User login
Trichodysplasia Spinulosa in the Setting of Colon Cancer
Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
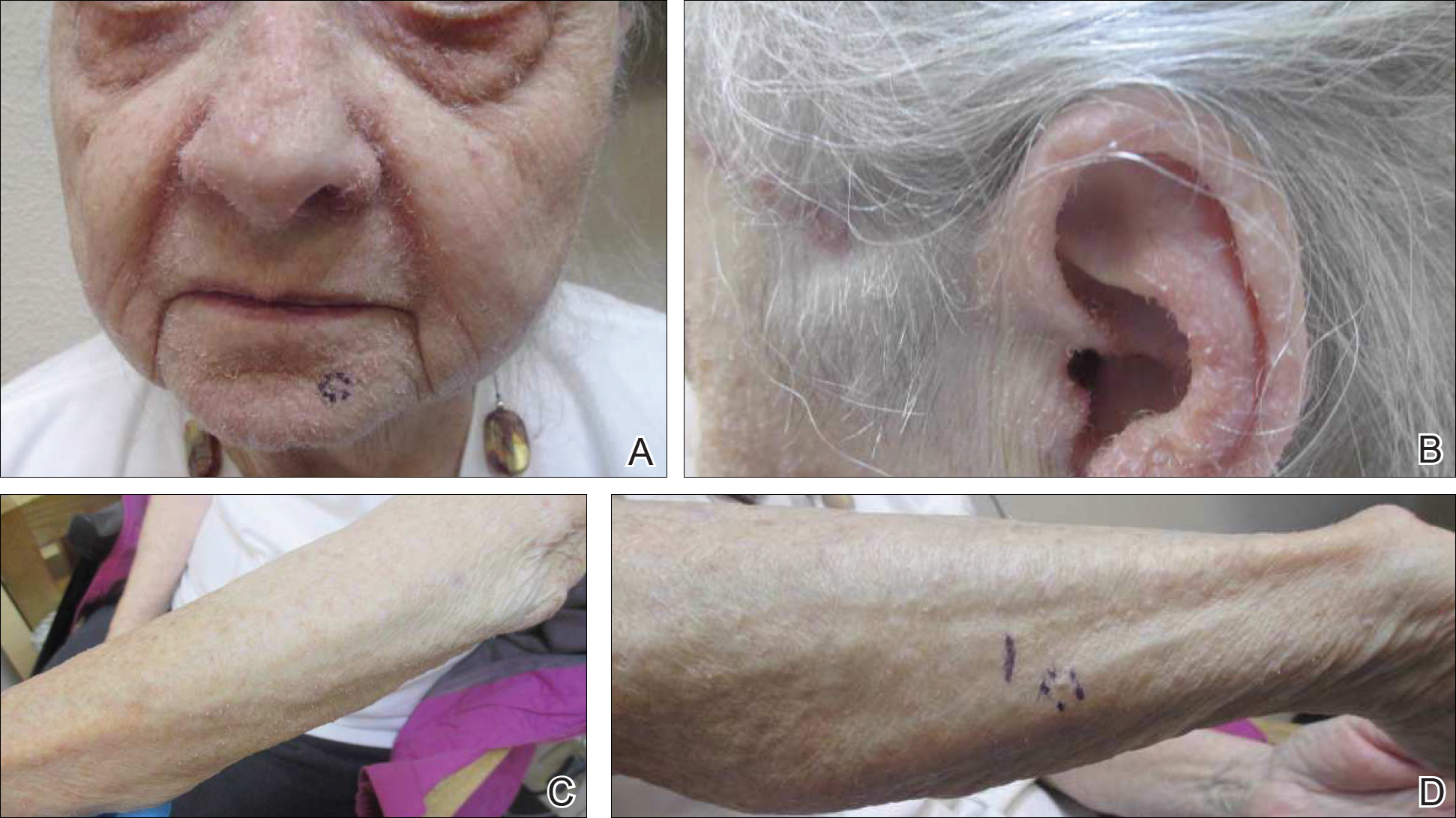
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).
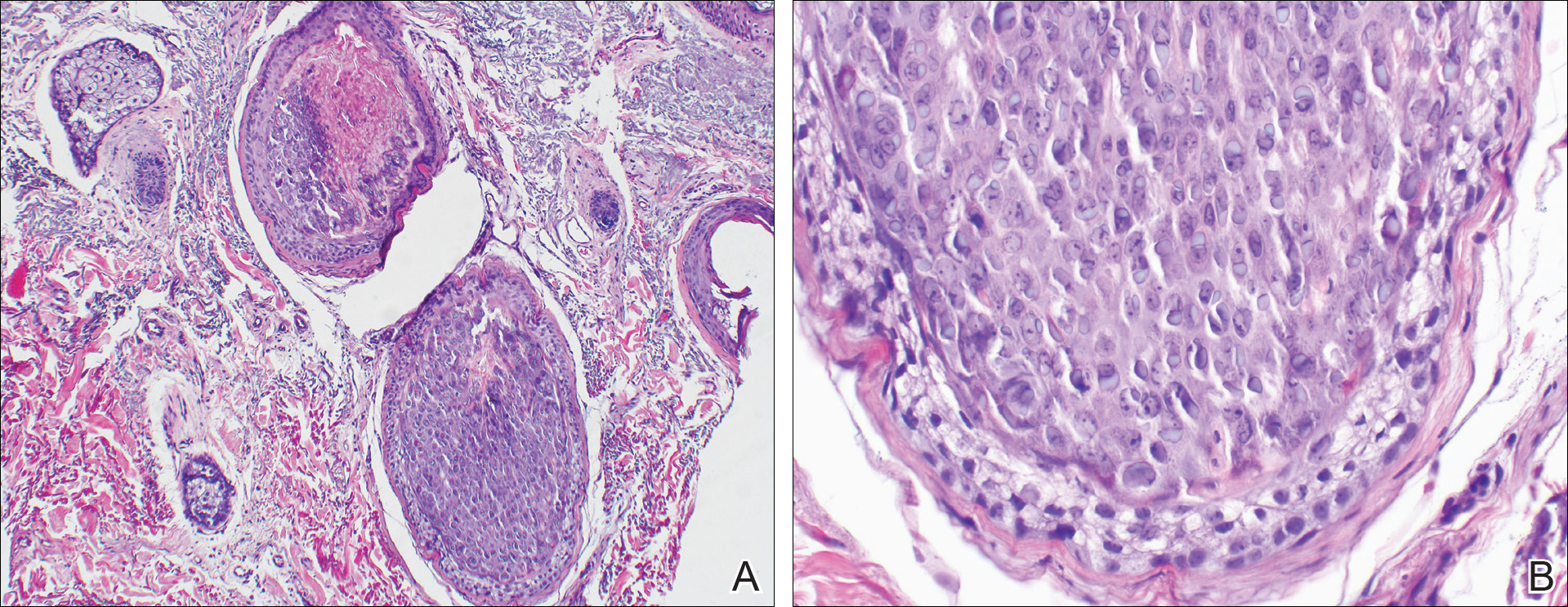
A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.
The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.
Comment
History and Presentation
Trichodysplasia spinulosa was first recognized as hairlike hyperkeratosis.1 The name by which it is currently known was later championed by Haycox et al.2 They reported a case of a 44-year-old man who underwent a combined renal-pancreas transplant and while taking immunosuppressive medication developed erythematous papules with follicular spinous processes and progressive alopecia.2 Other synonymous terms used for this condition include pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, virus-associated trichodysplasia,3 and follicular dystrophy of immunosuppression.4 Trichodysplasia spinulosa can affect both adult and pediatric immunocompromised patients, including organ transplant recipients on immunosuppressants and cancer patients on chemotherapy.3 The condition also has been reported to precede the recurrence of lymphoma.5
Etiology
The connection of TS with a viral etiology was first demonstrated in 1999, and subsequently it was confirmed to be a polyomavirus.2 The family name of Polyomaviridae possesses a Greek derivation with poly- meaning many and -oma meaning cancer.3 This name was given after the polyomavirus induced multiple tumors in mice.3,6 This viral family consists of multiple naked viruses with a surrounding icosahedral capsid containing 3 structural proteins known as VP1, VP2, and VP3. Their life cycle is characterized by early and late phases with respective early and late protein formation.3
Polyomavirus infections maintain an asymptomatic and latent course in immunocompetent patients.7 The prevalence and manifestation of these viruses change when the host’s immune system is altered. The first identified JC virus and BK virus of the same family have been found at increased frequencies in blood and lymphoid tissue during host immunosuppression.6 Moreover, the Merkel cell polyomavirus detected in Merkel cell carcinoma is well documented in the dermatologic literature.6,8
A specific polyomavirus has been implicated in the majority of TS cases and has subsequently received the name of TS polyomavirus.9 As a polyomavirus, it similarly produces capsid antigens and large/small T antigens. Among the viral protein antigens produced, the large tumor or LT antigen represents one of the most potent viral proteins. It has been postulated to inhibit the retinoblastoma family of proteins, leading to increased inner root sheath cells that allow for further viral replication.9,10
The disease presents with folliculocentric papules localized mainly on the central face and ears, which grow central keratin spines or spicules that can become 1 to 3 mm in length. Coinciding alopecia and madarosis also may be present.9
Diagnosis
Histologic examination reveals abnormal follicular maturation and distension. Additionally, increased proliferation and amount of trichohyalin is seen within the inner root sheath cells. Further testing via viral culture, polymerase chain reaction, electron microscopy, or immunohistochemical stains can confirm the diagnosis. Such testing may not be warranted in all cases given that classic clinical findings coupled with routine histopathology staining can provide enough evidence.10,11
Management
Currently, a universal successful treatment for TS does not exist. There have been anecdotal successes reported with topical medications such as cidofovir ointment 1%, acyclovir combined with 2-deoxy-D-glucose and epigallocatechin, corticosteroids, topical tacrolimus, topical retinoids, and imiquimod. Additionally, success has been seen with oral minocycline, oral retinoids, valacyclovir, and valganciclovir, with the latter showing the best results. Patients also have shown improvement after modifying their immunosuppressive treatment regimen.10,12
Conclusion
Given the previously published case of TS preceding the recurrence of lymphoma,5 we notified our patient’s oncologist of this potential risk. Her history of lymphoma and immunosuppressive treatment 4 years prior may represent the etiology of the cutaneous presentation; however, the TS with concurrent colon cancer presented prior to starting immunosuppressive therapy, suggesting that it also may have been a paraneoplastic process and not just a sign of immunosuppression. Therefore, we recommend that patients who present with TS should be evaluated for underlying malignancy if not already diagnosed.
- Linke M, Geraud C, Sauer C, et al. Follicular erythematous papules with keratotic spicules. Acta Derm Venereol . 2014;94:493-494.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases [published online September 12, 2011]. Patholog Res Int. 2011;2011:123491.
- Matthews MR, Wang RC, Reddick RL, et al. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa–associated human polyomavirus. J Cutan Pathol. 2011;38:420-431.
- Osswald SS, Kulick KB, Tomaszewski MM, et al. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721-725.
- Dalianis T, Hirsch HH. Human polyomavirus in disease and cancer. Virology. 2013;437:63-72.
- Tsuzuki S, Fukumoto H, Mine S, et al. Detection of trichodysplasia spinulosa–associated polyomavirus in a fatal case of myocarditis in a seven-month-old girl. Int J Clin Exp Pathol. 2014;7:5308-5312.
- Sadeghi M, Aronen M, Chen T, et al. Merkel cell polyomavirus and trichodysplasia spinulosa–associated polyomavirus DNAs and antibodies in blood among the elderly. BMC Infect Dis. 2012;12:383.
- Van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- Krichhof MG, Shojania K, Hull MW, et al. Trichodysplasia spinulosa: rare presentation of polyomavirus infection in immunocompromised patients. J Cutan Med Surg. 2014;18:430-435.
- Rianthavorn P, Posuwan N, Payungporn S, et al. Polyomavirus reactivation in pediatric patients with systemic lupus erythematosus. Tohoku J Exp Med. 2012;228:197-204.
- Wanat KA, Holler PD, Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219-223.
Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).

A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.

The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.
Comment
History and Presentation
Trichodysplasia spinulosa was first recognized as hairlike hyperkeratosis.1 The name by which it is currently known was later championed by Haycox et al.2 They reported a case of a 44-year-old man who underwent a combined renal-pancreas transplant and while taking immunosuppressive medication developed erythematous papules with follicular spinous processes and progressive alopecia.2 Other synonymous terms used for this condition include pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, virus-associated trichodysplasia,3 and follicular dystrophy of immunosuppression.4 Trichodysplasia spinulosa can affect both adult and pediatric immunocompromised patients, including organ transplant recipients on immunosuppressants and cancer patients on chemotherapy.3 The condition also has been reported to precede the recurrence of lymphoma.5
Etiology
The connection of TS with a viral etiology was first demonstrated in 1999, and subsequently it was confirmed to be a polyomavirus.2 The family name of Polyomaviridae possesses a Greek derivation with poly- meaning many and -oma meaning cancer.3 This name was given after the polyomavirus induced multiple tumors in mice.3,6 This viral family consists of multiple naked viruses with a surrounding icosahedral capsid containing 3 structural proteins known as VP1, VP2, and VP3. Their life cycle is characterized by early and late phases with respective early and late protein formation.3
Polyomavirus infections maintain an asymptomatic and latent course in immunocompetent patients.7 The prevalence and manifestation of these viruses change when the host’s immune system is altered. The first identified JC virus and BK virus of the same family have been found at increased frequencies in blood and lymphoid tissue during host immunosuppression.6 Moreover, the Merkel cell polyomavirus detected in Merkel cell carcinoma is well documented in the dermatologic literature.6,8
A specific polyomavirus has been implicated in the majority of TS cases and has subsequently received the name of TS polyomavirus.9 As a polyomavirus, it similarly produces capsid antigens and large/small T antigens. Among the viral protein antigens produced, the large tumor or LT antigen represents one of the most potent viral proteins. It has been postulated to inhibit the retinoblastoma family of proteins, leading to increased inner root sheath cells that allow for further viral replication.9,10
The disease presents with folliculocentric papules localized mainly on the central face and ears, which grow central keratin spines or spicules that can become 1 to 3 mm in length. Coinciding alopecia and madarosis also may be present.9
Diagnosis
Histologic examination reveals abnormal follicular maturation and distension. Additionally, increased proliferation and amount of trichohyalin is seen within the inner root sheath cells. Further testing via viral culture, polymerase chain reaction, electron microscopy, or immunohistochemical stains can confirm the diagnosis. Such testing may not be warranted in all cases given that classic clinical findings coupled with routine histopathology staining can provide enough evidence.10,11
Management
Currently, a universal successful treatment for TS does not exist. There have been anecdotal successes reported with topical medications such as cidofovir ointment 1%, acyclovir combined with 2-deoxy-D-glucose and epigallocatechin, corticosteroids, topical tacrolimus, topical retinoids, and imiquimod. Additionally, success has been seen with oral minocycline, oral retinoids, valacyclovir, and valganciclovir, with the latter showing the best results. Patients also have shown improvement after modifying their immunosuppressive treatment regimen.10,12
Conclusion
Given the previously published case of TS preceding the recurrence of lymphoma,5 we notified our patient’s oncologist of this potential risk. Her history of lymphoma and immunosuppressive treatment 4 years prior may represent the etiology of the cutaneous presentation; however, the TS with concurrent colon cancer presented prior to starting immunosuppressive therapy, suggesting that it also may have been a paraneoplastic process and not just a sign of immunosuppression. Therefore, we recommend that patients who present with TS should be evaluated for underlying malignancy if not already diagnosed.
Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).

A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.

The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.
Comment
History and Presentation
Trichodysplasia spinulosa was first recognized as hairlike hyperkeratosis.1 The name by which it is currently known was later championed by Haycox et al.2 They reported a case of a 44-year-old man who underwent a combined renal-pancreas transplant and while taking immunosuppressive medication developed erythematous papules with follicular spinous processes and progressive alopecia.2 Other synonymous terms used for this condition include pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, virus-associated trichodysplasia,3 and follicular dystrophy of immunosuppression.4 Trichodysplasia spinulosa can affect both adult and pediatric immunocompromised patients, including organ transplant recipients on immunosuppressants and cancer patients on chemotherapy.3 The condition also has been reported to precede the recurrence of lymphoma.5
Etiology
The connection of TS with a viral etiology was first demonstrated in 1999, and subsequently it was confirmed to be a polyomavirus.2 The family name of Polyomaviridae possesses a Greek derivation with poly- meaning many and -oma meaning cancer.3 This name was given after the polyomavirus induced multiple tumors in mice.3,6 This viral family consists of multiple naked viruses with a surrounding icosahedral capsid containing 3 structural proteins known as VP1, VP2, and VP3. Their life cycle is characterized by early and late phases with respective early and late protein formation.3
Polyomavirus infections maintain an asymptomatic and latent course in immunocompetent patients.7 The prevalence and manifestation of these viruses change when the host’s immune system is altered. The first identified JC virus and BK virus of the same family have been found at increased frequencies in blood and lymphoid tissue during host immunosuppression.6 Moreover, the Merkel cell polyomavirus detected in Merkel cell carcinoma is well documented in the dermatologic literature.6,8
A specific polyomavirus has been implicated in the majority of TS cases and has subsequently received the name of TS polyomavirus.9 As a polyomavirus, it similarly produces capsid antigens and large/small T antigens. Among the viral protein antigens produced, the large tumor or LT antigen represents one of the most potent viral proteins. It has been postulated to inhibit the retinoblastoma family of proteins, leading to increased inner root sheath cells that allow for further viral replication.9,10
The disease presents with folliculocentric papules localized mainly on the central face and ears, which grow central keratin spines or spicules that can become 1 to 3 mm in length. Coinciding alopecia and madarosis also may be present.9
Diagnosis
Histologic examination reveals abnormal follicular maturation and distension. Additionally, increased proliferation and amount of trichohyalin is seen within the inner root sheath cells. Further testing via viral culture, polymerase chain reaction, electron microscopy, or immunohistochemical stains can confirm the diagnosis. Such testing may not be warranted in all cases given that classic clinical findings coupled with routine histopathology staining can provide enough evidence.10,11
Management
Currently, a universal successful treatment for TS does not exist. There have been anecdotal successes reported with topical medications such as cidofovir ointment 1%, acyclovir combined with 2-deoxy-D-glucose and epigallocatechin, corticosteroids, topical tacrolimus, topical retinoids, and imiquimod. Additionally, success has been seen with oral minocycline, oral retinoids, valacyclovir, and valganciclovir, with the latter showing the best results. Patients also have shown improvement after modifying their immunosuppressive treatment regimen.10,12
Conclusion
Given the previously published case of TS preceding the recurrence of lymphoma,5 we notified our patient’s oncologist of this potential risk. Her history of lymphoma and immunosuppressive treatment 4 years prior may represent the etiology of the cutaneous presentation; however, the TS with concurrent colon cancer presented prior to starting immunosuppressive therapy, suggesting that it also may have been a paraneoplastic process and not just a sign of immunosuppression. Therefore, we recommend that patients who present with TS should be evaluated for underlying malignancy if not already diagnosed.
- Linke M, Geraud C, Sauer C, et al. Follicular erythematous papules with keratotic spicules. Acta Derm Venereol . 2014;94:493-494.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases [published online September 12, 2011]. Patholog Res Int. 2011;2011:123491.
- Matthews MR, Wang RC, Reddick RL, et al. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa–associated human polyomavirus. J Cutan Pathol. 2011;38:420-431.
- Osswald SS, Kulick KB, Tomaszewski MM, et al. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721-725.
- Dalianis T, Hirsch HH. Human polyomavirus in disease and cancer. Virology. 2013;437:63-72.
- Tsuzuki S, Fukumoto H, Mine S, et al. Detection of trichodysplasia spinulosa–associated polyomavirus in a fatal case of myocarditis in a seven-month-old girl. Int J Clin Exp Pathol. 2014;7:5308-5312.
- Sadeghi M, Aronen M, Chen T, et al. Merkel cell polyomavirus and trichodysplasia spinulosa–associated polyomavirus DNAs and antibodies in blood among the elderly. BMC Infect Dis. 2012;12:383.
- Van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- Krichhof MG, Shojania K, Hull MW, et al. Trichodysplasia spinulosa: rare presentation of polyomavirus infection in immunocompromised patients. J Cutan Med Surg. 2014;18:430-435.
- Rianthavorn P, Posuwan N, Payungporn S, et al. Polyomavirus reactivation in pediatric patients with systemic lupus erythematosus. Tohoku J Exp Med. 2012;228:197-204.
- Wanat KA, Holler PD, Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219-223.
- Linke M, Geraud C, Sauer C, et al. Follicular erythematous papules with keratotic spicules. Acta Derm Venereol . 2014;94:493-494.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases [published online September 12, 2011]. Patholog Res Int. 2011;2011:123491.
- Matthews MR, Wang RC, Reddick RL, et al. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa–associated human polyomavirus. J Cutan Pathol. 2011;38:420-431.
- Osswald SS, Kulick KB, Tomaszewski MM, et al. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721-725.
- Dalianis T, Hirsch HH. Human polyomavirus in disease and cancer. Virology. 2013;437:63-72.
- Tsuzuki S, Fukumoto H, Mine S, et al. Detection of trichodysplasia spinulosa–associated polyomavirus in a fatal case of myocarditis in a seven-month-old girl. Int J Clin Exp Pathol. 2014;7:5308-5312.
- Sadeghi M, Aronen M, Chen T, et al. Merkel cell polyomavirus and trichodysplasia spinulosa–associated polyomavirus DNAs and antibodies in blood among the elderly. BMC Infect Dis. 2012;12:383.
- Van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- Krichhof MG, Shojania K, Hull MW, et al. Trichodysplasia spinulosa: rare presentation of polyomavirus infection in immunocompromised patients. J Cutan Med Surg. 2014;18:430-435.
- Rianthavorn P, Posuwan N, Payungporn S, et al. Polyomavirus reactivation in pediatric patients with systemic lupus erythematosus. Tohoku J Exp Med. 2012;228:197-204.
- Wanat KA, Holler PD, Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219-223.
Practice Points
- Rashes have a life span and can evolve with time.
- If apparent straightforward conditions do not appear to respond to standard therapy, start to think outside the box for underlying potential causes.
Nausea and vomiting • sensitivity to smell • history of hypertension and alcohol abuse • Dx?
THE CASE
A 57-year-old woman presented to a family physician with acute encephalopathy and complaints of recent gastritis. She reported a 2-month history of nausea, vomiting, decreased oral intake, and extreme sensitivity to smell. The patient had a history of hypertension, and a family member privately disclosed to the FP that she also had a history of alcohol abuse. The patient was taking lorazepam daily, as needed, for anxiety.
On initial assessment, the patient was alert, but not oriented to time or situation. She was ataxic and agitated but did not exhibit pupillary constriction or tremor. The FP sent her to the emergency department (ED).
After being assessed in the ED, the patient was admitted. Over the course of several days, she showed worsening mentation; she persistently believed she was in Chicago, her childhood home. On memory testing, she was unable to recall any of 3 objects after 5 minutes. She exhibited horizontal nystagmus and dysmetria bilaterally and continued to be ataxic, requiring 2-point assistance. Her agitation was managed nonpharmacologically.
A work-up was performed, which included laboratory testing, a urinalysis, and computed tomography (CT) of the head. A comprehensive metabolic panel, complete blood count, and thyroid stimulating hormone test were unremarkable except for electrolyte disturbances, with a sodium level of 158 mEq/L and a potassium level of 2.6 mEq/L (reference ranges: 135-145 mEq/L and 3.5-5 mEq/L, respectively).
Her blood alcohol level was zero, and not surprisingly given her use of lorazepam, a urine drug screen was positive for benzodiazepines. The urinalysis results were consistent with a urinary tract infection (UTI), for which she was treated with an antibiotic. A carbohydrate-deficient transferrin test may have been useful to establish chronic alcohol abuse, but was not ordered. The head CT was negative.
After a few days with fluids and electrolyte replacement, the patient’s electrolytes normalized.
THE DIAGNOSIS
The differential diagnosis included sepsis, metabolic encephalopathy, and alcoholic encephalopathy. Given that the patient’s urine drug screen was positive, benzodiazepine withdrawal was also considered a plausible explanation for her continued cognitive disturbances. (It was surmised that she had likely taken her last lorazepam several days prior.) However, the lack of other signs of withdrawal prompted further investigation.
Continue to: Since her encephalopathy...
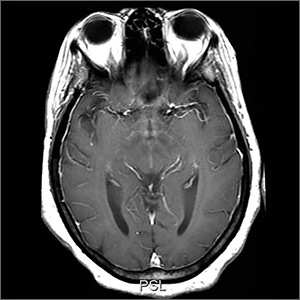
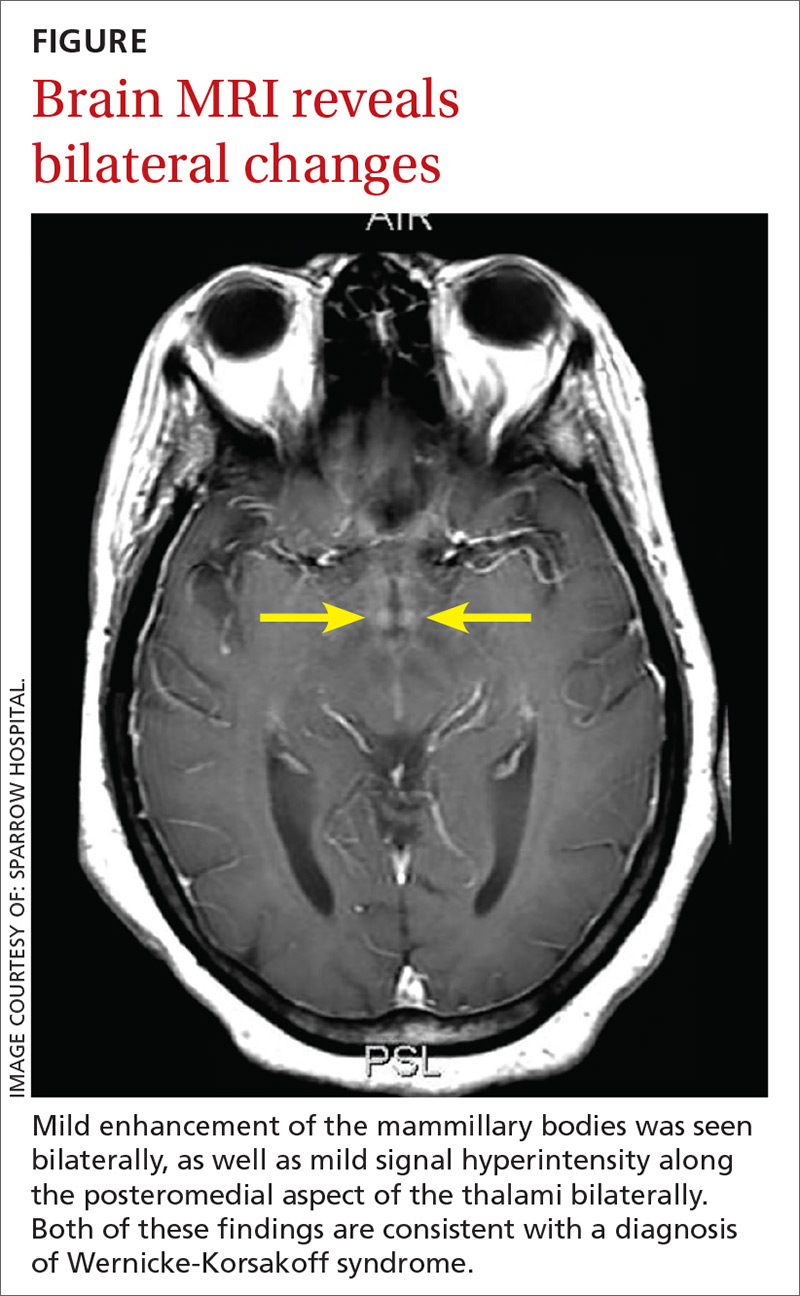
Since her encephalopathy, ataxia, and nystagmus persisted, magnetic resonance imaging (MRI) of the brain was performed on Day 3 of hospitalization (FIGURE). A lumbar puncture and an electroencephalogram were also considered but were not performed because the MRI results revealed bilateral enhancement of the m
DISCUSSION
WKS is the concurrence of Wernicke’s encephalopathy (an acute, life-threatening condition marked by ataxia, confusion, and ocular signs) and Korsakoff’s psychosis (a long-term, debilitating amnestic syndrome). WKS is a neuropsychiatric disorder in which patients experience profound short-term amnesia; it is precipitated by thiamine deficiency (defined as a whole blood thiamine level <0.7 ng/ml1).The link to thiamine was confirmed during World War II, when thiamine treatment resolved symptoms in starving prisoners. If recognized early, treatment of thiamine deficiency can prevent long-term morbidity from WKS.
Etiology of thiamine deficiency
Our patient’s alcohol abuse placed her at risk for WKS, and her olfactory aversion to certain foods was a diagnostic clue. In this case, we inadvertently administered dextrose with antibiotics for the UTI prior to administering thiamine; this exacerbated the thiamine deficiency because glucose and thiamine compete for the same substrate.
Is alcohol abuse always to blame for WKS?
The quantity and type of alcohol that results in the development of WKS has not been well studied, but the Caine diagnostic criteria defines chronic alcoholism as the consumption of 80 g/d of ethanol (8 drinks/d).2 While WKS is commonly associated with alcoholism, other causative conditions may be overlooked. Other associated illnesses include acquired immune deficiency syndrome (AIDS), cancer, hyperemesis gravidarum, prolonged total parenteral nutrition, and psychiatric illnesses such as eating disorders and schizophrenia. Procedures such as gastric bypass and dialysis can also precipitate WKS.3
Men and women are both at risk of developing WKS. A lack of consumption of thiamine-rich sources such as cereals, rice, and legumes puts patients at risk for WKS. The recommended dietary allowance of thiamine increases with age and may be higher for obese patients.4
Continue to: Suspect thiamine deficiency and obstain a thorough history
Suspect thiamine deficiency and obtain a thorough history
A high index of suspicion for thiamine deficiency is essential for diagnosis of WKS. History of alcohol use should be obtained, including quantity, frequency, pattern, duration, and time of last use. Physicians should assess nutrition and ask about vomiting and diarrhea. It is important to collaborate with the patient’s family and friends and inquire into other substance misuse.5
Since WKS targets the dorsomedial thalamus, which is responsible for olfactory processing, patients may complain of a distorted perception of smell.6 On physical examination, look for signs of protein-calorie malnutrition, including cheilitis, glossitis, and bleeding gums; signs of alcohol abuse, such as hepatomegaly; and evidence of injuries or poor self-care.5
Varied presentation leads to under- and misdiagnosis
Diagnosis of WKS can be difficult due to the varied presentation; there is a broad spectrum of clinical features. The clinical triad of mental status change, ophthalmoplegia, and gait ataxia is present in as few as 10% of cases.3 Mental status changes may include a global confusional state ranging from disorientation, apathy, anxiety, fear, and mild memory impairment to pronounced amnesia. Ophthalmoplegia can include nystagmus, ocular palsies, retinal hemorrhages, scotoma, or photophobia; and ataxia can range from a mild gait abnormality to an inability to stand.7 This varied presentation ultimately leads to underdiagnosis and misdiagnosis.
MRI findings are also varied in WKS. However, the mammillary bodies are involved in many cases, where atrophy of these structures have high specificity. The dorsomedial thalamus is associated with the reported impairment in memory and can be identified antemortem on MRI.3 There is no quantifiable evidence of how much thiamine should be used to prevent WKS. However, thiamine should be given before the administration of glucose whenever WKS is considered.
Our patient. Despite the administration of thiamine (100 mg parenterally for 5 d, followed by oral thiamine 300 mg/d indefinitely), our patient’s memory and cognition remained unchanged. She underwent intensive inpatient rehabilitation for 2 months and was eventually placed in long-term nursing care.
Continue to: THE TAKEAWAY
THE TAKEAWAY
A high index of suspicion is crucial to prevent possible long-term neurologic sequelae in WKS. Appropriate care starts at the beginning, with the patient’s story.
CORRESPONDENCE
Romith Naug, MD, 15 St. Andrew Street, Unit 601, Brockville, ON Canada K6V0B8; [email protected].
1. Doshi S, Velpandian T, Seth S, et al. Prevalence of thiamine deficiency in heart failure patients on long-term diuretic therapy. J Prac Cardiovasc Sci. 2015;1:25-29.
2. Caine D, Halliday GM, Kril JJ, et al. Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62:51-60.
3. Donnino MW, Vega J, Miller J, et al. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50:715-721.
4. Kerns J, Arundel C, Chawla LS. Thiamin deficiency in people with obesity. Adv Nutr. 2015;6:147-153.
5. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44:911-915.
6. Wilson DA, Xu W, Sadrian B, et al. Cortical odor processing in health and disease. Prog Brain Res. 2014;208:275-305.
7. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff syndrome: under-recognized and under-treated. Psychosomatics. 2012;53:507-516.
THE CASE
A 57-year-old woman presented to a family physician with acute encephalopathy and complaints of recent gastritis. She reported a 2-month history of nausea, vomiting, decreased oral intake, and extreme sensitivity to smell. The patient had a history of hypertension, and a family member privately disclosed to the FP that she also had a history of alcohol abuse. The patient was taking lorazepam daily, as needed, for anxiety.
On initial assessment, the patient was alert, but not oriented to time or situation. She was ataxic and agitated but did not exhibit pupillary constriction or tremor. The FP sent her to the emergency department (ED).
After being assessed in the ED, the patient was admitted. Over the course of several days, she showed worsening mentation; she persistently believed she was in Chicago, her childhood home. On memory testing, she was unable to recall any of 3 objects after 5 minutes. She exhibited horizontal nystagmus and dysmetria bilaterally and continued to be ataxic, requiring 2-point assistance. Her agitation was managed nonpharmacologically.
A work-up was performed, which included laboratory testing, a urinalysis, and computed tomography (CT) of the head. A comprehensive metabolic panel, complete blood count, and thyroid stimulating hormone test were unremarkable except for electrolyte disturbances, with a sodium level of 158 mEq/L and a potassium level of 2.6 mEq/L (reference ranges: 135-145 mEq/L and 3.5-5 mEq/L, respectively).
Her blood alcohol level was zero, and not surprisingly given her use of lorazepam, a urine drug screen was positive for benzodiazepines. The urinalysis results were consistent with a urinary tract infection (UTI), for which she was treated with an antibiotic. A carbohydrate-deficient transferrin test may have been useful to establish chronic alcohol abuse, but was not ordered. The head CT was negative.
After a few days with fluids and electrolyte replacement, the patient’s electrolytes normalized.
THE DIAGNOSIS
The differential diagnosis included sepsis, metabolic encephalopathy, and alcoholic encephalopathy. Given that the patient’s urine drug screen was positive, benzodiazepine withdrawal was also considered a plausible explanation for her continued cognitive disturbances. (It was surmised that she had likely taken her last lorazepam several days prior.) However, the lack of other signs of withdrawal prompted further investigation.
Continue to: Since her encephalopathy...
Since her encephalopathy, ataxia, and nystagmus persisted, magnetic resonance imaging (MRI) of the brain was performed on Day 3 of hospitalization (FIGURE). A lumbar puncture and an electroencephalogram were also considered but were not performed because the MRI results revealed bilateral enhancement of the m

DISCUSSION
WKS is the concurrence of Wernicke’s encephalopathy (an acute, life-threatening condition marked by ataxia, confusion, and ocular signs) and Korsakoff’s psychosis (a long-term, debilitating amnestic syndrome). WKS is a neuropsychiatric disorder in which patients experience profound short-term amnesia; it is precipitated by thiamine deficiency (defined as a whole blood thiamine level <0.7 ng/ml1).The link to thiamine was confirmed during World War II, when thiamine treatment resolved symptoms in starving prisoners. If recognized early, treatment of thiamine deficiency can prevent long-term morbidity from WKS.
Etiology of thiamine deficiency
Our patient’s alcohol abuse placed her at risk for WKS, and her olfactory aversion to certain foods was a diagnostic clue. In this case, we inadvertently administered dextrose with antibiotics for the UTI prior to administering thiamine; this exacerbated the thiamine deficiency because glucose and thiamine compete for the same substrate.
Is alcohol abuse always to blame for WKS?
The quantity and type of alcohol that results in the development of WKS has not been well studied, but the Caine diagnostic criteria defines chronic alcoholism as the consumption of 80 g/d of ethanol (8 drinks/d).2 While WKS is commonly associated with alcoholism, other causative conditions may be overlooked. Other associated illnesses include acquired immune deficiency syndrome (AIDS), cancer, hyperemesis gravidarum, prolonged total parenteral nutrition, and psychiatric illnesses such as eating disorders and schizophrenia. Procedures such as gastric bypass and dialysis can also precipitate WKS.3
Men and women are both at risk of developing WKS. A lack of consumption of thiamine-rich sources such as cereals, rice, and legumes puts patients at risk for WKS. The recommended dietary allowance of thiamine increases with age and may be higher for obese patients.4
Continue to: Suspect thiamine deficiency and obstain a thorough history
Suspect thiamine deficiency and obtain a thorough history
A high index of suspicion for thiamine deficiency is essential for diagnosis of WKS. History of alcohol use should be obtained, including quantity, frequency, pattern, duration, and time of last use. Physicians should assess nutrition and ask about vomiting and diarrhea. It is important to collaborate with the patient’s family and friends and inquire into other substance misuse.5
Since WKS targets the dorsomedial thalamus, which is responsible for olfactory processing, patients may complain of a distorted perception of smell.6 On physical examination, look for signs of protein-calorie malnutrition, including cheilitis, glossitis, and bleeding gums; signs of alcohol abuse, such as hepatomegaly; and evidence of injuries or poor self-care.5
Varied presentation leads to under- and misdiagnosis
Diagnosis of WKS can be difficult due to the varied presentation; there is a broad spectrum of clinical features. The clinical triad of mental status change, ophthalmoplegia, and gait ataxia is present in as few as 10% of cases.3 Mental status changes may include a global confusional state ranging from disorientation, apathy, anxiety, fear, and mild memory impairment to pronounced amnesia. Ophthalmoplegia can include nystagmus, ocular palsies, retinal hemorrhages, scotoma, or photophobia; and ataxia can range from a mild gait abnormality to an inability to stand.7 This varied presentation ultimately leads to underdiagnosis and misdiagnosis.
MRI findings are also varied in WKS. However, the mammillary bodies are involved in many cases, where atrophy of these structures have high specificity. The dorsomedial thalamus is associated with the reported impairment in memory and can be identified antemortem on MRI.3 There is no quantifiable evidence of how much thiamine should be used to prevent WKS. However, thiamine should be given before the administration of glucose whenever WKS is considered.
Our patient. Despite the administration of thiamine (100 mg parenterally for 5 d, followed by oral thiamine 300 mg/d indefinitely), our patient’s memory and cognition remained unchanged. She underwent intensive inpatient rehabilitation for 2 months and was eventually placed in long-term nursing care.
Continue to: THE TAKEAWAY
THE TAKEAWAY
A high index of suspicion is crucial to prevent possible long-term neurologic sequelae in WKS. Appropriate care starts at the beginning, with the patient’s story.
CORRESPONDENCE
Romith Naug, MD, 15 St. Andrew Street, Unit 601, Brockville, ON Canada K6V0B8; [email protected].
THE CASE
A 57-year-old woman presented to a family physician with acute encephalopathy and complaints of recent gastritis. She reported a 2-month history of nausea, vomiting, decreased oral intake, and extreme sensitivity to smell. The patient had a history of hypertension, and a family member privately disclosed to the FP that she also had a history of alcohol abuse. The patient was taking lorazepam daily, as needed, for anxiety.
On initial assessment, the patient was alert, but not oriented to time or situation. She was ataxic and agitated but did not exhibit pupillary constriction or tremor. The FP sent her to the emergency department (ED).
After being assessed in the ED, the patient was admitted. Over the course of several days, she showed worsening mentation; she persistently believed she was in Chicago, her childhood home. On memory testing, she was unable to recall any of 3 objects after 5 minutes. She exhibited horizontal nystagmus and dysmetria bilaterally and continued to be ataxic, requiring 2-point assistance. Her agitation was managed nonpharmacologically.
A work-up was performed, which included laboratory testing, a urinalysis, and computed tomography (CT) of the head. A comprehensive metabolic panel, complete blood count, and thyroid stimulating hormone test were unremarkable except for electrolyte disturbances, with a sodium level of 158 mEq/L and a potassium level of 2.6 mEq/L (reference ranges: 135-145 mEq/L and 3.5-5 mEq/L, respectively).
Her blood alcohol level was zero, and not surprisingly given her use of lorazepam, a urine drug screen was positive for benzodiazepines. The urinalysis results were consistent with a urinary tract infection (UTI), for which she was treated with an antibiotic. A carbohydrate-deficient transferrin test may have been useful to establish chronic alcohol abuse, but was not ordered. The head CT was negative.
After a few days with fluids and electrolyte replacement, the patient’s electrolytes normalized.
THE DIAGNOSIS
The differential diagnosis included sepsis, metabolic encephalopathy, and alcoholic encephalopathy. Given that the patient’s urine drug screen was positive, benzodiazepine withdrawal was also considered a plausible explanation for her continued cognitive disturbances. (It was surmised that she had likely taken her last lorazepam several days prior.) However, the lack of other signs of withdrawal prompted further investigation.
Continue to: Since her encephalopathy...
Since her encephalopathy, ataxia, and nystagmus persisted, magnetic resonance imaging (MRI) of the brain was performed on Day 3 of hospitalization (FIGURE). A lumbar puncture and an electroencephalogram were also considered but were not performed because the MRI results revealed bilateral enhancement of the m

DISCUSSION
WKS is the concurrence of Wernicke’s encephalopathy (an acute, life-threatening condition marked by ataxia, confusion, and ocular signs) and Korsakoff’s psychosis (a long-term, debilitating amnestic syndrome). WKS is a neuropsychiatric disorder in which patients experience profound short-term amnesia; it is precipitated by thiamine deficiency (defined as a whole blood thiamine level <0.7 ng/ml1).The link to thiamine was confirmed during World War II, when thiamine treatment resolved symptoms in starving prisoners. If recognized early, treatment of thiamine deficiency can prevent long-term morbidity from WKS.
Etiology of thiamine deficiency
Our patient’s alcohol abuse placed her at risk for WKS, and her olfactory aversion to certain foods was a diagnostic clue. In this case, we inadvertently administered dextrose with antibiotics for the UTI prior to administering thiamine; this exacerbated the thiamine deficiency because glucose and thiamine compete for the same substrate.
Is alcohol abuse always to blame for WKS?
The quantity and type of alcohol that results in the development of WKS has not been well studied, but the Caine diagnostic criteria defines chronic alcoholism as the consumption of 80 g/d of ethanol (8 drinks/d).2 While WKS is commonly associated with alcoholism, other causative conditions may be overlooked. Other associated illnesses include acquired immune deficiency syndrome (AIDS), cancer, hyperemesis gravidarum, prolonged total parenteral nutrition, and psychiatric illnesses such as eating disorders and schizophrenia. Procedures such as gastric bypass and dialysis can also precipitate WKS.3
Men and women are both at risk of developing WKS. A lack of consumption of thiamine-rich sources such as cereals, rice, and legumes puts patients at risk for WKS. The recommended dietary allowance of thiamine increases with age and may be higher for obese patients.4
Continue to: Suspect thiamine deficiency and obstain a thorough history
Suspect thiamine deficiency and obtain a thorough history
A high index of suspicion for thiamine deficiency is essential for diagnosis of WKS. History of alcohol use should be obtained, including quantity, frequency, pattern, duration, and time of last use. Physicians should assess nutrition and ask about vomiting and diarrhea. It is important to collaborate with the patient’s family and friends and inquire into other substance misuse.5
Since WKS targets the dorsomedial thalamus, which is responsible for olfactory processing, patients may complain of a distorted perception of smell.6 On physical examination, look for signs of protein-calorie malnutrition, including cheilitis, glossitis, and bleeding gums; signs of alcohol abuse, such as hepatomegaly; and evidence of injuries or poor self-care.5
Varied presentation leads to under- and misdiagnosis
Diagnosis of WKS can be difficult due to the varied presentation; there is a broad spectrum of clinical features. The clinical triad of mental status change, ophthalmoplegia, and gait ataxia is present in as few as 10% of cases.3 Mental status changes may include a global confusional state ranging from disorientation, apathy, anxiety, fear, and mild memory impairment to pronounced amnesia. Ophthalmoplegia can include nystagmus, ocular palsies, retinal hemorrhages, scotoma, or photophobia; and ataxia can range from a mild gait abnormality to an inability to stand.7 This varied presentation ultimately leads to underdiagnosis and misdiagnosis.
MRI findings are also varied in WKS. However, the mammillary bodies are involved in many cases, where atrophy of these structures have high specificity. The dorsomedial thalamus is associated with the reported impairment in memory and can be identified antemortem on MRI.3 There is no quantifiable evidence of how much thiamine should be used to prevent WKS. However, thiamine should be given before the administration of glucose whenever WKS is considered.
Our patient. Despite the administration of thiamine (100 mg parenterally for 5 d, followed by oral thiamine 300 mg/d indefinitely), our patient’s memory and cognition remained unchanged. She underwent intensive inpatient rehabilitation for 2 months and was eventually placed in long-term nursing care.
Continue to: THE TAKEAWAY
THE TAKEAWAY
A high index of suspicion is crucial to prevent possible long-term neurologic sequelae in WKS. Appropriate care starts at the beginning, with the patient’s story.
CORRESPONDENCE
Romith Naug, MD, 15 St. Andrew Street, Unit 601, Brockville, ON Canada K6V0B8; [email protected].
1. Doshi S, Velpandian T, Seth S, et al. Prevalence of thiamine deficiency in heart failure patients on long-term diuretic therapy. J Prac Cardiovasc Sci. 2015;1:25-29.
2. Caine D, Halliday GM, Kril JJ, et al. Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62:51-60.
3. Donnino MW, Vega J, Miller J, et al. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50:715-721.
4. Kerns J, Arundel C, Chawla LS. Thiamin deficiency in people with obesity. Adv Nutr. 2015;6:147-153.
5. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44:911-915.
6. Wilson DA, Xu W, Sadrian B, et al. Cortical odor processing in health and disease. Prog Brain Res. 2014;208:275-305.
7. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff syndrome: under-recognized and under-treated. Psychosomatics. 2012;53:507-516.
1. Doshi S, Velpandian T, Seth S, et al. Prevalence of thiamine deficiency in heart failure patients on long-term diuretic therapy. J Prac Cardiovasc Sci. 2015;1:25-29.
2. Caine D, Halliday GM, Kril JJ, et al. Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62:51-60.
3. Donnino MW, Vega J, Miller J, et al. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50:715-721.
4. Kerns J, Arundel C, Chawla LS. Thiamin deficiency in people with obesity. Adv Nutr. 2015;6:147-153.
5. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44:911-915.
6. Wilson DA, Xu W, Sadrian B, et al. Cortical odor processing in health and disease. Prog Brain Res. 2014;208:275-305.
7. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff syndrome: under-recognized and under-treated. Psychosomatics. 2012;53:507-516.
Adult-Onset Still Disease: Persistent Pruritic Papular Rash With Unique Histopathologic Findings
Adult-onset Still disease (AOSD) is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, evanescent skin rash, and lymphadenopathy. 1 The most commonly used criteria for diagnosing AOSD are the Yamaguchi criteria. 2 The major criteria include high fever for more than 1 week, arthralgia for more than 2 weeks, leukocytosis, and an evanescent skin rash. The minor criteria consist of sore throat, lymphadenopathy and/or splenomegaly, liver dysfunction, and negative rheumatoid factor and antinuclear antibodies. Classically, the skin rash is described as an evanescent, salmon-colored erythema involving the extremities. Nevertheless, unusual cutaneous eruptions have been reported in AOSD, including persistent pruritic papules and plaques. 3 Importantly, this atypical rash demonstrates specific histologic findings that are not found on routine histopathology of a typical evanescent rash. We describe 2 patients with this atypical cutaneous eruption along with the unique histopathologic findings of AOSD.
Case Reports
Patient 1
A 23-year-old Chinese woman presented with periodic fevers, persistent rash, and joint pain of 2 years’ duration. Her medical history included splenectomy for hepatosplenomegaly as well as evaluation by hematology for lymphadenopathy; a cervical lymph node biopsy showed lymphoid and follicular hyperplasia.
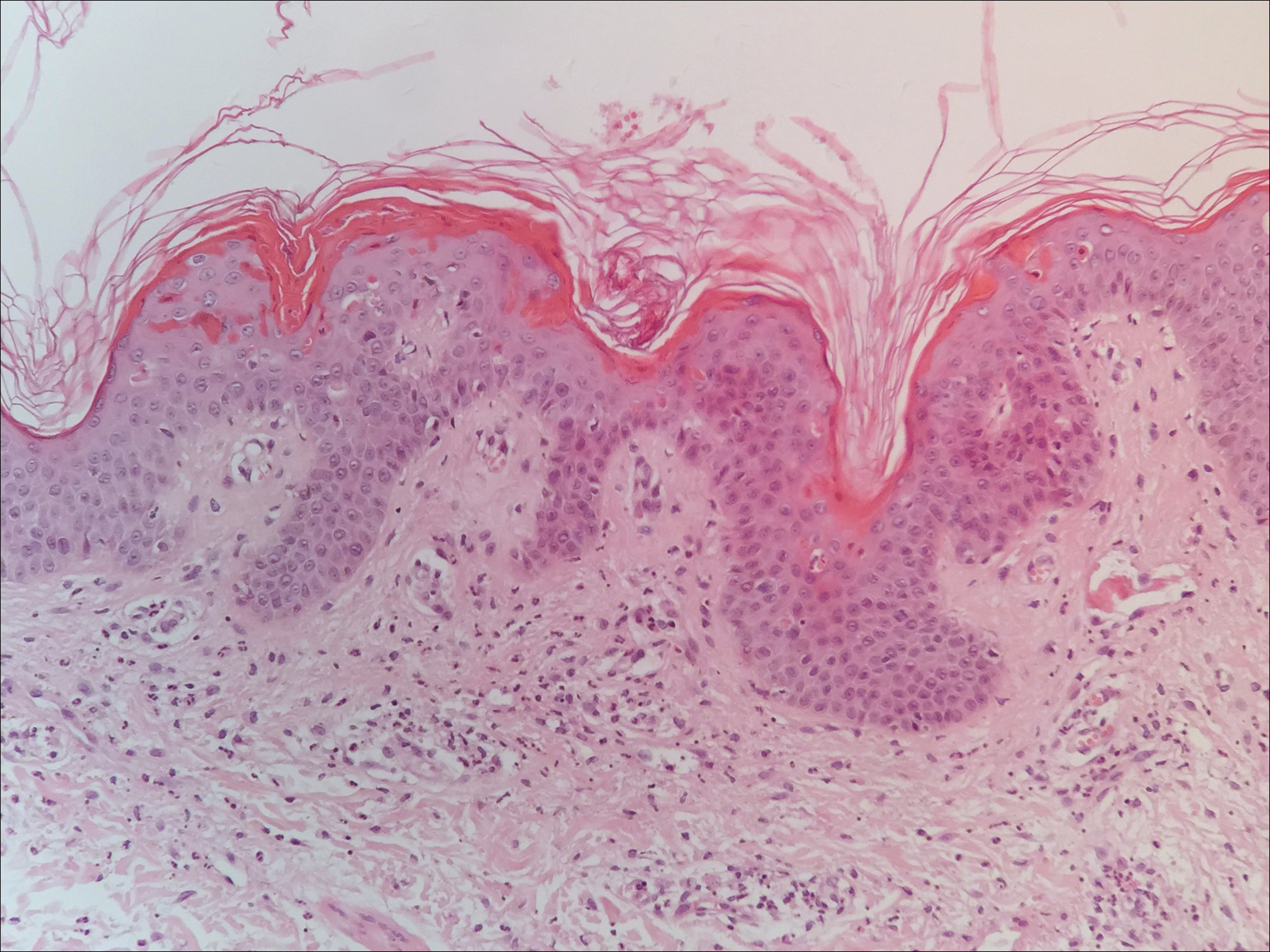
Twenty days later, the patient was referred to the dermatology department for evaluation of the persistent rash. The patient described a history of flushing of the face, severe joint pain in both arms and legs, aching muscles, and persistent sore throat. The patient did not report any history of drug ingestion. Physical examination revealed a fever (temperature, 39.2°C); swollen nontender lymph nodes in the neck, axillae, and groin; and salmon-colored and hyperpigmented patches and thin plaques over the neck, chest, abdomen, and arms (Figure 1). A splenectomy scar also was noted. Peripheral blood was collected for laboratory analyses, which revealed transaminitis and moderate hyperferritinemia (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. The patient was admitted to the hospital, and a skin biopsy was performed. Histology showed superficial dyskeratotic keratinocytes and sparse perivascular infiltration of neutrophils in the upper dermis (Figure 2).
The patient was diagnosed with AOSD based on fulfillment of the Yamaguchi criteria.2 She was treated with methylprednisolone 60 mg daily and was discharged 14 days later. At 16-month follow-up, the patient demonstrated complete resolution of symptoms with a maintenance dose of prednisolone (7.5 mg daily).
Patient 2
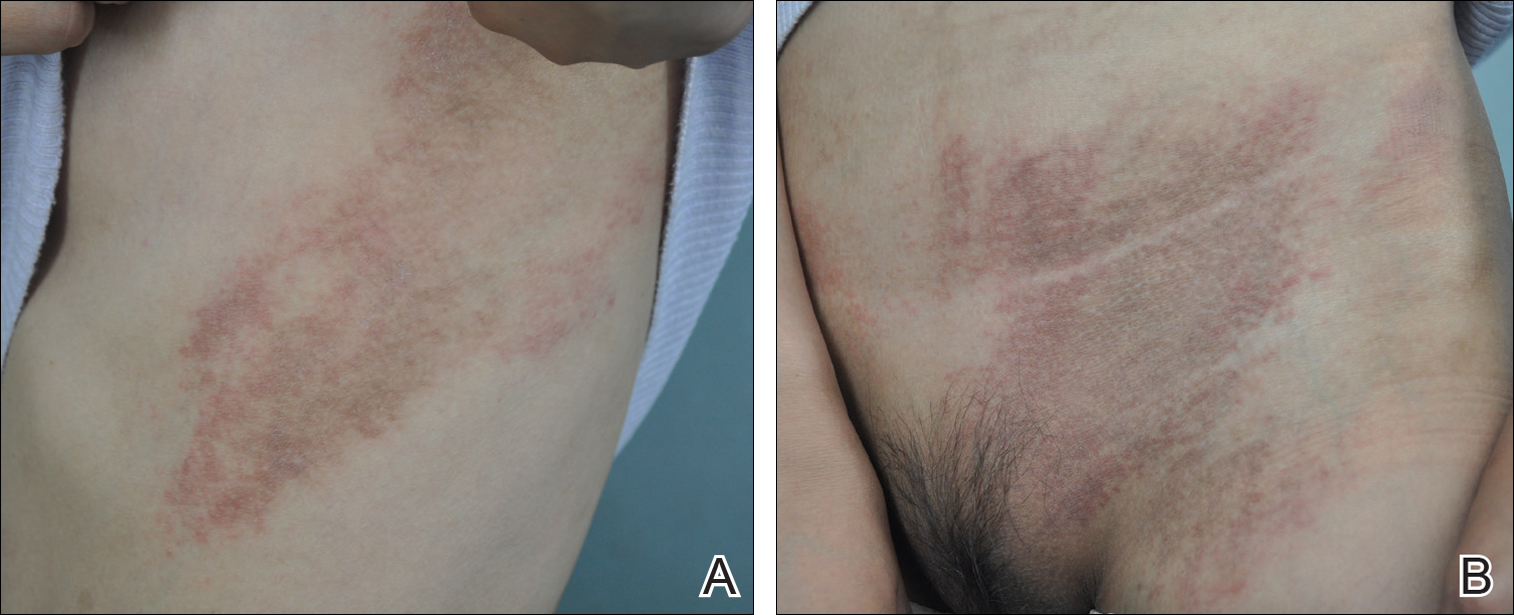
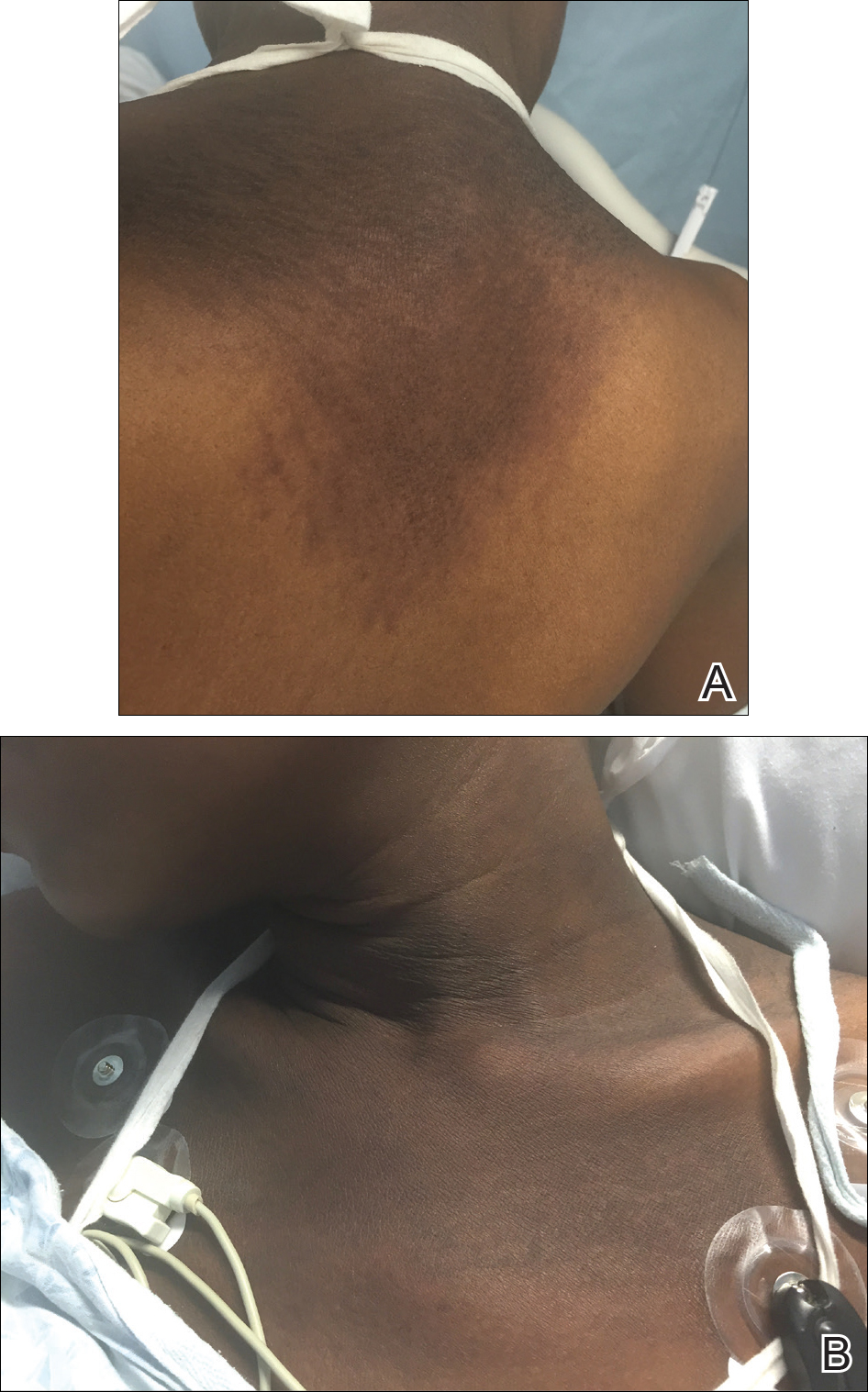
A 23-year-old black woman presented to the emergency department 3 months postpartum with recurrent high fevers, worsening joint pain, and persistent itchy rash of 2 months’ duration. The patient had no history of travel, autoimmune disease, or sick contacts. She occasionally took aspirin for joint pain. Physical examination revealed a fever (temperature, 39.1°C) along with hyperpigmented patches and thin scaly hyperpigmented papules coalescing into a poorly demarcated V-shaped plaque on the upper back and posterior neck, extending to the chest in a shawl-like distribution (Figure 3). Submental lymphadenopathy was present. The spleen was not palpable.
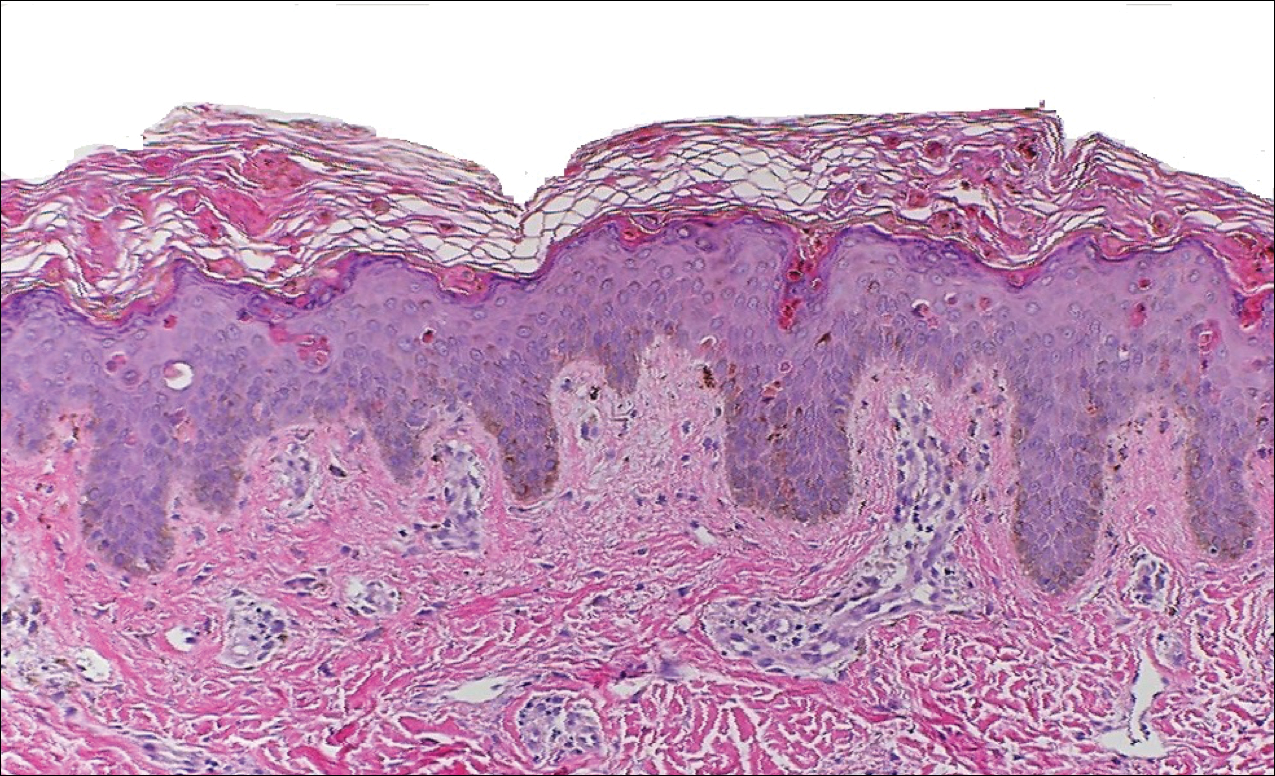
Peripheral blood was collected for laboratory analysis and demonstrated transaminitis and a markedly high ferritin level (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. Skin biopsy was performed and demonstrated many necrotic keratinocytes, singly and in aggregates, distributed from the spinous layer to the stratum corneum. A neutrophilic infiltrate was present in the papillary dermis (Figure 4).
The patient met the Yamaguchi criteria and was subsequently diagnosed with AOSD. She was treated with intravenous methylprednisolone 20 mg every 8 hours and was discharged 1 week later on oral prednisone 60 mg daily to be tapered over a period of months. At 2-week follow-up, the patient continued to experience rash and joint pain; oral methotrexate 10 mg weekly was added to her regimen, as well as vitamin D, calcium, and folic acid supplementation. At the next 2-week follow-up the patient noted improvement in the rash as well as the joint pain, but both still persisted. Prednisone was decreased to 50 mg daily and methotrexate was increased to 15 mg weekly. The patient continued to show improvement over the subsequent 3 months, during which prednisone was tapered to 10 mg daily and methotrexate was increased to 20 mg weekly. The patient showed resolution of symptoms at 3-month follow-up on this regimen, with plans to continue the prednisone taper and maintain methotrexate dosing.
Comment
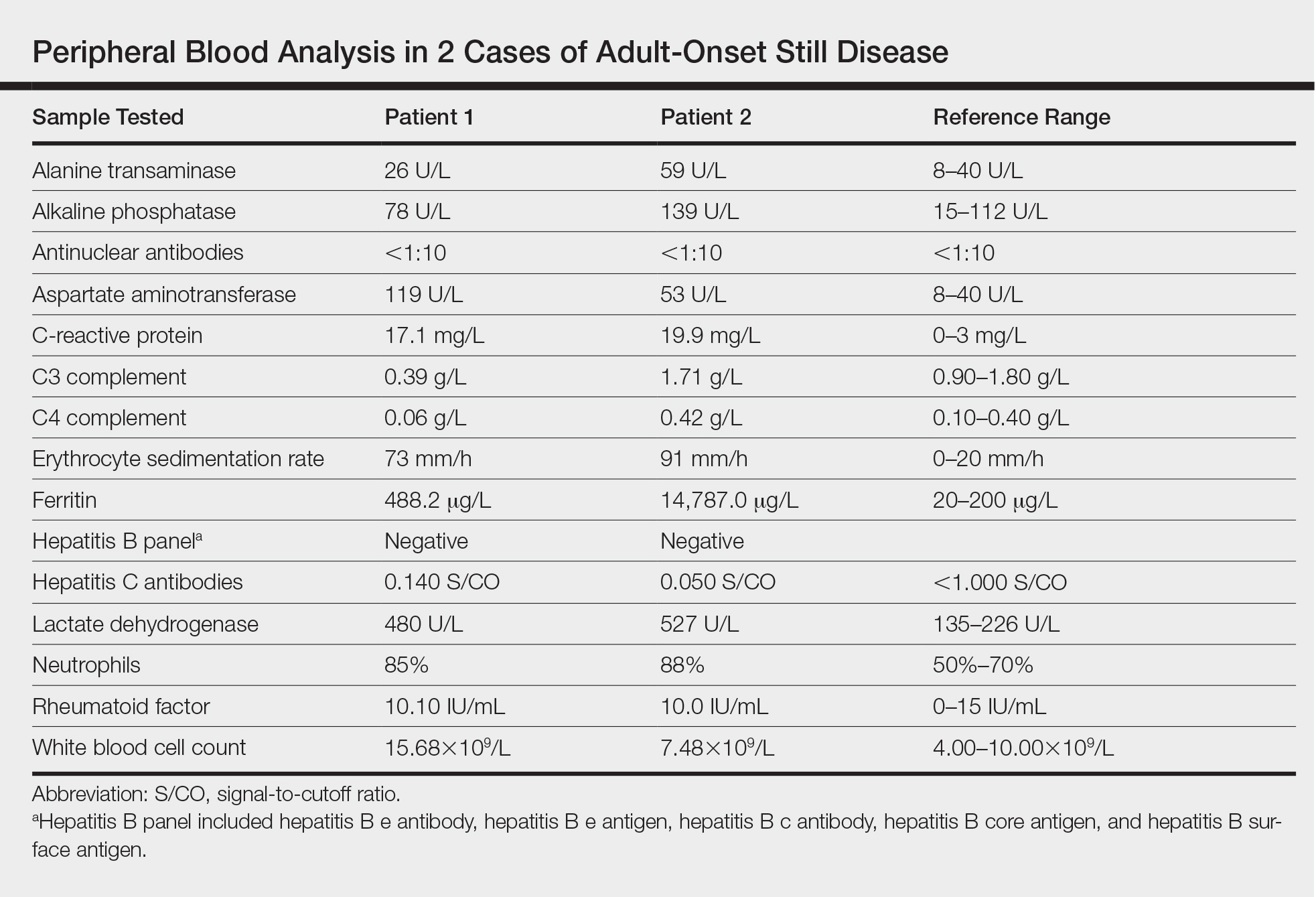
Adult-onset Still disease is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, salmon-pink evanescent erythema, and lymphadenopathy.2 The condition also can cause liver dysfunction, splenomegaly, pericarditis, pleuritis, renal dysfunction, and a reactive hemophagocytic syndrome.1 Furthermore, one review of the literature described an association with delayed-onset malignancy.4 Early diagnosis is important yet challenging, as AOSD is a diagnosis of exclusion. The Yamaguchi criteria are the most widely used method of diagnosis and demonstrate more than 90% sensitivity.In addition to the Yamaguchi criteria, marked hyperferritinemia is characteristic of AOSD and can act as an indicator of disease activity.5 Interestingly, both of our patients had elevated ferritin levels, with patient 2 showing marked elevation (Table). In both patients, all major criteria were fulfilled, except the typical skin rash.
The skin rash in AOSD, classically consisting of an evanescent, salmon-pink erythema predominantly involving the extremities, has been observed in up to 87% of AOSD patients.5 The histology of the typical evanescent rash is nonspecific, characterized by a relatively sparse, perivascular, mixed inflammatory infiltrate. Notably, other skin manifestations may be found in patients with AOSD.1,2,5-16 Persistent pruritic papules and plaques are the most commonly reported nonclassical rash, presenting as erythematous, slightly scaly papules and plaques with a linear configuration typically on the trunk.2 Both of our patients presented with this atypical eruption. Importantly, the histopathology of this unique rash displays distinctive features, which can aid in early diagnosis. Findings include dyskeratotic keratinocytes in the cornified layers as well as in the epidermis, and a sparse neutrophilic and/or lymphocytic infiltrate in the papillary dermis without vasculitis. These findings were evident in both histopathologic studies of our patients (Figures 2 and 4). Although not present in our patients, dermal mucin deposition has been demonstrated in some reports.1,13,15
A 2015 review of the literature yielded 30 cases of AOSD with pruritic persistent papules and plaques.4 The study confirmed a linear, erythematous or brown rash on the back and neck in the majority of cases. Histologic findings were congruent with those reported in our 2 cases: necrotic keratinocytes in the upper epidermis with a neutrophilic infiltrate in the upper dermis without vasculitis. Most patients showed rapid resolution of the rash and symptoms with the use of prednisone, prednisolone, or intravenous pulsed methylprednisolone. Interestingly, a range of presentations were noted, including prurigo pigmentosalike urticarial papules; lichenoid papules; and dermatographismlike, dermatomyositislike, and lichen amyloidosis–like rashes.4 In our report, patient 2 presented with a rash in a dermat-omyositislike shawl distribution. It has been suggested that patients with dermatomyositislike rashes require more potent immunotherapy as compared to patients with other rash morphologies.4 The need for methotrexate in addition to a prednisone taper in the clinical course of patient 2 lends further support to this observation.
Conclusion
A clinically and pathologically distinct form of cutaneous disease—AOSD with persistent pruritic papules and plaques—was observed in our 2 patients. These histopathologic findings facilitated timely diagnosis in both patients. A range of clinical morphologies may exist in AOSD, an awareness of which is paramount. Adult-onset Still disease should be included in the differential diagnosis of a dermatomyositislike presentation in a shawl distribution. Prompt diagnosis is essential to ensure adequate therapy.
- Yamamoto T. Cutaneous manifestations associated with adult-onset Still’s disease: important diagnostic values. Rheumatol Int. 2012;32:2233-2237.
- Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424-431.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Sun NZ, Brezinski EA, Berliner J, et al. Updates in adult-onset Still disease: atypical cutaneous manifestations and associates with delayed malignancy [published online June 6, 2015]. J Am Acad Dermatol. 2015;73:294-303.
- Schwarz-Eywill M, Heilig B, Bauer H, et al. Evaluation of serum ferritin as a marker for adult Still’s disease activity. Ann Rheum Dis. 1992;51:683-685.
- Ohta A, Yamaguchi M, Tsunematsu T, et al. Adult Still’s disease: a multicenter survey of Japanese patients. J Rheumatol. 1990;17:1058-1063.
- Kaur S, Bambery P, Dhar S. Persistent dermal plaque lesions in adult onset Still’s disease. Dermatology. 1994;188:241-242.
- Lübbe J, Hofer M, Chavaz P, et al. Adult onset Still’s disease with persistent plaques. Br J Dermatol. 1999;141:710-713.
- Suzuki K, Kimura Y, Aoki M, et al. Persistent plaques and linear pigmentation in adult-onset Still’s disease. Dermatology. 2001;202:333-335.
- Fujii K, Konishi K, Kanno Y, et al. Persistent generalized erythema in adult-onset Still’s disease. Int J Dermatol. 2003;42:824-825.
- Thien Huong NT, Pitche P, Minh Hoa T, et al. Persistent pigmented plaques in adult-onset Still’s disease. Ann Dermatol Venereol. 2005;132:693-696.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Wolgamot G, Yoo J, Hurst S, et al. Unique histopathologic findings in a patient with adult-onset Still’s disease. Am J Dermatopathol. 2007;49:194-196.
- Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still’s disease. J Cutan Pathol. 2010;37:932-937.
- Yang CC, Lee JY, Liu MF, et al. Adult-onset Still’s disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol. 2006;16:593-594.
- Azeck AG, Littlewood SM. Adult-onset Still’s disease with atypical cutaneous features. J Eur Acad Dermatol Venereol. 2005;19:360-363.
Adult-onset Still disease (AOSD) is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, evanescent skin rash, and lymphadenopathy. 1 The most commonly used criteria for diagnosing AOSD are the Yamaguchi criteria. 2 The major criteria include high fever for more than 1 week, arthralgia for more than 2 weeks, leukocytosis, and an evanescent skin rash. The minor criteria consist of sore throat, lymphadenopathy and/or splenomegaly, liver dysfunction, and negative rheumatoid factor and antinuclear antibodies. Classically, the skin rash is described as an evanescent, salmon-colored erythema involving the extremities. Nevertheless, unusual cutaneous eruptions have been reported in AOSD, including persistent pruritic papules and plaques. 3 Importantly, this atypical rash demonstrates specific histologic findings that are not found on routine histopathology of a typical evanescent rash. We describe 2 patients with this atypical cutaneous eruption along with the unique histopathologic findings of AOSD.
Case Reports
Patient 1
A 23-year-old Chinese woman presented with periodic fevers, persistent rash, and joint pain of 2 years’ duration. Her medical history included splenectomy for hepatosplenomegaly as well as evaluation by hematology for lymphadenopathy; a cervical lymph node biopsy showed lymphoid and follicular hyperplasia.
Twenty days later, the patient was referred to the dermatology department for evaluation of the persistent rash. The patient described a history of flushing of the face, severe joint pain in both arms and legs, aching muscles, and persistent sore throat. The patient did not report any history of drug ingestion. Physical examination revealed a fever (temperature, 39.2°C); swollen nontender lymph nodes in the neck, axillae, and groin; and salmon-colored and hyperpigmented patches and thin plaques over the neck, chest, abdomen, and arms (Figure 1). A splenectomy scar also was noted. Peripheral blood was collected for laboratory analyses, which revealed transaminitis and moderate hyperferritinemia (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. The patient was admitted to the hospital, and a skin biopsy was performed. Histology showed superficial dyskeratotic keratinocytes and sparse perivascular infiltration of neutrophils in the upper dermis (Figure 2).



The patient was diagnosed with AOSD based on fulfillment of the Yamaguchi criteria.2 She was treated with methylprednisolone 60 mg daily and was discharged 14 days later. At 16-month follow-up, the patient demonstrated complete resolution of symptoms with a maintenance dose of prednisolone (7.5 mg daily).
Patient 2
A 23-year-old black woman presented to the emergency department 3 months postpartum with recurrent high fevers, worsening joint pain, and persistent itchy rash of 2 months’ duration. The patient had no history of travel, autoimmune disease, or sick contacts. She occasionally took aspirin for joint pain. Physical examination revealed a fever (temperature, 39.1°C) along with hyperpigmented patches and thin scaly hyperpigmented papules coalescing into a poorly demarcated V-shaped plaque on the upper back and posterior neck, extending to the chest in a shawl-like distribution (Figure 3). Submental lymphadenopathy was present. The spleen was not palpable.

Peripheral blood was collected for laboratory analysis and demonstrated transaminitis and a markedly high ferritin level (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. Skin biopsy was performed and demonstrated many necrotic keratinocytes, singly and in aggregates, distributed from the spinous layer to the stratum corneum. A neutrophilic infiltrate was present in the papillary dermis (Figure 4).

The patient met the Yamaguchi criteria and was subsequently diagnosed with AOSD. She was treated with intravenous methylprednisolone 20 mg every 8 hours and was discharged 1 week later on oral prednisone 60 mg daily to be tapered over a period of months. At 2-week follow-up, the patient continued to experience rash and joint pain; oral methotrexate 10 mg weekly was added to her regimen, as well as vitamin D, calcium, and folic acid supplementation. At the next 2-week follow-up the patient noted improvement in the rash as well as the joint pain, but both still persisted. Prednisone was decreased to 50 mg daily and methotrexate was increased to 15 mg weekly. The patient continued to show improvement over the subsequent 3 months, during which prednisone was tapered to 10 mg daily and methotrexate was increased to 20 mg weekly. The patient showed resolution of symptoms at 3-month follow-up on this regimen, with plans to continue the prednisone taper and maintain methotrexate dosing.
Comment
Adult-onset Still disease is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, salmon-pink evanescent erythema, and lymphadenopathy.2 The condition also can cause liver dysfunction, splenomegaly, pericarditis, pleuritis, renal dysfunction, and a reactive hemophagocytic syndrome.1 Furthermore, one review of the literature described an association with delayed-onset malignancy.4 Early diagnosis is important yet challenging, as AOSD is a diagnosis of exclusion. The Yamaguchi criteria are the most widely used method of diagnosis and demonstrate more than 90% sensitivity.In addition to the Yamaguchi criteria, marked hyperferritinemia is characteristic of AOSD and can act as an indicator of disease activity.5 Interestingly, both of our patients had elevated ferritin levels, with patient 2 showing marked elevation (Table). In both patients, all major criteria were fulfilled, except the typical skin rash.
The skin rash in AOSD, classically consisting of an evanescent, salmon-pink erythema predominantly involving the extremities, has been observed in up to 87% of AOSD patients.5 The histology of the typical evanescent rash is nonspecific, characterized by a relatively sparse, perivascular, mixed inflammatory infiltrate. Notably, other skin manifestations may be found in patients with AOSD.1,2,5-16 Persistent pruritic papules and plaques are the most commonly reported nonclassical rash, presenting as erythematous, slightly scaly papules and plaques with a linear configuration typically on the trunk.2 Both of our patients presented with this atypical eruption. Importantly, the histopathology of this unique rash displays distinctive features, which can aid in early diagnosis. Findings include dyskeratotic keratinocytes in the cornified layers as well as in the epidermis, and a sparse neutrophilic and/or lymphocytic infiltrate in the papillary dermis without vasculitis. These findings were evident in both histopathologic studies of our patients (Figures 2 and 4). Although not present in our patients, dermal mucin deposition has been demonstrated in some reports.1,13,15
A 2015 review of the literature yielded 30 cases of AOSD with pruritic persistent papules and plaques.4 The study confirmed a linear, erythematous or brown rash on the back and neck in the majority of cases. Histologic findings were congruent with those reported in our 2 cases: necrotic keratinocytes in the upper epidermis with a neutrophilic infiltrate in the upper dermis without vasculitis. Most patients showed rapid resolution of the rash and symptoms with the use of prednisone, prednisolone, or intravenous pulsed methylprednisolone. Interestingly, a range of presentations were noted, including prurigo pigmentosalike urticarial papules; lichenoid papules; and dermatographismlike, dermatomyositislike, and lichen amyloidosis–like rashes.4 In our report, patient 2 presented with a rash in a dermat-omyositislike shawl distribution. It has been suggested that patients with dermatomyositislike rashes require more potent immunotherapy as compared to patients with other rash morphologies.4 The need for methotrexate in addition to a prednisone taper in the clinical course of patient 2 lends further support to this observation.
Conclusion
A clinically and pathologically distinct form of cutaneous disease—AOSD with persistent pruritic papules and plaques—was observed in our 2 patients. These histopathologic findings facilitated timely diagnosis in both patients. A range of clinical morphologies may exist in AOSD, an awareness of which is paramount. Adult-onset Still disease should be included in the differential diagnosis of a dermatomyositislike presentation in a shawl distribution. Prompt diagnosis is essential to ensure adequate therapy.
Adult-onset Still disease (AOSD) is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, evanescent skin rash, and lymphadenopathy. 1 The most commonly used criteria for diagnosing AOSD are the Yamaguchi criteria. 2 The major criteria include high fever for more than 1 week, arthralgia for more than 2 weeks, leukocytosis, and an evanescent skin rash. The minor criteria consist of sore throat, lymphadenopathy and/or splenomegaly, liver dysfunction, and negative rheumatoid factor and antinuclear antibodies. Classically, the skin rash is described as an evanescent, salmon-colored erythema involving the extremities. Nevertheless, unusual cutaneous eruptions have been reported in AOSD, including persistent pruritic papules and plaques. 3 Importantly, this atypical rash demonstrates specific histologic findings that are not found on routine histopathology of a typical evanescent rash. We describe 2 patients with this atypical cutaneous eruption along with the unique histopathologic findings of AOSD.
Case Reports
Patient 1
A 23-year-old Chinese woman presented with periodic fevers, persistent rash, and joint pain of 2 years’ duration. Her medical history included splenectomy for hepatosplenomegaly as well as evaluation by hematology for lymphadenopathy; a cervical lymph node biopsy showed lymphoid and follicular hyperplasia.
Twenty days later, the patient was referred to the dermatology department for evaluation of the persistent rash. The patient described a history of flushing of the face, severe joint pain in both arms and legs, aching muscles, and persistent sore throat. The patient did not report any history of drug ingestion. Physical examination revealed a fever (temperature, 39.2°C); swollen nontender lymph nodes in the neck, axillae, and groin; and salmon-colored and hyperpigmented patches and thin plaques over the neck, chest, abdomen, and arms (Figure 1). A splenectomy scar also was noted. Peripheral blood was collected for laboratory analyses, which revealed transaminitis and moderate hyperferritinemia (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. The patient was admitted to the hospital, and a skin biopsy was performed. Histology showed superficial dyskeratotic keratinocytes and sparse perivascular infiltration of neutrophils in the upper dermis (Figure 2).



The patient was diagnosed with AOSD based on fulfillment of the Yamaguchi criteria.2 She was treated with methylprednisolone 60 mg daily and was discharged 14 days later. At 16-month follow-up, the patient demonstrated complete resolution of symptoms with a maintenance dose of prednisolone (7.5 mg daily).
Patient 2
A 23-year-old black woman presented to the emergency department 3 months postpartum with recurrent high fevers, worsening joint pain, and persistent itchy rash of 2 months’ duration. The patient had no history of travel, autoimmune disease, or sick contacts. She occasionally took aspirin for joint pain. Physical examination revealed a fever (temperature, 39.1°C) along with hyperpigmented patches and thin scaly hyperpigmented papules coalescing into a poorly demarcated V-shaped plaque on the upper back and posterior neck, extending to the chest in a shawl-like distribution (Figure 3). Submental lymphadenopathy was present. The spleen was not palpable.

Peripheral blood was collected for laboratory analysis and demonstrated transaminitis and a markedly high ferritin level (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. Skin biopsy was performed and demonstrated many necrotic keratinocytes, singly and in aggregates, distributed from the spinous layer to the stratum corneum. A neutrophilic infiltrate was present in the papillary dermis (Figure 4).

The patient met the Yamaguchi criteria and was subsequently diagnosed with AOSD. She was treated with intravenous methylprednisolone 20 mg every 8 hours and was discharged 1 week later on oral prednisone 60 mg daily to be tapered over a period of months. At 2-week follow-up, the patient continued to experience rash and joint pain; oral methotrexate 10 mg weekly was added to her regimen, as well as vitamin D, calcium, and folic acid supplementation. At the next 2-week follow-up the patient noted improvement in the rash as well as the joint pain, but both still persisted. Prednisone was decreased to 50 mg daily and methotrexate was increased to 15 mg weekly. The patient continued to show improvement over the subsequent 3 months, during which prednisone was tapered to 10 mg daily and methotrexate was increased to 20 mg weekly. The patient showed resolution of symptoms at 3-month follow-up on this regimen, with plans to continue the prednisone taper and maintain methotrexate dosing.
Comment
Adult-onset Still disease is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, salmon-pink evanescent erythema, and lymphadenopathy.2 The condition also can cause liver dysfunction, splenomegaly, pericarditis, pleuritis, renal dysfunction, and a reactive hemophagocytic syndrome.1 Furthermore, one review of the literature described an association with delayed-onset malignancy.4 Early diagnosis is important yet challenging, as AOSD is a diagnosis of exclusion. The Yamaguchi criteria are the most widely used method of diagnosis and demonstrate more than 90% sensitivity.In addition to the Yamaguchi criteria, marked hyperferritinemia is characteristic of AOSD and can act as an indicator of disease activity.5 Interestingly, both of our patients had elevated ferritin levels, with patient 2 showing marked elevation (Table). In both patients, all major criteria were fulfilled, except the typical skin rash.
The skin rash in AOSD, classically consisting of an evanescent, salmon-pink erythema predominantly involving the extremities, has been observed in up to 87% of AOSD patients.5 The histology of the typical evanescent rash is nonspecific, characterized by a relatively sparse, perivascular, mixed inflammatory infiltrate. Notably, other skin manifestations may be found in patients with AOSD.1,2,5-16 Persistent pruritic papules and plaques are the most commonly reported nonclassical rash, presenting as erythematous, slightly scaly papules and plaques with a linear configuration typically on the trunk.2 Both of our patients presented with this atypical eruption. Importantly, the histopathology of this unique rash displays distinctive features, which can aid in early diagnosis. Findings include dyskeratotic keratinocytes in the cornified layers as well as in the epidermis, and a sparse neutrophilic and/or lymphocytic infiltrate in the papillary dermis without vasculitis. These findings were evident in both histopathologic studies of our patients (Figures 2 and 4). Although not present in our patients, dermal mucin deposition has been demonstrated in some reports.1,13,15
A 2015 review of the literature yielded 30 cases of AOSD with pruritic persistent papules and plaques.4 The study confirmed a linear, erythematous or brown rash on the back and neck in the majority of cases. Histologic findings were congruent with those reported in our 2 cases: necrotic keratinocytes in the upper epidermis with a neutrophilic infiltrate in the upper dermis without vasculitis. Most patients showed rapid resolution of the rash and symptoms with the use of prednisone, prednisolone, or intravenous pulsed methylprednisolone. Interestingly, a range of presentations were noted, including prurigo pigmentosalike urticarial papules; lichenoid papules; and dermatographismlike, dermatomyositislike, and lichen amyloidosis–like rashes.4 In our report, patient 2 presented with a rash in a dermat-omyositislike shawl distribution. It has been suggested that patients with dermatomyositislike rashes require more potent immunotherapy as compared to patients with other rash morphologies.4 The need for methotrexate in addition to a prednisone taper in the clinical course of patient 2 lends further support to this observation.
Conclusion
A clinically and pathologically distinct form of cutaneous disease—AOSD with persistent pruritic papules and plaques—was observed in our 2 patients. These histopathologic findings facilitated timely diagnosis in both patients. A range of clinical morphologies may exist in AOSD, an awareness of which is paramount. Adult-onset Still disease should be included in the differential diagnosis of a dermatomyositislike presentation in a shawl distribution. Prompt diagnosis is essential to ensure adequate therapy.
- Yamamoto T. Cutaneous manifestations associated with adult-onset Still’s disease: important diagnostic values. Rheumatol Int. 2012;32:2233-2237.
- Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424-431.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Sun NZ, Brezinski EA, Berliner J, et al. Updates in adult-onset Still disease: atypical cutaneous manifestations and associates with delayed malignancy [published online June 6, 2015]. J Am Acad Dermatol. 2015;73:294-303.
- Schwarz-Eywill M, Heilig B, Bauer H, et al. Evaluation of serum ferritin as a marker for adult Still’s disease activity. Ann Rheum Dis. 1992;51:683-685.
- Ohta A, Yamaguchi M, Tsunematsu T, et al. Adult Still’s disease: a multicenter survey of Japanese patients. J Rheumatol. 1990;17:1058-1063.
- Kaur S, Bambery P, Dhar S. Persistent dermal plaque lesions in adult onset Still’s disease. Dermatology. 1994;188:241-242.
- Lübbe J, Hofer M, Chavaz P, et al. Adult onset Still’s disease with persistent plaques. Br J Dermatol. 1999;141:710-713.
- Suzuki K, Kimura Y, Aoki M, et al. Persistent plaques and linear pigmentation in adult-onset Still’s disease. Dermatology. 2001;202:333-335.
- Fujii K, Konishi K, Kanno Y, et al. Persistent generalized erythema in adult-onset Still’s disease. Int J Dermatol. 2003;42:824-825.
- Thien Huong NT, Pitche P, Minh Hoa T, et al. Persistent pigmented plaques in adult-onset Still’s disease. Ann Dermatol Venereol. 2005;132:693-696.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Wolgamot G, Yoo J, Hurst S, et al. Unique histopathologic findings in a patient with adult-onset Still’s disease. Am J Dermatopathol. 2007;49:194-196.
- Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still’s disease. J Cutan Pathol. 2010;37:932-937.
- Yang CC, Lee JY, Liu MF, et al. Adult-onset Still’s disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol. 2006;16:593-594.
- Azeck AG, Littlewood SM. Adult-onset Still’s disease with atypical cutaneous features. J Eur Acad Dermatol Venereol. 2005;19:360-363.
- Yamamoto T. Cutaneous manifestations associated with adult-onset Still’s disease: important diagnostic values. Rheumatol Int. 2012;32:2233-2237.
- Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424-431.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Sun NZ, Brezinski EA, Berliner J, et al. Updates in adult-onset Still disease: atypical cutaneous manifestations and associates with delayed malignancy [published online June 6, 2015]. J Am Acad Dermatol. 2015;73:294-303.
- Schwarz-Eywill M, Heilig B, Bauer H, et al. Evaluation of serum ferritin as a marker for adult Still’s disease activity. Ann Rheum Dis. 1992;51:683-685.
- Ohta A, Yamaguchi M, Tsunematsu T, et al. Adult Still’s disease: a multicenter survey of Japanese patients. J Rheumatol. 1990;17:1058-1063.
- Kaur S, Bambery P, Dhar S. Persistent dermal plaque lesions in adult onset Still’s disease. Dermatology. 1994;188:241-242.
- Lübbe J, Hofer M, Chavaz P, et al. Adult onset Still’s disease with persistent plaques. Br J Dermatol. 1999;141:710-713.
- Suzuki K, Kimura Y, Aoki M, et al. Persistent plaques and linear pigmentation in adult-onset Still’s disease. Dermatology. 2001;202:333-335.
- Fujii K, Konishi K, Kanno Y, et al. Persistent generalized erythema in adult-onset Still’s disease. Int J Dermatol. 2003;42:824-825.
- Thien Huong NT, Pitche P, Minh Hoa T, et al. Persistent pigmented plaques in adult-onset Still’s disease. Ann Dermatol Venereol. 2005;132:693-696.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Wolgamot G, Yoo J, Hurst S, et al. Unique histopathologic findings in a patient with adult-onset Still’s disease. Am J Dermatopathol. 2007;49:194-196.
- Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still’s disease. J Cutan Pathol. 2010;37:932-937.
- Yang CC, Lee JY, Liu MF, et al. Adult-onset Still’s disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol. 2006;16:593-594.
- Azeck AG, Littlewood SM. Adult-onset Still’s disease with atypical cutaneous features. J Eur Acad Dermatol Venereol. 2005;19:360-363.
Practice Points
- Serologic testing and skin biopsy are necessary in the timely and appropriate diagnosis of adult-onset Still disease (AOSD).
- In patients with a persistent pruritic papular rash, consider AOSD if there is a supporting history.
- Skin biopsy is diagnostic of AOSD with the unique histopathologic findings of dyskeratotic keratinocytes in the cornified layers as well as in the epidermis and a sparse neutrophilic and/or lymphocytic infiltrate in the papillary dermis without vasculitis.
Acral Cutaneous Metastasis From a Primary Breast Carcinoma Following Chemotherapy With Bevacizumab and Paclitaxel
Cutaneous metastasis of internal malignancy is a relatively uncommon phenomenon, with an overall incidence of 5.3% in cancer patients.1 Cutaneous involvement typically occurs late in the course of disease but can occasionally be the first extranodal sign of metastatic disease. Breast cancer has the highest rate of cutaneous metastasis, most often involving the chest wall1; however, cutaneous metastasis to the acral sites is exceedingly rare. The hand is the site of 0.1% of all metastatic lesions, with only 10% of these being cutaneous lesions and the remaining 90% being osseous metastases.2 Herein, we report a case of multiple cutaneous metastases to acral sites involving the palmar and plantar surfaces of the hands and feet.
Case Report
A 54-year-old black woman with a history of stage IV carcinoma of the breast was admitted to the university medical center with exquisitely painful cutaneous nodules on the hands and feet of 5 weeks’ duration that had started to cause difficulty with walking and daily activities. The patient reported that the breast carcinoma had initially been diagnosed in Nigeria 2 years prior, but she did not receive treatment until moving to the United States. She received a total of 4 cycles of chemotherapy with paclitaxel and bevacizumab, which was discontinued 6 weeks prior to admission due to pain in the lower extremities that was thought to be secondary to neuropathy. One week after discontinuation of chemotherapy, the patient reported increasing pain in the extremities and new-onset painful nodules on the hands and feet. Treatment with gabapentin as well as several courses of antibiotics failed to improve the condition.
She was admitted for symptomatic pain control and a dermatology consultation. Physical examination revealed multiple firm, tender, subcutaneous nodules on the volar surfaces of the soles, toes, palms, and fingertips (Figure 1). A nodule also was noted on the scalp. A punch biopsy of a nodule on the right fourth finger revealed a dermal carcinoma (Figure 2). On immunohistochemistry, the tumor stained positive for cytokeratin 5/6, cytokeratin 7, and gross cystic disease fluid protein 15. It did not demonstrate connection to the epidermis or adnexal structures. Although the tumor did not express estrogen or progesterone receptors, the findings were compatible with metastasis from the patient’s primary breast carcinoma with poor differentiation. A biopsy of the primary breast carcinoma was not available for review from Nigeria.
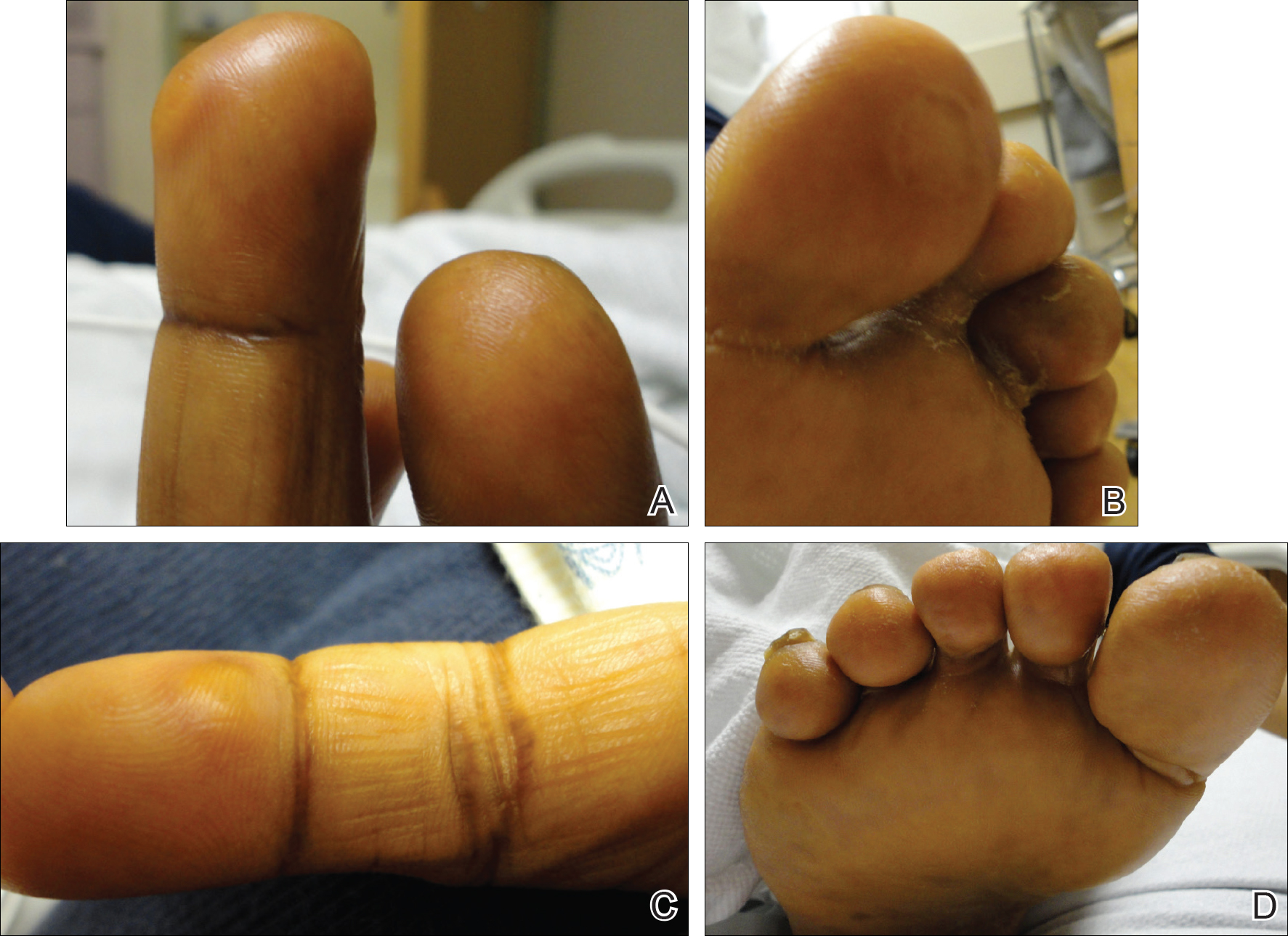
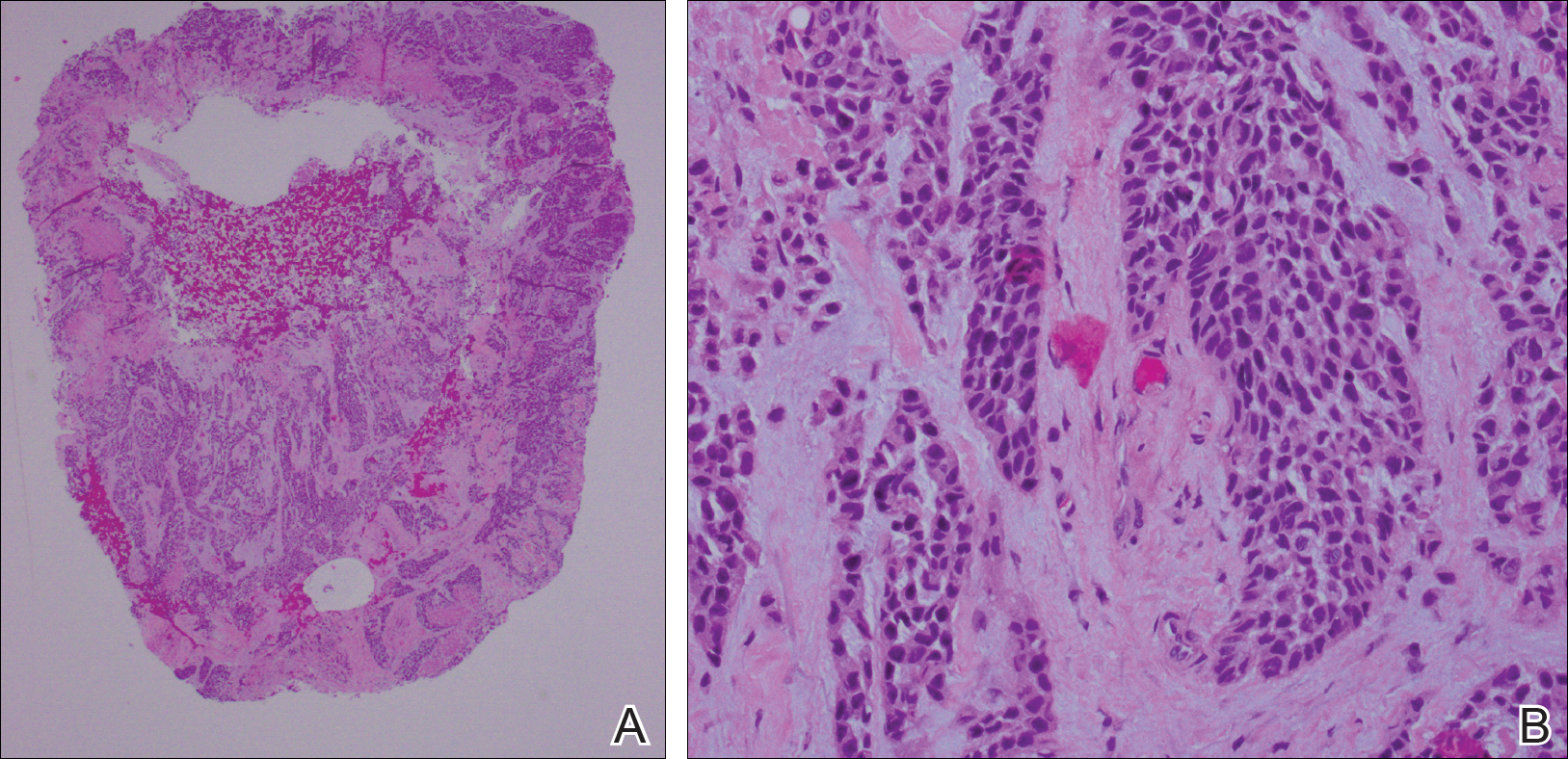
Comment
The majority of cases reporting acral cutaneous metastasis from internal malignancies are unilateral, involving only one extremity. Several hypotheses have been provided, including spread from localized trauma, which causes disruption of blood vessels and consequent extravasation and localization of tumor cells into the extravascular space.3 The distal extremities are particularly vulnerable to trauma, making this hypothesis plausible.
Considering the overall rarity of metastases to acral sites, it is interesting that our patient developed multiple distal nodules on both the hands and feet. The rapid onset of cutaneous nodules shortly after a course of chemotherapy led the team to consider the physiologic effects of paclitaxel and bevacizumab in the etiology of the acral cutaneous metastases. Karamouzis et al3 described a similar case of multiple cutaneous metastases with a bilateral acral distribution. This case also was associated with chemotherapy in the treatment of breast cancer. The authors proposed hand-foot syndrome, a chemotherapy-related eruption localized to acral skin, as a possible mechanism for hematogenous spread of malignant cells.3 The pathogenesis of hand-foot syndrome is not well understood, but the unique anatomy and physiology of acral skin including temperature gradients, rapidly dividing epidermal cells, absence of hair follicles and sebaceous glands, wide dermal papillae, and exposure to high pressures from carrying body weight and repetitive minor trauma may contribute to the localization of signs and symptoms.3,4 Our case supports a chemotherapy-related etiology of acral cutaneous metastasis of a primary breast cancer; however, our patient did not have apparent signs or symptoms of hand-foot syndrome during the course of treatment. We propose that effects of bevacizumab on acral skin may have contributed to the development of our patient’s metastatic pattern.
Bevacizumab, a monoclonal antibody to vascular endothelial growth factor A, has well-known vascular side effects. Unlike the inhibition of vascular endothelial growth factor A provided by the receptor tyrosine kinase inhibitors sorafenib and sunitinib, bevacizumab typically is not associated with hand-foot syndrome.5 However, several cases have been reported with chemotherapy-associated palmoplantar eruptions that resolved after withholding bevacizumab while continuing other chemotherapeutic agents, suggesting that bevacizumab-induced changes in acral skin contributed to the eruption.6 Specific factors that could contribute to acral metastasis in patients taking bevacizumab are endothelial dysfunction and capillary rarefaction of the acral skin, as well as hemorrhage, decreased wound healing, and changes in vascular permeability.5,7
We present a rare case of acral cutaneous metastasis associated with bevacizumab, one of few reported cases associated with a taxane chemotherapeutic agent.3 More cases need to be identified and reported to establish a causative association, if indeed existent, between acral cutaneous metastasis of breast carcinoma and the use of bevacizumab as well as other chemotherapeutic drugs.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
- Wu CY, Gao HW, Huang WH, et al. Infection-like acral cutaneous metastasis as the presenting sign of an occult breast cancer. Clin Exp Dermatol. 2009;34:409-410.
- Karamouzis MV, Ardavanis A, Alexopoulos A, et al. Multiple cutaneous acral metastases in a woman with breast adenocarcinoma treated with pegylated liposomal doxorubicin: incidental or aetiological association? Eur J Cancer Care (Engl). 2005;14:267-271.
- Nagore E, Insa A, Sanmartin O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome. incidence, recognition and management. Am J Clin Dermatol. 2000;1:225-234.
- Wozel G, Sticherling M, Schon MP. Cutaneous side effects of inhibition of VEGF signal transduction. J Dtsch Dermatol Ges. 2010;8:243-249.
- Munehiro A, Yoneda K, Nakai K, et al. Bevacizumab-induced hand-foot syndrome: circumscribed type. Br J Dermatol. 2010;162:1411-1413.
- Mourad JJ, des Guetz G, Debbabi H, et al. Blood pressure rise following angiogenesis inhibition by bevacizumab. a crucial role for microcirculation. Ann Oncol. 2008;19:927-934.
Cutaneous metastasis of internal malignancy is a relatively uncommon phenomenon, with an overall incidence of 5.3% in cancer patients.1 Cutaneous involvement typically occurs late in the course of disease but can occasionally be the first extranodal sign of metastatic disease. Breast cancer has the highest rate of cutaneous metastasis, most often involving the chest wall1; however, cutaneous metastasis to the acral sites is exceedingly rare. The hand is the site of 0.1% of all metastatic lesions, with only 10% of these being cutaneous lesions and the remaining 90% being osseous metastases.2 Herein, we report a case of multiple cutaneous metastases to acral sites involving the palmar and plantar surfaces of the hands and feet.
Case Report
A 54-year-old black woman with a history of stage IV carcinoma of the breast was admitted to the university medical center with exquisitely painful cutaneous nodules on the hands and feet of 5 weeks’ duration that had started to cause difficulty with walking and daily activities. The patient reported that the breast carcinoma had initially been diagnosed in Nigeria 2 years prior, but she did not receive treatment until moving to the United States. She received a total of 4 cycles of chemotherapy with paclitaxel and bevacizumab, which was discontinued 6 weeks prior to admission due to pain in the lower extremities that was thought to be secondary to neuropathy. One week after discontinuation of chemotherapy, the patient reported increasing pain in the extremities and new-onset painful nodules on the hands and feet. Treatment with gabapentin as well as several courses of antibiotics failed to improve the condition.
She was admitted for symptomatic pain control and a dermatology consultation. Physical examination revealed multiple firm, tender, subcutaneous nodules on the volar surfaces of the soles, toes, palms, and fingertips (Figure 1). A nodule also was noted on the scalp. A punch biopsy of a nodule on the right fourth finger revealed a dermal carcinoma (Figure 2). On immunohistochemistry, the tumor stained positive for cytokeratin 5/6, cytokeratin 7, and gross cystic disease fluid protein 15. It did not demonstrate connection to the epidermis or adnexal structures. Although the tumor did not express estrogen or progesterone receptors, the findings were compatible with metastasis from the patient’s primary breast carcinoma with poor differentiation. A biopsy of the primary breast carcinoma was not available for review from Nigeria.


Comment
The majority of cases reporting acral cutaneous metastasis from internal malignancies are unilateral, involving only one extremity. Several hypotheses have been provided, including spread from localized trauma, which causes disruption of blood vessels and consequent extravasation and localization of tumor cells into the extravascular space.3 The distal extremities are particularly vulnerable to trauma, making this hypothesis plausible.
Considering the overall rarity of metastases to acral sites, it is interesting that our patient developed multiple distal nodules on both the hands and feet. The rapid onset of cutaneous nodules shortly after a course of chemotherapy led the team to consider the physiologic effects of paclitaxel and bevacizumab in the etiology of the acral cutaneous metastases. Karamouzis et al3 described a similar case of multiple cutaneous metastases with a bilateral acral distribution. This case also was associated with chemotherapy in the treatment of breast cancer. The authors proposed hand-foot syndrome, a chemotherapy-related eruption localized to acral skin, as a possible mechanism for hematogenous spread of malignant cells.3 The pathogenesis of hand-foot syndrome is not well understood, but the unique anatomy and physiology of acral skin including temperature gradients, rapidly dividing epidermal cells, absence of hair follicles and sebaceous glands, wide dermal papillae, and exposure to high pressures from carrying body weight and repetitive minor trauma may contribute to the localization of signs and symptoms.3,4 Our case supports a chemotherapy-related etiology of acral cutaneous metastasis of a primary breast cancer; however, our patient did not have apparent signs or symptoms of hand-foot syndrome during the course of treatment. We propose that effects of bevacizumab on acral skin may have contributed to the development of our patient’s metastatic pattern.
Bevacizumab, a monoclonal antibody to vascular endothelial growth factor A, has well-known vascular side effects. Unlike the inhibition of vascular endothelial growth factor A provided by the receptor tyrosine kinase inhibitors sorafenib and sunitinib, bevacizumab typically is not associated with hand-foot syndrome.5 However, several cases have been reported with chemotherapy-associated palmoplantar eruptions that resolved after withholding bevacizumab while continuing other chemotherapeutic agents, suggesting that bevacizumab-induced changes in acral skin contributed to the eruption.6 Specific factors that could contribute to acral metastasis in patients taking bevacizumab are endothelial dysfunction and capillary rarefaction of the acral skin, as well as hemorrhage, decreased wound healing, and changes in vascular permeability.5,7
We present a rare case of acral cutaneous metastasis associated with bevacizumab, one of few reported cases associated with a taxane chemotherapeutic agent.3 More cases need to be identified and reported to establish a causative association, if indeed existent, between acral cutaneous metastasis of breast carcinoma and the use of bevacizumab as well as other chemotherapeutic drugs.
Cutaneous metastasis of internal malignancy is a relatively uncommon phenomenon, with an overall incidence of 5.3% in cancer patients.1 Cutaneous involvement typically occurs late in the course of disease but can occasionally be the first extranodal sign of metastatic disease. Breast cancer has the highest rate of cutaneous metastasis, most often involving the chest wall1; however, cutaneous metastasis to the acral sites is exceedingly rare. The hand is the site of 0.1% of all metastatic lesions, with only 10% of these being cutaneous lesions and the remaining 90% being osseous metastases.2 Herein, we report a case of multiple cutaneous metastases to acral sites involving the palmar and plantar surfaces of the hands and feet.
Case Report
A 54-year-old black woman with a history of stage IV carcinoma of the breast was admitted to the university medical center with exquisitely painful cutaneous nodules on the hands and feet of 5 weeks’ duration that had started to cause difficulty with walking and daily activities. The patient reported that the breast carcinoma had initially been diagnosed in Nigeria 2 years prior, but she did not receive treatment until moving to the United States. She received a total of 4 cycles of chemotherapy with paclitaxel and bevacizumab, which was discontinued 6 weeks prior to admission due to pain in the lower extremities that was thought to be secondary to neuropathy. One week after discontinuation of chemotherapy, the patient reported increasing pain in the extremities and new-onset painful nodules on the hands and feet. Treatment with gabapentin as well as several courses of antibiotics failed to improve the condition.
She was admitted for symptomatic pain control and a dermatology consultation. Physical examination revealed multiple firm, tender, subcutaneous nodules on the volar surfaces of the soles, toes, palms, and fingertips (Figure 1). A nodule also was noted on the scalp. A punch biopsy of a nodule on the right fourth finger revealed a dermal carcinoma (Figure 2). On immunohistochemistry, the tumor stained positive for cytokeratin 5/6, cytokeratin 7, and gross cystic disease fluid protein 15. It did not demonstrate connection to the epidermis or adnexal structures. Although the tumor did not express estrogen or progesterone receptors, the findings were compatible with metastasis from the patient’s primary breast carcinoma with poor differentiation. A biopsy of the primary breast carcinoma was not available for review from Nigeria.


Comment
The majority of cases reporting acral cutaneous metastasis from internal malignancies are unilateral, involving only one extremity. Several hypotheses have been provided, including spread from localized trauma, which causes disruption of blood vessels and consequent extravasation and localization of tumor cells into the extravascular space.3 The distal extremities are particularly vulnerable to trauma, making this hypothesis plausible.
Considering the overall rarity of metastases to acral sites, it is interesting that our patient developed multiple distal nodules on both the hands and feet. The rapid onset of cutaneous nodules shortly after a course of chemotherapy led the team to consider the physiologic effects of paclitaxel and bevacizumab in the etiology of the acral cutaneous metastases. Karamouzis et al3 described a similar case of multiple cutaneous metastases with a bilateral acral distribution. This case also was associated with chemotherapy in the treatment of breast cancer. The authors proposed hand-foot syndrome, a chemotherapy-related eruption localized to acral skin, as a possible mechanism for hematogenous spread of malignant cells.3 The pathogenesis of hand-foot syndrome is not well understood, but the unique anatomy and physiology of acral skin including temperature gradients, rapidly dividing epidermal cells, absence of hair follicles and sebaceous glands, wide dermal papillae, and exposure to high pressures from carrying body weight and repetitive minor trauma may contribute to the localization of signs and symptoms.3,4 Our case supports a chemotherapy-related etiology of acral cutaneous metastasis of a primary breast cancer; however, our patient did not have apparent signs or symptoms of hand-foot syndrome during the course of treatment. We propose that effects of bevacizumab on acral skin may have contributed to the development of our patient’s metastatic pattern.
Bevacizumab, a monoclonal antibody to vascular endothelial growth factor A, has well-known vascular side effects. Unlike the inhibition of vascular endothelial growth factor A provided by the receptor tyrosine kinase inhibitors sorafenib and sunitinib, bevacizumab typically is not associated with hand-foot syndrome.5 However, several cases have been reported with chemotherapy-associated palmoplantar eruptions that resolved after withholding bevacizumab while continuing other chemotherapeutic agents, suggesting that bevacizumab-induced changes in acral skin contributed to the eruption.6 Specific factors that could contribute to acral metastasis in patients taking bevacizumab are endothelial dysfunction and capillary rarefaction of the acral skin, as well as hemorrhage, decreased wound healing, and changes in vascular permeability.5,7
We present a rare case of acral cutaneous metastasis associated with bevacizumab, one of few reported cases associated with a taxane chemotherapeutic agent.3 More cases need to be identified and reported to establish a causative association, if indeed existent, between acral cutaneous metastasis of breast carcinoma and the use of bevacizumab as well as other chemotherapeutic drugs.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
- Wu CY, Gao HW, Huang WH, et al. Infection-like acral cutaneous metastasis as the presenting sign of an occult breast cancer. Clin Exp Dermatol. 2009;34:409-410.
- Karamouzis MV, Ardavanis A, Alexopoulos A, et al. Multiple cutaneous acral metastases in a woman with breast adenocarcinoma treated with pegylated liposomal doxorubicin: incidental or aetiological association? Eur J Cancer Care (Engl). 2005;14:267-271.
- Nagore E, Insa A, Sanmartin O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome. incidence, recognition and management. Am J Clin Dermatol. 2000;1:225-234.
- Wozel G, Sticherling M, Schon MP. Cutaneous side effects of inhibition of VEGF signal transduction. J Dtsch Dermatol Ges. 2010;8:243-249.
- Munehiro A, Yoneda K, Nakai K, et al. Bevacizumab-induced hand-foot syndrome: circumscribed type. Br J Dermatol. 2010;162:1411-1413.
- Mourad JJ, des Guetz G, Debbabi H, et al. Blood pressure rise following angiogenesis inhibition by bevacizumab. a crucial role for microcirculation. Ann Oncol. 2008;19:927-934.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
- Wu CY, Gao HW, Huang WH, et al. Infection-like acral cutaneous metastasis as the presenting sign of an occult breast cancer. Clin Exp Dermatol. 2009;34:409-410.
- Karamouzis MV, Ardavanis A, Alexopoulos A, et al. Multiple cutaneous acral metastases in a woman with breast adenocarcinoma treated with pegylated liposomal doxorubicin: incidental or aetiological association? Eur J Cancer Care (Engl). 2005;14:267-271.
- Nagore E, Insa A, Sanmartin O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome. incidence, recognition and management. Am J Clin Dermatol. 2000;1:225-234.
- Wozel G, Sticherling M, Schon MP. Cutaneous side effects of inhibition of VEGF signal transduction. J Dtsch Dermatol Ges. 2010;8:243-249.
- Munehiro A, Yoneda K, Nakai K, et al. Bevacizumab-induced hand-foot syndrome: circumscribed type. Br J Dermatol. 2010;162:1411-1413.
- Mourad JJ, des Guetz G, Debbabi H, et al. Blood pressure rise following angiogenesis inhibition by bevacizumab. a crucial role for microcirculation. Ann Oncol. 2008;19:927-934.
Practice Points
- Cutaneous involvement of internal malignancy typically occurs late in the disease course but can occasionally be the first extranodal sign of metastatic disease.
- Acral cutaneous metastasis from internal malignancies typically is unilateral, involving only one extremity; however, this case demonstrates involvement on both the hands and feet.
- This case support a chemotherapy-related etiology of acral cutaneous metastasis of a primary breast cancer.
Mycobacterium abscessus: A Rare Cause of Periprosthetic Knee Joint Infection
ABSTRACT
A 61-year-old woman with a periprosthetic knee joint infection caused by Mycobacterium abscessus was successfully treated with surgical débridement, multidrug antimicrobial therapy, and staged reimplantation. To the authors’ knowledge, this represents the first report of successfully treating this organism after knee arthroplasty.
M. abscessus knee infections are rare, and there are no specific guidelines to inform treatment or successful treatment regimens for periprosthetic knee infections. Medical management alone was not successful in this case and hence cannot be recommended. Using a collaborative multidisciplinary approach, including surgical débridement, staged reimplantation, and multidrug antimicrobials, successful eradication of the periprosthetic joint infection caused by M. abscessus was achieved.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) procedures are projected to increase by more than 6-fold by 2030, with concurrent increases in revision TKA for infection projected.1 Infection after TKA remains one of the most serious complications of the procedure, occurring in <2% of primary TKAs.2 The majority of prosthetic joint infections (PJIs) are caused by staphylococci and streptococci.3 Although infection and treatment of PJIs by mycobacterial species have been described, there are presently no established treatment guidelines for mycobacterial PJIs.4,5
Given the scarcity of clinical experience in dealing with these organisms, and the predicted increasing incidence of revision knee arthroplasty due to infection, we describe an unusual case of a PJI caused by Mycobacterium abscessus (M. abscessus), which was successfully treated using a combination of antimicrobial therapy and staged reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
BACKGROUND
Mycobacteria are common environmental organisms that can survive harsh conditions, including low pH and extreme temperatures. They form biofilms and may be difficult to eradicate in cases of infection.6 M. abscessus has proven to be difficult to eradicate due to limited antimicrobial susceptibility, lack of bactericidal options, and the variable presence of the erm gene, which yields inducible resistance to macrolides.7 Post-procedural outbreaks due to mycobacteria have been reported, often attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, or improper skin preparation.6,8-13
CASE REPORT
A 61-year-old woman was referred with a 3-year history of progressive left knee pain and swelling. Before 8 months, she had undergone knee arthroscopy and had been treated with multiple steroid and hyaluronic acid injections, as well as ultrasound-guided aspiration of a Baker’s cyst (Figures 1A, 1B).
She elected to proceed with TKA 1 month after her last steroid injection. There was no preoperative concern for native joint infection. At the time of arthroplasty, clear joint fluid was encountered, and a deep tissue culture was taken (Figures 2A-2C).
Routine screening cultures for acid-fast bacilli (AFB) returned positive 9 days after the index arthroplasty, with subsequent identification of a nontuberculous mycobacterium (NTM), M. abscessus, subspecies massiliense. Sensitivity tests revealed susceptibility to amikacin, cefoxitin, and tigecycline (Table 1). The isolate was found to have inducible macrolide resistance by erm gene testing.
Table 1. Initial Mycobacterium abscessus massiliense Susceptibilities
Medication | Minimum Inhibitory Concentration |
Amikacin | 16 (S) |
Cefoxitin | 16 (S) |
Imipenem | 8 (I) |
Linezolid | 16 (I) |
Clarithromycin | 2 (S)a |
Tigecycline | 1 (S) |
aAt 3 days; erm gene detected at 7 days.
Given no prior surgical suspicion for infection and the uncertain significance of the culture result, treatment options were debated. Medical management was selected based on the presumption that if infection was present, it was a native joint infection in which surgical débridement had already been undertaken at the time of primary arthroplasty. Similar reports for the treatment of M. tuberculosis infection in the knee have been reported with some success.14,15 Short-interval reassessment was planned. Antimicrobial therapy was selected based on susceptibility data and clinical experience and consisted of intravenous (IV) cefoxitin, oral clarithromycin, and thrice-weekly intravenous amikacin. Over the ensuing weeks, she developed fevers, knee swelling, and persistent elevation of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). With known potential of this organism for biofilm formation in other areas of the body and positive repeat cultures of the knee joint fluid, confirming the offending organism, a deep and resistant infection of the implant could not be excluded. Therefore, in an attempt to give the patient the best opportunity for clinical cure, the patient subsequently underwent a 2-stage antibiotic spacer explantation and exchange (Figures 3A, 3B). Moderate caseous material was present throughout the knee joint and the subcutaneous tissues. All bone was débrided, and complete synovectomy was undertaken, along with the removal of all implants. The antibiotic concentrations within the spacer were selected by guidance from the Infectious Disease and Pharmacy based on minimal inhibitory concentrations, with 3 packages of cement (40 g each) utilized and a total of 10 g of amikacin and 24 g of cefoxitin contained within the spacer. The patient continued systemic administration of amikacin, cefoxitin, and clarithromycin.
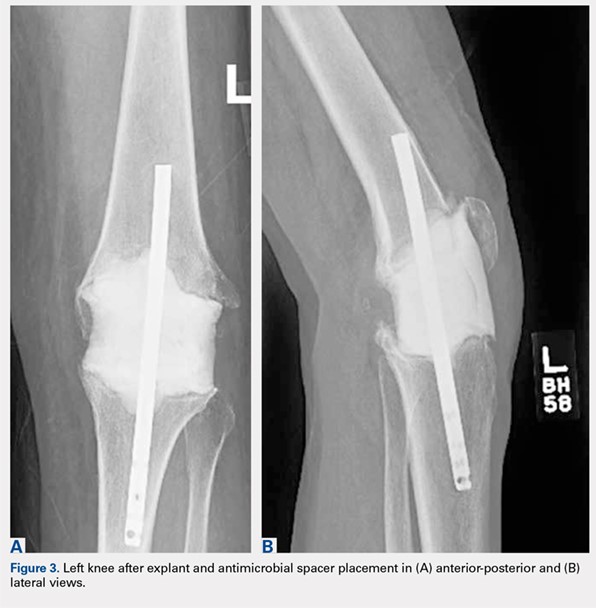
Continue to: One month postoperatively...
One month postoperatively, her constitutional symptoms, including fevers and night sweats, abated and inflammatory markers (ESR and CRP) had normalized. There were no clinical signs of infection. Amikacin was discontinued due to a 10-dB change on audiologic screening (4-6 kHz range), and tigecycline was substituted. Ultimately, she underwent 15 weeks of antimycobacterial therapy, 10 of which were after the explantation.
Eight weeks after cessation of her antibiotics, she underwent open biopsy. Multiple operative tissue samples showed negative results in pathology and culture tests.
Replantation was performed 14 weeks after stopping antimicrobials and 24 weeks after her explantation. The bone appeared healthy without evidence of osteomyelitis. A constrained reconstruction was secured with tobramycin-impregnated cement. One small island of necrotizing granuloma was observed within the bony cortex on histologic review; the granulomata appeared active with scattered neutrophils along with histiocytes and lymphocytes. AFB stains were negative. Intraoperative cultures, including mycobacterial cultures, were negative.
Based on the histologic evidence that infection may have persisted, and given the high stakes, antimicrobial treatment was reinitiated. Amikacin was again stopped after 3 weeks due to the development of tinnitus; tigecycline was substituted to complete the fourth and final week, at which point all antibiotics were discontinued. The patient was followed up uneventfully for 4 years (Figures 4A-4D and 5A-5C) with normal ESR and CRP. She continues to be ambulatory without assistive devices and walks an average of 30 miles per week without pain or constitutional symptoms.
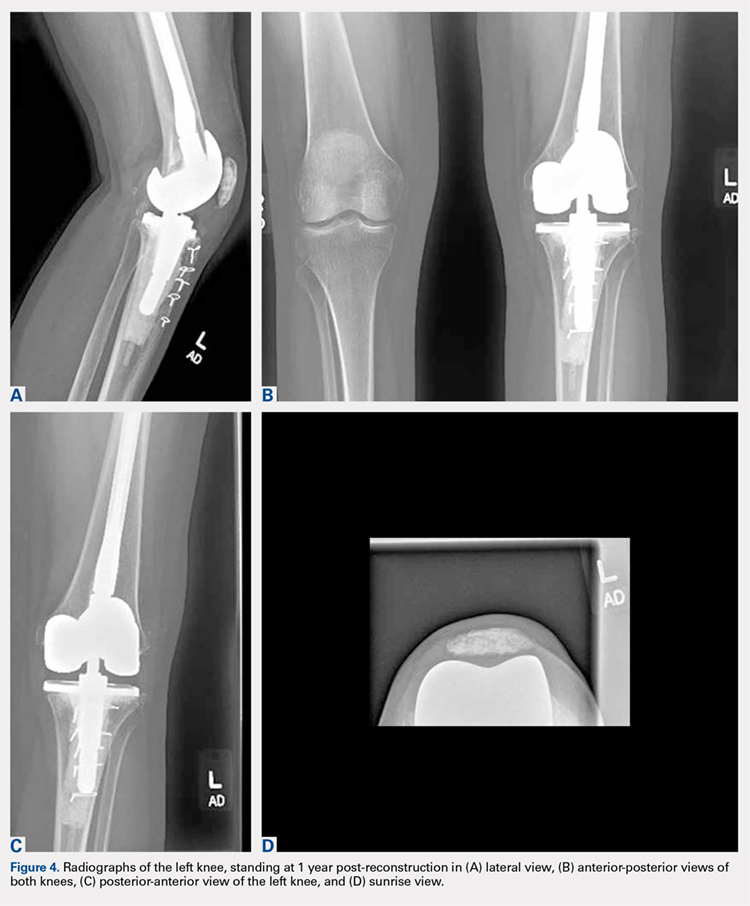
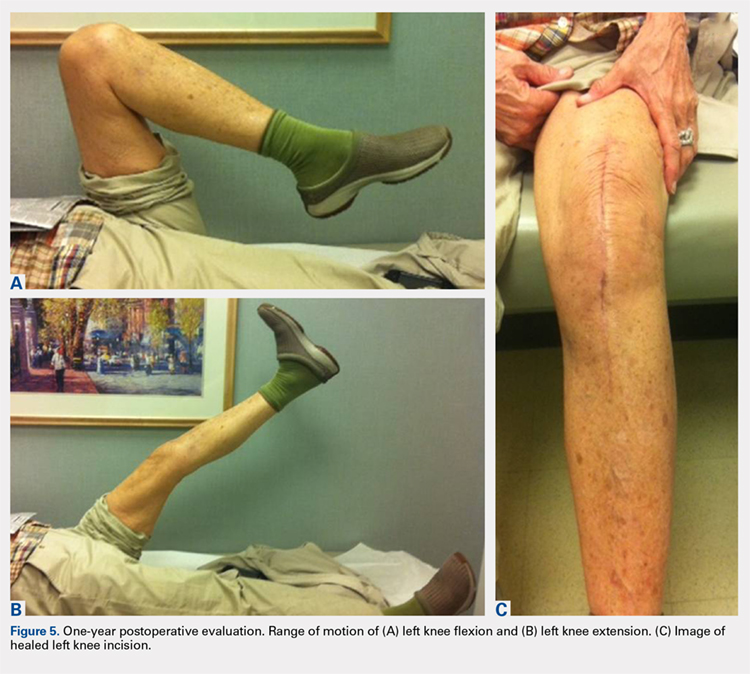
Continue to: DISCUSSION...
DISCUSSION
Diagnosis of acute infection after TKA remains challenging, as some degree of pain, swelling, and even postoperative fevers may be common in noninfected TKA patients. Synovial white blood cell count and differential as well as alpha-defensin levels have been cited as predictive factors of infection.16,17 Deep tissue and synovial fluid cultures offer the advantage of both identification and antimicrobial sensitivity testing of the offending organism. In this case, culture of the knee joint fluid at the time of TKA led to the unexpected finding of M. abscessus infection.
Preventable outbreaks due to M. abscessus have been reported and attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, and improper skin preparation.11-13 Rarely, M. abscessus has been reported as the cause of PJI. When an unusual organism is encountered after native joint instrumentation, an investigation should be undertaken to identify the source of contamination, with the assistance of infection control practitioners and/or the US Food and Drug Administration reporting. Reporting and investigation was undertaken in this case, though no suspect source could be identified.
Although there were no signs of infection prior to the TKA, there is an ongoing debate as to whether intra-articular corticosteroid injections increase the risk of PJIs, and if so, what the optimal amount of time to wait between procedures is. Although several earlier studies have been underpowered to answer these questions,18 this patient underwent TKA 1 month following the corticosteroid injection. Recent meta-analyses have shown no definitive evidence to indicate that this increased her risk of PJI.19,20
Continue to: Treatments for mycobacterial infections...
Treatments for mycobacterial infections have been described with variable efficacy,21,22 and only 2 cases of successfully treated PJIs have been reported after infection with M. abscessus. Both these cases were described in total hip arthroplasties,23,24 and to the authors’ knowledge, this report represents the first described successfully treated case after TKA. Staged reconstruction remains a standard treatment for invasive organisms chronically infecting prosthetic joint implants, with reimplantation pending joint sterility and improvement in inflammatory markers.3 Previous successful reports of treating M. abscessus describe either resection arthroplasty21 or staged reconstruction.23,24 The authors reported variable multidrug antimicrobial regimens, as summarized in Table 2, as guidelines for the treatment of mycobacterial PJI are currently not available.
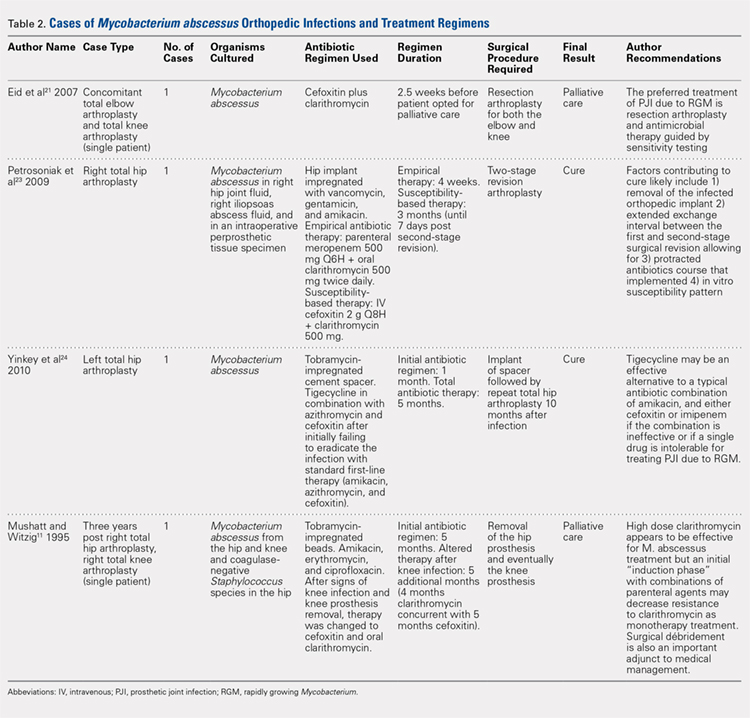
CONCLUSION
This case report represents an episode of iatrogenic septic arthritis caused by Mycobacteria of the native knee after previous history of instrumentation, corticosteroid, and hyaluronic acid injections, with an overall indolent clinical course until subsequent arthroplasty. There were several important lessons learned, which are as follows: 1) Multidrug combination with antimicrobial therapy combined with aggressive surgical débridement and staged reimplantation permitted successful eradication of TKA PJI caused by M. abscessus in this patient. 2) Initial medical management alone was not successful and cannot be recommended for the treatment of M. abscessus in the setting of PJI. 3) Delaying the surgical débridement and the reconstructive course for a trial of medical management contributed to the ultimate requirement of a tibial tubercle osteotomy for an ankylosed knee at replantation. In this case, we initially had a low index of suspicion for deep infection, contributing to delayed surgical débridement. Ideally, a high degree of clinical suspicion should be maintained for joint infection in the presence of positive culture isolates of M. abscessus, as it may have a delayed clinical presentation of the typical features of PJI (fevers, swelling, erythema, etc). In such cases, the authors recommend consideration of early surgical débridement. 4) Medical management of TKA PJI is not without risks. Careful monitoring of patient side effects during antimicrobial administration remains paramount, as this patient did sustain a degree of hearing loss associated with prolonged medical therapy. 5) In complicated PJIs involving rare and intrinsically resistant organisms, a collaborative multidisciplinary approach, including specialists in orthopedic surgery, infectious disease, microbiology, pharmacy, and pathology, may be the preferred path to clinical cure.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Cobo J, Del Pozo JL. Prosthetic joint infection: diagnosis and management. Expert Rev Anti Infect Ther. 2011;9(9):787-802. doi:10.1586/eri.11.95.
3. Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006;88(2):149-155. doi:10.1302/0301-620X.88B2.17058.
4. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803.
5. Restrepo C, Schmitt S, Backstein D, et al. Antibiotic treatment and timing of reimplantation. J Orthop Res. 2014;32 Suppl 1:S136-S140. doi:10.1002/jor.22557.
6. De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis. 2006;42(12):1756-1763. doi:10.1086/504381.
7. Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), Confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53(4):1367-1376. doi:10.1128/AAC.01275-08.
8. Furuya EY, Paez A, Srinivasan A, et al. Outbreak of Mycobacterium abscessus wound infections among "lipotourists" from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis. 2008;46(8):1181-1188. doi:10.1086/529191.
9. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52(5):565-571. doi:10.1093/cid/ciq237.
10. Mueller PS, Edson RS. Disseminated Mycobacterium abscessus infection manifesting as fever of unknown origin and intra-abdominal lymphadenitis: case report and literature review. Diagn Microbiol Infect Dis. 2001;39(1):33-37. doi:10.1016/S0732-8893(00)00211-X.
11. Mushatt DM, Witzig RS. Successful treatment of Mycobacterium abscessus infections with multidrug regimens containing clarithromycin. Clin Infect Dis. 1995;20(5):1441-1442. doi:10.1093/clinids/20.5.1441.
12. Tiwari TS, Ray B, Jost KC Jr, et al. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin Infect Dis. 2003;36(8):954-962. doi:10.1086/368192.
13. Villanueva A, Calderon RV, Vargas BA, et al. Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin Infect Dis. 1997;24(6):1147-1153. doi:10.1086/513656.
14. Gale DW, Harding ML. Total knee arthroplasty in the presence of active tuberculosis. J Bone Joint Surg Br. 1991;73(6):1006-1007. doi:10.1302/0301-620X.73B6.1955424.
15. Kim YH. Total knee arthroplasty for tuberculous arthritis. J Bone Joint Surg Am. 1988;70(9):1322-1330. doi:10.2106/00004623-198870090-00008.
16. Bedair H, Ting N, Jacovides C, et al. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469(1):34-40. doi:10.1007/s11999-010-1433-2.
17. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009. doi:10.1007/s11999-014-3900-7.
18. Marsland D, Mumith A, Barlow IW. Systematic review: the safety of intra-articular corticosteroid injection prior to total knee arthroplasty. Knee. 2014;21(1):6-11. doi:10.1016/j.knee.2013.07.003.
19. Charalambous CP, Prodromidis AD, Kwaees TA. Do intra-articular steroid injections increase infection rates in subsequent arthroplasty? A systematic review and meta-analysis of comparative studies. J Arthroplast. 2014;29(11):2175-2180. doi:10.1016/j.arth.2014.07.013.
20. Xing D, Yang Y, Ma X, Ma J, Ma B, Chen Y. Dose intraarticular steroid injection increase the rate of infection in subsequent arthroplasty: grading the evidence through a meta-analysis. J Orthop Surg Res. 2014;9:107. doi:10.1186/s13018-014-0107-2.
21. Eid AJ, Berbari EF, Sia IG, Wengenack NL, Osmon DR, Razonable RR. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;45(6):687-694. doi:10.1086/520982.
22. Herold RC, Lotke PA, MacGregor RR. Prosthetic joint infections secondary to rapidly growing Mycobacterium fortuitum. Clin Orthop Relat Res. 1987;216(216):183-186. doi:10.1097/00003086-198703000-00029.
23. Petrosoniak A, Kim P, Desjardins M, Lee BC. Successful treatment of a prosthetic joint infection due to Mycobacterium abscessus. Can J Infect Dis Med Microbiol. 2009;20(3):e94-e96.
24. Yinkey LM, Halsey ES, Lloyd BA. Successful tigecycline combination therapy for Mycobacterium abscessus infection of a total hip arthroplasty. Infect Dis Clin Practice. 2010;18(4):269-270. doi:10.1097/IPC.0b013e3181d04a09.
25. AAOS Guidelines: the diagnosis of periprosthetic joint infections of the hip and knee guideline and evidence report. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors; June 18th, 2010. AAOS Publication: 2010.
26. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcomittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
ABSTRACT
A 61-year-old woman with a periprosthetic knee joint infection caused by Mycobacterium abscessus was successfully treated with surgical débridement, multidrug antimicrobial therapy, and staged reimplantation. To the authors’ knowledge, this represents the first report of successfully treating this organism after knee arthroplasty.
M. abscessus knee infections are rare, and there are no specific guidelines to inform treatment or successful treatment regimens for periprosthetic knee infections. Medical management alone was not successful in this case and hence cannot be recommended. Using a collaborative multidisciplinary approach, including surgical débridement, staged reimplantation, and multidrug antimicrobials, successful eradication of the periprosthetic joint infection caused by M. abscessus was achieved.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) procedures are projected to increase by more than 6-fold by 2030, with concurrent increases in revision TKA for infection projected.1 Infection after TKA remains one of the most serious complications of the procedure, occurring in <2% of primary TKAs.2 The majority of prosthetic joint infections (PJIs) are caused by staphylococci and streptococci.3 Although infection and treatment of PJIs by mycobacterial species have been described, there are presently no established treatment guidelines for mycobacterial PJIs.4,5
Given the scarcity of clinical experience in dealing with these organisms, and the predicted increasing incidence of revision knee arthroplasty due to infection, we describe an unusual case of a PJI caused by Mycobacterium abscessus (M. abscessus), which was successfully treated using a combination of antimicrobial therapy and staged reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
BACKGROUND
Mycobacteria are common environmental organisms that can survive harsh conditions, including low pH and extreme temperatures. They form biofilms and may be difficult to eradicate in cases of infection.6 M. abscessus has proven to be difficult to eradicate due to limited antimicrobial susceptibility, lack of bactericidal options, and the variable presence of the erm gene, which yields inducible resistance to macrolides.7 Post-procedural outbreaks due to mycobacteria have been reported, often attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, or improper skin preparation.6,8-13
CASE REPORT
A 61-year-old woman was referred with a 3-year history of progressive left knee pain and swelling. Before 8 months, she had undergone knee arthroscopy and had been treated with multiple steroid and hyaluronic acid injections, as well as ultrasound-guided aspiration of a Baker’s cyst (Figures 1A, 1B).

She elected to proceed with TKA 1 month after her last steroid injection. There was no preoperative concern for native joint infection. At the time of arthroplasty, clear joint fluid was encountered, and a deep tissue culture was taken (Figures 2A-2C).

Routine screening cultures for acid-fast bacilli (AFB) returned positive 9 days after the index arthroplasty, with subsequent identification of a nontuberculous mycobacterium (NTM), M. abscessus, subspecies massiliense. Sensitivity tests revealed susceptibility to amikacin, cefoxitin, and tigecycline (Table 1). The isolate was found to have inducible macrolide resistance by erm gene testing.
Table 1. Initial Mycobacterium abscessus massiliense Susceptibilities
Medication | Minimum Inhibitory Concentration |
Amikacin | 16 (S) |
Cefoxitin | 16 (S) |
Imipenem | 8 (I) |
Linezolid | 16 (I) |
Clarithromycin | 2 (S)a |
Tigecycline | 1 (S) |
aAt 3 days; erm gene detected at 7 days.
Given no prior surgical suspicion for infection and the uncertain significance of the culture result, treatment options were debated. Medical management was selected based on the presumption that if infection was present, it was a native joint infection in which surgical débridement had already been undertaken at the time of primary arthroplasty. Similar reports for the treatment of M. tuberculosis infection in the knee have been reported with some success.14,15 Short-interval reassessment was planned. Antimicrobial therapy was selected based on susceptibility data and clinical experience and consisted of intravenous (IV) cefoxitin, oral clarithromycin, and thrice-weekly intravenous amikacin. Over the ensuing weeks, she developed fevers, knee swelling, and persistent elevation of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). With known potential of this organism for biofilm formation in other areas of the body and positive repeat cultures of the knee joint fluid, confirming the offending organism, a deep and resistant infection of the implant could not be excluded. Therefore, in an attempt to give the patient the best opportunity for clinical cure, the patient subsequently underwent a 2-stage antibiotic spacer explantation and exchange (Figures 3A, 3B). Moderate caseous material was present throughout the knee joint and the subcutaneous tissues. All bone was débrided, and complete synovectomy was undertaken, along with the removal of all implants. The antibiotic concentrations within the spacer were selected by guidance from the Infectious Disease and Pharmacy based on minimal inhibitory concentrations, with 3 packages of cement (40 g each) utilized and a total of 10 g of amikacin and 24 g of cefoxitin contained within the spacer. The patient continued systemic administration of amikacin, cefoxitin, and clarithromycin.

Continue to: One month postoperatively...
One month postoperatively, her constitutional symptoms, including fevers and night sweats, abated and inflammatory markers (ESR and CRP) had normalized. There were no clinical signs of infection. Amikacin was discontinued due to a 10-dB change on audiologic screening (4-6 kHz range), and tigecycline was substituted. Ultimately, she underwent 15 weeks of antimycobacterial therapy, 10 of which were after the explantation.
Eight weeks after cessation of her antibiotics, she underwent open biopsy. Multiple operative tissue samples showed negative results in pathology and culture tests.
Replantation was performed 14 weeks after stopping antimicrobials and 24 weeks after her explantation. The bone appeared healthy without evidence of osteomyelitis. A constrained reconstruction was secured with tobramycin-impregnated cement. One small island of necrotizing granuloma was observed within the bony cortex on histologic review; the granulomata appeared active with scattered neutrophils along with histiocytes and lymphocytes. AFB stains were negative. Intraoperative cultures, including mycobacterial cultures, were negative.
Based on the histologic evidence that infection may have persisted, and given the high stakes, antimicrobial treatment was reinitiated. Amikacin was again stopped after 3 weeks due to the development of tinnitus; tigecycline was substituted to complete the fourth and final week, at which point all antibiotics were discontinued. The patient was followed up uneventfully for 4 years (Figures 4A-4D and 5A-5C) with normal ESR and CRP. She continues to be ambulatory without assistive devices and walks an average of 30 miles per week without pain or constitutional symptoms.


Continue to: DISCUSSION...
DISCUSSION
Diagnosis of acute infection after TKA remains challenging, as some degree of pain, swelling, and even postoperative fevers may be common in noninfected TKA patients. Synovial white blood cell count and differential as well as alpha-defensin levels have been cited as predictive factors of infection.16,17 Deep tissue and synovial fluid cultures offer the advantage of both identification and antimicrobial sensitivity testing of the offending organism. In this case, culture of the knee joint fluid at the time of TKA led to the unexpected finding of M. abscessus infection.
Preventable outbreaks due to M. abscessus have been reported and attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, and improper skin preparation.11-13 Rarely, M. abscessus has been reported as the cause of PJI. When an unusual organism is encountered after native joint instrumentation, an investigation should be undertaken to identify the source of contamination, with the assistance of infection control practitioners and/or the US Food and Drug Administration reporting. Reporting and investigation was undertaken in this case, though no suspect source could be identified.
Although there were no signs of infection prior to the TKA, there is an ongoing debate as to whether intra-articular corticosteroid injections increase the risk of PJIs, and if so, what the optimal amount of time to wait between procedures is. Although several earlier studies have been underpowered to answer these questions,18 this patient underwent TKA 1 month following the corticosteroid injection. Recent meta-analyses have shown no definitive evidence to indicate that this increased her risk of PJI.19,20
Continue to: Treatments for mycobacterial infections...
Treatments for mycobacterial infections have been described with variable efficacy,21,22 and only 2 cases of successfully treated PJIs have been reported after infection with M. abscessus. Both these cases were described in total hip arthroplasties,23,24 and to the authors’ knowledge, this report represents the first described successfully treated case after TKA. Staged reconstruction remains a standard treatment for invasive organisms chronically infecting prosthetic joint implants, with reimplantation pending joint sterility and improvement in inflammatory markers.3 Previous successful reports of treating M. abscessus describe either resection arthroplasty21 or staged reconstruction.23,24 The authors reported variable multidrug antimicrobial regimens, as summarized in Table 2, as guidelines for the treatment of mycobacterial PJI are currently not available.

CONCLUSION
This case report represents an episode of iatrogenic septic arthritis caused by Mycobacteria of the native knee after previous history of instrumentation, corticosteroid, and hyaluronic acid injections, with an overall indolent clinical course until subsequent arthroplasty. There were several important lessons learned, which are as follows: 1) Multidrug combination with antimicrobial therapy combined with aggressive surgical débridement and staged reimplantation permitted successful eradication of TKA PJI caused by M. abscessus in this patient. 2) Initial medical management alone was not successful and cannot be recommended for the treatment of M. abscessus in the setting of PJI. 3) Delaying the surgical débridement and the reconstructive course for a trial of medical management contributed to the ultimate requirement of a tibial tubercle osteotomy for an ankylosed knee at replantation. In this case, we initially had a low index of suspicion for deep infection, contributing to delayed surgical débridement. Ideally, a high degree of clinical suspicion should be maintained for joint infection in the presence of positive culture isolates of M. abscessus, as it may have a delayed clinical presentation of the typical features of PJI (fevers, swelling, erythema, etc). In such cases, the authors recommend consideration of early surgical débridement. 4) Medical management of TKA PJI is not without risks. Careful monitoring of patient side effects during antimicrobial administration remains paramount, as this patient did sustain a degree of hearing loss associated with prolonged medical therapy. 5) In complicated PJIs involving rare and intrinsically resistant organisms, a collaborative multidisciplinary approach, including specialists in orthopedic surgery, infectious disease, microbiology, pharmacy, and pathology, may be the preferred path to clinical cure.
ABSTRACT
A 61-year-old woman with a periprosthetic knee joint infection caused by Mycobacterium abscessus was successfully treated with surgical débridement, multidrug antimicrobial therapy, and staged reimplantation. To the authors’ knowledge, this represents the first report of successfully treating this organism after knee arthroplasty.
M. abscessus knee infections are rare, and there are no specific guidelines to inform treatment or successful treatment regimens for periprosthetic knee infections. Medical management alone was not successful in this case and hence cannot be recommended. Using a collaborative multidisciplinary approach, including surgical débridement, staged reimplantation, and multidrug antimicrobials, successful eradication of the periprosthetic joint infection caused by M. abscessus was achieved.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) procedures are projected to increase by more than 6-fold by 2030, with concurrent increases in revision TKA for infection projected.1 Infection after TKA remains one of the most serious complications of the procedure, occurring in <2% of primary TKAs.2 The majority of prosthetic joint infections (PJIs) are caused by staphylococci and streptococci.3 Although infection and treatment of PJIs by mycobacterial species have been described, there are presently no established treatment guidelines for mycobacterial PJIs.4,5
Given the scarcity of clinical experience in dealing with these organisms, and the predicted increasing incidence of revision knee arthroplasty due to infection, we describe an unusual case of a PJI caused by Mycobacterium abscessus (M. abscessus), which was successfully treated using a combination of antimicrobial therapy and staged reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
BACKGROUND
Mycobacteria are common environmental organisms that can survive harsh conditions, including low pH and extreme temperatures. They form biofilms and may be difficult to eradicate in cases of infection.6 M. abscessus has proven to be difficult to eradicate due to limited antimicrobial susceptibility, lack of bactericidal options, and the variable presence of the erm gene, which yields inducible resistance to macrolides.7 Post-procedural outbreaks due to mycobacteria have been reported, often attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, or improper skin preparation.6,8-13
CASE REPORT
A 61-year-old woman was referred with a 3-year history of progressive left knee pain and swelling. Before 8 months, she had undergone knee arthroscopy and had been treated with multiple steroid and hyaluronic acid injections, as well as ultrasound-guided aspiration of a Baker’s cyst (Figures 1A, 1B).

She elected to proceed with TKA 1 month after her last steroid injection. There was no preoperative concern for native joint infection. At the time of arthroplasty, clear joint fluid was encountered, and a deep tissue culture was taken (Figures 2A-2C).

Routine screening cultures for acid-fast bacilli (AFB) returned positive 9 days after the index arthroplasty, with subsequent identification of a nontuberculous mycobacterium (NTM), M. abscessus, subspecies massiliense. Sensitivity tests revealed susceptibility to amikacin, cefoxitin, and tigecycline (Table 1). The isolate was found to have inducible macrolide resistance by erm gene testing.
Table 1. Initial Mycobacterium abscessus massiliense Susceptibilities
Medication | Minimum Inhibitory Concentration |
Amikacin | 16 (S) |
Cefoxitin | 16 (S) |
Imipenem | 8 (I) |
Linezolid | 16 (I) |
Clarithromycin | 2 (S)a |
Tigecycline | 1 (S) |
aAt 3 days; erm gene detected at 7 days.
Given no prior surgical suspicion for infection and the uncertain significance of the culture result, treatment options were debated. Medical management was selected based on the presumption that if infection was present, it was a native joint infection in which surgical débridement had already been undertaken at the time of primary arthroplasty. Similar reports for the treatment of M. tuberculosis infection in the knee have been reported with some success.14,15 Short-interval reassessment was planned. Antimicrobial therapy was selected based on susceptibility data and clinical experience and consisted of intravenous (IV) cefoxitin, oral clarithromycin, and thrice-weekly intravenous amikacin. Over the ensuing weeks, she developed fevers, knee swelling, and persistent elevation of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). With known potential of this organism for biofilm formation in other areas of the body and positive repeat cultures of the knee joint fluid, confirming the offending organism, a deep and resistant infection of the implant could not be excluded. Therefore, in an attempt to give the patient the best opportunity for clinical cure, the patient subsequently underwent a 2-stage antibiotic spacer explantation and exchange (Figures 3A, 3B). Moderate caseous material was present throughout the knee joint and the subcutaneous tissues. All bone was débrided, and complete synovectomy was undertaken, along with the removal of all implants. The antibiotic concentrations within the spacer were selected by guidance from the Infectious Disease and Pharmacy based on minimal inhibitory concentrations, with 3 packages of cement (40 g each) utilized and a total of 10 g of amikacin and 24 g of cefoxitin contained within the spacer. The patient continued systemic administration of amikacin, cefoxitin, and clarithromycin.

Continue to: One month postoperatively...
One month postoperatively, her constitutional symptoms, including fevers and night sweats, abated and inflammatory markers (ESR and CRP) had normalized. There were no clinical signs of infection. Amikacin was discontinued due to a 10-dB change on audiologic screening (4-6 kHz range), and tigecycline was substituted. Ultimately, she underwent 15 weeks of antimycobacterial therapy, 10 of which were after the explantation.
Eight weeks after cessation of her antibiotics, she underwent open biopsy. Multiple operative tissue samples showed negative results in pathology and culture tests.
Replantation was performed 14 weeks after stopping antimicrobials and 24 weeks after her explantation. The bone appeared healthy without evidence of osteomyelitis. A constrained reconstruction was secured with tobramycin-impregnated cement. One small island of necrotizing granuloma was observed within the bony cortex on histologic review; the granulomata appeared active with scattered neutrophils along with histiocytes and lymphocytes. AFB stains were negative. Intraoperative cultures, including mycobacterial cultures, were negative.
Based on the histologic evidence that infection may have persisted, and given the high stakes, antimicrobial treatment was reinitiated. Amikacin was again stopped after 3 weeks due to the development of tinnitus; tigecycline was substituted to complete the fourth and final week, at which point all antibiotics were discontinued. The patient was followed up uneventfully for 4 years (Figures 4A-4D and 5A-5C) with normal ESR and CRP. She continues to be ambulatory without assistive devices and walks an average of 30 miles per week without pain or constitutional symptoms.


Continue to: DISCUSSION...
DISCUSSION
Diagnosis of acute infection after TKA remains challenging, as some degree of pain, swelling, and even postoperative fevers may be common in noninfected TKA patients. Synovial white blood cell count and differential as well as alpha-defensin levels have been cited as predictive factors of infection.16,17 Deep tissue and synovial fluid cultures offer the advantage of both identification and antimicrobial sensitivity testing of the offending organism. In this case, culture of the knee joint fluid at the time of TKA led to the unexpected finding of M. abscessus infection.
Preventable outbreaks due to M. abscessus have been reported and attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, and improper skin preparation.11-13 Rarely, M. abscessus has been reported as the cause of PJI. When an unusual organism is encountered after native joint instrumentation, an investigation should be undertaken to identify the source of contamination, with the assistance of infection control practitioners and/or the US Food and Drug Administration reporting. Reporting and investigation was undertaken in this case, though no suspect source could be identified.
Although there were no signs of infection prior to the TKA, there is an ongoing debate as to whether intra-articular corticosteroid injections increase the risk of PJIs, and if so, what the optimal amount of time to wait between procedures is. Although several earlier studies have been underpowered to answer these questions,18 this patient underwent TKA 1 month following the corticosteroid injection. Recent meta-analyses have shown no definitive evidence to indicate that this increased her risk of PJI.19,20
Continue to: Treatments for mycobacterial infections...
Treatments for mycobacterial infections have been described with variable efficacy,21,22 and only 2 cases of successfully treated PJIs have been reported after infection with M. abscessus. Both these cases were described in total hip arthroplasties,23,24 and to the authors’ knowledge, this report represents the first described successfully treated case after TKA. Staged reconstruction remains a standard treatment for invasive organisms chronically infecting prosthetic joint implants, with reimplantation pending joint sterility and improvement in inflammatory markers.3 Previous successful reports of treating M. abscessus describe either resection arthroplasty21 or staged reconstruction.23,24 The authors reported variable multidrug antimicrobial regimens, as summarized in Table 2, as guidelines for the treatment of mycobacterial PJI are currently not available.

CONCLUSION
This case report represents an episode of iatrogenic septic arthritis caused by Mycobacteria of the native knee after previous history of instrumentation, corticosteroid, and hyaluronic acid injections, with an overall indolent clinical course until subsequent arthroplasty. There were several important lessons learned, which are as follows: 1) Multidrug combination with antimicrobial therapy combined with aggressive surgical débridement and staged reimplantation permitted successful eradication of TKA PJI caused by M. abscessus in this patient. 2) Initial medical management alone was not successful and cannot be recommended for the treatment of M. abscessus in the setting of PJI. 3) Delaying the surgical débridement and the reconstructive course for a trial of medical management contributed to the ultimate requirement of a tibial tubercle osteotomy for an ankylosed knee at replantation. In this case, we initially had a low index of suspicion for deep infection, contributing to delayed surgical débridement. Ideally, a high degree of clinical suspicion should be maintained for joint infection in the presence of positive culture isolates of M. abscessus, as it may have a delayed clinical presentation of the typical features of PJI (fevers, swelling, erythema, etc). In such cases, the authors recommend consideration of early surgical débridement. 4) Medical management of TKA PJI is not without risks. Careful monitoring of patient side effects during antimicrobial administration remains paramount, as this patient did sustain a degree of hearing loss associated with prolonged medical therapy. 5) In complicated PJIs involving rare and intrinsically resistant organisms, a collaborative multidisciplinary approach, including specialists in orthopedic surgery, infectious disease, microbiology, pharmacy, and pathology, may be the preferred path to clinical cure.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Cobo J, Del Pozo JL. Prosthetic joint infection: diagnosis and management. Expert Rev Anti Infect Ther. 2011;9(9):787-802. doi:10.1586/eri.11.95.
3. Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006;88(2):149-155. doi:10.1302/0301-620X.88B2.17058.
4. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803.
5. Restrepo C, Schmitt S, Backstein D, et al. Antibiotic treatment and timing of reimplantation. J Orthop Res. 2014;32 Suppl 1:S136-S140. doi:10.1002/jor.22557.
6. De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis. 2006;42(12):1756-1763. doi:10.1086/504381.
7. Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), Confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53(4):1367-1376. doi:10.1128/AAC.01275-08.
8. Furuya EY, Paez A, Srinivasan A, et al. Outbreak of Mycobacterium abscessus wound infections among "lipotourists" from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis. 2008;46(8):1181-1188. doi:10.1086/529191.
9. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52(5):565-571. doi:10.1093/cid/ciq237.
10. Mueller PS, Edson RS. Disseminated Mycobacterium abscessus infection manifesting as fever of unknown origin and intra-abdominal lymphadenitis: case report and literature review. Diagn Microbiol Infect Dis. 2001;39(1):33-37. doi:10.1016/S0732-8893(00)00211-X.
11. Mushatt DM, Witzig RS. Successful treatment of Mycobacterium abscessus infections with multidrug regimens containing clarithromycin. Clin Infect Dis. 1995;20(5):1441-1442. doi:10.1093/clinids/20.5.1441.
12. Tiwari TS, Ray B, Jost KC Jr, et al. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin Infect Dis. 2003;36(8):954-962. doi:10.1086/368192.
13. Villanueva A, Calderon RV, Vargas BA, et al. Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin Infect Dis. 1997;24(6):1147-1153. doi:10.1086/513656.
14. Gale DW, Harding ML. Total knee arthroplasty in the presence of active tuberculosis. J Bone Joint Surg Br. 1991;73(6):1006-1007. doi:10.1302/0301-620X.73B6.1955424.
15. Kim YH. Total knee arthroplasty for tuberculous arthritis. J Bone Joint Surg Am. 1988;70(9):1322-1330. doi:10.2106/00004623-198870090-00008.
16. Bedair H, Ting N, Jacovides C, et al. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469(1):34-40. doi:10.1007/s11999-010-1433-2.
17. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009. doi:10.1007/s11999-014-3900-7.
18. Marsland D, Mumith A, Barlow IW. Systematic review: the safety of intra-articular corticosteroid injection prior to total knee arthroplasty. Knee. 2014;21(1):6-11. doi:10.1016/j.knee.2013.07.003.
19. Charalambous CP, Prodromidis AD, Kwaees TA. Do intra-articular steroid injections increase infection rates in subsequent arthroplasty? A systematic review and meta-analysis of comparative studies. J Arthroplast. 2014;29(11):2175-2180. doi:10.1016/j.arth.2014.07.013.
20. Xing D, Yang Y, Ma X, Ma J, Ma B, Chen Y. Dose intraarticular steroid injection increase the rate of infection in subsequent arthroplasty: grading the evidence through a meta-analysis. J Orthop Surg Res. 2014;9:107. doi:10.1186/s13018-014-0107-2.
21. Eid AJ, Berbari EF, Sia IG, Wengenack NL, Osmon DR, Razonable RR. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;45(6):687-694. doi:10.1086/520982.
22. Herold RC, Lotke PA, MacGregor RR. Prosthetic joint infections secondary to rapidly growing Mycobacterium fortuitum. Clin Orthop Relat Res. 1987;216(216):183-186. doi:10.1097/00003086-198703000-00029.
23. Petrosoniak A, Kim P, Desjardins M, Lee BC. Successful treatment of a prosthetic joint infection due to Mycobacterium abscessus. Can J Infect Dis Med Microbiol. 2009;20(3):e94-e96.
24. Yinkey LM, Halsey ES, Lloyd BA. Successful tigecycline combination therapy for Mycobacterium abscessus infection of a total hip arthroplasty. Infect Dis Clin Practice. 2010;18(4):269-270. doi:10.1097/IPC.0b013e3181d04a09.
25. AAOS Guidelines: the diagnosis of periprosthetic joint infections of the hip and knee guideline and evidence report. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors; June 18th, 2010. AAOS Publication: 2010.
26. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcomittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Cobo J, Del Pozo JL. Prosthetic joint infection: diagnosis and management. Expert Rev Anti Infect Ther. 2011;9(9):787-802. doi:10.1586/eri.11.95.
3. Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006;88(2):149-155. doi:10.1302/0301-620X.88B2.17058.
4. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803.
5. Restrepo C, Schmitt S, Backstein D, et al. Antibiotic treatment and timing of reimplantation. J Orthop Res. 2014;32 Suppl 1:S136-S140. doi:10.1002/jor.22557.
6. De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis. 2006;42(12):1756-1763. doi:10.1086/504381.
7. Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), Confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53(4):1367-1376. doi:10.1128/AAC.01275-08.
8. Furuya EY, Paez A, Srinivasan A, et al. Outbreak of Mycobacterium abscessus wound infections among "lipotourists" from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis. 2008;46(8):1181-1188. doi:10.1086/529191.
9. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52(5):565-571. doi:10.1093/cid/ciq237.
10. Mueller PS, Edson RS. Disseminated Mycobacterium abscessus infection manifesting as fever of unknown origin and intra-abdominal lymphadenitis: case report and literature review. Diagn Microbiol Infect Dis. 2001;39(1):33-37. doi:10.1016/S0732-8893(00)00211-X.
11. Mushatt DM, Witzig RS. Successful treatment of Mycobacterium abscessus infections with multidrug regimens containing clarithromycin. Clin Infect Dis. 1995;20(5):1441-1442. doi:10.1093/clinids/20.5.1441.
12. Tiwari TS, Ray B, Jost KC Jr, et al. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin Infect Dis. 2003;36(8):954-962. doi:10.1086/368192.
13. Villanueva A, Calderon RV, Vargas BA, et al. Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin Infect Dis. 1997;24(6):1147-1153. doi:10.1086/513656.
14. Gale DW, Harding ML. Total knee arthroplasty in the presence of active tuberculosis. J Bone Joint Surg Br. 1991;73(6):1006-1007. doi:10.1302/0301-620X.73B6.1955424.
15. Kim YH. Total knee arthroplasty for tuberculous arthritis. J Bone Joint Surg Am. 1988;70(9):1322-1330. doi:10.2106/00004623-198870090-00008.
16. Bedair H, Ting N, Jacovides C, et al. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469(1):34-40. doi:10.1007/s11999-010-1433-2.
17. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009. doi:10.1007/s11999-014-3900-7.
18. Marsland D, Mumith A, Barlow IW. Systematic review: the safety of intra-articular corticosteroid injection prior to total knee arthroplasty. Knee. 2014;21(1):6-11. doi:10.1016/j.knee.2013.07.003.
19. Charalambous CP, Prodromidis AD, Kwaees TA. Do intra-articular steroid injections increase infection rates in subsequent arthroplasty? A systematic review and meta-analysis of comparative studies. J Arthroplast. 2014;29(11):2175-2180. doi:10.1016/j.arth.2014.07.013.
20. Xing D, Yang Y, Ma X, Ma J, Ma B, Chen Y. Dose intraarticular steroid injection increase the rate of infection in subsequent arthroplasty: grading the evidence through a meta-analysis. J Orthop Surg Res. 2014;9:107. doi:10.1186/s13018-014-0107-2.
21. Eid AJ, Berbari EF, Sia IG, Wengenack NL, Osmon DR, Razonable RR. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;45(6):687-694. doi:10.1086/520982.
22. Herold RC, Lotke PA, MacGregor RR. Prosthetic joint infections secondary to rapidly growing Mycobacterium fortuitum. Clin Orthop Relat Res. 1987;216(216):183-186. doi:10.1097/00003086-198703000-00029.
23. Petrosoniak A, Kim P, Desjardins M, Lee BC. Successful treatment of a prosthetic joint infection due to Mycobacterium abscessus. Can J Infect Dis Med Microbiol. 2009;20(3):e94-e96.
24. Yinkey LM, Halsey ES, Lloyd BA. Successful tigecycline combination therapy for Mycobacterium abscessus infection of a total hip arthroplasty. Infect Dis Clin Practice. 2010;18(4):269-270. doi:10.1097/IPC.0b013e3181d04a09.
25. AAOS Guidelines: the diagnosis of periprosthetic joint infections of the hip and knee guideline and evidence report. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors; June 18th, 2010. AAOS Publication: 2010.
26. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcomittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
TAKE-HOME POINTS:
- Periprosthetic joint infections due to Mycobacterium abscess have been rarely reported, and no specific guidlines exist to inform treatment.
- Medical management alone was not successful in our clinical case and cannot be recommended.
- Combination medical and surgical management may provide the best opportunity for clincal cure of periprosthetic infections.
- In complicated periprosthetic joint infections involving rare and intrinsically resistant organisms, a collaborative multidisciplinary approach likley represents the preferred path to clinical cure.
- Successful erradiation of periprosthetic infection with M. abscessus may not preclude acceptable outcomes after revision TKA.
Arthroscopically-Guided, Cannulated, Headless Compression Screw Fixation of the Symptomatic Os Acromiale
ABSTRACT
Os acromiale is a failure of fusion between 1 or more ossification centers of the scapula and the acromion process. Pain can be caused by motion and impingement of the unfused segment. Several methods for the management of os acromiale have been described. Internal fixation is the most common surgical technique, followed by excision and acromioplasty. We present a novel technique for treatment of symptomatic os acromiale using arthroscopically-guided headless compression screws. This is a viable technique in the management of symptomatic os acromiale due to preservation of the periosteal blood supply and less concern for symptomatic hardware.
Continue to: Os acromiale results from a failure of...
Os acromiale results from a failure of fusion between 1 or more ossification centers and the acromion process.1 The acromion consists of 4 different ossification centers, which appear by 14 years of age and fuse by age 25 years. The 4 ossification centers are the basi-acromion, meta-acromion, mesoacromion, and pre-acromion (Figure 1). Formation of an os acromiale occurs most often due to failure of fusion between the meta-acromion and mesoacromion. Os acromiale appears to occur in approximately 8% of the population, according to cadaveric studies.2 This anatomic variant occurs more commonly in African-Americans than Caucasians, and shows a preponderance for males over females.3
Plain radiographs are usually adequate for diagnosis. Axillary views are most sensitive for detection, which can be difficult to see on anteroposterior radiographs.4 In os acromiale, the unfused segment is connected to the acromioclavicular joint and the coracoid, which can lead to motion of the segment and impingement of the rotator cuff.2-4 Patients frequently experience localized tenderness and symptomatic pain with signs and symptoms of impingement. Rotator cuff tears may occur secondary to chronic impingement.5
Various forms of repair have been described. A recent meta-analysis showed that internal fixation (60%) was the most common surgical technique reported, followed by excision (27%) and acromioplasty (13%).6 Rotator cuff repair is a common concurrent surgical procedure.7-11 The available literature favors internal fixation through an open technique with or without bone grafting.5,7,8,12-15 Various forms of fixation have been presented in the literature, including Kirschner wire fixation, cannulated screw fixation alone, cannulated screw fixation with FiberWire Suture (Arthrex), and cannulated screw fixation with a stainless steel wire tension band technique. Based on the results of the meta-analysis, surgical fixation with cannulated screws has been shown to lead to a significantly greater rate of radiographic healing (23/24 patients) compared to Kirschner wire fixation (31/49 patients).6 Further, radiographic healing is significantly associated with improved clinical outcomes.12 Removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation cases (88%; 43/49) compared to cannulated screw fixation cases (38%; 9/24). However, hardware issues may also be encountered with screw fixation, with 1 case series reporting a 25% rate of hardware complication.16 The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
The patient is a 19-year-old right-hand-dominant woman who injured her right shoulder while diving into the bleachers during a volleyball game 4 years prior to presentation. She suffered a direct blow to her shoulder and immediately became symptomatic. She underwent a long period of nonoperative management, which included physical therapy, strengthening, nonsteroidal anti-inflammatory drug (NSAID) therapy, and narcotic pain medications. Her primary complaints upon presentation were pain with lifting, as well as mechanical symptoms. On examination, the patient had moderate tenderness directly over the acromion. She also had evidence of mild impingement symptoms. Plain radiographs revealed a mesoacromial-type os acromiale clearly seen on the axillary lateral film (Figure 2). She underwent magnetic resonance imaging, which suggested rotator cuff tendinosis and evidence of edema at the os acromiale site. She underwent a diagnostic injection directly into the site of maximal tenderness at the os, which provided complete transient relief of her pain. Despite the transient pain relief, the patient continued to be symptomatic after the local anesthetic effect wore off. Surgical options were then discussed with the patient.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
A standard diagnostic shoulder arthroscopy was performed using anterior, posterior and direct lateral portals. The rotator cuff was evaluated, and no evidence of a tear was found. The undersurface of the acromion was exposed, and the os acromiale was identified arthroscopically (Figure 3). This was found to be unstable under direct digital pressure.
We then elected to repair the unstable fibrous os acromiale (Figures 4A-4D). The fibrous nonunion was first debrided to bleeding bone with a 4.0-mm round burr aligned with the os using the direct lateral portal (Smith & Nephew Endoscopy). Through the anterior portal, two AcutrakTM guide wires (Acumed) were placed under arthroscopic visualization from the anterior margin of the acromion, across the os site, and into the posterior acromion. A 1-cm counter incision was made at the level of the posterior acromion to allow confirmation of the guide wire position and to permit placement of a large, pointed reduction clamp, used to reduce the mesoacromial fragment to the stable portion of the acromion. The calibrated, cannulated drill bit was passed over each guide wire to a depth of 34 mm, according to standard technique, and viewed arthroscopically from the subacromial space. Two 34-mm AcutrakTM cannulated headless compression screws (Acumed) were then placed across the defect. Direct arthroscopic visualization confirmed reduction and complete intraosseous placement of the screws (Figure 5). Screw position was also assessed with image intensification. Fluoroscopic views showed the repair to be stable when the shoulder was taken through range of motion. The os site was never exposed directly through an incision. The surgery was performed on an outpatient basis.
POSTOPERATIVE COURSE
The patient was maintained in a sling and small abduction pillow (Ultrasling IIITM, DonJoy). She was kept non-weight-bearing but was permitted unrestricted motion through the elbow, wrist, and hand for the first 6 weeks. She was permitted supine passive external rotation of the shoulder to 30° and forward flexion to 45° for the first 2 weeks, and 90° through 6 weeks. At her initial postoperative visit 2 weeks later, she noted minimal pain in the shoulder, much improved from her preoperative pain. She was no longer taking any pain medicine, including NSAIDs. Radiographs showed no change in fixation.
At her second visit (6 weeks), she was completely pain free. Clinical examination showed no tenderness at the acromion, healed incisions, and pain-free passive ROM. Radiographs demonstrated early evidence of consolidation and no sign of fixation failure (Figures 6-8). Her Single Assessment Numeric Evaluation (SANE) score was 85%, and her Simple Shoulder Test (SST) score was 3/12. She was permitted to discontinue the sling, to begin using the arm actively at the side, and progress with unloaded use above shoulder height over the next 6 weeks.
She was seen in follow-up at 4 months, where she was found to have no pain but had not yet returned to sports. At her 6-month follow-up, she showed continued improvement with no limitation of activity. At 1-year follow-up, her SANE score improved from 85% at 6 weeks postoperatively to 100%, and her SST improved from 3/12 at 6 weeks to 12/12. She demonstrated full function of her shoulder with no evidence of hardware loosening. At that time, her os acromiale had completely fused radiographically.
Continue to: DISCUSSION...
DISCUSSION
A variety of methods for the management of os acromiale have been described in the literature. Internal fixation is reported as the most common surgical technique, followed by excision and acromioplasty.6 Surgical fixation with cannulated screws is effective at achieving radiographic union.5,9,12,13,15
Excision is also an option in cases where there is a symptomatic pre-acromion with a relatively small fragment. In the case of a larger fragment, techniques that preserve the vascularity of the os acromiale appear more likely to be successful than excision.17 While excision can be performed arthroscopically to preserve the blood supply, a recent report showed that 35% of patients still had residual pain.18 Another study suggests that protecting the vascular supply with an arthroscopic technique would be a better option to promote healing to union.19
Given that removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation (88%; 43/49) than after cannulated screw fixation (38%; 9/24),6 and given that significant hardware complications can arise from screw tips,16 we chose headless, cannulated Acutrak compression screws for arthroscopic-assisted fixation. Performing the operation arthroscopically minimized soft-tissue violation, allowing us to directly visualize the reduction and also allowing confirmation that the screws were not at risk for impingement of the rotator cuff. The tapered nature of the Acutrak screws allowed for excellent compression at the reduction site without a prominent screw head.
CONCLUSION
Arthroscopic management of the symptomatic os acromiale has been documented in the literature. Cannulated screw fixation has shown to lead to a higher rate of radiographic union than Kirschner wire fixation. Arthroscopically guided placement of headless, cannulated compression screw fixation may be a viable repair alternative in the management of the symptomatic os acromiale with less concern for symptomatic hardware.6,20-27
1. Barbier O, Block D, Dezaly C, Sirveaux F, Mole D. Os acromiale, a cause of shoulder pain, not to be overlooked. Orthop Traumatol Surg Res. 2013;99(4):465-472. doi: 10.1016/j.otsr.2012.10.020.
2. Swain RA, Wilson FD, Harsha DM. The os acromiale: another cause of impingement. Med Sci Sports Exerc. 1996;28(12):1459-1462. doi:10.1097/00005768-199612000-00003.
3. Kurtz CA, Humble BJ, Rodosky MW, Sekiya JK. Symptomatic os acromiale. J Am Acad Orthop Surg. 2006;14(1):12-19. doi:10.5435/00124635-200601000-00004.
4. Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447-457.
5. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80(9):1320-1326. doi:10.2106/00004623-199809000-00011.
6. Harris JD, Griesser MJ, Jones GL. Systematic review of the surgical treatment for symptomatic os acromiale. Int J Shoulder Surg. 2011;5(1):9-16. doi:10.4103/0973-6042.80461.
7. Abboud JA, Silverberg D, Pepe M, et al. Surgical treatment of os acromiale with and without associated rotator cuff tears. J Shoulder Elbow Surg. 2006;15(3):265-270. doi:10.1016/j.jse.2005.08.024.
8. Boehm TD, Matzer M, Brazda D, Gohlke FE. Os acromiale associated with tear of the rotator cuff treated operatively Review of 33 patients. J Bone Joint Surg Br. 2003;85(4):545-549. doi:10.1302/0301-620X.85B4.13634.
9. Boehm TD, Rolf O, Martetschlaeger F, Kenn W, Gohlke F. Rotator cuff tears associated with os acromiale. Acta Orthop. 2005;76(2):241-244. doi:10.1080/00016470510030643.
10. Barbiera F, Bellissima G, Iovane A, De Maria M. OS acromiale producing rotator cuff impingement and rupture. A case report. Radiol Med. 2002;104(4):359-362.
11. Neer CS 2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
12. Hertel R, Windisch W, Schuster A, Ballmer FT. Transacromial approach to obtain fusion of unstable os acromiale. J Shoulder Elbow Surg. 1998;7(6):606-609. doi:10.1016/S1058-2746(98)90008-8.
13. Ozbaydar MU, Keriş I, Altun M, Yalaman O. Results of the surgical treatment for symptomatic mesoacromion. Acta Orthop Traumatol Turc. 2006;40(2):123-129.
14. Satterlee CC. Successful osteosynthesis of an unstable mesoacromion in 6 shoulders: a new technique. J Shoulder Elbow Surg. 1999;8(2):125-129. doi:10.1016/S1058-2746(99)90004-6.
15. Ryu RK, Fan RS, Dunbar WHt. The treatment of symptomatic os acromiale. Orthopedics. 1999;22(3):325-328.
16. Atoun E, van Tongel A, Narvani A, Rath E, Sforza G, Levy O. Arthroscopically assisted internal fixation of the symptomatic unstable os acromiale with absorbable screws. J Shoulder Elbow Surg. 2012;21(12):1740-1745. doi:10.1016/j.jse.2011.12.011.
17. Johnston PS, Paxton ES, Gordon V, Kraeutler MJ, Abboud JA, Williams GR. Os acromiale: a review and an introduction of a new surgical technique for management. Orthop Clin North Am. 2013;44(4):635-644. doi:10.1016/j.ocl.2013.06.015.
18. Campbell PT, Nizlan NM, Skirving AP. Arthroscopic excision of os acromiale: effects on deltoid function and strength. Orthopedics. 2012;35(11):e1601-e1605. doi:10.3928/01477447-20121023-16.
19. Yepes H, Al-Hibshi A, Tang M, Morris SF, Stanish WD. Vascular anatomy of the subacromial space: a map of bleeding points for the arthroscopic surgeon. Arthroscopy. 2007;23(9):978-984. doi:10.1016/j.arthro.2007.03.093.
20. Kummer FJ, Van Gelderen J, Meislin RJ. Two-screw, arthroscopic fixation of os acromiale compared to a similar, open procedure incorporating a tension band: a laboratory study. Shoulder Elbow. 2011;3(2):85-87. doi:10.1111/j.1758-5740.2011.00115.x.
21. Wright RW, Heller MA, Quick DC, Buss DD. Arthroscopic decompression for impingement syndrome secondary to an unstable os acromiale. Arthroscopy. 2000;16(6):595-599. doi:10.1053/jars.2000.9239.
22. Edelson JG, Zuckerman J, Hershkovitz I. Os acromiale: anatomy and surgical implications. J Bone Joint Surg Br. 1993;75(4):551-555. doi:10.1302/0301-620X.75B4.8331108.
23. Fery A, Sommelet J. Os acromiale: significance--diagnosis--pathology Apropos of 28 cases including 2 with fracture separation. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(2):160-172.
24. Lee DH. The double-density sign: a radiographic finding suggestive of an os acromiale. J Bone Joint Surg Am. 2004;86-A(12):2666-2670. doi:10.2106/00004623-200412000-00012.
25. Ortiguera CJ, Buss DD. Surgical management of the symptomatic os acromiale. J Shoulder Elbow Surg. 2002;11(5):521-528. doi:10.1067/mse.2002.122227.
26. Peckett WR, Gunther SB, Harper GD, Hughes JS, Sonnabend DH. Internal fixation of symptomatic os acromiale: a series of twenty-six cases. J Shoulder Elbow Surg. 2004;13(4):381-385. doi:10.1016/S1058274604000400.
27. Sahajpal D, Strauss EJ, Ishak C, Keyes JM, Joseph G, Jazrawi LM. Surgical management of os acromiale: a case report and review of the literature. Bull NYU Hosp Jt Dis. 2007;65(4):312-316.
ABSTRACT
Os acromiale is a failure of fusion between 1 or more ossification centers of the scapula and the acromion process. Pain can be caused by motion and impingement of the unfused segment. Several methods for the management of os acromiale have been described. Internal fixation is the most common surgical technique, followed by excision and acromioplasty. We present a novel technique for treatment of symptomatic os acromiale using arthroscopically-guided headless compression screws. This is a viable technique in the management of symptomatic os acromiale due to preservation of the periosteal blood supply and less concern for symptomatic hardware.
Continue to: Os acromiale results from a failure of...
Os acromiale results from a failure of fusion between 1 or more ossification centers and the acromion process.1 The acromion consists of 4 different ossification centers, which appear by 14 years of age and fuse by age 25 years. The 4 ossification centers are the basi-acromion, meta-acromion, mesoacromion, and pre-acromion (Figure 1). Formation of an os acromiale occurs most often due to failure of fusion between the meta-acromion and mesoacromion. Os acromiale appears to occur in approximately 8% of the population, according to cadaveric studies.2 This anatomic variant occurs more commonly in African-Americans than Caucasians, and shows a preponderance for males over females.3
Plain radiographs are usually adequate for diagnosis. Axillary views are most sensitive for detection, which can be difficult to see on anteroposterior radiographs.4 In os acromiale, the unfused segment is connected to the acromioclavicular joint and the coracoid, which can lead to motion of the segment and impingement of the rotator cuff.2-4 Patients frequently experience localized tenderness and symptomatic pain with signs and symptoms of impingement. Rotator cuff tears may occur secondary to chronic impingement.5
Various forms of repair have been described. A recent meta-analysis showed that internal fixation (60%) was the most common surgical technique reported, followed by excision (27%) and acromioplasty (13%).6 Rotator cuff repair is a common concurrent surgical procedure.7-11 The available literature favors internal fixation through an open technique with or without bone grafting.5,7,8,12-15 Various forms of fixation have been presented in the literature, including Kirschner wire fixation, cannulated screw fixation alone, cannulated screw fixation with FiberWire Suture (Arthrex), and cannulated screw fixation with a stainless steel wire tension band technique. Based on the results of the meta-analysis, surgical fixation with cannulated screws has been shown to lead to a significantly greater rate of radiographic healing (23/24 patients) compared to Kirschner wire fixation (31/49 patients).6 Further, radiographic healing is significantly associated with improved clinical outcomes.12 Removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation cases (88%; 43/49) compared to cannulated screw fixation cases (38%; 9/24). However, hardware issues may also be encountered with screw fixation, with 1 case series reporting a 25% rate of hardware complication.16 The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
The patient is a 19-year-old right-hand-dominant woman who injured her right shoulder while diving into the bleachers during a volleyball game 4 years prior to presentation. She suffered a direct blow to her shoulder and immediately became symptomatic. She underwent a long period of nonoperative management, which included physical therapy, strengthening, nonsteroidal anti-inflammatory drug (NSAID) therapy, and narcotic pain medications. Her primary complaints upon presentation were pain with lifting, as well as mechanical symptoms. On examination, the patient had moderate tenderness directly over the acromion. She also had evidence of mild impingement symptoms. Plain radiographs revealed a mesoacromial-type os acromiale clearly seen on the axillary lateral film (Figure 2). She underwent magnetic resonance imaging, which suggested rotator cuff tendinosis and evidence of edema at the os acromiale site. She underwent a diagnostic injection directly into the site of maximal tenderness at the os, which provided complete transient relief of her pain. Despite the transient pain relief, the patient continued to be symptomatic after the local anesthetic effect wore off. Surgical options were then discussed with the patient.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
A standard diagnostic shoulder arthroscopy was performed using anterior, posterior and direct lateral portals. The rotator cuff was evaluated, and no evidence of a tear was found. The undersurface of the acromion was exposed, and the os acromiale was identified arthroscopically (Figure 3). This was found to be unstable under direct digital pressure.
We then elected to repair the unstable fibrous os acromiale (Figures 4A-4D). The fibrous nonunion was first debrided to bleeding bone with a 4.0-mm round burr aligned with the os using the direct lateral portal (Smith & Nephew Endoscopy). Through the anterior portal, two AcutrakTM guide wires (Acumed) were placed under arthroscopic visualization from the anterior margin of the acromion, across the os site, and into the posterior acromion. A 1-cm counter incision was made at the level of the posterior acromion to allow confirmation of the guide wire position and to permit placement of a large, pointed reduction clamp, used to reduce the mesoacromial fragment to the stable portion of the acromion. The calibrated, cannulated drill bit was passed over each guide wire to a depth of 34 mm, according to standard technique, and viewed arthroscopically from the subacromial space. Two 34-mm AcutrakTM cannulated headless compression screws (Acumed) were then placed across the defect. Direct arthroscopic visualization confirmed reduction and complete intraosseous placement of the screws (Figure 5). Screw position was also assessed with image intensification. Fluoroscopic views showed the repair to be stable when the shoulder was taken through range of motion. The os site was never exposed directly through an incision. The surgery was performed on an outpatient basis.
POSTOPERATIVE COURSE
The patient was maintained in a sling and small abduction pillow (Ultrasling IIITM, DonJoy). She was kept non-weight-bearing but was permitted unrestricted motion through the elbow, wrist, and hand for the first 6 weeks. She was permitted supine passive external rotation of the shoulder to 30° and forward flexion to 45° for the first 2 weeks, and 90° through 6 weeks. At her initial postoperative visit 2 weeks later, she noted minimal pain in the shoulder, much improved from her preoperative pain. She was no longer taking any pain medicine, including NSAIDs. Radiographs showed no change in fixation.
At her second visit (6 weeks), she was completely pain free. Clinical examination showed no tenderness at the acromion, healed incisions, and pain-free passive ROM. Radiographs demonstrated early evidence of consolidation and no sign of fixation failure (Figures 6-8). Her Single Assessment Numeric Evaluation (SANE) score was 85%, and her Simple Shoulder Test (SST) score was 3/12. She was permitted to discontinue the sling, to begin using the arm actively at the side, and progress with unloaded use above shoulder height over the next 6 weeks.
She was seen in follow-up at 4 months, where she was found to have no pain but had not yet returned to sports. At her 6-month follow-up, she showed continued improvement with no limitation of activity. At 1-year follow-up, her SANE score improved from 85% at 6 weeks postoperatively to 100%, and her SST improved from 3/12 at 6 weeks to 12/12. She demonstrated full function of her shoulder with no evidence of hardware loosening. At that time, her os acromiale had completely fused radiographically.
Continue to: DISCUSSION...
DISCUSSION
A variety of methods for the management of os acromiale have been described in the literature. Internal fixation is reported as the most common surgical technique, followed by excision and acromioplasty.6 Surgical fixation with cannulated screws is effective at achieving radiographic union.5,9,12,13,15
Excision is also an option in cases where there is a symptomatic pre-acromion with a relatively small fragment. In the case of a larger fragment, techniques that preserve the vascularity of the os acromiale appear more likely to be successful than excision.17 While excision can be performed arthroscopically to preserve the blood supply, a recent report showed that 35% of patients still had residual pain.18 Another study suggests that protecting the vascular supply with an arthroscopic technique would be a better option to promote healing to union.19
Given that removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation (88%; 43/49) than after cannulated screw fixation (38%; 9/24),6 and given that significant hardware complications can arise from screw tips,16 we chose headless, cannulated Acutrak compression screws for arthroscopic-assisted fixation. Performing the operation arthroscopically minimized soft-tissue violation, allowing us to directly visualize the reduction and also allowing confirmation that the screws were not at risk for impingement of the rotator cuff. The tapered nature of the Acutrak screws allowed for excellent compression at the reduction site without a prominent screw head.
CONCLUSION
Arthroscopic management of the symptomatic os acromiale has been documented in the literature. Cannulated screw fixation has shown to lead to a higher rate of radiographic union than Kirschner wire fixation. Arthroscopically guided placement of headless, cannulated compression screw fixation may be a viable repair alternative in the management of the symptomatic os acromiale with less concern for symptomatic hardware.6,20-27
ABSTRACT
Os acromiale is a failure of fusion between 1 or more ossification centers of the scapula and the acromion process. Pain can be caused by motion and impingement of the unfused segment. Several methods for the management of os acromiale have been described. Internal fixation is the most common surgical technique, followed by excision and acromioplasty. We present a novel technique for treatment of symptomatic os acromiale using arthroscopically-guided headless compression screws. This is a viable technique in the management of symptomatic os acromiale due to preservation of the periosteal blood supply and less concern for symptomatic hardware.
Continue to: Os acromiale results from a failure of...
Os acromiale results from a failure of fusion between 1 or more ossification centers and the acromion process.1 The acromion consists of 4 different ossification centers, which appear by 14 years of age and fuse by age 25 years. The 4 ossification centers are the basi-acromion, meta-acromion, mesoacromion, and pre-acromion (Figure 1). Formation of an os acromiale occurs most often due to failure of fusion between the meta-acromion and mesoacromion. Os acromiale appears to occur in approximately 8% of the population, according to cadaveric studies.2 This anatomic variant occurs more commonly in African-Americans than Caucasians, and shows a preponderance for males over females.3
Plain radiographs are usually adequate for diagnosis. Axillary views are most sensitive for detection, which can be difficult to see on anteroposterior radiographs.4 In os acromiale, the unfused segment is connected to the acromioclavicular joint and the coracoid, which can lead to motion of the segment and impingement of the rotator cuff.2-4 Patients frequently experience localized tenderness and symptomatic pain with signs and symptoms of impingement. Rotator cuff tears may occur secondary to chronic impingement.5
Various forms of repair have been described. A recent meta-analysis showed that internal fixation (60%) was the most common surgical technique reported, followed by excision (27%) and acromioplasty (13%).6 Rotator cuff repair is a common concurrent surgical procedure.7-11 The available literature favors internal fixation through an open technique with or without bone grafting.5,7,8,12-15 Various forms of fixation have been presented in the literature, including Kirschner wire fixation, cannulated screw fixation alone, cannulated screw fixation with FiberWire Suture (Arthrex), and cannulated screw fixation with a stainless steel wire tension band technique. Based on the results of the meta-analysis, surgical fixation with cannulated screws has been shown to lead to a significantly greater rate of radiographic healing (23/24 patients) compared to Kirschner wire fixation (31/49 patients).6 Further, radiographic healing is significantly associated with improved clinical outcomes.12 Removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation cases (88%; 43/49) compared to cannulated screw fixation cases (38%; 9/24). However, hardware issues may also be encountered with screw fixation, with 1 case series reporting a 25% rate of hardware complication.16 The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
The patient is a 19-year-old right-hand-dominant woman who injured her right shoulder while diving into the bleachers during a volleyball game 4 years prior to presentation. She suffered a direct blow to her shoulder and immediately became symptomatic. She underwent a long period of nonoperative management, which included physical therapy, strengthening, nonsteroidal anti-inflammatory drug (NSAID) therapy, and narcotic pain medications. Her primary complaints upon presentation were pain with lifting, as well as mechanical symptoms. On examination, the patient had moderate tenderness directly over the acromion. She also had evidence of mild impingement symptoms. Plain radiographs revealed a mesoacromial-type os acromiale clearly seen on the axillary lateral film (Figure 2). She underwent magnetic resonance imaging, which suggested rotator cuff tendinosis and evidence of edema at the os acromiale site. She underwent a diagnostic injection directly into the site of maximal tenderness at the os, which provided complete transient relief of her pain. Despite the transient pain relief, the patient continued to be symptomatic after the local anesthetic effect wore off. Surgical options were then discussed with the patient.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
A standard diagnostic shoulder arthroscopy was performed using anterior, posterior and direct lateral portals. The rotator cuff was evaluated, and no evidence of a tear was found. The undersurface of the acromion was exposed, and the os acromiale was identified arthroscopically (Figure 3). This was found to be unstable under direct digital pressure.
We then elected to repair the unstable fibrous os acromiale (Figures 4A-4D). The fibrous nonunion was first debrided to bleeding bone with a 4.0-mm round burr aligned with the os using the direct lateral portal (Smith & Nephew Endoscopy). Through the anterior portal, two AcutrakTM guide wires (Acumed) were placed under arthroscopic visualization from the anterior margin of the acromion, across the os site, and into the posterior acromion. A 1-cm counter incision was made at the level of the posterior acromion to allow confirmation of the guide wire position and to permit placement of a large, pointed reduction clamp, used to reduce the mesoacromial fragment to the stable portion of the acromion. The calibrated, cannulated drill bit was passed over each guide wire to a depth of 34 mm, according to standard technique, and viewed arthroscopically from the subacromial space. Two 34-mm AcutrakTM cannulated headless compression screws (Acumed) were then placed across the defect. Direct arthroscopic visualization confirmed reduction and complete intraosseous placement of the screws (Figure 5). Screw position was also assessed with image intensification. Fluoroscopic views showed the repair to be stable when the shoulder was taken through range of motion. The os site was never exposed directly through an incision. The surgery was performed on an outpatient basis.
POSTOPERATIVE COURSE
The patient was maintained in a sling and small abduction pillow (Ultrasling IIITM, DonJoy). She was kept non-weight-bearing but was permitted unrestricted motion through the elbow, wrist, and hand for the first 6 weeks. She was permitted supine passive external rotation of the shoulder to 30° and forward flexion to 45° for the first 2 weeks, and 90° through 6 weeks. At her initial postoperative visit 2 weeks later, she noted minimal pain in the shoulder, much improved from her preoperative pain. She was no longer taking any pain medicine, including NSAIDs. Radiographs showed no change in fixation.
At her second visit (6 weeks), she was completely pain free. Clinical examination showed no tenderness at the acromion, healed incisions, and pain-free passive ROM. Radiographs demonstrated early evidence of consolidation and no sign of fixation failure (Figures 6-8). Her Single Assessment Numeric Evaluation (SANE) score was 85%, and her Simple Shoulder Test (SST) score was 3/12. She was permitted to discontinue the sling, to begin using the arm actively at the side, and progress with unloaded use above shoulder height over the next 6 weeks.
She was seen in follow-up at 4 months, where she was found to have no pain but had not yet returned to sports. At her 6-month follow-up, she showed continued improvement with no limitation of activity. At 1-year follow-up, her SANE score improved from 85% at 6 weeks postoperatively to 100%, and her SST improved from 3/12 at 6 weeks to 12/12. She demonstrated full function of her shoulder with no evidence of hardware loosening. At that time, her os acromiale had completely fused radiographically.
Continue to: DISCUSSION...
DISCUSSION
A variety of methods for the management of os acromiale have been described in the literature. Internal fixation is reported as the most common surgical technique, followed by excision and acromioplasty.6 Surgical fixation with cannulated screws is effective at achieving radiographic union.5,9,12,13,15
Excision is also an option in cases where there is a symptomatic pre-acromion with a relatively small fragment. In the case of a larger fragment, techniques that preserve the vascularity of the os acromiale appear more likely to be successful than excision.17 While excision can be performed arthroscopically to preserve the blood supply, a recent report showed that 35% of patients still had residual pain.18 Another study suggests that protecting the vascular supply with an arthroscopic technique would be a better option to promote healing to union.19
Given that removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation (88%; 43/49) than after cannulated screw fixation (38%; 9/24),6 and given that significant hardware complications can arise from screw tips,16 we chose headless, cannulated Acutrak compression screws for arthroscopic-assisted fixation. Performing the operation arthroscopically minimized soft-tissue violation, allowing us to directly visualize the reduction and also allowing confirmation that the screws were not at risk for impingement of the rotator cuff. The tapered nature of the Acutrak screws allowed for excellent compression at the reduction site without a prominent screw head.
CONCLUSION
Arthroscopic management of the symptomatic os acromiale has been documented in the literature. Cannulated screw fixation has shown to lead to a higher rate of radiographic union than Kirschner wire fixation. Arthroscopically guided placement of headless, cannulated compression screw fixation may be a viable repair alternative in the management of the symptomatic os acromiale with less concern for symptomatic hardware.6,20-27
1. Barbier O, Block D, Dezaly C, Sirveaux F, Mole D. Os acromiale, a cause of shoulder pain, not to be overlooked. Orthop Traumatol Surg Res. 2013;99(4):465-472. doi: 10.1016/j.otsr.2012.10.020.
2. Swain RA, Wilson FD, Harsha DM. The os acromiale: another cause of impingement. Med Sci Sports Exerc. 1996;28(12):1459-1462. doi:10.1097/00005768-199612000-00003.
3. Kurtz CA, Humble BJ, Rodosky MW, Sekiya JK. Symptomatic os acromiale. J Am Acad Orthop Surg. 2006;14(1):12-19. doi:10.5435/00124635-200601000-00004.
4. Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447-457.
5. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80(9):1320-1326. doi:10.2106/00004623-199809000-00011.
6. Harris JD, Griesser MJ, Jones GL. Systematic review of the surgical treatment for symptomatic os acromiale. Int J Shoulder Surg. 2011;5(1):9-16. doi:10.4103/0973-6042.80461.
7. Abboud JA, Silverberg D, Pepe M, et al. Surgical treatment of os acromiale with and without associated rotator cuff tears. J Shoulder Elbow Surg. 2006;15(3):265-270. doi:10.1016/j.jse.2005.08.024.
8. Boehm TD, Matzer M, Brazda D, Gohlke FE. Os acromiale associated with tear of the rotator cuff treated operatively Review of 33 patients. J Bone Joint Surg Br. 2003;85(4):545-549. doi:10.1302/0301-620X.85B4.13634.
9. Boehm TD, Rolf O, Martetschlaeger F, Kenn W, Gohlke F. Rotator cuff tears associated with os acromiale. Acta Orthop. 2005;76(2):241-244. doi:10.1080/00016470510030643.
10. Barbiera F, Bellissima G, Iovane A, De Maria M. OS acromiale producing rotator cuff impingement and rupture. A case report. Radiol Med. 2002;104(4):359-362.
11. Neer CS 2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
12. Hertel R, Windisch W, Schuster A, Ballmer FT. Transacromial approach to obtain fusion of unstable os acromiale. J Shoulder Elbow Surg. 1998;7(6):606-609. doi:10.1016/S1058-2746(98)90008-8.
13. Ozbaydar MU, Keriş I, Altun M, Yalaman O. Results of the surgical treatment for symptomatic mesoacromion. Acta Orthop Traumatol Turc. 2006;40(2):123-129.
14. Satterlee CC. Successful osteosynthesis of an unstable mesoacromion in 6 shoulders: a new technique. J Shoulder Elbow Surg. 1999;8(2):125-129. doi:10.1016/S1058-2746(99)90004-6.
15. Ryu RK, Fan RS, Dunbar WHt. The treatment of symptomatic os acromiale. Orthopedics. 1999;22(3):325-328.
16. Atoun E, van Tongel A, Narvani A, Rath E, Sforza G, Levy O. Arthroscopically assisted internal fixation of the symptomatic unstable os acromiale with absorbable screws. J Shoulder Elbow Surg. 2012;21(12):1740-1745. doi:10.1016/j.jse.2011.12.011.
17. Johnston PS, Paxton ES, Gordon V, Kraeutler MJ, Abboud JA, Williams GR. Os acromiale: a review and an introduction of a new surgical technique for management. Orthop Clin North Am. 2013;44(4):635-644. doi:10.1016/j.ocl.2013.06.015.
18. Campbell PT, Nizlan NM, Skirving AP. Arthroscopic excision of os acromiale: effects on deltoid function and strength. Orthopedics. 2012;35(11):e1601-e1605. doi:10.3928/01477447-20121023-16.
19. Yepes H, Al-Hibshi A, Tang M, Morris SF, Stanish WD. Vascular anatomy of the subacromial space: a map of bleeding points for the arthroscopic surgeon. Arthroscopy. 2007;23(9):978-984. doi:10.1016/j.arthro.2007.03.093.
20. Kummer FJ, Van Gelderen J, Meislin RJ. Two-screw, arthroscopic fixation of os acromiale compared to a similar, open procedure incorporating a tension band: a laboratory study. Shoulder Elbow. 2011;3(2):85-87. doi:10.1111/j.1758-5740.2011.00115.x.
21. Wright RW, Heller MA, Quick DC, Buss DD. Arthroscopic decompression for impingement syndrome secondary to an unstable os acromiale. Arthroscopy. 2000;16(6):595-599. doi:10.1053/jars.2000.9239.
22. Edelson JG, Zuckerman J, Hershkovitz I. Os acromiale: anatomy and surgical implications. J Bone Joint Surg Br. 1993;75(4):551-555. doi:10.1302/0301-620X.75B4.8331108.
23. Fery A, Sommelet J. Os acromiale: significance--diagnosis--pathology Apropos of 28 cases including 2 with fracture separation. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(2):160-172.
24. Lee DH. The double-density sign: a radiographic finding suggestive of an os acromiale. J Bone Joint Surg Am. 2004;86-A(12):2666-2670. doi:10.2106/00004623-200412000-00012.
25. Ortiguera CJ, Buss DD. Surgical management of the symptomatic os acromiale. J Shoulder Elbow Surg. 2002;11(5):521-528. doi:10.1067/mse.2002.122227.
26. Peckett WR, Gunther SB, Harper GD, Hughes JS, Sonnabend DH. Internal fixation of symptomatic os acromiale: a series of twenty-six cases. J Shoulder Elbow Surg. 2004;13(4):381-385. doi:10.1016/S1058274604000400.
27. Sahajpal D, Strauss EJ, Ishak C, Keyes JM, Joseph G, Jazrawi LM. Surgical management of os acromiale: a case report and review of the literature. Bull NYU Hosp Jt Dis. 2007;65(4):312-316.
1. Barbier O, Block D, Dezaly C, Sirveaux F, Mole D. Os acromiale, a cause of shoulder pain, not to be overlooked. Orthop Traumatol Surg Res. 2013;99(4):465-472. doi: 10.1016/j.otsr.2012.10.020.
2. Swain RA, Wilson FD, Harsha DM. The os acromiale: another cause of impingement. Med Sci Sports Exerc. 1996;28(12):1459-1462. doi:10.1097/00005768-199612000-00003.
3. Kurtz CA, Humble BJ, Rodosky MW, Sekiya JK. Symptomatic os acromiale. J Am Acad Orthop Surg. 2006;14(1):12-19. doi:10.5435/00124635-200601000-00004.
4. Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447-457.
5. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80(9):1320-1326. doi:10.2106/00004623-199809000-00011.
6. Harris JD, Griesser MJ, Jones GL. Systematic review of the surgical treatment for symptomatic os acromiale. Int J Shoulder Surg. 2011;5(1):9-16. doi:10.4103/0973-6042.80461.
7. Abboud JA, Silverberg D, Pepe M, et al. Surgical treatment of os acromiale with and without associated rotator cuff tears. J Shoulder Elbow Surg. 2006;15(3):265-270. doi:10.1016/j.jse.2005.08.024.
8. Boehm TD, Matzer M, Brazda D, Gohlke FE. Os acromiale associated with tear of the rotator cuff treated operatively Review of 33 patients. J Bone Joint Surg Br. 2003;85(4):545-549. doi:10.1302/0301-620X.85B4.13634.
9. Boehm TD, Rolf O, Martetschlaeger F, Kenn W, Gohlke F. Rotator cuff tears associated with os acromiale. Acta Orthop. 2005;76(2):241-244. doi:10.1080/00016470510030643.
10. Barbiera F, Bellissima G, Iovane A, De Maria M. OS acromiale producing rotator cuff impingement and rupture. A case report. Radiol Med. 2002;104(4):359-362.
11. Neer CS 2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
12. Hertel R, Windisch W, Schuster A, Ballmer FT. Transacromial approach to obtain fusion of unstable os acromiale. J Shoulder Elbow Surg. 1998;7(6):606-609. doi:10.1016/S1058-2746(98)90008-8.
13. Ozbaydar MU, Keriş I, Altun M, Yalaman O. Results of the surgical treatment for symptomatic mesoacromion. Acta Orthop Traumatol Turc. 2006;40(2):123-129.
14. Satterlee CC. Successful osteosynthesis of an unstable mesoacromion in 6 shoulders: a new technique. J Shoulder Elbow Surg. 1999;8(2):125-129. doi:10.1016/S1058-2746(99)90004-6.
15. Ryu RK, Fan RS, Dunbar WHt. The treatment of symptomatic os acromiale. Orthopedics. 1999;22(3):325-328.
16. Atoun E, van Tongel A, Narvani A, Rath E, Sforza G, Levy O. Arthroscopically assisted internal fixation of the symptomatic unstable os acromiale with absorbable screws. J Shoulder Elbow Surg. 2012;21(12):1740-1745. doi:10.1016/j.jse.2011.12.011.
17. Johnston PS, Paxton ES, Gordon V, Kraeutler MJ, Abboud JA, Williams GR. Os acromiale: a review and an introduction of a new surgical technique for management. Orthop Clin North Am. 2013;44(4):635-644. doi:10.1016/j.ocl.2013.06.015.
18. Campbell PT, Nizlan NM, Skirving AP. Arthroscopic excision of os acromiale: effects on deltoid function and strength. Orthopedics. 2012;35(11):e1601-e1605. doi:10.3928/01477447-20121023-16.
19. Yepes H, Al-Hibshi A, Tang M, Morris SF, Stanish WD. Vascular anatomy of the subacromial space: a map of bleeding points for the arthroscopic surgeon. Arthroscopy. 2007;23(9):978-984. doi:10.1016/j.arthro.2007.03.093.
20. Kummer FJ, Van Gelderen J, Meislin RJ. Two-screw, arthroscopic fixation of os acromiale compared to a similar, open procedure incorporating a tension band: a laboratory study. Shoulder Elbow. 2011;3(2):85-87. doi:10.1111/j.1758-5740.2011.00115.x.
21. Wright RW, Heller MA, Quick DC, Buss DD. Arthroscopic decompression for impingement syndrome secondary to an unstable os acromiale. Arthroscopy. 2000;16(6):595-599. doi:10.1053/jars.2000.9239.
22. Edelson JG, Zuckerman J, Hershkovitz I. Os acromiale: anatomy and surgical implications. J Bone Joint Surg Br. 1993;75(4):551-555. doi:10.1302/0301-620X.75B4.8331108.
23. Fery A, Sommelet J. Os acromiale: significance--diagnosis--pathology Apropos of 28 cases including 2 with fracture separation. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(2):160-172.
24. Lee DH. The double-density sign: a radiographic finding suggestive of an os acromiale. J Bone Joint Surg Am. 2004;86-A(12):2666-2670. doi:10.2106/00004623-200412000-00012.
25. Ortiguera CJ, Buss DD. Surgical management of the symptomatic os acromiale. J Shoulder Elbow Surg. 2002;11(5):521-528. doi:10.1067/mse.2002.122227.
26. Peckett WR, Gunther SB, Harper GD, Hughes JS, Sonnabend DH. Internal fixation of symptomatic os acromiale: a series of twenty-six cases. J Shoulder Elbow Surg. 2004;13(4):381-385. doi:10.1016/S1058274604000400.
27. Sahajpal D, Strauss EJ, Ishak C, Keyes JM, Joseph G, Jazrawi LM. Surgical management of os acromiale: a case report and review of the literature. Bull NYU Hosp Jt Dis. 2007;65(4):312-316.
TAKE-HOME POINTS
- Os acromiale is a failure of acromial ossification centers to fuse, and occurs in 8% of the population.
- Symptomatic os acromiale can be treated with repair, or sometimes excision or acromioplasty.
- Repair preserves the anterior deltoid origin and can result in less pain than excision of the fragment.
- Repair of larger fragments can be completed with cannulated screws to reliably achieve union.
- The arthroscope-assisted repair technique described in this article preserves vascularity and can reduce the risk of hardware-related complaints.
Amantadine-Induced Livedo Reticularis in a Child Treated Off Label for Neurobehavioral Disorders
Livedo reticularis (LR) is a common dermatologic finding consisting of diffuse, reticulated, violaceous patches. It often is a benign physical finding known as cutis marmorata; however, LR can be associated with other medical conditions as well as with the use of some medications.1,2 Amantadine is a common cause of LR in Parkinson disease patients.3,4 We present a rare case of amantadine-induced LR in a pediatric patient and highlight the off-label use of this medication in children.
Case Report
An 8-year-old boy presented with a diffuse rash on the trunk, arms, and legs of 9 months’ duration. The patient denied any associated symptoms as well as alleviating or exacerbating factors. He also denied any changes with temperature. He had no recent international travel and no prior drug allergies. His medical history was remarkable for attention deficit hyperactivity disorder (ADHD), bipolar disorder, and autism spectrum disorder. His previously prescribed medications included atomoxetine, quetiapine, and valproic acid. The only new medication that had been started within the last year was amantadine. Physical examination revealed a diffuse, reticulated, erythematous to violaceous, blanching rash that was most notable on the legs (Figure 1A) but also was present on the trunk (Figure 1B) and arms (Figure 1C). The clinical examination was consistent with LR, which was presumed to be secondary to amantadine use. Given the multiple psychiatric diagnoses and medication history in this young patient, a consultation with child psychiatry was facilitated. His medications and diagnosis were reviewed, and amantadine was discontinued. At a follow-up visit 5 months later, the patient’s LR had improved (Figure 2).
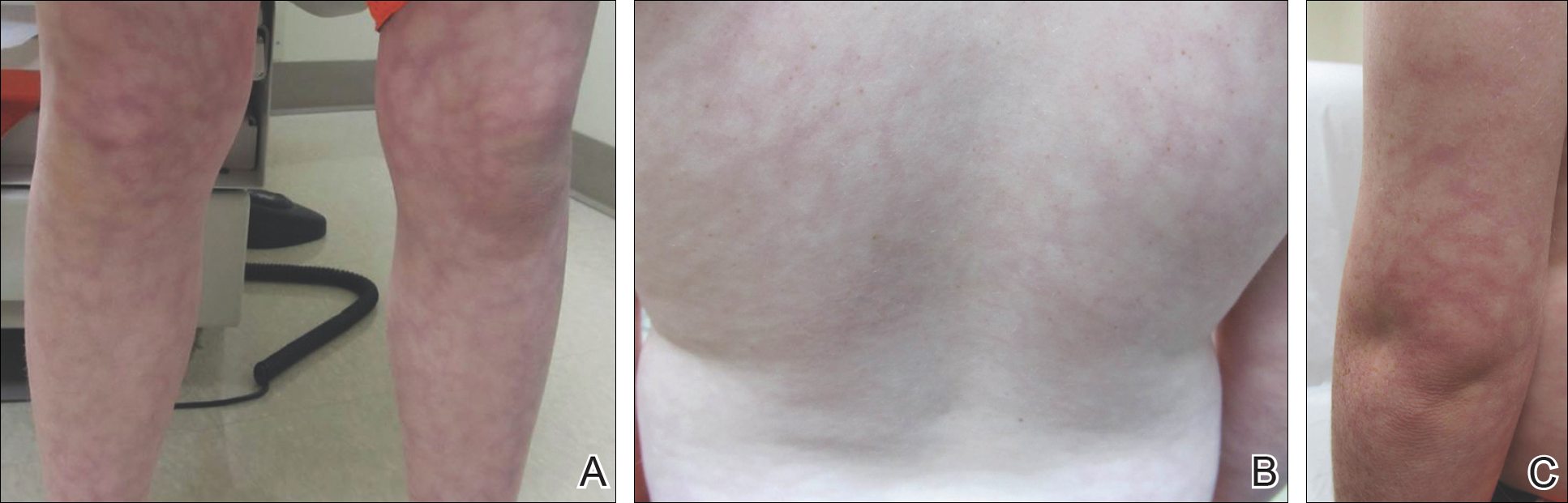
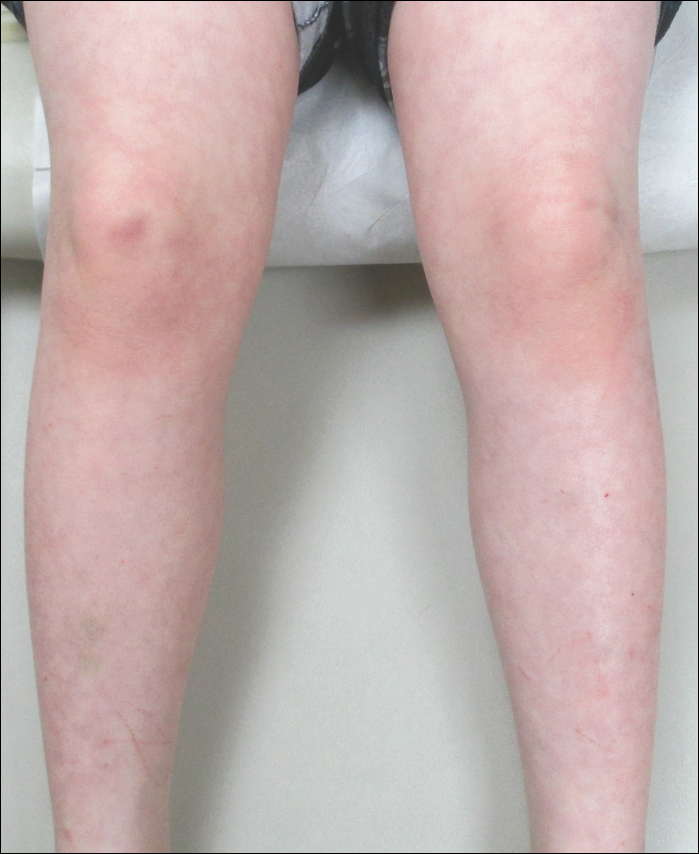
Comment
Amantadine has a well-documented association with LR in patients with Parkinson disease,3,4 which has been reported in up to 40% of those taking amantadine.2 More recently, amantadine has been used off label to treat neurobehavioral disorders in children due to beneficial effects including improvement in attention and concentration, distractibility, and fatigue.5 Our patient was being treated off label with amantadine for ADHD and bipolar disorder. Amantadine acts as a noncompetitive antagonist of the N-methyl-D-aspartate receptor, enhancing dopamine release to reduce symptoms of ADHD.5,6 Additionally, amantadine can cause a depletion of catecholamines in the peripheral nerve terminals, which may lead to dilatation of dermal vessels.4,6 This sequence of events has been proposed as a possible mechanism contributing to amantadine-induced LR, though the pathophysiology is not fully understood.1,3,4
Our case of LR likely was induced by amantadine given the temporal relationship between initiation of the medication, onset of the rash, and the considerable improvement of the rash upon discontinuation of amantadine. Barrera and Browning6 reported another case of amantadine-induced LR in a pediatric patient. Because amantadine is increasingly being used off label to treat childhood neurobehavioral disorders, amantadine-induced LR may become more prevalent in patients who do not have Parkinson disease; therefore, physicians who treat pediatric patients must be aware of this side effect.5
- Quaresma MV, Gomes-Dias AC, Serruya A, et al. Amantadine-induced livedo reticularis: a case report. An Bras Dermatol. 2015;90:745-747.
- Gibbs MB, English JC, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019.
- Silva SB, Miot HA. Case for diagnosis. amantadine-induced livedo reticularis. An Bras Dermatol. 2012;87:319-321.
- Vollum DI, Parkes JD, Doyle D. Livedo reticularis during amantadine treatment. Br Med J. 1971;2:627-628.
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55-60.
- Barrera F, Browning JC. Likely amantadine-induced livedo reticularis in a child. Pediatr Dermatol. 2012;29:329-330.
Livedo reticularis (LR) is a common dermatologic finding consisting of diffuse, reticulated, violaceous patches. It often is a benign physical finding known as cutis marmorata; however, LR can be associated with other medical conditions as well as with the use of some medications.1,2 Amantadine is a common cause of LR in Parkinson disease patients.3,4 We present a rare case of amantadine-induced LR in a pediatric patient and highlight the off-label use of this medication in children.
Case Report
An 8-year-old boy presented with a diffuse rash on the trunk, arms, and legs of 9 months’ duration. The patient denied any associated symptoms as well as alleviating or exacerbating factors. He also denied any changes with temperature. He had no recent international travel and no prior drug allergies. His medical history was remarkable for attention deficit hyperactivity disorder (ADHD), bipolar disorder, and autism spectrum disorder. His previously prescribed medications included atomoxetine, quetiapine, and valproic acid. The only new medication that had been started within the last year was amantadine. Physical examination revealed a diffuse, reticulated, erythematous to violaceous, blanching rash that was most notable on the legs (Figure 1A) but also was present on the trunk (Figure 1B) and arms (Figure 1C). The clinical examination was consistent with LR, which was presumed to be secondary to amantadine use. Given the multiple psychiatric diagnoses and medication history in this young patient, a consultation with child psychiatry was facilitated. His medications and diagnosis were reviewed, and amantadine was discontinued. At a follow-up visit 5 months later, the patient’s LR had improved (Figure 2).


Comment
Amantadine has a well-documented association with LR in patients with Parkinson disease,3,4 which has been reported in up to 40% of those taking amantadine.2 More recently, amantadine has been used off label to treat neurobehavioral disorders in children due to beneficial effects including improvement in attention and concentration, distractibility, and fatigue.5 Our patient was being treated off label with amantadine for ADHD and bipolar disorder. Amantadine acts as a noncompetitive antagonist of the N-methyl-D-aspartate receptor, enhancing dopamine release to reduce symptoms of ADHD.5,6 Additionally, amantadine can cause a depletion of catecholamines in the peripheral nerve terminals, which may lead to dilatation of dermal vessels.4,6 This sequence of events has been proposed as a possible mechanism contributing to amantadine-induced LR, though the pathophysiology is not fully understood.1,3,4
Our case of LR likely was induced by amantadine given the temporal relationship between initiation of the medication, onset of the rash, and the considerable improvement of the rash upon discontinuation of amantadine. Barrera and Browning6 reported another case of amantadine-induced LR in a pediatric patient. Because amantadine is increasingly being used off label to treat childhood neurobehavioral disorders, amantadine-induced LR may become more prevalent in patients who do not have Parkinson disease; therefore, physicians who treat pediatric patients must be aware of this side effect.5
Livedo reticularis (LR) is a common dermatologic finding consisting of diffuse, reticulated, violaceous patches. It often is a benign physical finding known as cutis marmorata; however, LR can be associated with other medical conditions as well as with the use of some medications.1,2 Amantadine is a common cause of LR in Parkinson disease patients.3,4 We present a rare case of amantadine-induced LR in a pediatric patient and highlight the off-label use of this medication in children.
Case Report
An 8-year-old boy presented with a diffuse rash on the trunk, arms, and legs of 9 months’ duration. The patient denied any associated symptoms as well as alleviating or exacerbating factors. He also denied any changes with temperature. He had no recent international travel and no prior drug allergies. His medical history was remarkable for attention deficit hyperactivity disorder (ADHD), bipolar disorder, and autism spectrum disorder. His previously prescribed medications included atomoxetine, quetiapine, and valproic acid. The only new medication that had been started within the last year was amantadine. Physical examination revealed a diffuse, reticulated, erythematous to violaceous, blanching rash that was most notable on the legs (Figure 1A) but also was present on the trunk (Figure 1B) and arms (Figure 1C). The clinical examination was consistent with LR, which was presumed to be secondary to amantadine use. Given the multiple psychiatric diagnoses and medication history in this young patient, a consultation with child psychiatry was facilitated. His medications and diagnosis were reviewed, and amantadine was discontinued. At a follow-up visit 5 months later, the patient’s LR had improved (Figure 2).


Comment
Amantadine has a well-documented association with LR in patients with Parkinson disease,3,4 which has been reported in up to 40% of those taking amantadine.2 More recently, amantadine has been used off label to treat neurobehavioral disorders in children due to beneficial effects including improvement in attention and concentration, distractibility, and fatigue.5 Our patient was being treated off label with amantadine for ADHD and bipolar disorder. Amantadine acts as a noncompetitive antagonist of the N-methyl-D-aspartate receptor, enhancing dopamine release to reduce symptoms of ADHD.5,6 Additionally, amantadine can cause a depletion of catecholamines in the peripheral nerve terminals, which may lead to dilatation of dermal vessels.4,6 This sequence of events has been proposed as a possible mechanism contributing to amantadine-induced LR, though the pathophysiology is not fully understood.1,3,4
Our case of LR likely was induced by amantadine given the temporal relationship between initiation of the medication, onset of the rash, and the considerable improvement of the rash upon discontinuation of amantadine. Barrera and Browning6 reported another case of amantadine-induced LR in a pediatric patient. Because amantadine is increasingly being used off label to treat childhood neurobehavioral disorders, amantadine-induced LR may become more prevalent in patients who do not have Parkinson disease; therefore, physicians who treat pediatric patients must be aware of this side effect.5
- Quaresma MV, Gomes-Dias AC, Serruya A, et al. Amantadine-induced livedo reticularis: a case report. An Bras Dermatol. 2015;90:745-747.
- Gibbs MB, English JC, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019.
- Silva SB, Miot HA. Case for diagnosis. amantadine-induced livedo reticularis. An Bras Dermatol. 2012;87:319-321.
- Vollum DI, Parkes JD, Doyle D. Livedo reticularis during amantadine treatment. Br Med J. 1971;2:627-628.
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55-60.
- Barrera F, Browning JC. Likely amantadine-induced livedo reticularis in a child. Pediatr Dermatol. 2012;29:329-330.
- Quaresma MV, Gomes-Dias AC, Serruya A, et al. Amantadine-induced livedo reticularis: a case report. An Bras Dermatol. 2015;90:745-747.
- Gibbs MB, English JC, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019.
- Silva SB, Miot HA. Case for diagnosis. amantadine-induced livedo reticularis. An Bras Dermatol. 2012;87:319-321.
- Vollum DI, Parkes JD, Doyle D. Livedo reticularis during amantadine treatment. Br Med J. 1971;2:627-628.
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55-60.
- Barrera F, Browning JC. Likely amantadine-induced livedo reticularis in a child. Pediatr Dermatol. 2012;29:329-330.
Practice Points
- Amantadine is a generally well-tolerated medication that is more commonly used for off-label treatment of several pediatric neurobehavioral conditions such as attention deficit hyperactivity disorder, autism spectrum disorders, obsessive compulsive disorder, depression, and others.
- Livedo reticularis has known associations with several medications and diseases; however, the most common presentation is cutis marmorata, a benign condition that typically affects newborns.
Nevus of Ota Associated With a Primary Uveal Melanoma and Intracranial Melanoma Metastasis
Nevus of Ota, originally referred to as nevus fusco-caeruleus ophthalmomaxillaris, initially was described in 1939 by Ota and Tanino.1 It is a dermal melanocytic hamartoma arising from incomplete migration of neural crest melanocytes to the epidermis during embryogenesis, resulting in nesting of subtle bands of dendritic melanocytes in the upper dermis. More common in Asians, Native Americans, and females, this hyperpigmented dermatosis most often is unilaterally distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.2 In some patients, nevus of Ota also is associated with ocular, orbital, and leptomeningeal melanocytosis. Approximately 15% of nevi of Ota have an activating guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or G protein subunit alpha 11 (GNAQ) mutation; 85% of uveal melanomas harbor one of these mutations.3 Although uncommon, neoplastic transformation with extension or metastasis to the brain has been reported in patients with nevus of Ota.4
We report the case of a 29-year-old woman with a long-standing history of nevus of Ota who presented acutely with an intracranial melanoma as an extension of a primary uveal melanoma.
Case Report
A 29-year-old woman with a history of a nevus of Ota involving the left inner canthus, eyelids, sclera, and superior malar cheek that had been present since birth presented to the emergency department with an acute onset of severe headache, blurred vision, and vomiting. Computed tomography (CT) and magnetic resonance imaging of the brain revealed a hemorrhagic mass in the left frontal lobe. Subsequent frontal craniotomy and resection revealed an intracranial melanoma.
Two weeks following surgery, the patient underwent magnetic resonance imaging and combined positron emission tomography and CT scans that demonstrated a fluorodeoxyglucose-avid left retro-orbital mass. Histopathology of a biopsy from the left retro-orbital mass that had been obtained intraoperatively demonstrated a pigmented, spindled to epithelioid neoplasm with areas of marked atypia and a high mitotic rate that was compatible with malignant melanoma (Figure 1). Intracranial biopsies were sent for genetic study and were found to harbor GNAQ (Q209P) and BRCA1-associated protein 1 (BAP1)(p.P324fs*11) mutations.
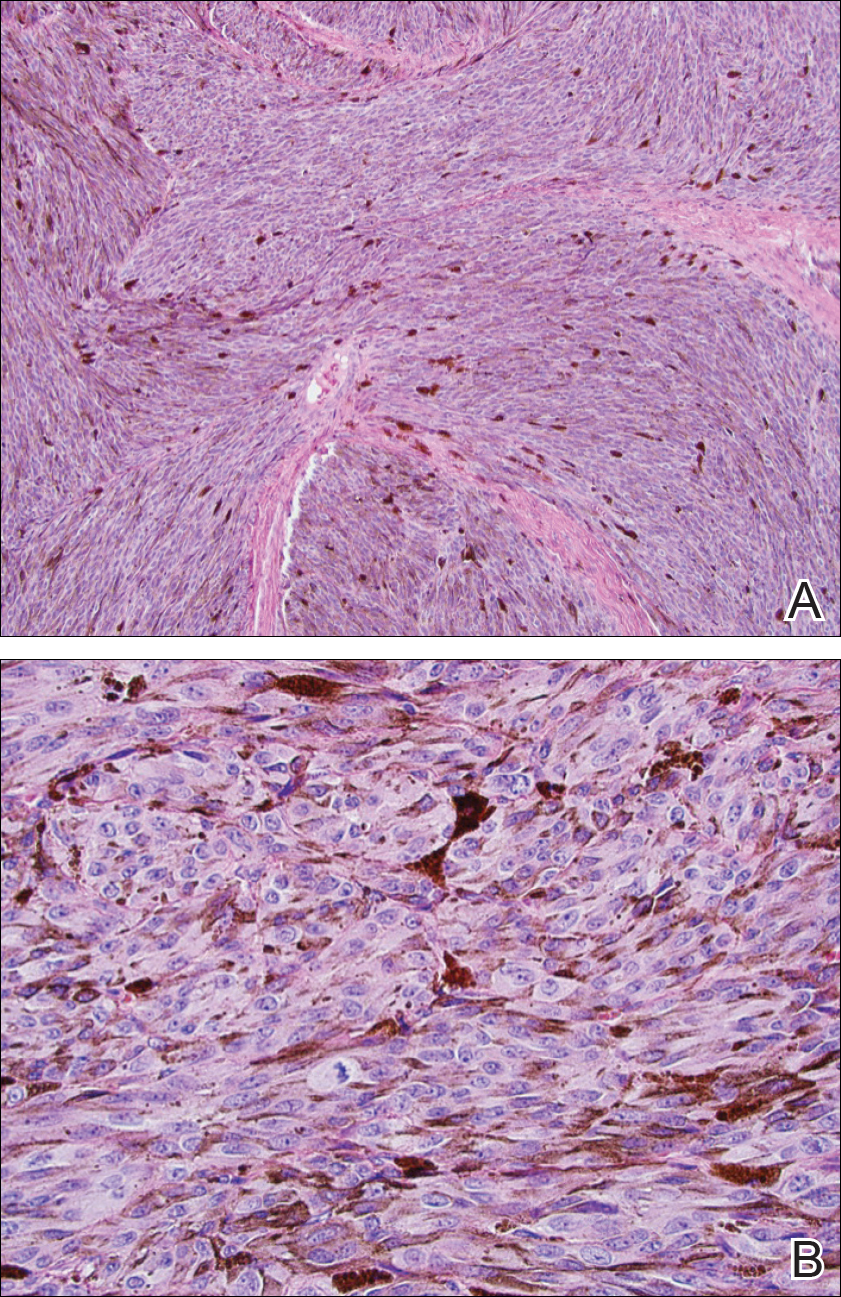
The patient was referred to dermatology by neurosurgery for evaluation of a suspected primary cutaneous melanoma.

The patient entered a clinical trial at an outside institution several weeks after initial presentation to our institution for treatment with a mitogen-activated protein kinase MEK1 inhibitor as well as radiation therapy. The patient was lost to follow-up.
Comment
It has been demonstrated that homozygous loss of BAP1, located on the chromosome 3p21.1 locus, allows for progression to metastatic disease in uveal melanoma. The BAP1 gene codes for ubiquitin carboxyl-terminal hydrolase 7, which is involved in the removal of ubiquitin from proteins. This enzyme binds to BRCA1 (BRCA1, DNA repair associated) via the RING (Really Interesting New Gene) finger domain and acts as a tumor suppressor.5 Biallelic BAP1 mutations allow the transition to malignancy in concert with other mutations, such as GNAQ. Identification of a BAP1 mutation may serve as a valuable diagnostic and future therapeutic target in uveal melanoma.
Currently, there are no drugs that directly target mutated GNA11 and GNAQ proteins. Because aberrant GNA11 and GNAQ proteins activate MEK1, several MEK1 inhibitors are being tested with the hope of achieving indirect suppression of GNA11/GNAQ.6
We present a rare case of BAP1 and GNAQ mutations in intracranial melanoma associated with nevus of Ota. Although the uveal melanoma was not confirmed on histopathology, the clear mention of foci within the eye by ophthalmology, positron emission tomography–CT scan showing a fluorodeoxyglucose-avid left retro-orbital mass, and genetic studies of the intracranial biopsies were highly suggestive of a primary uveal melanoma.
Our case highlights the importance of ongoing ocular screening in patients with nevus of Ota, noting the possibility of malignant transformation. Furthermore, patients with nevus of Ota with ocular involvement may benefit from testing of BAP1 protein expression by immunohistochemistry.7 Identification of BAP1 and GNAQ mutations in patients with nevus of Ota place them at markedly higher risk for malignant melanoma. Therefore, dermatologic evaluation of patients with nevus of Ota should include a thorough review of the patient’s history and skin examination as well as referral for ophthalmologic evaluation.
- Ota M, Tanino H. A variety of nevus, frequently encountered in Japan, nevus fusco-caeruleus ophthalmomaxillaris and its relationship to pigmentary changes in the eye. Tokyo Med J. 1939;63:1243-1244.
- Swann PG, Kwong E. The naevus of Ota. Clin Exp Optom. 2010;93:264-267.
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma [published online November 17, 2010]. N Engl J Med. 2010;363:2191-2199.
- Nitta K, Kashima T, Mayuzumi H, et al. Animal-type malignancy melanoma associated with nevus of Ota in the orbit of a Japanese woman: a case report. Melanoma Res. 2014;24:286-289.
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas [published online November 4, 2010]. Science. 2010;330:1410-1413.
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724-4734.
- Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing [published online July 24, 2010]. Br J Cancer. 2014;111:1373-1380.
Nevus of Ota, originally referred to as nevus fusco-caeruleus ophthalmomaxillaris, initially was described in 1939 by Ota and Tanino.1 It is a dermal melanocytic hamartoma arising from incomplete migration of neural crest melanocytes to the epidermis during embryogenesis, resulting in nesting of subtle bands of dendritic melanocytes in the upper dermis. More common in Asians, Native Americans, and females, this hyperpigmented dermatosis most often is unilaterally distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.2 In some patients, nevus of Ota also is associated with ocular, orbital, and leptomeningeal melanocytosis. Approximately 15% of nevi of Ota have an activating guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or G protein subunit alpha 11 (GNAQ) mutation; 85% of uveal melanomas harbor one of these mutations.3 Although uncommon, neoplastic transformation with extension or metastasis to the brain has been reported in patients with nevus of Ota.4
We report the case of a 29-year-old woman with a long-standing history of nevus of Ota who presented acutely with an intracranial melanoma as an extension of a primary uveal melanoma.
Case Report
A 29-year-old woman with a history of a nevus of Ota involving the left inner canthus, eyelids, sclera, and superior malar cheek that had been present since birth presented to the emergency department with an acute onset of severe headache, blurred vision, and vomiting. Computed tomography (CT) and magnetic resonance imaging of the brain revealed a hemorrhagic mass in the left frontal lobe. Subsequent frontal craniotomy and resection revealed an intracranial melanoma.
Two weeks following surgery, the patient underwent magnetic resonance imaging and combined positron emission tomography and CT scans that demonstrated a fluorodeoxyglucose-avid left retro-orbital mass. Histopathology of a biopsy from the left retro-orbital mass that had been obtained intraoperatively demonstrated a pigmented, spindled to epithelioid neoplasm with areas of marked atypia and a high mitotic rate that was compatible with malignant melanoma (Figure 1). Intracranial biopsies were sent for genetic study and were found to harbor GNAQ (Q209P) and BRCA1-associated protein 1 (BAP1)(p.P324fs*11) mutations.

The patient was referred to dermatology by neurosurgery for evaluation of a suspected primary cutaneous melanoma.


The patient entered a clinical trial at an outside institution several weeks after initial presentation to our institution for treatment with a mitogen-activated protein kinase MEK1 inhibitor as well as radiation therapy. The patient was lost to follow-up.
Comment
It has been demonstrated that homozygous loss of BAP1, located on the chromosome 3p21.1 locus, allows for progression to metastatic disease in uveal melanoma. The BAP1 gene codes for ubiquitin carboxyl-terminal hydrolase 7, which is involved in the removal of ubiquitin from proteins. This enzyme binds to BRCA1 (BRCA1, DNA repair associated) via the RING (Really Interesting New Gene) finger domain and acts as a tumor suppressor.5 Biallelic BAP1 mutations allow the transition to malignancy in concert with other mutations, such as GNAQ. Identification of a BAP1 mutation may serve as a valuable diagnostic and future therapeutic target in uveal melanoma.
Currently, there are no drugs that directly target mutated GNA11 and GNAQ proteins. Because aberrant GNA11 and GNAQ proteins activate MEK1, several MEK1 inhibitors are being tested with the hope of achieving indirect suppression of GNA11/GNAQ.6
We present a rare case of BAP1 and GNAQ mutations in intracranial melanoma associated with nevus of Ota. Although the uveal melanoma was not confirmed on histopathology, the clear mention of foci within the eye by ophthalmology, positron emission tomography–CT scan showing a fluorodeoxyglucose-avid left retro-orbital mass, and genetic studies of the intracranial biopsies were highly suggestive of a primary uveal melanoma.
Our case highlights the importance of ongoing ocular screening in patients with nevus of Ota, noting the possibility of malignant transformation. Furthermore, patients with nevus of Ota with ocular involvement may benefit from testing of BAP1 protein expression by immunohistochemistry.7 Identification of BAP1 and GNAQ mutations in patients with nevus of Ota place them at markedly higher risk for malignant melanoma. Therefore, dermatologic evaluation of patients with nevus of Ota should include a thorough review of the patient’s history and skin examination as well as referral for ophthalmologic evaluation.
Nevus of Ota, originally referred to as nevus fusco-caeruleus ophthalmomaxillaris, initially was described in 1939 by Ota and Tanino.1 It is a dermal melanocytic hamartoma arising from incomplete migration of neural crest melanocytes to the epidermis during embryogenesis, resulting in nesting of subtle bands of dendritic melanocytes in the upper dermis. More common in Asians, Native Americans, and females, this hyperpigmented dermatosis most often is unilaterally distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.2 In some patients, nevus of Ota also is associated with ocular, orbital, and leptomeningeal melanocytosis. Approximately 15% of nevi of Ota have an activating guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or G protein subunit alpha 11 (GNAQ) mutation; 85% of uveal melanomas harbor one of these mutations.3 Although uncommon, neoplastic transformation with extension or metastasis to the brain has been reported in patients with nevus of Ota.4
We report the case of a 29-year-old woman with a long-standing history of nevus of Ota who presented acutely with an intracranial melanoma as an extension of a primary uveal melanoma.
Case Report
A 29-year-old woman with a history of a nevus of Ota involving the left inner canthus, eyelids, sclera, and superior malar cheek that had been present since birth presented to the emergency department with an acute onset of severe headache, blurred vision, and vomiting. Computed tomography (CT) and magnetic resonance imaging of the brain revealed a hemorrhagic mass in the left frontal lobe. Subsequent frontal craniotomy and resection revealed an intracranial melanoma.
Two weeks following surgery, the patient underwent magnetic resonance imaging and combined positron emission tomography and CT scans that demonstrated a fluorodeoxyglucose-avid left retro-orbital mass. Histopathology of a biopsy from the left retro-orbital mass that had been obtained intraoperatively demonstrated a pigmented, spindled to epithelioid neoplasm with areas of marked atypia and a high mitotic rate that was compatible with malignant melanoma (Figure 1). Intracranial biopsies were sent for genetic study and were found to harbor GNAQ (Q209P) and BRCA1-associated protein 1 (BAP1)(p.P324fs*11) mutations.

The patient was referred to dermatology by neurosurgery for evaluation of a suspected primary cutaneous melanoma.


The patient entered a clinical trial at an outside institution several weeks after initial presentation to our institution for treatment with a mitogen-activated protein kinase MEK1 inhibitor as well as radiation therapy. The patient was lost to follow-up.
Comment
It has been demonstrated that homozygous loss of BAP1, located on the chromosome 3p21.1 locus, allows for progression to metastatic disease in uveal melanoma. The BAP1 gene codes for ubiquitin carboxyl-terminal hydrolase 7, which is involved in the removal of ubiquitin from proteins. This enzyme binds to BRCA1 (BRCA1, DNA repair associated) via the RING (Really Interesting New Gene) finger domain and acts as a tumor suppressor.5 Biallelic BAP1 mutations allow the transition to malignancy in concert with other mutations, such as GNAQ. Identification of a BAP1 mutation may serve as a valuable diagnostic and future therapeutic target in uveal melanoma.
Currently, there are no drugs that directly target mutated GNA11 and GNAQ proteins. Because aberrant GNA11 and GNAQ proteins activate MEK1, several MEK1 inhibitors are being tested with the hope of achieving indirect suppression of GNA11/GNAQ.6
We present a rare case of BAP1 and GNAQ mutations in intracranial melanoma associated with nevus of Ota. Although the uveal melanoma was not confirmed on histopathology, the clear mention of foci within the eye by ophthalmology, positron emission tomography–CT scan showing a fluorodeoxyglucose-avid left retro-orbital mass, and genetic studies of the intracranial biopsies were highly suggestive of a primary uveal melanoma.
Our case highlights the importance of ongoing ocular screening in patients with nevus of Ota, noting the possibility of malignant transformation. Furthermore, patients with nevus of Ota with ocular involvement may benefit from testing of BAP1 protein expression by immunohistochemistry.7 Identification of BAP1 and GNAQ mutations in patients with nevus of Ota place them at markedly higher risk for malignant melanoma. Therefore, dermatologic evaluation of patients with nevus of Ota should include a thorough review of the patient’s history and skin examination as well as referral for ophthalmologic evaluation.
- Ota M, Tanino H. A variety of nevus, frequently encountered in Japan, nevus fusco-caeruleus ophthalmomaxillaris and its relationship to pigmentary changes in the eye. Tokyo Med J. 1939;63:1243-1244.
- Swann PG, Kwong E. The naevus of Ota. Clin Exp Optom. 2010;93:264-267.
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma [published online November 17, 2010]. N Engl J Med. 2010;363:2191-2199.
- Nitta K, Kashima T, Mayuzumi H, et al. Animal-type malignancy melanoma associated with nevus of Ota in the orbit of a Japanese woman: a case report. Melanoma Res. 2014;24:286-289.
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas [published online November 4, 2010]. Science. 2010;330:1410-1413.
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724-4734.
- Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing [published online July 24, 2010]. Br J Cancer. 2014;111:1373-1380.
- Ota M, Tanino H. A variety of nevus, frequently encountered in Japan, nevus fusco-caeruleus ophthalmomaxillaris and its relationship to pigmentary changes in the eye. Tokyo Med J. 1939;63:1243-1244.
- Swann PG, Kwong E. The naevus of Ota. Clin Exp Optom. 2010;93:264-267.
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma [published online November 17, 2010]. N Engl J Med. 2010;363:2191-2199.
- Nitta K, Kashima T, Mayuzumi H, et al. Animal-type malignancy melanoma associated with nevus of Ota in the orbit of a Japanese woman: a case report. Melanoma Res. 2014;24:286-289.
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas [published online November 4, 2010]. Science. 2010;330:1410-1413.
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724-4734.
- Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing [published online July 24, 2010]. Br J Cancer. 2014;111:1373-1380.
Practice Points
- Nevus of Ota is a hyperpigmented dermatosis that typically is distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.
- GNAQ and BAP1 mutations in patients with nevus of Ota confer a greater risk for malignant melanoma and metastatic progression.
- Ongoing ophthalmologic screening is paramount in patients with nevus of Ota and may prevent devastating sequelae.
When Rodents Attack: A Review of Rabies and Post-Exposure Prophylaxis
In this case report of a 65-year-old woman who presented to the ED for evaluation of an animal bite, the authors review the literature about the treatment of rabies with post-exposure prophylaxis.
Case
A 65-year-old woman presented to the ED with a chief complaint of an animal bite, in Southwest Ohio. The patient reported picking up what she thought to be an injured bird, which in fact turned out to be a chipmunk. When she handled the creature, it jumped up and bit her on the right arm, latching on through the sleeve of her sweater. Her husband struck the chipmunk with a shovel, thus terminating the threat. The patient was brought to the ED by her husband for evaluation, along with the lifeless animal which had been placed in a plastic bag. On examination, the patient had a small superficial abrasion to her right forearm with shallow skin puncture. This was treated with local wound care, including copious irrigation with normal saline and chlorhexidine. Given that she sustained an animal bite wound, she was treated empirically with amoxicillin/clavulanic acid (Augmentin) orally. Her tetanus vaccine was up to date. The critically important question regarding management of the patient was whether she should be treated with post-exposure prophylaxis for rabies after being bitten by the rodent.
Discussion
According to the Centers for Disease Control and Prevention (CDC) website regarding the indications for rabies post-exposure prophylaxis, it was found that small rodents, including chipmunks, have not been known to transmit rabies to humans. While these small animals are rarely infected with rabies, there have been reports of squirrels infected with rabies in the United States.1,2 Therefore, it is recommended that in all cases of rodent bites, the state or local health department should be contacted prior to making a decision regarding post-exposure prophylaxis.1 The state health department was contacted and agreed that post-exposure prophylaxis was not indicated; however, they requested the animal be sent to them for testing, which was arranged. An in-depth literature review found a case report in India of a 7-year-old boy who contracted rabies after being bitten by a squirrel. Per this research, small rodents are rarely infected with rabies, with woodchucks being indicated as local vectors. This particular patient received a tetanus vaccine and wound care initially after the squirrel bite, but presented 2 months later with difficulty with oral feeding, fever, and cough. He succumbed to his illness just 4 hours after admission, and rabies was confirmed through a corneal impression smear.3 Birhane et al4 reported three human deaths in the United States from rabies during 2015. The deaths included one due to a rabid dog bite while abroad, one due to contact with a bat, and one from a mongoose bite. None of these patients had been treated with post-exposure prophylaxis.4
Rabies is an RNA virus in the genus Lyssavirus. Once contracted, the rabies virus initially binds to the nicotinic acetylcholine receptor in muscle, replicates, and ascends along the nerves until it reaches the central nervous system (CNS), then propagates outward via the peripheral nerves. Replication in the CNS occurs in the Negri bodies, which are highly specific for rabies. There are two forms of rabies, a paralytic form and an encephalitic form, with the encephalitic form occurring far more commonly. The encephalitic form presents with hallucinations, and disorientation intermixed with lucid intervals. The paralytic form presents with weakness in the affected limb(s), progressing to quadriparesis and facial weakness, eventually leading to organ failure. The classic hydrophobia that is thought of in connection with rabies is present in only 50% of cases.5Transmission occurs from an infected host via bite most commonly, but can occur through exposure to mucous membranes, aerosol transmission, or exposure in a laboratory setting. The rabies virus can be “shed in the saliva concomitantly with, before or after the development of clinical signs.”6 Lyssaviruses, such as the rabies virus, do not persist in the environment. Once outside the host, the virus is rapidly inactivated, therefore, fomites do not play a role in transmission.6 Rabies hosts vary significantly, and the virus has been found in almost all mammalian orders and on all continents except for Antarctica. The primary reservoir for rabies is the bat, followed by dogs; however, cats, foxes, coyotes, jackals, wolves, mongoose, and raccoons are all vectors of rabies. Animals infected with rabies typically show signs of CNS disturbance. According to Rupprecht,2 “the most reliable signs, regardless of species, are acute behavioral changes and unexplained progressive paralysis.” Notably, wild animals infected with rabies “may lose their fear of people, and nocturnal species may be seen wandering about during the daytime.”2According to Rupprecht et al,6 “Rodents and lagomorphs, although used heavily as laboratory models, are not important in the epidemiology of the disease [rabies], except in the public-health resources devoted to consultation or prophylaxis after routine contact with these ubiquitous small mammals.”
Post-exposure prophylaxis is indicated when someone has been in a room with a bat, even if direct contact with the animal is uncertain. Examples of this would include “a sleeping person [who] awakens to find a bat in the room or an adult witnesses a bat in the room with a previously unattended child, mentally disabled person, or intoxicated person.”7 Following a bite requiring post-exposure prophylaxis treatment, it is pertinent to note if the patient has had a previous immunization. Regardless of immunization status, all bite areas must be thoroughly cleansed and irrigated. The CDC recommends using a virucidal agent, such as a povidine-iodine solution, in the cleansing process. If an individual has been previously immunized to rabies, then the rabies immunoglobulin (RIG) should not be administered; rather, the patient should be given the rabies vaccine, such as the human diploid cell culture rabies vaccine (HDCV) or the purified chick embryo cell vaccine (PCECV). The dose is 1 mL intramuscularly on day 0 and day 3. If a patient has not had either pre-exposure or prior post-exposure vaccinations, then RIG is also indicated. The full dose of RIG should be given at the site of the bite; however, if this is not feasible due to the location of the wound, then any remainder should be given at a site distant from the vaccine. The rabies vaccine (HDCV or PCECV) should be administered intramuscularly on days 0, 3, 7, and 14. A fifth dose may be considered on day 28 for immunocompromised patients. In adults, the vaccine should be given in the deltoid region, whereas in children it can also be given in the anterolateral aspect of the thigh. It should never be administered in the gluteal region because it may result in lower antibody titers.8 It is extremely important to administer the RIG in unvaccinated persons. A case report was reviewed from India in which a 45-year-old woman presented with fever, headache, dizziness, and hearing loss 1 month after being bitten by a mongoose on her right leg. She was given 4 doses of a rabies vaccine on days 0, 3, 7, and 28 but was not given RIG. Rabies virus neutralizing antibody titers in the cerebral spinal fluid were initially 2,048 IU/mL and increased after 2 weeks to greater than 16,384 IU/mL confirming the diagnosis of rabies encephalitis. The patient died 1 month after admission.9 The incubation period for rabies is 1 to 3 months in general, but a range from days to years has been reported.6 Post-exposure prophylaxis should typically be initiated as soon as possible after a bite; however, it may be delayed up to 10 days after exposure if the animal has been captured and can be monitored for signs of rabies or euthanized and tested. It is recommended that anyone who presents for evaluation after possible exposure, regardless of timeline, should be treated as if the contact had just occurred.10
Case Conclusion
In this case of the chipmunk bite, in accordance with the state health department, rabies prophylaxis was not indicated. It was recommended that the chipmunk be sent off for a necropsy (noting to leave the chipmunk intact and not to behead it). Following shared decision making with the patient and her husband, the chipmunk was sent off for testing with the results to be sent to the patient. She was discharged from the ED with Augmentin, but without rabies post-exposure prophylaxis. According to review of outpatient medical records, the patient was doing well at primary care appointments after the injury.
1. Centers for Disease Control and Prevention. Rabies: Other Wild Animals. https://www.cdc.gov/rabies/exposure/animals/other.html. Published April 29, 2016. Accessed July 20, 2017.
2. Rupprecht CERE. Overview of Rabies – Nervous System. Merck Veterinary Manual. https://www.merckvetmanual.com/nervous-system/rabies/overview-of-rabies. Accessed June 15, 2018.
3. Kumari PL, Mohanan KR, Kailas L, Chacko KP. A case or rabies after squirrel bite. Indian J Pediatr. 2014;81(2):198. doi:10.1007/s12098-013-0990-2.
4. Birhane MG, Cleaton JM, Monroe BP, et al. Rabies surveillance in the United States during 2015. J Am Vet Med Assoc. 2017;250(10):1117-1130. doi:10.2460/javma.250.10.1117.
5. Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016.
6. Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002;2(6):327-343.
7. Centers for Disease Control and Prevention. Rabies: Bats. https://www.cdc.gov/rabies/exposure/animals/bats.html. Published July 5, 2017. Accessed June 15, 2018.
8. Centers for Disease Control and Prevention. Rabies: Rabies Vaccine. https://www.cdc.gov/rabies/medical_care/vaccine.html. Published September 24, 2014. Accessed July 20, 2017.
9. Mani RS, Moorkoth AP, Balasubramanian P, Devi KL, Madhusudana SN. Rabies following mongoose bite. Indian J Med Microbiol. 2016;34(2):256-257. doi:10.4103/0255-0857.176848.
10. Petersen B. Rabies: What’s an Exposure? Know When to Vaccinate. Medscape. https://www.medscape.com/viewarticle/877636. Published April 03, 2017. Accessed September 3, 2018.
In this case report of a 65-year-old woman who presented to the ED for evaluation of an animal bite, the authors review the literature about the treatment of rabies with post-exposure prophylaxis.
In this case report of a 65-year-old woman who presented to the ED for evaluation of an animal bite, the authors review the literature about the treatment of rabies with post-exposure prophylaxis.
Case
A 65-year-old woman presented to the ED with a chief complaint of an animal bite, in Southwest Ohio. The patient reported picking up what she thought to be an injured bird, which in fact turned out to be a chipmunk. When she handled the creature, it jumped up and bit her on the right arm, latching on through the sleeve of her sweater. Her husband struck the chipmunk with a shovel, thus terminating the threat. The patient was brought to the ED by her husband for evaluation, along with the lifeless animal which had been placed in a plastic bag. On examination, the patient had a small superficial abrasion to her right forearm with shallow skin puncture. This was treated with local wound care, including copious irrigation with normal saline and chlorhexidine. Given that she sustained an animal bite wound, she was treated empirically with amoxicillin/clavulanic acid (Augmentin) orally. Her tetanus vaccine was up to date. The critically important question regarding management of the patient was whether she should be treated with post-exposure prophylaxis for rabies after being bitten by the rodent.
Discussion
According to the Centers for Disease Control and Prevention (CDC) website regarding the indications for rabies post-exposure prophylaxis, it was found that small rodents, including chipmunks, have not been known to transmit rabies to humans. While these small animals are rarely infected with rabies, there have been reports of squirrels infected with rabies in the United States.1,2 Therefore, it is recommended that in all cases of rodent bites, the state or local health department should be contacted prior to making a decision regarding post-exposure prophylaxis.1 The state health department was contacted and agreed that post-exposure prophylaxis was not indicated; however, they requested the animal be sent to them for testing, which was arranged. An in-depth literature review found a case report in India of a 7-year-old boy who contracted rabies after being bitten by a squirrel. Per this research, small rodents are rarely infected with rabies, with woodchucks being indicated as local vectors. This particular patient received a tetanus vaccine and wound care initially after the squirrel bite, but presented 2 months later with difficulty with oral feeding, fever, and cough. He succumbed to his illness just 4 hours after admission, and rabies was confirmed through a corneal impression smear.3 Birhane et al4 reported three human deaths in the United States from rabies during 2015. The deaths included one due to a rabid dog bite while abroad, one due to contact with a bat, and one from a mongoose bite. None of these patients had been treated with post-exposure prophylaxis.4
Rabies is an RNA virus in the genus Lyssavirus. Once contracted, the rabies virus initially binds to the nicotinic acetylcholine receptor in muscle, replicates, and ascends along the nerves until it reaches the central nervous system (CNS), then propagates outward via the peripheral nerves. Replication in the CNS occurs in the Negri bodies, which are highly specific for rabies. There are two forms of rabies, a paralytic form and an encephalitic form, with the encephalitic form occurring far more commonly. The encephalitic form presents with hallucinations, and disorientation intermixed with lucid intervals. The paralytic form presents with weakness in the affected limb(s), progressing to quadriparesis and facial weakness, eventually leading to organ failure. The classic hydrophobia that is thought of in connection with rabies is present in only 50% of cases.5Transmission occurs from an infected host via bite most commonly, but can occur through exposure to mucous membranes, aerosol transmission, or exposure in a laboratory setting. The rabies virus can be “shed in the saliva concomitantly with, before or after the development of clinical signs.”6 Lyssaviruses, such as the rabies virus, do not persist in the environment. Once outside the host, the virus is rapidly inactivated, therefore, fomites do not play a role in transmission.6 Rabies hosts vary significantly, and the virus has been found in almost all mammalian orders and on all continents except for Antarctica. The primary reservoir for rabies is the bat, followed by dogs; however, cats, foxes, coyotes, jackals, wolves, mongoose, and raccoons are all vectors of rabies. Animals infected with rabies typically show signs of CNS disturbance. According to Rupprecht,2 “the most reliable signs, regardless of species, are acute behavioral changes and unexplained progressive paralysis.” Notably, wild animals infected with rabies “may lose their fear of people, and nocturnal species may be seen wandering about during the daytime.”2According to Rupprecht et al,6 “Rodents and lagomorphs, although used heavily as laboratory models, are not important in the epidemiology of the disease [rabies], except in the public-health resources devoted to consultation or prophylaxis after routine contact with these ubiquitous small mammals.”
Post-exposure prophylaxis is indicated when someone has been in a room with a bat, even if direct contact with the animal is uncertain. Examples of this would include “a sleeping person [who] awakens to find a bat in the room or an adult witnesses a bat in the room with a previously unattended child, mentally disabled person, or intoxicated person.”7 Following a bite requiring post-exposure prophylaxis treatment, it is pertinent to note if the patient has had a previous immunization. Regardless of immunization status, all bite areas must be thoroughly cleansed and irrigated. The CDC recommends using a virucidal agent, such as a povidine-iodine solution, in the cleansing process. If an individual has been previously immunized to rabies, then the rabies immunoglobulin (RIG) should not be administered; rather, the patient should be given the rabies vaccine, such as the human diploid cell culture rabies vaccine (HDCV) or the purified chick embryo cell vaccine (PCECV). The dose is 1 mL intramuscularly on day 0 and day 3. If a patient has not had either pre-exposure or prior post-exposure vaccinations, then RIG is also indicated. The full dose of RIG should be given at the site of the bite; however, if this is not feasible due to the location of the wound, then any remainder should be given at a site distant from the vaccine. The rabies vaccine (HDCV or PCECV) should be administered intramuscularly on days 0, 3, 7, and 14. A fifth dose may be considered on day 28 for immunocompromised patients. In adults, the vaccine should be given in the deltoid region, whereas in children it can also be given in the anterolateral aspect of the thigh. It should never be administered in the gluteal region because it may result in lower antibody titers.8 It is extremely important to administer the RIG in unvaccinated persons. A case report was reviewed from India in which a 45-year-old woman presented with fever, headache, dizziness, and hearing loss 1 month after being bitten by a mongoose on her right leg. She was given 4 doses of a rabies vaccine on days 0, 3, 7, and 28 but was not given RIG. Rabies virus neutralizing antibody titers in the cerebral spinal fluid were initially 2,048 IU/mL and increased after 2 weeks to greater than 16,384 IU/mL confirming the diagnosis of rabies encephalitis. The patient died 1 month after admission.9 The incubation period for rabies is 1 to 3 months in general, but a range from days to years has been reported.6 Post-exposure prophylaxis should typically be initiated as soon as possible after a bite; however, it may be delayed up to 10 days after exposure if the animal has been captured and can be monitored for signs of rabies or euthanized and tested. It is recommended that anyone who presents for evaluation after possible exposure, regardless of timeline, should be treated as if the contact had just occurred.10
Case Conclusion
In this case of the chipmunk bite, in accordance with the state health department, rabies prophylaxis was not indicated. It was recommended that the chipmunk be sent off for a necropsy (noting to leave the chipmunk intact and not to behead it). Following shared decision making with the patient and her husband, the chipmunk was sent off for testing with the results to be sent to the patient. She was discharged from the ED with Augmentin, but without rabies post-exposure prophylaxis. According to review of outpatient medical records, the patient was doing well at primary care appointments after the injury.
Case
A 65-year-old woman presented to the ED with a chief complaint of an animal bite, in Southwest Ohio. The patient reported picking up what she thought to be an injured bird, which in fact turned out to be a chipmunk. When she handled the creature, it jumped up and bit her on the right arm, latching on through the sleeve of her sweater. Her husband struck the chipmunk with a shovel, thus terminating the threat. The patient was brought to the ED by her husband for evaluation, along with the lifeless animal which had been placed in a plastic bag. On examination, the patient had a small superficial abrasion to her right forearm with shallow skin puncture. This was treated with local wound care, including copious irrigation with normal saline and chlorhexidine. Given that she sustained an animal bite wound, she was treated empirically with amoxicillin/clavulanic acid (Augmentin) orally. Her tetanus vaccine was up to date. The critically important question regarding management of the patient was whether she should be treated with post-exposure prophylaxis for rabies after being bitten by the rodent.
Discussion
According to the Centers for Disease Control and Prevention (CDC) website regarding the indications for rabies post-exposure prophylaxis, it was found that small rodents, including chipmunks, have not been known to transmit rabies to humans. While these small animals are rarely infected with rabies, there have been reports of squirrels infected with rabies in the United States.1,2 Therefore, it is recommended that in all cases of rodent bites, the state or local health department should be contacted prior to making a decision regarding post-exposure prophylaxis.1 The state health department was contacted and agreed that post-exposure prophylaxis was not indicated; however, they requested the animal be sent to them for testing, which was arranged. An in-depth literature review found a case report in India of a 7-year-old boy who contracted rabies after being bitten by a squirrel. Per this research, small rodents are rarely infected with rabies, with woodchucks being indicated as local vectors. This particular patient received a tetanus vaccine and wound care initially after the squirrel bite, but presented 2 months later with difficulty with oral feeding, fever, and cough. He succumbed to his illness just 4 hours after admission, and rabies was confirmed through a corneal impression smear.3 Birhane et al4 reported three human deaths in the United States from rabies during 2015. The deaths included one due to a rabid dog bite while abroad, one due to contact with a bat, and one from a mongoose bite. None of these patients had been treated with post-exposure prophylaxis.4
Rabies is an RNA virus in the genus Lyssavirus. Once contracted, the rabies virus initially binds to the nicotinic acetylcholine receptor in muscle, replicates, and ascends along the nerves until it reaches the central nervous system (CNS), then propagates outward via the peripheral nerves. Replication in the CNS occurs in the Negri bodies, which are highly specific for rabies. There are two forms of rabies, a paralytic form and an encephalitic form, with the encephalitic form occurring far more commonly. The encephalitic form presents with hallucinations, and disorientation intermixed with lucid intervals. The paralytic form presents with weakness in the affected limb(s), progressing to quadriparesis and facial weakness, eventually leading to organ failure. The classic hydrophobia that is thought of in connection with rabies is present in only 50% of cases.5Transmission occurs from an infected host via bite most commonly, but can occur through exposure to mucous membranes, aerosol transmission, or exposure in a laboratory setting. The rabies virus can be “shed in the saliva concomitantly with, before or after the development of clinical signs.”6 Lyssaviruses, such as the rabies virus, do not persist in the environment. Once outside the host, the virus is rapidly inactivated, therefore, fomites do not play a role in transmission.6 Rabies hosts vary significantly, and the virus has been found in almost all mammalian orders and on all continents except for Antarctica. The primary reservoir for rabies is the bat, followed by dogs; however, cats, foxes, coyotes, jackals, wolves, mongoose, and raccoons are all vectors of rabies. Animals infected with rabies typically show signs of CNS disturbance. According to Rupprecht,2 “the most reliable signs, regardless of species, are acute behavioral changes and unexplained progressive paralysis.” Notably, wild animals infected with rabies “may lose their fear of people, and nocturnal species may be seen wandering about during the daytime.”2According to Rupprecht et al,6 “Rodents and lagomorphs, although used heavily as laboratory models, are not important in the epidemiology of the disease [rabies], except in the public-health resources devoted to consultation or prophylaxis after routine contact with these ubiquitous small mammals.”
Post-exposure prophylaxis is indicated when someone has been in a room with a bat, even if direct contact with the animal is uncertain. Examples of this would include “a sleeping person [who] awakens to find a bat in the room or an adult witnesses a bat in the room with a previously unattended child, mentally disabled person, or intoxicated person.”7 Following a bite requiring post-exposure prophylaxis treatment, it is pertinent to note if the patient has had a previous immunization. Regardless of immunization status, all bite areas must be thoroughly cleansed and irrigated. The CDC recommends using a virucidal agent, such as a povidine-iodine solution, in the cleansing process. If an individual has been previously immunized to rabies, then the rabies immunoglobulin (RIG) should not be administered; rather, the patient should be given the rabies vaccine, such as the human diploid cell culture rabies vaccine (HDCV) or the purified chick embryo cell vaccine (PCECV). The dose is 1 mL intramuscularly on day 0 and day 3. If a patient has not had either pre-exposure or prior post-exposure vaccinations, then RIG is also indicated. The full dose of RIG should be given at the site of the bite; however, if this is not feasible due to the location of the wound, then any remainder should be given at a site distant from the vaccine. The rabies vaccine (HDCV or PCECV) should be administered intramuscularly on days 0, 3, 7, and 14. A fifth dose may be considered on day 28 for immunocompromised patients. In adults, the vaccine should be given in the deltoid region, whereas in children it can also be given in the anterolateral aspect of the thigh. It should never be administered in the gluteal region because it may result in lower antibody titers.8 It is extremely important to administer the RIG in unvaccinated persons. A case report was reviewed from India in which a 45-year-old woman presented with fever, headache, dizziness, and hearing loss 1 month after being bitten by a mongoose on her right leg. She was given 4 doses of a rabies vaccine on days 0, 3, 7, and 28 but was not given RIG. Rabies virus neutralizing antibody titers in the cerebral spinal fluid were initially 2,048 IU/mL and increased after 2 weeks to greater than 16,384 IU/mL confirming the diagnosis of rabies encephalitis. The patient died 1 month after admission.9 The incubation period for rabies is 1 to 3 months in general, but a range from days to years has been reported.6 Post-exposure prophylaxis should typically be initiated as soon as possible after a bite; however, it may be delayed up to 10 days after exposure if the animal has been captured and can be monitored for signs of rabies or euthanized and tested. It is recommended that anyone who presents for evaluation after possible exposure, regardless of timeline, should be treated as if the contact had just occurred.10
Case Conclusion
In this case of the chipmunk bite, in accordance with the state health department, rabies prophylaxis was not indicated. It was recommended that the chipmunk be sent off for a necropsy (noting to leave the chipmunk intact and not to behead it). Following shared decision making with the patient and her husband, the chipmunk was sent off for testing with the results to be sent to the patient. She was discharged from the ED with Augmentin, but without rabies post-exposure prophylaxis. According to review of outpatient medical records, the patient was doing well at primary care appointments after the injury.
1. Centers for Disease Control and Prevention. Rabies: Other Wild Animals. https://www.cdc.gov/rabies/exposure/animals/other.html. Published April 29, 2016. Accessed July 20, 2017.
2. Rupprecht CERE. Overview of Rabies – Nervous System. Merck Veterinary Manual. https://www.merckvetmanual.com/nervous-system/rabies/overview-of-rabies. Accessed June 15, 2018.
3. Kumari PL, Mohanan KR, Kailas L, Chacko KP. A case or rabies after squirrel bite. Indian J Pediatr. 2014;81(2):198. doi:10.1007/s12098-013-0990-2.
4. Birhane MG, Cleaton JM, Monroe BP, et al. Rabies surveillance in the United States during 2015. J Am Vet Med Assoc. 2017;250(10):1117-1130. doi:10.2460/javma.250.10.1117.
5. Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016.
6. Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002;2(6):327-343.
7. Centers for Disease Control and Prevention. Rabies: Bats. https://www.cdc.gov/rabies/exposure/animals/bats.html. Published July 5, 2017. Accessed June 15, 2018.
8. Centers for Disease Control and Prevention. Rabies: Rabies Vaccine. https://www.cdc.gov/rabies/medical_care/vaccine.html. Published September 24, 2014. Accessed July 20, 2017.
9. Mani RS, Moorkoth AP, Balasubramanian P, Devi KL, Madhusudana SN. Rabies following mongoose bite. Indian J Med Microbiol. 2016;34(2):256-257. doi:10.4103/0255-0857.176848.
10. Petersen B. Rabies: What’s an Exposure? Know When to Vaccinate. Medscape. https://www.medscape.com/viewarticle/877636. Published April 03, 2017. Accessed September 3, 2018.
1. Centers for Disease Control and Prevention. Rabies: Other Wild Animals. https://www.cdc.gov/rabies/exposure/animals/other.html. Published April 29, 2016. Accessed July 20, 2017.
2. Rupprecht CERE. Overview of Rabies – Nervous System. Merck Veterinary Manual. https://www.merckvetmanual.com/nervous-system/rabies/overview-of-rabies. Accessed June 15, 2018.
3. Kumari PL, Mohanan KR, Kailas L, Chacko KP. A case or rabies after squirrel bite. Indian J Pediatr. 2014;81(2):198. doi:10.1007/s12098-013-0990-2.
4. Birhane MG, Cleaton JM, Monroe BP, et al. Rabies surveillance in the United States during 2015. J Am Vet Med Assoc. 2017;250(10):1117-1130. doi:10.2460/javma.250.10.1117.
5. Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016.
6. Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002;2(6):327-343.
7. Centers for Disease Control and Prevention. Rabies: Bats. https://www.cdc.gov/rabies/exposure/animals/bats.html. Published July 5, 2017. Accessed June 15, 2018.
8. Centers for Disease Control and Prevention. Rabies: Rabies Vaccine. https://www.cdc.gov/rabies/medical_care/vaccine.html. Published September 24, 2014. Accessed July 20, 2017.
9. Mani RS, Moorkoth AP, Balasubramanian P, Devi KL, Madhusudana SN. Rabies following mongoose bite. Indian J Med Microbiol. 2016;34(2):256-257. doi:10.4103/0255-0857.176848.
10. Petersen B. Rabies: What’s an Exposure? Know When to Vaccinate. Medscape. https://www.medscape.com/viewarticle/877636. Published April 03, 2017. Accessed September 3, 2018.