User login
Plantar Ulcerative Lichen Planus: Rapid Improvement With a Novel Triple-Therapy Approach
Ulcerative lichen planus (ULP)(also called erosive) is a rare variant of lichen planus. Similar to classic lichen planus, the cause of ULP is largely unknown. Ulcerative lichen planus typically involves the oral mucosa or genitalia but rarely may present as ulcerations on the palms and soles. Clinical presentation usually involves a history of chronic ulcers that often have been previously misdiagnosed and resistant to treatment. Ulcerations on the plantar surfaces frequently cause severe pain and disability. Few cases have been reported and successful treatment is rare.
Case Report
A 56-year-old man was referred by podiatry to the dermatology clinic for evaluation of painful ulcerations involving the dorsal and plantar surfaces of the right great toe as well as the second to third digits. The ulcers had been ongoing for 8 years, treated mostly with local wound care without clinical improvement. His medical and family history was considered noncontributory as a possible etiology of the ulcers; however, he had been taking ibuprofen intermittently for years for general aches and pains, which raised the suspicion of a drug-induced etiology. Laboratory evaluation revealed positive hepatitis B serology but was otherwise unremarkable, including normal liver function tests and negative wound cultures.
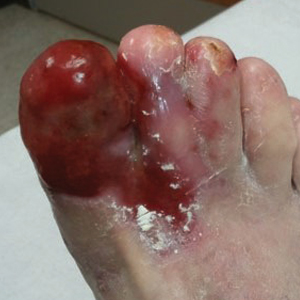
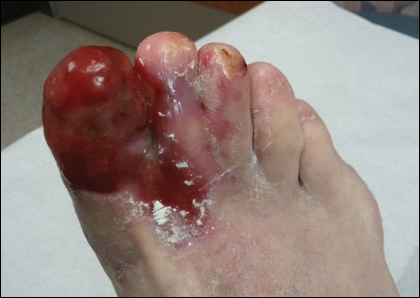
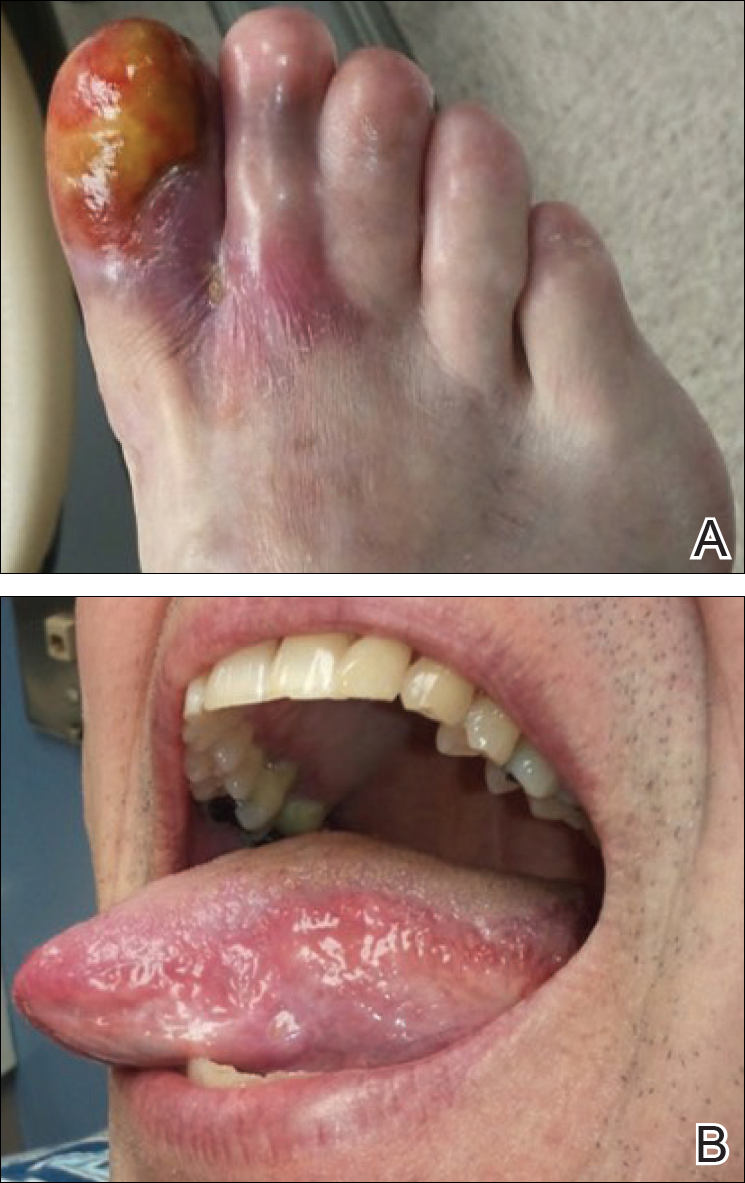
Physical examination revealed a beefy red, glazed ulceration involving the entire right great toe with extension onto the second and third toes. There was considerable scarring with syndactyly of the second and third toes and complete toenail loss of the right foot (Figure 1). On the insteps of the bilateral soles were a few scattered, pale, atrophic, violaceous papules with overlying thin lacy white streaks that were reflective of Wickham striae. Early dorsal pterygium formation also was noted on the bilateral third fingernails. Oral mucosal examination revealed lacy white plaques on the bilateral buccal mucosa with a large ulcer of the left lateral tongue (Figure 2). No genital or scalp lesions were present.

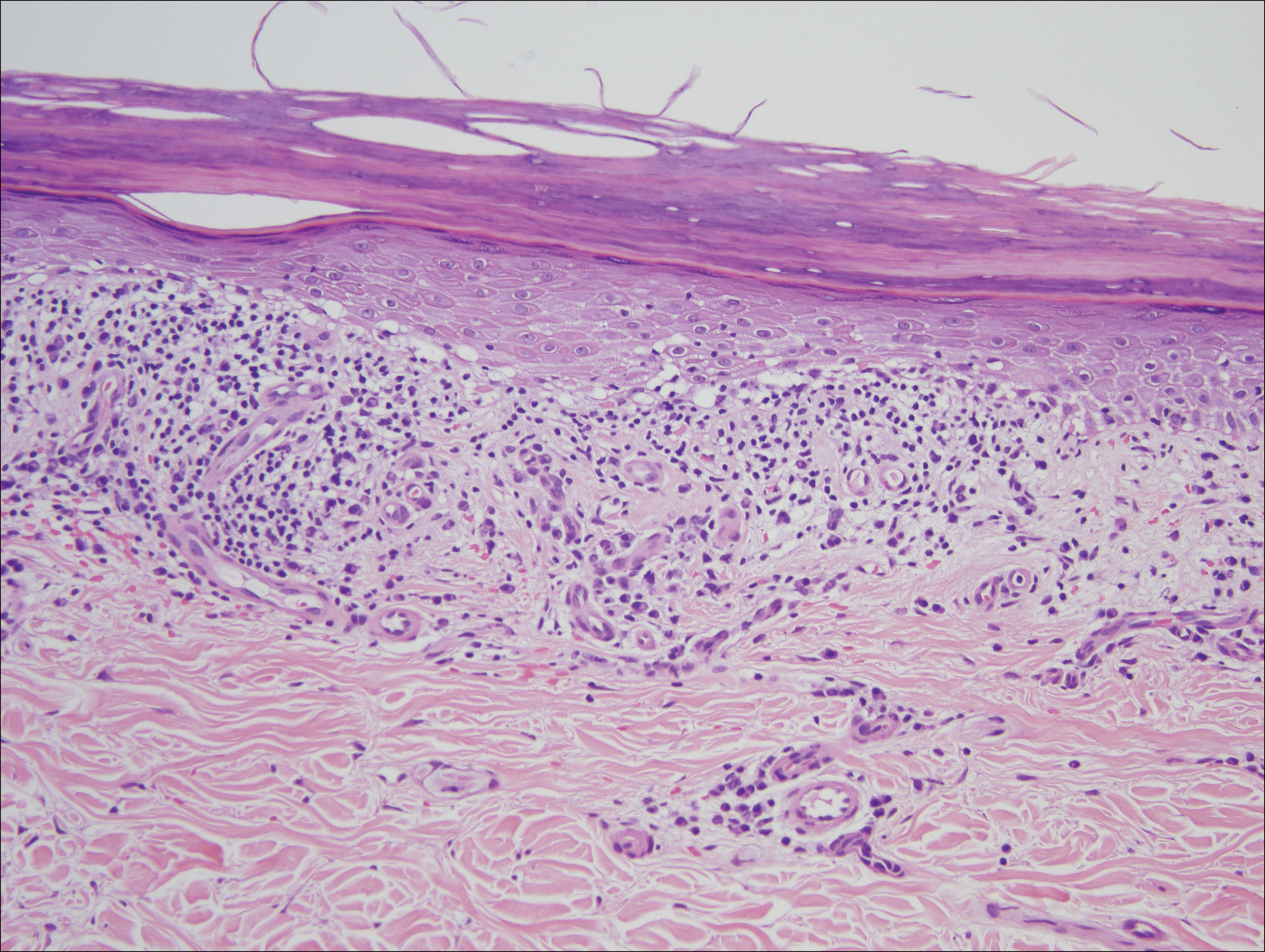
Histologic examination of a papule on the instep of the right sole demonstrated a dense lichenoid lymphocytic infiltrate in the papillary dermis with basal vacuolar degeneration and early focal Max-Joseph space formation. Additionally, there was epidermal atrophy with mild hypergranulosis and scattered necrotic keratinocytes (Figure 3). A similar histologic picture was noted on a biopsy of the buccal mucosa overlying the right molar, albeit with epithelial acanthosis rather than atrophy.
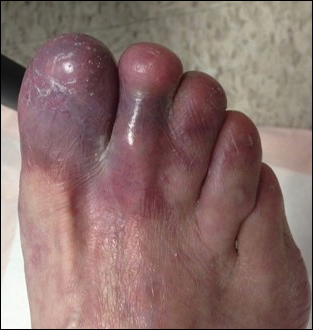
Based on initial clinical suspicion for ULP, we suggested that our patient discontinue ibuprofen and started him on a regimen of oral prednisone 40 mg once daily and clobetasol ointment 0.05% applied twice daily to the plantar ulceration, both for 2 weeks. Dramatic improvement was noted after only 2 weeks of treatment. This regimen was then switched to oral doxycycline 100 mg twice daily combined with tacrolimus ointment 0.1% applied twice daily to the plantar ulceration to avoid side effects of prolonged steroid use. Topical therapies were not used for the mucosal lesions. At 4-week follow-up, the patient continued to demonstrate notable clinical response with a greater than 70% physician-assessed improvement in ulcer severity (Figure 4) and near-complete resolution of the oral mucosal lesions. Our patient also reported almost complete resolution of pain. By 4-month follow-up, complete reepithelialization and resolution of the ulcers was noted (Figure 5). This improvement was sustained at additional follow-up 1 year after the initial presentation.
Comment
Ulcerative (or erosive) lichen planus is a rare form of lichen planus. Ulcerative lichen planus most commonly presents as erosive lesions of the oral and genital mucosae but rarely can involve other sites. The palms and soles are the most common sites of cutaneous involvement, with lesions frequently characterized by severe pain and limited mobility.2
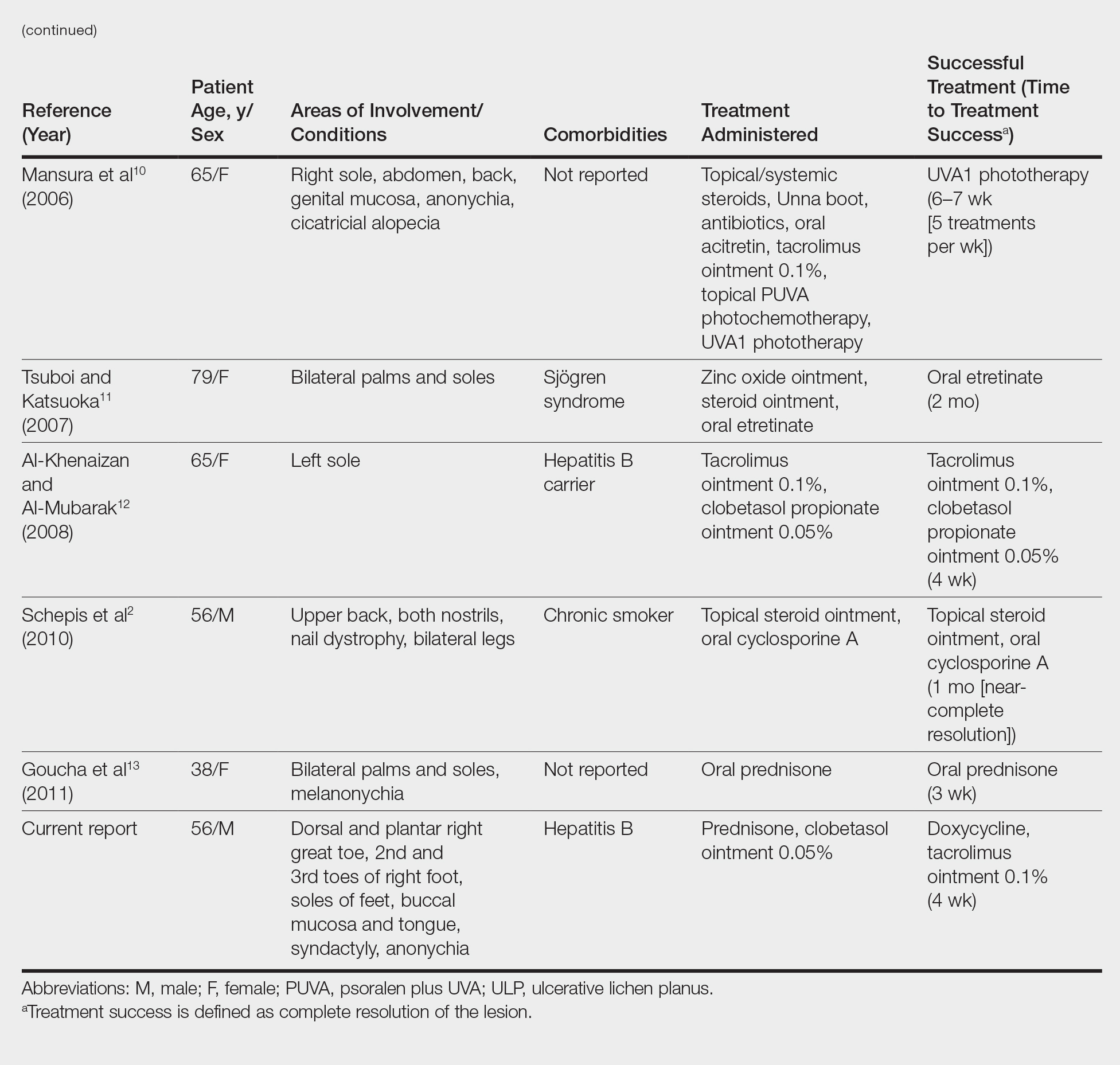
We conducted a review of the Ovid MEDLINE database using the search terms ulcerative lichen planus and erosive lichen planus for articles from the last 30 years, focusing specifically on articles that reported cases of cutaneous involvement of ULP and successful therapeutic modalities. The Table provides a detailed summary of the cases from 1985 to present, representing a spectrum of clinical manifestations and successful treatments of ULP.1-13
Hepatitis C is a comorbidity commonly associated with classic lichen planus, while hepatitis B immunization has a well-described association with classic and oral ULP.12,14 Although hepatitis C was negative in our patient, we did find a chronic inactive carrier state for hepatitis B infection. Al-Khenaizan and Al-Mubarak12 reported the only other known case of ULP of the sole associated with positive serology for hepatitis B surface antigen.
Ulcerative lichen planus of the soles can be difficult to diagnose, especially when it is an isolated finding. It should be differentiated from localized bullous pemphigoid, epidermolysis bullosa acquisita, ulcerative lupus erythematosus, and dermatitis artefacta.13 The characteristic associated clinical features of plantar ULP in our patient and lack of diagnostic immunofluorescence helped us to rule out these alternative diagnoses.4 Long-standing ulcerations of ULP also pose an increased risk for neoplastic transformation. Eisen15 noted a 0.4% to 5% frequency of malignant transformation into squamous cell carcinoma in those with oral ULP. Therefore, it is important to monitor previously ulcerated lesions long-term for such development.
Plantar ULP is difficult to treat and often is unresponsive to systemic and local treatment. Historically, surgical grafting of the affected areas was the treatment of choice, as reported by Patrone et al.6 Goucha et al13 reported complete healing of ulcerations within 3 weeks of starting oral prednisone 1 mg/kg once daily followed by a maintenance dosage of 5 mg once daily. Tacrolimus is a macrolide immunosuppressant that inhibits T-cell activation by forming a complex with FK506 binding protein in the cytoplasm of T cells that binds and inhibits calcineurin dephosphorylation of nuclear factor of activated T cells.12 Al-Khenaizan and Al-Mubarak12 reported resolution of plantar ULP ulcerations after 4 weeks of treatment with topical tacrolimus. Eisman and Orteu7 also achieved complete healing of ulcerations of plantar ULP using tacrolimus ointment 0.1%.
In our patient, doxycycline also was started at the time of initiating the topical tacrolimus. We chose this treatment to take advantage of its systemic anti-inflammatory, antiangiogenic, and antibacterial properties. Our case represents rapid and successful treatment of plantar ULP utilizing this specific combination of oral doxycycline and topical tacrolimus.
Conclusion
Ulcerative lichen planus is an uncommon variant of lichen planus, with cutaneous involvement only rarely reported in the literature. Physicians should be aware of this entity and should consider it in the differential diagnosis in patients presenting with chronic ulcers on the soles, especially when lesions have been unresponsive to appropriate wound care and antibiotic treatment or when cultures have been persistently negative for microbial growth. The possibility of drug-induced lichen planus also should not be overlooked, and one should consider discontinuation of all nonessential medications that could be potential culprits. In our patient ibuprofen was discontinued, but we can only speculate that it was contributory to his healing and only time will tell if resumption of this nonsteroidal anti-inflammatory drug causes a relapse in symptoms.
In our patient, a combination of systemic and topical steroids, topical tacrolimus, and oral doxycycline successfully treated his plantar ULP. Our findings provide further support for the use of topical tacrolimus as a steroid-sparing anti-inflammatory agent for the treatment of plantar ULP. We also introduce the combination of topical tacrolimus and oral doxycycline as a novel therapeutic combination and relatively safer alternative to conventional immunosuppressive agents for long-term systemic anti-inflammatory effects.
- Wollina U, Konrad H, Graefe T. Ulcerative lichen planus: a case responding to recombinant platelet-derived growth factor BB and immunosuppression. Acta Derm Venereol. 2001;81:364-383.
- Schepis C, Lentini M, Siragusa M. Erosive lichen planus on an atypical site mimicking a factitial dermatitis. Acta Derm Venereol. 2010;90:185-186.
- Falk DK, Latour DL, King EL. Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-570.
- Sonnex TS, Eady RA, Sparrow GP, et al. Ulcerative lichen planus associated with webbing of the toes. J R Soc Med. 1986;79:363-365.
- Dereure O, Basset-Sequin N, Guilhou JJ. Erosive lichen planus: dramatic response to thalidomide. Arch Dermatol. 1996;132:1392-1393.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8:243-244.
- Eisman S, Orteu C. Recalcitrant erosive flexural lichen planus: successful treatment with a combination of thalidomide and 0.1% tacrolimus ointment. Clin Exp Dermatol. 2004;29:268-270.
- Henderson RL Jr, Williford PM, Molnar JA. Cutaneous ulcerative lichen planus exhibiting pathergy, response to acitretin. J Drugs Dermatol. 2004;3:191-192.
- Meyer S, Burgdorf T, Szeimies R, et al. Management of erosive lichen planus with topical tacrolimus and recurrence secondary to metoprolol. J Eur Acad Dermatol Venereol. 2005;19:236-239.
- Mansura A, Alkalay R, Slodownik D, et al. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Pathomed. 2006;22:164-165.
- Tsuboi H, Katsuoka K. Ulcerative lichen planus associated with Sjögren’s syndrome. J Dermatol. 2007;34:131-134.
- Al-Khenaizan S, Al-Mubarak L. Ulcerative lichen planus of the sole: excellent response to topical tacrolimus. Int J Dermatol. 2008;47:626-628.
- Goucha S, Khaled A, Rammeh S, et al. Erosive lichen planus of the soles: effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Binesh F, Parichehr K. Erosive lichen planus of the scalp and hepatitis C infection. J Coll Physicians Surg Pak. 2013;23:169.
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
Ulcerative lichen planus (ULP)(also called erosive) is a rare variant of lichen planus. Similar to classic lichen planus, the cause of ULP is largely unknown. Ulcerative lichen planus typically involves the oral mucosa or genitalia but rarely may present as ulcerations on the palms and soles. Clinical presentation usually involves a history of chronic ulcers that often have been previously misdiagnosed and resistant to treatment. Ulcerations on the plantar surfaces frequently cause severe pain and disability. Few cases have been reported and successful treatment is rare.
Case Report
A 56-year-old man was referred by podiatry to the dermatology clinic for evaluation of painful ulcerations involving the dorsal and plantar surfaces of the right great toe as well as the second to third digits. The ulcers had been ongoing for 8 years, treated mostly with local wound care without clinical improvement. His medical and family history was considered noncontributory as a possible etiology of the ulcers; however, he had been taking ibuprofen intermittently for years for general aches and pains, which raised the suspicion of a drug-induced etiology. Laboratory evaluation revealed positive hepatitis B serology but was otherwise unremarkable, including normal liver function tests and negative wound cultures.
Physical examination revealed a beefy red, glazed ulceration involving the entire right great toe with extension onto the second and third toes. There was considerable scarring with syndactyly of the second and third toes and complete toenail loss of the right foot (Figure 1). On the insteps of the bilateral soles were a few scattered, pale, atrophic, violaceous papules with overlying thin lacy white streaks that were reflective of Wickham striae. Early dorsal pterygium formation also was noted on the bilateral third fingernails. Oral mucosal examination revealed lacy white plaques on the bilateral buccal mucosa with a large ulcer of the left lateral tongue (Figure 2). No genital or scalp lesions were present.


Histologic examination of a papule on the instep of the right sole demonstrated a dense lichenoid lymphocytic infiltrate in the papillary dermis with basal vacuolar degeneration and early focal Max-Joseph space formation. Additionally, there was epidermal atrophy with mild hypergranulosis and scattered necrotic keratinocytes (Figure 3). A similar histologic picture was noted on a biopsy of the buccal mucosa overlying the right molar, albeit with epithelial acanthosis rather than atrophy.

Based on initial clinical suspicion for ULP, we suggested that our patient discontinue ibuprofen and started him on a regimen of oral prednisone 40 mg once daily and clobetasol ointment 0.05% applied twice daily to the plantar ulceration, both for 2 weeks. Dramatic improvement was noted after only 2 weeks of treatment. This regimen was then switched to oral doxycycline 100 mg twice daily combined with tacrolimus ointment 0.1% applied twice daily to the plantar ulceration to avoid side effects of prolonged steroid use. Topical therapies were not used for the mucosal lesions. At 4-week follow-up, the patient continued to demonstrate notable clinical response with a greater than 70% physician-assessed improvement in ulcer severity (Figure 4) and near-complete resolution of the oral mucosal lesions. Our patient also reported almost complete resolution of pain. By 4-month follow-up, complete reepithelialization and resolution of the ulcers was noted (Figure 5). This improvement was sustained at additional follow-up 1 year after the initial presentation.


Comment
Ulcerative (or erosive) lichen planus is a rare form of lichen planus. Ulcerative lichen planus most commonly presents as erosive lesions of the oral and genital mucosae but rarely can involve other sites. The palms and soles are the most common sites of cutaneous involvement, with lesions frequently characterized by severe pain and limited mobility.2
We conducted a review of the Ovid MEDLINE database using the search terms ulcerative lichen planus and erosive lichen planus for articles from the last 30 years, focusing specifically on articles that reported cases of cutaneous involvement of ULP and successful therapeutic modalities. The Table provides a detailed summary of the cases from 1985 to present, representing a spectrum of clinical manifestations and successful treatments of ULP.1-13


Hepatitis C is a comorbidity commonly associated with classic lichen planus, while hepatitis B immunization has a well-described association with classic and oral ULP.12,14 Although hepatitis C was negative in our patient, we did find a chronic inactive carrier state for hepatitis B infection. Al-Khenaizan and Al-Mubarak12 reported the only other known case of ULP of the sole associated with positive serology for hepatitis B surface antigen.
Ulcerative lichen planus of the soles can be difficult to diagnose, especially when it is an isolated finding. It should be differentiated from localized bullous pemphigoid, epidermolysis bullosa acquisita, ulcerative lupus erythematosus, and dermatitis artefacta.13 The characteristic associated clinical features of plantar ULP in our patient and lack of diagnostic immunofluorescence helped us to rule out these alternative diagnoses.4 Long-standing ulcerations of ULP also pose an increased risk for neoplastic transformation. Eisen15 noted a 0.4% to 5% frequency of malignant transformation into squamous cell carcinoma in those with oral ULP. Therefore, it is important to monitor previously ulcerated lesions long-term for such development.
Plantar ULP is difficult to treat and often is unresponsive to systemic and local treatment. Historically, surgical grafting of the affected areas was the treatment of choice, as reported by Patrone et al.6 Goucha et al13 reported complete healing of ulcerations within 3 weeks of starting oral prednisone 1 mg/kg once daily followed by a maintenance dosage of 5 mg once daily. Tacrolimus is a macrolide immunosuppressant that inhibits T-cell activation by forming a complex with FK506 binding protein in the cytoplasm of T cells that binds and inhibits calcineurin dephosphorylation of nuclear factor of activated T cells.12 Al-Khenaizan and Al-Mubarak12 reported resolution of plantar ULP ulcerations after 4 weeks of treatment with topical tacrolimus. Eisman and Orteu7 also achieved complete healing of ulcerations of plantar ULP using tacrolimus ointment 0.1%.
In our patient, doxycycline also was started at the time of initiating the topical tacrolimus. We chose this treatment to take advantage of its systemic anti-inflammatory, antiangiogenic, and antibacterial properties. Our case represents rapid and successful treatment of plantar ULP utilizing this specific combination of oral doxycycline and topical tacrolimus.
Conclusion
Ulcerative lichen planus is an uncommon variant of lichen planus, with cutaneous involvement only rarely reported in the literature. Physicians should be aware of this entity and should consider it in the differential diagnosis in patients presenting with chronic ulcers on the soles, especially when lesions have been unresponsive to appropriate wound care and antibiotic treatment or when cultures have been persistently negative for microbial growth. The possibility of drug-induced lichen planus also should not be overlooked, and one should consider discontinuation of all nonessential medications that could be potential culprits. In our patient ibuprofen was discontinued, but we can only speculate that it was contributory to his healing and only time will tell if resumption of this nonsteroidal anti-inflammatory drug causes a relapse in symptoms.
In our patient, a combination of systemic and topical steroids, topical tacrolimus, and oral doxycycline successfully treated his plantar ULP. Our findings provide further support for the use of topical tacrolimus as a steroid-sparing anti-inflammatory agent for the treatment of plantar ULP. We also introduce the combination of topical tacrolimus and oral doxycycline as a novel therapeutic combination and relatively safer alternative to conventional immunosuppressive agents for long-term systemic anti-inflammatory effects.
Ulcerative lichen planus (ULP)(also called erosive) is a rare variant of lichen planus. Similar to classic lichen planus, the cause of ULP is largely unknown. Ulcerative lichen planus typically involves the oral mucosa or genitalia but rarely may present as ulcerations on the palms and soles. Clinical presentation usually involves a history of chronic ulcers that often have been previously misdiagnosed and resistant to treatment. Ulcerations on the plantar surfaces frequently cause severe pain and disability. Few cases have been reported and successful treatment is rare.
Case Report
A 56-year-old man was referred by podiatry to the dermatology clinic for evaluation of painful ulcerations involving the dorsal and plantar surfaces of the right great toe as well as the second to third digits. The ulcers had been ongoing for 8 years, treated mostly with local wound care without clinical improvement. His medical and family history was considered noncontributory as a possible etiology of the ulcers; however, he had been taking ibuprofen intermittently for years for general aches and pains, which raised the suspicion of a drug-induced etiology. Laboratory evaluation revealed positive hepatitis B serology but was otherwise unremarkable, including normal liver function tests and negative wound cultures.
Physical examination revealed a beefy red, glazed ulceration involving the entire right great toe with extension onto the second and third toes. There was considerable scarring with syndactyly of the second and third toes and complete toenail loss of the right foot (Figure 1). On the insteps of the bilateral soles were a few scattered, pale, atrophic, violaceous papules with overlying thin lacy white streaks that were reflective of Wickham striae. Early dorsal pterygium formation also was noted on the bilateral third fingernails. Oral mucosal examination revealed lacy white plaques on the bilateral buccal mucosa with a large ulcer of the left lateral tongue (Figure 2). No genital or scalp lesions were present.


Histologic examination of a papule on the instep of the right sole demonstrated a dense lichenoid lymphocytic infiltrate in the papillary dermis with basal vacuolar degeneration and early focal Max-Joseph space formation. Additionally, there was epidermal atrophy with mild hypergranulosis and scattered necrotic keratinocytes (Figure 3). A similar histologic picture was noted on a biopsy of the buccal mucosa overlying the right molar, albeit with epithelial acanthosis rather than atrophy.

Based on initial clinical suspicion for ULP, we suggested that our patient discontinue ibuprofen and started him on a regimen of oral prednisone 40 mg once daily and clobetasol ointment 0.05% applied twice daily to the plantar ulceration, both for 2 weeks. Dramatic improvement was noted after only 2 weeks of treatment. This regimen was then switched to oral doxycycline 100 mg twice daily combined with tacrolimus ointment 0.1% applied twice daily to the plantar ulceration to avoid side effects of prolonged steroid use. Topical therapies were not used for the mucosal lesions. At 4-week follow-up, the patient continued to demonstrate notable clinical response with a greater than 70% physician-assessed improvement in ulcer severity (Figure 4) and near-complete resolution of the oral mucosal lesions. Our patient also reported almost complete resolution of pain. By 4-month follow-up, complete reepithelialization and resolution of the ulcers was noted (Figure 5). This improvement was sustained at additional follow-up 1 year after the initial presentation.


Comment
Ulcerative (or erosive) lichen planus is a rare form of lichen planus. Ulcerative lichen planus most commonly presents as erosive lesions of the oral and genital mucosae but rarely can involve other sites. The palms and soles are the most common sites of cutaneous involvement, with lesions frequently characterized by severe pain and limited mobility.2
We conducted a review of the Ovid MEDLINE database using the search terms ulcerative lichen planus and erosive lichen planus for articles from the last 30 years, focusing specifically on articles that reported cases of cutaneous involvement of ULP and successful therapeutic modalities. The Table provides a detailed summary of the cases from 1985 to present, representing a spectrum of clinical manifestations and successful treatments of ULP.1-13


Hepatitis C is a comorbidity commonly associated with classic lichen planus, while hepatitis B immunization has a well-described association with classic and oral ULP.12,14 Although hepatitis C was negative in our patient, we did find a chronic inactive carrier state for hepatitis B infection. Al-Khenaizan and Al-Mubarak12 reported the only other known case of ULP of the sole associated with positive serology for hepatitis B surface antigen.
Ulcerative lichen planus of the soles can be difficult to diagnose, especially when it is an isolated finding. It should be differentiated from localized bullous pemphigoid, epidermolysis bullosa acquisita, ulcerative lupus erythematosus, and dermatitis artefacta.13 The characteristic associated clinical features of plantar ULP in our patient and lack of diagnostic immunofluorescence helped us to rule out these alternative diagnoses.4 Long-standing ulcerations of ULP also pose an increased risk for neoplastic transformation. Eisen15 noted a 0.4% to 5% frequency of malignant transformation into squamous cell carcinoma in those with oral ULP. Therefore, it is important to monitor previously ulcerated lesions long-term for such development.
Plantar ULP is difficult to treat and often is unresponsive to systemic and local treatment. Historically, surgical grafting of the affected areas was the treatment of choice, as reported by Patrone et al.6 Goucha et al13 reported complete healing of ulcerations within 3 weeks of starting oral prednisone 1 mg/kg once daily followed by a maintenance dosage of 5 mg once daily. Tacrolimus is a macrolide immunosuppressant that inhibits T-cell activation by forming a complex with FK506 binding protein in the cytoplasm of T cells that binds and inhibits calcineurin dephosphorylation of nuclear factor of activated T cells.12 Al-Khenaizan and Al-Mubarak12 reported resolution of plantar ULP ulcerations after 4 weeks of treatment with topical tacrolimus. Eisman and Orteu7 also achieved complete healing of ulcerations of plantar ULP using tacrolimus ointment 0.1%.
In our patient, doxycycline also was started at the time of initiating the topical tacrolimus. We chose this treatment to take advantage of its systemic anti-inflammatory, antiangiogenic, and antibacterial properties. Our case represents rapid and successful treatment of plantar ULP utilizing this specific combination of oral doxycycline and topical tacrolimus.
Conclusion
Ulcerative lichen planus is an uncommon variant of lichen planus, with cutaneous involvement only rarely reported in the literature. Physicians should be aware of this entity and should consider it in the differential diagnosis in patients presenting with chronic ulcers on the soles, especially when lesions have been unresponsive to appropriate wound care and antibiotic treatment or when cultures have been persistently negative for microbial growth. The possibility of drug-induced lichen planus also should not be overlooked, and one should consider discontinuation of all nonessential medications that could be potential culprits. In our patient ibuprofen was discontinued, but we can only speculate that it was contributory to his healing and only time will tell if resumption of this nonsteroidal anti-inflammatory drug causes a relapse in symptoms.
In our patient, a combination of systemic and topical steroids, topical tacrolimus, and oral doxycycline successfully treated his plantar ULP. Our findings provide further support for the use of topical tacrolimus as a steroid-sparing anti-inflammatory agent for the treatment of plantar ULP. We also introduce the combination of topical tacrolimus and oral doxycycline as a novel therapeutic combination and relatively safer alternative to conventional immunosuppressive agents for long-term systemic anti-inflammatory effects.
- Wollina U, Konrad H, Graefe T. Ulcerative lichen planus: a case responding to recombinant platelet-derived growth factor BB and immunosuppression. Acta Derm Venereol. 2001;81:364-383.
- Schepis C, Lentini M, Siragusa M. Erosive lichen planus on an atypical site mimicking a factitial dermatitis. Acta Derm Venereol. 2010;90:185-186.
- Falk DK, Latour DL, King EL. Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-570.
- Sonnex TS, Eady RA, Sparrow GP, et al. Ulcerative lichen planus associated with webbing of the toes. J R Soc Med. 1986;79:363-365.
- Dereure O, Basset-Sequin N, Guilhou JJ. Erosive lichen planus: dramatic response to thalidomide. Arch Dermatol. 1996;132:1392-1393.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8:243-244.
- Eisman S, Orteu C. Recalcitrant erosive flexural lichen planus: successful treatment with a combination of thalidomide and 0.1% tacrolimus ointment. Clin Exp Dermatol. 2004;29:268-270.
- Henderson RL Jr, Williford PM, Molnar JA. Cutaneous ulcerative lichen planus exhibiting pathergy, response to acitretin. J Drugs Dermatol. 2004;3:191-192.
- Meyer S, Burgdorf T, Szeimies R, et al. Management of erosive lichen planus with topical tacrolimus and recurrence secondary to metoprolol. J Eur Acad Dermatol Venereol. 2005;19:236-239.
- Mansura A, Alkalay R, Slodownik D, et al. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Pathomed. 2006;22:164-165.
- Tsuboi H, Katsuoka K. Ulcerative lichen planus associated with Sjögren’s syndrome. J Dermatol. 2007;34:131-134.
- Al-Khenaizan S, Al-Mubarak L. Ulcerative lichen planus of the sole: excellent response to topical tacrolimus. Int J Dermatol. 2008;47:626-628.
- Goucha S, Khaled A, Rammeh S, et al. Erosive lichen planus of the soles: effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Binesh F, Parichehr K. Erosive lichen planus of the scalp and hepatitis C infection. J Coll Physicians Surg Pak. 2013;23:169.
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
- Wollina U, Konrad H, Graefe T. Ulcerative lichen planus: a case responding to recombinant platelet-derived growth factor BB and immunosuppression. Acta Derm Venereol. 2001;81:364-383.
- Schepis C, Lentini M, Siragusa M. Erosive lichen planus on an atypical site mimicking a factitial dermatitis. Acta Derm Venereol. 2010;90:185-186.
- Falk DK, Latour DL, King EL. Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-570.
- Sonnex TS, Eady RA, Sparrow GP, et al. Ulcerative lichen planus associated with webbing of the toes. J R Soc Med. 1986;79:363-365.
- Dereure O, Basset-Sequin N, Guilhou JJ. Erosive lichen planus: dramatic response to thalidomide. Arch Dermatol. 1996;132:1392-1393.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8:243-244.
- Eisman S, Orteu C. Recalcitrant erosive flexural lichen planus: successful treatment with a combination of thalidomide and 0.1% tacrolimus ointment. Clin Exp Dermatol. 2004;29:268-270.
- Henderson RL Jr, Williford PM, Molnar JA. Cutaneous ulcerative lichen planus exhibiting pathergy, response to acitretin. J Drugs Dermatol. 2004;3:191-192.
- Meyer S, Burgdorf T, Szeimies R, et al. Management of erosive lichen planus with topical tacrolimus and recurrence secondary to metoprolol. J Eur Acad Dermatol Venereol. 2005;19:236-239.
- Mansura A, Alkalay R, Slodownik D, et al. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Pathomed. 2006;22:164-165.
- Tsuboi H, Katsuoka K. Ulcerative lichen planus associated with Sjögren’s syndrome. J Dermatol. 2007;34:131-134.
- Al-Khenaizan S, Al-Mubarak L. Ulcerative lichen planus of the sole: excellent response to topical tacrolimus. Int J Dermatol. 2008;47:626-628.
- Goucha S, Khaled A, Rammeh S, et al. Erosive lichen planus of the soles: effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Binesh F, Parichehr K. Erosive lichen planus of the scalp and hepatitis C infection. J Coll Physicians Surg Pak. 2013;23:169.
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
Practice Points
- Consider ulcerative lichen planus (ULP) for chronic wounds on the soles.
- Topical therapeutic options may present a rapidly effective and relatively safe alternative to conventional immunosuppressive agents for long-term management of plantar ULP.
Deer Ked: A Lyme-Carrying Ectoparasite on the Move
How to Prevent Mosquito and Tick-Borne Disease
Case Report
A 31-year-old man presented to the dermatology clinic 1 day after mountain biking in the woods in Hartford County, Connecticut. He stated that he found a tick attached to his shirt after riding (Figure). Careful examination of the patient showed no signs of a bite reaction. The insect was identified via microscopy as the deer ked Lipoptena cervi.

Comment
Lipoptena cervi, known as the deer ked, is an ectoparasite of cervids traditionally found in Norway, Sweden, and Finland.1 The deer ked was first reported in American deer in 2 independent sightings in Pennsylvania and New Hampshire in 1907.2 More recently deer keds have been reported in Massachusetts, New York, Pennsylvania, and New Hampshire.3 In the United States, L cervi is thought to be an invasive species transported from Europe in the 1800s.4,5 The main host is thought to be the white-tailed deer (Odocoileus viginianus). Once a suitable host is found, the deer ked sheds its wings and crawls into the fur. After engorging on a blood meal, it deposits prepupae that fall from the host and mature into winged adults during the late summer into the autumn. Adults may exhibit swarming behavior, and it is during this host-seeking activity that they land on humans.3
Following the bite of a deer ked, there are reports of long-lasting dermatitis in both humans and dogs.1,4,6 One case series involving 19 patients following deer ked bites reported pruritic bite papules.4 The reaction appeared to be treatment resistant and lasted from 2 weeks to 12 months. Histologic examination was typical for arthropod assault. Of 11 papules that were biopsied, most (7/11) showed C3 deposition in dermal vessel walls under direct immunofluorescence. Of 19 patients, 57% had elevated serum IgE levels.4
In addition to the associated dermatologic findings, the deer ked is a vector of various infectious agents. Bartonella schoenbuchensis has been isolated from deer ked in Massachusettes.7 A recent study found a 75% prevalence of Bartonella species in 217 deer keds collected from red deer in Poland.5 The first incidence of Borrelia burgdorferi and Anaplasma phagocytophylum in deer keds was reported in the United States in 2016. Of 48 adult deer keds collected from an unknown number of deer, 19 (40%), 14 (29%), and 3 (6%) were positive for B burgdorferi, A phagocytophylum, and both on polymerase chain reaction, respectively.3
A recent study from Europe showed deer keds are now more frequently found in regions where they had not previously been observed.8 It stands to reason that with climate change, L cervi and other disease-carrying vectors are likely to migrate to and inhabit new regions of the country. Even in the current climate, there are more disease-carrying arthropods than are routinely studied in medicine, and all patients who experience an arthropod assault should be monitored for signs of systemic disease.
- Mysterud A, Madslien K, Herland A, et al. Phenology of deer ked (Lipoptena cervi) host-seeking flight activity and its relationship with prevailing autumn weather. Parasit Vectors. 2016;9:95.
- Bequaert JC. A Monograph of the Melophaginae or Ked-flies of Sheep, Goats, Deer, and Antelopes (Diptera, Hippoboscidae). Brooklyn, NY: Brooklyn Entomological Society; 1942.
- Buss M, Case L, Kearney B, et al. Detection of Lyme disease and anaplasmosis pathogens via PCR in Pennsylvania deer ked. J Vector Ecol. 2016;41:292-294.
- Rantanen T, Reunala T, Vuojolahti P, et al. Persistent pruritic papules from deer ked bites. Acta Derm Venereol. 1982;62:307-311.
- Szewczyk T, Werszko J, Steiner-Bogdaszewska Ż, et al. Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasit Vectors. 2017;10:487.
- Hermosilla C, Pantchev N, Bachmann R, et al. Lipoptena cervi (deer ked) in two naturally infested dogs. Vet Rec. 2006;159:286-287.
- Matsumoto K, Berrada ZL, Klinger E, et al. Molecular detection of Bartonella schoenbuchensis from ectoparasites of deer in Massachusetts. Vector Borne Zoonotic Dis. 2008;8:549-554.
- Sokół R, Gałęcki R. Prevalence of keds on city dogs in central Poland. Med Vet Entomol. 2017;31:114-116.
How to Prevent Mosquito and Tick-Borne Disease
Case Report
A 31-year-old man presented to the dermatology clinic 1 day after mountain biking in the woods in Hartford County, Connecticut. He stated that he found a tick attached to his shirt after riding (Figure). Careful examination of the patient showed no signs of a bite reaction. The insect was identified via microscopy as the deer ked Lipoptena cervi.

Comment
Lipoptena cervi, known as the deer ked, is an ectoparasite of cervids traditionally found in Norway, Sweden, and Finland.1 The deer ked was first reported in American deer in 2 independent sightings in Pennsylvania and New Hampshire in 1907.2 More recently deer keds have been reported in Massachusetts, New York, Pennsylvania, and New Hampshire.3 In the United States, L cervi is thought to be an invasive species transported from Europe in the 1800s.4,5 The main host is thought to be the white-tailed deer (Odocoileus viginianus). Once a suitable host is found, the deer ked sheds its wings and crawls into the fur. After engorging on a blood meal, it deposits prepupae that fall from the host and mature into winged adults during the late summer into the autumn. Adults may exhibit swarming behavior, and it is during this host-seeking activity that they land on humans.3
Following the bite of a deer ked, there are reports of long-lasting dermatitis in both humans and dogs.1,4,6 One case series involving 19 patients following deer ked bites reported pruritic bite papules.4 The reaction appeared to be treatment resistant and lasted from 2 weeks to 12 months. Histologic examination was typical for arthropod assault. Of 11 papules that were biopsied, most (7/11) showed C3 deposition in dermal vessel walls under direct immunofluorescence. Of 19 patients, 57% had elevated serum IgE levels.4
In addition to the associated dermatologic findings, the deer ked is a vector of various infectious agents. Bartonella schoenbuchensis has been isolated from deer ked in Massachusettes.7 A recent study found a 75% prevalence of Bartonella species in 217 deer keds collected from red deer in Poland.5 The first incidence of Borrelia burgdorferi and Anaplasma phagocytophylum in deer keds was reported in the United States in 2016. Of 48 adult deer keds collected from an unknown number of deer, 19 (40%), 14 (29%), and 3 (6%) were positive for B burgdorferi, A phagocytophylum, and both on polymerase chain reaction, respectively.3
A recent study from Europe showed deer keds are now more frequently found in regions where they had not previously been observed.8 It stands to reason that with climate change, L cervi and other disease-carrying vectors are likely to migrate to and inhabit new regions of the country. Even in the current climate, there are more disease-carrying arthropods than are routinely studied in medicine, and all patients who experience an arthropod assault should be monitored for signs of systemic disease.
How to Prevent Mosquito and Tick-Borne Disease
Case Report
A 31-year-old man presented to the dermatology clinic 1 day after mountain biking in the woods in Hartford County, Connecticut. He stated that he found a tick attached to his shirt after riding (Figure). Careful examination of the patient showed no signs of a bite reaction. The insect was identified via microscopy as the deer ked Lipoptena cervi.

Comment
Lipoptena cervi, known as the deer ked, is an ectoparasite of cervids traditionally found in Norway, Sweden, and Finland.1 The deer ked was first reported in American deer in 2 independent sightings in Pennsylvania and New Hampshire in 1907.2 More recently deer keds have been reported in Massachusetts, New York, Pennsylvania, and New Hampshire.3 In the United States, L cervi is thought to be an invasive species transported from Europe in the 1800s.4,5 The main host is thought to be the white-tailed deer (Odocoileus viginianus). Once a suitable host is found, the deer ked sheds its wings and crawls into the fur. After engorging on a blood meal, it deposits prepupae that fall from the host and mature into winged adults during the late summer into the autumn. Adults may exhibit swarming behavior, and it is during this host-seeking activity that they land on humans.3
Following the bite of a deer ked, there are reports of long-lasting dermatitis in both humans and dogs.1,4,6 One case series involving 19 patients following deer ked bites reported pruritic bite papules.4 The reaction appeared to be treatment resistant and lasted from 2 weeks to 12 months. Histologic examination was typical for arthropod assault. Of 11 papules that were biopsied, most (7/11) showed C3 deposition in dermal vessel walls under direct immunofluorescence. Of 19 patients, 57% had elevated serum IgE levels.4
In addition to the associated dermatologic findings, the deer ked is a vector of various infectious agents. Bartonella schoenbuchensis has been isolated from deer ked in Massachusettes.7 A recent study found a 75% prevalence of Bartonella species in 217 deer keds collected from red deer in Poland.5 The first incidence of Borrelia burgdorferi and Anaplasma phagocytophylum in deer keds was reported in the United States in 2016. Of 48 adult deer keds collected from an unknown number of deer, 19 (40%), 14 (29%), and 3 (6%) were positive for B burgdorferi, A phagocytophylum, and both on polymerase chain reaction, respectively.3
A recent study from Europe showed deer keds are now more frequently found in regions where they had not previously been observed.8 It stands to reason that with climate change, L cervi and other disease-carrying vectors are likely to migrate to and inhabit new regions of the country. Even in the current climate, there are more disease-carrying arthropods than are routinely studied in medicine, and all patients who experience an arthropod assault should be monitored for signs of systemic disease.
- Mysterud A, Madslien K, Herland A, et al. Phenology of deer ked (Lipoptena cervi) host-seeking flight activity and its relationship with prevailing autumn weather. Parasit Vectors. 2016;9:95.
- Bequaert JC. A Monograph of the Melophaginae or Ked-flies of Sheep, Goats, Deer, and Antelopes (Diptera, Hippoboscidae). Brooklyn, NY: Brooklyn Entomological Society; 1942.
- Buss M, Case L, Kearney B, et al. Detection of Lyme disease and anaplasmosis pathogens via PCR in Pennsylvania deer ked. J Vector Ecol. 2016;41:292-294.
- Rantanen T, Reunala T, Vuojolahti P, et al. Persistent pruritic papules from deer ked bites. Acta Derm Venereol. 1982;62:307-311.
- Szewczyk T, Werszko J, Steiner-Bogdaszewska Ż, et al. Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasit Vectors. 2017;10:487.
- Hermosilla C, Pantchev N, Bachmann R, et al. Lipoptena cervi (deer ked) in two naturally infested dogs. Vet Rec. 2006;159:286-287.
- Matsumoto K, Berrada ZL, Klinger E, et al. Molecular detection of Bartonella schoenbuchensis from ectoparasites of deer in Massachusetts. Vector Borne Zoonotic Dis. 2008;8:549-554.
- Sokół R, Gałęcki R. Prevalence of keds on city dogs in central Poland. Med Vet Entomol. 2017;31:114-116.
- Mysterud A, Madslien K, Herland A, et al. Phenology of deer ked (Lipoptena cervi) host-seeking flight activity and its relationship with prevailing autumn weather. Parasit Vectors. 2016;9:95.
- Bequaert JC. A Monograph of the Melophaginae or Ked-flies of Sheep, Goats, Deer, and Antelopes (Diptera, Hippoboscidae). Brooklyn, NY: Brooklyn Entomological Society; 1942.
- Buss M, Case L, Kearney B, et al. Detection of Lyme disease and anaplasmosis pathogens via PCR in Pennsylvania deer ked. J Vector Ecol. 2016;41:292-294.
- Rantanen T, Reunala T, Vuojolahti P, et al. Persistent pruritic papules from deer ked bites. Acta Derm Venereol. 1982;62:307-311.
- Szewczyk T, Werszko J, Steiner-Bogdaszewska Ż, et al. Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasit Vectors. 2017;10:487.
- Hermosilla C, Pantchev N, Bachmann R, et al. Lipoptena cervi (deer ked) in two naturally infested dogs. Vet Rec. 2006;159:286-287.
- Matsumoto K, Berrada ZL, Klinger E, et al. Molecular detection of Bartonella schoenbuchensis from ectoparasites of deer in Massachusetts. Vector Borne Zoonotic Dis. 2008;8:549-554.
- Sokół R, Gałęcki R. Prevalence of keds on city dogs in central Poland. Med Vet Entomol. 2017;31:114-116.
Practice Points
- There are many more disease-carrying arthropods than are routinely studied by scientists and physicians.
- Even if the insect cannot be identified, it is important to monitor patients who have experienced arthropod assault for signs of clinical diseases.
Latex Hypersensitivity to Injection Devices for Biologic Therapies in Psoriasis Patients
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
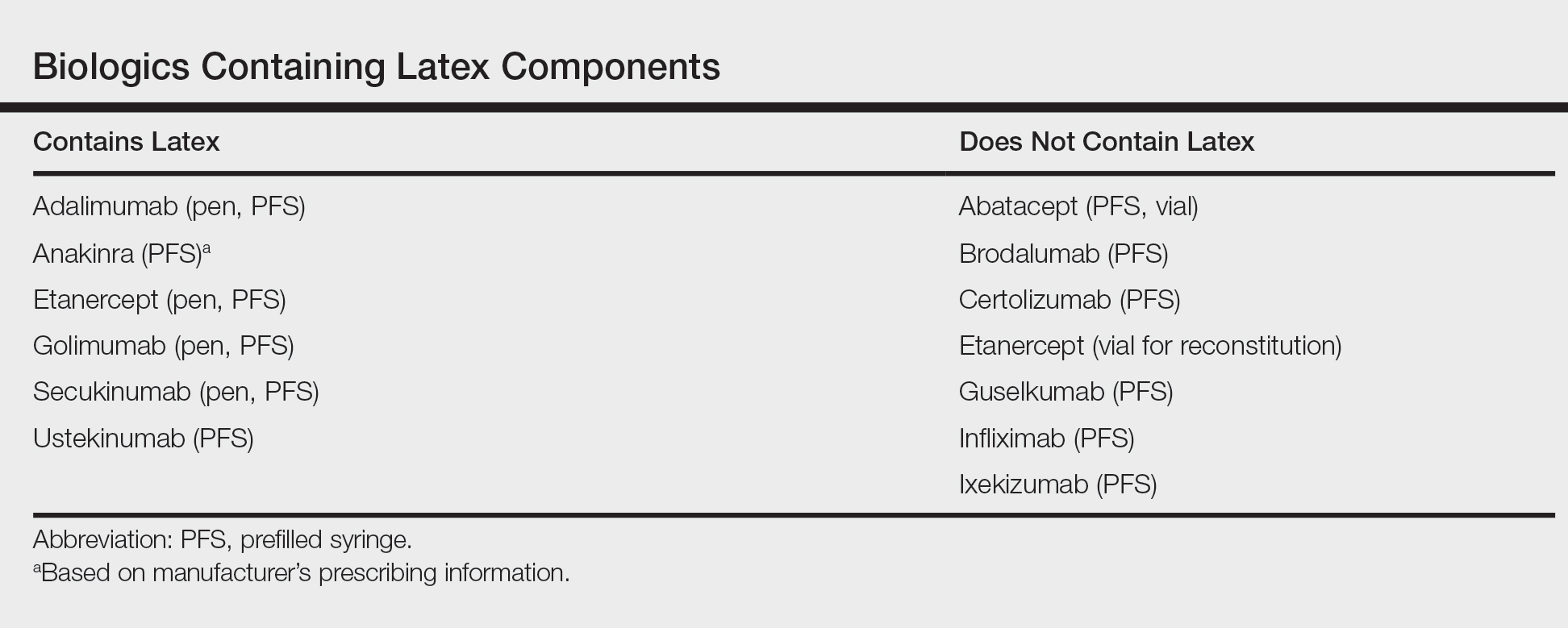
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4

Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4

Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
Inflammatory Linear Verrucous Epidermal Nevus Responsive to 308-nm Excimer Laser Treatment
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).
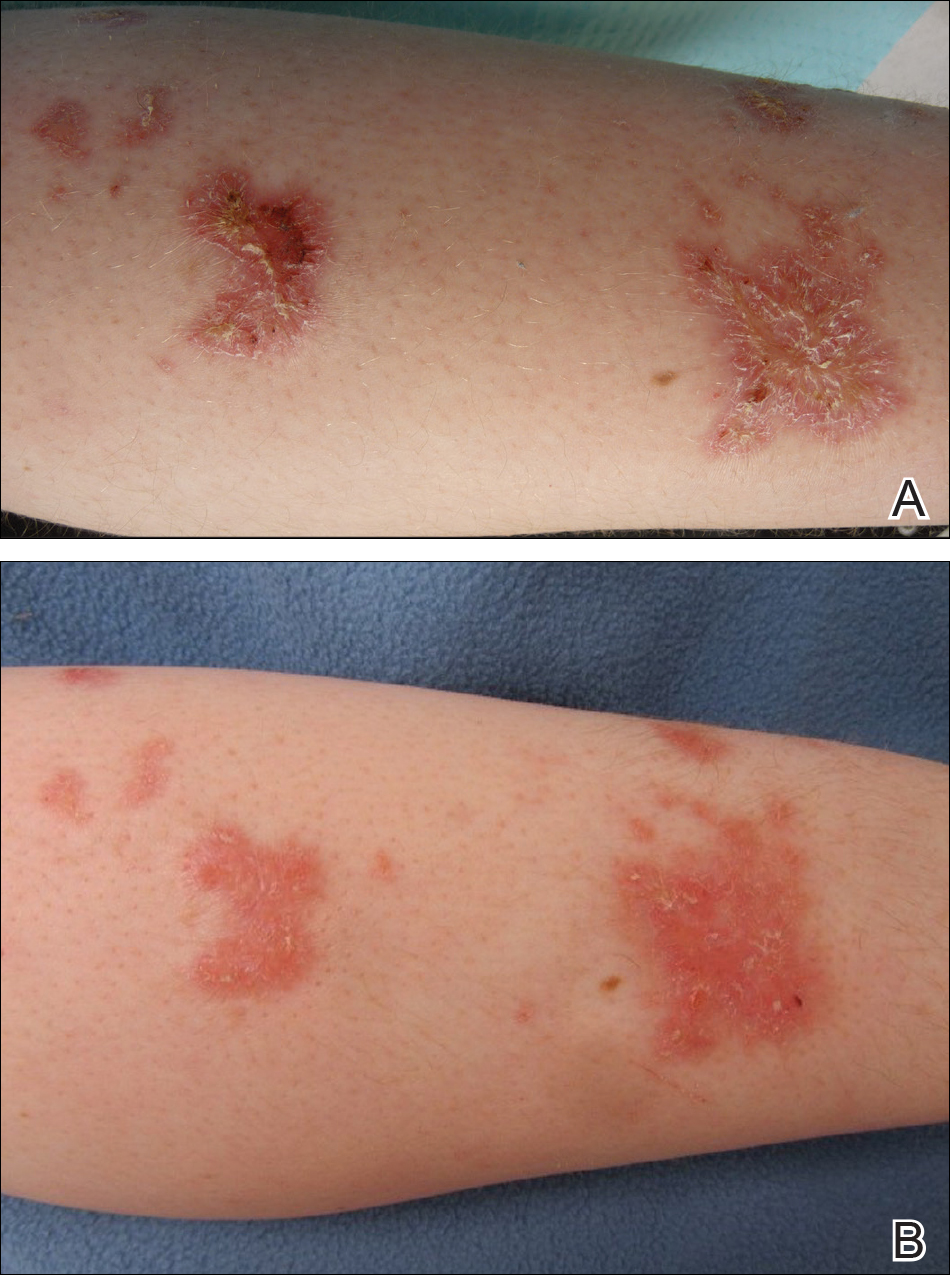
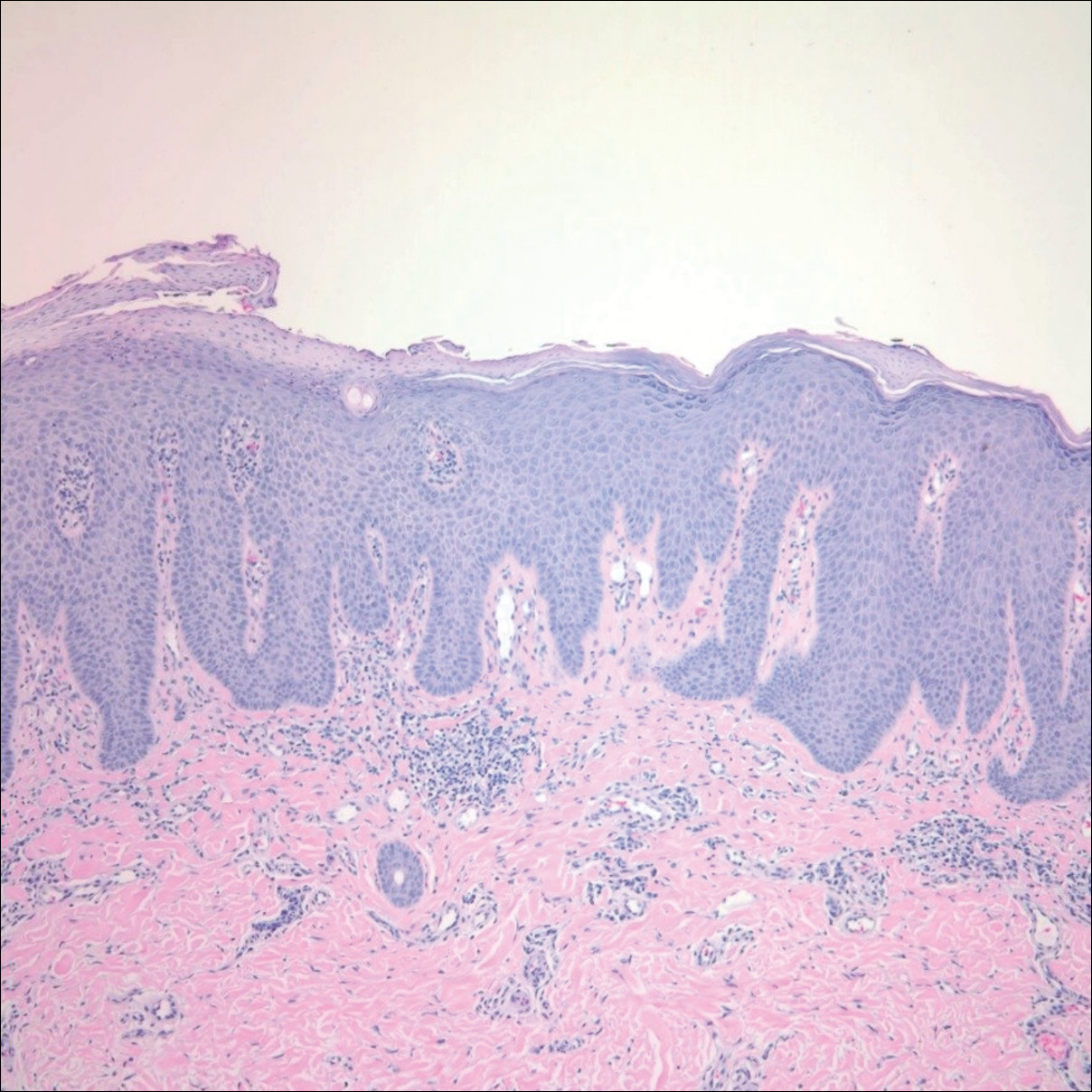
Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
13 weeks' gestation • heart palpitations • chest tightness • Dx?
THE CASE
A 29-year-old G1P0 woman at 13 weeks’ gestation came in for a routine prenatal visit complaining of sudden-onset heart palpitations that were occurring about once a week. Each episode lasted between 15 and 60 minutes and was accompanied by chest tightness, with no identifiable cause. The patient could inconsistently terminate the episodes with Valsalva maneuvers. She reported having had 2 similar incidents of palpitations within the past year. Her family history was significant for sudden cardiac death of her father and paternal grandfather in their fifth decades of life.
A cardiovascular exam was normal; heart auscultation revealed a regular rate and rhythm without murmurs, rubs, or gallops, and the peripheral pulses were normal. A thyroid-stimulating hormone (TSH) level, basic metabolic panel (BMP), and complete blood count (CBC) were within normal limits. A transthoracic echocardiogram was negative for structural heart disease.
THE DIAGNOSIS
An initial Holter monitor study failed to capture an episode of her palpitations. The frequency of her palpitations increased as her pregnancy progressed, occurring almost daily by the second half of the third trimester, and a repeat Holter monitor study in the third trimester was significant for a 3-minute episode of supraventricular tachycardia (SVT) that correlated with patient-recorded symptoms (FIGURE).
Based on these results, we diagnosed the patient with an atrioventricular nodal reentry tachycardia (AVNRT). Although atrioventricular reciprocating tachycardia (AVRT) remained a remote possibility, it is far less common, and a 12-lead electrocardiogram (EKG) showed no evidence of pre-excitation.
DISCUSSION
AVNRT is the most common form of paroxysmal supraventricular tachycardia (PSVT). It occurs more frequently in women and typically manifests in the second to fourth decades of life.1 AVNRT is a narrow complex tachycardia characterized by a heart rate of 120 to >200 beats/min.
Hemodynamic changes in pregnancy can trigger arrhythmias
During pregnancy, hemodynamic changes (including increased blood volume and cardiac output) are thought to stimulate stretch-activated ion channels within the walls of the heart.2-4 Such changes may exacerbate previously existing cardiac arrhythmias or (less commonly) cause new-onset arrhythmias.3,4 A family history positive for arrhythmias or sudden cardiac death increases the likelihood of developing tachyarrhythmia during pregnancy.3 Women with a known history of PSVT might experience symptom exacerbation despite being on prophylactic therapy.4
Detection and diagnosis
While AVNRT is relatively benign in pregnancy, other cardiac arrhythmias (eg, atrial fibrillation/flutter, ventricular tachycardia) carry a greater risk for fetal and maternal complications, underscoring the need to correctly identify the type of arrhythmia.2,3
Continue to: Physical exam findings
Physical exam findings are often unremarkable unless the patient is actively experiencing SVT in the office, in which case prominent jugular pulsations may be seen due to simultaneous contraction of the atria and ventricles.
The initial evaluation of a pregnant patient presenting with tachycardia should include a BMP, TSH, 12-lead EKG, and transthoracic echocardiography.3,5 In most patients with AVNRT, the results of these tests will be normal. A Holter monitor can be used to document an arrhythmia if the episodes are relatively frequent or an event monitor can be used if the episodes are infrequent.5
EKG findings. When patients are actively experiencing SVT, EKG findings include a P wave obscured by the QRS complex, sometimes manifesting as a pseudo-R wave in the V1 lead and a pseudo-S wave in leads II, III, and AVF. The QRS complex is narrow and the R-R interval is regular.6
Types of treatment
Valsalva maneuvers. Treatment of AVNRT in pregnancy should first involve addressing any precipitating causes, including metabolic and endocrine abnormalities.3 As virtually all antiarrhythmic drugs cross the placenta and are traceable in breast milk,2,3 patients should be counseled to try to stop episodes using Valsalva maneuvers before moving to pharmacologic treatment.
Antiarrhythmics. First-line pharmacologic treatment for the prevention of AVNRT in pregnancy is metoprolol or verapamil.2,5 Neither drug has been associated with adverse outcomes in infants, although there is a large body of evidence suggesting that low levels of metoprolol are present in breast milk.7
Continue to: Acute episodes of SVT that are refractory to...
Acute episodes of SVT that are refractory to vagal maneuvers or occur despite medical management can be treated acutely in pregnancy with adenosine, which effectively stops episodes about 90% of the time.2 (See the TABLE8,9 for a list of antiarrhythmics that may be used to treat AVNRT.)
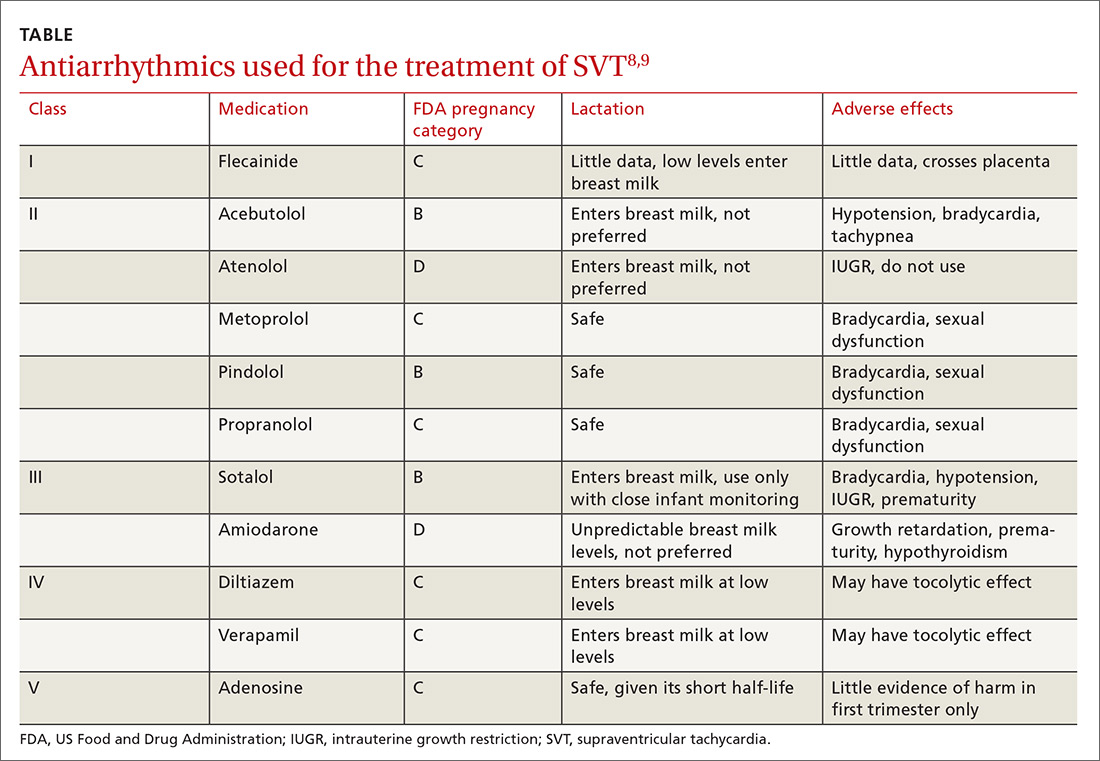
Catheter ablation is first-line treatment for AVNRT in nonpregnant patients.1,5 The risks of undergoing ablation during pregnancy include fetal exposure to radiation and anesthetic drugs.2,3 Therefore, this treatment should be used only when pharmacologic treatment is unsuccessful and risks to the mother and fetus due to the arrhythmia outweigh the risks of the procedure. Ablation can be offered postpartum as more definitive therapy.
Our patient was started on metoprolol tartrate 12.5 mg bid at 35 weeks’ gestation due to increasingly common and persistent palpitations. This helped control the episodes for 2 weeks, at which point they increased again in frequency. These were terminated using Valsalva maneuvers; increasing the metoprolol dosage was prohibitive due to patient intolerance.
Following an uncomplicated delivery, and discontinuation of metoprolol, the patient reported a decrease in both the number of episodes and the duration of SVT. Ultimately, she opted for a catheter ablation to prevent SVT exacerbation during subsequent pregnancies.
THE TAKEAWAY
AVNRT (and other tachyarrhythmias) may worsen or manifest with physiologic changes that occur during pregnancy. After establishing the diagnosis, effort should be made to manage the condition conservatively with Valsalva maneuvers and medication. Catheter ablation should be offered postpartum as a more definitive treatment option.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine Medical Director, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Kwaku KF, Josephson ME. Typical AVNRT—an update on mechanisms and therapy. Card Electrophysiol Rev. 2002;6:414-421.
2. Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961-967.
3. Knotts RJ, Garan H. Cardiac arrhythmias in pregnancy. Semin Perinatol. 2014;38:285-288.
4. Silversides CK, Harris L, Haberer K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tacharrhythmias and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206-1212.
5. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e471-e505.
6. Di Biase L, Gianni C, Bagliani G, et. al. Arrhythmias involving the atrioventricular junction. Card Electrophysiol Clin. 2017;9:435-452.
7. Fitzpatrick RB. LactMed: drugs and lactation database. J Electron Resour Med Libr. 2007;4:155.
8. Yaksh A, van der Does LJ, Lanters EA, et al. Pharmacological therapy of tachyarrhythmias during pregnancy. Arrhythm Electrophysiol Rev. 2016;5:41-44.
9. US National Library of Medicine. Drugs and lactation database (LactMed). Available at: toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed July 3, 2018.
THE CASE
A 29-year-old G1P0 woman at 13 weeks’ gestation came in for a routine prenatal visit complaining of sudden-onset heart palpitations that were occurring about once a week. Each episode lasted between 15 and 60 minutes and was accompanied by chest tightness, with no identifiable cause. The patient could inconsistently terminate the episodes with Valsalva maneuvers. She reported having had 2 similar incidents of palpitations within the past year. Her family history was significant for sudden cardiac death of her father and paternal grandfather in their fifth decades of life.
A cardiovascular exam was normal; heart auscultation revealed a regular rate and rhythm without murmurs, rubs, or gallops, and the peripheral pulses were normal. A thyroid-stimulating hormone (TSH) level, basic metabolic panel (BMP), and complete blood count (CBC) were within normal limits. A transthoracic echocardiogram was negative for structural heart disease.
THE DIAGNOSIS
An initial Holter monitor study failed to capture an episode of her palpitations. The frequency of her palpitations increased as her pregnancy progressed, occurring almost daily by the second half of the third trimester, and a repeat Holter monitor study in the third trimester was significant for a 3-minute episode of supraventricular tachycardia (SVT) that correlated with patient-recorded symptoms (FIGURE).

Based on these results, we diagnosed the patient with an atrioventricular nodal reentry tachycardia (AVNRT). Although atrioventricular reciprocating tachycardia (AVRT) remained a remote possibility, it is far less common, and a 12-lead electrocardiogram (EKG) showed no evidence of pre-excitation.
DISCUSSION
AVNRT is the most common form of paroxysmal supraventricular tachycardia (PSVT). It occurs more frequently in women and typically manifests in the second to fourth decades of life.1 AVNRT is a narrow complex tachycardia characterized by a heart rate of 120 to >200 beats/min.
Hemodynamic changes in pregnancy can trigger arrhythmias
During pregnancy, hemodynamic changes (including increased blood volume and cardiac output) are thought to stimulate stretch-activated ion channels within the walls of the heart.2-4 Such changes may exacerbate previously existing cardiac arrhythmias or (less commonly) cause new-onset arrhythmias.3,4 A family history positive for arrhythmias or sudden cardiac death increases the likelihood of developing tachyarrhythmia during pregnancy.3 Women with a known history of PSVT might experience symptom exacerbation despite being on prophylactic therapy.4
Detection and diagnosis
While AVNRT is relatively benign in pregnancy, other cardiac arrhythmias (eg, atrial fibrillation/flutter, ventricular tachycardia) carry a greater risk for fetal and maternal complications, underscoring the need to correctly identify the type of arrhythmia.2,3
Continue to: Physical exam findings
Physical exam findings are often unremarkable unless the patient is actively experiencing SVT in the office, in which case prominent jugular pulsations may be seen due to simultaneous contraction of the atria and ventricles.
The initial evaluation of a pregnant patient presenting with tachycardia should include a BMP, TSH, 12-lead EKG, and transthoracic echocardiography.3,5 In most patients with AVNRT, the results of these tests will be normal. A Holter monitor can be used to document an arrhythmia if the episodes are relatively frequent or an event monitor can be used if the episodes are infrequent.5
EKG findings. When patients are actively experiencing SVT, EKG findings include a P wave obscured by the QRS complex, sometimes manifesting as a pseudo-R wave in the V1 lead and a pseudo-S wave in leads II, III, and AVF. The QRS complex is narrow and the R-R interval is regular.6
Types of treatment
Valsalva maneuvers. Treatment of AVNRT in pregnancy should first involve addressing any precipitating causes, including metabolic and endocrine abnormalities.3 As virtually all antiarrhythmic drugs cross the placenta and are traceable in breast milk,2,3 patients should be counseled to try to stop episodes using Valsalva maneuvers before moving to pharmacologic treatment.
Antiarrhythmics. First-line pharmacologic treatment for the prevention of AVNRT in pregnancy is metoprolol or verapamil.2,5 Neither drug has been associated with adverse outcomes in infants, although there is a large body of evidence suggesting that low levels of metoprolol are present in breast milk.7
Continue to: Acute episodes of SVT that are refractory to...
Acute episodes of SVT that are refractory to vagal maneuvers or occur despite medical management can be treated acutely in pregnancy with adenosine, which effectively stops episodes about 90% of the time.2 (See the TABLE8,9 for a list of antiarrhythmics that may be used to treat AVNRT.)

Catheter ablation is first-line treatment for AVNRT in nonpregnant patients.1,5 The risks of undergoing ablation during pregnancy include fetal exposure to radiation and anesthetic drugs.2,3 Therefore, this treatment should be used only when pharmacologic treatment is unsuccessful and risks to the mother and fetus due to the arrhythmia outweigh the risks of the procedure. Ablation can be offered postpartum as more definitive therapy.
Our patient was started on metoprolol tartrate 12.5 mg bid at 35 weeks’ gestation due to increasingly common and persistent palpitations. This helped control the episodes for 2 weeks, at which point they increased again in frequency. These were terminated using Valsalva maneuvers; increasing the metoprolol dosage was prohibitive due to patient intolerance.
Following an uncomplicated delivery, and discontinuation of metoprolol, the patient reported a decrease in both the number of episodes and the duration of SVT. Ultimately, she opted for a catheter ablation to prevent SVT exacerbation during subsequent pregnancies.
THE TAKEAWAY
AVNRT (and other tachyarrhythmias) may worsen or manifest with physiologic changes that occur during pregnancy. After establishing the diagnosis, effort should be made to manage the condition conservatively with Valsalva maneuvers and medication. Catheter ablation should be offered postpartum as a more definitive treatment option.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine Medical Director, 101 Heart Drive, Greenville, NC 27834; [email protected].
THE CASE
A 29-year-old G1P0 woman at 13 weeks’ gestation came in for a routine prenatal visit complaining of sudden-onset heart palpitations that were occurring about once a week. Each episode lasted between 15 and 60 minutes and was accompanied by chest tightness, with no identifiable cause. The patient could inconsistently terminate the episodes with Valsalva maneuvers. She reported having had 2 similar incidents of palpitations within the past year. Her family history was significant for sudden cardiac death of her father and paternal grandfather in their fifth decades of life.
A cardiovascular exam was normal; heart auscultation revealed a regular rate and rhythm without murmurs, rubs, or gallops, and the peripheral pulses were normal. A thyroid-stimulating hormone (TSH) level, basic metabolic panel (BMP), and complete blood count (CBC) were within normal limits. A transthoracic echocardiogram was negative for structural heart disease.
THE DIAGNOSIS
An initial Holter monitor study failed to capture an episode of her palpitations. The frequency of her palpitations increased as her pregnancy progressed, occurring almost daily by the second half of the third trimester, and a repeat Holter monitor study in the third trimester was significant for a 3-minute episode of supraventricular tachycardia (SVT) that correlated with patient-recorded symptoms (FIGURE).

Based on these results, we diagnosed the patient with an atrioventricular nodal reentry tachycardia (AVNRT). Although atrioventricular reciprocating tachycardia (AVRT) remained a remote possibility, it is far less common, and a 12-lead electrocardiogram (EKG) showed no evidence of pre-excitation.
DISCUSSION
AVNRT is the most common form of paroxysmal supraventricular tachycardia (PSVT). It occurs more frequently in women and typically manifests in the second to fourth decades of life.1 AVNRT is a narrow complex tachycardia characterized by a heart rate of 120 to >200 beats/min.
Hemodynamic changes in pregnancy can trigger arrhythmias
During pregnancy, hemodynamic changes (including increased blood volume and cardiac output) are thought to stimulate stretch-activated ion channels within the walls of the heart.2-4 Such changes may exacerbate previously existing cardiac arrhythmias or (less commonly) cause new-onset arrhythmias.3,4 A family history positive for arrhythmias or sudden cardiac death increases the likelihood of developing tachyarrhythmia during pregnancy.3 Women with a known history of PSVT might experience symptom exacerbation despite being on prophylactic therapy.4
Detection and diagnosis
While AVNRT is relatively benign in pregnancy, other cardiac arrhythmias (eg, atrial fibrillation/flutter, ventricular tachycardia) carry a greater risk for fetal and maternal complications, underscoring the need to correctly identify the type of arrhythmia.2,3
Continue to: Physical exam findings
Physical exam findings are often unremarkable unless the patient is actively experiencing SVT in the office, in which case prominent jugular pulsations may be seen due to simultaneous contraction of the atria and ventricles.
The initial evaluation of a pregnant patient presenting with tachycardia should include a BMP, TSH, 12-lead EKG, and transthoracic echocardiography.3,5 In most patients with AVNRT, the results of these tests will be normal. A Holter monitor can be used to document an arrhythmia if the episodes are relatively frequent or an event monitor can be used if the episodes are infrequent.5
EKG findings. When patients are actively experiencing SVT, EKG findings include a P wave obscured by the QRS complex, sometimes manifesting as a pseudo-R wave in the V1 lead and a pseudo-S wave in leads II, III, and AVF. The QRS complex is narrow and the R-R interval is regular.6
Types of treatment
Valsalva maneuvers. Treatment of AVNRT in pregnancy should first involve addressing any precipitating causes, including metabolic and endocrine abnormalities.3 As virtually all antiarrhythmic drugs cross the placenta and are traceable in breast milk,2,3 patients should be counseled to try to stop episodes using Valsalva maneuvers before moving to pharmacologic treatment.
Antiarrhythmics. First-line pharmacologic treatment for the prevention of AVNRT in pregnancy is metoprolol or verapamil.2,5 Neither drug has been associated with adverse outcomes in infants, although there is a large body of evidence suggesting that low levels of metoprolol are present in breast milk.7
Continue to: Acute episodes of SVT that are refractory to...
Acute episodes of SVT that are refractory to vagal maneuvers or occur despite medical management can be treated acutely in pregnancy with adenosine, which effectively stops episodes about 90% of the time.2 (See the TABLE8,9 for a list of antiarrhythmics that may be used to treat AVNRT.)

Catheter ablation is first-line treatment for AVNRT in nonpregnant patients.1,5 The risks of undergoing ablation during pregnancy include fetal exposure to radiation and anesthetic drugs.2,3 Therefore, this treatment should be used only when pharmacologic treatment is unsuccessful and risks to the mother and fetus due to the arrhythmia outweigh the risks of the procedure. Ablation can be offered postpartum as more definitive therapy.
Our patient was started on metoprolol tartrate 12.5 mg bid at 35 weeks’ gestation due to increasingly common and persistent palpitations. This helped control the episodes for 2 weeks, at which point they increased again in frequency. These were terminated using Valsalva maneuvers; increasing the metoprolol dosage was prohibitive due to patient intolerance.
Following an uncomplicated delivery, and discontinuation of metoprolol, the patient reported a decrease in both the number of episodes and the duration of SVT. Ultimately, she opted for a catheter ablation to prevent SVT exacerbation during subsequent pregnancies.
THE TAKEAWAY
AVNRT (and other tachyarrhythmias) may worsen or manifest with physiologic changes that occur during pregnancy. After establishing the diagnosis, effort should be made to manage the condition conservatively with Valsalva maneuvers and medication. Catheter ablation should be offered postpartum as a more definitive treatment option.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine Medical Director, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Kwaku KF, Josephson ME. Typical AVNRT—an update on mechanisms and therapy. Card Electrophysiol Rev. 2002;6:414-421.
2. Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961-967.
3. Knotts RJ, Garan H. Cardiac arrhythmias in pregnancy. Semin Perinatol. 2014;38:285-288.
4. Silversides CK, Harris L, Haberer K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tacharrhythmias and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206-1212.
5. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e471-e505.
6. Di Biase L, Gianni C, Bagliani G, et. al. Arrhythmias involving the atrioventricular junction. Card Electrophysiol Clin. 2017;9:435-452.
7. Fitzpatrick RB. LactMed: drugs and lactation database. J Electron Resour Med Libr. 2007;4:155.
8. Yaksh A, van der Does LJ, Lanters EA, et al. Pharmacological therapy of tachyarrhythmias during pregnancy. Arrhythm Electrophysiol Rev. 2016;5:41-44.
9. US National Library of Medicine. Drugs and lactation database (LactMed). Available at: toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed July 3, 2018.
1. Kwaku KF, Josephson ME. Typical AVNRT—an update on mechanisms and therapy. Card Electrophysiol Rev. 2002;6:414-421.
2. Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961-967.
3. Knotts RJ, Garan H. Cardiac arrhythmias in pregnancy. Semin Perinatol. 2014;38:285-288.
4. Silversides CK, Harris L, Haberer K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tacharrhythmias and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206-1212.
5. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e471-e505.
6. Di Biase L, Gianni C, Bagliani G, et. al. Arrhythmias involving the atrioventricular junction. Card Electrophysiol Clin. 2017;9:435-452.
7. Fitzpatrick RB. LactMed: drugs and lactation database. J Electron Resour Med Libr. 2007;4:155.
8. Yaksh A, van der Does LJ, Lanters EA, et al. Pharmacological therapy of tachyarrhythmias during pregnancy. Arrhythm Electrophysiol Rev. 2016;5:41-44.
9. US National Library of Medicine. Drugs and lactation database (LactMed). Available at: toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed July 3, 2018.
Anterolateral hip pain • no specific injury • Dx?
THE CASE
A 22-year-old man presented to our family medicine clinic with hip pain of 2 weeks’ duration. The patient played hockey around the time of onset, but denied any specific injury. The pain, which affected the anterolateral aspect of the patient’s right hip, first started when he stood up after eating a meal. He rated the pain as an 8/10 on average and said that it was worse with movement. The patient had not shown improvement with conservative therapy (rest, ice, and ibuprofen). His medical and surgi
The physical exam revealed pain on active flexion and abduction of the hip. Passive range of motion (ROM) was negative for pain. The right hip was grossly normal with no pain on palpation or crepitus. There was no associated muscle tenderness. The patient was advised to continue to rest and ice the hip, as well as to take ibuprofen for pain relief. He was referred to Physical Therapy.
He returned to our clinic 4 weeks later with no improvement in his symptoms despite several sessions of physical therapy. We ordered radiographic images and magnetic resonance imaging (MRI) of the right hip.
THE DIAGNOSIS
Plain films (FIGURE 1A) showed bilateral avascular necrosis (AVN) of the femoral heads, which was worse on the right side than the left. An MRI (FIGURE 1B) further supported this diagnosis, revealing changes in the femoral neck consistent with a stress reaction and no significant collapse of the femoral head.
DISCUSSION
AVN of the hip has an incidence ranging from 10,000 to 20,000 new cases annually.1,2 It has many possible causes, including trauma, systemic lupus erythematosus, glucocorticoid use, and chronic excessive alcohol use. Although the underlying pathophysiology varies, experts hypothesize that most cases are caused by a disruption of the blood supply, which leads to hyperemia and cortical destruction and collapse.1,2
Certain medications can cause AVN
A more thorough history-taking at this patient’s initial visit would have prompted imaging at that time and ensured that the standard of care was met. Upon further investigation at his follow-up appointment, it was discovered that he had been diagnosed with acute pre-B cell lymphoblastic leukemia (ALL) 2 years earlier and had undergone chemotherapy with cytarabine, vincristine, L-asparaginase, daunorubicin, methotrexate, and glucocorticoids. This discovery, along with the lack of symptom improvement, prompted the ordering of his imaging studies. Long-term glucocorticoid therapy is the second leading cause of AVN, following traumatic events.3 High daily dosages (>40 mg/d) and high cumulative dosages of glucocorticoids are associated with a significantly increased risk for AVN.4,5
The other chemotherapy agents with which our patient had been treated (cytarabine, vincristine, L-asparaginase, daunorubicin, and methotrexate) have no reported links to AVN. When mentioned in the literature, however, they are usually coupled with the use of dexamethasone or prednisone.
Continue to: One case report described a patient with...
One case report described a patient with acute promyelocytic leukemia who was treated with all-transretinoic acid, daunomycin, cytarabine, and a short course of dexamethasone, and was diagnosed with AVN 2 years after the cessation of chemotherapy.6 This demonstrates that steroid use does not need to be recent to have a contributory effect.
Did leukemic burden play a role?
We also considered whether the patient’s leukemic burden contributed to his osteonecrosis. Leukemia and its therapy regimens have been reported to cause cerebrovascular complications,7 so it would be logical to postulate that they might also pose a risk to the vasculature of the femoral head. One case report describes hip pain and AVN as the initial manifestation of chronic myeloid leukemia (CML).8 But CML is more often associated with a severely increased white blood cell (WBC) count than is ALL, and our patient’s WBC count was in the expected range for a patient in the maintenance phase of chemotherapy, making leukemic burden a less likely culprit.
Know your patient’s history
Our patient had received an initial dose of approximately 120 mg/d prednisone alone during the first 28 days of his induction therapy for ALL. In addition, he received dexamethasone maintenance therapy, which can accumulate to >140 mg/m2 over the course of therapy.9 This information was ultimately integral to his diagnosis and treatment.
Our patient was referred to Orthopedics. He underwent therapy with alendronate and did not require surgical intervention.
THE TAKEAWAY
This case illustrates the importance of obtaining a thorough medical history, including previous drug exposures, as a means to raise or lower one’s index of suspicion appropriately.
CORRESPONDENCE
Patrick Basile, 7124 Bristol Boulevard, Edina, MN 55435; [email protected].
1. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250-261.
2. Vail TP, Covington DB. The incidence of osteonecrosis. In: Urbaniak JR, Jones JR, eds. Osteonecrosis: Etiology, Diagnosis, Treatment. Rosemont, IL: American Academy of Orthopedic Surgeons;1997:43-49.
3. Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183-190.
4. Shigemura T, Nakamura J, Kishida S, et al. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study. Rheumatology (Oxford). 2011;50:2023-2028.
5. Salem KH, Brockert AK, Mertens R, et al. Avascular necrosis after chemotherapy for haematological malignancy in childhood. Bone Joint J. 2013;95-B:1708-1713.
6. Abhyankar D, Nair R, Menon H, et al. Avascular necrosis of head of femur in a patient with acute promyelocytic leukemia. Leuk Lymphoma. 2000;37:635-637.
7. Muñiz AE. Myocardial infarction and stroke as the presenting symptoms of acute myeloid leukemia. J Emerg Med. 2012;42:651-654.
8. Gupta D, Gaiha M, Siddaraju N, et al. Chronic myeloid leukemia presenting with avascular necrosis of femur head. J Assoc Physicians, India. 2003;51:214-215.
9. Hunger SP, Loh ML, Whitlock JA, et al. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:957-963.
THE CASE
A 22-year-old man presented to our family medicine clinic with hip pain of 2 weeks’ duration. The patient played hockey around the time of onset, but denied any specific injury. The pain, which affected the anterolateral aspect of the patient’s right hip, first started when he stood up after eating a meal. He rated the pain as an 8/10 on average and said that it was worse with movement. The patient had not shown improvement with conservative therapy (rest, ice, and ibuprofen). His medical and surgi
The physical exam revealed pain on active flexion and abduction of the hip. Passive range of motion (ROM) was negative for pain. The right hip was grossly normal with no pain on palpation or crepitus. There was no associated muscle tenderness. The patient was advised to continue to rest and ice the hip, as well as to take ibuprofen for pain relief. He was referred to Physical Therapy.
He returned to our clinic 4 weeks later with no improvement in his symptoms despite several sessions of physical therapy. We ordered radiographic images and magnetic resonance imaging (MRI) of the right hip.
THE DIAGNOSIS
Plain films (FIGURE 1A) showed bilateral avascular necrosis (AVN) of the femoral heads, which was worse on the right side than the left. An MRI (FIGURE 1B) further supported this diagnosis, revealing changes in the femoral neck consistent with a stress reaction and no significant collapse of the femoral head.

DISCUSSION
AVN of the hip has an incidence ranging from 10,000 to 20,000 new cases annually.1,2 It has many possible causes, including trauma, systemic lupus erythematosus, glucocorticoid use, and chronic excessive alcohol use. Although the underlying pathophysiology varies, experts hypothesize that most cases are caused by a disruption of the blood supply, which leads to hyperemia and cortical destruction and collapse.1,2
Certain medications can cause AVN
A more thorough history-taking at this patient’s initial visit would have prompted imaging at that time and ensured that the standard of care was met. Upon further investigation at his follow-up appointment, it was discovered that he had been diagnosed with acute pre-B cell lymphoblastic leukemia (ALL) 2 years earlier and had undergone chemotherapy with cytarabine, vincristine, L-asparaginase, daunorubicin, methotrexate, and glucocorticoids. This discovery, along with the lack of symptom improvement, prompted the ordering of his imaging studies. Long-term glucocorticoid therapy is the second leading cause of AVN, following traumatic events.3 High daily dosages (>40 mg/d) and high cumulative dosages of glucocorticoids are associated with a significantly increased risk for AVN.4,5
The other chemotherapy agents with which our patient had been treated (cytarabine, vincristine, L-asparaginase, daunorubicin, and methotrexate) have no reported links to AVN. When mentioned in the literature, however, they are usually coupled with the use of dexamethasone or prednisone.
Continue to: One case report described a patient with...
One case report described a patient with acute promyelocytic leukemia who was treated with all-transretinoic acid, daunomycin, cytarabine, and a short course of dexamethasone, and was diagnosed with AVN 2 years after the cessation of chemotherapy.6 This demonstrates that steroid use does not need to be recent to have a contributory effect.
Did leukemic burden play a role?
We also considered whether the patient’s leukemic burden contributed to his osteonecrosis. Leukemia and its therapy regimens have been reported to cause cerebrovascular complications,7 so it would be logical to postulate that they might also pose a risk to the vasculature of the femoral head. One case report describes hip pain and AVN as the initial manifestation of chronic myeloid leukemia (CML).8 But CML is more often associated with a severely increased white blood cell (WBC) count than is ALL, and our patient’s WBC count was in the expected range for a patient in the maintenance phase of chemotherapy, making leukemic burden a less likely culprit.
Know your patient’s history
Our patient had received an initial dose of approximately 120 mg/d prednisone alone during the first 28 days of his induction therapy for ALL. In addition, he received dexamethasone maintenance therapy, which can accumulate to >140 mg/m2 over the course of therapy.9 This information was ultimately integral to his diagnosis and treatment.
Our patient was referred to Orthopedics. He underwent therapy with alendronate and did not require surgical intervention.
THE TAKEAWAY
This case illustrates the importance of obtaining a thorough medical history, including previous drug exposures, as a means to raise or lower one’s index of suspicion appropriately.
CORRESPONDENCE
Patrick Basile, 7124 Bristol Boulevard, Edina, MN 55435; [email protected].
THE CASE
A 22-year-old man presented to our family medicine clinic with hip pain of 2 weeks’ duration. The patient played hockey around the time of onset, but denied any specific injury. The pain, which affected the anterolateral aspect of the patient’s right hip, first started when he stood up after eating a meal. He rated the pain as an 8/10 on average and said that it was worse with movement. The patient had not shown improvement with conservative therapy (rest, ice, and ibuprofen). His medical and surgi
The physical exam revealed pain on active flexion and abduction of the hip. Passive range of motion (ROM) was negative for pain. The right hip was grossly normal with no pain on palpation or crepitus. There was no associated muscle tenderness. The patient was advised to continue to rest and ice the hip, as well as to take ibuprofen for pain relief. He was referred to Physical Therapy.
He returned to our clinic 4 weeks later with no improvement in his symptoms despite several sessions of physical therapy. We ordered radiographic images and magnetic resonance imaging (MRI) of the right hip.
THE DIAGNOSIS
Plain films (FIGURE 1A) showed bilateral avascular necrosis (AVN) of the femoral heads, which was worse on the right side than the left. An MRI (FIGURE 1B) further supported this diagnosis, revealing changes in the femoral neck consistent with a stress reaction and no significant collapse of the femoral head.

DISCUSSION
AVN of the hip has an incidence ranging from 10,000 to 20,000 new cases annually.1,2 It has many possible causes, including trauma, systemic lupus erythematosus, glucocorticoid use, and chronic excessive alcohol use. Although the underlying pathophysiology varies, experts hypothesize that most cases are caused by a disruption of the blood supply, which leads to hyperemia and cortical destruction and collapse.1,2
Certain medications can cause AVN
A more thorough history-taking at this patient’s initial visit would have prompted imaging at that time and ensured that the standard of care was met. Upon further investigation at his follow-up appointment, it was discovered that he had been diagnosed with acute pre-B cell lymphoblastic leukemia (ALL) 2 years earlier and had undergone chemotherapy with cytarabine, vincristine, L-asparaginase, daunorubicin, methotrexate, and glucocorticoids. This discovery, along with the lack of symptom improvement, prompted the ordering of his imaging studies. Long-term glucocorticoid therapy is the second leading cause of AVN, following traumatic events.3 High daily dosages (>40 mg/d) and high cumulative dosages of glucocorticoids are associated with a significantly increased risk for AVN.4,5
The other chemotherapy agents with which our patient had been treated (cytarabine, vincristine, L-asparaginase, daunorubicin, and methotrexate) have no reported links to AVN. When mentioned in the literature, however, they are usually coupled with the use of dexamethasone or prednisone.
Continue to: One case report described a patient with...
One case report described a patient with acute promyelocytic leukemia who was treated with all-transretinoic acid, daunomycin, cytarabine, and a short course of dexamethasone, and was diagnosed with AVN 2 years after the cessation of chemotherapy.6 This demonstrates that steroid use does not need to be recent to have a contributory effect.
Did leukemic burden play a role?
We also considered whether the patient’s leukemic burden contributed to his osteonecrosis. Leukemia and its therapy regimens have been reported to cause cerebrovascular complications,7 so it would be logical to postulate that they might also pose a risk to the vasculature of the femoral head. One case report describes hip pain and AVN as the initial manifestation of chronic myeloid leukemia (CML).8 But CML is more often associated with a severely increased white blood cell (WBC) count than is ALL, and our patient’s WBC count was in the expected range for a patient in the maintenance phase of chemotherapy, making leukemic burden a less likely culprit.
Know your patient’s history
Our patient had received an initial dose of approximately 120 mg/d prednisone alone during the first 28 days of his induction therapy for ALL. In addition, he received dexamethasone maintenance therapy, which can accumulate to >140 mg/m2 over the course of therapy.9 This information was ultimately integral to his diagnosis and treatment.
Our patient was referred to Orthopedics. He underwent therapy with alendronate and did not require surgical intervention.
THE TAKEAWAY
This case illustrates the importance of obtaining a thorough medical history, including previous drug exposures, as a means to raise or lower one’s index of suspicion appropriately.
CORRESPONDENCE
Patrick Basile, 7124 Bristol Boulevard, Edina, MN 55435; [email protected].
1. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250-261.
2. Vail TP, Covington DB. The incidence of osteonecrosis. In: Urbaniak JR, Jones JR, eds. Osteonecrosis: Etiology, Diagnosis, Treatment. Rosemont, IL: American Academy of Orthopedic Surgeons;1997:43-49.
3. Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183-190.
4. Shigemura T, Nakamura J, Kishida S, et al. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study. Rheumatology (Oxford). 2011;50:2023-2028.
5. Salem KH, Brockert AK, Mertens R, et al. Avascular necrosis after chemotherapy for haematological malignancy in childhood. Bone Joint J. 2013;95-B:1708-1713.
6. Abhyankar D, Nair R, Menon H, et al. Avascular necrosis of head of femur in a patient with acute promyelocytic leukemia. Leuk Lymphoma. 2000;37:635-637.
7. Muñiz AE. Myocardial infarction and stroke as the presenting symptoms of acute myeloid leukemia. J Emerg Med. 2012;42:651-654.
8. Gupta D, Gaiha M, Siddaraju N, et al. Chronic myeloid leukemia presenting with avascular necrosis of femur head. J Assoc Physicians, India. 2003;51:214-215.
9. Hunger SP, Loh ML, Whitlock JA, et al. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:957-963.
1. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250-261.
2. Vail TP, Covington DB. The incidence of osteonecrosis. In: Urbaniak JR, Jones JR, eds. Osteonecrosis: Etiology, Diagnosis, Treatment. Rosemont, IL: American Academy of Orthopedic Surgeons;1997:43-49.
3. Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183-190.
4. Shigemura T, Nakamura J, Kishida S, et al. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study. Rheumatology (Oxford). 2011;50:2023-2028.
5. Salem KH, Brockert AK, Mertens R, et al. Avascular necrosis after chemotherapy for haematological malignancy in childhood. Bone Joint J. 2013;95-B:1708-1713.
6. Abhyankar D, Nair R, Menon H, et al. Avascular necrosis of head of femur in a patient with acute promyelocytic leukemia. Leuk Lymphoma. 2000;37:635-637.
7. Muñiz AE. Myocardial infarction and stroke as the presenting symptoms of acute myeloid leukemia. J Emerg Med. 2012;42:651-654.
8. Gupta D, Gaiha M, Siddaraju N, et al. Chronic myeloid leukemia presenting with avascular necrosis of femur head. J Assoc Physicians, India. 2003;51:214-215.
9. Hunger SP, Loh ML, Whitlock JA, et al. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:957-963.
The Aberrant Anterior Tibial Artery and its Surgical Risk
ABSTRACT
Vascular injury to the popliteal artery during knee surgery is uncommon, but it has significant consequences not only for the patient but also to the surgeon since it poses the threat of malpractice litigation. The vascular anatomy of the lower extremity is variable especially when it involves both the popliteal artery and its branches. An aberrant vascular course may increase the risk of iatrogenic vascular injury during surgery. Careful preoperative planning with advanced imaging can decrease the risk of a devastating vascular injury.
Continue to: Most non-traumatic injuries...
Most non-traumatic injuries to the popliteal artery are iatrogenic and may occur during total knee replacement,1-8 high tibial osteotomy,2,3,5-7 anterior cruciate ligament reconstruction,2,6 posterior cruciate ligament reconstruction,2,6,9,10 and arthroscopic meniscectomy.2,6,9 Despite the rare occurrence of complications involving the popliteal artery during such procedures, results of vessel injuries can be devastating and may also lead to malpractice litigation. Anatomic variations of the distal popliteal artery and its significance in surgery have been well documented in the literature.2-6,8,11 However, due to lack of awareness, this issue is often unintentionally disregarded. We present the case of an aberrant anterior tibial artery that was found during the review of a magnetic resonance imaging study. The patient was provided written informed consent for print and electronic publication of this case report.
CASE
A 61-year-old woman presented with a history of right knee pain from osteoarthritis that had rapidly progressed over 1 week secondary to a fall. The patient had no history of previous knee surgery. After careful evaluation of her right knee pain, treatment options were discussed. The patient agreed to proceed with total knee arthroplasty (TKA). During preoperative planning, the patient’s previous magnetic resonance imaging (MRI) was reviewed. The MRI study revealed an aberrant anterior tibial artery. The popliteal artery bifurcated at the level of the knee joint (Figures 1A-1C). After the bifurcation, the anterior tibial artery coursed anteriorly to the tibioperoneal trunk. The anterior tibial artery is seen just anterior to the popliteus muscle and just posterior to the tibial plateau cortex (Figure 2). Intraoperatively, an oscillating saw was utilized for the tibial cut. Care was taken not to penetrate the posterior cortex. An osteotome was used to elevate the tibial cut and hinge it open, and with a small mallet, finish the tibial cut. The patient had a successful TKA without complication.
DISCUSSION
Emerging from the adductor hiatus (Hunter’s canal), the normal course of the popliteal artery is a position slightly lateral in the intercondylar fossa. It courses obliquely and posteriorly to the popliteus then bifurcates into the anterior tibial artery and the tibioperoneal trunk at the inferior border of the popliteus. The tibioperoneal trunk bifurcates into both the posterior tibial artery and the peroneal artery at the proximal tibia well below the knee joint.
There are many reported cases of popliteal artery variations.2,3,6,7,9,11-13 Variations in the popliteal artery are consequences of persistent embryonic vessels from primitive segments of the artery or abnormal fusions among them.14 According to Kim and colleagues,11 variations can be classified by the modified Lippert’s system. This system has 3 categories with 3 subtypes (Table). Variations are not uncommon and occur in 7.4% to 12% of the population.2,4,5,7,13
Table. Modified Lippert’s System11
Category (Subtype) |
|
I | Normal level of popliteal arterial branching |
IA | Usual pattern |
IB | Trifurcation- No true tibioperoneal trunk |
IC | Anterior tibioperoneal trunk- Posterior tibial artery is first branch |
II | High division of popliteal artery |
IIA | Anterior tibial artery arises at or above the knee joint |
IIB | Posterior tibial artery arises at or above the knee joint |
IIC | Peroneal artery arises at or above the knee joint |
III | Hypoplastic or aplastic branching with altered distal supply |
IIIA | Hypoplastic-aplastic posterior tibial artery |
IIIB | Hypoplastic-aplastic anterior tibial artery |
IIIC | Hypoplastic-aplastic posterior and anterior tibial artery |
Of these variations, type IIA, a high bifurcation of the anterior tibial artery, arising at or above the knee joint from the popliteal artery is the most significant. Forty-two percent of these vessels course anterior to the popliteus and make direct contact with the cortex of the posterior tibia.4 It is also the most frequent variant type reported in 1.2% to 6% of the population.3,7,11-13
Continue to: Injury to the popliteal artery...
Injury to the popliteal artery during an orthopedic procedure is believed to be under reported6 but is considered a rare complication. The incidence of popliteal artery injury in TKA is thought to be 0.03% to 0.2%.1,2,5,7,8 Vessel injury in both high tibial osteotomy and arthroscopic surgeries (lateral meniscal repair) have also been reported.5,6,8,10 Despite the rare occurrence of this complication, it may have devastating outcomes. The injury can be repaired with vascular grafting depending on its severity; however, it could also lead to compartment syndrome, loss of function, chronic ulcers, and necrosis of the affected limb resulting in below the knee amputation. The current consensus is that the popliteal artery moves posteriorly away from the tibia when the knee is in 90° of flexion,5 which is the standard position for many orthopedic knee surgeries. This position limits the risk of injuring the vessel. However, Metzdorf and colleagues,4 Smith and colleagues,6 and Zaidi and colleagues8 suggested that the vessel not be displaced posteriorly with flexion. These studies reported that the behavior of the popliteal artery varied among individuals since in some cases it had moved closer to the tibia in flexion when compared with extension.
Regardless of the behavior of the artery, it is protected by the popliteus muscle in most orthopedic knee surgeries since the majority course posterior to the muscle. However, in cases of Lippert’s type IIA variation, it not only loses protection as it courses beneath the popliteus but also is extremely vulnerable from the close relationship to the posterior tibial cortex. Klecker and colleagues2 described the aberrant artery locations related to common orthopedic procedures, which demonstrated its close proximity to various surgical plane levels. The position of the aberrant artery is approximately 1 to 1.5 cm distal to the posterior tibial joint line, just posterior to the posterior capsule, and close to the posterior cruciate ligament insertion site where the transverse tibial cut is made during TKA. This location also corresponds to the position for an inlay block and the tibial tunnel for posterior cruciate ligament reconstruction. A transverse cut for a high tibial osteotomy is approximately 1.5 to 2.5 cm distal to the posterior tibial joint line; the aberrant artery appeared directly posterior to the tibial cortex. These relationships were equivalent findings in this case. Such relationships of the aberrant anterior tibial artery to both the posterior tibial cortex and the posterior capsule increase the risk of vessel (anterior tibial artery) injury intraoperatively. The risk further increases in a revision of total knee replacement. This is secondary to limited flexibility of the vessel from scar formation which requires a more distal incision.1,4
CONCLUSION
Vascular injuries in knee surgeries are rare and often overlooked. Despite their low occurrence rate, outcomes of these injuries have grave consequences not only regarding medical but also legal matters. Variations in the popliteal artery are not uncommon and could potentially contribute to risks of vessel injury. Of these variations, the high originating anterior tibial artery poses a special risk. However, due to the low occurrence rate of these injuries, screening the general population may not be cost-effective. Since many patients already have obtained necessary imaging (preferably MRI), a careful review of these images along with preoperative planning and special care during surgery is recommended to identify popliteal artery variants and avoid iatrogenic vascular injury.
This paper will be judged for the Resident Writer’s Award.
- Abdel Karim MM, Anbar A, Keenan J. Position of the popliteal artery in revision total knee arthroplasty. Arch Orthop Trauma Surg. 2012;132(6):861-865. doi:10.1007/s00402-012-1479-6.
- Klecker RJ, Winalski CS, Aliabadi P, Minas T. The aberrant anterior tibial artery, magnetic resonance appearance, prevalence, and surgical implication. Am J Sports Medicine. 2008;36:720-727.
- Kropman RHJ, Kiela G, Moll FL, Vries JPM. Variations in anatomy of the popliteal artery and its side branches. Vasc Endovascular Surg. 2011;45:536-540.
- Metzdorf A, Jakob RP, Petropoulos P, Middleton R. Arterial injury during revision total knee replacement. A case report. Knee Surg Sports Traumatol Arthrosc. 1999;7:246-248.
- Shetty AA, Tindall AJ, Qureshi F, Divekar M, Fernando KWK. The Effect of knee flexion on the popliteal artery and its surgical significance. J Bone Joint Surg Br. 2003;85:218-222.
- Smith PN, Gelinas J, Kennedy K, Thain L, Rorabeck CH, Bourne B. Popliteal vessels in knee surgery; a magnetic resonance imaging study. Clin Orthop Rel Res. 1999;367:158-164
- Tindall AJ, Shetty AA, James KD, Middleton A, Fernando KWK. Prevalence and surgical significance of a high-origin anterior tibial artery. J Orthop Surg. 2006;14:13-16.
- Zaidi SHA, Cobb AG, Bentley G. Danger to the popliteal artery in high tibial osteotomy. J Bone Joint Surg Br. 1995;77:384-386.
- Keser S, Savranlar A, Bayar A, Ulukent SC, Ozer T, Tuncay I. Anatomic localization of the popliteal artery at the level of the knee joint: a magnetic resonance imaging study. Arthroscopy. 2006;22:656-659.
- Makino A, Costa-Paz M, Aponte-Tinao L, Ayerza MA, Muscolo L. Popliteal artery laceration during arthroscopic posterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1396.
- Kim D, Orron DE, Skillman JJ. Surgical significance of popliteal arterial variants, a unified angiographic classification. Ann Surg. 1989;210:776-781.
- Day CP, Orme R. Popliteal artery branching patterns-an angiographic study. Clin Radiol. 2006;61:696-699.
- Kil SW, Jung GS. Anatomical variations of the popliteal artery and its tibial branches: Analysis in 1242 extremities. Cardiovasc Intervent Radiol. 2009;32:233-240.
- Senior HD. The development of the arteries of the human lower extremity. Am J Anat. 1919;25:55-94.
ABSTRACT
Vascular injury to the popliteal artery during knee surgery is uncommon, but it has significant consequences not only for the patient but also to the surgeon since it poses the threat of malpractice litigation. The vascular anatomy of the lower extremity is variable especially when it involves both the popliteal artery and its branches. An aberrant vascular course may increase the risk of iatrogenic vascular injury during surgery. Careful preoperative planning with advanced imaging can decrease the risk of a devastating vascular injury.
Continue to: Most non-traumatic injuries...
Most non-traumatic injuries to the popliteal artery are iatrogenic and may occur during total knee replacement,1-8 high tibial osteotomy,2,3,5-7 anterior cruciate ligament reconstruction,2,6 posterior cruciate ligament reconstruction,2,6,9,10 and arthroscopic meniscectomy.2,6,9 Despite the rare occurrence of complications involving the popliteal artery during such procedures, results of vessel injuries can be devastating and may also lead to malpractice litigation. Anatomic variations of the distal popliteal artery and its significance in surgery have been well documented in the literature.2-6,8,11 However, due to lack of awareness, this issue is often unintentionally disregarded. We present the case of an aberrant anterior tibial artery that was found during the review of a magnetic resonance imaging study. The patient was provided written informed consent for print and electronic publication of this case report.
CASE
A 61-year-old woman presented with a history of right knee pain from osteoarthritis that had rapidly progressed over 1 week secondary to a fall. The patient had no history of previous knee surgery. After careful evaluation of her right knee pain, treatment options were discussed. The patient agreed to proceed with total knee arthroplasty (TKA). During preoperative planning, the patient’s previous magnetic resonance imaging (MRI) was reviewed. The MRI study revealed an aberrant anterior tibial artery. The popliteal artery bifurcated at the level of the knee joint (Figures 1A-1C). After the bifurcation, the anterior tibial artery coursed anteriorly to the tibioperoneal trunk. The anterior tibial artery is seen just anterior to the popliteus muscle and just posterior to the tibial plateau cortex (Figure 2). Intraoperatively, an oscillating saw was utilized for the tibial cut. Care was taken not to penetrate the posterior cortex. An osteotome was used to elevate the tibial cut and hinge it open, and with a small mallet, finish the tibial cut. The patient had a successful TKA without complication.


DISCUSSION
Emerging from the adductor hiatus (Hunter’s canal), the normal course of the popliteal artery is a position slightly lateral in the intercondylar fossa. It courses obliquely and posteriorly to the popliteus then bifurcates into the anterior tibial artery and the tibioperoneal trunk at the inferior border of the popliteus. The tibioperoneal trunk bifurcates into both the posterior tibial artery and the peroneal artery at the proximal tibia well below the knee joint.
There are many reported cases of popliteal artery variations.2,3,6,7,9,11-13 Variations in the popliteal artery are consequences of persistent embryonic vessels from primitive segments of the artery or abnormal fusions among them.14 According to Kim and colleagues,11 variations can be classified by the modified Lippert’s system. This system has 3 categories with 3 subtypes (Table). Variations are not uncommon and occur in 7.4% to 12% of the population.2,4,5,7,13
Table. Modified Lippert’s System11
Category (Subtype) |
|
I | Normal level of popliteal arterial branching |
IA | Usual pattern |
IB | Trifurcation- No true tibioperoneal trunk |
IC | Anterior tibioperoneal trunk- Posterior tibial artery is first branch |
II | High division of popliteal artery |
IIA | Anterior tibial artery arises at or above the knee joint |
IIB | Posterior tibial artery arises at or above the knee joint |
IIC | Peroneal artery arises at or above the knee joint |
III | Hypoplastic or aplastic branching with altered distal supply |
IIIA | Hypoplastic-aplastic posterior tibial artery |
IIIB | Hypoplastic-aplastic anterior tibial artery |
IIIC | Hypoplastic-aplastic posterior and anterior tibial artery |
Of these variations, type IIA, a high bifurcation of the anterior tibial artery, arising at or above the knee joint from the popliteal artery is the most significant. Forty-two percent of these vessels course anterior to the popliteus and make direct contact with the cortex of the posterior tibia.4 It is also the most frequent variant type reported in 1.2% to 6% of the population.3,7,11-13
Continue to: Injury to the popliteal artery...
Injury to the popliteal artery during an orthopedic procedure is believed to be under reported6 but is considered a rare complication. The incidence of popliteal artery injury in TKA is thought to be 0.03% to 0.2%.1,2,5,7,8 Vessel injury in both high tibial osteotomy and arthroscopic surgeries (lateral meniscal repair) have also been reported.5,6,8,10 Despite the rare occurrence of this complication, it may have devastating outcomes. The injury can be repaired with vascular grafting depending on its severity; however, it could also lead to compartment syndrome, loss of function, chronic ulcers, and necrosis of the affected limb resulting in below the knee amputation. The current consensus is that the popliteal artery moves posteriorly away from the tibia when the knee is in 90° of flexion,5 which is the standard position for many orthopedic knee surgeries. This position limits the risk of injuring the vessel. However, Metzdorf and colleagues,4 Smith and colleagues,6 and Zaidi and colleagues8 suggested that the vessel not be displaced posteriorly with flexion. These studies reported that the behavior of the popliteal artery varied among individuals since in some cases it had moved closer to the tibia in flexion when compared with extension.
Regardless of the behavior of the artery, it is protected by the popliteus muscle in most orthopedic knee surgeries since the majority course posterior to the muscle. However, in cases of Lippert’s type IIA variation, it not only loses protection as it courses beneath the popliteus but also is extremely vulnerable from the close relationship to the posterior tibial cortex. Klecker and colleagues2 described the aberrant artery locations related to common orthopedic procedures, which demonstrated its close proximity to various surgical plane levels. The position of the aberrant artery is approximately 1 to 1.5 cm distal to the posterior tibial joint line, just posterior to the posterior capsule, and close to the posterior cruciate ligament insertion site where the transverse tibial cut is made during TKA. This location also corresponds to the position for an inlay block and the tibial tunnel for posterior cruciate ligament reconstruction. A transverse cut for a high tibial osteotomy is approximately 1.5 to 2.5 cm distal to the posterior tibial joint line; the aberrant artery appeared directly posterior to the tibial cortex. These relationships were equivalent findings in this case. Such relationships of the aberrant anterior tibial artery to both the posterior tibial cortex and the posterior capsule increase the risk of vessel (anterior tibial artery) injury intraoperatively. The risk further increases in a revision of total knee replacement. This is secondary to limited flexibility of the vessel from scar formation which requires a more distal incision.1,4
CONCLUSION
Vascular injuries in knee surgeries are rare and often overlooked. Despite their low occurrence rate, outcomes of these injuries have grave consequences not only regarding medical but also legal matters. Variations in the popliteal artery are not uncommon and could potentially contribute to risks of vessel injury. Of these variations, the high originating anterior tibial artery poses a special risk. However, due to the low occurrence rate of these injuries, screening the general population may not be cost-effective. Since many patients already have obtained necessary imaging (preferably MRI), a careful review of these images along with preoperative planning and special care during surgery is recommended to identify popliteal artery variants and avoid iatrogenic vascular injury.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Vascular injury to the popliteal artery during knee surgery is uncommon, but it has significant consequences not only for the patient but also to the surgeon since it poses the threat of malpractice litigation. The vascular anatomy of the lower extremity is variable especially when it involves both the popliteal artery and its branches. An aberrant vascular course may increase the risk of iatrogenic vascular injury during surgery. Careful preoperative planning with advanced imaging can decrease the risk of a devastating vascular injury.
Continue to: Most non-traumatic injuries...
Most non-traumatic injuries to the popliteal artery are iatrogenic and may occur during total knee replacement,1-8 high tibial osteotomy,2,3,5-7 anterior cruciate ligament reconstruction,2,6 posterior cruciate ligament reconstruction,2,6,9,10 and arthroscopic meniscectomy.2,6,9 Despite the rare occurrence of complications involving the popliteal artery during such procedures, results of vessel injuries can be devastating and may also lead to malpractice litigation. Anatomic variations of the distal popliteal artery and its significance in surgery have been well documented in the literature.2-6,8,11 However, due to lack of awareness, this issue is often unintentionally disregarded. We present the case of an aberrant anterior tibial artery that was found during the review of a magnetic resonance imaging study. The patient was provided written informed consent for print and electronic publication of this case report.
CASE
A 61-year-old woman presented with a history of right knee pain from osteoarthritis that had rapidly progressed over 1 week secondary to a fall. The patient had no history of previous knee surgery. After careful evaluation of her right knee pain, treatment options were discussed. The patient agreed to proceed with total knee arthroplasty (TKA). During preoperative planning, the patient’s previous magnetic resonance imaging (MRI) was reviewed. The MRI study revealed an aberrant anterior tibial artery. The popliteal artery bifurcated at the level of the knee joint (Figures 1A-1C). After the bifurcation, the anterior tibial artery coursed anteriorly to the tibioperoneal trunk. The anterior tibial artery is seen just anterior to the popliteus muscle and just posterior to the tibial plateau cortex (Figure 2). Intraoperatively, an oscillating saw was utilized for the tibial cut. Care was taken not to penetrate the posterior cortex. An osteotome was used to elevate the tibial cut and hinge it open, and with a small mallet, finish the tibial cut. The patient had a successful TKA without complication.


DISCUSSION
Emerging from the adductor hiatus (Hunter’s canal), the normal course of the popliteal artery is a position slightly lateral in the intercondylar fossa. It courses obliquely and posteriorly to the popliteus then bifurcates into the anterior tibial artery and the tibioperoneal trunk at the inferior border of the popliteus. The tibioperoneal trunk bifurcates into both the posterior tibial artery and the peroneal artery at the proximal tibia well below the knee joint.
There are many reported cases of popliteal artery variations.2,3,6,7,9,11-13 Variations in the popliteal artery are consequences of persistent embryonic vessels from primitive segments of the artery or abnormal fusions among them.14 According to Kim and colleagues,11 variations can be classified by the modified Lippert’s system. This system has 3 categories with 3 subtypes (Table). Variations are not uncommon and occur in 7.4% to 12% of the population.2,4,5,7,13
Table. Modified Lippert’s System11
Category (Subtype) |
|
I | Normal level of popliteal arterial branching |
IA | Usual pattern |
IB | Trifurcation- No true tibioperoneal trunk |
IC | Anterior tibioperoneal trunk- Posterior tibial artery is first branch |
II | High division of popliteal artery |
IIA | Anterior tibial artery arises at or above the knee joint |
IIB | Posterior tibial artery arises at or above the knee joint |
IIC | Peroneal artery arises at or above the knee joint |
III | Hypoplastic or aplastic branching with altered distal supply |
IIIA | Hypoplastic-aplastic posterior tibial artery |
IIIB | Hypoplastic-aplastic anterior tibial artery |
IIIC | Hypoplastic-aplastic posterior and anterior tibial artery |
Of these variations, type IIA, a high bifurcation of the anterior tibial artery, arising at or above the knee joint from the popliteal artery is the most significant. Forty-two percent of these vessels course anterior to the popliteus and make direct contact with the cortex of the posterior tibia.4 It is also the most frequent variant type reported in 1.2% to 6% of the population.3,7,11-13
Continue to: Injury to the popliteal artery...
Injury to the popliteal artery during an orthopedic procedure is believed to be under reported6 but is considered a rare complication. The incidence of popliteal artery injury in TKA is thought to be 0.03% to 0.2%.1,2,5,7,8 Vessel injury in both high tibial osteotomy and arthroscopic surgeries (lateral meniscal repair) have also been reported.5,6,8,10 Despite the rare occurrence of this complication, it may have devastating outcomes. The injury can be repaired with vascular grafting depending on its severity; however, it could also lead to compartment syndrome, loss of function, chronic ulcers, and necrosis of the affected limb resulting in below the knee amputation. The current consensus is that the popliteal artery moves posteriorly away from the tibia when the knee is in 90° of flexion,5 which is the standard position for many orthopedic knee surgeries. This position limits the risk of injuring the vessel. However, Metzdorf and colleagues,4 Smith and colleagues,6 and Zaidi and colleagues8 suggested that the vessel not be displaced posteriorly with flexion. These studies reported that the behavior of the popliteal artery varied among individuals since in some cases it had moved closer to the tibia in flexion when compared with extension.
Regardless of the behavior of the artery, it is protected by the popliteus muscle in most orthopedic knee surgeries since the majority course posterior to the muscle. However, in cases of Lippert’s type IIA variation, it not only loses protection as it courses beneath the popliteus but also is extremely vulnerable from the close relationship to the posterior tibial cortex. Klecker and colleagues2 described the aberrant artery locations related to common orthopedic procedures, which demonstrated its close proximity to various surgical plane levels. The position of the aberrant artery is approximately 1 to 1.5 cm distal to the posterior tibial joint line, just posterior to the posterior capsule, and close to the posterior cruciate ligament insertion site where the transverse tibial cut is made during TKA. This location also corresponds to the position for an inlay block and the tibial tunnel for posterior cruciate ligament reconstruction. A transverse cut for a high tibial osteotomy is approximately 1.5 to 2.5 cm distal to the posterior tibial joint line; the aberrant artery appeared directly posterior to the tibial cortex. These relationships were equivalent findings in this case. Such relationships of the aberrant anterior tibial artery to both the posterior tibial cortex and the posterior capsule increase the risk of vessel (anterior tibial artery) injury intraoperatively. The risk further increases in a revision of total knee replacement. This is secondary to limited flexibility of the vessel from scar formation which requires a more distal incision.1,4
CONCLUSION
Vascular injuries in knee surgeries are rare and often overlooked. Despite their low occurrence rate, outcomes of these injuries have grave consequences not only regarding medical but also legal matters. Variations in the popliteal artery are not uncommon and could potentially contribute to risks of vessel injury. Of these variations, the high originating anterior tibial artery poses a special risk. However, due to the low occurrence rate of these injuries, screening the general population may not be cost-effective. Since many patients already have obtained necessary imaging (preferably MRI), a careful review of these images along with preoperative planning and special care during surgery is recommended to identify popliteal artery variants and avoid iatrogenic vascular injury.
This paper will be judged for the Resident Writer’s Award.
- Abdel Karim MM, Anbar A, Keenan J. Position of the popliteal artery in revision total knee arthroplasty. Arch Orthop Trauma Surg. 2012;132(6):861-865. doi:10.1007/s00402-012-1479-6.
- Klecker RJ, Winalski CS, Aliabadi P, Minas T. The aberrant anterior tibial artery, magnetic resonance appearance, prevalence, and surgical implication. Am J Sports Medicine. 2008;36:720-727.
- Kropman RHJ, Kiela G, Moll FL, Vries JPM. Variations in anatomy of the popliteal artery and its side branches. Vasc Endovascular Surg. 2011;45:536-540.
- Metzdorf A, Jakob RP, Petropoulos P, Middleton R. Arterial injury during revision total knee replacement. A case report. Knee Surg Sports Traumatol Arthrosc. 1999;7:246-248.
- Shetty AA, Tindall AJ, Qureshi F, Divekar M, Fernando KWK. The Effect of knee flexion on the popliteal artery and its surgical significance. J Bone Joint Surg Br. 2003;85:218-222.
- Smith PN, Gelinas J, Kennedy K, Thain L, Rorabeck CH, Bourne B. Popliteal vessels in knee surgery; a magnetic resonance imaging study. Clin Orthop Rel Res. 1999;367:158-164
- Tindall AJ, Shetty AA, James KD, Middleton A, Fernando KWK. Prevalence and surgical significance of a high-origin anterior tibial artery. J Orthop Surg. 2006;14:13-16.
- Zaidi SHA, Cobb AG, Bentley G. Danger to the popliteal artery in high tibial osteotomy. J Bone Joint Surg Br. 1995;77:384-386.
- Keser S, Savranlar A, Bayar A, Ulukent SC, Ozer T, Tuncay I. Anatomic localization of the popliteal artery at the level of the knee joint: a magnetic resonance imaging study. Arthroscopy. 2006;22:656-659.
- Makino A, Costa-Paz M, Aponte-Tinao L, Ayerza MA, Muscolo L. Popliteal artery laceration during arthroscopic posterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1396.
- Kim D, Orron DE, Skillman JJ. Surgical significance of popliteal arterial variants, a unified angiographic classification. Ann Surg. 1989;210:776-781.
- Day CP, Orme R. Popliteal artery branching patterns-an angiographic study. Clin Radiol. 2006;61:696-699.
- Kil SW, Jung GS. Anatomical variations of the popliteal artery and its tibial branches: Analysis in 1242 extremities. Cardiovasc Intervent Radiol. 2009;32:233-240.
- Senior HD. The development of the arteries of the human lower extremity. Am J Anat. 1919;25:55-94.
- Abdel Karim MM, Anbar A, Keenan J. Position of the popliteal artery in revision total knee arthroplasty. Arch Orthop Trauma Surg. 2012;132(6):861-865. doi:10.1007/s00402-012-1479-6.
- Klecker RJ, Winalski CS, Aliabadi P, Minas T. The aberrant anterior tibial artery, magnetic resonance appearance, prevalence, and surgical implication. Am J Sports Medicine. 2008;36:720-727.
- Kropman RHJ, Kiela G, Moll FL, Vries JPM. Variations in anatomy of the popliteal artery and its side branches. Vasc Endovascular Surg. 2011;45:536-540.
- Metzdorf A, Jakob RP, Petropoulos P, Middleton R. Arterial injury during revision total knee replacement. A case report. Knee Surg Sports Traumatol Arthrosc. 1999;7:246-248.
- Shetty AA, Tindall AJ, Qureshi F, Divekar M, Fernando KWK. The Effect of knee flexion on the popliteal artery and its surgical significance. J Bone Joint Surg Br. 2003;85:218-222.
- Smith PN, Gelinas J, Kennedy K, Thain L, Rorabeck CH, Bourne B. Popliteal vessels in knee surgery; a magnetic resonance imaging study. Clin Orthop Rel Res. 1999;367:158-164
- Tindall AJ, Shetty AA, James KD, Middleton A, Fernando KWK. Prevalence and surgical significance of a high-origin anterior tibial artery. J Orthop Surg. 2006;14:13-16.
- Zaidi SHA, Cobb AG, Bentley G. Danger to the popliteal artery in high tibial osteotomy. J Bone Joint Surg Br. 1995;77:384-386.
- Keser S, Savranlar A, Bayar A, Ulukent SC, Ozer T, Tuncay I. Anatomic localization of the popliteal artery at the level of the knee joint: a magnetic resonance imaging study. Arthroscopy. 2006;22:656-659.
- Makino A, Costa-Paz M, Aponte-Tinao L, Ayerza MA, Muscolo L. Popliteal artery laceration during arthroscopic posterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1396.
- Kim D, Orron DE, Skillman JJ. Surgical significance of popliteal arterial variants, a unified angiographic classification. Ann Surg. 1989;210:776-781.
- Day CP, Orme R. Popliteal artery branching patterns-an angiographic study. Clin Radiol. 2006;61:696-699.
- Kil SW, Jung GS. Anatomical variations of the popliteal artery and its tibial branches: Analysis in 1242 extremities. Cardiovasc Intervent Radiol. 2009;32:233-240.
- Senior HD. The development of the arteries of the human lower extremity. Am J Anat. 1919;25:55-94.
TAKE-HOME POINTS
- Surgeon must understand and be aware of aberrant vascular anatomy around the knee.
- Careful evaluation of advance imaging for aberrant vascular anatomy is required in surgeries around the knee.
- When aberrant vascular anatomy is recognized, appropriate preoperative planning is required.
Human T-Lymphotropic Virus 1 Associated With Adult T-Cell Leukemia/Lymphoma
Adult T-cell leukemia/lymphoma (ATLL) is an uncommon neoplasm of mature T lymphocytes associated with infection by human T-lymphotropic virus 1 (HTLV-1),1-3 which is increasing in incidence in areas of the United States with large immigrant populations.4 Human T-lymphotrophic virus 1 infection is asymptomatic in most patients and has been associated with ATLL as well as tropical spastic paraparesis.5 We present a case of rapid-onset ATLL in an 82-year-old Japanese man who had immigrated to the United States.
Case Report
An 82-year-old Japanese man who had immigrated to the United States presented with papules and nodules on the neck, trunk, and arms of 4 weeks’ duration. Minimal pruritus was associated with the lesions, which were otherwise asymptomatic. The patient reported that he was generally healthy, and a review of systems was negative.
Physical examination revealed numerous erythematous and violaceous papules and nodules on the right side of the neck (Figure 1A), chest, back, abdomen, groin, left arm (Figure 1B), and medial thighs. Bilateral axillary and inguinal lymphadenopathy also was noted.
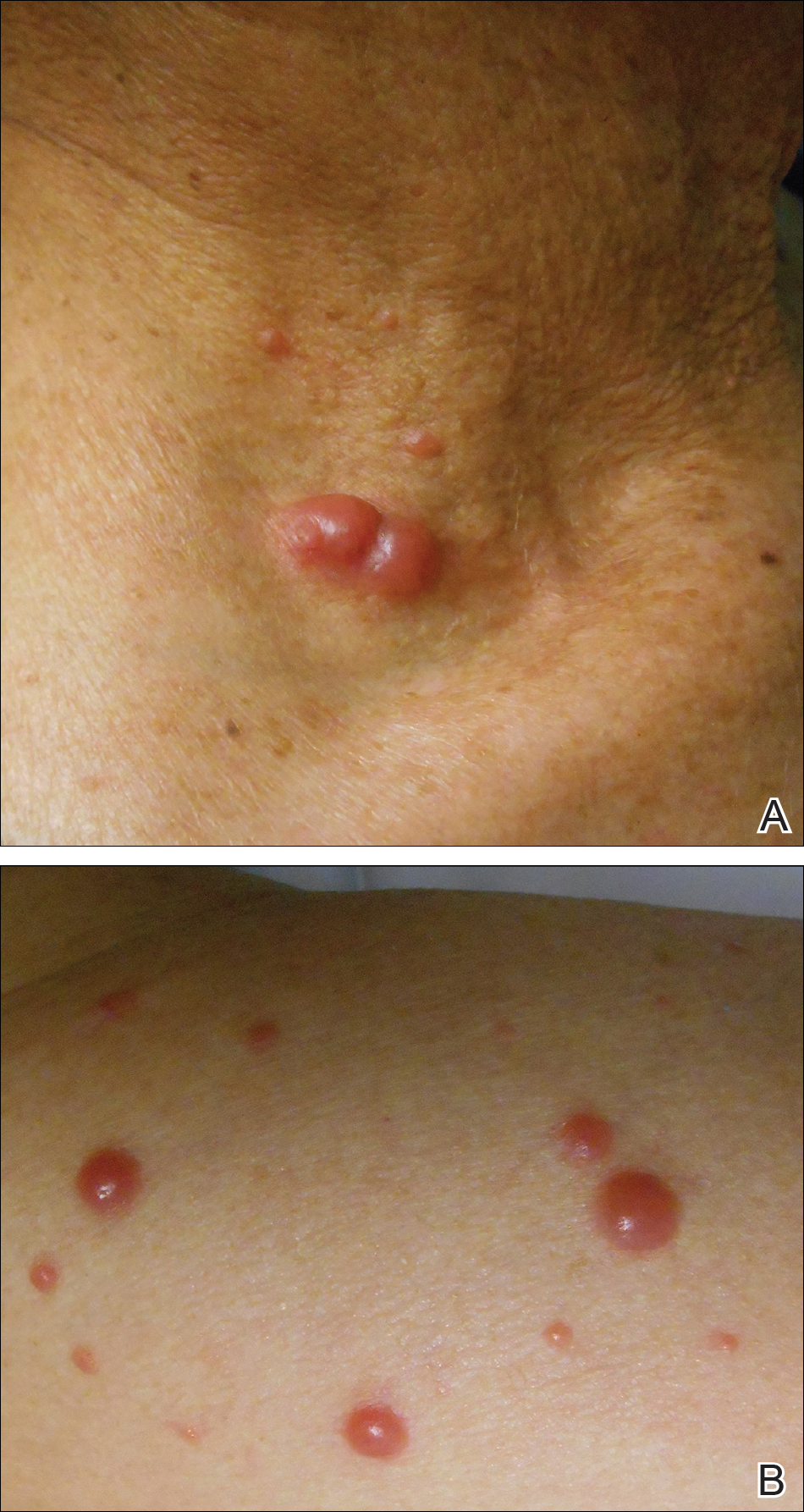
A biopsy from the abdomen revealed a dense, atypical, pandermal lymphoid infiltrate comprised of medium-sized lymphocytes with oval nuclei, fine chromatin, and pale cytoplasm (Figure 2). Mitotic figures and apoptotic cells also were observed. Immunostaining was strongly and diffusely positive for CD4 (Figure 3A), B-cell lymphoma 2 (Bcl-2)(Figure 3B), CD3, and programmed death 1, and was negative for CD8, CD10, CD20, CD30, and myeloperoxidase.
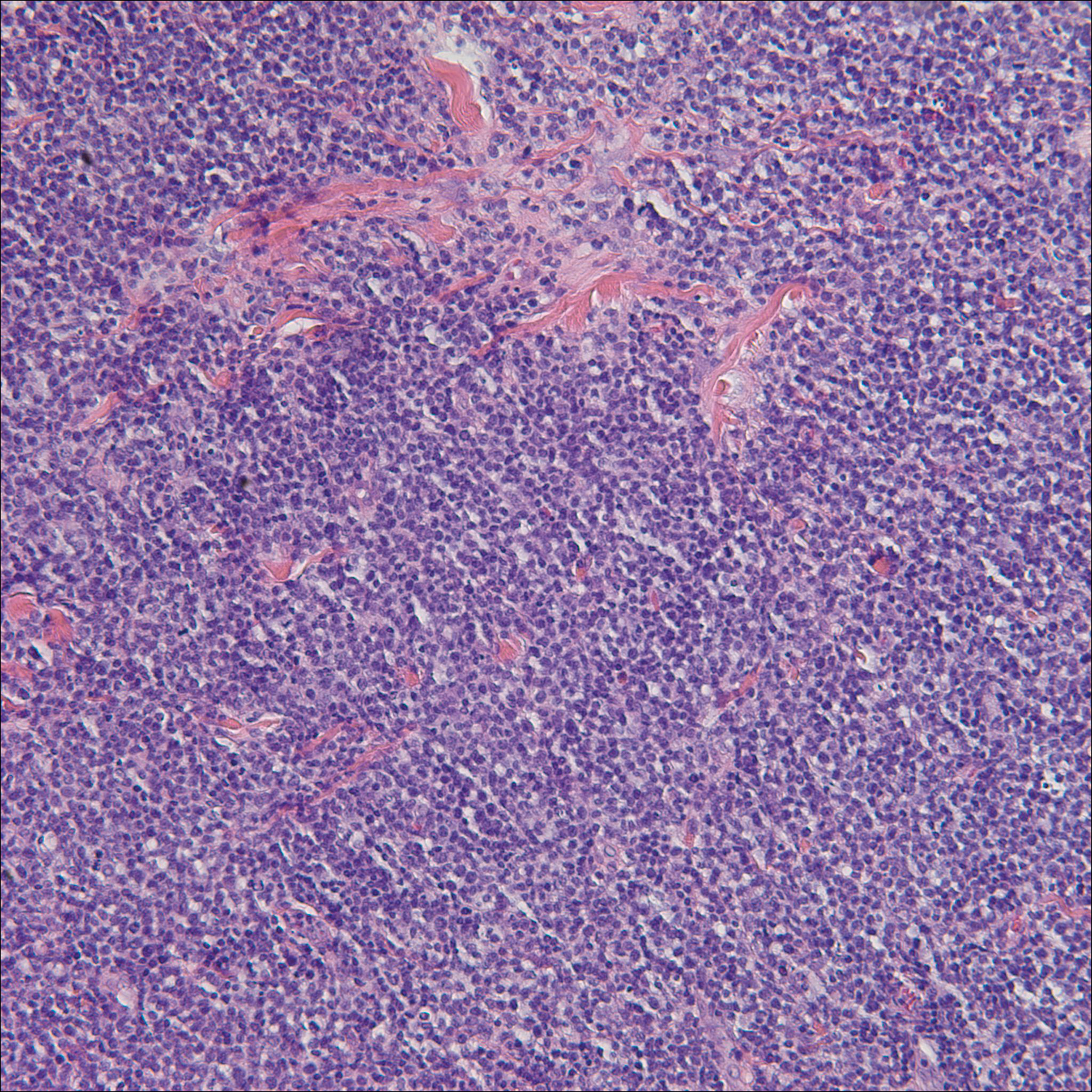
A bone marrow biopsy revealed an atypical T-cell population on flow cytometry. Western blot analysis for HTLV-1 antibodies was positive. Complete blood cell count and complete metabolic panel were within reference range.
Clinical and histopathologic findings fit the diagnosis of ATLL. The patient was referred to hematology/oncology, but the rapid progression of lesions continued, and the patient died within 4 months of initial presentation.
Comment
Etiology
First described in 1977, ATLL is an uncommon neoplasm of mature T cells.6 The etiology is associated with infection by the retrovirus HTLV-1, which is endemic in Southern Japan, the Caribbean, Central and West Africa, and Central and South America, with increasing incidence in areas of the United States with large immigrant populations.7 The incidence of ATLL among all registered lymphoma cases from 2003 to 2008 in Japan was 8.3% compared to 0.2% in the United States.7
Transmission of HTLV-1
Human T-lymphotropic virus 1 is a retrovirus most commonly found in CD4+T cells and can be transmitted through breast milk, sexual intercourse, and blood exposure (eg, blood transfusion), with breastfeeding and blood exposure being the most common.8-10 Human T-lymphotrophic virus 1 has been described as the causative agent for 3 entities: (1) ATLL, (2) a nervous system degenerative disorder known as HTLV-1–associated myelopathy or tropical spastic paraparesis, and (3) HTLV-1 uveitis.5,11 It is thought that 10 to 20 million individuals worldwide are infected with HTLV-1.12
The evolution from infection with HTLV-1 to ATLL is thought to involve multiple steps.13,14 Those who contract the virus later in life rarely, if ever, develop ATLL, suggesting that this progression requires considerable time to evolve to carcinogenesis. More than 90% of those infected with HTLV-1 remain asymptomatic, while only 2% to 3% of women and 6% to 7% of men develop ATLL with a median incubation period greater than 15 to 20 years.7
Subtypes
Adult T-cell leukemia/lymphoma has been divided into 4 clinical subtypes based on clinical presentation and prognosis.15 The acute type is more aggressive and has a poorer prognosis, while the chronic and smoldering types have a more indolent course. The smoldering variant largely has only cutaneous involvement with less than 1% of the peripheral leukocytes being atypical lymphocytes.16 A cutaneous subtype in which few to no leukemic cells are present also has been described and may overlap with the smoldering variant.The cutaneous variant has been further classified into 2 subtypes, tumoral and erythematopapular, with the tumoral subtype carrying a worse prognosis.17,18 Clinically, 39% to 57% of ATLL cases have skin involvement, with nearly one-third reporting skin manifestations as the first symptom.19,20 The cutaneous manifestations vary greatly and may include papules, plaques, nodules, tumors, erythematous patches, or erythroderma.4,21 In addition to skin manifestations, most patients with acute ATLL demonstrate leukemia, lymphadenopathy, organomegaly, and hypercalcemia.22
Histopathology
Histologically, both the smoldering and chronic forms of tumoral or erythematopapular ATLL demonstrate a cutaneous, dermal, or subcutaneous infiltrate of small- to medium-sized CD4+ T cells with histiocytes and admixed granulomas.4 Epidermotropism and Pautrier microabscesses often are limited or absent but can be seen.
Differential Diagnosis
The differential diagnosis includes other small- or medium-sized T-cell lymphomas. The chronic and smoldering types can be difficult to distinguish from mycosis fungoides.
Treatment
Treatment decisions should be made based on the subclassification and prognostic factors at the time of diagnosis. High doses of interferon alfa and zidovudine may show some benefit, but many cases require multiagent chemotherapy.22 The only possible curative treatment is allogeneic stem cell transplant. Mogamulizumab, an antichemokine receptor 4 monoclonal antibody, has demonstrated some ATLL antitumor activity.24
- Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481-492.
- Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retro-virus particles form fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415-7419.
- Hinuma Y, Gotoh Y, Sugamura K, et al. A retrovirus associated with human adult T-cell leukemia: in vitro activation. Gan. 1982;73:341-344.
- Marchetti MA, Pulitzer MP, Myskowski PL, et al. Cutaneous manifestations of human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a single-center, retrospective study. J Am Acad Dermatol. 2015;72:293-301.
- Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407-410.
- Takatsuki K, Uchiyama T, Sagawa K, et al. Adult T cell leukemia in Japan. In: Seno S, Takasu F, Irino S, eds. Topics in Hematology. Amsterdam, Netherlands: Excerpta Medica; 1977:73-77.
- Yoshida N, Chihara D. Incidence of adult T-cell leukemia/lymphoma in nonendemic areas. Curr Treat Options Oncol. 2015;16:7.
- Tajima K, Tominaga S, Suchi T, et al. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gan. 1982;73:893-901.
- Kajiyama W, Kashiwagi S, Ikematsu H, et al. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis. 1986;154:851-857.
- Ichimaru M, Ikeda S, Kinoshita K, et al. Mother-to-child transmission of HTLV-1. Cancer Detect Prev. 1991;15:177-181.
- Lyra-da-Silva JO, de Mello Gonzaga YB, de Melo Espíndola O, et al. Adult t-cell leukemia/lymphoma: a case report of primary cutaneous tumoral type. Dermatol Pract Concept. 2012;2:202a03.
- Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J Emerg Med. 2000;18:109-119.
- Magalhaes M, Oliveira PD, Bittencourt AL, et al. Microsatellite alterations are also present in the less aggressive types of adult T-cell leukemia-lymphoma. PLoS Negl Trop Dis. 2015;9:e0003403.
- Okamoto T, Ohno Y, Tsugane S, et al. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191-195.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991;79:428-437.
- Takahashi K, Tanaka T, Fujita M, et al. Cutaneous-type adult T-cell leukemia lymphoma. a unique clinical feature with monoclonal T-cell proliferation detected by Southern blot analysis Arch Dermatol. 1988;124:399-404.
- Amano M, Kurokawa M, Ogata K, et al. New entity, definition and diagnostic criteria of cutaneous adult T-cell leukemia/lymphoma: human T-lymphotropic virus type 1 proviral DNA load can distinguish between cutaneous and smoldering types. J Dermatol. 2008;35:270-275.
- Johno M, Ohishi M, Kojo Y, et al. Cutaneous manifestations of adult T-cell leukemia lymphoma. Gann Monogr Cancer Res. 1992;39:33-42.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma: a report from the Lymphoma Study Group (1984-87). Br J Haematol. 1991;79:428-437.
- Levine PH, Manns A, Jaffe ES, et al. The effect of ethnic differences on the pattern of HTLV-I-associated T-cell leukemia/lymphoma (HATL) in the United States. Int J Cancer. 1994;56:177-181.
- Pezeshkpoor F, Yazdanpanah MJ, Shirdel A. Specific cutaneous manifestations in adult T-cell leukemia/lymphoma. Int J Dermatol. 2008;47:359-362.
- Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453-459.
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124-4130.
- Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837-842.
Adult T-cell leukemia/lymphoma (ATLL) is an uncommon neoplasm of mature T lymphocytes associated with infection by human T-lymphotropic virus 1 (HTLV-1),1-3 which is increasing in incidence in areas of the United States with large immigrant populations.4 Human T-lymphotrophic virus 1 infection is asymptomatic in most patients and has been associated with ATLL as well as tropical spastic paraparesis.5 We present a case of rapid-onset ATLL in an 82-year-old Japanese man who had immigrated to the United States.
Case Report
An 82-year-old Japanese man who had immigrated to the United States presented with papules and nodules on the neck, trunk, and arms of 4 weeks’ duration. Minimal pruritus was associated with the lesions, which were otherwise asymptomatic. The patient reported that he was generally healthy, and a review of systems was negative.
Physical examination revealed numerous erythematous and violaceous papules and nodules on the right side of the neck (Figure 1A), chest, back, abdomen, groin, left arm (Figure 1B), and medial thighs. Bilateral axillary and inguinal lymphadenopathy also was noted.

A biopsy from the abdomen revealed a dense, atypical, pandermal lymphoid infiltrate comprised of medium-sized lymphocytes with oval nuclei, fine chromatin, and pale cytoplasm (Figure 2). Mitotic figures and apoptotic cells also were observed. Immunostaining was strongly and diffusely positive for CD4 (Figure 3A), B-cell lymphoma 2 (Bcl-2)(Figure 3B), CD3, and programmed death 1, and was negative for CD8, CD10, CD20, CD30, and myeloperoxidase.


A bone marrow biopsy revealed an atypical T-cell population on flow cytometry. Western blot analysis for HTLV-1 antibodies was positive. Complete blood cell count and complete metabolic panel were within reference range.
Clinical and histopathologic findings fit the diagnosis of ATLL. The patient was referred to hematology/oncology, but the rapid progression of lesions continued, and the patient died within 4 months of initial presentation.
Comment
Etiology
First described in 1977, ATLL is an uncommon neoplasm of mature T cells.6 The etiology is associated with infection by the retrovirus HTLV-1, which is endemic in Southern Japan, the Caribbean, Central and West Africa, and Central and South America, with increasing incidence in areas of the United States with large immigrant populations.7 The incidence of ATLL among all registered lymphoma cases from 2003 to 2008 in Japan was 8.3% compared to 0.2% in the United States.7
Transmission of HTLV-1
Human T-lymphotropic virus 1 is a retrovirus most commonly found in CD4+T cells and can be transmitted through breast milk, sexual intercourse, and blood exposure (eg, blood transfusion), with breastfeeding and blood exposure being the most common.8-10 Human T-lymphotrophic virus 1 has been described as the causative agent for 3 entities: (1) ATLL, (2) a nervous system degenerative disorder known as HTLV-1–associated myelopathy or tropical spastic paraparesis, and (3) HTLV-1 uveitis.5,11 It is thought that 10 to 20 million individuals worldwide are infected with HTLV-1.12
The evolution from infection with HTLV-1 to ATLL is thought to involve multiple steps.13,14 Those who contract the virus later in life rarely, if ever, develop ATLL, suggesting that this progression requires considerable time to evolve to carcinogenesis. More than 90% of those infected with HTLV-1 remain asymptomatic, while only 2% to 3% of women and 6% to 7% of men develop ATLL with a median incubation period greater than 15 to 20 years.7
Subtypes
Adult T-cell leukemia/lymphoma has been divided into 4 clinical subtypes based on clinical presentation and prognosis.15 The acute type is more aggressive and has a poorer prognosis, while the chronic and smoldering types have a more indolent course. The smoldering variant largely has only cutaneous involvement with less than 1% of the peripheral leukocytes being atypical lymphocytes.16 A cutaneous subtype in which few to no leukemic cells are present also has been described and may overlap with the smoldering variant.The cutaneous variant has been further classified into 2 subtypes, tumoral and erythematopapular, with the tumoral subtype carrying a worse prognosis.17,18 Clinically, 39% to 57% of ATLL cases have skin involvement, with nearly one-third reporting skin manifestations as the first symptom.19,20 The cutaneous manifestations vary greatly and may include papules, plaques, nodules, tumors, erythematous patches, or erythroderma.4,21 In addition to skin manifestations, most patients with acute ATLL demonstrate leukemia, lymphadenopathy, organomegaly, and hypercalcemia.22
Histopathology
Histologically, both the smoldering and chronic forms of tumoral or erythematopapular ATLL demonstrate a cutaneous, dermal, or subcutaneous infiltrate of small- to medium-sized CD4+ T cells with histiocytes and admixed granulomas.4 Epidermotropism and Pautrier microabscesses often are limited or absent but can be seen.
Differential Diagnosis
The differential diagnosis includes other small- or medium-sized T-cell lymphomas. The chronic and smoldering types can be difficult to distinguish from mycosis fungoides.
Treatment
Treatment decisions should be made based on the subclassification and prognostic factors at the time of diagnosis. High doses of interferon alfa and zidovudine may show some benefit, but many cases require multiagent chemotherapy.22 The only possible curative treatment is allogeneic stem cell transplant. Mogamulizumab, an antichemokine receptor 4 monoclonal antibody, has demonstrated some ATLL antitumor activity.24
Adult T-cell leukemia/lymphoma (ATLL) is an uncommon neoplasm of mature T lymphocytes associated with infection by human T-lymphotropic virus 1 (HTLV-1),1-3 which is increasing in incidence in areas of the United States with large immigrant populations.4 Human T-lymphotrophic virus 1 infection is asymptomatic in most patients and has been associated with ATLL as well as tropical spastic paraparesis.5 We present a case of rapid-onset ATLL in an 82-year-old Japanese man who had immigrated to the United States.
Case Report
An 82-year-old Japanese man who had immigrated to the United States presented with papules and nodules on the neck, trunk, and arms of 4 weeks’ duration. Minimal pruritus was associated with the lesions, which were otherwise asymptomatic. The patient reported that he was generally healthy, and a review of systems was negative.
Physical examination revealed numerous erythematous and violaceous papules and nodules on the right side of the neck (Figure 1A), chest, back, abdomen, groin, left arm (Figure 1B), and medial thighs. Bilateral axillary and inguinal lymphadenopathy also was noted.

A biopsy from the abdomen revealed a dense, atypical, pandermal lymphoid infiltrate comprised of medium-sized lymphocytes with oval nuclei, fine chromatin, and pale cytoplasm (Figure 2). Mitotic figures and apoptotic cells also were observed. Immunostaining was strongly and diffusely positive for CD4 (Figure 3A), B-cell lymphoma 2 (Bcl-2)(Figure 3B), CD3, and programmed death 1, and was negative for CD8, CD10, CD20, CD30, and myeloperoxidase.


A bone marrow biopsy revealed an atypical T-cell population on flow cytometry. Western blot analysis for HTLV-1 antibodies was positive. Complete blood cell count and complete metabolic panel were within reference range.
Clinical and histopathologic findings fit the diagnosis of ATLL. The patient was referred to hematology/oncology, but the rapid progression of lesions continued, and the patient died within 4 months of initial presentation.
Comment
Etiology
First described in 1977, ATLL is an uncommon neoplasm of mature T cells.6 The etiology is associated with infection by the retrovirus HTLV-1, which is endemic in Southern Japan, the Caribbean, Central and West Africa, and Central and South America, with increasing incidence in areas of the United States with large immigrant populations.7 The incidence of ATLL among all registered lymphoma cases from 2003 to 2008 in Japan was 8.3% compared to 0.2% in the United States.7
Transmission of HTLV-1
Human T-lymphotropic virus 1 is a retrovirus most commonly found in CD4+T cells and can be transmitted through breast milk, sexual intercourse, and blood exposure (eg, blood transfusion), with breastfeeding and blood exposure being the most common.8-10 Human T-lymphotrophic virus 1 has been described as the causative agent for 3 entities: (1) ATLL, (2) a nervous system degenerative disorder known as HTLV-1–associated myelopathy or tropical spastic paraparesis, and (3) HTLV-1 uveitis.5,11 It is thought that 10 to 20 million individuals worldwide are infected with HTLV-1.12
The evolution from infection with HTLV-1 to ATLL is thought to involve multiple steps.13,14 Those who contract the virus later in life rarely, if ever, develop ATLL, suggesting that this progression requires considerable time to evolve to carcinogenesis. More than 90% of those infected with HTLV-1 remain asymptomatic, while only 2% to 3% of women and 6% to 7% of men develop ATLL with a median incubation period greater than 15 to 20 years.7
Subtypes
Adult T-cell leukemia/lymphoma has been divided into 4 clinical subtypes based on clinical presentation and prognosis.15 The acute type is more aggressive and has a poorer prognosis, while the chronic and smoldering types have a more indolent course. The smoldering variant largely has only cutaneous involvement with less than 1% of the peripheral leukocytes being atypical lymphocytes.16 A cutaneous subtype in which few to no leukemic cells are present also has been described and may overlap with the smoldering variant.The cutaneous variant has been further classified into 2 subtypes, tumoral and erythematopapular, with the tumoral subtype carrying a worse prognosis.17,18 Clinically, 39% to 57% of ATLL cases have skin involvement, with nearly one-third reporting skin manifestations as the first symptom.19,20 The cutaneous manifestations vary greatly and may include papules, plaques, nodules, tumors, erythematous patches, or erythroderma.4,21 In addition to skin manifestations, most patients with acute ATLL demonstrate leukemia, lymphadenopathy, organomegaly, and hypercalcemia.22
Histopathology
Histologically, both the smoldering and chronic forms of tumoral or erythematopapular ATLL demonstrate a cutaneous, dermal, or subcutaneous infiltrate of small- to medium-sized CD4+ T cells with histiocytes and admixed granulomas.4 Epidermotropism and Pautrier microabscesses often are limited or absent but can be seen.
Differential Diagnosis
The differential diagnosis includes other small- or medium-sized T-cell lymphomas. The chronic and smoldering types can be difficult to distinguish from mycosis fungoides.
Treatment
Treatment decisions should be made based on the subclassification and prognostic factors at the time of diagnosis. High doses of interferon alfa and zidovudine may show some benefit, but many cases require multiagent chemotherapy.22 The only possible curative treatment is allogeneic stem cell transplant. Mogamulizumab, an antichemokine receptor 4 monoclonal antibody, has demonstrated some ATLL antitumor activity.24
- Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481-492.
- Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retro-virus particles form fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415-7419.
- Hinuma Y, Gotoh Y, Sugamura K, et al. A retrovirus associated with human adult T-cell leukemia: in vitro activation. Gan. 1982;73:341-344.
- Marchetti MA, Pulitzer MP, Myskowski PL, et al. Cutaneous manifestations of human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a single-center, retrospective study. J Am Acad Dermatol. 2015;72:293-301.
- Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407-410.
- Takatsuki K, Uchiyama T, Sagawa K, et al. Adult T cell leukemia in Japan. In: Seno S, Takasu F, Irino S, eds. Topics in Hematology. Amsterdam, Netherlands: Excerpta Medica; 1977:73-77.
- Yoshida N, Chihara D. Incidence of adult T-cell leukemia/lymphoma in nonendemic areas. Curr Treat Options Oncol. 2015;16:7.
- Tajima K, Tominaga S, Suchi T, et al. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gan. 1982;73:893-901.
- Kajiyama W, Kashiwagi S, Ikematsu H, et al. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis. 1986;154:851-857.
- Ichimaru M, Ikeda S, Kinoshita K, et al. Mother-to-child transmission of HTLV-1. Cancer Detect Prev. 1991;15:177-181.
- Lyra-da-Silva JO, de Mello Gonzaga YB, de Melo Espíndola O, et al. Adult t-cell leukemia/lymphoma: a case report of primary cutaneous tumoral type. Dermatol Pract Concept. 2012;2:202a03.
- Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J Emerg Med. 2000;18:109-119.
- Magalhaes M, Oliveira PD, Bittencourt AL, et al. Microsatellite alterations are also present in the less aggressive types of adult T-cell leukemia-lymphoma. PLoS Negl Trop Dis. 2015;9:e0003403.
- Okamoto T, Ohno Y, Tsugane S, et al. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191-195.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991;79:428-437.
- Takahashi K, Tanaka T, Fujita M, et al. Cutaneous-type adult T-cell leukemia lymphoma. a unique clinical feature with monoclonal T-cell proliferation detected by Southern blot analysis Arch Dermatol. 1988;124:399-404.
- Amano M, Kurokawa M, Ogata K, et al. New entity, definition and diagnostic criteria of cutaneous adult T-cell leukemia/lymphoma: human T-lymphotropic virus type 1 proviral DNA load can distinguish between cutaneous and smoldering types. J Dermatol. 2008;35:270-275.
- Johno M, Ohishi M, Kojo Y, et al. Cutaneous manifestations of adult T-cell leukemia lymphoma. Gann Monogr Cancer Res. 1992;39:33-42.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma: a report from the Lymphoma Study Group (1984-87). Br J Haematol. 1991;79:428-437.
- Levine PH, Manns A, Jaffe ES, et al. The effect of ethnic differences on the pattern of HTLV-I-associated T-cell leukemia/lymphoma (HATL) in the United States. Int J Cancer. 1994;56:177-181.
- Pezeshkpoor F, Yazdanpanah MJ, Shirdel A. Specific cutaneous manifestations in adult T-cell leukemia/lymphoma. Int J Dermatol. 2008;47:359-362.
- Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453-459.
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124-4130.
- Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837-842.
- Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481-492.
- Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retro-virus particles form fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415-7419.
- Hinuma Y, Gotoh Y, Sugamura K, et al. A retrovirus associated with human adult T-cell leukemia: in vitro activation. Gan. 1982;73:341-344.
- Marchetti MA, Pulitzer MP, Myskowski PL, et al. Cutaneous manifestations of human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a single-center, retrospective study. J Am Acad Dermatol. 2015;72:293-301.
- Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407-410.
- Takatsuki K, Uchiyama T, Sagawa K, et al. Adult T cell leukemia in Japan. In: Seno S, Takasu F, Irino S, eds. Topics in Hematology. Amsterdam, Netherlands: Excerpta Medica; 1977:73-77.
- Yoshida N, Chihara D. Incidence of adult T-cell leukemia/lymphoma in nonendemic areas. Curr Treat Options Oncol. 2015;16:7.
- Tajima K, Tominaga S, Suchi T, et al. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gan. 1982;73:893-901.
- Kajiyama W, Kashiwagi S, Ikematsu H, et al. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis. 1986;154:851-857.
- Ichimaru M, Ikeda S, Kinoshita K, et al. Mother-to-child transmission of HTLV-1. Cancer Detect Prev. 1991;15:177-181.
- Lyra-da-Silva JO, de Mello Gonzaga YB, de Melo Espíndola O, et al. Adult t-cell leukemia/lymphoma: a case report of primary cutaneous tumoral type. Dermatol Pract Concept. 2012;2:202a03.
- Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J Emerg Med. 2000;18:109-119.
- Magalhaes M, Oliveira PD, Bittencourt AL, et al. Microsatellite alterations are also present in the less aggressive types of adult T-cell leukemia-lymphoma. PLoS Negl Trop Dis. 2015;9:e0003403.
- Okamoto T, Ohno Y, Tsugane S, et al. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191-195.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991;79:428-437.
- Takahashi K, Tanaka T, Fujita M, et al. Cutaneous-type adult T-cell leukemia lymphoma. a unique clinical feature with monoclonal T-cell proliferation detected by Southern blot analysis Arch Dermatol. 1988;124:399-404.
- Amano M, Kurokawa M, Ogata K, et al. New entity, definition and diagnostic criteria of cutaneous adult T-cell leukemia/lymphoma: human T-lymphotropic virus type 1 proviral DNA load can distinguish between cutaneous and smoldering types. J Dermatol. 2008;35:270-275.
- Johno M, Ohishi M, Kojo Y, et al. Cutaneous manifestations of adult T-cell leukemia lymphoma. Gann Monogr Cancer Res. 1992;39:33-42.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma: a report from the Lymphoma Study Group (1984-87). Br J Haematol. 1991;79:428-437.
- Levine PH, Manns A, Jaffe ES, et al. The effect of ethnic differences on the pattern of HTLV-I-associated T-cell leukemia/lymphoma (HATL) in the United States. Int J Cancer. 1994;56:177-181.
- Pezeshkpoor F, Yazdanpanah MJ, Shirdel A. Specific cutaneous manifestations in adult T-cell leukemia/lymphoma. Int J Dermatol. 2008;47:359-362.
- Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453-459.
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124-4130.
- Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837-842.
Practice Points
- Adult T-cell leukemia/lymphoma (ATLL) is an uncommon neoplasm of mature T lymphocytes associated with infection by human T-lymphotropic virus 1.
- In the United States, ATLL is increasing in incidence in areas with large immigrant populations.
- High suspicion and clinical features must be present to make the diagnosis of ATLL due to considerable histologic overlap with other cutaneous T-cell lymphomas.
Snapping Biceps Femoris Tendon
ABSTRACT
A 23-year-old male active duty soldier presented with a biceps femoris tendon snapping over the fibular head with flexion of the knee beyond 90°. Surgical release of anomalous anterolateral tibial and lateral fibular insertions provided relief of snapping with no other repair or reconstruction required. The soldier quickly returned to full running and active duty.
Snapping biceps femoris tendon is a rare but potential cause of pain and dysfunction in the lateral knee. The possible anatomical variations and the cause of snapping must be considered when determining the operative approaches to this condition.
Continue to: Snapping in the knee...
Snapping in the knee is not as common as in other joints, such as the hip or ankle. The snapping sensation can occur from several pathologies, including the following: lateral meniscal tears, iliotibial band syndrome, proximal tibiofibular instability, snapping popliteus, peroneal nerve compression/neuritis, lateral discoid meniscus, rheumatoid nodules, plicae, congenital snapping knee, exostoses, or previous trauma.1,2 A detailed history must be provided, and physical examination and appropriate imaging must be performed to narrow down the differential diagnosis and prescribe the appropriate course of treatment for snapping.
Snapping biceps femoris syndrome is a rare cause of knee snapping. This condition has been described in various case reports.2-13 The reasons for a snapping biceps femoris can vary, and the treating provider must be ready to accommodate and treat these causes. The symptoms typically include an audible, and usually visual, lateral snapping distal to the knee joint and over the fibular head. Imaging may reveal bony abnormalities such as fibular exostoses. Magnetic resonance imaging (MRI) can aid in determining any anomalous or abnormal insertions of the biceps femoris tendon. The snapping can be debilitating, particularly in athletes or patients with high-demand occupations, and surgical intervention is often warranted.
We present a case of an active-duty military service member with symptomatic unilateral snapping biceps femoris and review the literature for treatment of this condition. Surgical release allowed the patient a quick and unrestricted return to full mission capabilities.
The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
A 23-year-old active-duty soldier presented to the orthopedic clinic with several months of noticeable snapping and pain over the lateral knee with attempted running and deep squatting activities, resulting in difficulty to perform his army duties. The patient reported no history of antecedent trauma. No locking of the knee or paresthesia distally into the leg or foot was observed.
The physical examination revealed a palpable and observable snapping of the long head of the biceps tendon over the fibular head with squatting beyond 90° in the left knee. The patient presented with full strength and no instability or joint line pain throughout the knee. Application of a posterior-to-anterior directed force over the biceps femoris proximal to the insertion allowed the patient to perform a deep squat without snapping. The radiographs demonstrated no abnormal fibular morphology (Figures 1A, 1B). Axial MRI images demonstrated an anomalous slip of the tendon inserting on the anterolateral aspect of the proximal tibia in addition to the normal insertion on the posterolateral and lateral edge of the fibular head (Figure 2) as described by Terry and LaPrade.14
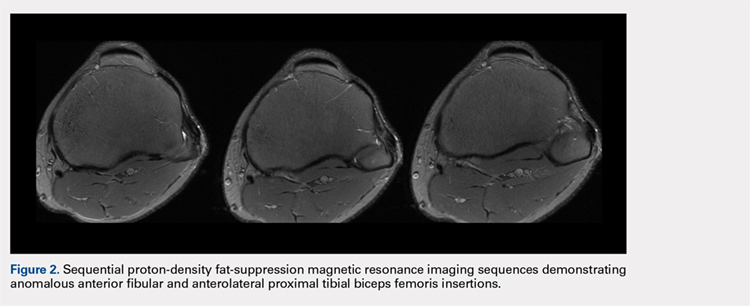
Continue to: A conservative treatment with physical therapy...
A conservative treatment with physical therapy, activity modification, and a Cho-Pat knee strap (to provide a posterior-to-anterior buttress and to prevent snapping) was attempted for 4 weeks. However, the patient could not tolerate the strap, and the activity restraints prevented him from performing his job as an active-duty soldier. Given the failure of conservative treatment, operative intervention was elected.
Upon exploration of the biceps femoris insertion, the accessory anterolateral tibial insertion was readily identified (Figure 3). Notably, the expected normal lateral edge insertion was thickened and extended beyond the lateral edge, distal, and anterior on to the fibular head (Figure 4). The anterolateral tibial band was released first. However, the snapping remained evident. The thickened anterior fibular accessory band was then released back to its normal, lateral edge, and at this point, no further snapping was observed with deep flexion of the knee. Inspection of the remaining posterolateral and lateral edge insertion demonstrated a healthy, 1-cm thick tendinous insertion. The accessory slips were completely excised, and the incision was closed without any additional repair or re-insertion (Figure 5). The patient presented no complications postoperatively. He was allowed to bear weight as tolerated and was limited to stretching and gravity resistance training for 4 weeks. At 1 month, the patient was released to progress back to full activity. By 8 weeks postoperative, he remained free of snapping and resumed his regular running routine and military duties without restriction or pain.
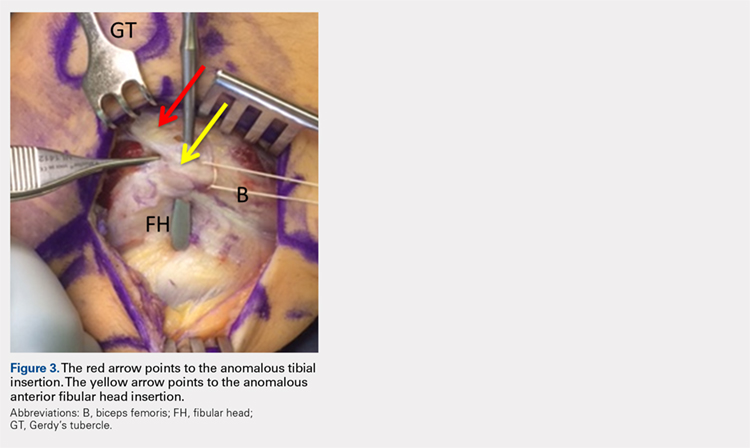
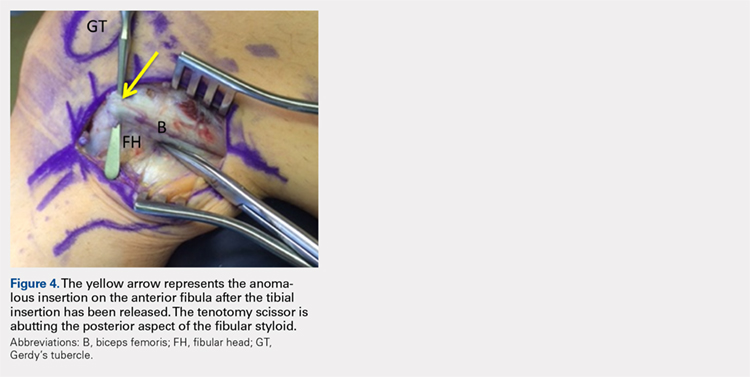

DISCUSSION
Release of the anomalous bands with no further repair or re-insertion of the biceps femoris allowed this active-duty soldier to resume full running and duty-related activities in <2 months. In this particular patient, given his anatomy, the treatment was successful. The literature indicates that optimal results and surgical approach depend upon the pathological anatomy encountered.
Date and colleagues4 described a similar anatomical anomaly as with our patient, whom after the release of tibial insertion, snapping was still observed, thus requiring the release of anterior fibular insertion. They noted the necessity of suturing the accessory limbs onto the periosteum of the fibular head to achieve a stable biceps femoris.
In other cases, abnormal bony anatomy of the fibula has been shown to cause snapping. Vavalle and Capozzi5 described a case of snapping biceps in a marathon runner, who needed partial resection of the fibular head to eliminate snapping. The runner made a full return to the sport. Fung and colleagues2 described a similar approach to a 17-year-old cyclist; however, this patient presented exostoses of the bilateral fibular heads. The exostoses were bilaterally excised, and the snapping ceased. Kristensen and colleagues13 described a patient with an anomalous tibial insertion. Rather than releasing the tibial insertion, a partial resection of the fibular head allowed for cessation of snapping.
Other authors advocate the detachment and anatomic re-insertion of the biceps femoris into the fibular head. Bernhardson and LaPrade6 reported a series of 3 patients requiring this approach with excellent results. Bansal and colleagues8 were the first to describe a soccer player with an isolated injury to the knee as a traumatic cause for a snapping biceps femoris. After failure of conservative treatment attempts, exploration and re-insertion through a bone tunnel allowed for return to the sport. Hernandez and colleagues11 and Lokiec and colleagues12 both described the reproduction of the normal biceps femoris anatomy through re-insertion procedures after identifying patients with abnormal anatomical insertions as causes for snapping.
CONCLUSION
We presented a case of an active military service member with a unilateral snapping biceps femoris tendon due to an anomalous distal insertion on both the proximal tibia and anterior fibular head. The release of abnormal insertions and maintenance of his normal anatomical insertion allowed for a quick and effective return to running and duty at full capacity. Although other surgical approaches have been described to include partial fibular head resection or anatomical re-insertion, we believe that the approach to this rare condition should be anatomy-based as the causes of snapping can significantly vary. We believe that if the normal posterolateral and lateral edge insertions of the biceps femoris are intact, removal of the abnormal anatomy without any repair or reconstruction can safely lead to successful surgical outcomes.
- Barker JU, Strauss EJ, Lodha S, Bach BR Jr. Extra-articular mimickers of lateral meniscal tears. Sports Health. 2011;3(1):82-88.
- Fung DA, Frey S, Markbreiter L. Bilateral symptomatic snapping biceps femoris tendon due to fibular exostosis. J Knee Surg. 2008;21(1):55-57.
- Mirchandani M, Gandhi P, Cai P. Poster 175 bilateral symptomatic snapping knee from biceps femoris tendon subluxation–an atypical cause of bilateral knee pain: a case report. PM R. 2016;8(9S):S218-S219.
- Date H, Hayakawa K, Yamada H. Snapping knee due to the biceps femoris tendon treated with repositioning of the anomalous tibial insertion. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1581-1583.
- Vavalle G, Capozzi M. Symptomatic snapping knee from biceps femoris tendon subluxation: an unusual case of lateral pain in a marathon runner. J Orthop Traumatol. 2010;11(4):263-266.
- Bernhardson AS, LaPrade RF. Snapping biceps femoris tendon treated with an anatomic repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1110-1112.
- Guillin R, Mendoza-Ruiz JJ, Moser T, Ropars M, Duvauferrier R, Cardinal E. Snapping biceps femoris tendon: a dynamic real-time sonographic evaluation. J Clin Ultrasound. 2010;38(8):435-437.
- Bansal R, Taylor C, Pimpalnerkar AL. Snapping knee: an unusual biceps femoris tendon injury. Knee. 2005;12(6):458-460.
- Bagchi K, Grelsamer RP. Partial fibular head resection for bilateral snapping biceps femoris tendon. Orthopedics. 2003;26(11):1147-1149.
- Kissenberth MJ, Wilckens JH. The snapping biceps femoris tendon. Am J Knee Surg. 2000;13(1):25-28.
- Hernandez JA, Rius M. Noonan KJ. Snapping knee from anomalous biceps femoris tendon insertion: a case report. Iowa Orthop J. 1996;16:161-163.
- Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop Relat Res. 1992;(283):205-206.
- Kristensen G, Nielsen K, Blyme PJ. Snapping knee from biceps femoris tendon. A case report. Acta Orthop Scand. 1989;60(5):621.
- Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee. Its anatomy and injury patterns associated with acute anterolateral-anteromedial rotator instability. Am J Sports Med. 1996;24:2-8.
ABSTRACT
A 23-year-old male active duty soldier presented with a biceps femoris tendon snapping over the fibular head with flexion of the knee beyond 90°. Surgical release of anomalous anterolateral tibial and lateral fibular insertions provided relief of snapping with no other repair or reconstruction required. The soldier quickly returned to full running and active duty.
Snapping biceps femoris tendon is a rare but potential cause of pain and dysfunction in the lateral knee. The possible anatomical variations and the cause of snapping must be considered when determining the operative approaches to this condition.
Continue to: Snapping in the knee...
Snapping in the knee is not as common as in other joints, such as the hip or ankle. The snapping sensation can occur from several pathologies, including the following: lateral meniscal tears, iliotibial band syndrome, proximal tibiofibular instability, snapping popliteus, peroneal nerve compression/neuritis, lateral discoid meniscus, rheumatoid nodules, plicae, congenital snapping knee, exostoses, or previous trauma.1,2 A detailed history must be provided, and physical examination and appropriate imaging must be performed to narrow down the differential diagnosis and prescribe the appropriate course of treatment for snapping.
Snapping biceps femoris syndrome is a rare cause of knee snapping. This condition has been described in various case reports.2-13 The reasons for a snapping biceps femoris can vary, and the treating provider must be ready to accommodate and treat these causes. The symptoms typically include an audible, and usually visual, lateral snapping distal to the knee joint and over the fibular head. Imaging may reveal bony abnormalities such as fibular exostoses. Magnetic resonance imaging (MRI) can aid in determining any anomalous or abnormal insertions of the biceps femoris tendon. The snapping can be debilitating, particularly in athletes or patients with high-demand occupations, and surgical intervention is often warranted.
We present a case of an active-duty military service member with symptomatic unilateral snapping biceps femoris and review the literature for treatment of this condition. Surgical release allowed the patient a quick and unrestricted return to full mission capabilities.
The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
A 23-year-old active-duty soldier presented to the orthopedic clinic with several months of noticeable snapping and pain over the lateral knee with attempted running and deep squatting activities, resulting in difficulty to perform his army duties. The patient reported no history of antecedent trauma. No locking of the knee or paresthesia distally into the leg or foot was observed.
The physical examination revealed a palpable and observable snapping of the long head of the biceps tendon over the fibular head with squatting beyond 90° in the left knee. The patient presented with full strength and no instability or joint line pain throughout the knee. Application of a posterior-to-anterior directed force over the biceps femoris proximal to the insertion allowed the patient to perform a deep squat without snapping. The radiographs demonstrated no abnormal fibular morphology (Figures 1A, 1B). Axial MRI images demonstrated an anomalous slip of the tendon inserting on the anterolateral aspect of the proximal tibia in addition to the normal insertion on the posterolateral and lateral edge of the fibular head (Figure 2) as described by Terry and LaPrade.14


Continue to: A conservative treatment with physical therapy...
A conservative treatment with physical therapy, activity modification, and a Cho-Pat knee strap (to provide a posterior-to-anterior buttress and to prevent snapping) was attempted for 4 weeks. However, the patient could not tolerate the strap, and the activity restraints prevented him from performing his job as an active-duty soldier. Given the failure of conservative treatment, operative intervention was elected.
Upon exploration of the biceps femoris insertion, the accessory anterolateral tibial insertion was readily identified (Figure 3). Notably, the expected normal lateral edge insertion was thickened and extended beyond the lateral edge, distal, and anterior on to the fibular head (Figure 4). The anterolateral tibial band was released first. However, the snapping remained evident. The thickened anterior fibular accessory band was then released back to its normal, lateral edge, and at this point, no further snapping was observed with deep flexion of the knee. Inspection of the remaining posterolateral and lateral edge insertion demonstrated a healthy, 1-cm thick tendinous insertion. The accessory slips were completely excised, and the incision was closed without any additional repair or re-insertion (Figure 5). The patient presented no complications postoperatively. He was allowed to bear weight as tolerated and was limited to stretching and gravity resistance training for 4 weeks. At 1 month, the patient was released to progress back to full activity. By 8 weeks postoperative, he remained free of snapping and resumed his regular running routine and military duties without restriction or pain.



DISCUSSION
Release of the anomalous bands with no further repair or re-insertion of the biceps femoris allowed this active-duty soldier to resume full running and duty-related activities in <2 months. In this particular patient, given his anatomy, the treatment was successful. The literature indicates that optimal results and surgical approach depend upon the pathological anatomy encountered.
Date and colleagues4 described a similar anatomical anomaly as with our patient, whom after the release of tibial insertion, snapping was still observed, thus requiring the release of anterior fibular insertion. They noted the necessity of suturing the accessory limbs onto the periosteum of the fibular head to achieve a stable biceps femoris.
In other cases, abnormal bony anatomy of the fibula has been shown to cause snapping. Vavalle and Capozzi5 described a case of snapping biceps in a marathon runner, who needed partial resection of the fibular head to eliminate snapping. The runner made a full return to the sport. Fung and colleagues2 described a similar approach to a 17-year-old cyclist; however, this patient presented exostoses of the bilateral fibular heads. The exostoses were bilaterally excised, and the snapping ceased. Kristensen and colleagues13 described a patient with an anomalous tibial insertion. Rather than releasing the tibial insertion, a partial resection of the fibular head allowed for cessation of snapping.
Other authors advocate the detachment and anatomic re-insertion of the biceps femoris into the fibular head. Bernhardson and LaPrade6 reported a series of 3 patients requiring this approach with excellent results. Bansal and colleagues8 were the first to describe a soccer player with an isolated injury to the knee as a traumatic cause for a snapping biceps femoris. After failure of conservative treatment attempts, exploration and re-insertion through a bone tunnel allowed for return to the sport. Hernandez and colleagues11 and Lokiec and colleagues12 both described the reproduction of the normal biceps femoris anatomy through re-insertion procedures after identifying patients with abnormal anatomical insertions as causes for snapping.
CONCLUSION
We presented a case of an active military service member with a unilateral snapping biceps femoris tendon due to an anomalous distal insertion on both the proximal tibia and anterior fibular head. The release of abnormal insertions and maintenance of his normal anatomical insertion allowed for a quick and effective return to running and duty at full capacity. Although other surgical approaches have been described to include partial fibular head resection or anatomical re-insertion, we believe that the approach to this rare condition should be anatomy-based as the causes of snapping can significantly vary. We believe that if the normal posterolateral and lateral edge insertions of the biceps femoris are intact, removal of the abnormal anatomy without any repair or reconstruction can safely lead to successful surgical outcomes.
ABSTRACT
A 23-year-old male active duty soldier presented with a biceps femoris tendon snapping over the fibular head with flexion of the knee beyond 90°. Surgical release of anomalous anterolateral tibial and lateral fibular insertions provided relief of snapping with no other repair or reconstruction required. The soldier quickly returned to full running and active duty.
Snapping biceps femoris tendon is a rare but potential cause of pain and dysfunction in the lateral knee. The possible anatomical variations and the cause of snapping must be considered when determining the operative approaches to this condition.
Continue to: Snapping in the knee...
Snapping in the knee is not as common as in other joints, such as the hip or ankle. The snapping sensation can occur from several pathologies, including the following: lateral meniscal tears, iliotibial band syndrome, proximal tibiofibular instability, snapping popliteus, peroneal nerve compression/neuritis, lateral discoid meniscus, rheumatoid nodules, plicae, congenital snapping knee, exostoses, or previous trauma.1,2 A detailed history must be provided, and physical examination and appropriate imaging must be performed to narrow down the differential diagnosis and prescribe the appropriate course of treatment for snapping.
Snapping biceps femoris syndrome is a rare cause of knee snapping. This condition has been described in various case reports.2-13 The reasons for a snapping biceps femoris can vary, and the treating provider must be ready to accommodate and treat these causes. The symptoms typically include an audible, and usually visual, lateral snapping distal to the knee joint and over the fibular head. Imaging may reveal bony abnormalities such as fibular exostoses. Magnetic resonance imaging (MRI) can aid in determining any anomalous or abnormal insertions of the biceps femoris tendon. The snapping can be debilitating, particularly in athletes or patients with high-demand occupations, and surgical intervention is often warranted.
We present a case of an active-duty military service member with symptomatic unilateral snapping biceps femoris and review the literature for treatment of this condition. Surgical release allowed the patient a quick and unrestricted return to full mission capabilities.
The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
A 23-year-old active-duty soldier presented to the orthopedic clinic with several months of noticeable snapping and pain over the lateral knee with attempted running and deep squatting activities, resulting in difficulty to perform his army duties. The patient reported no history of antecedent trauma. No locking of the knee or paresthesia distally into the leg or foot was observed.
The physical examination revealed a palpable and observable snapping of the long head of the biceps tendon over the fibular head with squatting beyond 90° in the left knee. The patient presented with full strength and no instability or joint line pain throughout the knee. Application of a posterior-to-anterior directed force over the biceps femoris proximal to the insertion allowed the patient to perform a deep squat without snapping. The radiographs demonstrated no abnormal fibular morphology (Figures 1A, 1B). Axial MRI images demonstrated an anomalous slip of the tendon inserting on the anterolateral aspect of the proximal tibia in addition to the normal insertion on the posterolateral and lateral edge of the fibular head (Figure 2) as described by Terry and LaPrade.14


Continue to: A conservative treatment with physical therapy...
A conservative treatment with physical therapy, activity modification, and a Cho-Pat knee strap (to provide a posterior-to-anterior buttress and to prevent snapping) was attempted for 4 weeks. However, the patient could not tolerate the strap, and the activity restraints prevented him from performing his job as an active-duty soldier. Given the failure of conservative treatment, operative intervention was elected.
Upon exploration of the biceps femoris insertion, the accessory anterolateral tibial insertion was readily identified (Figure 3). Notably, the expected normal lateral edge insertion was thickened and extended beyond the lateral edge, distal, and anterior on to the fibular head (Figure 4). The anterolateral tibial band was released first. However, the snapping remained evident. The thickened anterior fibular accessory band was then released back to its normal, lateral edge, and at this point, no further snapping was observed with deep flexion of the knee. Inspection of the remaining posterolateral and lateral edge insertion demonstrated a healthy, 1-cm thick tendinous insertion. The accessory slips were completely excised, and the incision was closed without any additional repair or re-insertion (Figure 5). The patient presented no complications postoperatively. He was allowed to bear weight as tolerated and was limited to stretching and gravity resistance training for 4 weeks. At 1 month, the patient was released to progress back to full activity. By 8 weeks postoperative, he remained free of snapping and resumed his regular running routine and military duties without restriction or pain.



DISCUSSION
Release of the anomalous bands with no further repair or re-insertion of the biceps femoris allowed this active-duty soldier to resume full running and duty-related activities in <2 months. In this particular patient, given his anatomy, the treatment was successful. The literature indicates that optimal results and surgical approach depend upon the pathological anatomy encountered.
Date and colleagues4 described a similar anatomical anomaly as with our patient, whom after the release of tibial insertion, snapping was still observed, thus requiring the release of anterior fibular insertion. They noted the necessity of suturing the accessory limbs onto the periosteum of the fibular head to achieve a stable biceps femoris.
In other cases, abnormal bony anatomy of the fibula has been shown to cause snapping. Vavalle and Capozzi5 described a case of snapping biceps in a marathon runner, who needed partial resection of the fibular head to eliminate snapping. The runner made a full return to the sport. Fung and colleagues2 described a similar approach to a 17-year-old cyclist; however, this patient presented exostoses of the bilateral fibular heads. The exostoses were bilaterally excised, and the snapping ceased. Kristensen and colleagues13 described a patient with an anomalous tibial insertion. Rather than releasing the tibial insertion, a partial resection of the fibular head allowed for cessation of snapping.
Other authors advocate the detachment and anatomic re-insertion of the biceps femoris into the fibular head. Bernhardson and LaPrade6 reported a series of 3 patients requiring this approach with excellent results. Bansal and colleagues8 were the first to describe a soccer player with an isolated injury to the knee as a traumatic cause for a snapping biceps femoris. After failure of conservative treatment attempts, exploration and re-insertion through a bone tunnel allowed for return to the sport. Hernandez and colleagues11 and Lokiec and colleagues12 both described the reproduction of the normal biceps femoris anatomy through re-insertion procedures after identifying patients with abnormal anatomical insertions as causes for snapping.
CONCLUSION
We presented a case of an active military service member with a unilateral snapping biceps femoris tendon due to an anomalous distal insertion on both the proximal tibia and anterior fibular head. The release of abnormal insertions and maintenance of his normal anatomical insertion allowed for a quick and effective return to running and duty at full capacity. Although other surgical approaches have been described to include partial fibular head resection or anatomical re-insertion, we believe that the approach to this rare condition should be anatomy-based as the causes of snapping can significantly vary. We believe that if the normal posterolateral and lateral edge insertions of the biceps femoris are intact, removal of the abnormal anatomy without any repair or reconstruction can safely lead to successful surgical outcomes.
- Barker JU, Strauss EJ, Lodha S, Bach BR Jr. Extra-articular mimickers of lateral meniscal tears. Sports Health. 2011;3(1):82-88.
- Fung DA, Frey S, Markbreiter L. Bilateral symptomatic snapping biceps femoris tendon due to fibular exostosis. J Knee Surg. 2008;21(1):55-57.
- Mirchandani M, Gandhi P, Cai P. Poster 175 bilateral symptomatic snapping knee from biceps femoris tendon subluxation–an atypical cause of bilateral knee pain: a case report. PM R. 2016;8(9S):S218-S219.
- Date H, Hayakawa K, Yamada H. Snapping knee due to the biceps femoris tendon treated with repositioning of the anomalous tibial insertion. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1581-1583.
- Vavalle G, Capozzi M. Symptomatic snapping knee from biceps femoris tendon subluxation: an unusual case of lateral pain in a marathon runner. J Orthop Traumatol. 2010;11(4):263-266.
- Bernhardson AS, LaPrade RF. Snapping biceps femoris tendon treated with an anatomic repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1110-1112.
- Guillin R, Mendoza-Ruiz JJ, Moser T, Ropars M, Duvauferrier R, Cardinal E. Snapping biceps femoris tendon: a dynamic real-time sonographic evaluation. J Clin Ultrasound. 2010;38(8):435-437.
- Bansal R, Taylor C, Pimpalnerkar AL. Snapping knee: an unusual biceps femoris tendon injury. Knee. 2005;12(6):458-460.
- Bagchi K, Grelsamer RP. Partial fibular head resection for bilateral snapping biceps femoris tendon. Orthopedics. 2003;26(11):1147-1149.
- Kissenberth MJ, Wilckens JH. The snapping biceps femoris tendon. Am J Knee Surg. 2000;13(1):25-28.
- Hernandez JA, Rius M. Noonan KJ. Snapping knee from anomalous biceps femoris tendon insertion: a case report. Iowa Orthop J. 1996;16:161-163.
- Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop Relat Res. 1992;(283):205-206.
- Kristensen G, Nielsen K, Blyme PJ. Snapping knee from biceps femoris tendon. A case report. Acta Orthop Scand. 1989;60(5):621.
- Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee. Its anatomy and injury patterns associated with acute anterolateral-anteromedial rotator instability. Am J Sports Med. 1996;24:2-8.
- Barker JU, Strauss EJ, Lodha S, Bach BR Jr. Extra-articular mimickers of lateral meniscal tears. Sports Health. 2011;3(1):82-88.
- Fung DA, Frey S, Markbreiter L. Bilateral symptomatic snapping biceps femoris tendon due to fibular exostosis. J Knee Surg. 2008;21(1):55-57.
- Mirchandani M, Gandhi P, Cai P. Poster 175 bilateral symptomatic snapping knee from biceps femoris tendon subluxation–an atypical cause of bilateral knee pain: a case report. PM R. 2016;8(9S):S218-S219.
- Date H, Hayakawa K, Yamada H. Snapping knee due to the biceps femoris tendon treated with repositioning of the anomalous tibial insertion. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1581-1583.
- Vavalle G, Capozzi M. Symptomatic snapping knee from biceps femoris tendon subluxation: an unusual case of lateral pain in a marathon runner. J Orthop Traumatol. 2010;11(4):263-266.
- Bernhardson AS, LaPrade RF. Snapping biceps femoris tendon treated with an anatomic repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1110-1112.
- Guillin R, Mendoza-Ruiz JJ, Moser T, Ropars M, Duvauferrier R, Cardinal E. Snapping biceps femoris tendon: a dynamic real-time sonographic evaluation. J Clin Ultrasound. 2010;38(8):435-437.
- Bansal R, Taylor C, Pimpalnerkar AL. Snapping knee: an unusual biceps femoris tendon injury. Knee. 2005;12(6):458-460.
- Bagchi K, Grelsamer RP. Partial fibular head resection for bilateral snapping biceps femoris tendon. Orthopedics. 2003;26(11):1147-1149.
- Kissenberth MJ, Wilckens JH. The snapping biceps femoris tendon. Am J Knee Surg. 2000;13(1):25-28.
- Hernandez JA, Rius M. Noonan KJ. Snapping knee from anomalous biceps femoris tendon insertion: a case report. Iowa Orthop J. 1996;16:161-163.
- Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop Relat Res. 1992;(283):205-206.
- Kristensen G, Nielsen K, Blyme PJ. Snapping knee from biceps femoris tendon. A case report. Acta Orthop Scand. 1989;60(5):621.
- Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee. Its anatomy and injury patterns associated with acute anterolateral-anteromedial rotator instability. Am J Sports Med. 1996;24:2-8.
TAKE-HOME POINTS
- Snapping biceps femoris is a rare, but debilitating condition.
- Understanding the pathology from an anatomical perspective is key.
- For bone abnormalities, correct the bony pathology to relieve the snapping.
- For soft tissue abnormalities, both excisional and reconstructive approaches can be utilized.
- Preservation of normal anatomy, when possible, can help expedite recovery.