User login
Evaluating fever in the first 90 days of life
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
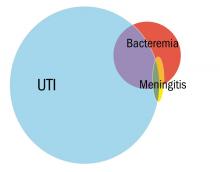
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
Point-of-care ultrasound: Coming soon to primary care?
Point-of-care ultrasound (POCUS) has been gaining greater traction in recent years as a way to quickly (and cost-effectively) assess for conditions including systolic dysfunction, pleural effusion, abdominal aortic aneurysms (AAAs), and deep vein thrombosis (DVT). It involves limited and specific ultrasound protocols performed at the bedside by the health care provider who is trying to answer a specific question and, thus, help guide treatment of the patient.
POCUS was first widely used by emergency physicians starting in the early 1990s with the widespread adoption of the Focused Assessment with Sonography in Trauma (FAST) scan.1,2 Since that time, POCUS has expanded beyond trauma applications and into family medicine.
One study assessed physicians’ perceptions of POCUS after its integration into a military family medicine clinic. The study showed that physicians perceived POCUS to be relatively easy to use, not overly time consuming, and of high value to the practice.3 In fact, the literature tells us that POCUS can help decrease the cost of health care and improve outcomes,4-7 while requiring a relatively brief training period.
If residencies are any indication, POCUS may be headed your way
Ultrasound units are becoming smaller and more affordable, and medical schools are increasingly incorporating ultrasound curricula into medical student training.8 As of 2016, only 6% of practicing FPs reported using non-obstetric POCUS in their practices.9 Similarly, a survey from 2015 reported that only 2% of family medicine residency programs had established POCUS curricula.10 However, 50% of respondents in the 2015 survey reported early-stage development or interest in developing a POCUS curriculum.
Since then a validated family medicine residency curriculum has been published,11 and the American Academy of Family Physicians (AAFP) recently released a POCUS Curriculum Guideline for residencies (https://www.aafp.org/dam/AAFP/documents/medical_education_residency/program_directors/Reprint290D_POCUS.pdf).
[polldaddy:9928416]
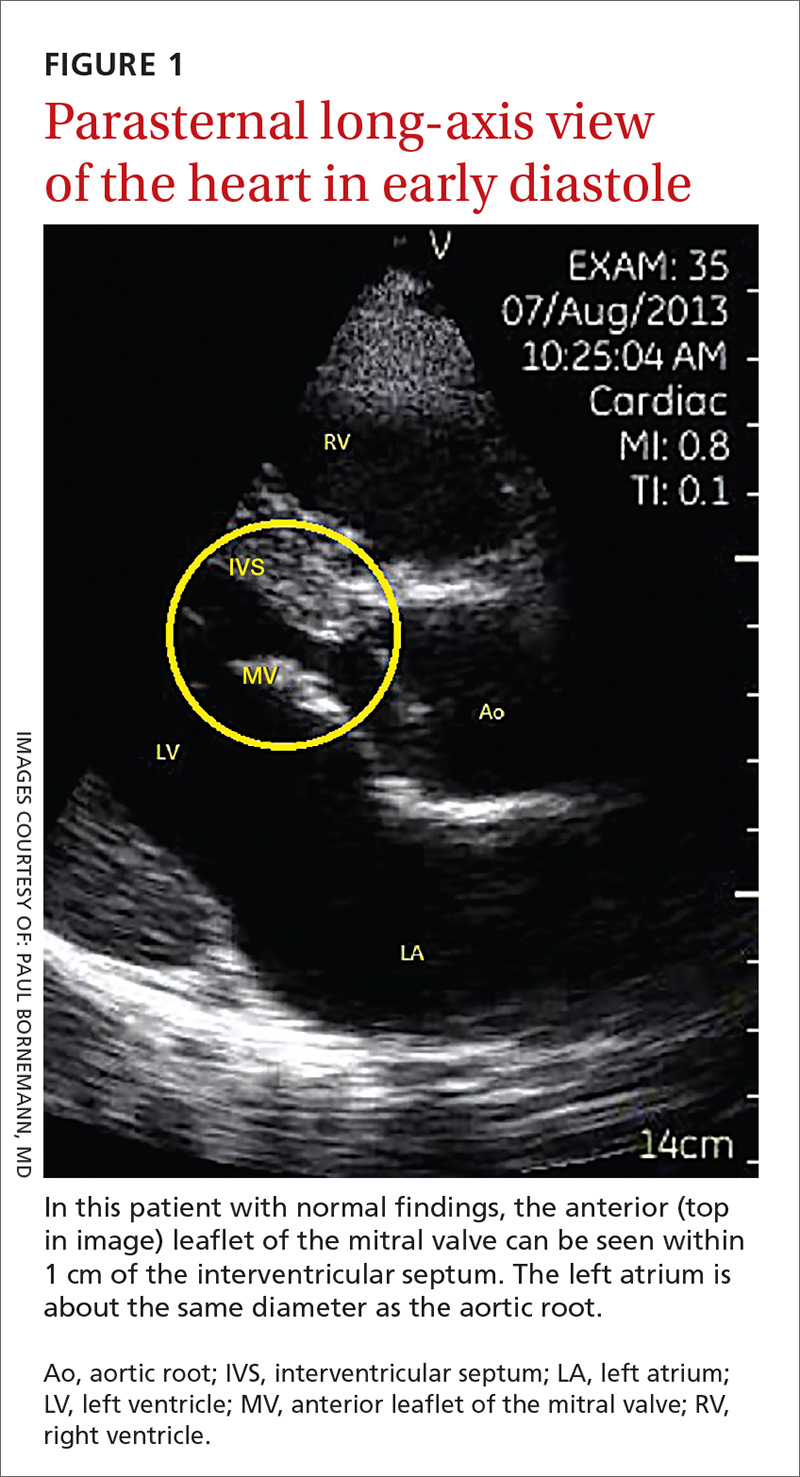
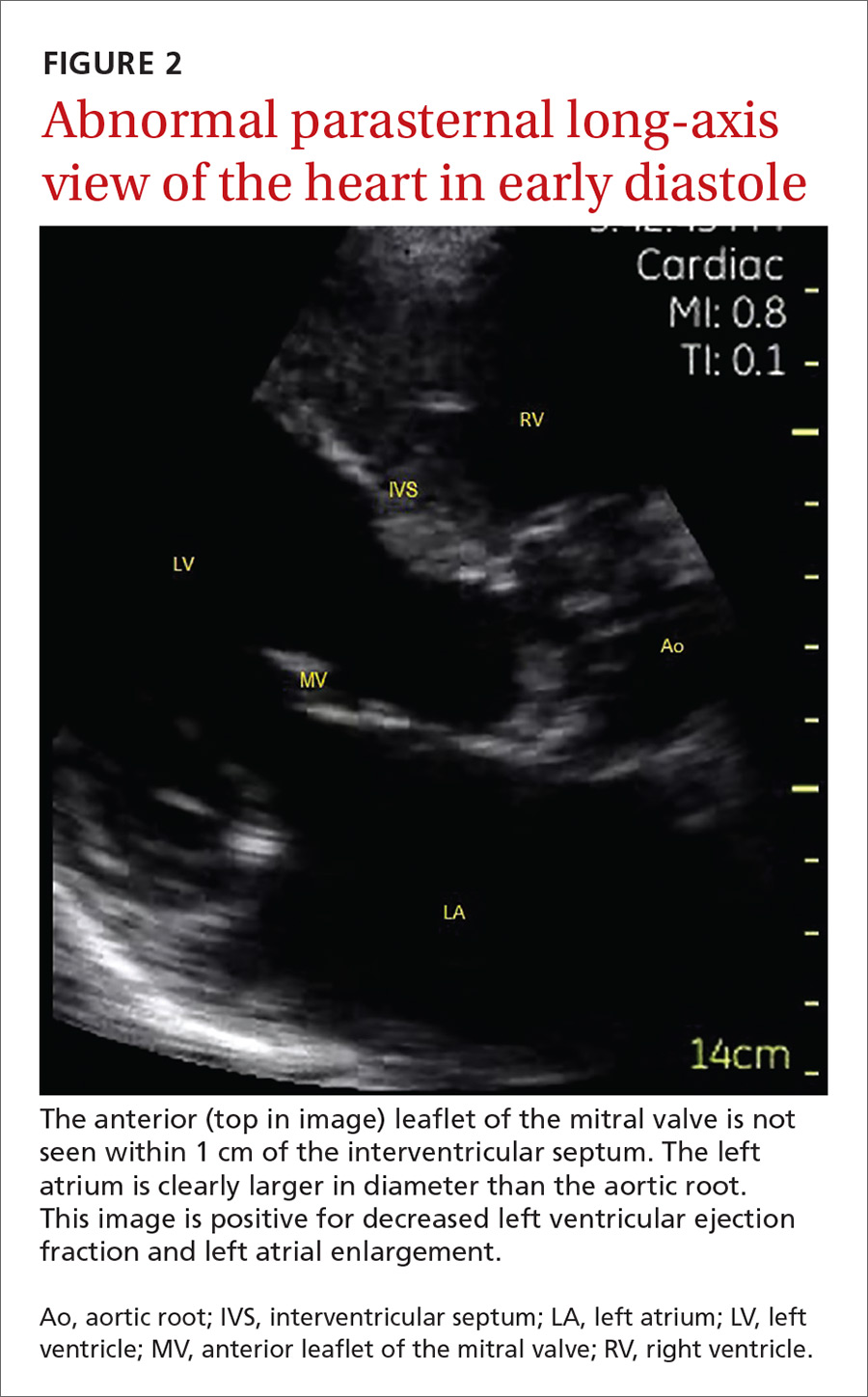
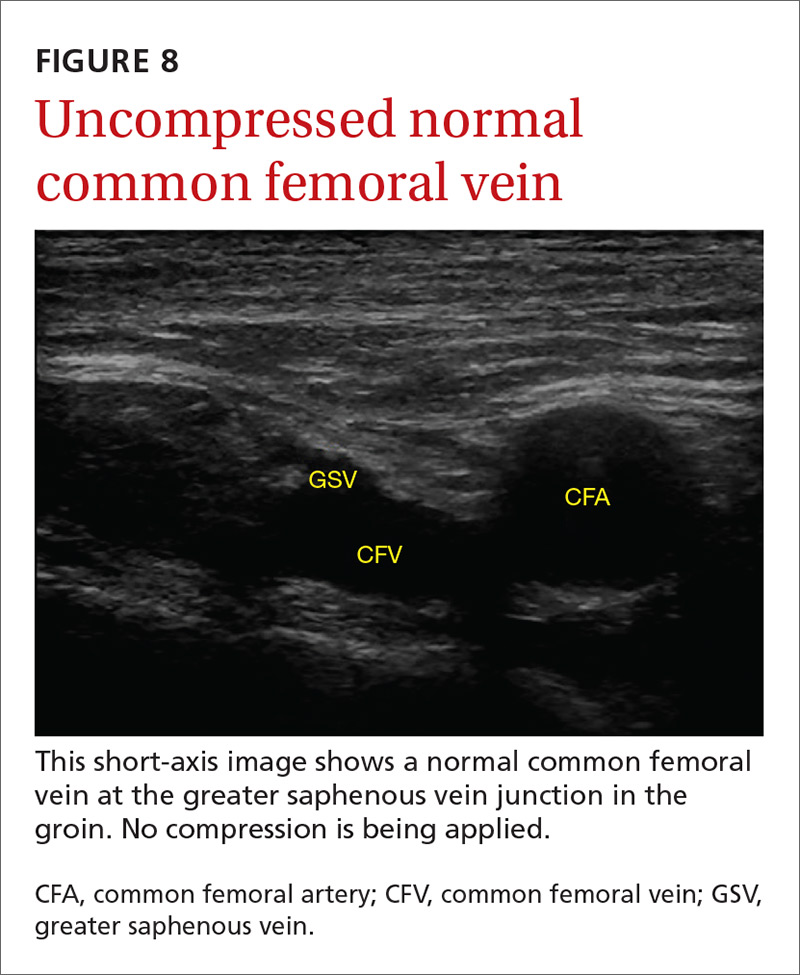
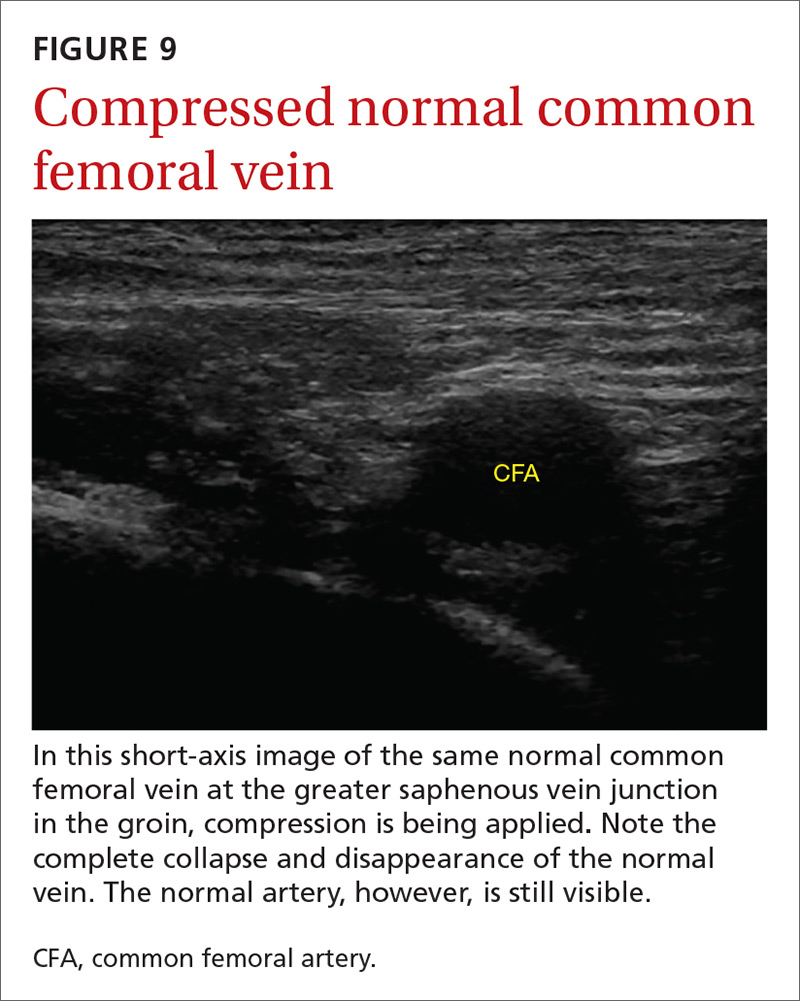
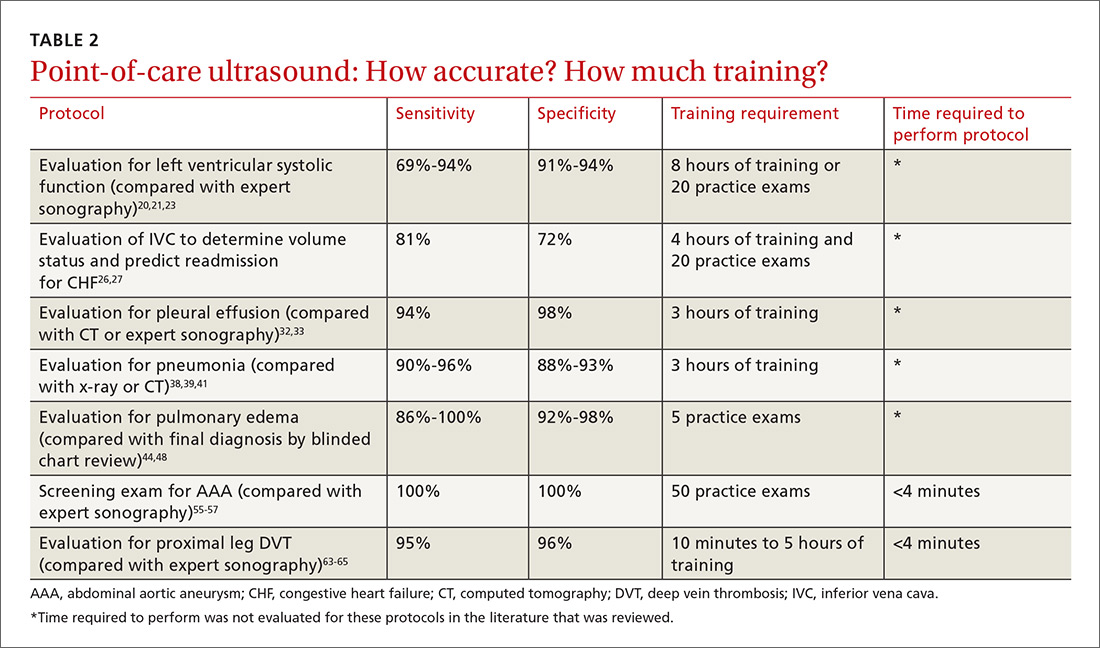
The potential applications of POCUS in family medicine are numerous and have been reviewed in several recent publications.12,13 In this article, we will review the evidence for the use of POCUS in 4 areas: the cardiovascular exam (FIGURES 1 and 2), the lung exam (FIGURES 3-6), the screening exam for AAAs (FIGURE 7), and the evaluation for DVT (FIGURES 8 and 9). (Obstetric and musculoskeletal applications have been sufficiently covered elsewhere.14-17) For all of these applications, POCUS is safe, accurate, and beneficial and can be performed with a relatively small amount of training by non-radiology specialists, including FPs (TABLEs 1 and 2).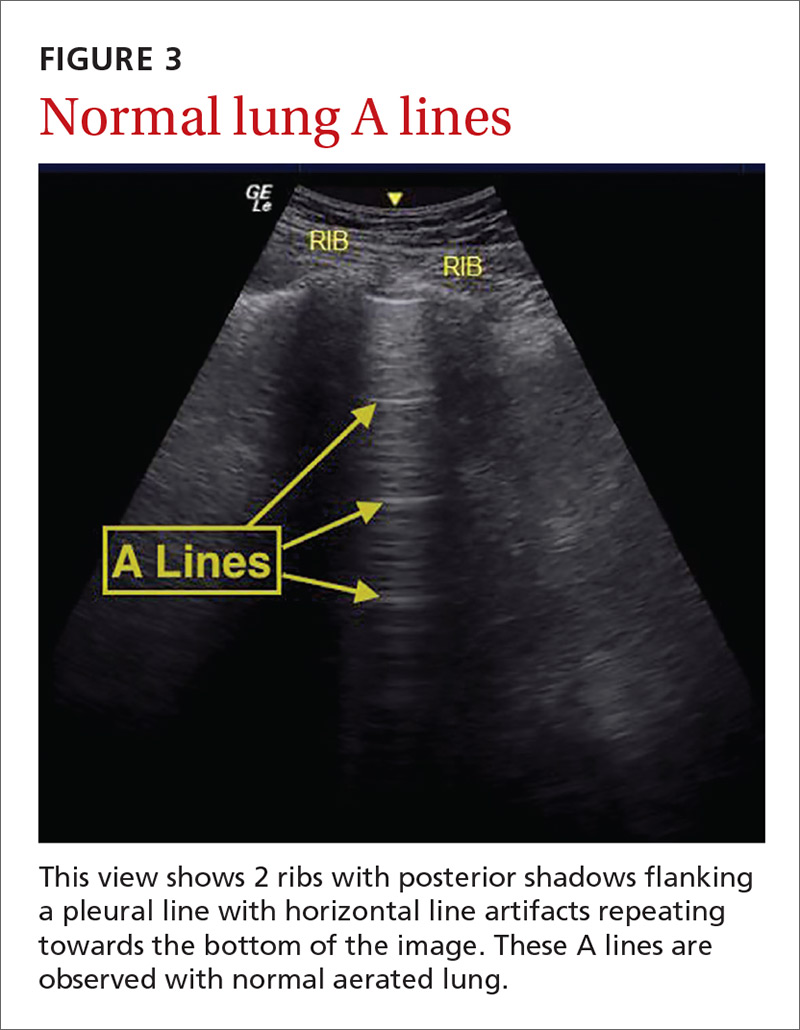
Just 2 hours of cardio POCUS training enhanced Dx accuracy
The American Society of Echocardiography (ASE) issued an expert consensus statement for focused cardiac ultrasound in 2013.18 The guideline supports non-cardiologists utilizing POCUS to assess for pericardial effusion and right and left ventricular enlargement, as well as to review global cardiac systolic function and intravascular volume status. Cardiovascular POCUS protocols are relatively easy to learn; even small amounts of training and practice can yield competency.
For example, a 2013 study showed that after 2 hours of training with a pocket ultrasound device, medical students and junior physicians inexperienced with POCUS were able to improve their diagnostic accuracy for heart failure from 50% to 75%.19 In another study, internal medicine residents with limited cardiac ultrasound training (ie, 20 practice exams) were able to detect decreased left ventricular ejection fraction using a handheld ultrasound device with 94% sensitivity and specificity in patients admitted to the hospital with acute decompensated heart failure.20 Similarly, after only 8 hours of training, a group of Norwegian general practitioners were able to obtain measurements of systolic function with a pocket ultrasound device that were not statistically different from a cardiologist’s measurements.21
In another study, rural FPs attended a 4-day course and then performed focused cardiac ultrasounds on primary care patients with a clinical indication for an echocardiogram.22 The scans were uploaded to a Web-based program for remote interpretation by a cardiologist. There was high concordance between the FPs’ interpretations of the focused cardiac ultrasounds and the cardiologist’s interpretations. Only 32% of the patients in the study group required a formal follow-up echocardiogram.
Kimura et al published a POCUS protocol for the rapid assessment of patients with heart failure, called the Cardiopulmonary Limited Ultrasound Exam (CLUE).23 The CLUE protocol utilizes 4 views to assess left ventricular systolic and diastolic function along with signs of pulmonary edema or systemic volume overload (TABLE 323). The presence of pulmonary edema or a plethoric inferior vena cava (IVC) was highly prognostic of in-hospital mortality. The CLUE protocol has been successfully used by novices including internal medicine residents after brief training (ie, up to 60 supervised scans) and can be performed in less than 5 minutes.24,25 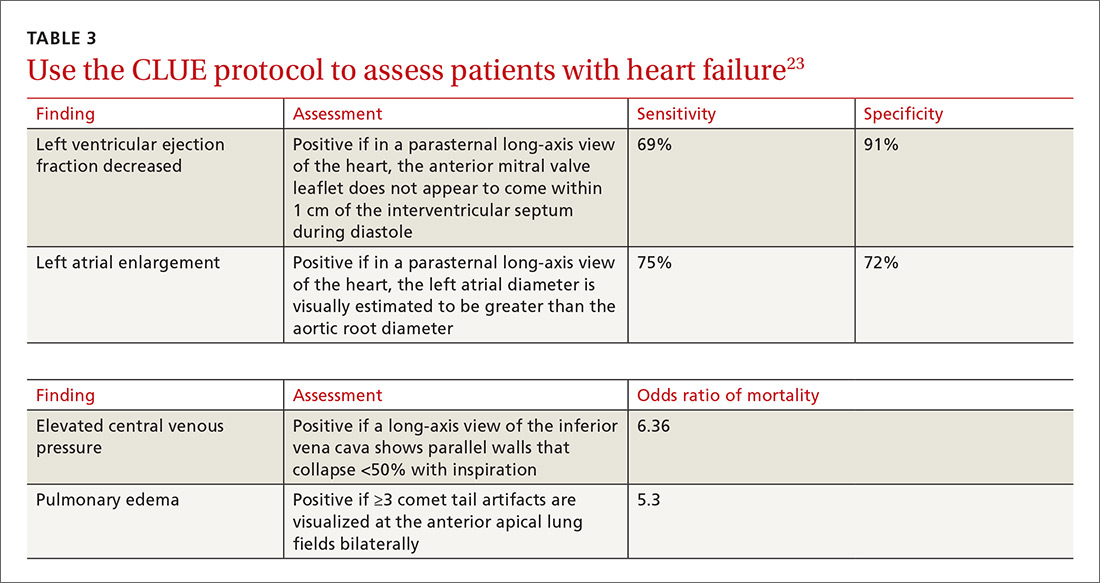
Inpatient use. In addition to its use as an outpatient diagnostic tool, POCUS may be able to help guide therapy in patients admitted to the hospital with heart failure. Increasing collapse of the IVC directly correlates with the amount of fluid volume removed during hemodialysis.26 Goonewardena et al showed that IVC collapsibility was an independent predictor of 30-day hospital readmission even when demographics, signs and symptoms, and volume of diuresis were otherwise equal.27 However, whether the use of IVC collapsibility to guide management improves outcomes in heart failure remains to be validated in a prospective trial.
More sensitive, specific than x-rays for pulmonary diagnoses
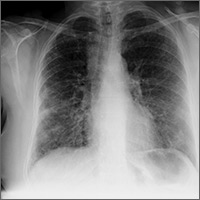
The chest x-ray has traditionally been the imaging modality of choice to evaluate primary care pulmonary complaints. However, POCUS can be more sensitive and specific than a chest x-ray for evaluating several pulmonary diagnoses including pleural effusion, pneumonia, and pulmonary edema.
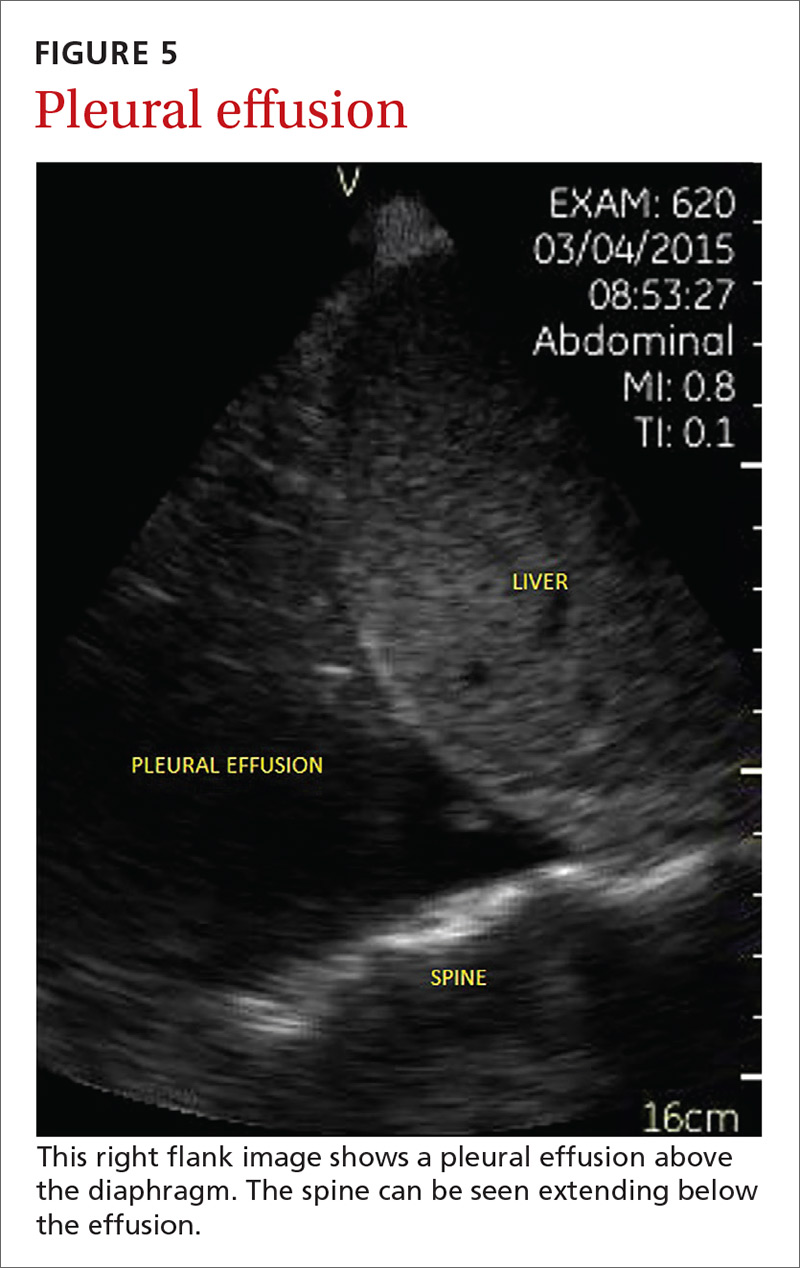
Pleural effusion can be difficult to detect with a physical exam alone. A systematic review showed that the physical exam is not sensitive for effusions <300 mL and can have even lower utility in obese patients.28 While an upright lateral chest x-ray can accurately detect effusions as small as 50 mL, portable x-rays have sensitivities of only 53% to 71% for small- or moderate-sized effusions.29,30 Ultrasound, however, has a sensitivity of 97% for small effusions.31
A 2016 meta-analysis showed that POCUS had a pooled sensitivity and specificity of 94% and 98%, respectively, for pleural effusions, while chest x-ray had a pooled sensitivity and specificity of 51% and 91%, respectively, when compared with computed tomography (CT) and expert sonography.32 POCUS evaluation for pleural effusion is technically simple, and at least one study showed that even novice users can achieve high diagnostic accuracy after only 3 hours of training.33
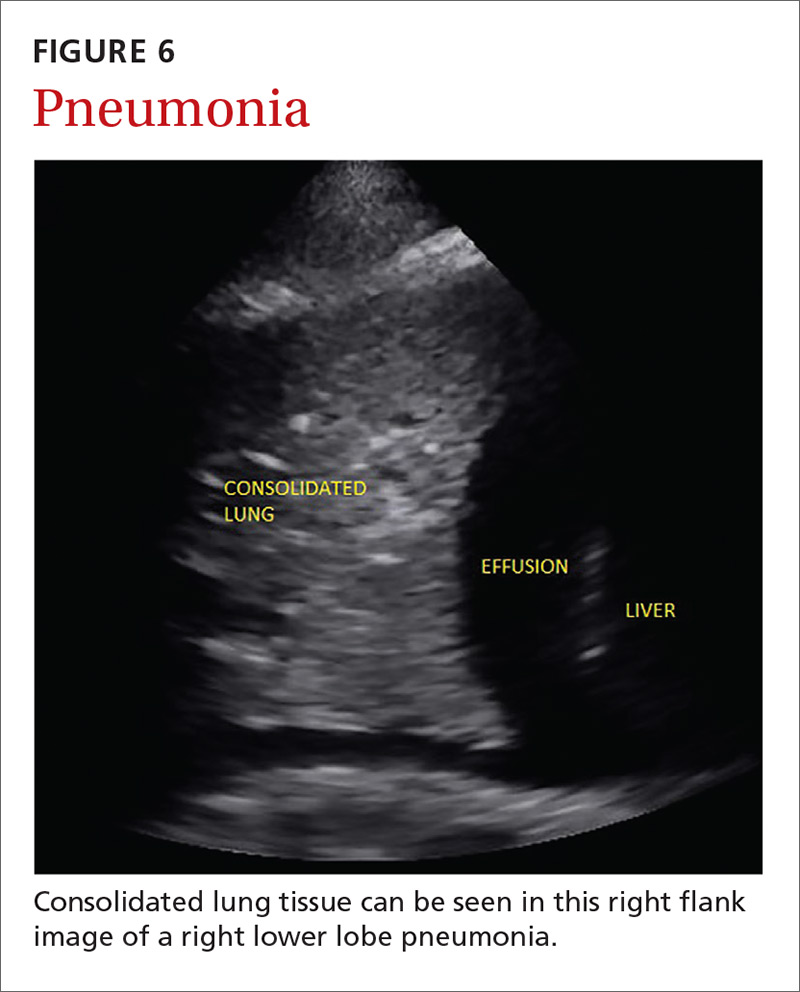
Pneumonia is the eighth leading cause of death in the United States and the single leading cause of infectious disease death in children worldwide.34-36 Pneumonia is a difficult diagnosis to make based on a history and physical examination alone, and the Infectious Diseases Society of America recommends diagnostic imaging to make the diagnosis.37
The adult and pediatric literature clearly demonstrate that lung ultrasound is accurate at diagnosing pneumonia. In a 2015 meta-analysis of the pediatric literature, lung ultrasound had a sensitivity of 96% and a specificity of 93% and positive and negative likelihood ratios of 15.3 and 0.06, respectively.38 In adults, a 2016 meta-analysis of lung ultrasound showed a pooled sensitivity and specificity of 90% and 88%, respectively, with positive and negative likelihood ratios of 6.6 and 0.08, respectively.39
In 2015, a prospective study compared the accuracy of lung ultrasound and chest x-ray using CT as the gold standard.40 Lung ultrasound had a significantly better sensitivity of 82% compared to a sensitivity of 64% for chest x-ray. Specificities were comparable at 94% for ultrasound and 90% for chest x-ray.40
At least one study found novice sonographers to be accurate with lung POCUS for the diagnosis of pneumonia after only two 90-minute training sessions.41 Moreover, ultrasound has a more favorable safety profile, greater portability, and lower cost compared with chest x-ray and CT.
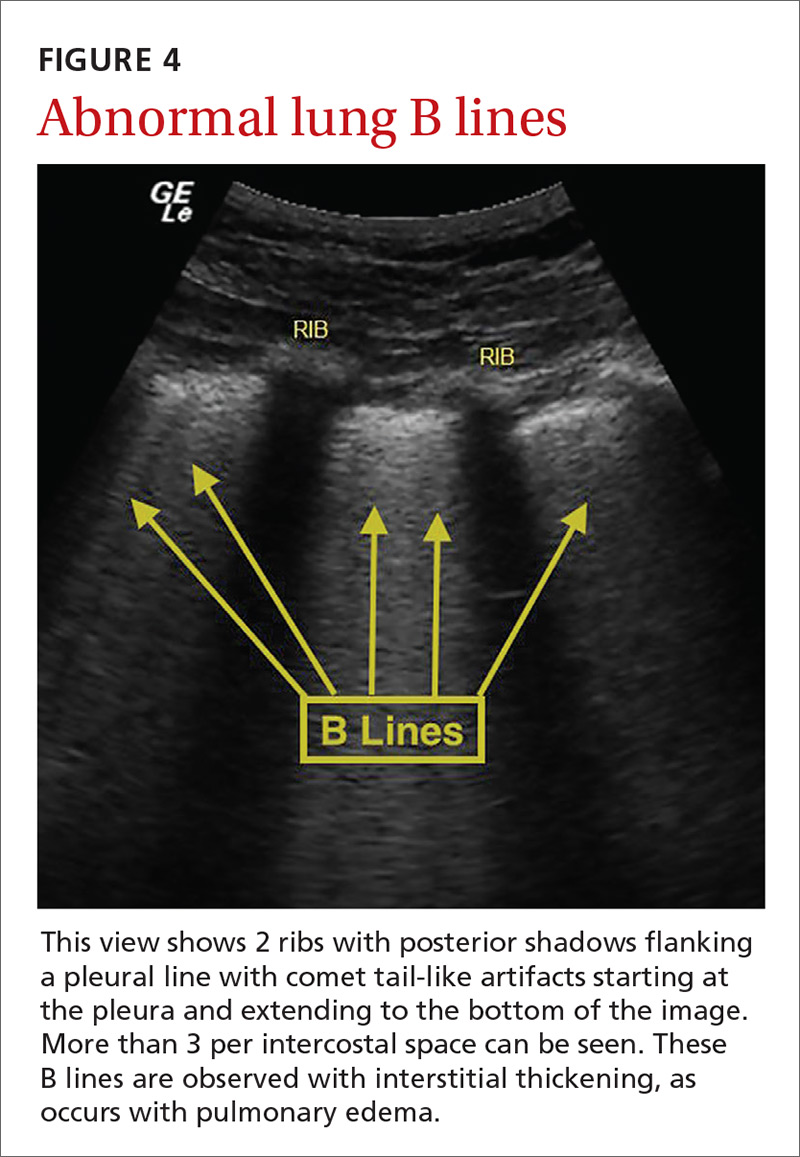
Pulmonary edema. Lung ultrasound can identify interstitial pulmonary edema via artifacts called B lines, which are produced by the reverberation of sound waves from the pleura due to the widening of the fluid-filled interlobular septa. These are distinctly different from the A-line pattern of repeating horizontal lines that is seen with normal lungs, making lung ultrasound more accurate than chest x-ray for identification of pulmonary edema.42,43 When final diagnosis via blinded chart review is used as the reference standard, bilateral B lines on a lung ultrasound image have a sensitivity of 86% to 100% and a specificity of 92% to 98% for the diagnosis of pulmonary edema compared to chest x-ray’s sensitivity of 56.9% and specificity of 89.2%.44 There is also a linear correlation between the number of B lines present and the extent of pulmonary edema.42,45,46 The number of B lines decreases in real time as volume is removed in dialysis patients.47
POCUS evaluation for B lines can be learned very quickly. Exams of novices who have performed only 5 prior exams correlate highly with those of experts who have performed more than 100 exams.48
Simple, efficient screening method for abdominal aortic aneurysm
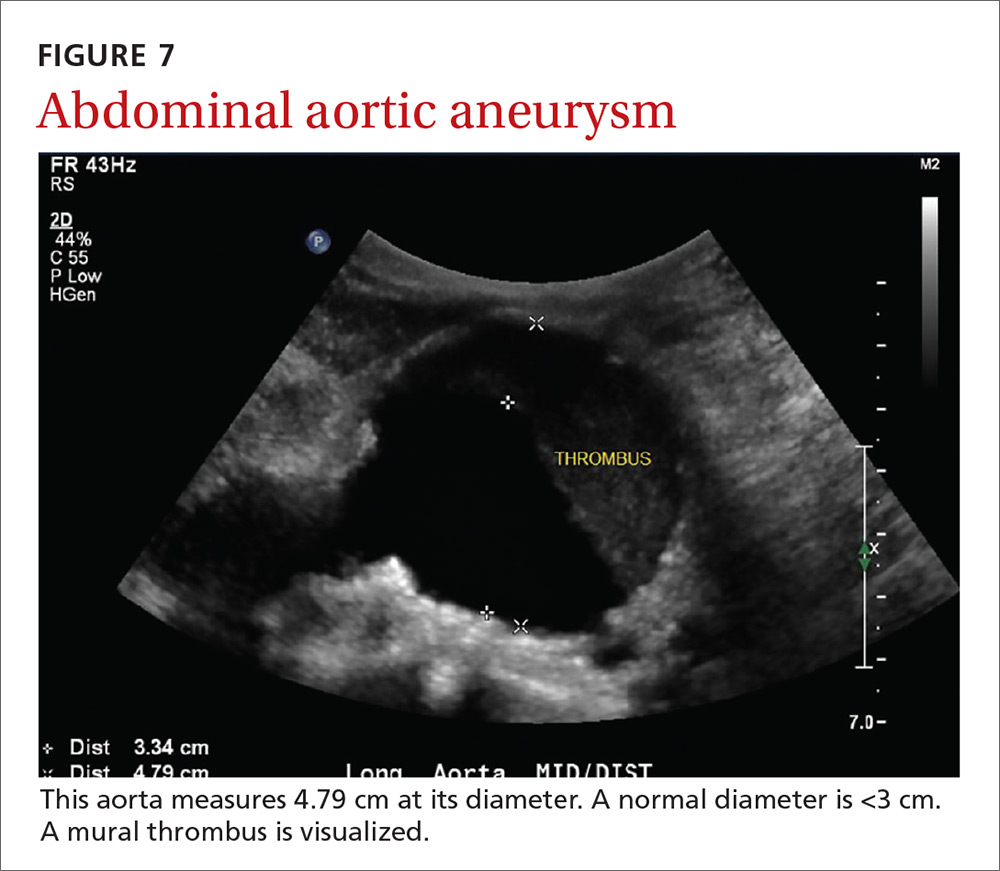
AAAs are present in up to 7% of men over the age of 50.49 The mortality rate of a ruptured AAA is as high as 80% to 95%.50 There is, however, a long prodromal period when interventions can make a significant difference, which is why accurate screening is so important.
AAA screening with ultrasound has been shown to decrease mortality.51 The current recommendation of the US Preventive Services Task Force (USPSTF) is a one-time AAA screening for all men ages 65 to 75 years who have ever smoked (Grade B).52 Despite the recommendations of the USPSTF, screening rates are low. One study found that only 9% of eligible patients in primary care practices received appropriate screening.51
Ultrasound performed by specialists is known to be an excellent screening test for AAA with a sensitivity of 98.9% and a specificity of 99.9%.53 POCUS use by emergency medicine physicians for the evaluation of symptomatic AAA is well established in the literature. A meta-analysis including 7 studies and 655 patients showed a pooled sensitivity of 99% and a specificity of 98%.54 Multiple studies also support primary care physicians performing POCUS AAA screening in the clinic setting.
For example, a 2012 prospective, observational study performed in Canada compared office-based ultrasound screening exams performed by a rural FP to scans performed in the hospital on the same patients.55 The physician completed 50 training examinations. The average discrepancy in aorta diameters between the 2 was only 2 mm, which is clinically insignificant, and the office-based scans had a sensitivity and specificity of 100%.
Similarly, a second FP study performed in Barcelona, showed that an FP who performed POCUS AAA screening had 100% concordance with a radiologist.56 Additionally, POCUS screening for AAA was not time consuming; it was performed in under 4 minutes per patient.55,57
Ruling out DVT
DVT is a relatively rare occurrence in the ambulatory setting. However, patients who present with a painful, swollen lower extremity are much more common, and DVT must be considered and ruled out in these situations.
Although isolated distal DVTs that occur in the calf veins are usually self-limited and have a very low risk of embolization, they can progress to proximal DVTs of the thigh veins up to 20% of time.58,59 Similarly, thrombophlebitis of the superficial lower extremity veins rarely embolizes, but can progress to a proximal DVT, especially if large segments are involved or if the segments are within 5 cm of the junction to the deep venous system.59 The risk of missing a proximal leg DVT is high because embolization occurs up to 60% of the time if the DVT is left untreated.60
The current standard for diagnosis of DVT is the lower extremity Doppler ultrasound examination, but obtaining same-day Doppler evaluations can be difficult in the ambulatory setting. In these instances, the American College of Chest Physicians (ACCP) recommends that even low-risk patients receive anticoagulation pending the evaluation if it cannot be obtained in the first 24 hours.59 This approach not only increases the cost of care, but also exposes patients—many of whom will not be diagnosed with thrombosis in the end—to the risks of anticoagulation.
D-dimer blood tests have drawbacks, too. While a negative high-sensitivity D-dimer blood test in a patient with a low pre-test probability of DVT can effectively rule out a DVT, laboratory testing is not always immediately available in the ambulatory setting either.61 Additionally, false-positive rates are high, and positive D-dimer exams still require evaluation by Doppler ultrasound.
Given these limitations, performing an ultrasound at the bedside or in the exam room can allow for more timely and cost-effective care. In fact, research shows that a limited ultrasound, called the 2-region compression exam, which follows along the course of the common femoral vein and popliteal vein only, ignoring the femoral and calf veins, is highly accurate in assessing for proximal leg DVTs. As such, it has been adopted for POCUS use by emergency medicine physicians.62
Multiple studies show that physicians with minimal training can perform the 2-region compression exam with a high degree of accuracy when full-leg Doppler ultrasound was used as the gold standard.63,64 In these studies, hands-on training times ranged from only 10 minutes to 5 hours, and the exam could be performed in less than 4 minutes. A systematic review of 6 studies comparing emergency physician-performed ultrasound with radiology-performed ultrasound calculated an overall sensitivity of 0.95 (95% CI, 0.87-0.99) and specificity of 0.96 (95% CI, 0.87-0.99) for those performed by emergency physicians.65
The main concern with the 2-region compression exam is that it can miss a distal leg DVT. As stated earlier, distal DVTs are relatively benign and tend to resolve without treatment; however, up to 20% can progress to become a dangerous proximal leg DVT.58 Researchers have validated several methods by prospective trials to address this limitation.
Specifically, researchers have demonstrated that patients with a low pre-test probability of DVT per the Wells scoring system could have DVT effectively ruled out with a single 2-region compression ultrasound without further evaluation.66 In another study, researchers evaluated all patients (regardless of pretest probability) with a 2-point compression exam and found that those with negative exams could be followed with a second exam in 7 to 10 days without initiating anticoagulation. If the second one was negative, no further evaluation was needed.67,68
And finally, researchers demonstrated that a negative 2-point compression ultrasound in combination with a concurrent negative D-dimer test was effective at ruling out DVT, regardless of pre-test probability.69,70
A preferred approach
Given this data and the fact that in the ambulatory setting it is often easier and faster to perform a 2-region compression examination than to obtain a D-dimer laboratory test or a formal full-leg Doppler ultrasound, what follows is our preferred approach to a patient with suspected DVT in the outpatient setting (FIGURE 10).
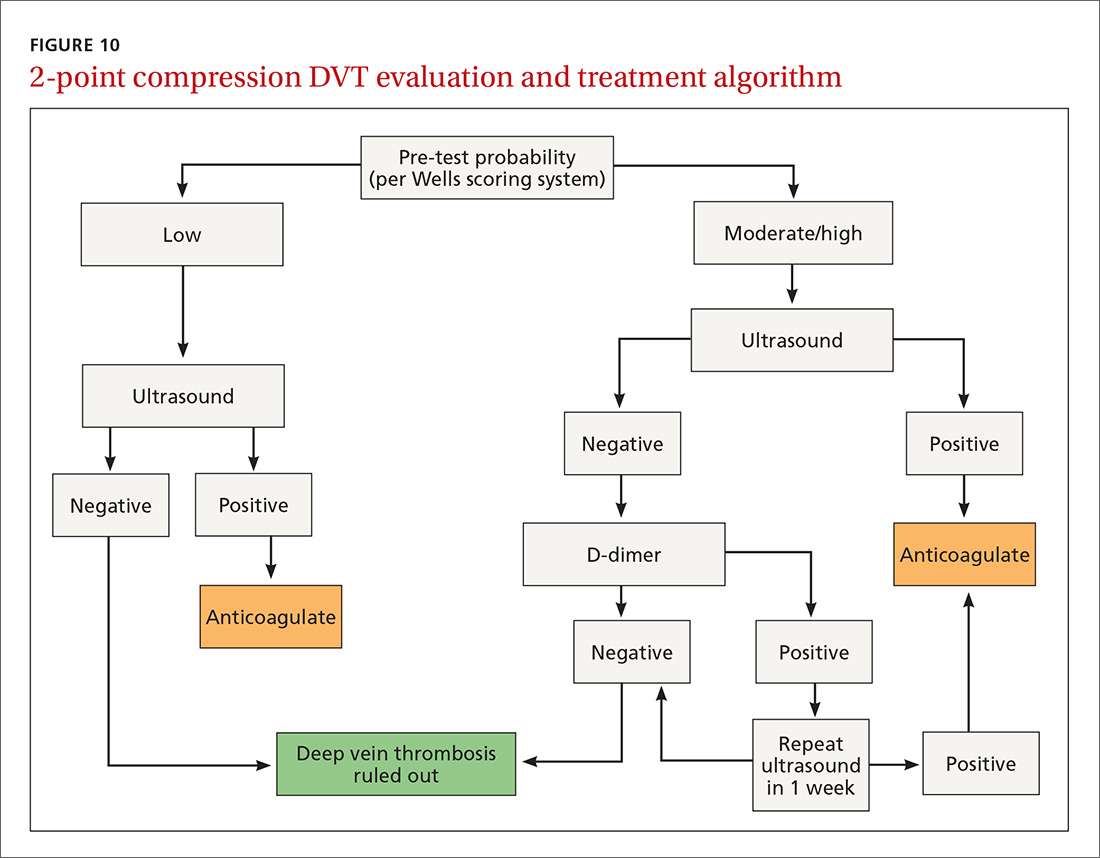
We first assess pre-test probability using the Wells scoring system. We then perform the 2-region compression ultrasound. If the patient has low pre-test risk according to the Wells score, we rule out DVT. If the patient has moderate or high risk with a negative 2-region compression ultrasound, the patient gets a D-dimer test. If the D-dimer test is negative, we rule out DVT. If the D-dimer test is positive, we schedule the patient for a repeat 2-region compression ultrasound in 7 to 10 days. If at any time the 2-region compression evaluation is positive, we treat the patient for DVT.
CORRESPONDENCE
Paul Bornemann, MD, Palmetto Health Family Medicine Residency, Department of Family and Preventive Medicine, University of South Carolina School of Medicine, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
2. Deutchman ME, Hahn RG, Rodney WMM. Diagnostic ultrasound imaging by physicians of first contact: extending the family medicine experience into emergency medicine. Ann Emerg Med. 1993;22:594-596.
3. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-1477.
4. Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100-1110.
5. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal imaging: medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5:182-188.
6. Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
7. Calvert N, Hind D, McWilliams RG, et al. The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation. Health Technol Assess. 2003;7:1-84.
8. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7:18.
9. Clinical procedures performed by physicians at their practice. American Academy of Family Physicians Member Census, December 31, 2016. Available at: http://www.aafp.org/about/the-aafp/family-medicine-facts/table-12(rev).html. Accessed June 26, 2017.
10. Hall JW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: a CERA study. Fam Med. 2015;47:706-711.
11. Bornemann P. Assessment of a novel point-of-care ultrasound curriculum’s effect on competency measures in family medicine graduate medical education. J Ultrasound Med. 2017;36:1205-1211.
12. Steinmetz P, Oleskevich S. The benefits of doing ultrasound exams in your office. J Fam Pract. 2016;65:517-523.
13. Flick D. Bedside ultrasound education in family medicine. J Ultrasound Med. 2016;35:1369-1371.
14. Dresang LT, Rodney WM, Rodney KM. Prenatal ultrasound: a tale of two cities. J Natl Med Assoc. 2006;98:167-171.
15. Dresang LT, Rodney WM, Dees J. Teaching prenatal ultrasound to family medicine residents. Fam Med. 2004;36:98-107.
16. Rodney WM, Deutchman ME, Hartman KJ, et al. Obstetric ultrasound by family physicians. J Fam Pract. 1992;34:186-194.
17. Broadhurst NA, Simmons N. Musculoskeletal ultrasound - used to best advantage. Aust Fam Physician. 2007;36:430-432.
18. Spencer KT, Kimura BJ, Korcarz CE, et al. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567-581.
19. Panoulas VF, Daigeler AL, Malaweera AS, et al. Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging. 2013;14:323-330.
20. Razi R, Estrada JR, Doll J, et al. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24:1319-1324.
21. Mjølstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
22. Evangelista A, Galuppo V, Méndez J, et al. Hand-held cardiac ultrasound screening performed by family doctors with remote expert support interpretation. Heart. 2016;102:376-382.
23. Kimura BJ, Yogo N, O’Connell CW, et al. Cardiopulmonary limited ultrasound examination for “quick-look” bedside application. Am J Cardiol. 2011;108:586-590.
24. Kimura BJ, Amundson SA, Phan JN, et al. Observations during development of an internal medicine residency training program in cardiovascular limited ultrasound examination. J Hosp Med. 2012;7:537-542.
25. Kimura BJ, Shaw DJ, Amundson SA, et al. Cardiac limited ultrasound examination techniques to augment the bedside cardiac physical examination. J Ultrasound Med. 2015;34:1683-1690.
26. Brennan JM, Ronan A, Goonewardena S, et al. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749-753.
27. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1:595-601.
28. Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA. 2009;301:309-317.
29. Blackmore CC, Black WC, Dallas RV, et al. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3:103-109.
30. Kitazono MT, Lau CT, Parada AN, et al. Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. Am J Roentgenol. 2010;194:407-412.
31. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12:12-16.
32. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran, Iran). 2016;4:1-10.
33. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40:1475-1480.
34. World Health Organization. Pneumonia. Fact Sheet No. 331. Available at: http://www.who.int/mediacentre/factsheets/fs331/en/. Accessed June 26, 2017.
35. Gereige RS, Laufer PM. Pneumonia. Pediatr Rev. 2013;34:438-456.
36. National Center for Health Statistics. Leading causes of death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed July 2, 2017.
37. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72.
38. Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714-722.
39. Xia Y, Ying Y, Wang S, et al. Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: a systematic review and meta-analysis. J Thorac Dis. 2016;8:2822-2831.
40. Nazerian P, Volpicelli G, Vanni S, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med. 2015;33:620-625.
41. Filopei J, Siedenburg H, Rattner P, et al. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9:594-597.
42. Lichtenstein D, Mezière G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331-1334.
43. Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014;12:25.
44. Martindale JL, Wakai A, Collins SP, et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:223-242.
45. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689-696.
46. Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356-363.
47. Noble VE, Murray AF, Capp R, et al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis: time course for resolution. Chest. 2009;135:1433-1439.
48. Gullett J, Donnelly JP, Sinert R, et al. Interobserver agreement in the evaluation of B-lines using bedside ultrasound. J Crit Care. 2015;30:1395-1399.
49. Guirguis-Blake JM, Beil TL, Sun X, et al. Primary Care Screening for Abdominal Aortic Aneurysm: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Syntheses No. 109. Rockville, MD; 2014.
50. Metcalfe D, Holt PJE, Thompson MM. The management of abdominal aortic aneurysms. BMJ. 2011;342:d1384.
51. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Brit J Surg. 2012;99:1649-1656.
52. LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:281-290.
53. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
54. Rubano E, Mehta N, Caputo W, et al. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;20:128-138.
55. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
56. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis. Med Clin (Barc). 2013;141:417-422.
57. Sisó-Almirall A, Kostov B, Navarro González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
58. Philbrick JT, Becker DM. Calf deep venous thrombosis: a wolf in sheep’s clothing? Arch Intern Med. 1988;148:2131-2138.
59. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e351S-418S.
60. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
61. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227-1235.
62. Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med. 1989;320:342-345.
63. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56:601-610.
64. Blaivas M, Lambert MJ, Harwood RA, et al. Lower-extremity doppler for deep venous thrombosis—can emergency physicians be accurate and fast? Acad Emerg Med. 2000;7:120-126.
65. Burnside PR, Brown MD, Kline JA. Systematic review of emergency physician-performed ultrasonography for lower-extremity deep vein thrombosis. Acad Emerg Med. 2008;15:493-498.
66. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795-1798.
67. Birdwell BG, Raskob GE, Whitsett TL, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1-7.
68. Cogo A, Lensing AW, Koopman MM, et al. Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: prospective cohort study. BMJ. 1998;316:17-20.
69. Tick LW, Ton E, Van Voorthuizen T, et al. Practical diagnostic management of patients with clinically suspected deep vein thrombosis by clinical probability test, compression ultrasonography, and D-dimer test. Am J Med. 2002;113:630-635.
70. Stevens
Point-of-care ultrasound (POCUS) has been gaining greater traction in recent years as a way to quickly (and cost-effectively) assess for conditions including systolic dysfunction, pleural effusion, abdominal aortic aneurysms (AAAs), and deep vein thrombosis (DVT). It involves limited and specific ultrasound protocols performed at the bedside by the health care provider who is trying to answer a specific question and, thus, help guide treatment of the patient.
POCUS was first widely used by emergency physicians starting in the early 1990s with the widespread adoption of the Focused Assessment with Sonography in Trauma (FAST) scan.1,2 Since that time, POCUS has expanded beyond trauma applications and into family medicine.
One study assessed physicians’ perceptions of POCUS after its integration into a military family medicine clinic. The study showed that physicians perceived POCUS to be relatively easy to use, not overly time consuming, and of high value to the practice.3 In fact, the literature tells us that POCUS can help decrease the cost of health care and improve outcomes,4-7 while requiring a relatively brief training period.

If residencies are any indication, POCUS may be headed your way
Ultrasound units are becoming smaller and more affordable, and medical schools are increasingly incorporating ultrasound curricula into medical student training.8 As of 2016, only 6% of practicing FPs reported using non-obstetric POCUS in their practices.9 Similarly, a survey from 2015 reported that only 2% of family medicine residency programs had established POCUS curricula.10 However, 50% of respondents in the 2015 survey reported early-stage development or interest in developing a POCUS curriculum.
Since then a validated family medicine residency curriculum has been published,11 and the American Academy of Family Physicians (AAFP) recently released a POCUS Curriculum Guideline for residencies (https://www.aafp.org/dam/AAFP/documents/medical_education_residency/program_directors/Reprint290D_POCUS.pdf).
[polldaddy:9928416]


The potential applications of POCUS in family medicine are numerous and have been reviewed in several recent publications.12,13 In this article, we will review the evidence for the use of POCUS in 4 areas: the cardiovascular exam (FIGURES 1 and 2), the lung exam (FIGURES 3-6), the screening exam for AAAs (FIGURE 7), and the evaluation for DVT (FIGURES 8 and 9). (Obstetric and musculoskeletal applications have been sufficiently covered elsewhere.14-17) For all of these applications, POCUS is safe, accurate, and beneficial and can be performed with a relatively small amount of training by non-radiology specialists, including FPs (TABLEs 1 and 2).








Just 2 hours of cardio POCUS training enhanced Dx accuracy
The American Society of Echocardiography (ASE) issued an expert consensus statement for focused cardiac ultrasound in 2013.18 The guideline supports non-cardiologists utilizing POCUS to assess for pericardial effusion and right and left ventricular enlargement, as well as to review global cardiac systolic function and intravascular volume status. Cardiovascular POCUS protocols are relatively easy to learn; even small amounts of training and practice can yield competency.
For example, a 2013 study showed that after 2 hours of training with a pocket ultrasound device, medical students and junior physicians inexperienced with POCUS were able to improve their diagnostic accuracy for heart failure from 50% to 75%.19 In another study, internal medicine residents with limited cardiac ultrasound training (ie, 20 practice exams) were able to detect decreased left ventricular ejection fraction using a handheld ultrasound device with 94% sensitivity and specificity in patients admitted to the hospital with acute decompensated heart failure.20 Similarly, after only 8 hours of training, a group of Norwegian general practitioners were able to obtain measurements of systolic function with a pocket ultrasound device that were not statistically different from a cardiologist’s measurements.21
In another study, rural FPs attended a 4-day course and then performed focused cardiac ultrasounds on primary care patients with a clinical indication for an echocardiogram.22 The scans were uploaded to a Web-based program for remote interpretation by a cardiologist. There was high concordance between the FPs’ interpretations of the focused cardiac ultrasounds and the cardiologist’s interpretations. Only 32% of the patients in the study group required a formal follow-up echocardiogram.
Kimura et al published a POCUS protocol for the rapid assessment of patients with heart failure, called the Cardiopulmonary Limited Ultrasound Exam (CLUE).23 The CLUE protocol utilizes 4 views to assess left ventricular systolic and diastolic function along with signs of pulmonary edema or systemic volume overload (TABLE 323). The presence of pulmonary edema or a plethoric inferior vena cava (IVC) was highly prognostic of in-hospital mortality. The CLUE protocol has been successfully used by novices including internal medicine residents after brief training (ie, up to 60 supervised scans) and can be performed in less than 5 minutes.24,25 
Inpatient use. In addition to its use as an outpatient diagnostic tool, POCUS may be able to help guide therapy in patients admitted to the hospital with heart failure. Increasing collapse of the IVC directly correlates with the amount of fluid volume removed during hemodialysis.26 Goonewardena et al showed that IVC collapsibility was an independent predictor of 30-day hospital readmission even when demographics, signs and symptoms, and volume of diuresis were otherwise equal.27 However, whether the use of IVC collapsibility to guide management improves outcomes in heart failure remains to be validated in a prospective trial.
More sensitive, specific than x-rays for pulmonary diagnoses
The chest x-ray has traditionally been the imaging modality of choice to evaluate primary care pulmonary complaints. However, POCUS can be more sensitive and specific than a chest x-ray for evaluating several pulmonary diagnoses including pleural effusion, pneumonia, and pulmonary edema.
Pleural effusion can be difficult to detect with a physical exam alone. A systematic review showed that the physical exam is not sensitive for effusions <300 mL and can have even lower utility in obese patients.28 While an upright lateral chest x-ray can accurately detect effusions as small as 50 mL, portable x-rays have sensitivities of only 53% to 71% for small- or moderate-sized effusions.29,30 Ultrasound, however, has a sensitivity of 97% for small effusions.31
A 2016 meta-analysis showed that POCUS had a pooled sensitivity and specificity of 94% and 98%, respectively, for pleural effusions, while chest x-ray had a pooled sensitivity and specificity of 51% and 91%, respectively, when compared with computed tomography (CT) and expert sonography.32 POCUS evaluation for pleural effusion is technically simple, and at least one study showed that even novice users can achieve high diagnostic accuracy after only 3 hours of training.33
Pneumonia is the eighth leading cause of death in the United States and the single leading cause of infectious disease death in children worldwide.34-36 Pneumonia is a difficult diagnosis to make based on a history and physical examination alone, and the Infectious Diseases Society of America recommends diagnostic imaging to make the diagnosis.37
The adult and pediatric literature clearly demonstrate that lung ultrasound is accurate at diagnosing pneumonia. In a 2015 meta-analysis of the pediatric literature, lung ultrasound had a sensitivity of 96% and a specificity of 93% and positive and negative likelihood ratios of 15.3 and 0.06, respectively.38 In adults, a 2016 meta-analysis of lung ultrasound showed a pooled sensitivity and specificity of 90% and 88%, respectively, with positive and negative likelihood ratios of 6.6 and 0.08, respectively.39
In 2015, a prospective study compared the accuracy of lung ultrasound and chest x-ray using CT as the gold standard.40 Lung ultrasound had a significantly better sensitivity of 82% compared to a sensitivity of 64% for chest x-ray. Specificities were comparable at 94% for ultrasound and 90% for chest x-ray.40
At least one study found novice sonographers to be accurate with lung POCUS for the diagnosis of pneumonia after only two 90-minute training sessions.41 Moreover, ultrasound has a more favorable safety profile, greater portability, and lower cost compared with chest x-ray and CT.
Pulmonary edema. Lung ultrasound can identify interstitial pulmonary edema via artifacts called B lines, which are produced by the reverberation of sound waves from the pleura due to the widening of the fluid-filled interlobular septa. These are distinctly different from the A-line pattern of repeating horizontal lines that is seen with normal lungs, making lung ultrasound more accurate than chest x-ray for identification of pulmonary edema.42,43 When final diagnosis via blinded chart review is used as the reference standard, bilateral B lines on a lung ultrasound image have a sensitivity of 86% to 100% and a specificity of 92% to 98% for the diagnosis of pulmonary edema compared to chest x-ray’s sensitivity of 56.9% and specificity of 89.2%.44 There is also a linear correlation between the number of B lines present and the extent of pulmonary edema.42,45,46 The number of B lines decreases in real time as volume is removed in dialysis patients.47
POCUS evaluation for B lines can be learned very quickly. Exams of novices who have performed only 5 prior exams correlate highly with those of experts who have performed more than 100 exams.48
Simple, efficient screening method for abdominal aortic aneurysm
AAAs are present in up to 7% of men over the age of 50.49 The mortality rate of a ruptured AAA is as high as 80% to 95%.50 There is, however, a long prodromal period when interventions can make a significant difference, which is why accurate screening is so important.
AAA screening with ultrasound has been shown to decrease mortality.51 The current recommendation of the US Preventive Services Task Force (USPSTF) is a one-time AAA screening for all men ages 65 to 75 years who have ever smoked (Grade B).52 Despite the recommendations of the USPSTF, screening rates are low. One study found that only 9% of eligible patients in primary care practices received appropriate screening.51
Ultrasound performed by specialists is known to be an excellent screening test for AAA with a sensitivity of 98.9% and a specificity of 99.9%.53 POCUS use by emergency medicine physicians for the evaluation of symptomatic AAA is well established in the literature. A meta-analysis including 7 studies and 655 patients showed a pooled sensitivity of 99% and a specificity of 98%.54 Multiple studies also support primary care physicians performing POCUS AAA screening in the clinic setting.
For example, a 2012 prospective, observational study performed in Canada compared office-based ultrasound screening exams performed by a rural FP to scans performed in the hospital on the same patients.55 The physician completed 50 training examinations. The average discrepancy in aorta diameters between the 2 was only 2 mm, which is clinically insignificant, and the office-based scans had a sensitivity and specificity of 100%.
Similarly, a second FP study performed in Barcelona, showed that an FP who performed POCUS AAA screening had 100% concordance with a radiologist.56 Additionally, POCUS screening for AAA was not time consuming; it was performed in under 4 minutes per patient.55,57
Ruling out DVT
DVT is a relatively rare occurrence in the ambulatory setting. However, patients who present with a painful, swollen lower extremity are much more common, and DVT must be considered and ruled out in these situations.
Although isolated distal DVTs that occur in the calf veins are usually self-limited and have a very low risk of embolization, they can progress to proximal DVTs of the thigh veins up to 20% of time.58,59 Similarly, thrombophlebitis of the superficial lower extremity veins rarely embolizes, but can progress to a proximal DVT, especially if large segments are involved or if the segments are within 5 cm of the junction to the deep venous system.59 The risk of missing a proximal leg DVT is high because embolization occurs up to 60% of the time if the DVT is left untreated.60
The current standard for diagnosis of DVT is the lower extremity Doppler ultrasound examination, but obtaining same-day Doppler evaluations can be difficult in the ambulatory setting. In these instances, the American College of Chest Physicians (ACCP) recommends that even low-risk patients receive anticoagulation pending the evaluation if it cannot be obtained in the first 24 hours.59 This approach not only increases the cost of care, but also exposes patients—many of whom will not be diagnosed with thrombosis in the end—to the risks of anticoagulation.
D-dimer blood tests have drawbacks, too. While a negative high-sensitivity D-dimer blood test in a patient with a low pre-test probability of DVT can effectively rule out a DVT, laboratory testing is not always immediately available in the ambulatory setting either.61 Additionally, false-positive rates are high, and positive D-dimer exams still require evaluation by Doppler ultrasound.
Given these limitations, performing an ultrasound at the bedside or in the exam room can allow for more timely and cost-effective care. In fact, research shows that a limited ultrasound, called the 2-region compression exam, which follows along the course of the common femoral vein and popliteal vein only, ignoring the femoral and calf veins, is highly accurate in assessing for proximal leg DVTs. As such, it has been adopted for POCUS use by emergency medicine physicians.62
Multiple studies show that physicians with minimal training can perform the 2-region compression exam with a high degree of accuracy when full-leg Doppler ultrasound was used as the gold standard.63,64 In these studies, hands-on training times ranged from only 10 minutes to 5 hours, and the exam could be performed in less than 4 minutes. A systematic review of 6 studies comparing emergency physician-performed ultrasound with radiology-performed ultrasound calculated an overall sensitivity of 0.95 (95% CI, 0.87-0.99) and specificity of 0.96 (95% CI, 0.87-0.99) for those performed by emergency physicians.65
The main concern with the 2-region compression exam is that it can miss a distal leg DVT. As stated earlier, distal DVTs are relatively benign and tend to resolve without treatment; however, up to 20% can progress to become a dangerous proximal leg DVT.58 Researchers have validated several methods by prospective trials to address this limitation.
Specifically, researchers have demonstrated that patients with a low pre-test probability of DVT per the Wells scoring system could have DVT effectively ruled out with a single 2-region compression ultrasound without further evaluation.66 In another study, researchers evaluated all patients (regardless of pretest probability) with a 2-point compression exam and found that those with negative exams could be followed with a second exam in 7 to 10 days without initiating anticoagulation. If the second one was negative, no further evaluation was needed.67,68
And finally, researchers demonstrated that a negative 2-point compression ultrasound in combination with a concurrent negative D-dimer test was effective at ruling out DVT, regardless of pre-test probability.69,70
A preferred approach
Given this data and the fact that in the ambulatory setting it is often easier and faster to perform a 2-region compression examination than to obtain a D-dimer laboratory test or a formal full-leg Doppler ultrasound, what follows is our preferred approach to a patient with suspected DVT in the outpatient setting (FIGURE 10).

We first assess pre-test probability using the Wells scoring system. We then perform the 2-region compression ultrasound. If the patient has low pre-test risk according to the Wells score, we rule out DVT. If the patient has moderate or high risk with a negative 2-region compression ultrasound, the patient gets a D-dimer test. If the D-dimer test is negative, we rule out DVT. If the D-dimer test is positive, we schedule the patient for a repeat 2-region compression ultrasound in 7 to 10 days. If at any time the 2-region compression evaluation is positive, we treat the patient for DVT.
CORRESPONDENCE
Paul Bornemann, MD, Palmetto Health Family Medicine Residency, Department of Family and Preventive Medicine, University of South Carolina School of Medicine, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
Point-of-care ultrasound (POCUS) has been gaining greater traction in recent years as a way to quickly (and cost-effectively) assess for conditions including systolic dysfunction, pleural effusion, abdominal aortic aneurysms (AAAs), and deep vein thrombosis (DVT). It involves limited and specific ultrasound protocols performed at the bedside by the health care provider who is trying to answer a specific question and, thus, help guide treatment of the patient.
POCUS was first widely used by emergency physicians starting in the early 1990s with the widespread adoption of the Focused Assessment with Sonography in Trauma (FAST) scan.1,2 Since that time, POCUS has expanded beyond trauma applications and into family medicine.
One study assessed physicians’ perceptions of POCUS after its integration into a military family medicine clinic. The study showed that physicians perceived POCUS to be relatively easy to use, not overly time consuming, and of high value to the practice.3 In fact, the literature tells us that POCUS can help decrease the cost of health care and improve outcomes,4-7 while requiring a relatively brief training period.

If residencies are any indication, POCUS may be headed your way
Ultrasound units are becoming smaller and more affordable, and medical schools are increasingly incorporating ultrasound curricula into medical student training.8 As of 2016, only 6% of practicing FPs reported using non-obstetric POCUS in their practices.9 Similarly, a survey from 2015 reported that only 2% of family medicine residency programs had established POCUS curricula.10 However, 50% of respondents in the 2015 survey reported early-stage development or interest in developing a POCUS curriculum.
Since then a validated family medicine residency curriculum has been published,11 and the American Academy of Family Physicians (AAFP) recently released a POCUS Curriculum Guideline for residencies (https://www.aafp.org/dam/AAFP/documents/medical_education_residency/program_directors/Reprint290D_POCUS.pdf).
[polldaddy:9928416]


The potential applications of POCUS in family medicine are numerous and have been reviewed in several recent publications.12,13 In this article, we will review the evidence for the use of POCUS in 4 areas: the cardiovascular exam (FIGURES 1 and 2), the lung exam (FIGURES 3-6), the screening exam for AAAs (FIGURE 7), and the evaluation for DVT (FIGURES 8 and 9). (Obstetric and musculoskeletal applications have been sufficiently covered elsewhere.14-17) For all of these applications, POCUS is safe, accurate, and beneficial and can be performed with a relatively small amount of training by non-radiology specialists, including FPs (TABLEs 1 and 2).








Just 2 hours of cardio POCUS training enhanced Dx accuracy
The American Society of Echocardiography (ASE) issued an expert consensus statement for focused cardiac ultrasound in 2013.18 The guideline supports non-cardiologists utilizing POCUS to assess for pericardial effusion and right and left ventricular enlargement, as well as to review global cardiac systolic function and intravascular volume status. Cardiovascular POCUS protocols are relatively easy to learn; even small amounts of training and practice can yield competency.
For example, a 2013 study showed that after 2 hours of training with a pocket ultrasound device, medical students and junior physicians inexperienced with POCUS were able to improve their diagnostic accuracy for heart failure from 50% to 75%.19 In another study, internal medicine residents with limited cardiac ultrasound training (ie, 20 practice exams) were able to detect decreased left ventricular ejection fraction using a handheld ultrasound device with 94% sensitivity and specificity in patients admitted to the hospital with acute decompensated heart failure.20 Similarly, after only 8 hours of training, a group of Norwegian general practitioners were able to obtain measurements of systolic function with a pocket ultrasound device that were not statistically different from a cardiologist’s measurements.21
In another study, rural FPs attended a 4-day course and then performed focused cardiac ultrasounds on primary care patients with a clinical indication for an echocardiogram.22 The scans were uploaded to a Web-based program for remote interpretation by a cardiologist. There was high concordance between the FPs’ interpretations of the focused cardiac ultrasounds and the cardiologist’s interpretations. Only 32% of the patients in the study group required a formal follow-up echocardiogram.
Kimura et al published a POCUS protocol for the rapid assessment of patients with heart failure, called the Cardiopulmonary Limited Ultrasound Exam (CLUE).23 The CLUE protocol utilizes 4 views to assess left ventricular systolic and diastolic function along with signs of pulmonary edema or systemic volume overload (TABLE 323). The presence of pulmonary edema or a plethoric inferior vena cava (IVC) was highly prognostic of in-hospital mortality. The CLUE protocol has been successfully used by novices including internal medicine residents after brief training (ie, up to 60 supervised scans) and can be performed in less than 5 minutes.24,25 
Inpatient use. In addition to its use as an outpatient diagnostic tool, POCUS may be able to help guide therapy in patients admitted to the hospital with heart failure. Increasing collapse of the IVC directly correlates with the amount of fluid volume removed during hemodialysis.26 Goonewardena et al showed that IVC collapsibility was an independent predictor of 30-day hospital readmission even when demographics, signs and symptoms, and volume of diuresis were otherwise equal.27 However, whether the use of IVC collapsibility to guide management improves outcomes in heart failure remains to be validated in a prospective trial.
More sensitive, specific than x-rays for pulmonary diagnoses
The chest x-ray has traditionally been the imaging modality of choice to evaluate primary care pulmonary complaints. However, POCUS can be more sensitive and specific than a chest x-ray for evaluating several pulmonary diagnoses including pleural effusion, pneumonia, and pulmonary edema.
Pleural effusion can be difficult to detect with a physical exam alone. A systematic review showed that the physical exam is not sensitive for effusions <300 mL and can have even lower utility in obese patients.28 While an upright lateral chest x-ray can accurately detect effusions as small as 50 mL, portable x-rays have sensitivities of only 53% to 71% for small- or moderate-sized effusions.29,30 Ultrasound, however, has a sensitivity of 97% for small effusions.31
A 2016 meta-analysis showed that POCUS had a pooled sensitivity and specificity of 94% and 98%, respectively, for pleural effusions, while chest x-ray had a pooled sensitivity and specificity of 51% and 91%, respectively, when compared with computed tomography (CT) and expert sonography.32 POCUS evaluation for pleural effusion is technically simple, and at least one study showed that even novice users can achieve high diagnostic accuracy after only 3 hours of training.33
Pneumonia is the eighth leading cause of death in the United States and the single leading cause of infectious disease death in children worldwide.34-36 Pneumonia is a difficult diagnosis to make based on a history and physical examination alone, and the Infectious Diseases Society of America recommends diagnostic imaging to make the diagnosis.37
The adult and pediatric literature clearly demonstrate that lung ultrasound is accurate at diagnosing pneumonia. In a 2015 meta-analysis of the pediatric literature, lung ultrasound had a sensitivity of 96% and a specificity of 93% and positive and negative likelihood ratios of 15.3 and 0.06, respectively.38 In adults, a 2016 meta-analysis of lung ultrasound showed a pooled sensitivity and specificity of 90% and 88%, respectively, with positive and negative likelihood ratios of 6.6 and 0.08, respectively.39
In 2015, a prospective study compared the accuracy of lung ultrasound and chest x-ray using CT as the gold standard.40 Lung ultrasound had a significantly better sensitivity of 82% compared to a sensitivity of 64% for chest x-ray. Specificities were comparable at 94% for ultrasound and 90% for chest x-ray.40
At least one study found novice sonographers to be accurate with lung POCUS for the diagnosis of pneumonia after only two 90-minute training sessions.41 Moreover, ultrasound has a more favorable safety profile, greater portability, and lower cost compared with chest x-ray and CT.
Pulmonary edema. Lung ultrasound can identify interstitial pulmonary edema via artifacts called B lines, which are produced by the reverberation of sound waves from the pleura due to the widening of the fluid-filled interlobular septa. These are distinctly different from the A-line pattern of repeating horizontal lines that is seen with normal lungs, making lung ultrasound more accurate than chest x-ray for identification of pulmonary edema.42,43 When final diagnosis via blinded chart review is used as the reference standard, bilateral B lines on a lung ultrasound image have a sensitivity of 86% to 100% and a specificity of 92% to 98% for the diagnosis of pulmonary edema compared to chest x-ray’s sensitivity of 56.9% and specificity of 89.2%.44 There is also a linear correlation between the number of B lines present and the extent of pulmonary edema.42,45,46 The number of B lines decreases in real time as volume is removed in dialysis patients.47
POCUS evaluation for B lines can be learned very quickly. Exams of novices who have performed only 5 prior exams correlate highly with those of experts who have performed more than 100 exams.48
Simple, efficient screening method for abdominal aortic aneurysm
AAAs are present in up to 7% of men over the age of 50.49 The mortality rate of a ruptured AAA is as high as 80% to 95%.50 There is, however, a long prodromal period when interventions can make a significant difference, which is why accurate screening is so important.
AAA screening with ultrasound has been shown to decrease mortality.51 The current recommendation of the US Preventive Services Task Force (USPSTF) is a one-time AAA screening for all men ages 65 to 75 years who have ever smoked (Grade B).52 Despite the recommendations of the USPSTF, screening rates are low. One study found that only 9% of eligible patients in primary care practices received appropriate screening.51
Ultrasound performed by specialists is known to be an excellent screening test for AAA with a sensitivity of 98.9% and a specificity of 99.9%.53 POCUS use by emergency medicine physicians for the evaluation of symptomatic AAA is well established in the literature. A meta-analysis including 7 studies and 655 patients showed a pooled sensitivity of 99% and a specificity of 98%.54 Multiple studies also support primary care physicians performing POCUS AAA screening in the clinic setting.
For example, a 2012 prospective, observational study performed in Canada compared office-based ultrasound screening exams performed by a rural FP to scans performed in the hospital on the same patients.55 The physician completed 50 training examinations. The average discrepancy in aorta diameters between the 2 was only 2 mm, which is clinically insignificant, and the office-based scans had a sensitivity and specificity of 100%.
Similarly, a second FP study performed in Barcelona, showed that an FP who performed POCUS AAA screening had 100% concordance with a radiologist.56 Additionally, POCUS screening for AAA was not time consuming; it was performed in under 4 minutes per patient.55,57
Ruling out DVT
DVT is a relatively rare occurrence in the ambulatory setting. However, patients who present with a painful, swollen lower extremity are much more common, and DVT must be considered and ruled out in these situations.
Although isolated distal DVTs that occur in the calf veins are usually self-limited and have a very low risk of embolization, they can progress to proximal DVTs of the thigh veins up to 20% of time.58,59 Similarly, thrombophlebitis of the superficial lower extremity veins rarely embolizes, but can progress to a proximal DVT, especially if large segments are involved or if the segments are within 5 cm of the junction to the deep venous system.59 The risk of missing a proximal leg DVT is high because embolization occurs up to 60% of the time if the DVT is left untreated.60
The current standard for diagnosis of DVT is the lower extremity Doppler ultrasound examination, but obtaining same-day Doppler evaluations can be difficult in the ambulatory setting. In these instances, the American College of Chest Physicians (ACCP) recommends that even low-risk patients receive anticoagulation pending the evaluation if it cannot be obtained in the first 24 hours.59 This approach not only increases the cost of care, but also exposes patients—many of whom will not be diagnosed with thrombosis in the end—to the risks of anticoagulation.
D-dimer blood tests have drawbacks, too. While a negative high-sensitivity D-dimer blood test in a patient with a low pre-test probability of DVT can effectively rule out a DVT, laboratory testing is not always immediately available in the ambulatory setting either.61 Additionally, false-positive rates are high, and positive D-dimer exams still require evaluation by Doppler ultrasound.
Given these limitations, performing an ultrasound at the bedside or in the exam room can allow for more timely and cost-effective care. In fact, research shows that a limited ultrasound, called the 2-region compression exam, which follows along the course of the common femoral vein and popliteal vein only, ignoring the femoral and calf veins, is highly accurate in assessing for proximal leg DVTs. As such, it has been adopted for POCUS use by emergency medicine physicians.62
Multiple studies show that physicians with minimal training can perform the 2-region compression exam with a high degree of accuracy when full-leg Doppler ultrasound was used as the gold standard.63,64 In these studies, hands-on training times ranged from only 10 minutes to 5 hours, and the exam could be performed in less than 4 minutes. A systematic review of 6 studies comparing emergency physician-performed ultrasound with radiology-performed ultrasound calculated an overall sensitivity of 0.95 (95% CI, 0.87-0.99) and specificity of 0.96 (95% CI, 0.87-0.99) for those performed by emergency physicians.65
The main concern with the 2-region compression exam is that it can miss a distal leg DVT. As stated earlier, distal DVTs are relatively benign and tend to resolve without treatment; however, up to 20% can progress to become a dangerous proximal leg DVT.58 Researchers have validated several methods by prospective trials to address this limitation.
Specifically, researchers have demonstrated that patients with a low pre-test probability of DVT per the Wells scoring system could have DVT effectively ruled out with a single 2-region compression ultrasound without further evaluation.66 In another study, researchers evaluated all patients (regardless of pretest probability) with a 2-point compression exam and found that those with negative exams could be followed with a second exam in 7 to 10 days without initiating anticoagulation. If the second one was negative, no further evaluation was needed.67,68
And finally, researchers demonstrated that a negative 2-point compression ultrasound in combination with a concurrent negative D-dimer test was effective at ruling out DVT, regardless of pre-test probability.69,70
A preferred approach
Given this data and the fact that in the ambulatory setting it is often easier and faster to perform a 2-region compression examination than to obtain a D-dimer laboratory test or a formal full-leg Doppler ultrasound, what follows is our preferred approach to a patient with suspected DVT in the outpatient setting (FIGURE 10).

We first assess pre-test probability using the Wells scoring system. We then perform the 2-region compression ultrasound. If the patient has low pre-test risk according to the Wells score, we rule out DVT. If the patient has moderate or high risk with a negative 2-region compression ultrasound, the patient gets a D-dimer test. If the D-dimer test is negative, we rule out DVT. If the D-dimer test is positive, we schedule the patient for a repeat 2-region compression ultrasound in 7 to 10 days. If at any time the 2-region compression evaluation is positive, we treat the patient for DVT.
CORRESPONDENCE
Paul Bornemann, MD, Palmetto Health Family Medicine Residency, Department of Family and Preventive Medicine, University of South Carolina School of Medicine, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
2. Deutchman ME, Hahn RG, Rodney WMM. Diagnostic ultrasound imaging by physicians of first contact: extending the family medicine experience into emergency medicine. Ann Emerg Med. 1993;22:594-596.
3. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-1477.
4. Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100-1110.
5. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal imaging: medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5:182-188.
6. Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
7. Calvert N, Hind D, McWilliams RG, et al. The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation. Health Technol Assess. 2003;7:1-84.
8. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7:18.
9. Clinical procedures performed by physicians at their practice. American Academy of Family Physicians Member Census, December 31, 2016. Available at: http://www.aafp.org/about/the-aafp/family-medicine-facts/table-12(rev).html. Accessed June 26, 2017.
10. Hall JW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: a CERA study. Fam Med. 2015;47:706-711.
11. Bornemann P. Assessment of a novel point-of-care ultrasound curriculum’s effect on competency measures in family medicine graduate medical education. J Ultrasound Med. 2017;36:1205-1211.
12. Steinmetz P, Oleskevich S. The benefits of doing ultrasound exams in your office. J Fam Pract. 2016;65:517-523.
13. Flick D. Bedside ultrasound education in family medicine. J Ultrasound Med. 2016;35:1369-1371.
14. Dresang LT, Rodney WM, Rodney KM. Prenatal ultrasound: a tale of two cities. J Natl Med Assoc. 2006;98:167-171.
15. Dresang LT, Rodney WM, Dees J. Teaching prenatal ultrasound to family medicine residents. Fam Med. 2004;36:98-107.
16. Rodney WM, Deutchman ME, Hartman KJ, et al. Obstetric ultrasound by family physicians. J Fam Pract. 1992;34:186-194.
17. Broadhurst NA, Simmons N. Musculoskeletal ultrasound - used to best advantage. Aust Fam Physician. 2007;36:430-432.
18. Spencer KT, Kimura BJ, Korcarz CE, et al. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567-581.
19. Panoulas VF, Daigeler AL, Malaweera AS, et al. Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging. 2013;14:323-330.
20. Razi R, Estrada JR, Doll J, et al. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24:1319-1324.
21. Mjølstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
22. Evangelista A, Galuppo V, Méndez J, et al. Hand-held cardiac ultrasound screening performed by family doctors with remote expert support interpretation. Heart. 2016;102:376-382.
23. Kimura BJ, Yogo N, O’Connell CW, et al. Cardiopulmonary limited ultrasound examination for “quick-look” bedside application. Am J Cardiol. 2011;108:586-590.
24. Kimura BJ, Amundson SA, Phan JN, et al. Observations during development of an internal medicine residency training program in cardiovascular limited ultrasound examination. J Hosp Med. 2012;7:537-542.
25. Kimura BJ, Shaw DJ, Amundson SA, et al. Cardiac limited ultrasound examination techniques to augment the bedside cardiac physical examination. J Ultrasound Med. 2015;34:1683-1690.
26. Brennan JM, Ronan A, Goonewardena S, et al. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749-753.
27. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1:595-601.
28. Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA. 2009;301:309-317.
29. Blackmore CC, Black WC, Dallas RV, et al. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3:103-109.
30. Kitazono MT, Lau CT, Parada AN, et al. Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. Am J Roentgenol. 2010;194:407-412.
31. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12:12-16.
32. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran, Iran). 2016;4:1-10.
33. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40:1475-1480.
34. World Health Organization. Pneumonia. Fact Sheet No. 331. Available at: http://www.who.int/mediacentre/factsheets/fs331/en/. Accessed June 26, 2017.
35. Gereige RS, Laufer PM. Pneumonia. Pediatr Rev. 2013;34:438-456.
36. National Center for Health Statistics. Leading causes of death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed July 2, 2017.
37. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72.
38. Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714-722.
39. Xia Y, Ying Y, Wang S, et al. Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: a systematic review and meta-analysis. J Thorac Dis. 2016;8:2822-2831.
40. Nazerian P, Volpicelli G, Vanni S, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med. 2015;33:620-625.
41. Filopei J, Siedenburg H, Rattner P, et al. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9:594-597.
42. Lichtenstein D, Mezière G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331-1334.
43. Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014;12:25.
44. Martindale JL, Wakai A, Collins SP, et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:223-242.
45. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689-696.
46. Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356-363.
47. Noble VE, Murray AF, Capp R, et al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis: time course for resolution. Chest. 2009;135:1433-1439.
48. Gullett J, Donnelly JP, Sinert R, et al. Interobserver agreement in the evaluation of B-lines using bedside ultrasound. J Crit Care. 2015;30:1395-1399.
49. Guirguis-Blake JM, Beil TL, Sun X, et al. Primary Care Screening for Abdominal Aortic Aneurysm: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Syntheses No. 109. Rockville, MD; 2014.
50. Metcalfe D, Holt PJE, Thompson MM. The management of abdominal aortic aneurysms. BMJ. 2011;342:d1384.
51. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Brit J Surg. 2012;99:1649-1656.
52. LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:281-290.
53. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
54. Rubano E, Mehta N, Caputo W, et al. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;20:128-138.
55. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
56. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis. Med Clin (Barc). 2013;141:417-422.
57. Sisó-Almirall A, Kostov B, Navarro González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
58. Philbrick JT, Becker DM. Calf deep venous thrombosis: a wolf in sheep’s clothing? Arch Intern Med. 1988;148:2131-2138.
59. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e351S-418S.
60. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
61. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227-1235.
62. Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med. 1989;320:342-345.
63. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56:601-610.
64. Blaivas M, Lambert MJ, Harwood RA, et al. Lower-extremity doppler for deep venous thrombosis—can emergency physicians be accurate and fast? Acad Emerg Med. 2000;7:120-126.
65. Burnside PR, Brown MD, Kline JA. Systematic review of emergency physician-performed ultrasonography for lower-extremity deep vein thrombosis. Acad Emerg Med. 2008;15:493-498.
66. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795-1798.
67. Birdwell BG, Raskob GE, Whitsett TL, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1-7.
68. Cogo A, Lensing AW, Koopman MM, et al. Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: prospective cohort study. BMJ. 1998;316:17-20.
69. Tick LW, Ton E, Van Voorthuizen T, et al. Practical diagnostic management of patients with clinically suspected deep vein thrombosis by clinical probability test, compression ultrasonography, and D-dimer test. Am J Med. 2002;113:630-635.
70. Stevens
1. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
2. Deutchman ME, Hahn RG, Rodney WMM. Diagnostic ultrasound imaging by physicians of first contact: extending the family medicine experience into emergency medicine. Ann Emerg Med. 1993;22:594-596.
3. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-1477.
4. Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100-1110.
5. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal imaging: medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5:182-188.
6. Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
7. Calvert N, Hind D, McWilliams RG, et al. The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation. Health Technol Assess. 2003;7:1-84.
8. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7:18.
9. Clinical procedures performed by physicians at their practice. American Academy of Family Physicians Member Census, December 31, 2016. Available at: http://www.aafp.org/about/the-aafp/family-medicine-facts/table-12(rev).html. Accessed June 26, 2017.
10. Hall JW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: a CERA study. Fam Med. 2015;47:706-711.
11. Bornemann P. Assessment of a novel point-of-care ultrasound curriculum’s effect on competency measures in family medicine graduate medical education. J Ultrasound Med. 2017;36:1205-1211.
12. Steinmetz P, Oleskevich S. The benefits of doing ultrasound exams in your office. J Fam Pract. 2016;65:517-523.
13. Flick D. Bedside ultrasound education in family medicine. J Ultrasound Med. 2016;35:1369-1371.
14. Dresang LT, Rodney WM, Rodney KM. Prenatal ultrasound: a tale of two cities. J Natl Med Assoc. 2006;98:167-171.
15. Dresang LT, Rodney WM, Dees J. Teaching prenatal ultrasound to family medicine residents. Fam Med. 2004;36:98-107.
16. Rodney WM, Deutchman ME, Hartman KJ, et al. Obstetric ultrasound by family physicians. J Fam Pract. 1992;34:186-194.
17. Broadhurst NA, Simmons N. Musculoskeletal ultrasound - used to best advantage. Aust Fam Physician. 2007;36:430-432.
18. Spencer KT, Kimura BJ, Korcarz CE, et al. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567-581.
19. Panoulas VF, Daigeler AL, Malaweera AS, et al. Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging. 2013;14:323-330.
20. Razi R, Estrada JR, Doll J, et al. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24:1319-1324.
21. Mjølstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
22. Evangelista A, Galuppo V, Méndez J, et al. Hand-held cardiac ultrasound screening performed by family doctors with remote expert support interpretation. Heart. 2016;102:376-382.
23. Kimura BJ, Yogo N, O’Connell CW, et al. Cardiopulmonary limited ultrasound examination for “quick-look” bedside application. Am J Cardiol. 2011;108:586-590.
24. Kimura BJ, Amundson SA, Phan JN, et al. Observations during development of an internal medicine residency training program in cardiovascular limited ultrasound examination. J Hosp Med. 2012;7:537-542.
25. Kimura BJ, Shaw DJ, Amundson SA, et al. Cardiac limited ultrasound examination techniques to augment the bedside cardiac physical examination. J Ultrasound Med. 2015;34:1683-1690.
26. Brennan JM, Ronan A, Goonewardena S, et al. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749-753.
27. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1:595-601.
28. Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA. 2009;301:309-317.
29. Blackmore CC, Black WC, Dallas RV, et al. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3:103-109.
30. Kitazono MT, Lau CT, Parada AN, et al. Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. Am J Roentgenol. 2010;194:407-412.
31. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12:12-16.
32. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran, Iran). 2016;4:1-10.
33. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40:1475-1480.
34. World Health Organization. Pneumonia. Fact Sheet No. 331. Available at: http://www.who.int/mediacentre/factsheets/fs331/en/. Accessed June 26, 2017.
35. Gereige RS, Laufer PM. Pneumonia. Pediatr Rev. 2013;34:438-456.
36. National Center for Health Statistics. Leading causes of death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed July 2, 2017.
37. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72.
38. Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714-722.
39. Xia Y, Ying Y, Wang S, et al. Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: a systematic review and meta-analysis. J Thorac Dis. 2016;8:2822-2831.
40. Nazerian P, Volpicelli G, Vanni S, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med. 2015;33:620-625.
41. Filopei J, Siedenburg H, Rattner P, et al. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9:594-597.
42. Lichtenstein D, Mezière G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331-1334.
43. Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014;12:25.
44. Martindale JL, Wakai A, Collins SP, et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:223-242.
45. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689-696.
46. Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356-363.
47. Noble VE, Murray AF, Capp R, et al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis: time course for resolution. Chest. 2009;135:1433-1439.
48. Gullett J, Donnelly JP, Sinert R, et al. Interobserver agreement in the evaluation of B-lines using bedside ultrasound. J Crit Care. 2015;30:1395-1399.
49. Guirguis-Blake JM, Beil TL, Sun X, et al. Primary Care Screening for Abdominal Aortic Aneurysm: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Syntheses No. 109. Rockville, MD; 2014.
50. Metcalfe D, Holt PJE, Thompson MM. The management of abdominal aortic aneurysms. BMJ. 2011;342:d1384.
51. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Brit J Surg. 2012;99:1649-1656.
52. LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:281-290.
53. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
54. Rubano E, Mehta N, Caputo W, et al. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;20:128-138.
55. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
56. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis. Med Clin (Barc). 2013;141:417-422.
57. Sisó-Almirall A, Kostov B, Navarro González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
58. Philbrick JT, Becker DM. Calf deep venous thrombosis: a wolf in sheep’s clothing? Arch Intern Med. 1988;148:2131-2138.
59. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e351S-418S.
60. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
61. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227-1235.
62. Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med. 1989;320:342-345.
63. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56:601-610.
64. Blaivas M, Lambert MJ, Harwood RA, et al. Lower-extremity doppler for deep venous thrombosis—can emergency physicians be accurate and fast? Acad Emerg Med. 2000;7:120-126.
65. Burnside PR, Brown MD, Kline JA. Systematic review of emergency physician-performed ultrasonography for lower-extremity deep vein thrombosis. Acad Emerg Med. 2008;15:493-498.
66. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795-1798.
67. Birdwell BG, Raskob GE, Whitsett TL, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1-7.
68. Cogo A, Lensing AW, Koopman MM, et al. Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: prospective cohort study. BMJ. 1998;316:17-20.
69. Tick LW, Ton E, Van Voorthuizen T, et al. Practical diagnostic management of patients with clinically suspected deep vein thrombosis by clinical probability test, compression ultrasonography, and D-dimer test. Am J Med. 2002;113:630-635.
70. Stevens
Mild cough • wheezing • loud heart sounds • Dx?
THE CASE
A 25-year-old man, who was an active duty US Navy sailor, went to his ship’s medical department complaining of a mild cough that he’d had for 2 days. He denied having any fevers, chills, night sweats, angina, or dyspnea. He said he hadn’t experienced any exertional fatigue or difficulty completing the rigorous physical tasks of his occupation as an engineman on the ship. The patient had no medical or surgical history of significance, and he wasn’t taking any medications or supplements.
On exam, he was not in acute distress and his vital signs were within normal limits. Auscultation revealed mild wheezing throughout the upper lung fields and loud heart sounds throughout his chest that were audible even with gentle contact of the stethoscope diaphragm. He had no discernible murmurs, rubs, or gallops.
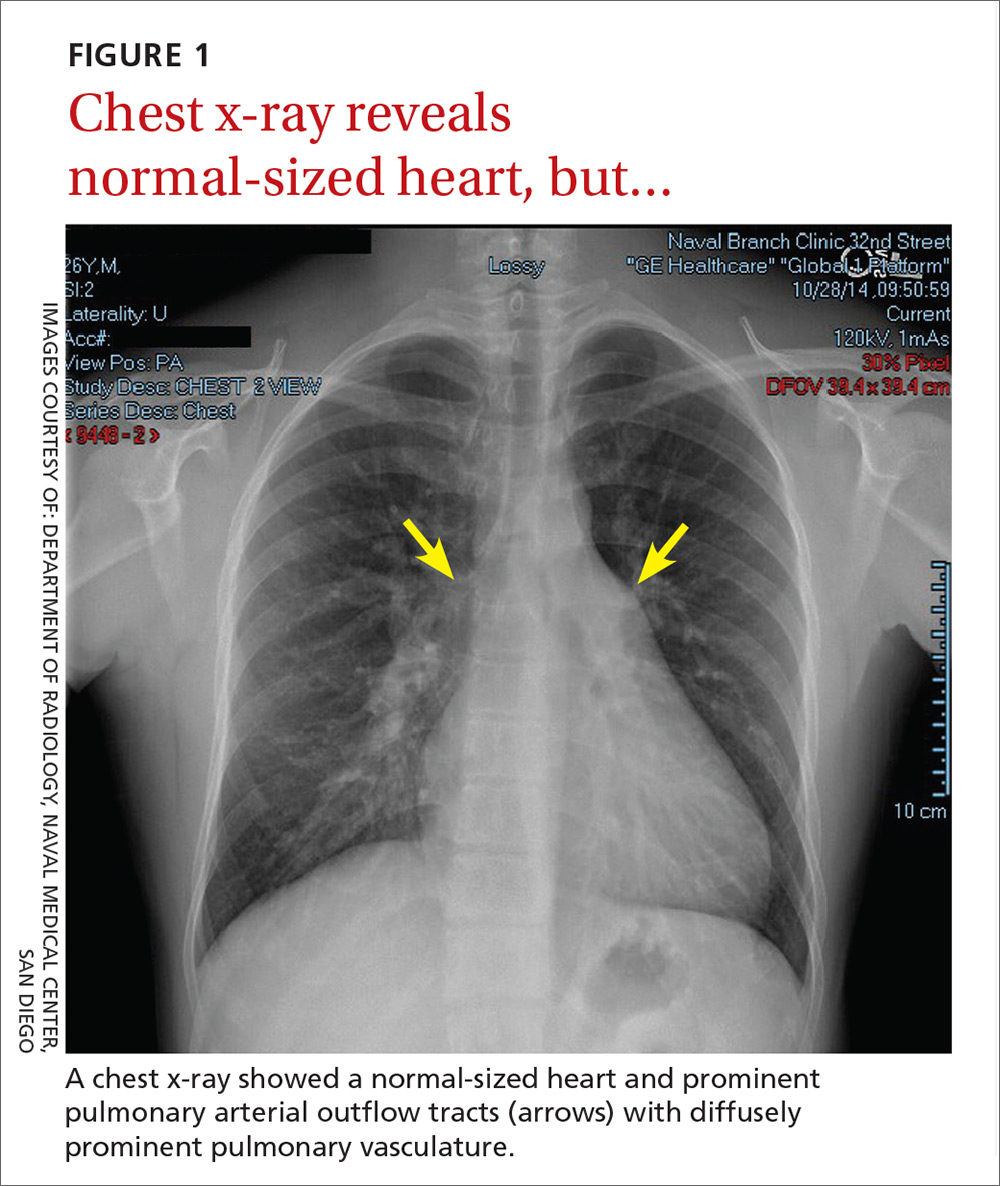
In light of the unusually loud heart sounds heard on exam, we performed an electrocardiogram. The EKG revealed a normal sinus rhythm, slight right axis deviation indicated by tall R-waves in V1 (also suggestive of right ventricular hypertrophy), an incomplete right bundle branch block, and a crochetage sign (a notch in the R-waves of the inferior leads).1 A chest x-ray (FIGURE 1) revealed a normal-sized heart and dilated pulmonary vasculature suggestive of pulmonary hypertension.

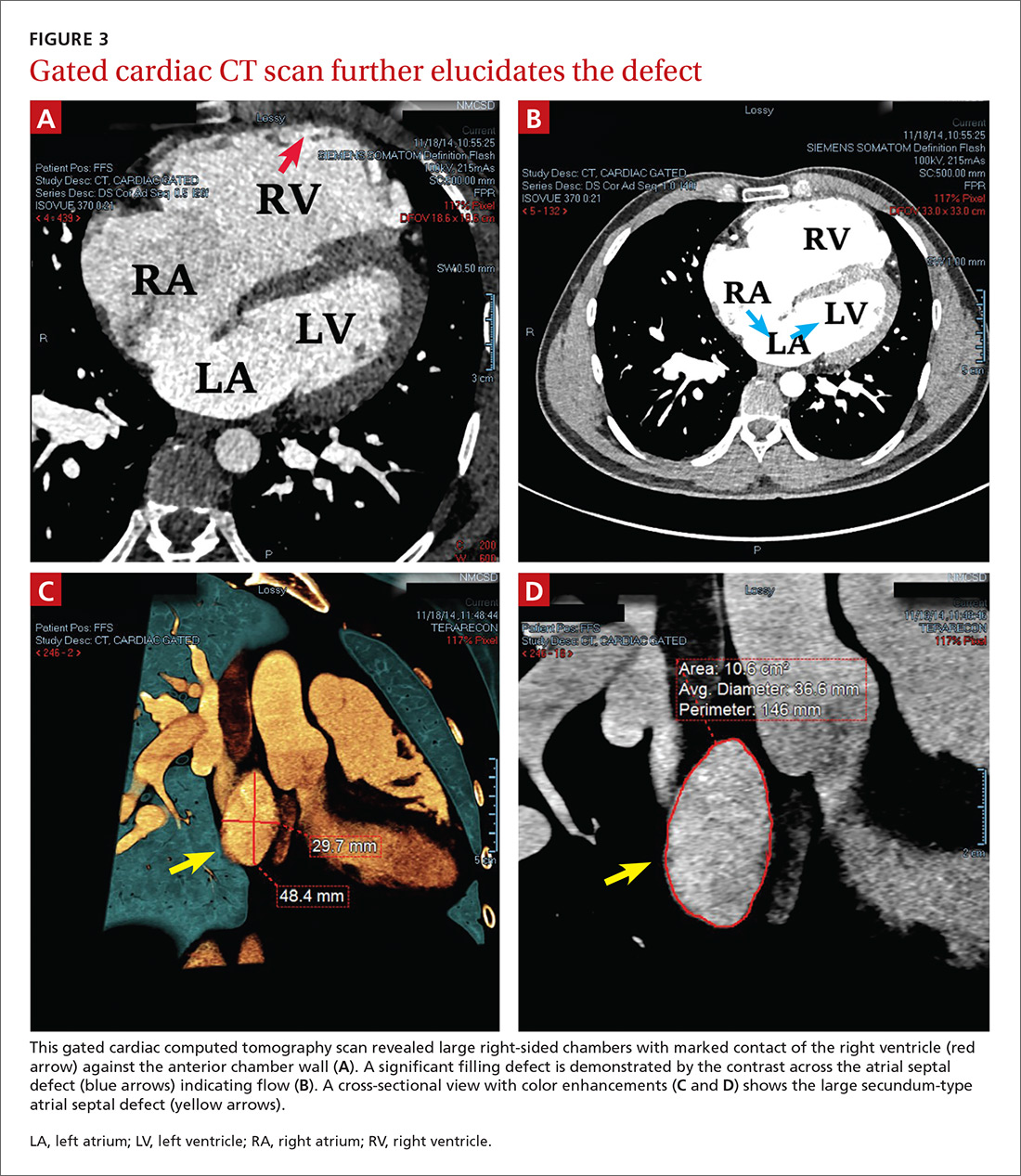
THE DIAGNOSIS
To further evaluate the cardiopulmonary findings, ultrasound studies (transthoracic and transesophageal echocardiography) were performed. These demonstrated a very large secundum-type atrial septal defect (ASD), measuring at its largest point about 30 × 48 mm (FIGURE 2 and FIGURE 3C). Doppler flow analysis and a bubble study (VIDEOS 1 and 2) demonstrated significant shunting across the ASD. Gated cardiac computed tomography (CT) was also used to characterize the ASD (FIGURE 3). It revealed that the superior and posterior rims of the ASD were essentially absent and that the right atrium and ventricle were severely enlarged, while the left chambers were normal in size and function with an ejection fraction >55%. The notching of the R-waves of the inferior leads, seen in our patient’s EKG, is typically seen with large ASDs.1,2
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with color Doppler flow (red) demonstrated significant shunting across a large atrial septal defect (white box). The largest white dot is positioned near the center of the defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with a bubble study showed injected air bubbles traversing the atrial septal defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
DISCUSSION
ASDs are typically uncovered on exam via auscultation of heart sounds, which might reveal a split of the second heart sound (S2) and diastolic murmurs. ASDs are typically classified by size, and their management depends on this factor, along with the patient’s age and symptoms. In children with small defects (<6 mm), treatment usually consists of conservative observation, as more than half of these ASDs will spontaneously close.3 But, as children age, they are more likely to engage in exertional activity (work, recreational sports) and an unrepaired ASD may yield symptoms (angina, dyspnea, fatigue, other cardiopulmonary strain). With such symptoms and when closure is not spontaneously achieved by adolescence or adulthood, an invasive approach is often necessary to correct the defect.
ASD repair. Traditionally, repair has involved some form of open thoracotomy. More recently, several minimally invasive techniques have been developed. Catheter-based device closure, in which a catheter is percutaneously guided to the defect and a patch is deployed to seal the ASD, is a technique that has been shown to successfully correct large ASDs of up to 40 mm in size.4 Robotic procedures have also been developed to correct ASDs through much smaller incisions.5 Both of these techniques require a significant rim of residual septal tissue around the defect.
Individualized approach. Since our patient had a rather large ASD that did not have sufficient residual septal rim tissue, percutaneous and robotic approaches were not feasible. Instead, he required more invasive cardiothoracic surgery. In cases such as this, the exact technique and type of incision (sternotomy vs access through the lateral chest wall) depend on age, gender, and the presence of other comorbidities.6
Our patient. Because there was concern that any approach other than a median one might not afford enough space to fix an ASD of such considerable size, our patient underwent a median sternotomy by a pediatric cardiothoracic surgeon who specialized in these repairs (in children as well as young adults). During the procedure, the ASD was accessed and confirmed to be as large as predicted by diagnostic imaging. A surgical patch was sutured in place to correct the defect. There were no intra-operative or postop complications.
Four weeks later, the patient had a mild pericardial effusion that was managed medically with daily furosemide and aspirin. At his 8-week postop appointment, the fluid accumulation had resolved, and he was completely asymptomatic. The patient returned to full-time active duty in the US Navy.
Adults with rather large ASDs can present in a relatively asymptomatic manner and report none of the classic complaints (angina, dyspnea, fatigue). They may even engage in heavy exertional activity with no difficulty. The underlying defect may be discovered incidentally on exam by noting a split of the S2 on auscultation. If pulmonary hypertension exists, the clinician may also note a loud S2. An exam that raises suspicion for an ASD can then be followed by tests that solidify the diagnosis. Surgery is usually necessary to correct an ASD in an adult who is symptomatic or exhibits significant cardiopulmonary strain.
1. Heller J, Hagège AA, Besse B, et al. “Crochetage” (notch) on R wave in inferior limb leads: a new independent electrocardiographic sign of atrial septal defect. J Am Coll Cardiol. 1996;27:877-882.
2. Kuijpers JM, Mulder BJM, Bouma BJ. Secundum atrial septal defect in adults: a practical review and recent developments. Neth Heart J. 2015;23:205-211.
3. McMahon CJ, Feltes TF, Fraley JK, et al. Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart. 2002;87:256-259.
4. Lopez K, Dalvi BV, Balzer D, et al. Transcatheter closure of large secundum atrial septal defects using the 40 mm amplatzer septal occluder: results of an international registry. Catheter Cardiovasc Interv. 2005;66:580-584.
5. Argenziano M, Oz MC, Kohmoto T, et al. Totally endoscopic atrial septal defect repair with robotic assistance. Circulation. 2003;108 Suppl 1:II191-II194.
6. Hopkins RA, Bert AA, Buchholz B, et al. Surgical patch closure of atrial septal defects. Ann Thorac Surg. 2004;77:2144-2149.
THE CASE
A 25-year-old man, who was an active duty US Navy sailor, went to his ship’s medical department complaining of a mild cough that he’d had for 2 days. He denied having any fevers, chills, night sweats, angina, or dyspnea. He said he hadn’t experienced any exertional fatigue or difficulty completing the rigorous physical tasks of his occupation as an engineman on the ship. The patient had no medical or surgical history of significance, and he wasn’t taking any medications or supplements.
On exam, he was not in acute distress and his vital signs were within normal limits. Auscultation revealed mild wheezing throughout the upper lung fields and loud heart sounds throughout his chest that were audible even with gentle contact of the stethoscope diaphragm. He had no discernible murmurs, rubs, or gallops.

In light of the unusually loud heart sounds heard on exam, we performed an electrocardiogram. The EKG revealed a normal sinus rhythm, slight right axis deviation indicated by tall R-waves in V1 (also suggestive of right ventricular hypertrophy), an incomplete right bundle branch block, and a crochetage sign (a notch in the R-waves of the inferior leads).1 A chest x-ray (FIGURE 1) revealed a normal-sized heart and dilated pulmonary vasculature suggestive of pulmonary hypertension.


THE DIAGNOSIS
To further evaluate the cardiopulmonary findings, ultrasound studies (transthoracic and transesophageal echocardiography) were performed. These demonstrated a very large secundum-type atrial septal defect (ASD), measuring at its largest point about 30 × 48 mm (FIGURE 2 and FIGURE 3C). Doppler flow analysis and a bubble study (VIDEOS 1 and 2) demonstrated significant shunting across the ASD. Gated cardiac computed tomography (CT) was also used to characterize the ASD (FIGURE 3). It revealed that the superior and posterior rims of the ASD were essentially absent and that the right atrium and ventricle were severely enlarged, while the left chambers were normal in size and function with an ejection fraction >55%. The notching of the R-waves of the inferior leads, seen in our patient’s EKG, is typically seen with large ASDs.1,2
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with color Doppler flow (red) demonstrated significant shunting across a large atrial septal defect (white box). The largest white dot is positioned near the center of the defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with a bubble study showed injected air bubbles traversing the atrial septal defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
DISCUSSION
ASDs are typically uncovered on exam via auscultation of heart sounds, which might reveal a split of the second heart sound (S2) and diastolic murmurs. ASDs are typically classified by size, and their management depends on this factor, along with the patient’s age and symptoms. In children with small defects (<6 mm), treatment usually consists of conservative observation, as more than half of these ASDs will spontaneously close.3 But, as children age, they are more likely to engage in exertional activity (work, recreational sports) and an unrepaired ASD may yield symptoms (angina, dyspnea, fatigue, other cardiopulmonary strain). With such symptoms and when closure is not spontaneously achieved by adolescence or adulthood, an invasive approach is often necessary to correct the defect.
ASD repair. Traditionally, repair has involved some form of open thoracotomy. More recently, several minimally invasive techniques have been developed. Catheter-based device closure, in which a catheter is percutaneously guided to the defect and a patch is deployed to seal the ASD, is a technique that has been shown to successfully correct large ASDs of up to 40 mm in size.4 Robotic procedures have also been developed to correct ASDs through much smaller incisions.5 Both of these techniques require a significant rim of residual septal tissue around the defect.
Individualized approach. Since our patient had a rather large ASD that did not have sufficient residual septal rim tissue, percutaneous and robotic approaches were not feasible. Instead, he required more invasive cardiothoracic surgery. In cases such as this, the exact technique and type of incision (sternotomy vs access through the lateral chest wall) depend on age, gender, and the presence of other comorbidities.6
Our patient. Because there was concern that any approach other than a median one might not afford enough space to fix an ASD of such considerable size, our patient underwent a median sternotomy by a pediatric cardiothoracic surgeon who specialized in these repairs (in children as well as young adults). During the procedure, the ASD was accessed and confirmed to be as large as predicted by diagnostic imaging. A surgical patch was sutured in place to correct the defect. There were no intra-operative or postop complications.
Four weeks later, the patient had a mild pericardial effusion that was managed medically with daily furosemide and aspirin. At his 8-week postop appointment, the fluid accumulation had resolved, and he was completely asymptomatic. The patient returned to full-time active duty in the US Navy.
Adults with rather large ASDs can present in a relatively asymptomatic manner and report none of the classic complaints (angina, dyspnea, fatigue). They may even engage in heavy exertional activity with no difficulty. The underlying defect may be discovered incidentally on exam by noting a split of the S2 on auscultation. If pulmonary hypertension exists, the clinician may also note a loud S2. An exam that raises suspicion for an ASD can then be followed by tests that solidify the diagnosis. Surgery is usually necessary to correct an ASD in an adult who is symptomatic or exhibits significant cardiopulmonary strain.
THE CASE
A 25-year-old man, who was an active duty US Navy sailor, went to his ship’s medical department complaining of a mild cough that he’d had for 2 days. He denied having any fevers, chills, night sweats, angina, or dyspnea. He said he hadn’t experienced any exertional fatigue or difficulty completing the rigorous physical tasks of his occupation as an engineman on the ship. The patient had no medical or surgical history of significance, and he wasn’t taking any medications or supplements.
On exam, he was not in acute distress and his vital signs were within normal limits. Auscultation revealed mild wheezing throughout the upper lung fields and loud heart sounds throughout his chest that were audible even with gentle contact of the stethoscope diaphragm. He had no discernible murmurs, rubs, or gallops.

In light of the unusually loud heart sounds heard on exam, we performed an electrocardiogram. The EKG revealed a normal sinus rhythm, slight right axis deviation indicated by tall R-waves in V1 (also suggestive of right ventricular hypertrophy), an incomplete right bundle branch block, and a crochetage sign (a notch in the R-waves of the inferior leads).1 A chest x-ray (FIGURE 1) revealed a normal-sized heart and dilated pulmonary vasculature suggestive of pulmonary hypertension.


THE DIAGNOSIS
To further evaluate the cardiopulmonary findings, ultrasound studies (transthoracic and transesophageal echocardiography) were performed. These demonstrated a very large secundum-type atrial septal defect (ASD), measuring at its largest point about 30 × 48 mm (FIGURE 2 and FIGURE 3C). Doppler flow analysis and a bubble study (VIDEOS 1 and 2) demonstrated significant shunting across the ASD. Gated cardiac computed tomography (CT) was also used to characterize the ASD (FIGURE 3). It revealed that the superior and posterior rims of the ASD were essentially absent and that the right atrium and ventricle were severely enlarged, while the left chambers were normal in size and function with an ejection fraction >55%. The notching of the R-waves of the inferior leads, seen in our patient’s EKG, is typically seen with large ASDs.1,2
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with color Doppler flow (red) demonstrated significant shunting across a large atrial septal defect (white box). The largest white dot is positioned near the center of the defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with a bubble study showed injected air bubbles traversing the atrial septal defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
DISCUSSION
ASDs are typically uncovered on exam via auscultation of heart sounds, which might reveal a split of the second heart sound (S2) and diastolic murmurs. ASDs are typically classified by size, and their management depends on this factor, along with the patient’s age and symptoms. In children with small defects (<6 mm), treatment usually consists of conservative observation, as more than half of these ASDs will spontaneously close.3 But, as children age, they are more likely to engage in exertional activity (work, recreational sports) and an unrepaired ASD may yield symptoms (angina, dyspnea, fatigue, other cardiopulmonary strain). With such symptoms and when closure is not spontaneously achieved by adolescence or adulthood, an invasive approach is often necessary to correct the defect.
ASD repair. Traditionally, repair has involved some form of open thoracotomy. More recently, several minimally invasive techniques have been developed. Catheter-based device closure, in which a catheter is percutaneously guided to the defect and a patch is deployed to seal the ASD, is a technique that has been shown to successfully correct large ASDs of up to 40 mm in size.4 Robotic procedures have also been developed to correct ASDs through much smaller incisions.5 Both of these techniques require a significant rim of residual septal tissue around the defect.
Individualized approach. Since our patient had a rather large ASD that did not have sufficient residual septal rim tissue, percutaneous and robotic approaches were not feasible. Instead, he required more invasive cardiothoracic surgery. In cases such as this, the exact technique and type of incision (sternotomy vs access through the lateral chest wall) depend on age, gender, and the presence of other comorbidities.6
Our patient. Because there was concern that any approach other than a median one might not afford enough space to fix an ASD of such considerable size, our patient underwent a median sternotomy by a pediatric cardiothoracic surgeon who specialized in these repairs (in children as well as young adults). During the procedure, the ASD was accessed and confirmed to be as large as predicted by diagnostic imaging. A surgical patch was sutured in place to correct the defect. There were no intra-operative or postop complications.
Four weeks later, the patient had a mild pericardial effusion that was managed medically with daily furosemide and aspirin. At his 8-week postop appointment, the fluid accumulation had resolved, and he was completely asymptomatic. The patient returned to full-time active duty in the US Navy.
Adults with rather large ASDs can present in a relatively asymptomatic manner and report none of the classic complaints (angina, dyspnea, fatigue). They may even engage in heavy exertional activity with no difficulty. The underlying defect may be discovered incidentally on exam by noting a split of the S2 on auscultation. If pulmonary hypertension exists, the clinician may also note a loud S2. An exam that raises suspicion for an ASD can then be followed by tests that solidify the diagnosis. Surgery is usually necessary to correct an ASD in an adult who is symptomatic or exhibits significant cardiopulmonary strain.
1. Heller J, Hagège AA, Besse B, et al. “Crochetage” (notch) on R wave in inferior limb leads: a new independent electrocardiographic sign of atrial septal defect. J Am Coll Cardiol. 1996;27:877-882.
2. Kuijpers JM, Mulder BJM, Bouma BJ. Secundum atrial septal defect in adults: a practical review and recent developments. Neth Heart J. 2015;23:205-211.
3. McMahon CJ, Feltes TF, Fraley JK, et al. Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart. 2002;87:256-259.
4. Lopez K, Dalvi BV, Balzer D, et al. Transcatheter closure of large secundum atrial septal defects using the 40 mm amplatzer septal occluder: results of an international registry. Catheter Cardiovasc Interv. 2005;66:580-584.
5. Argenziano M, Oz MC, Kohmoto T, et al. Totally endoscopic atrial septal defect repair with robotic assistance. Circulation. 2003;108 Suppl 1:II191-II194.
6. Hopkins RA, Bert AA, Buchholz B, et al. Surgical patch closure of atrial septal defects. Ann Thorac Surg. 2004;77:2144-2149.
1. Heller J, Hagège AA, Besse B, et al. “Crochetage” (notch) on R wave in inferior limb leads: a new independent electrocardiographic sign of atrial septal defect. J Am Coll Cardiol. 1996;27:877-882.
2. Kuijpers JM, Mulder BJM, Bouma BJ. Secundum atrial septal defect in adults: a practical review and recent developments. Neth Heart J. 2015;23:205-211.
3. McMahon CJ, Feltes TF, Fraley JK, et al. Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart. 2002;87:256-259.
4. Lopez K, Dalvi BV, Balzer D, et al. Transcatheter closure of large secundum atrial septal defects using the 40 mm amplatzer septal occluder: results of an international registry. Catheter Cardiovasc Interv. 2005;66:580-584.
5. Argenziano M, Oz MC, Kohmoto T, et al. Totally endoscopic atrial septal defect repair with robotic assistance. Circulation. 2003;108 Suppl 1:II191-II194.
6. Hopkins RA, Bert AA, Buchholz B, et al. Surgical patch closure of atrial septal defects. Ann Thorac Surg. 2004;77:2144-2149.
Does fish oil during pregnancy help prevent asthma in kids?
ILLUSTRATIVE CASE
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma and she is inquiring as to whether there is anything she can do to lower the risk of her second child developing asthma in the future. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 The Centers for Disease Control and Prevention (CDC) reported that 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreased the risk of atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial by Palmer et al, which included 706 women, showed no benefit for omega-3 supplementation.8 The second largest by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR]=0.37; 95% confidence interval [CI], 0.15-0.92; number needed to treat [NNT]=19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until 3 years of age, which is around the time that asthma can be formally diagnosed, potentially leading to under-reporting.8 In addition, the diagnosis of asthma was based on parent report of 3 episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (they did not report the rate without sensitization) was 1.8% in both arms, which is much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces incidence of asthma in children
This single-center, double-blinded RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after 3 years of age, the diagnosis of persistent wheeze was termed asthma) based on daily diary recordings of 5 episodes of troublesome lung symptoms within the last 6 months (each lasting for at least 3 consecutive days), rescue use of inhaled beta2-agonists, and/or relapse after a 3-month course of inhaled glucocorticoids. Secondary outcomes included lower respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study with 95.5% follow-up at 3 years and 93.1% follow-up at 5 years. The children had scheduled pediatric visits at 1 week; 1, 3, 6, 12, 18, 24, 30, and 36 months; and at 4 and 5 years, and acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of the mothers who received the fish oil had a lower risk of persistent wheeze or asthma at ages 3 to 5 years compared to those who received placebo (16.9% vs 23.7%; HR=0.69; 95% CI, 0.49-0.97; P=.035; NNT=14.7). But the effect of the fish oil supplementation was significant only in the children of the mothers with baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR=0.46; 95% CI, 0.25-0.83; P=.011; NNT=5.6). Similarly, in mothers who consumed the least EPA and DHA before the start of the study, fish oil supplementation had a greater benefit in terms of decreased wheeze and asthma (18.5% vs 32.4%; HR=0.55; 95% CI, 0.30-0.98; P=.043; NNT=7.2).
As for the secondary outcomes, only a reduction in lower respiratory tract infections was associated with the fish oil supplementation vs the control (38.8% vs 45.5%; HR=0.77; 95% CI, 0.61-0.99; P=.041; NNT=14.9). There was no reduction in asthma exacerbations, eczema, or risk of sensitization in the fish oil group.
WHAT'S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk of asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the United States.
CAVEATS
Questions remain: Ideal dose and which women to treat?
The US Food and Drug Administration currently recommends 8 to 12 ounces of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study,8 using 900 mg/d fish oil, did not show a benefit, whereas there did appear to be benefit in this study (2400 mg/d)1 and the Olsen study (2700 mg/d).9 Further research is needed to determine the optimal dosage.
The decreased risk of persistent wheeze or asthma was seen only in the children of the women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r=0.32; P<.001).1 Therefore, additional screening questions to determine fish consumption would be useful for identifying women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills and additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially-available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to an additional 4 pills/d for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dessemination Committee Report. Allergy. 2004;59:469-478.
3. Centers for Disease Control and Prevention. Asthma. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed October 9, 2017.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42:513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22:CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167-175.
10. Helgi Library. Fish consumption per capita by country. Available at: http://www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed September 27, 2017.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Federal Register.2017;82:6571-6574.
ILLUSTRATIVE CASE
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma and she is inquiring as to whether there is anything she can do to lower the risk of her second child developing asthma in the future. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 The Centers for Disease Control and Prevention (CDC) reported that 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreased the risk of atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial by Palmer et al, which included 706 women, showed no benefit for omega-3 supplementation.8 The second largest by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR]=0.37; 95% confidence interval [CI], 0.15-0.92; number needed to treat [NNT]=19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until 3 years of age, which is around the time that asthma can be formally diagnosed, potentially leading to under-reporting.8 In addition, the diagnosis of asthma was based on parent report of 3 episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (they did not report the rate without sensitization) was 1.8% in both arms, which is much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces incidence of asthma in children
This single-center, double-blinded RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after 3 years of age, the diagnosis of persistent wheeze was termed asthma) based on daily diary recordings of 5 episodes of troublesome lung symptoms within the last 6 months (each lasting for at least 3 consecutive days), rescue use of inhaled beta2-agonists, and/or relapse after a 3-month course of inhaled glucocorticoids. Secondary outcomes included lower respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study with 95.5% follow-up at 3 years and 93.1% follow-up at 5 years. The children had scheduled pediatric visits at 1 week; 1, 3, 6, 12, 18, 24, 30, and 36 months; and at 4 and 5 years, and acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of the mothers who received the fish oil had a lower risk of persistent wheeze or asthma at ages 3 to 5 years compared to those who received placebo (16.9% vs 23.7%; HR=0.69; 95% CI, 0.49-0.97; P=.035; NNT=14.7). But the effect of the fish oil supplementation was significant only in the children of the mothers with baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR=0.46; 95% CI, 0.25-0.83; P=.011; NNT=5.6). Similarly, in mothers who consumed the least EPA and DHA before the start of the study, fish oil supplementation had a greater benefit in terms of decreased wheeze and asthma (18.5% vs 32.4%; HR=0.55; 95% CI, 0.30-0.98; P=.043; NNT=7.2).
As for the secondary outcomes, only a reduction in lower respiratory tract infections was associated with the fish oil supplementation vs the control (38.8% vs 45.5%; HR=0.77; 95% CI, 0.61-0.99; P=.041; NNT=14.9). There was no reduction in asthma exacerbations, eczema, or risk of sensitization in the fish oil group.
WHAT'S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk of asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the United States.
CAVEATS
Questions remain: Ideal dose and which women to treat?
The US Food and Drug Administration currently recommends 8 to 12 ounces of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study,8 using 900 mg/d fish oil, did not show a benefit, whereas there did appear to be benefit in this study (2400 mg/d)1 and the Olsen study (2700 mg/d).9 Further research is needed to determine the optimal dosage.
The decreased risk of persistent wheeze or asthma was seen only in the children of the women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r=0.32; P<.001).1 Therefore, additional screening questions to determine fish consumption would be useful for identifying women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills and additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially-available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to an additional 4 pills/d for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma and she is inquiring as to whether there is anything she can do to lower the risk of her second child developing asthma in the future. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 The Centers for Disease Control and Prevention (CDC) reported that 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreased the risk of atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial by Palmer et al, which included 706 women, showed no benefit for omega-3 supplementation.8 The second largest by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR]=0.37; 95% confidence interval [CI], 0.15-0.92; number needed to treat [NNT]=19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until 3 years of age, which is around the time that asthma can be formally diagnosed, potentially leading to under-reporting.8 In addition, the diagnosis of asthma was based on parent report of 3 episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (they did not report the rate without sensitization) was 1.8% in both arms, which is much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces incidence of asthma in children
This single-center, double-blinded RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after 3 years of age, the diagnosis of persistent wheeze was termed asthma) based on daily diary recordings of 5 episodes of troublesome lung symptoms within the last 6 months (each lasting for at least 3 consecutive days), rescue use of inhaled beta2-agonists, and/or relapse after a 3-month course of inhaled glucocorticoids. Secondary outcomes included lower respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study with 95.5% follow-up at 3 years and 93.1% follow-up at 5 years. The children had scheduled pediatric visits at 1 week; 1, 3, 6, 12, 18, 24, 30, and 36 months; and at 4 and 5 years, and acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of the mothers who received the fish oil had a lower risk of persistent wheeze or asthma at ages 3 to 5 years compared to those who received placebo (16.9% vs 23.7%; HR=0.69; 95% CI, 0.49-0.97; P=.035; NNT=14.7). But the effect of the fish oil supplementation was significant only in the children of the mothers with baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR=0.46; 95% CI, 0.25-0.83; P=.011; NNT=5.6). Similarly, in mothers who consumed the least EPA and DHA before the start of the study, fish oil supplementation had a greater benefit in terms of decreased wheeze and asthma (18.5% vs 32.4%; HR=0.55; 95% CI, 0.30-0.98; P=.043; NNT=7.2).
As for the secondary outcomes, only a reduction in lower respiratory tract infections was associated with the fish oil supplementation vs the control (38.8% vs 45.5%; HR=0.77; 95% CI, 0.61-0.99; P=.041; NNT=14.9). There was no reduction in asthma exacerbations, eczema, or risk of sensitization in the fish oil group.
WHAT'S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk of asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the United States.
CAVEATS
Questions remain: Ideal dose and which women to treat?
The US Food and Drug Administration currently recommends 8 to 12 ounces of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study,8 using 900 mg/d fish oil, did not show a benefit, whereas there did appear to be benefit in this study (2400 mg/d)1 and the Olsen study (2700 mg/d).9 Further research is needed to determine the optimal dosage.
The decreased risk of persistent wheeze or asthma was seen only in the children of the women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r=0.32; P<.001).1 Therefore, additional screening questions to determine fish consumption would be useful for identifying women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills and additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially-available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to an additional 4 pills/d for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dessemination Committee Report. Allergy. 2004;59:469-478.
3. Centers for Disease Control and Prevention. Asthma. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed October 9, 2017.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42:513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22:CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167-175.
10. Helgi Library. Fish consumption per capita by country. Available at: http://www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed September 27, 2017.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Federal Register.2017;82:6571-6574.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dessemination Committee Report. Allergy. 2004;59:469-478.
3. Centers for Disease Control and Prevention. Asthma. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed October 9, 2017.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42:513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22:CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167-175.
10. Helgi Library. Fish consumption per capita by country. Available at: http://www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed September 27, 2017.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Federal Register.2017;82:6571-6574.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Fish oil supplementation taken by women in the third trimester of pregnancy can reduce the risk of persistent wheeze, asthma, and infections of the lower respiratory tract in their children.1
STRENGTH OF RECOMMENDATION
B: Based on 2 double-blinded randomized controlled trials (RCTs).
Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.1
Don’t give up on influenza vaccine
I suspect most health care providers have heard the complaint, “The vaccine doesn’t work. One year I got the vaccine, and I still came down with the flu.”
Over the years, I’ve polished my responses to vaccine naysayers.
Influenza vaccine doesn’t protect you against every virus that can cause cold and flu symptoms. It only prevents influenza. It’s possible you had a different virus, such as adenovirus, coronavirus, parainfluenza virus, or respiratory syncytial virus.
Some years, the vaccine works better than others because there is a mismatch between the viruses chosen for the vaccine, and the viruses that end up circulating. Even when it doesn’t prevent flu, the vaccine can potentially reduce the severity of illness.
The discussion became a little more complicated in 2016 when the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices withdrew its support for the live attenuated influenza virus vaccine (LAIV4) because of concerns about effectiveness. During the 2015-2016 influenza season, LAIV4 demonstrated no statistically significant effectiveness in children 2-17 years of age against H1N1pdm09, the predominant influenza strain. Fortunately, inactivated injectable vaccine did offer protection. An estimated 41.8 million children aged 6 months to 17 years ultimately received this vaccine during the 2016-2017 influenza season.
Now with the 2017-2018 influenza season in full swing, some media reports are proclaiming the influenza vaccine is only 10% effective this year. This claim is based on an interim analysis of data from the most recent flu season in Australia and the effectiveness of the vaccine against the circulating H3N2 virus strain. News from the U.S. CDC is more encouraging. The H3N2 virus contained in this year’s vaccine is the same as that used last year, and so far, circulating H3N2 viruses in the United States are similar to the vaccine virus. Public health officials suggest that we can hope that the vaccine works as well as it did last year, when overall vaccine effectiveness against all circulating flu viruses was 39%, and effectiveness against the H3N2 virus specifically was 32%.
I’m upping my game when talking to parents about flu vaccine. I mention one study conducted between 2010 and 2012 in which influenza immunization reduced a child’s risk of being admitted to an intensive care unit with flu by 74% (J Infect Dis. 2014 Sep 1;210[5]:674-83). I emphasize that flu vaccine reduces the chance that a child will die from flu. According to a study published in 2017, influenza vaccine reduced the risk of death from flu by 65% in healthy children and 51% in children with high-risk medical conditions (Pediatrics. 2017 May. doi: 10.1542/peds.2016-4244).
When I’m talking to trainees, I no longer just focus on the match between circulating strains of flu and vaccine strains. I mention that viruses used to produce most seasonal flu vaccines are grown in eggs, a process that can result in minor antigenic changes in the hemagglutinin protein, especially in H3N2 viruses. These “egg-adapted changes” may result in a vaccine that stimulates a less effective immune response, even with a good match between circulating strains and vaccine strains. For example, Zost et al. found that the H3N2 virus that emerged during the 2014-2015 season possessed a new hemagglutinin-associated glycosylation site (Proc Natl Acad Sci U S A. 2017 Nov 21;114[47]:12578-83). Although this virus was represented in the 2016-2017 influenza vaccine, the egg-adapted version lost the glycosylation site, resulting in decreased vaccine immunogenicity and less protection against H3N2 viruses circulating in the community.
The real take-home message here is that we need better flu vaccines. In the short term, cell-based flu vaccines that use virus grown in animal cells are a potential alternative to egg-based vaccines. In the long term, we need a universal flu vaccine. The National Institute of Allergy and Infectious Diseases is prioritizing work on a vaccine that could provide long-lasting protection against multiple subtypes of the virus. According to a report on the National Institutes of Health website, such a vaccine could “eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains,” including those with the potential to cause a pandemic. The NIH researchers acknowledge, however, that achieving this goal will require “a broad range of expertise and substantial resources.”
Until new vaccines are available, we need to do a better job of using available, albeit imperfect, flu vaccines. During the 2016-2017 season, only 59% of children 6 months to 17 years were immunized, and there were 110 influenza-associated deaths in children, according to the CDC. It’s likely that some of these were preventable.
The total magnitude of suffering associated with flu is more difficult to quantify, but anecdotes can be illuminating. A friend recently diagnosed with influenza shared her experience via Facebook. “Rough night. I’m seconds away from a meltdown. My body aches so bad that I can’t get comfortable on the couch or my bed. Can’t breathe, and I cough until I vomit. My head is about to burst along with my ears. Just took a hot bath hoping that would help. I don’t know what else to do. The flu really sucks.”
Indeed. Even a 1 in 10 chance of preventing the flu is better than no chance at all.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
I suspect most health care providers have heard the complaint, “The vaccine doesn’t work. One year I got the vaccine, and I still came down with the flu.”
Over the years, I’ve polished my responses to vaccine naysayers.
Influenza vaccine doesn’t protect you against every virus that can cause cold and flu symptoms. It only prevents influenza. It’s possible you had a different virus, such as adenovirus, coronavirus, parainfluenza virus, or respiratory syncytial virus.
Some years, the vaccine works better than others because there is a mismatch between the viruses chosen for the vaccine, and the viruses that end up circulating. Even when it doesn’t prevent flu, the vaccine can potentially reduce the severity of illness.
The discussion became a little more complicated in 2016 when the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices withdrew its support for the live attenuated influenza virus vaccine (LAIV4) because of concerns about effectiveness. During the 2015-2016 influenza season, LAIV4 demonstrated no statistically significant effectiveness in children 2-17 years of age against H1N1pdm09, the predominant influenza strain. Fortunately, inactivated injectable vaccine did offer protection. An estimated 41.8 million children aged 6 months to 17 years ultimately received this vaccine during the 2016-2017 influenza season.
Now with the 2017-2018 influenza season in full swing, some media reports are proclaiming the influenza vaccine is only 10% effective this year. This claim is based on an interim analysis of data from the most recent flu season in Australia and the effectiveness of the vaccine against the circulating H3N2 virus strain. News from the U.S. CDC is more encouraging. The H3N2 virus contained in this year’s vaccine is the same as that used last year, and so far, circulating H3N2 viruses in the United States are similar to the vaccine virus. Public health officials suggest that we can hope that the vaccine works as well as it did last year, when overall vaccine effectiveness against all circulating flu viruses was 39%, and effectiveness against the H3N2 virus specifically was 32%.
I’m upping my game when talking to parents about flu vaccine. I mention one study conducted between 2010 and 2012 in which influenza immunization reduced a child’s risk of being admitted to an intensive care unit with flu by 74% (J Infect Dis. 2014 Sep 1;210[5]:674-83). I emphasize that flu vaccine reduces the chance that a child will die from flu. According to a study published in 2017, influenza vaccine reduced the risk of death from flu by 65% in healthy children and 51% in children with high-risk medical conditions (Pediatrics. 2017 May. doi: 10.1542/peds.2016-4244).
When I’m talking to trainees, I no longer just focus on the match between circulating strains of flu and vaccine strains. I mention that viruses used to produce most seasonal flu vaccines are grown in eggs, a process that can result in minor antigenic changes in the hemagglutinin protein, especially in H3N2 viruses. These “egg-adapted changes” may result in a vaccine that stimulates a less effective immune response, even with a good match between circulating strains and vaccine strains. For example, Zost et al. found that the H3N2 virus that emerged during the 2014-2015 season possessed a new hemagglutinin-associated glycosylation site (Proc Natl Acad Sci U S A. 2017 Nov 21;114[47]:12578-83). Although this virus was represented in the 2016-2017 influenza vaccine, the egg-adapted version lost the glycosylation site, resulting in decreased vaccine immunogenicity and less protection against H3N2 viruses circulating in the community.
The real take-home message here is that we need better flu vaccines. In the short term, cell-based flu vaccines that use virus grown in animal cells are a potential alternative to egg-based vaccines. In the long term, we need a universal flu vaccine. The National Institute of Allergy and Infectious Diseases is prioritizing work on a vaccine that could provide long-lasting protection against multiple subtypes of the virus. According to a report on the National Institutes of Health website, such a vaccine could “eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains,” including those with the potential to cause a pandemic. The NIH researchers acknowledge, however, that achieving this goal will require “a broad range of expertise and substantial resources.”
Until new vaccines are available, we need to do a better job of using available, albeit imperfect, flu vaccines. During the 2016-2017 season, only 59% of children 6 months to 17 years were immunized, and there were 110 influenza-associated deaths in children, according to the CDC. It’s likely that some of these were preventable.
The total magnitude of suffering associated with flu is more difficult to quantify, but anecdotes can be illuminating. A friend recently diagnosed with influenza shared her experience via Facebook. “Rough night. I’m seconds away from a meltdown. My body aches so bad that I can’t get comfortable on the couch or my bed. Can’t breathe, and I cough until I vomit. My head is about to burst along with my ears. Just took a hot bath hoping that would help. I don’t know what else to do. The flu really sucks.”
Indeed. Even a 1 in 10 chance of preventing the flu is better than no chance at all.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
I suspect most health care providers have heard the complaint, “The vaccine doesn’t work. One year I got the vaccine, and I still came down with the flu.”
Over the years, I’ve polished my responses to vaccine naysayers.
Influenza vaccine doesn’t protect you against every virus that can cause cold and flu symptoms. It only prevents influenza. It’s possible you had a different virus, such as adenovirus, coronavirus, parainfluenza virus, or respiratory syncytial virus.
Some years, the vaccine works better than others because there is a mismatch between the viruses chosen for the vaccine, and the viruses that end up circulating. Even when it doesn’t prevent flu, the vaccine can potentially reduce the severity of illness.
The discussion became a little more complicated in 2016 when the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices withdrew its support for the live attenuated influenza virus vaccine (LAIV4) because of concerns about effectiveness. During the 2015-2016 influenza season, LAIV4 demonstrated no statistically significant effectiveness in children 2-17 years of age against H1N1pdm09, the predominant influenza strain. Fortunately, inactivated injectable vaccine did offer protection. An estimated 41.8 million children aged 6 months to 17 years ultimately received this vaccine during the 2016-2017 influenza season.
Now with the 2017-2018 influenza season in full swing, some media reports are proclaiming the influenza vaccine is only 10% effective this year. This claim is based on an interim analysis of data from the most recent flu season in Australia and the effectiveness of the vaccine against the circulating H3N2 virus strain. News from the U.S. CDC is more encouraging. The H3N2 virus contained in this year’s vaccine is the same as that used last year, and so far, circulating H3N2 viruses in the United States are similar to the vaccine virus. Public health officials suggest that we can hope that the vaccine works as well as it did last year, when overall vaccine effectiveness against all circulating flu viruses was 39%, and effectiveness against the H3N2 virus specifically was 32%.
I’m upping my game when talking to parents about flu vaccine. I mention one study conducted between 2010 and 2012 in which influenza immunization reduced a child’s risk of being admitted to an intensive care unit with flu by 74% (J Infect Dis. 2014 Sep 1;210[5]:674-83). I emphasize that flu vaccine reduces the chance that a child will die from flu. According to a study published in 2017, influenza vaccine reduced the risk of death from flu by 65% in healthy children and 51% in children with high-risk medical conditions (Pediatrics. 2017 May. doi: 10.1542/peds.2016-4244).
When I’m talking to trainees, I no longer just focus on the match between circulating strains of flu and vaccine strains. I mention that viruses used to produce most seasonal flu vaccines are grown in eggs, a process that can result in minor antigenic changes in the hemagglutinin protein, especially in H3N2 viruses. These “egg-adapted changes” may result in a vaccine that stimulates a less effective immune response, even with a good match between circulating strains and vaccine strains. For example, Zost et al. found that the H3N2 virus that emerged during the 2014-2015 season possessed a new hemagglutinin-associated glycosylation site (Proc Natl Acad Sci U S A. 2017 Nov 21;114[47]:12578-83). Although this virus was represented in the 2016-2017 influenza vaccine, the egg-adapted version lost the glycosylation site, resulting in decreased vaccine immunogenicity and less protection against H3N2 viruses circulating in the community.
The real take-home message here is that we need better flu vaccines. In the short term, cell-based flu vaccines that use virus grown in animal cells are a potential alternative to egg-based vaccines. In the long term, we need a universal flu vaccine. The National Institute of Allergy and Infectious Diseases is prioritizing work on a vaccine that could provide long-lasting protection against multiple subtypes of the virus. According to a report on the National Institutes of Health website, such a vaccine could “eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains,” including those with the potential to cause a pandemic. The NIH researchers acknowledge, however, that achieving this goal will require “a broad range of expertise and substantial resources.”
Until new vaccines are available, we need to do a better job of using available, albeit imperfect, flu vaccines. During the 2016-2017 season, only 59% of children 6 months to 17 years were immunized, and there were 110 influenza-associated deaths in children, according to the CDC. It’s likely that some of these were preventable.
The total magnitude of suffering associated with flu is more difficult to quantify, but anecdotes can be illuminating. A friend recently diagnosed with influenza shared her experience via Facebook. “Rough night. I’m seconds away from a meltdown. My body aches so bad that I can’t get comfortable on the couch or my bed. Can’t breathe, and I cough until I vomit. My head is about to burst along with my ears. Just took a hot bath hoping that would help. I don’t know what else to do. The flu really sucks.”
Indeed. Even a 1 in 10 chance of preventing the flu is better than no chance at all.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Heart failure treatment: Keeping up with best practices
Heart failure (HF) affects nearly 6 million Americans and accounts for one million hospital admissions each year.1 The condition, which results from a structural or functional disorder that impairs the ventricles’ ability to fill, empty, or both,2 is a major cause of morbidity and mortality. The 5-year mortality rate ranges from 44% to 77%.3,4
Growing evidence demonstrates reduced morbidity and mortality when patients with HF with reduced ejection fraction (HFrEF) are treated with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB); a beta-blocker; and a mineralocorticoid/aldosterone receptor antagonist (MRA) in appropriate doses.5 In addition, 2 new medications representing novel drug classes have recently entered the market and are recommended in select patients who remain symptomatic despite standard treatment.
The first is sacubitril, which is available in a combination pill with the ARB valsartan, and the second is ivabradine.6 Additionally, implanted medical devices are proving useful, particularly in the management of patients with refractory symptoms.
This article will briefly review the diagnosis and initial evaluation of the patient with suspected HF and then describe how newer treatments fit within HF management priorities and strategies. But first, a word about what causes HF.
Causes are many and diverse
HF has a variety of cardiac and non-cardiac etiologies.2,7,8 Some important cardiac causes include hypertension (HTN), coronary artery disease (CAD), valvular heart disease, arrhythmias, myocarditis, Takotsubo cardiomyopathy, and postpartum cardiomyopathy. Common and important non-cardiac causes of HF include alcoholic cardiomyopathy, pulmonary embolism, pulmonary hypertension, obstructive sleep apnea, anemia, hemochromatosis, amyloidosis, sarcoidosis, thyroid dysfunction, nephrotic syndrome, and cardiac toxins (especially stimulants and certain chemotherapy drugs).2,7,8
Diagnosing an elusive culprit
HF remains a clinical diagnosis. Common symptoms include dyspnea, cough, pedal edema, and decreased exercise tolerance, but these symptoms are not at all specific. Given the varied causes and manifestations of HF, the diagnosis can be somewhat elusive. Fortunately, there are a number of objective methods to help identify patients with HF.
Framingham criteria. One commonly used tool for making the diagnosis of HF is the Framingham criteria (see https://www.mdcalc.com/framingham-heart-failure-diagnostic-criteria),9 which diagnoses HF based on historical and physical exam findings. Another well-validated decision tool is the Heart Failure Diagnostic Rule (see http://circ.ahajournals.org/content/124/25/2865.long),10 which incorporates N-terminal pro–B-type natriuretic peptide (NT-proBNP) results, as well as exam findings.
Measurement of natriuretic peptides, either B-type natriuretic peptide (BNP) or NT-proBNP, aids in the diagnosis of HF.5 Although several factors (including age, weight, and renal function) can affect BNP levels, a normal BNP value effectively rules out HF5,7 and an elevated BNP can help to make the diagnosis in the context of a patient with corresponding symptoms.
The initial evaluation: Necessary lab work and imaging studies
The purpose of the initial evaluation of the patient with suspected HF is to establish the diagnosis, look for underlying etiologies of HF, identify comorbidities, and establish baseline values (eg, of potassium and creatinine) for elements monitored during treatment.5,7 TABLE 15,7 lists the lab work and imaging tests that are commonly ordered in the initial evaluation of the patient with HF.
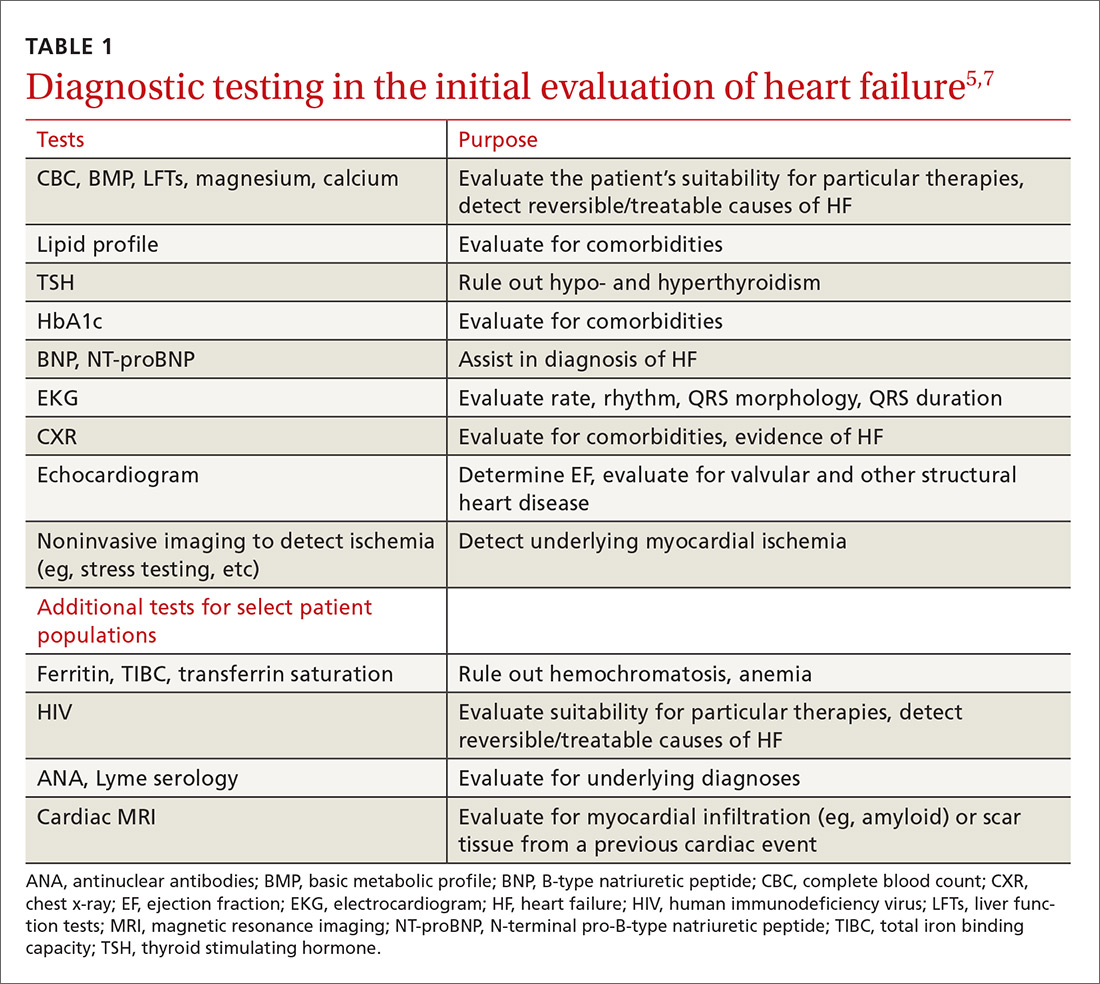
Echocardiography is useful in determining the ejection fraction (EF), which is essential in guiding treatment. Echocardiography can also identify important structural abnormalities including significant valvular disease. Refer patients with severe valvular disease for evaluation for valve repair/replacement, regardless of EF.8
Noninvasive testing (stress nuclear imaging or echocardiography) to evaluate for underlying CAD is reasonable in patients with unknown CAD status.8,11 Patients for whom there is a high suspicion of obstructive CAD should undergo coronary angiography if they are candidates for revascularization.5,7 Noninvasive testing may also be an acceptable option for assessing ischemia in patients presenting with HF who have known CAD and no angina.5
Classification of HF is determined by ejection fraction
Physicians have traditionally classified patients with HF as having either systolic or diastolic dysfunction. Patients with HF symptoms and a reduced EF were said to have systolic dysfunction; those with a normal EF were said to have diastolic dysfunction.
More recently, researchers have learned that patients with reduced EF and those with preserved EF can have both systolic and diastolic dysfunction simultaneously.8 Therefore, the current preferred terminology is HFpEF (heart failure with preserved ejection fraction) for those with an EF ≥50% and HFrEF (heart failure with reduced ejection fraction) for those with an EF ≤40%.5 Both the American Heart Association (AHA) and the European Society of Cardiology recognize a category of HF with moderately reduced ejection fraction defined as an EF between 40% and 50%.5,7 Practically speaking, this group is treated as per the guidelines for HFrEF.5
Treatment of HFrEF: The evidence is clear
The cornerstone of medical treatment for HFrEF is the combination of an ACE inhibitor or ARB with a beta-blocker.2,5,7,8 Several early trials showed clear benefits of these medications. For example, the Studies Of Left Ventricular Dysfunction trial (SOLVD), compared enalapril to placebo in patients receiving standard therapy (consisting chiefly of digitalis, diuretics, and nitrates). This study demonstrated a reduction in all-cause mortality or first hospitalization for HF (number needed to treat [NNT]=21) in the enalapril group vs the placebo group.12
Similarly, a subgroup analysis of the Valsartan Heart Failure Treatment (Val-HeFT) trial demonstrated morbidity (NNT=10) and all-cause mortality benefits (NNT=6) when valsartan (an ARB) was given to patients who were not receiving an ACE inhibitor.13
MERIT-HF (Metoprolol CR/XL Randomised Intervention Trial in congestive Heart Failure) compared the beta-blocker metoprolol succinate to placebo and found fewer deaths from HF and lower all-cause mortality (NNT=26) associated with the treatment group vs the placebo group.14
And a comparison of 2 beta-blockers—carvedilol and metoprolol tartrate—on clinical outcomes in patients with chronic HF in the Carvedilol Or Metoprolol European Trial (COMET) showed that carvedilol extended survival compared with metoprolol tartrate (NNT=19).15
Unlike ACE inhibitors and ARBs, which seem to show a class benefit, only 3 beta-blockers available in the United States have been proven to reduce mortality: sustained-release metoprolol succinate, carvedilol, and bisoprolol.2,7,8
Unless contraindicated, all patients with a reduced EF—even those without symptoms—should receive a beta-blocker and an ACE inhibitor or ARB.5,7,8
Cautionary notes
Remember the following caveats when treating patients with ACE inhibitors, ARBs, and beta-blockers:
- Use ACE inhibitors and ARBs with caution in patients with impaired renal function (serum creatinine >2.5 mg/dL) or elevated serum potassium (>5 mEq/L).16,17
- ARBs are associated with a much lower incidence of cough and angioedema than ACE inhibitors.18
- Although physicians frequently start patients on low doses of beta-blockers and ACE inhibitors or ARBs to minimize hypotension and other adverse effects, the goal of therapy is to titrate up to the therapeutic doses used in clinical trials.5-7 (For dosages of medications commonly used in the treatment of heart failure, see Table 3 in the American College of Cardiology/AHA/Heart Failure Society of America guidelines available at https://www.sciencedirect.com/science/article/pii/S0735109717370870?via%3Dihub#tbl3 and Table 7.2 in the European Society of Cardiology guidelines available at https://academic.oup.com/eurheartj/article/37/27/2129/1748921.)
- Because beta-blockers can exacerbate fluid retention, do not initiate them in patients with fluid overload unless such patients are being treated with diuretics.5,19
When more Tx is needed
For patients who remain symptomatic despite treatment with an ACE inhibitor or ARB and a beta-blocker, consider the following add-on therapies.
Diuretics are the only medications used in the treatment of HF that adequately reduce fluid overload.2,7 While thiazide diuretics confer greater blood pressure control, loop diuretics are generally preferred in the treatment of HF because they are more efficacious.5 Loop diuretics should be prescribed to all patients with fluid overload, as few patients can maintain their target (“dry”) weight without diuretic therapy.5,7 Common adverse effects include hypokalemia, dehydration, and azotemia.
Two MRAs are currently available in the United States: spironolactone and eplerenone. MRAs are used as add-on therapy for symptomatic patients with an EF ≤35% or an EF ≤40% following an acute myocardial infarction (MI).5 They significantly reduce all-cause mortality (NNT=26).20
Because hyperkalemia is a risk with MRAs, do not prescribe them for patients who are already taking both an ACE inhibitor and an ARB.5 Also, do not initiate MRA therapy in patients who have an elevated creatinine level (≥2.5 mg/dL in men; ≥2 mg/dL in women) or a potassium level ≥5 mEq/L.5,7,8 Discontinue MRA therapy if a patient’s potassium level rises to ≥5.5 mEq/L.5
Hydralazine combined with isosorbide dinitrate (H/ID) is an alternative in patients for whom ACE inhibitor/ARB therapy is contraindicated.5,8
H/ID is also an add-on option in African American patients. Trials have demonstrated that H/ID reduces both first hospitalization for HF (NNT=13) and all-cause mortality (NNT=25) when it is used as add-on therapy in African Americans already receiving standard therapy with an ACE inhibitor or ARB, a beta-blocker, and an MRA.21 Headache and dizziness are commonly reported adverse effects.
Digoxin does not reduce mortality, but it does improve both quality of life and exercise tolerance and reduces hospital admissions for patients with HF.5,7 Significant adverse effects of digoxin include anorexia, nausea, visual disturbances, and cardiac arrhythmias.22
Also, hypokalemia can intensify digoxin toxicity.23 Because of these concerns, digoxin is typically dosed at 0.125 mg/d (0.125 mg every other day in patients >70 years or patients with impaired renal function or low body weight) with a target therapeutic range of 0.5 to 0.9 ng/mL.5
New classes, new agents
Sacubitril, a neprilysin inhibitor, is the first drug from this class approved for use in the United States. Neprilysin is the enzyme responsible for the degradation of natriuretic peptides; as such it increases endogenous NPs, promoting diuresis and lowering blood pressure.24,25 Early trials with sacubitril alone showed limited clinical efficacy;25 however, when it was combined with the ARB, valsartan (the combination being called angiotensin receptor blocker + neprilysin inhibitor [ARNI] therapy), it was found to be of significant benefit.6,25
The PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trial compared outcomes in patients receiving ARNI therapy to those receiving enalapril.26 The authors stopped the trial early due to the overwhelming benefit seen in the ARNI arm.
After a median follow-up of 27 months, the researchers found a reduction in the primary outcomes of either cardiovascular death or first hospitalization for HF (26.5% in the enalapril-treated group vs 21.8% in the ARNI-treated group; NNT=21).26 There were slightly more cases of angioedema in the ARNI arm than in the enalapril arm (0.5% vs 0.2%), although there were no patients in the trial who required endotracheal intubation.26
Because of this increased risk, do not prescribe ARNI therapy for any patient with a history of angioedema.6 Hypotension was more common in the ARNI-treated group than in the enalapril group (14% vs 9.2%), but there were lower rates of hyperkalemia, elevated serum creatinine, and cough in the ARNI-treated group than in the enalapril group.26
Consider ARNI treatment for all patients with an EF ≤40% who remain symptomatic despite appropriate doses of an ACE inhibitor or ARB plus a beta-blocker. Do not administer ARNI therapy concomitantly with an ACE inhibitor or ARB. When switching, do not start ARNI therapy for at least 36 hours after the last dose of an ACE inhibitor or ARB.6
Ivabradine is a sinoatrial node modulator that provides additional heart rate reduction. It does not affect ventricular repolarization or myocardial contractility.27 Early trials with this medication have shown reduced cardiac mortality and an NNT to prevent one first HF hospitalization within one year of 27.28 Adverse effects include symptomatic and asymptomatic bradycardia and luminous phenomena.28
Recommend ivabradine as add-on therapy to all patients with an EF ≤35%, normal sinus rhythm, and resting heart rate ≥70 bpm who remain symptomatic despite taking the maximum-tolerated dose of a beta-blocker.6 The dose is adjusted to achieve a resting heart rate of 50 to 60 bpm.27
Nonpharmacologic options
Implantable cardioverter defibrillators (ICDs) are recommended as primary prevention in select HFrEF patients to reduce the risk of sudden cardiac death and all-cause mortality. The 2013 American College of Cardiology Foundation/AHA Guideline for the Management of Heart Failure recommends an ICD for primary prevention for: 1) patients with symptomatic HF and an LVEF ≤35% despite ≥3 months of optimal medical therapy, and 2) patients at least 40 days post-MI with an LVEF of ≤30%.5,29 ICDs are not recommended for patients who have a life expectancy of less than one year, and the devices are of unclear benefit for patients ≥75 years of age.5
Cardiac resynchronization therapy (CRT), although not new to the field of cardiology, is new to the treatment of heart failure. A number of patients with HFrEF have QRS prolongation and in particular, left bundle branch block (LBBB).5 CRT uses biventricular pacing to restore synchronous contraction of the left and right ventricles.30 It is strongly recommended for patients with an EF ≤35%, sinus rhythm, LBBB, QRS ≥150 ms, and a life expectancy of at least one year.5,7 It is weakly recommended for patients with an EF ≤35% and a QRS ≥150 ms but without LBBB. It’s also weakly recommended for patients with an EF ≤35% and LBBB with a QRS of 120 to 150 ms.5,31
Left ventricular assist devices (LVADs) and cardiac transplantation are considerations for patients with severe symptoms refractory to all other interventions.5 LVADs may be used either while awaiting cardiac transplantation (bridge therapy) or as definitive treatment (destination therapy). Appropriate patient selection for such therapies requires a team of experts that ideally includes HF and transplantation cardiologists, cardiothoracic surgeons, nurses, social workers, and palliative care clinicians.5
Treatment of HFpEF: Evidence is lacking
While HFpEF is common—affecting about half of all patients with HF—ideal treatment remains unclear.32 Some trials have shown promise, but to date no unequivocal evidence exists that any standard therapy reduces mortality in patients with HFpEF.33-37 Underlying mechanisms of action of HFpEF include cardiac rate and rhythm abnormalities, atrial dysfunction, and stiffening of the ventricles. In a sense, it represents an exaggerated expression of the pathophysiology seen with the normal aging of the heart and can be conceptualized as “presbycardia.”37 Indeed, HFpEF is more common in the elderly, but it is also more common in patients of African descent.38,39 Common contributing causes (which we’ll get to in a bit) include HTN, CAD, atrial fibrillation (AF), obesity, and obstructive sleep apnea (OSA).
Trials have failed to show clear benefit for ACE inhibitors, ARBs, or beta-blockers.7,33 The evidence for MRAs is somewhat unclear; however, they have recently been recommended as an option for patients who have been hospitalized in the last year to reduce the risk of subsequent hospitalizations.40 Digoxin is used primarily for rate control in the setting of AF, but otherwise is of unclear benefit.7 A low-sodium diet (ie, ≤2 g/d) may be useful in those patients who are prone to fluid overload.5,7 The cornerstone of treatment of HFpEF is the relief of volume overload with diuretics and the treatment of coexisting conditions.33
Common contributing causes of HFpEF
HTN is not only a common contributing cause, but also the most common comorbid condition affecting patients with HFpEF. As such, treatment of HTN represents the most important management goal.33,34 Based on recent data, the American College of Cardiology, the AHA, and the Heart Failure Society of America have recommended a systolic blood pressure goal <130 mm Hg for patients with HFpEF.40 Most patients with HFpEF and HTN will have some degree of fluid overload and, therefore, should receive a diuretic.
CAD. Patients with HFpEF should be evaluated for CAD and treated with medical management and coronary revascularization, as appropriate.
AF is poorly tolerated by patients with HFpEF.37 Patients with AF should receive anticoagulation and rate control medications, and those with persistent HF symptoms should be evaluated for rhythm control.33
Obesity is more prevalent in patients with HFpEF than in those with HFrEF.41 Although there is indirect evidence that weight loss improves cardiac function,34,42,43 and studies have shown bariatric surgery to improve diastolic function,44,45 there are no studies reporting clinical outcomes.
Treatment of OSA with continuous positive airway pressure appears to alleviate some symptoms of HF and to reduce all-cause mortality.46,47
Keeping HF patients out of the hospital
Many readmissions to the hospital for HF exacerbation are preventable. Patients often do not understand hospital discharge instructions or the nature of their chronic disease and its management.48-51 Routine follow-up in the office or clinic provides an opportunity to improve quality of life for patients and decrease admissions.7,52
A major role for the family physician is in the co-creation of, and adherence to, an individualized, comprehensive care plan. Make sure such a plan is easily understood not only by the patient with HF, but also by his or her care team. In addition, it should be evidence-based and reflect the patient’s culture, values, and goals of treatment.5,7
At each visit, the family physician or a member of the health care team should assess adherence to guideline-directed medical therapy, measure weight, evaluate fluid status, and provide ongoing patient education including information on the importance of activity, monitoring weight daily, and moderating fluid, salt, and alcohol intake.5,52
Research shows that cardiac rehabilitation improves functional capacity, exercise duration, quality of life, and mortality. Therefore, recommend it to all symptomatic patients with HF who are clinically stable.2
Consider collaboration with a subspecialist. Patients who remain symptomatic despite optimal medical management and patients with recurrent hospitalizations are best managed in conjunction with a subspecialist in HF treatment.2,5
CORRESPONDENCE
Darin Brink, MD, 420 Delaware St. SE, MMC 381, Minneapolis, MN 55455; [email protected].
1. Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000-2010. NCHS Data Brief. 2012;(108):1-8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23102190. Accessed April 26, 2017.
2. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
3. Passantino A, Guida P, Lagioia R, et al. Predictors of long-term mortality in older patients hospitalized for acutely decompensated heart failure: clinical relevance of natriuretic peptides. J Am Geriatr Soc. 2017;65:822-826.
4. Lassus JP, Siirilä-Waris K, Nieminen MS, et al. Long-term survival after hospitalization for acute heart failure—differences in prognosis of acutely decompensated chronic and new-onset acute heart failure. Int J Cardiol. 2013;168:458-462.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
6. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol. 2016;68:1476-1488.
7. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200.
8. Pinkerman CP, Sander JE, Breeding D, et al. Institute for Clinical Systems Improvement. Heart failure in adults. Available at: https://www.scribd.com/document/310893227/HeartFailure-pdf. Accessed December 6, 2017.
9. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441-1446.
10. Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation. 2011;124:2865-2873.
11. Heart Failure Society of America, Lindenfeld J, Albert NM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1-194.
12. Pouleur H, The SOLVD Investigators. Results of the treatment trial of the studies of left ventricular dysfunction (SOLVD). Am J Cardiol. 1992;70:135-136.
13. Maggioni AP, Anand I, Gottlieb SO, et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002;40:1414-1421.
14. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007.
15. Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7-13.
16. Gehr TW, Sica DA. Pharmacotherapy in congestive heart failure: Hyperkalemia in congestive heart failure. Congest Heart Fail. 2001;7:97-100.
17. National Institute for Health and Clinical Excellence (NICE). Chronic heart failure in adults: management. 2010. Available at: https://www.nice.org.uk/guidance/cg108. Accessed November 27, 2017.
18. Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent). 2003;16:123-126.
19. Epstein SE, Braunwald E. The effect of beta adrenergic blockade on patterns of urinary sodium excretion: studies in normal subjects and in patients with heart disease. Ann Intern Med. 1966;65:20-27.
20. Berbenetz NM, Mrkobrada M. Mineralocorticoid receptor antagonists for heart failure: systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:246.
21. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-2057.
22. Kelly RA, Smith TW. Recognition and management of digitalis toxicity. Am J Cardiol. 1992;69:108G-118G.
23. Sundar S, Burma DP, Vaish SK. Digoxin toxicity and electrolytes: a correlative study. Acta Cardiol. 1983;38:115-123.
24. McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8:79-84.
25. Prenner SB, Shah SJ, Yancy CW. Role of angiotensin receptor-neprilysin inhibition in heart failure. Curr Atheroscler Rep. 2016;18:48.
26. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
27. Corlanor package insert. Amgen Inc., Thousand Oaks, CA. Available at: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/corlanor/corlanor_pi.pdf. Accessed November 28, 2017.
28. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
29. Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130:94-125.
30. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:1047-1058.
31. Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS Focused Update Incorporated Into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283-e352.
32. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670-679.
33. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868-1877.
34. Nanayakkara S, Kaye DM. Management of heart failure with preserved ejection fraction: a review. Clin Ther. 2015;37:2186-2198.
35. Cleland JG, Pellicori P, Dierckx R. Clinical trials in patients with heart failure and preserved left ventricular ejection fraction. Heart Fail Clin. 2014;10:511-523.
36. Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665-671.
37. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507-515.
38. Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2008;52:1015-1021.
39. Zile MR. Heart failure with a preserved ejection fraction. In: Mann DL, Zipes D, Libby P BR, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 10th ed. Phila
40. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803.
41. Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281-2293.
42. de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376-2381.
43. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. JAMA. 2016;315:36-46.
44. Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718-726.
45. Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14:198-202.
46. Yoshihisa A, Suzuki S, Yamauchi H, et al. Beneficial effects of positive airway pressure therapy for sleep-disordered breathing in heart failure patients with preserved left ventricular ejection fraction. Clin Cardiol. 2015;38:413-421.
47. Shah RV, Abbasi SA, Heydari B, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014;167:620-626.
48. Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141-1163.
49. Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150:984.
50. Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure: update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3:459-467.
51. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407-413.
52. Cowie MR, Anker SD, Cleland JG, et al. Improving care for patients with acute heart failure: before, during and after hospitalization. Available at: http://www.oxfordhealthpolicyforum.org/files/reports/ahf-report.pdf. Accessed November 27, 2017.
Heart failure (HF) affects nearly 6 million Americans and accounts for one million hospital admissions each year.1 The condition, which results from a structural or functional disorder that impairs the ventricles’ ability to fill, empty, or both,2 is a major cause of morbidity and mortality. The 5-year mortality rate ranges from 44% to 77%.3,4
Growing evidence demonstrates reduced morbidity and mortality when patients with HF with reduced ejection fraction (HFrEF) are treated with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB); a beta-blocker; and a mineralocorticoid/aldosterone receptor antagonist (MRA) in appropriate doses.5 In addition, 2 new medications representing novel drug classes have recently entered the market and are recommended in select patients who remain symptomatic despite standard treatment.
The first is sacubitril, which is available in a combination pill with the ARB valsartan, and the second is ivabradine.6 Additionally, implanted medical devices are proving useful, particularly in the management of patients with refractory symptoms.
This article will briefly review the diagnosis and initial evaluation of the patient with suspected HF and then describe how newer treatments fit within HF management priorities and strategies. But first, a word about what causes HF.
Causes are many and diverse
HF has a variety of cardiac and non-cardiac etiologies.2,7,8 Some important cardiac causes include hypertension (HTN), coronary artery disease (CAD), valvular heart disease, arrhythmias, myocarditis, Takotsubo cardiomyopathy, and postpartum cardiomyopathy. Common and important non-cardiac causes of HF include alcoholic cardiomyopathy, pulmonary embolism, pulmonary hypertension, obstructive sleep apnea, anemia, hemochromatosis, amyloidosis, sarcoidosis, thyroid dysfunction, nephrotic syndrome, and cardiac toxins (especially stimulants and certain chemotherapy drugs).2,7,8
Diagnosing an elusive culprit
HF remains a clinical diagnosis. Common symptoms include dyspnea, cough, pedal edema, and decreased exercise tolerance, but these symptoms are not at all specific. Given the varied causes and manifestations of HF, the diagnosis can be somewhat elusive. Fortunately, there are a number of objective methods to help identify patients with HF.
Framingham criteria. One commonly used tool for making the diagnosis of HF is the Framingham criteria (see https://www.mdcalc.com/framingham-heart-failure-diagnostic-criteria),9 which diagnoses HF based on historical and physical exam findings. Another well-validated decision tool is the Heart Failure Diagnostic Rule (see http://circ.ahajournals.org/content/124/25/2865.long),10 which incorporates N-terminal pro–B-type natriuretic peptide (NT-proBNP) results, as well as exam findings.
Measurement of natriuretic peptides, either B-type natriuretic peptide (BNP) or NT-proBNP, aids in the diagnosis of HF.5 Although several factors (including age, weight, and renal function) can affect BNP levels, a normal BNP value effectively rules out HF5,7 and an elevated BNP can help to make the diagnosis in the context of a patient with corresponding symptoms.
The initial evaluation: Necessary lab work and imaging studies
The purpose of the initial evaluation of the patient with suspected HF is to establish the diagnosis, look for underlying etiologies of HF, identify comorbidities, and establish baseline values (eg, of potassium and creatinine) for elements monitored during treatment.5,7 TABLE 15,7 lists the lab work and imaging tests that are commonly ordered in the initial evaluation of the patient with HF.

Echocardiography is useful in determining the ejection fraction (EF), which is essential in guiding treatment. Echocardiography can also identify important structural abnormalities including significant valvular disease. Refer patients with severe valvular disease for evaluation for valve repair/replacement, regardless of EF.8
Noninvasive testing (stress nuclear imaging or echocardiography) to evaluate for underlying CAD is reasonable in patients with unknown CAD status.8,11 Patients for whom there is a high suspicion of obstructive CAD should undergo coronary angiography if they are candidates for revascularization.5,7 Noninvasive testing may also be an acceptable option for assessing ischemia in patients presenting with HF who have known CAD and no angina.5
Classification of HF is determined by ejection fraction
Physicians have traditionally classified patients with HF as having either systolic or diastolic dysfunction. Patients with HF symptoms and a reduced EF were said to have systolic dysfunction; those with a normal EF were said to have diastolic dysfunction.
More recently, researchers have learned that patients with reduced EF and those with preserved EF can have both systolic and diastolic dysfunction simultaneously.8 Therefore, the current preferred terminology is HFpEF (heart failure with preserved ejection fraction) for those with an EF ≥50% and HFrEF (heart failure with reduced ejection fraction) for those with an EF ≤40%.5 Both the American Heart Association (AHA) and the European Society of Cardiology recognize a category of HF with moderately reduced ejection fraction defined as an EF between 40% and 50%.5,7 Practically speaking, this group is treated as per the guidelines for HFrEF.5
Treatment of HFrEF: The evidence is clear
The cornerstone of medical treatment for HFrEF is the combination of an ACE inhibitor or ARB with a beta-blocker.2,5,7,8 Several early trials showed clear benefits of these medications. For example, the Studies Of Left Ventricular Dysfunction trial (SOLVD), compared enalapril to placebo in patients receiving standard therapy (consisting chiefly of digitalis, diuretics, and nitrates). This study demonstrated a reduction in all-cause mortality or first hospitalization for HF (number needed to treat [NNT]=21) in the enalapril group vs the placebo group.12
Similarly, a subgroup analysis of the Valsartan Heart Failure Treatment (Val-HeFT) trial demonstrated morbidity (NNT=10) and all-cause mortality benefits (NNT=6) when valsartan (an ARB) was given to patients who were not receiving an ACE inhibitor.13
MERIT-HF (Metoprolol CR/XL Randomised Intervention Trial in congestive Heart Failure) compared the beta-blocker metoprolol succinate to placebo and found fewer deaths from HF and lower all-cause mortality (NNT=26) associated with the treatment group vs the placebo group.14
And a comparison of 2 beta-blockers—carvedilol and metoprolol tartrate—on clinical outcomes in patients with chronic HF in the Carvedilol Or Metoprolol European Trial (COMET) showed that carvedilol extended survival compared with metoprolol tartrate (NNT=19).15
Unlike ACE inhibitors and ARBs, which seem to show a class benefit, only 3 beta-blockers available in the United States have been proven to reduce mortality: sustained-release metoprolol succinate, carvedilol, and bisoprolol.2,7,8
Unless contraindicated, all patients with a reduced EF—even those without symptoms—should receive a beta-blocker and an ACE inhibitor or ARB.5,7,8
Cautionary notes
Remember the following caveats when treating patients with ACE inhibitors, ARBs, and beta-blockers:
- Use ACE inhibitors and ARBs with caution in patients with impaired renal function (serum creatinine >2.5 mg/dL) or elevated serum potassium (>5 mEq/L).16,17
- ARBs are associated with a much lower incidence of cough and angioedema than ACE inhibitors.18
- Although physicians frequently start patients on low doses of beta-blockers and ACE inhibitors or ARBs to minimize hypotension and other adverse effects, the goal of therapy is to titrate up to the therapeutic doses used in clinical trials.5-7 (For dosages of medications commonly used in the treatment of heart failure, see Table 3 in the American College of Cardiology/AHA/Heart Failure Society of America guidelines available at https://www.sciencedirect.com/science/article/pii/S0735109717370870?via%3Dihub#tbl3 and Table 7.2 in the European Society of Cardiology guidelines available at https://academic.oup.com/eurheartj/article/37/27/2129/1748921.)
- Because beta-blockers can exacerbate fluid retention, do not initiate them in patients with fluid overload unless such patients are being treated with diuretics.5,19
When more Tx is needed
For patients who remain symptomatic despite treatment with an ACE inhibitor or ARB and a beta-blocker, consider the following add-on therapies.
Diuretics are the only medications used in the treatment of HF that adequately reduce fluid overload.2,7 While thiazide diuretics confer greater blood pressure control, loop diuretics are generally preferred in the treatment of HF because they are more efficacious.5 Loop diuretics should be prescribed to all patients with fluid overload, as few patients can maintain their target (“dry”) weight without diuretic therapy.5,7 Common adverse effects include hypokalemia, dehydration, and azotemia.
Two MRAs are currently available in the United States: spironolactone and eplerenone. MRAs are used as add-on therapy for symptomatic patients with an EF ≤35% or an EF ≤40% following an acute myocardial infarction (MI).5 They significantly reduce all-cause mortality (NNT=26).20
Because hyperkalemia is a risk with MRAs, do not prescribe them for patients who are already taking both an ACE inhibitor and an ARB.5 Also, do not initiate MRA therapy in patients who have an elevated creatinine level (≥2.5 mg/dL in men; ≥2 mg/dL in women) or a potassium level ≥5 mEq/L.5,7,8 Discontinue MRA therapy if a patient’s potassium level rises to ≥5.5 mEq/L.5
Hydralazine combined with isosorbide dinitrate (H/ID) is an alternative in patients for whom ACE inhibitor/ARB therapy is contraindicated.5,8
H/ID is also an add-on option in African American patients. Trials have demonstrated that H/ID reduces both first hospitalization for HF (NNT=13) and all-cause mortality (NNT=25) when it is used as add-on therapy in African Americans already receiving standard therapy with an ACE inhibitor or ARB, a beta-blocker, and an MRA.21 Headache and dizziness are commonly reported adverse effects.
Digoxin does not reduce mortality, but it does improve both quality of life and exercise tolerance and reduces hospital admissions for patients with HF.5,7 Significant adverse effects of digoxin include anorexia, nausea, visual disturbances, and cardiac arrhythmias.22
Also, hypokalemia can intensify digoxin toxicity.23 Because of these concerns, digoxin is typically dosed at 0.125 mg/d (0.125 mg every other day in patients >70 years or patients with impaired renal function or low body weight) with a target therapeutic range of 0.5 to 0.9 ng/mL.5
New classes, new agents
Sacubitril, a neprilysin inhibitor, is the first drug from this class approved for use in the United States. Neprilysin is the enzyme responsible for the degradation of natriuretic peptides; as such it increases endogenous NPs, promoting diuresis and lowering blood pressure.24,25 Early trials with sacubitril alone showed limited clinical efficacy;25 however, when it was combined with the ARB, valsartan (the combination being called angiotensin receptor blocker + neprilysin inhibitor [ARNI] therapy), it was found to be of significant benefit.6,25
The PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trial compared outcomes in patients receiving ARNI therapy to those receiving enalapril.26 The authors stopped the trial early due to the overwhelming benefit seen in the ARNI arm.
After a median follow-up of 27 months, the researchers found a reduction in the primary outcomes of either cardiovascular death or first hospitalization for HF (26.5% in the enalapril-treated group vs 21.8% in the ARNI-treated group; NNT=21).26 There were slightly more cases of angioedema in the ARNI arm than in the enalapril arm (0.5% vs 0.2%), although there were no patients in the trial who required endotracheal intubation.26
Because of this increased risk, do not prescribe ARNI therapy for any patient with a history of angioedema.6 Hypotension was more common in the ARNI-treated group than in the enalapril group (14% vs 9.2%), but there were lower rates of hyperkalemia, elevated serum creatinine, and cough in the ARNI-treated group than in the enalapril group.26
Consider ARNI treatment for all patients with an EF ≤40% who remain symptomatic despite appropriate doses of an ACE inhibitor or ARB plus a beta-blocker. Do not administer ARNI therapy concomitantly with an ACE inhibitor or ARB. When switching, do not start ARNI therapy for at least 36 hours after the last dose of an ACE inhibitor or ARB.6
Ivabradine is a sinoatrial node modulator that provides additional heart rate reduction. It does not affect ventricular repolarization or myocardial contractility.27 Early trials with this medication have shown reduced cardiac mortality and an NNT to prevent one first HF hospitalization within one year of 27.28 Adverse effects include symptomatic and asymptomatic bradycardia and luminous phenomena.28
Recommend ivabradine as add-on therapy to all patients with an EF ≤35%, normal sinus rhythm, and resting heart rate ≥70 bpm who remain symptomatic despite taking the maximum-tolerated dose of a beta-blocker.6 The dose is adjusted to achieve a resting heart rate of 50 to 60 bpm.27
Nonpharmacologic options
Implantable cardioverter defibrillators (ICDs) are recommended as primary prevention in select HFrEF patients to reduce the risk of sudden cardiac death and all-cause mortality. The 2013 American College of Cardiology Foundation/AHA Guideline for the Management of Heart Failure recommends an ICD for primary prevention for: 1) patients with symptomatic HF and an LVEF ≤35% despite ≥3 months of optimal medical therapy, and 2) patients at least 40 days post-MI with an LVEF of ≤30%.5,29 ICDs are not recommended for patients who have a life expectancy of less than one year, and the devices are of unclear benefit for patients ≥75 years of age.5
Cardiac resynchronization therapy (CRT), although not new to the field of cardiology, is new to the treatment of heart failure. A number of patients with HFrEF have QRS prolongation and in particular, left bundle branch block (LBBB).5 CRT uses biventricular pacing to restore synchronous contraction of the left and right ventricles.30 It is strongly recommended for patients with an EF ≤35%, sinus rhythm, LBBB, QRS ≥150 ms, and a life expectancy of at least one year.5,7 It is weakly recommended for patients with an EF ≤35% and a QRS ≥150 ms but without LBBB. It’s also weakly recommended for patients with an EF ≤35% and LBBB with a QRS of 120 to 150 ms.5,31
Left ventricular assist devices (LVADs) and cardiac transplantation are considerations for patients with severe symptoms refractory to all other interventions.5 LVADs may be used either while awaiting cardiac transplantation (bridge therapy) or as definitive treatment (destination therapy). Appropriate patient selection for such therapies requires a team of experts that ideally includes HF and transplantation cardiologists, cardiothoracic surgeons, nurses, social workers, and palliative care clinicians.5
Treatment of HFpEF: Evidence is lacking
While HFpEF is common—affecting about half of all patients with HF—ideal treatment remains unclear.32 Some trials have shown promise, but to date no unequivocal evidence exists that any standard therapy reduces mortality in patients with HFpEF.33-37 Underlying mechanisms of action of HFpEF include cardiac rate and rhythm abnormalities, atrial dysfunction, and stiffening of the ventricles. In a sense, it represents an exaggerated expression of the pathophysiology seen with the normal aging of the heart and can be conceptualized as “presbycardia.”37 Indeed, HFpEF is more common in the elderly, but it is also more common in patients of African descent.38,39 Common contributing causes (which we’ll get to in a bit) include HTN, CAD, atrial fibrillation (AF), obesity, and obstructive sleep apnea (OSA).
Trials have failed to show clear benefit for ACE inhibitors, ARBs, or beta-blockers.7,33 The evidence for MRAs is somewhat unclear; however, they have recently been recommended as an option for patients who have been hospitalized in the last year to reduce the risk of subsequent hospitalizations.40 Digoxin is used primarily for rate control in the setting of AF, but otherwise is of unclear benefit.7 A low-sodium diet (ie, ≤2 g/d) may be useful in those patients who are prone to fluid overload.5,7 The cornerstone of treatment of HFpEF is the relief of volume overload with diuretics and the treatment of coexisting conditions.33
Common contributing causes of HFpEF
HTN is not only a common contributing cause, but also the most common comorbid condition affecting patients with HFpEF. As such, treatment of HTN represents the most important management goal.33,34 Based on recent data, the American College of Cardiology, the AHA, and the Heart Failure Society of America have recommended a systolic blood pressure goal <130 mm Hg for patients with HFpEF.40 Most patients with HFpEF and HTN will have some degree of fluid overload and, therefore, should receive a diuretic.
CAD. Patients with HFpEF should be evaluated for CAD and treated with medical management and coronary revascularization, as appropriate.
AF is poorly tolerated by patients with HFpEF.37 Patients with AF should receive anticoagulation and rate control medications, and those with persistent HF symptoms should be evaluated for rhythm control.33
Obesity is more prevalent in patients with HFpEF than in those with HFrEF.41 Although there is indirect evidence that weight loss improves cardiac function,34,42,43 and studies have shown bariatric surgery to improve diastolic function,44,45 there are no studies reporting clinical outcomes.
Treatment of OSA with continuous positive airway pressure appears to alleviate some symptoms of HF and to reduce all-cause mortality.46,47
Keeping HF patients out of the hospital
Many readmissions to the hospital for HF exacerbation are preventable. Patients often do not understand hospital discharge instructions or the nature of their chronic disease and its management.48-51 Routine follow-up in the office or clinic provides an opportunity to improve quality of life for patients and decrease admissions.7,52
A major role for the family physician is in the co-creation of, and adherence to, an individualized, comprehensive care plan. Make sure such a plan is easily understood not only by the patient with HF, but also by his or her care team. In addition, it should be evidence-based and reflect the patient’s culture, values, and goals of treatment.5,7
At each visit, the family physician or a member of the health care team should assess adherence to guideline-directed medical therapy, measure weight, evaluate fluid status, and provide ongoing patient education including information on the importance of activity, monitoring weight daily, and moderating fluid, salt, and alcohol intake.5,52
Research shows that cardiac rehabilitation improves functional capacity, exercise duration, quality of life, and mortality. Therefore, recommend it to all symptomatic patients with HF who are clinically stable.2
Consider collaboration with a subspecialist. Patients who remain symptomatic despite optimal medical management and patients with recurrent hospitalizations are best managed in conjunction with a subspecialist in HF treatment.2,5
CORRESPONDENCE
Darin Brink, MD, 420 Delaware St. SE, MMC 381, Minneapolis, MN 55455; [email protected].
Heart failure (HF) affects nearly 6 million Americans and accounts for one million hospital admissions each year.1 The condition, which results from a structural or functional disorder that impairs the ventricles’ ability to fill, empty, or both,2 is a major cause of morbidity and mortality. The 5-year mortality rate ranges from 44% to 77%.3,4
Growing evidence demonstrates reduced morbidity and mortality when patients with HF with reduced ejection fraction (HFrEF) are treated with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB); a beta-blocker; and a mineralocorticoid/aldosterone receptor antagonist (MRA) in appropriate doses.5 In addition, 2 new medications representing novel drug classes have recently entered the market and are recommended in select patients who remain symptomatic despite standard treatment.
The first is sacubitril, which is available in a combination pill with the ARB valsartan, and the second is ivabradine.6 Additionally, implanted medical devices are proving useful, particularly in the management of patients with refractory symptoms.
This article will briefly review the diagnosis and initial evaluation of the patient with suspected HF and then describe how newer treatments fit within HF management priorities and strategies. But first, a word about what causes HF.
Causes are many and diverse
HF has a variety of cardiac and non-cardiac etiologies.2,7,8 Some important cardiac causes include hypertension (HTN), coronary artery disease (CAD), valvular heart disease, arrhythmias, myocarditis, Takotsubo cardiomyopathy, and postpartum cardiomyopathy. Common and important non-cardiac causes of HF include alcoholic cardiomyopathy, pulmonary embolism, pulmonary hypertension, obstructive sleep apnea, anemia, hemochromatosis, amyloidosis, sarcoidosis, thyroid dysfunction, nephrotic syndrome, and cardiac toxins (especially stimulants and certain chemotherapy drugs).2,7,8
Diagnosing an elusive culprit
HF remains a clinical diagnosis. Common symptoms include dyspnea, cough, pedal edema, and decreased exercise tolerance, but these symptoms are not at all specific. Given the varied causes and manifestations of HF, the diagnosis can be somewhat elusive. Fortunately, there are a number of objective methods to help identify patients with HF.
Framingham criteria. One commonly used tool for making the diagnosis of HF is the Framingham criteria (see https://www.mdcalc.com/framingham-heart-failure-diagnostic-criteria),9 which diagnoses HF based on historical and physical exam findings. Another well-validated decision tool is the Heart Failure Diagnostic Rule (see http://circ.ahajournals.org/content/124/25/2865.long),10 which incorporates N-terminal pro–B-type natriuretic peptide (NT-proBNP) results, as well as exam findings.
Measurement of natriuretic peptides, either B-type natriuretic peptide (BNP) or NT-proBNP, aids in the diagnosis of HF.5 Although several factors (including age, weight, and renal function) can affect BNP levels, a normal BNP value effectively rules out HF5,7 and an elevated BNP can help to make the diagnosis in the context of a patient with corresponding symptoms.
The initial evaluation: Necessary lab work and imaging studies
The purpose of the initial evaluation of the patient with suspected HF is to establish the diagnosis, look for underlying etiologies of HF, identify comorbidities, and establish baseline values (eg, of potassium and creatinine) for elements monitored during treatment.5,7 TABLE 15,7 lists the lab work and imaging tests that are commonly ordered in the initial evaluation of the patient with HF.

Echocardiography is useful in determining the ejection fraction (EF), which is essential in guiding treatment. Echocardiography can also identify important structural abnormalities including significant valvular disease. Refer patients with severe valvular disease for evaluation for valve repair/replacement, regardless of EF.8
Noninvasive testing (stress nuclear imaging or echocardiography) to evaluate for underlying CAD is reasonable in patients with unknown CAD status.8,11 Patients for whom there is a high suspicion of obstructive CAD should undergo coronary angiography if they are candidates for revascularization.5,7 Noninvasive testing may also be an acceptable option for assessing ischemia in patients presenting with HF who have known CAD and no angina.5
Classification of HF is determined by ejection fraction
Physicians have traditionally classified patients with HF as having either systolic or diastolic dysfunction. Patients with HF symptoms and a reduced EF were said to have systolic dysfunction; those with a normal EF were said to have diastolic dysfunction.
More recently, researchers have learned that patients with reduced EF and those with preserved EF can have both systolic and diastolic dysfunction simultaneously.8 Therefore, the current preferred terminology is HFpEF (heart failure with preserved ejection fraction) for those with an EF ≥50% and HFrEF (heart failure with reduced ejection fraction) for those with an EF ≤40%.5 Both the American Heart Association (AHA) and the European Society of Cardiology recognize a category of HF with moderately reduced ejection fraction defined as an EF between 40% and 50%.5,7 Practically speaking, this group is treated as per the guidelines for HFrEF.5
Treatment of HFrEF: The evidence is clear
The cornerstone of medical treatment for HFrEF is the combination of an ACE inhibitor or ARB with a beta-blocker.2,5,7,8 Several early trials showed clear benefits of these medications. For example, the Studies Of Left Ventricular Dysfunction trial (SOLVD), compared enalapril to placebo in patients receiving standard therapy (consisting chiefly of digitalis, diuretics, and nitrates). This study demonstrated a reduction in all-cause mortality or first hospitalization for HF (number needed to treat [NNT]=21) in the enalapril group vs the placebo group.12
Similarly, a subgroup analysis of the Valsartan Heart Failure Treatment (Val-HeFT) trial demonstrated morbidity (NNT=10) and all-cause mortality benefits (NNT=6) when valsartan (an ARB) was given to patients who were not receiving an ACE inhibitor.13
MERIT-HF (Metoprolol CR/XL Randomised Intervention Trial in congestive Heart Failure) compared the beta-blocker metoprolol succinate to placebo and found fewer deaths from HF and lower all-cause mortality (NNT=26) associated with the treatment group vs the placebo group.14
And a comparison of 2 beta-blockers—carvedilol and metoprolol tartrate—on clinical outcomes in patients with chronic HF in the Carvedilol Or Metoprolol European Trial (COMET) showed that carvedilol extended survival compared with metoprolol tartrate (NNT=19).15
Unlike ACE inhibitors and ARBs, which seem to show a class benefit, only 3 beta-blockers available in the United States have been proven to reduce mortality: sustained-release metoprolol succinate, carvedilol, and bisoprolol.2,7,8
Unless contraindicated, all patients with a reduced EF—even those without symptoms—should receive a beta-blocker and an ACE inhibitor or ARB.5,7,8
Cautionary notes
Remember the following caveats when treating patients with ACE inhibitors, ARBs, and beta-blockers:
- Use ACE inhibitors and ARBs with caution in patients with impaired renal function (serum creatinine >2.5 mg/dL) or elevated serum potassium (>5 mEq/L).16,17
- ARBs are associated with a much lower incidence of cough and angioedema than ACE inhibitors.18
- Although physicians frequently start patients on low doses of beta-blockers and ACE inhibitors or ARBs to minimize hypotension and other adverse effects, the goal of therapy is to titrate up to the therapeutic doses used in clinical trials.5-7 (For dosages of medications commonly used in the treatment of heart failure, see Table 3 in the American College of Cardiology/AHA/Heart Failure Society of America guidelines available at https://www.sciencedirect.com/science/article/pii/S0735109717370870?via%3Dihub#tbl3 and Table 7.2 in the European Society of Cardiology guidelines available at https://academic.oup.com/eurheartj/article/37/27/2129/1748921.)
- Because beta-blockers can exacerbate fluid retention, do not initiate them in patients with fluid overload unless such patients are being treated with diuretics.5,19
When more Tx is needed
For patients who remain symptomatic despite treatment with an ACE inhibitor or ARB and a beta-blocker, consider the following add-on therapies.
Diuretics are the only medications used in the treatment of HF that adequately reduce fluid overload.2,7 While thiazide diuretics confer greater blood pressure control, loop diuretics are generally preferred in the treatment of HF because they are more efficacious.5 Loop diuretics should be prescribed to all patients with fluid overload, as few patients can maintain their target (“dry”) weight without diuretic therapy.5,7 Common adverse effects include hypokalemia, dehydration, and azotemia.
Two MRAs are currently available in the United States: spironolactone and eplerenone. MRAs are used as add-on therapy for symptomatic patients with an EF ≤35% or an EF ≤40% following an acute myocardial infarction (MI).5 They significantly reduce all-cause mortality (NNT=26).20
Because hyperkalemia is a risk with MRAs, do not prescribe them for patients who are already taking both an ACE inhibitor and an ARB.5 Also, do not initiate MRA therapy in patients who have an elevated creatinine level (≥2.5 mg/dL in men; ≥2 mg/dL in women) or a potassium level ≥5 mEq/L.5,7,8 Discontinue MRA therapy if a patient’s potassium level rises to ≥5.5 mEq/L.5
Hydralazine combined with isosorbide dinitrate (H/ID) is an alternative in patients for whom ACE inhibitor/ARB therapy is contraindicated.5,8
H/ID is also an add-on option in African American patients. Trials have demonstrated that H/ID reduces both first hospitalization for HF (NNT=13) and all-cause mortality (NNT=25) when it is used as add-on therapy in African Americans already receiving standard therapy with an ACE inhibitor or ARB, a beta-blocker, and an MRA.21 Headache and dizziness are commonly reported adverse effects.
Digoxin does not reduce mortality, but it does improve both quality of life and exercise tolerance and reduces hospital admissions for patients with HF.5,7 Significant adverse effects of digoxin include anorexia, nausea, visual disturbances, and cardiac arrhythmias.22
Also, hypokalemia can intensify digoxin toxicity.23 Because of these concerns, digoxin is typically dosed at 0.125 mg/d (0.125 mg every other day in patients >70 years or patients with impaired renal function or low body weight) with a target therapeutic range of 0.5 to 0.9 ng/mL.5
New classes, new agents
Sacubitril, a neprilysin inhibitor, is the first drug from this class approved for use in the United States. Neprilysin is the enzyme responsible for the degradation of natriuretic peptides; as such it increases endogenous NPs, promoting diuresis and lowering blood pressure.24,25 Early trials with sacubitril alone showed limited clinical efficacy;25 however, when it was combined with the ARB, valsartan (the combination being called angiotensin receptor blocker + neprilysin inhibitor [ARNI] therapy), it was found to be of significant benefit.6,25
The PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trial compared outcomes in patients receiving ARNI therapy to those receiving enalapril.26 The authors stopped the trial early due to the overwhelming benefit seen in the ARNI arm.
After a median follow-up of 27 months, the researchers found a reduction in the primary outcomes of either cardiovascular death or first hospitalization for HF (26.5% in the enalapril-treated group vs 21.8% in the ARNI-treated group; NNT=21).26 There were slightly more cases of angioedema in the ARNI arm than in the enalapril arm (0.5% vs 0.2%), although there were no patients in the trial who required endotracheal intubation.26
Because of this increased risk, do not prescribe ARNI therapy for any patient with a history of angioedema.6 Hypotension was more common in the ARNI-treated group than in the enalapril group (14% vs 9.2%), but there were lower rates of hyperkalemia, elevated serum creatinine, and cough in the ARNI-treated group than in the enalapril group.26
Consider ARNI treatment for all patients with an EF ≤40% who remain symptomatic despite appropriate doses of an ACE inhibitor or ARB plus a beta-blocker. Do not administer ARNI therapy concomitantly with an ACE inhibitor or ARB. When switching, do not start ARNI therapy for at least 36 hours after the last dose of an ACE inhibitor or ARB.6
Ivabradine is a sinoatrial node modulator that provides additional heart rate reduction. It does not affect ventricular repolarization or myocardial contractility.27 Early trials with this medication have shown reduced cardiac mortality and an NNT to prevent one first HF hospitalization within one year of 27.28 Adverse effects include symptomatic and asymptomatic bradycardia and luminous phenomena.28
Recommend ivabradine as add-on therapy to all patients with an EF ≤35%, normal sinus rhythm, and resting heart rate ≥70 bpm who remain symptomatic despite taking the maximum-tolerated dose of a beta-blocker.6 The dose is adjusted to achieve a resting heart rate of 50 to 60 bpm.27
Nonpharmacologic options
Implantable cardioverter defibrillators (ICDs) are recommended as primary prevention in select HFrEF patients to reduce the risk of sudden cardiac death and all-cause mortality. The 2013 American College of Cardiology Foundation/AHA Guideline for the Management of Heart Failure recommends an ICD for primary prevention for: 1) patients with symptomatic HF and an LVEF ≤35% despite ≥3 months of optimal medical therapy, and 2) patients at least 40 days post-MI with an LVEF of ≤30%.5,29 ICDs are not recommended for patients who have a life expectancy of less than one year, and the devices are of unclear benefit for patients ≥75 years of age.5
Cardiac resynchronization therapy (CRT), although not new to the field of cardiology, is new to the treatment of heart failure. A number of patients with HFrEF have QRS prolongation and in particular, left bundle branch block (LBBB).5 CRT uses biventricular pacing to restore synchronous contraction of the left and right ventricles.30 It is strongly recommended for patients with an EF ≤35%, sinus rhythm, LBBB, QRS ≥150 ms, and a life expectancy of at least one year.5,7 It is weakly recommended for patients with an EF ≤35% and a QRS ≥150 ms but without LBBB. It’s also weakly recommended for patients with an EF ≤35% and LBBB with a QRS of 120 to 150 ms.5,31
Left ventricular assist devices (LVADs) and cardiac transplantation are considerations for patients with severe symptoms refractory to all other interventions.5 LVADs may be used either while awaiting cardiac transplantation (bridge therapy) or as definitive treatment (destination therapy). Appropriate patient selection for such therapies requires a team of experts that ideally includes HF and transplantation cardiologists, cardiothoracic surgeons, nurses, social workers, and palliative care clinicians.5
Treatment of HFpEF: Evidence is lacking
While HFpEF is common—affecting about half of all patients with HF—ideal treatment remains unclear.32 Some trials have shown promise, but to date no unequivocal evidence exists that any standard therapy reduces mortality in patients with HFpEF.33-37 Underlying mechanisms of action of HFpEF include cardiac rate and rhythm abnormalities, atrial dysfunction, and stiffening of the ventricles. In a sense, it represents an exaggerated expression of the pathophysiology seen with the normal aging of the heart and can be conceptualized as “presbycardia.”37 Indeed, HFpEF is more common in the elderly, but it is also more common in patients of African descent.38,39 Common contributing causes (which we’ll get to in a bit) include HTN, CAD, atrial fibrillation (AF), obesity, and obstructive sleep apnea (OSA).
Trials have failed to show clear benefit for ACE inhibitors, ARBs, or beta-blockers.7,33 The evidence for MRAs is somewhat unclear; however, they have recently been recommended as an option for patients who have been hospitalized in the last year to reduce the risk of subsequent hospitalizations.40 Digoxin is used primarily for rate control in the setting of AF, but otherwise is of unclear benefit.7 A low-sodium diet (ie, ≤2 g/d) may be useful in those patients who are prone to fluid overload.5,7 The cornerstone of treatment of HFpEF is the relief of volume overload with diuretics and the treatment of coexisting conditions.33
Common contributing causes of HFpEF
HTN is not only a common contributing cause, but also the most common comorbid condition affecting patients with HFpEF. As such, treatment of HTN represents the most important management goal.33,34 Based on recent data, the American College of Cardiology, the AHA, and the Heart Failure Society of America have recommended a systolic blood pressure goal <130 mm Hg for patients with HFpEF.40 Most patients with HFpEF and HTN will have some degree of fluid overload and, therefore, should receive a diuretic.
CAD. Patients with HFpEF should be evaluated for CAD and treated with medical management and coronary revascularization, as appropriate.
AF is poorly tolerated by patients with HFpEF.37 Patients with AF should receive anticoagulation and rate control medications, and those with persistent HF symptoms should be evaluated for rhythm control.33
Obesity is more prevalent in patients with HFpEF than in those with HFrEF.41 Although there is indirect evidence that weight loss improves cardiac function,34,42,43 and studies have shown bariatric surgery to improve diastolic function,44,45 there are no studies reporting clinical outcomes.
Treatment of OSA with continuous positive airway pressure appears to alleviate some symptoms of HF and to reduce all-cause mortality.46,47
Keeping HF patients out of the hospital
Many readmissions to the hospital for HF exacerbation are preventable. Patients often do not understand hospital discharge instructions or the nature of their chronic disease and its management.48-51 Routine follow-up in the office or clinic provides an opportunity to improve quality of life for patients and decrease admissions.7,52
A major role for the family physician is in the co-creation of, and adherence to, an individualized, comprehensive care plan. Make sure such a plan is easily understood not only by the patient with HF, but also by his or her care team. In addition, it should be evidence-based and reflect the patient’s culture, values, and goals of treatment.5,7
At each visit, the family physician or a member of the health care team should assess adherence to guideline-directed medical therapy, measure weight, evaluate fluid status, and provide ongoing patient education including information on the importance of activity, monitoring weight daily, and moderating fluid, salt, and alcohol intake.5,52
Research shows that cardiac rehabilitation improves functional capacity, exercise duration, quality of life, and mortality. Therefore, recommend it to all symptomatic patients with HF who are clinically stable.2
Consider collaboration with a subspecialist. Patients who remain symptomatic despite optimal medical management and patients with recurrent hospitalizations are best managed in conjunction with a subspecialist in HF treatment.2,5
CORRESPONDENCE
Darin Brink, MD, 420 Delaware St. SE, MMC 381, Minneapolis, MN 55455; [email protected].
1. Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000-2010. NCHS Data Brief. 2012;(108):1-8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23102190. Accessed April 26, 2017.
2. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
3. Passantino A, Guida P, Lagioia R, et al. Predictors of long-term mortality in older patients hospitalized for acutely decompensated heart failure: clinical relevance of natriuretic peptides. J Am Geriatr Soc. 2017;65:822-826.
4. Lassus JP, Siirilä-Waris K, Nieminen MS, et al. Long-term survival after hospitalization for acute heart failure—differences in prognosis of acutely decompensated chronic and new-onset acute heart failure. Int J Cardiol. 2013;168:458-462.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
6. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol. 2016;68:1476-1488.
7. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200.
8. Pinkerman CP, Sander JE, Breeding D, et al. Institute for Clinical Systems Improvement. Heart failure in adults. Available at: https://www.scribd.com/document/310893227/HeartFailure-pdf. Accessed December 6, 2017.
9. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441-1446.
10. Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation. 2011;124:2865-2873.
11. Heart Failure Society of America, Lindenfeld J, Albert NM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1-194.
12. Pouleur H, The SOLVD Investigators. Results of the treatment trial of the studies of left ventricular dysfunction (SOLVD). Am J Cardiol. 1992;70:135-136.
13. Maggioni AP, Anand I, Gottlieb SO, et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002;40:1414-1421.
14. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007.
15. Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7-13.
16. Gehr TW, Sica DA. Pharmacotherapy in congestive heart failure: Hyperkalemia in congestive heart failure. Congest Heart Fail. 2001;7:97-100.
17. National Institute for Health and Clinical Excellence (NICE). Chronic heart failure in adults: management. 2010. Available at: https://www.nice.org.uk/guidance/cg108. Accessed November 27, 2017.
18. Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent). 2003;16:123-126.
19. Epstein SE, Braunwald E. The effect of beta adrenergic blockade on patterns of urinary sodium excretion: studies in normal subjects and in patients with heart disease. Ann Intern Med. 1966;65:20-27.
20. Berbenetz NM, Mrkobrada M. Mineralocorticoid receptor antagonists for heart failure: systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:246.
21. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-2057.
22. Kelly RA, Smith TW. Recognition and management of digitalis toxicity. Am J Cardiol. 1992;69:108G-118G.
23. Sundar S, Burma DP, Vaish SK. Digoxin toxicity and electrolytes: a correlative study. Acta Cardiol. 1983;38:115-123.
24. McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8:79-84.
25. Prenner SB, Shah SJ, Yancy CW. Role of angiotensin receptor-neprilysin inhibition in heart failure. Curr Atheroscler Rep. 2016;18:48.
26. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
27. Corlanor package insert. Amgen Inc., Thousand Oaks, CA. Available at: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/corlanor/corlanor_pi.pdf. Accessed November 28, 2017.
28. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
29. Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130:94-125.
30. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:1047-1058.
31. Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS Focused Update Incorporated Into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283-e352.
32. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670-679.
33. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868-1877.
34. Nanayakkara S, Kaye DM. Management of heart failure with preserved ejection fraction: a review. Clin Ther. 2015;37:2186-2198.
35. Cleland JG, Pellicori P, Dierckx R. Clinical trials in patients with heart failure and preserved left ventricular ejection fraction. Heart Fail Clin. 2014;10:511-523.
36. Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665-671.
37. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507-515.
38. Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2008;52:1015-1021.
39. Zile MR. Heart failure with a preserved ejection fraction. In: Mann DL, Zipes D, Libby P BR, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 10th ed. Phila
40. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803.
41. Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281-2293.
42. de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376-2381.
43. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. JAMA. 2016;315:36-46.
44. Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718-726.
45. Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14:198-202.
46. Yoshihisa A, Suzuki S, Yamauchi H, et al. Beneficial effects of positive airway pressure therapy for sleep-disordered breathing in heart failure patients with preserved left ventricular ejection fraction. Clin Cardiol. 2015;38:413-421.
47. Shah RV, Abbasi SA, Heydari B, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014;167:620-626.
48. Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141-1163.
49. Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150:984.
50. Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure: update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3:459-467.
51. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407-413.
52. Cowie MR, Anker SD, Cleland JG, et al. Improving care for patients with acute heart failure: before, during and after hospitalization. Available at: http://www.oxfordhealthpolicyforum.org/files/reports/ahf-report.pdf. Accessed November 27, 2017.
1. Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000-2010. NCHS Data Brief. 2012;(108):1-8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23102190. Accessed April 26, 2017.
2. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
3. Passantino A, Guida P, Lagioia R, et al. Predictors of long-term mortality in older patients hospitalized for acutely decompensated heart failure: clinical relevance of natriuretic peptides. J Am Geriatr Soc. 2017;65:822-826.
4. Lassus JP, Siirilä-Waris K, Nieminen MS, et al. Long-term survival after hospitalization for acute heart failure—differences in prognosis of acutely decompensated chronic and new-onset acute heart failure. Int J Cardiol. 2013;168:458-462.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
6. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol. 2016;68:1476-1488.
7. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200.
8. Pinkerman CP, Sander JE, Breeding D, et al. Institute for Clinical Systems Improvement. Heart failure in adults. Available at: https://www.scribd.com/document/310893227/HeartFailure-pdf. Accessed December 6, 2017.
9. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441-1446.
10. Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation. 2011;124:2865-2873.
11. Heart Failure Society of America, Lindenfeld J, Albert NM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1-194.
12. Pouleur H, The SOLVD Investigators. Results of the treatment trial of the studies of left ventricular dysfunction (SOLVD). Am J Cardiol. 1992;70:135-136.
13. Maggioni AP, Anand I, Gottlieb SO, et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002;40:1414-1421.
14. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007.
15. Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7-13.
16. Gehr TW, Sica DA. Pharmacotherapy in congestive heart failure: Hyperkalemia in congestive heart failure. Congest Heart Fail. 2001;7:97-100.
17. National Institute for Health and Clinical Excellence (NICE). Chronic heart failure in adults: management. 2010. Available at: https://www.nice.org.uk/guidance/cg108. Accessed November 27, 2017.
18. Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent). 2003;16:123-126.
19. Epstein SE, Braunwald E. The effect of beta adrenergic blockade on patterns of urinary sodium excretion: studies in normal subjects and in patients with heart disease. Ann Intern Med. 1966;65:20-27.
20. Berbenetz NM, Mrkobrada M. Mineralocorticoid receptor antagonists for heart failure: systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:246.
21. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-2057.
22. Kelly RA, Smith TW. Recognition and management of digitalis toxicity. Am J Cardiol. 1992;69:108G-118G.
23. Sundar S, Burma DP, Vaish SK. Digoxin toxicity and electrolytes: a correlative study. Acta Cardiol. 1983;38:115-123.
24. McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8:79-84.
25. Prenner SB, Shah SJ, Yancy CW. Role of angiotensin receptor-neprilysin inhibition in heart failure. Curr Atheroscler Rep. 2016;18:48.
26. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
27. Corlanor package insert. Amgen Inc., Thousand Oaks, CA. Available at: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/corlanor/corlanor_pi.pdf. Accessed November 28, 2017.
28. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
29. Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130:94-125.
30. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:1047-1058.
31. Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS Focused Update Incorporated Into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283-e352.
32. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670-679.
33. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868-1877.
34. Nanayakkara S, Kaye DM. Management of heart failure with preserved ejection fraction: a review. Clin Ther. 2015;37:2186-2198.
35. Cleland JG, Pellicori P, Dierckx R. Clinical trials in patients with heart failure and preserved left ventricular ejection fraction. Heart Fail Clin. 2014;10:511-523.
36. Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665-671.
37. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507-515.
38. Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2008;52:1015-1021.
39. Zile MR. Heart failure with a preserved ejection fraction. In: Mann DL, Zipes D, Libby P BR, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 10th ed. Phila
40. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803.
41. Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281-2293.
42. de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376-2381.
43. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. JAMA. 2016;315:36-46.
44. Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718-726.
45. Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14:198-202.
46. Yoshihisa A, Suzuki S, Yamauchi H, et al. Beneficial effects of positive airway pressure therapy for sleep-disordered breathing in heart failure patients with preserved left ventricular ejection fraction. Clin Cardiol. 2015;38:413-421.
47. Shah RV, Abbasi SA, Heydari B, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014;167:620-626.
48. Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141-1163.
49. Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150:984.
50. Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure: update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3:459-467.
51. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407-413.
52. Cowie MR, Anker SD, Cleland JG, et al. Improving care for patients with acute heart failure: before, during and after hospitalization. Available at: http://www.oxfordhealthpolicyforum.org/files/reports/ahf-report.pdf. Accessed November 27, 2017.
From The Journal of Family Practice | 2018;67(1):18-26.
PRACTICE RECOMMENDATIONS
› Order a measurement of B-type natriuretic peptide or N-terminal pro-B-type natriuretic peptide in patients with dyspnea to help diagnose and manage heart failure (HF). A
› Refer patients with symptomatic HF and a left ventricular ejection fraction (LVEF) ≤35% that persists despite ≥3 months of optimal medical therapy for an implantable cardioverter defibrillator to reduce the risk of sudden death and all-cause mortality. A
› Consider cardiac resynchronization therapy for patients with an LVEF ≤35%, sinus rhythm, left bundle branch block, and a QRS duration ≥150 ms who remain symptomatic despite optimal medical therapy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Worsening dyspnea
A 62-year-old woman presented with a 2- to 3-week history of fatigue, nonproductive cough, dyspnea on exertion, and intermittent fever/chills. Her past medical history was significant for rheumatoid arthritis (RA) that had been treated with methotrexate and prednisone for the past 6 years. The patient was currently smoking half a pack a day with a 40-pack year history. The patient was a lifelong resident of Arizona and had previously worked in a stone mine.
On physical examination she appeared comfortable without any increased work of breathing. Her vital signs included a temperature of 36.6° C, a blood pressure of 110/54 mm Hg, a pulse of 90 beats/min, respirations of 16/min, and room-air oxygen saturation of 87%. Pulmonary examination revealed scattered wheezes with fine bibasilar crackles. The remainder of her physical exam was normal. Because she was hypoxic, she was admitted to the hospital.
At the hospital, a chest x-ray showed diffuse, bilateral interstitial changes (FIGURE 1). Laboratory tests revealed a white blood cell count of 13,800/mcL (normal: 4500-10,500/mcL) with 73% neutrophils (normal: 40%-60%), 3% bands (normal: 0-3%), 14% monocytes (normal: 2%-8%), 6% eosinophils (normal: 1%-4%), and 3% lymphocytes (normal: 20%-30%). Community-acquired pneumonia was suspected, and the patient was started on levofloxacin. Over the next 2 days, her dyspnea worsened. She became tachycardic, and her oxygen requirement increased to 15 L/min via a non-rebreather mask. She was transferred to the intensive care unit.
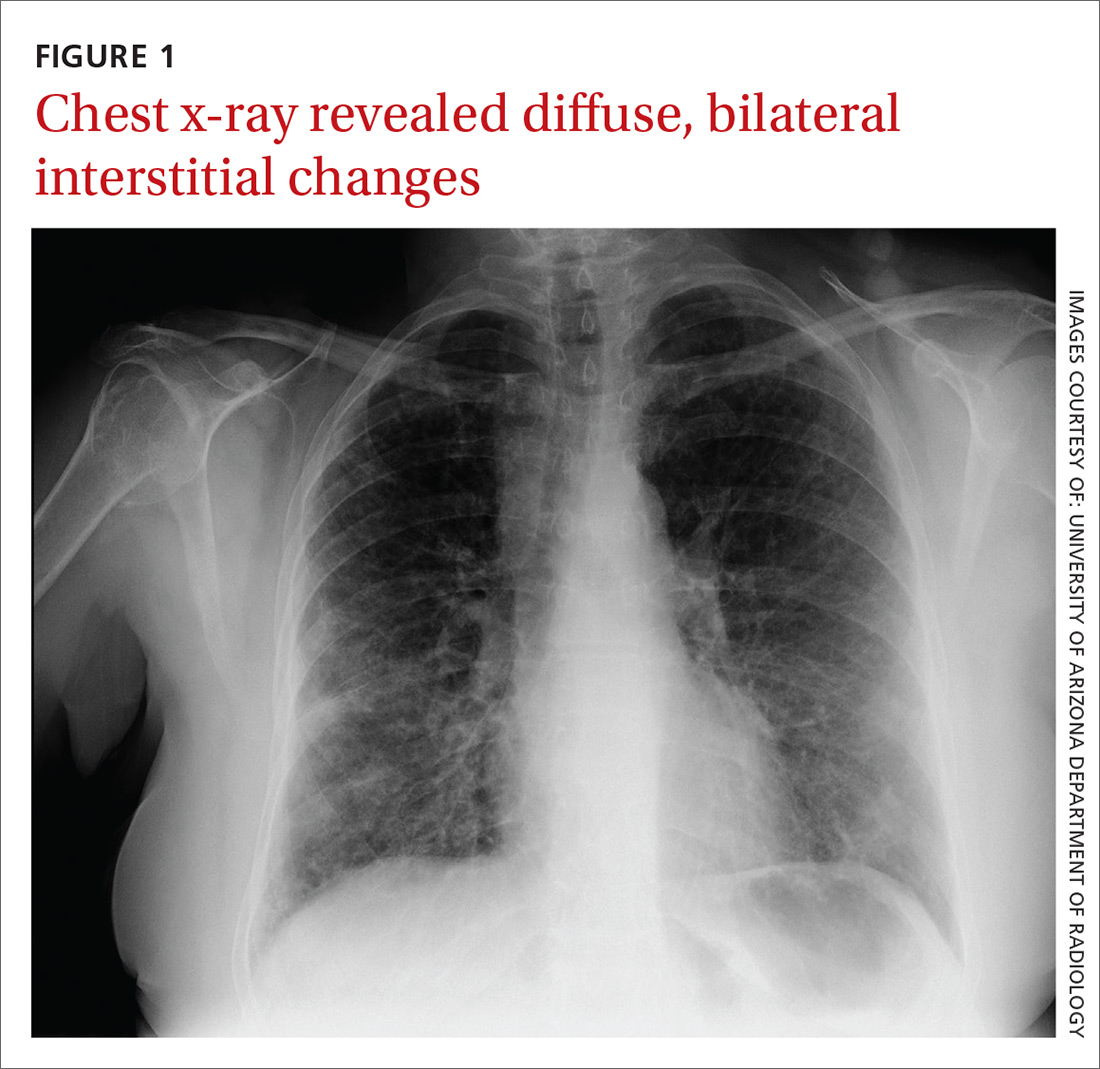
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Interstitial lung disease
Given the patient’s worsening respiratory status, a computed tomography (CT) scan was ordered (FIGURE 2). Review of the CT scan showed ground-glass opacification, mild subpleural honeycombing, reticularity, and traction bronchiectasis bilaterally at the lung bases. Bronchoscopy with lavage was performed to rule out infectious etiologies and was negative. These findings, along with the patient’s medical history of RA and use of methotrexate, led us to diagnose interstitial lung disease (ILD) in this patient.
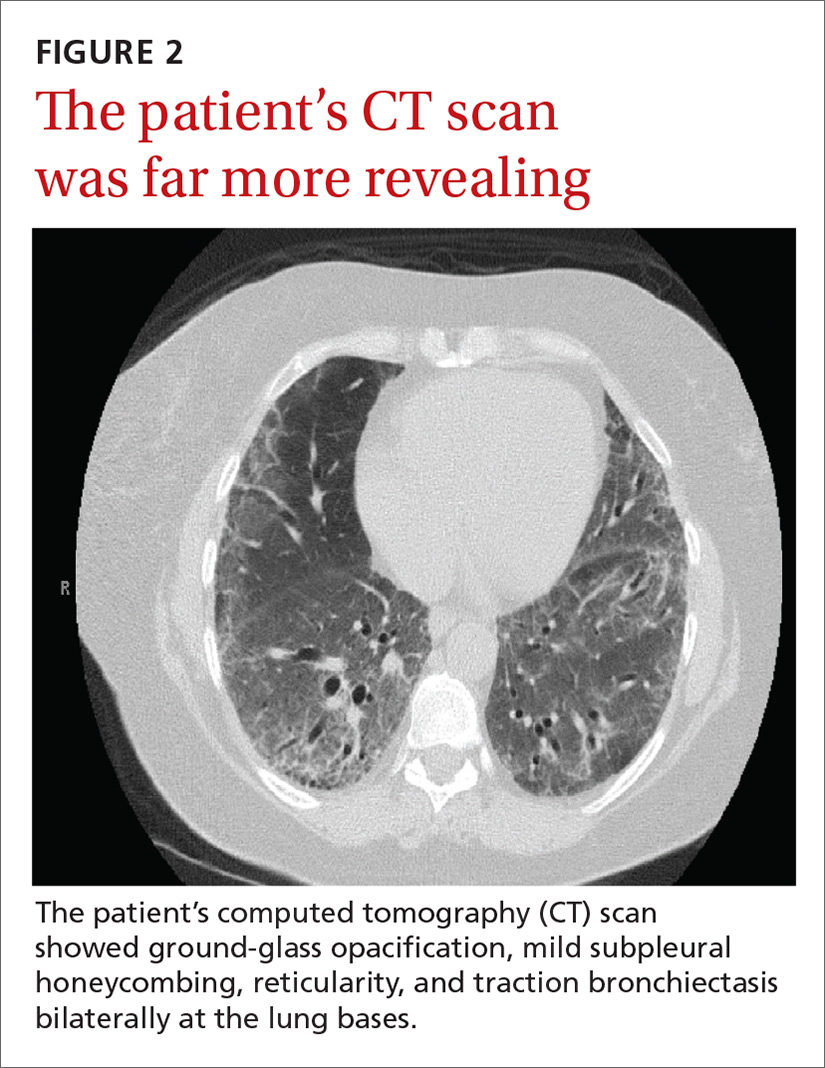
ILD refers to a group of disorders that primarily affects the pulmonary interstitium, rather than the alveolar spaces or pleura.1 The most common causes of ILD seen in primary care are idiopathic pulmonary fibrosis, connective tissue disease, and hypersensitivity pneumonitis secondary to drugs (such as methotrexate, citalopram, fluoxetine, nitrofurantoin, and cephalosporins), radiation, or occupational exposures. (Textile, metal, and plastic workers are at a heightened risk, as are painters and individuals who work with animals.)1 In 2010, idiopathic pulmonary fibrosis had a prevalence of 18.2 cases per 100,000 people.2 Determining the underlying cause of ILD is important, as it may influence prognosis and treatment decisions.
The most common presenting symptoms of ILD are exertional dyspnea, cough with insidious onset, fatigue, and weakness.1,3 Bear in mind, however, that patients with ILD associated with a connective tissue disease may have more subtle manifestations of exertional dyspnea, such as a change in activity level or low resting oxygen saturations. The pulmonary exam can be normal or can reveal fine end-inspiratory crackles, and may include high-pitched, inspiratory rhonchi, or “squeaks.”1
When a diagnosis of ILD is suspected, investigation should begin with high-resolution CT (HRCT).1.3-5 In patients for whom a potential cause of ILD is not identified or who have more than one potential cause, specific patterns seen on the HRCT can help determine the most likely etiology.5 Chest x-ray has low sensitivity and specificity for ILD and can frequently be misinterpreted, as occurred with our patient.1
Rule out other causes of dyspnea
The differential diagnosis for dyspnea includes:
Heart failure. Congestive heart failure can present with acutely worsening dyspnea and cough, but is also commonly associated with orthopnea and/or paroxysmal nocturnal dyspnea. On physical examination, findings of volume overload such as pulmonary crackles, lower extremity edema, and elevated jugular venous pressure are additional signs that heart failure is present.
Pulmonary embolism (PE). Patients with PE commonly present with acute dyspnea, chest pain, and may also have a cough. Additional risk factors for PE (prolonged immobility, fracture, recent hospitalization) may also be present. A Wells score and a D-dimer test can be used to determine the probability of a patient having PE.
Asthma/chronic obstructive pulmonary disease. COPD exacerbations commonly present with a productive cough and worsening dyspnea. Pulmonary exam findings include wheezing, tachypnea, increased respiratory effort, and poor air movement.
Infection (including coccidioidomycosis in the desert southwest, where this patient lived). Our patient was initially treated for pneumonia because she had reported fevers associated with dyspnea and cough along with an elevated white blood cell count. Chest x-ray findings in patients with pneumonia can reveal either lobar consolidation or interstitial infiltrates.
Failure to respond to treatment of the more common causes of dyspnea, as occurred with our patient, should prompt consideration of ILD, particularly in those who have a history of connective tissue disease. Once a diagnosis of ILD is made, referral to a pulmonary specialist is advised.1,3
A poor prognosis and a focus on quality of life
Immunosuppressive therapy is currently the standard treatment for ILD, although there is little evidence to support this practice.1,3,4 Therapy usually includes corticosteroids with or without the addition of a second immunosuppressive agent such as azathioprine, mycophenolate mofetil, or cyclophosphamide.1,4
In addition to drug therapy, the American College of Chest Physicians recommends routine assessment of quality-of-life (QOL) concerns in patients with ILD (TABLE).6,7 Additional QOL tools available to physicians include the Medical Outcomes Study Short-Form 36-Item Instrument8 and the St. George’s Respiratory Questionnaire.9
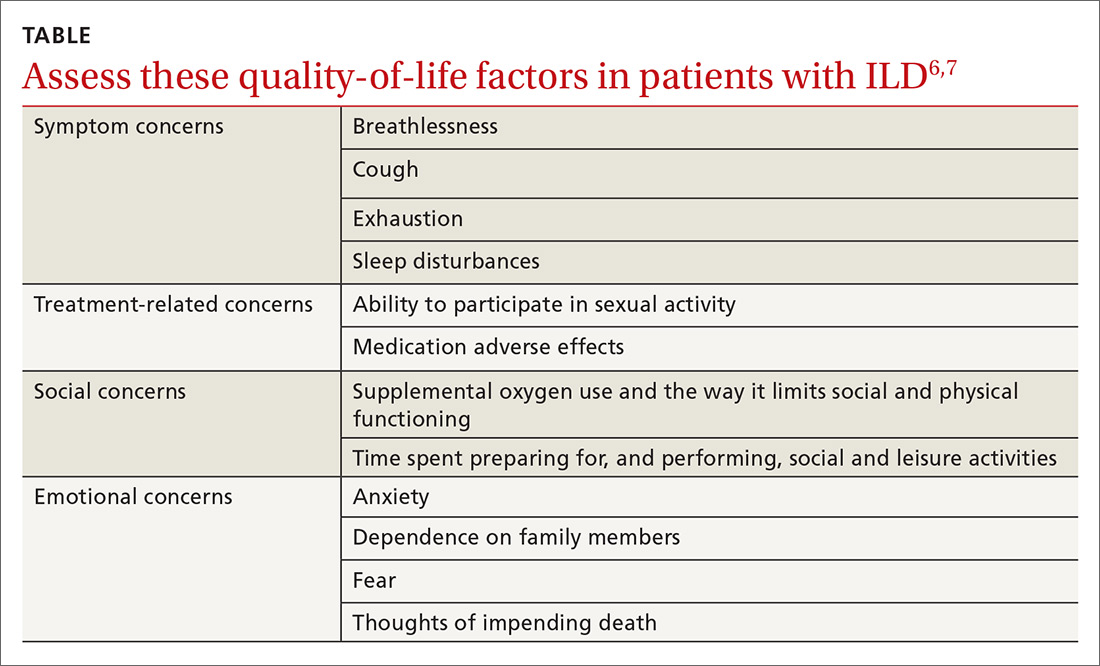
The prognosis is poor, even with treatment. Patients with ILD have a life expectancy that averages 2 to 4 years from diagnosis.6 Patients with ILD are frequently distressed about worsening control of dyspnea and becoming a burden to family members; they also have anxiety about dying.6 It’s important to allocate sufficient time for end-of-life discussions, as studies have shown that patients would like their physicians to address the issue more thoroughly.10
Our patient was started on high-flow oxygen and high-dose steroids. Azathioprine was later added. The patient’s methotrexate was stopped, in light of its association with ILD. Unfortunately, the treatments were not successful and the patient’s respiratory status continued to deteriorate. A family meeting was held with the patient to discuss end-of-life wishes, and the patient expressed a preference for hospice care. She died a few days after hospice enrollment.
CORRESPONDENCE
Karyn B. Kolman, MD, University of Arizona College of Medicine at South Campus Family Medicine Residency, 2800 E Ajo Way, Room 3006, Tucson, AZ 85713; [email protected].
1. Wallis A, Spinks K. The diagnosis and management of interstial lung disease. BMJ. 2015;350:h2072.
2. Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179-186.
3. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2015;41:225-236.
4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824.
5. Nair A, Walsh SL, Desai SR. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167-196.
6. Gilbert CR, Smith CM. Advanced parenchymal lung disease: quality of life and palliative care. Mt Sinai J Med. 2009;76:63-70.
7. Swigris JJ, Stewart AL, Gould MK, et al. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
8. RAND. Medical Outcomes Study 36-Item Short Form Survey (SF-36). Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. Accessed May 27, 2016.
9. St George’s Respiratory Questionnaire. Available at: http://www.healthstatus.sgul.ac.uk/. Accessed May 27, 2016.
10. Bajwah S, Koffman J, Higginson IJ, et. al. ‘I wish I knew more…’ the end-of-life planning and information needs for end-stage fibrotic interstitial lung disease: views of patients, carers, and health professionals. BMJ Support Palliat Care. 2013;3;84-90.
A 62-year-old woman presented with a 2- to 3-week history of fatigue, nonproductive cough, dyspnea on exertion, and intermittent fever/chills. Her past medical history was significant for rheumatoid arthritis (RA) that had been treated with methotrexate and prednisone for the past 6 years. The patient was currently smoking half a pack a day with a 40-pack year history. The patient was a lifelong resident of Arizona and had previously worked in a stone mine.
On physical examination she appeared comfortable without any increased work of breathing. Her vital signs included a temperature of 36.6° C, a blood pressure of 110/54 mm Hg, a pulse of 90 beats/min, respirations of 16/min, and room-air oxygen saturation of 87%. Pulmonary examination revealed scattered wheezes with fine bibasilar crackles. The remainder of her physical exam was normal. Because she was hypoxic, she was admitted to the hospital.
At the hospital, a chest x-ray showed diffuse, bilateral interstitial changes (FIGURE 1). Laboratory tests revealed a white blood cell count of 13,800/mcL (normal: 4500-10,500/mcL) with 73% neutrophils (normal: 40%-60%), 3% bands (normal: 0-3%), 14% monocytes (normal: 2%-8%), 6% eosinophils (normal: 1%-4%), and 3% lymphocytes (normal: 20%-30%). Community-acquired pneumonia was suspected, and the patient was started on levofloxacin. Over the next 2 days, her dyspnea worsened. She became tachycardic, and her oxygen requirement increased to 15 L/min via a non-rebreather mask. She was transferred to the intensive care unit.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Interstitial lung disease
Given the patient’s worsening respiratory status, a computed tomography (CT) scan was ordered (FIGURE 2). Review of the CT scan showed ground-glass opacification, mild subpleural honeycombing, reticularity, and traction bronchiectasis bilaterally at the lung bases. Bronchoscopy with lavage was performed to rule out infectious etiologies and was negative. These findings, along with the patient’s medical history of RA and use of methotrexate, led us to diagnose interstitial lung disease (ILD) in this patient.

ILD refers to a group of disorders that primarily affects the pulmonary interstitium, rather than the alveolar spaces or pleura.1 The most common causes of ILD seen in primary care are idiopathic pulmonary fibrosis, connective tissue disease, and hypersensitivity pneumonitis secondary to drugs (such as methotrexate, citalopram, fluoxetine, nitrofurantoin, and cephalosporins), radiation, or occupational exposures. (Textile, metal, and plastic workers are at a heightened risk, as are painters and individuals who work with animals.)1 In 2010, idiopathic pulmonary fibrosis had a prevalence of 18.2 cases per 100,000 people.2 Determining the underlying cause of ILD is important, as it may influence prognosis and treatment decisions.
The most common presenting symptoms of ILD are exertional dyspnea, cough with insidious onset, fatigue, and weakness.1,3 Bear in mind, however, that patients with ILD associated with a connective tissue disease may have more subtle manifestations of exertional dyspnea, such as a change in activity level or low resting oxygen saturations. The pulmonary exam can be normal or can reveal fine end-inspiratory crackles, and may include high-pitched, inspiratory rhonchi, or “squeaks.”1
When a diagnosis of ILD is suspected, investigation should begin with high-resolution CT (HRCT).1.3-5 In patients for whom a potential cause of ILD is not identified or who have more than one potential cause, specific patterns seen on the HRCT can help determine the most likely etiology.5 Chest x-ray has low sensitivity and specificity for ILD and can frequently be misinterpreted, as occurred with our patient.1
Rule out other causes of dyspnea
The differential diagnosis for dyspnea includes:
Heart failure. Congestive heart failure can present with acutely worsening dyspnea and cough, but is also commonly associated with orthopnea and/or paroxysmal nocturnal dyspnea. On physical examination, findings of volume overload such as pulmonary crackles, lower extremity edema, and elevated jugular venous pressure are additional signs that heart failure is present.
Pulmonary embolism (PE). Patients with PE commonly present with acute dyspnea, chest pain, and may also have a cough. Additional risk factors for PE (prolonged immobility, fracture, recent hospitalization) may also be present. A Wells score and a D-dimer test can be used to determine the probability of a patient having PE.
Asthma/chronic obstructive pulmonary disease. COPD exacerbations commonly present with a productive cough and worsening dyspnea. Pulmonary exam findings include wheezing, tachypnea, increased respiratory effort, and poor air movement.
Infection (including coccidioidomycosis in the desert southwest, where this patient lived). Our patient was initially treated for pneumonia because she had reported fevers associated with dyspnea and cough along with an elevated white blood cell count. Chest x-ray findings in patients with pneumonia can reveal either lobar consolidation or interstitial infiltrates.
Failure to respond to treatment of the more common causes of dyspnea, as occurred with our patient, should prompt consideration of ILD, particularly in those who have a history of connective tissue disease. Once a diagnosis of ILD is made, referral to a pulmonary specialist is advised.1,3
A poor prognosis and a focus on quality of life
Immunosuppressive therapy is currently the standard treatment for ILD, although there is little evidence to support this practice.1,3,4 Therapy usually includes corticosteroids with or without the addition of a second immunosuppressive agent such as azathioprine, mycophenolate mofetil, or cyclophosphamide.1,4
In addition to drug therapy, the American College of Chest Physicians recommends routine assessment of quality-of-life (QOL) concerns in patients with ILD (TABLE).6,7 Additional QOL tools available to physicians include the Medical Outcomes Study Short-Form 36-Item Instrument8 and the St. George’s Respiratory Questionnaire.9

The prognosis is poor, even with treatment. Patients with ILD have a life expectancy that averages 2 to 4 years from diagnosis.6 Patients with ILD are frequently distressed about worsening control of dyspnea and becoming a burden to family members; they also have anxiety about dying.6 It’s important to allocate sufficient time for end-of-life discussions, as studies have shown that patients would like their physicians to address the issue more thoroughly.10
Our patient was started on high-flow oxygen and high-dose steroids. Azathioprine was later added. The patient’s methotrexate was stopped, in light of its association with ILD. Unfortunately, the treatments were not successful and the patient’s respiratory status continued to deteriorate. A family meeting was held with the patient to discuss end-of-life wishes, and the patient expressed a preference for hospice care. She died a few days after hospice enrollment.
CORRESPONDENCE
Karyn B. Kolman, MD, University of Arizona College of Medicine at South Campus Family Medicine Residency, 2800 E Ajo Way, Room 3006, Tucson, AZ 85713; [email protected].
A 62-year-old woman presented with a 2- to 3-week history of fatigue, nonproductive cough, dyspnea on exertion, and intermittent fever/chills. Her past medical history was significant for rheumatoid arthritis (RA) that had been treated with methotrexate and prednisone for the past 6 years. The patient was currently smoking half a pack a day with a 40-pack year history. The patient was a lifelong resident of Arizona and had previously worked in a stone mine.
On physical examination she appeared comfortable without any increased work of breathing. Her vital signs included a temperature of 36.6° C, a blood pressure of 110/54 mm Hg, a pulse of 90 beats/min, respirations of 16/min, and room-air oxygen saturation of 87%. Pulmonary examination revealed scattered wheezes with fine bibasilar crackles. The remainder of her physical exam was normal. Because she was hypoxic, she was admitted to the hospital.
At the hospital, a chest x-ray showed diffuse, bilateral interstitial changes (FIGURE 1). Laboratory tests revealed a white blood cell count of 13,800/mcL (normal: 4500-10,500/mcL) with 73% neutrophils (normal: 40%-60%), 3% bands (normal: 0-3%), 14% monocytes (normal: 2%-8%), 6% eosinophils (normal: 1%-4%), and 3% lymphocytes (normal: 20%-30%). Community-acquired pneumonia was suspected, and the patient was started on levofloxacin. Over the next 2 days, her dyspnea worsened. She became tachycardic, and her oxygen requirement increased to 15 L/min via a non-rebreather mask. She was transferred to the intensive care unit.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Interstitial lung disease
Given the patient’s worsening respiratory status, a computed tomography (CT) scan was ordered (FIGURE 2). Review of the CT scan showed ground-glass opacification, mild subpleural honeycombing, reticularity, and traction bronchiectasis bilaterally at the lung bases. Bronchoscopy with lavage was performed to rule out infectious etiologies and was negative. These findings, along with the patient’s medical history of RA and use of methotrexate, led us to diagnose interstitial lung disease (ILD) in this patient.

ILD refers to a group of disorders that primarily affects the pulmonary interstitium, rather than the alveolar spaces or pleura.1 The most common causes of ILD seen in primary care are idiopathic pulmonary fibrosis, connective tissue disease, and hypersensitivity pneumonitis secondary to drugs (such as methotrexate, citalopram, fluoxetine, nitrofurantoin, and cephalosporins), radiation, or occupational exposures. (Textile, metal, and plastic workers are at a heightened risk, as are painters and individuals who work with animals.)1 In 2010, idiopathic pulmonary fibrosis had a prevalence of 18.2 cases per 100,000 people.2 Determining the underlying cause of ILD is important, as it may influence prognosis and treatment decisions.
The most common presenting symptoms of ILD are exertional dyspnea, cough with insidious onset, fatigue, and weakness.1,3 Bear in mind, however, that patients with ILD associated with a connective tissue disease may have more subtle manifestations of exertional dyspnea, such as a change in activity level or low resting oxygen saturations. The pulmonary exam can be normal or can reveal fine end-inspiratory crackles, and may include high-pitched, inspiratory rhonchi, or “squeaks.”1
When a diagnosis of ILD is suspected, investigation should begin with high-resolution CT (HRCT).1.3-5 In patients for whom a potential cause of ILD is not identified or who have more than one potential cause, specific patterns seen on the HRCT can help determine the most likely etiology.5 Chest x-ray has low sensitivity and specificity for ILD and can frequently be misinterpreted, as occurred with our patient.1
Rule out other causes of dyspnea
The differential diagnosis for dyspnea includes:
Heart failure. Congestive heart failure can present with acutely worsening dyspnea and cough, but is also commonly associated with orthopnea and/or paroxysmal nocturnal dyspnea. On physical examination, findings of volume overload such as pulmonary crackles, lower extremity edema, and elevated jugular venous pressure are additional signs that heart failure is present.
Pulmonary embolism (PE). Patients with PE commonly present with acute dyspnea, chest pain, and may also have a cough. Additional risk factors for PE (prolonged immobility, fracture, recent hospitalization) may also be present. A Wells score and a D-dimer test can be used to determine the probability of a patient having PE.
Asthma/chronic obstructive pulmonary disease. COPD exacerbations commonly present with a productive cough and worsening dyspnea. Pulmonary exam findings include wheezing, tachypnea, increased respiratory effort, and poor air movement.
Infection (including coccidioidomycosis in the desert southwest, where this patient lived). Our patient was initially treated for pneumonia because she had reported fevers associated with dyspnea and cough along with an elevated white blood cell count. Chest x-ray findings in patients with pneumonia can reveal either lobar consolidation or interstitial infiltrates.
Failure to respond to treatment of the more common causes of dyspnea, as occurred with our patient, should prompt consideration of ILD, particularly in those who have a history of connective tissue disease. Once a diagnosis of ILD is made, referral to a pulmonary specialist is advised.1,3
A poor prognosis and a focus on quality of life
Immunosuppressive therapy is currently the standard treatment for ILD, although there is little evidence to support this practice.1,3,4 Therapy usually includes corticosteroids with or without the addition of a second immunosuppressive agent such as azathioprine, mycophenolate mofetil, or cyclophosphamide.1,4
In addition to drug therapy, the American College of Chest Physicians recommends routine assessment of quality-of-life (QOL) concerns in patients with ILD (TABLE).6,7 Additional QOL tools available to physicians include the Medical Outcomes Study Short-Form 36-Item Instrument8 and the St. George’s Respiratory Questionnaire.9

The prognosis is poor, even with treatment. Patients with ILD have a life expectancy that averages 2 to 4 years from diagnosis.6 Patients with ILD are frequently distressed about worsening control of dyspnea and becoming a burden to family members; they also have anxiety about dying.6 It’s important to allocate sufficient time for end-of-life discussions, as studies have shown that patients would like their physicians to address the issue more thoroughly.10
Our patient was started on high-flow oxygen and high-dose steroids. Azathioprine was later added. The patient’s methotrexate was stopped, in light of its association with ILD. Unfortunately, the treatments were not successful and the patient’s respiratory status continued to deteriorate. A family meeting was held with the patient to discuss end-of-life wishes, and the patient expressed a preference for hospice care. She died a few days after hospice enrollment.
CORRESPONDENCE
Karyn B. Kolman, MD, University of Arizona College of Medicine at South Campus Family Medicine Residency, 2800 E Ajo Way, Room 3006, Tucson, AZ 85713; [email protected].
1. Wallis A, Spinks K. The diagnosis and management of interstial lung disease. BMJ. 2015;350:h2072.
2. Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179-186.
3. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2015;41:225-236.
4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824.
5. Nair A, Walsh SL, Desai SR. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167-196.
6. Gilbert CR, Smith CM. Advanced parenchymal lung disease: quality of life and palliative care. Mt Sinai J Med. 2009;76:63-70.
7. Swigris JJ, Stewart AL, Gould MK, et al. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
8. RAND. Medical Outcomes Study 36-Item Short Form Survey (SF-36). Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. Accessed May 27, 2016.
9. St George’s Respiratory Questionnaire. Available at: http://www.healthstatus.sgul.ac.uk/. Accessed May 27, 2016.
10. Bajwah S, Koffman J, Higginson IJ, et. al. ‘I wish I knew more…’ the end-of-life planning and information needs for end-stage fibrotic interstitial lung disease: views of patients, carers, and health professionals. BMJ Support Palliat Care. 2013;3;84-90.
1. Wallis A, Spinks K. The diagnosis and management of interstial lung disease. BMJ. 2015;350:h2072.
2. Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179-186.
3. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2015;41:225-236.
4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824.
5. Nair A, Walsh SL, Desai SR. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167-196.
6. Gilbert CR, Smith CM. Advanced parenchymal lung disease: quality of life and palliative care. Mt Sinai J Med. 2009;76:63-70.
7. Swigris JJ, Stewart AL, Gould MK, et al. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
8. RAND. Medical Outcomes Study 36-Item Short Form Survey (SF-36). Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. Accessed May 27, 2016.
9. St George’s Respiratory Questionnaire. Available at: http://www.healthstatus.sgul.ac.uk/. Accessed May 27, 2016.
10. Bajwah S, Koffman J, Higginson IJ, et. al. ‘I wish I knew more…’ the end-of-life planning and information needs for end-stage fibrotic interstitial lung disease: views of patients, carers, and health professionals. BMJ Support Palliat Care. 2013;3;84-90.