditions, comorbid fibromyalgia (indicative of central sensitization) is thought to occur in 15% to 30% of patients.5
FPs can utilize the underlying mechanisms to cut across diagnostic labels and tailor treatments to those that are most likely to be effective. For a patient with more prominent peripheral involvement, a procedural intervention such as injections or surgery alone may suffice, whereas a broader approach including psychotherapy, medications, exercise, and other lifestyle interventions may be necessary for a patient with pain caused predominantly by central sensitization.
Addressing both peripheral and central components is essential. One prospective, observational cohort study of more than 600 patients scheduled for unilateral total knee or total hip arthroplasty found that those patients with a higher degree of centralization of pain (as measured by widespread pain index and modified fibromyalgia screening scales6) were less likely to report improvement in the affected body part and in overall body pain following the surgery.7
There is a high degree of overlap among many of the chronic pain syndromes (fibromyalgia, irritable bowel syndrome, interstitial cystitis, chronic headaches) that have been found to have a central sensitization component.8 Providers of primary care are aptly positioned to recognize central sensitization as the underlying pathology and target treatment effectively.
Tailor the treatment plan to the underlying mechanisms of pain
As with any chronic condition, a thorough work-up (complete with history, physical exam, and diagnostic testing, as appropriate) is indicated. In the setting of chronic pain, it’s important to identify both the primary mechanism, as well as secondary factors that may be contributing to the patient’s pain, before developing your treatment plan. These secondary factors may include co-occurring affect disorders,9 a history of trauma,10 poor sleep,11 and tobacco use,12 among others. A history of trauma, for example, co-exists with many pain syndromes. For these patients, central sensitization is responsible for much of their pain. As a result, traditional cognitive behavioral therapy (CBT) may not be the best option because of its focus on accepting pain as a chronic diagnosis; more trauma-focused treatments such as those dealing in emotional awareness and understanding of the central nervous system’s role in chronic pain need to be considered.13
3 common conditions. Below we present evidence-based treatment approaches for 3 conditions that are typically associated with each of the major mechanisms of chronic pain generation: fibromyalgia (a central sensitization cause), OA (a peripheral nociceptive cause), and low back pain (a mixed pain state).
Fibromyalgia: A case of central sensitization
Fibromyalgia is a hallmark diagnosis for those patients in whom central sensitization is the dominant cause of pain. These patients usually present with widespread, diffuse pain, as well as somatic symptoms such as fatigue, memory difficulties, and poor sleep quality.8 When explaining the pain mechanism (ie, central sensitization) to patients, it may be useful to use the analogy of a volume control dial that is stuck in the “high” position and can’t be turned down.
Genes, the environment, and neurotransmitters play a role. The origin of the pain amplification process is believed to be multifactorial.
- Genetic factors are thought to contribute to a predisposition for amplification. To date, 5 sets of genes have been implicated in increased sensitivity to pain leading to increased risk of the development of chronic pain during a patient’s lifetime.14-19
- Environmental factors (eg, early life trauma, physical trauma especially to the trunk, certain infections such as Lyme disease and Epstein-Barr virus, and emotional stress) may trigger or exacerbate symptoms.8 Of note: Only about 5% to 10% of people who experience these triggers actually develop a chronic pain state, while the rest regain their baseline health.4 This raises the question of whether there is a point during an acute pain episode in which one can intervene and prevent the acute pain from becoming chronic in those at higher risk.4
- Imbalances of neurotransmitters (high glutamate;20 low norepinephrine, serotonin,21 and gamma-aminobutyric acid [GABA]22) play a role in central amplification. These substances not only affect sensory transmission, but also control levels of alertness, sleep, mood, and memory.
The diagnostic criteria for fibromyalgia were modified in 2011 to remove the tender point examination and to add in somatic symptoms.6 These criteria can be useful in the clinical setting in identifying not only fibromyalgia itself but also the degree of “fibromyalgianess” a patient has, which is an indicator of how large a role the centralization process plays in the maintenance of chronic pain.23,24
Treatment: Multimodal and patient empowering. Evidence-based treatment options for fibromyalgia, as well as other conditions for which there is a high degree of centralized pain, can be found in TABLE 2.25-36 Multimodal treatment, with an emphasis on patient knowledge and empowerment, is generally thought to be the most beneficial.25,37 Treatment should almost always include CBT and exercise/activity therapies,26,29 which have high degrees of efficacy with few adverse effects.
In terms of medication, centrally-acting agents (tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors [SNRIs], and alpha 2 delta ligands) are the most effective. There is little to no data showing benefit from anti-inflammatories or opioids in the setting of fibromyalgia. There is some data to suggest that combination therapy, for example with an SNRI (milnacipran) and an alpha 2 delta ligand (pregabalin), may provide more benefit than treating with pregabalin alone.38
Complementary and alternative therapies (eg, yoga, chiropractic care, acupuncture, massage) are being studied more, and while evidence is only preliminary in terms of efficacy, there is increasing emphasis being placed on the need for patients with chronic pain to shift their treatment expectations to greater acceptance of pain and the need for ongoing self-care.28 (For more advice on managing fibromyalgia, see the related videos at http://bit.ly/2lPEt0f and http://bit.ly/2lmjEcn.)
Osteoarthritis: An example of peripheral nociceptive pain
OA is a condition long thought to be characterized by damage to the cartilage and bone; however, as with many other pain diagnoses, there is frequently little correlation between damage seen on radiographs and the amount of pain that patients experience.
One study analyzed data on almost 7000 patients from the National Health and Nutrition Examination Survey (NHANES I) and found that between 30% and 50% of OA patients with moderate to severe radiographic changes were asymptomatic, and 10% of those with moderate to severe pain had normal radiographs or only mild changes.39 Research is showing that many factors may contribute to this discrepancy, including the typical “wear and tear” of the disease, subacute levels of inflammation that can lead to peripheral sensitization,40 and, in some patients, a centralized pain component. The patients with more centralized pain often have pain that is disproportionate to radiographic evidence, as well as more somatic symptoms such as fatigue, sleep disturbance, and memory issues.41
Treatment should be multimodal and include interventions targeted at halting the progression of damage as well as palliation of pain. All treatment plans for OA should also include exercise, weight reduction, and self-management, in addition to pharmacologic interventions, to reduce both the micro-inflammation and the centralized pain component (when present). Intra-articular injections of various types have been studied with some having more efficacy in pain reduction and functional improvement than others.42-45 See TABLE 342-61 for a summary of evidence-based treatment options.
Low back pain—a mixed pain state
Low back pain (LBP) has been recognized as a mixed pain state for quite some time. While some patients may experience purely nociceptive and/or neuropathic pain, most cases are nonspecific with patients experiencing varying degrees of nociceptive (myofascial low back pain), neuropathic (lumbar radiculopathy), and central sensitization pain.62,63 Evidence for centralized pain is demonstrated in studies showing hyperalgesia,64 augmented central pain processing,65 involvement of the emotional brain,66 and delayed recovery influenced by poor coping strategies.67
When developing a treatment plan for a patient with chronic low back pain, remember that the pain derives from a complex combination of pathophysiologic contributors. Identifying where a patient lies on the pain centralization spectrum can help you tailor treatment.
In one study of 548 patients presenting to a tertiary pain clinic with primary spine pain diagnoses, 42% met diagnostic criteria for fibromyalgia.68 Compared to criteria-negative patients, these patients tended to be younger, unemployed, and receiving compensation; they had greater pain intensity, pain interference, and used stronger words to describe their neuropathic pain; they also had higher levels of depression/anxiety and a lower level of physical function.
Because low back pain is a condition with high prevalence and associated disability, many clinical boards have created guidelines for management. These guidelines tend to vary in the strength of evidence used, and the extent to which they are followed in clinical practice remains largely unknown. Recommendations frequently discourage the use of ultrasound/electrotherapy, but many encourage short-term use of medications (see “How effective are opioids for chronic low back pain?” J Fam Pract. 2015;64:584-584), supervised exercise therapy, CBT, and multidisciplinary treatment.
Guidelines tend to differ most widely with regard to recommendations for spinal manipulation and specific drug therapies.69 The classes of drugs that may be most useful when centralized pain is present include the SNRIs and the alpha 2 delta calcium channel ligands.4 See TABLE 470-89 for a summary of evidence-based treatment options.
CASE 1 › Ms. A is started on amitriptyline 25 mg at bedtime, which improves her fatigue and cognitive symptoms. During monthly office visits, her FP educates her about the pathophysiology of fibromyalgia and uses motivational interviewing to get her slowly moving and increasing her activity level. She is weaned off the gabapentin previously prescribed, as her symptoms stabilize and improve.
CASE 2 › Mr. P is sent for a steroid injection, which decreases his pain temporarily. During this time, he begins physical therapy; slowly, with increased movement, his function improves. A trial of duloxetine provides pain relief; that combined with intermittent nonsteroidal anti-inflammatory drugs (NSAIDs) has allowed Mr. P to maintain his function and his job.
CASE 3 › Because Mr. B was only taking the narcotics intermittently and wasn’t certain they were helping, CBT was sufficient to wean Mr. B off the medication without any worsening of his pain in the process. By participating in physical therapy, he has learned how to perform certain tasks at his job without pain or injury. He uses NSAIDs as needed for pain.
CORRESPONDENCE
Jill Schneiderhan, MD, 24 Frank Lloyd Wright Dr., Lobby H, Suite 2300, Ann Arbor, MI 48105; [email protected].
ACKNOWLEDGEMENTS
We thank Drs. Daniel Clauw (University of Michigan, Ann Arbor) and Martha Rumschlag (Providence Family Medicine Residency Program, Southfield, Mich), for their valuable contributions to this article.
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US); 2011.
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
3. Aronoff GM. What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am. 2016;100:31-42.
4. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6-19.
5. Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335-342.
6. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113-1122.
7. Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386-1394.
8. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233-251.
9. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
10. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157:1799-1809.
11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539-1552.
12. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977-992.
13. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
14. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240-1243.
15. van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628-629.
16. McLean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101-107.
17. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519-2527.
18. Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269-1277.
19. Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852-12860.
20. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies abberrant brain chemistry, connectivity, and functional responses in chronic pain patients. Anesthesiology. 2013;119:1453-1464.
21. Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550-556.
22. Foerster BR, Petrou M, Edden RAE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579-583.
23. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
26. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
27. Porter NS, Jason LA, Boulton A, et al. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010;16:235-249.
28. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12.
29. Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991-2005.
30. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104-113.
31. Moldofsky H, Harris HW, Archambault WT, et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653-2663.
32. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;Sep 8 Suppl 2:S63-S74.
33. Häuser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69-81.
34. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;Jun 23:CD010692.
35. MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15:469-475.
36. Younger J, Noor N, McCue R, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529-538.
37. Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337-344.
38. Mease PJ, Farmer MV, Palmer RH, et al. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskeletal Dis. 2013;5:113-126.
39. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
40. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890-1896.
41. Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043-1056.
42. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
43. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328.
44. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505.
45. Wu T, Song HX, Dong Y, et al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil. 2016.
46. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956.
48. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:CD007912.
49. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.
50. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105.
51. Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:76.
52. Berthelot JM, Darrieutort-Lafitte C, Le Goff B, et al. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397-401.
53. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
54. Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
55. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
56. Wu D, Huang Y, Gu Y, et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013;67:585-594.
57. Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
58. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156-165.
59. Clinton CM, O’Brien S, Law J, et al. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152.
60. Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312.
61. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
62. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333-E346.
63. Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15:4-15.
64. Hübscher M, Moloney N, Rebbeck T, et al. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain. 2014;30:886-893.
65. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
66. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165-12173.
68. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285-3292.
69. Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075-2094.
70. Oliveira VC, Ferreira PH, Maher CG, et al. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res. 2012;64:1739-1748.
71. Engers A, Jellema P, Wensing M, et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008:CD004057.
72. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335.
73. French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006:CD004750.
74. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskeletal Disord. 2014;15:286.
75. Franke H, Fryer G, Ostelo RW, et al. Muscle energy technique for non-specific low back pain. Cochrane Database Syst Rev. 2015:CD009852.
76. Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004:197-210.
77. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015:CD001929.
78. Khadilkar A, Odebiyi DO, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low back pain. Cochrane Database Syst Rev. 2008:CD003008.
79. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014:CD009169.
80. Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005:CD001351.
81. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
82. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
83. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
84. Staal JB, de Bie R, de Vet HC, et al. Injection therapy for subacute and chronic low back pain. Cochrane Database Syst Rev. 2008:CD001824.
85. Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34:1094-1109.
86. Felson D. Paracetamol is ineffective for spinal pain and knee and hip osteoarthritis. Evid Based Med. 2015;20:205.
87. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
88. Enthoven WT, Roelofs PD, Deyo RA, et al. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087.
89. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143.
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press (US); 2011.
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
3. Aronoff GM. What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am. 2016;100:31-42.
4. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6-19.
5. Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335-342.
6. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113-1122.
7. Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386-1394.
8. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233-251.
9. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
10. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157:1799-1809.
11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539-1552.
12. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977-992.
13. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
14. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240-1243.
15. van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628-629.
16. McLean SA, Diatchenko L, Lee YM, et al. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12:101-107.
17. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519-2527.
18. Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269-1277.
19. Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852-12860.
20. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies abberrant brain chemistry, connectivity, and functional responses in chronic pain patients. Anesthesiology. 2013;119:1453-1464.
21. Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550-556.
22. Foerster BR, Petrou M, Edden RAE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579-583.
23. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
26. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
27. Porter NS, Jason LA, Boulton A, et al. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med. 2010;16:235-249.
28. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12.
29. Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome: a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991-2005.
30. Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104-113.
31. Moldofsky H, Harris HW, Archambault WT, et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653-2663.
32. Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;Sep 8 Suppl 2:S63-S74.
33. Häuser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69-81.
34. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;Jun 23:CD010692.
35. MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15:469-475.
36. Younger J, Noor N, McCue R, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529-538.
37. Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337-344.
38. Mease PJ, Farmer MV, Palmer RH, et al. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskeletal Dis. 2013;5:113-126.
39. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
40. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890-1896.
41. Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043-1056.
42. Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
43. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328.
44. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505.
45. Wu T, Song HX, Dong Y, et al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil. 2016.
46. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956.
48. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:CD007912.
49. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.
50. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093-2105.
51. Myers J, Wielage RC, Han B, et al. The efficacy of duloxetine, non-steroidal anti-inflammatory drugs, and opioids in osteoarthritis: a systematic literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:76.
52. Berthelot JM, Darrieutort-Lafitte C, Le Goff B, et al. Strong opioids for noncancer pain due to musculoskeletal diseases: not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397-401.
53. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
54. Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
55. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
56. Wu D, Huang Y, Gu Y, et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013;67:585-594.
57. Kahan A, Uebelhart D, De Vathaire F, et al. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-533.
58. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156-165.
59. Clinton CM, O’Brien S, Law J, et al. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152.
60. Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312.
61. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
62. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333-E346.
63. Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15:4-15.
64. Hübscher M, Moloney N, Rebbeck T, et al. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: a comparison with pain-free controls. Clin J Pain. 2014;30:886-893.
65. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
66. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165-12173.
68. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285-3292.
69. Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075-2094.
70. Oliveira VC, Ferreira PH, Maher CG, et al. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res. 2012;64:1739-1748.
71. Engers A, Jellema P, Wensing M, et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008:CD004057.
72. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335.
73. French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006:CD004750.
74. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskeletal Disord. 2014;15:286.
75. Franke H, Fryer G, Ostelo RW, et al. Muscle energy technique for non-specific low back pain. Cochrane Database Syst Rev. 2015:CD009852.
76. Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004:197-210.
77. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015:CD001929.
78. Khadilkar A, Odebiyi DO, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low back pain. Cochrane Database Syst Rev. 2008:CD003008.
79. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014:CD009169.
80. Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005:CD001351.
81. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
82. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
83. Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
84. Staal JB, de Bie R, de Vet HC, et al. Injection therapy for subacute and chronic low back pain. Cochrane Database Syst Rev. 2008:CD001824.
85. Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine. 2009;34:1094-1109.
86. Felson D. Paracetamol is ineffective for spinal pain and knee and hip osteoarthritis. Evid Based Med. 2015;20:205.
87. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
88. Enthoven WT, Roelofs PD, Deyo RA, et al. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087.
89. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36:S131-S143.
PRACTICE RECOMMENDATIONS
› Recommend cognitive behavioral therapy for most patients suffering from chronic pain. A
› Recommend movement and exercise therapies for all patients with chronic pain. A
› Prescribe anti-inflammatory medications for patients with peripheral nociceptive pain and centrally-acting agents, such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, and alpha 2 delta ligands, for patients with centralized pain. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What family physicians can do to combat bullying
CASE › Stacey, a 12-year-old girl with mild persistent asthma, presents to her family physician (FP) with her mother for her annual well visit. Stacey reports no complaints, but has visited twice recently for acute exacerbations of her asthma, which had previously been well-controlled. When reviewing her social history, Stacey reports that she started her second year of middle school 3 months ago. When asked if she enjoys school, Stacey looks down and says, “School is fine.” Her mother quickly adds that Stacey has quit the school cheerleading team—much to the coach’s dismay—and is having difficulty in her math class, a class in which she normally excels. Stacey appears embarrassed that her mother has brought these things up. Her mother says that at the beginning of the year, 2 girls began picking on Stacey, calling her names and making fun of her on social media and in front of other students.
For many years, bullying was trivialized. Some viewed it as a universal childhood experience; others considered it a rite of passage.1,2 It was not examined as a public health issue until the 1970s. In fact, no legislation addressing bullying or “peer abuse” existed in the United States until the mass shooting at Columbine High School in Littleton, Colo, in 1999. Within 3 years of the Columbine tragedy, the number of state laws that mentioned bullying went from zero to 15; within 10 years of Columbine, 41 states had laws addressing bullying,1 and by 2015, every state, the District of Columbia, and some territories had a bullying law in place.3
As research and advocacy regarding bullying has grown, its impact on the health of children, adolescents, and even adults has become more apparent. In a 2001 study of school-associated violent deaths in the United States between 1994 and 1999, the Centers for Disease Control and Prevention (CDC) found that among students, homicide perpetrators were more than twice as likely as homicide victims to have been bullied by peers.4 Given that homicide is the third leading cause of death in people ages 15 to 24,5 past exposure to bullying may be a significant contributing factor to mortality in this age group.4
In addition to a correlation with homicidal behavior, those involved in bullying—whether as the bully or victim—are at risk for a wide range of symptoms, conditions, and problems including poor psychosocial adjustment, depression, anxiety, suicide (the second leading cause of death in the 10-14 and 15-24 age groups5), academic decline, psychosomatic manifestations, fighting, alcohol use, smoking, and difficulty with the management of chronic diseases.6-10 Not only does being a victim of bullying have a direct impact on a child’s current mental and physical well-being, but it can have lasting psychological and behavioral effects that can follow children well into adulthood.7 The significant impact of bullying on individuals and society as a whole mandates a multifaceted approach that begins in your exam room. What follows is practical advice on screening, counseling, and working with schools and the community at large to curb the bullying epidemic.

Clarifying the problem: The CDC’s definition
Recognizing that varying definitions of bullying were being used in research studies that looked at violent or aggressive behaviors in youth, the CDC published a consensus statement in 2014 that proposed the following definition for bullying:11 any unwanted aggressive behavior by another youth or group of youths who are not siblings or current dating partners that involves an observed or perceived power imbalance and is repeated multiple times or is highly likely to be repeated. This expanded on an earlier definition by Olweus12,13 that also identified a longitudinal nature and power imbalance as key features.
Types of bullying. Direct bullying entails blatant attacks on a targeted young person, while indirect bullying involves communication with others about the targeted individual (eg, spreading harmful rumors). Bullying may be physical, verbal, or relational (eg, excluding someone from their usual social circle, denying friendship, the silent treatment, writing mean letters, eye rolling, etc.) and may involve damage to property. Boys tend toward more direct bullying behaviors, while girls more often engage in indirect bullying, which may be more challenging for both adults and other students to recognize.12,13 With increased use of technology and social media by adolescents, cyberbullying has become increasingly more prevalent, with its effects on adolescent health and academics being every bit as profound as those of traditional bullying.14
About 1 in 4/5 students suffer. The prevalence of bullying ranges by country and culture. The vast majority of early bullying research was conducted in Norway, which found that approximately 15% of students in elementary and secondary schools were involved in bullying in some capacity.12 In a study involving over 200,000 adolescents from 40 European countries, 26% of adolescents reported being involved in bullying, ranging from 8.6% to 45.2% for boys and 4.8% to 35.8% for girls.15 Variations in prevalence may be due to cultural differences in the acts of bullying or differences in interpretation of the term “bullying.”1,15
In the United States, a 2001 survey of more than 15,000 students in public and private schools (grades 6-10) asked the students about their involvement in bullying: 13% said they'd been a bully, 10.6% a victim, and 6.3% said they'd been both.6 There was no significant difference in the frequency of self-reported bullying among urban, suburban, or rural settings.
Despite efforts to educate the public about bullying and work with schools to intervene and prevent bullying, incidence remains largely unchanged. In 2013, the National Crime Victimization Survey reported that approximately 22% of adolescents ages 12 through 18 were victims of bullying.16 Similarly, the CDC's 2015 Youth Risk Behavior Surveillance System reported that 20.2% of high school students experienced bullying on school property.17
Screening: Best practices
The FP’s role begins with screening children at risk for bullying (TABLE 118-22) or those whose complaints suggest that they may be victims of bullying.
Start screening when children enter elementary school
Given that providers’ time is limited for every patient visit, it is important to address bullying at times that are most likely to yield impactful results. The American Academy of Pediatrics recommends that the topic of bullying be introduced at the 6-year-old well-child visit (a typical age for entry to elementary school).7 Views in the literature are inconsistent regarding when and how to address bullying at other time points. One approach is to pre-screen those with risk factors associated with bullying (TABLE 118-22), and to focus screening on those with warning signs of bullying, which include mood disorders, psychosomatic or behavioral symptoms, substance abuse, self-harm behaviors, suicidal ideation or a suicide attempt, a decline in academic performance, and reports of school truancy. Parental concerns, such as when a child suddenly needs more money for lunch, is having aggressive outbursts, or is exhibiting unexplained physical injuries, should also be regarded as cues to screen.9
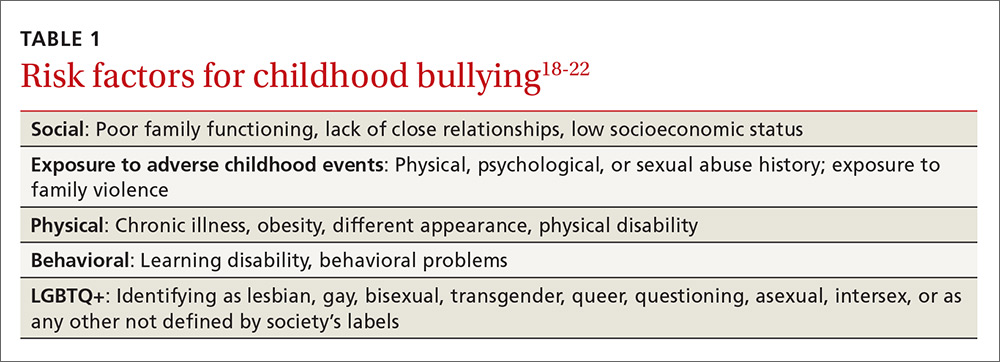
Screen patients in high-risk groups

A number of groups of children are at high risk for bullying and warrant targeted screening efforts.
- Children with special health needs. Research has shown that children with special health needs are at increased risk for being bullied.18 In fact, the presence of a chronic disease may increase the risk for bullying, and bullying often negatively impacts chronic disease management. As a result, it’s important to have a high index of suspicion with patients who have a chronic disease and who are not responding as expected to medical management or who experience deterioration after being previously well-controlled.18
- Children who are under- or overweight. Similarly, bullying based on a child's weight is a phenomenon that has been recognized to have a significant impact on children’s emotional health.19
- Youth who identify as lesbian, gay, bisexual, transgender, or queer/questioning (LGBTQ+) are more likely than non-LGBTQ+ peers to attempt suicide when exposed to a hostile social environment, such as that created by bullying.20
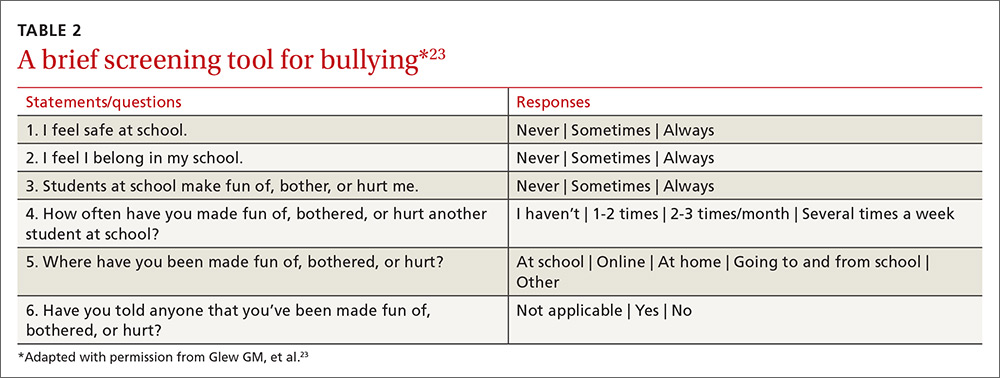
Screening need not be complicated

One screening approach is simply to ask patients, “Are you being bullied?” followed by such questions as, “How often are you bullied?” or “How long have you been bullied?” Asking about the setting of the bullying (Does it happen at school? Traveling to/from school? Online?) and other details may help guide interventions and the provision of resources.9 Another approach is to provide patients with some type of written survey (see TABLE 223 for an example) to encourage responses that patients might be reluctant to disclose verbally.23,24 (See “Barriers to screening.")
SIDEBAR
Barriers to screeningScreening for any condition presupposes a response. Ideally, family physicians should be prepared to provide basic counseling, resources, and, if necessary, treatment, if a patient screens positive for bullying. But screening for violence or bullying can be difficult, and evidence-based guidelines for screening and intervention are lacking, leaving many primary care practitioners feeling ill-equipped to meaningfully respond.
One study of the use of a screening tool aimed at intimate partner violence (IPV) showed that even with the availability of a screening tool, health care providers’ use of the tool was inconsistent and referral practices were ineffective.1 Providers cited the following limiting factors in screening for IPV: 1) a lack of immediate referral availability, 2) a lack of time during the office visit, and 3) a lack of confidence in the ability to screen.1 These same issues may be barriers to screening for bullying.
1. Ramachandran DV, Covarrubias L, Watson C, et al. How you screen is as important as whether you screen: a qualitative analysis of violence screening practices in reproductive health clinics. J Community Health. 2013;38:856-863.
Provider and parental interventions
Interventions often entail counseling the patient and the family about bullying and its effects, empowering victims and their caregivers, and screening for bullying comorbidities and correlates.2 Refer patients to behavioral health specialists when there is evidence of pervasive effects on mood, behavior, or social development, but keep in mind that counseling can begin in your own exam room.
Effective discussion starters. Affirming the problem and its unacceptability, talking about the different types of bullying and where bullying may occur, and asking about patient perceptions of bullying can be effective discussion starters. FPs should help patients identify bullying, open lines of communication between children and their parents and between parents and other caregivers, and demonstrate respect and kindness in their approach to discussing the topic. Encourage children to speak with trusted adults when exposed to bullying. Talk to them about standing up to bullies (saying “stop” confidently or walking away from difficult situations) and staying safe by staying near adults or groups of peers when bullies are present (TABLE 325).
Empowering caregivers. Encourage parents to spend time each day talking with their child about the child’s time away from home (TABLE 325). Counsel parents/caregivers to expand their role. Knowing a child’s friends, encouraging the child academically, and increasing communication are all associated with lower risks of bullying.26 Similarly, parental oversight of Internet and social media use is associated with decreased participation in cyberbullying.27
In addition, the Positive Parenting telephone-based parenting education curriculum has been shown to decrease bullying, physical fighting, physical injuries, and victimization of children.28 The research-based, family strengthening program emphasizes 3 core elements of authoritative parenting: nurturance, discipline, and respect or granting of psychologic autonomy. The program entails 15- to 30-minute weekly phone conversations between parents and educators, as well as videos and a manual.
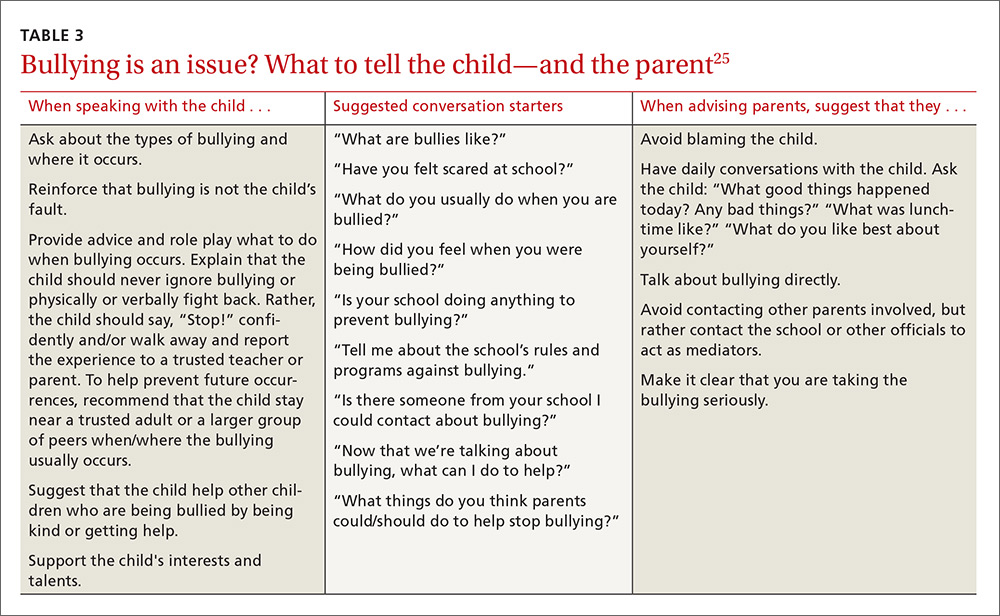
Are community programs in place—or are they needed?
Many schools have robust, state-mandated programs in place to identify bullying and provide support for students who are victims of bullying. (See “NJ’s harassment and bullying protocol: A case in point.”) Explaining this to victims and their families may help them come forward and seek assistance. FPs who want to advocate for their patients should start with local schools to support such programs and link students at risk with school counselors.
SIDEBAR
NJ's harassment and bullying protocol: A case in pointThere is no federal law that specifically applies to bullying, but all 50 states have some type of anti-bullying legislation on the books, and 40 of those states have additional detailed policies in place addressing the subject.1
New Jersey, for example, began enforcing one of the toughest harassment, intimidation, and bullying (HIB) protocols in the country back in September 20112 in the wake of the death of Rutgers University freshman Tyler Clementi, who committed suicide after his roommate allegedly shot a video of him with another man and posted it to the Internet.3 Among many other things, New Jersey’s legislation stipulates in its Anti-Bullying Bill of Rights4 that:
› Every school/district have plans in place that clearly define, prevent, prohibit, and promptly deal with acts of harassment, intimidation, or bullying, on school grounds, at school-sponsored functions, and on school buses.
› Plans must include a description of the type of behavior expected from each student and the consequences and remedial action for a person who commits an act of harassment, intimidation, or bullying. Student perpetrators may be suspended or expelled if convicted of any type of bullying, whether it be for teasing or something more severe.
› All school employees must act on any incidents of bullying reported to, or witnessed by, them and report such incidents on the same day to the school principal.
› Plans must include provisions and deadlines for investigating and resolving all matters in a timely fashion; investigations into allegations of bullying must be launched within one day.
› Every case of bullying must be reported to the state. Schools are graded by the state on their compliance with anti-bullying standards and policies and their handling of incidents.
› Schools must appoint safety teams made up of parents, teachers, and staff, and school personnel and students must receive extensive anti-bullying training.
1. US Department of Health and Human Services. Stopbullying.gov. Policies and laws. Available at: https://www.stopbullying.gov/laws/index.html.
Accessed January 5, 2017.
2. State of New Jersey Department of Education. An overview of amendments to laws on harassment, intimidation, and bullying. Available at: http://www.state.nj.us/education/students/safety/behavior/hib/overview.pdf. Accessed January 5, 2017.
3. Cohen A. Case study: Why New Jersey’s antibullying law should be a model for other states. Time. September 6, 2011. Available at: http://ideas.time.com/2011/09/06/why-new-jerseys-antibullying-law-should-be-a-model-for-other-states/. Accessed January 5, 2017.
4. New Jersey Legislature. Anti-bullying Bill of Rights Act. Available at: http://www.njleg.state.nj.us/2010/Bills/PL10/122.PDF. Accessed January 5, 2017.
If programs are lacking in your community, there is much you can do to educate yourself about successful programs and advise local community organizations and schools about them. Among the most successful and well-studied interventions for thwarting the bullying epidemic have been school-based community ones. The most studied of these is the Olweus Bullying Prevention Program (OBPP), which is based on 4 principles:1,29
- Adults both at home and at school should take a positive and encouraging interest in students.
- Unacceptable behavior should have strict and well-known limits.
- Sanctions should be applied consistently and should be non-hostile in nature.
- Adults both at home and in the educational environment should act as authorities.
In short, the program focuses on greater awareness and involvement on the part of adults, and employing measures at the school level (eg, surveys, better supervision during break and lunch times), the class level (eg, rules against bullying, regular class meetings with students), and the individual level (eg, serious talks with bullies, victims, parents of involved students).
Research has shown that the OBPP reduces bullying behaviors by as much as 50%, reduces vandalism and truancy, and reduces the number of new victims.12 Limits to the more widespread implementation of the OBPP have consisted mainly of the inability to appropriately train adults, including teachers and other school personnel in educational settings. Despite these limitations, the OBPP has been praised and endorsed by numerous groups, including the US Department of Justice.30
Other non-curricular, school-based programs exist, such as the School-Wide Positive Behavioral Interventions and Supports (SWPBIS). This program is a school-wide prevention strategy aimed at: 1) reducing behavior problems that lead to office discipline referrals and suspensions, and 2) changing perceptions of school safety. (For more information, see https://www.crimesolutions.gov/ProgramDetails.aspx?ID=385 and https://www.pbis.org/school/swpbis-for-beginners.)31
The research-based Second Step: Student Success Through Prevention (SS-SSTP) Middle School Program (http://www.cfchildren.org/second-step/middle-school)32 focuses on the often difficult middle school years. The program helps schools teach and model essential communication, coping, and decision-making skills to help adolescents navigate around common pitfalls such as peer pressure, substance abuse, and bullying (both in-person and online). The program aims to reduce aggression and provide support for a more inclusive environment that helps students stay in school, make good choices, and experience social and academic success.
The Positive Action Program (https://www.positiveaction.net/research/primer),33 which is predicated on the notion that we feel good about ourselves when we do positive things, features scripted lessons and kits of materials (eg, posters, games, worksheets, puzzles) appropriate for each grade level.
CASE › Stacey’s visit to her FP’s office has presented several clues that she may be a victim of bullying. Her mild persistent asthma appears to no longer be as well controlled as it was in the past. Direct questioning has revealed that 2 girls at school have been making fun of Stacey when she uses her inhaled corticosteroid in the morning before class, so she has stopped using it. These same students are on her cheerleading team, so she quit the team to avoid them. Her school-related anxiety is so great that she no longer pays attention in math class and is constantly worried that something is being posted about her online.
Stacy’s FP responds to this information with a multifaceted approach. In the exam room, he screens Stacy for depression. While she is negative and denies any suicidal ideation, Stacy is clearly having anxiety, so the FP refers Stacey to a counselor at a local mental health clinic. With Stacy’s permission, the FP discusses the issue with her mother and they decide together with Stacy that she should talk to a teacher at school about the ongoing bullying. Because this was not the first time that the FP has heard this from a child in the community, the FP plans to attend an upcoming school board meeting to advocate for an evidence-based bullying prevention program to help curb the ongoing problem facing his patients.
CORRESPONDENCE
Robert McClowry, MD, Department of Family and Community Medicine, Jefferson Family Medicine Associates, 833 Chestnut East, 3rd Floor, Suite 301, Philadelphia, PA, 19107-4414; [email protected].
1. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80:124-134.
2. Lyznicki JM, McCaffree MA, Robinowitz CB, et al. Childhood bullying: implications for physicians. Am Fam Physician. 2004;70:1723-1730.
3. Temkin D. All 50 states now have a bullying law. Now what? The Huffington Post. April 27, 2015. Available at: http://www.huffingtonpost.com/deborah-temkin/all-50-states-now-have-a_b_7153114.html. Accessed January 5, 2017.
4. Anderson M, Kaufman J, Simon TR, et al. School-associated violent deaths in the United States, 1994-1999. JAMA. 2001;286:2695-2702.
5. Centers for Disease Control and Prevention. Injury prevention and control: Data and statistics (WISQARS). Ten leading causes of death and injury. Available at: https://www.cdc.gov/injury/wisqars/leadingcauses.html. Accessed January 5, 2017.
6. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285:2094-2100.
7. Committee on Injury, Violence, and Poison Prevention. Policy statement—Role of the pediatrician in youth violence prevention. Pediatrics. 2009;124:393-402.
8. Klein DA, Myhre KK, Ahrendt DM. Bullying among adolescents: a challenge in primary care. Am Fam Physician. 2013;88:87-92.
9. Lamb J, Pepler DJ, Craig W. Approach to bullying and victimization. Can Fam Physician. 2009;55:356-360.
10. Spector ND, Kelly SF. Pediatrician’s role in screening and treatment: bullying, prediabetes, oral health. Curr Opin Pediatr. 2006;18:661-670.
11. Gladden RM, Vivolo-Kantor AM, Hamburger ME, et al. Bullying surveillance among youths: uniform definitions for public health and recommended data elements. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention and US Department of Education; 2014. Available at: https://www.cdc.gov/violenceprevention/pdf/bullying-definitions-final-a.pdf. Accessed June 8, 2016.
12. Olweus D. Bullying at school: basic facts and effects of a school based intervention program. J Child Psychol Psychiatry. 1994;35:1171-1190.
13. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
14. Kowalski RM, Limber SP. Psychological, physical, and academic correlates of cyberbullying and traditional bullying. J Adolesc Health. 2013;53:S13-S20.
15. Craig W, Harel-Fisch Y, Fogel-Grinvald H, et al. A cross-national profile of bullying and victimization among adolescents in 40 countries. Int J Public Health. 2009;54(Suppl 2):216-224.
16. US Department of Education, National Center for Educational Statistics (2015). Student reports of bullying and cyberbullying: results from the 2013 School Crime Supplement to the National Victimization Survey. Available at: http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2015056. Accessed November 9, 2016.
17. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1-174.
18. Van Cleave J, Davis MM. Bullying and peer victimization among children with special health care needs. Pediatrics. 2006;118:e1212-e1219.
19. Eisenberg ME, Neumark-Sztainer D, Story M. Associations of weight-based teasing and emotional well-being among adolescents. Arch Pediatr Adolesc Med. 2003;157:733-738.
20. Hatzenbuehler ML. The social environment and suicide attempts in lesbian, gay, and bisexual youth. Pediatrics. 2011;127:896-903.
21. Song LY, Singer MI, Anglin TM. Violence exposure and emotional trauma as contributors to adolescents’ violent behaviors. Arch Pediatr Adolesc Med. 1998;152:531-536.
22. Singer MI, Anglin TM, Song LY, et al. Adolescents’ exposure to violence and associated symptoms of psychological trauma. JAMA. 1995;273:477-482.
23. Glew GM, Fan MY, Katon W, et al. Bullying, psychosocial adjustment, and academic performance in elementary school. Arch Pediatr Adolesc Med. 2005;159:1026-1031.
24. Waseem M, Ryan M, Foster CB, et al. Assessment and management of bullied children in the emergency department. Pediatr Emerg Care. 2013;29:389-398.
25. US Department of Health and Human Services. stopbullying.gov. Available at: https//www.stopbullying.gov. Accessed January 5, 2017.
26. Shetgiri R, Lin H, Avila RM, et al. Parental characteristics associated with bullying perpetration in US children aged 10 to 17 years. Am J Public Health. 2012;102:2280-2286.
27. Hinduja S, Patchin JW. Social influences on cyberbullying behaviors among middle and high school students. J Youth Adolesc. 2013;42:711-722.
28. Borowsky IW, Mozayeny S, Stuenkel K, et al. Effects of a primary care-based intervention on violent behavior and injury in children. Pediatrics. 2004;114:e392-e399.
29. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
30. Mihalic SF. Blueprints for Violence Prevention: Report. US Department of Justice, Office of Justice Programs, Office of Juvenile Justice and Delinquency Prevention; 2004.
31. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med. 2012;166:149-156.
32. Espelage DL, Low S, Polanin JR, et al. The impact of a middle school program to reduce aggression, victimization, and sexual violence. J Adolesc Health. 2013;53:180-186.
33. Lewis KM, Schure MB, Bavarian N, et al. Problem behavior and urban, low-income youth: a randomized controlled trial of positive action in Chicago. Am J Prev Med. 2013;44:622-630.
CASE › Stacey, a 12-year-old girl with mild persistent asthma, presents to her family physician (FP) with her mother for her annual well visit. Stacey reports no complaints, but has visited twice recently for acute exacerbations of her asthma, which had previously been well-controlled. When reviewing her social history, Stacey reports that she started her second year of middle school 3 months ago. When asked if she enjoys school, Stacey looks down and says, “School is fine.” Her mother quickly adds that Stacey has quit the school cheerleading team—much to the coach’s dismay—and is having difficulty in her math class, a class in which she normally excels. Stacey appears embarrassed that her mother has brought these things up. Her mother says that at the beginning of the year, 2 girls began picking on Stacey, calling her names and making fun of her on social media and in front of other students.
For many years, bullying was trivialized. Some viewed it as a universal childhood experience; others considered it a rite of passage.1,2 It was not examined as a public health issue until the 1970s. In fact, no legislation addressing bullying or “peer abuse” existed in the United States until the mass shooting at Columbine High School in Littleton, Colo, in 1999. Within 3 years of the Columbine tragedy, the number of state laws that mentioned bullying went from zero to 15; within 10 years of Columbine, 41 states had laws addressing bullying,1 and by 2015, every state, the District of Columbia, and some territories had a bullying law in place.3
As research and advocacy regarding bullying has grown, its impact on the health of children, adolescents, and even adults has become more apparent. In a 2001 study of school-associated violent deaths in the United States between 1994 and 1999, the Centers for Disease Control and Prevention (CDC) found that among students, homicide perpetrators were more than twice as likely as homicide victims to have been bullied by peers.4 Given that homicide is the third leading cause of death in people ages 15 to 24,5 past exposure to bullying may be a significant contributing factor to mortality in this age group.4
In addition to a correlation with homicidal behavior, those involved in bullying—whether as the bully or victim—are at risk for a wide range of symptoms, conditions, and problems including poor psychosocial adjustment, depression, anxiety, suicide (the second leading cause of death in the 10-14 and 15-24 age groups5), academic decline, psychosomatic manifestations, fighting, alcohol use, smoking, and difficulty with the management of chronic diseases.6-10 Not only does being a victim of bullying have a direct impact on a child’s current mental and physical well-being, but it can have lasting psychological and behavioral effects that can follow children well into adulthood.7 The significant impact of bullying on individuals and society as a whole mandates a multifaceted approach that begins in your exam room. What follows is practical advice on screening, counseling, and working with schools and the community at large to curb the bullying epidemic.

Clarifying the problem: The CDC’s definition
Recognizing that varying definitions of bullying were being used in research studies that looked at violent or aggressive behaviors in youth, the CDC published a consensus statement in 2014 that proposed the following definition for bullying:11 any unwanted aggressive behavior by another youth or group of youths who are not siblings or current dating partners that involves an observed or perceived power imbalance and is repeated multiple times or is highly likely to be repeated. This expanded on an earlier definition by Olweus12,13 that also identified a longitudinal nature and power imbalance as key features.
Types of bullying. Direct bullying entails blatant attacks on a targeted young person, while indirect bullying involves communication with others about the targeted individual (eg, spreading harmful rumors). Bullying may be physical, verbal, or relational (eg, excluding someone from their usual social circle, denying friendship, the silent treatment, writing mean letters, eye rolling, etc.) and may involve damage to property. Boys tend toward more direct bullying behaviors, while girls more often engage in indirect bullying, which may be more challenging for both adults and other students to recognize.12,13 With increased use of technology and social media by adolescents, cyberbullying has become increasingly more prevalent, with its effects on adolescent health and academics being every bit as profound as those of traditional bullying.14
About 1 in 4/5 students suffer. The prevalence of bullying ranges by country and culture. The vast majority of early bullying research was conducted in Norway, which found that approximately 15% of students in elementary and secondary schools were involved in bullying in some capacity.12 In a study involving over 200,000 adolescents from 40 European countries, 26% of adolescents reported being involved in bullying, ranging from 8.6% to 45.2% for boys and 4.8% to 35.8% for girls.15 Variations in prevalence may be due to cultural differences in the acts of bullying or differences in interpretation of the term “bullying.”1,15
In the United States, a 2001 survey of more than 15,000 students in public and private schools (grades 6-10) asked the students about their involvement in bullying: 13% said they'd been a bully, 10.6% a victim, and 6.3% said they'd been both.6 There was no significant difference in the frequency of self-reported bullying among urban, suburban, or rural settings.
Despite efforts to educate the public about bullying and work with schools to intervene and prevent bullying, incidence remains largely unchanged. In 2013, the National Crime Victimization Survey reported that approximately 22% of adolescents ages 12 through 18 were victims of bullying.16 Similarly, the CDC's 2015 Youth Risk Behavior Surveillance System reported that 20.2% of high school students experienced bullying on school property.17
Screening: Best practices
The FP’s role begins with screening children at risk for bullying (TABLE 118-22) or those whose complaints suggest that they may be victims of bullying.
Start screening when children enter elementary school
Given that providers’ time is limited for every patient visit, it is important to address bullying at times that are most likely to yield impactful results. The American Academy of Pediatrics recommends that the topic of bullying be introduced at the 6-year-old well-child visit (a typical age for entry to elementary school).7 Views in the literature are inconsistent regarding when and how to address bullying at other time points. One approach is to pre-screen those with risk factors associated with bullying (TABLE 118-22), and to focus screening on those with warning signs of bullying, which include mood disorders, psychosomatic or behavioral symptoms, substance abuse, self-harm behaviors, suicidal ideation or a suicide attempt, a decline in academic performance, and reports of school truancy. Parental concerns, such as when a child suddenly needs more money for lunch, is having aggressive outbursts, or is exhibiting unexplained physical injuries, should also be regarded as cues to screen.9

Screen patients in high-risk groups

A number of groups of children are at high risk for bullying and warrant targeted screening efforts.
- Children with special health needs. Research has shown that children with special health needs are at increased risk for being bullied.18 In fact, the presence of a chronic disease may increase the risk for bullying, and bullying often negatively impacts chronic disease management. As a result, it’s important to have a high index of suspicion with patients who have a chronic disease and who are not responding as expected to medical management or who experience deterioration after being previously well-controlled.18
- Children who are under- or overweight. Similarly, bullying based on a child's weight is a phenomenon that has been recognized to have a significant impact on children’s emotional health.19
- Youth who identify as lesbian, gay, bisexual, transgender, or queer/questioning (LGBTQ+) are more likely than non-LGBTQ+ peers to attempt suicide when exposed to a hostile social environment, such as that created by bullying.20

Screening need not be complicated

One screening approach is simply to ask patients, “Are you being bullied?” followed by such questions as, “How often are you bullied?” or “How long have you been bullied?” Asking about the setting of the bullying (Does it happen at school? Traveling to/from school? Online?) and other details may help guide interventions and the provision of resources.9 Another approach is to provide patients with some type of written survey (see TABLE 223 for an example) to encourage responses that patients might be reluctant to disclose verbally.23,24 (See “Barriers to screening.")
SIDEBAR
Barriers to screeningScreening for any condition presupposes a response. Ideally, family physicians should be prepared to provide basic counseling, resources, and, if necessary, treatment, if a patient screens positive for bullying. But screening for violence or bullying can be difficult, and evidence-based guidelines for screening and intervention are lacking, leaving many primary care practitioners feeling ill-equipped to meaningfully respond.
One study of the use of a screening tool aimed at intimate partner violence (IPV) showed that even with the availability of a screening tool, health care providers’ use of the tool was inconsistent and referral practices were ineffective.1 Providers cited the following limiting factors in screening for IPV: 1) a lack of immediate referral availability, 2) a lack of time during the office visit, and 3) a lack of confidence in the ability to screen.1 These same issues may be barriers to screening for bullying.
1. Ramachandran DV, Covarrubias L, Watson C, et al. How you screen is as important as whether you screen: a qualitative analysis of violence screening practices in reproductive health clinics. J Community Health. 2013;38:856-863.
Provider and parental interventions
Interventions often entail counseling the patient and the family about bullying and its effects, empowering victims and their caregivers, and screening for bullying comorbidities and correlates.2 Refer patients to behavioral health specialists when there is evidence of pervasive effects on mood, behavior, or social development, but keep in mind that counseling can begin in your own exam room.
Effective discussion starters. Affirming the problem and its unacceptability, talking about the different types of bullying and where bullying may occur, and asking about patient perceptions of bullying can be effective discussion starters. FPs should help patients identify bullying, open lines of communication between children and their parents and between parents and other caregivers, and demonstrate respect and kindness in their approach to discussing the topic. Encourage children to speak with trusted adults when exposed to bullying. Talk to them about standing up to bullies (saying “stop” confidently or walking away from difficult situations) and staying safe by staying near adults or groups of peers when bullies are present (TABLE 325).
Empowering caregivers. Encourage parents to spend time each day talking with their child about the child’s time away from home (TABLE 325). Counsel parents/caregivers to expand their role. Knowing a child’s friends, encouraging the child academically, and increasing communication are all associated with lower risks of bullying.26 Similarly, parental oversight of Internet and social media use is associated with decreased participation in cyberbullying.27
In addition, the Positive Parenting telephone-based parenting education curriculum has been shown to decrease bullying, physical fighting, physical injuries, and victimization of children.28 The research-based, family strengthening program emphasizes 3 core elements of authoritative parenting: nurturance, discipline, and respect or granting of psychologic autonomy. The program entails 15- to 30-minute weekly phone conversations between parents and educators, as well as videos and a manual.

Are community programs in place—or are they needed?
Many schools have robust, state-mandated programs in place to identify bullying and provide support for students who are victims of bullying. (See “NJ’s harassment and bullying protocol: A case in point.”) Explaining this to victims and their families may help them come forward and seek assistance. FPs who want to advocate for their patients should start with local schools to support such programs and link students at risk with school counselors.
SIDEBAR
NJ's harassment and bullying protocol: A case in pointThere is no federal law that specifically applies to bullying, but all 50 states have some type of anti-bullying legislation on the books, and 40 of those states have additional detailed policies in place addressing the subject.1
New Jersey, for example, began enforcing one of the toughest harassment, intimidation, and bullying (HIB) protocols in the country back in September 20112 in the wake of the death of Rutgers University freshman Tyler Clementi, who committed suicide after his roommate allegedly shot a video of him with another man and posted it to the Internet.3 Among many other things, New Jersey’s legislation stipulates in its Anti-Bullying Bill of Rights4 that:
› Every school/district have plans in place that clearly define, prevent, prohibit, and promptly deal with acts of harassment, intimidation, or bullying, on school grounds, at school-sponsored functions, and on school buses.
› Plans must include a description of the type of behavior expected from each student and the consequences and remedial action for a person who commits an act of harassment, intimidation, or bullying. Student perpetrators may be suspended or expelled if convicted of any type of bullying, whether it be for teasing or something more severe.
› All school employees must act on any incidents of bullying reported to, or witnessed by, them and report such incidents on the same day to the school principal.
› Plans must include provisions and deadlines for investigating and resolving all matters in a timely fashion; investigations into allegations of bullying must be launched within one day.
› Every case of bullying must be reported to the state. Schools are graded by the state on their compliance with anti-bullying standards and policies and their handling of incidents.
› Schools must appoint safety teams made up of parents, teachers, and staff, and school personnel and students must receive extensive anti-bullying training.
1. US Department of Health and Human Services. Stopbullying.gov. Policies and laws. Available at: https://www.stopbullying.gov/laws/index.html.
Accessed January 5, 2017.
2. State of New Jersey Department of Education. An overview of amendments to laws on harassment, intimidation, and bullying. Available at: http://www.state.nj.us/education/students/safety/behavior/hib/overview.pdf. Accessed January 5, 2017.
3. Cohen A. Case study: Why New Jersey’s antibullying law should be a model for other states. Time. September 6, 2011. Available at: http://ideas.time.com/2011/09/06/why-new-jerseys-antibullying-law-should-be-a-model-for-other-states/. Accessed January 5, 2017.
4. New Jersey Legislature. Anti-bullying Bill of Rights Act. Available at: http://www.njleg.state.nj.us/2010/Bills/PL10/122.PDF. Accessed January 5, 2017.
If programs are lacking in your community, there is much you can do to educate yourself about successful programs and advise local community organizations and schools about them. Among the most successful and well-studied interventions for thwarting the bullying epidemic have been school-based community ones. The most studied of these is the Olweus Bullying Prevention Program (OBPP), which is based on 4 principles:1,29
- Adults both at home and at school should take a positive and encouraging interest in students.
- Unacceptable behavior should have strict and well-known limits.
- Sanctions should be applied consistently and should be non-hostile in nature.
- Adults both at home and in the educational environment should act as authorities.
In short, the program focuses on greater awareness and involvement on the part of adults, and employing measures at the school level (eg, surveys, better supervision during break and lunch times), the class level (eg, rules against bullying, regular class meetings with students), and the individual level (eg, serious talks with bullies, victims, parents of involved students).
Research has shown that the OBPP reduces bullying behaviors by as much as 50%, reduces vandalism and truancy, and reduces the number of new victims.12 Limits to the more widespread implementation of the OBPP have consisted mainly of the inability to appropriately train adults, including teachers and other school personnel in educational settings. Despite these limitations, the OBPP has been praised and endorsed by numerous groups, including the US Department of Justice.30
Other non-curricular, school-based programs exist, such as the School-Wide Positive Behavioral Interventions and Supports (SWPBIS). This program is a school-wide prevention strategy aimed at: 1) reducing behavior problems that lead to office discipline referrals and suspensions, and 2) changing perceptions of school safety. (For more information, see https://www.crimesolutions.gov/ProgramDetails.aspx?ID=385 and https://www.pbis.org/school/swpbis-for-beginners.)31
The research-based Second Step: Student Success Through Prevention (SS-SSTP) Middle School Program (http://www.cfchildren.org/second-step/middle-school)32 focuses on the often difficult middle school years. The program helps schools teach and model essential communication, coping, and decision-making skills to help adolescents navigate around common pitfalls such as peer pressure, substance abuse, and bullying (both in-person and online). The program aims to reduce aggression and provide support for a more inclusive environment that helps students stay in school, make good choices, and experience social and academic success.
The Positive Action Program (https://www.positiveaction.net/research/primer),33 which is predicated on the notion that we feel good about ourselves when we do positive things, features scripted lessons and kits of materials (eg, posters, games, worksheets, puzzles) appropriate for each grade level.
CASE › Stacey’s visit to her FP’s office has presented several clues that she may be a victim of bullying. Her mild persistent asthma appears to no longer be as well controlled as it was in the past. Direct questioning has revealed that 2 girls at school have been making fun of Stacey when she uses her inhaled corticosteroid in the morning before class, so she has stopped using it. These same students are on her cheerleading team, so she quit the team to avoid them. Her school-related anxiety is so great that she no longer pays attention in math class and is constantly worried that something is being posted about her online.
Stacy’s FP responds to this information with a multifaceted approach. In the exam room, he screens Stacy for depression. While she is negative and denies any suicidal ideation, Stacy is clearly having anxiety, so the FP refers Stacey to a counselor at a local mental health clinic. With Stacy’s permission, the FP discusses the issue with her mother and they decide together with Stacy that she should talk to a teacher at school about the ongoing bullying. Because this was not the first time that the FP has heard this from a child in the community, the FP plans to attend an upcoming school board meeting to advocate for an evidence-based bullying prevention program to help curb the ongoing problem facing his patients.
CORRESPONDENCE
Robert McClowry, MD, Department of Family and Community Medicine, Jefferson Family Medicine Associates, 833 Chestnut East, 3rd Floor, Suite 301, Philadelphia, PA, 19107-4414; [email protected].
CASE › Stacey, a 12-year-old girl with mild persistent asthma, presents to her family physician (FP) with her mother for her annual well visit. Stacey reports no complaints, but has visited twice recently for acute exacerbations of her asthma, which had previously been well-controlled. When reviewing her social history, Stacey reports that she started her second year of middle school 3 months ago. When asked if she enjoys school, Stacey looks down and says, “School is fine.” Her mother quickly adds that Stacey has quit the school cheerleading team—much to the coach’s dismay—and is having difficulty in her math class, a class in which she normally excels. Stacey appears embarrassed that her mother has brought these things up. Her mother says that at the beginning of the year, 2 girls began picking on Stacey, calling her names and making fun of her on social media and in front of other students.
For many years, bullying was trivialized. Some viewed it as a universal childhood experience; others considered it a rite of passage.1,2 It was not examined as a public health issue until the 1970s. In fact, no legislation addressing bullying or “peer abuse” existed in the United States until the mass shooting at Columbine High School in Littleton, Colo, in 1999. Within 3 years of the Columbine tragedy, the number of state laws that mentioned bullying went from zero to 15; within 10 years of Columbine, 41 states had laws addressing bullying,1 and by 2015, every state, the District of Columbia, and some territories had a bullying law in place.3
As research and advocacy regarding bullying has grown, its impact on the health of children, adolescents, and even adults has become more apparent. In a 2001 study of school-associated violent deaths in the United States between 1994 and 1999, the Centers for Disease Control and Prevention (CDC) found that among students, homicide perpetrators were more than twice as likely as homicide victims to have been bullied by peers.4 Given that homicide is the third leading cause of death in people ages 15 to 24,5 past exposure to bullying may be a significant contributing factor to mortality in this age group.4
In addition to a correlation with homicidal behavior, those involved in bullying—whether as the bully or victim—are at risk for a wide range of symptoms, conditions, and problems including poor psychosocial adjustment, depression, anxiety, suicide (the second leading cause of death in the 10-14 and 15-24 age groups5), academic decline, psychosomatic manifestations, fighting, alcohol use, smoking, and difficulty with the management of chronic diseases.6-10 Not only does being a victim of bullying have a direct impact on a child’s current mental and physical well-being, but it can have lasting psychological and behavioral effects that can follow children well into adulthood.7 The significant impact of bullying on individuals and society as a whole mandates a multifaceted approach that begins in your exam room. What follows is practical advice on screening, counseling, and working with schools and the community at large to curb the bullying epidemic.

Clarifying the problem: The CDC’s definition
Recognizing that varying definitions of bullying were being used in research studies that looked at violent or aggressive behaviors in youth, the CDC published a consensus statement in 2014 that proposed the following definition for bullying:11 any unwanted aggressive behavior by another youth or group of youths who are not siblings or current dating partners that involves an observed or perceived power imbalance and is repeated multiple times or is highly likely to be repeated. This expanded on an earlier definition by Olweus12,13 that also identified a longitudinal nature and power imbalance as key features.
Types of bullying. Direct bullying entails blatant attacks on a targeted young person, while indirect bullying involves communication with others about the targeted individual (eg, spreading harmful rumors). Bullying may be physical, verbal, or relational (eg, excluding someone from their usual social circle, denying friendship, the silent treatment, writing mean letters, eye rolling, etc.) and may involve damage to property. Boys tend toward more direct bullying behaviors, while girls more often engage in indirect bullying, which may be more challenging for both adults and other students to recognize.12,13 With increased use of technology and social media by adolescents, cyberbullying has become increasingly more prevalent, with its effects on adolescent health and academics being every bit as profound as those of traditional bullying.14
About 1 in 4/5 students suffer. The prevalence of bullying ranges by country and culture. The vast majority of early bullying research was conducted in Norway, which found that approximately 15% of students in elementary and secondary schools were involved in bullying in some capacity.12 In a study involving over 200,000 adolescents from 40 European countries, 26% of adolescents reported being involved in bullying, ranging from 8.6% to 45.2% for boys and 4.8% to 35.8% for girls.15 Variations in prevalence may be due to cultural differences in the acts of bullying or differences in interpretation of the term “bullying.”1,15
In the United States, a 2001 survey of more than 15,000 students in public and private schools (grades 6-10) asked the students about their involvement in bullying: 13% said they'd been a bully, 10.6% a victim, and 6.3% said they'd been both.6 There was no significant difference in the frequency of self-reported bullying among urban, suburban, or rural settings.
Despite efforts to educate the public about bullying and work with schools to intervene and prevent bullying, incidence remains largely unchanged. In 2013, the National Crime Victimization Survey reported that approximately 22% of adolescents ages 12 through 18 were victims of bullying.16 Similarly, the CDC's 2015 Youth Risk Behavior Surveillance System reported that 20.2% of high school students experienced bullying on school property.17
Screening: Best practices
The FP’s role begins with screening children at risk for bullying (TABLE 118-22) or those whose complaints suggest that they may be victims of bullying.
Start screening when children enter elementary school
Given that providers’ time is limited for every patient visit, it is important to address bullying at times that are most likely to yield impactful results. The American Academy of Pediatrics recommends that the topic of bullying be introduced at the 6-year-old well-child visit (a typical age for entry to elementary school).7 Views in the literature are inconsistent regarding when and how to address bullying at other time points. One approach is to pre-screen those with risk factors associated with bullying (TABLE 118-22), and to focus screening on those with warning signs of bullying, which include mood disorders, psychosomatic or behavioral symptoms, substance abuse, self-harm behaviors, suicidal ideation or a suicide attempt, a decline in academic performance, and reports of school truancy. Parental concerns, such as when a child suddenly needs more money for lunch, is having aggressive outbursts, or is exhibiting unexplained physical injuries, should also be regarded as cues to screen.9

Screen patients in high-risk groups

A number of groups of children are at high risk for bullying and warrant targeted screening efforts.
- Children with special health needs. Research has shown that children with special health needs are at increased risk for being bullied.18 In fact, the presence of a chronic disease may increase the risk for bullying, and bullying often negatively impacts chronic disease management. As a result, it’s important to have a high index of suspicion with patients who have a chronic disease and who are not responding as expected to medical management or who experience deterioration after being previously well-controlled.18
- Children who are under- or overweight. Similarly, bullying based on a child's weight is a phenomenon that has been recognized to have a significant impact on children’s emotional health.19
- Youth who identify as lesbian, gay, bisexual, transgender, or queer/questioning (LGBTQ+) are more likely than non-LGBTQ+ peers to attempt suicide when exposed to a hostile social environment, such as that created by bullying.20

Screening need not be complicated

One screening approach is simply to ask patients, “Are you being bullied?” followed by such questions as, “How often are you bullied?” or “How long have you been bullied?” Asking about the setting of the bullying (Does it happen at school? Traveling to/from school? Online?) and other details may help guide interventions and the provision of resources.9 Another approach is to provide patients with some type of written survey (see TABLE 223 for an example) to encourage responses that patients might be reluctant to disclose verbally.23,24 (See “Barriers to screening.")
SIDEBAR
Barriers to screeningScreening for any condition presupposes a response. Ideally, family physicians should be prepared to provide basic counseling, resources, and, if necessary, treatment, if a patient screens positive for bullying. But screening for violence or bullying can be difficult, and evidence-based guidelines for screening and intervention are lacking, leaving many primary care practitioners feeling ill-equipped to meaningfully respond.
One study of the use of a screening tool aimed at intimate partner violence (IPV) showed that even with the availability of a screening tool, health care providers’ use of the tool was inconsistent and referral practices were ineffective.1 Providers cited the following limiting factors in screening for IPV: 1) a lack of immediate referral availability, 2) a lack of time during the office visit, and 3) a lack of confidence in the ability to screen.1 These same issues may be barriers to screening for bullying.
1. Ramachandran DV, Covarrubias L, Watson C, et al. How you screen is as important as whether you screen: a qualitative analysis of violence screening practices in reproductive health clinics. J Community Health. 2013;38:856-863.
Provider and parental interventions
Interventions often entail counseling the patient and the family about bullying and its effects, empowering victims and their caregivers, and screening for bullying comorbidities and correlates.2 Refer patients to behavioral health specialists when there is evidence of pervasive effects on mood, behavior, or social development, but keep in mind that counseling can begin in your own exam room.
Effective discussion starters. Affirming the problem and its unacceptability, talking about the different types of bullying and where bullying may occur, and asking about patient perceptions of bullying can be effective discussion starters. FPs should help patients identify bullying, open lines of communication between children and their parents and between parents and other caregivers, and demonstrate respect and kindness in their approach to discussing the topic. Encourage children to speak with trusted adults when exposed to bullying. Talk to them about standing up to bullies (saying “stop” confidently or walking away from difficult situations) and staying safe by staying near adults or groups of peers when bullies are present (TABLE 325).
Empowering caregivers. Encourage parents to spend time each day talking with their child about the child’s time away from home (TABLE 325). Counsel parents/caregivers to expand their role. Knowing a child’s friends, encouraging the child academically, and increasing communication are all associated with lower risks of bullying.26 Similarly, parental oversight of Internet and social media use is associated with decreased participation in cyberbullying.27
In addition, the Positive Parenting telephone-based parenting education curriculum has been shown to decrease bullying, physical fighting, physical injuries, and victimization of children.28 The research-based, family strengthening program emphasizes 3 core elements of authoritative parenting: nurturance, discipline, and respect or granting of psychologic autonomy. The program entails 15- to 30-minute weekly phone conversations between parents and educators, as well as videos and a manual.

Are community programs in place—or are they needed?
Many schools have robust, state-mandated programs in place to identify bullying and provide support for students who are victims of bullying. (See “NJ’s harassment and bullying protocol: A case in point.”) Explaining this to victims and their families may help them come forward and seek assistance. FPs who want to advocate for their patients should start with local schools to support such programs and link students at risk with school counselors.
SIDEBAR
NJ's harassment and bullying protocol: A case in pointThere is no federal law that specifically applies to bullying, but all 50 states have some type of anti-bullying legislation on the books, and 40 of those states have additional detailed policies in place addressing the subject.1
New Jersey, for example, began enforcing one of the toughest harassment, intimidation, and bullying (HIB) protocols in the country back in September 20112 in the wake of the death of Rutgers University freshman Tyler Clementi, who committed suicide after his roommate allegedly shot a video of him with another man and posted it to the Internet.3 Among many other things, New Jersey’s legislation stipulates in its Anti-Bullying Bill of Rights4 that:
› Every school/district have plans in place that clearly define, prevent, prohibit, and promptly deal with acts of harassment, intimidation, or bullying, on school grounds, at school-sponsored functions, and on school buses.
› Plans must include a description of the type of behavior expected from each student and the consequences and remedial action for a person who commits an act of harassment, intimidation, or bullying. Student perpetrators may be suspended or expelled if convicted of any type of bullying, whether it be for teasing or something more severe.
› All school employees must act on any incidents of bullying reported to, or witnessed by, them and report such incidents on the same day to the school principal.
› Plans must include provisions and deadlines for investigating and resolving all matters in a timely fashion; investigations into allegations of bullying must be launched within one day.
› Every case of bullying must be reported to the state. Schools are graded by the state on their compliance with anti-bullying standards and policies and their handling of incidents.
› Schools must appoint safety teams made up of parents, teachers, and staff, and school personnel and students must receive extensive anti-bullying training.
1. US Department of Health and Human Services. Stopbullying.gov. Policies and laws. Available at: https://www.stopbullying.gov/laws/index.html.
Accessed January 5, 2017.
2. State of New Jersey Department of Education. An overview of amendments to laws on harassment, intimidation, and bullying. Available at: http://www.state.nj.us/education/students/safety/behavior/hib/overview.pdf. Accessed January 5, 2017.
3. Cohen A. Case study: Why New Jersey’s antibullying law should be a model for other states. Time. September 6, 2011. Available at: http://ideas.time.com/2011/09/06/why-new-jerseys-antibullying-law-should-be-a-model-for-other-states/. Accessed January 5, 2017.
4. New Jersey Legislature. Anti-bullying Bill of Rights Act. Available at: http://www.njleg.state.nj.us/2010/Bills/PL10/122.PDF. Accessed January 5, 2017.
If programs are lacking in your community, there is much you can do to educate yourself about successful programs and advise local community organizations and schools about them. Among the most successful and well-studied interventions for thwarting the bullying epidemic have been school-based community ones. The most studied of these is the Olweus Bullying Prevention Program (OBPP), which is based on 4 principles:1,29
- Adults both at home and at school should take a positive and encouraging interest in students.
- Unacceptable behavior should have strict and well-known limits.
- Sanctions should be applied consistently and should be non-hostile in nature.
- Adults both at home and in the educational environment should act as authorities.
In short, the program focuses on greater awareness and involvement on the part of adults, and employing measures at the school level (eg, surveys, better supervision during break and lunch times), the class level (eg, rules against bullying, regular class meetings with students), and the individual level (eg, serious talks with bullies, victims, parents of involved students).
Research has shown that the OBPP reduces bullying behaviors by as much as 50%, reduces vandalism and truancy, and reduces the number of new victims.12 Limits to the more widespread implementation of the OBPP have consisted mainly of the inability to appropriately train adults, including teachers and other school personnel in educational settings. Despite these limitations, the OBPP has been praised and endorsed by numerous groups, including the US Department of Justice.30
Other non-curricular, school-based programs exist, such as the School-Wide Positive Behavioral Interventions and Supports (SWPBIS). This program is a school-wide prevention strategy aimed at: 1) reducing behavior problems that lead to office discipline referrals and suspensions, and 2) changing perceptions of school safety. (For more information, see https://www.crimesolutions.gov/ProgramDetails.aspx?ID=385 and https://www.pbis.org/school/swpbis-for-beginners.)31
The research-based Second Step: Student Success Through Prevention (SS-SSTP) Middle School Program (http://www.cfchildren.org/second-step/middle-school)32 focuses on the often difficult middle school years. The program helps schools teach and model essential communication, coping, and decision-making skills to help adolescents navigate around common pitfalls such as peer pressure, substance abuse, and bullying (both in-person and online). The program aims to reduce aggression and provide support for a more inclusive environment that helps students stay in school, make good choices, and experience social and academic success.
The Positive Action Program (https://www.positiveaction.net/research/primer),33 which is predicated on the notion that we feel good about ourselves when we do positive things, features scripted lessons and kits of materials (eg, posters, games, worksheets, puzzles) appropriate for each grade level.
CASE › Stacey’s visit to her FP’s office has presented several clues that she may be a victim of bullying. Her mild persistent asthma appears to no longer be as well controlled as it was in the past. Direct questioning has revealed that 2 girls at school have been making fun of Stacey when she uses her inhaled corticosteroid in the morning before class, so she has stopped using it. These same students are on her cheerleading team, so she quit the team to avoid them. Her school-related anxiety is so great that she no longer pays attention in math class and is constantly worried that something is being posted about her online.
Stacy’s FP responds to this information with a multifaceted approach. In the exam room, he screens Stacy for depression. While she is negative and denies any suicidal ideation, Stacy is clearly having anxiety, so the FP refers Stacey to a counselor at a local mental health clinic. With Stacy’s permission, the FP discusses the issue with her mother and they decide together with Stacy that she should talk to a teacher at school about the ongoing bullying. Because this was not the first time that the FP has heard this from a child in the community, the FP plans to attend an upcoming school board meeting to advocate for an evidence-based bullying prevention program to help curb the ongoing problem facing his patients.
CORRESPONDENCE
Robert McClowry, MD, Department of Family and Community Medicine, Jefferson Family Medicine Associates, 833 Chestnut East, 3rd Floor, Suite 301, Philadelphia, PA, 19107-4414; [email protected].
1. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80:124-134.
2. Lyznicki JM, McCaffree MA, Robinowitz CB, et al. Childhood bullying: implications for physicians. Am Fam Physician. 2004;70:1723-1730.
3. Temkin D. All 50 states now have a bullying law. Now what? The Huffington Post. April 27, 2015. Available at: http://www.huffingtonpost.com/deborah-temkin/all-50-states-now-have-a_b_7153114.html. Accessed January 5, 2017.
4. Anderson M, Kaufman J, Simon TR, et al. School-associated violent deaths in the United States, 1994-1999. JAMA. 2001;286:2695-2702.
5. Centers for Disease Control and Prevention. Injury prevention and control: Data and statistics (WISQARS). Ten leading causes of death and injury. Available at: https://www.cdc.gov/injury/wisqars/leadingcauses.html. Accessed January 5, 2017.
6. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285:2094-2100.
7. Committee on Injury, Violence, and Poison Prevention. Policy statement—Role of the pediatrician in youth violence prevention. Pediatrics. 2009;124:393-402.
8. Klein DA, Myhre KK, Ahrendt DM. Bullying among adolescents: a challenge in primary care. Am Fam Physician. 2013;88:87-92.
9. Lamb J, Pepler DJ, Craig W. Approach to bullying and victimization. Can Fam Physician. 2009;55:356-360.
10. Spector ND, Kelly SF. Pediatrician’s role in screening and treatment: bullying, prediabetes, oral health. Curr Opin Pediatr. 2006;18:661-670.
11. Gladden RM, Vivolo-Kantor AM, Hamburger ME, et al. Bullying surveillance among youths: uniform definitions for public health and recommended data elements. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention and US Department of Education; 2014. Available at: https://www.cdc.gov/violenceprevention/pdf/bullying-definitions-final-a.pdf. Accessed June 8, 2016.
12. Olweus D. Bullying at school: basic facts and effects of a school based intervention program. J Child Psychol Psychiatry. 1994;35:1171-1190.
13. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
14. Kowalski RM, Limber SP. Psychological, physical, and academic correlates of cyberbullying and traditional bullying. J Adolesc Health. 2013;53:S13-S20.
15. Craig W, Harel-Fisch Y, Fogel-Grinvald H, et al. A cross-national profile of bullying and victimization among adolescents in 40 countries. Int J Public Health. 2009;54(Suppl 2):216-224.
16. US Department of Education, National Center for Educational Statistics (2015). Student reports of bullying and cyberbullying: results from the 2013 School Crime Supplement to the National Victimization Survey. Available at: http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2015056. Accessed November 9, 2016.
17. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1-174.
18. Van Cleave J, Davis MM. Bullying and peer victimization among children with special health care needs. Pediatrics. 2006;118:e1212-e1219.
19. Eisenberg ME, Neumark-Sztainer D, Story M. Associations of weight-based teasing and emotional well-being among adolescents. Arch Pediatr Adolesc Med. 2003;157:733-738.
20. Hatzenbuehler ML. The social environment and suicide attempts in lesbian, gay, and bisexual youth. Pediatrics. 2011;127:896-903.
21. Song LY, Singer MI, Anglin TM. Violence exposure and emotional trauma as contributors to adolescents’ violent behaviors. Arch Pediatr Adolesc Med. 1998;152:531-536.
22. Singer MI, Anglin TM, Song LY, et al. Adolescents’ exposure to violence and associated symptoms of psychological trauma. JAMA. 1995;273:477-482.
23. Glew GM, Fan MY, Katon W, et al. Bullying, psychosocial adjustment, and academic performance in elementary school. Arch Pediatr Adolesc Med. 2005;159:1026-1031.
24. Waseem M, Ryan M, Foster CB, et al. Assessment and management of bullied children in the emergency department. Pediatr Emerg Care. 2013;29:389-398.
25. US Department of Health and Human Services. stopbullying.gov. Available at: https//www.stopbullying.gov. Accessed January 5, 2017.
26. Shetgiri R, Lin H, Avila RM, et al. Parental characteristics associated with bullying perpetration in US children aged 10 to 17 years. Am J Public Health. 2012;102:2280-2286.
27. Hinduja S, Patchin JW. Social influences on cyberbullying behaviors among middle and high school students. J Youth Adolesc. 2013;42:711-722.
28. Borowsky IW, Mozayeny S, Stuenkel K, et al. Effects of a primary care-based intervention on violent behavior and injury in children. Pediatrics. 2004;114:e392-e399.
29. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
30. Mihalic SF. Blueprints for Violence Prevention: Report. US Department of Justice, Office of Justice Programs, Office of Juvenile Justice and Delinquency Prevention; 2004.
31. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med. 2012;166:149-156.
32. Espelage DL, Low S, Polanin JR, et al. The impact of a middle school program to reduce aggression, victimization, and sexual violence. J Adolesc Health. 2013;53:180-186.
33. Lewis KM, Schure MB, Bavarian N, et al. Problem behavior and urban, low-income youth: a randomized controlled trial of positive action in Chicago. Am J Prev Med. 2013;44:622-630.
1. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80:124-134.
2. Lyznicki JM, McCaffree MA, Robinowitz CB, et al. Childhood bullying: implications for physicians. Am Fam Physician. 2004;70:1723-1730.
3. Temkin D. All 50 states now have a bullying law. Now what? The Huffington Post. April 27, 2015. Available at: http://www.huffingtonpost.com/deborah-temkin/all-50-states-now-have-a_b_7153114.html. Accessed January 5, 2017.
4. Anderson M, Kaufman J, Simon TR, et al. School-associated violent deaths in the United States, 1994-1999. JAMA. 2001;286:2695-2702.
5. Centers for Disease Control and Prevention. Injury prevention and control: Data and statistics (WISQARS). Ten leading causes of death and injury. Available at: https://www.cdc.gov/injury/wisqars/leadingcauses.html. Accessed January 5, 2017.
6. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285:2094-2100.
7. Committee on Injury, Violence, and Poison Prevention. Policy statement—Role of the pediatrician in youth violence prevention. Pediatrics. 2009;124:393-402.
8. Klein DA, Myhre KK, Ahrendt DM. Bullying among adolescents: a challenge in primary care. Am Fam Physician. 2013;88:87-92.
9. Lamb J, Pepler DJ, Craig W. Approach to bullying and victimization. Can Fam Physician. 2009;55:356-360.
10. Spector ND, Kelly SF. Pediatrician’s role in screening and treatment: bullying, prediabetes, oral health. Curr Opin Pediatr. 2006;18:661-670.
11. Gladden RM, Vivolo-Kantor AM, Hamburger ME, et al. Bullying surveillance among youths: uniform definitions for public health and recommended data elements. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention and US Department of Education; 2014. Available at: https://www.cdc.gov/violenceprevention/pdf/bullying-definitions-final-a.pdf. Accessed June 8, 2016.
12. Olweus D. Bullying at school: basic facts and effects of a school based intervention program. J Child Psychol Psychiatry. 1994;35:1171-1190.
13. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
14. Kowalski RM, Limber SP. Psychological, physical, and academic correlates of cyberbullying and traditional bullying. J Adolesc Health. 2013;53:S13-S20.
15. Craig W, Harel-Fisch Y, Fogel-Grinvald H, et al. A cross-national profile of bullying and victimization among adolescents in 40 countries. Int J Public Health. 2009;54(Suppl 2):216-224.
16. US Department of Education, National Center for Educational Statistics (2015). Student reports of bullying and cyberbullying: results from the 2013 School Crime Supplement to the National Victimization Survey. Available at: http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2015056. Accessed November 9, 2016.
17. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1-174.
18. Van Cleave J, Davis MM. Bullying and peer victimization among children with special health care needs. Pediatrics. 2006;118:e1212-e1219.
19. Eisenberg ME, Neumark-Sztainer D, Story M. Associations of weight-based teasing and emotional well-being among adolescents. Arch Pediatr Adolesc Med. 2003;157:733-738.
20. Hatzenbuehler ML. The social environment and suicide attempts in lesbian, gay, and bisexual youth. Pediatrics. 2011;127:896-903.
21. Song LY, Singer MI, Anglin TM. Violence exposure and emotional trauma as contributors to adolescents’ violent behaviors. Arch Pediatr Adolesc Med. 1998;152:531-536.
22. Singer MI, Anglin TM, Song LY, et al. Adolescents’ exposure to violence and associated symptoms of psychological trauma. JAMA. 1995;273:477-482.
23. Glew GM, Fan MY, Katon W, et al. Bullying, psychosocial adjustment, and academic performance in elementary school. Arch Pediatr Adolesc Med. 2005;159:1026-1031.
24. Waseem M, Ryan M, Foster CB, et al. Assessment and management of bullied children in the emergency department. Pediatr Emerg Care. 2013;29:389-398.
25. US Department of Health and Human Services. stopbullying.gov. Available at: https//www.stopbullying.gov. Accessed January 5, 2017.
26. Shetgiri R, Lin H, Avila RM, et al. Parental characteristics associated with bullying perpetration in US children aged 10 to 17 years. Am J Public Health. 2012;102:2280-2286.
27. Hinduja S, Patchin JW. Social influences on cyberbullying behaviors among middle and high school students. J Youth Adolesc. 2013;42:711-722.
28. Borowsky IW, Mozayeny S, Stuenkel K, et al. Effects of a primary care-based intervention on violent behavior and injury in children. Pediatrics. 2004;114:e392-e399.
29. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
30. Mihalic SF. Blueprints for Violence Prevention: Report. US Department of Justice, Office of Justice Programs, Office of Juvenile Justice and Delinquency Prevention; 2004.
31. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med. 2012;166:149-156.
32. Espelage DL, Low S, Polanin JR, et al. The impact of a middle school program to reduce aggression, victimization, and sexual violence. J Adolesc Health. 2013;53:180-186.
33. Lewis KM, Schure MB, Bavarian N, et al. Problem behavior and urban, low-income youth: a randomized controlled trial of positive action in Chicago. Am J Prev Med. 2013;44:622-630.
PRACTICE RECOMMENDATIONS
› Suspect bullying when children with chronic conditions that were stable begin deteriorating for unexplained reasons or when children become non-adherent to medication regimens. C
› Empower not only patients, but also parents/caregivers, to take action and deter bullying behaviors. B
› Support school-based and community-oriented intervention programs, which have been shown to be among the most effective strategies for curbing bullying. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Recurrent UTIs in women: How you can refine your care
CASE › For the third time in 9 months, 28-year-old Joan B comes into the office with complaints of painful, frequent, and urgent urination. Ms B is sexually active and her medical history is otherwise unremarkable. In each of the previous 2 episodes, her urine culture grew Escherichia coli, and she was treated with a 5-day course of nitrofurantoin. At this current visit, she asks about the need for additional work-up, treatment for her symptoms, and whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women1 and account for an estimated 5.4 million primary care office visits and 2.3 million emergency room visits annually.2 For women, the lifetime risk of developing a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women under 55 and 53% of women over 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 105 colony forming units (ie, viable bacteria) per milliliter of urine collected midstream on 2 consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant, have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI, and the term applies when such an infection occurs within 6 months of the last UTI or when 3 or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within 2 weeks of the original treatment.9 Reinfection is a UTI that occurs more than 2 weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following 7 to 14 days of appropriate antibiotic treatment.9
Factors that increase the risk of recurrent UTI
Premenopausal women
Both modifiable and non-modifiable factors (TABLE 110-21) have been associated with increased risk of recurrent UTI in premenopausal women. Among women with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other non-modifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are women whose mothers had no such history.13
Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse during the week, sexually active college women who had engaged in intercourse 3 times had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk of UTI development.15 This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women,21 which is thought to predispose them to UTI. These risk factors are summarized in TABLE 1.10-21
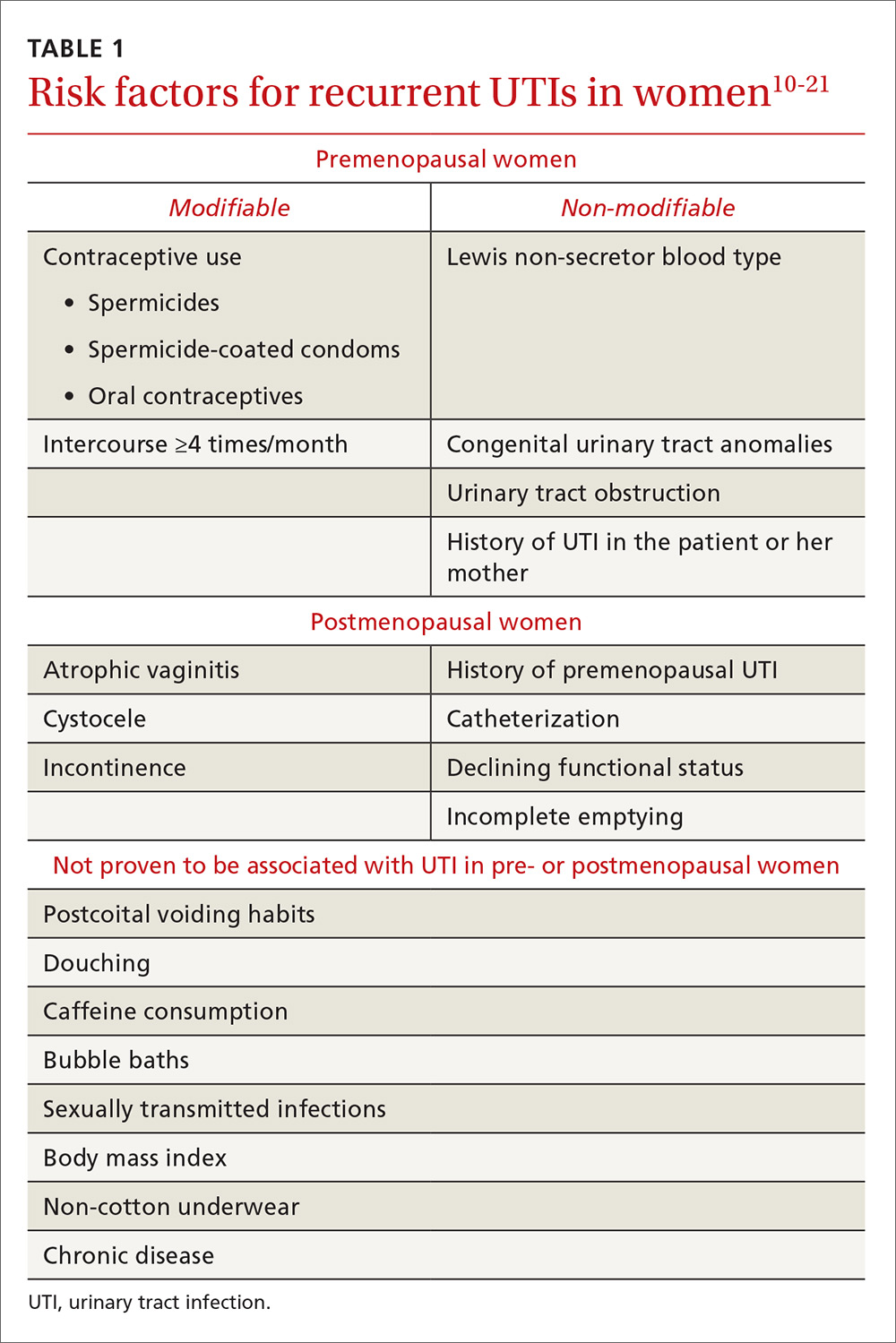
Initial evaluation of recurrent UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (TABLE 218). Likewise, perform a pelvic examination to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18
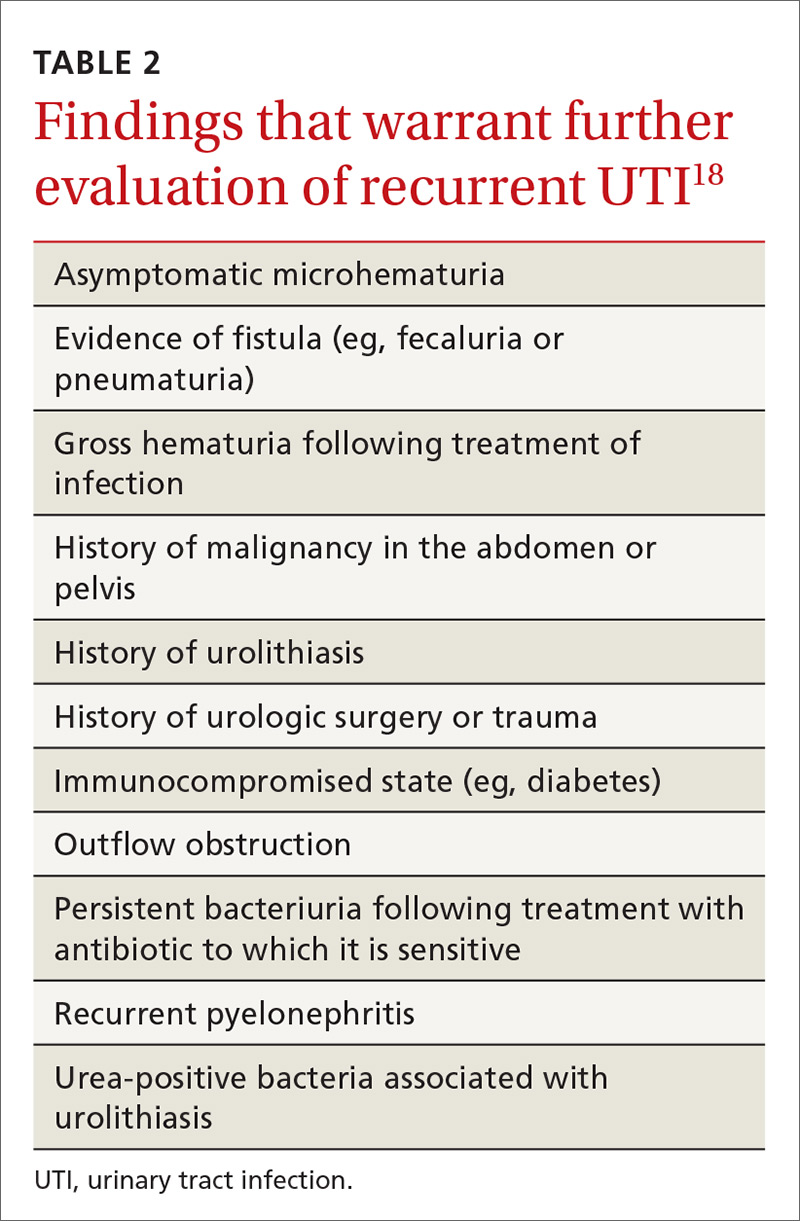
Treatment options and precautions
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX), 160 mg/800 mg twice daily for 3 days, has long been the mainstay of treatment for uncomplicated UTI. Over recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as first-line treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin 100 mg twice daily for 5 days has efficacy similar to that of TMP-SMX, but without significant bacterial resistance. While fosfomycin 3 g as a single dose is still recommended as first-line treatment, it is less effective than either TMP-SMX or nitrofurantoin. TABLE 324 summarizes these antibiotic choices and their efficacies.
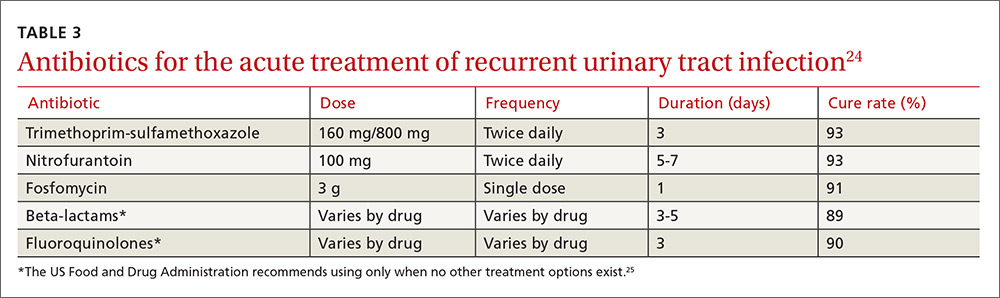
Agents to avoid or use only as a last resort. For patients unable to take any of the drugs above, consider beta-lactam antibiotics, although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the Food and Drug Administration issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 days) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 days). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for women who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (TABLE 324). Patient-initiated treatment yields similar rates of efficacy as physician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies28 (which we’ll discuss in a moment).
Time for imaging and referral?
For patients with a high risk of complicated UTI or a surgically amenable condition, either ultrasound or computerized tomography (CT) of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in TABLE 2.18
2 weeks later…and it’s back? Finally, for women who experience recurrent symptoms within 2 weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
Prevention dos and don’ts
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or who douche, consume caffeinated beverages, or wear non-cotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Physicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had 2 or more UTIs in the past 6 months29 or 3 or more UTIs in the past year.30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or post-coital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to 2 weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin31,32 (TABLE 431), none of which demonstrated superiority in a Cochrane review.33 Although the same review showed no optimal duration of treatment,33 6 to 24 months of treatment is usually recommended.29
A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence35,36 (TABLE 431).

Several non-pharmacologic strategies have been suggested for preventing recurrent UTI—eg, use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and D-mannose.
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a non-inferiority trial and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolysed to bactericidal ammonia and formaldehyde), and D-mannose (which inhibits bacterial adherence), have been shown—in limited studies—to decrease recurrence of UTIs.41-43 Further study is necessary to confirm their efficacy in preventing UTIs.
As noted, the only behavioral modifications that have been shown to decrease the risk of recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
CASE › Ms. B is started on a 3-day course of TMP-SMX. Further questioning reveals that each of her 3 UTIs followed sexual intercourse. Her physician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
CORRESPONDENCE
Jeffrey D. Quinlan, MD, FAAFP, Family Medicine, Room A-1038A, 4301 Jones Bridge Road, Bethesda, MD 20814-4712; [email protected].
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. Centers for Disease Control and Prevention. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. Available at: www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed August 31, 2016.
3. Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991;164:1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New Eng J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(Suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. U.S. Food and Drug Administration. FDA drug safety communication. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed November 7, 2016.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988;9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157:935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trail. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;(10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Kranjčec B, Papeš D, Altarac S. D-m
CASE › For the third time in 9 months, 28-year-old Joan B comes into the office with complaints of painful, frequent, and urgent urination. Ms B is sexually active and her medical history is otherwise unremarkable. In each of the previous 2 episodes, her urine culture grew Escherichia coli, and she was treated with a 5-day course of nitrofurantoin. At this current visit, she asks about the need for additional work-up, treatment for her symptoms, and whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women1 and account for an estimated 5.4 million primary care office visits and 2.3 million emergency room visits annually.2 For women, the lifetime risk of developing a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women under 55 and 53% of women over 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 105 colony forming units (ie, viable bacteria) per milliliter of urine collected midstream on 2 consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant, have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI, and the term applies when such an infection occurs within 6 months of the last UTI or when 3 or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within 2 weeks of the original treatment.9 Reinfection is a UTI that occurs more than 2 weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following 7 to 14 days of appropriate antibiotic treatment.9
Factors that increase the risk of recurrent UTI
Premenopausal women
Both modifiable and non-modifiable factors (TABLE 110-21) have been associated with increased risk of recurrent UTI in premenopausal women. Among women with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other non-modifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are women whose mothers had no such history.13
Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse during the week, sexually active college women who had engaged in intercourse 3 times had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk of UTI development.15 This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women,21 which is thought to predispose them to UTI. These risk factors are summarized in TABLE 1.10-21

Initial evaluation of recurrent UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (TABLE 218). Likewise, perform a pelvic examination to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

Treatment options and precautions
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX), 160 mg/800 mg twice daily for 3 days, has long been the mainstay of treatment for uncomplicated UTI. Over recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as first-line treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin 100 mg twice daily for 5 days has efficacy similar to that of TMP-SMX, but without significant bacterial resistance. While fosfomycin 3 g as a single dose is still recommended as first-line treatment, it is less effective than either TMP-SMX or nitrofurantoin. TABLE 324 summarizes these antibiotic choices and their efficacies.

Agents to avoid or use only as a last resort. For patients unable to take any of the drugs above, consider beta-lactam antibiotics, although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the Food and Drug Administration issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 days) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 days). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for women who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (TABLE 324). Patient-initiated treatment yields similar rates of efficacy as physician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies28 (which we’ll discuss in a moment).
Time for imaging and referral?
For patients with a high risk of complicated UTI or a surgically amenable condition, either ultrasound or computerized tomography (CT) of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in TABLE 2.18
2 weeks later…and it’s back? Finally, for women who experience recurrent symptoms within 2 weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
Prevention dos and don’ts
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or who douche, consume caffeinated beverages, or wear non-cotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Physicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had 2 or more UTIs in the past 6 months29 or 3 or more UTIs in the past year.30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or post-coital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to 2 weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin31,32 (TABLE 431), none of which demonstrated superiority in a Cochrane review.33 Although the same review showed no optimal duration of treatment,33 6 to 24 months of treatment is usually recommended.29
A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence35,36 (TABLE 431).

Several non-pharmacologic strategies have been suggested for preventing recurrent UTI—eg, use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and D-mannose.
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a non-inferiority trial and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolysed to bactericidal ammonia and formaldehyde), and D-mannose (which inhibits bacterial adherence), have been shown—in limited studies—to decrease recurrence of UTIs.41-43 Further study is necessary to confirm their efficacy in preventing UTIs.
As noted, the only behavioral modifications that have been shown to decrease the risk of recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
CASE › Ms. B is started on a 3-day course of TMP-SMX. Further questioning reveals that each of her 3 UTIs followed sexual intercourse. Her physician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
CORRESPONDENCE
Jeffrey D. Quinlan, MD, FAAFP, Family Medicine, Room A-1038A, 4301 Jones Bridge Road, Bethesda, MD 20814-4712; [email protected].
CASE › For the third time in 9 months, 28-year-old Joan B comes into the office with complaints of painful, frequent, and urgent urination. Ms B is sexually active and her medical history is otherwise unremarkable. In each of the previous 2 episodes, her urine culture grew Escherichia coli, and she was treated with a 5-day course of nitrofurantoin. At this current visit, she asks about the need for additional work-up, treatment for her symptoms, and whether there is a way to prevent further infections.
Urinary tract infections (UTIs) are the most common bacterial infection in women1 and account for an estimated 5.4 million primary care office visits and 2.3 million emergency room visits annually.2 For women, the lifetime risk of developing a UTI is greater than 50%.3 In one study of UTI in a primary care setting, 36% of women under 55 and 53% of women over 55 had a recurrent infection within a year.4 Most women with UTI are treated as outpatients, but 16.7% require hospitalization.5 In the United States, direct costs for evaluation and treatment of UTI total $1.6 billion each year.5
Accurately characterizing recurrent UTI
Bacteriuria is defined as the presence of 105 colony forming units (ie, viable bacteria) per milliliter of urine collected midstream on 2 consecutive urinations.6 UTIs are symptomatic infections of the urinary tract and may involve the urethra, bladder, ureters, or kidneys.7 Infections of the lower tract (bladder and urethra) are commonly referred to as cystitis; infections of the upper tract (kidney and ureters) are referred to as pyelonephritis.
Most UTIs are uncomplicated and do not progress to more serious infections. However, patients who are pregnant, have chronic medical conditions (eg, renal insufficiency or use of immunosuppressant medications), urinary obstruction, or calculi may develop complicated UTIs.8
Recurrent UTI is an infection that follows resolution of bacteriuria and symptoms of a prior UTI, and the term applies when such an infection occurs within 6 months of the last UTI or when 3 or more UTIs occur within a year.7 Recurrent infection can be further characterized as relapse or reinfection. Relapse occurs when the patient has a second UTI caused by the same pathogen within 2 weeks of the original treatment.9 Reinfection is a UTI that occurs more than 2 weeks after completion of treatment for the original UTI. The pathogen in a reinfection may be the same one that caused the original UTI or it may be a different agent.9
It’s also important to differentiate between recurrent and resistant UTI. In resistant UTI, bacteriuria fails to resolve following 7 to 14 days of appropriate antibiotic treatment.9
Factors that increase the risk of recurrent UTI
Premenopausal women
Both modifiable and non-modifiable factors (TABLE 110-21) have been associated with increased risk of recurrent UTI in premenopausal women. Among women with specific blood group phenotypes (Lewis non-secretor, in particular), rates of UTI rise secondary to increased adherence of bacteria to epithelial cells in the urinary tract.10 Other non-modifiable risk factors include congenital urinary tract anomalies, obstruction of the urinary tract, and a history of UTI.11,12 Women whose mothers had UTIs are at higher risk for recurrent UTI than are women whose mothers had no such history.13
Modifiable risk factors for recurrent UTI include contraceptive use (spermicides, spermicide-coated condoms, and oral contraceptives) and frequency of intercourse (≥4 times/month).13 Spermicides alter the normal vaginal flora and lead to increased colonization of E coli, which increases the risk for UTI.14 Women with recurrent UTIs were 1.27 to 1.45 times more likely to use oral contraceptives than those without recurrent UTIs.13 Compared with college women who had not had intercourse during the week, sexually active college women who had engaged in intercourse 3 times had a 2.6-fold increase in relative risk for UTI.15 Those who had daily intercourse had a 9-fold increase in relative risk of UTI development.15 This elevated risk is due to trauma to the lower urogenital tract (urethra) and introduction of bacteria into the urethra via mechanical factors.16,17
Postmenopausal women
Atrophic vaginitis, catheterization, declining functional status, cystocele, incomplete emptying, incontinence, and history of premenopausal UTIs are all risk factors for recurrent UTI in postmenopausal women.19,20 Decreased estrogen and resulting vaginal atrophy appear to be associated with increased rates of UTI in these women. Additionally, postmenopausal women’s vaginas are more likely to be colonized with E coli and have fewer lactobacilli than those of premenopausal women,21 which is thought to predispose them to UTI. These risk factors are summarized in TABLE 1.10-21

Initial evaluation of recurrent UTI
Patients with recurrent UTI experience signs and symptoms similar to those with isolated uncomplicated UTI: dysuria, frequency, urgency, and hematuria. Focus your history interview on potential causes of complicated UTI (TABLE 218). Likewise, perform a pelvic examination to evaluate for predisposing anatomic abnormalities.22 Finally, obtain a urine culture with antibiotic sensitivities to ensure that previous treatment was appropriate and to rule out microbes associated with infected uroliths.18 Given the low probability of finding abnormalities on cystoscopy or imaging, neither one is routinely recommended for the evaluation of recurrent UTI.18

Treatment options and precautions
Preferred antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX), 160 mg/800 mg twice daily for 3 days, has long been the mainstay of treatment for uncomplicated UTI. Over recent years, however, resistance to TMP-SMX has increased. While it is still appropriate for many situations as first-line treatment, it is not recommended for empiric treatment if local resistance rates are higher than 20%.24 Nitrofurantoin 100 mg twice daily for 5 days has efficacy similar to that of TMP-SMX, but without significant bacterial resistance. While fosfomycin 3 g as a single dose is still recommended as first-line treatment, it is less effective than either TMP-SMX or nitrofurantoin. TABLE 324 summarizes these antibiotic choices and their efficacies.

Agents to avoid or use only as a last resort. For patients unable to take any of the drugs above, consider beta-lactam antibiotics, although they are typically less effective for this indication. While fluoroquinolones are very effective and have low (but rising) resistance rates, they are also associated with serious and potentially permanent adverse effects. As a result, on May 12, 2016, the Food and Drug Administration issued a Drug Safety Communication recommending that fluoroquinolones be used only in patients without other treatment options.24,25
Shorter course of treatment? When deciding on the length of treatment for recurrent UTI, remember that shorter antibiotic courses (3-5 days) are associated with similar rates of cure and progression to systemic infections as longer courses (7-10 days). Also, patients adhere better to the shorter treatment regimen and experience fewer adverse effects.26,27
Standing prescription? Studies have shown that women know when they have a UTI. Therefore, for women who experience recurrent UTI, consider giving them a standing prescription for antibiotics that they can initiate when symptoms arise (TABLE 324). Patient-initiated treatment yields similar rates of efficacy as physician-initiated treatment, while avoiding the adverse effects and costs associated with preventive strategies28 (which we’ll discuss in a moment).
Time for imaging and referral?
For patients with a high risk of complicated UTI or a surgically amenable condition, either ultrasound or computerized tomography (CT) of the abdomen and pelvis with and without contrast is appropriate to evaluate for anatomic anomalies. While CT is the more sensitive imaging study to identify anomalies, ultrasound is less expensive and minimizes radiation exposure and is therefore also appropriate.18
Consider referring patients to a urologist if they have an underlying condition that may be amenable to surgery, such as bladder outlet obstruction, cystoceles, urinary tract diverticula, fistulae, pelvic floor dysfunction, ureteral stricture, urolithiasis, or vesicoureteral reflux.18 Additional risk factors for complicated UTI, which warrant referral as outlined by the Canadian Urologic Association, are summarized in TABLE 2.18
2 weeks later…and it’s back? Finally, for women who experience recurrent symptoms within 2 weeks of completing treatment, obtain a urine culture with antibiotic sensitivities to ensure that the infecting organism is not one typically associated with urolithiasis (Proteus and Yersinia) and that it is susceptible to planned antibiotic therapy.18Proteus and Yersinia are urease-positive bacteria that may cause stone formation in the urinary tract system. Evaluate any patient who has a UTI from either organism for urinary tract stones.
Prevention dos and don’ts
Popular myth suggests that recurrent UTIs are more common in patients who do not void after intercourse or who douche, consume caffeinated beverages, or wear non-cotton underwear. Research, however, has failed to show a relationship between any of these factors and recurrent UTIs.13,18 Physicians should therefore stop recommending that patients modify these behaviors to decrease recurrent infections.
Antibiotic prophylaxis decreases the rate of recurrent UTI by 95%.29 It has been recommended for women who have had 2 or more UTIs in the past 6 months29 or 3 or more UTIs in the past year.30 Effective strategies to prevent recurrent UTI are low-dose continuous antibiotic prophylaxis or post-coital antibiotic prophylaxis.
While a test-of-cure culture is not typically recommended following treatment for uncomplicated UTI, you will want to obtain a confirmatory urine culture one to 2 weeks before starting low-dose antibiotic prophylaxis. Base your choice of antibiotic on known patient allergies and previous culture results. Agents typically used are trimethoprim, TMP-SMX, or nitrofurantoin31,32 (TABLE 431), none of which demonstrated superiority in a Cochrane review.33 Although the same review showed no optimal duration of treatment,33 6 to 24 months of treatment is usually recommended.29
A single dose of antibiotic following intercourse may be as effective as daily low-dose prophylaxis for women whose UTIs are related to sexual activity.34 Studies have shown that single doses of TMP-SMX, nitrofurantoin, cephalexin, or a fluoroquinolone (see earlier notes about FDA warning on fluoroquinolone use) are similarly effective in decreasing the rate of recurrence35,36 (TABLE 431).

Several non-pharmacologic strategies have been suggested for preventing recurrent UTI—eg, use of cranberry products, lactobacillus, vaginal estrogen in postmenopausal women, methenamine salts, and D-mannose.
A 2012 Cochrane review of 24 studies found that cranberry products were less effective in preventing recurrent UTIs than previously thought, with no statistically significant difference between women who took them and those who did not.37
Results have been mixed in using lactobacilli or probiotics to prevent recurrent UTIs. One study examining the use of lactobacilli to colonize the vaginal flora found a reduction in the number of recurrent infections in premenopausal women taking intravaginal lactobacillus over 12 months.38 A second study, involving postmenopausal women, found that those who were randomized to take lactobacillus tablets for 12 months had more frequent recurrences of UTIs than women randomized to take daily TMP-SMX.39 However, this last study was designed as a non-inferiority trial and its results do not negate the prior study’s findings. Additionally, vaginal estrogen, which is thought to work through colonization of the vagina with lactobacilli, has prevented recurrent UTIs in postmenopausal women.40
Ascorbic acid (which is bacteriostatic), methenamine salts (which are hydrolysed to bactericidal ammonia and formaldehyde), and D-mannose (which inhibits bacterial adherence), have been shown—in limited studies—to decrease recurrence of UTIs.41-43 Further study is necessary to confirm their efficacy in preventing UTIs.
As noted, the only behavioral modifications that have been shown to decrease the risk of recurrent UTI are discontinuing the use of spermicides/spermicide-coated condoms or oral contraceptives, and decreasing the frequency of intercourse.13
CASE › Ms. B is started on a 3-day course of TMP-SMX. Further questioning reveals that each of her 3 UTIs followed sexual intercourse. Her physician discusses the options of self-directed therapy using continuous prophylaxis or postcoital prophylaxis, either of which would be an appropriate evidence-based intervention for her. After engaging in shared decision making, she is prescribed TMP-SMX to be taken as a single dose following intercourse in the future.
CORRESPONDENCE
Jeffrey D. Quinlan, MD, FAAFP, Family Medicine, Room A-1038A, 4301 Jones Bridge Road, Bethesda, MD 20814-4712; [email protected].
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. Centers for Disease Control and Prevention. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. Available at: www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed August 31, 2016.
3. Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991;164:1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New Eng J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(Suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. U.S. Food and Drug Administration. FDA drug safety communication. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed November 7, 2016.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988;9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157:935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trail. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;(10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Kranjčec B, Papeš D, Altarac S. D-m
1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001;18:153-162.
2. Centers for Disease Control and Prevention. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. Available at: www.cdc.gov/nchs/data/ahcd/combined_tables/2009-2010_combined_web_table01.pdf. Accessed August 31, 2016.
3. Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281-1287.
4. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;222:91-99.
5. Sammon JD, Sharma P, Rahbar H, et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World J Urol. 2014;32:813-819.
6. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654.
7. Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719-724.
8. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111:785-794.
10. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, et al. Association of the Lewis blood group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773-777.
11. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194-1205.
12. Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220-226.
13. Scholes D, Hooton TM, Roberts DL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182.
14. Hooton TM, Fennell CL, Clark AM, et al. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991;164:1216-1219.
15. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New Eng J Med. 1996;335:468-474.
16. Hooton TM, Hillier S, Johnson C, et al. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64-69.
17. Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
18. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316-322.
19. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(Suppl 1):1-7.
20. Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30:152-156.
21. Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446-450.
22. Neal DE. Complicated urinary tract infections. Urol Clin North Am. 2008;35:13-22.
23. Amna MA, Chazan B, Raz R, et al. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection. 2013;41:473-477.
24. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
25. U.S. Food and Drug Administration. FDA drug safety communication. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed November 7, 2016.
26. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196-1207.
27. Milo G, Katchman EA, Paul M, et al. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682.
28. Gupta K, Hooton TM, Roberts PL, et al. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16.
29. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:793-806.
30. Ronald AR, Conway B. An approach to urinary tract infections in ambulatory women. Curr Clin Top Infect Dis. 1988;9:76-125.
31. Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795-804.
32. McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417-429.
33. Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209.
34. Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157:935-939.
35. Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274-277.
36. Stapleton A, Latham RH, Johnson C, et al. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection: A randomized, double-blind, placebo-controlled trail. JAMA. 1990;264:703-706.
37. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;(10):CD001321.
38. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212-1217.
39. Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704-712.
40. Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.
41. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329-337.
42. Lee BB, Simpson JM, Craig JC, et al. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265.
43. Kranjčec B, Papeš D, Altarac S. D-m
PRACTICE RECOMMENDATIONS
› Avoid routine use of cystoscopy and imaging when evaluating women with recurrent urinary tract infection (UTI).
› Keep in mind that 3- to 5-day courses of antibiotics (nitrofurantoin, trimethoprim- sulfamethoxazole, fosfomycin, or beta-lactams) for UTIs are as effective as longer courses, and are associated with better compliance and fewer adverse effects.
› Assure patients considering prophylaxis for recurrent UTI that either continuous or postcoital antibiotics are effective.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Adult ADHD: Addressing a unique set of challenges
Attention-deficit/hyperactivity disorder (ADHD) in adults brings with it unique challenges, not the least of which are arriving at a proper diagnosis and ensuring that any psychostimulant drugs that you prescribe are not misused. A number of conditions such as anxiety, bipolar disorder, and substance abuse can mimic some of the symptoms of ADHD, and diagnostic criteria for the condition in adults changed with the latest edition of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).
Furthermore, for many of the estimated 4.4% of adults who have ADHD,1 psychostimulants provide necessary and effective treatment, but misuse and diversion of these agents are real concerns. In fact, recent research reveals that these issues are more common than previously thought.2 Data suggest that the prevalence of misuse and diversion of ADHD medication is 5% to 10% among high school students and 5% to 35% among college students.2,3
This is not meant to indicate that adults diagnosed with ADHD should go untreated. In fact, adults with ADHD often struggle in their professional and family lives because they do not receive the treatment they need.
Rather, family physicians should take certain steps, first to diagnose ADHD correctly, and then to ascertain and maintain correct use of psychostimulants and other treatments among their adult patient populations. Read on for several practical strategies.
Criteria for adult Dx differ from those in children
ADHD, a common behavioral disorder that often, but not always, begins in childhood, is characterized by deficits in paying attention, difficulty controlling impulses, and marked hyperactivity. Diagnosis of ADHD is based on the DSM-5 criteria and supplemented with historical data and clinical observations.4 Using self-report measures may also aid in the diagnosis, and psychological testing may be required for some individuals when the clinical presentation is unclear.
With the DSM-5 changes (TABLE 14), the diagnosis of ADHD in adults (people ≥18 years) requires fewer symptoms than the diagnosis of ADHD in children; just 5 symptoms from either of the 2 categories of diagnostic criteria are sufficient now, whereas 6 symptoms were required previously and still are required to make the diagnosis in young people. People may present with the inattentive profile (5 or more symptoms of inattention), the hyperactive-impulsive profile (5 or more hyperactive-impulsive symptoms), or a combination of the 2 (5 or more symptoms of inattention plus 5 or more symptoms of hyperactivity-impulsivity for a total of 10 or more symptoms). While children are more likely to present with the combined type of ADHD, adults of any age are more likely to present with the inattentive type.4
In addition, patients must meet the diagnostic criteria for ADHD for at least 6 months, have had some of the symptoms prior to age 12, and the symptoms must cause significant impairment in 2 or more environments (eg, home, work, school). When the diagnosis is unclear, it is important to obtain collateral information from the family, school, or workplace. The requirement regarding symptomatology before age 12 indicates the need for a review of the patient’s educational history. Research reveals that many adults with ADHD struggled in school and were considered “underachievers” as students.5
Common complaints and characteristics. Previous studies have shown the following to be common complaints and characteristics of adult patients diagnosed with ADHD:5
- difficulty meeting time limits
- vocational struggles, such as frequent job changes or nonpromotion at work
- anger issues
- addiction
- relationship/social strain
- comprehension problems, and
- a family history of ADHD.
Common correlates include low socioeconomic status, driving violations, frequent injuries, legal problems, alcohol and/or tobacco use, and self-reported maladjustment.5 People treated for ADHD have a comorbid DSM diagnosis 81% of the time with the most likely diagnoses being substance abuse, depression, and anxiety.6
Adult-onset ADHD? Even though the DSM-5 criteria for an ADHD diagnosis in adulthood require that some ADHD symptoms were manifest prior to age 12, recent longitudinal research on ADHD in Brazil and the United Kingdom reveals that a large portion of people who meet the criteria for ADHD in adulthood did not meet the criteria as children. The researchers in these studies proposed that there may be a form of ADHD that manifests later in life, a so-called “adult-onset ADHD.”7 While this information is something for clinicians to consider, further research is needed to justify a paradigm shift in how ADHD is diagnosed.
Self-rating measures can offer clarification. Whether or not history-gathering leaves the diagnosis murky, self-rating measures can be valuable in rounding out the clinical picture and alerting clinicians to any inconsistencies in symptoms.8 Four common ADHD self-rating measures are provided in TABLE 2. As one example, the Adult ADHD Self-Report Scale (ASRS) Symptom Checklist is a valuable ADHD screening tool that is free of charge and takes only 5 to 10 minutes.8 Other self-report measures require a similar amount of time, but are not available on a complimentary basis.
Psychological testing. Some adults who seem to have symptoms of ADHD may require a referral for psychological testing. These may be patients who present with complicated cases or whose histories and/or findings do not consistently indicate an ADHD diagnosis. In such cases, psychological testing can fill in the holes and provide a more complete picture of the patient’s neurocognitive abilities and deficits.9,10
Psychostimulant treatment: Opt for longer-acting agents
The standard treatment for ADHD is a psychostimulant. One controlled trial, for example, of a mixed amphetamine salts compound (Adderall) found that the compound effectively treated ADHD symptoms (hyperactivity, impulsivity, inattention) in adults and was well tolerated.11 While far fewer studies have been performed in adult vs youth populations, those that have been conducted in adults indicate that psychostimulants are largely safe and efficacious. In fact, the study mentioned above found that 70% of patients with ADHD ages 18 and older reported improvement of symptoms while on a short-acting psychostimulant, as compared to 7% who reported improvement on placebo.11
Similarly, a meta-analysis of 1991 participants in 11 studies found significant improvement in patients who received medication vs placebo, with stimulant medications demonstrating greater efficacy than non-stimulant treatments for ADHD.12 In general, psychostimulant treatment for adults is similar to that for children; the only difference is that adults tend to be more forthcoming with information regarding how the treatment is working and what adjustments might be needed.
Longer-acting stimulants (ie, extended release) tend to be preferred by patients to short-acting ones because they typically provide adequate control of symptoms over a longer period of time and thus may be taken less frequently.13 Also, the potential for abuse of psychostimulant medication tends to be lower with the longer-acting, extended-release formulations.14 A shorter acting formulation may be preferred if a patient has a specific window of time when their ADHD symptoms impact them. For example, a patient may request a short-acting form of medication for afternoons if he or she has to attend many business meetings at that time of day. A relatively new category is the intermediate-acting psychostimulants. For more on specific psychostimulants, see Table 3.15
Adverse effects lead many to discontinue treatment
Regardless of the length of action of the psychostimulant, studies show that about 30% of adults (and, incidentally, 10% to 30% of children) discontinue treatment due to uncontrolled/unwanted symptoms or adverse effects.16 These include decreased appetite, headache, insomnia, abdominal pain, and irritable mood.17 If you are prescribing a psychostimulant for an adult with ADHD, it is important to tell the patient that if the effects become intolerable, adjustments can be made, such as tweaking dosages, switching to a different medication, or adding an adjunctive therapy such as cognitive behavioral therapy. Keep in mind, too, that if a medication is to be discontinued, tapering is suggested for most psychostimulants; patients should take a lower (eg, half) dose for about a week prior to complete discontinuation. People who have difficulties with a number of treatments for ADHD should be reevaluated in a year to see if circumstances have changed.5
Combatting misuse and diversion
Perhaps the most controversial issues surrounding the treatment of adults with ADHD are abuse and diversion of psychostimulants. Abuse generally refers to misuse of the drug by the person prescribed the agent, whereas diversion refers to use of the drug by people for whom the drug was not intended—with or without the prescribee’s knowledge.3 Although rare, chronic abuse of psychostimulants can lead to serious problems such as aggression, suicidal thoughts/behaviors, psychosis, and mania.5
While earlier studies tended to downplay the likelihood of diversion, recent research indicates that physicians should not underestimate the possibility. In fact, a previous article in this journal about student athletes with ADHD (http://bit.ly/2k1a6TL) indicated that psychostimulants have “great potential for misuse” and that recently there has been “a surge in nonprescription stimulant use among adolescents and young adults.”17 The authors of the article concluded, however, that while physicians should be aware of the potential for misuse, fear should not preclude treatment.17
A national multi-cohort study of 4572 US high school seniors who had used psychostimulants either medically or nonmedically indicated that while one in 6 high school seniors had been exposed to psychostimulants, about half were appropriately exposed through prescription use, while the other half was not. The researchers also reported that current nonmedical users of psychostimulants and those with a history of nonmedical use had a greater risk of substance use and abuse when compared to medical users of psychostimulants.18
Young men are at higher risk for diversion. In a nationwide survey of a sample of adults ages 18 to 49 years who had a prescription for psychostimulant medication in the past month, 17% admitted diverting their medication.
In another study, 483 students ages 17 to 19 years were followed for one year and interviewed frequently regarding their use of medications.20 The researchers reported that the lifetime prevalence of diversion of any medication in those students was around 36%. They also found that of those who diverted medication, 62% diverted ADHD medications at least once. These were most commonly diverted by sharing (34%) and selling (9%) the psychostimulants. Interview analysis revealed that those students who diverted were more likely to have used illicit drugs and to have had conduct problems. The authors advised “vigilance regarding…stimulant medications for young adults.”
Psychostimulant prescriptions do not cause substance abuse. Nevertheless, a salient point in the literature is that there is no causal relationship between psychostimulant prescriptions that are properly prescribed for people who have ADHD and substance abuse. One study that followed young people with ADHD for 8 years into adulthood revealed that psychostimulant treatment did not make adolescents or young adults any more or less likely to abuse drugs.21 The study also found that alcohol use was common in young adults whether they were diagnosed with ADHD or not. Nonetheless, family physicians should urge those taking psychostimulants to refrain from alcohol use or at least to drink in moderation.
The bottom line is that adults diagnosed with ADHD carry about the same risk of substance abuse as the general population if they are effectively treated for their presenting attention problems.22 If they are taking a psychostimulant, however, they have access to a controlled substance, unlike most of their cohorts. So it's important to teach patients with ADHD that for safety and legal reasons, they should not share or sell their stimulant medication to anyone.
Minimize the risk for abuse, diversion using these strategies
As with any drug regimen, it is important to monitor the patient’s response to treatment and minimize adverse effects and outcomes. When the drug is a psychostimulant for adults diagnosed with ADHD, it’s also important to minimize the risk for abuse and diversion. The following steps can help:
- Obtain a signed controlled substance agreement.23 This agreement between the physician and the patient usually outlines such specifics as frequency of office visits, circumstances surrounding medication refills, urine drug monitoring, and pill counts. (For more on the specifics of a controlled substance agreement, see "Key points of a controlled substance agreement.")
- Schedule frequent follow-up appointments with open communication about abuse and diversion.20,23 The age-old adage, “Start low, go slow,” applies to stimulant medications for ADHD. Medication dosage may vary and necessitate titration depending on the person’s weight and tolerance. At the onset of treatment, frequent office visits allow the physician to gauge treatment response and the patient’s commitment to therapy.
- Review your state’s prescription drug monitoring program.24 It is imperative that providers check their state’s medical board rules for prescribing controlled medications to ensure practice compliance. As diversion rates of controlled medications have risen in this country, most states have established monitoring systems through their pharmacy boards.24 Although the names of the programs vary, these prescription drug monitoring programs provide information on any medication prescribed. This allows the prescribing physician to ensure patient compliance and ascertain that no other controlled medications are being prescribed that could interfere with treatment. (For more information about state prescription drug monitoring programs, see https://www.deadiversion.usdoj.gov/faq/rx_monitor.htm.)
- Perform random urine drug screenings (UDS).20,23 An important strategy for ensuring adherence to the treatment plan and the controlled substance contract is UDS. Explain to patients that this is a way of making sure they are taking the medication exactly as prescribed. If the UDS indicates that the patient has not been taking the medication, then the provider should intervene by either restricting or discontinuing the controlled substance to prevent or counteract potential diversion. Similarly, if a higher dose is requested by a patient, the provider can closely monitor the situation to determine whether the additional drug is actually being taken and whether the dose is optimal. (See JFP’s October 2016 “3 in 3” video on urine drug testing at: http://bit.ly/2iDnfgD.)
- Employ a team-based, multimodal approach.25 A referral to a mental health professional and multimodal treatment are often recommended in the literature as best practices.25 Behavioral therapies are a cornerstone of treatment in adults with ADHD and often serve as important adjuncts to pharmacotherapy. Also, a referral provides a second professional opinion about the patient’s motivations, adherence, and response to treatment.
Trained cognitive behavioral therapists (eg, psychologists, counselors) can be helpful with treatment for ADHD.23 Therapists can be useful in setting goals for the patient regarding adherence, organization, impulse control, and social skills training. Therapists may wish to involve the family in treatment, depending on the nature of the patient’s presenting issues.
SIDEBAR
Key points of a controlled substance agreementThe primary purpose of a controlled substance agreement is to provide clarity for the provider and the patient regarding the use of controlled medications. The document is meant to prevent potential problems and confusion down the road. There are generally 3 parts:
- a doctor/patient agreement
- information about medications
- patient consent to utilize controlled substances that the provider believes would be beneficial.
Patients are typically told of the potential value of controlled medications in helping them and are warned about the potential for problems should the medications be used in ways other than intended. While wording may differ, patients are generally asked to agree to variations of the following 10 guidelines:
- I will talk with my doctor before using more than the prescribed amount of the medicine or discontinuing its use.
- I will tell my doctor if new medications are prescribed by another provider.
- I will tell the doctor if I become pregnant, so that any necessary medication adjustments can be made.
- If I abuse this drug, I understand that the doctor may need to stop treatment.
- I will uphold the visit schedule to the office/clinic according to guidelines for controlled substances (eg, every 90 days).
- I will refrain from using illicit drugs including marijuana and excessive quantities of alcohol.
- I will refrain from sharing, trading, or selling controlled substances.
- I will submit to regular urine drug screens as requested by the doctor.
- I understand that a failed drug screen may mean discontinuation of treatment.
- I will be forthright and honest about how the treatment is going, adverse effects, and how I am taking the medication.
Adapted from: https://www.drugabuse.gov/sites/default/files/files/SamplePatientAgreementForms.pdf.
Don’t tempt fate. As with any controlled medication, safe storage of psychostimulants is paramount. Patients should be urged to keep their medication in a locked box or cupboard that is accessible to only the adult for whom the drug is prescribed. Prior research cautions that open access to controlled substances can lead to larger issues with abuse and diversion, particularly when adolescents are in the home.26
Consider atomoxetine. Research has also demonstrated that the non-stimulant medication atomoxetine has some benefit in the treatment of ADHD.12 Unlike psychostimulants that act on the neurotransmitter dopamine, atomoxetine acts on the neurotransmitter norepinephrine. This different mechanism of action results in a lower potential for abuse and diversion.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected].
1. Kessler RC, Adler LA, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
2. Clemow DB, Walker DJ. The potential for misuse and abuse of medications in ADHD: a review. Postgrad Med. 2014;126:64-81.
3. Novak SP, Kroutil LA, Williams RL, et al. The nonmedical use of prescription ADHD medications: results from a national internet panel. Subst Abuse Treat Prev Policy. 2007;2:32.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Press; 2013.
5. Kolar D, Keller A, Golfinopoulos M, et al. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2008;4:389-403.
6. McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry. 2005;162:1621-1627.
7. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry.
8. van de Glind G, van den Brink W, Koeter MWJ, et al. Validity of the Adult ADHD Self-Report Scale (ASRS) as a screener for adult ADHD in treatment seeking substance use disorder patients. Drug Alcohol Depend. 2013;132:587-596.
9. Gualtieri CT, Johnson LG. ADHD: Is objective diagnosis possible? Psychiatry. 2005;2:44-53.
10. Perrin AE, Jotwani VM. Addressing the unique issues of student athletes with ADHD. J Fam Pract. 2014;63:E1-E9.
11. Spencer T, Biederman J, Wilens T, et al. Efficacy of mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:775-782.
12. Mészáros A, Czobor P, Bálint S, et al. Pharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): a meta-analysis. Int J Neuropsychopharmacol. 2009;12:1137-1147.
13. Weiss M, Shingler T, Capone NM. Medication satisfaction among adults with ADHD: long term results from the Quality of Life, Effectiveness, Safety and Tolerability (Qu.S.T) study. New Orleans: Program and Abstracts of the 19th US Psychiatric and Mental Health Congress, Abstract 120, 2006.
14. Lopez FA, Leroux JR.
15. Felt BT, Biermann B, Christner JG, et al. Diagnosis and management of ADHD in children. Am Fam Physician. 2014;90:456-464.
16. Spencer T, Biederman J, Wilens T. Nonstimulant treatment of adult attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27:373-383.
17. Withrow LM, Hash PA, Holten KB. Managing ADHD in children: are you doing enough? J Fam Pract. 2011;60:E1-E3.
18. McCabe SE, West BT. Medical and nonmedical use of prescription stimulants: results from a national multicohort study. J Am Acad Child Adolesc Psychiatry. 2013;52:1272-1280.
19. Aldridge AP, Kroutil LA, Cowell AJ, et al. Medication costs to private insurers of diversion of medications for attention-deficit hyperactivity disorder. Pharmacoeconomics. 2011;29:621-635.
20. Garnier LM, Arria AM, Caldeira KM, et al. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71:262-269.
21. Molina BSG, Hinshaw SP, Arnold LE, et al. Adolescent substance use in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52:250-263.
22. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143.
23. Post RE, Kurlansik SL. Diagnosis and management of attention-deficit/hyperactivity disorder in adults. Am Fam Physician. 2012;85:890-896.
24. Cepeda MS, Fife D, Berwaerts J, et al. Doctor shopping for medications used in the treatment of attention deficit hyperactivity disorder: shoppers often pay in cash and cross state lines. Am J Drug Alcohol Abuse. 2015;41:226-229.
25. Safren SA, Sprich S, Mimiaga MJ, et al. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA. 2010;304:875-880.
26. Ross-Durow PL, McCabe SE, Boyd CJ. Adolescents’ access to their own prescription medication in the home. J Adolesc Health. 2013;53:260-264.
Attention-deficit/hyperactivity disorder (ADHD) in adults brings with it unique challenges, not the least of which are arriving at a proper diagnosis and ensuring that any psychostimulant drugs that you prescribe are not misused. A number of conditions such as anxiety, bipolar disorder, and substance abuse can mimic some of the symptoms of ADHD, and diagnostic criteria for the condition in adults changed with the latest edition of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).
Furthermore, for many of the estimated 4.4% of adults who have ADHD,1 psychostimulants provide necessary and effective treatment, but misuse and diversion of these agents are real concerns. In fact, recent research reveals that these issues are more common than previously thought.2 Data suggest that the prevalence of misuse and diversion of ADHD medication is 5% to 10% among high school students and 5% to 35% among college students.2,3
This is not meant to indicate that adults diagnosed with ADHD should go untreated. In fact, adults with ADHD often struggle in their professional and family lives because they do not receive the treatment they need.
Rather, family physicians should take certain steps, first to diagnose ADHD correctly, and then to ascertain and maintain correct use of psychostimulants and other treatments among their adult patient populations. Read on for several practical strategies.
Criteria for adult Dx differ from those in children
ADHD, a common behavioral disorder that often, but not always, begins in childhood, is characterized by deficits in paying attention, difficulty controlling impulses, and marked hyperactivity. Diagnosis of ADHD is based on the DSM-5 criteria and supplemented with historical data and clinical observations.4 Using self-report measures may also aid in the diagnosis, and psychological testing may be required for some individuals when the clinical presentation is unclear.
With the DSM-5 changes (TABLE 14), the diagnosis of ADHD in adults (people ≥18 years) requires fewer symptoms than the diagnosis of ADHD in children; just 5 symptoms from either of the 2 categories of diagnostic criteria are sufficient now, whereas 6 symptoms were required previously and still are required to make the diagnosis in young people. People may present with the inattentive profile (5 or more symptoms of inattention), the hyperactive-impulsive profile (5 or more hyperactive-impulsive symptoms), or a combination of the 2 (5 or more symptoms of inattention plus 5 or more symptoms of hyperactivity-impulsivity for a total of 10 or more symptoms). While children are more likely to present with the combined type of ADHD, adults of any age are more likely to present with the inattentive type.4

In addition, patients must meet the diagnostic criteria for ADHD for at least 6 months, have had some of the symptoms prior to age 12, and the symptoms must cause significant impairment in 2 or more environments (eg, home, work, school). When the diagnosis is unclear, it is important to obtain collateral information from the family, school, or workplace. The requirement regarding symptomatology before age 12 indicates the need for a review of the patient’s educational history. Research reveals that many adults with ADHD struggled in school and were considered “underachievers” as students.5
Common complaints and characteristics. Previous studies have shown the following to be common complaints and characteristics of adult patients diagnosed with ADHD:5
- difficulty meeting time limits
- vocational struggles, such as frequent job changes or nonpromotion at work
- anger issues
- addiction
- relationship/social strain
- comprehension problems, and
- a family history of ADHD.
Common correlates include low socioeconomic status, driving violations, frequent injuries, legal problems, alcohol and/or tobacco use, and self-reported maladjustment.5 People treated for ADHD have a comorbid DSM diagnosis 81% of the time with the most likely diagnoses being substance abuse, depression, and anxiety.6
Adult-onset ADHD? Even though the DSM-5 criteria for an ADHD diagnosis in adulthood require that some ADHD symptoms were manifest prior to age 12, recent longitudinal research on ADHD in Brazil and the United Kingdom reveals that a large portion of people who meet the criteria for ADHD in adulthood did not meet the criteria as children. The researchers in these studies proposed that there may be a form of ADHD that manifests later in life, a so-called “adult-onset ADHD.”7 While this information is something for clinicians to consider, further research is needed to justify a paradigm shift in how ADHD is diagnosed.
Self-rating measures can offer clarification. Whether or not history-gathering leaves the diagnosis murky, self-rating measures can be valuable in rounding out the clinical picture and alerting clinicians to any inconsistencies in symptoms.8 Four common ADHD self-rating measures are provided in TABLE 2. As one example, the Adult ADHD Self-Report Scale (ASRS) Symptom Checklist is a valuable ADHD screening tool that is free of charge and takes only 5 to 10 minutes.8 Other self-report measures require a similar amount of time, but are not available on a complimentary basis.

Psychological testing. Some adults who seem to have symptoms of ADHD may require a referral for psychological testing. These may be patients who present with complicated cases or whose histories and/or findings do not consistently indicate an ADHD diagnosis. In such cases, psychological testing can fill in the holes and provide a more complete picture of the patient’s neurocognitive abilities and deficits.9,10
Psychostimulant treatment: Opt for longer-acting agents
The standard treatment for ADHD is a psychostimulant. One controlled trial, for example, of a mixed amphetamine salts compound (Adderall) found that the compound effectively treated ADHD symptoms (hyperactivity, impulsivity, inattention) in adults and was well tolerated.11 While far fewer studies have been performed in adult vs youth populations, those that have been conducted in adults indicate that psychostimulants are largely safe and efficacious. In fact, the study mentioned above found that 70% of patients with ADHD ages 18 and older reported improvement of symptoms while on a short-acting psychostimulant, as compared to 7% who reported improvement on placebo.11
Similarly, a meta-analysis of 1991 participants in 11 studies found significant improvement in patients who received medication vs placebo, with stimulant medications demonstrating greater efficacy than non-stimulant treatments for ADHD.12 In general, psychostimulant treatment for adults is similar to that for children; the only difference is that adults tend to be more forthcoming with information regarding how the treatment is working and what adjustments might be needed.
Longer-acting stimulants (ie, extended release) tend to be preferred by patients to short-acting ones because they typically provide adequate control of symptoms over a longer period of time and thus may be taken less frequently.13 Also, the potential for abuse of psychostimulant medication tends to be lower with the longer-acting, extended-release formulations.14 A shorter acting formulation may be preferred if a patient has a specific window of time when their ADHD symptoms impact them. For example, a patient may request a short-acting form of medication for afternoons if he or she has to attend many business meetings at that time of day. A relatively new category is the intermediate-acting psychostimulants. For more on specific psychostimulants, see Table 3.15

Adverse effects lead many to discontinue treatment
Regardless of the length of action of the psychostimulant, studies show that about 30% of adults (and, incidentally, 10% to 30% of children) discontinue treatment due to uncontrolled/unwanted symptoms or adverse effects.16 These include decreased appetite, headache, insomnia, abdominal pain, and irritable mood.17 If you are prescribing a psychostimulant for an adult with ADHD, it is important to tell the patient that if the effects become intolerable, adjustments can be made, such as tweaking dosages, switching to a different medication, or adding an adjunctive therapy such as cognitive behavioral therapy. Keep in mind, too, that if a medication is to be discontinued, tapering is suggested for most psychostimulants; patients should take a lower (eg, half) dose for about a week prior to complete discontinuation. People who have difficulties with a number of treatments for ADHD should be reevaluated in a year to see if circumstances have changed.5
Combatting misuse and diversion
Perhaps the most controversial issues surrounding the treatment of adults with ADHD are abuse and diversion of psychostimulants. Abuse generally refers to misuse of the drug by the person prescribed the agent, whereas diversion refers to use of the drug by people for whom the drug was not intended—with or without the prescribee’s knowledge.3 Although rare, chronic abuse of psychostimulants can lead to serious problems such as aggression, suicidal thoughts/behaviors, psychosis, and mania.5
While earlier studies tended to downplay the likelihood of diversion, recent research indicates that physicians should not underestimate the possibility. In fact, a previous article in this journal about student athletes with ADHD (http://bit.ly/2k1a6TL) indicated that psychostimulants have “great potential for misuse” and that recently there has been “a surge in nonprescription stimulant use among adolescents and young adults.”17 The authors of the article concluded, however, that while physicians should be aware of the potential for misuse, fear should not preclude treatment.17
A national multi-cohort study of 4572 US high school seniors who had used psychostimulants either medically or nonmedically indicated that while one in 6 high school seniors had been exposed to psychostimulants, about half were appropriately exposed through prescription use, while the other half was not. The researchers also reported that current nonmedical users of psychostimulants and those with a history of nonmedical use had a greater risk of substance use and abuse when compared to medical users of psychostimulants.18
Young men are at higher risk for diversion. In a nationwide survey of a sample of adults ages 18 to 49 years who had a prescription for psychostimulant medication in the past month, 17% admitted diverting their medication.
In another study, 483 students ages 17 to 19 years were followed for one year and interviewed frequently regarding their use of medications.20 The researchers reported that the lifetime prevalence of diversion of any medication in those students was around 36%. They also found that of those who diverted medication, 62% diverted ADHD medications at least once. These were most commonly diverted by sharing (34%) and selling (9%) the psychostimulants. Interview analysis revealed that those students who diverted were more likely to have used illicit drugs and to have had conduct problems. The authors advised “vigilance regarding…stimulant medications for young adults.”
Psychostimulant prescriptions do not cause substance abuse. Nevertheless, a salient point in the literature is that there is no causal relationship between psychostimulant prescriptions that are properly prescribed for people who have ADHD and substance abuse. One study that followed young people with ADHD for 8 years into adulthood revealed that psychostimulant treatment did not make adolescents or young adults any more or less likely to abuse drugs.21 The study also found that alcohol use was common in young adults whether they were diagnosed with ADHD or not. Nonetheless, family physicians should urge those taking psychostimulants to refrain from alcohol use or at least to drink in moderation.
The bottom line is that adults diagnosed with ADHD carry about the same risk of substance abuse as the general population if they are effectively treated for their presenting attention problems.22 If they are taking a psychostimulant, however, they have access to a controlled substance, unlike most of their cohorts. So it's important to teach patients with ADHD that for safety and legal reasons, they should not share or sell their stimulant medication to anyone.
Minimize the risk for abuse, diversion using these strategies
As with any drug regimen, it is important to monitor the patient’s response to treatment and minimize adverse effects and outcomes. When the drug is a psychostimulant for adults diagnosed with ADHD, it’s also important to minimize the risk for abuse and diversion. The following steps can help:
- Obtain a signed controlled substance agreement.23 This agreement between the physician and the patient usually outlines such specifics as frequency of office visits, circumstances surrounding medication refills, urine drug monitoring, and pill counts. (For more on the specifics of a controlled substance agreement, see "Key points of a controlled substance agreement.")
- Schedule frequent follow-up appointments with open communication about abuse and diversion.20,23 The age-old adage, “Start low, go slow,” applies to stimulant medications for ADHD. Medication dosage may vary and necessitate titration depending on the person’s weight and tolerance. At the onset of treatment, frequent office visits allow the physician to gauge treatment response and the patient’s commitment to therapy.
- Review your state’s prescription drug monitoring program.24 It is imperative that providers check their state’s medical board rules for prescribing controlled medications to ensure practice compliance. As diversion rates of controlled medications have risen in this country, most states have established monitoring systems through their pharmacy boards.24 Although the names of the programs vary, these prescription drug monitoring programs provide information on any medication prescribed. This allows the prescribing physician to ensure patient compliance and ascertain that no other controlled medications are being prescribed that could interfere with treatment. (For more information about state prescription drug monitoring programs, see https://www.deadiversion.usdoj.gov/faq/rx_monitor.htm.)
- Perform random urine drug screenings (UDS).20,23 An important strategy for ensuring adherence to the treatment plan and the controlled substance contract is UDS. Explain to patients that this is a way of making sure they are taking the medication exactly as prescribed. If the UDS indicates that the patient has not been taking the medication, then the provider should intervene by either restricting or discontinuing the controlled substance to prevent or counteract potential diversion. Similarly, if a higher dose is requested by a patient, the provider can closely monitor the situation to determine whether the additional drug is actually being taken and whether the dose is optimal. (See JFP’s October 2016 “3 in 3” video on urine drug testing at: http://bit.ly/2iDnfgD.)
- Employ a team-based, multimodal approach.25 A referral to a mental health professional and multimodal treatment are often recommended in the literature as best practices.25 Behavioral therapies are a cornerstone of treatment in adults with ADHD and often serve as important adjuncts to pharmacotherapy. Also, a referral provides a second professional opinion about the patient’s motivations, adherence, and response to treatment.
Trained cognitive behavioral therapists (eg, psychologists, counselors) can be helpful with treatment for ADHD.23 Therapists can be useful in setting goals for the patient regarding adherence, organization, impulse control, and social skills training. Therapists may wish to involve the family in treatment, depending on the nature of the patient’s presenting issues.
SIDEBAR
Key points of a controlled substance agreementThe primary purpose of a controlled substance agreement is to provide clarity for the provider and the patient regarding the use of controlled medications. The document is meant to prevent potential problems and confusion down the road. There are generally 3 parts:
- a doctor/patient agreement
- information about medications
- patient consent to utilize controlled substances that the provider believes would be beneficial.
Patients are typically told of the potential value of controlled medications in helping them and are warned about the potential for problems should the medications be used in ways other than intended. While wording may differ, patients are generally asked to agree to variations of the following 10 guidelines:
- I will talk with my doctor before using more than the prescribed amount of the medicine or discontinuing its use.
- I will tell my doctor if new medications are prescribed by another provider.
- I will tell the doctor if I become pregnant, so that any necessary medication adjustments can be made.
- If I abuse this drug, I understand that the doctor may need to stop treatment.
- I will uphold the visit schedule to the office/clinic according to guidelines for controlled substances (eg, every 90 days).
- I will refrain from using illicit drugs including marijuana and excessive quantities of alcohol.
- I will refrain from sharing, trading, or selling controlled substances.
- I will submit to regular urine drug screens as requested by the doctor.
- I understand that a failed drug screen may mean discontinuation of treatment.
- I will be forthright and honest about how the treatment is going, adverse effects, and how I am taking the medication.
Adapted from: https://www.drugabuse.gov/sites/default/files/files/SamplePatientAgreementForms.pdf.
Don’t tempt fate. As with any controlled medication, safe storage of psychostimulants is paramount. Patients should be urged to keep their medication in a locked box or cupboard that is accessible to only the adult for whom the drug is prescribed. Prior research cautions that open access to controlled substances can lead to larger issues with abuse and diversion, particularly when adolescents are in the home.26
Consider atomoxetine. Research has also demonstrated that the non-stimulant medication atomoxetine has some benefit in the treatment of ADHD.12 Unlike psychostimulants that act on the neurotransmitter dopamine, atomoxetine acts on the neurotransmitter norepinephrine. This different mechanism of action results in a lower potential for abuse and diversion.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected].
Attention-deficit/hyperactivity disorder (ADHD) in adults brings with it unique challenges, not the least of which are arriving at a proper diagnosis and ensuring that any psychostimulant drugs that you prescribe are not misused. A number of conditions such as anxiety, bipolar disorder, and substance abuse can mimic some of the symptoms of ADHD, and diagnostic criteria for the condition in adults changed with the latest edition of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).
Furthermore, for many of the estimated 4.4% of adults who have ADHD,1 psychostimulants provide necessary and effective treatment, but misuse and diversion of these agents are real concerns. In fact, recent research reveals that these issues are more common than previously thought.2 Data suggest that the prevalence of misuse and diversion of ADHD medication is 5% to 10% among high school students and 5% to 35% among college students.2,3
This is not meant to indicate that adults diagnosed with ADHD should go untreated. In fact, adults with ADHD often struggle in their professional and family lives because they do not receive the treatment they need.
Rather, family physicians should take certain steps, first to diagnose ADHD correctly, and then to ascertain and maintain correct use of psychostimulants and other treatments among their adult patient populations. Read on for several practical strategies.
Criteria for adult Dx differ from those in children
ADHD, a common behavioral disorder that often, but not always, begins in childhood, is characterized by deficits in paying attention, difficulty controlling impulses, and marked hyperactivity. Diagnosis of ADHD is based on the DSM-5 criteria and supplemented with historical data and clinical observations.4 Using self-report measures may also aid in the diagnosis, and psychological testing may be required for some individuals when the clinical presentation is unclear.
With the DSM-5 changes (TABLE 14), the diagnosis of ADHD in adults (people ≥18 years) requires fewer symptoms than the diagnosis of ADHD in children; just 5 symptoms from either of the 2 categories of diagnostic criteria are sufficient now, whereas 6 symptoms were required previously and still are required to make the diagnosis in young people. People may present with the inattentive profile (5 or more symptoms of inattention), the hyperactive-impulsive profile (5 or more hyperactive-impulsive symptoms), or a combination of the 2 (5 or more symptoms of inattention plus 5 or more symptoms of hyperactivity-impulsivity for a total of 10 or more symptoms). While children are more likely to present with the combined type of ADHD, adults of any age are more likely to present with the inattentive type.4

In addition, patients must meet the diagnostic criteria for ADHD for at least 6 months, have had some of the symptoms prior to age 12, and the symptoms must cause significant impairment in 2 or more environments (eg, home, work, school). When the diagnosis is unclear, it is important to obtain collateral information from the family, school, or workplace. The requirement regarding symptomatology before age 12 indicates the need for a review of the patient’s educational history. Research reveals that many adults with ADHD struggled in school and were considered “underachievers” as students.5
Common complaints and characteristics. Previous studies have shown the following to be common complaints and characteristics of adult patients diagnosed with ADHD:5
- difficulty meeting time limits
- vocational struggles, such as frequent job changes or nonpromotion at work
- anger issues
- addiction
- relationship/social strain
- comprehension problems, and
- a family history of ADHD.
Common correlates include low socioeconomic status, driving violations, frequent injuries, legal problems, alcohol and/or tobacco use, and self-reported maladjustment.5 People treated for ADHD have a comorbid DSM diagnosis 81% of the time with the most likely diagnoses being substance abuse, depression, and anxiety.6
Adult-onset ADHD? Even though the DSM-5 criteria for an ADHD diagnosis in adulthood require that some ADHD symptoms were manifest prior to age 12, recent longitudinal research on ADHD in Brazil and the United Kingdom reveals that a large portion of people who meet the criteria for ADHD in adulthood did not meet the criteria as children. The researchers in these studies proposed that there may be a form of ADHD that manifests later in life, a so-called “adult-onset ADHD.”7 While this information is something for clinicians to consider, further research is needed to justify a paradigm shift in how ADHD is diagnosed.
Self-rating measures can offer clarification. Whether or not history-gathering leaves the diagnosis murky, self-rating measures can be valuable in rounding out the clinical picture and alerting clinicians to any inconsistencies in symptoms.8 Four common ADHD self-rating measures are provided in TABLE 2. As one example, the Adult ADHD Self-Report Scale (ASRS) Symptom Checklist is a valuable ADHD screening tool that is free of charge and takes only 5 to 10 minutes.8 Other self-report measures require a similar amount of time, but are not available on a complimentary basis.

Psychological testing. Some adults who seem to have symptoms of ADHD may require a referral for psychological testing. These may be patients who present with complicated cases or whose histories and/or findings do not consistently indicate an ADHD diagnosis. In such cases, psychological testing can fill in the holes and provide a more complete picture of the patient’s neurocognitive abilities and deficits.9,10
Psychostimulant treatment: Opt for longer-acting agents
The standard treatment for ADHD is a psychostimulant. One controlled trial, for example, of a mixed amphetamine salts compound (Adderall) found that the compound effectively treated ADHD symptoms (hyperactivity, impulsivity, inattention) in adults and was well tolerated.11 While far fewer studies have been performed in adult vs youth populations, those that have been conducted in adults indicate that psychostimulants are largely safe and efficacious. In fact, the study mentioned above found that 70% of patients with ADHD ages 18 and older reported improvement of symptoms while on a short-acting psychostimulant, as compared to 7% who reported improvement on placebo.11
Similarly, a meta-analysis of 1991 participants in 11 studies found significant improvement in patients who received medication vs placebo, with stimulant medications demonstrating greater efficacy than non-stimulant treatments for ADHD.12 In general, psychostimulant treatment for adults is similar to that for children; the only difference is that adults tend to be more forthcoming with information regarding how the treatment is working and what adjustments might be needed.
Longer-acting stimulants (ie, extended release) tend to be preferred by patients to short-acting ones because they typically provide adequate control of symptoms over a longer period of time and thus may be taken less frequently.13 Also, the potential for abuse of psychostimulant medication tends to be lower with the longer-acting, extended-release formulations.14 A shorter acting formulation may be preferred if a patient has a specific window of time when their ADHD symptoms impact them. For example, a patient may request a short-acting form of medication for afternoons if he or she has to attend many business meetings at that time of day. A relatively new category is the intermediate-acting psychostimulants. For more on specific psychostimulants, see Table 3.15

Adverse effects lead many to discontinue treatment
Regardless of the length of action of the psychostimulant, studies show that about 30% of adults (and, incidentally, 10% to 30% of children) discontinue treatment due to uncontrolled/unwanted symptoms or adverse effects.16 These include decreased appetite, headache, insomnia, abdominal pain, and irritable mood.17 If you are prescribing a psychostimulant for an adult with ADHD, it is important to tell the patient that if the effects become intolerable, adjustments can be made, such as tweaking dosages, switching to a different medication, or adding an adjunctive therapy such as cognitive behavioral therapy. Keep in mind, too, that if a medication is to be discontinued, tapering is suggested for most psychostimulants; patients should take a lower (eg, half) dose for about a week prior to complete discontinuation. People who have difficulties with a number of treatments for ADHD should be reevaluated in a year to see if circumstances have changed.5
Combatting misuse and diversion
Perhaps the most controversial issues surrounding the treatment of adults with ADHD are abuse and diversion of psychostimulants. Abuse generally refers to misuse of the drug by the person prescribed the agent, whereas diversion refers to use of the drug by people for whom the drug was not intended—with or without the prescribee’s knowledge.3 Although rare, chronic abuse of psychostimulants can lead to serious problems such as aggression, suicidal thoughts/behaviors, psychosis, and mania.5
While earlier studies tended to downplay the likelihood of diversion, recent research indicates that physicians should not underestimate the possibility. In fact, a previous article in this journal about student athletes with ADHD (http://bit.ly/2k1a6TL) indicated that psychostimulants have “great potential for misuse” and that recently there has been “a surge in nonprescription stimulant use among adolescents and young adults.”17 The authors of the article concluded, however, that while physicians should be aware of the potential for misuse, fear should not preclude treatment.17
A national multi-cohort study of 4572 US high school seniors who had used psychostimulants either medically or nonmedically indicated that while one in 6 high school seniors had been exposed to psychostimulants, about half were appropriately exposed through prescription use, while the other half was not. The researchers also reported that current nonmedical users of psychostimulants and those with a history of nonmedical use had a greater risk of substance use and abuse when compared to medical users of psychostimulants.18
Young men are at higher risk for diversion. In a nationwide survey of a sample of adults ages 18 to 49 years who had a prescription for psychostimulant medication in the past month, 17% admitted diverting their medication.
In another study, 483 students ages 17 to 19 years were followed for one year and interviewed frequently regarding their use of medications.20 The researchers reported that the lifetime prevalence of diversion of any medication in those students was around 36%. They also found that of those who diverted medication, 62% diverted ADHD medications at least once. These were most commonly diverted by sharing (34%) and selling (9%) the psychostimulants. Interview analysis revealed that those students who diverted were more likely to have used illicit drugs and to have had conduct problems. The authors advised “vigilance regarding…stimulant medications for young adults.”
Psychostimulant prescriptions do not cause substance abuse. Nevertheless, a salient point in the literature is that there is no causal relationship between psychostimulant prescriptions that are properly prescribed for people who have ADHD and substance abuse. One study that followed young people with ADHD for 8 years into adulthood revealed that psychostimulant treatment did not make adolescents or young adults any more or less likely to abuse drugs.21 The study also found that alcohol use was common in young adults whether they were diagnosed with ADHD or not. Nonetheless, family physicians should urge those taking psychostimulants to refrain from alcohol use or at least to drink in moderation.
The bottom line is that adults diagnosed with ADHD carry about the same risk of substance abuse as the general population if they are effectively treated for their presenting attention problems.22 If they are taking a psychostimulant, however, they have access to a controlled substance, unlike most of their cohorts. So it's important to teach patients with ADHD that for safety and legal reasons, they should not share or sell their stimulant medication to anyone.
Minimize the risk for abuse, diversion using these strategies
As with any drug regimen, it is important to monitor the patient’s response to treatment and minimize adverse effects and outcomes. When the drug is a psychostimulant for adults diagnosed with ADHD, it’s also important to minimize the risk for abuse and diversion. The following steps can help:
- Obtain a signed controlled substance agreement.23 This agreement between the physician and the patient usually outlines such specifics as frequency of office visits, circumstances surrounding medication refills, urine drug monitoring, and pill counts. (For more on the specifics of a controlled substance agreement, see "Key points of a controlled substance agreement.")
- Schedule frequent follow-up appointments with open communication about abuse and diversion.20,23 The age-old adage, “Start low, go slow,” applies to stimulant medications for ADHD. Medication dosage may vary and necessitate titration depending on the person’s weight and tolerance. At the onset of treatment, frequent office visits allow the physician to gauge treatment response and the patient’s commitment to therapy.
- Review your state’s prescription drug monitoring program.24 It is imperative that providers check their state’s medical board rules for prescribing controlled medications to ensure practice compliance. As diversion rates of controlled medications have risen in this country, most states have established monitoring systems through their pharmacy boards.24 Although the names of the programs vary, these prescription drug monitoring programs provide information on any medication prescribed. This allows the prescribing physician to ensure patient compliance and ascertain that no other controlled medications are being prescribed that could interfere with treatment. (For more information about state prescription drug monitoring programs, see https://www.deadiversion.usdoj.gov/faq/rx_monitor.htm.)
- Perform random urine drug screenings (UDS).20,23 An important strategy for ensuring adherence to the treatment plan and the controlled substance contract is UDS. Explain to patients that this is a way of making sure they are taking the medication exactly as prescribed. If the UDS indicates that the patient has not been taking the medication, then the provider should intervene by either restricting or discontinuing the controlled substance to prevent or counteract potential diversion. Similarly, if a higher dose is requested by a patient, the provider can closely monitor the situation to determine whether the additional drug is actually being taken and whether the dose is optimal. (See JFP’s October 2016 “3 in 3” video on urine drug testing at: http://bit.ly/2iDnfgD.)
- Employ a team-based, multimodal approach.25 A referral to a mental health professional and multimodal treatment are often recommended in the literature as best practices.25 Behavioral therapies are a cornerstone of treatment in adults with ADHD and often serve as important adjuncts to pharmacotherapy. Also, a referral provides a second professional opinion about the patient’s motivations, adherence, and response to treatment.
Trained cognitive behavioral therapists (eg, psychologists, counselors) can be helpful with treatment for ADHD.23 Therapists can be useful in setting goals for the patient regarding adherence, organization, impulse control, and social skills training. Therapists may wish to involve the family in treatment, depending on the nature of the patient’s presenting issues.
SIDEBAR
Key points of a controlled substance agreementThe primary purpose of a controlled substance agreement is to provide clarity for the provider and the patient regarding the use of controlled medications. The document is meant to prevent potential problems and confusion down the road. There are generally 3 parts:
- a doctor/patient agreement
- information about medications
- patient consent to utilize controlled substances that the provider believes would be beneficial.
Patients are typically told of the potential value of controlled medications in helping them and are warned about the potential for problems should the medications be used in ways other than intended. While wording may differ, patients are generally asked to agree to variations of the following 10 guidelines:
- I will talk with my doctor before using more than the prescribed amount of the medicine or discontinuing its use.
- I will tell my doctor if new medications are prescribed by another provider.
- I will tell the doctor if I become pregnant, so that any necessary medication adjustments can be made.
- If I abuse this drug, I understand that the doctor may need to stop treatment.
- I will uphold the visit schedule to the office/clinic according to guidelines for controlled substances (eg, every 90 days).
- I will refrain from using illicit drugs including marijuana and excessive quantities of alcohol.
- I will refrain from sharing, trading, or selling controlled substances.
- I will submit to regular urine drug screens as requested by the doctor.
- I understand that a failed drug screen may mean discontinuation of treatment.
- I will be forthright and honest about how the treatment is going, adverse effects, and how I am taking the medication.
Adapted from: https://www.drugabuse.gov/sites/default/files/files/SamplePatientAgreementForms.pdf.
Don’t tempt fate. As with any controlled medication, safe storage of psychostimulants is paramount. Patients should be urged to keep their medication in a locked box or cupboard that is accessible to only the adult for whom the drug is prescribed. Prior research cautions that open access to controlled substances can lead to larger issues with abuse and diversion, particularly when adolescents are in the home.26
Consider atomoxetine. Research has also demonstrated that the non-stimulant medication atomoxetine has some benefit in the treatment of ADHD.12 Unlike psychostimulants that act on the neurotransmitter dopamine, atomoxetine acts on the neurotransmitter norepinephrine. This different mechanism of action results in a lower potential for abuse and diversion.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected].
1. Kessler RC, Adler LA, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
2. Clemow DB, Walker DJ. The potential for misuse and abuse of medications in ADHD: a review. Postgrad Med. 2014;126:64-81.
3. Novak SP, Kroutil LA, Williams RL, et al. The nonmedical use of prescription ADHD medications: results from a national internet panel. Subst Abuse Treat Prev Policy. 2007;2:32.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Press; 2013.
5. Kolar D, Keller A, Golfinopoulos M, et al. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2008;4:389-403.
6. McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry. 2005;162:1621-1627.
7. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry.
8. van de Glind G, van den Brink W, Koeter MWJ, et al. Validity of the Adult ADHD Self-Report Scale (ASRS) as a screener for adult ADHD in treatment seeking substance use disorder patients. Drug Alcohol Depend. 2013;132:587-596.
9. Gualtieri CT, Johnson LG. ADHD: Is objective diagnosis possible? Psychiatry. 2005;2:44-53.
10. Perrin AE, Jotwani VM. Addressing the unique issues of student athletes with ADHD. J Fam Pract. 2014;63:E1-E9.
11. Spencer T, Biederman J, Wilens T, et al. Efficacy of mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:775-782.
12. Mészáros A, Czobor P, Bálint S, et al. Pharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): a meta-analysis. Int J Neuropsychopharmacol. 2009;12:1137-1147.
13. Weiss M, Shingler T, Capone NM. Medication satisfaction among adults with ADHD: long term results from the Quality of Life, Effectiveness, Safety and Tolerability (Qu.S.T) study. New Orleans: Program and Abstracts of the 19th US Psychiatric and Mental Health Congress, Abstract 120, 2006.
14. Lopez FA, Leroux JR.
15. Felt BT, Biermann B, Christner JG, et al. Diagnosis and management of ADHD in children. Am Fam Physician. 2014;90:456-464.
16. Spencer T, Biederman J, Wilens T. Nonstimulant treatment of adult attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27:373-383.
17. Withrow LM, Hash PA, Holten KB. Managing ADHD in children: are you doing enough? J Fam Pract. 2011;60:E1-E3.
18. McCabe SE, West BT. Medical and nonmedical use of prescription stimulants: results from a national multicohort study. J Am Acad Child Adolesc Psychiatry. 2013;52:1272-1280.
19. Aldridge AP, Kroutil LA, Cowell AJ, et al. Medication costs to private insurers of diversion of medications for attention-deficit hyperactivity disorder. Pharmacoeconomics. 2011;29:621-635.
20. Garnier LM, Arria AM, Caldeira KM, et al. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71:262-269.
21. Molina BSG, Hinshaw SP, Arnold LE, et al. Adolescent substance use in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52:250-263.
22. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143.
23. Post RE, Kurlansik SL. Diagnosis and management of attention-deficit/hyperactivity disorder in adults. Am Fam Physician. 2012;85:890-896.
24. Cepeda MS, Fife D, Berwaerts J, et al. Doctor shopping for medications used in the treatment of attention deficit hyperactivity disorder: shoppers often pay in cash and cross state lines. Am J Drug Alcohol Abuse. 2015;41:226-229.
25. Safren SA, Sprich S, Mimiaga MJ, et al. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA. 2010;304:875-880.
26. Ross-Durow PL, McCabe SE, Boyd CJ. Adolescents’ access to their own prescription medication in the home. J Adolesc Health. 2013;53:260-264.
1. Kessler RC, Adler LA, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
2. Clemow DB, Walker DJ. The potential for misuse and abuse of medications in ADHD: a review. Postgrad Med. 2014;126:64-81.
3. Novak SP, Kroutil LA, Williams RL, et al. The nonmedical use of prescription ADHD medications: results from a national internet panel. Subst Abuse Treat Prev Policy. 2007;2:32.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Press; 2013.
5. Kolar D, Keller A, Golfinopoulos M, et al. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2008;4:389-403.
6. McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry. 2005;162:1621-1627.
7. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry.
8. van de Glind G, van den Brink W, Koeter MWJ, et al. Validity of the Adult ADHD Self-Report Scale (ASRS) as a screener for adult ADHD in treatment seeking substance use disorder patients. Drug Alcohol Depend. 2013;132:587-596.
9. Gualtieri CT, Johnson LG. ADHD: Is objective diagnosis possible? Psychiatry. 2005;2:44-53.
10. Perrin AE, Jotwani VM. Addressing the unique issues of student athletes with ADHD. J Fam Pract. 2014;63:E1-E9.
11. Spencer T, Biederman J, Wilens T, et al. Efficacy of mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:775-782.
12. Mészáros A, Czobor P, Bálint S, et al. Pharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): a meta-analysis. Int J Neuropsychopharmacol. 2009;12:1137-1147.
13. Weiss M, Shingler T, Capone NM. Medication satisfaction among adults with ADHD: long term results from the Quality of Life, Effectiveness, Safety and Tolerability (Qu.S.T) study. New Orleans: Program and Abstracts of the 19th US Psychiatric and Mental Health Congress, Abstract 120, 2006.
14. Lopez FA, Leroux JR.
15. Felt BT, Biermann B, Christner JG, et al. Diagnosis and management of ADHD in children. Am Fam Physician. 2014;90:456-464.
16. Spencer T, Biederman J, Wilens T. Nonstimulant treatment of adult attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27:373-383.
17. Withrow LM, Hash PA, Holten KB. Managing ADHD in children: are you doing enough? J Fam Pract. 2011;60:E1-E3.
18. McCabe SE, West BT. Medical and nonmedical use of prescription stimulants: results from a national multicohort study. J Am Acad Child Adolesc Psychiatry. 2013;52:1272-1280.
19. Aldridge AP, Kroutil LA, Cowell AJ, et al. Medication costs to private insurers of diversion of medications for attention-deficit hyperactivity disorder. Pharmacoeconomics. 2011;29:621-635.
20. Garnier LM, Arria AM, Caldeira KM, et al. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71:262-269.
21. Molina BSG, Hinshaw SP, Arnold LE, et al. Adolescent substance use in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52:250-263.
22. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143.
23. Post RE, Kurlansik SL. Diagnosis and management of attention-deficit/hyperactivity disorder in adults. Am Fam Physician. 2012;85:890-896.
24. Cepeda MS, Fife D, Berwaerts J, et al. Doctor shopping for medications used in the treatment of attention deficit hyperactivity disorder: shoppers often pay in cash and cross state lines. Am J Drug Alcohol Abuse. 2015;41:226-229.
25. Safren SA, Sprich S, Mimiaga MJ, et al. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA. 2010;304:875-880.
26. Ross-Durow PL, McCabe SE, Boyd CJ. Adolescents’ access to their own prescription medication in the home. J Adolesc Health. 2013;53:260-264.
PRACTICE RECOMMENDATIONS
› Be sure to take steps, which include utilization of a self-report measure—to correctly diagnose attention-deficit/hyperactivity disorder (ADHD) in adult patients before beginning treatment.
› Consider prescribing stimulant medications, such as the short-acting dextroamphetamine/amphetamine or the long-acting lisdexamfetamine, for adults with ADHD.
› Don't underestimate the problems of misuse and diversion among patients taking psychostimulant medications, particularly among younger men.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series