User login
How to identify balance disorders and reduce fall risk
CASE Mr. J, a 75-year-old man, presents to your family practice reporting that he feels increasingly unsteady and slow while walking. He fell twice last year, without resulting injury. He now worries about tripping while walking around the house and relies on his spouse to run errands.
Clearly, Mr. J is experiencing a problem with balance. What management approach should you undertake to prevent him from falling?

Balance disorders are common in older people and drastically hinder quality of life.1-4 Patients often describe imbalance as vague symptoms: dizziness, unsteadiness, faintness, spinning sensations.5,6 Importantly, balance disorders disrupt normal gait and contribute to falls that are a major cause of disability and morbidity in older people. Almost 30% of people older than 65 years report 1 or more falls annually.7 Factors that increase the risk of falls include impaired mobility, previously reported falls, reduced psychological functioning, chronic medical conditions, and polypharmacy.7,8
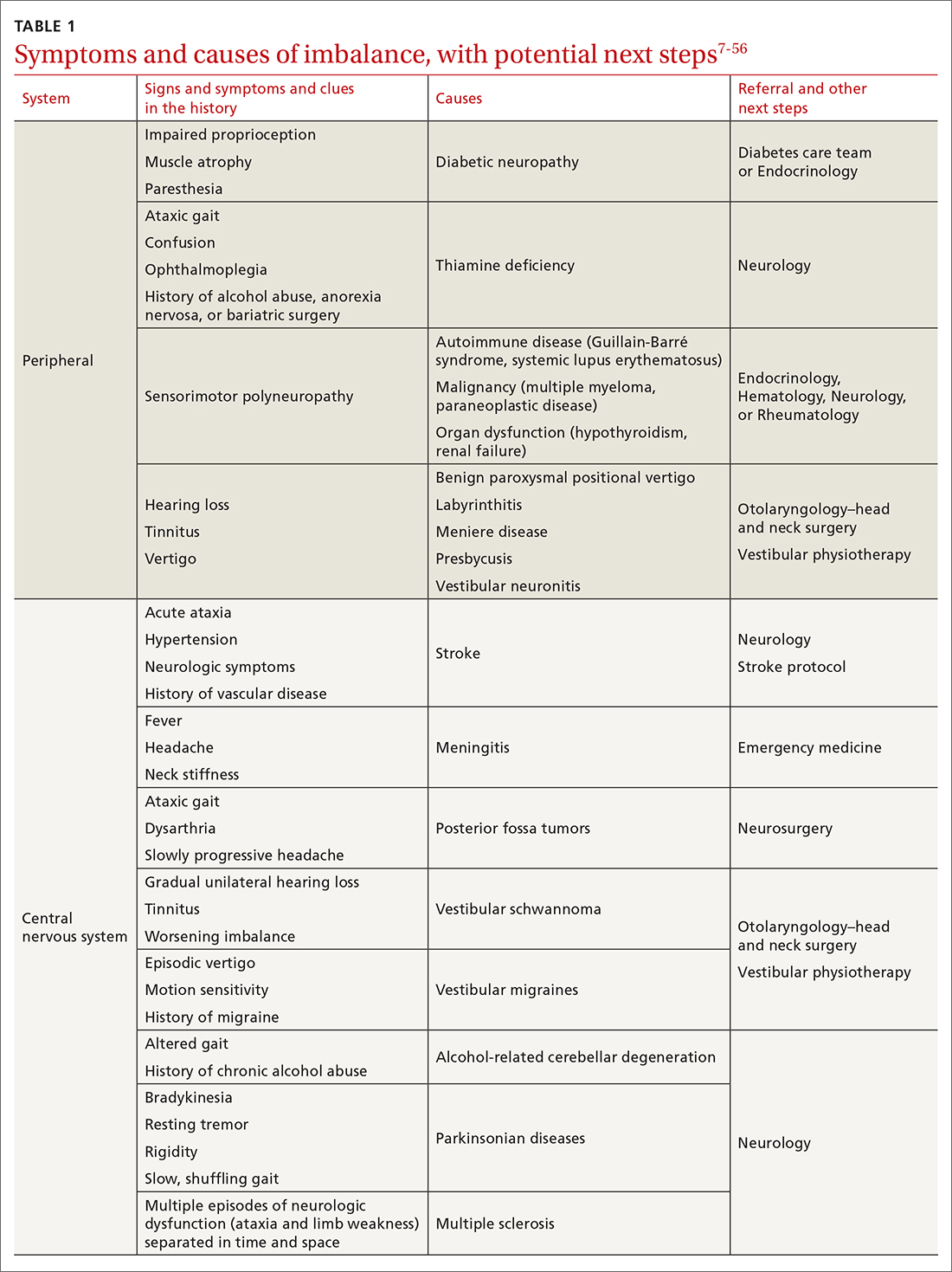
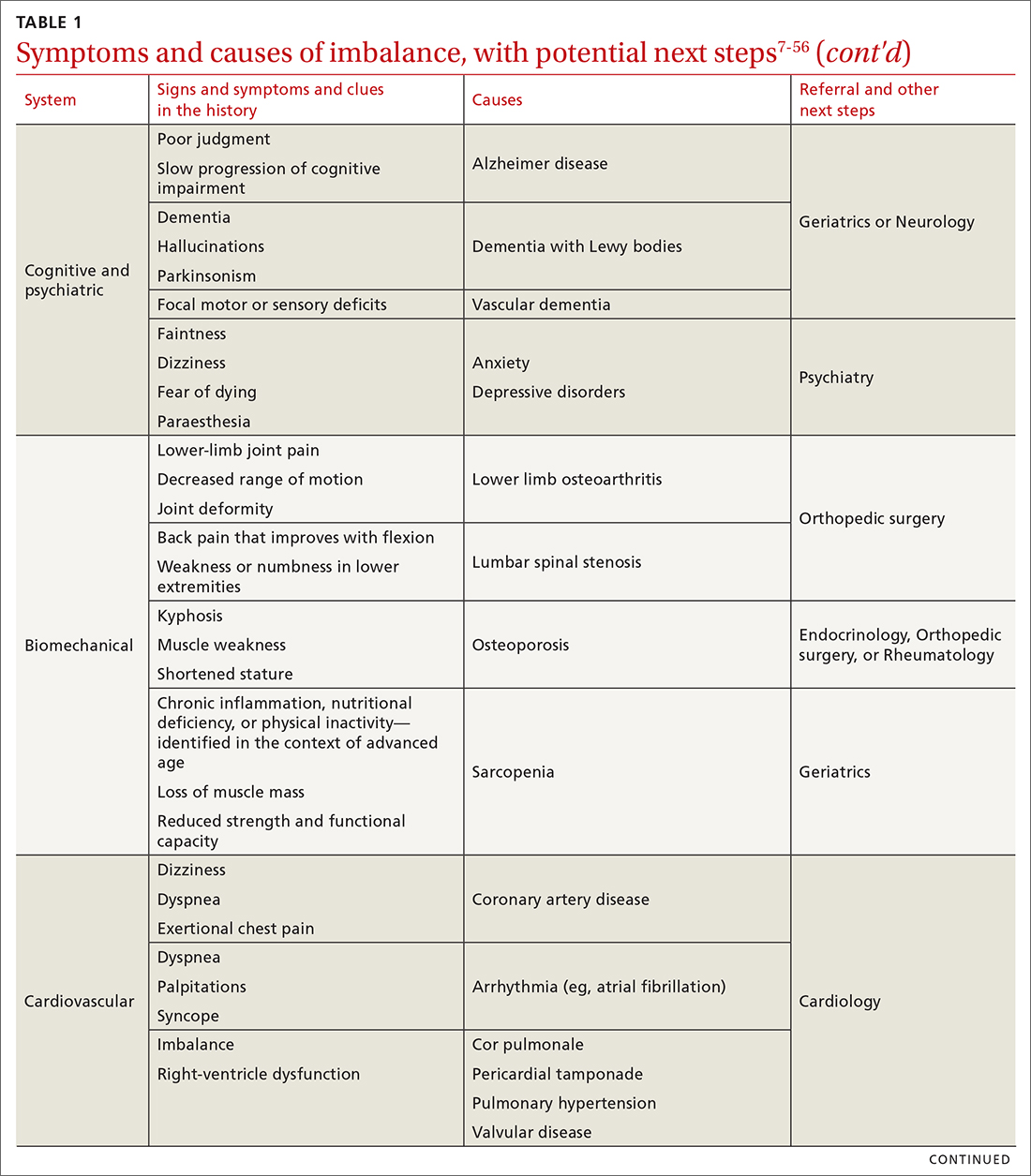
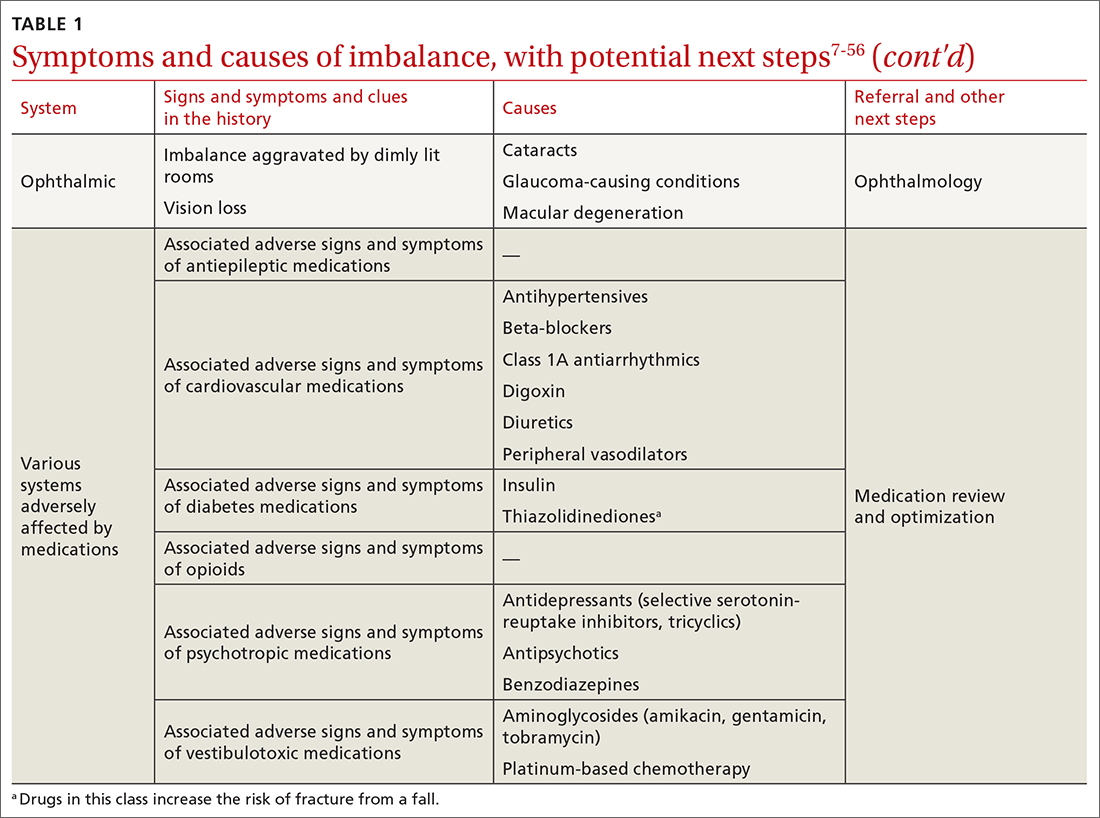
The cause of any single case of imbalance is often multifactorial, resulting from dysfunction of multiple body systems (TABLE 17-56); in our clinical experience, most patients with imbalance and who are at risk of falls do not have a detectable deficit of the vestibular system. These alterations in function arise in 3 key systems—vision, proprioception, and vestibular function—which signal to, and are incorporated by, the cerebellum to mediate balance. Cognitive and neurologic decline are also factors in imbalance.
Considering that 20% of falls result in serious injury in older populations, it is important to identify balance disorders and implement preventive strategies to mitigate harmful consequences of falls on patients’ health and independence.7,57 In this article, we answer the question that the case presentation raises about the proper management approach to imbalance in family practice, including assessment of risk and rehabilitation strategies to reduce the risk of falls. Our insights and recommendations are based on our clinical experience and a review of the medical literature from the past 40 years.
CASE Mr. J has a history of hypertension, age-related hearing loss, and osteoarthritis of the knees; he has not had surgery for the arthritis. His medications are antihypertensives and extra-strength acetaminophen for knee pain.
Making the diagnosis of a balance disorder
History
A thorough clinical history, often including a collateral history from caregivers, narrows the differential diagnosis. Information regarding onset, duration, timing, character, and previous episodes of imbalance is essential. Symptoms of imbalance are often challenging for the patient to describe: They might use terms such as vertigo or dizziness, when, in fact, on further questioning, they are describing balance difficulties. Inquiry into (1) their use of assistive walking devices and (2) development or exacerbation of neurologic, musculoskeletal, auditory, visual, and mood symptoms is necessary. Note the current level of their mobility, episodes of pain or fatigue, previous falls and associated injuries, fear of falling, balance confidence, and sensations that precede falls.58
Continue to: The medical and surgical histories
The medical and surgical histories are key pieces of information. The history of smoking, alcohol habits, and substance use is relevant.
A robust medication history is essential to evaluate a patient’s risk of falling. Polypharmacy—typically, defined as taking 4 or more medications—has been repeatedly associated with a heightened risk of falls.53,59-61 Moreover, a dose-dependent association between polypharmacy and hospitalization following falls has been identified, and demonstrates that taking 10 or more medications greatly increases the risk of hospitalization.59 Studies of polypharmacy cement the importance of inquiring about medication use when assessing imbalance, particularly in older patients.
Physical examination
A focused and detailed physical examination provides insight into systems that should be investigated:
- Obtain vital signs, including orthostatic vitals to test for orthostatic hypotension62; keep in mind that symptoms of orthostatic dizziness can occur without orthostatic hypotension.
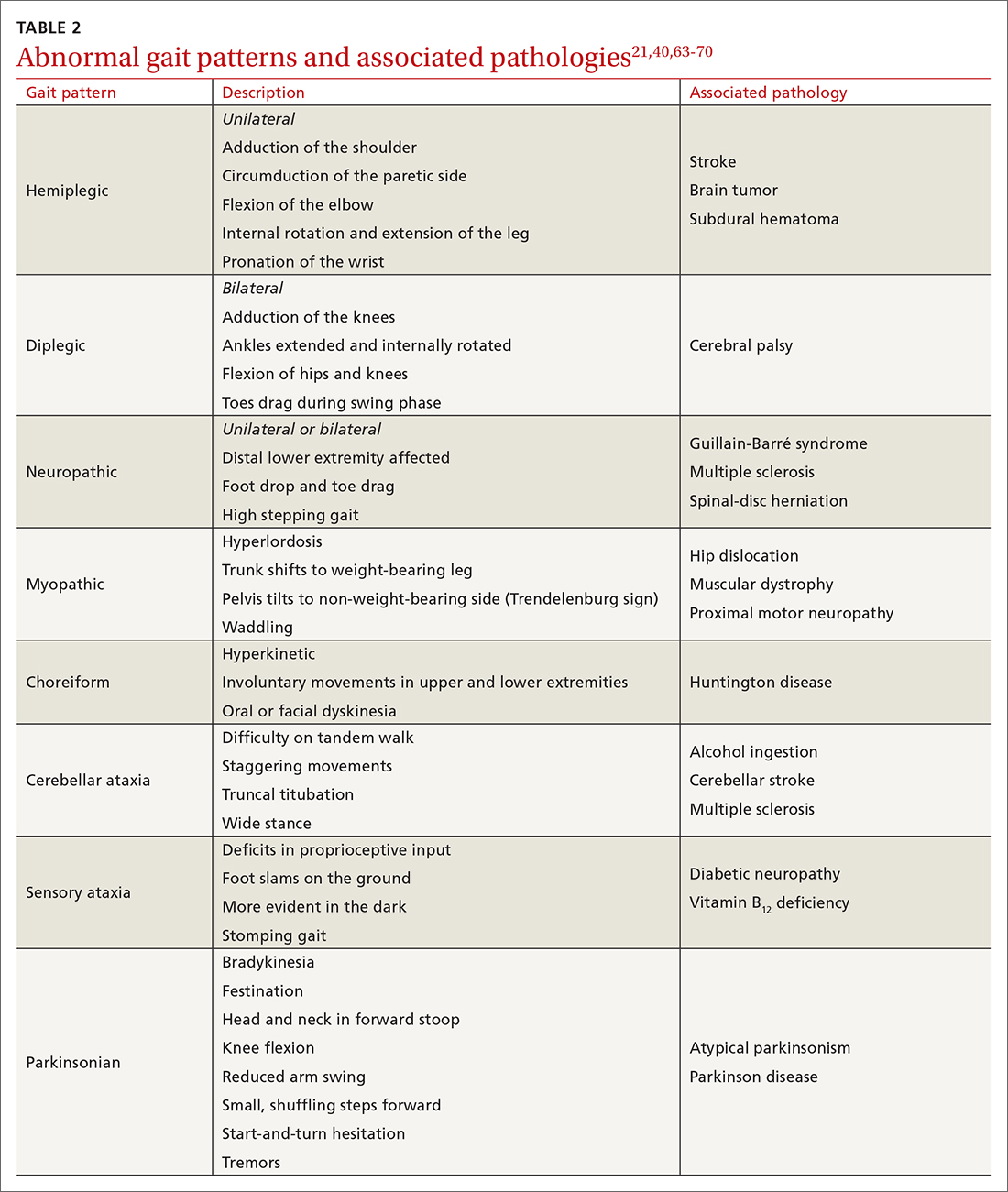
- Examine gait, which can distinguish between causes of imbalance (TABLE 2).21,40,63-70
- Perform a cardiac examination.
- Assess visual acuity and visual fields; test for nystagmus and identify any optic-nerve and retinal abnormalities.
- Evaluate lower-limb sensation, proprioception, and motor function.
- Evaluate suspected vestibular dysfunction, including dysfunction with positional testing (the Dix-Hallpike maneuver71). The patient is taken from sitting to supine while the head is rotated 45° to the tested side by the examiner. As the patient moves into a supine position, the neck is extended 30° off the table and held for at least 30 seconds. The maneuver is positive if torsional nystagmus is noted while the head is held rotated during neck extension. The maneuver is negative if the patient reports dizziness, vertigo, unsteadiness, or “pressure in the head.” Torsional nystagmus must be present to confirm a diagnosis of benign paroxysmal positional vertigo.
- If you suspect a central nervous system cause of imbalance, assess the cranial nerves, coordination, strength, and, of course, balance.
CASE
Mr. J’s physical examination showed normal vital signs without significant postural changes in blood pressure. Gait analysis revealed a slowed gait, with reduced range of motion in both knees over the entire gait cycle. Audiometry revealed symmetric moderate sensorineural hearing loss characteristic of presbycusis.
Diagnostic investigations
Consider focused investigations into imbalance based on the history and physical examination. We discourage overly broad testing and imaging; in primary care, cost and limited access to technology can bar robust investigations into causes of imbalance. However, identification of acute pathologies should prompt immediate referral to the emergency department. Furthermore, specific symptoms (TABLE 17-56) should prompt referral to specialists for assessment.
Continue to: In the emergency department...
In the emergency department and academic hospitals, key investigations can identify causes of imbalance:
- Electrocardiography and Holter monitoring test for cardiac arrhythmias.
- Echocardiography identifies structural abnormalities.
- Radiography and computed tomography are useful for detecting musculoskeletal abnormalities.
- Bone densitometry can identify osteoporosis.
- Head and spinal cord magnetic resonance imaging can be used to identify lesions of the central nervous system.
- Computed tomographic angiography of the head and neck is useful for identifying stroke, cerebral atrophy, and stenotic lesions of the carotid and vertebral arteries.
- Nerve conduction studies and levels of serum vitamin B12, hemoglobin A1C, thyroid-stimulating hormone, and random cortisol can uncover causes of peripheral neuropathy.
- Bedside cognitive screening tests can be used to measure cognitive decline.72
- Suspicion of vestibular disease requires audiometry and vestibular testing, including videonystagmography, head impulse testing, and vestibular evoked myogenic potentials.
In many cases of imbalance, no specific underlying correctable cause is discovered.
Management of imbalance
Pharmacotherapy
Targeted pharmacotherapy can be utilized in select clinical scenarios:
- Medical treatment of peripheral neuropathy should target the underlying condition.
- Cognitive behavioral therapy and antidepressants are useful for treating anxiety and depressive disorders.73
- Musculoskeletal pain can be managed with acetaminophen and topical nonsteroidal anti-inflammatory drugs (NSAIDs), using a short course of an oral NSAID when needed.74
- Cardiovascular disease management might include any of several classes of pharmacotherapy, including antiplatelet and lipid-lowering medications, antiarrhythmic drugs, and antihypertensive agents.
- Acute episodes of vertigo due to vestibular neuritis or labyrinthitis can be managed with an antiemetic.46
Surgical treatment
Surgery is infrequently considered for patients with imbalance. Examples of indications include microsurgical resection of vestibular schwannoma, resection of central nervous system tumors, lens replacement surgery for cataract, surgical management of severe spinal fracture, and hip or knee arthroplasty in select patients.
Tools for assessing the risk of falls
Scoring systems called falls risk assessment tools, or FRAT, have been developed to gauge a patient’s risk of falling. The various FRATs differ in specificity and sensitivity for predicting the risk of falls, and are typically designed for specific clinical environments, such as hospital inpatient care or long-term care facilities. Specifically, FRATs attempt to classify risk using sets of risk factors known to be associated with falls.
Continue to: Research abounds into...
Research abounds into the validity of commonly used FRATs across institutions, patient populations, and clinical environments:
The Johns Hopkins FRATa determines risk using metrics such as age, fall history, incontinence, cognition, mobility, and medications75; it is predominantly used for assessment in hospital inpatient units. This tool has been validated repeatedly.76,77
Peninsula Health FRATb stratifies patients in subacute and residential aged-care settings, based on risk factors that include recent falls, medications, psychological status, and cognition.78
FRAT-upc is a web-based tool that generates falls risk using risk factors that users input. This tool has been studied in the context of patients older than 65 years living in the community.79
Although FRATs are reasonably useful for predicting falls, their utility varies by patient population and clinical context. Moreover, it has been suggested that FRATs neglect environmental and personal factors when assessing risk by focusing primarily on bodily factors.80 Implementing a FRAT requires extensive consideration of the target population and should be accompanied by clinical judgment that is grounded in an individual patient’s circumstances.81
Continue to: Preventing falls in primary care
Preventing falls in primary care
An approach to preventing falls includes the development of individualized programs that account for frailty, a syndrome of physiologic decline associated with aging. Because frailty leads to diminished balance and mobility, a patient’s frailty index—determined using the 5 frailty phenotype criteria (exhaustion, weight loss, low physical activity, weakness, slowness)82 or the Canadian Study of Health and Aging Clinical Frailty Scale83—is a useful tool for predicting falls risk and readmission for falls following trauma-related injury. Prevention of falls in communities is critical for reducing mortality and allowing older people to maintain their independence and quality of life.
Exercise. In some areas, exercise and falls prevention programs are accessible to seniors.84 Community exercise programs that focus on balance retraining and muscle strengthening can reduce the risk of falls.73,85 The Choosing Wisely initiative of the ABIM [American Board of Internal Medicine] Foundation recommends that exercise programs be designed around an accurate functional baseline of the patient to avoid underdosed strength training.54
Multifactorial risk assessment in high-risk patients can reduce the rate of falls. Such an assessment includes examination of orthostatic blood pressure, vision and hearing, bone health, gait, activities of daily living, cognition, and environmental hazards, and enables provision of necessary interventions.73,86 Hearing amplification, specifically, correlates with enhanced postural control, slowed cognitive decline, and a reduced likelihood of falls.87-93 The mechanism behind improved balance performance might be reduced cognitive load through supporting a patient’s listening needs.88-90
Pharmacotherapy. Optimizing medications and performing a complete medication review before prescribing new medications is highly recommended to avoid unnecessary polypharmacy7,8,18,53-56 (TABLE 17-56).
Management of comorbidities associated with a higher risk of falls, including arthritis, cancer, stroke, diabetes, depression, kidney disease, chronic obstructive pulmonary disease, cognitive impairment, hypertension, and atrial fibrillation, is essential.94-96
Continue to: Home safety interventions
Home safety interventions, through occupational therapy, are important. These include removing unsafe mats and step-overs and installing nonslip strips on stairs, double-sided tape under mats, and handrails.73-97
Screening for risk of falls. The Centers for Disease Control and Prevention recommends that (1) all patients older than 65 years and (2) any patient presenting with an acute fall undergo screening for their risk of falls.98 When a patient is identified as at risk of falling, you can, when appropriate, assess modifiable risk factors and facilitate interventions.98 This strategy is supported by a 2018 statement from the US Preventive Services Task Force99 that recommends identifying high-risk patients who have:
- a history of falling
- a balance disturbance that causes a deficit of mobility or function
- poor performance on clinical tests, such as the 3-meter Timed Up and Go (TUG) assessment (www.cdc.gov/steadi/pdf/TUG_test-print.pdf).
An increased risk of falls should prompt you to refer the patient to community programs and physiotherapy in accordance with the individual’s personal goals99; a balance and vestibular physiotherapist is ideally positioned to accurately assess and manage patients at risk of falls. Specifically, the Task Force identified exercise programs and multifactorial interventions as being beneficial in preventing falls in high-risk older people.99
Balance assessment and rehabilitation in specialty centers
An individualized rehabilitation program aims to restore safe mobility by testing and addressing specific balance deficits, improving functional balance, and increasing balance confidence. Collaboration with colleagues from physiotherapy and occupational therapy aids in tailoring individualized programs.
Many tests are available to assess balance, determine the risk of falls, and guide rehabilitation:
- The timed 10-meter walk testd and the TUG test are simple assessments that measure functional mobility; both have normalized values for the risk of falls. A TUG time of ≥ 12 seconds suggests a high risk of falls.
- The 30-second chair stande evaluates functional lower-extremity strength in older patients. The test can indicate if lower-extremity strength is contributing to a patient’s imbalance.
- The modified clinical test of sensory interaction in balancef is a static balance test that measures the integrity of sensory inputs. The test can suggest if 1 or more sensory systems are compromised.
- The mini balance evaluation systems testg is similar: It can differentiate balance deficits by underlying system and allows individualization of a rehabilitation program.
- The functional gait assessmenth is a modification of the dynamic gait index that assesses postural stability during everyday dynamic activities, including tasks such as walking with head turns and pivots.
- The Berg Balance Scalei continues to be used extensively to assess balance.
Continue to: The mini balance evaluation systems test...
The mini balance evaluation systems test, functional gait index, and Berg Balance Scale all have normative age-graded values to predict fall risk.
CASE
Mr. J was referred for balance assessment and to a rehabilitation program. He underwent balance physiotherapy, including multifactorial balance assessment, joined a community exercise program, was fitted with hearing aids, and had his home environment optimized by an occupational therapist. (See examples of “home safety interventions” under “Preventing falls in primary care.”)
3 months later. Mr. J says he feels stronger on his feet. His knee pain has eased, and he is more confident walking around his home. He continues to engage in exercise programs and is comfortable running errands with his spouse.
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Division of OtolaryngologyHead and Neck Surgery, Queen’s University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected]
a www.hopkinsmedicine.org/institute_nursing/models_tools/jhfrat_acute%20care%20original_6_22_17.pdf
c www.ncbi.nlm.nih.gov/pmc/articles/PMC4376110/figure/figure14/?report=objectonly
e www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf
f www.mdapp.co/mctsib-modified-clinical-test-of-sensory-interaction-in-balance-calculator-404/
g www.sralab.org/sites/default/files/2017-07/MiniBEST_revised_final_3_8_13.pdf
1. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189-201. doi: 10.2165/11591150-000000000-00000
2. TB, ZF, ES, et al. The relationship of balance disorders with falling, the effect of health problems, and social life on postural balance in the elderly living in a district in Turkey. Geriatrics (Basel). 2019;4:37. doi: 10.3390/geriatrics4020037
3. R, Sixt E, Landahl S, et al. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47-52.
4. Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467-478. doi: 10.1016/j.neucli.2008.09.001
5. Boult C, Murphy J, Sloane P, et al. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858-861. doi: 10.1111/j.1532-5415.1991.tb04451.x
6. Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122:1858-1861. doi: 10.1002/lary.23376
7. Jia H, Lubetkin EI, DeMichele K, et al. Prevalence, risk factors, and burden of disease for falls and balance or walking problems among older adults in the U.S. Prev Med. 2019;126:105737. doi: 10.1016/j.ypmed.2019.05.025
8. Al-Momani M, Al-Momani F, Alghadir AH, et al. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043-1049. doi: 10.2147/CIA.S112282
9. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23:113-117. doi: 10.3233/VES-130498
10. Altinsoy B, Erboy F, Tanriverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag. 2016;12:1023-1028. doi: 10.2147/TCRM.S105722
11. Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687
12. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(suppl 3):S1-S47. doi: 10.1177/0194599816689667
13. S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19:33. doi: 10.1186/s12875-017-0695-0
14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492. doi: 10.1128/CMR.00070-09
15. Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418. doi: 10.1016/j.ncl.2007.01.003
16. Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987-992. doi: 10.1016/j.gaitpost.2013.05.010
17. de Luna RA, Mihailovic A, Nguyen AM, et al. The association of glaucomatous visual field loss and balance. Transl Vis Sci Technol. 2017;6:8. doi: 10.1167/tvst.6.3.8
18. DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40:104-107. doi: 10.1055/s-0039-1684040
19. Ebersbach G, Moreau C, Gandor F, et al. Clinical syndromes: parkinsonian gait. Mov Disord. 2013;28:1552-1559. doi: 10.1002/mds.25675
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. doi: 10.3945/ajcn.2010.28608A
21. Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep. 2018;8:4984. doi: 10.1038/s41598-018-22676-0
22. Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1-7. doi: 10.1016/j.gaitpost.2016.08.009
23. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9-26. doi: 10.1016/s0887-6185(00)00040-2
24. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706-715. doi: 10.1016/S1474-4422(13)70107-8
25. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. 1989;18:31-34. doi: 10.1093/ageing/18.1.31
26. Goudakos JK, Markou KD, Franco-Vidal V, et al. Corticosteroids in the treatment of vestibular neuritis: a systematic review and meta-analysis. Otol Neurotol. 2010;31:183-189. doi: 10.1097/MAO.0b013e3181ca843d
27. Green AD, CS, Bastian L, et al. Does this woman have osteoporosis? JAMA. 2004;292:2890-2900. doi: 10.1001/jama.292.23.2890
28. Hallemans A, Ortibus E, Meire F, et al. Low vision affects dynamic stability of gait. Gait Posture. 2010;32:547-551. doi: 10.1016/j.gaitpost.2010.07.018
29. Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol. 2018;57(suppl 4):S99-S107. doi: 10.1080/14992027.2018.1468092
30. Head VA, Wakerley BR. Guillain-Barré syndrome in general practice: clinical features suggestive of early diagnosis. Br J Gen Pract. 2016;66:218-219. doi: 10.3399/bjgp16X684733
31. Helbostad JL, Vereijken B, Hesseberg K, et al. Altered vision destabilizes gait in older persons. Gait Posture. 2009;30:233-238. doi: 10.1016/j.gaitpost.2009.05.004
32. Hsu W-L, Chen C-Y, Tsauo J-Y, et al. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334-339. doi: 10.1016/j.jfma.2014.02.006
33. Kim H-S, Yun DH, Yoo SD, et al. Balance control and knee osteoarthritis severity. Ann Rehabil Med. 2011;35:701-709. doi: 10.5535/arm.2011.35.5.701
34. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25-29.
35. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. doi: 10.1212/WNL.0000000000004058
36. Milner KA, Funk M, Richards S, et al. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399. doi: 10.1016/s0002-9149(99)00322-7
37. Paillard T, F, Bru N, et al. The impact of time of day on the gait and balance control of Alzheimer’s patients. Chronobiol Int. 2016;33:161-168. doi: 10.3109/07420528.2015.1124885
38. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1-8. doi: 10.1016/j.jocn.2016.05.003
39. Picorelli AMA, Hatton AL, Gane EM, et al. Balance performance in older adults with hip osteoarthritis: a systematic review. Gait Posture. 2018;65:89-99. doi: 10.1016/j.gaitpost.2018.07.001
40. Raccagni C, Nonnekes J, Bloem BR, et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169-3176. doi: 10.1007/s00415-019-09382-1
41. Shanmugarajah PD, Hoggard N, Currie S, et al. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. doi: 10.1186/s40673-016-0055-1
42. Shih RY, Smirniotopoulos JG. Posterior fossa tumors in adult patients. Neuroimaging Clin N Am. 2016;26:493-510. doi: 10.1016/j.nic.2016.06.003
43. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131:1059-1068. doi: 10.1042/CS20160607
44. Streur M, Ratcliffe SJ, Ball J, et al. Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. 2017;32:296-303. doi: 10.1097/JCN.0000000000000344
45. Strupp M, M, JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165-173. doi: 10.1097/WCO.0000000000000649
46. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
47. Timar B, Timar R, L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11:e0154654. doi: 10.1371/journal.pone.0154654
48. Walls R, Hockberger R, Gausche-Hill M. Peripheral nerve disorders. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Elsevier, Inc; 2018:1307-1320.
49. Watson JC, Dyck PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940-951. doi: 10.1016/j.mayocp.2015.05.004
50. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. doi: 10.1111/nyas.13919
51. Wu V, Sykes EA, Beyea MM, et al. Approach to Meniere disease management. Can Fam Physician. 2019;65:463-467.
52. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528-536.
53. Seppala LJ, van de Glind EMM, Daams JG, et al; . Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099
54. ABIM Foundation. Choosing wisely. Choosing Wisely website. 2021. Accessed November 11. 2021. www.choosingwisely.org/
55. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712-717. doi: 10.1345/aph.1M551
56. Hartikainen S, E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-1181. doi: 10.1093/gerona/62.10.1172
57. Khanuja K, Joki J, Bachmann G, et al. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas. 2018;110:51-56. doi: 10.1016/j.maturitas.2018.01.021
58. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82:61-68.
59. Zaninotto P, Huang YT, Di Gessa G, et al. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English Longitudinal Study of Ageing. BMC Public Health. 2020;20:1804. doi: 10.1186/s12889-020-09920-x
60. Morin L, Calderon A, Welmer AK, et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483-493. doi: 10.2147/CLEP.S201614
61. Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358
62. Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007
63. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689-703.
64. Baker JM. Gait disorders. Am J Med. 2018;131:602-607. doi: 10.1016/j.amjmed.2017.11.051
65. Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep. 2011;11:507-515. doi: 10.1007/s11910-011-0214-y
66. Chen C-L, Chen H-C, Tang SF-T, et al. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003;82:925-935. doi: 10.1097/01.PHM.0000098040.13355.B5
67. Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195-216. doi: 10.1177/0269215510382495
68. Mirek E, Filip M, W, et al. Three-dimensional trunk and lower limbs characteristics during gait in patients with Huntington’s disease. Front Neurosci. 2017;11:566. doi: 10.3389/fnins.2017.00566
69. Paramanandam V, Lizarraga KJ, Soh D, et al. Unusual gait disorders: a phenomenological approach and classification. Expert Rev Neurother. 2019;19:119-132. doi: 10.1080/14737175.2019.1562337
70. Sahyouni R, Goshtasbi K, Mahmoodi A, et al. Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg. 2017;108:948-953. doi: 10.1016/j.wneu.2017.09.064
71. Talmud JD, Coffey R, Edemekong PF. Dix Hallpike maneuver. StatPearls [Internet]. StatPearls Publishing Updated September 5, 2021. Accessed December 6, 2021. www.ncbi.nlm.nih.gov/books/NBK459307/
72. Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68:2207-2213. doi: 10.1111/jgs.16713
73. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:CD007146. doi: 10.1002/14651858.CD007146.pub3
74. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306
75. Poe SS, Cvach M, Dawson PB, Straus H, Hill EE. The Johns Hopkins Fall Risk Assessment Tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293-298. doi: 10.1097/01.NCQ.0000290408.74027.39
76. Poe SS, Dawson PB, Cvach M, et al. The Johns Hopkins Fall Risk Assessment Tool: a study of reliability and validity. J Nurs Care Qual. 2018;33:10-19. doi: 10.1097/NCQ.0000000000000301
77. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for predicting falls on inpatient medicine services. J Nurs Care Qual. 2017;32:108-113. doi: 10.1097/NCQ.0000000000000210
78. Stapleton C, Hough P, Oldmeadow L, et al. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28:139-143. doi: 10.1111/j.1741-6612.2009.00375.x
79. Cattelani L, Palumbo P, Palmerini L, et al. FRAT-up, a Web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res. 2015;17:e41. doi: 10.2196/jmir.4064
80. De Clercq H, Naudé A, Bornman J. Factors included in adult fall risk assessment tools (FRATs): a systematic review. Ageing Soc. 2020;41:2558-2582. doi: 10.1017/S0144686X2000046X
81. Yap G, Melder A. Accuracy of validated falls risk assessment tools and clinical judgement. Centre for Clinical Effectiveness, Monash Innovation and Quality. Monash Health. February 5, 2020. Accessed November 11, 2021. https://monashhealth.org/wp-content/uploads/2019/01/Rapid-Review_Falls-risk-tools-FINAL.pdf
82. Chittrakul J, Siviroj P, Sungkarat S, et al. Physical frailty and fall risk in community-dwelling older adults: a cross-sectional study. J Aging Res. 2020;2020:3964973. doi: 10.1155/2020/3964973
83. Hatcher VH, Galet C, Lilienthal M, et al. Association of clinical frailty scores with hospital readmission for falls after index admission for trauma-related injury. JAMA Netw Open. 2019;2:e1912409. doi: 10.1001/jamanetworkopen.2019.12409
84. Exercise and fall prevention programs. Government of Ontario Ministry of Health. Updated April 9, 2019. Accessed November 11. 2021. www.ontario.ca/page/exercise-and-falls-prevention-programs
85. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2
86. Hopewell S, Copsey B, Nicolson P, et al. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340-1350. doi: 10.1136/bjsports-2019-100732
87. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. doi: 10.1016/j.arr.2019.100963
88. Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108:401-412. doi: 10.1016/j.neuron.2020.08.003
89. Martini A, Castiglione A, Bovo R, et al. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol. 2014;19(suppl 1):2-5. doi: 10.1159/000371593
90. Vitkovic J, Le C, Lee S-L, et al. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195-202. doi: 10.1159/000445100
91. Maheu M, Behtani L, Nooristani M, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. 2019;40:1418-1424. doi: 10.1097/AUD.0000000000000720
92. Negahban H, Bavarsad Cheshmeh Ali M, Nassadj G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture. 2017;58:126-129. doi: 10.1016/j.gaitpost.2017.07.112
93. Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67:2362-2369. doi: 10.1111/jgs.16109
94. Paliwal Y, Slattum PW, Ratliff SM. Chronic health conditions as a risk factor for falls among the community-dwelling US older adults: a zero-inflated regression modeling approach. Biomed Res Int. 2017;2017:5146378. doi: 10.1155/2017/5146378
95. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658-668. doi: 10.1097/EDE.0b013e3181e89905
96. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. doi: 10.1016/j.maturitas.2013.02.009
97. Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc. 2001;49:1442-1447. doi: 10.1046/j.1532-5415.2001.4911235.x
98. Clinical resources. Centers for Disease Control and Prevention STEADI-Older Adult Fall Prevention website. 2020. Accessed November 12, 2021. www.cdc.gov/steadi/materials.html
99. ; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704. doi: 10.1001/jama.2018.3097
CASE Mr. J, a 75-year-old man, presents to your family practice reporting that he feels increasingly unsteady and slow while walking. He fell twice last year, without resulting injury. He now worries about tripping while walking around the house and relies on his spouse to run errands.
Clearly, Mr. J is experiencing a problem with balance. What management approach should you undertake to prevent him from falling?

Balance disorders are common in older people and drastically hinder quality of life.1-4 Patients often describe imbalance as vague symptoms: dizziness, unsteadiness, faintness, spinning sensations.5,6 Importantly, balance disorders disrupt normal gait and contribute to falls that are a major cause of disability and morbidity in older people. Almost 30% of people older than 65 years report 1 or more falls annually.7 Factors that increase the risk of falls include impaired mobility, previously reported falls, reduced psychological functioning, chronic medical conditions, and polypharmacy.7,8
The cause of any single case of imbalance is often multifactorial, resulting from dysfunction of multiple body systems (TABLE 17-56); in our clinical experience, most patients with imbalance and who are at risk of falls do not have a detectable deficit of the vestibular system. These alterations in function arise in 3 key systems—vision, proprioception, and vestibular function—which signal to, and are incorporated by, the cerebellum to mediate balance. Cognitive and neurologic decline are also factors in imbalance.

Considering that 20% of falls result in serious injury in older populations, it is important to identify balance disorders and implement preventive strategies to mitigate harmful consequences of falls on patients’ health and independence.7,57 In this article, we answer the question that the case presentation raises about the proper management approach to imbalance in family practice, including assessment of risk and rehabilitation strategies to reduce the risk of falls. Our insights and recommendations are based on our clinical experience and a review of the medical literature from the past 40 years.

CASE Mr. J has a history of hypertension, age-related hearing loss, and osteoarthritis of the knees; he has not had surgery for the arthritis. His medications are antihypertensives and extra-strength acetaminophen for knee pain.

Making the diagnosis of a balance disorder
History
A thorough clinical history, often including a collateral history from caregivers, narrows the differential diagnosis. Information regarding onset, duration, timing, character, and previous episodes of imbalance is essential. Symptoms of imbalance are often challenging for the patient to describe: They might use terms such as vertigo or dizziness, when, in fact, on further questioning, they are describing balance difficulties. Inquiry into (1) their use of assistive walking devices and (2) development or exacerbation of neurologic, musculoskeletal, auditory, visual, and mood symptoms is necessary. Note the current level of their mobility, episodes of pain or fatigue, previous falls and associated injuries, fear of falling, balance confidence, and sensations that precede falls.58
Continue to: The medical and surgical histories
The medical and surgical histories are key pieces of information. The history of smoking, alcohol habits, and substance use is relevant.
A robust medication history is essential to evaluate a patient’s risk of falling. Polypharmacy—typically, defined as taking 4 or more medications—has been repeatedly associated with a heightened risk of falls.53,59-61 Moreover, a dose-dependent association between polypharmacy and hospitalization following falls has been identified, and demonstrates that taking 10 or more medications greatly increases the risk of hospitalization.59 Studies of polypharmacy cement the importance of inquiring about medication use when assessing imbalance, particularly in older patients.
Physical examination
A focused and detailed physical examination provides insight into systems that should be investigated:
- Obtain vital signs, including orthostatic vitals to test for orthostatic hypotension62; keep in mind that symptoms of orthostatic dizziness can occur without orthostatic hypotension.
- Examine gait, which can distinguish between causes of imbalance (TABLE 2).21,40,63-70
- Perform a cardiac examination.
- Assess visual acuity and visual fields; test for nystagmus and identify any optic-nerve and retinal abnormalities.
- Evaluate lower-limb sensation, proprioception, and motor function.
- Evaluate suspected vestibular dysfunction, including dysfunction with positional testing (the Dix-Hallpike maneuver71). The patient is taken from sitting to supine while the head is rotated 45° to the tested side by the examiner. As the patient moves into a supine position, the neck is extended 30° off the table and held for at least 30 seconds. The maneuver is positive if torsional nystagmus is noted while the head is held rotated during neck extension. The maneuver is negative if the patient reports dizziness, vertigo, unsteadiness, or “pressure in the head.” Torsional nystagmus must be present to confirm a diagnosis of benign paroxysmal positional vertigo.
- If you suspect a central nervous system cause of imbalance, assess the cranial nerves, coordination, strength, and, of course, balance.

CASE
Mr. J’s physical examination showed normal vital signs without significant postural changes in blood pressure. Gait analysis revealed a slowed gait, with reduced range of motion in both knees over the entire gait cycle. Audiometry revealed symmetric moderate sensorineural hearing loss characteristic of presbycusis.
Diagnostic investigations
Consider focused investigations into imbalance based on the history and physical examination. We discourage overly broad testing and imaging; in primary care, cost and limited access to technology can bar robust investigations into causes of imbalance. However, identification of acute pathologies should prompt immediate referral to the emergency department. Furthermore, specific symptoms (TABLE 17-56) should prompt referral to specialists for assessment.
Continue to: In the emergency department...
In the emergency department and academic hospitals, key investigations can identify causes of imbalance:
- Electrocardiography and Holter monitoring test for cardiac arrhythmias.
- Echocardiography identifies structural abnormalities.
- Radiography and computed tomography are useful for detecting musculoskeletal abnormalities.
- Bone densitometry can identify osteoporosis.
- Head and spinal cord magnetic resonance imaging can be used to identify lesions of the central nervous system.
- Computed tomographic angiography of the head and neck is useful for identifying stroke, cerebral atrophy, and stenotic lesions of the carotid and vertebral arteries.
- Nerve conduction studies and levels of serum vitamin B12, hemoglobin A1C, thyroid-stimulating hormone, and random cortisol can uncover causes of peripheral neuropathy.
- Bedside cognitive screening tests can be used to measure cognitive decline.72
- Suspicion of vestibular disease requires audiometry and vestibular testing, including videonystagmography, head impulse testing, and vestibular evoked myogenic potentials.
In many cases of imbalance, no specific underlying correctable cause is discovered.
Management of imbalance
Pharmacotherapy
Targeted pharmacotherapy can be utilized in select clinical scenarios:
- Medical treatment of peripheral neuropathy should target the underlying condition.
- Cognitive behavioral therapy and antidepressants are useful for treating anxiety and depressive disorders.73
- Musculoskeletal pain can be managed with acetaminophen and topical nonsteroidal anti-inflammatory drugs (NSAIDs), using a short course of an oral NSAID when needed.74
- Cardiovascular disease management might include any of several classes of pharmacotherapy, including antiplatelet and lipid-lowering medications, antiarrhythmic drugs, and antihypertensive agents.
- Acute episodes of vertigo due to vestibular neuritis or labyrinthitis can be managed with an antiemetic.46
Surgical treatment
Surgery is infrequently considered for patients with imbalance. Examples of indications include microsurgical resection of vestibular schwannoma, resection of central nervous system tumors, lens replacement surgery for cataract, surgical management of severe spinal fracture, and hip or knee arthroplasty in select patients.
Tools for assessing the risk of falls
Scoring systems called falls risk assessment tools, or FRAT, have been developed to gauge a patient’s risk of falling. The various FRATs differ in specificity and sensitivity for predicting the risk of falls, and are typically designed for specific clinical environments, such as hospital inpatient care or long-term care facilities. Specifically, FRATs attempt to classify risk using sets of risk factors known to be associated with falls.
Continue to: Research abounds into...
Research abounds into the validity of commonly used FRATs across institutions, patient populations, and clinical environments:
The Johns Hopkins FRATa determines risk using metrics such as age, fall history, incontinence, cognition, mobility, and medications75; it is predominantly used for assessment in hospital inpatient units. This tool has been validated repeatedly.76,77
Peninsula Health FRATb stratifies patients in subacute and residential aged-care settings, based on risk factors that include recent falls, medications, psychological status, and cognition.78
FRAT-upc is a web-based tool that generates falls risk using risk factors that users input. This tool has been studied in the context of patients older than 65 years living in the community.79
Although FRATs are reasonably useful for predicting falls, their utility varies by patient population and clinical context. Moreover, it has been suggested that FRATs neglect environmental and personal factors when assessing risk by focusing primarily on bodily factors.80 Implementing a FRAT requires extensive consideration of the target population and should be accompanied by clinical judgment that is grounded in an individual patient’s circumstances.81
Continue to: Preventing falls in primary care
Preventing falls in primary care
An approach to preventing falls includes the development of individualized programs that account for frailty, a syndrome of physiologic decline associated with aging. Because frailty leads to diminished balance and mobility, a patient’s frailty index—determined using the 5 frailty phenotype criteria (exhaustion, weight loss, low physical activity, weakness, slowness)82 or the Canadian Study of Health and Aging Clinical Frailty Scale83—is a useful tool for predicting falls risk and readmission for falls following trauma-related injury. Prevention of falls in communities is critical for reducing mortality and allowing older people to maintain their independence and quality of life.
Exercise. In some areas, exercise and falls prevention programs are accessible to seniors.84 Community exercise programs that focus on balance retraining and muscle strengthening can reduce the risk of falls.73,85 The Choosing Wisely initiative of the ABIM [American Board of Internal Medicine] Foundation recommends that exercise programs be designed around an accurate functional baseline of the patient to avoid underdosed strength training.54
Multifactorial risk assessment in high-risk patients can reduce the rate of falls. Such an assessment includes examination of orthostatic blood pressure, vision and hearing, bone health, gait, activities of daily living, cognition, and environmental hazards, and enables provision of necessary interventions.73,86 Hearing amplification, specifically, correlates with enhanced postural control, slowed cognitive decline, and a reduced likelihood of falls.87-93 The mechanism behind improved balance performance might be reduced cognitive load through supporting a patient’s listening needs.88-90
Pharmacotherapy. Optimizing medications and performing a complete medication review before prescribing new medications is highly recommended to avoid unnecessary polypharmacy7,8,18,53-56 (TABLE 17-56).
Management of comorbidities associated with a higher risk of falls, including arthritis, cancer, stroke, diabetes, depression, kidney disease, chronic obstructive pulmonary disease, cognitive impairment, hypertension, and atrial fibrillation, is essential.94-96
Continue to: Home safety interventions
Home safety interventions, through occupational therapy, are important. These include removing unsafe mats and step-overs and installing nonslip strips on stairs, double-sided tape under mats, and handrails.73-97
Screening for risk of falls. The Centers for Disease Control and Prevention recommends that (1) all patients older than 65 years and (2) any patient presenting with an acute fall undergo screening for their risk of falls.98 When a patient is identified as at risk of falling, you can, when appropriate, assess modifiable risk factors and facilitate interventions.98 This strategy is supported by a 2018 statement from the US Preventive Services Task Force99 that recommends identifying high-risk patients who have:
- a history of falling
- a balance disturbance that causes a deficit of mobility or function
- poor performance on clinical tests, such as the 3-meter Timed Up and Go (TUG) assessment (www.cdc.gov/steadi/pdf/TUG_test-print.pdf).
An increased risk of falls should prompt you to refer the patient to community programs and physiotherapy in accordance with the individual’s personal goals99; a balance and vestibular physiotherapist is ideally positioned to accurately assess and manage patients at risk of falls. Specifically, the Task Force identified exercise programs and multifactorial interventions as being beneficial in preventing falls in high-risk older people.99
Balance assessment and rehabilitation in specialty centers
An individualized rehabilitation program aims to restore safe mobility by testing and addressing specific balance deficits, improving functional balance, and increasing balance confidence. Collaboration with colleagues from physiotherapy and occupational therapy aids in tailoring individualized programs.
Many tests are available to assess balance, determine the risk of falls, and guide rehabilitation:
- The timed 10-meter walk testd and the TUG test are simple assessments that measure functional mobility; both have normalized values for the risk of falls. A TUG time of ≥ 12 seconds suggests a high risk of falls.
- The 30-second chair stande evaluates functional lower-extremity strength in older patients. The test can indicate if lower-extremity strength is contributing to a patient’s imbalance.
- The modified clinical test of sensory interaction in balancef is a static balance test that measures the integrity of sensory inputs. The test can suggest if 1 or more sensory systems are compromised.
- The mini balance evaluation systems testg is similar: It can differentiate balance deficits by underlying system and allows individualization of a rehabilitation program.
- The functional gait assessmenth is a modification of the dynamic gait index that assesses postural stability during everyday dynamic activities, including tasks such as walking with head turns and pivots.
- The Berg Balance Scalei continues to be used extensively to assess balance.
Continue to: The mini balance evaluation systems test...
The mini balance evaluation systems test, functional gait index, and Berg Balance Scale all have normative age-graded values to predict fall risk.
CASE
Mr. J was referred for balance assessment and to a rehabilitation program. He underwent balance physiotherapy, including multifactorial balance assessment, joined a community exercise program, was fitted with hearing aids, and had his home environment optimized by an occupational therapist. (See examples of “home safety interventions” under “Preventing falls in primary care.”)
3 months later. Mr. J says he feels stronger on his feet. His knee pain has eased, and he is more confident walking around his home. He continues to engage in exercise programs and is comfortable running errands with his spouse.
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Division of OtolaryngologyHead and Neck Surgery, Queen’s University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected]
a www.hopkinsmedicine.org/institute_nursing/models_tools/jhfrat_acute%20care%20original_6_22_17.pdf
c www.ncbi.nlm.nih.gov/pmc/articles/PMC4376110/figure/figure14/?report=objectonly
e www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf
f www.mdapp.co/mctsib-modified-clinical-test-of-sensory-interaction-in-balance-calculator-404/
g www.sralab.org/sites/default/files/2017-07/MiniBEST_revised_final_3_8_13.pdf
CASE Mr. J, a 75-year-old man, presents to your family practice reporting that he feels increasingly unsteady and slow while walking. He fell twice last year, without resulting injury. He now worries about tripping while walking around the house and relies on his spouse to run errands.
Clearly, Mr. J is experiencing a problem with balance. What management approach should you undertake to prevent him from falling?

Balance disorders are common in older people and drastically hinder quality of life.1-4 Patients often describe imbalance as vague symptoms: dizziness, unsteadiness, faintness, spinning sensations.5,6 Importantly, balance disorders disrupt normal gait and contribute to falls that are a major cause of disability and morbidity in older people. Almost 30% of people older than 65 years report 1 or more falls annually.7 Factors that increase the risk of falls include impaired mobility, previously reported falls, reduced psychological functioning, chronic medical conditions, and polypharmacy.7,8
The cause of any single case of imbalance is often multifactorial, resulting from dysfunction of multiple body systems (TABLE 17-56); in our clinical experience, most patients with imbalance and who are at risk of falls do not have a detectable deficit of the vestibular system. These alterations in function arise in 3 key systems—vision, proprioception, and vestibular function—which signal to, and are incorporated by, the cerebellum to mediate balance. Cognitive and neurologic decline are also factors in imbalance.

Considering that 20% of falls result in serious injury in older populations, it is important to identify balance disorders and implement preventive strategies to mitigate harmful consequences of falls on patients’ health and independence.7,57 In this article, we answer the question that the case presentation raises about the proper management approach to imbalance in family practice, including assessment of risk and rehabilitation strategies to reduce the risk of falls. Our insights and recommendations are based on our clinical experience and a review of the medical literature from the past 40 years.

CASE Mr. J has a history of hypertension, age-related hearing loss, and osteoarthritis of the knees; he has not had surgery for the arthritis. His medications are antihypertensives and extra-strength acetaminophen for knee pain.

Making the diagnosis of a balance disorder
History
A thorough clinical history, often including a collateral history from caregivers, narrows the differential diagnosis. Information regarding onset, duration, timing, character, and previous episodes of imbalance is essential. Symptoms of imbalance are often challenging for the patient to describe: They might use terms such as vertigo or dizziness, when, in fact, on further questioning, they are describing balance difficulties. Inquiry into (1) their use of assistive walking devices and (2) development or exacerbation of neurologic, musculoskeletal, auditory, visual, and mood symptoms is necessary. Note the current level of their mobility, episodes of pain or fatigue, previous falls and associated injuries, fear of falling, balance confidence, and sensations that precede falls.58
Continue to: The medical and surgical histories
The medical and surgical histories are key pieces of information. The history of smoking, alcohol habits, and substance use is relevant.
A robust medication history is essential to evaluate a patient’s risk of falling. Polypharmacy—typically, defined as taking 4 or more medications—has been repeatedly associated with a heightened risk of falls.53,59-61 Moreover, a dose-dependent association between polypharmacy and hospitalization following falls has been identified, and demonstrates that taking 10 or more medications greatly increases the risk of hospitalization.59 Studies of polypharmacy cement the importance of inquiring about medication use when assessing imbalance, particularly in older patients.
Physical examination
A focused and detailed physical examination provides insight into systems that should be investigated:
- Obtain vital signs, including orthostatic vitals to test for orthostatic hypotension62; keep in mind that symptoms of orthostatic dizziness can occur without orthostatic hypotension.
- Examine gait, which can distinguish between causes of imbalance (TABLE 2).21,40,63-70
- Perform a cardiac examination.
- Assess visual acuity and visual fields; test for nystagmus and identify any optic-nerve and retinal abnormalities.
- Evaluate lower-limb sensation, proprioception, and motor function.
- Evaluate suspected vestibular dysfunction, including dysfunction with positional testing (the Dix-Hallpike maneuver71). The patient is taken from sitting to supine while the head is rotated 45° to the tested side by the examiner. As the patient moves into a supine position, the neck is extended 30° off the table and held for at least 30 seconds. The maneuver is positive if torsional nystagmus is noted while the head is held rotated during neck extension. The maneuver is negative if the patient reports dizziness, vertigo, unsteadiness, or “pressure in the head.” Torsional nystagmus must be present to confirm a diagnosis of benign paroxysmal positional vertigo.
- If you suspect a central nervous system cause of imbalance, assess the cranial nerves, coordination, strength, and, of course, balance.

CASE
Mr. J’s physical examination showed normal vital signs without significant postural changes in blood pressure. Gait analysis revealed a slowed gait, with reduced range of motion in both knees over the entire gait cycle. Audiometry revealed symmetric moderate sensorineural hearing loss characteristic of presbycusis.
Diagnostic investigations
Consider focused investigations into imbalance based on the history and physical examination. We discourage overly broad testing and imaging; in primary care, cost and limited access to technology can bar robust investigations into causes of imbalance. However, identification of acute pathologies should prompt immediate referral to the emergency department. Furthermore, specific symptoms (TABLE 17-56) should prompt referral to specialists for assessment.
Continue to: In the emergency department...
In the emergency department and academic hospitals, key investigations can identify causes of imbalance:
- Electrocardiography and Holter monitoring test for cardiac arrhythmias.
- Echocardiography identifies structural abnormalities.
- Radiography and computed tomography are useful for detecting musculoskeletal abnormalities.
- Bone densitometry can identify osteoporosis.
- Head and spinal cord magnetic resonance imaging can be used to identify lesions of the central nervous system.
- Computed tomographic angiography of the head and neck is useful for identifying stroke, cerebral atrophy, and stenotic lesions of the carotid and vertebral arteries.
- Nerve conduction studies and levels of serum vitamin B12, hemoglobin A1C, thyroid-stimulating hormone, and random cortisol can uncover causes of peripheral neuropathy.
- Bedside cognitive screening tests can be used to measure cognitive decline.72
- Suspicion of vestibular disease requires audiometry and vestibular testing, including videonystagmography, head impulse testing, and vestibular evoked myogenic potentials.
In many cases of imbalance, no specific underlying correctable cause is discovered.
Management of imbalance
Pharmacotherapy
Targeted pharmacotherapy can be utilized in select clinical scenarios:
- Medical treatment of peripheral neuropathy should target the underlying condition.
- Cognitive behavioral therapy and antidepressants are useful for treating anxiety and depressive disorders.73
- Musculoskeletal pain can be managed with acetaminophen and topical nonsteroidal anti-inflammatory drugs (NSAIDs), using a short course of an oral NSAID when needed.74
- Cardiovascular disease management might include any of several classes of pharmacotherapy, including antiplatelet and lipid-lowering medications, antiarrhythmic drugs, and antihypertensive agents.
- Acute episodes of vertigo due to vestibular neuritis or labyrinthitis can be managed with an antiemetic.46
Surgical treatment
Surgery is infrequently considered for patients with imbalance. Examples of indications include microsurgical resection of vestibular schwannoma, resection of central nervous system tumors, lens replacement surgery for cataract, surgical management of severe spinal fracture, and hip or knee arthroplasty in select patients.
Tools for assessing the risk of falls
Scoring systems called falls risk assessment tools, or FRAT, have been developed to gauge a patient’s risk of falling. The various FRATs differ in specificity and sensitivity for predicting the risk of falls, and are typically designed for specific clinical environments, such as hospital inpatient care or long-term care facilities. Specifically, FRATs attempt to classify risk using sets of risk factors known to be associated with falls.
Continue to: Research abounds into...
Research abounds into the validity of commonly used FRATs across institutions, patient populations, and clinical environments:
The Johns Hopkins FRATa determines risk using metrics such as age, fall history, incontinence, cognition, mobility, and medications75; it is predominantly used for assessment in hospital inpatient units. This tool has been validated repeatedly.76,77
Peninsula Health FRATb stratifies patients in subacute and residential aged-care settings, based on risk factors that include recent falls, medications, psychological status, and cognition.78
FRAT-upc is a web-based tool that generates falls risk using risk factors that users input. This tool has been studied in the context of patients older than 65 years living in the community.79
Although FRATs are reasonably useful for predicting falls, their utility varies by patient population and clinical context. Moreover, it has been suggested that FRATs neglect environmental and personal factors when assessing risk by focusing primarily on bodily factors.80 Implementing a FRAT requires extensive consideration of the target population and should be accompanied by clinical judgment that is grounded in an individual patient’s circumstances.81
Continue to: Preventing falls in primary care
Preventing falls in primary care
An approach to preventing falls includes the development of individualized programs that account for frailty, a syndrome of physiologic decline associated with aging. Because frailty leads to diminished balance and mobility, a patient’s frailty index—determined using the 5 frailty phenotype criteria (exhaustion, weight loss, low physical activity, weakness, slowness)82 or the Canadian Study of Health and Aging Clinical Frailty Scale83—is a useful tool for predicting falls risk and readmission for falls following trauma-related injury. Prevention of falls in communities is critical for reducing mortality and allowing older people to maintain their independence and quality of life.
Exercise. In some areas, exercise and falls prevention programs are accessible to seniors.84 Community exercise programs that focus on balance retraining and muscle strengthening can reduce the risk of falls.73,85 The Choosing Wisely initiative of the ABIM [American Board of Internal Medicine] Foundation recommends that exercise programs be designed around an accurate functional baseline of the patient to avoid underdosed strength training.54
Multifactorial risk assessment in high-risk patients can reduce the rate of falls. Such an assessment includes examination of orthostatic blood pressure, vision and hearing, bone health, gait, activities of daily living, cognition, and environmental hazards, and enables provision of necessary interventions.73,86 Hearing amplification, specifically, correlates with enhanced postural control, slowed cognitive decline, and a reduced likelihood of falls.87-93 The mechanism behind improved balance performance might be reduced cognitive load through supporting a patient’s listening needs.88-90
Pharmacotherapy. Optimizing medications and performing a complete medication review before prescribing new medications is highly recommended to avoid unnecessary polypharmacy7,8,18,53-56 (TABLE 17-56).
Management of comorbidities associated with a higher risk of falls, including arthritis, cancer, stroke, diabetes, depression, kidney disease, chronic obstructive pulmonary disease, cognitive impairment, hypertension, and atrial fibrillation, is essential.94-96
Continue to: Home safety interventions
Home safety interventions, through occupational therapy, are important. These include removing unsafe mats and step-overs and installing nonslip strips on stairs, double-sided tape under mats, and handrails.73-97
Screening for risk of falls. The Centers for Disease Control and Prevention recommends that (1) all patients older than 65 years and (2) any patient presenting with an acute fall undergo screening for their risk of falls.98 When a patient is identified as at risk of falling, you can, when appropriate, assess modifiable risk factors and facilitate interventions.98 This strategy is supported by a 2018 statement from the US Preventive Services Task Force99 that recommends identifying high-risk patients who have:
- a history of falling
- a balance disturbance that causes a deficit of mobility or function
- poor performance on clinical tests, such as the 3-meter Timed Up and Go (TUG) assessment (www.cdc.gov/steadi/pdf/TUG_test-print.pdf).
An increased risk of falls should prompt you to refer the patient to community programs and physiotherapy in accordance with the individual’s personal goals99; a balance and vestibular physiotherapist is ideally positioned to accurately assess and manage patients at risk of falls. Specifically, the Task Force identified exercise programs and multifactorial interventions as being beneficial in preventing falls in high-risk older people.99
Balance assessment and rehabilitation in specialty centers
An individualized rehabilitation program aims to restore safe mobility by testing and addressing specific balance deficits, improving functional balance, and increasing balance confidence. Collaboration with colleagues from physiotherapy and occupational therapy aids in tailoring individualized programs.
Many tests are available to assess balance, determine the risk of falls, and guide rehabilitation:
- The timed 10-meter walk testd and the TUG test are simple assessments that measure functional mobility; both have normalized values for the risk of falls. A TUG time of ≥ 12 seconds suggests a high risk of falls.
- The 30-second chair stande evaluates functional lower-extremity strength in older patients. The test can indicate if lower-extremity strength is contributing to a patient’s imbalance.
- The modified clinical test of sensory interaction in balancef is a static balance test that measures the integrity of sensory inputs. The test can suggest if 1 or more sensory systems are compromised.
- The mini balance evaluation systems testg is similar: It can differentiate balance deficits by underlying system and allows individualization of a rehabilitation program.
- The functional gait assessmenth is a modification of the dynamic gait index that assesses postural stability during everyday dynamic activities, including tasks such as walking with head turns and pivots.
- The Berg Balance Scalei continues to be used extensively to assess balance.
Continue to: The mini balance evaluation systems test...
The mini balance evaluation systems test, functional gait index, and Berg Balance Scale all have normative age-graded values to predict fall risk.
CASE
Mr. J was referred for balance assessment and to a rehabilitation program. He underwent balance physiotherapy, including multifactorial balance assessment, joined a community exercise program, was fitted with hearing aids, and had his home environment optimized by an occupational therapist. (See examples of “home safety interventions” under “Preventing falls in primary care.”)
3 months later. Mr. J says he feels stronger on his feet. His knee pain has eased, and he is more confident walking around his home. He continues to engage in exercise programs and is comfortable running errands with his spouse.
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Division of OtolaryngologyHead and Neck Surgery, Queen’s University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected]
a www.hopkinsmedicine.org/institute_nursing/models_tools/jhfrat_acute%20care%20original_6_22_17.pdf
c www.ncbi.nlm.nih.gov/pmc/articles/PMC4376110/figure/figure14/?report=objectonly
e www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf
f www.mdapp.co/mctsib-modified-clinical-test-of-sensory-interaction-in-balance-calculator-404/
g www.sralab.org/sites/default/files/2017-07/MiniBEST_revised_final_3_8_13.pdf
1. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189-201. doi: 10.2165/11591150-000000000-00000
2. TB, ZF, ES, et al. The relationship of balance disorders with falling, the effect of health problems, and social life on postural balance in the elderly living in a district in Turkey. Geriatrics (Basel). 2019;4:37. doi: 10.3390/geriatrics4020037
3. R, Sixt E, Landahl S, et al. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47-52.
4. Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467-478. doi: 10.1016/j.neucli.2008.09.001
5. Boult C, Murphy J, Sloane P, et al. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858-861. doi: 10.1111/j.1532-5415.1991.tb04451.x
6. Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122:1858-1861. doi: 10.1002/lary.23376
7. Jia H, Lubetkin EI, DeMichele K, et al. Prevalence, risk factors, and burden of disease for falls and balance or walking problems among older adults in the U.S. Prev Med. 2019;126:105737. doi: 10.1016/j.ypmed.2019.05.025
8. Al-Momani M, Al-Momani F, Alghadir AH, et al. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043-1049. doi: 10.2147/CIA.S112282
9. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23:113-117. doi: 10.3233/VES-130498
10. Altinsoy B, Erboy F, Tanriverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag. 2016;12:1023-1028. doi: 10.2147/TCRM.S105722
11. Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687
12. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(suppl 3):S1-S47. doi: 10.1177/0194599816689667
13. S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19:33. doi: 10.1186/s12875-017-0695-0
14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492. doi: 10.1128/CMR.00070-09
15. Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418. doi: 10.1016/j.ncl.2007.01.003
16. Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987-992. doi: 10.1016/j.gaitpost.2013.05.010
17. de Luna RA, Mihailovic A, Nguyen AM, et al. The association of glaucomatous visual field loss and balance. Transl Vis Sci Technol. 2017;6:8. doi: 10.1167/tvst.6.3.8
18. DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40:104-107. doi: 10.1055/s-0039-1684040
19. Ebersbach G, Moreau C, Gandor F, et al. Clinical syndromes: parkinsonian gait. Mov Disord. 2013;28:1552-1559. doi: 10.1002/mds.25675
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. doi: 10.3945/ajcn.2010.28608A
21. Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep. 2018;8:4984. doi: 10.1038/s41598-018-22676-0
22. Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1-7. doi: 10.1016/j.gaitpost.2016.08.009
23. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9-26. doi: 10.1016/s0887-6185(00)00040-2
24. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706-715. doi: 10.1016/S1474-4422(13)70107-8
25. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. 1989;18:31-34. doi: 10.1093/ageing/18.1.31
26. Goudakos JK, Markou KD, Franco-Vidal V, et al. Corticosteroids in the treatment of vestibular neuritis: a systematic review and meta-analysis. Otol Neurotol. 2010;31:183-189. doi: 10.1097/MAO.0b013e3181ca843d
27. Green AD, CS, Bastian L, et al. Does this woman have osteoporosis? JAMA. 2004;292:2890-2900. doi: 10.1001/jama.292.23.2890
28. Hallemans A, Ortibus E, Meire F, et al. Low vision affects dynamic stability of gait. Gait Posture. 2010;32:547-551. doi: 10.1016/j.gaitpost.2010.07.018
29. Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol. 2018;57(suppl 4):S99-S107. doi: 10.1080/14992027.2018.1468092
30. Head VA, Wakerley BR. Guillain-Barré syndrome in general practice: clinical features suggestive of early diagnosis. Br J Gen Pract. 2016;66:218-219. doi: 10.3399/bjgp16X684733
31. Helbostad JL, Vereijken B, Hesseberg K, et al. Altered vision destabilizes gait in older persons. Gait Posture. 2009;30:233-238. doi: 10.1016/j.gaitpost.2009.05.004
32. Hsu W-L, Chen C-Y, Tsauo J-Y, et al. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334-339. doi: 10.1016/j.jfma.2014.02.006
33. Kim H-S, Yun DH, Yoo SD, et al. Balance control and knee osteoarthritis severity. Ann Rehabil Med. 2011;35:701-709. doi: 10.5535/arm.2011.35.5.701
34. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25-29.
35. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. doi: 10.1212/WNL.0000000000004058
36. Milner KA, Funk M, Richards S, et al. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399. doi: 10.1016/s0002-9149(99)00322-7
37. Paillard T, F, Bru N, et al. The impact of time of day on the gait and balance control of Alzheimer’s patients. Chronobiol Int. 2016;33:161-168. doi: 10.3109/07420528.2015.1124885
38. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1-8. doi: 10.1016/j.jocn.2016.05.003
39. Picorelli AMA, Hatton AL, Gane EM, et al. Balance performance in older adults with hip osteoarthritis: a systematic review. Gait Posture. 2018;65:89-99. doi: 10.1016/j.gaitpost.2018.07.001
40. Raccagni C, Nonnekes J, Bloem BR, et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169-3176. doi: 10.1007/s00415-019-09382-1
41. Shanmugarajah PD, Hoggard N, Currie S, et al. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. doi: 10.1186/s40673-016-0055-1
42. Shih RY, Smirniotopoulos JG. Posterior fossa tumors in adult patients. Neuroimaging Clin N Am. 2016;26:493-510. doi: 10.1016/j.nic.2016.06.003
43. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131:1059-1068. doi: 10.1042/CS20160607
44. Streur M, Ratcliffe SJ, Ball J, et al. Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. 2017;32:296-303. doi: 10.1097/JCN.0000000000000344
45. Strupp M, M, JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165-173. doi: 10.1097/WCO.0000000000000649
46. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
47. Timar B, Timar R, L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11:e0154654. doi: 10.1371/journal.pone.0154654
48. Walls R, Hockberger R, Gausche-Hill M. Peripheral nerve disorders. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Elsevier, Inc; 2018:1307-1320.
49. Watson JC, Dyck PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940-951. doi: 10.1016/j.mayocp.2015.05.004
50. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. doi: 10.1111/nyas.13919
51. Wu V, Sykes EA, Beyea MM, et al. Approach to Meniere disease management. Can Fam Physician. 2019;65:463-467.
52. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528-536.
53. Seppala LJ, van de Glind EMM, Daams JG, et al; . Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099
54. ABIM Foundation. Choosing wisely. Choosing Wisely website. 2021. Accessed November 11. 2021. www.choosingwisely.org/
55. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712-717. doi: 10.1345/aph.1M551
56. Hartikainen S, E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-1181. doi: 10.1093/gerona/62.10.1172
57. Khanuja K, Joki J, Bachmann G, et al. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas. 2018;110:51-56. doi: 10.1016/j.maturitas.2018.01.021
58. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82:61-68.
59. Zaninotto P, Huang YT, Di Gessa G, et al. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English Longitudinal Study of Ageing. BMC Public Health. 2020;20:1804. doi: 10.1186/s12889-020-09920-x
60. Morin L, Calderon A, Welmer AK, et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483-493. doi: 10.2147/CLEP.S201614
61. Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358
62. Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007
63. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689-703.
64. Baker JM. Gait disorders. Am J Med. 2018;131:602-607. doi: 10.1016/j.amjmed.2017.11.051
65. Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep. 2011;11:507-515. doi: 10.1007/s11910-011-0214-y
66. Chen C-L, Chen H-C, Tang SF-T, et al. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003;82:925-935. doi: 10.1097/01.PHM.0000098040.13355.B5
67. Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195-216. doi: 10.1177/0269215510382495
68. Mirek E, Filip M, W, et al. Three-dimensional trunk and lower limbs characteristics during gait in patients with Huntington’s disease. Front Neurosci. 2017;11:566. doi: 10.3389/fnins.2017.00566
69. Paramanandam V, Lizarraga KJ, Soh D, et al. Unusual gait disorders: a phenomenological approach and classification. Expert Rev Neurother. 2019;19:119-132. doi: 10.1080/14737175.2019.1562337
70. Sahyouni R, Goshtasbi K, Mahmoodi A, et al. Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg. 2017;108:948-953. doi: 10.1016/j.wneu.2017.09.064
71. Talmud JD, Coffey R, Edemekong PF. Dix Hallpike maneuver. StatPearls [Internet]. StatPearls Publishing Updated September 5, 2021. Accessed December 6, 2021. www.ncbi.nlm.nih.gov/books/NBK459307/
72. Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68:2207-2213. doi: 10.1111/jgs.16713
73. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:CD007146. doi: 10.1002/14651858.CD007146.pub3
74. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306
75. Poe SS, Cvach M, Dawson PB, Straus H, Hill EE. The Johns Hopkins Fall Risk Assessment Tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293-298. doi: 10.1097/01.NCQ.0000290408.74027.39
76. Poe SS, Dawson PB, Cvach M, et al. The Johns Hopkins Fall Risk Assessment Tool: a study of reliability and validity. J Nurs Care Qual. 2018;33:10-19. doi: 10.1097/NCQ.0000000000000301
77. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for predicting falls on inpatient medicine services. J Nurs Care Qual. 2017;32:108-113. doi: 10.1097/NCQ.0000000000000210
78. Stapleton C, Hough P, Oldmeadow L, et al. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28:139-143. doi: 10.1111/j.1741-6612.2009.00375.x
79. Cattelani L, Palumbo P, Palmerini L, et al. FRAT-up, a Web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res. 2015;17:e41. doi: 10.2196/jmir.4064
80. De Clercq H, Naudé A, Bornman J. Factors included in adult fall risk assessment tools (FRATs): a systematic review. Ageing Soc. 2020;41:2558-2582. doi: 10.1017/S0144686X2000046X
81. Yap G, Melder A. Accuracy of validated falls risk assessment tools and clinical judgement. Centre for Clinical Effectiveness, Monash Innovation and Quality. Monash Health. February 5, 2020. Accessed November 11, 2021. https://monashhealth.org/wp-content/uploads/2019/01/Rapid-Review_Falls-risk-tools-FINAL.pdf
82. Chittrakul J, Siviroj P, Sungkarat S, et al. Physical frailty and fall risk in community-dwelling older adults: a cross-sectional study. J Aging Res. 2020;2020:3964973. doi: 10.1155/2020/3964973
83. Hatcher VH, Galet C, Lilienthal M, et al. Association of clinical frailty scores with hospital readmission for falls after index admission for trauma-related injury. JAMA Netw Open. 2019;2:e1912409. doi: 10.1001/jamanetworkopen.2019.12409
84. Exercise and fall prevention programs. Government of Ontario Ministry of Health. Updated April 9, 2019. Accessed November 11. 2021. www.ontario.ca/page/exercise-and-falls-prevention-programs
85. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2
86. Hopewell S, Copsey B, Nicolson P, et al. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340-1350. doi: 10.1136/bjsports-2019-100732
87. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. doi: 10.1016/j.arr.2019.100963
88. Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108:401-412. doi: 10.1016/j.neuron.2020.08.003
89. Martini A, Castiglione A, Bovo R, et al. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol. 2014;19(suppl 1):2-5. doi: 10.1159/000371593
90. Vitkovic J, Le C, Lee S-L, et al. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195-202. doi: 10.1159/000445100
91. Maheu M, Behtani L, Nooristani M, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. 2019;40:1418-1424. doi: 10.1097/AUD.0000000000000720
92. Negahban H, Bavarsad Cheshmeh Ali M, Nassadj G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture. 2017;58:126-129. doi: 10.1016/j.gaitpost.2017.07.112
93. Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67:2362-2369. doi: 10.1111/jgs.16109
94. Paliwal Y, Slattum PW, Ratliff SM. Chronic health conditions as a risk factor for falls among the community-dwelling US older adults: a zero-inflated regression modeling approach. Biomed Res Int. 2017;2017:5146378. doi: 10.1155/2017/5146378
95. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658-668. doi: 10.1097/EDE.0b013e3181e89905
96. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. doi: 10.1016/j.maturitas.2013.02.009
97. Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc. 2001;49:1442-1447. doi: 10.1046/j.1532-5415.2001.4911235.x
98. Clinical resources. Centers for Disease Control and Prevention STEADI-Older Adult Fall Prevention website. 2020. Accessed November 12, 2021. www.cdc.gov/steadi/materials.html
99. ; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704. doi: 10.1001/jama.2018.3097
1. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189-201. doi: 10.2165/11591150-000000000-00000
2. TB, ZF, ES, et al. The relationship of balance disorders with falling, the effect of health problems, and social life on postural balance in the elderly living in a district in Turkey. Geriatrics (Basel). 2019;4:37. doi: 10.3390/geriatrics4020037
3. R, Sixt E, Landahl S, et al. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47-52.
4. Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467-478. doi: 10.1016/j.neucli.2008.09.001
5. Boult C, Murphy J, Sloane P, et al. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858-861. doi: 10.1111/j.1532-5415.1991.tb04451.x
6. Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122:1858-1861. doi: 10.1002/lary.23376
7. Jia H, Lubetkin EI, DeMichele K, et al. Prevalence, risk factors, and burden of disease for falls and balance or walking problems among older adults in the U.S. Prev Med. 2019;126:105737. doi: 10.1016/j.ypmed.2019.05.025
8. Al-Momani M, Al-Momani F, Alghadir AH, et al. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043-1049. doi: 10.2147/CIA.S112282
9. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23:113-117. doi: 10.3233/VES-130498
10. Altinsoy B, Erboy F, Tanriverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag. 2016;12:1023-1028. doi: 10.2147/TCRM.S105722
11. Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687
12. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(suppl 3):S1-S47. doi: 10.1177/0194599816689667
13. S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19:33. doi: 10.1186/s12875-017-0695-0
14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492. doi: 10.1128/CMR.00070-09
15. Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418. doi: 10.1016/j.ncl.2007.01.003
16. Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987-992. doi: 10.1016/j.gaitpost.2013.05.010
17. de Luna RA, Mihailovic A, Nguyen AM, et al. The association of glaucomatous visual field loss and balance. Transl Vis Sci Technol. 2017;6:8. doi: 10.1167/tvst.6.3.8
18. DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40:104-107. doi: 10.1055/s-0039-1684040
19. Ebersbach G, Moreau C, Gandor F, et al. Clinical syndromes: parkinsonian gait. Mov Disord. 2013;28:1552-1559. doi: 10.1002/mds.25675
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. doi: 10.3945/ajcn.2010.28608A
21. Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep. 2018;8:4984. doi: 10.1038/s41598-018-22676-0
22. Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1-7. doi: 10.1016/j.gaitpost.2016.08.009
23. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9-26. doi: 10.1016/s0887-6185(00)00040-2
24. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706-715. doi: 10.1016/S1474-4422(13)70107-8
25. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. 1989;18:31-34. doi: 10.1093/ageing/18.1.31
26. Goudakos JK, Markou KD, Franco-Vidal V, et al. Corticosteroids in the treatment of vestibular neuritis: a systematic review and meta-analysis. Otol Neurotol. 2010;31:183-189. doi: 10.1097/MAO.0b013e3181ca843d
27. Green AD, CS, Bastian L, et al. Does this woman have osteoporosis? JAMA. 2004;292:2890-2900. doi: 10.1001/jama.292.23.2890
28. Hallemans A, Ortibus E, Meire F, et al. Low vision affects dynamic stability of gait. Gait Posture. 2010;32:547-551. doi: 10.1016/j.gaitpost.2010.07.018
29. Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol. 2018;57(suppl 4):S99-S107. doi: 10.1080/14992027.2018.1468092
30. Head VA, Wakerley BR. Guillain-Barré syndrome in general practice: clinical features suggestive of early diagnosis. Br J Gen Pract. 2016;66:218-219. doi: 10.3399/bjgp16X684733
31. Helbostad JL, Vereijken B, Hesseberg K, et al. Altered vision destabilizes gait in older persons. Gait Posture. 2009;30:233-238. doi: 10.1016/j.gaitpost.2009.05.004
32. Hsu W-L, Chen C-Y, Tsauo J-Y, et al. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334-339. doi: 10.1016/j.jfma.2014.02.006
33. Kim H-S, Yun DH, Yoo SD, et al. Balance control and knee osteoarthritis severity. Ann Rehabil Med. 2011;35:701-709. doi: 10.5535/arm.2011.35.5.701
34. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25-29.
35. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. doi: 10.1212/WNL.0000000000004058
36. Milner KA, Funk M, Richards S, et al. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399. doi: 10.1016/s0002-9149(99)00322-7
37. Paillard T, F, Bru N, et al. The impact of time of day on the gait and balance control of Alzheimer’s patients. Chronobiol Int. 2016;33:161-168. doi: 10.3109/07420528.2015.1124885
38. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1-8. doi: 10.1016/j.jocn.2016.05.003
39. Picorelli AMA, Hatton AL, Gane EM, et al. Balance performance in older adults with hip osteoarthritis: a systematic review. Gait Posture. 2018;65:89-99. doi: 10.1016/j.gaitpost.2018.07.001
40. Raccagni C, Nonnekes J, Bloem BR, et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169-3176. doi: 10.1007/s00415-019-09382-1
41. Shanmugarajah PD, Hoggard N, Currie S, et al. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. doi: 10.1186/s40673-016-0055-1
42. Shih RY, Smirniotopoulos JG. Posterior fossa tumors in adult patients. Neuroimaging Clin N Am. 2016;26:493-510. doi: 10.1016/j.nic.2016.06.003
43. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131:1059-1068. doi: 10.1042/CS20160607
44. Streur M, Ratcliffe SJ, Ball J, et al. Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. 2017;32:296-303. doi: 10.1097/JCN.0000000000000344
45. Strupp M, M, JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165-173. doi: 10.1097/WCO.0000000000000649
46. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
47. Timar B, Timar R, L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11:e0154654. doi: 10.1371/journal.pone.0154654
48. Walls R, Hockberger R, Gausche-Hill M. Peripheral nerve disorders. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Elsevier, Inc; 2018:1307-1320.
49. Watson JC, Dyck PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940-951. doi: 10.1016/j.mayocp.2015.05.004
50. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. doi: 10.1111/nyas.13919
51. Wu V, Sykes EA, Beyea MM, et al. Approach to Meniere disease management. Can Fam Physician. 2019;65:463-467.
52. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528-536.
53. Seppala LJ, van de Glind EMM, Daams JG, et al; . Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099
54. ABIM Foundation. Choosing wisely. Choosing Wisely website. 2021. Accessed November 11. 2021. www.choosingwisely.org/
55. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712-717. doi: 10.1345/aph.1M551
56. Hartikainen S, E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-1181. doi: 10.1093/gerona/62.10.1172
57. Khanuja K, Joki J, Bachmann G, et al. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas. 2018;110:51-56. doi: 10.1016/j.maturitas.2018.01.021
58. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82:61-68.
59. Zaninotto P, Huang YT, Di Gessa G, et al. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English Longitudinal Study of Ageing. BMC Public Health. 2020;20:1804. doi: 10.1186/s12889-020-09920-x
60. Morin L, Calderon A, Welmer AK, et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483-493. doi: 10.2147/CLEP.S201614
61. Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358
62. Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007
63. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689-703.
64. Baker JM. Gait disorders. Am J Med. 2018;131:602-607. doi: 10.1016/j.amjmed.2017.11.051
65. Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep. 2011;11:507-515. doi: 10.1007/s11910-011-0214-y
66. Chen C-L, Chen H-C, Tang SF-T, et al. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003;82:925-935. doi: 10.1097/01.PHM.0000098040.13355.B5
67. Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195-216. doi: 10.1177/0269215510382495
68. Mirek E, Filip M, W, et al. Three-dimensional trunk and lower limbs characteristics during gait in patients with Huntington’s disease. Front Neurosci. 2017;11:566. doi: 10.3389/fnins.2017.00566
69. Paramanandam V, Lizarraga KJ, Soh D, et al. Unusual gait disorders: a phenomenological approach and classification. Expert Rev Neurother. 2019;19:119-132. doi: 10.1080/14737175.2019.1562337
70. Sahyouni R, Goshtasbi K, Mahmoodi A, et al. Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg. 2017;108:948-953. doi: 10.1016/j.wneu.2017.09.064
71. Talmud JD, Coffey R, Edemekong PF. Dix Hallpike maneuver. StatPearls [Internet]. StatPearls Publishing Updated September 5, 2021. Accessed December 6, 2021. www.ncbi.nlm.nih.gov/books/NBK459307/
72. Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68:2207-2213. doi: 10.1111/jgs.16713
73. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:CD007146. doi: 10.1002/14651858.CD007146.pub3
74. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306
75. Poe SS, Cvach M, Dawson PB, Straus H, Hill EE. The Johns Hopkins Fall Risk Assessment Tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293-298. doi: 10.1097/01.NCQ.0000290408.74027.39
76. Poe SS, Dawson PB, Cvach M, et al. The Johns Hopkins Fall Risk Assessment Tool: a study of reliability and validity. J Nurs Care Qual. 2018;33:10-19. doi: 10.1097/NCQ.0000000000000301
77. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for predicting falls on inpatient medicine services. J Nurs Care Qual. 2017;32:108-113. doi: 10.1097/NCQ.0000000000000210
78. Stapleton C, Hough P, Oldmeadow L, et al. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28:139-143. doi: 10.1111/j.1741-6612.2009.00375.x
79. Cattelani L, Palumbo P, Palmerini L, et al. FRAT-up, a Web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res. 2015;17:e41. doi: 10.2196/jmir.4064
80. De Clercq H, Naudé A, Bornman J. Factors included in adult fall risk assessment tools (FRATs): a systematic review. Ageing Soc. 2020;41:2558-2582. doi: 10.1017/S0144686X2000046X
81. Yap G, Melder A. Accuracy of validated falls risk assessment tools and clinical judgement. Centre for Clinical Effectiveness, Monash Innovation and Quality. Monash Health. February 5, 2020. Accessed November 11, 2021. https://monashhealth.org/wp-content/uploads/2019/01/Rapid-Review_Falls-risk-tools-FINAL.pdf
82. Chittrakul J, Siviroj P, Sungkarat S, et al. Physical frailty and fall risk in community-dwelling older adults: a cross-sectional study. J Aging Res. 2020;2020:3964973. doi: 10.1155/2020/3964973
83. Hatcher VH, Galet C, Lilienthal M, et al. Association of clinical frailty scores with hospital readmission for falls after index admission for trauma-related injury. JAMA Netw Open. 2019;2:e1912409. doi: 10.1001/jamanetworkopen.2019.12409
84. Exercise and fall prevention programs. Government of Ontario Ministry of Health. Updated April 9, 2019. Accessed November 11. 2021. www.ontario.ca/page/exercise-and-falls-prevention-programs
85. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2
86. Hopewell S, Copsey B, Nicolson P, et al. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340-1350. doi: 10.1136/bjsports-2019-100732
87. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. doi: 10.1016/j.arr.2019.100963
88. Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108:401-412. doi: 10.1016/j.neuron.2020.08.003
89. Martini A, Castiglione A, Bovo R, et al. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol. 2014;19(suppl 1):2-5. doi: 10.1159/000371593
90. Vitkovic J, Le C, Lee S-L, et al. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195-202. doi: 10.1159/000445100
91. Maheu M, Behtani L, Nooristani M, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. 2019;40:1418-1424. doi: 10.1097/AUD.0000000000000720
92. Negahban H, Bavarsad Cheshmeh Ali M, Nassadj G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture. 2017;58:126-129. doi: 10.1016/j.gaitpost.2017.07.112
93. Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67:2362-2369. doi: 10.1111/jgs.16109
94. Paliwal Y, Slattum PW, Ratliff SM. Chronic health conditions as a risk factor for falls among the community-dwelling US older adults: a zero-inflated regression modeling approach. Biomed Res Int. 2017;2017:5146378. doi: 10.1155/2017/5146378
95. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658-668. doi: 10.1097/EDE.0b013e3181e89905
96. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. doi: 10.1016/j.maturitas.2013.02.009
97. Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc. 2001;49:1442-1447. doi: 10.1046/j.1532-5415.2001.4911235.x
98. Clinical resources. Centers for Disease Control and Prevention STEADI-Older Adult Fall Prevention website. 2020. Accessed November 12, 2021. www.cdc.gov/steadi/materials.html
99. ; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704. doi: 10.1001/jama.2018.3097
PRACTICE RECOMMENDATIONS
› Utilize a falls-prevention program for older patients that focuses on balance and functional exercises. A
› Perform a multifactorial assessment of the risk of falls in older patients that includes optimizing medications, managing comorbidities, and addressing environmental hazards. B
› Use a systems-based approach to presentations of imbalance to direct your clinical judgment and highlight the need for referral to specialists for management and rehabilitation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Did a switch to a generic antidepressant cause relapse?
Cervical cancer update: The latest on screening & management
The World Health Organization estimates that, in 2020, worldwide, there were 604,000 new cases of uterine cervical cancer and approximately 342,000 deaths, 84% of which occurred in developing countries.1 In the United States, as of 2018, the lifetime risk of death from cervical cancer was 2.2 for every 100,000
In this article, we summarize recent updates in the epidemiology, prevention, and treatment of cervical cancer. We emphasize recent information of value to family physicians, including updates in clinical guidelines and other pertinent national recommendations.
Spotlight continues to shine on HPV
It has been known for several decades that cervical cancer is caused by human papillomavirus (HPV). Of more than 100 known HPV types, 14 or 15 are classified as carcinogenic. HPV 16 is the most common oncogenic type, causing more than 60% of cases of cervical cancer3,4
HPV is the most common sexually transmitted infection, with as many as 80% of sexually active people becoming infected during their lifetime, generally before 50 years of age.5 HPV also causes other anogenital and oropharyngeal cancers; however, worldwide, more than 80% of HPV-associated cancers are cervical.6 Risk factors for cervical cancer are listed in TABLE 1.7 Cervical cancer is less common when partners are circumcised.7
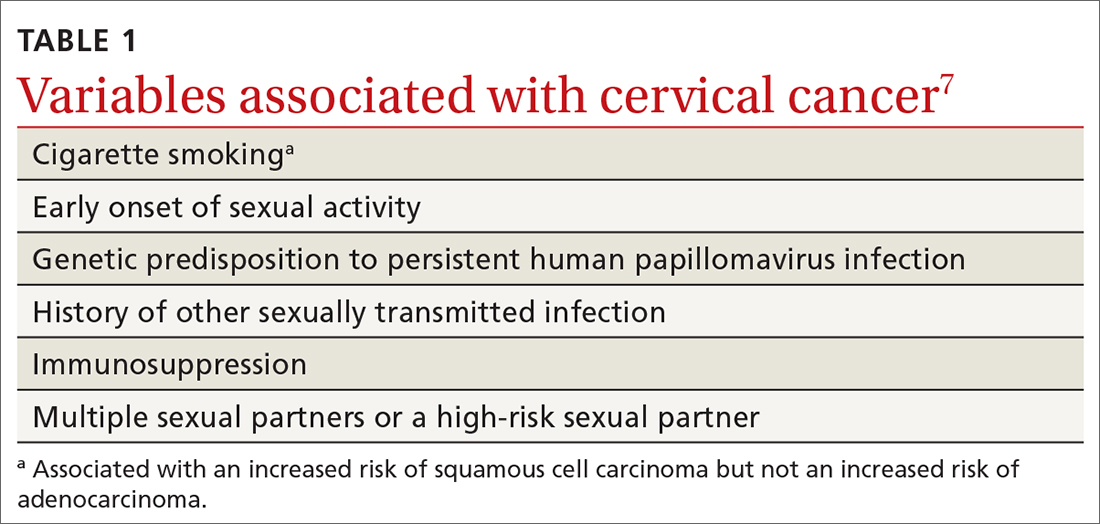
Most cases of HPV infection clear in 1 or 2 years
At least 70% of cervical cancers are squamous cell carcinoma (SCC); 20% to 25% are adenocarcinoma (ADC); and < 3% to 5% are adenosquamous carcinoma.10 Almost 100% of cervical SCCs are HPV+, as are 86% of cervical ADCs. The most common reason for HPV-negative status in patients with cervical cancer is false-negative testing because of inadequate methods.
Primary prevention through vaccination
HPV vaccination was introduced in 2006 in the United States for girls,a and for boysa in 2011. The primary reason for vaccinating boys is to reduce the rates of HPV-related anal and oropharyngeal cancer. The only available HPV vaccine in the United States is Gardasil 9 (9-valent vaccine, recombinant; Merck), which provides coverage for 7 high-risk HPV types that account for approximately 90% of cervical cancers and 2 types (6 and 11) that are the principal causes of condylomata acuminata (genital warts). Future generations of prophylactic vaccines are expected to cover additional strains.
Continue to: Vaccine studies...
Vaccine studies have been summarized in a Cochrane review,11 showing that vaccination is highly effective for prevention of cervical dysplasia, especially when given to young girls and womena previously unexposed to the virus. It has not been fully established how long protection lasts, but vaccination appears to be 70% to 90% effective for ≥ 10 years.
Dosing schedule. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends a 2-dose schedule 6 to 15 months apart, for both girls and boys between 9 and 14 years of age.12 A third dose is indicated if the first and second doses were given less than 5 months apart, or the person is older than 15 years or is immunocompromised. No recommendation has been made for revaccination after the primary series.
In 2018, the US Food and Drug Administration approved Gardasil 9 for adults 27 to 45 years of age. In June 2019, ACIP recommended vaccination for mena as old as 26 years, and adopted a recommendation that unvaccinated men and women between 27 and 45 years discuss HPV vaccination with their physician.13
The adolescent HPV vaccination rate varies by state; however, all states lag behind the CDC’s Healthy People 2020 goal of 80%.14 Barriers to vaccination include cost, infrastructure limitations, and social stigma.
Secondary prevention: Screening and Tx of precancerous lesions
Cervical cancer screening identifies patients at increased risk of cervical cancer and reassures the great majority of them that their risk of cervical cancer is very low. There are 3 general approaches to cervical cancer screening:
- cytology-based screening, which has been implemented for decades in many countries
- primary testing for DNA or RNA markers of high-risk HPV types
- co-testing with cytology-based screening plus HPV testing.
Continue to: USPSTF guidance
USPSTF guidance. Recommendations of the US Preventive Services Task Force (USPSTF) for cervical cancer screening were updated in 2018 (TABLE 215). The recommendations state that high-risk HPV screening alone is a strategy that is amenable to patient self-sampling and self-mailing for processing—a protocol that has the potential to improve access

ASCCP guidance. The American Society of Colposcopy and Cervical Pathology (ASCCP) makes nearly the same recommendations for cervical cancer screening. An exception is that ASCCP guidelines allow for the possibility of screening using primary high-risk HPV testing for patients starting at 25 years of age.16
Screening programs that can be initiated at a later age and longer intervals should be possible once the adolescent vaccination rate is optimized and vaccination registries are widely implemented.
Cervical cytology protocol
Cervical cytologic abnormalities are reported using the Bethesda system. Specimen adequacy is the most important component of quality assurance,17 and is determined primarily by sufficient cellularity. However, any specimen containing abnormal squamous cells of undetermined significance (ASCUS) or atypical glandular cells (AGCs) is considered satisfactory, regardless of the number of cells. Obscuring factors that impair quality include excessive blood; inflammation; air-drying artifact; and an interfering substance, such as lubricant. The presence of reactive changes resulting from inflammation does not require further evaluation unless the patient is immunosuppressed.
Abnormalities are most often of squamous cells, of 2 categories: low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs). HSILs are more likely to be associated with persistent HPV infection and higher risk of progression to cervical cancer.
Continue to: Cytologic findings...
Cytologic findings can be associated with histologic findings that are sometimes more, sometimes less, severe. LSIL cytology specimens that contain a few cells that are suspicious for HSIL, but that do not contain enough cells to be diagnostic, are reported as atypical squamous cells, and do not exclude a high-grade intraepithelial lesion.
Glandular-cell abnormalities usually originate from the glandular epithelium of the endocervix or the endometrium—most often, AGCs. Less frequent are AGCs, favor neoplasia; endocervical adenocarcinoma in situ; and ADC. Rarely, AGCs are associated with adenosquamous carcinoma. Endometrial polyps are a typical benign pathology that can be associated with AGCs.
In about 30% of cases, AGCs are associated with premalignant or malignant disease.18 The risk of malignancy in patients with AGCs increases with age, from < 2% among patients younger than 40 years to approximately 15% among those > 50 years.19 Endometrial malignancy is more common than cervical malignancy among patients > 40 years.
AGC cytology requires endocervical curettage, plus endometrial sampling for patients ≥ 35 years
Cytology-based screening has limitations. Sensitivity is relatively low and dependent on the expertise of the cytologist, although regular repeat testing has been used to overcome this limitation. A substantial subset of results are reported as equivocal—ie, ASCUS.
Continue to: Primary HPV screening
Primary HPV screening
Primary HPV testing was approved by the US Food and Drug Administration in 2015 and recommended as an appropriate screening option by professional societies
In contrast to cytology-based screening, HPV testing has high sensitivity (≥ 90%); the population-based negative likelihood ratio is near zero.20 This degree of sensitivity allows for extended screening intervals. However, primary HPV testing lacks specificity for persistent infection and high-grade or invasive lesions, which approximately doubles the number of patients who screen positive. The potential for excess patients to be referred for colposcopy led to the need for secondary triage.
Instituting secondary triage. Cytology is, currently, the primary method of secondary triage, reducing the number of referrals for colposcopy by nearly one-half, compared to referrals for all high-risk HPV results, and with better overall accuracy over cytology with high-risk HPV triage.21 When cytology shows ASCUS, or worse, refer the patient for colposcopy; alternatively, if so-called reflex testing for HPV types 16 and 18 is available and positive, direct referral to colposcopy without cytology is also appropriate.
In the future, secondary triage for cytology is likely to be replaced with improved technologies, such as immunostaining of the specimen for biomarkers associated with cervical precancer or cancer, or for viral genome methylation testing.22
Management of abnormal cervical cancer screening results
Routine screening applies to asymptomatic patients who do not require surveillance because they have not had prior abnormal screening results. In 2020, ASCCP published risk-based management consensus guidelines that were developed for abnormal cervical cancer screening tests and for cancer precursors.16 Guiding principles, and screening situations in which the guidelines can be applied, are summarized in TABLE 3
Continue to: ASCCP guidelines...
ASCCP guidelines provide a framework to incorporate new data and technologies without major revision
Some noteworthy scenarios in ASCCP risk-based management are:
- For unsatisfactory cytology with a negative HPV test or no HPV test, repeat age-based screening in 2 to 4 months. (Note: A negative HPV test might reflect an inadequate specimen; do not interpret this result as a true negative.)
- An absent transformation zone (ie, between glandular and squamous cervical cells) with an otherwise adequate specimen should be interpreted as satisfactory for screening in patients 21 to 29 years of age. For those ≥ 30 years and with no HPV testing in this circumstance, HPV testing is preferred; repeating cytology, in 3 years, is also acceptable.
- After a finding of LSIL/CIN1 without evidence of a high-grade abnormality, and after 2 negative annual screenings (including HPV testing), a return to 3-year (not 5-year) screening is recommended.
- A cytology result of an HSIL carries a risk of 26% for CIN3+, in which case colposcopy is recommended, regardless of HPV test results.
- For long-term management after treatment for CIN2+, continue surveillance testing every 3 years after 3 consecutive negative HPV tests or cytology findings, for at least 25 years. If the 25-year threshold is reached before 65 years of age, continuing surveillance every 3 years is optional, as long as the patient is in good health (ie, life expectancy ≥ 10 years).
- After hysterectomy for a high-grade abnormality, annual vaginal HPV testing is recommended until 3 negative tests are returned; after that, surveillance shifts to a 3-year interval until the 25-year threshold.
Treatment of cancer precursors
Treatment for cervical dysplasia is excisional or ablative.
Excisional therapy. In most cases, excisional therapy (either a loop electrosurgical excision procedure [LEEP; also known as large loop excision of the transformation zone, cold knife conization, and laser conization] or cone biopsy) is required, or preferred. Excisional treatment has the advantage of providing a diagnostic specimen.
The World Health Organization recommends LEEP over ablation in settings in which LEEP is available.23 ASCCP states that, in the relatively few cases in which treatment is needed and it is for CIN1, either excision or ablation is acceptable. TABLE 416 lists situations in which excisional treatment is required because a diagnostic specimen is needed.
Continue to: Ablative treatments
Ablative treatments are cryotherapy, CO2 laser ablation, and thermal ablation. Ablative therapy has the advantage of presenting less risk of adverse obstetric outcomes (eg, preterm birth); it can be used if the indication for therapy is:
- CIN1 or CIN2 and HPV type 16 or 18 positivity
- concordant cytology and histology
- satisfactory colposcopy
- negative endocervical curettage.
The most common ablative treatment is liquid nitrogen applied to a metal tip under local anesthesia.
Hysterectomy can be considered for patients with recurrent CIN2+ who have completed childbearing or for whom repeat excision is infeasible (eg, scarring or a short cervix), or both.
Cost, availability, and convenience might play a role in decision-making with regard to the treatment choice for cancer precursors.
Is care after treatment called for? Patients who continue to be at increased risk of (and thus mortality from) cervical and vaginal cancer require enhanced surveillance. The risk of cancer is more than triple for patients who were given their diagnosis, and treated, when they were > 60 years, compared to patients treated in their 30s.1 The excess period of risk covers at least 25 years after treatment, even among patients who have had 3 posttreatment screenings.
Continue to: Persistent HPV positivity...
Persistent HPV positivity is more challenging. Patients infected with HPV type 16 have an increased risk of residual disease.
Cancer management
Invasive cancer. Most cervical cancers (60%) occur among patients who have not been screened during the 5 years before their diagnosis.24 For patients who have a diagnosis of cancer, those detected through screening have a much better prognosis than those identified by symptoms (mean cure rate, 92% and 66%, respectively).25 The median 5-year survival for patients who were not screened during the 5 years before their diagnosis of cervical cancer is 66%.2
In unscreened patients, cervical cancer usually manifests as abnormal vaginal bleeding, especially postcoitally. In approximately 45% of cases, the patient has localized disease at diagnosis; in 36%, regional disease; and in 15%, distant metastases.26
For cancers marked by stromal invasion < 3 mm, appropriate treatment is cone biopsy or simple hysterectomy.27
Most patients with early-stage cervical cancer undergo modified radical hysterectomy. The ovaries are usually conserved, unless the cancer is adenocarcinoma. Sentinel-node dissection has become standard practice. Primary radiation therapy is most often used for patients who are a poor surgical candidate because of medical comorbidity or poor functional status. Antiangiogenic agents (eg, bevacizumab) can be used as adjuvant palliative therapy for advanced and recurrent disease
Continue to: After treatment for...
After treatment for invasive cervical cancer, the goal is early detection of recurrence, although there is no consensus on a protocol. Most recurrences are detected within the first 2 years.
Long-term sequelae after treatment for advanced cancer are considerable. Patients report significantly lower quality of life,
Hormone replacement therapy is generally considered acceptable after treatment of cervical cancer because it does not increase replication of HPV.
Recurrent or metastatic cancer. Recurrence or metastases will develop in 15% to 60% of patients,30 usually within the first 2 years after treatment.
Management depends on location and extent of disease, using mainly radiation therapy or surgical resection. Recurrence or metastasis is usually incurable.
Continue to: Last, there are promising...
Last, there are promising areas of research for more effective treatment for cervical cancer precursors and cancers, including gene editing tools31 and therapeutic
Prospects for better cervical cancer care
Prevention. HPV vaccination is likely to have a large impact on population-based risk of both cancer and cancer precursors in the next generation.
Screening in the foreseeable future will gravitate toward reliance on primary HPV screening, with a self-sampling option.
Surveillance after dysplastic disease. The 2019 ASCCP guidelines for surveillance and intervention decisions after abnormal cancer screening results will evolve to incorporate introduction of new technology into computerized algorithms.
Treatment. New biologic therapies, including monoclonal antibodies and therapeutic vaccines against HPV, will likely be introduced for treating cancer precursors and invasive cancer.
A NOTE FROM THE EDITORS The Editors of The Journal of Family Practice recognize the importance of addressing the reproductive health of gender-diverse individuals. In this article, we use the words “women,” “men,” “girls,” and “boys” in limited circumstances (1) for ease of reading and (2) to reflect the official language of the US Food and Drug Administration and the Advisory Committee on Immunization Practices. The reader should consider the information and guidance offered in this discussion of cervical cancer and other human papillomavirus-related cancers to speak to the care of people with a uterine cervix and people with a penis.
CORRESPONDENCE
Linda Speer, MD, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; [email protected]
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660
2. Cancer stat facts: cervical cancer. National Cancer Institute Surveillance, Epidemiology, and End Results [SEER] Program. Accessed November 14, 2021. https://seer.cancer.gov/statfacts/html/cervix.html
3. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349-2359. doi: 10.1002/ijc.27485
4. Winer RL, Hughes JP, Feng Q, et al. Early history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699-707. doi: 10.1158/1055-9965.EPI-10-1108
5. Chesson HW, Dunne EF, Hariri F, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-664. doi: 10.1097/OLQ.0000000000000193
6. Human papillomavirus (HPV) and cervical cancer. Fact sheet. Geneva, Switzerland: World Health Organization; November 11, 2020. Accessed November 14, 2021. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
7. International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120:885-891. doi: 10.1002/ijc.22357
8. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical cancer neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008:9:425-434. doi: 10.1016/S1470-2045(08)70103-7
9. de Sanjose S, Quint WG, Alemany I, et al; . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective, cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. doi: 10.1016/S1470-2045(10)70230-8
10. Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review 1975-2004. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2004/#citation
11. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3
12. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016:65;1405-1408. doi: 10.15585/mmwr.mm6549a5
13. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
14. State-level data: Female adolescents receiving 2 or 3 doses of HPV vaccine by age 13-15 years (percent). HealthyPeople.gov. Accessed November 14, 2021. www.healthypeople.gov/2020/data/map/4657?year=2018
15. United States Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674-686. doi: 10.1001/jama.2018.10897
16. Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
17. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123;271-281. doi: 10.1002/cncy.21521
18. Schnatz PF, Guile M, O’Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701-708. doi: 10.1097/01.AOG.0000202401.29145.68
19. Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383-389. doi: 10.1016/j.ygyno.2009.05.019
20. Zazove P, Reed BD, Gregoire L, et al. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708-2713. doi: 10.1128/JCM.36.9.2708-2713.1998
21. Richardson LA, El-Zein M, Ramankumar AV, et al; . HPV DNA testing with cytology triage in cervical cancer screening: influence of revealing HPV infection status. Cancer Cytopathol. 2015:123:745-754. doi: 10.1002/cncy.21596
22. Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016;76:S49-S55. doi: 10.1016/j.jcv.2015.11.015
23. WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: World Health Organization; 2011. Accessed November 14, 2021. www.ncbi.nlm.nih.gov/books/NBK138476/pdf/Bookshelf_NBK138476.pdf
24. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007:45:93-106. doi: 10.1016/j.ypmed.2007.06.007
25. Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000-2009. Cancer. 2014:120:2032-2038. doi: 10.1002/cncr.28548
26. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654
27. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cervical cancer. Accessed June 15, 2021. www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
28. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. doi: 10.1016/S0140-6736(17)31607-0
29. Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266-272. doi: 10.1016/j.ygyno.2014.08.036
30. Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975 to 2003. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2003/#citation
31. Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-5236. doi: 10.1002/cam4.1501
32. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88-102. doi: 10.1016/j.canlet.2019.11.039
The World Health Organization estimates that, in 2020, worldwide, there were 604,000 new cases of uterine cervical cancer and approximately 342,000 deaths, 84% of which occurred in developing countries.1 In the United States, as of 2018, the lifetime risk of death from cervical cancer was 2.2 for every 100,000
In this article, we summarize recent updates in the epidemiology, prevention, and treatment of cervical cancer. We emphasize recent information of value to family physicians, including updates in clinical guidelines and other pertinent national recommendations.
Spotlight continues to shine on HPV
It has been known for several decades that cervical cancer is caused by human papillomavirus (HPV). Of more than 100 known HPV types, 14 or 15 are classified as carcinogenic. HPV 16 is the most common oncogenic type, causing more than 60% of cases of cervical cancer3,4
HPV is the most common sexually transmitted infection, with as many as 80% of sexually active people becoming infected during their lifetime, generally before 50 years of age.5 HPV also causes other anogenital and oropharyngeal cancers; however, worldwide, more than 80% of HPV-associated cancers are cervical.6 Risk factors for cervical cancer are listed in TABLE 1.7 Cervical cancer is less common when partners are circumcised.7

Most cases of HPV infection clear in 1 or 2 years
At least 70% of cervical cancers are squamous cell carcinoma (SCC); 20% to 25% are adenocarcinoma (ADC); and < 3% to 5% are adenosquamous carcinoma.10 Almost 100% of cervical SCCs are HPV+, as are 86% of cervical ADCs. The most common reason for HPV-negative status in patients with cervical cancer is false-negative testing because of inadequate methods.
Primary prevention through vaccination
HPV vaccination was introduced in 2006 in the United States for girls,a and for boysa in 2011. The primary reason for vaccinating boys is to reduce the rates of HPV-related anal and oropharyngeal cancer. The only available HPV vaccine in the United States is Gardasil 9 (9-valent vaccine, recombinant; Merck), which provides coverage for 7 high-risk HPV types that account for approximately 90% of cervical cancers and 2 types (6 and 11) that are the principal causes of condylomata acuminata (genital warts). Future generations of prophylactic vaccines are expected to cover additional strains.
Continue to: Vaccine studies...
Vaccine studies have been summarized in a Cochrane review,11 showing that vaccination is highly effective for prevention of cervical dysplasia, especially when given to young girls and womena previously unexposed to the virus. It has not been fully established how long protection lasts, but vaccination appears to be 70% to 90% effective for ≥ 10 years.
Dosing schedule. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends a 2-dose schedule 6 to 15 months apart, for both girls and boys between 9 and 14 years of age.12 A third dose is indicated if the first and second doses were given less than 5 months apart, or the person is older than 15 years or is immunocompromised. No recommendation has been made for revaccination after the primary series.
In 2018, the US Food and Drug Administration approved Gardasil 9 for adults 27 to 45 years of age. In June 2019, ACIP recommended vaccination for mena as old as 26 years, and adopted a recommendation that unvaccinated men and women between 27 and 45 years discuss HPV vaccination with their physician.13
The adolescent HPV vaccination rate varies by state; however, all states lag behind the CDC’s Healthy People 2020 goal of 80%.14 Barriers to vaccination include cost, infrastructure limitations, and social stigma.
Secondary prevention: Screening and Tx of precancerous lesions
Cervical cancer screening identifies patients at increased risk of cervical cancer and reassures the great majority of them that their risk of cervical cancer is very low. There are 3 general approaches to cervical cancer screening:
- cytology-based screening, which has been implemented for decades in many countries
- primary testing for DNA or RNA markers of high-risk HPV types
- co-testing with cytology-based screening plus HPV testing.
Continue to: USPSTF guidance
USPSTF guidance. Recommendations of the US Preventive Services Task Force (USPSTF) for cervical cancer screening were updated in 2018 (TABLE 215). The recommendations state that high-risk HPV screening alone is a strategy that is amenable to patient self-sampling and self-mailing for processing—a protocol that has the potential to improve access

ASCCP guidance. The American Society of Colposcopy and Cervical Pathology (ASCCP) makes nearly the same recommendations for cervical cancer screening. An exception is that ASCCP guidelines allow for the possibility of screening using primary high-risk HPV testing for patients starting at 25 years of age.16
Screening programs that can be initiated at a later age and longer intervals should be possible once the adolescent vaccination rate is optimized and vaccination registries are widely implemented.
Cervical cytology protocol
Cervical cytologic abnormalities are reported using the Bethesda system. Specimen adequacy is the most important component of quality assurance,17 and is determined primarily by sufficient cellularity. However, any specimen containing abnormal squamous cells of undetermined significance (ASCUS) or atypical glandular cells (AGCs) is considered satisfactory, regardless of the number of cells. Obscuring factors that impair quality include excessive blood; inflammation; air-drying artifact; and an interfering substance, such as lubricant. The presence of reactive changes resulting from inflammation does not require further evaluation unless the patient is immunosuppressed.
Abnormalities are most often of squamous cells, of 2 categories: low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs). HSILs are more likely to be associated with persistent HPV infection and higher risk of progression to cervical cancer.
Continue to: Cytologic findings...
Cytologic findings can be associated with histologic findings that are sometimes more, sometimes less, severe. LSIL cytology specimens that contain a few cells that are suspicious for HSIL, but that do not contain enough cells to be diagnostic, are reported as atypical squamous cells, and do not exclude a high-grade intraepithelial lesion.
Glandular-cell abnormalities usually originate from the glandular epithelium of the endocervix or the endometrium—most often, AGCs. Less frequent are AGCs, favor neoplasia; endocervical adenocarcinoma in situ; and ADC. Rarely, AGCs are associated with adenosquamous carcinoma. Endometrial polyps are a typical benign pathology that can be associated with AGCs.
In about 30% of cases, AGCs are associated with premalignant or malignant disease.18 The risk of malignancy in patients with AGCs increases with age, from < 2% among patients younger than 40 years to approximately 15% among those > 50 years.19 Endometrial malignancy is more common than cervical malignancy among patients > 40 years.
AGC cytology requires endocervical curettage, plus endometrial sampling for patients ≥ 35 years
Cytology-based screening has limitations. Sensitivity is relatively low and dependent on the expertise of the cytologist, although regular repeat testing has been used to overcome this limitation. A substantial subset of results are reported as equivocal—ie, ASCUS.
Continue to: Primary HPV screening
Primary HPV screening
Primary HPV testing was approved by the US Food and Drug Administration in 2015 and recommended as an appropriate screening option by professional societies
In contrast to cytology-based screening, HPV testing has high sensitivity (≥ 90%); the population-based negative likelihood ratio is near zero.20 This degree of sensitivity allows for extended screening intervals. However, primary HPV testing lacks specificity for persistent infection and high-grade or invasive lesions, which approximately doubles the number of patients who screen positive. The potential for excess patients to be referred for colposcopy led to the need for secondary triage.
Instituting secondary triage. Cytology is, currently, the primary method of secondary triage, reducing the number of referrals for colposcopy by nearly one-half, compared to referrals for all high-risk HPV results, and with better overall accuracy over cytology with high-risk HPV triage.21 When cytology shows ASCUS, or worse, refer the patient for colposcopy; alternatively, if so-called reflex testing for HPV types 16 and 18 is available and positive, direct referral to colposcopy without cytology is also appropriate.
In the future, secondary triage for cytology is likely to be replaced with improved technologies, such as immunostaining of the specimen for biomarkers associated with cervical precancer or cancer, or for viral genome methylation testing.22

Management of abnormal cervical cancer screening results
Routine screening applies to asymptomatic patients who do not require surveillance because they have not had prior abnormal screening results. In 2020, ASCCP published risk-based management consensus guidelines that were developed for abnormal cervical cancer screening tests and for cancer precursors.16 Guiding principles, and screening situations in which the guidelines can be applied, are summarized in TABLE 3
Continue to: ASCCP guidelines...
ASCCP guidelines provide a framework to incorporate new data and technologies without major revision
Some noteworthy scenarios in ASCCP risk-based management are:
- For unsatisfactory cytology with a negative HPV test or no HPV test, repeat age-based screening in 2 to 4 months. (Note: A negative HPV test might reflect an inadequate specimen; do not interpret this result as a true negative.)
- An absent transformation zone (ie, between glandular and squamous cervical cells) with an otherwise adequate specimen should be interpreted as satisfactory for screening in patients 21 to 29 years of age. For those ≥ 30 years and with no HPV testing in this circumstance, HPV testing is preferred; repeating cytology, in 3 years, is also acceptable.
- After a finding of LSIL/CIN1 without evidence of a high-grade abnormality, and after 2 negative annual screenings (including HPV testing), a return to 3-year (not 5-year) screening is recommended.
- A cytology result of an HSIL carries a risk of 26% for CIN3+, in which case colposcopy is recommended, regardless of HPV test results.
- For long-term management after treatment for CIN2+, continue surveillance testing every 3 years after 3 consecutive negative HPV tests or cytology findings, for at least 25 years. If the 25-year threshold is reached before 65 years of age, continuing surveillance every 3 years is optional, as long as the patient is in good health (ie, life expectancy ≥ 10 years).
- After hysterectomy for a high-grade abnormality, annual vaginal HPV testing is recommended until 3 negative tests are returned; after that, surveillance shifts to a 3-year interval until the 25-year threshold.
Treatment of cancer precursors
Treatment for cervical dysplasia is excisional or ablative.
Excisional therapy. In most cases, excisional therapy (either a loop electrosurgical excision procedure [LEEP; also known as large loop excision of the transformation zone, cold knife conization, and laser conization] or cone biopsy) is required, or preferred. Excisional treatment has the advantage of providing a diagnostic specimen.
The World Health Organization recommends LEEP over ablation in settings in which LEEP is available.23 ASCCP states that, in the relatively few cases in which treatment is needed and it is for CIN1, either excision or ablation is acceptable. TABLE 416 lists situations in which excisional treatment is required because a diagnostic specimen is needed.

Continue to: Ablative treatments
Ablative treatments are cryotherapy, CO2 laser ablation, and thermal ablation. Ablative therapy has the advantage of presenting less risk of adverse obstetric outcomes (eg, preterm birth); it can be used if the indication for therapy is:
- CIN1 or CIN2 and HPV type 16 or 18 positivity
- concordant cytology and histology
- satisfactory colposcopy
- negative endocervical curettage.
The most common ablative treatment is liquid nitrogen applied to a metal tip under local anesthesia.
Hysterectomy can be considered for patients with recurrent CIN2+ who have completed childbearing or for whom repeat excision is infeasible (eg, scarring or a short cervix), or both.
Cost, availability, and convenience might play a role in decision-making with regard to the treatment choice for cancer precursors.
Is care after treatment called for? Patients who continue to be at increased risk of (and thus mortality from) cervical and vaginal cancer require enhanced surveillance. The risk of cancer is more than triple for patients who were given their diagnosis, and treated, when they were > 60 years, compared to patients treated in their 30s.1 The excess period of risk covers at least 25 years after treatment, even among patients who have had 3 posttreatment screenings.
Continue to: Persistent HPV positivity...
Persistent HPV positivity is more challenging. Patients infected with HPV type 16 have an increased risk of residual disease.
Cancer management
Invasive cancer. Most cervical cancers (60%) occur among patients who have not been screened during the 5 years before their diagnosis.24 For patients who have a diagnosis of cancer, those detected through screening have a much better prognosis than those identified by symptoms (mean cure rate, 92% and 66%, respectively).25 The median 5-year survival for patients who were not screened during the 5 years before their diagnosis of cervical cancer is 66%.2
In unscreened patients, cervical cancer usually manifests as abnormal vaginal bleeding, especially postcoitally. In approximately 45% of cases, the patient has localized disease at diagnosis; in 36%, regional disease; and in 15%, distant metastases.26
For cancers marked by stromal invasion < 3 mm, appropriate treatment is cone biopsy or simple hysterectomy.27
Most patients with early-stage cervical cancer undergo modified radical hysterectomy. The ovaries are usually conserved, unless the cancer is adenocarcinoma. Sentinel-node dissection has become standard practice. Primary radiation therapy is most often used for patients who are a poor surgical candidate because of medical comorbidity or poor functional status. Antiangiogenic agents (eg, bevacizumab) can be used as adjuvant palliative therapy for advanced and recurrent disease
Continue to: After treatment for...
After treatment for invasive cervical cancer, the goal is early detection of recurrence, although there is no consensus on a protocol. Most recurrences are detected within the first 2 years.
Long-term sequelae after treatment for advanced cancer are considerable. Patients report significantly lower quality of life,
Hormone replacement therapy is generally considered acceptable after treatment of cervical cancer because it does not increase replication of HPV.
Recurrent or metastatic cancer. Recurrence or metastases will develop in 15% to 60% of patients,30 usually within the first 2 years after treatment.
Management depends on location and extent of disease, using mainly radiation therapy or surgical resection. Recurrence or metastasis is usually incurable.
Continue to: Last, there are promising...
Last, there are promising areas of research for more effective treatment for cervical cancer precursors and cancers, including gene editing tools31 and therapeutic
Prospects for better cervical cancer care
Prevention. HPV vaccination is likely to have a large impact on population-based risk of both cancer and cancer precursors in the next generation.
Screening in the foreseeable future will gravitate toward reliance on primary HPV screening, with a self-sampling option.
Surveillance after dysplastic disease. The 2019 ASCCP guidelines for surveillance and intervention decisions after abnormal cancer screening results will evolve to incorporate introduction of new technology into computerized algorithms.
Treatment. New biologic therapies, including monoclonal antibodies and therapeutic vaccines against HPV, will likely be introduced for treating cancer precursors and invasive cancer.
A NOTE FROM THE EDITORS The Editors of The Journal of Family Practice recognize the importance of addressing the reproductive health of gender-diverse individuals. In this article, we use the words “women,” “men,” “girls,” and “boys” in limited circumstances (1) for ease of reading and (2) to reflect the official language of the US Food and Drug Administration and the Advisory Committee on Immunization Practices. The reader should consider the information and guidance offered in this discussion of cervical cancer and other human papillomavirus-related cancers to speak to the care of people with a uterine cervix and people with a penis.
CORRESPONDENCE
Linda Speer, MD, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; [email protected]
The World Health Organization estimates that, in 2020, worldwide, there were 604,000 new cases of uterine cervical cancer and approximately 342,000 deaths, 84% of which occurred in developing countries.1 In the United States, as of 2018, the lifetime risk of death from cervical cancer was 2.2 for every 100,000
In this article, we summarize recent updates in the epidemiology, prevention, and treatment of cervical cancer. We emphasize recent information of value to family physicians, including updates in clinical guidelines and other pertinent national recommendations.
Spotlight continues to shine on HPV
It has been known for several decades that cervical cancer is caused by human papillomavirus (HPV). Of more than 100 known HPV types, 14 or 15 are classified as carcinogenic. HPV 16 is the most common oncogenic type, causing more than 60% of cases of cervical cancer3,4
HPV is the most common sexually transmitted infection, with as many as 80% of sexually active people becoming infected during their lifetime, generally before 50 years of age.5 HPV also causes other anogenital and oropharyngeal cancers; however, worldwide, more than 80% of HPV-associated cancers are cervical.6 Risk factors for cervical cancer are listed in TABLE 1.7 Cervical cancer is less common when partners are circumcised.7

Most cases of HPV infection clear in 1 or 2 years
At least 70% of cervical cancers are squamous cell carcinoma (SCC); 20% to 25% are adenocarcinoma (ADC); and < 3% to 5% are adenosquamous carcinoma.10 Almost 100% of cervical SCCs are HPV+, as are 86% of cervical ADCs. The most common reason for HPV-negative status in patients with cervical cancer is false-negative testing because of inadequate methods.
Primary prevention through vaccination
HPV vaccination was introduced in 2006 in the United States for girls,a and for boysa in 2011. The primary reason for vaccinating boys is to reduce the rates of HPV-related anal and oropharyngeal cancer. The only available HPV vaccine in the United States is Gardasil 9 (9-valent vaccine, recombinant; Merck), which provides coverage for 7 high-risk HPV types that account for approximately 90% of cervical cancers and 2 types (6 and 11) that are the principal causes of condylomata acuminata (genital warts). Future generations of prophylactic vaccines are expected to cover additional strains.
Continue to: Vaccine studies...
Vaccine studies have been summarized in a Cochrane review,11 showing that vaccination is highly effective for prevention of cervical dysplasia, especially when given to young girls and womena previously unexposed to the virus. It has not been fully established how long protection lasts, but vaccination appears to be 70% to 90% effective for ≥ 10 years.
Dosing schedule. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends a 2-dose schedule 6 to 15 months apart, for both girls and boys between 9 and 14 years of age.12 A third dose is indicated if the first and second doses were given less than 5 months apart, or the person is older than 15 years or is immunocompromised. No recommendation has been made for revaccination after the primary series.
In 2018, the US Food and Drug Administration approved Gardasil 9 for adults 27 to 45 years of age. In June 2019, ACIP recommended vaccination for mena as old as 26 years, and adopted a recommendation that unvaccinated men and women between 27 and 45 years discuss HPV vaccination with their physician.13
The adolescent HPV vaccination rate varies by state; however, all states lag behind the CDC’s Healthy People 2020 goal of 80%.14 Barriers to vaccination include cost, infrastructure limitations, and social stigma.
Secondary prevention: Screening and Tx of precancerous lesions
Cervical cancer screening identifies patients at increased risk of cervical cancer and reassures the great majority of them that their risk of cervical cancer is very low. There are 3 general approaches to cervical cancer screening:
- cytology-based screening, which has been implemented for decades in many countries
- primary testing for DNA or RNA markers of high-risk HPV types
- co-testing with cytology-based screening plus HPV testing.
Continue to: USPSTF guidance
USPSTF guidance. Recommendations of the US Preventive Services Task Force (USPSTF) for cervical cancer screening were updated in 2018 (TABLE 215). The recommendations state that high-risk HPV screening alone is a strategy that is amenable to patient self-sampling and self-mailing for processing—a protocol that has the potential to improve access

ASCCP guidance. The American Society of Colposcopy and Cervical Pathology (ASCCP) makes nearly the same recommendations for cervical cancer screening. An exception is that ASCCP guidelines allow for the possibility of screening using primary high-risk HPV testing for patients starting at 25 years of age.16
Screening programs that can be initiated at a later age and longer intervals should be possible once the adolescent vaccination rate is optimized and vaccination registries are widely implemented.
Cervical cytology protocol
Cervical cytologic abnormalities are reported using the Bethesda system. Specimen adequacy is the most important component of quality assurance,17 and is determined primarily by sufficient cellularity. However, any specimen containing abnormal squamous cells of undetermined significance (ASCUS) or atypical glandular cells (AGCs) is considered satisfactory, regardless of the number of cells. Obscuring factors that impair quality include excessive blood; inflammation; air-drying artifact; and an interfering substance, such as lubricant. The presence of reactive changes resulting from inflammation does not require further evaluation unless the patient is immunosuppressed.
Abnormalities are most often of squamous cells, of 2 categories: low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs). HSILs are more likely to be associated with persistent HPV infection and higher risk of progression to cervical cancer.
Continue to: Cytologic findings...
Cytologic findings can be associated with histologic findings that are sometimes more, sometimes less, severe. LSIL cytology specimens that contain a few cells that are suspicious for HSIL, but that do not contain enough cells to be diagnostic, are reported as atypical squamous cells, and do not exclude a high-grade intraepithelial lesion.
Glandular-cell abnormalities usually originate from the glandular epithelium of the endocervix or the endometrium—most often, AGCs. Less frequent are AGCs, favor neoplasia; endocervical adenocarcinoma in situ; and ADC. Rarely, AGCs are associated with adenosquamous carcinoma. Endometrial polyps are a typical benign pathology that can be associated with AGCs.
In about 30% of cases, AGCs are associated with premalignant or malignant disease.18 The risk of malignancy in patients with AGCs increases with age, from < 2% among patients younger than 40 years to approximately 15% among those > 50 years.19 Endometrial malignancy is more common than cervical malignancy among patients > 40 years.
AGC cytology requires endocervical curettage, plus endometrial sampling for patients ≥ 35 years
Cytology-based screening has limitations. Sensitivity is relatively low and dependent on the expertise of the cytologist, although regular repeat testing has been used to overcome this limitation. A substantial subset of results are reported as equivocal—ie, ASCUS.
Continue to: Primary HPV screening
Primary HPV screening
Primary HPV testing was approved by the US Food and Drug Administration in 2015 and recommended as an appropriate screening option by professional societies
In contrast to cytology-based screening, HPV testing has high sensitivity (≥ 90%); the population-based negative likelihood ratio is near zero.20 This degree of sensitivity allows for extended screening intervals. However, primary HPV testing lacks specificity for persistent infection and high-grade or invasive lesions, which approximately doubles the number of patients who screen positive. The potential for excess patients to be referred for colposcopy led to the need for secondary triage.
Instituting secondary triage. Cytology is, currently, the primary method of secondary triage, reducing the number of referrals for colposcopy by nearly one-half, compared to referrals for all high-risk HPV results, and with better overall accuracy over cytology with high-risk HPV triage.21 When cytology shows ASCUS, or worse, refer the patient for colposcopy; alternatively, if so-called reflex testing for HPV types 16 and 18 is available and positive, direct referral to colposcopy without cytology is also appropriate.
In the future, secondary triage for cytology is likely to be replaced with improved technologies, such as immunostaining of the specimen for biomarkers associated with cervical precancer or cancer, or for viral genome methylation testing.22

Management of abnormal cervical cancer screening results
Routine screening applies to asymptomatic patients who do not require surveillance because they have not had prior abnormal screening results. In 2020, ASCCP published risk-based management consensus guidelines that were developed for abnormal cervical cancer screening tests and for cancer precursors.16 Guiding principles, and screening situations in which the guidelines can be applied, are summarized in TABLE 3
Continue to: ASCCP guidelines...
ASCCP guidelines provide a framework to incorporate new data and technologies without major revision
Some noteworthy scenarios in ASCCP risk-based management are:
- For unsatisfactory cytology with a negative HPV test or no HPV test, repeat age-based screening in 2 to 4 months. (Note: A negative HPV test might reflect an inadequate specimen; do not interpret this result as a true negative.)
- An absent transformation zone (ie, between glandular and squamous cervical cells) with an otherwise adequate specimen should be interpreted as satisfactory for screening in patients 21 to 29 years of age. For those ≥ 30 years and with no HPV testing in this circumstance, HPV testing is preferred; repeating cytology, in 3 years, is also acceptable.
- After a finding of LSIL/CIN1 without evidence of a high-grade abnormality, and after 2 negative annual screenings (including HPV testing), a return to 3-year (not 5-year) screening is recommended.
- A cytology result of an HSIL carries a risk of 26% for CIN3+, in which case colposcopy is recommended, regardless of HPV test results.
- For long-term management after treatment for CIN2+, continue surveillance testing every 3 years after 3 consecutive negative HPV tests or cytology findings, for at least 25 years. If the 25-year threshold is reached before 65 years of age, continuing surveillance every 3 years is optional, as long as the patient is in good health (ie, life expectancy ≥ 10 years).
- After hysterectomy for a high-grade abnormality, annual vaginal HPV testing is recommended until 3 negative tests are returned; after that, surveillance shifts to a 3-year interval until the 25-year threshold.
Treatment of cancer precursors
Treatment for cervical dysplasia is excisional or ablative.
Excisional therapy. In most cases, excisional therapy (either a loop electrosurgical excision procedure [LEEP; also known as large loop excision of the transformation zone, cold knife conization, and laser conization] or cone biopsy) is required, or preferred. Excisional treatment has the advantage of providing a diagnostic specimen.
The World Health Organization recommends LEEP over ablation in settings in which LEEP is available.23 ASCCP states that, in the relatively few cases in which treatment is needed and it is for CIN1, either excision or ablation is acceptable. TABLE 416 lists situations in which excisional treatment is required because a diagnostic specimen is needed.

Continue to: Ablative treatments
Ablative treatments are cryotherapy, CO2 laser ablation, and thermal ablation. Ablative therapy has the advantage of presenting less risk of adverse obstetric outcomes (eg, preterm birth); it can be used if the indication for therapy is:
- CIN1 or CIN2 and HPV type 16 or 18 positivity
- concordant cytology and histology
- satisfactory colposcopy
- negative endocervical curettage.
The most common ablative treatment is liquid nitrogen applied to a metal tip under local anesthesia.
Hysterectomy can be considered for patients with recurrent CIN2+ who have completed childbearing or for whom repeat excision is infeasible (eg, scarring or a short cervix), or both.
Cost, availability, and convenience might play a role in decision-making with regard to the treatment choice for cancer precursors.
Is care after treatment called for? Patients who continue to be at increased risk of (and thus mortality from) cervical and vaginal cancer require enhanced surveillance. The risk of cancer is more than triple for patients who were given their diagnosis, and treated, when they were > 60 years, compared to patients treated in their 30s.1 The excess period of risk covers at least 25 years after treatment, even among patients who have had 3 posttreatment screenings.
Continue to: Persistent HPV positivity...
Persistent HPV positivity is more challenging. Patients infected with HPV type 16 have an increased risk of residual disease.
Cancer management
Invasive cancer. Most cervical cancers (60%) occur among patients who have not been screened during the 5 years before their diagnosis.24 For patients who have a diagnosis of cancer, those detected through screening have a much better prognosis than those identified by symptoms (mean cure rate, 92% and 66%, respectively).25 The median 5-year survival for patients who were not screened during the 5 years before their diagnosis of cervical cancer is 66%.2
In unscreened patients, cervical cancer usually manifests as abnormal vaginal bleeding, especially postcoitally. In approximately 45% of cases, the patient has localized disease at diagnosis; in 36%, regional disease; and in 15%, distant metastases.26
For cancers marked by stromal invasion < 3 mm, appropriate treatment is cone biopsy or simple hysterectomy.27
Most patients with early-stage cervical cancer undergo modified radical hysterectomy. The ovaries are usually conserved, unless the cancer is adenocarcinoma. Sentinel-node dissection has become standard practice. Primary radiation therapy is most often used for patients who are a poor surgical candidate because of medical comorbidity or poor functional status. Antiangiogenic agents (eg, bevacizumab) can be used as adjuvant palliative therapy for advanced and recurrent disease
Continue to: After treatment for...
After treatment for invasive cervical cancer, the goal is early detection of recurrence, although there is no consensus on a protocol. Most recurrences are detected within the first 2 years.
Long-term sequelae after treatment for advanced cancer are considerable. Patients report significantly lower quality of life,
Hormone replacement therapy is generally considered acceptable after treatment of cervical cancer because it does not increase replication of HPV.
Recurrent or metastatic cancer. Recurrence or metastases will develop in 15% to 60% of patients,30 usually within the first 2 years after treatment.
Management depends on location and extent of disease, using mainly radiation therapy or surgical resection. Recurrence or metastasis is usually incurable.
Continue to: Last, there are promising...
Last, there are promising areas of research for more effective treatment for cervical cancer precursors and cancers, including gene editing tools31 and therapeutic
Prospects for better cervical cancer care
Prevention. HPV vaccination is likely to have a large impact on population-based risk of both cancer and cancer precursors in the next generation.
Screening in the foreseeable future will gravitate toward reliance on primary HPV screening, with a self-sampling option.
Surveillance after dysplastic disease. The 2019 ASCCP guidelines for surveillance and intervention decisions after abnormal cancer screening results will evolve to incorporate introduction of new technology into computerized algorithms.
Treatment. New biologic therapies, including monoclonal antibodies and therapeutic vaccines against HPV, will likely be introduced for treating cancer precursors and invasive cancer.
A NOTE FROM THE EDITORS The Editors of The Journal of Family Practice recognize the importance of addressing the reproductive health of gender-diverse individuals. In this article, we use the words “women,” “men,” “girls,” and “boys” in limited circumstances (1) for ease of reading and (2) to reflect the official language of the US Food and Drug Administration and the Advisory Committee on Immunization Practices. The reader should consider the information and guidance offered in this discussion of cervical cancer and other human papillomavirus-related cancers to speak to the care of people with a uterine cervix and people with a penis.
CORRESPONDENCE
Linda Speer, MD, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; [email protected]
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660
2. Cancer stat facts: cervical cancer. National Cancer Institute Surveillance, Epidemiology, and End Results [SEER] Program. Accessed November 14, 2021. https://seer.cancer.gov/statfacts/html/cervix.html
3. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349-2359. doi: 10.1002/ijc.27485
4. Winer RL, Hughes JP, Feng Q, et al. Early history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699-707. doi: 10.1158/1055-9965.EPI-10-1108
5. Chesson HW, Dunne EF, Hariri F, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-664. doi: 10.1097/OLQ.0000000000000193
6. Human papillomavirus (HPV) and cervical cancer. Fact sheet. Geneva, Switzerland: World Health Organization; November 11, 2020. Accessed November 14, 2021. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
7. International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120:885-891. doi: 10.1002/ijc.22357
8. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical cancer neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008:9:425-434. doi: 10.1016/S1470-2045(08)70103-7
9. de Sanjose S, Quint WG, Alemany I, et al; . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective, cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. doi: 10.1016/S1470-2045(10)70230-8
10. Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review 1975-2004. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2004/#citation
11. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3
12. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016:65;1405-1408. doi: 10.15585/mmwr.mm6549a5
13. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
14. State-level data: Female adolescents receiving 2 or 3 doses of HPV vaccine by age 13-15 years (percent). HealthyPeople.gov. Accessed November 14, 2021. www.healthypeople.gov/2020/data/map/4657?year=2018
15. United States Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674-686. doi: 10.1001/jama.2018.10897
16. Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
17. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123;271-281. doi: 10.1002/cncy.21521
18. Schnatz PF, Guile M, O’Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701-708. doi: 10.1097/01.AOG.0000202401.29145.68
19. Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383-389. doi: 10.1016/j.ygyno.2009.05.019
20. Zazove P, Reed BD, Gregoire L, et al. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708-2713. doi: 10.1128/JCM.36.9.2708-2713.1998
21. Richardson LA, El-Zein M, Ramankumar AV, et al; . HPV DNA testing with cytology triage in cervical cancer screening: influence of revealing HPV infection status. Cancer Cytopathol. 2015:123:745-754. doi: 10.1002/cncy.21596
22. Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016;76:S49-S55. doi: 10.1016/j.jcv.2015.11.015
23. WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: World Health Organization; 2011. Accessed November 14, 2021. www.ncbi.nlm.nih.gov/books/NBK138476/pdf/Bookshelf_NBK138476.pdf
24. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007:45:93-106. doi: 10.1016/j.ypmed.2007.06.007
25. Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000-2009. Cancer. 2014:120:2032-2038. doi: 10.1002/cncr.28548
26. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654
27. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cervical cancer. Accessed June 15, 2021. www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
28. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. doi: 10.1016/S0140-6736(17)31607-0
29. Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266-272. doi: 10.1016/j.ygyno.2014.08.036
30. Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975 to 2003. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2003/#citation
31. Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-5236. doi: 10.1002/cam4.1501
32. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88-102. doi: 10.1016/j.canlet.2019.11.039
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660
2. Cancer stat facts: cervical cancer. National Cancer Institute Surveillance, Epidemiology, and End Results [SEER] Program. Accessed November 14, 2021. https://seer.cancer.gov/statfacts/html/cervix.html
3. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349-2359. doi: 10.1002/ijc.27485
4. Winer RL, Hughes JP, Feng Q, et al. Early history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699-707. doi: 10.1158/1055-9965.EPI-10-1108
5. Chesson HW, Dunne EF, Hariri F, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-664. doi: 10.1097/OLQ.0000000000000193
6. Human papillomavirus (HPV) and cervical cancer. Fact sheet. Geneva, Switzerland: World Health Organization; November 11, 2020. Accessed November 14, 2021. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
7. International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120:885-891. doi: 10.1002/ijc.22357
8. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical cancer neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008:9:425-434. doi: 10.1016/S1470-2045(08)70103-7
9. de Sanjose S, Quint WG, Alemany I, et al; . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective, cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. doi: 10.1016/S1470-2045(10)70230-8
10. Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review 1975-2004. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2004/#citation
11. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3
12. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016:65;1405-1408. doi: 10.15585/mmwr.mm6549a5
13. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
14. State-level data: Female adolescents receiving 2 or 3 doses of HPV vaccine by age 13-15 years (percent). HealthyPeople.gov. Accessed November 14, 2021. www.healthypeople.gov/2020/data/map/4657?year=2018
15. United States Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674-686. doi: 10.1001/jama.2018.10897
16. Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
17. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123;271-281. doi: 10.1002/cncy.21521
18. Schnatz PF, Guile M, O’Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701-708. doi: 10.1097/01.AOG.0000202401.29145.68
19. Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383-389. doi: 10.1016/j.ygyno.2009.05.019
20. Zazove P, Reed BD, Gregoire L, et al. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708-2713. doi: 10.1128/JCM.36.9.2708-2713.1998
21. Richardson LA, El-Zein M, Ramankumar AV, et al; . HPV DNA testing with cytology triage in cervical cancer screening: influence of revealing HPV infection status. Cancer Cytopathol. 2015:123:745-754. doi: 10.1002/cncy.21596
22. Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016;76:S49-S55. doi: 10.1016/j.jcv.2015.11.015
23. WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: World Health Organization; 2011. Accessed November 14, 2021. www.ncbi.nlm.nih.gov/books/NBK138476/pdf/Bookshelf_NBK138476.pdf
24. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007:45:93-106. doi: 10.1016/j.ypmed.2007.06.007
25. Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000-2009. Cancer. 2014:120:2032-2038. doi: 10.1002/cncr.28548
26. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654
27. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cervical cancer. Accessed June 15, 2021. www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
28. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. doi: 10.1016/S0140-6736(17)31607-0
29. Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266-272. doi: 10.1016/j.ygyno.2014.08.036
30. Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975 to 2003. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2003/#citation
31. Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-5236. doi: 10.1002/cam4.1501
32. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88-102. doi: 10.1016/j.canlet.2019.11.039
PRACTICE RECOMMENDATIONS
› Encourage eligible patients to be vaccinated against human papillomavirus (HPV) because the vaccine is highly effective for preventing cervical dysplasia, especially when given to patients previously unexposed to the virus. A
› Screen for cervical disease with either cytology plus HPV testing or primary HPV testing with secondary triage for cytology; both protocols are more accurate than screening with cervical cytology alone, and allow you to widen the screening interval. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Write an exercise Rx to improve patients' cardiorespiratory fitness
It is well-known that per capita health care spending in the United States is more than twice the average in other developed countries1; nevertheless, the overall health care ranking of the US is near the bottom compared to other countries in this group.2 Much of the reason for this poor relative showing lies in the fact that the US has employed a somewhat traditional fee-for-service health care model that does not incentivize efforts to promote health and wellness or prevent chronic disease. The paradigm of promoting physical activity for its disease-preventing and treatment benefits has not been well-integrated in the US health care system.
In this article, we endeavor to provide better understanding of the barriers that keep family physicians from routinely promoting physical activity in clinical practice; define tools and resources that can be used in the clinical setting to promote physical activity; and delineate areas for future work.
Glaring hole in US physical activity education
Many primary care physicians feel underprepared to prescribe or motivate patients to exercise. The reason for that lack of preparedness likely relates to a medical education system that does not spend time preparing physicians to perform this critical task. A study showed that, on average, medical schools require only 8 hours of physical activity education in their curriculum during the 4 years of schooling.3 Likewise, the average primary care residency program offers only 3 hours of didactic training on physical activity, nutrition, and obesity.4 The problem extends to sports medicine fellowship training, in which a 2019 survey showed that 63% of fellows were never taught how to write an exercise prescription in their training program.5
Without education on physical activity, medical students, residents, and fellows are woefully underprepared to realize the therapeutic value of physical activity in patient care, comprehend current physical activity guidelines, appropriately motivate patients to engage in exercise, and competently discuss exercise prescriptions in different disease states. Throughout their training, it is imperative for medical professionals to be educated on the social determinants of health, which include the conditions in which people live, work, and play. These environmental variables can contribute to health inequities that create additional barriers to improvement in physical fitness.6
National guidelines on physical activity
The 2018 National Physical Activity Guidelines detail recommendations for children, adolescents, adults, and special populations.7 The guidelines define physical activity as bodily movement produced by skeletal muscles that result in energy expenditure above resting baseline levels, and includes all types, intensities, and domains of activity. Exercise is a subset of physical activity characterized as planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health.
Highlights from the 2018 guidelines include7:
- Preschool-aged children (3 to 5 years of age) should be physically active throughout the day, with as much as 3 hours per day of physical activity of all intensities—light, moderate, and vigorous.
- Older children and adolescents (6 to 17 years) should accumulate 60 minutes per day of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening activities.
- Adults of all ages should achieve approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week, along with at least 2 days per week of muscle-strengthening activities. Other types of physical activity include flexibility, balance, bone-strengthening, and mind–body exercises.
3-step framework for enhancing physical activity counseling
Merely knowing that physical activity is healthy is not enough, during a patient encounter, to increase the level of physical activity. Therefore, it is imperative to learn and adopt a framework that has proved to yield successful outcomes. The Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework, which has predominantly been used to change patient behavior related to alcohol and substance use, is now being utilized by some providers to promote physical activity.8 We apply the SBIRT approach in this article, although research is lacking on its clinical utility and outcome measures.
Continue to: SBIRT
SBIRT: Screening
An office visit provides an opportunity to understand a patient’s level of physical activity. Often, understanding a patient’s baseline level of activity is only asked during a thorough social history, which might not be performed during patient encounters. As physical activity is the primary determinant of cardiorespiratory fitness (CRF), some health care systems have begun delineating physical activity levels as a vital sign to ensure that the assessment of physical activity is a standard part of every clinical encounter. At a minimum, this serves as a prompt and provides an opportunity to start a conversation around improving physical activity levels when guidelines are not being met.
The exercise vital sign. Assessment and documentation of physical activity in the electronic health record are not yet standardized; however, Kaiser Permanente health plans have implemented the exercise vital sign, or EVS, in its HealthConnect (Epic Systems) electronic health record. The EVS incorporates information about a patient’s:
- days per week of moderate-to-strenuous exercise (eg, a brisk walk)
- minutes per day, on average, of exercise at this level.
The physical activity vital sign. Intermountain Healthcare implemented the physical activity vital sign, or PAVS, in its iCentra (Cerner Corp.) electronic health record. The 3-question PAVS assessment asks:
- On average, how many days of the week do you perform physical activity or exercise?
- On average, how many total minutes of physical activity or exercise do you perform on those days?
- How would you describe the intensity of your physical activity or exercise: Light (ie, a casual walk)? Moderate (a brisk walk)? Or vigorous (jogging)?
PAVS includes a fourth data point: The physician–user documents whether the patient was counseled to start, increase, maintain, or modify physical activity or exercise.
EVS and the PAVS have demonstrated validity.9-11
Continue to: Cardiorespiratory fitness as a vital sign
Cardiorespiratory fitness as a vital sign. In 2016, the American Heart Association (AHA) asserted the importance of assessing CRF as a clinical vital sign.12 CRF is commonly expressed as maximal oxygen consumption (VO2max = O2 mL/kg/min) and measured through cardiopulmonary exercise testing (CPET), considered the gold standard by combining conventional graded exercise testing with ventilatory expired gas analysis. CPET is more objective and precise than equations estimating CRF that are derived from peak work rate. AHA recommended that efforts to improve CRF should become standard in clinical encounters, explaining that even a small increase in CRF (eg, 1 or 2 metabolic equivalentsa [METs]) is associated with a considerably (10% to 30%) lower rate of adverse cardiovascular events.12
De Souza de Silva and colleagues revealed an association between each 1-MET increase in CRF and per-person annual health care cost savings (adjusted for age and presence of cardiovascular disease) of $3272 (normal-weight patients), $4252 (overweight), and $6103 (obese).13 In its 2016 scientific statement on CRF as a vital sign, AHA listed several methods of estimating CRF and concluded that, although CPET involves a higher level of training, proficiency, equipment, and, therefore, cost, the independent and additive information obtained justifies its use in many patients.12
CASE
Mary Q, 68 years of age, presents for an annual well-woman examination. Body mass index is 32; resting heart rate (HR), 73 bpm; and blood pressure, 126/74 mm Hg. She reports being inactive, except for light walking every day with her dog around the neighborhood, which takes them approximately 15 minutes. She denies any history or signs and symptoms of cardiovascular, metabolic, or renal disease.
You consider 3 questions before taking next steps regarding increasing Ms. Q’s activity level:
- What is her PAVS?
- Does she need medical clearance before starting an exercise program?
- What would an evidence-based cardiovascular exercise prescription for Ms. Q look like?
SBIRT: Brief intervention
When a patient does not meet the recommended level of physical activity, you have an opportunity to deliver a brief intervention. To do this effectively, you must have adequate understanding of the patient’s receptivity for change. The transtheoretical, or Stages of Change, model proposes that a person typically goes through 5 stages of growth—pre-contemplation, contemplation, preparation, action, and maintenance—in the process of lifestyle modification. This model highlights the different approaches to exercise adoption and maintenance that need to be taken, based on a given patient’s stage at the moment.
Continue to: Using this framework...
Using this framework, you can help patients realize intrinsic motivation that can facilitate progression through each stage, utilizing techniques such as motivational interviewing—so-called change talk—to increase self-efficacy.14TABLE 115 provides examples of motivational interviewing techniques that can be used during a patient encounter to improve health behaviors, such as physical activity.
Writing the exercise prescription
A patient who wants to increase their level of physical activity should be offered a formal exercise prescription, which has been shown to increase the level of physical activity, particularly in older patients. In fact, a study conducted in Spain in the practices of family physicians found that older patients who received a physical activity prescription increased their activity by 131 minutes per week; and compared to control patients, they doubled the minutes per week devoted to moderate or vigorous physical activity.16
FITT-VP. The basics of a cardiovascular exercise prescription can be found in the FITT-VP (Frequency, Intensity, Time, Type, Volume, and [monitoring of] Progression) framework (TABLE 217-19). For most patients, this model includes 3 to 5 days per week of moderate-to-vigorous physical activity for 30 to 60 minutes per session. For patients with established chronic disease, physical activity provides health benefits but might require modification. Disease-specific patient handouts for exercise can be downloaded, at no cost, through the American College of Sports Medicine (ACSM) “Exercise Is Medicine” program, which can be found at: www.exerciseismedicine.org/support_page.php/rx-for-health-series.
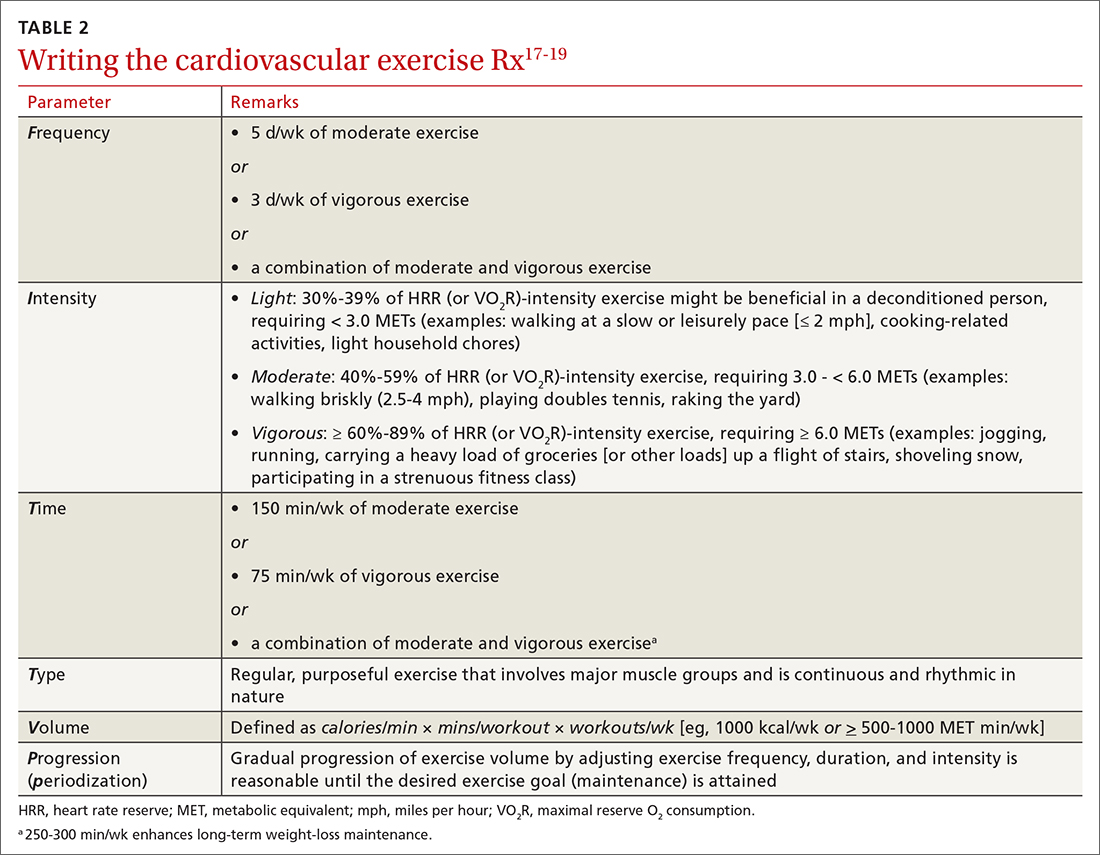
Determining intensity level. Although CPET is the gold standard for determining a patient’s target intensity level, such a test might be impracticable for a given patient. Surrogate markers of target intensity level can be obtained by measuring maximum HR (HRmax), using a well-known equation20:
HRmax = 220 – age
which is then multiplied by intensity range:
- light: 30%-39%
- moderate: 40%-59%
- vigorous: 60%-89%
or, more preferably, by calculating the HR training zone while accounting for HR at rest (HRrest). This is accomplished by calculating the HR reserve (HRR) (ie, HRR = HRmax – HRrest) and then calculating the target heart rate (THR)21:
THR = [HRR × %intensity] + HRrest
Continue to: The THR calculation...
The THR calculation is performed twice, once with a lower %intensity and again with a higher %intensity to develop a training zone based on HRR.
The HRR equation is more accurate than calculating HRmax from 220 – age, because HRR accounts for resting HR, which is often lower in people who are better conditioned.
Another method of calculating intensity for patients who are beginning a physical activity program is the rating of perceived exertion (RPE), which is graded on a scale of 6 to 20: Moderate exercise correlates with an RPE of 12 to 13 (“somewhat hard”); vigorous exercise correlates with an RPE of 14 to 16 (“hard”). By adding a zero to the rating on the RPE scale, the corresponding HR in a healthy adult can be estimated when they are performing an activity at that perceived intensity.22 Moderate exercise therefore correlates with a HR of 120 and 130 bpm.
The so-called talk test can also guide exercise intensity: Light-intensity activity correlates with an ability to sing; moderate-intensity physical activity likely allows the patient to still hold a conversation; and vigorous-intensity activity correlates with an inability to carry on a conversation while exercising.
An exercise prescription should be accompanied by a patient-derived goal, which can be reassessed during a follow-up visit. So-called SMART goals (Specific, Measurable, Achievable, Relevant, and Time-bound) are tools to help patients set personalized and realistic expectations for physical activity. Meeting the goal of approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week is ideal, but a patient needs to start where they are, at the moment, and gradually increase activity by setting what for them are realistic and sustainable goals.
Continue to: CASE
CASE
With a PAVS of 105 minutes (ie, 15 minutes per day × 7 days) of weekly light-to-moderate exercise walking her dog, Ms. Q does not satisfy current physical activity guidelines. She needs an exercise prescription to incorporate into her lifestyle (see “Cardiovascular exercise prescription,” at left).
First, based on ACSM pre-participation guidelines, Ms. Q does not need medical clearance before initiating light-to-moderate exercise and gradually progressing to vigorous-intensity exercise.
Second, in addition to walking the dog for 105 minutes a week, you:
- advise her to start walking for 10 minutes, 3 times per week, at a pace that keeps her HR at 97-104 bpm.
- encourage her to gradually increase the frequency or duration of her walks by no more than 10% per week.

SBIRT: Referral for treatment
When referring a patient to a fitness program or professional, it is essential to consider their preferences, resources, and environment.23 Community fitness partners are often an excellent referral option for a patient seeking guidance or structure for their exercise program. Using the ACSM ProFinder service, (www.acsm.org/get-stay-certified/find-a-pro) you can search for exercise professionals who have achieved the College’s Gold Standard credential.
Gym memberships or fitness programs might be part of the extra coverage offered by Medicare Advantage Plans, other Medicare health plans, or Medicare Supplement Insurance (Medigap) plans.24
Continue to: CASE
CASE
After providing Ms. Q with her exercise prescription, you refer her to a local gym that participates in the Silver Sneakers fitness and wellness program (for adults ≥ 65 years of age in eligible Medicare plans) to determine whether she qualifies to begin resistance and flexibility training, for which you will write a second exercise prescription (TABLE 317-19).
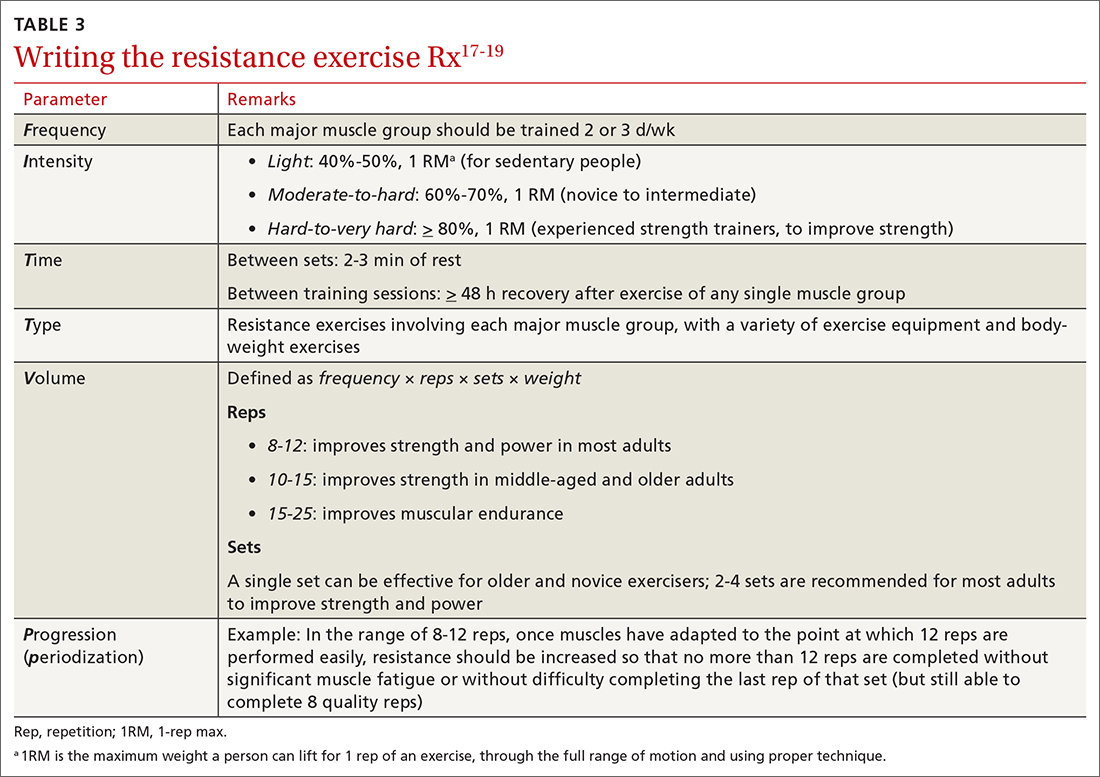
Pre-participation screening
Updated 2015 ACSM exercise pre-participation health screening recommendations attempt to decrease possible barriers to people who are becoming more physically active, by minimizing unnecessary referral to health care providers before they change their level of physical activity. ACSM recommendations on exercise clearance include this guidance25:
- For a patient who is asymptomatic and already physically active—regardless of whether they have known cardiovascular, metabolic, or renal disease—medical clearance is unnecessary for moderate-intensity exercise.
- Any patient who has been physically active and asymptomatic but who becomes symptomatic during exercise should immediately discontinue such activity and undergo medical evaluation.
- For a patient who is inactive, asymptomatic, and who does not have known cardiovascular, metabolic, or renal disease, medical clearance for light- or moderate-intensity exercise is unnecessary.
- For inactive, asymptomatic patients who have known cardiovascular, metabolic, or renal disease, medical clearance is recommended.
Digital health
Smartwatches and health apps (eg, CardioCoach, Fitbit, Garmin Connect, Nike Training Club, Strava, and Training Peaks) can provide workouts and offer patients the ability to collect information and even connect with other users through social media platforms. This information can be synced to Apple Health platforms for iPhones (www.apple.com/ios/health/) or through Google Fit (www.google.com/fit/) on Android devices. Primary care physicians who become familiar with health apps might find them useful for select patients who want to use technology to improve their physical activity level.
However, data on the value of using digital apps for increasing physical activity, in relation to their cost, are limited. Additional research is needed to assess their validity.
Billing and coding
For most patients, the physical activity assessment, prescription, and referral are performed in the context of treating another condition (eg, hypertension, type 2 diabetes, obesity, depression) or during a preventive health examination, and are typically covered without additional charge to the patient. An evaluation and management visit for an established patient could be used to bill if > 50% of the office visit was spent face-to-face with a physician, with patient counseling and coordination of care.
Continue to: Physicians and physical therapists...
Physicians and physical therapists can use the therapeutic exercise code (Current Procedural Terminology code 97110) when teaching patients exercises to develop muscle strength and endurance, joint range of motion, and flexibility26 (TABLE 426).
Conclusion
Physical activity and CRF are strong predictors of premature mortality, even compared to other risk factors, such as cigarette smoking, hypertension, hypercholesterolemia, and type 2 diabetes.27 Brief physical activity assessment and counseling is an efficient, effective, and cost-effective means to increase physical activity, and presents a unique opportunity for you to encourage lifestyle-based strategies for reducing cardiovascular risk.28
However, it is essential to meet patients where they are before trying to have them progress; it is therefore imperative to assess the individual patient’s level of activity using PAVS. With that information in hand, you can personalize physical activity advice; determine readiness for change and potential barriers for change; assist the patient in setting SMART goals; and arrange follow-up to assess adherence to the exercise prescription. Encourage the patient to call their health insurance plan to determine whether a gym membership or fitness program is covered.
Research is needed to evaluate the value of using digital apps, in light of their cost, to increase physical activity and improve CRF in a clinical setting. Prospective trials should be initiated to determine how routine implementation of CRF assessment in primary care alters the trajectory of clinical care. It is hoped that future research will answer the question: Would such an approach improve clinical outcomes and reduce health care expenditures?12
a Defined as O2 consumed while sitting at rest; equivalent to 3.5 mL of O2 × kg of body weight × min.
CORRESPONDENCE
Matthew Kampert, DO, MS, Sports Medicine, 5555 Transportation Boulevard, Cleveland, OH 44125; [email protected]
1. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
2. Tikkanen R, Abrams MK. U.S. health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund Website. January 30, 2020. Accessed November 16, 2021. www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
3. Stoutenberg M, Stasi S, Stamatakis E, et al. Physical activity training in US medical schools: preparing future physicians to engage in primary prevention. Phys Sportsmed. 2015;43:388-394. doi: 10.1080/00913847.2015.1084868
4. Antognoli EL, Seeholzer EL, Gullett H, et al. Primary care resident training for obesity, nutrition, and physical activity counseling: a mixed-methods study. Health Promot Pract. 2017;18:672-680. doi: 10.1177/1524839916658025
5. Asif IM, Drezner JA. Sports and exercise medicine education in the USA: call to action. Br J Sports Med. 2020;54:195-196. doi: 10.1136/bjsports-2019-101104
6. Douglas JA, Briones MD, Bauer EZ, et al. Social and environmental determinants of physical activity in urban parks: testing a neighborhood disorder model. Prev Med. 2018;109:119-124. doi: 10.1016/j.ypmed.2018.01.013
7. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health & Human Services; 2018. Accessed November 15, 2021. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
8. Avis JL, Cave AL, Donaldson S, et al. Working with parents to prevent childhood obesity: protocol for a primary care-based ehealth study. JMIR Res Protoc. 2015;4:e35. doi:10.2196/resprot.4147
9. Ball TJ, Joy EA, Gren LH, et al. Concurrent validity of a self-reported physical activity ‘vital sign’ questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:e16. doi: 10.5888/pcd13.150228
10. Ball TJ, Joy EA, Gren LH, et al. Predictive validity of an adult physical activity “vital sign” recorded in electronic health records. J Phys Act Health. 2016;13:403-408. doi: 10.1123/jpah.2015-0210
11. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071-2076. doi: 10.1249/MSS.0b013e3182630ec1
12. Ross R, Blair SN, Arena R, et al; ; ; ; ; ; . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653-e699. doi: 10.1161/CIR.0000000000000461
13. de Souza de Silva CG, Kokkinos PP, Doom R, et al. Association between cardiorespiratory fitness, obesity, and health care costs: The Veterans Exercise Testing Study. Int J Obes (Lond). 2019;43:2225-2232. doi: 10.1038/s41366-018-0257-0
14. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48. doi: 10.4278/0890-1171-12.1.38
15. Riebe D, Ehrman JK, Liguori G, et al. Methods for evoking change talk. In: ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018.
16. Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
17. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011-1022. doi: 10.1016/j.socscimed.2006.03.012
18. Garber CE, Blissmer BE, Deschenes MR, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Position stand. Med Sci Sport Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb
19. Donnelly JE, Blair SN, Jakicic JM, et al; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Position stand. Med Sci Sport Exerc. 2009;41:459-471. doi: 10.1249/MSS.0b013e3181949333
20. Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404-432.
21. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307-315.
22. The Borg RPE scale. In: Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998:29-38.
23. Ratamess NA, Alvar BA, Evetoch TK, et al; American College of Sports Medicine. Progression models in resistance training for healthy adults. Position stand. Med Sci Sport Exerc. 2009;41:687-708. doi: 10.1249/MSS.0b013e3181915670
24. Gym memberships & fitness programs. Medicare.gov. Baltimore, MD: US Centers for Medicare and Medicaid Services. Accessed November 16, 2021. www.medicare.gov/coverage/gym-memberships-fitness-programs
25. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. doi: 10.1249/MSS.0000000000000664
26. Physical Activity Related Current Procedural Terminology (CPT®) Codes. Physical Activity Alliance website. Accessed November 16, 2021. https://paamovewithus.org/wp-content/uploads/2020/11/PAA-Physical-Activity-CPT-Codes-Nov-2020-AMA-Approved-Final-1.pdf
27. Blair SN. Physical inactivity: the biggest public health problem of the 21st century Br J Sports Med. 2009;43:1-2.
28. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020
It is well-known that per capita health care spending in the United States is more than twice the average in other developed countries1; nevertheless, the overall health care ranking of the US is near the bottom compared to other countries in this group.2 Much of the reason for this poor relative showing lies in the fact that the US has employed a somewhat traditional fee-for-service health care model that does not incentivize efforts to promote health and wellness or prevent chronic disease. The paradigm of promoting physical activity for its disease-preventing and treatment benefits has not been well-integrated in the US health care system.
In this article, we endeavor to provide better understanding of the barriers that keep family physicians from routinely promoting physical activity in clinical practice; define tools and resources that can be used in the clinical setting to promote physical activity; and delineate areas for future work.
Glaring hole in US physical activity education
Many primary care physicians feel underprepared to prescribe or motivate patients to exercise. The reason for that lack of preparedness likely relates to a medical education system that does not spend time preparing physicians to perform this critical task. A study showed that, on average, medical schools require only 8 hours of physical activity education in their curriculum during the 4 years of schooling.3 Likewise, the average primary care residency program offers only 3 hours of didactic training on physical activity, nutrition, and obesity.4 The problem extends to sports medicine fellowship training, in which a 2019 survey showed that 63% of fellows were never taught how to write an exercise prescription in their training program.5
Without education on physical activity, medical students, residents, and fellows are woefully underprepared to realize the therapeutic value of physical activity in patient care, comprehend current physical activity guidelines, appropriately motivate patients to engage in exercise, and competently discuss exercise prescriptions in different disease states. Throughout their training, it is imperative for medical professionals to be educated on the social determinants of health, which include the conditions in which people live, work, and play. These environmental variables can contribute to health inequities that create additional barriers to improvement in physical fitness.6
National guidelines on physical activity
The 2018 National Physical Activity Guidelines detail recommendations for children, adolescents, adults, and special populations.7 The guidelines define physical activity as bodily movement produced by skeletal muscles that result in energy expenditure above resting baseline levels, and includes all types, intensities, and domains of activity. Exercise is a subset of physical activity characterized as planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health.
Highlights from the 2018 guidelines include7:
- Preschool-aged children (3 to 5 years of age) should be physically active throughout the day, with as much as 3 hours per day of physical activity of all intensities—light, moderate, and vigorous.
- Older children and adolescents (6 to 17 years) should accumulate 60 minutes per day of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening activities.
- Adults of all ages should achieve approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week, along with at least 2 days per week of muscle-strengthening activities. Other types of physical activity include flexibility, balance, bone-strengthening, and mind–body exercises.
3-step framework for enhancing physical activity counseling
Merely knowing that physical activity is healthy is not enough, during a patient encounter, to increase the level of physical activity. Therefore, it is imperative to learn and adopt a framework that has proved to yield successful outcomes. The Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework, which has predominantly been used to change patient behavior related to alcohol and substance use, is now being utilized by some providers to promote physical activity.8 We apply the SBIRT approach in this article, although research is lacking on its clinical utility and outcome measures.
Continue to: SBIRT
SBIRT: Screening
An office visit provides an opportunity to understand a patient’s level of physical activity. Often, understanding a patient’s baseline level of activity is only asked during a thorough social history, which might not be performed during patient encounters. As physical activity is the primary determinant of cardiorespiratory fitness (CRF), some health care systems have begun delineating physical activity levels as a vital sign to ensure that the assessment of physical activity is a standard part of every clinical encounter. At a minimum, this serves as a prompt and provides an opportunity to start a conversation around improving physical activity levels when guidelines are not being met.
The exercise vital sign. Assessment and documentation of physical activity in the electronic health record are not yet standardized; however, Kaiser Permanente health plans have implemented the exercise vital sign, or EVS, in its HealthConnect (Epic Systems) electronic health record. The EVS incorporates information about a patient’s:
- days per week of moderate-to-strenuous exercise (eg, a brisk walk)
- minutes per day, on average, of exercise at this level.
The physical activity vital sign. Intermountain Healthcare implemented the physical activity vital sign, or PAVS, in its iCentra (Cerner Corp.) electronic health record. The 3-question PAVS assessment asks:
- On average, how many days of the week do you perform physical activity or exercise?
- On average, how many total minutes of physical activity or exercise do you perform on those days?
- How would you describe the intensity of your physical activity or exercise: Light (ie, a casual walk)? Moderate (a brisk walk)? Or vigorous (jogging)?
PAVS includes a fourth data point: The physician–user documents whether the patient was counseled to start, increase, maintain, or modify physical activity or exercise.
EVS and the PAVS have demonstrated validity.9-11
Continue to: Cardiorespiratory fitness as a vital sign
Cardiorespiratory fitness as a vital sign. In 2016, the American Heart Association (AHA) asserted the importance of assessing CRF as a clinical vital sign.12 CRF is commonly expressed as maximal oxygen consumption (VO2max = O2 mL/kg/min) and measured through cardiopulmonary exercise testing (CPET), considered the gold standard by combining conventional graded exercise testing with ventilatory expired gas analysis. CPET is more objective and precise than equations estimating CRF that are derived from peak work rate. AHA recommended that efforts to improve CRF should become standard in clinical encounters, explaining that even a small increase in CRF (eg, 1 or 2 metabolic equivalentsa [METs]) is associated with a considerably (10% to 30%) lower rate of adverse cardiovascular events.12
De Souza de Silva and colleagues revealed an association between each 1-MET increase in CRF and per-person annual health care cost savings (adjusted for age and presence of cardiovascular disease) of $3272 (normal-weight patients), $4252 (overweight), and $6103 (obese).13 In its 2016 scientific statement on CRF as a vital sign, AHA listed several methods of estimating CRF and concluded that, although CPET involves a higher level of training, proficiency, equipment, and, therefore, cost, the independent and additive information obtained justifies its use in many patients.12
CASE
Mary Q, 68 years of age, presents for an annual well-woman examination. Body mass index is 32; resting heart rate (HR), 73 bpm; and blood pressure, 126/74 mm Hg. She reports being inactive, except for light walking every day with her dog around the neighborhood, which takes them approximately 15 minutes. She denies any history or signs and symptoms of cardiovascular, metabolic, or renal disease.
You consider 3 questions before taking next steps regarding increasing Ms. Q’s activity level:
- What is her PAVS?
- Does she need medical clearance before starting an exercise program?
- What would an evidence-based cardiovascular exercise prescription for Ms. Q look like?
SBIRT: Brief intervention
When a patient does not meet the recommended level of physical activity, you have an opportunity to deliver a brief intervention. To do this effectively, you must have adequate understanding of the patient’s receptivity for change. The transtheoretical, or Stages of Change, model proposes that a person typically goes through 5 stages of growth—pre-contemplation, contemplation, preparation, action, and maintenance—in the process of lifestyle modification. This model highlights the different approaches to exercise adoption and maintenance that need to be taken, based on a given patient’s stage at the moment.
Continue to: Using this framework...
Using this framework, you can help patients realize intrinsic motivation that can facilitate progression through each stage, utilizing techniques such as motivational interviewing—so-called change talk—to increase self-efficacy.14TABLE 115 provides examples of motivational interviewing techniques that can be used during a patient encounter to improve health behaviors, such as physical activity.

Writing the exercise prescription
A patient who wants to increase their level of physical activity should be offered a formal exercise prescription, which has been shown to increase the level of physical activity, particularly in older patients. In fact, a study conducted in Spain in the practices of family physicians found that older patients who received a physical activity prescription increased their activity by 131 minutes per week; and compared to control patients, they doubled the minutes per week devoted to moderate or vigorous physical activity.16
FITT-VP. The basics of a cardiovascular exercise prescription can be found in the FITT-VP (Frequency, Intensity, Time, Type, Volume, and [monitoring of] Progression) framework (TABLE 217-19). For most patients, this model includes 3 to 5 days per week of moderate-to-vigorous physical activity for 30 to 60 minutes per session. For patients with established chronic disease, physical activity provides health benefits but might require modification. Disease-specific patient handouts for exercise can be downloaded, at no cost, through the American College of Sports Medicine (ACSM) “Exercise Is Medicine” program, which can be found at: www.exerciseismedicine.org/support_page.php/rx-for-health-series.

Determining intensity level. Although CPET is the gold standard for determining a patient’s target intensity level, such a test might be impracticable for a given patient. Surrogate markers of target intensity level can be obtained by measuring maximum HR (HRmax), using a well-known equation20:
HRmax = 220 – age
which is then multiplied by intensity range:
- light: 30%-39%
- moderate: 40%-59%
- vigorous: 60%-89%
or, more preferably, by calculating the HR training zone while accounting for HR at rest (HRrest). This is accomplished by calculating the HR reserve (HRR) (ie, HRR = HRmax – HRrest) and then calculating the target heart rate (THR)21:
THR = [HRR × %intensity] + HRrest
Continue to: The THR calculation...
The THR calculation is performed twice, once with a lower %intensity and again with a higher %intensity to develop a training zone based on HRR.
The HRR equation is more accurate than calculating HRmax from 220 – age, because HRR accounts for resting HR, which is often lower in people who are better conditioned.
Another method of calculating intensity for patients who are beginning a physical activity program is the rating of perceived exertion (RPE), which is graded on a scale of 6 to 20: Moderate exercise correlates with an RPE of 12 to 13 (“somewhat hard”); vigorous exercise correlates with an RPE of 14 to 16 (“hard”). By adding a zero to the rating on the RPE scale, the corresponding HR in a healthy adult can be estimated when they are performing an activity at that perceived intensity.22 Moderate exercise therefore correlates with a HR of 120 and 130 bpm.
The so-called talk test can also guide exercise intensity: Light-intensity activity correlates with an ability to sing; moderate-intensity physical activity likely allows the patient to still hold a conversation; and vigorous-intensity activity correlates with an inability to carry on a conversation while exercising.
An exercise prescription should be accompanied by a patient-derived goal, which can be reassessed during a follow-up visit. So-called SMART goals (Specific, Measurable, Achievable, Relevant, and Time-bound) are tools to help patients set personalized and realistic expectations for physical activity. Meeting the goal of approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week is ideal, but a patient needs to start where they are, at the moment, and gradually increase activity by setting what for them are realistic and sustainable goals.
Continue to: CASE
CASE
With a PAVS of 105 minutes (ie, 15 minutes per day × 7 days) of weekly light-to-moderate exercise walking her dog, Ms. Q does not satisfy current physical activity guidelines. She needs an exercise prescription to incorporate into her lifestyle (see “Cardiovascular exercise prescription,” at left).
First, based on ACSM pre-participation guidelines, Ms. Q does not need medical clearance before initiating light-to-moderate exercise and gradually progressing to vigorous-intensity exercise.
Second, in addition to walking the dog for 105 minutes a week, you:
- advise her to start walking for 10 minutes, 3 times per week, at a pace that keeps her HR at 97-104 bpm.
- encourage her to gradually increase the frequency or duration of her walks by no more than 10% per week.

SBIRT: Referral for treatment
When referring a patient to a fitness program or professional, it is essential to consider their preferences, resources, and environment.23 Community fitness partners are often an excellent referral option for a patient seeking guidance or structure for their exercise program. Using the ACSM ProFinder service, (www.acsm.org/get-stay-certified/find-a-pro) you can search for exercise professionals who have achieved the College’s Gold Standard credential.
Gym memberships or fitness programs might be part of the extra coverage offered by Medicare Advantage Plans, other Medicare health plans, or Medicare Supplement Insurance (Medigap) plans.24
Continue to: CASE
CASE
After providing Ms. Q with her exercise prescription, you refer her to a local gym that participates in the Silver Sneakers fitness and wellness program (for adults ≥ 65 years of age in eligible Medicare plans) to determine whether she qualifies to begin resistance and flexibility training, for which you will write a second exercise prescription (TABLE 317-19).

Pre-participation screening
Updated 2015 ACSM exercise pre-participation health screening recommendations attempt to decrease possible barriers to people who are becoming more physically active, by minimizing unnecessary referral to health care providers before they change their level of physical activity. ACSM recommendations on exercise clearance include this guidance25:
- For a patient who is asymptomatic and already physically active—regardless of whether they have known cardiovascular, metabolic, or renal disease—medical clearance is unnecessary for moderate-intensity exercise.
- Any patient who has been physically active and asymptomatic but who becomes symptomatic during exercise should immediately discontinue such activity and undergo medical evaluation.
- For a patient who is inactive, asymptomatic, and who does not have known cardiovascular, metabolic, or renal disease, medical clearance for light- or moderate-intensity exercise is unnecessary.
- For inactive, asymptomatic patients who have known cardiovascular, metabolic, or renal disease, medical clearance is recommended.
Digital health
Smartwatches and health apps (eg, CardioCoach, Fitbit, Garmin Connect, Nike Training Club, Strava, and Training Peaks) can provide workouts and offer patients the ability to collect information and even connect with other users through social media platforms. This information can be synced to Apple Health platforms for iPhones (www.apple.com/ios/health/) or through Google Fit (www.google.com/fit/) on Android devices. Primary care physicians who become familiar with health apps might find them useful for select patients who want to use technology to improve their physical activity level.
However, data on the value of using digital apps for increasing physical activity, in relation to their cost, are limited. Additional research is needed to assess their validity.
Billing and coding
For most patients, the physical activity assessment, prescription, and referral are performed in the context of treating another condition (eg, hypertension, type 2 diabetes, obesity, depression) or during a preventive health examination, and are typically covered without additional charge to the patient. An evaluation and management visit for an established patient could be used to bill if > 50% of the office visit was spent face-to-face with a physician, with patient counseling and coordination of care.
Continue to: Physicians and physical therapists...
Physicians and physical therapists can use the therapeutic exercise code (Current Procedural Terminology code 97110) when teaching patients exercises to develop muscle strength and endurance, joint range of motion, and flexibility26 (TABLE 426).

Conclusion
Physical activity and CRF are strong predictors of premature mortality, even compared to other risk factors, such as cigarette smoking, hypertension, hypercholesterolemia, and type 2 diabetes.27 Brief physical activity assessment and counseling is an efficient, effective, and cost-effective means to increase physical activity, and presents a unique opportunity for you to encourage lifestyle-based strategies for reducing cardiovascular risk.28
However, it is essential to meet patients where they are before trying to have them progress; it is therefore imperative to assess the individual patient’s level of activity using PAVS. With that information in hand, you can personalize physical activity advice; determine readiness for change and potential barriers for change; assist the patient in setting SMART goals; and arrange follow-up to assess adherence to the exercise prescription. Encourage the patient to call their health insurance plan to determine whether a gym membership or fitness program is covered.
Research is needed to evaluate the value of using digital apps, in light of their cost, to increase physical activity and improve CRF in a clinical setting. Prospective trials should be initiated to determine how routine implementation of CRF assessment in primary care alters the trajectory of clinical care. It is hoped that future research will answer the question: Would such an approach improve clinical outcomes and reduce health care expenditures?12
a Defined as O2 consumed while sitting at rest; equivalent to 3.5 mL of O2 × kg of body weight × min.
CORRESPONDENCE
Matthew Kampert, DO, MS, Sports Medicine, 5555 Transportation Boulevard, Cleveland, OH 44125; [email protected]
It is well-known that per capita health care spending in the United States is more than twice the average in other developed countries1; nevertheless, the overall health care ranking of the US is near the bottom compared to other countries in this group.2 Much of the reason for this poor relative showing lies in the fact that the US has employed a somewhat traditional fee-for-service health care model that does not incentivize efforts to promote health and wellness or prevent chronic disease. The paradigm of promoting physical activity for its disease-preventing and treatment benefits has not been well-integrated in the US health care system.
In this article, we endeavor to provide better understanding of the barriers that keep family physicians from routinely promoting physical activity in clinical practice; define tools and resources that can be used in the clinical setting to promote physical activity; and delineate areas for future work.
Glaring hole in US physical activity education
Many primary care physicians feel underprepared to prescribe or motivate patients to exercise. The reason for that lack of preparedness likely relates to a medical education system that does not spend time preparing physicians to perform this critical task. A study showed that, on average, medical schools require only 8 hours of physical activity education in their curriculum during the 4 years of schooling.3 Likewise, the average primary care residency program offers only 3 hours of didactic training on physical activity, nutrition, and obesity.4 The problem extends to sports medicine fellowship training, in which a 2019 survey showed that 63% of fellows were never taught how to write an exercise prescription in their training program.5
Without education on physical activity, medical students, residents, and fellows are woefully underprepared to realize the therapeutic value of physical activity in patient care, comprehend current physical activity guidelines, appropriately motivate patients to engage in exercise, and competently discuss exercise prescriptions in different disease states. Throughout their training, it is imperative for medical professionals to be educated on the social determinants of health, which include the conditions in which people live, work, and play. These environmental variables can contribute to health inequities that create additional barriers to improvement in physical fitness.6
National guidelines on physical activity
The 2018 National Physical Activity Guidelines detail recommendations for children, adolescents, adults, and special populations.7 The guidelines define physical activity as bodily movement produced by skeletal muscles that result in energy expenditure above resting baseline levels, and includes all types, intensities, and domains of activity. Exercise is a subset of physical activity characterized as planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health.
Highlights from the 2018 guidelines include7:
- Preschool-aged children (3 to 5 years of age) should be physically active throughout the day, with as much as 3 hours per day of physical activity of all intensities—light, moderate, and vigorous.
- Older children and adolescents (6 to 17 years) should accumulate 60 minutes per day of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening activities.
- Adults of all ages should achieve approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week, along with at least 2 days per week of muscle-strengthening activities. Other types of physical activity include flexibility, balance, bone-strengthening, and mind–body exercises.
3-step framework for enhancing physical activity counseling
Merely knowing that physical activity is healthy is not enough, during a patient encounter, to increase the level of physical activity. Therefore, it is imperative to learn and adopt a framework that has proved to yield successful outcomes. The Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework, which has predominantly been used to change patient behavior related to alcohol and substance use, is now being utilized by some providers to promote physical activity.8 We apply the SBIRT approach in this article, although research is lacking on its clinical utility and outcome measures.
Continue to: SBIRT
SBIRT: Screening
An office visit provides an opportunity to understand a patient’s level of physical activity. Often, understanding a patient’s baseline level of activity is only asked during a thorough social history, which might not be performed during patient encounters. As physical activity is the primary determinant of cardiorespiratory fitness (CRF), some health care systems have begun delineating physical activity levels as a vital sign to ensure that the assessment of physical activity is a standard part of every clinical encounter. At a minimum, this serves as a prompt and provides an opportunity to start a conversation around improving physical activity levels when guidelines are not being met.
The exercise vital sign. Assessment and documentation of physical activity in the electronic health record are not yet standardized; however, Kaiser Permanente health plans have implemented the exercise vital sign, or EVS, in its HealthConnect (Epic Systems) electronic health record. The EVS incorporates information about a patient’s:
- days per week of moderate-to-strenuous exercise (eg, a brisk walk)
- minutes per day, on average, of exercise at this level.
The physical activity vital sign. Intermountain Healthcare implemented the physical activity vital sign, or PAVS, in its iCentra (Cerner Corp.) electronic health record. The 3-question PAVS assessment asks:
- On average, how many days of the week do you perform physical activity or exercise?
- On average, how many total minutes of physical activity or exercise do you perform on those days?
- How would you describe the intensity of your physical activity or exercise: Light (ie, a casual walk)? Moderate (a brisk walk)? Or vigorous (jogging)?
PAVS includes a fourth data point: The physician–user documents whether the patient was counseled to start, increase, maintain, or modify physical activity or exercise.
EVS and the PAVS have demonstrated validity.9-11
Continue to: Cardiorespiratory fitness as a vital sign
Cardiorespiratory fitness as a vital sign. In 2016, the American Heart Association (AHA) asserted the importance of assessing CRF as a clinical vital sign.12 CRF is commonly expressed as maximal oxygen consumption (VO2max = O2 mL/kg/min) and measured through cardiopulmonary exercise testing (CPET), considered the gold standard by combining conventional graded exercise testing with ventilatory expired gas analysis. CPET is more objective and precise than equations estimating CRF that are derived from peak work rate. AHA recommended that efforts to improve CRF should become standard in clinical encounters, explaining that even a small increase in CRF (eg, 1 or 2 metabolic equivalentsa [METs]) is associated with a considerably (10% to 30%) lower rate of adverse cardiovascular events.12
De Souza de Silva and colleagues revealed an association between each 1-MET increase in CRF and per-person annual health care cost savings (adjusted for age and presence of cardiovascular disease) of $3272 (normal-weight patients), $4252 (overweight), and $6103 (obese).13 In its 2016 scientific statement on CRF as a vital sign, AHA listed several methods of estimating CRF and concluded that, although CPET involves a higher level of training, proficiency, equipment, and, therefore, cost, the independent and additive information obtained justifies its use in many patients.12
CASE
Mary Q, 68 years of age, presents for an annual well-woman examination. Body mass index is 32; resting heart rate (HR), 73 bpm; and blood pressure, 126/74 mm Hg. She reports being inactive, except for light walking every day with her dog around the neighborhood, which takes them approximately 15 minutes. She denies any history or signs and symptoms of cardiovascular, metabolic, or renal disease.
You consider 3 questions before taking next steps regarding increasing Ms. Q’s activity level:
- What is her PAVS?
- Does she need medical clearance before starting an exercise program?
- What would an evidence-based cardiovascular exercise prescription for Ms. Q look like?
SBIRT: Brief intervention
When a patient does not meet the recommended level of physical activity, you have an opportunity to deliver a brief intervention. To do this effectively, you must have adequate understanding of the patient’s receptivity for change. The transtheoretical, or Stages of Change, model proposes that a person typically goes through 5 stages of growth—pre-contemplation, contemplation, preparation, action, and maintenance—in the process of lifestyle modification. This model highlights the different approaches to exercise adoption and maintenance that need to be taken, based on a given patient’s stage at the moment.
Continue to: Using this framework...
Using this framework, you can help patients realize intrinsic motivation that can facilitate progression through each stage, utilizing techniques such as motivational interviewing—so-called change talk—to increase self-efficacy.14TABLE 115 provides examples of motivational interviewing techniques that can be used during a patient encounter to improve health behaviors, such as physical activity.

Writing the exercise prescription
A patient who wants to increase their level of physical activity should be offered a formal exercise prescription, which has been shown to increase the level of physical activity, particularly in older patients. In fact, a study conducted in Spain in the practices of family physicians found that older patients who received a physical activity prescription increased their activity by 131 minutes per week; and compared to control patients, they doubled the minutes per week devoted to moderate or vigorous physical activity.16
FITT-VP. The basics of a cardiovascular exercise prescription can be found in the FITT-VP (Frequency, Intensity, Time, Type, Volume, and [monitoring of] Progression) framework (TABLE 217-19). For most patients, this model includes 3 to 5 days per week of moderate-to-vigorous physical activity for 30 to 60 minutes per session. For patients with established chronic disease, physical activity provides health benefits but might require modification. Disease-specific patient handouts for exercise can be downloaded, at no cost, through the American College of Sports Medicine (ACSM) “Exercise Is Medicine” program, which can be found at: www.exerciseismedicine.org/support_page.php/rx-for-health-series.

Determining intensity level. Although CPET is the gold standard for determining a patient’s target intensity level, such a test might be impracticable for a given patient. Surrogate markers of target intensity level can be obtained by measuring maximum HR (HRmax), using a well-known equation20:
HRmax = 220 – age
which is then multiplied by intensity range:
- light: 30%-39%
- moderate: 40%-59%
- vigorous: 60%-89%
or, more preferably, by calculating the HR training zone while accounting for HR at rest (HRrest). This is accomplished by calculating the HR reserve (HRR) (ie, HRR = HRmax – HRrest) and then calculating the target heart rate (THR)21:
THR = [HRR × %intensity] + HRrest
Continue to: The THR calculation...
The THR calculation is performed twice, once with a lower %intensity and again with a higher %intensity to develop a training zone based on HRR.
The HRR equation is more accurate than calculating HRmax from 220 – age, because HRR accounts for resting HR, which is often lower in people who are better conditioned.
Another method of calculating intensity for patients who are beginning a physical activity program is the rating of perceived exertion (RPE), which is graded on a scale of 6 to 20: Moderate exercise correlates with an RPE of 12 to 13 (“somewhat hard”); vigorous exercise correlates with an RPE of 14 to 16 (“hard”). By adding a zero to the rating on the RPE scale, the corresponding HR in a healthy adult can be estimated when they are performing an activity at that perceived intensity.22 Moderate exercise therefore correlates with a HR of 120 and 130 bpm.
The so-called talk test can also guide exercise intensity: Light-intensity activity correlates with an ability to sing; moderate-intensity physical activity likely allows the patient to still hold a conversation; and vigorous-intensity activity correlates with an inability to carry on a conversation while exercising.
An exercise prescription should be accompanied by a patient-derived goal, which can be reassessed during a follow-up visit. So-called SMART goals (Specific, Measurable, Achievable, Relevant, and Time-bound) are tools to help patients set personalized and realistic expectations for physical activity. Meeting the goal of approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week is ideal, but a patient needs to start where they are, at the moment, and gradually increase activity by setting what for them are realistic and sustainable goals.
Continue to: CASE
CASE
With a PAVS of 105 minutes (ie, 15 minutes per day × 7 days) of weekly light-to-moderate exercise walking her dog, Ms. Q does not satisfy current physical activity guidelines. She needs an exercise prescription to incorporate into her lifestyle (see “Cardiovascular exercise prescription,” at left).
First, based on ACSM pre-participation guidelines, Ms. Q does not need medical clearance before initiating light-to-moderate exercise and gradually progressing to vigorous-intensity exercise.
Second, in addition to walking the dog for 105 minutes a week, you:
- advise her to start walking for 10 minutes, 3 times per week, at a pace that keeps her HR at 97-104 bpm.
- encourage her to gradually increase the frequency or duration of her walks by no more than 10% per week.

SBIRT: Referral for treatment
When referring a patient to a fitness program or professional, it is essential to consider their preferences, resources, and environment.23 Community fitness partners are often an excellent referral option for a patient seeking guidance or structure for their exercise program. Using the ACSM ProFinder service, (www.acsm.org/get-stay-certified/find-a-pro) you can search for exercise professionals who have achieved the College’s Gold Standard credential.
Gym memberships or fitness programs might be part of the extra coverage offered by Medicare Advantage Plans, other Medicare health plans, or Medicare Supplement Insurance (Medigap) plans.24
Continue to: CASE
CASE
After providing Ms. Q with her exercise prescription, you refer her to a local gym that participates in the Silver Sneakers fitness and wellness program (for adults ≥ 65 years of age in eligible Medicare plans) to determine whether she qualifies to begin resistance and flexibility training, for which you will write a second exercise prescription (TABLE 317-19).

Pre-participation screening
Updated 2015 ACSM exercise pre-participation health screening recommendations attempt to decrease possible barriers to people who are becoming more physically active, by minimizing unnecessary referral to health care providers before they change their level of physical activity. ACSM recommendations on exercise clearance include this guidance25:
- For a patient who is asymptomatic and already physically active—regardless of whether they have known cardiovascular, metabolic, or renal disease—medical clearance is unnecessary for moderate-intensity exercise.
- Any patient who has been physically active and asymptomatic but who becomes symptomatic during exercise should immediately discontinue such activity and undergo medical evaluation.
- For a patient who is inactive, asymptomatic, and who does not have known cardiovascular, metabolic, or renal disease, medical clearance for light- or moderate-intensity exercise is unnecessary.
- For inactive, asymptomatic patients who have known cardiovascular, metabolic, or renal disease, medical clearance is recommended.
Digital health
Smartwatches and health apps (eg, CardioCoach, Fitbit, Garmin Connect, Nike Training Club, Strava, and Training Peaks) can provide workouts and offer patients the ability to collect information and even connect with other users through social media platforms. This information can be synced to Apple Health platforms for iPhones (www.apple.com/ios/health/) or through Google Fit (www.google.com/fit/) on Android devices. Primary care physicians who become familiar with health apps might find them useful for select patients who want to use technology to improve their physical activity level.
However, data on the value of using digital apps for increasing physical activity, in relation to their cost, are limited. Additional research is needed to assess their validity.
Billing and coding
For most patients, the physical activity assessment, prescription, and referral are performed in the context of treating another condition (eg, hypertension, type 2 diabetes, obesity, depression) or during a preventive health examination, and are typically covered without additional charge to the patient. An evaluation and management visit for an established patient could be used to bill if > 50% of the office visit was spent face-to-face with a physician, with patient counseling and coordination of care.
Continue to: Physicians and physical therapists...
Physicians and physical therapists can use the therapeutic exercise code (Current Procedural Terminology code 97110) when teaching patients exercises to develop muscle strength and endurance, joint range of motion, and flexibility26 (TABLE 426).

Conclusion
Physical activity and CRF are strong predictors of premature mortality, even compared to other risk factors, such as cigarette smoking, hypertension, hypercholesterolemia, and type 2 diabetes.27 Brief physical activity assessment and counseling is an efficient, effective, and cost-effective means to increase physical activity, and presents a unique opportunity for you to encourage lifestyle-based strategies for reducing cardiovascular risk.28
However, it is essential to meet patients where they are before trying to have them progress; it is therefore imperative to assess the individual patient’s level of activity using PAVS. With that information in hand, you can personalize physical activity advice; determine readiness for change and potential barriers for change; assist the patient in setting SMART goals; and arrange follow-up to assess adherence to the exercise prescription. Encourage the patient to call their health insurance plan to determine whether a gym membership or fitness program is covered.
Research is needed to evaluate the value of using digital apps, in light of their cost, to increase physical activity and improve CRF in a clinical setting. Prospective trials should be initiated to determine how routine implementation of CRF assessment in primary care alters the trajectory of clinical care. It is hoped that future research will answer the question: Would such an approach improve clinical outcomes and reduce health care expenditures?12
a Defined as O2 consumed while sitting at rest; equivalent to 3.5 mL of O2 × kg of body weight × min.
CORRESPONDENCE
Matthew Kampert, DO, MS, Sports Medicine, 5555 Transportation Boulevard, Cleveland, OH 44125; [email protected]
1. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
2. Tikkanen R, Abrams MK. U.S. health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund Website. January 30, 2020. Accessed November 16, 2021. www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
3. Stoutenberg M, Stasi S, Stamatakis E, et al. Physical activity training in US medical schools: preparing future physicians to engage in primary prevention. Phys Sportsmed. 2015;43:388-394. doi: 10.1080/00913847.2015.1084868
4. Antognoli EL, Seeholzer EL, Gullett H, et al. Primary care resident training for obesity, nutrition, and physical activity counseling: a mixed-methods study. Health Promot Pract. 2017;18:672-680. doi: 10.1177/1524839916658025
5. Asif IM, Drezner JA. Sports and exercise medicine education in the USA: call to action. Br J Sports Med. 2020;54:195-196. doi: 10.1136/bjsports-2019-101104
6. Douglas JA, Briones MD, Bauer EZ, et al. Social and environmental determinants of physical activity in urban parks: testing a neighborhood disorder model. Prev Med. 2018;109:119-124. doi: 10.1016/j.ypmed.2018.01.013
7. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health & Human Services; 2018. Accessed November 15, 2021. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
8. Avis JL, Cave AL, Donaldson S, et al. Working with parents to prevent childhood obesity: protocol for a primary care-based ehealth study. JMIR Res Protoc. 2015;4:e35. doi:10.2196/resprot.4147
9. Ball TJ, Joy EA, Gren LH, et al. Concurrent validity of a self-reported physical activity ‘vital sign’ questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:e16. doi: 10.5888/pcd13.150228
10. Ball TJ, Joy EA, Gren LH, et al. Predictive validity of an adult physical activity “vital sign” recorded in electronic health records. J Phys Act Health. 2016;13:403-408. doi: 10.1123/jpah.2015-0210
11. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071-2076. doi: 10.1249/MSS.0b013e3182630ec1
12. Ross R, Blair SN, Arena R, et al; ; ; ; ; ; . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653-e699. doi: 10.1161/CIR.0000000000000461
13. de Souza de Silva CG, Kokkinos PP, Doom R, et al. Association between cardiorespiratory fitness, obesity, and health care costs: The Veterans Exercise Testing Study. Int J Obes (Lond). 2019;43:2225-2232. doi: 10.1038/s41366-018-0257-0
14. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48. doi: 10.4278/0890-1171-12.1.38
15. Riebe D, Ehrman JK, Liguori G, et al. Methods for evoking change talk. In: ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018.
16. Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
17. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011-1022. doi: 10.1016/j.socscimed.2006.03.012
18. Garber CE, Blissmer BE, Deschenes MR, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Position stand. Med Sci Sport Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb
19. Donnelly JE, Blair SN, Jakicic JM, et al; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Position stand. Med Sci Sport Exerc. 2009;41:459-471. doi: 10.1249/MSS.0b013e3181949333
20. Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404-432.
21. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307-315.
22. The Borg RPE scale. In: Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998:29-38.
23. Ratamess NA, Alvar BA, Evetoch TK, et al; American College of Sports Medicine. Progression models in resistance training for healthy adults. Position stand. Med Sci Sport Exerc. 2009;41:687-708. doi: 10.1249/MSS.0b013e3181915670
24. Gym memberships & fitness programs. Medicare.gov. Baltimore, MD: US Centers for Medicare and Medicaid Services. Accessed November 16, 2021. www.medicare.gov/coverage/gym-memberships-fitness-programs
25. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. doi: 10.1249/MSS.0000000000000664
26. Physical Activity Related Current Procedural Terminology (CPT®) Codes. Physical Activity Alliance website. Accessed November 16, 2021. https://paamovewithus.org/wp-content/uploads/2020/11/PAA-Physical-Activity-CPT-Codes-Nov-2020-AMA-Approved-Final-1.pdf
27. Blair SN. Physical inactivity: the biggest public health problem of the 21st century Br J Sports Med. 2009;43:1-2.
28. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020
1. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
2. Tikkanen R, Abrams MK. U.S. health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund Website. January 30, 2020. Accessed November 16, 2021. www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
3. Stoutenberg M, Stasi S, Stamatakis E, et al. Physical activity training in US medical schools: preparing future physicians to engage in primary prevention. Phys Sportsmed. 2015;43:388-394. doi: 10.1080/00913847.2015.1084868
4. Antognoli EL, Seeholzer EL, Gullett H, et al. Primary care resident training for obesity, nutrition, and physical activity counseling: a mixed-methods study. Health Promot Pract. 2017;18:672-680. doi: 10.1177/1524839916658025
5. Asif IM, Drezner JA. Sports and exercise medicine education in the USA: call to action. Br J Sports Med. 2020;54:195-196. doi: 10.1136/bjsports-2019-101104
6. Douglas JA, Briones MD, Bauer EZ, et al. Social and environmental determinants of physical activity in urban parks: testing a neighborhood disorder model. Prev Med. 2018;109:119-124. doi: 10.1016/j.ypmed.2018.01.013
7. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health & Human Services; 2018. Accessed November 15, 2021. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
8. Avis JL, Cave AL, Donaldson S, et al. Working with parents to prevent childhood obesity: protocol for a primary care-based ehealth study. JMIR Res Protoc. 2015;4:e35. doi:10.2196/resprot.4147
9. Ball TJ, Joy EA, Gren LH, et al. Concurrent validity of a self-reported physical activity ‘vital sign’ questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:e16. doi: 10.5888/pcd13.150228
10. Ball TJ, Joy EA, Gren LH, et al. Predictive validity of an adult physical activity “vital sign” recorded in electronic health records. J Phys Act Health. 2016;13:403-408. doi: 10.1123/jpah.2015-0210
11. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071-2076. doi: 10.1249/MSS.0b013e3182630ec1
12. Ross R, Blair SN, Arena R, et al; ; ; ; ; ; . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653-e699. doi: 10.1161/CIR.0000000000000461
13. de Souza de Silva CG, Kokkinos PP, Doom R, et al. Association between cardiorespiratory fitness, obesity, and health care costs: The Veterans Exercise Testing Study. Int J Obes (Lond). 2019;43:2225-2232. doi: 10.1038/s41366-018-0257-0
14. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48. doi: 10.4278/0890-1171-12.1.38
15. Riebe D, Ehrman JK, Liguori G, et al. Methods for evoking change talk. In: ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018.
16. Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
17. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011-1022. doi: 10.1016/j.socscimed.2006.03.012
18. Garber CE, Blissmer BE, Deschenes MR, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Position stand. Med Sci Sport Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb
19. Donnelly JE, Blair SN, Jakicic JM, et al; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Position stand. Med Sci Sport Exerc. 2009;41:459-471. doi: 10.1249/MSS.0b013e3181949333
20. Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404-432.
21. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307-315.
22. The Borg RPE scale. In: Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998:29-38.
23. Ratamess NA, Alvar BA, Evetoch TK, et al; American College of Sports Medicine. Progression models in resistance training for healthy adults. Position stand. Med Sci Sport Exerc. 2009;41:687-708. doi: 10.1249/MSS.0b013e3181915670
24. Gym memberships & fitness programs. Medicare.gov. Baltimore, MD: US Centers for Medicare and Medicaid Services. Accessed November 16, 2021. www.medicare.gov/coverage/gym-memberships-fitness-programs
25. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. doi: 10.1249/MSS.0000000000000664
26. Physical Activity Related Current Procedural Terminology (CPT®) Codes. Physical Activity Alliance website. Accessed November 16, 2021. https://paamovewithus.org/wp-content/uploads/2020/11/PAA-Physical-Activity-CPT-Codes-Nov-2020-AMA-Approved-Final-1.pdf
27. Blair SN. Physical inactivity: the biggest public health problem of the 21st century Br J Sports Med. 2009;43:1-2.
28. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020
PRACTICE RECOMMENDATIONS
› Encourage children and adolescents (6 to 17 years of age) to engage in 60 min of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening endeavors on most, if not all, days of the week. A
› Encourage adults to perform approximately 150 to 300 min of moderate or 75 to 150 min of vigorous physical activity (or an equivalent combination) per week, along with moderate-intensity muscle-strengthening activities on ≥ 2 days per week. A
› Counsel patients that even a small (eg, 1-2 metabolic equivalents) increase in cardiorespiratory fitness is associated with a 10% to 30% lower rate of adverse events. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Functional medicine: Focusing on imbalances in core metabolic processes
Could screening patients for cytokine markers help direct interventions to prevent quality-of-life deterioration? What evidence is there that a patient’s methylenetetrahydrofolate reductase (MTHFR) genotype and baseline folate level can determine whether folate therapy will be needed to prevent stroke?
Favorable findings in these areas and others—eg, that specific probiotics benefit those with various gastrointestinal, respiratory, and lipid disorders—are strengthening support for the clinical approach of functional medicine (FM), which focuses on core functional processes: assimilation, defense and repair, energy, biotransformation and elimination, transport, communication, and structural integrity.
In this article, we describe the paradigm of FM, review its origins, and present the evidence base of selected topics: diagnostic testing, nutrition and supplements, probiotics, and chelation. As FM’s popularity increases, a better understanding of it will help us educate our patients on this approach and implement some of its evidence-backed practices. In preparing this review we used keyword searches of PubMed, Embase, Web of Science, Cochrane Library, University of Florida Health Science Center Library eJournals, the Institute for Functional Medicine website,1 and Lifestyle Medicine textbook.2
The core of functional medicine
Complementary and alternative medicine (CAM) refers to medical practices diverging from standards of care and not generally taught at US medical schools or available at US hospitals.3 Integrative Medicine encompasses evidence-informed CAM, conventional Western medicine, and whole systems like FM.4
FM aims to identify root causes of disease, emphasizing function as a dynamic process that can move back and forth on a continuum between health and disorder.5,6 There are 7 defining characteristics of FM: (1) patient centered vs disease centered; (2) systems biology approach acknowledging web-like interconnections of physiologic factors; (3) dynamic balance of gene–environment interactions; (4) personalized care based on biochemical individuality; (5) promotion of organ reserve and sustained health span; (6) health as a positive vitality—not merely the absence of disease; and (7) function vs pathology focused.5
The concept of FM is not new. Its origins can be traced to the 19th century. Jeffrey Bland, PhD, credits the term’s first use to Sir Willoughby F. Wade, MD, in an 1871 Lancet editorial, “Clinical lecture on functional medicine.” Bland formulated the FM paradigm and in 1991 founded the Institute for Functional Medicine (the Institute), its main educational and certifying organization.7 The Institute certifies masters- or doctorate-prepared health professionals from both conventional and complementary health fields who complete 7 courses (Applying FM in Clinical Practice, Gastrointestinal, Environmental Health, Immune, Hormone, Cardiometabolic, and Bioenergetics), present 1 case report, and pass a written exam. In a retrospective cohort study, researchers found that the FM model was associated with greater improvements in patient-reported, health-related quality of life (QOL) compared with usual care.8
Clinical model
FM uses a comprehensive yet practical matrix for obtaining patient histories and for guiding diagnostic testing.9 Tools that support history-taking include a timeline; weeklong dietary survey; daily activity log; exercise, sleep and self-care questionnaires; and an environmental risk assessment. Instead of a review of symptoms arranged by organ system as is typical with conventional Western medicine, FM assesses the balance of core functional processes: assimilation, defense and repair, energy, biotransformation and elimination, transport, communication, and structural integrity.
Continue to: Within the matrix...
Within the matrix, FM also recognizes 5 modifiable personal lifestyle factors: sleep and relaxation, exercise and movement, nutrition, stress, and relationships. When these lifestyle elements are influenced by specific predisposing factors (antecedents), discrete events precipitating illness (triggers), and ongoing physiologic processes (mediators), fundamental imbalances eventually result in the signs and symptoms characterizing diagnosable diseases.
The essence of the FM therapeutic plan is a discussion of lifestyle changes, personal strengths, and potential adherence challenges so the clinician can better offer assistive resources, which may include multidisciplinary referrals to personalized counselors such as a nutritionist, health coach, mind-body therapist, personal trainer, exercise physiologist, or physical therapist. While these professionals are not unique to FM, they are more frequently used by FM practitioners as part of the health care team.
Diagnostic testing through a different lens
A proposed solution to complex diseases recalcitrant to conventional modern treatments is a systems biology approach to health care.10 Current laboratory testing such as liver enzymes, C-reactive protein (CRP), and leukocyte count provide some information on organ system homeostasis and molecular pathology, but even high-sensitivity CRP, which measures inflammation across the human system, is not adequate in cardiovascular disease (CVD) risk assessment.11 Genome-wide association studies suggest an association between biological pathways, genes, molecular markers, and QOL domains, and screening patients for cytokine markers may help provide prophylactic interventions to prevent QOL deterioration.12
With the purpose of being more systems-biology centric rather than disease centric,13 FM practitioners use tests to guide dietary recommendations and supplement selection, the evidence for which is found mostly in symptom-specific case series.14-16 Commonly used tests include MTHFR genotyping, comprehensive stool profiles, hormone and heavy metal panels, allergy panels, lactulose breath testing, micronutrient and advanced lipid panels, omega 3:6 ratios, and oxidative stress tests.
Some of these tests are performed by standard labs and are covered by insurance. Certain assays are primarily performed by specialized functional laboratories, such as comprehensive stool profiles, provoked heavy metal tests, non-IgE allergy panels, and oxidative stress tests. These laboratories also frequently offer direct-to-consumer orders. The latter situation raises a potential conflict of interest from supplement sales linked to laboratory testing and makes it imperative for health care professionals to understand the reliability and clinical utility of these tests in order to counsel patients accordingly.
Continue to: The remainder of this article...
The remainder of this article focuses on the evidence behind a subset of FM treatments, which typically include various dietary interventions (elimination, cardiometabolic, detox) and multidomain lifestyle modifications. The practitioner’s selection of dietary interventions, nutraceuticals (vitamins, minerals, essential fatty acids, botanicals), and probiotics is informed by results of different diagnostic tests.
Nutrition and supplements
Nearly one-third of older Americans are affected by at least 1 vitamin deficiency or anemia, and even those consuming an adequate diet have a substantial risk of any deficiency (16%), although less so than those with an inadequate diet (57%).17 The Western diet is known to be nutrient deficient, particularly in vitamin D, thiamine, riboflavin, calcium, magnesium, and selenium.18 Dietary supplement nonusers have the highest risk of any deficiency (40%) compared with users of “full-spectrum multivitamin-multimineral supplements” (14%) and other dietary supplement users (28%).17
Nevertheless, the US Preventive Services Task Force (USPSTF) concluded in 2014 that there are not enough data to make a recommendation for or against taking vitamins A, C, or E; multivitamins with folic acid; or combinations of these vitamins for the primary prevention of CVD or cancer.19 USPSTF also does not recommend daily vitamin D and calcium supplementation in community-dwelling, postmenopausal women for primary prevention of fracture.20 Notwithstanding the lack of supplement recommendations for primary prevention, their benefits in patients with chronic disease is still being investigated. For example, a polyphenol-rich antioxidant may reduce cardiovascular complications in those with diabetes.21
Rethinking how nutrition studies are designed. Drawing on studies to determine the benefits of nutrition in chronic disease has been challenging. Factors that must be taken into account include the types of vitamin and mineral supplements patients use, nutrient absorption and utilization, and differing dietary assessment methods and reference values used.18 For example, vitamin and mineral absorption work best with whole food or fortified diets wherein specific nutrients are consumed together (eg, vitamin D and vitamin E with fat; non-heme iron with vitamin C).22-24 Foods with competing nutrients or “inhibitors” may even require absorption enhancers at minimum molar ratios.24
Adding to the complexity of vitamin and mineral absorption, botanical dietary supplements have their own modifying effects on micronutrient absorption.25 These are just some of the reasons randomized controlled trials (RCTs) are fundamentally limited when investigating the health outcomes of diet and supplementation, and alternative study methods should be considered for future nutrition clinical trials to better inform clinicians who are prescribing supplements.26 In the meantime, nutrition plans can be individualized to patients’ biological and cultural needs, ideally in conjunction with a multidisplinary team that includes dietitians, behaviorists, and exercise specialists.27
Continue to: Nutrition is one of the most...
Nutrition is one of the most important environmental factors modulating genes and phenotypes (nutrigenomics),28,29 and recent studies on single nucleotide polymorphisms (SNPs) have reinforced the importance of considering the effect of genetic variation on dietary response (nutrigenetics).29,30 While nutrigenomics and nutrigenetics have the potential to improve the health of large populations by personalizing dietary advice based on genotype and phenotype, genetic variation occurring within a specific biochemical pathway makes generalizing genotype-based dietary advice to other populations complex.
For instance, Greenlandic Inuit, the indigenous people inhabiting the Arctic regions of Greenland, have a low incidence of CVD, largely due to “non-European” genetic variants that lower their LDL, protecting them from the oxidative stress of their diet high in polyunsaturated fats.31,32 In a study of European adults, phenotypic and phenotypic-plus-genotypic information did not enhance the effectiveness of the personalized nutrition advice, demonstrating that more research is needed in larger populations.33
Another specific, complex example of gene-nutrient interaction is the pathophysiologic outcome of polymorphisms in the MTHFR gene.30 MTHFR is a rate-limiting enzyme involved in folate and homocysteine metabolism, DNA and RNA biosynthesis, and DNA and protein methylation. MTHFR polymorphisms are common in otherwise healthy people, but some have been reported to increase chronic disease susceptibility via MTHFR deficiency. The most common MTHFR variant is the SNP rs1801133 that reduces enzyme activity to ~30% in homozygotes (677TT), which can lead to reduced folate bioavailability and mild-to-moderate hyperhomocysteinemia depending on one’s dietary folate intake and food fortification.
One FM focus at variance with current recommendations. FM’s approach to homocysteine and MTHFR genotyping for nutritional assessment, dietary counseling, and supplement advice contrasts with recommendations from meta-analyses34-36 and guidelines from the American Heart Association and USPSTF.37,38 While USPSTF does recommend all women planning or capable of pregnancy take 0.4 to 0.8 mg of folic acid daily to prevent neural tube defects,39MTHFR polymorphism screening to guide supplementation is not recommended during pregnancy given conflicting studies and uncertain clinical significance.40 Moreover, while it is generally agreed that the MTHFR C677T polymorphism, and in particular the homozygous MTHFR 677TT genotype, is an independent risk factor for hyperhomocysteinemia, neither variant genotype was an independent coronary artery disease risk factor in several large studies.41-43
However, study populations with generally high folate consumption or fortified foods may be a main confounding variable.44 For example, in a recent meta-analysis of an elderly population, the T-allele of the MTHFR C677T variant increased pooled stroke risk. While this increased stroke risk is highest in homozygotes, the association was statistically significant only in the Chinese cohort—a group that generally has poor dietary intake of folate and B12.45 Therefore, both MTHFR genotype and baseline folate level are important determinants of folate therapy efficacy in stroke prevention, and this may explain why US studies have not clearly identified a patient subset most likely to benefit.46 Future high-quality studies should measure baseline folate, target populations with moderate-to-severe hyperhomocysteinemia with specific MTHFR polymorphisms, and compare high- and low-dose B vitamins or use 5-methylfolate (the active form of folate).
Continue to: Probiotics
Probiotics
Probiotics are used extensively in FM, and there are very few fields in conventional Western medicine where probiotics have not been researched. Interestingly, gut microbiota (microflora) change rapidly and individually to therapeutic dietary changes, both in composition (community) and function (metabolic plasticity), implicating gut microbiota as a mediator of dietary impact on host metabolism.47 This highlights the potential for tailored probiotics to transform dietary nonresponders (eg, those who do not routinely consume a high-fiber diet) into responders whose metabolism becomes enabled to counter such conditions as obesity and type 2 diabetes.48
Caveats with probiotic administration. While the strength of recommendation for probiotic therapy is increased by their safe use in pregnancy, infants, and immunocompromised populations,49-51 various harms (ranging from mild to severe) may be underreported.52-54 In clinical practice, the effective probiotic strain(s), formulation, and dosage will vary not only by disease, but also from patient to patient, and may be dependent on nutritional factors such as vitamin D,55 diet, and even epigenetics.56
As we come to more fully recognize commensal microbiota as a major player in overall health with crosstalk in signaling pathways between intestinal bacterial and epithelial immune cells, future research is needed to help optimize probiotic dose, duration, route of administration, and whether microbial communities outperform single-species probiotics.50 Furthermore, much of a bacterial community’s effect on the host is through its metabolites, and since certain species can be used interchangeably given similar metabolic activity and function, studies to understand how prebiotics and probiotics affect the host must analyze the metabolome, in addition to the bacterial community composition.51
Alternative research methods could be informative. Similar to their limited evaluation of health outcomes in nutrition and supplement research, many RCTs examining the health benefits of probiotics often yield ambiguous results or fall short of valid conclusions because the underlying presuppositions are not met.26 For example, assuming the RCT uses a well-defined probiotic formulation, efficacy and generalizability can still be confounded by patient microbiota, diet, mucosa, immune system, and emotional status, which all affect probiotic activity/potency.50 Under these circumstances, other research methods may be more suitable or supplemental—eg, Phase II trials, epidemiologic studies, single-case experiments (n-of-1 trials) and their meta-analyses, using historical controls, preclinical studies for conceptual and theoretical development, and clinical experience.26,57-59
It is currently unknown how nonvaginal microbiota affect the health of menopausal women;63,64 and while a multicenter RCT is underway to examine a novel probiotic’s effect on menopausal symptoms and bone health, the supplemental study methods listed above could be employed as well.
Continue to: Evidence of probiotic effectiveness
Evidence of probiotic effectiveness. Positive evidence in RCTs and meta-analyses suggests that strain-specific probiotics are beneficial for infectious gastroenteritis, persistent antibiotic-associated diarrhea, infant colic,50 irritable bowel syndrome (IBS),50,65Clostridioides difficile prevention,66 inflammatory bowel disease (IBD),67 radiation-induced diarrhea,68,69 and nongastrointestinal conditions such as multiple sclerosis,70 upper respiratory infections,50 atopic dermatitis,50,71,72 surgical site infections,73,74 hyperlipidemia,50,75,76 and nonalcoholic steatohepatitis.77
However, because of knowledge gaps and low-quality evidence, the American Gastroenterological Association’s (AGA) 2020 Clinical Practice Guidelines recommend “conditional” use of strain-specific probiotics only for necrotizing enterocolitis prevention (in pre-term, low-birth-weight infants), antibiotic-induced C difficile prevention, and pouchitis.53,54 Additionally, the AGA’s guidelines state that probiotics should only be used for C difficile infection, IBD, or IBS in a clinical trial and not used at all for infectious gastroenteritis.53,54
Chelation
Chelation therapy is thought to inhibit metal-catalyzed oxidation reactions and inflammatory processes in tissues that lead to, and result from, accumulation of oxidative damage. While many FM practitioners recommend some form of chelation therapy, there is much controversy surrounding its use. Well-designed, large-scale clinical trials continue to emerge for what appears to be an old intervention with still uncertain clinical applications.
The first chelation agent, disodium ethylenediaminetetraacetic acid (EDTA), was synthesized in the early 1930s, but it was not until World War II that chelation therapy began with use of dimercaprol, a potent antidote for the arsenical chemical weapon lewisite. Disodium EDTA infusions were first used to treat angina pectoris in the mid-20th century,78 and use accelerated in the early 21st century without a clear indication to treat CVD besides decades of anecdotes and case reports.79 Strong epidemiologic data support a causal association between heavy metals (arsenic, lead, cadmium, mercury, and copper) and CVD,80,81 but a distinct mechanism of action is not yet known.
Disodium EDTA was FDA approved in the 1950s for use in patients with hypercalcemia or ventricular arrhythmias from digitalis toxicity, but it was eventually withdrawn from the market in 2008, in part due to safety concerns following 3 deaths from severe hypocalcemia.82 These deaths were caused by inappropriate use related to rapid infusions, pediatric use, and in one case inadvertently administering disodium EDTA to a child instead of calcium EDTA (which can safely be bolused to treat pediatric lead toxicity).82-85
Continue to: The Trial to Assess Chelation Therapy...
The Trial to Assess Chelation Therapy (TACT) was the first large, double-blinded, placebo-controlled RCT to test post-myocardial infarction (MI) disodium EDTA infusions (weekly 3-hour infusions for 30 weeks followed by 10 infusions 2 to 8 weeks apart).86 Despite the chelation group having a modest reduction in the primary composite endpoint (hazard ratio [HR] = 0.82; 95% CI, 0.69-0.99; P = .035),86 especially in those with diabetes (HR = 0.59; 95% CI, 0.44-0.79; P < .001; number needed to treat [NNT] = 6.5),87 the authors concluded these results were insufficient to support routine disodium EDTA chelation post MI. Moreover, the 2014 Guidelines for Chronic Ischemic Heart Disease only upgraded chelation therapy from “not recommended” to “uncertain.”88
Nevertheless, to put into perspective the magnitude of chelation’s treatment effect post MI in patients with diabetes, it can be compared to standard-of-care statin RCTs for secondary prevention, reported as follows. The absolute risk reduction (ARR) of coronary events with statin therapy in a high genetic risk group (the group with the greatest ARR) was 6.03% in the CARE trial and 6.87% in the PROVE IT-TIMI 22 trial, corresponding to a calculated NNT (1/ARR) of 16.6 and 14.6 respectively.89
In order to understand the hesitancy to accept chelation therapy as a reasonable CVD treatment,59 one must balance the modest effect demonstrated by TACT with its limitations, the greatest of which was the unusually large proportion of patients who withdrew their consent or were lost to follow-up (~18%).86 However, it is important to note that some patients withdrew after experiencing a primary endpoint and that at least 50% more were from the placebo group than the chelation group,86 rather than the reverse that was erroneously reported by Fihn et al.88 Unblinding was possible, but there were no significant differences in serious adverse events between groups.
Furthermore, since withdrawn subjects had similar CVD risk profiles, more withdrawals in the placebo arm increases the likelihood of more unrecorded primary outcome events in the placebo arm than treatment arm. One possibility is that these could have accentuated the benefit of chelation therapy, and that missing data reduced the reported efficacy. Because of TACT’s profound findings for those with diabetes post MI, the TACT2 Phase 3 clinical trial underway through 2022 will further clarify chelation’s utility for treating stable ischemic heart disease specifically in this group.
Interestingly, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldose reductase inhibitors themselves inhibit advanced glycation end products (AGE) formation in diabetes, most likely via chelation. Increased protein glycation and accumulation of AGEs on tissue proteins during hyperglycemia (the Maillard reaction) are hypothesized to be fundamental in the pathogenesis of diabetic vascular complications. Consequently, chronic, low-dose chelation therapy may have a role in preventing and treating diabetic CVD and nephropathy.90
ACKNOWLEDGEMENT
The authors thank Ariel Pomputius, MLIS, for contributing to the literature searches during manuscript preparation.
CORRESPONDENCE
Frank A. Orlando, MD, UF Health Family Medicine – Springhill, 4197 NW 86th Terrace; [email protected]
1. The Institute for Functional Medicine. 2020. Accessed November 19, 2021. www.ifm.org/
2. Rippe JM, ed. Lifestyle Medicine. 3rd ed. CRC Press, Taylor & Francis Group; 2019.
3. Eisenberg DM, Kessler RC, Foster C, et al. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246-252.
4. Ali A, Katz DL. Disease prevention and health promotion: how integrative medicine fits. Am J Prev Med. 2015;49(5 suppl 3):S230-S240.
5. Bland J. Defining function in the functional medicine model. Integr Med (Encinitas). 2017;16:22-25.
6. ABPS. Integrative medicine examination description. 2020. Accessed November 19, 2021. www.abpsus.org/integrative-medicine-description/
7. Bland JS. The natural roots of functional medicine. Integr Med (Encinitas). 2018;17:12-17.
8. Beidelschies M, Alejandro-Rodriguez M, Ji X, et al. Association of the functional medicine model of care with patient-reported health-related quality-of-life outcomes. JAMA Netw Open. 2019;2:e1914017.
9. The Institute for Functional Medicine. Functional medicine matrix: organizing clinical imbalances. 2020. Accessed November 19, 2021. www.ifm.org/news-insights/toolkit-functional-medicine-matrix/
10. Schadt EE, Björkegren JL. NEW: Network-enabled wisdom in biology, medicine, and health care. Sci Transl Med. 2012;4:115rv1.
11. Curry SJ, Krist AH, Owens DK, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:272-280.
12. Sprangers MA, Thong MS, Bartels M, et al. Biological pathways, candidate genes, and molecular markers associated with quality-of-life domains: an update. Qual Life Res. 2014;23:1997-2013.
13. Bland J. Functional medicine: an operating system for integrative medicine. Integr Med (Encinitas). 2015;14:18-20.
14. Cutshall SM, Bergstrom LR, Kalish DJ. Evaluation of a functional medicine approach to treating fatigue, stress, and digestive issues in women. Complement Ther Clin Pract. 2016;23:75-81.
15. Jaffe R. First line comprehensive care. Part II: Anthropogenic xenobiotics in functional medicine. Managing persisting bioaccumulating pollutants: toxic minerals, biocides, hormone mimics, solvents, and chemical disruptors. Semin Integr Med. 2005;3:79-92.
16. Muran PJ, Muran SY, Beseler CL, et al. Breast health and reducing breast cancer risk: a functional medicine approach. J Altern Complement Med. 2015;21:321-326.
17. Bird JK, Murphy RA, Ciappio ED, et al. Risk of deficiency in multiple concurrent micronutrients in children and adults in the United States. Nutrients. 2017;9:655.
18. ter Borg S, Verlaan S, Hemsworth J, et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr. 2015;113:1195-1206.
19. Moyer VA, on behalf of the USPSTF. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:558-564.
20. Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;319:1592-1599.
21. Fenercioglu AK, Saler T, Genc E, et al. The effects of polyphenol-containing antioxidants on oxidative stress and lipid peroxidation in Type 2 diabetes mellitus without complications. J Endocrinol Invest. 2010;33:118-124.
22. Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: A systematic review. Nutr Rev. 2018;76:60-76.
23. Schmölz L, Birringer M, Lorkowski S, et al. Complexity of vitamin E metabolism. World J Biol Chem. 2016;7:14-43.
24. Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403-419.
25. Gurley BJ, Tonsing-Carter A, Thomas SL, et al. Clinically relevant herb-micronutrient interactions: when botanicals, minerals, and vitamins collide. Adv Nutr. 2018;9:524s-532s.
26. Zeilstra D, Younes JA, Brummer RJ, et al. Perspective: fundamental limitations of the randomized controlled trial method in nutritional research: the example of probiotics. Adv Nutr. 2018;9:561-571.
27. Kimokoti RW, Millen BE. Nutrition for the prevention of chronic diseases. Med Clin North Am. 2016;100:1185-1198.
28. Tucker KL, Smith CE, Lai CQ, et al. Quantifying diet for nutrigenomic studies. Annu Rev Nutr. 2013;33:349-371.
29. Fenech M, El-Sohemy A, Cahill L, et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J Nutrigenet Nutrigenomics. 2011;4:69-89.
30. van Ommen B, van den Broek T, de Hoogh I, et al. Systems biology of personalized nutrition. Nutr Rev. 2017;75:579-599.
31. Fumagalli M, Moltke I, Grarup N, et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343-1347.
32. Mathieson I, Lazaridis I, Rohland N, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499-503.
33. Celis-Morales C, Livingstone KM, Marsaux CF, et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46:578-588.
34. Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009;60:39-54.
35. Li Y, Huang T, Zheng Y, et al. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5:e003768.
36. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;8:CD006612.
37. Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S73.
38. USPSTF. Using nontraditional risk factors in coronary heart disease risk assessment: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:474-482.
39. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. JAMA. 2017;317:183-189.
40. Levin BL, Varga E. MTHFR: addressing genetic counseling dilemmas using evidence-based literature. J Genet Couns. 2016;25:901-911.
41. Luo Z, Lu Z, Muhammad I, et al. Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: a systematic review and updated meta-analysis. Lipids Health Dis. 2018;17:191.
42. Clarke R, Bennett DA, Parish S, et al. Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012;9:e1001177.
43. Kölling K, Ndrepepa G, Koch W, et al. Methylenetetrahydrofolate reductase gene C677T and A1298C polymorphisms, plasma homocysteine, folate, and vitamin B12 levels and the extent of coronary artery disease. Am J Cardiol. 2004;93:1201-1206.
44. Holmes MV, Newcombe P, Hubacek JA, et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet. 2011;378:584-594.
45. Chang G, Kuai Z, Wang J, et al. The association of MTHFR C677T variant with increased risk of ischemic stroke in the elderly population: a meta-analysis of observational studies. BMC Geriatr. 2019;19:331.
46. Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325-1335.
47. Reid G. Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol. 2016;30:17-25.
48. Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56-64.
49. Van den Nieuwboer M, Brummer RJ, Guarner F, et al. The administration of probiotics and synbiotics in immune compromised adults: Is it safe? Benef Microbes. 2015;6:3-17.
50. Liu Y, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment. J Clin Pharmacol. 2018;58(suppl 10):S164-S179.
51. O’Connell TM. The application of metabolomics to probiotic and prebiotic interventions in human clinical studies. Metabolites. 2020;10:120.
52. Lerner A, Shoenfeld Y, Matthias T. Probiotics: if it does not help it does not do any harm. Really? Microorganisms. 2019;7:104.
53. Su G, Ko C, Bercik P, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterol. 2020;159:697-705.
54. Preidis GA, Weizman AV, Kashyap PC, et al. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159:708-738.e4.
55. Charoenngam N, Shirvani A, Kalajian TA, et al. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: A randomized, double-blinded, dose-response study. Anticancer Res. 2020;40:551-556.
56. Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, et al. Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv Nutr. 2019;10(suppl1):S17-S30.
57. APA. Clay RA. More than one way to measure. Monitor Psychol. 2010;4:52. Accessed November 19, 2021. www.apa.org/monitor/2010/09/trials
58. Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2-21.
59. Bauchner H, Fontanarosa PB, Golub RM. Evaluation of the trial to assess chelation therapy (TACT): the scientific process, peer review, and editorial scrutiny. JAMA. 2013;309:1291-1292.
60. Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019;216:20-40.
61. Matsumoto M, Kitada Y, Naito Y. Endothelial function is improved by inducing microbial polyamine production in the gut: a randomized placebo-controlled trial. Nutrients. 2019;11:1188.
62. Bagga D, Reichert JL, Koschutnig K, et al. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes. 2018;9:486-496.
63. Vieira AT, Castelo PM, Ribeiro DA, et al. Influence of oral and gut microbiota in the health of menopausal women. Front Microbiol. 2017;8:1884.
64. Ribeiro AE, Monteiro NES, Moraes AVG, et al. Can the use of probiotics in association with isoflavone improve the symptoms of genitourinary syndrome of menopause? Results from a randomized controlled trial. Menopause. 2018;26:643-652.
65. Hong YS, Hong KS, Park MH, et al. Metabonomic understanding of probiotic effects in humans with irritable bowel syndrome. J Clin Gastroenterol. 2011;45:415-425.
66. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterol. 2017;152:1889-1900.e9.
67. Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol. 2018;233:2091-2103.
68. Linn YH, Thu KK, Win NHH. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiotics Antimicrob Proteins. 2019;11:638-647.
69. Liu M-M, Li S-T, Shu Y, et al. Probiotics for prevention of radiation-induced diarrhea: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0178870.
70. Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36:1245-1249.
71. Navarro-López V, Ramírez-Boscá A, Ramón-Vidal D, et al. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2018;154:37-43.
72. Wang HT, Anvari S, Anagnostou K. The role of probiotics in preventing allergic disease. Children (Basel). 2019;6:24.
73. Kasatpibal N, Whitney JD, Saokaew S, et al. Effectiveness of probiotic, prebiotic, and synbiotic therapies in reducing postoperative complications: a systematic review and network meta-analysis. Clin Infect Dis. 2017;64(suppl2):S153-S160.
74. Liu PC, Yan YK, Ma YJ, et al. Probiotics reduce postoperative infections in patients undergoing colorectal surgery: a systematic review and meta-analysis. Gastroenterol Res Pract. 2017;2017:6029075.
75. Hendijani F, Akbari V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: a systematic review and meta-analysis. Clin Nutr. 2018;37:532-541.
76. Wu Y, Zhang Q, Ren Y, et al. Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PLoS One. 2017;12:e0178868.
77. Ferolla SM, Couto CA, Costa-Silva L, et al. Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with nonalcoholic steatohepatitis. Nutrients. 2016;8:397.
78. Clarke CN, Clarke NE, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetraacetic acid. Am J Med Sci. 1956;232:654-666.
79. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1-23.
80. Chowdhury R, Ramond A, O’Keeffe LM, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:k3310.
81. Zhuang X, Ni A, Liao L, et al. Environment-wide association study to identify novel factors associated with peripheral arterial disease: evidence from the National Health and Nutrition Examination Survey (1999–2004). Atherosclerosis. 2018;269:172-177.
82. Wax PM. Current use of chelation in American health care. J Med Toxicol. 2013:9;303-307.
83. CDC. Deaths associated with hypocalcemia from chelation therapy—Texas, Pennsylvania, and Oregon, 2003-2005. MMWR Morb Mortal Wkly Rep. 2006;55:204-207.
84. Atwood KC, Woeckner E. In pediatric fatality, edetate disodium was no accident. Clin Toxicol (Phila). 2009;47:256.
85. Baxter AJ, Krenzelok EP. Pediatric fatality secondary to EDTA chelation. Clin Toxicol (Phila). 2008;46:1083-1084.
86. Lamas GA, Goertz C, Boineau R, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309:1241-1250.
87. Escolar E, Lamas GA, Mark DB, et al. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014;7:15-24.
88. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929-1949.
89. Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264-2271.
90. Nagai R, Murray DB, Metz TO, et al. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61:549-559.
Could screening patients for cytokine markers help direct interventions to prevent quality-of-life deterioration? What evidence is there that a patient’s methylenetetrahydrofolate reductase (MTHFR) genotype and baseline folate level can determine whether folate therapy will be needed to prevent stroke?
Favorable findings in these areas and others—eg, that specific probiotics benefit those with various gastrointestinal, respiratory, and lipid disorders—are strengthening support for the clinical approach of functional medicine (FM), which focuses on core functional processes: assimilation, defense and repair, energy, biotransformation and elimination, transport, communication, and structural integrity.
In this article, we describe the paradigm of FM, review its origins, and present the evidence base of selected topics: diagnostic testing, nutrition and supplements, probiotics, and chelation. As FM’s popularity increases, a better understanding of it will help us educate our patients on this approach and implement some of its evidence-backed practices. In preparing this review we used keyword searches of PubMed, Embase, Web of Science, Cochrane Library, University of Florida Health Science Center Library eJournals, the Institute for Functional Medicine website,1 and Lifestyle Medicine textbook.2
The core of functional medicine
Complementary and alternative medicine (CAM) refers to medical practices diverging from standards of care and not generally taught at US medical schools or available at US hospitals.3 Integrative Medicine encompasses evidence-informed CAM, conventional Western medicine, and whole systems like FM.4
FM aims to identify root causes of disease, emphasizing function as a dynamic process that can move back and forth on a continuum between health and disorder.5,6 There are 7 defining characteristics of FM: (1) patient centered vs disease centered; (2) systems biology approach acknowledging web-like interconnections of physiologic factors; (3) dynamic balance of gene–environment interactions; (4) personalized care based on biochemical individuality; (5) promotion of organ reserve and sustained health span; (6) health as a positive vitality—not merely the absence of disease; and (7) function vs pathology focused.5
The concept of FM is not new. Its origins can be traced to the 19th century. Jeffrey Bland, PhD, credits the term’s first use to Sir Willoughby F. Wade, MD, in an 1871 Lancet editorial, “Clinical lecture on functional medicine.” Bland formulated the FM paradigm and in 1991 founded the Institute for Functional Medicine (the Institute), its main educational and certifying organization.7 The Institute certifies masters- or doctorate-prepared health professionals from both conventional and complementary health fields who complete 7 courses (Applying FM in Clinical Practice, Gastrointestinal, Environmental Health, Immune, Hormone, Cardiometabolic, and Bioenergetics), present 1 case report, and pass a written exam. In a retrospective cohort study, researchers found that the FM model was associated with greater improvements in patient-reported, health-related quality of life (QOL) compared with usual care.8
Clinical model
FM uses a comprehensive yet practical matrix for obtaining patient histories and for guiding diagnostic testing.9 Tools that support history-taking include a timeline; weeklong dietary survey; daily activity log; exercise, sleep and self-care questionnaires; and an environmental risk assessment. Instead of a review of symptoms arranged by organ system as is typical with conventional Western medicine, FM assesses the balance of core functional processes: assimilation, defense and repair, energy, biotransformation and elimination, transport, communication, and structural integrity.
Continue to: Within the matrix...
Within the matrix, FM also recognizes 5 modifiable personal lifestyle factors: sleep and relaxation, exercise and movement, nutrition, stress, and relationships. When these lifestyle elements are influenced by specific predisposing factors (antecedents), discrete events precipitating illness (triggers), and ongoing physiologic processes (mediators), fundamental imbalances eventually result in the signs and symptoms characterizing diagnosable diseases.
The essence of the FM therapeutic plan is a discussion of lifestyle changes, personal strengths, and potential adherence challenges so the clinician can better offer assistive resources, which may include multidisciplinary referrals to personalized counselors such as a nutritionist, health coach, mind-body therapist, personal trainer, exercise physiologist, or physical therapist. While these professionals are not unique to FM, they are more frequently used by FM practitioners as part of the health care team.
Diagnostic testing through a different lens
A proposed solution to complex diseases recalcitrant to conventional modern treatments is a systems biology approach to health care.10 Current laboratory testing such as liver enzymes, C-reactive protein (CRP), and leukocyte count provide some information on organ system homeostasis and molecular pathology, but even high-sensitivity CRP, which measures inflammation across the human system, is not adequate in cardiovascular disease (CVD) risk assessment.11 Genome-wide association studies suggest an association between biological pathways, genes, molecular markers, and QOL domains, and screening patients for cytokine markers may help provide prophylactic interventions to prevent QOL deterioration.12
With the purpose of being more systems-biology centric rather than disease centric,13 FM practitioners use tests to guide dietary recommendations and supplement selection, the evidence for which is found mostly in symptom-specific case series.14-16 Commonly used tests include MTHFR genotyping, comprehensive stool profiles, hormone and heavy metal panels, allergy panels, lactulose breath testing, micronutrient and advanced lipid panels, omega 3:6 ratios, and oxidative stress tests.
Some of these tests are performed by standard labs and are covered by insurance. Certain assays are primarily performed by specialized functional laboratories, such as comprehensive stool profiles, provoked heavy metal tests, non-IgE allergy panels, and oxidative stress tests. These laboratories also frequently offer direct-to-consumer orders. The latter situation raises a potential conflict of interest from supplement sales linked to laboratory testing and makes it imperative for health care professionals to understand the reliability and clinical utility of these tests in order to counsel patients accordingly.
Continue to: The remainder of this article...
The remainder of this article focuses on the evidence behind a subset of FM treatments, which typically include various dietary interventions (elimination, cardiometabolic, detox) and multidomain lifestyle modifications. The practitioner’s selection of dietary interventions, nutraceuticals (vitamins, minerals, essential fatty acids, botanicals), and probiotics is informed by results of different diagnostic tests.
Nutrition and supplements
Nearly one-third of older Americans are affected by at least 1 vitamin deficiency or anemia, and even those consuming an adequate diet have a substantial risk of any deficiency (16%), although less so than those with an inadequate diet (57%).17 The Western diet is known to be nutrient deficient, particularly in vitamin D, thiamine, riboflavin, calcium, magnesium, and selenium.18 Dietary supplement nonusers have the highest risk of any deficiency (40%) compared with users of “full-spectrum multivitamin-multimineral supplements” (14%) and other dietary supplement users (28%).17
Nevertheless, the US Preventive Services Task Force (USPSTF) concluded in 2014 that there are not enough data to make a recommendation for or against taking vitamins A, C, or E; multivitamins with folic acid; or combinations of these vitamins for the primary prevention of CVD or cancer.19 USPSTF also does not recommend daily vitamin D and calcium supplementation in community-dwelling, postmenopausal women for primary prevention of fracture.20 Notwithstanding the lack of supplement recommendations for primary prevention, their benefits in patients with chronic disease is still being investigated. For example, a polyphenol-rich antioxidant may reduce cardiovascular complications in those with diabetes.21
Rethinking how nutrition studies are designed. Drawing on studies to determine the benefits of nutrition in chronic disease has been challenging. Factors that must be taken into account include the types of vitamin and mineral supplements patients use, nutrient absorption and utilization, and differing dietary assessment methods and reference values used.18 For example, vitamin and mineral absorption work best with whole food or fortified diets wherein specific nutrients are consumed together (eg, vitamin D and vitamin E with fat; non-heme iron with vitamin C).22-24 Foods with competing nutrients or “inhibitors” may even require absorption enhancers at minimum molar ratios.24
Adding to the complexity of vitamin and mineral absorption, botanical dietary supplements have their own modifying effects on micronutrient absorption.25 These are just some of the reasons randomized controlled trials (RCTs) are fundamentally limited when investigating the health outcomes of diet and supplementation, and alternative study methods should be considered for future nutrition clinical trials to better inform clinicians who are prescribing supplements.26 In the meantime, nutrition plans can be individualized to patients’ biological and cultural needs, ideally in conjunction with a multidisplinary team that includes dietitians, behaviorists, and exercise specialists.27
Continue to: Nutrition is one of the most...
Nutrition is one of the most important environmental factors modulating genes and phenotypes (nutrigenomics),28,29 and recent studies on single nucleotide polymorphisms (SNPs) have reinforced the importance of considering the effect of genetic variation on dietary response (nutrigenetics).29,30 While nutrigenomics and nutrigenetics have the potential to improve the health of large populations by personalizing dietary advice based on genotype and phenotype, genetic variation occurring within a specific biochemical pathway makes generalizing genotype-based dietary advice to other populations complex.
For instance, Greenlandic Inuit, the indigenous people inhabiting the Arctic regions of Greenland, have a low incidence of CVD, largely due to “non-European” genetic variants that lower their LDL, protecting them from the oxidative stress of their diet high in polyunsaturated fats.31,32 In a study of European adults, phenotypic and phenotypic-plus-genotypic information did not enhance the effectiveness of the personalized nutrition advice, demonstrating that more research is needed in larger populations.33
Another specific, complex example of gene-nutrient interaction is the pathophysiologic outcome of polymorphisms in the MTHFR gene.30 MTHFR is a rate-limiting enzyme involved in folate and homocysteine metabolism, DNA and RNA biosynthesis, and DNA and protein methylation. MTHFR polymorphisms are common in otherwise healthy people, but some have been reported to increase chronic disease susceptibility via MTHFR deficiency. The most common MTHFR variant is the SNP rs1801133 that reduces enzyme activity to ~30% in homozygotes (677TT), which can lead to reduced folate bioavailability and mild-to-moderate hyperhomocysteinemia depending on one’s dietary folate intake and food fortification.
One FM focus at variance with current recommendations. FM’s approach to homocysteine and MTHFR genotyping for nutritional assessment, dietary counseling, and supplement advice contrasts with recommendations from meta-analyses34-36 and guidelines from the American Heart Association and USPSTF.37,38 While USPSTF does recommend all women planning or capable of pregnancy take 0.4 to 0.8 mg of folic acid daily to prevent neural tube defects,39MTHFR polymorphism screening to guide supplementation is not recommended during pregnancy given conflicting studies and uncertain clinical significance.40 Moreover, while it is generally agreed that the MTHFR C677T polymorphism, and in particular the homozygous MTHFR 677TT genotype, is an independent risk factor for hyperhomocysteinemia, neither variant genotype was an independent coronary artery disease risk factor in several large studies.41-43
However, study populations with generally high folate consumption or fortified foods may be a main confounding variable.44 For example, in a recent meta-analysis of an elderly population, the T-allele of the MTHFR C677T variant increased pooled stroke risk. While this increased stroke risk is highest in homozygotes, the association was statistically significant only in the Chinese cohort—a group that generally has poor dietary intake of folate and B12.45 Therefore, both MTHFR genotype and baseline folate level are important determinants of folate therapy efficacy in stroke prevention, and this may explain why US studies have not clearly identified a patient subset most likely to benefit.46 Future high-quality studies should measure baseline folate, target populations with moderate-to-severe hyperhomocysteinemia with specific MTHFR polymorphisms, and compare high- and low-dose B vitamins or use 5-methylfolate (the active form of folate).
Continue to: Probiotics
Probiotics
Probiotics are used extensively in FM, and there are very few fields in conventional Western medicine where probiotics have not been researched. Interestingly, gut microbiota (microflora) change rapidly and individually to therapeutic dietary changes, both in composition (community) and function (metabolic plasticity), implicating gut microbiota as a mediator of dietary impact on host metabolism.47 This highlights the potential for tailored probiotics to transform dietary nonresponders (eg, those who do not routinely consume a high-fiber diet) into responders whose metabolism becomes enabled to counter such conditions as obesity and type 2 diabetes.48
Caveats with probiotic administration. While the strength of recommendation for probiotic therapy is increased by their safe use in pregnancy, infants, and immunocompromised populations,49-51 various harms (ranging from mild to severe) may be underreported.52-54 In clinical practice, the effective probiotic strain(s), formulation, and dosage will vary not only by disease, but also from patient to patient, and may be dependent on nutritional factors such as vitamin D,55 diet, and even epigenetics.56
As we come to more fully recognize commensal microbiota as a major player in overall health with crosstalk in signaling pathways between intestinal bacterial and epithelial immune cells, future research is needed to help optimize probiotic dose, duration, route of administration, and whether microbial communities outperform single-species probiotics.50 Furthermore, much of a bacterial community’s effect on the host is through its metabolites, and since certain species can be used interchangeably given similar metabolic activity and function, studies to understand how prebiotics and probiotics affect the host must analyze the metabolome, in addition to the bacterial community composition.51
Alternative research methods could be informative. Similar to their limited evaluation of health outcomes in nutrition and supplement research, many RCTs examining the health benefits of probiotics often yield ambiguous results or fall short of valid conclusions because the underlying presuppositions are not met.26 For example, assuming the RCT uses a well-defined probiotic formulation, efficacy and generalizability can still be confounded by patient microbiota, diet, mucosa, immune system, and emotional status, which all affect probiotic activity/potency.50 Under these circumstances, other research methods may be more suitable or supplemental—eg, Phase II trials, epidemiologic studies, single-case experiments (n-of-1 trials) and their meta-analyses, using historical controls, preclinical studies for conceptual and theoretical development, and clinical experience.26,57-59
It is currently unknown how nonvaginal microbiota affect the health of menopausal women;63,64 and while a multicenter RCT is underway to examine a novel probiotic’s effect on menopausal symptoms and bone health, the supplemental study methods listed above could be employed as well.
Continue to: Evidence of probiotic effectiveness
Evidence of probiotic effectiveness. Positive evidence in RCTs and meta-analyses suggests that strain-specific probiotics are beneficial for infectious gastroenteritis, persistent antibiotic-associated diarrhea, infant colic,50 irritable bowel syndrome (IBS),50,65Clostridioides difficile prevention,66 inflammatory bowel disease (IBD),67 radiation-induced diarrhea,68,69 and nongastrointestinal conditions such as multiple sclerosis,70 upper respiratory infections,50 atopic dermatitis,50,71,72 surgical site infections,73,74 hyperlipidemia,50,75,76 and nonalcoholic steatohepatitis.77
However, because of knowledge gaps and low-quality evidence, the American Gastroenterological Association’s (AGA) 2020 Clinical Practice Guidelines recommend “conditional” use of strain-specific probiotics only for necrotizing enterocolitis prevention (in pre-term, low-birth-weight infants), antibiotic-induced C difficile prevention, and pouchitis.53,54 Additionally, the AGA’s guidelines state that probiotics should only be used for C difficile infection, IBD, or IBS in a clinical trial and not used at all for infectious gastroenteritis.53,54
Chelation
Chelation therapy is thought to inhibit metal-catalyzed oxidation reactions and inflammatory processes in tissues that lead to, and result from, accumulation of oxidative damage. While many FM practitioners recommend some form of chelation therapy, there is much controversy surrounding its use. Well-designed, large-scale clinical trials continue to emerge for what appears to be an old intervention with still uncertain clinical applications.
The first chelation agent, disodium ethylenediaminetetraacetic acid (EDTA), was synthesized in the early 1930s, but it was not until World War II that chelation therapy began with use of dimercaprol, a potent antidote for the arsenical chemical weapon lewisite. Disodium EDTA infusions were first used to treat angina pectoris in the mid-20th century,78 and use accelerated in the early 21st century without a clear indication to treat CVD besides decades of anecdotes and case reports.79 Strong epidemiologic data support a causal association between heavy metals (arsenic, lead, cadmium, mercury, and copper) and CVD,80,81 but a distinct mechanism of action is not yet known.
Disodium EDTA was FDA approved in the 1950s for use in patients with hypercalcemia or ventricular arrhythmias from digitalis toxicity, but it was eventually withdrawn from the market in 2008, in part due to safety concerns following 3 deaths from severe hypocalcemia.82 These deaths were caused by inappropriate use related to rapid infusions, pediatric use, and in one case inadvertently administering disodium EDTA to a child instead of calcium EDTA (which can safely be bolused to treat pediatric lead toxicity).82-85
Continue to: The Trial to Assess Chelation Therapy...
The Trial to Assess Chelation Therapy (TACT) was the first large, double-blinded, placebo-controlled RCT to test post-myocardial infarction (MI) disodium EDTA infusions (weekly 3-hour infusions for 30 weeks followed by 10 infusions 2 to 8 weeks apart).86 Despite the chelation group having a modest reduction in the primary composite endpoint (hazard ratio [HR] = 0.82; 95% CI, 0.69-0.99; P = .035),86 especially in those with diabetes (HR = 0.59; 95% CI, 0.44-0.79; P < .001; number needed to treat [NNT] = 6.5),87 the authors concluded these results were insufficient to support routine disodium EDTA chelation post MI. Moreover, the 2014 Guidelines for Chronic Ischemic Heart Disease only upgraded chelation therapy from “not recommended” to “uncertain.”88
Nevertheless, to put into perspective the magnitude of chelation’s treatment effect post MI in patients with diabetes, it can be compared to standard-of-care statin RCTs for secondary prevention, reported as follows. The absolute risk reduction (ARR) of coronary events with statin therapy in a high genetic risk group (the group with the greatest ARR) was 6.03% in the CARE trial and 6.87% in the PROVE IT-TIMI 22 trial, corresponding to a calculated NNT (1/ARR) of 16.6 and 14.6 respectively.89
In order to understand the hesitancy to accept chelation therapy as a reasonable CVD treatment,59 one must balance the modest effect demonstrated by TACT with its limitations, the greatest of which was the unusually large proportion of patients who withdrew their consent or were lost to follow-up (~18%).86 However, it is important to note that some patients withdrew after experiencing a primary endpoint and that at least 50% more were from the placebo group than the chelation group,86 rather than the reverse that was erroneously reported by Fihn et al.88 Unblinding was possible, but there were no significant differences in serious adverse events between groups.
Furthermore, since withdrawn subjects had similar CVD risk profiles, more withdrawals in the placebo arm increases the likelihood of more unrecorded primary outcome events in the placebo arm than treatment arm. One possibility is that these could have accentuated the benefit of chelation therapy, and that missing data reduced the reported efficacy. Because of TACT’s profound findings for those with diabetes post MI, the TACT2 Phase 3 clinical trial underway through 2022 will further clarify chelation’s utility for treating stable ischemic heart disease specifically in this group.
Interestingly, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldose reductase inhibitors themselves inhibit advanced glycation end products (AGE) formation in diabetes, most likely via chelation. Increased protein glycation and accumulation of AGEs on tissue proteins during hyperglycemia (the Maillard reaction) are hypothesized to be fundamental in the pathogenesis of diabetic vascular complications. Consequently, chronic, low-dose chelation therapy may have a role in preventing and treating diabetic CVD and nephropathy.90
ACKNOWLEDGEMENT
The authors thank Ariel Pomputius, MLIS, for contributing to the literature searches during manuscript preparation.
CORRESPONDENCE
Frank A. Orlando, MD, UF Health Family Medicine – Springhill, 4197 NW 86th Terrace; [email protected]
Could screening patients for cytokine markers help direct interventions to prevent quality-of-life deterioration? What evidence is there that a patient’s methylenetetrahydrofolate reductase (MTHFR) genotype and baseline folate level can determine whether folate therapy will be needed to prevent stroke?
Favorable findings in these areas and others—eg, that specific probiotics benefit those with various gastrointestinal, respiratory, and lipid disorders—are strengthening support for the clinical approach of functional medicine (FM), which focuses on core functional processes: assimilation, defense and repair, energy, biotransformation and elimination, transport, communication, and structural integrity.
In this article, we describe the paradigm of FM, review its origins, and present the evidence base of selected topics: diagnostic testing, nutrition and supplements, probiotics, and chelation. As FM’s popularity increases, a better understanding of it will help us educate our patients on this approach and implement some of its evidence-backed practices. In preparing this review we used keyword searches of PubMed, Embase, Web of Science, Cochrane Library, University of Florida Health Science Center Library eJournals, the Institute for Functional Medicine website,1 and Lifestyle Medicine textbook.2
The core of functional medicine
Complementary and alternative medicine (CAM) refers to medical practices diverging from standards of care and not generally taught at US medical schools or available at US hospitals.3 Integrative Medicine encompasses evidence-informed CAM, conventional Western medicine, and whole systems like FM.4
FM aims to identify root causes of disease, emphasizing function as a dynamic process that can move back and forth on a continuum between health and disorder.5,6 There are 7 defining characteristics of FM: (1) patient centered vs disease centered; (2) systems biology approach acknowledging web-like interconnections of physiologic factors; (3) dynamic balance of gene–environment interactions; (4) personalized care based on biochemical individuality; (5) promotion of organ reserve and sustained health span; (6) health as a positive vitality—not merely the absence of disease; and (7) function vs pathology focused.5
The concept of FM is not new. Its origins can be traced to the 19th century. Jeffrey Bland, PhD, credits the term’s first use to Sir Willoughby F. Wade, MD, in an 1871 Lancet editorial, “Clinical lecture on functional medicine.” Bland formulated the FM paradigm and in 1991 founded the Institute for Functional Medicine (the Institute), its main educational and certifying organization.7 The Institute certifies masters- or doctorate-prepared health professionals from both conventional and complementary health fields who complete 7 courses (Applying FM in Clinical Practice, Gastrointestinal, Environmental Health, Immune, Hormone, Cardiometabolic, and Bioenergetics), present 1 case report, and pass a written exam. In a retrospective cohort study, researchers found that the FM model was associated with greater improvements in patient-reported, health-related quality of life (QOL) compared with usual care.8
Clinical model
FM uses a comprehensive yet practical matrix for obtaining patient histories and for guiding diagnostic testing.9 Tools that support history-taking include a timeline; weeklong dietary survey; daily activity log; exercise, sleep and self-care questionnaires; and an environmental risk assessment. Instead of a review of symptoms arranged by organ system as is typical with conventional Western medicine, FM assesses the balance of core functional processes: assimilation, defense and repair, energy, biotransformation and elimination, transport, communication, and structural integrity.
Continue to: Within the matrix...
Within the matrix, FM also recognizes 5 modifiable personal lifestyle factors: sleep and relaxation, exercise and movement, nutrition, stress, and relationships. When these lifestyle elements are influenced by specific predisposing factors (antecedents), discrete events precipitating illness (triggers), and ongoing physiologic processes (mediators), fundamental imbalances eventually result in the signs and symptoms characterizing diagnosable diseases.
The essence of the FM therapeutic plan is a discussion of lifestyle changes, personal strengths, and potential adherence challenges so the clinician can better offer assistive resources, which may include multidisciplinary referrals to personalized counselors such as a nutritionist, health coach, mind-body therapist, personal trainer, exercise physiologist, or physical therapist. While these professionals are not unique to FM, they are more frequently used by FM practitioners as part of the health care team.
Diagnostic testing through a different lens
A proposed solution to complex diseases recalcitrant to conventional modern treatments is a systems biology approach to health care.10 Current laboratory testing such as liver enzymes, C-reactive protein (CRP), and leukocyte count provide some information on organ system homeostasis and molecular pathology, but even high-sensitivity CRP, which measures inflammation across the human system, is not adequate in cardiovascular disease (CVD) risk assessment.11 Genome-wide association studies suggest an association between biological pathways, genes, molecular markers, and QOL domains, and screening patients for cytokine markers may help provide prophylactic interventions to prevent QOL deterioration.12
With the purpose of being more systems-biology centric rather than disease centric,13 FM practitioners use tests to guide dietary recommendations and supplement selection, the evidence for which is found mostly in symptom-specific case series.14-16 Commonly used tests include MTHFR genotyping, comprehensive stool profiles, hormone and heavy metal panels, allergy panels, lactulose breath testing, micronutrient and advanced lipid panels, omega 3:6 ratios, and oxidative stress tests.
Some of these tests are performed by standard labs and are covered by insurance. Certain assays are primarily performed by specialized functional laboratories, such as comprehensive stool profiles, provoked heavy metal tests, non-IgE allergy panels, and oxidative stress tests. These laboratories also frequently offer direct-to-consumer orders. The latter situation raises a potential conflict of interest from supplement sales linked to laboratory testing and makes it imperative for health care professionals to understand the reliability and clinical utility of these tests in order to counsel patients accordingly.
Continue to: The remainder of this article...
The remainder of this article focuses on the evidence behind a subset of FM treatments, which typically include various dietary interventions (elimination, cardiometabolic, detox) and multidomain lifestyle modifications. The practitioner’s selection of dietary interventions, nutraceuticals (vitamins, minerals, essential fatty acids, botanicals), and probiotics is informed by results of different diagnostic tests.
Nutrition and supplements
Nearly one-third of older Americans are affected by at least 1 vitamin deficiency or anemia, and even those consuming an adequate diet have a substantial risk of any deficiency (16%), although less so than those with an inadequate diet (57%).17 The Western diet is known to be nutrient deficient, particularly in vitamin D, thiamine, riboflavin, calcium, magnesium, and selenium.18 Dietary supplement nonusers have the highest risk of any deficiency (40%) compared with users of “full-spectrum multivitamin-multimineral supplements” (14%) and other dietary supplement users (28%).17
Nevertheless, the US Preventive Services Task Force (USPSTF) concluded in 2014 that there are not enough data to make a recommendation for or against taking vitamins A, C, or E; multivitamins with folic acid; or combinations of these vitamins for the primary prevention of CVD or cancer.19 USPSTF also does not recommend daily vitamin D and calcium supplementation in community-dwelling, postmenopausal women for primary prevention of fracture.20 Notwithstanding the lack of supplement recommendations for primary prevention, their benefits in patients with chronic disease is still being investigated. For example, a polyphenol-rich antioxidant may reduce cardiovascular complications in those with diabetes.21
Rethinking how nutrition studies are designed. Drawing on studies to determine the benefits of nutrition in chronic disease has been challenging. Factors that must be taken into account include the types of vitamin and mineral supplements patients use, nutrient absorption and utilization, and differing dietary assessment methods and reference values used.18 For example, vitamin and mineral absorption work best with whole food or fortified diets wherein specific nutrients are consumed together (eg, vitamin D and vitamin E with fat; non-heme iron with vitamin C).22-24 Foods with competing nutrients or “inhibitors” may even require absorption enhancers at minimum molar ratios.24
Adding to the complexity of vitamin and mineral absorption, botanical dietary supplements have their own modifying effects on micronutrient absorption.25 These are just some of the reasons randomized controlled trials (RCTs) are fundamentally limited when investigating the health outcomes of diet and supplementation, and alternative study methods should be considered for future nutrition clinical trials to better inform clinicians who are prescribing supplements.26 In the meantime, nutrition plans can be individualized to patients’ biological and cultural needs, ideally in conjunction with a multidisplinary team that includes dietitians, behaviorists, and exercise specialists.27
Continue to: Nutrition is one of the most...
Nutrition is one of the most important environmental factors modulating genes and phenotypes (nutrigenomics),28,29 and recent studies on single nucleotide polymorphisms (SNPs) have reinforced the importance of considering the effect of genetic variation on dietary response (nutrigenetics).29,30 While nutrigenomics and nutrigenetics have the potential to improve the health of large populations by personalizing dietary advice based on genotype and phenotype, genetic variation occurring within a specific biochemical pathway makes generalizing genotype-based dietary advice to other populations complex.
For instance, Greenlandic Inuit, the indigenous people inhabiting the Arctic regions of Greenland, have a low incidence of CVD, largely due to “non-European” genetic variants that lower their LDL, protecting them from the oxidative stress of their diet high in polyunsaturated fats.31,32 In a study of European adults, phenotypic and phenotypic-plus-genotypic information did not enhance the effectiveness of the personalized nutrition advice, demonstrating that more research is needed in larger populations.33
Another specific, complex example of gene-nutrient interaction is the pathophysiologic outcome of polymorphisms in the MTHFR gene.30 MTHFR is a rate-limiting enzyme involved in folate and homocysteine metabolism, DNA and RNA biosynthesis, and DNA and protein methylation. MTHFR polymorphisms are common in otherwise healthy people, but some have been reported to increase chronic disease susceptibility via MTHFR deficiency. The most common MTHFR variant is the SNP rs1801133 that reduces enzyme activity to ~30% in homozygotes (677TT), which can lead to reduced folate bioavailability and mild-to-moderate hyperhomocysteinemia depending on one’s dietary folate intake and food fortification.
One FM focus at variance with current recommendations. FM’s approach to homocysteine and MTHFR genotyping for nutritional assessment, dietary counseling, and supplement advice contrasts with recommendations from meta-analyses34-36 and guidelines from the American Heart Association and USPSTF.37,38 While USPSTF does recommend all women planning or capable of pregnancy take 0.4 to 0.8 mg of folic acid daily to prevent neural tube defects,39MTHFR polymorphism screening to guide supplementation is not recommended during pregnancy given conflicting studies and uncertain clinical significance.40 Moreover, while it is generally agreed that the MTHFR C677T polymorphism, and in particular the homozygous MTHFR 677TT genotype, is an independent risk factor for hyperhomocysteinemia, neither variant genotype was an independent coronary artery disease risk factor in several large studies.41-43
However, study populations with generally high folate consumption or fortified foods may be a main confounding variable.44 For example, in a recent meta-analysis of an elderly population, the T-allele of the MTHFR C677T variant increased pooled stroke risk. While this increased stroke risk is highest in homozygotes, the association was statistically significant only in the Chinese cohort—a group that generally has poor dietary intake of folate and B12.45 Therefore, both MTHFR genotype and baseline folate level are important determinants of folate therapy efficacy in stroke prevention, and this may explain why US studies have not clearly identified a patient subset most likely to benefit.46 Future high-quality studies should measure baseline folate, target populations with moderate-to-severe hyperhomocysteinemia with specific MTHFR polymorphisms, and compare high- and low-dose B vitamins or use 5-methylfolate (the active form of folate).
Continue to: Probiotics
Probiotics
Probiotics are used extensively in FM, and there are very few fields in conventional Western medicine where probiotics have not been researched. Interestingly, gut microbiota (microflora) change rapidly and individually to therapeutic dietary changes, both in composition (community) and function (metabolic plasticity), implicating gut microbiota as a mediator of dietary impact on host metabolism.47 This highlights the potential for tailored probiotics to transform dietary nonresponders (eg, those who do not routinely consume a high-fiber diet) into responders whose metabolism becomes enabled to counter such conditions as obesity and type 2 diabetes.48
Caveats with probiotic administration. While the strength of recommendation for probiotic therapy is increased by their safe use in pregnancy, infants, and immunocompromised populations,49-51 various harms (ranging from mild to severe) may be underreported.52-54 In clinical practice, the effective probiotic strain(s), formulation, and dosage will vary not only by disease, but also from patient to patient, and may be dependent on nutritional factors such as vitamin D,55 diet, and even epigenetics.56
As we come to more fully recognize commensal microbiota as a major player in overall health with crosstalk in signaling pathways between intestinal bacterial and epithelial immune cells, future research is needed to help optimize probiotic dose, duration, route of administration, and whether microbial communities outperform single-species probiotics.50 Furthermore, much of a bacterial community’s effect on the host is through its metabolites, and since certain species can be used interchangeably given similar metabolic activity and function, studies to understand how prebiotics and probiotics affect the host must analyze the metabolome, in addition to the bacterial community composition.51
Alternative research methods could be informative. Similar to their limited evaluation of health outcomes in nutrition and supplement research, many RCTs examining the health benefits of probiotics often yield ambiguous results or fall short of valid conclusions because the underlying presuppositions are not met.26 For example, assuming the RCT uses a well-defined probiotic formulation, efficacy and generalizability can still be confounded by patient microbiota, diet, mucosa, immune system, and emotional status, which all affect probiotic activity/potency.50 Under these circumstances, other research methods may be more suitable or supplemental—eg, Phase II trials, epidemiologic studies, single-case experiments (n-of-1 trials) and their meta-analyses, using historical controls, preclinical studies for conceptual and theoretical development, and clinical experience.26,57-59
It is currently unknown how nonvaginal microbiota affect the health of menopausal women;63,64 and while a multicenter RCT is underway to examine a novel probiotic’s effect on menopausal symptoms and bone health, the supplemental study methods listed above could be employed as well.
Continue to: Evidence of probiotic effectiveness
Evidence of probiotic effectiveness. Positive evidence in RCTs and meta-analyses suggests that strain-specific probiotics are beneficial for infectious gastroenteritis, persistent antibiotic-associated diarrhea, infant colic,50 irritable bowel syndrome (IBS),50,65Clostridioides difficile prevention,66 inflammatory bowel disease (IBD),67 radiation-induced diarrhea,68,69 and nongastrointestinal conditions such as multiple sclerosis,70 upper respiratory infections,50 atopic dermatitis,50,71,72 surgical site infections,73,74 hyperlipidemia,50,75,76 and nonalcoholic steatohepatitis.77
However, because of knowledge gaps and low-quality evidence, the American Gastroenterological Association’s (AGA) 2020 Clinical Practice Guidelines recommend “conditional” use of strain-specific probiotics only for necrotizing enterocolitis prevention (in pre-term, low-birth-weight infants), antibiotic-induced C difficile prevention, and pouchitis.53,54 Additionally, the AGA’s guidelines state that probiotics should only be used for C difficile infection, IBD, or IBS in a clinical trial and not used at all for infectious gastroenteritis.53,54
Chelation
Chelation therapy is thought to inhibit metal-catalyzed oxidation reactions and inflammatory processes in tissues that lead to, and result from, accumulation of oxidative damage. While many FM practitioners recommend some form of chelation therapy, there is much controversy surrounding its use. Well-designed, large-scale clinical trials continue to emerge for what appears to be an old intervention with still uncertain clinical applications.
The first chelation agent, disodium ethylenediaminetetraacetic acid (EDTA), was synthesized in the early 1930s, but it was not until World War II that chelation therapy began with use of dimercaprol, a potent antidote for the arsenical chemical weapon lewisite. Disodium EDTA infusions were first used to treat angina pectoris in the mid-20th century,78 and use accelerated in the early 21st century without a clear indication to treat CVD besides decades of anecdotes and case reports.79 Strong epidemiologic data support a causal association between heavy metals (arsenic, lead, cadmium, mercury, and copper) and CVD,80,81 but a distinct mechanism of action is not yet known.
Disodium EDTA was FDA approved in the 1950s for use in patients with hypercalcemia or ventricular arrhythmias from digitalis toxicity, but it was eventually withdrawn from the market in 2008, in part due to safety concerns following 3 deaths from severe hypocalcemia.82 These deaths were caused by inappropriate use related to rapid infusions, pediatric use, and in one case inadvertently administering disodium EDTA to a child instead of calcium EDTA (which can safely be bolused to treat pediatric lead toxicity).82-85
Continue to: The Trial to Assess Chelation Therapy...
The Trial to Assess Chelation Therapy (TACT) was the first large, double-blinded, placebo-controlled RCT to test post-myocardial infarction (MI) disodium EDTA infusions (weekly 3-hour infusions for 30 weeks followed by 10 infusions 2 to 8 weeks apart).86 Despite the chelation group having a modest reduction in the primary composite endpoint (hazard ratio [HR] = 0.82; 95% CI, 0.69-0.99; P = .035),86 especially in those with diabetes (HR = 0.59; 95% CI, 0.44-0.79; P < .001; number needed to treat [NNT] = 6.5),87 the authors concluded these results were insufficient to support routine disodium EDTA chelation post MI. Moreover, the 2014 Guidelines for Chronic Ischemic Heart Disease only upgraded chelation therapy from “not recommended” to “uncertain.”88
Nevertheless, to put into perspective the magnitude of chelation’s treatment effect post MI in patients with diabetes, it can be compared to standard-of-care statin RCTs for secondary prevention, reported as follows. The absolute risk reduction (ARR) of coronary events with statin therapy in a high genetic risk group (the group with the greatest ARR) was 6.03% in the CARE trial and 6.87% in the PROVE IT-TIMI 22 trial, corresponding to a calculated NNT (1/ARR) of 16.6 and 14.6 respectively.89
In order to understand the hesitancy to accept chelation therapy as a reasonable CVD treatment,59 one must balance the modest effect demonstrated by TACT with its limitations, the greatest of which was the unusually large proportion of patients who withdrew their consent or were lost to follow-up (~18%).86 However, it is important to note that some patients withdrew after experiencing a primary endpoint and that at least 50% more were from the placebo group than the chelation group,86 rather than the reverse that was erroneously reported by Fihn et al.88 Unblinding was possible, but there were no significant differences in serious adverse events between groups.
Furthermore, since withdrawn subjects had similar CVD risk profiles, more withdrawals in the placebo arm increases the likelihood of more unrecorded primary outcome events in the placebo arm than treatment arm. One possibility is that these could have accentuated the benefit of chelation therapy, and that missing data reduced the reported efficacy. Because of TACT’s profound findings for those with diabetes post MI, the TACT2 Phase 3 clinical trial underway through 2022 will further clarify chelation’s utility for treating stable ischemic heart disease specifically in this group.
Interestingly, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldose reductase inhibitors themselves inhibit advanced glycation end products (AGE) formation in diabetes, most likely via chelation. Increased protein glycation and accumulation of AGEs on tissue proteins during hyperglycemia (the Maillard reaction) are hypothesized to be fundamental in the pathogenesis of diabetic vascular complications. Consequently, chronic, low-dose chelation therapy may have a role in preventing and treating diabetic CVD and nephropathy.90
ACKNOWLEDGEMENT
The authors thank Ariel Pomputius, MLIS, for contributing to the literature searches during manuscript preparation.
CORRESPONDENCE
Frank A. Orlando, MD, UF Health Family Medicine – Springhill, 4197 NW 86th Terrace; [email protected]
1. The Institute for Functional Medicine. 2020. Accessed November 19, 2021. www.ifm.org/
2. Rippe JM, ed. Lifestyle Medicine. 3rd ed. CRC Press, Taylor & Francis Group; 2019.
3. Eisenberg DM, Kessler RC, Foster C, et al. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246-252.
4. Ali A, Katz DL. Disease prevention and health promotion: how integrative medicine fits. Am J Prev Med. 2015;49(5 suppl 3):S230-S240.
5. Bland J. Defining function in the functional medicine model. Integr Med (Encinitas). 2017;16:22-25.
6. ABPS. Integrative medicine examination description. 2020. Accessed November 19, 2021. www.abpsus.org/integrative-medicine-description/
7. Bland JS. The natural roots of functional medicine. Integr Med (Encinitas). 2018;17:12-17.
8. Beidelschies M, Alejandro-Rodriguez M, Ji X, et al. Association of the functional medicine model of care with patient-reported health-related quality-of-life outcomes. JAMA Netw Open. 2019;2:e1914017.
9. The Institute for Functional Medicine. Functional medicine matrix: organizing clinical imbalances. 2020. Accessed November 19, 2021. www.ifm.org/news-insights/toolkit-functional-medicine-matrix/
10. Schadt EE, Björkegren JL. NEW: Network-enabled wisdom in biology, medicine, and health care. Sci Transl Med. 2012;4:115rv1.
11. Curry SJ, Krist AH, Owens DK, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:272-280.
12. Sprangers MA, Thong MS, Bartels M, et al. Biological pathways, candidate genes, and molecular markers associated with quality-of-life domains: an update. Qual Life Res. 2014;23:1997-2013.
13. Bland J. Functional medicine: an operating system for integrative medicine. Integr Med (Encinitas). 2015;14:18-20.
14. Cutshall SM, Bergstrom LR, Kalish DJ. Evaluation of a functional medicine approach to treating fatigue, stress, and digestive issues in women. Complement Ther Clin Pract. 2016;23:75-81.
15. Jaffe R. First line comprehensive care. Part II: Anthropogenic xenobiotics in functional medicine. Managing persisting bioaccumulating pollutants: toxic minerals, biocides, hormone mimics, solvents, and chemical disruptors. Semin Integr Med. 2005;3:79-92.
16. Muran PJ, Muran SY, Beseler CL, et al. Breast health and reducing breast cancer risk: a functional medicine approach. J Altern Complement Med. 2015;21:321-326.
17. Bird JK, Murphy RA, Ciappio ED, et al. Risk of deficiency in multiple concurrent micronutrients in children and adults in the United States. Nutrients. 2017;9:655.
18. ter Borg S, Verlaan S, Hemsworth J, et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr. 2015;113:1195-1206.
19. Moyer VA, on behalf of the USPSTF. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:558-564.
20. Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;319:1592-1599.
21. Fenercioglu AK, Saler T, Genc E, et al. The effects of polyphenol-containing antioxidants on oxidative stress and lipid peroxidation in Type 2 diabetes mellitus without complications. J Endocrinol Invest. 2010;33:118-124.
22. Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: A systematic review. Nutr Rev. 2018;76:60-76.
23. Schmölz L, Birringer M, Lorkowski S, et al. Complexity of vitamin E metabolism. World J Biol Chem. 2016;7:14-43.
24. Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403-419.
25. Gurley BJ, Tonsing-Carter A, Thomas SL, et al. Clinically relevant herb-micronutrient interactions: when botanicals, minerals, and vitamins collide. Adv Nutr. 2018;9:524s-532s.
26. Zeilstra D, Younes JA, Brummer RJ, et al. Perspective: fundamental limitations of the randomized controlled trial method in nutritional research: the example of probiotics. Adv Nutr. 2018;9:561-571.
27. Kimokoti RW, Millen BE. Nutrition for the prevention of chronic diseases. Med Clin North Am. 2016;100:1185-1198.
28. Tucker KL, Smith CE, Lai CQ, et al. Quantifying diet for nutrigenomic studies. Annu Rev Nutr. 2013;33:349-371.
29. Fenech M, El-Sohemy A, Cahill L, et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J Nutrigenet Nutrigenomics. 2011;4:69-89.
30. van Ommen B, van den Broek T, de Hoogh I, et al. Systems biology of personalized nutrition. Nutr Rev. 2017;75:579-599.
31. Fumagalli M, Moltke I, Grarup N, et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343-1347.
32. Mathieson I, Lazaridis I, Rohland N, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499-503.
33. Celis-Morales C, Livingstone KM, Marsaux CF, et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46:578-588.
34. Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009;60:39-54.
35. Li Y, Huang T, Zheng Y, et al. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5:e003768.
36. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;8:CD006612.
37. Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S73.
38. USPSTF. Using nontraditional risk factors in coronary heart disease risk assessment: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:474-482.
39. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. JAMA. 2017;317:183-189.
40. Levin BL, Varga E. MTHFR: addressing genetic counseling dilemmas using evidence-based literature. J Genet Couns. 2016;25:901-911.
41. Luo Z, Lu Z, Muhammad I, et al. Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: a systematic review and updated meta-analysis. Lipids Health Dis. 2018;17:191.
42. Clarke R, Bennett DA, Parish S, et al. Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012;9:e1001177.
43. Kölling K, Ndrepepa G, Koch W, et al. Methylenetetrahydrofolate reductase gene C677T and A1298C polymorphisms, plasma homocysteine, folate, and vitamin B12 levels and the extent of coronary artery disease. Am J Cardiol. 2004;93:1201-1206.
44. Holmes MV, Newcombe P, Hubacek JA, et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet. 2011;378:584-594.
45. Chang G, Kuai Z, Wang J, et al. The association of MTHFR C677T variant with increased risk of ischemic stroke in the elderly population: a meta-analysis of observational studies. BMC Geriatr. 2019;19:331.
46. Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325-1335.
47. Reid G. Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol. 2016;30:17-25.
48. Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56-64.
49. Van den Nieuwboer M, Brummer RJ, Guarner F, et al. The administration of probiotics and synbiotics in immune compromised adults: Is it safe? Benef Microbes. 2015;6:3-17.
50. Liu Y, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment. J Clin Pharmacol. 2018;58(suppl 10):S164-S179.
51. O’Connell TM. The application of metabolomics to probiotic and prebiotic interventions in human clinical studies. Metabolites. 2020;10:120.
52. Lerner A, Shoenfeld Y, Matthias T. Probiotics: if it does not help it does not do any harm. Really? Microorganisms. 2019;7:104.
53. Su G, Ko C, Bercik P, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterol. 2020;159:697-705.
54. Preidis GA, Weizman AV, Kashyap PC, et al. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159:708-738.e4.
55. Charoenngam N, Shirvani A, Kalajian TA, et al. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: A randomized, double-blinded, dose-response study. Anticancer Res. 2020;40:551-556.
56. Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, et al. Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv Nutr. 2019;10(suppl1):S17-S30.
57. APA. Clay RA. More than one way to measure. Monitor Psychol. 2010;4:52. Accessed November 19, 2021. www.apa.org/monitor/2010/09/trials
58. Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2-21.
59. Bauchner H, Fontanarosa PB, Golub RM. Evaluation of the trial to assess chelation therapy (TACT): the scientific process, peer review, and editorial scrutiny. JAMA. 2013;309:1291-1292.
60. Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019;216:20-40.
61. Matsumoto M, Kitada Y, Naito Y. Endothelial function is improved by inducing microbial polyamine production in the gut: a randomized placebo-controlled trial. Nutrients. 2019;11:1188.
62. Bagga D, Reichert JL, Koschutnig K, et al. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes. 2018;9:486-496.
63. Vieira AT, Castelo PM, Ribeiro DA, et al. Influence of oral and gut microbiota in the health of menopausal women. Front Microbiol. 2017;8:1884.
64. Ribeiro AE, Monteiro NES, Moraes AVG, et al. Can the use of probiotics in association with isoflavone improve the symptoms of genitourinary syndrome of menopause? Results from a randomized controlled trial. Menopause. 2018;26:643-652.
65. Hong YS, Hong KS, Park MH, et al. Metabonomic understanding of probiotic effects in humans with irritable bowel syndrome. J Clin Gastroenterol. 2011;45:415-425.
66. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterol. 2017;152:1889-1900.e9.
67. Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol. 2018;233:2091-2103.
68. Linn YH, Thu KK, Win NHH. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiotics Antimicrob Proteins. 2019;11:638-647.
69. Liu M-M, Li S-T, Shu Y, et al. Probiotics for prevention of radiation-induced diarrhea: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0178870.
70. Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36:1245-1249.
71. Navarro-López V, Ramírez-Boscá A, Ramón-Vidal D, et al. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2018;154:37-43.
72. Wang HT, Anvari S, Anagnostou K. The role of probiotics in preventing allergic disease. Children (Basel). 2019;6:24.
73. Kasatpibal N, Whitney JD, Saokaew S, et al. Effectiveness of probiotic, prebiotic, and synbiotic therapies in reducing postoperative complications: a systematic review and network meta-analysis. Clin Infect Dis. 2017;64(suppl2):S153-S160.
74. Liu PC, Yan YK, Ma YJ, et al. Probiotics reduce postoperative infections in patients undergoing colorectal surgery: a systematic review and meta-analysis. Gastroenterol Res Pract. 2017;2017:6029075.
75. Hendijani F, Akbari V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: a systematic review and meta-analysis. Clin Nutr. 2018;37:532-541.
76. Wu Y, Zhang Q, Ren Y, et al. Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PLoS One. 2017;12:e0178868.
77. Ferolla SM, Couto CA, Costa-Silva L, et al. Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with nonalcoholic steatohepatitis. Nutrients. 2016;8:397.
78. Clarke CN, Clarke NE, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetraacetic acid. Am J Med Sci. 1956;232:654-666.
79. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1-23.
80. Chowdhury R, Ramond A, O’Keeffe LM, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:k3310.
81. Zhuang X, Ni A, Liao L, et al. Environment-wide association study to identify novel factors associated with peripheral arterial disease: evidence from the National Health and Nutrition Examination Survey (1999–2004). Atherosclerosis. 2018;269:172-177.
82. Wax PM. Current use of chelation in American health care. J Med Toxicol. 2013:9;303-307.
83. CDC. Deaths associated with hypocalcemia from chelation therapy—Texas, Pennsylvania, and Oregon, 2003-2005. MMWR Morb Mortal Wkly Rep. 2006;55:204-207.
84. Atwood KC, Woeckner E. In pediatric fatality, edetate disodium was no accident. Clin Toxicol (Phila). 2009;47:256.
85. Baxter AJ, Krenzelok EP. Pediatric fatality secondary to EDTA chelation. Clin Toxicol (Phila). 2008;46:1083-1084.
86. Lamas GA, Goertz C, Boineau R, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309:1241-1250.
87. Escolar E, Lamas GA, Mark DB, et al. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014;7:15-24.
88. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929-1949.
89. Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264-2271.
90. Nagai R, Murray DB, Metz TO, et al. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61:549-559.
1. The Institute for Functional Medicine. 2020. Accessed November 19, 2021. www.ifm.org/
2. Rippe JM, ed. Lifestyle Medicine. 3rd ed. CRC Press, Taylor & Francis Group; 2019.
3. Eisenberg DM, Kessler RC, Foster C, et al. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246-252.
4. Ali A, Katz DL. Disease prevention and health promotion: how integrative medicine fits. Am J Prev Med. 2015;49(5 suppl 3):S230-S240.
5. Bland J. Defining function in the functional medicine model. Integr Med (Encinitas). 2017;16:22-25.
6. ABPS. Integrative medicine examination description. 2020. Accessed November 19, 2021. www.abpsus.org/integrative-medicine-description/
7. Bland JS. The natural roots of functional medicine. Integr Med (Encinitas). 2018;17:12-17.
8. Beidelschies M, Alejandro-Rodriguez M, Ji X, et al. Association of the functional medicine model of care with patient-reported health-related quality-of-life outcomes. JAMA Netw Open. 2019;2:e1914017.
9. The Institute for Functional Medicine. Functional medicine matrix: organizing clinical imbalances. 2020. Accessed November 19, 2021. www.ifm.org/news-insights/toolkit-functional-medicine-matrix/
10. Schadt EE, Björkegren JL. NEW: Network-enabled wisdom in biology, medicine, and health care. Sci Transl Med. 2012;4:115rv1.
11. Curry SJ, Krist AH, Owens DK, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:272-280.
12. Sprangers MA, Thong MS, Bartels M, et al. Biological pathways, candidate genes, and molecular markers associated with quality-of-life domains: an update. Qual Life Res. 2014;23:1997-2013.
13. Bland J. Functional medicine: an operating system for integrative medicine. Integr Med (Encinitas). 2015;14:18-20.
14. Cutshall SM, Bergstrom LR, Kalish DJ. Evaluation of a functional medicine approach to treating fatigue, stress, and digestive issues in women. Complement Ther Clin Pract. 2016;23:75-81.
15. Jaffe R. First line comprehensive care. Part II: Anthropogenic xenobiotics in functional medicine. Managing persisting bioaccumulating pollutants: toxic minerals, biocides, hormone mimics, solvents, and chemical disruptors. Semin Integr Med. 2005;3:79-92.
16. Muran PJ, Muran SY, Beseler CL, et al. Breast health and reducing breast cancer risk: a functional medicine approach. J Altern Complement Med. 2015;21:321-326.
17. Bird JK, Murphy RA, Ciappio ED, et al. Risk of deficiency in multiple concurrent micronutrients in children and adults in the United States. Nutrients. 2017;9:655.
18. ter Borg S, Verlaan S, Hemsworth J, et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr. 2015;113:1195-1206.
19. Moyer VA, on behalf of the USPSTF. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:558-564.
20. Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;319:1592-1599.
21. Fenercioglu AK, Saler T, Genc E, et al. The effects of polyphenol-containing antioxidants on oxidative stress and lipid peroxidation in Type 2 diabetes mellitus without complications. J Endocrinol Invest. 2010;33:118-124.
22. Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: A systematic review. Nutr Rev. 2018;76:60-76.
23. Schmölz L, Birringer M, Lorkowski S, et al. Complexity of vitamin E metabolism. World J Biol Chem. 2016;7:14-43.
24. Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403-419.
25. Gurley BJ, Tonsing-Carter A, Thomas SL, et al. Clinically relevant herb-micronutrient interactions: when botanicals, minerals, and vitamins collide. Adv Nutr. 2018;9:524s-532s.
26. Zeilstra D, Younes JA, Brummer RJ, et al. Perspective: fundamental limitations of the randomized controlled trial method in nutritional research: the example of probiotics. Adv Nutr. 2018;9:561-571.
27. Kimokoti RW, Millen BE. Nutrition for the prevention of chronic diseases. Med Clin North Am. 2016;100:1185-1198.
28. Tucker KL, Smith CE, Lai CQ, et al. Quantifying diet for nutrigenomic studies. Annu Rev Nutr. 2013;33:349-371.
29. Fenech M, El-Sohemy A, Cahill L, et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J Nutrigenet Nutrigenomics. 2011;4:69-89.
30. van Ommen B, van den Broek T, de Hoogh I, et al. Systems biology of personalized nutrition. Nutr Rev. 2017;75:579-599.
31. Fumagalli M, Moltke I, Grarup N, et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343-1347.
32. Mathieson I, Lazaridis I, Rohland N, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499-503.
33. Celis-Morales C, Livingstone KM, Marsaux CF, et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46:578-588.
34. Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009;60:39-54.
35. Li Y, Huang T, Zheng Y, et al. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5:e003768.
36. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;8:CD006612.
37. Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S73.
38. USPSTF. Using nontraditional risk factors in coronary heart disease risk assessment: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:474-482.
39. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. JAMA. 2017;317:183-189.
40. Levin BL, Varga E. MTHFR: addressing genetic counseling dilemmas using evidence-based literature. J Genet Couns. 2016;25:901-911.
41. Luo Z, Lu Z, Muhammad I, et al. Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: a systematic review and updated meta-analysis. Lipids Health Dis. 2018;17:191.
42. Clarke R, Bennett DA, Parish S, et al. Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012;9:e1001177.
43. Kölling K, Ndrepepa G, Koch W, et al. Methylenetetrahydrofolate reductase gene C677T and A1298C polymorphisms, plasma homocysteine, folate, and vitamin B12 levels and the extent of coronary artery disease. Am J Cardiol. 2004;93:1201-1206.
44. Holmes MV, Newcombe P, Hubacek JA, et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet. 2011;378:584-594.
45. Chang G, Kuai Z, Wang J, et al. The association of MTHFR C677T variant with increased risk of ischemic stroke in the elderly population: a meta-analysis of observational studies. BMC Geriatr. 2019;19:331.
46. Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325-1335.
47. Reid G. Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol. 2016;30:17-25.
48. Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56-64.
49. Van den Nieuwboer M, Brummer RJ, Guarner F, et al. The administration of probiotics and synbiotics in immune compromised adults: Is it safe? Benef Microbes. 2015;6:3-17.
50. Liu Y, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment. J Clin Pharmacol. 2018;58(suppl 10):S164-S179.
51. O’Connell TM. The application of metabolomics to probiotic and prebiotic interventions in human clinical studies. Metabolites. 2020;10:120.
52. Lerner A, Shoenfeld Y, Matthias T. Probiotics: if it does not help it does not do any harm. Really? Microorganisms. 2019;7:104.
53. Su G, Ko C, Bercik P, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterol. 2020;159:697-705.
54. Preidis GA, Weizman AV, Kashyap PC, et al. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159:708-738.e4.
55. Charoenngam N, Shirvani A, Kalajian TA, et al. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: A randomized, double-blinded, dose-response study. Anticancer Res. 2020;40:551-556.
56. Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, et al. Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv Nutr. 2019;10(suppl1):S17-S30.
57. APA. Clay RA. More than one way to measure. Monitor Psychol. 2010;4:52. Accessed November 19, 2021. www.apa.org/monitor/2010/09/trials
58. Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2-21.
59. Bauchner H, Fontanarosa PB, Golub RM. Evaluation of the trial to assess chelation therapy (TACT): the scientific process, peer review, and editorial scrutiny. JAMA. 2013;309:1291-1292.
60. Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019;216:20-40.
61. Matsumoto M, Kitada Y, Naito Y. Endothelial function is improved by inducing microbial polyamine production in the gut: a randomized placebo-controlled trial. Nutrients. 2019;11:1188.
62. Bagga D, Reichert JL, Koschutnig K, et al. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes. 2018;9:486-496.
63. Vieira AT, Castelo PM, Ribeiro DA, et al. Influence of oral and gut microbiota in the health of menopausal women. Front Microbiol. 2017;8:1884.
64. Ribeiro AE, Monteiro NES, Moraes AVG, et al. Can the use of probiotics in association with isoflavone improve the symptoms of genitourinary syndrome of menopause? Results from a randomized controlled trial. Menopause. 2018;26:643-652.
65. Hong YS, Hong KS, Park MH, et al. Metabonomic understanding of probiotic effects in humans with irritable bowel syndrome. J Clin Gastroenterol. 2011;45:415-425.
66. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterol. 2017;152:1889-1900.e9.
67. Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol. 2018;233:2091-2103.
68. Linn YH, Thu KK, Win NHH. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiotics Antimicrob Proteins. 2019;11:638-647.
69. Liu M-M, Li S-T, Shu Y, et al. Probiotics for prevention of radiation-induced diarrhea: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0178870.
70. Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36:1245-1249.
71. Navarro-López V, Ramírez-Boscá A, Ramón-Vidal D, et al. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2018;154:37-43.
72. Wang HT, Anvari S, Anagnostou K. The role of probiotics in preventing allergic disease. Children (Basel). 2019;6:24.
73. Kasatpibal N, Whitney JD, Saokaew S, et al. Effectiveness of probiotic, prebiotic, and synbiotic therapies in reducing postoperative complications: a systematic review and network meta-analysis. Clin Infect Dis. 2017;64(suppl2):S153-S160.
74. Liu PC, Yan YK, Ma YJ, et al. Probiotics reduce postoperative infections in patients undergoing colorectal surgery: a systematic review and meta-analysis. Gastroenterol Res Pract. 2017;2017:6029075.
75. Hendijani F, Akbari V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: a systematic review and meta-analysis. Clin Nutr. 2018;37:532-541.
76. Wu Y, Zhang Q, Ren Y, et al. Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PLoS One. 2017;12:e0178868.
77. Ferolla SM, Couto CA, Costa-Silva L, et al. Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with nonalcoholic steatohepatitis. Nutrients. 2016;8:397.
78. Clarke CN, Clarke NE, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetraacetic acid. Am J Med Sci. 1956;232:654-666.
79. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1-23.
80. Chowdhury R, Ramond A, O’Keeffe LM, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:k3310.
81. Zhuang X, Ni A, Liao L, et al. Environment-wide association study to identify novel factors associated with peripheral arterial disease: evidence from the National Health and Nutrition Examination Survey (1999–2004). Atherosclerosis. 2018;269:172-177.
82. Wax PM. Current use of chelation in American health care. J Med Toxicol. 2013:9;303-307.
83. CDC. Deaths associated with hypocalcemia from chelation therapy—Texas, Pennsylvania, and Oregon, 2003-2005. MMWR Morb Mortal Wkly Rep. 2006;55:204-207.
84. Atwood KC, Woeckner E. In pediatric fatality, edetate disodium was no accident. Clin Toxicol (Phila). 2009;47:256.
85. Baxter AJ, Krenzelok EP. Pediatric fatality secondary to EDTA chelation. Clin Toxicol (Phila). 2008;46:1083-1084.
86. Lamas GA, Goertz C, Boineau R, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309:1241-1250.
87. Escolar E, Lamas GA, Mark DB, et al. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014;7:15-24.
88. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929-1949.
89. Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264-2271.
90. Nagai R, Murray DB, Metz TO, et al. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61:549-559.
Using biomarkers to quantify problematic alcohol use
CASE A 34-year-old woman presents with fatigue. She appears defensive when asked about her alcohol use. She answers No to all questions on the CAGE (cut down, annoyed, guilty, eye-opener) screening tool, but acknowledges drinking excessively on rare occasions. Her physician has a high suspicion for alcohol use disorder (AUD) and recommends further testing. The patient agrees but denies having used alcohol over the past several days. Which of the following is most likely to help support the suspicion of a heavy drinking pattern?
- Routine lab tests (liver panel and complete blood count).
- Blood or urine alcohol level.
- Phosphatidylethanol (PEth) level in the blood.
- Ethyl glucuronide (EtG) in the urine.
- Carbohydrate-deficient transferrin (CDT) in the blood.
(See "Case answer.").
About 1 in 12 Americans have AUD,1 and 1 in 10 children live in a home with a parent who has a drinking problem.2 While the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) succinctly defines AUD with specific criteria,1 the term generally refers to an inability to control or stop drinking despite adverse social or health consequences. AUD is regarded as > 4 drinks per day for men and > 3 drinks per day for women.3 A “standard drink” would be a 12-oz bottle of beer, a 5-oz glass of wine, or 1.5 oz of distilled spirits. Effects of chronic alcohol use are vast and include malnutrition, alcohol withdrawal syndrome, alcoholic liver disease, pancreatitis/pancreatic cancer, cardiomyopathy, and stroke.4-6 Alcohol use by a pregnant woman can lead to fetal alcohol syndrome in her child.7
AUD may be more prevalent in the wake of COVID-19. Primary care practitioners tend to miss a large fraction of patients with AUD in their practice, especially younger patients and those without somatic comorbidities.8 Systematic screening for AUD can identify many of these people.8 Particularly as the COVID-19 pandemic continues to unfold and increases stress for everyone, risk of worsening drinking increases both in individuals with current AUD and for those in remission.9 Contrary to common belief, patients visiting primary care favor screening for at-risk drinking.10 Thus, awareness of the prevalence of AUD and patient acceptance of screening should encourage wider testing.
Screening tools. The 2014 guidelines published by the Centers for Disease Control and Prevention recommend using quick screening tools—ie, single question or AUDIT 1-3 (TABLE 111-18)—as an objective means of determining whether patients’ drinking creates a risk for themselves or others.11 Excessive drinking identified using alcohol questionnaires can help reduce medical complications and health care costs.19 The questionnaires we review do not provide a diagnosis but help identify individuals who might benefit from more thorough assessment.20 Following up, as needed, by testing for alcohol biomarkers can provide quantitative insight into problematic alcohol use.2
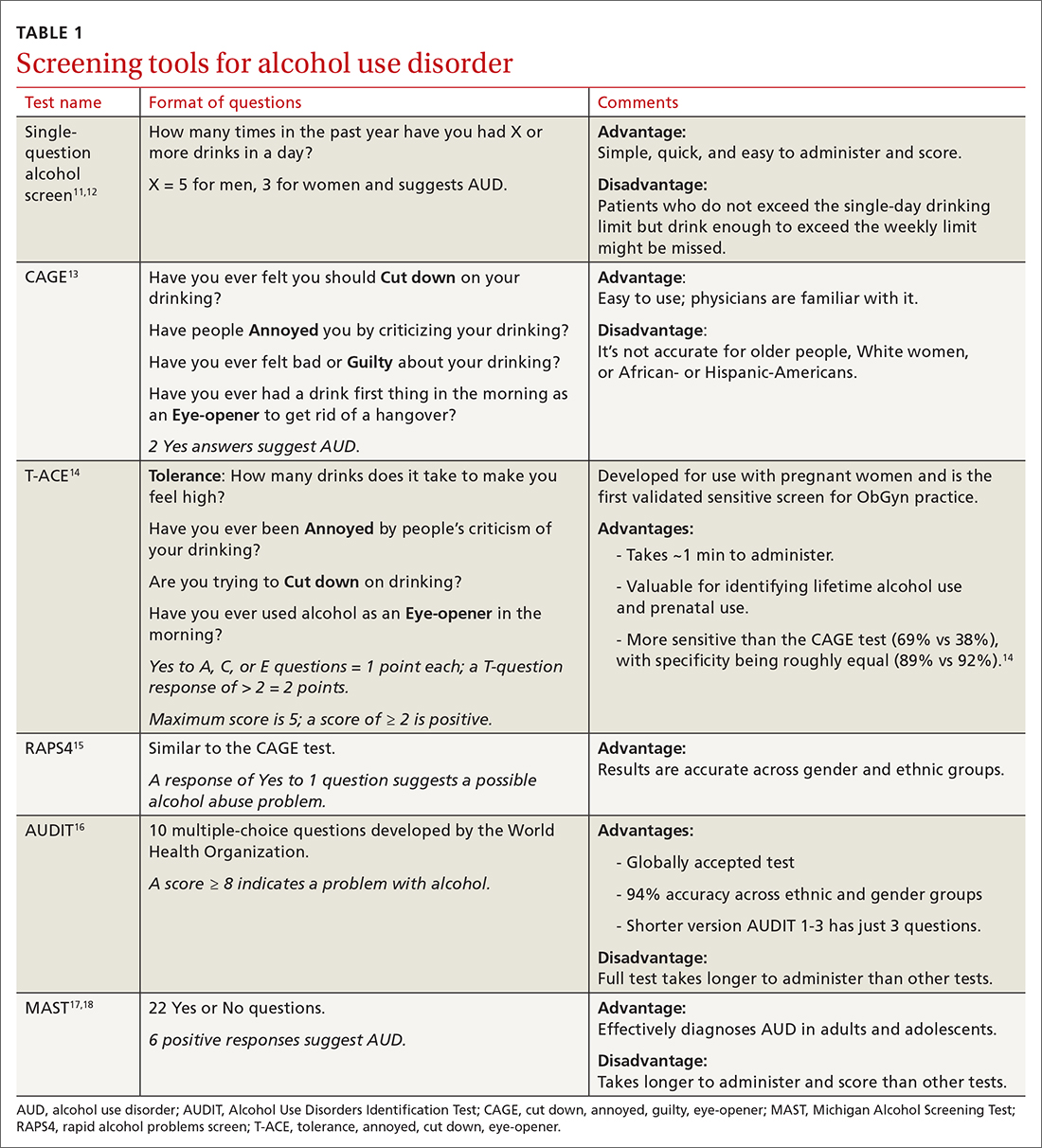
But before we discuss the utility of biomarkers, it’s important to quickly review how alcohol is eliminated from the body.
Alcohol elimination
The stomach and small intestine are the primary sites for alcohol absorption. Alcohol elimination from the body occurs through 3 pathways. The first involves oxidative metabolism, which eliminates most ethanol (95%) through the actions of alcohol dehydrogenase, cytochrome P4502E1, or catalase. A lesser amount of alcohol (2%-5%) is eliminated, unchanged, via the second pathway, which includes urine, sweat, and breath. Nonoxidative metabolism makes up the third pathway. Nonoxidative metabolism removes a very small amount (0.1%) of alcohol and involves the direct ethanol biomarkers PEth, EtG, ethyl sulfate (EtS), and fatty acid ethyl esters (FAEEs).21 Our emphasis in this article is on assays of direct metabolites of alcohol—particularly PEth.
Continue to: To understand the utility...
To understand the utility of these direct biomarkers, it is helpful to look at the indirect biomarkers first.
Indirect biomarkers have limited sensitivity and specificity
When alcohol is consumed in large enough quantities over time, indirect biomarkers of alcohol can become abnormal.22 The major indirect biomarkers are the liver enzymes aspartate and alanine aminotransferase (AST and ALT), gamma-glutamyl transferase (GGT), mean corpuscular volume (MCV) of red blood cells, and carbohydrate-deficient transferrin (CDT). Indirect biomarkers have limited sensitivity and specificity for AUD. (For specifics on sensitivity and specificity of indirect and direct biomarkers, see TABLE 2.23-31)
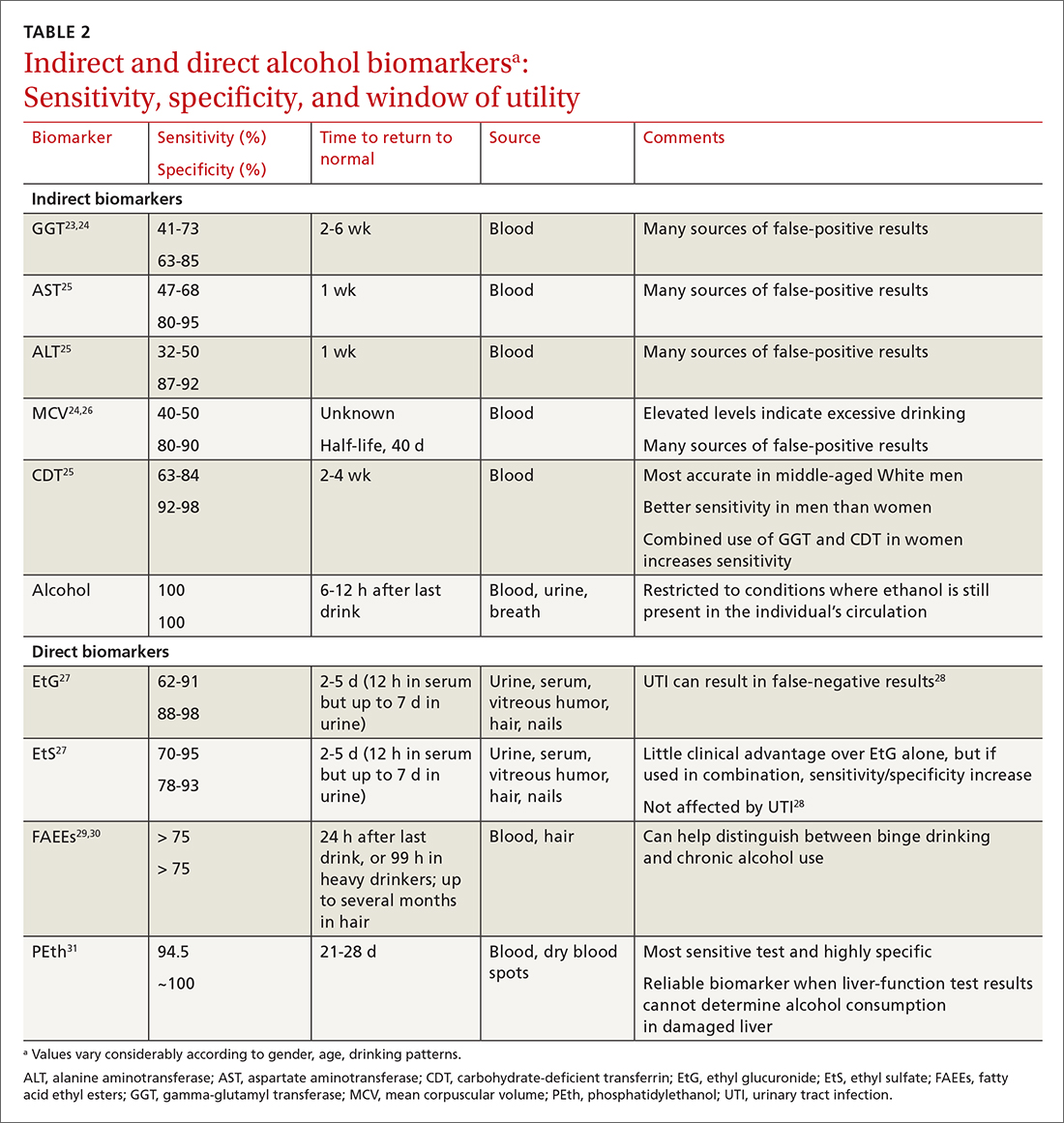
Liver enzymes. AST and ALT are also present in the heart, muscle, and kidneys. Elevated levels usually imply injury to hepatocytes, with ALT being more reflective of liver involvement than AST. Both AST and ALT are elevated in other common liver conditions including hepatitis C virus infection and fatty liver disease. In alcoholic liver disease (ALD), AST is elevated more than ALT; an AST-to-ALT ratio > 3 suggests ALD. An elevated GGT often indicates hepatic injury and is used to confirm that elevated alkaline phosphatase is of hepatic origin.3
MCV is the average volume of erythrocytes,33 and an elevated MCV is a potential indicator of excessive alcohol intake. Macrocytosis requires sustained alcohol use, and the test has low sensitivity. Other diseases such as vitamin B12 or folic acid deficiency, hypothyroidism, hematologic diseases (eg, cold agglutinin disease, multiple myeloma, amyloidosis), and certain medications can also increase MCV.34 Moreover, MCV responds slowly to alcohol use, abstinence, and relapse because red cells have a life span of 120 days.35
CDT. Transferrin is a glycoprotein produced in the liver. The level of transferrin with sialic acid chains increases with alcohol consumption as well as in the carbohydrate deficient glycoprotein syndrome, leading to so-called carbohydrate deficient transferrin.36 It is a sensitive marker for detecting alcohol relapse and monitoring sobriety. Moderate-to-heavy alcohol use, averaging ≥ 40 g of alcohol per day for 2 weeks,36 can decrease the amount of carbohydrate attached to transferrin. Two weeks after complete alcohol cessation, CDT levels will return to normal.37
Continue to: CDT is approved...
CDT is approved by the FDA as an assay for alcohol consumption.37 While CDT is felt to be one of the better indirect markers of AUD and can extend the window of detection, there are still issues with its sensitivity and specificity.38 This biomarker can be elevated with other liver diseases and can be affected by the patient’s age, body mass index, gender, and tobacco use.39,40 Testing for CDT has never achieved widespread clinical use and has been largely supplanted by the more accurate PEth test (described in a bit).
Direct biomarkers offer insight into recent alcohol use
Other than ethanol itself, direct biomarkers of alcohol use are minor ethanol metabolites created through biochemical reactions when ethanol is coupled to endogenous compounds. Hence, the presence of these metabolites is usually directly related to ethanol consumption.41 Direct alcohol biomarkers are EtG, EtS, FAEEs, and PEth (TABLE 223-31). They reflect alcohol consumption over a period of several days, making them useful when paired with questionnaire data, especially for identifying young adults who engage in binge drinking.42
Ethanol can be measured in blood, urine, and breath and is detectable a bit longer in urine than in blood. However, alcohol is detectable in the blood only for 6 to 12 hours after drinking. After alcohol consumption, concentrations peak in the blood within 2 hours. The window for detecting ethanol in the blood depends on the amount of alcohol consumed and the elimination rate of alcohol, which is about 12 mg/dL/h (or 0.012%)—approximately the same amount of alcohol contained in a standard drink (14 g).
Checking the blood alcohol level might be helpful in the office if a patient appears intoxicated but denies alcohol use. A blood alcohol level > 300 mg/dL, or > 150 mg/dL without gross evidence of intoxication, or > 100 mg/dL upon routine examination indicates AUD with a high degree of reliability.33,43 But the short half-life of ethanol in blood limits its use as a biomarker,33 and it is not a good indicator of chronic drinking.44
EtG and EtS. Less than 0.1% of ethanol is secreted as the metabolites EtG and EtS, which are generated, respectively, by the enzymes uridine diphosphate glucuronosyltransferase and sulfotransferase.45 They have value in the diagnosis of AUD because of the length of time in which they can be detected. Urinary EtG and EtS have been especially important biomarkers for monitoring relapse in outpatients treated for alcohol-related problems.46 Generally, EtG and EtS can be detected in urine for 13 to 20 hours after a single drink (0.1 g/kg), and for up to 4 to 5 days following ingestion of large amounts of alcohol.47
Continue to: EtG has been detectable...
EtG has been detectable in urine for ≥ 24 hours following only 1 or 2 drinks, and for up to 4 days following heavy consumption.48 Shortly after alcohol intake, even in small amounts, EtG is detectable. Analysis of EtG in urine is helpful in monitoring alcohol consumption during withdrawal treatment, for workplace testing, and to check for abstinence in legal matters. The EtG urine test is useful in detecting alcohol consumption in a person who claims to be abstinent but who drank 2 or 3 days before the evaluation. Although accurate, EtG’s window for detection is narrower than that of the PEth assay.
EtS is a good marker of acute short-term alcohol use, up to 12 hours in the blood (or longer in heavier drinkers) and up to 5 days in urine.49 Its sensitivity is highest in heavy drinkers. Post-sampling formation and degradation of EtS have not been known to occur in urine samples. Testing for this second metabolite of ethanol can slightly improve the sensitivity and specificity of the EtG test. A urine test for EtS has a wider detection window. But it has little practical advantage compared with EtG.50
For better clinical specificity, a combination of both EtG and EtS testing has been recommended. However, the EtS assay is more cumbersome and provides little advantage over EtG. EtG values do not correlate precisely with the amount or frequency of ethanol use, but the magnitude of the EtG finding roughly corresponds to the amount of alcohol recently consumed.
False-positive and false-negative results for EtG and EtS are uncommon in practice. However, false-positive results are possible with the EtG test in certain circumstances: presence of Escherichia coli in the specimen, use of ethanol-based hand sanitizers (> 20 times a day) or mouthwashes, and the consumption of substances like pralines, nonalcoholic beer, pharmaceutical products, and fruit juice. Similarly, false-negative results of EtG can occur from degradation if the samples are contaminated with other bacteria, transported without cooling, or stored improperly.51 In practice, this is uncommon, and the test is believed to be specific with few false-positive results. Commercially available EtG colorimetric test strips permit on-site analysis of urine samples.
FAEEs are a combination of different esters and products of alcohol metabolism through a nonoxidative pathway. They are formed by esterification of endogenous free fatty acids and ethanol in blood and several tissues.29 These are sensitive and specific markers of alcohol ingestion and can differentiate chronic alcohol consumption from binge drinking.29 It is elevated for up to 99 hours in heavy alcohol drinkers.30 It can be detected in hair for a longer period than in blood.52 Detection of FAEEs in meconium can help establish fetal alcohol exposure.53
Continue to: PEth
PEth. Use of the PEth assay has increased in recent years and its accuracy has had a transformative effect on the diagnosis of AUD.54 PEth is a phospholipid found in erythrocyte membranes, formed by an interaction between ethanol and phosphatidylcholine, catalyzed by phospholipase D.55,56 Major advantages of PEth include an unusually long half-life and specificity. Red cells lack enzymes to degrade PEth, therefore PEth accumulates in red cells and has a half-life of 4 to 10 days57,58 allowing for detection of significant ethanol consumption extending back 3 to 4 weeks.59 There is no evidence that PEth is formed in the absence of ethanol, making the test essentially 100% specific, particularly at higher cutoff values of ≥ 150 ng/mL.31,60
PEth levels are not affected by age, gender, or underlying liver or renal disease.61 PEth can differentiate between heavy alcohol use and social drinking and can therefore identify chronic excessive use.62 With chronic excessive alcohol consumption, PEth is detectable in blood up to 28 days after sobriety.63 A correlation exists between PEth concentrations in blood and the amount of consumed ethanol. PEth has increased specificity and sensitivity for the detection of latent ethanol use compared with other direct biomarkers.21 It can identify recent heavy drinking earlier than indirect biomarkers, as it does not rely on hepatic injury.
Using a cutoff level of 20 ng/mL, PEth assays have a sensitivity of 73% for any alcohol use in the past month; at 80 ng/mL, the sensitivity is 91% for > 4 drinks/d.61 PEth is considered semi-quantitative. The World Health Organization defines acceptable social alcohol use at a PEth value < 40 ng/dL for men and < 20 ng/dL for women. Chronic excessive use is defined by a level > 60 ng/dL.55 The cutoff levels tend to be arbitrary and vary with different guidelines.
Although false-positive PEth test results may be possible, most experts believe that dishonesty in self-reporting by test subjects is more likely. That said, the true specificity of PEth remains unknown; a lower value detected should not be regarded as absolute proof of relapse or chronic alcoholism.
Studies have shown a positive correlation between the AUDIT-C score and PEth values combined with self-reported alcohol consumption, indicating that PEth may be a useful marker in difficult-to-assess settings, or in confirming or invalidating self-reported alcohol consumption.61,64,65 The PEth test is now widely available and, in the authors’ experience, usually costs $100 to $200. Analysis typically costs $40 to $100,66 and costs could decrease as the test becomes more widely used. Turnaround time for PEth is 5 to 10 days. It is now the recommended assay by transplant hepatologists for detecting alcohol use.67

Continue to: CASE ANSWER
CASE
CORRESPONDENCE
Frederick Nunes, MD, Pennsylvania Hospital of University of Pennsylvania, 230 West Washington Square, 4th Floor, Philadelphia, PA 19104; [email protected]
1. APA. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. American Psychiatric Publishing. 2013:490-497.
2. Fleming MF, Smith MJ, Oslakovic E, et al. Phosphatidylethanol detects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol Clin Exp Res. 2017;41:857-862.
3. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed November 12, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
4. Herreros-Villanueva M, Hijona E, Bañales JM, et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19:638-647.
5. Rocco A, Compare D, Angrisani D, et al. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652-14659.
6.
7. Sebastiani G, Borrás-Novell C, Casanova MA, et al. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients. 2018;10:1008.
8. Rehm J, Anderson P, Manthey J, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51:422-427. doi: 10.1093/alcalc/agv127
9. ASAM. Caring for patients during the COVID-19 pandemic. Accessed November 12, 2021. www.asam.org/docs/default-source/covid-19/acute-care_062620.pdf?sfvrsn=e66d54c2_10
10. Miller PM, Thomas SE, Mallin R. Patient attitudes towards self-report and biomarker alcohol screening by primary care physicians. Alcohol Alcohol. 2006;41:306-310. doi: 10.1093/alcalc/agl022
11. Zoorob R, Snell H, Kihlberg C, et al. Screening and brief intervention for risky alcohol use. Curr Probl Pediatr Adolesc Health Care. 2014;44:82-87.
12. Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783-788.
13. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905-1907.
14. Sokol RJ, Martier SS, Ager JW. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160:863-868.
15. Cherpitel CJ. A brief screening instrument for problem drinking in the emergency room: the RAPS4. Rapid Alcohol Problems Screen. J Stud Alcohol. 2000;61:447-449.
16. WHO. AUDIT: The alcohol use identification test. Accessed November 14, 2021. http://apps.who.int/iris/bitstream/handle/10665/67205/WHO_MSD_MSB_01.6a.pdf?sequence=1
17. Westermeyer J, Yargic I, Thuras P. Michigan assessment-screening test for alcohol and drugs (MAST/AD): evaluation in a clinical sample. Am J Addict. 2004;13:151-162.
18. Powers JS, Spickard A. Michigan Alcoholism Screening Test to diagnose early alcoholism in a general practice. South Med J. 1984;77:852-856.
19. NIH. Treatment for alcohol problems: finding and getting help. Accessed November 12, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/treatment-alcohol-problems-finding-and-getting-help
20. Kitchens JM. Does this patient have an alcohol problem? JAMA. 1994;272:1782-1787.
21. Kummer N, Lambert WE, Samyn N, et al. Alternative sampling strategies for the assessment of alcohol intake of living persons. Clin Biochem. 2016;49:1078-1091.
22. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63:1634-1640.
23. Mundle G, Ackermann K, Munkes J, et al. Influence of age, alcohol consumption and abstinence on the sensitivity of carbohydrate‐deficient transferrin, gamma‐glutamyltransferase and mean corpuscular volume. Alcohol Alcohol. 1999;34:760-766.
24. Neumann T, Spies C. Use of biomarkers for alcohol use disorders in clinical practice. Addiction. 2003;98(suppl 2):81-91.
25. Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:11684-11699.
26. Helander A. Biological markers of alcohol use and abuse in theory and practice. In: Agarwal DP, Seitz HK, eds. Alcohol in Health and Disease. Marcel Dekker. 2001:177-205.
27. Stewart SH, Koch DG, Burgess DM, et al. Sensitivity and specificity of urinary ethyl glucuronide and ethyl sulfate in liver disease patients. Alcohol Clin Exp Res. 2013;37:150-155.
28. Helander A, Dahl H. Urinary tract infection: a risk factor for false-negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clin Chem. 2005;51:1728-1730.
29. Ghosh S, Jain R, Jhanjee S, et al. Alcohol biomarkers and their relevance in detection of alcohol consumption in clinical settings. Accessed November 12, 2021. https://www.clinmedjournals.org/articles/iasar/international-archives-of-substance-abuse-and-rehabilitation-iasar-1-002.php?jid=iasar
30. Borucki K, Dierkes J, Wartberg J, et al. In heavy drinkers, fatty acid ethyl esters remain elevated for up to 99 hours. Alcohol Clin Exp Res. 2007;31:423-427.
31. Hartmann S, Aradottir S, Graf M, et al. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12:81-84.
32. Choe YM, Lee BC, Choi IG, et al. Combination of the CAGE and serum gamma-glutamyl transferase: an effective screening tool for alcohol use disorder and alcohol dependence. Neuropsychiatr Dis Treat. 2019 31;15:1507-1515.
33. Niemelä O. Biomarkers in alcoholism. Clin Chim Acta. 2007;377:39-49.
34. Kauffmann T, Evans DS. Macrocytosis. Accessed November 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK560908/
35. Maenhout TM, De Buyzere ML, Delanghe JR. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin Chim Acta. 2013;415:322-329.
36. Solomons HD. Carbohydrate deficient transferrin and alcoholism. Germs. 2012;2:75-78.
37. Allen JP, Wurst FM, Thon N, et al. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl. 2013;19:369-376.
38. Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001-2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841:96-109.
39. Hannuksela ML, Liisanantti MK, Nissinen AE, et al. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953-961.
40. Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem. 2001;47:13-27.
41. Cabarcos P, Hassan HM, Tabernero MJ, et al. Analysis of ethyl glucuronide in hair samples by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). J Appl Toxicol. 2013;33:638-643.
42. Piano MR, Mazzuco A, Kang M, et al. Binge drinking episodes in young adults: how should we measure them in a research setting? J Stud Alcohol Drugs. 2017;78:502-511.
43. Adinoff B, Bone GH, Linnoila M. Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol Adverse Drug Exp. 1988;3:172-196.
44. Cabezas J, Lucey MR, Bataller R. Biomarkers for monitoring alcohol use. Clin Liver Dis (Hoboken). 2016;8:59-63.
45. Wurst FM, Alling C, Aradottir S, et al. Emerging biomarkers: new directions and clinical applications. Alcohol Clin Exp Res. 2005;29:465-473.
46. Helander A, Péter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552-557.
47. Helander A, Böttcher M, Fehr C, et al. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44:55-61.
48. Jatlow P, O’Malley SS. Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin Exp Res. 2010;34:968-975.
49. Jatlow PI, Agro A, Wu R, et al. Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: results of a dose ranging alcohol challenge study and 2 clinical trials. Alcohol Clin Exp Res. 2014;38:2056-2065.
50. Gonzalo P, Radenne S, Gonzalo S. Biomarkers of chronic alcohol misuse. Curr Biomark Find. 2014;4:9-22.
51. Bornhorst JA, Mbughuni MM. Alcohol biomarkers: clinical issues and analytical methods. In: Critical Issues in Alcohol and Drugs of Abuse Testing. 2nd ed. Academic Press. 2019:25-42.
52. Soderberg BL, Salem RO, Best CA, et al. Fatty acid ethyl esters. Ethanol metabolites that reflect ethanol intake. Am J Clin Pathol. 2003;119(suppl):S94-S99.
53. Cheng CT, Ostrea EM Jr, Alviedo JN, et al. Fatty acid ethyl esters in meconium: a biomarker of fetal alcohol exposure and effect. Exp Biol Med (Maywood). 2021;246:380-386.
54. Andresen-Streichert H, Beres Y, Weinmann W, et al. Improved detection of alcohol consumption using the novel marker phosphatidylethanol in the transplant setting: results of a prospective study. Transpl Int. 2017;30:611-620.
55. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13:14788-14812.
56. Gnann H, Weinmann W, Thierauf A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36:1507-1511.
57. Aradóttir S, Moller K, Alling C. Phosphatidylethanol formation and degradation in human and rat blood. Alcohol Alcohol. 2004;39:8-13.
58. Varga A, Alling C. Formation of phosphatidylethanol in vitro in red blood cells from healthy volunteers and chronic alcoholics. J Lab Clin Med. 2002;140:79-83.
59. Javors MA, Hill-Kapturczak N, Roache JD, et al. Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcohol Clin Exp Res. 2016;40:1228-1234.
60. Schröck A, Pfäffli M, König S, et al. Application of phosphatidylethanol (PEth) in whole blood in comparison to ethyl glucuronide in hair (hEtG) in driving aptitude assessment (DAA). Int J Legal Med. 2016;130:1527-1533.
61. Stewart SH, Koch DG, Willner IR, et al. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706-1711.
62. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015 29;5:1339-1385.
63. Hill-Kapturczak N, Dougherty DM, Roache JD, et al. Phosphatidylethanol homologs in blood as biomarkers for the time frame and amount of recent alcohol consumption. In: Preedy VR (ed) Neuroscience of Alcohol. Academic Press; 2019:567-576.
64. Jain J, Evans JL, Briceño A, et al. Comparison of phosphatidylethanol results to self-reported alcohol consumption among young injection drug users. Alcohol Alcohol. 2014;49:520-524.
65. Schröck A, Wurst FM, Thon N, et al. Assessing phosphatidylethanol (PEth) levels reflecting different drinking habits in comparison to the alcohol use disorders identification test - C (AUDIT-C). Drug Alcohol Depend. 2017;178:80-86.
66. McDonnell MG, Skalisky J, Leickly E, et al. Pilot investigation of a phosphatidylethanol-based contingency management intervention targeting alcohol use. Psychol Addict Behav. 2017;31:608-613.
67. Asrani SK, Trotter J, Lake J, et al. Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis. Liver Transpl. 2020;26:127-140.
68. WHO. International Guide for Monitoring Alcohol Consumption and Harm. 2000. Accessed November 12, 2021. http://apps.who.int/iris/bitstream/handle/10665/66529/WHO_MSD_MSB_00.4.pdf?sequence=1
CASE A 34-year-old woman presents with fatigue. She appears defensive when asked about her alcohol use. She answers No to all questions on the CAGE (cut down, annoyed, guilty, eye-opener) screening tool, but acknowledges drinking excessively on rare occasions. Her physician has a high suspicion for alcohol use disorder (AUD) and recommends further testing. The patient agrees but denies having used alcohol over the past several days. Which of the following is most likely to help support the suspicion of a heavy drinking pattern?
- Routine lab tests (liver panel and complete blood count).
- Blood or urine alcohol level.
- Phosphatidylethanol (PEth) level in the blood.
- Ethyl glucuronide (EtG) in the urine.
- Carbohydrate-deficient transferrin (CDT) in the blood.
(See "Case answer.").
About 1 in 12 Americans have AUD,1 and 1 in 10 children live in a home with a parent who has a drinking problem.2 While the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) succinctly defines AUD with specific criteria,1 the term generally refers to an inability to control or stop drinking despite adverse social or health consequences. AUD is regarded as > 4 drinks per day for men and > 3 drinks per day for women.3 A “standard drink” would be a 12-oz bottle of beer, a 5-oz glass of wine, or 1.5 oz of distilled spirits. Effects of chronic alcohol use are vast and include malnutrition, alcohol withdrawal syndrome, alcoholic liver disease, pancreatitis/pancreatic cancer, cardiomyopathy, and stroke.4-6 Alcohol use by a pregnant woman can lead to fetal alcohol syndrome in her child.7
AUD may be more prevalent in the wake of COVID-19. Primary care practitioners tend to miss a large fraction of patients with AUD in their practice, especially younger patients and those without somatic comorbidities.8 Systematic screening for AUD can identify many of these people.8 Particularly as the COVID-19 pandemic continues to unfold and increases stress for everyone, risk of worsening drinking increases both in individuals with current AUD and for those in remission.9 Contrary to common belief, patients visiting primary care favor screening for at-risk drinking.10 Thus, awareness of the prevalence of AUD and patient acceptance of screening should encourage wider testing.
Screening tools. The 2014 guidelines published by the Centers for Disease Control and Prevention recommend using quick screening tools—ie, single question or AUDIT 1-3 (TABLE 111-18)—as an objective means of determining whether patients’ drinking creates a risk for themselves or others.11 Excessive drinking identified using alcohol questionnaires can help reduce medical complications and health care costs.19 The questionnaires we review do not provide a diagnosis but help identify individuals who might benefit from more thorough assessment.20 Following up, as needed, by testing for alcohol biomarkers can provide quantitative insight into problematic alcohol use.2

But before we discuss the utility of biomarkers, it’s important to quickly review how alcohol is eliminated from the body.
Alcohol elimination
The stomach and small intestine are the primary sites for alcohol absorption. Alcohol elimination from the body occurs through 3 pathways. The first involves oxidative metabolism, which eliminates most ethanol (95%) through the actions of alcohol dehydrogenase, cytochrome P4502E1, or catalase. A lesser amount of alcohol (2%-5%) is eliminated, unchanged, via the second pathway, which includes urine, sweat, and breath. Nonoxidative metabolism makes up the third pathway. Nonoxidative metabolism removes a very small amount (0.1%) of alcohol and involves the direct ethanol biomarkers PEth, EtG, ethyl sulfate (EtS), and fatty acid ethyl esters (FAEEs).21 Our emphasis in this article is on assays of direct metabolites of alcohol—particularly PEth.
Continue to: To understand the utility...
To understand the utility of these direct biomarkers, it is helpful to look at the indirect biomarkers first.
Indirect biomarkers have limited sensitivity and specificity
When alcohol is consumed in large enough quantities over time, indirect biomarkers of alcohol can become abnormal.22 The major indirect biomarkers are the liver enzymes aspartate and alanine aminotransferase (AST and ALT), gamma-glutamyl transferase (GGT), mean corpuscular volume (MCV) of red blood cells, and carbohydrate-deficient transferrin (CDT). Indirect biomarkers have limited sensitivity and specificity for AUD. (For specifics on sensitivity and specificity of indirect and direct biomarkers, see TABLE 2.23-31)

Liver enzymes. AST and ALT are also present in the heart, muscle, and kidneys. Elevated levels usually imply injury to hepatocytes, with ALT being more reflective of liver involvement than AST. Both AST and ALT are elevated in other common liver conditions including hepatitis C virus infection and fatty liver disease. In alcoholic liver disease (ALD), AST is elevated more than ALT; an AST-to-ALT ratio > 3 suggests ALD. An elevated GGT often indicates hepatic injury and is used to confirm that elevated alkaline phosphatase is of hepatic origin.3
MCV is the average volume of erythrocytes,33 and an elevated MCV is a potential indicator of excessive alcohol intake. Macrocytosis requires sustained alcohol use, and the test has low sensitivity. Other diseases such as vitamin B12 or folic acid deficiency, hypothyroidism, hematologic diseases (eg, cold agglutinin disease, multiple myeloma, amyloidosis), and certain medications can also increase MCV.34 Moreover, MCV responds slowly to alcohol use, abstinence, and relapse because red cells have a life span of 120 days.35
CDT. Transferrin is a glycoprotein produced in the liver. The level of transferrin with sialic acid chains increases with alcohol consumption as well as in the carbohydrate deficient glycoprotein syndrome, leading to so-called carbohydrate deficient transferrin.36 It is a sensitive marker for detecting alcohol relapse and monitoring sobriety. Moderate-to-heavy alcohol use, averaging ≥ 40 g of alcohol per day for 2 weeks,36 can decrease the amount of carbohydrate attached to transferrin. Two weeks after complete alcohol cessation, CDT levels will return to normal.37
Continue to: CDT is approved...
CDT is approved by the FDA as an assay for alcohol consumption.37 While CDT is felt to be one of the better indirect markers of AUD and can extend the window of detection, there are still issues with its sensitivity and specificity.38 This biomarker can be elevated with other liver diseases and can be affected by the patient’s age, body mass index, gender, and tobacco use.39,40 Testing for CDT has never achieved widespread clinical use and has been largely supplanted by the more accurate PEth test (described in a bit).
Direct biomarkers offer insight into recent alcohol use
Other than ethanol itself, direct biomarkers of alcohol use are minor ethanol metabolites created through biochemical reactions when ethanol is coupled to endogenous compounds. Hence, the presence of these metabolites is usually directly related to ethanol consumption.41 Direct alcohol biomarkers are EtG, EtS, FAEEs, and PEth (TABLE 223-31). They reflect alcohol consumption over a period of several days, making them useful when paired with questionnaire data, especially for identifying young adults who engage in binge drinking.42
Ethanol can be measured in blood, urine, and breath and is detectable a bit longer in urine than in blood. However, alcohol is detectable in the blood only for 6 to 12 hours after drinking. After alcohol consumption, concentrations peak in the blood within 2 hours. The window for detecting ethanol in the blood depends on the amount of alcohol consumed and the elimination rate of alcohol, which is about 12 mg/dL/h (or 0.012%)—approximately the same amount of alcohol contained in a standard drink (14 g).
Checking the blood alcohol level might be helpful in the office if a patient appears intoxicated but denies alcohol use. A blood alcohol level > 300 mg/dL, or > 150 mg/dL without gross evidence of intoxication, or > 100 mg/dL upon routine examination indicates AUD with a high degree of reliability.33,43 But the short half-life of ethanol in blood limits its use as a biomarker,33 and it is not a good indicator of chronic drinking.44
EtG and EtS. Less than 0.1% of ethanol is secreted as the metabolites EtG and EtS, which are generated, respectively, by the enzymes uridine diphosphate glucuronosyltransferase and sulfotransferase.45 They have value in the diagnosis of AUD because of the length of time in which they can be detected. Urinary EtG and EtS have been especially important biomarkers for monitoring relapse in outpatients treated for alcohol-related problems.46 Generally, EtG and EtS can be detected in urine for 13 to 20 hours after a single drink (0.1 g/kg), and for up to 4 to 5 days following ingestion of large amounts of alcohol.47
Continue to: EtG has been detectable...
EtG has been detectable in urine for ≥ 24 hours following only 1 or 2 drinks, and for up to 4 days following heavy consumption.48 Shortly after alcohol intake, even in small amounts, EtG is detectable. Analysis of EtG in urine is helpful in monitoring alcohol consumption during withdrawal treatment, for workplace testing, and to check for abstinence in legal matters. The EtG urine test is useful in detecting alcohol consumption in a person who claims to be abstinent but who drank 2 or 3 days before the evaluation. Although accurate, EtG’s window for detection is narrower than that of the PEth assay.
EtS is a good marker of acute short-term alcohol use, up to 12 hours in the blood (or longer in heavier drinkers) and up to 5 days in urine.49 Its sensitivity is highest in heavy drinkers. Post-sampling formation and degradation of EtS have not been known to occur in urine samples. Testing for this second metabolite of ethanol can slightly improve the sensitivity and specificity of the EtG test. A urine test for EtS has a wider detection window. But it has little practical advantage compared with EtG.50
For better clinical specificity, a combination of both EtG and EtS testing has been recommended. However, the EtS assay is more cumbersome and provides little advantage over EtG. EtG values do not correlate precisely with the amount or frequency of ethanol use, but the magnitude of the EtG finding roughly corresponds to the amount of alcohol recently consumed.
False-positive and false-negative results for EtG and EtS are uncommon in practice. However, false-positive results are possible with the EtG test in certain circumstances: presence of Escherichia coli in the specimen, use of ethanol-based hand sanitizers (> 20 times a day) or mouthwashes, and the consumption of substances like pralines, nonalcoholic beer, pharmaceutical products, and fruit juice. Similarly, false-negative results of EtG can occur from degradation if the samples are contaminated with other bacteria, transported without cooling, or stored improperly.51 In practice, this is uncommon, and the test is believed to be specific with few false-positive results. Commercially available EtG colorimetric test strips permit on-site analysis of urine samples.
FAEEs are a combination of different esters and products of alcohol metabolism through a nonoxidative pathway. They are formed by esterification of endogenous free fatty acids and ethanol in blood and several tissues.29 These are sensitive and specific markers of alcohol ingestion and can differentiate chronic alcohol consumption from binge drinking.29 It is elevated for up to 99 hours in heavy alcohol drinkers.30 It can be detected in hair for a longer period than in blood.52 Detection of FAEEs in meconium can help establish fetal alcohol exposure.53
Continue to: PEth
PEth. Use of the PEth assay has increased in recent years and its accuracy has had a transformative effect on the diagnosis of AUD.54 PEth is a phospholipid found in erythrocyte membranes, formed by an interaction between ethanol and phosphatidylcholine, catalyzed by phospholipase D.55,56 Major advantages of PEth include an unusually long half-life and specificity. Red cells lack enzymes to degrade PEth, therefore PEth accumulates in red cells and has a half-life of 4 to 10 days57,58 allowing for detection of significant ethanol consumption extending back 3 to 4 weeks.59 There is no evidence that PEth is formed in the absence of ethanol, making the test essentially 100% specific, particularly at higher cutoff values of ≥ 150 ng/mL.31,60
PEth levels are not affected by age, gender, or underlying liver or renal disease.61 PEth can differentiate between heavy alcohol use and social drinking and can therefore identify chronic excessive use.62 With chronic excessive alcohol consumption, PEth is detectable in blood up to 28 days after sobriety.63 A correlation exists between PEth concentrations in blood and the amount of consumed ethanol. PEth has increased specificity and sensitivity for the detection of latent ethanol use compared with other direct biomarkers.21 It can identify recent heavy drinking earlier than indirect biomarkers, as it does not rely on hepatic injury.
Using a cutoff level of 20 ng/mL, PEth assays have a sensitivity of 73% for any alcohol use in the past month; at 80 ng/mL, the sensitivity is 91% for > 4 drinks/d.61 PEth is considered semi-quantitative. The World Health Organization defines acceptable social alcohol use at a PEth value < 40 ng/dL for men and < 20 ng/dL for women. Chronic excessive use is defined by a level > 60 ng/dL.55 The cutoff levels tend to be arbitrary and vary with different guidelines.
Although false-positive PEth test results may be possible, most experts believe that dishonesty in self-reporting by test subjects is more likely. That said, the true specificity of PEth remains unknown; a lower value detected should not be regarded as absolute proof of relapse or chronic alcoholism.
Studies have shown a positive correlation between the AUDIT-C score and PEth values combined with self-reported alcohol consumption, indicating that PEth may be a useful marker in difficult-to-assess settings, or in confirming or invalidating self-reported alcohol consumption.61,64,65 The PEth test is now widely available and, in the authors’ experience, usually costs $100 to $200. Analysis typically costs $40 to $100,66 and costs could decrease as the test becomes more widely used. Turnaround time for PEth is 5 to 10 days. It is now the recommended assay by transplant hepatologists for detecting alcohol use.67

Continue to: CASE ANSWER
CASE
CORRESPONDENCE
Frederick Nunes, MD, Pennsylvania Hospital of University of Pennsylvania, 230 West Washington Square, 4th Floor, Philadelphia, PA 19104; [email protected]
CASE A 34-year-old woman presents with fatigue. She appears defensive when asked about her alcohol use. She answers No to all questions on the CAGE (cut down, annoyed, guilty, eye-opener) screening tool, but acknowledges drinking excessively on rare occasions. Her physician has a high suspicion for alcohol use disorder (AUD) and recommends further testing. The patient agrees but denies having used alcohol over the past several days. Which of the following is most likely to help support the suspicion of a heavy drinking pattern?
- Routine lab tests (liver panel and complete blood count).
- Blood or urine alcohol level.
- Phosphatidylethanol (PEth) level in the blood.
- Ethyl glucuronide (EtG) in the urine.
- Carbohydrate-deficient transferrin (CDT) in the blood.
(See "Case answer.").
About 1 in 12 Americans have AUD,1 and 1 in 10 children live in a home with a parent who has a drinking problem.2 While the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) succinctly defines AUD with specific criteria,1 the term generally refers to an inability to control or stop drinking despite adverse social or health consequences. AUD is regarded as > 4 drinks per day for men and > 3 drinks per day for women.3 A “standard drink” would be a 12-oz bottle of beer, a 5-oz glass of wine, or 1.5 oz of distilled spirits. Effects of chronic alcohol use are vast and include malnutrition, alcohol withdrawal syndrome, alcoholic liver disease, pancreatitis/pancreatic cancer, cardiomyopathy, and stroke.4-6 Alcohol use by a pregnant woman can lead to fetal alcohol syndrome in her child.7
AUD may be more prevalent in the wake of COVID-19. Primary care practitioners tend to miss a large fraction of patients with AUD in their practice, especially younger patients and those without somatic comorbidities.8 Systematic screening for AUD can identify many of these people.8 Particularly as the COVID-19 pandemic continues to unfold and increases stress for everyone, risk of worsening drinking increases both in individuals with current AUD and for those in remission.9 Contrary to common belief, patients visiting primary care favor screening for at-risk drinking.10 Thus, awareness of the prevalence of AUD and patient acceptance of screening should encourage wider testing.
Screening tools. The 2014 guidelines published by the Centers for Disease Control and Prevention recommend using quick screening tools—ie, single question or AUDIT 1-3 (TABLE 111-18)—as an objective means of determining whether patients’ drinking creates a risk for themselves or others.11 Excessive drinking identified using alcohol questionnaires can help reduce medical complications and health care costs.19 The questionnaires we review do not provide a diagnosis but help identify individuals who might benefit from more thorough assessment.20 Following up, as needed, by testing for alcohol biomarkers can provide quantitative insight into problematic alcohol use.2

But before we discuss the utility of biomarkers, it’s important to quickly review how alcohol is eliminated from the body.
Alcohol elimination
The stomach and small intestine are the primary sites for alcohol absorption. Alcohol elimination from the body occurs through 3 pathways. The first involves oxidative metabolism, which eliminates most ethanol (95%) through the actions of alcohol dehydrogenase, cytochrome P4502E1, or catalase. A lesser amount of alcohol (2%-5%) is eliminated, unchanged, via the second pathway, which includes urine, sweat, and breath. Nonoxidative metabolism makes up the third pathway. Nonoxidative metabolism removes a very small amount (0.1%) of alcohol and involves the direct ethanol biomarkers PEth, EtG, ethyl sulfate (EtS), and fatty acid ethyl esters (FAEEs).21 Our emphasis in this article is on assays of direct metabolites of alcohol—particularly PEth.
Continue to: To understand the utility...
To understand the utility of these direct biomarkers, it is helpful to look at the indirect biomarkers first.
Indirect biomarkers have limited sensitivity and specificity
When alcohol is consumed in large enough quantities over time, indirect biomarkers of alcohol can become abnormal.22 The major indirect biomarkers are the liver enzymes aspartate and alanine aminotransferase (AST and ALT), gamma-glutamyl transferase (GGT), mean corpuscular volume (MCV) of red blood cells, and carbohydrate-deficient transferrin (CDT). Indirect biomarkers have limited sensitivity and specificity for AUD. (For specifics on sensitivity and specificity of indirect and direct biomarkers, see TABLE 2.23-31)

Liver enzymes. AST and ALT are also present in the heart, muscle, and kidneys. Elevated levels usually imply injury to hepatocytes, with ALT being more reflective of liver involvement than AST. Both AST and ALT are elevated in other common liver conditions including hepatitis C virus infection and fatty liver disease. In alcoholic liver disease (ALD), AST is elevated more than ALT; an AST-to-ALT ratio > 3 suggests ALD. An elevated GGT often indicates hepatic injury and is used to confirm that elevated alkaline phosphatase is of hepatic origin.3
MCV is the average volume of erythrocytes,33 and an elevated MCV is a potential indicator of excessive alcohol intake. Macrocytosis requires sustained alcohol use, and the test has low sensitivity. Other diseases such as vitamin B12 or folic acid deficiency, hypothyroidism, hematologic diseases (eg, cold agglutinin disease, multiple myeloma, amyloidosis), and certain medications can also increase MCV.34 Moreover, MCV responds slowly to alcohol use, abstinence, and relapse because red cells have a life span of 120 days.35
CDT. Transferrin is a glycoprotein produced in the liver. The level of transferrin with sialic acid chains increases with alcohol consumption as well as in the carbohydrate deficient glycoprotein syndrome, leading to so-called carbohydrate deficient transferrin.36 It is a sensitive marker for detecting alcohol relapse and monitoring sobriety. Moderate-to-heavy alcohol use, averaging ≥ 40 g of alcohol per day for 2 weeks,36 can decrease the amount of carbohydrate attached to transferrin. Two weeks after complete alcohol cessation, CDT levels will return to normal.37
Continue to: CDT is approved...
CDT is approved by the FDA as an assay for alcohol consumption.37 While CDT is felt to be one of the better indirect markers of AUD and can extend the window of detection, there are still issues with its sensitivity and specificity.38 This biomarker can be elevated with other liver diseases and can be affected by the patient’s age, body mass index, gender, and tobacco use.39,40 Testing for CDT has never achieved widespread clinical use and has been largely supplanted by the more accurate PEth test (described in a bit).
Direct biomarkers offer insight into recent alcohol use
Other than ethanol itself, direct biomarkers of alcohol use are minor ethanol metabolites created through biochemical reactions when ethanol is coupled to endogenous compounds. Hence, the presence of these metabolites is usually directly related to ethanol consumption.41 Direct alcohol biomarkers are EtG, EtS, FAEEs, and PEth (TABLE 223-31). They reflect alcohol consumption over a period of several days, making them useful when paired with questionnaire data, especially for identifying young adults who engage in binge drinking.42
Ethanol can be measured in blood, urine, and breath and is detectable a bit longer in urine than in blood. However, alcohol is detectable in the blood only for 6 to 12 hours after drinking. After alcohol consumption, concentrations peak in the blood within 2 hours. The window for detecting ethanol in the blood depends on the amount of alcohol consumed and the elimination rate of alcohol, which is about 12 mg/dL/h (or 0.012%)—approximately the same amount of alcohol contained in a standard drink (14 g).
Checking the blood alcohol level might be helpful in the office if a patient appears intoxicated but denies alcohol use. A blood alcohol level > 300 mg/dL, or > 150 mg/dL without gross evidence of intoxication, or > 100 mg/dL upon routine examination indicates AUD with a high degree of reliability.33,43 But the short half-life of ethanol in blood limits its use as a biomarker,33 and it is not a good indicator of chronic drinking.44
EtG and EtS. Less than 0.1% of ethanol is secreted as the metabolites EtG and EtS, which are generated, respectively, by the enzymes uridine diphosphate glucuronosyltransferase and sulfotransferase.45 They have value in the diagnosis of AUD because of the length of time in which they can be detected. Urinary EtG and EtS have been especially important biomarkers for monitoring relapse in outpatients treated for alcohol-related problems.46 Generally, EtG and EtS can be detected in urine for 13 to 20 hours after a single drink (0.1 g/kg), and for up to 4 to 5 days following ingestion of large amounts of alcohol.47
Continue to: EtG has been detectable...
EtG has been detectable in urine for ≥ 24 hours following only 1 or 2 drinks, and for up to 4 days following heavy consumption.48 Shortly after alcohol intake, even in small amounts, EtG is detectable. Analysis of EtG in urine is helpful in monitoring alcohol consumption during withdrawal treatment, for workplace testing, and to check for abstinence in legal matters. The EtG urine test is useful in detecting alcohol consumption in a person who claims to be abstinent but who drank 2 or 3 days before the evaluation. Although accurate, EtG’s window for detection is narrower than that of the PEth assay.
EtS is a good marker of acute short-term alcohol use, up to 12 hours in the blood (or longer in heavier drinkers) and up to 5 days in urine.49 Its sensitivity is highest in heavy drinkers. Post-sampling formation and degradation of EtS have not been known to occur in urine samples. Testing for this second metabolite of ethanol can slightly improve the sensitivity and specificity of the EtG test. A urine test for EtS has a wider detection window. But it has little practical advantage compared with EtG.50
For better clinical specificity, a combination of both EtG and EtS testing has been recommended. However, the EtS assay is more cumbersome and provides little advantage over EtG. EtG values do not correlate precisely with the amount or frequency of ethanol use, but the magnitude of the EtG finding roughly corresponds to the amount of alcohol recently consumed.
False-positive and false-negative results for EtG and EtS are uncommon in practice. However, false-positive results are possible with the EtG test in certain circumstances: presence of Escherichia coli in the specimen, use of ethanol-based hand sanitizers (> 20 times a day) or mouthwashes, and the consumption of substances like pralines, nonalcoholic beer, pharmaceutical products, and fruit juice. Similarly, false-negative results of EtG can occur from degradation if the samples are contaminated with other bacteria, transported without cooling, or stored improperly.51 In practice, this is uncommon, and the test is believed to be specific with few false-positive results. Commercially available EtG colorimetric test strips permit on-site analysis of urine samples.
FAEEs are a combination of different esters and products of alcohol metabolism through a nonoxidative pathway. They are formed by esterification of endogenous free fatty acids and ethanol in blood and several tissues.29 These are sensitive and specific markers of alcohol ingestion and can differentiate chronic alcohol consumption from binge drinking.29 It is elevated for up to 99 hours in heavy alcohol drinkers.30 It can be detected in hair for a longer period than in blood.52 Detection of FAEEs in meconium can help establish fetal alcohol exposure.53
Continue to: PEth
PEth. Use of the PEth assay has increased in recent years and its accuracy has had a transformative effect on the diagnosis of AUD.54 PEth is a phospholipid found in erythrocyte membranes, formed by an interaction between ethanol and phosphatidylcholine, catalyzed by phospholipase D.55,56 Major advantages of PEth include an unusually long half-life and specificity. Red cells lack enzymes to degrade PEth, therefore PEth accumulates in red cells and has a half-life of 4 to 10 days57,58 allowing for detection of significant ethanol consumption extending back 3 to 4 weeks.59 There is no evidence that PEth is formed in the absence of ethanol, making the test essentially 100% specific, particularly at higher cutoff values of ≥ 150 ng/mL.31,60
PEth levels are not affected by age, gender, or underlying liver or renal disease.61 PEth can differentiate between heavy alcohol use and social drinking and can therefore identify chronic excessive use.62 With chronic excessive alcohol consumption, PEth is detectable in blood up to 28 days after sobriety.63 A correlation exists between PEth concentrations in blood and the amount of consumed ethanol. PEth has increased specificity and sensitivity for the detection of latent ethanol use compared with other direct biomarkers.21 It can identify recent heavy drinking earlier than indirect biomarkers, as it does not rely on hepatic injury.
Using a cutoff level of 20 ng/mL, PEth assays have a sensitivity of 73% for any alcohol use in the past month; at 80 ng/mL, the sensitivity is 91% for > 4 drinks/d.61 PEth is considered semi-quantitative. The World Health Organization defines acceptable social alcohol use at a PEth value < 40 ng/dL for men and < 20 ng/dL for women. Chronic excessive use is defined by a level > 60 ng/dL.55 The cutoff levels tend to be arbitrary and vary with different guidelines.
Although false-positive PEth test results may be possible, most experts believe that dishonesty in self-reporting by test subjects is more likely. That said, the true specificity of PEth remains unknown; a lower value detected should not be regarded as absolute proof of relapse or chronic alcoholism.
Studies have shown a positive correlation between the AUDIT-C score and PEth values combined with self-reported alcohol consumption, indicating that PEth may be a useful marker in difficult-to-assess settings, or in confirming or invalidating self-reported alcohol consumption.61,64,65 The PEth test is now widely available and, in the authors’ experience, usually costs $100 to $200. Analysis typically costs $40 to $100,66 and costs could decrease as the test becomes more widely used. Turnaround time for PEth is 5 to 10 days. It is now the recommended assay by transplant hepatologists for detecting alcohol use.67

Continue to: CASE ANSWER
CASE
CORRESPONDENCE
Frederick Nunes, MD, Pennsylvania Hospital of University of Pennsylvania, 230 West Washington Square, 4th Floor, Philadelphia, PA 19104; [email protected]
1. APA. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. American Psychiatric Publishing. 2013:490-497.
2. Fleming MF, Smith MJ, Oslakovic E, et al. Phosphatidylethanol detects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol Clin Exp Res. 2017;41:857-862.
3. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed November 12, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
4. Herreros-Villanueva M, Hijona E, Bañales JM, et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19:638-647.
5. Rocco A, Compare D, Angrisani D, et al. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652-14659.
6.
7. Sebastiani G, Borrás-Novell C, Casanova MA, et al. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients. 2018;10:1008.
8. Rehm J, Anderson P, Manthey J, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51:422-427. doi: 10.1093/alcalc/agv127
9. ASAM. Caring for patients during the COVID-19 pandemic. Accessed November 12, 2021. www.asam.org/docs/default-source/covid-19/acute-care_062620.pdf?sfvrsn=e66d54c2_10
10. Miller PM, Thomas SE, Mallin R. Patient attitudes towards self-report and biomarker alcohol screening by primary care physicians. Alcohol Alcohol. 2006;41:306-310. doi: 10.1093/alcalc/agl022
11. Zoorob R, Snell H, Kihlberg C, et al. Screening and brief intervention for risky alcohol use. Curr Probl Pediatr Adolesc Health Care. 2014;44:82-87.
12. Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783-788.
13. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905-1907.
14. Sokol RJ, Martier SS, Ager JW. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160:863-868.
15. Cherpitel CJ. A brief screening instrument for problem drinking in the emergency room: the RAPS4. Rapid Alcohol Problems Screen. J Stud Alcohol. 2000;61:447-449.
16. WHO. AUDIT: The alcohol use identification test. Accessed November 14, 2021. http://apps.who.int/iris/bitstream/handle/10665/67205/WHO_MSD_MSB_01.6a.pdf?sequence=1
17. Westermeyer J, Yargic I, Thuras P. Michigan assessment-screening test for alcohol and drugs (MAST/AD): evaluation in a clinical sample. Am J Addict. 2004;13:151-162.
18. Powers JS, Spickard A. Michigan Alcoholism Screening Test to diagnose early alcoholism in a general practice. South Med J. 1984;77:852-856.
19. NIH. Treatment for alcohol problems: finding and getting help. Accessed November 12, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/treatment-alcohol-problems-finding-and-getting-help
20. Kitchens JM. Does this patient have an alcohol problem? JAMA. 1994;272:1782-1787.
21. Kummer N, Lambert WE, Samyn N, et al. Alternative sampling strategies for the assessment of alcohol intake of living persons. Clin Biochem. 2016;49:1078-1091.
22. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63:1634-1640.
23. Mundle G, Ackermann K, Munkes J, et al. Influence of age, alcohol consumption and abstinence on the sensitivity of carbohydrate‐deficient transferrin, gamma‐glutamyltransferase and mean corpuscular volume. Alcohol Alcohol. 1999;34:760-766.
24. Neumann T, Spies C. Use of biomarkers for alcohol use disorders in clinical practice. Addiction. 2003;98(suppl 2):81-91.
25. Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:11684-11699.
26. Helander A. Biological markers of alcohol use and abuse in theory and practice. In: Agarwal DP, Seitz HK, eds. Alcohol in Health and Disease. Marcel Dekker. 2001:177-205.
27. Stewart SH, Koch DG, Burgess DM, et al. Sensitivity and specificity of urinary ethyl glucuronide and ethyl sulfate in liver disease patients. Alcohol Clin Exp Res. 2013;37:150-155.
28. Helander A, Dahl H. Urinary tract infection: a risk factor for false-negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clin Chem. 2005;51:1728-1730.
29. Ghosh S, Jain R, Jhanjee S, et al. Alcohol biomarkers and their relevance in detection of alcohol consumption in clinical settings. Accessed November 12, 2021. https://www.clinmedjournals.org/articles/iasar/international-archives-of-substance-abuse-and-rehabilitation-iasar-1-002.php?jid=iasar
30. Borucki K, Dierkes J, Wartberg J, et al. In heavy drinkers, fatty acid ethyl esters remain elevated for up to 99 hours. Alcohol Clin Exp Res. 2007;31:423-427.
31. Hartmann S, Aradottir S, Graf M, et al. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12:81-84.
32. Choe YM, Lee BC, Choi IG, et al. Combination of the CAGE and serum gamma-glutamyl transferase: an effective screening tool for alcohol use disorder and alcohol dependence. Neuropsychiatr Dis Treat. 2019 31;15:1507-1515.
33. Niemelä O. Biomarkers in alcoholism. Clin Chim Acta. 2007;377:39-49.
34. Kauffmann T, Evans DS. Macrocytosis. Accessed November 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK560908/
35. Maenhout TM, De Buyzere ML, Delanghe JR. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin Chim Acta. 2013;415:322-329.
36. Solomons HD. Carbohydrate deficient transferrin and alcoholism. Germs. 2012;2:75-78.
37. Allen JP, Wurst FM, Thon N, et al. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl. 2013;19:369-376.
38. Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001-2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841:96-109.
39. Hannuksela ML, Liisanantti MK, Nissinen AE, et al. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953-961.
40. Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem. 2001;47:13-27.
41. Cabarcos P, Hassan HM, Tabernero MJ, et al. Analysis of ethyl glucuronide in hair samples by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). J Appl Toxicol. 2013;33:638-643.
42. Piano MR, Mazzuco A, Kang M, et al. Binge drinking episodes in young adults: how should we measure them in a research setting? J Stud Alcohol Drugs. 2017;78:502-511.
43. Adinoff B, Bone GH, Linnoila M. Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol Adverse Drug Exp. 1988;3:172-196.
44. Cabezas J, Lucey MR, Bataller R. Biomarkers for monitoring alcohol use. Clin Liver Dis (Hoboken). 2016;8:59-63.
45. Wurst FM, Alling C, Aradottir S, et al. Emerging biomarkers: new directions and clinical applications. Alcohol Clin Exp Res. 2005;29:465-473.
46. Helander A, Péter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552-557.
47. Helander A, Böttcher M, Fehr C, et al. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44:55-61.
48. Jatlow P, O’Malley SS. Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin Exp Res. 2010;34:968-975.
49. Jatlow PI, Agro A, Wu R, et al. Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: results of a dose ranging alcohol challenge study and 2 clinical trials. Alcohol Clin Exp Res. 2014;38:2056-2065.
50. Gonzalo P, Radenne S, Gonzalo S. Biomarkers of chronic alcohol misuse. Curr Biomark Find. 2014;4:9-22.
51. Bornhorst JA, Mbughuni MM. Alcohol biomarkers: clinical issues and analytical methods. In: Critical Issues in Alcohol and Drugs of Abuse Testing. 2nd ed. Academic Press. 2019:25-42.
52. Soderberg BL, Salem RO, Best CA, et al. Fatty acid ethyl esters. Ethanol metabolites that reflect ethanol intake. Am J Clin Pathol. 2003;119(suppl):S94-S99.
53. Cheng CT, Ostrea EM Jr, Alviedo JN, et al. Fatty acid ethyl esters in meconium: a biomarker of fetal alcohol exposure and effect. Exp Biol Med (Maywood). 2021;246:380-386.
54. Andresen-Streichert H, Beres Y, Weinmann W, et al. Improved detection of alcohol consumption using the novel marker phosphatidylethanol in the transplant setting: results of a prospective study. Transpl Int. 2017;30:611-620.
55. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13:14788-14812.
56. Gnann H, Weinmann W, Thierauf A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36:1507-1511.
57. Aradóttir S, Moller K, Alling C. Phosphatidylethanol formation and degradation in human and rat blood. Alcohol Alcohol. 2004;39:8-13.
58. Varga A, Alling C. Formation of phosphatidylethanol in vitro in red blood cells from healthy volunteers and chronic alcoholics. J Lab Clin Med. 2002;140:79-83.
59. Javors MA, Hill-Kapturczak N, Roache JD, et al. Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcohol Clin Exp Res. 2016;40:1228-1234.
60. Schröck A, Pfäffli M, König S, et al. Application of phosphatidylethanol (PEth) in whole blood in comparison to ethyl glucuronide in hair (hEtG) in driving aptitude assessment (DAA). Int J Legal Med. 2016;130:1527-1533.
61. Stewart SH, Koch DG, Willner IR, et al. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706-1711.
62. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015 29;5:1339-1385.
63. Hill-Kapturczak N, Dougherty DM, Roache JD, et al. Phosphatidylethanol homologs in blood as biomarkers for the time frame and amount of recent alcohol consumption. In: Preedy VR (ed) Neuroscience of Alcohol. Academic Press; 2019:567-576.
64. Jain J, Evans JL, Briceño A, et al. Comparison of phosphatidylethanol results to self-reported alcohol consumption among young injection drug users. Alcohol Alcohol. 2014;49:520-524.
65. Schröck A, Wurst FM, Thon N, et al. Assessing phosphatidylethanol (PEth) levels reflecting different drinking habits in comparison to the alcohol use disorders identification test - C (AUDIT-C). Drug Alcohol Depend. 2017;178:80-86.
66. McDonnell MG, Skalisky J, Leickly E, et al. Pilot investigation of a phosphatidylethanol-based contingency management intervention targeting alcohol use. Psychol Addict Behav. 2017;31:608-613.
67. Asrani SK, Trotter J, Lake J, et al. Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis. Liver Transpl. 2020;26:127-140.
68. WHO. International Guide for Monitoring Alcohol Consumption and Harm. 2000. Accessed November 12, 2021. http://apps.who.int/iris/bitstream/handle/10665/66529/WHO_MSD_MSB_00.4.pdf?sequence=1
1. APA. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. American Psychiatric Publishing. 2013:490-497.
2. Fleming MF, Smith MJ, Oslakovic E, et al. Phosphatidylethanol detects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol Clin Exp Res. 2017;41:857-862.
3. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed November 12, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
4. Herreros-Villanueva M, Hijona E, Bañales JM, et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19:638-647.
5. Rocco A, Compare D, Angrisani D, et al. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652-14659.
6.
7. Sebastiani G, Borrás-Novell C, Casanova MA, et al. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients. 2018;10:1008.
8. Rehm J, Anderson P, Manthey J, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51:422-427. doi: 10.1093/alcalc/agv127
9. ASAM. Caring for patients during the COVID-19 pandemic. Accessed November 12, 2021. www.asam.org/docs/default-source/covid-19/acute-care_062620.pdf?sfvrsn=e66d54c2_10
10. Miller PM, Thomas SE, Mallin R. Patient attitudes towards self-report and biomarker alcohol screening by primary care physicians. Alcohol Alcohol. 2006;41:306-310. doi: 10.1093/alcalc/agl022
11. Zoorob R, Snell H, Kihlberg C, et al. Screening and brief intervention for risky alcohol use. Curr Probl Pediatr Adolesc Health Care. 2014;44:82-87.
12. Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783-788.
13. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905-1907.
14. Sokol RJ, Martier SS, Ager JW. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160:863-868.
15. Cherpitel CJ. A brief screening instrument for problem drinking in the emergency room: the RAPS4. Rapid Alcohol Problems Screen. J Stud Alcohol. 2000;61:447-449.
16. WHO. AUDIT: The alcohol use identification test. Accessed November 14, 2021. http://apps.who.int/iris/bitstream/handle/10665/67205/WHO_MSD_MSB_01.6a.pdf?sequence=1
17. Westermeyer J, Yargic I, Thuras P. Michigan assessment-screening test for alcohol and drugs (MAST/AD): evaluation in a clinical sample. Am J Addict. 2004;13:151-162.
18. Powers JS, Spickard A. Michigan Alcoholism Screening Test to diagnose early alcoholism in a general practice. South Med J. 1984;77:852-856.
19. NIH. Treatment for alcohol problems: finding and getting help. Accessed November 12, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/treatment-alcohol-problems-finding-and-getting-help
20. Kitchens JM. Does this patient have an alcohol problem? JAMA. 1994;272:1782-1787.
21. Kummer N, Lambert WE, Samyn N, et al. Alternative sampling strategies for the assessment of alcohol intake of living persons. Clin Biochem. 2016;49:1078-1091.
22. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63:1634-1640.
23. Mundle G, Ackermann K, Munkes J, et al. Influence of age, alcohol consumption and abstinence on the sensitivity of carbohydrate‐deficient transferrin, gamma‐glutamyltransferase and mean corpuscular volume. Alcohol Alcohol. 1999;34:760-766.
24. Neumann T, Spies C. Use of biomarkers for alcohol use disorders in clinical practice. Addiction. 2003;98(suppl 2):81-91.
25. Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:11684-11699.
26. Helander A. Biological markers of alcohol use and abuse in theory and practice. In: Agarwal DP, Seitz HK, eds. Alcohol in Health and Disease. Marcel Dekker. 2001:177-205.
27. Stewart SH, Koch DG, Burgess DM, et al. Sensitivity and specificity of urinary ethyl glucuronide and ethyl sulfate in liver disease patients. Alcohol Clin Exp Res. 2013;37:150-155.
28. Helander A, Dahl H. Urinary tract infection: a risk factor for false-negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clin Chem. 2005;51:1728-1730.
29. Ghosh S, Jain R, Jhanjee S, et al. Alcohol biomarkers and their relevance in detection of alcohol consumption in clinical settings. Accessed November 12, 2021. https://www.clinmedjournals.org/articles/iasar/international-archives-of-substance-abuse-and-rehabilitation-iasar-1-002.php?jid=iasar
30. Borucki K, Dierkes J, Wartberg J, et al. In heavy drinkers, fatty acid ethyl esters remain elevated for up to 99 hours. Alcohol Clin Exp Res. 2007;31:423-427.
31. Hartmann S, Aradottir S, Graf M, et al. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12:81-84.
32. Choe YM, Lee BC, Choi IG, et al. Combination of the CAGE and serum gamma-glutamyl transferase: an effective screening tool for alcohol use disorder and alcohol dependence. Neuropsychiatr Dis Treat. 2019 31;15:1507-1515.
33. Niemelä O. Biomarkers in alcoholism. Clin Chim Acta. 2007;377:39-49.
34. Kauffmann T, Evans DS. Macrocytosis. Accessed November 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK560908/
35. Maenhout TM, De Buyzere ML, Delanghe JR. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin Chim Acta. 2013;415:322-329.
36. Solomons HD. Carbohydrate deficient transferrin and alcoholism. Germs. 2012;2:75-78.
37. Allen JP, Wurst FM, Thon N, et al. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl. 2013;19:369-376.
38. Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001-2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841:96-109.
39. Hannuksela ML, Liisanantti MK, Nissinen AE, et al. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953-961.
40. Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem. 2001;47:13-27.
41. Cabarcos P, Hassan HM, Tabernero MJ, et al. Analysis of ethyl glucuronide in hair samples by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). J Appl Toxicol. 2013;33:638-643.
42. Piano MR, Mazzuco A, Kang M, et al. Binge drinking episodes in young adults: how should we measure them in a research setting? J Stud Alcohol Drugs. 2017;78:502-511.
43. Adinoff B, Bone GH, Linnoila M. Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol Adverse Drug Exp. 1988;3:172-196.
44. Cabezas J, Lucey MR, Bataller R. Biomarkers for monitoring alcohol use. Clin Liver Dis (Hoboken). 2016;8:59-63.
45. Wurst FM, Alling C, Aradottir S, et al. Emerging biomarkers: new directions and clinical applications. Alcohol Clin Exp Res. 2005;29:465-473.
46. Helander A, Péter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552-557.
47. Helander A, Böttcher M, Fehr C, et al. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44:55-61.
48. Jatlow P, O’Malley SS. Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin Exp Res. 2010;34:968-975.
49. Jatlow PI, Agro A, Wu R, et al. Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: results of a dose ranging alcohol challenge study and 2 clinical trials. Alcohol Clin Exp Res. 2014;38:2056-2065.
50. Gonzalo P, Radenne S, Gonzalo S. Biomarkers of chronic alcohol misuse. Curr Biomark Find. 2014;4:9-22.
51. Bornhorst JA, Mbughuni MM. Alcohol biomarkers: clinical issues and analytical methods. In: Critical Issues in Alcohol and Drugs of Abuse Testing. 2nd ed. Academic Press. 2019:25-42.
52. Soderberg BL, Salem RO, Best CA, et al. Fatty acid ethyl esters. Ethanol metabolites that reflect ethanol intake. Am J Clin Pathol. 2003;119(suppl):S94-S99.
53. Cheng CT, Ostrea EM Jr, Alviedo JN, et al. Fatty acid ethyl esters in meconium: a biomarker of fetal alcohol exposure and effect. Exp Biol Med (Maywood). 2021;246:380-386.
54. Andresen-Streichert H, Beres Y, Weinmann W, et al. Improved detection of alcohol consumption using the novel marker phosphatidylethanol in the transplant setting: results of a prospective study. Transpl Int. 2017;30:611-620.
55. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13:14788-14812.
56. Gnann H, Weinmann W, Thierauf A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36:1507-1511.
57. Aradóttir S, Moller K, Alling C. Phosphatidylethanol formation and degradation in human and rat blood. Alcohol Alcohol. 2004;39:8-13.
58. Varga A, Alling C. Formation of phosphatidylethanol in vitro in red blood cells from healthy volunteers and chronic alcoholics. J Lab Clin Med. 2002;140:79-83.
59. Javors MA, Hill-Kapturczak N, Roache JD, et al. Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcohol Clin Exp Res. 2016;40:1228-1234.
60. Schröck A, Pfäffli M, König S, et al. Application of phosphatidylethanol (PEth) in whole blood in comparison to ethyl glucuronide in hair (hEtG) in driving aptitude assessment (DAA). Int J Legal Med. 2016;130:1527-1533.
61. Stewart SH, Koch DG, Willner IR, et al. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706-1711.
62. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015 29;5:1339-1385.
63. Hill-Kapturczak N, Dougherty DM, Roache JD, et al. Phosphatidylethanol homologs in blood as biomarkers for the time frame and amount of recent alcohol consumption. In: Preedy VR (ed) Neuroscience of Alcohol. Academic Press; 2019:567-576.
64. Jain J, Evans JL, Briceño A, et al. Comparison of phosphatidylethanol results to self-reported alcohol consumption among young injection drug users. Alcohol Alcohol. 2014;49:520-524.
65. Schröck A, Wurst FM, Thon N, et al. Assessing phosphatidylethanol (PEth) levels reflecting different drinking habits in comparison to the alcohol use disorders identification test - C (AUDIT-C). Drug Alcohol Depend. 2017;178:80-86.
66. McDonnell MG, Skalisky J, Leickly E, et al. Pilot investigation of a phosphatidylethanol-based contingency management intervention targeting alcohol use. Psychol Addict Behav. 2017;31:608-613.
67. Asrani SK, Trotter J, Lake J, et al. Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis. Liver Transpl. 2020;26:127-140.
68. WHO. International Guide for Monitoring Alcohol Consumption and Harm. 2000. Accessed November 12, 2021. http://apps.who.int/iris/bitstream/handle/10665/66529/WHO_MSD_MSB_00.4.pdf?sequence=1
PRACTICE RECOMMENDATIONS
› Use a quick screening instrument such as the single-question tool or the AUDIT 1-3 to objectively determine whether patients’ drinking is risky for themselves or for others. C
› Suspect alcoholic liver disease if the ratio of aspartate aminotransferase to alanine aminotransferase is > 3. C
› Consider using the PEth assay in high-risk patients to differentiate between heavy alcohol use and social drinking. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Botulinum toxin for chronic pain: What's on the horizon?
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.
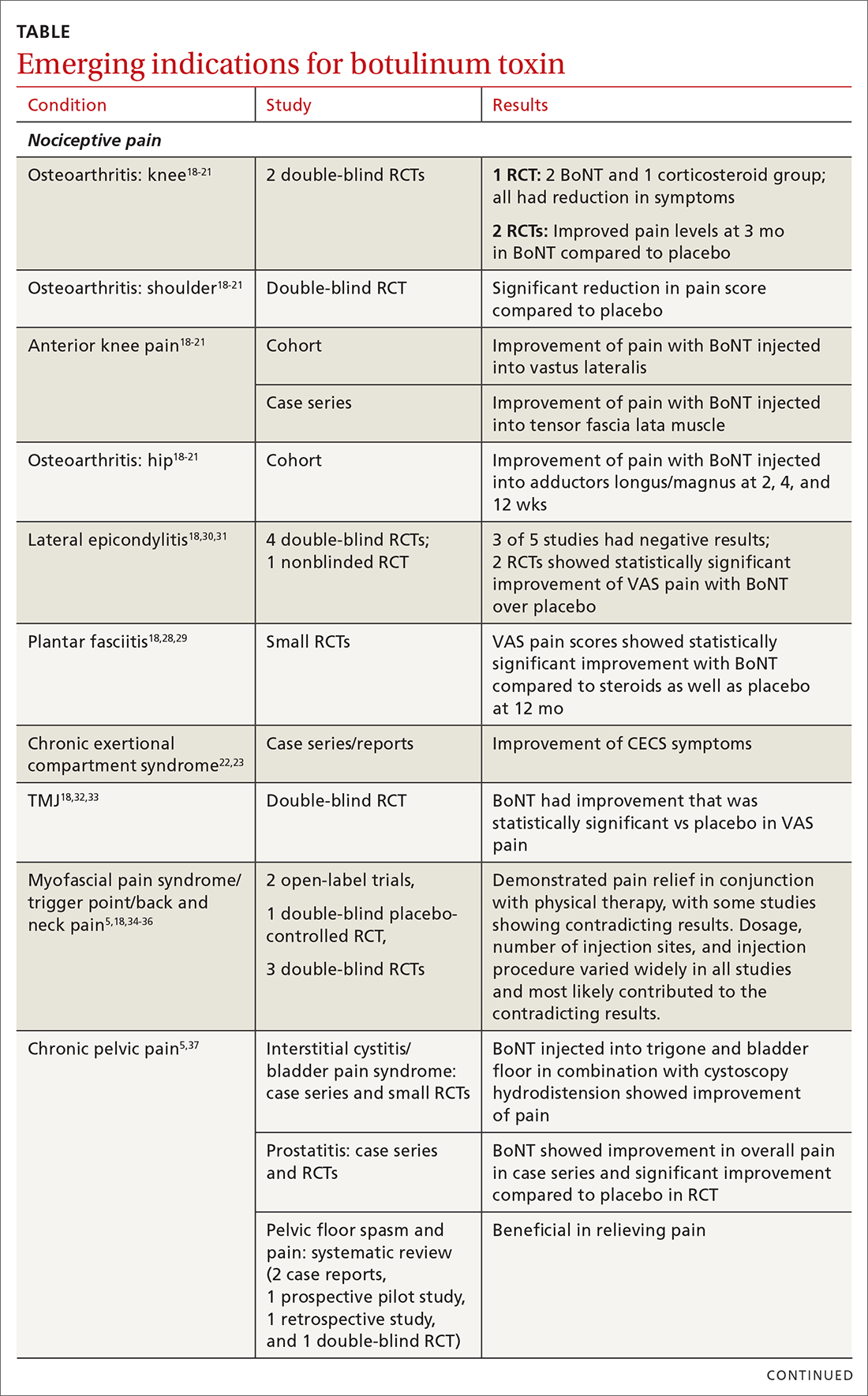
The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.
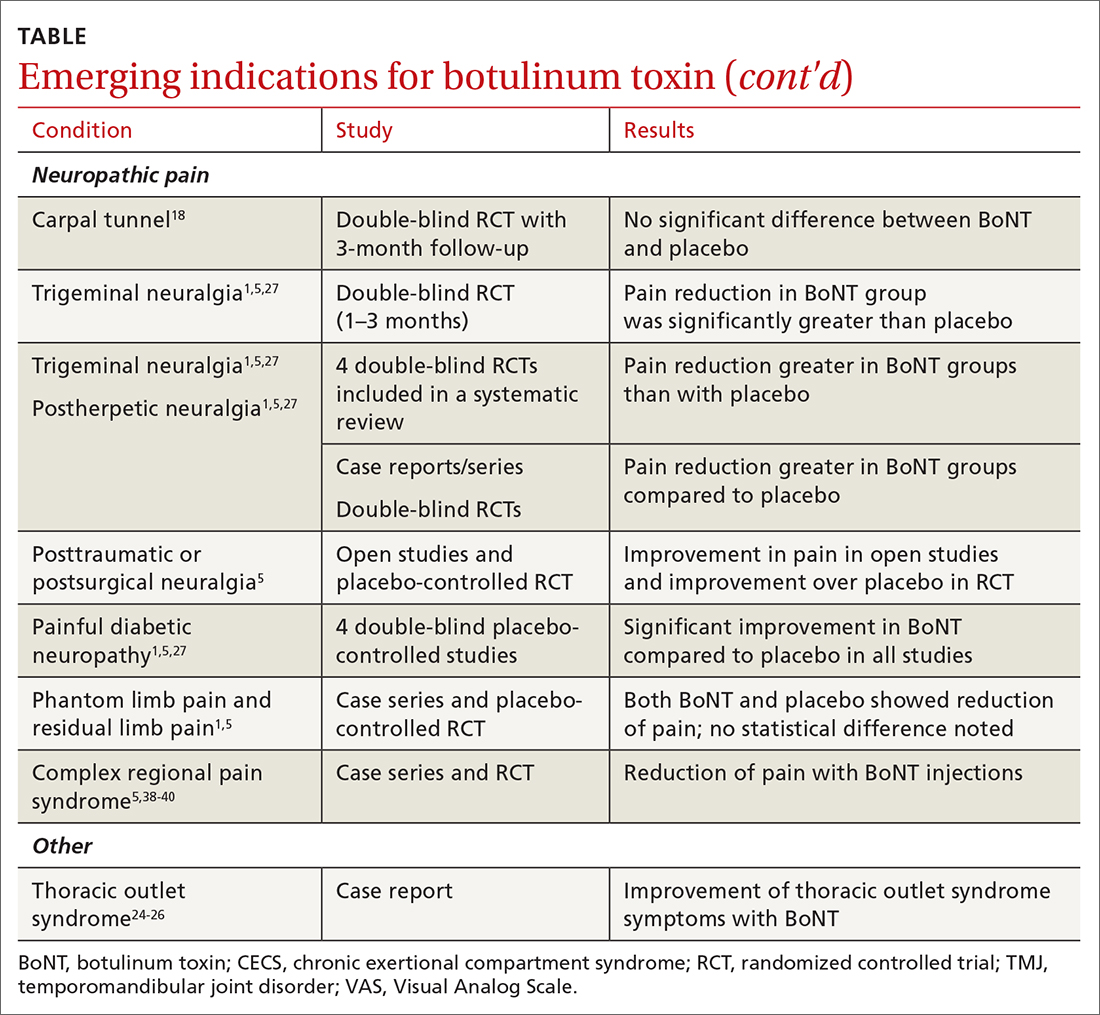
Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.

The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.

Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.

The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.

Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
PRACTICE RECOMMENDATIONS
› Consider botulinum toxin (BoNT) for patients with headache, spasticity, or cervical dystonia, as the FDA has approved BoNT for pain relief in these conditions. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How to meet the challenges of managing patients with IBS
Irritable bowel syndrome (IBS) continues to pose a diagnostic and therapeutic challenge to clinicians and patients—a challenge that arises from the varying manifestations of the condition, its complex pathophysiology, lack of effective treatment, and psychological consequences for patients. In this article, I explore new findings related to the pathophysiology, diagnosis, and management of IBS subtypes.
Start with the Rome IV classification of IBS
The Rome Foundation published its latest IBS classification and diagnostic criteria (known as Rome IV) in 2016.1 IBS is defined as abdominal pain that (1) has recurred, on average, ≥ 1 time per week during the past 3 months and (2) is associated with ≥ 2 of these criteria1:
- related to defecation
- associated with a change in stool frequency
- associated with a change in the appearance of stool.
Onset of symptoms should be present for 6 months before a diagnosis of IBS is made.1
IBS subtypes—constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), mixed (IBS-M), and unclassified (IBS-U) (TABLE 1)1—are based on the frequency of specific stool forms, as described and illustrated in the Bristol Stool Scale (www.webmd.com/digestive-disorders/poop-chart-bristol-stool-scale).2
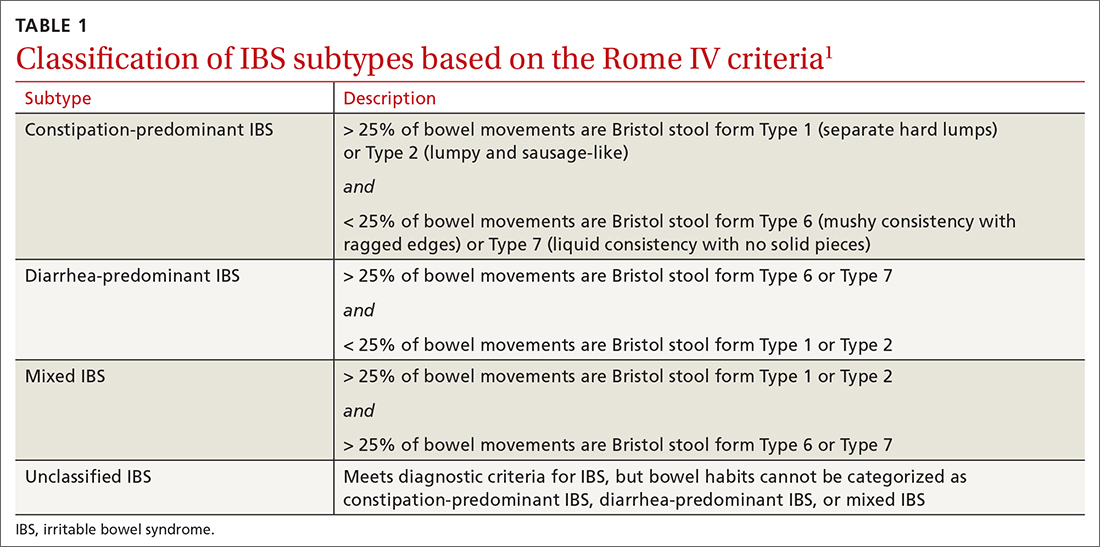
A widespread, costly, potentially debilitating disorder
IBS affects 10% to 12% of adults worldwide. The condition is more common among women and people younger than 50 years.1,3 Women with IBS tend to have more constipation symptoms (IBS-C); men with IBS, more diarrhea symptoms (IBS-D).4
The financial burden of IBS on the health care system and patients is significant. In a 2013 appraisal of 35 studies, the authors note that estimates of the direct cost of IBS care in the United States vary considerably—from $1562 to $7547 for a patient annually.5
A recent study found that almost 25% of IBS patients report absenteeism from work due to IBS symptoms.6 A Danish study that followed 7278 patients for 5 years found that IBS patients utilized more health care, sick days, and disability pension benefits than non-IBS patients, and had increased utilization of medical resources because of psychiatric conditions.7
Continue to: IBS patients also have comorbidities
IBS patients also have comorbidities:
- More than 20% of IBS patients have functional dyspepsia, gastroesophageal reflux disease, incontinence, or pelvic floor dyssynergia.4
- The frequency of fibromyalgia syndrome in IBS patients is reported to be 20% to 65%.8
- 14% of IBS patients meet criteria for chronic fatigue syndrome.8
- Interstitial cystitis and dyspareunia are common among IBS patients.9
Pathophysiology is complex
Models describing the pathophysiology of IBS have evolved through the years. Recent models describe it as a combination of altered gastrointestinal motility, visceral hyperalgesia, increased intestinal permeability, immune activation, altered intestinal microbiota, and dysfunction in the brain–gut axis. Certain environmental and psychological variables (eg, previous gastroenteritis, food intolerance, chronic stress, diverticulitis, and surgery) increase the risk of IBS.1,10,11
In the past several years, considerable attention has been paid to the roles played by the immune system, brain–gut axis function, and intestinal microbiota in IBS manifestations. Research focus in these areas might assist in the development of specific treatment modalities targeting IBS subtypes.
Immune system. A recent meta-analysis of the records of 706 IBS patients found an increased number of mast cells and CD3 T cells in biopsy specimens from the rectosigmoid and descending colon of IBS patients.12 Another study found a significant increase in mast cells in the ileum of IBS patients13; this increase is evident not only on intestinal biopsy but also at the serologic level. IBS-D patients have a higher plasma interleukin (IL)-6 level than the general population.14 Another meta-analysis found an imbalance in the serum level of tumor necrosis factor-α and IL-10 in IBS patients.15
Brain–gut axis. A 2016 meta-analysis showed that patients with anxiety and depression have a 2-fold increased risk of IBS.16 A more recent study, using data from the National Health Insurance Research Database that included 22,356 patients with IBS, found a 3.6-fold increased risk of psychiatric disorders in IBS.17 These findings reflect the complex interaction between the brain and the intestinal tract in IBS.
Continue to: Intestinal microbiota
Intestinal microbiota. Research evaluating the role of altered intestinal microbiota in IBS has yielded mixed results. A meta-analysis of 777 IBS patients showed an increase in Firmicutes spp, a decrease in Bacteroidetes spp, and an increase in the ratio of Firmicutes spp to Bacteroidetes spp in subjects’ fecal specimens.18 Another study, of 1340 patients, found no difference in Bacteroides spp and Enterococcus spp between healthy controls and IBS patients, but did find (1) lower fecal counts of Lactobacillus spp and Bifidobacterium spp and (2) higher fecal counts of Escherichia coli and Enterobacter spp in IBS patients.19
Postinfectious IBS. The Rome Foundation introduced the diagnosis of postinfectious IBS (PI-IBS) in 2019. PI-IBS develops in 10% of patients who have had infectious enteritis. Female gender, younger age, psychological distress during or before the enteritis episode, and severity of the acute episode are risk factors for this IBS variant.20 A study of 21,421 enteritis patients found that 42% with protozoal or parasitic infection and 14% with bacterial infection developed IBS.
Patients with nonviral enteritis often have a more severe course of enteritis, typically requiring antibiotics. It is believed that the resulting irregularities in the intestinal microbiota make these patients more likely to develop PI-IBS.21 PI-IBS patients are likely to improve or fully recover over time. Symptoms of PI-IBS are managed in a manner similar to how non-PI-IBS patients are managed.20
Challenges in making the IBS diagnosis
Historically, the diagnosis of IBS has been made clinically after excluding red flags (ie, signs or symptoms that might reflect other underlying medical problems) in the clinical presentation. For this reason, obtain a thorough clinical history that includes the course of symptoms, triggers, and alleviating factors. Any of the following are considered red flags1,22,23:
- age > 50 years at onset of symptoms
- new-onset constipation in the elderly
- rectal bleeding
- unexplained weight loss or anemia
- family history of organic gastrointestinal disease
- palpable abdominal or rectal mass
- nocturnal symptoms.
New studies demonstrate that several inflammatory markers can help exclude inflammatory bowel disease from the differential diagnosis in patients in whom IBS is suspected and being investigated.24 In 2019, the American Gastroenterological Association published a clinical practice guideline updating the laboratory evaluation of functional diarrhea and IBS-D in adults,25 and made several recommendations:
- Obtain the level of fecal calprotectin (normal level, ≤ 50 mcg/g) or fecal lactoferrin (≤ 4.0-7.25 mcg/g); if these tests are not available or results are not accessible, the C-reactive protein level is a reasonable option.
- Do not routinely use the erythrocyte sedimentation rate or C-reactive protein level to screen for inflammatory bowel disease.
- Test for Giardia lamblia with an antigen or polymerase chain reaction test.
- Do not test for ova and parasites (other than Giardia) in patients who do not have a history of travel or who have not emigrated from a high-risk area recently.
- Obtain testing for celiac disease with immunoglobulin A (IgA) tissue transglutaminase and with a second test, of immunoglobulin G (IgG) tissue transglutaminase and IgG or IgA deaminated gliadin peptides, to detect celiac disease in IgA-deficient patients.
- Order testing for bile-acid diarrhea with selenium homotaurocholic acid nuclear medicine scanning (if available in your region; the test is available in Europe); measurement of bile acid from a 48-hour stool collection; or an assay of fibroblast growth factor 19, which measures defective feedback of bile-acid synthesis. If these tests are unavailable, consider an empiric trial of a bile-acid binder.
- Do not use available serologic IBS testing.
Continue to: Continue to obtain a...
Continue to obtain a complete blood count for the evaluation of anemia. Endoscopic procedures are indicated in patients with a red flag.1
Treat based on subtype
The first step in the treatment of all IBS patients (TABLE 21,3,4,9,26,27) is for you to develop a strong relationship with the patient: You must acknowledge the disease and empower the patient to manage their symptoms. A strong physician–patient relationship leads to more effective outcomes.4
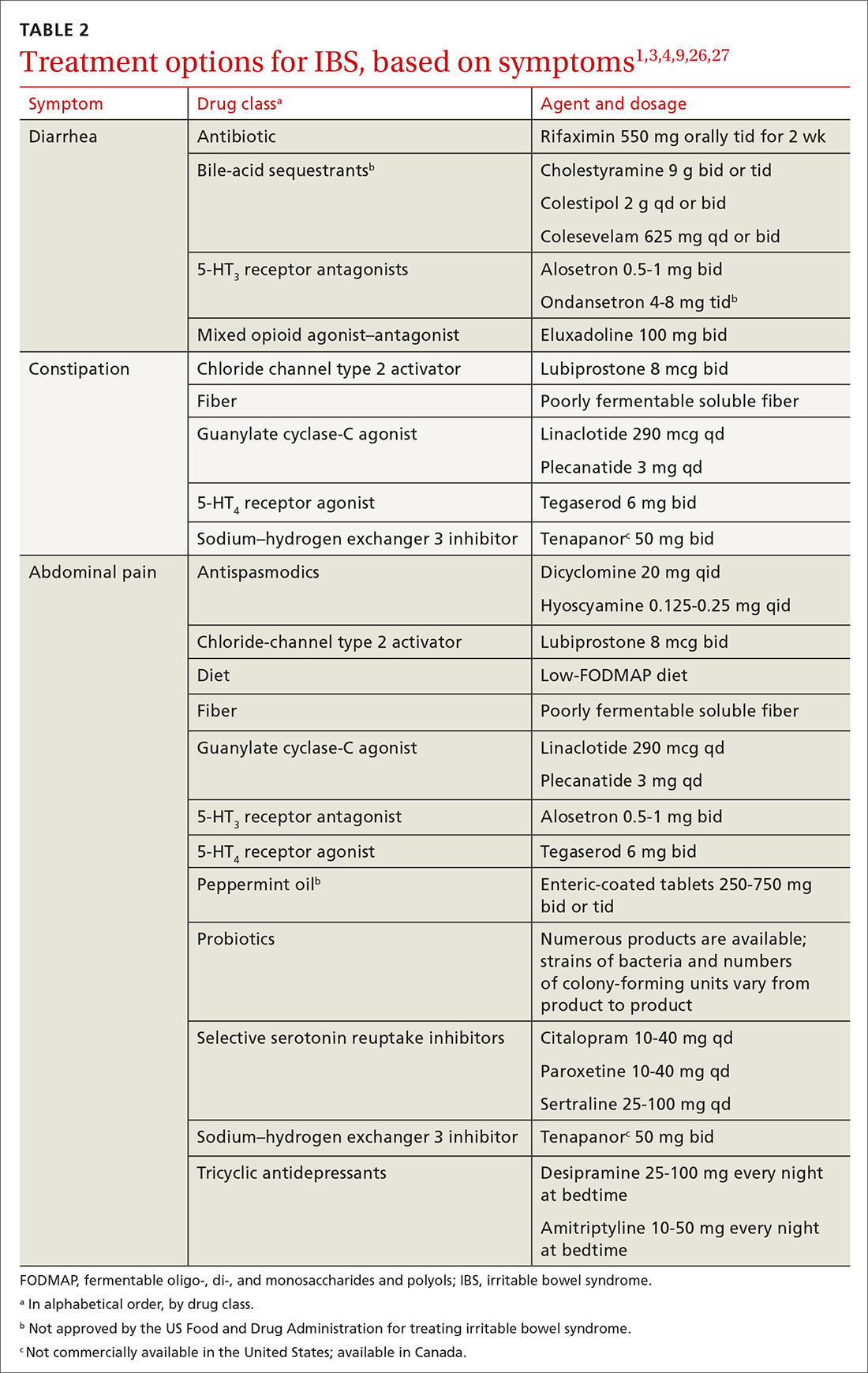
IBS treatment modalities target abdominal pain, bloating, abdominal distention, and altered bowel function—described in the literature as global symptoms. IBS-M patients should direct their treatment to the predominant symptom (constipation or diarrhea). The following sections describe available treatment options. The FIGURE1,3,4,9,25 shows a treatment workflow based on IBS subtype and symptom severity.
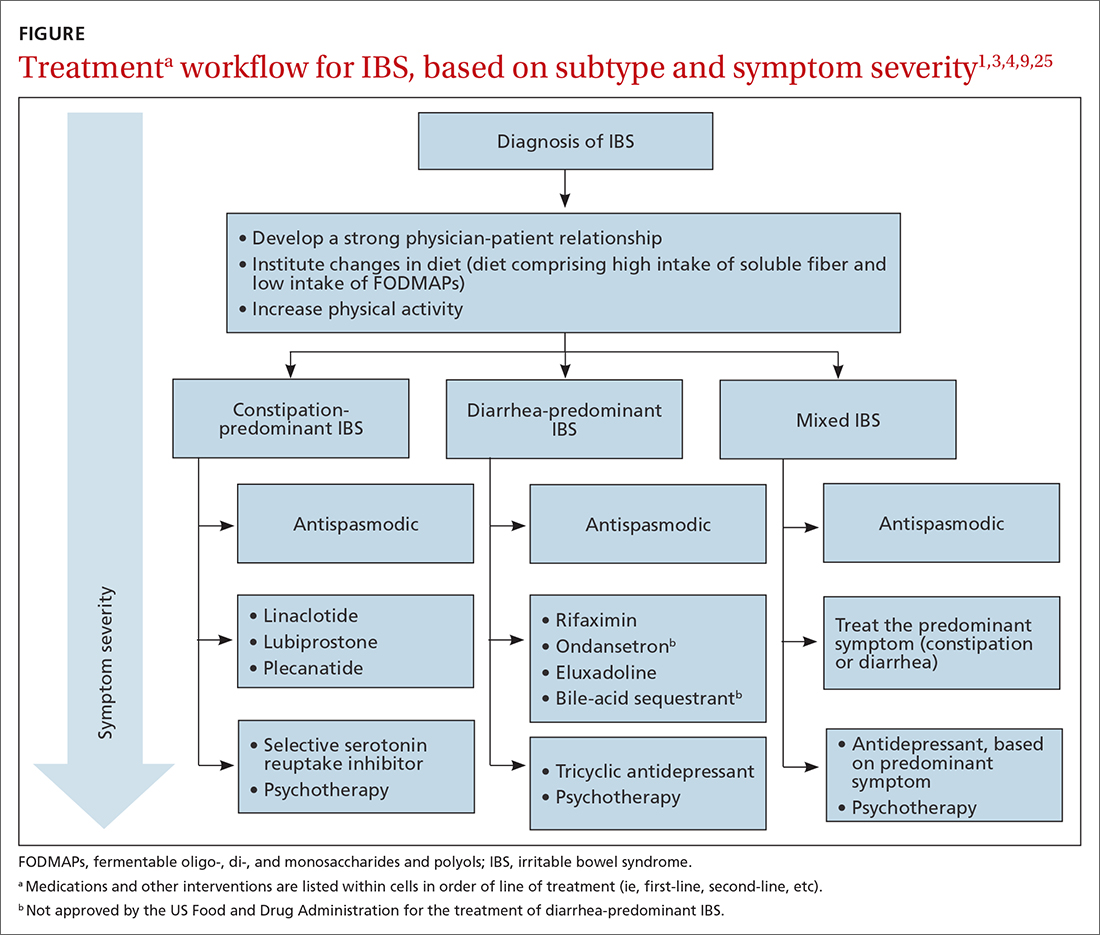
Treatments for all IBS subtypes
Lifestyle modification. Exercise provides overall positive health benefits. With such a variety of exercise forms, however, it is difficult to identify specific exercises that are better for IBS patients.28 A study of 305 IBS patients found that exercise alleviated constipation but not other IBS symptoms, and did not improve quality of life.3 Based on low cost and low risk of adverse effects, exercise should be recommended to all IBS patients.
Dietary restriction therapies have become an area of focus for patients, clinicians, and researchers. Modification of the diet is thought to improve global symptoms and intestinal health through modification of gut microbiota, immune activation, and a decrease in levels of fecal short-chain fatty acids.29
Continue to: The 2 main diets...
The 2 main diets studied for the treatment of IBS are a diet low in fermentable oligo-, di- and monosaccharides and polyols—the so-called low-FODMAP diet (TABLE 330)—and a gluten-free diet. Evidence behind the benefits of both diets conflicts; trials of the low-FODMAP diet are more favorable.
A small study with 20 patients with IBS-D and IBS-M who followed a low-FODMAP diet found improvement in IBS symptoms and a reduction in serum levels of proinflammatory cytokines, fecal bacteria, and total fecal short-chain fatty acid levels.29 Several meta-analyses have shown improvement in overall IBS symptoms for patients who follow a low-FODMAP diet. Because of the heterogeneity of the studies, however, the quality of the data is low.31-34
Data supporting the use of a gluten-free diet for IBS patients are insufficient.31
The American College of Gastroenterology (ACG) gave a weak recommendation for the low-FODMAP diet and recommended against the gluten-free diet in IBS patients.3 More data are needed regarding the safety profile of using a low-FODMAP diet for an extended period: There is concern about the risk of nutritional deficiencies associated with long-term use of this diet.3
Supplementation with poorly fermentable soluble fiber has been shown to alleviate global IBS symptoms; insoluble fiber does not yield improvement of symptoms. Psyllium fiber is recommended over wheat bran.3,35
Continue to: Consider a low-FODMAP diet...
Consider a low-FODMAP diet and soluble fiber as initial treatment for all IBS patients.
Modification of intestinal microbiota. Understanding the difference between prebiotics and probiotics is important when considering treatment for IBS. Prebiotics are foods or dietary supplements that generate changes in the composition and activity of intestinal microbiota. Probiotics are live microorganisms that can improve intestinal health.3
A meta-analysis of 729 IBS patients found that prebiotics do not reduce gastrointestinal symptoms or improve the quality of life of IBS patients.36 Evidence supporting the benefit of probiotics is favorable; however, data in these studies have significant heterogeneity. Several meta-analyses studied the benefits of Lactobacillus spp and Bifidobacterium spp in alleviating IBS symptoms. The studies found improvement in abdominal pain, bloating and distention, and flatulence.3,37-40 Consider recommending probiotics for all IBS patients; for some, however, the high cost of some of these products might be an obstacle.
Researchers are also studying the use of fecal microbiota transplantation (FMT) to treat IBS. Studies have evaluated the delivery of FMT orally (as capsules) and endoscopically. Evidence does not show improvement in global IBS symptoms with FMT. More studies, with larger sample populations, are needed.41-43
Antispasmodic medications and peppermint oil. Antispasmodic medications have been considered a mainstay therapy for IBS because of their effect on intestinal dysmotility. Hyoscine and dicyclomine are commonly used. Meta-analyses have shown improvement in global symptoms and abdominal pain, but effects were modest.3,44 Use this class of drugs as first-line treatment for mild IBS symptoms.
Continue to: Peppermint oil has been...
Peppermint oil has been found useful in improving IBS global symptoms and abdominal pain in several studies.44-46 A common adverse effect of peppermint oil is heartburn, resulting from relaxation of esophageal muscle.3 Peppermint oil can be considered an adjuvant agent in treating IBS.
Antidepressants. Tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) have been studied for the treatment of IBS. Meta-analyses show that both are effective in reducing pain and overall IBS symptoms.1,3,47 The number needed to treat (NNT) for TCAs is 4.5; for SSRIs, 5.47 Data do not show that either drug class is superior to the other for IBS. Based on the adverse effect profile, TCAs are more suitable for IBS-D patients; SSRIs are better for IBS-C patients.47
New data show that serotonin-norepinephrine reuptake inhibitors, such as duloxetine and milnacipran, can alleviate IBS symptoms through their pain-modifying properties.47
Based on the adverse effect profile and stigma associated with antidepressant medications, patients might be less likely to take them for IBS symptoms than for these drugs’ primary indications. Clinicians should still consider this drug class if other first-line treatments do not provide full resolution of symptoms.
Psychotherapy. Several psychotherapeutic modalities have been evaluated for efficacy in reducing global IBS symptoms. The approaches studied most often were provider-directed cognitive behavioral therapy, relaxation therapy, hypnotherapy, and multicomponent psychological therapy. The NNT for these modalities is 4, but studies had significant heterogeneity.3 Consider referring patients for psychotherapeutic intervention if they have not responded to medical therapy after 12 months.4
Continue to Treatment of IBS-C
Treatment of IBS-C
Prosecretory agents. Linaclotide and plecanatide are amino-acid peptides that act as a guanylate cyclase C agonist. Both increase gastrointestinal transit rate by increasing electrolyte and fluid transport into the intestinal lumen. They also decrease the activity of pain-sensing nerves by increasing extracellular cyclic guanosine-3'5'-monophosphate levels.3,48 In a recent meta-analysis, both treatments produced improvement in global symptoms. However, linaclotide showed superior improvement in abdominal pain and global symptoms compared to other secretory agents.48,49 Diarrhea was the most common adverse effect of linaclotide and plecanatide, although less so with plecanatide.49
Lubiprostone activates the intestinal chloride channel type 2 on the small intestine, leading to an increase in chloride and water efflux into the intestinal lumen, in turn accelerating gastrointestinal transit.3 A meta-analysis with 1468 IBS patients found that lubiprostone improved constipation, stool consistency, abdominal pain, degree of straining, and abdominal bloating.50 Diarrhea and nausea are commonly reported adverse effects of lubiprostone.49,50
Linaclotide, plecanatide, and lubiprostone should be considered first-line therapies for patients with IBS-C. High cost is still a roadblock to the use of these agents.
The US Food and Drug Administration (FDA) approved tenapanor in September 2019; however, the drug is not commercially available in the United States (it is available in Canada). Tenapanor is a sodium–hydrogen exchanger 3 inhibitor that reduces sodium absorption from the intestine and colon. The drug increases water secretion into the intestinal lumen, thus accelerating gut transit time. It also inhibits active absorption of phosphate in the intestine.
Tenapanor was approved for treating both IBS-C and hyperphosphatemia in patients with chronic kidney disease on dialysis or end-stage renal disease.26 In a recent meta-analysis, the drug showed benefit in alleviating global IBS symptoms, and ranked first in reducing bloating.49 It is too soon to know if tenapanor will perform clinically better than other prosecretory agents.
Continue to: Serotonergic agents
Serotonergic agents. Serotonin (5-hydroxytryptamine [5-HT]) modulates gastrointestinal secretions, gut motility, and visceral sensation. Researchers have developed IBS treatments that target receptors involved in these functions.
Tegaserod is a partial, selective 5-HT4 agonist indicated for the treatment of IBS-C in women. A study with 661 women with IBS-M and IBS-C showed that tegaserod increased the number of bowel movement episodes. Patients also reported higher stool consistency scores and fewer days with straining compared to placebo.27 The medication was removed from the market in 2007 because of its potential for cardiovascular adverse effects3; however, it was reintroduced in 2019 for women < 65 years of age with IBS-C. Consider prescribing tegaserod if other treatment options fail to alleviate symptoms.
Treatment of IBS-D
Antibiotics. The nonabsorbable antibiotic rifaximin is approved by the FDA for IBS-D at a dosage of 550 mg tid for 2 weeks.1 Several studies show improvement in IBS global symptoms with the recommended treatment course51-53; benefit persisted for the 10-week follow-up study period.1 A meta-analysis found that the NNT for rifaximin is 8-11.54 Preliminary data indicate that the rates of Clostridioides difficile infection and microbial resistance among rifaximin users are low.3 Consider using rifaximin as a first-line treatment option for patients with IBS-D. Retreatment might be necessary because the drug’s effect gradually disappears.9
Antidiarrheals. Eluxadoline is a µ-opioid and κ-opioid receptor agonist and δ-opioid receptor antagonist with effects on the intestinal nervous system.3 Several meta-analyses demonstrated that eluxadoline improves abdominal pain scores and daily stool consistency in IBS-D patients.53,54 Eluxadoline should be considered early in the management of IBS-D patients. The most common adverse effect is constipation.
The FDA issued a safety warning in 2017 regarding an increased risk of pancreatitis in patients taking eluxadoline who do not have a gallbladder. In addition, eluxadoline should be avoided in patients with a history of sphincter of Oddi dysfunction, alcohol abuse, or severe liver problems.3,54
Continue to: The high cost of...
The high cost of eluxadoline can be a significant barrier to use.
Serotonergic agents. Alosetron is a selective
Ondansetron has also been used to treat IBS-D. In a meta-analysis with 294 patients, ondansetron showed improvement in stool consistency.55 Ondansetron does not improve abdominal pain.4 It can be used in patients who have mild-to-moderate symptoms.9 Ondansetron is not FDA approved for the treatment of IBS-D.
Bile-acid sequestrants. Traditionally, bile-acid sequestrants have been used to treat bile-acid diarrhea. A meta-analysis of 6 studies of 908 patients with IBS-D found that 28.1% were affected by bile-acid malabsorption. Two small studies that evaluated the benefits of colesevelam for IBS-D found significant improvement in stool consistency.54 Another study, which evaluated the benefits of cholestyramine, found improvement in stool consistency, but findings were not significant.54 Many patients taking a bile-acid sequestrant stop taking the medication because of considerable adverse effects (constipation, nausea, bloating, flatulence, and abdominal pain).54 For that reason, this class of medication is not recommended as first-line treatment for IBS-D and is not FDA approved for IBS-D.
SIDEBAR
KEY POINTS The challenge of, and a needed framework for, managing IBS
- IBS is a complex, chronic condition affecting a considerable number of people worldwide.
- Because of the substantial disease burden associated with IBS, patients are at higher risk of mental health disorders.
- Physicians who care for IBS patients must build a strong physician–patient relationship; their mutual trust will ensure development of an effective treatment plan.
- Family physicians and other primary care providers are equipped to help IBS patients navigate the complex health care system and the IBS disease process. They can help coordinate care with specialists and behavioral health clinicians, which will help patients improve quality of life and manage symptoms appropriately.
A role for complementaryand integrative medicine?
Recently, complementary and integrative modalities for treating IBS have sparked the interest of researchers.
Continue to: Acupuncture
Acupuncture. In a meta-analysis with 3440 patients, acupuncture was more effective than Western medicine in alleviating IBS symptoms for as long as 3 months. The authors concluded that acupuncture could be used in combination with other therapies to reduce the severity of IBS symptoms.56
Concomitant acupuncture and Chinese herbal medicine. In a systematic review and meta-analysis comprising 21 randomized controlled trials, researchers reported that acupuncture combined with Chinese herbal medicine improved IBS symptoms, compared to what was noted in matched controls who were treated with Western medicine or with Western medicine combined with Chinese herbal medicine. The authors were cautious about the results of the meta-analysis, however, because the studies examined were small and of low quality, and presented a high risk of bias.57
Agents not to be used routinely for IBS
Loperamide. This peripheral µ-opioid receptor agonist controls diarrhea. However, recent studies showed no significant benefit to loperamide over placebo in IBS-M and IBS-D. In 2018, the FDA issued a safety alert regarding an elevated risk of serious cardiac adverse effects in patients taking loperamide. The ACG recommends against using loperamide to treat IBS symptoms.3,54
Polyethylene glycol. An osmotic laxative that is not absorbed in the intestinal lumen, polyethylene glycol is highly efficacious for alleviating constipation, but it does not reduce pain or other IBS symptoms. For that reason, the ACG recommends against its use.3
CORRESPONDENCE
Jose M. Villalon-Gomez, MD, MPH, Emory Healthcare Family Medicine, 4500 North Shallowford Road, Dunwoody, GA 30338; [email protected]
1. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393-1407.e5. doi:10.1053/j.gastro.2016.02.031
2. What kind of poop do I have? WebMD. January 16, 2020. Accessed September 20, 2021. www.webmd.com/digestive-disorders/poop-chart-bristol-stool-scale
3. Ford AC, Moayyedi P, Chey WD, et al; . American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol. 2018;113(suppl 2):1-18. doi:10.1038/s41395-018-0084-x
4. Ferreira AI, Garrido M, Castro-Poças F. Irritable bowel syndrome: news from an old disorder. GE Port J Gastroenterol. 2020;27:255-268. doi:10.1159/000503757
5. Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473-486. doi: 10.1038/s41575-020-0286-8
6. Frändemark Å, Törnblom H, Jakobsson S, et al. Work productivity and activity impairment in irritable bowel syndrome (IBS): a multifaceted problem: Am J Gastroenterol. 2018;113:1540-1549. doi:10.1038/s41395-018-0262-x
7. Poulsen CH, Eplov LF, Hjorthøj C, et al. Irritable bowel symptoms, use of healthcare, costs, sickness and disability pension benefits: a long-term population-based study. Scand J Public Health. 2019;47:867-875. doi:10.1177/1403494818776168
8. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20:6024-6030. doi:10.3748/wjg.v20.i20.6024
9. Moayyedi P, Mearin F, Azpiroz F, et al. Irritable bowel syndrome diagnosis and management: a simplified algorithm for clinical practice. United European Gastroenterol J. 2017;5:773-788. doi:10.1177/2050640617731968
10. Zhu S, Wang B, Jia Q, et al. Candidate single nucleotide polymorphisms of irritable bowel syndrome: a [systematic] review and meta-analysis. BMC Gastroenterology. 2019;19:165. doi:10.1186/s12876-019-1084-z
11. Simrén M, Törnblom H, Palsson OS, et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2018;67:255-262. doi:10.1136/gutjnl-2016-312361
12. Bashashati M, Moossavi S, Cremon C, et al. Colonic immune cells in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2018;30:e13192. doi:10.1111/nmo.13192
13. Robles A, Ingles DP, Myneedu K, et al. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2019;31:e13718. doi:10.1111/nmo.13718
14. Bashashati M, Moradi M, Sarosiek I. Interleukin-6 in irritable bowel syndrome: A systematic review and meta-analysis of IL-6 (-G174C) and circulating IL-6 levels. Cytokine. 2017;99:132-138. doi:10.1016/j.cyto.2017.08.017
15. Bashashati M, Rezaei N, Shafieyoun A, et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2014;26:1036-1048. doi:10.1111/nmo.12358
16. Sibelli A, Chalder T, Everitt H, et al. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol Med. 2016;46:3065-3080. doi:10.1017/S0033291716001987
17. Yeh H-W, Chien W-C, Chung C-H, et al. Risk of psychiatric disorders in irritable bowel syndrome—a nationwide, population-based, cohort study. Int J Clin Pract. 2018;72:e13212. doi:10.1111/ijcp.13212
18. Duan R, Zhu S, Wang B, et al. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clin Transl Gastroenterol. 2019;10:e00012. doi:10.14309/ctg.0000000000000012
19. Wang L, Alammar N, Singh R, et al. Gut microbial dysbiosis in the irritable bowel syndrome: a systematic review and meta-analysis of case-controlled studies. J Acad Nutr Diet. 2020;120:565-586. doi:10.1016/j.jand.2019.05.015
20. Barbara G, Grover M, Bercik P, et al. Rome Foundation working team report on post-infection irritable bowel syndrome. Gastroenterology. 2019;156:46-58.e7. doi:10.1053/j.gastro.2018.07.011
21. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology. 2017;152:1042-1054.e1. doi:10.1053/j.gastro.2016.12.039
22. Heidelbaugh JJ. These 3 tools can help you streamline management of IBS. J Fam Pract. 2017;66:346-353.
23. American College of Gastroenterology Task Force on Irritable Bowel Syndrome; Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(suppl 1):S1-S35. doi:10.1038/ajg.2008.122
24. Menees SB, Powell C, Kurlander J, et al. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol. 2015;110:444-454. doi:10.1038/ajg.2015.6
25. Smalley W, Falck-Ytter C, Carrasco-Labra A, et al. AGA clinical practice guidelines on the laboratory evaluation of functional diarrhea and diarrhea-predominant irritable bowel syndrome in adults (IBS-D). Gastroenterology. 2019;157:851-854. doi:10.1053/j.gastro.2019.07.004
26. Markham A. Tenapanor: first approval. Drugs. 2019;79:1897-1903. doi:10.1007/s40265-019-01215-9
27. Chey WD, Paré P, Viegas A, et al. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008;103:1217-1225. doi:10.1111/j.1572-0241.2008.01808.x
28. Zhou C, Zhao E, Li Y, et al. Exercise therapy of patients with irritable bowel syndrome: a systematic review of randomized controlled trials. Neurogastroenterol Motil. 2019;31:e13461. doi:10.1111/nmo.13461
29. Hustoft TN, Hausken T, Ystad SO, et al. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017;29:e12969. doi:10.1111/nmo.12969
30. Zegarac JP. The low-FODMAP diet for IBS: what you need to know. Medscape. August 13, 2019. Accessed September 20, 2021. www.medscape.com/viewarticle/917069
31. Dionne J, Ford AC, Yuan Y, et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low
32. Su H, Li Y-T, Heitkemper MM, et al. Effects of low-FODMAPS diet on irritable bowel syndrome symptoms and gut microbiome: Gastroenterol Nurs. 2019;42:150-158. doi:10.1097/SGA.0000000000000428
33. Nawawi KNM, Belov M, Goulding C. Low FODMAP diet significantly improves IBS symptoms: an Irish retrospective cohort study. Eur J Nutr. 2020;59:2237-2248. doi: 10.1007/s00394-019-02074-6
34. Altobelli E, Del Negro V, Angeletti PM, et al. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients. 2017;9:940. doi:10.3390/nu9090940
35. Nagarajan N, Morden A, Bischof D, et al. The role of fiber supplementation in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2015;27:1002-1010. doi:10.1097/MEG.0000000000000425
36. Wilson B, Rossi M, Dimidi E, et al. Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019;109:1098-1111. doi:10.1093/ajcn/nqy376
37. Yuan F, Ni H, Asche CV, et al. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: a meta-analysis. Curr Med Res Opin. 2017;33:1191-1197. doi:10.1080/03007995.2017.1292230
38. Liang D, Longgui N, Guoqiang X. Efficacy of different probiotic protocols in irritable bowel syndrome: a network meta-analysis. Medicine (Baltimore). 2019;98:16068. doi:10.1097/MD.0000000000016068
39. Dale HF, Rasmussen SH, Asiller ÖÖ, et al. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11:2048. doi:10.3390/nu11092048
40. Pratt C, Campbell MD. The effect of Bifidobacterium on reducing symptomatic abdominal pain in patients with irritable bowel syndrome: a systematic review. Probiotics Antimicrob Proteins. 2020;12:834-839. doi:10.1007/s12602-019-09609-7
41. Ianiro G, Eusebi LH, Black CJ, et al. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:240-248. doi:10.1111/apt.15330
42. Myneedu K, Deoker A, Schmulson MJ, et al. Fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. United European Gastroenterol J. 2019;7:1033-1041. doi:10.1177/2050640619866990
43. Xu D, Chen VL, Steiner CA, et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114:1043-1050. doi:10.14309/ajg.0000000000000198
44. Black CJ, Yuan Y, Selinger CP, et al. Efficacy of soluble fibre, antispasmodic drugs, and gut–brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:117-131. doi:10.1016/S2468-1253(19)30324-3
45. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512. doi:10.1097/MCG.0b013e3182a88357
46. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi:10.1186/s12906-018-2409-0
47. Ford AC, Lacy BE, Harris LA, et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. 2019;114:21-39. doi: 10.1038/s41395-018-0222-5
48. Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-c agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol. 2018;113:329-338. doi:10.1038/ajg.2017.495
49. Black CJ, Burr NE, Quigley EMM, et al. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology. 2018;155:1753-1763. doi:10.1053/j.gastro.2018.08.021
50. Li F, Fu T, Tong W-D, et al. Lubiprostone is effective in the treatment of chronic idiopathic constipation and irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2016;91:456-468. doi:10.1016/j.mayocp.2016.01.015
51. Ford AC, Harris LA, Lacy BE, et al. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044-1060. doi:10.1111/apt.15001
52. Yoon K, Kim N, Lee JY, et al. Clinical response of rifaximin treatment in patients with abdominal bloating. Korean J Gastroenterol. 2018;72:121-127. doi:10.4166/kjg.2018.72.3.121
53. Black CJ, Burr NE, Camilleri M, et al. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut. 2020;69:74-82. doi:10.1136/gutjnl-2018-318160
54. Lacy BE. Review article: an analysis of safety profiles of treatments for diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:817-830. doi:10.1111/apt.14948
55. Zheng Y, Yu T, Tang Y, et al. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. PLOS ONE. 2017;12:e0172846. doi:10.1371/journal.pone.0172846
56. Zheng H, Chen R, Zhao X, et al. Comparison between the effects of acupuncture relative to other controls on irritable bowel syndrome: a meta-analysis. Pain Research and Management. 2019;2019:1-13. doi:https://doi.org/10.1155/2019/2871505
57. Yan J, Miao Z-W, Lu J, et al. Acupuncture plus Chinese herbal medicine for irritable bowel syndrome with diarrhea: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019:1-16. https://doi.org/10.1155/2019/7680963
Irritable bowel syndrome (IBS) continues to pose a diagnostic and therapeutic challenge to clinicians and patients—a challenge that arises from the varying manifestations of the condition, its complex pathophysiology, lack of effective treatment, and psychological consequences for patients. In this article, I explore new findings related to the pathophysiology, diagnosis, and management of IBS subtypes.
Start with the Rome IV classification of IBS
The Rome Foundation published its latest IBS classification and diagnostic criteria (known as Rome IV) in 2016.1 IBS is defined as abdominal pain that (1) has recurred, on average, ≥ 1 time per week during the past 3 months and (2) is associated with ≥ 2 of these criteria1:
- related to defecation
- associated with a change in stool frequency
- associated with a change in the appearance of stool.
Onset of symptoms should be present for 6 months before a diagnosis of IBS is made.1
IBS subtypes—constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), mixed (IBS-M), and unclassified (IBS-U) (TABLE 1)1—are based on the frequency of specific stool forms, as described and illustrated in the Bristol Stool Scale (www.webmd.com/digestive-disorders/poop-chart-bristol-stool-scale).2

A widespread, costly, potentially debilitating disorder
IBS affects 10% to 12% of adults worldwide. The condition is more common among women and people younger than 50 years.1,3 Women with IBS tend to have more constipation symptoms (IBS-C); men with IBS, more diarrhea symptoms (IBS-D).4
The financial burden of IBS on the health care system and patients is significant. In a 2013 appraisal of 35 studies, the authors note that estimates of the direct cost of IBS care in the United States vary considerably—from $1562 to $7547 for a patient annually.5
A recent study found that almost 25% of IBS patients report absenteeism from work due to IBS symptoms.6 A Danish study that followed 7278 patients for 5 years found that IBS patients utilized more health care, sick days, and disability pension benefits than non-IBS patients, and had increased utilization of medical resources because of psychiatric conditions.7
Continue to: IBS patients also have comorbidities
IBS patients also have comorbidities:
- More than 20% of IBS patients have functional dyspepsia, gastroesophageal reflux disease, incontinence, or pelvic floor dyssynergia.4
- The frequency of fibromyalgia syndrome in IBS patients is reported to be 20% to 65%.8
- 14% of IBS patients meet criteria for chronic fatigue syndrome.8
- Interstitial cystitis and dyspareunia are common among IBS patients.9
Pathophysiology is complex
Models describing the pathophysiology of IBS have evolved through the years. Recent models describe it as a combination of altered gastrointestinal motility, visceral hyperalgesia, increased intestinal permeability, immune activation, altered intestinal microbiota, and dysfunction in the brain–gut axis. Certain environmental and psychological variables (eg, previous gastroenteritis, food intolerance, chronic stress, diverticulitis, and surgery) increase the risk of IBS.1,10,11
In the past several years, considerable attention has been paid to the roles played by the immune system, brain–gut axis function, and intestinal microbiota in IBS manifestations. Research focus in these areas might assist in the development of specific treatment modalities targeting IBS subtypes.
Immune system. A recent meta-analysis of the records of 706 IBS patients found an increased number of mast cells and CD3 T cells in biopsy specimens from the rectosigmoid and descending colon of IBS patients.12 Another study found a significant increase in mast cells in the ileum of IBS patients13; this increase is evident not only on intestinal biopsy but also at the serologic level. IBS-D patients have a higher plasma interleukin (IL)-6 level than the general population.14 Another meta-analysis found an imbalance in the serum level of tumor necrosis factor-α and IL-10 in IBS patients.15
Brain–gut axis. A 2016 meta-analysis showed that patients with anxiety and depression have a 2-fold increased risk of IBS.16 A more recent study, using data from the National Health Insurance Research Database that included 22,356 patients with IBS, found a 3.6-fold increased risk of psychiatric disorders in IBS.17 These findings reflect the complex interaction between the brain and the intestinal tract in IBS.
Continue to: Intestinal microbiota
Intestinal microbiota. Research evaluating the role of altered intestinal microbiota in IBS has yielded mixed results. A meta-analysis of 777 IBS patients showed an increase in Firmicutes spp, a decrease in Bacteroidetes spp, and an increase in the ratio of Firmicutes spp to Bacteroidetes spp in subjects’ fecal specimens.18 Another study, of 1340 patients, found no difference in Bacteroides spp and Enterococcus spp between healthy controls and IBS patients, but did find (1) lower fecal counts of Lactobacillus spp and Bifidobacterium spp and (2) higher fecal counts of Escherichia coli and Enterobacter spp in IBS patients.19
Postinfectious IBS. The Rome Foundation introduced the diagnosis of postinfectious IBS (PI-IBS) in 2019. PI-IBS develops in 10% of patients who have had infectious enteritis. Female gender, younger age, psychological distress during or before the enteritis episode, and severity of the acute episode are risk factors for this IBS variant.20 A study of 21,421 enteritis patients found that 42% with protozoal or parasitic infection and 14% with bacterial infection developed IBS.
Patients with nonviral enteritis often have a more severe course of enteritis, typically requiring antibiotics. It is believed that the resulting irregularities in the intestinal microbiota make these patients more likely to develop PI-IBS.21 PI-IBS patients are likely to improve or fully recover over time. Symptoms of PI-IBS are managed in a manner similar to how non-PI-IBS patients are managed.20
Challenges in making the IBS diagnosis
Historically, the diagnosis of IBS has been made clinically after excluding red flags (ie, signs or symptoms that might reflect other underlying medical problems) in the clinical presentation. For this reason, obtain a thorough clinical history that includes the course of symptoms, triggers, and alleviating factors. Any of the following are considered red flags1,22,23:
- age > 50 years at onset of symptoms
- new-onset constipation in the elderly
- rectal bleeding
- unexplained weight loss or anemia
- family history of organic gastrointestinal disease
- palpable abdominal or rectal mass
- nocturnal symptoms.
New studies demonstrate that several inflammatory markers can help exclude inflammatory bowel disease from the differential diagnosis in patients in whom IBS is suspected and being investigated.24 In 2019, the American Gastroenterological Association published a clinical practice guideline updating the laboratory evaluation of functional diarrhea and IBS-D in adults,25 and made several recommendations:
- Obtain the level of fecal calprotectin (normal level, ≤ 50 mcg/g) or fecal lactoferrin (≤ 4.0-7.25 mcg/g); if these tests are not available or results are not accessible, the C-reactive protein level is a reasonable option.
- Do not routinely use the erythrocyte sedimentation rate or C-reactive protein level to screen for inflammatory bowel disease.
- Test for Giardia lamblia with an antigen or polymerase chain reaction test.
- Do not test for ova and parasites (other than Giardia) in patients who do not have a history of travel or who have not emigrated from a high-risk area recently.
- Obtain testing for celiac disease with immunoglobulin A (IgA) tissue transglutaminase and with a second test, of immunoglobulin G (IgG) tissue transglutaminase and IgG or IgA deaminated gliadin peptides, to detect celiac disease in IgA-deficient patients.
- Order testing for bile-acid diarrhea with selenium homotaurocholic acid nuclear medicine scanning (if available in your region; the test is available in Europe); measurement of bile acid from a 48-hour stool collection; or an assay of fibroblast growth factor 19, which measures defective feedback of bile-acid synthesis. If these tests are unavailable, consider an empiric trial of a bile-acid binder.
- Do not use available serologic IBS testing.
Continue to: Continue to obtain a...
Continue to obtain a complete blood count for the evaluation of anemia. Endoscopic procedures are indicated in patients with a red flag.1
Treat based on subtype
The first step in the treatment of all IBS patients (TABLE 21,3,4,9,26,27) is for you to develop a strong relationship with the patient: You must acknowledge the disease and empower the patient to manage their symptoms. A strong physician–patient relationship leads to more effective outcomes.4

IBS treatment modalities target abdominal pain, bloating, abdominal distention, and altered bowel function—described in the literature as global symptoms. IBS-M patients should direct their treatment to the predominant symptom (constipation or diarrhea). The following sections describe available treatment options. The FIGURE1,3,4,9,25 shows a treatment workflow based on IBS subtype and symptom severity.

Treatments for all IBS subtypes
Lifestyle modification. Exercise provides overall positive health benefits. With such a variety of exercise forms, however, it is difficult to identify specific exercises that are better for IBS patients.28 A study of 305 IBS patients found that exercise alleviated constipation but not other IBS symptoms, and did not improve quality of life.3 Based on low cost and low risk of adverse effects, exercise should be recommended to all IBS patients.
Dietary restriction therapies have become an area of focus for patients, clinicians, and researchers. Modification of the diet is thought to improve global symptoms and intestinal health through modification of gut microbiota, immune activation, and a decrease in levels of fecal short-chain fatty acids.29
Continue to: The 2 main diets...
The 2 main diets studied for the treatment of IBS are a diet low in fermentable oligo-, di- and monosaccharides and polyols—the so-called low-FODMAP diet (TABLE 330)—and a gluten-free diet. Evidence behind the benefits of both diets conflicts; trials of the low-FODMAP diet are more favorable.

A small study with 20 patients with IBS-D and IBS-M who followed a low-FODMAP diet found improvement in IBS symptoms and a reduction in serum levels of proinflammatory cytokines, fecal bacteria, and total fecal short-chain fatty acid levels.29 Several meta-analyses have shown improvement in overall IBS symptoms for patients who follow a low-FODMAP diet. Because of the heterogeneity of the studies, however, the quality of the data is low.31-34
Data supporting the use of a gluten-free diet for IBS patients are insufficient.31
The American College of Gastroenterology (ACG) gave a weak recommendation for the low-FODMAP diet and recommended against the gluten-free diet in IBS patients.3 More data are needed regarding the safety profile of using a low-FODMAP diet for an extended period: There is concern about the risk of nutritional deficiencies associated with long-term use of this diet.3
Supplementation with poorly fermentable soluble fiber has been shown to alleviate global IBS symptoms; insoluble fiber does not yield improvement of symptoms. Psyllium fiber is recommended over wheat bran.3,35
Continue to: Consider a low-FODMAP diet...
Consider a low-FODMAP diet and soluble fiber as initial treatment for all IBS patients.
Modification of intestinal microbiota. Understanding the difference between prebiotics and probiotics is important when considering treatment for IBS. Prebiotics are foods or dietary supplements that generate changes in the composition and activity of intestinal microbiota. Probiotics are live microorganisms that can improve intestinal health.3
A meta-analysis of 729 IBS patients found that prebiotics do not reduce gastrointestinal symptoms or improve the quality of life of IBS patients.36 Evidence supporting the benefit of probiotics is favorable; however, data in these studies have significant heterogeneity. Several meta-analyses studied the benefits of Lactobacillus spp and Bifidobacterium spp in alleviating IBS symptoms. The studies found improvement in abdominal pain, bloating and distention, and flatulence.3,37-40 Consider recommending probiotics for all IBS patients; for some, however, the high cost of some of these products might be an obstacle.
Researchers are also studying the use of fecal microbiota transplantation (FMT) to treat IBS. Studies have evaluated the delivery of FMT orally (as capsules) and endoscopically. Evidence does not show improvement in global IBS symptoms with FMT. More studies, with larger sample populations, are needed.41-43
Antispasmodic medications and peppermint oil. Antispasmodic medications have been considered a mainstay therapy for IBS because of their effect on intestinal dysmotility. Hyoscine and dicyclomine are commonly used. Meta-analyses have shown improvement in global symptoms and abdominal pain, but effects were modest.3,44 Use this class of drugs as first-line treatment for mild IBS symptoms.
Continue to: Peppermint oil has been...
Peppermint oil has been found useful in improving IBS global symptoms and abdominal pain in several studies.44-46 A common adverse effect of peppermint oil is heartburn, resulting from relaxation of esophageal muscle.3 Peppermint oil can be considered an adjuvant agent in treating IBS.
Antidepressants. Tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) have been studied for the treatment of IBS. Meta-analyses show that both are effective in reducing pain and overall IBS symptoms.1,3,47 The number needed to treat (NNT) for TCAs is 4.5; for SSRIs, 5.47 Data do not show that either drug class is superior to the other for IBS. Based on the adverse effect profile, TCAs are more suitable for IBS-D patients; SSRIs are better for IBS-C patients.47
New data show that serotonin-norepinephrine reuptake inhibitors, such as duloxetine and milnacipran, can alleviate IBS symptoms through their pain-modifying properties.47
Based on the adverse effect profile and stigma associated with antidepressant medications, patients might be less likely to take them for IBS symptoms than for these drugs’ primary indications. Clinicians should still consider this drug class if other first-line treatments do not provide full resolution of symptoms.
Psychotherapy. Several psychotherapeutic modalities have been evaluated for efficacy in reducing global IBS symptoms. The approaches studied most often were provider-directed cognitive behavioral therapy, relaxation therapy, hypnotherapy, and multicomponent psychological therapy. The NNT for these modalities is 4, but studies had significant heterogeneity.3 Consider referring patients for psychotherapeutic intervention if they have not responded to medical therapy after 12 months.4
Continue to Treatment of IBS-C
Treatment of IBS-C
Prosecretory agents. Linaclotide and plecanatide are amino-acid peptides that act as a guanylate cyclase C agonist. Both increase gastrointestinal transit rate by increasing electrolyte and fluid transport into the intestinal lumen. They also decrease the activity of pain-sensing nerves by increasing extracellular cyclic guanosine-3'5'-monophosphate levels.3,48 In a recent meta-analysis, both treatments produced improvement in global symptoms. However, linaclotide showed superior improvement in abdominal pain and global symptoms compared to other secretory agents.48,49 Diarrhea was the most common adverse effect of linaclotide and plecanatide, although less so with plecanatide.49
Lubiprostone activates the intestinal chloride channel type 2 on the small intestine, leading to an increase in chloride and water efflux into the intestinal lumen, in turn accelerating gastrointestinal transit.3 A meta-analysis with 1468 IBS patients found that lubiprostone improved constipation, stool consistency, abdominal pain, degree of straining, and abdominal bloating.50 Diarrhea and nausea are commonly reported adverse effects of lubiprostone.49,50
Linaclotide, plecanatide, and lubiprostone should be considered first-line therapies for patients with IBS-C. High cost is still a roadblock to the use of these agents.
The US Food and Drug Administration (FDA) approved tenapanor in September 2019; however, the drug is not commercially available in the United States (it is available in Canada). Tenapanor is a sodium–hydrogen exchanger 3 inhibitor that reduces sodium absorption from the intestine and colon. The drug increases water secretion into the intestinal lumen, thus accelerating gut transit time. It also inhibits active absorption of phosphate in the intestine.
Tenapanor was approved for treating both IBS-C and hyperphosphatemia in patients with chronic kidney disease on dialysis or end-stage renal disease.26 In a recent meta-analysis, the drug showed benefit in alleviating global IBS symptoms, and ranked first in reducing bloating.49 It is too soon to know if tenapanor will perform clinically better than other prosecretory agents.
Continue to: Serotonergic agents
Serotonergic agents. Serotonin (5-hydroxytryptamine [5-HT]) modulates gastrointestinal secretions, gut motility, and visceral sensation. Researchers have developed IBS treatments that target receptors involved in these functions.
Tegaserod is a partial, selective 5-HT4 agonist indicated for the treatment of IBS-C in women. A study with 661 women with IBS-M and IBS-C showed that tegaserod increased the number of bowel movement episodes. Patients also reported higher stool consistency scores and fewer days with straining compared to placebo.27 The medication was removed from the market in 2007 because of its potential for cardiovascular adverse effects3; however, it was reintroduced in 2019 for women < 65 years of age with IBS-C. Consider prescribing tegaserod if other treatment options fail to alleviate symptoms.
Treatment of IBS-D
Antibiotics. The nonabsorbable antibiotic rifaximin is approved by the FDA for IBS-D at a dosage of 550 mg tid for 2 weeks.1 Several studies show improvement in IBS global symptoms with the recommended treatment course51-53; benefit persisted for the 10-week follow-up study period.1 A meta-analysis found that the NNT for rifaximin is 8-11.54 Preliminary data indicate that the rates of Clostridioides difficile infection and microbial resistance among rifaximin users are low.3 Consider using rifaximin as a first-line treatment option for patients with IBS-D. Retreatment might be necessary because the drug’s effect gradually disappears.9
Antidiarrheals. Eluxadoline is a µ-opioid and κ-opioid receptor agonist and δ-opioid receptor antagonist with effects on the intestinal nervous system.3 Several meta-analyses demonstrated that eluxadoline improves abdominal pain scores and daily stool consistency in IBS-D patients.53,54 Eluxadoline should be considered early in the management of IBS-D patients. The most common adverse effect is constipation.
The FDA issued a safety warning in 2017 regarding an increased risk of pancreatitis in patients taking eluxadoline who do not have a gallbladder. In addition, eluxadoline should be avoided in patients with a history of sphincter of Oddi dysfunction, alcohol abuse, or severe liver problems.3,54
Continue to: The high cost of...
The high cost of eluxadoline can be a significant barrier to use.
Serotonergic agents. Alosetron is a selective
Ondansetron has also been used to treat IBS-D. In a meta-analysis with 294 patients, ondansetron showed improvement in stool consistency.55 Ondansetron does not improve abdominal pain.4 It can be used in patients who have mild-to-moderate symptoms.9 Ondansetron is not FDA approved for the treatment of IBS-D.
Bile-acid sequestrants. Traditionally, bile-acid sequestrants have been used to treat bile-acid diarrhea. A meta-analysis of 6 studies of 908 patients with IBS-D found that 28.1% were affected by bile-acid malabsorption. Two small studies that evaluated the benefits of colesevelam for IBS-D found significant improvement in stool consistency.54 Another study, which evaluated the benefits of cholestyramine, found improvement in stool consistency, but findings were not significant.54 Many patients taking a bile-acid sequestrant stop taking the medication because of considerable adverse effects (constipation, nausea, bloating, flatulence, and abdominal pain).54 For that reason, this class of medication is not recommended as first-line treatment for IBS-D and is not FDA approved for IBS-D.
SIDEBAR
KEY POINTS The challenge of, and a needed framework for, managing IBS
- IBS is a complex, chronic condition affecting a considerable number of people worldwide.
- Because of the substantial disease burden associated with IBS, patients are at higher risk of mental health disorders.
- Physicians who care for IBS patients must build a strong physician–patient relationship; their mutual trust will ensure development of an effective treatment plan.
- Family physicians and other primary care providers are equipped to help IBS patients navigate the complex health care system and the IBS disease process. They can help coordinate care with specialists and behavioral health clinicians, which will help patients improve quality of life and manage symptoms appropriately.
A role for complementaryand integrative medicine?
Recently, complementary and integrative modalities for treating IBS have sparked the interest of researchers.
Continue to: Acupuncture
Acupuncture. In a meta-analysis with 3440 patients, acupuncture was more effective than Western medicine in alleviating IBS symptoms for as long as 3 months. The authors concluded that acupuncture could be used in combination with other therapies to reduce the severity of IBS symptoms.56
Concomitant acupuncture and Chinese herbal medicine. In a systematic review and meta-analysis comprising 21 randomized controlled trials, researchers reported that acupuncture combined with Chinese herbal medicine improved IBS symptoms, compared to what was noted in matched controls who were treated with Western medicine or with Western medicine combined with Chinese herbal medicine. The authors were cautious about the results of the meta-analysis, however, because the studies examined were small and of low quality, and presented a high risk of bias.57
Agents not to be used routinely for IBS
Loperamide. This peripheral µ-opioid receptor agonist controls diarrhea. However, recent studies showed no significant benefit to loperamide over placebo in IBS-M and IBS-D. In 2018, the FDA issued a safety alert regarding an elevated risk of serious cardiac adverse effects in patients taking loperamide. The ACG recommends against using loperamide to treat IBS symptoms.3,54
Polyethylene glycol. An osmotic laxative that is not absorbed in the intestinal lumen, polyethylene glycol is highly efficacious for alleviating constipation, but it does not reduce pain or other IBS symptoms. For that reason, the ACG recommends against its use.3
CORRESPONDENCE
Jose M. Villalon-Gomez, MD, MPH, Emory Healthcare Family Medicine, 4500 North Shallowford Road, Dunwoody, GA 30338; [email protected]
Irritable bowel syndrome (IBS) continues to pose a diagnostic and therapeutic challenge to clinicians and patients—a challenge that arises from the varying manifestations of the condition, its complex pathophysiology, lack of effective treatment, and psychological consequences for patients. In this article, I explore new findings related to the pathophysiology, diagnosis, and management of IBS subtypes.
Start with the Rome IV classification of IBS
The Rome Foundation published its latest IBS classification and diagnostic criteria (known as Rome IV) in 2016.1 IBS is defined as abdominal pain that (1) has recurred, on average, ≥ 1 time per week during the past 3 months and (2) is associated with ≥ 2 of these criteria1:
- related to defecation
- associated with a change in stool frequency
- associated with a change in the appearance of stool.
Onset of symptoms should be present for 6 months before a diagnosis of IBS is made.1
IBS subtypes—constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), mixed (IBS-M), and unclassified (IBS-U) (TABLE 1)1—are based on the frequency of specific stool forms, as described and illustrated in the Bristol Stool Scale (www.webmd.com/digestive-disorders/poop-chart-bristol-stool-scale).2

A widespread, costly, potentially debilitating disorder
IBS affects 10% to 12% of adults worldwide. The condition is more common among women and people younger than 50 years.1,3 Women with IBS tend to have more constipation symptoms (IBS-C); men with IBS, more diarrhea symptoms (IBS-D).4
The financial burden of IBS on the health care system and patients is significant. In a 2013 appraisal of 35 studies, the authors note that estimates of the direct cost of IBS care in the United States vary considerably—from $1562 to $7547 for a patient annually.5
A recent study found that almost 25% of IBS patients report absenteeism from work due to IBS symptoms.6 A Danish study that followed 7278 patients for 5 years found that IBS patients utilized more health care, sick days, and disability pension benefits than non-IBS patients, and had increased utilization of medical resources because of psychiatric conditions.7
Continue to: IBS patients also have comorbidities
IBS patients also have comorbidities:
- More than 20% of IBS patients have functional dyspepsia, gastroesophageal reflux disease, incontinence, or pelvic floor dyssynergia.4
- The frequency of fibromyalgia syndrome in IBS patients is reported to be 20% to 65%.8
- 14% of IBS patients meet criteria for chronic fatigue syndrome.8
- Interstitial cystitis and dyspareunia are common among IBS patients.9
Pathophysiology is complex
Models describing the pathophysiology of IBS have evolved through the years. Recent models describe it as a combination of altered gastrointestinal motility, visceral hyperalgesia, increased intestinal permeability, immune activation, altered intestinal microbiota, and dysfunction in the brain–gut axis. Certain environmental and psychological variables (eg, previous gastroenteritis, food intolerance, chronic stress, diverticulitis, and surgery) increase the risk of IBS.1,10,11
In the past several years, considerable attention has been paid to the roles played by the immune system, brain–gut axis function, and intestinal microbiota in IBS manifestations. Research focus in these areas might assist in the development of specific treatment modalities targeting IBS subtypes.
Immune system. A recent meta-analysis of the records of 706 IBS patients found an increased number of mast cells and CD3 T cells in biopsy specimens from the rectosigmoid and descending colon of IBS patients.12 Another study found a significant increase in mast cells in the ileum of IBS patients13; this increase is evident not only on intestinal biopsy but also at the serologic level. IBS-D patients have a higher plasma interleukin (IL)-6 level than the general population.14 Another meta-analysis found an imbalance in the serum level of tumor necrosis factor-α and IL-10 in IBS patients.15
Brain–gut axis. A 2016 meta-analysis showed that patients with anxiety and depression have a 2-fold increased risk of IBS.16 A more recent study, using data from the National Health Insurance Research Database that included 22,356 patients with IBS, found a 3.6-fold increased risk of psychiatric disorders in IBS.17 These findings reflect the complex interaction between the brain and the intestinal tract in IBS.
Continue to: Intestinal microbiota
Intestinal microbiota. Research evaluating the role of altered intestinal microbiota in IBS has yielded mixed results. A meta-analysis of 777 IBS patients showed an increase in Firmicutes spp, a decrease in Bacteroidetes spp, and an increase in the ratio of Firmicutes spp to Bacteroidetes spp in subjects’ fecal specimens.18 Another study, of 1340 patients, found no difference in Bacteroides spp and Enterococcus spp between healthy controls and IBS patients, but did find (1) lower fecal counts of Lactobacillus spp and Bifidobacterium spp and (2) higher fecal counts of Escherichia coli and Enterobacter spp in IBS patients.19
Postinfectious IBS. The Rome Foundation introduced the diagnosis of postinfectious IBS (PI-IBS) in 2019. PI-IBS develops in 10% of patients who have had infectious enteritis. Female gender, younger age, psychological distress during or before the enteritis episode, and severity of the acute episode are risk factors for this IBS variant.20 A study of 21,421 enteritis patients found that 42% with protozoal or parasitic infection and 14% with bacterial infection developed IBS.
Patients with nonviral enteritis often have a more severe course of enteritis, typically requiring antibiotics. It is believed that the resulting irregularities in the intestinal microbiota make these patients more likely to develop PI-IBS.21 PI-IBS patients are likely to improve or fully recover over time. Symptoms of PI-IBS are managed in a manner similar to how non-PI-IBS patients are managed.20
Challenges in making the IBS diagnosis
Historically, the diagnosis of IBS has been made clinically after excluding red flags (ie, signs or symptoms that might reflect other underlying medical problems) in the clinical presentation. For this reason, obtain a thorough clinical history that includes the course of symptoms, triggers, and alleviating factors. Any of the following are considered red flags1,22,23:
- age > 50 years at onset of symptoms
- new-onset constipation in the elderly
- rectal bleeding
- unexplained weight loss or anemia
- family history of organic gastrointestinal disease
- palpable abdominal or rectal mass
- nocturnal symptoms.
New studies demonstrate that several inflammatory markers can help exclude inflammatory bowel disease from the differential diagnosis in patients in whom IBS is suspected and being investigated.24 In 2019, the American Gastroenterological Association published a clinical practice guideline updating the laboratory evaluation of functional diarrhea and IBS-D in adults,25 and made several recommendations:
- Obtain the level of fecal calprotectin (normal level, ≤ 50 mcg/g) or fecal lactoferrin (≤ 4.0-7.25 mcg/g); if these tests are not available or results are not accessible, the C-reactive protein level is a reasonable option.
- Do not routinely use the erythrocyte sedimentation rate or C-reactive protein level to screen for inflammatory bowel disease.
- Test for Giardia lamblia with an antigen or polymerase chain reaction test.
- Do not test for ova and parasites (other than Giardia) in patients who do not have a history of travel or who have not emigrated from a high-risk area recently.
- Obtain testing for celiac disease with immunoglobulin A (IgA) tissue transglutaminase and with a second test, of immunoglobulin G (IgG) tissue transglutaminase and IgG or IgA deaminated gliadin peptides, to detect celiac disease in IgA-deficient patients.
- Order testing for bile-acid diarrhea with selenium homotaurocholic acid nuclear medicine scanning (if available in your region; the test is available in Europe); measurement of bile acid from a 48-hour stool collection; or an assay of fibroblast growth factor 19, which measures defective feedback of bile-acid synthesis. If these tests are unavailable, consider an empiric trial of a bile-acid binder.
- Do not use available serologic IBS testing.
Continue to: Continue to obtain a...
Continue to obtain a complete blood count for the evaluation of anemia. Endoscopic procedures are indicated in patients with a red flag.1
Treat based on subtype
The first step in the treatment of all IBS patients (TABLE 21,3,4,9,26,27) is for you to develop a strong relationship with the patient: You must acknowledge the disease and empower the patient to manage their symptoms. A strong physician–patient relationship leads to more effective outcomes.4

IBS treatment modalities target abdominal pain, bloating, abdominal distention, and altered bowel function—described in the literature as global symptoms. IBS-M patients should direct their treatment to the predominant symptom (constipation or diarrhea). The following sections describe available treatment options. The FIGURE1,3,4,9,25 shows a treatment workflow based on IBS subtype and symptom severity.

Treatments for all IBS subtypes
Lifestyle modification. Exercise provides overall positive health benefits. With such a variety of exercise forms, however, it is difficult to identify specific exercises that are better for IBS patients.28 A study of 305 IBS patients found that exercise alleviated constipation but not other IBS symptoms, and did not improve quality of life.3 Based on low cost and low risk of adverse effects, exercise should be recommended to all IBS patients.
Dietary restriction therapies have become an area of focus for patients, clinicians, and researchers. Modification of the diet is thought to improve global symptoms and intestinal health through modification of gut microbiota, immune activation, and a decrease in levels of fecal short-chain fatty acids.29
Continue to: The 2 main diets...
The 2 main diets studied for the treatment of IBS are a diet low in fermentable oligo-, di- and monosaccharides and polyols—the so-called low-FODMAP diet (TABLE 330)—and a gluten-free diet. Evidence behind the benefits of both diets conflicts; trials of the low-FODMAP diet are more favorable.

A small study with 20 patients with IBS-D and IBS-M who followed a low-FODMAP diet found improvement in IBS symptoms and a reduction in serum levels of proinflammatory cytokines, fecal bacteria, and total fecal short-chain fatty acid levels.29 Several meta-analyses have shown improvement in overall IBS symptoms for patients who follow a low-FODMAP diet. Because of the heterogeneity of the studies, however, the quality of the data is low.31-34
Data supporting the use of a gluten-free diet for IBS patients are insufficient.31
The American College of Gastroenterology (ACG) gave a weak recommendation for the low-FODMAP diet and recommended against the gluten-free diet in IBS patients.3 More data are needed regarding the safety profile of using a low-FODMAP diet for an extended period: There is concern about the risk of nutritional deficiencies associated with long-term use of this diet.3
Supplementation with poorly fermentable soluble fiber has been shown to alleviate global IBS symptoms; insoluble fiber does not yield improvement of symptoms. Psyllium fiber is recommended over wheat bran.3,35
Continue to: Consider a low-FODMAP diet...
Consider a low-FODMAP diet and soluble fiber as initial treatment for all IBS patients.
Modification of intestinal microbiota. Understanding the difference between prebiotics and probiotics is important when considering treatment for IBS. Prebiotics are foods or dietary supplements that generate changes in the composition and activity of intestinal microbiota. Probiotics are live microorganisms that can improve intestinal health.3
A meta-analysis of 729 IBS patients found that prebiotics do not reduce gastrointestinal symptoms or improve the quality of life of IBS patients.36 Evidence supporting the benefit of probiotics is favorable; however, data in these studies have significant heterogeneity. Several meta-analyses studied the benefits of Lactobacillus spp and Bifidobacterium spp in alleviating IBS symptoms. The studies found improvement in abdominal pain, bloating and distention, and flatulence.3,37-40 Consider recommending probiotics for all IBS patients; for some, however, the high cost of some of these products might be an obstacle.
Researchers are also studying the use of fecal microbiota transplantation (FMT) to treat IBS. Studies have evaluated the delivery of FMT orally (as capsules) and endoscopically. Evidence does not show improvement in global IBS symptoms with FMT. More studies, with larger sample populations, are needed.41-43
Antispasmodic medications and peppermint oil. Antispasmodic medications have been considered a mainstay therapy for IBS because of their effect on intestinal dysmotility. Hyoscine and dicyclomine are commonly used. Meta-analyses have shown improvement in global symptoms and abdominal pain, but effects were modest.3,44 Use this class of drugs as first-line treatment for mild IBS symptoms.
Continue to: Peppermint oil has been...
Peppermint oil has been found useful in improving IBS global symptoms and abdominal pain in several studies.44-46 A common adverse effect of peppermint oil is heartburn, resulting from relaxation of esophageal muscle.3 Peppermint oil can be considered an adjuvant agent in treating IBS.
Antidepressants. Tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) have been studied for the treatment of IBS. Meta-analyses show that both are effective in reducing pain and overall IBS symptoms.1,3,47 The number needed to treat (NNT) for TCAs is 4.5; for SSRIs, 5.47 Data do not show that either drug class is superior to the other for IBS. Based on the adverse effect profile, TCAs are more suitable for IBS-D patients; SSRIs are better for IBS-C patients.47
New data show that serotonin-norepinephrine reuptake inhibitors, such as duloxetine and milnacipran, can alleviate IBS symptoms through their pain-modifying properties.47
Based on the adverse effect profile and stigma associated with antidepressant medications, patients might be less likely to take them for IBS symptoms than for these drugs’ primary indications. Clinicians should still consider this drug class if other first-line treatments do not provide full resolution of symptoms.
Psychotherapy. Several psychotherapeutic modalities have been evaluated for efficacy in reducing global IBS symptoms. The approaches studied most often were provider-directed cognitive behavioral therapy, relaxation therapy, hypnotherapy, and multicomponent psychological therapy. The NNT for these modalities is 4, but studies had significant heterogeneity.3 Consider referring patients for psychotherapeutic intervention if they have not responded to medical therapy after 12 months.4
Continue to Treatment of IBS-C
Treatment of IBS-C
Prosecretory agents. Linaclotide and plecanatide are amino-acid peptides that act as a guanylate cyclase C agonist. Both increase gastrointestinal transit rate by increasing electrolyte and fluid transport into the intestinal lumen. They also decrease the activity of pain-sensing nerves by increasing extracellular cyclic guanosine-3'5'-monophosphate levels.3,48 In a recent meta-analysis, both treatments produced improvement in global symptoms. However, linaclotide showed superior improvement in abdominal pain and global symptoms compared to other secretory agents.48,49 Diarrhea was the most common adverse effect of linaclotide and plecanatide, although less so with plecanatide.49
Lubiprostone activates the intestinal chloride channel type 2 on the small intestine, leading to an increase in chloride and water efflux into the intestinal lumen, in turn accelerating gastrointestinal transit.3 A meta-analysis with 1468 IBS patients found that lubiprostone improved constipation, stool consistency, abdominal pain, degree of straining, and abdominal bloating.50 Diarrhea and nausea are commonly reported adverse effects of lubiprostone.49,50
Linaclotide, plecanatide, and lubiprostone should be considered first-line therapies for patients with IBS-C. High cost is still a roadblock to the use of these agents.
The US Food and Drug Administration (FDA) approved tenapanor in September 2019; however, the drug is not commercially available in the United States (it is available in Canada). Tenapanor is a sodium–hydrogen exchanger 3 inhibitor that reduces sodium absorption from the intestine and colon. The drug increases water secretion into the intestinal lumen, thus accelerating gut transit time. It also inhibits active absorption of phosphate in the intestine.
Tenapanor was approved for treating both IBS-C and hyperphosphatemia in patients with chronic kidney disease on dialysis or end-stage renal disease.26 In a recent meta-analysis, the drug showed benefit in alleviating global IBS symptoms, and ranked first in reducing bloating.49 It is too soon to know if tenapanor will perform clinically better than other prosecretory agents.
Continue to: Serotonergic agents
Serotonergic agents. Serotonin (5-hydroxytryptamine [5-HT]) modulates gastrointestinal secretions, gut motility, and visceral sensation. Researchers have developed IBS treatments that target receptors involved in these functions.
Tegaserod is a partial, selective 5-HT4 agonist indicated for the treatment of IBS-C in women. A study with 661 women with IBS-M and IBS-C showed that tegaserod increased the number of bowel movement episodes. Patients also reported higher stool consistency scores and fewer days with straining compared to placebo.27 The medication was removed from the market in 2007 because of its potential for cardiovascular adverse effects3; however, it was reintroduced in 2019 for women < 65 years of age with IBS-C. Consider prescribing tegaserod if other treatment options fail to alleviate symptoms.
Treatment of IBS-D
Antibiotics. The nonabsorbable antibiotic rifaximin is approved by the FDA for IBS-D at a dosage of 550 mg tid for 2 weeks.1 Several studies show improvement in IBS global symptoms with the recommended treatment course51-53; benefit persisted for the 10-week follow-up study period.1 A meta-analysis found that the NNT for rifaximin is 8-11.54 Preliminary data indicate that the rates of Clostridioides difficile infection and microbial resistance among rifaximin users are low.3 Consider using rifaximin as a first-line treatment option for patients with IBS-D. Retreatment might be necessary because the drug’s effect gradually disappears.9
Antidiarrheals. Eluxadoline is a µ-opioid and κ-opioid receptor agonist and δ-opioid receptor antagonist with effects on the intestinal nervous system.3 Several meta-analyses demonstrated that eluxadoline improves abdominal pain scores and daily stool consistency in IBS-D patients.53,54 Eluxadoline should be considered early in the management of IBS-D patients. The most common adverse effect is constipation.
The FDA issued a safety warning in 2017 regarding an increased risk of pancreatitis in patients taking eluxadoline who do not have a gallbladder. In addition, eluxadoline should be avoided in patients with a history of sphincter of Oddi dysfunction, alcohol abuse, or severe liver problems.3,54
Continue to: The high cost of...
The high cost of eluxadoline can be a significant barrier to use.
Serotonergic agents. Alosetron is a selective
Ondansetron has also been used to treat IBS-D. In a meta-analysis with 294 patients, ondansetron showed improvement in stool consistency.55 Ondansetron does not improve abdominal pain.4 It can be used in patients who have mild-to-moderate symptoms.9 Ondansetron is not FDA approved for the treatment of IBS-D.
Bile-acid sequestrants. Traditionally, bile-acid sequestrants have been used to treat bile-acid diarrhea. A meta-analysis of 6 studies of 908 patients with IBS-D found that 28.1% were affected by bile-acid malabsorption. Two small studies that evaluated the benefits of colesevelam for IBS-D found significant improvement in stool consistency.54 Another study, which evaluated the benefits of cholestyramine, found improvement in stool consistency, but findings were not significant.54 Many patients taking a bile-acid sequestrant stop taking the medication because of considerable adverse effects (constipation, nausea, bloating, flatulence, and abdominal pain).54 For that reason, this class of medication is not recommended as first-line treatment for IBS-D and is not FDA approved for IBS-D.
SIDEBAR
KEY POINTS The challenge of, and a needed framework for, managing IBS
- IBS is a complex, chronic condition affecting a considerable number of people worldwide.
- Because of the substantial disease burden associated with IBS, patients are at higher risk of mental health disorders.
- Physicians who care for IBS patients must build a strong physician–patient relationship; their mutual trust will ensure development of an effective treatment plan.
- Family physicians and other primary care providers are equipped to help IBS patients navigate the complex health care system and the IBS disease process. They can help coordinate care with specialists and behavioral health clinicians, which will help patients improve quality of life and manage symptoms appropriately.
A role for complementaryand integrative medicine?
Recently, complementary and integrative modalities for treating IBS have sparked the interest of researchers.
Continue to: Acupuncture
Acupuncture. In a meta-analysis with 3440 patients, acupuncture was more effective than Western medicine in alleviating IBS symptoms for as long as 3 months. The authors concluded that acupuncture could be used in combination with other therapies to reduce the severity of IBS symptoms.56
Concomitant acupuncture and Chinese herbal medicine. In a systematic review and meta-analysis comprising 21 randomized controlled trials, researchers reported that acupuncture combined with Chinese herbal medicine improved IBS symptoms, compared to what was noted in matched controls who were treated with Western medicine or with Western medicine combined with Chinese herbal medicine. The authors were cautious about the results of the meta-analysis, however, because the studies examined were small and of low quality, and presented a high risk of bias.57
Agents not to be used routinely for IBS
Loperamide. This peripheral µ-opioid receptor agonist controls diarrhea. However, recent studies showed no significant benefit to loperamide over placebo in IBS-M and IBS-D. In 2018, the FDA issued a safety alert regarding an elevated risk of serious cardiac adverse effects in patients taking loperamide. The ACG recommends against using loperamide to treat IBS symptoms.3,54
Polyethylene glycol. An osmotic laxative that is not absorbed in the intestinal lumen, polyethylene glycol is highly efficacious for alleviating constipation, but it does not reduce pain or other IBS symptoms. For that reason, the ACG recommends against its use.3
CORRESPONDENCE
Jose M. Villalon-Gomez, MD, MPH, Emory Healthcare Family Medicine, 4500 North Shallowford Road, Dunwoody, GA 30338; [email protected]
1. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393-1407.e5. doi:10.1053/j.gastro.2016.02.031
2. What kind of poop do I have? WebMD. January 16, 2020. Accessed September 20, 2021. www.webmd.com/digestive-disorders/poop-chart-bristol-stool-scale
3. Ford AC, Moayyedi P, Chey WD, et al; . American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol. 2018;113(suppl 2):1-18. doi:10.1038/s41395-018-0084-x
4. Ferreira AI, Garrido M, Castro-Poças F. Irritable bowel syndrome: news from an old disorder. GE Port J Gastroenterol. 2020;27:255-268. doi:10.1159/000503757
5. Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473-486. doi: 10.1038/s41575-020-0286-8
6. Frändemark Å, Törnblom H, Jakobsson S, et al. Work productivity and activity impairment in irritable bowel syndrome (IBS): a multifaceted problem: Am J Gastroenterol. 2018;113:1540-1549. doi:10.1038/s41395-018-0262-x
7. Poulsen CH, Eplov LF, Hjorthøj C, et al. Irritable bowel symptoms, use of healthcare, costs, sickness and disability pension benefits: a long-term population-based study. Scand J Public Health. 2019;47:867-875. doi:10.1177/1403494818776168
8. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20:6024-6030. doi:10.3748/wjg.v20.i20.6024
9. Moayyedi P, Mearin F, Azpiroz F, et al. Irritable bowel syndrome diagnosis and management: a simplified algorithm for clinical practice. United European Gastroenterol J. 2017;5:773-788. doi:10.1177/2050640617731968
10. Zhu S, Wang B, Jia Q, et al. Candidate single nucleotide polymorphisms of irritable bowel syndrome: a [systematic] review and meta-analysis. BMC Gastroenterology. 2019;19:165. doi:10.1186/s12876-019-1084-z
11. Simrén M, Törnblom H, Palsson OS, et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2018;67:255-262. doi:10.1136/gutjnl-2016-312361
12. Bashashati M, Moossavi S, Cremon C, et al. Colonic immune cells in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2018;30:e13192. doi:10.1111/nmo.13192
13. Robles A, Ingles DP, Myneedu K, et al. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2019;31:e13718. doi:10.1111/nmo.13718
14. Bashashati M, Moradi M, Sarosiek I. Interleukin-6 in irritable bowel syndrome: A systematic review and meta-analysis of IL-6 (-G174C) and circulating IL-6 levels. Cytokine. 2017;99:132-138. doi:10.1016/j.cyto.2017.08.017
15. Bashashati M, Rezaei N, Shafieyoun A, et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2014;26:1036-1048. doi:10.1111/nmo.12358
16. Sibelli A, Chalder T, Everitt H, et al. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol Med. 2016;46:3065-3080. doi:10.1017/S0033291716001987
17. Yeh H-W, Chien W-C, Chung C-H, et al. Risk of psychiatric disorders in irritable bowel syndrome—a nationwide, population-based, cohort study. Int J Clin Pract. 2018;72:e13212. doi:10.1111/ijcp.13212
18. Duan R, Zhu S, Wang B, et al. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clin Transl Gastroenterol. 2019;10:e00012. doi:10.14309/ctg.0000000000000012
19. Wang L, Alammar N, Singh R, et al. Gut microbial dysbiosis in the irritable bowel syndrome: a systematic review and meta-analysis of case-controlled studies. J Acad Nutr Diet. 2020;120:565-586. doi:10.1016/j.jand.2019.05.015
20. Barbara G, Grover M, Bercik P, et al. Rome Foundation working team report on post-infection irritable bowel syndrome. Gastroenterology. 2019;156:46-58.e7. doi:10.1053/j.gastro.2018.07.011
21. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology. 2017;152:1042-1054.e1. doi:10.1053/j.gastro.2016.12.039
22. Heidelbaugh JJ. These 3 tools can help you streamline management of IBS. J Fam Pract. 2017;66:346-353.
23. American College of Gastroenterology Task Force on Irritable Bowel Syndrome; Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(suppl 1):S1-S35. doi:10.1038/ajg.2008.122
24. Menees SB, Powell C, Kurlander J, et al. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol. 2015;110:444-454. doi:10.1038/ajg.2015.6
25. Smalley W, Falck-Ytter C, Carrasco-Labra A, et al. AGA clinical practice guidelines on the laboratory evaluation of functional diarrhea and diarrhea-predominant irritable bowel syndrome in adults (IBS-D). Gastroenterology. 2019;157:851-854. doi:10.1053/j.gastro.2019.07.004
26. Markham A. Tenapanor: first approval. Drugs. 2019;79:1897-1903. doi:10.1007/s40265-019-01215-9
27. Chey WD, Paré P, Viegas A, et al. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008;103:1217-1225. doi:10.1111/j.1572-0241.2008.01808.x
28. Zhou C, Zhao E, Li Y, et al. Exercise therapy of patients with irritable bowel syndrome: a systematic review of randomized controlled trials. Neurogastroenterol Motil. 2019;31:e13461. doi:10.1111/nmo.13461
29. Hustoft TN, Hausken T, Ystad SO, et al. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017;29:e12969. doi:10.1111/nmo.12969
30. Zegarac JP. The low-FODMAP diet for IBS: what you need to know. Medscape. August 13, 2019. Accessed September 20, 2021. www.medscape.com/viewarticle/917069
31. Dionne J, Ford AC, Yuan Y, et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low
32. Su H, Li Y-T, Heitkemper MM, et al. Effects of low-FODMAPS diet on irritable bowel syndrome symptoms and gut microbiome: Gastroenterol Nurs. 2019;42:150-158. doi:10.1097/SGA.0000000000000428
33. Nawawi KNM, Belov M, Goulding C. Low FODMAP diet significantly improves IBS symptoms: an Irish retrospective cohort study. Eur J Nutr. 2020;59:2237-2248. doi: 10.1007/s00394-019-02074-6
34. Altobelli E, Del Negro V, Angeletti PM, et al. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients. 2017;9:940. doi:10.3390/nu9090940
35. Nagarajan N, Morden A, Bischof D, et al. The role of fiber supplementation in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2015;27:1002-1010. doi:10.1097/MEG.0000000000000425
36. Wilson B, Rossi M, Dimidi E, et al. Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019;109:1098-1111. doi:10.1093/ajcn/nqy376
37. Yuan F, Ni H, Asche CV, et al. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: a meta-analysis. Curr Med Res Opin. 2017;33:1191-1197. doi:10.1080/03007995.2017.1292230
38. Liang D, Longgui N, Guoqiang X. Efficacy of different probiotic protocols in irritable bowel syndrome: a network meta-analysis. Medicine (Baltimore). 2019;98:16068. doi:10.1097/MD.0000000000016068
39. Dale HF, Rasmussen SH, Asiller ÖÖ, et al. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11:2048. doi:10.3390/nu11092048
40. Pratt C, Campbell MD. The effect of Bifidobacterium on reducing symptomatic abdominal pain in patients with irritable bowel syndrome: a systematic review. Probiotics Antimicrob Proteins. 2020;12:834-839. doi:10.1007/s12602-019-09609-7
41. Ianiro G, Eusebi LH, Black CJ, et al. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:240-248. doi:10.1111/apt.15330
42. Myneedu K, Deoker A, Schmulson MJ, et al. Fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. United European Gastroenterol J. 2019;7:1033-1041. doi:10.1177/2050640619866990
43. Xu D, Chen VL, Steiner CA, et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114:1043-1050. doi:10.14309/ajg.0000000000000198
44. Black CJ, Yuan Y, Selinger CP, et al. Efficacy of soluble fibre, antispasmodic drugs, and gut–brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:117-131. doi:10.1016/S2468-1253(19)30324-3
45. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512. doi:10.1097/MCG.0b013e3182a88357
46. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi:10.1186/s12906-018-2409-0
47. Ford AC, Lacy BE, Harris LA, et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. 2019;114:21-39. doi: 10.1038/s41395-018-0222-5
48. Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-c agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol. 2018;113:329-338. doi:10.1038/ajg.2017.495
49. Black CJ, Burr NE, Quigley EMM, et al. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology. 2018;155:1753-1763. doi:10.1053/j.gastro.2018.08.021
50. Li F, Fu T, Tong W-D, et al. Lubiprostone is effective in the treatment of chronic idiopathic constipation and irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2016;91:456-468. doi:10.1016/j.mayocp.2016.01.015
51. Ford AC, Harris LA, Lacy BE, et al. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044-1060. doi:10.1111/apt.15001
52. Yoon K, Kim N, Lee JY, et al. Clinical response of rifaximin treatment in patients with abdominal bloating. Korean J Gastroenterol. 2018;72:121-127. doi:10.4166/kjg.2018.72.3.121
53. Black CJ, Burr NE, Camilleri M, et al. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut. 2020;69:74-82. doi:10.1136/gutjnl-2018-318160
54. Lacy BE. Review article: an analysis of safety profiles of treatments for diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:817-830. doi:10.1111/apt.14948
55. Zheng Y, Yu T, Tang Y, et al. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. PLOS ONE. 2017;12:e0172846. doi:10.1371/journal.pone.0172846
56. Zheng H, Chen R, Zhao X, et al. Comparison between the effects of acupuncture relative to other controls on irritable bowel syndrome: a meta-analysis. Pain Research and Management. 2019;2019:1-13. doi:https://doi.org/10.1155/2019/2871505
57. Yan J, Miao Z-W, Lu J, et al. Acupuncture plus Chinese herbal medicine for irritable bowel syndrome with diarrhea: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019:1-16. https://doi.org/10.1155/2019/7680963
1. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393-1407.e5. doi:10.1053/j.gastro.2016.02.031
2. What kind of poop do I have? WebMD. January 16, 2020. Accessed September 20, 2021. www.webmd.com/digestive-disorders/poop-chart-bristol-stool-scale
3. Ford AC, Moayyedi P, Chey WD, et al; . American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol. 2018;113(suppl 2):1-18. doi:10.1038/s41395-018-0084-x
4. Ferreira AI, Garrido M, Castro-Poças F. Irritable bowel syndrome: news from an old disorder. GE Port J Gastroenterol. 2020;27:255-268. doi:10.1159/000503757
5. Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473-486. doi: 10.1038/s41575-020-0286-8
6. Frändemark Å, Törnblom H, Jakobsson S, et al. Work productivity and activity impairment in irritable bowel syndrome (IBS): a multifaceted problem: Am J Gastroenterol. 2018;113:1540-1549. doi:10.1038/s41395-018-0262-x
7. Poulsen CH, Eplov LF, Hjorthøj C, et al. Irritable bowel symptoms, use of healthcare, costs, sickness and disability pension benefits: a long-term population-based study. Scand J Public Health. 2019;47:867-875. doi:10.1177/1403494818776168
8. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20:6024-6030. doi:10.3748/wjg.v20.i20.6024
9. Moayyedi P, Mearin F, Azpiroz F, et al. Irritable bowel syndrome diagnosis and management: a simplified algorithm for clinical practice. United European Gastroenterol J. 2017;5:773-788. doi:10.1177/2050640617731968
10. Zhu S, Wang B, Jia Q, et al. Candidate single nucleotide polymorphisms of irritable bowel syndrome: a [systematic] review and meta-analysis. BMC Gastroenterology. 2019;19:165. doi:10.1186/s12876-019-1084-z
11. Simrén M, Törnblom H, Palsson OS, et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2018;67:255-262. doi:10.1136/gutjnl-2016-312361
12. Bashashati M, Moossavi S, Cremon C, et al. Colonic immune cells in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2018;30:e13192. doi:10.1111/nmo.13192
13. Robles A, Ingles DP, Myneedu K, et al. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2019;31:e13718. doi:10.1111/nmo.13718
14. Bashashati M, Moradi M, Sarosiek I. Interleukin-6 in irritable bowel syndrome: A systematic review and meta-analysis of IL-6 (-G174C) and circulating IL-6 levels. Cytokine. 2017;99:132-138. doi:10.1016/j.cyto.2017.08.017
15. Bashashati M, Rezaei N, Shafieyoun A, et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2014;26:1036-1048. doi:10.1111/nmo.12358
16. Sibelli A, Chalder T, Everitt H, et al. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol Med. 2016;46:3065-3080. doi:10.1017/S0033291716001987
17. Yeh H-W, Chien W-C, Chung C-H, et al. Risk of psychiatric disorders in irritable bowel syndrome—a nationwide, population-based, cohort study. Int J Clin Pract. 2018;72:e13212. doi:10.1111/ijcp.13212
18. Duan R, Zhu S, Wang B, et al. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clin Transl Gastroenterol. 2019;10:e00012. doi:10.14309/ctg.0000000000000012
19. Wang L, Alammar N, Singh R, et al. Gut microbial dysbiosis in the irritable bowel syndrome: a systematic review and meta-analysis of case-controlled studies. J Acad Nutr Diet. 2020;120:565-586. doi:10.1016/j.jand.2019.05.015
20. Barbara G, Grover M, Bercik P, et al. Rome Foundation working team report on post-infection irritable bowel syndrome. Gastroenterology. 2019;156:46-58.e7. doi:10.1053/j.gastro.2018.07.011
21. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology. 2017;152:1042-1054.e1. doi:10.1053/j.gastro.2016.12.039
22. Heidelbaugh JJ. These 3 tools can help you streamline management of IBS. J Fam Pract. 2017;66:346-353.
23. American College of Gastroenterology Task Force on Irritable Bowel Syndrome; Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(suppl 1):S1-S35. doi:10.1038/ajg.2008.122
24. Menees SB, Powell C, Kurlander J, et al. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol. 2015;110:444-454. doi:10.1038/ajg.2015.6
25. Smalley W, Falck-Ytter C, Carrasco-Labra A, et al. AGA clinical practice guidelines on the laboratory evaluation of functional diarrhea and diarrhea-predominant irritable bowel syndrome in adults (IBS-D). Gastroenterology. 2019;157:851-854. doi:10.1053/j.gastro.2019.07.004
26. Markham A. Tenapanor: first approval. Drugs. 2019;79:1897-1903. doi:10.1007/s40265-019-01215-9
27. Chey WD, Paré P, Viegas A, et al. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008;103:1217-1225. doi:10.1111/j.1572-0241.2008.01808.x
28. Zhou C, Zhao E, Li Y, et al. Exercise therapy of patients with irritable bowel syndrome: a systematic review of randomized controlled trials. Neurogastroenterol Motil. 2019;31:e13461. doi:10.1111/nmo.13461
29. Hustoft TN, Hausken T, Ystad SO, et al. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017;29:e12969. doi:10.1111/nmo.12969
30. Zegarac JP. The low-FODMAP diet for IBS: what you need to know. Medscape. August 13, 2019. Accessed September 20, 2021. www.medscape.com/viewarticle/917069
31. Dionne J, Ford AC, Yuan Y, et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low
32. Su H, Li Y-T, Heitkemper MM, et al. Effects of low-FODMAPS diet on irritable bowel syndrome symptoms and gut microbiome: Gastroenterol Nurs. 2019;42:150-158. doi:10.1097/SGA.0000000000000428
33. Nawawi KNM, Belov M, Goulding C. Low FODMAP diet significantly improves IBS symptoms: an Irish retrospective cohort study. Eur J Nutr. 2020;59:2237-2248. doi: 10.1007/s00394-019-02074-6
34. Altobelli E, Del Negro V, Angeletti PM, et al. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients. 2017;9:940. doi:10.3390/nu9090940
35. Nagarajan N, Morden A, Bischof D, et al. The role of fiber supplementation in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2015;27:1002-1010. doi:10.1097/MEG.0000000000000425
36. Wilson B, Rossi M, Dimidi E, et al. Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019;109:1098-1111. doi:10.1093/ajcn/nqy376
37. Yuan F, Ni H, Asche CV, et al. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: a meta-analysis. Curr Med Res Opin. 2017;33:1191-1197. doi:10.1080/03007995.2017.1292230
38. Liang D, Longgui N, Guoqiang X. Efficacy of different probiotic protocols in irritable bowel syndrome: a network meta-analysis. Medicine (Baltimore). 2019;98:16068. doi:10.1097/MD.0000000000016068
39. Dale HF, Rasmussen SH, Asiller ÖÖ, et al. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11:2048. doi:10.3390/nu11092048
40. Pratt C, Campbell MD. The effect of Bifidobacterium on reducing symptomatic abdominal pain in patients with irritable bowel syndrome: a systematic review. Probiotics Antimicrob Proteins. 2020;12:834-839. doi:10.1007/s12602-019-09609-7
41. Ianiro G, Eusebi LH, Black CJ, et al. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:240-248. doi:10.1111/apt.15330
42. Myneedu K, Deoker A, Schmulson MJ, et al. Fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. United European Gastroenterol J. 2019;7:1033-1041. doi:10.1177/2050640619866990
43. Xu D, Chen VL, Steiner CA, et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114:1043-1050. doi:10.14309/ajg.0000000000000198
44. Black CJ, Yuan Y, Selinger CP, et al. Efficacy of soluble fibre, antispasmodic drugs, and gut–brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:117-131. doi:10.1016/S2468-1253(19)30324-3
45. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512. doi:10.1097/MCG.0b013e3182a88357
46. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi:10.1186/s12906-018-2409-0
47. Ford AC, Lacy BE, Harris LA, et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. 2019;114:21-39. doi: 10.1038/s41395-018-0222-5
48. Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-c agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol. 2018;113:329-338. doi:10.1038/ajg.2017.495
49. Black CJ, Burr NE, Quigley EMM, et al. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology. 2018;155:1753-1763. doi:10.1053/j.gastro.2018.08.021
50. Li F, Fu T, Tong W-D, et al. Lubiprostone is effective in the treatment of chronic idiopathic constipation and irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2016;91:456-468. doi:10.1016/j.mayocp.2016.01.015
51. Ford AC, Harris LA, Lacy BE, et al. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044-1060. doi:10.1111/apt.15001
52. Yoon K, Kim N, Lee JY, et al. Clinical response of rifaximin treatment in patients with abdominal bloating. Korean J Gastroenterol. 2018;72:121-127. doi:10.4166/kjg.2018.72.3.121
53. Black CJ, Burr NE, Camilleri M, et al. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut. 2020;69:74-82. doi:10.1136/gutjnl-2018-318160
54. Lacy BE. Review article: an analysis of safety profiles of treatments for diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:817-830. doi:10.1111/apt.14948
55. Zheng Y, Yu T, Tang Y, et al. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. PLOS ONE. 2017;12:e0172846. doi:10.1371/journal.pone.0172846
56. Zheng H, Chen R, Zhao X, et al. Comparison between the effects of acupuncture relative to other controls on irritable bowel syndrome: a meta-analysis. Pain Research and Management. 2019;2019:1-13. doi:https://doi.org/10.1155/2019/2871505
57. Yan J, Miao Z-W, Lu J, et al. Acupuncture plus Chinese herbal medicine for irritable bowel syndrome with diarrhea: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019:1-16. https://doi.org/10.1155/2019/7680963
PRACTICE RECOMMENDATIONS
› Make the diagnosis of irritable bowel syndrome (IBS) based on clinical findings, after excluding red flags in the presentation. C
› Screen patients with diarrhea-predominant IBS with fecal and serologic studies to rule out inflammatory bowel disease and celiac disease. B
› Counsel all IBS patients to increase their intake of soluble fiber, follow a low-FODMAP (fermentable oligo-, di-, and monosaccharide, and polyol) diet, and increase physical activity. B
› Prescribe an antispasmodic to treat mild IBS of all subtypes. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series