User login
Skin infections in athletes: Treating the patient, protecting the team
• Do not permit athletes with wet, weeping lesions to return to play. C
• Familiarize yourself with the rules governing the team sports your patients participate in and use them to guide return-to-play decisions. C
• Explain to athletes with herpes or bacterial infections that they cannot participate while the lesions are active, even if they are covered with occlusive dressings. C
Strength of recommendation (SOR)
A: Good-quality patient-oriented evidence
B: Inconsistent or limited-quality patient-oriented evidence
C: Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Shane O, a 16-year-old on his high school wrestling team, presents with red, scaly, well-defined plaques on his forehead. The coach spotted the lesions and removed him from play. The patient reports that he’s training for a competitive event and is anxious to return to practice.
You take skin scrapings for a potassium chloride (KOH) test, which is negative, and for a dermatophyte culture. The culture will take 7 to 14 days, however. What should you do in the meantime?
Chances are you’ve cared for countless patients with cutaneous infections. But when the individual who’s infected participates in a team sport, it’s a game changer. In addition to treating the infection, it is necessary to take steps to prevent an outbreak among other players.
More than half of the infections incurred by athletes are cutaneous lesions1—not surprising considering the numerous opportunities sports play provides for common skin conditions to spread. Predisposing factors, in addition to skin-to-skin contact and abrasions, include shared close quarters (eg, locker rooms and showers), shared sports equipment, and poor hygiene.2-5
For a competitive athlete like Shane, the loss of practice may be the most worrisome aspect of a cutaneous infection. From a medical perspective, there are far more pressing concerns. Contact with a teammate’s active herpes simplex lesion (HSV-1), for example, could result in a lifelong infection, and a methicillin-resistant Staphylococcus aureus (MRSA) infection can be a source of significant morbidity for anyone who is infected. There is also the potential for a public health hazard. In addition to prompt, accurate diagnosis, a treatment approach that considers both the need to protect others and the importance of getting the player back in the game as soon as it is safe to do so is critical.
Athlete’s foot, ringworm, jock itch—fungal infections are easily spread
Fungal infections are a common cause of skin disease in athletes. Dermatophytes can spread from fomites such as mats, floors, laundry items, and shared clothing, combs, and brushes, as well as in swimming pools and from direct contact with carriers.2
National Collegiate Athletic Association (NCAA) data show that fungi account for 22% of the skin infections that athletes involved in competitive wrestling develop.6 Sports with skin-to-skin contact, such as wrestling, pose the greatest risk. Fungal infection is the No. 1 reason cited for missing wrestling practice, according to the NCAA.6
Fungal infections are not just a wrestler’s problem, however. Tinea pedis (athlete’s foot) is common among runners and swimmers, and tinea corporis (ringworm), in prepubertal athletes.5
Fungal skin infections are caused by 3 dermatophyte genera: Trichophyton, Epidermophyton, and Microsporum. In the United States, T rubrum is responsible for most cutaneous fungal infections, including tinea corporis, tinea unguium (nail), tinea pedis, and tinea cruris (commonly called jock itch).5
Tinea corporis (Figure 1) may present with areas of central hypopigmentation that give it a ring-like appearance. This type of fungal infection also includes tinea gladiatorum, so named for its high prevalence among wrestlers and characterized by well-defined, red scaling plaques on the head, neck, and upper extremities, often with an irregular border.7
Tinea pedis typically develops in the web spaces of the toes, with skin maceration often accompanied by thick scaling or desquamation. Tinea cruris presents as erythematous plaques in the pubic and inguinal areas.8
Onychomycosis can affect the nail plate, resulting in thickening, change in color, and alteration of nail texture.9
T tonsurans is the dominant pathogen for tinea capitis—fungal infection of the scalp,5 which can be inflammatory or noninflammatory. The noninflammatory form may present with gray-patch scaling, seborrheic dermatitis-like scale, hair thinning without significant scaling, or patches of “black-dot” alopecia. Inflammatory forms of tinea capitis may present as anything from localized pustules to widespread abscesses, or may remain in an asymptomatic carrier state.10
When to confirm a clinical diagnosis
Fungal infections can often be diagnosed based on clinical presentation, but confirmation is important when systemic therapy is required—for tinea capitis, in particular. The traditional method is an examination of skin scraped from the edge of a lesion and treated with 10% to 20% KOH, gently heated, and viewed under light microscopy.8
Alternatively, fungal infection can be confirmed retrospectively, by culture on selective media. Dermatophyte test medium (DTM) is convenient and easy to use, and usually reveals fungal growth within 7 days. A newer selective media, DBM (bromothymol blue is the pH indicator) is a modification of the DTM formulation that may offer earlier and more accurate identification of fungi.11
Keep in mind, however, that a negative KOH preparation or culture does not necessarily rule out a fungal infection.12 Polymerase chain reaction (PCR), an emerging technology performed on a specimen swab, has a greater sensitivity than either KOH or culture in identifying fungal pathogens. PCR can identify the presence of fungi even if the dermatophyte growth on culture is hidden by the overgrowth of Candida albicans.13
In patients with onychomycosis, dermatophytes may be exceedingly difficult to isolate on either KOH or culture medium, and the results of these tests often conflict. Onychomycosis is best diagnosed histopathologically by examination of periodic acid-Schiff-stained nail clippings.14
Topical or systemic treatment?
Treatment of dermatophyte infections is site-dependent. For simple epidermal infection, other than scalp or nail, topical therapy is first-line treatment. In a recent Cochrane review, topical allylamines, azoles, butenafine, ciclopirox olamine, tolciclate, and tolnaftate were all found to be effective. However, the allylamines had the greatest efficacy, which increased with duration of use.15
Topical terbinafine was found to be effective in as little as one to 3 days of treat-ment for tinea pedis. Mycological cure with near total symptom elimination at 28 days was reported in 61% and 78% of those receiving topical treatment for one day and 3 days, respectively; the difference was not statistically significant.16
Use this topical when bacterial infection complicates care. Although 1% naftifine gel requires a prescription and costs more than many other topicals, its advantages may offset the higher costs. Once-daily naftifine gel is as effective as other allylamines that require twice-daily application, and has both antihistamine and corticosteroid effects to offset inflammation. What’s more, naftifine is active against both gram-positive and gram-negative bacteria; therefore, it should be considered in instances in which bacterial superinfection is a possibility, as suggested by a high degree of inflammation with bright red and yellow crusts.17,18
Scalp, nail, and complicated foot infections typically require systemic therapy. Griseofulvin is the most widely used systemic treatment for tinea capitis.10 While terbinafine requires a shorter duration of treatment (4-6 weeks) and is similar in efficacy—except in cases of microsporum infection of the scalp, for which griseofulvin has been found to have higher cure rates19—it is often not used because it has a higher cost.
Tinea pedis and onychomycosis often occur concurrently, making eradication difficult and increasing the potential for reinfection. For recalcitrant cases of onychomycosis, a combination of topical and systemic therapy may be required, along with trimming, debridement, nail abrasion, and partial nail avulsion.20 A recent study found laser therapy to be a promising treatment for onychomycosis, but randomized controlled trials have yet to be done.21
NCAA and NFHS rules. Both the NCAA and the National Federation of State
High School Associations (NFHS) mandate that a wrestler with tinea corporis receive a minimum of 72 hours of topical therapy prior to participation; 14 days of systemic antifungal therapy are required for athletes with tinea capitis.22,23 The NCAA allows wrestlers to be cleared to participate on an individual basis, at the discretion of the examining physician or certified trainer.
The degree of disease involvement, the activity of disease as judged by KOH preparation, or the review of therapeutic regimen and the ability to properly cover lesions securely are taken into account.22 Proper coverage could consist of a semiocclusive or occlusive dressing such as film, foam, hydrogel, or hydrocolloid covered with stretch tape.24 Similarly, the NFHS permits a wrestler to participate once the lesion is deemed to be no longer contagious and can be covered with a bio-occlusive dressing.23
Prophylactic oral fluconazole, given in a 3-day regimen twice during the season to all team members, has been shown to be successful in reducing a high burden of tinea gladiatorum in a high school wrestling setting.25
CASE You presumptively diagnose tinea gladiatorum based on both the presentation and the patient’s history as a wrestler and prescribe topical terbinafine therapy twice daily. You schedule a follow-up appointment in 3 days, and tell Shane he must refrain from wrestling practice at least until then.
Staph and strep infections
Bacterial skin infections are also common among athletes, with S aureus reported to be responsible for 22% of infectious disease outbreaks.26 Here, too, infections occur primarily in contact sports such as football, rugby, and soccer, as well as wrestling.
There are several types of bacterial dermatoses: impetigo, folliculitis, furuncles, carbuncles, abscesses, and cellulitis. Most are caused by group A beta-hemolytic Streptococcus or S aureus and can be easily treated, but identification of the pathogen is needed to facilitate healing and a safe return to play (TABLE 1).27
When to suspect CA-MRSA
Community-acquired methicillin-resistant S aureus (CA-MRSA) was first reported in an athletic population in the 1960s, and in 1993 the first documented outbreak of CA-MRSA in a sports setting—involving 6 high school wrestlers from Vermont—was reported.28
The athlete with CA-MRSA usually presents with a painful, purulent, swollen, red abscess-like lesion, sometimes described as (or mistaken for) a spider bite. The patient may also develop fever, fatigue, and malaise. Culturing the wound for identification of the bacterium and for susceptibility to antibiotic therapy is needed for a definitive diagnosis of CA-MRSA.
Is incision and drainage sufficient treatment? The standard treatment for uncomplicated CA-MRSA lesions is incision and drainage. Several studies have found this to be adequate for simple lesions.29 Others have reported increased treatment failure with simple incision and drainage and shown that the addition of antimicrobial therapy helps decrease further tissue damage and morbidity.29
With no clear consensus as to when and whether to add oral antibiotic therapy after incision and drainage of a CA-MRSA lesion, decisions should be based on the severity of the lesion, the presence or absence of systemic symptoms, and the potential risk of bacterial spread to other team members.
Choosing an antimicrobial agent. Antimicrobial treatment should be guided by culture and sensitivity results, as well as the regional incidence of CA-MRSA. Empiric treatments for CA-MRSA are trimethoprim-sulfamethoxazole, doxycycline, and clindamycin, taken for 7 to 14 days. If you’re considering the use of clindamycin and there is known resistance to erythromycin from antimicrobial sensitivities, a double disc diffusion (D-test) should be ordered to detect inducible macrolide resistance that can occur in some strains of CA-MRSA.29,30
Fluoroquinolones and certain macrolides should not be used, due to resistance to these antibiotics.1
Topicals for superficial lesions. Topical antibiotic therapy with mupirocin or retapamulin should be reserved for superficial CA-MRSA lesions, such as impetigo.31,32 Caution must be used when mupirocin is prescribed, however, due to recent studies showing increasing resistance to mupirocin, especially when used for nasal decolonizing purposes.29
Once treatment is initiated, see the athlete every 2 to 3 days. Resolution usually occurs in 10 to 14 days. The athlete can return to play after 72 hours of treatment, however, provided there is evidence of clinical improvement, no further drainage from the infected lesion, and no new lesions have developed.1,30
Containing CA-MRSA. Mass nasal decolonization—applying topical mupirocin in the nares of infected athletes as well as their teammates—has been attempted to prevent the spread of CA-MRSA. But there is no evidence to suggest that mupirocin or any other intranasal antimicrobial is effective in preventing the spread of CA-MRSA, and nasal decolonization should not be attempted in any community setting.29 In fact, studies have found that attempts at nasal decolonization can actually lead to increased bacterial resistance and a recurrence of colonization.29,31,32
HSV-1 is highly prevalent
Herpes simplex virus-1 (HSV-1) is a common problem in athletes who play team sports that involve skin-to-skin contact. It is particularly prevalent among competitive wrestlers—earning it the name herpes gladiatorum.
In fact, HSV-1 is widespread throughout the country: Its prevalence in the general population is 58%.33 It is estimated that nearly half (47%) of cutaneous infections in collegiate athletes are caused by the herpes virus, making HSV-1 the most common pathogen of skin infections in this group.34 The clinical presentation of active HSV-1 depends on whether the infection is primary or recurrent.
So which form of HSV-1 is it?
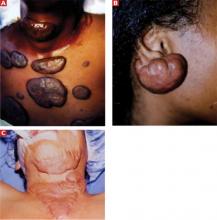
Primary lesions may be preceded by a prodromal period with systemic symptoms, such as fever. Oral lesions and enlarged cervical and submandibular lymph nodes follow.35 The lesions, which are typically painful, can be found on the lips, buccal mucosa, or tongue—nearly anywhere in and around the oral cavity (Figure 2). They are vesicular at first, then ulcerate. Healing typically takes 10 to 14 days.36
In recurrent HSV-1, clusters of vesicles with erythematous borders typically occur. In those who play contact sports, HSV lesions can be found not only in the typical facial and oral areas, but anywhere on the head, face, torso, or extremities, as well.
Identifying herpetic whitlow. Presenting as a cluster of herpetic vesicles on the hands, fingers, or toes, herpetic whitlow is a common presentation of recurrent HSV-1. Recognition of these lesions is usually adequate for a diagnosis of recurrent infection, but confirmation can be obtained by laboratory or serologic testing.
Testing should be considered when you suspect that a patient has a primary herpes infection, as HSV-1 is a lifelong diagnosis. Viral culture is technique dependent and limited by 50% sensitivity, as cultures can take from 2 to 4 days to grow.35 The Tzanck test (direct microscopic examination of skin scrapings) has fallen out of favor, and PCR is now the gold standard.
Compared with viral culture, PCR has been shown to detect 80% of positive cases.35 However, PCR—using swab specimens taken from the lesions—is expensive and must be sent to a lab for analysis. One study suggests that while less sensitive than PCR, the Tzanck test can still be a reliably sensitive method of diagnosis when done properly, at less cost and with quicker
results.37
Serologic tests of HSV-1 IgM and IgG antibodies are also available. Serum IgG levels remain elevated in patients with previous infections. In primary infections, IgM is most useful as it can detect recent or active infection, but results may be falsely negative for several days after infection.38
Once diagnosed, management of HSV-1 in athletes is based on whether the infection is primary or recurrent. In both cases, oral antivirals are needed. Acyclovir is the gold standard, but famciclovir and valacyclovir have been proven to be equally effective.39 Both the NCAA and NFHS have published guidelines addressing the question of return to play (TABLE 2).22,23
In addition to the management of primary and recurrent infections of HSV-1, it is recommended that athletes and coaches known to be HSV-1 seropositive be treated with prophylactic suppressive therapy.40
The Centers for Disease Control and Prevention recommends acyclovir 400 mg bid or valacyclovir 500 mg/d as prophylaxis for anyone with ≤10 recurrences per year. For those with >10 recurrences annually, valacyclovir 1000 mg/d is recommended for the rest of the season.40 This has been shown to be effective in the reduction of outbreaks among wrestlers after a large outbreak occurred in 2007 in Minnesota,40,41 and NCAA and NFHS guidelines now support prophylactic therapy during competitive season.22,23 Before prescribing it, clinicians need to consider both the benefits of prophylactic antiviral therapy and the risks of promoting HSV-1 resistance.
Focus on prevention and squelching outbreaks
For cutaneous infections, as with so many medical conditions, prevention is paramount. With good preventive practices, many, if not all, of the skin infections common among athletes can be eliminated and outbreaks can be squelched.
Good hygienic practice is the cornerstone of prevention. Patients who participate in team sports should be advised to:
- shower immediately after practice
- refrain from sharing personal equipment like uniforms, towels, razors, and headgear
- launder workout clothes and towels after each use
- immediately cleanse and cover any abrasions that occur during practice.
Athletes should also be advised to ask their trainer or coach to check their skin for lesions on a regular basis. Surveillance should be instituted to prohibit athletes with cutaneous lesions from participating until they are sufficiently treated.
Although the role that environmental contamination plays in transmission of infection is uncertain, it is recommended that all sports equipment, playing surfaces, and locker rooms be disinfected daily with either a freshly made bactericidal (1/100 bleach/water solution)23,24 or an appropriate product. The Environmental Protection Agency provides a list of commercial products that have been proven to prevent the spread of MRSA on such surfaces (http://epa.gov/oppad001/chemregindex.htm) on its Web site.42
CASE When Shane returned 3 days later, the erythema had resolved, and minimal scaling remained. You tell him to continue to use the topical terbinafine twice a day for 10 days. You also show him how to apply a bio-occlusive dressing and clear him for practice, provided the lesions are fully covered.
You also talk to Shane about prevention, recommending that he immediately clean and cover any abrasion or other skin trauma that occurs during practice and suggesting that he ask his trainer to regularly check team members for skin lesions.
At Day 7, the DTM culture is positive, confirming a dermatophyte infection.
CORRESPONDENCE
Nilesh Shah, MD, 20 Olive Street, Suite 201, Akron, OH 44310;
[email protected]
ACKNOWLEDGEMENT
The authors would like to thank Tom Bartsokas, MD, for his help with this manuscript.
1. Zinder SM, Basler RS, Foley J, et al. National Athletic Trainers Association position statement: skin diseases. J Athletic Training. 2010;45:411-428.
2. Brandi G, Sisti M, Paparini A, et al. Swimming pools and fungi: an environmental epidemiology survey in Italian indoor swimming facilities. Int J Environ Health Res. 2007;17:197-206.
3. Dienst WL Jr, Dightman L, Dworkin MS. Diagnosis, treatment and pinning down skin infections: diagnosis, treatment and prevention in wrestlers. Physician Sportsmed. 2005;25:45-56.
4. Ilkit M, GÜmral R, Saraçli MA, et al. Trichophyton tonsurans scalp carriage among wrestlers in a national competition in Turkey. Mycopathologia. 2011;172:215-222.
5. Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
6. Agel J, Ransone J, Dick R, et al. Descriptive epidemiology of collegiate men’s wrestling injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Training. 2007;42:303-310.
7. Adams BB. Tinea corporis gladiatorum. J Am Acad Dermatol. 2002;47:286-297.
8. Pecci M, Comeau D, Chawla V. Skin conditions in the athlete. Am J Sport Med. 2009;37:406-418.
9. Beaven DW, Brooks SE. Color Atlas of the Nail in Clinical Diagnosis. 2nd ed. London: Mosby-Wolfe; 1994.
10. Gupta AK, Summerbell RC. Tinea capitis. Med Mycol. 2000;38:255-287.
11. Li XF, Shen YN, Chen W, et al. A new medium for diagnosis of dermatophyte infection. Eur J Dermatol. 2009;19:34-37.
12. Akcaglar S, Ener B, Toker SC, et al. A comparative study of dermatophyte infections in Bursa Turkey. Med Mycol. 2011;49:602-607.
13. Garg J, Tilak R, Garg A, et al. Rapid detection of dermatophytes from skin and hair. BMC Res Notes. 2009;18:60.
14. Reisberger EM, Abels C, Landthaler M, et al. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br J Dermatol. 2003;148:749-754.
15. Crawford F, Hollis S. Topical treatments for fungal infections of the ski and nails of the foot. Cochrane Database Syst Rev. 2007;(3):CD001434.
16. Evans EGV, Seaman RAJ, James IGV. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol. 1994;130:83-88.
17. Gupta AK, Ryder JE, Cooper EA. Naftifine: a review. J Cutan Med Surg. 2008;12:51-58.
18. Friedrich M. Inflammatory tinea pedis with bacterial superinfection effectively treated with isoconazole nitrate and diflucortolone valerate combination therapy. Mycoses. 2013;56(suppl 1):S23-S25.
19. González U, Seaton T, Bergus G, et al. Systemic antifungal therapy for tinea capitis in children. Cochrane Database Syst Rev. 2007;(4):CD004685.
20. Baran R, Hay RJ, Garduno JI. Review of antifungal therapy, part II: treatment rationale, including specific patient populations. J Dermatol Treat. 2008;19:168-175.
21. Zhang RN, Wang DK, Zhuo FL, et al. Long-pulse Nd:YAG 1064- nm laser treatment for onychomycosis. Chin Med J (Engl). 2012;125:3288-3291.
22. Guideline 2j: Skin infections in athletics. In: National Collegiate Athletic Association Sports Medicine Handbook. 22nd ed. Indianapolis, IN: National Collegiate Athletic Association; 2011:63.
23. National Federation of State High School Associations, Sports Medicine Advisory Committee. General guidelines for sports hygiene, skin infections and communicable diseases. Revised October 2012. Available at: http://www.nfhs.org/search. aspx?searchtext=skin%20infection. Accessed May 15, 2013.
24. Zinder SM, Basler RS, Foley J, et al. National Athletic Trainers’ Association position statement: skin diseases. J Athl Training. 2010;45:411-428.
25. Brickman K, Einstein E, Sinha S, et al. Fluconazole as a prophylactic measure for tinea gladiatorum in high school wrestlers. Clin J Sport Med. 2009;19:412-414.
26. Turbeville S, Cowan L, Greenfield R. Infectious disease outbreaks in competitive sports. Am J Sports Med. 2006;34:1860-1865.
27. Sedgwick P, Dexter W, Smith C. Bacterial dermatoses in sports. Clin Sports Med. 2007;26:383-396.
28. Patel A, Fischer S, Calfee R, et al. Locker room acquired methicillin-resistance Staphylococcus aureus. Orthopedics. 2007;30:532-535.
29. David M, Daum R. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616-687.
30. Benjamin H, Nikore V, Takagishi J. Practical management: community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA): the latest sports epidemic. Clin J Sport Med. 2007;17:393-397.
31. Rhin JA, Posfay-Barbe K, Harner CD, et al. Community-acquired methicillin-resistant Staphylococcus aureus outbreak in a local high school football team unsuccessful interventions. Pediatr Infect Dis J. 2005;24:841-843.
32. Loeb M, Main C, Walker-Dilks C, et al. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev. 2003;(4):CD003340.
33. Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964-973.
34. Yard EE, Collins CL, Dick RW, et al. An epidemiologic comparison of high school and college wrestling injuries. Am J Sports Med. 2008;36:57-64.
35. Usatine RP, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
36. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
37. Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:541-555.
38. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9:86-90.
39. Ozcan A, Senol M, Saglam H, et al. Comparison of the Tzanck test and polymerase chain reaction in the diagnosis of cutaneous herpes simplex and varicella zoster virus infections. Int J Dermatol. 2007;46:1177-1179.
40. Morrow R, Friedrich D. Performance of a novel test for IgM and IgG antibodies in subjects with culture-documented genital herpes simplex virus-1 or -2 infection. Clin Microbiol Infect. 2006;12:463-469.
41. Anderson BJ. Managing herpes gladiatorum outbreaks in competitive wrestling: the 2007 Minnesota experience. Curr Sports Med Rep. 2008;7:323-327.
42. Environmental Protection Agency. EPA’s registered sterilizers, tuberculocides, and antimicrobial products against certain human public health bacteria and viruses. October 2012. Available at: http://epa.gov/oppad001/chemregindex.htm. Accessed November 13, 2012.
• Do not permit athletes with wet, weeping lesions to return to play. C
• Familiarize yourself with the rules governing the team sports your patients participate in and use them to guide return-to-play decisions. C
• Explain to athletes with herpes or bacterial infections that they cannot participate while the lesions are active, even if they are covered with occlusive dressings. C
Strength of recommendation (SOR)
A: Good-quality patient-oriented evidence
B: Inconsistent or limited-quality patient-oriented evidence
C: Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Shane O, a 16-year-old on his high school wrestling team, presents with red, scaly, well-defined plaques on his forehead. The coach spotted the lesions and removed him from play. The patient reports that he’s training for a competitive event and is anxious to return to practice.
You take skin scrapings for a potassium chloride (KOH) test, which is negative, and for a dermatophyte culture. The culture will take 7 to 14 days, however. What should you do in the meantime?
Chances are you’ve cared for countless patients with cutaneous infections. But when the individual who’s infected participates in a team sport, it’s a game changer. In addition to treating the infection, it is necessary to take steps to prevent an outbreak among other players.
More than half of the infections incurred by athletes are cutaneous lesions1—not surprising considering the numerous opportunities sports play provides for common skin conditions to spread. Predisposing factors, in addition to skin-to-skin contact and abrasions, include shared close quarters (eg, locker rooms and showers), shared sports equipment, and poor hygiene.2-5
For a competitive athlete like Shane, the loss of practice may be the most worrisome aspect of a cutaneous infection. From a medical perspective, there are far more pressing concerns. Contact with a teammate’s active herpes simplex lesion (HSV-1), for example, could result in a lifelong infection, and a methicillin-resistant Staphylococcus aureus (MRSA) infection can be a source of significant morbidity for anyone who is infected. There is also the potential for a public health hazard. In addition to prompt, accurate diagnosis, a treatment approach that considers both the need to protect others and the importance of getting the player back in the game as soon as it is safe to do so is critical.
Athlete’s foot, ringworm, jock itch—fungal infections are easily spread
Fungal infections are a common cause of skin disease in athletes. Dermatophytes can spread from fomites such as mats, floors, laundry items, and shared clothing, combs, and brushes, as well as in swimming pools and from direct contact with carriers.2
National Collegiate Athletic Association (NCAA) data show that fungi account for 22% of the skin infections that athletes involved in competitive wrestling develop.6 Sports with skin-to-skin contact, such as wrestling, pose the greatest risk. Fungal infection is the No. 1 reason cited for missing wrestling practice, according to the NCAA.6
Fungal infections are not just a wrestler’s problem, however. Tinea pedis (athlete’s foot) is common among runners and swimmers, and tinea corporis (ringworm), in prepubertal athletes.5
Fungal skin infections are caused by 3 dermatophyte genera: Trichophyton, Epidermophyton, and Microsporum. In the United States, T rubrum is responsible for most cutaneous fungal infections, including tinea corporis, tinea unguium (nail), tinea pedis, and tinea cruris (commonly called jock itch).5
Tinea corporis (Figure 1) may present with areas of central hypopigmentation that give it a ring-like appearance. This type of fungal infection also includes tinea gladiatorum, so named for its high prevalence among wrestlers and characterized by well-defined, red scaling plaques on the head, neck, and upper extremities, often with an irregular border.7
Tinea pedis typically develops in the web spaces of the toes, with skin maceration often accompanied by thick scaling or desquamation. Tinea cruris presents as erythematous plaques in the pubic and inguinal areas.8
Onychomycosis can affect the nail plate, resulting in thickening, change in color, and alteration of nail texture.9
T tonsurans is the dominant pathogen for tinea capitis—fungal infection of the scalp,5 which can be inflammatory or noninflammatory. The noninflammatory form may present with gray-patch scaling, seborrheic dermatitis-like scale, hair thinning without significant scaling, or patches of “black-dot” alopecia. Inflammatory forms of tinea capitis may present as anything from localized pustules to widespread abscesses, or may remain in an asymptomatic carrier state.10
When to confirm a clinical diagnosis
Fungal infections can often be diagnosed based on clinical presentation, but confirmation is important when systemic therapy is required—for tinea capitis, in particular. The traditional method is an examination of skin scraped from the edge of a lesion and treated with 10% to 20% KOH, gently heated, and viewed under light microscopy.8
Alternatively, fungal infection can be confirmed retrospectively, by culture on selective media. Dermatophyte test medium (DTM) is convenient and easy to use, and usually reveals fungal growth within 7 days. A newer selective media, DBM (bromothymol blue is the pH indicator) is a modification of the DTM formulation that may offer earlier and more accurate identification of fungi.11
Keep in mind, however, that a negative KOH preparation or culture does not necessarily rule out a fungal infection.12 Polymerase chain reaction (PCR), an emerging technology performed on a specimen swab, has a greater sensitivity than either KOH or culture in identifying fungal pathogens. PCR can identify the presence of fungi even if the dermatophyte growth on culture is hidden by the overgrowth of Candida albicans.13
In patients with onychomycosis, dermatophytes may be exceedingly difficult to isolate on either KOH or culture medium, and the results of these tests often conflict. Onychomycosis is best diagnosed histopathologically by examination of periodic acid-Schiff-stained nail clippings.14
Topical or systemic treatment?
Treatment of dermatophyte infections is site-dependent. For simple epidermal infection, other than scalp or nail, topical therapy is first-line treatment. In a recent Cochrane review, topical allylamines, azoles, butenafine, ciclopirox olamine, tolciclate, and tolnaftate were all found to be effective. However, the allylamines had the greatest efficacy, which increased with duration of use.15
Topical terbinafine was found to be effective in as little as one to 3 days of treat-ment for tinea pedis. Mycological cure with near total symptom elimination at 28 days was reported in 61% and 78% of those receiving topical treatment for one day and 3 days, respectively; the difference was not statistically significant.16
Use this topical when bacterial infection complicates care. Although 1% naftifine gel requires a prescription and costs more than many other topicals, its advantages may offset the higher costs. Once-daily naftifine gel is as effective as other allylamines that require twice-daily application, and has both antihistamine and corticosteroid effects to offset inflammation. What’s more, naftifine is active against both gram-positive and gram-negative bacteria; therefore, it should be considered in instances in which bacterial superinfection is a possibility, as suggested by a high degree of inflammation with bright red and yellow crusts.17,18
Scalp, nail, and complicated foot infections typically require systemic therapy. Griseofulvin is the most widely used systemic treatment for tinea capitis.10 While terbinafine requires a shorter duration of treatment (4-6 weeks) and is similar in efficacy—except in cases of microsporum infection of the scalp, for which griseofulvin has been found to have higher cure rates19—it is often not used because it has a higher cost.
Tinea pedis and onychomycosis often occur concurrently, making eradication difficult and increasing the potential for reinfection. For recalcitrant cases of onychomycosis, a combination of topical and systemic therapy may be required, along with trimming, debridement, nail abrasion, and partial nail avulsion.20 A recent study found laser therapy to be a promising treatment for onychomycosis, but randomized controlled trials have yet to be done.21
NCAA and NFHS rules. Both the NCAA and the National Federation of State
High School Associations (NFHS) mandate that a wrestler with tinea corporis receive a minimum of 72 hours of topical therapy prior to participation; 14 days of systemic antifungal therapy are required for athletes with tinea capitis.22,23 The NCAA allows wrestlers to be cleared to participate on an individual basis, at the discretion of the examining physician or certified trainer.
The degree of disease involvement, the activity of disease as judged by KOH preparation, or the review of therapeutic regimen and the ability to properly cover lesions securely are taken into account.22 Proper coverage could consist of a semiocclusive or occlusive dressing such as film, foam, hydrogel, or hydrocolloid covered with stretch tape.24 Similarly, the NFHS permits a wrestler to participate once the lesion is deemed to be no longer contagious and can be covered with a bio-occlusive dressing.23
Prophylactic oral fluconazole, given in a 3-day regimen twice during the season to all team members, has been shown to be successful in reducing a high burden of tinea gladiatorum in a high school wrestling setting.25
CASE You presumptively diagnose tinea gladiatorum based on both the presentation and the patient’s history as a wrestler and prescribe topical terbinafine therapy twice daily. You schedule a follow-up appointment in 3 days, and tell Shane he must refrain from wrestling practice at least until then.
Staph and strep infections
Bacterial skin infections are also common among athletes, with S aureus reported to be responsible for 22% of infectious disease outbreaks.26 Here, too, infections occur primarily in contact sports such as football, rugby, and soccer, as well as wrestling.
There are several types of bacterial dermatoses: impetigo, folliculitis, furuncles, carbuncles, abscesses, and cellulitis. Most are caused by group A beta-hemolytic Streptococcus or S aureus and can be easily treated, but identification of the pathogen is needed to facilitate healing and a safe return to play (TABLE 1).27
When to suspect CA-MRSA
Community-acquired methicillin-resistant S aureus (CA-MRSA) was first reported in an athletic population in the 1960s, and in 1993 the first documented outbreak of CA-MRSA in a sports setting—involving 6 high school wrestlers from Vermont—was reported.28
The athlete with CA-MRSA usually presents with a painful, purulent, swollen, red abscess-like lesion, sometimes described as (or mistaken for) a spider bite. The patient may also develop fever, fatigue, and malaise. Culturing the wound for identification of the bacterium and for susceptibility to antibiotic therapy is needed for a definitive diagnosis of CA-MRSA.
Is incision and drainage sufficient treatment? The standard treatment for uncomplicated CA-MRSA lesions is incision and drainage. Several studies have found this to be adequate for simple lesions.29 Others have reported increased treatment failure with simple incision and drainage and shown that the addition of antimicrobial therapy helps decrease further tissue damage and morbidity.29
With no clear consensus as to when and whether to add oral antibiotic therapy after incision and drainage of a CA-MRSA lesion, decisions should be based on the severity of the lesion, the presence or absence of systemic symptoms, and the potential risk of bacterial spread to other team members.
Choosing an antimicrobial agent. Antimicrobial treatment should be guided by culture and sensitivity results, as well as the regional incidence of CA-MRSA. Empiric treatments for CA-MRSA are trimethoprim-sulfamethoxazole, doxycycline, and clindamycin, taken for 7 to 14 days. If you’re considering the use of clindamycin and there is known resistance to erythromycin from antimicrobial sensitivities, a double disc diffusion (D-test) should be ordered to detect inducible macrolide resistance that can occur in some strains of CA-MRSA.29,30
Fluoroquinolones and certain macrolides should not be used, due to resistance to these antibiotics.1
Topicals for superficial lesions. Topical antibiotic therapy with mupirocin or retapamulin should be reserved for superficial CA-MRSA lesions, such as impetigo.31,32 Caution must be used when mupirocin is prescribed, however, due to recent studies showing increasing resistance to mupirocin, especially when used for nasal decolonizing purposes.29
Once treatment is initiated, see the athlete every 2 to 3 days. Resolution usually occurs in 10 to 14 days. The athlete can return to play after 72 hours of treatment, however, provided there is evidence of clinical improvement, no further drainage from the infected lesion, and no new lesions have developed.1,30
Containing CA-MRSA. Mass nasal decolonization—applying topical mupirocin in the nares of infected athletes as well as their teammates—has been attempted to prevent the spread of CA-MRSA. But there is no evidence to suggest that mupirocin or any other intranasal antimicrobial is effective in preventing the spread of CA-MRSA, and nasal decolonization should not be attempted in any community setting.29 In fact, studies have found that attempts at nasal decolonization can actually lead to increased bacterial resistance and a recurrence of colonization.29,31,32
HSV-1 is highly prevalent
Herpes simplex virus-1 (HSV-1) is a common problem in athletes who play team sports that involve skin-to-skin contact. It is particularly prevalent among competitive wrestlers—earning it the name herpes gladiatorum.
In fact, HSV-1 is widespread throughout the country: Its prevalence in the general population is 58%.33 It is estimated that nearly half (47%) of cutaneous infections in collegiate athletes are caused by the herpes virus, making HSV-1 the most common pathogen of skin infections in this group.34 The clinical presentation of active HSV-1 depends on whether the infection is primary or recurrent.
So which form of HSV-1 is it?
Primary lesions may be preceded by a prodromal period with systemic symptoms, such as fever. Oral lesions and enlarged cervical and submandibular lymph nodes follow.35 The lesions, which are typically painful, can be found on the lips, buccal mucosa, or tongue—nearly anywhere in and around the oral cavity (Figure 2). They are vesicular at first, then ulcerate. Healing typically takes 10 to 14 days.36
In recurrent HSV-1, clusters of vesicles with erythematous borders typically occur. In those who play contact sports, HSV lesions can be found not only in the typical facial and oral areas, but anywhere on the head, face, torso, or extremities, as well.
Identifying herpetic whitlow. Presenting as a cluster of herpetic vesicles on the hands, fingers, or toes, herpetic whitlow is a common presentation of recurrent HSV-1. Recognition of these lesions is usually adequate for a diagnosis of recurrent infection, but confirmation can be obtained by laboratory or serologic testing.
Testing should be considered when you suspect that a patient has a primary herpes infection, as HSV-1 is a lifelong diagnosis. Viral culture is technique dependent and limited by 50% sensitivity, as cultures can take from 2 to 4 days to grow.35 The Tzanck test (direct microscopic examination of skin scrapings) has fallen out of favor, and PCR is now the gold standard.
Compared with viral culture, PCR has been shown to detect 80% of positive cases.35 However, PCR—using swab specimens taken from the lesions—is expensive and must be sent to a lab for analysis. One study suggests that while less sensitive than PCR, the Tzanck test can still be a reliably sensitive method of diagnosis when done properly, at less cost and with quicker
results.37
Serologic tests of HSV-1 IgM and IgG antibodies are also available. Serum IgG levels remain elevated in patients with previous infections. In primary infections, IgM is most useful as it can detect recent or active infection, but results may be falsely negative for several days after infection.38
Once diagnosed, management of HSV-1 in athletes is based on whether the infection is primary or recurrent. In both cases, oral antivirals are needed. Acyclovir is the gold standard, but famciclovir and valacyclovir have been proven to be equally effective.39 Both the NCAA and NFHS have published guidelines addressing the question of return to play (TABLE 2).22,23
In addition to the management of primary and recurrent infections of HSV-1, it is recommended that athletes and coaches known to be HSV-1 seropositive be treated with prophylactic suppressive therapy.40
The Centers for Disease Control and Prevention recommends acyclovir 400 mg bid or valacyclovir 500 mg/d as prophylaxis for anyone with ≤10 recurrences per year. For those with >10 recurrences annually, valacyclovir 1000 mg/d is recommended for the rest of the season.40 This has been shown to be effective in the reduction of outbreaks among wrestlers after a large outbreak occurred in 2007 in Minnesota,40,41 and NCAA and NFHS guidelines now support prophylactic therapy during competitive season.22,23 Before prescribing it, clinicians need to consider both the benefits of prophylactic antiviral therapy and the risks of promoting HSV-1 resistance.
Focus on prevention and squelching outbreaks
For cutaneous infections, as with so many medical conditions, prevention is paramount. With good preventive practices, many, if not all, of the skin infections common among athletes can be eliminated and outbreaks can be squelched.
Good hygienic practice is the cornerstone of prevention. Patients who participate in team sports should be advised to:
- shower immediately after practice
- refrain from sharing personal equipment like uniforms, towels, razors, and headgear
- launder workout clothes and towels after each use
- immediately cleanse and cover any abrasions that occur during practice.
Athletes should also be advised to ask their trainer or coach to check their skin for lesions on a regular basis. Surveillance should be instituted to prohibit athletes with cutaneous lesions from participating until they are sufficiently treated.
Although the role that environmental contamination plays in transmission of infection is uncertain, it is recommended that all sports equipment, playing surfaces, and locker rooms be disinfected daily with either a freshly made bactericidal (1/100 bleach/water solution)23,24 or an appropriate product. The Environmental Protection Agency provides a list of commercial products that have been proven to prevent the spread of MRSA on such surfaces (http://epa.gov/oppad001/chemregindex.htm) on its Web site.42
CASE When Shane returned 3 days later, the erythema had resolved, and minimal scaling remained. You tell him to continue to use the topical terbinafine twice a day for 10 days. You also show him how to apply a bio-occlusive dressing and clear him for practice, provided the lesions are fully covered.
You also talk to Shane about prevention, recommending that he immediately clean and cover any abrasion or other skin trauma that occurs during practice and suggesting that he ask his trainer to regularly check team members for skin lesions.
At Day 7, the DTM culture is positive, confirming a dermatophyte infection.
CORRESPONDENCE
Nilesh Shah, MD, 20 Olive Street, Suite 201, Akron, OH 44310;
[email protected]
ACKNOWLEDGEMENT
The authors would like to thank Tom Bartsokas, MD, for his help with this manuscript.
• Do not permit athletes with wet, weeping lesions to return to play. C
• Familiarize yourself with the rules governing the team sports your patients participate in and use them to guide return-to-play decisions. C
• Explain to athletes with herpes or bacterial infections that they cannot participate while the lesions are active, even if they are covered with occlusive dressings. C
Strength of recommendation (SOR)
A: Good-quality patient-oriented evidence
B: Inconsistent or limited-quality patient-oriented evidence
C: Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Shane O, a 16-year-old on his high school wrestling team, presents with red, scaly, well-defined plaques on his forehead. The coach spotted the lesions and removed him from play. The patient reports that he’s training for a competitive event and is anxious to return to practice.
You take skin scrapings for a potassium chloride (KOH) test, which is negative, and for a dermatophyte culture. The culture will take 7 to 14 days, however. What should you do in the meantime?
Chances are you’ve cared for countless patients with cutaneous infections. But when the individual who’s infected participates in a team sport, it’s a game changer. In addition to treating the infection, it is necessary to take steps to prevent an outbreak among other players.
More than half of the infections incurred by athletes are cutaneous lesions1—not surprising considering the numerous opportunities sports play provides for common skin conditions to spread. Predisposing factors, in addition to skin-to-skin contact and abrasions, include shared close quarters (eg, locker rooms and showers), shared sports equipment, and poor hygiene.2-5
For a competitive athlete like Shane, the loss of practice may be the most worrisome aspect of a cutaneous infection. From a medical perspective, there are far more pressing concerns. Contact with a teammate’s active herpes simplex lesion (HSV-1), for example, could result in a lifelong infection, and a methicillin-resistant Staphylococcus aureus (MRSA) infection can be a source of significant morbidity for anyone who is infected. There is also the potential for a public health hazard. In addition to prompt, accurate diagnosis, a treatment approach that considers both the need to protect others and the importance of getting the player back in the game as soon as it is safe to do so is critical.
Athlete’s foot, ringworm, jock itch—fungal infections are easily spread
Fungal infections are a common cause of skin disease in athletes. Dermatophytes can spread from fomites such as mats, floors, laundry items, and shared clothing, combs, and brushes, as well as in swimming pools and from direct contact with carriers.2
National Collegiate Athletic Association (NCAA) data show that fungi account for 22% of the skin infections that athletes involved in competitive wrestling develop.6 Sports with skin-to-skin contact, such as wrestling, pose the greatest risk. Fungal infection is the No. 1 reason cited for missing wrestling practice, according to the NCAA.6
Fungal infections are not just a wrestler’s problem, however. Tinea pedis (athlete’s foot) is common among runners and swimmers, and tinea corporis (ringworm), in prepubertal athletes.5
Fungal skin infections are caused by 3 dermatophyte genera: Trichophyton, Epidermophyton, and Microsporum. In the United States, T rubrum is responsible for most cutaneous fungal infections, including tinea corporis, tinea unguium (nail), tinea pedis, and tinea cruris (commonly called jock itch).5
Tinea corporis (Figure 1) may present with areas of central hypopigmentation that give it a ring-like appearance. This type of fungal infection also includes tinea gladiatorum, so named for its high prevalence among wrestlers and characterized by well-defined, red scaling plaques on the head, neck, and upper extremities, often with an irregular border.7
Tinea pedis typically develops in the web spaces of the toes, with skin maceration often accompanied by thick scaling or desquamation. Tinea cruris presents as erythematous plaques in the pubic and inguinal areas.8
Onychomycosis can affect the nail plate, resulting in thickening, change in color, and alteration of nail texture.9
T tonsurans is the dominant pathogen for tinea capitis—fungal infection of the scalp,5 which can be inflammatory or noninflammatory. The noninflammatory form may present with gray-patch scaling, seborrheic dermatitis-like scale, hair thinning without significant scaling, or patches of “black-dot” alopecia. Inflammatory forms of tinea capitis may present as anything from localized pustules to widespread abscesses, or may remain in an asymptomatic carrier state.10
When to confirm a clinical diagnosis
Fungal infections can often be diagnosed based on clinical presentation, but confirmation is important when systemic therapy is required—for tinea capitis, in particular. The traditional method is an examination of skin scraped from the edge of a lesion and treated with 10% to 20% KOH, gently heated, and viewed under light microscopy.8
Alternatively, fungal infection can be confirmed retrospectively, by culture on selective media. Dermatophyte test medium (DTM) is convenient and easy to use, and usually reveals fungal growth within 7 days. A newer selective media, DBM (bromothymol blue is the pH indicator) is a modification of the DTM formulation that may offer earlier and more accurate identification of fungi.11
Keep in mind, however, that a negative KOH preparation or culture does not necessarily rule out a fungal infection.12 Polymerase chain reaction (PCR), an emerging technology performed on a specimen swab, has a greater sensitivity than either KOH or culture in identifying fungal pathogens. PCR can identify the presence of fungi even if the dermatophyte growth on culture is hidden by the overgrowth of Candida albicans.13
In patients with onychomycosis, dermatophytes may be exceedingly difficult to isolate on either KOH or culture medium, and the results of these tests often conflict. Onychomycosis is best diagnosed histopathologically by examination of periodic acid-Schiff-stained nail clippings.14
Topical or systemic treatment?
Treatment of dermatophyte infections is site-dependent. For simple epidermal infection, other than scalp or nail, topical therapy is first-line treatment. In a recent Cochrane review, topical allylamines, azoles, butenafine, ciclopirox olamine, tolciclate, and tolnaftate were all found to be effective. However, the allylamines had the greatest efficacy, which increased with duration of use.15
Topical terbinafine was found to be effective in as little as one to 3 days of treat-ment for tinea pedis. Mycological cure with near total symptom elimination at 28 days was reported in 61% and 78% of those receiving topical treatment for one day and 3 days, respectively; the difference was not statistically significant.16
Use this topical when bacterial infection complicates care. Although 1% naftifine gel requires a prescription and costs more than many other topicals, its advantages may offset the higher costs. Once-daily naftifine gel is as effective as other allylamines that require twice-daily application, and has both antihistamine and corticosteroid effects to offset inflammation. What’s more, naftifine is active against both gram-positive and gram-negative bacteria; therefore, it should be considered in instances in which bacterial superinfection is a possibility, as suggested by a high degree of inflammation with bright red and yellow crusts.17,18
Scalp, nail, and complicated foot infections typically require systemic therapy. Griseofulvin is the most widely used systemic treatment for tinea capitis.10 While terbinafine requires a shorter duration of treatment (4-6 weeks) and is similar in efficacy—except in cases of microsporum infection of the scalp, for which griseofulvin has been found to have higher cure rates19—it is often not used because it has a higher cost.
Tinea pedis and onychomycosis often occur concurrently, making eradication difficult and increasing the potential for reinfection. For recalcitrant cases of onychomycosis, a combination of topical and systemic therapy may be required, along with trimming, debridement, nail abrasion, and partial nail avulsion.20 A recent study found laser therapy to be a promising treatment for onychomycosis, but randomized controlled trials have yet to be done.21
NCAA and NFHS rules. Both the NCAA and the National Federation of State
High School Associations (NFHS) mandate that a wrestler with tinea corporis receive a minimum of 72 hours of topical therapy prior to participation; 14 days of systemic antifungal therapy are required for athletes with tinea capitis.22,23 The NCAA allows wrestlers to be cleared to participate on an individual basis, at the discretion of the examining physician or certified trainer.
The degree of disease involvement, the activity of disease as judged by KOH preparation, or the review of therapeutic regimen and the ability to properly cover lesions securely are taken into account.22 Proper coverage could consist of a semiocclusive or occlusive dressing such as film, foam, hydrogel, or hydrocolloid covered with stretch tape.24 Similarly, the NFHS permits a wrestler to participate once the lesion is deemed to be no longer contagious and can be covered with a bio-occlusive dressing.23
Prophylactic oral fluconazole, given in a 3-day regimen twice during the season to all team members, has been shown to be successful in reducing a high burden of tinea gladiatorum in a high school wrestling setting.25
CASE You presumptively diagnose tinea gladiatorum based on both the presentation and the patient’s history as a wrestler and prescribe topical terbinafine therapy twice daily. You schedule a follow-up appointment in 3 days, and tell Shane he must refrain from wrestling practice at least until then.
Staph and strep infections
Bacterial skin infections are also common among athletes, with S aureus reported to be responsible for 22% of infectious disease outbreaks.26 Here, too, infections occur primarily in contact sports such as football, rugby, and soccer, as well as wrestling.
There are several types of bacterial dermatoses: impetigo, folliculitis, furuncles, carbuncles, abscesses, and cellulitis. Most are caused by group A beta-hemolytic Streptococcus or S aureus and can be easily treated, but identification of the pathogen is needed to facilitate healing and a safe return to play (TABLE 1).27
When to suspect CA-MRSA
Community-acquired methicillin-resistant S aureus (CA-MRSA) was first reported in an athletic population in the 1960s, and in 1993 the first documented outbreak of CA-MRSA in a sports setting—involving 6 high school wrestlers from Vermont—was reported.28
The athlete with CA-MRSA usually presents with a painful, purulent, swollen, red abscess-like lesion, sometimes described as (or mistaken for) a spider bite. The patient may also develop fever, fatigue, and malaise. Culturing the wound for identification of the bacterium and for susceptibility to antibiotic therapy is needed for a definitive diagnosis of CA-MRSA.
Is incision and drainage sufficient treatment? The standard treatment for uncomplicated CA-MRSA lesions is incision and drainage. Several studies have found this to be adequate for simple lesions.29 Others have reported increased treatment failure with simple incision and drainage and shown that the addition of antimicrobial therapy helps decrease further tissue damage and morbidity.29
With no clear consensus as to when and whether to add oral antibiotic therapy after incision and drainage of a CA-MRSA lesion, decisions should be based on the severity of the lesion, the presence or absence of systemic symptoms, and the potential risk of bacterial spread to other team members.
Choosing an antimicrobial agent. Antimicrobial treatment should be guided by culture and sensitivity results, as well as the regional incidence of CA-MRSA. Empiric treatments for CA-MRSA are trimethoprim-sulfamethoxazole, doxycycline, and clindamycin, taken for 7 to 14 days. If you’re considering the use of clindamycin and there is known resistance to erythromycin from antimicrobial sensitivities, a double disc diffusion (D-test) should be ordered to detect inducible macrolide resistance that can occur in some strains of CA-MRSA.29,30
Fluoroquinolones and certain macrolides should not be used, due to resistance to these antibiotics.1
Topicals for superficial lesions. Topical antibiotic therapy with mupirocin or retapamulin should be reserved for superficial CA-MRSA lesions, such as impetigo.31,32 Caution must be used when mupirocin is prescribed, however, due to recent studies showing increasing resistance to mupirocin, especially when used for nasal decolonizing purposes.29
Once treatment is initiated, see the athlete every 2 to 3 days. Resolution usually occurs in 10 to 14 days. The athlete can return to play after 72 hours of treatment, however, provided there is evidence of clinical improvement, no further drainage from the infected lesion, and no new lesions have developed.1,30
Containing CA-MRSA. Mass nasal decolonization—applying topical mupirocin in the nares of infected athletes as well as their teammates—has been attempted to prevent the spread of CA-MRSA. But there is no evidence to suggest that mupirocin or any other intranasal antimicrobial is effective in preventing the spread of CA-MRSA, and nasal decolonization should not be attempted in any community setting.29 In fact, studies have found that attempts at nasal decolonization can actually lead to increased bacterial resistance and a recurrence of colonization.29,31,32
HSV-1 is highly prevalent
Herpes simplex virus-1 (HSV-1) is a common problem in athletes who play team sports that involve skin-to-skin contact. It is particularly prevalent among competitive wrestlers—earning it the name herpes gladiatorum.
In fact, HSV-1 is widespread throughout the country: Its prevalence in the general population is 58%.33 It is estimated that nearly half (47%) of cutaneous infections in collegiate athletes are caused by the herpes virus, making HSV-1 the most common pathogen of skin infections in this group.34 The clinical presentation of active HSV-1 depends on whether the infection is primary or recurrent.
So which form of HSV-1 is it?
Primary lesions may be preceded by a prodromal period with systemic symptoms, such as fever. Oral lesions and enlarged cervical and submandibular lymph nodes follow.35 The lesions, which are typically painful, can be found on the lips, buccal mucosa, or tongue—nearly anywhere in and around the oral cavity (Figure 2). They are vesicular at first, then ulcerate. Healing typically takes 10 to 14 days.36
In recurrent HSV-1, clusters of vesicles with erythematous borders typically occur. In those who play contact sports, HSV lesions can be found not only in the typical facial and oral areas, but anywhere on the head, face, torso, or extremities, as well.
Identifying herpetic whitlow. Presenting as a cluster of herpetic vesicles on the hands, fingers, or toes, herpetic whitlow is a common presentation of recurrent HSV-1. Recognition of these lesions is usually adequate for a diagnosis of recurrent infection, but confirmation can be obtained by laboratory or serologic testing.
Testing should be considered when you suspect that a patient has a primary herpes infection, as HSV-1 is a lifelong diagnosis. Viral culture is technique dependent and limited by 50% sensitivity, as cultures can take from 2 to 4 days to grow.35 The Tzanck test (direct microscopic examination of skin scrapings) has fallen out of favor, and PCR is now the gold standard.
Compared with viral culture, PCR has been shown to detect 80% of positive cases.35 However, PCR—using swab specimens taken from the lesions—is expensive and must be sent to a lab for analysis. One study suggests that while less sensitive than PCR, the Tzanck test can still be a reliably sensitive method of diagnosis when done properly, at less cost and with quicker
results.37
Serologic tests of HSV-1 IgM and IgG antibodies are also available. Serum IgG levels remain elevated in patients with previous infections. In primary infections, IgM is most useful as it can detect recent or active infection, but results may be falsely negative for several days after infection.38
Once diagnosed, management of HSV-1 in athletes is based on whether the infection is primary or recurrent. In both cases, oral antivirals are needed. Acyclovir is the gold standard, but famciclovir and valacyclovir have been proven to be equally effective.39 Both the NCAA and NFHS have published guidelines addressing the question of return to play (TABLE 2).22,23
In addition to the management of primary and recurrent infections of HSV-1, it is recommended that athletes and coaches known to be HSV-1 seropositive be treated with prophylactic suppressive therapy.40
The Centers for Disease Control and Prevention recommends acyclovir 400 mg bid or valacyclovir 500 mg/d as prophylaxis for anyone with ≤10 recurrences per year. For those with >10 recurrences annually, valacyclovir 1000 mg/d is recommended for the rest of the season.40 This has been shown to be effective in the reduction of outbreaks among wrestlers after a large outbreak occurred in 2007 in Minnesota,40,41 and NCAA and NFHS guidelines now support prophylactic therapy during competitive season.22,23 Before prescribing it, clinicians need to consider both the benefits of prophylactic antiviral therapy and the risks of promoting HSV-1 resistance.
Focus on prevention and squelching outbreaks
For cutaneous infections, as with so many medical conditions, prevention is paramount. With good preventive practices, many, if not all, of the skin infections common among athletes can be eliminated and outbreaks can be squelched.
Good hygienic practice is the cornerstone of prevention. Patients who participate in team sports should be advised to:
- shower immediately after practice
- refrain from sharing personal equipment like uniforms, towels, razors, and headgear
- launder workout clothes and towels after each use
- immediately cleanse and cover any abrasions that occur during practice.
Athletes should also be advised to ask their trainer or coach to check their skin for lesions on a regular basis. Surveillance should be instituted to prohibit athletes with cutaneous lesions from participating until they are sufficiently treated.
Although the role that environmental contamination plays in transmission of infection is uncertain, it is recommended that all sports equipment, playing surfaces, and locker rooms be disinfected daily with either a freshly made bactericidal (1/100 bleach/water solution)23,24 or an appropriate product. The Environmental Protection Agency provides a list of commercial products that have been proven to prevent the spread of MRSA on such surfaces (http://epa.gov/oppad001/chemregindex.htm) on its Web site.42
CASE When Shane returned 3 days later, the erythema had resolved, and minimal scaling remained. You tell him to continue to use the topical terbinafine twice a day for 10 days. You also show him how to apply a bio-occlusive dressing and clear him for practice, provided the lesions are fully covered.
You also talk to Shane about prevention, recommending that he immediately clean and cover any abrasion or other skin trauma that occurs during practice and suggesting that he ask his trainer to regularly check team members for skin lesions.
At Day 7, the DTM culture is positive, confirming a dermatophyte infection.
CORRESPONDENCE
Nilesh Shah, MD, 20 Olive Street, Suite 201, Akron, OH 44310;
[email protected]
ACKNOWLEDGEMENT
The authors would like to thank Tom Bartsokas, MD, for his help with this manuscript.
1. Zinder SM, Basler RS, Foley J, et al. National Athletic Trainers Association position statement: skin diseases. J Athletic Training. 2010;45:411-428.
2. Brandi G, Sisti M, Paparini A, et al. Swimming pools and fungi: an environmental epidemiology survey in Italian indoor swimming facilities. Int J Environ Health Res. 2007;17:197-206.
3. Dienst WL Jr, Dightman L, Dworkin MS. Diagnosis, treatment and pinning down skin infections: diagnosis, treatment and prevention in wrestlers. Physician Sportsmed. 2005;25:45-56.
4. Ilkit M, GÜmral R, Saraçli MA, et al. Trichophyton tonsurans scalp carriage among wrestlers in a national competition in Turkey. Mycopathologia. 2011;172:215-222.
5. Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
6. Agel J, Ransone J, Dick R, et al. Descriptive epidemiology of collegiate men’s wrestling injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Training. 2007;42:303-310.
7. Adams BB. Tinea corporis gladiatorum. J Am Acad Dermatol. 2002;47:286-297.
8. Pecci M, Comeau D, Chawla V. Skin conditions in the athlete. Am J Sport Med. 2009;37:406-418.
9. Beaven DW, Brooks SE. Color Atlas of the Nail in Clinical Diagnosis. 2nd ed. London: Mosby-Wolfe; 1994.
10. Gupta AK, Summerbell RC. Tinea capitis. Med Mycol. 2000;38:255-287.
11. Li XF, Shen YN, Chen W, et al. A new medium for diagnosis of dermatophyte infection. Eur J Dermatol. 2009;19:34-37.
12. Akcaglar S, Ener B, Toker SC, et al. A comparative study of dermatophyte infections in Bursa Turkey. Med Mycol. 2011;49:602-607.
13. Garg J, Tilak R, Garg A, et al. Rapid detection of dermatophytes from skin and hair. BMC Res Notes. 2009;18:60.
14. Reisberger EM, Abels C, Landthaler M, et al. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br J Dermatol. 2003;148:749-754.
15. Crawford F, Hollis S. Topical treatments for fungal infections of the ski and nails of the foot. Cochrane Database Syst Rev. 2007;(3):CD001434.
16. Evans EGV, Seaman RAJ, James IGV. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol. 1994;130:83-88.
17. Gupta AK, Ryder JE, Cooper EA. Naftifine: a review. J Cutan Med Surg. 2008;12:51-58.
18. Friedrich M. Inflammatory tinea pedis with bacterial superinfection effectively treated with isoconazole nitrate and diflucortolone valerate combination therapy. Mycoses. 2013;56(suppl 1):S23-S25.
19. González U, Seaton T, Bergus G, et al. Systemic antifungal therapy for tinea capitis in children. Cochrane Database Syst Rev. 2007;(4):CD004685.
20. Baran R, Hay RJ, Garduno JI. Review of antifungal therapy, part II: treatment rationale, including specific patient populations. J Dermatol Treat. 2008;19:168-175.
21. Zhang RN, Wang DK, Zhuo FL, et al. Long-pulse Nd:YAG 1064- nm laser treatment for onychomycosis. Chin Med J (Engl). 2012;125:3288-3291.
22. Guideline 2j: Skin infections in athletics. In: National Collegiate Athletic Association Sports Medicine Handbook. 22nd ed. Indianapolis, IN: National Collegiate Athletic Association; 2011:63.
23. National Federation of State High School Associations, Sports Medicine Advisory Committee. General guidelines for sports hygiene, skin infections and communicable diseases. Revised October 2012. Available at: http://www.nfhs.org/search. aspx?searchtext=skin%20infection. Accessed May 15, 2013.
24. Zinder SM, Basler RS, Foley J, et al. National Athletic Trainers’ Association position statement: skin diseases. J Athl Training. 2010;45:411-428.
25. Brickman K, Einstein E, Sinha S, et al. Fluconazole as a prophylactic measure for tinea gladiatorum in high school wrestlers. Clin J Sport Med. 2009;19:412-414.
26. Turbeville S, Cowan L, Greenfield R. Infectious disease outbreaks in competitive sports. Am J Sports Med. 2006;34:1860-1865.
27. Sedgwick P, Dexter W, Smith C. Bacterial dermatoses in sports. Clin Sports Med. 2007;26:383-396.
28. Patel A, Fischer S, Calfee R, et al. Locker room acquired methicillin-resistance Staphylococcus aureus. Orthopedics. 2007;30:532-535.
29. David M, Daum R. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616-687.
30. Benjamin H, Nikore V, Takagishi J. Practical management: community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA): the latest sports epidemic. Clin J Sport Med. 2007;17:393-397.
31. Rhin JA, Posfay-Barbe K, Harner CD, et al. Community-acquired methicillin-resistant Staphylococcus aureus outbreak in a local high school football team unsuccessful interventions. Pediatr Infect Dis J. 2005;24:841-843.
32. Loeb M, Main C, Walker-Dilks C, et al. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev. 2003;(4):CD003340.
33. Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964-973.
34. Yard EE, Collins CL, Dick RW, et al. An epidemiologic comparison of high school and college wrestling injuries. Am J Sports Med. 2008;36:57-64.
35. Usatine RP, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
36. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
37. Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:541-555.
38. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9:86-90.
39. Ozcan A, Senol M, Saglam H, et al. Comparison of the Tzanck test and polymerase chain reaction in the diagnosis of cutaneous herpes simplex and varicella zoster virus infections. Int J Dermatol. 2007;46:1177-1179.
40. Morrow R, Friedrich D. Performance of a novel test for IgM and IgG antibodies in subjects with culture-documented genital herpes simplex virus-1 or -2 infection. Clin Microbiol Infect. 2006;12:463-469.
41. Anderson BJ. Managing herpes gladiatorum outbreaks in competitive wrestling: the 2007 Minnesota experience. Curr Sports Med Rep. 2008;7:323-327.
42. Environmental Protection Agency. EPA’s registered sterilizers, tuberculocides, and antimicrobial products against certain human public health bacteria and viruses. October 2012. Available at: http://epa.gov/oppad001/chemregindex.htm. Accessed November 13, 2012.
1. Zinder SM, Basler RS, Foley J, et al. National Athletic Trainers Association position statement: skin diseases. J Athletic Training. 2010;45:411-428.
2. Brandi G, Sisti M, Paparini A, et al. Swimming pools and fungi: an environmental epidemiology survey in Italian indoor swimming facilities. Int J Environ Health Res. 2007;17:197-206.
3. Dienst WL Jr, Dightman L, Dworkin MS. Diagnosis, treatment and pinning down skin infections: diagnosis, treatment and prevention in wrestlers. Physician Sportsmed. 2005;25:45-56.
4. Ilkit M, GÜmral R, Saraçli MA, et al. Trichophyton tonsurans scalp carriage among wrestlers in a national competition in Turkey. Mycopathologia. 2011;172:215-222.
5. Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
6. Agel J, Ransone J, Dick R, et al. Descriptive epidemiology of collegiate men’s wrestling injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Training. 2007;42:303-310.
7. Adams BB. Tinea corporis gladiatorum. J Am Acad Dermatol. 2002;47:286-297.
8. Pecci M, Comeau D, Chawla V. Skin conditions in the athlete. Am J Sport Med. 2009;37:406-418.
9. Beaven DW, Brooks SE. Color Atlas of the Nail in Clinical Diagnosis. 2nd ed. London: Mosby-Wolfe; 1994.
10. Gupta AK, Summerbell RC. Tinea capitis. Med Mycol. 2000;38:255-287.
11. Li XF, Shen YN, Chen W, et al. A new medium for diagnosis of dermatophyte infection. Eur J Dermatol. 2009;19:34-37.
12. Akcaglar S, Ener B, Toker SC, et al. A comparative study of dermatophyte infections in Bursa Turkey. Med Mycol. 2011;49:602-607.
13. Garg J, Tilak R, Garg A, et al. Rapid detection of dermatophytes from skin and hair. BMC Res Notes. 2009;18:60.
14. Reisberger EM, Abels C, Landthaler M, et al. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br J Dermatol. 2003;148:749-754.
15. Crawford F, Hollis S. Topical treatments for fungal infections of the ski and nails of the foot. Cochrane Database Syst Rev. 2007;(3):CD001434.
16. Evans EGV, Seaman RAJ, James IGV. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol. 1994;130:83-88.
17. Gupta AK, Ryder JE, Cooper EA. Naftifine: a review. J Cutan Med Surg. 2008;12:51-58.
18. Friedrich M. Inflammatory tinea pedis with bacterial superinfection effectively treated with isoconazole nitrate and diflucortolone valerate combination therapy. Mycoses. 2013;56(suppl 1):S23-S25.
19. González U, Seaton T, Bergus G, et al. Systemic antifungal therapy for tinea capitis in children. Cochrane Database Syst Rev. 2007;(4):CD004685.
20. Baran R, Hay RJ, Garduno JI. Review of antifungal therapy, part II: treatment rationale, including specific patient populations. J Dermatol Treat. 2008;19:168-175.
21. Zhang RN, Wang DK, Zhuo FL, et al. Long-pulse Nd:YAG 1064- nm laser treatment for onychomycosis. Chin Med J (Engl). 2012;125:3288-3291.
22. Guideline 2j: Skin infections in athletics. In: National Collegiate Athletic Association Sports Medicine Handbook. 22nd ed. Indianapolis, IN: National Collegiate Athletic Association; 2011:63.
23. National Federation of State High School Associations, Sports Medicine Advisory Committee. General guidelines for sports hygiene, skin infections and communicable diseases. Revised October 2012. Available at: http://www.nfhs.org/search. aspx?searchtext=skin%20infection. Accessed May 15, 2013.
24. Zinder SM, Basler RS, Foley J, et al. National Athletic Trainers’ Association position statement: skin diseases. J Athl Training. 2010;45:411-428.
25. Brickman K, Einstein E, Sinha S, et al. Fluconazole as a prophylactic measure for tinea gladiatorum in high school wrestlers. Clin J Sport Med. 2009;19:412-414.
26. Turbeville S, Cowan L, Greenfield R. Infectious disease outbreaks in competitive sports. Am J Sports Med. 2006;34:1860-1865.
27. Sedgwick P, Dexter W, Smith C. Bacterial dermatoses in sports. Clin Sports Med. 2007;26:383-396.
28. Patel A, Fischer S, Calfee R, et al. Locker room acquired methicillin-resistance Staphylococcus aureus. Orthopedics. 2007;30:532-535.
29. David M, Daum R. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616-687.
30. Benjamin H, Nikore V, Takagishi J. Practical management: community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA): the latest sports epidemic. Clin J Sport Med. 2007;17:393-397.
31. Rhin JA, Posfay-Barbe K, Harner CD, et al. Community-acquired methicillin-resistant Staphylococcus aureus outbreak in a local high school football team unsuccessful interventions. Pediatr Infect Dis J. 2005;24:841-843.
32. Loeb M, Main C, Walker-Dilks C, et al. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev. 2003;(4):CD003340.
33. Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964-973.
34. Yard EE, Collins CL, Dick RW, et al. An epidemiologic comparison of high school and college wrestling injuries. Am J Sports Med. 2008;36:57-64.
35. Usatine RP, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
36. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
37. Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:541-555.
38. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9:86-90.
39. Ozcan A, Senol M, Saglam H, et al. Comparison of the Tzanck test and polymerase chain reaction in the diagnosis of cutaneous herpes simplex and varicella zoster virus infections. Int J Dermatol. 2007;46:1177-1179.
40. Morrow R, Friedrich D. Performance of a novel test for IgM and IgG antibodies in subjects with culture-documented genital herpes simplex virus-1 or -2 infection. Clin Microbiol Infect. 2006;12:463-469.
41. Anderson BJ. Managing herpes gladiatorum outbreaks in competitive wrestling: the 2007 Minnesota experience. Curr Sports Med Rep. 2008;7:323-327.
42. Environmental Protection Agency. EPA’s registered sterilizers, tuberculocides, and antimicrobial products against certain human public health bacteria and viruses. October 2012. Available at: http://epa.gov/oppad001/chemregindex.htm. Accessed November 13, 2012.
Self-management of type 2 diabetes: A good idea—or not?
• Recommend self- monitoring of blood glucose to anyone using insulin. B
• Consider self-monitoring of blood glucose in non-insulin-treated diabetes, but recognize that its effect on glycemic control is limited. B
• Consider self-management programs to promote patient involvement, but keep in mind that there is insufficient evidence to recommend for or against them. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Donna M is a 53-year-old woman with type 2 diabetes mellitus, who maintains fair glycemic control with metformin and glipizide. Her HbA1c level is 8.7%, but she has mixed feelings about initiating insulin treatment. Many of her family members also struggle with diabetes, and they frequently accompany Ms. M on her office visits. Ms. M is motivated to do whatever she can—in addition to taking her medications—to improve her diabetes. Her family asks if there is anything they can do to help. If you were Ms. M’s physician, what would you recommend?
The Centers for Disease Control and Prevention (CDC) estimates that diabetes affects 25.8 million people (or 8.3% of the population) in the United States, and that 7 million of them are undiagnosed.1 Based on the known prevalence of prediabetes, the CDC estimates that 79 million Americans ≥20 years of age are at risk for diabetes. Approximately 5.7 million people with diabetes take insulin, with or without oral medications.2
As the spotlight shines brighter on efforts to promote patient-centeredness in health care—especially with respect to chronic illness—attention to the role of self-management has also grown. And family physicians have begun to reconsider how best to engage and motivate patients to manage their illness.
In this article, we review “what else” patients can do—and perhaps need not do—based on the evidence.
What is self-management anyway?
The concept of self-management is not foreign to most family physicians, yet they and their patients probably do not share a common understanding of what it entails. The American Diabetes Association (ADA) defines diabetes self-management as “the ongoing process of facilitating the knowledge, skill, and ability necessary for diabetes self-care. Self-management should incorporate the needs, goals, and life experiences of the person with diabetes and should be guided by evidence-based standards. The overall objectives of DSME (diabetes self-management education) are to support informed decision-making, self-care behaviors, problem solving, and active collaboration with the health care team and improve clinical outcomes, health status, and quality of life.”3
Few family physicians would disagree that self-management is a good thing for patients, but many would be surprised to find that the evidence for self-management is not as convincing as one might expect. The CDC reports that 57.4% of patients with diabetes have attended a self-management class for diabetes, and 63.6% perform daily self-monitoring of blood glucose (SMBG).4 Yet, there is only indirect evidence that self-management programs are associated with modest improvements in HbA1c.5 SMBG has long been considered a mainstay of diabetes self-care, yet a growing body of evidence has shown that this practice is not universally beneficial.6 Although self-management education may reduce HbA1c levels in the short term, the long-term clinical effectiveness of SMBG has not been established.7-11
Know when to recommend SMBG
With clinical interventions, we want to give priority to those that significantly improve patient outcomes. Checking blood glucose makes good sense for insulin-treated patients to monitor for and prevent asymptomatic hypoglycemia or hyperglycemia, especially when the risk for these complications is high. In a large database study of almost 27,000 children and adolescents with type 1 diabetes, increased daily frequency of SMBG, after adjustment for multiple confounders, was significantly associated with lower HbA1c levels (–0.2% per additional test per day, leveling off at 5 tests per day) and fewer acute complications.12
Although it has been suggested that more frequent SMBG improves long-term glycemic control among patients with insulin-treated type 1 and type 2 diabetes, the benefits are modest.13 The ADA recommends SMBG 3 or more times daily for patients using multiple insulin injections or insulin pump therapy.14
In patients with type 2 diabetes who are not taking insulin, the benefits of SMBG are less clear. A meta-analysis of SMBG in non-insulin-treated patients with type 2 diabetes showed that it was associated with a reduction of HbA1c of –0.4%.15 A Cochrane review added that SMBG leads to small but significant decreases after 6 months, but that these improvements are not sustained at 12 months. The same review noted no improvements in patient satisfaction or general health-related quality of life resulting from SMBG.6 But many of the studies in this analysis included other interventions, making it difficult to isolate the impact of SMBG on glycemic control. Other studies show that SMBG does not improve glycemic control at all.16
For patients using less-frequent insulin injections, non-insulin therapies, or medical nutrition therapy alone, the ADA suggests that SMBG may be useful as a guide to management. Continuous glucose monitoring for patients with type 2 diabetes might improve glycemic control, but the evidence for this is inconsistent.17
Why wouldn’t you want to recommend self-monitoring? Despite the fact that the benefits of SMBG are unclear in patients with type 2 diabetes not treated with insulin, it’s hard to imagine why this practice could be harmful to patients. After all, it’s natural to assume that more knowledge must be a good thing. Unfortunately, it is not that simple. Even in newly diagnosed patients with type 2 diabetes not taking insulin, self-monitoring does not improve glycemic control and may increase depression.16
It is also important to remember that self-monitoring comes at considerable cost, monetarily for the health care system and in impaired quality of life for patients.18 While there is scant evidence in the empiric literature about patient attitudes toward self-monitoring, the available evidence suggests that patients are ambivalent about it. One qualitative study concluded that patients tended not to act on the results of self-monitoring, in part because of a lack of education about the appropriate response to readings.19 With better knowledge, it is possible that patients might find more value in SMBG.
Self-management programs: Not all are created equal
The driving principle in patient-centered care is engaging patients to be active participants in the management of their chronic conditions. At face value, this would seem to be a good thing. But although individual trials of self-management are promising, the balance of evidence for self-management is limited and inconclusive. In a systematic review of 72 randomized trials of DSME in patients ≥18 years with type 2 diabetes, short-term improvements in diabetes knowledge, frequency and accuracy of glucose self-monitoring, self-reported dietary habits, and better glycemic control were possible, but long-term clinical effectiveness was not shown. In this analysis, there was no significant effect on cardiovascular events or mortality.20 In another systematic review and critique of the literature on self-management, investigators again found small to moderate effects, but with significant evidence of publication bias in the included trials.21
The uncertainty about self-management exists because not all self-management interventions have equal impact on patient outcomes. Motivational interviewing, collaborative problem solving, and negotiating individualized goals for each patient, for example, may have longer-standing benefit than those focused on education alone.22
A 2009 meta-analysis of DSME and its efficacy differentiated teaching, behavioral, psychological, and “mixed” or combination approaches. Most of the interventions were behaviorally oriented, sometimes combined with one other format. Psychological interventions targeting negative or self-defeating moods and social and emotional coping skills yielded moderate effects on metabolic control and self-care behaviors.23
Clinic-based self-management. One randomized prospective study compared intensive clinic-based education on complications of diabetes with standard care. After 4 years, patients exhibited significant reductions in HbA1c, blood pressure, and low-density lipoprotein cholesterol levels.24
A large meta-analysis examining a range of self-management programs for multiple chronic conditions showed a statistically and clinically significant improvement in glycemic control equivalent to a 0.81% reduction in HbA1c. Features of self-management addressed in this meta-analysis included various forms of nurse- and provider-driven education about medications, diet and exercise, motivational interviewing, and biofeedback.25
Nurse-led DSME has been associated with improvements in HbA1c and cardiovascular risk factors.26 Dietician-led DSME has been associated improvements in HbA1c when compared with routine care.26
Cognitive behavioral therapy. Overall, the most frequently reported and most widely used psychosocial intervention is cognitive behavioral therapy (CBT); it is often short term and skills based, targeting unhelpful negative thinking and increasing positive behavior, including problem solving and relaxation, which have been shown to be effective in treating depression.27 An older randomized control trial (RCT) specifically focused on type 2 diabetes explored the impact of CBT on both diabetes and depression among patients with diabetes and comorbid major depressive disorder (MDD). Improvements in depression seen at the end of the intervention were still evident 6 months later. And while there was no difference in HbA1c levels immediately following the intervention, after 6 months the mean HbA1c level was significantly better in the CBT group than in the control group (9.5% vs 10.9%; P=.03). There was no statistically significant difference in SMBG between the groups.28
Group-based vs individual training. The evidence comparing group-based and individual self-management support is inconsistent. In one RCT focused on personalized action-oriented goals for healthy eating, SMBG, taking medications, problem solving, risk reduction, healthy coping, and physical activity, individual education led to reductions in HbA1c levels (–0.51%) after 6 months that were not observed in the group-based education and usual care groups.10 On the other hand, a Cochrane review of trials comparing group-based and individual routine care suggested greater benefits overall in group-based approaches, but with the caveat that many of the included trials had methodological limitations.29
Mobile phone and online interventions? Stay tuned
The jury is still out on interventions like peer advising and telephone, telemedicine, and online support. In a systematic review of 22 trials evaluating mobile phone interventions for self-management (eg, text messaging, phone reminders, and coaching interventions), investigators observed a 0.5% decrease in HbA1c levels over a median followup period of 6 months.30 Various telephone interventions have shown modest and short-term improvements in HbA1c levels, but none of these interventions has improved clinical outcomes.31-33 Combinations of telephone and online self-management are beginning to show promise, but so far the evidence shows only short-term benefit, and clinical outcomes have not been studied.34,35
CASE Based on the available evidence, a number of ways to support Ms. M’s efforts at self-management would be justified. Eliciting her perspective on the options would be well worth the effort. She is not taking insulin, so we would not recommend daily SMBG, but we’d support her if she expressed a strong preference for self-monitoring. Once insulin treatment enters the picture, however, we would strongly recommend daily SMBG to promote patient engagement and safety. And although there is limited evidence to support referral to self-management programs, if a particular program fit Ms. M’s lifestyle, we would refer her nonetheless.
CORRESPONDENCE
Michael Mendoza, MD, MPH, Highland Family Medicine, 777 S. Clinton Avenue, Rochester, NY 14620; [email protected]
1. CDC. National diabetes fact sheet, 2011. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed July 20, 2012.
2. CDC. Diabetes data and trends. Available at: http://www.cdc.gov/diabetes/statistics/meduse/fig1.htm. Accessed November 20, 2012.
3. ADA. National standards for diabetes self-management education. Available at: http://care.diabetesjournals.org/content/31/Supplement_1/S97.extract. Accessed July 20, 2012.
4. CDC. Diabetes report card, 2012: national and state profile of diabetes and its complications. Available at: http://www.cdc.gov/diabetes/pubs/reportcard.htm. Accessed November 20, 2012.
5. Chodosh J, Morton SC, Mojica W, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143:427-438.
6. Malanda UL, Welschen LM, Riphagen II, et al. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012;(1):CD005060.-
7. Tshiananga JK, Kocher S, Weber C, et al. The effect of nurse-led diabetes self-management education on glycosylated hemoglobin and cardiovascular risk factors: a meta-analysis. Diabetes Educ. 2012;38:108-123.
8. Warsi A, Wang PS, LaValley MP, et al. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164:1641-1649.
9. Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561-587.
10. Sperl-Hillen J, Beaton S, Fernandes O, et al. Comparative effectiveness of patient education methods for type 2 diabetes: a randomized controlled trial. Arch Intern Med. 2011;171:2001-2010.
11. Rosal MC, Ockene IS, Restrepo A, et al. Randomized trial of a literacy-sensitive, culturally tailored diabetes self-management intervention for low-income latinos: latinos en control. Diabetes Care. 2011;34:838-844.
12. Ziegler R, Heidtmann B, Hilgard D, et al. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12:11-17.
13. Schütt M, Kern W, Krause U, et al. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114:384-388.
14. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
15. Welschen LM, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care. 2005;28:1510-1517.
16. O’Kane MJ, Bunting B, Copeland M, et al. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336:1174-1177.
17. Meade LT. The use of continuous glucose monitoring in patients with type 2 diabetes. Diabetes Technol Ther. 2012;14:190-195.
18. Simon J, Gray A, Clarke P, et al. Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ. 2008;336:1177-1180.
19. Peel E, Douglas M, Lawton J. Self-monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493-498.
20. Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561-587.
21. Warsi A, Wang PS, LaValley MP, et al. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164:1641-1649.
22. Clark M, Hampson SE. Implementing a psychological intervention to improve lifestyle self-management in patients with type 2 diabetes. Patient Educ Couns. 2001;42:247-256.
23. Fan L, Sidani S. Effectiveness of diabetes self-management education intervention elements: a meta-analysis. Can J Diabetes. 2009;33:18-26.
24. Rachmani R, Levi Z, Slavachevski I, et al. Teaching patients to monitor their risk factors retards the progression of vascular complications in high-risk patients with type 2 diabetes mellitus—a randomized prospective study. Diabet Med. 2002;19:385-392.
25. Chodosh J, Morton SC, Mojica W, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143:427-438.
26. Tshiananga JK, Kocher S, Weber C, et al. The effect of nurse-led diabetes self-management education on glycosylated hemoglobin and cardiovascular risk factors: a meta-analysis. Diabetes Educ. 2012;38:108-123.
27. Cuijpers P, van Straten A, Andersson G, et al. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol. 2008;76:909-922.
28. Lustman PJ, Griffith LS, Freedland KE, et al. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;129:613-621.
29. Deakin T, McShane CE, Cade JE, et al. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2):CD003417.-
30. Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabet Med. 2011;28:455-463.
31. Walker EA, Shmukler C, Ullman R, et al. Results of a successful telephonic intervention to improve diabetes control in urban adults: a randomized trial. Diabetes Care. 2011;34:2-7.
32. Wu L, Forbes A, Griffiths P, et al. Telephone follow-up to improve glycaemic control in patients with type 2 diabetes: systematic review and meta-analysis of controlled trials. Diabet Med. 2010;27:1217-1225.
33. Amoako E, Skelly AH, Rossen EK. Outcomes of an intervention to reduce uncertainty among African American women with diabetes. West J Nurs Res. 2008;30:928-942.
34. Quinn CC, Shardell MD, Terrin ML, et al. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34:1934-1942.
35. Yoo HJ, Park MS, Kim TN, et al. A ubiquitous chronic disease care system using cellular phones and the internet. Diabet Med. 2009;26:628-635.
• Recommend self- monitoring of blood glucose to anyone using insulin. B
• Consider self-monitoring of blood glucose in non-insulin-treated diabetes, but recognize that its effect on glycemic control is limited. B
• Consider self-management programs to promote patient involvement, but keep in mind that there is insufficient evidence to recommend for or against them. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Donna M is a 53-year-old woman with type 2 diabetes mellitus, who maintains fair glycemic control with metformin and glipizide. Her HbA1c level is 8.7%, but she has mixed feelings about initiating insulin treatment. Many of her family members also struggle with diabetes, and they frequently accompany Ms. M on her office visits. Ms. M is motivated to do whatever she can—in addition to taking her medications—to improve her diabetes. Her family asks if there is anything they can do to help. If you were Ms. M’s physician, what would you recommend?
The Centers for Disease Control and Prevention (CDC) estimates that diabetes affects 25.8 million people (or 8.3% of the population) in the United States, and that 7 million of them are undiagnosed.1 Based on the known prevalence of prediabetes, the CDC estimates that 79 million Americans ≥20 years of age are at risk for diabetes. Approximately 5.7 million people with diabetes take insulin, with or without oral medications.2
As the spotlight shines brighter on efforts to promote patient-centeredness in health care—especially with respect to chronic illness—attention to the role of self-management has also grown. And family physicians have begun to reconsider how best to engage and motivate patients to manage their illness.
In this article, we review “what else” patients can do—and perhaps need not do—based on the evidence.
What is self-management anyway?
The concept of self-management is not foreign to most family physicians, yet they and their patients probably do not share a common understanding of what it entails. The American Diabetes Association (ADA) defines diabetes self-management as “the ongoing process of facilitating the knowledge, skill, and ability necessary for diabetes self-care. Self-management should incorporate the needs, goals, and life experiences of the person with diabetes and should be guided by evidence-based standards. The overall objectives of DSME (diabetes self-management education) are to support informed decision-making, self-care behaviors, problem solving, and active collaboration with the health care team and improve clinical outcomes, health status, and quality of life.”3
Few family physicians would disagree that self-management is a good thing for patients, but many would be surprised to find that the evidence for self-management is not as convincing as one might expect. The CDC reports that 57.4% of patients with diabetes have attended a self-management class for diabetes, and 63.6% perform daily self-monitoring of blood glucose (SMBG).4 Yet, there is only indirect evidence that self-management programs are associated with modest improvements in HbA1c.5 SMBG has long been considered a mainstay of diabetes self-care, yet a growing body of evidence has shown that this practice is not universally beneficial.6 Although self-management education may reduce HbA1c levels in the short term, the long-term clinical effectiveness of SMBG has not been established.7-11
Know when to recommend SMBG
With clinical interventions, we want to give priority to those that significantly improve patient outcomes. Checking blood glucose makes good sense for insulin-treated patients to monitor for and prevent asymptomatic hypoglycemia or hyperglycemia, especially when the risk for these complications is high. In a large database study of almost 27,000 children and adolescents with type 1 diabetes, increased daily frequency of SMBG, after adjustment for multiple confounders, was significantly associated with lower HbA1c levels (–0.2% per additional test per day, leveling off at 5 tests per day) and fewer acute complications.12
Although it has been suggested that more frequent SMBG improves long-term glycemic control among patients with insulin-treated type 1 and type 2 diabetes, the benefits are modest.13 The ADA recommends SMBG 3 or more times daily for patients using multiple insulin injections or insulin pump therapy.14
In patients with type 2 diabetes who are not taking insulin, the benefits of SMBG are less clear. A meta-analysis of SMBG in non-insulin-treated patients with type 2 diabetes showed that it was associated with a reduction of HbA1c of –0.4%.15 A Cochrane review added that SMBG leads to small but significant decreases after 6 months, but that these improvements are not sustained at 12 months. The same review noted no improvements in patient satisfaction or general health-related quality of life resulting from SMBG.6 But many of the studies in this analysis included other interventions, making it difficult to isolate the impact of SMBG on glycemic control. Other studies show that SMBG does not improve glycemic control at all.16
For patients using less-frequent insulin injections, non-insulin therapies, or medical nutrition therapy alone, the ADA suggests that SMBG may be useful as a guide to management. Continuous glucose monitoring for patients with type 2 diabetes might improve glycemic control, but the evidence for this is inconsistent.17
Why wouldn’t you want to recommend self-monitoring? Despite the fact that the benefits of SMBG are unclear in patients with type 2 diabetes not treated with insulin, it’s hard to imagine why this practice could be harmful to patients. After all, it’s natural to assume that more knowledge must be a good thing. Unfortunately, it is not that simple. Even in newly diagnosed patients with type 2 diabetes not taking insulin, self-monitoring does not improve glycemic control and may increase depression.16
It is also important to remember that self-monitoring comes at considerable cost, monetarily for the health care system and in impaired quality of life for patients.18 While there is scant evidence in the empiric literature about patient attitudes toward self-monitoring, the available evidence suggests that patients are ambivalent about it. One qualitative study concluded that patients tended not to act on the results of self-monitoring, in part because of a lack of education about the appropriate response to readings.19 With better knowledge, it is possible that patients might find more value in SMBG.
Self-management programs: Not all are created equal
The driving principle in patient-centered care is engaging patients to be active participants in the management of their chronic conditions. At face value, this would seem to be a good thing. But although individual trials of self-management are promising, the balance of evidence for self-management is limited and inconclusive. In a systematic review of 72 randomized trials of DSME in patients ≥18 years with type 2 diabetes, short-term improvements in diabetes knowledge, frequency and accuracy of glucose self-monitoring, self-reported dietary habits, and better glycemic control were possible, but long-term clinical effectiveness was not shown. In this analysis, there was no significant effect on cardiovascular events or mortality.20 In another systematic review and critique of the literature on self-management, investigators again found small to moderate effects, but with significant evidence of publication bias in the included trials.21
The uncertainty about self-management exists because not all self-management interventions have equal impact on patient outcomes. Motivational interviewing, collaborative problem solving, and negotiating individualized goals for each patient, for example, may have longer-standing benefit than those focused on education alone.22
A 2009 meta-analysis of DSME and its efficacy differentiated teaching, behavioral, psychological, and “mixed” or combination approaches. Most of the interventions were behaviorally oriented, sometimes combined with one other format. Psychological interventions targeting negative or self-defeating moods and social and emotional coping skills yielded moderate effects on metabolic control and self-care behaviors.23
Clinic-based self-management. One randomized prospective study compared intensive clinic-based education on complications of diabetes with standard care. After 4 years, patients exhibited significant reductions in HbA1c, blood pressure, and low-density lipoprotein cholesterol levels.24
A large meta-analysis examining a range of self-management programs for multiple chronic conditions showed a statistically and clinically significant improvement in glycemic control equivalent to a 0.81% reduction in HbA1c. Features of self-management addressed in this meta-analysis included various forms of nurse- and provider-driven education about medications, diet and exercise, motivational interviewing, and biofeedback.25
Nurse-led DSME has been associated with improvements in HbA1c and cardiovascular risk factors.26 Dietician-led DSME has been associated improvements in HbA1c when compared with routine care.26
Cognitive behavioral therapy. Overall, the most frequently reported and most widely used psychosocial intervention is cognitive behavioral therapy (CBT); it is often short term and skills based, targeting unhelpful negative thinking and increasing positive behavior, including problem solving and relaxation, which have been shown to be effective in treating depression.27 An older randomized control trial (RCT) specifically focused on type 2 diabetes explored the impact of CBT on both diabetes and depression among patients with diabetes and comorbid major depressive disorder (MDD). Improvements in depression seen at the end of the intervention were still evident 6 months later. And while there was no difference in HbA1c levels immediately following the intervention, after 6 months the mean HbA1c level was significantly better in the CBT group than in the control group (9.5% vs 10.9%; P=.03). There was no statistically significant difference in SMBG between the groups.28
Group-based vs individual training. The evidence comparing group-based and individual self-management support is inconsistent. In one RCT focused on personalized action-oriented goals for healthy eating, SMBG, taking medications, problem solving, risk reduction, healthy coping, and physical activity, individual education led to reductions in HbA1c levels (–0.51%) after 6 months that were not observed in the group-based education and usual care groups.10 On the other hand, a Cochrane review of trials comparing group-based and individual routine care suggested greater benefits overall in group-based approaches, but with the caveat that many of the included trials had methodological limitations.29
Mobile phone and online interventions? Stay tuned
The jury is still out on interventions like peer advising and telephone, telemedicine, and online support. In a systematic review of 22 trials evaluating mobile phone interventions for self-management (eg, text messaging, phone reminders, and coaching interventions), investigators observed a 0.5% decrease in HbA1c levels over a median followup period of 6 months.30 Various telephone interventions have shown modest and short-term improvements in HbA1c levels, but none of these interventions has improved clinical outcomes.31-33 Combinations of telephone and online self-management are beginning to show promise, but so far the evidence shows only short-term benefit, and clinical outcomes have not been studied.34,35
CASE Based on the available evidence, a number of ways to support Ms. M’s efforts at self-management would be justified. Eliciting her perspective on the options would be well worth the effort. She is not taking insulin, so we would not recommend daily SMBG, but we’d support her if she expressed a strong preference for self-monitoring. Once insulin treatment enters the picture, however, we would strongly recommend daily SMBG to promote patient engagement and safety. And although there is limited evidence to support referral to self-management programs, if a particular program fit Ms. M’s lifestyle, we would refer her nonetheless.
CORRESPONDENCE
Michael Mendoza, MD, MPH, Highland Family Medicine, 777 S. Clinton Avenue, Rochester, NY 14620; [email protected]
• Recommend self- monitoring of blood glucose to anyone using insulin. B
• Consider self-monitoring of blood glucose in non-insulin-treated diabetes, but recognize that its effect on glycemic control is limited. B
• Consider self-management programs to promote patient involvement, but keep in mind that there is insufficient evidence to recommend for or against them. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Donna M is a 53-year-old woman with type 2 diabetes mellitus, who maintains fair glycemic control with metformin and glipizide. Her HbA1c level is 8.7%, but she has mixed feelings about initiating insulin treatment. Many of her family members also struggle with diabetes, and they frequently accompany Ms. M on her office visits. Ms. M is motivated to do whatever she can—in addition to taking her medications—to improve her diabetes. Her family asks if there is anything they can do to help. If you were Ms. M’s physician, what would you recommend?
The Centers for Disease Control and Prevention (CDC) estimates that diabetes affects 25.8 million people (or 8.3% of the population) in the United States, and that 7 million of them are undiagnosed.1 Based on the known prevalence of prediabetes, the CDC estimates that 79 million Americans ≥20 years of age are at risk for diabetes. Approximately 5.7 million people with diabetes take insulin, with or without oral medications.2
As the spotlight shines brighter on efforts to promote patient-centeredness in health care—especially with respect to chronic illness—attention to the role of self-management has also grown. And family physicians have begun to reconsider how best to engage and motivate patients to manage their illness.
In this article, we review “what else” patients can do—and perhaps need not do—based on the evidence.
What is self-management anyway?
The concept of self-management is not foreign to most family physicians, yet they and their patients probably do not share a common understanding of what it entails. The American Diabetes Association (ADA) defines diabetes self-management as “the ongoing process of facilitating the knowledge, skill, and ability necessary for diabetes self-care. Self-management should incorporate the needs, goals, and life experiences of the person with diabetes and should be guided by evidence-based standards. The overall objectives of DSME (diabetes self-management education) are to support informed decision-making, self-care behaviors, problem solving, and active collaboration with the health care team and improve clinical outcomes, health status, and quality of life.”3
Few family physicians would disagree that self-management is a good thing for patients, but many would be surprised to find that the evidence for self-management is not as convincing as one might expect. The CDC reports that 57.4% of patients with diabetes have attended a self-management class for diabetes, and 63.6% perform daily self-monitoring of blood glucose (SMBG).4 Yet, there is only indirect evidence that self-management programs are associated with modest improvements in HbA1c.5 SMBG has long been considered a mainstay of diabetes self-care, yet a growing body of evidence has shown that this practice is not universally beneficial.6 Although self-management education may reduce HbA1c levels in the short term, the long-term clinical effectiveness of SMBG has not been established.7-11
Know when to recommend SMBG
With clinical interventions, we want to give priority to those that significantly improve patient outcomes. Checking blood glucose makes good sense for insulin-treated patients to monitor for and prevent asymptomatic hypoglycemia or hyperglycemia, especially when the risk for these complications is high. In a large database study of almost 27,000 children and adolescents with type 1 diabetes, increased daily frequency of SMBG, after adjustment for multiple confounders, was significantly associated with lower HbA1c levels (–0.2% per additional test per day, leveling off at 5 tests per day) and fewer acute complications.12
Although it has been suggested that more frequent SMBG improves long-term glycemic control among patients with insulin-treated type 1 and type 2 diabetes, the benefits are modest.13 The ADA recommends SMBG 3 or more times daily for patients using multiple insulin injections or insulin pump therapy.14
In patients with type 2 diabetes who are not taking insulin, the benefits of SMBG are less clear. A meta-analysis of SMBG in non-insulin-treated patients with type 2 diabetes showed that it was associated with a reduction of HbA1c of –0.4%.15 A Cochrane review added that SMBG leads to small but significant decreases after 6 months, but that these improvements are not sustained at 12 months. The same review noted no improvements in patient satisfaction or general health-related quality of life resulting from SMBG.6 But many of the studies in this analysis included other interventions, making it difficult to isolate the impact of SMBG on glycemic control. Other studies show that SMBG does not improve glycemic control at all.16
For patients using less-frequent insulin injections, non-insulin therapies, or medical nutrition therapy alone, the ADA suggests that SMBG may be useful as a guide to management. Continuous glucose monitoring for patients with type 2 diabetes might improve glycemic control, but the evidence for this is inconsistent.17
Why wouldn’t you want to recommend self-monitoring? Despite the fact that the benefits of SMBG are unclear in patients with type 2 diabetes not treated with insulin, it’s hard to imagine why this practice could be harmful to patients. After all, it’s natural to assume that more knowledge must be a good thing. Unfortunately, it is not that simple. Even in newly diagnosed patients with type 2 diabetes not taking insulin, self-monitoring does not improve glycemic control and may increase depression.16
It is also important to remember that self-monitoring comes at considerable cost, monetarily for the health care system and in impaired quality of life for patients.18 While there is scant evidence in the empiric literature about patient attitudes toward self-monitoring, the available evidence suggests that patients are ambivalent about it. One qualitative study concluded that patients tended not to act on the results of self-monitoring, in part because of a lack of education about the appropriate response to readings.19 With better knowledge, it is possible that patients might find more value in SMBG.
Self-management programs: Not all are created equal
The driving principle in patient-centered care is engaging patients to be active participants in the management of their chronic conditions. At face value, this would seem to be a good thing. But although individual trials of self-management are promising, the balance of evidence for self-management is limited and inconclusive. In a systematic review of 72 randomized trials of DSME in patients ≥18 years with type 2 diabetes, short-term improvements in diabetes knowledge, frequency and accuracy of glucose self-monitoring, self-reported dietary habits, and better glycemic control were possible, but long-term clinical effectiveness was not shown. In this analysis, there was no significant effect on cardiovascular events or mortality.20 In another systematic review and critique of the literature on self-management, investigators again found small to moderate effects, but with significant evidence of publication bias in the included trials.21
The uncertainty about self-management exists because not all self-management interventions have equal impact on patient outcomes. Motivational interviewing, collaborative problem solving, and negotiating individualized goals for each patient, for example, may have longer-standing benefit than those focused on education alone.22
A 2009 meta-analysis of DSME and its efficacy differentiated teaching, behavioral, psychological, and “mixed” or combination approaches. Most of the interventions were behaviorally oriented, sometimes combined with one other format. Psychological interventions targeting negative or self-defeating moods and social and emotional coping skills yielded moderate effects on metabolic control and self-care behaviors.23
Clinic-based self-management. One randomized prospective study compared intensive clinic-based education on complications of diabetes with standard care. After 4 years, patients exhibited significant reductions in HbA1c, blood pressure, and low-density lipoprotein cholesterol levels.24
A large meta-analysis examining a range of self-management programs for multiple chronic conditions showed a statistically and clinically significant improvement in glycemic control equivalent to a 0.81% reduction in HbA1c. Features of self-management addressed in this meta-analysis included various forms of nurse- and provider-driven education about medications, diet and exercise, motivational interviewing, and biofeedback.25
Nurse-led DSME has been associated with improvements in HbA1c and cardiovascular risk factors.26 Dietician-led DSME has been associated improvements in HbA1c when compared with routine care.26
Cognitive behavioral therapy. Overall, the most frequently reported and most widely used psychosocial intervention is cognitive behavioral therapy (CBT); it is often short term and skills based, targeting unhelpful negative thinking and increasing positive behavior, including problem solving and relaxation, which have been shown to be effective in treating depression.27 An older randomized control trial (RCT) specifically focused on type 2 diabetes explored the impact of CBT on both diabetes and depression among patients with diabetes and comorbid major depressive disorder (MDD). Improvements in depression seen at the end of the intervention were still evident 6 months later. And while there was no difference in HbA1c levels immediately following the intervention, after 6 months the mean HbA1c level was significantly better in the CBT group than in the control group (9.5% vs 10.9%; P=.03). There was no statistically significant difference in SMBG between the groups.28
Group-based vs individual training. The evidence comparing group-based and individual self-management support is inconsistent. In one RCT focused on personalized action-oriented goals for healthy eating, SMBG, taking medications, problem solving, risk reduction, healthy coping, and physical activity, individual education led to reductions in HbA1c levels (–0.51%) after 6 months that were not observed in the group-based education and usual care groups.10 On the other hand, a Cochrane review of trials comparing group-based and individual routine care suggested greater benefits overall in group-based approaches, but with the caveat that many of the included trials had methodological limitations.29
Mobile phone and online interventions? Stay tuned
The jury is still out on interventions like peer advising and telephone, telemedicine, and online support. In a systematic review of 22 trials evaluating mobile phone interventions for self-management (eg, text messaging, phone reminders, and coaching interventions), investigators observed a 0.5% decrease in HbA1c levels over a median followup period of 6 months.30 Various telephone interventions have shown modest and short-term improvements in HbA1c levels, but none of these interventions has improved clinical outcomes.31-33 Combinations of telephone and online self-management are beginning to show promise, but so far the evidence shows only short-term benefit, and clinical outcomes have not been studied.34,35
CASE Based on the available evidence, a number of ways to support Ms. M’s efforts at self-management would be justified. Eliciting her perspective on the options would be well worth the effort. She is not taking insulin, so we would not recommend daily SMBG, but we’d support her if she expressed a strong preference for self-monitoring. Once insulin treatment enters the picture, however, we would strongly recommend daily SMBG to promote patient engagement and safety. And although there is limited evidence to support referral to self-management programs, if a particular program fit Ms. M’s lifestyle, we would refer her nonetheless.
CORRESPONDENCE
Michael Mendoza, MD, MPH, Highland Family Medicine, 777 S. Clinton Avenue, Rochester, NY 14620; [email protected]
1. CDC. National diabetes fact sheet, 2011. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed July 20, 2012.
2. CDC. Diabetes data and trends. Available at: http://www.cdc.gov/diabetes/statistics/meduse/fig1.htm. Accessed November 20, 2012.
3. ADA. National standards for diabetes self-management education. Available at: http://care.diabetesjournals.org/content/31/Supplement_1/S97.extract. Accessed July 20, 2012.
4. CDC. Diabetes report card, 2012: national and state profile of diabetes and its complications. Available at: http://www.cdc.gov/diabetes/pubs/reportcard.htm. Accessed November 20, 2012.
5. Chodosh J, Morton SC, Mojica W, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143:427-438.
6. Malanda UL, Welschen LM, Riphagen II, et al. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012;(1):CD005060.-
7. Tshiananga JK, Kocher S, Weber C, et al. The effect of nurse-led diabetes self-management education on glycosylated hemoglobin and cardiovascular risk factors: a meta-analysis. Diabetes Educ. 2012;38:108-123.
8. Warsi A, Wang PS, LaValley MP, et al. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164:1641-1649.
9. Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561-587.
10. Sperl-Hillen J, Beaton S, Fernandes O, et al. Comparative effectiveness of patient education methods for type 2 diabetes: a randomized controlled trial. Arch Intern Med. 2011;171:2001-2010.
11. Rosal MC, Ockene IS, Restrepo A, et al. Randomized trial of a literacy-sensitive, culturally tailored diabetes self-management intervention for low-income latinos: latinos en control. Diabetes Care. 2011;34:838-844.
12. Ziegler R, Heidtmann B, Hilgard D, et al. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12:11-17.
13. Schütt M, Kern W, Krause U, et al. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114:384-388.
14. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
15. Welschen LM, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care. 2005;28:1510-1517.
16. O’Kane MJ, Bunting B, Copeland M, et al. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336:1174-1177.
17. Meade LT. The use of continuous glucose monitoring in patients with type 2 diabetes. Diabetes Technol Ther. 2012;14:190-195.
18. Simon J, Gray A, Clarke P, et al. Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ. 2008;336:1177-1180.
19. Peel E, Douglas M, Lawton J. Self-monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493-498.
20. Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561-587.
21. Warsi A, Wang PS, LaValley MP, et al. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164:1641-1649.
22. Clark M, Hampson SE. Implementing a psychological intervention to improve lifestyle self-management in patients with type 2 diabetes. Patient Educ Couns. 2001;42:247-256.
23. Fan L, Sidani S. Effectiveness of diabetes self-management education intervention elements: a meta-analysis. Can J Diabetes. 2009;33:18-26.
24. Rachmani R, Levi Z, Slavachevski I, et al. Teaching patients to monitor their risk factors retards the progression of vascular complications in high-risk patients with type 2 diabetes mellitus—a randomized prospective study. Diabet Med. 2002;19:385-392.
25. Chodosh J, Morton SC, Mojica W, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143:427-438.
26. Tshiananga JK, Kocher S, Weber C, et al. The effect of nurse-led diabetes self-management education on glycosylated hemoglobin and cardiovascular risk factors: a meta-analysis. Diabetes Educ. 2012;38:108-123.
27. Cuijpers P, van Straten A, Andersson G, et al. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol. 2008;76:909-922.
28. Lustman PJ, Griffith LS, Freedland KE, et al. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;129:613-621.
29. Deakin T, McShane CE, Cade JE, et al. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2):CD003417.-
30. Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabet Med. 2011;28:455-463.
31. Walker EA, Shmukler C, Ullman R, et al. Results of a successful telephonic intervention to improve diabetes control in urban adults: a randomized trial. Diabetes Care. 2011;34:2-7.
32. Wu L, Forbes A, Griffiths P, et al. Telephone follow-up to improve glycaemic control in patients with type 2 diabetes: systematic review and meta-analysis of controlled trials. Diabet Med. 2010;27:1217-1225.
33. Amoako E, Skelly AH, Rossen EK. Outcomes of an intervention to reduce uncertainty among African American women with diabetes. West J Nurs Res. 2008;30:928-942.
34. Quinn CC, Shardell MD, Terrin ML, et al. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34:1934-1942.
35. Yoo HJ, Park MS, Kim TN, et al. A ubiquitous chronic disease care system using cellular phones and the internet. Diabet Med. 2009;26:628-635.
1. CDC. National diabetes fact sheet, 2011. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed July 20, 2012.
2. CDC. Diabetes data and trends. Available at: http://www.cdc.gov/diabetes/statistics/meduse/fig1.htm. Accessed November 20, 2012.
3. ADA. National standards for diabetes self-management education. Available at: http://care.diabetesjournals.org/content/31/Supplement_1/S97.extract. Accessed July 20, 2012.
4. CDC. Diabetes report card, 2012: national and state profile of diabetes and its complications. Available at: http://www.cdc.gov/diabetes/pubs/reportcard.htm. Accessed November 20, 2012.
5. Chodosh J, Morton SC, Mojica W, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143:427-438.
6. Malanda UL, Welschen LM, Riphagen II, et al. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012;(1):CD005060.-
7. Tshiananga JK, Kocher S, Weber C, et al. The effect of nurse-led diabetes self-management education on glycosylated hemoglobin and cardiovascular risk factors: a meta-analysis. Diabetes Educ. 2012;38:108-123.
8. Warsi A, Wang PS, LaValley MP, et al. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164:1641-1649.
9. Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561-587.
10. Sperl-Hillen J, Beaton S, Fernandes O, et al. Comparative effectiveness of patient education methods for type 2 diabetes: a randomized controlled trial. Arch Intern Med. 2011;171:2001-2010.
11. Rosal MC, Ockene IS, Restrepo A, et al. Randomized trial of a literacy-sensitive, culturally tailored diabetes self-management intervention for low-income latinos: latinos en control. Diabetes Care. 2011;34:838-844.
12. Ziegler R, Heidtmann B, Hilgard D, et al. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12:11-17.
13. Schütt M, Kern W, Krause U, et al. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114:384-388.
14. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
15. Welschen LM, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care. 2005;28:1510-1517.
16. O’Kane MJ, Bunting B, Copeland M, et al. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336:1174-1177.
17. Meade LT. The use of continuous glucose monitoring in patients with type 2 diabetes. Diabetes Technol Ther. 2012;14:190-195.
18. Simon J, Gray A, Clarke P, et al. Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ. 2008;336:1177-1180.
19. Peel E, Douglas M, Lawton J. Self-monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493-498.
20. Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561-587.
21. Warsi A, Wang PS, LaValley MP, et al. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164:1641-1649.
22. Clark M, Hampson SE. Implementing a psychological intervention to improve lifestyle self-management in patients with type 2 diabetes. Patient Educ Couns. 2001;42:247-256.
23. Fan L, Sidani S. Effectiveness of diabetes self-management education intervention elements: a meta-analysis. Can J Diabetes. 2009;33:18-26.
24. Rachmani R, Levi Z, Slavachevski I, et al. Teaching patients to monitor their risk factors retards the progression of vascular complications in high-risk patients with type 2 diabetes mellitus—a randomized prospective study. Diabet Med. 2002;19:385-392.
25. Chodosh J, Morton SC, Mojica W, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143:427-438.
26. Tshiananga JK, Kocher S, Weber C, et al. The effect of nurse-led diabetes self-management education on glycosylated hemoglobin and cardiovascular risk factors: a meta-analysis. Diabetes Educ. 2012;38:108-123.
27. Cuijpers P, van Straten A, Andersson G, et al. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol. 2008;76:909-922.
28. Lustman PJ, Griffith LS, Freedland KE, et al. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;129:613-621.
29. Deakin T, McShane CE, Cade JE, et al. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2):CD003417.-
30. Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabet Med. 2011;28:455-463.
31. Walker EA, Shmukler C, Ullman R, et al. Results of a successful telephonic intervention to improve diabetes control in urban adults: a randomized trial. Diabetes Care. 2011;34:2-7.
32. Wu L, Forbes A, Griffiths P, et al. Telephone follow-up to improve glycaemic control in patients with type 2 diabetes: systematic review and meta-analysis of controlled trials. Diabet Med. 2010;27:1217-1225.
33. Amoako E, Skelly AH, Rossen EK. Outcomes of an intervention to reduce uncertainty among African American women with diabetes. West J Nurs Res. 2008;30:928-942.
34. Quinn CC, Shardell MD, Terrin ML, et al. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34:1934-1942.
35. Yoo HJ, Park MS, Kim TN, et al. A ubiquitous chronic disease care system using cellular phones and the internet. Diabet Med. 2009;26:628-635.
Heart failure: Best options when ejection fraction is preserved
• Suspect diastolic heart failure in patients who have symptoms of heart failure but a normal ejection fraction, with or without evidence of diastolic abnormalities. B
• Treatment goals for patients who have heart failure with preserved ejection fraction (HFPEF) include normalization of blood pressure, prevention of tachycardia and ischemia, reduction of congestion, and improvement in exercise capacity. B
• Initiate beta-blocker therapy without delay for patients who have acute decompensated HFPEF and tachycardia; consider cardioversion for those with atrial fibrillation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most studies of heart failure (HF)—the most common cause of hospitalization in patients older than 65 years1—have focused on patients with reduced ejection fraction (EF). Yet half of those hospitalized for acute decompensated HF have a normal left ventricular EF.2 For these patients, contractility is not the problem—impaired relaxation during diastole is.
Commonly called diastolic HF, heart failure with preserved ejection fraction (HFPEF) is a more precise name for this condition. Patients are usually older than those with a reduced EF.3 Thus, as the US population ages, the prevalence of HFPEF increases, as well.4
Diagnostic criteria have been developed for HFPEF, but there are few large, high-quality studies to guide its treatment. Yet family physicians need to be familiar with HFPEF and know how best to treat it. With extrapolation from studies of patients with reduced EF, as well as expert consensus and our own experience, we offer an evidence-based approach to the management of both stable and acute decompensated HFPEF.
A closer look at diastolic dysfunction
Defined as an abnormality of diastolic compliance, filling, or relaxation of the ventricle, diastolic dysfunction can occur whether EF is normal or abnormal.3 Ventricular diastole includes isovolumic relaxation, early passive filling after mitral valve opening, and active filling during atrial contraction. Transmission of high ventricular pressure to the pulmonary circulation leads to pulmonary edema, dyspnea, and other symptoms of HF. Factors other than abnormal diastolic physiology, such as chronic volume overload, ventricular coupling dyssynchrony, increased autonomic tone leading to reduced venous capacitance, and chronotropic intolerance, may also be involved.5
Patient history: What to look for
A variety of conditions, including ischemia, tachycardia, impaired myocardial relaxation, and age-related loss of myocardial compliance, can contribute to abnormal diastolic function, but the major causes of HFPEF are chronic hypertension, hypertrophic cardiomyopathy, and coronary artery disease (CAD).3 Rarely, infiltrative or restrictive cardiomyopathy (eg, amyloidosis or sarcoidosis) is implicated.6 Noncardiovascular comorbidities such as diabetes, renal impairment, anemia, and chronic lung disease are more prevalent among those with HFPEF, and more women are affected than men.1
Mortality risk. In a study of more than 100,000 hospitalizations for acute decompensated HF, patients with preserved EF had lower in-hospital mortality (3% vs 4% for those with reduced EF).2 Patients with both diabetes and CAD commonly develop HFPEF,7 and the presence of these comorbidities are an independent predictor of 5-year mortality.8
Population studies suggest that 5-year mortality rates for African Americans with HFPEF are higher than for Caucasians with this condition.9 Other predictors of mortality include older age, male sex, lower left ventricular EF, ischemic disease, impaired renal function, and peripheral arterial disease.10-12
Diagnosing HFPEF: What you’ll see, when to test
The presentation of patients with HFPEF is similar to that of individuals with reduced EF. In an outpatient setting, both groups will have reduced exercise capacity; increased neuroendocrine activation, which may cause chronic fluid retention, vasoconstriction, and tachycardia; and a reduced quality of life.5
Neither the American College of Cardiology/American Heart Association (ACC/AHA) nor the Heart Failure Society of America (HFSA)13,14 recommends screening for asymptomatic left ventricular dysfunction. For those with signs and symptoms of HF, however, echocardiography is a key component of the initial evaluation. Echocardiography provides information about left ventricular systolic function, including EF, regional wall motion abnormalities, and wall thickness. Echocardiographic evidence of diastolic abnormalities is found for some patients with HFPEF, while others have no demonstrable diastolic dysfunction.3
While an electrocardiogram (EKG) cannot distinguish between HF with reduced EF and HFPEF, common findings might include signs of ventricular hypertrophy or tachycardia during acute exacerbations. An EKG should be obtained in patients with suspected HF to screen for antecedent causes such as hypertrophy, atrial fibrillation, and ischemia.15
What Doppler echocardiography and the E/A ratio reveal
Doppler echocardiography is used to further evaluate the characteristics of blood flow, showing the relationship among left ventricular (LV) relaxation, atrial pressure, atrial contraction, and blood flow velocity across the mitral valve during diastole. The peak velocity of blood flow during early diastole (called the “E wave”) and late diastole (the atrial contraction, or “A wave”) is measured and the E/A ratio (reflecting the transmitral blood flow pattern) is calculated (FIGURE).3
FIGURE
The E/A ratio* and what it reveals
A, atrial contraction; E, early passive filling; MVC, mitral valve closes; MVO, mitral valve opens.
*E/A ratio represents the relationship between the peak velocity of blood flow during early diastole (E wave) and late diastole (A wave).
Adapted from: Aurigemma GP, Gaasch WH. N Engl J Med. 2004.3
Normally, transmitral flow velocity is greater during early diastole than during atrial contraction, and the E/A ratio is approximately 1.5 (E>A). With early diastolic dysfunction, impaired relaxation prevents blood from flowing passively into the LV during early diastole. This causes reversal of the E/A ratio, which drops to <1 (E<A). As diastolic function worsens, atrial contraction is impaired, and left atrial pressure rises. The result: A reduction in the A wave amplitude and proportionally more blood flow during early diastole and a “pseudonormal” (E>A) ratio, with a greater difference between the E and A than is normally observed. This finding is an independent predictor of all-cause mortality in patients with asymptomatic HF.16
Cardiac catheterization. Invasive measurement of LV filling pressures is the gold standard for diagnosing HFPEF. If echocardiography does not lead to a clear diagnosis, cardiac catheterization can provide information about concomitant pulmonary hypertension and mechanical asynchrony that may contribute to symptomatic HF.1 When the diagnosis is uncertain, additional testing—eg, plasma brain natriuretic peptide (BNP), chest x-ray, or exercise testing—may be necessary to establish a diagnosis of symptomatic HF.
The diagnostic criteria developed by HFSA include clinical evidence of HF and:
- echocardiographic evidence of LV hypertrophy or left atrial enlargement (without atrial fibrillation) or
- evidence of diastolic dysfunction on Doppler echocardiography or cardiac catheterization.14
It is important to note that the diagnostic criteria have not been validated, and the sensitivity and specificity of the various clinical findings are not known.
CASE Carrie W, a 76-year-old woman referred to you by a colleague, presents for follow-up after being hospitalized for HF. She recalls feeling fatigue, chest pain, and out of breath with even minimal exertion before being admitted to the hospital.
You obtain her hospital records, which show that echocardiography found impaired LV relaxation based on a reversed E/A ratio and an EF of 65%. In addition, BNP was elevated, and a chest x-ray showed pulmonary vascular congestion. You note that her blood pressure was 175/103 mm Hg on admission and an EKG showed LV hypertrophy and sinus tachycardia, but no ischemia.
Before being hospitalized, Ms. W was taking extended-release metoprolol, aspirin, and lisinopril. The hospitalist added lovastatin and increased the daily dose of extended-release metoprolol from 25 to 100 mg.
What changes, if any, would you make in her medication regimen?
Diastolic dysfunction as chronic disease
Often asymptomatic, diastolic dysfunction should be thought of as a chronic progressive disease characterized by complex physiologic adaptations that vary over time (See “Staging heart failure: The clinical course of HFPEF”.13) Patients with HFPEF have a difficult time tolerating hemodynamic stress and any perturbation of afterload, heart rate, or ventricular function can precipitate an acute exacerbation.2 Clinical factors that precipitate acute decompensation of HFPEF—which we’ll discuss a bit later—include uncontrolled hypertension; atrial fibrillation; and noncardiovascular comorbidities such as lung disease, renal impairment, or sepsis.2
The ACC/AHA staging system for HF can be applied to patients with HFPEF, both to classify disease severity and to track the progression of the disease. Patients at Stage A are at high risk of developing HF, but early and aggressive treatment of hypertension and other cardiovascular risk factors may delay or potentially prevent the onset of overt disease. Stage B refers to patients with known structural disease, such as a history of myocardial infarction or systolic or diastolic dysfunction, but no symptoms of HF.
Patients at Stage C have evidence of structural disease and symptoms of HF, such as fatigue, shortness of breath, or reduced exercise tolerance. This stage represents the spectrum of patients falling into New York Heart Association (NYHA) Class 1 through 3 categories. Finally, patients at Stage D—analogous to NYHA Class 4—have refractory HF, with marked symptoms even at rest despite maximal medical therapy.
The Acute Decompensated HEart failure national REgistry (ADHERE), in which the records of well over 80,000 Medicare patients were reviewed, found that more than 60% of those hospitalized with HFPEF had uncontrolled hypertension, with a systolic pressure >140 mm Hg; 21% had atrial fibrillation.2 These findings emphasize the importance of aggressive blood pressure (BP) and heart rate control.
Management of HFPEF is goal directed
The aim of pharmacologic treatment of HFPEF is to maintain fluid balance, prevent tachycardia, treat and prevent ischemia, and control hypertension (TABLE).14,17-30 While the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and beta-blockers, among other pharmacologic agents, is well studied for patients with reduced EF, there is limited evidence to guide the treatment of those with HFPEF. Although no single agent or drug class has been shown to be superior for such patients, there are a number of pharmacologic treatments to consider.
TABLE
Management of heart failure with preserved ejection fraction—matching treatment and goals14,17-30
| Treatment goal | Modality |
|---|---|
| Reduce congestion | Diuretics Salt restriction |
| Maintain atrial contraction | A-V pacing Cardioversion |
| Prevent tachycardia | A-V pacing Beta-blockers Calcium channel blockers |
| Prevent/treat ischemia | Antiplatelet therapy Beta-blockers Calcium channel blockers Revascularization Statins |
| Control hypertension | Antihypertensive agents:
|
| Promote regression of LV remodeling | ACE inhibitors ARBs |
| Improve exercise capacity | Supervised exercise program |
| ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; LV, left ventricle. | |
Inhibition of the renin-angiotensin-aldosterone system
Pathologic activation of the renin-angiotensin-aldosterone system (RAAS) contributes to elevated systolic and diastolic pressure, LV hypertrophy, and LV fibrosis. Inhibition of this system is a promising treatment modality for HFPEF.31
ACE inhibitors. Experimental studies suggest that ACE inhibitors benefit the diastolic properties of the heart, in both short- and long-term use. The PEP-CHF trial found that for older patients with diastolic dysfunction, perindopril led to significant improvements in functional class and exercise capacity but failed to show a statistically significant reduction in all-cause mortality or hospitalization for acute decompensated HF.17
ARBs. There is no evidence to show that ARB therapy improves morbidity or mortality in HFPEF. Using surrogate end points, ARBs have been associated with regression of LV hypertrophy, and losartan was found to improve exercise tolerance and quality of life, compared with hydrochlorothiazide.18,19 In the CHARM-Preserved trial, candesartan showed an insignificant reduction in cardiovascular mortality and hospitalization for HF.
These results must be viewed with caution, however, because adverse effects led to high rates of medication discontinuation.32 In the I-PRESERVE trial, irbesartan conferred no benefit with respect to mortality, hospitalization, or quality of life on patients with HFPEF.33
ACE inhibitor or ARB—not both. ACE inhibitors and ARBs are good choices for BP control in patients with HFPEF, especially if LV hypertrophy is present, but periodic testing of renal function and potassium levels is needed. ACE inhibitors and ARBs should not be used concurrently, as the combination increases the risk of acute renal failure and has no benefit in clinical outcomes.34
BP and rate control
In small trials, beta-blockers have been found to improve diastolic function as seen on echocardiography, but data on morbidity and mortality are lacking.20 A secondary analysis of the OPTIMIZE-HF registry found that beta-blocker therapy was associated with reduced mortality and readmission in patients with reduced EF, but not in those with normal EF.21
Findings from the SENIORS trial were more promising: Treatment with nebivolol reduced both mortality and readmission rates for elderly patients with HF, with similar benefits for those with reduced and preserved EF.22 Overall, beta-blockers appear to be a reasonable choice for heart rate and/or BP control in patients who have HFPEF and atrial fibrillation or hypertension. Carvedilol, long-acting metoprolol, and bisoprolol have been shown to reduce mortality in HF with reduced EF, and it is reasonable to choose one of these agents for patients with preserved EF, as well.23
Calcium channel blockers (CCBs) may be useful in treating patients with HFPEF for both BP and heart rate control, as well. Theoretically, CCBs may also improve the process of relaxation by altering intracellular calcium cycling during the contractile cycle in myocytes. This contrasts with the management of HF patients with reduced EF, for whom the use of nonselective CCBs such as diltiazem and verapamil may adversely affect contractility.
In small RCTs, verapamil has been found to improve HF symptoms and exercise tolerance in patients with HFPEF,24 but no evidence of improved outcomes or mortality rates with CCB use has been found.
Other pharmacologic options to consider
Aldosterone antagonist therapy is an important component of treatment for patients with HF with reduced EF. Data supporting the use of spironolactone use from the RALES trial and eplerenone in the EPHESUS and EMPHASIS-HF trials suggest a reduction in mortality in patients with low (<35%) LVEF.25-27 For patients with preserved EF, however, spironolactone is not generally recommended.
A large National Institutes of Health-sponsored trial is underway to determine if the drug is beneficial for patients with preserved LVEF, and will build on a small study in which 30 patients with HFPEF showed improved myocardial function after treatment with spironolactone.35 Until more data become available, the risks of using aldosterone antagonists outweigh the evidence to support their use in this patient population.
Diuretics are an important component of treatment for all patients with HF and fluid overload. The Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) showed a reduced incidence of symptomatic HFPEF in patients taking diuretics.28 As is the case with patients with reduced EF, those with preserved EF should be treated with diuretics if they have symptoms of fluid overload.
Statins. Intensive lipid lowering with statin therapy has been shown in observational studies to benefit patients with HFPEF with respect to mortality, independent of baseline low-density lipoprotein cholesterol.29 RCTs are needed to confirm these observations, but statin therapy is recommended for the secondary prevention of cardiovascular disease, independent of the presence of diastolic dysfunction or HFPEF.
Guard against hypotension. Patients with diastolic dysfunction are susceptible to hypotension if there is a rapid reduction in preload with diuretics, nonselective CCBs, or nitrates, so it is important that doses be titrated slowly.
Nonpharmacologic measures are important, too
In addition to optimizing treatment of comorbid conditions, patients with HFPEF should be advised that lifestyle modifications such as weight loss, smoking cessation, and dietary changes can do much to reduce the risk. You can help by providing an exercise “prescription” (with a specified intensity, frequency, and duration) and dietary guidelines, with emphasis on the importance of a low-sodium diet to prevent fluid overload.14,30 Recommend local programs for patients with HF, which many hospitals and health systems offer as part of their efforts to reduce readmission rates.
Consider cardioversion
Tachycardia shortens the time for filling during diastole; thus, it is poorly tolerated in patients with diastolic dysfunction and could trigger acute decompensation. To avoid the risk, restoration of sinus rhythm should be considered for patients with HFPEF and atrial fibrillation. Patients with known paroxysmal or permanent atrial fibrillation and preserved EF should be seen by a cardiologist to determine whether direct current cardioversion or ablation with a permanent pacemaker is appropriate.11 When cardioversion is contraindicated, a beta-blocker is needed to control heart rate and improve hemodynamics.
Patients with stable angina and HFPEF should be evaluated for revascularization when medical therapy alone is not sufficient for symptom relief.10 Here, too, a cardiology consult is indicated for any patient who has HF and an abnormal noninvasive stress test or persistent symptoms despite optimal drug therapy.
Recognizing and responding to acute decompensated HFPEF
The initial response to acute decompensated HFPEF, like that of HF with reduced EF, should be focused on restoring volume status and providing oxygenation, ventilation, and vasodilator therapy in some cases.11 Unlike those with acute decompensated HF with reduced EF, however, patients with HFPEF can safely tolerate the initiation of beta-blockers in the acute phase, especially when rate control is needed.3 Inotropic agents like digoxin and dobutamine, however, are contraindicated.3
Guidelines recommend hospitalization for patients with abnormal vital signs, arrhythmia, and suspected acute coronary syndromes, and consideration of hospitalization for those with associated comorbid conditions, new HF, or progressive fluid overload.13
CASE Because Ms. W has a normal BP and heart rate and is feeling well, you decline to alter her medication regimen. You do, however, recommend that she begin an exercise program, adopt a low-sodium diet, and maintain regular contact with your office so you can evaluate any changes in status.
You introduce Ms. W to the nurse case manager in your office. The nurse works with the patient to develop an action plan that includes daily tracking of her weight and sodium intake; a progressive walking program, starting with 2-minute sessions and progressing to 15 to 30 minutes 3 to 5 times a week; weekly telephone checkins; and immediate calls to report any weight increase or symptoms of HF.
At follow-up 6 months later, Ms. W has improved BP and reports that she enjoys her new exercise routine. She has more energy and denies any edema or breathing difficulties.
1. Lam CSP, Donal E, Kraigher-Krainer E, et al. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18-28.
2. Yancy CW, Lopatin M, Stevenson LW, et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the acute decompensated heart failure national registry (ADHERE) database. J Am Coll Cardiol. 2006;47:76-84.
3. Aurigemma GP, Gaasch WH. Diastolic heart failure. N Engl J Med. 2004;351:1097-1105.
4. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251-259.
5. Bench T, Burkhoff D, O’Connell JB, et al. Heart failure with normal ejection fraction: consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep. 2009;6:57-64.
6. Ammash NM, Seward JB, Bailey KR, et al. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101:2490-2496.
7. Bell DSH. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949-2951.
8. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction: a population-based study. J Am Coll Cardiol. 2010;55:300-305.
9. East MA, Peterson ED, Shaw LK, et al. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J. 2004;148:151-156.
10. Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444-450.
11. Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671-678.
12. Somaratne JB, Berry C, McMurray JJ, et al. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail. 2009;11:855-862.
13. 2005 Writing committee members; Hunt SA, Abraham WT, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. Circulation. 2009;119:e391-e479.
14. Heart Failure Society of America. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1-e2.
15. Davie AP, Francis CM, Love MP, et al. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ. 1996;312:222.-
16. Halley CM, Houghtaling PL, Khalil MK, et al. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082-1087.
17. Cleland JGF, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338-2345.
18. Wachtell K, Bella JN, Rokkedal J, et al. Change in diastolic left ventricular filling after one year of antihypertensive treatment. Circulation. 2002;105:1071-1076.
19. Little WC, Zile MR, Klein A, et al. Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and left ventricular diastolic dysfunction. Am J Cardiol. 2006;98:383-385.
20. Bonow RO, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mechanisms and management Ann Intern Med. 1992;117:502-510.
21. Hernandez AF, Hammill BG, O’Connor CM, et al. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (organized program to initiate lifesaving treatment in hospitalized patients with heart failure) registry. J Am Coll Cardiol. 2009;53:184-192.
22. Flather MD, Shibata MC, Coats AJS, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215-225.
23. Chavey WE, Bleske BE, Van Harrison R, et al. Pharmacologic management of heart failure caused by systolic dysfunction. Am Fam Physician. 2008;77:957-964.
24. Setaro JF, Zaret BL, Schulman DS, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981-986.
25. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-717.
26. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321.
27. Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11-21.
28. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981-2997.
29. Fukuta H, Sane DC, Brucks S, et al. Statin therapy may be associated with lower mortality in patients with diastolic heart failure. Circulation. 2005;112:357-363.
30. Arcand JAL, Brazel S, Joliffe C, et al. Education by a dietitian in patients with heart failure results in improved adherence with a sodium-restricted diet: a randomized trial. Am Heart J. 2005;150:716.e1-716.e5.
31. Bernal J, Pitta SR, Thatai D. Role of the renin-angiotensin-aldosterone system in diastolic heart failure: potential for pharmacologic intervention. Am J Cardiovasc Drugs. 2006;6:373-381.
32. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362:777-781.
33. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456-2467.
34. Heran BS, Musini VM, Bassett K, et al. Angiotensin receptor blockers for heart failure. Cochrane Database Syst Rev. 2012;(4):CD003040.-
35. Mottram PM, Haluska B, Leano R, et al. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;0110:558-565.
CORRESPONDENCE Geoffrey D. Mills, MD, PhD, Department of Family and Community Medicine, Jefferson Medical College, 833 Chestnut Street, Suite 301, Philadelphia, PA 19107; [email protected]
• Suspect diastolic heart failure in patients who have symptoms of heart failure but a normal ejection fraction, with or without evidence of diastolic abnormalities. B
• Treatment goals for patients who have heart failure with preserved ejection fraction (HFPEF) include normalization of blood pressure, prevention of tachycardia and ischemia, reduction of congestion, and improvement in exercise capacity. B
• Initiate beta-blocker therapy without delay for patients who have acute decompensated HFPEF and tachycardia; consider cardioversion for those with atrial fibrillation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most studies of heart failure (HF)—the most common cause of hospitalization in patients older than 65 years1—have focused on patients with reduced ejection fraction (EF). Yet half of those hospitalized for acute decompensated HF have a normal left ventricular EF.2 For these patients, contractility is not the problem—impaired relaxation during diastole is.
Commonly called diastolic HF, heart failure with preserved ejection fraction (HFPEF) is a more precise name for this condition. Patients are usually older than those with a reduced EF.3 Thus, as the US population ages, the prevalence of HFPEF increases, as well.4
Diagnostic criteria have been developed for HFPEF, but there are few large, high-quality studies to guide its treatment. Yet family physicians need to be familiar with HFPEF and know how best to treat it. With extrapolation from studies of patients with reduced EF, as well as expert consensus and our own experience, we offer an evidence-based approach to the management of both stable and acute decompensated HFPEF.
A closer look at diastolic dysfunction
Defined as an abnormality of diastolic compliance, filling, or relaxation of the ventricle, diastolic dysfunction can occur whether EF is normal or abnormal.3 Ventricular diastole includes isovolumic relaxation, early passive filling after mitral valve opening, and active filling during atrial contraction. Transmission of high ventricular pressure to the pulmonary circulation leads to pulmonary edema, dyspnea, and other symptoms of HF. Factors other than abnormal diastolic physiology, such as chronic volume overload, ventricular coupling dyssynchrony, increased autonomic tone leading to reduced venous capacitance, and chronotropic intolerance, may also be involved.5
Patient history: What to look for
A variety of conditions, including ischemia, tachycardia, impaired myocardial relaxation, and age-related loss of myocardial compliance, can contribute to abnormal diastolic function, but the major causes of HFPEF are chronic hypertension, hypertrophic cardiomyopathy, and coronary artery disease (CAD).3 Rarely, infiltrative or restrictive cardiomyopathy (eg, amyloidosis or sarcoidosis) is implicated.6 Noncardiovascular comorbidities such as diabetes, renal impairment, anemia, and chronic lung disease are more prevalent among those with HFPEF, and more women are affected than men.1
Mortality risk. In a study of more than 100,000 hospitalizations for acute decompensated HF, patients with preserved EF had lower in-hospital mortality (3% vs 4% for those with reduced EF).2 Patients with both diabetes and CAD commonly develop HFPEF,7 and the presence of these comorbidities are an independent predictor of 5-year mortality.8
Population studies suggest that 5-year mortality rates for African Americans with HFPEF are higher than for Caucasians with this condition.9 Other predictors of mortality include older age, male sex, lower left ventricular EF, ischemic disease, impaired renal function, and peripheral arterial disease.10-12
Diagnosing HFPEF: What you’ll see, when to test
The presentation of patients with HFPEF is similar to that of individuals with reduced EF. In an outpatient setting, both groups will have reduced exercise capacity; increased neuroendocrine activation, which may cause chronic fluid retention, vasoconstriction, and tachycardia; and a reduced quality of life.5
Neither the American College of Cardiology/American Heart Association (ACC/AHA) nor the Heart Failure Society of America (HFSA)13,14 recommends screening for asymptomatic left ventricular dysfunction. For those with signs and symptoms of HF, however, echocardiography is a key component of the initial evaluation. Echocardiography provides information about left ventricular systolic function, including EF, regional wall motion abnormalities, and wall thickness. Echocardiographic evidence of diastolic abnormalities is found for some patients with HFPEF, while others have no demonstrable diastolic dysfunction.3
While an electrocardiogram (EKG) cannot distinguish between HF with reduced EF and HFPEF, common findings might include signs of ventricular hypertrophy or tachycardia during acute exacerbations. An EKG should be obtained in patients with suspected HF to screen for antecedent causes such as hypertrophy, atrial fibrillation, and ischemia.15
What Doppler echocardiography and the E/A ratio reveal
Doppler echocardiography is used to further evaluate the characteristics of blood flow, showing the relationship among left ventricular (LV) relaxation, atrial pressure, atrial contraction, and blood flow velocity across the mitral valve during diastole. The peak velocity of blood flow during early diastole (called the “E wave”) and late diastole (the atrial contraction, or “A wave”) is measured and the E/A ratio (reflecting the transmitral blood flow pattern) is calculated (FIGURE).3
FIGURE
The E/A ratio* and what it reveals
A, atrial contraction; E, early passive filling; MVC, mitral valve closes; MVO, mitral valve opens.
*E/A ratio represents the relationship between the peak velocity of blood flow during early diastole (E wave) and late diastole (A wave).
Adapted from: Aurigemma GP, Gaasch WH. N Engl J Med. 2004.3
Normally, transmitral flow velocity is greater during early diastole than during atrial contraction, and the E/A ratio is approximately 1.5 (E>A). With early diastolic dysfunction, impaired relaxation prevents blood from flowing passively into the LV during early diastole. This causes reversal of the E/A ratio, which drops to <1 (E<A). As diastolic function worsens, atrial contraction is impaired, and left atrial pressure rises. The result: A reduction in the A wave amplitude and proportionally more blood flow during early diastole and a “pseudonormal” (E>A) ratio, with a greater difference between the E and A than is normally observed. This finding is an independent predictor of all-cause mortality in patients with asymptomatic HF.16
Cardiac catheterization. Invasive measurement of LV filling pressures is the gold standard for diagnosing HFPEF. If echocardiography does not lead to a clear diagnosis, cardiac catheterization can provide information about concomitant pulmonary hypertension and mechanical asynchrony that may contribute to symptomatic HF.1 When the diagnosis is uncertain, additional testing—eg, plasma brain natriuretic peptide (BNP), chest x-ray, or exercise testing—may be necessary to establish a diagnosis of symptomatic HF.
The diagnostic criteria developed by HFSA include clinical evidence of HF and:
- echocardiographic evidence of LV hypertrophy or left atrial enlargement (without atrial fibrillation) or
- evidence of diastolic dysfunction on Doppler echocardiography or cardiac catheterization.14
It is important to note that the diagnostic criteria have not been validated, and the sensitivity and specificity of the various clinical findings are not known.
CASE Carrie W, a 76-year-old woman referred to you by a colleague, presents for follow-up after being hospitalized for HF. She recalls feeling fatigue, chest pain, and out of breath with even minimal exertion before being admitted to the hospital.
You obtain her hospital records, which show that echocardiography found impaired LV relaxation based on a reversed E/A ratio and an EF of 65%. In addition, BNP was elevated, and a chest x-ray showed pulmonary vascular congestion. You note that her blood pressure was 175/103 mm Hg on admission and an EKG showed LV hypertrophy and sinus tachycardia, but no ischemia.
Before being hospitalized, Ms. W was taking extended-release metoprolol, aspirin, and lisinopril. The hospitalist added lovastatin and increased the daily dose of extended-release metoprolol from 25 to 100 mg.
What changes, if any, would you make in her medication regimen?
Diastolic dysfunction as chronic disease
Often asymptomatic, diastolic dysfunction should be thought of as a chronic progressive disease characterized by complex physiologic adaptations that vary over time (See “Staging heart failure: The clinical course of HFPEF”.13) Patients with HFPEF have a difficult time tolerating hemodynamic stress and any perturbation of afterload, heart rate, or ventricular function can precipitate an acute exacerbation.2 Clinical factors that precipitate acute decompensation of HFPEF—which we’ll discuss a bit later—include uncontrolled hypertension; atrial fibrillation; and noncardiovascular comorbidities such as lung disease, renal impairment, or sepsis.2
The ACC/AHA staging system for HF can be applied to patients with HFPEF, both to classify disease severity and to track the progression of the disease. Patients at Stage A are at high risk of developing HF, but early and aggressive treatment of hypertension and other cardiovascular risk factors may delay or potentially prevent the onset of overt disease. Stage B refers to patients with known structural disease, such as a history of myocardial infarction or systolic or diastolic dysfunction, but no symptoms of HF.
Patients at Stage C have evidence of structural disease and symptoms of HF, such as fatigue, shortness of breath, or reduced exercise tolerance. This stage represents the spectrum of patients falling into New York Heart Association (NYHA) Class 1 through 3 categories. Finally, patients at Stage D—analogous to NYHA Class 4—have refractory HF, with marked symptoms even at rest despite maximal medical therapy.
The Acute Decompensated HEart failure national REgistry (ADHERE), in which the records of well over 80,000 Medicare patients were reviewed, found that more than 60% of those hospitalized with HFPEF had uncontrolled hypertension, with a systolic pressure >140 mm Hg; 21% had atrial fibrillation.2 These findings emphasize the importance of aggressive blood pressure (BP) and heart rate control.
Management of HFPEF is goal directed
The aim of pharmacologic treatment of HFPEF is to maintain fluid balance, prevent tachycardia, treat and prevent ischemia, and control hypertension (TABLE).14,17-30 While the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and beta-blockers, among other pharmacologic agents, is well studied for patients with reduced EF, there is limited evidence to guide the treatment of those with HFPEF. Although no single agent or drug class has been shown to be superior for such patients, there are a number of pharmacologic treatments to consider.
TABLE
Management of heart failure with preserved ejection fraction—matching treatment and goals14,17-30
| Treatment goal | Modality |
|---|---|
| Reduce congestion | Diuretics Salt restriction |
| Maintain atrial contraction | A-V pacing Cardioversion |
| Prevent tachycardia | A-V pacing Beta-blockers Calcium channel blockers |
| Prevent/treat ischemia | Antiplatelet therapy Beta-blockers Calcium channel blockers Revascularization Statins |
| Control hypertension | Antihypertensive agents:
|
| Promote regression of LV remodeling | ACE inhibitors ARBs |
| Improve exercise capacity | Supervised exercise program |
| ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; LV, left ventricle. | |
Inhibition of the renin-angiotensin-aldosterone system
Pathologic activation of the renin-angiotensin-aldosterone system (RAAS) contributes to elevated systolic and diastolic pressure, LV hypertrophy, and LV fibrosis. Inhibition of this system is a promising treatment modality for HFPEF.31
ACE inhibitors. Experimental studies suggest that ACE inhibitors benefit the diastolic properties of the heart, in both short- and long-term use. The PEP-CHF trial found that for older patients with diastolic dysfunction, perindopril led to significant improvements in functional class and exercise capacity but failed to show a statistically significant reduction in all-cause mortality or hospitalization for acute decompensated HF.17
ARBs. There is no evidence to show that ARB therapy improves morbidity or mortality in HFPEF. Using surrogate end points, ARBs have been associated with regression of LV hypertrophy, and losartan was found to improve exercise tolerance and quality of life, compared with hydrochlorothiazide.18,19 In the CHARM-Preserved trial, candesartan showed an insignificant reduction in cardiovascular mortality and hospitalization for HF.
These results must be viewed with caution, however, because adverse effects led to high rates of medication discontinuation.32 In the I-PRESERVE trial, irbesartan conferred no benefit with respect to mortality, hospitalization, or quality of life on patients with HFPEF.33
ACE inhibitor or ARB—not both. ACE inhibitors and ARBs are good choices for BP control in patients with HFPEF, especially if LV hypertrophy is present, but periodic testing of renal function and potassium levels is needed. ACE inhibitors and ARBs should not be used concurrently, as the combination increases the risk of acute renal failure and has no benefit in clinical outcomes.34
BP and rate control
In small trials, beta-blockers have been found to improve diastolic function as seen on echocardiography, but data on morbidity and mortality are lacking.20 A secondary analysis of the OPTIMIZE-HF registry found that beta-blocker therapy was associated with reduced mortality and readmission in patients with reduced EF, but not in those with normal EF.21
Findings from the SENIORS trial were more promising: Treatment with nebivolol reduced both mortality and readmission rates for elderly patients with HF, with similar benefits for those with reduced and preserved EF.22 Overall, beta-blockers appear to be a reasonable choice for heart rate and/or BP control in patients who have HFPEF and atrial fibrillation or hypertension. Carvedilol, long-acting metoprolol, and bisoprolol have been shown to reduce mortality in HF with reduced EF, and it is reasonable to choose one of these agents for patients with preserved EF, as well.23
Calcium channel blockers (CCBs) may be useful in treating patients with HFPEF for both BP and heart rate control, as well. Theoretically, CCBs may also improve the process of relaxation by altering intracellular calcium cycling during the contractile cycle in myocytes. This contrasts with the management of HF patients with reduced EF, for whom the use of nonselective CCBs such as diltiazem and verapamil may adversely affect contractility.
In small RCTs, verapamil has been found to improve HF symptoms and exercise tolerance in patients with HFPEF,24 but no evidence of improved outcomes or mortality rates with CCB use has been found.
Other pharmacologic options to consider
Aldosterone antagonist therapy is an important component of treatment for patients with HF with reduced EF. Data supporting the use of spironolactone use from the RALES trial and eplerenone in the EPHESUS and EMPHASIS-HF trials suggest a reduction in mortality in patients with low (<35%) LVEF.25-27 For patients with preserved EF, however, spironolactone is not generally recommended.
A large National Institutes of Health-sponsored trial is underway to determine if the drug is beneficial for patients with preserved LVEF, and will build on a small study in which 30 patients with HFPEF showed improved myocardial function after treatment with spironolactone.35 Until more data become available, the risks of using aldosterone antagonists outweigh the evidence to support their use in this patient population.
Diuretics are an important component of treatment for all patients with HF and fluid overload. The Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) showed a reduced incidence of symptomatic HFPEF in patients taking diuretics.28 As is the case with patients with reduced EF, those with preserved EF should be treated with diuretics if they have symptoms of fluid overload.
Statins. Intensive lipid lowering with statin therapy has been shown in observational studies to benefit patients with HFPEF with respect to mortality, independent of baseline low-density lipoprotein cholesterol.29 RCTs are needed to confirm these observations, but statin therapy is recommended for the secondary prevention of cardiovascular disease, independent of the presence of diastolic dysfunction or HFPEF.
Guard against hypotension. Patients with diastolic dysfunction are susceptible to hypotension if there is a rapid reduction in preload with diuretics, nonselective CCBs, or nitrates, so it is important that doses be titrated slowly.
Nonpharmacologic measures are important, too
In addition to optimizing treatment of comorbid conditions, patients with HFPEF should be advised that lifestyle modifications such as weight loss, smoking cessation, and dietary changes can do much to reduce the risk. You can help by providing an exercise “prescription” (with a specified intensity, frequency, and duration) and dietary guidelines, with emphasis on the importance of a low-sodium diet to prevent fluid overload.14,30 Recommend local programs for patients with HF, which many hospitals and health systems offer as part of their efforts to reduce readmission rates.
Consider cardioversion
Tachycardia shortens the time for filling during diastole; thus, it is poorly tolerated in patients with diastolic dysfunction and could trigger acute decompensation. To avoid the risk, restoration of sinus rhythm should be considered for patients with HFPEF and atrial fibrillation. Patients with known paroxysmal or permanent atrial fibrillation and preserved EF should be seen by a cardiologist to determine whether direct current cardioversion or ablation with a permanent pacemaker is appropriate.11 When cardioversion is contraindicated, a beta-blocker is needed to control heart rate and improve hemodynamics.
Patients with stable angina and HFPEF should be evaluated for revascularization when medical therapy alone is not sufficient for symptom relief.10 Here, too, a cardiology consult is indicated for any patient who has HF and an abnormal noninvasive stress test or persistent symptoms despite optimal drug therapy.
Recognizing and responding to acute decompensated HFPEF
The initial response to acute decompensated HFPEF, like that of HF with reduced EF, should be focused on restoring volume status and providing oxygenation, ventilation, and vasodilator therapy in some cases.11 Unlike those with acute decompensated HF with reduced EF, however, patients with HFPEF can safely tolerate the initiation of beta-blockers in the acute phase, especially when rate control is needed.3 Inotropic agents like digoxin and dobutamine, however, are contraindicated.3
Guidelines recommend hospitalization for patients with abnormal vital signs, arrhythmia, and suspected acute coronary syndromes, and consideration of hospitalization for those with associated comorbid conditions, new HF, or progressive fluid overload.13
CASE Because Ms. W has a normal BP and heart rate and is feeling well, you decline to alter her medication regimen. You do, however, recommend that she begin an exercise program, adopt a low-sodium diet, and maintain regular contact with your office so you can evaluate any changes in status.
You introduce Ms. W to the nurse case manager in your office. The nurse works with the patient to develop an action plan that includes daily tracking of her weight and sodium intake; a progressive walking program, starting with 2-minute sessions and progressing to 15 to 30 minutes 3 to 5 times a week; weekly telephone checkins; and immediate calls to report any weight increase or symptoms of HF.
At follow-up 6 months later, Ms. W has improved BP and reports that she enjoys her new exercise routine. She has more energy and denies any edema or breathing difficulties.
• Suspect diastolic heart failure in patients who have symptoms of heart failure but a normal ejection fraction, with or without evidence of diastolic abnormalities. B
• Treatment goals for patients who have heart failure with preserved ejection fraction (HFPEF) include normalization of blood pressure, prevention of tachycardia and ischemia, reduction of congestion, and improvement in exercise capacity. B
• Initiate beta-blocker therapy without delay for patients who have acute decompensated HFPEF and tachycardia; consider cardioversion for those with atrial fibrillation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most studies of heart failure (HF)—the most common cause of hospitalization in patients older than 65 years1—have focused on patients with reduced ejection fraction (EF). Yet half of those hospitalized for acute decompensated HF have a normal left ventricular EF.2 For these patients, contractility is not the problem—impaired relaxation during diastole is.
Commonly called diastolic HF, heart failure with preserved ejection fraction (HFPEF) is a more precise name for this condition. Patients are usually older than those with a reduced EF.3 Thus, as the US population ages, the prevalence of HFPEF increases, as well.4
Diagnostic criteria have been developed for HFPEF, but there are few large, high-quality studies to guide its treatment. Yet family physicians need to be familiar with HFPEF and know how best to treat it. With extrapolation from studies of patients with reduced EF, as well as expert consensus and our own experience, we offer an evidence-based approach to the management of both stable and acute decompensated HFPEF.
A closer look at diastolic dysfunction
Defined as an abnormality of diastolic compliance, filling, or relaxation of the ventricle, diastolic dysfunction can occur whether EF is normal or abnormal.3 Ventricular diastole includes isovolumic relaxation, early passive filling after mitral valve opening, and active filling during atrial contraction. Transmission of high ventricular pressure to the pulmonary circulation leads to pulmonary edema, dyspnea, and other symptoms of HF. Factors other than abnormal diastolic physiology, such as chronic volume overload, ventricular coupling dyssynchrony, increased autonomic tone leading to reduced venous capacitance, and chronotropic intolerance, may also be involved.5
Patient history: What to look for
A variety of conditions, including ischemia, tachycardia, impaired myocardial relaxation, and age-related loss of myocardial compliance, can contribute to abnormal diastolic function, but the major causes of HFPEF are chronic hypertension, hypertrophic cardiomyopathy, and coronary artery disease (CAD).3 Rarely, infiltrative or restrictive cardiomyopathy (eg, amyloidosis or sarcoidosis) is implicated.6 Noncardiovascular comorbidities such as diabetes, renal impairment, anemia, and chronic lung disease are more prevalent among those with HFPEF, and more women are affected than men.1
Mortality risk. In a study of more than 100,000 hospitalizations for acute decompensated HF, patients with preserved EF had lower in-hospital mortality (3% vs 4% for those with reduced EF).2 Patients with both diabetes and CAD commonly develop HFPEF,7 and the presence of these comorbidities are an independent predictor of 5-year mortality.8
Population studies suggest that 5-year mortality rates for African Americans with HFPEF are higher than for Caucasians with this condition.9 Other predictors of mortality include older age, male sex, lower left ventricular EF, ischemic disease, impaired renal function, and peripheral arterial disease.10-12
Diagnosing HFPEF: What you’ll see, when to test
The presentation of patients with HFPEF is similar to that of individuals with reduced EF. In an outpatient setting, both groups will have reduced exercise capacity; increased neuroendocrine activation, which may cause chronic fluid retention, vasoconstriction, and tachycardia; and a reduced quality of life.5
Neither the American College of Cardiology/American Heart Association (ACC/AHA) nor the Heart Failure Society of America (HFSA)13,14 recommends screening for asymptomatic left ventricular dysfunction. For those with signs and symptoms of HF, however, echocardiography is a key component of the initial evaluation. Echocardiography provides information about left ventricular systolic function, including EF, regional wall motion abnormalities, and wall thickness. Echocardiographic evidence of diastolic abnormalities is found for some patients with HFPEF, while others have no demonstrable diastolic dysfunction.3
While an electrocardiogram (EKG) cannot distinguish between HF with reduced EF and HFPEF, common findings might include signs of ventricular hypertrophy or tachycardia during acute exacerbations. An EKG should be obtained in patients with suspected HF to screen for antecedent causes such as hypertrophy, atrial fibrillation, and ischemia.15
What Doppler echocardiography and the E/A ratio reveal
Doppler echocardiography is used to further evaluate the characteristics of blood flow, showing the relationship among left ventricular (LV) relaxation, atrial pressure, atrial contraction, and blood flow velocity across the mitral valve during diastole. The peak velocity of blood flow during early diastole (called the “E wave”) and late diastole (the atrial contraction, or “A wave”) is measured and the E/A ratio (reflecting the transmitral blood flow pattern) is calculated (FIGURE).3
FIGURE
The E/A ratio* and what it reveals
A, atrial contraction; E, early passive filling; MVC, mitral valve closes; MVO, mitral valve opens.
*E/A ratio represents the relationship between the peak velocity of blood flow during early diastole (E wave) and late diastole (A wave).
Adapted from: Aurigemma GP, Gaasch WH. N Engl J Med. 2004.3
Normally, transmitral flow velocity is greater during early diastole than during atrial contraction, and the E/A ratio is approximately 1.5 (E>A). With early diastolic dysfunction, impaired relaxation prevents blood from flowing passively into the LV during early diastole. This causes reversal of the E/A ratio, which drops to <1 (E<A). As diastolic function worsens, atrial contraction is impaired, and left atrial pressure rises. The result: A reduction in the A wave amplitude and proportionally more blood flow during early diastole and a “pseudonormal” (E>A) ratio, with a greater difference between the E and A than is normally observed. This finding is an independent predictor of all-cause mortality in patients with asymptomatic HF.16
Cardiac catheterization. Invasive measurement of LV filling pressures is the gold standard for diagnosing HFPEF. If echocardiography does not lead to a clear diagnosis, cardiac catheterization can provide information about concomitant pulmonary hypertension and mechanical asynchrony that may contribute to symptomatic HF.1 When the diagnosis is uncertain, additional testing—eg, plasma brain natriuretic peptide (BNP), chest x-ray, or exercise testing—may be necessary to establish a diagnosis of symptomatic HF.
The diagnostic criteria developed by HFSA include clinical evidence of HF and:
- echocardiographic evidence of LV hypertrophy or left atrial enlargement (without atrial fibrillation) or
- evidence of diastolic dysfunction on Doppler echocardiography or cardiac catheterization.14
It is important to note that the diagnostic criteria have not been validated, and the sensitivity and specificity of the various clinical findings are not known.
CASE Carrie W, a 76-year-old woman referred to you by a colleague, presents for follow-up after being hospitalized for HF. She recalls feeling fatigue, chest pain, and out of breath with even minimal exertion before being admitted to the hospital.
You obtain her hospital records, which show that echocardiography found impaired LV relaxation based on a reversed E/A ratio and an EF of 65%. In addition, BNP was elevated, and a chest x-ray showed pulmonary vascular congestion. You note that her blood pressure was 175/103 mm Hg on admission and an EKG showed LV hypertrophy and sinus tachycardia, but no ischemia.
Before being hospitalized, Ms. W was taking extended-release metoprolol, aspirin, and lisinopril. The hospitalist added lovastatin and increased the daily dose of extended-release metoprolol from 25 to 100 mg.
What changes, if any, would you make in her medication regimen?
Diastolic dysfunction as chronic disease
Often asymptomatic, diastolic dysfunction should be thought of as a chronic progressive disease characterized by complex physiologic adaptations that vary over time (See “Staging heart failure: The clinical course of HFPEF”.13) Patients with HFPEF have a difficult time tolerating hemodynamic stress and any perturbation of afterload, heart rate, or ventricular function can precipitate an acute exacerbation.2 Clinical factors that precipitate acute decompensation of HFPEF—which we’ll discuss a bit later—include uncontrolled hypertension; atrial fibrillation; and noncardiovascular comorbidities such as lung disease, renal impairment, or sepsis.2
The ACC/AHA staging system for HF can be applied to patients with HFPEF, both to classify disease severity and to track the progression of the disease. Patients at Stage A are at high risk of developing HF, but early and aggressive treatment of hypertension and other cardiovascular risk factors may delay or potentially prevent the onset of overt disease. Stage B refers to patients with known structural disease, such as a history of myocardial infarction or systolic or diastolic dysfunction, but no symptoms of HF.
Patients at Stage C have evidence of structural disease and symptoms of HF, such as fatigue, shortness of breath, or reduced exercise tolerance. This stage represents the spectrum of patients falling into New York Heart Association (NYHA) Class 1 through 3 categories. Finally, patients at Stage D—analogous to NYHA Class 4—have refractory HF, with marked symptoms even at rest despite maximal medical therapy.
The Acute Decompensated HEart failure national REgistry (ADHERE), in which the records of well over 80,000 Medicare patients were reviewed, found that more than 60% of those hospitalized with HFPEF had uncontrolled hypertension, with a systolic pressure >140 mm Hg; 21% had atrial fibrillation.2 These findings emphasize the importance of aggressive blood pressure (BP) and heart rate control.
Management of HFPEF is goal directed
The aim of pharmacologic treatment of HFPEF is to maintain fluid balance, prevent tachycardia, treat and prevent ischemia, and control hypertension (TABLE).14,17-30 While the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and beta-blockers, among other pharmacologic agents, is well studied for patients with reduced EF, there is limited evidence to guide the treatment of those with HFPEF. Although no single agent or drug class has been shown to be superior for such patients, there are a number of pharmacologic treatments to consider.
TABLE
Management of heart failure with preserved ejection fraction—matching treatment and goals14,17-30
| Treatment goal | Modality |
|---|---|
| Reduce congestion | Diuretics Salt restriction |
| Maintain atrial contraction | A-V pacing Cardioversion |
| Prevent tachycardia | A-V pacing Beta-blockers Calcium channel blockers |
| Prevent/treat ischemia | Antiplatelet therapy Beta-blockers Calcium channel blockers Revascularization Statins |
| Control hypertension | Antihypertensive agents:
|
| Promote regression of LV remodeling | ACE inhibitors ARBs |
| Improve exercise capacity | Supervised exercise program |
| ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; LV, left ventricle. | |
Inhibition of the renin-angiotensin-aldosterone system
Pathologic activation of the renin-angiotensin-aldosterone system (RAAS) contributes to elevated systolic and diastolic pressure, LV hypertrophy, and LV fibrosis. Inhibition of this system is a promising treatment modality for HFPEF.31
ACE inhibitors. Experimental studies suggest that ACE inhibitors benefit the diastolic properties of the heart, in both short- and long-term use. The PEP-CHF trial found that for older patients with diastolic dysfunction, perindopril led to significant improvements in functional class and exercise capacity but failed to show a statistically significant reduction in all-cause mortality or hospitalization for acute decompensated HF.17
ARBs. There is no evidence to show that ARB therapy improves morbidity or mortality in HFPEF. Using surrogate end points, ARBs have been associated with regression of LV hypertrophy, and losartan was found to improve exercise tolerance and quality of life, compared with hydrochlorothiazide.18,19 In the CHARM-Preserved trial, candesartan showed an insignificant reduction in cardiovascular mortality and hospitalization for HF.
These results must be viewed with caution, however, because adverse effects led to high rates of medication discontinuation.32 In the I-PRESERVE trial, irbesartan conferred no benefit with respect to mortality, hospitalization, or quality of life on patients with HFPEF.33
ACE inhibitor or ARB—not both. ACE inhibitors and ARBs are good choices for BP control in patients with HFPEF, especially if LV hypertrophy is present, but periodic testing of renal function and potassium levels is needed. ACE inhibitors and ARBs should not be used concurrently, as the combination increases the risk of acute renal failure and has no benefit in clinical outcomes.34
BP and rate control
In small trials, beta-blockers have been found to improve diastolic function as seen on echocardiography, but data on morbidity and mortality are lacking.20 A secondary analysis of the OPTIMIZE-HF registry found that beta-blocker therapy was associated with reduced mortality and readmission in patients with reduced EF, but not in those with normal EF.21
Findings from the SENIORS trial were more promising: Treatment with nebivolol reduced both mortality and readmission rates for elderly patients with HF, with similar benefits for those with reduced and preserved EF.22 Overall, beta-blockers appear to be a reasonable choice for heart rate and/or BP control in patients who have HFPEF and atrial fibrillation or hypertension. Carvedilol, long-acting metoprolol, and bisoprolol have been shown to reduce mortality in HF with reduced EF, and it is reasonable to choose one of these agents for patients with preserved EF, as well.23
Calcium channel blockers (CCBs) may be useful in treating patients with HFPEF for both BP and heart rate control, as well. Theoretically, CCBs may also improve the process of relaxation by altering intracellular calcium cycling during the contractile cycle in myocytes. This contrasts with the management of HF patients with reduced EF, for whom the use of nonselective CCBs such as diltiazem and verapamil may adversely affect contractility.
In small RCTs, verapamil has been found to improve HF symptoms and exercise tolerance in patients with HFPEF,24 but no evidence of improved outcomes or mortality rates with CCB use has been found.
Other pharmacologic options to consider
Aldosterone antagonist therapy is an important component of treatment for patients with HF with reduced EF. Data supporting the use of spironolactone use from the RALES trial and eplerenone in the EPHESUS and EMPHASIS-HF trials suggest a reduction in mortality in patients with low (<35%) LVEF.25-27 For patients with preserved EF, however, spironolactone is not generally recommended.
A large National Institutes of Health-sponsored trial is underway to determine if the drug is beneficial for patients with preserved LVEF, and will build on a small study in which 30 patients with HFPEF showed improved myocardial function after treatment with spironolactone.35 Until more data become available, the risks of using aldosterone antagonists outweigh the evidence to support their use in this patient population.
Diuretics are an important component of treatment for all patients with HF and fluid overload. The Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) showed a reduced incidence of symptomatic HFPEF in patients taking diuretics.28 As is the case with patients with reduced EF, those with preserved EF should be treated with diuretics if they have symptoms of fluid overload.
Statins. Intensive lipid lowering with statin therapy has been shown in observational studies to benefit patients with HFPEF with respect to mortality, independent of baseline low-density lipoprotein cholesterol.29 RCTs are needed to confirm these observations, but statin therapy is recommended for the secondary prevention of cardiovascular disease, independent of the presence of diastolic dysfunction or HFPEF.
Guard against hypotension. Patients with diastolic dysfunction are susceptible to hypotension if there is a rapid reduction in preload with diuretics, nonselective CCBs, or nitrates, so it is important that doses be titrated slowly.
Nonpharmacologic measures are important, too
In addition to optimizing treatment of comorbid conditions, patients with HFPEF should be advised that lifestyle modifications such as weight loss, smoking cessation, and dietary changes can do much to reduce the risk. You can help by providing an exercise “prescription” (with a specified intensity, frequency, and duration) and dietary guidelines, with emphasis on the importance of a low-sodium diet to prevent fluid overload.14,30 Recommend local programs for patients with HF, which many hospitals and health systems offer as part of their efforts to reduce readmission rates.
Consider cardioversion
Tachycardia shortens the time for filling during diastole; thus, it is poorly tolerated in patients with diastolic dysfunction and could trigger acute decompensation. To avoid the risk, restoration of sinus rhythm should be considered for patients with HFPEF and atrial fibrillation. Patients with known paroxysmal or permanent atrial fibrillation and preserved EF should be seen by a cardiologist to determine whether direct current cardioversion or ablation with a permanent pacemaker is appropriate.11 When cardioversion is contraindicated, a beta-blocker is needed to control heart rate and improve hemodynamics.
Patients with stable angina and HFPEF should be evaluated for revascularization when medical therapy alone is not sufficient for symptom relief.10 Here, too, a cardiology consult is indicated for any patient who has HF and an abnormal noninvasive stress test or persistent symptoms despite optimal drug therapy.
Recognizing and responding to acute decompensated HFPEF
The initial response to acute decompensated HFPEF, like that of HF with reduced EF, should be focused on restoring volume status and providing oxygenation, ventilation, and vasodilator therapy in some cases.11 Unlike those with acute decompensated HF with reduced EF, however, patients with HFPEF can safely tolerate the initiation of beta-blockers in the acute phase, especially when rate control is needed.3 Inotropic agents like digoxin and dobutamine, however, are contraindicated.3
Guidelines recommend hospitalization for patients with abnormal vital signs, arrhythmia, and suspected acute coronary syndromes, and consideration of hospitalization for those with associated comorbid conditions, new HF, or progressive fluid overload.13
CASE Because Ms. W has a normal BP and heart rate and is feeling well, you decline to alter her medication regimen. You do, however, recommend that she begin an exercise program, adopt a low-sodium diet, and maintain regular contact with your office so you can evaluate any changes in status.
You introduce Ms. W to the nurse case manager in your office. The nurse works with the patient to develop an action plan that includes daily tracking of her weight and sodium intake; a progressive walking program, starting with 2-minute sessions and progressing to 15 to 30 minutes 3 to 5 times a week; weekly telephone checkins; and immediate calls to report any weight increase or symptoms of HF.
At follow-up 6 months later, Ms. W has improved BP and reports that she enjoys her new exercise routine. She has more energy and denies any edema or breathing difficulties.
1. Lam CSP, Donal E, Kraigher-Krainer E, et al. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18-28.
2. Yancy CW, Lopatin M, Stevenson LW, et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the acute decompensated heart failure national registry (ADHERE) database. J Am Coll Cardiol. 2006;47:76-84.
3. Aurigemma GP, Gaasch WH. Diastolic heart failure. N Engl J Med. 2004;351:1097-1105.
4. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251-259.
5. Bench T, Burkhoff D, O’Connell JB, et al. Heart failure with normal ejection fraction: consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep. 2009;6:57-64.
6. Ammash NM, Seward JB, Bailey KR, et al. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101:2490-2496.
7. Bell DSH. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949-2951.
8. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction: a population-based study. J Am Coll Cardiol. 2010;55:300-305.
9. East MA, Peterson ED, Shaw LK, et al. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J. 2004;148:151-156.
10. Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444-450.
11. Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671-678.
12. Somaratne JB, Berry C, McMurray JJ, et al. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail. 2009;11:855-862.
13. 2005 Writing committee members; Hunt SA, Abraham WT, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. Circulation. 2009;119:e391-e479.
14. Heart Failure Society of America. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1-e2.
15. Davie AP, Francis CM, Love MP, et al. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ. 1996;312:222.-
16. Halley CM, Houghtaling PL, Khalil MK, et al. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082-1087.
17. Cleland JGF, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338-2345.
18. Wachtell K, Bella JN, Rokkedal J, et al. Change in diastolic left ventricular filling after one year of antihypertensive treatment. Circulation. 2002;105:1071-1076.
19. Little WC, Zile MR, Klein A, et al. Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and left ventricular diastolic dysfunction. Am J Cardiol. 2006;98:383-385.
20. Bonow RO, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mechanisms and management Ann Intern Med. 1992;117:502-510.
21. Hernandez AF, Hammill BG, O’Connor CM, et al. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (organized program to initiate lifesaving treatment in hospitalized patients with heart failure) registry. J Am Coll Cardiol. 2009;53:184-192.
22. Flather MD, Shibata MC, Coats AJS, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215-225.
23. Chavey WE, Bleske BE, Van Harrison R, et al. Pharmacologic management of heart failure caused by systolic dysfunction. Am Fam Physician. 2008;77:957-964.
24. Setaro JF, Zaret BL, Schulman DS, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981-986.
25. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-717.
26. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321.
27. Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11-21.
28. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981-2997.
29. Fukuta H, Sane DC, Brucks S, et al. Statin therapy may be associated with lower mortality in patients with diastolic heart failure. Circulation. 2005;112:357-363.
30. Arcand JAL, Brazel S, Joliffe C, et al. Education by a dietitian in patients with heart failure results in improved adherence with a sodium-restricted diet: a randomized trial. Am Heart J. 2005;150:716.e1-716.e5.
31. Bernal J, Pitta SR, Thatai D. Role of the renin-angiotensin-aldosterone system in diastolic heart failure: potential for pharmacologic intervention. Am J Cardiovasc Drugs. 2006;6:373-381.
32. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362:777-781.
33. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456-2467.
34. Heran BS, Musini VM, Bassett K, et al. Angiotensin receptor blockers for heart failure. Cochrane Database Syst Rev. 2012;(4):CD003040.-
35. Mottram PM, Haluska B, Leano R, et al. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;0110:558-565.
CORRESPONDENCE Geoffrey D. Mills, MD, PhD, Department of Family and Community Medicine, Jefferson Medical College, 833 Chestnut Street, Suite 301, Philadelphia, PA 19107; [email protected]
1. Lam CSP, Donal E, Kraigher-Krainer E, et al. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18-28.
2. Yancy CW, Lopatin M, Stevenson LW, et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the acute decompensated heart failure national registry (ADHERE) database. J Am Coll Cardiol. 2006;47:76-84.
3. Aurigemma GP, Gaasch WH. Diastolic heart failure. N Engl J Med. 2004;351:1097-1105.
4. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251-259.
5. Bench T, Burkhoff D, O’Connell JB, et al. Heart failure with normal ejection fraction: consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep. 2009;6:57-64.
6. Ammash NM, Seward JB, Bailey KR, et al. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101:2490-2496.
7. Bell DSH. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949-2951.
8. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction: a population-based study. J Am Coll Cardiol. 2010;55:300-305.
9. East MA, Peterson ED, Shaw LK, et al. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J. 2004;148:151-156.
10. Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444-450.
11. Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671-678.
12. Somaratne JB, Berry C, McMurray JJ, et al. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail. 2009;11:855-862.
13. 2005 Writing committee members; Hunt SA, Abraham WT, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. Circulation. 2009;119:e391-e479.
14. Heart Failure Society of America. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1-e2.
15. Davie AP, Francis CM, Love MP, et al. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ. 1996;312:222.-
16. Halley CM, Houghtaling PL, Khalil MK, et al. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082-1087.
17. Cleland JGF, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338-2345.
18. Wachtell K, Bella JN, Rokkedal J, et al. Change in diastolic left ventricular filling after one year of antihypertensive treatment. Circulation. 2002;105:1071-1076.
19. Little WC, Zile MR, Klein A, et al. Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and left ventricular diastolic dysfunction. Am J Cardiol. 2006;98:383-385.
20. Bonow RO, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mechanisms and management Ann Intern Med. 1992;117:502-510.
21. Hernandez AF, Hammill BG, O’Connor CM, et al. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (organized program to initiate lifesaving treatment in hospitalized patients with heart failure) registry. J Am Coll Cardiol. 2009;53:184-192.
22. Flather MD, Shibata MC, Coats AJS, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215-225.
23. Chavey WE, Bleske BE, Van Harrison R, et al. Pharmacologic management of heart failure caused by systolic dysfunction. Am Fam Physician. 2008;77:957-964.
24. Setaro JF, Zaret BL, Schulman DS, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981-986.
25. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-717.
26. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321.
27. Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11-21.
28. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981-2997.
29. Fukuta H, Sane DC, Brucks S, et al. Statin therapy may be associated with lower mortality in patients with diastolic heart failure. Circulation. 2005;112:357-363.
30. Arcand JAL, Brazel S, Joliffe C, et al. Education by a dietitian in patients with heart failure results in improved adherence with a sodium-restricted diet: a randomized trial. Am Heart J. 2005;150:716.e1-716.e5.
31. Bernal J, Pitta SR, Thatai D. Role of the renin-angiotensin-aldosterone system in diastolic heart failure: potential for pharmacologic intervention. Am J Cardiovasc Drugs. 2006;6:373-381.
32. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362:777-781.
33. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456-2467.
34. Heran BS, Musini VM, Bassett K, et al. Angiotensin receptor blockers for heart failure. Cochrane Database Syst Rev. 2012;(4):CD003040.-
35. Mottram PM, Haluska B, Leano R, et al. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;0110:558-565.
CORRESPONDENCE Geoffrey D. Mills, MD, PhD, Department of Family and Community Medicine, Jefferson Medical College, 833 Chestnut Street, Suite 301, Philadelphia, PA 19107; [email protected]
Keloids: Which treatment is best for your patient?
• Consider using corticosteroid injections to inhibit collagen synthesis and stimulate enzymatic degradation of existing keloid collagen. B
• Turn to treatment combinations for refractory keloids; regimens may include corticosteroid injections, surgical excision, pressure, occlusive dressings, or radiation. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Keloids are an ongoing clinical challenge. Despite the availability of multiple treatments, choosing an effective therapeutic regimen for these fibroproliferative scars can be vexing, as keloids usually recur.
This review provides a guide to determining which regimen is best for your patient. But first, a word about the causes of these claw-like growths and which patients are at highest risk of developing them.
Even superficial injuries can cause keloids
Keloids arise after a disruption of skin integrity following superficial or deep injuries (FIGURES 1A-C). Causes include physical trauma such as cuts, scratches, and insect bites; iatrogenic trauma as in vaccinations or surgical procedures; thermal or chemical burns; and skin eruptions such as chicken pox.
FIGURE 1
Keloids induced by scratches (A), ear piercing (B), and thermal burns (C)
Common sites of keloid development include the ears, jaw, neck, clavicle and sternum, and shoulders. Less commonly, keloids occur on the back, abdomen, and extremities. Rarely, keloids develop on the palms and soles, face, or mucous membranes.1
A minor keloid is smaller than 0.5 cm, while a major keloid is larger. No upper growth limit has been identified, as keloids seem to enlarge indefinitely.2 Keloid scars have a firm and inflexible texture, a shiny appearance, and are elevated above skin level. They are usually flesh-colored, but may be erythematous or hyperpigmented.1 (A similar aberrant wound healing condition often confused with keloids is hypertrophic scarring. See “Differentiating keloids from hypertrophic scars”).
Distinguishing between keloids and hypertrophic scars can be challenging, because both types of scars arise histologically from excessive collagen buildup during the wound healing process. However, there are 3 distinctive characteristics of keloids that hypertrophic scars lack:
- Keloids enlarge beyond wound margins, often with irregular shapes, while hypertrophic scars remain within the confines of the original wound and tend to be linear along scars.
- Keloids do not regress on their own; hypertrophic scars tend to regress spontaneously within a few years.
- Keloids may take months to years to develop, while hypertrophic scars usually appear within 4 to 6 weeks of a traumatic incident.
Source: Gauglitz G, Korting H, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113-125.
Although keloids have an aggressive growth pattern, they are considered benign tumors, because the development of malignant cells in a keloid is rare.1 Most patients seek medical attention for cosmetic concerns. However, tissue bulk can cause functional problems, and some keloids are symptomatic with pruritus (27%) or pain (19%).1,3
Who is affected?
The most consistent risk factor for keloid development is a previous keloid. Individuals with deeply pigmented skin appear to be at higher risk, with an estimated prevalence of 4% to 16% among individuals of African, Asian, and Hispanic ancestry.3 Although the rate of keloid occurrence among Caucasians has not been established, keloids have been reported in individuals of nearly every skin color, except albinos.3 Keloids occur in both sexes with similar frequency, and are most likely to develop during puberty and early adulthood—between the ages of 10 and 30 years—but can occur at any age.1,3
Although no clear familial inheritance pattern has been identified, keloids are associated with blood type A and human leukocyte antigens (HLA)-B14, -B21, -BW35, -DR5, -DRB1, -DQA1, -DQB1, and -DQW3.3,4 Emerging research indicates that mutations of the CDC2L1 gene—which encodes a protein kinase essential to cell cycle control—correlate with keloid formation.4
Wound healing gone awry
Normal wound healing involves an overlapping series of processes, including hemostasis, inflammation, and granulation and remodeling. Various mechanisms have been identified for keloid pathology, although no single definitive trigger is known.
Hemostasis. In normal hemostasis, platelets aggregate around a fibrin clot in a wound, stimulating the release of growth factors,3 which precipitate the migration of fibroblasts to the granulation-tissue scaffolding for wound healing. Keloids have increased fibroblast proliferation, with up to 20-fold increased production of Type 1 collagen.1
Inflammation. During the inflammation phase, cytokine cascades stimulate cell proliferation from inflammatory, endothelial, epithelial, and fibroblast cell lines. Keloids have altered levels of many cytokines, resulting in increased inflammatory activity, such as histamine release by mast cells.3
Granulation and remodeling. During normal granulation and remodeling, fibroblasts aggregate and produce extracellular matrix components. The keloid fibroblasts produce higher levels of collagen, elastin, fibronectin, and proteoglycans, but lower levels of hyaluronic acid.3,5 Keloid fibroblasts also make lower levels of tissue plasminogen activator inhibitor (TPA-I), with inferior breakdown of scaffold structures and collagens. While collagen bundles run parallel to the epithelial surface in normal skin, keloid collagen fibers are larger, thicker, and randomly oriented in dense sheets, swirls, or nodules.1
In normal tissue, wound healing stops after the wound has been fully epithelialized by keratinocytes, after which the scar tissue contracts to minimal size. However, keloid growth can continue for years.1
Numerous studies have inspired various hypotheses for keloid formation. Studies demonstrating that keloids contain an increased level of immunoglobulins suggest that they may be produced by an abnormal immune reaction. Microscopic examinations of keloids have shown that overabundant endothelial cells occlude microvessels, suggesting that keloids may occur in the context of wound hypoxia. In vitro studies showing that fibroblasts proliferate when cultured with keloidal keratinocytes suggest that keloids may be a result of abnormal epithelial–mesenchymal interactions.6,7 A hypothesis based on the body sites where keloids are observed is that mechanical stretch and tension across a wound may overstimulate collagen production, possibly as a result of mechanoreceptor damage or disorders.8
Treatment: Weighing the options
Due to the complex mechanisms of pathogenesis, many modalities are available for managing keloids, yet no definitive treatment protocols exist. Intradermal, extradermal, or systemic therapies may be used singly, although combination regimens are the most effective in treating keloids and preventing recurrences.5 The TABLE1,3,5,9-14 provides an overview of monotherapeutic treatment options, including efficacy and risks.
TABLE
Treatment options for keloids
| Treatment | Anticipated effects | Dosing and frequency | Efficacy in reducing keloid size | Adverse effects | SOR |
|---|---|---|---|---|---|
| Hygienic relief: washing & drying | Relief of pruritus, pain, general discomfort | As needed | N/A | Minimal | C |
| Antihistamines | Relief of pruritus, pain, burning sensation9 | As needed | N/A | Minimal | C |
| Corticosteroid injections | Reduction in keloid size, pruritus10 | Injections given every 4-8 wk for several months10 | >80% report moderate to marked regression10 | Local or systemic infection and allergy or anaphylaxis10 | B |
| Surgical excision | Temporary reduction in keloid size11 | Not successful as monotherapy; combine with alternative therapies as needed11 | Temporary; keloids always recur after excision11 | Chance of infection, excessive bleeding, or injury to adjacent tissues11 | C |
| Pressure therapy | Reduction in keloid size3,12 | Pressure of 25-40 mm Hg for 23-24 h/d over several months3,12 | 60%-80% of patients report at least partial improvement3 | Minimal; rarely, skin thinning and redness3,12 | B |
| Radiation therapy | Reduction in keloid size3 | 10-20 Gy fractionated over several weeks5 | Up to 94% of patients report improvement, but recurrence is common3 | Minimal; theoretical risk of malignancy13 | B |
| Silicone occlusive dressings | Reduction in pain, pruritus, and keloid size1,12,14 | Applied topically for 12-24 h/d for 18 mo1,12,14 | 68%-86% of patients report improvement in keloid texture, color, and size14; may be useful as a preventive measure14 | Skin breakdown, rash, pruritus12 | B |
| N/A, not applicable; SOR, strength of recommendation. | |||||
Offer symptomatic therapy routinely
Some patients find symptomatic relief through hygienic measures, such as regular washing and drying. Antihistamines can relieve symptoms of burning or pruritus,9 but are not expected to reduce keloid size.
Use corticosteroids for first-line treatment
Corticosteroids are the most widely used therapy for keloids.3 They decrease keloid bulking by inhibiting collagen synthesis1 and stimulate tissue collagenases and collagen degradation.5 Commonly used in the office setting are intralesional injections of triamcinolone acetonide, a potent anti-inflammatory and highly atrophogenic agent.
Administer injections every 4 to 8 weeks until clinical improvement (usually requiring several months) or prohibitive adverse effects occur. A general dosing guideline is about 0.1 to 0.2 mL of corticosteroid for every square centimeter of keloid tissue. Injecting higher doses can lead to accretions or deposits beneath the skin that might need to be unroofed. Additionally, excess corticosteroid can cause an atrophic contour deformity in the adjacent subcutaneous tissue. FIGURE 2 details steroid injection procedures and follow-up based on the author’s (SPD) experience.
FIGURE 2
A 5-step procedure for injecting keloids with corticosteroids*
*Based on the author’s (SPD) experience.
Contraindications to injections include local or systemic infection and known allergy or anaphylactic reaction to corticosteroids. Although injecting keloids can be difficult initially, the tissue tends to soften and become easier to inject with each successive treatment. Some clinicians advocate cryotherapy to soften the keloid before giving injections. Large trials, in which more than 80% of patients had moderate to marked keloid regression,10 have demonstrated the efficacy of this technique in reducing keloid size and itching.
Surgery alone is ineffective
The oldest remedy, simple scalpel excision of keloidal tissue, invariably results in regrowth, because the aberrant wound healing process is not remedied.11 Although surgical excision is a poor monotherapy, it may be useful in combination with other modalities. Repairs using meticulous atraumatic surgical techniques with minimal wound tension can lessen the risk of keloid recurrence. Absorbable monofilament sutures with a subcuticular stitch help to minimize further epidermal damage.6
Pressure is useful in certain cases
Applying pressure is a noninvasive method that produces initial tissue thinning and pliability, with 60% to 80% of patients reporting at least partial improvement.3 Pressure reduces keloids by decreasing tissue metabolism, so long-term therapy is required, with application of pressure 23 to 24 hours daily over several months.12 Pressure of 25 to 40 mm Hg is needed to exceed capillary pressure without damaging peripheral circulation.3
Custom pressure garments for burn patients have been used in specialized cases involving limbs and torso, face and neck, and hands and feet. For earlobe keloids, clip-on earring devices are the only practical solution. Compliance tends to wane after months of therapy, and cessation may be followed by rebound hypertrophy. The therapeutic effect of regular pressure massage has been poorly studied. Because pressure has minimal adverse effects, it is likely most useful as an adjunct therapy where practical.
Radiation has variable effectiveness
Radiation damages fibroblasts, inhibits proliferation, and may improve local oxygenation.13 Low megavolt electron beam radiation is used to limit depth of penetration, usually with a total dose of 10 to 20 Gy fractionated over several weeks.5 A dose-response effect is likely.3 Response rates vary from 10% to 94%,3 and keloid recurrence is common, making radiation a poor choice for primary treatment except in cases of a large-volume unresectable keloid (eg, post-burn keloids).13
Adverse effects can include nodule formation, hyperpigmentation, ulceration, pruritus, paresthesia, and wound dehiscence. Theoretical risks of malignancy with radiation treatment of keloids have not been borne out in large studies, which have shown a zero percent malignancy rate despite rare anecdotal reports.15 Nevertheless, radiation therapy for benign disease is not recommended for children or pregnant women, or in breast, thyroid, or other cancer-prone body sites.5
Occlusive dressings require patient compliance
An innovation in keloid treatment is the use of occlusive dressings, which started in 1981 and gained popularity in the 1990s.16 Gel, fluid, or rubbery sheeting, usually made from silicone, is applied topically for 12 to 24 hours daily for up to 18 months.1,12,14 Gel may be practical along creases or in areas of motion where silicone sheets are obtrusive. Because long-term therapy is recommended, occlusive dressings require active patient participation. Occlusive dressings can decrease pruritic symptoms by decreasing mast cell activity,12 and may do so by warmth, hydration, or occlusion effects.3
Although study methodology has been suboptimal and further research is required, some practitioners have reported efficacy of 86% for texture reduction, 84% improvement in color, and 68% in diminishing height of scars.14 However, silicone gel and sheeting may be most successful when applied to a wound before a keloid has formed, as a preventive measure for keloid-prone patients.14 Occlusive dressings are a relatively benign treatment because silicone rubber is inert, but adverse effects may include skin breakdown, rash, or pruritus that may require discontinuation of therapy for a few days or more.
Combination regimens may be most effective
If a keloid remains unresponsive to first-line therapies, combined therapies may reduce keloid size and prevent recurrence. Select regimens according to keloid characteristics and patient preferences.17 Corticosteroids used after surgical excision can produce cure rates exceeding 80%, making this a consistently successful management regimen for keloids and the standard of care in many primary care practices.3
Surgery and pressure are the preferred combination for earlobe keloids, as adverse effects are minimal and compliant patients may achieve response rates exceeding 80%.3,5
Surgery followed by immediate radiation reportedly has a response rate of 65% or higher.18 This regimen may be most successful in low-tension body areas, like the neck or lower limbs, where keloids are less likely to recur.18
A combination of surgery and occlusive dressings is also promising. Early studies have had recurrence-free rates of more than 80% when patients applied the dressings for up to 24 hours daily over 4 to 6 months.6
Emerging therapies that require further testing
Many promising treatments lack sufficiently rigorous evidence of efficacy.
- Intralesional calcium channel blockers, which depolymerize actin19 and inhibit protein synthesis,5 have shown promising results in nonrandomized early clinical trials.19
- Ultraviolet light is a potentially successful noninvasive method.
- Topical retinoids (vitamin A derivatives) may be effective but must be applied twice daily for several months to reduce collagen metabolism by fibroblasts5; they may also cause photosensitivity and skin irritation.7
- Intralesional fluorouracil (5-FU) (as an antimetabolite) successfully reduced keloid size after one year of treatment in a few small trials.5,20
- Oral lathyrogen, such as penicillamine with colchicine, can interfere with collagen cross-linking; in a small case series there was no keloid recurrence after treatment.5
- Topical imiquimod (Aldara) cream, an immune response modifier, showed promise in a few case reports.5,21
- Laser procedures are less likely than scalpel procedures to produce keloid scars and have been reported to successfully improve scar color, size, and texture, although the risk of hyperpigmentation ranges from 1% to 24%.2,3,22 (Lasers induce ischemia through blood vessel destruction and cause decreased fibroblast production and histamine release.23,24) Red flat scars (which triamcinolone injections may cause) can be lightened with yellow light laser, which has been used in the treatment of capillary malformations.25
Other methods may produce suboptimal results. Cryotherapy causes ischemic damage that reduces tumor bulk at least temporarily, but is inappropriate for darker skin due to a high risk of persistent hypopigmentation.1,26,27 Intralesional antifibrotic cytokines, such as interferon, reduce collagen synthesis in vitro,1 but lead to considerable systemic adverse effects including headache, myalgias, and influenza-like symptoms.3,5 Skin grafting is inappropriate as it may lead to keloid wave formation at the graft edges.
CORRESPONDENCE
Stephen P. Daane, MD, 2186 Geary Boulevard #212, San Francisco, CA 94115; [email protected]
1. Shaffer JJ, Taylor SC, Cook-Bolden F. Keloid scars: a review with a critical look at therapeutic options. J Am Acad Dermatol. 2002;46(suppl):S63-S97.
2. Mutoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560-571.
3. Niessen FB, Pauwen PH, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435-1458.
4. Zhang G, Jiang J, Luo S, et al. Analyses of CDC2L1 gene mutations in keloid tissue. Clin Exp Dermatol. 2012;37:277-283.
5. Al Attar A, Mess S, Thomassen JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117:286-300.
6. Yang GP, Lim IJ, Phan TT, et al. From scarless fetal wounds to keloids: molecular studies in wound healing. Wound Repair Regen. 2003;11:411-418.
7. Lim IJ, Phan TT, Bay BH, et al. Fibroblast cocultured with keloid keratinocytes: normal fibroblasts secrete collagen in a keloid like manner. Am J Physiol Cell Physiol. 2002;283:C212-C222.
8. Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses. 2008;71:493-500.
9. Davidson S, Aziz N, Rashid R, et al. A primary care perspective on keloids. Medscape J Med. 2009;11:18 [Epub].-Available at: http://www.medscape.com/viewarticle/582445. Accessed March 8, 2013.
10. Ketchum LD, Smith J, Robinson DW, et al. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg. 1996;38:209-218.
11. Cosman B, Crikelair GF, Ju MC, et al. The surgical treatment of keloidal scars. Plast Reconstr Surg. 1961;27:335-358.
12. Eishi K, Bae SJ, Ogawa F, et al. Silicone gel sheets relieve pain and pruritus with clinical improvement of keloid: possible target mast cells. J Dermatol Treat. 2003;14:248-252.
13. Malaker K, Vijayraghavan K, Hodson I, et al. Retrospective analysis of treatment of unresectable keloids with primary radiation over 25 years. Clin Oncol. 2004;26:290-298.
14. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic and keloid scars. J Cutan Aesthet Surg. 2009;2:104-106.
15. Botwood N, Lewanski C, Lowdell C. The risks of treating keloids with radiotherapy. Br J Radiol. 1999;72:1222-1224.
16. O’Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2006;(1):CD003826.-
17. Juckett G, Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician. 2009;80:253-260.
18. Ogawa R, Mitsuhasi K, Hyakusoku H, et al. Postoperative electron beam irradiation therapy for keloid scars. Plast Reconstr Surg. 2003;111:547-555.
19. D’Andrea F, Brongo S, Ferrano G, et al. Prevention and treatment of keloids with intralesional verapamil. Dermatology. 2002;204:60-62.
20. Kontochristopoulus G, Stefanaki C, Panagiotopoulos A, et al. Intralesional 5-fluorouracil in the treatment of keloids: an open clinical and histopathological study. J Am Acad Dermatol. 2005;52:474-479.
21. Berman B, Kaufman J. Pilot study of the effect of postoperative imiquimod 5% cream on the recurrence rate of excised keloids. J Am Acad Dermatol. 2002;47:2209-2211.
22. Fickerstrand EJ, Svaasand LO, Volden G. Pigmentary changes after pulsed dye laser treatment in 125 northern European patients with port wine stains. Br J Dermatol. 1998;138:477-479.
23. Alster TS. Laser treatment of hypertrophic scars, keloids and striae. Dermatol Clin. 1997;15:419-429.
24. Alster TS. Laser scar revision: comparison study of 585nm pulsed dye laser with and without intralesional corticosteroids. Dermatol Surg. 2003;29:25-29.
25. Astner S, Anderson RR. Treating vascular lesions. Dermatol Ther. 2005;18:267-281.
26. Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg. 2003;111:1841-1852.
27. Williams C, De Groote S. What treatment is best for hypertrophic scars and keloids? J Fam Pract. 2011;60:757-758.
• Consider using corticosteroid injections to inhibit collagen synthesis and stimulate enzymatic degradation of existing keloid collagen. B
• Turn to treatment combinations for refractory keloids; regimens may include corticosteroid injections, surgical excision, pressure, occlusive dressings, or radiation. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Keloids are an ongoing clinical challenge. Despite the availability of multiple treatments, choosing an effective therapeutic regimen for these fibroproliferative scars can be vexing, as keloids usually recur.
This review provides a guide to determining which regimen is best for your patient. But first, a word about the causes of these claw-like growths and which patients are at highest risk of developing them.
Even superficial injuries can cause keloids
Keloids arise after a disruption of skin integrity following superficial or deep injuries (FIGURES 1A-C). Causes include physical trauma such as cuts, scratches, and insect bites; iatrogenic trauma as in vaccinations or surgical procedures; thermal or chemical burns; and skin eruptions such as chicken pox.
FIGURE 1
Keloids induced by scratches (A), ear piercing (B), and thermal burns (C)
Common sites of keloid development include the ears, jaw, neck, clavicle and sternum, and shoulders. Less commonly, keloids occur on the back, abdomen, and extremities. Rarely, keloids develop on the palms and soles, face, or mucous membranes.1
A minor keloid is smaller than 0.5 cm, while a major keloid is larger. No upper growth limit has been identified, as keloids seem to enlarge indefinitely.2 Keloid scars have a firm and inflexible texture, a shiny appearance, and are elevated above skin level. They are usually flesh-colored, but may be erythematous or hyperpigmented.1 (A similar aberrant wound healing condition often confused with keloids is hypertrophic scarring. See “Differentiating keloids from hypertrophic scars”).
Distinguishing between keloids and hypertrophic scars can be challenging, because both types of scars arise histologically from excessive collagen buildup during the wound healing process. However, there are 3 distinctive characteristics of keloids that hypertrophic scars lack:
- Keloids enlarge beyond wound margins, often with irregular shapes, while hypertrophic scars remain within the confines of the original wound and tend to be linear along scars.
- Keloids do not regress on their own; hypertrophic scars tend to regress spontaneously within a few years.
- Keloids may take months to years to develop, while hypertrophic scars usually appear within 4 to 6 weeks of a traumatic incident.
Source: Gauglitz G, Korting H, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113-125.
Although keloids have an aggressive growth pattern, they are considered benign tumors, because the development of malignant cells in a keloid is rare.1 Most patients seek medical attention for cosmetic concerns. However, tissue bulk can cause functional problems, and some keloids are symptomatic with pruritus (27%) or pain (19%).1,3
Who is affected?
The most consistent risk factor for keloid development is a previous keloid. Individuals with deeply pigmented skin appear to be at higher risk, with an estimated prevalence of 4% to 16% among individuals of African, Asian, and Hispanic ancestry.3 Although the rate of keloid occurrence among Caucasians has not been established, keloids have been reported in individuals of nearly every skin color, except albinos.3 Keloids occur in both sexes with similar frequency, and are most likely to develop during puberty and early adulthood—between the ages of 10 and 30 years—but can occur at any age.1,3
Although no clear familial inheritance pattern has been identified, keloids are associated with blood type A and human leukocyte antigens (HLA)-B14, -B21, -BW35, -DR5, -DRB1, -DQA1, -DQB1, and -DQW3.3,4 Emerging research indicates that mutations of the CDC2L1 gene—which encodes a protein kinase essential to cell cycle control—correlate with keloid formation.4
Wound healing gone awry
Normal wound healing involves an overlapping series of processes, including hemostasis, inflammation, and granulation and remodeling. Various mechanisms have been identified for keloid pathology, although no single definitive trigger is known.
Hemostasis. In normal hemostasis, platelets aggregate around a fibrin clot in a wound, stimulating the release of growth factors,3 which precipitate the migration of fibroblasts to the granulation-tissue scaffolding for wound healing. Keloids have increased fibroblast proliferation, with up to 20-fold increased production of Type 1 collagen.1
Inflammation. During the inflammation phase, cytokine cascades stimulate cell proliferation from inflammatory, endothelial, epithelial, and fibroblast cell lines. Keloids have altered levels of many cytokines, resulting in increased inflammatory activity, such as histamine release by mast cells.3
Granulation and remodeling. During normal granulation and remodeling, fibroblasts aggregate and produce extracellular matrix components. The keloid fibroblasts produce higher levels of collagen, elastin, fibronectin, and proteoglycans, but lower levels of hyaluronic acid.3,5 Keloid fibroblasts also make lower levels of tissue plasminogen activator inhibitor (TPA-I), with inferior breakdown of scaffold structures and collagens. While collagen bundles run parallel to the epithelial surface in normal skin, keloid collagen fibers are larger, thicker, and randomly oriented in dense sheets, swirls, or nodules.1
In normal tissue, wound healing stops after the wound has been fully epithelialized by keratinocytes, after which the scar tissue contracts to minimal size. However, keloid growth can continue for years.1
Numerous studies have inspired various hypotheses for keloid formation. Studies demonstrating that keloids contain an increased level of immunoglobulins suggest that they may be produced by an abnormal immune reaction. Microscopic examinations of keloids have shown that overabundant endothelial cells occlude microvessels, suggesting that keloids may occur in the context of wound hypoxia. In vitro studies showing that fibroblasts proliferate when cultured with keloidal keratinocytes suggest that keloids may be a result of abnormal epithelial–mesenchymal interactions.6,7 A hypothesis based on the body sites where keloids are observed is that mechanical stretch and tension across a wound may overstimulate collagen production, possibly as a result of mechanoreceptor damage or disorders.8
Treatment: Weighing the options
Due to the complex mechanisms of pathogenesis, many modalities are available for managing keloids, yet no definitive treatment protocols exist. Intradermal, extradermal, or systemic therapies may be used singly, although combination regimens are the most effective in treating keloids and preventing recurrences.5 The TABLE1,3,5,9-14 provides an overview of monotherapeutic treatment options, including efficacy and risks.
TABLE
Treatment options for keloids
| Treatment | Anticipated effects | Dosing and frequency | Efficacy in reducing keloid size | Adverse effects | SOR |
|---|---|---|---|---|---|
| Hygienic relief: washing & drying | Relief of pruritus, pain, general discomfort | As needed | N/A | Minimal | C |
| Antihistamines | Relief of pruritus, pain, burning sensation9 | As needed | N/A | Minimal | C |
| Corticosteroid injections | Reduction in keloid size, pruritus10 | Injections given every 4-8 wk for several months10 | >80% report moderate to marked regression10 | Local or systemic infection and allergy or anaphylaxis10 | B |
| Surgical excision | Temporary reduction in keloid size11 | Not successful as monotherapy; combine with alternative therapies as needed11 | Temporary; keloids always recur after excision11 | Chance of infection, excessive bleeding, or injury to adjacent tissues11 | C |
| Pressure therapy | Reduction in keloid size3,12 | Pressure of 25-40 mm Hg for 23-24 h/d over several months3,12 | 60%-80% of patients report at least partial improvement3 | Minimal; rarely, skin thinning and redness3,12 | B |
| Radiation therapy | Reduction in keloid size3 | 10-20 Gy fractionated over several weeks5 | Up to 94% of patients report improvement, but recurrence is common3 | Minimal; theoretical risk of malignancy13 | B |
| Silicone occlusive dressings | Reduction in pain, pruritus, and keloid size1,12,14 | Applied topically for 12-24 h/d for 18 mo1,12,14 | 68%-86% of patients report improvement in keloid texture, color, and size14; may be useful as a preventive measure14 | Skin breakdown, rash, pruritus12 | B |
| N/A, not applicable; SOR, strength of recommendation. | |||||
Offer symptomatic therapy routinely
Some patients find symptomatic relief through hygienic measures, such as regular washing and drying. Antihistamines can relieve symptoms of burning or pruritus,9 but are not expected to reduce keloid size.
Use corticosteroids for first-line treatment
Corticosteroids are the most widely used therapy for keloids.3 They decrease keloid bulking by inhibiting collagen synthesis1 and stimulate tissue collagenases and collagen degradation.5 Commonly used in the office setting are intralesional injections of triamcinolone acetonide, a potent anti-inflammatory and highly atrophogenic agent.
Administer injections every 4 to 8 weeks until clinical improvement (usually requiring several months) or prohibitive adverse effects occur. A general dosing guideline is about 0.1 to 0.2 mL of corticosteroid for every square centimeter of keloid tissue. Injecting higher doses can lead to accretions or deposits beneath the skin that might need to be unroofed. Additionally, excess corticosteroid can cause an atrophic contour deformity in the adjacent subcutaneous tissue. FIGURE 2 details steroid injection procedures and follow-up based on the author’s (SPD) experience.
FIGURE 2
A 5-step procedure for injecting keloids with corticosteroids*
*Based on the author’s (SPD) experience.
Contraindications to injections include local or systemic infection and known allergy or anaphylactic reaction to corticosteroids. Although injecting keloids can be difficult initially, the tissue tends to soften and become easier to inject with each successive treatment. Some clinicians advocate cryotherapy to soften the keloid before giving injections. Large trials, in which more than 80% of patients had moderate to marked keloid regression,10 have demonstrated the efficacy of this technique in reducing keloid size and itching.
Surgery alone is ineffective
The oldest remedy, simple scalpel excision of keloidal tissue, invariably results in regrowth, because the aberrant wound healing process is not remedied.11 Although surgical excision is a poor monotherapy, it may be useful in combination with other modalities. Repairs using meticulous atraumatic surgical techniques with minimal wound tension can lessen the risk of keloid recurrence. Absorbable monofilament sutures with a subcuticular stitch help to minimize further epidermal damage.6
Pressure is useful in certain cases
Applying pressure is a noninvasive method that produces initial tissue thinning and pliability, with 60% to 80% of patients reporting at least partial improvement.3 Pressure reduces keloids by decreasing tissue metabolism, so long-term therapy is required, with application of pressure 23 to 24 hours daily over several months.12 Pressure of 25 to 40 mm Hg is needed to exceed capillary pressure without damaging peripheral circulation.3
Custom pressure garments for burn patients have been used in specialized cases involving limbs and torso, face and neck, and hands and feet. For earlobe keloids, clip-on earring devices are the only practical solution. Compliance tends to wane after months of therapy, and cessation may be followed by rebound hypertrophy. The therapeutic effect of regular pressure massage has been poorly studied. Because pressure has minimal adverse effects, it is likely most useful as an adjunct therapy where practical.
Radiation has variable effectiveness
Radiation damages fibroblasts, inhibits proliferation, and may improve local oxygenation.13 Low megavolt electron beam radiation is used to limit depth of penetration, usually with a total dose of 10 to 20 Gy fractionated over several weeks.5 A dose-response effect is likely.3 Response rates vary from 10% to 94%,3 and keloid recurrence is common, making radiation a poor choice for primary treatment except in cases of a large-volume unresectable keloid (eg, post-burn keloids).13
Adverse effects can include nodule formation, hyperpigmentation, ulceration, pruritus, paresthesia, and wound dehiscence. Theoretical risks of malignancy with radiation treatment of keloids have not been borne out in large studies, which have shown a zero percent malignancy rate despite rare anecdotal reports.15 Nevertheless, radiation therapy for benign disease is not recommended for children or pregnant women, or in breast, thyroid, or other cancer-prone body sites.5
Occlusive dressings require patient compliance
An innovation in keloid treatment is the use of occlusive dressings, which started in 1981 and gained popularity in the 1990s.16 Gel, fluid, or rubbery sheeting, usually made from silicone, is applied topically for 12 to 24 hours daily for up to 18 months.1,12,14 Gel may be practical along creases or in areas of motion where silicone sheets are obtrusive. Because long-term therapy is recommended, occlusive dressings require active patient participation. Occlusive dressings can decrease pruritic symptoms by decreasing mast cell activity,12 and may do so by warmth, hydration, or occlusion effects.3
Although study methodology has been suboptimal and further research is required, some practitioners have reported efficacy of 86% for texture reduction, 84% improvement in color, and 68% in diminishing height of scars.14 However, silicone gel and sheeting may be most successful when applied to a wound before a keloid has formed, as a preventive measure for keloid-prone patients.14 Occlusive dressings are a relatively benign treatment because silicone rubber is inert, but adverse effects may include skin breakdown, rash, or pruritus that may require discontinuation of therapy for a few days or more.
Combination regimens may be most effective
If a keloid remains unresponsive to first-line therapies, combined therapies may reduce keloid size and prevent recurrence. Select regimens according to keloid characteristics and patient preferences.17 Corticosteroids used after surgical excision can produce cure rates exceeding 80%, making this a consistently successful management regimen for keloids and the standard of care in many primary care practices.3
Surgery and pressure are the preferred combination for earlobe keloids, as adverse effects are minimal and compliant patients may achieve response rates exceeding 80%.3,5
Surgery followed by immediate radiation reportedly has a response rate of 65% or higher.18 This regimen may be most successful in low-tension body areas, like the neck or lower limbs, where keloids are less likely to recur.18
A combination of surgery and occlusive dressings is also promising. Early studies have had recurrence-free rates of more than 80% when patients applied the dressings for up to 24 hours daily over 4 to 6 months.6
Emerging therapies that require further testing
Many promising treatments lack sufficiently rigorous evidence of efficacy.
- Intralesional calcium channel blockers, which depolymerize actin19 and inhibit protein synthesis,5 have shown promising results in nonrandomized early clinical trials.19
- Ultraviolet light is a potentially successful noninvasive method.
- Topical retinoids (vitamin A derivatives) may be effective but must be applied twice daily for several months to reduce collagen metabolism by fibroblasts5; they may also cause photosensitivity and skin irritation.7
- Intralesional fluorouracil (5-FU) (as an antimetabolite) successfully reduced keloid size after one year of treatment in a few small trials.5,20
- Oral lathyrogen, such as penicillamine with colchicine, can interfere with collagen cross-linking; in a small case series there was no keloid recurrence after treatment.5
- Topical imiquimod (Aldara) cream, an immune response modifier, showed promise in a few case reports.5,21
- Laser procedures are less likely than scalpel procedures to produce keloid scars and have been reported to successfully improve scar color, size, and texture, although the risk of hyperpigmentation ranges from 1% to 24%.2,3,22 (Lasers induce ischemia through blood vessel destruction and cause decreased fibroblast production and histamine release.23,24) Red flat scars (which triamcinolone injections may cause) can be lightened with yellow light laser, which has been used in the treatment of capillary malformations.25
Other methods may produce suboptimal results. Cryotherapy causes ischemic damage that reduces tumor bulk at least temporarily, but is inappropriate for darker skin due to a high risk of persistent hypopigmentation.1,26,27 Intralesional antifibrotic cytokines, such as interferon, reduce collagen synthesis in vitro,1 but lead to considerable systemic adverse effects including headache, myalgias, and influenza-like symptoms.3,5 Skin grafting is inappropriate as it may lead to keloid wave formation at the graft edges.
CORRESPONDENCE
Stephen P. Daane, MD, 2186 Geary Boulevard #212, San Francisco, CA 94115; [email protected]
• Consider using corticosteroid injections to inhibit collagen synthesis and stimulate enzymatic degradation of existing keloid collagen. B
• Turn to treatment combinations for refractory keloids; regimens may include corticosteroid injections, surgical excision, pressure, occlusive dressings, or radiation. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Keloids are an ongoing clinical challenge. Despite the availability of multiple treatments, choosing an effective therapeutic regimen for these fibroproliferative scars can be vexing, as keloids usually recur.
This review provides a guide to determining which regimen is best for your patient. But first, a word about the causes of these claw-like growths and which patients are at highest risk of developing them.
Even superficial injuries can cause keloids
Keloids arise after a disruption of skin integrity following superficial or deep injuries (FIGURES 1A-C). Causes include physical trauma such as cuts, scratches, and insect bites; iatrogenic trauma as in vaccinations or surgical procedures; thermal or chemical burns; and skin eruptions such as chicken pox.
FIGURE 1
Keloids induced by scratches (A), ear piercing (B), and thermal burns (C)
Common sites of keloid development include the ears, jaw, neck, clavicle and sternum, and shoulders. Less commonly, keloids occur on the back, abdomen, and extremities. Rarely, keloids develop on the palms and soles, face, or mucous membranes.1
A minor keloid is smaller than 0.5 cm, while a major keloid is larger. No upper growth limit has been identified, as keloids seem to enlarge indefinitely.2 Keloid scars have a firm and inflexible texture, a shiny appearance, and are elevated above skin level. They are usually flesh-colored, but may be erythematous or hyperpigmented.1 (A similar aberrant wound healing condition often confused with keloids is hypertrophic scarring. See “Differentiating keloids from hypertrophic scars”).
Distinguishing between keloids and hypertrophic scars can be challenging, because both types of scars arise histologically from excessive collagen buildup during the wound healing process. However, there are 3 distinctive characteristics of keloids that hypertrophic scars lack:
- Keloids enlarge beyond wound margins, often with irregular shapes, while hypertrophic scars remain within the confines of the original wound and tend to be linear along scars.
- Keloids do not regress on their own; hypertrophic scars tend to regress spontaneously within a few years.
- Keloids may take months to years to develop, while hypertrophic scars usually appear within 4 to 6 weeks of a traumatic incident.
Source: Gauglitz G, Korting H, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113-125.
Although keloids have an aggressive growth pattern, they are considered benign tumors, because the development of malignant cells in a keloid is rare.1 Most patients seek medical attention for cosmetic concerns. However, tissue bulk can cause functional problems, and some keloids are symptomatic with pruritus (27%) or pain (19%).1,3
Who is affected?
The most consistent risk factor for keloid development is a previous keloid. Individuals with deeply pigmented skin appear to be at higher risk, with an estimated prevalence of 4% to 16% among individuals of African, Asian, and Hispanic ancestry.3 Although the rate of keloid occurrence among Caucasians has not been established, keloids have been reported in individuals of nearly every skin color, except albinos.3 Keloids occur in both sexes with similar frequency, and are most likely to develop during puberty and early adulthood—between the ages of 10 and 30 years—but can occur at any age.1,3
Although no clear familial inheritance pattern has been identified, keloids are associated with blood type A and human leukocyte antigens (HLA)-B14, -B21, -BW35, -DR5, -DRB1, -DQA1, -DQB1, and -DQW3.3,4 Emerging research indicates that mutations of the CDC2L1 gene—which encodes a protein kinase essential to cell cycle control—correlate with keloid formation.4
Wound healing gone awry
Normal wound healing involves an overlapping series of processes, including hemostasis, inflammation, and granulation and remodeling. Various mechanisms have been identified for keloid pathology, although no single definitive trigger is known.
Hemostasis. In normal hemostasis, platelets aggregate around a fibrin clot in a wound, stimulating the release of growth factors,3 which precipitate the migration of fibroblasts to the granulation-tissue scaffolding for wound healing. Keloids have increased fibroblast proliferation, with up to 20-fold increased production of Type 1 collagen.1
Inflammation. During the inflammation phase, cytokine cascades stimulate cell proliferation from inflammatory, endothelial, epithelial, and fibroblast cell lines. Keloids have altered levels of many cytokines, resulting in increased inflammatory activity, such as histamine release by mast cells.3
Granulation and remodeling. During normal granulation and remodeling, fibroblasts aggregate and produce extracellular matrix components. The keloid fibroblasts produce higher levels of collagen, elastin, fibronectin, and proteoglycans, but lower levels of hyaluronic acid.3,5 Keloid fibroblasts also make lower levels of tissue plasminogen activator inhibitor (TPA-I), with inferior breakdown of scaffold structures and collagens. While collagen bundles run parallel to the epithelial surface in normal skin, keloid collagen fibers are larger, thicker, and randomly oriented in dense sheets, swirls, or nodules.1
In normal tissue, wound healing stops after the wound has been fully epithelialized by keratinocytes, after which the scar tissue contracts to minimal size. However, keloid growth can continue for years.1
Numerous studies have inspired various hypotheses for keloid formation. Studies demonstrating that keloids contain an increased level of immunoglobulins suggest that they may be produced by an abnormal immune reaction. Microscopic examinations of keloids have shown that overabundant endothelial cells occlude microvessels, suggesting that keloids may occur in the context of wound hypoxia. In vitro studies showing that fibroblasts proliferate when cultured with keloidal keratinocytes suggest that keloids may be a result of abnormal epithelial–mesenchymal interactions.6,7 A hypothesis based on the body sites where keloids are observed is that mechanical stretch and tension across a wound may overstimulate collagen production, possibly as a result of mechanoreceptor damage or disorders.8
Treatment: Weighing the options
Due to the complex mechanisms of pathogenesis, many modalities are available for managing keloids, yet no definitive treatment protocols exist. Intradermal, extradermal, or systemic therapies may be used singly, although combination regimens are the most effective in treating keloids and preventing recurrences.5 The TABLE1,3,5,9-14 provides an overview of monotherapeutic treatment options, including efficacy and risks.
TABLE
Treatment options for keloids
| Treatment | Anticipated effects | Dosing and frequency | Efficacy in reducing keloid size | Adverse effects | SOR |
|---|---|---|---|---|---|
| Hygienic relief: washing & drying | Relief of pruritus, pain, general discomfort | As needed | N/A | Minimal | C |
| Antihistamines | Relief of pruritus, pain, burning sensation9 | As needed | N/A | Minimal | C |
| Corticosteroid injections | Reduction in keloid size, pruritus10 | Injections given every 4-8 wk for several months10 | >80% report moderate to marked regression10 | Local or systemic infection and allergy or anaphylaxis10 | B |
| Surgical excision | Temporary reduction in keloid size11 | Not successful as monotherapy; combine with alternative therapies as needed11 | Temporary; keloids always recur after excision11 | Chance of infection, excessive bleeding, or injury to adjacent tissues11 | C |
| Pressure therapy | Reduction in keloid size3,12 | Pressure of 25-40 mm Hg for 23-24 h/d over several months3,12 | 60%-80% of patients report at least partial improvement3 | Minimal; rarely, skin thinning and redness3,12 | B |
| Radiation therapy | Reduction in keloid size3 | 10-20 Gy fractionated over several weeks5 | Up to 94% of patients report improvement, but recurrence is common3 | Minimal; theoretical risk of malignancy13 | B |
| Silicone occlusive dressings | Reduction in pain, pruritus, and keloid size1,12,14 | Applied topically for 12-24 h/d for 18 mo1,12,14 | 68%-86% of patients report improvement in keloid texture, color, and size14; may be useful as a preventive measure14 | Skin breakdown, rash, pruritus12 | B |
| N/A, not applicable; SOR, strength of recommendation. | |||||
Offer symptomatic therapy routinely
Some patients find symptomatic relief through hygienic measures, such as regular washing and drying. Antihistamines can relieve symptoms of burning or pruritus,9 but are not expected to reduce keloid size.
Use corticosteroids for first-line treatment
Corticosteroids are the most widely used therapy for keloids.3 They decrease keloid bulking by inhibiting collagen synthesis1 and stimulate tissue collagenases and collagen degradation.5 Commonly used in the office setting are intralesional injections of triamcinolone acetonide, a potent anti-inflammatory and highly atrophogenic agent.
Administer injections every 4 to 8 weeks until clinical improvement (usually requiring several months) or prohibitive adverse effects occur. A general dosing guideline is about 0.1 to 0.2 mL of corticosteroid for every square centimeter of keloid tissue. Injecting higher doses can lead to accretions or deposits beneath the skin that might need to be unroofed. Additionally, excess corticosteroid can cause an atrophic contour deformity in the adjacent subcutaneous tissue. FIGURE 2 details steroid injection procedures and follow-up based on the author’s (SPD) experience.
FIGURE 2
A 5-step procedure for injecting keloids with corticosteroids*
*Based on the author’s (SPD) experience.
Contraindications to injections include local or systemic infection and known allergy or anaphylactic reaction to corticosteroids. Although injecting keloids can be difficult initially, the tissue tends to soften and become easier to inject with each successive treatment. Some clinicians advocate cryotherapy to soften the keloid before giving injections. Large trials, in which more than 80% of patients had moderate to marked keloid regression,10 have demonstrated the efficacy of this technique in reducing keloid size and itching.
Surgery alone is ineffective
The oldest remedy, simple scalpel excision of keloidal tissue, invariably results in regrowth, because the aberrant wound healing process is not remedied.11 Although surgical excision is a poor monotherapy, it may be useful in combination with other modalities. Repairs using meticulous atraumatic surgical techniques with minimal wound tension can lessen the risk of keloid recurrence. Absorbable monofilament sutures with a subcuticular stitch help to minimize further epidermal damage.6
Pressure is useful in certain cases
Applying pressure is a noninvasive method that produces initial tissue thinning and pliability, with 60% to 80% of patients reporting at least partial improvement.3 Pressure reduces keloids by decreasing tissue metabolism, so long-term therapy is required, with application of pressure 23 to 24 hours daily over several months.12 Pressure of 25 to 40 mm Hg is needed to exceed capillary pressure without damaging peripheral circulation.3
Custom pressure garments for burn patients have been used in specialized cases involving limbs and torso, face and neck, and hands and feet. For earlobe keloids, clip-on earring devices are the only practical solution. Compliance tends to wane after months of therapy, and cessation may be followed by rebound hypertrophy. The therapeutic effect of regular pressure massage has been poorly studied. Because pressure has minimal adverse effects, it is likely most useful as an adjunct therapy where practical.
Radiation has variable effectiveness
Radiation damages fibroblasts, inhibits proliferation, and may improve local oxygenation.13 Low megavolt electron beam radiation is used to limit depth of penetration, usually with a total dose of 10 to 20 Gy fractionated over several weeks.5 A dose-response effect is likely.3 Response rates vary from 10% to 94%,3 and keloid recurrence is common, making radiation a poor choice for primary treatment except in cases of a large-volume unresectable keloid (eg, post-burn keloids).13
Adverse effects can include nodule formation, hyperpigmentation, ulceration, pruritus, paresthesia, and wound dehiscence. Theoretical risks of malignancy with radiation treatment of keloids have not been borne out in large studies, which have shown a zero percent malignancy rate despite rare anecdotal reports.15 Nevertheless, radiation therapy for benign disease is not recommended for children or pregnant women, or in breast, thyroid, or other cancer-prone body sites.5
Occlusive dressings require patient compliance
An innovation in keloid treatment is the use of occlusive dressings, which started in 1981 and gained popularity in the 1990s.16 Gel, fluid, or rubbery sheeting, usually made from silicone, is applied topically for 12 to 24 hours daily for up to 18 months.1,12,14 Gel may be practical along creases or in areas of motion where silicone sheets are obtrusive. Because long-term therapy is recommended, occlusive dressings require active patient participation. Occlusive dressings can decrease pruritic symptoms by decreasing mast cell activity,12 and may do so by warmth, hydration, or occlusion effects.3
Although study methodology has been suboptimal and further research is required, some practitioners have reported efficacy of 86% for texture reduction, 84% improvement in color, and 68% in diminishing height of scars.14 However, silicone gel and sheeting may be most successful when applied to a wound before a keloid has formed, as a preventive measure for keloid-prone patients.14 Occlusive dressings are a relatively benign treatment because silicone rubber is inert, but adverse effects may include skin breakdown, rash, or pruritus that may require discontinuation of therapy for a few days or more.
Combination regimens may be most effective
If a keloid remains unresponsive to first-line therapies, combined therapies may reduce keloid size and prevent recurrence. Select regimens according to keloid characteristics and patient preferences.17 Corticosteroids used after surgical excision can produce cure rates exceeding 80%, making this a consistently successful management regimen for keloids and the standard of care in many primary care practices.3
Surgery and pressure are the preferred combination for earlobe keloids, as adverse effects are minimal and compliant patients may achieve response rates exceeding 80%.3,5
Surgery followed by immediate radiation reportedly has a response rate of 65% or higher.18 This regimen may be most successful in low-tension body areas, like the neck or lower limbs, where keloids are less likely to recur.18
A combination of surgery and occlusive dressings is also promising. Early studies have had recurrence-free rates of more than 80% when patients applied the dressings for up to 24 hours daily over 4 to 6 months.6
Emerging therapies that require further testing
Many promising treatments lack sufficiently rigorous evidence of efficacy.
- Intralesional calcium channel blockers, which depolymerize actin19 and inhibit protein synthesis,5 have shown promising results in nonrandomized early clinical trials.19
- Ultraviolet light is a potentially successful noninvasive method.
- Topical retinoids (vitamin A derivatives) may be effective but must be applied twice daily for several months to reduce collagen metabolism by fibroblasts5; they may also cause photosensitivity and skin irritation.7
- Intralesional fluorouracil (5-FU) (as an antimetabolite) successfully reduced keloid size after one year of treatment in a few small trials.5,20
- Oral lathyrogen, such as penicillamine with colchicine, can interfere with collagen cross-linking; in a small case series there was no keloid recurrence after treatment.5
- Topical imiquimod (Aldara) cream, an immune response modifier, showed promise in a few case reports.5,21
- Laser procedures are less likely than scalpel procedures to produce keloid scars and have been reported to successfully improve scar color, size, and texture, although the risk of hyperpigmentation ranges from 1% to 24%.2,3,22 (Lasers induce ischemia through blood vessel destruction and cause decreased fibroblast production and histamine release.23,24) Red flat scars (which triamcinolone injections may cause) can be lightened with yellow light laser, which has been used in the treatment of capillary malformations.25
Other methods may produce suboptimal results. Cryotherapy causes ischemic damage that reduces tumor bulk at least temporarily, but is inappropriate for darker skin due to a high risk of persistent hypopigmentation.1,26,27 Intralesional antifibrotic cytokines, such as interferon, reduce collagen synthesis in vitro,1 but lead to considerable systemic adverse effects including headache, myalgias, and influenza-like symptoms.3,5 Skin grafting is inappropriate as it may lead to keloid wave formation at the graft edges.
CORRESPONDENCE
Stephen P. Daane, MD, 2186 Geary Boulevard #212, San Francisco, CA 94115; [email protected]
1. Shaffer JJ, Taylor SC, Cook-Bolden F. Keloid scars: a review with a critical look at therapeutic options. J Am Acad Dermatol. 2002;46(suppl):S63-S97.
2. Mutoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560-571.
3. Niessen FB, Pauwen PH, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435-1458.
4. Zhang G, Jiang J, Luo S, et al. Analyses of CDC2L1 gene mutations in keloid tissue. Clin Exp Dermatol. 2012;37:277-283.
5. Al Attar A, Mess S, Thomassen JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117:286-300.
6. Yang GP, Lim IJ, Phan TT, et al. From scarless fetal wounds to keloids: molecular studies in wound healing. Wound Repair Regen. 2003;11:411-418.
7. Lim IJ, Phan TT, Bay BH, et al. Fibroblast cocultured with keloid keratinocytes: normal fibroblasts secrete collagen in a keloid like manner. Am J Physiol Cell Physiol. 2002;283:C212-C222.
8. Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses. 2008;71:493-500.
9. Davidson S, Aziz N, Rashid R, et al. A primary care perspective on keloids. Medscape J Med. 2009;11:18 [Epub].-Available at: http://www.medscape.com/viewarticle/582445. Accessed March 8, 2013.
10. Ketchum LD, Smith J, Robinson DW, et al. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg. 1996;38:209-218.
11. Cosman B, Crikelair GF, Ju MC, et al. The surgical treatment of keloidal scars. Plast Reconstr Surg. 1961;27:335-358.
12. Eishi K, Bae SJ, Ogawa F, et al. Silicone gel sheets relieve pain and pruritus with clinical improvement of keloid: possible target mast cells. J Dermatol Treat. 2003;14:248-252.
13. Malaker K, Vijayraghavan K, Hodson I, et al. Retrospective analysis of treatment of unresectable keloids with primary radiation over 25 years. Clin Oncol. 2004;26:290-298.
14. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic and keloid scars. J Cutan Aesthet Surg. 2009;2:104-106.
15. Botwood N, Lewanski C, Lowdell C. The risks of treating keloids with radiotherapy. Br J Radiol. 1999;72:1222-1224.
16. O’Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2006;(1):CD003826.-
17. Juckett G, Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician. 2009;80:253-260.
18. Ogawa R, Mitsuhasi K, Hyakusoku H, et al. Postoperative electron beam irradiation therapy for keloid scars. Plast Reconstr Surg. 2003;111:547-555.
19. D’Andrea F, Brongo S, Ferrano G, et al. Prevention and treatment of keloids with intralesional verapamil. Dermatology. 2002;204:60-62.
20. Kontochristopoulus G, Stefanaki C, Panagiotopoulos A, et al. Intralesional 5-fluorouracil in the treatment of keloids: an open clinical and histopathological study. J Am Acad Dermatol. 2005;52:474-479.
21. Berman B, Kaufman J. Pilot study of the effect of postoperative imiquimod 5% cream on the recurrence rate of excised keloids. J Am Acad Dermatol. 2002;47:2209-2211.
22. Fickerstrand EJ, Svaasand LO, Volden G. Pigmentary changes after pulsed dye laser treatment in 125 northern European patients with port wine stains. Br J Dermatol. 1998;138:477-479.
23. Alster TS. Laser treatment of hypertrophic scars, keloids and striae. Dermatol Clin. 1997;15:419-429.
24. Alster TS. Laser scar revision: comparison study of 585nm pulsed dye laser with and without intralesional corticosteroids. Dermatol Surg. 2003;29:25-29.
25. Astner S, Anderson RR. Treating vascular lesions. Dermatol Ther. 2005;18:267-281.
26. Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg. 2003;111:1841-1852.
27. Williams C, De Groote S. What treatment is best for hypertrophic scars and keloids? J Fam Pract. 2011;60:757-758.
1. Shaffer JJ, Taylor SC, Cook-Bolden F. Keloid scars: a review with a critical look at therapeutic options. J Am Acad Dermatol. 2002;46(suppl):S63-S97.
2. Mutoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560-571.
3. Niessen FB, Pauwen PH, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435-1458.
4. Zhang G, Jiang J, Luo S, et al. Analyses of CDC2L1 gene mutations in keloid tissue. Clin Exp Dermatol. 2012;37:277-283.
5. Al Attar A, Mess S, Thomassen JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117:286-300.
6. Yang GP, Lim IJ, Phan TT, et al. From scarless fetal wounds to keloids: molecular studies in wound healing. Wound Repair Regen. 2003;11:411-418.
7. Lim IJ, Phan TT, Bay BH, et al. Fibroblast cocultured with keloid keratinocytes: normal fibroblasts secrete collagen in a keloid like manner. Am J Physiol Cell Physiol. 2002;283:C212-C222.
8. Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses. 2008;71:493-500.
9. Davidson S, Aziz N, Rashid R, et al. A primary care perspective on keloids. Medscape J Med. 2009;11:18 [Epub].-Available at: http://www.medscape.com/viewarticle/582445. Accessed March 8, 2013.
10. Ketchum LD, Smith J, Robinson DW, et al. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg. 1996;38:209-218.
11. Cosman B, Crikelair GF, Ju MC, et al. The surgical treatment of keloidal scars. Plast Reconstr Surg. 1961;27:335-358.
12. Eishi K, Bae SJ, Ogawa F, et al. Silicone gel sheets relieve pain and pruritus with clinical improvement of keloid: possible target mast cells. J Dermatol Treat. 2003;14:248-252.
13. Malaker K, Vijayraghavan K, Hodson I, et al. Retrospective analysis of treatment of unresectable keloids with primary radiation over 25 years. Clin Oncol. 2004;26:290-298.
14. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic and keloid scars. J Cutan Aesthet Surg. 2009;2:104-106.
15. Botwood N, Lewanski C, Lowdell C. The risks of treating keloids with radiotherapy. Br J Radiol. 1999;72:1222-1224.
16. O’Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2006;(1):CD003826.-
17. Juckett G, Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician. 2009;80:253-260.
18. Ogawa R, Mitsuhasi K, Hyakusoku H, et al. Postoperative electron beam irradiation therapy for keloid scars. Plast Reconstr Surg. 2003;111:547-555.
19. D’Andrea F, Brongo S, Ferrano G, et al. Prevention and treatment of keloids with intralesional verapamil. Dermatology. 2002;204:60-62.
20. Kontochristopoulus G, Stefanaki C, Panagiotopoulos A, et al. Intralesional 5-fluorouracil in the treatment of keloids: an open clinical and histopathological study. J Am Acad Dermatol. 2005;52:474-479.
21. Berman B, Kaufman J. Pilot study of the effect of postoperative imiquimod 5% cream on the recurrence rate of excised keloids. J Am Acad Dermatol. 2002;47:2209-2211.
22. Fickerstrand EJ, Svaasand LO, Volden G. Pigmentary changes after pulsed dye laser treatment in 125 northern European patients with port wine stains. Br J Dermatol. 1998;138:477-479.
23. Alster TS. Laser treatment of hypertrophic scars, keloids and striae. Dermatol Clin. 1997;15:419-429.
24. Alster TS. Laser scar revision: comparison study of 585nm pulsed dye laser with and without intralesional corticosteroids. Dermatol Surg. 2003;29:25-29.
25. Astner S, Anderson RR. Treating vascular lesions. Dermatol Ther. 2005;18:267-281.
26. Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg. 2003;111:1841-1852.
27. Williams C, De Groote S. What treatment is best for hypertrophic scars and keloids? J Fam Pract. 2011;60:757-758.
Head off complications in late preterm infants
• Delay discharge of late preterm infants to a minimum of 48 hours to prevent readmission. B
• Perform transcutaneous or total serum bilirubin testing before discharging late preterm infants. C
• Perform a formal feeding assessment of breastfed infants prior to discharge. C
• Ensure that a follow-up appointment is made for 24 to 48 hours after discharge. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Laura M delivers her third baby at 35 weeks 4 days after an uneventful spontaneous labor. Julia, 5 lb 1 oz, has Apgar scores of 8 and 9. You see them on the mother-baby unit Friday afternoon, 36 hours after the delivery.
Ms. M has successfully breastfed her other 2 children and is planning to breastfeed Julia as well. She says the infant is nursing well today but seemed sleepy yesterday. The nursing notes say that Julia went to the nursery overnight because her mother was tired, and the infant spit up after receiving a formula bottle. Ms. M is asking you to discharge her because tomorrow is her son’s birthday. After considering this and her experience with breastfeeding, you decide to discharge her with follow-up on Monday instead of checking her out to your partner for the weekend.
Ms. M returns to your office on Monday with Julia, whose weight is down 10% and is “glowing yellow.” The baby is not latching well at the breast and is spitting up when Ms. M tries to supplement with formula. After examining Julia and checking lab work (bilirubin, 20 mg/dL), you decide to readmit her for feeding difficulties and hyperbilirubinemia.
Complications are common with late preterm infants, which refers to babies born between 34 weeks 0 days and 36 weeks 6 days of pregnancy. Between 1990 and 2006, there was a dramatic (25%) increase in the rate of late preterm infants in the United States, although more recently this number has leveled off.1
Several factors have been linked to increased late preterm births: maternal obesity, increased maternal age, and the increasing rate of multiple gestation pregnancies that have resulted from the expanded use of reproductive technology.2 Similarly, treatment of severe preeclampsia and premature rupture of membranes often includes delivery after 34 weeks of gestation,3 further contributing to the problem. Finally, higher rates of antenatal screening have contributed to more inductions and cesarean sections at earlier gestational ages. One study even found a correlation between higher malpractice premiums and more frequent late preterm inductions.4
Don’t let their appearance fool you
At first appearance, late preterm infants are similar to term infants in terms of Apgar scores,5 size, and weight.2 However, the care of these infants can be complex. They are often placed in well-infant nurseries under the same protocols as term infants and discharged before an adequate observation period. These infants have both increased short- and long-term morbidity and mortality and use a significant amount of health care resources.5 Morbidity in these infants decreases with each week of gestation from 34 weeks to a nadir at 39 weeks and can be unrelated to maternal and pregnancy complications.6
The following are some important issues to keep in mind when caring for these infants.
Hypothermia and hypoglycemia
Late preterm infants experience increased cold stress because of their limited fat stores, reduced brown fat, an immature epidermal barrier, and increased surface area to body mass ratio.2 Hypothermia increases the metabolic demands on the neonate and can worsen hypoglycemia as well as respiratory distress.7
Ideally, clinicians should dry these infants with warm blankets, place them skin-to-skin with their mother, and cover them with a warm blanket and cap to avoid excess energy expenditure.8 If conditions necessitate, neonates can be placed in a radiant warmer. Infants’ temperature needs to be monitored within the first 30 minutes of life and frequently reassessed during the first 12 hours of life—the “transition period.”8 The infant’s axillary temperature should be maintained between 36.5°C and 37.4°C (97.7-99.3°F); the temperature should remain stable in an open crib for the 12 hours before discharge.2
Decreased glycogen stores, increased glucose utilization, and immature hepatic enzymes help to explain the fact that hypoglycemia is 3 times more common in late preterm infants compared with full-term neonates.7 In all newborns, glucose levels decrease to their nadir between 30 and 90 minutes of life and normally trigger the breakdown of glycogen if the infant does not eat.7 Hypoglycemia can manifest as a change in level of consciousness, apnea, cyanosis, tachypnea, hypothermia, and seizures.9 The evidence is limited and there is controversy as to what level of hypoglycemia and over what duration of time is harmful.9
Most experts agree that breastfeeding should be started in the delivery room, and plasma glucose checked at 60 minutes of life and any time an infant is symptomatic. A level less than 45 mg/dL during the transition period should prompt feeding.9 Glucose levels should be rechecked within an hour of feeding and every 3 hours thereafter. If the level remains low or the infant is not interested in feeding, give a bolus of D10W and consider an infusion.7,10 TABLE 110 highlights one proposed method of glucose management.
TABLE 1
How best to manage glucose in a late preterm newborn10
| Asymptomatic late preterm infants | Glucose level (mg/dL) | Response |
|---|---|---|
| Check plasma glucose at 1 hour of life | <45 | Feed, recheck 1 h |
| Subsequent glucose monitoring | <35 | IV infusion* |
| >35 | Rescreen q3h for 36 h | |
| Symptomatic late preterm infants | ||
| Infusion for goal >55 mg/dL | <45 | Minibolus;† IV infusion* |
| *IV infusion=D10W at 6-8 mg/kg/min. †Minibolus=200 mg/kg D10W (2 mL/kg). Adapted with permission from: Macmillan Publishers Ltd. Adamkin DH. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29:S12-S17. Copyright 2009. | ||
Respiratory distress
At least 30% of late preterm infants will have some evidence of respiratory distress,5 which is defined as the need for oxygen supplementation due to tachypnea, grunting, nasal flaring, retractions, or cyanosis. Those at highest risk are white males born via cesarean section.11 Transient tachypnea of the newborn (TTN) appears to be the cause of almost half the cases of respiratory distress in these young patients.12 As this is a diagnosis by exclusion, consider other common causes: respiratory distress syndrome, neonatal pneumonia, meconium aspiration syndrome, and persistent pulmonary hypertension.12 The need for ventilator support is a significant concern in this group and increases exponentially with decreasing gestational age.6
Hyperbilirubinemia
More than half of all late preterm infants will present with clinically significant jaundice.5 Late preterm infants have an increased bilirubin load, decreased uptake of bilirubin, and delayed conjugation. They are less able to bind bilirubin to albumin, which increases their predisposition to bilirubin-induced neurological dysfunction and kernicterus.13 They also have more difficulty with breastfeeding, which exacerbates their hyperbilirubinemia.14
Infants born at 36 weeks have an 8-fold increased risk of developing severe hyperbilirubinemia (total serum bilirubin >20 mg/dL) than those born at 41 weeks,13 which explains why they are disproportionately represented in the US Pilot Kernicterus Registry.14 These infants can develop neurological sequelae at lower levels of bilirubin than their term counterparts and have less chance of complete recovery once intensive therapy has been implemented.14
The 2 main risk factors for severe hyperbilirubinemia have to do with discharge time and breastfeeding. Discharge at less than 48 hours is a risk factor for both term and late preterm infants.14,15 This is likely due to the fact that bilirubin peaks in late preterm infants between Days 5 and 7, and in term infants between Days 3 and 5.16 Difficulties with breastfeeding, the second risk factor,17 can be due to a combination of delayed maternal lactogenesis and ineffective milk removal on the part of the infant.18 When infants ingest smaller volumes of milk, bowel movements are infrequent and the bilirubin in the gut gets reabsorbed instead of excreted.
American Academy of Pediatrics (AAP) guidelines suggest measuring total serum bilirubin or transcutaneous bilirubin and plotting the value on an hour-specific, gestational-age-specific, risk-specific nomogram (FIGURE).19 Alternately, BiliTool (http://www.bilitool.org) can be used to individualize an infant’s risk.
FIGURE
Bilirubin levels and risk of significant hyperbilirubinemia19
Bilirubin levels should be plotted according to the infant’s hours of life and assessed according to risk factors on the appropriate risk line. If infants are 34 weeks 0 days to 34 weeks 6 days, they should be assessed on the high risk line.
- Use total bilirubin. Do not subtract direct reacting or conjugated bilirubin.
- Risk factors include isoimmune hemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis, or albumin <3.0 g/dL (if measured).
- For well infants 35-37 6/7 weeks, you can adjust the total serum bilirubin (TSB) levels for intervention around the medium risk line. It is an option to intervene at lower TSB levels for infants closer to 35 weeks and at higher TSB levels for those closer to 37 6/7 weeks.
- It is an option to provide conventional phototherapy in the hospital or at home at TSB levels of 2-3 mg/dL (35-50 mmol/L) below those shown, but home phototherapy should not be used in any infant with risk factors.
Reprinted with permission from: American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316. Copyright © 2004 by the American Academy of Pediatrics.
Feeding difficulties
The advantages of breast milk feeding for these babies are great. Unfortunately, it is breastfed infants who are more likely to have feeding difficulties and are at higher risk for readmission.17 Early feeding skills are complex and challenging. Because of their immaturity, late preterm infants have less effective suck and swallow coordination. They can be sleepier and have less stamina.10 Infants require coordinated oral motor movements, breathing, and swallowing to avoid desaturation and aspiration.20 It is also important to note that almost every reported case of kernicterus in the past 20 years has been in breastfed infants whose feeding was not well established.13 First-born infants are especially at risk, as it takes longer to establish an adequate supply of milk.
For late preterm infants, it is imperative to establish successful breastfeeding to avoid dehydration and jaundice. Lactation consultation, education, and close follow-up are essential to successful breastfeeding in this group.18 The AAP Committee on Fetus and Newborn recommends a formal breastfeeding evaluation by trained caregivers at least twice daily prior to discharge.2
Brain injury
Late gestation is a critical period of brain growth. The 34-week-old brain weighs 65% of the term brain and increases linearly with each week. Fifty percent of the increase in cortical volume happens after 34 weeks.21 The risk of intraventricular hemorrhage and periventricular leukomalacia, while common in earlier preemies, is rare in infants born after 34 weeks. However, there is evidence to suggest other complications. Late preterm infants are more likely to be diagnosed with developmental delay and require special resources in preschool and less likely to be ready for school.22
Sepsis
While late preterm neonates do have a small but significant increase in culture-proven sepsis and pneumonia compared with term babies,6 the work-up for possible sepsis is 3 times more likely. When these infants have poor feeding, mild respiratory distress, or TTN, physicians become concerned about sepsis and initiate a work-up.5 Currently there are no management guidelines for sepsis evaluation in this subset of preterm infants.23
Readmission is a distinct possibility
One study of healthy late preterm infants showed a readmission rate of 4.8%. The most common reasons for readmission were jaundice and infection.17 Risk factors for readmission were breastfeeding, primiparity, labor and delivery complications, public payer source at delivery, and a mother of Asian/Pacific Islander ethnicity.17 Another study showed that discharge at less than 48 hours significantly increased the likelihood of readmission, even more so if the infant was breastfeeding.15
Several recent studies have highlighted a relationship between decreasing gestational age and a wide range of long-term adverse outcomes. In the early years of childhood, there is an increased risk for developmental delay and decreased kindergarten readiness.22 There is also a significant risk for disability, including cerebral palsy, mental retardation, and behavioral disorders.
Late preterm infants are at greater risk for several complications and the mortality rate is high in this group, when compared with term infants. By initial appearance and even weight, they rival their term counterparts. However, while they may look much like term babies and not weigh much less, they need more intense monitoring and should meet stringent discharge criteria (TABLE 22).
TABLE 2
Minimum discharge criteria for late preterm infants2
All criteria should be met prior to discharge.
|
| Adapted with permission from: Engle WA, Tomashek KM, Wallman C, Committee on Fetus and Newborn. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390-1401. Copyright © 2007 by the American Academy of Pediatrics. |
Clearly, more studies need to be done to address the unique needs and specific treatment guidelines for these infants. In fact, hospitals may need to consider introducing neonatal observation nurseries with protocols specifically tailored for late preterm infants.
CASE Ms. M and Julia likely would have been better served with an extra day in the hospital to address feeding issues and monitor for hyperbilirubinemia. Julia received phototherapy, IV fluids, and intensive lactation support, which included pumped breast milk given through a supplemental nursing system.
Over the course of 48 hours, her bilirubin decreased to 14 mg/dL and she was able to feed for longer periods of time without tiring. While both Ms. M and Julia’s initial outcomes were good, an extra day of feeding support may have prevented a readmission, thousands of dollars in care, and unnecessary stress on both mother and baby.
CORRESPONDENCE
Kimberly Stuckey-Schrock, MD, IU Health Goshen, Lincoln Avenue Family Medicine, 400 W Lincoln Avenue, Goshen, IN 46526; [email protected]
1. Martin JA, Hamilton BE, Sutton PD, et al. Birth: final data for 2008. Natl Vital Stat Rep. 2010;59:1, 3-71.
2. Engle WA, Tomashek KM, Wallman C. Committee on Fetus and Newborn. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390-1401.
3. Gabbe SG, Niebyl JR, Simpson JL. eds. Obstetrics Normal and Problem Pregnancies. Philadelphia, Pa: Churchill Livingstone; 2007.
4. Murthy K, Grobman WA, Lee TA, et al. Obstetricians’ rising liability insurance premiums and inductions at late preterm gestations. Med Care. 2009;47:425-430.
5. Wang ML, Dorer DJ, Fleming MP, et al. Clinical outcomes of near-term infants. Pediatrics. 2004;114:372-376.
6. Melamed N, Klinger G, Tenenbaum-Gavish K, et al. Short-term neonatal outcome in low-risk, spontaneous, singleton, late preterm deliveries. Obstet Gynecol. 2009;114:253-260.
7. Garg M, Devaskar SU. Glucose metabolism in the late preterm infant. Clin Perinatol. 2006;33:853-870.
8. Laptook A, Jackson G. Cold stress and hypoglycemia in the late preterm (“near-term”) infant: impact on nursery of admission. Semin Perinatol. 2006;30:24-27.
9. Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105:1141-1145.
10. Adamkin D. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29(suppl):S12-S17.
11. Clark R. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol. 2005;25:251-257.
12. Kalyoncu O, Aygun C, Cetinoglu E, et al. Neonatal morbidity and mortality of late-preterm babies. J Matern Fetal Neonatal Med. 2010;23:607-612.
13. Watchko J. Hyperbilirubinemia and bilirubin toxicity in the late preterm infant. Clin Perinatol. 2006;33:839-852.
14. Bhutani V. Kernicterus in late preterm infants cared for as term healthy infants. Semin Perinatol. 2006;30:89-97.
15. Tomashek KM, Shapiro-Mendoza CK, Weiss J, et al. Early discharge among late preterm and term newborns and risk of neonatal morbidity. Semin Perinatol. 2006;30:61-68.
16. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
17. Shapiro-Mendoza CK, Tomashek K, Kotelchuck M, et al. Risk factors for neonatal morbidity and mortality among “healthy” late preterm newborns. Semin Perinatol. 2006;30:54-60.
18. Meier PP, Furman LM, Degenhardt M. Increased lactation risk for late preterm infants and mothers: evidence and management strategies to protect breastfeeding. J Midwifery Women’s Health. 2007;52:579-587.
19. American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
20. Thoyre S, Shaker CS, Pridham KF. The early feeding skills assessment for preterm infants. Neonatal Netw. 2005;24:7-16.
21. Adams-Chapman I. Neurodevelopmental outcomes of the late preterm infant. Clin Perinatol. 2006;33:947-964.
22. Morse SB, Zheng H, Tang Y, et al. Early school age outcomes of late preterm infants. Pediatrics. 2009;123:622-629.
23. Cohen-Wolkowiez M, Moran C, Benjamin D, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052-1056.
• Delay discharge of late preterm infants to a minimum of 48 hours to prevent readmission. B
• Perform transcutaneous or total serum bilirubin testing before discharging late preterm infants. C
• Perform a formal feeding assessment of breastfed infants prior to discharge. C
• Ensure that a follow-up appointment is made for 24 to 48 hours after discharge. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Laura M delivers her third baby at 35 weeks 4 days after an uneventful spontaneous labor. Julia, 5 lb 1 oz, has Apgar scores of 8 and 9. You see them on the mother-baby unit Friday afternoon, 36 hours after the delivery.
Ms. M has successfully breastfed her other 2 children and is planning to breastfeed Julia as well. She says the infant is nursing well today but seemed sleepy yesterday. The nursing notes say that Julia went to the nursery overnight because her mother was tired, and the infant spit up after receiving a formula bottle. Ms. M is asking you to discharge her because tomorrow is her son’s birthday. After considering this and her experience with breastfeeding, you decide to discharge her with follow-up on Monday instead of checking her out to your partner for the weekend.
Ms. M returns to your office on Monday with Julia, whose weight is down 10% and is “glowing yellow.” The baby is not latching well at the breast and is spitting up when Ms. M tries to supplement with formula. After examining Julia and checking lab work (bilirubin, 20 mg/dL), you decide to readmit her for feeding difficulties and hyperbilirubinemia.
Complications are common with late preterm infants, which refers to babies born between 34 weeks 0 days and 36 weeks 6 days of pregnancy. Between 1990 and 2006, there was a dramatic (25%) increase in the rate of late preterm infants in the United States, although more recently this number has leveled off.1
Several factors have been linked to increased late preterm births: maternal obesity, increased maternal age, and the increasing rate of multiple gestation pregnancies that have resulted from the expanded use of reproductive technology.2 Similarly, treatment of severe preeclampsia and premature rupture of membranes often includes delivery after 34 weeks of gestation,3 further contributing to the problem. Finally, higher rates of antenatal screening have contributed to more inductions and cesarean sections at earlier gestational ages. One study even found a correlation between higher malpractice premiums and more frequent late preterm inductions.4
Don’t let their appearance fool you
At first appearance, late preterm infants are similar to term infants in terms of Apgar scores,5 size, and weight.2 However, the care of these infants can be complex. They are often placed in well-infant nurseries under the same protocols as term infants and discharged before an adequate observation period. These infants have both increased short- and long-term morbidity and mortality and use a significant amount of health care resources.5 Morbidity in these infants decreases with each week of gestation from 34 weeks to a nadir at 39 weeks and can be unrelated to maternal and pregnancy complications.6
The following are some important issues to keep in mind when caring for these infants.
Hypothermia and hypoglycemia
Late preterm infants experience increased cold stress because of their limited fat stores, reduced brown fat, an immature epidermal barrier, and increased surface area to body mass ratio.2 Hypothermia increases the metabolic demands on the neonate and can worsen hypoglycemia as well as respiratory distress.7
Ideally, clinicians should dry these infants with warm blankets, place them skin-to-skin with their mother, and cover them with a warm blanket and cap to avoid excess energy expenditure.8 If conditions necessitate, neonates can be placed in a radiant warmer. Infants’ temperature needs to be monitored within the first 30 minutes of life and frequently reassessed during the first 12 hours of life—the “transition period.”8 The infant’s axillary temperature should be maintained between 36.5°C and 37.4°C (97.7-99.3°F); the temperature should remain stable in an open crib for the 12 hours before discharge.2
Decreased glycogen stores, increased glucose utilization, and immature hepatic enzymes help to explain the fact that hypoglycemia is 3 times more common in late preterm infants compared with full-term neonates.7 In all newborns, glucose levels decrease to their nadir between 30 and 90 minutes of life and normally trigger the breakdown of glycogen if the infant does not eat.7 Hypoglycemia can manifest as a change in level of consciousness, apnea, cyanosis, tachypnea, hypothermia, and seizures.9 The evidence is limited and there is controversy as to what level of hypoglycemia and over what duration of time is harmful.9
Most experts agree that breastfeeding should be started in the delivery room, and plasma glucose checked at 60 minutes of life and any time an infant is symptomatic. A level less than 45 mg/dL during the transition period should prompt feeding.9 Glucose levels should be rechecked within an hour of feeding and every 3 hours thereafter. If the level remains low or the infant is not interested in feeding, give a bolus of D10W and consider an infusion.7,10 TABLE 110 highlights one proposed method of glucose management.
TABLE 1
How best to manage glucose in a late preterm newborn10
| Asymptomatic late preterm infants | Glucose level (mg/dL) | Response |
|---|---|---|
| Check plasma glucose at 1 hour of life | <45 | Feed, recheck 1 h |
| Subsequent glucose monitoring | <35 | IV infusion* |
| >35 | Rescreen q3h for 36 h | |
| Symptomatic late preterm infants | ||
| Infusion for goal >55 mg/dL | <45 | Minibolus;† IV infusion* |
| *IV infusion=D10W at 6-8 mg/kg/min. †Minibolus=200 mg/kg D10W (2 mL/kg). Adapted with permission from: Macmillan Publishers Ltd. Adamkin DH. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29:S12-S17. Copyright 2009. | ||
Respiratory distress
At least 30% of late preterm infants will have some evidence of respiratory distress,5 which is defined as the need for oxygen supplementation due to tachypnea, grunting, nasal flaring, retractions, or cyanosis. Those at highest risk are white males born via cesarean section.11 Transient tachypnea of the newborn (TTN) appears to be the cause of almost half the cases of respiratory distress in these young patients.12 As this is a diagnosis by exclusion, consider other common causes: respiratory distress syndrome, neonatal pneumonia, meconium aspiration syndrome, and persistent pulmonary hypertension.12 The need for ventilator support is a significant concern in this group and increases exponentially with decreasing gestational age.6
Hyperbilirubinemia
More than half of all late preterm infants will present with clinically significant jaundice.5 Late preterm infants have an increased bilirubin load, decreased uptake of bilirubin, and delayed conjugation. They are less able to bind bilirubin to albumin, which increases their predisposition to bilirubin-induced neurological dysfunction and kernicterus.13 They also have more difficulty with breastfeeding, which exacerbates their hyperbilirubinemia.14
Infants born at 36 weeks have an 8-fold increased risk of developing severe hyperbilirubinemia (total serum bilirubin >20 mg/dL) than those born at 41 weeks,13 which explains why they are disproportionately represented in the US Pilot Kernicterus Registry.14 These infants can develop neurological sequelae at lower levels of bilirubin than their term counterparts and have less chance of complete recovery once intensive therapy has been implemented.14
The 2 main risk factors for severe hyperbilirubinemia have to do with discharge time and breastfeeding. Discharge at less than 48 hours is a risk factor for both term and late preterm infants.14,15 This is likely due to the fact that bilirubin peaks in late preterm infants between Days 5 and 7, and in term infants between Days 3 and 5.16 Difficulties with breastfeeding, the second risk factor,17 can be due to a combination of delayed maternal lactogenesis and ineffective milk removal on the part of the infant.18 When infants ingest smaller volumes of milk, bowel movements are infrequent and the bilirubin in the gut gets reabsorbed instead of excreted.
American Academy of Pediatrics (AAP) guidelines suggest measuring total serum bilirubin or transcutaneous bilirubin and plotting the value on an hour-specific, gestational-age-specific, risk-specific nomogram (FIGURE).19 Alternately, BiliTool (http://www.bilitool.org) can be used to individualize an infant’s risk.
FIGURE
Bilirubin levels and risk of significant hyperbilirubinemia19
Bilirubin levels should be plotted according to the infant’s hours of life and assessed according to risk factors on the appropriate risk line. If infants are 34 weeks 0 days to 34 weeks 6 days, they should be assessed on the high risk line.
- Use total bilirubin. Do not subtract direct reacting or conjugated bilirubin.
- Risk factors include isoimmune hemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis, or albumin <3.0 g/dL (if measured).
- For well infants 35-37 6/7 weeks, you can adjust the total serum bilirubin (TSB) levels for intervention around the medium risk line. It is an option to intervene at lower TSB levels for infants closer to 35 weeks and at higher TSB levels for those closer to 37 6/7 weeks.
- It is an option to provide conventional phototherapy in the hospital or at home at TSB levels of 2-3 mg/dL (35-50 mmol/L) below those shown, but home phototherapy should not be used in any infant with risk factors.
Reprinted with permission from: American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316. Copyright © 2004 by the American Academy of Pediatrics.
Feeding difficulties
The advantages of breast milk feeding for these babies are great. Unfortunately, it is breastfed infants who are more likely to have feeding difficulties and are at higher risk for readmission.17 Early feeding skills are complex and challenging. Because of their immaturity, late preterm infants have less effective suck and swallow coordination. They can be sleepier and have less stamina.10 Infants require coordinated oral motor movements, breathing, and swallowing to avoid desaturation and aspiration.20 It is also important to note that almost every reported case of kernicterus in the past 20 years has been in breastfed infants whose feeding was not well established.13 First-born infants are especially at risk, as it takes longer to establish an adequate supply of milk.
For late preterm infants, it is imperative to establish successful breastfeeding to avoid dehydration and jaundice. Lactation consultation, education, and close follow-up are essential to successful breastfeeding in this group.18 The AAP Committee on Fetus and Newborn recommends a formal breastfeeding evaluation by trained caregivers at least twice daily prior to discharge.2
Brain injury
Late gestation is a critical period of brain growth. The 34-week-old brain weighs 65% of the term brain and increases linearly with each week. Fifty percent of the increase in cortical volume happens after 34 weeks.21 The risk of intraventricular hemorrhage and periventricular leukomalacia, while common in earlier preemies, is rare in infants born after 34 weeks. However, there is evidence to suggest other complications. Late preterm infants are more likely to be diagnosed with developmental delay and require special resources in preschool and less likely to be ready for school.22
Sepsis
While late preterm neonates do have a small but significant increase in culture-proven sepsis and pneumonia compared with term babies,6 the work-up for possible sepsis is 3 times more likely. When these infants have poor feeding, mild respiratory distress, or TTN, physicians become concerned about sepsis and initiate a work-up.5 Currently there are no management guidelines for sepsis evaluation in this subset of preterm infants.23
Readmission is a distinct possibility
One study of healthy late preterm infants showed a readmission rate of 4.8%. The most common reasons for readmission were jaundice and infection.17 Risk factors for readmission were breastfeeding, primiparity, labor and delivery complications, public payer source at delivery, and a mother of Asian/Pacific Islander ethnicity.17 Another study showed that discharge at less than 48 hours significantly increased the likelihood of readmission, even more so if the infant was breastfeeding.15
Several recent studies have highlighted a relationship between decreasing gestational age and a wide range of long-term adverse outcomes. In the early years of childhood, there is an increased risk for developmental delay and decreased kindergarten readiness.22 There is also a significant risk for disability, including cerebral palsy, mental retardation, and behavioral disorders.
Late preterm infants are at greater risk for several complications and the mortality rate is high in this group, when compared with term infants. By initial appearance and even weight, they rival their term counterparts. However, while they may look much like term babies and not weigh much less, they need more intense monitoring and should meet stringent discharge criteria (TABLE 22).
TABLE 2
Minimum discharge criteria for late preterm infants2
All criteria should be met prior to discharge.
|
| Adapted with permission from: Engle WA, Tomashek KM, Wallman C, Committee on Fetus and Newborn. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390-1401. Copyright © 2007 by the American Academy of Pediatrics. |
Clearly, more studies need to be done to address the unique needs and specific treatment guidelines for these infants. In fact, hospitals may need to consider introducing neonatal observation nurseries with protocols specifically tailored for late preterm infants.
CASE Ms. M and Julia likely would have been better served with an extra day in the hospital to address feeding issues and monitor for hyperbilirubinemia. Julia received phototherapy, IV fluids, and intensive lactation support, which included pumped breast milk given through a supplemental nursing system.
Over the course of 48 hours, her bilirubin decreased to 14 mg/dL and she was able to feed for longer periods of time without tiring. While both Ms. M and Julia’s initial outcomes were good, an extra day of feeding support may have prevented a readmission, thousands of dollars in care, and unnecessary stress on both mother and baby.
CORRESPONDENCE
Kimberly Stuckey-Schrock, MD, IU Health Goshen, Lincoln Avenue Family Medicine, 400 W Lincoln Avenue, Goshen, IN 46526; [email protected]
• Delay discharge of late preterm infants to a minimum of 48 hours to prevent readmission. B
• Perform transcutaneous or total serum bilirubin testing before discharging late preterm infants. C
• Perform a formal feeding assessment of breastfed infants prior to discharge. C
• Ensure that a follow-up appointment is made for 24 to 48 hours after discharge. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Laura M delivers her third baby at 35 weeks 4 days after an uneventful spontaneous labor. Julia, 5 lb 1 oz, has Apgar scores of 8 and 9. You see them on the mother-baby unit Friday afternoon, 36 hours after the delivery.
Ms. M has successfully breastfed her other 2 children and is planning to breastfeed Julia as well. She says the infant is nursing well today but seemed sleepy yesterday. The nursing notes say that Julia went to the nursery overnight because her mother was tired, and the infant spit up after receiving a formula bottle. Ms. M is asking you to discharge her because tomorrow is her son’s birthday. After considering this and her experience with breastfeeding, you decide to discharge her with follow-up on Monday instead of checking her out to your partner for the weekend.
Ms. M returns to your office on Monday with Julia, whose weight is down 10% and is “glowing yellow.” The baby is not latching well at the breast and is spitting up when Ms. M tries to supplement with formula. After examining Julia and checking lab work (bilirubin, 20 mg/dL), you decide to readmit her for feeding difficulties and hyperbilirubinemia.
Complications are common with late preterm infants, which refers to babies born between 34 weeks 0 days and 36 weeks 6 days of pregnancy. Between 1990 and 2006, there was a dramatic (25%) increase in the rate of late preterm infants in the United States, although more recently this number has leveled off.1
Several factors have been linked to increased late preterm births: maternal obesity, increased maternal age, and the increasing rate of multiple gestation pregnancies that have resulted from the expanded use of reproductive technology.2 Similarly, treatment of severe preeclampsia and premature rupture of membranes often includes delivery after 34 weeks of gestation,3 further contributing to the problem. Finally, higher rates of antenatal screening have contributed to more inductions and cesarean sections at earlier gestational ages. One study even found a correlation between higher malpractice premiums and more frequent late preterm inductions.4
Don’t let their appearance fool you
At first appearance, late preterm infants are similar to term infants in terms of Apgar scores,5 size, and weight.2 However, the care of these infants can be complex. They are often placed in well-infant nurseries under the same protocols as term infants and discharged before an adequate observation period. These infants have both increased short- and long-term morbidity and mortality and use a significant amount of health care resources.5 Morbidity in these infants decreases with each week of gestation from 34 weeks to a nadir at 39 weeks and can be unrelated to maternal and pregnancy complications.6
The following are some important issues to keep in mind when caring for these infants.
Hypothermia and hypoglycemia
Late preterm infants experience increased cold stress because of their limited fat stores, reduced brown fat, an immature epidermal barrier, and increased surface area to body mass ratio.2 Hypothermia increases the metabolic demands on the neonate and can worsen hypoglycemia as well as respiratory distress.7
Ideally, clinicians should dry these infants with warm blankets, place them skin-to-skin with their mother, and cover them with a warm blanket and cap to avoid excess energy expenditure.8 If conditions necessitate, neonates can be placed in a radiant warmer. Infants’ temperature needs to be monitored within the first 30 minutes of life and frequently reassessed during the first 12 hours of life—the “transition period.”8 The infant’s axillary temperature should be maintained between 36.5°C and 37.4°C (97.7-99.3°F); the temperature should remain stable in an open crib for the 12 hours before discharge.2
Decreased glycogen stores, increased glucose utilization, and immature hepatic enzymes help to explain the fact that hypoglycemia is 3 times more common in late preterm infants compared with full-term neonates.7 In all newborns, glucose levels decrease to their nadir between 30 and 90 minutes of life and normally trigger the breakdown of glycogen if the infant does not eat.7 Hypoglycemia can manifest as a change in level of consciousness, apnea, cyanosis, tachypnea, hypothermia, and seizures.9 The evidence is limited and there is controversy as to what level of hypoglycemia and over what duration of time is harmful.9
Most experts agree that breastfeeding should be started in the delivery room, and plasma glucose checked at 60 minutes of life and any time an infant is symptomatic. A level less than 45 mg/dL during the transition period should prompt feeding.9 Glucose levels should be rechecked within an hour of feeding and every 3 hours thereafter. If the level remains low or the infant is not interested in feeding, give a bolus of D10W and consider an infusion.7,10 TABLE 110 highlights one proposed method of glucose management.
TABLE 1
How best to manage glucose in a late preterm newborn10
| Asymptomatic late preterm infants | Glucose level (mg/dL) | Response |
|---|---|---|
| Check plasma glucose at 1 hour of life | <45 | Feed, recheck 1 h |
| Subsequent glucose monitoring | <35 | IV infusion* |
| >35 | Rescreen q3h for 36 h | |
| Symptomatic late preterm infants | ||
| Infusion for goal >55 mg/dL | <45 | Minibolus;† IV infusion* |
| *IV infusion=D10W at 6-8 mg/kg/min. †Minibolus=200 mg/kg D10W (2 mL/kg). Adapted with permission from: Macmillan Publishers Ltd. Adamkin DH. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29:S12-S17. Copyright 2009. | ||
Respiratory distress
At least 30% of late preterm infants will have some evidence of respiratory distress,5 which is defined as the need for oxygen supplementation due to tachypnea, grunting, nasal flaring, retractions, or cyanosis. Those at highest risk are white males born via cesarean section.11 Transient tachypnea of the newborn (TTN) appears to be the cause of almost half the cases of respiratory distress in these young patients.12 As this is a diagnosis by exclusion, consider other common causes: respiratory distress syndrome, neonatal pneumonia, meconium aspiration syndrome, and persistent pulmonary hypertension.12 The need for ventilator support is a significant concern in this group and increases exponentially with decreasing gestational age.6
Hyperbilirubinemia
More than half of all late preterm infants will present with clinically significant jaundice.5 Late preterm infants have an increased bilirubin load, decreased uptake of bilirubin, and delayed conjugation. They are less able to bind bilirubin to albumin, which increases their predisposition to bilirubin-induced neurological dysfunction and kernicterus.13 They also have more difficulty with breastfeeding, which exacerbates their hyperbilirubinemia.14
Infants born at 36 weeks have an 8-fold increased risk of developing severe hyperbilirubinemia (total serum bilirubin >20 mg/dL) than those born at 41 weeks,13 which explains why they are disproportionately represented in the US Pilot Kernicterus Registry.14 These infants can develop neurological sequelae at lower levels of bilirubin than their term counterparts and have less chance of complete recovery once intensive therapy has been implemented.14
The 2 main risk factors for severe hyperbilirubinemia have to do with discharge time and breastfeeding. Discharge at less than 48 hours is a risk factor for both term and late preterm infants.14,15 This is likely due to the fact that bilirubin peaks in late preterm infants between Days 5 and 7, and in term infants between Days 3 and 5.16 Difficulties with breastfeeding, the second risk factor,17 can be due to a combination of delayed maternal lactogenesis and ineffective milk removal on the part of the infant.18 When infants ingest smaller volumes of milk, bowel movements are infrequent and the bilirubin in the gut gets reabsorbed instead of excreted.
American Academy of Pediatrics (AAP) guidelines suggest measuring total serum bilirubin or transcutaneous bilirubin and plotting the value on an hour-specific, gestational-age-specific, risk-specific nomogram (FIGURE).19 Alternately, BiliTool (http://www.bilitool.org) can be used to individualize an infant’s risk.
FIGURE
Bilirubin levels and risk of significant hyperbilirubinemia19
Bilirubin levels should be plotted according to the infant’s hours of life and assessed according to risk factors on the appropriate risk line. If infants are 34 weeks 0 days to 34 weeks 6 days, they should be assessed on the high risk line.
- Use total bilirubin. Do not subtract direct reacting or conjugated bilirubin.
- Risk factors include isoimmune hemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis, or albumin <3.0 g/dL (if measured).
- For well infants 35-37 6/7 weeks, you can adjust the total serum bilirubin (TSB) levels for intervention around the medium risk line. It is an option to intervene at lower TSB levels for infants closer to 35 weeks and at higher TSB levels for those closer to 37 6/7 weeks.
- It is an option to provide conventional phototherapy in the hospital or at home at TSB levels of 2-3 mg/dL (35-50 mmol/L) below those shown, but home phototherapy should not be used in any infant with risk factors.
Reprinted with permission from: American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316. Copyright © 2004 by the American Academy of Pediatrics.
Feeding difficulties
The advantages of breast milk feeding for these babies are great. Unfortunately, it is breastfed infants who are more likely to have feeding difficulties and are at higher risk for readmission.17 Early feeding skills are complex and challenging. Because of their immaturity, late preterm infants have less effective suck and swallow coordination. They can be sleepier and have less stamina.10 Infants require coordinated oral motor movements, breathing, and swallowing to avoid desaturation and aspiration.20 It is also important to note that almost every reported case of kernicterus in the past 20 years has been in breastfed infants whose feeding was not well established.13 First-born infants are especially at risk, as it takes longer to establish an adequate supply of milk.
For late preterm infants, it is imperative to establish successful breastfeeding to avoid dehydration and jaundice. Lactation consultation, education, and close follow-up are essential to successful breastfeeding in this group.18 The AAP Committee on Fetus and Newborn recommends a formal breastfeeding evaluation by trained caregivers at least twice daily prior to discharge.2
Brain injury
Late gestation is a critical period of brain growth. The 34-week-old brain weighs 65% of the term brain and increases linearly with each week. Fifty percent of the increase in cortical volume happens after 34 weeks.21 The risk of intraventricular hemorrhage and periventricular leukomalacia, while common in earlier preemies, is rare in infants born after 34 weeks. However, there is evidence to suggest other complications. Late preterm infants are more likely to be diagnosed with developmental delay and require special resources in preschool and less likely to be ready for school.22
Sepsis
While late preterm neonates do have a small but significant increase in culture-proven sepsis and pneumonia compared with term babies,6 the work-up for possible sepsis is 3 times more likely. When these infants have poor feeding, mild respiratory distress, or TTN, physicians become concerned about sepsis and initiate a work-up.5 Currently there are no management guidelines for sepsis evaluation in this subset of preterm infants.23
Readmission is a distinct possibility
One study of healthy late preterm infants showed a readmission rate of 4.8%. The most common reasons for readmission were jaundice and infection.17 Risk factors for readmission were breastfeeding, primiparity, labor and delivery complications, public payer source at delivery, and a mother of Asian/Pacific Islander ethnicity.17 Another study showed that discharge at less than 48 hours significantly increased the likelihood of readmission, even more so if the infant was breastfeeding.15
Several recent studies have highlighted a relationship between decreasing gestational age and a wide range of long-term adverse outcomes. In the early years of childhood, there is an increased risk for developmental delay and decreased kindergarten readiness.22 There is also a significant risk for disability, including cerebral palsy, mental retardation, and behavioral disorders.
Late preterm infants are at greater risk for several complications and the mortality rate is high in this group, when compared with term infants. By initial appearance and even weight, they rival their term counterparts. However, while they may look much like term babies and not weigh much less, they need more intense monitoring and should meet stringent discharge criteria (TABLE 22).
TABLE 2
Minimum discharge criteria for late preterm infants2
All criteria should be met prior to discharge.
|
| Adapted with permission from: Engle WA, Tomashek KM, Wallman C, Committee on Fetus and Newborn. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390-1401. Copyright © 2007 by the American Academy of Pediatrics. |
Clearly, more studies need to be done to address the unique needs and specific treatment guidelines for these infants. In fact, hospitals may need to consider introducing neonatal observation nurseries with protocols specifically tailored for late preterm infants.
CASE Ms. M and Julia likely would have been better served with an extra day in the hospital to address feeding issues and monitor for hyperbilirubinemia. Julia received phototherapy, IV fluids, and intensive lactation support, which included pumped breast milk given through a supplemental nursing system.
Over the course of 48 hours, her bilirubin decreased to 14 mg/dL and she was able to feed for longer periods of time without tiring. While both Ms. M and Julia’s initial outcomes were good, an extra day of feeding support may have prevented a readmission, thousands of dollars in care, and unnecessary stress on both mother and baby.
CORRESPONDENCE
Kimberly Stuckey-Schrock, MD, IU Health Goshen, Lincoln Avenue Family Medicine, 400 W Lincoln Avenue, Goshen, IN 46526; [email protected]
1. Martin JA, Hamilton BE, Sutton PD, et al. Birth: final data for 2008. Natl Vital Stat Rep. 2010;59:1, 3-71.
2. Engle WA, Tomashek KM, Wallman C. Committee on Fetus and Newborn. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390-1401.
3. Gabbe SG, Niebyl JR, Simpson JL. eds. Obstetrics Normal and Problem Pregnancies. Philadelphia, Pa: Churchill Livingstone; 2007.
4. Murthy K, Grobman WA, Lee TA, et al. Obstetricians’ rising liability insurance premiums and inductions at late preterm gestations. Med Care. 2009;47:425-430.
5. Wang ML, Dorer DJ, Fleming MP, et al. Clinical outcomes of near-term infants. Pediatrics. 2004;114:372-376.
6. Melamed N, Klinger G, Tenenbaum-Gavish K, et al. Short-term neonatal outcome in low-risk, spontaneous, singleton, late preterm deliveries. Obstet Gynecol. 2009;114:253-260.
7. Garg M, Devaskar SU. Glucose metabolism in the late preterm infant. Clin Perinatol. 2006;33:853-870.
8. Laptook A, Jackson G. Cold stress and hypoglycemia in the late preterm (“near-term”) infant: impact on nursery of admission. Semin Perinatol. 2006;30:24-27.
9. Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105:1141-1145.
10. Adamkin D. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29(suppl):S12-S17.
11. Clark R. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol. 2005;25:251-257.
12. Kalyoncu O, Aygun C, Cetinoglu E, et al. Neonatal morbidity and mortality of late-preterm babies. J Matern Fetal Neonatal Med. 2010;23:607-612.
13. Watchko J. Hyperbilirubinemia and bilirubin toxicity in the late preterm infant. Clin Perinatol. 2006;33:839-852.
14. Bhutani V. Kernicterus in late preterm infants cared for as term healthy infants. Semin Perinatol. 2006;30:89-97.
15. Tomashek KM, Shapiro-Mendoza CK, Weiss J, et al. Early discharge among late preterm and term newborns and risk of neonatal morbidity. Semin Perinatol. 2006;30:61-68.
16. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
17. Shapiro-Mendoza CK, Tomashek K, Kotelchuck M, et al. Risk factors for neonatal morbidity and mortality among “healthy” late preterm newborns. Semin Perinatol. 2006;30:54-60.
18. Meier PP, Furman LM, Degenhardt M. Increased lactation risk for late preterm infants and mothers: evidence and management strategies to protect breastfeeding. J Midwifery Women’s Health. 2007;52:579-587.
19. American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
20. Thoyre S, Shaker CS, Pridham KF. The early feeding skills assessment for preterm infants. Neonatal Netw. 2005;24:7-16.
21. Adams-Chapman I. Neurodevelopmental outcomes of the late preterm infant. Clin Perinatol. 2006;33:947-964.
22. Morse SB, Zheng H, Tang Y, et al. Early school age outcomes of late preterm infants. Pediatrics. 2009;123:622-629.
23. Cohen-Wolkowiez M, Moran C, Benjamin D, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052-1056.
1. Martin JA, Hamilton BE, Sutton PD, et al. Birth: final data for 2008. Natl Vital Stat Rep. 2010;59:1, 3-71.
2. Engle WA, Tomashek KM, Wallman C. Committee on Fetus and Newborn. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390-1401.
3. Gabbe SG, Niebyl JR, Simpson JL. eds. Obstetrics Normal and Problem Pregnancies. Philadelphia, Pa: Churchill Livingstone; 2007.
4. Murthy K, Grobman WA, Lee TA, et al. Obstetricians’ rising liability insurance premiums and inductions at late preterm gestations. Med Care. 2009;47:425-430.
5. Wang ML, Dorer DJ, Fleming MP, et al. Clinical outcomes of near-term infants. Pediatrics. 2004;114:372-376.
6. Melamed N, Klinger G, Tenenbaum-Gavish K, et al. Short-term neonatal outcome in low-risk, spontaneous, singleton, late preterm deliveries. Obstet Gynecol. 2009;114:253-260.
7. Garg M, Devaskar SU. Glucose metabolism in the late preterm infant. Clin Perinatol. 2006;33:853-870.
8. Laptook A, Jackson G. Cold stress and hypoglycemia in the late preterm (“near-term”) infant: impact on nursery of admission. Semin Perinatol. 2006;30:24-27.
9. Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105:1141-1145.
10. Adamkin D. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29(suppl):S12-S17.
11. Clark R. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol. 2005;25:251-257.
12. Kalyoncu O, Aygun C, Cetinoglu E, et al. Neonatal morbidity and mortality of late-preterm babies. J Matern Fetal Neonatal Med. 2010;23:607-612.
13. Watchko J. Hyperbilirubinemia and bilirubin toxicity in the late preterm infant. Clin Perinatol. 2006;33:839-852.
14. Bhutani V. Kernicterus in late preterm infants cared for as term healthy infants. Semin Perinatol. 2006;30:89-97.
15. Tomashek KM, Shapiro-Mendoza CK, Weiss J, et al. Early discharge among late preterm and term newborns and risk of neonatal morbidity. Semin Perinatol. 2006;30:61-68.
16. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
17. Shapiro-Mendoza CK, Tomashek K, Kotelchuck M, et al. Risk factors for neonatal morbidity and mortality among “healthy” late preterm newborns. Semin Perinatol. 2006;30:54-60.
18. Meier PP, Furman LM, Degenhardt M. Increased lactation risk for late preterm infants and mothers: evidence and management strategies to protect breastfeeding. J Midwifery Women’s Health. 2007;52:579-587.
19. American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
20. Thoyre S, Shaker CS, Pridham KF. The early feeding skills assessment for preterm infants. Neonatal Netw. 2005;24:7-16.
21. Adams-Chapman I. Neurodevelopmental outcomes of the late preterm infant. Clin Perinatol. 2006;33:947-964.
22. Morse SB, Zheng H, Tang Y, et al. Early school age outcomes of late preterm infants. Pediatrics. 2009;123:622-629.
23. Cohen-Wolkowiez M, Moran C, Benjamin D, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052-1056.
Cholestyramine for thyrotoxicosis?
CASE A 44-year-old Korean woman who was receiving pegylated interferon (IFN) and ribavirin for chronic hepatitis C infection came into our facility with complaints of gradual onset of fatigue, unintentional weight loss of 15 pounds within a month, palpitations, and heat intolerance. She said her symptoms had worsened a week earlier, after she’d undergone an abdominal computed tomography scan with intravenous contrast.
Her physical exam revealed a blood pressure of 90/60 mm Hg, heart rate of 120 beats per minute, body mass index (BMI) of 17 kg/m2, and visibly symmetric thyroid enlargement without any nodules, tenderness, or bruits. Despite her low systolic reading, she was asymptomatic and not septic or in thyroid storm. Laboratory data showed pancytopenia with a white blood cell count of 700/mcL (absolute neutrophil count, 189), hemoglobin of 9.6 g/L, platelet count of 96,000 cell/mcL, elevated free thyroxine (T4) of 5.7 ng/dL (normal, 0.6-1.6 ng/dL), total triiodothyronine (T3) of 301 ng/dL (normal, 90-180 ng/dL), and suppressed thyroid-stimulating hormone (TSH) of 0.01 mcU/mL (normal, 0.27-4.20 mcU/mL). The thyroid peroxidase antibody level was elevated at 9420 U/mL (normal, <35 U/mL), with a normal level of thyroid-stimulating immunoglobulin. Neither of these latter tests was obtained before IFN treatment. The patient had no history of thyroid dysfunction.
We discontinued the patient’s pegylated IFN and ribavirin. Antithyroid medication and beta-blockade were contraindicated due to pancytopenia and hypotension, respectively. Given her recent iodine exposure from contrast media, radioactive iodine treatment would be ineffective. We started the patient on cholestyramine 4 g orally 3 times a day and she improved rapidly. Her thyroid function normalized after 2.5 weeks of treatment.
After 6 weeks, when her TSH increased to 13 mcU/mL, we discontinued cholestyramine. Two weeks later, her TSH was >50 mcU/mL and we started the patient on levothyroxine replacement.
Discussion
The association between IFN-alpha and thyroid disease was recognized as early as 1985.1 Between 5% and 10% of patients receiving IFN-alpha therapy may develop clinical thyroid disease, including painless thyroiditis, Hashimoto’s thyroiditis, and Graves’ disease,2 although a recent prospective study in Australia showed a thyroid disease prevalence of just 1% up to 6 months after treatment with IFN-alpha and ribavirin.3
The cause of our patient’s thyrotoxicosis. IFN-alpha therapy likely caused our patient’s thyrotoxicosis, but iodide-induced hyperthyroidism may occur after use of iodinated contrast media.4 Prompt treatment of thyrotoxicosis with beta-blockers, anti-thyroid drugs, or radioiodine is necessary to reduce morbidity and the risk of developing thyroid storm, a condition associated with significant mortality.2,5
Cholestyramine, a bile salt sequestrant, has been used chiefly to lower cholesterol since the 1960s.6,7 However, studies also found that cholestyramine’s ionic exchange properties could decrease excessive thyroid hormone levels by enhancing hormone clearance from the enterohepatic circulation and thereby increase hormone fecal excretion.6,7 In one study, radioactive iodine was given to patients simultaneously receiving levothyroxine for thyroid hormone deficiency and cholestyramine for hyperlipidemia.6 Results included increased stool radioactivity, decreased urine radioactivity, and decreased thyroid uptake consistent with enhanced clearance of thyroid hormones.6 The study also showed that 50 mg of cholestyramine can bind about 3000 mcg of T4.6
Cholestyramine as adjunctive therapy has rapidly decreased thyroid hormone levels compared with standard therapy alone, maintaining its effect for about 4 weeks.2,5,8,9 Complete normalization of free T4 and free T3 levels and notable symptom improvement have occurred within one week of instituting cholestyramine therapy.5 The optimal dosage is 4 g cholestyramine orally, 2 to 4 times daily for 4 weeks.2,5,8,9 Primary adverse effects of cholestyramine are constipation and abdominal discomfort. In contrast to methimazole or propylthiouracil, which only treat conditions of excess thyroid hormone production, cholestyramine is effective for any condition with excessive thyroid hormone levels—eg, subacute thyroiditis, Jod-Basdow phenomenon, and factitious thyroid hormone disorder.
Resolution. In our patient, the clinical picture was consistent with an autoimmune form of thyrotoxicosis induced by IFN therapy and possibly exacerbated by iodine exposure. In the absence of a thyroid scan at the time of thyrotoxicosis, we cannot exclude destructive thyroiditis; in fact, the ensuing hypothyroidism strengthens this possibility. Nevertheless, we believe that the oral administration of cholestyramine as monotherapy contributed to the resolution of our patient’s thyrotoxic symptoms when first-line medications were contraindicated.
CORRESPONDENCE
Dagmar Lin, MD, Jackson Memorial Hospital, University of Miami, 1611 NW 12th Avenue, MICU, Central Room 455, Miami, FL 33136; [email protected]
1. Fentiman IS, Thomas BS, Balkwill FR, et al. Primary hypothyroidism associated with interferon therapy of breast cancer. Lancet. 1985;1:1166.-
2. Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am. 2006;35:663-686, vii.
3. Tran HA, Reeves GE, Ianna EA, et al. Thyroid function outcomes following pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Endocr Pract. 2010;16:934-939.
4. Nayak B, Hodak SP. Hyperthyroidism. Endocrinol Metab Clin North Am. 2007;36:617-656, v.
5. Sebastian-Ochoa A, Quesada-Charneco M, Fernandez-Garcia D, et al. Dramatic response to cholestyramine in a patient with Graves’ disease resistant to conventional therapy. Thyroid. 2008;18:1115-1117.
6. Northcutt RC, Stiel JN, Hollifield JW, et al. The influence of cholestyramine on thyroxine absorption. JAMA. 1969;208:1857-1861.
7. Bergman F, Heedman PA, van der Linden W. Influence of cholestyramine on absorption and excretion of thyroxine in Syrian hamster. Acta Endocrinol (Copenh). 1966;53:256-263.
8. Kaykhaei MA, Shams M, Sadegholvad A, et al. Low doses of cholestyramine in the treatment of hyperthyroidism. Endocrine. 2008;34:52-55.
9. Solomon BL, Wartofsky L, Burman KD. Adjunctive cholestyramine therapy for thyrotoxicosis. Clin Endocrinol (Oxf). 1993;38:39-43.
CASE A 44-year-old Korean woman who was receiving pegylated interferon (IFN) and ribavirin for chronic hepatitis C infection came into our facility with complaints of gradual onset of fatigue, unintentional weight loss of 15 pounds within a month, palpitations, and heat intolerance. She said her symptoms had worsened a week earlier, after she’d undergone an abdominal computed tomography scan with intravenous contrast.
Her physical exam revealed a blood pressure of 90/60 mm Hg, heart rate of 120 beats per minute, body mass index (BMI) of 17 kg/m2, and visibly symmetric thyroid enlargement without any nodules, tenderness, or bruits. Despite her low systolic reading, she was asymptomatic and not septic or in thyroid storm. Laboratory data showed pancytopenia with a white blood cell count of 700/mcL (absolute neutrophil count, 189), hemoglobin of 9.6 g/L, platelet count of 96,000 cell/mcL, elevated free thyroxine (T4) of 5.7 ng/dL (normal, 0.6-1.6 ng/dL), total triiodothyronine (T3) of 301 ng/dL (normal, 90-180 ng/dL), and suppressed thyroid-stimulating hormone (TSH) of 0.01 mcU/mL (normal, 0.27-4.20 mcU/mL). The thyroid peroxidase antibody level was elevated at 9420 U/mL (normal, <35 U/mL), with a normal level of thyroid-stimulating immunoglobulin. Neither of these latter tests was obtained before IFN treatment. The patient had no history of thyroid dysfunction.
We discontinued the patient’s pegylated IFN and ribavirin. Antithyroid medication and beta-blockade were contraindicated due to pancytopenia and hypotension, respectively. Given her recent iodine exposure from contrast media, radioactive iodine treatment would be ineffective. We started the patient on cholestyramine 4 g orally 3 times a day and she improved rapidly. Her thyroid function normalized after 2.5 weeks of treatment.
After 6 weeks, when her TSH increased to 13 mcU/mL, we discontinued cholestyramine. Two weeks later, her TSH was >50 mcU/mL and we started the patient on levothyroxine replacement.
Discussion
The association between IFN-alpha and thyroid disease was recognized as early as 1985.1 Between 5% and 10% of patients receiving IFN-alpha therapy may develop clinical thyroid disease, including painless thyroiditis, Hashimoto’s thyroiditis, and Graves’ disease,2 although a recent prospective study in Australia showed a thyroid disease prevalence of just 1% up to 6 months after treatment with IFN-alpha and ribavirin.3
The cause of our patient’s thyrotoxicosis. IFN-alpha therapy likely caused our patient’s thyrotoxicosis, but iodide-induced hyperthyroidism may occur after use of iodinated contrast media.4 Prompt treatment of thyrotoxicosis with beta-blockers, anti-thyroid drugs, or radioiodine is necessary to reduce morbidity and the risk of developing thyroid storm, a condition associated with significant mortality.2,5
Cholestyramine, a bile salt sequestrant, has been used chiefly to lower cholesterol since the 1960s.6,7 However, studies also found that cholestyramine’s ionic exchange properties could decrease excessive thyroid hormone levels by enhancing hormone clearance from the enterohepatic circulation and thereby increase hormone fecal excretion.6,7 In one study, radioactive iodine was given to patients simultaneously receiving levothyroxine for thyroid hormone deficiency and cholestyramine for hyperlipidemia.6 Results included increased stool radioactivity, decreased urine radioactivity, and decreased thyroid uptake consistent with enhanced clearance of thyroid hormones.6 The study also showed that 50 mg of cholestyramine can bind about 3000 mcg of T4.6
Cholestyramine as adjunctive therapy has rapidly decreased thyroid hormone levels compared with standard therapy alone, maintaining its effect for about 4 weeks.2,5,8,9 Complete normalization of free T4 and free T3 levels and notable symptom improvement have occurred within one week of instituting cholestyramine therapy.5 The optimal dosage is 4 g cholestyramine orally, 2 to 4 times daily for 4 weeks.2,5,8,9 Primary adverse effects of cholestyramine are constipation and abdominal discomfort. In contrast to methimazole or propylthiouracil, which only treat conditions of excess thyroid hormone production, cholestyramine is effective for any condition with excessive thyroid hormone levels—eg, subacute thyroiditis, Jod-Basdow phenomenon, and factitious thyroid hormone disorder.
Resolution. In our patient, the clinical picture was consistent with an autoimmune form of thyrotoxicosis induced by IFN therapy and possibly exacerbated by iodine exposure. In the absence of a thyroid scan at the time of thyrotoxicosis, we cannot exclude destructive thyroiditis; in fact, the ensuing hypothyroidism strengthens this possibility. Nevertheless, we believe that the oral administration of cholestyramine as monotherapy contributed to the resolution of our patient’s thyrotoxic symptoms when first-line medications were contraindicated.
CORRESPONDENCE
Dagmar Lin, MD, Jackson Memorial Hospital, University of Miami, 1611 NW 12th Avenue, MICU, Central Room 455, Miami, FL 33136; [email protected]
CASE A 44-year-old Korean woman who was receiving pegylated interferon (IFN) and ribavirin for chronic hepatitis C infection came into our facility with complaints of gradual onset of fatigue, unintentional weight loss of 15 pounds within a month, palpitations, and heat intolerance. She said her symptoms had worsened a week earlier, after she’d undergone an abdominal computed tomography scan with intravenous contrast.
Her physical exam revealed a blood pressure of 90/60 mm Hg, heart rate of 120 beats per minute, body mass index (BMI) of 17 kg/m2, and visibly symmetric thyroid enlargement without any nodules, tenderness, or bruits. Despite her low systolic reading, she was asymptomatic and not septic or in thyroid storm. Laboratory data showed pancytopenia with a white blood cell count of 700/mcL (absolute neutrophil count, 189), hemoglobin of 9.6 g/L, platelet count of 96,000 cell/mcL, elevated free thyroxine (T4) of 5.7 ng/dL (normal, 0.6-1.6 ng/dL), total triiodothyronine (T3) of 301 ng/dL (normal, 90-180 ng/dL), and suppressed thyroid-stimulating hormone (TSH) of 0.01 mcU/mL (normal, 0.27-4.20 mcU/mL). The thyroid peroxidase antibody level was elevated at 9420 U/mL (normal, <35 U/mL), with a normal level of thyroid-stimulating immunoglobulin. Neither of these latter tests was obtained before IFN treatment. The patient had no history of thyroid dysfunction.
We discontinued the patient’s pegylated IFN and ribavirin. Antithyroid medication and beta-blockade were contraindicated due to pancytopenia and hypotension, respectively. Given her recent iodine exposure from contrast media, radioactive iodine treatment would be ineffective. We started the patient on cholestyramine 4 g orally 3 times a day and she improved rapidly. Her thyroid function normalized after 2.5 weeks of treatment.
After 6 weeks, when her TSH increased to 13 mcU/mL, we discontinued cholestyramine. Two weeks later, her TSH was >50 mcU/mL and we started the patient on levothyroxine replacement.
Discussion
The association between IFN-alpha and thyroid disease was recognized as early as 1985.1 Between 5% and 10% of patients receiving IFN-alpha therapy may develop clinical thyroid disease, including painless thyroiditis, Hashimoto’s thyroiditis, and Graves’ disease,2 although a recent prospective study in Australia showed a thyroid disease prevalence of just 1% up to 6 months after treatment with IFN-alpha and ribavirin.3
The cause of our patient’s thyrotoxicosis. IFN-alpha therapy likely caused our patient’s thyrotoxicosis, but iodide-induced hyperthyroidism may occur after use of iodinated contrast media.4 Prompt treatment of thyrotoxicosis with beta-blockers, anti-thyroid drugs, or radioiodine is necessary to reduce morbidity and the risk of developing thyroid storm, a condition associated with significant mortality.2,5
Cholestyramine, a bile salt sequestrant, has been used chiefly to lower cholesterol since the 1960s.6,7 However, studies also found that cholestyramine’s ionic exchange properties could decrease excessive thyroid hormone levels by enhancing hormone clearance from the enterohepatic circulation and thereby increase hormone fecal excretion.6,7 In one study, radioactive iodine was given to patients simultaneously receiving levothyroxine for thyroid hormone deficiency and cholestyramine for hyperlipidemia.6 Results included increased stool radioactivity, decreased urine radioactivity, and decreased thyroid uptake consistent with enhanced clearance of thyroid hormones.6 The study also showed that 50 mg of cholestyramine can bind about 3000 mcg of T4.6
Cholestyramine as adjunctive therapy has rapidly decreased thyroid hormone levels compared with standard therapy alone, maintaining its effect for about 4 weeks.2,5,8,9 Complete normalization of free T4 and free T3 levels and notable symptom improvement have occurred within one week of instituting cholestyramine therapy.5 The optimal dosage is 4 g cholestyramine orally, 2 to 4 times daily for 4 weeks.2,5,8,9 Primary adverse effects of cholestyramine are constipation and abdominal discomfort. In contrast to methimazole or propylthiouracil, which only treat conditions of excess thyroid hormone production, cholestyramine is effective for any condition with excessive thyroid hormone levels—eg, subacute thyroiditis, Jod-Basdow phenomenon, and factitious thyroid hormone disorder.
Resolution. In our patient, the clinical picture was consistent with an autoimmune form of thyrotoxicosis induced by IFN therapy and possibly exacerbated by iodine exposure. In the absence of a thyroid scan at the time of thyrotoxicosis, we cannot exclude destructive thyroiditis; in fact, the ensuing hypothyroidism strengthens this possibility. Nevertheless, we believe that the oral administration of cholestyramine as monotherapy contributed to the resolution of our patient’s thyrotoxic symptoms when first-line medications were contraindicated.
CORRESPONDENCE
Dagmar Lin, MD, Jackson Memorial Hospital, University of Miami, 1611 NW 12th Avenue, MICU, Central Room 455, Miami, FL 33136; [email protected]
1. Fentiman IS, Thomas BS, Balkwill FR, et al. Primary hypothyroidism associated with interferon therapy of breast cancer. Lancet. 1985;1:1166.-
2. Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am. 2006;35:663-686, vii.
3. Tran HA, Reeves GE, Ianna EA, et al. Thyroid function outcomes following pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Endocr Pract. 2010;16:934-939.
4. Nayak B, Hodak SP. Hyperthyroidism. Endocrinol Metab Clin North Am. 2007;36:617-656, v.
5. Sebastian-Ochoa A, Quesada-Charneco M, Fernandez-Garcia D, et al. Dramatic response to cholestyramine in a patient with Graves’ disease resistant to conventional therapy. Thyroid. 2008;18:1115-1117.
6. Northcutt RC, Stiel JN, Hollifield JW, et al. The influence of cholestyramine on thyroxine absorption. JAMA. 1969;208:1857-1861.
7. Bergman F, Heedman PA, van der Linden W. Influence of cholestyramine on absorption and excretion of thyroxine in Syrian hamster. Acta Endocrinol (Copenh). 1966;53:256-263.
8. Kaykhaei MA, Shams M, Sadegholvad A, et al. Low doses of cholestyramine in the treatment of hyperthyroidism. Endocrine. 2008;34:52-55.
9. Solomon BL, Wartofsky L, Burman KD. Adjunctive cholestyramine therapy for thyrotoxicosis. Clin Endocrinol (Oxf). 1993;38:39-43.
1. Fentiman IS, Thomas BS, Balkwill FR, et al. Primary hypothyroidism associated with interferon therapy of breast cancer. Lancet. 1985;1:1166.-
2. Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am. 2006;35:663-686, vii.
3. Tran HA, Reeves GE, Ianna EA, et al. Thyroid function outcomes following pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Endocr Pract. 2010;16:934-939.
4. Nayak B, Hodak SP. Hyperthyroidism. Endocrinol Metab Clin North Am. 2007;36:617-656, v.
5. Sebastian-Ochoa A, Quesada-Charneco M, Fernandez-Garcia D, et al. Dramatic response to cholestyramine in a patient with Graves’ disease resistant to conventional therapy. Thyroid. 2008;18:1115-1117.
6. Northcutt RC, Stiel JN, Hollifield JW, et al. The influence of cholestyramine on thyroxine absorption. JAMA. 1969;208:1857-1861.
7. Bergman F, Heedman PA, van der Linden W. Influence of cholestyramine on absorption and excretion of thyroxine in Syrian hamster. Acta Endocrinol (Copenh). 1966;53:256-263.
8. Kaykhaei MA, Shams M, Sadegholvad A, et al. Low doses of cholestyramine in the treatment of hyperthyroidism. Endocrine. 2008;34:52-55.
9. Solomon BL, Wartofsky L, Burman KD. Adjunctive cholestyramine therapy for thyrotoxicosis. Clin Endocrinol (Oxf). 1993;38:39-43.
The perils of prescribing fluoroquinolones
• Evaluate liver function before initiating fluoroquinolone (FQ) therapy, and avoid prescribing these antibiotics for patients at increased risk for hepatotoxicity. C
• Avoid prescribing FQs for patients with a history of prolonged QT syndrome. C
• Closely monitor older patients being treated with FQs, particularly if they have atherosclerosis or epilepsy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
PATIENT HANDOUT
Taking a fluoroquinolone antibiotic?
CASE Sara Z, a 62-year-old patient with a history of chronic urinary tract infections, presents with a 3-day history of dysuria and urinary frequency. Her last 2 urine cultures found Escherichia coli resistant to trimethoprim-sulfamethoxazole, amoxicillin, and cephalosporins. So her family physician ordered a urine culture and prescribed a 7-day course of ciprofloxacin empirically.
Five days later, Ms. Z returned, suffering from nonbloody diarrhea and bilateral Achilles tendon pain.
If you were treating Ms. Z, what would your next step be?
Widely used to treat urinary tract, skin, and pulmonary infections and to fight infections resistant to other antibiotics, fluoroquinolones (FQ) are generally regarded as safe in both inpatient and outpatient settings. Yet these broad-spectrum antibiotics are associated with both common and rare adverse effects, as well as a number of drug-drug interactions.
The Centers for Disease Control and Prevention estimates that adverse events from FQs leading to emergency department (ED) visits occur at a rate of 9.2 for every 10,000 prescriptions. That’s higher than the ED rates for cephalosporins (6.1 per 10,000) and macrolides (5.1 per 10,000), but far lower than for penicillins (13 per 10,000), clindamycin (18.5 per 10,000), sulfonamides (18.9 per 10,000), and vancomycin (24.1 per 10,000).1
In fact, adverse events associated with FQs range from mild and self-limiting to rare and severe. This review discusses both. Relatively common adverse effects and drug-drug interactions are discussed in the text, while the TABLE2 includes a broader range of potential adverse effects. You’ll also find a handout for patients taking FQs on page 195 that clearly describes signs and symptoms that need to be reported right away.
TABLE
Fluoroquinolones: Adverse effects to guard against*2
Cardiovascular
| Immunologic
|
Dermatologic
| Musculoskeletal
|
Drug-drug interactions
| Neurologic
|
Endocrine/Metabolic
| Ocular
|
Gastrointestinal
| Psychiatric
|
Hematologic
| Respiratory
|
| *This is not a complete list of potential adverse effects associated with fluoroquinolones. †Fluoroquinolones may potentiate warfarin. | |
A black box warning of tendinopathies
FQs exhibit an affinity for connective tissue, with higher concentrations found in bone and cartilage than in serum. While FQs are therefore well suited for treating orthopedic-related infections,3 they also increase the risk of tendinopathies.
In the last 2 decades, numerous case reports linking tendinitis and FQs have been published.4-6 In 2008, the US Food and Drug Administration (FDA) issued a black box warning of tendinitis and tendon rupture. Patients on FQ therapy should be advised to stop taking the antibiotic at the first sign of pain, swelling, or inflammation in a tendon, the FDA advises.7
How common is this adverse effect? A case-control study of 22,194 patients with a diagnosis of nontraumatic tendiopathy determined that FQ use resulted in a 1.3-fold risk of tendon rupture and more than a 4-fold risk of rupture of the Achilles tendon. One Achilles tendon rupture would occur for every 5958 patients treated with FQs, the researchers estimated.8
The precise mechanism by which FQs lead to tendinopathies is not completely understood. Studies suggest that the antibiotics cause a decrease in the synthesis of type I collagen, elastin, fibronectin, and beta (1)-integrin, and time- and concentration-dependent increases of cellular apoptosis.9 In vitro studies have shown inhibition of both cell proliferation and fibroblast metabolism when tissue is exposed to FQs, which may impede tissue healing.10
Which patients are at higher risk? The risk of FQ-associated tendinopathies is greatest in patients older than 60 years; in kidney, heart, and lung transplant recipients; and in patients taking an FQ with concomitant corticosteroid therapy. Decreased renal clearance of the medication may play a role in the increased risk.11
GI problems are common, especially in kids and older patients
Gastrointestinal (GI) disturbances are common in patients taking FQs, and typically occur more frequently in children and older adults, and in those taking higher doses. Reactions attributable to ciprofloxacin, for example, include nausea (affecting 1.4%-4% of adults and 2.7% of children taking the drug), vomiting (1%-2% of adults and 4.8% of children), diarrhea (<1%-2% of adults and 4.8% of children), and abdominal pain or discomfort (<1%-1.7% of adults and 3.3% of children).12
C difficile and FQ resistance. The extent to which Clostridium difficile-associated diarrhea (CDAD) is attributable to FQs has been subject to controversy in recent years. A previously uncommon strain of C difficile (B1/NAP1) with variations that have become more resistant to FQs has been linked to an increased incidence of CDAD across both the United States and Europe.13 A systematic review suggested that FQs predispose patients to CDAD,14 while a retrospective case-control study of 174 adult inpatients with CDAD determined that FQ administration did not significantly increase the rate of complications from C difficile (odds ratio [OR]=1.37; 95% confidence interval [CI], 0.72-2.61).15
Factors that affect risk of hepatotoxicity
Hepatitis/transaminitis, pancreatitis, jaundice, liver injury, and hepatic failure have all been reported in patients taking FQs, with the extent of hepatotoxicity varying based on the particular FQ taken, the dosage, and the patient’s baseline hepatic function.16,17 Comorbidities, including renal failure, may increase the potential for FQ-associated hepatotoxicity, as well. Thus, some experts recommend that clinicians evaluate a patient’s liver function before initiating FQ therapy and avoid prescribing FQs for those at added risk.
The exact mechanism by which FQ-induced hepatotoxicity occurs is unknown. One theory posits that the drugs generate oxidative radicals involved in mitochondrial damage, RNA processing, transcription, and inflammation;18 another suggests that FQs generate oxidative radicals in the liver as a result of cytochrome P450 metabolism.16 Case reports have shown that hepatitis resolves when the drug is discontinued, but often recurs in patients who are switched to a different FQ.16,17
Torsades de pointes is the key cardiovascular risk
FQs prolong the QT interval by blocking voltage-gated potassium channels, causing a reduction of the rapid component of the delayed rectifier potassium current in a dose-dependent fashion.19 But the average QT interval prolongation caused by FQs over a 3- to 6-month period does not appear to have clinical significance, nor is it associated with any discernible cardiac symptoms or impairment.19
For most, risk is minimal. There appears to be considerable variation in QT interval prolongation among FQs. A retrospective database analysis of published case reports of patients who received FQs over a 15-year period found 25 cases of torsades de pointes; moxifloxacin (highest), levofloxacin, and gatifloxacin (which was taken off the market by the FDA in 2006)20 were associated with a higher incidence than ciprofloxacin.21 Ciprofloxacin appears to be the safest FQ for cardiovascular events, with the lowest reported risk of torsades de pointes.22 However, several small randomized controlled trials have found that levofloxacin, like ciprofloxacin, did not significantly affect the QT interval.23,24
These patients face a higher risk. Notably, individuals with abnormal baseline QT prolongation (>440 ms in men; >460 ms in women) are at increased risk of developing torsades de pointes from the use of FQs, regardless of the dose.19 In fact, anyone with a history of prolonged QT syndrome should avoid these antibiotics, particularly if he or she is taking class Ia (eg, procainamide, quinidine) or class III (eg, amiodarone, sotalol) antiarrhythmics.19 Patients taking warfarin may be candidates for FQ therapy, but because the antibiotics may potentiate the anticoagulant, close monitoring is required. (Other potential drug-drug interactions are detailed in the TABLE.)
Evaluation of risk vs benefit is imperative prior to prescribing FQs for patients with increased risk for adverse cardiovascular events. An electrocardiogram is advisable, as well.
Mild neurologic and psychiatric effects not uncommon
Studies examining central nervous system (CNS) effects have estimated that neurotoxicity occurs in approximately 1% to 4.4% of patients taking FQs, with serious adverse effects occurring less than 0.5% of the time.25 Common—and milder—CNS effects include headache, dizziness, and insomnia. More severe CNS effects include tremors, restlessness, anxiety, light-headedness, confusion, hallucinations, paranoia, depression, nightmares, insomnia, and suicidal thoughts or attempts.25,26 Case reports have documented FQ-induced psychosis, catatonia, seizures, and delirium, with a higher incidence associated with higher doses of the antibiotic.26
A literature review aimed at identifying case reports yielded reports of 232 adverse psychiatric and neurologic drug reactions attributable to FQs in 145 patients.27 Nearly half were related to ciprofloxacin, with psychiatric reactions such as mania and acute psychosis being the most common. Most adverse CNS events (eg, convulsion, confusional state, agitation) developed rather quickly—in some cases within a few minutes of FQ administration and in others, within the first one to 8 days. In most reported cases, the patients had no known underlying psychiatric diseases or concomitant medication likely to have precipitated the development of delirium, psychosis, or seizures.28
Monitor older adults taking FQs. Because the risk of psychiatric adverse events is greatest in older individuals, especially those with known atherosclerotic disease or epilepsy, FQ therapy should be used cautiously—and with close monitoring—in this patient population. Symptoms such as weakness, confusion, tremor, loss of appetite, and depression are often incorrectly attributed simply to age, and thus go unreported as potential adverse effects of FQs.29 The exact mechanism by which FQs may induce seizures is unknown, but it may be related to excitatory effects at GABA receptors in the hippocampus.30
FQs may affect glucose levels
FQs have been reported to have varying effects on glucose metabolism, and have been implicated in both hypo- and hyperglycemia. FQ-related hypoglycemia has been thought to occur as a result of an increase in insulin secretion through a sulfonylurea-like action on pancreatic beta cells,31 via drug-drug interactions in patients with renal impairment,32 or via cytochrome P450 interactions.33 The mechanism of action relating to hyperglycemia is less well understood.
One retrospective cohort study in outpatients at a Veterans Administration facility sought to identify outcomes of hospitalization with a primary diagnosis of either hypo- or hyperglycemia in patients with a new prescription for either an FQ or azithromycin.34 In patients with diabetes, the OR for FQ-associated hypoglycemia (compared with azithromycin) was 2.1 for levofloxacin (95% CI, 1.4-3.3) and 1.1 for ciprofloxacin (95% CI, 0.6-2.0). The ORs for hyperglycemia were 1.8 for levofloxacin (95%, CI 1.2-2.7) and 1.0 for ciprofloxacin (95% CI, 0.6-1.8).
A retrospective chart review of more than 17,000 hospitalized patients who were receiving either an FQ or ceftriaxone revealed that 101 patients had either high (>200 mg/dL) or low (<50 mg/dL) glucose levels within 72 hours of receiving the antibiotic.35 Nearly 89% of those studied had diabetes and 40% had prescriptions for oral hypoglycemic agents. While most of these patients had underlying renal insufficiency, rates of hyperglycemia were greater with levofloxacin than with ceftriaxone. (In this study and the VA study, gatifloxacin had greater effects on glucose levels than the non-FQ antibiotics they were compared with; as noted earlier, however, gatifloxacin was removed from the US market in 2006.)
Diplopia is the most common ophthalmologic effect
A database review found 171 case reports of diplopia associated with FQs; ciprofloxacin was the most commonly implicated FQ, with 75 cases. The median time between medication initiation and the development of diplopia was 9.6 days. Most FQ-associated diplopia is completely reversible upon cessation of drug therapy, as evidenced by 53 published reports in which that was the case.36
Adverse effects of intraocular FQs. Ocular keratitis, corneal infiltrates and precipitates, and delayed corneal epithelial healing have been linked to the administration of intraocular FQs.36-38 In addition, retinal detachment has been found to occur in 3.3% of patients being treated with intraocular FQs, compared with 0.6% of controls (adjusted rate ratio=4.50; number needed to harm= 2500).39
CASE Suspecting CDAD and Achilles tendinitis secondary to ciprofloxacin, you stop the medication. Ms. Z’s urine culture is positive for Klebsiella pneumoniae, which is also sensitive to nitrofurantoin, so a 7-day course is prescribed. And, because a stool test for C difficile is positive, you prescribe a 7-day course of metronidazole, as well. Within 4 weeks of stopping the ciprofloxacin, the Achilles tendinitis had completely resolved.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected]
1. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-743.
2. Poisindex System. Ann Arbor, Mich: Truven Health Analytics.
3. Melhus A, Apelqvist J, Larsson J, et al. Levofloxacin-associated Achilles tendon rupture and tendinopathy. Scand J Infect Dis. 2003;35:768-770.
4. Damuth E, Heidelbaugh JJ, Malani PN, et al. Case report: an elderly subject with fluoroquinolone-associated Achilles tendinitis. Am J Geriatr Pharmacother. 2008;6:264-268.
5. Gold L, Igra H. Levofloxacin-induced tendon rupture: a case report and review of the literature. J Am Board Fam Pract. 2003;16:458-460.
6. Haddow LJ, Chandra Sekhar M, Hajela V, et al. Spontaneous Achilles tendon rupture in patients treated with levofloxacin. J Antimicrob Chemother. 2003;51:747-748.
7. US Food and Drug Administration. Information for healthcare professionals: fluoroquinolone antimicrobial drugs Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126085.htm. Accessed September 30, 2012.
8. Corrao G, Zambon A, Bertu L, et al. Evidence of tendinitis provoked by fluoroquinolone treatment: a case-control study. Drug Saf. 2006;29:889-896.
9. Sendzik J, Shakibaei M, Schafer-Korting M, et al. Fluoroquinolones cause changes in extracellular matrix, signaling proteins, metalloproteinases and caspase-3 in cultured human tendon cells. Toxicology. 2005;212:24-36.
10. Corps AN, Harrall RL, Curry VA, et al. Ciprofloxacin enhances the stimulation of matrix metalloproteinase 3 expression by interleukin-1beta in human tendon-derived cells: a potential mechanism of fluoroquinolone-induced tendinopathy. Arthritis Rheum. 2002;46:3034-3040.
11. Muzi F, Gravanta G, Tati E, et al. Fluoroquinolones-induced tendinitis and tendon rupture in kidney transplant recipients: 2 cases and a review of the literature. Transplant Proc. 2007;39:1673-1675.
12. Cholongitas E, Georgousaki C, Spyrou S, et al. Ciprofloxacin-induced acute cholestatic hepatitis. Ann Hepatol. 2009;8:400-401.
13. Denève C, Janoir C, Poilane I, et al. New trends in Clostridium difficile virulence and pathogenesis. Int J Antimicrob Agents. 2009;33(suppl 1):S24-S28.
14. Deshpande A, Pant C, Jain A, et al. Do fluoroquinolones predispose patients to Clostridium difficile associated disease? A review of the evidence. Curr Med Res Opin. 2008;24:329-333.
15. Novelle M, Morreale CA. The relationship between inpatient fluoroquinolone use and Clostridium difficile-associated diarrhea. Ann Pharmacother. 2010;44:826-831.
16. Adikwu E, Deo O. Fluoroquinolones reported hepatotoxicity. Pharmacology Pharmacy. 2012;3:328-336.
17. Nicholson SC, Webb CD, Moellering RC. Antimicrobial-associated acute hepatitis. Pharmacotherapy. 2002;22:794-797.
18. Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondria dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol. 2008;22:335-353.
19. Mitcheson JS, Chen J, Lim M, et al. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci. 2000;97:12329-12333.
20. US Food and Drug Administration. Determination that Tequin (gatifloxacin) was withdrawn from sale for reasons of safety or effectiveness. Fed Regist. 2008;73:52357-52358.
21. Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother. 2002;49:593-596.
22. Briasoulis A, Agarwal V, Pierce WJ. QT prolongation and torsade de pointes induced by fluoroquinolones: infrequent side effects from commonly used medications. Cardiology. 2011;120:103-110.
23. Prabhakar M, Krahn AD. Ciprofloxacin-induced acquired long QT syndrome. Heart Rhythm. 2004;1:624-626.
24. Demolis JL, Kubitza D, Tenneze L, et al. Effect of a single oral dose of moxifloxacin (400 and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther. 2000;68:658-666.
25. Tome AM, Filipe A. Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf. 2011;34:465-488.
26. Kiangkitiwan B. Levofloxacin-induced delirium with psychotic features. Gen Hosp Psychiatry. 2008;30:381-383.
27. Carbon C. Comparison of side effects of levofloxacin versus other fluoroquinolones. Chemotherapy. 2001;47(suppl 3):S9-S14.
28. LaSalvia EA, Domek GJ, Gitlin DF. Fluoroquinolone-induced suicidal ideation. Gen Hosp Psychiatry. 2010;32:108-110.
29. Stahlmann R, Lode H. Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging. 2010;27:193-209.
30. Owens RC, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl):S144-S157.
31. Smith KM, Lomaestro BM. What role do fluoroquinolone antimicrobial agents play in cardiac dysfunction and altered glycemic control? J Pharm Pract. 2003;16:349-360.
32. LeBlanc M, Belanger C, Cossette P. Severe and resistant hypoglycemia associated with concomitant gatifloxacin and glyburide therapy. Pharmacotherapy. 2004;24:926-931.
33. Graumlich JF, Habis S, Avelino RR, et al. Hypoglycemia in inpatients after gatifloxacin or levofloxacin therapy: nested case control study. Pharmacotherapy. 2005;25:1296-1302.
34. Aspinall SL, Good CB, Jiang R, et al. Severe dysglycemia with the fluoroquinolones: a class effect? Clin Infect Dis. 2009;49:402-408.
35. Mohr JF, McKinnon PS, Peymann PJ, et al. A retrospective, comparative evaluation of dysglycemias in hospitalized patients receiving gatifloxacin, levofloxacin, ciprofloxacin, or ceftriaxone. Pharmacotherapy. 2005;25:1303-1309.
36. Fraunfelder FW, Fraunfelder FT. Diplopia and fluoroquinolones. Ophthalmology. 2009;116:1814-1817.
37. Eiferman RA, Snyder JP, Nordquist RE. Ciprofloxacin microprecipitates and macroprecipitates in the human corneal epithelium. J Cataract Refract Surg. 2001;27:1701-1702.
38. Fraunfelder FW. Corneal toxicity from topical ocular and systemic medications. Cornea. 2006;25:1133-1138.
39. Etminan M, Forooghian F, Brophy JM, et al. Oral fluoroquinolones and the risk of retinal detachment. JAMA. 2012;307:1414-1419.
• Evaluate liver function before initiating fluoroquinolone (FQ) therapy, and avoid prescribing these antibiotics for patients at increased risk for hepatotoxicity. C
• Avoid prescribing FQs for patients with a history of prolonged QT syndrome. C
• Closely monitor older patients being treated with FQs, particularly if they have atherosclerosis or epilepsy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
PATIENT HANDOUT
Taking a fluoroquinolone antibiotic?
CASE Sara Z, a 62-year-old patient with a history of chronic urinary tract infections, presents with a 3-day history of dysuria and urinary frequency. Her last 2 urine cultures found Escherichia coli resistant to trimethoprim-sulfamethoxazole, amoxicillin, and cephalosporins. So her family physician ordered a urine culture and prescribed a 7-day course of ciprofloxacin empirically.
Five days later, Ms. Z returned, suffering from nonbloody diarrhea and bilateral Achilles tendon pain.
If you were treating Ms. Z, what would your next step be?
Widely used to treat urinary tract, skin, and pulmonary infections and to fight infections resistant to other antibiotics, fluoroquinolones (FQ) are generally regarded as safe in both inpatient and outpatient settings. Yet these broad-spectrum antibiotics are associated with both common and rare adverse effects, as well as a number of drug-drug interactions.
The Centers for Disease Control and Prevention estimates that adverse events from FQs leading to emergency department (ED) visits occur at a rate of 9.2 for every 10,000 prescriptions. That’s higher than the ED rates for cephalosporins (6.1 per 10,000) and macrolides (5.1 per 10,000), but far lower than for penicillins (13 per 10,000), clindamycin (18.5 per 10,000), sulfonamides (18.9 per 10,000), and vancomycin (24.1 per 10,000).1
In fact, adverse events associated with FQs range from mild and self-limiting to rare and severe. This review discusses both. Relatively common adverse effects and drug-drug interactions are discussed in the text, while the TABLE2 includes a broader range of potential adverse effects. You’ll also find a handout for patients taking FQs on page 195 that clearly describes signs and symptoms that need to be reported right away.
TABLE
Fluoroquinolones: Adverse effects to guard against*2
Cardiovascular
| Immunologic
|
Dermatologic
| Musculoskeletal
|
Drug-drug interactions
| Neurologic
|
Endocrine/Metabolic
| Ocular
|
Gastrointestinal
| Psychiatric
|
Hematologic
| Respiratory
|
| *This is not a complete list of potential adverse effects associated with fluoroquinolones. †Fluoroquinolones may potentiate warfarin. | |
A black box warning of tendinopathies
FQs exhibit an affinity for connective tissue, with higher concentrations found in bone and cartilage than in serum. While FQs are therefore well suited for treating orthopedic-related infections,3 they also increase the risk of tendinopathies.
In the last 2 decades, numerous case reports linking tendinitis and FQs have been published.4-6 In 2008, the US Food and Drug Administration (FDA) issued a black box warning of tendinitis and tendon rupture. Patients on FQ therapy should be advised to stop taking the antibiotic at the first sign of pain, swelling, or inflammation in a tendon, the FDA advises.7
How common is this adverse effect? A case-control study of 22,194 patients with a diagnosis of nontraumatic tendiopathy determined that FQ use resulted in a 1.3-fold risk of tendon rupture and more than a 4-fold risk of rupture of the Achilles tendon. One Achilles tendon rupture would occur for every 5958 patients treated with FQs, the researchers estimated.8
The precise mechanism by which FQs lead to tendinopathies is not completely understood. Studies suggest that the antibiotics cause a decrease in the synthesis of type I collagen, elastin, fibronectin, and beta (1)-integrin, and time- and concentration-dependent increases of cellular apoptosis.9 In vitro studies have shown inhibition of both cell proliferation and fibroblast metabolism when tissue is exposed to FQs, which may impede tissue healing.10
Which patients are at higher risk? The risk of FQ-associated tendinopathies is greatest in patients older than 60 years; in kidney, heart, and lung transplant recipients; and in patients taking an FQ with concomitant corticosteroid therapy. Decreased renal clearance of the medication may play a role in the increased risk.11
GI problems are common, especially in kids and older patients
Gastrointestinal (GI) disturbances are common in patients taking FQs, and typically occur more frequently in children and older adults, and in those taking higher doses. Reactions attributable to ciprofloxacin, for example, include nausea (affecting 1.4%-4% of adults and 2.7% of children taking the drug), vomiting (1%-2% of adults and 4.8% of children), diarrhea (<1%-2% of adults and 4.8% of children), and abdominal pain or discomfort (<1%-1.7% of adults and 3.3% of children).12
C difficile and FQ resistance. The extent to which Clostridium difficile-associated diarrhea (CDAD) is attributable to FQs has been subject to controversy in recent years. A previously uncommon strain of C difficile (B1/NAP1) with variations that have become more resistant to FQs has been linked to an increased incidence of CDAD across both the United States and Europe.13 A systematic review suggested that FQs predispose patients to CDAD,14 while a retrospective case-control study of 174 adult inpatients with CDAD determined that FQ administration did not significantly increase the rate of complications from C difficile (odds ratio [OR]=1.37; 95% confidence interval [CI], 0.72-2.61).15
Factors that affect risk of hepatotoxicity
Hepatitis/transaminitis, pancreatitis, jaundice, liver injury, and hepatic failure have all been reported in patients taking FQs, with the extent of hepatotoxicity varying based on the particular FQ taken, the dosage, and the patient’s baseline hepatic function.16,17 Comorbidities, including renal failure, may increase the potential for FQ-associated hepatotoxicity, as well. Thus, some experts recommend that clinicians evaluate a patient’s liver function before initiating FQ therapy and avoid prescribing FQs for those at added risk.
The exact mechanism by which FQ-induced hepatotoxicity occurs is unknown. One theory posits that the drugs generate oxidative radicals involved in mitochondrial damage, RNA processing, transcription, and inflammation;18 another suggests that FQs generate oxidative radicals in the liver as a result of cytochrome P450 metabolism.16 Case reports have shown that hepatitis resolves when the drug is discontinued, but often recurs in patients who are switched to a different FQ.16,17
Torsades de pointes is the key cardiovascular risk
FQs prolong the QT interval by blocking voltage-gated potassium channels, causing a reduction of the rapid component of the delayed rectifier potassium current in a dose-dependent fashion.19 But the average QT interval prolongation caused by FQs over a 3- to 6-month period does not appear to have clinical significance, nor is it associated with any discernible cardiac symptoms or impairment.19
For most, risk is minimal. There appears to be considerable variation in QT interval prolongation among FQs. A retrospective database analysis of published case reports of patients who received FQs over a 15-year period found 25 cases of torsades de pointes; moxifloxacin (highest), levofloxacin, and gatifloxacin (which was taken off the market by the FDA in 2006)20 were associated with a higher incidence than ciprofloxacin.21 Ciprofloxacin appears to be the safest FQ for cardiovascular events, with the lowest reported risk of torsades de pointes.22 However, several small randomized controlled trials have found that levofloxacin, like ciprofloxacin, did not significantly affect the QT interval.23,24
These patients face a higher risk. Notably, individuals with abnormal baseline QT prolongation (>440 ms in men; >460 ms in women) are at increased risk of developing torsades de pointes from the use of FQs, regardless of the dose.19 In fact, anyone with a history of prolonged QT syndrome should avoid these antibiotics, particularly if he or she is taking class Ia (eg, procainamide, quinidine) or class III (eg, amiodarone, sotalol) antiarrhythmics.19 Patients taking warfarin may be candidates for FQ therapy, but because the antibiotics may potentiate the anticoagulant, close monitoring is required. (Other potential drug-drug interactions are detailed in the TABLE.)
Evaluation of risk vs benefit is imperative prior to prescribing FQs for patients with increased risk for adverse cardiovascular events. An electrocardiogram is advisable, as well.
Mild neurologic and psychiatric effects not uncommon
Studies examining central nervous system (CNS) effects have estimated that neurotoxicity occurs in approximately 1% to 4.4% of patients taking FQs, with serious adverse effects occurring less than 0.5% of the time.25 Common—and milder—CNS effects include headache, dizziness, and insomnia. More severe CNS effects include tremors, restlessness, anxiety, light-headedness, confusion, hallucinations, paranoia, depression, nightmares, insomnia, and suicidal thoughts or attempts.25,26 Case reports have documented FQ-induced psychosis, catatonia, seizures, and delirium, with a higher incidence associated with higher doses of the antibiotic.26
A literature review aimed at identifying case reports yielded reports of 232 adverse psychiatric and neurologic drug reactions attributable to FQs in 145 patients.27 Nearly half were related to ciprofloxacin, with psychiatric reactions such as mania and acute psychosis being the most common. Most adverse CNS events (eg, convulsion, confusional state, agitation) developed rather quickly—in some cases within a few minutes of FQ administration and in others, within the first one to 8 days. In most reported cases, the patients had no known underlying psychiatric diseases or concomitant medication likely to have precipitated the development of delirium, psychosis, or seizures.28
Monitor older adults taking FQs. Because the risk of psychiatric adverse events is greatest in older individuals, especially those with known atherosclerotic disease or epilepsy, FQ therapy should be used cautiously—and with close monitoring—in this patient population. Symptoms such as weakness, confusion, tremor, loss of appetite, and depression are often incorrectly attributed simply to age, and thus go unreported as potential adverse effects of FQs.29 The exact mechanism by which FQs may induce seizures is unknown, but it may be related to excitatory effects at GABA receptors in the hippocampus.30
FQs may affect glucose levels
FQs have been reported to have varying effects on glucose metabolism, and have been implicated in both hypo- and hyperglycemia. FQ-related hypoglycemia has been thought to occur as a result of an increase in insulin secretion through a sulfonylurea-like action on pancreatic beta cells,31 via drug-drug interactions in patients with renal impairment,32 or via cytochrome P450 interactions.33 The mechanism of action relating to hyperglycemia is less well understood.
One retrospective cohort study in outpatients at a Veterans Administration facility sought to identify outcomes of hospitalization with a primary diagnosis of either hypo- or hyperglycemia in patients with a new prescription for either an FQ or azithromycin.34 In patients with diabetes, the OR for FQ-associated hypoglycemia (compared with azithromycin) was 2.1 for levofloxacin (95% CI, 1.4-3.3) and 1.1 for ciprofloxacin (95% CI, 0.6-2.0). The ORs for hyperglycemia were 1.8 for levofloxacin (95%, CI 1.2-2.7) and 1.0 for ciprofloxacin (95% CI, 0.6-1.8).
A retrospective chart review of more than 17,000 hospitalized patients who were receiving either an FQ or ceftriaxone revealed that 101 patients had either high (>200 mg/dL) or low (<50 mg/dL) glucose levels within 72 hours of receiving the antibiotic.35 Nearly 89% of those studied had diabetes and 40% had prescriptions for oral hypoglycemic agents. While most of these patients had underlying renal insufficiency, rates of hyperglycemia were greater with levofloxacin than with ceftriaxone. (In this study and the VA study, gatifloxacin had greater effects on glucose levels than the non-FQ antibiotics they were compared with; as noted earlier, however, gatifloxacin was removed from the US market in 2006.)
Diplopia is the most common ophthalmologic effect
A database review found 171 case reports of diplopia associated with FQs; ciprofloxacin was the most commonly implicated FQ, with 75 cases. The median time between medication initiation and the development of diplopia was 9.6 days. Most FQ-associated diplopia is completely reversible upon cessation of drug therapy, as evidenced by 53 published reports in which that was the case.36
Adverse effects of intraocular FQs. Ocular keratitis, corneal infiltrates and precipitates, and delayed corneal epithelial healing have been linked to the administration of intraocular FQs.36-38 In addition, retinal detachment has been found to occur in 3.3% of patients being treated with intraocular FQs, compared with 0.6% of controls (adjusted rate ratio=4.50; number needed to harm= 2500).39
CASE Suspecting CDAD and Achilles tendinitis secondary to ciprofloxacin, you stop the medication. Ms. Z’s urine culture is positive for Klebsiella pneumoniae, which is also sensitive to nitrofurantoin, so a 7-day course is prescribed. And, because a stool test for C difficile is positive, you prescribe a 7-day course of metronidazole, as well. Within 4 weeks of stopping the ciprofloxacin, the Achilles tendinitis had completely resolved.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected]
• Evaluate liver function before initiating fluoroquinolone (FQ) therapy, and avoid prescribing these antibiotics for patients at increased risk for hepatotoxicity. C
• Avoid prescribing FQs for patients with a history of prolonged QT syndrome. C
• Closely monitor older patients being treated with FQs, particularly if they have atherosclerosis or epilepsy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
PATIENT HANDOUT
Taking a fluoroquinolone antibiotic?
CASE Sara Z, a 62-year-old patient with a history of chronic urinary tract infections, presents with a 3-day history of dysuria and urinary frequency. Her last 2 urine cultures found Escherichia coli resistant to trimethoprim-sulfamethoxazole, amoxicillin, and cephalosporins. So her family physician ordered a urine culture and prescribed a 7-day course of ciprofloxacin empirically.
Five days later, Ms. Z returned, suffering from nonbloody diarrhea and bilateral Achilles tendon pain.
If you were treating Ms. Z, what would your next step be?
Widely used to treat urinary tract, skin, and pulmonary infections and to fight infections resistant to other antibiotics, fluoroquinolones (FQ) are generally regarded as safe in both inpatient and outpatient settings. Yet these broad-spectrum antibiotics are associated with both common and rare adverse effects, as well as a number of drug-drug interactions.
The Centers for Disease Control and Prevention estimates that adverse events from FQs leading to emergency department (ED) visits occur at a rate of 9.2 for every 10,000 prescriptions. That’s higher than the ED rates for cephalosporins (6.1 per 10,000) and macrolides (5.1 per 10,000), but far lower than for penicillins (13 per 10,000), clindamycin (18.5 per 10,000), sulfonamides (18.9 per 10,000), and vancomycin (24.1 per 10,000).1
In fact, adverse events associated with FQs range from mild and self-limiting to rare and severe. This review discusses both. Relatively common adverse effects and drug-drug interactions are discussed in the text, while the TABLE2 includes a broader range of potential adverse effects. You’ll also find a handout for patients taking FQs on page 195 that clearly describes signs and symptoms that need to be reported right away.
TABLE
Fluoroquinolones: Adverse effects to guard against*2
Cardiovascular
| Immunologic
|
Dermatologic
| Musculoskeletal
|
Drug-drug interactions
| Neurologic
|
Endocrine/Metabolic
| Ocular
|
Gastrointestinal
| Psychiatric
|
Hematologic
| Respiratory
|
| *This is not a complete list of potential adverse effects associated with fluoroquinolones. †Fluoroquinolones may potentiate warfarin. | |
A black box warning of tendinopathies
FQs exhibit an affinity for connective tissue, with higher concentrations found in bone and cartilage than in serum. While FQs are therefore well suited for treating orthopedic-related infections,3 they also increase the risk of tendinopathies.
In the last 2 decades, numerous case reports linking tendinitis and FQs have been published.4-6 In 2008, the US Food and Drug Administration (FDA) issued a black box warning of tendinitis and tendon rupture. Patients on FQ therapy should be advised to stop taking the antibiotic at the first sign of pain, swelling, or inflammation in a tendon, the FDA advises.7
How common is this adverse effect? A case-control study of 22,194 patients with a diagnosis of nontraumatic tendiopathy determined that FQ use resulted in a 1.3-fold risk of tendon rupture and more than a 4-fold risk of rupture of the Achilles tendon. One Achilles tendon rupture would occur for every 5958 patients treated with FQs, the researchers estimated.8
The precise mechanism by which FQs lead to tendinopathies is not completely understood. Studies suggest that the antibiotics cause a decrease in the synthesis of type I collagen, elastin, fibronectin, and beta (1)-integrin, and time- and concentration-dependent increases of cellular apoptosis.9 In vitro studies have shown inhibition of both cell proliferation and fibroblast metabolism when tissue is exposed to FQs, which may impede tissue healing.10
Which patients are at higher risk? The risk of FQ-associated tendinopathies is greatest in patients older than 60 years; in kidney, heart, and lung transplant recipients; and in patients taking an FQ with concomitant corticosteroid therapy. Decreased renal clearance of the medication may play a role in the increased risk.11
GI problems are common, especially in kids and older patients
Gastrointestinal (GI) disturbances are common in patients taking FQs, and typically occur more frequently in children and older adults, and in those taking higher doses. Reactions attributable to ciprofloxacin, for example, include nausea (affecting 1.4%-4% of adults and 2.7% of children taking the drug), vomiting (1%-2% of adults and 4.8% of children), diarrhea (<1%-2% of adults and 4.8% of children), and abdominal pain or discomfort (<1%-1.7% of adults and 3.3% of children).12
C difficile and FQ resistance. The extent to which Clostridium difficile-associated diarrhea (CDAD) is attributable to FQs has been subject to controversy in recent years. A previously uncommon strain of C difficile (B1/NAP1) with variations that have become more resistant to FQs has been linked to an increased incidence of CDAD across both the United States and Europe.13 A systematic review suggested that FQs predispose patients to CDAD,14 while a retrospective case-control study of 174 adult inpatients with CDAD determined that FQ administration did not significantly increase the rate of complications from C difficile (odds ratio [OR]=1.37; 95% confidence interval [CI], 0.72-2.61).15
Factors that affect risk of hepatotoxicity
Hepatitis/transaminitis, pancreatitis, jaundice, liver injury, and hepatic failure have all been reported in patients taking FQs, with the extent of hepatotoxicity varying based on the particular FQ taken, the dosage, and the patient’s baseline hepatic function.16,17 Comorbidities, including renal failure, may increase the potential for FQ-associated hepatotoxicity, as well. Thus, some experts recommend that clinicians evaluate a patient’s liver function before initiating FQ therapy and avoid prescribing FQs for those at added risk.
The exact mechanism by which FQ-induced hepatotoxicity occurs is unknown. One theory posits that the drugs generate oxidative radicals involved in mitochondrial damage, RNA processing, transcription, and inflammation;18 another suggests that FQs generate oxidative radicals in the liver as a result of cytochrome P450 metabolism.16 Case reports have shown that hepatitis resolves when the drug is discontinued, but often recurs in patients who are switched to a different FQ.16,17
Torsades de pointes is the key cardiovascular risk
FQs prolong the QT interval by blocking voltage-gated potassium channels, causing a reduction of the rapid component of the delayed rectifier potassium current in a dose-dependent fashion.19 But the average QT interval prolongation caused by FQs over a 3- to 6-month period does not appear to have clinical significance, nor is it associated with any discernible cardiac symptoms or impairment.19
For most, risk is minimal. There appears to be considerable variation in QT interval prolongation among FQs. A retrospective database analysis of published case reports of patients who received FQs over a 15-year period found 25 cases of torsades de pointes; moxifloxacin (highest), levofloxacin, and gatifloxacin (which was taken off the market by the FDA in 2006)20 were associated with a higher incidence than ciprofloxacin.21 Ciprofloxacin appears to be the safest FQ for cardiovascular events, with the lowest reported risk of torsades de pointes.22 However, several small randomized controlled trials have found that levofloxacin, like ciprofloxacin, did not significantly affect the QT interval.23,24
These patients face a higher risk. Notably, individuals with abnormal baseline QT prolongation (>440 ms in men; >460 ms in women) are at increased risk of developing torsades de pointes from the use of FQs, regardless of the dose.19 In fact, anyone with a history of prolonged QT syndrome should avoid these antibiotics, particularly if he or she is taking class Ia (eg, procainamide, quinidine) or class III (eg, amiodarone, sotalol) antiarrhythmics.19 Patients taking warfarin may be candidates for FQ therapy, but because the antibiotics may potentiate the anticoagulant, close monitoring is required. (Other potential drug-drug interactions are detailed in the TABLE.)
Evaluation of risk vs benefit is imperative prior to prescribing FQs for patients with increased risk for adverse cardiovascular events. An electrocardiogram is advisable, as well.
Mild neurologic and psychiatric effects not uncommon
Studies examining central nervous system (CNS) effects have estimated that neurotoxicity occurs in approximately 1% to 4.4% of patients taking FQs, with serious adverse effects occurring less than 0.5% of the time.25 Common—and milder—CNS effects include headache, dizziness, and insomnia. More severe CNS effects include tremors, restlessness, anxiety, light-headedness, confusion, hallucinations, paranoia, depression, nightmares, insomnia, and suicidal thoughts or attempts.25,26 Case reports have documented FQ-induced psychosis, catatonia, seizures, and delirium, with a higher incidence associated with higher doses of the antibiotic.26
A literature review aimed at identifying case reports yielded reports of 232 adverse psychiatric and neurologic drug reactions attributable to FQs in 145 patients.27 Nearly half were related to ciprofloxacin, with psychiatric reactions such as mania and acute psychosis being the most common. Most adverse CNS events (eg, convulsion, confusional state, agitation) developed rather quickly—in some cases within a few minutes of FQ administration and in others, within the first one to 8 days. In most reported cases, the patients had no known underlying psychiatric diseases or concomitant medication likely to have precipitated the development of delirium, psychosis, or seizures.28
Monitor older adults taking FQs. Because the risk of psychiatric adverse events is greatest in older individuals, especially those with known atherosclerotic disease or epilepsy, FQ therapy should be used cautiously—and with close monitoring—in this patient population. Symptoms such as weakness, confusion, tremor, loss of appetite, and depression are often incorrectly attributed simply to age, and thus go unreported as potential adverse effects of FQs.29 The exact mechanism by which FQs may induce seizures is unknown, but it may be related to excitatory effects at GABA receptors in the hippocampus.30
FQs may affect glucose levels
FQs have been reported to have varying effects on glucose metabolism, and have been implicated in both hypo- and hyperglycemia. FQ-related hypoglycemia has been thought to occur as a result of an increase in insulin secretion through a sulfonylurea-like action on pancreatic beta cells,31 via drug-drug interactions in patients with renal impairment,32 or via cytochrome P450 interactions.33 The mechanism of action relating to hyperglycemia is less well understood.
One retrospective cohort study in outpatients at a Veterans Administration facility sought to identify outcomes of hospitalization with a primary diagnosis of either hypo- or hyperglycemia in patients with a new prescription for either an FQ or azithromycin.34 In patients with diabetes, the OR for FQ-associated hypoglycemia (compared with azithromycin) was 2.1 for levofloxacin (95% CI, 1.4-3.3) and 1.1 for ciprofloxacin (95% CI, 0.6-2.0). The ORs for hyperglycemia were 1.8 for levofloxacin (95%, CI 1.2-2.7) and 1.0 for ciprofloxacin (95% CI, 0.6-1.8).
A retrospective chart review of more than 17,000 hospitalized patients who were receiving either an FQ or ceftriaxone revealed that 101 patients had either high (>200 mg/dL) or low (<50 mg/dL) glucose levels within 72 hours of receiving the antibiotic.35 Nearly 89% of those studied had diabetes and 40% had prescriptions for oral hypoglycemic agents. While most of these patients had underlying renal insufficiency, rates of hyperglycemia were greater with levofloxacin than with ceftriaxone. (In this study and the VA study, gatifloxacin had greater effects on glucose levels than the non-FQ antibiotics they were compared with; as noted earlier, however, gatifloxacin was removed from the US market in 2006.)
Diplopia is the most common ophthalmologic effect
A database review found 171 case reports of diplopia associated with FQs; ciprofloxacin was the most commonly implicated FQ, with 75 cases. The median time between medication initiation and the development of diplopia was 9.6 days. Most FQ-associated diplopia is completely reversible upon cessation of drug therapy, as evidenced by 53 published reports in which that was the case.36
Adverse effects of intraocular FQs. Ocular keratitis, corneal infiltrates and precipitates, and delayed corneal epithelial healing have been linked to the administration of intraocular FQs.36-38 In addition, retinal detachment has been found to occur in 3.3% of patients being treated with intraocular FQs, compared with 0.6% of controls (adjusted rate ratio=4.50; number needed to harm= 2500).39
CASE Suspecting CDAD and Achilles tendinitis secondary to ciprofloxacin, you stop the medication. Ms. Z’s urine culture is positive for Klebsiella pneumoniae, which is also sensitive to nitrofurantoin, so a 7-day course is prescribed. And, because a stool test for C difficile is positive, you prescribe a 7-day course of metronidazole, as well. Within 4 weeks of stopping the ciprofloxacin, the Achilles tendinitis had completely resolved.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected]
1. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-743.
2. Poisindex System. Ann Arbor, Mich: Truven Health Analytics.
3. Melhus A, Apelqvist J, Larsson J, et al. Levofloxacin-associated Achilles tendon rupture and tendinopathy. Scand J Infect Dis. 2003;35:768-770.
4. Damuth E, Heidelbaugh JJ, Malani PN, et al. Case report: an elderly subject with fluoroquinolone-associated Achilles tendinitis. Am J Geriatr Pharmacother. 2008;6:264-268.
5. Gold L, Igra H. Levofloxacin-induced tendon rupture: a case report and review of the literature. J Am Board Fam Pract. 2003;16:458-460.
6. Haddow LJ, Chandra Sekhar M, Hajela V, et al. Spontaneous Achilles tendon rupture in patients treated with levofloxacin. J Antimicrob Chemother. 2003;51:747-748.
7. US Food and Drug Administration. Information for healthcare professionals: fluoroquinolone antimicrobial drugs Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126085.htm. Accessed September 30, 2012.
8. Corrao G, Zambon A, Bertu L, et al. Evidence of tendinitis provoked by fluoroquinolone treatment: a case-control study. Drug Saf. 2006;29:889-896.
9. Sendzik J, Shakibaei M, Schafer-Korting M, et al. Fluoroquinolones cause changes in extracellular matrix, signaling proteins, metalloproteinases and caspase-3 in cultured human tendon cells. Toxicology. 2005;212:24-36.
10. Corps AN, Harrall RL, Curry VA, et al. Ciprofloxacin enhances the stimulation of matrix metalloproteinase 3 expression by interleukin-1beta in human tendon-derived cells: a potential mechanism of fluoroquinolone-induced tendinopathy. Arthritis Rheum. 2002;46:3034-3040.
11. Muzi F, Gravanta G, Tati E, et al. Fluoroquinolones-induced tendinitis and tendon rupture in kidney transplant recipients: 2 cases and a review of the literature. Transplant Proc. 2007;39:1673-1675.
12. Cholongitas E, Georgousaki C, Spyrou S, et al. Ciprofloxacin-induced acute cholestatic hepatitis. Ann Hepatol. 2009;8:400-401.
13. Denève C, Janoir C, Poilane I, et al. New trends in Clostridium difficile virulence and pathogenesis. Int J Antimicrob Agents. 2009;33(suppl 1):S24-S28.
14. Deshpande A, Pant C, Jain A, et al. Do fluoroquinolones predispose patients to Clostridium difficile associated disease? A review of the evidence. Curr Med Res Opin. 2008;24:329-333.
15. Novelle M, Morreale CA. The relationship between inpatient fluoroquinolone use and Clostridium difficile-associated diarrhea. Ann Pharmacother. 2010;44:826-831.
16. Adikwu E, Deo O. Fluoroquinolones reported hepatotoxicity. Pharmacology Pharmacy. 2012;3:328-336.
17. Nicholson SC, Webb CD, Moellering RC. Antimicrobial-associated acute hepatitis. Pharmacotherapy. 2002;22:794-797.
18. Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondria dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol. 2008;22:335-353.
19. Mitcheson JS, Chen J, Lim M, et al. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci. 2000;97:12329-12333.
20. US Food and Drug Administration. Determination that Tequin (gatifloxacin) was withdrawn from sale for reasons of safety or effectiveness. Fed Regist. 2008;73:52357-52358.
21. Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother. 2002;49:593-596.
22. Briasoulis A, Agarwal V, Pierce WJ. QT prolongation and torsade de pointes induced by fluoroquinolones: infrequent side effects from commonly used medications. Cardiology. 2011;120:103-110.
23. Prabhakar M, Krahn AD. Ciprofloxacin-induced acquired long QT syndrome. Heart Rhythm. 2004;1:624-626.
24. Demolis JL, Kubitza D, Tenneze L, et al. Effect of a single oral dose of moxifloxacin (400 and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther. 2000;68:658-666.
25. Tome AM, Filipe A. Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf. 2011;34:465-488.
26. Kiangkitiwan B. Levofloxacin-induced delirium with psychotic features. Gen Hosp Psychiatry. 2008;30:381-383.
27. Carbon C. Comparison of side effects of levofloxacin versus other fluoroquinolones. Chemotherapy. 2001;47(suppl 3):S9-S14.
28. LaSalvia EA, Domek GJ, Gitlin DF. Fluoroquinolone-induced suicidal ideation. Gen Hosp Psychiatry. 2010;32:108-110.
29. Stahlmann R, Lode H. Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging. 2010;27:193-209.
30. Owens RC, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl):S144-S157.
31. Smith KM, Lomaestro BM. What role do fluoroquinolone antimicrobial agents play in cardiac dysfunction and altered glycemic control? J Pharm Pract. 2003;16:349-360.
32. LeBlanc M, Belanger C, Cossette P. Severe and resistant hypoglycemia associated with concomitant gatifloxacin and glyburide therapy. Pharmacotherapy. 2004;24:926-931.
33. Graumlich JF, Habis S, Avelino RR, et al. Hypoglycemia in inpatients after gatifloxacin or levofloxacin therapy: nested case control study. Pharmacotherapy. 2005;25:1296-1302.
34. Aspinall SL, Good CB, Jiang R, et al. Severe dysglycemia with the fluoroquinolones: a class effect? Clin Infect Dis. 2009;49:402-408.
35. Mohr JF, McKinnon PS, Peymann PJ, et al. A retrospective, comparative evaluation of dysglycemias in hospitalized patients receiving gatifloxacin, levofloxacin, ciprofloxacin, or ceftriaxone. Pharmacotherapy. 2005;25:1303-1309.
36. Fraunfelder FW, Fraunfelder FT. Diplopia and fluoroquinolones. Ophthalmology. 2009;116:1814-1817.
37. Eiferman RA, Snyder JP, Nordquist RE. Ciprofloxacin microprecipitates and macroprecipitates in the human corneal epithelium. J Cataract Refract Surg. 2001;27:1701-1702.
38. Fraunfelder FW. Corneal toxicity from topical ocular and systemic medications. Cornea. 2006;25:1133-1138.
39. Etminan M, Forooghian F, Brophy JM, et al. Oral fluoroquinolones and the risk of retinal detachment. JAMA. 2012;307:1414-1419.
1. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-743.
2. Poisindex System. Ann Arbor, Mich: Truven Health Analytics.
3. Melhus A, Apelqvist J, Larsson J, et al. Levofloxacin-associated Achilles tendon rupture and tendinopathy. Scand J Infect Dis. 2003;35:768-770.
4. Damuth E, Heidelbaugh JJ, Malani PN, et al. Case report: an elderly subject with fluoroquinolone-associated Achilles tendinitis. Am J Geriatr Pharmacother. 2008;6:264-268.
5. Gold L, Igra H. Levofloxacin-induced tendon rupture: a case report and review of the literature. J Am Board Fam Pract. 2003;16:458-460.
6. Haddow LJ, Chandra Sekhar M, Hajela V, et al. Spontaneous Achilles tendon rupture in patients treated with levofloxacin. J Antimicrob Chemother. 2003;51:747-748.
7. US Food and Drug Administration. Information for healthcare professionals: fluoroquinolone antimicrobial drugs Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126085.htm. Accessed September 30, 2012.
8. Corrao G, Zambon A, Bertu L, et al. Evidence of tendinitis provoked by fluoroquinolone treatment: a case-control study. Drug Saf. 2006;29:889-896.
9. Sendzik J, Shakibaei M, Schafer-Korting M, et al. Fluoroquinolones cause changes in extracellular matrix, signaling proteins, metalloproteinases and caspase-3 in cultured human tendon cells. Toxicology. 2005;212:24-36.
10. Corps AN, Harrall RL, Curry VA, et al. Ciprofloxacin enhances the stimulation of matrix metalloproteinase 3 expression by interleukin-1beta in human tendon-derived cells: a potential mechanism of fluoroquinolone-induced tendinopathy. Arthritis Rheum. 2002;46:3034-3040.
11. Muzi F, Gravanta G, Tati E, et al. Fluoroquinolones-induced tendinitis and tendon rupture in kidney transplant recipients: 2 cases and a review of the literature. Transplant Proc. 2007;39:1673-1675.
12. Cholongitas E, Georgousaki C, Spyrou S, et al. Ciprofloxacin-induced acute cholestatic hepatitis. Ann Hepatol. 2009;8:400-401.
13. Denève C, Janoir C, Poilane I, et al. New trends in Clostridium difficile virulence and pathogenesis. Int J Antimicrob Agents. 2009;33(suppl 1):S24-S28.
14. Deshpande A, Pant C, Jain A, et al. Do fluoroquinolones predispose patients to Clostridium difficile associated disease? A review of the evidence. Curr Med Res Opin. 2008;24:329-333.
15. Novelle M, Morreale CA. The relationship between inpatient fluoroquinolone use and Clostridium difficile-associated diarrhea. Ann Pharmacother. 2010;44:826-831.
16. Adikwu E, Deo O. Fluoroquinolones reported hepatotoxicity. Pharmacology Pharmacy. 2012;3:328-336.
17. Nicholson SC, Webb CD, Moellering RC. Antimicrobial-associated acute hepatitis. Pharmacotherapy. 2002;22:794-797.
18. Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondria dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol. 2008;22:335-353.
19. Mitcheson JS, Chen J, Lim M, et al. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci. 2000;97:12329-12333.
20. US Food and Drug Administration. Determination that Tequin (gatifloxacin) was withdrawn from sale for reasons of safety or effectiveness. Fed Regist. 2008;73:52357-52358.
21. Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother. 2002;49:593-596.
22. Briasoulis A, Agarwal V, Pierce WJ. QT prolongation and torsade de pointes induced by fluoroquinolones: infrequent side effects from commonly used medications. Cardiology. 2011;120:103-110.
23. Prabhakar M, Krahn AD. Ciprofloxacin-induced acquired long QT syndrome. Heart Rhythm. 2004;1:624-626.
24. Demolis JL, Kubitza D, Tenneze L, et al. Effect of a single oral dose of moxifloxacin (400 and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther. 2000;68:658-666.
25. Tome AM, Filipe A. Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf. 2011;34:465-488.
26. Kiangkitiwan B. Levofloxacin-induced delirium with psychotic features. Gen Hosp Psychiatry. 2008;30:381-383.
27. Carbon C. Comparison of side effects of levofloxacin versus other fluoroquinolones. Chemotherapy. 2001;47(suppl 3):S9-S14.
28. LaSalvia EA, Domek GJ, Gitlin DF. Fluoroquinolone-induced suicidal ideation. Gen Hosp Psychiatry. 2010;32:108-110.
29. Stahlmann R, Lode H. Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging. 2010;27:193-209.
30. Owens RC, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl):S144-S157.
31. Smith KM, Lomaestro BM. What role do fluoroquinolone antimicrobial agents play in cardiac dysfunction and altered glycemic control? J Pharm Pract. 2003;16:349-360.
32. LeBlanc M, Belanger C, Cossette P. Severe and resistant hypoglycemia associated with concomitant gatifloxacin and glyburide therapy. Pharmacotherapy. 2004;24:926-931.
33. Graumlich JF, Habis S, Avelino RR, et al. Hypoglycemia in inpatients after gatifloxacin or levofloxacin therapy: nested case control study. Pharmacotherapy. 2005;25:1296-1302.
34. Aspinall SL, Good CB, Jiang R, et al. Severe dysglycemia with the fluoroquinolones: a class effect? Clin Infect Dis. 2009;49:402-408.
35. Mohr JF, McKinnon PS, Peymann PJ, et al. A retrospective, comparative evaluation of dysglycemias in hospitalized patients receiving gatifloxacin, levofloxacin, ciprofloxacin, or ceftriaxone. Pharmacotherapy. 2005;25:1303-1309.
36. Fraunfelder FW, Fraunfelder FT. Diplopia and fluoroquinolones. Ophthalmology. 2009;116:1814-1817.
37. Eiferman RA, Snyder JP, Nordquist RE. Ciprofloxacin microprecipitates and macroprecipitates in the human corneal epithelium. J Cataract Refract Surg. 2001;27:1701-1702.
38. Fraunfelder FW. Corneal toxicity from topical ocular and systemic medications. Cornea. 2006;25:1133-1138.
39. Etminan M, Forooghian F, Brophy JM, et al. Oral fluoroquinolones and the risk of retinal detachment. JAMA. 2012;307:1414-1419.
Help patients control their asthma
• Classify and treat asthma based on the patient’s worst symptom, whether or not it is the symptom that occurs most frequently. C
• Treat patients with poorly controlled asthma aggressively to gain quick control, then scale back slowly to the fewest medications and lowest doses needed to maintain control. A
• Reserve long-acting beta-agonists for use as an adjunct to inhaled corticosteroids for adults with poor baseline pulmonary function tests. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Angela D, a 34-year-old patient, has asthma with recurrent exacerbations. She uses a low-dose inhaled corticosteroid (ICS) daily and an albuterol inhaler, as needed, for shortness of breath or wheezing. She also has allergic rhinitis, for which she uses nasal fluticasone. Yet despite this regimen, Ms. D reports she still experiences wheezing, chest tightness, and shortness of breath 3 to 4 times a week and is awak-ened by coughing at least twice a week. In the past 6 months, she has had one emergency department (ED) visit and completed 2 courses of oral steroids.
Ms. D has gained weight since her last visit 3 months ago; her body mass index has gone from 27.5 to 29 kg/m2. And, while she has always been somewhat anxious, Ms. D notes that her anxiety has gotten progressively worse, as well.
About 25 million Americans—approximately one in 12—suffer from asthma1 and, despite improvements in asthma guidelines and treatment in the last 20 years,2 many still struggle with uncontrolled symptoms.3 The consequences can be severe.
Suboptimal control of asthma is associated with a significant decrease in quality of life, a greater likelihood of absence from work or school, and an increased risk for life-threatening events, trips to the ED, hospital admissions, and death.1 A multifaceted approach, including regular assessment, aggressive medication management, and attention to comorbidities, is needed to alleviate the suffering of patients with persistent asthma. This evidence-based review can help you provide such broad-based treatment.
Diagnosis and classification go hand in hand
The cornerstones of asthma management are accurate diagnosis and assessment of disease severity, based on both qualitative and quantitative measures. Start with a patient history, eliciting information about symptoms, triggers, risk factors, and most importantly, how often symptoms occur. Classic high-pitched wheezing sounds during exhalation, a cough that often worsens at night, shortness of breath, and chest tightness should raise suspicion for an asthma diagnosis.2 But frequency (and timing) of symptoms and exacerbations, as well as changes in the patient’s ability to function normally, help to determine whether asthma is classified as mild intermittent, mild persistent, moderate persistent, or severe persistent (TABLE).2
TABLE
Classifying asthma severity2
| Findings | Mild intermittent | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|
| Frequency | ≤2/wk | >2/wk, but <1/d | Daily | Continuous |
| Exacerbations | Rare | <2/wk | ≥2/wk | Frequent |
| Activity level | Normal | May decrease with exacerbation | Frequently limited | Significantly limited |
| Nighttime symptoms | ≤2/mo | >2/mo | >1/wk | Frequent |
| FEV1 (or PEF) predicted | >80% | >80% | >60% to <80% | ≤60% |
| PEF variability | <20% | 20%-30% | >30% | >30% |
| FEV1, forced expiratory volume in one second; PEF, peak expiratory flow. | ||||
Because asthma treatment should be based on its classification, an accurate assessment of disease severity is especially important for patients like Ms. D, who have been treated for asthma but continue to have unresolved symptoms. Keep in mind that asthma classification should be based on the worst symptom a patient has, not necessarily the symptom that occurs most frequently. Thus, a patient who has daytime symptoms requiring use of a rescue inhaler 2 to 3 times a week but is awakened at night with shortness of breath 2 times a week would receive a diagnosis of moderate persistent asthma on the basis of the night-time symptoms.
In assessing asthma severity, it is also important to ask specifically about recent events, including ED visits, hospitalizations, and intubations. This information, as well as answers to questions about smoking status, mental health problems, quality of life, and treatment compliance—and whether the patient can afford to purchase the asthma medications you’ve prescribed—can be used to assess the likelihood of poor outcomes.2
Factor in spirometry findings
History and physical examination alone cannot adequately diagnose and classify asthma severity.4,5 Spirometry, a reimbursable office test that can be administered by trained staff members, can be beneficial for any patient older than 5 years for whom a diagnosis of asthma is being considered or disease severity being determined.2 Other objective measures, such as the Mini Asthma Quality of Life questionnaire (http://erj.ersjournals.com/content/14/1/32.full.pdf+html) and peak expiratory flow measurement, may be helpful, as well.2,6
Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.
A dose increase avoids both the additional risk of adverse drug reactions and the added cost associated with another medication. But the easiest solution is not necessarily the best. Consider the evidence detailed below, which includes findings from studies published after the NHLBI’s guidelines.
The research on LABAs
LABAs have been widely used as adjunctive therapy for adults with asthma. However, a 2006 study raised safety concerns.16
The Salmeterol Multicenter Asthma Research Trial (SMART) compared the safety of the LABA salmeterol with a placebo added to usual asthma care over a 28-week treatment period. Overall, the primary composite end point—the number of respiratory-related deaths or life-threatening events—was low, and not statistically significant for salmeterol (50 vs 36; relative risk [RR]=1.40; 95% confidence interval [CI], 0.91-2.14).16 However, individual outcomes—respiratory-related deaths, asthma-related deaths, and asthma-related deaths or life-threatening episodes—were significantly more likely in the salmeterol group compared with the placebo group. In subgroup analysis, African American patients were found to be at greatest risk.16
It is hard to draw general conclusions from these data because the study was terminated early and poor outcomes were limited to a particular study year. Nonetheless, many physicians remain wary of LABAs as adjunctive therapy because of these findings and the media publicity they generated.
A 2010 Cochrane review provided additional data on the safety and efficacy of the combination of a LABA and ICS compared with a higher dose of ICS.17 The review, which included 48 randomized controlled trials, found that combination therapy had a lower risk of exacerbations for which oral corticosteroids were required than a higher dose of ICS (RR=0.88; 95% CI, 0.78-0.98; P=.02). The median number needed to treat (NNT) was 73. No significant difference in the risk of overall adverse events (RR=0.99; 95% CI, 0.95-1.03) was found, but there was an increase in the risk of tremor (RR=1.84; 95% CI, 1.20-2.82) and a decrease in risk for oral thrush (RR=0.58; 95% CI, 0.40-0.86) in the combination therapy group.
While the Cochrane review did not show a combination of LABA and ICS to be less safe overall than higher doses of ICS alone, the findings were less favorable for children and patients with higher baseline lung function, in circumstances in which the combination therapy was taken for a longer duration, and when the LABA being studied was formoterol.17
Overall, it is when a LABA is delivered via separate inhaler that adverse outcomes have been reported. Findings have been positive when the LABA is combined with ICS, and this combination is recommended as maintenance therapy for moderate to severe asthma.
Two new studies, published in March 2013, reported successful use of a LABA-ICS combination not only for maintenance via scheduled dosing, but also for early phases of exacerbation via extra dosing—an approach called Single inhaler Maintenance and Reliever Therapy (SMART).18,19 In both studies, SMART resulted in less excessive use of SABAs and less need for oral steroids, fewer hospitalizations for asthma, and fewer cases of progression to a full-blown exacerbation.
The takeaway: LABAs should be reserved for use as an adjunct to ICS in adults with poor baseline pulmonary function tests or severe asthma, and delivered as a combination product with ICS, not as a separate inhaled medication. SMART is a safe and effective means of administering LABA-ICS therapy for some patients at risk for frequent severe exacerbations.
When to consider LTRAs
LTRAs can be valuable medications in asthma management and there are extensive data on their use, particularly in the treatment of children with asthma. A Cochrane review published in 2012, however, supported current guideline recommendations, finding that as monotherapy, ICS are superior to LTRAs.20
When LTRAs as an adjunctive therapy to ICS were compared with ICS monotherapy, researchers found a modest improvement in PEF (weighted mean difference [WMD] =7.7 L/min; 95% CI, 3.6-11.8) in the group receiving combination therapy and a decrease in the need for a SABA as rescue therapy (WMD=1 puff/week; 95% CI, 0.5-2.0).21 There was no significant reduction in the risk of exacerbations requiring systemic steroids (RR=0.64; 95% CI, 0.38-1.07).
LABAs and LTRAs go head to head. A 2010 Cochrane review compared the efficacy and safety of a daily LABA vs a LTRA as add-on therapy for patients whose asthma was not well controlled with ICS monotherapy.22 The LABA/ICS combination was significantly better at reducing the risk of exacerbations requiring systemic corticosteroids than monotherapy with either a LTRA or ICS, reducing the risk from 11% to 9% (RR=0.83; 95% CI, 0.71-0.97). The NNT to prevent one exacerbation over 48 weeks was 38 (95% CI, 22-244).22
The safety of LABAs continues to be a concern, however, as serious adverse events were more common in the LABA group. The number needed to harm (NNH) with LABA therapy vs LTRA over 48 weeks was 78; 95% CI, 33 to infinity.22 (The width of the CI indicates that while harm is possible in as few as 33 patients, it is also possible that an infinite number of patients would need to be treated for one individual to incur harm.) Overall, the evidence suggests that LABAs are superior add-on therapy to ICS for the treatment of uncontrolled asthma compared with LTRAs, but their use nonetheless requires caution and close monitoring in African American and pediatric patients.17
Is there a role for a long-acting anticholinergic inhaler?
Long-acting anticholinergic medication (LAAM)—tiotropium is the only drug in this class on the market, but there are others in clinical trials—is the mainstay of therapy for chronic obstructive pulmonary disease. This drug class was not widely available or studied as an asthma treatment when the NHLBI guidelines were drafted.
A 2010 study of tiotropium challenged the notion that there is no place for LAAMs in asthma therapy. Using a 3-way crossover design, the study compared the addition of tiotropium to ICS with a double dose of ICS or a LABA/ICS combination.23
The results suggest that LAAMs could be useful in treating uncontrolled asthma. Compared with the double dose of ICS, the tiotropium/ICS combination increased PEF by a mean difference of 25.8 L/min (P<.001) and resulted in a statistically significant improvement in the proportion of asthma control days, FEV1, and daily symptom scores.23 As an adjunctive treatment to ICS, tiotropium was not inferior to a LABA.
CASE After a detailed history, physical exam, and diagnostic testing, Ms. D was given a diagnosis of moderate persistent asthma. We recognized the need to step up her treatment. Prior to making any changes in her medication regimen, however, our team, which included a clinical pharmacist, observed her use of inhaled medications and verified that she was using the inhaler properly. We then initiated combination therapy, pairing a LABA and ICS.
Comorbidities complicate asthma management
Asthma management is often complicated by other uncontrolled coexisting medical problems. Common comorbidities that can affect asthma severity include allergic rhinitis, chronic sinusitis, gastroesophageal reflux disease (GERD), obesity, obstructive sleep apnea (OSA), mental health disorders, tobacco use, and hormonal disturbances.2
Allergic rhinitis. Allergic rhinitis has been associated with worse asthma control and a negative impact on quality of life, and the upper airway inflammation associated with it should be treated.24
Antihistamines and nasal steroids are the most effective medical management. Some patients with allergic rhinitis benefit from blood or skin allergy testing for confirmation or to aid in avoidance. Referral to an allergist may be necessary if symptoms are recalcitrant, a food allergy is in question, or the diagnosis is unclear.
GERD. Compared with the general population, patients with asthma have a much higher risk of GERD, although it is not always symptomatic. While results are inconsistent and difficult to predict, treating symptomatic GERD with acid-blocking medications can result in better asthma control for some patients. However, proton pump inhibitors should not be used to treat asthma symptoms in patients with asymptomatic GERD.25,26
Obesity and OSA. Weight loss can significantly improve asthma control, decrease medication use, and improve quality of life.27,28 Obese patients are less likely to respond to treatment with ICS.2 Weight loss also benefits those who suffer from OSA, which may contribute to airway hyperresponsiveness.29
Mental health disorders. Compared with the general population, patients with asthma are more likely to have depression, anxiety, and panic disorders.30 Diagnosis and treatment of these comorbid conditions can lead to better asthma management, increased medication adherence, decreased health care utilization—including fewer ED visits and hospitalizations—and a better quality of life.30
CASE We also addressed our patient’s comorbidities—weight gain, allergic rhinitis, and anxiety. The allergic rhinitis was already well-controlled with a nasal steroid, but we suspected a relationship between Ms. D’s weight gain and increasing anxiety associated with some recent life events. We suggested she see a counselor, and she agreed.
When the patient returned in 12 weeks, she reported that she hardly needed her rescue inhaler anymore and that she was managing her anxiety more effectively. She also told us that she had begun a low-fat dietary regimen, and we confirmed that she had already lost 5 pounds.
CORRESPONDENCE
Stephen A. Wilson, MD, MPH, FAAFP, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; [email protected]
1. American Academy of Allergy, Asthma, and Immunology. Asthma statistics. Available at: http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx. Accessed March 7, 2012.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for diagnosis and management of asthma. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Centers for Disease Control and Prevention. National surveillance for asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54.
4. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children. Arch Pediatr Adolesc Med. 2006;160:844-850.
5. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children. Am J Respir Crit Care Med. 2004;170:426-432.
6. Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32-38.
7. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179-187.
8. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
9. Bateman ED, Hurd SS, Barnes PJ, et al. Global Strategy for Asthma Management and Prevention. Eur Respir J. 2008;31:143-178.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. February 2013. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 7, 2013.
11. AsthmaMD. Available at: http://www.asthmamd.org/#resources/iphone_chart.jpg. Accessed March 7, 2013.
12. Indiegogo. Huff & Puff. Available at: http://www.indiegogo.com/projects/the-best-asthma-education-app-in-the-world-period. Accessed March 7, 2013.
13. Vimukti Technologies Pvt Ltd. Peak flow calculator. Available at: http://appworld.blackberry.com/webstore/content/7615. Accessed March 7, 2013,
14. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;35:1068-1080.
15. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375-387.
16. Nelson HS, Weiss ST, Bleeker ER, et al. The Salmeterol Multicenter Asthma Research Trial. Chest. 2006;129:15-26.
17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533.-
18. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma. Lancet Respir Med. 2013;1:23-31.
19. Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerba-tions. Lancet Respir Med. 2013;1:32-42.
20. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.-
21. Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004;(1):CD003133.-
22. Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011;(5):CD003137.-
23. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
24. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65:1290-1297.
25. Gibson PG, Henry RL, Coughlan JL. Gastroesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003;(2):CD001496.-
26. The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1499.
27. Eneli IU, Skybo T, Camargo CA, Jr. Weight loss and asthma. Thorax. 2008;63:671-676.
28. Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma. BMJ. 2000;320:827-832.
29. Sariman N, Levent E, Cubuk R, et al. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath. 2011;15:341-50.
30. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22-28.
• Classify and treat asthma based on the patient’s worst symptom, whether or not it is the symptom that occurs most frequently. C
• Treat patients with poorly controlled asthma aggressively to gain quick control, then scale back slowly to the fewest medications and lowest doses needed to maintain control. A
• Reserve long-acting beta-agonists for use as an adjunct to inhaled corticosteroids for adults with poor baseline pulmonary function tests. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Angela D, a 34-year-old patient, has asthma with recurrent exacerbations. She uses a low-dose inhaled corticosteroid (ICS) daily and an albuterol inhaler, as needed, for shortness of breath or wheezing. She also has allergic rhinitis, for which she uses nasal fluticasone. Yet despite this regimen, Ms. D reports she still experiences wheezing, chest tightness, and shortness of breath 3 to 4 times a week and is awak-ened by coughing at least twice a week. In the past 6 months, she has had one emergency department (ED) visit and completed 2 courses of oral steroids.
Ms. D has gained weight since her last visit 3 months ago; her body mass index has gone from 27.5 to 29 kg/m2. And, while she has always been somewhat anxious, Ms. D notes that her anxiety has gotten progressively worse, as well.
About 25 million Americans—approximately one in 12—suffer from asthma1 and, despite improvements in asthma guidelines and treatment in the last 20 years,2 many still struggle with uncontrolled symptoms.3 The consequences can be severe.
Suboptimal control of asthma is associated with a significant decrease in quality of life, a greater likelihood of absence from work or school, and an increased risk for life-threatening events, trips to the ED, hospital admissions, and death.1 A multifaceted approach, including regular assessment, aggressive medication management, and attention to comorbidities, is needed to alleviate the suffering of patients with persistent asthma. This evidence-based review can help you provide such broad-based treatment.
Diagnosis and classification go hand in hand
The cornerstones of asthma management are accurate diagnosis and assessment of disease severity, based on both qualitative and quantitative measures. Start with a patient history, eliciting information about symptoms, triggers, risk factors, and most importantly, how often symptoms occur. Classic high-pitched wheezing sounds during exhalation, a cough that often worsens at night, shortness of breath, and chest tightness should raise suspicion for an asthma diagnosis.2 But frequency (and timing) of symptoms and exacerbations, as well as changes in the patient’s ability to function normally, help to determine whether asthma is classified as mild intermittent, mild persistent, moderate persistent, or severe persistent (TABLE).2
TABLE
Classifying asthma severity2
| Findings | Mild intermittent | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|
| Frequency | ≤2/wk | >2/wk, but <1/d | Daily | Continuous |
| Exacerbations | Rare | <2/wk | ≥2/wk | Frequent |
| Activity level | Normal | May decrease with exacerbation | Frequently limited | Significantly limited |
| Nighttime symptoms | ≤2/mo | >2/mo | >1/wk | Frequent |
| FEV1 (or PEF) predicted | >80% | >80% | >60% to <80% | ≤60% |
| PEF variability | <20% | 20%-30% | >30% | >30% |
| FEV1, forced expiratory volume in one second; PEF, peak expiratory flow. | ||||
Because asthma treatment should be based on its classification, an accurate assessment of disease severity is especially important for patients like Ms. D, who have been treated for asthma but continue to have unresolved symptoms. Keep in mind that asthma classification should be based on the worst symptom a patient has, not necessarily the symptom that occurs most frequently. Thus, a patient who has daytime symptoms requiring use of a rescue inhaler 2 to 3 times a week but is awakened at night with shortness of breath 2 times a week would receive a diagnosis of moderate persistent asthma on the basis of the night-time symptoms.
In assessing asthma severity, it is also important to ask specifically about recent events, including ED visits, hospitalizations, and intubations. This information, as well as answers to questions about smoking status, mental health problems, quality of life, and treatment compliance—and whether the patient can afford to purchase the asthma medications you’ve prescribed—can be used to assess the likelihood of poor outcomes.2
Factor in spirometry findings
History and physical examination alone cannot adequately diagnose and classify asthma severity.4,5 Spirometry, a reimbursable office test that can be administered by trained staff members, can be beneficial for any patient older than 5 years for whom a diagnosis of asthma is being considered or disease severity being determined.2 Other objective measures, such as the Mini Asthma Quality of Life questionnaire (http://erj.ersjournals.com/content/14/1/32.full.pdf+html) and peak expiratory flow measurement, may be helpful, as well.2,6
Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.
A dose increase avoids both the additional risk of adverse drug reactions and the added cost associated with another medication. But the easiest solution is not necessarily the best. Consider the evidence detailed below, which includes findings from studies published after the NHLBI’s guidelines.
The research on LABAs
LABAs have been widely used as adjunctive therapy for adults with asthma. However, a 2006 study raised safety concerns.16
The Salmeterol Multicenter Asthma Research Trial (SMART) compared the safety of the LABA salmeterol with a placebo added to usual asthma care over a 28-week treatment period. Overall, the primary composite end point—the number of respiratory-related deaths or life-threatening events—was low, and not statistically significant for salmeterol (50 vs 36; relative risk [RR]=1.40; 95% confidence interval [CI], 0.91-2.14).16 However, individual outcomes—respiratory-related deaths, asthma-related deaths, and asthma-related deaths or life-threatening episodes—were significantly more likely in the salmeterol group compared with the placebo group. In subgroup analysis, African American patients were found to be at greatest risk.16
It is hard to draw general conclusions from these data because the study was terminated early and poor outcomes were limited to a particular study year. Nonetheless, many physicians remain wary of LABAs as adjunctive therapy because of these findings and the media publicity they generated.
A 2010 Cochrane review provided additional data on the safety and efficacy of the combination of a LABA and ICS compared with a higher dose of ICS.17 The review, which included 48 randomized controlled trials, found that combination therapy had a lower risk of exacerbations for which oral corticosteroids were required than a higher dose of ICS (RR=0.88; 95% CI, 0.78-0.98; P=.02). The median number needed to treat (NNT) was 73. No significant difference in the risk of overall adverse events (RR=0.99; 95% CI, 0.95-1.03) was found, but there was an increase in the risk of tremor (RR=1.84; 95% CI, 1.20-2.82) and a decrease in risk for oral thrush (RR=0.58; 95% CI, 0.40-0.86) in the combination therapy group.
While the Cochrane review did not show a combination of LABA and ICS to be less safe overall than higher doses of ICS alone, the findings were less favorable for children and patients with higher baseline lung function, in circumstances in which the combination therapy was taken for a longer duration, and when the LABA being studied was formoterol.17
Overall, it is when a LABA is delivered via separate inhaler that adverse outcomes have been reported. Findings have been positive when the LABA is combined with ICS, and this combination is recommended as maintenance therapy for moderate to severe asthma.
Two new studies, published in March 2013, reported successful use of a LABA-ICS combination not only for maintenance via scheduled dosing, but also for early phases of exacerbation via extra dosing—an approach called Single inhaler Maintenance and Reliever Therapy (SMART).18,19 In both studies, SMART resulted in less excessive use of SABAs and less need for oral steroids, fewer hospitalizations for asthma, and fewer cases of progression to a full-blown exacerbation.
The takeaway: LABAs should be reserved for use as an adjunct to ICS in adults with poor baseline pulmonary function tests or severe asthma, and delivered as a combination product with ICS, not as a separate inhaled medication. SMART is a safe and effective means of administering LABA-ICS therapy for some patients at risk for frequent severe exacerbations.
When to consider LTRAs
LTRAs can be valuable medications in asthma management and there are extensive data on their use, particularly in the treatment of children with asthma. A Cochrane review published in 2012, however, supported current guideline recommendations, finding that as monotherapy, ICS are superior to LTRAs.20
When LTRAs as an adjunctive therapy to ICS were compared with ICS monotherapy, researchers found a modest improvement in PEF (weighted mean difference [WMD] =7.7 L/min; 95% CI, 3.6-11.8) in the group receiving combination therapy and a decrease in the need for a SABA as rescue therapy (WMD=1 puff/week; 95% CI, 0.5-2.0).21 There was no significant reduction in the risk of exacerbations requiring systemic steroids (RR=0.64; 95% CI, 0.38-1.07).
LABAs and LTRAs go head to head. A 2010 Cochrane review compared the efficacy and safety of a daily LABA vs a LTRA as add-on therapy for patients whose asthma was not well controlled with ICS monotherapy.22 The LABA/ICS combination was significantly better at reducing the risk of exacerbations requiring systemic corticosteroids than monotherapy with either a LTRA or ICS, reducing the risk from 11% to 9% (RR=0.83; 95% CI, 0.71-0.97). The NNT to prevent one exacerbation over 48 weeks was 38 (95% CI, 22-244).22
The safety of LABAs continues to be a concern, however, as serious adverse events were more common in the LABA group. The number needed to harm (NNH) with LABA therapy vs LTRA over 48 weeks was 78; 95% CI, 33 to infinity.22 (The width of the CI indicates that while harm is possible in as few as 33 patients, it is also possible that an infinite number of patients would need to be treated for one individual to incur harm.) Overall, the evidence suggests that LABAs are superior add-on therapy to ICS for the treatment of uncontrolled asthma compared with LTRAs, but their use nonetheless requires caution and close monitoring in African American and pediatric patients.17
Is there a role for a long-acting anticholinergic inhaler?
Long-acting anticholinergic medication (LAAM)—tiotropium is the only drug in this class on the market, but there are others in clinical trials—is the mainstay of therapy for chronic obstructive pulmonary disease. This drug class was not widely available or studied as an asthma treatment when the NHLBI guidelines were drafted.
A 2010 study of tiotropium challenged the notion that there is no place for LAAMs in asthma therapy. Using a 3-way crossover design, the study compared the addition of tiotropium to ICS with a double dose of ICS or a LABA/ICS combination.23
The results suggest that LAAMs could be useful in treating uncontrolled asthma. Compared with the double dose of ICS, the tiotropium/ICS combination increased PEF by a mean difference of 25.8 L/min (P<.001) and resulted in a statistically significant improvement in the proportion of asthma control days, FEV1, and daily symptom scores.23 As an adjunctive treatment to ICS, tiotropium was not inferior to a LABA.
CASE After a detailed history, physical exam, and diagnostic testing, Ms. D was given a diagnosis of moderate persistent asthma. We recognized the need to step up her treatment. Prior to making any changes in her medication regimen, however, our team, which included a clinical pharmacist, observed her use of inhaled medications and verified that she was using the inhaler properly. We then initiated combination therapy, pairing a LABA and ICS.
Comorbidities complicate asthma management
Asthma management is often complicated by other uncontrolled coexisting medical problems. Common comorbidities that can affect asthma severity include allergic rhinitis, chronic sinusitis, gastroesophageal reflux disease (GERD), obesity, obstructive sleep apnea (OSA), mental health disorders, tobacco use, and hormonal disturbances.2
Allergic rhinitis. Allergic rhinitis has been associated with worse asthma control and a negative impact on quality of life, and the upper airway inflammation associated with it should be treated.24
Antihistamines and nasal steroids are the most effective medical management. Some patients with allergic rhinitis benefit from blood or skin allergy testing for confirmation or to aid in avoidance. Referral to an allergist may be necessary if symptoms are recalcitrant, a food allergy is in question, or the diagnosis is unclear.
GERD. Compared with the general population, patients with asthma have a much higher risk of GERD, although it is not always symptomatic. While results are inconsistent and difficult to predict, treating symptomatic GERD with acid-blocking medications can result in better asthma control for some patients. However, proton pump inhibitors should not be used to treat asthma symptoms in patients with asymptomatic GERD.25,26
Obesity and OSA. Weight loss can significantly improve asthma control, decrease medication use, and improve quality of life.27,28 Obese patients are less likely to respond to treatment with ICS.2 Weight loss also benefits those who suffer from OSA, which may contribute to airway hyperresponsiveness.29
Mental health disorders. Compared with the general population, patients with asthma are more likely to have depression, anxiety, and panic disorders.30 Diagnosis and treatment of these comorbid conditions can lead to better asthma management, increased medication adherence, decreased health care utilization—including fewer ED visits and hospitalizations—and a better quality of life.30
CASE We also addressed our patient’s comorbidities—weight gain, allergic rhinitis, and anxiety. The allergic rhinitis was already well-controlled with a nasal steroid, but we suspected a relationship between Ms. D’s weight gain and increasing anxiety associated with some recent life events. We suggested she see a counselor, and she agreed.
When the patient returned in 12 weeks, she reported that she hardly needed her rescue inhaler anymore and that she was managing her anxiety more effectively. She also told us that she had begun a low-fat dietary regimen, and we confirmed that she had already lost 5 pounds.
CORRESPONDENCE
Stephen A. Wilson, MD, MPH, FAAFP, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; [email protected]
• Classify and treat asthma based on the patient’s worst symptom, whether or not it is the symptom that occurs most frequently. C
• Treat patients with poorly controlled asthma aggressively to gain quick control, then scale back slowly to the fewest medications and lowest doses needed to maintain control. A
• Reserve long-acting beta-agonists for use as an adjunct to inhaled corticosteroids for adults with poor baseline pulmonary function tests. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Angela D, a 34-year-old patient, has asthma with recurrent exacerbations. She uses a low-dose inhaled corticosteroid (ICS) daily and an albuterol inhaler, as needed, for shortness of breath or wheezing. She also has allergic rhinitis, for which she uses nasal fluticasone. Yet despite this regimen, Ms. D reports she still experiences wheezing, chest tightness, and shortness of breath 3 to 4 times a week and is awak-ened by coughing at least twice a week. In the past 6 months, she has had one emergency department (ED) visit and completed 2 courses of oral steroids.
Ms. D has gained weight since her last visit 3 months ago; her body mass index has gone from 27.5 to 29 kg/m2. And, while she has always been somewhat anxious, Ms. D notes that her anxiety has gotten progressively worse, as well.
About 25 million Americans—approximately one in 12—suffer from asthma1 and, despite improvements in asthma guidelines and treatment in the last 20 years,2 many still struggle with uncontrolled symptoms.3 The consequences can be severe.
Suboptimal control of asthma is associated with a significant decrease in quality of life, a greater likelihood of absence from work or school, and an increased risk for life-threatening events, trips to the ED, hospital admissions, and death.1 A multifaceted approach, including regular assessment, aggressive medication management, and attention to comorbidities, is needed to alleviate the suffering of patients with persistent asthma. This evidence-based review can help you provide such broad-based treatment.
Diagnosis and classification go hand in hand
The cornerstones of asthma management are accurate diagnosis and assessment of disease severity, based on both qualitative and quantitative measures. Start with a patient history, eliciting information about symptoms, triggers, risk factors, and most importantly, how often symptoms occur. Classic high-pitched wheezing sounds during exhalation, a cough that often worsens at night, shortness of breath, and chest tightness should raise suspicion for an asthma diagnosis.2 But frequency (and timing) of symptoms and exacerbations, as well as changes in the patient’s ability to function normally, help to determine whether asthma is classified as mild intermittent, mild persistent, moderate persistent, or severe persistent (TABLE).2
TABLE
Classifying asthma severity2
| Findings | Mild intermittent | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|
| Frequency | ≤2/wk | >2/wk, but <1/d | Daily | Continuous |
| Exacerbations | Rare | <2/wk | ≥2/wk | Frequent |
| Activity level | Normal | May decrease with exacerbation | Frequently limited | Significantly limited |
| Nighttime symptoms | ≤2/mo | >2/mo | >1/wk | Frequent |
| FEV1 (or PEF) predicted | >80% | >80% | >60% to <80% | ≤60% |
| PEF variability | <20% | 20%-30% | >30% | >30% |
| FEV1, forced expiratory volume in one second; PEF, peak expiratory flow. | ||||
Because asthma treatment should be based on its classification, an accurate assessment of disease severity is especially important for patients like Ms. D, who have been treated for asthma but continue to have unresolved symptoms. Keep in mind that asthma classification should be based on the worst symptom a patient has, not necessarily the symptom that occurs most frequently. Thus, a patient who has daytime symptoms requiring use of a rescue inhaler 2 to 3 times a week but is awakened at night with shortness of breath 2 times a week would receive a diagnosis of moderate persistent asthma on the basis of the night-time symptoms.
In assessing asthma severity, it is also important to ask specifically about recent events, including ED visits, hospitalizations, and intubations. This information, as well as answers to questions about smoking status, mental health problems, quality of life, and treatment compliance—and whether the patient can afford to purchase the asthma medications you’ve prescribed—can be used to assess the likelihood of poor outcomes.2
Factor in spirometry findings
History and physical examination alone cannot adequately diagnose and classify asthma severity.4,5 Spirometry, a reimbursable office test that can be administered by trained staff members, can be beneficial for any patient older than 5 years for whom a diagnosis of asthma is being considered or disease severity being determined.2 Other objective measures, such as the Mini Asthma Quality of Life questionnaire (http://erj.ersjournals.com/content/14/1/32.full.pdf+html) and peak expiratory flow measurement, may be helpful, as well.2,6
Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.
A dose increase avoids both the additional risk of adverse drug reactions and the added cost associated with another medication. But the easiest solution is not necessarily the best. Consider the evidence detailed below, which includes findings from studies published after the NHLBI’s guidelines.
The research on LABAs
LABAs have been widely used as adjunctive therapy for adults with asthma. However, a 2006 study raised safety concerns.16
The Salmeterol Multicenter Asthma Research Trial (SMART) compared the safety of the LABA salmeterol with a placebo added to usual asthma care over a 28-week treatment period. Overall, the primary composite end point—the number of respiratory-related deaths or life-threatening events—was low, and not statistically significant for salmeterol (50 vs 36; relative risk [RR]=1.40; 95% confidence interval [CI], 0.91-2.14).16 However, individual outcomes—respiratory-related deaths, asthma-related deaths, and asthma-related deaths or life-threatening episodes—were significantly more likely in the salmeterol group compared with the placebo group. In subgroup analysis, African American patients were found to be at greatest risk.16
It is hard to draw general conclusions from these data because the study was terminated early and poor outcomes were limited to a particular study year. Nonetheless, many physicians remain wary of LABAs as adjunctive therapy because of these findings and the media publicity they generated.
A 2010 Cochrane review provided additional data on the safety and efficacy of the combination of a LABA and ICS compared with a higher dose of ICS.17 The review, which included 48 randomized controlled trials, found that combination therapy had a lower risk of exacerbations for which oral corticosteroids were required than a higher dose of ICS (RR=0.88; 95% CI, 0.78-0.98; P=.02). The median number needed to treat (NNT) was 73. No significant difference in the risk of overall adverse events (RR=0.99; 95% CI, 0.95-1.03) was found, but there was an increase in the risk of tremor (RR=1.84; 95% CI, 1.20-2.82) and a decrease in risk for oral thrush (RR=0.58; 95% CI, 0.40-0.86) in the combination therapy group.
While the Cochrane review did not show a combination of LABA and ICS to be less safe overall than higher doses of ICS alone, the findings were less favorable for children and patients with higher baseline lung function, in circumstances in which the combination therapy was taken for a longer duration, and when the LABA being studied was formoterol.17
Overall, it is when a LABA is delivered via separate inhaler that adverse outcomes have been reported. Findings have been positive when the LABA is combined with ICS, and this combination is recommended as maintenance therapy for moderate to severe asthma.
Two new studies, published in March 2013, reported successful use of a LABA-ICS combination not only for maintenance via scheduled dosing, but also for early phases of exacerbation via extra dosing—an approach called Single inhaler Maintenance and Reliever Therapy (SMART).18,19 In both studies, SMART resulted in less excessive use of SABAs and less need for oral steroids, fewer hospitalizations for asthma, and fewer cases of progression to a full-blown exacerbation.
The takeaway: LABAs should be reserved for use as an adjunct to ICS in adults with poor baseline pulmonary function tests or severe asthma, and delivered as a combination product with ICS, not as a separate inhaled medication. SMART is a safe and effective means of administering LABA-ICS therapy for some patients at risk for frequent severe exacerbations.
When to consider LTRAs
LTRAs can be valuable medications in asthma management and there are extensive data on their use, particularly in the treatment of children with asthma. A Cochrane review published in 2012, however, supported current guideline recommendations, finding that as monotherapy, ICS are superior to LTRAs.20
When LTRAs as an adjunctive therapy to ICS were compared with ICS monotherapy, researchers found a modest improvement in PEF (weighted mean difference [WMD] =7.7 L/min; 95% CI, 3.6-11.8) in the group receiving combination therapy and a decrease in the need for a SABA as rescue therapy (WMD=1 puff/week; 95% CI, 0.5-2.0).21 There was no significant reduction in the risk of exacerbations requiring systemic steroids (RR=0.64; 95% CI, 0.38-1.07).
LABAs and LTRAs go head to head. A 2010 Cochrane review compared the efficacy and safety of a daily LABA vs a LTRA as add-on therapy for patients whose asthma was not well controlled with ICS monotherapy.22 The LABA/ICS combination was significantly better at reducing the risk of exacerbations requiring systemic corticosteroids than monotherapy with either a LTRA or ICS, reducing the risk from 11% to 9% (RR=0.83; 95% CI, 0.71-0.97). The NNT to prevent one exacerbation over 48 weeks was 38 (95% CI, 22-244).22
The safety of LABAs continues to be a concern, however, as serious adverse events were more common in the LABA group. The number needed to harm (NNH) with LABA therapy vs LTRA over 48 weeks was 78; 95% CI, 33 to infinity.22 (The width of the CI indicates that while harm is possible in as few as 33 patients, it is also possible that an infinite number of patients would need to be treated for one individual to incur harm.) Overall, the evidence suggests that LABAs are superior add-on therapy to ICS for the treatment of uncontrolled asthma compared with LTRAs, but their use nonetheless requires caution and close monitoring in African American and pediatric patients.17
Is there a role for a long-acting anticholinergic inhaler?
Long-acting anticholinergic medication (LAAM)—tiotropium is the only drug in this class on the market, but there are others in clinical trials—is the mainstay of therapy for chronic obstructive pulmonary disease. This drug class was not widely available or studied as an asthma treatment when the NHLBI guidelines were drafted.
A 2010 study of tiotropium challenged the notion that there is no place for LAAMs in asthma therapy. Using a 3-way crossover design, the study compared the addition of tiotropium to ICS with a double dose of ICS or a LABA/ICS combination.23
The results suggest that LAAMs could be useful in treating uncontrolled asthma. Compared with the double dose of ICS, the tiotropium/ICS combination increased PEF by a mean difference of 25.8 L/min (P<.001) and resulted in a statistically significant improvement in the proportion of asthma control days, FEV1, and daily symptom scores.23 As an adjunctive treatment to ICS, tiotropium was not inferior to a LABA.
CASE After a detailed history, physical exam, and diagnostic testing, Ms. D was given a diagnosis of moderate persistent asthma. We recognized the need to step up her treatment. Prior to making any changes in her medication regimen, however, our team, which included a clinical pharmacist, observed her use of inhaled medications and verified that she was using the inhaler properly. We then initiated combination therapy, pairing a LABA and ICS.
Comorbidities complicate asthma management
Asthma management is often complicated by other uncontrolled coexisting medical problems. Common comorbidities that can affect asthma severity include allergic rhinitis, chronic sinusitis, gastroesophageal reflux disease (GERD), obesity, obstructive sleep apnea (OSA), mental health disorders, tobacco use, and hormonal disturbances.2
Allergic rhinitis. Allergic rhinitis has been associated with worse asthma control and a negative impact on quality of life, and the upper airway inflammation associated with it should be treated.24
Antihistamines and nasal steroids are the most effective medical management. Some patients with allergic rhinitis benefit from blood or skin allergy testing for confirmation or to aid in avoidance. Referral to an allergist may be necessary if symptoms are recalcitrant, a food allergy is in question, or the diagnosis is unclear.
GERD. Compared with the general population, patients with asthma have a much higher risk of GERD, although it is not always symptomatic. While results are inconsistent and difficult to predict, treating symptomatic GERD with acid-blocking medications can result in better asthma control for some patients. However, proton pump inhibitors should not be used to treat asthma symptoms in patients with asymptomatic GERD.25,26
Obesity and OSA. Weight loss can significantly improve asthma control, decrease medication use, and improve quality of life.27,28 Obese patients are less likely to respond to treatment with ICS.2 Weight loss also benefits those who suffer from OSA, which may contribute to airway hyperresponsiveness.29
Mental health disorders. Compared with the general population, patients with asthma are more likely to have depression, anxiety, and panic disorders.30 Diagnosis and treatment of these comorbid conditions can lead to better asthma management, increased medication adherence, decreased health care utilization—including fewer ED visits and hospitalizations—and a better quality of life.30
CASE We also addressed our patient’s comorbidities—weight gain, allergic rhinitis, and anxiety. The allergic rhinitis was already well-controlled with a nasal steroid, but we suspected a relationship between Ms. D’s weight gain and increasing anxiety associated with some recent life events. We suggested she see a counselor, and she agreed.
When the patient returned in 12 weeks, she reported that she hardly needed her rescue inhaler anymore and that she was managing her anxiety more effectively. She also told us that she had begun a low-fat dietary regimen, and we confirmed that she had already lost 5 pounds.
CORRESPONDENCE
Stephen A. Wilson, MD, MPH, FAAFP, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; [email protected]
1. American Academy of Allergy, Asthma, and Immunology. Asthma statistics. Available at: http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx. Accessed March 7, 2012.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for diagnosis and management of asthma. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Centers for Disease Control and Prevention. National surveillance for asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54.
4. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children. Arch Pediatr Adolesc Med. 2006;160:844-850.
5. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children. Am J Respir Crit Care Med. 2004;170:426-432.
6. Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32-38.
7. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179-187.
8. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
9. Bateman ED, Hurd SS, Barnes PJ, et al. Global Strategy for Asthma Management and Prevention. Eur Respir J. 2008;31:143-178.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. February 2013. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 7, 2013.
11. AsthmaMD. Available at: http://www.asthmamd.org/#resources/iphone_chart.jpg. Accessed March 7, 2013.
12. Indiegogo. Huff & Puff. Available at: http://www.indiegogo.com/projects/the-best-asthma-education-app-in-the-world-period. Accessed March 7, 2013.
13. Vimukti Technologies Pvt Ltd. Peak flow calculator. Available at: http://appworld.blackberry.com/webstore/content/7615. Accessed March 7, 2013,
14. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;35:1068-1080.
15. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375-387.
16. Nelson HS, Weiss ST, Bleeker ER, et al. The Salmeterol Multicenter Asthma Research Trial. Chest. 2006;129:15-26.
17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533.-
18. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma. Lancet Respir Med. 2013;1:23-31.
19. Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerba-tions. Lancet Respir Med. 2013;1:32-42.
20. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.-
21. Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004;(1):CD003133.-
22. Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011;(5):CD003137.-
23. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
24. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65:1290-1297.
25. Gibson PG, Henry RL, Coughlan JL. Gastroesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003;(2):CD001496.-
26. The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1499.
27. Eneli IU, Skybo T, Camargo CA, Jr. Weight loss and asthma. Thorax. 2008;63:671-676.
28. Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma. BMJ. 2000;320:827-832.
29. Sariman N, Levent E, Cubuk R, et al. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath. 2011;15:341-50.
30. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22-28.
1. American Academy of Allergy, Asthma, and Immunology. Asthma statistics. Available at: http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx. Accessed March 7, 2012.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for diagnosis and management of asthma. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Centers for Disease Control and Prevention. National surveillance for asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54.
4. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children. Arch Pediatr Adolesc Med. 2006;160:844-850.
5. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children. Am J Respir Crit Care Med. 2004;170:426-432.
6. Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32-38.
7. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179-187.
8. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
9. Bateman ED, Hurd SS, Barnes PJ, et al. Global Strategy for Asthma Management and Prevention. Eur Respir J. 2008;31:143-178.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. February 2013. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 7, 2013.
11. AsthmaMD. Available at: http://www.asthmamd.org/#resources/iphone_chart.jpg. Accessed March 7, 2013.
12. Indiegogo. Huff & Puff. Available at: http://www.indiegogo.com/projects/the-best-asthma-education-app-in-the-world-period. Accessed March 7, 2013.
13. Vimukti Technologies Pvt Ltd. Peak flow calculator. Available at: http://appworld.blackberry.com/webstore/content/7615. Accessed March 7, 2013,
14. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;35:1068-1080.
15. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375-387.
16. Nelson HS, Weiss ST, Bleeker ER, et al. The Salmeterol Multicenter Asthma Research Trial. Chest. 2006;129:15-26.
17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533.-
18. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma. Lancet Respir Med. 2013;1:23-31.
19. Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerba-tions. Lancet Respir Med. 2013;1:32-42.
20. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.-
21. Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004;(1):CD003133.-
22. Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011;(5):CD003137.-
23. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
24. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65:1290-1297.
25. Gibson PG, Henry RL, Coughlan JL. Gastroesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003;(2):CD001496.-
26. The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1499.
27. Eneli IU, Skybo T, Camargo CA, Jr. Weight loss and asthma. Thorax. 2008;63:671-676.
28. Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma. BMJ. 2000;320:827-832.
29. Sariman N, Levent E, Cubuk R, et al. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath. 2011;15:341-50.
30. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22-28.
A practical guide to shoulder injuries in the throwing athlete
• Manage most throwing injuries with relative rest and physical therapy. A
• Evaluate patients for total loss of range of motion, which is a predictor of increased injury. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Baseball players and other athletes who spend much of their time throwing a ball risk a variety of shoulder injuries because the repetitive motion causes repeated microtraumatic stress in the area. Injuries result from overuse of the muscles involved, improper technique—or both.
The review that follows will help you zero in on the correct diagnosis and identify the treatment that’s best for your patient.
First step: Cover these points in the history
In order to gather a detailed history of a patient with shoulder pain, you’ll want to do the following:
Ask about the location of the pain. Anterior shoulder pain is associated with subluxation, multidirectional instability, subacromial bursitis, and injury to the biceps, supraspinatus or subscapularis.1 Posterior shoulder pain has been linked to infraspinatus injuries.
Assess the severity of the pain. Ask patients: “On a scale of one to 10, where 10 is the worst pain you have ever felt, how would you rate the pain you are feeling?”
Pinpoint the timing of the pain. Determine the phase of the throwing process that reproduces the primary symptoms. The 6 phases are wind up, early cocking, late cocking, acceleration, deceleration, and follow-through (FIGURE 1). So if, for instance, the patient tells you that his arm “went dead” during the late cocking, or early acceleration phase, it should prompt you to suspect subluxation.2
See how the Neer’s and Hawkin’s tests are done
Christopher Faubel, MD, Thepainsource.com
Neer’s Impingement test
Hawkins test
FIGURE 1
The 6 phases of throwing
Ascertain the nature of the patient’s pain after activity. Does the patient experience the pain at night? If he answers Yes, you’ll want to consider the possibility of a rotator cuff tear.
Ask these targeted questions:
- When you raise your arm, do you feel a pinching pain in your shoulder? This may suggest the presence of impingement.
- Is your shoulder “catching” or “locking up”? If so, consider a labral tear or loose body, eg, a piece of cartilage or bone floating around in the joint.
- Does your shoulder feel like it is coming out of its socket—either partially or completely? This suggests shoulder instability.
- Is it difficult for you to reach behind your back, or do you have shoulder pain when you try to do this? This may indicate glenohumeral internal rotation deficit.
- Do you feel like you are throwing the ball slower, or with less accuracy? This may be an indication that there is something wrong with the rotator cuff muscles, the innervation around the shoulders, or the labrum that partly holds the shoulder together. Sometimes, a tear of the labrum presents simply as a “loss of power” in throwing, as defined by the athlete who is used to throwing the ball faster or farther.
Diagnoses to consider
Based on the patient’s history and responses to your questions, you’ll likely consider one of the following diagnoses as part of the differential.
External or internal impingement syndromes
What you’ll see. External or “subacromial” impingement syndrome results from compression of the rotator cuff between the coracoacromial arch and the humeral head. A sloping or hooked acromion or osteophyte may contribute to the syndrome.3 Neer’s and Hawkin’s tests are often positive, and there may be pain with the arc of motion. (For more on these and the other tests mentioned here, see “Athlete has shoulder problems? Consider these tests”.)
While a full review of provocative shoulder testing is beyond the scope of this article, specific tests for impingement, labral tears, instability, and rotator cuff tears should be included when examining the throwing athlete.
Impingement
Neer’s test. The clinician uses one hand to passively flex the arm of a patient whose thumb is pointing down, as the clinician’s other hand stabilizes the patient’s scapula. The test is positive for impingement if the patient feels pain in the shoulder with this maneuver.
Hawkin’s test. This test involves stabilizing the scapula, passively abducting the shoulder to 90°, flexing the shoulder to 30°, flexing the elbow to 90°, and internally rotating the shoulder. Pain with this maneuver suggests rotator cuff impingement.
Labral tears
O’Brien’s test. The physician asks the patient to adduct his arm across the midline of his body while keeping his shoulder flexed at 90° and his thumb down. As he does this, the physician pushes downward to resist the patient’s shoulder flexion and to see if the patient feels pain. Then, the same motion is done by the patient, but this time with the thumb up. If the pain is not present—or diminishes—with the thumb up, the test is considered positive for a labral tear.
Instability
Load and shift test. The physician uses force to push the humeral head centrally onto the glenoid fossa and then attempts to move the humeral head backward and forward, while keeping the scapula stable, to see how far it can go. Displacement <1 cm is mild; 1 to 2 cm is moderate; and >2 cm is severe.
Sulcus sign. With the patient’s arm in a relaxed position at his side, the physician pulls it downward. If a gap more than 1 cm wide develops between the humeral head and the acromion, the test is positive for inferior glenohumeral instability.
Apprehension-relocation test. The physician asks the patient to lie down on his back and abduct his shoulder at 90°. The physician then externally rotates the patient’s arm and places stress on the glenohumeral joint. A patient with shoulder instability will often stop the physician and say that he feels as if his shoulder is going to “pop out.”
The relocation part of the test is done by the physician applying a posteriorly directed force on the front of the shoulder. If the patient says that the almost popping out feeling of his shoulder has disappeared (and experiences a sense of relief), the test is considered positive.
Rotator cuff tears
Drop arm test. The shoulder is passively abducted to 90° and flexed to 30° while the thumb is pointing down. The test is considered positive for a supraspinatus muscle tear if the patient is unable to keep the arm elevated after the physician releases the arm.
Empty can test (Jobe test). The shoulder is passively abducted to 90° and flexed to 30° while the thumb is pointing down. In this position, resistance is provided as the patient tries to lift the arm upward. Pain with weakness suggests a tear of the supraspinatus muscle.
Push-off test. The clinician asks the patient to adduct and internally rotate his arm behind the back. The examiner provides resistance as the patient tries to push the arm away from the body. Pain with weakness suggests a tear of the subscapularis muscle.
Internal impingement results from pinching of the rotator cuff between the posterosuperior labrum and the greater tuberosity. The pain usually occurs with repetitive maximal shoulder internal rotation and abduction, which leads to cumulative microtrauma and eventual articular-sided rotator cuff pathology.4 The patient will complain of pain with shoulder internal rotation and abduction. Neer’s and Hawkin’s tests are helpful in detecting internal impingement.
Shoulder girdle fatigue from lack of conditioning and overthrowing—or the tight posterior capsule often seen in throwing athletes—may also contribute to the disorder.4
Treatment. Proper management involves relative rest from overhead activities, and an individualized rehabilitation program that includes dynamic stretching/strengthening through the rotator cuff, posterior capsule, and scapular stabilizers. Injecting a corticosteroid-analgesic solution into the subacromial space may help you arrive at a diagnosis and also offers symptomatic relief.1,3 Consider bursectomy, arthroscopic acromioplasty, capsulotomy and/or debridement for recalcitrant cases.4,5
Shoulder labrum pathology
What you’ll see. Overhead-throwing athletes are at risk of labral tears. The externally rotated, abducted arm of a thrower causes posterior rotation of the biceps anchor, peeling the biceps from its superior labrum attachment,6 a superior labrum anterior and posterior (SLAP) tear, or a type II tear from anterior to posterior. SLAP tears may lead to shoulder catching and locking. The patient may complain of vague shoulder pain,7 which is worse in the late cocking phase. O’Brien’s test will be positive.
Magnetic resonance imaging (MRI) with arthrogram can reveal a labral tear. Consider ordering an MRI when the athlete’s pain is accompanied by mechanical symptoms, such as locking, catching, or instability, or if the shoulder signs and symptoms do not appear to be responding to appropriate physical therapy interventions after a period of time—usually 4 to 6 weeks.
Treatment. For small tears, conservative management includes relative rest and physical therapy.3 Depending on the tear morphology, consider arthroscopic labral debridement or repair if conservative measures fail. The literature offers mixed conclusions on the benefits of surgery, with varying rates of full return to play.8-10
Shoulder instability
What you’ll see. Instability in throwing athletes is multifactorial, and rarely due to an isolated shoulder structure injury.11 Patients will complain that their shoulder feels as if it is going to come out of its socket, even when they are not throwing. To help detect instability, look for the sulcus sign, and do a load and shift test and an apprehension-relocation test.
Two categories of injury. Instability injuries fall into 2 primary categories: TUBS (Traumatic, Unilateral, associated with Bankart lesion, treated with Surgery) and AMBRI (Atraumatic, Multidirectional, Bilateral, treated with Rehabilitation, Inferior capsular shift).
As its name makes clear, TUBS is associated with a Bankart lesion (an avulsion of the anteroinferior glenoid labrum to its attachment to the humerus). Shoulder x-rays, including outlet, axillary lateral, and anteroposterior views,3 may reveal a bony Bankart lesion. You may also see a Hill-Sachs lesion here, which is noted on the humeral posterolateral head as a depression in the bony cortex.
AMBRI is more common than TUBS in throwers. Athletes often gain a competitive edge by increasing external rotation. However, when overdone, this results in the excessive laxity seen in AMBRI. While rare, acute traumatic dislocation can occur in those with AMBRI-type instability.
Treatment. Scapular stabilization exercises, dynamic rotator cuff strengthening, relative rest, and a short course (7-10 days) of nonsteroidal anti-inflammatory drugs are the mainstays of shoulder instability treatment in the throwing athlete.1,3 A throwing program may be started when the athlete is asymptomatic and has rested. You may also need to prescribe a longer rest period of 4 to 6 weeks if the symptoms return after commencing activity. For recalcitrant cases, consider surgery (via open or arthroscopic approaches6) to treat the associated underlying pathology.
Glenohumeral internal rotation deficit
What you’ll see. Posterior capsular contracture, common in the throwing athlete’s shoulder, causes decreased internal rotation and posterior shift of the total arc of glenohumeral motion.3 The patient may complain of decreased ability to reach backwards or pain when attempting to do so.
The anterior aspect of the shoulder’s capsule also lengthens, allowing anterior capsular laxity that causes additional problems, including internal impingement, SLAP tears, articular-sided, partial-thickness rotator cuff tears, and posterosuperior rotator cuff impingement. The risk for this cascade of complications increases in patients with throwing-shoulder internal rotation deficits ≥25° compared with the nonthrowing side, and a total arc of motion <180°.12
Treatment. Stretching the tight posterior capsule using the sleeper stretch (FIGURE 2) or the cross-body stretch (FIGURE 3) has proven very successful, with 90% of athletes seeing their symptoms resolve within 2 weeks.13,14 If conservative treatment is ineffective, consider selective arthroscopic capsular release of the posterior inferior glenohumeral ligament.
FIGURE 2
The sleeper stretch
FIGURE 3
The cross-body stretch
Rotator cuff tears
What you’ll see. Partial-thickness, articular-sided tears of the supraspinatus, infraspinatus, or both—found posterosuperiorly at the posterior rotator interval—are common in throwing athletes. The patient may complain of weakness when trying to do overhead tasks or movements requiring shoulder abduction. The supraspinatus is usually the muscle affected, and so testing of this muscle with the “empty can test” will show pain with weakness if there is a tear. However, full-thickness rotator cuff tears are rare;3 consider a diagnosis of instability or a partial tear in such cases. An MRI can reveal a rotator cuff tear. In fact, the imaging may be necessary for any suspicion of a tear in an athlete.
Treatment. Recommend strengthening exercises to patients before considering surgery. Nonoperative treatment is preferred, and should be given a fair trial before surgery; studies have not consistently supported the operative approach to rotator cuff tears.5,15 However, if conservative management fails, arthroscopic debridement of torn tissues is recommended over open procedures.3
Scapular dyskinesis and “SICK syndrome”
What you’ll see. Poor development of, or fatigue in, the scapular stabilizers leads to scapular dyskinesis (poor scapular control and motion). Scapulothoracic dyskinesis can progress to an overuse muscular fatigue syndrome called the “SICK syndrome” (Scapular malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).16 The most common symptoms include anterior shoulder pain, posterior/superior scapular pain with or without radiation,16 and a “dead arm” sensation. If not treated, this can result in rotator cuff lesions, impingement, and labral pathology.
Treatment. Both treatment and prevention are dependent on the proper biomechanics to retract and rotate the scapula correctly during throwing.1 Strengthening the scapular stabilizers and stretching tight posterior structures help to promote proper biomechanics, and enable a successful return to throwing.14
Help patients prevent injuries in the first place
To reduce the risk of shoulder injuries, athletes need to maintain an appropriate “thrower’s motion” at the glenohumeral joint.17 Overhead throwing athletes often exhibit excessive external rotation in their dominant shoulders,18 while internal rotation is reduced.19
Frequent gentle stretching may help maintain equal total motion in both the throwing shoulder and the nondominant shoulder. However, warn patients to avoid overaggressive stretching to gain mobility; the goal should be to maintain mobility.17
Strengthening of the entire upper extremity (shoulder, scapula, elbow, and wrist) is essential. While the individual needs of each athlete must be addressed, electromyographic studies of the throwing motion suggest that stretching, strengthening, and retraining of the muscles that allow the shoulders to rotate upwards and backwards help the shoulder blade keep close to the rib cage at the back. These are the most important initial steps in rehabilitating shoulder injuries in a throwing athlete.
Prevention and treatment programs for the throwing athlete should always incorporate dynamic stabilization and neuromuscular control.17 Additionally, the transfer of kinetic energy, as well as proximal stability with distal mobility of the upper extremity, are enhanced by core stabilization drills, including planks and side planks, as well as lower body training. As such, core strengthening is a very important component of injury prevention exercise regimens for throwing athletes.
Lastly, throwing programs incorporating maximum pitch counts per day, rest days, and gentle throwing are key to injury prevention. Direct young throwing athletes and their parents to resources such as http://pediatrics.aappublications.org/content/129/3/e842.full.pdf+html. (Tell them to see the recommendations at the end of the document.) Keep in mind, however, that there are no clear recommendations for college and professional pitching.
Young athletes. It is important to note that athletes with immature skeletons are at particular risk of injury due to the relative weakness of the open growth plate and the development of muscle imbalance. It is essential to appropriately apply the principles discussed here to young athletes to prevent injury.
CORRESPONDENCE
George Guntur A. Pujalte, MD, Penn State Milton S. Hershey Medical Center, 500 Hershey Center Drive, Hershey, PA 17033; [email protected]
1. Altcheck DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3:159-165.
2. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63:863-872.
3. Jancosko JJ, Kazanjian JE. Shoulder injuries in the throwing athlete. Phys Sportsmed. 2012;40:84-90.
4. Jobe CM. Posterior superior glenoid impingement: expanded spectrum. Arthroscopy. 1995;11:530-536.
5. Riand N, Boulahia A, Walch G. Posterosuperior impingement of the shoulder in the athlete: results of arthroscopic debridement in 75 patients. Rev Chir Orthop Reparatrice Appar Mot. 2002;88:19-27.
6. Bottoni CR, Smith EL, Berkowitz MJ, et al. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized controlled trial. Am J Sports Med. 2006;34:1730-1737.
7. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14:637-640.
8. Glascow SG, Bruce RA, Yacobucci GN, et al. Arthroscopic resection of glenoid labral tears in the athlete: a report of 29 cases. Arthroscopy. 1992;8:48-54.
9. Altcheck DW, Warren RF, Wickiewicz TL, et al. Arthroscopic labral debridement: a three-year follow-up study. Am J Sports Med. 1992;20:702-706.
10. Kim SH, Ha KI, Kim SH, et al. Results of arthroscopic treatment of superior labral lesions. J Bone Joint Surg Am. 2002;84:981-985.
11. Pouliart N, Marmor S, Gagey O. Simulated capsulolabral lesion in cadavers: dislocation does not result from a Bankart lesion only. Arthroscopy. 2006;22:748-754.
12. Verna C. Shoulder flexibility to reduce impingement. Paper presented at: 3rd Annual Professional Baseball Athletic Trainers Society Meeting; March 1991; Mesa, Ariz.
13. Lintner D, Mayol M, Uzodinma O, et al. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35:617-621.
14. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19:404-420.
15. Mazoue CG, Andrews JR. Repair of full thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34:182-189.
16. Cheung S. Shoulder injuries in the throwing athlete. Orth Sports Med. 2011;4:173-184.
17. Reinold MM, Gill TJ, Wilk KE, et al. Current concepts in the evaluation and treatment of the shoulder in overhead throwing athletes, part 2: injury prevention and treatment. Sports Health. 2010;2:101-115.
18. Reinold MM, Gill TJ. Current concepts in the evaluation and treatment of the shoulder in overhead throwing athletes, part 1: physical characteristics and clinical examination. Sports Health. 2010;2:39-50.
19. Reinold MM, Wilk KE, Macrina LC, et al. Changes in shoulder and elbow passive range of motion after pitching in professional baseball players. Am J Sports Med. 2008;36:523-527.
• Manage most throwing injuries with relative rest and physical therapy. A
• Evaluate patients for total loss of range of motion, which is a predictor of increased injury. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Baseball players and other athletes who spend much of their time throwing a ball risk a variety of shoulder injuries because the repetitive motion causes repeated microtraumatic stress in the area. Injuries result from overuse of the muscles involved, improper technique—or both.
The review that follows will help you zero in on the correct diagnosis and identify the treatment that’s best for your patient.
First step: Cover these points in the history
In order to gather a detailed history of a patient with shoulder pain, you’ll want to do the following:
Ask about the location of the pain. Anterior shoulder pain is associated with subluxation, multidirectional instability, subacromial bursitis, and injury to the biceps, supraspinatus or subscapularis.1 Posterior shoulder pain has been linked to infraspinatus injuries.
Assess the severity of the pain. Ask patients: “On a scale of one to 10, where 10 is the worst pain you have ever felt, how would you rate the pain you are feeling?”
Pinpoint the timing of the pain. Determine the phase of the throwing process that reproduces the primary symptoms. The 6 phases are wind up, early cocking, late cocking, acceleration, deceleration, and follow-through (FIGURE 1). So if, for instance, the patient tells you that his arm “went dead” during the late cocking, or early acceleration phase, it should prompt you to suspect subluxation.2
See how the Neer’s and Hawkin’s tests are done
Christopher Faubel, MD, Thepainsource.com
Neer’s Impingement test
Hawkins test
FIGURE 1
The 6 phases of throwing
Ascertain the nature of the patient’s pain after activity. Does the patient experience the pain at night? If he answers Yes, you’ll want to consider the possibility of a rotator cuff tear.
Ask these targeted questions:
- When you raise your arm, do you feel a pinching pain in your shoulder? This may suggest the presence of impingement.
- Is your shoulder “catching” or “locking up”? If so, consider a labral tear or loose body, eg, a piece of cartilage or bone floating around in the joint.
- Does your shoulder feel like it is coming out of its socket—either partially or completely? This suggests shoulder instability.
- Is it difficult for you to reach behind your back, or do you have shoulder pain when you try to do this? This may indicate glenohumeral internal rotation deficit.
- Do you feel like you are throwing the ball slower, or with less accuracy? This may be an indication that there is something wrong with the rotator cuff muscles, the innervation around the shoulders, or the labrum that partly holds the shoulder together. Sometimes, a tear of the labrum presents simply as a “loss of power” in throwing, as defined by the athlete who is used to throwing the ball faster or farther.
Diagnoses to consider
Based on the patient’s history and responses to your questions, you’ll likely consider one of the following diagnoses as part of the differential.
External or internal impingement syndromes
What you’ll see. External or “subacromial” impingement syndrome results from compression of the rotator cuff between the coracoacromial arch and the humeral head. A sloping or hooked acromion or osteophyte may contribute to the syndrome.3 Neer’s and Hawkin’s tests are often positive, and there may be pain with the arc of motion. (For more on these and the other tests mentioned here, see “Athlete has shoulder problems? Consider these tests”.)
While a full review of provocative shoulder testing is beyond the scope of this article, specific tests for impingement, labral tears, instability, and rotator cuff tears should be included when examining the throwing athlete.
Impingement
Neer’s test. The clinician uses one hand to passively flex the arm of a patient whose thumb is pointing down, as the clinician’s other hand stabilizes the patient’s scapula. The test is positive for impingement if the patient feels pain in the shoulder with this maneuver.
Hawkin’s test. This test involves stabilizing the scapula, passively abducting the shoulder to 90°, flexing the shoulder to 30°, flexing the elbow to 90°, and internally rotating the shoulder. Pain with this maneuver suggests rotator cuff impingement.
Labral tears
O’Brien’s test. The physician asks the patient to adduct his arm across the midline of his body while keeping his shoulder flexed at 90° and his thumb down. As he does this, the physician pushes downward to resist the patient’s shoulder flexion and to see if the patient feels pain. Then, the same motion is done by the patient, but this time with the thumb up. If the pain is not present—or diminishes—with the thumb up, the test is considered positive for a labral tear.
Instability
Load and shift test. The physician uses force to push the humeral head centrally onto the glenoid fossa and then attempts to move the humeral head backward and forward, while keeping the scapula stable, to see how far it can go. Displacement <1 cm is mild; 1 to 2 cm is moderate; and >2 cm is severe.
Sulcus sign. With the patient’s arm in a relaxed position at his side, the physician pulls it downward. If a gap more than 1 cm wide develops between the humeral head and the acromion, the test is positive for inferior glenohumeral instability.
Apprehension-relocation test. The physician asks the patient to lie down on his back and abduct his shoulder at 90°. The physician then externally rotates the patient’s arm and places stress on the glenohumeral joint. A patient with shoulder instability will often stop the physician and say that he feels as if his shoulder is going to “pop out.”
The relocation part of the test is done by the physician applying a posteriorly directed force on the front of the shoulder. If the patient says that the almost popping out feeling of his shoulder has disappeared (and experiences a sense of relief), the test is considered positive.
Rotator cuff tears
Drop arm test. The shoulder is passively abducted to 90° and flexed to 30° while the thumb is pointing down. The test is considered positive for a supraspinatus muscle tear if the patient is unable to keep the arm elevated after the physician releases the arm.
Empty can test (Jobe test). The shoulder is passively abducted to 90° and flexed to 30° while the thumb is pointing down. In this position, resistance is provided as the patient tries to lift the arm upward. Pain with weakness suggests a tear of the supraspinatus muscle.
Push-off test. The clinician asks the patient to adduct and internally rotate his arm behind the back. The examiner provides resistance as the patient tries to push the arm away from the body. Pain with weakness suggests a tear of the subscapularis muscle.
Internal impingement results from pinching of the rotator cuff between the posterosuperior labrum and the greater tuberosity. The pain usually occurs with repetitive maximal shoulder internal rotation and abduction, which leads to cumulative microtrauma and eventual articular-sided rotator cuff pathology.4 The patient will complain of pain with shoulder internal rotation and abduction. Neer’s and Hawkin’s tests are helpful in detecting internal impingement.
Shoulder girdle fatigue from lack of conditioning and overthrowing—or the tight posterior capsule often seen in throwing athletes—may also contribute to the disorder.4
Treatment. Proper management involves relative rest from overhead activities, and an individualized rehabilitation program that includes dynamic stretching/strengthening through the rotator cuff, posterior capsule, and scapular stabilizers. Injecting a corticosteroid-analgesic solution into the subacromial space may help you arrive at a diagnosis and also offers symptomatic relief.1,3 Consider bursectomy, arthroscopic acromioplasty, capsulotomy and/or debridement for recalcitrant cases.4,5
Shoulder labrum pathology
What you’ll see. Overhead-throwing athletes are at risk of labral tears. The externally rotated, abducted arm of a thrower causes posterior rotation of the biceps anchor, peeling the biceps from its superior labrum attachment,6 a superior labrum anterior and posterior (SLAP) tear, or a type II tear from anterior to posterior. SLAP tears may lead to shoulder catching and locking. The patient may complain of vague shoulder pain,7 which is worse in the late cocking phase. O’Brien’s test will be positive.
Magnetic resonance imaging (MRI) with arthrogram can reveal a labral tear. Consider ordering an MRI when the athlete’s pain is accompanied by mechanical symptoms, such as locking, catching, or instability, or if the shoulder signs and symptoms do not appear to be responding to appropriate physical therapy interventions after a period of time—usually 4 to 6 weeks.
Treatment. For small tears, conservative management includes relative rest and physical therapy.3 Depending on the tear morphology, consider arthroscopic labral debridement or repair if conservative measures fail. The literature offers mixed conclusions on the benefits of surgery, with varying rates of full return to play.8-10
Shoulder instability
What you’ll see. Instability in throwing athletes is multifactorial, and rarely due to an isolated shoulder structure injury.11 Patients will complain that their shoulder feels as if it is going to come out of its socket, even when they are not throwing. To help detect instability, look for the sulcus sign, and do a load and shift test and an apprehension-relocation test.
Two categories of injury. Instability injuries fall into 2 primary categories: TUBS (Traumatic, Unilateral, associated with Bankart lesion, treated with Surgery) and AMBRI (Atraumatic, Multidirectional, Bilateral, treated with Rehabilitation, Inferior capsular shift).
As its name makes clear, TUBS is associated with a Bankart lesion (an avulsion of the anteroinferior glenoid labrum to its attachment to the humerus). Shoulder x-rays, including outlet, axillary lateral, and anteroposterior views,3 may reveal a bony Bankart lesion. You may also see a Hill-Sachs lesion here, which is noted on the humeral posterolateral head as a depression in the bony cortex.
AMBRI is more common than TUBS in throwers. Athletes often gain a competitive edge by increasing external rotation. However, when overdone, this results in the excessive laxity seen in AMBRI. While rare, acute traumatic dislocation can occur in those with AMBRI-type instability.
Treatment. Scapular stabilization exercises, dynamic rotator cuff strengthening, relative rest, and a short course (7-10 days) of nonsteroidal anti-inflammatory drugs are the mainstays of shoulder instability treatment in the throwing athlete.1,3 A throwing program may be started when the athlete is asymptomatic and has rested. You may also need to prescribe a longer rest period of 4 to 6 weeks if the symptoms return after commencing activity. For recalcitrant cases, consider surgery (via open or arthroscopic approaches6) to treat the associated underlying pathology.
Glenohumeral internal rotation deficit
What you’ll see. Posterior capsular contracture, common in the throwing athlete’s shoulder, causes decreased internal rotation and posterior shift of the total arc of glenohumeral motion.3 The patient may complain of decreased ability to reach backwards or pain when attempting to do so.
The anterior aspect of the shoulder’s capsule also lengthens, allowing anterior capsular laxity that causes additional problems, including internal impingement, SLAP tears, articular-sided, partial-thickness rotator cuff tears, and posterosuperior rotator cuff impingement. The risk for this cascade of complications increases in patients with throwing-shoulder internal rotation deficits ≥25° compared with the nonthrowing side, and a total arc of motion <180°.12
Treatment. Stretching the tight posterior capsule using the sleeper stretch (FIGURE 2) or the cross-body stretch (FIGURE 3) has proven very successful, with 90% of athletes seeing their symptoms resolve within 2 weeks.13,14 If conservative treatment is ineffective, consider selective arthroscopic capsular release of the posterior inferior glenohumeral ligament.
FIGURE 2
The sleeper stretch
FIGURE 3
The cross-body stretch
Rotator cuff tears
What you’ll see. Partial-thickness, articular-sided tears of the supraspinatus, infraspinatus, or both—found posterosuperiorly at the posterior rotator interval—are common in throwing athletes. The patient may complain of weakness when trying to do overhead tasks or movements requiring shoulder abduction. The supraspinatus is usually the muscle affected, and so testing of this muscle with the “empty can test” will show pain with weakness if there is a tear. However, full-thickness rotator cuff tears are rare;3 consider a diagnosis of instability or a partial tear in such cases. An MRI can reveal a rotator cuff tear. In fact, the imaging may be necessary for any suspicion of a tear in an athlete.
Treatment. Recommend strengthening exercises to patients before considering surgery. Nonoperative treatment is preferred, and should be given a fair trial before surgery; studies have not consistently supported the operative approach to rotator cuff tears.5,15 However, if conservative management fails, arthroscopic debridement of torn tissues is recommended over open procedures.3
Scapular dyskinesis and “SICK syndrome”
What you’ll see. Poor development of, or fatigue in, the scapular stabilizers leads to scapular dyskinesis (poor scapular control and motion). Scapulothoracic dyskinesis can progress to an overuse muscular fatigue syndrome called the “SICK syndrome” (Scapular malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).16 The most common symptoms include anterior shoulder pain, posterior/superior scapular pain with or without radiation,16 and a “dead arm” sensation. If not treated, this can result in rotator cuff lesions, impingement, and labral pathology.
Treatment. Both treatment and prevention are dependent on the proper biomechanics to retract and rotate the scapula correctly during throwing.1 Strengthening the scapular stabilizers and stretching tight posterior structures help to promote proper biomechanics, and enable a successful return to throwing.14
Help patients prevent injuries in the first place
To reduce the risk of shoulder injuries, athletes need to maintain an appropriate “thrower’s motion” at the glenohumeral joint.17 Overhead throwing athletes often exhibit excessive external rotation in their dominant shoulders,18 while internal rotation is reduced.19
Frequent gentle stretching may help maintain equal total motion in both the throwing shoulder and the nondominant shoulder. However, warn patients to avoid overaggressive stretching to gain mobility; the goal should be to maintain mobility.17
Strengthening of the entire upper extremity (shoulder, scapula, elbow, and wrist) is essential. While the individual needs of each athlete must be addressed, electromyographic studies of the throwing motion suggest that stretching, strengthening, and retraining of the muscles that allow the shoulders to rotate upwards and backwards help the shoulder blade keep close to the rib cage at the back. These are the most important initial steps in rehabilitating shoulder injuries in a throwing athlete.
Prevention and treatment programs for the throwing athlete should always incorporate dynamic stabilization and neuromuscular control.17 Additionally, the transfer of kinetic energy, as well as proximal stability with distal mobility of the upper extremity, are enhanced by core stabilization drills, including planks and side planks, as well as lower body training. As such, core strengthening is a very important component of injury prevention exercise regimens for throwing athletes.
Lastly, throwing programs incorporating maximum pitch counts per day, rest days, and gentle throwing are key to injury prevention. Direct young throwing athletes and their parents to resources such as http://pediatrics.aappublications.org/content/129/3/e842.full.pdf+html. (Tell them to see the recommendations at the end of the document.) Keep in mind, however, that there are no clear recommendations for college and professional pitching.
Young athletes. It is important to note that athletes with immature skeletons are at particular risk of injury due to the relative weakness of the open growth plate and the development of muscle imbalance. It is essential to appropriately apply the principles discussed here to young athletes to prevent injury.
CORRESPONDENCE
George Guntur A. Pujalte, MD, Penn State Milton S. Hershey Medical Center, 500 Hershey Center Drive, Hershey, PA 17033; [email protected]
• Manage most throwing injuries with relative rest and physical therapy. A
• Evaluate patients for total loss of range of motion, which is a predictor of increased injury. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Baseball players and other athletes who spend much of their time throwing a ball risk a variety of shoulder injuries because the repetitive motion causes repeated microtraumatic stress in the area. Injuries result from overuse of the muscles involved, improper technique—or both.
The review that follows will help you zero in on the correct diagnosis and identify the treatment that’s best for your patient.
First step: Cover these points in the history
In order to gather a detailed history of a patient with shoulder pain, you’ll want to do the following:
Ask about the location of the pain. Anterior shoulder pain is associated with subluxation, multidirectional instability, subacromial bursitis, and injury to the biceps, supraspinatus or subscapularis.1 Posterior shoulder pain has been linked to infraspinatus injuries.
Assess the severity of the pain. Ask patients: “On a scale of one to 10, where 10 is the worst pain you have ever felt, how would you rate the pain you are feeling?”
Pinpoint the timing of the pain. Determine the phase of the throwing process that reproduces the primary symptoms. The 6 phases are wind up, early cocking, late cocking, acceleration, deceleration, and follow-through (FIGURE 1). So if, for instance, the patient tells you that his arm “went dead” during the late cocking, or early acceleration phase, it should prompt you to suspect subluxation.2
See how the Neer’s and Hawkin’s tests are done
Christopher Faubel, MD, Thepainsource.com
Neer’s Impingement test
Hawkins test
FIGURE 1
The 6 phases of throwing
Ascertain the nature of the patient’s pain after activity. Does the patient experience the pain at night? If he answers Yes, you’ll want to consider the possibility of a rotator cuff tear.
Ask these targeted questions:
- When you raise your arm, do you feel a pinching pain in your shoulder? This may suggest the presence of impingement.
- Is your shoulder “catching” or “locking up”? If so, consider a labral tear or loose body, eg, a piece of cartilage or bone floating around in the joint.
- Does your shoulder feel like it is coming out of its socket—either partially or completely? This suggests shoulder instability.
- Is it difficult for you to reach behind your back, or do you have shoulder pain when you try to do this? This may indicate glenohumeral internal rotation deficit.
- Do you feel like you are throwing the ball slower, or with less accuracy? This may be an indication that there is something wrong with the rotator cuff muscles, the innervation around the shoulders, or the labrum that partly holds the shoulder together. Sometimes, a tear of the labrum presents simply as a “loss of power” in throwing, as defined by the athlete who is used to throwing the ball faster or farther.
Diagnoses to consider
Based on the patient’s history and responses to your questions, you’ll likely consider one of the following diagnoses as part of the differential.
External or internal impingement syndromes
What you’ll see. External or “subacromial” impingement syndrome results from compression of the rotator cuff between the coracoacromial arch and the humeral head. A sloping or hooked acromion or osteophyte may contribute to the syndrome.3 Neer’s and Hawkin’s tests are often positive, and there may be pain with the arc of motion. (For more on these and the other tests mentioned here, see “Athlete has shoulder problems? Consider these tests”.)
While a full review of provocative shoulder testing is beyond the scope of this article, specific tests for impingement, labral tears, instability, and rotator cuff tears should be included when examining the throwing athlete.
Impingement
Neer’s test. The clinician uses one hand to passively flex the arm of a patient whose thumb is pointing down, as the clinician’s other hand stabilizes the patient’s scapula. The test is positive for impingement if the patient feels pain in the shoulder with this maneuver.
Hawkin’s test. This test involves stabilizing the scapula, passively abducting the shoulder to 90°, flexing the shoulder to 30°, flexing the elbow to 90°, and internally rotating the shoulder. Pain with this maneuver suggests rotator cuff impingement.
Labral tears
O’Brien’s test. The physician asks the patient to adduct his arm across the midline of his body while keeping his shoulder flexed at 90° and his thumb down. As he does this, the physician pushes downward to resist the patient’s shoulder flexion and to see if the patient feels pain. Then, the same motion is done by the patient, but this time with the thumb up. If the pain is not present—or diminishes—with the thumb up, the test is considered positive for a labral tear.
Instability
Load and shift test. The physician uses force to push the humeral head centrally onto the glenoid fossa and then attempts to move the humeral head backward and forward, while keeping the scapula stable, to see how far it can go. Displacement <1 cm is mild; 1 to 2 cm is moderate; and >2 cm is severe.
Sulcus sign. With the patient’s arm in a relaxed position at his side, the physician pulls it downward. If a gap more than 1 cm wide develops between the humeral head and the acromion, the test is positive for inferior glenohumeral instability.
Apprehension-relocation test. The physician asks the patient to lie down on his back and abduct his shoulder at 90°. The physician then externally rotates the patient’s arm and places stress on the glenohumeral joint. A patient with shoulder instability will often stop the physician and say that he feels as if his shoulder is going to “pop out.”
The relocation part of the test is done by the physician applying a posteriorly directed force on the front of the shoulder. If the patient says that the almost popping out feeling of his shoulder has disappeared (and experiences a sense of relief), the test is considered positive.
Rotator cuff tears
Drop arm test. The shoulder is passively abducted to 90° and flexed to 30° while the thumb is pointing down. The test is considered positive for a supraspinatus muscle tear if the patient is unable to keep the arm elevated after the physician releases the arm.
Empty can test (Jobe test). The shoulder is passively abducted to 90° and flexed to 30° while the thumb is pointing down. In this position, resistance is provided as the patient tries to lift the arm upward. Pain with weakness suggests a tear of the supraspinatus muscle.
Push-off test. The clinician asks the patient to adduct and internally rotate his arm behind the back. The examiner provides resistance as the patient tries to push the arm away from the body. Pain with weakness suggests a tear of the subscapularis muscle.
Internal impingement results from pinching of the rotator cuff between the posterosuperior labrum and the greater tuberosity. The pain usually occurs with repetitive maximal shoulder internal rotation and abduction, which leads to cumulative microtrauma and eventual articular-sided rotator cuff pathology.4 The patient will complain of pain with shoulder internal rotation and abduction. Neer’s and Hawkin’s tests are helpful in detecting internal impingement.
Shoulder girdle fatigue from lack of conditioning and overthrowing—or the tight posterior capsule often seen in throwing athletes—may also contribute to the disorder.4
Treatment. Proper management involves relative rest from overhead activities, and an individualized rehabilitation program that includes dynamic stretching/strengthening through the rotator cuff, posterior capsule, and scapular stabilizers. Injecting a corticosteroid-analgesic solution into the subacromial space may help you arrive at a diagnosis and also offers symptomatic relief.1,3 Consider bursectomy, arthroscopic acromioplasty, capsulotomy and/or debridement for recalcitrant cases.4,5
Shoulder labrum pathology
What you’ll see. Overhead-throwing athletes are at risk of labral tears. The externally rotated, abducted arm of a thrower causes posterior rotation of the biceps anchor, peeling the biceps from its superior labrum attachment,6 a superior labrum anterior and posterior (SLAP) tear, or a type II tear from anterior to posterior. SLAP tears may lead to shoulder catching and locking. The patient may complain of vague shoulder pain,7 which is worse in the late cocking phase. O’Brien’s test will be positive.
Magnetic resonance imaging (MRI) with arthrogram can reveal a labral tear. Consider ordering an MRI when the athlete’s pain is accompanied by mechanical symptoms, such as locking, catching, or instability, or if the shoulder signs and symptoms do not appear to be responding to appropriate physical therapy interventions after a period of time—usually 4 to 6 weeks.
Treatment. For small tears, conservative management includes relative rest and physical therapy.3 Depending on the tear morphology, consider arthroscopic labral debridement or repair if conservative measures fail. The literature offers mixed conclusions on the benefits of surgery, with varying rates of full return to play.8-10
Shoulder instability
What you’ll see. Instability in throwing athletes is multifactorial, and rarely due to an isolated shoulder structure injury.11 Patients will complain that their shoulder feels as if it is going to come out of its socket, even when they are not throwing. To help detect instability, look for the sulcus sign, and do a load and shift test and an apprehension-relocation test.
Two categories of injury. Instability injuries fall into 2 primary categories: TUBS (Traumatic, Unilateral, associated with Bankart lesion, treated with Surgery) and AMBRI (Atraumatic, Multidirectional, Bilateral, treated with Rehabilitation, Inferior capsular shift).
As its name makes clear, TUBS is associated with a Bankart lesion (an avulsion of the anteroinferior glenoid labrum to its attachment to the humerus). Shoulder x-rays, including outlet, axillary lateral, and anteroposterior views,3 may reveal a bony Bankart lesion. You may also see a Hill-Sachs lesion here, which is noted on the humeral posterolateral head as a depression in the bony cortex.
AMBRI is more common than TUBS in throwers. Athletes often gain a competitive edge by increasing external rotation. However, when overdone, this results in the excessive laxity seen in AMBRI. While rare, acute traumatic dislocation can occur in those with AMBRI-type instability.
Treatment. Scapular stabilization exercises, dynamic rotator cuff strengthening, relative rest, and a short course (7-10 days) of nonsteroidal anti-inflammatory drugs are the mainstays of shoulder instability treatment in the throwing athlete.1,3 A throwing program may be started when the athlete is asymptomatic and has rested. You may also need to prescribe a longer rest period of 4 to 6 weeks if the symptoms return after commencing activity. For recalcitrant cases, consider surgery (via open or arthroscopic approaches6) to treat the associated underlying pathology.
Glenohumeral internal rotation deficit
What you’ll see. Posterior capsular contracture, common in the throwing athlete’s shoulder, causes decreased internal rotation and posterior shift of the total arc of glenohumeral motion.3 The patient may complain of decreased ability to reach backwards or pain when attempting to do so.
The anterior aspect of the shoulder’s capsule also lengthens, allowing anterior capsular laxity that causes additional problems, including internal impingement, SLAP tears, articular-sided, partial-thickness rotator cuff tears, and posterosuperior rotator cuff impingement. The risk for this cascade of complications increases in patients with throwing-shoulder internal rotation deficits ≥25° compared with the nonthrowing side, and a total arc of motion <180°.12
Treatment. Stretching the tight posterior capsule using the sleeper stretch (FIGURE 2) or the cross-body stretch (FIGURE 3) has proven very successful, with 90% of athletes seeing their symptoms resolve within 2 weeks.13,14 If conservative treatment is ineffective, consider selective arthroscopic capsular release of the posterior inferior glenohumeral ligament.
FIGURE 2
The sleeper stretch
FIGURE 3
The cross-body stretch
Rotator cuff tears
What you’ll see. Partial-thickness, articular-sided tears of the supraspinatus, infraspinatus, or both—found posterosuperiorly at the posterior rotator interval—are common in throwing athletes. The patient may complain of weakness when trying to do overhead tasks or movements requiring shoulder abduction. The supraspinatus is usually the muscle affected, and so testing of this muscle with the “empty can test” will show pain with weakness if there is a tear. However, full-thickness rotator cuff tears are rare;3 consider a diagnosis of instability or a partial tear in such cases. An MRI can reveal a rotator cuff tear. In fact, the imaging may be necessary for any suspicion of a tear in an athlete.
Treatment. Recommend strengthening exercises to patients before considering surgery. Nonoperative treatment is preferred, and should be given a fair trial before surgery; studies have not consistently supported the operative approach to rotator cuff tears.5,15 However, if conservative management fails, arthroscopic debridement of torn tissues is recommended over open procedures.3
Scapular dyskinesis and “SICK syndrome”
What you’ll see. Poor development of, or fatigue in, the scapular stabilizers leads to scapular dyskinesis (poor scapular control and motion). Scapulothoracic dyskinesis can progress to an overuse muscular fatigue syndrome called the “SICK syndrome” (Scapular malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).16 The most common symptoms include anterior shoulder pain, posterior/superior scapular pain with or without radiation,16 and a “dead arm” sensation. If not treated, this can result in rotator cuff lesions, impingement, and labral pathology.
Treatment. Both treatment and prevention are dependent on the proper biomechanics to retract and rotate the scapula correctly during throwing.1 Strengthening the scapular stabilizers and stretching tight posterior structures help to promote proper biomechanics, and enable a successful return to throwing.14
Help patients prevent injuries in the first place
To reduce the risk of shoulder injuries, athletes need to maintain an appropriate “thrower’s motion” at the glenohumeral joint.17 Overhead throwing athletes often exhibit excessive external rotation in their dominant shoulders,18 while internal rotation is reduced.19
Frequent gentle stretching may help maintain equal total motion in both the throwing shoulder and the nondominant shoulder. However, warn patients to avoid overaggressive stretching to gain mobility; the goal should be to maintain mobility.17
Strengthening of the entire upper extremity (shoulder, scapula, elbow, and wrist) is essential. While the individual needs of each athlete must be addressed, electromyographic studies of the throwing motion suggest that stretching, strengthening, and retraining of the muscles that allow the shoulders to rotate upwards and backwards help the shoulder blade keep close to the rib cage at the back. These are the most important initial steps in rehabilitating shoulder injuries in a throwing athlete.
Prevention and treatment programs for the throwing athlete should always incorporate dynamic stabilization and neuromuscular control.17 Additionally, the transfer of kinetic energy, as well as proximal stability with distal mobility of the upper extremity, are enhanced by core stabilization drills, including planks and side planks, as well as lower body training. As such, core strengthening is a very important component of injury prevention exercise regimens for throwing athletes.
Lastly, throwing programs incorporating maximum pitch counts per day, rest days, and gentle throwing are key to injury prevention. Direct young throwing athletes and their parents to resources such as http://pediatrics.aappublications.org/content/129/3/e842.full.pdf+html. (Tell them to see the recommendations at the end of the document.) Keep in mind, however, that there are no clear recommendations for college and professional pitching.
Young athletes. It is important to note that athletes with immature skeletons are at particular risk of injury due to the relative weakness of the open growth plate and the development of muscle imbalance. It is essential to appropriately apply the principles discussed here to young athletes to prevent injury.
CORRESPONDENCE
George Guntur A. Pujalte, MD, Penn State Milton S. Hershey Medical Center, 500 Hershey Center Drive, Hershey, PA 17033; [email protected]
1. Altcheck DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3:159-165.
2. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63:863-872.
3. Jancosko JJ, Kazanjian JE. Shoulder injuries in the throwing athlete. Phys Sportsmed. 2012;40:84-90.
4. Jobe CM. Posterior superior glenoid impingement: expanded spectrum. Arthroscopy. 1995;11:530-536.
5. Riand N, Boulahia A, Walch G. Posterosuperior impingement of the shoulder in the athlete: results of arthroscopic debridement in 75 patients. Rev Chir Orthop Reparatrice Appar Mot. 2002;88:19-27.
6. Bottoni CR, Smith EL, Berkowitz MJ, et al. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized controlled trial. Am J Sports Med. 2006;34:1730-1737.
7. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14:637-640.
8. Glascow SG, Bruce RA, Yacobucci GN, et al. Arthroscopic resection of glenoid labral tears in the athlete: a report of 29 cases. Arthroscopy. 1992;8:48-54.
9. Altcheck DW, Warren RF, Wickiewicz TL, et al. Arthroscopic labral debridement: a three-year follow-up study. Am J Sports Med. 1992;20:702-706.
10. Kim SH, Ha KI, Kim SH, et al. Results of arthroscopic treatment of superior labral lesions. J Bone Joint Surg Am. 2002;84:981-985.
11. Pouliart N, Marmor S, Gagey O. Simulated capsulolabral lesion in cadavers: dislocation does not result from a Bankart lesion only. Arthroscopy. 2006;22:748-754.
12. Verna C. Shoulder flexibility to reduce impingement. Paper presented at: 3rd Annual Professional Baseball Athletic Trainers Society Meeting; March 1991; Mesa, Ariz.
13. Lintner D, Mayol M, Uzodinma O, et al. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35:617-621.
14. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19:404-420.
15. Mazoue CG, Andrews JR. Repair of full thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34:182-189.
16. Cheung S. Shoulder injuries in the throwing athlete. Orth Sports Med. 2011;4:173-184.
17. Reinold MM, Gill TJ, Wilk KE, et al. Current concepts in the evaluation and treatment of the shoulder in overhead throwing athletes, part 2: injury prevention and treatment. Sports Health. 2010;2:101-115.
18. Reinold MM, Gill TJ. Current concepts in the evaluation and treatment of the shoulder in overhead throwing athletes, part 1: physical characteristics and clinical examination. Sports Health. 2010;2:39-50.
19. Reinold MM, Wilk KE, Macrina LC, et al. Changes in shoulder and elbow passive range of motion after pitching in professional baseball players. Am J Sports Med. 2008;36:523-527.
1. Altcheck DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3:159-165.
2. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63:863-872.
3. Jancosko JJ, Kazanjian JE. Shoulder injuries in the throwing athlete. Phys Sportsmed. 2012;40:84-90.
4. Jobe CM. Posterior superior glenoid impingement: expanded spectrum. Arthroscopy. 1995;11:530-536.
5. Riand N, Boulahia A, Walch G. Posterosuperior impingement of the shoulder in the athlete: results of arthroscopic debridement in 75 patients. Rev Chir Orthop Reparatrice Appar Mot. 2002;88:19-27.
6. Bottoni CR, Smith EL, Berkowitz MJ, et al. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized controlled trial. Am J Sports Med. 2006;34:1730-1737.
7. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14:637-640.
8. Glascow SG, Bruce RA, Yacobucci GN, et al. Arthroscopic resection of glenoid labral tears in the athlete: a report of 29 cases. Arthroscopy. 1992;8:48-54.
9. Altcheck DW, Warren RF, Wickiewicz TL, et al. Arthroscopic labral debridement: a three-year follow-up study. Am J Sports Med. 1992;20:702-706.
10. Kim SH, Ha KI, Kim SH, et al. Results of arthroscopic treatment of superior labral lesions. J Bone Joint Surg Am. 2002;84:981-985.
11. Pouliart N, Marmor S, Gagey O. Simulated capsulolabral lesion in cadavers: dislocation does not result from a Bankart lesion only. Arthroscopy. 2006;22:748-754.
12. Verna C. Shoulder flexibility to reduce impingement. Paper presented at: 3rd Annual Professional Baseball Athletic Trainers Society Meeting; March 1991; Mesa, Ariz.
13. Lintner D, Mayol M, Uzodinma O, et al. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35:617-621.
14. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19:404-420.
15. Mazoue CG, Andrews JR. Repair of full thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34:182-189.
16. Cheung S. Shoulder injuries in the throwing athlete. Orth Sports Med. 2011;4:173-184.
17. Reinold MM, Gill TJ, Wilk KE, et al. Current concepts in the evaluation and treatment of the shoulder in overhead throwing athletes, part 2: injury prevention and treatment. Sports Health. 2010;2:101-115.
18. Reinold MM, Gill TJ. Current concepts in the evaluation and treatment of the shoulder in overhead throwing athletes, part 1: physical characteristics and clinical examination. Sports Health. 2010;2:39-50.
19. Reinold MM, Wilk KE, Macrina LC, et al. Changes in shoulder and elbow passive range of motion after pitching in professional baseball players. Am J Sports Med. 2008;36:523-527.
Allergic rhinitis: What’s best for your patient?
• Use nasal steroids to treat allergic rhinitis (AR) in adults. A
• Recommend nasal saline irrigation to reduce symptoms in children and adults with seasonal rhinitis. A
• Consider immunotherapy for adults and children with severe AR that does not respond to conventional pharmacotherapy or allergen avoidance measures. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A man in his 30s with allergic rhinitis (AR) at predictable times of the year with high pollen counts reports only modest symptom relief with a nasal steroid preparation after 3 weeks of use. He comes to see you because he’s “tired of feeling lousy all of the time.”
What management options would you consider?
There is a plethora of treatment options for patients like this one, and considerable variation in clinical practice when it comes to AR.1 The good news is that there are several recent guidelines for treating AR patients, whose symptoms (and underlying cause) can vary widely.
The following review—and accompanying algorithm—provides evidence-based recommendations that can help you refine your approach to AR.
Two guidelines, and several Cochrane reviews
Allergic Rhinitis and its Impact on Asthma (ARIA), a sentinel rhinitis treatment guideline, was published in 2001 and updated in 2008 and 2010.2-4 The British Society for Allergy and Clinical Immunology Standards of Care Committee (BSACI) published guidelines for rhinitis management in 2008 and guidelines for immunotherapy in 2011.5,6 In addition, several Cochrane reviews have been performed.7-12 The ALGORITHM1-6 combines these recommendations. The TABLE2-12 itemizes the recommendations made by each guideline.
ALGORITHM
An evidence-based approach to treating allergic rhinitis1-6
Based on recommendations from ARIA and BSACI guidelines and Cochrane reviews
ARIA, Allergic Rhinitis and its Impact on Asthma; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee.
TABLE
Treatment recommendations/suggestions for allergic rhinitis2-12
| TREATMENT RECOMMENDATIONS/SUGGESTIONS | ARIA 2001 | ARIA 2008 | ARIA 2010 | BSACI 2008 | BSACI 2011 | COCHRANE REVIEWS |
|---|---|---|---|---|---|---|
| General principles of treatment | ||||||
| Maintenance therapy is required for persistent AR as medications have little effect after cessation. | X | |||||
| Patient education | ||||||
| Standardized patient education improves disease-specific quality of life. | X | |||||
| Nasal steroids | ||||||
| NS are the most effective monotherapy for all symptoms of AR, seasonal and perennial,* including nasal congestion. | X | |||||
| NS are recommended for AR treatment in adults and suggested for children. | X | |||||
| NS are the treatment of choice for moderate to severe persistent* AR and for treatment failures with antihistamines alone. | X | |||||
| NS are suggested over oral antihistamines in adults and children for seasonal AR. | X | |||||
| NS are suggested over oral antihistamines for adults and children with persistent AR. | X | |||||
| NS are recommended rather than nasal antihistamines. | X | |||||
| NS are recommended over oral leukotriene receptor antagonists for seasonal AR. | X | |||||
| NS are the most effective treatment of AR for children. | X | |||||
| There is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR. | X (2010) | |||||
| Intermittent* NS use may be beneficial in children. | X | |||||
| Avoid NS with high bioavailability (betamethasone) in children, as regular use for >1 year may decrease growth rate. | X | |||||
| Antihistamines | ||||||
| New-generation oral nonsedating antihistamines that do not affect cytochrome P450 are recommended for the treatment of patients with AR. | X | |||||
| Oral or topical antihistamines are first-line treatment for mild to moderate intermittent and moderate persistent AR. | X | |||||
| When NS alone do not control moderate to severe persistent AR, may add oral or topical antihistamines. | X | |||||
| New-generation oral antihistamines are suggested over nasal antihistamines for children and adults, and for children with seasonal or persistent AR. | X | |||||
| Oral antihistamines are suggested over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR. | X | |||||
| Nasal antihistamines are suggested over nasal chromones (the need to use chromones 4 times daily may limit adherence). | X | |||||
| Nasal antihistamine use is suggested for children and adults with seasonal AR. | X | |||||
| Patients with persistent AR should avoid using nasal antihistamines until more data on efficacy and safety are available. | X | |||||
| In children, weigh adverse effects of antihistamines against the general malaise caused by AR. | X | |||||
| Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children. | X | |||||
| Continuous administration of antihistamines is optimal in children, rather than as needed. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for patients with ocular symptoms. | X | |||||
| Oral leukotriene receptor antagonists | ||||||
| Oral leukotriene receptor antagonists are suggested for children and adults with seasonal AR and for preschool children with persistent AR. | X | |||||
| Avoid oral leukotriene receptor antagonists in adults with persistent AR. | X | |||||
| Decongestants | ||||||
| For adults with severe nasal obstruction, a short course (<5 days) of a nasal decongestant, along with other drugs, is suggested. | X | |||||
| Nasal decongestants may be useful for eustachian tube dysfunction when flying, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency prior to NS use. | X | |||||
| Regular oral decongestant use is not suggested. | X | X | ||||
| Avoid decongestants in pregnant patients. | X | |||||
| Avoid using nasal decongestants in preschool children. | X | |||||
| Chromones | ||||||
| Limited use of chromones is recommended for children and adults with mild symptoms. | X | |||||
| Chromones are less effective than NS or antihistamines. | X | |||||
| Nasal antihistamines are suggested over nasal chromones. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for ocular symptoms. Due to the excellent safety of these agents, chromones may be tried before antihistamines. | X | |||||
| Nasal saline | ||||||
| Nasal saline irrigation reduces symptoms in children and adults with seasonal rhinitis. | X | |||||
| Oral, intramuscular steroids | ||||||
| A short course of oral glucocorticosteroids is suggested for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments. | X | |||||
| Oral steroids are rarely indicated, but a short course (5-10 days) may be used for severe nasal congestion, uncontrolled symptoms on conventional pharmacotherapy, or important social/work events. | X | |||||
| Avoid intramuscular steroids. | X | X | ||||
| Ipratropium | ||||||
| Nasal ipratropium is suggested for treatment of rhinorrhea for patients with persistent AR. | X | |||||
| Allergen-specific immunotherapy | ||||||
| Immunotherapy is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy or allergen avoidance measures. | X | |||||
| SCIT is suggested for adults with seasonal AR and those with persistent AR due to house dust mites. | X | |||||
| SCIT is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few severe adverse reactions. | X (2007) | |||||
| SLIT is suggested for adults with AR due to pollen, although other alternatives may be equally reasonable. | X | |||||
| SLIT is safe and efficacious for AR treatment, decreasing symptoms and medication requirements. | X (2003) | |||||
| Nasal immunotherapy is suggested for adults with AR due to pollens. | X | |||||
| For pregnant patients, maintenance ASI may be continued, but starting ASI or increasing the dose is contraindicated. | X | |||||
| SCIT is suggested for children with AR. | X | |||||
| SCIT should not be started before 5 years of age. | X | |||||
| Based on preliminary studies, SLIT is safe, but more studies are needed in children. | X | |||||
| SLIT and NIT are suggested for children with AR due to pollens, acknowledging that other alternatives may be equally reasonable. SLIT should not be given to children with AR due to HDM unless being done for research. | X | |||||
| Lifestyle changes | ||||||
| Avoid single chemical or physical preventive and combination preventive methods to reduce HDM exposure. | X | |||||
| Allergen avoidance may decrease AR symptoms, but more research is needed. | X (2010) | |||||
| Achieving substantial reductions in HDM load may decrease AR symptoms. | X (2012) | |||||
| Avoidance of mold or animal dander is recommended for patients who are allergic to them. | X | |||||
| Nasal filters can reduce symptoms of AR during ragweed and grass pollen seasons. | X | |||||
| Complementary and alternative medicine | ||||||
| Avoid homeopathy, acupuncture, butterbur, herbal medicines, and phototherapy. | X | |||||
| AR, allergic rhinitis; ARIA, Allergic Rhinitis and its Impact on Asthma; ASI, allergen-specific immunotherapy; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee; HDM, house dust mites; NIT, nasal immunotherapy; NS, nasal steroids; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy. *ARIA 2008 recommended changing the classification of AR from seasonal and perennial (frequent nonseasonal nasal or ocular symptoms) to intermittent (symptoms lasting <4 days per week or <4 weeks per year) or persistent (symptoms >4 days per week and >4 weeks per year).3 AR severity is classified as mild or moderate to severe.2,3 | ||||||
The summary that follows provides a more detailed look at the recommendations, with a review of the pathophysiology of AR (“Phases of allergic rhinitis”2,3,5,8,13-15).
The early phase of allergic rhinitis (AR) occurs within minutes of allergen exposure. Mast cell degranulation releases histamine and other inflammatory mediators that cause sneezing, pruritus, rhinorrhea, and nasal congestion.3,8,13 The late phase, beginning at 4 hours and peaking 6 to 12 hours after exposure, is believed to be due to recruitment of circulating leukocytes—particularly eosinophils. Leukocyte activation causes additional inflammatory mediators to be released, which primarily causes nasal congestion—often the most bothersome symptom of AR.2,5,8,13,14 Other presenting symptoms may include feeling “fuzzy” or tired, chronic viral infections, sniffing, eye rubbing, blinking, congested voice, snoring, or dark skin beneath the eyes (allergic shiners).15
Of note: This summary preserves the terminology used in ARIA 2010. Specifically, the ARIA guideline uses the term suggest for conditional recommendations and recommend for strong recommendations.4 That same language is used here.
Nasal steroids: First-line Tx for moderate to severe symptoms
BSACI indicates that nasal steroids (NS) are the treatment of choice for moderate to severe persistent AR (symptoms lasting >4 days per week or >4 weeks per year).5 ARIA 2010 suggests NS as first-line treatment rather than oral antihistamines for adults and children with seasonal (related to outdoor allergens such as pollens or molds) and persistent AR.4 ARIA 2008 finds NS are the most effective treatment for children.3 Steroids reduce inflammation by decreasing inflammatory cell migration and inhibiting cytokine release.16 They are the most effective monotherapy for all symptoms of AR, including nasal congestion, which antihistamines do not treat effectively.13,16 NS also treat ocular symptoms of allergy effectively.15,17
The ARIA 2010 guideline also recommends using NS rather than nasal antihistamines and leukotriene receptor antagonists.4 Combination therapy (eg, NS with the addition of nasal antihistamines) is an option for severe or persistent AR, but it appears to be no more effective than monotherapy with NS.16 A 2010 Cochrane review determined there is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR.7 Intermittent steroid use may be beneficial in children.5
Steroids begin working 6 to 8 hours after the first dose, although symptom reduction may take days and maximal effect up to 2 weeks.5 Treatment failure may be due to poor technique that can cause local adverse effects (ie, dryness, irritation, epistaxis). Technique-related failure occurs in up to 10% of users.5,15 Educating patients and families about correct technique with steroid spray may decrease nonadherence due to irritation and epistaxis.18 Tell them to shake the bottle well, look down, aim the nozzle toward the outside wall of the nostril using the opposite hand, and spray while sniffing lightly.5
Any steroid is appropriate for adults. For children ≥2 years of age, consider fluticasone propionate, mometasone furoate, or triamcinolone acetonide.3 These medications have lower systemic bioavailability and a decreased risk of such adverse effects as hypothalamic-pituitary-adrenal axis suppression and growth retardation.15 Budesonide is appropriate for those ≥6 years.19-21 Avoid regular use of betamethasone, which has high bioavailability, for >1 year in children, as it may decrease their growth rate.3 Beclomethasone, fluticasone, and budesonide have been used widely and safely for pregnant women with asthma.5
Antihistamines are first-line Tx for mild symptoms
ARIA 2010 recommends new-generation oral nonsedating antihistamines that do not affect cytochrome P450 for mild AR,4 such as cetirizine, levocetirizine, loratadine, desloratadine, and fexofenadine. First-generation antihistamines can reduce symptoms, but are not first-line treatment as they cause sedation, fatigue, decreased cognitive function, and reduced academic and work performance.3-5 ARIA 2010 further suggests choosing oral antihistamines over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR.4
BSACI recommends oral or topical antihistamines as first-line treatment for mild to moderate symptoms lasting <4 days per week or <4 weeks per year and moderate persistent AR.5 When steroids alone do not control moderate to severe persistent AR, BSACI recommends adding oral or topical antihistamines.5 Oral and topical antihistamines decrease histamine-related symptoms of itching, rhinorrhea, and sneezing, but do not significantly decrease nasal congestion.15
Nasal antihistamines (levocabastine, azelastine) have a rapid onset of action and few adverse effects.3 ARIA 2010 suggests nasal antihistamines over nasal chromones (inhibitors of mast cell degranulation) and notes that the need to use chromones 4 times daily may limit adherence.4 The same guidelines suggest nasal antihistamine use for children and adults with seasonal AR and suggest not using nasal antihistamines for patients with persistent AR until more data on efficacy and safety are available.4
Alezastine is approved for individuals ≥5 years, and olopatadine is approved for individuals ≥6 years for the treatment of AR.16,22,23 A pediatric review article noted nasal antihistamine (azelastine) plus nasal fluticasone was more efficacious than NS alone.15
In children, weigh adverse effects of antihistamines against the general malaise caused by AR.3 Do not use first-generation antihistamines due to the sedation that may interfere with learning.15 Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children.5 Continuous administration, rather than as needed, is optimal treatment in children.5 Cetirizine, loratadine, and levocetirizine have been studied and are effective and safe in children.3 Levocetirizine has proven safe and efficacious for children ≥2 years.24 Fexofenadine was found to be effective and safe for those ≥6 years.25
For children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones.4 Due to the safety of these agents, chromones may be used first, then antihistamines.4 Just as with nasal chromones, the need to use intraocular chromones 4 times daily may limit their use in children.4
Pregnant patients. Antihistamines do cross the placenta.5 Agents that appear to be safe for pregnant patients are chlorphenamine (first-generation), loratadine, and cetirizine.5
Leukotriene receptor antagonists: Always pair with antihistamines
As adjunctive therapy for additional symptom control, ARIA 2010 suggests oral leukotriene receptor antagonists for children and adults with seasonal AR, and for preschool children with persistent AR. These agents may also be helpful in children with concurrent asthma.15 Always pair leukotriene receptor antagonists with antihistamines. Montelukast is approved for seasonal AR in children ≥2 years and for frequent nonseasonal nasal or ocular AR symptoms in children ≥6 months.26
ARIA 2010 recommends against the use of oral leukotriene receptor antagonists in adults with persistent AR.4
Decongestants are for limited use only
For adults with severe nasal obstruction, ARIA 2010 suggests a short course (<5 days) of nasal decongestant along with other drugs.4 Limiting use of nasal decongestants to <10 days helps prevent rhinitis medicamentosa.5,27 BSACI notes nasal decongestants may be useful for eustachian tube dysfunction experienced aboard airplanes, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency before NS use.5 Both guidelines suggest against regular oral decongestant use.4,5
Avoid decongestants in pregnant patients.5 ARIA 2010 suggests against nasal decongestant use in preschool children.4
Chromones may help, but require multiple daily dosing
Chromones inhibit mast cell degranulation, are weakly effective for reducing nasal obstruction in AR, and have a high safety profile.3-5,28 As noted earlier, they must be used 4 times daily, which may reduce adherence—particularly in children.4
ARIA 2008 notes that disodium cromoglycate is less effective than NS or antihistamines.3 The 2010 update suggests nasal antihistamines over nasal chromones.4 For adults as well as children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones. BSACI recommends limited use of chromones for children and adults with mild symptoms.5
Nasal saline helpful as adjunct to medication
Nasal saline irrigation improves symptoms of AR, clears nasal passages, and is helpful for pregnant patients, for whom medications should be used with caution.2,3,5 Nasal irrigation using a neti pot or squeeze bottle is efficacious for chronic rhinorrhea, as solo or complementary treatment, and for children.5,16,27
Oral steroids: Use only rarely
ARIA 2010 suggests a short course of oral glucocorticosteroids for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments.4 BSACI notes oral steroids are rarely indicated, but that their use over 5 to 10 days may help with severe nasal congestion, symptoms uncontrolled by conventional pharmacotherapy, or before important social or work events.5 Both guidelines recommend against intramuscular steroids.4 ARIA 2008 notes oral and depot preparations of steroids affect growth in young children.3
Ipratropium when rhinorrhea is severe
Nasal ipratropium bromide, a topical anticholinergic, is helpful for excessive or refractory rhinorrhea. Consider using ipratropium with NS for patients for whom rhinorrhea is the dominant symptom.5,16,28 ARIA 2010 suggests using nasal ipratropium to treat rhinorrhea in patients with persistent AR.4
Allergen-specific immunotherapy: When other treatments fail
Allergen-specific immunotherapy (ASI) consists of repeated exposure to an allergen to induce immunomodulation, which prevents or reduces allergy symptoms and actually changes the natural course of AR. (For more on identifying the offending agent, see “Time for allergen testing?”2,5,15,18,29.) This treatment process decreases medication needs, prevents new allergen sensitization, and results in long-lasting improvement.2,5,6,30 BSACI 2011 notes that ASI is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy and allergen avoidance measures.6
When a patient’s symptoms are poorly controlled or persist after treatment, consider allergen testing.29 Skin prick testing (SPT) is the best means of eliciting specific allergen sensitization. However, limit testing to allergens most likely causing the patient’s symptoms rather than ordering a random panel; 15% of those with a positive SPT to specific allergens do not have symptoms when exposed to those allergens in their environment.5 And always interpret results of allergy testing in light of the patient’s history.2,15,18
SPT has a high negative predictive value, which can prevent unnecessary lifestyle changes.29 However, keep in mind that SPT results may be suppressed if the patient is using antihistamines, tricyclic antidepressants, or topical steroids.29 If SPT is not feasible or the patient is taking medications that may suppress results, consider arranging for serum-specific IgE testing, also known as radioallergosorbent testing, or RAST.5 RAST and SPT have similar sensitivities for house dust mites, but RAST is not as sensitive as SPT for other inhalants (eg, cat epithelium, mold, grass pollen).5
ASI methods developed to date use subcutaneous, sublingual, or nasal routes of administration. However, the US Food and Drug Administration has yet to approve commercial sublingual or nasal products for use in the United States.16
Subcutaneous immunotherapy may cause local adverse reactions (pruritus and swelling) and systemic reactions that can be severe or life threatening (anaphylaxis) and thus must be given in a doctor’s office prepared to treat anaphylaxis.6,16,30 Adrenaline administration has been necessary in 0.13% of those being treated.9 Subcutaneous immunotherapy must be done for 3 to 5 years for sustained effective treatment.15
ARIA 2010 suggests subcutaneous immunotherapy for adults with seasonal AR and with persistent AR due to house dust mites.4 A 2007 Cochrane review found subcutaneous immunotherapy is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few significant severe adverse reactions.9 A meta-analysis showed subcutaneous immunotherapy is as potent as pharmacotherapy in controlling seasonal AR symptoms as early as the first season of treatment.31
What if the patient is pregnant—or a child? BSACI notes that maintenance ASI may be continued in a patient who becomes pregnant, but starting ASI or increasing the dose is contraindicated.5
Based on ARIA 2008 and 2010, consider subcutaneous immunotherapy for children—but not for those <5 years.3,4 Care must be used in selecting patients, as 3 to 5 years of treatment are necessary for sustained benefit.15
Lifestyle changes: Limited benefit may be achievable
ARIA 2010 recommends mold avoidance and animal dander avoidance for patients so affected.4 Allergens from pets can persist in homes for months after pet removal.15 BSACI found that commercially available nasal filters (filters or screens placed over or within both nares) reduced symptoms of AR during ragweed and grass pollen seasons.5 Allergen avoidance for children with persistent AR has not shown consistent benefit.15 A 2010 Cochrane review concluded that allergen avoidance may decrease AR symptoms, but more research is needed.11
House dust mites. The 2010 Cochrane review also reported on 2 trials that assessed high-efficiency particulate air (HEPA) filters specifically for patients allergic to house dust mites.11 The studies, which had methodological limitations (inconsistent randomization, small sample size, and short duration), concluded that HEPA filters alone will not likely reduce symptoms of house dust mite allergy. But HEPA filters may be beneficial as one component of an extensive bedroom-based environmental control program.11
Impermeable bedding has been shown to reduce dust mite load by 50% to 70%, leaving residual allergen that may still trigger symptoms.11 A 2012 Cochrane review concluded that achieving substantial reductions in house dust mite load using a combination approach of multiple interventions, including acaricides and extensive bedroom-based environmental control programs, may decrease AR symptoms.12 However, ARIA 2010 recommends against single chemical or physical preventive methods and against combination preventive methods to reduce house dust mite exposure.4
Total elimination of house dust mites may be impossible, and recommending use of impermeable covers and HEPA filters, removal of rugs and curtains, and frequent cleaning must take into account a patient’s symptoms and a family’s motivation and finances.11,18
Complementary and alternative medicine: Too little evidence
ARIA 2010 suggests against patients using homeopathy, acupuncture, butterbur, herbal medicines, or phototherapy for AR.4 While one systematic review of acupuncture for AR demonstrated mixed results with no specific effects for seasonal AR and some improvement of frequent nonseasonal symptoms,32 another review concluded evidence was insufficient to make any recommendation.32,33 The benefit of ear acupressure is unknown, as supporting studies are of low methodological quality, although it appeared to provide some benefit for AR.34
Due to lack of data, probiotics should not be recommended.27 A pediatric review article noted that probiotics may alter cytokine production in patients with seasonal AR and may be more helpful in AR than in asthma, although more research was needed.15 Another review showed that probiotics may reduce AR symptoms and medication use.35
CASE Since the nasal steroid you prescribed for your patient did not provide adequate relief, you opt to add cetirizine 10 mg to his NS regimen. This step relieved his symptoms within 2 to 3 days. Had his symptoms persisted, the patient would have been a candidate for a one-week course of oral decongestant, such as pseudoephedrine 120 mg orally every 12 hours, as needed; and then for allergen testing, specifically for pollens corresponding to the seasonality of his AR. Appropriate follow-up would be to monitor the patient until his symptoms resolved or became manageable.
1. Bousquet J, Schünemann HJ, Zuberbier T, et al. Development and implementation of guidelines in allergic rhinitis – an ARIA-GA2LEN paper. Allergy. 2010;65:1212-1221.
2. Bousquet J, Van-Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(suppl):S147-S334.
3. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy. 2008;63(suppl 86):S8-S160.
4. Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466-476.
5. Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38:19-42.
6. Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177-1200.
7. Al Sayyad JJ, Fedorowicz Z, Alhashimi D, et al. Topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2007;(1):CD003163.-
8. Nasser M, Fedorowicz, Alijufairi H, et al. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2010;(7):CD006989.-
9. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936.-
10. Wilson D, Torres-Lima M, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;(2):CD002893.-
11. Sheikh A, Hurwitz B, Nurmatov U, et al. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010;(7):CD001563.-
12. Nurmatov U, van Schayck CP, Hurwitz B, et al. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158-165.
13. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(suppl):S2-S8.
14. Nathan RA. The pathophysiology, clinical impact, and management of nasal congestion in allergic rhinitis. Clin Ther. 2008;30:573-586.
15. Kemp AS. Allergic rhinitis. Paediatric Respir Rev. 2009;10:63-68.
16. Sur DK, Scandale S. Treatment of allergic rhinitis. Am Fam Physician. 2010;81:1440-1446.
17. Hong J, Bielory B, Rosenberg JL, et al. Efficacy of intranasal corticosteroids for the ocular symptoms of allergic rhinitis: a systematic review. Allergy Asthma Proc. 2011;32:22-35.
18. Hu W, Katelaris CH, Kemp AS. Allergic rhinitis – practical management strategies. Aust Fam Physician. 2008;37:214-220.
19. Veramyst (fluticasone furoate) nasal spray [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; August 2012. Available at: http://us.gsk.com/products/assets/us_veramyst.pdf. Accessed January 16, 2013.
20. Nasacort AQ (triamcinolone acetonide) nasal spray [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/nasacort_aq/nasacort_aq.html. Accessed January 16, 2013.
21. Rhinocort AQUA 32 mcg (budesonide) nasal spray [prescribing information]. Wilmington, Del: AstraZeneca; revised December 2010. Available at: http://www1.astrazeneca-us.com/pi/Rhinocort_Aqua.pdf. Accessed January 16, 2013.
22. Astelin (azelastine hydrochloride) spray, metered [prescribing information]. Somerset, NJ: Meda Pharmaceuticals; revised July 2011. Available at: http://www.astelin.com/pdf/astelin_pi.pdf. Accessed January 16, 2013.
23. Patanase (olopatadine hydrochloride) nasal spray [prescribing information]. Fort Worth, Tex: Alcon Laboratories; revised February 2012. Available at: http://ecatalog.alcon.com/PI/Patanase_us_en.pdf. Accessed January 16, 2013.
24. Xyzal (levocetirizine dihydrochloride) tablets and oral solution [prescribing information]. Smyrna, Ga: UCB and Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/xyzal/xyzal.pdf. Accessed January 16, 2013.
25. Allegra (fexofenadine hydrochloride) tablets, ODT, and oral suspension [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2007. Available at: http://products.sanofi.us/allegra/allegra.html. Accessed January 16, 2013.
26. Singulair (montelukast sodium) tablets, chewable tablets, and oral granules [prescribing information]. Whitehouse Station, NJ: Merck; revised November 2012. Available at: http://www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf. Accessed January 16, 2013.
27. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(suppl):S12-S16.
28. Lim MY, Leong JM. Allergic rhinitis: evidence-based practice. Singapore Med J. 2010;51:542-550.
29. Angier E, Willington J, Scadding G, et al. Management of allergic and non-allergic rhinitis: a primary care summary of the BSACI guideline. Prim Care Respir J. 2010;19:217-222.
30. Radulovic S, Wilson D, Calderon M, et al. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66:740-752.
31. Matricardi PM, Kuna P, Panetta V, et al. Subcutaneous immunotherapy and pharmacology in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011;128:791-799.
32. Lee MS, Pittler MH, Shin B, et al. Acupuncture for allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2009;102:269-279.
33. Roberts J, Huissoon A, Dretzke J, et al. A systematic review of the clinical effectiveness of acupuncture for allergic rhinitis. BMC Complement Altern Med. 2008;8:13.-
34. Zhang CS, Yang AW, Zhang AL, et al. Ear-acupressure for allergic rhinitis: a systematic review. Clin Otolaryngol. 2010;35:6-12.
35. Vliagoftis H, Kouranos VD, Betsi GI, et al. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:570-579.
CORRESPONDENCE Suzanne Minor, MD, Florida International University Herbert Wertheim College of Medicine; 11200 SW 8th Street, AHC II 361A, Miami, FL 33199; [email protected]
• Use nasal steroids to treat allergic rhinitis (AR) in adults. A
• Recommend nasal saline irrigation to reduce symptoms in children and adults with seasonal rhinitis. A
• Consider immunotherapy for adults and children with severe AR that does not respond to conventional pharmacotherapy or allergen avoidance measures. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A man in his 30s with allergic rhinitis (AR) at predictable times of the year with high pollen counts reports only modest symptom relief with a nasal steroid preparation after 3 weeks of use. He comes to see you because he’s “tired of feeling lousy all of the time.”
What management options would you consider?
There is a plethora of treatment options for patients like this one, and considerable variation in clinical practice when it comes to AR.1 The good news is that there are several recent guidelines for treating AR patients, whose symptoms (and underlying cause) can vary widely.
The following review—and accompanying algorithm—provides evidence-based recommendations that can help you refine your approach to AR.
Two guidelines, and several Cochrane reviews
Allergic Rhinitis and its Impact on Asthma (ARIA), a sentinel rhinitis treatment guideline, was published in 2001 and updated in 2008 and 2010.2-4 The British Society for Allergy and Clinical Immunology Standards of Care Committee (BSACI) published guidelines for rhinitis management in 2008 and guidelines for immunotherapy in 2011.5,6 In addition, several Cochrane reviews have been performed.7-12 The ALGORITHM1-6 combines these recommendations. The TABLE2-12 itemizes the recommendations made by each guideline.
ALGORITHM
An evidence-based approach to treating allergic rhinitis1-6
Based on recommendations from ARIA and BSACI guidelines and Cochrane reviews
ARIA, Allergic Rhinitis and its Impact on Asthma; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee.
TABLE
Treatment recommendations/suggestions for allergic rhinitis2-12
| TREATMENT RECOMMENDATIONS/SUGGESTIONS | ARIA 2001 | ARIA 2008 | ARIA 2010 | BSACI 2008 | BSACI 2011 | COCHRANE REVIEWS |
|---|---|---|---|---|---|---|
| General principles of treatment | ||||||
| Maintenance therapy is required for persistent AR as medications have little effect after cessation. | X | |||||
| Patient education | ||||||
| Standardized patient education improves disease-specific quality of life. | X | |||||
| Nasal steroids | ||||||
| NS are the most effective monotherapy for all symptoms of AR, seasonal and perennial,* including nasal congestion. | X | |||||
| NS are recommended for AR treatment in adults and suggested for children. | X | |||||
| NS are the treatment of choice for moderate to severe persistent* AR and for treatment failures with antihistamines alone. | X | |||||
| NS are suggested over oral antihistamines in adults and children for seasonal AR. | X | |||||
| NS are suggested over oral antihistamines for adults and children with persistent AR. | X | |||||
| NS are recommended rather than nasal antihistamines. | X | |||||
| NS are recommended over oral leukotriene receptor antagonists for seasonal AR. | X | |||||
| NS are the most effective treatment of AR for children. | X | |||||
| There is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR. | X (2010) | |||||
| Intermittent* NS use may be beneficial in children. | X | |||||
| Avoid NS with high bioavailability (betamethasone) in children, as regular use for >1 year may decrease growth rate. | X | |||||
| Antihistamines | ||||||
| New-generation oral nonsedating antihistamines that do not affect cytochrome P450 are recommended for the treatment of patients with AR. | X | |||||
| Oral or topical antihistamines are first-line treatment for mild to moderate intermittent and moderate persistent AR. | X | |||||
| When NS alone do not control moderate to severe persistent AR, may add oral or topical antihistamines. | X | |||||
| New-generation oral antihistamines are suggested over nasal antihistamines for children and adults, and for children with seasonal or persistent AR. | X | |||||
| Oral antihistamines are suggested over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR. | X | |||||
| Nasal antihistamines are suggested over nasal chromones (the need to use chromones 4 times daily may limit adherence). | X | |||||
| Nasal antihistamine use is suggested for children and adults with seasonal AR. | X | |||||
| Patients with persistent AR should avoid using nasal antihistamines until more data on efficacy and safety are available. | X | |||||
| In children, weigh adverse effects of antihistamines against the general malaise caused by AR. | X | |||||
| Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children. | X | |||||
| Continuous administration of antihistamines is optimal in children, rather than as needed. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for patients with ocular symptoms. | X | |||||
| Oral leukotriene receptor antagonists | ||||||
| Oral leukotriene receptor antagonists are suggested for children and adults with seasonal AR and for preschool children with persistent AR. | X | |||||
| Avoid oral leukotriene receptor antagonists in adults with persistent AR. | X | |||||
| Decongestants | ||||||
| For adults with severe nasal obstruction, a short course (<5 days) of a nasal decongestant, along with other drugs, is suggested. | X | |||||
| Nasal decongestants may be useful for eustachian tube dysfunction when flying, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency prior to NS use. | X | |||||
| Regular oral decongestant use is not suggested. | X | X | ||||
| Avoid decongestants in pregnant patients. | X | |||||
| Avoid using nasal decongestants in preschool children. | X | |||||
| Chromones | ||||||
| Limited use of chromones is recommended for children and adults with mild symptoms. | X | |||||
| Chromones are less effective than NS or antihistamines. | X | |||||
| Nasal antihistamines are suggested over nasal chromones. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for ocular symptoms. Due to the excellent safety of these agents, chromones may be tried before antihistamines. | X | |||||
| Nasal saline | ||||||
| Nasal saline irrigation reduces symptoms in children and adults with seasonal rhinitis. | X | |||||
| Oral, intramuscular steroids | ||||||
| A short course of oral glucocorticosteroids is suggested for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments. | X | |||||
| Oral steroids are rarely indicated, but a short course (5-10 days) may be used for severe nasal congestion, uncontrolled symptoms on conventional pharmacotherapy, or important social/work events. | X | |||||
| Avoid intramuscular steroids. | X | X | ||||
| Ipratropium | ||||||
| Nasal ipratropium is suggested for treatment of rhinorrhea for patients with persistent AR. | X | |||||
| Allergen-specific immunotherapy | ||||||
| Immunotherapy is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy or allergen avoidance measures. | X | |||||
| SCIT is suggested for adults with seasonal AR and those with persistent AR due to house dust mites. | X | |||||
| SCIT is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few severe adverse reactions. | X (2007) | |||||
| SLIT is suggested for adults with AR due to pollen, although other alternatives may be equally reasonable. | X | |||||
| SLIT is safe and efficacious for AR treatment, decreasing symptoms and medication requirements. | X (2003) | |||||
| Nasal immunotherapy is suggested for adults with AR due to pollens. | X | |||||
| For pregnant patients, maintenance ASI may be continued, but starting ASI or increasing the dose is contraindicated. | X | |||||
| SCIT is suggested for children with AR. | X | |||||
| SCIT should not be started before 5 years of age. | X | |||||
| Based on preliminary studies, SLIT is safe, but more studies are needed in children. | X | |||||
| SLIT and NIT are suggested for children with AR due to pollens, acknowledging that other alternatives may be equally reasonable. SLIT should not be given to children with AR due to HDM unless being done for research. | X | |||||
| Lifestyle changes | ||||||
| Avoid single chemical or physical preventive and combination preventive methods to reduce HDM exposure. | X | |||||
| Allergen avoidance may decrease AR symptoms, but more research is needed. | X (2010) | |||||
| Achieving substantial reductions in HDM load may decrease AR symptoms. | X (2012) | |||||
| Avoidance of mold or animal dander is recommended for patients who are allergic to them. | X | |||||
| Nasal filters can reduce symptoms of AR during ragweed and grass pollen seasons. | X | |||||
| Complementary and alternative medicine | ||||||
| Avoid homeopathy, acupuncture, butterbur, herbal medicines, and phototherapy. | X | |||||
| AR, allergic rhinitis; ARIA, Allergic Rhinitis and its Impact on Asthma; ASI, allergen-specific immunotherapy; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee; HDM, house dust mites; NIT, nasal immunotherapy; NS, nasal steroids; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy. *ARIA 2008 recommended changing the classification of AR from seasonal and perennial (frequent nonseasonal nasal or ocular symptoms) to intermittent (symptoms lasting <4 days per week or <4 weeks per year) or persistent (symptoms >4 days per week and >4 weeks per year).3 AR severity is classified as mild or moderate to severe.2,3 | ||||||
The summary that follows provides a more detailed look at the recommendations, with a review of the pathophysiology of AR (“Phases of allergic rhinitis”2,3,5,8,13-15).
The early phase of allergic rhinitis (AR) occurs within minutes of allergen exposure. Mast cell degranulation releases histamine and other inflammatory mediators that cause sneezing, pruritus, rhinorrhea, and nasal congestion.3,8,13 The late phase, beginning at 4 hours and peaking 6 to 12 hours after exposure, is believed to be due to recruitment of circulating leukocytes—particularly eosinophils. Leukocyte activation causes additional inflammatory mediators to be released, which primarily causes nasal congestion—often the most bothersome symptom of AR.2,5,8,13,14 Other presenting symptoms may include feeling “fuzzy” or tired, chronic viral infections, sniffing, eye rubbing, blinking, congested voice, snoring, or dark skin beneath the eyes (allergic shiners).15
Of note: This summary preserves the terminology used in ARIA 2010. Specifically, the ARIA guideline uses the term suggest for conditional recommendations and recommend for strong recommendations.4 That same language is used here.
Nasal steroids: First-line Tx for moderate to severe symptoms
BSACI indicates that nasal steroids (NS) are the treatment of choice for moderate to severe persistent AR (symptoms lasting >4 days per week or >4 weeks per year).5 ARIA 2010 suggests NS as first-line treatment rather than oral antihistamines for adults and children with seasonal (related to outdoor allergens such as pollens or molds) and persistent AR.4 ARIA 2008 finds NS are the most effective treatment for children.3 Steroids reduce inflammation by decreasing inflammatory cell migration and inhibiting cytokine release.16 They are the most effective monotherapy for all symptoms of AR, including nasal congestion, which antihistamines do not treat effectively.13,16 NS also treat ocular symptoms of allergy effectively.15,17
The ARIA 2010 guideline also recommends using NS rather than nasal antihistamines and leukotriene receptor antagonists.4 Combination therapy (eg, NS with the addition of nasal antihistamines) is an option for severe or persistent AR, but it appears to be no more effective than monotherapy with NS.16 A 2010 Cochrane review determined there is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR.7 Intermittent steroid use may be beneficial in children.5
Steroids begin working 6 to 8 hours after the first dose, although symptom reduction may take days and maximal effect up to 2 weeks.5 Treatment failure may be due to poor technique that can cause local adverse effects (ie, dryness, irritation, epistaxis). Technique-related failure occurs in up to 10% of users.5,15 Educating patients and families about correct technique with steroid spray may decrease nonadherence due to irritation and epistaxis.18 Tell them to shake the bottle well, look down, aim the nozzle toward the outside wall of the nostril using the opposite hand, and spray while sniffing lightly.5
Any steroid is appropriate for adults. For children ≥2 years of age, consider fluticasone propionate, mometasone furoate, or triamcinolone acetonide.3 These medications have lower systemic bioavailability and a decreased risk of such adverse effects as hypothalamic-pituitary-adrenal axis suppression and growth retardation.15 Budesonide is appropriate for those ≥6 years.19-21 Avoid regular use of betamethasone, which has high bioavailability, for >1 year in children, as it may decrease their growth rate.3 Beclomethasone, fluticasone, and budesonide have been used widely and safely for pregnant women with asthma.5
Antihistamines are first-line Tx for mild symptoms
ARIA 2010 recommends new-generation oral nonsedating antihistamines that do not affect cytochrome P450 for mild AR,4 such as cetirizine, levocetirizine, loratadine, desloratadine, and fexofenadine. First-generation antihistamines can reduce symptoms, but are not first-line treatment as they cause sedation, fatigue, decreased cognitive function, and reduced academic and work performance.3-5 ARIA 2010 further suggests choosing oral antihistamines over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR.4
BSACI recommends oral or topical antihistamines as first-line treatment for mild to moderate symptoms lasting <4 days per week or <4 weeks per year and moderate persistent AR.5 When steroids alone do not control moderate to severe persistent AR, BSACI recommends adding oral or topical antihistamines.5 Oral and topical antihistamines decrease histamine-related symptoms of itching, rhinorrhea, and sneezing, but do not significantly decrease nasal congestion.15
Nasal antihistamines (levocabastine, azelastine) have a rapid onset of action and few adverse effects.3 ARIA 2010 suggests nasal antihistamines over nasal chromones (inhibitors of mast cell degranulation) and notes that the need to use chromones 4 times daily may limit adherence.4 The same guidelines suggest nasal antihistamine use for children and adults with seasonal AR and suggest not using nasal antihistamines for patients with persistent AR until more data on efficacy and safety are available.4
Alezastine is approved for individuals ≥5 years, and olopatadine is approved for individuals ≥6 years for the treatment of AR.16,22,23 A pediatric review article noted nasal antihistamine (azelastine) plus nasal fluticasone was more efficacious than NS alone.15
In children, weigh adverse effects of antihistamines against the general malaise caused by AR.3 Do not use first-generation antihistamines due to the sedation that may interfere with learning.15 Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children.5 Continuous administration, rather than as needed, is optimal treatment in children.5 Cetirizine, loratadine, and levocetirizine have been studied and are effective and safe in children.3 Levocetirizine has proven safe and efficacious for children ≥2 years.24 Fexofenadine was found to be effective and safe for those ≥6 years.25
For children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones.4 Due to the safety of these agents, chromones may be used first, then antihistamines.4 Just as with nasal chromones, the need to use intraocular chromones 4 times daily may limit their use in children.4
Pregnant patients. Antihistamines do cross the placenta.5 Agents that appear to be safe for pregnant patients are chlorphenamine (first-generation), loratadine, and cetirizine.5
Leukotriene receptor antagonists: Always pair with antihistamines
As adjunctive therapy for additional symptom control, ARIA 2010 suggests oral leukotriene receptor antagonists for children and adults with seasonal AR, and for preschool children with persistent AR. These agents may also be helpful in children with concurrent asthma.15 Always pair leukotriene receptor antagonists with antihistamines. Montelukast is approved for seasonal AR in children ≥2 years and for frequent nonseasonal nasal or ocular AR symptoms in children ≥6 months.26
ARIA 2010 recommends against the use of oral leukotriene receptor antagonists in adults with persistent AR.4
Decongestants are for limited use only
For adults with severe nasal obstruction, ARIA 2010 suggests a short course (<5 days) of nasal decongestant along with other drugs.4 Limiting use of nasal decongestants to <10 days helps prevent rhinitis medicamentosa.5,27 BSACI notes nasal decongestants may be useful for eustachian tube dysfunction experienced aboard airplanes, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency before NS use.5 Both guidelines suggest against regular oral decongestant use.4,5
Avoid decongestants in pregnant patients.5 ARIA 2010 suggests against nasal decongestant use in preschool children.4
Chromones may help, but require multiple daily dosing
Chromones inhibit mast cell degranulation, are weakly effective for reducing nasal obstruction in AR, and have a high safety profile.3-5,28 As noted earlier, they must be used 4 times daily, which may reduce adherence—particularly in children.4
ARIA 2008 notes that disodium cromoglycate is less effective than NS or antihistamines.3 The 2010 update suggests nasal antihistamines over nasal chromones.4 For adults as well as children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones. BSACI recommends limited use of chromones for children and adults with mild symptoms.5
Nasal saline helpful as adjunct to medication
Nasal saline irrigation improves symptoms of AR, clears nasal passages, and is helpful for pregnant patients, for whom medications should be used with caution.2,3,5 Nasal irrigation using a neti pot or squeeze bottle is efficacious for chronic rhinorrhea, as solo or complementary treatment, and for children.5,16,27
Oral steroids: Use only rarely
ARIA 2010 suggests a short course of oral glucocorticosteroids for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments.4 BSACI notes oral steroids are rarely indicated, but that their use over 5 to 10 days may help with severe nasal congestion, symptoms uncontrolled by conventional pharmacotherapy, or before important social or work events.5 Both guidelines recommend against intramuscular steroids.4 ARIA 2008 notes oral and depot preparations of steroids affect growth in young children.3
Ipratropium when rhinorrhea is severe
Nasal ipratropium bromide, a topical anticholinergic, is helpful for excessive or refractory rhinorrhea. Consider using ipratropium with NS for patients for whom rhinorrhea is the dominant symptom.5,16,28 ARIA 2010 suggests using nasal ipratropium to treat rhinorrhea in patients with persistent AR.4
Allergen-specific immunotherapy: When other treatments fail
Allergen-specific immunotherapy (ASI) consists of repeated exposure to an allergen to induce immunomodulation, which prevents or reduces allergy symptoms and actually changes the natural course of AR. (For more on identifying the offending agent, see “Time for allergen testing?”2,5,15,18,29.) This treatment process decreases medication needs, prevents new allergen sensitization, and results in long-lasting improvement.2,5,6,30 BSACI 2011 notes that ASI is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy and allergen avoidance measures.6
When a patient’s symptoms are poorly controlled or persist after treatment, consider allergen testing.29 Skin prick testing (SPT) is the best means of eliciting specific allergen sensitization. However, limit testing to allergens most likely causing the patient’s symptoms rather than ordering a random panel; 15% of those with a positive SPT to specific allergens do not have symptoms when exposed to those allergens in their environment.5 And always interpret results of allergy testing in light of the patient’s history.2,15,18
SPT has a high negative predictive value, which can prevent unnecessary lifestyle changes.29 However, keep in mind that SPT results may be suppressed if the patient is using antihistamines, tricyclic antidepressants, or topical steroids.29 If SPT is not feasible or the patient is taking medications that may suppress results, consider arranging for serum-specific IgE testing, also known as radioallergosorbent testing, or RAST.5 RAST and SPT have similar sensitivities for house dust mites, but RAST is not as sensitive as SPT for other inhalants (eg, cat epithelium, mold, grass pollen).5
ASI methods developed to date use subcutaneous, sublingual, or nasal routes of administration. However, the US Food and Drug Administration has yet to approve commercial sublingual or nasal products for use in the United States.16
Subcutaneous immunotherapy may cause local adverse reactions (pruritus and swelling) and systemic reactions that can be severe or life threatening (anaphylaxis) and thus must be given in a doctor’s office prepared to treat anaphylaxis.6,16,30 Adrenaline administration has been necessary in 0.13% of those being treated.9 Subcutaneous immunotherapy must be done for 3 to 5 years for sustained effective treatment.15
ARIA 2010 suggests subcutaneous immunotherapy for adults with seasonal AR and with persistent AR due to house dust mites.4 A 2007 Cochrane review found subcutaneous immunotherapy is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few significant severe adverse reactions.9 A meta-analysis showed subcutaneous immunotherapy is as potent as pharmacotherapy in controlling seasonal AR symptoms as early as the first season of treatment.31
What if the patient is pregnant—or a child? BSACI notes that maintenance ASI may be continued in a patient who becomes pregnant, but starting ASI or increasing the dose is contraindicated.5
Based on ARIA 2008 and 2010, consider subcutaneous immunotherapy for children—but not for those <5 years.3,4 Care must be used in selecting patients, as 3 to 5 years of treatment are necessary for sustained benefit.15
Lifestyle changes: Limited benefit may be achievable
ARIA 2010 recommends mold avoidance and animal dander avoidance for patients so affected.4 Allergens from pets can persist in homes for months after pet removal.15 BSACI found that commercially available nasal filters (filters or screens placed over or within both nares) reduced symptoms of AR during ragweed and grass pollen seasons.5 Allergen avoidance for children with persistent AR has not shown consistent benefit.15 A 2010 Cochrane review concluded that allergen avoidance may decrease AR symptoms, but more research is needed.11
House dust mites. The 2010 Cochrane review also reported on 2 trials that assessed high-efficiency particulate air (HEPA) filters specifically for patients allergic to house dust mites.11 The studies, which had methodological limitations (inconsistent randomization, small sample size, and short duration), concluded that HEPA filters alone will not likely reduce symptoms of house dust mite allergy. But HEPA filters may be beneficial as one component of an extensive bedroom-based environmental control program.11
Impermeable bedding has been shown to reduce dust mite load by 50% to 70%, leaving residual allergen that may still trigger symptoms.11 A 2012 Cochrane review concluded that achieving substantial reductions in house dust mite load using a combination approach of multiple interventions, including acaricides and extensive bedroom-based environmental control programs, may decrease AR symptoms.12 However, ARIA 2010 recommends against single chemical or physical preventive methods and against combination preventive methods to reduce house dust mite exposure.4
Total elimination of house dust mites may be impossible, and recommending use of impermeable covers and HEPA filters, removal of rugs and curtains, and frequent cleaning must take into account a patient’s symptoms and a family’s motivation and finances.11,18
Complementary and alternative medicine: Too little evidence
ARIA 2010 suggests against patients using homeopathy, acupuncture, butterbur, herbal medicines, or phototherapy for AR.4 While one systematic review of acupuncture for AR demonstrated mixed results with no specific effects for seasonal AR and some improvement of frequent nonseasonal symptoms,32 another review concluded evidence was insufficient to make any recommendation.32,33 The benefit of ear acupressure is unknown, as supporting studies are of low methodological quality, although it appeared to provide some benefit for AR.34
Due to lack of data, probiotics should not be recommended.27 A pediatric review article noted that probiotics may alter cytokine production in patients with seasonal AR and may be more helpful in AR than in asthma, although more research was needed.15 Another review showed that probiotics may reduce AR symptoms and medication use.35
CASE Since the nasal steroid you prescribed for your patient did not provide adequate relief, you opt to add cetirizine 10 mg to his NS regimen. This step relieved his symptoms within 2 to 3 days. Had his symptoms persisted, the patient would have been a candidate for a one-week course of oral decongestant, such as pseudoephedrine 120 mg orally every 12 hours, as needed; and then for allergen testing, specifically for pollens corresponding to the seasonality of his AR. Appropriate follow-up would be to monitor the patient until his symptoms resolved or became manageable.
• Use nasal steroids to treat allergic rhinitis (AR) in adults. A
• Recommend nasal saline irrigation to reduce symptoms in children and adults with seasonal rhinitis. A
• Consider immunotherapy for adults and children with severe AR that does not respond to conventional pharmacotherapy or allergen avoidance measures. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A man in his 30s with allergic rhinitis (AR) at predictable times of the year with high pollen counts reports only modest symptom relief with a nasal steroid preparation after 3 weeks of use. He comes to see you because he’s “tired of feeling lousy all of the time.”
What management options would you consider?
There is a plethora of treatment options for patients like this one, and considerable variation in clinical practice when it comes to AR.1 The good news is that there are several recent guidelines for treating AR patients, whose symptoms (and underlying cause) can vary widely.
The following review—and accompanying algorithm—provides evidence-based recommendations that can help you refine your approach to AR.
Two guidelines, and several Cochrane reviews
Allergic Rhinitis and its Impact on Asthma (ARIA), a sentinel rhinitis treatment guideline, was published in 2001 and updated in 2008 and 2010.2-4 The British Society for Allergy and Clinical Immunology Standards of Care Committee (BSACI) published guidelines for rhinitis management in 2008 and guidelines for immunotherapy in 2011.5,6 In addition, several Cochrane reviews have been performed.7-12 The ALGORITHM1-6 combines these recommendations. The TABLE2-12 itemizes the recommendations made by each guideline.
ALGORITHM
An evidence-based approach to treating allergic rhinitis1-6
Based on recommendations from ARIA and BSACI guidelines and Cochrane reviews
ARIA, Allergic Rhinitis and its Impact on Asthma; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee.
TABLE
Treatment recommendations/suggestions for allergic rhinitis2-12
| TREATMENT RECOMMENDATIONS/SUGGESTIONS | ARIA 2001 | ARIA 2008 | ARIA 2010 | BSACI 2008 | BSACI 2011 | COCHRANE REVIEWS |
|---|---|---|---|---|---|---|
| General principles of treatment | ||||||
| Maintenance therapy is required for persistent AR as medications have little effect after cessation. | X | |||||
| Patient education | ||||||
| Standardized patient education improves disease-specific quality of life. | X | |||||
| Nasal steroids | ||||||
| NS are the most effective monotherapy for all symptoms of AR, seasonal and perennial,* including nasal congestion. | X | |||||
| NS are recommended for AR treatment in adults and suggested for children. | X | |||||
| NS are the treatment of choice for moderate to severe persistent* AR and for treatment failures with antihistamines alone. | X | |||||
| NS are suggested over oral antihistamines in adults and children for seasonal AR. | X | |||||
| NS are suggested over oral antihistamines for adults and children with persistent AR. | X | |||||
| NS are recommended rather than nasal antihistamines. | X | |||||
| NS are recommended over oral leukotriene receptor antagonists for seasonal AR. | X | |||||
| NS are the most effective treatment of AR for children. | X | |||||
| There is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR. | X (2010) | |||||
| Intermittent* NS use may be beneficial in children. | X | |||||
| Avoid NS with high bioavailability (betamethasone) in children, as regular use for >1 year may decrease growth rate. | X | |||||
| Antihistamines | ||||||
| New-generation oral nonsedating antihistamines that do not affect cytochrome P450 are recommended for the treatment of patients with AR. | X | |||||
| Oral or topical antihistamines are first-line treatment for mild to moderate intermittent and moderate persistent AR. | X | |||||
| When NS alone do not control moderate to severe persistent AR, may add oral or topical antihistamines. | X | |||||
| New-generation oral antihistamines are suggested over nasal antihistamines for children and adults, and for children with seasonal or persistent AR. | X | |||||
| Oral antihistamines are suggested over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR. | X | |||||
| Nasal antihistamines are suggested over nasal chromones (the need to use chromones 4 times daily may limit adherence). | X | |||||
| Nasal antihistamine use is suggested for children and adults with seasonal AR. | X | |||||
| Patients with persistent AR should avoid using nasal antihistamines until more data on efficacy and safety are available. | X | |||||
| In children, weigh adverse effects of antihistamines against the general malaise caused by AR. | X | |||||
| Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children. | X | |||||
| Continuous administration of antihistamines is optimal in children, rather than as needed. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for patients with ocular symptoms. | X | |||||
| Oral leukotriene receptor antagonists | ||||||
| Oral leukotriene receptor antagonists are suggested for children and adults with seasonal AR and for preschool children with persistent AR. | X | |||||
| Avoid oral leukotriene receptor antagonists in adults with persistent AR. | X | |||||
| Decongestants | ||||||
| For adults with severe nasal obstruction, a short course (<5 days) of a nasal decongestant, along with other drugs, is suggested. | X | |||||
| Nasal decongestants may be useful for eustachian tube dysfunction when flying, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency prior to NS use. | X | |||||
| Regular oral decongestant use is not suggested. | X | X | ||||
| Avoid decongestants in pregnant patients. | X | |||||
| Avoid using nasal decongestants in preschool children. | X | |||||
| Chromones | ||||||
| Limited use of chromones is recommended for children and adults with mild symptoms. | X | |||||
| Chromones are less effective than NS or antihistamines. | X | |||||
| Nasal antihistamines are suggested over nasal chromones. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for ocular symptoms. Due to the excellent safety of these agents, chromones may be tried before antihistamines. | X | |||||
| Nasal saline | ||||||
| Nasal saline irrigation reduces symptoms in children and adults with seasonal rhinitis. | X | |||||
| Oral, intramuscular steroids | ||||||
| A short course of oral glucocorticosteroids is suggested for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments. | X | |||||
| Oral steroids are rarely indicated, but a short course (5-10 days) may be used for severe nasal congestion, uncontrolled symptoms on conventional pharmacotherapy, or important social/work events. | X | |||||
| Avoid intramuscular steroids. | X | X | ||||
| Ipratropium | ||||||
| Nasal ipratropium is suggested for treatment of rhinorrhea for patients with persistent AR. | X | |||||
| Allergen-specific immunotherapy | ||||||
| Immunotherapy is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy or allergen avoidance measures. | X | |||||
| SCIT is suggested for adults with seasonal AR and those with persistent AR due to house dust mites. | X | |||||
| SCIT is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few severe adverse reactions. | X (2007) | |||||
| SLIT is suggested for adults with AR due to pollen, although other alternatives may be equally reasonable. | X | |||||
| SLIT is safe and efficacious for AR treatment, decreasing symptoms and medication requirements. | X (2003) | |||||
| Nasal immunotherapy is suggested for adults with AR due to pollens. | X | |||||
| For pregnant patients, maintenance ASI may be continued, but starting ASI or increasing the dose is contraindicated. | X | |||||
| SCIT is suggested for children with AR. | X | |||||
| SCIT should not be started before 5 years of age. | X | |||||
| Based on preliminary studies, SLIT is safe, but more studies are needed in children. | X | |||||
| SLIT and NIT are suggested for children with AR due to pollens, acknowledging that other alternatives may be equally reasonable. SLIT should not be given to children with AR due to HDM unless being done for research. | X | |||||
| Lifestyle changes | ||||||
| Avoid single chemical or physical preventive and combination preventive methods to reduce HDM exposure. | X | |||||
| Allergen avoidance may decrease AR symptoms, but more research is needed. | X (2010) | |||||
| Achieving substantial reductions in HDM load may decrease AR symptoms. | X (2012) | |||||
| Avoidance of mold or animal dander is recommended for patients who are allergic to them. | X | |||||
| Nasal filters can reduce symptoms of AR during ragweed and grass pollen seasons. | X | |||||
| Complementary and alternative medicine | ||||||
| Avoid homeopathy, acupuncture, butterbur, herbal medicines, and phototherapy. | X | |||||
| AR, allergic rhinitis; ARIA, Allergic Rhinitis and its Impact on Asthma; ASI, allergen-specific immunotherapy; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee; HDM, house dust mites; NIT, nasal immunotherapy; NS, nasal steroids; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy. *ARIA 2008 recommended changing the classification of AR from seasonal and perennial (frequent nonseasonal nasal or ocular symptoms) to intermittent (symptoms lasting <4 days per week or <4 weeks per year) or persistent (symptoms >4 days per week and >4 weeks per year).3 AR severity is classified as mild or moderate to severe.2,3 | ||||||
The summary that follows provides a more detailed look at the recommendations, with a review of the pathophysiology of AR (“Phases of allergic rhinitis”2,3,5,8,13-15).
The early phase of allergic rhinitis (AR) occurs within minutes of allergen exposure. Mast cell degranulation releases histamine and other inflammatory mediators that cause sneezing, pruritus, rhinorrhea, and nasal congestion.3,8,13 The late phase, beginning at 4 hours and peaking 6 to 12 hours after exposure, is believed to be due to recruitment of circulating leukocytes—particularly eosinophils. Leukocyte activation causes additional inflammatory mediators to be released, which primarily causes nasal congestion—often the most bothersome symptom of AR.2,5,8,13,14 Other presenting symptoms may include feeling “fuzzy” or tired, chronic viral infections, sniffing, eye rubbing, blinking, congested voice, snoring, or dark skin beneath the eyes (allergic shiners).15
Of note: This summary preserves the terminology used in ARIA 2010. Specifically, the ARIA guideline uses the term suggest for conditional recommendations and recommend for strong recommendations.4 That same language is used here.
Nasal steroids: First-line Tx for moderate to severe symptoms
BSACI indicates that nasal steroids (NS) are the treatment of choice for moderate to severe persistent AR (symptoms lasting >4 days per week or >4 weeks per year).5 ARIA 2010 suggests NS as first-line treatment rather than oral antihistamines for adults and children with seasonal (related to outdoor allergens such as pollens or molds) and persistent AR.4 ARIA 2008 finds NS are the most effective treatment for children.3 Steroids reduce inflammation by decreasing inflammatory cell migration and inhibiting cytokine release.16 They are the most effective monotherapy for all symptoms of AR, including nasal congestion, which antihistamines do not treat effectively.13,16 NS also treat ocular symptoms of allergy effectively.15,17
The ARIA 2010 guideline also recommends using NS rather than nasal antihistamines and leukotriene receptor antagonists.4 Combination therapy (eg, NS with the addition of nasal antihistamines) is an option for severe or persistent AR, but it appears to be no more effective than monotherapy with NS.16 A 2010 Cochrane review determined there is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR.7 Intermittent steroid use may be beneficial in children.5
Steroids begin working 6 to 8 hours after the first dose, although symptom reduction may take days and maximal effect up to 2 weeks.5 Treatment failure may be due to poor technique that can cause local adverse effects (ie, dryness, irritation, epistaxis). Technique-related failure occurs in up to 10% of users.5,15 Educating patients and families about correct technique with steroid spray may decrease nonadherence due to irritation and epistaxis.18 Tell them to shake the bottle well, look down, aim the nozzle toward the outside wall of the nostril using the opposite hand, and spray while sniffing lightly.5
Any steroid is appropriate for adults. For children ≥2 years of age, consider fluticasone propionate, mometasone furoate, or triamcinolone acetonide.3 These medications have lower systemic bioavailability and a decreased risk of such adverse effects as hypothalamic-pituitary-adrenal axis suppression and growth retardation.15 Budesonide is appropriate for those ≥6 years.19-21 Avoid regular use of betamethasone, which has high bioavailability, for >1 year in children, as it may decrease their growth rate.3 Beclomethasone, fluticasone, and budesonide have been used widely and safely for pregnant women with asthma.5
Antihistamines are first-line Tx for mild symptoms
ARIA 2010 recommends new-generation oral nonsedating antihistamines that do not affect cytochrome P450 for mild AR,4 such as cetirizine, levocetirizine, loratadine, desloratadine, and fexofenadine. First-generation antihistamines can reduce symptoms, but are not first-line treatment as they cause sedation, fatigue, decreased cognitive function, and reduced academic and work performance.3-5 ARIA 2010 further suggests choosing oral antihistamines over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR.4
BSACI recommends oral or topical antihistamines as first-line treatment for mild to moderate symptoms lasting <4 days per week or <4 weeks per year and moderate persistent AR.5 When steroids alone do not control moderate to severe persistent AR, BSACI recommends adding oral or topical antihistamines.5 Oral and topical antihistamines decrease histamine-related symptoms of itching, rhinorrhea, and sneezing, but do not significantly decrease nasal congestion.15
Nasal antihistamines (levocabastine, azelastine) have a rapid onset of action and few adverse effects.3 ARIA 2010 suggests nasal antihistamines over nasal chromones (inhibitors of mast cell degranulation) and notes that the need to use chromones 4 times daily may limit adherence.4 The same guidelines suggest nasal antihistamine use for children and adults with seasonal AR and suggest not using nasal antihistamines for patients with persistent AR until more data on efficacy and safety are available.4
Alezastine is approved for individuals ≥5 years, and olopatadine is approved for individuals ≥6 years for the treatment of AR.16,22,23 A pediatric review article noted nasal antihistamine (azelastine) plus nasal fluticasone was more efficacious than NS alone.15
In children, weigh adverse effects of antihistamines against the general malaise caused by AR.3 Do not use first-generation antihistamines due to the sedation that may interfere with learning.15 Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children.5 Continuous administration, rather than as needed, is optimal treatment in children.5 Cetirizine, loratadine, and levocetirizine have been studied and are effective and safe in children.3 Levocetirizine has proven safe and efficacious for children ≥2 years.24 Fexofenadine was found to be effective and safe for those ≥6 years.25
For children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones.4 Due to the safety of these agents, chromones may be used first, then antihistamines.4 Just as with nasal chromones, the need to use intraocular chromones 4 times daily may limit their use in children.4
Pregnant patients. Antihistamines do cross the placenta.5 Agents that appear to be safe for pregnant patients are chlorphenamine (first-generation), loratadine, and cetirizine.5
Leukotriene receptor antagonists: Always pair with antihistamines
As adjunctive therapy for additional symptom control, ARIA 2010 suggests oral leukotriene receptor antagonists for children and adults with seasonal AR, and for preschool children with persistent AR. These agents may also be helpful in children with concurrent asthma.15 Always pair leukotriene receptor antagonists with antihistamines. Montelukast is approved for seasonal AR in children ≥2 years and for frequent nonseasonal nasal or ocular AR symptoms in children ≥6 months.26
ARIA 2010 recommends against the use of oral leukotriene receptor antagonists in adults with persistent AR.4
Decongestants are for limited use only
For adults with severe nasal obstruction, ARIA 2010 suggests a short course (<5 days) of nasal decongestant along with other drugs.4 Limiting use of nasal decongestants to <10 days helps prevent rhinitis medicamentosa.5,27 BSACI notes nasal decongestants may be useful for eustachian tube dysfunction experienced aboard airplanes, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency before NS use.5 Both guidelines suggest against regular oral decongestant use.4,5
Avoid decongestants in pregnant patients.5 ARIA 2010 suggests against nasal decongestant use in preschool children.4
Chromones may help, but require multiple daily dosing
Chromones inhibit mast cell degranulation, are weakly effective for reducing nasal obstruction in AR, and have a high safety profile.3-5,28 As noted earlier, they must be used 4 times daily, which may reduce adherence—particularly in children.4
ARIA 2008 notes that disodium cromoglycate is less effective than NS or antihistamines.3 The 2010 update suggests nasal antihistamines over nasal chromones.4 For adults as well as children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones. BSACI recommends limited use of chromones for children and adults with mild symptoms.5
Nasal saline helpful as adjunct to medication
Nasal saline irrigation improves symptoms of AR, clears nasal passages, and is helpful for pregnant patients, for whom medications should be used with caution.2,3,5 Nasal irrigation using a neti pot or squeeze bottle is efficacious for chronic rhinorrhea, as solo or complementary treatment, and for children.5,16,27
Oral steroids: Use only rarely
ARIA 2010 suggests a short course of oral glucocorticosteroids for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments.4 BSACI notes oral steroids are rarely indicated, but that their use over 5 to 10 days may help with severe nasal congestion, symptoms uncontrolled by conventional pharmacotherapy, or before important social or work events.5 Both guidelines recommend against intramuscular steroids.4 ARIA 2008 notes oral and depot preparations of steroids affect growth in young children.3
Ipratropium when rhinorrhea is severe
Nasal ipratropium bromide, a topical anticholinergic, is helpful for excessive or refractory rhinorrhea. Consider using ipratropium with NS for patients for whom rhinorrhea is the dominant symptom.5,16,28 ARIA 2010 suggests using nasal ipratropium to treat rhinorrhea in patients with persistent AR.4
Allergen-specific immunotherapy: When other treatments fail
Allergen-specific immunotherapy (ASI) consists of repeated exposure to an allergen to induce immunomodulation, which prevents or reduces allergy symptoms and actually changes the natural course of AR. (For more on identifying the offending agent, see “Time for allergen testing?”2,5,15,18,29.) This treatment process decreases medication needs, prevents new allergen sensitization, and results in long-lasting improvement.2,5,6,30 BSACI 2011 notes that ASI is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy and allergen avoidance measures.6
When a patient’s symptoms are poorly controlled or persist after treatment, consider allergen testing.29 Skin prick testing (SPT) is the best means of eliciting specific allergen sensitization. However, limit testing to allergens most likely causing the patient’s symptoms rather than ordering a random panel; 15% of those with a positive SPT to specific allergens do not have symptoms when exposed to those allergens in their environment.5 And always interpret results of allergy testing in light of the patient’s history.2,15,18
SPT has a high negative predictive value, which can prevent unnecessary lifestyle changes.29 However, keep in mind that SPT results may be suppressed if the patient is using antihistamines, tricyclic antidepressants, or topical steroids.29 If SPT is not feasible or the patient is taking medications that may suppress results, consider arranging for serum-specific IgE testing, also known as radioallergosorbent testing, or RAST.5 RAST and SPT have similar sensitivities for house dust mites, but RAST is not as sensitive as SPT for other inhalants (eg, cat epithelium, mold, grass pollen).5
ASI methods developed to date use subcutaneous, sublingual, or nasal routes of administration. However, the US Food and Drug Administration has yet to approve commercial sublingual or nasal products for use in the United States.16
Subcutaneous immunotherapy may cause local adverse reactions (pruritus and swelling) and systemic reactions that can be severe or life threatening (anaphylaxis) and thus must be given in a doctor’s office prepared to treat anaphylaxis.6,16,30 Adrenaline administration has been necessary in 0.13% of those being treated.9 Subcutaneous immunotherapy must be done for 3 to 5 years for sustained effective treatment.15
ARIA 2010 suggests subcutaneous immunotherapy for adults with seasonal AR and with persistent AR due to house dust mites.4 A 2007 Cochrane review found subcutaneous immunotherapy is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few significant severe adverse reactions.9 A meta-analysis showed subcutaneous immunotherapy is as potent as pharmacotherapy in controlling seasonal AR symptoms as early as the first season of treatment.31
What if the patient is pregnant—or a child? BSACI notes that maintenance ASI may be continued in a patient who becomes pregnant, but starting ASI or increasing the dose is contraindicated.5
Based on ARIA 2008 and 2010, consider subcutaneous immunotherapy for children—but not for those <5 years.3,4 Care must be used in selecting patients, as 3 to 5 years of treatment are necessary for sustained benefit.15
Lifestyle changes: Limited benefit may be achievable
ARIA 2010 recommends mold avoidance and animal dander avoidance for patients so affected.4 Allergens from pets can persist in homes for months after pet removal.15 BSACI found that commercially available nasal filters (filters or screens placed over or within both nares) reduced symptoms of AR during ragweed and grass pollen seasons.5 Allergen avoidance for children with persistent AR has not shown consistent benefit.15 A 2010 Cochrane review concluded that allergen avoidance may decrease AR symptoms, but more research is needed.11
House dust mites. The 2010 Cochrane review also reported on 2 trials that assessed high-efficiency particulate air (HEPA) filters specifically for patients allergic to house dust mites.11 The studies, which had methodological limitations (inconsistent randomization, small sample size, and short duration), concluded that HEPA filters alone will not likely reduce symptoms of house dust mite allergy. But HEPA filters may be beneficial as one component of an extensive bedroom-based environmental control program.11
Impermeable bedding has been shown to reduce dust mite load by 50% to 70%, leaving residual allergen that may still trigger symptoms.11 A 2012 Cochrane review concluded that achieving substantial reductions in house dust mite load using a combination approach of multiple interventions, including acaricides and extensive bedroom-based environmental control programs, may decrease AR symptoms.12 However, ARIA 2010 recommends against single chemical or physical preventive methods and against combination preventive methods to reduce house dust mite exposure.4
Total elimination of house dust mites may be impossible, and recommending use of impermeable covers and HEPA filters, removal of rugs and curtains, and frequent cleaning must take into account a patient’s symptoms and a family’s motivation and finances.11,18
Complementary and alternative medicine: Too little evidence
ARIA 2010 suggests against patients using homeopathy, acupuncture, butterbur, herbal medicines, or phototherapy for AR.4 While one systematic review of acupuncture for AR demonstrated mixed results with no specific effects for seasonal AR and some improvement of frequent nonseasonal symptoms,32 another review concluded evidence was insufficient to make any recommendation.32,33 The benefit of ear acupressure is unknown, as supporting studies are of low methodological quality, although it appeared to provide some benefit for AR.34
Due to lack of data, probiotics should not be recommended.27 A pediatric review article noted that probiotics may alter cytokine production in patients with seasonal AR and may be more helpful in AR than in asthma, although more research was needed.15 Another review showed that probiotics may reduce AR symptoms and medication use.35
CASE Since the nasal steroid you prescribed for your patient did not provide adequate relief, you opt to add cetirizine 10 mg to his NS regimen. This step relieved his symptoms within 2 to 3 days. Had his symptoms persisted, the patient would have been a candidate for a one-week course of oral decongestant, such as pseudoephedrine 120 mg orally every 12 hours, as needed; and then for allergen testing, specifically for pollens corresponding to the seasonality of his AR. Appropriate follow-up would be to monitor the patient until his symptoms resolved or became manageable.
1. Bousquet J, Schünemann HJ, Zuberbier T, et al. Development and implementation of guidelines in allergic rhinitis – an ARIA-GA2LEN paper. Allergy. 2010;65:1212-1221.
2. Bousquet J, Van-Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(suppl):S147-S334.
3. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy. 2008;63(suppl 86):S8-S160.
4. Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466-476.
5. Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38:19-42.
6. Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177-1200.
7. Al Sayyad JJ, Fedorowicz Z, Alhashimi D, et al. Topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2007;(1):CD003163.-
8. Nasser M, Fedorowicz, Alijufairi H, et al. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2010;(7):CD006989.-
9. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936.-
10. Wilson D, Torres-Lima M, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;(2):CD002893.-
11. Sheikh A, Hurwitz B, Nurmatov U, et al. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010;(7):CD001563.-
12. Nurmatov U, van Schayck CP, Hurwitz B, et al. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158-165.
13. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(suppl):S2-S8.
14. Nathan RA. The pathophysiology, clinical impact, and management of nasal congestion in allergic rhinitis. Clin Ther. 2008;30:573-586.
15. Kemp AS. Allergic rhinitis. Paediatric Respir Rev. 2009;10:63-68.
16. Sur DK, Scandale S. Treatment of allergic rhinitis. Am Fam Physician. 2010;81:1440-1446.
17. Hong J, Bielory B, Rosenberg JL, et al. Efficacy of intranasal corticosteroids for the ocular symptoms of allergic rhinitis: a systematic review. Allergy Asthma Proc. 2011;32:22-35.
18. Hu W, Katelaris CH, Kemp AS. Allergic rhinitis – practical management strategies. Aust Fam Physician. 2008;37:214-220.
19. Veramyst (fluticasone furoate) nasal spray [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; August 2012. Available at: http://us.gsk.com/products/assets/us_veramyst.pdf. Accessed January 16, 2013.
20. Nasacort AQ (triamcinolone acetonide) nasal spray [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/nasacort_aq/nasacort_aq.html. Accessed January 16, 2013.
21. Rhinocort AQUA 32 mcg (budesonide) nasal spray [prescribing information]. Wilmington, Del: AstraZeneca; revised December 2010. Available at: http://www1.astrazeneca-us.com/pi/Rhinocort_Aqua.pdf. Accessed January 16, 2013.
22. Astelin (azelastine hydrochloride) spray, metered [prescribing information]. Somerset, NJ: Meda Pharmaceuticals; revised July 2011. Available at: http://www.astelin.com/pdf/astelin_pi.pdf. Accessed January 16, 2013.
23. Patanase (olopatadine hydrochloride) nasal spray [prescribing information]. Fort Worth, Tex: Alcon Laboratories; revised February 2012. Available at: http://ecatalog.alcon.com/PI/Patanase_us_en.pdf. Accessed January 16, 2013.
24. Xyzal (levocetirizine dihydrochloride) tablets and oral solution [prescribing information]. Smyrna, Ga: UCB and Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/xyzal/xyzal.pdf. Accessed January 16, 2013.
25. Allegra (fexofenadine hydrochloride) tablets, ODT, and oral suspension [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2007. Available at: http://products.sanofi.us/allegra/allegra.html. Accessed January 16, 2013.
26. Singulair (montelukast sodium) tablets, chewable tablets, and oral granules [prescribing information]. Whitehouse Station, NJ: Merck; revised November 2012. Available at: http://www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf. Accessed January 16, 2013.
27. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(suppl):S12-S16.
28. Lim MY, Leong JM. Allergic rhinitis: evidence-based practice. Singapore Med J. 2010;51:542-550.
29. Angier E, Willington J, Scadding G, et al. Management of allergic and non-allergic rhinitis: a primary care summary of the BSACI guideline. Prim Care Respir J. 2010;19:217-222.
30. Radulovic S, Wilson D, Calderon M, et al. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66:740-752.
31. Matricardi PM, Kuna P, Panetta V, et al. Subcutaneous immunotherapy and pharmacology in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011;128:791-799.
32. Lee MS, Pittler MH, Shin B, et al. Acupuncture for allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2009;102:269-279.
33. Roberts J, Huissoon A, Dretzke J, et al. A systematic review of the clinical effectiveness of acupuncture for allergic rhinitis. BMC Complement Altern Med. 2008;8:13.-
34. Zhang CS, Yang AW, Zhang AL, et al. Ear-acupressure for allergic rhinitis: a systematic review. Clin Otolaryngol. 2010;35:6-12.
35. Vliagoftis H, Kouranos VD, Betsi GI, et al. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:570-579.
CORRESPONDENCE Suzanne Minor, MD, Florida International University Herbert Wertheim College of Medicine; 11200 SW 8th Street, AHC II 361A, Miami, FL 33199; [email protected]
1. Bousquet J, Schünemann HJ, Zuberbier T, et al. Development and implementation of guidelines in allergic rhinitis – an ARIA-GA2LEN paper. Allergy. 2010;65:1212-1221.
2. Bousquet J, Van-Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(suppl):S147-S334.
3. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy. 2008;63(suppl 86):S8-S160.
4. Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466-476.
5. Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38:19-42.
6. Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177-1200.
7. Al Sayyad JJ, Fedorowicz Z, Alhashimi D, et al. Topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2007;(1):CD003163.-
8. Nasser M, Fedorowicz, Alijufairi H, et al. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2010;(7):CD006989.-
9. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936.-
10. Wilson D, Torres-Lima M, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;(2):CD002893.-
11. Sheikh A, Hurwitz B, Nurmatov U, et al. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010;(7):CD001563.-
12. Nurmatov U, van Schayck CP, Hurwitz B, et al. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158-165.
13. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(suppl):S2-S8.
14. Nathan RA. The pathophysiology, clinical impact, and management of nasal congestion in allergic rhinitis. Clin Ther. 2008;30:573-586.
15. Kemp AS. Allergic rhinitis. Paediatric Respir Rev. 2009;10:63-68.
16. Sur DK, Scandale S. Treatment of allergic rhinitis. Am Fam Physician. 2010;81:1440-1446.
17. Hong J, Bielory B, Rosenberg JL, et al. Efficacy of intranasal corticosteroids for the ocular symptoms of allergic rhinitis: a systematic review. Allergy Asthma Proc. 2011;32:22-35.
18. Hu W, Katelaris CH, Kemp AS. Allergic rhinitis – practical management strategies. Aust Fam Physician. 2008;37:214-220.
19. Veramyst (fluticasone furoate) nasal spray [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; August 2012. Available at: http://us.gsk.com/products/assets/us_veramyst.pdf. Accessed January 16, 2013.
20. Nasacort AQ (triamcinolone acetonide) nasal spray [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/nasacort_aq/nasacort_aq.html. Accessed January 16, 2013.
21. Rhinocort AQUA 32 mcg (budesonide) nasal spray [prescribing information]. Wilmington, Del: AstraZeneca; revised December 2010. Available at: http://www1.astrazeneca-us.com/pi/Rhinocort_Aqua.pdf. Accessed January 16, 2013.
22. Astelin (azelastine hydrochloride) spray, metered [prescribing information]. Somerset, NJ: Meda Pharmaceuticals; revised July 2011. Available at: http://www.astelin.com/pdf/astelin_pi.pdf. Accessed January 16, 2013.
23. Patanase (olopatadine hydrochloride) nasal spray [prescribing information]. Fort Worth, Tex: Alcon Laboratories; revised February 2012. Available at: http://ecatalog.alcon.com/PI/Patanase_us_en.pdf. Accessed January 16, 2013.
24. Xyzal (levocetirizine dihydrochloride) tablets and oral solution [prescribing information]. Smyrna, Ga: UCB and Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/xyzal/xyzal.pdf. Accessed January 16, 2013.
25. Allegra (fexofenadine hydrochloride) tablets, ODT, and oral suspension [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2007. Available at: http://products.sanofi.us/allegra/allegra.html. Accessed January 16, 2013.
26. Singulair (montelukast sodium) tablets, chewable tablets, and oral granules [prescribing information]. Whitehouse Station, NJ: Merck; revised November 2012. Available at: http://www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf. Accessed January 16, 2013.
27. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(suppl):S12-S16.
28. Lim MY, Leong JM. Allergic rhinitis: evidence-based practice. Singapore Med J. 2010;51:542-550.
29. Angier E, Willington J, Scadding G, et al. Management of allergic and non-allergic rhinitis: a primary care summary of the BSACI guideline. Prim Care Respir J. 2010;19:217-222.
30. Radulovic S, Wilson D, Calderon M, et al. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66:740-752.
31. Matricardi PM, Kuna P, Panetta V, et al. Subcutaneous immunotherapy and pharmacology in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011;128:791-799.
32. Lee MS, Pittler MH, Shin B, et al. Acupuncture for allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2009;102:269-279.
33. Roberts J, Huissoon A, Dretzke J, et al. A systematic review of the clinical effectiveness of acupuncture for allergic rhinitis. BMC Complement Altern Med. 2008;8:13.-
34. Zhang CS, Yang AW, Zhang AL, et al. Ear-acupressure for allergic rhinitis: a systematic review. Clin Otolaryngol. 2010;35:6-12.
35. Vliagoftis H, Kouranos VD, Betsi GI, et al. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:570-579.
CORRESPONDENCE Suzanne Minor, MD, Florida International University Herbert Wertheim College of Medicine; 11200 SW 8th Street, AHC II 361A, Miami, FL 33199; [email protected]