User login
Tips and tools to help refine your approach to chest pain
One of the most concerning and challenging patient complaints presented to physicians is chest pain. Chest pain is a ubiquitous complaint in primary care settings and in the emergency department (ED), accounting for 8 million ED visits and 0.4% of all primary care visits in North America annually.1,2
Despite the great number of chest-pain encounters, early identification of life-threatening causes and prompt treatment remain a challenge. In this article, we examine how the approach to a complaint of chest pain in a primary care practice (and, likewise, in the ED) must first, rest on the clinical evaluation and second, employ risk-stratification tools to aid in evaluation, appropriate diagnosis, triage, and treatment.
Chest pain by the numbers
Acute coronary syndrome (ACS) is the cause of chest pain in 5.1% of patients with chest pain who present to the ED, compared with 1.5% to 3.1% of chest-pain patients seen in ambulatory care.1,3 “Nonspecific chest pain” is the most frequent diagnosis of chest pain in the ED for all age groups (47.5% to 55.8%).3 In contrast, the most common cause of chest pain in primary care is musculoskeletal (36%), followed by gastrointestinal disease (18% to 19%); serious cardiac causes (15%), including ACS (1.5%); nonspecific causes (16%); psychiatric causes (8%); and pulmonary causes (5% to 10%).4 Among patients seen in the ED because of chest pain, 57.4% are discharged, 30.6% are admitted for further evaluation, and 0.4% die in the ED or after admission.3

First challenge: The scale of the differential Dx
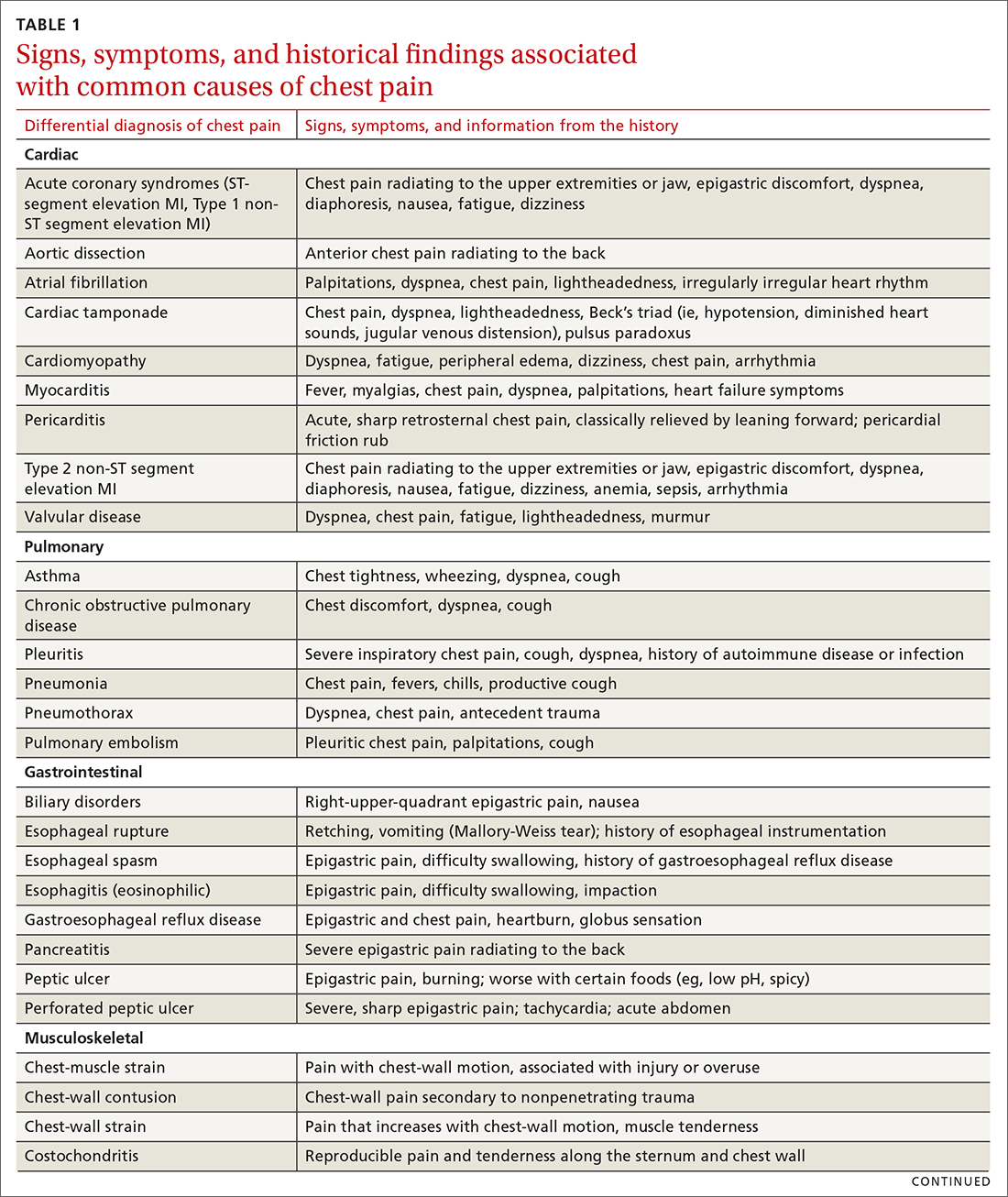
The differential diagnosis of chest pain is broad. It includes life-threatening causes, such as ACS (from ST-segment elevation myocardial infarction [STEMI], Type 1 non-STEMI, and unstable angina), acute aortic dissection, pulmonary embolism (PE), esophageal rupture, and tension pneumothorax, as well as non-life-threatening causes (TABLE 1).
History and physical exam guide early decisions
Triage assessment of the patient with chest pain, including vital signs, general appearance, and basic symptom questions, can guide you as to whether they require transfer to a higher level of care. Although an individual’s findings cannot, alone, accurately exclude or diagnose ACS, the findings can be used in combination in clinical decision tools to distinguish noncardiac chest pain from ACS.
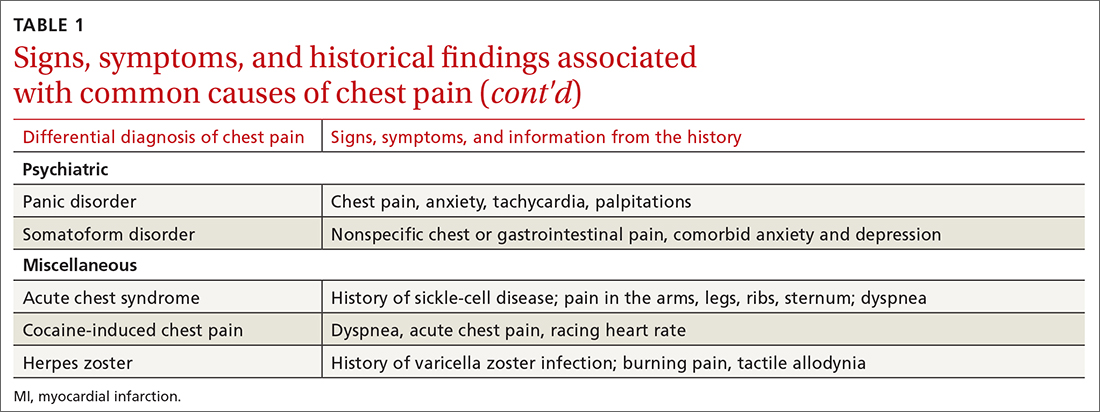
History. Features in the history (TABLE 25-9) that are most helpful at increasing the probability (ie, a positive likelihood ratio [LR] ≥ 2) of chest pain being caused by ACS are:
- pain radiating to both arms or the right arm
- pain that is worse upon exertion
- a history of peripheral artery disease or coronary artery disease (CAD)
- a previously abnormal stress test.
The presence of any prior normal stress test is unhelpful: Such patients have a similar risk of a 30-day adverse cardiac event as a patient who has never had a stress test.5
Continue to: A history of tobacco use...
A history of tobacco use, hyperlipidemia, hypertension, obesity, acute myocardial infarction (AMI), coronary artery bypass grafting, or a family history of CAD does not significantly increase the risk of ACS.6 However, exploring each of these risk factors further is important, because genetic links between these risk factors can lead to an increased risk of CAD (eg, familial hypercholesterolemia).7
A history of normal or near-normal coronary angiography (< 25% stenosis) is associated with a lower likelihood of ACS, because 98% of such patients are free of AMI and 90% are without single-vessel coronary disease nearly 10 years out.6 A history of coronary artery bypass grafting is not necessarily predictive of ACS (LR = 1-3).5,6
Historical features classically associated with ACS, but that have an LR < 2, are pain radiating to the neck or jaw, nausea or vomiting, dyspnea, and pain that is relieved with nitroglycerin.5,6 Pain described as pleuritic, sharp, positional, or reproduced with palpation is less likely due to AMI.5
Physical exam findings are not independently diagnostic when evaluating chest pain. However, a third heart sound is the most likely finding associated with AMI and hypotension is the clinical sign most likely associated with ACS.5
Consider the diagnosis of PE in all patients with chest pain. In PE, chest pain might be associated with dyspnea, presyncope, syncope, or hemoptysis.8 On examination, 40% of patients have tachycardia.8 If PE is suspected; the patient should be risk-stratified using a validated prediction rule (see the discussion of PE that follows).
Continue to: Other historical features...
Other historical features or physical exam findings correlate with aortic dissection, pneumonia, and psychiatric causes of chest pain (TABLE 25-9).
Useful EKG findings
Among patients in whom ACS or PE is suspected, 12-lead electrocardiography (EKG) should be performed.
AMI. EKG findings most predictive of AMI are new ST-segment elevation or depression > 1 mm (LR = 6-54), new left bundle branch block (LR = 6.3), Q wave (positive LR = 3.9), and prominent, wide-based (hyperacute) T wave (LR = 3.1).10
ACS. Useful EKG findings to predict ACS are ST-segment depression (LR = 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia, defined as ST-segment depression, T-wave inversion, or Q wave (LR = 3.6 [95% CI, 1.6-5.7]).10
PE. The most common abnormal finding on EKG in the setting of PE is sinus tachycardia.
Continue to: Right ventricular strain
Right ventricular strain. Other findings that reflect right ventricular strain, but are much less common, are complete or incomplete right bundle branch block, prominent S wave in lead I, Q wave in lead III, and T-wave inversion in lead III (S1Q3T3; the McGinn-White sign) and in leads V1-V4.8
The utility of troponin and high-sensitivity troponin testing
Clinical evaluation and EKG findings are unable to diagnose or exclude ACS without the use of the cardiac biomarker troponin. In the past decade, high-sensitivity troponin assays have been used to stratify patients at risk of ACS.11,12 Many protocols now exist using short interval (2-3 hours), high-sensitivity troponin testing to identify patients at low risk of myocardial infarction who can be safely discharged from the ED after 2 normal tests of the troponin level.13-16
An elevated troponin value alone, however, is not a specific indicator of ACS; troponin can be elevated in the settings of myocardial ischemia related to increased oxygen demand (Type 2 non-STEMI) and decreased renal clearance. Consideration of the rate of rising and falling levels of troponin, its absolute value > 99th percentile, and other findings is critical to interpreting an elevated troponin level.17 Studies in which the HEART score (History, Electrocardiography, Age, Risk factors, Troponin) was combined with high-sensitivity troponin measurement show that this pairing is promising in reducing unnecessary admissions for chest pain.18 (For a description of this tool, see the discussion of the HEART score that follows.) Carlton and colleagues18 showed that a HEART score ≤ 3 and a negative high-sensitivity troponin I level had a negative predictive value of ≥ 99.5% for AMI.
Clinical decision tools: Who needs care? Who can go home?
Given the varied presentations of patients with life-threatening causes of chest pain, it is challenging to confidently determine who is safe to send home after initial assessment. Guidance in 2014 from the American Heart Association and American College of Cardiology recommends risk-stratifying patients for ACS using clinical decision tools to help guide management.19,20 The American College of Physicians, in its 2015 guidelines, also recommends using a clinical decision tool to assess patients when there is suspicion of PE.21 Clinical application of these tools identifies patients at low risk of life-threatening conditions and can help avoid unnecessary intervention and a higher level of care.
Tools for investigating ACS
The Marburg Heart Score22 assesses the likelihood of CAD in ambulatory settings while the HEART score assesses the risk of major adverse cardiac events in ED patients.23 The Diamond Forrester criteria can be used to assess the pretest probability of CAD in both settings.24
Continue to: Marburg Heart Score
Marburg Heart Score. Validated in patients older than 35 years of age in 2 different outpatient populations in 201022 and 2012,25 the Marburg score is determined by answering 5 questions:
- Female ≥ 65 years? Or male ≥ 55 years of age? (No, 0; Yes, +1)
- Known CAD, cerebrovascular disease, or peripheral vascular disease? (No, 0; Yes, +1)
- Is pain worse with exercise? (No, 0; Yes, +1)
- Is pain reproducible with palpation? (No, +1, Yes, 0)
- Does the patient assume that the pain is cardiac in nature? (No, 0; Yes, +1)
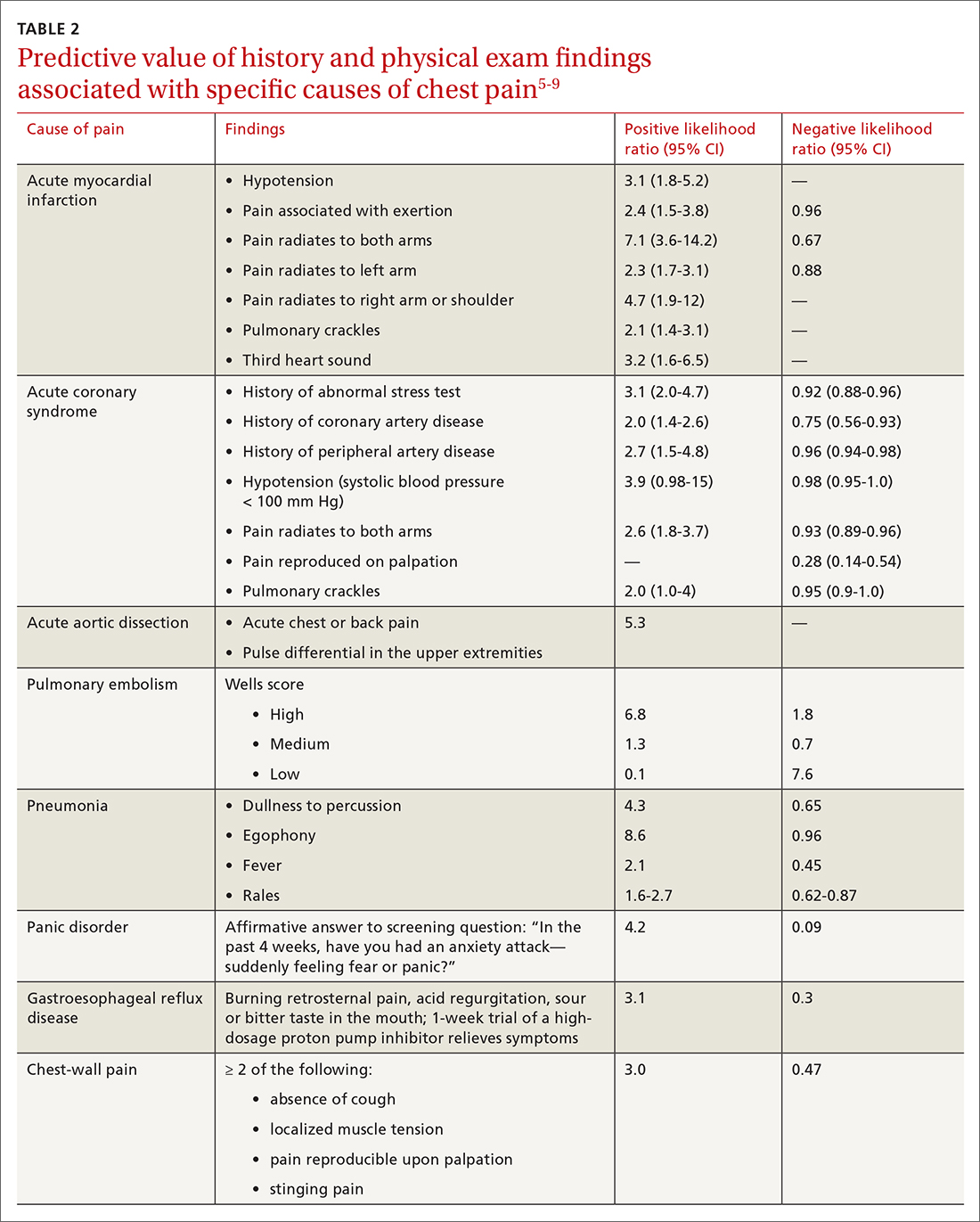
A Marburg Heart Score of 0 or 1 means CAD is highly unlikely in a patient with chest pain (negative predictive value = 99%-100%; positive predictive value = 0.6%)4 (TABLE 34,26-28). A score of ≤ 2 has a negative predictive value of 98%. A Marburg Heart Score of 4 or 5 has a relatively low positive predictive value (63%).4
This tool does not accurately diagnose acute MI, but it does help identify patients at low risk of ACS, thus reducing unnecessary subsequent testing. Although no clinical decision tool can rule out AMI with absolute certainty, the Marburg Heart Score is considered one of the most extensively tested and sensitive tools to predict low risk of CAD in outpatient primary care.29
INTERCHEST rule (in outpatient primary care) is a newer prediction rule using data from 5 primary care–based studies of chest pain.30 For a score ≤ 2, the negative predictive value for CAD causing chest pain is 97% to 98% and the positive predictive value is 43%. INTERCHEST incorporates studies used to validate the Marburg Heart Score, but has not been validated beyond initial pooled studies. Concerns have been raised about the quality of these pooled studies, however, and this rule has not been widely accepted for clinical use at this time.29
The HEART score has been validated in patients older than 12 years in multiple institutions and across multiple ED populations.23,31,32 It is widely used in the ED to assess a patient’s risk of major adverse cardiac events (MACE) over the next 6 weeks. MACE is defined as AMI, percutaneous coronary intervention, coronary artery bypass grafting, or death.
Continue to: The HEART score...
The HEART score is calculated based on 5 components:
- History of chest pain (slightly [0], moderately [+1], or highly [+2]) suspicious for ACS)
- EKG (normal [0], nonspecific ST changes [+1], significant ST deviations [+2])
- Age (< 45 y [0], 45-64 y [+1], ≥ 65 y [+2])
- Risk factors (none [0], 1 or 2 [+1], ≥ 3 or a history of atherosclerotic disease [+2]) a
- Initial troponin assay, standard sensitivity (≤ normal [0], 1-3× normal [+1], > 3× normal [+2]).
For patients with a HEART score of 0-3 (ie, at low risk), the pooled positive predictive value of a MACE was determined to be 0.19 (95% CI, 0.14-0.24), and the negative predictive value was 0.99 (95% CI, 0.98-0.99)—making it an effective tool to rule out a MACE over the short term26 (TABLE 34,26-28).
Because the HEART Score was published in 2008, multiple systematic reviews and meta-analyses have compared it to the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Events) scores for predicting short-term (30-day to 6-week) MACE in ED patients.27,28,33,34 These studies have all shown that the HEART score is relatively superior to the TIMI and GRACE tools.
Characteristics of these tools are summarized in TABLE 3.4,26-28
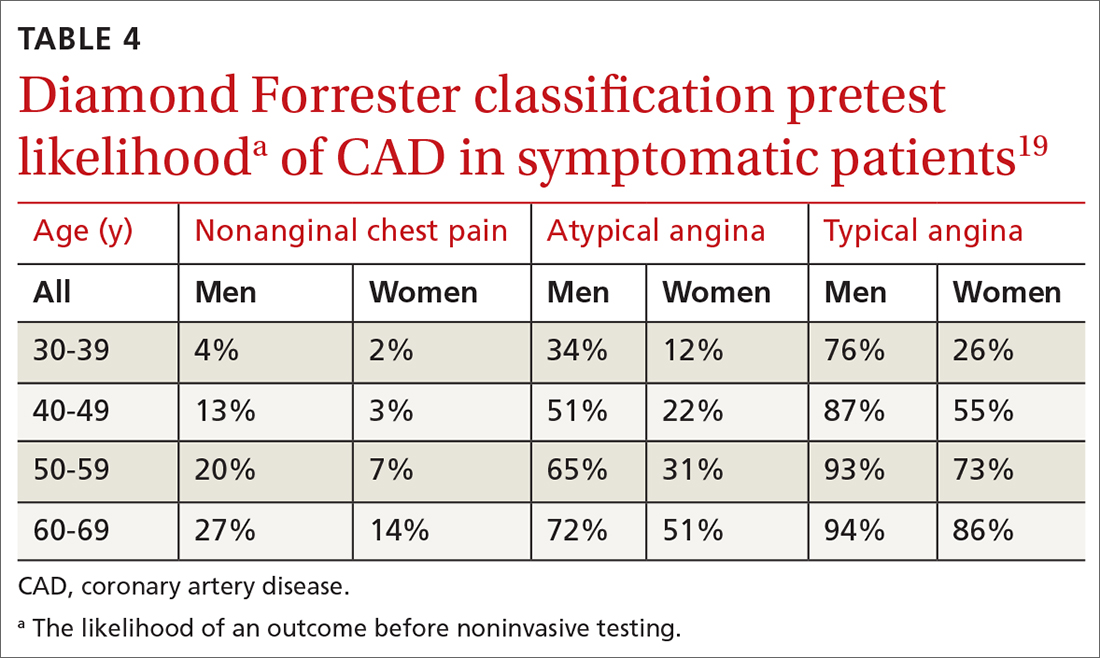
Diamond Forrester classification (in ED and outpatient settings). This tool uses 3 criteria—substernal chest pain, pain that increases upon exertion or with stress, and pain relieved by nitroglycerin or rest—to classify chest pain as typical angina (all 3 criteria), atypical angina (2 criteria), or noncardiac chest pain (0 criteria or 1 criterion).24 Pretest probability (ie, the likelihood of an outcome before noninvasive testing) of the pain being due to CAD can then be determined from the type of chest pain and the patient’s gender and age19 (TABLE 419). Recent studies have found that the Diamond Forrester criteria might overestimate the probability of CAD.35
Continue to: Noninvasive imaging-based diagnostic methods
Noninvasive imaging-based diagnostic methods
Positron-emission tomography stress testing, stress echocardiography, myocardial perfusion scanning, exercise treadmill testing. The first 3 of these imaging tests have a sensitivity and specificity ranging from 74% to 87%36; exercise treadmill testing is less sensitive (68%) and specific (77%).37
In a patient with a very low (< 5%) probability of CAD, a positive stress test (of any modality) is likely to be a false-positive; conversely, in a patient with a very high (> 90%) probability of CAD, a negative stress test is likely to be a false-negative.19 The American Heart Association, therefore, does not recommend any of these modalities for patients who have a < 5% or > 90% probability of CAD.19
Noninvasive testing to rule out ACS in low- and intermediate-risk patients who present to the ED with chest pain provides no clinical benefit over clinical evaluation alone.38 Therefore, these tests are rarely used in the initial evaluation of chest pain in an acute setting.
Coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA). These tests have demonstrated promise in the risk stratification of chest pain, given their high sensitivity and negative predictive value in low- and intermediate-risk patients.39,40 However, their application remains unclear in the evaluation of acute chest pain: Appropriate-use criteria do not favor CACS or CCTA alone to evaluate acute chest pain when there is suspicion of ACS.41 The Choosing Wisely initiative (for “avoiding unnecessary medical tests, treatments, and procedures”; www.choosingwisely.org) recommends against CCTA for high-risk patients presenting to the ED with acute chest pain.42
Cardiac magnetic resonance imaging does not have an established role in the evaluation of patients with suspected ACS.43
Continue to: Tools for investigating PE
Tools for investigating PE
Three clinical decision tools have been validated to predict the risk of PE: the Wells score, the Geneva score, and Pulmonary Embolism Rule Out Criteria (PERC).44,45
Wells score is more sensitive than the Geneva score and has been validated in ambulatory1 and ED46-48 settings. Based on Wells criteria, high-risk patients need further evaluation with imaging. In low-risk patients, a normal D-dimer level effectively excludes PE, with a < 1% risk of subsequent thromboembolism in the following 3 months. Positive predictive value of the Wells decision tool is low because it is intended to rule out, not confirm, PE.
PERC can be used in a low-probability setting (defined as the treating physician arriving at the conclusion that PE is not the most likely diagnosis and can be excluded with a negative D-dimer test). In that setting, if the patient meets the 8 clinical variables in PERC, the diagnosis of PE is, effectively, ruled out.48
Summing up: Evaluation of chest pain guided by risk of CAD
Patients who present in an outpatient setting with a potentially life-threatening cause of chest pain (TABLE 1) and patients with unstable vital signs should be sent to the ED for urgent evaluation. In the remaining outpatients, use the Marburg Heart Score or Diamond Forrester classification to assess the likelihood that pain is due to CAD (in the ED, the HEART score can be used for this purpose) (FIGURE).
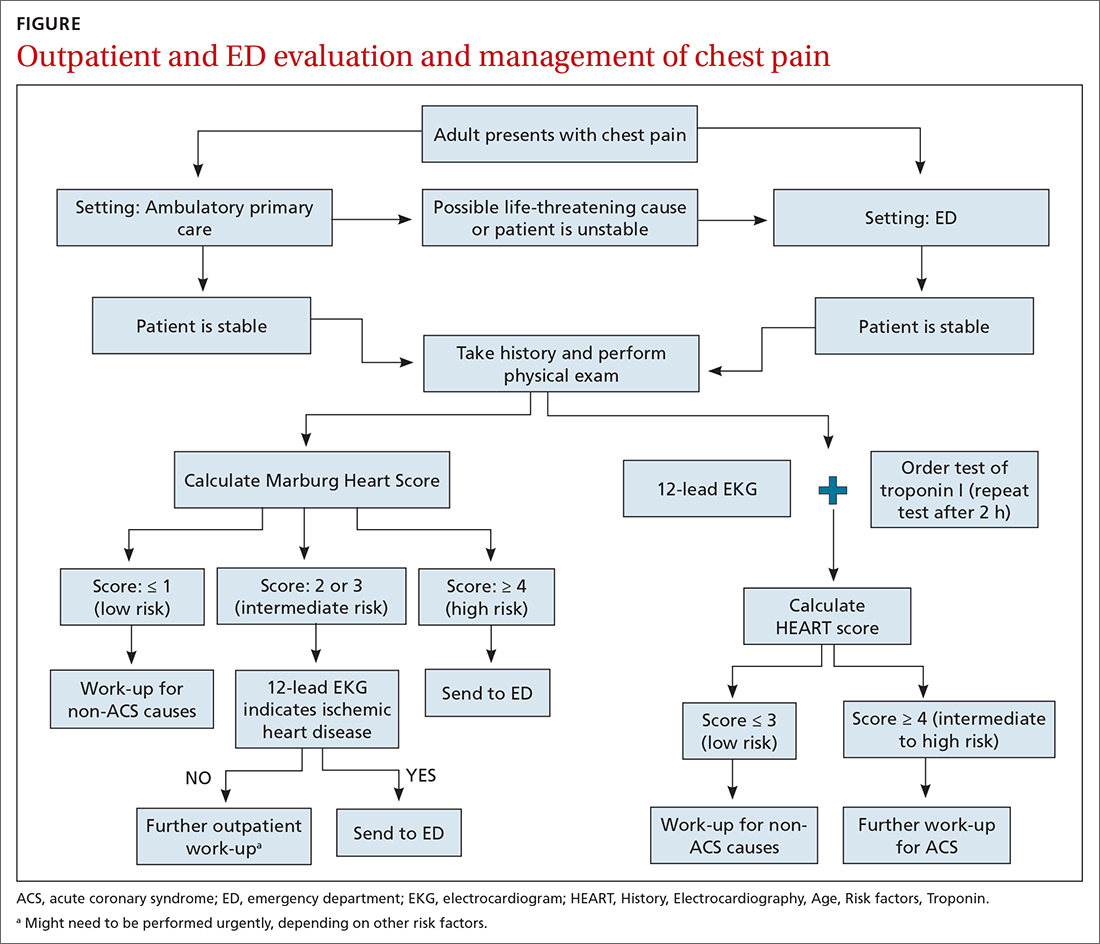
When the risk is low. No further cardiac testing is indicated in patients with a risk of CAD < 5%, based on a Marburg score of 0 or 1, or on Diamond Forrester criteria; an abnormal stress test is likely to be a false-positive.19
Continue to: Moderate risk
Moderate risk. However, further testing is indicated, with a stress test (with or without myocardial imaging), in patients whose risk of CAD is 5% to 70%, based on the Diamond Forrester classification or an intermediate Marburg Heart Score (ie, a score of 2 or 3 but a normal EKG). This further testing can be performed urgently in patients who have multiple other risk factors that are not assessed by the Marburg Heart Score.
High risk. In patients whose risk is > 70%, invasive testing with angiography should be considered.35,49
EKG abnormalities. Patients with a Marburg Score of 2 or 3 and an abnormal EKG should be sent to the ED (FIGURE). There, patients with a HEART score < 4 and a negative 2-3–hour troponin test have a < 1% chance of ACS and can be safely discharged.31
CORRESPONDENCE
Anne Mounsey, MD, UNC Family Medicine, 590 Manning Drive, Chapel Hill, NC 27599; [email protected]
1. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36:1-12. doi: 10.1016/j.ccl.2017.08.001
2. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed February 16, 2021. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
3. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029-1032. doi: 10.1001/jamainternmed.2016.2498
4. Ebell MH. Evaluation of chest pain in primary care patients. Am Fam Physician. 2011;83:603-605.
5. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547-564. doi: 10.1161/CIRCULATIONAHA.116.021886
6. Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome? The rational clinical examination systematic review. JAMA. 2015;314:1955-1965. doi: 10.1001/jama.2015.12735
7. Kolminsky J, Choxi R, Mahmoud AR, et al. Familial hypercholesterolemia: cardiovascular risk stratification and clinical management. American College of Cardiology. June 1, 2020. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2020/06/01/13/54/familial-hypercholesterolemia
8. Konstantinides SV, Meyer G, Becattini C, et al; . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
9. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-182.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877. doi: 10.1056/NEJMoa0903515
12. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867. doi: 10.1056/NEJMoa0900428
13. Tada M, Azuma H, Yamada N, et al. A comprehensive validation of very early rule-out strategies for non-ST-segment elevation myocardial infarction in emergency departments: protocol for a multicentre prospective cohort study. BMJ Open. 2019;9:e026985. doi: 10.1136/bmjopen-2018-026985
14. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-1218. doi: 10.1001/archinternmed.2012.3698
15. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481-2488. doi: 10.1016/S0140-6736(15)00391-8
16. Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913-1924. doi: 10.1001/jama.2017.17488
17. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology. July 16, 2018. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-in-the-evaluation-of-possible-am
18. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66:635-645.e1. doi: 10.1016/j.annemergmed.2015.07.006
19. Qaseem A, Fihn SD, Williams S, et al; . Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/Preventative Cardiovascular nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157:729-734. doi: 10.7326/0003-4819-157-10-201211200-00010
20. Amsterdam EA, Wenger NK, Brindis RG, et al; . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-2394. doi: 10.1161/CIR.0000000000000133
21. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163:701-711. doi: 10.7326/M14-1772
22. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300. doi: 10.1503/cmaj.100212
23. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196. doi: 10.1007/BF03086144
24. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350-1358. doi: 10.1056/NEJM197906143002402
25. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e21. doi: 10.3399/bjgp12X649106
26. Laureano-Phillips J, Robinson RD, Aryal S, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2019;74:187-203. doi: 10.1016/j.annemergmed.2018.12.010
27. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26:140-151. doi: 10.1111/acem.13649
28. Sakamoto JT, Liu N, Koh ZX, et al. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016;221:759-764. doi: 10.1016/j.ijcard.2016.07.147
29. Harskamp RE, Laeven SC, Himmelreich JCL, et al. Chest pain in general practice: a systematic review of prediction rules. BMJ Open. 2019;9:e027081. doi: 10.1136/bmjopen-2018-027081
30. Aerts M, Minalu G, Bösner S, et al. Internal Working Group on Chest Pain in Primary Care (INTERCHEST). Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J. Clin Epidemiol. 2017;81:120-128. doi: 10.1016/j.jclinepi.2016.09.011
31. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients in the emergency department. Int J Cardiol. 2013;168:2153-2158. doi: 10.1016/j.ijcard.2013.01.255
32. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164-169. doi: 10.1097/HPC.0b013e3181ec36d8
33. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661. doi: 10.1016/j.ijcard.2016.10.080
34. Reaney PDW, Elliott HI, Noman A, et al. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018;35:420-427. doi: 10.1136/emermed-2017-207172
35. Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond Forrester score: The Partners Registry. Circulation. 2016;134:201-211. doi: 10.1161/CIRCULATIONAHA.116.023396
36. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427-437. doi: 10.2147/VHRM.S106838
37. Borque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. doi: 10.1016/j.jcmg.2015.09.006
38. Reinhardt SW, Lin C-J, Novak E, et al. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178:212-219. doi: 10.1001/jamainternmed.2017.7360
39. Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, et al. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17-23. doi: 10.1016/j.amjcard.2010.08.037
40. Linde JJ, H, Hansen TF, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J AM Coll Cardiol 2020;75:453-463. doi: 10.1016/j.jacc.2019.12.012
41. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525-e555. doi: 10.1161/CIR.0b013e3181fcae66
42. Society of Cardiovascular Computed Tomography. Five things physicians and patients should question. Choosing Wisely Campaign. February 21, 2013. Accessed September 28, 2021. www.choosingwisely.org/wp-content/uploads/2015/02/SCCT-Choosing-Wisely-List.pdf
43. Hamm CW, Bassand J-P, Agewall S, et al; . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. doi: 10.1093/eurheartj/ehr236
44. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
45. Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. doi: 10.1111/j.1538-7836.2010.03801.x
46. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in the emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255. doi: 10.1111/j.1538-7836.2004.00790.x
47. Hendriksen JMT, Geersing G-J, Lucassen WAM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438. doi: 10.1136/bmj.h4438
48. Shen J-H, Chen H-L, Chen J-R, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482-492. doi: 10.1007/s11239-015-1250-2
49. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventative Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. doi: 10.1016/j.jacc.2012.07.013
One of the most concerning and challenging patient complaints presented to physicians is chest pain. Chest pain is a ubiquitous complaint in primary care settings and in the emergency department (ED), accounting for 8 million ED visits and 0.4% of all primary care visits in North America annually.1,2
Despite the great number of chest-pain encounters, early identification of life-threatening causes and prompt treatment remain a challenge. In this article, we examine how the approach to a complaint of chest pain in a primary care practice (and, likewise, in the ED) must first, rest on the clinical evaluation and second, employ risk-stratification tools to aid in evaluation, appropriate diagnosis, triage, and treatment.
Chest pain by the numbers
Acute coronary syndrome (ACS) is the cause of chest pain in 5.1% of patients with chest pain who present to the ED, compared with 1.5% to 3.1% of chest-pain patients seen in ambulatory care.1,3 “Nonspecific chest pain” is the most frequent diagnosis of chest pain in the ED for all age groups (47.5% to 55.8%).3 In contrast, the most common cause of chest pain in primary care is musculoskeletal (36%), followed by gastrointestinal disease (18% to 19%); serious cardiac causes (15%), including ACS (1.5%); nonspecific causes (16%); psychiatric causes (8%); and pulmonary causes (5% to 10%).4 Among patients seen in the ED because of chest pain, 57.4% are discharged, 30.6% are admitted for further evaluation, and 0.4% die in the ED or after admission.3

First challenge: The scale of the differential Dx
The differential diagnosis of chest pain is broad. It includes life-threatening causes, such as ACS (from ST-segment elevation myocardial infarction [STEMI], Type 1 non-STEMI, and unstable angina), acute aortic dissection, pulmonary embolism (PE), esophageal rupture, and tension pneumothorax, as well as non-life-threatening causes (TABLE 1).

History and physical exam guide early decisions
Triage assessment of the patient with chest pain, including vital signs, general appearance, and basic symptom questions, can guide you as to whether they require transfer to a higher level of care. Although an individual’s findings cannot, alone, accurately exclude or diagnose ACS, the findings can be used in combination in clinical decision tools to distinguish noncardiac chest pain from ACS.

History. Features in the history (TABLE 25-9) that are most helpful at increasing the probability (ie, a positive likelihood ratio [LR] ≥ 2) of chest pain being caused by ACS are:
- pain radiating to both arms or the right arm
- pain that is worse upon exertion
- a history of peripheral artery disease or coronary artery disease (CAD)
- a previously abnormal stress test.

The presence of any prior normal stress test is unhelpful: Such patients have a similar risk of a 30-day adverse cardiac event as a patient who has never had a stress test.5
Continue to: A history of tobacco use...
A history of tobacco use, hyperlipidemia, hypertension, obesity, acute myocardial infarction (AMI), coronary artery bypass grafting, or a family history of CAD does not significantly increase the risk of ACS.6 However, exploring each of these risk factors further is important, because genetic links between these risk factors can lead to an increased risk of CAD (eg, familial hypercholesterolemia).7
A history of normal or near-normal coronary angiography (< 25% stenosis) is associated with a lower likelihood of ACS, because 98% of such patients are free of AMI and 90% are without single-vessel coronary disease nearly 10 years out.6 A history of coronary artery bypass grafting is not necessarily predictive of ACS (LR = 1-3).5,6
Historical features classically associated with ACS, but that have an LR < 2, are pain radiating to the neck or jaw, nausea or vomiting, dyspnea, and pain that is relieved with nitroglycerin.5,6 Pain described as pleuritic, sharp, positional, or reproduced with palpation is less likely due to AMI.5
Physical exam findings are not independently diagnostic when evaluating chest pain. However, a third heart sound is the most likely finding associated with AMI and hypotension is the clinical sign most likely associated with ACS.5
Consider the diagnosis of PE in all patients with chest pain. In PE, chest pain might be associated with dyspnea, presyncope, syncope, or hemoptysis.8 On examination, 40% of patients have tachycardia.8 If PE is suspected; the patient should be risk-stratified using a validated prediction rule (see the discussion of PE that follows).
Continue to: Other historical features...
Other historical features or physical exam findings correlate with aortic dissection, pneumonia, and psychiatric causes of chest pain (TABLE 25-9).
Useful EKG findings
Among patients in whom ACS or PE is suspected, 12-lead electrocardiography (EKG) should be performed.
AMI. EKG findings most predictive of AMI are new ST-segment elevation or depression > 1 mm (LR = 6-54), new left bundle branch block (LR = 6.3), Q wave (positive LR = 3.9), and prominent, wide-based (hyperacute) T wave (LR = 3.1).10
ACS. Useful EKG findings to predict ACS are ST-segment depression (LR = 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia, defined as ST-segment depression, T-wave inversion, or Q wave (LR = 3.6 [95% CI, 1.6-5.7]).10
PE. The most common abnormal finding on EKG in the setting of PE is sinus tachycardia.
Continue to: Right ventricular strain
Right ventricular strain. Other findings that reflect right ventricular strain, but are much less common, are complete or incomplete right bundle branch block, prominent S wave in lead I, Q wave in lead III, and T-wave inversion in lead III (S1Q3T3; the McGinn-White sign) and in leads V1-V4.8
The utility of troponin and high-sensitivity troponin testing
Clinical evaluation and EKG findings are unable to diagnose or exclude ACS without the use of the cardiac biomarker troponin. In the past decade, high-sensitivity troponin assays have been used to stratify patients at risk of ACS.11,12 Many protocols now exist using short interval (2-3 hours), high-sensitivity troponin testing to identify patients at low risk of myocardial infarction who can be safely discharged from the ED after 2 normal tests of the troponin level.13-16
An elevated troponin value alone, however, is not a specific indicator of ACS; troponin can be elevated in the settings of myocardial ischemia related to increased oxygen demand (Type 2 non-STEMI) and decreased renal clearance. Consideration of the rate of rising and falling levels of troponin, its absolute value > 99th percentile, and other findings is critical to interpreting an elevated troponin level.17 Studies in which the HEART score (History, Electrocardiography, Age, Risk factors, Troponin) was combined with high-sensitivity troponin measurement show that this pairing is promising in reducing unnecessary admissions for chest pain.18 (For a description of this tool, see the discussion of the HEART score that follows.) Carlton and colleagues18 showed that a HEART score ≤ 3 and a negative high-sensitivity troponin I level had a negative predictive value of ≥ 99.5% for AMI.
Clinical decision tools: Who needs care? Who can go home?
Given the varied presentations of patients with life-threatening causes of chest pain, it is challenging to confidently determine who is safe to send home after initial assessment. Guidance in 2014 from the American Heart Association and American College of Cardiology recommends risk-stratifying patients for ACS using clinical decision tools to help guide management.19,20 The American College of Physicians, in its 2015 guidelines, also recommends using a clinical decision tool to assess patients when there is suspicion of PE.21 Clinical application of these tools identifies patients at low risk of life-threatening conditions and can help avoid unnecessary intervention and a higher level of care.
Tools for investigating ACS
The Marburg Heart Score22 assesses the likelihood of CAD in ambulatory settings while the HEART score assesses the risk of major adverse cardiac events in ED patients.23 The Diamond Forrester criteria can be used to assess the pretest probability of CAD in both settings.24
Continue to: Marburg Heart Score
Marburg Heart Score. Validated in patients older than 35 years of age in 2 different outpatient populations in 201022 and 2012,25 the Marburg score is determined by answering 5 questions:
- Female ≥ 65 years? Or male ≥ 55 years of age? (No, 0; Yes, +1)
- Known CAD, cerebrovascular disease, or peripheral vascular disease? (No, 0; Yes, +1)
- Is pain worse with exercise? (No, 0; Yes, +1)
- Is pain reproducible with palpation? (No, +1, Yes, 0)
- Does the patient assume that the pain is cardiac in nature? (No, 0; Yes, +1)
A Marburg Heart Score of 0 or 1 means CAD is highly unlikely in a patient with chest pain (negative predictive value = 99%-100%; positive predictive value = 0.6%)4 (TABLE 34,26-28). A score of ≤ 2 has a negative predictive value of 98%. A Marburg Heart Score of 4 or 5 has a relatively low positive predictive value (63%).4

This tool does not accurately diagnose acute MI, but it does help identify patients at low risk of ACS, thus reducing unnecessary subsequent testing. Although no clinical decision tool can rule out AMI with absolute certainty, the Marburg Heart Score is considered one of the most extensively tested and sensitive tools to predict low risk of CAD in outpatient primary care.29
INTERCHEST rule (in outpatient primary care) is a newer prediction rule using data from 5 primary care–based studies of chest pain.30 For a score ≤ 2, the negative predictive value for CAD causing chest pain is 97% to 98% and the positive predictive value is 43%. INTERCHEST incorporates studies used to validate the Marburg Heart Score, but has not been validated beyond initial pooled studies. Concerns have been raised about the quality of these pooled studies, however, and this rule has not been widely accepted for clinical use at this time.29
The HEART score has been validated in patients older than 12 years in multiple institutions and across multiple ED populations.23,31,32 It is widely used in the ED to assess a patient’s risk of major adverse cardiac events (MACE) over the next 6 weeks. MACE is defined as AMI, percutaneous coronary intervention, coronary artery bypass grafting, or death.
Continue to: The HEART score...
The HEART score is calculated based on 5 components:
- History of chest pain (slightly [0], moderately [+1], or highly [+2]) suspicious for ACS)
- EKG (normal [0], nonspecific ST changes [+1], significant ST deviations [+2])
- Age (< 45 y [0], 45-64 y [+1], ≥ 65 y [+2])
- Risk factors (none [0], 1 or 2 [+1], ≥ 3 or a history of atherosclerotic disease [+2]) a
- Initial troponin assay, standard sensitivity (≤ normal [0], 1-3× normal [+1], > 3× normal [+2]).
For patients with a HEART score of 0-3 (ie, at low risk), the pooled positive predictive value of a MACE was determined to be 0.19 (95% CI, 0.14-0.24), and the negative predictive value was 0.99 (95% CI, 0.98-0.99)—making it an effective tool to rule out a MACE over the short term26 (TABLE 34,26-28).
Because the HEART Score was published in 2008, multiple systematic reviews and meta-analyses have compared it to the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Events) scores for predicting short-term (30-day to 6-week) MACE in ED patients.27,28,33,34 These studies have all shown that the HEART score is relatively superior to the TIMI and GRACE tools.
Characteristics of these tools are summarized in TABLE 3.4,26-28
Diamond Forrester classification (in ED and outpatient settings). This tool uses 3 criteria—substernal chest pain, pain that increases upon exertion or with stress, and pain relieved by nitroglycerin or rest—to classify chest pain as typical angina (all 3 criteria), atypical angina (2 criteria), or noncardiac chest pain (0 criteria or 1 criterion).24 Pretest probability (ie, the likelihood of an outcome before noninvasive testing) of the pain being due to CAD can then be determined from the type of chest pain and the patient’s gender and age19 (TABLE 419). Recent studies have found that the Diamond Forrester criteria might overestimate the probability of CAD.35

Continue to: Noninvasive imaging-based diagnostic methods
Noninvasive imaging-based diagnostic methods
Positron-emission tomography stress testing, stress echocardiography, myocardial perfusion scanning, exercise treadmill testing. The first 3 of these imaging tests have a sensitivity and specificity ranging from 74% to 87%36; exercise treadmill testing is less sensitive (68%) and specific (77%).37
In a patient with a very low (< 5%) probability of CAD, a positive stress test (of any modality) is likely to be a false-positive; conversely, in a patient with a very high (> 90%) probability of CAD, a negative stress test is likely to be a false-negative.19 The American Heart Association, therefore, does not recommend any of these modalities for patients who have a < 5% or > 90% probability of CAD.19
Noninvasive testing to rule out ACS in low- and intermediate-risk patients who present to the ED with chest pain provides no clinical benefit over clinical evaluation alone.38 Therefore, these tests are rarely used in the initial evaluation of chest pain in an acute setting.
Coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA). These tests have demonstrated promise in the risk stratification of chest pain, given their high sensitivity and negative predictive value in low- and intermediate-risk patients.39,40 However, their application remains unclear in the evaluation of acute chest pain: Appropriate-use criteria do not favor CACS or CCTA alone to evaluate acute chest pain when there is suspicion of ACS.41 The Choosing Wisely initiative (for “avoiding unnecessary medical tests, treatments, and procedures”; www.choosingwisely.org) recommends against CCTA for high-risk patients presenting to the ED with acute chest pain.42
Cardiac magnetic resonance imaging does not have an established role in the evaluation of patients with suspected ACS.43
Continue to: Tools for investigating PE
Tools for investigating PE
Three clinical decision tools have been validated to predict the risk of PE: the Wells score, the Geneva score, and Pulmonary Embolism Rule Out Criteria (PERC).44,45
Wells score is more sensitive than the Geneva score and has been validated in ambulatory1 and ED46-48 settings. Based on Wells criteria, high-risk patients need further evaluation with imaging. In low-risk patients, a normal D-dimer level effectively excludes PE, with a < 1% risk of subsequent thromboembolism in the following 3 months. Positive predictive value of the Wells decision tool is low because it is intended to rule out, not confirm, PE.
PERC can be used in a low-probability setting (defined as the treating physician arriving at the conclusion that PE is not the most likely diagnosis and can be excluded with a negative D-dimer test). In that setting, if the patient meets the 8 clinical variables in PERC, the diagnosis of PE is, effectively, ruled out.48
Summing up: Evaluation of chest pain guided by risk of CAD
Patients who present in an outpatient setting with a potentially life-threatening cause of chest pain (TABLE 1) and patients with unstable vital signs should be sent to the ED for urgent evaluation. In the remaining outpatients, use the Marburg Heart Score or Diamond Forrester classification to assess the likelihood that pain is due to CAD (in the ED, the HEART score can be used for this purpose) (FIGURE).

When the risk is low. No further cardiac testing is indicated in patients with a risk of CAD < 5%, based on a Marburg score of 0 or 1, or on Diamond Forrester criteria; an abnormal stress test is likely to be a false-positive.19
Continue to: Moderate risk
Moderate risk. However, further testing is indicated, with a stress test (with or without myocardial imaging), in patients whose risk of CAD is 5% to 70%, based on the Diamond Forrester classification or an intermediate Marburg Heart Score (ie, a score of 2 or 3 but a normal EKG). This further testing can be performed urgently in patients who have multiple other risk factors that are not assessed by the Marburg Heart Score.
High risk. In patients whose risk is > 70%, invasive testing with angiography should be considered.35,49
EKG abnormalities. Patients with a Marburg Score of 2 or 3 and an abnormal EKG should be sent to the ED (FIGURE). There, patients with a HEART score < 4 and a negative 2-3–hour troponin test have a < 1% chance of ACS and can be safely discharged.31
CORRESPONDENCE
Anne Mounsey, MD, UNC Family Medicine, 590 Manning Drive, Chapel Hill, NC 27599; [email protected]
One of the most concerning and challenging patient complaints presented to physicians is chest pain. Chest pain is a ubiquitous complaint in primary care settings and in the emergency department (ED), accounting for 8 million ED visits and 0.4% of all primary care visits in North America annually.1,2
Despite the great number of chest-pain encounters, early identification of life-threatening causes and prompt treatment remain a challenge. In this article, we examine how the approach to a complaint of chest pain in a primary care practice (and, likewise, in the ED) must first, rest on the clinical evaluation and second, employ risk-stratification tools to aid in evaluation, appropriate diagnosis, triage, and treatment.
Chest pain by the numbers
Acute coronary syndrome (ACS) is the cause of chest pain in 5.1% of patients with chest pain who present to the ED, compared with 1.5% to 3.1% of chest-pain patients seen in ambulatory care.1,3 “Nonspecific chest pain” is the most frequent diagnosis of chest pain in the ED for all age groups (47.5% to 55.8%).3 In contrast, the most common cause of chest pain in primary care is musculoskeletal (36%), followed by gastrointestinal disease (18% to 19%); serious cardiac causes (15%), including ACS (1.5%); nonspecific causes (16%); psychiatric causes (8%); and pulmonary causes (5% to 10%).4 Among patients seen in the ED because of chest pain, 57.4% are discharged, 30.6% are admitted for further evaluation, and 0.4% die in the ED or after admission.3

First challenge: The scale of the differential Dx
The differential diagnosis of chest pain is broad. It includes life-threatening causes, such as ACS (from ST-segment elevation myocardial infarction [STEMI], Type 1 non-STEMI, and unstable angina), acute aortic dissection, pulmonary embolism (PE), esophageal rupture, and tension pneumothorax, as well as non-life-threatening causes (TABLE 1).

History and physical exam guide early decisions
Triage assessment of the patient with chest pain, including vital signs, general appearance, and basic symptom questions, can guide you as to whether they require transfer to a higher level of care. Although an individual’s findings cannot, alone, accurately exclude or diagnose ACS, the findings can be used in combination in clinical decision tools to distinguish noncardiac chest pain from ACS.

History. Features in the history (TABLE 25-9) that are most helpful at increasing the probability (ie, a positive likelihood ratio [LR] ≥ 2) of chest pain being caused by ACS are:
- pain radiating to both arms or the right arm
- pain that is worse upon exertion
- a history of peripheral artery disease or coronary artery disease (CAD)
- a previously abnormal stress test.

The presence of any prior normal stress test is unhelpful: Such patients have a similar risk of a 30-day adverse cardiac event as a patient who has never had a stress test.5
Continue to: A history of tobacco use...
A history of tobacco use, hyperlipidemia, hypertension, obesity, acute myocardial infarction (AMI), coronary artery bypass grafting, or a family history of CAD does not significantly increase the risk of ACS.6 However, exploring each of these risk factors further is important, because genetic links between these risk factors can lead to an increased risk of CAD (eg, familial hypercholesterolemia).7
A history of normal or near-normal coronary angiography (< 25% stenosis) is associated with a lower likelihood of ACS, because 98% of such patients are free of AMI and 90% are without single-vessel coronary disease nearly 10 years out.6 A history of coronary artery bypass grafting is not necessarily predictive of ACS (LR = 1-3).5,6
Historical features classically associated with ACS, but that have an LR < 2, are pain radiating to the neck or jaw, nausea or vomiting, dyspnea, and pain that is relieved with nitroglycerin.5,6 Pain described as pleuritic, sharp, positional, or reproduced with palpation is less likely due to AMI.5
Physical exam findings are not independently diagnostic when evaluating chest pain. However, a third heart sound is the most likely finding associated with AMI and hypotension is the clinical sign most likely associated with ACS.5
Consider the diagnosis of PE in all patients with chest pain. In PE, chest pain might be associated with dyspnea, presyncope, syncope, or hemoptysis.8 On examination, 40% of patients have tachycardia.8 If PE is suspected; the patient should be risk-stratified using a validated prediction rule (see the discussion of PE that follows).
Continue to: Other historical features...
Other historical features or physical exam findings correlate with aortic dissection, pneumonia, and psychiatric causes of chest pain (TABLE 25-9).
Useful EKG findings
Among patients in whom ACS or PE is suspected, 12-lead electrocardiography (EKG) should be performed.
AMI. EKG findings most predictive of AMI are new ST-segment elevation or depression > 1 mm (LR = 6-54), new left bundle branch block (LR = 6.3), Q wave (positive LR = 3.9), and prominent, wide-based (hyperacute) T wave (LR = 3.1).10
ACS. Useful EKG findings to predict ACS are ST-segment depression (LR = 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia, defined as ST-segment depression, T-wave inversion, or Q wave (LR = 3.6 [95% CI, 1.6-5.7]).10
PE. The most common abnormal finding on EKG in the setting of PE is sinus tachycardia.
Continue to: Right ventricular strain
Right ventricular strain. Other findings that reflect right ventricular strain, but are much less common, are complete or incomplete right bundle branch block, prominent S wave in lead I, Q wave in lead III, and T-wave inversion in lead III (S1Q3T3; the McGinn-White sign) and in leads V1-V4.8
The utility of troponin and high-sensitivity troponin testing
Clinical evaluation and EKG findings are unable to diagnose or exclude ACS without the use of the cardiac biomarker troponin. In the past decade, high-sensitivity troponin assays have been used to stratify patients at risk of ACS.11,12 Many protocols now exist using short interval (2-3 hours), high-sensitivity troponin testing to identify patients at low risk of myocardial infarction who can be safely discharged from the ED after 2 normal tests of the troponin level.13-16
An elevated troponin value alone, however, is not a specific indicator of ACS; troponin can be elevated in the settings of myocardial ischemia related to increased oxygen demand (Type 2 non-STEMI) and decreased renal clearance. Consideration of the rate of rising and falling levels of troponin, its absolute value > 99th percentile, and other findings is critical to interpreting an elevated troponin level.17 Studies in which the HEART score (History, Electrocardiography, Age, Risk factors, Troponin) was combined with high-sensitivity troponin measurement show that this pairing is promising in reducing unnecessary admissions for chest pain.18 (For a description of this tool, see the discussion of the HEART score that follows.) Carlton and colleagues18 showed that a HEART score ≤ 3 and a negative high-sensitivity troponin I level had a negative predictive value of ≥ 99.5% for AMI.
Clinical decision tools: Who needs care? Who can go home?
Given the varied presentations of patients with life-threatening causes of chest pain, it is challenging to confidently determine who is safe to send home after initial assessment. Guidance in 2014 from the American Heart Association and American College of Cardiology recommends risk-stratifying patients for ACS using clinical decision tools to help guide management.19,20 The American College of Physicians, in its 2015 guidelines, also recommends using a clinical decision tool to assess patients when there is suspicion of PE.21 Clinical application of these tools identifies patients at low risk of life-threatening conditions and can help avoid unnecessary intervention and a higher level of care.
Tools for investigating ACS
The Marburg Heart Score22 assesses the likelihood of CAD in ambulatory settings while the HEART score assesses the risk of major adverse cardiac events in ED patients.23 The Diamond Forrester criteria can be used to assess the pretest probability of CAD in both settings.24
Continue to: Marburg Heart Score
Marburg Heart Score. Validated in patients older than 35 years of age in 2 different outpatient populations in 201022 and 2012,25 the Marburg score is determined by answering 5 questions:
- Female ≥ 65 years? Or male ≥ 55 years of age? (No, 0; Yes, +1)
- Known CAD, cerebrovascular disease, or peripheral vascular disease? (No, 0; Yes, +1)
- Is pain worse with exercise? (No, 0; Yes, +1)
- Is pain reproducible with palpation? (No, +1, Yes, 0)
- Does the patient assume that the pain is cardiac in nature? (No, 0; Yes, +1)
A Marburg Heart Score of 0 or 1 means CAD is highly unlikely in a patient with chest pain (negative predictive value = 99%-100%; positive predictive value = 0.6%)4 (TABLE 34,26-28). A score of ≤ 2 has a negative predictive value of 98%. A Marburg Heart Score of 4 or 5 has a relatively low positive predictive value (63%).4

This tool does not accurately diagnose acute MI, but it does help identify patients at low risk of ACS, thus reducing unnecessary subsequent testing. Although no clinical decision tool can rule out AMI with absolute certainty, the Marburg Heart Score is considered one of the most extensively tested and sensitive tools to predict low risk of CAD in outpatient primary care.29
INTERCHEST rule (in outpatient primary care) is a newer prediction rule using data from 5 primary care–based studies of chest pain.30 For a score ≤ 2, the negative predictive value for CAD causing chest pain is 97% to 98% and the positive predictive value is 43%. INTERCHEST incorporates studies used to validate the Marburg Heart Score, but has not been validated beyond initial pooled studies. Concerns have been raised about the quality of these pooled studies, however, and this rule has not been widely accepted for clinical use at this time.29
The HEART score has been validated in patients older than 12 years in multiple institutions and across multiple ED populations.23,31,32 It is widely used in the ED to assess a patient’s risk of major adverse cardiac events (MACE) over the next 6 weeks. MACE is defined as AMI, percutaneous coronary intervention, coronary artery bypass grafting, or death.
Continue to: The HEART score...
The HEART score is calculated based on 5 components:
- History of chest pain (slightly [0], moderately [+1], or highly [+2]) suspicious for ACS)
- EKG (normal [0], nonspecific ST changes [+1], significant ST deviations [+2])
- Age (< 45 y [0], 45-64 y [+1], ≥ 65 y [+2])
- Risk factors (none [0], 1 or 2 [+1], ≥ 3 or a history of atherosclerotic disease [+2]) a
- Initial troponin assay, standard sensitivity (≤ normal [0], 1-3× normal [+1], > 3× normal [+2]).
For patients with a HEART score of 0-3 (ie, at low risk), the pooled positive predictive value of a MACE was determined to be 0.19 (95% CI, 0.14-0.24), and the negative predictive value was 0.99 (95% CI, 0.98-0.99)—making it an effective tool to rule out a MACE over the short term26 (TABLE 34,26-28).
Because the HEART Score was published in 2008, multiple systematic reviews and meta-analyses have compared it to the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Events) scores for predicting short-term (30-day to 6-week) MACE in ED patients.27,28,33,34 These studies have all shown that the HEART score is relatively superior to the TIMI and GRACE tools.
Characteristics of these tools are summarized in TABLE 3.4,26-28
Diamond Forrester classification (in ED and outpatient settings). This tool uses 3 criteria—substernal chest pain, pain that increases upon exertion or with stress, and pain relieved by nitroglycerin or rest—to classify chest pain as typical angina (all 3 criteria), atypical angina (2 criteria), or noncardiac chest pain (0 criteria or 1 criterion).24 Pretest probability (ie, the likelihood of an outcome before noninvasive testing) of the pain being due to CAD can then be determined from the type of chest pain and the patient’s gender and age19 (TABLE 419). Recent studies have found that the Diamond Forrester criteria might overestimate the probability of CAD.35

Continue to: Noninvasive imaging-based diagnostic methods
Noninvasive imaging-based diagnostic methods
Positron-emission tomography stress testing, stress echocardiography, myocardial perfusion scanning, exercise treadmill testing. The first 3 of these imaging tests have a sensitivity and specificity ranging from 74% to 87%36; exercise treadmill testing is less sensitive (68%) and specific (77%).37
In a patient with a very low (< 5%) probability of CAD, a positive stress test (of any modality) is likely to be a false-positive; conversely, in a patient with a very high (> 90%) probability of CAD, a negative stress test is likely to be a false-negative.19 The American Heart Association, therefore, does not recommend any of these modalities for patients who have a < 5% or > 90% probability of CAD.19
Noninvasive testing to rule out ACS in low- and intermediate-risk patients who present to the ED with chest pain provides no clinical benefit over clinical evaluation alone.38 Therefore, these tests are rarely used in the initial evaluation of chest pain in an acute setting.
Coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA). These tests have demonstrated promise in the risk stratification of chest pain, given their high sensitivity and negative predictive value in low- and intermediate-risk patients.39,40 However, their application remains unclear in the evaluation of acute chest pain: Appropriate-use criteria do not favor CACS or CCTA alone to evaluate acute chest pain when there is suspicion of ACS.41 The Choosing Wisely initiative (for “avoiding unnecessary medical tests, treatments, and procedures”; www.choosingwisely.org) recommends against CCTA for high-risk patients presenting to the ED with acute chest pain.42
Cardiac magnetic resonance imaging does not have an established role in the evaluation of patients with suspected ACS.43
Continue to: Tools for investigating PE
Tools for investigating PE
Three clinical decision tools have been validated to predict the risk of PE: the Wells score, the Geneva score, and Pulmonary Embolism Rule Out Criteria (PERC).44,45
Wells score is more sensitive than the Geneva score and has been validated in ambulatory1 and ED46-48 settings. Based on Wells criteria, high-risk patients need further evaluation with imaging. In low-risk patients, a normal D-dimer level effectively excludes PE, with a < 1% risk of subsequent thromboembolism in the following 3 months. Positive predictive value of the Wells decision tool is low because it is intended to rule out, not confirm, PE.
PERC can be used in a low-probability setting (defined as the treating physician arriving at the conclusion that PE is not the most likely diagnosis and can be excluded with a negative D-dimer test). In that setting, if the patient meets the 8 clinical variables in PERC, the diagnosis of PE is, effectively, ruled out.48
Summing up: Evaluation of chest pain guided by risk of CAD
Patients who present in an outpatient setting with a potentially life-threatening cause of chest pain (TABLE 1) and patients with unstable vital signs should be sent to the ED for urgent evaluation. In the remaining outpatients, use the Marburg Heart Score or Diamond Forrester classification to assess the likelihood that pain is due to CAD (in the ED, the HEART score can be used for this purpose) (FIGURE).

When the risk is low. No further cardiac testing is indicated in patients with a risk of CAD < 5%, based on a Marburg score of 0 or 1, or on Diamond Forrester criteria; an abnormal stress test is likely to be a false-positive.19
Continue to: Moderate risk
Moderate risk. However, further testing is indicated, with a stress test (with or without myocardial imaging), in patients whose risk of CAD is 5% to 70%, based on the Diamond Forrester classification or an intermediate Marburg Heart Score (ie, a score of 2 or 3 but a normal EKG). This further testing can be performed urgently in patients who have multiple other risk factors that are not assessed by the Marburg Heart Score.
High risk. In patients whose risk is > 70%, invasive testing with angiography should be considered.35,49
EKG abnormalities. Patients with a Marburg Score of 2 or 3 and an abnormal EKG should be sent to the ED (FIGURE). There, patients with a HEART score < 4 and a negative 2-3–hour troponin test have a < 1% chance of ACS and can be safely discharged.31
CORRESPONDENCE
Anne Mounsey, MD, UNC Family Medicine, 590 Manning Drive, Chapel Hill, NC 27599; [email protected]
1. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36:1-12. doi: 10.1016/j.ccl.2017.08.001
2. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed February 16, 2021. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
3. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029-1032. doi: 10.1001/jamainternmed.2016.2498
4. Ebell MH. Evaluation of chest pain in primary care patients. Am Fam Physician. 2011;83:603-605.
5. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547-564. doi: 10.1161/CIRCULATIONAHA.116.021886
6. Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome? The rational clinical examination systematic review. JAMA. 2015;314:1955-1965. doi: 10.1001/jama.2015.12735
7. Kolminsky J, Choxi R, Mahmoud AR, et al. Familial hypercholesterolemia: cardiovascular risk stratification and clinical management. American College of Cardiology. June 1, 2020. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2020/06/01/13/54/familial-hypercholesterolemia
8. Konstantinides SV, Meyer G, Becattini C, et al; . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
9. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-182.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877. doi: 10.1056/NEJMoa0903515
12. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867. doi: 10.1056/NEJMoa0900428
13. Tada M, Azuma H, Yamada N, et al. A comprehensive validation of very early rule-out strategies for non-ST-segment elevation myocardial infarction in emergency departments: protocol for a multicentre prospective cohort study. BMJ Open. 2019;9:e026985. doi: 10.1136/bmjopen-2018-026985
14. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-1218. doi: 10.1001/archinternmed.2012.3698
15. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481-2488. doi: 10.1016/S0140-6736(15)00391-8
16. Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913-1924. doi: 10.1001/jama.2017.17488
17. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology. July 16, 2018. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-in-the-evaluation-of-possible-am
18. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66:635-645.e1. doi: 10.1016/j.annemergmed.2015.07.006
19. Qaseem A, Fihn SD, Williams S, et al; . Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/Preventative Cardiovascular nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157:729-734. doi: 10.7326/0003-4819-157-10-201211200-00010
20. Amsterdam EA, Wenger NK, Brindis RG, et al; . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-2394. doi: 10.1161/CIR.0000000000000133
21. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163:701-711. doi: 10.7326/M14-1772
22. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300. doi: 10.1503/cmaj.100212
23. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196. doi: 10.1007/BF03086144
24. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350-1358. doi: 10.1056/NEJM197906143002402
25. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e21. doi: 10.3399/bjgp12X649106
26. Laureano-Phillips J, Robinson RD, Aryal S, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2019;74:187-203. doi: 10.1016/j.annemergmed.2018.12.010
27. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26:140-151. doi: 10.1111/acem.13649
28. Sakamoto JT, Liu N, Koh ZX, et al. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016;221:759-764. doi: 10.1016/j.ijcard.2016.07.147
29. Harskamp RE, Laeven SC, Himmelreich JCL, et al. Chest pain in general practice: a systematic review of prediction rules. BMJ Open. 2019;9:e027081. doi: 10.1136/bmjopen-2018-027081
30. Aerts M, Minalu G, Bösner S, et al. Internal Working Group on Chest Pain in Primary Care (INTERCHEST). Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J. Clin Epidemiol. 2017;81:120-128. doi: 10.1016/j.jclinepi.2016.09.011
31. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients in the emergency department. Int J Cardiol. 2013;168:2153-2158. doi: 10.1016/j.ijcard.2013.01.255
32. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164-169. doi: 10.1097/HPC.0b013e3181ec36d8
33. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661. doi: 10.1016/j.ijcard.2016.10.080
34. Reaney PDW, Elliott HI, Noman A, et al. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018;35:420-427. doi: 10.1136/emermed-2017-207172
35. Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond Forrester score: The Partners Registry. Circulation. 2016;134:201-211. doi: 10.1161/CIRCULATIONAHA.116.023396
36. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427-437. doi: 10.2147/VHRM.S106838
37. Borque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. doi: 10.1016/j.jcmg.2015.09.006
38. Reinhardt SW, Lin C-J, Novak E, et al. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178:212-219. doi: 10.1001/jamainternmed.2017.7360
39. Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, et al. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17-23. doi: 10.1016/j.amjcard.2010.08.037
40. Linde JJ, H, Hansen TF, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J AM Coll Cardiol 2020;75:453-463. doi: 10.1016/j.jacc.2019.12.012
41. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525-e555. doi: 10.1161/CIR.0b013e3181fcae66
42. Society of Cardiovascular Computed Tomography. Five things physicians and patients should question. Choosing Wisely Campaign. February 21, 2013. Accessed September 28, 2021. www.choosingwisely.org/wp-content/uploads/2015/02/SCCT-Choosing-Wisely-List.pdf
43. Hamm CW, Bassand J-P, Agewall S, et al; . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. doi: 10.1093/eurheartj/ehr236
44. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
45. Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. doi: 10.1111/j.1538-7836.2010.03801.x
46. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in the emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255. doi: 10.1111/j.1538-7836.2004.00790.x
47. Hendriksen JMT, Geersing G-J, Lucassen WAM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438. doi: 10.1136/bmj.h4438
48. Shen J-H, Chen H-L, Chen J-R, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482-492. doi: 10.1007/s11239-015-1250-2
49. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventative Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. doi: 10.1016/j.jacc.2012.07.013
1. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36:1-12. doi: 10.1016/j.ccl.2017.08.001
2. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed February 16, 2021. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
3. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029-1032. doi: 10.1001/jamainternmed.2016.2498
4. Ebell MH. Evaluation of chest pain in primary care patients. Am Fam Physician. 2011;83:603-605.
5. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547-564. doi: 10.1161/CIRCULATIONAHA.116.021886
6. Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome? The rational clinical examination systematic review. JAMA. 2015;314:1955-1965. doi: 10.1001/jama.2015.12735
7. Kolminsky J, Choxi R, Mahmoud AR, et al. Familial hypercholesterolemia: cardiovascular risk stratification and clinical management. American College of Cardiology. June 1, 2020. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2020/06/01/13/54/familial-hypercholesterolemia
8. Konstantinides SV, Meyer G, Becattini C, et al; . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
9. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-182.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877. doi: 10.1056/NEJMoa0903515
12. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867. doi: 10.1056/NEJMoa0900428
13. Tada M, Azuma H, Yamada N, et al. A comprehensive validation of very early rule-out strategies for non-ST-segment elevation myocardial infarction in emergency departments: protocol for a multicentre prospective cohort study. BMJ Open. 2019;9:e026985. doi: 10.1136/bmjopen-2018-026985
14. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-1218. doi: 10.1001/archinternmed.2012.3698
15. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481-2488. doi: 10.1016/S0140-6736(15)00391-8
16. Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913-1924. doi: 10.1001/jama.2017.17488
17. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology. July 16, 2018. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-in-the-evaluation-of-possible-am
18. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66:635-645.e1. doi: 10.1016/j.annemergmed.2015.07.006
19. Qaseem A, Fihn SD, Williams S, et al; . Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/Preventative Cardiovascular nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157:729-734. doi: 10.7326/0003-4819-157-10-201211200-00010
20. Amsterdam EA, Wenger NK, Brindis RG, et al; . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-2394. doi: 10.1161/CIR.0000000000000133
21. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163:701-711. doi: 10.7326/M14-1772
22. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300. doi: 10.1503/cmaj.100212
23. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196. doi: 10.1007/BF03086144
24. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350-1358. doi: 10.1056/NEJM197906143002402
25. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e21. doi: 10.3399/bjgp12X649106
26. Laureano-Phillips J, Robinson RD, Aryal S, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2019;74:187-203. doi: 10.1016/j.annemergmed.2018.12.010
27. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26:140-151. doi: 10.1111/acem.13649
28. Sakamoto JT, Liu N, Koh ZX, et al. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016;221:759-764. doi: 10.1016/j.ijcard.2016.07.147
29. Harskamp RE, Laeven SC, Himmelreich JCL, et al. Chest pain in general practice: a systematic review of prediction rules. BMJ Open. 2019;9:e027081. doi: 10.1136/bmjopen-2018-027081
30. Aerts M, Minalu G, Bösner S, et al. Internal Working Group on Chest Pain in Primary Care (INTERCHEST). Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J. Clin Epidemiol. 2017;81:120-128. doi: 10.1016/j.jclinepi.2016.09.011
31. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients in the emergency department. Int J Cardiol. 2013;168:2153-2158. doi: 10.1016/j.ijcard.2013.01.255
32. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164-169. doi: 10.1097/HPC.0b013e3181ec36d8
33. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661. doi: 10.1016/j.ijcard.2016.10.080
34. Reaney PDW, Elliott HI, Noman A, et al. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018;35:420-427. doi: 10.1136/emermed-2017-207172
35. Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond Forrester score: The Partners Registry. Circulation. 2016;134:201-211. doi: 10.1161/CIRCULATIONAHA.116.023396
36. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427-437. doi: 10.2147/VHRM.S106838
37. Borque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. doi: 10.1016/j.jcmg.2015.09.006
38. Reinhardt SW, Lin C-J, Novak E, et al. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178:212-219. doi: 10.1001/jamainternmed.2017.7360
39. Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, et al. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17-23. doi: 10.1016/j.amjcard.2010.08.037
40. Linde JJ, H, Hansen TF, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J AM Coll Cardiol 2020;75:453-463. doi: 10.1016/j.jacc.2019.12.012
41. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525-e555. doi: 10.1161/CIR.0b013e3181fcae66
42. Society of Cardiovascular Computed Tomography. Five things physicians and patients should question. Choosing Wisely Campaign. February 21, 2013. Accessed September 28, 2021. www.choosingwisely.org/wp-content/uploads/2015/02/SCCT-Choosing-Wisely-List.pdf
43. Hamm CW, Bassand J-P, Agewall S, et al; . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. doi: 10.1093/eurheartj/ehr236
44. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
45. Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. doi: 10.1111/j.1538-7836.2010.03801.x
46. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in the emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255. doi: 10.1111/j.1538-7836.2004.00790.x
47. Hendriksen JMT, Geersing G-J, Lucassen WAM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438. doi: 10.1136/bmj.h4438
48. Shen J-H, Chen H-L, Chen J-R, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482-492. doi: 10.1007/s11239-015-1250-2
49. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventative Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. doi: 10.1016/j.jacc.2012.07.013
PRACTICE RECOMMENDATIONS
› Use the highly sensitive Marburg Heart Score to rule out coronary artery disease as a cause of chest pain in the ambulatory care setting. B
› Consider a prior normal stress test result nonpredictive of outcome in a patient presenting with chest pain. Patients with such a history of testing have a risk of a 30-day adverse cardiac event that is similar to the risk seen in patients who have never had a stress test. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
a Risk factors include hypertension, hypercholesterolemia, diabetes, obesity (body mass index > 30), smoking (current, or smoking cessation for ≤ 3 mo), and family history of CAD (ie, parent or sibling affected before 65 years of age). Atherosclerotic disease includes history of AMI, percutaneous coronary intervention or coronary artery bypass grafting, stroke, or peripheral artery disease.
Vitamin supplementation in healthy patients: What does the evidence support?
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1
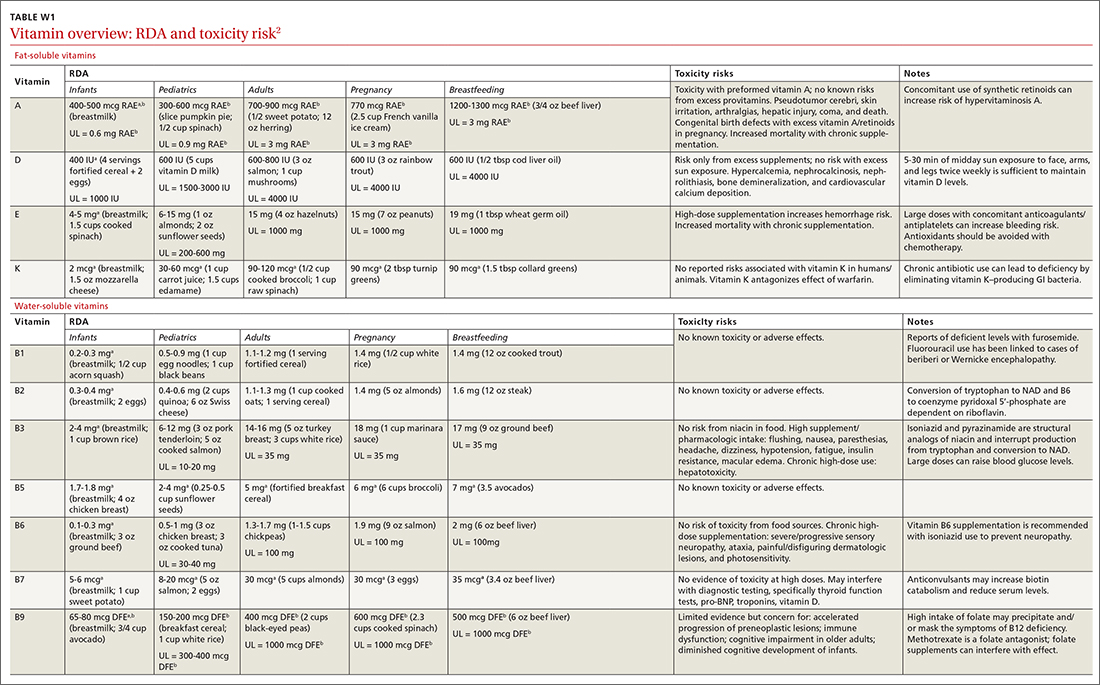
While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.
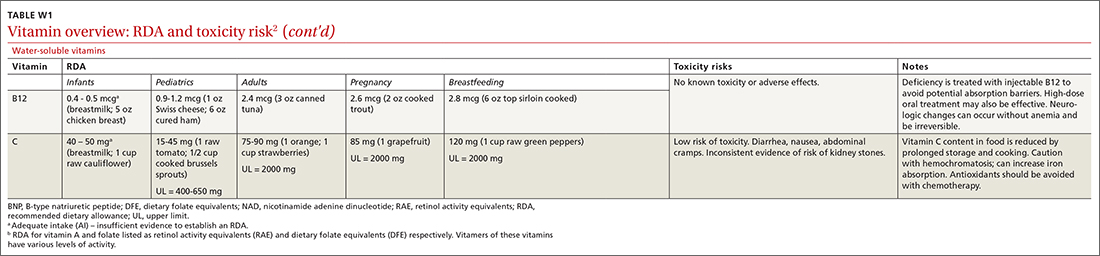
Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE W12 (available at mdedge.com/familymedicine) lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96

B COMPLEX VITAMINS
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5
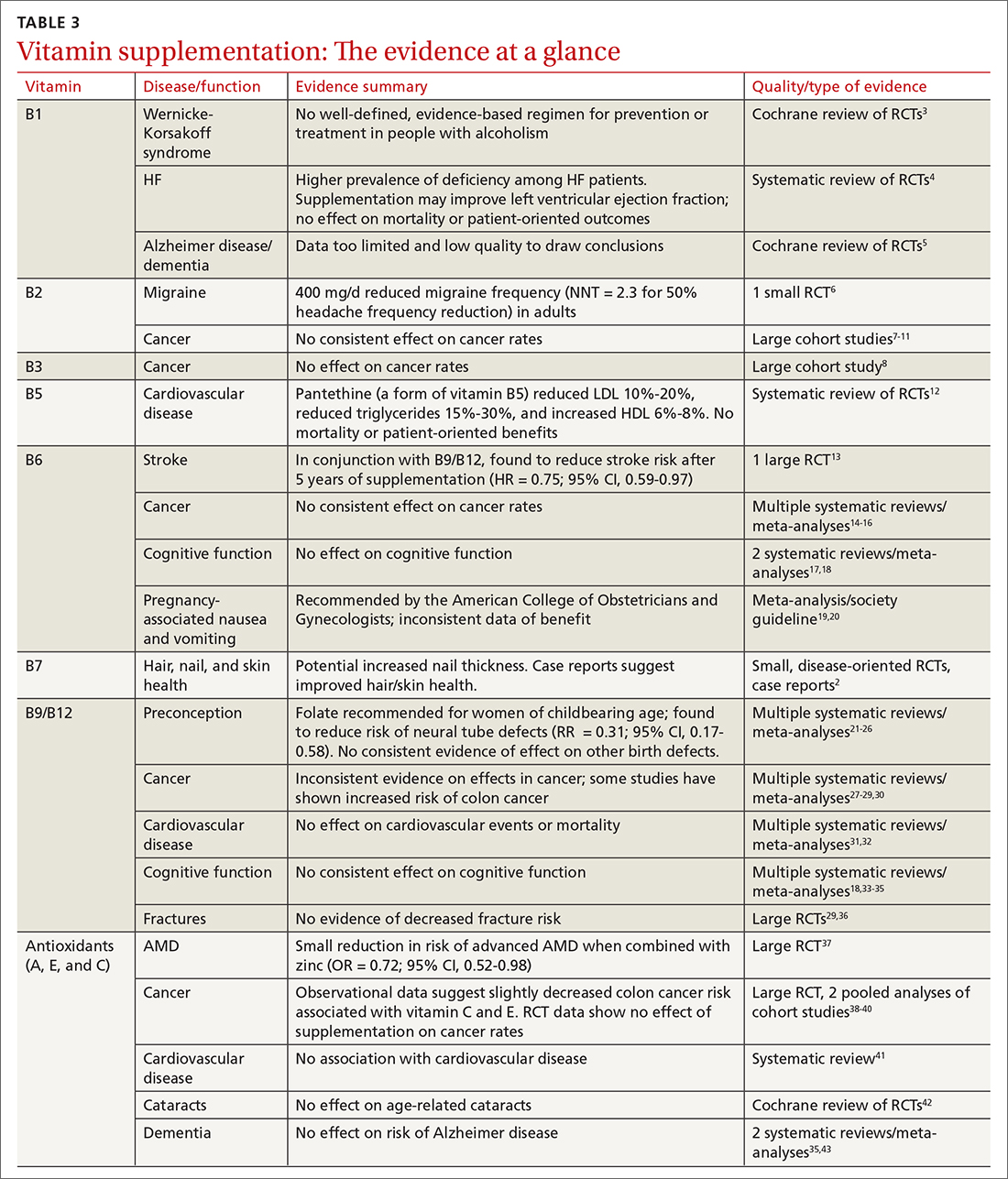
Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains
Riboflavin is essential to energy production, cellular growth, and metabolism.2
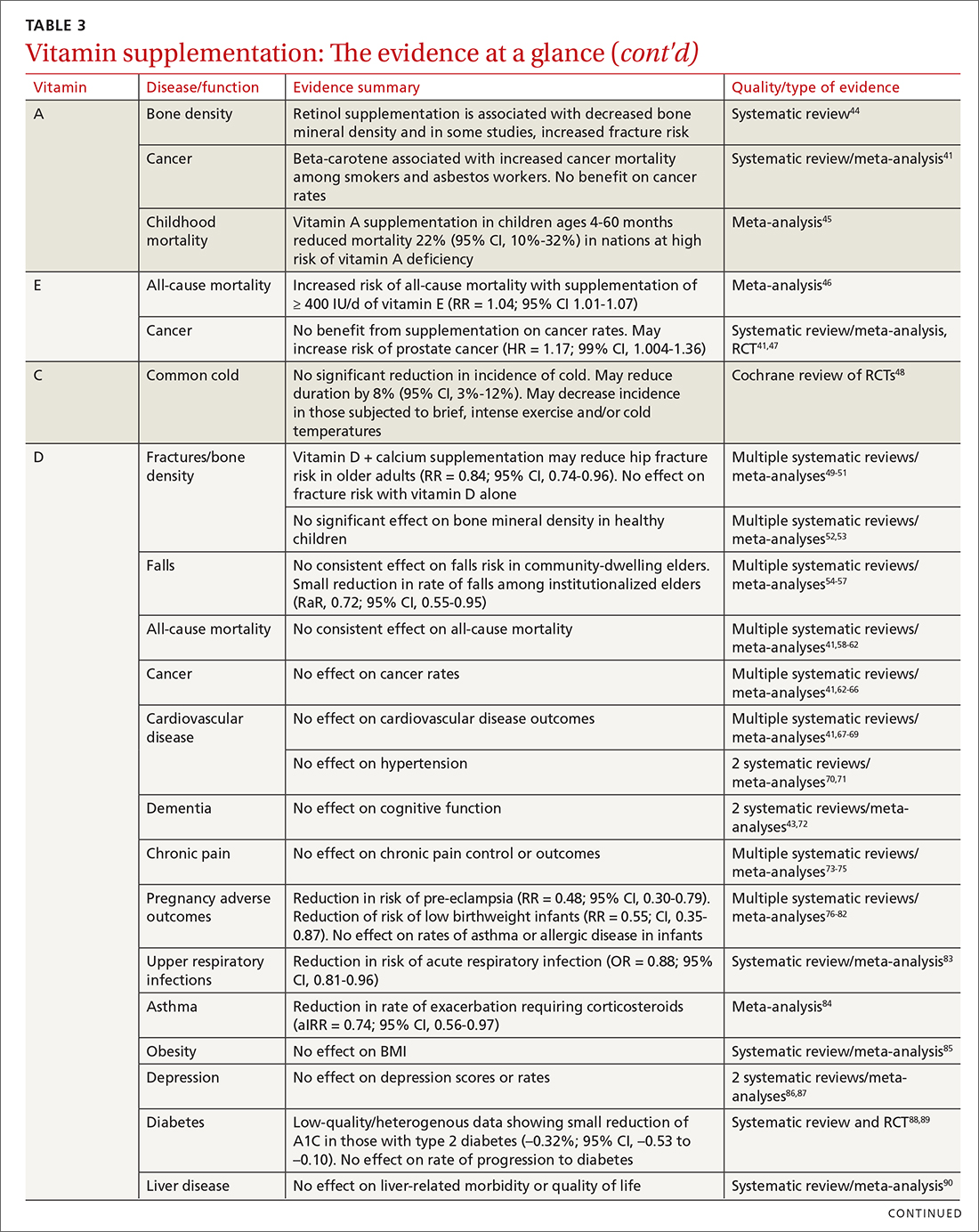
Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.
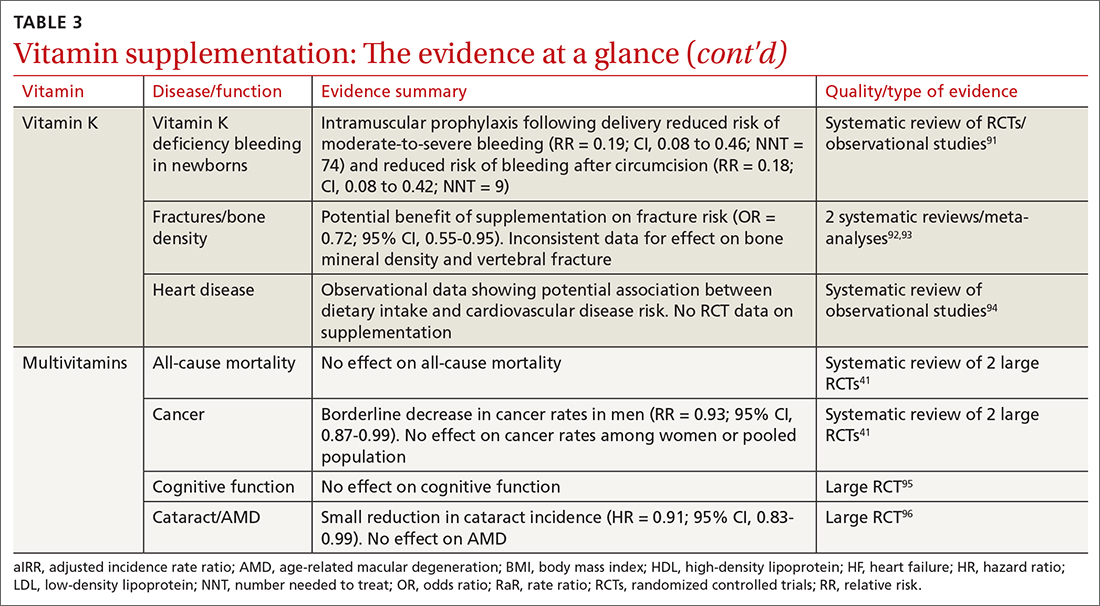
Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
Continue to: Vitamin B6
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
Continue to: ANTIOXIDANTS
ANTIOXIDANTS
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils
Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
Continue to: Vitamin D
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
Continue to: MULTIVITAMINS
MULTIVITAMINS
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
SUMMARY
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
CORRESPONDENCE
Joel Herness, MD, 4700 North Las Vegas Boulevard, Nellis AFB, NV 89191; [email protected]
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1

While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.

Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE W12 (available at mdedge.com/familymedicine) lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96

B COMPLEX VITAMINS
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5

Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains
Riboflavin is essential to energy production, cellular growth, and metabolism.2

Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.

Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
Continue to: Vitamin B6
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
Continue to: ANTIOXIDANTS
ANTIOXIDANTS
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils
Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
Continue to: Vitamin D
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
Continue to: MULTIVITAMINS
MULTIVITAMINS
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
SUMMARY
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
CORRESPONDENCE
Joel Herness, MD, 4700 North Las Vegas Boulevard, Nellis AFB, NV 89191; [email protected]
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1

While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.

Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE W12 (available at mdedge.com/familymedicine) lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96

B COMPLEX VITAMINS
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5

Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains
Riboflavin is essential to energy production, cellular growth, and metabolism.2

Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.

Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
Continue to: Vitamin B6
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
Continue to: ANTIOXIDANTS
ANTIOXIDANTS
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils
Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
Continue to: Vitamin D
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
Continue to: MULTIVITAMINS
MULTIVITAMINS
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
SUMMARY
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
CORRESPONDENCE
Joel Herness, MD, 4700 North Las Vegas Boulevard, Nellis AFB, NV 89191; [email protected]
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
GRADE DEFINITIONS
For an explanation of USPSTF grade definitions, see www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/grade-definitions
Step-by-step evaluation and treatment of shoulder dislocation
The architecture of the glenohumeral joint makes it the most common large joint to become dislocated, accounting for approximately 45% of all dislocations. Anterior dislocation constitutes more than 95% of glenohumeral joint dislocations; posterior dislocation, only 2% to 5%.1,2
For the family physician, determining appropriate follow-up after emergent reduction depends on several distinct variables, which we review here; subsequent treatment might involve, as we outline, physical therapy, immobilization, surgical intervention, or a combination of several modalities. Treatment decisions can make the difference between successful rehabilitation and potential disability, particularly in typically young and active patients.
Numerous mechanisms of injury
Anterior shoulder dislocations typically occur with the affected shoulder in a position of abduction and external rotation; 90% of patients are 21 to 30 years of age, and men are affected 3 times more often than women.2 Unsurprisingly, athletes are affected most frequently, with the common sports-related mechanism of injury being either sudden pressure exerted on the abducted and externally rotated arm or a fall onto an outstretched hand with the arm elevated. Repetitive microtrauma from such sports as swimming, baseball, and volleyball can also lead to instability.
Bankart lesion. This tear of the anterior or inferior section of the labrum is the most characteristic lesion noted in anterior dislocations, found in 73% of first-time dislocations and 100% of recurrent dislocations.3,4
Hills-Sachs lesion is often associated with a Bankart lesion. The Hills-Sachs lesion is an impaction fracture of the posterolateral aspect of the humeral head resulting from its displacement over the anterior lip of the glenoid. Hill-Sachs lesions are seen in 71% of first-time and recurrent dislocations.3
Less common concomitant injuries during anterior shoulder dislocation include rupture of the rotator-cuff tendons (particularly in patients older than 40 years), glenoid and proximal humerus fractures, a tear of the superior labrum (known as a “SLAP lesion”), cartilage injury, and neurovascular injury.
Posterior instability typically occurs as a result of a strong muscle contraction, as seen in electrocution or seizure; however, it can be caused by athletic trauma, particularly in football.5 Repetitive forces exerted on the forward-flexed and internally rotated shoulder position during blocking puts football players at increased risk of posterior instability.5
Continue to: Multidirectional instability
Multidirectional instability is more frequently attributable to congenital hyperlaxity of the glenohumeral joint capsule, rather than to acute injury. However, athletes can also develop capsular laxity from repetitive microtrauma to the shoulder.5
Emergent reduction: Prompt action needed
Acute dislocation of the shoulder should be reduced as soon as possible to minimize neurovascular injury and patient discomfort. (Typically, but not always, this is done in the emergency department.) It is crucial to have effective muscle relaxation before any attempt at reduction, to minimize the risk of iatrogenic injury to bone, cartilage, tendons, and neurovascular structures.
Muscle relaxation can be facilitated with intravenous midazolam or other agents, as specified by institutional protocol. Intra-articular lidocaine injection or intravenous fentanyl is often utilized in conjunction with the sedating agent to reduce pain and further accommodate relaxation.
Anterior reduction. Any one of several techniques can be used to perform emergent reduction of anterior shoulder dislocations, all of which have demonstrated success. The Milch technique is among the least traumatic for effective reduction.6 In this technique (FIGURE 1), the patient is supine; gentle but firm downward traction is applied to the humerus at the elbow of the affected arm while the arm is in abduction and external rotation. The provider can manipulate the humeral head at that point by placing a thumb in the patient’s axilla; the arm can also be further internally rotated and adducted until reduction is achieved.
Posterior reduction of a dislocation is performed while the patient is supine, with the body stabilized. Traction is applied on the adducted and internally rotated arm in conjunction with direct pressure on the posterior aspect of the humeral head (FIGURE 2).
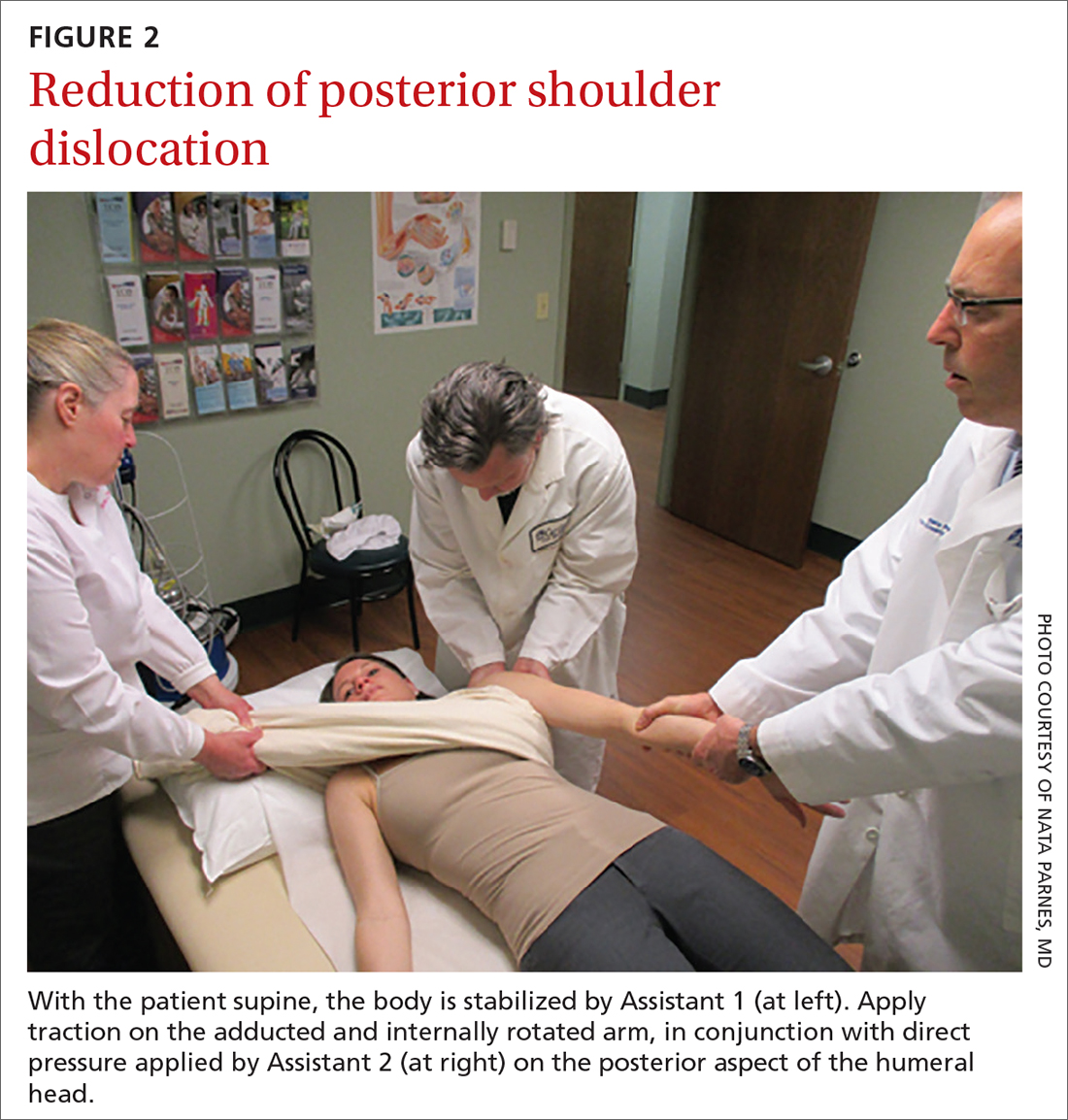
Continue to: Follow-up actions
Follow-up actions. Before discharging the patient after reduction of a dislocation, it is essential to:
- perform post-reduction evaluation of shoulder stability at different levels of abduction
- perform a thorough neurovascular assessment
- obtain an anteroposterior (AP) radiograph to ensure proper positioning of the glenohumeral joint.
The reduced shoulder should be immobilized in a sling. The discharge plan should include pain management for several days and a follow-up appointment in 5 to 8 days with the primary care provider2 (FIGURE 3).
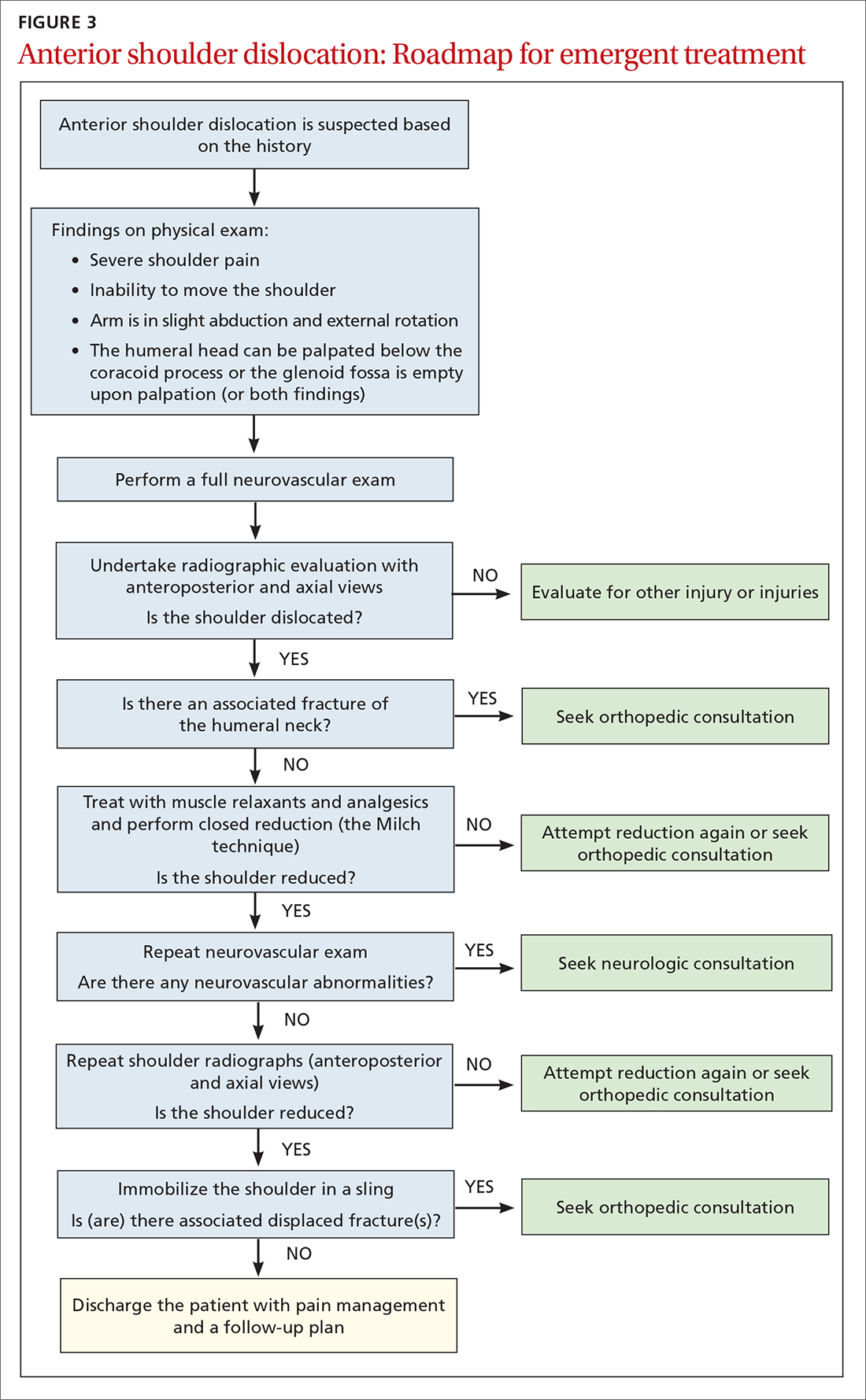
Follow-up evaluation by the primary care provider
History. Prior to the initial examination at follow-up, obtain a comprehensive history that includes the nature of the injury and the direction of force that was placed on the shoulder. Determine whether the shoulder was reduced spontaneously or required manual reduction in the field or an emergency department. Note any associated injury sustained concurrently and the presence (or absence) of neck pain, numbness, tingling, or weakness in the affected arm.
Physical exam starts with thorough inspection of the affected shoulder, with comparison to the contralateral side, at rest and during shoulder motion. Palpation to reveal points of tenderness should include the anterior joint line, acromioclavicular joint, bicipital groove, subacromial space, acromion, and greater tuberosity.
Following inspection and palpation, assess active and passive range of motion in forward elevation, abduction, internal and external rotation at the side of the body, and internal and external rotation in shoulder abduction. Assessment might be limited by pain and apprehension, and should be performed within the patient’s comfortable range of motion.
Continue to: Once range of motion...
Once range of motion is determined, assess7:
- muscle power of the rotator cuff in abduction (for the supraspinatus muscle)
- resisted external rotation at the side of the body (the infraspinatus)
- resisted external rotation in abduction > 60° (the teres minor)
- resisted internal rotation (the subscapularis).
Specific tests for shoulder laxity and stability
It is important during the primary care follow-up examination to differentiate true instability and shoulder hyperlaxity, particularly in young, flexible patients (TABLE). Many of these patients present with painless hypermobility of the shoulder without true injury to the labrum or ligamentous structures. It might appear to the patient, or to family, that the shoulder is subluxating; however, the humeral head returns to a centered position on the glenoid in a hypermobile state—typically, without pain. Actual shoulder instability is defined as loss of the ability of the humeral head to re-center, accompanied by pain—pathology that is frequently associated with damage to the capsulolabral complex.
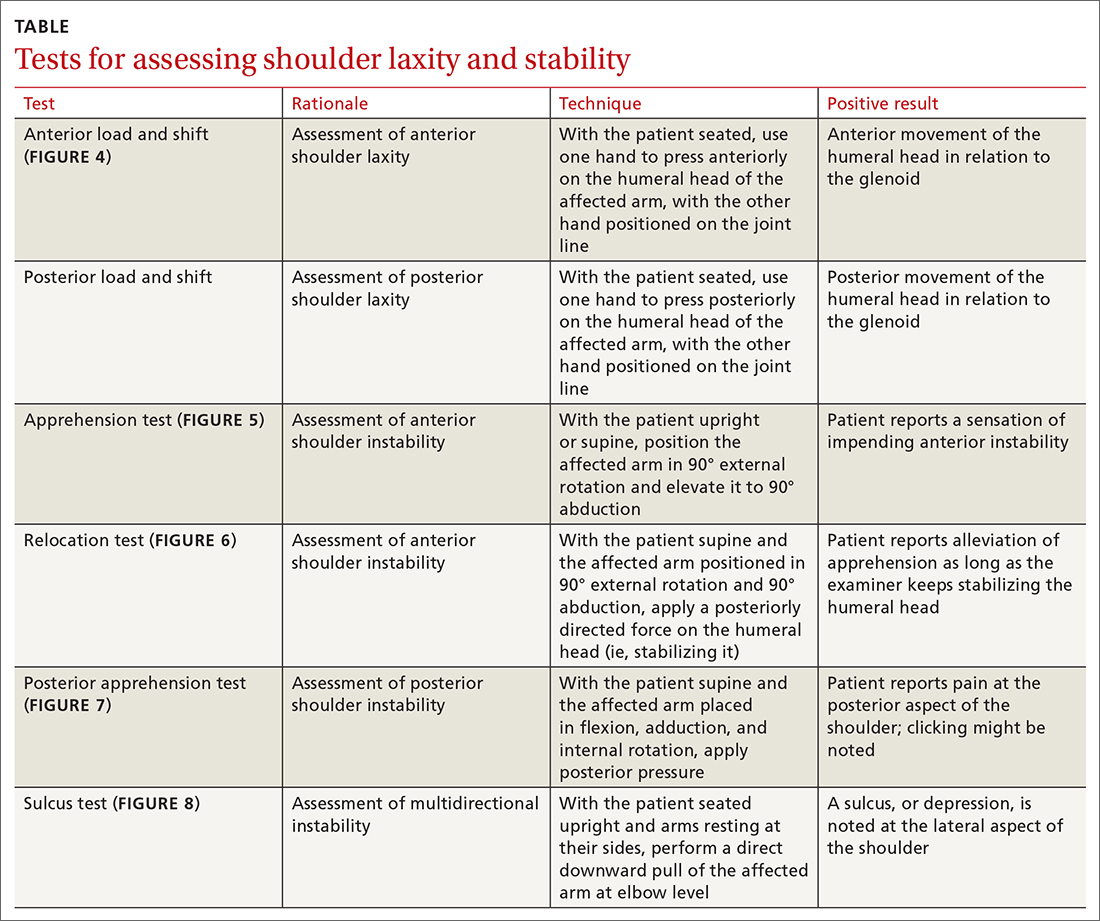
The load and shift test is used to assess anterior and posterior laxity. The patient is seated, and the forearm is allowed to rest on the thigh. Examination is performed using 1 hand to press anteriorly or posteriorly on the humeral head; the other hand is simultaneously positioned on the joint line to feel movement of the humeral head in relation to the glenoid (FIGURE 4).
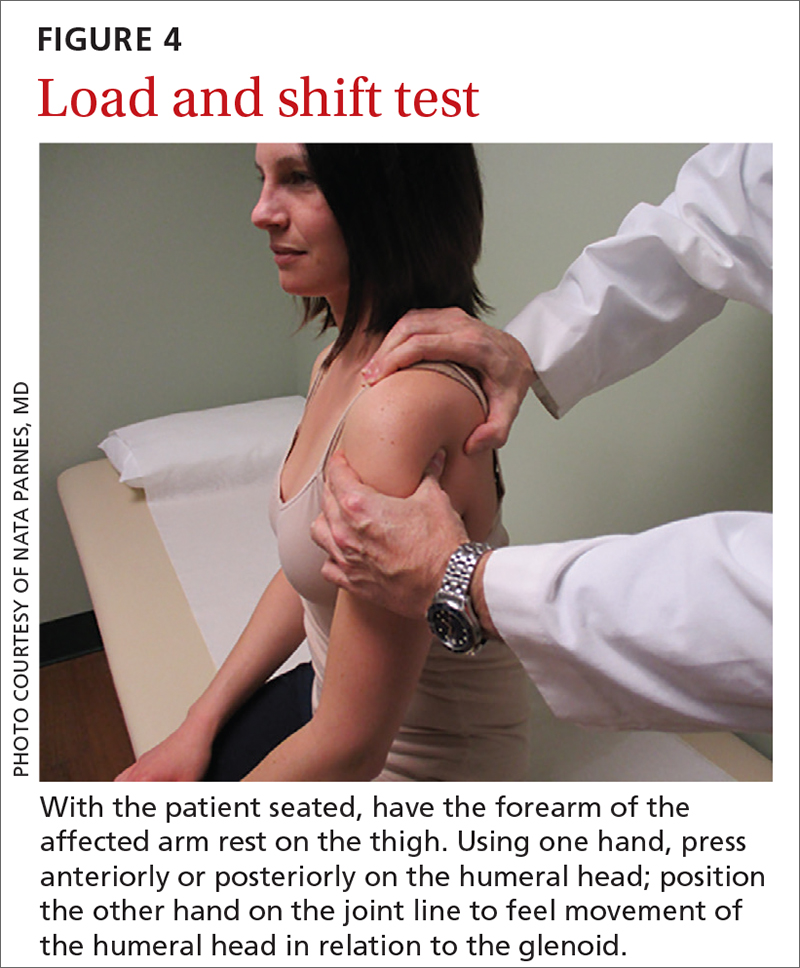
The apprehension test is a common maneuver used to assess anterior shoulder instability. It is performed by positioning the affected arm to 90° external rotation and then elevating it to 90° abduction. Although this maneuver can be performed with the patient upright, it is beneficial to have them supine, to more easily control the arm (FIGURE 5). A positive test is noted when the patient reports a sensation of impending instability (apprehension), rather than pain alone.
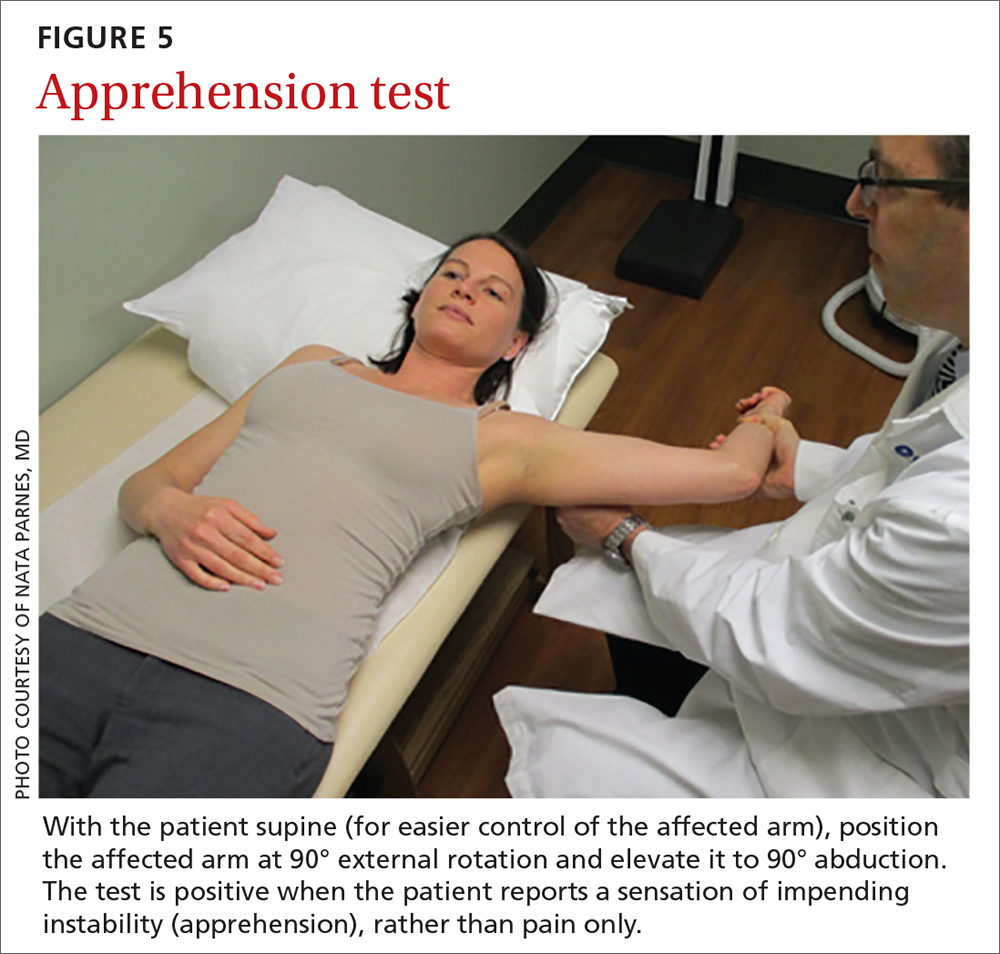
Relocation test. When the apprehension test is positive, the supine position can be exploited to further perform the relocation test, in 2 stages (FIGURE 6):
- Apply a posteriorly directed force on the humeral head, which stabilizes the shoulder and typically alleviates symptoms.
- Release pressure quickly from the humeral head to assess recurrence of pain and apprehension as the humeral head snaps back against the anterior labrum.
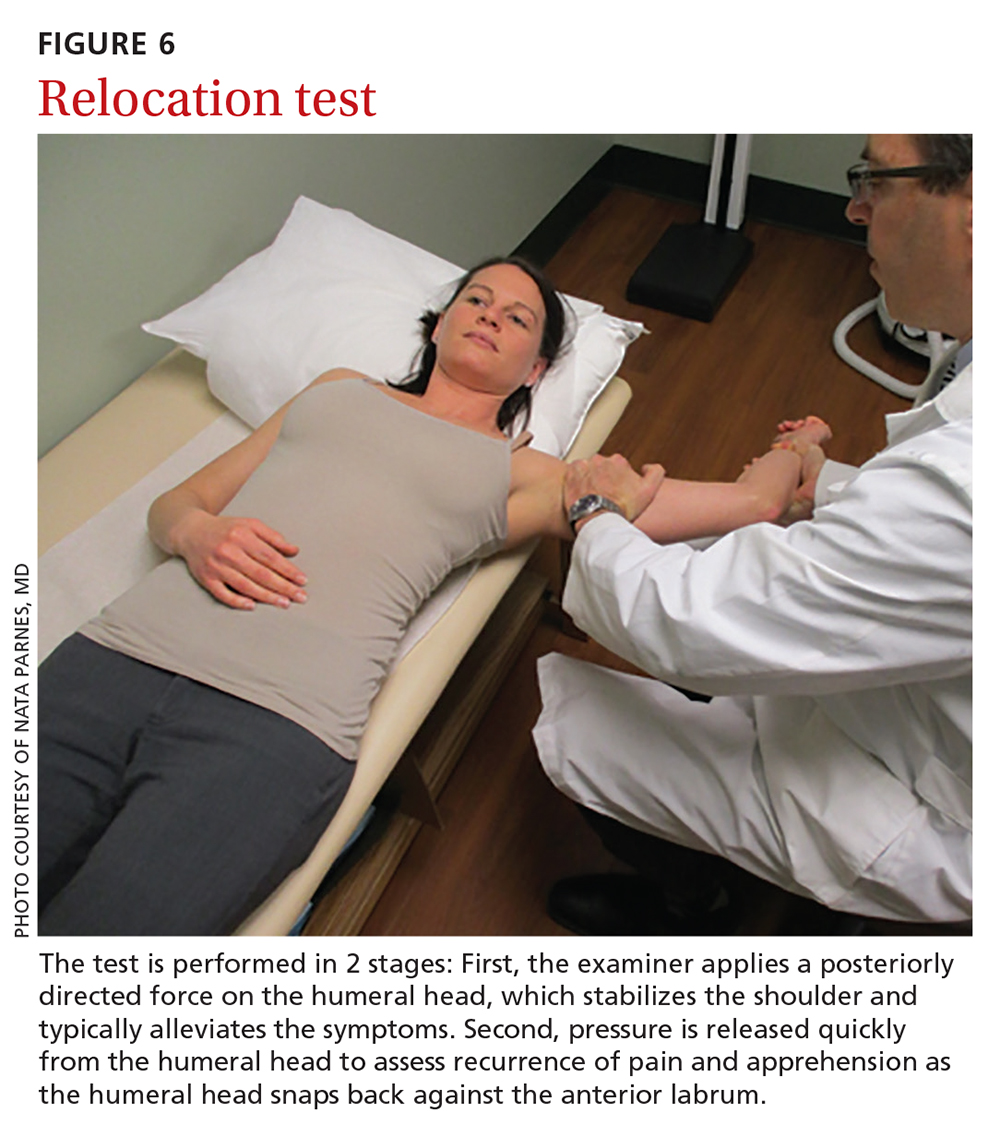
Continue to: Combined, apprehension and relocation...
Combined, apprehension and relocation tests to identify anterior shoulder instability have been shown to significantly improve specificity while maintaining sensitivity.8
The posterior apprehension test is used to assess posterior instability. The patient is supine; the affected arm is placed in flexion, adduction, and internal rotation; and posterior pressure is applied (FIGURE 7). A positive test is noted when pain is reported at the posterior aspect of the shoulder. Clicking might be noted as the humeral head dislocates rearward.1
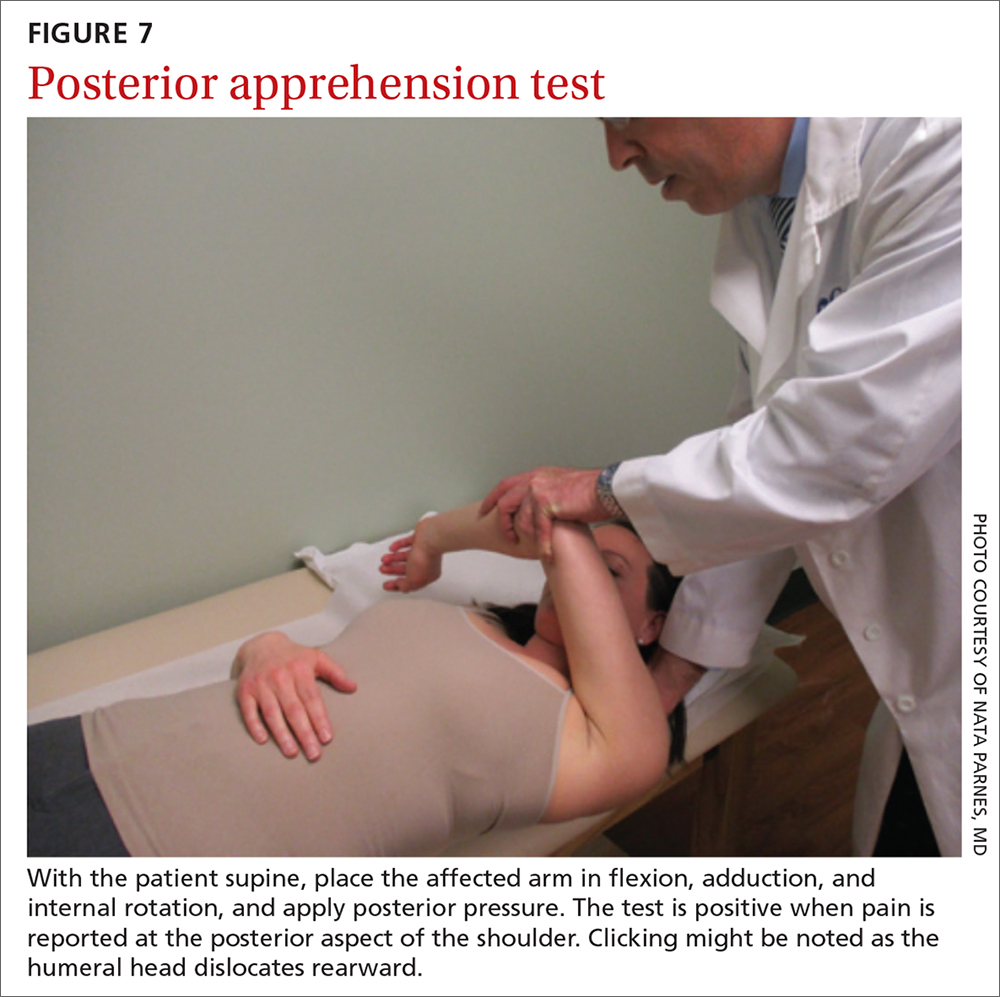
Sulcus sign. Multidirectional instability is elicited using the sulcus sign. While the patient is seated upright, arms resting at their sides, a direct downward pull at elbow level will, when positive, reveal a depression (sulcus) at the lateral aspect of the affected shoulder as the humeral head translates inferiorly (FIGURE 8). A positive sulcus sign is documented in 3 grades, according to the amount of translation1:
- Grade I: < 1 cm
- Grade II: 1-2 cm
- Grade III: > 2 cm.
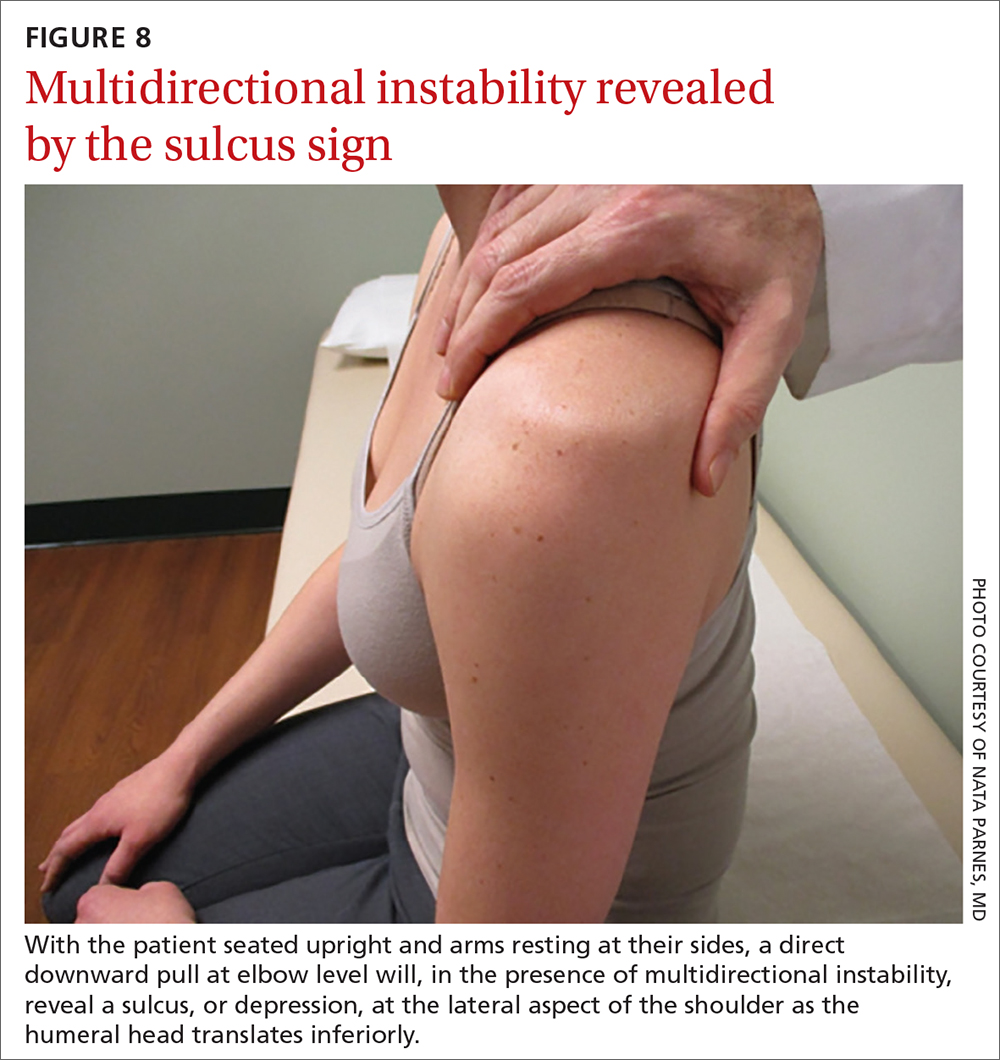
Neurovascular status should be verified at every physical evaluation, with motor and sensory function tested in the axillary, musculocutaneous, median, radial, and ulnar nerve distributions. If nerve injury is suspected, electromyography and nerve-conduction testing is indicated.9-13 Vascular compromise is much less common but equally important to assess.11
Use of imaging
Post-reduction radiographs, including internal and external AP—and especially axillary—views are invaluable. Not only do they help to ensure reduction, but they also help to assess for fracture. A magnetic resonance imaging (MRI) arthrogram is the preferred imaging modality if a labral tear is suspected (FIGURE 9). Other concomitant shoulder injuries, such as subtle bone fracture, rotator cuff tear, and biceps pathology can also be reliably diagnosed with noncontrast MRI.
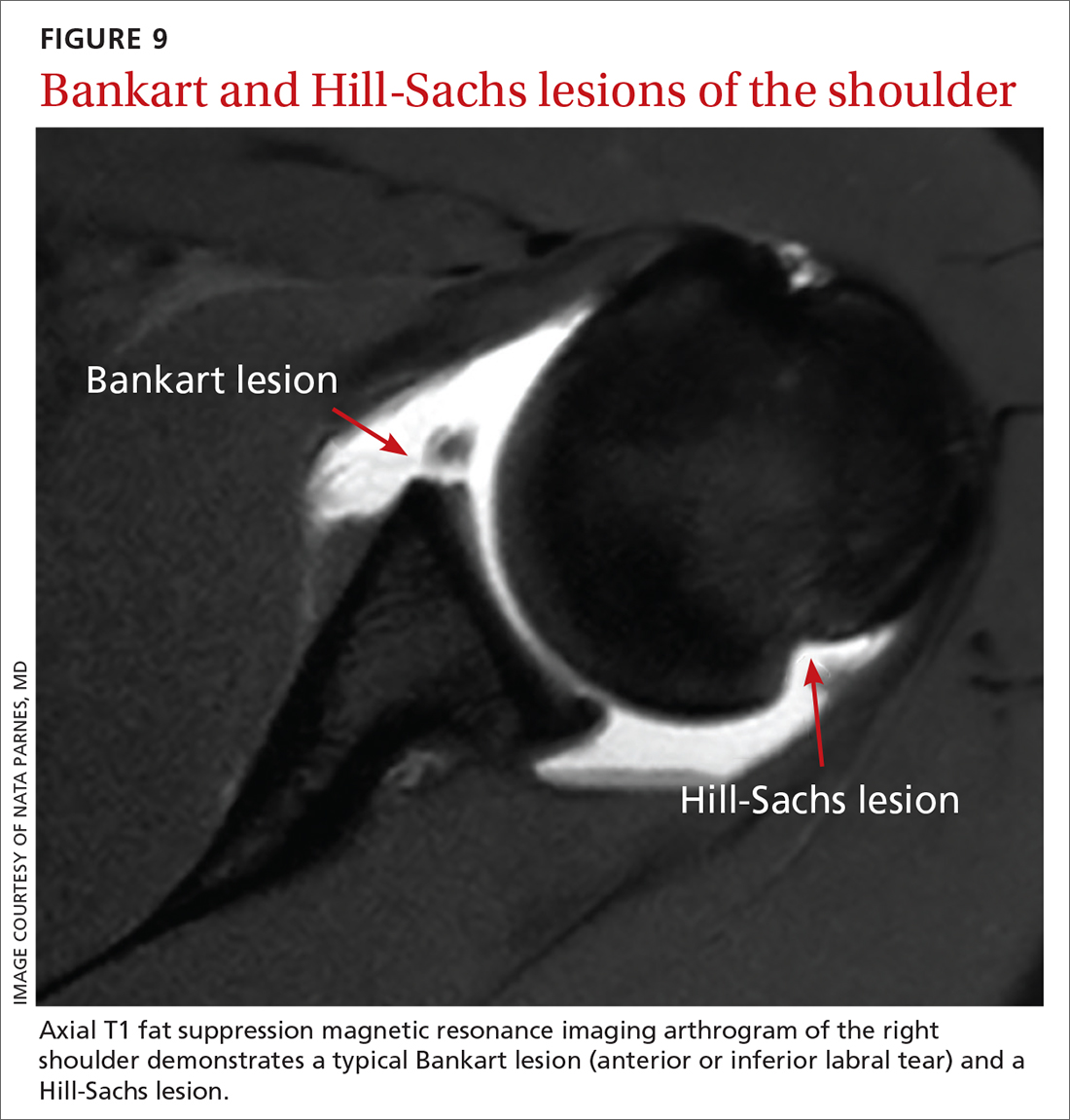
Continue to: Roadmap for treatment
Roadmap for treatment
The rate of recurrence after a first anterior shoulder dislocation is strongly associated with a person’s age and level of activity. Active patients younger than 20 years have a 92% to 96% recurrence rate14; patients 20 to 40 years, 25% to 48%; and patients older than 40 years, < 10%.15
Young, athletic patients who are treated nonoperatively are left at an unacceptably high risk of recurrence, leading to progressive damage to bony and soft-tissue structures.16,17 Surgical labral repair after a first-time anterior dislocation produced improved outcomes in terms of recurrent dislocation (7.9%), compared to outcomes after nonsurgical treatment (52.9%),14 and has been associated with a lower incidence of future glenohumeral osteoarthritis.18 For those reasons, we recommend referral to an orthopedic surgeon for all patients younger than 20 years who sustain an anterior shoulder dislocation.
Patients older than 20 years who do not have concomitant shoulder injury, and who demonstrate full strength in abduction, external rotation, and internal rotation of the shoulder on clinical examination, have a low probability of associated rotator-cuff tear. They can be immobilized in a sling for 1 to 3 weeks, followed by a 6 to 12–week regimen of physical therapy.
Concomitant tear of the rotator cuff. Weakness on examination requires MRI or a magnetic resonance arthrogram for evaluation of associated rotator-cuff tear. A tear identified on MRI should be referred to an orthopedic surgeon because timely repair can be crucial to attaining best outcomes. Conservative treatment of traumatic full-tendon rotator-cuff tear is associated with poor results, progression in the size of the tear, and advancement of muscle atrophy.19,20 For patients younger than 40 years, arthroscopic rotator-cuff repair, with or without labral repair, produces excellent clinical outcomes, carries a low risk of complications, and results in a > 95% rate of return to a preoperative level of recreational and job activities.21
Patients who demonstrate weakness of the rotator-cuff muscles on examination, but who do not have a tear noted on MRI, should be evaluated by electromyography and nerve-conduction testing to assess nerve injury as an alternative cause of weakness.10,11 If a neurologic deficit is found on nerve-conduction testing, the patient should be referred for neurologic evaluation.10
Continue to: Patients with negative findings...
Patients with negative findings on MRI and nerve-conduction studies should be offered physical therapy. Patients with recurrent anterior shoulder dislocation should be referred to an orthopedic surgeon for surgical repair. Frequently, improper or delayed treatment with chronic instability results in degenerative arthropathy of the joint22 (FIGURE 10).

Posterior and multidirectional instability can typically be treated conservatively; however, whereas posterior dislocation typically must be immobilized for 3 to 6 weeks post reduction, multidirectional instability does not require immobilization. Instead, physical therapy should start as soon as possible. In these cases, recurrent dislocation or subluxation that persists after conservative treatment should be referred for possible surgical intervention.5
Instability with associated fracture
Fracture concomitant with dislocation most commonly involves the humeral neck, humeral head, greater tuberosity, or the glenoid itself.2 Clinical variables that predict a fracture associated with shoulder dislocation include23:
- first episode of dislocation
- age ≥ 40 years
- fall from higher than 1 flight of stairs
- fight or assault
- motor vehicle crash.
A computed tomography scan with 3-dimensional reconstruction can help characterize associated fracture accurately—including location, size, and displacement—and can play an important role in treatment planning and prognosis in these complicated injuries. Displaced fracture should be referred to an orthopedic surgeon. Nondisplaced fracture of the humeral head or greater tuberosity (FIGURE 11) poses less risk of complications and can be treated conservatively with 6 weeks in an arm sling, followed by physical therapy.24
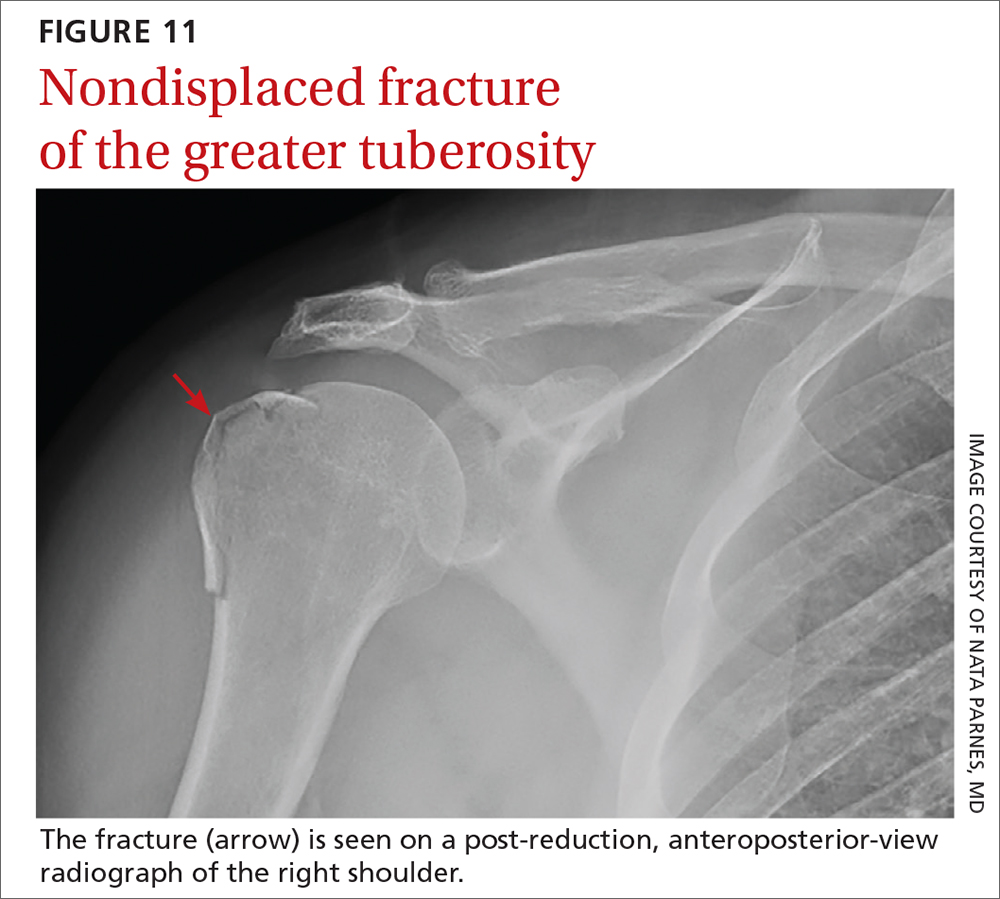
Summing up
Management of shoulder dislocation must, first, be tailored to the individual and, second, account for several interactive factors—including age, direction of instability, functional demands, risk of recurrence, and associated injuries. In many patients, conservative treatment produces a favorable long-term outcome. Particularly in young, active patients with anterior shoulder instability, most surgeons consider open or arthroscopic reconstruction to be the treatment of choice.2,18
Continue to: Pre-reduction and post-reduction...
Pre-reduction and post-reduction imaging should be carefully examined for the presence of concomitant injury, which might change the preferred treatment modality appreciably.
Last, communication among emergency department providers, the primary care provider, orthopedist, radiologist, and neurologist is crucial for determining an appropriate patient-centered approach to initial and long-term management.
CORRESPONDENCE
Nata Parnes, MD, Carthage Area Hospital, 3 Bridge Street, Carthage, NY; [email protected]
1. Valencia Mora M, Ruiz Ibán MA, Heredia JD, et al. Physical exam and evaluation of the unstable shoulder. Open Orthop J. 2017;11(suppl 6, M12):946-956. doi: 10.2174/1874325001711010946
2. Khiami F, A, Loriaut P. Management of recent first-time anterior shoulder dislocation. Orthop Traumatol Surg Res. 2015;101(1 suppl):S51-S57. doi: 10.1016/j.otsr.2014.06.027
3. Antonio GE, Griffith JF, Yu AB, et al. First-time shoulder dislocation: high prevalence of labral injury and age-related differences revealed by MR arthrography. J Magn Reson Imaging. 2007;26:983-991. doi: 10.1002/jmri.21092
4. Carrazzone OL, Tamaoki MJS, Ambra LFM, et al. Prevalence of lesions associated with traumatic recurrent shoulder dislocation. Rev Bras Ortop. 2015;46:281-287. doi: 10.1016/S2255-4971(15)30196-8
5. Mahaffey BL, Smith PA. Shoulder instability in young athletes. Am Fam Physician. 1999;59:2773-2787.
6. Amar E, Maman E, Khashan M, et al. Milch versus Stimson technique for nonsedated reduction of anterior shoulder dislocation: a prospective randomized trial and analysis of factors affecting success. J Shoulder Elbow Surg. 2012;21:1443-1449. doi: 10.1016/j.jse.2012.01.004
7. Jain NB, Wilcox RB 3rd, Katz JN, et al. Clinical examination of the rotator cuff. PM R. 2013;5:45-56. doi: 10.1016/j.pmrj.2012.08.019
8. Lizzio VA, Meta F, Fidai M, et al. Clinical evaluation and physical exam findings in patients with anterior shoulder instability. Curr Rev Musculoskelet Med. 2017;10:434-441. doi: 10.1007/s12178-017-9434-3
9. Farber AJ, Castillo R, Clough M, et al. Clinical assessment of three common tests for traumatic anterior shoulder instability. J Bone Joint Surg Am. 2006;88:1467-1474. doi: 10.2106/JBJS.E.00594
10. Robinson CM, Shur N, Sharpe T, et al. Injuries associated with traumatic anterior glenohumeral dislocations. J Bone Joint Surg Am. 2012;94:18-26. doi: 10.2106/JBJS.J.01795
11. de Laat EA, Visser CP, Coene LN, et al. Nerve lesions in primary shoulder dislocations and humeral neck fractures. A prospective clinical and EMG study. J Bone Joint Surg Br. 1994;76:381-383.
12. Avis D, Power D. Axillary nerve injury associated with glenohumeral dislocation: a review and algorithm for management. EFORT Open Rev. 2018;3:70-77. doi: 10.1302/2058-5241.3.170003
13. Drury JK, Scullion JE. Vascular complications of anterior dislocation of the shoulder. Br J Surg. 1980;67:579-581. doi: 10.1002/bjs.1800670817
14. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010. doi: 10.2174/1874325001711011001
15. te Slaa RL, Brand R, Marti RK. A prospective arthroscopic study of acute first-time anterior shoulder dislocation in the young: a five-year follow-up study. J Shoulder Elbow Surg. 2003;12:529-534. doi: 10.1016/s1058-2746(03)00218-0
16. Kavaja L, T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506. doi: 10.1136/bjsports-2017-098539
17. Krych AJ, Sousa PL, King AH, et al. The effect of cartilage injury after arthroscopic stabilization for shoulder instability. Orthopedics. 2015;38:e965-e969. doi: 10.3928/01477447-20151020-03
18. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
19. Maman E, Harris C, White L, et al. Outcome of nonoperative treatment of symptomatic rotator cuff tears monitored by magnetic resonance imaging. J Bone Joint Surg Am. 2009;91:1898-1906. doi: 10.2106/JBJS.G.01335
20. Safran O, Schroeder J, Bloom R, et al. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39:710-714. doi: 10.1177/0363546510393944
21. Parnes N, Bartoszewski NR, Defranco MJ. Arthroscopic repair of full-thickness rotator cuff tears in active patients younger than 40 years: 2- to 5-year clinical outcomes. Orthopedics 2018;41:e52-e57. doi: 10.3928/01477447-20171114-02
22. Sofu H, Gürsu S, Koçkara N, et al. Recurrent anterior shoulder instability: review of the literature and current concepts. World J Clin Cases. 2014;2:676-682. doi: 10.12998/wjcc.v2.i11.676
23. Emond M, Le Sage N, Lavoie A, et al. Clinical factors predicting fractures associated with an anterior shoulder dislocation. Acad Emerg Med. 2004;11:853-858. doi: 10.1111/j.1553-2712.2004.tb00768.x
24. Parnes N, Jupiter JB. Fixed-angle locking plating of displaced proximal humerus fractures. Instr Course Lect. 2010;59:539-552.
The architecture of the glenohumeral joint makes it the most common large joint to become dislocated, accounting for approximately 45% of all dislocations. Anterior dislocation constitutes more than 95% of glenohumeral joint dislocations; posterior dislocation, only 2% to 5%.1,2
For the family physician, determining appropriate follow-up after emergent reduction depends on several distinct variables, which we review here; subsequent treatment might involve, as we outline, physical therapy, immobilization, surgical intervention, or a combination of several modalities. Treatment decisions can make the difference between successful rehabilitation and potential disability, particularly in typically young and active patients.
Numerous mechanisms of injury
Anterior shoulder dislocations typically occur with the affected shoulder in a position of abduction and external rotation; 90% of patients are 21 to 30 years of age, and men are affected 3 times more often than women.2 Unsurprisingly, athletes are affected most frequently, with the common sports-related mechanism of injury being either sudden pressure exerted on the abducted and externally rotated arm or a fall onto an outstretched hand with the arm elevated. Repetitive microtrauma from such sports as swimming, baseball, and volleyball can also lead to instability.
Bankart lesion. This tear of the anterior or inferior section of the labrum is the most characteristic lesion noted in anterior dislocations, found in 73% of first-time dislocations and 100% of recurrent dislocations.3,4
Hills-Sachs lesion is often associated with a Bankart lesion. The Hills-Sachs lesion is an impaction fracture of the posterolateral aspect of the humeral head resulting from its displacement over the anterior lip of the glenoid. Hill-Sachs lesions are seen in 71% of first-time and recurrent dislocations.3
Less common concomitant injuries during anterior shoulder dislocation include rupture of the rotator-cuff tendons (particularly in patients older than 40 years), glenoid and proximal humerus fractures, a tear of the superior labrum (known as a “SLAP lesion”), cartilage injury, and neurovascular injury.
Posterior instability typically occurs as a result of a strong muscle contraction, as seen in electrocution or seizure; however, it can be caused by athletic trauma, particularly in football.5 Repetitive forces exerted on the forward-flexed and internally rotated shoulder position during blocking puts football players at increased risk of posterior instability.5
Continue to: Multidirectional instability
Multidirectional instability is more frequently attributable to congenital hyperlaxity of the glenohumeral joint capsule, rather than to acute injury. However, athletes can also develop capsular laxity from repetitive microtrauma to the shoulder.5
Emergent reduction: Prompt action needed
Acute dislocation of the shoulder should be reduced as soon as possible to minimize neurovascular injury and patient discomfort. (Typically, but not always, this is done in the emergency department.) It is crucial to have effective muscle relaxation before any attempt at reduction, to minimize the risk of iatrogenic injury to bone, cartilage, tendons, and neurovascular structures.
Muscle relaxation can be facilitated with intravenous midazolam or other agents, as specified by institutional protocol. Intra-articular lidocaine injection or intravenous fentanyl is often utilized in conjunction with the sedating agent to reduce pain and further accommodate relaxation.
Anterior reduction. Any one of several techniques can be used to perform emergent reduction of anterior shoulder dislocations, all of which have demonstrated success. The Milch technique is among the least traumatic for effective reduction.6 In this technique (FIGURE 1), the patient is supine; gentle but firm downward traction is applied to the humerus at the elbow of the affected arm while the arm is in abduction and external rotation. The provider can manipulate the humeral head at that point by placing a thumb in the patient’s axilla; the arm can also be further internally rotated and adducted until reduction is achieved.

Posterior reduction of a dislocation is performed while the patient is supine, with the body stabilized. Traction is applied on the adducted and internally rotated arm in conjunction with direct pressure on the posterior aspect of the humeral head (FIGURE 2).

Continue to: Follow-up actions
Follow-up actions. Before discharging the patient after reduction of a dislocation, it is essential to:
- perform post-reduction evaluation of shoulder stability at different levels of abduction
- perform a thorough neurovascular assessment
- obtain an anteroposterior (AP) radiograph to ensure proper positioning of the glenohumeral joint.
The reduced shoulder should be immobilized in a sling. The discharge plan should include pain management for several days and a follow-up appointment in 5 to 8 days with the primary care provider2 (FIGURE 3).

Follow-up evaluation by the primary care provider
History. Prior to the initial examination at follow-up, obtain a comprehensive history that includes the nature of the injury and the direction of force that was placed on the shoulder. Determine whether the shoulder was reduced spontaneously or required manual reduction in the field or an emergency department. Note any associated injury sustained concurrently and the presence (or absence) of neck pain, numbness, tingling, or weakness in the affected arm.
Physical exam starts with thorough inspection of the affected shoulder, with comparison to the contralateral side, at rest and during shoulder motion. Palpation to reveal points of tenderness should include the anterior joint line, acromioclavicular joint, bicipital groove, subacromial space, acromion, and greater tuberosity.
Following inspection and palpation, assess active and passive range of motion in forward elevation, abduction, internal and external rotation at the side of the body, and internal and external rotation in shoulder abduction. Assessment might be limited by pain and apprehension, and should be performed within the patient’s comfortable range of motion.
Continue to: Once range of motion...
Once range of motion is determined, assess7:
- muscle power of the rotator cuff in abduction (for the supraspinatus muscle)
- resisted external rotation at the side of the body (the infraspinatus)
- resisted external rotation in abduction > 60° (the teres minor)
- resisted internal rotation (the subscapularis).
Specific tests for shoulder laxity and stability
It is important during the primary care follow-up examination to differentiate true instability and shoulder hyperlaxity, particularly in young, flexible patients (TABLE). Many of these patients present with painless hypermobility of the shoulder without true injury to the labrum or ligamentous structures. It might appear to the patient, or to family, that the shoulder is subluxating; however, the humeral head returns to a centered position on the glenoid in a hypermobile state—typically, without pain. Actual shoulder instability is defined as loss of the ability of the humeral head to re-center, accompanied by pain—pathology that is frequently associated with damage to the capsulolabral complex.

The load and shift test is used to assess anterior and posterior laxity. The patient is seated, and the forearm is allowed to rest on the thigh. Examination is performed using 1 hand to press anteriorly or posteriorly on the humeral head; the other hand is simultaneously positioned on the joint line to feel movement of the humeral head in relation to the glenoid (FIGURE 4).

The apprehension test is a common maneuver used to assess anterior shoulder instability. It is performed by positioning the affected arm to 90° external rotation and then elevating it to 90° abduction. Although this maneuver can be performed with the patient upright, it is beneficial to have them supine, to more easily control the arm (FIGURE 5). A positive test is noted when the patient reports a sensation of impending instability (apprehension), rather than pain alone.

Relocation test. When the apprehension test is positive, the supine position can be exploited to further perform the relocation test, in 2 stages (FIGURE 6):
- Apply a posteriorly directed force on the humeral head, which stabilizes the shoulder and typically alleviates symptoms.
- Release pressure quickly from the humeral head to assess recurrence of pain and apprehension as the humeral head snaps back against the anterior labrum.

Continue to: Combined, apprehension and relocation...
Combined, apprehension and relocation tests to identify anterior shoulder instability have been shown to significantly improve specificity while maintaining sensitivity.8
The posterior apprehension test is used to assess posterior instability. The patient is supine; the affected arm is placed in flexion, adduction, and internal rotation; and posterior pressure is applied (FIGURE 7). A positive test is noted when pain is reported at the posterior aspect of the shoulder. Clicking might be noted as the humeral head dislocates rearward.1

Sulcus sign. Multidirectional instability is elicited using the sulcus sign. While the patient is seated upright, arms resting at their sides, a direct downward pull at elbow level will, when positive, reveal a depression (sulcus) at the lateral aspect of the affected shoulder as the humeral head translates inferiorly (FIGURE 8). A positive sulcus sign is documented in 3 grades, according to the amount of translation1:
- Grade I: < 1 cm
- Grade II: 1-2 cm
- Grade III: > 2 cm.

Neurovascular status should be verified at every physical evaluation, with motor and sensory function tested in the axillary, musculocutaneous, median, radial, and ulnar nerve distributions. If nerve injury is suspected, electromyography and nerve-conduction testing is indicated.9-13 Vascular compromise is much less common but equally important to assess.11
Use of imaging
Post-reduction radiographs, including internal and external AP—and especially axillary—views are invaluable. Not only do they help to ensure reduction, but they also help to assess for fracture. A magnetic resonance imaging (MRI) arthrogram is the preferred imaging modality if a labral tear is suspected (FIGURE 9). Other concomitant shoulder injuries, such as subtle bone fracture, rotator cuff tear, and biceps pathology can also be reliably diagnosed with noncontrast MRI.

Continue to: Roadmap for treatment
Roadmap for treatment
The rate of recurrence after a first anterior shoulder dislocation is strongly associated with a person’s age and level of activity. Active patients younger than 20 years have a 92% to 96% recurrence rate14; patients 20 to 40 years, 25% to 48%; and patients older than 40 years, < 10%.15
Young, athletic patients who are treated nonoperatively are left at an unacceptably high risk of recurrence, leading to progressive damage to bony and soft-tissue structures.16,17 Surgical labral repair after a first-time anterior dislocation produced improved outcomes in terms of recurrent dislocation (7.9%), compared to outcomes after nonsurgical treatment (52.9%),14 and has been associated with a lower incidence of future glenohumeral osteoarthritis.18 For those reasons, we recommend referral to an orthopedic surgeon for all patients younger than 20 years who sustain an anterior shoulder dislocation.
Patients older than 20 years who do not have concomitant shoulder injury, and who demonstrate full strength in abduction, external rotation, and internal rotation of the shoulder on clinical examination, have a low probability of associated rotator-cuff tear. They can be immobilized in a sling for 1 to 3 weeks, followed by a 6 to 12–week regimen of physical therapy.
Concomitant tear of the rotator cuff. Weakness on examination requires MRI or a magnetic resonance arthrogram for evaluation of associated rotator-cuff tear. A tear identified on MRI should be referred to an orthopedic surgeon because timely repair can be crucial to attaining best outcomes. Conservative treatment of traumatic full-tendon rotator-cuff tear is associated with poor results, progression in the size of the tear, and advancement of muscle atrophy.19,20 For patients younger than 40 years, arthroscopic rotator-cuff repair, with or without labral repair, produces excellent clinical outcomes, carries a low risk of complications, and results in a > 95% rate of return to a preoperative level of recreational and job activities.21
Patients who demonstrate weakness of the rotator-cuff muscles on examination, but who do not have a tear noted on MRI, should be evaluated by electromyography and nerve-conduction testing to assess nerve injury as an alternative cause of weakness.10,11 If a neurologic deficit is found on nerve-conduction testing, the patient should be referred for neurologic evaluation.10
Continue to: Patients with negative findings...
Patients with negative findings on MRI and nerve-conduction studies should be offered physical therapy. Patients with recurrent anterior shoulder dislocation should be referred to an orthopedic surgeon for surgical repair. Frequently, improper or delayed treatment with chronic instability results in degenerative arthropathy of the joint22 (FIGURE 10).

Posterior and multidirectional instability can typically be treated conservatively; however, whereas posterior dislocation typically must be immobilized for 3 to 6 weeks post reduction, multidirectional instability does not require immobilization. Instead, physical therapy should start as soon as possible. In these cases, recurrent dislocation or subluxation that persists after conservative treatment should be referred for possible surgical intervention.5
Instability with associated fracture
Fracture concomitant with dislocation most commonly involves the humeral neck, humeral head, greater tuberosity, or the glenoid itself.2 Clinical variables that predict a fracture associated with shoulder dislocation include23:
- first episode of dislocation
- age ≥ 40 years
- fall from higher than 1 flight of stairs
- fight or assault
- motor vehicle crash.
A computed tomography scan with 3-dimensional reconstruction can help characterize associated fracture accurately—including location, size, and displacement—and can play an important role in treatment planning and prognosis in these complicated injuries. Displaced fracture should be referred to an orthopedic surgeon. Nondisplaced fracture of the humeral head or greater tuberosity (FIGURE 11) poses less risk of complications and can be treated conservatively with 6 weeks in an arm sling, followed by physical therapy.24

Summing up
Management of shoulder dislocation must, first, be tailored to the individual and, second, account for several interactive factors—including age, direction of instability, functional demands, risk of recurrence, and associated injuries. In many patients, conservative treatment produces a favorable long-term outcome. Particularly in young, active patients with anterior shoulder instability, most surgeons consider open or arthroscopic reconstruction to be the treatment of choice.2,18
Continue to: Pre-reduction and post-reduction...
Pre-reduction and post-reduction imaging should be carefully examined for the presence of concomitant injury, which might change the preferred treatment modality appreciably.
Last, communication among emergency department providers, the primary care provider, orthopedist, radiologist, and neurologist is crucial for determining an appropriate patient-centered approach to initial and long-term management.
CORRESPONDENCE
Nata Parnes, MD, Carthage Area Hospital, 3 Bridge Street, Carthage, NY; [email protected]
The architecture of the glenohumeral joint makes it the most common large joint to become dislocated, accounting for approximately 45% of all dislocations. Anterior dislocation constitutes more than 95% of glenohumeral joint dislocations; posterior dislocation, only 2% to 5%.1,2
For the family physician, determining appropriate follow-up after emergent reduction depends on several distinct variables, which we review here; subsequent treatment might involve, as we outline, physical therapy, immobilization, surgical intervention, or a combination of several modalities. Treatment decisions can make the difference between successful rehabilitation and potential disability, particularly in typically young and active patients.
Numerous mechanisms of injury
Anterior shoulder dislocations typically occur with the affected shoulder in a position of abduction and external rotation; 90% of patients are 21 to 30 years of age, and men are affected 3 times more often than women.2 Unsurprisingly, athletes are affected most frequently, with the common sports-related mechanism of injury being either sudden pressure exerted on the abducted and externally rotated arm or a fall onto an outstretched hand with the arm elevated. Repetitive microtrauma from such sports as swimming, baseball, and volleyball can also lead to instability.
Bankart lesion. This tear of the anterior or inferior section of the labrum is the most characteristic lesion noted in anterior dislocations, found in 73% of first-time dislocations and 100% of recurrent dislocations.3,4
Hills-Sachs lesion is often associated with a Bankart lesion. The Hills-Sachs lesion is an impaction fracture of the posterolateral aspect of the humeral head resulting from its displacement over the anterior lip of the glenoid. Hill-Sachs lesions are seen in 71% of first-time and recurrent dislocations.3
Less common concomitant injuries during anterior shoulder dislocation include rupture of the rotator-cuff tendons (particularly in patients older than 40 years), glenoid and proximal humerus fractures, a tear of the superior labrum (known as a “SLAP lesion”), cartilage injury, and neurovascular injury.
Posterior instability typically occurs as a result of a strong muscle contraction, as seen in electrocution or seizure; however, it can be caused by athletic trauma, particularly in football.5 Repetitive forces exerted on the forward-flexed and internally rotated shoulder position during blocking puts football players at increased risk of posterior instability.5
Continue to: Multidirectional instability
Multidirectional instability is more frequently attributable to congenital hyperlaxity of the glenohumeral joint capsule, rather than to acute injury. However, athletes can also develop capsular laxity from repetitive microtrauma to the shoulder.5
Emergent reduction: Prompt action needed
Acute dislocation of the shoulder should be reduced as soon as possible to minimize neurovascular injury and patient discomfort. (Typically, but not always, this is done in the emergency department.) It is crucial to have effective muscle relaxation before any attempt at reduction, to minimize the risk of iatrogenic injury to bone, cartilage, tendons, and neurovascular structures.
Muscle relaxation can be facilitated with intravenous midazolam or other agents, as specified by institutional protocol. Intra-articular lidocaine injection or intravenous fentanyl is often utilized in conjunction with the sedating agent to reduce pain and further accommodate relaxation.
Anterior reduction. Any one of several techniques can be used to perform emergent reduction of anterior shoulder dislocations, all of which have demonstrated success. The Milch technique is among the least traumatic for effective reduction.6 In this technique (FIGURE 1), the patient is supine; gentle but firm downward traction is applied to the humerus at the elbow of the affected arm while the arm is in abduction and external rotation. The provider can manipulate the humeral head at that point by placing a thumb in the patient’s axilla; the arm can also be further internally rotated and adducted until reduction is achieved.

Posterior reduction of a dislocation is performed while the patient is supine, with the body stabilized. Traction is applied on the adducted and internally rotated arm in conjunction with direct pressure on the posterior aspect of the humeral head (FIGURE 2).

Continue to: Follow-up actions
Follow-up actions. Before discharging the patient after reduction of a dislocation, it is essential to:
- perform post-reduction evaluation of shoulder stability at different levels of abduction
- perform a thorough neurovascular assessment
- obtain an anteroposterior (AP) radiograph to ensure proper positioning of the glenohumeral joint.
The reduced shoulder should be immobilized in a sling. The discharge plan should include pain management for several days and a follow-up appointment in 5 to 8 days with the primary care provider2 (FIGURE 3).

Follow-up evaluation by the primary care provider
History. Prior to the initial examination at follow-up, obtain a comprehensive history that includes the nature of the injury and the direction of force that was placed on the shoulder. Determine whether the shoulder was reduced spontaneously or required manual reduction in the field or an emergency department. Note any associated injury sustained concurrently and the presence (or absence) of neck pain, numbness, tingling, or weakness in the affected arm.
Physical exam starts with thorough inspection of the affected shoulder, with comparison to the contralateral side, at rest and during shoulder motion. Palpation to reveal points of tenderness should include the anterior joint line, acromioclavicular joint, bicipital groove, subacromial space, acromion, and greater tuberosity.
Following inspection and palpation, assess active and passive range of motion in forward elevation, abduction, internal and external rotation at the side of the body, and internal and external rotation in shoulder abduction. Assessment might be limited by pain and apprehension, and should be performed within the patient’s comfortable range of motion.
Continue to: Once range of motion...
Once range of motion is determined, assess7:
- muscle power of the rotator cuff in abduction (for the supraspinatus muscle)
- resisted external rotation at the side of the body (the infraspinatus)
- resisted external rotation in abduction > 60° (the teres minor)
- resisted internal rotation (the subscapularis).
Specific tests for shoulder laxity and stability
It is important during the primary care follow-up examination to differentiate true instability and shoulder hyperlaxity, particularly in young, flexible patients (TABLE). Many of these patients present with painless hypermobility of the shoulder without true injury to the labrum or ligamentous structures. It might appear to the patient, or to family, that the shoulder is subluxating; however, the humeral head returns to a centered position on the glenoid in a hypermobile state—typically, without pain. Actual shoulder instability is defined as loss of the ability of the humeral head to re-center, accompanied by pain—pathology that is frequently associated with damage to the capsulolabral complex.

The load and shift test is used to assess anterior and posterior laxity. The patient is seated, and the forearm is allowed to rest on the thigh. Examination is performed using 1 hand to press anteriorly or posteriorly on the humeral head; the other hand is simultaneously positioned on the joint line to feel movement of the humeral head in relation to the glenoid (FIGURE 4).

The apprehension test is a common maneuver used to assess anterior shoulder instability. It is performed by positioning the affected arm to 90° external rotation and then elevating it to 90° abduction. Although this maneuver can be performed with the patient upright, it is beneficial to have them supine, to more easily control the arm (FIGURE 5). A positive test is noted when the patient reports a sensation of impending instability (apprehension), rather than pain alone.

Relocation test. When the apprehension test is positive, the supine position can be exploited to further perform the relocation test, in 2 stages (FIGURE 6):
- Apply a posteriorly directed force on the humeral head, which stabilizes the shoulder and typically alleviates symptoms.
- Release pressure quickly from the humeral head to assess recurrence of pain and apprehension as the humeral head snaps back against the anterior labrum.

Continue to: Combined, apprehension and relocation...
Combined, apprehension and relocation tests to identify anterior shoulder instability have been shown to significantly improve specificity while maintaining sensitivity.8
The posterior apprehension test is used to assess posterior instability. The patient is supine; the affected arm is placed in flexion, adduction, and internal rotation; and posterior pressure is applied (FIGURE 7). A positive test is noted when pain is reported at the posterior aspect of the shoulder. Clicking might be noted as the humeral head dislocates rearward.1

Sulcus sign. Multidirectional instability is elicited using the sulcus sign. While the patient is seated upright, arms resting at their sides, a direct downward pull at elbow level will, when positive, reveal a depression (sulcus) at the lateral aspect of the affected shoulder as the humeral head translates inferiorly (FIGURE 8). A positive sulcus sign is documented in 3 grades, according to the amount of translation1:
- Grade I: < 1 cm
- Grade II: 1-2 cm
- Grade III: > 2 cm.

Neurovascular status should be verified at every physical evaluation, with motor and sensory function tested in the axillary, musculocutaneous, median, radial, and ulnar nerve distributions. If nerve injury is suspected, electromyography and nerve-conduction testing is indicated.9-13 Vascular compromise is much less common but equally important to assess.11
Use of imaging
Post-reduction radiographs, including internal and external AP—and especially axillary—views are invaluable. Not only do they help to ensure reduction, but they also help to assess for fracture. A magnetic resonance imaging (MRI) arthrogram is the preferred imaging modality if a labral tear is suspected (FIGURE 9). Other concomitant shoulder injuries, such as subtle bone fracture, rotator cuff tear, and biceps pathology can also be reliably diagnosed with noncontrast MRI.

Continue to: Roadmap for treatment
Roadmap for treatment
The rate of recurrence after a first anterior shoulder dislocation is strongly associated with a person’s age and level of activity. Active patients younger than 20 years have a 92% to 96% recurrence rate14; patients 20 to 40 years, 25% to 48%; and patients older than 40 years, < 10%.15
Young, athletic patients who are treated nonoperatively are left at an unacceptably high risk of recurrence, leading to progressive damage to bony and soft-tissue structures.16,17 Surgical labral repair after a first-time anterior dislocation produced improved outcomes in terms of recurrent dislocation (7.9%), compared to outcomes after nonsurgical treatment (52.9%),14 and has been associated with a lower incidence of future glenohumeral osteoarthritis.18 For those reasons, we recommend referral to an orthopedic surgeon for all patients younger than 20 years who sustain an anterior shoulder dislocation.
Patients older than 20 years who do not have concomitant shoulder injury, and who demonstrate full strength in abduction, external rotation, and internal rotation of the shoulder on clinical examination, have a low probability of associated rotator-cuff tear. They can be immobilized in a sling for 1 to 3 weeks, followed by a 6 to 12–week regimen of physical therapy.
Concomitant tear of the rotator cuff. Weakness on examination requires MRI or a magnetic resonance arthrogram for evaluation of associated rotator-cuff tear. A tear identified on MRI should be referred to an orthopedic surgeon because timely repair can be crucial to attaining best outcomes. Conservative treatment of traumatic full-tendon rotator-cuff tear is associated with poor results, progression in the size of the tear, and advancement of muscle atrophy.19,20 For patients younger than 40 years, arthroscopic rotator-cuff repair, with or without labral repair, produces excellent clinical outcomes, carries a low risk of complications, and results in a > 95% rate of return to a preoperative level of recreational and job activities.21
Patients who demonstrate weakness of the rotator-cuff muscles on examination, but who do not have a tear noted on MRI, should be evaluated by electromyography and nerve-conduction testing to assess nerve injury as an alternative cause of weakness.10,11 If a neurologic deficit is found on nerve-conduction testing, the patient should be referred for neurologic evaluation.10
Continue to: Patients with negative findings...
Patients with negative findings on MRI and nerve-conduction studies should be offered physical therapy. Patients with recurrent anterior shoulder dislocation should be referred to an orthopedic surgeon for surgical repair. Frequently, improper or delayed treatment with chronic instability results in degenerative arthropathy of the joint22 (FIGURE 10).

Posterior and multidirectional instability can typically be treated conservatively; however, whereas posterior dislocation typically must be immobilized for 3 to 6 weeks post reduction, multidirectional instability does not require immobilization. Instead, physical therapy should start as soon as possible. In these cases, recurrent dislocation or subluxation that persists after conservative treatment should be referred for possible surgical intervention.5
Instability with associated fracture
Fracture concomitant with dislocation most commonly involves the humeral neck, humeral head, greater tuberosity, or the glenoid itself.2 Clinical variables that predict a fracture associated with shoulder dislocation include23:
- first episode of dislocation
- age ≥ 40 years
- fall from higher than 1 flight of stairs
- fight or assault
- motor vehicle crash.
A computed tomography scan with 3-dimensional reconstruction can help characterize associated fracture accurately—including location, size, and displacement—and can play an important role in treatment planning and prognosis in these complicated injuries. Displaced fracture should be referred to an orthopedic surgeon. Nondisplaced fracture of the humeral head or greater tuberosity (FIGURE 11) poses less risk of complications and can be treated conservatively with 6 weeks in an arm sling, followed by physical therapy.24

Summing up
Management of shoulder dislocation must, first, be tailored to the individual and, second, account for several interactive factors—including age, direction of instability, functional demands, risk of recurrence, and associated injuries. In many patients, conservative treatment produces a favorable long-term outcome. Particularly in young, active patients with anterior shoulder instability, most surgeons consider open or arthroscopic reconstruction to be the treatment of choice.2,18
Continue to: Pre-reduction and post-reduction...
Pre-reduction and post-reduction imaging should be carefully examined for the presence of concomitant injury, which might change the preferred treatment modality appreciably.
Last, communication among emergency department providers, the primary care provider, orthopedist, radiologist, and neurologist is crucial for determining an appropriate patient-centered approach to initial and long-term management.
CORRESPONDENCE
Nata Parnes, MD, Carthage Area Hospital, 3 Bridge Street, Carthage, NY; [email protected]
1. Valencia Mora M, Ruiz Ibán MA, Heredia JD, et al. Physical exam and evaluation of the unstable shoulder. Open Orthop J. 2017;11(suppl 6, M12):946-956. doi: 10.2174/1874325001711010946
2. Khiami F, A, Loriaut P. Management of recent first-time anterior shoulder dislocation. Orthop Traumatol Surg Res. 2015;101(1 suppl):S51-S57. doi: 10.1016/j.otsr.2014.06.027
3. Antonio GE, Griffith JF, Yu AB, et al. First-time shoulder dislocation: high prevalence of labral injury and age-related differences revealed by MR arthrography. J Magn Reson Imaging. 2007;26:983-991. doi: 10.1002/jmri.21092
4. Carrazzone OL, Tamaoki MJS, Ambra LFM, et al. Prevalence of lesions associated with traumatic recurrent shoulder dislocation. Rev Bras Ortop. 2015;46:281-287. doi: 10.1016/S2255-4971(15)30196-8
5. Mahaffey BL, Smith PA. Shoulder instability in young athletes. Am Fam Physician. 1999;59:2773-2787.
6. Amar E, Maman E, Khashan M, et al. Milch versus Stimson technique for nonsedated reduction of anterior shoulder dislocation: a prospective randomized trial and analysis of factors affecting success. J Shoulder Elbow Surg. 2012;21:1443-1449. doi: 10.1016/j.jse.2012.01.004
7. Jain NB, Wilcox RB 3rd, Katz JN, et al. Clinical examination of the rotator cuff. PM R. 2013;5:45-56. doi: 10.1016/j.pmrj.2012.08.019
8. Lizzio VA, Meta F, Fidai M, et al. Clinical evaluation and physical exam findings in patients with anterior shoulder instability. Curr Rev Musculoskelet Med. 2017;10:434-441. doi: 10.1007/s12178-017-9434-3
9. Farber AJ, Castillo R, Clough M, et al. Clinical assessment of three common tests for traumatic anterior shoulder instability. J Bone Joint Surg Am. 2006;88:1467-1474. doi: 10.2106/JBJS.E.00594
10. Robinson CM, Shur N, Sharpe T, et al. Injuries associated with traumatic anterior glenohumeral dislocations. J Bone Joint Surg Am. 2012;94:18-26. doi: 10.2106/JBJS.J.01795
11. de Laat EA, Visser CP, Coene LN, et al. Nerve lesions in primary shoulder dislocations and humeral neck fractures. A prospective clinical and EMG study. J Bone Joint Surg Br. 1994;76:381-383.
12. Avis D, Power D. Axillary nerve injury associated with glenohumeral dislocation: a review and algorithm for management. EFORT Open Rev. 2018;3:70-77. doi: 10.1302/2058-5241.3.170003
13. Drury JK, Scullion JE. Vascular complications of anterior dislocation of the shoulder. Br J Surg. 1980;67:579-581. doi: 10.1002/bjs.1800670817
14. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010. doi: 10.2174/1874325001711011001
15. te Slaa RL, Brand R, Marti RK. A prospective arthroscopic study of acute first-time anterior shoulder dislocation in the young: a five-year follow-up study. J Shoulder Elbow Surg. 2003;12:529-534. doi: 10.1016/s1058-2746(03)00218-0
16. Kavaja L, T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506. doi: 10.1136/bjsports-2017-098539
17. Krych AJ, Sousa PL, King AH, et al. The effect of cartilage injury after arthroscopic stabilization for shoulder instability. Orthopedics. 2015;38:e965-e969. doi: 10.3928/01477447-20151020-03
18. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
19. Maman E, Harris C, White L, et al. Outcome of nonoperative treatment of symptomatic rotator cuff tears monitored by magnetic resonance imaging. J Bone Joint Surg Am. 2009;91:1898-1906. doi: 10.2106/JBJS.G.01335
20. Safran O, Schroeder J, Bloom R, et al. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39:710-714. doi: 10.1177/0363546510393944
21. Parnes N, Bartoszewski NR, Defranco MJ. Arthroscopic repair of full-thickness rotator cuff tears in active patients younger than 40 years: 2- to 5-year clinical outcomes. Orthopedics 2018;41:e52-e57. doi: 10.3928/01477447-20171114-02
22. Sofu H, Gürsu S, Koçkara N, et al. Recurrent anterior shoulder instability: review of the literature and current concepts. World J Clin Cases. 2014;2:676-682. doi: 10.12998/wjcc.v2.i11.676
23. Emond M, Le Sage N, Lavoie A, et al. Clinical factors predicting fractures associated with an anterior shoulder dislocation. Acad Emerg Med. 2004;11:853-858. doi: 10.1111/j.1553-2712.2004.tb00768.x
24. Parnes N, Jupiter JB. Fixed-angle locking plating of displaced proximal humerus fractures. Instr Course Lect. 2010;59:539-552.
1. Valencia Mora M, Ruiz Ibán MA, Heredia JD, et al. Physical exam and evaluation of the unstable shoulder. Open Orthop J. 2017;11(suppl 6, M12):946-956. doi: 10.2174/1874325001711010946
2. Khiami F, A, Loriaut P. Management of recent first-time anterior shoulder dislocation. Orthop Traumatol Surg Res. 2015;101(1 suppl):S51-S57. doi: 10.1016/j.otsr.2014.06.027
3. Antonio GE, Griffith JF, Yu AB, et al. First-time shoulder dislocation: high prevalence of labral injury and age-related differences revealed by MR arthrography. J Magn Reson Imaging. 2007;26:983-991. doi: 10.1002/jmri.21092
4. Carrazzone OL, Tamaoki MJS, Ambra LFM, et al. Prevalence of lesions associated with traumatic recurrent shoulder dislocation. Rev Bras Ortop. 2015;46:281-287. doi: 10.1016/S2255-4971(15)30196-8
5. Mahaffey BL, Smith PA. Shoulder instability in young athletes. Am Fam Physician. 1999;59:2773-2787.
6. Amar E, Maman E, Khashan M, et al. Milch versus Stimson technique for nonsedated reduction of anterior shoulder dislocation: a prospective randomized trial and analysis of factors affecting success. J Shoulder Elbow Surg. 2012;21:1443-1449. doi: 10.1016/j.jse.2012.01.004
7. Jain NB, Wilcox RB 3rd, Katz JN, et al. Clinical examination of the rotator cuff. PM R. 2013;5:45-56. doi: 10.1016/j.pmrj.2012.08.019
8. Lizzio VA, Meta F, Fidai M, et al. Clinical evaluation and physical exam findings in patients with anterior shoulder instability. Curr Rev Musculoskelet Med. 2017;10:434-441. doi: 10.1007/s12178-017-9434-3
9. Farber AJ, Castillo R, Clough M, et al. Clinical assessment of three common tests for traumatic anterior shoulder instability. J Bone Joint Surg Am. 2006;88:1467-1474. doi: 10.2106/JBJS.E.00594
10. Robinson CM, Shur N, Sharpe T, et al. Injuries associated with traumatic anterior glenohumeral dislocations. J Bone Joint Surg Am. 2012;94:18-26. doi: 10.2106/JBJS.J.01795
11. de Laat EA, Visser CP, Coene LN, et al. Nerve lesions in primary shoulder dislocations and humeral neck fractures. A prospective clinical and EMG study. J Bone Joint Surg Br. 1994;76:381-383.
12. Avis D, Power D. Axillary nerve injury associated with glenohumeral dislocation: a review and algorithm for management. EFORT Open Rev. 2018;3:70-77. doi: 10.1302/2058-5241.3.170003
13. Drury JK, Scullion JE. Vascular complications of anterior dislocation of the shoulder. Br J Surg. 1980;67:579-581. doi: 10.1002/bjs.1800670817
14. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010. doi: 10.2174/1874325001711011001
15. te Slaa RL, Brand R, Marti RK. A prospective arthroscopic study of acute first-time anterior shoulder dislocation in the young: a five-year follow-up study. J Shoulder Elbow Surg. 2003;12:529-534. doi: 10.1016/s1058-2746(03)00218-0
16. Kavaja L, T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506. doi: 10.1136/bjsports-2017-098539
17. Krych AJ, Sousa PL, King AH, et al. The effect of cartilage injury after arthroscopic stabilization for shoulder instability. Orthopedics. 2015;38:e965-e969. doi: 10.3928/01477447-20151020-03
18. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
19. Maman E, Harris C, White L, et al. Outcome of nonoperative treatment of symptomatic rotator cuff tears monitored by magnetic resonance imaging. J Bone Joint Surg Am. 2009;91:1898-1906. doi: 10.2106/JBJS.G.01335
20. Safran O, Schroeder J, Bloom R, et al. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39:710-714. doi: 10.1177/0363546510393944
21. Parnes N, Bartoszewski NR, Defranco MJ. Arthroscopic repair of full-thickness rotator cuff tears in active patients younger than 40 years: 2- to 5-year clinical outcomes. Orthopedics 2018;41:e52-e57. doi: 10.3928/01477447-20171114-02
22. Sofu H, Gürsu S, Koçkara N, et al. Recurrent anterior shoulder instability: review of the literature and current concepts. World J Clin Cases. 2014;2:676-682. doi: 10.12998/wjcc.v2.i11.676
23. Emond M, Le Sage N, Lavoie A, et al. Clinical factors predicting fractures associated with an anterior shoulder dislocation. Acad Emerg Med. 2004;11:853-858. doi: 10.1111/j.1553-2712.2004.tb00768.x
24. Parnes N, Jupiter JB. Fixed-angle locking plating of displaced proximal humerus fractures. Instr Course Lect. 2010;59:539-552.
PRACTICE RECOMMENDATIONS
› Refer first-time dislocation in patients younger than 20 years or who have a displaced fracture to an orthopedic surgeon. A
› Order magnetic resonance imaging (MRI) for all patients with a suspected rotator cuff tear. A
› Send patients with weakness of the rotator cuff—but no tear on MRI—for evaluation by electromyography and nerve-conduction studies. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 4-pronged approach to foster healthy aging in older adults
Our approach to caring for the growing number of community-dwelling US adults ages ≥ 65 years has shifted. Although we continue to manage disease and disability, there is an increasing emphasis on the promotion of healthy aging by optimizing health care needs and quality of life (QOL).
The American Geriatric Society (AGS) uses the term “healthy aging” to reflect a dedication to improving the health, independence, and QOL of older people.1 The World Health Organization (WHO) defines healthy aging as “the process of developing and maintaining the functional ability that enables well-being in older age.”2 Functional ability encompasses capabilities that align with a person’s values, including meeting basic needs; learning, growing, and making independent decisions; being mobile; building and maintaining healthy relationships; and contributing to society.2 Similarly, the US Department of Health and Human Services has adopted a multidimensional approach to support people in creating “a productive and meaningful life” as they grow older.3
Numerous theoretical models have emerged from research on aging as a multidimensional construct, as evidenced by a 2016 citation analysis that identified 1755 articles written between 1902 and 2015 relating to “successful aging.”4 The analysis revealed 609 definitions operationalized by researchers’ measurement tools (mostly focused on physical function and other health metrics) and 1146 descriptions created by older adults, many emphasizing psychosocial strategies and cultural factors as key to successful aging.4

One approach that is likely to be useful for family physicians is the Age-Friendly Health System. This is an initiative of The John A. Hartford Foundation and the Institute for Healthcare Improvement that uses a multidisciplinary approach to create environments that foster inclusivity and address the needs of older people.5 Following this guidance, primary care providers use evidence-informed strategies that promote safety and address what matters most to older adults and their family caregivers.
The Age-Friendly Health System, as well as AGS and WHO, recognize that there are multiple aspects to well-being as one grows older. By using focused, evidence-based screening, assessments, and interventions, family physicians can best support aging patients in living their most fulfilling lives.
Here we present a review of evidence-based strategies that promote safety and address what matters most to older adults and their family caregivers using a 4-pronged framework, in the style of the Age-Friendly Health System model. However, the literature on healthy aging includes important messages about patient context and lifelong health behaviors, which we capture in an expanded set of thematic guidance. As such, we encourage family physicians to approach healthy aging as follows: (1) monitor health (screening and prevention), (2) promote mobility (physical function), (3) manage mentation (emotional health and cognitive function), and (4) encourage maintenance of social connections (social networks and QOL).
Monitoring health
Leverage Medicare annual wellness visits. A systematic approach is needed to prevent frailty and functional decline, and thus increase the QOL of older adults. To do this, it is important to focus on health promotion and disease prevention, while addressing existing ailments. One method is to leverage the Medicare annual wellness visit (AWV), which provides an opportunity to assess current health status as well as discuss behavior-change and risk-reduction strategies with patients.
Continue to: Although AWVs...
Although AWVs are an opportunity to improve patient outcomes, we are not taking full advantage of them.6 While AWVs have gained traction since their introduction in 2011, usage rates among ethnoracial minority groups has lagged behind.6 A 2018 cohort study examined reasons for disparate utilization rates among individuals ages ≥ 66 years (N = 14,687).7 Researchers found that differences in utilization between ethnoracial groups were explained by socioeconomic factors. Lower education and lower income, as well as rural living, were associated with lower rates of AWV completion.7 In addition, having a usual, nonemergent place to obtain medical care served as a powerful predictor of AWV utilization for all groups.7
Strategies to increase AWV completion rates among all eligible adults include increasing staff awareness of health literacy challenges and ensuring communication strategies are inclusive by providing printed materials in multiple languages, Braille, or larger typefaces; using accessible vocabulary that does not include medical jargon; and making medical interpreters accessible. In addition, training clinicians about unconscious bias and cultural humility can help foster empathy and awareness of differences in health beliefs and behaviors within diverse patient populations.
A 2019 scoping review of 11 studies (N > 60 million) focused on outcomes from Medicare AWVs for patients ages ≥ 65 years.8 This included uptake of preventive services, such as vaccinations or cancer screenings; advice, education, or referrals offered during the AWV; medication use; and hospitalization rates. Overall findings showed that older adults who received a Medicare AWV were more likely to receive referrals for preventive screenings and follow-through on these recommendations compared with those who did not undergo an AWV.8
Completion rates for vaccines, while remaining low overall, were higher among those who completed an AWV. Additionally, these studies showed improved completion of screenings for breast cancer, bone density, and colon cancer. Several studies in the scoping review supported the use of AWVs as an effective means by which to offer health education and advice related to health promotion and risk reduction, such as diet and lifestyle modifications.8 Little evidence exists on long-term outcomes related to AWV completion.8
Utilize shared decision-making to determine whether preventive screening makes sense for your patient. Although cancer remains the second leading cause of death among Americans ages ≥ 65 years,9 clear screening guidelines for this age group remain elusive.10 Physicians and patients often are reluctant to stop cancer screening despite lower life expectancy and fewer potential benefits of diagnosis in this population.9 Some recent studies reinforce the heterogeneity of the older adult population and further underscore the importance of individual-level decision-making.11-14 It is important to let older adult patients and their caregivers know about the potential risks of screening tests, especially the possibility that incidental findings may lead to unwarranted additional care or monitoring.9
Continue to: Avoid these screening conversation missteps
Avoid these screening conversation missteps. A 2017 qualitative study asked 40 community-dwelling older adults (mean age = 76 years) about their preferences for discussing screening cessation with their physicians.13 Three themes emerged.First, they were open to stopping their screenings, especially when suggested by a trusted physician. Second, health status and physical function made sense as decision points, but life expectancy did not. Finally, lengthy discussions with expanded details about risks and benefits were not appreciated, especially if coupled with comments on the limited benefits for those nearing the end of life. When discussing life expectancy, patients preferred phrasing that focused on how the screening was unnecessary because it would not help them live longer.13
Ensure that your message is understood—and culturally relevant. Recent studies on lower health literacy among older adults15,16 and ethnic and racial minorities17-21—as revealed in the 2003 National Assessment of Adult Literacy22—might offer clues to patient receptivity to discussions about preventive screening and other health decisions.
One study found a significant correlation between higher self-rated health literacy and higher engagement in health behaviors such as mammography screening, moderate physical activity, and tobacco avoidance.16 Perceptions of personal control over health status, as well as perceived social standing, also correlated with health literacy score levels.16 Another study concluded that lower health literacy combined with lower self-efficacy, cultural beliefs about health topics (eg, diet and exercise), and distrust in the health care system contributed to lower rates of preventive care utilization among ethnocultural minority older adults in Canada, the United Kingdom, the United States, and Australia.18
Ensuring that easy-to-understand information is equitably shared with older adults of all races and ethnicities is critical. A 2018 study showed that distrust of the health system and cultural issues contributed to the lower incidence of colorectal cancer screenings in Hispanic and Asian American patients ages 50 to 75 years.21 Patients whose physicians engaged in “health literate practices” (eg, offering clear explanations of diagnostic plans and asking patients to describe what they understood) were more likely to obtain recommended breast and colorectal cancer screenings.20 In particular, researchers found that non-Hispanic Blacks were nearly twice as likely to follow through on colorectal cancer screening if their physicians engaged in health literate practices.20 In addition, receiving clear instructions from physicians increased the odds of completing breast cancer screening among Hispanic and non-Hispanic White women.20
Overall, screening information and recommendations should be standardized for all patients. This is particularly important in light of research that found that older non-Hispanic Black patients were less likely than their non-Hispanic White counterparts to receive information from their physicians about colorectal cancer screening.20
Continue to: Mobility
Mobility
Encourage physical activity. Frequent exercise and other forms of physical activity are associated with healthy aging, as shown in a 2017 systematic review and meta-analysis of 23 studies (N = 174,114).23 Despite considerable heterogeneity between studies in how researchers defined healthy aging and physical activity, they found that adults who incorporate regular movement and exercise into daily life are likely to continue to benefit from it into older age.23 In addition, a 2016 secondary analysis of data from the InCHIANTI longitudinal aging study concluded that adults ages ≥ 65 years (N = 1149) who had maintained higher physical activity levels throughout adulthood had less physical function decline and reduced rates of mobility disability and premature death compared with those who reported being less active.24
Preserve gait speed (and bolster health) with these activities. Walking speed, or gait, measured on a level surface has been used as a predictor for various aspects of well-being in older age, such as daily function, mobility, independence, falls, mortality, and hospitalization risk.25 Reduced gait speed is also one of the key indicators of functional impairment in older adults.
A 2015 systematic review sought to determine which type of exercise intervention (resistance, coordination, or multimodal training) would be most effective in preserving gait speed in healthy older adults (N = 2495; mean age = 74.2 years).25 While the 42 included studies were deemed to be fairly low quality, the review revealed (with large effect size [0.84]) that a number of exercise modalities might stave off loss of gait speed in older adults. Patients in the resistance training group had the greatest improvement in gait speed (0.11 m/s), followed by those in the coordination training group (0.09 m/s) and the multimodal training group (0.05 m/s).25
Finally, muscle mass and strength offer a measure of physical performance and functionality. A 2020 systematic review of 83 studies (N = 108,428) showed that low muscle mass and strength, reduced handgrip strength, and lower physical performance were predictive of reduced capacities in activities of daily living and instrumental activities of daily living.26 It is important to counsel adults to remain active throughout their lives and to include resistance training to maintain muscle mass and strength to preserve their motor function, mobility, independence, and QOL.
Use 1 of these scales to identify frailty. Frailty is a distinct clinical syndrome, in which an individual has low reserves and is highly vulnerable to internal and external stressors. It affects many community-dwelling older adults. Within the literature, there has been ongoing discussion regarding the definition of frailty27 (TABLE 128-31).
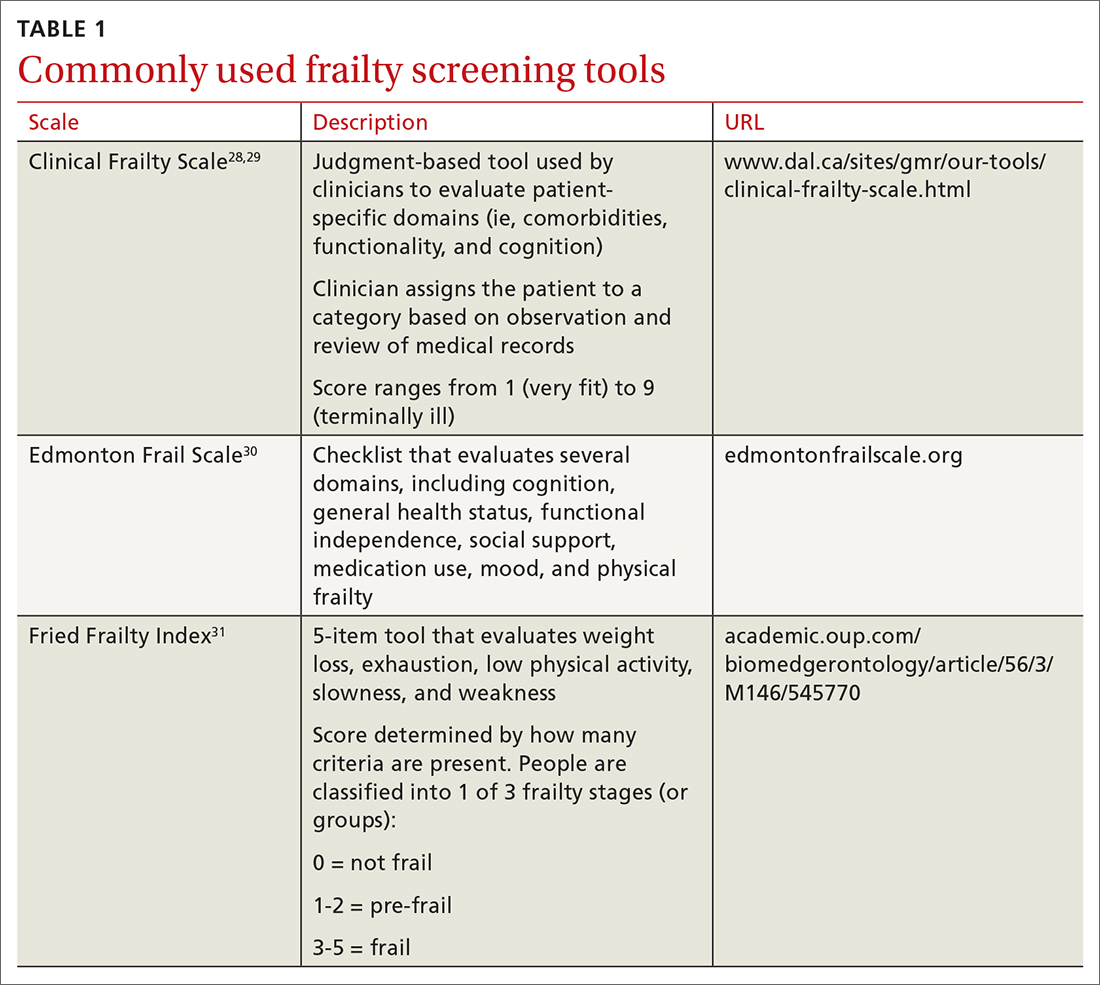
Continue to: The Fried Frailty Index...
The Fried Frailty Index defines frailty as a purely physical condition; patients need to exhibit 3 of 5 components (ie, weight loss, exhaustion, weakness, slowness, and low physical activity) to be deemed frail.31 The Edmonton Frail Scale is commonly used in geriatric assessments and counts impairments across several domains including physical activity, mood, cognition, and incontinence.30,32,33 Physicians need to complete a training course prior to its use. Finally, the definition of frailty used by Rockwood et al28, 29 was used to develop the Clinical Frailty Scale, which relies on broader criteria that include social and psychological elements in addition to physical elements.The Clinical Frailty Scale uses clinician judgment to evaluate patient-specific domains (eg, comorbidities, functionality, and cognition) and to generate a score ranging from 1 (very fit) to 9 (terminally ill).29 This scale is accessible and easy to implement. As a result, use of this scale has increased during the COVID-19 pandemic. All definitions include a pre-frail state, indicating the dynamic nature of frailty over time.
It is important to identify pre-frail and frail older adults using 1 of these screening tools. Interventions to reverse frailty that can be initiated in the primary care setting include identifying treatable medical conditions, assessing medication appropriateness, providing nutritional advice, and developing an exercise plan.34
Conduct a nutritional assessment; consider this diet. Studies show that nutritional status can predict physical function and frailty risk in older adults. A 2017 systematic review of 19 studies (N = 22,270) of frail adults ages ≥ 65 years found associations related to specific dietary constructs (ie, micronutrients, macronutrients, antioxidants, overall diet quality, and timing of consumption).35 Plant-based diets with higher levels of micronutrients, such as vitamins C and E and beta-carotene, or diets with more protein or macronutrients, regardless of source foods, all resulted in inverse associations with frailty syndrome.35
A 2017 study showed that physical exercise and maintaining good nutritional status may be effective for preventing frailty in community-dwelling pre-frail older individuals.36 A 2019 study showed that a combination of muscle strength training and protein supplementation was the most effective intervention to delay or reverse frailty and was easiest to implement in primary care.37 A 2020 meta-analysis of 31 studies (N = 4794) addressing frailty among primary care patients > 60 years showed that interventions using predominantly resistance-based exercise and nutrition supplementation improved frailty status over the control.38 Researchers also found that a comprehensive geriatric assessment or exercise—alone or in combination with nutrition education—reduced physical frailty.
Mentation
Screen and treat cognitive impairments. Cognitive function and autonomy in decision-making are important factors in healthy aging. Aspects of mental health (eg, depression and anxiety), sensory impairment (eg, visual and auditory impairment), and mentation issues (eg, delirium, dementia, and related conditions), as well as diet, physical exercise, and mobility, can all impede cognitive functionality. The long-term effects of depression, anxiety,39 sensory deficits,40 mobility,41 diet,42 and, ultimately, aging may impact Alzheimer disease (AD). The risk of an AD diagnosis increases with age.39
Continue to: A 2018 prospective cohort study...
A 2018 prospective cohort study using data from the National Alzheimer’s Coordinating Center followed individuals (N = 12,053) who were cognitively asymptomatic at their initial visits to determine who developed clinical signs of AD.39 Survival analysis showed several psychosocial factors—anxiety, sleep disturbances, and depressive episodes of any type (occurring within the past 2 years, clinician verified, lifetime report)—were significantly associated with an eventual AD diagnosis and increased the risk of AD.39 More research is needed to verify the impact of early intervention for these conditions on neurodegenerative disease; however, screening and treating psychosocial factors such as anxiety and depression should be maintained.
Researchers evaluated the impact of a dual sensory impairment (DSI) on dementia risk using data from 2051 participants in the Ginkgo Evaluation of Memory Study.40 Hearing and visual impairments (defined as DSI when these conditions coexist) or visual impairment alone were significantly associated with increased risk of dementia in older adults. The researchers reported that DSI was significantly associated with a higher risk of all-cause dementia (hazard ratio [HR] = 1.86; 95% CI, 1.25-2.76) and AD (HR = 2.12; 95% CI, 1.34-3.36).40 Visual impairment alone was associated with an increased risk of all-cause dementia (HR = 1.32; 95% CI, 1.02-1.71).40 These results suggest that screening of DSI or visual impairment earlier in the patient’s lifespan may identify those at high risk of dementia in older adulthood.
The American Academy of Ophthalmology recommends patients with healthy eyes be screened once during their 20s and twice in their 30s; a full examination is recommended by age 40. For patients ages ≥ 65 years, it is recommended that eye examinations occur every 1 to 2 years.43
Diet and mobility play a big role in cognition. Diet43 and exercise41,42,44 are believed to have an impact on mentation, and recent studies show memory and global cognition could be malleable later in life. A 2015 meta-analysis of 490 treatment arms of 24 randomized controlled studies showed improvement in global cognition with consumption of a Mediterranean diet plus olive oil (effect size [ES] standardized mean difference [SMD] = 0.22; 95% CI, 0.16-0.27) and tai chi exercises (ES SMD = 0.18; 95% CI, 0.06-0.29).42 The analysis also found improved memory among participants who consumed the Mediterranean diet/olive oil combination (ES SMD = 0.22; 95% CI, 0.12-0.32) and soy isoflavone supplements (ES SMD = 0.11; 95% CI, 0.04-0.17). Although the ESs are small, they are significant and offer promising evidence that individual choices related to nutrition or exercise may influence cognition and memory.
A 2018 systematic review found that all domains of cognition showed improvement with 45 to 60 minutes of moderate-to-vigorous physical exercise.44 Attention, executive function, memory, and working memory showed significant increases, whereas global cognition improvements were not statistically significant.44 A 2016 meta-analysis of 26 studies (N = 26,355) found a positive association between an objective mobility measure (gait, lower-extremity function, and balance) and cognitive function (global, executive function, memory, and processing speed) in older adults.41 These results highlight that diet, mobility, and physical exercise impact cognitive functioning.
Continue to: Maintaining social connections
Maintaining social connections
Social isolation and loneliness—compounded by a pandemic. The US Department of Health and Human Services notes that “community connections” are among the key factors required for healthy aging.3 Similarly, the WHO definition of healthy aging considers whether individuals can build and sustain relationships with other people and find ways to engender their personal values through these connections.2
As people age, their social connections often decrease due to the death of friends and family, shifts in living arrangements, loss of mobility or eyesight (and thus self-transport), and the onset or increased acuity of illness or chronic conditions.45 This has been exacerbated by the COVID-19 pandemic, which has spurred shelter-in-place and stay-at-home orders along with recommendations for physical distancing (also known as social distancing), especially for older adults who are at higher risk.46 Smith et al47 calls this the COVID-19 Social Connectivity Paradox, in which older adults limit their interactions with others to protect their physical health and reduce their risk of contracting the virus, but as a result they may undermine their psychosocial health by placing themselves at risk of social isolation and loneliness.47
The double threat. Social isolation and loneliness have been shown to negatively impact physical health and well-being, resulting in an increased risk of early death48-50; higher likelihood of specific diagnoses, including dementia and cardiovascular conditions48,50; and more frequent use of health care services.50 These concepts, while related, represent different mechanisms for negative health outcomes. Social isolation is an objective condition when one has a lack of opportunities for interaction with other people; loneliness refers to the emotional disconnect one feels when separated from others. Few studies have compared outcomes between these concepts, but in those that have, social isolation appears to be more strongly associated with early death.48-50
A 2013 observational study using data from the English Longitudinal Study on Aging found that both social isolation and loneliness were associated with increased mortality among men and women ages ≥ 52 years (N = 6500).48 However, when studied independently, loneliness was not found to be a significant risk factor. In contrast, social isolation significantly impacted mortality risk, even after adjusting for demographic factors and baseline health status.48 These findings are supported by a 2018 cohort study of individuals (N = 479,054) with a history of an acute cardiovascular event that concluded social isolation was a predictor of mortality, whereas loneliness was not.50
A large 2015 meta-analysis (70 studies, N = 3,407,134) of mortality causes among community-dwelling older adults (average age, 66) confirmed that both objective measures of isolation, as well as subjective measures (such as feelings of loneliness or living alone), have a significant predictive effect in longer-term studies. Each measure shows an approximately 30% increase in the likelihood of death after an average of 7 years.49
Continue to: Health care remains a connection point
Health care remains a connection point. Even when life course events and conditions (eg, death of loved ones, loss of transportation or financial resources, or disengagement from community activities) reduce social connections, most older adults engage in some way with the health care system. A 2020 consensus report by the National Academies of Sciences, Engineering, and Medicine suggests health care professionals capitalize on these connection points with adults ages ≥ 50 years by periodically screening for social isolation and loneliness, documenting social status updates in medical records, and piloting and evaluating interventions in the clinical setting.51
The report offered information about potential avenues for intervention by primary care professionals beyond screening, such as participating in research studies that investigate screening tools and multisystem interventions; social prescribing (linking patients to embedded social work services or community-based organizations); referring patients to support groups; initiating cognitive-based therapy or other behavioral health interventions; or recommending mindfulness practices.51 However, most of the cited intervention studies were not specific to primary care settings and contained poor-quality evidence related to efficacy.
Isolation creates a greater reliance on health services due to a lack of a social support system, while a feeling of emotional disconnection (loneliness) seems to be a barrier to accessing care. A 2017 cohort study linking data from the Health and Retirement Study and Medicare claims revealed that social isolation predicts higher annual health expenditures (> $1600 per beneficiary) driven by hospitalization and skilled nursing facility usage, along with greater mortality, whereas individuals who are lonely result in reduced costs (a reduction of $770 annually) due to lower usage of inpatient and outpatient services.52 Prioritizing interventions that identify and connect isolated older adults to social support, therefore, may increase survivability by ensuring they have access to resources and health care interventions when needed.
In addition, these findings underscore the importance of looking at quality—not just quantity—of older adults’ social connections. A number of validated screening tools exist for social isolation and loneliness (TABLE 253-59); however, concerns exist about assessing risk using a unidimensional tool for a complex concern,47 as well as identifying a problem without having evidence-based interventions to offer as solutions.47,51 Until future studies resolve these concerns, leveraging the physician-patient relationship to broach these difficult subjects may help normalize the issues and create safe spaces to identify individuals who are at risk.
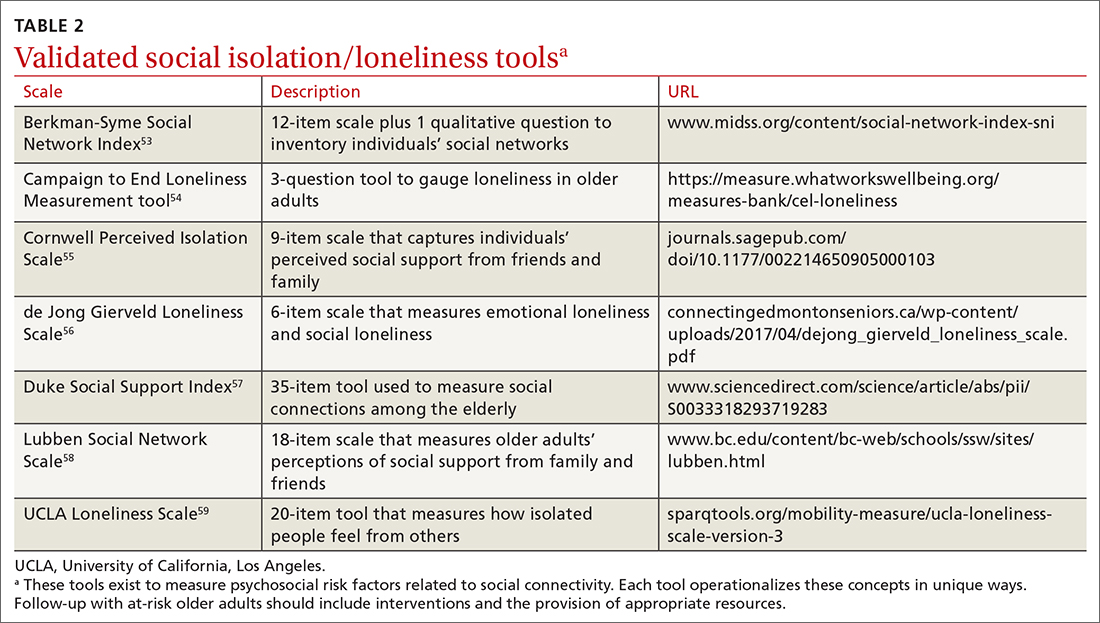
QOL is key to healthy aging. As Kusumastuti et al4 state, “successful ageing lies in the eyes of the beholder.” A 2019 systematic review of 48 qualitative studies revealed that community-dwelling older adults ages ≥ 50 years in 11 countries (N > 4175) perceive well-being by considering QOL within 9 domains: health perception, autonomy, role and activity, relationships, emotional comfort, attitude and adaptation, spirituality, financial security, and home and neighborhood.60 Researchers found that as engagement in any one of these domains declines, older adults may shift their definition of health toward their remaining abilities.60 This offers an explanation as to why patients might rate their health status much higher than their physicians do: older adults tend to have a more holistic concept of health.
Continue to: Take a multidimensional approach to healthy aging
Take a multidimensional approach to healthy aging
Although we have separately examined each of the 4 components of managing healthy aging in a community-dwelling adult, applying a multidimensional approach is most effective. Increasing use of the Medicare AWV provides an opportunity to assess patient health status, determine care preferences, and improve follow-through on preventive screening. It is also important to encourage older adults to engage in regular physical activity—especially muscle-strengthening exercises—and to discuss nutrition and caloric intake to prevent frailty and functional decline.
Assessing and treating vision and hearing impairments and mental health issues, including anxiety and depression, may guard against losses in cognition. When speaking with older adult patients about their social connections, consider asking not only about frequency of contact and access to resources such as food and transportation, but also about whether they are finding ways to bring their own values into those relationships to bolster their QOL. This guidance also may be useful for primary care practices and health care networks when planning future quality-improvement initiatives.
Additional research is needed to support the evidence base for aligning older adult preferences in health care interventions, such as preventive screenings. Also, clinical decision-making requires more clarity about the efficacy of specific diet and exercise interventions for older adults; the impact of early intervention for depression, anxiety, and sleep disorders on neurodegenerative disease; whether loneliness predicts mortality; and how health care delivery systems can be effective at building social connectivity.
For now, it is essential to recognize that initiating health education, screening, and prevention throughout the patient’s lifespan can promote healthy aging outcomes. As family physicians, it is important to capitalize on longitudinal relationships with patients and begin educating younger patients using this multidimensional framework to promote living “a productive and meaningful life”at any age.3
Lynn M. Wilson, DO, 707 Hamilton Street, 8th floor, Department of Family Medicine, Lehigh Valley Health Network, Allentown, PA 18101; [email protected]
1. Friedman S, Mulhausen P, Cleveland M, et al. Healthy aging: American Geriatrics Society white paper executive summary. J Am Geriatr Soc. 2018;67:17-20. doi: 10.1111/jgs.15644
2. World Health Organization. World report on ageing and health. 2015. Accessed June 29, 2020. https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf?sequence=1
3. U.S. Department of Health & Human Services. Healthy aging. Accessed June 29, 2020. www.hhs.gov/aging/healthy-aging
4. Kusumastuti S, Derks MGM, Tellier S, et al. Successful ageing: a study of the literature using citation network analysis. Maturitas. 2016;93:4-12. doi: 10.1016/j.maturitas.2016.04.010
5. Institute for Healthcare Improvement. Age-friendly health systems: guide to using the 4Ms in the care of older adults [white paper]. 2020. Accessed June 29, 2020. www.ihi.org/Engage/Initiatives/Age-Friendly-Health-systems/Documents/IHIAgeFriendlyHealthSystems_GuidetoUsing4MsCare.pdf
6. Lind KE, Hildreth KL, Perraillon MC. Persistent disparities in Medicare’s annual wellness visit utilization. Med Care. 2019;57:984-989. doi: 10.1097/MLR.0000000000001229
7. Lind KE, Hildreth K, Lindrooth R, et al. Ethnoracial disparities in Medicare annual wellness visit utilization: evidence from a nationally representative database. Med Care. 2018;56:761-766. doi: 10.1097/MLR.0000000000000962
8. Simpson VL, Kovich M. Outcomes of primary care-based Medicare annual wellness visits with older adults: a scoping review. Geriatr Nurs. 2019;40:590-596. doi: 10.1016/j.gerinurse.2019.06.001
9. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68:1-77.
10. Salzman B, Beldowski K, de la Paz A. Cancer screening in older patients. Am Fam Physician. 2016;93:659-667.
11. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177:399-406. doi: 10.1001/jamainternmed.2016.9022
12. Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. 2014;311:1336-1347. doi: 10.1001/jama.2014.2834
13. Schoenborn NL, Lee K, Pollack CE, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177:1121-1128. doi: 10.1001/jamainternmed.2017.1778
14. Butterworth JE, Hays R, McDonagh ST, et al. Interventions for involving older patients with multi-morbidity in decision-making during primary care consultations. Cochrane Database Syst Rev. 2019;10:CD013124. doi: 10.1002/14651858.CD013124.pub2
15. Bostock S, Steptoe A. Association between low functional health literacy and mortality in older adults: longitudinal cohort study. BMJ. 2012;344:e1602. doi: 10.1136/bmj.e1602
16. Fernandez DM, Larson JL, Zikmund-Fisher BJ. Associations between health literacy and preventive health behaviors among older adults: findings from the health and retirement study. BMC Public Health. 2016;16:596. doi: 10.1186/s12889-016-3267-7
17. Weekes CV. African Americans and health literacy: a systematic review. ABNF J. 2012;23:76-80.
18. Mantwill S, Monestel-Umaña S, Schulz PJ. The relationship between health literacy and health disparities: a systematic review. PLoS One. 2015;10:e0145455. doi: 10.1371/journal.pone.0145455
19. Khan MM, Kobayashi K. Optimizing health promotion among ethnocultural minority older adults (EMOA). Int J Migration Health Soc Care. 2015;11:268-281. doi: 10.1108/IJMHSC-12-2014-0047
20. Kindratt TB, Dallo FJ, Allicock M, et al. The influence of patient-provider communication on cancer screenings differs among racial and ethnic groups. Prev Med Rep. 2020;18:101086. doi: 10.1016/j.pmedr.2020.101086
21. Hong Y-R, Tauscher J, Cardel M. Distrust in health care and cultural factors are associated with uptake of colorectal cancer screening in Hispanic and Asian Americans. Cancer. 2018;124:335-345. doi: 10.1002/cncr.31052
22. Kutner M, Greenberg E, Jin Y, et al. Literacy in everyday life: results from the 2003 National Assessment of Adult Literacy. NCES 2007-480. U.S. Department of Education, National Center for Education Statistics. April 2007. Accessed August 27, 2021. http://nces.ed.gov/Pubs2007/2007480_1.pdf
23. Daskalopoulou C, Stubbs B, Kralj C, et al. Physical activity and healthy ageing: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2017;38:6-17. doi: 10.1016/j.arr.2017.06.003
24. Stenholm S, Koster A, Valkeinen H, et al. Association of physical activity history with physical function and mortality in old age. J Gerontol A Biol Sci Med Sci. 2016;71:496-501. doi: 10.1093/gerona/glv111
25. Hortobágyi T, Lesinski M, Gäbler M, et al. Effects of three types of exercise interventions on healthy old adults’ gait speed: a systematic review and meta-analysis. Sports Med. 2015;45:1627‐1643. Published correction appears in Sports Med. 2016;46:453. doi: 10.1007/s40279-015-0371-2
26. Wang DXM, Yao J, Zirek Y, et al. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11:3‐25. doi: 10.1002/jcsm.12502
27. Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129-2138. doi: 10.1111/j.1532-5415.2011.03597.x
28. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-495. doi: 10.1503/cmaj.050051
29. Church S, Rogers E, Rockwood K, et al. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20:393. doi: 10.1186/s12877-020-01801-7
30. Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526-529. doi: 10.1093/ageing/afl041
31. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146
32. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Euro J Intern Med. 2016;31:3-10. doi: 10.1016/j.ejim.2016.03.007
33. Perna S, Francis MD, Bologna C, et al. Performance of Edmonton Frail Scale on frailty assessment: its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr. 2017;17:2. doi: 10.1186/s12877-016-0382-3
34. Chen CY, Gan P, How CH. Approach to frailty in the elderly in primary care and the community. Singapore Med J. 2018;59:338. doi: 10.11622/smedj.2018052
35. Lorenzo-López L, Maseda A, de Labra C, et al. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. 2017;17:108. doi: 10.1186/s12877-017-0496-2
36. Serra-Prat M, Sist X, Domenich R, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing. 2017;46:401-407. doi: 10.1093/ageing/afw242
37. Travers J, Romero-Ortuno R, Bailey J, et al. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69:e61-e69. doi: 10.3399/bjgp18X700241
38. Macdonald SHF, Travers J, Shé ÉN, et al. Primary care interventions to address physical frailty among community-dwelling adults aged 60 years or older: a meta-analysis. PLoS One. 2020;15:e0228821. doi: 10.1371/journal.pone.0228821
39. Burke SL, Cadet T, Alcide A, et al. Psychosocial risk factors and Alzheimer’s disease: the associative effect of depression, sleep disturbance, and anxiety. Aging Ment Health. 2018;22:1577-1584. doi: 10.1080/13607863.2017.1387760
40. Hwang PH, Longstreth WT Jr, Brenowitz WD, et al. Dual sensory impairment in older adults and risk of dementia from the GEM Study. Alzheimers Dement (Amst). 2020;12:e12054. doi: 10.1002/dad2.12054
41. Demnitz N, Esser P, Dawes H, et al. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164‐174. doi: 10.1016/j.gaitpost.2016.08.028
42. Lehert P, Villaseca P, Hogervorst E, et al. Individually modifiable risk factors to ameliorate cognitive aging: a systematic review and meta-analysis. Climacteric. 2015;18:678-689. doi: 10.3109/13697137.2015.1078106
43. Turbert D. Eye exam and vision testing basics. American Academy of Ophthalmology Web site. January 14, 2021. Accessed March 5, 2021. www.aao.org/eye-health/tips-prevention/eye-exams-101
44. Northey JM, Cherbuin N, Pumpa KL, et al. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52:154-160. doi: 10.1136/bjsports-2016-096587
45. CDC. Percent of U.S. adults 55 and over with chronic conditions. November 6, 2015. Accessed April 29, 2021. www.cdc.gov/nchs/health_policy/adult_chronic_conditions.htm
46. National Council on Aging. COVID-driven isolation can be dangerous for older adults. March 31, 2021. Accessed April 29, 2021. www.ncoa.org/article/covid-driven-isolation-can-be-dangerous-for-older-adults
47. Smith ML, Steinman LE, Casey EA. Combatting social isolation among older adults in a time of physical distancing: the COVID-19 social connectivity paradox. Front Public Health. 2020;8:403. doi: 10.3389/fpubh.2020.00403
48. Steptoe A, Shankar A, Demakakos P, et al. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110:5797-5801. doi: 10.1073/pnas.1219686110
49. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10:227-237. doi: 10.1177/1745691614568352
50. Hakulinen C, Pulkki-Råback L, Virtanen M, et al. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart. 2018;104:1536-1542. doi: 10.1136/heartjnl-2017-312663
51. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. The National Academies Press; 2020. doi: 10.17226/25663
52. Shaw JG, Farid M, Noel-Miller C, et al. Social isolation and Medicare spending: among older adults, objective isolation increases expenditures while loneliness does not. J Aging Health. 2017;29:1119-1143. doi: 10.1177/0898264317703559
53. Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186-204. doi: 10.1093/oxfordjournals.aje.a112674
54. Campaign to End Loneliness. Measuring your impact on loneliness in later life. Accessed April 29, 2021. www.campaigntoendloneliness.org/wp-content/uploads/Loneliness-Measurement-Guidance1-1.pdf
55. Cornwell EY, Waite LJ. Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav. 2009;50:31-48. doi: 10.1177/002214650905000103
56. Gierveld JDJ, Van Tilburg T. A 6-item scale for overall, emotional, and social loneliness: confirmatory tests on survey data. Res Aging. 2006;28:582-598. doi: 10.1177/0164027506289723
57. Koenig HG, Westlund RE, George LK, et al. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics. 1993;34:61-69. doi: 10.1016/S0033-3182(93)71928-3
58. Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 2006;46:503-513. doi: 10.1093/geront/46.4.503
59. Russell DW. UCLA Loneliness Scale (version 3): reliability, validity, factor structure. J Pers Assess. 1996;66:20-40. doi: 10.1207/s15327752jpa6601_2
60. van Leeuwen KM, van Loon MS, van Nes FA, et al. What does quality of life mean to older adults? A thematic synthesis. PLoS One. 2019;14:e0213263. doi: 10.1371/journal.pone.0213263
Our approach to caring for the growing number of community-dwelling US adults ages ≥ 65 years has shifted. Although we continue to manage disease and disability, there is an increasing emphasis on the promotion of healthy aging by optimizing health care needs and quality of life (QOL).
The American Geriatric Society (AGS) uses the term “healthy aging” to reflect a dedication to improving the health, independence, and QOL of older people.1 The World Health Organization (WHO) defines healthy aging as “the process of developing and maintaining the functional ability that enables well-being in older age.”2 Functional ability encompasses capabilities that align with a person’s values, including meeting basic needs; learning, growing, and making independent decisions; being mobile; building and maintaining healthy relationships; and contributing to society.2 Similarly, the US Department of Health and Human Services has adopted a multidimensional approach to support people in creating “a productive and meaningful life” as they grow older.3
Numerous theoretical models have emerged from research on aging as a multidimensional construct, as evidenced by a 2016 citation analysis that identified 1755 articles written between 1902 and 2015 relating to “successful aging.”4 The analysis revealed 609 definitions operationalized by researchers’ measurement tools (mostly focused on physical function and other health metrics) and 1146 descriptions created by older adults, many emphasizing psychosocial strategies and cultural factors as key to successful aging.4

One approach that is likely to be useful for family physicians is the Age-Friendly Health System. This is an initiative of The John A. Hartford Foundation and the Institute for Healthcare Improvement that uses a multidisciplinary approach to create environments that foster inclusivity and address the needs of older people.5 Following this guidance, primary care providers use evidence-informed strategies that promote safety and address what matters most to older adults and their family caregivers.
The Age-Friendly Health System, as well as AGS and WHO, recognize that there are multiple aspects to well-being as one grows older. By using focused, evidence-based screening, assessments, and interventions, family physicians can best support aging patients in living their most fulfilling lives.
Here we present a review of evidence-based strategies that promote safety and address what matters most to older adults and their family caregivers using a 4-pronged framework, in the style of the Age-Friendly Health System model. However, the literature on healthy aging includes important messages about patient context and lifelong health behaviors, which we capture in an expanded set of thematic guidance. As such, we encourage family physicians to approach healthy aging as follows: (1) monitor health (screening and prevention), (2) promote mobility (physical function), (3) manage mentation (emotional health and cognitive function), and (4) encourage maintenance of social connections (social networks and QOL).
Monitoring health
Leverage Medicare annual wellness visits. A systematic approach is needed to prevent frailty and functional decline, and thus increase the QOL of older adults. To do this, it is important to focus on health promotion and disease prevention, while addressing existing ailments. One method is to leverage the Medicare annual wellness visit (AWV), which provides an opportunity to assess current health status as well as discuss behavior-change and risk-reduction strategies with patients.
Continue to: Although AWVs...
Although AWVs are an opportunity to improve patient outcomes, we are not taking full advantage of them.6 While AWVs have gained traction since their introduction in 2011, usage rates among ethnoracial minority groups has lagged behind.6 A 2018 cohort study examined reasons for disparate utilization rates among individuals ages ≥ 66 years (N = 14,687).7 Researchers found that differences in utilization between ethnoracial groups were explained by socioeconomic factors. Lower education and lower income, as well as rural living, were associated with lower rates of AWV completion.7 In addition, having a usual, nonemergent place to obtain medical care served as a powerful predictor of AWV utilization for all groups.7
Strategies to increase AWV completion rates among all eligible adults include increasing staff awareness of health literacy challenges and ensuring communication strategies are inclusive by providing printed materials in multiple languages, Braille, or larger typefaces; using accessible vocabulary that does not include medical jargon; and making medical interpreters accessible. In addition, training clinicians about unconscious bias and cultural humility can help foster empathy and awareness of differences in health beliefs and behaviors within diverse patient populations.
A 2019 scoping review of 11 studies (N > 60 million) focused on outcomes from Medicare AWVs for patients ages ≥ 65 years.8 This included uptake of preventive services, such as vaccinations or cancer screenings; advice, education, or referrals offered during the AWV; medication use; and hospitalization rates. Overall findings showed that older adults who received a Medicare AWV were more likely to receive referrals for preventive screenings and follow-through on these recommendations compared with those who did not undergo an AWV.8
Completion rates for vaccines, while remaining low overall, were higher among those who completed an AWV. Additionally, these studies showed improved completion of screenings for breast cancer, bone density, and colon cancer. Several studies in the scoping review supported the use of AWVs as an effective means by which to offer health education and advice related to health promotion and risk reduction, such as diet and lifestyle modifications.8 Little evidence exists on long-term outcomes related to AWV completion.8
Utilize shared decision-making to determine whether preventive screening makes sense for your patient. Although cancer remains the second leading cause of death among Americans ages ≥ 65 years,9 clear screening guidelines for this age group remain elusive.10 Physicians and patients often are reluctant to stop cancer screening despite lower life expectancy and fewer potential benefits of diagnosis in this population.9 Some recent studies reinforce the heterogeneity of the older adult population and further underscore the importance of individual-level decision-making.11-14 It is important to let older adult patients and their caregivers know about the potential risks of screening tests, especially the possibility that incidental findings may lead to unwarranted additional care or monitoring.9
Continue to: Avoid these screening conversation missteps
Avoid these screening conversation missteps. A 2017 qualitative study asked 40 community-dwelling older adults (mean age = 76 years) about their preferences for discussing screening cessation with their physicians.13 Three themes emerged.First, they were open to stopping their screenings, especially when suggested by a trusted physician. Second, health status and physical function made sense as decision points, but life expectancy did not. Finally, lengthy discussions with expanded details about risks and benefits were not appreciated, especially if coupled with comments on the limited benefits for those nearing the end of life. When discussing life expectancy, patients preferred phrasing that focused on how the screening was unnecessary because it would not help them live longer.13
Ensure that your message is understood—and culturally relevant. Recent studies on lower health literacy among older adults15,16 and ethnic and racial minorities17-21—as revealed in the 2003 National Assessment of Adult Literacy22—might offer clues to patient receptivity to discussions about preventive screening and other health decisions.
One study found a significant correlation between higher self-rated health literacy and higher engagement in health behaviors such as mammography screening, moderate physical activity, and tobacco avoidance.16 Perceptions of personal control over health status, as well as perceived social standing, also correlated with health literacy score levels.16 Another study concluded that lower health literacy combined with lower self-efficacy, cultural beliefs about health topics (eg, diet and exercise), and distrust in the health care system contributed to lower rates of preventive care utilization among ethnocultural minority older adults in Canada, the United Kingdom, the United States, and Australia.18
Ensuring that easy-to-understand information is equitably shared with older adults of all races and ethnicities is critical. A 2018 study showed that distrust of the health system and cultural issues contributed to the lower incidence of colorectal cancer screenings in Hispanic and Asian American patients ages 50 to 75 years.21 Patients whose physicians engaged in “health literate practices” (eg, offering clear explanations of diagnostic plans and asking patients to describe what they understood) were more likely to obtain recommended breast and colorectal cancer screenings.20 In particular, researchers found that non-Hispanic Blacks were nearly twice as likely to follow through on colorectal cancer screening if their physicians engaged in health literate practices.20 In addition, receiving clear instructions from physicians increased the odds of completing breast cancer screening among Hispanic and non-Hispanic White women.20
Overall, screening information and recommendations should be standardized for all patients. This is particularly important in light of research that found that older non-Hispanic Black patients were less likely than their non-Hispanic White counterparts to receive information from their physicians about colorectal cancer screening.20
Continue to: Mobility
Mobility
Encourage physical activity. Frequent exercise and other forms of physical activity are associated with healthy aging, as shown in a 2017 systematic review and meta-analysis of 23 studies (N = 174,114).23 Despite considerable heterogeneity between studies in how researchers defined healthy aging and physical activity, they found that adults who incorporate regular movement and exercise into daily life are likely to continue to benefit from it into older age.23 In addition, a 2016 secondary analysis of data from the InCHIANTI longitudinal aging study concluded that adults ages ≥ 65 years (N = 1149) who had maintained higher physical activity levels throughout adulthood had less physical function decline and reduced rates of mobility disability and premature death compared with those who reported being less active.24
Preserve gait speed (and bolster health) with these activities. Walking speed, or gait, measured on a level surface has been used as a predictor for various aspects of well-being in older age, such as daily function, mobility, independence, falls, mortality, and hospitalization risk.25 Reduced gait speed is also one of the key indicators of functional impairment in older adults.
A 2015 systematic review sought to determine which type of exercise intervention (resistance, coordination, or multimodal training) would be most effective in preserving gait speed in healthy older adults (N = 2495; mean age = 74.2 years).25 While the 42 included studies were deemed to be fairly low quality, the review revealed (with large effect size [0.84]) that a number of exercise modalities might stave off loss of gait speed in older adults. Patients in the resistance training group had the greatest improvement in gait speed (0.11 m/s), followed by those in the coordination training group (0.09 m/s) and the multimodal training group (0.05 m/s).25
Finally, muscle mass and strength offer a measure of physical performance and functionality. A 2020 systematic review of 83 studies (N = 108,428) showed that low muscle mass and strength, reduced handgrip strength, and lower physical performance were predictive of reduced capacities in activities of daily living and instrumental activities of daily living.26 It is important to counsel adults to remain active throughout their lives and to include resistance training to maintain muscle mass and strength to preserve their motor function, mobility, independence, and QOL.
Use 1 of these scales to identify frailty. Frailty is a distinct clinical syndrome, in which an individual has low reserves and is highly vulnerable to internal and external stressors. It affects many community-dwelling older adults. Within the literature, there has been ongoing discussion regarding the definition of frailty27 (TABLE 128-31).

Continue to: The Fried Frailty Index...
The Fried Frailty Index defines frailty as a purely physical condition; patients need to exhibit 3 of 5 components (ie, weight loss, exhaustion, weakness, slowness, and low physical activity) to be deemed frail.31 The Edmonton Frail Scale is commonly used in geriatric assessments and counts impairments across several domains including physical activity, mood, cognition, and incontinence.30,32,33 Physicians need to complete a training course prior to its use. Finally, the definition of frailty used by Rockwood et al28, 29 was used to develop the Clinical Frailty Scale, which relies on broader criteria that include social and psychological elements in addition to physical elements.The Clinical Frailty Scale uses clinician judgment to evaluate patient-specific domains (eg, comorbidities, functionality, and cognition) and to generate a score ranging from 1 (very fit) to 9 (terminally ill).29 This scale is accessible and easy to implement. As a result, use of this scale has increased during the COVID-19 pandemic. All definitions include a pre-frail state, indicating the dynamic nature of frailty over time.
It is important to identify pre-frail and frail older adults using 1 of these screening tools. Interventions to reverse frailty that can be initiated in the primary care setting include identifying treatable medical conditions, assessing medication appropriateness, providing nutritional advice, and developing an exercise plan.34
Conduct a nutritional assessment; consider this diet. Studies show that nutritional status can predict physical function and frailty risk in older adults. A 2017 systematic review of 19 studies (N = 22,270) of frail adults ages ≥ 65 years found associations related to specific dietary constructs (ie, micronutrients, macronutrients, antioxidants, overall diet quality, and timing of consumption).35 Plant-based diets with higher levels of micronutrients, such as vitamins C and E and beta-carotene, or diets with more protein or macronutrients, regardless of source foods, all resulted in inverse associations with frailty syndrome.35
A 2017 study showed that physical exercise and maintaining good nutritional status may be effective for preventing frailty in community-dwelling pre-frail older individuals.36 A 2019 study showed that a combination of muscle strength training and protein supplementation was the most effective intervention to delay or reverse frailty and was easiest to implement in primary care.37 A 2020 meta-analysis of 31 studies (N = 4794) addressing frailty among primary care patients > 60 years showed that interventions using predominantly resistance-based exercise and nutrition supplementation improved frailty status over the control.38 Researchers also found that a comprehensive geriatric assessment or exercise—alone or in combination with nutrition education—reduced physical frailty.
Mentation
Screen and treat cognitive impairments. Cognitive function and autonomy in decision-making are important factors in healthy aging. Aspects of mental health (eg, depression and anxiety), sensory impairment (eg, visual and auditory impairment), and mentation issues (eg, delirium, dementia, and related conditions), as well as diet, physical exercise, and mobility, can all impede cognitive functionality. The long-term effects of depression, anxiety,39 sensory deficits,40 mobility,41 diet,42 and, ultimately, aging may impact Alzheimer disease (AD). The risk of an AD diagnosis increases with age.39
Continue to: A 2018 prospective cohort study...
A 2018 prospective cohort study using data from the National Alzheimer’s Coordinating Center followed individuals (N = 12,053) who were cognitively asymptomatic at their initial visits to determine who developed clinical signs of AD.39 Survival analysis showed several psychosocial factors—anxiety, sleep disturbances, and depressive episodes of any type (occurring within the past 2 years, clinician verified, lifetime report)—were significantly associated with an eventual AD diagnosis and increased the risk of AD.39 More research is needed to verify the impact of early intervention for these conditions on neurodegenerative disease; however, screening and treating psychosocial factors such as anxiety and depression should be maintained.
Researchers evaluated the impact of a dual sensory impairment (DSI) on dementia risk using data from 2051 participants in the Ginkgo Evaluation of Memory Study.40 Hearing and visual impairments (defined as DSI when these conditions coexist) or visual impairment alone were significantly associated with increased risk of dementia in older adults. The researchers reported that DSI was significantly associated with a higher risk of all-cause dementia (hazard ratio [HR] = 1.86; 95% CI, 1.25-2.76) and AD (HR = 2.12; 95% CI, 1.34-3.36).40 Visual impairment alone was associated with an increased risk of all-cause dementia (HR = 1.32; 95% CI, 1.02-1.71).40 These results suggest that screening of DSI or visual impairment earlier in the patient’s lifespan may identify those at high risk of dementia in older adulthood.
The American Academy of Ophthalmology recommends patients with healthy eyes be screened once during their 20s and twice in their 30s; a full examination is recommended by age 40. For patients ages ≥ 65 years, it is recommended that eye examinations occur every 1 to 2 years.43
Diet and mobility play a big role in cognition. Diet43 and exercise41,42,44 are believed to have an impact on mentation, and recent studies show memory and global cognition could be malleable later in life. A 2015 meta-analysis of 490 treatment arms of 24 randomized controlled studies showed improvement in global cognition with consumption of a Mediterranean diet plus olive oil (effect size [ES] standardized mean difference [SMD] = 0.22; 95% CI, 0.16-0.27) and tai chi exercises (ES SMD = 0.18; 95% CI, 0.06-0.29).42 The analysis also found improved memory among participants who consumed the Mediterranean diet/olive oil combination (ES SMD = 0.22; 95% CI, 0.12-0.32) and soy isoflavone supplements (ES SMD = 0.11; 95% CI, 0.04-0.17). Although the ESs are small, they are significant and offer promising evidence that individual choices related to nutrition or exercise may influence cognition and memory.
A 2018 systematic review found that all domains of cognition showed improvement with 45 to 60 minutes of moderate-to-vigorous physical exercise.44 Attention, executive function, memory, and working memory showed significant increases, whereas global cognition improvements were not statistically significant.44 A 2016 meta-analysis of 26 studies (N = 26,355) found a positive association between an objective mobility measure (gait, lower-extremity function, and balance) and cognitive function (global, executive function, memory, and processing speed) in older adults.41 These results highlight that diet, mobility, and physical exercise impact cognitive functioning.
Continue to: Maintaining social connections
Maintaining social connections
Social isolation and loneliness—compounded by a pandemic. The US Department of Health and Human Services notes that “community connections” are among the key factors required for healthy aging.3 Similarly, the WHO definition of healthy aging considers whether individuals can build and sustain relationships with other people and find ways to engender their personal values through these connections.2
As people age, their social connections often decrease due to the death of friends and family, shifts in living arrangements, loss of mobility or eyesight (and thus self-transport), and the onset or increased acuity of illness or chronic conditions.45 This has been exacerbated by the COVID-19 pandemic, which has spurred shelter-in-place and stay-at-home orders along with recommendations for physical distancing (also known as social distancing), especially for older adults who are at higher risk.46 Smith et al47 calls this the COVID-19 Social Connectivity Paradox, in which older adults limit their interactions with others to protect their physical health and reduce their risk of contracting the virus, but as a result they may undermine their psychosocial health by placing themselves at risk of social isolation and loneliness.47
The double threat. Social isolation and loneliness have been shown to negatively impact physical health and well-being, resulting in an increased risk of early death48-50; higher likelihood of specific diagnoses, including dementia and cardiovascular conditions48,50; and more frequent use of health care services.50 These concepts, while related, represent different mechanisms for negative health outcomes. Social isolation is an objective condition when one has a lack of opportunities for interaction with other people; loneliness refers to the emotional disconnect one feels when separated from others. Few studies have compared outcomes between these concepts, but in those that have, social isolation appears to be more strongly associated with early death.48-50
A 2013 observational study using data from the English Longitudinal Study on Aging found that both social isolation and loneliness were associated with increased mortality among men and women ages ≥ 52 years (N = 6500).48 However, when studied independently, loneliness was not found to be a significant risk factor. In contrast, social isolation significantly impacted mortality risk, even after adjusting for demographic factors and baseline health status.48 These findings are supported by a 2018 cohort study of individuals (N = 479,054) with a history of an acute cardiovascular event that concluded social isolation was a predictor of mortality, whereas loneliness was not.50
A large 2015 meta-analysis (70 studies, N = 3,407,134) of mortality causes among community-dwelling older adults (average age, 66) confirmed that both objective measures of isolation, as well as subjective measures (such as feelings of loneliness or living alone), have a significant predictive effect in longer-term studies. Each measure shows an approximately 30% increase in the likelihood of death after an average of 7 years.49
Continue to: Health care remains a connection point
Health care remains a connection point. Even when life course events and conditions (eg, death of loved ones, loss of transportation or financial resources, or disengagement from community activities) reduce social connections, most older adults engage in some way with the health care system. A 2020 consensus report by the National Academies of Sciences, Engineering, and Medicine suggests health care professionals capitalize on these connection points with adults ages ≥ 50 years by periodically screening for social isolation and loneliness, documenting social status updates in medical records, and piloting and evaluating interventions in the clinical setting.51
The report offered information about potential avenues for intervention by primary care professionals beyond screening, such as participating in research studies that investigate screening tools and multisystem interventions; social prescribing (linking patients to embedded social work services or community-based organizations); referring patients to support groups; initiating cognitive-based therapy or other behavioral health interventions; or recommending mindfulness practices.51 However, most of the cited intervention studies were not specific to primary care settings and contained poor-quality evidence related to efficacy.
Isolation creates a greater reliance on health services due to a lack of a social support system, while a feeling of emotional disconnection (loneliness) seems to be a barrier to accessing care. A 2017 cohort study linking data from the Health and Retirement Study and Medicare claims revealed that social isolation predicts higher annual health expenditures (> $1600 per beneficiary) driven by hospitalization and skilled nursing facility usage, along with greater mortality, whereas individuals who are lonely result in reduced costs (a reduction of $770 annually) due to lower usage of inpatient and outpatient services.52 Prioritizing interventions that identify and connect isolated older adults to social support, therefore, may increase survivability by ensuring they have access to resources and health care interventions when needed.
In addition, these findings underscore the importance of looking at quality—not just quantity—of older adults’ social connections. A number of validated screening tools exist for social isolation and loneliness (TABLE 253-59); however, concerns exist about assessing risk using a unidimensional tool for a complex concern,47 as well as identifying a problem without having evidence-based interventions to offer as solutions.47,51 Until future studies resolve these concerns, leveraging the physician-patient relationship to broach these difficult subjects may help normalize the issues and create safe spaces to identify individuals who are at risk.

QOL is key to healthy aging. As Kusumastuti et al4 state, “successful ageing lies in the eyes of the beholder.” A 2019 systematic review of 48 qualitative studies revealed that community-dwelling older adults ages ≥ 50 years in 11 countries (N > 4175) perceive well-being by considering QOL within 9 domains: health perception, autonomy, role and activity, relationships, emotional comfort, attitude and adaptation, spirituality, financial security, and home and neighborhood.60 Researchers found that as engagement in any one of these domains declines, older adults may shift their definition of health toward their remaining abilities.60 This offers an explanation as to why patients might rate their health status much higher than their physicians do: older adults tend to have a more holistic concept of health.
Continue to: Take a multidimensional approach to healthy aging
Take a multidimensional approach to healthy aging
Although we have separately examined each of the 4 components of managing healthy aging in a community-dwelling adult, applying a multidimensional approach is most effective. Increasing use of the Medicare AWV provides an opportunity to assess patient health status, determine care preferences, and improve follow-through on preventive screening. It is also important to encourage older adults to engage in regular physical activity—especially muscle-strengthening exercises—and to discuss nutrition and caloric intake to prevent frailty and functional decline.
Assessing and treating vision and hearing impairments and mental health issues, including anxiety and depression, may guard against losses in cognition. When speaking with older adult patients about their social connections, consider asking not only about frequency of contact and access to resources such as food and transportation, but also about whether they are finding ways to bring their own values into those relationships to bolster their QOL. This guidance also may be useful for primary care practices and health care networks when planning future quality-improvement initiatives.
Additional research is needed to support the evidence base for aligning older adult preferences in health care interventions, such as preventive screenings. Also, clinical decision-making requires more clarity about the efficacy of specific diet and exercise interventions for older adults; the impact of early intervention for depression, anxiety, and sleep disorders on neurodegenerative disease; whether loneliness predicts mortality; and how health care delivery systems can be effective at building social connectivity.
For now, it is essential to recognize that initiating health education, screening, and prevention throughout the patient’s lifespan can promote healthy aging outcomes. As family physicians, it is important to capitalize on longitudinal relationships with patients and begin educating younger patients using this multidimensional framework to promote living “a productive and meaningful life”at any age.3
Lynn M. Wilson, DO, 707 Hamilton Street, 8th floor, Department of Family Medicine, Lehigh Valley Health Network, Allentown, PA 18101; [email protected]
Our approach to caring for the growing number of community-dwelling US adults ages ≥ 65 years has shifted. Although we continue to manage disease and disability, there is an increasing emphasis on the promotion of healthy aging by optimizing health care needs and quality of life (QOL).
The American Geriatric Society (AGS) uses the term “healthy aging” to reflect a dedication to improving the health, independence, and QOL of older people.1 The World Health Organization (WHO) defines healthy aging as “the process of developing and maintaining the functional ability that enables well-being in older age.”2 Functional ability encompasses capabilities that align with a person’s values, including meeting basic needs; learning, growing, and making independent decisions; being mobile; building and maintaining healthy relationships; and contributing to society.2 Similarly, the US Department of Health and Human Services has adopted a multidimensional approach to support people in creating “a productive and meaningful life” as they grow older.3
Numerous theoretical models have emerged from research on aging as a multidimensional construct, as evidenced by a 2016 citation analysis that identified 1755 articles written between 1902 and 2015 relating to “successful aging.”4 The analysis revealed 609 definitions operationalized by researchers’ measurement tools (mostly focused on physical function and other health metrics) and 1146 descriptions created by older adults, many emphasizing psychosocial strategies and cultural factors as key to successful aging.4

One approach that is likely to be useful for family physicians is the Age-Friendly Health System. This is an initiative of The John A. Hartford Foundation and the Institute for Healthcare Improvement that uses a multidisciplinary approach to create environments that foster inclusivity and address the needs of older people.5 Following this guidance, primary care providers use evidence-informed strategies that promote safety and address what matters most to older adults and their family caregivers.
The Age-Friendly Health System, as well as AGS and WHO, recognize that there are multiple aspects to well-being as one grows older. By using focused, evidence-based screening, assessments, and interventions, family physicians can best support aging patients in living their most fulfilling lives.
Here we present a review of evidence-based strategies that promote safety and address what matters most to older adults and their family caregivers using a 4-pronged framework, in the style of the Age-Friendly Health System model. However, the literature on healthy aging includes important messages about patient context and lifelong health behaviors, which we capture in an expanded set of thematic guidance. As such, we encourage family physicians to approach healthy aging as follows: (1) monitor health (screening and prevention), (2) promote mobility (physical function), (3) manage mentation (emotional health and cognitive function), and (4) encourage maintenance of social connections (social networks and QOL).
Monitoring health
Leverage Medicare annual wellness visits. A systematic approach is needed to prevent frailty and functional decline, and thus increase the QOL of older adults. To do this, it is important to focus on health promotion and disease prevention, while addressing existing ailments. One method is to leverage the Medicare annual wellness visit (AWV), which provides an opportunity to assess current health status as well as discuss behavior-change and risk-reduction strategies with patients.
Continue to: Although AWVs...
Although AWVs are an opportunity to improve patient outcomes, we are not taking full advantage of them.6 While AWVs have gained traction since their introduction in 2011, usage rates among ethnoracial minority groups has lagged behind.6 A 2018 cohort study examined reasons for disparate utilization rates among individuals ages ≥ 66 years (N = 14,687).7 Researchers found that differences in utilization between ethnoracial groups were explained by socioeconomic factors. Lower education and lower income, as well as rural living, were associated with lower rates of AWV completion.7 In addition, having a usual, nonemergent place to obtain medical care served as a powerful predictor of AWV utilization for all groups.7
Strategies to increase AWV completion rates among all eligible adults include increasing staff awareness of health literacy challenges and ensuring communication strategies are inclusive by providing printed materials in multiple languages, Braille, or larger typefaces; using accessible vocabulary that does not include medical jargon; and making medical interpreters accessible. In addition, training clinicians about unconscious bias and cultural humility can help foster empathy and awareness of differences in health beliefs and behaviors within diverse patient populations.
A 2019 scoping review of 11 studies (N > 60 million) focused on outcomes from Medicare AWVs for patients ages ≥ 65 years.8 This included uptake of preventive services, such as vaccinations or cancer screenings; advice, education, or referrals offered during the AWV; medication use; and hospitalization rates. Overall findings showed that older adults who received a Medicare AWV were more likely to receive referrals for preventive screenings and follow-through on these recommendations compared with those who did not undergo an AWV.8
Completion rates for vaccines, while remaining low overall, were higher among those who completed an AWV. Additionally, these studies showed improved completion of screenings for breast cancer, bone density, and colon cancer. Several studies in the scoping review supported the use of AWVs as an effective means by which to offer health education and advice related to health promotion and risk reduction, such as diet and lifestyle modifications.8 Little evidence exists on long-term outcomes related to AWV completion.8
Utilize shared decision-making to determine whether preventive screening makes sense for your patient. Although cancer remains the second leading cause of death among Americans ages ≥ 65 years,9 clear screening guidelines for this age group remain elusive.10 Physicians and patients often are reluctant to stop cancer screening despite lower life expectancy and fewer potential benefits of diagnosis in this population.9 Some recent studies reinforce the heterogeneity of the older adult population and further underscore the importance of individual-level decision-making.11-14 It is important to let older adult patients and their caregivers know about the potential risks of screening tests, especially the possibility that incidental findings may lead to unwarranted additional care or monitoring.9
Continue to: Avoid these screening conversation missteps
Avoid these screening conversation missteps. A 2017 qualitative study asked 40 community-dwelling older adults (mean age = 76 years) about their preferences for discussing screening cessation with their physicians.13 Three themes emerged.First, they were open to stopping their screenings, especially when suggested by a trusted physician. Second, health status and physical function made sense as decision points, but life expectancy did not. Finally, lengthy discussions with expanded details about risks and benefits were not appreciated, especially if coupled with comments on the limited benefits for those nearing the end of life. When discussing life expectancy, patients preferred phrasing that focused on how the screening was unnecessary because it would not help them live longer.13
Ensure that your message is understood—and culturally relevant. Recent studies on lower health literacy among older adults15,16 and ethnic and racial minorities17-21—as revealed in the 2003 National Assessment of Adult Literacy22—might offer clues to patient receptivity to discussions about preventive screening and other health decisions.
One study found a significant correlation between higher self-rated health literacy and higher engagement in health behaviors such as mammography screening, moderate physical activity, and tobacco avoidance.16 Perceptions of personal control over health status, as well as perceived social standing, also correlated with health literacy score levels.16 Another study concluded that lower health literacy combined with lower self-efficacy, cultural beliefs about health topics (eg, diet and exercise), and distrust in the health care system contributed to lower rates of preventive care utilization among ethnocultural minority older adults in Canada, the United Kingdom, the United States, and Australia.18
Ensuring that easy-to-understand information is equitably shared with older adults of all races and ethnicities is critical. A 2018 study showed that distrust of the health system and cultural issues contributed to the lower incidence of colorectal cancer screenings in Hispanic and Asian American patients ages 50 to 75 years.21 Patients whose physicians engaged in “health literate practices” (eg, offering clear explanations of diagnostic plans and asking patients to describe what they understood) were more likely to obtain recommended breast and colorectal cancer screenings.20 In particular, researchers found that non-Hispanic Blacks were nearly twice as likely to follow through on colorectal cancer screening if their physicians engaged in health literate practices.20 In addition, receiving clear instructions from physicians increased the odds of completing breast cancer screening among Hispanic and non-Hispanic White women.20
Overall, screening information and recommendations should be standardized for all patients. This is particularly important in light of research that found that older non-Hispanic Black patients were less likely than their non-Hispanic White counterparts to receive information from their physicians about colorectal cancer screening.20
Continue to: Mobility
Mobility
Encourage physical activity. Frequent exercise and other forms of physical activity are associated with healthy aging, as shown in a 2017 systematic review and meta-analysis of 23 studies (N = 174,114).23 Despite considerable heterogeneity between studies in how researchers defined healthy aging and physical activity, they found that adults who incorporate regular movement and exercise into daily life are likely to continue to benefit from it into older age.23 In addition, a 2016 secondary analysis of data from the InCHIANTI longitudinal aging study concluded that adults ages ≥ 65 years (N = 1149) who had maintained higher physical activity levels throughout adulthood had less physical function decline and reduced rates of mobility disability and premature death compared with those who reported being less active.24
Preserve gait speed (and bolster health) with these activities. Walking speed, or gait, measured on a level surface has been used as a predictor for various aspects of well-being in older age, such as daily function, mobility, independence, falls, mortality, and hospitalization risk.25 Reduced gait speed is also one of the key indicators of functional impairment in older adults.
A 2015 systematic review sought to determine which type of exercise intervention (resistance, coordination, or multimodal training) would be most effective in preserving gait speed in healthy older adults (N = 2495; mean age = 74.2 years).25 While the 42 included studies were deemed to be fairly low quality, the review revealed (with large effect size [0.84]) that a number of exercise modalities might stave off loss of gait speed in older adults. Patients in the resistance training group had the greatest improvement in gait speed (0.11 m/s), followed by those in the coordination training group (0.09 m/s) and the multimodal training group (0.05 m/s).25
Finally, muscle mass and strength offer a measure of physical performance and functionality. A 2020 systematic review of 83 studies (N = 108,428) showed that low muscle mass and strength, reduced handgrip strength, and lower physical performance were predictive of reduced capacities in activities of daily living and instrumental activities of daily living.26 It is important to counsel adults to remain active throughout their lives and to include resistance training to maintain muscle mass and strength to preserve their motor function, mobility, independence, and QOL.
Use 1 of these scales to identify frailty. Frailty is a distinct clinical syndrome, in which an individual has low reserves and is highly vulnerable to internal and external stressors. It affects many community-dwelling older adults. Within the literature, there has been ongoing discussion regarding the definition of frailty27 (TABLE 128-31).

Continue to: The Fried Frailty Index...
The Fried Frailty Index defines frailty as a purely physical condition; patients need to exhibit 3 of 5 components (ie, weight loss, exhaustion, weakness, slowness, and low physical activity) to be deemed frail.31 The Edmonton Frail Scale is commonly used in geriatric assessments and counts impairments across several domains including physical activity, mood, cognition, and incontinence.30,32,33 Physicians need to complete a training course prior to its use. Finally, the definition of frailty used by Rockwood et al28, 29 was used to develop the Clinical Frailty Scale, which relies on broader criteria that include social and psychological elements in addition to physical elements.The Clinical Frailty Scale uses clinician judgment to evaluate patient-specific domains (eg, comorbidities, functionality, and cognition) and to generate a score ranging from 1 (very fit) to 9 (terminally ill).29 This scale is accessible and easy to implement. As a result, use of this scale has increased during the COVID-19 pandemic. All definitions include a pre-frail state, indicating the dynamic nature of frailty over time.
It is important to identify pre-frail and frail older adults using 1 of these screening tools. Interventions to reverse frailty that can be initiated in the primary care setting include identifying treatable medical conditions, assessing medication appropriateness, providing nutritional advice, and developing an exercise plan.34
Conduct a nutritional assessment; consider this diet. Studies show that nutritional status can predict physical function and frailty risk in older adults. A 2017 systematic review of 19 studies (N = 22,270) of frail adults ages ≥ 65 years found associations related to specific dietary constructs (ie, micronutrients, macronutrients, antioxidants, overall diet quality, and timing of consumption).35 Plant-based diets with higher levels of micronutrients, such as vitamins C and E and beta-carotene, or diets with more protein or macronutrients, regardless of source foods, all resulted in inverse associations with frailty syndrome.35
A 2017 study showed that physical exercise and maintaining good nutritional status may be effective for preventing frailty in community-dwelling pre-frail older individuals.36 A 2019 study showed that a combination of muscle strength training and protein supplementation was the most effective intervention to delay or reverse frailty and was easiest to implement in primary care.37 A 2020 meta-analysis of 31 studies (N = 4794) addressing frailty among primary care patients > 60 years showed that interventions using predominantly resistance-based exercise and nutrition supplementation improved frailty status over the control.38 Researchers also found that a comprehensive geriatric assessment or exercise—alone or in combination with nutrition education—reduced physical frailty.
Mentation
Screen and treat cognitive impairments. Cognitive function and autonomy in decision-making are important factors in healthy aging. Aspects of mental health (eg, depression and anxiety), sensory impairment (eg, visual and auditory impairment), and mentation issues (eg, delirium, dementia, and related conditions), as well as diet, physical exercise, and mobility, can all impede cognitive functionality. The long-term effects of depression, anxiety,39 sensory deficits,40 mobility,41 diet,42 and, ultimately, aging may impact Alzheimer disease (AD). The risk of an AD diagnosis increases with age.39
Continue to: A 2018 prospective cohort study...
A 2018 prospective cohort study using data from the National Alzheimer’s Coordinating Center followed individuals (N = 12,053) who were cognitively asymptomatic at their initial visits to determine who developed clinical signs of AD.39 Survival analysis showed several psychosocial factors—anxiety, sleep disturbances, and depressive episodes of any type (occurring within the past 2 years, clinician verified, lifetime report)—were significantly associated with an eventual AD diagnosis and increased the risk of AD.39 More research is needed to verify the impact of early intervention for these conditions on neurodegenerative disease; however, screening and treating psychosocial factors such as anxiety and depression should be maintained.
Researchers evaluated the impact of a dual sensory impairment (DSI) on dementia risk using data from 2051 participants in the Ginkgo Evaluation of Memory Study.40 Hearing and visual impairments (defined as DSI when these conditions coexist) or visual impairment alone were significantly associated with increased risk of dementia in older adults. The researchers reported that DSI was significantly associated with a higher risk of all-cause dementia (hazard ratio [HR] = 1.86; 95% CI, 1.25-2.76) and AD (HR = 2.12; 95% CI, 1.34-3.36).40 Visual impairment alone was associated with an increased risk of all-cause dementia (HR = 1.32; 95% CI, 1.02-1.71).40 These results suggest that screening of DSI or visual impairment earlier in the patient’s lifespan may identify those at high risk of dementia in older adulthood.
The American Academy of Ophthalmology recommends patients with healthy eyes be screened once during their 20s and twice in their 30s; a full examination is recommended by age 40. For patients ages ≥ 65 years, it is recommended that eye examinations occur every 1 to 2 years.43
Diet and mobility play a big role in cognition. Diet43 and exercise41,42,44 are believed to have an impact on mentation, and recent studies show memory and global cognition could be malleable later in life. A 2015 meta-analysis of 490 treatment arms of 24 randomized controlled studies showed improvement in global cognition with consumption of a Mediterranean diet plus olive oil (effect size [ES] standardized mean difference [SMD] = 0.22; 95% CI, 0.16-0.27) and tai chi exercises (ES SMD = 0.18; 95% CI, 0.06-0.29).42 The analysis also found improved memory among participants who consumed the Mediterranean diet/olive oil combination (ES SMD = 0.22; 95% CI, 0.12-0.32) and soy isoflavone supplements (ES SMD = 0.11; 95% CI, 0.04-0.17). Although the ESs are small, they are significant and offer promising evidence that individual choices related to nutrition or exercise may influence cognition and memory.
A 2018 systematic review found that all domains of cognition showed improvement with 45 to 60 minutes of moderate-to-vigorous physical exercise.44 Attention, executive function, memory, and working memory showed significant increases, whereas global cognition improvements were not statistically significant.44 A 2016 meta-analysis of 26 studies (N = 26,355) found a positive association between an objective mobility measure (gait, lower-extremity function, and balance) and cognitive function (global, executive function, memory, and processing speed) in older adults.41 These results highlight that diet, mobility, and physical exercise impact cognitive functioning.
Continue to: Maintaining social connections
Maintaining social connections
Social isolation and loneliness—compounded by a pandemic. The US Department of Health and Human Services notes that “community connections” are among the key factors required for healthy aging.3 Similarly, the WHO definition of healthy aging considers whether individuals can build and sustain relationships with other people and find ways to engender their personal values through these connections.2
As people age, their social connections often decrease due to the death of friends and family, shifts in living arrangements, loss of mobility or eyesight (and thus self-transport), and the onset or increased acuity of illness or chronic conditions.45 This has been exacerbated by the COVID-19 pandemic, which has spurred shelter-in-place and stay-at-home orders along with recommendations for physical distancing (also known as social distancing), especially for older adults who are at higher risk.46 Smith et al47 calls this the COVID-19 Social Connectivity Paradox, in which older adults limit their interactions with others to protect their physical health and reduce their risk of contracting the virus, but as a result they may undermine their psychosocial health by placing themselves at risk of social isolation and loneliness.47
The double threat. Social isolation and loneliness have been shown to negatively impact physical health and well-being, resulting in an increased risk of early death48-50; higher likelihood of specific diagnoses, including dementia and cardiovascular conditions48,50; and more frequent use of health care services.50 These concepts, while related, represent different mechanisms for negative health outcomes. Social isolation is an objective condition when one has a lack of opportunities for interaction with other people; loneliness refers to the emotional disconnect one feels when separated from others. Few studies have compared outcomes between these concepts, but in those that have, social isolation appears to be more strongly associated with early death.48-50
A 2013 observational study using data from the English Longitudinal Study on Aging found that both social isolation and loneliness were associated with increased mortality among men and women ages ≥ 52 years (N = 6500).48 However, when studied independently, loneliness was not found to be a significant risk factor. In contrast, social isolation significantly impacted mortality risk, even after adjusting for demographic factors and baseline health status.48 These findings are supported by a 2018 cohort study of individuals (N = 479,054) with a history of an acute cardiovascular event that concluded social isolation was a predictor of mortality, whereas loneliness was not.50
A large 2015 meta-analysis (70 studies, N = 3,407,134) of mortality causes among community-dwelling older adults (average age, 66) confirmed that both objective measures of isolation, as well as subjective measures (such as feelings of loneliness or living alone), have a significant predictive effect in longer-term studies. Each measure shows an approximately 30% increase in the likelihood of death after an average of 7 years.49
Continue to: Health care remains a connection point
Health care remains a connection point. Even when life course events and conditions (eg, death of loved ones, loss of transportation or financial resources, or disengagement from community activities) reduce social connections, most older adults engage in some way with the health care system. A 2020 consensus report by the National Academies of Sciences, Engineering, and Medicine suggests health care professionals capitalize on these connection points with adults ages ≥ 50 years by periodically screening for social isolation and loneliness, documenting social status updates in medical records, and piloting and evaluating interventions in the clinical setting.51
The report offered information about potential avenues for intervention by primary care professionals beyond screening, such as participating in research studies that investigate screening tools and multisystem interventions; social prescribing (linking patients to embedded social work services or community-based organizations); referring patients to support groups; initiating cognitive-based therapy or other behavioral health interventions; or recommending mindfulness practices.51 However, most of the cited intervention studies were not specific to primary care settings and contained poor-quality evidence related to efficacy.
Isolation creates a greater reliance on health services due to a lack of a social support system, while a feeling of emotional disconnection (loneliness) seems to be a barrier to accessing care. A 2017 cohort study linking data from the Health and Retirement Study and Medicare claims revealed that social isolation predicts higher annual health expenditures (> $1600 per beneficiary) driven by hospitalization and skilled nursing facility usage, along with greater mortality, whereas individuals who are lonely result in reduced costs (a reduction of $770 annually) due to lower usage of inpatient and outpatient services.52 Prioritizing interventions that identify and connect isolated older adults to social support, therefore, may increase survivability by ensuring they have access to resources and health care interventions when needed.
In addition, these findings underscore the importance of looking at quality—not just quantity—of older adults’ social connections. A number of validated screening tools exist for social isolation and loneliness (TABLE 253-59); however, concerns exist about assessing risk using a unidimensional tool for a complex concern,47 as well as identifying a problem without having evidence-based interventions to offer as solutions.47,51 Until future studies resolve these concerns, leveraging the physician-patient relationship to broach these difficult subjects may help normalize the issues and create safe spaces to identify individuals who are at risk.

QOL is key to healthy aging. As Kusumastuti et al4 state, “successful ageing lies in the eyes of the beholder.” A 2019 systematic review of 48 qualitative studies revealed that community-dwelling older adults ages ≥ 50 years in 11 countries (N > 4175) perceive well-being by considering QOL within 9 domains: health perception, autonomy, role and activity, relationships, emotional comfort, attitude and adaptation, spirituality, financial security, and home and neighborhood.60 Researchers found that as engagement in any one of these domains declines, older adults may shift their definition of health toward their remaining abilities.60 This offers an explanation as to why patients might rate their health status much higher than their physicians do: older adults tend to have a more holistic concept of health.
Continue to: Take a multidimensional approach to healthy aging
Take a multidimensional approach to healthy aging
Although we have separately examined each of the 4 components of managing healthy aging in a community-dwelling adult, applying a multidimensional approach is most effective. Increasing use of the Medicare AWV provides an opportunity to assess patient health status, determine care preferences, and improve follow-through on preventive screening. It is also important to encourage older adults to engage in regular physical activity—especially muscle-strengthening exercises—and to discuss nutrition and caloric intake to prevent frailty and functional decline.
Assessing and treating vision and hearing impairments and mental health issues, including anxiety and depression, may guard against losses in cognition. When speaking with older adult patients about their social connections, consider asking not only about frequency of contact and access to resources such as food and transportation, but also about whether they are finding ways to bring their own values into those relationships to bolster their QOL. This guidance also may be useful for primary care practices and health care networks when planning future quality-improvement initiatives.
Additional research is needed to support the evidence base for aligning older adult preferences in health care interventions, such as preventive screenings. Also, clinical decision-making requires more clarity about the efficacy of specific diet and exercise interventions for older adults; the impact of early intervention for depression, anxiety, and sleep disorders on neurodegenerative disease; whether loneliness predicts mortality; and how health care delivery systems can be effective at building social connectivity.
For now, it is essential to recognize that initiating health education, screening, and prevention throughout the patient’s lifespan can promote healthy aging outcomes. As family physicians, it is important to capitalize on longitudinal relationships with patients and begin educating younger patients using this multidimensional framework to promote living “a productive and meaningful life”at any age.3
Lynn M. Wilson, DO, 707 Hamilton Street, 8th floor, Department of Family Medicine, Lehigh Valley Health Network, Allentown, PA 18101; [email protected]
1. Friedman S, Mulhausen P, Cleveland M, et al. Healthy aging: American Geriatrics Society white paper executive summary. J Am Geriatr Soc. 2018;67:17-20. doi: 10.1111/jgs.15644
2. World Health Organization. World report on ageing and health. 2015. Accessed June 29, 2020. https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf?sequence=1
3. U.S. Department of Health & Human Services. Healthy aging. Accessed June 29, 2020. www.hhs.gov/aging/healthy-aging
4. Kusumastuti S, Derks MGM, Tellier S, et al. Successful ageing: a study of the literature using citation network analysis. Maturitas. 2016;93:4-12. doi: 10.1016/j.maturitas.2016.04.010
5. Institute for Healthcare Improvement. Age-friendly health systems: guide to using the 4Ms in the care of older adults [white paper]. 2020. Accessed June 29, 2020. www.ihi.org/Engage/Initiatives/Age-Friendly-Health-systems/Documents/IHIAgeFriendlyHealthSystems_GuidetoUsing4MsCare.pdf
6. Lind KE, Hildreth KL, Perraillon MC. Persistent disparities in Medicare’s annual wellness visit utilization. Med Care. 2019;57:984-989. doi: 10.1097/MLR.0000000000001229
7. Lind KE, Hildreth K, Lindrooth R, et al. Ethnoracial disparities in Medicare annual wellness visit utilization: evidence from a nationally representative database. Med Care. 2018;56:761-766. doi: 10.1097/MLR.0000000000000962
8. Simpson VL, Kovich M. Outcomes of primary care-based Medicare annual wellness visits with older adults: a scoping review. Geriatr Nurs. 2019;40:590-596. doi: 10.1016/j.gerinurse.2019.06.001
9. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68:1-77.
10. Salzman B, Beldowski K, de la Paz A. Cancer screening in older patients. Am Fam Physician. 2016;93:659-667.
11. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177:399-406. doi: 10.1001/jamainternmed.2016.9022
12. Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. 2014;311:1336-1347. doi: 10.1001/jama.2014.2834
13. Schoenborn NL, Lee K, Pollack CE, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177:1121-1128. doi: 10.1001/jamainternmed.2017.1778
14. Butterworth JE, Hays R, McDonagh ST, et al. Interventions for involving older patients with multi-morbidity in decision-making during primary care consultations. Cochrane Database Syst Rev. 2019;10:CD013124. doi: 10.1002/14651858.CD013124.pub2
15. Bostock S, Steptoe A. Association between low functional health literacy and mortality in older adults: longitudinal cohort study. BMJ. 2012;344:e1602. doi: 10.1136/bmj.e1602
16. Fernandez DM, Larson JL, Zikmund-Fisher BJ. Associations between health literacy and preventive health behaviors among older adults: findings from the health and retirement study. BMC Public Health. 2016;16:596. doi: 10.1186/s12889-016-3267-7
17. Weekes CV. African Americans and health literacy: a systematic review. ABNF J. 2012;23:76-80.
18. Mantwill S, Monestel-Umaña S, Schulz PJ. The relationship between health literacy and health disparities: a systematic review. PLoS One. 2015;10:e0145455. doi: 10.1371/journal.pone.0145455
19. Khan MM, Kobayashi K. Optimizing health promotion among ethnocultural minority older adults (EMOA). Int J Migration Health Soc Care. 2015;11:268-281. doi: 10.1108/IJMHSC-12-2014-0047
20. Kindratt TB, Dallo FJ, Allicock M, et al. The influence of patient-provider communication on cancer screenings differs among racial and ethnic groups. Prev Med Rep. 2020;18:101086. doi: 10.1016/j.pmedr.2020.101086
21. Hong Y-R, Tauscher J, Cardel M. Distrust in health care and cultural factors are associated with uptake of colorectal cancer screening in Hispanic and Asian Americans. Cancer. 2018;124:335-345. doi: 10.1002/cncr.31052
22. Kutner M, Greenberg E, Jin Y, et al. Literacy in everyday life: results from the 2003 National Assessment of Adult Literacy. NCES 2007-480. U.S. Department of Education, National Center for Education Statistics. April 2007. Accessed August 27, 2021. http://nces.ed.gov/Pubs2007/2007480_1.pdf
23. Daskalopoulou C, Stubbs B, Kralj C, et al. Physical activity and healthy ageing: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2017;38:6-17. doi: 10.1016/j.arr.2017.06.003
24. Stenholm S, Koster A, Valkeinen H, et al. Association of physical activity history with physical function and mortality in old age. J Gerontol A Biol Sci Med Sci. 2016;71:496-501. doi: 10.1093/gerona/glv111
25. Hortobágyi T, Lesinski M, Gäbler M, et al. Effects of three types of exercise interventions on healthy old adults’ gait speed: a systematic review and meta-analysis. Sports Med. 2015;45:1627‐1643. Published correction appears in Sports Med. 2016;46:453. doi: 10.1007/s40279-015-0371-2
26. Wang DXM, Yao J, Zirek Y, et al. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11:3‐25. doi: 10.1002/jcsm.12502
27. Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129-2138. doi: 10.1111/j.1532-5415.2011.03597.x
28. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-495. doi: 10.1503/cmaj.050051
29. Church S, Rogers E, Rockwood K, et al. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20:393. doi: 10.1186/s12877-020-01801-7
30. Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526-529. doi: 10.1093/ageing/afl041
31. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146
32. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Euro J Intern Med. 2016;31:3-10. doi: 10.1016/j.ejim.2016.03.007
33. Perna S, Francis MD, Bologna C, et al. Performance of Edmonton Frail Scale on frailty assessment: its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr. 2017;17:2. doi: 10.1186/s12877-016-0382-3
34. Chen CY, Gan P, How CH. Approach to frailty in the elderly in primary care and the community. Singapore Med J. 2018;59:338. doi: 10.11622/smedj.2018052
35. Lorenzo-López L, Maseda A, de Labra C, et al. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. 2017;17:108. doi: 10.1186/s12877-017-0496-2
36. Serra-Prat M, Sist X, Domenich R, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing. 2017;46:401-407. doi: 10.1093/ageing/afw242
37. Travers J, Romero-Ortuno R, Bailey J, et al. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69:e61-e69. doi: 10.3399/bjgp18X700241
38. Macdonald SHF, Travers J, Shé ÉN, et al. Primary care interventions to address physical frailty among community-dwelling adults aged 60 years or older: a meta-analysis. PLoS One. 2020;15:e0228821. doi: 10.1371/journal.pone.0228821
39. Burke SL, Cadet T, Alcide A, et al. Psychosocial risk factors and Alzheimer’s disease: the associative effect of depression, sleep disturbance, and anxiety. Aging Ment Health. 2018;22:1577-1584. doi: 10.1080/13607863.2017.1387760
40. Hwang PH, Longstreth WT Jr, Brenowitz WD, et al. Dual sensory impairment in older adults and risk of dementia from the GEM Study. Alzheimers Dement (Amst). 2020;12:e12054. doi: 10.1002/dad2.12054
41. Demnitz N, Esser P, Dawes H, et al. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164‐174. doi: 10.1016/j.gaitpost.2016.08.028
42. Lehert P, Villaseca P, Hogervorst E, et al. Individually modifiable risk factors to ameliorate cognitive aging: a systematic review and meta-analysis. Climacteric. 2015;18:678-689. doi: 10.3109/13697137.2015.1078106
43. Turbert D. Eye exam and vision testing basics. American Academy of Ophthalmology Web site. January 14, 2021. Accessed March 5, 2021. www.aao.org/eye-health/tips-prevention/eye-exams-101
44. Northey JM, Cherbuin N, Pumpa KL, et al. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52:154-160. doi: 10.1136/bjsports-2016-096587
45. CDC. Percent of U.S. adults 55 and over with chronic conditions. November 6, 2015. Accessed April 29, 2021. www.cdc.gov/nchs/health_policy/adult_chronic_conditions.htm
46. National Council on Aging. COVID-driven isolation can be dangerous for older adults. March 31, 2021. Accessed April 29, 2021. www.ncoa.org/article/covid-driven-isolation-can-be-dangerous-for-older-adults
47. Smith ML, Steinman LE, Casey EA. Combatting social isolation among older adults in a time of physical distancing: the COVID-19 social connectivity paradox. Front Public Health. 2020;8:403. doi: 10.3389/fpubh.2020.00403
48. Steptoe A, Shankar A, Demakakos P, et al. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110:5797-5801. doi: 10.1073/pnas.1219686110
49. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10:227-237. doi: 10.1177/1745691614568352
50. Hakulinen C, Pulkki-Råback L, Virtanen M, et al. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart. 2018;104:1536-1542. doi: 10.1136/heartjnl-2017-312663
51. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. The National Academies Press; 2020. doi: 10.17226/25663
52. Shaw JG, Farid M, Noel-Miller C, et al. Social isolation and Medicare spending: among older adults, objective isolation increases expenditures while loneliness does not. J Aging Health. 2017;29:1119-1143. doi: 10.1177/0898264317703559
53. Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186-204. doi: 10.1093/oxfordjournals.aje.a112674
54. Campaign to End Loneliness. Measuring your impact on loneliness in later life. Accessed April 29, 2021. www.campaigntoendloneliness.org/wp-content/uploads/Loneliness-Measurement-Guidance1-1.pdf
55. Cornwell EY, Waite LJ. Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav. 2009;50:31-48. doi: 10.1177/002214650905000103
56. Gierveld JDJ, Van Tilburg T. A 6-item scale for overall, emotional, and social loneliness: confirmatory tests on survey data. Res Aging. 2006;28:582-598. doi: 10.1177/0164027506289723
57. Koenig HG, Westlund RE, George LK, et al. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics. 1993;34:61-69. doi: 10.1016/S0033-3182(93)71928-3
58. Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 2006;46:503-513. doi: 10.1093/geront/46.4.503
59. Russell DW. UCLA Loneliness Scale (version 3): reliability, validity, factor structure. J Pers Assess. 1996;66:20-40. doi: 10.1207/s15327752jpa6601_2
60. van Leeuwen KM, van Loon MS, van Nes FA, et al. What does quality of life mean to older adults? A thematic synthesis. PLoS One. 2019;14:e0213263. doi: 10.1371/journal.pone.0213263
1. Friedman S, Mulhausen P, Cleveland M, et al. Healthy aging: American Geriatrics Society white paper executive summary. J Am Geriatr Soc. 2018;67:17-20. doi: 10.1111/jgs.15644
2. World Health Organization. World report on ageing and health. 2015. Accessed June 29, 2020. https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf?sequence=1
3. U.S. Department of Health & Human Services. Healthy aging. Accessed June 29, 2020. www.hhs.gov/aging/healthy-aging
4. Kusumastuti S, Derks MGM, Tellier S, et al. Successful ageing: a study of the literature using citation network analysis. Maturitas. 2016;93:4-12. doi: 10.1016/j.maturitas.2016.04.010
5. Institute for Healthcare Improvement. Age-friendly health systems: guide to using the 4Ms in the care of older adults [white paper]. 2020. Accessed June 29, 2020. www.ihi.org/Engage/Initiatives/Age-Friendly-Health-systems/Documents/IHIAgeFriendlyHealthSystems_GuidetoUsing4MsCare.pdf
6. Lind KE, Hildreth KL, Perraillon MC. Persistent disparities in Medicare’s annual wellness visit utilization. Med Care. 2019;57:984-989. doi: 10.1097/MLR.0000000000001229
7. Lind KE, Hildreth K, Lindrooth R, et al. Ethnoracial disparities in Medicare annual wellness visit utilization: evidence from a nationally representative database. Med Care. 2018;56:761-766. doi: 10.1097/MLR.0000000000000962
8. Simpson VL, Kovich M. Outcomes of primary care-based Medicare annual wellness visits with older adults: a scoping review. Geriatr Nurs. 2019;40:590-596. doi: 10.1016/j.gerinurse.2019.06.001
9. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68:1-77.
10. Salzman B, Beldowski K, de la Paz A. Cancer screening in older patients. Am Fam Physician. 2016;93:659-667.
11. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177:399-406. doi: 10.1001/jamainternmed.2016.9022
12. Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. 2014;311:1336-1347. doi: 10.1001/jama.2014.2834
13. Schoenborn NL, Lee K, Pollack CE, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177:1121-1128. doi: 10.1001/jamainternmed.2017.1778
14. Butterworth JE, Hays R, McDonagh ST, et al. Interventions for involving older patients with multi-morbidity in decision-making during primary care consultations. Cochrane Database Syst Rev. 2019;10:CD013124. doi: 10.1002/14651858.CD013124.pub2
15. Bostock S, Steptoe A. Association between low functional health literacy and mortality in older adults: longitudinal cohort study. BMJ. 2012;344:e1602. doi: 10.1136/bmj.e1602
16. Fernandez DM, Larson JL, Zikmund-Fisher BJ. Associations between health literacy and preventive health behaviors among older adults: findings from the health and retirement study. BMC Public Health. 2016;16:596. doi: 10.1186/s12889-016-3267-7
17. Weekes CV. African Americans and health literacy: a systematic review. ABNF J. 2012;23:76-80.
18. Mantwill S, Monestel-Umaña S, Schulz PJ. The relationship between health literacy and health disparities: a systematic review. PLoS One. 2015;10:e0145455. doi: 10.1371/journal.pone.0145455
19. Khan MM, Kobayashi K. Optimizing health promotion among ethnocultural minority older adults (EMOA). Int J Migration Health Soc Care. 2015;11:268-281. doi: 10.1108/IJMHSC-12-2014-0047
20. Kindratt TB, Dallo FJ, Allicock M, et al. The influence of patient-provider communication on cancer screenings differs among racial and ethnic groups. Prev Med Rep. 2020;18:101086. doi: 10.1016/j.pmedr.2020.101086
21. Hong Y-R, Tauscher J, Cardel M. Distrust in health care and cultural factors are associated with uptake of colorectal cancer screening in Hispanic and Asian Americans. Cancer. 2018;124:335-345. doi: 10.1002/cncr.31052
22. Kutner M, Greenberg E, Jin Y, et al. Literacy in everyday life: results from the 2003 National Assessment of Adult Literacy. NCES 2007-480. U.S. Department of Education, National Center for Education Statistics. April 2007. Accessed August 27, 2021. http://nces.ed.gov/Pubs2007/2007480_1.pdf
23. Daskalopoulou C, Stubbs B, Kralj C, et al. Physical activity and healthy ageing: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2017;38:6-17. doi: 10.1016/j.arr.2017.06.003
24. Stenholm S, Koster A, Valkeinen H, et al. Association of physical activity history with physical function and mortality in old age. J Gerontol A Biol Sci Med Sci. 2016;71:496-501. doi: 10.1093/gerona/glv111
25. Hortobágyi T, Lesinski M, Gäbler M, et al. Effects of three types of exercise interventions on healthy old adults’ gait speed: a systematic review and meta-analysis. Sports Med. 2015;45:1627‐1643. Published correction appears in Sports Med. 2016;46:453. doi: 10.1007/s40279-015-0371-2
26. Wang DXM, Yao J, Zirek Y, et al. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11:3‐25. doi: 10.1002/jcsm.12502
27. Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129-2138. doi: 10.1111/j.1532-5415.2011.03597.x
28. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-495. doi: 10.1503/cmaj.050051
29. Church S, Rogers E, Rockwood K, et al. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20:393. doi: 10.1186/s12877-020-01801-7
30. Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526-529. doi: 10.1093/ageing/afl041
31. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146
32. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Euro J Intern Med. 2016;31:3-10. doi: 10.1016/j.ejim.2016.03.007
33. Perna S, Francis MD, Bologna C, et al. Performance of Edmonton Frail Scale on frailty assessment: its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr. 2017;17:2. doi: 10.1186/s12877-016-0382-3
34. Chen CY, Gan P, How CH. Approach to frailty in the elderly in primary care and the community. Singapore Med J. 2018;59:338. doi: 10.11622/smedj.2018052
35. Lorenzo-López L, Maseda A, de Labra C, et al. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. 2017;17:108. doi: 10.1186/s12877-017-0496-2
36. Serra-Prat M, Sist X, Domenich R, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing. 2017;46:401-407. doi: 10.1093/ageing/afw242
37. Travers J, Romero-Ortuno R, Bailey J, et al. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69:e61-e69. doi: 10.3399/bjgp18X700241
38. Macdonald SHF, Travers J, Shé ÉN, et al. Primary care interventions to address physical frailty among community-dwelling adults aged 60 years or older: a meta-analysis. PLoS One. 2020;15:e0228821. doi: 10.1371/journal.pone.0228821
39. Burke SL, Cadet T, Alcide A, et al. Psychosocial risk factors and Alzheimer’s disease: the associative effect of depression, sleep disturbance, and anxiety. Aging Ment Health. 2018;22:1577-1584. doi: 10.1080/13607863.2017.1387760
40. Hwang PH, Longstreth WT Jr, Brenowitz WD, et al. Dual sensory impairment in older adults and risk of dementia from the GEM Study. Alzheimers Dement (Amst). 2020;12:e12054. doi: 10.1002/dad2.12054
41. Demnitz N, Esser P, Dawes H, et al. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164‐174. doi: 10.1016/j.gaitpost.2016.08.028
42. Lehert P, Villaseca P, Hogervorst E, et al. Individually modifiable risk factors to ameliorate cognitive aging: a systematic review and meta-analysis. Climacteric. 2015;18:678-689. doi: 10.3109/13697137.2015.1078106
43. Turbert D. Eye exam and vision testing basics. American Academy of Ophthalmology Web site. January 14, 2021. Accessed March 5, 2021. www.aao.org/eye-health/tips-prevention/eye-exams-101
44. Northey JM, Cherbuin N, Pumpa KL, et al. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52:154-160. doi: 10.1136/bjsports-2016-096587
45. CDC. Percent of U.S. adults 55 and over with chronic conditions. November 6, 2015. Accessed April 29, 2021. www.cdc.gov/nchs/health_policy/adult_chronic_conditions.htm
46. National Council on Aging. COVID-driven isolation can be dangerous for older adults. March 31, 2021. Accessed April 29, 2021. www.ncoa.org/article/covid-driven-isolation-can-be-dangerous-for-older-adults
47. Smith ML, Steinman LE, Casey EA. Combatting social isolation among older adults in a time of physical distancing: the COVID-19 social connectivity paradox. Front Public Health. 2020;8:403. doi: 10.3389/fpubh.2020.00403
48. Steptoe A, Shankar A, Demakakos P, et al. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110:5797-5801. doi: 10.1073/pnas.1219686110
49. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10:227-237. doi: 10.1177/1745691614568352
50. Hakulinen C, Pulkki-Råback L, Virtanen M, et al. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart. 2018;104:1536-1542. doi: 10.1136/heartjnl-2017-312663
51. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. The National Academies Press; 2020. doi: 10.17226/25663
52. Shaw JG, Farid M, Noel-Miller C, et al. Social isolation and Medicare spending: among older adults, objective isolation increases expenditures while loneliness does not. J Aging Health. 2017;29:1119-1143. doi: 10.1177/0898264317703559
53. Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186-204. doi: 10.1093/oxfordjournals.aje.a112674
54. Campaign to End Loneliness. Measuring your impact on loneliness in later life. Accessed April 29, 2021. www.campaigntoendloneliness.org/wp-content/uploads/Loneliness-Measurement-Guidance1-1.pdf
55. Cornwell EY, Waite LJ. Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav. 2009;50:31-48. doi: 10.1177/002214650905000103
56. Gierveld JDJ, Van Tilburg T. A 6-item scale for overall, emotional, and social loneliness: confirmatory tests on survey data. Res Aging. 2006;28:582-598. doi: 10.1177/0164027506289723
57. Koenig HG, Westlund RE, George LK, et al. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics. 1993;34:61-69. doi: 10.1016/S0033-3182(93)71928-3
58. Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 2006;46:503-513. doi: 10.1093/geront/46.4.503
59. Russell DW. UCLA Loneliness Scale (version 3): reliability, validity, factor structure. J Pers Assess. 1996;66:20-40. doi: 10.1207/s15327752jpa6601_2
60. van Leeuwen KM, van Loon MS, van Nes FA, et al. What does quality of life mean to older adults? A thematic synthesis. PLoS One. 2019;14:e0213263. doi: 10.1371/journal.pone.0213263
PRACTICE RECOMMENDATIONS
› Prioritize annual wellness visits to improve patient follow-through on recommended services. B
› Encourage physical activity, especially musclestrengthening exercises, to prevent frailty and to mediate decline in the ability to perform activities of daily living. A
› Assess and treat older adults for visual and hearing impairments A , as well as anxiety, depression, and mobility impairments. C They are all associated with cognitive function.
› Ask patients about the frequency of their social interactions A and quality of their relationships B to determine their access to resources, such as food and transportation, as well as their perceptions about their quality of life.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Recognizing and treating trigger finger
CASE
A 55-year-old right-hand-dominant woman presented to the clinic with a chief complaint of right ring finger pain and stiffness. There was no history of trauma or prior surgery. She had no tingling or numbness. She had a history of type 2 diabetes that was well controlled. She worked as a clerk for a government office for many years, and her painful, limited finger motion interfered with keyboarding and picking up items. Physical examination revealed tenderness to palpation over the palmar aspect of the metacarpophalangeal joint (MCPJ) of the ring finger with no other joint tenderness or swelling. When she made a fist, her ring finger MCPJ, proximal interphalangeal joint (PIPJ), and distal interphalangeal joint (DIPJ) locked in a flexed position that required manipulation to extend the finger. A firm mass was palpated in the palm with finger flexion that moved into the finger with extension.
Stenosing tenosynovitis, also known as trigger finger (TF), is an inflammatory condition that causes pain in the distal palm and proximal digit with associated limited motion. The most commonly affected digits are the middle and ring fingers of the dominant hand.1 The disorder is particularly noticeable when it inhibits day-to-day functioning.
TF affects 2% to 3% of the general population and up to 20% of patients with diabetes.2,3 Patient age and duration of diabetes are commonly cited as contributing factors, although the effect of well-controlled blood glucose and A1C on the frequency and cure rate of TF has not been established.3,4 TF is most commonly seen in individuals ages 40 to 60 years, with a 6 times’ greater frequency in females than males.5
In the United States, there are an estimated 200,000 cases of TF each year, with initial presentation typically being to a primary care physician.6 For this reason, it is essential for primary care physicians to recognize this common pathology and treat symptoms early to prevent progression and the need for surgical intervention.
An impaired gliding motion of the flexor tendons
In each finger, a tendon sheath, consisting of 5 annular pulleys and 3 cruciate pulleys, forms a tunnel around the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). The tendon sheath allows for maximum force by eliminating bowstringing of the tendons when the digit is flexed. Deep to the tendons and surrounding the tendons is a synovial membrane that provides nutrition and reduces friction between the tendons and the tendon sheath.7
The FDP is longer and assists in flexion of the MCPJ and the PIPJ. It is the sole flexor of the DIPJ.
In the thumb, the flexor pollicis longus (FPL) is the only flexor within its tendon sheath. The FPL assists in flexion of the MCPJ and flexes the thumb interphalangeal joint (IPJ). The intrinsic muscles (lumbricals and interossei) do not extend into the tendon sheath and do not contribute to TF.
Continue to: TF occurs when
TF occurs when the tendon sheath, most commonly at the first annular pulley (A1), or the flexor tendons thicken due to fibrocartilaginous metaplasia. This results in impaired gliding motion of the flexor tendons.8 The stenosed A1 pulley can lead to pinching of the flexor tendons and cause the formation of a nodule on the FDS tendon at its bifurcation.9 The nodule of the FDS bifurcation moves proximal to the A1 pulley when the finger is flexed. Upon extension, the tendon nodule may get caught on the A1 pulley. This prevents smooth extension and is the source of pain and triggering (FIGURE 1). In a similar manner, thumb triggering is the result of a stenosed A1 pulley creating a nodule on the FPL tendon, which prevents smooth gliding of the FPL.
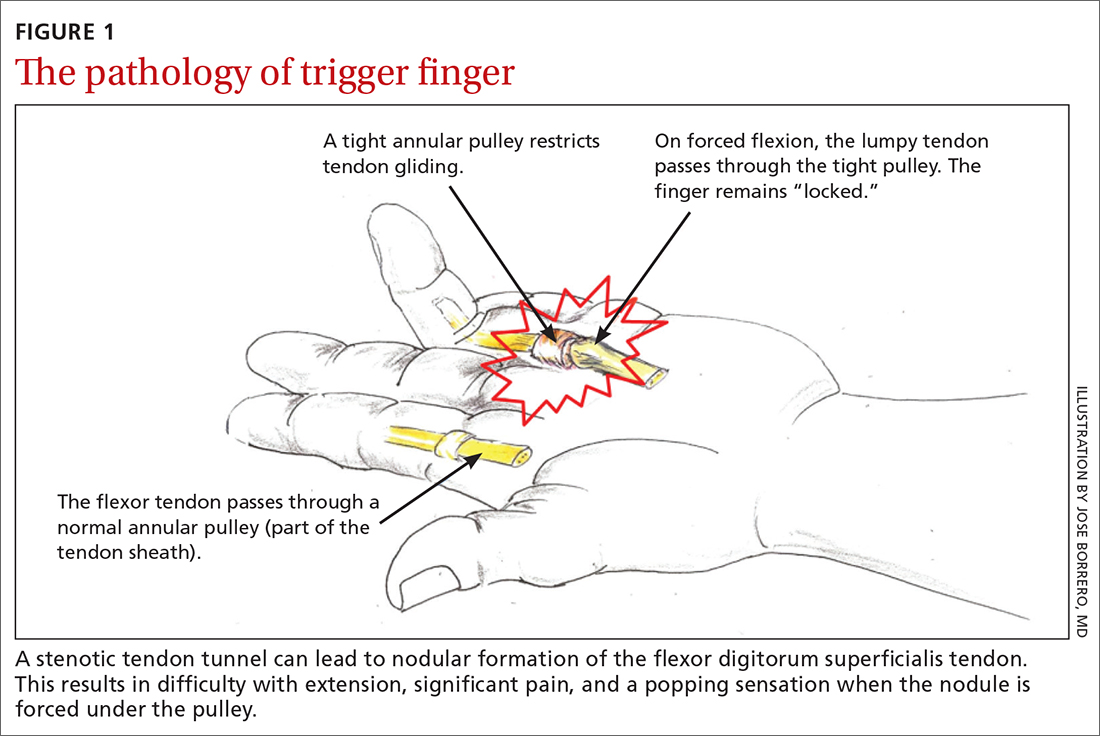
What you’ll see
TF is characterized by locking, popping, or clicking at the base of the finger or thumb.7,10 A small nodule may be palpated on the palmar aspect of the MCPJ when the finger is flexed. This nodule will then move distally when the finger is extended. Patients will present with the affected digit in a flexed position and will have difficulty extending the digit. In some cases, the patient may have to use the other hand to straighten the affected digit. In more severe cases, the digit may be fixed in a position of flexion or extension. The severity of triggering is commonly graded by the Green’s classification system (see TABLE11).
Is it Dupuytren contracture, trigger finger, or something else?
The differential diagnosis for TF includes Dupuytren contracture, MCPJ sprain, calcific peritendinitis, flexor tenosynovitis, diabetic cheiroarthropathy (DCA), rheumatoid arthritis (RA), osteoarthritis (OA), and crystalline arthropathy (gout).5
Dupuytren contracture is usually nonpainful and manifests with a palpable cord in the palm and a fixed flexion contracture that has progressed over time, with no history of catching.
MCPJ sprain is diagnosed with tenderness of the MCPJ and a history of trauma.
Continue to: Calcific peritendinitis
Calcific peritendinitis is characterized by pain, tenderness, and edema near a joint with calcified deposits seen on radiographs.
Flexor tenosynovitis manifests with fusiform swelling of the digit, tenderness over the flexor tendon sheath, and pain with passive extension of the digit; it is more commonly associated with RA.
DCA, RA, OA, and gout usually affect more than 1 digit. DCA is associated with both type 1 and type 2 diabetes and is characterized by thickened, waxy skin and painless, limited extension of the digits. RA and OA are diagnosed by medical history, lab work, and radiographs. Gout is diagnosed with lab work and aspiration of joint fluid.
A thorough history, physical exam, and review of radiographs must be performed to rule out these other disorders. Once the diagnosis of TF is made, available treatment options should be pursued.
Treatment: A conservative or surgical approach?
Current treatment options include both nonsurgical (conservative) and surgical interventions. Nonsurgical interventions include activity modification, splinting, and corticosteroid injections. While nonsteroidal anti-inflammatory drugs are commonly recommended to resolve the local inflammation secondary to triggering, there is no scientific evidence to support their use at this time.7 Surgical interventions, utilized in more severe cases or after conservative treatment has failed, include percutaneous and open release of the tendon sheath.2,7
Continue to: Conservative treatments
Conservative treatments
Splinting is only an option for digits that retain flexibility (Green’s classification grades I, II, and III). The goal of splinting is to keep the affected digit in extension to avoid repeated friction between the tendon and the tendon sheath.12 This ideally allows any cartilaginous metaplasia or inflammation to resolve, subsequently alleviating symptoms. The recommended length of treatment with splinting ranges from 3 to 12 weeks, with an average of 6 weeks.1
Multiple studies have shown long-term alleviation of symptoms with the use of orthotic devices. A retrospective analysis found that 87% of patients who wore their PIPJ orthotic device both day and night for a minimum of 6 weeks required no further treatment at 1-year follow-up.13 In contrast, MCPJ splinting only at night has been shown to resolve symptoms in just 55% of patients after 6 weeks.14 From a practical standpoint, however, patients are more likely to be compliant with night-only splinting, making it a reasonable option. Splinting does remain efficacious for patients even after 6 months of symptomatology.15
Day and night splinting for approximately 8 weeks using a PIPJ orthotic could be considered as an effective first-line intervention.16 Notably, PIPJ splinting is more functional, as it allows motion of the MCPJ and DIPJ.
An adjunct treatment to splinting is tendon-gliding exercises, including passive IPJ flexion, full finger flexion and extension, and hooking.13 Patients may remove the orthotic device to perform these exercises 3 times a day for 5 repetitions, as well as for activities that are not conducive to splinting.13
Corticosteroid injections. Injections of a corticosteroid and 1% lidocaine in a 1:1 mixture for a total volume of 1 cc can be inserted into the tendon sheath, A1 pulley, or adjacent tissue.17 Steroid injections help to decrease inflammation and pain in the affected area, giving symptom relief lasting a few months in as many as 57% to 87% of patients.18
Continue to: While the location of the injection...
While the location of the injection has been debated, recent literature suggests that symptoms can be effectively alleviated regardless of the specific anatomic injection site, such as intra-sheath or extra-sheath (FIGURE 2).19 This allows flexibility for the clinician, as the injection does not have to be placed within the tendon sheath. Corticosteroids should not be injected into the tendon itself, and the needle tip should be slightly withdrawn if there is resistance while injecting. Patients who are averse to injections have been shown to benefit from needle-free jet lidocaine (J-tip) administration prior to the actual steroid injection.20
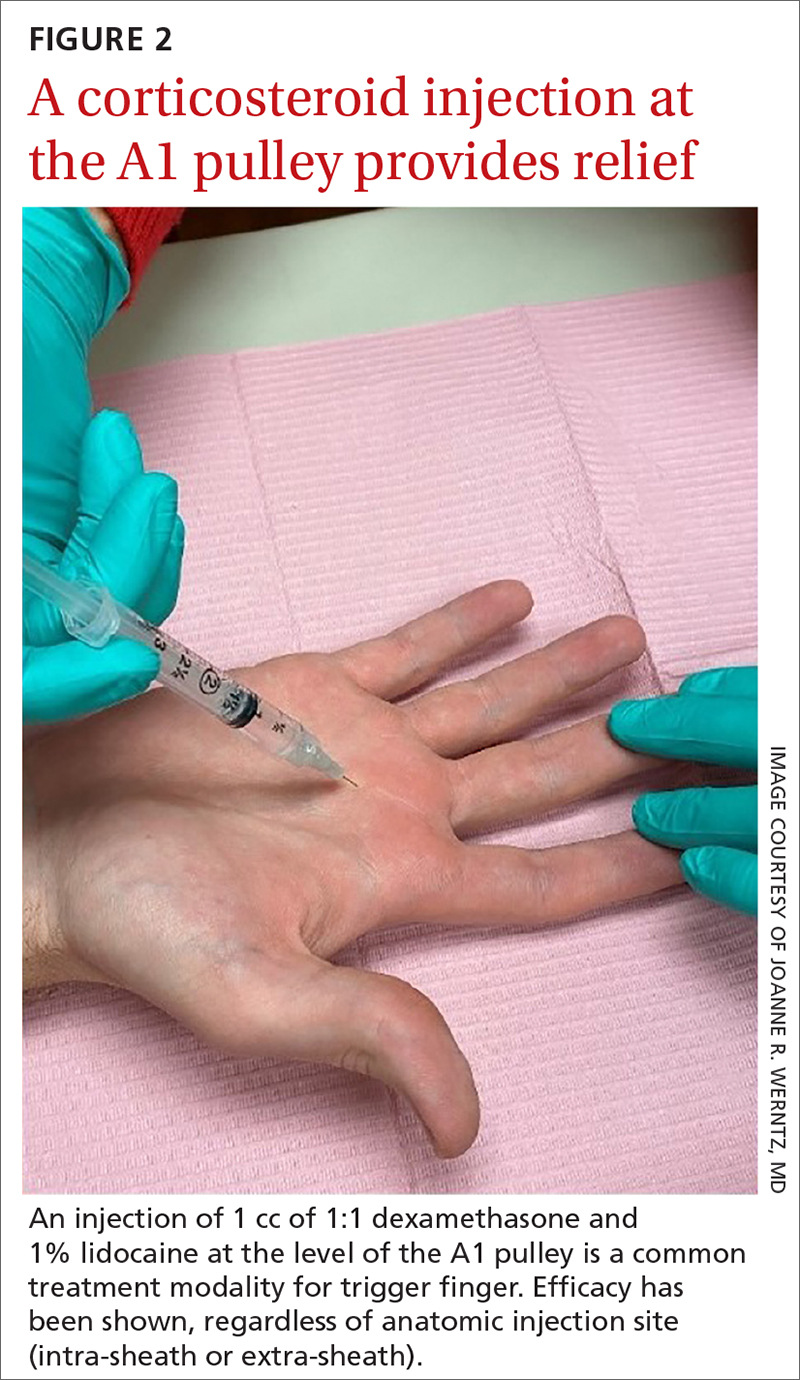
A randomized controlled trial comparing dexamethasone to triamcinolone injections found no difference in outcome at the 3-month follow-up (n = 84).17 This may suggest that the choice of corticosteroid is at the clinician’s discretion. In terms of long-term efficacy of steroid injections, it has been shown that 70% of trigger digits had complete resolution of symptoms at a mean follow-up of 8 years after just 1 injection (n = 43).21
Some patients, though, may require additional corticosteroid injections to maintain symptom control. If multiple injections are performed, they should not be given in intervals shorter than 4 months between treatments.5 Furthermore, steroids can be administered safely up to 3 times in the same digit before surgery is recommended.22
A patient’s options should be reconsidered if efficacy is not demonstrated with prior injections. Notably, a lower success rate has been shown in patients with type 2 diabetes (66%) compared to those without diabetes (90%).4,23 This difference in success rates is not well understood, as there is no causal relationship between well-controlled diabetes and TF.4 Complications of corticosteroid injections include local pain, fat atrophy, and hypopigmentation at the site of the injection, as well as short-term elevations in blood glucose levels in patients with diabetes.5,24
Surgical correction (to be discussed) remains superior to steroid injections in terms of cure rate and resolution of symptoms. A randomized controlled trial (n = 165) found that an injection-only group reported 86% and 49% success at 3-month and 12-month follow-up, respectively, compared to 99% success at both 3- and 12-month follow-up for the surgical group. Further, at 12-month follow-up, the median pain scores were significantly higher in the injection group (3; range, 1-9) than in the surgery group (1; range, 1-7).25 If conservative treatment modalities lead to unresolved symptoms or recurrence, referral to a hand specialist for surgery is recommended.
Continue to: Surgical treatments in an office setting
Surgical treatments in an office setting
Procedures for TF can be safely performed under conscious sedation or local anesthesia, with or without a tourniquet.26 Wide-awake procedures with local anesthesia and no tourniquet (WALANT) can be performed in an office-based procedure room rather than the operating room. This increases efficiency for the surgeon, reduces the amount of preparation and recovery time for the patient, and helps to keep costs down.
Percutaneous release involves the insertion of a 16-gauge hypodermic needle into the affected A1 pulley. The needle is used to fray and disrupt the pulley by moving the needle tip over the fibrotic A1 pulley.
However, it is not without possible complications.27 Inadvertent A2 pulley damage is particularly troublesome, as it leads to “bowstringing” or protrusion of the flexor tendon into the palm upon flexion. This can cause pain and failure to fully extend or flex the finger.10 Because the anatomy is not well visualized during the percutaneous approach, incomplete release, neurovascular injury, and iatrogenic injury to the A2 pulley or deep tendon may occur.28 Ultrasound-guided percutaneous release techniques have shown effective clinical outcomes with minimal complications compared to nonguided percutaneous release techniques.29,30
Open release is the gold standard surgical treatment for trigger finger (FIGURE 3). A small incision (1-2 cm) is made directly over or proximal to the A1 pulley in the distal palmar crease at the base of the affected digit. After blunt dissection through the subcutaneous tissue, the A1 pulley is sharply incised. An open approach has the clear benefit of avoiding the digital neurovascular bundles, as well as visualizing the resolution of triggering upon flexion and extension prior to closure. The WALANT procedure has the advantage of allowing the awake patient to actively flex and extend the digit to determine if the A1 release has been successful prior to closure of the incision.
Outcomes and complications of surgery. A recent systematic review and meta-analysis has shown percutaneous techniques to be successful in 94% of cases.27 The success rate of open surgery has been reported at 99% to 100% at varying follow-up intervals up to 1 year.25,30,31 The complication rate for percutaneous release (guided and nonguided) was calculated at 2.2% (n = 2114).27 In another study, the overall complication rate of open releases was calculated at 1% (n = 999).32 When comparing percutaneous release (guided and nonguided) and open release, a meta-analysis found no significant difference in complication rate (RR = 0.84) or failure rate (RR = 0.94).32
Continue to: Several risk factors...
Several risk factors have been associated with postoperative surgical infection, including recent steroid injection (< 80 d), smoking status, increasing age, and pre-operative use of lidocaine with epinephrine.33 Open release has been shown to be an effective and safe treatment modality for patients with and without diabetes alike.34 Overall, definitive surgical correction has been demonstrated to be superior to conservative measures due to a significantly lower rate of recurrence.35
CASE
Given the patient’s presentation with triggering of the digit, tenderness over the A1 pulley, and lack of trauma history, we diagnosed trigger finger in this patient. Potential treatments included splinting, corticosteroid injections, and surgery. After discussion of the risks and benefits of each treatment option, the patient elected to undergo a corticosteroid injection. She was also given a neoprene finger sleeve to wear every night, and in the daytime when possible.
At 12-week follow-up, she noted early improvement in her triggering, which had since recurred. Due to her history of diabetes, the patient was then referred for surgery. She had an open release under local anesthesia. The surgery was uncomplicated, and the abnormality was corrected. At the patient’s 1-year postoperative follow-up visit, there was no evidence of recurrence, and she had regained full active and passive range of motion of her finger.
Acknowledgements
The authors wish to thank Jose Borrero, MD, for contributing his time and creative talents to produce the illustrations in this article.
CORRESPONDENCE
Evan P. Johnson, MD; 506 South Greer Street, Memphis, TN 38111; [email protected]
1. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
2. Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92-96. doi: 10.1007/s12178-007-9012-1
3. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775. doi: 10.1016/j.jhsa.2008.01.038
4. Junot HSN, Anderson Hertz AFL, Gustavo Vasconcelos GR, et al. Epidemiology of trigger finger: metabolic syndrome as a new perspective of associated disease. Hand (N Y). 2019:1558944719867135. doi: 10.1177/1558944719867135.
5. Matthews A, Smith K, Read L, et al. Trigger finger: an overview of the treatment options. JAAPA. 2019;32:17-21. doi: 10.1097/01.Jaa.0000550281.42592.97
6. Pencle FJ, Waheed A, Molnar JA. Trigger thumb. StatPearls [Internet]. StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK441854/
7. Giugale JM, Fowler JR. Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 2015;46:561-569. doi: 10.1016/j.ocl.2015.06.014
8. Bianchi S, Gitto S, Draghi F. Ultrasound features of trigger finger: review of the literature. J Ultrasound Med. 2019;38:3141-3154. doi: 10.1002/jum.15025
9. Chuang XL, Ooi CC, Chin ST, et al. What triggers in trigger finger? The flexor tendons at the flexor digitorum superficialis bifurcation. J Plast Reconstr Aesthet Surg. 2017;70:1411-1419. doi: 10.1016/j.bjps.2017.05.037
10. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31:135-146. doi: 10.1016/j.jhsa.2005.10.013
11. Chapter 56: Tendinoapthy. In: Wolfe SW, Peterson WC, Kozin SH, Cohen MS. Green’s Operative Hand Surgery. Vol 2. 7th ed. Elsevier; 2017: 1904-1925.
12. Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012;37:243-249, 249.e241. doi: 10.1016/j.jhsa.2011.10.038
13. Valdes K. A retrospective review to determine the long-term efficacy of orthotic devices for trigger finger. J Hand Ther. 2012;25:89-95. doi: 10.1016/j.jht.2011.09.005
14. Drijkoningen T, van Berckel M, Becker SJE, et al. Night splinting for idiopathic trigger digits. Hand (N Y). 2018;13:558-562. doi: 10.1177/1558944717725374
15. Colbourn J, Heath N, Manary S, et al. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008;21:336-343. doi: 10.1197/j.jht.2008.05.001
16. Teo SH, Ng DCL, Wong YKY. Effectiveness of proximal interphalangeal joint-blocking orthosis vs metacarpophalangeal joint-blocking orthosis in trigger digit management: A randomized clinical trial. J Hand Ther. 2018;32:444-451. doi: 10.1016/j.jht.2018.02.007
17. Ring D, Lozano-Calderon S, Shin R, et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33:516-522; discussion 523-514. doi: 10.1016/j.jhsa.2008.01.001
18. Fleisch SB, Spindler KP, Lee DH. Corticosteroid injections in the treatment of trigger finger: A level I and II systematic review. J Am Acad Orthop Surg. 2007;15:166-171. doi: 10.5435/00124635-200703000-00006
19. Shinomiya R, Sunagawa T, Nakashima Y, et al. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016;42:2203-2208. doi: 10.1016/j.ultrasmedbio.2016.05.015
20. Earp BE, Stanbury SJ, Mora AN, et al. Needle-free jet lidocaine administration for preinjection anesthesia in trigger finger injection: a randomized controlled trial. J Hand Surg Am. 2017;42:618-622. doi: 10.1016/j.jhsa.2017.05.001
21. Castellanos J, Munoz-Mahamud E, Dominguez E, et al. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121-126. doi: 10.1016/j.jhsa.2014.09.006
22. Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263-268. doi: 10.4055/cios.2012.4.4.263
23. Griggs SM, Weiss AP, Lane LB, et al. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20:787-789. doi: 10.1016/s0363-5023(05)80432-0
24. Stepan JG, London DA, Boyer MI, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39:706-712. doi: 10.1016/j.jhsa.2014.01.014
25. Hansen RL, Sondergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am. 2017;42:359-366. doi: 10.1016/j.jhsa.2017.02.011
26. Mohd Rashid MZ, Sapuan J, Abdullah S. A randomized controlled trial of trigger finger release under digital anesthesia with (WALANT) and without adrenaline. J Orthop Surg (Hong Kong). 2019;27:2309499019833002. doi: 10.1177/2309499019833002
27. Zhao J-G, Kan S-L, Zhao L, et al. Percutaneous first annular pulley release for trigger digits: a systematic review and meta-analysis of current evidence. J Hand Surg Am. 2014;39:2192-2202. doi: 10.1016/j.jhsa.2014.07.044
28. Guler F, Kose O, Ercan EC, et al. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36:e1290-1294. doi: 10.3928/01477447-20130920-22
29. Wu KC, Chern TC, Jou IM. Ultrasound-assisted percutaneous trigger finger release: it is safe [letter]. Hand (N Y). 2009;4:339. doi: 10.1007/s11552-009-9179-6
30. Nikolaou VS, Malahias M-A, Kaseta M-K, et al. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop. 2017;8:163-169. doi: 10.5312/wjo.v8.i2.163
31. Lim M-H, Lim K-K, Rasheed MZ, et al. Outcome of open trigger digit release. J Hand Surg Eur Vol. 2007;32:457-459. doi: 10.1016/j.Jhsb.2007.02.016
32. Wang J, Zhao J-G, Liang C-C. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res. 2013;471:1879-1886. doi: 10.1007/s11999-012-2716-6
33. Ng WKY, Olmscheid N, Worhacz K, et al. Steroid injection and open trigger finger release outcomes: a retrospective review of 999 digits. Hand (N Y). 2018;15:399-406. doi: 10.1177/1558944718796559
34. Ho SWL, Chia CY, Rajaratnam V. Characteristics and clinical outcomes of open surgery for trigger digits in diabetes. J Hand Microsurg. 2019;11:80-83. doi: 10.1055/s-0038-1670927
35. Sato ES, dos Santos JB, Belloti JC, et al. Percutaneous release of trigger fingers. Hand Clin. 2014;30:39-45. doi: 10.1016/j.hcl.2013.08.017
CASE
A 55-year-old right-hand-dominant woman presented to the clinic with a chief complaint of right ring finger pain and stiffness. There was no history of trauma or prior surgery. She had no tingling or numbness. She had a history of type 2 diabetes that was well controlled. She worked as a clerk for a government office for many years, and her painful, limited finger motion interfered with keyboarding and picking up items. Physical examination revealed tenderness to palpation over the palmar aspect of the metacarpophalangeal joint (MCPJ) of the ring finger with no other joint tenderness or swelling. When she made a fist, her ring finger MCPJ, proximal interphalangeal joint (PIPJ), and distal interphalangeal joint (DIPJ) locked in a flexed position that required manipulation to extend the finger. A firm mass was palpated in the palm with finger flexion that moved into the finger with extension.
Stenosing tenosynovitis, also known as trigger finger (TF), is an inflammatory condition that causes pain in the distal palm and proximal digit with associated limited motion. The most commonly affected digits are the middle and ring fingers of the dominant hand.1 The disorder is particularly noticeable when it inhibits day-to-day functioning.
TF affects 2% to 3% of the general population and up to 20% of patients with diabetes.2,3 Patient age and duration of diabetes are commonly cited as contributing factors, although the effect of well-controlled blood glucose and A1C on the frequency and cure rate of TF has not been established.3,4 TF is most commonly seen in individuals ages 40 to 60 years, with a 6 times’ greater frequency in females than males.5
In the United States, there are an estimated 200,000 cases of TF each year, with initial presentation typically being to a primary care physician.6 For this reason, it is essential for primary care physicians to recognize this common pathology and treat symptoms early to prevent progression and the need for surgical intervention.
An impaired gliding motion of the flexor tendons
In each finger, a tendon sheath, consisting of 5 annular pulleys and 3 cruciate pulleys, forms a tunnel around the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). The tendon sheath allows for maximum force by eliminating bowstringing of the tendons when the digit is flexed. Deep to the tendons and surrounding the tendons is a synovial membrane that provides nutrition and reduces friction between the tendons and the tendon sheath.7
The FDP is longer and assists in flexion of the MCPJ and the PIPJ. It is the sole flexor of the DIPJ.
In the thumb, the flexor pollicis longus (FPL) is the only flexor within its tendon sheath. The FPL assists in flexion of the MCPJ and flexes the thumb interphalangeal joint (IPJ). The intrinsic muscles (lumbricals and interossei) do not extend into the tendon sheath and do not contribute to TF.
Continue to: TF occurs when
TF occurs when the tendon sheath, most commonly at the first annular pulley (A1), or the flexor tendons thicken due to fibrocartilaginous metaplasia. This results in impaired gliding motion of the flexor tendons.8 The stenosed A1 pulley can lead to pinching of the flexor tendons and cause the formation of a nodule on the FDS tendon at its bifurcation.9 The nodule of the FDS bifurcation moves proximal to the A1 pulley when the finger is flexed. Upon extension, the tendon nodule may get caught on the A1 pulley. This prevents smooth extension and is the source of pain and triggering (FIGURE 1). In a similar manner, thumb triggering is the result of a stenosed A1 pulley creating a nodule on the FPL tendon, which prevents smooth gliding of the FPL.

What you’ll see
TF is characterized by locking, popping, or clicking at the base of the finger or thumb.7,10 A small nodule may be palpated on the palmar aspect of the MCPJ when the finger is flexed. This nodule will then move distally when the finger is extended. Patients will present with the affected digit in a flexed position and will have difficulty extending the digit. In some cases, the patient may have to use the other hand to straighten the affected digit. In more severe cases, the digit may be fixed in a position of flexion or extension. The severity of triggering is commonly graded by the Green’s classification system (see TABLE11).

Is it Dupuytren contracture, trigger finger, or something else?
The differential diagnosis for TF includes Dupuytren contracture, MCPJ sprain, calcific peritendinitis, flexor tenosynovitis, diabetic cheiroarthropathy (DCA), rheumatoid arthritis (RA), osteoarthritis (OA), and crystalline arthropathy (gout).5
Dupuytren contracture is usually nonpainful and manifests with a palpable cord in the palm and a fixed flexion contracture that has progressed over time, with no history of catching.
MCPJ sprain is diagnosed with tenderness of the MCPJ and a history of trauma.
Continue to: Calcific peritendinitis
Calcific peritendinitis is characterized by pain, tenderness, and edema near a joint with calcified deposits seen on radiographs.
Flexor tenosynovitis manifests with fusiform swelling of the digit, tenderness over the flexor tendon sheath, and pain with passive extension of the digit; it is more commonly associated with RA.
DCA, RA, OA, and gout usually affect more than 1 digit. DCA is associated with both type 1 and type 2 diabetes and is characterized by thickened, waxy skin and painless, limited extension of the digits. RA and OA are diagnosed by medical history, lab work, and radiographs. Gout is diagnosed with lab work and aspiration of joint fluid.
A thorough history, physical exam, and review of radiographs must be performed to rule out these other disorders. Once the diagnosis of TF is made, available treatment options should be pursued.
Treatment: A conservative or surgical approach?
Current treatment options include both nonsurgical (conservative) and surgical interventions. Nonsurgical interventions include activity modification, splinting, and corticosteroid injections. While nonsteroidal anti-inflammatory drugs are commonly recommended to resolve the local inflammation secondary to triggering, there is no scientific evidence to support their use at this time.7 Surgical interventions, utilized in more severe cases or after conservative treatment has failed, include percutaneous and open release of the tendon sheath.2,7
Continue to: Conservative treatments
Conservative treatments
Splinting is only an option for digits that retain flexibility (Green’s classification grades I, II, and III). The goal of splinting is to keep the affected digit in extension to avoid repeated friction between the tendon and the tendon sheath.12 This ideally allows any cartilaginous metaplasia or inflammation to resolve, subsequently alleviating symptoms. The recommended length of treatment with splinting ranges from 3 to 12 weeks, with an average of 6 weeks.1
Multiple studies have shown long-term alleviation of symptoms with the use of orthotic devices. A retrospective analysis found that 87% of patients who wore their PIPJ orthotic device both day and night for a minimum of 6 weeks required no further treatment at 1-year follow-up.13 In contrast, MCPJ splinting only at night has been shown to resolve symptoms in just 55% of patients after 6 weeks.14 From a practical standpoint, however, patients are more likely to be compliant with night-only splinting, making it a reasonable option. Splinting does remain efficacious for patients even after 6 months of symptomatology.15
Day and night splinting for approximately 8 weeks using a PIPJ orthotic could be considered as an effective first-line intervention.16 Notably, PIPJ splinting is more functional, as it allows motion of the MCPJ and DIPJ.
An adjunct treatment to splinting is tendon-gliding exercises, including passive IPJ flexion, full finger flexion and extension, and hooking.13 Patients may remove the orthotic device to perform these exercises 3 times a day for 5 repetitions, as well as for activities that are not conducive to splinting.13
Corticosteroid injections. Injections of a corticosteroid and 1% lidocaine in a 1:1 mixture for a total volume of 1 cc can be inserted into the tendon sheath, A1 pulley, or adjacent tissue.17 Steroid injections help to decrease inflammation and pain in the affected area, giving symptom relief lasting a few months in as many as 57% to 87% of patients.18
Continue to: While the location of the injection...
While the location of the injection has been debated, recent literature suggests that symptoms can be effectively alleviated regardless of the specific anatomic injection site, such as intra-sheath or extra-sheath (FIGURE 2).19 This allows flexibility for the clinician, as the injection does not have to be placed within the tendon sheath. Corticosteroids should not be injected into the tendon itself, and the needle tip should be slightly withdrawn if there is resistance while injecting. Patients who are averse to injections have been shown to benefit from needle-free jet lidocaine (J-tip) administration prior to the actual steroid injection.20

A randomized controlled trial comparing dexamethasone to triamcinolone injections found no difference in outcome at the 3-month follow-up (n = 84).17 This may suggest that the choice of corticosteroid is at the clinician’s discretion. In terms of long-term efficacy of steroid injections, it has been shown that 70% of trigger digits had complete resolution of symptoms at a mean follow-up of 8 years after just 1 injection (n = 43).21
Some patients, though, may require additional corticosteroid injections to maintain symptom control. If multiple injections are performed, they should not be given in intervals shorter than 4 months between treatments.5 Furthermore, steroids can be administered safely up to 3 times in the same digit before surgery is recommended.22
A patient’s options should be reconsidered if efficacy is not demonstrated with prior injections. Notably, a lower success rate has been shown in patients with type 2 diabetes (66%) compared to those without diabetes (90%).4,23 This difference in success rates is not well understood, as there is no causal relationship between well-controlled diabetes and TF.4 Complications of corticosteroid injections include local pain, fat atrophy, and hypopigmentation at the site of the injection, as well as short-term elevations in blood glucose levels in patients with diabetes.5,24
Surgical correction (to be discussed) remains superior to steroid injections in terms of cure rate and resolution of symptoms. A randomized controlled trial (n = 165) found that an injection-only group reported 86% and 49% success at 3-month and 12-month follow-up, respectively, compared to 99% success at both 3- and 12-month follow-up for the surgical group. Further, at 12-month follow-up, the median pain scores were significantly higher in the injection group (3; range, 1-9) than in the surgery group (1; range, 1-7).25 If conservative treatment modalities lead to unresolved symptoms or recurrence, referral to a hand specialist for surgery is recommended.
Continue to: Surgical treatments in an office setting
Surgical treatments in an office setting
Procedures for TF can be safely performed under conscious sedation or local anesthesia, with or without a tourniquet.26 Wide-awake procedures with local anesthesia and no tourniquet (WALANT) can be performed in an office-based procedure room rather than the operating room. This increases efficiency for the surgeon, reduces the amount of preparation and recovery time for the patient, and helps to keep costs down.
Percutaneous release involves the insertion of a 16-gauge hypodermic needle into the affected A1 pulley. The needle is used to fray and disrupt the pulley by moving the needle tip over the fibrotic A1 pulley.
However, it is not without possible complications.27 Inadvertent A2 pulley damage is particularly troublesome, as it leads to “bowstringing” or protrusion of the flexor tendon into the palm upon flexion. This can cause pain and failure to fully extend or flex the finger.10 Because the anatomy is not well visualized during the percutaneous approach, incomplete release, neurovascular injury, and iatrogenic injury to the A2 pulley or deep tendon may occur.28 Ultrasound-guided percutaneous release techniques have shown effective clinical outcomes with minimal complications compared to nonguided percutaneous release techniques.29,30
Open release is the gold standard surgical treatment for trigger finger (FIGURE 3). A small incision (1-2 cm) is made directly over or proximal to the A1 pulley in the distal palmar crease at the base of the affected digit. After blunt dissection through the subcutaneous tissue, the A1 pulley is sharply incised. An open approach has the clear benefit of avoiding the digital neurovascular bundles, as well as visualizing the resolution of triggering upon flexion and extension prior to closure. The WALANT procedure has the advantage of allowing the awake patient to actively flex and extend the digit to determine if the A1 release has been successful prior to closure of the incision.

Outcomes and complications of surgery. A recent systematic review and meta-analysis has shown percutaneous techniques to be successful in 94% of cases.27 The success rate of open surgery has been reported at 99% to 100% at varying follow-up intervals up to 1 year.25,30,31 The complication rate for percutaneous release (guided and nonguided) was calculated at 2.2% (n = 2114).27 In another study, the overall complication rate of open releases was calculated at 1% (n = 999).32 When comparing percutaneous release (guided and nonguided) and open release, a meta-analysis found no significant difference in complication rate (RR = 0.84) or failure rate (RR = 0.94).32
Continue to: Several risk factors...
Several risk factors have been associated with postoperative surgical infection, including recent steroid injection (< 80 d), smoking status, increasing age, and pre-operative use of lidocaine with epinephrine.33 Open release has been shown to be an effective and safe treatment modality for patients with and without diabetes alike.34 Overall, definitive surgical correction has been demonstrated to be superior to conservative measures due to a significantly lower rate of recurrence.35
CASE
Given the patient’s presentation with triggering of the digit, tenderness over the A1 pulley, and lack of trauma history, we diagnosed trigger finger in this patient. Potential treatments included splinting, corticosteroid injections, and surgery. After discussion of the risks and benefits of each treatment option, the patient elected to undergo a corticosteroid injection. She was also given a neoprene finger sleeve to wear every night, and in the daytime when possible.
At 12-week follow-up, she noted early improvement in her triggering, which had since recurred. Due to her history of diabetes, the patient was then referred for surgery. She had an open release under local anesthesia. The surgery was uncomplicated, and the abnormality was corrected. At the patient’s 1-year postoperative follow-up visit, there was no evidence of recurrence, and she had regained full active and passive range of motion of her finger.
Acknowledgements
The authors wish to thank Jose Borrero, MD, for contributing his time and creative talents to produce the illustrations in this article.
CORRESPONDENCE
Evan P. Johnson, MD; 506 South Greer Street, Memphis, TN 38111; [email protected]
CASE
A 55-year-old right-hand-dominant woman presented to the clinic with a chief complaint of right ring finger pain and stiffness. There was no history of trauma or prior surgery. She had no tingling or numbness. She had a history of type 2 diabetes that was well controlled. She worked as a clerk for a government office for many years, and her painful, limited finger motion interfered with keyboarding and picking up items. Physical examination revealed tenderness to palpation over the palmar aspect of the metacarpophalangeal joint (MCPJ) of the ring finger with no other joint tenderness or swelling. When she made a fist, her ring finger MCPJ, proximal interphalangeal joint (PIPJ), and distal interphalangeal joint (DIPJ) locked in a flexed position that required manipulation to extend the finger. A firm mass was palpated in the palm with finger flexion that moved into the finger with extension.
Stenosing tenosynovitis, also known as trigger finger (TF), is an inflammatory condition that causes pain in the distal palm and proximal digit with associated limited motion. The most commonly affected digits are the middle and ring fingers of the dominant hand.1 The disorder is particularly noticeable when it inhibits day-to-day functioning.
TF affects 2% to 3% of the general population and up to 20% of patients with diabetes.2,3 Patient age and duration of diabetes are commonly cited as contributing factors, although the effect of well-controlled blood glucose and A1C on the frequency and cure rate of TF has not been established.3,4 TF is most commonly seen in individuals ages 40 to 60 years, with a 6 times’ greater frequency in females than males.5
In the United States, there are an estimated 200,000 cases of TF each year, with initial presentation typically being to a primary care physician.6 For this reason, it is essential for primary care physicians to recognize this common pathology and treat symptoms early to prevent progression and the need for surgical intervention.
An impaired gliding motion of the flexor tendons
In each finger, a tendon sheath, consisting of 5 annular pulleys and 3 cruciate pulleys, forms a tunnel around the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). The tendon sheath allows for maximum force by eliminating bowstringing of the tendons when the digit is flexed. Deep to the tendons and surrounding the tendons is a synovial membrane that provides nutrition and reduces friction between the tendons and the tendon sheath.7
The FDP is longer and assists in flexion of the MCPJ and the PIPJ. It is the sole flexor of the DIPJ.
In the thumb, the flexor pollicis longus (FPL) is the only flexor within its tendon sheath. The FPL assists in flexion of the MCPJ and flexes the thumb interphalangeal joint (IPJ). The intrinsic muscles (lumbricals and interossei) do not extend into the tendon sheath and do not contribute to TF.
Continue to: TF occurs when
TF occurs when the tendon sheath, most commonly at the first annular pulley (A1), or the flexor tendons thicken due to fibrocartilaginous metaplasia. This results in impaired gliding motion of the flexor tendons.8 The stenosed A1 pulley can lead to pinching of the flexor tendons and cause the formation of a nodule on the FDS tendon at its bifurcation.9 The nodule of the FDS bifurcation moves proximal to the A1 pulley when the finger is flexed. Upon extension, the tendon nodule may get caught on the A1 pulley. This prevents smooth extension and is the source of pain and triggering (FIGURE 1). In a similar manner, thumb triggering is the result of a stenosed A1 pulley creating a nodule on the FPL tendon, which prevents smooth gliding of the FPL.

What you’ll see
TF is characterized by locking, popping, or clicking at the base of the finger or thumb.7,10 A small nodule may be palpated on the palmar aspect of the MCPJ when the finger is flexed. This nodule will then move distally when the finger is extended. Patients will present with the affected digit in a flexed position and will have difficulty extending the digit. In some cases, the patient may have to use the other hand to straighten the affected digit. In more severe cases, the digit may be fixed in a position of flexion or extension. The severity of triggering is commonly graded by the Green’s classification system (see TABLE11).

Is it Dupuytren contracture, trigger finger, or something else?
The differential diagnosis for TF includes Dupuytren contracture, MCPJ sprain, calcific peritendinitis, flexor tenosynovitis, diabetic cheiroarthropathy (DCA), rheumatoid arthritis (RA), osteoarthritis (OA), and crystalline arthropathy (gout).5
Dupuytren contracture is usually nonpainful and manifests with a palpable cord in the palm and a fixed flexion contracture that has progressed over time, with no history of catching.
MCPJ sprain is diagnosed with tenderness of the MCPJ and a history of trauma.
Continue to: Calcific peritendinitis
Calcific peritendinitis is characterized by pain, tenderness, and edema near a joint with calcified deposits seen on radiographs.
Flexor tenosynovitis manifests with fusiform swelling of the digit, tenderness over the flexor tendon sheath, and pain with passive extension of the digit; it is more commonly associated with RA.
DCA, RA, OA, and gout usually affect more than 1 digit. DCA is associated with both type 1 and type 2 diabetes and is characterized by thickened, waxy skin and painless, limited extension of the digits. RA and OA are diagnosed by medical history, lab work, and radiographs. Gout is diagnosed with lab work and aspiration of joint fluid.
A thorough history, physical exam, and review of radiographs must be performed to rule out these other disorders. Once the diagnosis of TF is made, available treatment options should be pursued.
Treatment: A conservative or surgical approach?
Current treatment options include both nonsurgical (conservative) and surgical interventions. Nonsurgical interventions include activity modification, splinting, and corticosteroid injections. While nonsteroidal anti-inflammatory drugs are commonly recommended to resolve the local inflammation secondary to triggering, there is no scientific evidence to support their use at this time.7 Surgical interventions, utilized in more severe cases or after conservative treatment has failed, include percutaneous and open release of the tendon sheath.2,7
Continue to: Conservative treatments
Conservative treatments
Splinting is only an option for digits that retain flexibility (Green’s classification grades I, II, and III). The goal of splinting is to keep the affected digit in extension to avoid repeated friction between the tendon and the tendon sheath.12 This ideally allows any cartilaginous metaplasia or inflammation to resolve, subsequently alleviating symptoms. The recommended length of treatment with splinting ranges from 3 to 12 weeks, with an average of 6 weeks.1
Multiple studies have shown long-term alleviation of symptoms with the use of orthotic devices. A retrospective analysis found that 87% of patients who wore their PIPJ orthotic device both day and night for a minimum of 6 weeks required no further treatment at 1-year follow-up.13 In contrast, MCPJ splinting only at night has been shown to resolve symptoms in just 55% of patients after 6 weeks.14 From a practical standpoint, however, patients are more likely to be compliant with night-only splinting, making it a reasonable option. Splinting does remain efficacious for patients even after 6 months of symptomatology.15
Day and night splinting for approximately 8 weeks using a PIPJ orthotic could be considered as an effective first-line intervention.16 Notably, PIPJ splinting is more functional, as it allows motion of the MCPJ and DIPJ.
An adjunct treatment to splinting is tendon-gliding exercises, including passive IPJ flexion, full finger flexion and extension, and hooking.13 Patients may remove the orthotic device to perform these exercises 3 times a day for 5 repetitions, as well as for activities that are not conducive to splinting.13
Corticosteroid injections. Injections of a corticosteroid and 1% lidocaine in a 1:1 mixture for a total volume of 1 cc can be inserted into the tendon sheath, A1 pulley, or adjacent tissue.17 Steroid injections help to decrease inflammation and pain in the affected area, giving symptom relief lasting a few months in as many as 57% to 87% of patients.18
Continue to: While the location of the injection...
While the location of the injection has been debated, recent literature suggests that symptoms can be effectively alleviated regardless of the specific anatomic injection site, such as intra-sheath or extra-sheath (FIGURE 2).19 This allows flexibility for the clinician, as the injection does not have to be placed within the tendon sheath. Corticosteroids should not be injected into the tendon itself, and the needle tip should be slightly withdrawn if there is resistance while injecting. Patients who are averse to injections have been shown to benefit from needle-free jet lidocaine (J-tip) administration prior to the actual steroid injection.20

A randomized controlled trial comparing dexamethasone to triamcinolone injections found no difference in outcome at the 3-month follow-up (n = 84).17 This may suggest that the choice of corticosteroid is at the clinician’s discretion. In terms of long-term efficacy of steroid injections, it has been shown that 70% of trigger digits had complete resolution of symptoms at a mean follow-up of 8 years after just 1 injection (n = 43).21
Some patients, though, may require additional corticosteroid injections to maintain symptom control. If multiple injections are performed, they should not be given in intervals shorter than 4 months between treatments.5 Furthermore, steroids can be administered safely up to 3 times in the same digit before surgery is recommended.22
A patient’s options should be reconsidered if efficacy is not demonstrated with prior injections. Notably, a lower success rate has been shown in patients with type 2 diabetes (66%) compared to those without diabetes (90%).4,23 This difference in success rates is not well understood, as there is no causal relationship between well-controlled diabetes and TF.4 Complications of corticosteroid injections include local pain, fat atrophy, and hypopigmentation at the site of the injection, as well as short-term elevations in blood glucose levels in patients with diabetes.5,24
Surgical correction (to be discussed) remains superior to steroid injections in terms of cure rate and resolution of symptoms. A randomized controlled trial (n = 165) found that an injection-only group reported 86% and 49% success at 3-month and 12-month follow-up, respectively, compared to 99% success at both 3- and 12-month follow-up for the surgical group. Further, at 12-month follow-up, the median pain scores were significantly higher in the injection group (3; range, 1-9) than in the surgery group (1; range, 1-7).25 If conservative treatment modalities lead to unresolved symptoms or recurrence, referral to a hand specialist for surgery is recommended.
Continue to: Surgical treatments in an office setting
Surgical treatments in an office setting
Procedures for TF can be safely performed under conscious sedation or local anesthesia, with or without a tourniquet.26 Wide-awake procedures with local anesthesia and no tourniquet (WALANT) can be performed in an office-based procedure room rather than the operating room. This increases efficiency for the surgeon, reduces the amount of preparation and recovery time for the patient, and helps to keep costs down.
Percutaneous release involves the insertion of a 16-gauge hypodermic needle into the affected A1 pulley. The needle is used to fray and disrupt the pulley by moving the needle tip over the fibrotic A1 pulley.
However, it is not without possible complications.27 Inadvertent A2 pulley damage is particularly troublesome, as it leads to “bowstringing” or protrusion of the flexor tendon into the palm upon flexion. This can cause pain and failure to fully extend or flex the finger.10 Because the anatomy is not well visualized during the percutaneous approach, incomplete release, neurovascular injury, and iatrogenic injury to the A2 pulley or deep tendon may occur.28 Ultrasound-guided percutaneous release techniques have shown effective clinical outcomes with minimal complications compared to nonguided percutaneous release techniques.29,30
Open release is the gold standard surgical treatment for trigger finger (FIGURE 3). A small incision (1-2 cm) is made directly over or proximal to the A1 pulley in the distal palmar crease at the base of the affected digit. After blunt dissection through the subcutaneous tissue, the A1 pulley is sharply incised. An open approach has the clear benefit of avoiding the digital neurovascular bundles, as well as visualizing the resolution of triggering upon flexion and extension prior to closure. The WALANT procedure has the advantage of allowing the awake patient to actively flex and extend the digit to determine if the A1 release has been successful prior to closure of the incision.

Outcomes and complications of surgery. A recent systematic review and meta-analysis has shown percutaneous techniques to be successful in 94% of cases.27 The success rate of open surgery has been reported at 99% to 100% at varying follow-up intervals up to 1 year.25,30,31 The complication rate for percutaneous release (guided and nonguided) was calculated at 2.2% (n = 2114).27 In another study, the overall complication rate of open releases was calculated at 1% (n = 999).32 When comparing percutaneous release (guided and nonguided) and open release, a meta-analysis found no significant difference in complication rate (RR = 0.84) or failure rate (RR = 0.94).32
Continue to: Several risk factors...
Several risk factors have been associated with postoperative surgical infection, including recent steroid injection (< 80 d), smoking status, increasing age, and pre-operative use of lidocaine with epinephrine.33 Open release has been shown to be an effective and safe treatment modality for patients with and without diabetes alike.34 Overall, definitive surgical correction has been demonstrated to be superior to conservative measures due to a significantly lower rate of recurrence.35
CASE
Given the patient’s presentation with triggering of the digit, tenderness over the A1 pulley, and lack of trauma history, we diagnosed trigger finger in this patient. Potential treatments included splinting, corticosteroid injections, and surgery. After discussion of the risks and benefits of each treatment option, the patient elected to undergo a corticosteroid injection. She was also given a neoprene finger sleeve to wear every night, and in the daytime when possible.
At 12-week follow-up, she noted early improvement in her triggering, which had since recurred. Due to her history of diabetes, the patient was then referred for surgery. She had an open release under local anesthesia. The surgery was uncomplicated, and the abnormality was corrected. At the patient’s 1-year postoperative follow-up visit, there was no evidence of recurrence, and she had regained full active and passive range of motion of her finger.
Acknowledgements
The authors wish to thank Jose Borrero, MD, for contributing his time and creative talents to produce the illustrations in this article.
CORRESPONDENCE
Evan P. Johnson, MD; 506 South Greer Street, Memphis, TN 38111; [email protected]
1. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
2. Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92-96. doi: 10.1007/s12178-007-9012-1
3. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775. doi: 10.1016/j.jhsa.2008.01.038
4. Junot HSN, Anderson Hertz AFL, Gustavo Vasconcelos GR, et al. Epidemiology of trigger finger: metabolic syndrome as a new perspective of associated disease. Hand (N Y). 2019:1558944719867135. doi: 10.1177/1558944719867135.
5. Matthews A, Smith K, Read L, et al. Trigger finger: an overview of the treatment options. JAAPA. 2019;32:17-21. doi: 10.1097/01.Jaa.0000550281.42592.97
6. Pencle FJ, Waheed A, Molnar JA. Trigger thumb. StatPearls [Internet]. StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK441854/
7. Giugale JM, Fowler JR. Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 2015;46:561-569. doi: 10.1016/j.ocl.2015.06.014
8. Bianchi S, Gitto S, Draghi F. Ultrasound features of trigger finger: review of the literature. J Ultrasound Med. 2019;38:3141-3154. doi: 10.1002/jum.15025
9. Chuang XL, Ooi CC, Chin ST, et al. What triggers in trigger finger? The flexor tendons at the flexor digitorum superficialis bifurcation. J Plast Reconstr Aesthet Surg. 2017;70:1411-1419. doi: 10.1016/j.bjps.2017.05.037
10. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31:135-146. doi: 10.1016/j.jhsa.2005.10.013
11. Chapter 56: Tendinoapthy. In: Wolfe SW, Peterson WC, Kozin SH, Cohen MS. Green’s Operative Hand Surgery. Vol 2. 7th ed. Elsevier; 2017: 1904-1925.
12. Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012;37:243-249, 249.e241. doi: 10.1016/j.jhsa.2011.10.038
13. Valdes K. A retrospective review to determine the long-term efficacy of orthotic devices for trigger finger. J Hand Ther. 2012;25:89-95. doi: 10.1016/j.jht.2011.09.005
14. Drijkoningen T, van Berckel M, Becker SJE, et al. Night splinting for idiopathic trigger digits. Hand (N Y). 2018;13:558-562. doi: 10.1177/1558944717725374
15. Colbourn J, Heath N, Manary S, et al. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008;21:336-343. doi: 10.1197/j.jht.2008.05.001
16. Teo SH, Ng DCL, Wong YKY. Effectiveness of proximal interphalangeal joint-blocking orthosis vs metacarpophalangeal joint-blocking orthosis in trigger digit management: A randomized clinical trial. J Hand Ther. 2018;32:444-451. doi: 10.1016/j.jht.2018.02.007
17. Ring D, Lozano-Calderon S, Shin R, et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33:516-522; discussion 523-514. doi: 10.1016/j.jhsa.2008.01.001
18. Fleisch SB, Spindler KP, Lee DH. Corticosteroid injections in the treatment of trigger finger: A level I and II systematic review. J Am Acad Orthop Surg. 2007;15:166-171. doi: 10.5435/00124635-200703000-00006
19. Shinomiya R, Sunagawa T, Nakashima Y, et al. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016;42:2203-2208. doi: 10.1016/j.ultrasmedbio.2016.05.015
20. Earp BE, Stanbury SJ, Mora AN, et al. Needle-free jet lidocaine administration for preinjection anesthesia in trigger finger injection: a randomized controlled trial. J Hand Surg Am. 2017;42:618-622. doi: 10.1016/j.jhsa.2017.05.001
21. Castellanos J, Munoz-Mahamud E, Dominguez E, et al. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121-126. doi: 10.1016/j.jhsa.2014.09.006
22. Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263-268. doi: 10.4055/cios.2012.4.4.263
23. Griggs SM, Weiss AP, Lane LB, et al. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20:787-789. doi: 10.1016/s0363-5023(05)80432-0
24. Stepan JG, London DA, Boyer MI, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39:706-712. doi: 10.1016/j.jhsa.2014.01.014
25. Hansen RL, Sondergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am. 2017;42:359-366. doi: 10.1016/j.jhsa.2017.02.011
26. Mohd Rashid MZ, Sapuan J, Abdullah S. A randomized controlled trial of trigger finger release under digital anesthesia with (WALANT) and without adrenaline. J Orthop Surg (Hong Kong). 2019;27:2309499019833002. doi: 10.1177/2309499019833002
27. Zhao J-G, Kan S-L, Zhao L, et al. Percutaneous first annular pulley release for trigger digits: a systematic review and meta-analysis of current evidence. J Hand Surg Am. 2014;39:2192-2202. doi: 10.1016/j.jhsa.2014.07.044
28. Guler F, Kose O, Ercan EC, et al. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36:e1290-1294. doi: 10.3928/01477447-20130920-22
29. Wu KC, Chern TC, Jou IM. Ultrasound-assisted percutaneous trigger finger release: it is safe [letter]. Hand (N Y). 2009;4:339. doi: 10.1007/s11552-009-9179-6
30. Nikolaou VS, Malahias M-A, Kaseta M-K, et al. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop. 2017;8:163-169. doi: 10.5312/wjo.v8.i2.163
31. Lim M-H, Lim K-K, Rasheed MZ, et al. Outcome of open trigger digit release. J Hand Surg Eur Vol. 2007;32:457-459. doi: 10.1016/j.Jhsb.2007.02.016
32. Wang J, Zhao J-G, Liang C-C. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res. 2013;471:1879-1886. doi: 10.1007/s11999-012-2716-6
33. Ng WKY, Olmscheid N, Worhacz K, et al. Steroid injection and open trigger finger release outcomes: a retrospective review of 999 digits. Hand (N Y). 2018;15:399-406. doi: 10.1177/1558944718796559
34. Ho SWL, Chia CY, Rajaratnam V. Characteristics and clinical outcomes of open surgery for trigger digits in diabetes. J Hand Microsurg. 2019;11:80-83. doi: 10.1055/s-0038-1670927
35. Sato ES, dos Santos JB, Belloti JC, et al. Percutaneous release of trigger fingers. Hand Clin. 2014;30:39-45. doi: 10.1016/j.hcl.2013.08.017
1. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
2. Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92-96. doi: 10.1007/s12178-007-9012-1
3. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775. doi: 10.1016/j.jhsa.2008.01.038
4. Junot HSN, Anderson Hertz AFL, Gustavo Vasconcelos GR, et al. Epidemiology of trigger finger: metabolic syndrome as a new perspective of associated disease. Hand (N Y). 2019:1558944719867135. doi: 10.1177/1558944719867135.
5. Matthews A, Smith K, Read L, et al. Trigger finger: an overview of the treatment options. JAAPA. 2019;32:17-21. doi: 10.1097/01.Jaa.0000550281.42592.97
6. Pencle FJ, Waheed A, Molnar JA. Trigger thumb. StatPearls [Internet]. StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK441854/
7. Giugale JM, Fowler JR. Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 2015;46:561-569. doi: 10.1016/j.ocl.2015.06.014
8. Bianchi S, Gitto S, Draghi F. Ultrasound features of trigger finger: review of the literature. J Ultrasound Med. 2019;38:3141-3154. doi: 10.1002/jum.15025
9. Chuang XL, Ooi CC, Chin ST, et al. What triggers in trigger finger? The flexor tendons at the flexor digitorum superficialis bifurcation. J Plast Reconstr Aesthet Surg. 2017;70:1411-1419. doi: 10.1016/j.bjps.2017.05.037
10. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31:135-146. doi: 10.1016/j.jhsa.2005.10.013
11. Chapter 56: Tendinoapthy. In: Wolfe SW, Peterson WC, Kozin SH, Cohen MS. Green’s Operative Hand Surgery. Vol 2. 7th ed. Elsevier; 2017: 1904-1925.
12. Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012;37:243-249, 249.e241. doi: 10.1016/j.jhsa.2011.10.038
13. Valdes K. A retrospective review to determine the long-term efficacy of orthotic devices for trigger finger. J Hand Ther. 2012;25:89-95. doi: 10.1016/j.jht.2011.09.005
14. Drijkoningen T, van Berckel M, Becker SJE, et al. Night splinting for idiopathic trigger digits. Hand (N Y). 2018;13:558-562. doi: 10.1177/1558944717725374
15. Colbourn J, Heath N, Manary S, et al. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008;21:336-343. doi: 10.1197/j.jht.2008.05.001
16. Teo SH, Ng DCL, Wong YKY. Effectiveness of proximal interphalangeal joint-blocking orthosis vs metacarpophalangeal joint-blocking orthosis in trigger digit management: A randomized clinical trial. J Hand Ther. 2018;32:444-451. doi: 10.1016/j.jht.2018.02.007
17. Ring D, Lozano-Calderon S, Shin R, et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33:516-522; discussion 523-514. doi: 10.1016/j.jhsa.2008.01.001
18. Fleisch SB, Spindler KP, Lee DH. Corticosteroid injections in the treatment of trigger finger: A level I and II systematic review. J Am Acad Orthop Surg. 2007;15:166-171. doi: 10.5435/00124635-200703000-00006
19. Shinomiya R, Sunagawa T, Nakashima Y, et al. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016;42:2203-2208. doi: 10.1016/j.ultrasmedbio.2016.05.015
20. Earp BE, Stanbury SJ, Mora AN, et al. Needle-free jet lidocaine administration for preinjection anesthesia in trigger finger injection: a randomized controlled trial. J Hand Surg Am. 2017;42:618-622. doi: 10.1016/j.jhsa.2017.05.001
21. Castellanos J, Munoz-Mahamud E, Dominguez E, et al. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121-126. doi: 10.1016/j.jhsa.2014.09.006
22. Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263-268. doi: 10.4055/cios.2012.4.4.263
23. Griggs SM, Weiss AP, Lane LB, et al. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20:787-789. doi: 10.1016/s0363-5023(05)80432-0
24. Stepan JG, London DA, Boyer MI, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39:706-712. doi: 10.1016/j.jhsa.2014.01.014
25. Hansen RL, Sondergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am. 2017;42:359-366. doi: 10.1016/j.jhsa.2017.02.011
26. Mohd Rashid MZ, Sapuan J, Abdullah S. A randomized controlled trial of trigger finger release under digital anesthesia with (WALANT) and without adrenaline. J Orthop Surg (Hong Kong). 2019;27:2309499019833002. doi: 10.1177/2309499019833002
27. Zhao J-G, Kan S-L, Zhao L, et al. Percutaneous first annular pulley release for trigger digits: a systematic review and meta-analysis of current evidence. J Hand Surg Am. 2014;39:2192-2202. doi: 10.1016/j.jhsa.2014.07.044
28. Guler F, Kose O, Ercan EC, et al. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36:e1290-1294. doi: 10.3928/01477447-20130920-22
29. Wu KC, Chern TC, Jou IM. Ultrasound-assisted percutaneous trigger finger release: it is safe [letter]. Hand (N Y). 2009;4:339. doi: 10.1007/s11552-009-9179-6
30. Nikolaou VS, Malahias M-A, Kaseta M-K, et al. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop. 2017;8:163-169. doi: 10.5312/wjo.v8.i2.163
31. Lim M-H, Lim K-K, Rasheed MZ, et al. Outcome of open trigger digit release. J Hand Surg Eur Vol. 2007;32:457-459. doi: 10.1016/j.Jhsb.2007.02.016
32. Wang J, Zhao J-G, Liang C-C. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res. 2013;471:1879-1886. doi: 10.1007/s11999-012-2716-6
33. Ng WKY, Olmscheid N, Worhacz K, et al. Steroid injection and open trigger finger release outcomes: a retrospective review of 999 digits. Hand (N Y). 2018;15:399-406. doi: 10.1177/1558944718796559
34. Ho SWL, Chia CY, Rajaratnam V. Characteristics and clinical outcomes of open surgery for trigger digits in diabetes. J Hand Microsurg. 2019;11:80-83. doi: 10.1055/s-0038-1670927
35. Sato ES, dos Santos JB, Belloti JC, et al. Percutaneous release of trigger fingers. Hand Clin. 2014;30:39-45. doi: 10.1016/j.hcl.2013.08.017
PRACTICE RECOMMENDATIONS
› Recommend splinting as a first-line conservative treatment for trigger finger if there is not a fixed contracture. B
› Prescribe corticosteroids, which may completely resolve trigger finger in the majority of patients without diabetes. A
› Refer patients for surgical release if they do not respond to conservative management. The surgical success rate is as high as 99%. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Dyspepsia: A stepwise approach to evaluation and management
The global prevalence of dyspepsia is approximately 20%,1 and it is often associated with other comorbidities and overlapping gastrointestinal complaints. The effects on the patient’s quality of life, including societal impacts, are considerable. Symptoms and their response to treatment are highly variable, necessitating individualized management. While some patients’ symptoms may be refractory to standard medical treatment initially, evidence suggests that the strategies summarized in our guidance here—including the use of tricyclic antidepressants (TCAs), prokinetics, and adjunctive therapies—may alleviate symptoms and improve patients’ quality of life.
What dyspepsia is—and what it isn’t
Dyspepsia is a poorly characterized disorder often associated with nausea, heartburn, early satiety, and bloating. The American College of Gastroenterology (ACG) now advocates using a clinically relevant definition of dyspepsia as “predominant epigastric pain lasting at least a month” as long as epigastric pain is the patient's primary complaint.2 Causes of dyspepsia are listed in TABLE 1.
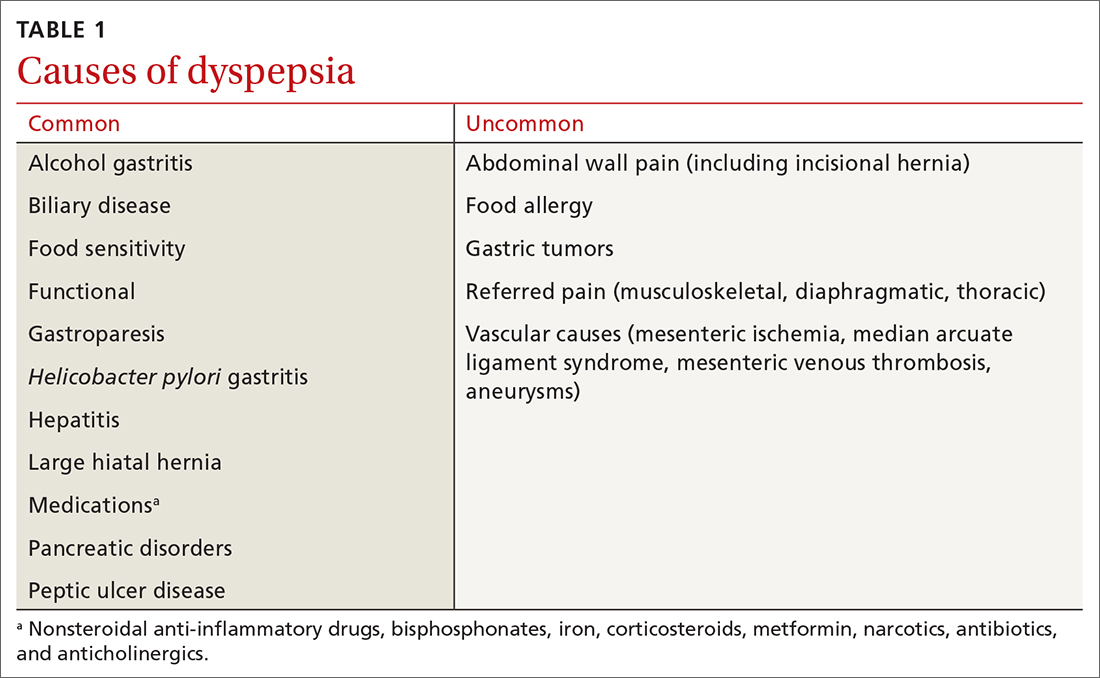
Heartburn, a burning sensation in the chest, is not a dyspeptic symptom but the 2 may often coexist. In general, dyspepsia does not have a colicky or postural component. Symptoms that are relieved by evacuation of feces or gas generally should not be considered a part of dyspepsia.
Functional dyspepsia (FD) is a subset for which no structural pathology has been identified, accounting for up to 70% of all patients with dyspepsia.3 The Rome Foundation, in its recent update (Rome IV), has highlighted 4 key symptoms and 2 proposed subtypes (TABLE 2).4 The comorbidities of anxiety, depression, and somatization appear to be more prevalent in these dyspepsia patients than in those with organic issues. The incidence of gastric malignancy is low in this cohort.3,5 Dyspepsia occurring after an acute infection is referred to as postinfectious functional dyspepsia.

Pathophysiology of functional dyspepsia. Dysmotility, visceral hypersensitivity, mucosal immune dysfunction, altered gut microbiota, and disturbed central nervous system processing contribute in varying degrees to the pathophysiology of FD. There is evidence that luminal factors have the potential to trigger local neuronal excitability.6,7 Early life psychosocial factors may further influence illness behaviors, coping strategies, stress responses, and the intensity of symptoms perceived by the patient.8
Clues in the history and physical examination
Patients describe their discomfort using a variety of terms, including pain, gnawing, burning, gassiness, or queasiness. Although allergic reactions to food (swelling of lips and tongue with a rash) are rare in adults, food intolerances are common in patients with dyspepsia.9 Consumption of nonsteroidal anti-inflammatory drugs is a common cause of dyspepsia, even at over-the-counter strength, and may cause ulceration, gastrointestinal bleeding, and anemia. Narcotic and marijuana use and the anticholinergic effects of antidepressant medications are associated with gastrointestinal dysmotility, including gastroparesis.
Patients with FD often exhibit symptoms of other functional abdominal disorders including irritable bowel syndrome, functional heartburn, bloating, or chronic nausea, and may have been previously diagnosed with overlapping conditions suggestive of visceral hypersensitivity, including depression, anxiety, fibromyalgia, migraine, and pelvic pain. During the patient’s office visit, be alert to any indication of an underlying psychological issue.
Continue to: The initial diagnostic challenge
The initial diagnostic challenge is to identify those patients who may have a structural disorder requiring expedited and targeted investigation. Weight loss, night waking, and vomiting are unusual in the setting of either FD or Helicobacter pylori gastritis. These and other features of concern (TABLE 3) make a diagnosis of a functional disorder less likely and should prompt immediate consideration of abdominal imaging or endoscopic examination. Epigastric tenderness on palpation is common in patients with FD and is not necessarily predictive of structural pathology—unless accompanied by other findings of concern. Abdominal scars or a history of trauma may be suggestive of abdominal wall pain. Abdominal pain that remains unchanged or increases when the muscles of the abdominal wall are tensed (Carnett sign) suggests abdominal wall pain.
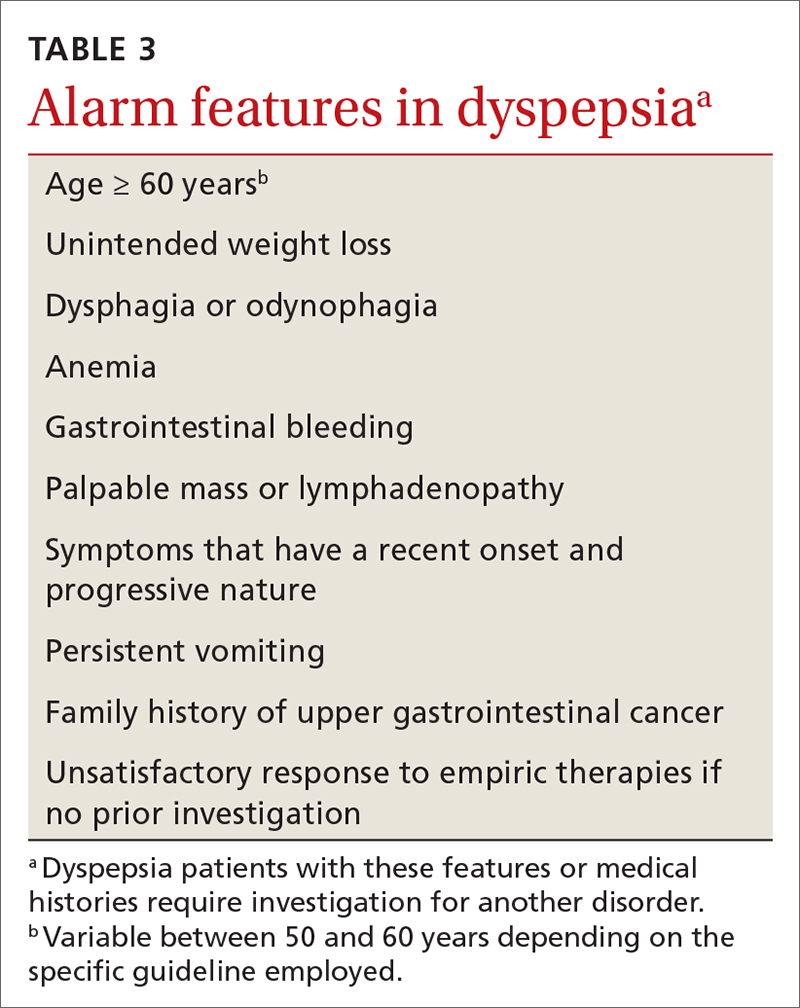
Initial testing and Tx assessments focus on H pylori
All 3 of the major US gastroenterology organizations recommend a stepwise approach in patients without alarm symptoms, generally beginning (in those < 60 years) by testing for H pylori with either the stool antigen or urea breath test (UBT)—and initiating appropriate treatment if results are positive.5,10 (The first step for those ≥ 60 years is discussed later.) Since the serum antibody test cannot differentiate between active and past infection, it is not recommended if other options are available.11 The stool antigen test is preferred; it is a cost-effective option used for both diagnosis and confirmation of H pylori eradication.
The UBT identifies active infection with a sensitivity and specificity of > 95%12 but is more labor intensive, employs an isotope, and is relatively expensive. Because proton pump inhibitors (PPIs), bismuth, and antibiotics may increase the false-negative rate for both the UBT and stool antigen test, we recommend that these medications be held for 2 to 4 weeks prior to testing.11 H2-receptor antagonists do not need to be restricted.
Treatment regimens containing clarithromycin have fallen into disfavor given the high rates of resistance that are now encountered. Fourteen-day regimens that can be used empirically (without susceptibility testing) are bismuth quadruple therapy (bismuth, metronidazole, tetracycline, and PPI) or rifabutin triple therapy (rifabutin, amoxicillin, and PPI).13 To confirm eradication, perform repeat testing with either stool antigen or UBT no sooner than 4 weeks after completion of therapy. If the first treatment fails, try a second regimen using different antibiotics.14 Although the impact of H pylori eradication on dyspeptic symptoms is only modest, this strategy is recommended also to reduce the risk of peptic ulceration and gastric neoplasia.
Next-step testing and Tx considerations
Given the heterogeneity of presenting symptoms of dyspepsia, some clinicians may be hesitant to diagnose a functional disorder at the first visit, preferring instead to conduct a limited range of investigations in concert with initial medical management. In these circumstances it would be reasonable, in addition to testing for H pylori, to order a complete blood count (CBC) and to measure serum lipase and liver enzymes. Keep in mind that liver enzymes may not be elevated in uncomplicated biliary colic.
Continue to: Consider ultrasound imaging...
Consider ultrasound imaging if gallstones are a consideration. A computerized tomography scan may not exclude uncomplicated and noncalcified gallstones, but it is an excellent modality for detecting suspected retroperitoneal pathology. Consider working with a gastroenterologist if the patient exhibits alarm features.
Empiric PPI therapy. A trial of daily PPI use over 4 weeks is recommended for patients without H pylori and for those whose symptoms continue despite eradication of the bacterium. A Cochrane meta-analysis found that PPI therapy was more effective than placebo (31% vs 26%; risk ratio, 0.88; number needed to treat [NNT] = 11; 95% CI 0.82 to 0.94; P < .001).15 PPI therapy appears to be slightly more effective than treatment with H2-receptor antagonists. Both are proposed in the United Kingdom guideline.16 Both are generally safe and well tolerated but are not without potential adverse effects when used long term.
Dietary modification. Patients with dyspepsia commonly report that meals exacerbate symptoms. This is likely due to a combination of gastric distension and underlying visceral hypersensitivity rather than food composition.
There is no reliable “dyspepsia diet,” although a systematic review implicated wheat and high-fat foods as the 2 most common contributors to symptom onset.17 Recommended dietary modifications would be to consume smaller, more frequent meals and to eliminate recognized trigger foods. Patients with postprandial distress syndrome, a subset of FD, may want to consider reducing fat intake to help alleviate discomfort. If symptoms continue, evaluate for lactose intolerance. Also, consider a trial of a gluten-free diet. The low-FODMAP diet (restricting fermentable oligo-, di- and monosaccharides, as well as polyols) has shown benefit in patients with irritable bowel syndrome and may be considered in those with intractable FD, given the overlap in physiology of the disorders.
Upper gastrointestinal endoscopy. The ACG has suggested that esophagogastroduodenoscopy (EGD) be performed as the first investigative step for patients ≥ 60 years, while testing for H pylori be considered as the first step in younger patients, even if alarm symptoms are present2 (FIGURE). This decision must be individualized, particularly in patients of Asian, Central or South American, or Caribbean descent, in whom the incidence of gastric cancer is higher with earlier onset.18
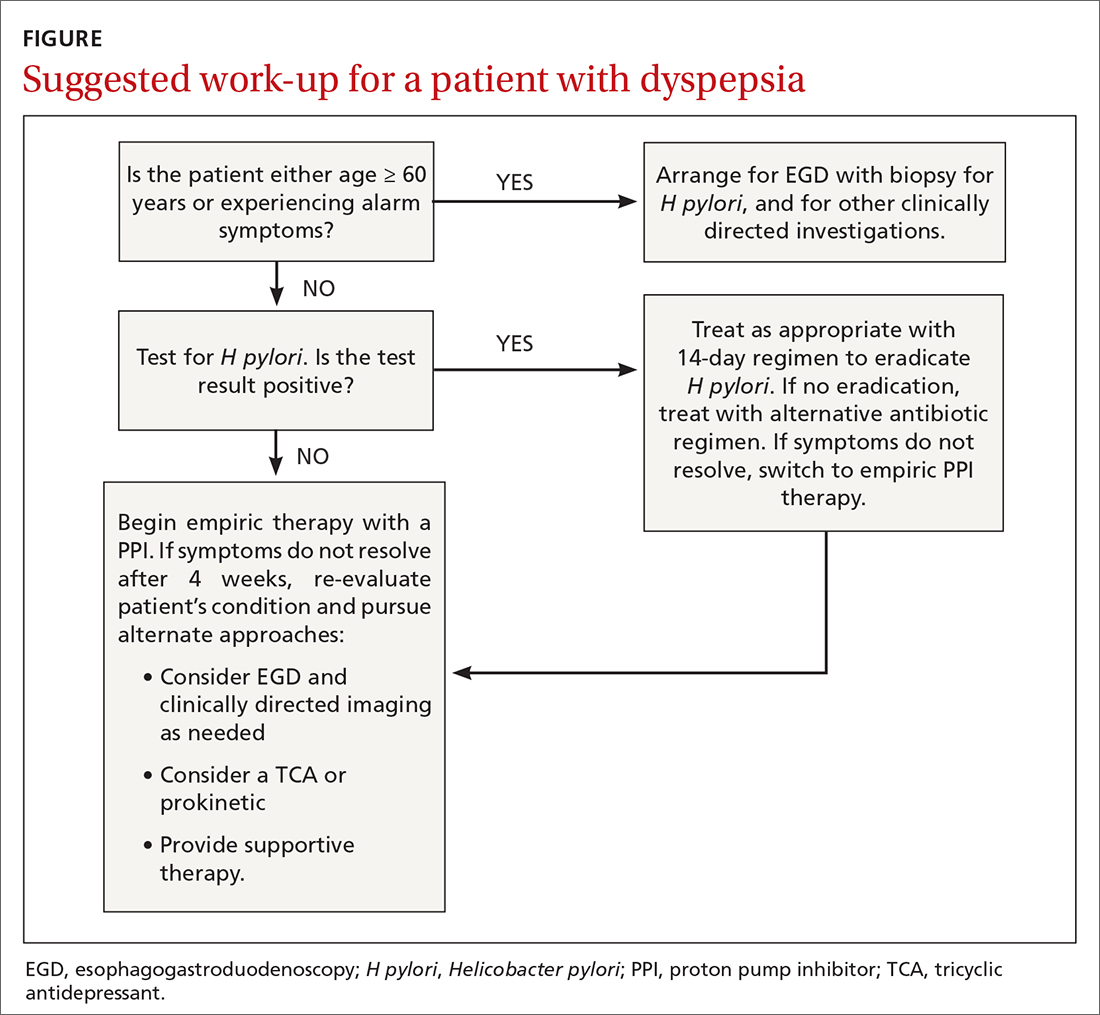
Continue to: Also consider EGD...
Also consider EGD for patients whose symptoms have not improved despite eradication of H pylori or an adequate trial of PPI therapy. While some guidelines do not require EGD in low-risk patients at this stage, other authorities would consider this step prudent, particularly when quality of life has been significantly impaired. An underlying organic cause, mainly erosive esophagitis or peptic ulcer disease, is found in 20% to 30% of patients with dyspepsia.5
Most patients without alarm features, with normal findings on upper endoscopy, who do not have H pylori gastritis, and whose symptoms continue despite a trial of PPI therapy, will have FD (FIGURE).2
Offer patients with functional dyspepsia supportive therapy
Neuromodulators
TCAs are superior to placebo in reducing dyspeptic symptoms with an NNT of 6 and are recommended for patients with ongoing symptoms despite PPI therapy or H pylori eradication.2 Begin with a low dose and increase as tolerated. It may take a few weeks for improvement to be seen. Exercise caution in the presence of cardiac arrhythmias.
Mirtazapine, 7.5 to 15 mg every night at bedtime, reduces fullness and bloating in postprandial distress syndrome and is useful for patients who have lost weight. It’s important to note that TCAs and mirtazapine both have the potential for QT prolongation, as well as depression and suicidality in younger patients.19 The anxiolytic buspirone, 10 mg before meals, augments fundic relaxation, improves overall symptom severity, and helps alleviate early satiety, postprandial fullness, and upper abdominal bloating.20
Prokinetics
A recent meta-analysis demonstrated significant benefit in symptom control in dyspeptic patients treated with prokinetics (NNT = 7).21 However, the benefit was predominantly due to cisapride, a drug that was withdrawn from the US market due to adverse effects. There are no clinical trials of metoclopramide or domperidone (not available in the United States) in FD. Nonetheless, the ACG has given a conditional recommendation, based on low-quality evidence, for the use of prokinetics in patients with FD not responding to PPI therapy, H pylori eradication, or TCA therapy.2
Continue to: A shortcoming of the established guidelines
A shortcoming of the established guidelines is that they do not provide guidance as to long-term management of those patients who respond to prescription medications. Our practice has been to continue medications for a minimum of 3 months, then begin a slow taper in order to establish the lowest efficacious dose. Some patients may relapse and require full dosage for a longer period of time.
Adjunctive therapies are worth considering
Complementary and alternative medicines. Products containing ginger, carraway oil, artichoke leaf extract, turmeric, and red pepper are readily available without prescription and have long been used with variable results for dyspepsia.22 The 9-herb combination STW-5 has demonstrated superiority over placebo in a number of studies and has a favorable safety profile.23 The recommended dose is 10 to 20 drops tid. The European manufacturer has recently modified the package insert noting rare cases of hepatotoxicity.24
A commercially available formulation (FDgard) containing L-menthol (a key component of peppermint oil) and caraway has been found to reduce the intensity of symptoms in patients with FD. Potential adverse effects include nausea, contact dermatitis, bronchospasm, and atrial fibrillation. Cayenne, a red pepper extract, is available over the counter for the benefit for epigastric pain and bloating. Begin with a 500-mg dose before breakfast and a 1000-mg dose before dinner, increasing to 2500 mg/d as tolerated. Cayenne preparations may trigger drug toxicities and are best avoided in patients taking antihypertensives, theophylline, or anticoagulants.
Cognitive behavioral therapy, acupuncture, and hypnosis. These modalities are time consuming, are often expensive, are not always covered by insurance, and require significant motivation. A systematic review found no benefit.25 Subsequent studies summarized in the ACG guidelines2 reported benefit; however, a lack of blinding and significant heterogeneity among the groups detract from the quality of the data. It remains unclear whether these are effective strategies for FD, and therefore they cannot be recommended on a routine basis.
CORRESPONDENCE
Norman H. Gilinsky, MD, Division of Digestive Diseases, University of Cincinnati College of Medicine, 231 Albert Sabin Way, Cincinnati, OH 45267-0595; norman.gilinsky@ uc.edu
1. Ford AC, Marwaha A, Sood R, et al. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015;64:1049-1057.
2. Moayyedi P, Lacy BE, Andrews CN, et al. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017;112:988-1013.
3. Ford AC, Marwaha A, Lim A, et al. What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8:830-837.
4. Stanghellini V, Chan FKL, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology. 2016;150:1380-1392.
5. Shaukat A, Wang A, Acosta RD, et al. The role of endoscopy in dyspepsia. Gastrointest Endosc. 2015;82:227-232.
6. Wauters L, Talley NJ, Walker MM, et al. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut. 2020;69:591-600.
7. Weinstock LB, Pace LA, Rezaie A, et al. Mast cell activation syndrome: a primer for the gastroenterologist. Dig Dis Sci. 2021;66:965-982.
8. Drossman DA. Functional gastrointestinal disorders. History, pathophysiology, clinical features and Rome IV. 2016. Accessed August 16, 2021. www.gastrojournal.org/article/S0016-5085(16)00223-7/fulltext
9. Boettcher E, Crowe SE. Dietary proteins and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:728-736.
10. Talley NJ, AGA. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterol. 2005;129:1753-1755.
11. El-Serag HB, Kao JY, Kanwal F, et al. Houston Consensus Conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol. 2018;16:992-1002.
12. Ferwana M, Abdulmajeed I, Alhajiahmed A, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. 2015;21:1305-1314.
13. Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1-3.
14. Chey WD, Leontiadis G, Howden W, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239.
15. Pinto-Sanchez MI, Yuan Y, Hassan A, et al. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017;11:CD011194.
16. National Institute for Health and Care Excellence. Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management. [Clinical guideline] Accessed August 6, 2021. www.ncbi.nlm.nih.gov/books/NBK552570/
17. Duncanson KR, Talley NJ, Walker MM, et al. Food and functional dyspepsia: a systematic review. J Hum Nutr Diet. 2018;31:390-407.
18. Lin JT. Screening of gastric cancer: who, when, and how. Clin Gastroenterol Hepatol. 2014;12:135-138.
19. Spielmans GI, Spence-Sing T, Parry P. Duty to warn: antidepressant black box suicidality warning is empirically justified. Front Psychiatry. 2020;11:1-18.
20. Tack J, Janssen P, Masaoka T, et al. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2012;10:1239-1245.
21. Pittayanon R, Yuan Y, Bollegala NP, et al. Prokinetics for functional dyspepsia: a systemic review and meta-analysis of randomized controlled trials. Am J Gastroenterol. 2019;114:233-243.
22. Deutsch JK, Levitt J, Hass DJ. Complementary and alternative medicine for functional gastrointestinal disorders. Am J Gastroenterol. 2020;115:350-364.
23. Malfertheiner P. STW 5 (iberogast) therapy in gastrointestinal functional disorders. Dig Dis. 2017;35:25-29.
24. Sáez-González E, Conde I, Díaz-Jaime FC, et al. Iberogast-induced severe hepatotoxicity leading to liver transplantation. Am J Gastroenterol. 2016;111:1364-1365.
25. Soo S, Forman D, Delaney B, et al. A systematic review of psychological therapies for nonulcer dyspepsia. Am J Gastroenterol. 2004;99:1817-1822.
The global prevalence of dyspepsia is approximately 20%,1 and it is often associated with other comorbidities and overlapping gastrointestinal complaints. The effects on the patient’s quality of life, including societal impacts, are considerable. Symptoms and their response to treatment are highly variable, necessitating individualized management. While some patients’ symptoms may be refractory to standard medical treatment initially, evidence suggests that the strategies summarized in our guidance here—including the use of tricyclic antidepressants (TCAs), prokinetics, and adjunctive therapies—may alleviate symptoms and improve patients’ quality of life.
What dyspepsia is—and what it isn’t
Dyspepsia is a poorly characterized disorder often associated with nausea, heartburn, early satiety, and bloating. The American College of Gastroenterology (ACG) now advocates using a clinically relevant definition of dyspepsia as “predominant epigastric pain lasting at least a month” as long as epigastric pain is the patient's primary complaint.2 Causes of dyspepsia are listed in TABLE 1.

Heartburn, a burning sensation in the chest, is not a dyspeptic symptom but the 2 may often coexist. In general, dyspepsia does not have a colicky or postural component. Symptoms that are relieved by evacuation of feces or gas generally should not be considered a part of dyspepsia.
Functional dyspepsia (FD) is a subset for which no structural pathology has been identified, accounting for up to 70% of all patients with dyspepsia.3 The Rome Foundation, in its recent update (Rome IV), has highlighted 4 key symptoms and 2 proposed subtypes (TABLE 2).4 The comorbidities of anxiety, depression, and somatization appear to be more prevalent in these dyspepsia patients than in those with organic issues. The incidence of gastric malignancy is low in this cohort.3,5 Dyspepsia occurring after an acute infection is referred to as postinfectious functional dyspepsia.

Pathophysiology of functional dyspepsia. Dysmotility, visceral hypersensitivity, mucosal immune dysfunction, altered gut microbiota, and disturbed central nervous system processing contribute in varying degrees to the pathophysiology of FD. There is evidence that luminal factors have the potential to trigger local neuronal excitability.6,7 Early life psychosocial factors may further influence illness behaviors, coping strategies, stress responses, and the intensity of symptoms perceived by the patient.8
Clues in the history and physical examination
Patients describe their discomfort using a variety of terms, including pain, gnawing, burning, gassiness, or queasiness. Although allergic reactions to food (swelling of lips and tongue with a rash) are rare in adults, food intolerances are common in patients with dyspepsia.9 Consumption of nonsteroidal anti-inflammatory drugs is a common cause of dyspepsia, even at over-the-counter strength, and may cause ulceration, gastrointestinal bleeding, and anemia. Narcotic and marijuana use and the anticholinergic effects of antidepressant medications are associated with gastrointestinal dysmotility, including gastroparesis.
Patients with FD often exhibit symptoms of other functional abdominal disorders including irritable bowel syndrome, functional heartburn, bloating, or chronic nausea, and may have been previously diagnosed with overlapping conditions suggestive of visceral hypersensitivity, including depression, anxiety, fibromyalgia, migraine, and pelvic pain. During the patient’s office visit, be alert to any indication of an underlying psychological issue.
Continue to: The initial diagnostic challenge
The initial diagnostic challenge is to identify those patients who may have a structural disorder requiring expedited and targeted investigation. Weight loss, night waking, and vomiting are unusual in the setting of either FD or Helicobacter pylori gastritis. These and other features of concern (TABLE 3) make a diagnosis of a functional disorder less likely and should prompt immediate consideration of abdominal imaging or endoscopic examination. Epigastric tenderness on palpation is common in patients with FD and is not necessarily predictive of structural pathology—unless accompanied by other findings of concern. Abdominal scars or a history of trauma may be suggestive of abdominal wall pain. Abdominal pain that remains unchanged or increases when the muscles of the abdominal wall are tensed (Carnett sign) suggests abdominal wall pain.

Initial testing and Tx assessments focus on H pylori
All 3 of the major US gastroenterology organizations recommend a stepwise approach in patients without alarm symptoms, generally beginning (in those < 60 years) by testing for H pylori with either the stool antigen or urea breath test (UBT)—and initiating appropriate treatment if results are positive.5,10 (The first step for those ≥ 60 years is discussed later.) Since the serum antibody test cannot differentiate between active and past infection, it is not recommended if other options are available.11 The stool antigen test is preferred; it is a cost-effective option used for both diagnosis and confirmation of H pylori eradication.
The UBT identifies active infection with a sensitivity and specificity of > 95%12 but is more labor intensive, employs an isotope, and is relatively expensive. Because proton pump inhibitors (PPIs), bismuth, and antibiotics may increase the false-negative rate for both the UBT and stool antigen test, we recommend that these medications be held for 2 to 4 weeks prior to testing.11 H2-receptor antagonists do not need to be restricted.
Treatment regimens containing clarithromycin have fallen into disfavor given the high rates of resistance that are now encountered. Fourteen-day regimens that can be used empirically (without susceptibility testing) are bismuth quadruple therapy (bismuth, metronidazole, tetracycline, and PPI) or rifabutin triple therapy (rifabutin, amoxicillin, and PPI).13 To confirm eradication, perform repeat testing with either stool antigen or UBT no sooner than 4 weeks after completion of therapy. If the first treatment fails, try a second regimen using different antibiotics.14 Although the impact of H pylori eradication on dyspeptic symptoms is only modest, this strategy is recommended also to reduce the risk of peptic ulceration and gastric neoplasia.
Next-step testing and Tx considerations
Given the heterogeneity of presenting symptoms of dyspepsia, some clinicians may be hesitant to diagnose a functional disorder at the first visit, preferring instead to conduct a limited range of investigations in concert with initial medical management. In these circumstances it would be reasonable, in addition to testing for H pylori, to order a complete blood count (CBC) and to measure serum lipase and liver enzymes. Keep in mind that liver enzymes may not be elevated in uncomplicated biliary colic.
Continue to: Consider ultrasound imaging...
Consider ultrasound imaging if gallstones are a consideration. A computerized tomography scan may not exclude uncomplicated and noncalcified gallstones, but it is an excellent modality for detecting suspected retroperitoneal pathology. Consider working with a gastroenterologist if the patient exhibits alarm features.
Empiric PPI therapy. A trial of daily PPI use over 4 weeks is recommended for patients without H pylori and for those whose symptoms continue despite eradication of the bacterium. A Cochrane meta-analysis found that PPI therapy was more effective than placebo (31% vs 26%; risk ratio, 0.88; number needed to treat [NNT] = 11; 95% CI 0.82 to 0.94; P < .001).15 PPI therapy appears to be slightly more effective than treatment with H2-receptor antagonists. Both are proposed in the United Kingdom guideline.16 Both are generally safe and well tolerated but are not without potential adverse effects when used long term.
Dietary modification. Patients with dyspepsia commonly report that meals exacerbate symptoms. This is likely due to a combination of gastric distension and underlying visceral hypersensitivity rather than food composition.
There is no reliable “dyspepsia diet,” although a systematic review implicated wheat and high-fat foods as the 2 most common contributors to symptom onset.17 Recommended dietary modifications would be to consume smaller, more frequent meals and to eliminate recognized trigger foods. Patients with postprandial distress syndrome, a subset of FD, may want to consider reducing fat intake to help alleviate discomfort. If symptoms continue, evaluate for lactose intolerance. Also, consider a trial of a gluten-free diet. The low-FODMAP diet (restricting fermentable oligo-, di- and monosaccharides, as well as polyols) has shown benefit in patients with irritable bowel syndrome and may be considered in those with intractable FD, given the overlap in physiology of the disorders.
Upper gastrointestinal endoscopy. The ACG has suggested that esophagogastroduodenoscopy (EGD) be performed as the first investigative step for patients ≥ 60 years, while testing for H pylori be considered as the first step in younger patients, even if alarm symptoms are present2 (FIGURE). This decision must be individualized, particularly in patients of Asian, Central or South American, or Caribbean descent, in whom the incidence of gastric cancer is higher with earlier onset.18

Continue to: Also consider EGD...
Also consider EGD for patients whose symptoms have not improved despite eradication of H pylori or an adequate trial of PPI therapy. While some guidelines do not require EGD in low-risk patients at this stage, other authorities would consider this step prudent, particularly when quality of life has been significantly impaired. An underlying organic cause, mainly erosive esophagitis or peptic ulcer disease, is found in 20% to 30% of patients with dyspepsia.5
Most patients without alarm features, with normal findings on upper endoscopy, who do not have H pylori gastritis, and whose symptoms continue despite a trial of PPI therapy, will have FD (FIGURE).2
Offer patients with functional dyspepsia supportive therapy
Neuromodulators
TCAs are superior to placebo in reducing dyspeptic symptoms with an NNT of 6 and are recommended for patients with ongoing symptoms despite PPI therapy or H pylori eradication.2 Begin with a low dose and increase as tolerated. It may take a few weeks for improvement to be seen. Exercise caution in the presence of cardiac arrhythmias.
Mirtazapine, 7.5 to 15 mg every night at bedtime, reduces fullness and bloating in postprandial distress syndrome and is useful for patients who have lost weight. It’s important to note that TCAs and mirtazapine both have the potential for QT prolongation, as well as depression and suicidality in younger patients.19 The anxiolytic buspirone, 10 mg before meals, augments fundic relaxation, improves overall symptom severity, and helps alleviate early satiety, postprandial fullness, and upper abdominal bloating.20
Prokinetics
A recent meta-analysis demonstrated significant benefit in symptom control in dyspeptic patients treated with prokinetics (NNT = 7).21 However, the benefit was predominantly due to cisapride, a drug that was withdrawn from the US market due to adverse effects. There are no clinical trials of metoclopramide or domperidone (not available in the United States) in FD. Nonetheless, the ACG has given a conditional recommendation, based on low-quality evidence, for the use of prokinetics in patients with FD not responding to PPI therapy, H pylori eradication, or TCA therapy.2
Continue to: A shortcoming of the established guidelines
A shortcoming of the established guidelines is that they do not provide guidance as to long-term management of those patients who respond to prescription medications. Our practice has been to continue medications for a minimum of 3 months, then begin a slow taper in order to establish the lowest efficacious dose. Some patients may relapse and require full dosage for a longer period of time.
Adjunctive therapies are worth considering
Complementary and alternative medicines. Products containing ginger, carraway oil, artichoke leaf extract, turmeric, and red pepper are readily available without prescription and have long been used with variable results for dyspepsia.22 The 9-herb combination STW-5 has demonstrated superiority over placebo in a number of studies and has a favorable safety profile.23 The recommended dose is 10 to 20 drops tid. The European manufacturer has recently modified the package insert noting rare cases of hepatotoxicity.24
A commercially available formulation (FDgard) containing L-menthol (a key component of peppermint oil) and caraway has been found to reduce the intensity of symptoms in patients with FD. Potential adverse effects include nausea, contact dermatitis, bronchospasm, and atrial fibrillation. Cayenne, a red pepper extract, is available over the counter for the benefit for epigastric pain and bloating. Begin with a 500-mg dose before breakfast and a 1000-mg dose before dinner, increasing to 2500 mg/d as tolerated. Cayenne preparations may trigger drug toxicities and are best avoided in patients taking antihypertensives, theophylline, or anticoagulants.
Cognitive behavioral therapy, acupuncture, and hypnosis. These modalities are time consuming, are often expensive, are not always covered by insurance, and require significant motivation. A systematic review found no benefit.25 Subsequent studies summarized in the ACG guidelines2 reported benefit; however, a lack of blinding and significant heterogeneity among the groups detract from the quality of the data. It remains unclear whether these are effective strategies for FD, and therefore they cannot be recommended on a routine basis.
CORRESPONDENCE
Norman H. Gilinsky, MD, Division of Digestive Diseases, University of Cincinnati College of Medicine, 231 Albert Sabin Way, Cincinnati, OH 45267-0595; norman.gilinsky@ uc.edu
The global prevalence of dyspepsia is approximately 20%,1 and it is often associated with other comorbidities and overlapping gastrointestinal complaints. The effects on the patient’s quality of life, including societal impacts, are considerable. Symptoms and their response to treatment are highly variable, necessitating individualized management. While some patients’ symptoms may be refractory to standard medical treatment initially, evidence suggests that the strategies summarized in our guidance here—including the use of tricyclic antidepressants (TCAs), prokinetics, and adjunctive therapies—may alleviate symptoms and improve patients’ quality of life.
What dyspepsia is—and what it isn’t
Dyspepsia is a poorly characterized disorder often associated with nausea, heartburn, early satiety, and bloating. The American College of Gastroenterology (ACG) now advocates using a clinically relevant definition of dyspepsia as “predominant epigastric pain lasting at least a month” as long as epigastric pain is the patient's primary complaint.2 Causes of dyspepsia are listed in TABLE 1.

Heartburn, a burning sensation in the chest, is not a dyspeptic symptom but the 2 may often coexist. In general, dyspepsia does not have a colicky or postural component. Symptoms that are relieved by evacuation of feces or gas generally should not be considered a part of dyspepsia.
Functional dyspepsia (FD) is a subset for which no structural pathology has been identified, accounting for up to 70% of all patients with dyspepsia.3 The Rome Foundation, in its recent update (Rome IV), has highlighted 4 key symptoms and 2 proposed subtypes (TABLE 2).4 The comorbidities of anxiety, depression, and somatization appear to be more prevalent in these dyspepsia patients than in those with organic issues. The incidence of gastric malignancy is low in this cohort.3,5 Dyspepsia occurring after an acute infection is referred to as postinfectious functional dyspepsia.

Pathophysiology of functional dyspepsia. Dysmotility, visceral hypersensitivity, mucosal immune dysfunction, altered gut microbiota, and disturbed central nervous system processing contribute in varying degrees to the pathophysiology of FD. There is evidence that luminal factors have the potential to trigger local neuronal excitability.6,7 Early life psychosocial factors may further influence illness behaviors, coping strategies, stress responses, and the intensity of symptoms perceived by the patient.8
Clues in the history and physical examination
Patients describe their discomfort using a variety of terms, including pain, gnawing, burning, gassiness, or queasiness. Although allergic reactions to food (swelling of lips and tongue with a rash) are rare in adults, food intolerances are common in patients with dyspepsia.9 Consumption of nonsteroidal anti-inflammatory drugs is a common cause of dyspepsia, even at over-the-counter strength, and may cause ulceration, gastrointestinal bleeding, and anemia. Narcotic and marijuana use and the anticholinergic effects of antidepressant medications are associated with gastrointestinal dysmotility, including gastroparesis.
Patients with FD often exhibit symptoms of other functional abdominal disorders including irritable bowel syndrome, functional heartburn, bloating, or chronic nausea, and may have been previously diagnosed with overlapping conditions suggestive of visceral hypersensitivity, including depression, anxiety, fibromyalgia, migraine, and pelvic pain. During the patient’s office visit, be alert to any indication of an underlying psychological issue.
Continue to: The initial diagnostic challenge
The initial diagnostic challenge is to identify those patients who may have a structural disorder requiring expedited and targeted investigation. Weight loss, night waking, and vomiting are unusual in the setting of either FD or Helicobacter pylori gastritis. These and other features of concern (TABLE 3) make a diagnosis of a functional disorder less likely and should prompt immediate consideration of abdominal imaging or endoscopic examination. Epigastric tenderness on palpation is common in patients with FD and is not necessarily predictive of structural pathology—unless accompanied by other findings of concern. Abdominal scars or a history of trauma may be suggestive of abdominal wall pain. Abdominal pain that remains unchanged or increases when the muscles of the abdominal wall are tensed (Carnett sign) suggests abdominal wall pain.

Initial testing and Tx assessments focus on H pylori
All 3 of the major US gastroenterology organizations recommend a stepwise approach in patients without alarm symptoms, generally beginning (in those < 60 years) by testing for H pylori with either the stool antigen or urea breath test (UBT)—and initiating appropriate treatment if results are positive.5,10 (The first step for those ≥ 60 years is discussed later.) Since the serum antibody test cannot differentiate between active and past infection, it is not recommended if other options are available.11 The stool antigen test is preferred; it is a cost-effective option used for both diagnosis and confirmation of H pylori eradication.
The UBT identifies active infection with a sensitivity and specificity of > 95%12 but is more labor intensive, employs an isotope, and is relatively expensive. Because proton pump inhibitors (PPIs), bismuth, and antibiotics may increase the false-negative rate for both the UBT and stool antigen test, we recommend that these medications be held for 2 to 4 weeks prior to testing.11 H2-receptor antagonists do not need to be restricted.
Treatment regimens containing clarithromycin have fallen into disfavor given the high rates of resistance that are now encountered. Fourteen-day regimens that can be used empirically (without susceptibility testing) are bismuth quadruple therapy (bismuth, metronidazole, tetracycline, and PPI) or rifabutin triple therapy (rifabutin, amoxicillin, and PPI).13 To confirm eradication, perform repeat testing with either stool antigen or UBT no sooner than 4 weeks after completion of therapy. If the first treatment fails, try a second regimen using different antibiotics.14 Although the impact of H pylori eradication on dyspeptic symptoms is only modest, this strategy is recommended also to reduce the risk of peptic ulceration and gastric neoplasia.
Next-step testing and Tx considerations
Given the heterogeneity of presenting symptoms of dyspepsia, some clinicians may be hesitant to diagnose a functional disorder at the first visit, preferring instead to conduct a limited range of investigations in concert with initial medical management. In these circumstances it would be reasonable, in addition to testing for H pylori, to order a complete blood count (CBC) and to measure serum lipase and liver enzymes. Keep in mind that liver enzymes may not be elevated in uncomplicated biliary colic.
Continue to: Consider ultrasound imaging...
Consider ultrasound imaging if gallstones are a consideration. A computerized tomography scan may not exclude uncomplicated and noncalcified gallstones, but it is an excellent modality for detecting suspected retroperitoneal pathology. Consider working with a gastroenterologist if the patient exhibits alarm features.
Empiric PPI therapy. A trial of daily PPI use over 4 weeks is recommended for patients without H pylori and for those whose symptoms continue despite eradication of the bacterium. A Cochrane meta-analysis found that PPI therapy was more effective than placebo (31% vs 26%; risk ratio, 0.88; number needed to treat [NNT] = 11; 95% CI 0.82 to 0.94; P < .001).15 PPI therapy appears to be slightly more effective than treatment with H2-receptor antagonists. Both are proposed in the United Kingdom guideline.16 Both are generally safe and well tolerated but are not without potential adverse effects when used long term.
Dietary modification. Patients with dyspepsia commonly report that meals exacerbate symptoms. This is likely due to a combination of gastric distension and underlying visceral hypersensitivity rather than food composition.
There is no reliable “dyspepsia diet,” although a systematic review implicated wheat and high-fat foods as the 2 most common contributors to symptom onset.17 Recommended dietary modifications would be to consume smaller, more frequent meals and to eliminate recognized trigger foods. Patients with postprandial distress syndrome, a subset of FD, may want to consider reducing fat intake to help alleviate discomfort. If symptoms continue, evaluate for lactose intolerance. Also, consider a trial of a gluten-free diet. The low-FODMAP diet (restricting fermentable oligo-, di- and monosaccharides, as well as polyols) has shown benefit in patients with irritable bowel syndrome and may be considered in those with intractable FD, given the overlap in physiology of the disorders.
Upper gastrointestinal endoscopy. The ACG has suggested that esophagogastroduodenoscopy (EGD) be performed as the first investigative step for patients ≥ 60 years, while testing for H pylori be considered as the first step in younger patients, even if alarm symptoms are present2 (FIGURE). This decision must be individualized, particularly in patients of Asian, Central or South American, or Caribbean descent, in whom the incidence of gastric cancer is higher with earlier onset.18

Continue to: Also consider EGD...
Also consider EGD for patients whose symptoms have not improved despite eradication of H pylori or an adequate trial of PPI therapy. While some guidelines do not require EGD in low-risk patients at this stage, other authorities would consider this step prudent, particularly when quality of life has been significantly impaired. An underlying organic cause, mainly erosive esophagitis or peptic ulcer disease, is found in 20% to 30% of patients with dyspepsia.5
Most patients without alarm features, with normal findings on upper endoscopy, who do not have H pylori gastritis, and whose symptoms continue despite a trial of PPI therapy, will have FD (FIGURE).2
Offer patients with functional dyspepsia supportive therapy
Neuromodulators
TCAs are superior to placebo in reducing dyspeptic symptoms with an NNT of 6 and are recommended for patients with ongoing symptoms despite PPI therapy or H pylori eradication.2 Begin with a low dose and increase as tolerated. It may take a few weeks for improvement to be seen. Exercise caution in the presence of cardiac arrhythmias.
Mirtazapine, 7.5 to 15 mg every night at bedtime, reduces fullness and bloating in postprandial distress syndrome and is useful for patients who have lost weight. It’s important to note that TCAs and mirtazapine both have the potential for QT prolongation, as well as depression and suicidality in younger patients.19 The anxiolytic buspirone, 10 mg before meals, augments fundic relaxation, improves overall symptom severity, and helps alleviate early satiety, postprandial fullness, and upper abdominal bloating.20
Prokinetics
A recent meta-analysis demonstrated significant benefit in symptom control in dyspeptic patients treated with prokinetics (NNT = 7).21 However, the benefit was predominantly due to cisapride, a drug that was withdrawn from the US market due to adverse effects. There are no clinical trials of metoclopramide or domperidone (not available in the United States) in FD. Nonetheless, the ACG has given a conditional recommendation, based on low-quality evidence, for the use of prokinetics in patients with FD not responding to PPI therapy, H pylori eradication, or TCA therapy.2
Continue to: A shortcoming of the established guidelines
A shortcoming of the established guidelines is that they do not provide guidance as to long-term management of those patients who respond to prescription medications. Our practice has been to continue medications for a minimum of 3 months, then begin a slow taper in order to establish the lowest efficacious dose. Some patients may relapse and require full dosage for a longer period of time.
Adjunctive therapies are worth considering
Complementary and alternative medicines. Products containing ginger, carraway oil, artichoke leaf extract, turmeric, and red pepper are readily available without prescription and have long been used with variable results for dyspepsia.22 The 9-herb combination STW-5 has demonstrated superiority over placebo in a number of studies and has a favorable safety profile.23 The recommended dose is 10 to 20 drops tid. The European manufacturer has recently modified the package insert noting rare cases of hepatotoxicity.24
A commercially available formulation (FDgard) containing L-menthol (a key component of peppermint oil) and caraway has been found to reduce the intensity of symptoms in patients with FD. Potential adverse effects include nausea, contact dermatitis, bronchospasm, and atrial fibrillation. Cayenne, a red pepper extract, is available over the counter for the benefit for epigastric pain and bloating. Begin with a 500-mg dose before breakfast and a 1000-mg dose before dinner, increasing to 2500 mg/d as tolerated. Cayenne preparations may trigger drug toxicities and are best avoided in patients taking antihypertensives, theophylline, or anticoagulants.
Cognitive behavioral therapy, acupuncture, and hypnosis. These modalities are time consuming, are often expensive, are not always covered by insurance, and require significant motivation. A systematic review found no benefit.25 Subsequent studies summarized in the ACG guidelines2 reported benefit; however, a lack of blinding and significant heterogeneity among the groups detract from the quality of the data. It remains unclear whether these are effective strategies for FD, and therefore they cannot be recommended on a routine basis.
CORRESPONDENCE
Norman H. Gilinsky, MD, Division of Digestive Diseases, University of Cincinnati College of Medicine, 231 Albert Sabin Way, Cincinnati, OH 45267-0595; norman.gilinsky@ uc.edu
1. Ford AC, Marwaha A, Sood R, et al. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015;64:1049-1057.
2. Moayyedi P, Lacy BE, Andrews CN, et al. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017;112:988-1013.
3. Ford AC, Marwaha A, Lim A, et al. What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8:830-837.
4. Stanghellini V, Chan FKL, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology. 2016;150:1380-1392.
5. Shaukat A, Wang A, Acosta RD, et al. The role of endoscopy in dyspepsia. Gastrointest Endosc. 2015;82:227-232.
6. Wauters L, Talley NJ, Walker MM, et al. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut. 2020;69:591-600.
7. Weinstock LB, Pace LA, Rezaie A, et al. Mast cell activation syndrome: a primer for the gastroenterologist. Dig Dis Sci. 2021;66:965-982.
8. Drossman DA. Functional gastrointestinal disorders. History, pathophysiology, clinical features and Rome IV. 2016. Accessed August 16, 2021. www.gastrojournal.org/article/S0016-5085(16)00223-7/fulltext
9. Boettcher E, Crowe SE. Dietary proteins and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:728-736.
10. Talley NJ, AGA. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterol. 2005;129:1753-1755.
11. El-Serag HB, Kao JY, Kanwal F, et al. Houston Consensus Conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol. 2018;16:992-1002.
12. Ferwana M, Abdulmajeed I, Alhajiahmed A, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. 2015;21:1305-1314.
13. Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1-3.
14. Chey WD, Leontiadis G, Howden W, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239.
15. Pinto-Sanchez MI, Yuan Y, Hassan A, et al. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017;11:CD011194.
16. National Institute for Health and Care Excellence. Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management. [Clinical guideline] Accessed August 6, 2021. www.ncbi.nlm.nih.gov/books/NBK552570/
17. Duncanson KR, Talley NJ, Walker MM, et al. Food and functional dyspepsia: a systematic review. J Hum Nutr Diet. 2018;31:390-407.
18. Lin JT. Screening of gastric cancer: who, when, and how. Clin Gastroenterol Hepatol. 2014;12:135-138.
19. Spielmans GI, Spence-Sing T, Parry P. Duty to warn: antidepressant black box suicidality warning is empirically justified. Front Psychiatry. 2020;11:1-18.
20. Tack J, Janssen P, Masaoka T, et al. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2012;10:1239-1245.
21. Pittayanon R, Yuan Y, Bollegala NP, et al. Prokinetics for functional dyspepsia: a systemic review and meta-analysis of randomized controlled trials. Am J Gastroenterol. 2019;114:233-243.
22. Deutsch JK, Levitt J, Hass DJ. Complementary and alternative medicine for functional gastrointestinal disorders. Am J Gastroenterol. 2020;115:350-364.
23. Malfertheiner P. STW 5 (iberogast) therapy in gastrointestinal functional disorders. Dig Dis. 2017;35:25-29.
24. Sáez-González E, Conde I, Díaz-Jaime FC, et al. Iberogast-induced severe hepatotoxicity leading to liver transplantation. Am J Gastroenterol. 2016;111:1364-1365.
25. Soo S, Forman D, Delaney B, et al. A systematic review of psychological therapies for nonulcer dyspepsia. Am J Gastroenterol. 2004;99:1817-1822.
1. Ford AC, Marwaha A, Sood R, et al. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015;64:1049-1057.
2. Moayyedi P, Lacy BE, Andrews CN, et al. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017;112:988-1013.
3. Ford AC, Marwaha A, Lim A, et al. What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8:830-837.
4. Stanghellini V, Chan FKL, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology. 2016;150:1380-1392.
5. Shaukat A, Wang A, Acosta RD, et al. The role of endoscopy in dyspepsia. Gastrointest Endosc. 2015;82:227-232.
6. Wauters L, Talley NJ, Walker MM, et al. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut. 2020;69:591-600.
7. Weinstock LB, Pace LA, Rezaie A, et al. Mast cell activation syndrome: a primer for the gastroenterologist. Dig Dis Sci. 2021;66:965-982.
8. Drossman DA. Functional gastrointestinal disorders. History, pathophysiology, clinical features and Rome IV. 2016. Accessed August 16, 2021. www.gastrojournal.org/article/S0016-5085(16)00223-7/fulltext
9. Boettcher E, Crowe SE. Dietary proteins and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:728-736.
10. Talley NJ, AGA. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterol. 2005;129:1753-1755.
11. El-Serag HB, Kao JY, Kanwal F, et al. Houston Consensus Conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol. 2018;16:992-1002.
12. Ferwana M, Abdulmajeed I, Alhajiahmed A, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. 2015;21:1305-1314.
13. Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1-3.
14. Chey WD, Leontiadis G, Howden W, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239.
15. Pinto-Sanchez MI, Yuan Y, Hassan A, et al. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017;11:CD011194.
16. National Institute for Health and Care Excellence. Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management. [Clinical guideline] Accessed August 6, 2021. www.ncbi.nlm.nih.gov/books/NBK552570/
17. Duncanson KR, Talley NJ, Walker MM, et al. Food and functional dyspepsia: a systematic review. J Hum Nutr Diet. 2018;31:390-407.
18. Lin JT. Screening of gastric cancer: who, when, and how. Clin Gastroenterol Hepatol. 2014;12:135-138.
19. Spielmans GI, Spence-Sing T, Parry P. Duty to warn: antidepressant black box suicidality warning is empirically justified. Front Psychiatry. 2020;11:1-18.
20. Tack J, Janssen P, Masaoka T, et al. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2012;10:1239-1245.
21. Pittayanon R, Yuan Y, Bollegala NP, et al. Prokinetics for functional dyspepsia: a systemic review and meta-analysis of randomized controlled trials. Am J Gastroenterol. 2019;114:233-243.
22. Deutsch JK, Levitt J, Hass DJ. Complementary and alternative medicine for functional gastrointestinal disorders. Am J Gastroenterol. 2020;115:350-364.
23. Malfertheiner P. STW 5 (iberogast) therapy in gastrointestinal functional disorders. Dig Dis. 2017;35:25-29.
24. Sáez-González E, Conde I, Díaz-Jaime FC, et al. Iberogast-induced severe hepatotoxicity leading to liver transplantation. Am J Gastroenterol. 2016;111:1364-1365.
25. Soo S, Forman D, Delaney B, et al. A systematic review of psychological therapies for nonulcer dyspepsia. Am J Gastroenterol. 2004;99:1817-1822.
PRACTICE RECOMMENDATIONS
› Test for Helicobacter pylori in patients who are < 60 years of age or who have no alarm symptoms. If results are negative, consider a trial of proton pump inhibitor therapy. C
› Arrange for esophagogastroduodenoscopy in individuals ≥ 60 years of age and all patients with alarm symptoms, to identify or rule out a structural cause. C
› Consider a diagnosis of functional dyspepsia if the work-up is negative. Supportive therapy, including the use of tricyclic antidepressants, prokinetics, and a holistic approach to lifestyle changes in select patients have shown encouraging results. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Strategies to identify and prevent penicillin allergy mislabeling and appropriately de-label patients
In North America and Europe, penicillin allergy is the most common drug-allergy label.1 Carrying a penicillin-allergy label, which has recently gained more attention in health care systems, leads to suboptimal outcomes, increased use of broad-spectrum antibiotics, increased risk of adverse reactions, and increased cost of care.2,3 Despite the high rate of reported reactions, clinically significant immunoglobulin E (IgE)-mediated and T cell–mediated hypersensitivity reactions to penicillins are uncommon.2
Through the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, the American Academy of Allergy, Asthma, and Immunology has issued a recommendation: “Don’t overuse non-beta lactam antibiotics in patients with a history of penicillin allergy without an appropriate evaluation.”4 The primary care physician (PCP) plays a critical role in the appropriate evaluation and accurate initial labeling of penicillin allergy. Furthermore, the PCP plays an integral part, in conjunction with the allergist, in removing the “penicillin allergy” label from a patient’s chart when feasible.
The history of penicillin and prevalence of allergy
History. Penicillin, the first antibiotic, was discovered in 1928 by physician and microbiologist Alexander Fleming when he observed that a mold of the Penicillium genus inhibited growth of gram-positive pathogens.5 Along with pharmacologist Howard Florey and chemist Ernst Chain, both of whom assisted in the large-scale isolation and production of the antibiotic, Fleming won the Nobel Prize in Physiology or Medicine in 1945 for this discovery.5
Antibiotics transformed the practice of medicine across a spectrum, including safer childbirth, surgical procedures, and transplantation.6 Penicillin remains first-line therapy for many infections, such as streptococcal pharyngitis,7 and is the only recommended medication for treating syphilis during pregnancy.8 Continued effectiveness of penicillin in these cases allows broad-spectrum antibiotics to be reserved for more severe infections. Regrettably, incorrect antibiotic allergy labeling poses a significant risk to the patient and health care system.
Epidemiology. As with all medications, the potential for anaphylaxis exists after administration of penicillin. Because its use is widespread, penicillin is the most common cause of drug-induced anaphylaxis. However, the incidence of penicillin-induced anaphylaxis is low9: A 1968 World Health Organization report stated that the rate of penicillin anaphylaxis was between 0.015% and 0.04%.10 A more recent study reported an incidence of 1 in 207,191 patients after an oral dose and 1 in 95,298 after a parenteral dose.11 The most common reactions to penicillins are urticaria and delayed maculopapular rash.8
In the United States, the prevalence of reported penicillin allergy is approximately 10% (estimated range, 8% to 12%)3,12-15; among hospitalized patients, that prevalence is estimated to be as high as 15%.13,15 However, the prevalence of confirmed penicillin allergy is low and has decreased over time—demonstrated in a longitudinal study in which the rate of a positive skin test fell from 15% in 1995 to 0.8% in 2013.16,17
Studies have confirmed that as many as 90% of patients who report penicillin allergy are, in fact, able to tolerate penicillins.14,18-20 This finding might be a consequence of initial mislabeling of penicillin allergy; often, adverse reactions are documented as “allergy” when no risk of anaphylaxis exists. Furthermore, patients can outgrow IgE-mediated penicillin allergy because the presence of penicillin IgE antibodies wanes over time.14,15
Continue to: Consequences of mislabeling
Consequences of mislabeling
Clinical consequences. A multitude of clinical consequences result from carrying a “penicillin allergy” label.
Use of broad-spectrum antibiotics leads to increased risk of Clostridium difficile infection and to development of resistant bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus.2,15
Alternative antibiotics used in the setting of a “penicillin allergy” label might be less efficacious and result in suboptimal outcomes. For example, vancomycin is less effective against methicillin-sensitive S aureus bacteremia than nafcillin or cefazolin.2,21 Beta-lactam antibiotics—in particular, cefazolin—are often first-line for perioperative prophylaxis; patients with reported penicillin allergy often receive a less-optimal alternative, such as clindamycin, vancomycin, or gentamicin.22 These patients are at increased risk of surgical site infection.2,22
In addition, using penicillin alternatives can result in greater risk of drug reactions and adverse effects.2
Increased health care costs. Primarily through observational studies, penicillin allergy has been associated with higher health care costs.23 Patients with reported penicillin allergy had, on average, a longer inpatient stay than patients without penicillin allergy, at a 3-year total estimated additional cost of $64.6 million.24 Inpatients with a listed penicillin allergy had direct drug costs ranging from “no difference” to $609 per patient more than patients without a listed penicillin allergy. Outpatient prescription costs were $14 to $193 higher per patient for patients with a listed penicillin allergy.23
Continue to: Considerations in special populations
Considerations in special populations. Evaluating penicillin allergy during routine care is key to decreasing the necessity for urgent penicillin evaluation and possible desensitization at the time of serious infection. Certain patient populations pose specific challenges:
- Pregnant patients. Unverified penicillin allergy during pregnancy is associated with an increased rate of cesarean section and longer postpartum hospitalization.25 Additionally, group B streptococcus-positive women have increased exposure to alternative antibiotics and an increased incidence of adverse drug reactions.25
- Elderly patients. Drug allergy increases with aging.1 Elderly patients in a long-term care facility are more likely to experience adverse drug effects or drug–drug interactions from the use of penicillin alternatives, such as clindamycin, vancomycin, and fluoroquinolones.2
- Oncology patients often require antibiotic prophylaxis as well as treatment for illnesses, such as neutropenic fever, for which beta-lactam antibiotics are often used as initial treatment.2,26
- Other important populations that present specific challenges include hospitalized patients, pediatric patients, and patients with a sexually transmitted infection.2
Active management of a penicillin-allergy label
Greater recognition of the consequences of penicillin allergy in recent years has led to efforts by hospitals and other health care organizations to develop processes by which patients can be successfully de-labeled as part of antibiotic stewardship programs9 and other initiatives. Ideally, every patient who has a “penicillin allergy” label would be referred to an allergist for evaluation; however, the number of allergy specialists is limited, and access to such specialists might be restricted in some areas, making this approach impracticable. Active management of penicillin allergy requires strategies to both test and de-label patients, as well as proactive approaches to prevent incorrect labeling. These proactive approaches require involvement of all members of the health care team—especially PCPs.
Preventing incorrect labeling. PCPs are the most likely to initially label a patient as allergic to penicillin.27 Most physicians rely on a reported history of allergy alone when selecting medication12; once a patient has been labeled “penicillin allergic,” they often retain that mislabel through adulthood.27,28 A qualitative study of PCPs’ views on prescribing penicillin found that many were aware that documented allergies were incorrect but were uncomfortable using their clinical judgment to prescribe a penicillin or change the record, for fear of a future anaphylactic reaction.29 The first step in the case of any reported reaction should be for you to elicit an accurate drug allergy history (TABLE 1).
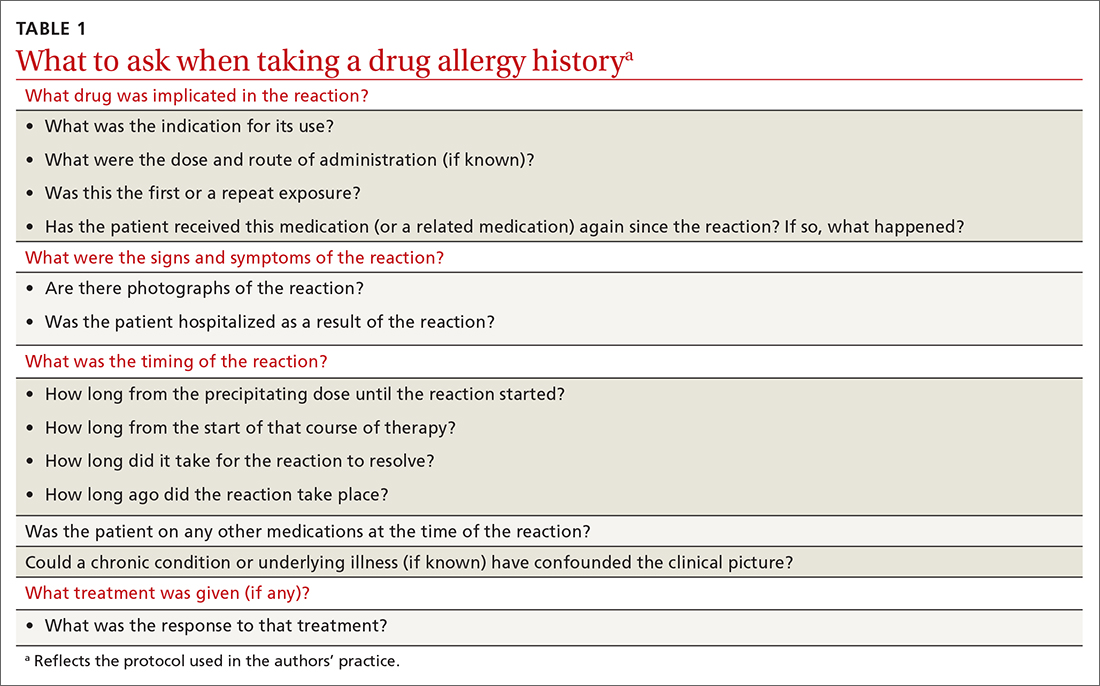
As with other drug reactions, you should consider the context surrounding the reaction to a penicillin. Take care to review signs and symptoms of the reaction to look for clues that make a true allergic reaction more, or less, likely.
Symptoms can generally be divided into low-risk and high-risk categories27 (TABLE 2). An example of a commonly reported low-risk symptom is diarrhea that develops after several doses of a penicillin. In the absence of other symptoms, this finding is most likely due to elimination of normal gut flora,30 not to an allergic reaction to the medication. Symptoms of intolerance to the medication, such as headache and nausea, are also low risk.27,31 In contrast, immediate onset of abdominal pain after a dose of penicillin and lip or throat swelling are considered high risk.
Continue to: Patients presenting with urticaria...
Patients presenting with urticaria or maculopapular rash after taking penicillin are particularly challenging.30 A study of patients in a primary care pediatrics practice found that 7.4% of children receiving a prescription for a penicillin reported a rash.32 Here, timing of onset of symptoms provides some clarity about the likelihood of true allergy. Rashes that manifest during the first hours after exposure are more likely to be IgE mediated, particularly when accompanied by other systemic symptoms; they should be considered high risk. Delayed-onset rashes (> 72 hours after exposure) are usually non-IgE mediated and therefore are generally lower risk,8,30,33 except when associated with certain features, such as mucosal involvement and skin peeling.
Despite acknowledging viral exanthems in the differential, many physicians still label patients presenting with any rash as “allergic.”28 Take care to look for other potential causes of a rash; for example, patients taking amoxicillin who have concurrent Epstein-Barr virus infection frequently develop a maculopapular rash.34 Caubet and colleagues found that 56% of pediatric patients with a history of nonimmediate rash and a negative oral challenge to amoxicillin tested positive for viral infection.28
A family history of penicillin allergy alone should not preclude the use of penicillin.8,27,31 Similarly, if a patient has already received and tolerated a subsequent course of the same penicillin derivative after the initial reaction, the “penicillin allergy” label can be removed. If the reaction history is unknown, refer the patient to an allergist for further evaluation.
Accurate charting is key. With most hospital systems and physician practices now documenting in an electronic health record, there exists the ability to document, in great detail, patients’ reactions to medications. Previous studies have found, however, that such documentation is often done poorly, or not done at all. One such study found that (1) > 20% of patients with a “penicillin allergy” label did not have reaction details listed and (2) when reactions were listed, many were incorrectly labeled as “allergy,” not “intolerance.”35
Many electronic health record systems lump drug allergies, adverse effects, and food and environmental allergies into a single section, leading to a lack of distinction between adverse reactions and true allergy.31 Although many PCPs report that it is easy to change a patient’s allergy label in the record,29 more often, a nurse, resident, or consultant actually documents the reaction.35
Continue to: Documentation at the time of the reaction...
Documentation at the time of the reaction, within the encounter note and the allergy tab, is essential, so that other physicians caring for the patient, in the future, will be knowledgeable about the details of the reaction. Make it your responsibility to accurately document penicillin allergy in patients’ charts, including removing the “penicillin allergy” label from the chart of patients whose history is inconsistent with allergy, who have tolerated subsequent courses of the same penicillin derivative, or who have passed testing in an allergist’s office. In a study of 639 patients who tested negative for penicillin allergy, 51% still had a “penicillin allergy” label in their chart more than 4 years later.36
Penicillin allergy evaluation. When a patient cannot be cleared of a “penicillin allergy” label by history alone, and in the absence of severe features such as mucous membrane involvement, they should be further evaluated through objective testing for potential IgE-mediated allergy. This assessment includes penicillin skin testing or an oral challenge, or both.
Skin testing involves skin-prick testing of major and minor determinants of penicillin; when skin-prick testing is negative, intradermal testing of major and minor determinants should follow. The negative predictive value of penicillin skin testing is high: In a prospective, multicenter investigation, researchers demonstrated that, when both the major penicillin determinant and a minor determinant mixture were used, negative predictive value was 97.9%.37
However, a minor determinant mixture is not commercially available in the United States; therefore, penicillin G is often used alone as the minor determinant. Typically, if a patient passes skin testing, a challenge dose of penicillin or amoxicillin is administered, followed by an observation period. The risk of re-sensitization after oral penicillin is thought to be low and does not preclude future use.38
Although drug testing is most often performed in an allergist’s office, several groups have developed protocols that allow for limited testing of low-risk patients in a primary care setting.8,31 For example, several studies have demonstrated that patients presenting with low-risk skin rash can be safely tested with a supervised oral challenge alone.18,28 The FIGURE8,27,30,31,33,34 outlines our proposed workflow for risk stratification and subsequent management of patients with a “penicillin allergy” label.
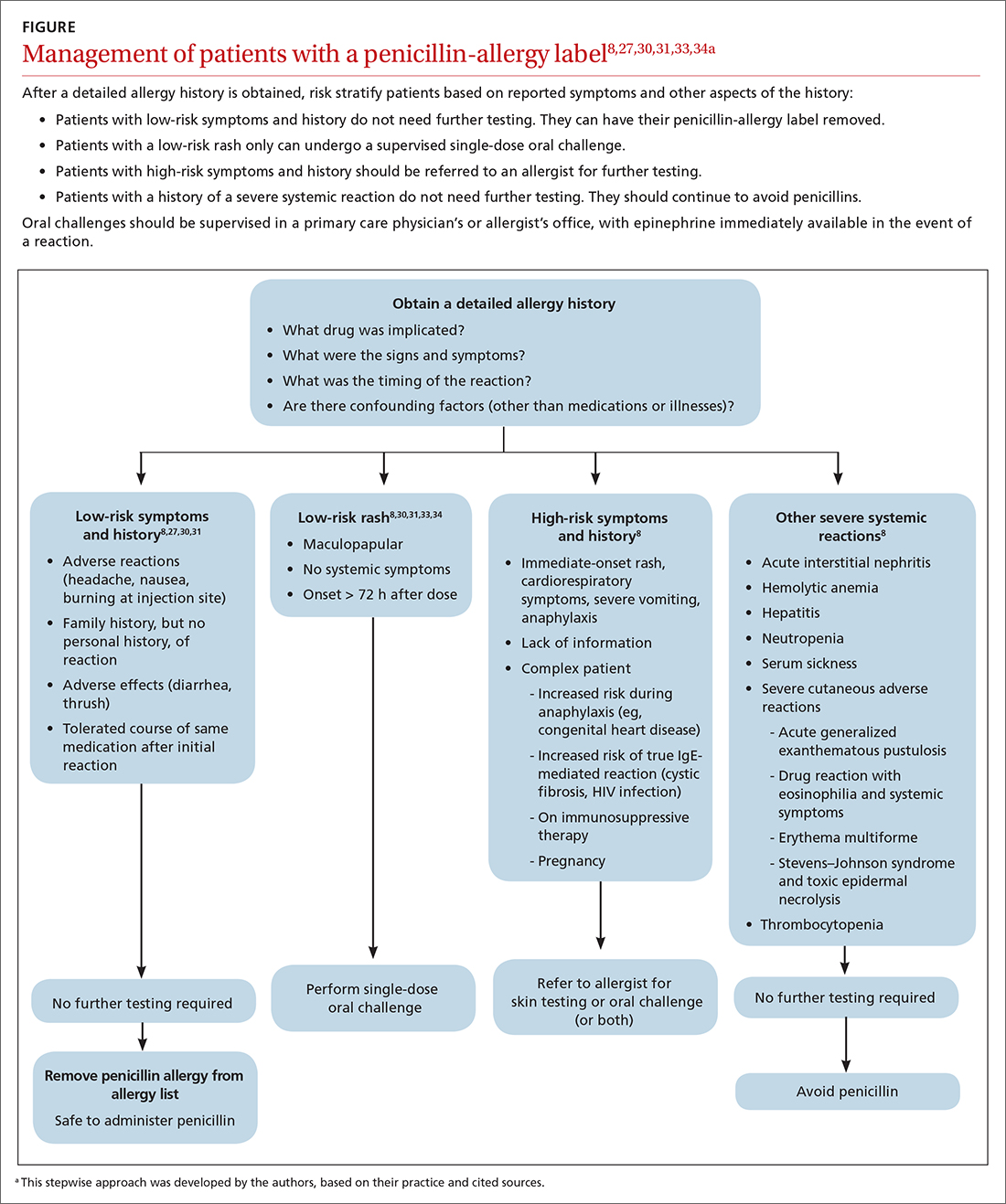
Continue to: De-labeling requires a systems approach
De-labeling requires a systems approach. Given the mismatch between the large number of patients labeled “penicillin allergic” and the few allergy specialists, referral alone is not enough to solve the problem of mislabeling. Targeting specific populations for testing, such as patients presenting to an inner-city sexually transmitted infection clinic19 or preoperative patients, as is done at the Mayo Clinic,9 has been successful. Skin testing in an inpatient setting has also been shown to be safe and effective,13 allowing for protocol-driven testing under the supervision of trained pharmacists (and others), to relieve the burden on allergy specialists.9
CORRESPONDENCE
Andrew Lutzkanin, MD, 500 University Drive, PO Box 850, Hershey, PA 17033; [email protected]
1. Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available? Clin Exp Allergy. 2011;41:1498-1501. doi: 10.1111/j.1365-2222.2011.03837.x
2. Shenoy ES, Macy E, Rowe T, et al. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321:188-199. doi: 10.1001/jama.2018.19283
3. Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6:1019-1027.e2. doi: 10.1016/j.jaip.2017.08.006
4. American Academy of Allergy, Asthma & Immunology: Ten things physicians and patients should question. American Board of Medicine Foundation Choosing Wisely website. 2018. Accessed July 7, 2021. www.choosingwisely.org/doctor-patient-lists/american-academy-of-allergy-asthma-immunology
5. Tan SY, Tatsumura Y. Alexander Fleming (1881-1955): discoverer of penicillin. Singapore Med J. 2015;56:366-367. doi: 10.11622/smedj.2015105
6. Marston HD, Dixon DM, Knisely JM, et al. Antimicrobial resistance. JAMA. 2016;316:1193-1204. doi: 10.1001/jama.2016.11764
7. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;2013:CD000023. doi: 10.1002/14651858.CD000023.pub4
8. Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381:2338-2351. doi: 10.1056/NEJMra1807761
9. Khan DA. Proactive management of penicillin and other antibiotic allergies. Allergy Asthma Proc. 2020;41:82-89. doi: 10.2500/aap.2020.41.190024
10. Idsoe O, Guthe T, Willcox RR, et al. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159-188.
11. Chiriac AM, Macy E. Large health system databases and drug hypersensitivity. J Allergy Clin Immunol Pract. 2019;7:2125-2131. doi: 10.1016/j.jaip.2019.04.014
12. Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35:489-494. doi: 10.2500/aap.2014.35.3791
13. Sacco KA, Bates A, Brigham TJ, et al. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72:1288-1296. doi: 10.1111/all.13168
14. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S126-S137. doi: 10.1016/j.jaci.2009.10.028
15. Blumenthal KG, Shenoy ES, Varughese CA, et al. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol. 2015;115:294-300.e2. doi: 10.1016/j.anai.2015.05.011
16. Macy E, Schatz M, Lin C, et al. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13:12-18. doi: 10.7812/tpp/08-073
17. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1:258-263. doi: 10.1016/j.jaip.2013.02.002
18. Bourke J, Pavlos R, James I, et al. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol Pract. 2015;3:365-374.e1. doi: 10.1016/j.jaip.2014.11.002
19. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456-2463.
20. Klaustermeyer WB, Gowda VC. Penicillin skin testing: a 20-year study at the West Los Angeles Veterans Affairs Medical Center. Mil Med. 2005;170:701-704. doi: 10.7205/milmed.170.8.701.
21. McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61:361-367. doi: 10.1093/cid/civ308
22. Blumenthal KG, Ryan EE, Li Y, et al. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66:329-336. doi: 10.1093/cid/cix794
23. Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6:1649-1654.e4. doi: 10.1016/j.jaip.2017.12.033
24. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133:790-796. doi: 10.1016/j.jaci.2013.09.021
25. Desai SH, Kaplan MS, Chen Q, et al. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B streptococcus infections. Perm J. 2017;21:16-80. doi: 10.7812/TPP/16-080
26. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-e93. doi: 10.1093/cid/cir073
27. Vyles D, Mistry RD, Heffner V, et al. Reported knowledge and management of potential penicillin allergy in children. Acad Pediatr. 2019;19:684-690. doi: 10.1016/j.acap.2019.01.002
28. Caubet J-C, Kaiser L, Lemaître B, et al. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218-222. doi: 10.1016/j.jaci.2010.08.025
29. Wanat M, Anthierens S, Butler CC, et al. Patient and primary care physician perceptions of penicillin allergy testing and subsequent use of penicillin-containing antibiotics: a qualitative study. J Allergy Clin Immunol Pract. 2019;7:1888-1893.e1. doi: 10.1016/j.jaip.2019.02.036
30. Norton AE, Konvinse K, Phillips EJ, et al. Antibiotic allergy in pediatrics. Pediatrics. 2018;141: e20172497. doi: 10.1542/peds.2017-2497
31. Collins C. The low risks and high rewards of penicillin allergy delabeling: an algorithm to expedite the evaluation. J Pediatr. 2019;212:216-223. doi: 10.1016/j.jpeds.2019.05.060
32. Ibia EO, Schwartz RH, Wiedermann BL. Antibiotic rashes in children: a survey in a private practice setting. Arch Dermatol. 2000;136:849-854. doi: 10.1001/archderm.136.7.849
33. Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505. doi: 10.1001/jama.285.19.2498
34. Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics. 1967;40:910-911.
35. Inglis JM, Caughey GE, Smith W, et al. Documentation of penicillin adverse drug reactions in electronic health records: inconsistent use of allergy and intolerance labels. Intern Med J. 2017;47:1292-1297. doi: 10.1111/imj.13558
36. Lachover-Roth I, Sharon S, Rosman Y, et al. Long-term follow-up after penicillin allergy delabeling in ambulatory patients. J Allergy Clin Immunol Pract. 2019;7:231-235.e1. doi: 10.1016/j.jaip.2018.04.042
37. Solensky R, Jacobs J, Lester M, et al. Penicillin allergy evaluation: a prospective, multicenter, open-label evaluation of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract. 2019;7:1876-1885.e3. doi: 10.1016/j.jaip.2019.02.040
38. A; ; . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273. doi: 10.1016/j.anai.2010.08.002
In North America and Europe, penicillin allergy is the most common drug-allergy label.1 Carrying a penicillin-allergy label, which has recently gained more attention in health care systems, leads to suboptimal outcomes, increased use of broad-spectrum antibiotics, increased risk of adverse reactions, and increased cost of care.2,3 Despite the high rate of reported reactions, clinically significant immunoglobulin E (IgE)-mediated and T cell–mediated hypersensitivity reactions to penicillins are uncommon.2
Through the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, the American Academy of Allergy, Asthma, and Immunology has issued a recommendation: “Don’t overuse non-beta lactam antibiotics in patients with a history of penicillin allergy without an appropriate evaluation.”4 The primary care physician (PCP) plays a critical role in the appropriate evaluation and accurate initial labeling of penicillin allergy. Furthermore, the PCP plays an integral part, in conjunction with the allergist, in removing the “penicillin allergy” label from a patient’s chart when feasible.

The history of penicillin and prevalence of allergy
History. Penicillin, the first antibiotic, was discovered in 1928 by physician and microbiologist Alexander Fleming when he observed that a mold of the Penicillium genus inhibited growth of gram-positive pathogens.5 Along with pharmacologist Howard Florey and chemist Ernst Chain, both of whom assisted in the large-scale isolation and production of the antibiotic, Fleming won the Nobel Prize in Physiology or Medicine in 1945 for this discovery.5
Antibiotics transformed the practice of medicine across a spectrum, including safer childbirth, surgical procedures, and transplantation.6 Penicillin remains first-line therapy for many infections, such as streptococcal pharyngitis,7 and is the only recommended medication for treating syphilis during pregnancy.8 Continued effectiveness of penicillin in these cases allows broad-spectrum antibiotics to be reserved for more severe infections. Regrettably, incorrect antibiotic allergy labeling poses a significant risk to the patient and health care system.
Epidemiology. As with all medications, the potential for anaphylaxis exists after administration of penicillin. Because its use is widespread, penicillin is the most common cause of drug-induced anaphylaxis. However, the incidence of penicillin-induced anaphylaxis is low9: A 1968 World Health Organization report stated that the rate of penicillin anaphylaxis was between 0.015% and 0.04%.10 A more recent study reported an incidence of 1 in 207,191 patients after an oral dose and 1 in 95,298 after a parenteral dose.11 The most common reactions to penicillins are urticaria and delayed maculopapular rash.8
In the United States, the prevalence of reported penicillin allergy is approximately 10% (estimated range, 8% to 12%)3,12-15; among hospitalized patients, that prevalence is estimated to be as high as 15%.13,15 However, the prevalence of confirmed penicillin allergy is low and has decreased over time—demonstrated in a longitudinal study in which the rate of a positive skin test fell from 15% in 1995 to 0.8% in 2013.16,17
Studies have confirmed that as many as 90% of patients who report penicillin allergy are, in fact, able to tolerate penicillins.14,18-20 This finding might be a consequence of initial mislabeling of penicillin allergy; often, adverse reactions are documented as “allergy” when no risk of anaphylaxis exists. Furthermore, patients can outgrow IgE-mediated penicillin allergy because the presence of penicillin IgE antibodies wanes over time.14,15
Continue to: Consequences of mislabeling
Consequences of mislabeling
Clinical consequences. A multitude of clinical consequences result from carrying a “penicillin allergy” label.
Use of broad-spectrum antibiotics leads to increased risk of Clostridium difficile infection and to development of resistant bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus.2,15
Alternative antibiotics used in the setting of a “penicillin allergy” label might be less efficacious and result in suboptimal outcomes. For example, vancomycin is less effective against methicillin-sensitive S aureus bacteremia than nafcillin or cefazolin.2,21 Beta-lactam antibiotics—in particular, cefazolin—are often first-line for perioperative prophylaxis; patients with reported penicillin allergy often receive a less-optimal alternative, such as clindamycin, vancomycin, or gentamicin.22 These patients are at increased risk of surgical site infection.2,22
In addition, using penicillin alternatives can result in greater risk of drug reactions and adverse effects.2
Increased health care costs. Primarily through observational studies, penicillin allergy has been associated with higher health care costs.23 Patients with reported penicillin allergy had, on average, a longer inpatient stay than patients without penicillin allergy, at a 3-year total estimated additional cost of $64.6 million.24 Inpatients with a listed penicillin allergy had direct drug costs ranging from “no difference” to $609 per patient more than patients without a listed penicillin allergy. Outpatient prescription costs were $14 to $193 higher per patient for patients with a listed penicillin allergy.23
Continue to: Considerations in special populations
Considerations in special populations. Evaluating penicillin allergy during routine care is key to decreasing the necessity for urgent penicillin evaluation and possible desensitization at the time of serious infection. Certain patient populations pose specific challenges:
- Pregnant patients. Unverified penicillin allergy during pregnancy is associated with an increased rate of cesarean section and longer postpartum hospitalization.25 Additionally, group B streptococcus-positive women have increased exposure to alternative antibiotics and an increased incidence of adverse drug reactions.25
- Elderly patients. Drug allergy increases with aging.1 Elderly patients in a long-term care facility are more likely to experience adverse drug effects or drug–drug interactions from the use of penicillin alternatives, such as clindamycin, vancomycin, and fluoroquinolones.2
- Oncology patients often require antibiotic prophylaxis as well as treatment for illnesses, such as neutropenic fever, for which beta-lactam antibiotics are often used as initial treatment.2,26
- Other important populations that present specific challenges include hospitalized patients, pediatric patients, and patients with a sexually transmitted infection.2
Active management of a penicillin-allergy label
Greater recognition of the consequences of penicillin allergy in recent years has led to efforts by hospitals and other health care organizations to develop processes by which patients can be successfully de-labeled as part of antibiotic stewardship programs9 and other initiatives. Ideally, every patient who has a “penicillin allergy” label would be referred to an allergist for evaluation; however, the number of allergy specialists is limited, and access to such specialists might be restricted in some areas, making this approach impracticable. Active management of penicillin allergy requires strategies to both test and de-label patients, as well as proactive approaches to prevent incorrect labeling. These proactive approaches require involvement of all members of the health care team—especially PCPs.
Preventing incorrect labeling. PCPs are the most likely to initially label a patient as allergic to penicillin.27 Most physicians rely on a reported history of allergy alone when selecting medication12; once a patient has been labeled “penicillin allergic,” they often retain that mislabel through adulthood.27,28 A qualitative study of PCPs’ views on prescribing penicillin found that many were aware that documented allergies were incorrect but were uncomfortable using their clinical judgment to prescribe a penicillin or change the record, for fear of a future anaphylactic reaction.29 The first step in the case of any reported reaction should be for you to elicit an accurate drug allergy history (TABLE 1).

As with other drug reactions, you should consider the context surrounding the reaction to a penicillin. Take care to review signs and symptoms of the reaction to look for clues that make a true allergic reaction more, or less, likely.
Symptoms can generally be divided into low-risk and high-risk categories27 (TABLE 2). An example of a commonly reported low-risk symptom is diarrhea that develops after several doses of a penicillin. In the absence of other symptoms, this finding is most likely due to elimination of normal gut flora,30 not to an allergic reaction to the medication. Symptoms of intolerance to the medication, such as headache and nausea, are also low risk.27,31 In contrast, immediate onset of abdominal pain after a dose of penicillin and lip or throat swelling are considered high risk.

Continue to: Patients presenting with urticaria...
Patients presenting with urticaria or maculopapular rash after taking penicillin are particularly challenging.30 A study of patients in a primary care pediatrics practice found that 7.4% of children receiving a prescription for a penicillin reported a rash.32 Here, timing of onset of symptoms provides some clarity about the likelihood of true allergy. Rashes that manifest during the first hours after exposure are more likely to be IgE mediated, particularly when accompanied by other systemic symptoms; they should be considered high risk. Delayed-onset rashes (> 72 hours after exposure) are usually non-IgE mediated and therefore are generally lower risk,8,30,33 except when associated with certain features, such as mucosal involvement and skin peeling.
Despite acknowledging viral exanthems in the differential, many physicians still label patients presenting with any rash as “allergic.”28 Take care to look for other potential causes of a rash; for example, patients taking amoxicillin who have concurrent Epstein-Barr virus infection frequently develop a maculopapular rash.34 Caubet and colleagues found that 56% of pediatric patients with a history of nonimmediate rash and a negative oral challenge to amoxicillin tested positive for viral infection.28
A family history of penicillin allergy alone should not preclude the use of penicillin.8,27,31 Similarly, if a patient has already received and tolerated a subsequent course of the same penicillin derivative after the initial reaction, the “penicillin allergy” label can be removed. If the reaction history is unknown, refer the patient to an allergist for further evaluation.
Accurate charting is key. With most hospital systems and physician practices now documenting in an electronic health record, there exists the ability to document, in great detail, patients’ reactions to medications. Previous studies have found, however, that such documentation is often done poorly, or not done at all. One such study found that (1) > 20% of patients with a “penicillin allergy” label did not have reaction details listed and (2) when reactions were listed, many were incorrectly labeled as “allergy,” not “intolerance.”35
Many electronic health record systems lump drug allergies, adverse effects, and food and environmental allergies into a single section, leading to a lack of distinction between adverse reactions and true allergy.31 Although many PCPs report that it is easy to change a patient’s allergy label in the record,29 more often, a nurse, resident, or consultant actually documents the reaction.35
Continue to: Documentation at the time of the reaction...
Documentation at the time of the reaction, within the encounter note and the allergy tab, is essential, so that other physicians caring for the patient, in the future, will be knowledgeable about the details of the reaction. Make it your responsibility to accurately document penicillin allergy in patients’ charts, including removing the “penicillin allergy” label from the chart of patients whose history is inconsistent with allergy, who have tolerated subsequent courses of the same penicillin derivative, or who have passed testing in an allergist’s office. In a study of 639 patients who tested negative for penicillin allergy, 51% still had a “penicillin allergy” label in their chart more than 4 years later.36
Penicillin allergy evaluation. When a patient cannot be cleared of a “penicillin allergy” label by history alone, and in the absence of severe features such as mucous membrane involvement, they should be further evaluated through objective testing for potential IgE-mediated allergy. This assessment includes penicillin skin testing or an oral challenge, or both.
Skin testing involves skin-prick testing of major and minor determinants of penicillin; when skin-prick testing is negative, intradermal testing of major and minor determinants should follow. The negative predictive value of penicillin skin testing is high: In a prospective, multicenter investigation, researchers demonstrated that, when both the major penicillin determinant and a minor determinant mixture were used, negative predictive value was 97.9%.37
However, a minor determinant mixture is not commercially available in the United States; therefore, penicillin G is often used alone as the minor determinant. Typically, if a patient passes skin testing, a challenge dose of penicillin or amoxicillin is administered, followed by an observation period. The risk of re-sensitization after oral penicillin is thought to be low and does not preclude future use.38
Although drug testing is most often performed in an allergist’s office, several groups have developed protocols that allow for limited testing of low-risk patients in a primary care setting.8,31 For example, several studies have demonstrated that patients presenting with low-risk skin rash can be safely tested with a supervised oral challenge alone.18,28 The FIGURE8,27,30,31,33,34 outlines our proposed workflow for risk stratification and subsequent management of patients with a “penicillin allergy” label.

Continue to: De-labeling requires a systems approach
De-labeling requires a systems approach. Given the mismatch between the large number of patients labeled “penicillin allergic” and the few allergy specialists, referral alone is not enough to solve the problem of mislabeling. Targeting specific populations for testing, such as patients presenting to an inner-city sexually transmitted infection clinic19 or preoperative patients, as is done at the Mayo Clinic,9 has been successful. Skin testing in an inpatient setting has also been shown to be safe and effective,13 allowing for protocol-driven testing under the supervision of trained pharmacists (and others), to relieve the burden on allergy specialists.9
CORRESPONDENCE
Andrew Lutzkanin, MD, 500 University Drive, PO Box 850, Hershey, PA 17033; [email protected]
In North America and Europe, penicillin allergy is the most common drug-allergy label.1 Carrying a penicillin-allergy label, which has recently gained more attention in health care systems, leads to suboptimal outcomes, increased use of broad-spectrum antibiotics, increased risk of adverse reactions, and increased cost of care.2,3 Despite the high rate of reported reactions, clinically significant immunoglobulin E (IgE)-mediated and T cell–mediated hypersensitivity reactions to penicillins are uncommon.2
Through the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, the American Academy of Allergy, Asthma, and Immunology has issued a recommendation: “Don’t overuse non-beta lactam antibiotics in patients with a history of penicillin allergy without an appropriate evaluation.”4 The primary care physician (PCP) plays a critical role in the appropriate evaluation and accurate initial labeling of penicillin allergy. Furthermore, the PCP plays an integral part, in conjunction with the allergist, in removing the “penicillin allergy” label from a patient’s chart when feasible.

The history of penicillin and prevalence of allergy
History. Penicillin, the first antibiotic, was discovered in 1928 by physician and microbiologist Alexander Fleming when he observed that a mold of the Penicillium genus inhibited growth of gram-positive pathogens.5 Along with pharmacologist Howard Florey and chemist Ernst Chain, both of whom assisted in the large-scale isolation and production of the antibiotic, Fleming won the Nobel Prize in Physiology or Medicine in 1945 for this discovery.5
Antibiotics transformed the practice of medicine across a spectrum, including safer childbirth, surgical procedures, and transplantation.6 Penicillin remains first-line therapy for many infections, such as streptococcal pharyngitis,7 and is the only recommended medication for treating syphilis during pregnancy.8 Continued effectiveness of penicillin in these cases allows broad-spectrum antibiotics to be reserved for more severe infections. Regrettably, incorrect antibiotic allergy labeling poses a significant risk to the patient and health care system.
Epidemiology. As with all medications, the potential for anaphylaxis exists after administration of penicillin. Because its use is widespread, penicillin is the most common cause of drug-induced anaphylaxis. However, the incidence of penicillin-induced anaphylaxis is low9: A 1968 World Health Organization report stated that the rate of penicillin anaphylaxis was between 0.015% and 0.04%.10 A more recent study reported an incidence of 1 in 207,191 patients after an oral dose and 1 in 95,298 after a parenteral dose.11 The most common reactions to penicillins are urticaria and delayed maculopapular rash.8
In the United States, the prevalence of reported penicillin allergy is approximately 10% (estimated range, 8% to 12%)3,12-15; among hospitalized patients, that prevalence is estimated to be as high as 15%.13,15 However, the prevalence of confirmed penicillin allergy is low and has decreased over time—demonstrated in a longitudinal study in which the rate of a positive skin test fell from 15% in 1995 to 0.8% in 2013.16,17
Studies have confirmed that as many as 90% of patients who report penicillin allergy are, in fact, able to tolerate penicillins.14,18-20 This finding might be a consequence of initial mislabeling of penicillin allergy; often, adverse reactions are documented as “allergy” when no risk of anaphylaxis exists. Furthermore, patients can outgrow IgE-mediated penicillin allergy because the presence of penicillin IgE antibodies wanes over time.14,15
Continue to: Consequences of mislabeling
Consequences of mislabeling
Clinical consequences. A multitude of clinical consequences result from carrying a “penicillin allergy” label.
Use of broad-spectrum antibiotics leads to increased risk of Clostridium difficile infection and to development of resistant bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus.2,15
Alternative antibiotics used in the setting of a “penicillin allergy” label might be less efficacious and result in suboptimal outcomes. For example, vancomycin is less effective against methicillin-sensitive S aureus bacteremia than nafcillin or cefazolin.2,21 Beta-lactam antibiotics—in particular, cefazolin—are often first-line for perioperative prophylaxis; patients with reported penicillin allergy often receive a less-optimal alternative, such as clindamycin, vancomycin, or gentamicin.22 These patients are at increased risk of surgical site infection.2,22
In addition, using penicillin alternatives can result in greater risk of drug reactions and adverse effects.2
Increased health care costs. Primarily through observational studies, penicillin allergy has been associated with higher health care costs.23 Patients with reported penicillin allergy had, on average, a longer inpatient stay than patients without penicillin allergy, at a 3-year total estimated additional cost of $64.6 million.24 Inpatients with a listed penicillin allergy had direct drug costs ranging from “no difference” to $609 per patient more than patients without a listed penicillin allergy. Outpatient prescription costs were $14 to $193 higher per patient for patients with a listed penicillin allergy.23
Continue to: Considerations in special populations
Considerations in special populations. Evaluating penicillin allergy during routine care is key to decreasing the necessity for urgent penicillin evaluation and possible desensitization at the time of serious infection. Certain patient populations pose specific challenges:
- Pregnant patients. Unverified penicillin allergy during pregnancy is associated with an increased rate of cesarean section and longer postpartum hospitalization.25 Additionally, group B streptococcus-positive women have increased exposure to alternative antibiotics and an increased incidence of adverse drug reactions.25
- Elderly patients. Drug allergy increases with aging.1 Elderly patients in a long-term care facility are more likely to experience adverse drug effects or drug–drug interactions from the use of penicillin alternatives, such as clindamycin, vancomycin, and fluoroquinolones.2
- Oncology patients often require antibiotic prophylaxis as well as treatment for illnesses, such as neutropenic fever, for which beta-lactam antibiotics are often used as initial treatment.2,26
- Other important populations that present specific challenges include hospitalized patients, pediatric patients, and patients with a sexually transmitted infection.2
Active management of a penicillin-allergy label
Greater recognition of the consequences of penicillin allergy in recent years has led to efforts by hospitals and other health care organizations to develop processes by which patients can be successfully de-labeled as part of antibiotic stewardship programs9 and other initiatives. Ideally, every patient who has a “penicillin allergy” label would be referred to an allergist for evaluation; however, the number of allergy specialists is limited, and access to such specialists might be restricted in some areas, making this approach impracticable. Active management of penicillin allergy requires strategies to both test and de-label patients, as well as proactive approaches to prevent incorrect labeling. These proactive approaches require involvement of all members of the health care team—especially PCPs.
Preventing incorrect labeling. PCPs are the most likely to initially label a patient as allergic to penicillin.27 Most physicians rely on a reported history of allergy alone when selecting medication12; once a patient has been labeled “penicillin allergic,” they often retain that mislabel through adulthood.27,28 A qualitative study of PCPs’ views on prescribing penicillin found that many were aware that documented allergies were incorrect but were uncomfortable using their clinical judgment to prescribe a penicillin or change the record, for fear of a future anaphylactic reaction.29 The first step in the case of any reported reaction should be for you to elicit an accurate drug allergy history (TABLE 1).

As with other drug reactions, you should consider the context surrounding the reaction to a penicillin. Take care to review signs and symptoms of the reaction to look for clues that make a true allergic reaction more, or less, likely.
Symptoms can generally be divided into low-risk and high-risk categories27 (TABLE 2). An example of a commonly reported low-risk symptom is diarrhea that develops after several doses of a penicillin. In the absence of other symptoms, this finding is most likely due to elimination of normal gut flora,30 not to an allergic reaction to the medication. Symptoms of intolerance to the medication, such as headache and nausea, are also low risk.27,31 In contrast, immediate onset of abdominal pain after a dose of penicillin and lip or throat swelling are considered high risk.

Continue to: Patients presenting with urticaria...
Patients presenting with urticaria or maculopapular rash after taking penicillin are particularly challenging.30 A study of patients in a primary care pediatrics practice found that 7.4% of children receiving a prescription for a penicillin reported a rash.32 Here, timing of onset of symptoms provides some clarity about the likelihood of true allergy. Rashes that manifest during the first hours after exposure are more likely to be IgE mediated, particularly when accompanied by other systemic symptoms; they should be considered high risk. Delayed-onset rashes (> 72 hours after exposure) are usually non-IgE mediated and therefore are generally lower risk,8,30,33 except when associated with certain features, such as mucosal involvement and skin peeling.
Despite acknowledging viral exanthems in the differential, many physicians still label patients presenting with any rash as “allergic.”28 Take care to look for other potential causes of a rash; for example, patients taking amoxicillin who have concurrent Epstein-Barr virus infection frequently develop a maculopapular rash.34 Caubet and colleagues found that 56% of pediatric patients with a history of nonimmediate rash and a negative oral challenge to amoxicillin tested positive for viral infection.28
A family history of penicillin allergy alone should not preclude the use of penicillin.8,27,31 Similarly, if a patient has already received and tolerated a subsequent course of the same penicillin derivative after the initial reaction, the “penicillin allergy” label can be removed. If the reaction history is unknown, refer the patient to an allergist for further evaluation.
Accurate charting is key. With most hospital systems and physician practices now documenting in an electronic health record, there exists the ability to document, in great detail, patients’ reactions to medications. Previous studies have found, however, that such documentation is often done poorly, or not done at all. One such study found that (1) > 20% of patients with a “penicillin allergy” label did not have reaction details listed and (2) when reactions were listed, many were incorrectly labeled as “allergy,” not “intolerance.”35
Many electronic health record systems lump drug allergies, adverse effects, and food and environmental allergies into a single section, leading to a lack of distinction between adverse reactions and true allergy.31 Although many PCPs report that it is easy to change a patient’s allergy label in the record,29 more often, a nurse, resident, or consultant actually documents the reaction.35
Continue to: Documentation at the time of the reaction...
Documentation at the time of the reaction, within the encounter note and the allergy tab, is essential, so that other physicians caring for the patient, in the future, will be knowledgeable about the details of the reaction. Make it your responsibility to accurately document penicillin allergy in patients’ charts, including removing the “penicillin allergy” label from the chart of patients whose history is inconsistent with allergy, who have tolerated subsequent courses of the same penicillin derivative, or who have passed testing in an allergist’s office. In a study of 639 patients who tested negative for penicillin allergy, 51% still had a “penicillin allergy” label in their chart more than 4 years later.36
Penicillin allergy evaluation. When a patient cannot be cleared of a “penicillin allergy” label by history alone, and in the absence of severe features such as mucous membrane involvement, they should be further evaluated through objective testing for potential IgE-mediated allergy. This assessment includes penicillin skin testing or an oral challenge, or both.
Skin testing involves skin-prick testing of major and minor determinants of penicillin; when skin-prick testing is negative, intradermal testing of major and minor determinants should follow. The negative predictive value of penicillin skin testing is high: In a prospective, multicenter investigation, researchers demonstrated that, when both the major penicillin determinant and a minor determinant mixture were used, negative predictive value was 97.9%.37
However, a minor determinant mixture is not commercially available in the United States; therefore, penicillin G is often used alone as the minor determinant. Typically, if a patient passes skin testing, a challenge dose of penicillin or amoxicillin is administered, followed by an observation period. The risk of re-sensitization after oral penicillin is thought to be low and does not preclude future use.38
Although drug testing is most often performed in an allergist’s office, several groups have developed protocols that allow for limited testing of low-risk patients in a primary care setting.8,31 For example, several studies have demonstrated that patients presenting with low-risk skin rash can be safely tested with a supervised oral challenge alone.18,28 The FIGURE8,27,30,31,33,34 outlines our proposed workflow for risk stratification and subsequent management of patients with a “penicillin allergy” label.

Continue to: De-labeling requires a systems approach
De-labeling requires a systems approach. Given the mismatch between the large number of patients labeled “penicillin allergic” and the few allergy specialists, referral alone is not enough to solve the problem of mislabeling. Targeting specific populations for testing, such as patients presenting to an inner-city sexually transmitted infection clinic19 or preoperative patients, as is done at the Mayo Clinic,9 has been successful. Skin testing in an inpatient setting has also been shown to be safe and effective,13 allowing for protocol-driven testing under the supervision of trained pharmacists (and others), to relieve the burden on allergy specialists.9
CORRESPONDENCE
Andrew Lutzkanin, MD, 500 University Drive, PO Box 850, Hershey, PA 17033; [email protected]
1. Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available? Clin Exp Allergy. 2011;41:1498-1501. doi: 10.1111/j.1365-2222.2011.03837.x
2. Shenoy ES, Macy E, Rowe T, et al. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321:188-199. doi: 10.1001/jama.2018.19283
3. Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6:1019-1027.e2. doi: 10.1016/j.jaip.2017.08.006
4. American Academy of Allergy, Asthma & Immunology: Ten things physicians and patients should question. American Board of Medicine Foundation Choosing Wisely website. 2018. Accessed July 7, 2021. www.choosingwisely.org/doctor-patient-lists/american-academy-of-allergy-asthma-immunology
5. Tan SY, Tatsumura Y. Alexander Fleming (1881-1955): discoverer of penicillin. Singapore Med J. 2015;56:366-367. doi: 10.11622/smedj.2015105
6. Marston HD, Dixon DM, Knisely JM, et al. Antimicrobial resistance. JAMA. 2016;316:1193-1204. doi: 10.1001/jama.2016.11764
7. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;2013:CD000023. doi: 10.1002/14651858.CD000023.pub4
8. Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381:2338-2351. doi: 10.1056/NEJMra1807761
9. Khan DA. Proactive management of penicillin and other antibiotic allergies. Allergy Asthma Proc. 2020;41:82-89. doi: 10.2500/aap.2020.41.190024
10. Idsoe O, Guthe T, Willcox RR, et al. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159-188.
11. Chiriac AM, Macy E. Large health system databases and drug hypersensitivity. J Allergy Clin Immunol Pract. 2019;7:2125-2131. doi: 10.1016/j.jaip.2019.04.014
12. Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35:489-494. doi: 10.2500/aap.2014.35.3791
13. Sacco KA, Bates A, Brigham TJ, et al. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72:1288-1296. doi: 10.1111/all.13168
14. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S126-S137. doi: 10.1016/j.jaci.2009.10.028
15. Blumenthal KG, Shenoy ES, Varughese CA, et al. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol. 2015;115:294-300.e2. doi: 10.1016/j.anai.2015.05.011
16. Macy E, Schatz M, Lin C, et al. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13:12-18. doi: 10.7812/tpp/08-073
17. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1:258-263. doi: 10.1016/j.jaip.2013.02.002
18. Bourke J, Pavlos R, James I, et al. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol Pract. 2015;3:365-374.e1. doi: 10.1016/j.jaip.2014.11.002
19. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456-2463.
20. Klaustermeyer WB, Gowda VC. Penicillin skin testing: a 20-year study at the West Los Angeles Veterans Affairs Medical Center. Mil Med. 2005;170:701-704. doi: 10.7205/milmed.170.8.701.
21. McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61:361-367. doi: 10.1093/cid/civ308
22. Blumenthal KG, Ryan EE, Li Y, et al. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66:329-336. doi: 10.1093/cid/cix794
23. Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6:1649-1654.e4. doi: 10.1016/j.jaip.2017.12.033
24. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133:790-796. doi: 10.1016/j.jaci.2013.09.021
25. Desai SH, Kaplan MS, Chen Q, et al. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B streptococcus infections. Perm J. 2017;21:16-80. doi: 10.7812/TPP/16-080
26. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-e93. doi: 10.1093/cid/cir073
27. Vyles D, Mistry RD, Heffner V, et al. Reported knowledge and management of potential penicillin allergy in children. Acad Pediatr. 2019;19:684-690. doi: 10.1016/j.acap.2019.01.002
28. Caubet J-C, Kaiser L, Lemaître B, et al. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218-222. doi: 10.1016/j.jaci.2010.08.025
29. Wanat M, Anthierens S, Butler CC, et al. Patient and primary care physician perceptions of penicillin allergy testing and subsequent use of penicillin-containing antibiotics: a qualitative study. J Allergy Clin Immunol Pract. 2019;7:1888-1893.e1. doi: 10.1016/j.jaip.2019.02.036
30. Norton AE, Konvinse K, Phillips EJ, et al. Antibiotic allergy in pediatrics. Pediatrics. 2018;141: e20172497. doi: 10.1542/peds.2017-2497
31. Collins C. The low risks and high rewards of penicillin allergy delabeling: an algorithm to expedite the evaluation. J Pediatr. 2019;212:216-223. doi: 10.1016/j.jpeds.2019.05.060
32. Ibia EO, Schwartz RH, Wiedermann BL. Antibiotic rashes in children: a survey in a private practice setting. Arch Dermatol. 2000;136:849-854. doi: 10.1001/archderm.136.7.849
33. Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505. doi: 10.1001/jama.285.19.2498
34. Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics. 1967;40:910-911.
35. Inglis JM, Caughey GE, Smith W, et al. Documentation of penicillin adverse drug reactions in electronic health records: inconsistent use of allergy and intolerance labels. Intern Med J. 2017;47:1292-1297. doi: 10.1111/imj.13558
36. Lachover-Roth I, Sharon S, Rosman Y, et al. Long-term follow-up after penicillin allergy delabeling in ambulatory patients. J Allergy Clin Immunol Pract. 2019;7:231-235.e1. doi: 10.1016/j.jaip.2018.04.042
37. Solensky R, Jacobs J, Lester M, et al. Penicillin allergy evaluation: a prospective, multicenter, open-label evaluation of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract. 2019;7:1876-1885.e3. doi: 10.1016/j.jaip.2019.02.040
38. A; ; . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273. doi: 10.1016/j.anai.2010.08.002
1. Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available? Clin Exp Allergy. 2011;41:1498-1501. doi: 10.1111/j.1365-2222.2011.03837.x
2. Shenoy ES, Macy E, Rowe T, et al. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321:188-199. doi: 10.1001/jama.2018.19283
3. Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6:1019-1027.e2. doi: 10.1016/j.jaip.2017.08.006
4. American Academy of Allergy, Asthma & Immunology: Ten things physicians and patients should question. American Board of Medicine Foundation Choosing Wisely website. 2018. Accessed July 7, 2021. www.choosingwisely.org/doctor-patient-lists/american-academy-of-allergy-asthma-immunology
5. Tan SY, Tatsumura Y. Alexander Fleming (1881-1955): discoverer of penicillin. Singapore Med J. 2015;56:366-367. doi: 10.11622/smedj.2015105
6. Marston HD, Dixon DM, Knisely JM, et al. Antimicrobial resistance. JAMA. 2016;316:1193-1204. doi: 10.1001/jama.2016.11764
7. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;2013:CD000023. doi: 10.1002/14651858.CD000023.pub4
8. Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381:2338-2351. doi: 10.1056/NEJMra1807761
9. Khan DA. Proactive management of penicillin and other antibiotic allergies. Allergy Asthma Proc. 2020;41:82-89. doi: 10.2500/aap.2020.41.190024
10. Idsoe O, Guthe T, Willcox RR, et al. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159-188.
11. Chiriac AM, Macy E. Large health system databases and drug hypersensitivity. J Allergy Clin Immunol Pract. 2019;7:2125-2131. doi: 10.1016/j.jaip.2019.04.014
12. Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35:489-494. doi: 10.2500/aap.2014.35.3791
13. Sacco KA, Bates A, Brigham TJ, et al. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72:1288-1296. doi: 10.1111/all.13168
14. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S126-S137. doi: 10.1016/j.jaci.2009.10.028
15. Blumenthal KG, Shenoy ES, Varughese CA, et al. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol. 2015;115:294-300.e2. doi: 10.1016/j.anai.2015.05.011
16. Macy E, Schatz M, Lin C, et al. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13:12-18. doi: 10.7812/tpp/08-073
17. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1:258-263. doi: 10.1016/j.jaip.2013.02.002
18. Bourke J, Pavlos R, James I, et al. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol Pract. 2015;3:365-374.e1. doi: 10.1016/j.jaip.2014.11.002
19. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456-2463.
20. Klaustermeyer WB, Gowda VC. Penicillin skin testing: a 20-year study at the West Los Angeles Veterans Affairs Medical Center. Mil Med. 2005;170:701-704. doi: 10.7205/milmed.170.8.701.
21. McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61:361-367. doi: 10.1093/cid/civ308
22. Blumenthal KG, Ryan EE, Li Y, et al. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66:329-336. doi: 10.1093/cid/cix794
23. Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6:1649-1654.e4. doi: 10.1016/j.jaip.2017.12.033
24. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133:790-796. doi: 10.1016/j.jaci.2013.09.021
25. Desai SH, Kaplan MS, Chen Q, et al. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B streptococcus infections. Perm J. 2017;21:16-80. doi: 10.7812/TPP/16-080
26. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-e93. doi: 10.1093/cid/cir073
27. Vyles D, Mistry RD, Heffner V, et al. Reported knowledge and management of potential penicillin allergy in children. Acad Pediatr. 2019;19:684-690. doi: 10.1016/j.acap.2019.01.002
28. Caubet J-C, Kaiser L, Lemaître B, et al. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218-222. doi: 10.1016/j.jaci.2010.08.025
29. Wanat M, Anthierens S, Butler CC, et al. Patient and primary care physician perceptions of penicillin allergy testing and subsequent use of penicillin-containing antibiotics: a qualitative study. J Allergy Clin Immunol Pract. 2019;7:1888-1893.e1. doi: 10.1016/j.jaip.2019.02.036
30. Norton AE, Konvinse K, Phillips EJ, et al. Antibiotic allergy in pediatrics. Pediatrics. 2018;141: e20172497. doi: 10.1542/peds.2017-2497
31. Collins C. The low risks and high rewards of penicillin allergy delabeling: an algorithm to expedite the evaluation. J Pediatr. 2019;212:216-223. doi: 10.1016/j.jpeds.2019.05.060
32. Ibia EO, Schwartz RH, Wiedermann BL. Antibiotic rashes in children: a survey in a private practice setting. Arch Dermatol. 2000;136:849-854. doi: 10.1001/archderm.136.7.849
33. Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505. doi: 10.1001/jama.285.19.2498
34. Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics. 1967;40:910-911.
35. Inglis JM, Caughey GE, Smith W, et al. Documentation of penicillin adverse drug reactions in electronic health records: inconsistent use of allergy and intolerance labels. Intern Med J. 2017;47:1292-1297. doi: 10.1111/imj.13558
36. Lachover-Roth I, Sharon S, Rosman Y, et al. Long-term follow-up after penicillin allergy delabeling in ambulatory patients. J Allergy Clin Immunol Pract. 2019;7:231-235.e1. doi: 10.1016/j.jaip.2018.04.042
37. Solensky R, Jacobs J, Lester M, et al. Penicillin allergy evaluation: a prospective, multicenter, open-label evaluation of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract. 2019;7:1876-1885.e3. doi: 10.1016/j.jaip.2019.02.040
38. A; ; . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273. doi: 10.1016/j.anai.2010.08.002
PRACTICE RECOMMENDATIONS
› Obtain an accurate drug allergy history from all patients who have a listed penicillin allergy. B
› De-label penicillin allergy in patients who report symptoms of an adverse reaction (diarrhea, headache, or nausea) but who (1) do not have other systemic symptoms; (2) do have a family history, but no personal history, of a reaction; or (3) have tolerated the same penicillin derivative since the initial reaction. B
› Refer patients whose reaction history includes hives, shortness of breath, or other allergic-type signs and symptoms for potential skin testing or oral challenge, or both. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series