User login
Eruptive Melanocytic Nevi During Azathioprine Therapy for Antisynthetase Syndrome
Case Report
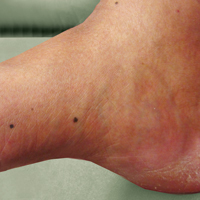
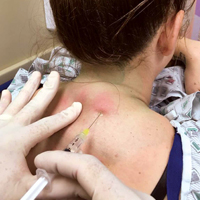
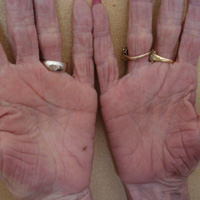
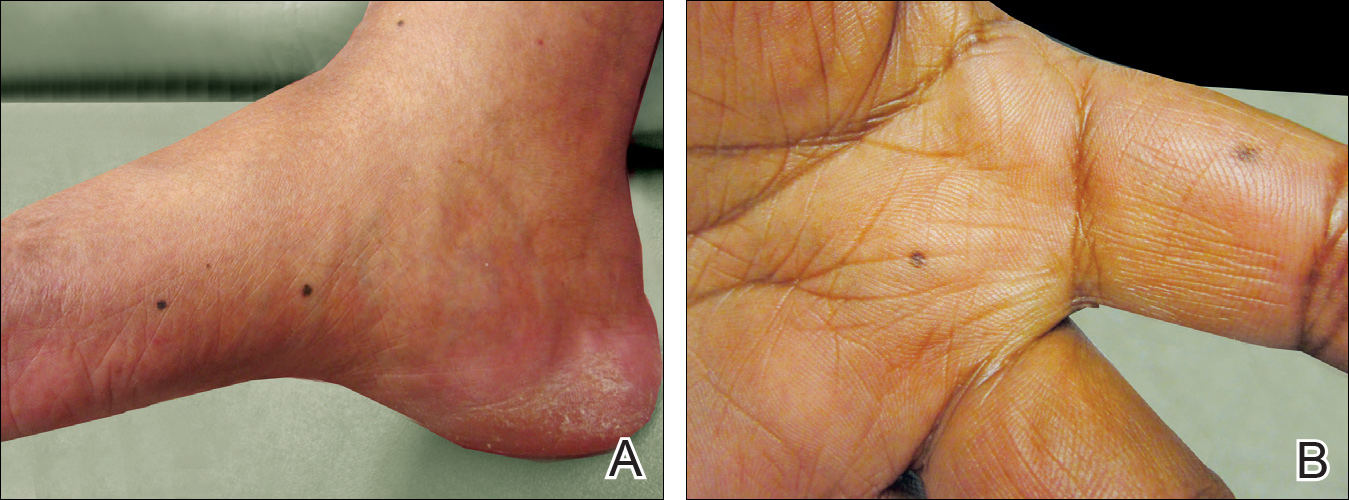
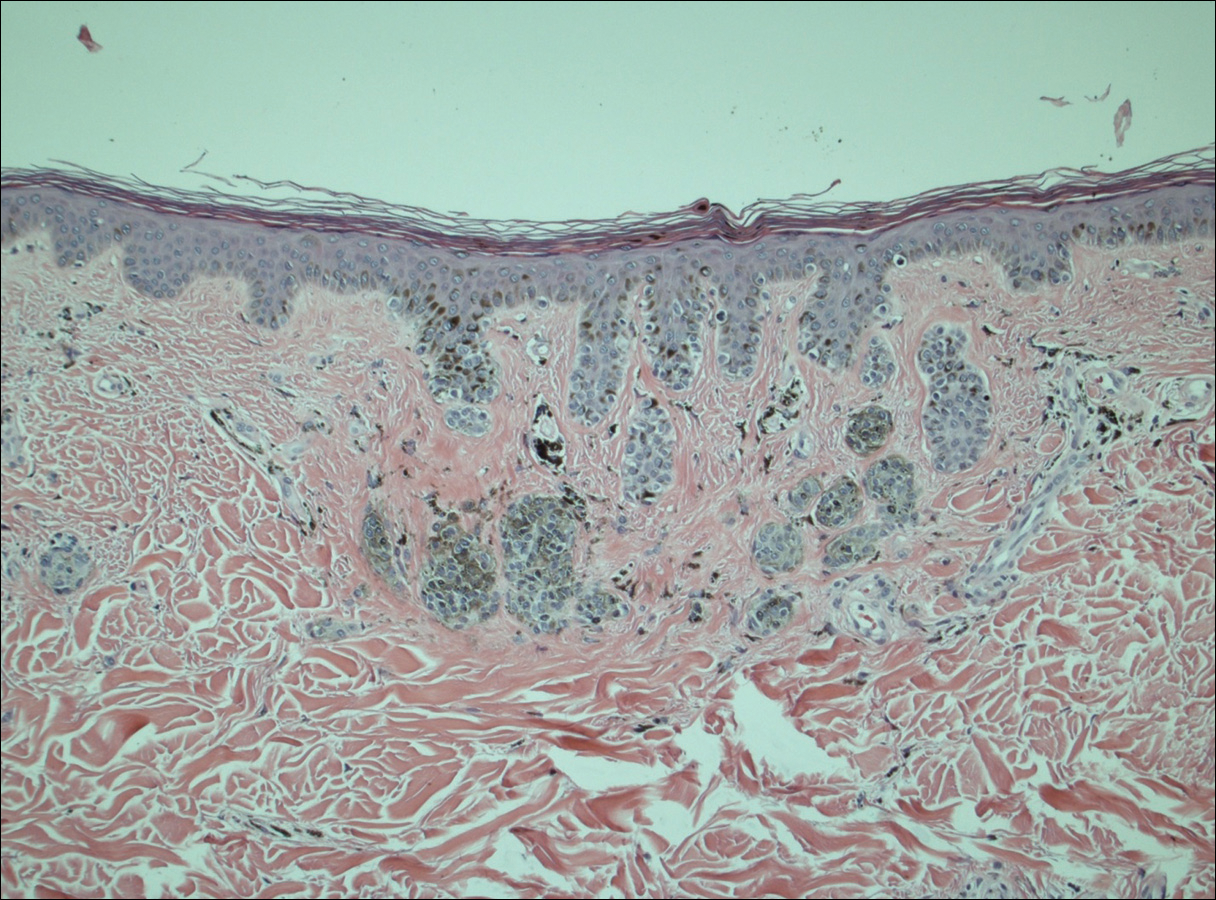
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.
Comment
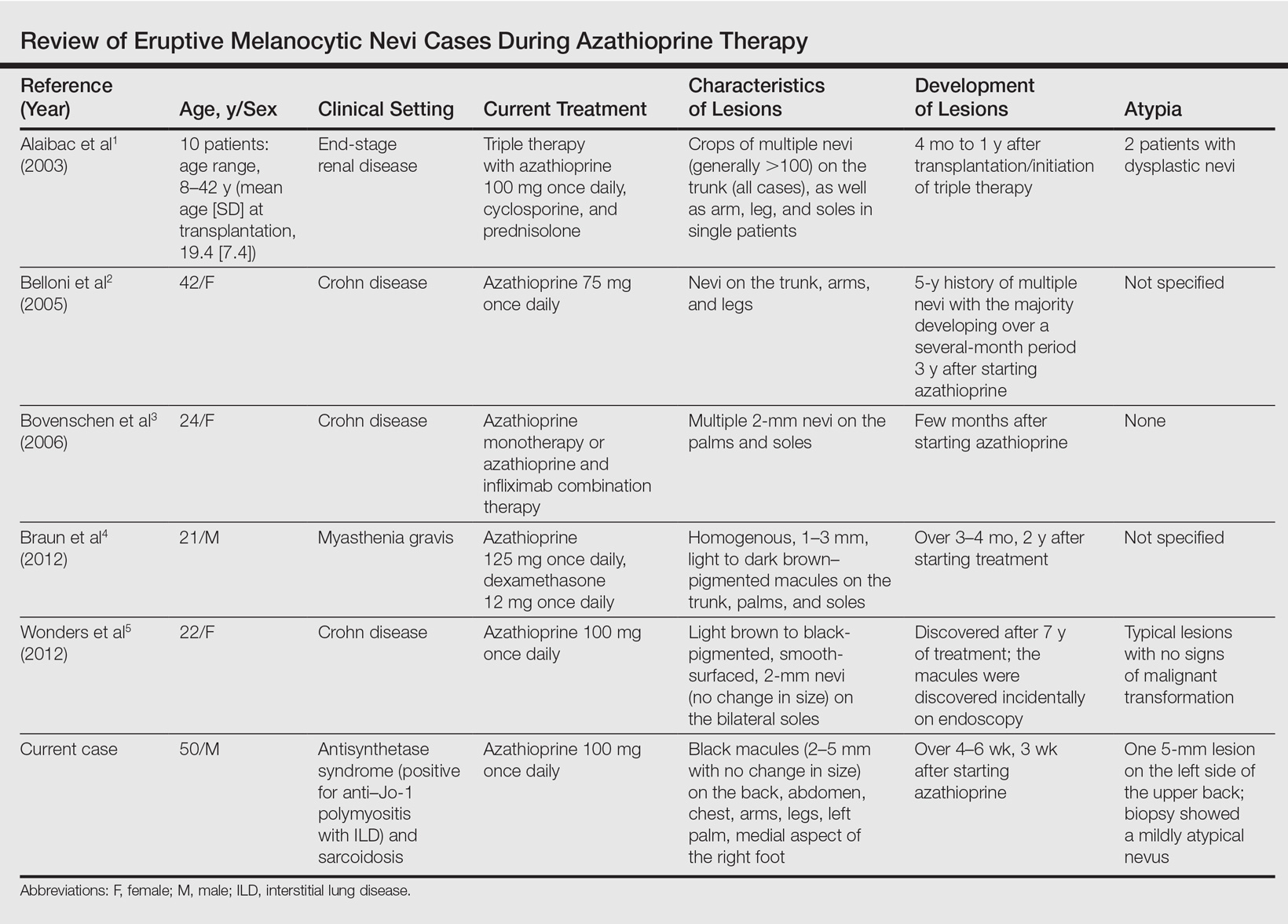
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.
Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.


Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.

Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.


Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.

Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
Practice Points
- A theoretical risk exists in the setting of eruptive melanocytic nevi (EMN) given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.
- Follow patients with EMN with regular skin examinations and biopsies of atypical-appearing lesions given the increased risk for melanoma in this population.
Muscle spasms, twitches in arm upon throwing • Dx?
THE CASE
A 31-year-old right-handed college baseball coach presented to his family physician (FP) with concerns about the “yips” in his right arm. His ability to throw a baseball had been gradually deteriorating. Involuntary upper right arm muscle contractions and spasms, which began intermittently when he was a teenager, were now a real problem for him as an adult. (See the video below.) The patient was having difficulty rolling a baseball underhand to players as part of infield practice and he was experiencing muscle spasms when lifting his right arm over his head. “Twitches” in the patient’s upper arm were making drinking difficult, but he had no problems feeding himself, writing, or performing other basic activities of daily living.
The patient experienced the same symptoms whether it was baseball season or not. He hadn’t noticed a change in symptoms with caffeine and denied use of any other stimulants in the last 4 years. His symptoms didn’t improve or worsen with greater or lesser quantity or quality of sleep or when he concentrated on stifling the involuntary movements. He had attempted to learn to throw left-handed to overcome the impairment, but was concerned that the same problem would occur in his left arm.
The patient had previously worked with a sports psychologist and hypnotherapist to overcome any potential subconscious performance anxiety, but this hadn’t helped. Stretching and strengthening with a physical therapist and numerous sessions with an acupuncturist hadn’t helped either. Despite this, he believed the problem to be primarily psychological.
The patient’s history included mild attention deficit disorder and exercise-induced asthma; his family history was negative for any movement or psychiatric disorders. He had 2 dislocation repairs on his left, non-throwing shoulder in his early twenties. His medications included fluticasone-salmeterol twice daily and albuterol, as needed.
The patient denied myalgia or arthralgia, decreased passive range of motion, shoulder or arm weakness, swelling, or muscle atrophy. He also didn’t have paresthesias in his right arm or hand, a resting tremor, difficulty moving (other than drinking from a cup), difficulty moving other extremities, dizziness, imbalance, or seizures.
The patient’s vital signs were normal. He had full range of motion and 5 out of 5 strength without pain during right shoulder abduction, external and internal rotation, an empty can test, a lower back lift off (Gerber’s) test, and a test of bicep and tricep strength, along with negative Neer and Hawkins tests.
There was no evidence of muscle wasting or asymmetry in the bilateral upper extremities. The patient’s deep tendon reflex grade was 2+ out of 4 in both of his arms. He didn’t have a sensory deficit to light touch in areas of C5 to T1 and he had normal cranial nerves II to XII. He had normal rapid alternating movements, heel-to-shin testing, and finger-to-nose testing, as well as a normal gait and Romberg test.
The patient provided a video showing the abnormal involuntary flexion of his shoulder when attempting to throw a baseball.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
THE DIAGNOSIS
The patient’s FP was aware of the “yips,” a condition that is commonly viewed as psychological or related to performance anxiety. (The “yips” are colloquially known as “Steve Blass Disease”—named after a Pittsburgh Pirates pitcher who suddenly lost the ability to control his pitches.1) But based on the patient’s clinical presentation and history of seeing a number of mental health care providers—in addition to his worsening symptoms—the FP ordered magnetic resonance imaging (MRI) of the brain. The MRI turned out to be unremarkable, so the patient was referred to Neurology.
In the general neurology clinic, a diagnosis of Wilson’s disease (a condition that leads to excess copper deposition in multiple organ systems, including the nervous system) was considered, as it can cause symptoms similar to those our patient was experiencing. However, a complete blood count, complete metabolic panel, antinuclear antibody test, ceruloplasmin test, and copper level were all normal, effectively ruling it out. An MRI of the cervical spine showed mild to moderate right foraminal stenosis at C3-4 and C5-6, but this did not explain the patient’s symptoms.
A diagnosis of paroxysmal exercise-induced dystonia was also considered at the time of the initial work-up, as our patient’s symptoms were most pronounced during physical activity. But this condition usually responds to antiepileptics, and carbamazepine and phenytoin were each tried for multiple months early in his evaluation without benefit.
3 factors led to a diagnosis of focal limb dystonia: Only our patient’s right arm was affected, his laboratory and imaging work-ups were negative, and he didn’t respond to antiepileptic treatment. Characterization of a movement disorder is based upon phenomenology. In this case, the patient had sustained abnormal posturing at the shoulder during right upper limb activation, which was only triggered with specific voluntary actions. This was consistent with dystonia, a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal movements and/or postures—often initiated or worsened by voluntary action.2
DISCUSSION
The “yips,” or intermittent, transient tremors, jerks, or spasms3 that are seen in athletes, are well-documented in the lay press, but haven’t been significantly addressed in the medical literature.4 Stigma surrounding the condition among athletes likely leads to under-reporting. Athletes typically experience yips with fine motor movements, such as short putts in golf and pitching in baseball. In fact, while the majority of the medical literature on yips revolves around golfers, many talented baseball players have had their careers altered by the condition. The yips may also affect movements in sports like darts, cricket, table tennis, and billiards.
In 1984, dystonia was defined as a disorder of sensorimotor integration that results in co-contraction of agonist/antagonist muscles, and may be characterized by state dependence (exacerbation with specific activities) or sensory tricks (amelioration with specific types of sensory input).5 In 2013, the definition was revised to remove “co-contraction” from the definition because phenomenology alone is sufficient to make the diagnosis.1
Many athletes and sports fans believe the yips are caused by performance anxiety or related phobias, but evidence suggests that many athletes with the movement disorder may actually have focal limb dystonia.6,7 The yips can, however, lead to performance anxiety,3 but there has been no difference noted between the anxiety level of golfers with or without the yips.7 Psychological treatment approaches are commonly employed, but surface electromyograms have shown abnormal co-contraction of wrist flexor and extensor muscles in 5 out of 10 golfers with the yips (but 0 of those without) while putting—which is consistent with focal limb dystonia.8
Botulinum toxin injections are Tx of choice, but can cause weakness
Muscle relaxers, such as baclofen and benzodiazepines, as well as dopamine antagonists, can ameliorate dystonia.9 Focal limb dystonia may also respond to the antispasmodic trihexyphenidyl, but the dose must often be limited due to adverse effects such as nausea, dizziness, and anxiety.10
Botulinum toxin injections have proven effective for focal limb dystonia11 and are considered the treatment of choice. However, there are few reports on their use in athletes, where the adverse effect of weakness could affect performance. One case report also showed improvement of yips with acupuncture, although this has not been extensively studied.12
Our patient didn’t respond to low-dose (2 mg twice a day) trihexyphenidyl. Tetrabenazine, a dopamine depletor frequently used for hyperkinetic disorders, was not effective at 25 mg taken prior to coaching sessions. Higher doses of an anticholinergic could have been effective, but the patient declined our recommendation to pursue this (or botulinum toxin injections). He decided instead to train himself to use his left arm while coaching.
THE TAKEAWAY
Athletes who play sports that require precision movements commonly develop the yips. While the prevailing theory among athletes is that this is a psychological phenomenon, evidence shows that this may in fact be a neurologic focal dystonia caused by repetitive use. Greater awareness of yips as a possible organic, treatable neurologic condition is needed in order to stimulate more research on this topic.
1. Baseball’s head cases often prove baffling. USA Today Baseball Weekly. 2001. Available at: http://usatoday30.usatoday.com/sports/bbw/2001-02-07/2001-02-07-head.htm. Accessed March 15, 2017.
2. Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863-873.
3. Dhungana S, Jankovic J. Yips and other movement disorders in golfers. Mov Disord. 2013;28:576-581.
4. Stacy MA, ed. Handbook of dystonia. New York, NY: Informa Healthcare USA, Inc; 2007.
5. Fahn S, Marsden CD, Calne DB. Classification and investigation of dystonia. In: Marsden CD, Fahn S, eds. Movement disorders 2. London: Butterworths; 1987:332-358.
6. Smith AM, Adler CH, Crews D, et al. The ‘yips’ in golf: a continuum between a focal dystonia and choking. Sports Med. 2003;33:13-31.
7. Sachdev P. Golfers’ cramp: clinical characteristics and evidence against it being an anxiety disorder. Mov Disord. 1992;7:326-332.
8. Adler CH, Crews D, Hentz JG, et al. Abnormal co-contraction in yips-affected but not unaffected golfers: evidence for focal dystonia. Neurology. 2005;64:1813-1814.
9. Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8:844-856.
10. Jankovic J. Treatment of dystonia. Lancet Neurol. 2006;5:864-872.
11. Lungu C, Karp BI, Alter K, et al. Long-term follow-up of botulinum toxin therapy for focal hand dystonia: outcome at 10 years or more. Mov Disord. 2011;26:750-753.
12. Rosted P. Acupuncture for treatment of the yips?—a case report. Acupunct Med. 2005;23:188-189.
THE CASE
A 31-year-old right-handed college baseball coach presented to his family physician (FP) with concerns about the “yips” in his right arm. His ability to throw a baseball had been gradually deteriorating. Involuntary upper right arm muscle contractions and spasms, which began intermittently when he was a teenager, were now a real problem for him as an adult. (See the video below.) The patient was having difficulty rolling a baseball underhand to players as part of infield practice and he was experiencing muscle spasms when lifting his right arm over his head. “Twitches” in the patient’s upper arm were making drinking difficult, but he had no problems feeding himself, writing, or performing other basic activities of daily living.
The patient experienced the same symptoms whether it was baseball season or not. He hadn’t noticed a change in symptoms with caffeine and denied use of any other stimulants in the last 4 years. His symptoms didn’t improve or worsen with greater or lesser quantity or quality of sleep or when he concentrated on stifling the involuntary movements. He had attempted to learn to throw left-handed to overcome the impairment, but was concerned that the same problem would occur in his left arm.
The patient had previously worked with a sports psychologist and hypnotherapist to overcome any potential subconscious performance anxiety, but this hadn’t helped. Stretching and strengthening with a physical therapist and numerous sessions with an acupuncturist hadn’t helped either. Despite this, he believed the problem to be primarily psychological.
The patient’s history included mild attention deficit disorder and exercise-induced asthma; his family history was negative for any movement or psychiatric disorders. He had 2 dislocation repairs on his left, non-throwing shoulder in his early twenties. His medications included fluticasone-salmeterol twice daily and albuterol, as needed.
The patient denied myalgia or arthralgia, decreased passive range of motion, shoulder or arm weakness, swelling, or muscle atrophy. He also didn’t have paresthesias in his right arm or hand, a resting tremor, difficulty moving (other than drinking from a cup), difficulty moving other extremities, dizziness, imbalance, or seizures.
The patient’s vital signs were normal. He had full range of motion and 5 out of 5 strength without pain during right shoulder abduction, external and internal rotation, an empty can test, a lower back lift off (Gerber’s) test, and a test of bicep and tricep strength, along with negative Neer and Hawkins tests.
There was no evidence of muscle wasting or asymmetry in the bilateral upper extremities. The patient’s deep tendon reflex grade was 2+ out of 4 in both of his arms. He didn’t have a sensory deficit to light touch in areas of C5 to T1 and he had normal cranial nerves II to XII. He had normal rapid alternating movements, heel-to-shin testing, and finger-to-nose testing, as well as a normal gait and Romberg test.
The patient provided a video showing the abnormal involuntary flexion of his shoulder when attempting to throw a baseball.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
THE DIAGNOSIS
The patient’s FP was aware of the “yips,” a condition that is commonly viewed as psychological or related to performance anxiety. (The “yips” are colloquially known as “Steve Blass Disease”—named after a Pittsburgh Pirates pitcher who suddenly lost the ability to control his pitches.1) But based on the patient’s clinical presentation and history of seeing a number of mental health care providers—in addition to his worsening symptoms—the FP ordered magnetic resonance imaging (MRI) of the brain. The MRI turned out to be unremarkable, so the patient was referred to Neurology.
In the general neurology clinic, a diagnosis of Wilson’s disease (a condition that leads to excess copper deposition in multiple organ systems, including the nervous system) was considered, as it can cause symptoms similar to those our patient was experiencing. However, a complete blood count, complete metabolic panel, antinuclear antibody test, ceruloplasmin test, and copper level were all normal, effectively ruling it out. An MRI of the cervical spine showed mild to moderate right foraminal stenosis at C3-4 and C5-6, but this did not explain the patient’s symptoms.
A diagnosis of paroxysmal exercise-induced dystonia was also considered at the time of the initial work-up, as our patient’s symptoms were most pronounced during physical activity. But this condition usually responds to antiepileptics, and carbamazepine and phenytoin were each tried for multiple months early in his evaluation without benefit.
3 factors led to a diagnosis of focal limb dystonia: Only our patient’s right arm was affected, his laboratory and imaging work-ups were negative, and he didn’t respond to antiepileptic treatment. Characterization of a movement disorder is based upon phenomenology. In this case, the patient had sustained abnormal posturing at the shoulder during right upper limb activation, which was only triggered with specific voluntary actions. This was consistent with dystonia, a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal movements and/or postures—often initiated or worsened by voluntary action.2
DISCUSSION
The “yips,” or intermittent, transient tremors, jerks, or spasms3 that are seen in athletes, are well-documented in the lay press, but haven’t been significantly addressed in the medical literature.4 Stigma surrounding the condition among athletes likely leads to under-reporting. Athletes typically experience yips with fine motor movements, such as short putts in golf and pitching in baseball. In fact, while the majority of the medical literature on yips revolves around golfers, many talented baseball players have had their careers altered by the condition. The yips may also affect movements in sports like darts, cricket, table tennis, and billiards.
In 1984, dystonia was defined as a disorder of sensorimotor integration that results in co-contraction of agonist/antagonist muscles, and may be characterized by state dependence (exacerbation with specific activities) or sensory tricks (amelioration with specific types of sensory input).5 In 2013, the definition was revised to remove “co-contraction” from the definition because phenomenology alone is sufficient to make the diagnosis.1
Many athletes and sports fans believe the yips are caused by performance anxiety or related phobias, but evidence suggests that many athletes with the movement disorder may actually have focal limb dystonia.6,7 The yips can, however, lead to performance anxiety,3 but there has been no difference noted between the anxiety level of golfers with or without the yips.7 Psychological treatment approaches are commonly employed, but surface electromyograms have shown abnormal co-contraction of wrist flexor and extensor muscles in 5 out of 10 golfers with the yips (but 0 of those without) while putting—which is consistent with focal limb dystonia.8
Botulinum toxin injections are Tx of choice, but can cause weakness
Muscle relaxers, such as baclofen and benzodiazepines, as well as dopamine antagonists, can ameliorate dystonia.9 Focal limb dystonia may also respond to the antispasmodic trihexyphenidyl, but the dose must often be limited due to adverse effects such as nausea, dizziness, and anxiety.10
Botulinum toxin injections have proven effective for focal limb dystonia11 and are considered the treatment of choice. However, there are few reports on their use in athletes, where the adverse effect of weakness could affect performance. One case report also showed improvement of yips with acupuncture, although this has not been extensively studied.12
Our patient didn’t respond to low-dose (2 mg twice a day) trihexyphenidyl. Tetrabenazine, a dopamine depletor frequently used for hyperkinetic disorders, was not effective at 25 mg taken prior to coaching sessions. Higher doses of an anticholinergic could have been effective, but the patient declined our recommendation to pursue this (or botulinum toxin injections). He decided instead to train himself to use his left arm while coaching.
THE TAKEAWAY
Athletes who play sports that require precision movements commonly develop the yips. While the prevailing theory among athletes is that this is a psychological phenomenon, evidence shows that this may in fact be a neurologic focal dystonia caused by repetitive use. Greater awareness of yips as a possible organic, treatable neurologic condition is needed in order to stimulate more research on this topic.
THE CASE
A 31-year-old right-handed college baseball coach presented to his family physician (FP) with concerns about the “yips” in his right arm. His ability to throw a baseball had been gradually deteriorating. Involuntary upper right arm muscle contractions and spasms, which began intermittently when he was a teenager, were now a real problem for him as an adult. (See the video below.) The patient was having difficulty rolling a baseball underhand to players as part of infield practice and he was experiencing muscle spasms when lifting his right arm over his head. “Twitches” in the patient’s upper arm were making drinking difficult, but he had no problems feeding himself, writing, or performing other basic activities of daily living.
The patient experienced the same symptoms whether it was baseball season or not. He hadn’t noticed a change in symptoms with caffeine and denied use of any other stimulants in the last 4 years. His symptoms didn’t improve or worsen with greater or lesser quantity or quality of sleep or when he concentrated on stifling the involuntary movements. He had attempted to learn to throw left-handed to overcome the impairment, but was concerned that the same problem would occur in his left arm.
The patient had previously worked with a sports psychologist and hypnotherapist to overcome any potential subconscious performance anxiety, but this hadn’t helped. Stretching and strengthening with a physical therapist and numerous sessions with an acupuncturist hadn’t helped either. Despite this, he believed the problem to be primarily psychological.
The patient’s history included mild attention deficit disorder and exercise-induced asthma; his family history was negative for any movement or psychiatric disorders. He had 2 dislocation repairs on his left, non-throwing shoulder in his early twenties. His medications included fluticasone-salmeterol twice daily and albuterol, as needed.
The patient denied myalgia or arthralgia, decreased passive range of motion, shoulder or arm weakness, swelling, or muscle atrophy. He also didn’t have paresthesias in his right arm or hand, a resting tremor, difficulty moving (other than drinking from a cup), difficulty moving other extremities, dizziness, imbalance, or seizures.
The patient’s vital signs were normal. He had full range of motion and 5 out of 5 strength without pain during right shoulder abduction, external and internal rotation, an empty can test, a lower back lift off (Gerber’s) test, and a test of bicep and tricep strength, along with negative Neer and Hawkins tests.
There was no evidence of muscle wasting or asymmetry in the bilateral upper extremities. The patient’s deep tendon reflex grade was 2+ out of 4 in both of his arms. He didn’t have a sensory deficit to light touch in areas of C5 to T1 and he had normal cranial nerves II to XII. He had normal rapid alternating movements, heel-to-shin testing, and finger-to-nose testing, as well as a normal gait and Romberg test.
The patient provided a video showing the abnormal involuntary flexion of his shoulder when attempting to throw a baseball.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
THE DIAGNOSIS
The patient’s FP was aware of the “yips,” a condition that is commonly viewed as psychological or related to performance anxiety. (The “yips” are colloquially known as “Steve Blass Disease”—named after a Pittsburgh Pirates pitcher who suddenly lost the ability to control his pitches.1) But based on the patient’s clinical presentation and history of seeing a number of mental health care providers—in addition to his worsening symptoms—the FP ordered magnetic resonance imaging (MRI) of the brain. The MRI turned out to be unremarkable, so the patient was referred to Neurology.
In the general neurology clinic, a diagnosis of Wilson’s disease (a condition that leads to excess copper deposition in multiple organ systems, including the nervous system) was considered, as it can cause symptoms similar to those our patient was experiencing. However, a complete blood count, complete metabolic panel, antinuclear antibody test, ceruloplasmin test, and copper level were all normal, effectively ruling it out. An MRI of the cervical spine showed mild to moderate right foraminal stenosis at C3-4 and C5-6, but this did not explain the patient’s symptoms.
A diagnosis of paroxysmal exercise-induced dystonia was also considered at the time of the initial work-up, as our patient’s symptoms were most pronounced during physical activity. But this condition usually responds to antiepileptics, and carbamazepine and phenytoin were each tried for multiple months early in his evaluation without benefit.
3 factors led to a diagnosis of focal limb dystonia: Only our patient’s right arm was affected, his laboratory and imaging work-ups were negative, and he didn’t respond to antiepileptic treatment. Characterization of a movement disorder is based upon phenomenology. In this case, the patient had sustained abnormal posturing at the shoulder during right upper limb activation, which was only triggered with specific voluntary actions. This was consistent with dystonia, a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal movements and/or postures—often initiated or worsened by voluntary action.2
DISCUSSION
The “yips,” or intermittent, transient tremors, jerks, or spasms3 that are seen in athletes, are well-documented in the lay press, but haven’t been significantly addressed in the medical literature.4 Stigma surrounding the condition among athletes likely leads to under-reporting. Athletes typically experience yips with fine motor movements, such as short putts in golf and pitching in baseball. In fact, while the majority of the medical literature on yips revolves around golfers, many talented baseball players have had their careers altered by the condition. The yips may also affect movements in sports like darts, cricket, table tennis, and billiards.
In 1984, dystonia was defined as a disorder of sensorimotor integration that results in co-contraction of agonist/antagonist muscles, and may be characterized by state dependence (exacerbation with specific activities) or sensory tricks (amelioration with specific types of sensory input).5 In 2013, the definition was revised to remove “co-contraction” from the definition because phenomenology alone is sufficient to make the diagnosis.1
Many athletes and sports fans believe the yips are caused by performance anxiety or related phobias, but evidence suggests that many athletes with the movement disorder may actually have focal limb dystonia.6,7 The yips can, however, lead to performance anxiety,3 but there has been no difference noted between the anxiety level of golfers with or without the yips.7 Psychological treatment approaches are commonly employed, but surface electromyograms have shown abnormal co-contraction of wrist flexor and extensor muscles in 5 out of 10 golfers with the yips (but 0 of those without) while putting—which is consistent with focal limb dystonia.8
Botulinum toxin injections are Tx of choice, but can cause weakness
Muscle relaxers, such as baclofen and benzodiazepines, as well as dopamine antagonists, can ameliorate dystonia.9 Focal limb dystonia may also respond to the antispasmodic trihexyphenidyl, but the dose must often be limited due to adverse effects such as nausea, dizziness, and anxiety.10
Botulinum toxin injections have proven effective for focal limb dystonia11 and are considered the treatment of choice. However, there are few reports on their use in athletes, where the adverse effect of weakness could affect performance. One case report also showed improvement of yips with acupuncture, although this has not been extensively studied.12
Our patient didn’t respond to low-dose (2 mg twice a day) trihexyphenidyl. Tetrabenazine, a dopamine depletor frequently used for hyperkinetic disorders, was not effective at 25 mg taken prior to coaching sessions. Higher doses of an anticholinergic could have been effective, but the patient declined our recommendation to pursue this (or botulinum toxin injections). He decided instead to train himself to use his left arm while coaching.
THE TAKEAWAY
Athletes who play sports that require precision movements commonly develop the yips. While the prevailing theory among athletes is that this is a psychological phenomenon, evidence shows that this may in fact be a neurologic focal dystonia caused by repetitive use. Greater awareness of yips as a possible organic, treatable neurologic condition is needed in order to stimulate more research on this topic.
1. Baseball’s head cases often prove baffling. USA Today Baseball Weekly. 2001. Available at: http://usatoday30.usatoday.com/sports/bbw/2001-02-07/2001-02-07-head.htm. Accessed March 15, 2017.
2. Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863-873.
3. Dhungana S, Jankovic J. Yips and other movement disorders in golfers. Mov Disord. 2013;28:576-581.
4. Stacy MA, ed. Handbook of dystonia. New York, NY: Informa Healthcare USA, Inc; 2007.
5. Fahn S, Marsden CD, Calne DB. Classification and investigation of dystonia. In: Marsden CD, Fahn S, eds. Movement disorders 2. London: Butterworths; 1987:332-358.
6. Smith AM, Adler CH, Crews D, et al. The ‘yips’ in golf: a continuum between a focal dystonia and choking. Sports Med. 2003;33:13-31.
7. Sachdev P. Golfers’ cramp: clinical characteristics and evidence against it being an anxiety disorder. Mov Disord. 1992;7:326-332.
8. Adler CH, Crews D, Hentz JG, et al. Abnormal co-contraction in yips-affected but not unaffected golfers: evidence for focal dystonia. Neurology. 2005;64:1813-1814.
9. Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8:844-856.
10. Jankovic J. Treatment of dystonia. Lancet Neurol. 2006;5:864-872.
11. Lungu C, Karp BI, Alter K, et al. Long-term follow-up of botulinum toxin therapy for focal hand dystonia: outcome at 10 years or more. Mov Disord. 2011;26:750-753.
12. Rosted P. Acupuncture for treatment of the yips?—a case report. Acupunct Med. 2005;23:188-189.
1. Baseball’s head cases often prove baffling. USA Today Baseball Weekly. 2001. Available at: http://usatoday30.usatoday.com/sports/bbw/2001-02-07/2001-02-07-head.htm. Accessed March 15, 2017.
2. Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863-873.
3. Dhungana S, Jankovic J. Yips and other movement disorders in golfers. Mov Disord. 2013;28:576-581.
4. Stacy MA, ed. Handbook of dystonia. New York, NY: Informa Healthcare USA, Inc; 2007.
5. Fahn S, Marsden CD, Calne DB. Classification and investigation of dystonia. In: Marsden CD, Fahn S, eds. Movement disorders 2. London: Butterworths; 1987:332-358.
6. Smith AM, Adler CH, Crews D, et al. The ‘yips’ in golf: a continuum between a focal dystonia and choking. Sports Med. 2003;33:13-31.
7. Sachdev P. Golfers’ cramp: clinical characteristics and evidence against it being an anxiety disorder. Mov Disord. 1992;7:326-332.
8. Adler CH, Crews D, Hentz JG, et al. Abnormal co-contraction in yips-affected but not unaffected golfers: evidence for focal dystonia. Neurology. 2005;64:1813-1814.
9. Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8:844-856.
10. Jankovic J. Treatment of dystonia. Lancet Neurol. 2006;5:864-872.
11. Lungu C, Karp BI, Alter K, et al. Long-term follow-up of botulinum toxin therapy for focal hand dystonia: outcome at 10 years or more. Mov Disord. 2011;26:750-753.
12. Rosted P. Acupuncture for treatment of the yips?—a case report. Acupunct Med. 2005;23:188-189.
Paraspinous Cervical Nerve Block for Primary Headache
Headaches—pain or discomfort in the head, scalp, or neck—are a very common reason for ED visits.1 In 2011, the World Health Organization estimated that 46.5% of the population in North and South America aged 18 to 65 years old experienced at least one headache within the previous year.1
Migraine is a recurrent headache disorder that afflicts 18% of US women and 9% of US men,2 resulting in at least 1.2 million visits to US EDs annually.1 The economic cost resulting from migraine-related loss of productive time in the US workforce is more than $13 billion per year, most of which is in the form of reduced work productivity.3 Management and treatment for migraine headache in the ED commonly include intravenous (IV) or intramuscular (IM) medications, fluids, or oxygen. While ultimately effective, these methods require nursing care and additional time for posttreatment monitoring, both of which adversely affect patient flow.
In 2006, Mellick et al4 described the safety and effectiveness of paraspinous cervical nerve block (PCNB) to abort migraine headaches. Despite its demonstrated efficacy and safety, a decade later, PCNB is still rarely used. Friedman et al5 ranked peripheral nerve blocks as the fourth step in management suggestions for primary headache.
Case Reports of Headache Patients
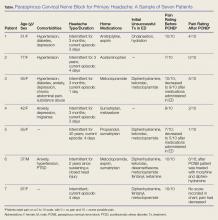
We report on seven headache patients we treated in our ED with PCNB who had good-to-complete resolution of pain, suggesting that PCNB is efficacious and can potentially shorten the ED length of stay. This series of seven patients (six female, one male) was a convenience sample of primary headache patients who presented over a 10-month period and were safely and rapidly treated with PCNB (Table).
In each case, the PCNB procedure was explained to the patient and consent was obtained. Each patient was treated with a total of 3 cc of 0.5% bupivacaine with epinephrine injected into the posterior neck according to the method described by Mellick et al.4 Our seven patients achieved an average 5-point reduction in pain on a 10-point pain scale, with 0 = no pain and 10 = worse possible pain.
Other than the provision of medications, no nursing assistance was required. Only one of the patients required further treatment after the PCNB, and none had an adverse reaction. All of the patients reported that their headaches were similar in nature to past headaches. Based on their history and physical examination, none were diagnosed to be experiencing a secondary, more serious cause of headache, and none subsequently returned to our institution with a secondary type of headache.
The Paraspinal Cervical Nerve Block
Paraspinous cervical nerve block requires less time to administer and recovery is shorter than that from IM or IV opioids, sedatives, or neuroleptics. It is an easy technique to teach since it requires bilateral injections.
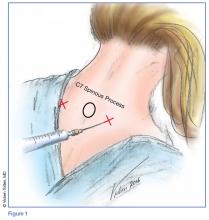
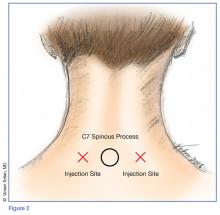
Technique
Prior to the procedure, cleanse the bilateral paravertebral zones surrounding C6 and C7 with chlorhexidine. Next, fill a 3 cc syringe using 0.5% bupivacaine with epinephrine.
Once the injection is complete, withdraw the needle completely, and compress and massage the injection site to facilitate anesthetic diffusion to surrounding tissues.
Indications
Paraspinous cervical nerve block is an appropriate treatment only for patients who are having a typical episode of chronic, recurring headaches, whose history and physical examination do not suggest the need for any further diagnostic work-up, and who, in the judgment of the treating clinician, require only pain relief.
Contraindications
A patient should not be considered for PCNB if he or she has a new-onset headache, fever, altered mental status, focal neurological deficits, meningismus, findings suggestive of meningitis, papilledema, increased intracranial pressure from a space-occupying lesion, recent head trauma with concern for intracranial hemorrhage, or suspicion of an alternate diagnosis.
Efficacy and Patient Response
Paraspinous cervical nerve block has been shown to decrease pain in patients who had failed standard migraine therapy and patients reported no complications. Of the seven patients in this case report, only one patient received opioids in the ED and none received prescriptions for opioids upon discharge for outpatient use.
Mellick and Mellick6 have postulated that pain may be modified due to the PCNB effect on the convergence of the trigeminal nerve with sensory fibers from the upper cervical roots. Since cervical innervation provides feeling to the head and upper neck, blocking this input can ameliorate pain.6
Summary
This series of seven patients provides further evidence of the effectiveness of PCNB in relieving headache symptoms for patients with recurrent, primary headaches when a secondary, more serious cause has been clinically excluded. Each of the seven patients had marked improvement of their pain and required only minimal nursing attention; moreover, all stated they would willingly undergo the procedure for future painful episodes.
Although there were no reported complications, this series is too small to demonstrate complete safety of the procedure. While this report is limited by a small sample size, it demonstrates that this is a quick, effective, and easily learned method of addressing a common ED complaint that obviates the need for parenteral medications and offers a potentially decreased patient length of stay.
Paraspinous cervical nerve block is a promising modality of treatment of ED patients who present with headache and migraine symptoms who do not respond to their outpatient “rescue” therapy. This procedure should be considered as an early treatment for migraine and other primary headaches unless contraindicated.
1. World Health Organization. Atlas of headache disorders and resources in the world 2011. http://www.who.int/mental_health/management/who_atlas_headache_disorders_results.pdf. Accessed February 9, 2017.
2. Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. 2010;30(9):1065-1072. doi:10.1177/0333102409355601.
3. Chawla J. Migraine headache. http://emedicine.medscape.com/article/1142556-overview. Accessed February 9, 2017.
4. Mellick LB, McIlrath ST, Mellick GA. Treatment of headaches in the ED with lower cervical intramuscular bupivacaine injections: a 1-year retrospective review of 417 patients. Headache. 2006;46(9):1441-1449.
5. Friedman BW, West J, Vinson DR, et al. Current management of migraine in US emergency departments: an analysis of the National Hospital Ambulatory Medical Care Survey. Cephalalgia. 2015;35:301-309.
6. Mellick GA, Mellick LB. Lower cervical intramuscular bupivacaine injections—another treatment option for headaches. http://www.neurologist-doctor.com/images/Mellick_Headache_injections.pdf. Accessed February 9, 2017.
Headaches—pain or discomfort in the head, scalp, or neck—are a very common reason for ED visits.1 In 2011, the World Health Organization estimated that 46.5% of the population in North and South America aged 18 to 65 years old experienced at least one headache within the previous year.1
Migraine is a recurrent headache disorder that afflicts 18% of US women and 9% of US men,2 resulting in at least 1.2 million visits to US EDs annually.1 The economic cost resulting from migraine-related loss of productive time in the US workforce is more than $13 billion per year, most of which is in the form of reduced work productivity.3 Management and treatment for migraine headache in the ED commonly include intravenous (IV) or intramuscular (IM) medications, fluids, or oxygen. While ultimately effective, these methods require nursing care and additional time for posttreatment monitoring, both of which adversely affect patient flow.
In 2006, Mellick et al4 described the safety and effectiveness of paraspinous cervical nerve block (PCNB) to abort migraine headaches. Despite its demonstrated efficacy and safety, a decade later, PCNB is still rarely used. Friedman et al5 ranked peripheral nerve blocks as the fourth step in management suggestions for primary headache.
Case Reports of Headache Patients
We report on seven headache patients we treated in our ED with PCNB who had good-to-complete resolution of pain, suggesting that PCNB is efficacious and can potentially shorten the ED length of stay. This series of seven patients (six female, one male) was a convenience sample of primary headache patients who presented over a 10-month period and were safely and rapidly treated with PCNB (Table).
In each case, the PCNB procedure was explained to the patient and consent was obtained. Each patient was treated with a total of 3 cc of 0.5% bupivacaine with epinephrine injected into the posterior neck according to the method described by Mellick et al.4 Our seven patients achieved an average 5-point reduction in pain on a 10-point pain scale, with 0 = no pain and 10 = worse possible pain.
Other than the provision of medications, no nursing assistance was required. Only one of the patients required further treatment after the PCNB, and none had an adverse reaction. All of the patients reported that their headaches were similar in nature to past headaches. Based on their history and physical examination, none were diagnosed to be experiencing a secondary, more serious cause of headache, and none subsequently returned to our institution with a secondary type of headache.
The Paraspinal Cervical Nerve Block
Paraspinous cervical nerve block requires less time to administer and recovery is shorter than that from IM or IV opioids, sedatives, or neuroleptics. It is an easy technique to teach since it requires bilateral injections.
Technique
Prior to the procedure, cleanse the bilateral paravertebral zones surrounding C6 and C7 with chlorhexidine. Next, fill a 3 cc syringe using 0.5% bupivacaine with epinephrine.
Once the injection is complete, withdraw the needle completely, and compress and massage the injection site to facilitate anesthetic diffusion to surrounding tissues.
Indications
Paraspinous cervical nerve block is an appropriate treatment only for patients who are having a typical episode of chronic, recurring headaches, whose history and physical examination do not suggest the need for any further diagnostic work-up, and who, in the judgment of the treating clinician, require only pain relief.
Contraindications
A patient should not be considered for PCNB if he or she has a new-onset headache, fever, altered mental status, focal neurological deficits, meningismus, findings suggestive of meningitis, papilledema, increased intracranial pressure from a space-occupying lesion, recent head trauma with concern for intracranial hemorrhage, or suspicion of an alternate diagnosis.
Efficacy and Patient Response
Paraspinous cervical nerve block has been shown to decrease pain in patients who had failed standard migraine therapy and patients reported no complications. Of the seven patients in this case report, only one patient received opioids in the ED and none received prescriptions for opioids upon discharge for outpatient use.
Mellick and Mellick6 have postulated that pain may be modified due to the PCNB effect on the convergence of the trigeminal nerve with sensory fibers from the upper cervical roots. Since cervical innervation provides feeling to the head and upper neck, blocking this input can ameliorate pain.6
Summary
This series of seven patients provides further evidence of the effectiveness of PCNB in relieving headache symptoms for patients with recurrent, primary headaches when a secondary, more serious cause has been clinically excluded. Each of the seven patients had marked improvement of their pain and required only minimal nursing attention; moreover, all stated they would willingly undergo the procedure for future painful episodes.
Although there were no reported complications, this series is too small to demonstrate complete safety of the procedure. While this report is limited by a small sample size, it demonstrates that this is a quick, effective, and easily learned method of addressing a common ED complaint that obviates the need for parenteral medications and offers a potentially decreased patient length of stay.
Paraspinous cervical nerve block is a promising modality of treatment of ED patients who present with headache and migraine symptoms who do not respond to their outpatient “rescue” therapy. This procedure should be considered as an early treatment for migraine and other primary headaches unless contraindicated.
Headaches—pain or discomfort in the head, scalp, or neck—are a very common reason for ED visits.1 In 2011, the World Health Organization estimated that 46.5% of the population in North and South America aged 18 to 65 years old experienced at least one headache within the previous year.1
Migraine is a recurrent headache disorder that afflicts 18% of US women and 9% of US men,2 resulting in at least 1.2 million visits to US EDs annually.1 The economic cost resulting from migraine-related loss of productive time in the US workforce is more than $13 billion per year, most of which is in the form of reduced work productivity.3 Management and treatment for migraine headache in the ED commonly include intravenous (IV) or intramuscular (IM) medications, fluids, or oxygen. While ultimately effective, these methods require nursing care and additional time for posttreatment monitoring, both of which adversely affect patient flow.
In 2006, Mellick et al4 described the safety and effectiveness of paraspinous cervical nerve block (PCNB) to abort migraine headaches. Despite its demonstrated efficacy and safety, a decade later, PCNB is still rarely used. Friedman et al5 ranked peripheral nerve blocks as the fourth step in management suggestions for primary headache.
Case Reports of Headache Patients
We report on seven headache patients we treated in our ED with PCNB who had good-to-complete resolution of pain, suggesting that PCNB is efficacious and can potentially shorten the ED length of stay. This series of seven patients (six female, one male) was a convenience sample of primary headache patients who presented over a 10-month period and were safely and rapidly treated with PCNB (Table).
In each case, the PCNB procedure was explained to the patient and consent was obtained. Each patient was treated with a total of 3 cc of 0.5% bupivacaine with epinephrine injected into the posterior neck according to the method described by Mellick et al.4 Our seven patients achieved an average 5-point reduction in pain on a 10-point pain scale, with 0 = no pain and 10 = worse possible pain.
Other than the provision of medications, no nursing assistance was required. Only one of the patients required further treatment after the PCNB, and none had an adverse reaction. All of the patients reported that their headaches were similar in nature to past headaches. Based on their history and physical examination, none were diagnosed to be experiencing a secondary, more serious cause of headache, and none subsequently returned to our institution with a secondary type of headache.
The Paraspinal Cervical Nerve Block
Paraspinous cervical nerve block requires less time to administer and recovery is shorter than that from IM or IV opioids, sedatives, or neuroleptics. It is an easy technique to teach since it requires bilateral injections.
Technique
Prior to the procedure, cleanse the bilateral paravertebral zones surrounding C6 and C7 with chlorhexidine. Next, fill a 3 cc syringe using 0.5% bupivacaine with epinephrine.
Once the injection is complete, withdraw the needle completely, and compress and massage the injection site to facilitate anesthetic diffusion to surrounding tissues.
Indications
Paraspinous cervical nerve block is an appropriate treatment only for patients who are having a typical episode of chronic, recurring headaches, whose history and physical examination do not suggest the need for any further diagnostic work-up, and who, in the judgment of the treating clinician, require only pain relief.
Contraindications
A patient should not be considered for PCNB if he or she has a new-onset headache, fever, altered mental status, focal neurological deficits, meningismus, findings suggestive of meningitis, papilledema, increased intracranial pressure from a space-occupying lesion, recent head trauma with concern for intracranial hemorrhage, or suspicion of an alternate diagnosis.
Efficacy and Patient Response
Paraspinous cervical nerve block has been shown to decrease pain in patients who had failed standard migraine therapy and patients reported no complications. Of the seven patients in this case report, only one patient received opioids in the ED and none received prescriptions for opioids upon discharge for outpatient use.
Mellick and Mellick6 have postulated that pain may be modified due to the PCNB effect on the convergence of the trigeminal nerve with sensory fibers from the upper cervical roots. Since cervical innervation provides feeling to the head and upper neck, blocking this input can ameliorate pain.6
Summary
This series of seven patients provides further evidence of the effectiveness of PCNB in relieving headache symptoms for patients with recurrent, primary headaches when a secondary, more serious cause has been clinically excluded. Each of the seven patients had marked improvement of their pain and required only minimal nursing attention; moreover, all stated they would willingly undergo the procedure for future painful episodes.
Although there were no reported complications, this series is too small to demonstrate complete safety of the procedure. While this report is limited by a small sample size, it demonstrates that this is a quick, effective, and easily learned method of addressing a common ED complaint that obviates the need for parenteral medications and offers a potentially decreased patient length of stay.
Paraspinous cervical nerve block is a promising modality of treatment of ED patients who present with headache and migraine symptoms who do not respond to their outpatient “rescue” therapy. This procedure should be considered as an early treatment for migraine and other primary headaches unless contraindicated.
1. World Health Organization. Atlas of headache disorders and resources in the world 2011. http://www.who.int/mental_health/management/who_atlas_headache_disorders_results.pdf. Accessed February 9, 2017.
2. Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. 2010;30(9):1065-1072. doi:10.1177/0333102409355601.
3. Chawla J. Migraine headache. http://emedicine.medscape.com/article/1142556-overview. Accessed February 9, 2017.
4. Mellick LB, McIlrath ST, Mellick GA. Treatment of headaches in the ED with lower cervical intramuscular bupivacaine injections: a 1-year retrospective review of 417 patients. Headache. 2006;46(9):1441-1449.
5. Friedman BW, West J, Vinson DR, et al. Current management of migraine in US emergency departments: an analysis of the National Hospital Ambulatory Medical Care Survey. Cephalalgia. 2015;35:301-309.
6. Mellick GA, Mellick LB. Lower cervical intramuscular bupivacaine injections—another treatment option for headaches. http://www.neurologist-doctor.com/images/Mellick_Headache_injections.pdf. Accessed February 9, 2017.
1. World Health Organization. Atlas of headache disorders and resources in the world 2011. http://www.who.int/mental_health/management/who_atlas_headache_disorders_results.pdf. Accessed February 9, 2017.
2. Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. 2010;30(9):1065-1072. doi:10.1177/0333102409355601.
3. Chawla J. Migraine headache. http://emedicine.medscape.com/article/1142556-overview. Accessed February 9, 2017.
4. Mellick LB, McIlrath ST, Mellick GA. Treatment of headaches in the ED with lower cervical intramuscular bupivacaine injections: a 1-year retrospective review of 417 patients. Headache. 2006;46(9):1441-1449.
5. Friedman BW, West J, Vinson DR, et al. Current management of migraine in US emergency departments: an analysis of the National Hospital Ambulatory Medical Care Survey. Cephalalgia. 2015;35:301-309.
6. Mellick GA, Mellick LB. Lower cervical intramuscular bupivacaine injections—another treatment option for headaches. http://www.neurologist-doctor.com/images/Mellick_Headache_injections.pdf. Accessed February 9, 2017.
Hypothyroidism-Induced Stercoral Sigmoid Colonic Perforation
According to the Centers for Disease Control and Prevention, abdominal pain is the leading reason for ED visits in the United States, with approximately 10 million visits per year.1 Though a large number of presentations are due to nontraumatic causes of abdominal pain, one etiology is among the most time-sensitive and critical diagnoses: acute colonic perforation.
Colonic perforations can be caused by diverticulitis, trauma, malignancy, ulcerative colitis, and other etiologies.2 A rare, yet life-threatening cause of colonic perforation, of which only a few cases have been documented in the literature, is stercoral colonic perforation.2
Regardless of the etiology, the critical actions for any colonic perforation are quick recognition, medical stabilization, and surgical evaluation. This case report highlights the diagnosis and treatment of acute stercoral colonic perforation with peritonitis secondary to hypothyroidism.
Case
A 49-year-old woman with a medical history significant for hypothyroidism presented to the ED for evaluation of diffuse abdominal pain, nausea, and nonbilious, nonbloody vomiting that started in the early evening of presentation. The patient denied any previous pain or associated symptoms, and said she had a small, hard bowel movement 1 day prior to arrival. She began experiencing mild abdominal pain on the morning of presentation. Her symptoms acutely worsened at approximately 5:00
On physical examination, her vital signs were: heart rate, 156 beats/min; blood pressure, 134/84 mm Hg; respiratory rate, 20 breaths/min, and temperature, 97.4°F. The patient appeared ill and diaphoretic, writhing on the stretcher. Abdominal examination was significant for diminished bowel sounds, diffuse abdominal distension, rigidity, and tenderness with light palpation.
Laboratory evaluation showed an elevated lactic acid level of 7.7 mmol/L, a white blood cell count of 7,200 cells/mm3 (segment form, 69.5%), and the following abnormal blood chemistry results: creatinine, 2.08 mg/dL; aspartate aminotransferase, 176 U/L; alanine aminotransferase, 138 U/L; and thyroid-stimulating hormone (TSH), 225.3 mcIU/mL. Other laboratory results were within normal range. Her electrocardiogram showed sinus tachycardia with a rate of 154 beats/min, a QTc within normal limits, and no ST elevations or depressions.
An abdominopelvic computed tomography (CT) scan revealed free air, free fluid, and possibly stool within the abdomen and pelvis. The findings were consistent with a ruptured hollow viscus, possibly a sigmoid colonic perforation. The radiologist also noted hepatomegaly and significant hepatic steatosis. A surgeon was immediately notified and evaluated the patient in the ED. The working diagnosis was stercoral colonic perforation secondary to severe hypothyroidism, and the patient was taken emergently to the operating room for repair.
Intraoperatively, the patient underwent exploratory laparotomy, which revealed gross fecal contamination of the abdomen. The surgeon noted that there was fecal staining along the serosal surface of the small bowel and throughout the pelvis. There were also large, hard stool balls outside of the colon. The perforation was along the mesenteric surface of the sigmoid just above the rectosigmoid junction.
The abdomen was copiously irrigated, the perforated segment was resected, and a Hartmann colostomy was created. The diagnosis was stercoral sigmoid perforation with peritonitis, and the patient was transferred to the intensive care unit for antibiotic treatment and further medical care, including intravenous (IV) levothyroxine.
She was extubated uneventfully on postoperative day 2, and the acute renal failure improved with supportive care only. Her bowel function slowly returned without complication. She was switched to oral levothyroxine on postoperative day 3. On day 13, she was given strict instructions for continuation of her thyroid medication and close monitoring for postsurgical complications, and was discharged home with appropriate follow-up.
Discussion
Multiple contributing factors can lead to bowel perforation. In this case, severe hypothyroidism with constipation caused a colonic perforation. Our patient had severe constipation that increased intraluminal pressure, causing the bowel wall to become ischemic and subsequently perforate.3 Any disease that causes significant constipation or obstruction of transit could lead to the same catastrophic result.
According to Huang et al,4 as of 2002, fewer than 90 cases of general stercoral bowel perforation had been reported, with no clear age range. However, patients in their mid-50s to mid-60s appear to be the most commonly affected age group.4 Our patient was younger than this age group, making identification of the problem by age alone difficult.
Hypothyroidism
The incidence of hypothyroidism in iodine-replete communities varies between 1% to 2% of the general population.5 The condition is more common in older women, affecting approximately 10% of those over age 65 years. In the United States, the prevalence of biochemical hypothyroidism is 4.6%; however, clinically evident hypothyroidism is present in only 0.3%.6 Common causes for hypothyroidism are listed in the Table.7,8
Myxedema Coma
Untreated, hypothyroidism can lead to potentially fatal conditions, such as myxedema coma, which is characterized by hypothermia, hypotension, bradycardia, respiratory depression, and altered mental status.7 Severe myxedema coma can result in cardiovascular collapse, and eventual death. Electrocardiography findings of severe hypothyroidism include bradycardia, low-voltage QRS, and widespread T-wave inversions.7 Our patient was tachycardic and did not have any acute findings to suggest myxedema coma.
Treatment for myxedema coma includes supportive care with ventilatory support and pressor support if necessary. Patients should be given IV hydrocortisone, 100 mg, to treat possible adrenal insufficiency and T4, 4 mcg/kg by slow IV infusion.7 Caution should be taken if giving a patient T3 due to the risk of dysrhythmias and myocardial infarction (MI).7 As our patient was not displaying myxedema coma, the surgeon elected not to start IV thyroid replacement to avoid exacerbating the patient’s tachycardia and possibly precipitating an MI intraoperatively.
Conclusion
Our case underscores the importance of promptly recognizing the signs and symptoms of stercoral colonic perforation in patients who present with nontraumatic abdominal pain accompanied by nausea and nonbilious, nonbloody vomiting. Although stercoral colonic perforation is a rare cause of nontraumatic abdominal pain, as with any type of colonic perforation, it constitutes a life-threatening medical emergency. As our case illustrates, prompt diagnosis through a thorough history taking, physical examination, and laboratory and imaging studies is critical to ensure medical stabilization and surgical management to reduce morbidity and mortality.
1. Centers for Disease Control and Prevention. Table 10. Ten leading principal reasons for emergency department visits, by patient age and sex: United States, 2013. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf. Accessed March 3, 2017.
2. Nam JK, Kim BS, Kim KS, Moon DJ. Clinical analysis of stercoral perforation of the colon. Korean J Gastroenterol. 2010;55:46-51.
3. Heffernan C, Pachter HL, Megibow AJ, Macari M. Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol. 2005;184(4):1189-1193. doi:10.2214/ajr.184.4.01841189.
4. Huang WS, Wang CS, Hsieh CC, Lin PY, Chin CC, Wang JY. Management of patients with stercoral perforation of the sigmoid colon: Report of five cases. World J Gastroenterol. 2006;12(3):500-503.
5. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526-534.
6. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
7. Idrose AM. Hypothyroidism. In: Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th Edition. New York, NY: McGraw-Hill Education; 2016:1469-1472.
8. Skugor M. Hypothyroidism and Hyperthyroidism. Cleveland Clinic Center for Continuing Education. August 2014. http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/endocrinology/hypothyroidism-and-hyperthyroidism/. Accessed March 3, 2017.
According to the Centers for Disease Control and Prevention, abdominal pain is the leading reason for ED visits in the United States, with approximately 10 million visits per year.1 Though a large number of presentations are due to nontraumatic causes of abdominal pain, one etiology is among the most time-sensitive and critical diagnoses: acute colonic perforation.
Colonic perforations can be caused by diverticulitis, trauma, malignancy, ulcerative colitis, and other etiologies.2 A rare, yet life-threatening cause of colonic perforation, of which only a few cases have been documented in the literature, is stercoral colonic perforation.2
Regardless of the etiology, the critical actions for any colonic perforation are quick recognition, medical stabilization, and surgical evaluation. This case report highlights the diagnosis and treatment of acute stercoral colonic perforation with peritonitis secondary to hypothyroidism.
Case
A 49-year-old woman with a medical history significant for hypothyroidism presented to the ED for evaluation of diffuse abdominal pain, nausea, and nonbilious, nonbloody vomiting that started in the early evening of presentation. The patient denied any previous pain or associated symptoms, and said she had a small, hard bowel movement 1 day prior to arrival. She began experiencing mild abdominal pain on the morning of presentation. Her symptoms acutely worsened at approximately 5:00
On physical examination, her vital signs were: heart rate, 156 beats/min; blood pressure, 134/84 mm Hg; respiratory rate, 20 breaths/min, and temperature, 97.4°F. The patient appeared ill and diaphoretic, writhing on the stretcher. Abdominal examination was significant for diminished bowel sounds, diffuse abdominal distension, rigidity, and tenderness with light palpation.
Laboratory evaluation showed an elevated lactic acid level of 7.7 mmol/L, a white blood cell count of 7,200 cells/mm3 (segment form, 69.5%), and the following abnormal blood chemistry results: creatinine, 2.08 mg/dL; aspartate aminotransferase, 176 U/L; alanine aminotransferase, 138 U/L; and thyroid-stimulating hormone (TSH), 225.3 mcIU/mL. Other laboratory results were within normal range. Her electrocardiogram showed sinus tachycardia with a rate of 154 beats/min, a QTc within normal limits, and no ST elevations or depressions.
An abdominopelvic computed tomography (CT) scan revealed free air, free fluid, and possibly stool within the abdomen and pelvis. The findings were consistent with a ruptured hollow viscus, possibly a sigmoid colonic perforation. The radiologist also noted hepatomegaly and significant hepatic steatosis. A surgeon was immediately notified and evaluated the patient in the ED. The working diagnosis was stercoral colonic perforation secondary to severe hypothyroidism, and the patient was taken emergently to the operating room for repair.
Intraoperatively, the patient underwent exploratory laparotomy, which revealed gross fecal contamination of the abdomen. The surgeon noted that there was fecal staining along the serosal surface of the small bowel and throughout the pelvis. There were also large, hard stool balls outside of the colon. The perforation was along the mesenteric surface of the sigmoid just above the rectosigmoid junction.
The abdomen was copiously irrigated, the perforated segment was resected, and a Hartmann colostomy was created. The diagnosis was stercoral sigmoid perforation with peritonitis, and the patient was transferred to the intensive care unit for antibiotic treatment and further medical care, including intravenous (IV) levothyroxine.
She was extubated uneventfully on postoperative day 2, and the acute renal failure improved with supportive care only. Her bowel function slowly returned without complication. She was switched to oral levothyroxine on postoperative day 3. On day 13, she was given strict instructions for continuation of her thyroid medication and close monitoring for postsurgical complications, and was discharged home with appropriate follow-up.
Discussion
Multiple contributing factors can lead to bowel perforation. In this case, severe hypothyroidism with constipation caused a colonic perforation. Our patient had severe constipation that increased intraluminal pressure, causing the bowel wall to become ischemic and subsequently perforate.3 Any disease that causes significant constipation or obstruction of transit could lead to the same catastrophic result.
According to Huang et al,4 as of 2002, fewer than 90 cases of general stercoral bowel perforation had been reported, with no clear age range. However, patients in their mid-50s to mid-60s appear to be the most commonly affected age group.4 Our patient was younger than this age group, making identification of the problem by age alone difficult.
Hypothyroidism
The incidence of hypothyroidism in iodine-replete communities varies between 1% to 2% of the general population.5 The condition is more common in older women, affecting approximately 10% of those over age 65 years. In the United States, the prevalence of biochemical hypothyroidism is 4.6%; however, clinically evident hypothyroidism is present in only 0.3%.6 Common causes for hypothyroidism are listed in the Table.7,8
Myxedema Coma
Untreated, hypothyroidism can lead to potentially fatal conditions, such as myxedema coma, which is characterized by hypothermia, hypotension, bradycardia, respiratory depression, and altered mental status.7 Severe myxedema coma can result in cardiovascular collapse, and eventual death. Electrocardiography findings of severe hypothyroidism include bradycardia, low-voltage QRS, and widespread T-wave inversions.7 Our patient was tachycardic and did not have any acute findings to suggest myxedema coma.
Treatment for myxedema coma includes supportive care with ventilatory support and pressor support if necessary. Patients should be given IV hydrocortisone, 100 mg, to treat possible adrenal insufficiency and T4, 4 mcg/kg by slow IV infusion.7 Caution should be taken if giving a patient T3 due to the risk of dysrhythmias and myocardial infarction (MI).7 As our patient was not displaying myxedema coma, the surgeon elected not to start IV thyroid replacement to avoid exacerbating the patient’s tachycardia and possibly precipitating an MI intraoperatively.
Conclusion
Our case underscores the importance of promptly recognizing the signs and symptoms of stercoral colonic perforation in patients who present with nontraumatic abdominal pain accompanied by nausea and nonbilious, nonbloody vomiting. Although stercoral colonic perforation is a rare cause of nontraumatic abdominal pain, as with any type of colonic perforation, it constitutes a life-threatening medical emergency. As our case illustrates, prompt diagnosis through a thorough history taking, physical examination, and laboratory and imaging studies is critical to ensure medical stabilization and surgical management to reduce morbidity and mortality.
According to the Centers for Disease Control and Prevention, abdominal pain is the leading reason for ED visits in the United States, with approximately 10 million visits per year.1 Though a large number of presentations are due to nontraumatic causes of abdominal pain, one etiology is among the most time-sensitive and critical diagnoses: acute colonic perforation.
Colonic perforations can be caused by diverticulitis, trauma, malignancy, ulcerative colitis, and other etiologies.2 A rare, yet life-threatening cause of colonic perforation, of which only a few cases have been documented in the literature, is stercoral colonic perforation.2
Regardless of the etiology, the critical actions for any colonic perforation are quick recognition, medical stabilization, and surgical evaluation. This case report highlights the diagnosis and treatment of acute stercoral colonic perforation with peritonitis secondary to hypothyroidism.
Case
A 49-year-old woman with a medical history significant for hypothyroidism presented to the ED for evaluation of diffuse abdominal pain, nausea, and nonbilious, nonbloody vomiting that started in the early evening of presentation. The patient denied any previous pain or associated symptoms, and said she had a small, hard bowel movement 1 day prior to arrival. She began experiencing mild abdominal pain on the morning of presentation. Her symptoms acutely worsened at approximately 5:00
On physical examination, her vital signs were: heart rate, 156 beats/min; blood pressure, 134/84 mm Hg; respiratory rate, 20 breaths/min, and temperature, 97.4°F. The patient appeared ill and diaphoretic, writhing on the stretcher. Abdominal examination was significant for diminished bowel sounds, diffuse abdominal distension, rigidity, and tenderness with light palpation.
Laboratory evaluation showed an elevated lactic acid level of 7.7 mmol/L, a white blood cell count of 7,200 cells/mm3 (segment form, 69.5%), and the following abnormal blood chemistry results: creatinine, 2.08 mg/dL; aspartate aminotransferase, 176 U/L; alanine aminotransferase, 138 U/L; and thyroid-stimulating hormone (TSH), 225.3 mcIU/mL. Other laboratory results were within normal range. Her electrocardiogram showed sinus tachycardia with a rate of 154 beats/min, a QTc within normal limits, and no ST elevations or depressions.
An abdominopelvic computed tomography (CT) scan revealed free air, free fluid, and possibly stool within the abdomen and pelvis. The findings were consistent with a ruptured hollow viscus, possibly a sigmoid colonic perforation. The radiologist also noted hepatomegaly and significant hepatic steatosis. A surgeon was immediately notified and evaluated the patient in the ED. The working diagnosis was stercoral colonic perforation secondary to severe hypothyroidism, and the patient was taken emergently to the operating room for repair.
Intraoperatively, the patient underwent exploratory laparotomy, which revealed gross fecal contamination of the abdomen. The surgeon noted that there was fecal staining along the serosal surface of the small bowel and throughout the pelvis. There were also large, hard stool balls outside of the colon. The perforation was along the mesenteric surface of the sigmoid just above the rectosigmoid junction.
The abdomen was copiously irrigated, the perforated segment was resected, and a Hartmann colostomy was created. The diagnosis was stercoral sigmoid perforation with peritonitis, and the patient was transferred to the intensive care unit for antibiotic treatment and further medical care, including intravenous (IV) levothyroxine.
She was extubated uneventfully on postoperative day 2, and the acute renal failure improved with supportive care only. Her bowel function slowly returned without complication. She was switched to oral levothyroxine on postoperative day 3. On day 13, she was given strict instructions for continuation of her thyroid medication and close monitoring for postsurgical complications, and was discharged home with appropriate follow-up.
Discussion
Multiple contributing factors can lead to bowel perforation. In this case, severe hypothyroidism with constipation caused a colonic perforation. Our patient had severe constipation that increased intraluminal pressure, causing the bowel wall to become ischemic and subsequently perforate.3 Any disease that causes significant constipation or obstruction of transit could lead to the same catastrophic result.
According to Huang et al,4 as of 2002, fewer than 90 cases of general stercoral bowel perforation had been reported, with no clear age range. However, patients in their mid-50s to mid-60s appear to be the most commonly affected age group.4 Our patient was younger than this age group, making identification of the problem by age alone difficult.
Hypothyroidism
The incidence of hypothyroidism in iodine-replete communities varies between 1% to 2% of the general population.5 The condition is more common in older women, affecting approximately 10% of those over age 65 years. In the United States, the prevalence of biochemical hypothyroidism is 4.6%; however, clinically evident hypothyroidism is present in only 0.3%.6 Common causes for hypothyroidism are listed in the Table.7,8
Myxedema Coma
Untreated, hypothyroidism can lead to potentially fatal conditions, such as myxedema coma, which is characterized by hypothermia, hypotension, bradycardia, respiratory depression, and altered mental status.7 Severe myxedema coma can result in cardiovascular collapse, and eventual death. Electrocardiography findings of severe hypothyroidism include bradycardia, low-voltage QRS, and widespread T-wave inversions.7 Our patient was tachycardic and did not have any acute findings to suggest myxedema coma.
Treatment for myxedema coma includes supportive care with ventilatory support and pressor support if necessary. Patients should be given IV hydrocortisone, 100 mg, to treat possible adrenal insufficiency and T4, 4 mcg/kg by slow IV infusion.7 Caution should be taken if giving a patient T3 due to the risk of dysrhythmias and myocardial infarction (MI).7 As our patient was not displaying myxedema coma, the surgeon elected not to start IV thyroid replacement to avoid exacerbating the patient’s tachycardia and possibly precipitating an MI intraoperatively.
Conclusion
Our case underscores the importance of promptly recognizing the signs and symptoms of stercoral colonic perforation in patients who present with nontraumatic abdominal pain accompanied by nausea and nonbilious, nonbloody vomiting. Although stercoral colonic perforation is a rare cause of nontraumatic abdominal pain, as with any type of colonic perforation, it constitutes a life-threatening medical emergency. As our case illustrates, prompt diagnosis through a thorough history taking, physical examination, and laboratory and imaging studies is critical to ensure medical stabilization and surgical management to reduce morbidity and mortality.
1. Centers for Disease Control and Prevention. Table 10. Ten leading principal reasons for emergency department visits, by patient age and sex: United States, 2013. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf. Accessed March 3, 2017.
2. Nam JK, Kim BS, Kim KS, Moon DJ. Clinical analysis of stercoral perforation of the colon. Korean J Gastroenterol. 2010;55:46-51.
3. Heffernan C, Pachter HL, Megibow AJ, Macari M. Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol. 2005;184(4):1189-1193. doi:10.2214/ajr.184.4.01841189.
4. Huang WS, Wang CS, Hsieh CC, Lin PY, Chin CC, Wang JY. Management of patients with stercoral perforation of the sigmoid colon: Report of five cases. World J Gastroenterol. 2006;12(3):500-503.
5. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526-534.
6. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
7. Idrose AM. Hypothyroidism. In: Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th Edition. New York, NY: McGraw-Hill Education; 2016:1469-1472.
8. Skugor M. Hypothyroidism and Hyperthyroidism. Cleveland Clinic Center for Continuing Education. August 2014. http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/endocrinology/hypothyroidism-and-hyperthyroidism/. Accessed March 3, 2017.
1. Centers for Disease Control and Prevention. Table 10. Ten leading principal reasons for emergency department visits, by patient age and sex: United States, 2013. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf. Accessed March 3, 2017.
2. Nam JK, Kim BS, Kim KS, Moon DJ. Clinical analysis of stercoral perforation of the colon. Korean J Gastroenterol. 2010;55:46-51.
3. Heffernan C, Pachter HL, Megibow AJ, Macari M. Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol. 2005;184(4):1189-1193. doi:10.2214/ajr.184.4.01841189.
4. Huang WS, Wang CS, Hsieh CC, Lin PY, Chin CC, Wang JY. Management of patients with stercoral perforation of the sigmoid colon: Report of five cases. World J Gastroenterol. 2006;12(3):500-503.
5. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526-534.
6. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
7. Idrose AM. Hypothyroidism. In: Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th Edition. New York, NY: McGraw-Hill Education; 2016:1469-1472.
8. Skugor M. Hypothyroidism and Hyperthyroidism. Cleveland Clinic Center for Continuing Education. August 2014. http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/endocrinology/hypothyroidism-and-hyperthyroidism/. Accessed March 3, 2017.
Emergency Imaging: Multiple Comorbidities With Fever and Nonproductive Cough
Laboratory studies revealed leukocytosis with a left shift. Chest radiographs were negative for pneumonia. A magnetic resonance image (MRI) of the lumbar spine was obtained to evaluate for diskitis osteomyelitis. A radiograph of the pelvis was also obtained to evaluate the patient’s THAs, and a computed tomography scan (CT) of the abdomen and pelvis with contrast was obtained for further evaluation. Representative CT, radiographic, and MRI images are shown at left (Figures 1-3).
What is the suspected diagnosis?
Answer
The MRI of the lumbar spine demonstrated no evidence of diskitis osteomyelitis. However, T2-weighted axial images showed enlarged heterogeneous bilateral psoas muscles with bright signal, indicating the presence of fluid (white arrows, Figure 4).
On the pelvic radiographs, both femoral heads appeared off-center within the acetabular cups (red arrows, Figure 5). This eccentric positioning indicated wear of the polyethylene in the THAs that normally occupies the space between the acetabular cup and the femoral head. In addition, focal lucency in the right acetabulum indicated breakdown of the bone, a condition referred to as osteolysis (white asterisk, Figure 5).
An abdominopelvic CT scan with contrast was performed and confirmed the findings of polyethylene wear and osteolysis. The CT scan also demonstrated large bilateral hip joint effusions (white arrows, Figure 6), decompressed along distended bilateral iliopsoas bursae (red asterisks, Figure 6), and communicating with the bilateral psoas muscle collections (red arrows, Figure 6).
Osteolysis With Iliopsoas Bursitis
Bursae are fluid-filled sacs lined by synovial tissue located throughout the body to reduce friction at sites of movement between muscles, bones, and tendons. Bursitis develops when these sacs become inflamed and/or infected and fill with fluid. The iliopsoas bursa lies between the anterior capsule of the hip and the psoas tendon, iliacus tendon, and muscle fibers.1,2 This bursa frequently communicates with the hip joint.3,4 Iliopsoas bursal distension has been reported following THA in the setting of polyethylene wear,5 and aseptic bursitis is a commonly seen incidental finding at the time of revision surgery.6
In this patient, long-standing polyethylene-induced synovitis had markedly expanded the hip joints and iliopsoas bursae, eventually resulting in superinfection, which accounted for the patient’s symptoms.
Treatment
Based on the imaging findings, interventional radiology services were contacted. The interventional radiologist drained the bilateral psoas abscesses. Cultures of the fluid were positive for both methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S aureus (MSSA). The patient was admitted to the hospital for treatment of MRSA and MSSA with intravenous antibiotic therapy. He recovered from the infection and was discharged on hospital day 2, with instructions to follow up with an orthopedic surgeon to discuss eventual revision of the bilateral THAs.
1. Chandler SB. The iliopsoas bursa in man. Anatom Record. 1934;58(3),235-240. doi:10.1002/ar.1090580304.
2. Tatu L, Parratte B, Vuillier F, Diop M, Monnier G. Descriptive anatomy of the femoral portion of the iliopsoas muscle. Anatomical basis of anterior snapping of the hip. Surg Radiol Anat. 2001;23(6):371-374.
3. Meaney JF, Cassar-Pullicino VN, Etherington R, Ritchie DA, McCall IW, Whitehouse GH. Ilio-psoas bursa enlargement. Clin Radiol. 1992;45(3):161-168.
4. Warren R, Kaye JJ, Salvati EA. Arthrographic demonstration of an enlarged iliopsoas bursa complicating osteoarthritis of the hip. A case report. J Bone Joint Surg Am. 1975;57(3):413-415.
5. Cheung YM, Gupte CM, Beverly MJ. Iliopsoas bursitis following total hip replacement. Arch Orthop Trauma Surg. 2004;124(10):720-723. Epub 2004 Oct 23. doi:10.1007/s00402-004-0751-9.
6. Howie DW, Cain CM, Cornish BL. Pseudo-abscess of the psoas bursa in failed double-cup arthroplasty of the hip. J Bone Joint Surg Br. 1991;73:29-32.
Laboratory studies revealed leukocytosis with a left shift. Chest radiographs were negative for pneumonia. A magnetic resonance image (MRI) of the lumbar spine was obtained to evaluate for diskitis osteomyelitis. A radiograph of the pelvis was also obtained to evaluate the patient’s THAs, and a computed tomography scan (CT) of the abdomen and pelvis with contrast was obtained for further evaluation. Representative CT, radiographic, and MRI images are shown at left (Figures 1-3).
What is the suspected diagnosis?
Answer
The MRI of the lumbar spine demonstrated no evidence of diskitis osteomyelitis. However, T2-weighted axial images showed enlarged heterogeneous bilateral psoas muscles with bright signal, indicating the presence of fluid (white arrows, Figure 4).
On the pelvic radiographs, both femoral heads appeared off-center within the acetabular cups (red arrows, Figure 5). This eccentric positioning indicated wear of the polyethylene in the THAs that normally occupies the space between the acetabular cup and the femoral head. In addition, focal lucency in the right acetabulum indicated breakdown of the bone, a condition referred to as osteolysis (white asterisk, Figure 5).
An abdominopelvic CT scan with contrast was performed and confirmed the findings of polyethylene wear and osteolysis. The CT scan also demonstrated large bilateral hip joint effusions (white arrows, Figure 6), decompressed along distended bilateral iliopsoas bursae (red asterisks, Figure 6), and communicating with the bilateral psoas muscle collections (red arrows, Figure 6).
Osteolysis With Iliopsoas Bursitis
Bursae are fluid-filled sacs lined by synovial tissue located throughout the body to reduce friction at sites of movement between muscles, bones, and tendons. Bursitis develops when these sacs become inflamed and/or infected and fill with fluid. The iliopsoas bursa lies between the anterior capsule of the hip and the psoas tendon, iliacus tendon, and muscle fibers.1,2 This bursa frequently communicates with the hip joint.3,4 Iliopsoas bursal distension has been reported following THA in the setting of polyethylene wear,5 and aseptic bursitis is a commonly seen incidental finding at the time of revision surgery.6
In this patient, long-standing polyethylene-induced synovitis had markedly expanded the hip joints and iliopsoas bursae, eventually resulting in superinfection, which accounted for the patient’s symptoms.
Treatment
Based on the imaging findings, interventional radiology services were contacted. The interventional radiologist drained the bilateral psoas abscesses. Cultures of the fluid were positive for both methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S aureus (MSSA). The patient was admitted to the hospital for treatment of MRSA and MSSA with intravenous antibiotic therapy. He recovered from the infection and was discharged on hospital day 2, with instructions to follow up with an orthopedic surgeon to discuss eventual revision of the bilateral THAs.
Laboratory studies revealed leukocytosis with a left shift. Chest radiographs were negative for pneumonia. A magnetic resonance image (MRI) of the lumbar spine was obtained to evaluate for diskitis osteomyelitis. A radiograph of the pelvis was also obtained to evaluate the patient’s THAs, and a computed tomography scan (CT) of the abdomen and pelvis with contrast was obtained for further evaluation. Representative CT, radiographic, and MRI images are shown at left (Figures 1-3).
What is the suspected diagnosis?
Answer
The MRI of the lumbar spine demonstrated no evidence of diskitis osteomyelitis. However, T2-weighted axial images showed enlarged heterogeneous bilateral psoas muscles with bright signal, indicating the presence of fluid (white arrows, Figure 4).
On the pelvic radiographs, both femoral heads appeared off-center within the acetabular cups (red arrows, Figure 5). This eccentric positioning indicated wear of the polyethylene in the THAs that normally occupies the space between the acetabular cup and the femoral head. In addition, focal lucency in the right acetabulum indicated breakdown of the bone, a condition referred to as osteolysis (white asterisk, Figure 5).
An abdominopelvic CT scan with contrast was performed and confirmed the findings of polyethylene wear and osteolysis. The CT scan also demonstrated large bilateral hip joint effusions (white arrows, Figure 6), decompressed along distended bilateral iliopsoas bursae (red asterisks, Figure 6), and communicating with the bilateral psoas muscle collections (red arrows, Figure 6).
Osteolysis With Iliopsoas Bursitis
Bursae are fluid-filled sacs lined by synovial tissue located throughout the body to reduce friction at sites of movement between muscles, bones, and tendons. Bursitis develops when these sacs become inflamed and/or infected and fill with fluid. The iliopsoas bursa lies between the anterior capsule of the hip and the psoas tendon, iliacus tendon, and muscle fibers.1,2 This bursa frequently communicates with the hip joint.3,4 Iliopsoas bursal distension has been reported following THA in the setting of polyethylene wear,5 and aseptic bursitis is a commonly seen incidental finding at the time of revision surgery.6
In this patient, long-standing polyethylene-induced synovitis had markedly expanded the hip joints and iliopsoas bursae, eventually resulting in superinfection, which accounted for the patient’s symptoms.
Treatment
Based on the imaging findings, interventional radiology services were contacted. The interventional radiologist drained the bilateral psoas abscesses. Cultures of the fluid were positive for both methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S aureus (MSSA). The patient was admitted to the hospital for treatment of MRSA and MSSA with intravenous antibiotic therapy. He recovered from the infection and was discharged on hospital day 2, with instructions to follow up with an orthopedic surgeon to discuss eventual revision of the bilateral THAs.
1. Chandler SB. The iliopsoas bursa in man. Anatom Record. 1934;58(3),235-240. doi:10.1002/ar.1090580304.
2. Tatu L, Parratte B, Vuillier F, Diop M, Monnier G. Descriptive anatomy of the femoral portion of the iliopsoas muscle. Anatomical basis of anterior snapping of the hip. Surg Radiol Anat. 2001;23(6):371-374.
3. Meaney JF, Cassar-Pullicino VN, Etherington R, Ritchie DA, McCall IW, Whitehouse GH. Ilio-psoas bursa enlargement. Clin Radiol. 1992;45(3):161-168.
4. Warren R, Kaye JJ, Salvati EA. Arthrographic demonstration of an enlarged iliopsoas bursa complicating osteoarthritis of the hip. A case report. J Bone Joint Surg Am. 1975;57(3):413-415.
5. Cheung YM, Gupte CM, Beverly MJ. Iliopsoas bursitis following total hip replacement. Arch Orthop Trauma Surg. 2004;124(10):720-723. Epub 2004 Oct 23. doi:10.1007/s00402-004-0751-9.
6. Howie DW, Cain CM, Cornish BL. Pseudo-abscess of the psoas bursa in failed double-cup arthroplasty of the hip. J Bone Joint Surg Br. 1991;73:29-32.
1. Chandler SB. The iliopsoas bursa in man. Anatom Record. 1934;58(3),235-240. doi:10.1002/ar.1090580304.
2. Tatu L, Parratte B, Vuillier F, Diop M, Monnier G. Descriptive anatomy of the femoral portion of the iliopsoas muscle. Anatomical basis of anterior snapping of the hip. Surg Radiol Anat. 2001;23(6):371-374.
3. Meaney JF, Cassar-Pullicino VN, Etherington R, Ritchie DA, McCall IW, Whitehouse GH. Ilio-psoas bursa enlargement. Clin Radiol. 1992;45(3):161-168.
4. Warren R, Kaye JJ, Salvati EA. Arthrographic demonstration of an enlarged iliopsoas bursa complicating osteoarthritis of the hip. A case report. J Bone Joint Surg Am. 1975;57(3):413-415.
5. Cheung YM, Gupte CM, Beverly MJ. Iliopsoas bursitis following total hip replacement. Arch Orthop Trauma Surg. 2004;124(10):720-723. Epub 2004 Oct 23. doi:10.1007/s00402-004-0751-9.
6. Howie DW, Cain CM, Cornish BL. Pseudo-abscess of the psoas bursa in failed double-cup arthroplasty of the hip. J Bone Joint Surg Br. 1991;73:29-32.
Superior Mesenteric Artery Syndrome as a Complication of Scoliosis Surgery
Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
Discussion
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. Review of the literature and report of eighteen cases. J Bone Joint Surg Am. 1971;53(3):431-444.
2. Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307-3310.
3. Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;(250):250-257.
4. Willet A. Fatal vomiting following application of plaster-of-Paris bandage in case of spinal curvature. St Barth Hosp Rep. 1878;14:333-335.
5. Dorph MH. The cast syndrome; review of the literature and report of a case. N Engl J Med. 1950;243(12):440-442.
6. Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312-318.
7. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9.
8. Akin JT Jr, Skandalakis JE, Gray SW. The anatomic basis of vascular compression of the duodenum. Surg Clin North Am. 1974;54(6):1361-1370.
9. Amy BW, Priebe CJ Jr, King A. Superior mesenteric artery syndrome associated with scoliosis treated by a modified Ladd procedure. J Pediatr Orthop. 1985;5(3):361-363.
10. Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377-378.
11. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
12. Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am. 2006;88(10):2252-2257.
13. Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144-148.
14. Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 suppl):S37-S45.
15. Kennedy RH, Cooper MJ. An unusually severe case of the cast syndrome. Postgrad Med J. 1983;59(694):539-540.
16. Keskin M, Akgül T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431.
17. Amarawickrama H, Harikrishnan A, Krijgsman B. Superior mesenteric artery syndrome in a young girl following spinal surgery for scoliosis. Br J Hosp Med. 2005;66(12):700-701.
18. Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-E533.
19. Moskovich R, Cheong-Leen P. Vascular compression of the duodenum. J R Soc Med. 1986;79(8):465-467.
20. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
Discussion
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
Discussion
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. Review of the literature and report of eighteen cases. J Bone Joint Surg Am. 1971;53(3):431-444.
2. Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307-3310.
3. Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;(250):250-257.
4. Willet A. Fatal vomiting following application of plaster-of-Paris bandage in case of spinal curvature. St Barth Hosp Rep. 1878;14:333-335.
5. Dorph MH. The cast syndrome; review of the literature and report of a case. N Engl J Med. 1950;243(12):440-442.
6. Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312-318.
7. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9.
8. Akin JT Jr, Skandalakis JE, Gray SW. The anatomic basis of vascular compression of the duodenum. Surg Clin North Am. 1974;54(6):1361-1370.
9. Amy BW, Priebe CJ Jr, King A. Superior mesenteric artery syndrome associated with scoliosis treated by a modified Ladd procedure. J Pediatr Orthop. 1985;5(3):361-363.
10. Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377-378.
11. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
12. Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am. 2006;88(10):2252-2257.
13. Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144-148.
14. Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 suppl):S37-S45.
15. Kennedy RH, Cooper MJ. An unusually severe case of the cast syndrome. Postgrad Med J. 1983;59(694):539-540.
16. Keskin M, Akgül T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431.
17. Amarawickrama H, Harikrishnan A, Krijgsman B. Superior mesenteric artery syndrome in a young girl following spinal surgery for scoliosis. Br J Hosp Med. 2005;66(12):700-701.
18. Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-E533.
19. Moskovich R, Cheong-Leen P. Vascular compression of the duodenum. J R Soc Med. 1986;79(8):465-467.
20. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
1. Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. Review of the literature and report of eighteen cases. J Bone Joint Surg Am. 1971;53(3):431-444.
2. Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307-3310.
3. Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;(250):250-257.
4. Willet A. Fatal vomiting following application of plaster-of-Paris bandage in case of spinal curvature. St Barth Hosp Rep. 1878;14:333-335.
5. Dorph MH. The cast syndrome; review of the literature and report of a case. N Engl J Med. 1950;243(12):440-442.
6. Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312-318.
7. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9.
8. Akin JT Jr, Skandalakis JE, Gray SW. The anatomic basis of vascular compression of the duodenum. Surg Clin North Am. 1974;54(6):1361-1370.
9. Amy BW, Priebe CJ Jr, King A. Superior mesenteric artery syndrome associated with scoliosis treated by a modified Ladd procedure. J Pediatr Orthop. 1985;5(3):361-363.
10. Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377-378.
11. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
12. Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am. 2006;88(10):2252-2257.
13. Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144-148.
14. Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 suppl):S37-S45.
15. Kennedy RH, Cooper MJ. An unusually severe case of the cast syndrome. Postgrad Med J. 1983;59(694):539-540.
16. Keskin M, Akgül T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431.
17. Amarawickrama H, Harikrishnan A, Krijgsman B. Superior mesenteric artery syndrome in a young girl following spinal surgery for scoliosis. Br J Hosp Med. 2005;66(12):700-701.
18. Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-E533.
19. Moskovich R, Cheong-Leen P. Vascular compression of the duodenum. J R Soc Med. 1986;79(8):465-467.
20. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
Paraneoplastic Palmoplantar Keratoderma Secondary to Metastatic Uterine Adenocarcinoma
Paraneoplastic palmoplantar keratoderma (PPK) is an acquired dermatosis that presents with hyperkeratosis of the palms and soles in association with visceral malignancies, such as esophageal, gastric, pulmonary, and bladder carcinomas. This condition may either be acquired or inherited.1
Case Report
A 72-year-old woman was referred to our dermatology clinic for evaluation of a nonpruritic hyperkeratotic eruption predominantly on the palms and soles of 2 to 3 months’ duration (Figure 1A). Review of systems was remarkable for chronic anxiety, unintentional weight loss of 10 lb over the last 6 months, and a mild cough of 10 days’ duration. The differential diagnosis included eczematous dermatitis, tinea manuum, new-onset palmoplantar psoriasis, and PPK.
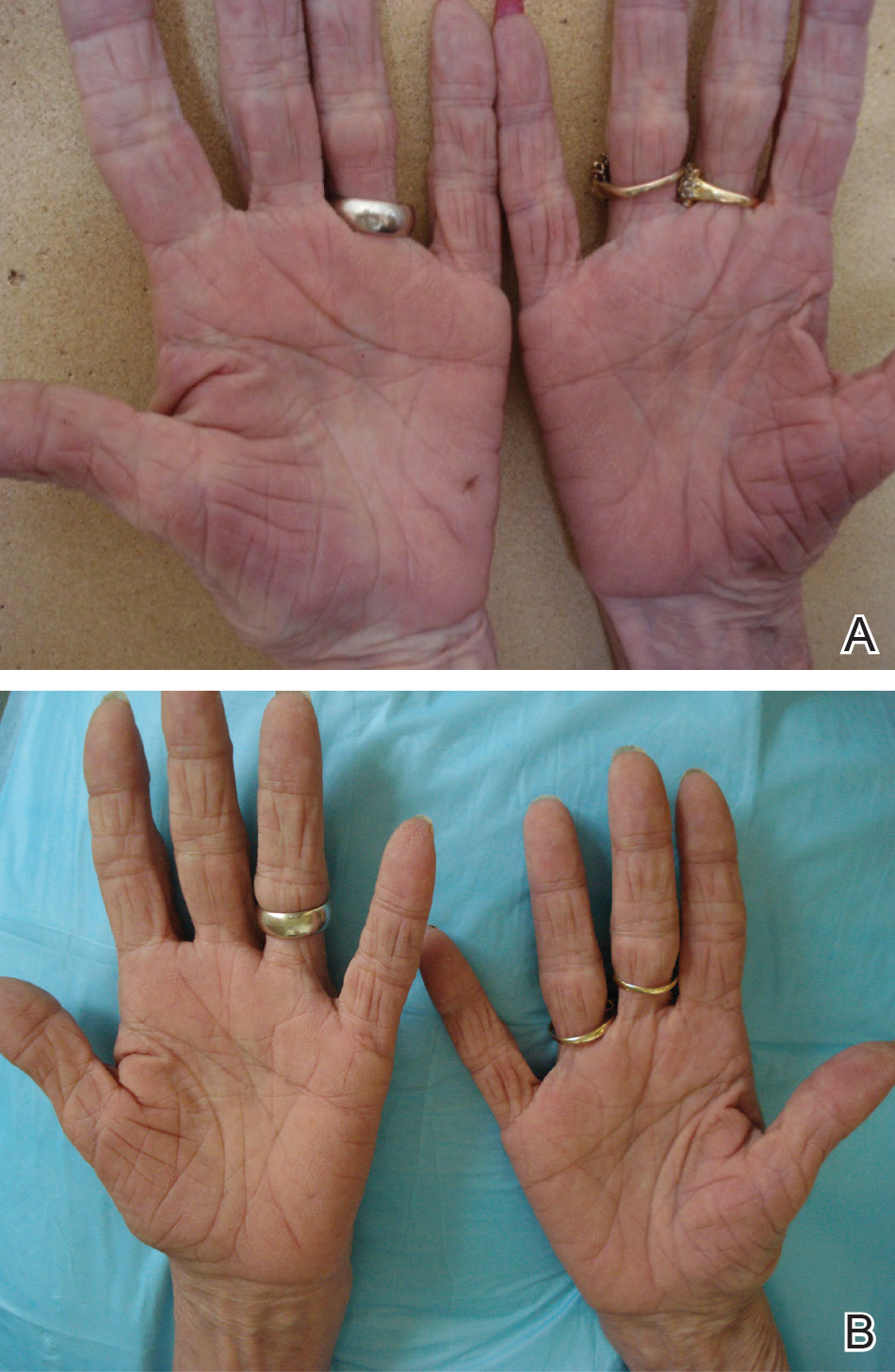
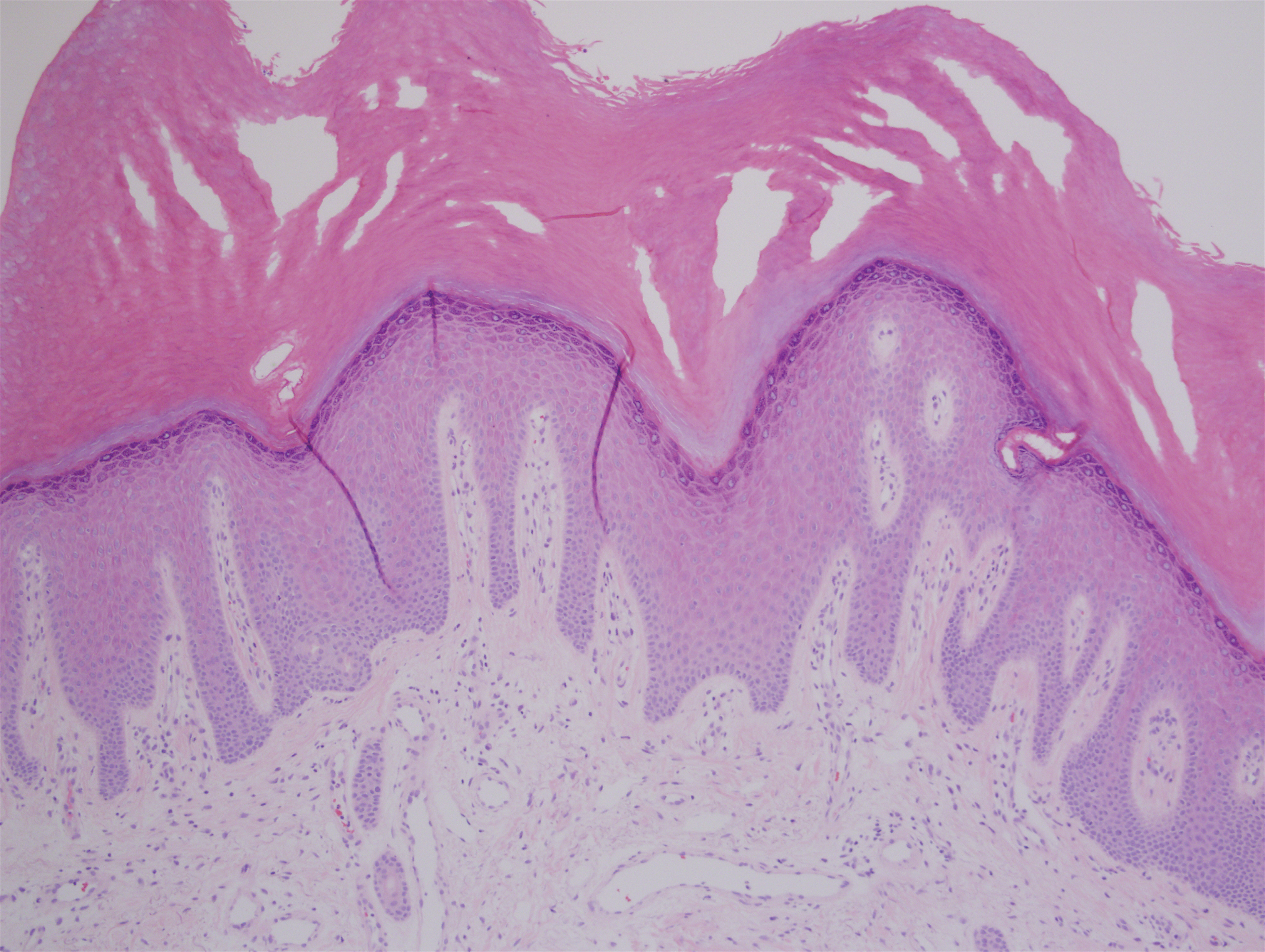
A punch biopsy of the medial hypothenar eminence of the left hand was performed, revealing notable lichenified hyperkeratosis with vascular ectasia (Figure 2). Periodic acid–Schiff staining was negative for fungal elements. Given the suspicion of PPK, multiple carcinoma markers were ordered. Cancer antigen 125 measured at 68 U/mL (reference range upper limit, 21 U/mL). Cancer antigen 27-29 was 50 U/mL (reference range, <38 U/mL) and cancer antigen 19-9 was 24 U/mL (reference range, <37 U/mL). Computed tomography of the chest revealed a large mass in the left lower lung associated with hilar lymphadenopathy. The patient was referred to oncology for further evaluation. Computed tomography–guided biopsy revealed metastatic uterine adenocarcinoma, which prompted subsequent chemotherapy. The combination of visceral malignancy with PPK led to the diagnosis of acquired PPK secondary to uterine cancer. After the completion of chemotherapy, the palmar dermatosis notably decreased (Figure 1B).
Comment
Paraneoplastic PPK is not uncommon. Ninety percent of acquired diffuse PPK is secondary to cancer,2 which occurs more frequently in male patients. Associated visceral malignancies include localized esophageal,3 myeloma,4 pulmonary, urinary/bladder,5 and gastric carcinoma.6 Paraneoplastic PPK in women is rare but has been linked to ovarian and breast carcinoma.7
The findings under light microscopy include thickening of any or all of the cell layers of the epidermis, which can include hyperkeratosis, acanthosis, and papillomatosis (Figure 2). A moderate amount of mononuclear cell infiltrates also can be visualized.
Palmoplantar keratoderma associated with uterine malignancy is rare. However, many other paraneoplastic dermatoses resulting from uterine cancer have been described as well as nonuterine gynecological malignancies (Table).8-17
The first step in managing acquired PPK is to determine its etiology via a complete history and a total-body skin examination. If findings are consistent with a hereditary PPK, then genetic workup is advised. Other suspected etiologies should be investigated via imaging and laboratory analysis.18
The first approach in managing acquired PPK is to treat the underlying cause. In prior cases, complete resolution of skin findings resulted once the malignancy or associated dermatosis had been treated.8-17 Adjunctive medication includes topical keratolytics (eg, urea, salicylic acid, lactic acid), topical retinoids, topical psoralen plus UVA, and topical corticosteroids.18 Vitamin A analogues have been found to be an effective treatment of many hyperkeratotic dermatoses.19 Isotretinoin and etretinate have been used to treat the cutaneous findings and prevent the onset and progression of esophageal malignancy of the inherited forms of PPK. The oral retinoid acitretin has been shown to rapidly resolve lesions, have persistent effects after 5 months of cessation, and have minimal side effects. Thus, it has been suggested as the first-line treatment of chronic PPK.19 One study found no response to topical keratolytics (urea cream and salicylic acid ointment) and a 2-week course of oral prednisone; however, low-dose oral acitretin 10 mg once daily resulted in notable improvement over several weeks.7 Physical debridement also may be necessary.18
Conclusion
Palmoplantar keratoderma is a condition that presents with hyperkeratosis of the palms and soles. Acquired PPK often occurs as a paraneoplastic response as well as a stigma of other dermatoses. It occurs more frequently in male patients. Reports of PPK secondary to uterine cancer are not common in the literature. Management of PPK includes a complete history and total-body skin examination. After appropriate imaging and laboratory analysis, treatment of the underlying cause is the best approach. Adjunctive medications include topical keratolytics, topical retinoids, topical psoralen plus UVA, and topical corticosteroids. Oral isotretinoin and etretinate have demonstrated promising results.
- Zamiri M, van Steensel MA, Munro CS. Inherited palmoplantar keratodermas. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:538-548.
- Cohen PR, Grossman ME, Silvers DN. Tripe palms and cancer. Clin Dermatol. 1993;11:165-173.
- Belmar P, Marquet A, Martín-Sáez E. Symmetric palmar hyperkeratosis and esophageal carcinoma [in Spanish]. Actas Dermosifiliogr. 2008;99:149-150.
- Smith CH, Barker JN, Hay RJ. Diffuse plane xanthomatosis and acquired palmoplantar keratoderma in association with myeloma. Br J Dermatol. 1995;132:286-289.
- Küchmeister B, Rasokat H. Acquired disseminated papulous palmar keratoses—a paraneoplastic syndrome in cancers of the urinary bladder and lung? [in German]. Z Hautkr. 1984;59:1123-1124.
- Stieler K, Blume-Peytavi U, Vogel A, et al. Hyperkeratoses as paraneoplastic syndrome [published online June 1, 2012]. J Dtsch Dermatol Ges. 2012;10:593-595.
- Vignale RA, Espasandín J, Paciel J, et al. Diagnostic value of keratosis palmaris as indicative sign of visceral cancer [in Spanish]. Med Cutan Ibero Lat Am. 1983;11:287-292.
- Blanchet-Bardon C, Nazzaro V, Chevrant-Breton J, et al. Hereditary epidermolytic palmoplantar keratoderma associated with breast and ovarian cancer in a large kindred. Br J Dermatol. 1987;117:363-370.
- Champion GD, Saxon JA, Kossard S. The syndrome of palmar fibromatosis (fasciitis) and polyarthritis. J Rheumatol. 1987;14:1196-1198.
- Requena L, Aguilar A, Renedo G, et al. Tripe palms: a cutaneous marker of internal malignancy. J Dermatol. 1995;22:492-495.
- Mahler V, Neureiter D, Kirchner T, et al. Digital ischemia as paraneoplastic marker of metastatic endometrial carcinoma [in German]. Hautarzt. 1999;50:748-752.
- Docquier Ch, Majois F, Mitine C. Palmar fasciitis and arthritis: association with endometrial adenocarcinoma. Clin Rheumatol. 2002;21:63-65.
- Shimizu Y, Uchiyama S, Mori G, et al. A young patient with endometrioid adenocarcinoma who suffered Trousseau’s syndrome associated with vasculitis [in Japanese]. Rinsho Shinkeigaku. 2002;42:227-232.
- Chandiramani M, Joynson C, Panchal R, et al. Dermatomyositis as a paraneoplastic syndrome in carcinosarcoma of uterine origin. Clin Oncol (R Coll Radiol). 2006;18:641-648.
- Kebria MM, Belinson J, Kim R, et al. Malignant acanthosis nigricans, tripe palms and the sign of Leser-Trélat, a hint to the diagnosis of early stage ovarian cancer: a case report and review of the literature [published online January 27, 2006]. Gynecol Oncol. 2006;101:353-355.
- Valverde R, Sánchez-Caminero MP, Calzado L, et al. Dermatomyositis and punctate porokeratotic keratoderma as paraneoplastic syndrome of ovarian carcinoma [in Spanish]. Actas Dermosifiliogr. 2007;98:358-360.
- Abakka S, Elhalouat H, Khoummane N, et al. Uterine leiomyosarcoma and Leser-Trélat sign. Lancet. 2013;381:88.
- Patel S, Zirwas M, English JC 3rd. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Capella GL, Fracchiolla C, Frigerio E, et al. A controlled study of comparative efficacy of oral retinoids and topical betamethasone/salicylic acid for chronic hyperkeratotic palmoplantar dermatitis. J Dermatolog Treat. 2004;15:88-93.
Paraneoplastic palmoplantar keratoderma (PPK) is an acquired dermatosis that presents with hyperkeratosis of the palms and soles in association with visceral malignancies, such as esophageal, gastric, pulmonary, and bladder carcinomas. This condition may either be acquired or inherited.1
Case Report
A 72-year-old woman was referred to our dermatology clinic for evaluation of a nonpruritic hyperkeratotic eruption predominantly on the palms and soles of 2 to 3 months’ duration (Figure 1A). Review of systems was remarkable for chronic anxiety, unintentional weight loss of 10 lb over the last 6 months, and a mild cough of 10 days’ duration. The differential diagnosis included eczematous dermatitis, tinea manuum, new-onset palmoplantar psoriasis, and PPK.


A punch biopsy of the medial hypothenar eminence of the left hand was performed, revealing notable lichenified hyperkeratosis with vascular ectasia (Figure 2). Periodic acid–Schiff staining was negative for fungal elements. Given the suspicion of PPK, multiple carcinoma markers were ordered. Cancer antigen 125 measured at 68 U/mL (reference range upper limit, 21 U/mL). Cancer antigen 27-29 was 50 U/mL (reference range, <38 U/mL) and cancer antigen 19-9 was 24 U/mL (reference range, <37 U/mL). Computed tomography of the chest revealed a large mass in the left lower lung associated with hilar lymphadenopathy. The patient was referred to oncology for further evaluation. Computed tomography–guided biopsy revealed metastatic uterine adenocarcinoma, which prompted subsequent chemotherapy. The combination of visceral malignancy with PPK led to the diagnosis of acquired PPK secondary to uterine cancer. After the completion of chemotherapy, the palmar dermatosis notably decreased (Figure 1B).
Comment
Paraneoplastic PPK is not uncommon. Ninety percent of acquired diffuse PPK is secondary to cancer,2 which occurs more frequently in male patients. Associated visceral malignancies include localized esophageal,3 myeloma,4 pulmonary, urinary/bladder,5 and gastric carcinoma.6 Paraneoplastic PPK in women is rare but has been linked to ovarian and breast carcinoma.7
The findings under light microscopy include thickening of any or all of the cell layers of the epidermis, which can include hyperkeratosis, acanthosis, and papillomatosis (Figure 2). A moderate amount of mononuclear cell infiltrates also can be visualized.
Palmoplantar keratoderma associated with uterine malignancy is rare. However, many other paraneoplastic dermatoses resulting from uterine cancer have been described as well as nonuterine gynecological malignancies (Table).8-17

The first step in managing acquired PPK is to determine its etiology via a complete history and a total-body skin examination. If findings are consistent with a hereditary PPK, then genetic workup is advised. Other suspected etiologies should be investigated via imaging and laboratory analysis.18
The first approach in managing acquired PPK is to treat the underlying cause. In prior cases, complete resolution of skin findings resulted once the malignancy or associated dermatosis had been treated.8-17 Adjunctive medication includes topical keratolytics (eg, urea, salicylic acid, lactic acid), topical retinoids, topical psoralen plus UVA, and topical corticosteroids.18 Vitamin A analogues have been found to be an effective treatment of many hyperkeratotic dermatoses.19 Isotretinoin and etretinate have been used to treat the cutaneous findings and prevent the onset and progression of esophageal malignancy of the inherited forms of PPK. The oral retinoid acitretin has been shown to rapidly resolve lesions, have persistent effects after 5 months of cessation, and have minimal side effects. Thus, it has been suggested as the first-line treatment of chronic PPK.19 One study found no response to topical keratolytics (urea cream and salicylic acid ointment) and a 2-week course of oral prednisone; however, low-dose oral acitretin 10 mg once daily resulted in notable improvement over several weeks.7 Physical debridement also may be necessary.18
Conclusion
Palmoplantar keratoderma is a condition that presents with hyperkeratosis of the palms and soles. Acquired PPK often occurs as a paraneoplastic response as well as a stigma of other dermatoses. It occurs more frequently in male patients. Reports of PPK secondary to uterine cancer are not common in the literature. Management of PPK includes a complete history and total-body skin examination. After appropriate imaging and laboratory analysis, treatment of the underlying cause is the best approach. Adjunctive medications include topical keratolytics, topical retinoids, topical psoralen plus UVA, and topical corticosteroids. Oral isotretinoin and etretinate have demonstrated promising results.
Paraneoplastic palmoplantar keratoderma (PPK) is an acquired dermatosis that presents with hyperkeratosis of the palms and soles in association with visceral malignancies, such as esophageal, gastric, pulmonary, and bladder carcinomas. This condition may either be acquired or inherited.1
Case Report
A 72-year-old woman was referred to our dermatology clinic for evaluation of a nonpruritic hyperkeratotic eruption predominantly on the palms and soles of 2 to 3 months’ duration (Figure 1A). Review of systems was remarkable for chronic anxiety, unintentional weight loss of 10 lb over the last 6 months, and a mild cough of 10 days’ duration. The differential diagnosis included eczematous dermatitis, tinea manuum, new-onset palmoplantar psoriasis, and PPK.


A punch biopsy of the medial hypothenar eminence of the left hand was performed, revealing notable lichenified hyperkeratosis with vascular ectasia (Figure 2). Periodic acid–Schiff staining was negative for fungal elements. Given the suspicion of PPK, multiple carcinoma markers were ordered. Cancer antigen 125 measured at 68 U/mL (reference range upper limit, 21 U/mL). Cancer antigen 27-29 was 50 U/mL (reference range, <38 U/mL) and cancer antigen 19-9 was 24 U/mL (reference range, <37 U/mL). Computed tomography of the chest revealed a large mass in the left lower lung associated with hilar lymphadenopathy. The patient was referred to oncology for further evaluation. Computed tomography–guided biopsy revealed metastatic uterine adenocarcinoma, which prompted subsequent chemotherapy. The combination of visceral malignancy with PPK led to the diagnosis of acquired PPK secondary to uterine cancer. After the completion of chemotherapy, the palmar dermatosis notably decreased (Figure 1B).
Comment
Paraneoplastic PPK is not uncommon. Ninety percent of acquired diffuse PPK is secondary to cancer,2 which occurs more frequently in male patients. Associated visceral malignancies include localized esophageal,3 myeloma,4 pulmonary, urinary/bladder,5 and gastric carcinoma.6 Paraneoplastic PPK in women is rare but has been linked to ovarian and breast carcinoma.7
The findings under light microscopy include thickening of any or all of the cell layers of the epidermis, which can include hyperkeratosis, acanthosis, and papillomatosis (Figure 2). A moderate amount of mononuclear cell infiltrates also can be visualized.
Palmoplantar keratoderma associated with uterine malignancy is rare. However, many other paraneoplastic dermatoses resulting from uterine cancer have been described as well as nonuterine gynecological malignancies (Table).8-17

The first step in managing acquired PPK is to determine its etiology via a complete history and a total-body skin examination. If findings are consistent with a hereditary PPK, then genetic workup is advised. Other suspected etiologies should be investigated via imaging and laboratory analysis.18
The first approach in managing acquired PPK is to treat the underlying cause. In prior cases, complete resolution of skin findings resulted once the malignancy or associated dermatosis had been treated.8-17 Adjunctive medication includes topical keratolytics (eg, urea, salicylic acid, lactic acid), topical retinoids, topical psoralen plus UVA, and topical corticosteroids.18 Vitamin A analogues have been found to be an effective treatment of many hyperkeratotic dermatoses.19 Isotretinoin and etretinate have been used to treat the cutaneous findings and prevent the onset and progression of esophageal malignancy of the inherited forms of PPK. The oral retinoid acitretin has been shown to rapidly resolve lesions, have persistent effects after 5 months of cessation, and have minimal side effects. Thus, it has been suggested as the first-line treatment of chronic PPK.19 One study found no response to topical keratolytics (urea cream and salicylic acid ointment) and a 2-week course of oral prednisone; however, low-dose oral acitretin 10 mg once daily resulted in notable improvement over several weeks.7 Physical debridement also may be necessary.18
Conclusion
Palmoplantar keratoderma is a condition that presents with hyperkeratosis of the palms and soles. Acquired PPK often occurs as a paraneoplastic response as well as a stigma of other dermatoses. It occurs more frequently in male patients. Reports of PPK secondary to uterine cancer are not common in the literature. Management of PPK includes a complete history and total-body skin examination. After appropriate imaging and laboratory analysis, treatment of the underlying cause is the best approach. Adjunctive medications include topical keratolytics, topical retinoids, topical psoralen plus UVA, and topical corticosteroids. Oral isotretinoin and etretinate have demonstrated promising results.
- Zamiri M, van Steensel MA, Munro CS. Inherited palmoplantar keratodermas. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:538-548.
- Cohen PR, Grossman ME, Silvers DN. Tripe palms and cancer. Clin Dermatol. 1993;11:165-173.
- Belmar P, Marquet A, Martín-Sáez E. Symmetric palmar hyperkeratosis and esophageal carcinoma [in Spanish]. Actas Dermosifiliogr. 2008;99:149-150.
- Smith CH, Barker JN, Hay RJ. Diffuse plane xanthomatosis and acquired palmoplantar keratoderma in association with myeloma. Br J Dermatol. 1995;132:286-289.
- Küchmeister B, Rasokat H. Acquired disseminated papulous palmar keratoses—a paraneoplastic syndrome in cancers of the urinary bladder and lung? [in German]. Z Hautkr. 1984;59:1123-1124.
- Stieler K, Blume-Peytavi U, Vogel A, et al. Hyperkeratoses as paraneoplastic syndrome [published online June 1, 2012]. J Dtsch Dermatol Ges. 2012;10:593-595.
- Vignale RA, Espasandín J, Paciel J, et al. Diagnostic value of keratosis palmaris as indicative sign of visceral cancer [in Spanish]. Med Cutan Ibero Lat Am. 1983;11:287-292.
- Blanchet-Bardon C, Nazzaro V, Chevrant-Breton J, et al. Hereditary epidermolytic palmoplantar keratoderma associated with breast and ovarian cancer in a large kindred. Br J Dermatol. 1987;117:363-370.
- Champion GD, Saxon JA, Kossard S. The syndrome of palmar fibromatosis (fasciitis) and polyarthritis. J Rheumatol. 1987;14:1196-1198.
- Requena L, Aguilar A, Renedo G, et al. Tripe palms: a cutaneous marker of internal malignancy. J Dermatol. 1995;22:492-495.
- Mahler V, Neureiter D, Kirchner T, et al. Digital ischemia as paraneoplastic marker of metastatic endometrial carcinoma [in German]. Hautarzt. 1999;50:748-752.
- Docquier Ch, Majois F, Mitine C. Palmar fasciitis and arthritis: association with endometrial adenocarcinoma. Clin Rheumatol. 2002;21:63-65.
- Shimizu Y, Uchiyama S, Mori G, et al. A young patient with endometrioid adenocarcinoma who suffered Trousseau’s syndrome associated with vasculitis [in Japanese]. Rinsho Shinkeigaku. 2002;42:227-232.
- Chandiramani M, Joynson C, Panchal R, et al. Dermatomyositis as a paraneoplastic syndrome in carcinosarcoma of uterine origin. Clin Oncol (R Coll Radiol). 2006;18:641-648.
- Kebria MM, Belinson J, Kim R, et al. Malignant acanthosis nigricans, tripe palms and the sign of Leser-Trélat, a hint to the diagnosis of early stage ovarian cancer: a case report and review of the literature [published online January 27, 2006]. Gynecol Oncol. 2006;101:353-355.
- Valverde R, Sánchez-Caminero MP, Calzado L, et al. Dermatomyositis and punctate porokeratotic keratoderma as paraneoplastic syndrome of ovarian carcinoma [in Spanish]. Actas Dermosifiliogr. 2007;98:358-360.
- Abakka S, Elhalouat H, Khoummane N, et al. Uterine leiomyosarcoma and Leser-Trélat sign. Lancet. 2013;381:88.
- Patel S, Zirwas M, English JC 3rd. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Capella GL, Fracchiolla C, Frigerio E, et al. A controlled study of comparative efficacy of oral retinoids and topical betamethasone/salicylic acid for chronic hyperkeratotic palmoplantar dermatitis. J Dermatolog Treat. 2004;15:88-93.
- Zamiri M, van Steensel MA, Munro CS. Inherited palmoplantar keratodermas. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:538-548.
- Cohen PR, Grossman ME, Silvers DN. Tripe palms and cancer. Clin Dermatol. 1993;11:165-173.
- Belmar P, Marquet A, Martín-Sáez E. Symmetric palmar hyperkeratosis and esophageal carcinoma [in Spanish]. Actas Dermosifiliogr. 2008;99:149-150.
- Smith CH, Barker JN, Hay RJ. Diffuse plane xanthomatosis and acquired palmoplantar keratoderma in association with myeloma. Br J Dermatol. 1995;132:286-289.
- Küchmeister B, Rasokat H. Acquired disseminated papulous palmar keratoses—a paraneoplastic syndrome in cancers of the urinary bladder and lung? [in German]. Z Hautkr. 1984;59:1123-1124.
- Stieler K, Blume-Peytavi U, Vogel A, et al. Hyperkeratoses as paraneoplastic syndrome [published online June 1, 2012]. J Dtsch Dermatol Ges. 2012;10:593-595.
- Vignale RA, Espasandín J, Paciel J, et al. Diagnostic value of keratosis palmaris as indicative sign of visceral cancer [in Spanish]. Med Cutan Ibero Lat Am. 1983;11:287-292.
- Blanchet-Bardon C, Nazzaro V, Chevrant-Breton J, et al. Hereditary epidermolytic palmoplantar keratoderma associated with breast and ovarian cancer in a large kindred. Br J Dermatol. 1987;117:363-370.
- Champion GD, Saxon JA, Kossard S. The syndrome of palmar fibromatosis (fasciitis) and polyarthritis. J Rheumatol. 1987;14:1196-1198.
- Requena L, Aguilar A, Renedo G, et al. Tripe palms: a cutaneous marker of internal malignancy. J Dermatol. 1995;22:492-495.
- Mahler V, Neureiter D, Kirchner T, et al. Digital ischemia as paraneoplastic marker of metastatic endometrial carcinoma [in German]. Hautarzt. 1999;50:748-752.
- Docquier Ch, Majois F, Mitine C. Palmar fasciitis and arthritis: association with endometrial adenocarcinoma. Clin Rheumatol. 2002;21:63-65.
- Shimizu Y, Uchiyama S, Mori G, et al. A young patient with endometrioid adenocarcinoma who suffered Trousseau’s syndrome associated with vasculitis [in Japanese]. Rinsho Shinkeigaku. 2002;42:227-232.
- Chandiramani M, Joynson C, Panchal R, et al. Dermatomyositis as a paraneoplastic syndrome in carcinosarcoma of uterine origin. Clin Oncol (R Coll Radiol). 2006;18:641-648.
- Kebria MM, Belinson J, Kim R, et al. Malignant acanthosis nigricans, tripe palms and the sign of Leser-Trélat, a hint to the diagnosis of early stage ovarian cancer: a case report and review of the literature [published online January 27, 2006]. Gynecol Oncol. 2006;101:353-355.
- Valverde R, Sánchez-Caminero MP, Calzado L, et al. Dermatomyositis and punctate porokeratotic keratoderma as paraneoplastic syndrome of ovarian carcinoma [in Spanish]. Actas Dermosifiliogr. 2007;98:358-360.
- Abakka S, Elhalouat H, Khoummane N, et al. Uterine leiomyosarcoma and Leser-Trélat sign. Lancet. 2013;381:88.
- Patel S, Zirwas M, English JC 3rd. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Capella GL, Fracchiolla C, Frigerio E, et al. A controlled study of comparative efficacy of oral retinoids and topical betamethasone/salicylic acid for chronic hyperkeratotic palmoplantar dermatitis. J Dermatolog Treat. 2004;15:88-93.
Practice Points
- Paraneoplastic palmoplantar keratoderma (PPK) is an acquired dermatosis that presents with hyperkeratosis of the palms and soles in association with visceral malignancies (eg, esophageal, gastric, pulmonary, and urinary/bladder carcinomas).
- Palmoplantar keratoderma secondary to uterine cancer is rare.
- Light microscopy shows thickening of any or all of the cell layers of the epidermis (hyperkeratosis, acanthosis, and papillomatosis) and mononuclear cells.
- Management of acquired PPK includes treatment of the underlying malignancy. Adjunctive vitamin A analogues may be of additional utility.
Removal of the Distal Aspect of a Broken Tibial Nail
Take-Home Points
- Nail breakage is a known complication of intramedullary nail (IMN) fixation of tibial fractures.
- Several techniques have been described for broken IMN extraction.
Intramedullary nail (IMN) fixation is reliably used to manage tibial fractures and has become very popular for managing fractures of varying complexity.1-4 An occasional complication of intramedullary nailing is nail breakage,5-7 which can result from a fatigue fracture (from excessive fracture site instability caused by inadequate nail diameter, delayed fracture healing, or fracture nonunion) and direct traumatic impact.5-7 Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from tibias and femurs.4,8-16 In this article, we describe an efficient technique for extracting broken tibial IMNs—a technique that can be used before attempting more invasive extraction methods. The patient provided written informed consent for print and electronic publication of this case report.
Case Report and Surgical Technique
A 34-year-old male logger presented to our facility (Department of Orthopaedics, Warren Alpert School of Medicine, Brown University) with a new fracture of the left tibia and fibula with an associated broken IMN after a tree fell on his leg at work (Figures 1A, 1B).
The original IMN had been placed through a paramedian incision, with lateral to medial distal locking screws. The tibial shaft fracture and broken nail were displaced in the coronal plane (Figures 1A, 1B). For restoration of the central canal of the nail, closed reduction was performed in the operating room (Figure 2A). Once the fracture was reduced, the more proximal of the 2 distal interlocking screws was partially backed out so the extraction hook could be passed antegrade into the distal segment of the nail (Figure 2A).
A ball-tipped guide wire was then passed down again, and reaming was carried out distally to 11.5 mm. A new tibial nail (10 mm × 315 mm) was placed down the intramedullary canal over the guide wire. The tibia was derotated to obtain better anatomical alignment using the fracture as an osteotomy, and 2 new distal interlocking screws were placed. The nail was then back-slapped to obtain impaction, and a single proximal dynamic interlocking screw was placed.
After surgery, the patient was allowed a gradual weight-bearing protocol.
Discussion
IMN fixation of tibial fractures is reliable.1-4 An occasional complication of intramedullary nailing is nail breakage. Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from knees and femurs.4,8-16
Our patient’s case involved a cannulated tibial IMN that broke secondary to an acute traumatic event. Several techniques have been used to remove the distal segment of broken cannulated tibial IMNs.8,9,14,17 Abdelgawad and Kanlic8 described a technique in which a small distractor hook was introduced past the distal end of the broken distal piece, and a small (~2 in) piece of flexible nail was introduced into the slot of the distal interlocking screw hole. The hook was pulled back and became incarcerated in the nail by the flexible nail piece, allowing the hook to extract the distal segment of the nail.
Charnley and Farrington9 used Petelin laparoscopic grasping forceps to extract the distal segment of a broken cannulated tibial IMN under fluoroscopic guidance. This tibial canal was initially reamed before inserting the instrument and removing the distal segment of the nail.
Levine and Georgiadis14 used a 4.5-mm bit to drill a hole in the distal aspect of the medial malleolus. A smooth Steinmann pin was used to engage the tip of the IMN. The nail was hammered several centimeters up the medullary canal of the tibia. A 3.0-mm ball-tipped guide wire was inserted in the hole in the medial malleolus and advanced through the distal aspect of the nail under fluoroscopic guidance. The guide wire was advanced through the extent of the nail proximally until it emerged through the knee incision. The distal segment of the broken nail was extracted with the guide wire; the end of the guide wire with the ball engaged the distal aspect of the nail.
Our technique allowed us to use a nail extraction device to extract the distal segment of a broken tibial IMN. This device is usually on hand for routine nail extraction. We used the more distal of the 2 distal interlocking screws to push the extraction hook over the distal lip of the nail, allowing for extraction without additional incisions or additional drill holes in bone. Our technique was efficient in this particular situation and avoided more time-consuming extraction methods. In cases in which the extraction hook does not engage the distal aspect of the nail secondary to bone ingrowth, our technique should be used before attempting other extraction methods.
Am J Orthop. 2017;46(2):E112-E115. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bone LB, Kassman S, Stegemann P, France J. Prospective study of union rate of open tibial fractures treated with locked, unreamed intramedullary nails. J Orthop Trauma. 1994;8(1):45-49.
2. Blachut PA, O’Brien PJ, Meek RN, Broekhuyse HM. Interlocking intramedullary nailing with and without reaming for the treatment of closed fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg Am. 1997;79(5):640-646.
3. Bonnevialle P, Savorit L, Combes JM, Rongières M, Bellumore Y, Mansat M. Value of intramedullary locked nailing in distal fractures of the tibia [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1996;82(5):428-436.
4. Polat A, Kose O, Canbora K, Yanık S, Guler F. Intramedullary nailing versus minimally invasive plate osteosynthesis for distal extra-articular tibial fractures: a prospective randomized clinical trial. J Orthop Sci. 2015;20(4):695-701.
5. Bucholz RW, Ross SE, Lawrence KL. Fatigue fracture of the interlocking nail in the treatment of fractures of the distal part of the femoral shaft. J Bone Joint Surg Am. 1987;69(9):1391-1399.
6. Zimmerman KW, Klasen HJ. Mechanical failure of intramedullary nails after fracture union. J Bone Joint Surg Br. 1983;65(3):274-275.
7. Hahn D, Bradbury N, Hartley R, Radford PJ. Intramedullary nail breakage in distal fractures of the tibia. Injury. 1996;27(5):323-327.
8. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
9. Charnley GJ, Farrington WJ. Laparoscopic forceps removal of a broken tibial intramedullary nail. Injury. 1998;29(6):489-490.
10. Georgilas I, Mouzopoulos G, Neila C, Morakis E, Tzurbakis M. Removal of broken distal intramedullary nail with a simple method: a case report. Arch Orthop Trauma Surg. 2008;129(2):203-205.
11. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
12. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
13. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
14. Levine JW, Georgiadis GM. Removal of a broken cannulated tibial nail: a simple intramedullary technique. J Orthop Trauma. 2004;18(4):247-249.
15. Schmidgen A, Naumann O, Wentzensen A. A simple and rapid method for removal of broken unreamed tibial nails [in German]. Unfallchirurg. 1999;102(12):975-978.
16. Steinberg EL, Luger E, Menahem A, Helfet DL. Removal of a broken distal closed section intramedullary nail: report of a case using a simple method. J Orthop Trauma. 2004;18(4):233-235.
17. Marwan M, Ibrahim M. Simple method for retrieval of distal segment of the broken interlocking intramedullary nail. Injury. 1999;30(5):333-335.
Take-Home Points
- Nail breakage is a known complication of intramedullary nail (IMN) fixation of tibial fractures.
- Several techniques have been described for broken IMN extraction.
Intramedullary nail (IMN) fixation is reliably used to manage tibial fractures and has become very popular for managing fractures of varying complexity.1-4 An occasional complication of intramedullary nailing is nail breakage,5-7 which can result from a fatigue fracture (from excessive fracture site instability caused by inadequate nail diameter, delayed fracture healing, or fracture nonunion) and direct traumatic impact.5-7 Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from tibias and femurs.4,8-16 In this article, we describe an efficient technique for extracting broken tibial IMNs—a technique that can be used before attempting more invasive extraction methods. The patient provided written informed consent for print and electronic publication of this case report.
Case Report and Surgical Technique
A 34-year-old male logger presented to our facility (Department of Orthopaedics, Warren Alpert School of Medicine, Brown University) with a new fracture of the left tibia and fibula with an associated broken IMN after a tree fell on his leg at work (Figures 1A, 1B).
The original IMN had been placed through a paramedian incision, with lateral to medial distal locking screws. The tibial shaft fracture and broken nail were displaced in the coronal plane (Figures 1A, 1B). For restoration of the central canal of the nail, closed reduction was performed in the operating room (Figure 2A). Once the fracture was reduced, the more proximal of the 2 distal interlocking screws was partially backed out so the extraction hook could be passed antegrade into the distal segment of the nail (Figure 2A).
A ball-tipped guide wire was then passed down again, and reaming was carried out distally to 11.5 mm. A new tibial nail (10 mm × 315 mm) was placed down the intramedullary canal over the guide wire. The tibia was derotated to obtain better anatomical alignment using the fracture as an osteotomy, and 2 new distal interlocking screws were placed. The nail was then back-slapped to obtain impaction, and a single proximal dynamic interlocking screw was placed.
After surgery, the patient was allowed a gradual weight-bearing protocol.
Discussion
IMN fixation of tibial fractures is reliable.1-4 An occasional complication of intramedullary nailing is nail breakage. Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from knees and femurs.4,8-16
Our patient’s case involved a cannulated tibial IMN that broke secondary to an acute traumatic event. Several techniques have been used to remove the distal segment of broken cannulated tibial IMNs.8,9,14,17 Abdelgawad and Kanlic8 described a technique in which a small distractor hook was introduced past the distal end of the broken distal piece, and a small (~2 in) piece of flexible nail was introduced into the slot of the distal interlocking screw hole. The hook was pulled back and became incarcerated in the nail by the flexible nail piece, allowing the hook to extract the distal segment of the nail.
Charnley and Farrington9 used Petelin laparoscopic grasping forceps to extract the distal segment of a broken cannulated tibial IMN under fluoroscopic guidance. This tibial canal was initially reamed before inserting the instrument and removing the distal segment of the nail.
Levine and Georgiadis14 used a 4.5-mm bit to drill a hole in the distal aspect of the medial malleolus. A smooth Steinmann pin was used to engage the tip of the IMN. The nail was hammered several centimeters up the medullary canal of the tibia. A 3.0-mm ball-tipped guide wire was inserted in the hole in the medial malleolus and advanced through the distal aspect of the nail under fluoroscopic guidance. The guide wire was advanced through the extent of the nail proximally until it emerged through the knee incision. The distal segment of the broken nail was extracted with the guide wire; the end of the guide wire with the ball engaged the distal aspect of the nail.
Our technique allowed us to use a nail extraction device to extract the distal segment of a broken tibial IMN. This device is usually on hand for routine nail extraction. We used the more distal of the 2 distal interlocking screws to push the extraction hook over the distal lip of the nail, allowing for extraction without additional incisions or additional drill holes in bone. Our technique was efficient in this particular situation and avoided more time-consuming extraction methods. In cases in which the extraction hook does not engage the distal aspect of the nail secondary to bone ingrowth, our technique should be used before attempting other extraction methods.
Am J Orthop. 2017;46(2):E112-E115. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Nail breakage is a known complication of intramedullary nail (IMN) fixation of tibial fractures.
- Several techniques have been described for broken IMN extraction.
Intramedullary nail (IMN) fixation is reliably used to manage tibial fractures and has become very popular for managing fractures of varying complexity.1-4 An occasional complication of intramedullary nailing is nail breakage,5-7 which can result from a fatigue fracture (from excessive fracture site instability caused by inadequate nail diameter, delayed fracture healing, or fracture nonunion) and direct traumatic impact.5-7 Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from tibias and femurs.4,8-16 In this article, we describe an efficient technique for extracting broken tibial IMNs—a technique that can be used before attempting more invasive extraction methods. The patient provided written informed consent for print and electronic publication of this case report.
Case Report and Surgical Technique
A 34-year-old male logger presented to our facility (Department of Orthopaedics, Warren Alpert School of Medicine, Brown University) with a new fracture of the left tibia and fibula with an associated broken IMN after a tree fell on his leg at work (Figures 1A, 1B).
The original IMN had been placed through a paramedian incision, with lateral to medial distal locking screws. The tibial shaft fracture and broken nail were displaced in the coronal plane (Figures 1A, 1B). For restoration of the central canal of the nail, closed reduction was performed in the operating room (Figure 2A). Once the fracture was reduced, the more proximal of the 2 distal interlocking screws was partially backed out so the extraction hook could be passed antegrade into the distal segment of the nail (Figure 2A).
A ball-tipped guide wire was then passed down again, and reaming was carried out distally to 11.5 mm. A new tibial nail (10 mm × 315 mm) was placed down the intramedullary canal over the guide wire. The tibia was derotated to obtain better anatomical alignment using the fracture as an osteotomy, and 2 new distal interlocking screws were placed. The nail was then back-slapped to obtain impaction, and a single proximal dynamic interlocking screw was placed.
After surgery, the patient was allowed a gradual weight-bearing protocol.
Discussion
IMN fixation of tibial fractures is reliable.1-4 An occasional complication of intramedullary nailing is nail breakage. Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from knees and femurs.4,8-16
Our patient’s case involved a cannulated tibial IMN that broke secondary to an acute traumatic event. Several techniques have been used to remove the distal segment of broken cannulated tibial IMNs.8,9,14,17 Abdelgawad and Kanlic8 described a technique in which a small distractor hook was introduced past the distal end of the broken distal piece, and a small (~2 in) piece of flexible nail was introduced into the slot of the distal interlocking screw hole. The hook was pulled back and became incarcerated in the nail by the flexible nail piece, allowing the hook to extract the distal segment of the nail.
Charnley and Farrington9 used Petelin laparoscopic grasping forceps to extract the distal segment of a broken cannulated tibial IMN under fluoroscopic guidance. This tibial canal was initially reamed before inserting the instrument and removing the distal segment of the nail.
Levine and Georgiadis14 used a 4.5-mm bit to drill a hole in the distal aspect of the medial malleolus. A smooth Steinmann pin was used to engage the tip of the IMN. The nail was hammered several centimeters up the medullary canal of the tibia. A 3.0-mm ball-tipped guide wire was inserted in the hole in the medial malleolus and advanced through the distal aspect of the nail under fluoroscopic guidance. The guide wire was advanced through the extent of the nail proximally until it emerged through the knee incision. The distal segment of the broken nail was extracted with the guide wire; the end of the guide wire with the ball engaged the distal aspect of the nail.
Our technique allowed us to use a nail extraction device to extract the distal segment of a broken tibial IMN. This device is usually on hand for routine nail extraction. We used the more distal of the 2 distal interlocking screws to push the extraction hook over the distal lip of the nail, allowing for extraction without additional incisions or additional drill holes in bone. Our technique was efficient in this particular situation and avoided more time-consuming extraction methods. In cases in which the extraction hook does not engage the distal aspect of the nail secondary to bone ingrowth, our technique should be used before attempting other extraction methods.
Am J Orthop. 2017;46(2):E112-E115. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bone LB, Kassman S, Stegemann P, France J. Prospective study of union rate of open tibial fractures treated with locked, unreamed intramedullary nails. J Orthop Trauma. 1994;8(1):45-49.
2. Blachut PA, O’Brien PJ, Meek RN, Broekhuyse HM. Interlocking intramedullary nailing with and without reaming for the treatment of closed fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg Am. 1997;79(5):640-646.
3. Bonnevialle P, Savorit L, Combes JM, Rongières M, Bellumore Y, Mansat M. Value of intramedullary locked nailing in distal fractures of the tibia [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1996;82(5):428-436.
4. Polat A, Kose O, Canbora K, Yanık S, Guler F. Intramedullary nailing versus minimally invasive plate osteosynthesis for distal extra-articular tibial fractures: a prospective randomized clinical trial. J Orthop Sci. 2015;20(4):695-701.
5. Bucholz RW, Ross SE, Lawrence KL. Fatigue fracture of the interlocking nail in the treatment of fractures of the distal part of the femoral shaft. J Bone Joint Surg Am. 1987;69(9):1391-1399.
6. Zimmerman KW, Klasen HJ. Mechanical failure of intramedullary nails after fracture union. J Bone Joint Surg Br. 1983;65(3):274-275.
7. Hahn D, Bradbury N, Hartley R, Radford PJ. Intramedullary nail breakage in distal fractures of the tibia. Injury. 1996;27(5):323-327.
8. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
9. Charnley GJ, Farrington WJ. Laparoscopic forceps removal of a broken tibial intramedullary nail. Injury. 1998;29(6):489-490.
10. Georgilas I, Mouzopoulos G, Neila C, Morakis E, Tzurbakis M. Removal of broken distal intramedullary nail with a simple method: a case report. Arch Orthop Trauma Surg. 2008;129(2):203-205.
11. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
12. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
13. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
14. Levine JW, Georgiadis GM. Removal of a broken cannulated tibial nail: a simple intramedullary technique. J Orthop Trauma. 2004;18(4):247-249.
15. Schmidgen A, Naumann O, Wentzensen A. A simple and rapid method for removal of broken unreamed tibial nails [in German]. Unfallchirurg. 1999;102(12):975-978.
16. Steinberg EL, Luger E, Menahem A, Helfet DL. Removal of a broken distal closed section intramedullary nail: report of a case using a simple method. J Orthop Trauma. 2004;18(4):233-235.
17. Marwan M, Ibrahim M. Simple method for retrieval of distal segment of the broken interlocking intramedullary nail. Injury. 1999;30(5):333-335.
1. Bone LB, Kassman S, Stegemann P, France J. Prospective study of union rate of open tibial fractures treated with locked, unreamed intramedullary nails. J Orthop Trauma. 1994;8(1):45-49.
2. Blachut PA, O’Brien PJ, Meek RN, Broekhuyse HM. Interlocking intramedullary nailing with and without reaming for the treatment of closed fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg Am. 1997;79(5):640-646.
3. Bonnevialle P, Savorit L, Combes JM, Rongières M, Bellumore Y, Mansat M. Value of intramedullary locked nailing in distal fractures of the tibia [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1996;82(5):428-436.
4. Polat A, Kose O, Canbora K, Yanık S, Guler F. Intramedullary nailing versus minimally invasive plate osteosynthesis for distal extra-articular tibial fractures: a prospective randomized clinical trial. J Orthop Sci. 2015;20(4):695-701.
5. Bucholz RW, Ross SE, Lawrence KL. Fatigue fracture of the interlocking nail in the treatment of fractures of the distal part of the femoral shaft. J Bone Joint Surg Am. 1987;69(9):1391-1399.
6. Zimmerman KW, Klasen HJ. Mechanical failure of intramedullary nails after fracture union. J Bone Joint Surg Br. 1983;65(3):274-275.
7. Hahn D, Bradbury N, Hartley R, Radford PJ. Intramedullary nail breakage in distal fractures of the tibia. Injury. 1996;27(5):323-327.
8. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
9. Charnley GJ, Farrington WJ. Laparoscopic forceps removal of a broken tibial intramedullary nail. Injury. 1998;29(6):489-490.
10. Georgilas I, Mouzopoulos G, Neila C, Morakis E, Tzurbakis M. Removal of broken distal intramedullary nail with a simple method: a case report. Arch Orthop Trauma Surg. 2008;129(2):203-205.
11. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
12. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
13. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
14. Levine JW, Georgiadis GM. Removal of a broken cannulated tibial nail: a simple intramedullary technique. J Orthop Trauma. 2004;18(4):247-249.
15. Schmidgen A, Naumann O, Wentzensen A. A simple and rapid method for removal of broken unreamed tibial nails [in German]. Unfallchirurg. 1999;102(12):975-978.
16. Steinberg EL, Luger E, Menahem A, Helfet DL. Removal of a broken distal closed section intramedullary nail: report of a case using a simple method. J Orthop Trauma. 2004;18(4):233-235.
17. Marwan M, Ibrahim M. Simple method for retrieval of distal segment of the broken interlocking intramedullary nail. Injury. 1999;30(5):333-335.
Severe polyarthralgia, high-grade fever, diffuse maculopapular rash on trunk and extremities • Dx?
THE CASE
A 30-year-old woman presented to our emergency department with severe polyarthralgia, a high-grade fever (102.6º F), and a diffuse maculopapular rash on her trunk and extremities. She had returned from her honeymoon in Jamaica 6 days earlier. During her time there, she ate local cuisine, hiked in the jungle, and was bitten by many mosquitoes. The patient was nauseous, and had been experiencing headaches, generalized weakness, and fatigue. Her physical exam revealed a maculopapular rash on her trunk and upper extremities. She had tenderness and pain, as well as decreased range of motion in her ankles, knees, and wrists. The patient had no erythema, swelling, petechiae, or bruising. She had a past medical history of Graves’ disease and had received all of her childhood immunizations.
THE DIAGNOSIS
Our lab work-up included a complete blood count, liver function tests, blood pathogens for malaria, and serologic tests for dengue fever, measles, mumps, rubella, Lyme disease, human immunodeficiency virus (HIV), and parvovirus. We ruled out dengue fever because the patient had no evidence of hemorrhage, thrombocytopenia, rising hematocrit, or neutropenia. HIV antibody screening tests were performed, although, in retrospect, confirmatory HIV quantitative RNA testing should’ve been obtained because of the acute nature of the patient’s symptoms. Regardless, the work-up was negative, with the exception of a positive parvovirus immunoglobulin G (IgG).
Given our patient’s travel history, unremarkable lab results, and physical exam (notably the rash and joint pain), we suspected that she was infected with the chikungunya virus and tested for it. The results of chikungunya serum titers returned 13 days later and were positive for both immunoglobulin M (IgM) and IgG, confirming our suspicion.
DISCUSSION
Chikungunya is a viral infection that is most commonly transmitted to humans via mosquitoes. The infection was first identified in West Africa in the mid-1900s and predominantly occurs in tropical and subtropical regions due to the numbers of mosquitoes in those areas. Since 2000, outbreaks have been most common in Africa and Asia, with the largest outbreaks occurring from 2005 to 2006.1,2 Chikungunya has since become more widespread in the Indian Ocean islands, and in 2013, it was first identified in the Caribbean islands. As of March 2017, over one million cases have been reported in the Americas, according to the Pan American Health Organization.3 In 2014, 12 locally transmitted cases were reported in Florida.4
The most common mosquito vectors for chikungunya are the Aedes albopictus (Asian tiger mosquito) and Aedes aegypti mosquitoes. These mosquitoes also transmit dengue fever, yellow fever, and Zika virus. Both of these mosquitoes are well-adapted to urban areas and can breed in standing water. A aegypti mosquitoes are found only in the southern United States, while A albopictus mosquitoes can be found in more temperate climates—areas like New York, New Jersey, and Pennsylvania.4 Both species are daytime biters, so their activity peaks during the dawn and dusk periods. Because local mosquito vectors exist as far north as New York, local transmission and outbreaks are possible in many parts of the United States.
When to suspect chikungunya, and what to look for
Suspect chikungunya in patients returning from endemic areas. After a 3- to 7-day incubation period, the clinical presentation of chikungunya typically begins with fever and malaise, followed by polyarthralgia that starts 2 to 5 days after the onset of fever. Headache, nausea, and conjunctivitis may also occur. Polyarthralgia and arthritis usually present in symmetrical distal joints and are accompanied by face and trunk flushing that is followed by a maculopapular rash. The rash predominantly appears on the trunk and limbs, but can also occur on the face, palms, and soles. Tendons and ligaments—especially the Achilles tendon—may become inflamed, as well. Symptoms typically resolve within 2 to 3 weeks, although polyarthralgia may last for months or even years.
There is no known risk of transmission through breast milk or in utero, although vertical transmission through vaginal or cesarean birth is common in viremic women. Blood-borne transmission can occur in the laboratory, nosocomially, or through the transfusion of blood products if exposure occurs during the early viremic phase.1,2 Dual infections are possible (typically with yellow fever, malaria, Zika virus, or dengue fever) and should be considered based on the patient’s travel history.5 Abnormal laboratory findings are less common, but may include lymphopenia (most common), thrombocytopenia, elevations in blood urea nitrogen and creatinine (indicating an acute kidney injury), and elevated liver transaminases.
Chikungunya is generally considered a self-limiting disease, but severe atypical manifestations can lead to meningoencephalitis, respiratory failure, and even death. Severe disease is more commonly seen in infants, patients over age 65 years, and in those with chronic medical conditions.
The differential diagnosis for chikungunya virus includes dengue fever, Zika virus, malaria, measles, rubella, parvovirus, primary HIV infection, Lyme disease, and other inflammatory joint conditions. The differential depends on where a patient lives, their travel history, and exposures.
Dengue and chikungunya have similar features, which often make them difficult to distinguish. However, patients with dengue fever present more often with neutropenia, thrombocytopenia, and signs or symptoms of shock or hemorrhage.6 The chikungunya rash is typically a maculopapular rash on the trunk, hands, and feet that appears within the first 2 days of illness, as opposed to dengue, which has a similar rash, but appears later in the disease (Days 2-5). Avoid nonsteroidal anti-inflammatory drugs (NSAIDs) if dengue fever is suspected, as they can worsen hemorrhaging.7
Zika virus typically presents with milder symptoms compared to chikungunya. Patients may have a skin rash and occasionally, conjunctivitis, but limited high fevers or joint pain. Zika rash is maculopapular, but typically starts on the face on the first day of the illness. Zika has been associated with neurological complications such as Guillain-Barré syndrome and microcephaly in fetuses of infected pregnant woman.8
Choice of testing modality depends on when symptoms began
Laboratory diagnosis of chikungunya can be accomplished 3 ways: viral culture, reverse transcriptase-polymerase chain reaction (RT-PCR) viral RNA, and serology.1 The choice of which modality to use depends on the time between the onset of symptoms and the date on which a serum sample is drawn.
- If the patient presents within the first 3 days of illness, viral culture can detect chikungunya. Chikungunya virus testing is available through the Centers for Disease Control and Prevention (CDC), some state laboratories, and one commercial lab.8 Viral cultures are considered the gold standard for diagnosis, but a requirement for an elevated biosafety level, as well as a longer incubation time, make them less useful in the clinical setting.1,9
- If symptoms started less than 5 to 8 days prior, serum should be sent for RT-PCR for viral RNA.
- If symptoms occurred more than 8 days prior, serum should be sent for IgM and IgG serologic testing.
If the acute sample was negative (and chikungunya is still suspected), a second serum sample should be drawn 2 to 3 weeks later during the convalescent phase and sent for IgG serologic testing.
There are no specific treatments or vaccines available for chikungunya, but both live and inactivated vaccines are being tested. To prevent chikungunya virus infection, advise patients traveling to endemic areas to reduce mosquito exposure by avoiding outdoor activities during dusk and dawn, wearing long-sleeved shirts and long pants, and using an insect repellent that contains DEET. Recommend that patients who will be sleeping in a high-risk area use bed netting treated with permethrin. The CDC Web site has excellent additional information, available at http://www.cdc.gov/chikungunya.10
Our patient was briefly hospitalized due to intractable pain. She was discharged after 2 days and given prescriptions for motrin 800 mg and percocet 5/325 mg for breakthrough pain. Her wrist and ankle pain persisted after discharge, but slowly resolved after 2 to 3 months.
THE TAKEAWAY
Chikungunya is becoming more common among travelers returning to the United States from the Caribbean islands, and mosquito vectors found in parts of the United States are enhancing the possibility of local outbreaks. Travel to endemic regions—and the classic symptoms of fever, polyarthralgia, and a maculopapular ras
1. Pan American Health Organization. Preparedness and response for chikungunya virus: Introduction in the Americas. Washington, DC: Pan American Health Organization; 2011:1-149. Available at: http://www1.paho.org/hq/dmdocuments/CHIKV_English.pdf. Accessed March 8, 2017.
2. Enserink M. Infectious diseases. Chikungunya: no longer a third world disease. Science. 2007;318:1860-1861.
3. Pan American Health Organization. Number of reported cases of chikungunya fever in the Americas, by country or territory, 2017. Available at: http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&Itemid=270&gid=38489&lang=en. Accessed March 7, 2017.
4. Centers for Disease Control and Prevention. 2014 final data for the United States. Centers for Disease Control and Prevention Web site. Available at: https://www.cdc.gov/chikungunya/geo/united-states-2014.html. Accessed March 16, 2017.
5. Nayar SK, Noridah O, Paranthaman V, et al. Co-infection of dengue virus and chikungunya virus in two patients with acute febrile illness. Med J Malaysia. 2007;62:335-336.
6. Staples JE, Fischer M. Chikungunya virus in the Americas—what a vectorborne pathogen can do. N Engl J Med. 2014;371:887-889.
7. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva: World Health Organization; 2009. Available at: http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf?ua=1. Accessed March 8, 2017.
8. Centers for Disease Control and Prevention. Chikungunya information for healthcare providers. 2014. Available at: https://www.cdc.gov/chikungunya/pdfs/chikv_clinicians.pdf. Accessed March 8, 2017.
9. Parida MM, Santhosh SR, Dash PK, et al. Rapid and real-time assays for detection and quantification of chikungunya virus. Future Virology. 2008;3:179-192.
10. Centers for Disease Control and Prevention. Chikungunya virus. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/chikungunya. Accessed February 26, 2015.
THE CASE
A 30-year-old woman presented to our emergency department with severe polyarthralgia, a high-grade fever (102.6º F), and a diffuse maculopapular rash on her trunk and extremities. She had returned from her honeymoon in Jamaica 6 days earlier. During her time there, she ate local cuisine, hiked in the jungle, and was bitten by many mosquitoes. The patient was nauseous, and had been experiencing headaches, generalized weakness, and fatigue. Her physical exam revealed a maculopapular rash on her trunk and upper extremities. She had tenderness and pain, as well as decreased range of motion in her ankles, knees, and wrists. The patient had no erythema, swelling, petechiae, or bruising. She had a past medical history of Graves’ disease and had received all of her childhood immunizations.
THE DIAGNOSIS
Our lab work-up included a complete blood count, liver function tests, blood pathogens for malaria, and serologic tests for dengue fever, measles, mumps, rubella, Lyme disease, human immunodeficiency virus (HIV), and parvovirus. We ruled out dengue fever because the patient had no evidence of hemorrhage, thrombocytopenia, rising hematocrit, or neutropenia. HIV antibody screening tests were performed, although, in retrospect, confirmatory HIV quantitative RNA testing should’ve been obtained because of the acute nature of the patient’s symptoms. Regardless, the work-up was negative, with the exception of a positive parvovirus immunoglobulin G (IgG).
Given our patient’s travel history, unremarkable lab results, and physical exam (notably the rash and joint pain), we suspected that she was infected with the chikungunya virus and tested for it. The results of chikungunya serum titers returned 13 days later and were positive for both immunoglobulin M (IgM) and IgG, confirming our suspicion.
DISCUSSION
Chikungunya is a viral infection that is most commonly transmitted to humans via mosquitoes. The infection was first identified in West Africa in the mid-1900s and predominantly occurs in tropical and subtropical regions due to the numbers of mosquitoes in those areas. Since 2000, outbreaks have been most common in Africa and Asia, with the largest outbreaks occurring from 2005 to 2006.1,2 Chikungunya has since become more widespread in the Indian Ocean islands, and in 2013, it was first identified in the Caribbean islands. As of March 2017, over one million cases have been reported in the Americas, according to the Pan American Health Organization.3 In 2014, 12 locally transmitted cases were reported in Florida.4
The most common mosquito vectors for chikungunya are the Aedes albopictus (Asian tiger mosquito) and Aedes aegypti mosquitoes. These mosquitoes also transmit dengue fever, yellow fever, and Zika virus. Both of these mosquitoes are well-adapted to urban areas and can breed in standing water. A aegypti mosquitoes are found only in the southern United States, while A albopictus mosquitoes can be found in more temperate climates—areas like New York, New Jersey, and Pennsylvania.4 Both species are daytime biters, so their activity peaks during the dawn and dusk periods. Because local mosquito vectors exist as far north as New York, local transmission and outbreaks are possible in many parts of the United States.
When to suspect chikungunya, and what to look for
Suspect chikungunya in patients returning from endemic areas. After a 3- to 7-day incubation period, the clinical presentation of chikungunya typically begins with fever and malaise, followed by polyarthralgia that starts 2 to 5 days after the onset of fever. Headache, nausea, and conjunctivitis may also occur. Polyarthralgia and arthritis usually present in symmetrical distal joints and are accompanied by face and trunk flushing that is followed by a maculopapular rash. The rash predominantly appears on the trunk and limbs, but can also occur on the face, palms, and soles. Tendons and ligaments—especially the Achilles tendon—may become inflamed, as well. Symptoms typically resolve within 2 to 3 weeks, although polyarthralgia may last for months or even years.
There is no known risk of transmission through breast milk or in utero, although vertical transmission through vaginal or cesarean birth is common in viremic women. Blood-borne transmission can occur in the laboratory, nosocomially, or through the transfusion of blood products if exposure occurs during the early viremic phase.1,2 Dual infections are possible (typically with yellow fever, malaria, Zika virus, or dengue fever) and should be considered based on the patient’s travel history.5 Abnormal laboratory findings are less common, but may include lymphopenia (most common), thrombocytopenia, elevations in blood urea nitrogen and creatinine (indicating an acute kidney injury), and elevated liver transaminases.
Chikungunya is generally considered a self-limiting disease, but severe atypical manifestations can lead to meningoencephalitis, respiratory failure, and even death. Severe disease is more commonly seen in infants, patients over age 65 years, and in those with chronic medical conditions.
The differential diagnosis for chikungunya virus includes dengue fever, Zika virus, malaria, measles, rubella, parvovirus, primary HIV infection, Lyme disease, and other inflammatory joint conditions. The differential depends on where a patient lives, their travel history, and exposures.
Dengue and chikungunya have similar features, which often make them difficult to distinguish. However, patients with dengue fever present more often with neutropenia, thrombocytopenia, and signs or symptoms of shock or hemorrhage.6 The chikungunya rash is typically a maculopapular rash on the trunk, hands, and feet that appears within the first 2 days of illness, as opposed to dengue, which has a similar rash, but appears later in the disease (Days 2-5). Avoid nonsteroidal anti-inflammatory drugs (NSAIDs) if dengue fever is suspected, as they can worsen hemorrhaging.7
Zika virus typically presents with milder symptoms compared to chikungunya. Patients may have a skin rash and occasionally, conjunctivitis, but limited high fevers or joint pain. Zika rash is maculopapular, but typically starts on the face on the first day of the illness. Zika has been associated with neurological complications such as Guillain-Barré syndrome and microcephaly in fetuses of infected pregnant woman.8
Choice of testing modality depends on when symptoms began
Laboratory diagnosis of chikungunya can be accomplished 3 ways: viral culture, reverse transcriptase-polymerase chain reaction (RT-PCR) viral RNA, and serology.1 The choice of which modality to use depends on the time between the onset of symptoms and the date on which a serum sample is drawn.
- If the patient presents within the first 3 days of illness, viral culture can detect chikungunya. Chikungunya virus testing is available through the Centers for Disease Control and Prevention (CDC), some state laboratories, and one commercial lab.8 Viral cultures are considered the gold standard for diagnosis, but a requirement for an elevated biosafety level, as well as a longer incubation time, make them less useful in the clinical setting.1,9
- If symptoms started less than 5 to 8 days prior, serum should be sent for RT-PCR for viral RNA.
- If symptoms occurred more than 8 days prior, serum should be sent for IgM and IgG serologic testing.
If the acute sample was negative (and chikungunya is still suspected), a second serum sample should be drawn 2 to 3 weeks later during the convalescent phase and sent for IgG serologic testing.
There are no specific treatments or vaccines available for chikungunya, but both live and inactivated vaccines are being tested. To prevent chikungunya virus infection, advise patients traveling to endemic areas to reduce mosquito exposure by avoiding outdoor activities during dusk and dawn, wearing long-sleeved shirts and long pants, and using an insect repellent that contains DEET. Recommend that patients who will be sleeping in a high-risk area use bed netting treated with permethrin. The CDC Web site has excellent additional information, available at http://www.cdc.gov/chikungunya.10
Our patient was briefly hospitalized due to intractable pain. She was discharged after 2 days and given prescriptions for motrin 800 mg and percocet 5/325 mg for breakthrough pain. Her wrist and ankle pain persisted after discharge, but slowly resolved after 2 to 3 months.
THE TAKEAWAY
Chikungunya is becoming more common among travelers returning to the United States from the Caribbean islands, and mosquito vectors found in parts of the United States are enhancing the possibility of local outbreaks. Travel to endemic regions—and the classic symptoms of fever, polyarthralgia, and a maculopapular ras
THE CASE
A 30-year-old woman presented to our emergency department with severe polyarthralgia, a high-grade fever (102.6º F), and a diffuse maculopapular rash on her trunk and extremities. She had returned from her honeymoon in Jamaica 6 days earlier. During her time there, she ate local cuisine, hiked in the jungle, and was bitten by many mosquitoes. The patient was nauseous, and had been experiencing headaches, generalized weakness, and fatigue. Her physical exam revealed a maculopapular rash on her trunk and upper extremities. She had tenderness and pain, as well as decreased range of motion in her ankles, knees, and wrists. The patient had no erythema, swelling, petechiae, or bruising. She had a past medical history of Graves’ disease and had received all of her childhood immunizations.
THE DIAGNOSIS
Our lab work-up included a complete blood count, liver function tests, blood pathogens for malaria, and serologic tests for dengue fever, measles, mumps, rubella, Lyme disease, human immunodeficiency virus (HIV), and parvovirus. We ruled out dengue fever because the patient had no evidence of hemorrhage, thrombocytopenia, rising hematocrit, or neutropenia. HIV antibody screening tests were performed, although, in retrospect, confirmatory HIV quantitative RNA testing should’ve been obtained because of the acute nature of the patient’s symptoms. Regardless, the work-up was negative, with the exception of a positive parvovirus immunoglobulin G (IgG).
Given our patient’s travel history, unremarkable lab results, and physical exam (notably the rash and joint pain), we suspected that she was infected with the chikungunya virus and tested for it. The results of chikungunya serum titers returned 13 days later and were positive for both immunoglobulin M (IgM) and IgG, confirming our suspicion.
DISCUSSION
Chikungunya is a viral infection that is most commonly transmitted to humans via mosquitoes. The infection was first identified in West Africa in the mid-1900s and predominantly occurs in tropical and subtropical regions due to the numbers of mosquitoes in those areas. Since 2000, outbreaks have been most common in Africa and Asia, with the largest outbreaks occurring from 2005 to 2006.1,2 Chikungunya has since become more widespread in the Indian Ocean islands, and in 2013, it was first identified in the Caribbean islands. As of March 2017, over one million cases have been reported in the Americas, according to the Pan American Health Organization.3 In 2014, 12 locally transmitted cases were reported in Florida.4
The most common mosquito vectors for chikungunya are the Aedes albopictus (Asian tiger mosquito) and Aedes aegypti mosquitoes. These mosquitoes also transmit dengue fever, yellow fever, and Zika virus. Both of these mosquitoes are well-adapted to urban areas and can breed in standing water. A aegypti mosquitoes are found only in the southern United States, while A albopictus mosquitoes can be found in more temperate climates—areas like New York, New Jersey, and Pennsylvania.4 Both species are daytime biters, so their activity peaks during the dawn and dusk periods. Because local mosquito vectors exist as far north as New York, local transmission and outbreaks are possible in many parts of the United States.
When to suspect chikungunya, and what to look for
Suspect chikungunya in patients returning from endemic areas. After a 3- to 7-day incubation period, the clinical presentation of chikungunya typically begins with fever and malaise, followed by polyarthralgia that starts 2 to 5 days after the onset of fever. Headache, nausea, and conjunctivitis may also occur. Polyarthralgia and arthritis usually present in symmetrical distal joints and are accompanied by face and trunk flushing that is followed by a maculopapular rash. The rash predominantly appears on the trunk and limbs, but can also occur on the face, palms, and soles. Tendons and ligaments—especially the Achilles tendon—may become inflamed, as well. Symptoms typically resolve within 2 to 3 weeks, although polyarthralgia may last for months or even years.
There is no known risk of transmission through breast milk or in utero, although vertical transmission through vaginal or cesarean birth is common in viremic women. Blood-borne transmission can occur in the laboratory, nosocomially, or through the transfusion of blood products if exposure occurs during the early viremic phase.1,2 Dual infections are possible (typically with yellow fever, malaria, Zika virus, or dengue fever) and should be considered based on the patient’s travel history.5 Abnormal laboratory findings are less common, but may include lymphopenia (most common), thrombocytopenia, elevations in blood urea nitrogen and creatinine (indicating an acute kidney injury), and elevated liver transaminases.
Chikungunya is generally considered a self-limiting disease, but severe atypical manifestations can lead to meningoencephalitis, respiratory failure, and even death. Severe disease is more commonly seen in infants, patients over age 65 years, and in those with chronic medical conditions.
The differential diagnosis for chikungunya virus includes dengue fever, Zika virus, malaria, measles, rubella, parvovirus, primary HIV infection, Lyme disease, and other inflammatory joint conditions. The differential depends on where a patient lives, their travel history, and exposures.
Dengue and chikungunya have similar features, which often make them difficult to distinguish. However, patients with dengue fever present more often with neutropenia, thrombocytopenia, and signs or symptoms of shock or hemorrhage.6 The chikungunya rash is typically a maculopapular rash on the trunk, hands, and feet that appears within the first 2 days of illness, as opposed to dengue, which has a similar rash, but appears later in the disease (Days 2-5). Avoid nonsteroidal anti-inflammatory drugs (NSAIDs) if dengue fever is suspected, as they can worsen hemorrhaging.7
Zika virus typically presents with milder symptoms compared to chikungunya. Patients may have a skin rash and occasionally, conjunctivitis, but limited high fevers or joint pain. Zika rash is maculopapular, but typically starts on the face on the first day of the illness. Zika has been associated with neurological complications such as Guillain-Barré syndrome and microcephaly in fetuses of infected pregnant woman.8
Choice of testing modality depends on when symptoms began
Laboratory diagnosis of chikungunya can be accomplished 3 ways: viral culture, reverse transcriptase-polymerase chain reaction (RT-PCR) viral RNA, and serology.1 The choice of which modality to use depends on the time between the onset of symptoms and the date on which a serum sample is drawn.
- If the patient presents within the first 3 days of illness, viral culture can detect chikungunya. Chikungunya virus testing is available through the Centers for Disease Control and Prevention (CDC), some state laboratories, and one commercial lab.8 Viral cultures are considered the gold standard for diagnosis, but a requirement for an elevated biosafety level, as well as a longer incubation time, make them less useful in the clinical setting.1,9
- If symptoms started less than 5 to 8 days prior, serum should be sent for RT-PCR for viral RNA.
- If symptoms occurred more than 8 days prior, serum should be sent for IgM and IgG serologic testing.
If the acute sample was negative (and chikungunya is still suspected), a second serum sample should be drawn 2 to 3 weeks later during the convalescent phase and sent for IgG serologic testing.
There are no specific treatments or vaccines available for chikungunya, but both live and inactivated vaccines are being tested. To prevent chikungunya virus infection, advise patients traveling to endemic areas to reduce mosquito exposure by avoiding outdoor activities during dusk and dawn, wearing long-sleeved shirts and long pants, and using an insect repellent that contains DEET. Recommend that patients who will be sleeping in a high-risk area use bed netting treated with permethrin. The CDC Web site has excellent additional information, available at http://www.cdc.gov/chikungunya.10
Our patient was briefly hospitalized due to intractable pain. She was discharged after 2 days and given prescriptions for motrin 800 mg and percocet 5/325 mg for breakthrough pain. Her wrist and ankle pain persisted after discharge, but slowly resolved after 2 to 3 months.
THE TAKEAWAY
Chikungunya is becoming more common among travelers returning to the United States from the Caribbean islands, and mosquito vectors found in parts of the United States are enhancing the possibility of local outbreaks. Travel to endemic regions—and the classic symptoms of fever, polyarthralgia, and a maculopapular ras
1. Pan American Health Organization. Preparedness and response for chikungunya virus: Introduction in the Americas. Washington, DC: Pan American Health Organization; 2011:1-149. Available at: http://www1.paho.org/hq/dmdocuments/CHIKV_English.pdf. Accessed March 8, 2017.
2. Enserink M. Infectious diseases. Chikungunya: no longer a third world disease. Science. 2007;318:1860-1861.
3. Pan American Health Organization. Number of reported cases of chikungunya fever in the Americas, by country or territory, 2017. Available at: http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&Itemid=270&gid=38489&lang=en. Accessed March 7, 2017.
4. Centers for Disease Control and Prevention. 2014 final data for the United States. Centers for Disease Control and Prevention Web site. Available at: https://www.cdc.gov/chikungunya/geo/united-states-2014.html. Accessed March 16, 2017.
5. Nayar SK, Noridah O, Paranthaman V, et al. Co-infection of dengue virus and chikungunya virus in two patients with acute febrile illness. Med J Malaysia. 2007;62:335-336.
6. Staples JE, Fischer M. Chikungunya virus in the Americas—what a vectorborne pathogen can do. N Engl J Med. 2014;371:887-889.
7. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva: World Health Organization; 2009. Available at: http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf?ua=1. Accessed March 8, 2017.
8. Centers for Disease Control and Prevention. Chikungunya information for healthcare providers. 2014. Available at: https://www.cdc.gov/chikungunya/pdfs/chikv_clinicians.pdf. Accessed March 8, 2017.
9. Parida MM, Santhosh SR, Dash PK, et al. Rapid and real-time assays for detection and quantification of chikungunya virus. Future Virology. 2008;3:179-192.
10. Centers for Disease Control and Prevention. Chikungunya virus. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/chikungunya. Accessed February 26, 2015.
1. Pan American Health Organization. Preparedness and response for chikungunya virus: Introduction in the Americas. Washington, DC: Pan American Health Organization; 2011:1-149. Available at: http://www1.paho.org/hq/dmdocuments/CHIKV_English.pdf. Accessed March 8, 2017.
2. Enserink M. Infectious diseases. Chikungunya: no longer a third world disease. Science. 2007;318:1860-1861.
3. Pan American Health Organization. Number of reported cases of chikungunya fever in the Americas, by country or territory, 2017. Available at: http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&Itemid=270&gid=38489&lang=en. Accessed March 7, 2017.
4. Centers for Disease Control and Prevention. 2014 final data for the United States. Centers for Disease Control and Prevention Web site. Available at: https://www.cdc.gov/chikungunya/geo/united-states-2014.html. Accessed March 16, 2017.
5. Nayar SK, Noridah O, Paranthaman V, et al. Co-infection of dengue virus and chikungunya virus in two patients with acute febrile illness. Med J Malaysia. 2007;62:335-336.
6. Staples JE, Fischer M. Chikungunya virus in the Americas—what a vectorborne pathogen can do. N Engl J Med. 2014;371:887-889.
7. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva: World Health Organization; 2009. Available at: http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf?ua=1. Accessed March 8, 2017.
8. Centers for Disease Control and Prevention. Chikungunya information for healthcare providers. 2014. Available at: https://www.cdc.gov/chikungunya/pdfs/chikv_clinicians.pdf. Accessed March 8, 2017.
9. Parida MM, Santhosh SR, Dash PK, et al. Rapid and real-time assays for detection and quantification of chikungunya virus. Future Virology. 2008;3:179-192.
10. Centers for Disease Control and Prevention. Chikungunya virus. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/chikungunya. Accessed February 26, 2015.