User login
Subacute loss of vision in one eye • rash on hands and feet • plaques with scaling on genitals • Dx?
THE CASE
A 67-year-old man presented to the hospital with subacute loss of vision in his left eye. The visual changes began 2 weeks earlier, with a central area of visual loss that had since progressed to near complete vision loss in the left eye.

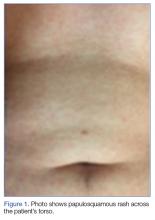
Physical examination revealed patchy alopecia, a scaling and hyperkeratotic rash of his hands and feet (FIGURE 1), and blanching, erythematous plaques with associated scaling on the scrotum and glans penis. Ophthalmologic examination revealed 1/200 vision in his left eye with a large plaque occupying a substantial portion of the superior quadrant, smaller perifoveal plaques in both of his eyes, and a small infiltrate above the left optic nerve head (FIGURE 2). The patient also described fatigue, loss of taste, and an unintentional weight loss of 7 to 10 kg over the previous 6 months. He had seen his primary care provider 3 months prior for a burning sensation and scaling rash on his feet and hands, and was prescribed a topical steroid.

The patient’s social history was relevant for intermittent condom use with 6 lifetime female partners, but it was negative for new sexual partners, sexual contact with men, intravenous drug use, tattoos, blood transfusions, or travel outside the state. His medical history was significant for hypertension.
Routine laboratory tests were remarkable for an elevated erythrocyte sedimentation rate of 53 mm/hr (normal: 0-15 mm/hr) and a C-reactive protein of 5.3 mg/dL (normal: <0.5 mg/dL). Lumbar puncture revealed a white blood cell count of 133 cells/mcL (normal: 0-5 cells/mcL) with 87% lymphocytes and protein elevated to 63 mg/dL (normal: 15-40 mg/dL).
Other tests were ordered and included a serum fourth-generation ELISA to screen for human immunodeficiency virus (HIV)-1 and HIV-2, a cerebrospinal fluid venereal disease research laboratory (CSF-VDRL) test, a syphilis IgG screen and reflexive rapid plasma reagin (RPR) quantitation, and tests for cytomegalovirus antibodies, antinuclear antibody, rheumatoid factor, and Toxoplasma antibodies. Punch biopsy of the patient’s palmar skin changes was also performed; Steiner stain and spirochete immunohistochemical stain were applied to the sample. Magnetic resonance imaging of the brain and orbit was unremarkable.
THE DIAGNOSIS
The patient’s HIV screening test came back positive and was followed by confirmation of HIV-1 antibody, with an HIV viral load of 61,000 copies/mL and a CD4 count of 383 cells/mm3. The CSF-VDRL test and serum syphilis IgG were also positive, and the RPR titer was 1:16. The Steiner and spirochete immunohistochemical stains confirmed the presence of treponemes in the epidermis (FIGURE 3). Taken together, these findings confirmed a unifying diagnosis of ocular syphilis and syphilitic keratoderma with concomitant HIV.

DISCUSSION
After reaching an all-time low in the mid-1990s, several recent reports indicate that the incidence of syphilis is again increasing in North America.1-3 In the United States, annual incidence rates have increased from 2.1/100,000 in 2000 to 5.3/100,000 in 2013.3 The increase has been most notable in younger men, men who have sex with men (MSM), and those with HIV infection.1
A 2015 Centers for Disease Control and Prevention advisory highlights an unusual collection of cases of ocular syphilis, predominantly in HIV-infected MSM, from California and Washington.4 Disease sequelae in this outbreak have resulted in blindness.
HIV coinfection has been reported in 27.5% of males and 12.4% of females with new diagnoses of syphilis.1 Patients with HIV are more likely to have asymptomatic primary syphilitic infection, and may have an earlier onset of secondary syphilis and neurosyphilis.1,5,6 Cutaneous findings such as malignant syphilis (characterized by ulcerating, pustular, or rupioid lesions), as well as other atypical rashes mimicking eczema, leprosy, mycosis fungoides, or keratoderma blenorrhagicum, may all be more common in those with HIV coinfection.6 Ageusia or dysgeusia is rare in syphilis, and to our knowledge has only been described with concomitant oral lesions.7
MANAGEMENT
Our patient was treated with a continuous daily infusion of 20 million units of penicillin G for 14 days, one drop of 1% ocular prednisolone in each eye 4 times daily for 4 weeks, one drop of 2% cyclopentoate in each eye 2 times daily for 4 weeks, and 60 mg/d of oral prednisone tapered over 3 months. For the HIV infection, he was started on antiretroviral therapy soon after diagnosis.
Within 48 hours of initiating penicillin, he reported a marked improvement in vision and regained the ability to taste. After one week of therapy, near resolution of the palmoplantar rash was noted and the patient was discharged on hospital Day 8. At a 3-month follow-up visit, he was asymptomatic, with return of normal sensation. Repeat ophthalmologic examination showed no evidence of disease.
THE TAKEAWAY
This case complements other sporadic reports of symptoms of ocular and cutaneous syphilis serving as the initial presentation of HIV infection.5,8,9 Risk-factor based screening for HIV often leads to missed diagnoses, and early recognition of this constellation of symptoms may aid in prompt diagnosis and treatment of syphilis and HIV.10
1. Lynn WA, Lightman S. Syphilis and HIV: a dangerous combination. Lancet Infect Dis. 2004;4:456-466.
2. Butler JN, Throne JE. Current status of HIV infection and ocular disease. Curr Opin Ophthalmol. 2012;23:517-522.
3. Patton ME, Su JR, Nelson R, et al. Primary and secondary syphilis–United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:402-406.
4. Woolston S, Cohen SE, Fanfare RN, et al. A cluster of ocular syphilis cases–Seattle, Washington, and San Francisco, California, 2014-2015. MMWR Morb Mortal Wkly Rep. 2015;64:1150-1151.
5. Kirby JS, Goreshi R, Mahoney N. Syphilitic palmoplantar keratoderma and ocular disease: a rare combination in an HIV-positive patient. Cutis. 2009;84:305-310.
6. Shimizu S, Yasui C, Tajima Y, et al. Unusual cutaneous features of syphilis in patients positive for human immunodeficiency virus. Clin Exp Dermatol. 2009;35:169-172.
7. Giovani EM, de Paula Neto ER, Vieira BC, et al. Conventional systemic treatments associated with therapeutic sites of local lesions of secondary syphilis in the oral cavity in patients with AIDS. Indian J Dent Res. 2012;23:670-673.
8. Kunkel J, Schürmann D, Pleyer U, et al. Ocular syphilis–indicator of previously unknown HIV-infection. J Infect. 2009;58:32-36.
9. Kishimoto M, Lee MJ, Mor A, et al. Syphilis mimicking Reiter’s syndrome in an HIV-positive patient. Am J Med Sci. 2006;332:90-92.
10. Jenkins TC, Gardner EM, Thrun MW, et al. Risk-based human immunodeficiency virus (HIV) testing fails to detect the majority of HIV-infected persons in medical care settings. Sex Transm Dis. 2006;33:329-333.
THE CASE
A 67-year-old man presented to the hospital with subacute loss of vision in his left eye. The visual changes began 2 weeks earlier, with a central area of visual loss that had since progressed to near complete vision loss in the left eye.

Physical examination revealed patchy alopecia, a scaling and hyperkeratotic rash of his hands and feet (FIGURE 1), and blanching, erythematous plaques with associated scaling on the scrotum and glans penis. Ophthalmologic examination revealed 1/200 vision in his left eye with a large plaque occupying a substantial portion of the superior quadrant, smaller perifoveal plaques in both of his eyes, and a small infiltrate above the left optic nerve head (FIGURE 2). The patient also described fatigue, loss of taste, and an unintentional weight loss of 7 to 10 kg over the previous 6 months. He had seen his primary care provider 3 months prior for a burning sensation and scaling rash on his feet and hands, and was prescribed a topical steroid.

The patient’s social history was relevant for intermittent condom use with 6 lifetime female partners, but it was negative for new sexual partners, sexual contact with men, intravenous drug use, tattoos, blood transfusions, or travel outside the state. His medical history was significant for hypertension.
Routine laboratory tests were remarkable for an elevated erythrocyte sedimentation rate of 53 mm/hr (normal: 0-15 mm/hr) and a C-reactive protein of 5.3 mg/dL (normal: <0.5 mg/dL). Lumbar puncture revealed a white blood cell count of 133 cells/mcL (normal: 0-5 cells/mcL) with 87% lymphocytes and protein elevated to 63 mg/dL (normal: 15-40 mg/dL).
Other tests were ordered and included a serum fourth-generation ELISA to screen for human immunodeficiency virus (HIV)-1 and HIV-2, a cerebrospinal fluid venereal disease research laboratory (CSF-VDRL) test, a syphilis IgG screen and reflexive rapid plasma reagin (RPR) quantitation, and tests for cytomegalovirus antibodies, antinuclear antibody, rheumatoid factor, and Toxoplasma antibodies. Punch biopsy of the patient’s palmar skin changes was also performed; Steiner stain and spirochete immunohistochemical stain were applied to the sample. Magnetic resonance imaging of the brain and orbit was unremarkable.
THE DIAGNOSIS
The patient’s HIV screening test came back positive and was followed by confirmation of HIV-1 antibody, with an HIV viral load of 61,000 copies/mL and a CD4 count of 383 cells/mm3. The CSF-VDRL test and serum syphilis IgG were also positive, and the RPR titer was 1:16. The Steiner and spirochete immunohistochemical stains confirmed the presence of treponemes in the epidermis (FIGURE 3). Taken together, these findings confirmed a unifying diagnosis of ocular syphilis and syphilitic keratoderma with concomitant HIV.

DISCUSSION
After reaching an all-time low in the mid-1990s, several recent reports indicate that the incidence of syphilis is again increasing in North America.1-3 In the United States, annual incidence rates have increased from 2.1/100,000 in 2000 to 5.3/100,000 in 2013.3 The increase has been most notable in younger men, men who have sex with men (MSM), and those with HIV infection.1
A 2015 Centers for Disease Control and Prevention advisory highlights an unusual collection of cases of ocular syphilis, predominantly in HIV-infected MSM, from California and Washington.4 Disease sequelae in this outbreak have resulted in blindness.
HIV coinfection has been reported in 27.5% of males and 12.4% of females with new diagnoses of syphilis.1 Patients with HIV are more likely to have asymptomatic primary syphilitic infection, and may have an earlier onset of secondary syphilis and neurosyphilis.1,5,6 Cutaneous findings such as malignant syphilis (characterized by ulcerating, pustular, or rupioid lesions), as well as other atypical rashes mimicking eczema, leprosy, mycosis fungoides, or keratoderma blenorrhagicum, may all be more common in those with HIV coinfection.6 Ageusia or dysgeusia is rare in syphilis, and to our knowledge has only been described with concomitant oral lesions.7
MANAGEMENT
Our patient was treated with a continuous daily infusion of 20 million units of penicillin G for 14 days, one drop of 1% ocular prednisolone in each eye 4 times daily for 4 weeks, one drop of 2% cyclopentoate in each eye 2 times daily for 4 weeks, and 60 mg/d of oral prednisone tapered over 3 months. For the HIV infection, he was started on antiretroviral therapy soon after diagnosis.
Within 48 hours of initiating penicillin, he reported a marked improvement in vision and regained the ability to taste. After one week of therapy, near resolution of the palmoplantar rash was noted and the patient was discharged on hospital Day 8. At a 3-month follow-up visit, he was asymptomatic, with return of normal sensation. Repeat ophthalmologic examination showed no evidence of disease.
THE TAKEAWAY
This case complements other sporadic reports of symptoms of ocular and cutaneous syphilis serving as the initial presentation of HIV infection.5,8,9 Risk-factor based screening for HIV often leads to missed diagnoses, and early recognition of this constellation of symptoms may aid in prompt diagnosis and treatment of syphilis and HIV.10
THE CASE
A 67-year-old man presented to the hospital with subacute loss of vision in his left eye. The visual changes began 2 weeks earlier, with a central area of visual loss that had since progressed to near complete vision loss in the left eye.

Physical examination revealed patchy alopecia, a scaling and hyperkeratotic rash of his hands and feet (FIGURE 1), and blanching, erythematous plaques with associated scaling on the scrotum and glans penis. Ophthalmologic examination revealed 1/200 vision in his left eye with a large plaque occupying a substantial portion of the superior quadrant, smaller perifoveal plaques in both of his eyes, and a small infiltrate above the left optic nerve head (FIGURE 2). The patient also described fatigue, loss of taste, and an unintentional weight loss of 7 to 10 kg over the previous 6 months. He had seen his primary care provider 3 months prior for a burning sensation and scaling rash on his feet and hands, and was prescribed a topical steroid.

The patient’s social history was relevant for intermittent condom use with 6 lifetime female partners, but it was negative for new sexual partners, sexual contact with men, intravenous drug use, tattoos, blood transfusions, or travel outside the state. His medical history was significant for hypertension.
Routine laboratory tests were remarkable for an elevated erythrocyte sedimentation rate of 53 mm/hr (normal: 0-15 mm/hr) and a C-reactive protein of 5.3 mg/dL (normal: <0.5 mg/dL). Lumbar puncture revealed a white blood cell count of 133 cells/mcL (normal: 0-5 cells/mcL) with 87% lymphocytes and protein elevated to 63 mg/dL (normal: 15-40 mg/dL).
Other tests were ordered and included a serum fourth-generation ELISA to screen for human immunodeficiency virus (HIV)-1 and HIV-2, a cerebrospinal fluid venereal disease research laboratory (CSF-VDRL) test, a syphilis IgG screen and reflexive rapid plasma reagin (RPR) quantitation, and tests for cytomegalovirus antibodies, antinuclear antibody, rheumatoid factor, and Toxoplasma antibodies. Punch biopsy of the patient’s palmar skin changes was also performed; Steiner stain and spirochete immunohistochemical stain were applied to the sample. Magnetic resonance imaging of the brain and orbit was unremarkable.
THE DIAGNOSIS
The patient’s HIV screening test came back positive and was followed by confirmation of HIV-1 antibody, with an HIV viral load of 61,000 copies/mL and a CD4 count of 383 cells/mm3. The CSF-VDRL test and serum syphilis IgG were also positive, and the RPR titer was 1:16. The Steiner and spirochete immunohistochemical stains confirmed the presence of treponemes in the epidermis (FIGURE 3). Taken together, these findings confirmed a unifying diagnosis of ocular syphilis and syphilitic keratoderma with concomitant HIV.

DISCUSSION
After reaching an all-time low in the mid-1990s, several recent reports indicate that the incidence of syphilis is again increasing in North America.1-3 In the United States, annual incidence rates have increased from 2.1/100,000 in 2000 to 5.3/100,000 in 2013.3 The increase has been most notable in younger men, men who have sex with men (MSM), and those with HIV infection.1
A 2015 Centers for Disease Control and Prevention advisory highlights an unusual collection of cases of ocular syphilis, predominantly in HIV-infected MSM, from California and Washington.4 Disease sequelae in this outbreak have resulted in blindness.
HIV coinfection has been reported in 27.5% of males and 12.4% of females with new diagnoses of syphilis.1 Patients with HIV are more likely to have asymptomatic primary syphilitic infection, and may have an earlier onset of secondary syphilis and neurosyphilis.1,5,6 Cutaneous findings such as malignant syphilis (characterized by ulcerating, pustular, or rupioid lesions), as well as other atypical rashes mimicking eczema, leprosy, mycosis fungoides, or keratoderma blenorrhagicum, may all be more common in those with HIV coinfection.6 Ageusia or dysgeusia is rare in syphilis, and to our knowledge has only been described with concomitant oral lesions.7
MANAGEMENT
Our patient was treated with a continuous daily infusion of 20 million units of penicillin G for 14 days, one drop of 1% ocular prednisolone in each eye 4 times daily for 4 weeks, one drop of 2% cyclopentoate in each eye 2 times daily for 4 weeks, and 60 mg/d of oral prednisone tapered over 3 months. For the HIV infection, he was started on antiretroviral therapy soon after diagnosis.
Within 48 hours of initiating penicillin, he reported a marked improvement in vision and regained the ability to taste. After one week of therapy, near resolution of the palmoplantar rash was noted and the patient was discharged on hospital Day 8. At a 3-month follow-up visit, he was asymptomatic, with return of normal sensation. Repeat ophthalmologic examination showed no evidence of disease.
THE TAKEAWAY
This case complements other sporadic reports of symptoms of ocular and cutaneous syphilis serving as the initial presentation of HIV infection.5,8,9 Risk-factor based screening for HIV often leads to missed diagnoses, and early recognition of this constellation of symptoms may aid in prompt diagnosis and treatment of syphilis and HIV.10
1. Lynn WA, Lightman S. Syphilis and HIV: a dangerous combination. Lancet Infect Dis. 2004;4:456-466.
2. Butler JN, Throne JE. Current status of HIV infection and ocular disease. Curr Opin Ophthalmol. 2012;23:517-522.
3. Patton ME, Su JR, Nelson R, et al. Primary and secondary syphilis–United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:402-406.
4. Woolston S, Cohen SE, Fanfare RN, et al. A cluster of ocular syphilis cases–Seattle, Washington, and San Francisco, California, 2014-2015. MMWR Morb Mortal Wkly Rep. 2015;64:1150-1151.
5. Kirby JS, Goreshi R, Mahoney N. Syphilitic palmoplantar keratoderma and ocular disease: a rare combination in an HIV-positive patient. Cutis. 2009;84:305-310.
6. Shimizu S, Yasui C, Tajima Y, et al. Unusual cutaneous features of syphilis in patients positive for human immunodeficiency virus. Clin Exp Dermatol. 2009;35:169-172.
7. Giovani EM, de Paula Neto ER, Vieira BC, et al. Conventional systemic treatments associated with therapeutic sites of local lesions of secondary syphilis in the oral cavity in patients with AIDS. Indian J Dent Res. 2012;23:670-673.
8. Kunkel J, Schürmann D, Pleyer U, et al. Ocular syphilis–indicator of previously unknown HIV-infection. J Infect. 2009;58:32-36.
9. Kishimoto M, Lee MJ, Mor A, et al. Syphilis mimicking Reiter’s syndrome in an HIV-positive patient. Am J Med Sci. 2006;332:90-92.
10. Jenkins TC, Gardner EM, Thrun MW, et al. Risk-based human immunodeficiency virus (HIV) testing fails to detect the majority of HIV-infected persons in medical care settings. Sex Transm Dis. 2006;33:329-333.
1. Lynn WA, Lightman S. Syphilis and HIV: a dangerous combination. Lancet Infect Dis. 2004;4:456-466.
2. Butler JN, Throne JE. Current status of HIV infection and ocular disease. Curr Opin Ophthalmol. 2012;23:517-522.
3. Patton ME, Su JR, Nelson R, et al. Primary and secondary syphilis–United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:402-406.
4. Woolston S, Cohen SE, Fanfare RN, et al. A cluster of ocular syphilis cases–Seattle, Washington, and San Francisco, California, 2014-2015. MMWR Morb Mortal Wkly Rep. 2015;64:1150-1151.
5. Kirby JS, Goreshi R, Mahoney N. Syphilitic palmoplantar keratoderma and ocular disease: a rare combination in an HIV-positive patient. Cutis. 2009;84:305-310.
6. Shimizu S, Yasui C, Tajima Y, et al. Unusual cutaneous features of syphilis in patients positive for human immunodeficiency virus. Clin Exp Dermatol. 2009;35:169-172.
7. Giovani EM, de Paula Neto ER, Vieira BC, et al. Conventional systemic treatments associated with therapeutic sites of local lesions of secondary syphilis in the oral cavity in patients with AIDS. Indian J Dent Res. 2012;23:670-673.
8. Kunkel J, Schürmann D, Pleyer U, et al. Ocular syphilis–indicator of previously unknown HIV-infection. J Infect. 2009;58:32-36.
9. Kishimoto M, Lee MJ, Mor A, et al. Syphilis mimicking Reiter’s syndrome in an HIV-positive patient. Am J Med Sci. 2006;332:90-92.
10. Jenkins TC, Gardner EM, Thrun MW, et al. Risk-based human immunodeficiency virus (HIV) testing fails to detect the majority of HIV-infected persons in medical care settings. Sex Transm Dis. 2006;33:329-333.
Erythematous, friable nipple with loss of protrusion • history of breastfeeding • Dx?
THE CASE
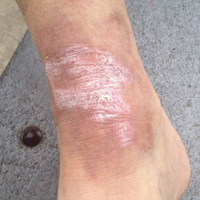
A 34-year-old healthy woman presented to the breast surgical oncology clinic with skin changes to her left nipple after being referred by her primary care provider. She attributed the skin changes to shearing from breastfeeding her third child 5 years earlier. Physical examination revealed an erythematous and friable nipple with loss of protrusion (FIGURE 1). The patient reported routine bleeding from her nipple, but said the skin changes had remained stable and denied any breast masses. The patient’s last mammogram was 2.5 years earlier and had only been remarkable for bilateral benign calcifications.

THE DIAGNOSIS
A screening mammogram showed flattening and retraction of the left nipple, as well as suspicious left breast calcifications (BIRADS [Breast Imaging Reporting and Data System] 4 classification, FIGURE 2). A subsequent diagnostic mammogram showed a cluster of fine pleomorphic calcifications in the upper inner quadrant of the left breast (FIGURE 3). A stereotactic core needle biopsy was performed, and results confirmed a diagnosis of high-grade, estrogen receptor-negative, ductal carcinoma in situ (DCIS).


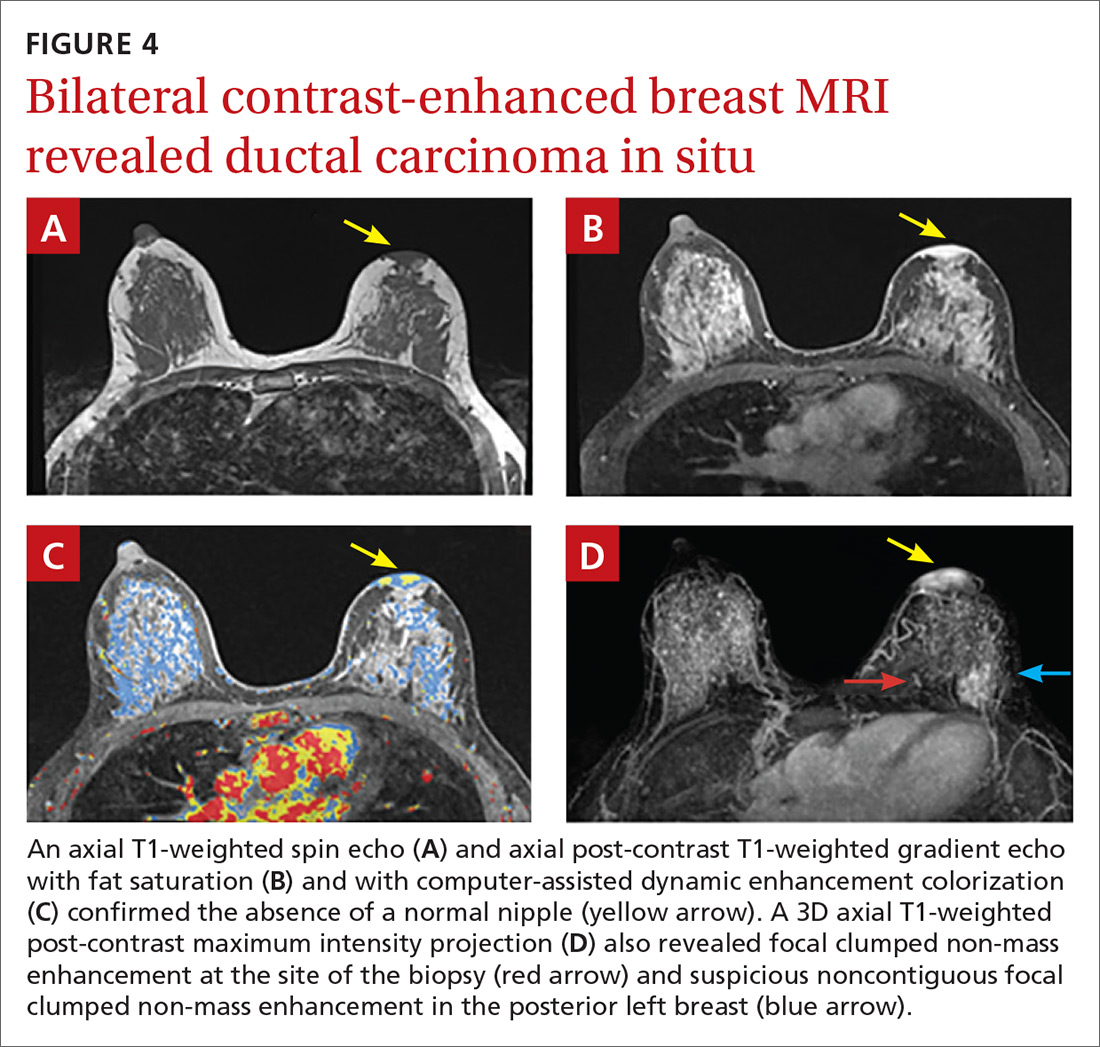
Subsequent work-up included a staging magnetic resonance imaging (MRI) and a left areola punch biopsy. MRI revealed an absence of a normal left nipple and extensive focal clumped non-mass enhancement in the area of the known DCIS (FIGURE 4). Biopsy results revealed enlarged atypical single cells within the epidermis. The cells stained positive for mucicarmine and cytokeratin 7 and negative for carcinoembryonic antigen and S-100 protein. This ruled out a pagetoid spread of melanoma and confirmed a diagnosis of Paget’s disease (PD) of the breast.
DISCUSSION
PD of the breast is a rare disorder (accounting for 0.5%-5% of all breast cancers) that is clinically characterized by erythematous, eczematous changes of the nipple-areolar complex (NAC).1-7 PD is almost always unilateral and symptoms include pain, burning, and itching of the nipple, often with bloody nipple discharge.1,3-8
PD can be mistaken for benign skin changes and diagnosed as dermatitis or eczema.3,5 Because such changes often resolve temporarily with the use of topical corticosteroids or no treatment at all,2 diagnosis is often delayed. PD of the breast is associated with underlying ductal carcinoma in 90% to 100% of cases,1,2,5,8 so any skin pathology involving the nipple should be assumed to be PD until proven otherwise.
When no palpable mass is noted on physical exam, DCIS is usually found centrally behind the nipple.1 In addition, lymph node involvement is noted in about 60% of cases.1
Confirm the diagnosis with these tests
Diagnosis of PD of the breast is primarily clinical, with pathologic confirmation. All patients with clinically suspected PD should be evaluated using the following tests to determine the need for biopsy.
Mammography with magnification views of the NAC will show thickening, retraction, or flattening of the nipple, microcalcifications of the retroareolar region, and/or a subareolar mass.3 However, because breast tissue appears normal on mammography in 22% to 71% of patients,1,5 the use of ultrasound and potentially MRI to delineate the extent of disease is warranted.
Ultrasound. While there are no characteristic findings on ultrasound, it can be used to detect dilation of the subareolar ducts, calcification, or a mass.4
MRI has a higher sensitivity for detection of occult disease.2,5 MRI is also useful in the evaluation of axillary node asymmetry, which may indicate nodal involvement.2
Treatment is variable and has not been widely studied
Due to the rarity of PD, there are no randomized studies to point toward optimal treatment strategies.7 Treatment for PD is typically surgical and often involves mastectomy, with or without axillary node dissection.1 Retrospective analyses have demonstrated that central lumpectomy (complete resection of the NAC and underlying disease) with radiation therapy has outcomes similar to mastectomy;2 however, the cosmetic result is sometimes unfavorable.
In cases where there is no palpable mass nor mammographic findings of disease, breast conserving therapy may be considered. If chemotherapy is considered, it should be chosen based on the receptor profile of the disease and subsequent oncotype scoring.
The prognosis for patients with PD who are adequately treated and remain disease free after 5 years is excellent. These patients are likely to have achieved cure.2
Our patient underwent left simple mastectomy with sentinel node biopsy and tissue expander placement. Her postoperative course was uncomplicated, and she was discharged home on postoperative Day 1. On final pathology, the 2 sentinel nodes were disease free. The left mastectomy specimen was found to have high-grade DCIS with clear surgical margins. The area of involvement was found to be 3.5 cm × 3 cm in size and had clear skin margins. At follow-up one year later, the patient was doing well with no evidence of disease. She subsequently underwent implant insertion.
THE TAKEAWAY
This case highlights the unique progression of undiagnosed PD of the breast. It also highlights the importance of ruling out PD when skin changes involving the nipple are present, despite other possible explanations for those changes. This case in particular was complicated by a proximal history of breastfeeding, which erroneously provided an explanation and false reassurance for the primary care provider and patient.
Due to the common association of PD of the breast with underlying DCIS or invasive cancer, the most important aspect of care is early diagnostic work-up and appropriate referral. Primary care physicians have a unique role in obtaining appropriate early diagnostic tests (including mammogram and ultrasound) and making the necessary referral to a breast specialist in the presence of an abnormal physical exam involving the NAC, even in the absence of a palpable mass. In our patient’s case, punch biopsy of the NAC would have been appropriate at the first signs of friable, erythematous changes.
1. Kollmorgen DR, Varanasi JS, Edge SB, et al. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg. 1998;187:171-177.
2. Sakorafas GH, Blanchard K, Sarr MG, et al. Paget’s disease of the breast. Cancer Treat Rev. 2001;27:9-18.
3. Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, et al. Paget’s disease of the nipple. Breast Cancer Res Treat. 2013;141:1-12.
4. Soler T, Lerin A, Serrano T, et al. Pigmented paget disease of the breast nipple with underlying infiltrating carcinoma: a case report and review of the literature. Am J Dermatopathol. 2011;33:e54-e57.
5. Trebska-McGowan K, Terracina KP, Takabe K. Update on the surgical management of Paget’s disease. Gland Surg. 2013;2:137-142.
6. Sakorafas GH, Blanchard DK, Sarr MG, et al. Paget’s disease of the breast: a clinical perspective. Langenbecks Arch Surg. 2001;386;444-450.
7. Durkan B, Bresee C, Bose S, et al. Paget’s disease of the nipple with parenchymal ductal carcinoma in situ is associated with worse prognosis than Paget’s disease alone. Am Surg. 2013;79:1009-1012.
8. Ward KA, Burton JL. Dermatologic diseases of the breast in young women. Clin Dermatol. 1997;15:45-52.
THE CASE
A 34-year-old healthy woman presented to the breast surgical oncology clinic with skin changes to her left nipple after being referred by her primary care provider. She attributed the skin changes to shearing from breastfeeding her third child 5 years earlier. Physical examination revealed an erythematous and friable nipple with loss of protrusion (FIGURE 1). The patient reported routine bleeding from her nipple, but said the skin changes had remained stable and denied any breast masses. The patient’s last mammogram was 2.5 years earlier and had only been remarkable for bilateral benign calcifications.

THE DIAGNOSIS
A screening mammogram showed flattening and retraction of the left nipple, as well as suspicious left breast calcifications (BIRADS [Breast Imaging Reporting and Data System] 4 classification, FIGURE 2). A subsequent diagnostic mammogram showed a cluster of fine pleomorphic calcifications in the upper inner quadrant of the left breast (FIGURE 3). A stereotactic core needle biopsy was performed, and results confirmed a diagnosis of high-grade, estrogen receptor-negative, ductal carcinoma in situ (DCIS).


Subsequent work-up included a staging magnetic resonance imaging (MRI) and a left areola punch biopsy. MRI revealed an absence of a normal left nipple and extensive focal clumped non-mass enhancement in the area of the known DCIS (FIGURE 4). Biopsy results revealed enlarged atypical single cells within the epidermis. The cells stained positive for mucicarmine and cytokeratin 7 and negative for carcinoembryonic antigen and S-100 protein. This ruled out a pagetoid spread of melanoma and confirmed a diagnosis of Paget’s disease (PD) of the breast.

DISCUSSION
PD of the breast is a rare disorder (accounting for 0.5%-5% of all breast cancers) that is clinically characterized by erythematous, eczematous changes of the nipple-areolar complex (NAC).1-7 PD is almost always unilateral and symptoms include pain, burning, and itching of the nipple, often with bloody nipple discharge.1,3-8
PD can be mistaken for benign skin changes and diagnosed as dermatitis or eczema.3,5 Because such changes often resolve temporarily with the use of topical corticosteroids or no treatment at all,2 diagnosis is often delayed. PD of the breast is associated with underlying ductal carcinoma in 90% to 100% of cases,1,2,5,8 so any skin pathology involving the nipple should be assumed to be PD until proven otherwise.
When no palpable mass is noted on physical exam, DCIS is usually found centrally behind the nipple.1 In addition, lymph node involvement is noted in about 60% of cases.1
Confirm the diagnosis with these tests
Diagnosis of PD of the breast is primarily clinical, with pathologic confirmation. All patients with clinically suspected PD should be evaluated using the following tests to determine the need for biopsy.
Mammography with magnification views of the NAC will show thickening, retraction, or flattening of the nipple, microcalcifications of the retroareolar region, and/or a subareolar mass.3 However, because breast tissue appears normal on mammography in 22% to 71% of patients,1,5 the use of ultrasound and potentially MRI to delineate the extent of disease is warranted.
Ultrasound. While there are no characteristic findings on ultrasound, it can be used to detect dilation of the subareolar ducts, calcification, or a mass.4
MRI has a higher sensitivity for detection of occult disease.2,5 MRI is also useful in the evaluation of axillary node asymmetry, which may indicate nodal involvement.2
Treatment is variable and has not been widely studied
Due to the rarity of PD, there are no randomized studies to point toward optimal treatment strategies.7 Treatment for PD is typically surgical and often involves mastectomy, with or without axillary node dissection.1 Retrospective analyses have demonstrated that central lumpectomy (complete resection of the NAC and underlying disease) with radiation therapy has outcomes similar to mastectomy;2 however, the cosmetic result is sometimes unfavorable.
In cases where there is no palpable mass nor mammographic findings of disease, breast conserving therapy may be considered. If chemotherapy is considered, it should be chosen based on the receptor profile of the disease and subsequent oncotype scoring.
The prognosis for patients with PD who are adequately treated and remain disease free after 5 years is excellent. These patients are likely to have achieved cure.2
Our patient underwent left simple mastectomy with sentinel node biopsy and tissue expander placement. Her postoperative course was uncomplicated, and she was discharged home on postoperative Day 1. On final pathology, the 2 sentinel nodes were disease free. The left mastectomy specimen was found to have high-grade DCIS with clear surgical margins. The area of involvement was found to be 3.5 cm × 3 cm in size and had clear skin margins. At follow-up one year later, the patient was doing well with no evidence of disease. She subsequently underwent implant insertion.
THE TAKEAWAY
This case highlights the unique progression of undiagnosed PD of the breast. It also highlights the importance of ruling out PD when skin changes involving the nipple are present, despite other possible explanations for those changes. This case in particular was complicated by a proximal history of breastfeeding, which erroneously provided an explanation and false reassurance for the primary care provider and patient.
Due to the common association of PD of the breast with underlying DCIS or invasive cancer, the most important aspect of care is early diagnostic work-up and appropriate referral. Primary care physicians have a unique role in obtaining appropriate early diagnostic tests (including mammogram and ultrasound) and making the necessary referral to a breast specialist in the presence of an abnormal physical exam involving the NAC, even in the absence of a palpable mass. In our patient’s case, punch biopsy of the NAC would have been appropriate at the first signs of friable, erythematous changes.
THE CASE
A 34-year-old healthy woman presented to the breast surgical oncology clinic with skin changes to her left nipple after being referred by her primary care provider. She attributed the skin changes to shearing from breastfeeding her third child 5 years earlier. Physical examination revealed an erythematous and friable nipple with loss of protrusion (FIGURE 1). The patient reported routine bleeding from her nipple, but said the skin changes had remained stable and denied any breast masses. The patient’s last mammogram was 2.5 years earlier and had only been remarkable for bilateral benign calcifications.

THE DIAGNOSIS
A screening mammogram showed flattening and retraction of the left nipple, as well as suspicious left breast calcifications (BIRADS [Breast Imaging Reporting and Data System] 4 classification, FIGURE 2). A subsequent diagnostic mammogram showed a cluster of fine pleomorphic calcifications in the upper inner quadrant of the left breast (FIGURE 3). A stereotactic core needle biopsy was performed, and results confirmed a diagnosis of high-grade, estrogen receptor-negative, ductal carcinoma in situ (DCIS).


Subsequent work-up included a staging magnetic resonance imaging (MRI) and a left areola punch biopsy. MRI revealed an absence of a normal left nipple and extensive focal clumped non-mass enhancement in the area of the known DCIS (FIGURE 4). Biopsy results revealed enlarged atypical single cells within the epidermis. The cells stained positive for mucicarmine and cytokeratin 7 and negative for carcinoembryonic antigen and S-100 protein. This ruled out a pagetoid spread of melanoma and confirmed a diagnosis of Paget’s disease (PD) of the breast.

DISCUSSION
PD of the breast is a rare disorder (accounting for 0.5%-5% of all breast cancers) that is clinically characterized by erythematous, eczematous changes of the nipple-areolar complex (NAC).1-7 PD is almost always unilateral and symptoms include pain, burning, and itching of the nipple, often with bloody nipple discharge.1,3-8
PD can be mistaken for benign skin changes and diagnosed as dermatitis or eczema.3,5 Because such changes often resolve temporarily with the use of topical corticosteroids or no treatment at all,2 diagnosis is often delayed. PD of the breast is associated with underlying ductal carcinoma in 90% to 100% of cases,1,2,5,8 so any skin pathology involving the nipple should be assumed to be PD until proven otherwise.
When no palpable mass is noted on physical exam, DCIS is usually found centrally behind the nipple.1 In addition, lymph node involvement is noted in about 60% of cases.1
Confirm the diagnosis with these tests
Diagnosis of PD of the breast is primarily clinical, with pathologic confirmation. All patients with clinically suspected PD should be evaluated using the following tests to determine the need for biopsy.
Mammography with magnification views of the NAC will show thickening, retraction, or flattening of the nipple, microcalcifications of the retroareolar region, and/or a subareolar mass.3 However, because breast tissue appears normal on mammography in 22% to 71% of patients,1,5 the use of ultrasound and potentially MRI to delineate the extent of disease is warranted.
Ultrasound. While there are no characteristic findings on ultrasound, it can be used to detect dilation of the subareolar ducts, calcification, or a mass.4
MRI has a higher sensitivity for detection of occult disease.2,5 MRI is also useful in the evaluation of axillary node asymmetry, which may indicate nodal involvement.2
Treatment is variable and has not been widely studied
Due to the rarity of PD, there are no randomized studies to point toward optimal treatment strategies.7 Treatment for PD is typically surgical and often involves mastectomy, with or without axillary node dissection.1 Retrospective analyses have demonstrated that central lumpectomy (complete resection of the NAC and underlying disease) with radiation therapy has outcomes similar to mastectomy;2 however, the cosmetic result is sometimes unfavorable.
In cases where there is no palpable mass nor mammographic findings of disease, breast conserving therapy may be considered. If chemotherapy is considered, it should be chosen based on the receptor profile of the disease and subsequent oncotype scoring.
The prognosis for patients with PD who are adequately treated and remain disease free after 5 years is excellent. These patients are likely to have achieved cure.2
Our patient underwent left simple mastectomy with sentinel node biopsy and tissue expander placement. Her postoperative course was uncomplicated, and she was discharged home on postoperative Day 1. On final pathology, the 2 sentinel nodes were disease free. The left mastectomy specimen was found to have high-grade DCIS with clear surgical margins. The area of involvement was found to be 3.5 cm × 3 cm in size and had clear skin margins. At follow-up one year later, the patient was doing well with no evidence of disease. She subsequently underwent implant insertion.
THE TAKEAWAY
This case highlights the unique progression of undiagnosed PD of the breast. It also highlights the importance of ruling out PD when skin changes involving the nipple are present, despite other possible explanations for those changes. This case in particular was complicated by a proximal history of breastfeeding, which erroneously provided an explanation and false reassurance for the primary care provider and patient.
Due to the common association of PD of the breast with underlying DCIS or invasive cancer, the most important aspect of care is early diagnostic work-up and appropriate referral. Primary care physicians have a unique role in obtaining appropriate early diagnostic tests (including mammogram and ultrasound) and making the necessary referral to a breast specialist in the presence of an abnormal physical exam involving the NAC, even in the absence of a palpable mass. In our patient’s case, punch biopsy of the NAC would have been appropriate at the first signs of friable, erythematous changes.
1. Kollmorgen DR, Varanasi JS, Edge SB, et al. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg. 1998;187:171-177.
2. Sakorafas GH, Blanchard K, Sarr MG, et al. Paget’s disease of the breast. Cancer Treat Rev. 2001;27:9-18.
3. Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, et al. Paget’s disease of the nipple. Breast Cancer Res Treat. 2013;141:1-12.
4. Soler T, Lerin A, Serrano T, et al. Pigmented paget disease of the breast nipple with underlying infiltrating carcinoma: a case report and review of the literature. Am J Dermatopathol. 2011;33:e54-e57.
5. Trebska-McGowan K, Terracina KP, Takabe K. Update on the surgical management of Paget’s disease. Gland Surg. 2013;2:137-142.
6. Sakorafas GH, Blanchard DK, Sarr MG, et al. Paget’s disease of the breast: a clinical perspective. Langenbecks Arch Surg. 2001;386;444-450.
7. Durkan B, Bresee C, Bose S, et al. Paget’s disease of the nipple with parenchymal ductal carcinoma in situ is associated with worse prognosis than Paget’s disease alone. Am Surg. 2013;79:1009-1012.
8. Ward KA, Burton JL. Dermatologic diseases of the breast in young women. Clin Dermatol. 1997;15:45-52.
1. Kollmorgen DR, Varanasi JS, Edge SB, et al. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg. 1998;187:171-177.
2. Sakorafas GH, Blanchard K, Sarr MG, et al. Paget’s disease of the breast. Cancer Treat Rev. 2001;27:9-18.
3. Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, et al. Paget’s disease of the nipple. Breast Cancer Res Treat. 2013;141:1-12.
4. Soler T, Lerin A, Serrano T, et al. Pigmented paget disease of the breast nipple with underlying infiltrating carcinoma: a case report and review of the literature. Am J Dermatopathol. 2011;33:e54-e57.
5. Trebska-McGowan K, Terracina KP, Takabe K. Update on the surgical management of Paget’s disease. Gland Surg. 2013;2:137-142.
6. Sakorafas GH, Blanchard DK, Sarr MG, et al. Paget’s disease of the breast: a clinical perspective. Langenbecks Arch Surg. 2001;386;444-450.
7. Durkan B, Bresee C, Bose S, et al. Paget’s disease of the nipple with parenchymal ductal carcinoma in situ is associated with worse prognosis than Paget’s disease alone. Am Surg. 2013;79:1009-1012.
8. Ward KA, Burton JL. Dermatologic diseases of the breast in young women. Clin Dermatol. 1997;15:45-52.
Linear Porokeratosis Associated With Multiple Squamous Cell Carcinomas
Lesions of porokeratosis are thought to arise from disordered keratinization, though the exact pathogenesis remains uncertain. At least 5 clinical subtypes of porokeratosis have been identified: porokeratosis of Mibelli, disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, punctuate porokeratosis, and porokeratosis palmaris et plantaris disseminata (PPPD).1,2 Linear porokeratosis is a rare subtype with a clinical differential diagnosis that includes lichen striatus, linear lichen planus, linear verrucous epidermal nevus, segmental Darier disease, and incontinentia pigmenti.3 Definitive diagnosis of linear porokeratosis is made by histopathologic examination demonstrating a cornoid lamella, defined as a column of parakeratotic cells that lies at 45°to the surface of the epidermis and contains pyknotic basophilic nuclei.4 Patients with linear porokeratosis typically develop lesions along the lines of Blaschko in infancy or childhood.5,6 Among the different subtypes of porokeratosis, linear porokeratosis demonstrates the highest rate of malignant transformation, therefore requiring close clinical observation.7
Case Report
An 83-year-old woman presented to the outpatient clinic with a large linear plaque on the right leg that had been present since birth. Ten years prior to presentation, a portion of the lesion started to bleed; biopsy of the area was performed by an outside provider demonstrating squamous cell carcinoma (SCC), which was treated with wide local excision. One year prior to presentation, a separate portion of the plaque was biopsied by an outside provider and another diagnosis of SCC was made.
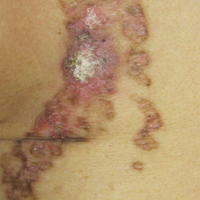
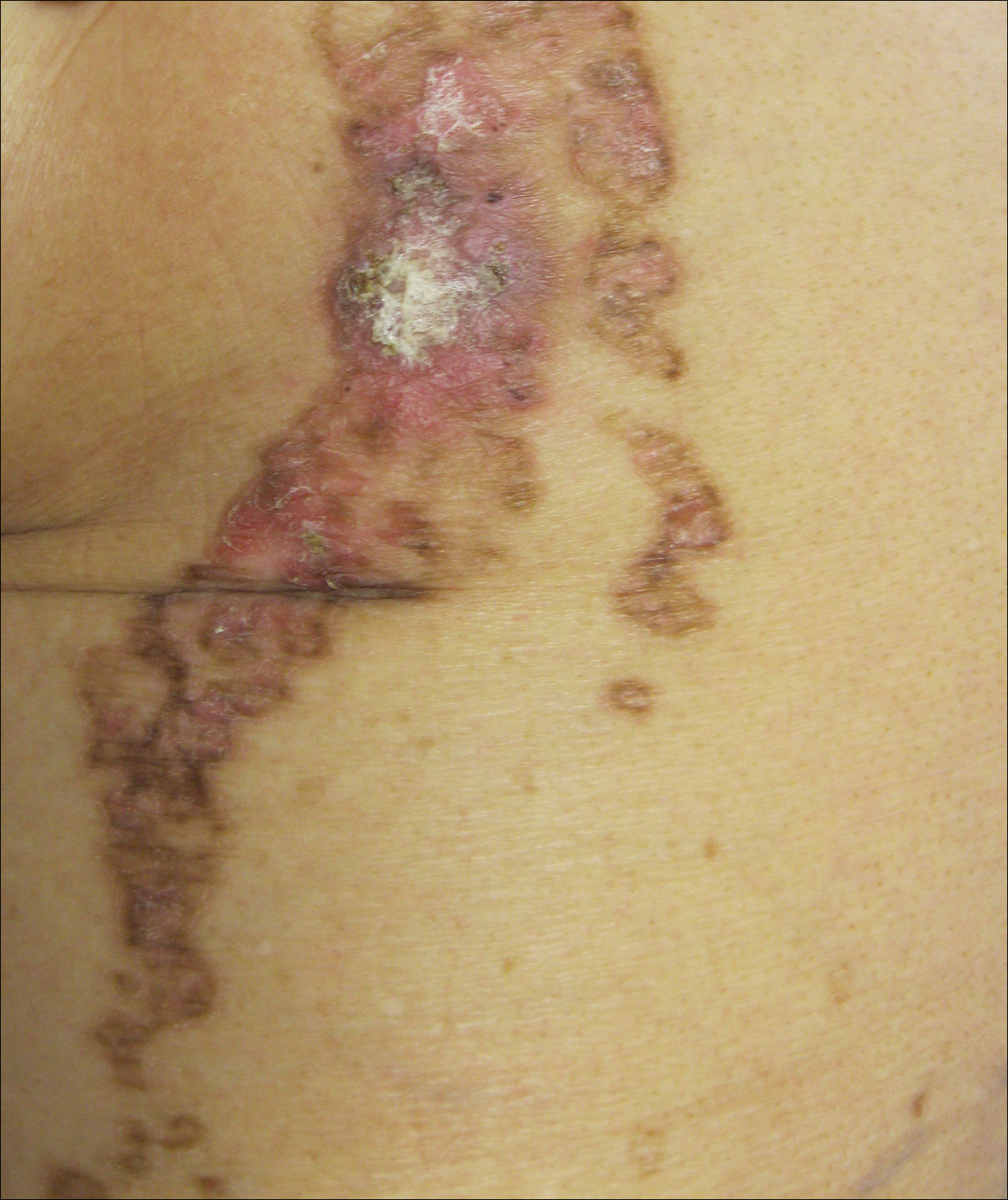
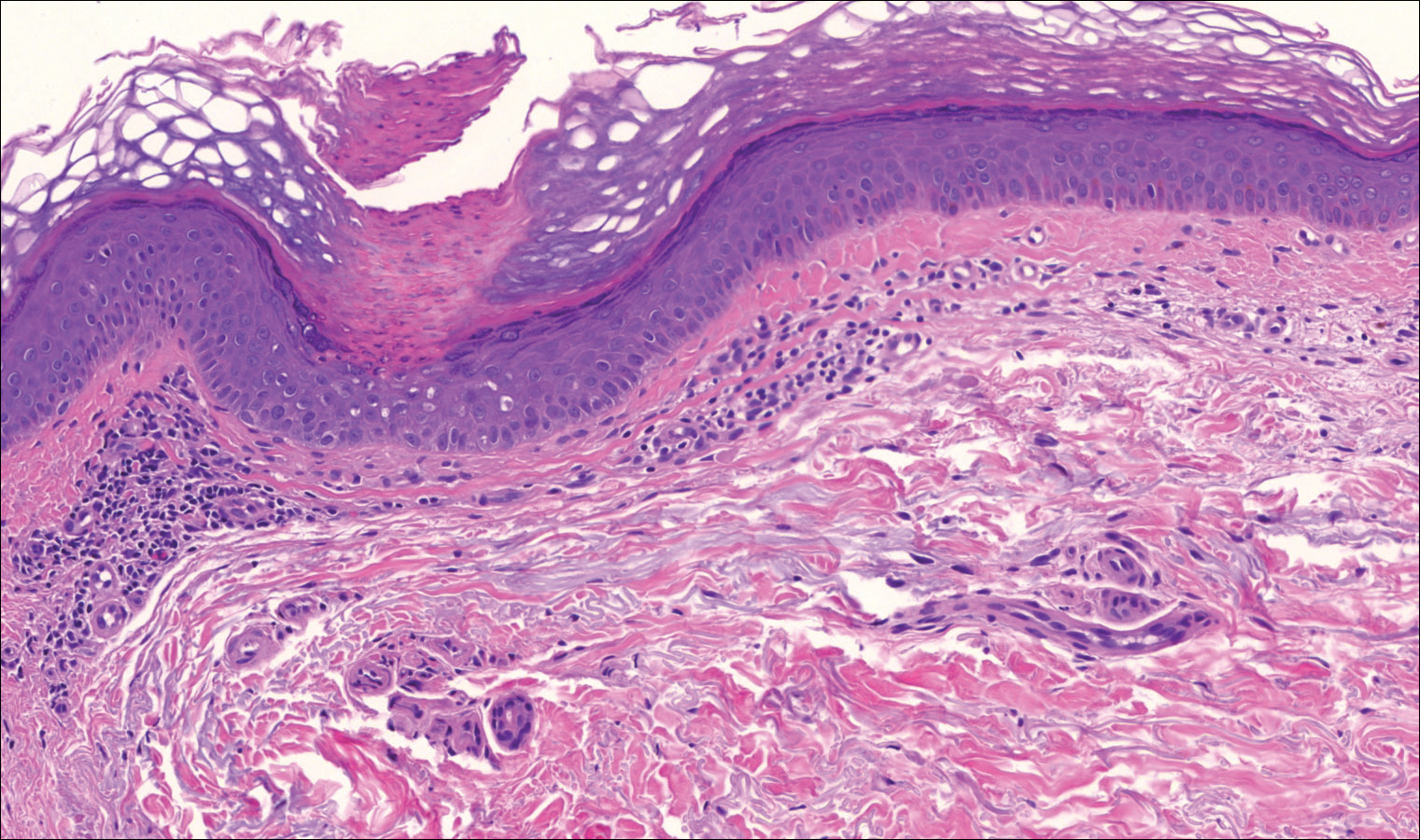
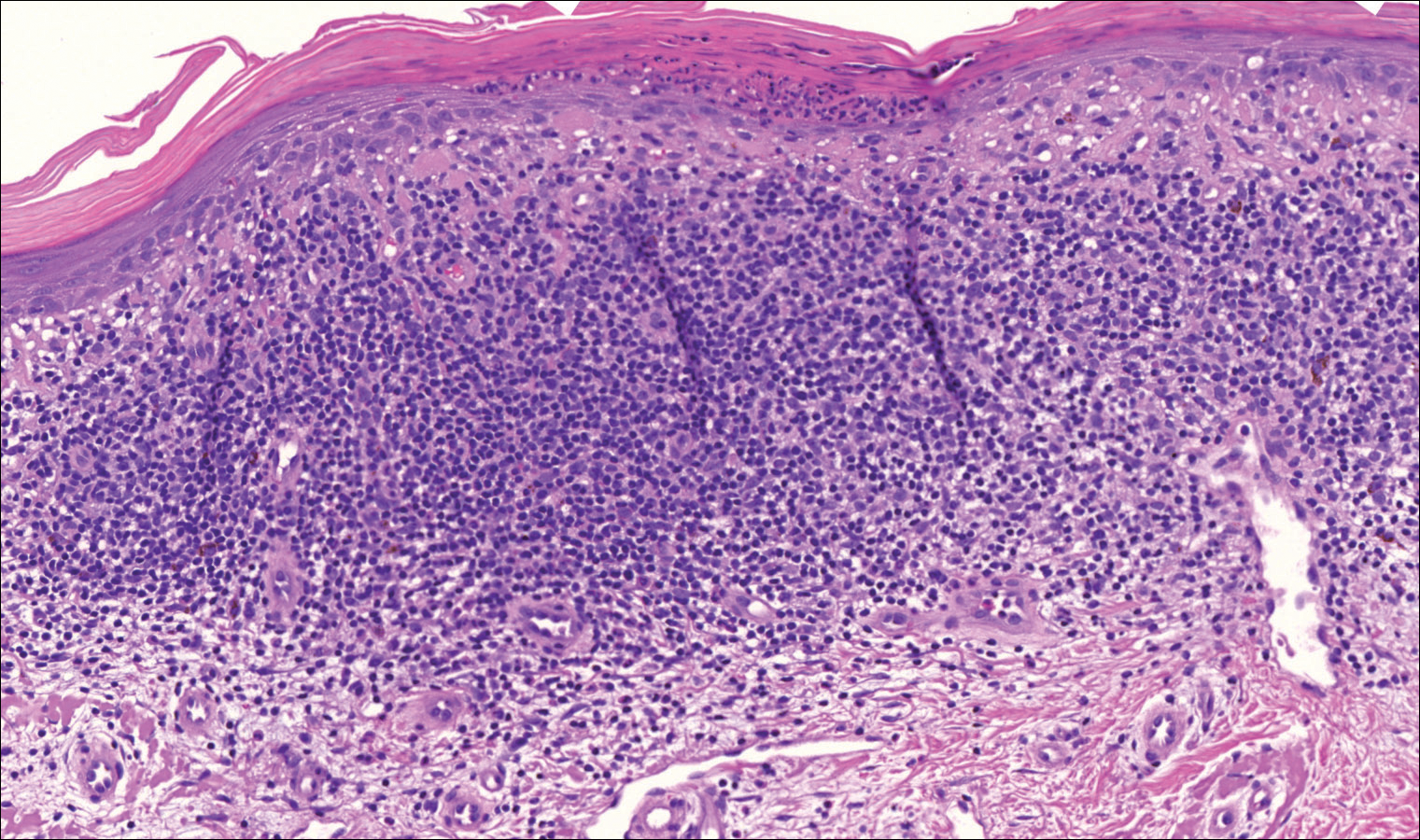
On examination performed during the initial presentation to our clinic, there was a well-demarcated tan to violaceous linear plaque present at the lower buttock and extending along the posterior leg to the skin overlying the Achilles tendon and dorsal aspect of the right foot. Within the plaque, there were areas of atrophy and areas of inflammation, induration, and hyperkeratosis (Figures 1 and 2). Two punch biopsies were performed: one from the edge of the plaque and one from a hyperkeratotic region within the plaque. Histology from the edge of the plaque demonstrated a cornoid lamella, consistent with a porokeratosis (Figure 3), whereas the histology from the hyperkeratotic region demonstrated a lichenoid infiltrate (Figure 4).

Several treatment options directed at the entire lesion were offered to the patient, but she declined these therapies and opted to address only those areas with clinical features of SCC, such as hyperkeratosis, bleeding, and rapid growth. Although biopsies performed by an outside provider were consistent with SCC, it had not been detected on biopsy performed during her initial visit to our clinic.
The patient was educated on the risk associated with her condition and instructed to follow up every 6 months to monitor for the development of SCC.
Comment
Porokeratosis is a disorder of keratinization with at least 5 clinical subtypes that share histologic similarities: porokeratosis of Mibelli, disseminated superficial porokeratosis and DSAP, linear porokeratosis, punctate porokeratosis, and PPPD.1,2 Other less common variants of porokeratosis include porokeratosis ptychotropica (a verrucous variant confined to the perianal area) and congenital unilateral linear porokeratosis.8,9
Linear porokeratosis appears in infancy or childhood with plaques that follow the lines of Blaschko.5,6 Most commonly, it presents unilaterally with annular plaques and linear hyperkeratotic papules that preferentially affect the extremities, though it also may present in a more generalized form or appear in a zosteriform pattern.10,11 Linear porokeratosis affects fewer than 20,000 individuals in the United States and accounts for fewer than 13% of all porokeratosis cases.12,13
Despite its relatively low prevalence, early identification of linear porokeratosis is important due to its high oncogenic potential, with malignant transformation to basal cell carcinoma or, more commonly, SCC reported in 19% of reported cases.1,5,7,14 The malignant transformation rate of linear porokeratosis is reported to be higher than rates seen in other porokeratosis subtypes (9.5%, 7.6%, and 3.4% for PPPD, porokeratosis of Mibelli, and DSAP, respectively).7 The risk of malignant transformation from porokeratosis increases with exposure to ionizing radiation, duration of the lesion, larger or coalescing lesions, and advanced age.7,15,16 Histologic studies have provided support for correlation between lesion size and oncogenic potential, with greater numbers of mitotic cells and more abnormal DNA ploidy seen in larger lesions.17
Histopathology
All subtypes of porokeratosis share certain histopathologic features that aid in the diagnosis of the disorder.18 Identification of the clinically observed hyperkeratotic ridged border or cornoid lamella is the primary means of definitively diagnosing porokeratosis; however, cornoid lamellae may be observed in other conditions, including verruca vulgaris and actinic keratosis.4,14
The cornoid lamella appears as a skewed column of densely packed parakeratotic cells with pyknotic basophilic nuclei extending through the stratum corneum from an epidermal invagination.4 Directly beneath the cornoid lamella, the granular layer is markedly diminished or absent, and cells of the stratum spinosum may demonstrate vacuolar changes or dyskeratosis.4,19 The superficial layer of the cornoid lamella may appear to be more centrifugally located and the cornoid lamella may be seen in several locations throughout the lesion.2,20 The degree of epidermal invagination, which is present under the cornoid lamella, varies by porokeratosis subtype; the central portion of the lesion may contain epidermis that ranges from hyperplastic to atrophic.2 Shumack et al21 noted that histologic changes under the cornoid lamella may include a lichenoid tissue reaction, papillary dermal lymphocytic infiltrate, vacuolar changes, dyskeratosis, and liquefaction degeneration of the basal layer. Because many of these histologic features also can be identified in lichen planus, a biopsy of the edge of lesions of porokeratosis is essential for making the correct diagnosis.
Heritability
Although linear porokeratosis has no identified pattern of inheritance and appears sporadic in onset, reports have described concomitant occurrence of linear porokeratosis and DSAP as well as linear porokeratosis arising in children of parents who have a diagnosis of DSAP.5,18,22,23 Based on these findings, it has been hypothesized that linear porokeratosis may represent a mosaic or segmental form of autosomal-dominant inherited subtypes of porokeratosis, such as DSAP.5 According to this hypothesis, loss of heterozygosity in patients with a DSAP mutation during early embryogenesis leads to proliferation of cells that are homozygous or hemizygous for the underlying mutation along lines of Blaschko.24 It has been suggested that the allelic loss implicated in the development of linear porokeratosis is the first step in a multistage process of carcinogenesis, which may help to explain the higher rates of malignant transformation that can be seen in linear porokeratosis.24
Management
Several treatment options exist for porokeratosis, including cryotherapy, topical 5-fluorouracil with or without adjunctive retinoid treatment, topical imiquimod, CO2 laser, shave and linear excision, curettage, dermabrasion, and oral acitretin for widespread lesions.1,25-29 One case report detailed successful treatment of adult-onset linear porokeratosis with tacrolimus ointment 0.1%.30 Treatments for porokeratosis demonstrate variable degrees of success, with the aim of eradicating the clonal population of mutant keratinocytes.2 Additionally, protection from UV radiation should be encouraged, especially in patients who have lesions that occur in areas of high actinic damage.1
Conclusion
We report of a case of linear porokeratosis with associated multiple SCCs that developed within the lesion. Definitive diagnosis of linear porokeratosis is important due to the higher rate of malignant transformation than the rate seen in other porokeratoses. In larger lesions, appropriate sampling and orientation of the pathology specimen is essential for identifying cornoid lamellae, thus allowing for appropriate follow-up and management. Several treatment options are available, though evidence for the effectiveness of any particular therapy is lacking. Research has shed light on possible genetic and molecular abnormalities in linear porokeratosis, but the exact pathogenesis of the disorder remains unclear.
- Curkova AK, Hegyi J, Kozub P, et al. A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol Ther. 2014;27:144-147.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Wade TR, Ackerman AB. Cornoid lamellation. a histologic reaction pattern. Am J Dermatopathol. 1980;2:5-15.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Rahbari H, Cordero AA, Mehregan AH. Linear porokeratosis. a distinctive clinical variant of porokeratosis of Mibelli. Arch Dermatol. 1974;109:526-528.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Yeo J, Winhoven S, Tallon B. Porokeratosis ptychotropica: a rare and evolving variant of porokeratosis. J Cutan Pathol. 2013;40:1042-1047.
- Scola N, Skrygan M, Wieland U, et al. Altered gene expression in squamous cell carcinoma arising from congenital unilateral linear porokeratosis. Clin Exp Dermatol. 2012;37:781-785.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Goldner R. Zosteriform porokeratosis of Mibelli. Arch Dermatol. 1971;104:425-426.
- Malhotra SK, Puri KJ, Goyal T, et al. Linear porokeratosis. Dermatol Online J. 2007;13:15.
- Leow YH, Soon YH, Tham SN. A report of 31 cases of porokeratosis at the National Skin Centre. Ann Acad Med Singapore. 1996;25:837-841.
- Vivas AC, Maderal AD, Kirsner RS. Giant ulcerating squamous cell carcinoma arising from linear porokeratosis: a case study. Ostomy Wound Manage. 2012;58:18-20.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Otsuka F, Someya T, Ishibashi Y. Porokeratosis and malignant skin tumors. J Cancer Res Clin Oncol. 1991;117:55-60.
- Otsuka F, Umebayashi Y, Watanabe S, et al. Porokeratosis large skin lesions are susceptible to skin cancer development: histological and cytological explanation for the susceptibility. J Cancer Res Clin Oncol. 1993;119:395-400.
- Lohrer R, Neumann-Acikel A, Eming R, et al. A case of linear porokeratosis superimposed on disseminated superficial actinic porokeratosis. Case Rep Dermatol. 2010;2:130-134.
- Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37:145-155.
- Reed RJ, Leone P. Porokeratosis—a mutant clonal keratosis of the epidermis. I. histogenesis. Arch Dermatol. 1970;101:340-347.
- Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. a histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991;13:26-31.
- Murase J, Gilliam AC. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Commens CA, Shumack SP. Linear porokeratosis in two families with disseminated superficial actinic porokeratosis. Pediatr Dermatol. 1987;4:209-214.
- Happle R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatology. 1997;195:20-25.
- Rabbin PE, Baldwin HE. Treatment of porokeratosis of Mibelli with CO2 laser vaporization versus surgical excision with split-thickness skin graft. a comparison. J Dermatol Surg Oncol. 1993;19:199-202.
- Spencer JM, Katz BE. Successful treatment of porokeratosis of Mibelli with diamond fraise dermabrasion. Arch Dermatol. 1992;128:1187-1188.
- Venkatarajan S, LeLeux TM, Yang D, et al. Porokeratosis of Mibelli: successful treatment with 5 percent topical imiquimod and topical 5 percent 5-fluorouracil. Dermatol Online J. 2010;16:10.
- McDonald SG, Peterka ES. Porokeratosis (Mibelli): treatment with topical 5-fluorouracil. J Am Acad Dermatol. 1983;8:107-110.
- Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989;20:1015-1022.
- Parks AC, Conner KJ, Armstrong CA. Long-term clearance of linear porokeratosis with tacrolimus, 0.1%, ointment. JAMA Dermatol. 2014;150:194-196.
Lesions of porokeratosis are thought to arise from disordered keratinization, though the exact pathogenesis remains uncertain. At least 5 clinical subtypes of porokeratosis have been identified: porokeratosis of Mibelli, disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, punctuate porokeratosis, and porokeratosis palmaris et plantaris disseminata (PPPD).1,2 Linear porokeratosis is a rare subtype with a clinical differential diagnosis that includes lichen striatus, linear lichen planus, linear verrucous epidermal nevus, segmental Darier disease, and incontinentia pigmenti.3 Definitive diagnosis of linear porokeratosis is made by histopathologic examination demonstrating a cornoid lamella, defined as a column of parakeratotic cells that lies at 45°to the surface of the epidermis and contains pyknotic basophilic nuclei.4 Patients with linear porokeratosis typically develop lesions along the lines of Blaschko in infancy or childhood.5,6 Among the different subtypes of porokeratosis, linear porokeratosis demonstrates the highest rate of malignant transformation, therefore requiring close clinical observation.7
Case Report
An 83-year-old woman presented to the outpatient clinic with a large linear plaque on the right leg that had been present since birth. Ten years prior to presentation, a portion of the lesion started to bleed; biopsy of the area was performed by an outside provider demonstrating squamous cell carcinoma (SCC), which was treated with wide local excision. One year prior to presentation, a separate portion of the plaque was biopsied by an outside provider and another diagnosis of SCC was made.
On examination performed during the initial presentation to our clinic, there was a well-demarcated tan to violaceous linear plaque present at the lower buttock and extending along the posterior leg to the skin overlying the Achilles tendon and dorsal aspect of the right foot. Within the plaque, there were areas of atrophy and areas of inflammation, induration, and hyperkeratosis (Figures 1 and 2). Two punch biopsies were performed: one from the edge of the plaque and one from a hyperkeratotic region within the plaque. Histology from the edge of the plaque demonstrated a cornoid lamella, consistent with a porokeratosis (Figure 3), whereas the histology from the hyperkeratotic region demonstrated a lichenoid infiltrate (Figure 4).




Several treatment options directed at the entire lesion were offered to the patient, but she declined these therapies and opted to address only those areas with clinical features of SCC, such as hyperkeratosis, bleeding, and rapid growth. Although biopsies performed by an outside provider were consistent with SCC, it had not been detected on biopsy performed during her initial visit to our clinic.
The patient was educated on the risk associated with her condition and instructed to follow up every 6 months to monitor for the development of SCC.
Comment
Porokeratosis is a disorder of keratinization with at least 5 clinical subtypes that share histologic similarities: porokeratosis of Mibelli, disseminated superficial porokeratosis and DSAP, linear porokeratosis, punctate porokeratosis, and PPPD.1,2 Other less common variants of porokeratosis include porokeratosis ptychotropica (a verrucous variant confined to the perianal area) and congenital unilateral linear porokeratosis.8,9
Linear porokeratosis appears in infancy or childhood with plaques that follow the lines of Blaschko.5,6 Most commonly, it presents unilaterally with annular plaques and linear hyperkeratotic papules that preferentially affect the extremities, though it also may present in a more generalized form or appear in a zosteriform pattern.10,11 Linear porokeratosis affects fewer than 20,000 individuals in the United States and accounts for fewer than 13% of all porokeratosis cases.12,13
Despite its relatively low prevalence, early identification of linear porokeratosis is important due to its high oncogenic potential, with malignant transformation to basal cell carcinoma or, more commonly, SCC reported in 19% of reported cases.1,5,7,14 The malignant transformation rate of linear porokeratosis is reported to be higher than rates seen in other porokeratosis subtypes (9.5%, 7.6%, and 3.4% for PPPD, porokeratosis of Mibelli, and DSAP, respectively).7 The risk of malignant transformation from porokeratosis increases with exposure to ionizing radiation, duration of the lesion, larger or coalescing lesions, and advanced age.7,15,16 Histologic studies have provided support for correlation between lesion size and oncogenic potential, with greater numbers of mitotic cells and more abnormal DNA ploidy seen in larger lesions.17
Histopathology
All subtypes of porokeratosis share certain histopathologic features that aid in the diagnosis of the disorder.18 Identification of the clinically observed hyperkeratotic ridged border or cornoid lamella is the primary means of definitively diagnosing porokeratosis; however, cornoid lamellae may be observed in other conditions, including verruca vulgaris and actinic keratosis.4,14
The cornoid lamella appears as a skewed column of densely packed parakeratotic cells with pyknotic basophilic nuclei extending through the stratum corneum from an epidermal invagination.4 Directly beneath the cornoid lamella, the granular layer is markedly diminished or absent, and cells of the stratum spinosum may demonstrate vacuolar changes or dyskeratosis.4,19 The superficial layer of the cornoid lamella may appear to be more centrifugally located and the cornoid lamella may be seen in several locations throughout the lesion.2,20 The degree of epidermal invagination, which is present under the cornoid lamella, varies by porokeratosis subtype; the central portion of the lesion may contain epidermis that ranges from hyperplastic to atrophic.2 Shumack et al21 noted that histologic changes under the cornoid lamella may include a lichenoid tissue reaction, papillary dermal lymphocytic infiltrate, vacuolar changes, dyskeratosis, and liquefaction degeneration of the basal layer. Because many of these histologic features also can be identified in lichen planus, a biopsy of the edge of lesions of porokeratosis is essential for making the correct diagnosis.
Heritability
Although linear porokeratosis has no identified pattern of inheritance and appears sporadic in onset, reports have described concomitant occurrence of linear porokeratosis and DSAP as well as linear porokeratosis arising in children of parents who have a diagnosis of DSAP.5,18,22,23 Based on these findings, it has been hypothesized that linear porokeratosis may represent a mosaic or segmental form of autosomal-dominant inherited subtypes of porokeratosis, such as DSAP.5 According to this hypothesis, loss of heterozygosity in patients with a DSAP mutation during early embryogenesis leads to proliferation of cells that are homozygous or hemizygous for the underlying mutation along lines of Blaschko.24 It has been suggested that the allelic loss implicated in the development of linear porokeratosis is the first step in a multistage process of carcinogenesis, which may help to explain the higher rates of malignant transformation that can be seen in linear porokeratosis.24
Management
Several treatment options exist for porokeratosis, including cryotherapy, topical 5-fluorouracil with or without adjunctive retinoid treatment, topical imiquimod, CO2 laser, shave and linear excision, curettage, dermabrasion, and oral acitretin for widespread lesions.1,25-29 One case report detailed successful treatment of adult-onset linear porokeratosis with tacrolimus ointment 0.1%.30 Treatments for porokeratosis demonstrate variable degrees of success, with the aim of eradicating the clonal population of mutant keratinocytes.2 Additionally, protection from UV radiation should be encouraged, especially in patients who have lesions that occur in areas of high actinic damage.1
Conclusion
We report of a case of linear porokeratosis with associated multiple SCCs that developed within the lesion. Definitive diagnosis of linear porokeratosis is important due to the higher rate of malignant transformation than the rate seen in other porokeratoses. In larger lesions, appropriate sampling and orientation of the pathology specimen is essential for identifying cornoid lamellae, thus allowing for appropriate follow-up and management. Several treatment options are available, though evidence for the effectiveness of any particular therapy is lacking. Research has shed light on possible genetic and molecular abnormalities in linear porokeratosis, but the exact pathogenesis of the disorder remains unclear.
Lesions of porokeratosis are thought to arise from disordered keratinization, though the exact pathogenesis remains uncertain. At least 5 clinical subtypes of porokeratosis have been identified: porokeratosis of Mibelli, disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, punctuate porokeratosis, and porokeratosis palmaris et plantaris disseminata (PPPD).1,2 Linear porokeratosis is a rare subtype with a clinical differential diagnosis that includes lichen striatus, linear lichen planus, linear verrucous epidermal nevus, segmental Darier disease, and incontinentia pigmenti.3 Definitive diagnosis of linear porokeratosis is made by histopathologic examination demonstrating a cornoid lamella, defined as a column of parakeratotic cells that lies at 45°to the surface of the epidermis and contains pyknotic basophilic nuclei.4 Patients with linear porokeratosis typically develop lesions along the lines of Blaschko in infancy or childhood.5,6 Among the different subtypes of porokeratosis, linear porokeratosis demonstrates the highest rate of malignant transformation, therefore requiring close clinical observation.7
Case Report
An 83-year-old woman presented to the outpatient clinic with a large linear plaque on the right leg that had been present since birth. Ten years prior to presentation, a portion of the lesion started to bleed; biopsy of the area was performed by an outside provider demonstrating squamous cell carcinoma (SCC), which was treated with wide local excision. One year prior to presentation, a separate portion of the plaque was biopsied by an outside provider and another diagnosis of SCC was made.
On examination performed during the initial presentation to our clinic, there was a well-demarcated tan to violaceous linear plaque present at the lower buttock and extending along the posterior leg to the skin overlying the Achilles tendon and dorsal aspect of the right foot. Within the plaque, there were areas of atrophy and areas of inflammation, induration, and hyperkeratosis (Figures 1 and 2). Two punch biopsies were performed: one from the edge of the plaque and one from a hyperkeratotic region within the plaque. Histology from the edge of the plaque demonstrated a cornoid lamella, consistent with a porokeratosis (Figure 3), whereas the histology from the hyperkeratotic region demonstrated a lichenoid infiltrate (Figure 4).




Several treatment options directed at the entire lesion were offered to the patient, but she declined these therapies and opted to address only those areas with clinical features of SCC, such as hyperkeratosis, bleeding, and rapid growth. Although biopsies performed by an outside provider were consistent with SCC, it had not been detected on biopsy performed during her initial visit to our clinic.
The patient was educated on the risk associated with her condition and instructed to follow up every 6 months to monitor for the development of SCC.
Comment
Porokeratosis is a disorder of keratinization with at least 5 clinical subtypes that share histologic similarities: porokeratosis of Mibelli, disseminated superficial porokeratosis and DSAP, linear porokeratosis, punctate porokeratosis, and PPPD.1,2 Other less common variants of porokeratosis include porokeratosis ptychotropica (a verrucous variant confined to the perianal area) and congenital unilateral linear porokeratosis.8,9
Linear porokeratosis appears in infancy or childhood with plaques that follow the lines of Blaschko.5,6 Most commonly, it presents unilaterally with annular plaques and linear hyperkeratotic papules that preferentially affect the extremities, though it also may present in a more generalized form or appear in a zosteriform pattern.10,11 Linear porokeratosis affects fewer than 20,000 individuals in the United States and accounts for fewer than 13% of all porokeratosis cases.12,13
Despite its relatively low prevalence, early identification of linear porokeratosis is important due to its high oncogenic potential, with malignant transformation to basal cell carcinoma or, more commonly, SCC reported in 19% of reported cases.1,5,7,14 The malignant transformation rate of linear porokeratosis is reported to be higher than rates seen in other porokeratosis subtypes (9.5%, 7.6%, and 3.4% for PPPD, porokeratosis of Mibelli, and DSAP, respectively).7 The risk of malignant transformation from porokeratosis increases with exposure to ionizing radiation, duration of the lesion, larger or coalescing lesions, and advanced age.7,15,16 Histologic studies have provided support for correlation between lesion size and oncogenic potential, with greater numbers of mitotic cells and more abnormal DNA ploidy seen in larger lesions.17
Histopathology
All subtypes of porokeratosis share certain histopathologic features that aid in the diagnosis of the disorder.18 Identification of the clinically observed hyperkeratotic ridged border or cornoid lamella is the primary means of definitively diagnosing porokeratosis; however, cornoid lamellae may be observed in other conditions, including verruca vulgaris and actinic keratosis.4,14
The cornoid lamella appears as a skewed column of densely packed parakeratotic cells with pyknotic basophilic nuclei extending through the stratum corneum from an epidermal invagination.4 Directly beneath the cornoid lamella, the granular layer is markedly diminished or absent, and cells of the stratum spinosum may demonstrate vacuolar changes or dyskeratosis.4,19 The superficial layer of the cornoid lamella may appear to be more centrifugally located and the cornoid lamella may be seen in several locations throughout the lesion.2,20 The degree of epidermal invagination, which is present under the cornoid lamella, varies by porokeratosis subtype; the central portion of the lesion may contain epidermis that ranges from hyperplastic to atrophic.2 Shumack et al21 noted that histologic changes under the cornoid lamella may include a lichenoid tissue reaction, papillary dermal lymphocytic infiltrate, vacuolar changes, dyskeratosis, and liquefaction degeneration of the basal layer. Because many of these histologic features also can be identified in lichen planus, a biopsy of the edge of lesions of porokeratosis is essential for making the correct diagnosis.
Heritability
Although linear porokeratosis has no identified pattern of inheritance and appears sporadic in onset, reports have described concomitant occurrence of linear porokeratosis and DSAP as well as linear porokeratosis arising in children of parents who have a diagnosis of DSAP.5,18,22,23 Based on these findings, it has been hypothesized that linear porokeratosis may represent a mosaic or segmental form of autosomal-dominant inherited subtypes of porokeratosis, such as DSAP.5 According to this hypothesis, loss of heterozygosity in patients with a DSAP mutation during early embryogenesis leads to proliferation of cells that are homozygous or hemizygous for the underlying mutation along lines of Blaschko.24 It has been suggested that the allelic loss implicated in the development of linear porokeratosis is the first step in a multistage process of carcinogenesis, which may help to explain the higher rates of malignant transformation that can be seen in linear porokeratosis.24
Management
Several treatment options exist for porokeratosis, including cryotherapy, topical 5-fluorouracil with or without adjunctive retinoid treatment, topical imiquimod, CO2 laser, shave and linear excision, curettage, dermabrasion, and oral acitretin for widespread lesions.1,25-29 One case report detailed successful treatment of adult-onset linear porokeratosis with tacrolimus ointment 0.1%.30 Treatments for porokeratosis demonstrate variable degrees of success, with the aim of eradicating the clonal population of mutant keratinocytes.2 Additionally, protection from UV radiation should be encouraged, especially in patients who have lesions that occur in areas of high actinic damage.1
Conclusion
We report of a case of linear porokeratosis with associated multiple SCCs that developed within the lesion. Definitive diagnosis of linear porokeratosis is important due to the higher rate of malignant transformation than the rate seen in other porokeratoses. In larger lesions, appropriate sampling and orientation of the pathology specimen is essential for identifying cornoid lamellae, thus allowing for appropriate follow-up and management. Several treatment options are available, though evidence for the effectiveness of any particular therapy is lacking. Research has shed light on possible genetic and molecular abnormalities in linear porokeratosis, but the exact pathogenesis of the disorder remains unclear.
- Curkova AK, Hegyi J, Kozub P, et al. A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol Ther. 2014;27:144-147.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Wade TR, Ackerman AB. Cornoid lamellation. a histologic reaction pattern. Am J Dermatopathol. 1980;2:5-15.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Rahbari H, Cordero AA, Mehregan AH. Linear porokeratosis. a distinctive clinical variant of porokeratosis of Mibelli. Arch Dermatol. 1974;109:526-528.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Yeo J, Winhoven S, Tallon B. Porokeratosis ptychotropica: a rare and evolving variant of porokeratosis. J Cutan Pathol. 2013;40:1042-1047.
- Scola N, Skrygan M, Wieland U, et al. Altered gene expression in squamous cell carcinoma arising from congenital unilateral linear porokeratosis. Clin Exp Dermatol. 2012;37:781-785.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Goldner R. Zosteriform porokeratosis of Mibelli. Arch Dermatol. 1971;104:425-426.
- Malhotra SK, Puri KJ, Goyal T, et al. Linear porokeratosis. Dermatol Online J. 2007;13:15.
- Leow YH, Soon YH, Tham SN. A report of 31 cases of porokeratosis at the National Skin Centre. Ann Acad Med Singapore. 1996;25:837-841.
- Vivas AC, Maderal AD, Kirsner RS. Giant ulcerating squamous cell carcinoma arising from linear porokeratosis: a case study. Ostomy Wound Manage. 2012;58:18-20.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Otsuka F, Someya T, Ishibashi Y. Porokeratosis and malignant skin tumors. J Cancer Res Clin Oncol. 1991;117:55-60.
- Otsuka F, Umebayashi Y, Watanabe S, et al. Porokeratosis large skin lesions are susceptible to skin cancer development: histological and cytological explanation for the susceptibility. J Cancer Res Clin Oncol. 1993;119:395-400.
- Lohrer R, Neumann-Acikel A, Eming R, et al. A case of linear porokeratosis superimposed on disseminated superficial actinic porokeratosis. Case Rep Dermatol. 2010;2:130-134.
- Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37:145-155.
- Reed RJ, Leone P. Porokeratosis—a mutant clonal keratosis of the epidermis. I. histogenesis. Arch Dermatol. 1970;101:340-347.
- Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. a histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991;13:26-31.
- Murase J, Gilliam AC. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Commens CA, Shumack SP. Linear porokeratosis in two families with disseminated superficial actinic porokeratosis. Pediatr Dermatol. 1987;4:209-214.
- Happle R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatology. 1997;195:20-25.
- Rabbin PE, Baldwin HE. Treatment of porokeratosis of Mibelli with CO2 laser vaporization versus surgical excision with split-thickness skin graft. a comparison. J Dermatol Surg Oncol. 1993;19:199-202.
- Spencer JM, Katz BE. Successful treatment of porokeratosis of Mibelli with diamond fraise dermabrasion. Arch Dermatol. 1992;128:1187-1188.
- Venkatarajan S, LeLeux TM, Yang D, et al. Porokeratosis of Mibelli: successful treatment with 5 percent topical imiquimod and topical 5 percent 5-fluorouracil. Dermatol Online J. 2010;16:10.
- McDonald SG, Peterka ES. Porokeratosis (Mibelli): treatment with topical 5-fluorouracil. J Am Acad Dermatol. 1983;8:107-110.
- Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989;20:1015-1022.
- Parks AC, Conner KJ, Armstrong CA. Long-term clearance of linear porokeratosis with tacrolimus, 0.1%, ointment. JAMA Dermatol. 2014;150:194-196.
- Curkova AK, Hegyi J, Kozub P, et al. A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol Ther. 2014;27:144-147.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Wade TR, Ackerman AB. Cornoid lamellation. a histologic reaction pattern. Am J Dermatopathol. 1980;2:5-15.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Rahbari H, Cordero AA, Mehregan AH. Linear porokeratosis. a distinctive clinical variant of porokeratosis of Mibelli. Arch Dermatol. 1974;109:526-528.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Yeo J, Winhoven S, Tallon B. Porokeratosis ptychotropica: a rare and evolving variant of porokeratosis. J Cutan Pathol. 2013;40:1042-1047.
- Scola N, Skrygan M, Wieland U, et al. Altered gene expression in squamous cell carcinoma arising from congenital unilateral linear porokeratosis. Clin Exp Dermatol. 2012;37:781-785.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Goldner R. Zosteriform porokeratosis of Mibelli. Arch Dermatol. 1971;104:425-426.
- Malhotra SK, Puri KJ, Goyal T, et al. Linear porokeratosis. Dermatol Online J. 2007;13:15.
- Leow YH, Soon YH, Tham SN. A report of 31 cases of porokeratosis at the National Skin Centre. Ann Acad Med Singapore. 1996;25:837-841.
- Vivas AC, Maderal AD, Kirsner RS. Giant ulcerating squamous cell carcinoma arising from linear porokeratosis: a case study. Ostomy Wound Manage. 2012;58:18-20.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Otsuka F, Someya T, Ishibashi Y. Porokeratosis and malignant skin tumors. J Cancer Res Clin Oncol. 1991;117:55-60.
- Otsuka F, Umebayashi Y, Watanabe S, et al. Porokeratosis large skin lesions are susceptible to skin cancer development: histological and cytological explanation for the susceptibility. J Cancer Res Clin Oncol. 1993;119:395-400.
- Lohrer R, Neumann-Acikel A, Eming R, et al. A case of linear porokeratosis superimposed on disseminated superficial actinic porokeratosis. Case Rep Dermatol. 2010;2:130-134.
- Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37:145-155.
- Reed RJ, Leone P. Porokeratosis—a mutant clonal keratosis of the epidermis. I. histogenesis. Arch Dermatol. 1970;101:340-347.
- Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. a histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991;13:26-31.
- Murase J, Gilliam AC. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Commens CA, Shumack SP. Linear porokeratosis in two families with disseminated superficial actinic porokeratosis. Pediatr Dermatol. 1987;4:209-214.
- Happle R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatology. 1997;195:20-25.
- Rabbin PE, Baldwin HE. Treatment of porokeratosis of Mibelli with CO2 laser vaporization versus surgical excision with split-thickness skin graft. a comparison. J Dermatol Surg Oncol. 1993;19:199-202.
- Spencer JM, Katz BE. Successful treatment of porokeratosis of Mibelli with diamond fraise dermabrasion. Arch Dermatol. 1992;128:1187-1188.
- Venkatarajan S, LeLeux TM, Yang D, et al. Porokeratosis of Mibelli: successful treatment with 5 percent topical imiquimod and topical 5 percent 5-fluorouracil. Dermatol Online J. 2010;16:10.
- McDonald SG, Peterka ES. Porokeratosis (Mibelli): treatment with topical 5-fluorouracil. J Am Acad Dermatol. 1983;8:107-110.
- Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989;20:1015-1022.
- Parks AC, Conner KJ, Armstrong CA. Long-term clearance of linear porokeratosis with tacrolimus, 0.1%, ointment. JAMA Dermatol. 2014;150:194-196.
Practice Points
- Porokeratosis represents a heterogeneous group of skin disorders.
- Porokeratosis can be inherited in an autosomal-dominant pattern, though many patients lack a family history.
- The presence of a cornoid lamella is the characteristic finding of porokeratosis on histology.
- The rate of malignant transformation to squamous cell carcinoma is highest in linear porokeratosis, lowest in disseminated superficial actinic porokeratosis, and unreported in the punctate type.
Shoulder Pain—Is It From the Shoulder, Neck, or Both?
Neck and shoulder pain are common presenting symptoms in the general adult population with a 41.7% and 50.9% lifetime incidence in males and females, respectively.1 Generally, a single diagnosis is sought to explain a patient’s signs and symptoms, but occasionally 2 or more different causes are responsible. Only by conducting a thorough history and physical examination with proper follow-up will all contributing diseases be discovered. The following case illustrates 2 distinct etiologies responsible for the patient’s pain, one with an extremely unusual presentation.
Case Presentation
A 23-year-old male presented with a 3-month history of pain, spasm, and tightness of his right upper extremity along his posterior neck, shoulder, and triceps area. The patient reported no history of trauma, but he revealed increasing the amount of weight lifting, and his symptoms were especially worse when bench pressing or performing overhead exercises. No paresthesias were reported.
The initial examination revealed a well-developed muscular male with no visible atrophy or tenderness to palpation. He had full range of motion (ROM) of his neck, a normal motor and sensory examination of the C5-T1 nerve roots, and a negative Spurling maneuver. The patient had full ROM of both shoulders but had pain with right shoulder abduction starting at about 120° to 140°. He had pain with resisted supraspinatus muscle testing as well as pain with the liftoff test. The results of the patient’s Hawkins and Neer tests were negative (Table, Figures 1 to 6).2-4 A point-of-care shoulder ultrasound examination revealed no abnormalities.
The working diagnosis was external shoulder impingement with a differential diagnosis of internal impingement and/or cervical radiculopathy. A diagnostic/therapeutic injection of 40 mg of triamcinolone and 4 mL of 1% xylocaine without epinephrine was administered into the right subacromial bursa. The patient experienced immediate and complete relief of pain with repeat shoulder abduction, supraspinatus muscle testing, and the liftoff test. Although this procedure temporarily relieved the pain with movement, a sensation of tightness, pain, and spasm in the posterior shoulder and right posterior arm was still present. The patient was asked to perform therapeutic rotator cuff and scapular strengthening exercises, annotated on a patient information handout, for 15 minutes a day, every other day and to follow-up in 4 weeks.
At follow-up the external impingement symptoms (pain with shoulder abduction, resisted supraspinatus testing and the liftoff test) were fully resolved, but the patient reported persistent pain, spasm, and sensation of tightness in his right posterior shoulder and arm with intermittent extension into forearm and hand. A review of the history reminded the patient of a wrestling episode that caused neck pain months earlier. The patient reported that his current symptoms began after the wrestling episode.
Physical examination at this time revealed pain in the right posterior arm with left lateral neck movement but no neck pain with right lateral neck movement or flexion and extension. There was again a normal motor and sensory examination in the C5-T1 nerve distribution. Of note, there was full painless abduction in the right shoulder, which had improved from the previous examination, and there was no pain with resisted supraspinatus testing or the liftoff test, both of which had been abnormal at the initial encounter.
Due to the patient’s persistent posterior shoulder pain and exacerbation of symptoms with neck movement and the now revealed antecedent event of neck trauma, a higher concern for cervical disc pathology was entertained. A cervical magnetic resonance imaging examination (MRI) was ordered. A moderately sized left paracentral herniation of the disk at C5-C6 was found. The disk herniation was compressing the left ventral hemi-cord with narrowing of the left neuronal foramina. Additionally, there was a mild posterior disc osteophyte complex that caused mild left foraminal narrowing at C6-C7.
Neurosurgical consultation was obtained. Extensive discussion of nonsurgical vs surgical options were conducted, and a trial of nonsurgical therapy was agreed on. Physical therapy with cervical traction was prescribed with 2 sessions a week for 4 weeks. The patient also continued his therapeutic rotator cuff and scapular stabilizing exercises and decreased the amount and intensity of his weight lifting.
At the next 4-week follow-up, his symptoms were greatly reduced. He was discharged from supervised physical therapy and continued his at-home neck and shoulder strengthening regimen. At the 1-year follow-up, the patient reported that the radiating pain had essentially resolved—only occasionally being present with heavy upper-extremity weight lifting or grappling activities. He continues to be symptom free of his external impingement symptoms as well.
The final diagnosis was cervical radiculopathy of C5/6 nerve root due to left paracentral disc herniation with concomitant cord compression as well as external impingement (rotator cuff dysfunction) of the right shoulder. It is unclear whether the disc herniation contributed to the external shoulder impingement due to alterations in biomechanics or whether the 2 diseases were unrelated.
Discussion
The patient’s cervical disc herniation most likely was due to his earlier grappling episode when he had acute trauma to the neck or an exacerbation of an older asymptomatic herniation. His external shoulder impingement likely was due to overuse with heavy weight lifting, which also caused enough mechanical strain to exacerbate the patient’s cervical disc herniation symptoms. What is most unusual about this case is the right-sided cervical radicular symptoms due to a left-sided cervical disc herniation.
With an annual incidence of 107.3 in men and 63.5 in women per 100,000 patients, cervical radiculopathy is caused by compression or irritation of the cervical nerve roots as they exit the spine. The most common cause of cervical radiculopathy is spondylosis followed by disc herniation, but both can be present in the same patient. Spondylosis refers to degeneration of the discs and facet joints but generally without frank disc herniation.5
External shoulder impingement and cervical radiculopathy can have nearly identical symptoms of shoulder and upper arm pain as in this illustrated case. Patients with cervical radiculopathy generally present with neck, shoulder, and arm pain or neurologic deficits. These symptoms alone are very broad and present a wide differential diagnosis. One must determine whether the pain is from the neck or shoulder region.1 The Table and Figures 1 to 6 describe the physical examination maneuvers used to differentiate the etiology.
The decision to pursue imaging should be based on injury severity and patient treatment goals. Although plain radiographic imaging may reveal spondylotic changes, such as degenerative joint changes at the vertebral facets and uncovertebral joints as well as decreased disc space, MRI is the imaging modality of choice for viewing disc herniations.6Nonoperative management of cervical radiculopathy focuses on restoration of full pain-free neck ROM, cervical muscle strengthening, and consideration for cervical traction. The use of either topical or oral medications can be considered if needed to aid in sleep and/or participation in active rehabilitation. Complimentary methods, such as acupuncture, yoga, or therapeutic massage also should be considered. Additionally, corticosteroid epidural injections can be considered, but these have increased risk compared with lumbar epidural injections.7 Surgical indications include persistent symptoms after 6 to 12 weeks of conservative therapy with no improvement of symptoms or progressively worsening motor/neurologic deficits.8
Conclusion
This case illustrates how 2 different conditions can present similarly and lead to diagnostic uncertainty. In this case, both the shoulder impingement and cervical radiculopathy manifested as shoulder and upper arm pain and could be separated only once the impingement had been treated. In addition the left-sided disc herniation causing right-sided symptoms was very unusual. To the best of the authors’ knowledge, this is only the second report of cervical disc herniation causing contralateral symptoms. In the only other available case report on cervical disc herniation with contralateral symptoms, the symptoms occurred in both the contralateral arm and leg.9
1. Briggs AM, Straker LM, Bear NL, Smith AJ. Neck/shoulder pain in adolescents is not related to the level or nature of self-reported physical activity or type of sedentary activity in an Australian pregnancy cohort. BMC Musculoskelet Disord. 2009;10(1):87.
2. Hermans J, Luime JJ, Meuffels DE, Reijman M, Simel DL, Bierma-Zeinstra SM. Does this patient with shoulder pain have rotator cuff disease? JAMA. 2013;310(8):837-847.
3. Rubinstein SM, Pool JJ, van Tulder MW, Riphagen I, Riphagen II, de Vet HC. A systematic review of the diagnostic accuracy of provocative tests of the neck for diagnosing cervical radiculopathy. Eur Spine J. 2007;16(3):307-319.
4. Ghasemi M, Golabchi K, Mousavi SA, et al. The value of provocative tests in diagnosis of cervical radiculopathy. J Res Med Sci. 2013;18(suppl 1):S35-S38.
5. Woods BI, Hilibrand AS. Cervical radiculopathy: epidemiology, etiology, diagnosis, and treatment. J Spinal Disord Tech. 2015;28(5):E251-E259.
6. Green C, Butler J, Eustace S, Poynton A, O’Byrne JM. Imaging modalities for cervical spondylotic stenosis and myelopathy. Adv Orthop. 2012;2012:908324. [Epub July 20, 2011].
7. Childress MA, Beckers BA. Nonoperative management of cervical radiculopathy. Am Fam Physician. 2016;93(9):746-754.
8. Carette S, Fehlings MG. Clinical practice. Cervical radiculopathy. N Engl J Med. 2005;353(4):392-399.
9. Yeung JT, Johnson JI, Karim AS. Cervical disc herniation presenting with neck pain and contralateral symptoms: a case report. J Med Case Rep. 2012;6:166.
Neck and shoulder pain are common presenting symptoms in the general adult population with a 41.7% and 50.9% lifetime incidence in males and females, respectively.1 Generally, a single diagnosis is sought to explain a patient’s signs and symptoms, but occasionally 2 or more different causes are responsible. Only by conducting a thorough history and physical examination with proper follow-up will all contributing diseases be discovered. The following case illustrates 2 distinct etiologies responsible for the patient’s pain, one with an extremely unusual presentation.
Case Presentation
A 23-year-old male presented with a 3-month history of pain, spasm, and tightness of his right upper extremity along his posterior neck, shoulder, and triceps area. The patient reported no history of trauma, but he revealed increasing the amount of weight lifting, and his symptoms were especially worse when bench pressing or performing overhead exercises. No paresthesias were reported.
The initial examination revealed a well-developed muscular male with no visible atrophy or tenderness to palpation. He had full range of motion (ROM) of his neck, a normal motor and sensory examination of the C5-T1 nerve roots, and a negative Spurling maneuver. The patient had full ROM of both shoulders but had pain with right shoulder abduction starting at about 120° to 140°. He had pain with resisted supraspinatus muscle testing as well as pain with the liftoff test. The results of the patient’s Hawkins and Neer tests were negative (Table, Figures 1 to 6).2-4 A point-of-care shoulder ultrasound examination revealed no abnormalities.
The working diagnosis was external shoulder impingement with a differential diagnosis of internal impingement and/or cervical radiculopathy. A diagnostic/therapeutic injection of 40 mg of triamcinolone and 4 mL of 1% xylocaine without epinephrine was administered into the right subacromial bursa. The patient experienced immediate and complete relief of pain with repeat shoulder abduction, supraspinatus muscle testing, and the liftoff test. Although this procedure temporarily relieved the pain with movement, a sensation of tightness, pain, and spasm in the posterior shoulder and right posterior arm was still present. The patient was asked to perform therapeutic rotator cuff and scapular strengthening exercises, annotated on a patient information handout, for 15 minutes a day, every other day and to follow-up in 4 weeks.
At follow-up the external impingement symptoms (pain with shoulder abduction, resisted supraspinatus testing and the liftoff test) were fully resolved, but the patient reported persistent pain, spasm, and sensation of tightness in his right posterior shoulder and arm with intermittent extension into forearm and hand. A review of the history reminded the patient of a wrestling episode that caused neck pain months earlier. The patient reported that his current symptoms began after the wrestling episode.
Physical examination at this time revealed pain in the right posterior arm with left lateral neck movement but no neck pain with right lateral neck movement or flexion and extension. There was again a normal motor and sensory examination in the C5-T1 nerve distribution. Of note, there was full painless abduction in the right shoulder, which had improved from the previous examination, and there was no pain with resisted supraspinatus testing or the liftoff test, both of which had been abnormal at the initial encounter.
Due to the patient’s persistent posterior shoulder pain and exacerbation of symptoms with neck movement and the now revealed antecedent event of neck trauma, a higher concern for cervical disc pathology was entertained. A cervical magnetic resonance imaging examination (MRI) was ordered. A moderately sized left paracentral herniation of the disk at C5-C6 was found. The disk herniation was compressing the left ventral hemi-cord with narrowing of the left neuronal foramina. Additionally, there was a mild posterior disc osteophyte complex that caused mild left foraminal narrowing at C6-C7.
Neurosurgical consultation was obtained. Extensive discussion of nonsurgical vs surgical options were conducted, and a trial of nonsurgical therapy was agreed on. Physical therapy with cervical traction was prescribed with 2 sessions a week for 4 weeks. The patient also continued his therapeutic rotator cuff and scapular stabilizing exercises and decreased the amount and intensity of his weight lifting.
At the next 4-week follow-up, his symptoms were greatly reduced. He was discharged from supervised physical therapy and continued his at-home neck and shoulder strengthening regimen. At the 1-year follow-up, the patient reported that the radiating pain had essentially resolved—only occasionally being present with heavy upper-extremity weight lifting or grappling activities. He continues to be symptom free of his external impingement symptoms as well.
The final diagnosis was cervical radiculopathy of C5/6 nerve root due to left paracentral disc herniation with concomitant cord compression as well as external impingement (rotator cuff dysfunction) of the right shoulder. It is unclear whether the disc herniation contributed to the external shoulder impingement due to alterations in biomechanics or whether the 2 diseases were unrelated.
Discussion
The patient’s cervical disc herniation most likely was due to his earlier grappling episode when he had acute trauma to the neck or an exacerbation of an older asymptomatic herniation. His external shoulder impingement likely was due to overuse with heavy weight lifting, which also caused enough mechanical strain to exacerbate the patient’s cervical disc herniation symptoms. What is most unusual about this case is the right-sided cervical radicular symptoms due to a left-sided cervical disc herniation.
With an annual incidence of 107.3 in men and 63.5 in women per 100,000 patients, cervical radiculopathy is caused by compression or irritation of the cervical nerve roots as they exit the spine. The most common cause of cervical radiculopathy is spondylosis followed by disc herniation, but both can be present in the same patient. Spondylosis refers to degeneration of the discs and facet joints but generally without frank disc herniation.5
External shoulder impingement and cervical radiculopathy can have nearly identical symptoms of shoulder and upper arm pain as in this illustrated case. Patients with cervical radiculopathy generally present with neck, shoulder, and arm pain or neurologic deficits. These symptoms alone are very broad and present a wide differential diagnosis. One must determine whether the pain is from the neck or shoulder region.1 The Table and Figures 1 to 6 describe the physical examination maneuvers used to differentiate the etiology.
The decision to pursue imaging should be based on injury severity and patient treatment goals. Although plain radiographic imaging may reveal spondylotic changes, such as degenerative joint changes at the vertebral facets and uncovertebral joints as well as decreased disc space, MRI is the imaging modality of choice for viewing disc herniations.6Nonoperative management of cervical radiculopathy focuses on restoration of full pain-free neck ROM, cervical muscle strengthening, and consideration for cervical traction. The use of either topical or oral medications can be considered if needed to aid in sleep and/or participation in active rehabilitation. Complimentary methods, such as acupuncture, yoga, or therapeutic massage also should be considered. Additionally, corticosteroid epidural injections can be considered, but these have increased risk compared with lumbar epidural injections.7 Surgical indications include persistent symptoms after 6 to 12 weeks of conservative therapy with no improvement of symptoms or progressively worsening motor/neurologic deficits.8
Conclusion
This case illustrates how 2 different conditions can present similarly and lead to diagnostic uncertainty. In this case, both the shoulder impingement and cervical radiculopathy manifested as shoulder and upper arm pain and could be separated only once the impingement had been treated. In addition the left-sided disc herniation causing right-sided symptoms was very unusual. To the best of the authors’ knowledge, this is only the second report of cervical disc herniation causing contralateral symptoms. In the only other available case report on cervical disc herniation with contralateral symptoms, the symptoms occurred in both the contralateral arm and leg.9
Neck and shoulder pain are common presenting symptoms in the general adult population with a 41.7% and 50.9% lifetime incidence in males and females, respectively.1 Generally, a single diagnosis is sought to explain a patient’s signs and symptoms, but occasionally 2 or more different causes are responsible. Only by conducting a thorough history and physical examination with proper follow-up will all contributing diseases be discovered. The following case illustrates 2 distinct etiologies responsible for the patient’s pain, one with an extremely unusual presentation.
Case Presentation
A 23-year-old male presented with a 3-month history of pain, spasm, and tightness of his right upper extremity along his posterior neck, shoulder, and triceps area. The patient reported no history of trauma, but he revealed increasing the amount of weight lifting, and his symptoms were especially worse when bench pressing or performing overhead exercises. No paresthesias were reported.
The initial examination revealed a well-developed muscular male with no visible atrophy or tenderness to palpation. He had full range of motion (ROM) of his neck, a normal motor and sensory examination of the C5-T1 nerve roots, and a negative Spurling maneuver. The patient had full ROM of both shoulders but had pain with right shoulder abduction starting at about 120° to 140°. He had pain with resisted supraspinatus muscle testing as well as pain with the liftoff test. The results of the patient’s Hawkins and Neer tests were negative (Table, Figures 1 to 6).2-4 A point-of-care shoulder ultrasound examination revealed no abnormalities.
The working diagnosis was external shoulder impingement with a differential diagnosis of internal impingement and/or cervical radiculopathy. A diagnostic/therapeutic injection of 40 mg of triamcinolone and 4 mL of 1% xylocaine without epinephrine was administered into the right subacromial bursa. The patient experienced immediate and complete relief of pain with repeat shoulder abduction, supraspinatus muscle testing, and the liftoff test. Although this procedure temporarily relieved the pain with movement, a sensation of tightness, pain, and spasm in the posterior shoulder and right posterior arm was still present. The patient was asked to perform therapeutic rotator cuff and scapular strengthening exercises, annotated on a patient information handout, for 15 minutes a day, every other day and to follow-up in 4 weeks.
At follow-up the external impingement symptoms (pain with shoulder abduction, resisted supraspinatus testing and the liftoff test) were fully resolved, but the patient reported persistent pain, spasm, and sensation of tightness in his right posterior shoulder and arm with intermittent extension into forearm and hand. A review of the history reminded the patient of a wrestling episode that caused neck pain months earlier. The patient reported that his current symptoms began after the wrestling episode.
Physical examination at this time revealed pain in the right posterior arm with left lateral neck movement but no neck pain with right lateral neck movement or flexion and extension. There was again a normal motor and sensory examination in the C5-T1 nerve distribution. Of note, there was full painless abduction in the right shoulder, which had improved from the previous examination, and there was no pain with resisted supraspinatus testing or the liftoff test, both of which had been abnormal at the initial encounter.
Due to the patient’s persistent posterior shoulder pain and exacerbation of symptoms with neck movement and the now revealed antecedent event of neck trauma, a higher concern for cervical disc pathology was entertained. A cervical magnetic resonance imaging examination (MRI) was ordered. A moderately sized left paracentral herniation of the disk at C5-C6 was found. The disk herniation was compressing the left ventral hemi-cord with narrowing of the left neuronal foramina. Additionally, there was a mild posterior disc osteophyte complex that caused mild left foraminal narrowing at C6-C7.
Neurosurgical consultation was obtained. Extensive discussion of nonsurgical vs surgical options were conducted, and a trial of nonsurgical therapy was agreed on. Physical therapy with cervical traction was prescribed with 2 sessions a week for 4 weeks. The patient also continued his therapeutic rotator cuff and scapular stabilizing exercises and decreased the amount and intensity of his weight lifting.
At the next 4-week follow-up, his symptoms were greatly reduced. He was discharged from supervised physical therapy and continued his at-home neck and shoulder strengthening regimen. At the 1-year follow-up, the patient reported that the radiating pain had essentially resolved—only occasionally being present with heavy upper-extremity weight lifting or grappling activities. He continues to be symptom free of his external impingement symptoms as well.
The final diagnosis was cervical radiculopathy of C5/6 nerve root due to left paracentral disc herniation with concomitant cord compression as well as external impingement (rotator cuff dysfunction) of the right shoulder. It is unclear whether the disc herniation contributed to the external shoulder impingement due to alterations in biomechanics or whether the 2 diseases were unrelated.
Discussion
The patient’s cervical disc herniation most likely was due to his earlier grappling episode when he had acute trauma to the neck or an exacerbation of an older asymptomatic herniation. His external shoulder impingement likely was due to overuse with heavy weight lifting, which also caused enough mechanical strain to exacerbate the patient’s cervical disc herniation symptoms. What is most unusual about this case is the right-sided cervical radicular symptoms due to a left-sided cervical disc herniation.
With an annual incidence of 107.3 in men and 63.5 in women per 100,000 patients, cervical radiculopathy is caused by compression or irritation of the cervical nerve roots as they exit the spine. The most common cause of cervical radiculopathy is spondylosis followed by disc herniation, but both can be present in the same patient. Spondylosis refers to degeneration of the discs and facet joints but generally without frank disc herniation.5
External shoulder impingement and cervical radiculopathy can have nearly identical symptoms of shoulder and upper arm pain as in this illustrated case. Patients with cervical radiculopathy generally present with neck, shoulder, and arm pain or neurologic deficits. These symptoms alone are very broad and present a wide differential diagnosis. One must determine whether the pain is from the neck or shoulder region.1 The Table and Figures 1 to 6 describe the physical examination maneuvers used to differentiate the etiology.
The decision to pursue imaging should be based on injury severity and patient treatment goals. Although plain radiographic imaging may reveal spondylotic changes, such as degenerative joint changes at the vertebral facets and uncovertebral joints as well as decreased disc space, MRI is the imaging modality of choice for viewing disc herniations.6Nonoperative management of cervical radiculopathy focuses on restoration of full pain-free neck ROM, cervical muscle strengthening, and consideration for cervical traction. The use of either topical or oral medications can be considered if needed to aid in sleep and/or participation in active rehabilitation. Complimentary methods, such as acupuncture, yoga, or therapeutic massage also should be considered. Additionally, corticosteroid epidural injections can be considered, but these have increased risk compared with lumbar epidural injections.7 Surgical indications include persistent symptoms after 6 to 12 weeks of conservative therapy with no improvement of symptoms or progressively worsening motor/neurologic deficits.8
Conclusion
This case illustrates how 2 different conditions can present similarly and lead to diagnostic uncertainty. In this case, both the shoulder impingement and cervical radiculopathy manifested as shoulder and upper arm pain and could be separated only once the impingement had been treated. In addition the left-sided disc herniation causing right-sided symptoms was very unusual. To the best of the authors’ knowledge, this is only the second report of cervical disc herniation causing contralateral symptoms. In the only other available case report on cervical disc herniation with contralateral symptoms, the symptoms occurred in both the contralateral arm and leg.9
1. Briggs AM, Straker LM, Bear NL, Smith AJ. Neck/shoulder pain in adolescents is not related to the level or nature of self-reported physical activity or type of sedentary activity in an Australian pregnancy cohort. BMC Musculoskelet Disord. 2009;10(1):87.
2. Hermans J, Luime JJ, Meuffels DE, Reijman M, Simel DL, Bierma-Zeinstra SM. Does this patient with shoulder pain have rotator cuff disease? JAMA. 2013;310(8):837-847.
3. Rubinstein SM, Pool JJ, van Tulder MW, Riphagen I, Riphagen II, de Vet HC. A systematic review of the diagnostic accuracy of provocative tests of the neck for diagnosing cervical radiculopathy. Eur Spine J. 2007;16(3):307-319.
4. Ghasemi M, Golabchi K, Mousavi SA, et al. The value of provocative tests in diagnosis of cervical radiculopathy. J Res Med Sci. 2013;18(suppl 1):S35-S38.
5. Woods BI, Hilibrand AS. Cervical radiculopathy: epidemiology, etiology, diagnosis, and treatment. J Spinal Disord Tech. 2015;28(5):E251-E259.
6. Green C, Butler J, Eustace S, Poynton A, O’Byrne JM. Imaging modalities for cervical spondylotic stenosis and myelopathy. Adv Orthop. 2012;2012:908324. [Epub July 20, 2011].
7. Childress MA, Beckers BA. Nonoperative management of cervical radiculopathy. Am Fam Physician. 2016;93(9):746-754.
8. Carette S, Fehlings MG. Clinical practice. Cervical radiculopathy. N Engl J Med. 2005;353(4):392-399.
9. Yeung JT, Johnson JI, Karim AS. Cervical disc herniation presenting with neck pain and contralateral symptoms: a case report. J Med Case Rep. 2012;6:166.
1. Briggs AM, Straker LM, Bear NL, Smith AJ. Neck/shoulder pain in adolescents is not related to the level or nature of self-reported physical activity or type of sedentary activity in an Australian pregnancy cohort. BMC Musculoskelet Disord. 2009;10(1):87.
2. Hermans J, Luime JJ, Meuffels DE, Reijman M, Simel DL, Bierma-Zeinstra SM. Does this patient with shoulder pain have rotator cuff disease? JAMA. 2013;310(8):837-847.
3. Rubinstein SM, Pool JJ, van Tulder MW, Riphagen I, Riphagen II, de Vet HC. A systematic review of the diagnostic accuracy of provocative tests of the neck for diagnosing cervical radiculopathy. Eur Spine J. 2007;16(3):307-319.
4. Ghasemi M, Golabchi K, Mousavi SA, et al. The value of provocative tests in diagnosis of cervical radiculopathy. J Res Med Sci. 2013;18(suppl 1):S35-S38.
5. Woods BI, Hilibrand AS. Cervical radiculopathy: epidemiology, etiology, diagnosis, and treatment. J Spinal Disord Tech. 2015;28(5):E251-E259.
6. Green C, Butler J, Eustace S, Poynton A, O’Byrne JM. Imaging modalities for cervical spondylotic stenosis and myelopathy. Adv Orthop. 2012;2012:908324. [Epub July 20, 2011].
7. Childress MA, Beckers BA. Nonoperative management of cervical radiculopathy. Am Fam Physician. 2016;93(9):746-754.
8. Carette S, Fehlings MG. Clinical practice. Cervical radiculopathy. N Engl J Med. 2005;353(4):392-399.
9. Yeung JT, Johnson JI, Karim AS. Cervical disc herniation presenting with neck pain and contralateral symptoms: a case report. J Med Case Rep. 2012;6:166.
Emergency Ultrasound: Pericardial Effusion and Tamponade: Making the Diagnosis at Bedside With Point-of-Care Echocardiography
Background
Pericardial effusions are usually an incidental finding on bedside echocardiogram—unless a patient shows clinical signs of tamponade physiology and obstructive shock. Echocardiogram is both sensitive and specific to detecting pericardial effusions.1 Although there are many causes of pericardial effusions, the most common causes of symptomatic effusions in the Western World are due to neoplasm, pericarditis, traumatic pathology, or idiopathic etiology. In developing countries, however, pericardial effusions are predominantly due to tuberculosis in an area where it is endemic.2,3 The size of the effusion is classified based on measurements of a fluid pocket during diastole. Mild effusion is defined as less than 10 mm; moderate effusion, 10 to 20 mm; and large effusion, greater than 20 mm.3
It is important to note that the pericardial space contains up to 50 mL of physiological fluid which may be seen on echocardiography during systole. Small effusions usually contain less than 100 mL of fluid; moderate effusions contain 100 to 500 mL of fluid; and large effusions, over 500 mL of fluid. Fluid of smaller effusions typically layer posteriorly, while the fluid of some moderate and most large effusions may be seen circumferentially.
Clinical Signs and Symptoms
Patients with large chronic effusions are often asymptomatic, and clinical symptoms usually correlate to the acuity of pericardial accumulation. Patients with symptomatic effusions present with dyspnea on exertion that is followed by orthopnea, chest pain, and sometimes dysphagia, hoarseness, or hiccups due to irritation of surrounding structures until they exhibit tamponade physiology leading to hypotension secondary to obstructive shock. The most recognized signs of tamponade physiology on bedside echocardiography are early diastolic collapse of the right atrium and right ventricle, as well as ventricular interdependence.4
Imaging Technique
Pericardial effusion and cardiac tamponade can be detected in any of the standard echocardiographic views, with fluid usually appearing as an anechoic stripe. The fluid will first appear in the dependent portion of the pericardial space, but may become circumferential as it grows (Figure 1).
After evaluating for signs of tamponade on examination, the next step is to evaluate the inferior vena cava (IVC). A simple way to identify the IVC on echocardiography is to visualize the heart in the subxiphoid view, then rotate the probe so that the marker is pointing toward the patient’s head. As demonstrated in Figure 5, the IVC should be seen entering the right atrium; if the patient is truly in obstructive shock, the IVC should be plethoric with minimal respiratory variation.
Pericardiocentesis
In the event of obstructive shock or pulseless electric activity with visualized or suspected tamponade, pericardiocentesis is considered standard of care. There are many approaches to performing a pericardiocentesis, including the classically taught blind subxiphoid approach, which is associated with high rates of morbidity and mortality.5 More recent image-guided approaches employ echocardiography-guided techniques that identify the location and distribution of fluid, and perform pericardiocentesis closest to the area largest fluid accumulation.
Most of these guided techniques involve in-plane visualization of the needle in either a subxiphoid, apical, or parasternal approach. Studies have shown that the subxiphoid approach has a higher risk of injury to the liver, heart, and IVC, with complication rates up to 20% depending on the study.6
The apical approach involves locating the cardiac apex and inserting the needle 1 cm lateral to the apex, with the point directed toward the effusion and in-line with the ultrasound probe, taking care to avoid the lingula. Studies have shown that complication rates with this approach are around 3%.7
Recent studies also suggest that in-line medial-to-lateral parasternal approaches may have minimal complications. However, when employing this approach, care must be taken to avoid the internal mammary artery, which can be identified using color-flow Doppler echocardiology.6
Conclusion
In general, bedside ultrasound is a quick and useful tool to evaluate for pericardial effusion and signs of tamponade physiology. When present, tamponade, a clinical diagnosis, is the likely cause of shock in the hemodynamically unstable patient with circumferential pericardial effusion.
While most cases of pericardial effusion are found incidentally, a stepwise approach to evaluate for tamponade is to quickly look for signs of early right-sided diastolic collapse or ventricular interdependence, as well as a plethoric IVC. For patients who have tamponade requiring pericardiocentesis, the ultrasound-guided apical or parasternal approaches have been shown to have fewer complications compared to the subxiphoid approach.
1. Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J. 2013;34(16):1186-1197. doi:10.1093/eurheartj/ehs372.
2. Ben-Horin S, Bank I, Guetta V, Livneh A. Large symptomatic pericardial effusion as the presentation of unrecognized cancer: a study in 173 consecutive patients undergoing pericardiocentesis. Medicine. 2006;85(1):49-53. doi:10.1097/01.md.0000199556.69588.8e
3. Adler Y, Charron P, Imazio M, et al; European Society of Cardiology (ESC). 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921-2964. doi:10.1093/eurheartj/ehv318.
4. Nagdev A, Stone MB. Point-of-care ultrasound evaluation of pericardial effusions: does this patient have cardiac tamponade? Resuscitation. 2011;82(6):671-673. doi:10.1016/j.resuscitation.2011.02.004.
5. Kumar R, Sinha A, Lin MJ. Complications of pericardiocentesis: a clinical synopsis. Int J Crit Illn Inj Sci. 2015;5(3):206-212. doi:10.4103/2229-5151.165007.
6. Osman A, Wan Chuan T, Ab Rahman J, Via G, Tavazzi G. Ultrasound-guided pericardiocentesis: a novel parasternal approach. Eur J Emerg Med. 2017;5. doi:10.1097/MEJ.0000000000000471.
7. Ozer HO, Davutoğlu V, Cakici M. Echocardiography-guided pericardiocentesis with the apical approach. Turk Kardiyol Dern Ars. 2009;37(3):177-181.
Background
Pericardial effusions are usually an incidental finding on bedside echocardiogram—unless a patient shows clinical signs of tamponade physiology and obstructive shock. Echocardiogram is both sensitive and specific to detecting pericardial effusions.1 Although there are many causes of pericardial effusions, the most common causes of symptomatic effusions in the Western World are due to neoplasm, pericarditis, traumatic pathology, or idiopathic etiology. In developing countries, however, pericardial effusions are predominantly due to tuberculosis in an area where it is endemic.2,3 The size of the effusion is classified based on measurements of a fluid pocket during diastole. Mild effusion is defined as less than 10 mm; moderate effusion, 10 to 20 mm; and large effusion, greater than 20 mm.3
It is important to note that the pericardial space contains up to 50 mL of physiological fluid which may be seen on echocardiography during systole. Small effusions usually contain less than 100 mL of fluid; moderate effusions contain 100 to 500 mL of fluid; and large effusions, over 500 mL of fluid. Fluid of smaller effusions typically layer posteriorly, while the fluid of some moderate and most large effusions may be seen circumferentially.
Clinical Signs and Symptoms
Patients with large chronic effusions are often asymptomatic, and clinical symptoms usually correlate to the acuity of pericardial accumulation. Patients with symptomatic effusions present with dyspnea on exertion that is followed by orthopnea, chest pain, and sometimes dysphagia, hoarseness, or hiccups due to irritation of surrounding structures until they exhibit tamponade physiology leading to hypotension secondary to obstructive shock. The most recognized signs of tamponade physiology on bedside echocardiography are early diastolic collapse of the right atrium and right ventricle, as well as ventricular interdependence.4
Imaging Technique
Pericardial effusion and cardiac tamponade can be detected in any of the standard echocardiographic views, with fluid usually appearing as an anechoic stripe. The fluid will first appear in the dependent portion of the pericardial space, but may become circumferential as it grows (Figure 1).
After evaluating for signs of tamponade on examination, the next step is to evaluate the inferior vena cava (IVC). A simple way to identify the IVC on echocardiography is to visualize the heart in the subxiphoid view, then rotate the probe so that the marker is pointing toward the patient’s head. As demonstrated in Figure 5, the IVC should be seen entering the right atrium; if the patient is truly in obstructive shock, the IVC should be plethoric with minimal respiratory variation.
Pericardiocentesis
In the event of obstructive shock or pulseless electric activity with visualized or suspected tamponade, pericardiocentesis is considered standard of care. There are many approaches to performing a pericardiocentesis, including the classically taught blind subxiphoid approach, which is associated with high rates of morbidity and mortality.5 More recent image-guided approaches employ echocardiography-guided techniques that identify the location and distribution of fluid, and perform pericardiocentesis closest to the area largest fluid accumulation.
Most of these guided techniques involve in-plane visualization of the needle in either a subxiphoid, apical, or parasternal approach. Studies have shown that the subxiphoid approach has a higher risk of injury to the liver, heart, and IVC, with complication rates up to 20% depending on the study.6
The apical approach involves locating the cardiac apex and inserting the needle 1 cm lateral to the apex, with the point directed toward the effusion and in-line with the ultrasound probe, taking care to avoid the lingula. Studies have shown that complication rates with this approach are around 3%.7
Recent studies also suggest that in-line medial-to-lateral parasternal approaches may have minimal complications. However, when employing this approach, care must be taken to avoid the internal mammary artery, which can be identified using color-flow Doppler echocardiology.6
Conclusion
In general, bedside ultrasound is a quick and useful tool to evaluate for pericardial effusion and signs of tamponade physiology. When present, tamponade, a clinical diagnosis, is the likely cause of shock in the hemodynamically unstable patient with circumferential pericardial effusion.
While most cases of pericardial effusion are found incidentally, a stepwise approach to evaluate for tamponade is to quickly look for signs of early right-sided diastolic collapse or ventricular interdependence, as well as a plethoric IVC. For patients who have tamponade requiring pericardiocentesis, the ultrasound-guided apical or parasternal approaches have been shown to have fewer complications compared to the subxiphoid approach.
Background
Pericardial effusions are usually an incidental finding on bedside echocardiogram—unless a patient shows clinical signs of tamponade physiology and obstructive shock. Echocardiogram is both sensitive and specific to detecting pericardial effusions.1 Although there are many causes of pericardial effusions, the most common causes of symptomatic effusions in the Western World are due to neoplasm, pericarditis, traumatic pathology, or idiopathic etiology. In developing countries, however, pericardial effusions are predominantly due to tuberculosis in an area where it is endemic.2,3 The size of the effusion is classified based on measurements of a fluid pocket during diastole. Mild effusion is defined as less than 10 mm; moderate effusion, 10 to 20 mm; and large effusion, greater than 20 mm.3
It is important to note that the pericardial space contains up to 50 mL of physiological fluid which may be seen on echocardiography during systole. Small effusions usually contain less than 100 mL of fluid; moderate effusions contain 100 to 500 mL of fluid; and large effusions, over 500 mL of fluid. Fluid of smaller effusions typically layer posteriorly, while the fluid of some moderate and most large effusions may be seen circumferentially.
Clinical Signs and Symptoms
Patients with large chronic effusions are often asymptomatic, and clinical symptoms usually correlate to the acuity of pericardial accumulation. Patients with symptomatic effusions present with dyspnea on exertion that is followed by orthopnea, chest pain, and sometimes dysphagia, hoarseness, or hiccups due to irritation of surrounding structures until they exhibit tamponade physiology leading to hypotension secondary to obstructive shock. The most recognized signs of tamponade physiology on bedside echocardiography are early diastolic collapse of the right atrium and right ventricle, as well as ventricular interdependence.4
Imaging Technique
Pericardial effusion and cardiac tamponade can be detected in any of the standard echocardiographic views, with fluid usually appearing as an anechoic stripe. The fluid will first appear in the dependent portion of the pericardial space, but may become circumferential as it grows (Figure 1).
After evaluating for signs of tamponade on examination, the next step is to evaluate the inferior vena cava (IVC). A simple way to identify the IVC on echocardiography is to visualize the heart in the subxiphoid view, then rotate the probe so that the marker is pointing toward the patient’s head. As demonstrated in Figure 5, the IVC should be seen entering the right atrium; if the patient is truly in obstructive shock, the IVC should be plethoric with minimal respiratory variation.
Pericardiocentesis
In the event of obstructive shock or pulseless electric activity with visualized or suspected tamponade, pericardiocentesis is considered standard of care. There are many approaches to performing a pericardiocentesis, including the classically taught blind subxiphoid approach, which is associated with high rates of morbidity and mortality.5 More recent image-guided approaches employ echocardiography-guided techniques that identify the location and distribution of fluid, and perform pericardiocentesis closest to the area largest fluid accumulation.
Most of these guided techniques involve in-plane visualization of the needle in either a subxiphoid, apical, or parasternal approach. Studies have shown that the subxiphoid approach has a higher risk of injury to the liver, heart, and IVC, with complication rates up to 20% depending on the study.6
The apical approach involves locating the cardiac apex and inserting the needle 1 cm lateral to the apex, with the point directed toward the effusion and in-line with the ultrasound probe, taking care to avoid the lingula. Studies have shown that complication rates with this approach are around 3%.7
Recent studies also suggest that in-line medial-to-lateral parasternal approaches may have minimal complications. However, when employing this approach, care must be taken to avoid the internal mammary artery, which can be identified using color-flow Doppler echocardiology.6
Conclusion
In general, bedside ultrasound is a quick and useful tool to evaluate for pericardial effusion and signs of tamponade physiology. When present, tamponade, a clinical diagnosis, is the likely cause of shock in the hemodynamically unstable patient with circumferential pericardial effusion.
While most cases of pericardial effusion are found incidentally, a stepwise approach to evaluate for tamponade is to quickly look for signs of early right-sided diastolic collapse or ventricular interdependence, as well as a plethoric IVC. For patients who have tamponade requiring pericardiocentesis, the ultrasound-guided apical or parasternal approaches have been shown to have fewer complications compared to the subxiphoid approach.
1. Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J. 2013;34(16):1186-1197. doi:10.1093/eurheartj/ehs372.
2. Ben-Horin S, Bank I, Guetta V, Livneh A. Large symptomatic pericardial effusion as the presentation of unrecognized cancer: a study in 173 consecutive patients undergoing pericardiocentesis. Medicine. 2006;85(1):49-53. doi:10.1097/01.md.0000199556.69588.8e
3. Adler Y, Charron P, Imazio M, et al; European Society of Cardiology (ESC). 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921-2964. doi:10.1093/eurheartj/ehv318.
4. Nagdev A, Stone MB. Point-of-care ultrasound evaluation of pericardial effusions: does this patient have cardiac tamponade? Resuscitation. 2011;82(6):671-673. doi:10.1016/j.resuscitation.2011.02.004.
5. Kumar R, Sinha A, Lin MJ. Complications of pericardiocentesis: a clinical synopsis. Int J Crit Illn Inj Sci. 2015;5(3):206-212. doi:10.4103/2229-5151.165007.
6. Osman A, Wan Chuan T, Ab Rahman J, Via G, Tavazzi G. Ultrasound-guided pericardiocentesis: a novel parasternal approach. Eur J Emerg Med. 2017;5. doi:10.1097/MEJ.0000000000000471.
7. Ozer HO, Davutoğlu V, Cakici M. Echocardiography-guided pericardiocentesis with the apical approach. Turk Kardiyol Dern Ars. 2009;37(3):177-181.
1. Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J. 2013;34(16):1186-1197. doi:10.1093/eurheartj/ehs372.
2. Ben-Horin S, Bank I, Guetta V, Livneh A. Large symptomatic pericardial effusion as the presentation of unrecognized cancer: a study in 173 consecutive patients undergoing pericardiocentesis. Medicine. 2006;85(1):49-53. doi:10.1097/01.md.0000199556.69588.8e
3. Adler Y, Charron P, Imazio M, et al; European Society of Cardiology (ESC). 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921-2964. doi:10.1093/eurheartj/ehv318.
4. Nagdev A, Stone MB. Point-of-care ultrasound evaluation of pericardial effusions: does this patient have cardiac tamponade? Resuscitation. 2011;82(6):671-673. doi:10.1016/j.resuscitation.2011.02.004.
5. Kumar R, Sinha A, Lin MJ. Complications of pericardiocentesis: a clinical synopsis. Int J Crit Illn Inj Sci. 2015;5(3):206-212. doi:10.4103/2229-5151.165007.
6. Osman A, Wan Chuan T, Ab Rahman J, Via G, Tavazzi G. Ultrasound-guided pericardiocentesis: a novel parasternal approach. Eur J Emerg Med. 2017;5. doi:10.1097/MEJ.0000000000000471.
7. Ozer HO, Davutoğlu V, Cakici M. Echocardiography-guided pericardiocentesis with the apical approach. Turk Kardiyol Dern Ars. 2009;37(3):177-181.
A Case of Streptococcus pyogenes Sepsis of Possible Oral Origin
Sepsis can be the result of single or multiple factors and sources of infection. Oral sources of sepsis and systemic infection are not commonly considered as the first potential source of infection when evaluating a septic patient. Oral infections of odontogenic or periodontal origin are frequently associated with localized or diffuse cellulitis of the head and neck region. 1 The patient’s health status and complicating problems, such as an immunocompromising condition, can further reduce the immune response for controlling chronic sources of infection. This in turn can lead to acute manifestations such as cellulitis, sepsis, or necrotizing fasciitis. Necrotizing fasciitis is caused by a polymicrobial or mixed aerobic-anaerobic infection from a variety of sources, including Streptococcus pyogenes .
Case
A 57-year-old woman with a history of major depressive disorder, paroxysmal atrial fibrillation, and opioid dependence that was in remission for more than 3 years was brought to the ED by a family member after the patient developed confusion and lethargy. She was primarily experiencing right breast pain and swelling. The breast pain was associated with high fevers, nausea, vomiting, and chills.
On examination, the patient’s vital signs were: blood pressure (BP), 109/58 mm Hg; heart rate, 160 beats/min; respiratory rate, 22 breaths/min; and temperature, 104°F. Laboratory evaluation was significant for a white blood cell count (WBC) of 8.7 x 103. There was a noted skin abrasion on the patient’s right hand. She was lethargic and confused. Blood cultures were positive for S pyogenes, and a swab of the right breast was negative for bacterial growth.
The patient was admitted to the medical intensive care unit (MICU) and placed on two vasopressors for control of low BP and assistance with low urine output. After a 6-L fluid resuscitation, the patient was started on vancomycin and piperacillin/tazobactam for possible cellulitis causing sepsis. An echocardiogram was negative for endocarditis. The patient continued to decline the following day with continuing tachycardia and tachypnea with hypotension and was intubated. Pulmonology services was consulted for possible acute respiratory distress syndrome secondary to sepsis; general surgery services was consulted for possible necrotizing fasciitis of the chest wall; and cardiology services was consulted for low-cardiac output.
On hospital day 4, the patient was taken to surgery for exploration, drainage, and debridement of the right axilla and breast; cultures were taken, and lack of organism growth was noted. While in the MICU, she was followed by the infectious disease service as her WBC remained elevated and peaking at 32.6 x 103, while blood cultures were negative for bacterial growth.
The dental service was consulted on hospital day 5 to evaluate for other possible sources of infection. Upon examination, the patient’s oral condition was noted as having advanced chronic periodontal disease that required full-mouth extraction. The patient remained hemodynamically unstable with platelet counts below 50 x 109/L until hospital day 7, at which time she was taken for surgery for full mouth extraction and associated alveoloplasty. Upon extraction, the patient continued to improve and was extubated on day 11 with platelets and WBC returning to normal levels by day 13 of her hospital stay. The patient remained hospitalized for a total MICU stay of 20 days and rehabilitation stay of more than 2 weeks.
Discussion
Oral infections most often present with acute onset and noted oral-facial cellulitis or abscess. Oral source of septicemia often are considered after ruling out most other potential sources. Although it is not certain that this case is directly related to the advanced chronic periodontal disease, S pyogenes has been noted to be a pathogen in periodontal disease progression.
According to the American Dental Association in 2012, dental visits to the ED cost the US health care system $1.6 billion and an average cost of $749 per visit. There are more than 2 million ED visits each year for dental pain and infection, and 39% return due to nonresolution of the dental problem. Patients return to the ED due to lack of access and resources to routine and emergent dental care. 2 The average daily cost of an MICU stay with mechanical ventilation was $2,193 in 2002. This particular case consisted of 11 days of mechanical ventilation, 20 MICU days, and an additional 20 days of inpatient rehabilitation which resulted in costs that exceeded $50,000. 3
1. Krishnan V, Johnson JV, Helfric JF. Management of maxillofacial infections: a review of 50 cases. J Oral Maxillofac Surg. 1993;51(8):868-873; discussion 873-874.
2. Wall T, Vujicic M. Emergency department use for dental conditions continues to increase. American Dental Association: Health Policy Institute. http://www.ada.org/~/media/ADA/Science%20and%20Research/HPI/Files/HPIBrief_0415_2.ashx. Published April 2015. Accessed November 6, 2017.
3. Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266-1271.
Sepsis can be the result of single or multiple factors and sources of infection. Oral sources of sepsis and systemic infection are not commonly considered as the first potential source of infection when evaluating a septic patient. Oral infections of odontogenic or periodontal origin are frequently associated with localized or diffuse cellulitis of the head and neck region. 1 The patient’s health status and complicating problems, such as an immunocompromising condition, can further reduce the immune response for controlling chronic sources of infection. This in turn can lead to acute manifestations such as cellulitis, sepsis, or necrotizing fasciitis. Necrotizing fasciitis is caused by a polymicrobial or mixed aerobic-anaerobic infection from a variety of sources, including Streptococcus pyogenes .
Case
A 57-year-old woman with a history of major depressive disorder, paroxysmal atrial fibrillation, and opioid dependence that was in remission for more than 3 years was brought to the ED by a family member after the patient developed confusion and lethargy. She was primarily experiencing right breast pain and swelling. The breast pain was associated with high fevers, nausea, vomiting, and chills.
On examination, the patient’s vital signs were: blood pressure (BP), 109/58 mm Hg; heart rate, 160 beats/min; respiratory rate, 22 breaths/min; and temperature, 104°F. Laboratory evaluation was significant for a white blood cell count (WBC) of 8.7 x 103. There was a noted skin abrasion on the patient’s right hand. She was lethargic and confused. Blood cultures were positive for S pyogenes, and a swab of the right breast was negative for bacterial growth.
The patient was admitted to the medical intensive care unit (MICU) and placed on two vasopressors for control of low BP and assistance with low urine output. After a 6-L fluid resuscitation, the patient was started on vancomycin and piperacillin/tazobactam for possible cellulitis causing sepsis. An echocardiogram was negative for endocarditis. The patient continued to decline the following day with continuing tachycardia and tachypnea with hypotension and was intubated. Pulmonology services was consulted for possible acute respiratory distress syndrome secondary to sepsis; general surgery services was consulted for possible necrotizing fasciitis of the chest wall; and cardiology services was consulted for low-cardiac output.
On hospital day 4, the patient was taken to surgery for exploration, drainage, and debridement of the right axilla and breast; cultures were taken, and lack of organism growth was noted. While in the MICU, she was followed by the infectious disease service as her WBC remained elevated and peaking at 32.6 x 103, while blood cultures were negative for bacterial growth.
The dental service was consulted on hospital day 5 to evaluate for other possible sources of infection. Upon examination, the patient’s oral condition was noted as having advanced chronic periodontal disease that required full-mouth extraction. The patient remained hemodynamically unstable with platelet counts below 50 x 109/L until hospital day 7, at which time she was taken for surgery for full mouth extraction and associated alveoloplasty. Upon extraction, the patient continued to improve and was extubated on day 11 with platelets and WBC returning to normal levels by day 13 of her hospital stay. The patient remained hospitalized for a total MICU stay of 20 days and rehabilitation stay of more than 2 weeks.
Discussion
Oral infections most often present with acute onset and noted oral-facial cellulitis or abscess. Oral source of septicemia often are considered after ruling out most other potential sources. Although it is not certain that this case is directly related to the advanced chronic periodontal disease, S pyogenes has been noted to be a pathogen in periodontal disease progression.
According to the American Dental Association in 2012, dental visits to the ED cost the US health care system $1.6 billion and an average cost of $749 per visit. There are more than 2 million ED visits each year for dental pain and infection, and 39% return due to nonresolution of the dental problem. Patients return to the ED due to lack of access and resources to routine and emergent dental care. 2 The average daily cost of an MICU stay with mechanical ventilation was $2,193 in 2002. This particular case consisted of 11 days of mechanical ventilation, 20 MICU days, and an additional 20 days of inpatient rehabilitation which resulted in costs that exceeded $50,000. 3
Sepsis can be the result of single or multiple factors and sources of infection. Oral sources of sepsis and systemic infection are not commonly considered as the first potential source of infection when evaluating a septic patient. Oral infections of odontogenic or periodontal origin are frequently associated with localized or diffuse cellulitis of the head and neck region. 1 The patient’s health status and complicating problems, such as an immunocompromising condition, can further reduce the immune response for controlling chronic sources of infection. This in turn can lead to acute manifestations such as cellulitis, sepsis, or necrotizing fasciitis. Necrotizing fasciitis is caused by a polymicrobial or mixed aerobic-anaerobic infection from a variety of sources, including Streptococcus pyogenes .
Case
A 57-year-old woman with a history of major depressive disorder, paroxysmal atrial fibrillation, and opioid dependence that was in remission for more than 3 years was brought to the ED by a family member after the patient developed confusion and lethargy. She was primarily experiencing right breast pain and swelling. The breast pain was associated with high fevers, nausea, vomiting, and chills.
On examination, the patient’s vital signs were: blood pressure (BP), 109/58 mm Hg; heart rate, 160 beats/min; respiratory rate, 22 breaths/min; and temperature, 104°F. Laboratory evaluation was significant for a white blood cell count (WBC) of 8.7 x 103. There was a noted skin abrasion on the patient’s right hand. She was lethargic and confused. Blood cultures were positive for S pyogenes, and a swab of the right breast was negative for bacterial growth.
The patient was admitted to the medical intensive care unit (MICU) and placed on two vasopressors for control of low BP and assistance with low urine output. After a 6-L fluid resuscitation, the patient was started on vancomycin and piperacillin/tazobactam for possible cellulitis causing sepsis. An echocardiogram was negative for endocarditis. The patient continued to decline the following day with continuing tachycardia and tachypnea with hypotension and was intubated. Pulmonology services was consulted for possible acute respiratory distress syndrome secondary to sepsis; general surgery services was consulted for possible necrotizing fasciitis of the chest wall; and cardiology services was consulted for low-cardiac output.
On hospital day 4, the patient was taken to surgery for exploration, drainage, and debridement of the right axilla and breast; cultures were taken, and lack of organism growth was noted. While in the MICU, she was followed by the infectious disease service as her WBC remained elevated and peaking at 32.6 x 103, while blood cultures were negative for bacterial growth.
The dental service was consulted on hospital day 5 to evaluate for other possible sources of infection. Upon examination, the patient’s oral condition was noted as having advanced chronic periodontal disease that required full-mouth extraction. The patient remained hemodynamically unstable with platelet counts below 50 x 109/L until hospital day 7, at which time she was taken for surgery for full mouth extraction and associated alveoloplasty. Upon extraction, the patient continued to improve and was extubated on day 11 with platelets and WBC returning to normal levels by day 13 of her hospital stay. The patient remained hospitalized for a total MICU stay of 20 days and rehabilitation stay of more than 2 weeks.
Discussion
Oral infections most often present with acute onset and noted oral-facial cellulitis or abscess. Oral source of septicemia often are considered after ruling out most other potential sources. Although it is not certain that this case is directly related to the advanced chronic periodontal disease, S pyogenes has been noted to be a pathogen in periodontal disease progression.
According to the American Dental Association in 2012, dental visits to the ED cost the US health care system $1.6 billion and an average cost of $749 per visit. There are more than 2 million ED visits each year for dental pain and infection, and 39% return due to nonresolution of the dental problem. Patients return to the ED due to lack of access and resources to routine and emergent dental care. 2 The average daily cost of an MICU stay with mechanical ventilation was $2,193 in 2002. This particular case consisted of 11 days of mechanical ventilation, 20 MICU days, and an additional 20 days of inpatient rehabilitation which resulted in costs that exceeded $50,000. 3
1. Krishnan V, Johnson JV, Helfric JF. Management of maxillofacial infections: a review of 50 cases. J Oral Maxillofac Surg. 1993;51(8):868-873; discussion 873-874.
2. Wall T, Vujicic M. Emergency department use for dental conditions continues to increase. American Dental Association: Health Policy Institute. http://www.ada.org/~/media/ADA/Science%20and%20Research/HPI/Files/HPIBrief_0415_2.ashx. Published April 2015. Accessed November 6, 2017.
3. Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266-1271.
1. Krishnan V, Johnson JV, Helfric JF. Management of maxillofacial infections: a review of 50 cases. J Oral Maxillofac Surg. 1993;51(8):868-873; discussion 873-874.
2. Wall T, Vujicic M. Emergency department use for dental conditions continues to increase. American Dental Association: Health Policy Institute. http://www.ada.org/~/media/ADA/Science%20and%20Research/HPI/Files/HPIBrief_0415_2.ashx. Published April 2015. Accessed November 6, 2017.
3. Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266-1271.
Case Studies in Toxicology: DILI Dally
Case
A 50-year-old Hispanic woman with a history of rheumatoid arthritis (RA), for which she was not currently taking medication, was referred to the ED by her primary care physician (PCP) for evaluation of generalized pruritus and jaundice, and an abnormal hepatic function panel.
The patient’s recent history was significant for a positive tuberculosis test (purified protein derivative [PPD], 13 mm), for which she had been on prophylactic medication. Laboratory evaluation taken during the patient’s recent follow-up visit with her PCP revealed the following significant hepatic abnormalities: total bilirubin, 20.0 mg/dL; direct bilirubin, 16.4 mg/dL; international normalized ratio, 2.9; aspartate aminotransferase, greater than 2,000 IU/L; and alanine aminotransferase, greater than 2,000 IU/L. The patient had no history of hepatic disease, and a hepatitis panel obtained in the ED was unremarkable.
Can this be drug-induced liver injury?
Drug-induced liver injury (DILI) accounts for nearly 50% of cases of acute liver failure in the United States.1 According to the National Institutes of Health database of drugs, supplements, and herbal medications acetaminophen is the most common drug associated with hepatotoxicity in the United States, whereas amoxicillin-clavulanate is the most common implicated drug worldwide.1,2 The histological pattern of DILI varies by drug (Table).3
Who is susceptible to drug-induced liver injury?
The factors that help predict DILI include drug pharmacokinetics and metabolism, as well as patient age, sex, and comorbidities. Although some patients are at an increased risk of DILI, it is extraordinarily difficult to accurately predict which patients will develop it. In general, there is a positive correlation between age and risk of developing DILI. For example, in a large US-based tuberculosis study, the incidence of isoniazid (INH)-induced hepatotoxicity was 4.4 per 1,000 patients aged 25 to 34 years. Patients older than age 50 years had a 20.83 per 1,000 incidence of DILI, and women also appear to be at increased risk.4
Pharmacogenetic factors affecting drug metabolism such as the specific cytochrome profile and acetylator status of an individual also influence a patient’s risk of developing DILI. Although our understanding of these issues is growing rapidly, our ability to apply this knowledge to the clinical venue is limited by the available technology, regulatory requirements, and cost.
Case Continuation
A detailed, careful history-taking in the ED revealed that, 2 months prior, the patient had been started on INH, rifampin, and pyridoxine for latent tuberculosis. She had been taking methotrexate for the RA but discontinued it 3 months ago because of the positive PPD. When routine outpatient laboratory testing results demonstrated significant hepatic dysfunction, the patient’s PCP advised her to immediately discontinue her medications and referred her to the ED for further evaluation and management.
By what mechanism does INH cause DILI?
Acute INH-associated hepatitis primarily results from the direct hepatotoxic effects of INH metabolites. Isoniazid is metabolized in the liver via N-acetylation to acetylisoniazid (Figure). Oxidation of this compound in the liver leads to an accumulation of the hepatotoxic metabolites acetylhydrazine and hydrazine.5,6
Is there a role for N-acetylcysteine in INH hepatotoxicity?
No antidote is specifically designed to treat INH-induced hepatotoxicity, and management is largely supportive. Observation for progressive liver failure is indicated and evaluation for liver transplant may become necessary.
N-acetylcysteine (NAC) has a clear role in preventing hepatotoxicity from acetaminophen overdose through its ability to act as a precursor for the synthesis of glutathione—a compound that protects hepatocytes from oxidative damage. In advanced acetaminophen-toxic patients and those with non-acetaminophen toxicity, NAC has nonspecific effects that promote healing through several mechanisms, including anti-inflammatory effect and enhanced hepatic perfusion. Though there are no studies that specifically evaluate the role of NAC in patients with INH-induced hepatotoxicity, it is commonly and appropriately administered for its aforementioned nonspecific effects.8 Common side effects from NAC administration include nausea, vomiting, and diarrhea, which are generally treatable with symptomatic and supportive care.
Case Conclusion
The patient was admitted to the hepatology service for continued clinical care. Although she received NAC, hepatic function testing showed only mild improvement. Additional etiologies of liver failure were investigated, including a computed tomography scan of the abdomen/pelvis and an abdominal ultrasound with Doppler. Both studies were negative for any pathology, and autoimmune laboratory studies were likewise unremarkable.
The patient underwent a liver biopsy, which revealed inflammation and scattered eosinophils suggestive of drug-induced hepatic injury. Her clinical condition continued to deteriorate, and she was transferred to another hospital for transplant evaluation.
1. Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575-586, viii. doi:10.1016/j.cld.2013.07.001.
2. National Institutes of Health Web site. LiverTox: Clinical and research information on drug-induced liver injury. https://livertox.nlm.nih.gov/. Updated February 10, 2017. Accessed October 12, 2017.
3. Ansari JA, Sayyed M, Sayeed F. Management of non alcoholic fatty liver diseases and their complications. Int J Pharmacol. 2011;7:579-588. doi:10.3923/ijp.2011.579.588.
4. Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128(1):116-123. doi:10.1378/chest.128.1.116.
5. Hernon CH. Antituberculous medications. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:787-796.
6. Teixeira RL, Morato RG, Cabello PH, et al. Genetic polymorphisms of NAT2, CYP2E1 and GST enzymes and the occurrence of antituberculosis drug-induced hepatitis in Brazilian TB patients. Mem Inst Oswaldo Cruz. 2011;106(6):716-724.
7. Mitchell JR, Thorgeirsson UP, Black M, et al. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydranize metabolites. Clin Pharmacol Ther. 1975;18(1):70-79.
8. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864. doi:10.1053/j.gastro.2009.06.006.
Case
A 50-year-old Hispanic woman with a history of rheumatoid arthritis (RA), for which she was not currently taking medication, was referred to the ED by her primary care physician (PCP) for evaluation of generalized pruritus and jaundice, and an abnormal hepatic function panel.
The patient’s recent history was significant for a positive tuberculosis test (purified protein derivative [PPD], 13 mm), for which she had been on prophylactic medication. Laboratory evaluation taken during the patient’s recent follow-up visit with her PCP revealed the following significant hepatic abnormalities: total bilirubin, 20.0 mg/dL; direct bilirubin, 16.4 mg/dL; international normalized ratio, 2.9; aspartate aminotransferase, greater than 2,000 IU/L; and alanine aminotransferase, greater than 2,000 IU/L. The patient had no history of hepatic disease, and a hepatitis panel obtained in the ED was unremarkable.
Can this be drug-induced liver injury?
Drug-induced liver injury (DILI) accounts for nearly 50% of cases of acute liver failure in the United States.1 According to the National Institutes of Health database of drugs, supplements, and herbal medications acetaminophen is the most common drug associated with hepatotoxicity in the United States, whereas amoxicillin-clavulanate is the most common implicated drug worldwide.1,2 The histological pattern of DILI varies by drug (Table).3
Who is susceptible to drug-induced liver injury?
The factors that help predict DILI include drug pharmacokinetics and metabolism, as well as patient age, sex, and comorbidities. Although some patients are at an increased risk of DILI, it is extraordinarily difficult to accurately predict which patients will develop it. In general, there is a positive correlation between age and risk of developing DILI. For example, in a large US-based tuberculosis study, the incidence of isoniazid (INH)-induced hepatotoxicity was 4.4 per 1,000 patients aged 25 to 34 years. Patients older than age 50 years had a 20.83 per 1,000 incidence of DILI, and women also appear to be at increased risk.4
Pharmacogenetic factors affecting drug metabolism such as the specific cytochrome profile and acetylator status of an individual also influence a patient’s risk of developing DILI. Although our understanding of these issues is growing rapidly, our ability to apply this knowledge to the clinical venue is limited by the available technology, regulatory requirements, and cost.
Case Continuation
A detailed, careful history-taking in the ED revealed that, 2 months prior, the patient had been started on INH, rifampin, and pyridoxine for latent tuberculosis. She had been taking methotrexate for the RA but discontinued it 3 months ago because of the positive PPD. When routine outpatient laboratory testing results demonstrated significant hepatic dysfunction, the patient’s PCP advised her to immediately discontinue her medications and referred her to the ED for further evaluation and management.
By what mechanism does INH cause DILI?
Acute INH-associated hepatitis primarily results from the direct hepatotoxic effects of INH metabolites. Isoniazid is metabolized in the liver via N-acetylation to acetylisoniazid (Figure). Oxidation of this compound in the liver leads to an accumulation of the hepatotoxic metabolites acetylhydrazine and hydrazine.5,6
Is there a role for N-acetylcysteine in INH hepatotoxicity?
No antidote is specifically designed to treat INH-induced hepatotoxicity, and management is largely supportive. Observation for progressive liver failure is indicated and evaluation for liver transplant may become necessary.
N-acetylcysteine (NAC) has a clear role in preventing hepatotoxicity from acetaminophen overdose through its ability to act as a precursor for the synthesis of glutathione—a compound that protects hepatocytes from oxidative damage. In advanced acetaminophen-toxic patients and those with non-acetaminophen toxicity, NAC has nonspecific effects that promote healing through several mechanisms, including anti-inflammatory effect and enhanced hepatic perfusion. Though there are no studies that specifically evaluate the role of NAC in patients with INH-induced hepatotoxicity, it is commonly and appropriately administered for its aforementioned nonspecific effects.8 Common side effects from NAC administration include nausea, vomiting, and diarrhea, which are generally treatable with symptomatic and supportive care.
Case Conclusion
The patient was admitted to the hepatology service for continued clinical care. Although she received NAC, hepatic function testing showed only mild improvement. Additional etiologies of liver failure were investigated, including a computed tomography scan of the abdomen/pelvis and an abdominal ultrasound with Doppler. Both studies were negative for any pathology, and autoimmune laboratory studies were likewise unremarkable.
The patient underwent a liver biopsy, which revealed inflammation and scattered eosinophils suggestive of drug-induced hepatic injury. Her clinical condition continued to deteriorate, and she was transferred to another hospital for transplant evaluation.
Case
A 50-year-old Hispanic woman with a history of rheumatoid arthritis (RA), for which she was not currently taking medication, was referred to the ED by her primary care physician (PCP) for evaluation of generalized pruritus and jaundice, and an abnormal hepatic function panel.
The patient’s recent history was significant for a positive tuberculosis test (purified protein derivative [PPD], 13 mm), for which she had been on prophylactic medication. Laboratory evaluation taken during the patient’s recent follow-up visit with her PCP revealed the following significant hepatic abnormalities: total bilirubin, 20.0 mg/dL; direct bilirubin, 16.4 mg/dL; international normalized ratio, 2.9; aspartate aminotransferase, greater than 2,000 IU/L; and alanine aminotransferase, greater than 2,000 IU/L. The patient had no history of hepatic disease, and a hepatitis panel obtained in the ED was unremarkable.
Can this be drug-induced liver injury?
Drug-induced liver injury (DILI) accounts for nearly 50% of cases of acute liver failure in the United States.1 According to the National Institutes of Health database of drugs, supplements, and herbal medications acetaminophen is the most common drug associated with hepatotoxicity in the United States, whereas amoxicillin-clavulanate is the most common implicated drug worldwide.1,2 The histological pattern of DILI varies by drug (Table).3
Who is susceptible to drug-induced liver injury?
The factors that help predict DILI include drug pharmacokinetics and metabolism, as well as patient age, sex, and comorbidities. Although some patients are at an increased risk of DILI, it is extraordinarily difficult to accurately predict which patients will develop it. In general, there is a positive correlation between age and risk of developing DILI. For example, in a large US-based tuberculosis study, the incidence of isoniazid (INH)-induced hepatotoxicity was 4.4 per 1,000 patients aged 25 to 34 years. Patients older than age 50 years had a 20.83 per 1,000 incidence of DILI, and women also appear to be at increased risk.4
Pharmacogenetic factors affecting drug metabolism such as the specific cytochrome profile and acetylator status of an individual also influence a patient’s risk of developing DILI. Although our understanding of these issues is growing rapidly, our ability to apply this knowledge to the clinical venue is limited by the available technology, regulatory requirements, and cost.
Case Continuation
A detailed, careful history-taking in the ED revealed that, 2 months prior, the patient had been started on INH, rifampin, and pyridoxine for latent tuberculosis. She had been taking methotrexate for the RA but discontinued it 3 months ago because of the positive PPD. When routine outpatient laboratory testing results demonstrated significant hepatic dysfunction, the patient’s PCP advised her to immediately discontinue her medications and referred her to the ED for further evaluation and management.
By what mechanism does INH cause DILI?
Acute INH-associated hepatitis primarily results from the direct hepatotoxic effects of INH metabolites. Isoniazid is metabolized in the liver via N-acetylation to acetylisoniazid (Figure). Oxidation of this compound in the liver leads to an accumulation of the hepatotoxic metabolites acetylhydrazine and hydrazine.5,6
Is there a role for N-acetylcysteine in INH hepatotoxicity?
No antidote is specifically designed to treat INH-induced hepatotoxicity, and management is largely supportive. Observation for progressive liver failure is indicated and evaluation for liver transplant may become necessary.
N-acetylcysteine (NAC) has a clear role in preventing hepatotoxicity from acetaminophen overdose through its ability to act as a precursor for the synthesis of glutathione—a compound that protects hepatocytes from oxidative damage. In advanced acetaminophen-toxic patients and those with non-acetaminophen toxicity, NAC has nonspecific effects that promote healing through several mechanisms, including anti-inflammatory effect and enhanced hepatic perfusion. Though there are no studies that specifically evaluate the role of NAC in patients with INH-induced hepatotoxicity, it is commonly and appropriately administered for its aforementioned nonspecific effects.8 Common side effects from NAC administration include nausea, vomiting, and diarrhea, which are generally treatable with symptomatic and supportive care.
Case Conclusion
The patient was admitted to the hepatology service for continued clinical care. Although she received NAC, hepatic function testing showed only mild improvement. Additional etiologies of liver failure were investigated, including a computed tomography scan of the abdomen/pelvis and an abdominal ultrasound with Doppler. Both studies were negative for any pathology, and autoimmune laboratory studies were likewise unremarkable.
The patient underwent a liver biopsy, which revealed inflammation and scattered eosinophils suggestive of drug-induced hepatic injury. Her clinical condition continued to deteriorate, and she was transferred to another hospital for transplant evaluation.
1. Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575-586, viii. doi:10.1016/j.cld.2013.07.001.
2. National Institutes of Health Web site. LiverTox: Clinical and research information on drug-induced liver injury. https://livertox.nlm.nih.gov/. Updated February 10, 2017. Accessed October 12, 2017.
3. Ansari JA, Sayyed M, Sayeed F. Management of non alcoholic fatty liver diseases and their complications. Int J Pharmacol. 2011;7:579-588. doi:10.3923/ijp.2011.579.588.
4. Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128(1):116-123. doi:10.1378/chest.128.1.116.
5. Hernon CH. Antituberculous medications. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:787-796.
6. Teixeira RL, Morato RG, Cabello PH, et al. Genetic polymorphisms of NAT2, CYP2E1 and GST enzymes and the occurrence of antituberculosis drug-induced hepatitis in Brazilian TB patients. Mem Inst Oswaldo Cruz. 2011;106(6):716-724.
7. Mitchell JR, Thorgeirsson UP, Black M, et al. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydranize metabolites. Clin Pharmacol Ther. 1975;18(1):70-79.
8. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864. doi:10.1053/j.gastro.2009.06.006.
1. Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575-586, viii. doi:10.1016/j.cld.2013.07.001.
2. National Institutes of Health Web site. LiverTox: Clinical and research information on drug-induced liver injury. https://livertox.nlm.nih.gov/. Updated February 10, 2017. Accessed October 12, 2017.
3. Ansari JA, Sayyed M, Sayeed F. Management of non alcoholic fatty liver diseases and their complications. Int J Pharmacol. 2011;7:579-588. doi:10.3923/ijp.2011.579.588.
4. Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128(1):116-123. doi:10.1378/chest.128.1.116.
5. Hernon CH. Antituberculous medications. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:787-796.
6. Teixeira RL, Morato RG, Cabello PH, et al. Genetic polymorphisms of NAT2, CYP2E1 and GST enzymes and the occurrence of antituberculosis drug-induced hepatitis in Brazilian TB patients. Mem Inst Oswaldo Cruz. 2011;106(6):716-724.
7. Mitchell JR, Thorgeirsson UP, Black M, et al. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydranize metabolites. Clin Pharmacol Ther. 1975;18(1):70-79.
8. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864. doi:10.1053/j.gastro.2009.06.006.
Back to Basics: An Uncommon, Unrelated Presentation of a Common Disease
The early initial ulcerative lesion (chancre) caused by Treponema pallidum infection, has a median incubation period of 21 days (primary syphilis). When untreated, secondary syphilis will develop within weeks to months and is characterized by generalized symptoms such as malaise, fevers, headaches, sore throat, and myalgia. However, the most characteristic finding in secondary syphilis remains a rash that is classically identified as symmetric, macular, or papular, and involving the entire trunk and extremities, including the palms and soles.
When secondary syphilis is left untreated, late syphilis or tertiary syphilis can develop, which is characterized by cardiovascular involvement, including aortitis, gummatous syphilis (granulomatous nodules in a variety of organs but typically the skin and bones), or central nervous system involvement.1-3 The following case describes a patient with nondescript symptoms, including malaise and cough, who had a characteristic rash of secondary syphilis that was diagnosed and treated in our Houston-area community hospital.
Case
In late autumn, a 30-year-old man presented to our community ED for evaluation of a cough productive of green sputum along with mild chest discomfort, malaise, and generalized myalgia, which were intermittent over the course of the past month. The patient denied rhinorrhea, fevers, chills, dyspnea, or any other systemic complaints. He also denied any sick contacts, but noted that his influenza vaccine was not up to date.
The patient denied any remote or recent medical or surgical history. He further denied taking any medications, and noted that his only medical allergy was to penicillin. His family history was noncontributory. Regarding his social history, the patient admitted to smoking one pack of cigarettes per day and to a daily alcohol intake of approximately one 6-pack of beer. He also admitted to frequently smoking crystal methamphetamine, which he stated he had last used 2 days prior to presentation. The patient said his current chest pain was similar to prior episodes, noting that when the pain occurred, he would temporarily stop smoking crystal methamphetamine.
Plain chest radiography, electrocardiogram, complete metabolic panel, complete blood count, B-natriuretic peptide, and troponin levels were all unremarkable. Due to the presence and nature of the patient’s rash, a rapid plasma reagin (RPR) screen was also taken, the results of which were reactive.
On further questioning, the patient admitted to having multiple female sexual partners with whom he used barrier protection sporadically. A more detailed physical examination revealed multiple painless ulcerations/chancres over the penile shaft and scrotum, without urethral drainage or inguinal lymphadenopathy. The patient denied dysuria or hematuria.
Since the patient was allergic to penicillin, he was given a single oral dose of azithromycin 2 g, and started on a 2-week course of oral doxycycline 100 mg. Further laboratory studies included gonorrhea and chlamydia cultures, both of which were negative. He was instructed to follow-up with his primary care physician for extended sexually transmitted infection (STI) panel-testing, including HIV, hepatitis, and confirmatory syphilis testing. Unfortunately, it is not known whether the patient complied with discharge instructions as he was lost to follow-up.
Discussion
Diagnostic algorithms for syphilis, one of the best studied STIs, have changed with technological advancement, but diagnosis and treatment for the most part has remained mostly the same. The uniqueness of this case is really focused around the patient’s chief complaint. While it is classic to present with malaise, headache, and rash, our patient complained of cough productive of sputum with chest pain—a rare presentation of secondary syphilis. The fortuitous key finding of the truncal rash directed the emergency physician toward the appropriate diagnosis.
Diagnosis
In the ED, where patients such as the one in our case are often lost to follow-up, and consistent infectious disease and primary care follow-up is unavailable, prompt treatment based on history and physical examination alone is recommended. Patients should be tested for syphilis, as well as other STIs including chlamydia, gonorrhea, hepatitis, and HIV as an outpatient. In addition, any partners with whom the patient has had sexual contact within the last 90 days should also undergo STI testing; sexual partners from over 90 days should be notified of the patient’s status and evaluated with testing as indicated.4 All positive test results should be reported to the Centers for Disease Control and Prevention (CDC).5
Nontreponemal and Treponemal Testing
For patients with clinical signs and symptoms of syphilis, recommended laboratory evaluation includes both nontreponemal and treponemal testing. Nontreponemal tests include RPR, venereal disease research laboratory test, and toluidine red unheated serum test. Treponemal tests include fluorescent treponemal antibody absorption, microhemagglutination test for antibodies to T pallidum, T pallidum particle agglutination assay, T pallidum enzyme immunoassay, and chemiluminescence immunoassay. Patients who test positive for treponemal antibody will typically remain reactive for life.5,6
In the setting of discordant test results, patients with a nonreactive treponemal result are generally considered to be negative for syphilis. Discordant results with a negative nontreponemal test are more complicated, and recommendations are based on symptomatology and repeat testing.5
Treatment
When a patient has a positive nontreponemal and treponemal test, treatment is usually indicated. As with the patient in this case, treatment is always indicated for patients who have no prior history of syphilis. For patients who have a history of treated syphilis, attention must be given to titer levels on previous testing and to patient symptomatology.
The treatment for early (primary and secondary) syphilis in patients with no penicillin allergy is a single dose of penicillin G benzathine intramuscularly, at a dose of 2.4 million U. Alternative regimens include doxycycline 100 mg orally twice daily for 14 days, and azithromycin 2 g orally as a single dose; however, there is an association of treatment failure with azithromycin due to macrolide resistance.5 The patient in this case received empiric treatment targeting syphilis, gonorrhea, and chlamydia.
Conclusion
Ten years ago, the rates of primary and secondary syphilis were low, leading the infectious disease community to believe that preventive efforts had been effective. According to the CDC, however, “[current] rates…are the highest they have been in more than 20 years.”5Figure 2 demonstrates the geographic distribution of syphilis cases in the United States in 2016.7
Heightened concern has prompted the CDC to promote the theme “Syphilis Strikes Back” in April 2017, which was STI Awareness Month.8 Identification of disease is critical in the ED, especially when a previously common disease has become uncommon, like the resurgence of syphilis we are now seeing.
1. Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: An epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
2. Rockwell DH, Yobs AR, Moore MB Jr. The Tuskegee study of untreated syphilis; the 30th year of observation. Arch Intern Med. 1964;114:792-798.
3. Sparling PF, Swartz MN, Musher DM, Healy BP. Clinical manifestations of syphilis. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. New York, NY: McGraw-Hill; 1999:661-684.
4. Birnbaumer DM. Sexually transmitted diseases. In: Marx JA, Hockberger RS, Walls RM, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Vol 2. 8th ed. Philadelphia, PA: Saunders; 2014:1312-1325.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
6. Larsen SA. Syphilis. Clin Lab Med. 1989;9(3):545-557.
7. Centers for Disease Control Prevention. Primary and secondary syphilis—rates of reported cases by county, United States, 2016. https://www.cdc.gov/std/stats16/figures/33.htm. Updated September 26, 2017. Accessed October 31 2017.]
8. Centers for Disease Control and Prevention. STD Awareness Month. Syphilis Strikes Back. https://www.cdc.gov/std/sam/index.htm?s_cid=tw_SAM_17001. Updated April 6, 2017. Accessed October 31, 2017.
The early initial ulcerative lesion (chancre) caused by Treponema pallidum infection, has a median incubation period of 21 days (primary syphilis). When untreated, secondary syphilis will develop within weeks to months and is characterized by generalized symptoms such as malaise, fevers, headaches, sore throat, and myalgia. However, the most characteristic finding in secondary syphilis remains a rash that is classically identified as symmetric, macular, or papular, and involving the entire trunk and extremities, including the palms and soles.
When secondary syphilis is left untreated, late syphilis or tertiary syphilis can develop, which is characterized by cardiovascular involvement, including aortitis, gummatous syphilis (granulomatous nodules in a variety of organs but typically the skin and bones), or central nervous system involvement.1-3 The following case describes a patient with nondescript symptoms, including malaise and cough, who had a characteristic rash of secondary syphilis that was diagnosed and treated in our Houston-area community hospital.
Case
In late autumn, a 30-year-old man presented to our community ED for evaluation of a cough productive of green sputum along with mild chest discomfort, malaise, and generalized myalgia, which were intermittent over the course of the past month. The patient denied rhinorrhea, fevers, chills, dyspnea, or any other systemic complaints. He also denied any sick contacts, but noted that his influenza vaccine was not up to date.
The patient denied any remote or recent medical or surgical history. He further denied taking any medications, and noted that his only medical allergy was to penicillin. His family history was noncontributory. Regarding his social history, the patient admitted to smoking one pack of cigarettes per day and to a daily alcohol intake of approximately one 6-pack of beer. He also admitted to frequently smoking crystal methamphetamine, which he stated he had last used 2 days prior to presentation. The patient said his current chest pain was similar to prior episodes, noting that when the pain occurred, he would temporarily stop smoking crystal methamphetamine.
Plain chest radiography, electrocardiogram, complete metabolic panel, complete blood count, B-natriuretic peptide, and troponin levels were all unremarkable. Due to the presence and nature of the patient’s rash, a rapid plasma reagin (RPR) screen was also taken, the results of which were reactive.
On further questioning, the patient admitted to having multiple female sexual partners with whom he used barrier protection sporadically. A more detailed physical examination revealed multiple painless ulcerations/chancres over the penile shaft and scrotum, without urethral drainage or inguinal lymphadenopathy. The patient denied dysuria or hematuria.
Since the patient was allergic to penicillin, he was given a single oral dose of azithromycin 2 g, and started on a 2-week course of oral doxycycline 100 mg. Further laboratory studies included gonorrhea and chlamydia cultures, both of which were negative. He was instructed to follow-up with his primary care physician for extended sexually transmitted infection (STI) panel-testing, including HIV, hepatitis, and confirmatory syphilis testing. Unfortunately, it is not known whether the patient complied with discharge instructions as he was lost to follow-up.
Discussion
Diagnostic algorithms for syphilis, one of the best studied STIs, have changed with technological advancement, but diagnosis and treatment for the most part has remained mostly the same. The uniqueness of this case is really focused around the patient’s chief complaint. While it is classic to present with malaise, headache, and rash, our patient complained of cough productive of sputum with chest pain—a rare presentation of secondary syphilis. The fortuitous key finding of the truncal rash directed the emergency physician toward the appropriate diagnosis.
Diagnosis
In the ED, where patients such as the one in our case are often lost to follow-up, and consistent infectious disease and primary care follow-up is unavailable, prompt treatment based on history and physical examination alone is recommended. Patients should be tested for syphilis, as well as other STIs including chlamydia, gonorrhea, hepatitis, and HIV as an outpatient. In addition, any partners with whom the patient has had sexual contact within the last 90 days should also undergo STI testing; sexual partners from over 90 days should be notified of the patient’s status and evaluated with testing as indicated.4 All positive test results should be reported to the Centers for Disease Control and Prevention (CDC).5
Nontreponemal and Treponemal Testing
For patients with clinical signs and symptoms of syphilis, recommended laboratory evaluation includes both nontreponemal and treponemal testing. Nontreponemal tests include RPR, venereal disease research laboratory test, and toluidine red unheated serum test. Treponemal tests include fluorescent treponemal antibody absorption, microhemagglutination test for antibodies to T pallidum, T pallidum particle agglutination assay, T pallidum enzyme immunoassay, and chemiluminescence immunoassay. Patients who test positive for treponemal antibody will typically remain reactive for life.5,6
In the setting of discordant test results, patients with a nonreactive treponemal result are generally considered to be negative for syphilis. Discordant results with a negative nontreponemal test are more complicated, and recommendations are based on symptomatology and repeat testing.5
Treatment
When a patient has a positive nontreponemal and treponemal test, treatment is usually indicated. As with the patient in this case, treatment is always indicated for patients who have no prior history of syphilis. For patients who have a history of treated syphilis, attention must be given to titer levels on previous testing and to patient symptomatology.
The treatment for early (primary and secondary) syphilis in patients with no penicillin allergy is a single dose of penicillin G benzathine intramuscularly, at a dose of 2.4 million U. Alternative regimens include doxycycline 100 mg orally twice daily for 14 days, and azithromycin 2 g orally as a single dose; however, there is an association of treatment failure with azithromycin due to macrolide resistance.5 The patient in this case received empiric treatment targeting syphilis, gonorrhea, and chlamydia.
Conclusion
Ten years ago, the rates of primary and secondary syphilis were low, leading the infectious disease community to believe that preventive efforts had been effective. According to the CDC, however, “[current] rates…are the highest they have been in more than 20 years.”5Figure 2 demonstrates the geographic distribution of syphilis cases in the United States in 2016.7
Heightened concern has prompted the CDC to promote the theme “Syphilis Strikes Back” in April 2017, which was STI Awareness Month.8 Identification of disease is critical in the ED, especially when a previously common disease has become uncommon, like the resurgence of syphilis we are now seeing.
The early initial ulcerative lesion (chancre) caused by Treponema pallidum infection, has a median incubation period of 21 days (primary syphilis). When untreated, secondary syphilis will develop within weeks to months and is characterized by generalized symptoms such as malaise, fevers, headaches, sore throat, and myalgia. However, the most characteristic finding in secondary syphilis remains a rash that is classically identified as symmetric, macular, or papular, and involving the entire trunk and extremities, including the palms and soles.
When secondary syphilis is left untreated, late syphilis or tertiary syphilis can develop, which is characterized by cardiovascular involvement, including aortitis, gummatous syphilis (granulomatous nodules in a variety of organs but typically the skin and bones), or central nervous system involvement.1-3 The following case describes a patient with nondescript symptoms, including malaise and cough, who had a characteristic rash of secondary syphilis that was diagnosed and treated in our Houston-area community hospital.
Case
In late autumn, a 30-year-old man presented to our community ED for evaluation of a cough productive of green sputum along with mild chest discomfort, malaise, and generalized myalgia, which were intermittent over the course of the past month. The patient denied rhinorrhea, fevers, chills, dyspnea, or any other systemic complaints. He also denied any sick contacts, but noted that his influenza vaccine was not up to date.
The patient denied any remote or recent medical or surgical history. He further denied taking any medications, and noted that his only medical allergy was to penicillin. His family history was noncontributory. Regarding his social history, the patient admitted to smoking one pack of cigarettes per day and to a daily alcohol intake of approximately one 6-pack of beer. He also admitted to frequently smoking crystal methamphetamine, which he stated he had last used 2 days prior to presentation. The patient said his current chest pain was similar to prior episodes, noting that when the pain occurred, he would temporarily stop smoking crystal methamphetamine.
Plain chest radiography, electrocardiogram, complete metabolic panel, complete blood count, B-natriuretic peptide, and troponin levels were all unremarkable. Due to the presence and nature of the patient’s rash, a rapid plasma reagin (RPR) screen was also taken, the results of which were reactive.
On further questioning, the patient admitted to having multiple female sexual partners with whom he used barrier protection sporadically. A more detailed physical examination revealed multiple painless ulcerations/chancres over the penile shaft and scrotum, without urethral drainage or inguinal lymphadenopathy. The patient denied dysuria or hematuria.
Since the patient was allergic to penicillin, he was given a single oral dose of azithromycin 2 g, and started on a 2-week course of oral doxycycline 100 mg. Further laboratory studies included gonorrhea and chlamydia cultures, both of which were negative. He was instructed to follow-up with his primary care physician for extended sexually transmitted infection (STI) panel-testing, including HIV, hepatitis, and confirmatory syphilis testing. Unfortunately, it is not known whether the patient complied with discharge instructions as he was lost to follow-up.
Discussion
Diagnostic algorithms for syphilis, one of the best studied STIs, have changed with technological advancement, but diagnosis and treatment for the most part has remained mostly the same. The uniqueness of this case is really focused around the patient’s chief complaint. While it is classic to present with malaise, headache, and rash, our patient complained of cough productive of sputum with chest pain—a rare presentation of secondary syphilis. The fortuitous key finding of the truncal rash directed the emergency physician toward the appropriate diagnosis.
Diagnosis
In the ED, where patients such as the one in our case are often lost to follow-up, and consistent infectious disease and primary care follow-up is unavailable, prompt treatment based on history and physical examination alone is recommended. Patients should be tested for syphilis, as well as other STIs including chlamydia, gonorrhea, hepatitis, and HIV as an outpatient. In addition, any partners with whom the patient has had sexual contact within the last 90 days should also undergo STI testing; sexual partners from over 90 days should be notified of the patient’s status and evaluated with testing as indicated.4 All positive test results should be reported to the Centers for Disease Control and Prevention (CDC).5
Nontreponemal and Treponemal Testing
For patients with clinical signs and symptoms of syphilis, recommended laboratory evaluation includes both nontreponemal and treponemal testing. Nontreponemal tests include RPR, venereal disease research laboratory test, and toluidine red unheated serum test. Treponemal tests include fluorescent treponemal antibody absorption, microhemagglutination test for antibodies to T pallidum, T pallidum particle agglutination assay, T pallidum enzyme immunoassay, and chemiluminescence immunoassay. Patients who test positive for treponemal antibody will typically remain reactive for life.5,6
In the setting of discordant test results, patients with a nonreactive treponemal result are generally considered to be negative for syphilis. Discordant results with a negative nontreponemal test are more complicated, and recommendations are based on symptomatology and repeat testing.5
Treatment
When a patient has a positive nontreponemal and treponemal test, treatment is usually indicated. As with the patient in this case, treatment is always indicated for patients who have no prior history of syphilis. For patients who have a history of treated syphilis, attention must be given to titer levels on previous testing and to patient symptomatology.
The treatment for early (primary and secondary) syphilis in patients with no penicillin allergy is a single dose of penicillin G benzathine intramuscularly, at a dose of 2.4 million U. Alternative regimens include doxycycline 100 mg orally twice daily for 14 days, and azithromycin 2 g orally as a single dose; however, there is an association of treatment failure with azithromycin due to macrolide resistance.5 The patient in this case received empiric treatment targeting syphilis, gonorrhea, and chlamydia.
Conclusion
Ten years ago, the rates of primary and secondary syphilis were low, leading the infectious disease community to believe that preventive efforts had been effective. According to the CDC, however, “[current] rates…are the highest they have been in more than 20 years.”5Figure 2 demonstrates the geographic distribution of syphilis cases in the United States in 2016.7
Heightened concern has prompted the CDC to promote the theme “Syphilis Strikes Back” in April 2017, which was STI Awareness Month.8 Identification of disease is critical in the ED, especially when a previously common disease has become uncommon, like the resurgence of syphilis we are now seeing.
1. Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: An epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
2. Rockwell DH, Yobs AR, Moore MB Jr. The Tuskegee study of untreated syphilis; the 30th year of observation. Arch Intern Med. 1964;114:792-798.
3. Sparling PF, Swartz MN, Musher DM, Healy BP. Clinical manifestations of syphilis. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. New York, NY: McGraw-Hill; 1999:661-684.
4. Birnbaumer DM. Sexually transmitted diseases. In: Marx JA, Hockberger RS, Walls RM, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Vol 2. 8th ed. Philadelphia, PA: Saunders; 2014:1312-1325.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
6. Larsen SA. Syphilis. Clin Lab Med. 1989;9(3):545-557.
7. Centers for Disease Control Prevention. Primary and secondary syphilis—rates of reported cases by county, United States, 2016. https://www.cdc.gov/std/stats16/figures/33.htm. Updated September 26, 2017. Accessed October 31 2017.]
8. Centers for Disease Control and Prevention. STD Awareness Month. Syphilis Strikes Back. https://www.cdc.gov/std/sam/index.htm?s_cid=tw_SAM_17001. Updated April 6, 2017. Accessed October 31, 2017.
1. Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: An epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
2. Rockwell DH, Yobs AR, Moore MB Jr. The Tuskegee study of untreated syphilis; the 30th year of observation. Arch Intern Med. 1964;114:792-798.
3. Sparling PF, Swartz MN, Musher DM, Healy BP. Clinical manifestations of syphilis. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. New York, NY: McGraw-Hill; 1999:661-684.
4. Birnbaumer DM. Sexually transmitted diseases. In: Marx JA, Hockberger RS, Walls RM, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Vol 2. 8th ed. Philadelphia, PA: Saunders; 2014:1312-1325.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
6. Larsen SA. Syphilis. Clin Lab Med. 1989;9(3):545-557.
7. Centers for Disease Control Prevention. Primary and secondary syphilis—rates of reported cases by county, United States, 2016. https://www.cdc.gov/std/stats16/figures/33.htm. Updated September 26, 2017. Accessed October 31 2017.]
8. Centers for Disease Control and Prevention. STD Awareness Month. Syphilis Strikes Back. https://www.cdc.gov/std/sam/index.htm?s_cid=tw_SAM_17001. Updated April 6, 2017. Accessed October 31, 2017.
Duodenal Perforation After Endoscopic Procedure
Tension pneumoperitoneum (TPP), also known as hyperacute abdominal compartment syndrome or abdominal tamponade, is a rare condition most commonly associated with gastrointestinal (GI) perforation during endoscopy and iatrogenic insufflation of gas into the peritoneal cavity.1 Other reported causes of TPP include gastric rupture after cardiopulmonary resuscitation, barotrauma during scuba diving, positive pressure ventilation through pleural-peritoneal channels, and spontaneous TPP of uncertain mechanism.1-4
Case Presentation
A 76-year-old male with a history of ischemic cardiomyopathy, hypertension, and diabetes mellitus presented to the VA Puget Sound Health Care System in Seattle, Washington emergency department with painless jaundice, hematemesis, melena, and acute renal failure. On esophagogastroduodenoscopy (EGD), he was found to have an ulcer on the posterior wall of the duodenal bulb. The ulcer was coagulated and injected with epinephrine. The patient’s subsequent hospital course was complicated by worsening liver function, the need for renal replacement therapy, and recurrence of upper GI bleeding that required a transcatheter embolization of 2 separate superior pancreaticoduodenal arteries (SPDA) and the inferior pancreaticoduodenal artery (IPDA).
Once clinically stable, an endoscopic retrograde cholangiopancreatography (ERCP) was performed to evaluate for cholangiocarcinoma. A stricture was discovered in the common hepatic duct, brushings were taken, and a 15 cm, 7 Fr stent was placed in the common hepatic duct. The procedure was performed with an Olympus TJF Type Q180V duodenovideoscope (Tokyo, Japan) with an external diameter of 13.7 mm. The patient became hypotensive during the procedure and was treated with phenylephrine and ephedrine boluses. There was no endoscopic evidence of bleeding or bowel trauma.
After completion of the procedure, in the recovery area the patient became severely hypotensive and unresponsive. The physical examination was noteworthy for gross abdominal distention. Arterial blood gas analysis revealed severe metabolic and respiratory acidosis. Chest radiography demonstrated massive pneumoperitoneum, low lung volumes, and diaphragmatic compression (Figure).
A diagnosis of tension pneumoperitoneum was made, and as the patient was transported to the operating room he became bradycardic without a pulse, requiring initiation of cardiopulmonary resuscitation. The abdomen was decompressed with a 14-gauge needle, followed by insertion of a laparoscopic trocar as a decompressive maneuver. This procedure resulted in return of spontaneous circulation.
An exploratory laparotomy was performed, and a massive rush of air was noted on opening the peritoneum. A pinhole perforation of the anterior wall of the second portion of the duodenum was found along with large-volume bilious ascites. This perforation was repaired with a Graham patch, and the patient was taken to the intensive care unit. Postoperatively, the patient developed disseminated intravascular coagulation, shock liver, and acute respiratory distress syndrome, expiring 10 days later from sequelae of multiorgan failure.
Discussion
In relation to upper GI endoscopic procedures, TPP has been reported after diagnostic EGD, endoscopic sphincterotomy, and submucosal tumor dissection.5-7 During these interventions, clinically apparent or overt iatrogenic perforations can occur either in the stomach or duodenum. These perforations may function as one-way valves that cause massive air accumulation and marked elevation of the diaphragm, which severely decreases lung volumes, pulmonary compliance, and limits gas exchange. Hemodynamically, compression of the inferior vena cava restricts venous return to the heart, resulting in decreased cardiac output.8
Patients with TPP present in acute distress with dyspnea, abdominal pain, and shock. On physical examination the abdomen is markedly distended, tympanic, and rigid. Rectal prolapse and subcutaneous emphysema also may be present.9 Roentographic features of TPP include findings of intraperitoneal air with elevation of the diaphragm, medial displacement of the liver (saddlebag sign), and juxtaposition of air in visceral interfaces, making intra-abdominal structures (spleen and gallbladder) appear more discrete.10 Abdominal computer tomography may show massive pneumoperitoneum with bowel loop compression and centralization of abdominal organs.4
Treatment strategies include emergent decompression either with percutaneous catheter insertion or abdominal drain placement followed by a definitive surgical repair. As with management of tension pneumothorax, treatment should not be delayed while awaiting confirmatory radiologic studies.9 When percutaneous needle decompression is undertaken, it is preferable to use a large bore (14-gauge venous catheter) and to advance a catheter over a needle to minimize the risk of visceral injury with egress of air and return of abdominal organs to their normal anatomical positions. The needle should be inserted directly above or below the umbilicus or in the left or right lower quadrants to avoid solid organ (ie, liver or spleen) injury.
Etiologic possibilities for the duodenal perforation in this case include mechanical trauma from the endoscope and duodenal tissue infarction after embolization of a bleeding duodenal ulcer. The duodenum and pancreatic head have a dual blood supply from the SPDA, a branch of the gastroduodenal artery, and the IPDA, a branch of the superior mesenteric artery.11 After failed endoscopic management of persistent duodenal hemorrhage, the patient underwent synchronous embolization of 2 separate SPDAs and the IPDA. This might have put the first 2 segments of the duodenum at risk for ischemic damage and caused it to perforate at some point during the patient’s hospitalization (as evidenced by the bilious ascitis) or rendered them vulnerable to perforation from intraluminal insufflation during endoscopy.12
During the laparotomy, a pinhole-sized perforation was noted in the anterior wall of the second part of the duodenum, distinct form the duodenal ulcer present on the posterior wall. This perforation likely provided a pathway for the intraluminal gas to escape into the peritoneal cavity, culminating in abdominal tamponade, cardiopulmonary deterioration, and arrest. Needle decompression of the abdominal cavity provided an effective, though temporizing relief of this pressure, enabling return of spontaneous circulation.
This case highlights the need for a high index of suspicion for TPP in a patient with cardiopulmonary compromise and abdominal distension after upper GI endoscopic procedures even in the absence of identifiable perforations. Close coordination among gastroenterologists, anesthesiologists, and surgeons is key in prevention, recognition, and management of this rare but catastrophic complication.
1. Bunni J, Bryson PJ, Higgs SM. Abdominal compartment syndrome caused by tension pneumoperitoneum in a scuba diver. Ann R Coll Surg Engl. 2012;94(8):e237-e239.
2. Cameron PA, Rosengarten PL, Johnson WR, Dziukas L. Tension pneumoperitoneum after cardiopulmonary resuscitation. Med J Aust. 1991;155(1):44-47.
3. Burdett-Smith P, Jaffey L. Tension pneumoperitoneum. J Accid Emerg Med. 1996;13(3):220-221.
4. Joshi D, Ganai B. Radiological features of tension pneumoperitoneum. BMJ Case Rep. 2015;2015.
5. Rai A, Iftikhar S. Tension pneumothorax complicating diagnostic upper endoscopy: a case report. Am J Gastroenterol. 1999;94(3):845-847.
6. Iyilikci L, Akarsu M, Duran E, et al. Duodenal perforation and bilateral tension pneumothorax following endoscopic sphincterotomy. J Anesth. 2009;23(1):164-165.
7. Siboni S, Bona D, Abate E, Bonavina L. Tension pneumoperitoneum following endoscopic submucosal dissection of leiomyoma of the cardia. Endoscopy. 2010;42(suppl 2):E152.
8. Deenichin GP. Abdominal compartment syndrome. Surg Today. 2008;38(1):5-19.
9. Chiapponi C, Stocker U, Korner M, et al. Emergency percutaneous needle decompression for tension pneumoperitoneum. BMC Gastroenterol. 2011;11:48.
10. Lin BW, Thanassi W. Tension pneumoperitoneum. J Emerg Med. 2010;38(1):57-59.
11. Bell SD, Lau KY, Sniderman KW. Synchronous embolization of the gastroduodenal artery and the inferior pancreaticoduodenal artery in patients with massive duodenal hemorrhage. J Vasc Interv Radiol. 1995;6(4):531-536.
12. Wang YL, Cheng YS, Liu LZ, He ZH, Ding KH. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18(34):4765-4770.
Tension pneumoperitoneum (TPP), also known as hyperacute abdominal compartment syndrome or abdominal tamponade, is a rare condition most commonly associated with gastrointestinal (GI) perforation during endoscopy and iatrogenic insufflation of gas into the peritoneal cavity.1 Other reported causes of TPP include gastric rupture after cardiopulmonary resuscitation, barotrauma during scuba diving, positive pressure ventilation through pleural-peritoneal channels, and spontaneous TPP of uncertain mechanism.1-4
Case Presentation
A 76-year-old male with a history of ischemic cardiomyopathy, hypertension, and diabetes mellitus presented to the VA Puget Sound Health Care System in Seattle, Washington emergency department with painless jaundice, hematemesis, melena, and acute renal failure. On esophagogastroduodenoscopy (EGD), he was found to have an ulcer on the posterior wall of the duodenal bulb. The ulcer was coagulated and injected with epinephrine. The patient’s subsequent hospital course was complicated by worsening liver function, the need for renal replacement therapy, and recurrence of upper GI bleeding that required a transcatheter embolization of 2 separate superior pancreaticoduodenal arteries (SPDA) and the inferior pancreaticoduodenal artery (IPDA).
Once clinically stable, an endoscopic retrograde cholangiopancreatography (ERCP) was performed to evaluate for cholangiocarcinoma. A stricture was discovered in the common hepatic duct, brushings were taken, and a 15 cm, 7 Fr stent was placed in the common hepatic duct. The procedure was performed with an Olympus TJF Type Q180V duodenovideoscope (Tokyo, Japan) with an external diameter of 13.7 mm. The patient became hypotensive during the procedure and was treated with phenylephrine and ephedrine boluses. There was no endoscopic evidence of bleeding or bowel trauma.
After completion of the procedure, in the recovery area the patient became severely hypotensive and unresponsive. The physical examination was noteworthy for gross abdominal distention. Arterial blood gas analysis revealed severe metabolic and respiratory acidosis. Chest radiography demonstrated massive pneumoperitoneum, low lung volumes, and diaphragmatic compression (Figure).
A diagnosis of tension pneumoperitoneum was made, and as the patient was transported to the operating room he became bradycardic without a pulse, requiring initiation of cardiopulmonary resuscitation. The abdomen was decompressed with a 14-gauge needle, followed by insertion of a laparoscopic trocar as a decompressive maneuver. This procedure resulted in return of spontaneous circulation.
An exploratory laparotomy was performed, and a massive rush of air was noted on opening the peritoneum. A pinhole perforation of the anterior wall of the second portion of the duodenum was found along with large-volume bilious ascites. This perforation was repaired with a Graham patch, and the patient was taken to the intensive care unit. Postoperatively, the patient developed disseminated intravascular coagulation, shock liver, and acute respiratory distress syndrome, expiring 10 days later from sequelae of multiorgan failure.
Discussion
In relation to upper GI endoscopic procedures, TPP has been reported after diagnostic EGD, endoscopic sphincterotomy, and submucosal tumor dissection.5-7 During these interventions, clinically apparent or overt iatrogenic perforations can occur either in the stomach or duodenum. These perforations may function as one-way valves that cause massive air accumulation and marked elevation of the diaphragm, which severely decreases lung volumes, pulmonary compliance, and limits gas exchange. Hemodynamically, compression of the inferior vena cava restricts venous return to the heart, resulting in decreased cardiac output.8
Patients with TPP present in acute distress with dyspnea, abdominal pain, and shock. On physical examination the abdomen is markedly distended, tympanic, and rigid. Rectal prolapse and subcutaneous emphysema also may be present.9 Roentographic features of TPP include findings of intraperitoneal air with elevation of the diaphragm, medial displacement of the liver (saddlebag sign), and juxtaposition of air in visceral interfaces, making intra-abdominal structures (spleen and gallbladder) appear more discrete.10 Abdominal computer tomography may show massive pneumoperitoneum with bowel loop compression and centralization of abdominal organs.4
Treatment strategies include emergent decompression either with percutaneous catheter insertion or abdominal drain placement followed by a definitive surgical repair. As with management of tension pneumothorax, treatment should not be delayed while awaiting confirmatory radiologic studies.9 When percutaneous needle decompression is undertaken, it is preferable to use a large bore (14-gauge venous catheter) and to advance a catheter over a needle to minimize the risk of visceral injury with egress of air and return of abdominal organs to their normal anatomical positions. The needle should be inserted directly above or below the umbilicus or in the left or right lower quadrants to avoid solid organ (ie, liver or spleen) injury.
Etiologic possibilities for the duodenal perforation in this case include mechanical trauma from the endoscope and duodenal tissue infarction after embolization of a bleeding duodenal ulcer. The duodenum and pancreatic head have a dual blood supply from the SPDA, a branch of the gastroduodenal artery, and the IPDA, a branch of the superior mesenteric artery.11 After failed endoscopic management of persistent duodenal hemorrhage, the patient underwent synchronous embolization of 2 separate SPDAs and the IPDA. This might have put the first 2 segments of the duodenum at risk for ischemic damage and caused it to perforate at some point during the patient’s hospitalization (as evidenced by the bilious ascitis) or rendered them vulnerable to perforation from intraluminal insufflation during endoscopy.12
During the laparotomy, a pinhole-sized perforation was noted in the anterior wall of the second part of the duodenum, distinct form the duodenal ulcer present on the posterior wall. This perforation likely provided a pathway for the intraluminal gas to escape into the peritoneal cavity, culminating in abdominal tamponade, cardiopulmonary deterioration, and arrest. Needle decompression of the abdominal cavity provided an effective, though temporizing relief of this pressure, enabling return of spontaneous circulation.
This case highlights the need for a high index of suspicion for TPP in a patient with cardiopulmonary compromise and abdominal distension after upper GI endoscopic procedures even in the absence of identifiable perforations. Close coordination among gastroenterologists, anesthesiologists, and surgeons is key in prevention, recognition, and management of this rare but catastrophic complication.
Tension pneumoperitoneum (TPP), also known as hyperacute abdominal compartment syndrome or abdominal tamponade, is a rare condition most commonly associated with gastrointestinal (GI) perforation during endoscopy and iatrogenic insufflation of gas into the peritoneal cavity.1 Other reported causes of TPP include gastric rupture after cardiopulmonary resuscitation, barotrauma during scuba diving, positive pressure ventilation through pleural-peritoneal channels, and spontaneous TPP of uncertain mechanism.1-4
Case Presentation
A 76-year-old male with a history of ischemic cardiomyopathy, hypertension, and diabetes mellitus presented to the VA Puget Sound Health Care System in Seattle, Washington emergency department with painless jaundice, hematemesis, melena, and acute renal failure. On esophagogastroduodenoscopy (EGD), he was found to have an ulcer on the posterior wall of the duodenal bulb. The ulcer was coagulated and injected with epinephrine. The patient’s subsequent hospital course was complicated by worsening liver function, the need for renal replacement therapy, and recurrence of upper GI bleeding that required a transcatheter embolization of 2 separate superior pancreaticoduodenal arteries (SPDA) and the inferior pancreaticoduodenal artery (IPDA).
Once clinically stable, an endoscopic retrograde cholangiopancreatography (ERCP) was performed to evaluate for cholangiocarcinoma. A stricture was discovered in the common hepatic duct, brushings were taken, and a 15 cm, 7 Fr stent was placed in the common hepatic duct. The procedure was performed with an Olympus TJF Type Q180V duodenovideoscope (Tokyo, Japan) with an external diameter of 13.7 mm. The patient became hypotensive during the procedure and was treated with phenylephrine and ephedrine boluses. There was no endoscopic evidence of bleeding or bowel trauma.
After completion of the procedure, in the recovery area the patient became severely hypotensive and unresponsive. The physical examination was noteworthy for gross abdominal distention. Arterial blood gas analysis revealed severe metabolic and respiratory acidosis. Chest radiography demonstrated massive pneumoperitoneum, low lung volumes, and diaphragmatic compression (Figure).
A diagnosis of tension pneumoperitoneum was made, and as the patient was transported to the operating room he became bradycardic without a pulse, requiring initiation of cardiopulmonary resuscitation. The abdomen was decompressed with a 14-gauge needle, followed by insertion of a laparoscopic trocar as a decompressive maneuver. This procedure resulted in return of spontaneous circulation.
An exploratory laparotomy was performed, and a massive rush of air was noted on opening the peritoneum. A pinhole perforation of the anterior wall of the second portion of the duodenum was found along with large-volume bilious ascites. This perforation was repaired with a Graham patch, and the patient was taken to the intensive care unit. Postoperatively, the patient developed disseminated intravascular coagulation, shock liver, and acute respiratory distress syndrome, expiring 10 days later from sequelae of multiorgan failure.
Discussion
In relation to upper GI endoscopic procedures, TPP has been reported after diagnostic EGD, endoscopic sphincterotomy, and submucosal tumor dissection.5-7 During these interventions, clinically apparent or overt iatrogenic perforations can occur either in the stomach or duodenum. These perforations may function as one-way valves that cause massive air accumulation and marked elevation of the diaphragm, which severely decreases lung volumes, pulmonary compliance, and limits gas exchange. Hemodynamically, compression of the inferior vena cava restricts venous return to the heart, resulting in decreased cardiac output.8
Patients with TPP present in acute distress with dyspnea, abdominal pain, and shock. On physical examination the abdomen is markedly distended, tympanic, and rigid. Rectal prolapse and subcutaneous emphysema also may be present.9 Roentographic features of TPP include findings of intraperitoneal air with elevation of the diaphragm, medial displacement of the liver (saddlebag sign), and juxtaposition of air in visceral interfaces, making intra-abdominal structures (spleen and gallbladder) appear more discrete.10 Abdominal computer tomography may show massive pneumoperitoneum with bowel loop compression and centralization of abdominal organs.4
Treatment strategies include emergent decompression either with percutaneous catheter insertion or abdominal drain placement followed by a definitive surgical repair. As with management of tension pneumothorax, treatment should not be delayed while awaiting confirmatory radiologic studies.9 When percutaneous needle decompression is undertaken, it is preferable to use a large bore (14-gauge venous catheter) and to advance a catheter over a needle to minimize the risk of visceral injury with egress of air and return of abdominal organs to their normal anatomical positions. The needle should be inserted directly above or below the umbilicus or in the left or right lower quadrants to avoid solid organ (ie, liver or spleen) injury.
Etiologic possibilities for the duodenal perforation in this case include mechanical trauma from the endoscope and duodenal tissue infarction after embolization of a bleeding duodenal ulcer. The duodenum and pancreatic head have a dual blood supply from the SPDA, a branch of the gastroduodenal artery, and the IPDA, a branch of the superior mesenteric artery.11 After failed endoscopic management of persistent duodenal hemorrhage, the patient underwent synchronous embolization of 2 separate SPDAs and the IPDA. This might have put the first 2 segments of the duodenum at risk for ischemic damage and caused it to perforate at some point during the patient’s hospitalization (as evidenced by the bilious ascitis) or rendered them vulnerable to perforation from intraluminal insufflation during endoscopy.12
During the laparotomy, a pinhole-sized perforation was noted in the anterior wall of the second part of the duodenum, distinct form the duodenal ulcer present on the posterior wall. This perforation likely provided a pathway for the intraluminal gas to escape into the peritoneal cavity, culminating in abdominal tamponade, cardiopulmonary deterioration, and arrest. Needle decompression of the abdominal cavity provided an effective, though temporizing relief of this pressure, enabling return of spontaneous circulation.
This case highlights the need for a high index of suspicion for TPP in a patient with cardiopulmonary compromise and abdominal distension after upper GI endoscopic procedures even in the absence of identifiable perforations. Close coordination among gastroenterologists, anesthesiologists, and surgeons is key in prevention, recognition, and management of this rare but catastrophic complication.
1. Bunni J, Bryson PJ, Higgs SM. Abdominal compartment syndrome caused by tension pneumoperitoneum in a scuba diver. Ann R Coll Surg Engl. 2012;94(8):e237-e239.
2. Cameron PA, Rosengarten PL, Johnson WR, Dziukas L. Tension pneumoperitoneum after cardiopulmonary resuscitation. Med J Aust. 1991;155(1):44-47.
3. Burdett-Smith P, Jaffey L. Tension pneumoperitoneum. J Accid Emerg Med. 1996;13(3):220-221.
4. Joshi D, Ganai B. Radiological features of tension pneumoperitoneum. BMJ Case Rep. 2015;2015.
5. Rai A, Iftikhar S. Tension pneumothorax complicating diagnostic upper endoscopy: a case report. Am J Gastroenterol. 1999;94(3):845-847.
6. Iyilikci L, Akarsu M, Duran E, et al. Duodenal perforation and bilateral tension pneumothorax following endoscopic sphincterotomy. J Anesth. 2009;23(1):164-165.
7. Siboni S, Bona D, Abate E, Bonavina L. Tension pneumoperitoneum following endoscopic submucosal dissection of leiomyoma of the cardia. Endoscopy. 2010;42(suppl 2):E152.
8. Deenichin GP. Abdominal compartment syndrome. Surg Today. 2008;38(1):5-19.
9. Chiapponi C, Stocker U, Korner M, et al. Emergency percutaneous needle decompression for tension pneumoperitoneum. BMC Gastroenterol. 2011;11:48.
10. Lin BW, Thanassi W. Tension pneumoperitoneum. J Emerg Med. 2010;38(1):57-59.
11. Bell SD, Lau KY, Sniderman KW. Synchronous embolization of the gastroduodenal artery and the inferior pancreaticoduodenal artery in patients with massive duodenal hemorrhage. J Vasc Interv Radiol. 1995;6(4):531-536.
12. Wang YL, Cheng YS, Liu LZ, He ZH, Ding KH. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18(34):4765-4770.
1. Bunni J, Bryson PJ, Higgs SM. Abdominal compartment syndrome caused by tension pneumoperitoneum in a scuba diver. Ann R Coll Surg Engl. 2012;94(8):e237-e239.
2. Cameron PA, Rosengarten PL, Johnson WR, Dziukas L. Tension pneumoperitoneum after cardiopulmonary resuscitation. Med J Aust. 1991;155(1):44-47.
3. Burdett-Smith P, Jaffey L. Tension pneumoperitoneum. J Accid Emerg Med. 1996;13(3):220-221.
4. Joshi D, Ganai B. Radiological features of tension pneumoperitoneum. BMJ Case Rep. 2015;2015.
5. Rai A, Iftikhar S. Tension pneumothorax complicating diagnostic upper endoscopy: a case report. Am J Gastroenterol. 1999;94(3):845-847.
6. Iyilikci L, Akarsu M, Duran E, et al. Duodenal perforation and bilateral tension pneumothorax following endoscopic sphincterotomy. J Anesth. 2009;23(1):164-165.
7. Siboni S, Bona D, Abate E, Bonavina L. Tension pneumoperitoneum following endoscopic submucosal dissection of leiomyoma of the cardia. Endoscopy. 2010;42(suppl 2):E152.
8. Deenichin GP. Abdominal compartment syndrome. Surg Today. 2008;38(1):5-19.
9. Chiapponi C, Stocker U, Korner M, et al. Emergency percutaneous needle decompression for tension pneumoperitoneum. BMC Gastroenterol. 2011;11:48.
10. Lin BW, Thanassi W. Tension pneumoperitoneum. J Emerg Med. 2010;38(1):57-59.
11. Bell SD, Lau KY, Sniderman KW. Synchronous embolization of the gastroduodenal artery and the inferior pancreaticoduodenal artery in patients with massive duodenal hemorrhage. J Vasc Interv Radiol. 1995;6(4):531-536.
12. Wang YL, Cheng YS, Liu LZ, He ZH, Ding KH. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18(34):4765-4770.
A Case of Leprosy in Central Florida
Case Report
A 65-year-old man presented with multiple anesthetic, annular, erythematous, scaly plaques with a raised border of 6 weeks’ duration that were unresponsive to topical steroid therapy. Four plaques were noted on the lower back ranging from 2 to 4 cm in diameter as well as a fifth plaque on the anterior portion of the right ankle that was approximately 6×6 cm. He denied fever, malaise, muscle weakness, changes in vision, or sensory deficits outside of the lesions themselves. The patient also denied any recent travel to endemic areas or exposure to armadillos.
Biopsies were taken from lesions on the lumbar back and anterior aspect of the right ankle (Figure 1A). Hematoxylin and eosin staining revealed a granulomatous infiltrate spreading along neurovascular structures (Figure 2). Granulomas also were identified in the dermal interstitium exhibiting partial necrosis (Figure 2 inset). Conspicuous distension of lymphovascular and perineural areas also was noted. Immunohistochemical studies with S-100 and neurofilament stains allowed insight into the pathomechanism of the clinically observed anesthesia, as nerve fibers were identified showing different stages of damage elicited by the granulomatous inflammatory infiltrate (Figure 3). Fite staining was positive for occasional bacilli within histiocytes (Figure 3A inset). Despite the clinical, histologic, and immunohistochemical evidence, the patient had no known exposure to leprosy; consequently, a polymerase chain reaction (PCR) assay was ordered for confirmation of the diagnosis. Surprisingly, the PCR was positive for Mycobacterium leprae DNA. These findings were consistent with borderline tuberculoid leprosy.
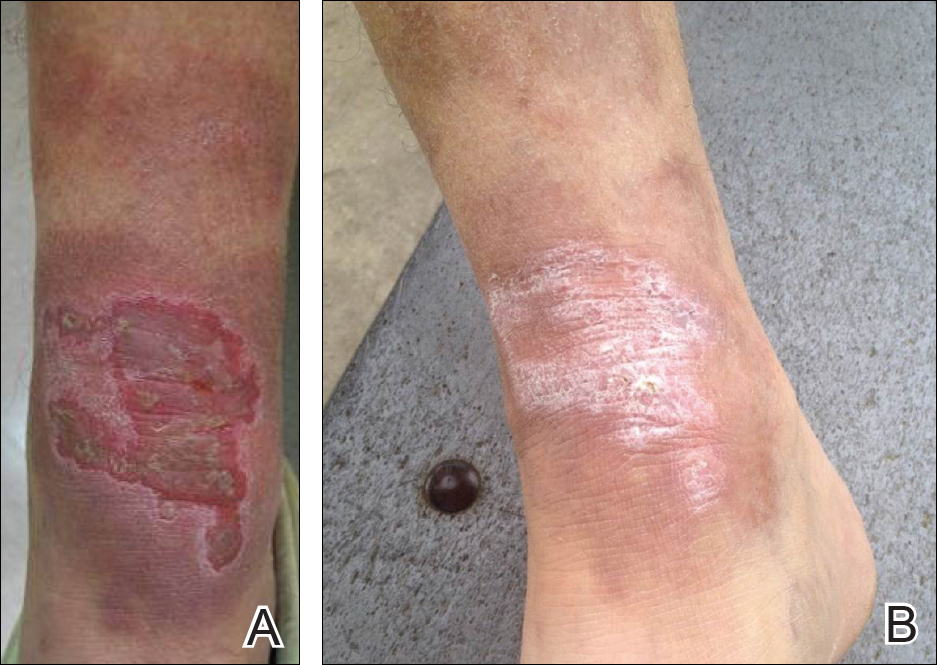
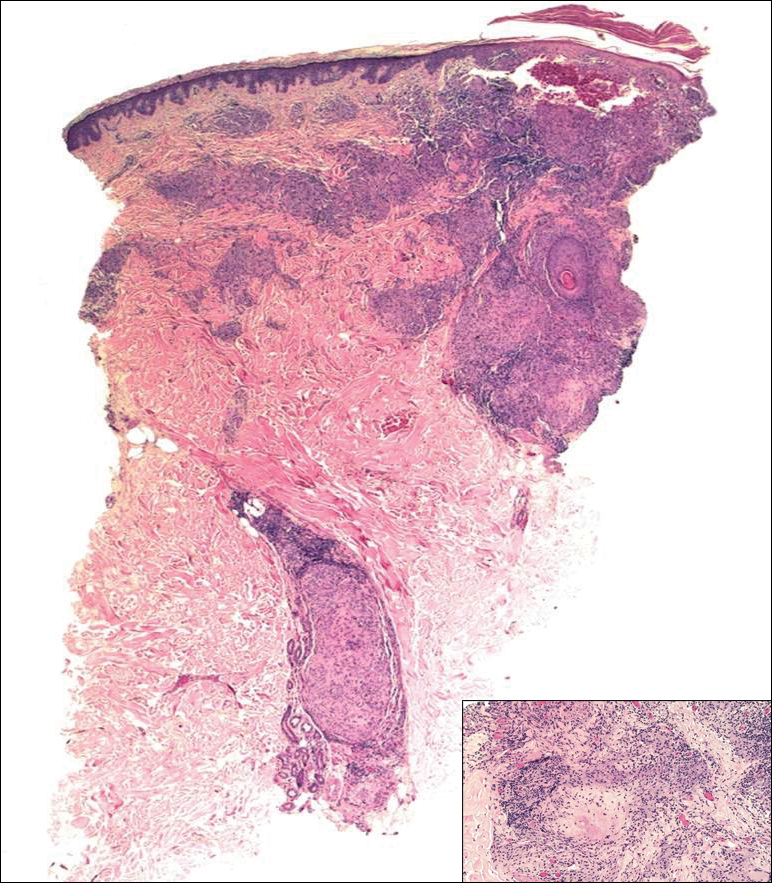
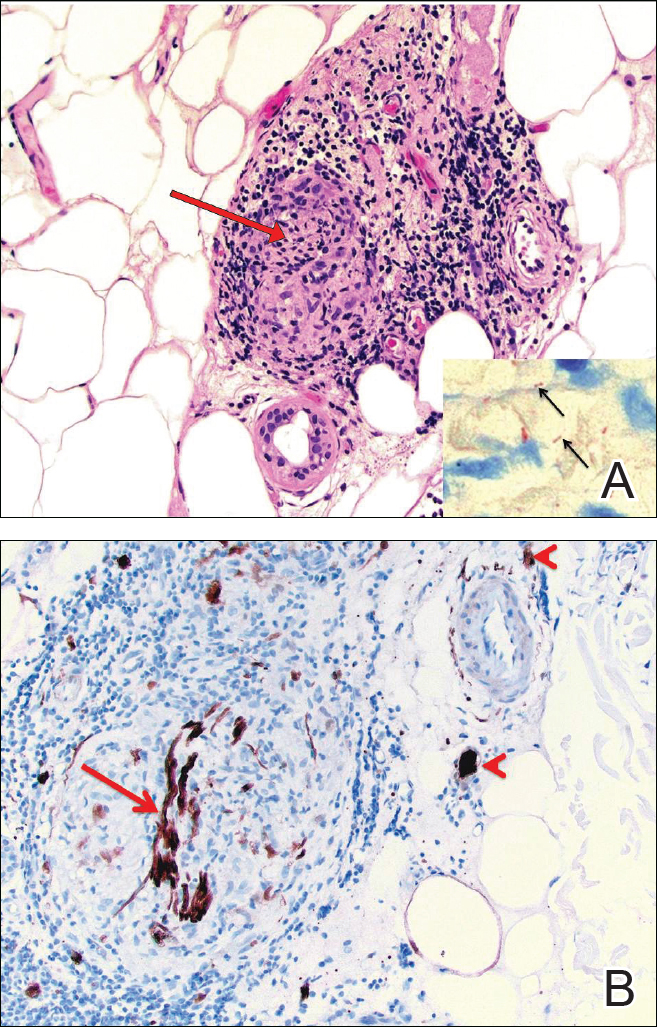
The case was reported to the National Hansen’s Disease Program (Baton Rouge, Louisiana). The patient was started on rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The lesions exhibited marked improvement after completion of therapy (Figure 1B).
Comment
Disease Transmission
Hansen disease, also known as leprosy, is a chronic granulomatous infectious disease that is caused by M leprae, an obligate intracellular bacillus aerobe.1 The mechanism of spread of M leprae is not clear. It is thought to be transmitted via respiratory droplets, though it may occur through injured skin.2 Studies have suggested that in addition to humans, nine-banded armadillos are a source of infection.2,3 Exposure to infected individuals, particularly multibacillary patients, increases the likelihood of contracting leprosy.2
According to the Centers for Disease Control and Prevention, 81 cases of Hansen disease were diagnosed in the United States in 2013,4 compared to 178 cases registered in 2015.5 Cases from Hawaii, Texas, California, Louisiana, New York, and Florida made up 72% (129/178) of the reported cases. There was an increase from 34 cases to 49 cases in Florida from 2014 to 2015.5 The spread of leprosy throughout Florida may be from the merge of 2 armadillo populations, an M leprae–infected population migrating east from Texas and one from south central Florida that historically had not been infected with M leprae until recently.3,6 Our patient did not have any known exposures to armadillos.
Classification and Presentation
The clinical presentation of Hansen disease is widely variable, as it can present at any point along a spectrum ranging from tuberculoid leprosy to lepromatous leprosy with borderline conditions in between, according to the Ridley-Jopling critera.7 The World Health Organization also classifies leprosy based on the number of acid-fast bacilli seen in a skin smear as either paucibacillary or multibacillary.2 The paucibacillary classification correlates with tuberculoid, borderline tuberculoid, and indeterminate leprosy, and multibacillary correlates with borderline lepromatous and lepromatous leprosy. Paucibacillary leprosy usually presents with a less dramatic clinical picture than multibacillary leprosy. The clinical presentation is dependent on the magnitude of immune response to M leprae.2
Paucibacillary infection occurs when the body generates a strong cell-mediated immune response against the bacteria,8 which causes the activation and proliferation of CD4 and CD8 T cells, limiting the spread of the mycobacterium. Subsequently, the patient typically presents with a mild clinical picture with few skin lesions and limited nerve involvement.8 The skin lesions are papules or plaques with raised borders that are usually hypopigmented on dark skin and erythematous on light skin. Nerve involvement in paucibacillary forms of leprosy include sensory impairment and anhidrosis within the lesions. Nerve enlargement usually affects superficial nerves, with the posterior tibial nerve being most commonly affected.
Multibacillary leprosy presents with systemic involvement due to a weak cell-mediated immune response. Patients generally present with diffuse, poorly defined nodules; greater nerve impairment; and other systemic symptoms such as blindness, swelling of the fingers and toes, and testicular atrophy (in men). Additionally, enlargement of the earlobes and widening of the nasal bridge may contribute to the appearance of leonine facies. Nerve impairment in multibacillary forms of leprosy may be more severe, including more diffuse sensory involvement (eg, stocking glove–pattern neuropathy, nerve-trunk palsies), which ultimately may lead to foot drop, claw toe, and lagophthalmos.8
In addition to the clinical presentation, the histology of the paucibacillary and multibacillary types differ. Multibacillary leprosy shows diffuse histiocytes without granulomas and multiple bacilli seen on Fite staining.8 In the paucibacillary form, there are well-formed granulomas with Langerhans giant cells and a perineural lymphocytic infiltrate seen on hematoxylin and eosin staining with rare acid-fast bacilli seen on Fite staining.
To diagnose leprosy, at least one of the following 3 clinical signs must be present: (1) a hypopigmented or erythematous lesion with loss of sensation, (2) thickened peripheral nerve, or (3) acid-fast bacilli on slit-skin smear.2
Management
The World Health Organization guidelines involve multidrug therapy over an extended period of time.2 For adults, the paucibacillary regimen includes rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The adult regimen for multibacillary leprosy includes clofazimine 300 mg once monthly and 50 mg once daily, in addition to rifampicin 600 mg once monthly and dapsone 100 mg once daily for 12 months. If classification cannot be determined, it is recommended the patient be treated for multibacillary disease.2
Reversal Reactions
During the course of the disease, patients may upgrade (to a less severe form) or downgrade (to a more severe form) between the tuberculoid, borderline, and lepromatous forms.8 The patient’s clinical picture also may change with complications of leprosy, which include type 1 and type 2 reactions. Type 1 reaction is a reversal reaction seen in 15% to 30% of patients at risk, usually those with borderline forms of leprosy.9 Reversal reactions usually manifest as erythema and edema of current skin lesions, formation of new tumid lesions, and tenderness of peripheral nerves with loss of nerve function.8 Treatment of reversal reactions involves systemic corticosteroids.10 Type 2 reaction is classified as erythema nodosum leprosum. It presents within the first 2 years of treatment in approximately 20% of lepromatous patients and approximately 10% of borderline lepromatous patients but is rare in paucibacillary infections.11 It presents with fever and crops of pink nodules and may include iritis, neuritis, lymphadenitis, orchitis, dactylitis, arthritis, and proteinuria.8 Treatment options for erythema nodosum leprosum include corticosteroids, clofazimine, and thalidomide.12,13
Conclusion
Hansen disease is a rare condition in the United States. This case is unique because, to our knowledge, it is the first known PCR-confirmed case of Hansen disease in Okeechobee County, Florida. Additionally, the patient had no known exposure to M leprae. Exposure is increasing due to the increased geographical range of infected armadillos. Infection rates also may rise due to travel to endemic countries. Initially lesions may appear as innocuous erythematous plaques. When they do not respond to standard therapy, infectious agents such as M leprae should be part of the differential diagnosis. Because hematoxylin and eosin staining does not always yield results, if clinical suspicion is present, PCR should be performed. If a patient meets the clinical and histological diagnosis, the case should be reported to the National Hansen’s Disease Program.
After completion of treatment, our patient has shown excellent results. He has not yet demonstrated a reversal reaction; however, he may still be at risk, as it most commonly presents 2 months after starting treatment but can present years after treatment has been initiated.8 Cutaneous leprosy must be considered in the differential diagnosis for steroid-nonresponsive skin lesions, particularly in states such as Florida with a documented increase in incidence.
Acknowledgment
We thank Sharon Barrineau, ARNP (Okeechobee, Florida), for her acumen, contributions, and support on this case.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- World Health Organization. WHO Expert Committee on Leprosy, 8th Report. Geneva, Switzerland: World Health Organization; 2010.
- Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626-1633.
- Adams DA, Fullerton K, Jajosky R, et al; Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC. Summary of notifiable diseases—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1-122.
- A summary of Hansen’s disease in the United States—2015. Department of Health and Human Services, Health Resources and Services Administration, National Hansen’s Disease Program. https://www.hrsa.gov/sites/default/files/hansensdisease/pdfs/hansens2015report.pdf. Accessed October 23, 2017.
- Loughry WJ, Truman RW, McDonough CM, et al. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45:144-152.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:225-273.
- Lee DJ, Rea TH, Modlin RL. Leprosy. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- Britton WJ. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225-234.
- Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1999;67:270-278.
- Lockwood DN. The management of erythema nodosum leprosum: current and future options. Lepr Rev. 1996;67:253-259.
- Jakeman P, Smith WC. Thalidomide in leprosy reaction. Lancet. 1994;343:432-433.
Case Report
A 65-year-old man presented with multiple anesthetic, annular, erythematous, scaly plaques with a raised border of 6 weeks’ duration that were unresponsive to topical steroid therapy. Four plaques were noted on the lower back ranging from 2 to 4 cm in diameter as well as a fifth plaque on the anterior portion of the right ankle that was approximately 6×6 cm. He denied fever, malaise, muscle weakness, changes in vision, or sensory deficits outside of the lesions themselves. The patient also denied any recent travel to endemic areas or exposure to armadillos.
Biopsies were taken from lesions on the lumbar back and anterior aspect of the right ankle (Figure 1A). Hematoxylin and eosin staining revealed a granulomatous infiltrate spreading along neurovascular structures (Figure 2). Granulomas also were identified in the dermal interstitium exhibiting partial necrosis (Figure 2 inset). Conspicuous distension of lymphovascular and perineural areas also was noted. Immunohistochemical studies with S-100 and neurofilament stains allowed insight into the pathomechanism of the clinically observed anesthesia, as nerve fibers were identified showing different stages of damage elicited by the granulomatous inflammatory infiltrate (Figure 3). Fite staining was positive for occasional bacilli within histiocytes (Figure 3A inset). Despite the clinical, histologic, and immunohistochemical evidence, the patient had no known exposure to leprosy; consequently, a polymerase chain reaction (PCR) assay was ordered for confirmation of the diagnosis. Surprisingly, the PCR was positive for Mycobacterium leprae DNA. These findings were consistent with borderline tuberculoid leprosy.



The case was reported to the National Hansen’s Disease Program (Baton Rouge, Louisiana). The patient was started on rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The lesions exhibited marked improvement after completion of therapy (Figure 1B).
Comment
Disease Transmission
Hansen disease, also known as leprosy, is a chronic granulomatous infectious disease that is caused by M leprae, an obligate intracellular bacillus aerobe.1 The mechanism of spread of M leprae is not clear. It is thought to be transmitted via respiratory droplets, though it may occur through injured skin.2 Studies have suggested that in addition to humans, nine-banded armadillos are a source of infection.2,3 Exposure to infected individuals, particularly multibacillary patients, increases the likelihood of contracting leprosy.2
According to the Centers for Disease Control and Prevention, 81 cases of Hansen disease were diagnosed in the United States in 2013,4 compared to 178 cases registered in 2015.5 Cases from Hawaii, Texas, California, Louisiana, New York, and Florida made up 72% (129/178) of the reported cases. There was an increase from 34 cases to 49 cases in Florida from 2014 to 2015.5 The spread of leprosy throughout Florida may be from the merge of 2 armadillo populations, an M leprae–infected population migrating east from Texas and one from south central Florida that historically had not been infected with M leprae until recently.3,6 Our patient did not have any known exposures to armadillos.
Classification and Presentation
The clinical presentation of Hansen disease is widely variable, as it can present at any point along a spectrum ranging from tuberculoid leprosy to lepromatous leprosy with borderline conditions in between, according to the Ridley-Jopling critera.7 The World Health Organization also classifies leprosy based on the number of acid-fast bacilli seen in a skin smear as either paucibacillary or multibacillary.2 The paucibacillary classification correlates with tuberculoid, borderline tuberculoid, and indeterminate leprosy, and multibacillary correlates with borderline lepromatous and lepromatous leprosy. Paucibacillary leprosy usually presents with a less dramatic clinical picture than multibacillary leprosy. The clinical presentation is dependent on the magnitude of immune response to M leprae.2
Paucibacillary infection occurs when the body generates a strong cell-mediated immune response against the bacteria,8 which causes the activation and proliferation of CD4 and CD8 T cells, limiting the spread of the mycobacterium. Subsequently, the patient typically presents with a mild clinical picture with few skin lesions and limited nerve involvement.8 The skin lesions are papules or plaques with raised borders that are usually hypopigmented on dark skin and erythematous on light skin. Nerve involvement in paucibacillary forms of leprosy include sensory impairment and anhidrosis within the lesions. Nerve enlargement usually affects superficial nerves, with the posterior tibial nerve being most commonly affected.
Multibacillary leprosy presents with systemic involvement due to a weak cell-mediated immune response. Patients generally present with diffuse, poorly defined nodules; greater nerve impairment; and other systemic symptoms such as blindness, swelling of the fingers and toes, and testicular atrophy (in men). Additionally, enlargement of the earlobes and widening of the nasal bridge may contribute to the appearance of leonine facies. Nerve impairment in multibacillary forms of leprosy may be more severe, including more diffuse sensory involvement (eg, stocking glove–pattern neuropathy, nerve-trunk palsies), which ultimately may lead to foot drop, claw toe, and lagophthalmos.8
In addition to the clinical presentation, the histology of the paucibacillary and multibacillary types differ. Multibacillary leprosy shows diffuse histiocytes without granulomas and multiple bacilli seen on Fite staining.8 In the paucibacillary form, there are well-formed granulomas with Langerhans giant cells and a perineural lymphocytic infiltrate seen on hematoxylin and eosin staining with rare acid-fast bacilli seen on Fite staining.
To diagnose leprosy, at least one of the following 3 clinical signs must be present: (1) a hypopigmented or erythematous lesion with loss of sensation, (2) thickened peripheral nerve, or (3) acid-fast bacilli on slit-skin smear.2
Management
The World Health Organization guidelines involve multidrug therapy over an extended period of time.2 For adults, the paucibacillary regimen includes rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The adult regimen for multibacillary leprosy includes clofazimine 300 mg once monthly and 50 mg once daily, in addition to rifampicin 600 mg once monthly and dapsone 100 mg once daily for 12 months. If classification cannot be determined, it is recommended the patient be treated for multibacillary disease.2
Reversal Reactions
During the course of the disease, patients may upgrade (to a less severe form) or downgrade (to a more severe form) between the tuberculoid, borderline, and lepromatous forms.8 The patient’s clinical picture also may change with complications of leprosy, which include type 1 and type 2 reactions. Type 1 reaction is a reversal reaction seen in 15% to 30% of patients at risk, usually those with borderline forms of leprosy.9 Reversal reactions usually manifest as erythema and edema of current skin lesions, formation of new tumid lesions, and tenderness of peripheral nerves with loss of nerve function.8 Treatment of reversal reactions involves systemic corticosteroids.10 Type 2 reaction is classified as erythema nodosum leprosum. It presents within the first 2 years of treatment in approximately 20% of lepromatous patients and approximately 10% of borderline lepromatous patients but is rare in paucibacillary infections.11 It presents with fever and crops of pink nodules and may include iritis, neuritis, lymphadenitis, orchitis, dactylitis, arthritis, and proteinuria.8 Treatment options for erythema nodosum leprosum include corticosteroids, clofazimine, and thalidomide.12,13
Conclusion
Hansen disease is a rare condition in the United States. This case is unique because, to our knowledge, it is the first known PCR-confirmed case of Hansen disease in Okeechobee County, Florida. Additionally, the patient had no known exposure to M leprae. Exposure is increasing due to the increased geographical range of infected armadillos. Infection rates also may rise due to travel to endemic countries. Initially lesions may appear as innocuous erythematous plaques. When they do not respond to standard therapy, infectious agents such as M leprae should be part of the differential diagnosis. Because hematoxylin and eosin staining does not always yield results, if clinical suspicion is present, PCR should be performed. If a patient meets the clinical and histological diagnosis, the case should be reported to the National Hansen’s Disease Program.
After completion of treatment, our patient has shown excellent results. He has not yet demonstrated a reversal reaction; however, he may still be at risk, as it most commonly presents 2 months after starting treatment but can present years after treatment has been initiated.8 Cutaneous leprosy must be considered in the differential diagnosis for steroid-nonresponsive skin lesions, particularly in states such as Florida with a documented increase in incidence.
Acknowledgment
We thank Sharon Barrineau, ARNP (Okeechobee, Florida), for her acumen, contributions, and support on this case.
Case Report
A 65-year-old man presented with multiple anesthetic, annular, erythematous, scaly plaques with a raised border of 6 weeks’ duration that were unresponsive to topical steroid therapy. Four plaques were noted on the lower back ranging from 2 to 4 cm in diameter as well as a fifth plaque on the anterior portion of the right ankle that was approximately 6×6 cm. He denied fever, malaise, muscle weakness, changes in vision, or sensory deficits outside of the lesions themselves. The patient also denied any recent travel to endemic areas or exposure to armadillos.
Biopsies were taken from lesions on the lumbar back and anterior aspect of the right ankle (Figure 1A). Hematoxylin and eosin staining revealed a granulomatous infiltrate spreading along neurovascular structures (Figure 2). Granulomas also were identified in the dermal interstitium exhibiting partial necrosis (Figure 2 inset). Conspicuous distension of lymphovascular and perineural areas also was noted. Immunohistochemical studies with S-100 and neurofilament stains allowed insight into the pathomechanism of the clinically observed anesthesia, as nerve fibers were identified showing different stages of damage elicited by the granulomatous inflammatory infiltrate (Figure 3). Fite staining was positive for occasional bacilli within histiocytes (Figure 3A inset). Despite the clinical, histologic, and immunohistochemical evidence, the patient had no known exposure to leprosy; consequently, a polymerase chain reaction (PCR) assay was ordered for confirmation of the diagnosis. Surprisingly, the PCR was positive for Mycobacterium leprae DNA. These findings were consistent with borderline tuberculoid leprosy.



The case was reported to the National Hansen’s Disease Program (Baton Rouge, Louisiana). The patient was started on rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The lesions exhibited marked improvement after completion of therapy (Figure 1B).
Comment
Disease Transmission
Hansen disease, also known as leprosy, is a chronic granulomatous infectious disease that is caused by M leprae, an obligate intracellular bacillus aerobe.1 The mechanism of spread of M leprae is not clear. It is thought to be transmitted via respiratory droplets, though it may occur through injured skin.2 Studies have suggested that in addition to humans, nine-banded armadillos are a source of infection.2,3 Exposure to infected individuals, particularly multibacillary patients, increases the likelihood of contracting leprosy.2
According to the Centers for Disease Control and Prevention, 81 cases of Hansen disease were diagnosed in the United States in 2013,4 compared to 178 cases registered in 2015.5 Cases from Hawaii, Texas, California, Louisiana, New York, and Florida made up 72% (129/178) of the reported cases. There was an increase from 34 cases to 49 cases in Florida from 2014 to 2015.5 The spread of leprosy throughout Florida may be from the merge of 2 armadillo populations, an M leprae–infected population migrating east from Texas and one from south central Florida that historically had not been infected with M leprae until recently.3,6 Our patient did not have any known exposures to armadillos.
Classification and Presentation
The clinical presentation of Hansen disease is widely variable, as it can present at any point along a spectrum ranging from tuberculoid leprosy to lepromatous leprosy with borderline conditions in between, according to the Ridley-Jopling critera.7 The World Health Organization also classifies leprosy based on the number of acid-fast bacilli seen in a skin smear as either paucibacillary or multibacillary.2 The paucibacillary classification correlates with tuberculoid, borderline tuberculoid, and indeterminate leprosy, and multibacillary correlates with borderline lepromatous and lepromatous leprosy. Paucibacillary leprosy usually presents with a less dramatic clinical picture than multibacillary leprosy. The clinical presentation is dependent on the magnitude of immune response to M leprae.2
Paucibacillary infection occurs when the body generates a strong cell-mediated immune response against the bacteria,8 which causes the activation and proliferation of CD4 and CD8 T cells, limiting the spread of the mycobacterium. Subsequently, the patient typically presents with a mild clinical picture with few skin lesions and limited nerve involvement.8 The skin lesions are papules or plaques with raised borders that are usually hypopigmented on dark skin and erythematous on light skin. Nerve involvement in paucibacillary forms of leprosy include sensory impairment and anhidrosis within the lesions. Nerve enlargement usually affects superficial nerves, with the posterior tibial nerve being most commonly affected.
Multibacillary leprosy presents with systemic involvement due to a weak cell-mediated immune response. Patients generally present with diffuse, poorly defined nodules; greater nerve impairment; and other systemic symptoms such as blindness, swelling of the fingers and toes, and testicular atrophy (in men). Additionally, enlargement of the earlobes and widening of the nasal bridge may contribute to the appearance of leonine facies. Nerve impairment in multibacillary forms of leprosy may be more severe, including more diffuse sensory involvement (eg, stocking glove–pattern neuropathy, nerve-trunk palsies), which ultimately may lead to foot drop, claw toe, and lagophthalmos.8
In addition to the clinical presentation, the histology of the paucibacillary and multibacillary types differ. Multibacillary leprosy shows diffuse histiocytes without granulomas and multiple bacilli seen on Fite staining.8 In the paucibacillary form, there are well-formed granulomas with Langerhans giant cells and a perineural lymphocytic infiltrate seen on hematoxylin and eosin staining with rare acid-fast bacilli seen on Fite staining.
To diagnose leprosy, at least one of the following 3 clinical signs must be present: (1) a hypopigmented or erythematous lesion with loss of sensation, (2) thickened peripheral nerve, or (3) acid-fast bacilli on slit-skin smear.2
Management
The World Health Organization guidelines involve multidrug therapy over an extended period of time.2 For adults, the paucibacillary regimen includes rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The adult regimen for multibacillary leprosy includes clofazimine 300 mg once monthly and 50 mg once daily, in addition to rifampicin 600 mg once monthly and dapsone 100 mg once daily for 12 months. If classification cannot be determined, it is recommended the patient be treated for multibacillary disease.2
Reversal Reactions
During the course of the disease, patients may upgrade (to a less severe form) or downgrade (to a more severe form) between the tuberculoid, borderline, and lepromatous forms.8 The patient’s clinical picture also may change with complications of leprosy, which include type 1 and type 2 reactions. Type 1 reaction is a reversal reaction seen in 15% to 30% of patients at risk, usually those with borderline forms of leprosy.9 Reversal reactions usually manifest as erythema and edema of current skin lesions, formation of new tumid lesions, and tenderness of peripheral nerves with loss of nerve function.8 Treatment of reversal reactions involves systemic corticosteroids.10 Type 2 reaction is classified as erythema nodosum leprosum. It presents within the first 2 years of treatment in approximately 20% of lepromatous patients and approximately 10% of borderline lepromatous patients but is rare in paucibacillary infections.11 It presents with fever and crops of pink nodules and may include iritis, neuritis, lymphadenitis, orchitis, dactylitis, arthritis, and proteinuria.8 Treatment options for erythema nodosum leprosum include corticosteroids, clofazimine, and thalidomide.12,13
Conclusion
Hansen disease is a rare condition in the United States. This case is unique because, to our knowledge, it is the first known PCR-confirmed case of Hansen disease in Okeechobee County, Florida. Additionally, the patient had no known exposure to M leprae. Exposure is increasing due to the increased geographical range of infected armadillos. Infection rates also may rise due to travel to endemic countries. Initially lesions may appear as innocuous erythematous plaques. When they do not respond to standard therapy, infectious agents such as M leprae should be part of the differential diagnosis. Because hematoxylin and eosin staining does not always yield results, if clinical suspicion is present, PCR should be performed. If a patient meets the clinical and histological diagnosis, the case should be reported to the National Hansen’s Disease Program.
After completion of treatment, our patient has shown excellent results. He has not yet demonstrated a reversal reaction; however, he may still be at risk, as it most commonly presents 2 months after starting treatment but can present years after treatment has been initiated.8 Cutaneous leprosy must be considered in the differential diagnosis for steroid-nonresponsive skin lesions, particularly in states such as Florida with a documented increase in incidence.
Acknowledgment
We thank Sharon Barrineau, ARNP (Okeechobee, Florida), for her acumen, contributions, and support on this case.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- World Health Organization. WHO Expert Committee on Leprosy, 8th Report. Geneva, Switzerland: World Health Organization; 2010.
- Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626-1633.
- Adams DA, Fullerton K, Jajosky R, et al; Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC. Summary of notifiable diseases—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1-122.
- A summary of Hansen’s disease in the United States—2015. Department of Health and Human Services, Health Resources and Services Administration, National Hansen’s Disease Program. https://www.hrsa.gov/sites/default/files/hansensdisease/pdfs/hansens2015report.pdf. Accessed October 23, 2017.
- Loughry WJ, Truman RW, McDonough CM, et al. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45:144-152.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:225-273.
- Lee DJ, Rea TH, Modlin RL. Leprosy. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- Britton WJ. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225-234.
- Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1999;67:270-278.
- Lockwood DN. The management of erythema nodosum leprosum: current and future options. Lepr Rev. 1996;67:253-259.
- Jakeman P, Smith WC. Thalidomide in leprosy reaction. Lancet. 1994;343:432-433.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- World Health Organization. WHO Expert Committee on Leprosy, 8th Report. Geneva, Switzerland: World Health Organization; 2010.
- Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626-1633.
- Adams DA, Fullerton K, Jajosky R, et al; Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC. Summary of notifiable diseases—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1-122.
- A summary of Hansen’s disease in the United States—2015. Department of Health and Human Services, Health Resources and Services Administration, National Hansen’s Disease Program. https://www.hrsa.gov/sites/default/files/hansensdisease/pdfs/hansens2015report.pdf. Accessed October 23, 2017.
- Loughry WJ, Truman RW, McDonough CM, et al. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45:144-152.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:225-273.
- Lee DJ, Rea TH, Modlin RL. Leprosy. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- Britton WJ. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225-234.
- Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1999;67:270-278.
- Lockwood DN. The management of erythema nodosum leprosum: current and future options. Lepr Rev. 1996;67:253-259.
- Jakeman P, Smith WC. Thalidomide in leprosy reaction. Lancet. 1994;343:432-433.
Practice Points
- A majority of leprosy cases in the United States have been reported in Florida, California, Texas, Louisiana, Hawaii, and New York.
- Leprosy should be included in the differential diagnosis for annular plaques, particularly those not responding to traditional treatment.