User login
An Imposter Twice Over: A Case of IgG4-Related Disease
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory condition that involves multiple organs and appears as syndromes that were once thought to be unrelated. This disease leads to mass lesions, fibrosis, and subsequent organ failure if allowed to progress untreated.1 Involvement of gastrointestinal (GI) organs, salivary glands, lacrimal glands, lymph, prostate, pulmonary, and vascular system have all been reported.2 Elevated IgG4 serum levels are common, but about one-third of patients with biopsy-proven IgG4-RD do not manifest this characteristic.3,4
Diagnostic confirmation is with biopsy, and all patients with symptomatic, active IgG4-RD require treatment. Glucocorticoids are first-line treatment and are utilized for relapse of symptoms. In addition to glucocorticoids, steroid-sparing medications, including rituximab, azathioprine, mycophenolate mofetil, tacrolimus, and cyclophosphamide have all been used with successful remission.5,6 Here, the authors discuss a case of IgG4-RD that presented with intrahepatic biliary obstruction (mimicking cholangiocarcinoma) and subsequent development of coronary arteritis despite treatment.
Case Presentation
In June 2015, a 57-year-old Air Force veteran presented to Eglin AFB Hospital with pruritic jaundice and acute abdominal pain. He was found to have elevated bilirubin levels (total bilirubin 10 mg/dL [normal range 0.2-1.3 mg/dL], direct bilirubin 6.6 mg/dL [normal range 0.1-0.4 mg/dL]). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also were moderately elevated (147 U/L and 337 U/L, respectively).
Prior to this presentation, the patient had been in his usual state of health. His past medical history was notable only for minimal change kidney disease (MCD). MCD is defined as effacement of the podocyte seen on electron microscopy, which allows the passage of large amounts of protein.
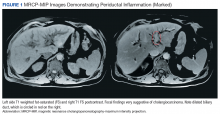
A cholangiogram showed abnormal filling into the left main intrahepatic duct and obvious obstruction at the bifurcation of the bile duct. A biliary drainage catheter was placed, and a repeat cholangiogram 2 days later showed involvement of both right and left intrahepatic ducts. The distal common bile duct appeared uninvolved as did the pancreas. Lymphadenopathy was noted at the liver hilum. Klatskin cholangiocarcinoma (type IIIB) was the presumed diagnosis. Based on these findings, tumor resection was performed 3 weeks later, including left hepatectomy, caudate lobe resection, complete bile duct resection, cholecystectomy, with reconstruction by Roux-en-Y intrahepaticojejunostomy. In addition, portal and hepatic artery lymph node dissection was completed.
Surgical specimens were sent for pathologic evaluation and were found negative for malignancy. Patchy areas of storiform fibrosis, obliterative phlebitis, and lymphoplasmacytic infiltrate were noted. IgG4 immunostain highlighted the presence of IgG4 positive plasma cells with a peak count of 145 IgG4 positive plasma cells/hpf. About 80% of the plasma cells were positive for IgG4. Unusually dense eosinophilic infiltrate with plasma cells and regions of dense fibrosis that strongly contributed to the masslike appearance on CT imaging also were noted. Final histology confirmed the diagnosis of IgG4-RD. Elevated levels of total IgG in the serum were observed without elevation in serum IgG4 (Table).
The patient was started on prednisone 40 mg and azathioprine 150 mg daily, with subsequent taper of prednisone over the next 6 months. After prednisone was discontinued, the patient reported new symptoms of lower extremity pain, neuropathy, and swelling of his face. Laboratory results were notable for elevated erythrocyte sedimentation rate. The patient was restarted on prednisone 40 mg daily. Azathioprine was replaced with a regimen of 4 doses every 6 months of IV rituximab 700 mg q week and mycophenolate mofetil (1,000 mg bid). After remission was induced, the patient was slowly weaned off prednisone again.
Following 6 months of successful discontinuation of prednisone and continued rituximab and mycophenolate mofetil therapy, the patient presented to the emergency department with new onset chest pain and shortness of breath. A CT angiography of the chest showed right upper and middle lobe infiltrate, and he was treated for community acquired pneumonia. Additionally, he was noted to have elevated troponin levels suggestive of myocardial infarction (MI). Initial troponin was 1.23 ng/mL (normal range < 0.015 ng/mL), which trended down over the next 18 hours. A bedside echocardiogram showed a normal left ventricular ejection fraction without wall motion abnormalities. Etiology for his acute MI was presumed to be demand ischemia from fixed atherosclerotic plaque. Further inpatient cardiac risk stratification was changed to the outpatient setting, and he was started on medical management for coronary artery disease with a beta blocker, a statin, and aspirin. He was discharged home on 10 mg prednisone daily, which was subsequently tapered over several weeks.
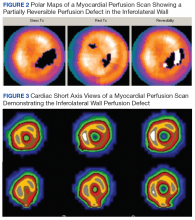
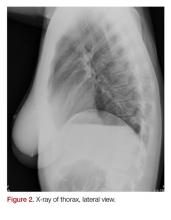
In follow-up, a Lexiscan myocardial perfusion imaging was conducted that demonstrated an inferolateral defect and associated wall motion abnormalities (Figures 2 and 3).
Discussion
IgG4-related disease has been found to be a systemic disorder. Typical characteristics include predominance in men aged > 50 years, elevated IgG4 levels, and findings on histology.1 It has been reported to involve many organs, including pancreas, liver, gallbladder, salivary glands, thyroid, and pleura of the lung.2,5
This case report begins with a presumptive diagnosis of cholangiocarcinoma, which was treated aggressively with extensive surgery. Several case reports of complex tumefactive lesions in the GI area (mostly pancreatic and biliary) have detailed IgG4-RD as both a risk factor for subsequent development of cholangiocarcinoma and as a separate entity of IgG4-related sclerosing cholangitis.7-9 It is hypothesized that the induction of IgG4- positive plasma cells has been intertwined with the development of cholangiocarcinoma. Differentiation between IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy is challenging. It has been documented that both elevated IgG4 levels and hilar hepatic lesions that resemble cholangiocarcinoma frequently accompany those cases of IgG4 sclerosing chlolangitis without pancreatic involvement.9 The histologic features of IgG4-RD need to be identified with multiple biopsies and cytology, and superficial biopsy from biliary mucosa cannot reliably exclude cholangiocarcinoma.
Lymphoplasmacytic aortitis and arteritis have been documented in IgG4-RD. In 2017, Barbu and colleagues described how one such case of coronary arteritis presented with typical angina and coronary catheterization revealing coronary artery stenosis.10 However, during coronary artery bypass surgery, the aorta and coronary vessels were noted to be abnormally stiff. A diffuse fibrotic tissue was identified to be causing the significant stenosis without evidence of atherosclerosis. Pathology showed typical findings of IgG4-RD, and there was a rapid response to immunosuppressive therapy. Involvement of coronary arteries has been described in a small number of cases at this time and is associated with progressive fibrotic changes resulting in an MI, aneurysms, and sudden cardiac death.2,10,11
IgG4-RD can be an extensively systemic disease. All presentations of fibrosis or vasculitis should be viewed with heightened suspicion in the future as being a facet of his IgG4-RD. Pleural involvement has been reported in 12% of cases presenting with systemic presentation, kidney involvement in 13%.2,12
Unfortunately, there is no standard laboratory parameter to date that is diagnostic for IgG4-RD. The gold standard remains confirmation of histologic findings with biopsy. According to an international consensus from 2015, 2 out of the 3 major findings need to be present: (1) dense lymphoplasmacytic infiltrate; (2) storiform fibrosis; and (3) obliterative phlebitis in veins and arteries.1,5 Most patients present with symptoms related to either tumefaction or fibrosis of an organ system.1 Peripheral eosinophilia and elevated serum IgE are often present in IgG4-RD.13 Although IgG4 values are elevated in 51% of biopsy-proven cases, flow cytometry of CD19lowCD38+CD20-CD27+ plasmablasts has been explored recently as a correlation with disease flare.3,14 These particular plasmablasts mark a stage between B cells and plasma cells and have been reported to have a sensitivity of 95% and a specificity of 82% in association with actual IgG4-RD.14 Furthermore, blood plasmablast concentrations decrease in response to glucocorticoid treatment, thereby providing a possible quantifiable value by which to measure success of IgG4 treatment.5,12
Treatment for this disease consists of immunosuppressive therapy. There is documentation of successful remission with rituximab and azathioprine, as well as methotrexate.1,5 Both 2015 consensus guidelines and a recent small single-center retrospective study support addition of second-line steroid sparing agents such as mycophenolate mofetil.5,6 For acute flairs, however, glucocorticoids with slow taper are usually utilized. In these cases, they should be tapered as soon as clinically feasible to avoid long-term adverse effects. Untreated IgG4-RD, even asymptomatic, has been shown to progress to fibrosis.5
Conclusion
IgG4-RD is a complicated disease process that requires a high index of suspicion to diagnose. In addition, for patients who are diagnosed with this condition, its ability to mimic other pathologic conditions should be taken into account with manifestation of any new illness. This case emphasizes the ability of this disease to localize in multiple organs over time and the need for lifetime surveillance in patients with IgG4-RD disease.
1. Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189-199.
2. Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
3. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466-2475.
4. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-RD. Ann Rheum Dis. 2015;74(1):14-18.
5. Khosroshahi A, Wallace ZS, Crowe JL, et al; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688-1699.
6. Gupta N, Mathew J, Mohan H, et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of immunoglobulin G4-related disease (IgG4-RD): a series from a tertiary care teaching hospital in South India. Rheumatol Int. 2017;38(2):203-209.
7. Lin HP, Lin KT, Ho WC, Chen CB, Kuo, CY, Lin YC. IgG4-associated cholangitis mimicking cholangiocarcinoma-report of a case. J Intern Med Taiwan. 2013;24:137-141.
8. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol. 2013;5(8):181-185.
9. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol. 2014;2014:803876.
10. Barbu M, Lindström U, Nordborg C, Martinsson A, Dworeck C, Jeppsson A. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. 2017;103(6):e487-e489.
11. Kim YJ, Park YS, Koo BS, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17(8):451-452.
12. Khosroshahi A, Digumarthy SR, Gibbons FK, Deshpande V. Case 34-2015: A 36-year-old woman with a lung mass, pleural effusion and hip pain. N Engl J Med. 2015;373(18):1762-1772.
13. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):191-206.
14. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195.
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory condition that involves multiple organs and appears as syndromes that were once thought to be unrelated. This disease leads to mass lesions, fibrosis, and subsequent organ failure if allowed to progress untreated.1 Involvement of gastrointestinal (GI) organs, salivary glands, lacrimal glands, lymph, prostate, pulmonary, and vascular system have all been reported.2 Elevated IgG4 serum levels are common, but about one-third of patients with biopsy-proven IgG4-RD do not manifest this characteristic.3,4
Diagnostic confirmation is with biopsy, and all patients with symptomatic, active IgG4-RD require treatment. Glucocorticoids are first-line treatment and are utilized for relapse of symptoms. In addition to glucocorticoids, steroid-sparing medications, including rituximab, azathioprine, mycophenolate mofetil, tacrolimus, and cyclophosphamide have all been used with successful remission.5,6 Here, the authors discuss a case of IgG4-RD that presented with intrahepatic biliary obstruction (mimicking cholangiocarcinoma) and subsequent development of coronary arteritis despite treatment.
Case Presentation
In June 2015, a 57-year-old Air Force veteran presented to Eglin AFB Hospital with pruritic jaundice and acute abdominal pain. He was found to have elevated bilirubin levels (total bilirubin 10 mg/dL [normal range 0.2-1.3 mg/dL], direct bilirubin 6.6 mg/dL [normal range 0.1-0.4 mg/dL]). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also were moderately elevated (147 U/L and 337 U/L, respectively).
Prior to this presentation, the patient had been in his usual state of health. His past medical history was notable only for minimal change kidney disease (MCD). MCD is defined as effacement of the podocyte seen on electron microscopy, which allows the passage of large amounts of protein.
A cholangiogram showed abnormal filling into the left main intrahepatic duct and obvious obstruction at the bifurcation of the bile duct. A biliary drainage catheter was placed, and a repeat cholangiogram 2 days later showed involvement of both right and left intrahepatic ducts. The distal common bile duct appeared uninvolved as did the pancreas. Lymphadenopathy was noted at the liver hilum. Klatskin cholangiocarcinoma (type IIIB) was the presumed diagnosis. Based on these findings, tumor resection was performed 3 weeks later, including left hepatectomy, caudate lobe resection, complete bile duct resection, cholecystectomy, with reconstruction by Roux-en-Y intrahepaticojejunostomy. In addition, portal and hepatic artery lymph node dissection was completed.
Surgical specimens were sent for pathologic evaluation and were found negative for malignancy. Patchy areas of storiform fibrosis, obliterative phlebitis, and lymphoplasmacytic infiltrate were noted. IgG4 immunostain highlighted the presence of IgG4 positive plasma cells with a peak count of 145 IgG4 positive plasma cells/hpf. About 80% of the plasma cells were positive for IgG4. Unusually dense eosinophilic infiltrate with plasma cells and regions of dense fibrosis that strongly contributed to the masslike appearance on CT imaging also were noted. Final histology confirmed the diagnosis of IgG4-RD. Elevated levels of total IgG in the serum were observed without elevation in serum IgG4 (Table).
The patient was started on prednisone 40 mg and azathioprine 150 mg daily, with subsequent taper of prednisone over the next 6 months. After prednisone was discontinued, the patient reported new symptoms of lower extremity pain, neuropathy, and swelling of his face. Laboratory results were notable for elevated erythrocyte sedimentation rate. The patient was restarted on prednisone 40 mg daily. Azathioprine was replaced with a regimen of 4 doses every 6 months of IV rituximab 700 mg q week and mycophenolate mofetil (1,000 mg bid). After remission was induced, the patient was slowly weaned off prednisone again.
Following 6 months of successful discontinuation of prednisone and continued rituximab and mycophenolate mofetil therapy, the patient presented to the emergency department with new onset chest pain and shortness of breath. A CT angiography of the chest showed right upper and middle lobe infiltrate, and he was treated for community acquired pneumonia. Additionally, he was noted to have elevated troponin levels suggestive of myocardial infarction (MI). Initial troponin was 1.23 ng/mL (normal range < 0.015 ng/mL), which trended down over the next 18 hours. A bedside echocardiogram showed a normal left ventricular ejection fraction without wall motion abnormalities. Etiology for his acute MI was presumed to be demand ischemia from fixed atherosclerotic plaque. Further inpatient cardiac risk stratification was changed to the outpatient setting, and he was started on medical management for coronary artery disease with a beta blocker, a statin, and aspirin. He was discharged home on 10 mg prednisone daily, which was subsequently tapered over several weeks.
In follow-up, a Lexiscan myocardial perfusion imaging was conducted that demonstrated an inferolateral defect and associated wall motion abnormalities (Figures 2 and 3).
Discussion
IgG4-related disease has been found to be a systemic disorder. Typical characteristics include predominance in men aged > 50 years, elevated IgG4 levels, and findings on histology.1 It has been reported to involve many organs, including pancreas, liver, gallbladder, salivary glands, thyroid, and pleura of the lung.2,5
This case report begins with a presumptive diagnosis of cholangiocarcinoma, which was treated aggressively with extensive surgery. Several case reports of complex tumefactive lesions in the GI area (mostly pancreatic and biliary) have detailed IgG4-RD as both a risk factor for subsequent development of cholangiocarcinoma and as a separate entity of IgG4-related sclerosing cholangitis.7-9 It is hypothesized that the induction of IgG4- positive plasma cells has been intertwined with the development of cholangiocarcinoma. Differentiation between IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy is challenging. It has been documented that both elevated IgG4 levels and hilar hepatic lesions that resemble cholangiocarcinoma frequently accompany those cases of IgG4 sclerosing chlolangitis without pancreatic involvement.9 The histologic features of IgG4-RD need to be identified with multiple biopsies and cytology, and superficial biopsy from biliary mucosa cannot reliably exclude cholangiocarcinoma.
Lymphoplasmacytic aortitis and arteritis have been documented in IgG4-RD. In 2017, Barbu and colleagues described how one such case of coronary arteritis presented with typical angina and coronary catheterization revealing coronary artery stenosis.10 However, during coronary artery bypass surgery, the aorta and coronary vessels were noted to be abnormally stiff. A diffuse fibrotic tissue was identified to be causing the significant stenosis without evidence of atherosclerosis. Pathology showed typical findings of IgG4-RD, and there was a rapid response to immunosuppressive therapy. Involvement of coronary arteries has been described in a small number of cases at this time and is associated with progressive fibrotic changes resulting in an MI, aneurysms, and sudden cardiac death.2,10,11
IgG4-RD can be an extensively systemic disease. All presentations of fibrosis or vasculitis should be viewed with heightened suspicion in the future as being a facet of his IgG4-RD. Pleural involvement has been reported in 12% of cases presenting with systemic presentation, kidney involvement in 13%.2,12
Unfortunately, there is no standard laboratory parameter to date that is diagnostic for IgG4-RD. The gold standard remains confirmation of histologic findings with biopsy. According to an international consensus from 2015, 2 out of the 3 major findings need to be present: (1) dense lymphoplasmacytic infiltrate; (2) storiform fibrosis; and (3) obliterative phlebitis in veins and arteries.1,5 Most patients present with symptoms related to either tumefaction or fibrosis of an organ system.1 Peripheral eosinophilia and elevated serum IgE are often present in IgG4-RD.13 Although IgG4 values are elevated in 51% of biopsy-proven cases, flow cytometry of CD19lowCD38+CD20-CD27+ plasmablasts has been explored recently as a correlation with disease flare.3,14 These particular plasmablasts mark a stage between B cells and plasma cells and have been reported to have a sensitivity of 95% and a specificity of 82% in association with actual IgG4-RD.14 Furthermore, blood plasmablast concentrations decrease in response to glucocorticoid treatment, thereby providing a possible quantifiable value by which to measure success of IgG4 treatment.5,12
Treatment for this disease consists of immunosuppressive therapy. There is documentation of successful remission with rituximab and azathioprine, as well as methotrexate.1,5 Both 2015 consensus guidelines and a recent small single-center retrospective study support addition of second-line steroid sparing agents such as mycophenolate mofetil.5,6 For acute flairs, however, glucocorticoids with slow taper are usually utilized. In these cases, they should be tapered as soon as clinically feasible to avoid long-term adverse effects. Untreated IgG4-RD, even asymptomatic, has been shown to progress to fibrosis.5
Conclusion
IgG4-RD is a complicated disease process that requires a high index of suspicion to diagnose. In addition, for patients who are diagnosed with this condition, its ability to mimic other pathologic conditions should be taken into account with manifestation of any new illness. This case emphasizes the ability of this disease to localize in multiple organs over time and the need for lifetime surveillance in patients with IgG4-RD disease.
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory condition that involves multiple organs and appears as syndromes that were once thought to be unrelated. This disease leads to mass lesions, fibrosis, and subsequent organ failure if allowed to progress untreated.1 Involvement of gastrointestinal (GI) organs, salivary glands, lacrimal glands, lymph, prostate, pulmonary, and vascular system have all been reported.2 Elevated IgG4 serum levels are common, but about one-third of patients with biopsy-proven IgG4-RD do not manifest this characteristic.3,4
Diagnostic confirmation is with biopsy, and all patients with symptomatic, active IgG4-RD require treatment. Glucocorticoids are first-line treatment and are utilized for relapse of symptoms. In addition to glucocorticoids, steroid-sparing medications, including rituximab, azathioprine, mycophenolate mofetil, tacrolimus, and cyclophosphamide have all been used with successful remission.5,6 Here, the authors discuss a case of IgG4-RD that presented with intrahepatic biliary obstruction (mimicking cholangiocarcinoma) and subsequent development of coronary arteritis despite treatment.
Case Presentation
In June 2015, a 57-year-old Air Force veteran presented to Eglin AFB Hospital with pruritic jaundice and acute abdominal pain. He was found to have elevated bilirubin levels (total bilirubin 10 mg/dL [normal range 0.2-1.3 mg/dL], direct bilirubin 6.6 mg/dL [normal range 0.1-0.4 mg/dL]). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also were moderately elevated (147 U/L and 337 U/L, respectively).
Prior to this presentation, the patient had been in his usual state of health. His past medical history was notable only for minimal change kidney disease (MCD). MCD is defined as effacement of the podocyte seen on electron microscopy, which allows the passage of large amounts of protein.
A cholangiogram showed abnormal filling into the left main intrahepatic duct and obvious obstruction at the bifurcation of the bile duct. A biliary drainage catheter was placed, and a repeat cholangiogram 2 days later showed involvement of both right and left intrahepatic ducts. The distal common bile duct appeared uninvolved as did the pancreas. Lymphadenopathy was noted at the liver hilum. Klatskin cholangiocarcinoma (type IIIB) was the presumed diagnosis. Based on these findings, tumor resection was performed 3 weeks later, including left hepatectomy, caudate lobe resection, complete bile duct resection, cholecystectomy, with reconstruction by Roux-en-Y intrahepaticojejunostomy. In addition, portal and hepatic artery lymph node dissection was completed.
Surgical specimens were sent for pathologic evaluation and were found negative for malignancy. Patchy areas of storiform fibrosis, obliterative phlebitis, and lymphoplasmacytic infiltrate were noted. IgG4 immunostain highlighted the presence of IgG4 positive plasma cells with a peak count of 145 IgG4 positive plasma cells/hpf. About 80% of the plasma cells were positive for IgG4. Unusually dense eosinophilic infiltrate with plasma cells and regions of dense fibrosis that strongly contributed to the masslike appearance on CT imaging also were noted. Final histology confirmed the diagnosis of IgG4-RD. Elevated levels of total IgG in the serum were observed without elevation in serum IgG4 (Table).
The patient was started on prednisone 40 mg and azathioprine 150 mg daily, with subsequent taper of prednisone over the next 6 months. After prednisone was discontinued, the patient reported new symptoms of lower extremity pain, neuropathy, and swelling of his face. Laboratory results were notable for elevated erythrocyte sedimentation rate. The patient was restarted on prednisone 40 mg daily. Azathioprine was replaced with a regimen of 4 doses every 6 months of IV rituximab 700 mg q week and mycophenolate mofetil (1,000 mg bid). After remission was induced, the patient was slowly weaned off prednisone again.
Following 6 months of successful discontinuation of prednisone and continued rituximab and mycophenolate mofetil therapy, the patient presented to the emergency department with new onset chest pain and shortness of breath. A CT angiography of the chest showed right upper and middle lobe infiltrate, and he was treated for community acquired pneumonia. Additionally, he was noted to have elevated troponin levels suggestive of myocardial infarction (MI). Initial troponin was 1.23 ng/mL (normal range < 0.015 ng/mL), which trended down over the next 18 hours. A bedside echocardiogram showed a normal left ventricular ejection fraction without wall motion abnormalities. Etiology for his acute MI was presumed to be demand ischemia from fixed atherosclerotic plaque. Further inpatient cardiac risk stratification was changed to the outpatient setting, and he was started on medical management for coronary artery disease with a beta blocker, a statin, and aspirin. He was discharged home on 10 mg prednisone daily, which was subsequently tapered over several weeks.
In follow-up, a Lexiscan myocardial perfusion imaging was conducted that demonstrated an inferolateral defect and associated wall motion abnormalities (Figures 2 and 3).
Discussion
IgG4-related disease has been found to be a systemic disorder. Typical characteristics include predominance in men aged > 50 years, elevated IgG4 levels, and findings on histology.1 It has been reported to involve many organs, including pancreas, liver, gallbladder, salivary glands, thyroid, and pleura of the lung.2,5
This case report begins with a presumptive diagnosis of cholangiocarcinoma, which was treated aggressively with extensive surgery. Several case reports of complex tumefactive lesions in the GI area (mostly pancreatic and biliary) have detailed IgG4-RD as both a risk factor for subsequent development of cholangiocarcinoma and as a separate entity of IgG4-related sclerosing cholangitis.7-9 It is hypothesized that the induction of IgG4- positive plasma cells has been intertwined with the development of cholangiocarcinoma. Differentiation between IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy is challenging. It has been documented that both elevated IgG4 levels and hilar hepatic lesions that resemble cholangiocarcinoma frequently accompany those cases of IgG4 sclerosing chlolangitis without pancreatic involvement.9 The histologic features of IgG4-RD need to be identified with multiple biopsies and cytology, and superficial biopsy from biliary mucosa cannot reliably exclude cholangiocarcinoma.
Lymphoplasmacytic aortitis and arteritis have been documented in IgG4-RD. In 2017, Barbu and colleagues described how one such case of coronary arteritis presented with typical angina and coronary catheterization revealing coronary artery stenosis.10 However, during coronary artery bypass surgery, the aorta and coronary vessels were noted to be abnormally stiff. A diffuse fibrotic tissue was identified to be causing the significant stenosis without evidence of atherosclerosis. Pathology showed typical findings of IgG4-RD, and there was a rapid response to immunosuppressive therapy. Involvement of coronary arteries has been described in a small number of cases at this time and is associated with progressive fibrotic changes resulting in an MI, aneurysms, and sudden cardiac death.2,10,11
IgG4-RD can be an extensively systemic disease. All presentations of fibrosis or vasculitis should be viewed with heightened suspicion in the future as being a facet of his IgG4-RD. Pleural involvement has been reported in 12% of cases presenting with systemic presentation, kidney involvement in 13%.2,12
Unfortunately, there is no standard laboratory parameter to date that is diagnostic for IgG4-RD. The gold standard remains confirmation of histologic findings with biopsy. According to an international consensus from 2015, 2 out of the 3 major findings need to be present: (1) dense lymphoplasmacytic infiltrate; (2) storiform fibrosis; and (3) obliterative phlebitis in veins and arteries.1,5 Most patients present with symptoms related to either tumefaction or fibrosis of an organ system.1 Peripheral eosinophilia and elevated serum IgE are often present in IgG4-RD.13 Although IgG4 values are elevated in 51% of biopsy-proven cases, flow cytometry of CD19lowCD38+CD20-CD27+ plasmablasts has been explored recently as a correlation with disease flare.3,14 These particular plasmablasts mark a stage between B cells and plasma cells and have been reported to have a sensitivity of 95% and a specificity of 82% in association with actual IgG4-RD.14 Furthermore, blood plasmablast concentrations decrease in response to glucocorticoid treatment, thereby providing a possible quantifiable value by which to measure success of IgG4 treatment.5,12
Treatment for this disease consists of immunosuppressive therapy. There is documentation of successful remission with rituximab and azathioprine, as well as methotrexate.1,5 Both 2015 consensus guidelines and a recent small single-center retrospective study support addition of second-line steroid sparing agents such as mycophenolate mofetil.5,6 For acute flairs, however, glucocorticoids with slow taper are usually utilized. In these cases, they should be tapered as soon as clinically feasible to avoid long-term adverse effects. Untreated IgG4-RD, even asymptomatic, has been shown to progress to fibrosis.5
Conclusion
IgG4-RD is a complicated disease process that requires a high index of suspicion to diagnose. In addition, for patients who are diagnosed with this condition, its ability to mimic other pathologic conditions should be taken into account with manifestation of any new illness. This case emphasizes the ability of this disease to localize in multiple organs over time and the need for lifetime surveillance in patients with IgG4-RD disease.
1. Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189-199.
2. Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
3. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466-2475.
4. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-RD. Ann Rheum Dis. 2015;74(1):14-18.
5. Khosroshahi A, Wallace ZS, Crowe JL, et al; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688-1699.
6. Gupta N, Mathew J, Mohan H, et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of immunoglobulin G4-related disease (IgG4-RD): a series from a tertiary care teaching hospital in South India. Rheumatol Int. 2017;38(2):203-209.
7. Lin HP, Lin KT, Ho WC, Chen CB, Kuo, CY, Lin YC. IgG4-associated cholangitis mimicking cholangiocarcinoma-report of a case. J Intern Med Taiwan. 2013;24:137-141.
8. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol. 2013;5(8):181-185.
9. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol. 2014;2014:803876.
10. Barbu M, Lindström U, Nordborg C, Martinsson A, Dworeck C, Jeppsson A. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. 2017;103(6):e487-e489.
11. Kim YJ, Park YS, Koo BS, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17(8):451-452.
12. Khosroshahi A, Digumarthy SR, Gibbons FK, Deshpande V. Case 34-2015: A 36-year-old woman with a lung mass, pleural effusion and hip pain. N Engl J Med. 2015;373(18):1762-1772.
13. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):191-206.
14. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195.
1. Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189-199.
2. Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
3. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466-2475.
4. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-RD. Ann Rheum Dis. 2015;74(1):14-18.
5. Khosroshahi A, Wallace ZS, Crowe JL, et al; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688-1699.
6. Gupta N, Mathew J, Mohan H, et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of immunoglobulin G4-related disease (IgG4-RD): a series from a tertiary care teaching hospital in South India. Rheumatol Int. 2017;38(2):203-209.
7. Lin HP, Lin KT, Ho WC, Chen CB, Kuo, CY, Lin YC. IgG4-associated cholangitis mimicking cholangiocarcinoma-report of a case. J Intern Med Taiwan. 2013;24:137-141.
8. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol. 2013;5(8):181-185.
9. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol. 2014;2014:803876.
10. Barbu M, Lindström U, Nordborg C, Martinsson A, Dworeck C, Jeppsson A. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. 2017;103(6):e487-e489.
11. Kim YJ, Park YS, Koo BS, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17(8):451-452.
12. Khosroshahi A, Digumarthy SR, Gibbons FK, Deshpande V. Case 34-2015: A 36-year-old woman with a lung mass, pleural effusion and hip pain. N Engl J Med. 2015;373(18):1762-1772.
13. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):191-206.
14. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195.
Primary renal synovial sarcoma – a diagnostic dilemma
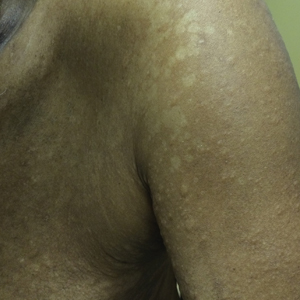
Soft tissue sarcomas are rare mesenchymal tumors that comprise 1% of all malignancies. Synovial sarcoma accounts for 5% to 10% of adult soft tissue sarcomas and usually occurs in close association with joint capsules, tendon sheaths, and bursa in the extremities of young and middle-aged adults.1 Synovial sarcomas have been reported in other unusual sites, including the head and neck, thoracic and abdominal wall, retroperitoneum, bone, pleura, and visceral organs such as the lung, prostate, or kidney.2 Primary renal synovial sarcoma is an extremely rare tumor accounting for <2% of all malignant renal tumors.3 To the best of our knowledge, fewer than 50 cases of primary renal synovial sarcoma have been described in the English literature.4 It presents as a diagnostic dilemma because of the dearth of specific clinical and imaging findings and is often confused with benign and malignant tumors. The differential diagnosis includes angiomyolipoma, renal cell carcinoma with sarcomatoid differentiation, metastatic sarcoma, hemangiopericytoma, malignant solitary fibrous tumor, Wilms tumor, and malignant peripheral nerve sheath tumor. Hence, a combination of histomorphologic, immunohistochemical, cytogenetic, and molecular studies that show a unique chromosomal translocation t(X;18) (p11;q11) is imperative in the diagnosis of primary renal synovial sarcoma.4 In the present report, we present the case of a 38-year-old man who was diagnosed with primary renal synovial sarcoma.
Case presentation and summary
A 38-year-old man with a medical history of gastroesophageal reflux disease and Barrett’s esophagus presented to our hospital for the first time with persistent and progressive right-sided flank and abdominal pain that was aggravated after a minor trauma to the back. There was no associated hematuria or dysuria.
Of note is that he had experienced intermittent flank pain for 2 years before this transfer. He had initially been diagnosed at his local hospital close to his home by ultrasound with an angiomyolipoma of 2 × 3 cm arising from the upper pole of his right kidney, which remained stable on repeat sonograms. About 22 months after his initial presentation at his local hospital, the flank pain increased, and a computed-tomographic (CT) scan revealed a perinephric hematoma that was thought to originate from a ruptured angiomyolipoma. He subsequently underwent embolization, but his symptoms recurred soon after. He presented again to his local hospital where CT imaging revealed a significant increase in the size of the retroperitoneal mass, and findings were suggestive of a hematoma. Subsequent angiogram did not reveal active extravasation, so a biopsy was performed.
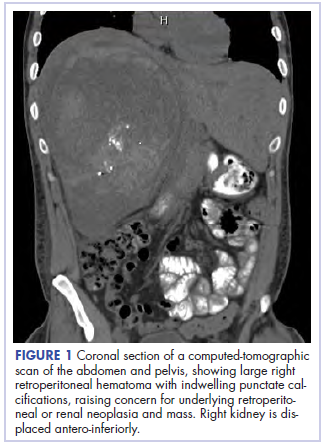
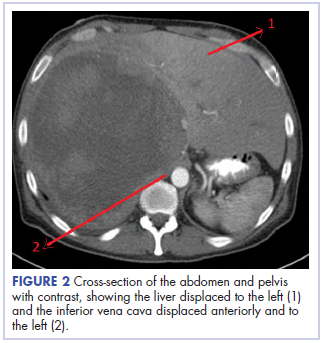
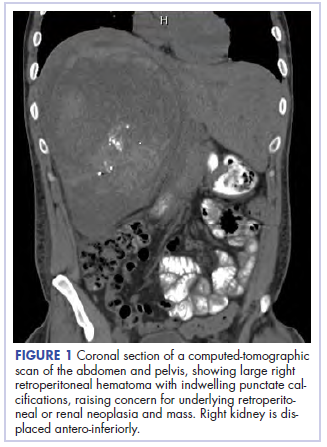
Before confirmatory pathologic evaluation could be completed, the patient presented to his local hospital again in excruciating pain. A CT scan of his abdomen and pelvis demonstrated a massive subacute on chronic hematoma in the right retroperitoneum measuring 22 × 19 × 18 cm, with calcifications originating from an upper pole right renal neoplasm. The right kidney was displaced antero-inferiorly, and the inferior vena cava was displaced anteriorly and to the left. The preliminary pathology returned with findings suggestive of sarcoma (Figures 1 and 2).
The patient was then transferred to our institution, where he was evaluated by medical and surgical oncology. A CT scan of the chest and magnetic-resonance imaging (MRI) of the brain did not reveal metastatic disease. He underwent exploratory laparotomy that involved the resection of a 22-cm retroperitoneal mass, right nephrectomy, right adrenalectomy, partial right hepatectomy, and a full thickness resection of the right postero-inferior diaphragm followed by mesh repair because of involvement by the tumor.
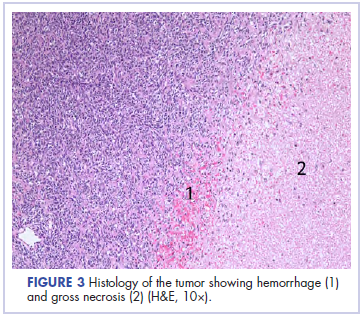
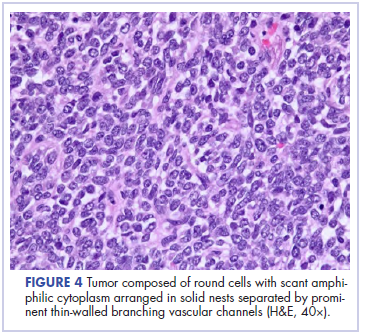
In its entirety, the specimen was a mass of 26 × 24 × 14 cm. It was sectioned to show extensively necrotic and hemorrhagic variegated white to tan-red parenchyma (Figure 3). Histology revealed a poorly differentiated malignant neoplasm composed of round cells with scant amphophilic cytoplasm arranged in solid, variably sized nests separated by prominent thin-walled branching vascular channels (Figure 4). The mitotic rate was high. It was determined to be a histologically ungraded sarcoma according to the French Federation of Comprehensive Cancer Centers system of grading soft tissue sarcomas; the margins were indeterminate. Immunohistochemistry was positive for EMA, TLE1, and negative for AE1/AE3, S100, STAT6, and Nkx2.2. Molecular pathology fluorescent in situ hybridization (FISH) analysis demonstrated positivity for SS18 gene rearrangement (SS18-SSX1 fusion).
After recovering from surgery, the patient received adjuvant chemotherapy with doxorubicin and ifosfamide. It has been almost 16 months since we first saw this patient. He was started on doxorubicin 20 mg/m2 on days 1 to 4, ifosfamide 2,500 mg on days 1 to 4, and mesna 800 mg on days 1 to 4, for a total of 6 cycles. He did well for the first 5 months, after which he developed disease recurrence in the postoperative nephrectomy bed (a biopsy showed it to be recurrent synovial sarcoma) as well as pulmonary nodules, for which he was started on trabectedin 1.5 mg/m2 every 3 weeks. Two months later, a CT scan showed an increase in the size of his retroperitoneal mass, and the treatment was changed to pazopanib 400 mg daily orally, on which he remained at the time of publication.
Discussion
Synovial sarcoma is the fourth most common type of soft tissue sarcoma, accounting for 2.5% to 10.5% of all primary soft tissue malignancies worldwide. It occurs most frequently in adolescents and young adults, with most patients presenting between the ages of 15 and 40 years. Median age of presentation is 36 years. Despite the nomenclature, synovial sarcoma does not arise in intra-articular locations but typically occurs in proximity to joints in the extremities. Synovial sarcomas are less commonly described in other sites, including the head and neck, mediastinum, intraperitoneum, retroperitoneum, lung, pleura, and kidney.4,5 Renal synovial sarcoma was first described in a published article by Argani and colleagues in 2000.5
Adult renal mesenchymal tumors are classified into benign and malignant tumors on the basis of the histologic features and clinicobiologic behavior.6,7 The benign esenchymal renal tumors include angiomyolipoma, leiomyoma, hemangioma, lymphangioma, juxtaglomerular cell tumor, renomedullary interstitial cell tumor (medullary fibroma), lipoma, solitary fibrous tumor, and schwannoma. Malignant renal tumors of mesenchymal origin include leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, osteosarcoma, fibrosarcoma, malignant fibrous histiocytoma, solitary fibrous tumor, and synovial sarcoma.
Most of these tumor types cause the same nonspecific symptoms in patients – abdominal pain, flank pain, abdominal fullness, a palpable mass, and hematuria – although they can be clinically silent. The average duration of symptoms in synovial sarcoma is 2 to 4 years.8 The long duration of symptoms and initial slow growth of synovial sarcomas may give a false impression of a benign process.
A preoperative radiological diagnosis of primary renal synovial sarcoma may be suspected by analyzing the tumor’s growth patterns on CT scans.9 Renal synovial sarcomas often appear as large, well-defined soft tissue masses that can extend into the renal pelvis or into the perinephric region.9 A CT scan may identify soft tissue calcifications, especially subtle ones in areas where the tumor anatomy is complex. A CT scan may also reveal areas of hemorrhage, necrosis, or cyst formation within the tumor, and can easily confirm bone involvement. Intravenous contrast may help in differentiating the mass from adjacent muscle and neurovascular complex.9,10 On MRI, renal synovial sarcomas are often described as nonspecific heterogeneous masses, although they may also exhibit heterogeneous enhancement of hemorrhagic areas, calcifications, and air-fluid levels (known as “triple sign”) as well as septae. The triple sign may be identified as areas of low, intermediate, and high signal intensity, correlating with areas of hemorrhage, calcification, and air-fluid level.9,10 Signal intensity is about equal to that of skeletal muscle on T1-weighted MRI and higher than that of subcutaneous fat on T2-weighted MRI.
In the present case, the tumor was initially misdiagnosed as an angiomyolipoma, the most common benign tumor of the kidney. Angiomyolipomas are usually solid triphasic tumors arising from the renal cortex and are composed of 3 major elements: dysmorphic blood vessels, smooth muscle components, and adipose tissue. When angiomyolipomas are large enough, they are readily recognized by the identification of macroscopic fat within the tumor, either by CT scan or MRI.11 When they are small, they may be difficult to distinguish from a small cyst on CT because of volume averaging.
On pathology, synovial sarcoma has dual epithelial and mesenchymal differentiation. They are frequently multi-lobulated, and areas of necrosis, hemorrhage, and cyst formation are also common. There are 3 main histologic subtypes of synovial sarcoma: biphasic (20%-30%), monophasic (50%-60%), and poorly differentiated (15%-25%). Poorly differentiated synovial sarcomas are generally epithelioid in morphology, have high mitotic activity (usually 10-20 mitoses/10 high-power field; range is <5 for well differentiated, low-grade tumors), and can be confused with round cell tumors such as Ewing sarcoma. Poorly differentiated synovial sarcomas are high-grade tumors.
Immunohistochemical studies can confirm the pathological diagnosis. Synovial sarcomas usually stain positive for Bcl2, CD99/Mic2, CD56, Vim, and focally for EMA but negatively for desmin, actin, WT1, S-100, CD34, and CD31.5 Currently, the gold standard for diagnosis and hallmark for synovial sarcomas are the t (X;18) translocation and SYT-SSX gene fusion products (SYT-SSX1 in 67% and SYT-SSX2 in 33% of cases). These can be detected either by FISH or reverse-transcription polymerase chain reaction. This genetic alteration is identified in more than 90% of synovial sarcomas and is highly specific.
The role of SYT-SSX gene fusion in the pathogenesis of synovial sarcoma is an active area of investigation. The fusion of SYT with SSX translates into a fusion protein that binds to the transcription activator SMARCA4 that is involved in chromatin remodeling, thus displacing both the wildtype SYT and the tumor suppressor gene SMARCB1. The modified protein complex then binds at several super-enhancer loci, unlocking suppressed genes such as Sox2, which is known to be necessary for synovial sarcoma proliferation. Alterations in SMARCB1 are involved in several cancer types, implicating this event as a driver of these malignancies.12 This results in a global alteration in chromatin remodeling that needs to be better understood to design targeted therapies.
The clinical course of synovial sarcoma, regardless of the tissue of origin, is typically poor. Multiple clinical and pathologic factors, including tumor size, location, patient age, and presence of poorly differentiated areas, are thought to have prognostic significance. A tumor size of more than 5 cm at presentation has the greatest impact on prognosis, with studies showing 5-year survival rates of 64% for patients with tumors smaller than 5 cm and 26% for patients with masses greater than 5 cm.13,14 High-grade synovial sarcoma is favored in tumors that have cystic components, hemorrhage, and fluid levels and the triple sign.
Patients with tumors in the extremities have a more favorable prognosis than those with lesions in the head and neck area or axially, a feature that likely reflects better surgical control available for extremity lesions. Patient age of less than 15 to 20 years is also associated with a better long-term prognosis.15,16 Varela-Duran and Enzinger17 reported that the presence of extensive calcifications suggests improved long-term survival, with 5-year survival rates of 82% and decreased rates of local recurrence (32%) and metastatic disease (29%). The poorly differentiated subtype is associated with a worsened prognosis, with a 5-year survival rate of 20% through 30%.18,19 Other pathologic factors associated with worsened prognosis include presence of rhabdoid cells, extensive tumor necrosis, high nuclear grade, p53 mutations, and high mitotic rate (>10 mitoses/10 high-power field). More recently, the gene fusion type SYT-SSX2 (more common in monophasic lesions) has been associated with an improved prognosis, compared with that for SYT-SSX1, and an 89% metastasis-free survival.20
Although there are no guidelines for the treatment of primary renal synovial sarcoma because of the limited number of cases reported, surgery is considered the first choice. Adjuvant chemotherapy with an anthracycline (doxorubicin or epirubicin) combined with ifosfamide has been the most frequently used regimen in published cases, especially in those in which patients have poor prognostic factors as mentioned above.
Overall, the 5-year survival rate ranges from 36% to 76%.14 The clinical course of synovial sarcoma is characterized by a high rate of local recurrence (30%-50%) and metastatic disease (41%). Most metastases occur within the first 2 to 5 years after treatment cessation. Metastases are present in 16% to 25% of patients at their initial presentation, with the most frequent metastatic site being the lung, followed by the lymph nodes (4%-18%) and bone (8%-11%).
Conclusion
Primary renal synovial sarcoma is extremely rare, and preoperative diagnosis is difficult in the absence of specific clinical or imaging findings. A high index of suspicion combined with pathologic, immunohistochemical, cytogenetic, and molecular studies is essential for accurate diagnosis and subsequent treatment planning. The differential diagnosis of renal synovial sarcoma can be extensive, and our experience with this patient illustrates the diagnostic dilemma associated with renal synovial sarcoma.
1. Majumder A, Dey S, Khandakar B, Medda S, Chandra Paul P. Primary renal synovial sarcoma: a rare tumor with an atypical presentation. Arch Iran Med. 2014;17(10):726-728.
2. Fetsch JF, Meis JM. Synovial sarcoma of the abdominal wall. Cancer. 1993;72(2):469 477.
3. Wang Z, Zhong Z, Zhu L, et al. Primary synovial sarcoma of the kidney: a case report. Oncol Lett. 2015;10(6):3542-3544.
4. Abbas M, Dämmrich ME, Braubach P, et al. Synovial sarcoma of the kidney in a young patient with a review of the literature. Rare tumors. 2014;6(2):5393
5. Argani P, Faria PA, Epstein JI, et al. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol. 2000;24(8):1087-1096.
6. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, eds. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC; 2004.
7. Tamboli P, Ro JY, Amin MB, Ligato S, Ayala AG. Benign tumors and tumor-like lesions of the adult kidney. Part II: benign mesenchymal and mixed neoplasms, and tumor-like lesions. Adv Anat Pathol. 2000;7(1):47-66.
8. Weiss SW, Goldblum JR. Malignant soft tissue tumors of uncertain type. In: Weiss SW, Goldblum JR, eds. Enzinger and Weiss’s soft tissue tumors. 4th ed. St. Louis, MO: Mosby, 2001; 1483-1565.
9. Lacovelli R, Altavilla A, Ciardi A, et al. Clinical and pathological features of primary renal synovial sarcoma: analysis of 64 cases from 11 years of medical literature. BJU Int. 2012;110(10):1449-1454.
10. Alhazzani AR, El-Sharkawy MS, Hassan H. Primary retroperitoneal synovial sarcoma in CT and MRI. Urol Ann. 2010;2(1):39-41.
11. Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525-1540.
12. Sápi Z, Papp G, Szendrői M, et al. Epigenetic regulation of SMARCB1 by miR-206, -381 and -671- 5p is evident in a variety of SMARCB1 immunonegative soft tissue sarcomas, while miR-765 appears specific for epithelioid sarcoma. A miRNA study of 223 soft tissue sarcomas. Genes Chromosomes Cancer. 2016;55(10):786-802.
13. Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627-634.
14. Rangheard AS, Vanel D, Viala J, Schwaab G, Casiraghi O, Sigal R. Synovial sarcomas of the head and neck: CT and MR imaging findings of eight patients. Am J Neuroradiol. 2001;22(5):851-857.
15. Oda Y, Hashimoto H, Tsuneyoshi M, Takeshita S. Survival in synovial sarcoma: a multivariate study of prognostic factors with special emphasis on the comparison between early death and long-term survival. Am J Surg Pathol. 1993;17(1):35-44.
16. Raney RB. Synovial sarcoma in young people: background, prognostic factors and therapeutic questions. J Pediatr Hematol Oncol. 2005;27(4):207-211.
17. Varela-Duran J, Enzinger FM. Calcifying synovial sarcoma. Cancer. 1982;50(2):345-352.
18. Cagle LA, Mirra JM, Storm FK, Roe DJ, Eilber FR. Histologic features relating to prognosis in synovial sarcoma. Cancer. 1987;59(10):1810-1814.
19. Skytting B, Meis-Kindblom JM, Larsson O, et al. Synovial sarcoma – identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand. 1999:70(6):543-554.
20. Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006;26(5):1543-1565.
Soft tissue sarcomas are rare mesenchymal tumors that comprise 1% of all malignancies. Synovial sarcoma accounts for 5% to 10% of adult soft tissue sarcomas and usually occurs in close association with joint capsules, tendon sheaths, and bursa in the extremities of young and middle-aged adults.1 Synovial sarcomas have been reported in other unusual sites, including the head and neck, thoracic and abdominal wall, retroperitoneum, bone, pleura, and visceral organs such as the lung, prostate, or kidney.2 Primary renal synovial sarcoma is an extremely rare tumor accounting for <2% of all malignant renal tumors.3 To the best of our knowledge, fewer than 50 cases of primary renal synovial sarcoma have been described in the English literature.4 It presents as a diagnostic dilemma because of the dearth of specific clinical and imaging findings and is often confused with benign and malignant tumors. The differential diagnosis includes angiomyolipoma, renal cell carcinoma with sarcomatoid differentiation, metastatic sarcoma, hemangiopericytoma, malignant solitary fibrous tumor, Wilms tumor, and malignant peripheral nerve sheath tumor. Hence, a combination of histomorphologic, immunohistochemical, cytogenetic, and molecular studies that show a unique chromosomal translocation t(X;18) (p11;q11) is imperative in the diagnosis of primary renal synovial sarcoma.4 In the present report, we present the case of a 38-year-old man who was diagnosed with primary renal synovial sarcoma.
Case presentation and summary
A 38-year-old man with a medical history of gastroesophageal reflux disease and Barrett’s esophagus presented to our hospital for the first time with persistent and progressive right-sided flank and abdominal pain that was aggravated after a minor trauma to the back. There was no associated hematuria or dysuria.
Of note is that he had experienced intermittent flank pain for 2 years before this transfer. He had initially been diagnosed at his local hospital close to his home by ultrasound with an angiomyolipoma of 2 × 3 cm arising from the upper pole of his right kidney, which remained stable on repeat sonograms. About 22 months after his initial presentation at his local hospital, the flank pain increased, and a computed-tomographic (CT) scan revealed a perinephric hematoma that was thought to originate from a ruptured angiomyolipoma. He subsequently underwent embolization, but his symptoms recurred soon after. He presented again to his local hospital where CT imaging revealed a significant increase in the size of the retroperitoneal mass, and findings were suggestive of a hematoma. Subsequent angiogram did not reveal active extravasation, so a biopsy was performed.
Before confirmatory pathologic evaluation could be completed, the patient presented to his local hospital again in excruciating pain. A CT scan of his abdomen and pelvis demonstrated a massive subacute on chronic hematoma in the right retroperitoneum measuring 22 × 19 × 18 cm, with calcifications originating from an upper pole right renal neoplasm. The right kidney was displaced antero-inferiorly, and the inferior vena cava was displaced anteriorly and to the left. The preliminary pathology returned with findings suggestive of sarcoma (Figures 1 and 2).
The patient was then transferred to our institution, where he was evaluated by medical and surgical oncology. A CT scan of the chest and magnetic-resonance imaging (MRI) of the brain did not reveal metastatic disease. He underwent exploratory laparotomy that involved the resection of a 22-cm retroperitoneal mass, right nephrectomy, right adrenalectomy, partial right hepatectomy, and a full thickness resection of the right postero-inferior diaphragm followed by mesh repair because of involvement by the tumor.
In its entirety, the specimen was a mass of 26 × 24 × 14 cm. It was sectioned to show extensively necrotic and hemorrhagic variegated white to tan-red parenchyma (Figure 3). Histology revealed a poorly differentiated malignant neoplasm composed of round cells with scant amphophilic cytoplasm arranged in solid, variably sized nests separated by prominent thin-walled branching vascular channels (Figure 4). The mitotic rate was high. It was determined to be a histologically ungraded sarcoma according to the French Federation of Comprehensive Cancer Centers system of grading soft tissue sarcomas; the margins were indeterminate. Immunohistochemistry was positive for EMA, TLE1, and negative for AE1/AE3, S100, STAT6, and Nkx2.2. Molecular pathology fluorescent in situ hybridization (FISH) analysis demonstrated positivity for SS18 gene rearrangement (SS18-SSX1 fusion).
After recovering from surgery, the patient received adjuvant chemotherapy with doxorubicin and ifosfamide. It has been almost 16 months since we first saw this patient. He was started on doxorubicin 20 mg/m2 on days 1 to 4, ifosfamide 2,500 mg on days 1 to 4, and mesna 800 mg on days 1 to 4, for a total of 6 cycles. He did well for the first 5 months, after which he developed disease recurrence in the postoperative nephrectomy bed (a biopsy showed it to be recurrent synovial sarcoma) as well as pulmonary nodules, for which he was started on trabectedin 1.5 mg/m2 every 3 weeks. Two months later, a CT scan showed an increase in the size of his retroperitoneal mass, and the treatment was changed to pazopanib 400 mg daily orally, on which he remained at the time of publication.
Discussion
Synovial sarcoma is the fourth most common type of soft tissue sarcoma, accounting for 2.5% to 10.5% of all primary soft tissue malignancies worldwide. It occurs most frequently in adolescents and young adults, with most patients presenting between the ages of 15 and 40 years. Median age of presentation is 36 years. Despite the nomenclature, synovial sarcoma does not arise in intra-articular locations but typically occurs in proximity to joints in the extremities. Synovial sarcomas are less commonly described in other sites, including the head and neck, mediastinum, intraperitoneum, retroperitoneum, lung, pleura, and kidney.4,5 Renal synovial sarcoma was first described in a published article by Argani and colleagues in 2000.5
Adult renal mesenchymal tumors are classified into benign and malignant tumors on the basis of the histologic features and clinicobiologic behavior.6,7 The benign esenchymal renal tumors include angiomyolipoma, leiomyoma, hemangioma, lymphangioma, juxtaglomerular cell tumor, renomedullary interstitial cell tumor (medullary fibroma), lipoma, solitary fibrous tumor, and schwannoma. Malignant renal tumors of mesenchymal origin include leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, osteosarcoma, fibrosarcoma, malignant fibrous histiocytoma, solitary fibrous tumor, and synovial sarcoma.
Most of these tumor types cause the same nonspecific symptoms in patients – abdominal pain, flank pain, abdominal fullness, a palpable mass, and hematuria – although they can be clinically silent. The average duration of symptoms in synovial sarcoma is 2 to 4 years.8 The long duration of symptoms and initial slow growth of synovial sarcomas may give a false impression of a benign process.
A preoperative radiological diagnosis of primary renal synovial sarcoma may be suspected by analyzing the tumor’s growth patterns on CT scans.9 Renal synovial sarcomas often appear as large, well-defined soft tissue masses that can extend into the renal pelvis or into the perinephric region.9 A CT scan may identify soft tissue calcifications, especially subtle ones in areas where the tumor anatomy is complex. A CT scan may also reveal areas of hemorrhage, necrosis, or cyst formation within the tumor, and can easily confirm bone involvement. Intravenous contrast may help in differentiating the mass from adjacent muscle and neurovascular complex.9,10 On MRI, renal synovial sarcomas are often described as nonspecific heterogeneous masses, although they may also exhibit heterogeneous enhancement of hemorrhagic areas, calcifications, and air-fluid levels (known as “triple sign”) as well as septae. The triple sign may be identified as areas of low, intermediate, and high signal intensity, correlating with areas of hemorrhage, calcification, and air-fluid level.9,10 Signal intensity is about equal to that of skeletal muscle on T1-weighted MRI and higher than that of subcutaneous fat on T2-weighted MRI.
In the present case, the tumor was initially misdiagnosed as an angiomyolipoma, the most common benign tumor of the kidney. Angiomyolipomas are usually solid triphasic tumors arising from the renal cortex and are composed of 3 major elements: dysmorphic blood vessels, smooth muscle components, and adipose tissue. When angiomyolipomas are large enough, they are readily recognized by the identification of macroscopic fat within the tumor, either by CT scan or MRI.11 When they are small, they may be difficult to distinguish from a small cyst on CT because of volume averaging.
On pathology, synovial sarcoma has dual epithelial and mesenchymal differentiation. They are frequently multi-lobulated, and areas of necrosis, hemorrhage, and cyst formation are also common. There are 3 main histologic subtypes of synovial sarcoma: biphasic (20%-30%), monophasic (50%-60%), and poorly differentiated (15%-25%). Poorly differentiated synovial sarcomas are generally epithelioid in morphology, have high mitotic activity (usually 10-20 mitoses/10 high-power field; range is <5 for well differentiated, low-grade tumors), and can be confused with round cell tumors such as Ewing sarcoma. Poorly differentiated synovial sarcomas are high-grade tumors.
Immunohistochemical studies can confirm the pathological diagnosis. Synovial sarcomas usually stain positive for Bcl2, CD99/Mic2, CD56, Vim, and focally for EMA but negatively for desmin, actin, WT1, S-100, CD34, and CD31.5 Currently, the gold standard for diagnosis and hallmark for synovial sarcomas are the t (X;18) translocation and SYT-SSX gene fusion products (SYT-SSX1 in 67% and SYT-SSX2 in 33% of cases). These can be detected either by FISH or reverse-transcription polymerase chain reaction. This genetic alteration is identified in more than 90% of synovial sarcomas and is highly specific.
The role of SYT-SSX gene fusion in the pathogenesis of synovial sarcoma is an active area of investigation. The fusion of SYT with SSX translates into a fusion protein that binds to the transcription activator SMARCA4 that is involved in chromatin remodeling, thus displacing both the wildtype SYT and the tumor suppressor gene SMARCB1. The modified protein complex then binds at several super-enhancer loci, unlocking suppressed genes such as Sox2, which is known to be necessary for synovial sarcoma proliferation. Alterations in SMARCB1 are involved in several cancer types, implicating this event as a driver of these malignancies.12 This results in a global alteration in chromatin remodeling that needs to be better understood to design targeted therapies.
The clinical course of synovial sarcoma, regardless of the tissue of origin, is typically poor. Multiple clinical and pathologic factors, including tumor size, location, patient age, and presence of poorly differentiated areas, are thought to have prognostic significance. A tumor size of more than 5 cm at presentation has the greatest impact on prognosis, with studies showing 5-year survival rates of 64% for patients with tumors smaller than 5 cm and 26% for patients with masses greater than 5 cm.13,14 High-grade synovial sarcoma is favored in tumors that have cystic components, hemorrhage, and fluid levels and the triple sign.
Patients with tumors in the extremities have a more favorable prognosis than those with lesions in the head and neck area or axially, a feature that likely reflects better surgical control available for extremity lesions. Patient age of less than 15 to 20 years is also associated with a better long-term prognosis.15,16 Varela-Duran and Enzinger17 reported that the presence of extensive calcifications suggests improved long-term survival, with 5-year survival rates of 82% and decreased rates of local recurrence (32%) and metastatic disease (29%). The poorly differentiated subtype is associated with a worsened prognosis, with a 5-year survival rate of 20% through 30%.18,19 Other pathologic factors associated with worsened prognosis include presence of rhabdoid cells, extensive tumor necrosis, high nuclear grade, p53 mutations, and high mitotic rate (>10 mitoses/10 high-power field). More recently, the gene fusion type SYT-SSX2 (more common in monophasic lesions) has been associated with an improved prognosis, compared with that for SYT-SSX1, and an 89% metastasis-free survival.20
Although there are no guidelines for the treatment of primary renal synovial sarcoma because of the limited number of cases reported, surgery is considered the first choice. Adjuvant chemotherapy with an anthracycline (doxorubicin or epirubicin) combined with ifosfamide has been the most frequently used regimen in published cases, especially in those in which patients have poor prognostic factors as mentioned above.
Overall, the 5-year survival rate ranges from 36% to 76%.14 The clinical course of synovial sarcoma is characterized by a high rate of local recurrence (30%-50%) and metastatic disease (41%). Most metastases occur within the first 2 to 5 years after treatment cessation. Metastases are present in 16% to 25% of patients at their initial presentation, with the most frequent metastatic site being the lung, followed by the lymph nodes (4%-18%) and bone (8%-11%).
Conclusion
Primary renal synovial sarcoma is extremely rare, and preoperative diagnosis is difficult in the absence of specific clinical or imaging findings. A high index of suspicion combined with pathologic, immunohistochemical, cytogenetic, and molecular studies is essential for accurate diagnosis and subsequent treatment planning. The differential diagnosis of renal synovial sarcoma can be extensive, and our experience with this patient illustrates the diagnostic dilemma associated with renal synovial sarcoma.
Soft tissue sarcomas are rare mesenchymal tumors that comprise 1% of all malignancies. Synovial sarcoma accounts for 5% to 10% of adult soft tissue sarcomas and usually occurs in close association with joint capsules, tendon sheaths, and bursa in the extremities of young and middle-aged adults.1 Synovial sarcomas have been reported in other unusual sites, including the head and neck, thoracic and abdominal wall, retroperitoneum, bone, pleura, and visceral organs such as the lung, prostate, or kidney.2 Primary renal synovial sarcoma is an extremely rare tumor accounting for <2% of all malignant renal tumors.3 To the best of our knowledge, fewer than 50 cases of primary renal synovial sarcoma have been described in the English literature.4 It presents as a diagnostic dilemma because of the dearth of specific clinical and imaging findings and is often confused with benign and malignant tumors. The differential diagnosis includes angiomyolipoma, renal cell carcinoma with sarcomatoid differentiation, metastatic sarcoma, hemangiopericytoma, malignant solitary fibrous tumor, Wilms tumor, and malignant peripheral nerve sheath tumor. Hence, a combination of histomorphologic, immunohistochemical, cytogenetic, and molecular studies that show a unique chromosomal translocation t(X;18) (p11;q11) is imperative in the diagnosis of primary renal synovial sarcoma.4 In the present report, we present the case of a 38-year-old man who was diagnosed with primary renal synovial sarcoma.
Case presentation and summary
A 38-year-old man with a medical history of gastroesophageal reflux disease and Barrett’s esophagus presented to our hospital for the first time with persistent and progressive right-sided flank and abdominal pain that was aggravated after a minor trauma to the back. There was no associated hematuria or dysuria.
Of note is that he had experienced intermittent flank pain for 2 years before this transfer. He had initially been diagnosed at his local hospital close to his home by ultrasound with an angiomyolipoma of 2 × 3 cm arising from the upper pole of his right kidney, which remained stable on repeat sonograms. About 22 months after his initial presentation at his local hospital, the flank pain increased, and a computed-tomographic (CT) scan revealed a perinephric hematoma that was thought to originate from a ruptured angiomyolipoma. He subsequently underwent embolization, but his symptoms recurred soon after. He presented again to his local hospital where CT imaging revealed a significant increase in the size of the retroperitoneal mass, and findings were suggestive of a hematoma. Subsequent angiogram did not reveal active extravasation, so a biopsy was performed.
Before confirmatory pathologic evaluation could be completed, the patient presented to his local hospital again in excruciating pain. A CT scan of his abdomen and pelvis demonstrated a massive subacute on chronic hematoma in the right retroperitoneum measuring 22 × 19 × 18 cm, with calcifications originating from an upper pole right renal neoplasm. The right kidney was displaced antero-inferiorly, and the inferior vena cava was displaced anteriorly and to the left. The preliminary pathology returned with findings suggestive of sarcoma (Figures 1 and 2).
The patient was then transferred to our institution, where he was evaluated by medical and surgical oncology. A CT scan of the chest and magnetic-resonance imaging (MRI) of the brain did not reveal metastatic disease. He underwent exploratory laparotomy that involved the resection of a 22-cm retroperitoneal mass, right nephrectomy, right adrenalectomy, partial right hepatectomy, and a full thickness resection of the right postero-inferior diaphragm followed by mesh repair because of involvement by the tumor.
In its entirety, the specimen was a mass of 26 × 24 × 14 cm. It was sectioned to show extensively necrotic and hemorrhagic variegated white to tan-red parenchyma (Figure 3). Histology revealed a poorly differentiated malignant neoplasm composed of round cells with scant amphophilic cytoplasm arranged in solid, variably sized nests separated by prominent thin-walled branching vascular channels (Figure 4). The mitotic rate was high. It was determined to be a histologically ungraded sarcoma according to the French Federation of Comprehensive Cancer Centers system of grading soft tissue sarcomas; the margins were indeterminate. Immunohistochemistry was positive for EMA, TLE1, and negative for AE1/AE3, S100, STAT6, and Nkx2.2. Molecular pathology fluorescent in situ hybridization (FISH) analysis demonstrated positivity for SS18 gene rearrangement (SS18-SSX1 fusion).
After recovering from surgery, the patient received adjuvant chemotherapy with doxorubicin and ifosfamide. It has been almost 16 months since we first saw this patient. He was started on doxorubicin 20 mg/m2 on days 1 to 4, ifosfamide 2,500 mg on days 1 to 4, and mesna 800 mg on days 1 to 4, for a total of 6 cycles. He did well for the first 5 months, after which he developed disease recurrence in the postoperative nephrectomy bed (a biopsy showed it to be recurrent synovial sarcoma) as well as pulmonary nodules, for which he was started on trabectedin 1.5 mg/m2 every 3 weeks. Two months later, a CT scan showed an increase in the size of his retroperitoneal mass, and the treatment was changed to pazopanib 400 mg daily orally, on which he remained at the time of publication.
Discussion
Synovial sarcoma is the fourth most common type of soft tissue sarcoma, accounting for 2.5% to 10.5% of all primary soft tissue malignancies worldwide. It occurs most frequently in adolescents and young adults, with most patients presenting between the ages of 15 and 40 years. Median age of presentation is 36 years. Despite the nomenclature, synovial sarcoma does not arise in intra-articular locations but typically occurs in proximity to joints in the extremities. Synovial sarcomas are less commonly described in other sites, including the head and neck, mediastinum, intraperitoneum, retroperitoneum, lung, pleura, and kidney.4,5 Renal synovial sarcoma was first described in a published article by Argani and colleagues in 2000.5
Adult renal mesenchymal tumors are classified into benign and malignant tumors on the basis of the histologic features and clinicobiologic behavior.6,7 The benign esenchymal renal tumors include angiomyolipoma, leiomyoma, hemangioma, lymphangioma, juxtaglomerular cell tumor, renomedullary interstitial cell tumor (medullary fibroma), lipoma, solitary fibrous tumor, and schwannoma. Malignant renal tumors of mesenchymal origin include leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, osteosarcoma, fibrosarcoma, malignant fibrous histiocytoma, solitary fibrous tumor, and synovial sarcoma.
Most of these tumor types cause the same nonspecific symptoms in patients – abdominal pain, flank pain, abdominal fullness, a palpable mass, and hematuria – although they can be clinically silent. The average duration of symptoms in synovial sarcoma is 2 to 4 years.8 The long duration of symptoms and initial slow growth of synovial sarcomas may give a false impression of a benign process.
A preoperative radiological diagnosis of primary renal synovial sarcoma may be suspected by analyzing the tumor’s growth patterns on CT scans.9 Renal synovial sarcomas often appear as large, well-defined soft tissue masses that can extend into the renal pelvis or into the perinephric region.9 A CT scan may identify soft tissue calcifications, especially subtle ones in areas where the tumor anatomy is complex. A CT scan may also reveal areas of hemorrhage, necrosis, or cyst formation within the tumor, and can easily confirm bone involvement. Intravenous contrast may help in differentiating the mass from adjacent muscle and neurovascular complex.9,10 On MRI, renal synovial sarcomas are often described as nonspecific heterogeneous masses, although they may also exhibit heterogeneous enhancement of hemorrhagic areas, calcifications, and air-fluid levels (known as “triple sign”) as well as septae. The triple sign may be identified as areas of low, intermediate, and high signal intensity, correlating with areas of hemorrhage, calcification, and air-fluid level.9,10 Signal intensity is about equal to that of skeletal muscle on T1-weighted MRI and higher than that of subcutaneous fat on T2-weighted MRI.
In the present case, the tumor was initially misdiagnosed as an angiomyolipoma, the most common benign tumor of the kidney. Angiomyolipomas are usually solid triphasic tumors arising from the renal cortex and are composed of 3 major elements: dysmorphic blood vessels, smooth muscle components, and adipose tissue. When angiomyolipomas are large enough, they are readily recognized by the identification of macroscopic fat within the tumor, either by CT scan or MRI.11 When they are small, they may be difficult to distinguish from a small cyst on CT because of volume averaging.
On pathology, synovial sarcoma has dual epithelial and mesenchymal differentiation. They are frequently multi-lobulated, and areas of necrosis, hemorrhage, and cyst formation are also common. There are 3 main histologic subtypes of synovial sarcoma: biphasic (20%-30%), monophasic (50%-60%), and poorly differentiated (15%-25%). Poorly differentiated synovial sarcomas are generally epithelioid in morphology, have high mitotic activity (usually 10-20 mitoses/10 high-power field; range is <5 for well differentiated, low-grade tumors), and can be confused with round cell tumors such as Ewing sarcoma. Poorly differentiated synovial sarcomas are high-grade tumors.
Immunohistochemical studies can confirm the pathological diagnosis. Synovial sarcomas usually stain positive for Bcl2, CD99/Mic2, CD56, Vim, and focally for EMA but negatively for desmin, actin, WT1, S-100, CD34, and CD31.5 Currently, the gold standard for diagnosis and hallmark for synovial sarcomas are the t (X;18) translocation and SYT-SSX gene fusion products (SYT-SSX1 in 67% and SYT-SSX2 in 33% of cases). These can be detected either by FISH or reverse-transcription polymerase chain reaction. This genetic alteration is identified in more than 90% of synovial sarcomas and is highly specific.
The role of SYT-SSX gene fusion in the pathogenesis of synovial sarcoma is an active area of investigation. The fusion of SYT with SSX translates into a fusion protein that binds to the transcription activator SMARCA4 that is involved in chromatin remodeling, thus displacing both the wildtype SYT and the tumor suppressor gene SMARCB1. The modified protein complex then binds at several super-enhancer loci, unlocking suppressed genes such as Sox2, which is known to be necessary for synovial sarcoma proliferation. Alterations in SMARCB1 are involved in several cancer types, implicating this event as a driver of these malignancies.12 This results in a global alteration in chromatin remodeling that needs to be better understood to design targeted therapies.
The clinical course of synovial sarcoma, regardless of the tissue of origin, is typically poor. Multiple clinical and pathologic factors, including tumor size, location, patient age, and presence of poorly differentiated areas, are thought to have prognostic significance. A tumor size of more than 5 cm at presentation has the greatest impact on prognosis, with studies showing 5-year survival rates of 64% for patients with tumors smaller than 5 cm and 26% for patients with masses greater than 5 cm.13,14 High-grade synovial sarcoma is favored in tumors that have cystic components, hemorrhage, and fluid levels and the triple sign.
Patients with tumors in the extremities have a more favorable prognosis than those with lesions in the head and neck area or axially, a feature that likely reflects better surgical control available for extremity lesions. Patient age of less than 15 to 20 years is also associated with a better long-term prognosis.15,16 Varela-Duran and Enzinger17 reported that the presence of extensive calcifications suggests improved long-term survival, with 5-year survival rates of 82% and decreased rates of local recurrence (32%) and metastatic disease (29%). The poorly differentiated subtype is associated with a worsened prognosis, with a 5-year survival rate of 20% through 30%.18,19 Other pathologic factors associated with worsened prognosis include presence of rhabdoid cells, extensive tumor necrosis, high nuclear grade, p53 mutations, and high mitotic rate (>10 mitoses/10 high-power field). More recently, the gene fusion type SYT-SSX2 (more common in monophasic lesions) has been associated with an improved prognosis, compared with that for SYT-SSX1, and an 89% metastasis-free survival.20
Although there are no guidelines for the treatment of primary renal synovial sarcoma because of the limited number of cases reported, surgery is considered the first choice. Adjuvant chemotherapy with an anthracycline (doxorubicin or epirubicin) combined with ifosfamide has been the most frequently used regimen in published cases, especially in those in which patients have poor prognostic factors as mentioned above.
Overall, the 5-year survival rate ranges from 36% to 76%.14 The clinical course of synovial sarcoma is characterized by a high rate of local recurrence (30%-50%) and metastatic disease (41%). Most metastases occur within the first 2 to 5 years after treatment cessation. Metastases are present in 16% to 25% of patients at their initial presentation, with the most frequent metastatic site being the lung, followed by the lymph nodes (4%-18%) and bone (8%-11%).
Conclusion
Primary renal synovial sarcoma is extremely rare, and preoperative diagnosis is difficult in the absence of specific clinical or imaging findings. A high index of suspicion combined with pathologic, immunohistochemical, cytogenetic, and molecular studies is essential for accurate diagnosis and subsequent treatment planning. The differential diagnosis of renal synovial sarcoma can be extensive, and our experience with this patient illustrates the diagnostic dilemma associated with renal synovial sarcoma.
1. Majumder A, Dey S, Khandakar B, Medda S, Chandra Paul P. Primary renal synovial sarcoma: a rare tumor with an atypical presentation. Arch Iran Med. 2014;17(10):726-728.
2. Fetsch JF, Meis JM. Synovial sarcoma of the abdominal wall. Cancer. 1993;72(2):469 477.
3. Wang Z, Zhong Z, Zhu L, et al. Primary synovial sarcoma of the kidney: a case report. Oncol Lett. 2015;10(6):3542-3544.
4. Abbas M, Dämmrich ME, Braubach P, et al. Synovial sarcoma of the kidney in a young patient with a review of the literature. Rare tumors. 2014;6(2):5393
5. Argani P, Faria PA, Epstein JI, et al. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol. 2000;24(8):1087-1096.
6. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, eds. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC; 2004.
7. Tamboli P, Ro JY, Amin MB, Ligato S, Ayala AG. Benign tumors and tumor-like lesions of the adult kidney. Part II: benign mesenchymal and mixed neoplasms, and tumor-like lesions. Adv Anat Pathol. 2000;7(1):47-66.
8. Weiss SW, Goldblum JR. Malignant soft tissue tumors of uncertain type. In: Weiss SW, Goldblum JR, eds. Enzinger and Weiss’s soft tissue tumors. 4th ed. St. Louis, MO: Mosby, 2001; 1483-1565.
9. Lacovelli R, Altavilla A, Ciardi A, et al. Clinical and pathological features of primary renal synovial sarcoma: analysis of 64 cases from 11 years of medical literature. BJU Int. 2012;110(10):1449-1454.
10. Alhazzani AR, El-Sharkawy MS, Hassan H. Primary retroperitoneal synovial sarcoma in CT and MRI. Urol Ann. 2010;2(1):39-41.
11. Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525-1540.
12. Sápi Z, Papp G, Szendrői M, et al. Epigenetic regulation of SMARCB1 by miR-206, -381 and -671- 5p is evident in a variety of SMARCB1 immunonegative soft tissue sarcomas, while miR-765 appears specific for epithelioid sarcoma. A miRNA study of 223 soft tissue sarcomas. Genes Chromosomes Cancer. 2016;55(10):786-802.
13. Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627-634.
14. Rangheard AS, Vanel D, Viala J, Schwaab G, Casiraghi O, Sigal R. Synovial sarcomas of the head and neck: CT and MR imaging findings of eight patients. Am J Neuroradiol. 2001;22(5):851-857.
15. Oda Y, Hashimoto H, Tsuneyoshi M, Takeshita S. Survival in synovial sarcoma: a multivariate study of prognostic factors with special emphasis on the comparison between early death and long-term survival. Am J Surg Pathol. 1993;17(1):35-44.
16. Raney RB. Synovial sarcoma in young people: background, prognostic factors and therapeutic questions. J Pediatr Hematol Oncol. 2005;27(4):207-211.
17. Varela-Duran J, Enzinger FM. Calcifying synovial sarcoma. Cancer. 1982;50(2):345-352.
18. Cagle LA, Mirra JM, Storm FK, Roe DJ, Eilber FR. Histologic features relating to prognosis in synovial sarcoma. Cancer. 1987;59(10):1810-1814.
19. Skytting B, Meis-Kindblom JM, Larsson O, et al. Synovial sarcoma – identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand. 1999:70(6):543-554.
20. Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006;26(5):1543-1565.
1. Majumder A, Dey S, Khandakar B, Medda S, Chandra Paul P. Primary renal synovial sarcoma: a rare tumor with an atypical presentation. Arch Iran Med. 2014;17(10):726-728.
2. Fetsch JF, Meis JM. Synovial sarcoma of the abdominal wall. Cancer. 1993;72(2):469 477.
3. Wang Z, Zhong Z, Zhu L, et al. Primary synovial sarcoma of the kidney: a case report. Oncol Lett. 2015;10(6):3542-3544.
4. Abbas M, Dämmrich ME, Braubach P, et al. Synovial sarcoma of the kidney in a young patient with a review of the literature. Rare tumors. 2014;6(2):5393
5. Argani P, Faria PA, Epstein JI, et al. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol. 2000;24(8):1087-1096.
6. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, eds. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC; 2004.
7. Tamboli P, Ro JY, Amin MB, Ligato S, Ayala AG. Benign tumors and tumor-like lesions of the adult kidney. Part II: benign mesenchymal and mixed neoplasms, and tumor-like lesions. Adv Anat Pathol. 2000;7(1):47-66.
8. Weiss SW, Goldblum JR. Malignant soft tissue tumors of uncertain type. In: Weiss SW, Goldblum JR, eds. Enzinger and Weiss’s soft tissue tumors. 4th ed. St. Louis, MO: Mosby, 2001; 1483-1565.
9. Lacovelli R, Altavilla A, Ciardi A, et al. Clinical and pathological features of primary renal synovial sarcoma: analysis of 64 cases from 11 years of medical literature. BJU Int. 2012;110(10):1449-1454.
10. Alhazzani AR, El-Sharkawy MS, Hassan H. Primary retroperitoneal synovial sarcoma in CT and MRI. Urol Ann. 2010;2(1):39-41.
11. Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525-1540.
12. Sápi Z, Papp G, Szendrői M, et al. Epigenetic regulation of SMARCB1 by miR-206, -381 and -671- 5p is evident in a variety of SMARCB1 immunonegative soft tissue sarcomas, while miR-765 appears specific for epithelioid sarcoma. A miRNA study of 223 soft tissue sarcomas. Genes Chromosomes Cancer. 2016;55(10):786-802.
13. Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627-634.
14. Rangheard AS, Vanel D, Viala J, Schwaab G, Casiraghi O, Sigal R. Synovial sarcomas of the head and neck: CT and MR imaging findings of eight patients. Am J Neuroradiol. 2001;22(5):851-857.
15. Oda Y, Hashimoto H, Tsuneyoshi M, Takeshita S. Survival in synovial sarcoma: a multivariate study of prognostic factors with special emphasis on the comparison between early death and long-term survival. Am J Surg Pathol. 1993;17(1):35-44.
16. Raney RB. Synovial sarcoma in young people: background, prognostic factors and therapeutic questions. J Pediatr Hematol Oncol. 2005;27(4):207-211.
17. Varela-Duran J, Enzinger FM. Calcifying synovial sarcoma. Cancer. 1982;50(2):345-352.
18. Cagle LA, Mirra JM, Storm FK, Roe DJ, Eilber FR. Histologic features relating to prognosis in synovial sarcoma. Cancer. 1987;59(10):1810-1814.
19. Skytting B, Meis-Kindblom JM, Larsson O, et al. Synovial sarcoma – identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand. 1999:70(6):543-554.
20. Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006;26(5):1543-1565.
Cutaneous Angiosarcoma of the Lower Leg
Angiosarcoma is a rare and aggressive vascular malignant neoplasm derived from endothelial cells. In general, sarcomas account for approximately 1% of all malignancies, with approximately 2% being angiosarcomas.1 The risk of recurrence at 5 years is estimated to be 84%, and 5-year survival is estimated at 15% to 30%. Poor prognostic factors for angiosarcoma include large tumor size, depth of invasion greater than 3 mm, high mitotic rate, positive surgical margins, and metastasis.2 Approximately 20% to 40% of patients who are diagnosed with angiosarcoma already have distant metastasis, contributing to the aggressive nature of this neoplasm.3
Angiosarcoma can affect various anatomic locations, including the skin, soft tissue, breasts, and liver. Cutaneous angiosarcoma is the most common clinical manifestation, accounting for approximately 50% to 60% of all cases, and typically is known to occur in 3 distinct settings.2 Primary or idiopathic cutaneous angiosarcoma is most commonly seen in elderly individuals, with a peak incidence in the seventh to eighth decades of life, and presents as a bruiselike lesion predominantly on the head and neck. Angiosarcoma also is seen clinically in patients exposed to radiation treatment, with a median onset of symptoms occurring 5 to 10 years posttreatment, and in patients with chronic lymphedema, usually on the arm following radical mastectomy, which also is known as Stewart-Treves syndrome.2
With any sarcoma, treatment typically first involves surgical excision; however, there is no direct approach for treatment of cutaneous angiosarcoma, as an individual plan typically is needed for each patient. Treatment options include surgical excision, radiation, chemotherapy, or a combination of these therapies.2,4
We present a rare case of cutaneous angiosarcoma of the left leg in the setting of chronic venous insufficiency with some degree of lymphedema and a nonhealing ulcer. This case is unique in that it does not fit the classic presentation of cutaneous angiosarcoma previously described.
Case Report
An 83-year-old woman with a medical history of advanced dementia, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, type 2 diabetes mellitus, hypertension, and chronic venous insufficiency with stasis dermatitis presented to the emergency department following a mechanical fall. Most of her medical history was obtained from the patient’s family. She had a history of multiple falls originally thought to be related to a chronic leg ulcer that had been managed with wound care. Recently, however, the lesion was noted to have increasing erythema surrounding the wound margins. An 8×8-cm erythematous plaque on the anterior lateral left leg with a firm central nodule with hemorrhagic crust that measured approximately 4 cm in diameter was noted by the emergency department physicians (Figure 1). In the emergency department, vitals and other laboratory values were within reference range, and a radiograph of the left tibia/fibula was unremarkable. Cellulitis initially was considered in the emergency department and cephalexin was started; however, since the patient was afebrile and had no leukocytosis, plastic surgery also was consulted. Biopsies were obtained from the superior and inferior parts of the lesion. Histologic analysis revealed a poorly differentiated vascular neoplasm of epithelioid endothelial cells with considerable cell atypia that extended through the entirety of the dermis (Figure 2). The tumor cells stained positive with vimentin and CD34. Pathology noted no immunohistochemistry stains to synaptophysin, S-100, human melanoma black 45, MART-1, CK20, CK7, CK8/18, CK5/6, and p63. The pathologic diagnosis was consistent with cutaneous angiosarcoma. Computed tomography of the chest, abdomen, and pelvis revealed no local or distant metastases.

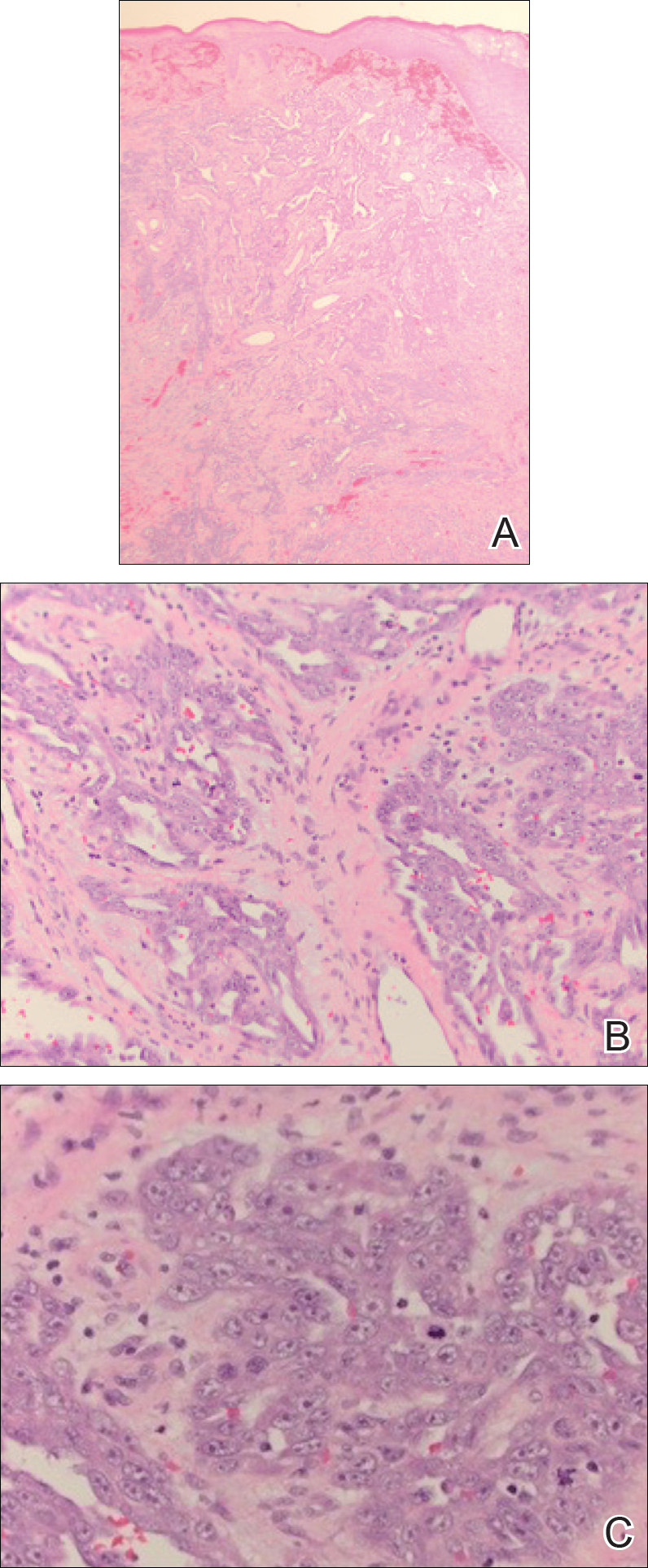
A wide excision of the cutaneous angiosarcoma was performed. The initial frozen section analysis revealed positive margins. Three additional excisions still showed positive margins, and further excision was held after obtaining family consent due to the extensive nature of the neoplasm and lengthy operating room time. The final defect after excision measured 15×10×2.5 cm (Figure 3A), and subsequent application of a split-thickness graft was performed. Additional treatment options were discussed with the family, including radiation therapy, amputation of the left lower leg, or no treatment. The family opted not to proceed with further treatment. The graft healed without signs of reoccurrence approximately 3 months later (Figure 3B), and the patient received physical therapy, which allowed her to gain strength and some independence.

Comment
Clinical Manifestation
Cutaneous angiosarcoma is a rare malignant vascular neoplasm that when clinically diagnosed is typically seen in 3 settings: (1) idiopathic (commonly on the face and neck), (2) following radiation treatment, and (3) classically following mastectomy with subsequent chronic lymphedema. Our patient did not classically fit these settings of cutaneous angiosarcoma due to the location of the lesion on the lower leg as well as its occurrence in the setting of a chronic nonhealing ulcer and lymphedema.
Chronic lymphedema is a common clinical manifestation that is likely secondary to other medical conditions, such as in our patient. As a result, these patients are at increased risk for developing chronic ulcers due to poor wound healing; however, as seen in our patient, chronic nonhealing ulcers require a broad differential because they may clinically mimic many processes. Patient history and visual presentation were crucial in this case because a biopsy was obtained that ultimately led to the patient’s diagnosis.
Differential Diagnosis
Initially, a venous ulcer secondary to chronic venous insufficiency was considered in the differential for our patient. She had a history of congestive heart failure, kidney disease, and type 2 diabetes mellitus, all of which contribute to lymphedema and/or poor wound healing. However, venous ulcers usually are located on the medial ankles and are irregularly shaped with an erythematous border and fibrinous exudate with central depression, making it a less likely diagnosis in our patient. Additionally, an infectious process was considered, but the patient was afebrile and laboratory values demonstrated no leukocytosis.
Marjolin ulcer was highly suspected because the clinical presentation revealed a nodule with hemorrhagic crust and induration in the setting of a chronic nonhealing ulcer. The pathogenesis of malignancy in chronic ulcers is thought to be due to continuous mitotic activity from regeneration and repair of the wound, especially in the setting of repeated trauma to the area.5 In our patient, the history of multiple falls with possible multitrauma injury to the chronic ulcer further increased suspicion of malignancy. The most common and frequently seen malignancy that develops in chronic ulcers is squamous cell carcinoma (SCC) followed by basal cell carcinoma. Plastic surgery suspected an SCC for the working diagnosis, which prompted a punch biopsy; however, the histologic analysis was not consistent with SCC or basal cell carcinoma. Marjolin ulcer also may demonstrate a periosteal reaction,5 which was not the case with our patient after a radiograph of the left tibia/fibula was unremarkable.
Another potential malignancy to consider is melanoma. There are few case reports of biopsy-proven melanoma from an enlarging chronic ulcer.6,7 Additionally, poorly differentiated angiosarcoma can mimic melanoma2; however, immunohistochemistry stain was negative for S-100, human melanoma black 45, and MART-1, making melanoma unlikely.
Kaposi sarcoma (KS) and angiosarcoma are both malignant vascular tumors that similarly present with red to purple patches, plaques, or nodules, making it difficult to distinguish between the two conditions. It is important to note that KS usually is lower grade, and the pathogenesis is linked to human herpesvirus 8, which can be identified on immunohistochemistry staining. There have been cases of KS reported in patients who have no history of human immunodeficiency virus/AIDS, thus the classic subtype of KS may have been considered in this patient.8 The histologic appearance of KS may vary from dilated irregular endothelial cells lining the vascular space to mild endothelial cell atypia. Histology also shows hemosiderin-laden macrophages, extravasated red blood cells, and an inflammatory infiltrate. An additional malignant vascular neoplasm that needs to be differentiated is epithelioid hemangioendothelioma. Cutaneous presentation of an epithelioid hemangioendothelioma may be similar to what was seen in our patient but histologically will usually show neoplastic cells with pale eosinophilic cytoplasm and vesicular nuclei of plump, oval, polygonal cells in cords or aggregates surrounding vascular channels. These neoplasms also tend to occur around medium- to large-sized veins.1,9 With our patient, even though human herpesvirus 8 was not tested with immunohistochemistry, gold standard immunohistochemistry confirmation with CD34 and vimentin staining combined with poorly differentiated endothelial atypia with mitotic figures on histologic analysis favored angiosarcoma versus KS or epithelioid hemangioendothelioma.10,11
Management
Cutaneous angiosarcoma is a rare and aggressive vascular neoplasm accounting for approximately 2% of all combined sarcomas, with an estimated 20% to 40% having distant metastasis at diagnosis.1,3 For this reason, computed tomography was performed in our patient and revealed no local or distant metastasis. Therefore, chemotherapy was not an appropriate adjuvant treatment option.12 With no evidence of metastasis, initial treatment began with surgical removal but proved to be difficult in our patient. Although the implications of positive surgical margins remain unclear with regard to overall patient survival, surgical resection followed by radiation therapy has been shown to be optimal, as it reduces the risk of local reoccurrence.3 There have been reported cases of cutaneous angiosarcoma of the leg that were treated with amputation without signs of reoccurrence or metastasis.10,13,14 Given the results from these cases and considering that our patient had no metastasis, amputation seemed to be a good prognostic option; however, considering other factors regarding the patient’s comorbidities and quality of life, her family decided not to pursue any further treatment with amputation or radiation therapy.
Conclusion
There should be low threshold for biopsy in patients who present with nonhealing wounds that do not progress in the normal phase of wound healing with suspicion for malignancy. As seen with our patient, cutaneous angiosarcoma can clinically mimic many disease processes, and although rare in nature, it should always be considered when a patient presents with a rapidly growing lesion in the setting of chronic lymphedema or venous ulcer.
- Kumar V, Abbas A, Aster J. Robbins Basic Pathology. 9th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Dossett LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64.
- Gerslova A, Pokorna A, Stukavcova A, et al. Rare cause of non-healing foot wound—acral lentiginous melanoma. Neuro Endocrinol Lett. 2012;37:12-17.
- Turk BG, Bozkurt A, Yaman B, et al. Melanoma arising in chronic ulceration associated with lymphoedema. J Wound Care. 2013;22:74-75.
- Phavixay L, Raynolds D, Simman R. Non AIDS Kaposi’s sarcoma leading to lower extremities wounds, case presentations and discussion.J Am Coll Clin Wound Spec. 2012;4:13-15.
- Requena L, Kutzner H. Hemangioendothelioma. Semin Diagn Pathol. 2013;30:29-44.
- Harrison WD, Chandrasekar CR. Stewart-Treves syndrome following idiopathic leg lymphoedema: remember sarcoma. J Wound Care. 2015;24(6 suppl):S5-S7.
- Kak I, Salama S, Gohla G, et al. A case of patch stage of Kaposi’s sarcoma and discussion of the differential diagnosis. Rare Tumors. 2016;8:6123.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Linda DD, Harish S, Alowami S, et al. Radiology-pathology conference: cutaneous angiosarcoma of the leg. Clin Imaging. 2013;37:602-607.
- Roy P, Clark MA, Thomas JM. Stewart-Treves syndrome—treatment and outcome in six patients from a single centre. Eur J Surg Oncol. 2004;30:982-986.
Angiosarcoma is a rare and aggressive vascular malignant neoplasm derived from endothelial cells. In general, sarcomas account for approximately 1% of all malignancies, with approximately 2% being angiosarcomas.1 The risk of recurrence at 5 years is estimated to be 84%, and 5-year survival is estimated at 15% to 30%. Poor prognostic factors for angiosarcoma include large tumor size, depth of invasion greater than 3 mm, high mitotic rate, positive surgical margins, and metastasis.2 Approximately 20% to 40% of patients who are diagnosed with angiosarcoma already have distant metastasis, contributing to the aggressive nature of this neoplasm.3
Angiosarcoma can affect various anatomic locations, including the skin, soft tissue, breasts, and liver. Cutaneous angiosarcoma is the most common clinical manifestation, accounting for approximately 50% to 60% of all cases, and typically is known to occur in 3 distinct settings.2 Primary or idiopathic cutaneous angiosarcoma is most commonly seen in elderly individuals, with a peak incidence in the seventh to eighth decades of life, and presents as a bruiselike lesion predominantly on the head and neck. Angiosarcoma also is seen clinically in patients exposed to radiation treatment, with a median onset of symptoms occurring 5 to 10 years posttreatment, and in patients with chronic lymphedema, usually on the arm following radical mastectomy, which also is known as Stewart-Treves syndrome.2
With any sarcoma, treatment typically first involves surgical excision; however, there is no direct approach for treatment of cutaneous angiosarcoma, as an individual plan typically is needed for each patient. Treatment options include surgical excision, radiation, chemotherapy, or a combination of these therapies.2,4
We present a rare case of cutaneous angiosarcoma of the left leg in the setting of chronic venous insufficiency with some degree of lymphedema and a nonhealing ulcer. This case is unique in that it does not fit the classic presentation of cutaneous angiosarcoma previously described.
Case Report
An 83-year-old woman with a medical history of advanced dementia, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, type 2 diabetes mellitus, hypertension, and chronic venous insufficiency with stasis dermatitis presented to the emergency department following a mechanical fall. Most of her medical history was obtained from the patient’s family. She had a history of multiple falls originally thought to be related to a chronic leg ulcer that had been managed with wound care. Recently, however, the lesion was noted to have increasing erythema surrounding the wound margins. An 8×8-cm erythematous plaque on the anterior lateral left leg with a firm central nodule with hemorrhagic crust that measured approximately 4 cm in diameter was noted by the emergency department physicians (Figure 1). In the emergency department, vitals and other laboratory values were within reference range, and a radiograph of the left tibia/fibula was unremarkable. Cellulitis initially was considered in the emergency department and cephalexin was started; however, since the patient was afebrile and had no leukocytosis, plastic surgery also was consulted. Biopsies were obtained from the superior and inferior parts of the lesion. Histologic analysis revealed a poorly differentiated vascular neoplasm of epithelioid endothelial cells with considerable cell atypia that extended through the entirety of the dermis (Figure 2). The tumor cells stained positive with vimentin and CD34. Pathology noted no immunohistochemistry stains to synaptophysin, S-100, human melanoma black 45, MART-1, CK20, CK7, CK8/18, CK5/6, and p63. The pathologic diagnosis was consistent with cutaneous angiosarcoma. Computed tomography of the chest, abdomen, and pelvis revealed no local or distant metastases.


A wide excision of the cutaneous angiosarcoma was performed. The initial frozen section analysis revealed positive margins. Three additional excisions still showed positive margins, and further excision was held after obtaining family consent due to the extensive nature of the neoplasm and lengthy operating room time. The final defect after excision measured 15×10×2.5 cm (Figure 3A), and subsequent application of a split-thickness graft was performed. Additional treatment options were discussed with the family, including radiation therapy, amputation of the left lower leg, or no treatment. The family opted not to proceed with further treatment. The graft healed without signs of reoccurrence approximately 3 months later (Figure 3B), and the patient received physical therapy, which allowed her to gain strength and some independence.

Comment
Clinical Manifestation
Cutaneous angiosarcoma is a rare malignant vascular neoplasm that when clinically diagnosed is typically seen in 3 settings: (1) idiopathic (commonly on the face and neck), (2) following radiation treatment, and (3) classically following mastectomy with subsequent chronic lymphedema. Our patient did not classically fit these settings of cutaneous angiosarcoma due to the location of the lesion on the lower leg as well as its occurrence in the setting of a chronic nonhealing ulcer and lymphedema.
Chronic lymphedema is a common clinical manifestation that is likely secondary to other medical conditions, such as in our patient. As a result, these patients are at increased risk for developing chronic ulcers due to poor wound healing; however, as seen in our patient, chronic nonhealing ulcers require a broad differential because they may clinically mimic many processes. Patient history and visual presentation were crucial in this case because a biopsy was obtained that ultimately led to the patient’s diagnosis.
Differential Diagnosis
Initially, a venous ulcer secondary to chronic venous insufficiency was considered in the differential for our patient. She had a history of congestive heart failure, kidney disease, and type 2 diabetes mellitus, all of which contribute to lymphedema and/or poor wound healing. However, venous ulcers usually are located on the medial ankles and are irregularly shaped with an erythematous border and fibrinous exudate with central depression, making it a less likely diagnosis in our patient. Additionally, an infectious process was considered, but the patient was afebrile and laboratory values demonstrated no leukocytosis.
Marjolin ulcer was highly suspected because the clinical presentation revealed a nodule with hemorrhagic crust and induration in the setting of a chronic nonhealing ulcer. The pathogenesis of malignancy in chronic ulcers is thought to be due to continuous mitotic activity from regeneration and repair of the wound, especially in the setting of repeated trauma to the area.5 In our patient, the history of multiple falls with possible multitrauma injury to the chronic ulcer further increased suspicion of malignancy. The most common and frequently seen malignancy that develops in chronic ulcers is squamous cell carcinoma (SCC) followed by basal cell carcinoma. Plastic surgery suspected an SCC for the working diagnosis, which prompted a punch biopsy; however, the histologic analysis was not consistent with SCC or basal cell carcinoma. Marjolin ulcer also may demonstrate a periosteal reaction,5 which was not the case with our patient after a radiograph of the left tibia/fibula was unremarkable.
Another potential malignancy to consider is melanoma. There are few case reports of biopsy-proven melanoma from an enlarging chronic ulcer.6,7 Additionally, poorly differentiated angiosarcoma can mimic melanoma2; however, immunohistochemistry stain was negative for S-100, human melanoma black 45, and MART-1, making melanoma unlikely.
Kaposi sarcoma (KS) and angiosarcoma are both malignant vascular tumors that similarly present with red to purple patches, plaques, or nodules, making it difficult to distinguish between the two conditions. It is important to note that KS usually is lower grade, and the pathogenesis is linked to human herpesvirus 8, which can be identified on immunohistochemistry staining. There have been cases of KS reported in patients who have no history of human immunodeficiency virus/AIDS, thus the classic subtype of KS may have been considered in this patient.8 The histologic appearance of KS may vary from dilated irregular endothelial cells lining the vascular space to mild endothelial cell atypia. Histology also shows hemosiderin-laden macrophages, extravasated red blood cells, and an inflammatory infiltrate. An additional malignant vascular neoplasm that needs to be differentiated is epithelioid hemangioendothelioma. Cutaneous presentation of an epithelioid hemangioendothelioma may be similar to what was seen in our patient but histologically will usually show neoplastic cells with pale eosinophilic cytoplasm and vesicular nuclei of plump, oval, polygonal cells in cords or aggregates surrounding vascular channels. These neoplasms also tend to occur around medium- to large-sized veins.1,9 With our patient, even though human herpesvirus 8 was not tested with immunohistochemistry, gold standard immunohistochemistry confirmation with CD34 and vimentin staining combined with poorly differentiated endothelial atypia with mitotic figures on histologic analysis favored angiosarcoma versus KS or epithelioid hemangioendothelioma.10,11
Management
Cutaneous angiosarcoma is a rare and aggressive vascular neoplasm accounting for approximately 2% of all combined sarcomas, with an estimated 20% to 40% having distant metastasis at diagnosis.1,3 For this reason, computed tomography was performed in our patient and revealed no local or distant metastasis. Therefore, chemotherapy was not an appropriate adjuvant treatment option.12 With no evidence of metastasis, initial treatment began with surgical removal but proved to be difficult in our patient. Although the implications of positive surgical margins remain unclear with regard to overall patient survival, surgical resection followed by radiation therapy has been shown to be optimal, as it reduces the risk of local reoccurrence.3 There have been reported cases of cutaneous angiosarcoma of the leg that were treated with amputation without signs of reoccurrence or metastasis.10,13,14 Given the results from these cases and considering that our patient had no metastasis, amputation seemed to be a good prognostic option; however, considering other factors regarding the patient’s comorbidities and quality of life, her family decided not to pursue any further treatment with amputation or radiation therapy.
Conclusion
There should be low threshold for biopsy in patients who present with nonhealing wounds that do not progress in the normal phase of wound healing with suspicion for malignancy. As seen with our patient, cutaneous angiosarcoma can clinically mimic many disease processes, and although rare in nature, it should always be considered when a patient presents with a rapidly growing lesion in the setting of chronic lymphedema or venous ulcer.
Angiosarcoma is a rare and aggressive vascular malignant neoplasm derived from endothelial cells. In general, sarcomas account for approximately 1% of all malignancies, with approximately 2% being angiosarcomas.1 The risk of recurrence at 5 years is estimated to be 84%, and 5-year survival is estimated at 15% to 30%. Poor prognostic factors for angiosarcoma include large tumor size, depth of invasion greater than 3 mm, high mitotic rate, positive surgical margins, and metastasis.2 Approximately 20% to 40% of patients who are diagnosed with angiosarcoma already have distant metastasis, contributing to the aggressive nature of this neoplasm.3
Angiosarcoma can affect various anatomic locations, including the skin, soft tissue, breasts, and liver. Cutaneous angiosarcoma is the most common clinical manifestation, accounting for approximately 50% to 60% of all cases, and typically is known to occur in 3 distinct settings.2 Primary or idiopathic cutaneous angiosarcoma is most commonly seen in elderly individuals, with a peak incidence in the seventh to eighth decades of life, and presents as a bruiselike lesion predominantly on the head and neck. Angiosarcoma also is seen clinically in patients exposed to radiation treatment, with a median onset of symptoms occurring 5 to 10 years posttreatment, and in patients with chronic lymphedema, usually on the arm following radical mastectomy, which also is known as Stewart-Treves syndrome.2
With any sarcoma, treatment typically first involves surgical excision; however, there is no direct approach for treatment of cutaneous angiosarcoma, as an individual plan typically is needed for each patient. Treatment options include surgical excision, radiation, chemotherapy, or a combination of these therapies.2,4
We present a rare case of cutaneous angiosarcoma of the left leg in the setting of chronic venous insufficiency with some degree of lymphedema and a nonhealing ulcer. This case is unique in that it does not fit the classic presentation of cutaneous angiosarcoma previously described.
Case Report
An 83-year-old woman with a medical history of advanced dementia, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, type 2 diabetes mellitus, hypertension, and chronic venous insufficiency with stasis dermatitis presented to the emergency department following a mechanical fall. Most of her medical history was obtained from the patient’s family. She had a history of multiple falls originally thought to be related to a chronic leg ulcer that had been managed with wound care. Recently, however, the lesion was noted to have increasing erythema surrounding the wound margins. An 8×8-cm erythematous plaque on the anterior lateral left leg with a firm central nodule with hemorrhagic crust that measured approximately 4 cm in diameter was noted by the emergency department physicians (Figure 1). In the emergency department, vitals and other laboratory values were within reference range, and a radiograph of the left tibia/fibula was unremarkable. Cellulitis initially was considered in the emergency department and cephalexin was started; however, since the patient was afebrile and had no leukocytosis, plastic surgery also was consulted. Biopsies were obtained from the superior and inferior parts of the lesion. Histologic analysis revealed a poorly differentiated vascular neoplasm of epithelioid endothelial cells with considerable cell atypia that extended through the entirety of the dermis (Figure 2). The tumor cells stained positive with vimentin and CD34. Pathology noted no immunohistochemistry stains to synaptophysin, S-100, human melanoma black 45, MART-1, CK20, CK7, CK8/18, CK5/6, and p63. The pathologic diagnosis was consistent with cutaneous angiosarcoma. Computed tomography of the chest, abdomen, and pelvis revealed no local or distant metastases.


A wide excision of the cutaneous angiosarcoma was performed. The initial frozen section analysis revealed positive margins. Three additional excisions still showed positive margins, and further excision was held after obtaining family consent due to the extensive nature of the neoplasm and lengthy operating room time. The final defect after excision measured 15×10×2.5 cm (Figure 3A), and subsequent application of a split-thickness graft was performed. Additional treatment options were discussed with the family, including radiation therapy, amputation of the left lower leg, or no treatment. The family opted not to proceed with further treatment. The graft healed without signs of reoccurrence approximately 3 months later (Figure 3B), and the patient received physical therapy, which allowed her to gain strength and some independence.

Comment
Clinical Manifestation
Cutaneous angiosarcoma is a rare malignant vascular neoplasm that when clinically diagnosed is typically seen in 3 settings: (1) idiopathic (commonly on the face and neck), (2) following radiation treatment, and (3) classically following mastectomy with subsequent chronic lymphedema. Our patient did not classically fit these settings of cutaneous angiosarcoma due to the location of the lesion on the lower leg as well as its occurrence in the setting of a chronic nonhealing ulcer and lymphedema.
Chronic lymphedema is a common clinical manifestation that is likely secondary to other medical conditions, such as in our patient. As a result, these patients are at increased risk for developing chronic ulcers due to poor wound healing; however, as seen in our patient, chronic nonhealing ulcers require a broad differential because they may clinically mimic many processes. Patient history and visual presentation were crucial in this case because a biopsy was obtained that ultimately led to the patient’s diagnosis.
Differential Diagnosis
Initially, a venous ulcer secondary to chronic venous insufficiency was considered in the differential for our patient. She had a history of congestive heart failure, kidney disease, and type 2 diabetes mellitus, all of which contribute to lymphedema and/or poor wound healing. However, venous ulcers usually are located on the medial ankles and are irregularly shaped with an erythematous border and fibrinous exudate with central depression, making it a less likely diagnosis in our patient. Additionally, an infectious process was considered, but the patient was afebrile and laboratory values demonstrated no leukocytosis.
Marjolin ulcer was highly suspected because the clinical presentation revealed a nodule with hemorrhagic crust and induration in the setting of a chronic nonhealing ulcer. The pathogenesis of malignancy in chronic ulcers is thought to be due to continuous mitotic activity from regeneration and repair of the wound, especially in the setting of repeated trauma to the area.5 In our patient, the history of multiple falls with possible multitrauma injury to the chronic ulcer further increased suspicion of malignancy. The most common and frequently seen malignancy that develops in chronic ulcers is squamous cell carcinoma (SCC) followed by basal cell carcinoma. Plastic surgery suspected an SCC for the working diagnosis, which prompted a punch biopsy; however, the histologic analysis was not consistent with SCC or basal cell carcinoma. Marjolin ulcer also may demonstrate a periosteal reaction,5 which was not the case with our patient after a radiograph of the left tibia/fibula was unremarkable.
Another potential malignancy to consider is melanoma. There are few case reports of biopsy-proven melanoma from an enlarging chronic ulcer.6,7 Additionally, poorly differentiated angiosarcoma can mimic melanoma2; however, immunohistochemistry stain was negative for S-100, human melanoma black 45, and MART-1, making melanoma unlikely.
Kaposi sarcoma (KS) and angiosarcoma are both malignant vascular tumors that similarly present with red to purple patches, plaques, or nodules, making it difficult to distinguish between the two conditions. It is important to note that KS usually is lower grade, and the pathogenesis is linked to human herpesvirus 8, which can be identified on immunohistochemistry staining. There have been cases of KS reported in patients who have no history of human immunodeficiency virus/AIDS, thus the classic subtype of KS may have been considered in this patient.8 The histologic appearance of KS may vary from dilated irregular endothelial cells lining the vascular space to mild endothelial cell atypia. Histology also shows hemosiderin-laden macrophages, extravasated red blood cells, and an inflammatory infiltrate. An additional malignant vascular neoplasm that needs to be differentiated is epithelioid hemangioendothelioma. Cutaneous presentation of an epithelioid hemangioendothelioma may be similar to what was seen in our patient but histologically will usually show neoplastic cells with pale eosinophilic cytoplasm and vesicular nuclei of plump, oval, polygonal cells in cords or aggregates surrounding vascular channels. These neoplasms also tend to occur around medium- to large-sized veins.1,9 With our patient, even though human herpesvirus 8 was not tested with immunohistochemistry, gold standard immunohistochemistry confirmation with CD34 and vimentin staining combined with poorly differentiated endothelial atypia with mitotic figures on histologic analysis favored angiosarcoma versus KS or epithelioid hemangioendothelioma.10,11
Management
Cutaneous angiosarcoma is a rare and aggressive vascular neoplasm accounting for approximately 2% of all combined sarcomas, with an estimated 20% to 40% having distant metastasis at diagnosis.1,3 For this reason, computed tomography was performed in our patient and revealed no local or distant metastasis. Therefore, chemotherapy was not an appropriate adjuvant treatment option.12 With no evidence of metastasis, initial treatment began with surgical removal but proved to be difficult in our patient. Although the implications of positive surgical margins remain unclear with regard to overall patient survival, surgical resection followed by radiation therapy has been shown to be optimal, as it reduces the risk of local reoccurrence.3 There have been reported cases of cutaneous angiosarcoma of the leg that were treated with amputation without signs of reoccurrence or metastasis.10,13,14 Given the results from these cases and considering that our patient had no metastasis, amputation seemed to be a good prognostic option; however, considering other factors regarding the patient’s comorbidities and quality of life, her family decided not to pursue any further treatment with amputation or radiation therapy.
Conclusion
There should be low threshold for biopsy in patients who present with nonhealing wounds that do not progress in the normal phase of wound healing with suspicion for malignancy. As seen with our patient, cutaneous angiosarcoma can clinically mimic many disease processes, and although rare in nature, it should always be considered when a patient presents with a rapidly growing lesion in the setting of chronic lymphedema or venous ulcer.
- Kumar V, Abbas A, Aster J. Robbins Basic Pathology. 9th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Dossett LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64.
- Gerslova A, Pokorna A, Stukavcova A, et al. Rare cause of non-healing foot wound—acral lentiginous melanoma. Neuro Endocrinol Lett. 2012;37:12-17.
- Turk BG, Bozkurt A, Yaman B, et al. Melanoma arising in chronic ulceration associated with lymphoedema. J Wound Care. 2013;22:74-75.
- Phavixay L, Raynolds D, Simman R. Non AIDS Kaposi’s sarcoma leading to lower extremities wounds, case presentations and discussion.J Am Coll Clin Wound Spec. 2012;4:13-15.
- Requena L, Kutzner H. Hemangioendothelioma. Semin Diagn Pathol. 2013;30:29-44.
- Harrison WD, Chandrasekar CR. Stewart-Treves syndrome following idiopathic leg lymphoedema: remember sarcoma. J Wound Care. 2015;24(6 suppl):S5-S7.
- Kak I, Salama S, Gohla G, et al. A case of patch stage of Kaposi’s sarcoma and discussion of the differential diagnosis. Rare Tumors. 2016;8:6123.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Linda DD, Harish S, Alowami S, et al. Radiology-pathology conference: cutaneous angiosarcoma of the leg. Clin Imaging. 2013;37:602-607.
- Roy P, Clark MA, Thomas JM. Stewart-Treves syndrome—treatment and outcome in six patients from a single centre. Eur J Surg Oncol. 2004;30:982-986.
- Kumar V, Abbas A, Aster J. Robbins Basic Pathology. 9th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Dossett LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64.
- Gerslova A, Pokorna A, Stukavcova A, et al. Rare cause of non-healing foot wound—acral lentiginous melanoma. Neuro Endocrinol Lett. 2012;37:12-17.
- Turk BG, Bozkurt A, Yaman B, et al. Melanoma arising in chronic ulceration associated with lymphoedema. J Wound Care. 2013;22:74-75.
- Phavixay L, Raynolds D, Simman R. Non AIDS Kaposi’s sarcoma leading to lower extremities wounds, case presentations and discussion.J Am Coll Clin Wound Spec. 2012;4:13-15.
- Requena L, Kutzner H. Hemangioendothelioma. Semin Diagn Pathol. 2013;30:29-44.
- Harrison WD, Chandrasekar CR. Stewart-Treves syndrome following idiopathic leg lymphoedema: remember sarcoma. J Wound Care. 2015;24(6 suppl):S5-S7.
- Kak I, Salama S, Gohla G, et al. A case of patch stage of Kaposi’s sarcoma and discussion of the differential diagnosis. Rare Tumors. 2016;8:6123.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Linda DD, Harish S, Alowami S, et al. Radiology-pathology conference: cutaneous angiosarcoma of the leg. Clin Imaging. 2013;37:602-607.
- Roy P, Clark MA, Thomas JM. Stewart-Treves syndrome—treatment and outcome in six patients from a single centre. Eur J Surg Oncol. 2004;30:982-986.
Practice Points
- Cutaneous angiosarcoma is a rare malignant vascular neoplasm typically seen in 3 settings: (1) idiopathic (commonly on the face and neck), (2) following radiation treatment, and (3) classically in the setting of chronic lymphedema following mastectomy (Stewart-Treves syndrome).
- There should be a low threshold for biopsy in patients who present with nonhealing wounds that do not progress in the normal phase of wound healing with suspicion for malignancy.
- Histologic analysis of angiosarcoma shows positive staining for CD34 and vimentin with poorly differentiated endothelial atypia with mitotic figures.
Acute Superior Mesenteric Venous Thrombosis in a Young Patient Without Risk Factors
In this case report, the authors address the diagnostic challenges of a young, healthy patient who presented to the ED with unrelenting abdominal pain.
Acute mesenteric ischemia (AMI) results when oxygen delivery to the mesenteric artery is compromised, and is a serious diagnosis that should be considered in patients of all ages to avoid significant morbidity and mortality. The majority of cases are due to arterial embolism, arterial thrombus, or intestinal hypoperfusion (non-occlusive). Acute mesenteric venous thrombosis (MVT) accounts for only 2% to 10% of AMI cases, and only 0.01% of emergency surgery admissions.1 A large systematic review showed a 44% mortality rate for MVT, in contrast to 66% to 89% for all other forms of AMI.2 The typical age range for MVT is reported between 45 and 60 years, with a slight male predominance.3 Dull, central abdominal pain is the most frequently reported symptom of MVT, although it is generally less impressive than the pain described in other forms of AMI.3Along with the hallmark of abdominal pain out of proportion to the examination, other gastrointestinal symptoms include weight loss and non-specific altered bowel function (constipation, diarrhea, abdominal distention, and bloating), which are present in half of all patients with MVT.1 Peritoneal signs and bloody stools portend poor outcomes, as they often occur with disease progression.4
Case
A 26-year-old man presented to the ED with periumbilical and lower abdominal pain for 1 week. The pain was described as constant and dull, worsened by movement and oral intake, and improved with lying flat. He described bloating and decreased volume of bowel movements. He denied nausea, vomiting, fever, colicky pain, blood in stool, testicular pain, urinary complaints, trauma, or any similar episodes in the past. The patient had no known medical conditions or surgical history, except for a remote history of alcohol dependence (in remission) and tobacco use. There was no personal or family history of coagulopathy. Of note, he was seen by his primary care physician a few days prior to his ED presentation and had been instructed to take acetaminophen, which did not provide relief.
The patient’s vital signs at presentation were: blood pressure, 122/70 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 37.5°C (99.5°F). Oxygen saturation was 99% on room air. The physical examination was remarkable only for mild abdominal tenderness diffusely, greater in the lower and central abdomen than in the upper abdomen. The remainder of the physical examination was unremarkable.
Laboratory studies ordered included a complete blood count, comprehensive metabolic profile, lipase, and urinalysis. The patient did have a mild transaminitis (aspartate aminotransferase, 48 U/L; alanine aminotransferase, 84 U/L); the remainder of the studies were normal. A serum lactate, drawn after the 1 L of normal saline was administered intravenously (IV), was within normal limits (0.7 mmol/L). No prior laboratory studies were available for comparison.
The patient’s continued abdominal pain and transaminitis prompted an ED bedside right upper quadrant ultrasound, which showed a small gallbladder polyp; no signs of gallbladder disease were present. The patient required three doses of morphine 4 mg IV without complete pain relief. Given the concern for pain out of proportion to physical examination, a computed tomography (CT) scan of the abdomen/pelvis with IV and oral contrast was ordered. The radiologist interpreted the scan as showing a superior mesenteric vein (SMV) thrombus extending into the splenic/portal vein confluence and the intrahepatic portal veins (Figures 1 and 2).
Ciprofloxacin and metronidazole were administered IV for antibiotic prophylaxis, and the patient was placed on bowel rest with advancement to regular diet as tolerated. Propranolol was given for variceal prophylaxis. The patient was discharged home the following day in stable condition. Although he still had mild abdominal tenderness, the vital signs and physical examination were within normal limits. The patient was placed on a 6-month course of rivaroxaban therapy. Coagulopathy testing was scheduled at a later date, since ongoing anticoagulation treatment could interfere with test results. Unfortunately, the patient did not attend follow-up appointments to obtain testing.
Discussion
Mesenteric venous thrombosis is seen predominantly in middle-aged patients presenting with vague symptoms, which makes this a challenging diagnosis to make in the acute care setting. Risk factors for MVT include recent injury (causing trauma to the vasculature), recent surgery (causing stagnant blood flow), inflammatory conditions, and hypercoagulable states.1 In this patient’s case, no risk factors were identified; although the majority of cases of MVT will have an identifiable risk factor.2 Still, 21% to 49% of cases of MVT are considered idiopathic.1,3It is possible that our patient had a prior undiagnosed pancreatitis associated with his history of alcoholism that contributed to his thrombosis. Pancreatitis and other inflammatory conditions, including diverticulitis or inflammatory bowel disease, are more commonly associated with thrombus formation in the large veins, as opposed to an undiagnosed hypercoagulable state, which would more likely affect distal venuoles, vasa recta, or venous arcades.1,5 The patient’s mild transaminitis was likely secondary to hepatic congestion from the venous thrombus extending to the splenic-portal vein confluence and intrahepatic portal vein. One study looked at patients with pancreatitis and found that 16.7% of their study population had an SMV thrombus, while 4.1% had a SMV thrombus with a concomitant portal vein thrombus.6
Although there are no pathognomonic laboratory findings of MVT, elevated lactate, leukocytosis, and elevated D-dimer levels may be helpful in supporting the diagnosis.7,8 A recent study found that elevated D-dimer levels may be a specific marker in the early recognition of acute SMV thrombosis, as well as predicting risk, outcomes, and treatment options.8 However, emergency physicians should maintain a high index of suspicion in patients with concerning features of the disease, since normal laboratory values, including lactate, do not reliably exclude the diagnosis.
Computed tomography scanning and CT angiography (CTA) are quite helpful in diagnosing MVT. Ultrasound of the upper abdomen may also play a role, noting dilated or thickened bowel wall with intraluminal air or echogenic material in the superior mesenteric vein or portal vein.9 Although magnetic resonance venography most reliably demonstrates thrombi, its lack of widespread availability makes CT with IV contrast the preferred initial study.3Computed tomography not only has high sensitivity, but also offers alternative diagnoses in the undifferentiated presentation.1One study found CT to be 100% sensitive in detecting any abnormality associated with MVT or bowel ischemia.10 Common CT findings of MVT include dilated and thickened bowel loops, mesenteric fat standing, ascites, a halo or target appearance of bowel, vessel filling defects from a thrombus, and pneumatosis intestinalis.11 The latter usually indicates transmural infarction, and can extend as portomesenteric vein gas.11 Of note, if the initial CT scan is non-diagnostic and a high clinical suspicion for mesenteric ischemia remains with no alternative diagnosis, CTA is the gold standard.3,7Expeditious diagnosis of MVT is imperative, given the potential complications of intestinal infarction, submucosal hemorrhage secondary to edema, and third spacing of the venous outflow into the bowel wall due to collateral vessels being unable to redirect blood flow in conjunction with complete venous occlusion.12Not all MVTs progress to infarction, given the extensive collateral circulation. Early diagnosis, however, is crucial for conservative management to be effective.9Acute MVT without signs of infarction necessitates anticoagulation therapy to decrease clot propagation and recurrence.1 In addition, prophylactic antibiotics to limit bacterial translocation, and bowel rest are advised.13,14 If the patient is unresponsive to anticoagulation, thrombolytic and endovascular therapies may be of benefit in select patients.15 Once intestinal ischemia or infarction develops, the prognosis is poor: mortality approaches 75% with infarction.1 If signs of bowel infarction are present, a laparotomy must be performed promptly, although in most cases, delayed patient presentation makes small bowel resection unavoidable.9 Further testing for hypercoagulability is recommended, particularly in isolated thrombosis, since long-term anticoagulation therapy may be necessary if a coagulopathy is discovered.1
Conclusion
Mesenteric venous thrombosis is atypical in a young, healthy patient. However, due to high mortality rates with disease progression, it is important to consider in any patient with unrelenting abdominal pain and vague gastrointestinal symptoms of uncertain cause, even in those without risk factors. Early detection and management of MVT before progression to mesenteric ischemia and infarction considerably lowers the mortality rate. Emergency physicians must be vigilant when treating a patient with abdominal pain out of proportion to physical examination, unrelenting pain despite analgesic medications, or repeat ED visits for the same abdominal complaints.
1. Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15(5):407-418. doi:10.1177/1358863x10379673.
2. Tilsed JV, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(2):253-270. doi:10.1007/s00068-016-0634-0.
3. Tendler DA, Lamont JT, Grubel P. Mesenteric venous thrombosis in adults. UpToDate Web site. https://www.uptodate.com/contents/mesenteric-venous-thrombosis-in-adults. Accessed November 16, 2017.
4. Al-Zahrani HA, Lindsay T. Mesenteric ischemia. In: Hall JB, Schmidt GA, Kress JP, eds. Principles of Critical Care. 4th ed. New York, NY: McGraw Hill; 2015:1036-1044.
5. Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345(23):1683-1688. doi:10.1056/nejmra010076.
6. Al-Khazraji A, Hasan AQ, Patel I, Alkhawam H, Ghrair F, Lieber J. The role of abdominal computed tomography scan in acute pancreatitis. Pancreas. 2017;46(6):e52-e54. doi:10.1097/mpa.0000000000000837.
7. Bradbury MS, Kavanagh PV, Bechtold RE, et al. Mesenteric venous thrombosis: diagnosis and noninvasive imaging. Radiographics. 2002;22(3):527-541.
8. Yang S, Fan X, Ding W, et al. D-dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore). 2014;93(29):e270. doi:10.1097/md.0000000000000270.
9. Matos C, Van Gansbeke D, Zalcman M, et al. Mesenteric vein thrombosis: early CT and US diagnosis and conservative management. Gastrointest Radiol. 1986;11(4):322-325.
10. Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20(5):688-697.
11. Furukawa A, Kanasaki S, Kono N, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192(2):408-416. doi:10.2214/ajr.08.1138.
12. Johnson CC, Baggenstoss AH. Mesenteric vascular occlusion; study of 99 cases of occlusion of veins. Proc Staff Meet Mayo Clin. 1949;24(25):628-636.13. Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4(3):257-263. doi:10.1016/j.jceh.2014.03.052.
14. Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91(1):17-27.
15. Yang S, Fan X, Ding W, et al. Multidisciplinary stepwise management strategy for acute superior mesenteric venous thrombosis: an intestinal stroke center experience. Thromb Res. 2015;135(1):36-45. doi:10.1016/j.thromres.2014.10.018.
In this case report, the authors address the diagnostic challenges of a young, healthy patient who presented to the ED with unrelenting abdominal pain.
In this case report, the authors address the diagnostic challenges of a young, healthy patient who presented to the ED with unrelenting abdominal pain.
Acute mesenteric ischemia (AMI) results when oxygen delivery to the mesenteric artery is compromised, and is a serious diagnosis that should be considered in patients of all ages to avoid significant morbidity and mortality. The majority of cases are due to arterial embolism, arterial thrombus, or intestinal hypoperfusion (non-occlusive). Acute mesenteric venous thrombosis (MVT) accounts for only 2% to 10% of AMI cases, and only 0.01% of emergency surgery admissions.1 A large systematic review showed a 44% mortality rate for MVT, in contrast to 66% to 89% for all other forms of AMI.2 The typical age range for MVT is reported between 45 and 60 years, with a slight male predominance.3 Dull, central abdominal pain is the most frequently reported symptom of MVT, although it is generally less impressive than the pain described in other forms of AMI.3Along with the hallmark of abdominal pain out of proportion to the examination, other gastrointestinal symptoms include weight loss and non-specific altered bowel function (constipation, diarrhea, abdominal distention, and bloating), which are present in half of all patients with MVT.1 Peritoneal signs and bloody stools portend poor outcomes, as they often occur with disease progression.4
Case
A 26-year-old man presented to the ED with periumbilical and lower abdominal pain for 1 week. The pain was described as constant and dull, worsened by movement and oral intake, and improved with lying flat. He described bloating and decreased volume of bowel movements. He denied nausea, vomiting, fever, colicky pain, blood in stool, testicular pain, urinary complaints, trauma, or any similar episodes in the past. The patient had no known medical conditions or surgical history, except for a remote history of alcohol dependence (in remission) and tobacco use. There was no personal or family history of coagulopathy. Of note, he was seen by his primary care physician a few days prior to his ED presentation and had been instructed to take acetaminophen, which did not provide relief.
The patient’s vital signs at presentation were: blood pressure, 122/70 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 37.5°C (99.5°F). Oxygen saturation was 99% on room air. The physical examination was remarkable only for mild abdominal tenderness diffusely, greater in the lower and central abdomen than in the upper abdomen. The remainder of the physical examination was unremarkable.
Laboratory studies ordered included a complete blood count, comprehensive metabolic profile, lipase, and urinalysis. The patient did have a mild transaminitis (aspartate aminotransferase, 48 U/L; alanine aminotransferase, 84 U/L); the remainder of the studies were normal. A serum lactate, drawn after the 1 L of normal saline was administered intravenously (IV), was within normal limits (0.7 mmol/L). No prior laboratory studies were available for comparison.
The patient’s continued abdominal pain and transaminitis prompted an ED bedside right upper quadrant ultrasound, which showed a small gallbladder polyp; no signs of gallbladder disease were present. The patient required three doses of morphine 4 mg IV without complete pain relief. Given the concern for pain out of proportion to physical examination, a computed tomography (CT) scan of the abdomen/pelvis with IV and oral contrast was ordered. The radiologist interpreted the scan as showing a superior mesenteric vein (SMV) thrombus extending into the splenic/portal vein confluence and the intrahepatic portal veins (Figures 1 and 2).
Ciprofloxacin and metronidazole were administered IV for antibiotic prophylaxis, and the patient was placed on bowel rest with advancement to regular diet as tolerated. Propranolol was given for variceal prophylaxis. The patient was discharged home the following day in stable condition. Although he still had mild abdominal tenderness, the vital signs and physical examination were within normal limits. The patient was placed on a 6-month course of rivaroxaban therapy. Coagulopathy testing was scheduled at a later date, since ongoing anticoagulation treatment could interfere with test results. Unfortunately, the patient did not attend follow-up appointments to obtain testing.
Discussion
Mesenteric venous thrombosis is seen predominantly in middle-aged patients presenting with vague symptoms, which makes this a challenging diagnosis to make in the acute care setting. Risk factors for MVT include recent injury (causing trauma to the vasculature), recent surgery (causing stagnant blood flow), inflammatory conditions, and hypercoagulable states.1 In this patient’s case, no risk factors were identified; although the majority of cases of MVT will have an identifiable risk factor.2 Still, 21% to 49% of cases of MVT are considered idiopathic.1,3It is possible that our patient had a prior undiagnosed pancreatitis associated with his history of alcoholism that contributed to his thrombosis. Pancreatitis and other inflammatory conditions, including diverticulitis or inflammatory bowel disease, are more commonly associated with thrombus formation in the large veins, as opposed to an undiagnosed hypercoagulable state, which would more likely affect distal venuoles, vasa recta, or venous arcades.1,5 The patient’s mild transaminitis was likely secondary to hepatic congestion from the venous thrombus extending to the splenic-portal vein confluence and intrahepatic portal vein. One study looked at patients with pancreatitis and found that 16.7% of their study population had an SMV thrombus, while 4.1% had a SMV thrombus with a concomitant portal vein thrombus.6
Although there are no pathognomonic laboratory findings of MVT, elevated lactate, leukocytosis, and elevated D-dimer levels may be helpful in supporting the diagnosis.7,8 A recent study found that elevated D-dimer levels may be a specific marker in the early recognition of acute SMV thrombosis, as well as predicting risk, outcomes, and treatment options.8 However, emergency physicians should maintain a high index of suspicion in patients with concerning features of the disease, since normal laboratory values, including lactate, do not reliably exclude the diagnosis.
Computed tomography scanning and CT angiography (CTA) are quite helpful in diagnosing MVT. Ultrasound of the upper abdomen may also play a role, noting dilated or thickened bowel wall with intraluminal air or echogenic material in the superior mesenteric vein or portal vein.9 Although magnetic resonance venography most reliably demonstrates thrombi, its lack of widespread availability makes CT with IV contrast the preferred initial study.3Computed tomography not only has high sensitivity, but also offers alternative diagnoses in the undifferentiated presentation.1One study found CT to be 100% sensitive in detecting any abnormality associated with MVT or bowel ischemia.10 Common CT findings of MVT include dilated and thickened bowel loops, mesenteric fat standing, ascites, a halo or target appearance of bowel, vessel filling defects from a thrombus, and pneumatosis intestinalis.11 The latter usually indicates transmural infarction, and can extend as portomesenteric vein gas.11 Of note, if the initial CT scan is non-diagnostic and a high clinical suspicion for mesenteric ischemia remains with no alternative diagnosis, CTA is the gold standard.3,7Expeditious diagnosis of MVT is imperative, given the potential complications of intestinal infarction, submucosal hemorrhage secondary to edema, and third spacing of the venous outflow into the bowel wall due to collateral vessels being unable to redirect blood flow in conjunction with complete venous occlusion.12Not all MVTs progress to infarction, given the extensive collateral circulation. Early diagnosis, however, is crucial for conservative management to be effective.9Acute MVT without signs of infarction necessitates anticoagulation therapy to decrease clot propagation and recurrence.1 In addition, prophylactic antibiotics to limit bacterial translocation, and bowel rest are advised.13,14 If the patient is unresponsive to anticoagulation, thrombolytic and endovascular therapies may be of benefit in select patients.15 Once intestinal ischemia or infarction develops, the prognosis is poor: mortality approaches 75% with infarction.1 If signs of bowel infarction are present, a laparotomy must be performed promptly, although in most cases, delayed patient presentation makes small bowel resection unavoidable.9 Further testing for hypercoagulability is recommended, particularly in isolated thrombosis, since long-term anticoagulation therapy may be necessary if a coagulopathy is discovered.1
Conclusion
Mesenteric venous thrombosis is atypical in a young, healthy patient. However, due to high mortality rates with disease progression, it is important to consider in any patient with unrelenting abdominal pain and vague gastrointestinal symptoms of uncertain cause, even in those without risk factors. Early detection and management of MVT before progression to mesenteric ischemia and infarction considerably lowers the mortality rate. Emergency physicians must be vigilant when treating a patient with abdominal pain out of proportion to physical examination, unrelenting pain despite analgesic medications, or repeat ED visits for the same abdominal complaints.
Acute mesenteric ischemia (AMI) results when oxygen delivery to the mesenteric artery is compromised, and is a serious diagnosis that should be considered in patients of all ages to avoid significant morbidity and mortality. The majority of cases are due to arterial embolism, arterial thrombus, or intestinal hypoperfusion (non-occlusive). Acute mesenteric venous thrombosis (MVT) accounts for only 2% to 10% of AMI cases, and only 0.01% of emergency surgery admissions.1 A large systematic review showed a 44% mortality rate for MVT, in contrast to 66% to 89% for all other forms of AMI.2 The typical age range for MVT is reported between 45 and 60 years, with a slight male predominance.3 Dull, central abdominal pain is the most frequently reported symptom of MVT, although it is generally less impressive than the pain described in other forms of AMI.3Along with the hallmark of abdominal pain out of proportion to the examination, other gastrointestinal symptoms include weight loss and non-specific altered bowel function (constipation, diarrhea, abdominal distention, and bloating), which are present in half of all patients with MVT.1 Peritoneal signs and bloody stools portend poor outcomes, as they often occur with disease progression.4
Case
A 26-year-old man presented to the ED with periumbilical and lower abdominal pain for 1 week. The pain was described as constant and dull, worsened by movement and oral intake, and improved with lying flat. He described bloating and decreased volume of bowel movements. He denied nausea, vomiting, fever, colicky pain, blood in stool, testicular pain, urinary complaints, trauma, or any similar episodes in the past. The patient had no known medical conditions or surgical history, except for a remote history of alcohol dependence (in remission) and tobacco use. There was no personal or family history of coagulopathy. Of note, he was seen by his primary care physician a few days prior to his ED presentation and had been instructed to take acetaminophen, which did not provide relief.
The patient’s vital signs at presentation were: blood pressure, 122/70 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 37.5°C (99.5°F). Oxygen saturation was 99% on room air. The physical examination was remarkable only for mild abdominal tenderness diffusely, greater in the lower and central abdomen than in the upper abdomen. The remainder of the physical examination was unremarkable.
Laboratory studies ordered included a complete blood count, comprehensive metabolic profile, lipase, and urinalysis. The patient did have a mild transaminitis (aspartate aminotransferase, 48 U/L; alanine aminotransferase, 84 U/L); the remainder of the studies were normal. A serum lactate, drawn after the 1 L of normal saline was administered intravenously (IV), was within normal limits (0.7 mmol/L). No prior laboratory studies were available for comparison.
The patient’s continued abdominal pain and transaminitis prompted an ED bedside right upper quadrant ultrasound, which showed a small gallbladder polyp; no signs of gallbladder disease were present. The patient required three doses of morphine 4 mg IV without complete pain relief. Given the concern for pain out of proportion to physical examination, a computed tomography (CT) scan of the abdomen/pelvis with IV and oral contrast was ordered. The radiologist interpreted the scan as showing a superior mesenteric vein (SMV) thrombus extending into the splenic/portal vein confluence and the intrahepatic portal veins (Figures 1 and 2).
Ciprofloxacin and metronidazole were administered IV for antibiotic prophylaxis, and the patient was placed on bowel rest with advancement to regular diet as tolerated. Propranolol was given for variceal prophylaxis. The patient was discharged home the following day in stable condition. Although he still had mild abdominal tenderness, the vital signs and physical examination were within normal limits. The patient was placed on a 6-month course of rivaroxaban therapy. Coagulopathy testing was scheduled at a later date, since ongoing anticoagulation treatment could interfere with test results. Unfortunately, the patient did not attend follow-up appointments to obtain testing.
Discussion
Mesenteric venous thrombosis is seen predominantly in middle-aged patients presenting with vague symptoms, which makes this a challenging diagnosis to make in the acute care setting. Risk factors for MVT include recent injury (causing trauma to the vasculature), recent surgery (causing stagnant blood flow), inflammatory conditions, and hypercoagulable states.1 In this patient’s case, no risk factors were identified; although the majority of cases of MVT will have an identifiable risk factor.2 Still, 21% to 49% of cases of MVT are considered idiopathic.1,3It is possible that our patient had a prior undiagnosed pancreatitis associated with his history of alcoholism that contributed to his thrombosis. Pancreatitis and other inflammatory conditions, including diverticulitis or inflammatory bowel disease, are more commonly associated with thrombus formation in the large veins, as opposed to an undiagnosed hypercoagulable state, which would more likely affect distal venuoles, vasa recta, or venous arcades.1,5 The patient’s mild transaminitis was likely secondary to hepatic congestion from the venous thrombus extending to the splenic-portal vein confluence and intrahepatic portal vein. One study looked at patients with pancreatitis and found that 16.7% of their study population had an SMV thrombus, while 4.1% had a SMV thrombus with a concomitant portal vein thrombus.6
Although there are no pathognomonic laboratory findings of MVT, elevated lactate, leukocytosis, and elevated D-dimer levels may be helpful in supporting the diagnosis.7,8 A recent study found that elevated D-dimer levels may be a specific marker in the early recognition of acute SMV thrombosis, as well as predicting risk, outcomes, and treatment options.8 However, emergency physicians should maintain a high index of suspicion in patients with concerning features of the disease, since normal laboratory values, including lactate, do not reliably exclude the diagnosis.
Computed tomography scanning and CT angiography (CTA) are quite helpful in diagnosing MVT. Ultrasound of the upper abdomen may also play a role, noting dilated or thickened bowel wall with intraluminal air or echogenic material in the superior mesenteric vein or portal vein.9 Although magnetic resonance venography most reliably demonstrates thrombi, its lack of widespread availability makes CT with IV contrast the preferred initial study.3Computed tomography not only has high sensitivity, but also offers alternative diagnoses in the undifferentiated presentation.1One study found CT to be 100% sensitive in detecting any abnormality associated with MVT or bowel ischemia.10 Common CT findings of MVT include dilated and thickened bowel loops, mesenteric fat standing, ascites, a halo or target appearance of bowel, vessel filling defects from a thrombus, and pneumatosis intestinalis.11 The latter usually indicates transmural infarction, and can extend as portomesenteric vein gas.11 Of note, if the initial CT scan is non-diagnostic and a high clinical suspicion for mesenteric ischemia remains with no alternative diagnosis, CTA is the gold standard.3,7Expeditious diagnosis of MVT is imperative, given the potential complications of intestinal infarction, submucosal hemorrhage secondary to edema, and third spacing of the venous outflow into the bowel wall due to collateral vessels being unable to redirect blood flow in conjunction with complete venous occlusion.12Not all MVTs progress to infarction, given the extensive collateral circulation. Early diagnosis, however, is crucial for conservative management to be effective.9Acute MVT without signs of infarction necessitates anticoagulation therapy to decrease clot propagation and recurrence.1 In addition, prophylactic antibiotics to limit bacterial translocation, and bowel rest are advised.13,14 If the patient is unresponsive to anticoagulation, thrombolytic and endovascular therapies may be of benefit in select patients.15 Once intestinal ischemia or infarction develops, the prognosis is poor: mortality approaches 75% with infarction.1 If signs of bowel infarction are present, a laparotomy must be performed promptly, although in most cases, delayed patient presentation makes small bowel resection unavoidable.9 Further testing for hypercoagulability is recommended, particularly in isolated thrombosis, since long-term anticoagulation therapy may be necessary if a coagulopathy is discovered.1
Conclusion
Mesenteric venous thrombosis is atypical in a young, healthy patient. However, due to high mortality rates with disease progression, it is important to consider in any patient with unrelenting abdominal pain and vague gastrointestinal symptoms of uncertain cause, even in those without risk factors. Early detection and management of MVT before progression to mesenteric ischemia and infarction considerably lowers the mortality rate. Emergency physicians must be vigilant when treating a patient with abdominal pain out of proportion to physical examination, unrelenting pain despite analgesic medications, or repeat ED visits for the same abdominal complaints.
1. Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15(5):407-418. doi:10.1177/1358863x10379673.
2. Tilsed JV, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(2):253-270. doi:10.1007/s00068-016-0634-0.
3. Tendler DA, Lamont JT, Grubel P. Mesenteric venous thrombosis in adults. UpToDate Web site. https://www.uptodate.com/contents/mesenteric-venous-thrombosis-in-adults. Accessed November 16, 2017.
4. Al-Zahrani HA, Lindsay T. Mesenteric ischemia. In: Hall JB, Schmidt GA, Kress JP, eds. Principles of Critical Care. 4th ed. New York, NY: McGraw Hill; 2015:1036-1044.
5. Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345(23):1683-1688. doi:10.1056/nejmra010076.
6. Al-Khazraji A, Hasan AQ, Patel I, Alkhawam H, Ghrair F, Lieber J. The role of abdominal computed tomography scan in acute pancreatitis. Pancreas. 2017;46(6):e52-e54. doi:10.1097/mpa.0000000000000837.
7. Bradbury MS, Kavanagh PV, Bechtold RE, et al. Mesenteric venous thrombosis: diagnosis and noninvasive imaging. Radiographics. 2002;22(3):527-541.
8. Yang S, Fan X, Ding W, et al. D-dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore). 2014;93(29):e270. doi:10.1097/md.0000000000000270.
9. Matos C, Van Gansbeke D, Zalcman M, et al. Mesenteric vein thrombosis: early CT and US diagnosis and conservative management. Gastrointest Radiol. 1986;11(4):322-325.
10. Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20(5):688-697.
11. Furukawa A, Kanasaki S, Kono N, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192(2):408-416. doi:10.2214/ajr.08.1138.
12. Johnson CC, Baggenstoss AH. Mesenteric vascular occlusion; study of 99 cases of occlusion of veins. Proc Staff Meet Mayo Clin. 1949;24(25):628-636.13. Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4(3):257-263. doi:10.1016/j.jceh.2014.03.052.
14. Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91(1):17-27.
15. Yang S, Fan X, Ding W, et al. Multidisciplinary stepwise management strategy for acute superior mesenteric venous thrombosis: an intestinal stroke center experience. Thromb Res. 2015;135(1):36-45. doi:10.1016/j.thromres.2014.10.018.
1. Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15(5):407-418. doi:10.1177/1358863x10379673.
2. Tilsed JV, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(2):253-270. doi:10.1007/s00068-016-0634-0.
3. Tendler DA, Lamont JT, Grubel P. Mesenteric venous thrombosis in adults. UpToDate Web site. https://www.uptodate.com/contents/mesenteric-venous-thrombosis-in-adults. Accessed November 16, 2017.
4. Al-Zahrani HA, Lindsay T. Mesenteric ischemia. In: Hall JB, Schmidt GA, Kress JP, eds. Principles of Critical Care. 4th ed. New York, NY: McGraw Hill; 2015:1036-1044.
5. Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345(23):1683-1688. doi:10.1056/nejmra010076.
6. Al-Khazraji A, Hasan AQ, Patel I, Alkhawam H, Ghrair F, Lieber J. The role of abdominal computed tomography scan in acute pancreatitis. Pancreas. 2017;46(6):e52-e54. doi:10.1097/mpa.0000000000000837.
7. Bradbury MS, Kavanagh PV, Bechtold RE, et al. Mesenteric venous thrombosis: diagnosis and noninvasive imaging. Radiographics. 2002;22(3):527-541.
8. Yang S, Fan X, Ding W, et al. D-dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore). 2014;93(29):e270. doi:10.1097/md.0000000000000270.
9. Matos C, Van Gansbeke D, Zalcman M, et al. Mesenteric vein thrombosis: early CT and US diagnosis and conservative management. Gastrointest Radiol. 1986;11(4):322-325.
10. Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20(5):688-697.
11. Furukawa A, Kanasaki S, Kono N, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192(2):408-416. doi:10.2214/ajr.08.1138.
12. Johnson CC, Baggenstoss AH. Mesenteric vascular occlusion; study of 99 cases of occlusion of veins. Proc Staff Meet Mayo Clin. 1949;24(25):628-636.13. Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4(3):257-263. doi:10.1016/j.jceh.2014.03.052.
14. Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91(1):17-27.
15. Yang S, Fan X, Ding W, et al. Multidisciplinary stepwise management strategy for acute superior mesenteric venous thrombosis: an intestinal stroke center experience. Thromb Res. 2015;135(1):36-45. doi:10.1016/j.thromres.2014.10.018.
A Ticking Noise From the Chest: Recognition of the Hamman Sign
The authors describe a case of a 21-year-old woman who presented with shortness of breath and exhibited a Hamman sign, an uncommon clinical finding.
Traditionally, a physician develops a differential diagnosis based primarily (>70%) on the history and the physical examination of a patient.1 While modern medicine has developed with new technological devices and a growing number of diagnostic tests, one must not forget the value of a thorough physical examination.
Case
A 21-year-old woman, in previous good health, presented to the ED with the chief complaint of shortness of breath. She stated that she woke up with acute dyspnea and a stabbing pain on the left side of her thorax, related to her breathing. The patient looked distressed upon presentation.
Her vital signs at presentation were: blood pressure, 150/85 mm Hg; heart rate, 120 beats/min; respiratory
During physical examination, a loud ticking noise was heard originating from the thorax, even without a stethoscope (an example of the sound can be heard at https://www.youtube.com/watch?v=mXJHtJeL1mM). During auscultation, the ticking noise was prominent in early systole and audible over all parts of the thorax. The sound was only heard when the patient was in the supine position and disappeared when she sat up. It persisted when the patient was holding her breath. Breath sounds were equal and clear bilaterally. There was no subcutaneous emphysema palpable over the thorax or neck region.
The electrocardiogram and blood results, including D-dimer, were normal. The chest X-ray showed an apical pneumothorax of 1.5 cm on the left side (Figures 1 and 2). There was no evidence of pneumomediastinum or pneumopericardium. The patient received acetaminophen and ibuprofen tablets for pain, and she was discharged home. At the follow-up 2 weeks later, she had no remaining symptoms and the ticking sound had disappeared.
Discussion
These loud intermittent noises originating from the thorax were described for the first time at the beginning of the 19th century.2,3 However, it was Louis Virgil Hamman whose name would be linked to this physical examination finding. In 1937 he described typical clicking, crackling, and popping sounds over the precordium, synchronized with the heartbeat. This was usually in combination with subcutaneous emphysema in the neck region. Hamman presumed that the symptoms were due to mediastinal air caused by rupture alveoli or bronchioles, resulting in interstitial emphysema of the lung parenchyma. In addition, air could leak into the pleural space, causing a pneumothorax. He concluded that the clinical findings were pathognomonic for spontaneous mediastinal emphysema, and this physical examination finding became known as the “Hamman sign”.4-11
However, in the following years it was demonstrated that the appearance of loud, systolic clicking noises over the precordium could also be present in patients with a small spontaneous left-sided pneumothorax.5-9 It was assumed the pneumothorax caused a small amount of air to accumulate in the pleural space in the major fissure inferiorly, which shifted with the cardiac contraction. This results in the noise being present while in the supine position. In the sitting position, the air moves cranially above the heart, meaning it is not influenced by the cardiac contractions and the noise disappears.5-8 The Hamman sign is absent in right-sided pneumothorax, presumably because of the smaller contact surface between the lung pleura and the mediastinal pleura overlying the heart in comparison to the left side. Also, the contractions of the right side of the heart are much weaker and generate less pressure in comparison to the left atrium and ventricle.8,11Only a small amount of air, approximately 25 mL, is enough to produce the typical sound. In larger pneumothorax’s, with more than 125 mL of intrapleural air, these sounds are absent, because the contractions of the heart cannot create enough pressure to cause the accumulated air to shift in the pleural space.5,8-9Sound analysis in left-sided pneumothorax by Roelandt et al7 showed multiple murmurs which can be present in both systole and diastole. In contrast to pulmonic noises, the Hamman sign persists when the patient is holding their breath and disappears with sitting or standing.3,6-10 Furthermore, it must not be confused with extra heart sounds, which present as a “gallop rhythm”, with a strong resemblance in quality to the first normal heart sound (S1). In addition, extra heart sounds are uncommon in healthy patients and do not appear suddenly or temporarily.5,8
The Hamman sign is a rare physical examination finding, only identified in less than 1% of all patients with a pneumothorax.9 However, its presence is so specific that it is strong evidence for an underlying pneumothorax or pneumomediastinum, even if radiographic imaging is normal.10 As previously stated, since the Hamman sign is mostly commonly associated with a pneumothorax consisting of less than 125 mL of air, these can usually be treated conservatively, without the necessity of placing a chest tube or aspiration. However, when a patient experiences significant shortness of breath, the emergency physician should consider ordering additional imaging, in the form of an ultrasound or a computed tomography scan to identify the underlying cause of the Hamman sign and place a chest tube when clinically indicated.
Conclusion
The Hamman sign is a rare clinical examination finding in left-sided pneumothorax or pneumomediastinum, in which a ticking or crackling noise is heard over the thorax. This is mostly synchronous with the heartbeat and not related to respiration. It is caused by a small amount of accumulated air in the pleural space, which is being displaced by cardiac contractions during the cardiac cycle. Although typically small, pneumothoraces have a good prognosis. Recognition of the Hamman sign is important, and physicians must realize that even a normal chest X-ray does not rule out the diagnosis.
1. Sandler G. Costs of unnecessary tests. Br Med J. 1979;2(6181):21-24.
2. Laennec RTH, Andral G, Laennec M. Traité De L’auscultation Médiate, Et Des Maladies Des Poumons Et Du Coeur. Paris, France: Paris, J.S.Chaudé, 1826; 1837.
3. Lister WA, Camb MB, Lond MRCP. A case of pericardial knock associated with spontaneous pneumothorax. Lancet. 1928;211(5468):1225-1226.
4. Hamman L. Spontaneous interstitial emphysema of the lungs. Tr A Am Physicians. 1937;52:311-319.
5. Scadding JG, Lond MRCP, Wood P. Systolic clicks due to left-sided pneumothorax. Lancet. 1939;234(6067):1208-1211.
6. Scott JT. Mediastinal emphysema and left pneumothorax. Dist Chest. 1957;32(4):421-434.
7. Roelandt J, Willems J, van der Hauwaert LG, de Geest H. Clicks and sounds (whoops) in left-sided pneumothorax. Clinical and phonocardiographic study. Dis Chest. 1969;56(1):31-36.
8. Smit FW, van Embden Andresen GH, Ubbens R. “Hammans’s sign”, pneumomediastinum and pneumothorax. Ned Tijdschr Geneeskd. 1974;118(22):828-833.
9. Baumann MH, Sahn SA. Hamman’s sign revisited. Pneumothorax or pneumomediastinum? Chest. 1992;102(4):1281-1282.
10. Remmelts HH, Banga JD. Popping pneumothorax. Neth J Med. 2010;68(4):187.
11. Jaiganesh T, Wright K, Sadana A. Mobile diagnosis: Hamman’s crunch in a primary spontaneous pneumothorax. Emerg Med J. 2010;27(6):482-483. doi:10.1136/emj.2009.079681.
The authors describe a case of a 21-year-old woman who presented with shortness of breath and exhibited a Hamman sign, an uncommon clinical finding.
The authors describe a case of a 21-year-old woman who presented with shortness of breath and exhibited a Hamman sign, an uncommon clinical finding.
Traditionally, a physician develops a differential diagnosis based primarily (>70%) on the history and the physical examination of a patient.1 While modern medicine has developed with new technological devices and a growing number of diagnostic tests, one must not forget the value of a thorough physical examination.
Case
A 21-year-old woman, in previous good health, presented to the ED with the chief complaint of shortness of breath. She stated that she woke up with acute dyspnea and a stabbing pain on the left side of her thorax, related to her breathing. The patient looked distressed upon presentation.
Her vital signs at presentation were: blood pressure, 150/85 mm Hg; heart rate, 120 beats/min; respiratory
During physical examination, a loud ticking noise was heard originating from the thorax, even without a stethoscope (an example of the sound can be heard at https://www.youtube.com/watch?v=mXJHtJeL1mM). During auscultation, the ticking noise was prominent in early systole and audible over all parts of the thorax. The sound was only heard when the patient was in the supine position and disappeared when she sat up. It persisted when the patient was holding her breath. Breath sounds were equal and clear bilaterally. There was no subcutaneous emphysema palpable over the thorax or neck region.
The electrocardiogram and blood results, including D-dimer, were normal. The chest X-ray showed an apical pneumothorax of 1.5 cm on the left side (Figures 1 and 2). There was no evidence of pneumomediastinum or pneumopericardium. The patient received acetaminophen and ibuprofen tablets for pain, and she was discharged home. At the follow-up 2 weeks later, she had no remaining symptoms and the ticking sound had disappeared.
Discussion
These loud intermittent noises originating from the thorax were described for the first time at the beginning of the 19th century.2,3 However, it was Louis Virgil Hamman whose name would be linked to this physical examination finding. In 1937 he described typical clicking, crackling, and popping sounds over the precordium, synchronized with the heartbeat. This was usually in combination with subcutaneous emphysema in the neck region. Hamman presumed that the symptoms were due to mediastinal air caused by rupture alveoli or bronchioles, resulting in interstitial emphysema of the lung parenchyma. In addition, air could leak into the pleural space, causing a pneumothorax. He concluded that the clinical findings were pathognomonic for spontaneous mediastinal emphysema, and this physical examination finding became known as the “Hamman sign”.4-11
However, in the following years it was demonstrated that the appearance of loud, systolic clicking noises over the precordium could also be present in patients with a small spontaneous left-sided pneumothorax.5-9 It was assumed the pneumothorax caused a small amount of air to accumulate in the pleural space in the major fissure inferiorly, which shifted with the cardiac contraction. This results in the noise being present while in the supine position. In the sitting position, the air moves cranially above the heart, meaning it is not influenced by the cardiac contractions and the noise disappears.5-8 The Hamman sign is absent in right-sided pneumothorax, presumably because of the smaller contact surface between the lung pleura and the mediastinal pleura overlying the heart in comparison to the left side. Also, the contractions of the right side of the heart are much weaker and generate less pressure in comparison to the left atrium and ventricle.8,11Only a small amount of air, approximately 25 mL, is enough to produce the typical sound. In larger pneumothorax’s, with more than 125 mL of intrapleural air, these sounds are absent, because the contractions of the heart cannot create enough pressure to cause the accumulated air to shift in the pleural space.5,8-9Sound analysis in left-sided pneumothorax by Roelandt et al7 showed multiple murmurs which can be present in both systole and diastole. In contrast to pulmonic noises, the Hamman sign persists when the patient is holding their breath and disappears with sitting or standing.3,6-10 Furthermore, it must not be confused with extra heart sounds, which present as a “gallop rhythm”, with a strong resemblance in quality to the first normal heart sound (S1). In addition, extra heart sounds are uncommon in healthy patients and do not appear suddenly or temporarily.5,8
The Hamman sign is a rare physical examination finding, only identified in less than 1% of all patients with a pneumothorax.9 However, its presence is so specific that it is strong evidence for an underlying pneumothorax or pneumomediastinum, even if radiographic imaging is normal.10 As previously stated, since the Hamman sign is mostly commonly associated with a pneumothorax consisting of less than 125 mL of air, these can usually be treated conservatively, without the necessity of placing a chest tube or aspiration. However, when a patient experiences significant shortness of breath, the emergency physician should consider ordering additional imaging, in the form of an ultrasound or a computed tomography scan to identify the underlying cause of the Hamman sign and place a chest tube when clinically indicated.
Conclusion
The Hamman sign is a rare clinical examination finding in left-sided pneumothorax or pneumomediastinum, in which a ticking or crackling noise is heard over the thorax. This is mostly synchronous with the heartbeat and not related to respiration. It is caused by a small amount of accumulated air in the pleural space, which is being displaced by cardiac contractions during the cardiac cycle. Although typically small, pneumothoraces have a good prognosis. Recognition of the Hamman sign is important, and physicians must realize that even a normal chest X-ray does not rule out the diagnosis.
Traditionally, a physician develops a differential diagnosis based primarily (>70%) on the history and the physical examination of a patient.1 While modern medicine has developed with new technological devices and a growing number of diagnostic tests, one must not forget the value of a thorough physical examination.
Case
A 21-year-old woman, in previous good health, presented to the ED with the chief complaint of shortness of breath. She stated that she woke up with acute dyspnea and a stabbing pain on the left side of her thorax, related to her breathing. The patient looked distressed upon presentation.
Her vital signs at presentation were: blood pressure, 150/85 mm Hg; heart rate, 120 beats/min; respiratory
During physical examination, a loud ticking noise was heard originating from the thorax, even without a stethoscope (an example of the sound can be heard at https://www.youtube.com/watch?v=mXJHtJeL1mM). During auscultation, the ticking noise was prominent in early systole and audible over all parts of the thorax. The sound was only heard when the patient was in the supine position and disappeared when she sat up. It persisted when the patient was holding her breath. Breath sounds were equal and clear bilaterally. There was no subcutaneous emphysema palpable over the thorax or neck region.
The electrocardiogram and blood results, including D-dimer, were normal. The chest X-ray showed an apical pneumothorax of 1.5 cm on the left side (Figures 1 and 2). There was no evidence of pneumomediastinum or pneumopericardium. The patient received acetaminophen and ibuprofen tablets for pain, and she was discharged home. At the follow-up 2 weeks later, she had no remaining symptoms and the ticking sound had disappeared.
Discussion
These loud intermittent noises originating from the thorax were described for the first time at the beginning of the 19th century.2,3 However, it was Louis Virgil Hamman whose name would be linked to this physical examination finding. In 1937 he described typical clicking, crackling, and popping sounds over the precordium, synchronized with the heartbeat. This was usually in combination with subcutaneous emphysema in the neck region. Hamman presumed that the symptoms were due to mediastinal air caused by rupture alveoli or bronchioles, resulting in interstitial emphysema of the lung parenchyma. In addition, air could leak into the pleural space, causing a pneumothorax. He concluded that the clinical findings were pathognomonic for spontaneous mediastinal emphysema, and this physical examination finding became known as the “Hamman sign”.4-11
However, in the following years it was demonstrated that the appearance of loud, systolic clicking noises over the precordium could also be present in patients with a small spontaneous left-sided pneumothorax.5-9 It was assumed the pneumothorax caused a small amount of air to accumulate in the pleural space in the major fissure inferiorly, which shifted with the cardiac contraction. This results in the noise being present while in the supine position. In the sitting position, the air moves cranially above the heart, meaning it is not influenced by the cardiac contractions and the noise disappears.5-8 The Hamman sign is absent in right-sided pneumothorax, presumably because of the smaller contact surface between the lung pleura and the mediastinal pleura overlying the heart in comparison to the left side. Also, the contractions of the right side of the heart are much weaker and generate less pressure in comparison to the left atrium and ventricle.8,11Only a small amount of air, approximately 25 mL, is enough to produce the typical sound. In larger pneumothorax’s, with more than 125 mL of intrapleural air, these sounds are absent, because the contractions of the heart cannot create enough pressure to cause the accumulated air to shift in the pleural space.5,8-9Sound analysis in left-sided pneumothorax by Roelandt et al7 showed multiple murmurs which can be present in both systole and diastole. In contrast to pulmonic noises, the Hamman sign persists when the patient is holding their breath and disappears with sitting or standing.3,6-10 Furthermore, it must not be confused with extra heart sounds, which present as a “gallop rhythm”, with a strong resemblance in quality to the first normal heart sound (S1). In addition, extra heart sounds are uncommon in healthy patients and do not appear suddenly or temporarily.5,8
The Hamman sign is a rare physical examination finding, only identified in less than 1% of all patients with a pneumothorax.9 However, its presence is so specific that it is strong evidence for an underlying pneumothorax or pneumomediastinum, even if radiographic imaging is normal.10 As previously stated, since the Hamman sign is mostly commonly associated with a pneumothorax consisting of less than 125 mL of air, these can usually be treated conservatively, without the necessity of placing a chest tube or aspiration. However, when a patient experiences significant shortness of breath, the emergency physician should consider ordering additional imaging, in the form of an ultrasound or a computed tomography scan to identify the underlying cause of the Hamman sign and place a chest tube when clinically indicated.
Conclusion
The Hamman sign is a rare clinical examination finding in left-sided pneumothorax or pneumomediastinum, in which a ticking or crackling noise is heard over the thorax. This is mostly synchronous with the heartbeat and not related to respiration. It is caused by a small amount of accumulated air in the pleural space, which is being displaced by cardiac contractions during the cardiac cycle. Although typically small, pneumothoraces have a good prognosis. Recognition of the Hamman sign is important, and physicians must realize that even a normal chest X-ray does not rule out the diagnosis.
1. Sandler G. Costs of unnecessary tests. Br Med J. 1979;2(6181):21-24.
2. Laennec RTH, Andral G, Laennec M. Traité De L’auscultation Médiate, Et Des Maladies Des Poumons Et Du Coeur. Paris, France: Paris, J.S.Chaudé, 1826; 1837.
3. Lister WA, Camb MB, Lond MRCP. A case of pericardial knock associated with spontaneous pneumothorax. Lancet. 1928;211(5468):1225-1226.
4. Hamman L. Spontaneous interstitial emphysema of the lungs. Tr A Am Physicians. 1937;52:311-319.
5. Scadding JG, Lond MRCP, Wood P. Systolic clicks due to left-sided pneumothorax. Lancet. 1939;234(6067):1208-1211.
6. Scott JT. Mediastinal emphysema and left pneumothorax. Dist Chest. 1957;32(4):421-434.
7. Roelandt J, Willems J, van der Hauwaert LG, de Geest H. Clicks and sounds (whoops) in left-sided pneumothorax. Clinical and phonocardiographic study. Dis Chest. 1969;56(1):31-36.
8. Smit FW, van Embden Andresen GH, Ubbens R. “Hammans’s sign”, pneumomediastinum and pneumothorax. Ned Tijdschr Geneeskd. 1974;118(22):828-833.
9. Baumann MH, Sahn SA. Hamman’s sign revisited. Pneumothorax or pneumomediastinum? Chest. 1992;102(4):1281-1282.
10. Remmelts HH, Banga JD. Popping pneumothorax. Neth J Med. 2010;68(4):187.
11. Jaiganesh T, Wright K, Sadana A. Mobile diagnosis: Hamman’s crunch in a primary spontaneous pneumothorax. Emerg Med J. 2010;27(6):482-483. doi:10.1136/emj.2009.079681.
1. Sandler G. Costs of unnecessary tests. Br Med J. 1979;2(6181):21-24.
2. Laennec RTH, Andral G, Laennec M. Traité De L’auscultation Médiate, Et Des Maladies Des Poumons Et Du Coeur. Paris, France: Paris, J.S.Chaudé, 1826; 1837.
3. Lister WA, Camb MB, Lond MRCP. A case of pericardial knock associated with spontaneous pneumothorax. Lancet. 1928;211(5468):1225-1226.
4. Hamman L. Spontaneous interstitial emphysema of the lungs. Tr A Am Physicians. 1937;52:311-319.
5. Scadding JG, Lond MRCP, Wood P. Systolic clicks due to left-sided pneumothorax. Lancet. 1939;234(6067):1208-1211.
6. Scott JT. Mediastinal emphysema and left pneumothorax. Dist Chest. 1957;32(4):421-434.
7. Roelandt J, Willems J, van der Hauwaert LG, de Geest H. Clicks and sounds (whoops) in left-sided pneumothorax. Clinical and phonocardiographic study. Dis Chest. 1969;56(1):31-36.
8. Smit FW, van Embden Andresen GH, Ubbens R. “Hammans’s sign”, pneumomediastinum and pneumothorax. Ned Tijdschr Geneeskd. 1974;118(22):828-833.
9. Baumann MH, Sahn SA. Hamman’s sign revisited. Pneumothorax or pneumomediastinum? Chest. 1992;102(4):1281-1282.
10. Remmelts HH, Banga JD. Popping pneumothorax. Neth J Med. 2010;68(4):187.
11. Jaiganesh T, Wright K, Sadana A. Mobile diagnosis: Hamman’s crunch in a primary spontaneous pneumothorax. Emerg Med J. 2010;27(6):482-483. doi:10.1136/emj.2009.079681.
Primary Cutaneous Apocrine Carcinoma Arising Within a Nevus Sebaceus
Nevus sebaceus (NS) is a benign hair follicle neoplasm present in approximately 1.3% of the population, typically involving the scalp, neck, or face.1 These lesions usually are present at birth or identified soon after, during the first year. They present as a yellowish hairless patch or plaque but can develop a more papillomatous appearance, especially after puberty. Historically, the concern with NS was its tendency to transform into basal cell carcinoma (BCC), which prompted surgical excision of the lesion during childhood. This theory has been discounted more recently, as further research has suggested that what was once thought to be BCC may have been confused with the similarly appearing trichoblastoma; however, malignant transformation of NS does still occur, with BCC still being the most common.2 We present the case of a long-standing NS with rare transformation to apocrine carcinoma.
Case Report
A 76-year-old woman presented with several new lesions within a previously diagnosed NS. She reported having the large plaque for as long as she could recall but reported that several new growths developed within the plaque over the last 2 months, slowly increasing in size. She reported a prior biopsy within the growth several years prior, which she described as an irritated seborrheic keratosis.
Physical examination demonstrated 4 distinct lesions within the flesh-colored, verrucous plaque located on the left side of the temporal scalp (Figure 1). The first lesion was a 2.5-cm pearly, pink, exophytic tumor (labeled as A in Figure 1). The next 2 lesions were brown, pedunculated, verrucous papules (labeled as B and C in Figure 1). The last lesion was a purple papule (labeled as D in Figure 1). Four shave biopsies were performed for histologic analysis of the lesions. Lesions B, C, and D were consistent with trichoblastomas, as pathology showed basaloid epithelial tumors that displayed primitive follicular structures, areas of stromal induction, and some pigmentation. Lesion A, originally thought to be suspicious for a BCC, was determined to be a primary cutaneous apocrine adenocarcinoma upon pathologic review. The pathology showed a dermal tumor displaying solid and tubular areas with decapitation secretion. Nuclear pleomorphism and mitoses were present (Figure 2), and staining for carcinoembryonic antigen was positive (Figure 3). Immunoreactivity with epithelial membrane antigen and cytokeratin 7 was noted as well as focal positivity for mammaglobin. Primary apocrine carcinoma was favored over metastatic carcinoma due to the location of the lesion within an NS along with a negative history of internal malignancy. Dermatopathology recommended complete removal of all lesions within the NS.

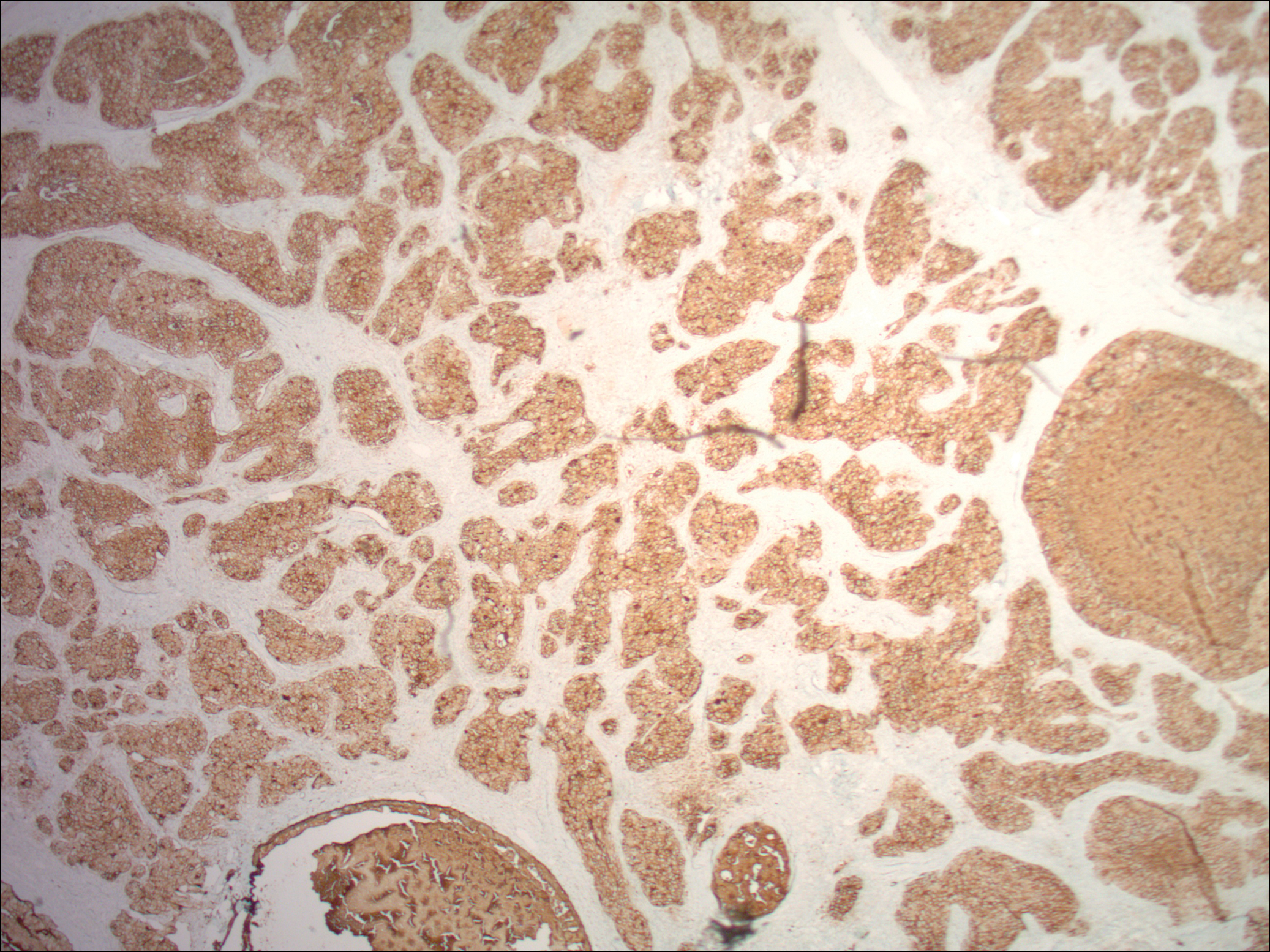
Upon discussing biopsy results and recommendations with our patient, she agreed to undergo excision with intraoperative pathology by a plastic surgeon within our practice to ensure clear margins. The surgical defect following excision was sizeable and closed utilizing a rhomboid flap, full-thickness skin graft, and a split-thickness skin graft. At surgical follow-up, she was doing well and there have been no signs of local recurrence for 10 months since excision.
Comment
Presentation
Nevus sebaceus is the most common adnexal tumor and is classified as a benign congenital hair follicle tumor that is located most commonly on the scalp but also occurs on the face and neck.1 The lesions usually are present at birth but also can develop during the first year of life.2 Diagnosis may be later, during adolescence, when patients seek medical attention during the lesion’s rapid growth phase.1 Nevus sebaceus also is known as an organoid nevus because it may contain all components of the skin. It was originally identified by Jadassohn in 1895.3 It presents as a yellowish, smooth, hairless patch or plaque in prepubertal patients. During adolescence, the lesion typically becomes more yellowish, as well as papillomatous, scaly, or warty. The reported incidence of NS is 0.05% to 1% in dermatology patients.2
Differential
Nevus sebaceus also is a component of several syndromes that should be kept in mind, including Schimmelpenning-Feuerstein-Mims syndrome, which presents with neurologic, skeletal, genitourinary, cardiovascular, and ophthalmic disorders, in addition to cutaneous features. Others include phacomatosis pigmentokeratotica, didmyosis aplasticosebacea, SCALP syndrome (sebaceus nevus, central nervous system malformations, aplasia cutis congenita, limbal dermoid, and pigmented nevus), and more.4,5
Etiology
The etiology of NS has not been completely determined. One study that evaluated 44 NS tissue samples suggested the presence of human papillomavirus (HPV) in NS formation, finding that 82% of NS lesions studied contained HPV DNA. From these results, Carlson et al6 suggested a possible maternal transmission of HPV and infection of ectodermal cells as a potential cause of NS; however, this hypothesis was soon challenged by a study that showed a complete absence of HPV in 16 samples via histological evaluation and polymerase chain reaction for a broad range of HPV types.7 There were investigations into a patched (PTCH) deletion as the cause of NS and thus explained the historically high rate of secondary BCC.8 Further studies showed no mutations at the PTCH locus in trichoblastomas or other tumors arising from NS.9,10
More recent studies have recognized HRAS and KRAS mutations as a causative factor in NS.11 Nevus sebaceus belongs to a group of syndromes resulting from lethal mutations that survive via mosaicism. Nevus sebaceus is caused by postzygotic HRAS or KRAS mutations and is known as a mosaic RASopathy.12 In fact, there is growing evidence to suggest that other nevoid proliferations including keratinocytic epidermal nevi and melanocytic nevi also fall into the spectrum of mosaic RASopathies.13
Staging
There are 3 clinical stages of NS, originally described by Mehregan and Pinkus.14 In stage I (historically known as the infantile stage), the lesion presents as a yellow to pink, smooth, hairless patch. Histologic features include immature hair follicles and hypoplastic sebaceous glands. In stage II (also known as the puberty stage), the lesion becomes more pronounced. Firmer plaques can develop with hyperkeratosis. Hormonal changes cause sebaceous glands to develop, accompanied by epidermal hyperplasia and maturation of apocrine glands. Stage III (the tumoral stage) is a period that various neoplasms have the highest likelihood of occurring. Nevus sebaceus in an adolescent or adult demonstrates mature adnexal structures and greater epidermal hyperplasia.2,4,15
Malignancy
By virtue of these stages of NS development, malignant transformation is expected most often during stage III. However, cases have been reported of malignant tumor development in NS in children before puberty. Two case reports described a 7-year-old boy and a 10-year-old boy diagnosed with a BCC arising from an NS.16,17 However, secondary BCC formation before 16 years of age is rare. Basal cell carcinoma arising from an NS has been commonly reported and is the most common malignant neoplasm in NS (1.1%).2,3 However, the most common neoplasm overall is trichoblastoma (7.4%). The second most common tumor was syringocystadenoma papilliferum, occurring in approximately 5.2% of NS cases. The neoplasm rate in NS was found to be proportional to the patient age.2,18 Multiple studies have shown the overall rate of secondary neoplasms in NS to be 13% to 21.4%, with malignant tumors composing 0.8% to 2.5%.2,15,19 Other neoplasms that have been reported include keratoacanthoma, trichilemmoma, sebaceoma, nevocellular nevus, squamous cell carcinoma, adnexal carcinoma, apocrine adenocarcinoma, and malignant melanoma.19-21
It is argued that the reported rate of BCC formation is overestimated, as prior studies incorrectly labeled trichoblastomas as BCCs. In fact, the largest studies of NS from the 1990s revealed lower rates of malignant secondary tumors than previously determined.4
The identification of apocrine adenocarcinoma tumors arising from NS is exceedingly rare. A study performed by Cribier et al19 in 2000 retrospect
Histopathology
Histopathologic examination reveals considerable variation in morphology, and an underlying pattern has been difficult to recognize. Unfortunately, some authors have concluded that the diagnosis of apocrine carcinoma is relatively subjective.26 Robson et al26 identified 3 general architectural patterns: tubular, tubulopapillary, and solid. Tubular structures consisted of glands and ducts lined by a single or multilayered epithelium. Tubulopapillary architecture was characterized by epithelium forming papillary folds without a fibrovascular core. The solid morphology showed sheets of cells with limited ductal or tubular formation.26 The most specific criteria of these apocrine carcinomas are identification of decapitation secretion, periodic acid–Schiff–positive diastase-resistant material present in the cells or lumen, and positive immunostaining for gross cystic disease fluid protein-15.27
Robson et al26 reported estrogen receptor positivity and androgen receptor positivity in 62% and 64% of 24 primary apocrine carcinoma cases, respectively. However, whether these markers are as common in NS-related apocrine carcinomas has yet to be noted in the literature. One study reports a case of apocrine carcinoma from NS with positive staining for human epidermal growth factor-2, a cell membrane receptor tyrosine kinase commonly investigated in breast cancers and extramammary Paget disease.22
These apocrine carcinomas do have the potential for lymphatic metastasis, as seen with multiple studies. Domingo and Helwig21 identified regional lymph node metastasis in 2 of its 4 apocrine carcinoma patients. Robson et al26 reported lymphovascular invasion in 4 cases and perineural invasion in 2 of 24 patients studied. However, even in the context of recurrence and regional metastasis, the prognosis was good and seldom fatal.26
Treatment
The most effective treatment of NS is excision of dermal and epidermal components. Excision should be completed with a minimum of 2- to 3-mm margins and full thickness down to the underlying supporting fat.28 Historically, the practice of prophylactic excision of NS was supported by the potential for malignant transformation; however, early excision of NS may be less reasonable in light of these more recent studies showing lower incidence of BCC (0.8%), replaced by benign trichoblastomas.19 In the case of apocrine carcinoma development, excision is undoubtedly recommended, with unclear recommendations regarding further evaluation for metastasis.
Excision also may be favored for cosmetic purposes, given the visible regions where NS tends to develop. Chepla and Gosain29 argued that surgical intervention should be based on other factors such as location on the scalp, alopecia, and other issues affecting appearance and monitoring rather than incidence of malignant transformation. Close monitoring and biopsy of suspicious areas is a more conservative option.
Other therapies include CO2 laser, as demonstrated by Kiedrowicz et al,30 on linear NS in a patient with Schimmelpenning-Feuerstein-Mims syndrome.31 However, this approach is palliative and not effective in removing the entire lesion. Electrodesiccation and curettage and dermabrasion also are not good options for the same reason.4
Occurrence in Children
Nevus sebaceus in children, accompanied by other findings suggestive of epidermal nevus syndromes, should prompt further investigation. Schimmelpenning-Feuerstein-Mims syndrome includes major neurological abnormalities including hemimegalencephaly and seizures.32
Conclusion
Apocrine carcinomas are malignant neoplasms that may rarely arise within an NS. Their clinical identification is difficult and requires histopathologic evaluation. Upon recognition, prompt excision with tumor-free margins is recommended. As a rare entity, little data is available regarding its metastatic potential or overall survival rates. Further investigation is clearly necessary as new cases arise.
- Kamyab-Hesari K, Balochi K, Afshar N, et al. Clinicopathological study of 1016 consecutive adnexal skin tumors. Acta Med Iran. 2013;51:879-885.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Ball EA, Hussain M, Moss AL. Squamous cell carcinoma and basal cell carcinoma arising in a naevus sebaceous of Jadassohn: case report and literature review. Clin Exp Dermatol. 2005;30:259-260.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22; quiz 23-24.
- Carlson JA, Cribier B, Nuovo G, et al. Epidermodysplasia verruciformis-associated and genital-mucosal high-risk human papillomavirus DNA are prevalent in nevus sebaceus of Jadassohn. J Am Acad Dermatol. 2008;59:279-294.
- Kim D, Benjamin LT, Sahoo MK, et al. Human papilloma virus is not prevalent in nevus sebaceus [published online November 14, 2013]. Pediatr Dermatol. 2014;31:326-330.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Hafner C, Schmiemann V, Ruetten A, et al. PTCH mutations are not mainly involved in the pathogenesis of sporadic trichoblastomas. Hum Pathol. 2007;38:1496-1500.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus [published online October 25, 2012]. J Invest Dermatol. 2013;133:827-830.
- Happle R. Nevus sebaceus is a mosaic RASopathy. J Invest Dermatol. 2013;133:597-600.
- Luo S, Tsao H. Epidermal, sebaceous, and melanocytic nevoid proliferations are spectrums of mosaic RASopathies. J Invest Dermatol. 2014;134:2493-2496.
- Mehregan AH, Pinkus H. Life history of organoid nevi. special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Altaykan A, Ersoy-Evans S, Erkin G, et al. Basal cell carcinoma arising in nevus sebaceous during childhood. Pediatr Dermatol. 2008;25:616-619.
- Turner CD, Shea CR, Rosoff PM. Basal cell carcinoma originating from a nevus sebaceus on the scalp of a 7-year-old boy. J Pediatr Hematol Oncol. 2001;23:247-249.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Paudel U, Jha A, Pokhrel DB, et al. Apocrine carcinoma developing in a naevus sebaceous of scalp. Kathmandu Univ Med J (KUMJ). 2012;10:103-105.
- Domingo J, Helwig EB. Malignant neoplasms associated with nevus sebaceus of Jadassohn. J Am Acad Dermatol. 1979;1:545-556.
- Tanese K, Wakabayashi A, Suzuki T, et al. Immunoexpression of human epidermal growth factor receptor-2 in apocrine carcinoma arising in naevus sebaceous, case report [published online August 23, 2009]. J Eur Acad Dermatol Venereol. 2010;24:360-362.
- Dalle S, Skowron F, Balme B, et al. Apocrine carcinoma developed in nevus sebaceus of Jadassohn. Eur J Dermatol. 2003;13:487-489.
- Jacyk WK, Requena L, Sánchez Yus E, et al. Tubular apocrine carcinoma arising in a nevus sebaceus of Jadassohn. Am J Dermatopathol. 1998;20:389-392.
- Ansai S, Koseki S, Hashimoto H, et al. A case of ductal sweat gland carcinoma connected to syringocystadenoma papilliferum arising in nevus sebaceus. J Cutan Pathol. 1994;21:557-563.
- Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008;32:682-690.
- Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. a clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;71:375-381.
- Davison SP, Khachemoune A, Yu D, et al. Nevus sebaceus of Jadassohn revisited with reconstruction options. Int J Dermatol. 2005;44:145-150.
- Chepla KJ, Gosain AK. Giant nevus sebaceus: definition, surgical techniques, and rationale for treatment. Plast Reconstr Surg. 2012;130:296E-304E.
- Kiedrowicz M, Kacalak-Rzepka A, Królicki A et al. Therapeutic effects of CO2 laser therapy of linear nevus sebaceous in the course of the Schimmelpenning-Feuerstein-Mims syndrome. Postepy Dermatol Allergol. 2013;30:320-323.
- Ashinoff R. Linear nevus sebaceus of Jadassohn treated with the carbon dioxide laser. Pediatr Dermatol. 1993;10:189-191.
- van de Warrenburg BP, van Gulik S, Renier WO, et al. The linear naevus sebaceus syndrome. Clin Neurol Neurosurg. 1998;100:126-132.
Nevus sebaceus (NS) is a benign hair follicle neoplasm present in approximately 1.3% of the population, typically involving the scalp, neck, or face.1 These lesions usually are present at birth or identified soon after, during the first year. They present as a yellowish hairless patch or plaque but can develop a more papillomatous appearance, especially after puberty. Historically, the concern with NS was its tendency to transform into basal cell carcinoma (BCC), which prompted surgical excision of the lesion during childhood. This theory has been discounted more recently, as further research has suggested that what was once thought to be BCC may have been confused with the similarly appearing trichoblastoma; however, malignant transformation of NS does still occur, with BCC still being the most common.2 We present the case of a long-standing NS with rare transformation to apocrine carcinoma.
Case Report
A 76-year-old woman presented with several new lesions within a previously diagnosed NS. She reported having the large plaque for as long as she could recall but reported that several new growths developed within the plaque over the last 2 months, slowly increasing in size. She reported a prior biopsy within the growth several years prior, which she described as an irritated seborrheic keratosis.
Physical examination demonstrated 4 distinct lesions within the flesh-colored, verrucous plaque located on the left side of the temporal scalp (Figure 1). The first lesion was a 2.5-cm pearly, pink, exophytic tumor (labeled as A in Figure 1). The next 2 lesions were brown, pedunculated, verrucous papules (labeled as B and C in Figure 1). The last lesion was a purple papule (labeled as D in Figure 1). Four shave biopsies were performed for histologic analysis of the lesions. Lesions B, C, and D were consistent with trichoblastomas, as pathology showed basaloid epithelial tumors that displayed primitive follicular structures, areas of stromal induction, and some pigmentation. Lesion A, originally thought to be suspicious for a BCC, was determined to be a primary cutaneous apocrine adenocarcinoma upon pathologic review. The pathology showed a dermal tumor displaying solid and tubular areas with decapitation secretion. Nuclear pleomorphism and mitoses were present (Figure 2), and staining for carcinoembryonic antigen was positive (Figure 3). Immunoreactivity with epithelial membrane antigen and cytokeratin 7 was noted as well as focal positivity for mammaglobin. Primary apocrine carcinoma was favored over metastatic carcinoma due to the location of the lesion within an NS along with a negative history of internal malignancy. Dermatopathology recommended complete removal of all lesions within the NS.



Upon discussing biopsy results and recommendations with our patient, she agreed to undergo excision with intraoperative pathology by a plastic surgeon within our practice to ensure clear margins. The surgical defect following excision was sizeable and closed utilizing a rhomboid flap, full-thickness skin graft, and a split-thickness skin graft. At surgical follow-up, she was doing well and there have been no signs of local recurrence for 10 months since excision.
Comment
Presentation
Nevus sebaceus is the most common adnexal tumor and is classified as a benign congenital hair follicle tumor that is located most commonly on the scalp but also occurs on the face and neck.1 The lesions usually are present at birth but also can develop during the first year of life.2 Diagnosis may be later, during adolescence, when patients seek medical attention during the lesion’s rapid growth phase.1 Nevus sebaceus also is known as an organoid nevus because it may contain all components of the skin. It was originally identified by Jadassohn in 1895.3 It presents as a yellowish, smooth, hairless patch or plaque in prepubertal patients. During adolescence, the lesion typically becomes more yellowish, as well as papillomatous, scaly, or warty. The reported incidence of NS is 0.05% to 1% in dermatology patients.2
Differential
Nevus sebaceus also is a component of several syndromes that should be kept in mind, including Schimmelpenning-Feuerstein-Mims syndrome, which presents with neurologic, skeletal, genitourinary, cardiovascular, and ophthalmic disorders, in addition to cutaneous features. Others include phacomatosis pigmentokeratotica, didmyosis aplasticosebacea, SCALP syndrome (sebaceus nevus, central nervous system malformations, aplasia cutis congenita, limbal dermoid, and pigmented nevus), and more.4,5
Etiology
The etiology of NS has not been completely determined. One study that evaluated 44 NS tissue samples suggested the presence of human papillomavirus (HPV) in NS formation, finding that 82% of NS lesions studied contained HPV DNA. From these results, Carlson et al6 suggested a possible maternal transmission of HPV and infection of ectodermal cells as a potential cause of NS; however, this hypothesis was soon challenged by a study that showed a complete absence of HPV in 16 samples via histological evaluation and polymerase chain reaction for a broad range of HPV types.7 There were investigations into a patched (PTCH) deletion as the cause of NS and thus explained the historically high rate of secondary BCC.8 Further studies showed no mutations at the PTCH locus in trichoblastomas or other tumors arising from NS.9,10
More recent studies have recognized HRAS and KRAS mutations as a causative factor in NS.11 Nevus sebaceus belongs to a group of syndromes resulting from lethal mutations that survive via mosaicism. Nevus sebaceus is caused by postzygotic HRAS or KRAS mutations and is known as a mosaic RASopathy.12 In fact, there is growing evidence to suggest that other nevoid proliferations including keratinocytic epidermal nevi and melanocytic nevi also fall into the spectrum of mosaic RASopathies.13
Staging
There are 3 clinical stages of NS, originally described by Mehregan and Pinkus.14 In stage I (historically known as the infantile stage), the lesion presents as a yellow to pink, smooth, hairless patch. Histologic features include immature hair follicles and hypoplastic sebaceous glands. In stage II (also known as the puberty stage), the lesion becomes more pronounced. Firmer plaques can develop with hyperkeratosis. Hormonal changes cause sebaceous glands to develop, accompanied by epidermal hyperplasia and maturation of apocrine glands. Stage III (the tumoral stage) is a period that various neoplasms have the highest likelihood of occurring. Nevus sebaceus in an adolescent or adult demonstrates mature adnexal structures and greater epidermal hyperplasia.2,4,15
Malignancy
By virtue of these stages of NS development, malignant transformation is expected most often during stage III. However, cases have been reported of malignant tumor development in NS in children before puberty. Two case reports described a 7-year-old boy and a 10-year-old boy diagnosed with a BCC arising from an NS.16,17 However, secondary BCC formation before 16 years of age is rare. Basal cell carcinoma arising from an NS has been commonly reported and is the most common malignant neoplasm in NS (1.1%).2,3 However, the most common neoplasm overall is trichoblastoma (7.4%). The second most common tumor was syringocystadenoma papilliferum, occurring in approximately 5.2% of NS cases. The neoplasm rate in NS was found to be proportional to the patient age.2,18 Multiple studies have shown the overall rate of secondary neoplasms in NS to be 13% to 21.4%, with malignant tumors composing 0.8% to 2.5%.2,15,19 Other neoplasms that have been reported include keratoacanthoma, trichilemmoma, sebaceoma, nevocellular nevus, squamous cell carcinoma, adnexal carcinoma, apocrine adenocarcinoma, and malignant melanoma.19-21
It is argued that the reported rate of BCC formation is overestimated, as prior studies incorrectly labeled trichoblastomas as BCCs. In fact, the largest studies of NS from the 1990s revealed lower rates of malignant secondary tumors than previously determined.4
The identification of apocrine adenocarcinoma tumors arising from NS is exceedingly rare. A study performed by Cribier et al19 in 2000 retrospect
Histopathology
Histopathologic examination reveals considerable variation in morphology, and an underlying pattern has been difficult to recognize. Unfortunately, some authors have concluded that the diagnosis of apocrine carcinoma is relatively subjective.26 Robson et al26 identified 3 general architectural patterns: tubular, tubulopapillary, and solid. Tubular structures consisted of glands and ducts lined by a single or multilayered epithelium. Tubulopapillary architecture was characterized by epithelium forming papillary folds without a fibrovascular core. The solid morphology showed sheets of cells with limited ductal or tubular formation.26 The most specific criteria of these apocrine carcinomas are identification of decapitation secretion, periodic acid–Schiff–positive diastase-resistant material present in the cells or lumen, and positive immunostaining for gross cystic disease fluid protein-15.27
Robson et al26 reported estrogen receptor positivity and androgen receptor positivity in 62% and 64% of 24 primary apocrine carcinoma cases, respectively. However, whether these markers are as common in NS-related apocrine carcinomas has yet to be noted in the literature. One study reports a case of apocrine carcinoma from NS with positive staining for human epidermal growth factor-2, a cell membrane receptor tyrosine kinase commonly investigated in breast cancers and extramammary Paget disease.22
These apocrine carcinomas do have the potential for lymphatic metastasis, as seen with multiple studies. Domingo and Helwig21 identified regional lymph node metastasis in 2 of its 4 apocrine carcinoma patients. Robson et al26 reported lymphovascular invasion in 4 cases and perineural invasion in 2 of 24 patients studied. However, even in the context of recurrence and regional metastasis, the prognosis was good and seldom fatal.26
Treatment
The most effective treatment of NS is excision of dermal and epidermal components. Excision should be completed with a minimum of 2- to 3-mm margins and full thickness down to the underlying supporting fat.28 Historically, the practice of prophylactic excision of NS was supported by the potential for malignant transformation; however, early excision of NS may be less reasonable in light of these more recent studies showing lower incidence of BCC (0.8%), replaced by benign trichoblastomas.19 In the case of apocrine carcinoma development, excision is undoubtedly recommended, with unclear recommendations regarding further evaluation for metastasis.
Excision also may be favored for cosmetic purposes, given the visible regions where NS tends to develop. Chepla and Gosain29 argued that surgical intervention should be based on other factors such as location on the scalp, alopecia, and other issues affecting appearance and monitoring rather than incidence of malignant transformation. Close monitoring and biopsy of suspicious areas is a more conservative option.
Other therapies include CO2 laser, as demonstrated by Kiedrowicz et al,30 on linear NS in a patient with Schimmelpenning-Feuerstein-Mims syndrome.31 However, this approach is palliative and not effective in removing the entire lesion. Electrodesiccation and curettage and dermabrasion also are not good options for the same reason.4
Occurrence in Children
Nevus sebaceus in children, accompanied by other findings suggestive of epidermal nevus syndromes, should prompt further investigation. Schimmelpenning-Feuerstein-Mims syndrome includes major neurological abnormalities including hemimegalencephaly and seizures.32
Conclusion
Apocrine carcinomas are malignant neoplasms that may rarely arise within an NS. Their clinical identification is difficult and requires histopathologic evaluation. Upon recognition, prompt excision with tumor-free margins is recommended. As a rare entity, little data is available regarding its metastatic potential or overall survival rates. Further investigation is clearly necessary as new cases arise.
Nevus sebaceus (NS) is a benign hair follicle neoplasm present in approximately 1.3% of the population, typically involving the scalp, neck, or face.1 These lesions usually are present at birth or identified soon after, during the first year. They present as a yellowish hairless patch or plaque but can develop a more papillomatous appearance, especially after puberty. Historically, the concern with NS was its tendency to transform into basal cell carcinoma (BCC), which prompted surgical excision of the lesion during childhood. This theory has been discounted more recently, as further research has suggested that what was once thought to be BCC may have been confused with the similarly appearing trichoblastoma; however, malignant transformation of NS does still occur, with BCC still being the most common.2 We present the case of a long-standing NS with rare transformation to apocrine carcinoma.
Case Report
A 76-year-old woman presented with several new lesions within a previously diagnosed NS. She reported having the large plaque for as long as she could recall but reported that several new growths developed within the plaque over the last 2 months, slowly increasing in size. She reported a prior biopsy within the growth several years prior, which she described as an irritated seborrheic keratosis.
Physical examination demonstrated 4 distinct lesions within the flesh-colored, verrucous plaque located on the left side of the temporal scalp (Figure 1). The first lesion was a 2.5-cm pearly, pink, exophytic tumor (labeled as A in Figure 1). The next 2 lesions were brown, pedunculated, verrucous papules (labeled as B and C in Figure 1). The last lesion was a purple papule (labeled as D in Figure 1). Four shave biopsies were performed for histologic analysis of the lesions. Lesions B, C, and D were consistent with trichoblastomas, as pathology showed basaloid epithelial tumors that displayed primitive follicular structures, areas of stromal induction, and some pigmentation. Lesion A, originally thought to be suspicious for a BCC, was determined to be a primary cutaneous apocrine adenocarcinoma upon pathologic review. The pathology showed a dermal tumor displaying solid and tubular areas with decapitation secretion. Nuclear pleomorphism and mitoses were present (Figure 2), and staining for carcinoembryonic antigen was positive (Figure 3). Immunoreactivity with epithelial membrane antigen and cytokeratin 7 was noted as well as focal positivity for mammaglobin. Primary apocrine carcinoma was favored over metastatic carcinoma due to the location of the lesion within an NS along with a negative history of internal malignancy. Dermatopathology recommended complete removal of all lesions within the NS.



Upon discussing biopsy results and recommendations with our patient, she agreed to undergo excision with intraoperative pathology by a plastic surgeon within our practice to ensure clear margins. The surgical defect following excision was sizeable and closed utilizing a rhomboid flap, full-thickness skin graft, and a split-thickness skin graft. At surgical follow-up, she was doing well and there have been no signs of local recurrence for 10 months since excision.
Comment
Presentation
Nevus sebaceus is the most common adnexal tumor and is classified as a benign congenital hair follicle tumor that is located most commonly on the scalp but also occurs on the face and neck.1 The lesions usually are present at birth but also can develop during the first year of life.2 Diagnosis may be later, during adolescence, when patients seek medical attention during the lesion’s rapid growth phase.1 Nevus sebaceus also is known as an organoid nevus because it may contain all components of the skin. It was originally identified by Jadassohn in 1895.3 It presents as a yellowish, smooth, hairless patch or plaque in prepubertal patients. During adolescence, the lesion typically becomes more yellowish, as well as papillomatous, scaly, or warty. The reported incidence of NS is 0.05% to 1% in dermatology patients.2
Differential
Nevus sebaceus also is a component of several syndromes that should be kept in mind, including Schimmelpenning-Feuerstein-Mims syndrome, which presents with neurologic, skeletal, genitourinary, cardiovascular, and ophthalmic disorders, in addition to cutaneous features. Others include phacomatosis pigmentokeratotica, didmyosis aplasticosebacea, SCALP syndrome (sebaceus nevus, central nervous system malformations, aplasia cutis congenita, limbal dermoid, and pigmented nevus), and more.4,5
Etiology
The etiology of NS has not been completely determined. One study that evaluated 44 NS tissue samples suggested the presence of human papillomavirus (HPV) in NS formation, finding that 82% of NS lesions studied contained HPV DNA. From these results, Carlson et al6 suggested a possible maternal transmission of HPV and infection of ectodermal cells as a potential cause of NS; however, this hypothesis was soon challenged by a study that showed a complete absence of HPV in 16 samples via histological evaluation and polymerase chain reaction for a broad range of HPV types.7 There were investigations into a patched (PTCH) deletion as the cause of NS and thus explained the historically high rate of secondary BCC.8 Further studies showed no mutations at the PTCH locus in trichoblastomas or other tumors arising from NS.9,10
More recent studies have recognized HRAS and KRAS mutations as a causative factor in NS.11 Nevus sebaceus belongs to a group of syndromes resulting from lethal mutations that survive via mosaicism. Nevus sebaceus is caused by postzygotic HRAS or KRAS mutations and is known as a mosaic RASopathy.12 In fact, there is growing evidence to suggest that other nevoid proliferations including keratinocytic epidermal nevi and melanocytic nevi also fall into the spectrum of mosaic RASopathies.13
Staging
There are 3 clinical stages of NS, originally described by Mehregan and Pinkus.14 In stage I (historically known as the infantile stage), the lesion presents as a yellow to pink, smooth, hairless patch. Histologic features include immature hair follicles and hypoplastic sebaceous glands. In stage II (also known as the puberty stage), the lesion becomes more pronounced. Firmer plaques can develop with hyperkeratosis. Hormonal changes cause sebaceous glands to develop, accompanied by epidermal hyperplasia and maturation of apocrine glands. Stage III (the tumoral stage) is a period that various neoplasms have the highest likelihood of occurring. Nevus sebaceus in an adolescent or adult demonstrates mature adnexal structures and greater epidermal hyperplasia.2,4,15
Malignancy
By virtue of these stages of NS development, malignant transformation is expected most often during stage III. However, cases have been reported of malignant tumor development in NS in children before puberty. Two case reports described a 7-year-old boy and a 10-year-old boy diagnosed with a BCC arising from an NS.16,17 However, secondary BCC formation before 16 years of age is rare. Basal cell carcinoma arising from an NS has been commonly reported and is the most common malignant neoplasm in NS (1.1%).2,3 However, the most common neoplasm overall is trichoblastoma (7.4%). The second most common tumor was syringocystadenoma papilliferum, occurring in approximately 5.2% of NS cases. The neoplasm rate in NS was found to be proportional to the patient age.2,18 Multiple studies have shown the overall rate of secondary neoplasms in NS to be 13% to 21.4%, with malignant tumors composing 0.8% to 2.5%.2,15,19 Other neoplasms that have been reported include keratoacanthoma, trichilemmoma, sebaceoma, nevocellular nevus, squamous cell carcinoma, adnexal carcinoma, apocrine adenocarcinoma, and malignant melanoma.19-21
It is argued that the reported rate of BCC formation is overestimated, as prior studies incorrectly labeled trichoblastomas as BCCs. In fact, the largest studies of NS from the 1990s revealed lower rates of malignant secondary tumors than previously determined.4
The identification of apocrine adenocarcinoma tumors arising from NS is exceedingly rare. A study performed by Cribier et al19 in 2000 retrospect
Histopathology
Histopathologic examination reveals considerable variation in morphology, and an underlying pattern has been difficult to recognize. Unfortunately, some authors have concluded that the diagnosis of apocrine carcinoma is relatively subjective.26 Robson et al26 identified 3 general architectural patterns: tubular, tubulopapillary, and solid. Tubular structures consisted of glands and ducts lined by a single or multilayered epithelium. Tubulopapillary architecture was characterized by epithelium forming papillary folds without a fibrovascular core. The solid morphology showed sheets of cells with limited ductal or tubular formation.26 The most specific criteria of these apocrine carcinomas are identification of decapitation secretion, periodic acid–Schiff–positive diastase-resistant material present in the cells or lumen, and positive immunostaining for gross cystic disease fluid protein-15.27
Robson et al26 reported estrogen receptor positivity and androgen receptor positivity in 62% and 64% of 24 primary apocrine carcinoma cases, respectively. However, whether these markers are as common in NS-related apocrine carcinomas has yet to be noted in the literature. One study reports a case of apocrine carcinoma from NS with positive staining for human epidermal growth factor-2, a cell membrane receptor tyrosine kinase commonly investigated in breast cancers and extramammary Paget disease.22
These apocrine carcinomas do have the potential for lymphatic metastasis, as seen with multiple studies. Domingo and Helwig21 identified regional lymph node metastasis in 2 of its 4 apocrine carcinoma patients. Robson et al26 reported lymphovascular invasion in 4 cases and perineural invasion in 2 of 24 patients studied. However, even in the context of recurrence and regional metastasis, the prognosis was good and seldom fatal.26
Treatment
The most effective treatment of NS is excision of dermal and epidermal components. Excision should be completed with a minimum of 2- to 3-mm margins and full thickness down to the underlying supporting fat.28 Historically, the practice of prophylactic excision of NS was supported by the potential for malignant transformation; however, early excision of NS may be less reasonable in light of these more recent studies showing lower incidence of BCC (0.8%), replaced by benign trichoblastomas.19 In the case of apocrine carcinoma development, excision is undoubtedly recommended, with unclear recommendations regarding further evaluation for metastasis.
Excision also may be favored for cosmetic purposes, given the visible regions where NS tends to develop. Chepla and Gosain29 argued that surgical intervention should be based on other factors such as location on the scalp, alopecia, and other issues affecting appearance and monitoring rather than incidence of malignant transformation. Close monitoring and biopsy of suspicious areas is a more conservative option.
Other therapies include CO2 laser, as demonstrated by Kiedrowicz et al,30 on linear NS in a patient with Schimmelpenning-Feuerstein-Mims syndrome.31 However, this approach is palliative and not effective in removing the entire lesion. Electrodesiccation and curettage and dermabrasion also are not good options for the same reason.4
Occurrence in Children
Nevus sebaceus in children, accompanied by other findings suggestive of epidermal nevus syndromes, should prompt further investigation. Schimmelpenning-Feuerstein-Mims syndrome includes major neurological abnormalities including hemimegalencephaly and seizures.32
Conclusion
Apocrine carcinomas are malignant neoplasms that may rarely arise within an NS. Their clinical identification is difficult and requires histopathologic evaluation. Upon recognition, prompt excision with tumor-free margins is recommended. As a rare entity, little data is available regarding its metastatic potential or overall survival rates. Further investigation is clearly necessary as new cases arise.
- Kamyab-Hesari K, Balochi K, Afshar N, et al. Clinicopathological study of 1016 consecutive adnexal skin tumors. Acta Med Iran. 2013;51:879-885.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Ball EA, Hussain M, Moss AL. Squamous cell carcinoma and basal cell carcinoma arising in a naevus sebaceous of Jadassohn: case report and literature review. Clin Exp Dermatol. 2005;30:259-260.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22; quiz 23-24.
- Carlson JA, Cribier B, Nuovo G, et al. Epidermodysplasia verruciformis-associated and genital-mucosal high-risk human papillomavirus DNA are prevalent in nevus sebaceus of Jadassohn. J Am Acad Dermatol. 2008;59:279-294.
- Kim D, Benjamin LT, Sahoo MK, et al. Human papilloma virus is not prevalent in nevus sebaceus [published online November 14, 2013]. Pediatr Dermatol. 2014;31:326-330.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Hafner C, Schmiemann V, Ruetten A, et al. PTCH mutations are not mainly involved in the pathogenesis of sporadic trichoblastomas. Hum Pathol. 2007;38:1496-1500.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus [published online October 25, 2012]. J Invest Dermatol. 2013;133:827-830.
- Happle R. Nevus sebaceus is a mosaic RASopathy. J Invest Dermatol. 2013;133:597-600.
- Luo S, Tsao H. Epidermal, sebaceous, and melanocytic nevoid proliferations are spectrums of mosaic RASopathies. J Invest Dermatol. 2014;134:2493-2496.
- Mehregan AH, Pinkus H. Life history of organoid nevi. special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Altaykan A, Ersoy-Evans S, Erkin G, et al. Basal cell carcinoma arising in nevus sebaceous during childhood. Pediatr Dermatol. 2008;25:616-619.
- Turner CD, Shea CR, Rosoff PM. Basal cell carcinoma originating from a nevus sebaceus on the scalp of a 7-year-old boy. J Pediatr Hematol Oncol. 2001;23:247-249.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Paudel U, Jha A, Pokhrel DB, et al. Apocrine carcinoma developing in a naevus sebaceous of scalp. Kathmandu Univ Med J (KUMJ). 2012;10:103-105.
- Domingo J, Helwig EB. Malignant neoplasms associated with nevus sebaceus of Jadassohn. J Am Acad Dermatol. 1979;1:545-556.
- Tanese K, Wakabayashi A, Suzuki T, et al. Immunoexpression of human epidermal growth factor receptor-2 in apocrine carcinoma arising in naevus sebaceous, case report [published online August 23, 2009]. J Eur Acad Dermatol Venereol. 2010;24:360-362.
- Dalle S, Skowron F, Balme B, et al. Apocrine carcinoma developed in nevus sebaceus of Jadassohn. Eur J Dermatol. 2003;13:487-489.
- Jacyk WK, Requena L, Sánchez Yus E, et al. Tubular apocrine carcinoma arising in a nevus sebaceus of Jadassohn. Am J Dermatopathol. 1998;20:389-392.
- Ansai S, Koseki S, Hashimoto H, et al. A case of ductal sweat gland carcinoma connected to syringocystadenoma papilliferum arising in nevus sebaceus. J Cutan Pathol. 1994;21:557-563.
- Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008;32:682-690.
- Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. a clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;71:375-381.
- Davison SP, Khachemoune A, Yu D, et al. Nevus sebaceus of Jadassohn revisited with reconstruction options. Int J Dermatol. 2005;44:145-150.
- Chepla KJ, Gosain AK. Giant nevus sebaceus: definition, surgical techniques, and rationale for treatment. Plast Reconstr Surg. 2012;130:296E-304E.
- Kiedrowicz M, Kacalak-Rzepka A, Królicki A et al. Therapeutic effects of CO2 laser therapy of linear nevus sebaceous in the course of the Schimmelpenning-Feuerstein-Mims syndrome. Postepy Dermatol Allergol. 2013;30:320-323.
- Ashinoff R. Linear nevus sebaceus of Jadassohn treated with the carbon dioxide laser. Pediatr Dermatol. 1993;10:189-191.
- van de Warrenburg BP, van Gulik S, Renier WO, et al. The linear naevus sebaceus syndrome. Clin Neurol Neurosurg. 1998;100:126-132.
- Kamyab-Hesari K, Balochi K, Afshar N, et al. Clinicopathological study of 1016 consecutive adnexal skin tumors. Acta Med Iran. 2013;51:879-885.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Ball EA, Hussain M, Moss AL. Squamous cell carcinoma and basal cell carcinoma arising in a naevus sebaceous of Jadassohn: case report and literature review. Clin Exp Dermatol. 2005;30:259-260.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22; quiz 23-24.
- Carlson JA, Cribier B, Nuovo G, et al. Epidermodysplasia verruciformis-associated and genital-mucosal high-risk human papillomavirus DNA are prevalent in nevus sebaceus of Jadassohn. J Am Acad Dermatol. 2008;59:279-294.
- Kim D, Benjamin LT, Sahoo MK, et al. Human papilloma virus is not prevalent in nevus sebaceus [published online November 14, 2013]. Pediatr Dermatol. 2014;31:326-330.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Hafner C, Schmiemann V, Ruetten A, et al. PTCH mutations are not mainly involved in the pathogenesis of sporadic trichoblastomas. Hum Pathol. 2007;38:1496-1500.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus [published online October 25, 2012]. J Invest Dermatol. 2013;133:827-830.
- Happle R. Nevus sebaceus is a mosaic RASopathy. J Invest Dermatol. 2013;133:597-600.
- Luo S, Tsao H. Epidermal, sebaceous, and melanocytic nevoid proliferations are spectrums of mosaic RASopathies. J Invest Dermatol. 2014;134:2493-2496.
- Mehregan AH, Pinkus H. Life history of organoid nevi. special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Altaykan A, Ersoy-Evans S, Erkin G, et al. Basal cell carcinoma arising in nevus sebaceous during childhood. Pediatr Dermatol. 2008;25:616-619.
- Turner CD, Shea CR, Rosoff PM. Basal cell carcinoma originating from a nevus sebaceus on the scalp of a 7-year-old boy. J Pediatr Hematol Oncol. 2001;23:247-249.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Paudel U, Jha A, Pokhrel DB, et al. Apocrine carcinoma developing in a naevus sebaceous of scalp. Kathmandu Univ Med J (KUMJ). 2012;10:103-105.
- Domingo J, Helwig EB. Malignant neoplasms associated with nevus sebaceus of Jadassohn. J Am Acad Dermatol. 1979;1:545-556.
- Tanese K, Wakabayashi A, Suzuki T, et al. Immunoexpression of human epidermal growth factor receptor-2 in apocrine carcinoma arising in naevus sebaceous, case report [published online August 23, 2009]. J Eur Acad Dermatol Venereol. 2010;24:360-362.
- Dalle S, Skowron F, Balme B, et al. Apocrine carcinoma developed in nevus sebaceus of Jadassohn. Eur J Dermatol. 2003;13:487-489.
- Jacyk WK, Requena L, Sánchez Yus E, et al. Tubular apocrine carcinoma arising in a nevus sebaceus of Jadassohn. Am J Dermatopathol. 1998;20:389-392.
- Ansai S, Koseki S, Hashimoto H, et al. A case of ductal sweat gland carcinoma connected to syringocystadenoma papilliferum arising in nevus sebaceus. J Cutan Pathol. 1994;21:557-563.
- Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008;32:682-690.
- Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. a clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;71:375-381.
- Davison SP, Khachemoune A, Yu D, et al. Nevus sebaceus of Jadassohn revisited with reconstruction options. Int J Dermatol. 2005;44:145-150.
- Chepla KJ, Gosain AK. Giant nevus sebaceus: definition, surgical techniques, and rationale for treatment. Plast Reconstr Surg. 2012;130:296E-304E.
- Kiedrowicz M, Kacalak-Rzepka A, Królicki A et al. Therapeutic effects of CO2 laser therapy of linear nevus sebaceous in the course of the Schimmelpenning-Feuerstein-Mims syndrome. Postepy Dermatol Allergol. 2013;30:320-323.
- Ashinoff R. Linear nevus sebaceus of Jadassohn treated with the carbon dioxide laser. Pediatr Dermatol. 1993;10:189-191.
- van de Warrenburg BP, van Gulik S, Renier WO, et al. The linear naevus sebaceus syndrome. Clin Neurol Neurosurg. 1998;100:126-132.
Practice Points
- Nevus sebaceus (NS) in the centrofacial region has been correlated with a higher risk for neurological abnormalities, including intellectual disability and seizures.
- Historically, basal cell carcinomas (BCCs) were considered a common occurrence arising from an NS, prompting prophylactic surgical excision of such lesions.
- More recently, it has been recognized that the most common tumor to arise from NS is trichoblastoma rather than BCC; in fact, BCC and other malignancies have been found to be relatively rare compared to their benign counterparts.
- In light of this discovery, observation of NS may be a more prudent course of treatment versus prophylactic surgical excision.
Xanthogranulomatous Reaction to Trametinib for Metastatic Malignant Melanoma
A decade ago, the few agents approved by the US Food and Drug Administration for treatment of metastatic melanoma demonstrated low therapeutic success rates (ie, <15%–20%).1 Since then, advances in molecular biology have identified oncogenes that contribute to melanoma progression.2 Inhibition of the mitogen-activated protein kinase (MAPK) pathway by targeting mutant BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) has created promising pharmacologic treatment opportunities.3 Due to the recent US Food and Drug Administration approval of these therapies for treatment of melanoma, it is important to better characterize these adverse events (AEs) so that we can manage them. We present the development of an unusual cutaneous reaction to trametinib, a MEK inhibitor, in a man with stage IV M1b malignant melanoma.
Case Report
A 66-year-old man with stage IV M1b malignant melanoma with metastases to the brain and lungs presented with recurring pruritic erythematous papules on the face and bilateral forearms that began shortly after initiating therapy with trametinib. The cutaneous eruption had initially presented on the face, forearms, and dorsal hands when trametinib was used in combination with vemurafenib, a BRAF inhibitor, and ipilimumab, a human cytotoxic T-lymphocyte antigen 4–blocking antibody; however, lesions initially were minimal and self-resolving. When trametinib was reintroduced as monotherapy due to fever attributed to the combination treatment regimen, the cutaneous eruption recurred more severely. Physical examination revealed erythematous scaly papules limited to the face and bilateral upper extremities, including the flexural surfaces.
A biopsy from the flexural surface of the right forearm revealed a dense perivascular lymphoid and xanthomatous infiltrate in the dermis (Figure 1). Poorly formed granulomas within the mid reticular dermis demonstrated focal palisading of histiocytes with prominent giant cells at the periphery. Histiocytes and giant cells showed foamy or xanthomatous cytoplasm. Within the reaction, degenerative and swollen collagen fibers were noted with no mucin deposition, which was confirmed with negative colloidal iron staining.
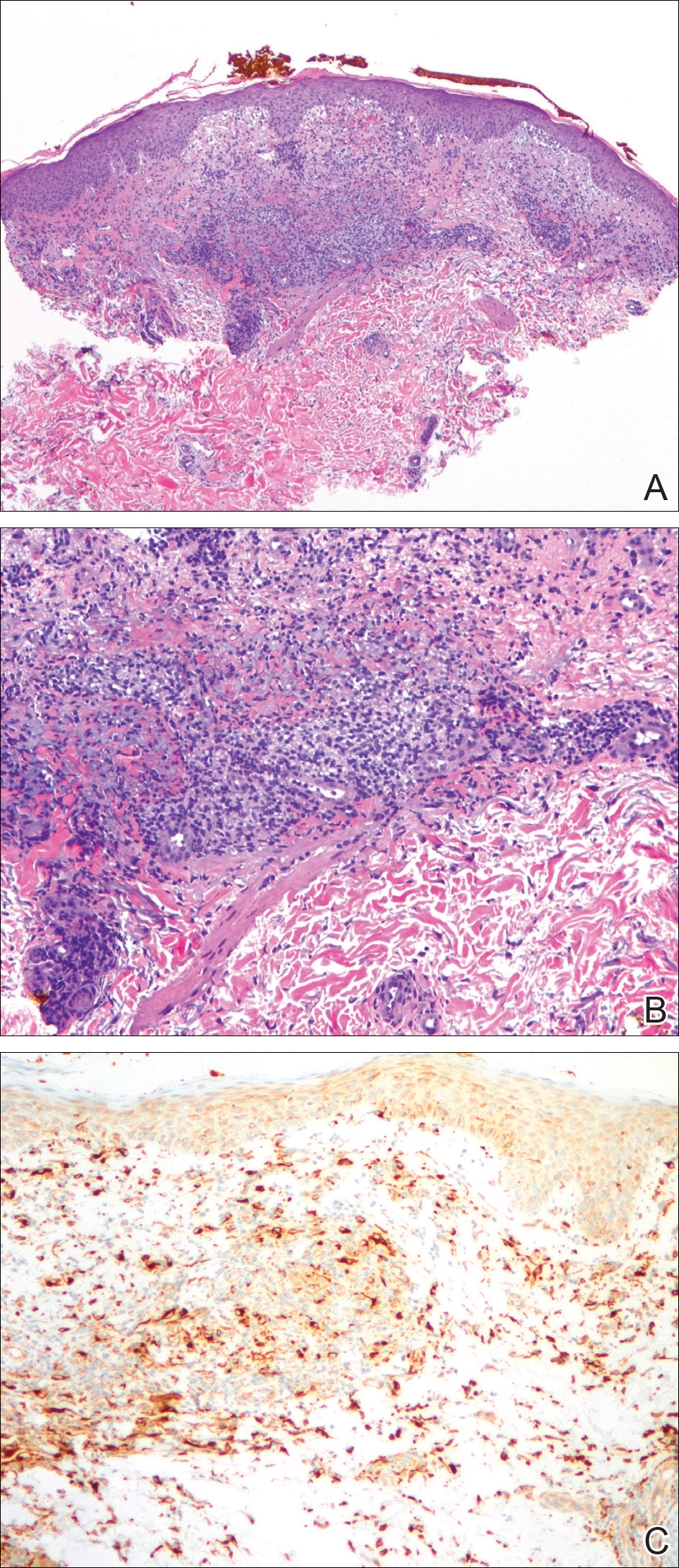
Brief cessation of trametinib along with application of clobetasol propionate ointment 0.05% resulted in resolution of the cutaneous eruption. Later, trametinib was reintroduced in combination with vemurafenib, though therapy was intermittently discontinued due to various side effects. Skin lesions continued to recur (Figure 2) while the patient was on trametinib but remained minimal and continued to respond to topical clobetasol propionate. One year later, the patient continues to tolerate combination therapy with trametinib and vemurafenib.
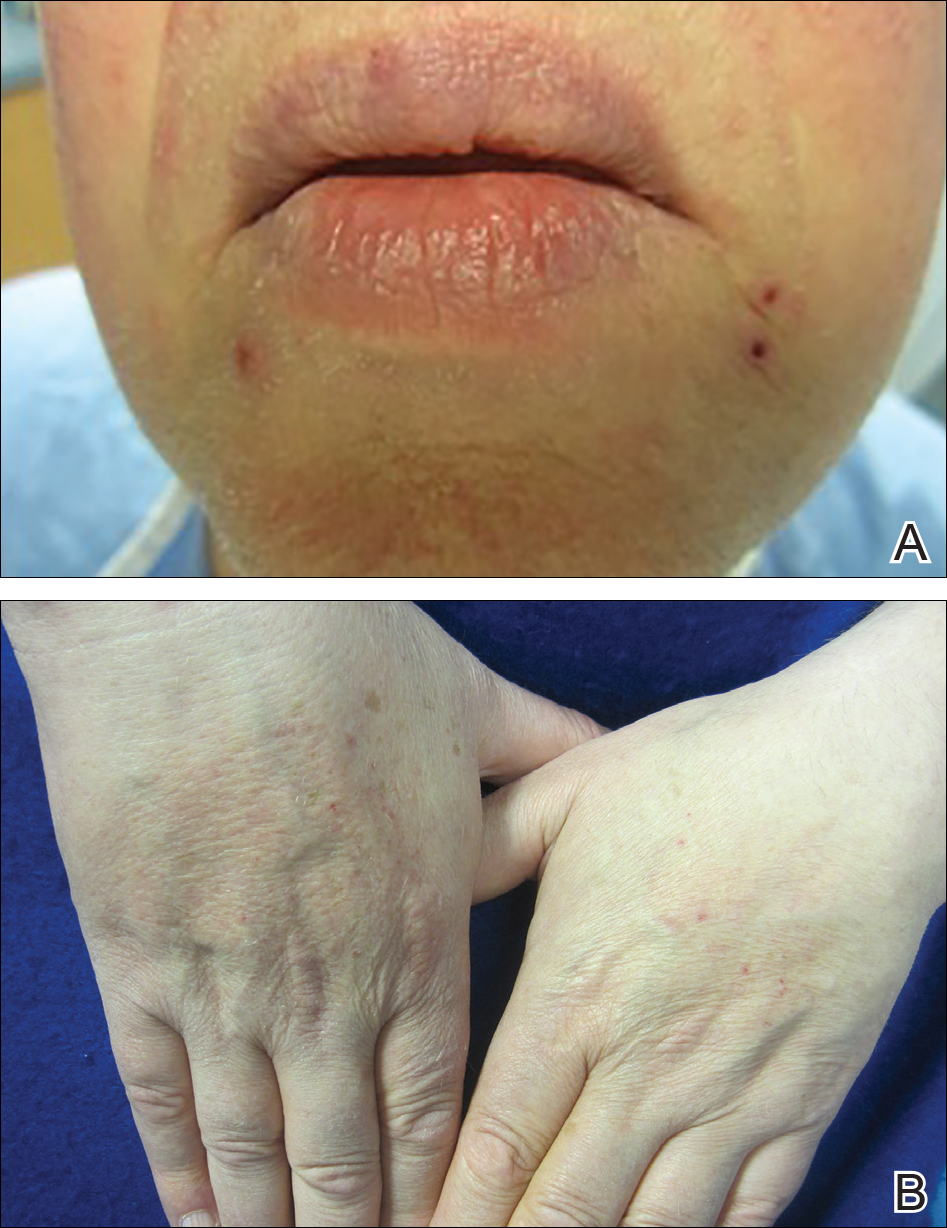
Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9
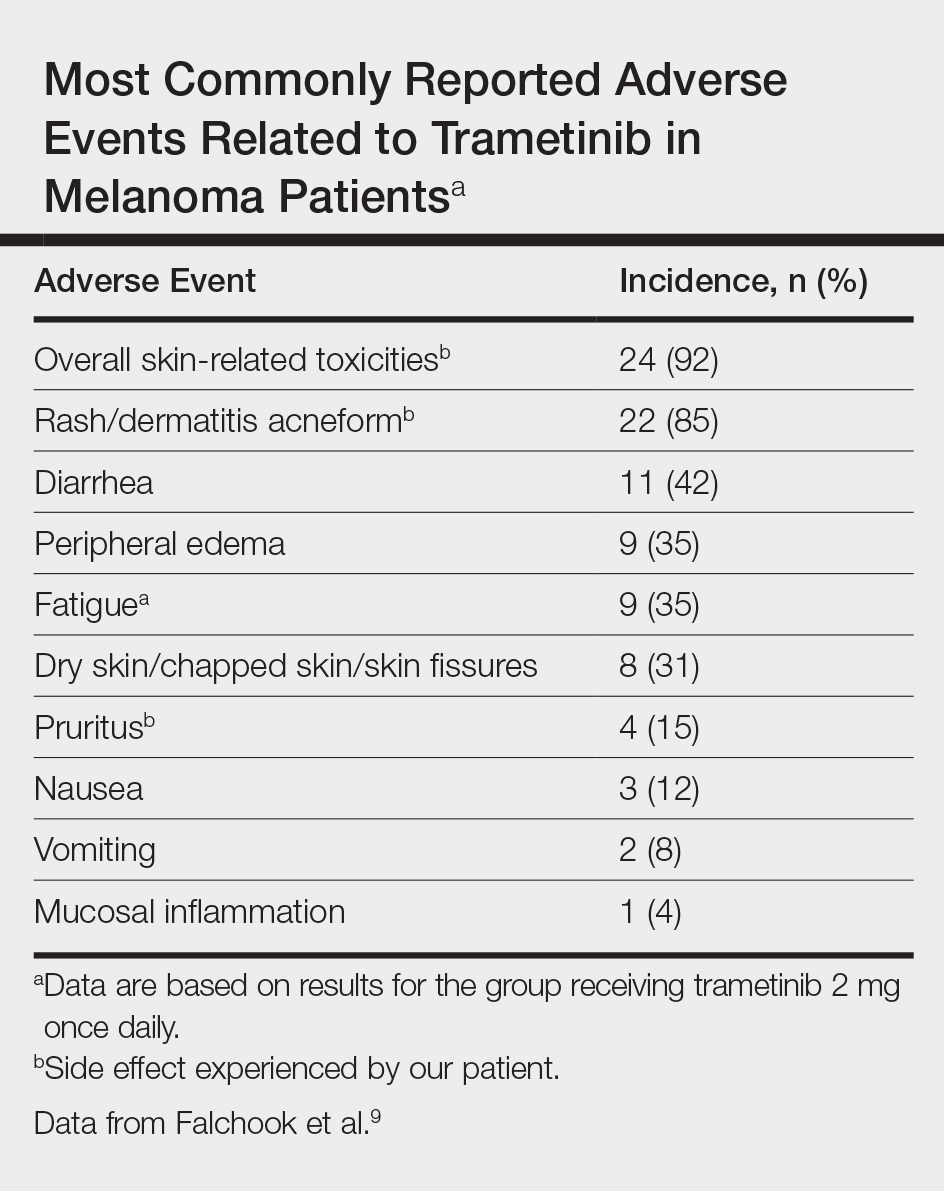
Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547-564.
- Chung C, Reilly S. Trametinib: a novel signal transduction inhibitors for the treatment of metastatic cutaneous melanoma. Am J Health Syst Pharm. 2015;72:101-110.
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy [published online February 12, 2009]. Cancer Lett. 2009;283:125-134.
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy [published online December 8, 2013]. Pharmacol Ther. 2014;142:176-182.
- Flaherty KT, Robert C, Hersey P, et al; METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma [published online June 4, 2012]. N Engl J Med. 2012;367:107-114.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer [published online June 9, 2002]. Nature. 2002;417:949-954.
- Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6.
- Roberts PF, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310.
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 2 dose-escalation trial [published online July 16, 2012]. Lancet Oncol. 2012;13:782-789.
- Anforth R, Liu M, Nguyen B, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib [published online December 9, 2013]. Australas J Dermatol. 2014;55:250-254.
- Gadiot J, Hooijkaas AI, Deken MA, et al. Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity. Onco Targets Ther. 2013;6:1649-1658.
- Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks [published online November 21, 2014]. Br J Dermatol. 2015;172:239-243.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307-311.
- Green JS, Norris DA, Wisell K. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
A decade ago, the few agents approved by the US Food and Drug Administration for treatment of metastatic melanoma demonstrated low therapeutic success rates (ie, <15%–20%).1 Since then, advances in molecular biology have identified oncogenes that contribute to melanoma progression.2 Inhibition of the mitogen-activated protein kinase (MAPK) pathway by targeting mutant BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) has created promising pharmacologic treatment opportunities.3 Due to the recent US Food and Drug Administration approval of these therapies for treatment of melanoma, it is important to better characterize these adverse events (AEs) so that we can manage them. We present the development of an unusual cutaneous reaction to trametinib, a MEK inhibitor, in a man with stage IV M1b malignant melanoma.
Case Report
A 66-year-old man with stage IV M1b malignant melanoma with metastases to the brain and lungs presented with recurring pruritic erythematous papules on the face and bilateral forearms that began shortly after initiating therapy with trametinib. The cutaneous eruption had initially presented on the face, forearms, and dorsal hands when trametinib was used in combination with vemurafenib, a BRAF inhibitor, and ipilimumab, a human cytotoxic T-lymphocyte antigen 4–blocking antibody; however, lesions initially were minimal and self-resolving. When trametinib was reintroduced as monotherapy due to fever attributed to the combination treatment regimen, the cutaneous eruption recurred more severely. Physical examination revealed erythematous scaly papules limited to the face and bilateral upper extremities, including the flexural surfaces.
A biopsy from the flexural surface of the right forearm revealed a dense perivascular lymphoid and xanthomatous infiltrate in the dermis (Figure 1). Poorly formed granulomas within the mid reticular dermis demonstrated focal palisading of histiocytes with prominent giant cells at the periphery. Histiocytes and giant cells showed foamy or xanthomatous cytoplasm. Within the reaction, degenerative and swollen collagen fibers were noted with no mucin deposition, which was confirmed with negative colloidal iron staining.

Brief cessation of trametinib along with application of clobetasol propionate ointment 0.05% resulted in resolution of the cutaneous eruption. Later, trametinib was reintroduced in combination with vemurafenib, though therapy was intermittently discontinued due to various side effects. Skin lesions continued to recur (Figure 2) while the patient was on trametinib but remained minimal and continued to respond to topical clobetasol propionate. One year later, the patient continues to tolerate combination therapy with trametinib and vemurafenib.

Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9

Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.
A decade ago, the few agents approved by the US Food and Drug Administration for treatment of metastatic melanoma demonstrated low therapeutic success rates (ie, <15%–20%).1 Since then, advances in molecular biology have identified oncogenes that contribute to melanoma progression.2 Inhibition of the mitogen-activated protein kinase (MAPK) pathway by targeting mutant BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) has created promising pharmacologic treatment opportunities.3 Due to the recent US Food and Drug Administration approval of these therapies for treatment of melanoma, it is important to better characterize these adverse events (AEs) so that we can manage them. We present the development of an unusual cutaneous reaction to trametinib, a MEK inhibitor, in a man with stage IV M1b malignant melanoma.
Case Report
A 66-year-old man with stage IV M1b malignant melanoma with metastases to the brain and lungs presented with recurring pruritic erythematous papules on the face and bilateral forearms that began shortly after initiating therapy with trametinib. The cutaneous eruption had initially presented on the face, forearms, and dorsal hands when trametinib was used in combination with vemurafenib, a BRAF inhibitor, and ipilimumab, a human cytotoxic T-lymphocyte antigen 4–blocking antibody; however, lesions initially were minimal and self-resolving. When trametinib was reintroduced as monotherapy due to fever attributed to the combination treatment regimen, the cutaneous eruption recurred more severely. Physical examination revealed erythematous scaly papules limited to the face and bilateral upper extremities, including the flexural surfaces.
A biopsy from the flexural surface of the right forearm revealed a dense perivascular lymphoid and xanthomatous infiltrate in the dermis (Figure 1). Poorly formed granulomas within the mid reticular dermis demonstrated focal palisading of histiocytes with prominent giant cells at the periphery. Histiocytes and giant cells showed foamy or xanthomatous cytoplasm. Within the reaction, degenerative and swollen collagen fibers were noted with no mucin deposition, which was confirmed with negative colloidal iron staining.

Brief cessation of trametinib along with application of clobetasol propionate ointment 0.05% resulted in resolution of the cutaneous eruption. Later, trametinib was reintroduced in combination with vemurafenib, though therapy was intermittently discontinued due to various side effects. Skin lesions continued to recur (Figure 2) while the patient was on trametinib but remained minimal and continued to respond to topical clobetasol propionate. One year later, the patient continues to tolerate combination therapy with trametinib and vemurafenib.

Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9

Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547-564.
- Chung C, Reilly S. Trametinib: a novel signal transduction inhibitors for the treatment of metastatic cutaneous melanoma. Am J Health Syst Pharm. 2015;72:101-110.
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy [published online February 12, 2009]. Cancer Lett. 2009;283:125-134.
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy [published online December 8, 2013]. Pharmacol Ther. 2014;142:176-182.
- Flaherty KT, Robert C, Hersey P, et al; METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma [published online June 4, 2012]. N Engl J Med. 2012;367:107-114.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer [published online June 9, 2002]. Nature. 2002;417:949-954.
- Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6.
- Roberts PF, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310.
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 2 dose-escalation trial [published online July 16, 2012]. Lancet Oncol. 2012;13:782-789.
- Anforth R, Liu M, Nguyen B, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib [published online December 9, 2013]. Australas J Dermatol. 2014;55:250-254.
- Gadiot J, Hooijkaas AI, Deken MA, et al. Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity. Onco Targets Ther. 2013;6:1649-1658.
- Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks [published online November 21, 2014]. Br J Dermatol. 2015;172:239-243.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307-311.
- Green JS, Norris DA, Wisell K. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547-564.
- Chung C, Reilly S. Trametinib: a novel signal transduction inhibitors for the treatment of metastatic cutaneous melanoma. Am J Health Syst Pharm. 2015;72:101-110.
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy [published online February 12, 2009]. Cancer Lett. 2009;283:125-134.
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy [published online December 8, 2013]. Pharmacol Ther. 2014;142:176-182.
- Flaherty KT, Robert C, Hersey P, et al; METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma [published online June 4, 2012]. N Engl J Med. 2012;367:107-114.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer [published online June 9, 2002]. Nature. 2002;417:949-954.
- Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6.
- Roberts PF, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310.
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 2 dose-escalation trial [published online July 16, 2012]. Lancet Oncol. 2012;13:782-789.
- Anforth R, Liu M, Nguyen B, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib [published online December 9, 2013]. Australas J Dermatol. 2014;55:250-254.
- Gadiot J, Hooijkaas AI, Deken MA, et al. Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity. Onco Targets Ther. 2013;6:1649-1658.
- Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks [published online November 21, 2014]. Br J Dermatol. 2015;172:239-243.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307-311.
- Green JS, Norris DA, Wisell K. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
Practice Points
- With the discovery of molecular targeting in melanoma, BRAF and MEK inhibitors have been increasingly utilized as therapies in metastatic melanoma management.
- Trametinib, a MEK inhibitor, is commonly associated with cutaneous adverse reactions, particularly acneform eruptions.
- We report a patient on trametinib who developed an eruption with an unusual xanthogranulomatous reaction pattern noted on histology.
Acquired Perforating Dermatosis in a Skin Graft
Case Report
A 57-year-old black woman with a history of dialysis-dependent end-stage renal disease, diabetes mellitus (DM), hypertension, diastolic congestive heart failure, and chronic bronchitis was admitted to Howard University Hospital (Washington, DC) for acute chest pain and shortness of breath. During her hospital stay the dermatology team was consulted for evaluation of two 1.6-cm teardrop-shaped, yellow-white-chalky plaques noted in the center of an atrophic, hyperpigmented, shiny, contracted split-thickness skin graft (STSG) on the right posterior forearm (Figure 1). Twenty years prior, the patient received STSGs on the right and left forearm secondary to caustic burns. Two months before the current admission she noticed 2 adjacent teardrop-shaped white plaques within the center of the STSG on the right forearm. At a 3-month follow-up, she had developed more lesions within both graft sites of the bilateral forearm. There was no notable pruritus associated with the lesions.
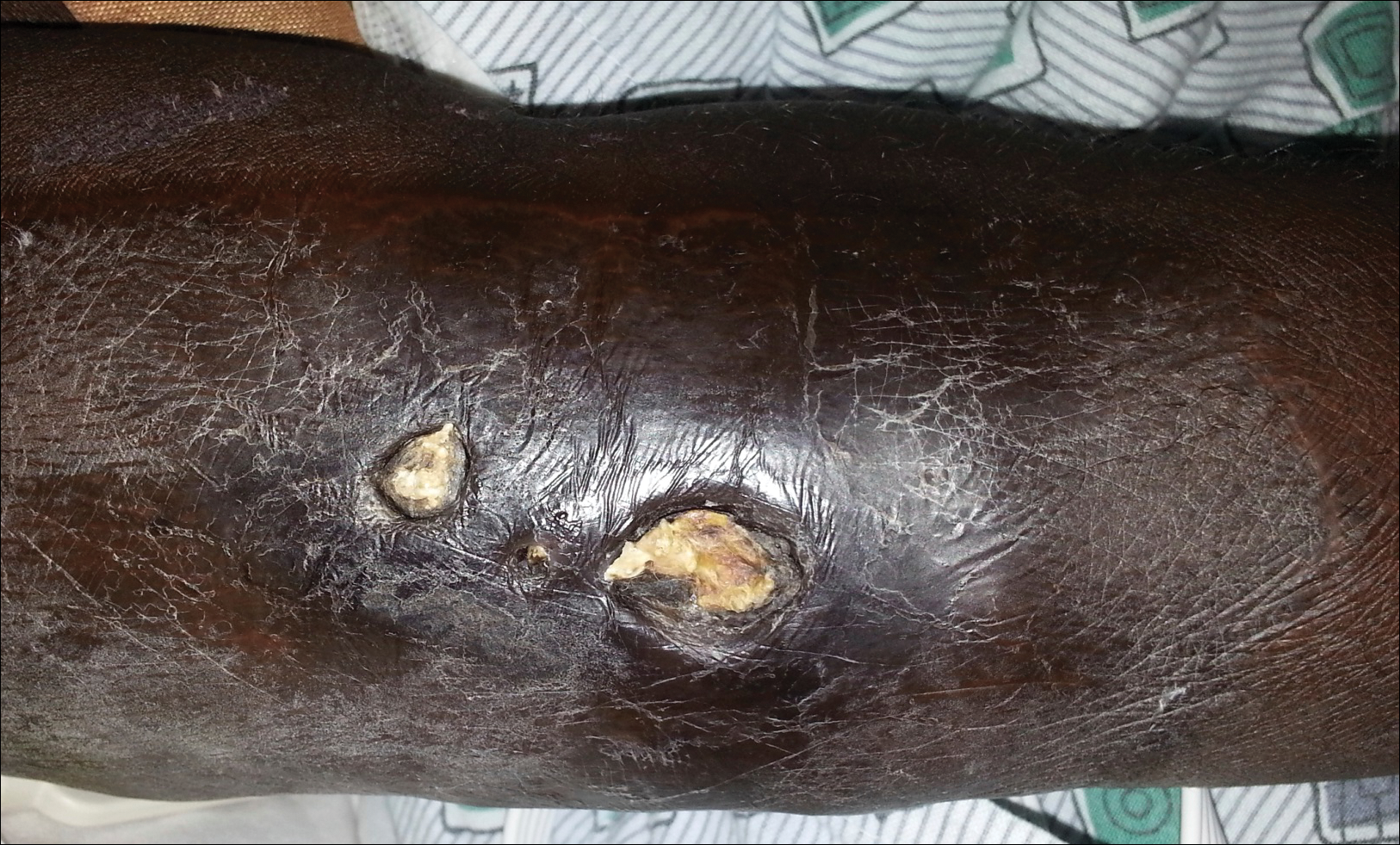
A 4-mm punch biopsy showed an orthokeratotic plug with basophilic inflammatory debris adjacent to acanthotic epidermis, necrotic basophilic debris at the superficial dermis with epidermal canals extending from the base of the lesion superiorly, and transepidermal elimination of elastic fibers (Figure 2A). A Verhoeff-van Gieson stain revealed the necrotic basophilic debris located in the superficial dermis admixed with a cluster of black wavy elastic fibers establishing the identity of the perforating substance (Figure 2B). Masson trichrome stain revealed loss of collagen structure within the aggregate of elastic fibers adjacent to the epidermis and no collagen within epidermal canals (Figure 2C). These histopathologic findings together with the clinical presentation were consistent with a diagnosis of acquired perforating dermatosis (APD).
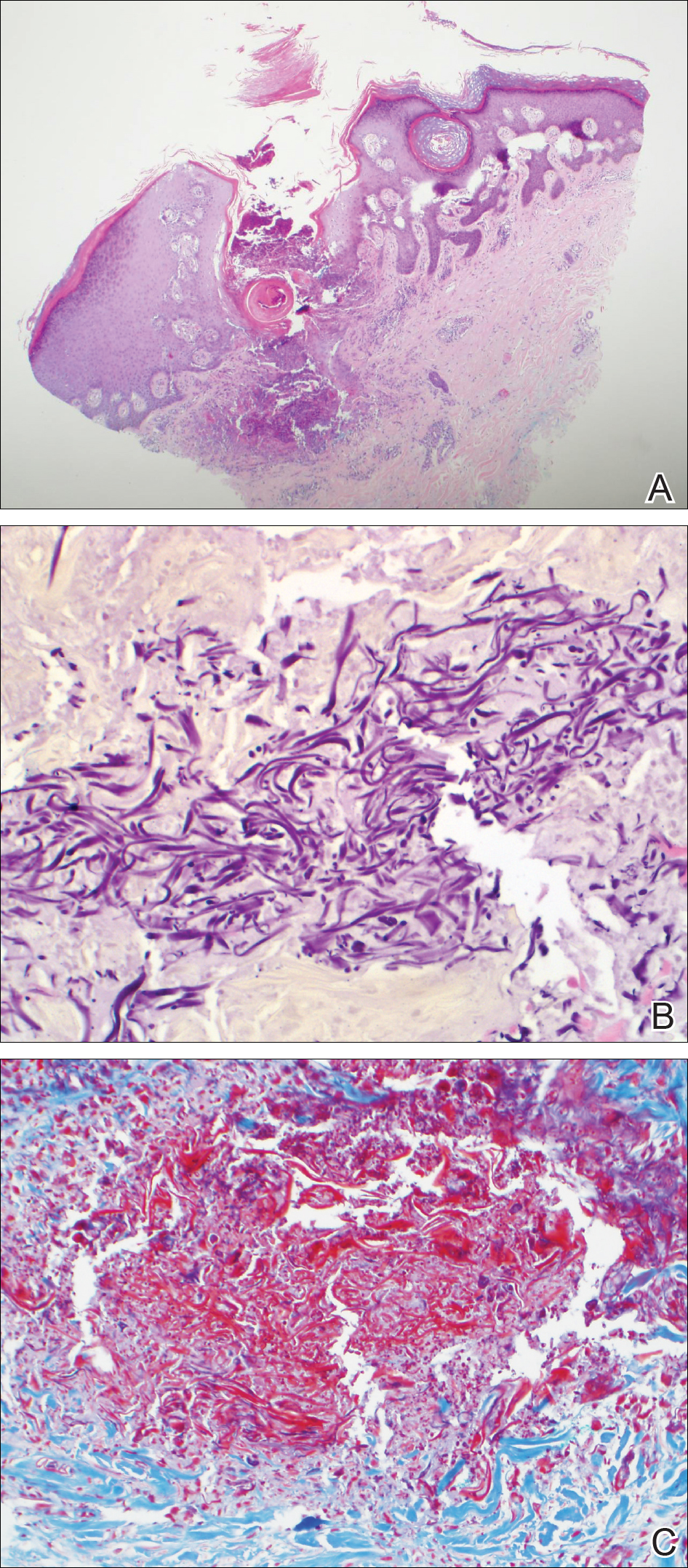
Comment
Presentation
Acquired perforating dermatosis is a dermatologic condition characterized by multiple pruritic, dome-shaped papules and plaques with central keratotic plugs giving a craterlike appearance.1-4 A green-brown or black crust with an erythematous border typically surrounds the primary lesions.4 Acquired perforating dermatosis favors a distribution over the trunk, gluteal region, and the extensor surfaces of the upper and lower extremities. Palmoplantar, intertriginous, and mucous membrane regions typically are spared.4 Occasionally, APD may present as generalized nodules and papules. Our case consisting of lesions that were localized to STSGs on the forearms supports the typical distribution; however, the presentation of APD occurring within a skin graft is unique.
From an epidemiologic standpoint, APD is more likely to affect men than women (1.5:1 ratio). Additionally, APD’s affected age range is 29 to 96 years (mean, 56.8 years),5 which is consistent with our patient’s age. Acquired perforating dermatosis has no racial predilection, though there is a predominance among black patients with concomitant chronic renal failure, as seen in our patient.3
Pathogenesis
The etiology of APD remains unknown.6 Some believe that the uremic or calcium deposits on the skin of patients with chronic kidney disease may trigger chronic pruritus, leading to epithelial hyperplasia and the development of perforating lesions.1,3 A prominent theory in the literature is that superficial trauma, such as scratching, induces necrosis of tissue, facilitating transepidermal elimination of connective tissue components.7 The Köbner phenomenon, which can easily be induced by scratching the skin, supports this idea.8 Fujimoto et al9 suggested that scratching exposes keratinocytes to advanced glycation end product–modified extracellular matrix proteins, particularly types I and III collagen. This exposure leads to the terminal differentiation of keratinocytes with the advanced glycation end receptor (CD36) followed by the upward movement of keratinocytes with glycated collagen. Others postulate fibronectin, involved in epidermal cell signaling, locomotion, and differentiation, is an antigenic trigger because patients with DM and uremia have increased levels of fibronectin in the serum and at sites of perforating skin lesions.10
Diseases Associated With APD
Acquired perforating dermatosis is an umbrella term for perforating disease found in adults. It is associated with systemic diseases, such as DM and pruritus of renal failure.11 Our patient had both dialysis-dependent end-stage renal disease and DM. Acquired perforating dermatosis is observed in 4.5% to 11% of patients on hemodialysis12,13; however, APD may occur prior to or in the absence of dialysis.3 Other examples of systemic conditions associated with APD include obstructive uropathy, chronic nephritis, anuria, and hypertensive nephrosclerosis. Koebnerization also may trigger lesions to manifest in a linear pattern after localized trauma to the skin.7 Acquired perforating dermatosis is associated with other types of trauma, such as healing herpes zoster, or following exposure to drugs, such as tumor necrosis factor α inhibitors, bevacizumab, telaprevir, sorafenib, sirolimus, and indinavir.14-16 Rarely, there have been associations with a history of insect bites, scabies, lymphoma, and hepatobiliary disease.1-3
Histopathology
Acquired perforating dermatosis is classified as a perforating disease, along with reactive perforating collagenosis, elastosis perforans serpiginosa (EPS), perforating folliculitis, and perforating calcific elastosis. Perforating diseases are histologically characterized by the transepidermal penetration and elimination of altered connective tissue and inflammatory cells.5 Each disease differs based on their clinical and histological characteristics.
Histologic sections of APD show a plug of crusting or hyperkeratosis with variable parakeratosis, acanthosis, and occasional dyskeratotic keratinocytes. In the dermis, aggregates of neutrophils, lymphocytes, macrophages, or multinucleated giant cells may be found.17 The histologic findings vary depending on the stage of evolution of the individual lesion. Early lesions show a concave depression with acanthosis, vacuolation of basal keratinocytes, and dermal inflammation.4 Additionally, transepidermal channels filled with keratin, pyknotic nuclear debris, inflammatory cells, elastin, or collagen can be noted.3 Over time, the elastic fibers, as detected by the Verhoeff-van Gieson stain, dissipate and the collagen acquires a basophilic staining. Adjacent to the channels, the basement membrane remains intact in early lesions but later shows discontinuities and electron-dense fibrinlike material.3 Occasionally, amorphous degenerated material within the perforations is the major histologic finding.11 Usually, the material cannot be clearly identified as collagen or elastin, but sometimes both are present.
In our case, we identified elastin as the perforating substance, which is less common than collagen, the typical perforating substance in APD. Elastin has occasionally been seen to serve as the only perforating substance from APD lesions among patients. Abe et al18 reported that the biopsy of a Japanese patient with keratotic follicular papules and serpiginous-arranged papules demonstrated elimination of atypical elastin fibers from the transepidermal channels. This patient was diagnosed with APD as well as EPS and perforating folliculitis based on the clinical presentation.18 Kim et al19 studied 30 Korean patients with APD. One had serpiginous hyperkeratotic plaques along the upper extremity and trunk that revealed transepidermal channels containing coarse elastic fibers and basophilic debris; however, due to the serpiginous morphology of lesions, both Abe et al18 and Kim et al19 favored a diagnosis of acquired EPS. Saray et al20 conducted a retrospective study of 22 Turkish patients with APD; 1 patient had a painful hyperkeratotic papule on the auricle that on histopathology showed degenerated elastin perforating through the keratotic plug, features similar to our case.
Differential Diagnosis
The differential diagnoses include perforating diseases14,19 as well as other disorders that exhibit the Köbner phenomenon, such as psoriasis, lichen planus, and verruca vulgaris.21,22 Also, it is not uncommon for patients with APD to have coexisting folliculitis or prurigo nodularis.22
Treatment
Management is focused on treating the symptoms. For pruritus, sedating antihistamines and other antipruritic agents are efficacious.23 Topical, intra-lesional, or systemic corticosteroids and topical retinoids have shown variable resolution in APD lesions.24 Some case reports describe topical menthol, salicylic acid, sulfur, benzoyl peroxide, systemic antibiotics (eg, clindamycin, doxycycline), and allopurinol for elevated uric acid levels as effective treatment methods.6 Narrowband UVB phototherapy is beneficial for APD and renal disease.25,26 Renal transplantation has been curative for some patients with APD.27 Given that our patient’s lesions were asymptomatic, no treatment was offered at the time.
Conclusion
Our patient presented with APD localized exclusively to the site of a skin graft, and histologic examination identified elastin as the primary perforating substance. A medical history of DM and chronic kidney disease predisposes patients to APD. This case suggests that skin graft sites may be predisposed to the development of APD.
- Rodney IJ, Taylor CS, Cohen G. Derm Dx: what are these pruritic nodules? The Dermatologist. October 15, 2009. http://www.the-dermatologist.com/content/derm-dx-what-are-these-pruritic-nodules. Accessed September 18, 2018.
- Gagnon, AL, Desai T. Dermatological diseases in patients with chronic kidney disease. J Nephropathol. 2013;2:104-109.
- Kurban MS, Boueiz A, Kibbi AG. Cutaneous manifestations of chronic kidney disease. Clin Dermatol. 2008;26:255-264.
- Wagner G, Sachse MM. Acquired reactive perforating dermatosis [published online May 29, 2013]. J Dtsch Dermatol Ges. 2013;11:723-729; 723-730.
- Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592.
- Healy R, Cerio R, Hollingsworth A, et al. Acquired perforating dermatosis associated with pregnancy. Clin Exp Dermatol. 2010;35:621-623.
- Cordova KB, Oberg TJ, Malik M, et al. Dermatologic conditions seen in end-stage renal disease. Semin Dial. 2009;22:45-55.
- Satchell AC, Crotty K, Lee S. Reactive perforating collagenosis: a condition that may be underdiagnosed. Australas J Dermatol. 2001;42:284-287.
- Fujimoto E, Kobayashi T, Fujimoto N, et al. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Invest Dermatol. 2010;130:405-414.
- Bilezikci B, Sechkin D, Demirhan B. Acquired perforating dermatosis in patients with chronic renal failure: a possible role for fibronectin. J Eur Acad Dermatol Venereol. 2003;17:230-232.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Hurwitz RM, Melton ME, Creech FT, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Lübbe J, Sorg O, Malé PJ, et al. Sirolimus-induced inflammatory papules with acquired reactive perforating collagenosis [published online January 9, 2008]. Dermatology. 2008;216:239-242.
- Pernet C, Pageaux GP, Guillot B, et al. Telaprevir-induced acquired perforating dermatosis. JAMA Dermatol. 2014;150:1371-1372.
- Severino-Freire M, Sibaud V, Tournier E, et al. Acquired perforating dermatosis associated with sorafenib therapy [published online September 11, 2014]. J Eur Acad Dermatol Venereol. 2016;30:328-330.
- Zelger B, Hintner H, Auböck J, et al. Acquired perforating dermatosis. transepidermal elimination of DNA material and possible role of leukocytes in pathogenesis. Arch Dermatol. 1991;127:695-700.
- Abe R, Murase S, Nomura Y, et al. Acquired perforating dermatosis appearing as elastosis perforans serpiginosa and perforating folliculitis. Clin Exp Dermatol. 2008;33:653-654.
- Kim SW, Kim MS, Lee JH, et al. A clinicopathologic study of thirty cases of acquired perforating dermatosis in Korea. Ann Dermatol. 2014;26:162-171.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006;20:679-688.
- Carter VH, Constantine VS. Kyrle’s disease. I. clinical findings in five cases and review of literature. Arch Dermatol. 1968;97:624-632.
- Robinson-Bostom L, Digiovanna JJ. Cutaneous manifestations of end-stage renal disease. J Am Acad Dermatol. 2000;43:975-986.
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Ohe S, Danno K, Sasaki H, et al. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2004;50:892-894.
- Sezer E, Erkek E. Acquired perforating dermatosis successfully treated with photodynamic therapy. Photodermatol Photoimmunol Photomed. 2012;28:50-52.
- Saldanha LF, Gonick HC, Rodriguez HJ, et al. Silicon-related syndrome in dialysis patients. Nephron. 1997;77:48-56.
Case Report
A 57-year-old black woman with a history of dialysis-dependent end-stage renal disease, diabetes mellitus (DM), hypertension, diastolic congestive heart failure, and chronic bronchitis was admitted to Howard University Hospital (Washington, DC) for acute chest pain and shortness of breath. During her hospital stay the dermatology team was consulted for evaluation of two 1.6-cm teardrop-shaped, yellow-white-chalky plaques noted in the center of an atrophic, hyperpigmented, shiny, contracted split-thickness skin graft (STSG) on the right posterior forearm (Figure 1). Twenty years prior, the patient received STSGs on the right and left forearm secondary to caustic burns. Two months before the current admission she noticed 2 adjacent teardrop-shaped white plaques within the center of the STSG on the right forearm. At a 3-month follow-up, she had developed more lesions within both graft sites of the bilateral forearm. There was no notable pruritus associated with the lesions.

A 4-mm punch biopsy showed an orthokeratotic plug with basophilic inflammatory debris adjacent to acanthotic epidermis, necrotic basophilic debris at the superficial dermis with epidermal canals extending from the base of the lesion superiorly, and transepidermal elimination of elastic fibers (Figure 2A). A Verhoeff-van Gieson stain revealed the necrotic basophilic debris located in the superficial dermis admixed with a cluster of black wavy elastic fibers establishing the identity of the perforating substance (Figure 2B). Masson trichrome stain revealed loss of collagen structure within the aggregate of elastic fibers adjacent to the epidermis and no collagen within epidermal canals (Figure 2C). These histopathologic findings together with the clinical presentation were consistent with a diagnosis of acquired perforating dermatosis (APD).

Comment
Presentation
Acquired perforating dermatosis is a dermatologic condition characterized by multiple pruritic, dome-shaped papules and plaques with central keratotic plugs giving a craterlike appearance.1-4 A green-brown or black crust with an erythematous border typically surrounds the primary lesions.4 Acquired perforating dermatosis favors a distribution over the trunk, gluteal region, and the extensor surfaces of the upper and lower extremities. Palmoplantar, intertriginous, and mucous membrane regions typically are spared.4 Occasionally, APD may present as generalized nodules and papules. Our case consisting of lesions that were localized to STSGs on the forearms supports the typical distribution; however, the presentation of APD occurring within a skin graft is unique.
From an epidemiologic standpoint, APD is more likely to affect men than women (1.5:1 ratio). Additionally, APD’s affected age range is 29 to 96 years (mean, 56.8 years),5 which is consistent with our patient’s age. Acquired perforating dermatosis has no racial predilection, though there is a predominance among black patients with concomitant chronic renal failure, as seen in our patient.3
Pathogenesis
The etiology of APD remains unknown.6 Some believe that the uremic or calcium deposits on the skin of patients with chronic kidney disease may trigger chronic pruritus, leading to epithelial hyperplasia and the development of perforating lesions.1,3 A prominent theory in the literature is that superficial trauma, such as scratching, induces necrosis of tissue, facilitating transepidermal elimination of connective tissue components.7 The Köbner phenomenon, which can easily be induced by scratching the skin, supports this idea.8 Fujimoto et al9 suggested that scratching exposes keratinocytes to advanced glycation end product–modified extracellular matrix proteins, particularly types I and III collagen. This exposure leads to the terminal differentiation of keratinocytes with the advanced glycation end receptor (CD36) followed by the upward movement of keratinocytes with glycated collagen. Others postulate fibronectin, involved in epidermal cell signaling, locomotion, and differentiation, is an antigenic trigger because patients with DM and uremia have increased levels of fibronectin in the serum and at sites of perforating skin lesions.10
Diseases Associated With APD
Acquired perforating dermatosis is an umbrella term for perforating disease found in adults. It is associated with systemic diseases, such as DM and pruritus of renal failure.11 Our patient had both dialysis-dependent end-stage renal disease and DM. Acquired perforating dermatosis is observed in 4.5% to 11% of patients on hemodialysis12,13; however, APD may occur prior to or in the absence of dialysis.3 Other examples of systemic conditions associated with APD include obstructive uropathy, chronic nephritis, anuria, and hypertensive nephrosclerosis. Koebnerization also may trigger lesions to manifest in a linear pattern after localized trauma to the skin.7 Acquired perforating dermatosis is associated with other types of trauma, such as healing herpes zoster, or following exposure to drugs, such as tumor necrosis factor α inhibitors, bevacizumab, telaprevir, sorafenib, sirolimus, and indinavir.14-16 Rarely, there have been associations with a history of insect bites, scabies, lymphoma, and hepatobiliary disease.1-3
Histopathology
Acquired perforating dermatosis is classified as a perforating disease, along with reactive perforating collagenosis, elastosis perforans serpiginosa (EPS), perforating folliculitis, and perforating calcific elastosis. Perforating diseases are histologically characterized by the transepidermal penetration and elimination of altered connective tissue and inflammatory cells.5 Each disease differs based on their clinical and histological characteristics.
Histologic sections of APD show a plug of crusting or hyperkeratosis with variable parakeratosis, acanthosis, and occasional dyskeratotic keratinocytes. In the dermis, aggregates of neutrophils, lymphocytes, macrophages, or multinucleated giant cells may be found.17 The histologic findings vary depending on the stage of evolution of the individual lesion. Early lesions show a concave depression with acanthosis, vacuolation of basal keratinocytes, and dermal inflammation.4 Additionally, transepidermal channels filled with keratin, pyknotic nuclear debris, inflammatory cells, elastin, or collagen can be noted.3 Over time, the elastic fibers, as detected by the Verhoeff-van Gieson stain, dissipate and the collagen acquires a basophilic staining. Adjacent to the channels, the basement membrane remains intact in early lesions but later shows discontinuities and electron-dense fibrinlike material.3 Occasionally, amorphous degenerated material within the perforations is the major histologic finding.11 Usually, the material cannot be clearly identified as collagen or elastin, but sometimes both are present.
In our case, we identified elastin as the perforating substance, which is less common than collagen, the typical perforating substance in APD. Elastin has occasionally been seen to serve as the only perforating substance from APD lesions among patients. Abe et al18 reported that the biopsy of a Japanese patient with keratotic follicular papules and serpiginous-arranged papules demonstrated elimination of atypical elastin fibers from the transepidermal channels. This patient was diagnosed with APD as well as EPS and perforating folliculitis based on the clinical presentation.18 Kim et al19 studied 30 Korean patients with APD. One had serpiginous hyperkeratotic plaques along the upper extremity and trunk that revealed transepidermal channels containing coarse elastic fibers and basophilic debris; however, due to the serpiginous morphology of lesions, both Abe et al18 and Kim et al19 favored a diagnosis of acquired EPS. Saray et al20 conducted a retrospective study of 22 Turkish patients with APD; 1 patient had a painful hyperkeratotic papule on the auricle that on histopathology showed degenerated elastin perforating through the keratotic plug, features similar to our case.
Differential Diagnosis
The differential diagnoses include perforating diseases14,19 as well as other disorders that exhibit the Köbner phenomenon, such as psoriasis, lichen planus, and verruca vulgaris.21,22 Also, it is not uncommon for patients with APD to have coexisting folliculitis or prurigo nodularis.22
Treatment
Management is focused on treating the symptoms. For pruritus, sedating antihistamines and other antipruritic agents are efficacious.23 Topical, intra-lesional, or systemic corticosteroids and topical retinoids have shown variable resolution in APD lesions.24 Some case reports describe topical menthol, salicylic acid, sulfur, benzoyl peroxide, systemic antibiotics (eg, clindamycin, doxycycline), and allopurinol for elevated uric acid levels as effective treatment methods.6 Narrowband UVB phototherapy is beneficial for APD and renal disease.25,26 Renal transplantation has been curative for some patients with APD.27 Given that our patient’s lesions were asymptomatic, no treatment was offered at the time.
Conclusion
Our patient presented with APD localized exclusively to the site of a skin graft, and histologic examination identified elastin as the primary perforating substance. A medical history of DM and chronic kidney disease predisposes patients to APD. This case suggests that skin graft sites may be predisposed to the development of APD.
Case Report
A 57-year-old black woman with a history of dialysis-dependent end-stage renal disease, diabetes mellitus (DM), hypertension, diastolic congestive heart failure, and chronic bronchitis was admitted to Howard University Hospital (Washington, DC) for acute chest pain and shortness of breath. During her hospital stay the dermatology team was consulted for evaluation of two 1.6-cm teardrop-shaped, yellow-white-chalky plaques noted in the center of an atrophic, hyperpigmented, shiny, contracted split-thickness skin graft (STSG) on the right posterior forearm (Figure 1). Twenty years prior, the patient received STSGs on the right and left forearm secondary to caustic burns. Two months before the current admission she noticed 2 adjacent teardrop-shaped white plaques within the center of the STSG on the right forearm. At a 3-month follow-up, she had developed more lesions within both graft sites of the bilateral forearm. There was no notable pruritus associated with the lesions.

A 4-mm punch biopsy showed an orthokeratotic plug with basophilic inflammatory debris adjacent to acanthotic epidermis, necrotic basophilic debris at the superficial dermis with epidermal canals extending from the base of the lesion superiorly, and transepidermal elimination of elastic fibers (Figure 2A). A Verhoeff-van Gieson stain revealed the necrotic basophilic debris located in the superficial dermis admixed with a cluster of black wavy elastic fibers establishing the identity of the perforating substance (Figure 2B). Masson trichrome stain revealed loss of collagen structure within the aggregate of elastic fibers adjacent to the epidermis and no collagen within epidermal canals (Figure 2C). These histopathologic findings together with the clinical presentation were consistent with a diagnosis of acquired perforating dermatosis (APD).

Comment
Presentation
Acquired perforating dermatosis is a dermatologic condition characterized by multiple pruritic, dome-shaped papules and plaques with central keratotic plugs giving a craterlike appearance.1-4 A green-brown or black crust with an erythematous border typically surrounds the primary lesions.4 Acquired perforating dermatosis favors a distribution over the trunk, gluteal region, and the extensor surfaces of the upper and lower extremities. Palmoplantar, intertriginous, and mucous membrane regions typically are spared.4 Occasionally, APD may present as generalized nodules and papules. Our case consisting of lesions that were localized to STSGs on the forearms supports the typical distribution; however, the presentation of APD occurring within a skin graft is unique.
From an epidemiologic standpoint, APD is more likely to affect men than women (1.5:1 ratio). Additionally, APD’s affected age range is 29 to 96 years (mean, 56.8 years),5 which is consistent with our patient’s age. Acquired perforating dermatosis has no racial predilection, though there is a predominance among black patients with concomitant chronic renal failure, as seen in our patient.3
Pathogenesis
The etiology of APD remains unknown.6 Some believe that the uremic or calcium deposits on the skin of patients with chronic kidney disease may trigger chronic pruritus, leading to epithelial hyperplasia and the development of perforating lesions.1,3 A prominent theory in the literature is that superficial trauma, such as scratching, induces necrosis of tissue, facilitating transepidermal elimination of connective tissue components.7 The Köbner phenomenon, which can easily be induced by scratching the skin, supports this idea.8 Fujimoto et al9 suggested that scratching exposes keratinocytes to advanced glycation end product–modified extracellular matrix proteins, particularly types I and III collagen. This exposure leads to the terminal differentiation of keratinocytes with the advanced glycation end receptor (CD36) followed by the upward movement of keratinocytes with glycated collagen. Others postulate fibronectin, involved in epidermal cell signaling, locomotion, and differentiation, is an antigenic trigger because patients with DM and uremia have increased levels of fibronectin in the serum and at sites of perforating skin lesions.10
Diseases Associated With APD
Acquired perforating dermatosis is an umbrella term for perforating disease found in adults. It is associated with systemic diseases, such as DM and pruritus of renal failure.11 Our patient had both dialysis-dependent end-stage renal disease and DM. Acquired perforating dermatosis is observed in 4.5% to 11% of patients on hemodialysis12,13; however, APD may occur prior to or in the absence of dialysis.3 Other examples of systemic conditions associated with APD include obstructive uropathy, chronic nephritis, anuria, and hypertensive nephrosclerosis. Koebnerization also may trigger lesions to manifest in a linear pattern after localized trauma to the skin.7 Acquired perforating dermatosis is associated with other types of trauma, such as healing herpes zoster, or following exposure to drugs, such as tumor necrosis factor α inhibitors, bevacizumab, telaprevir, sorafenib, sirolimus, and indinavir.14-16 Rarely, there have been associations with a history of insect bites, scabies, lymphoma, and hepatobiliary disease.1-3
Histopathology
Acquired perforating dermatosis is classified as a perforating disease, along with reactive perforating collagenosis, elastosis perforans serpiginosa (EPS), perforating folliculitis, and perforating calcific elastosis. Perforating diseases are histologically characterized by the transepidermal penetration and elimination of altered connective tissue and inflammatory cells.5 Each disease differs based on their clinical and histological characteristics.
Histologic sections of APD show a plug of crusting or hyperkeratosis with variable parakeratosis, acanthosis, and occasional dyskeratotic keratinocytes. In the dermis, aggregates of neutrophils, lymphocytes, macrophages, or multinucleated giant cells may be found.17 The histologic findings vary depending on the stage of evolution of the individual lesion. Early lesions show a concave depression with acanthosis, vacuolation of basal keratinocytes, and dermal inflammation.4 Additionally, transepidermal channels filled with keratin, pyknotic nuclear debris, inflammatory cells, elastin, or collagen can be noted.3 Over time, the elastic fibers, as detected by the Verhoeff-van Gieson stain, dissipate and the collagen acquires a basophilic staining. Adjacent to the channels, the basement membrane remains intact in early lesions but later shows discontinuities and electron-dense fibrinlike material.3 Occasionally, amorphous degenerated material within the perforations is the major histologic finding.11 Usually, the material cannot be clearly identified as collagen or elastin, but sometimes both are present.
In our case, we identified elastin as the perforating substance, which is less common than collagen, the typical perforating substance in APD. Elastin has occasionally been seen to serve as the only perforating substance from APD lesions among patients. Abe et al18 reported that the biopsy of a Japanese patient with keratotic follicular papules and serpiginous-arranged papules demonstrated elimination of atypical elastin fibers from the transepidermal channels. This patient was diagnosed with APD as well as EPS and perforating folliculitis based on the clinical presentation.18 Kim et al19 studied 30 Korean patients with APD. One had serpiginous hyperkeratotic plaques along the upper extremity and trunk that revealed transepidermal channels containing coarse elastic fibers and basophilic debris; however, due to the serpiginous morphology of lesions, both Abe et al18 and Kim et al19 favored a diagnosis of acquired EPS. Saray et al20 conducted a retrospective study of 22 Turkish patients with APD; 1 patient had a painful hyperkeratotic papule on the auricle that on histopathology showed degenerated elastin perforating through the keratotic plug, features similar to our case.
Differential Diagnosis
The differential diagnoses include perforating diseases14,19 as well as other disorders that exhibit the Köbner phenomenon, such as psoriasis, lichen planus, and verruca vulgaris.21,22 Also, it is not uncommon for patients with APD to have coexisting folliculitis or prurigo nodularis.22
Treatment
Management is focused on treating the symptoms. For pruritus, sedating antihistamines and other antipruritic agents are efficacious.23 Topical, intra-lesional, or systemic corticosteroids and topical retinoids have shown variable resolution in APD lesions.24 Some case reports describe topical menthol, salicylic acid, sulfur, benzoyl peroxide, systemic antibiotics (eg, clindamycin, doxycycline), and allopurinol for elevated uric acid levels as effective treatment methods.6 Narrowband UVB phototherapy is beneficial for APD and renal disease.25,26 Renal transplantation has been curative for some patients with APD.27 Given that our patient’s lesions were asymptomatic, no treatment was offered at the time.
Conclusion
Our patient presented with APD localized exclusively to the site of a skin graft, and histologic examination identified elastin as the primary perforating substance. A medical history of DM and chronic kidney disease predisposes patients to APD. This case suggests that skin graft sites may be predisposed to the development of APD.
- Rodney IJ, Taylor CS, Cohen G. Derm Dx: what are these pruritic nodules? The Dermatologist. October 15, 2009. http://www.the-dermatologist.com/content/derm-dx-what-are-these-pruritic-nodules. Accessed September 18, 2018.
- Gagnon, AL, Desai T. Dermatological diseases in patients with chronic kidney disease. J Nephropathol. 2013;2:104-109.
- Kurban MS, Boueiz A, Kibbi AG. Cutaneous manifestations of chronic kidney disease. Clin Dermatol. 2008;26:255-264.
- Wagner G, Sachse MM. Acquired reactive perforating dermatosis [published online May 29, 2013]. J Dtsch Dermatol Ges. 2013;11:723-729; 723-730.
- Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592.
- Healy R, Cerio R, Hollingsworth A, et al. Acquired perforating dermatosis associated with pregnancy. Clin Exp Dermatol. 2010;35:621-623.
- Cordova KB, Oberg TJ, Malik M, et al. Dermatologic conditions seen in end-stage renal disease. Semin Dial. 2009;22:45-55.
- Satchell AC, Crotty K, Lee S. Reactive perforating collagenosis: a condition that may be underdiagnosed. Australas J Dermatol. 2001;42:284-287.
- Fujimoto E, Kobayashi T, Fujimoto N, et al. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Invest Dermatol. 2010;130:405-414.
- Bilezikci B, Sechkin D, Demirhan B. Acquired perforating dermatosis in patients with chronic renal failure: a possible role for fibronectin. J Eur Acad Dermatol Venereol. 2003;17:230-232.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Hurwitz RM, Melton ME, Creech FT, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Lübbe J, Sorg O, Malé PJ, et al. Sirolimus-induced inflammatory papules with acquired reactive perforating collagenosis [published online January 9, 2008]. Dermatology. 2008;216:239-242.
- Pernet C, Pageaux GP, Guillot B, et al. Telaprevir-induced acquired perforating dermatosis. JAMA Dermatol. 2014;150:1371-1372.
- Severino-Freire M, Sibaud V, Tournier E, et al. Acquired perforating dermatosis associated with sorafenib therapy [published online September 11, 2014]. J Eur Acad Dermatol Venereol. 2016;30:328-330.
- Zelger B, Hintner H, Auböck J, et al. Acquired perforating dermatosis. transepidermal elimination of DNA material and possible role of leukocytes in pathogenesis. Arch Dermatol. 1991;127:695-700.
- Abe R, Murase S, Nomura Y, et al. Acquired perforating dermatosis appearing as elastosis perforans serpiginosa and perforating folliculitis. Clin Exp Dermatol. 2008;33:653-654.
- Kim SW, Kim MS, Lee JH, et al. A clinicopathologic study of thirty cases of acquired perforating dermatosis in Korea. Ann Dermatol. 2014;26:162-171.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006;20:679-688.
- Carter VH, Constantine VS. Kyrle’s disease. I. clinical findings in five cases and review of literature. Arch Dermatol. 1968;97:624-632.
- Robinson-Bostom L, Digiovanna JJ. Cutaneous manifestations of end-stage renal disease. J Am Acad Dermatol. 2000;43:975-986.
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Ohe S, Danno K, Sasaki H, et al. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2004;50:892-894.
- Sezer E, Erkek E. Acquired perforating dermatosis successfully treated with photodynamic therapy. Photodermatol Photoimmunol Photomed. 2012;28:50-52.
- Saldanha LF, Gonick HC, Rodriguez HJ, et al. Silicon-related syndrome in dialysis patients. Nephron. 1997;77:48-56.
- Rodney IJ, Taylor CS, Cohen G. Derm Dx: what are these pruritic nodules? The Dermatologist. October 15, 2009. http://www.the-dermatologist.com/content/derm-dx-what-are-these-pruritic-nodules. Accessed September 18, 2018.
- Gagnon, AL, Desai T. Dermatological diseases in patients with chronic kidney disease. J Nephropathol. 2013;2:104-109.
- Kurban MS, Boueiz A, Kibbi AG. Cutaneous manifestations of chronic kidney disease. Clin Dermatol. 2008;26:255-264.
- Wagner G, Sachse MM. Acquired reactive perforating dermatosis [published online May 29, 2013]. J Dtsch Dermatol Ges. 2013;11:723-729; 723-730.
- Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592.
- Healy R, Cerio R, Hollingsworth A, et al. Acquired perforating dermatosis associated with pregnancy. Clin Exp Dermatol. 2010;35:621-623.
- Cordova KB, Oberg TJ, Malik M, et al. Dermatologic conditions seen in end-stage renal disease. Semin Dial. 2009;22:45-55.
- Satchell AC, Crotty K, Lee S. Reactive perforating collagenosis: a condition that may be underdiagnosed. Australas J Dermatol. 2001;42:284-287.
- Fujimoto E, Kobayashi T, Fujimoto N, et al. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Invest Dermatol. 2010;130:405-414.
- Bilezikci B, Sechkin D, Demirhan B. Acquired perforating dermatosis in patients with chronic renal failure: a possible role for fibronectin. J Eur Acad Dermatol Venereol. 2003;17:230-232.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Hurwitz RM, Melton ME, Creech FT, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Lübbe J, Sorg O, Malé PJ, et al. Sirolimus-induced inflammatory papules with acquired reactive perforating collagenosis [published online January 9, 2008]. Dermatology. 2008;216:239-242.
- Pernet C, Pageaux GP, Guillot B, et al. Telaprevir-induced acquired perforating dermatosis. JAMA Dermatol. 2014;150:1371-1372.
- Severino-Freire M, Sibaud V, Tournier E, et al. Acquired perforating dermatosis associated with sorafenib therapy [published online September 11, 2014]. J Eur Acad Dermatol Venereol. 2016;30:328-330.
- Zelger B, Hintner H, Auböck J, et al. Acquired perforating dermatosis. transepidermal elimination of DNA material and possible role of leukocytes in pathogenesis. Arch Dermatol. 1991;127:695-700.
- Abe R, Murase S, Nomura Y, et al. Acquired perforating dermatosis appearing as elastosis perforans serpiginosa and perforating folliculitis. Clin Exp Dermatol. 2008;33:653-654.
- Kim SW, Kim MS, Lee JH, et al. A clinicopathologic study of thirty cases of acquired perforating dermatosis in Korea. Ann Dermatol. 2014;26:162-171.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006;20:679-688.
- Carter VH, Constantine VS. Kyrle’s disease. I. clinical findings in five cases and review of literature. Arch Dermatol. 1968;97:624-632.
- Robinson-Bostom L, Digiovanna JJ. Cutaneous manifestations of end-stage renal disease. J Am Acad Dermatol. 2000;43:975-986.
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Ohe S, Danno K, Sasaki H, et al. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2004;50:892-894.
- Sezer E, Erkek E. Acquired perforating dermatosis successfully treated with photodynamic therapy. Photodermatol Photoimmunol Photomed. 2012;28:50-52.
- Saldanha LF, Gonick HC, Rodriguez HJ, et al. Silicon-related syndrome in dialysis patients. Nephron. 1997;77:48-56.
Practice Points
- Acquired perforating dermatosis (APD) presents as pruritic crateriform papules and plaques with central keratotic plugs.
- A medical history of diabetes mellitus and chronic kidney disease predisposes patients to APD. This case suggests that skin graft sites may be predisposed to the development of APD.
Leukemia Cutis in Acute Myeloid Leukemia Signifies a Poor Prognosis
Case Report
A 66-year-old man with a history of type 2 diabetes mellitus presented with considerable muscle weakness and infiltrative, flesh-colored plaques on the face, trunk, and arms of 3 months’ duration. The patient required the use of a wheelchair due to muscle weakness. On physical examination he had diffuse, infiltrative, flesh-colored plaques on the entire face (Figure 1A), trunk, and arms. The eyelids and lips were swollen, and the nose was distorted due to the infiltrative plaques (Figure 1B). Additionally, there were hypopigmented macules and patches scattered among the infiltrative plaques on the face, trunk, and arms (Figure 1C).
Punch biopsy specimens were obtained from the left cheek and left upper arm and were submitted for histologic examination with routine hematoxylin and eosin staining (Figure 2). Histopathology showed infiltrating and diffuse monomorphic cells in the dermis with large and hyperchromatic nuclei. Some nuclei were cleaved or folded in configuration. The cells displayed ample surrounding cytoplasm, which was finely granular or vacuolated. The infiltrate was accentuated in the perifollicular adventitial dermis. Immunohistochemistry was positive for CD33 and negative for CD3, CD20, and myeloperoxidase. Additionally, periodic acid–Schiff and Fite stains were negative for microorganisms. These morphologic and immunohistochemical findings were consistent with acute myeloid leukemia (AML). Further testing with complete blood cell count, peripheral blood smear, and bone marrow biopsy confirmed the diagnosis of AML. The patient subsequently died 5 weeks later.
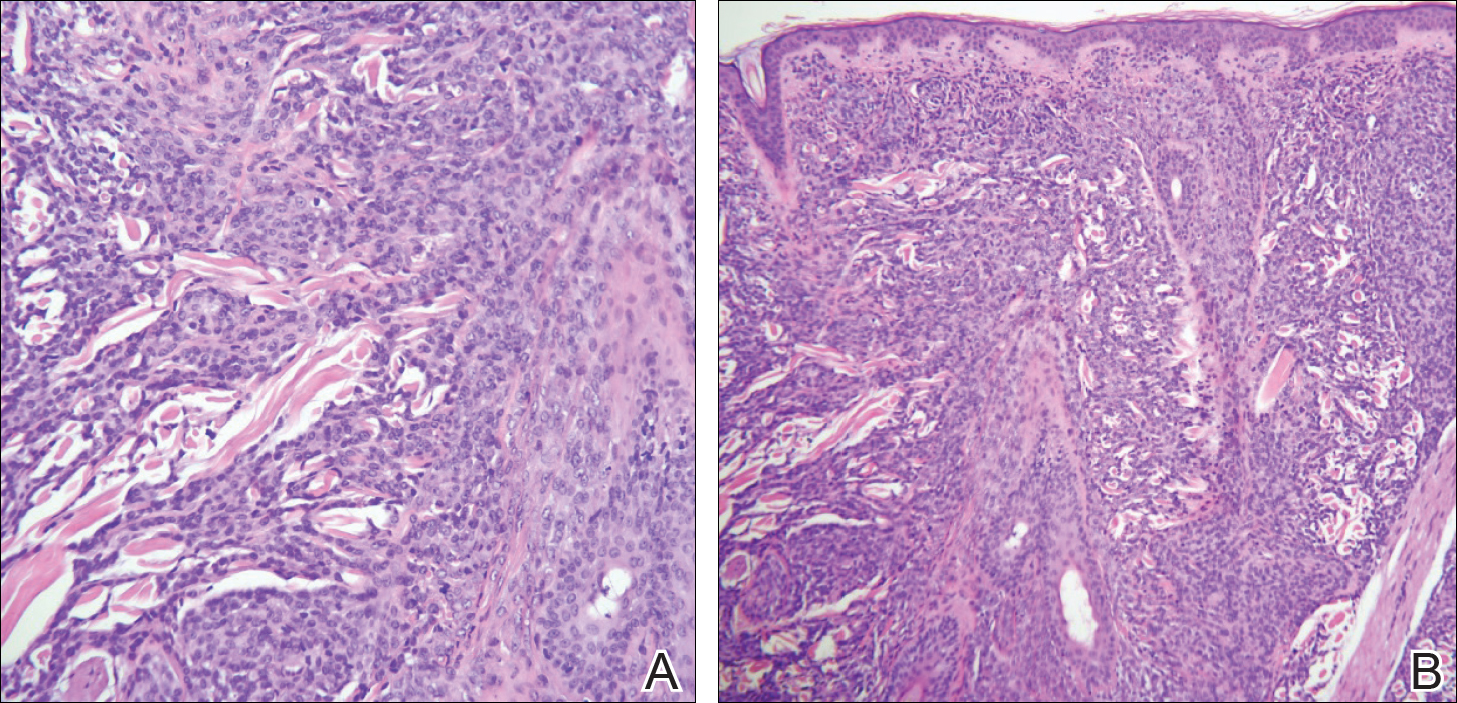
Comment
Presentation of LC
Thirty percent to 40% of leukemia patients present with a variety of nonspecific cutaneous signs, including those related to hemorrhage, infection, and drug eruptions, as well as paraneoplastic lesions.1 Cutaneous signs of leukemia are less commonly due to leukemia cutis (LC), defined as the neoplastic infiltration of the skin or subcutaneous tissue by leukemic cells. The clinical presentation of LC varies, making it difficult to diagnose without immunohistochemistry. It can pre-sent as single or multiple erythematous papules and/or nodules, infiltrated plaques, macules, palpable purpura, ulcers, ecchymoses, and/or vesicles.2 Leukemia cutis most often presents on the head, neck, trunk, and sites of current or prior trauma. Gingival hyperplasia is another associated finding in the acute monocytic and myelomonocytic types of AML.3 Additionally, chloromas or granulocytic sarcomas are dermal nodules that can pre-sent in myelogenous leukemia.4
LC and AML
Leukemia cutis most commonly is observed in AML compared to the other types of leukemia. The myelomonocytic and monocytic subtypes of AML are most often implicated.5,6 The majority of patients with LC present with a pre-established (55%–77%) or simultaneous diagnosis of systemic leukemia (23%–38%).
Histopathology
In LC, histology typically reveals a normal epidermis and nodular or diffuse infiltrating cells in the dermis. The cells can appear monomorphic, atypical, or immature, and there is occasional single-filing between collagen bundles. Causative types of neoplasms can be distinguished based on their morphologic, immunophenotypic, and cytogenetic properties.8-10
Incidence
Of the acute leukemias, AML accounts for the highest prevalence in adults,11 with an annual incidence of 14,590 cases in the United States.12 The incidence of AML increases with age; the mean age of patients diagnosed with AML is 67 years.12 Risk is increased with a history of exposure to radiotherapy, chemotherapy, or cigarette smoke; preexisting myeloproliferative or myelodysplastic syndromes and mutations in DNA repair (eg, Fanconi anemia); neutropenia (eg, from elastase mutations); and Down syndrome.13
Diagnosis
More than 20% blasts in the bone marrow is required for a diagnosis of AML.14 Specific to AML is the presence of large immature precursor cells with a granular cytoplasm and, when present, highly diagnostic Auer rods.12Acute myeloid leukemia can be distinguished by staining for myeloperoxidase; Sudan Black B; or the antigens CD13, CD33, or c-kit.15
In our case, CD33 was positive, which is a characteristic finding in AML. Myeloperoxidase also can be positive in AML; however, in our case it was negative, and it can be an insensitive marker in the context of LC. Although most cases of LC present concurrently with bone marrow infiltration, some cases present before systemic involvement; for example, granulocytic sarcomas can occur months earlier than the development of systemic leukemia. Thus, early detection by a dermatologist is essential. Depending on the lesion’s appearance, the differential diagnoses can include lymphoma, drug eruptions, infectious etiologies, sarcoidosis, metastases from other malignancies, and blistering dermatoses.
Management
Systemic therapy should be the cornerstone of therapy. Induction therapy includes the combined use of cytarabine (except in acute promyelocytic leukemia [M3], for which all-trans retinoic acid is indicated) and anthracycline derivatives in a “7+3” regimen to achieve complete remission. Specifically, cytarabine (100–200 mg/m2) typically is continuously administered intravenously for 7 days combined with intravenous administration of either daunorubicin (60–90 mg/m2) or idarubicin (12 mg/m2) on days 1, 2, and 3. Postremission therapy is highly individualized depending on patients’ prognostic factors and is indicated to reduce the likelihood of relapse and to improve patient mortality. High doses of cytarabine and hematopoietic stem cell transplantation commonly are utilized.12 Resolution of hematologic atypia may result in complete or partial resolution of LC.10
Conclusion
We diagnosed AML with systemic involvement in our patient based on the cutaneous manifestation of LC. Diagnosis of LC relies on immunohistochemistry and strong clinical suspicion, as cutaneous findings are diverse and nonspecific. Early recognition is essential, as LC in the context of systemic involvement portends a poor prognosis. Our patient died 5 weeks following initial presentation.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Su WPD, Buechner SA, Chin-Yang L. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
- Kumar M, Nair V, Mishra L, et al. Gingival hyperplasia—a clue to the diagnosis of acute leukemia? Arch Oral Sci Res. 2012;2:165-168.
- Winfield H, Smoller B. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Mosby/Elsevier; 2012:2037-2048.
- Babina T, Miller L, Thomas B. Leukemia cutis. J Drugs Dermatol. 2012;11:416-417.
- Tziotzios C, Makrygeorgou A. Leukemia cutis. Cleve Clin J Med. 2011;78:226-227.
- Ratnam KV, Khor CJL, Su WPD. Leukemia cutis. Dermatol Clin 1994;12:419-431.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Buechner SA, Li CY, Su WP. Leukemia cutis. a histopathologic study of 42 cases. Am J Dermatopathol. 1985;7:109-119.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- O’Donnell MR, Abboud CN, Altman J, et al. NCCN Clinical Practice Guidelines acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984-1021.
- Marcucci G, Bloomfield CD. Acute myeloid leukemia. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015:678-686.
- Aster JC, DeAngelo DJ. Acute leukemias. In: Bunn HF, Aster JC, eds. Pathophysiology of Blood Disorders. New York, NY: McGraw-Hill; 2010:244-259.
- Damon LE, Andreadis C. Blood disorders. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment 2016. New York, NY: McGraw-Hill; 2016:495-541.
- Parikh SA, Jabbour E, Koller CA. Adult acute myeloid leukemia. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 2nd ed. New York, NY: McGraw-Hill; 2011:15-32.
Case Report
A 66-year-old man with a history of type 2 diabetes mellitus presented with considerable muscle weakness and infiltrative, flesh-colored plaques on the face, trunk, and arms of 3 months’ duration. The patient required the use of a wheelchair due to muscle weakness. On physical examination he had diffuse, infiltrative, flesh-colored plaques on the entire face (Figure 1A), trunk, and arms. The eyelids and lips were swollen, and the nose was distorted due to the infiltrative plaques (Figure 1B). Additionally, there were hypopigmented macules and patches scattered among the infiltrative plaques on the face, trunk, and arms (Figure 1C).

Punch biopsy specimens were obtained from the left cheek and left upper arm and were submitted for histologic examination with routine hematoxylin and eosin staining (Figure 2). Histopathology showed infiltrating and diffuse monomorphic cells in the dermis with large and hyperchromatic nuclei. Some nuclei were cleaved or folded in configuration. The cells displayed ample surrounding cytoplasm, which was finely granular or vacuolated. The infiltrate was accentuated in the perifollicular adventitial dermis. Immunohistochemistry was positive for CD33 and negative for CD3, CD20, and myeloperoxidase. Additionally, periodic acid–Schiff and Fite stains were negative for microorganisms. These morphologic and immunohistochemical findings were consistent with acute myeloid leukemia (AML). Further testing with complete blood cell count, peripheral blood smear, and bone marrow biopsy confirmed the diagnosis of AML. The patient subsequently died 5 weeks later.

Comment
Presentation of LC
Thirty percent to 40% of leukemia patients present with a variety of nonspecific cutaneous signs, including those related to hemorrhage, infection, and drug eruptions, as well as paraneoplastic lesions.1 Cutaneous signs of leukemia are less commonly due to leukemia cutis (LC), defined as the neoplastic infiltration of the skin or subcutaneous tissue by leukemic cells. The clinical presentation of LC varies, making it difficult to diagnose without immunohistochemistry. It can pre-sent as single or multiple erythematous papules and/or nodules, infiltrated plaques, macules, palpable purpura, ulcers, ecchymoses, and/or vesicles.2 Leukemia cutis most often presents on the head, neck, trunk, and sites of current or prior trauma. Gingival hyperplasia is another associated finding in the acute monocytic and myelomonocytic types of AML.3 Additionally, chloromas or granulocytic sarcomas are dermal nodules that can pre-sent in myelogenous leukemia.4
LC and AML
Leukemia cutis most commonly is observed in AML compared to the other types of leukemia. The myelomonocytic and monocytic subtypes of AML are most often implicated.5,6 The majority of patients with LC present with a pre-established (55%–77%) or simultaneous diagnosis of systemic leukemia (23%–38%).
Histopathology
In LC, histology typically reveals a normal epidermis and nodular or diffuse infiltrating cells in the dermis. The cells can appear monomorphic, atypical, or immature, and there is occasional single-filing between collagen bundles. Causative types of neoplasms can be distinguished based on their morphologic, immunophenotypic, and cytogenetic properties.8-10
Incidence
Of the acute leukemias, AML accounts for the highest prevalence in adults,11 with an annual incidence of 14,590 cases in the United States.12 The incidence of AML increases with age; the mean age of patients diagnosed with AML is 67 years.12 Risk is increased with a history of exposure to radiotherapy, chemotherapy, or cigarette smoke; preexisting myeloproliferative or myelodysplastic syndromes and mutations in DNA repair (eg, Fanconi anemia); neutropenia (eg, from elastase mutations); and Down syndrome.13
Diagnosis
More than 20% blasts in the bone marrow is required for a diagnosis of AML.14 Specific to AML is the presence of large immature precursor cells with a granular cytoplasm and, when present, highly diagnostic Auer rods.12Acute myeloid leukemia can be distinguished by staining for myeloperoxidase; Sudan Black B; or the antigens CD13, CD33, or c-kit.15
In our case, CD33 was positive, which is a characteristic finding in AML. Myeloperoxidase also can be positive in AML; however, in our case it was negative, and it can be an insensitive marker in the context of LC. Although most cases of LC present concurrently with bone marrow infiltration, some cases present before systemic involvement; for example, granulocytic sarcomas can occur months earlier than the development of systemic leukemia. Thus, early detection by a dermatologist is essential. Depending on the lesion’s appearance, the differential diagnoses can include lymphoma, drug eruptions, infectious etiologies, sarcoidosis, metastases from other malignancies, and blistering dermatoses.
Management
Systemic therapy should be the cornerstone of therapy. Induction therapy includes the combined use of cytarabine (except in acute promyelocytic leukemia [M3], for which all-trans retinoic acid is indicated) and anthracycline derivatives in a “7+3” regimen to achieve complete remission. Specifically, cytarabine (100–200 mg/m2) typically is continuously administered intravenously for 7 days combined with intravenous administration of either daunorubicin (60–90 mg/m2) or idarubicin (12 mg/m2) on days 1, 2, and 3. Postremission therapy is highly individualized depending on patients’ prognostic factors and is indicated to reduce the likelihood of relapse and to improve patient mortality. High doses of cytarabine and hematopoietic stem cell transplantation commonly are utilized.12 Resolution of hematologic atypia may result in complete or partial resolution of LC.10
Conclusion
We diagnosed AML with systemic involvement in our patient based on the cutaneous manifestation of LC. Diagnosis of LC relies on immunohistochemistry and strong clinical suspicion, as cutaneous findings are diverse and nonspecific. Early recognition is essential, as LC in the context of systemic involvement portends a poor prognosis. Our patient died 5 weeks following initial presentation.
Case Report
A 66-year-old man with a history of type 2 diabetes mellitus presented with considerable muscle weakness and infiltrative, flesh-colored plaques on the face, trunk, and arms of 3 months’ duration. The patient required the use of a wheelchair due to muscle weakness. On physical examination he had diffuse, infiltrative, flesh-colored plaques on the entire face (Figure 1A), trunk, and arms. The eyelids and lips were swollen, and the nose was distorted due to the infiltrative plaques (Figure 1B). Additionally, there were hypopigmented macules and patches scattered among the infiltrative plaques on the face, trunk, and arms (Figure 1C).

Punch biopsy specimens were obtained from the left cheek and left upper arm and were submitted for histologic examination with routine hematoxylin and eosin staining (Figure 2). Histopathology showed infiltrating and diffuse monomorphic cells in the dermis with large and hyperchromatic nuclei. Some nuclei were cleaved or folded in configuration. The cells displayed ample surrounding cytoplasm, which was finely granular or vacuolated. The infiltrate was accentuated in the perifollicular adventitial dermis. Immunohistochemistry was positive for CD33 and negative for CD3, CD20, and myeloperoxidase. Additionally, periodic acid–Schiff and Fite stains were negative for microorganisms. These morphologic and immunohistochemical findings were consistent with acute myeloid leukemia (AML). Further testing with complete blood cell count, peripheral blood smear, and bone marrow biopsy confirmed the diagnosis of AML. The patient subsequently died 5 weeks later.

Comment
Presentation of LC
Thirty percent to 40% of leukemia patients present with a variety of nonspecific cutaneous signs, including those related to hemorrhage, infection, and drug eruptions, as well as paraneoplastic lesions.1 Cutaneous signs of leukemia are less commonly due to leukemia cutis (LC), defined as the neoplastic infiltration of the skin or subcutaneous tissue by leukemic cells. The clinical presentation of LC varies, making it difficult to diagnose without immunohistochemistry. It can pre-sent as single or multiple erythematous papules and/or nodules, infiltrated plaques, macules, palpable purpura, ulcers, ecchymoses, and/or vesicles.2 Leukemia cutis most often presents on the head, neck, trunk, and sites of current or prior trauma. Gingival hyperplasia is another associated finding in the acute monocytic and myelomonocytic types of AML.3 Additionally, chloromas or granulocytic sarcomas are dermal nodules that can pre-sent in myelogenous leukemia.4
LC and AML
Leukemia cutis most commonly is observed in AML compared to the other types of leukemia. The myelomonocytic and monocytic subtypes of AML are most often implicated.5,6 The majority of patients with LC present with a pre-established (55%–77%) or simultaneous diagnosis of systemic leukemia (23%–38%).
Histopathology
In LC, histology typically reveals a normal epidermis and nodular or diffuse infiltrating cells in the dermis. The cells can appear monomorphic, atypical, or immature, and there is occasional single-filing between collagen bundles. Causative types of neoplasms can be distinguished based on their morphologic, immunophenotypic, and cytogenetic properties.8-10
Incidence
Of the acute leukemias, AML accounts for the highest prevalence in adults,11 with an annual incidence of 14,590 cases in the United States.12 The incidence of AML increases with age; the mean age of patients diagnosed with AML is 67 years.12 Risk is increased with a history of exposure to radiotherapy, chemotherapy, or cigarette smoke; preexisting myeloproliferative or myelodysplastic syndromes and mutations in DNA repair (eg, Fanconi anemia); neutropenia (eg, from elastase mutations); and Down syndrome.13
Diagnosis
More than 20% blasts in the bone marrow is required for a diagnosis of AML.14 Specific to AML is the presence of large immature precursor cells with a granular cytoplasm and, when present, highly diagnostic Auer rods.12Acute myeloid leukemia can be distinguished by staining for myeloperoxidase; Sudan Black B; or the antigens CD13, CD33, or c-kit.15
In our case, CD33 was positive, which is a characteristic finding in AML. Myeloperoxidase also can be positive in AML; however, in our case it was negative, and it can be an insensitive marker in the context of LC. Although most cases of LC present concurrently with bone marrow infiltration, some cases present before systemic involvement; for example, granulocytic sarcomas can occur months earlier than the development of systemic leukemia. Thus, early detection by a dermatologist is essential. Depending on the lesion’s appearance, the differential diagnoses can include lymphoma, drug eruptions, infectious etiologies, sarcoidosis, metastases from other malignancies, and blistering dermatoses.
Management
Systemic therapy should be the cornerstone of therapy. Induction therapy includes the combined use of cytarabine (except in acute promyelocytic leukemia [M3], for which all-trans retinoic acid is indicated) and anthracycline derivatives in a “7+3” regimen to achieve complete remission. Specifically, cytarabine (100–200 mg/m2) typically is continuously administered intravenously for 7 days combined with intravenous administration of either daunorubicin (60–90 mg/m2) or idarubicin (12 mg/m2) on days 1, 2, and 3. Postremission therapy is highly individualized depending on patients’ prognostic factors and is indicated to reduce the likelihood of relapse and to improve patient mortality. High doses of cytarabine and hematopoietic stem cell transplantation commonly are utilized.12 Resolution of hematologic atypia may result in complete or partial resolution of LC.10
Conclusion
We diagnosed AML with systemic involvement in our patient based on the cutaneous manifestation of LC. Diagnosis of LC relies on immunohistochemistry and strong clinical suspicion, as cutaneous findings are diverse and nonspecific. Early recognition is essential, as LC in the context of systemic involvement portends a poor prognosis. Our patient died 5 weeks following initial presentation.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Su WPD, Buechner SA, Chin-Yang L. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
- Kumar M, Nair V, Mishra L, et al. Gingival hyperplasia—a clue to the diagnosis of acute leukemia? Arch Oral Sci Res. 2012;2:165-168.
- Winfield H, Smoller B. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Mosby/Elsevier; 2012:2037-2048.
- Babina T, Miller L, Thomas B. Leukemia cutis. J Drugs Dermatol. 2012;11:416-417.
- Tziotzios C, Makrygeorgou A. Leukemia cutis. Cleve Clin J Med. 2011;78:226-227.
- Ratnam KV, Khor CJL, Su WPD. Leukemia cutis. Dermatol Clin 1994;12:419-431.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Buechner SA, Li CY, Su WP. Leukemia cutis. a histopathologic study of 42 cases. Am J Dermatopathol. 1985;7:109-119.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- O’Donnell MR, Abboud CN, Altman J, et al. NCCN Clinical Practice Guidelines acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984-1021.
- Marcucci G, Bloomfield CD. Acute myeloid leukemia. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015:678-686.
- Aster JC, DeAngelo DJ. Acute leukemias. In: Bunn HF, Aster JC, eds. Pathophysiology of Blood Disorders. New York, NY: McGraw-Hill; 2010:244-259.
- Damon LE, Andreadis C. Blood disorders. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment 2016. New York, NY: McGraw-Hill; 2016:495-541.
- Parikh SA, Jabbour E, Koller CA. Adult acute myeloid leukemia. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 2nd ed. New York, NY: McGraw-Hill; 2011:15-32.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Su WPD, Buechner SA, Chin-Yang L. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
- Kumar M, Nair V, Mishra L, et al. Gingival hyperplasia—a clue to the diagnosis of acute leukemia? Arch Oral Sci Res. 2012;2:165-168.
- Winfield H, Smoller B. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Mosby/Elsevier; 2012:2037-2048.
- Babina T, Miller L, Thomas B. Leukemia cutis. J Drugs Dermatol. 2012;11:416-417.
- Tziotzios C, Makrygeorgou A. Leukemia cutis. Cleve Clin J Med. 2011;78:226-227.
- Ratnam KV, Khor CJL, Su WPD. Leukemia cutis. Dermatol Clin 1994;12:419-431.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Buechner SA, Li CY, Su WP. Leukemia cutis. a histopathologic study of 42 cases. Am J Dermatopathol. 1985;7:109-119.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- O’Donnell MR, Abboud CN, Altman J, et al. NCCN Clinical Practice Guidelines acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984-1021.
- Marcucci G, Bloomfield CD. Acute myeloid leukemia. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015:678-686.
- Aster JC, DeAngelo DJ. Acute leukemias. In: Bunn HF, Aster JC, eds. Pathophysiology of Blood Disorders. New York, NY: McGraw-Hill; 2010:244-259.
- Damon LE, Andreadis C. Blood disorders. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment 2016. New York, NY: McGraw-Hill; 2016:495-541.
- Parikh SA, Jabbour E, Koller CA. Adult acute myeloid leukemia. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 2nd ed. New York, NY: McGraw-Hill; 2011:15-32.
Practice Points
- Leukemia cutis (LC) describes cutaneous and/or subcutaneous infiltration by leukemic cells and most commonly occurs in patients with acute myeloid leukemia.
- The vast majority of patients presenting with LC already have systemic involvement.
- Cutaneous presentation of LC is diverse, thus diagnosis often is dependent on immunohisto-chemical findings.